Abstract
Background
Bauerane is a triterpenoid derived from the dandelion root (Taraxacum officinale). This study aimed to investigate the effects of bauerane on cell proliferation of A549 human lung cancer cells and the molecular mechanisms involved.
Material/Methods
A549 human lung adenocarcinoma cells and normal MRC-5 lung fibroblasts were grown in culture and treated with increasing doses of bauerane at 0, 2.5, 5, 10, 20, 40, 80, and 160 μM. The MTT assay was used to measure cell proliferation. Cell apoptosis was assessed by 4′, 6-diamidino-2-phenylindole (DAPI), and acridine orange/ethidium bromide (AO/EB) staining. The cell cycle was evaluated by flow cytometry. Western blot measured the protein expression levels of cytochrome c, Bax, cyclin B1, Bcl-2, PI3K, p-PI3K, Akt, p-Akt, and STAT3 proteins.
Results
Bauerane inhibited the proliferation of A549 lung cancer cells in a dose-dependent manner, with an IC50 of 10 μM, with no cytotoxicity for MRC-5 cells. Bauerane treatment induced apoptosis of A549 cells, which was associated with the upregulation of Bax and down-regulation of Bcl-2. Bauerane induced S-phase arrest of A549 cells, which was dose-dependent and associated with reduced expression of cyclin B1. The findings from Western blot showed that bauerane inhibited the phosphorylation of PI3K/AKT and STAT3 signaling pathways.
Conclusions
Bauerane inhibited the proliferation of A549 lung cancer cells in vitro and induced cell apoptosis and cell cycle arrest in a dose-dependent manner.
MeSH Keywords: Apoptosis, Cell Cycle Checkpoints, Lung Neoplasms
Background
Plants synthesize many natural compounds in response to changing environmental conditions [1,2]. These natural compounds are the products of primary and secondary metabolism and are termed primary and secondary metabolites [3]. Primary metabolic pathways in plants are highly conserved and include the generalized pathways of photosynthesis and gas exchange. However, secondary metabolites are more specific to plant species [4]. Secondary plant metabolites include the terpenoids, which are a dominant class of plant secondary metabolites [5]. Terpenoids help the plants to interact with the environment [6]. Also, plant terpenoids have been used in herbal medicines and traditional Chinese medicine [7,8]. Plant terpenoids that are currently used as antimicrobial agents include artimisinin, derived from Artimisia annua, which is used in the treatment of malaria [9,10].
Plant terpenoids have been studied for their effects on human cancer cells [11]. Taxol is a terpenoid derived from the bark of Taxus brevifolia, or the Pacific yew tree, and is the active constituent of the anticancer drug, paclitaxel [12]. Bauerane is a triterpenoid derived from the dandelion root (Taraxacum officinale). There have been few studies that have reported the anticancer effects of bauerane and other related compounds [13]. However, the antifungal activity of bauerane has been reported [14]. There have been no previous studies on the effects of bauerane on human lung cancer cells.
Worldwide, lung cancer results in a high morbidity and mortality rate. The incidence of lung cancer is higher in developing countries, and the mortality rate of lung cancer is higher in men than women [15]. Despite recent advances in the treatment of lung cancer, the overall 5-year survival rate for lung cancer patients is only 19% [16]. Continued studies are required to identify diagnostic, prognostic, and therapeutic targets in human lung cancer. Therefore, this study aimed to investigate the effects of bauerane on cell proliferation of A549 human lung cancer cells and the molecular mechanisms involved.
Material and Methods
Cell culture
A549 human lung adenocarcinoma cells and normal MRC-5 lung fibroblasts were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific) containing fetal bovine serum (FBS) (10%) in a humidified incubator at 37°C with 5% CO2.
MTT cell viability assay
A549 cells and the normal MRC-5 cells were cultured at a density of approximately 3×103 cells per well in 96-well plates for 24 h with increasing concentrations of bauerane (0, 2.5, 5, 10, 20, 40, 80, and 160 μM). MTT solution (20 μL) was added, and the cells were cultured for a further 4 h. The formazan crystals that formed from MTT were dissolved in dimethyl sulfoxide (DMSO). Optical density measurements were performed at 570 nm using a microplate reader.
Apoptosis assays
The 4′, 6-diamidino-2-phenylindole (DAPI) cell nuclear DNA stain (Thermofisher Scientific, Waltham, MA, USA) was used to examine the levels of cell apoptosis after treating the A549 lung cancer cells with 0, 2.5, 5, 10, 20, 40, 80, and 160 μM of bauerane for 24 h. The A549 cells were placed into the wells of a six-well plate at a density of 2×105 cells per well. The cell pellets were obtained by centrifugation at 600 rpm for 5 min. The cell pellets were washed three times with phosphate-buffered saline (PBS). The cells were fixed with 70% ethanol and then stained with DAPI. Nuclear morphology was examined using a fluorescence microscope. A similar method was used for acridine orange/ethidium bromide (AO/EB) staining.
Flow cytometry analysis of the cell cycle
The A549 cells were treated with 0, 5, 10, and 20 μM of bauerane and then seeded into six-well plates for 24 h. The cells were centrifuged, and the cell pellets were washed in PBS. The pellets were fixed in 70% alcohol, and propidium iodide (PI) was used to stain the cells. The stained cells were examined by flow cytometry to determine the cells in different phases of the cell cycle.
Western blot
The A549 cells treated with bauerane were lysed, and the protein extracts were prepared. Cells were centrifuged at 12000×g for 10 min. The protein concentration of each extract was assessed using the bicinchoninic acid (BCA) assay. Each sample was incubated for 10 min at 99°C. Then, 30 μg of protein from each sample underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and were loaded onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with blocking buffer for 55 min at 22°C, followed by incubation with specific primary antibodies overnight at 4°C and then with secondary antibody. The primary antibodies were to cytochrome c, Bax, cyclin B1, Bcl-2, PI3K, p-PI3K, Akt, p-Akt, and STAT3. The Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA) was used to detect the protein bands of interest.
Statistical analysis
All experiments were performed in triplicate. Data were presented as the mean±standard deviation (SD). Student’s t-test was used for comparison between two samples. One-way analysis of variance (ANOVA) and Tukey’s post hoc test were used to compare multiple samples. GraphPad Prism version 7 software was used (GraphPad Software, La Jolla, CA, USA). A P-value <0.05 was considered to be statistically significant.
Results
Proliferation of A549 human lung cancer cells was inhibited by bauerane
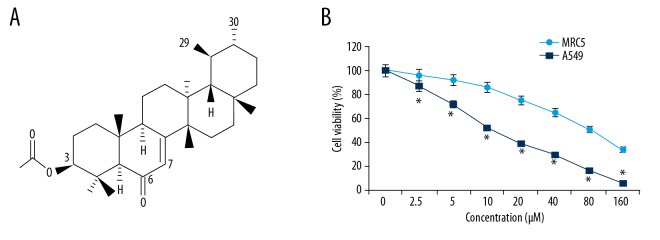
In this study, the effects of bauerane, a terpenoid that is a secondary plant metabolite, was studied (Figure 1A). The MRC-5 normal lung fibroblasts and A549 human adenocarcinoma cells were treated with 0, 2.5, 5, 10, 20, 40, 80, and 160 μM of bauerane. The proliferation curve plotted from the absorbance values of the MTT assay, showed a greater decline in cell growth of A549 cells with increasing doses of bauerane, but with little effect on MRC-5 cells (Figure 1B). The half-maximal inhibitory concentration (IC50) concentration of bauerane was 10 μM for the A549 cells. However, the IC50 of bauerane for the normal MRC-5 cells was 80 μM, which indicated cancer cell-specific for the activity of bauerane (Figure 1B).
Figure 1.
Bauerane treatment resulted in dose-dependent inhibition of A549 cell proliferation. (A) The chemical structure of bauerane. (B) The percentage of viable A549 cells following treatment with increasing concentrations of bauerane. A549 cells were treated with 0, 5, 10, and 20 μM of bauerane. The experiments were performed in triplicate. The results are expressed as the mean±SD (* P<0.05)
Bauerane induced apoptosis in A549 cells
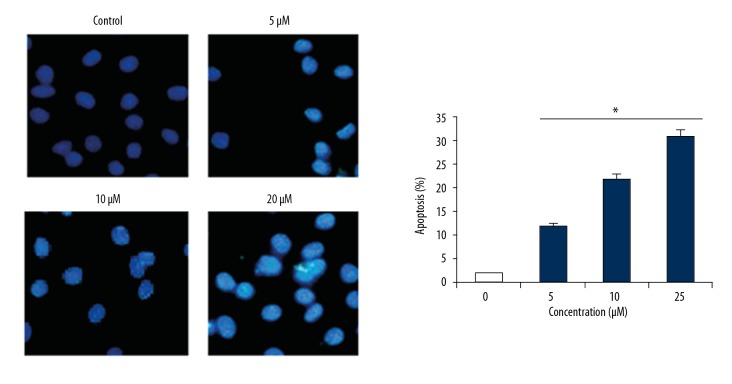
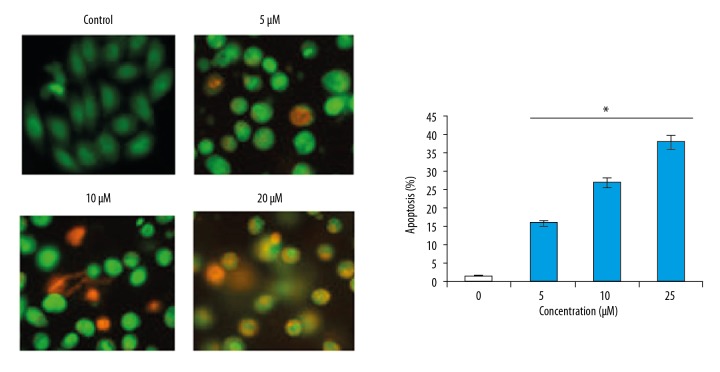
The A549 cells treated with 0, 5, 10, or 20 μM of bauerane were assessed using 4′, 6-diamidino-2-phenylindole (DAPI), and acridine orange/ethidium bromide (AO/EB) staining methods. The cell nuclear morphology was analyzed by fluorescence microscopy. The DAPI stained cells showed significant nuclear changes with higher concentrations of bauerane (Figure 2). The apoptosis rates were 2%, 12%, 22%, and 31% at 0, 5, 10, and 20 μM concentrations of bauerane. Flow cytometry showed cell apoptosis and nuclear changes (Figure 3). Apoptosis increased with increased doses of bauerane. To further confirm the role of bauerane in inducing apoptosis, the Western blot study was performed. Cytochrome c protein, which is an activator of caspase-9, was upregulated when cancer cells were treated with bauerane, and the Bax protein concentration increased with increasing concentrations of bauerane. The apoptosis protein, Bcl-2 decreased when cancer cells were treated with bauerane (Figure 4). These results support the role of bauerane in inducing apoptosis in A549 lung cancer cells in vitro.
Figure 2.
Bauerane treatment resulted in changes in the morphology of the cell nuclei of A549 cells. The 4′, 6-diamidino-2-phenylindole (DAPI) staining of A549 cancer cells. A549 cells were treated with 0, 5, 10, and 20 μM of bauerane. The experiments were performed in triplicate. The results are expressed as the mean±SD (* P<0.05).
Figure 3.
Bauerane induces the apoptosis in A549 cells. Dual acridine orange/ethidium bromide (AO/EB) staining of A549 cells. A549 cells were treated with 0, 5, 10, and 20 μM of bauerane. The experiments were performed in triplicate. The results are expressed as the mean±SD (* P<0.05)
Figure 4.
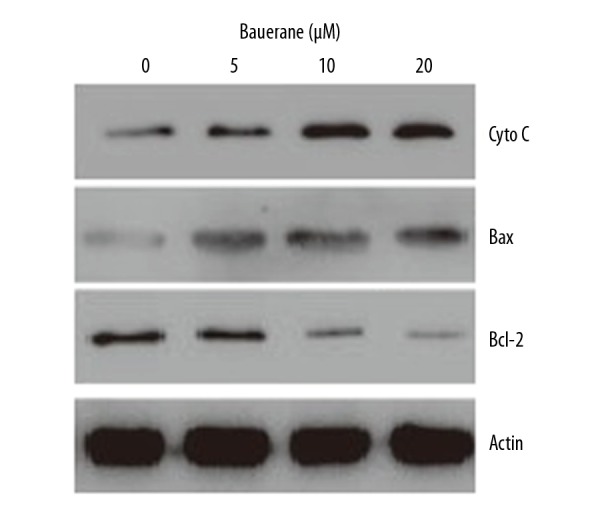
Bauerane induced apoptosis in A549 cells by upregulating cytochrome c and increasing the Bax/Bcl-2 ratio. Western blot findings for cytochrome c, Bax, and Bcl-2 proteins, with human actin as the normal control. A549 cells were treated with 0, 5, 10, and 20 μM of bauerane. The experiments were performed in triplicate.
Bauerane induced S-phase cell cycle arrest in A549 cells
The flow cytometric study of bauerane administered (0, 5, 10, and 20 μM) lung cancer cells showed that the percentage of cells in the S-phase increased significantly with bauerane treatment (Figure 5). The percentage of cells in the S-phase was 20%, 40%, 55%, and 72% with bauerane treatment at concentrations of 0, 5, 10, and 20 μM, respectively. Cyclin B1 protein levels were significantly reduced with bauerane administration, indicative of the cell cycle arrest in lung cancer cells (Figure 6). Bauerane promotes the S-phase cell cycle arrest in A549 lung cancer cells by reducing the protein concentrations of cyclin B1.
Figure 5.
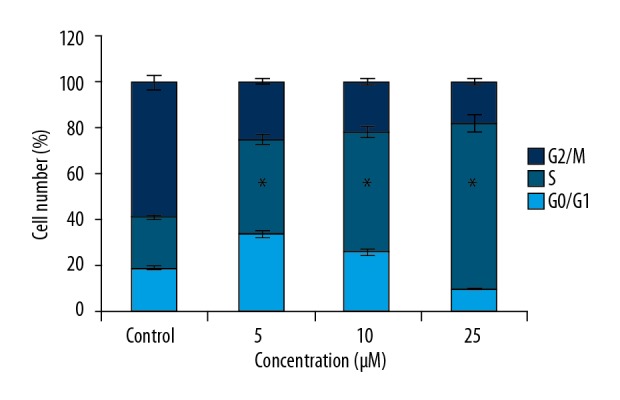
Bauerane arrested A549 cells in the S-phase of the cell cycle. Flow cytometry of the cell cycle distribution in A549 cells. The experiments were performed in triplicate.
Figure 6.
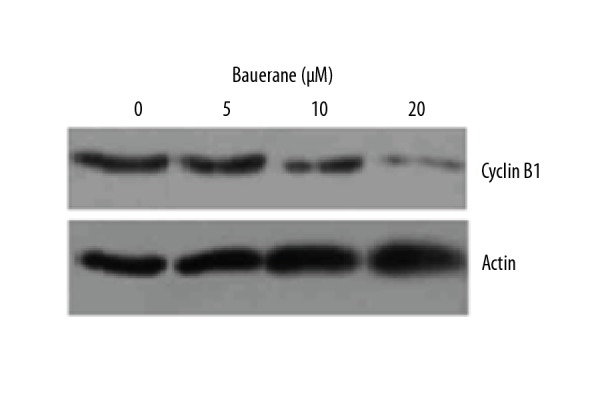
Bauerane down-regulated cyclin B1 expression in A549 cells. Western blot of cyclin B1 protein, with human actin as the normal control. A549 cells were treated with 0, 5, 10, and 20 μM of bauerane. The experiments were performed in triplicate.
Bauerane exerted its effects on A549 cells via PI3K/AKT and STAT3 signaling pathways
To understand the mechanism of action of bauerane to restrict the proliferation and to promote the cell cycle arrest in lung cancer cells, A549 cancer cells were treated with 0, 5, 10 and 20 μM bauerane and studied for PI3K/AKT and STAT3 signaling pathways which positively regulate the proliferation and cell cycle progression of human cells. The counts of phosphorylated phosphoinositide 3-kinase (p-PI3K) and protein kinase B (p-Akt), active transducers of PI3/AKT signaling pathway, were seen to decrease in a dose-dependent manner (Figure 7). However, there was no effect on the protein concentrations of non-phosphorylated PI3K and Akt indicating that Bauerane negatively regulates the PI3K/AKT signaling pathway by inhibiting the phosphorylation of PI3K and Akt proteins. The signal transducer and activator of transcription 3 (STAT3) transcription factor was also down-regulated when cancer cells were treated with bauerane (Figure 8). These results showed that the effects of bauerane on A549 lung cancer cells in vitro were through the down-regulation of the PI3K/AKT and STAT3 cellular signaling pathways.
Figure 7.
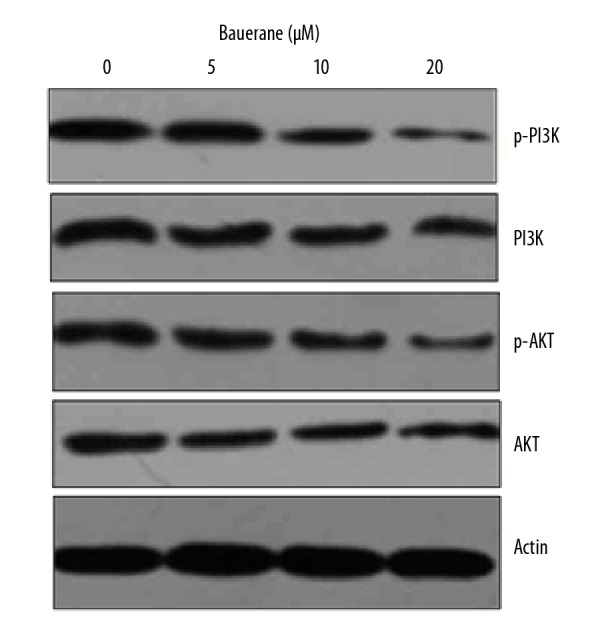
Bauerane inhibited the PI3K/AKT signaling pathway in A549 cells. Western blot of PI3K, p-PI3K, Akt and p-Akt proteins, with human actin as the normal control. A549 cells were treated with 0, 5, 10, and 20 μM of bauerane. The experiments were performed in triplicate.
Figure 8.
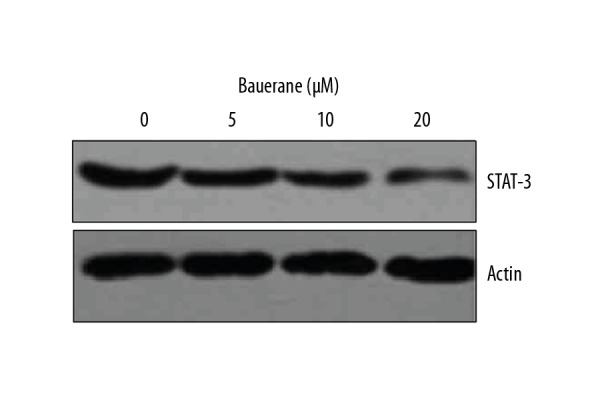
Bauerane inhibited the STAT3 signaling pathway in A549 cells. Western blot of STAT3 protein, with human actin as the normal control. A549 cells were treated with 0, 5, 10, and 20 μM of bauerane. The experiments were performed in triplicate.
Discussion
Human lung cancer is highly aggressive and has a high mortality rate [15,16]. Despite the recent advances in cancer treatment strategies, mortality and morbidity resulting from lung cancer are still very high [17]. Scientists have focused on the discovery of more potent anticancer drugs in recent years. For this purpose, the secondary plant metabolites have been studied for their effects against human cancers [18]. Plant phenolic compounds have been screened for their anticancer effects, and several studies have shown that these compounds inhibit the growth and proliferation of human cancers [19–21].
The present study aimed to investigate the effects of bauerane on cell proliferation of A549 human lung cancer cells and the molecular mechanisms involved. Anti-proliferative effects were exerted through the induction of apoptosis in A549 cells, and these effects were dependent on increasing concentrations of cytochrome c protein. Cytochrome c induces the expression of the caspase-9 apoptotic protein [22]. Also, the increase in Bax/Bcl-2 apoptosis-associated proteins occurred when A549 cells were treated with bauerane. An increase in the Bax/Bcl-2 ratio indicated cell apoptosis [23]. Several natural compounds have previously been shown to cause cell cycle arrest to exert their anti-proliferative effects [24,25]. in the present study, bauerane arrested A549 cells in the S-phase, as the mitotic regulating protein, cyclin B1 was down-regulated following treatment with bauerane. The PI3K/AKT signaling pathway was down-regulated in A549 cells following bauerane treatment, as was the STAT 3 transcription factor.
The PI3K/AKT and STAT3 pathways have previously been shown to have roles in the proliferation and metastasis of human cancers [27]. These pathways are dysregulated in human cancer, which raises the possibility that these signaling pathway components have a potential role as therapeutic targets for the treatment of human cancer. Both the PI3K/AKT and STAT3 pathways are modulators of cell growth and proliferation [26,27]. In this in vitro study of the A549 human lung adenocarcinoma cell line, bauerane negatively regulated cell proliferation by down-regulating the PI3K/AKT and STAT 3 signaling pathways.
Conclusions
This study aimed to investigate the effects of bauerane on cell proliferation of A549 human lung cancer cells and the molecular mechanisms involved. Bauerane inhibited the growth of A549 human lung cancer cells without toxicity for normal MRC-5 human lung fibroblasts. The effects of bauerane on cell proliferation and apoptosis of A549 cells was dose-dependent. Bauerane was associated with cell cycle arrest of A549 cells in the S-phase. These effects were shown to act through the P13K/AKT and STAT3 signaling pathways. The findings from this preliminary in vitro study indicate that the potential anticancer effects of bauerane require further study.
Footnotes
Source of support: Departmental sources
References
- 1.Olson ME. Plant evolutionary ecology in the age of the extended evolutionary synthesis. Integr Comp Biol. 2019;59(3):493–502. doi: 10.1093/icb/icz042. [DOI] [PubMed] [Google Scholar]
- 2.Uddin M. Environmental factors on secondary metabolism of medicinal plants. Acta Scientific Pharmaceutical Science. 2019;3:34–46. [Google Scholar]
- 3.Delfin JC, Watanabe M, Tohge T. Understanding the function and regulation of plant secondary metabolism through metabolomics approaches. Theoretical and Experimental Plant Physiol. 2019;31(1):127–38. [Google Scholar]
- 4.Seigler DS. Plant secondary metabolism. Springer Science & Business Media Berlin; Germany: 2012. [Google Scholar]
- 5.Moore BD, Andrew RL, Külheim C, Foley WJ. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014;201(3):733–50. doi: 10.1111/nph.12526. [DOI] [PubMed] [Google Scholar]
- 6.Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol. 2009;5(5):283–91. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- 7.Wagner KH, Elmadfa I. Biological relevance of terpenoids. Ann Nutr Metabol. 2003;47:95–106. doi: 10.1159/000070030. [DOI] [PubMed] [Google Scholar]
- 8.Paduch R, Kandefer-Szerszeń M, Trytek M, Fiedurek J. Terpenes: Substances useful in human healthcare. Arch Immunol Ther Exp. 2007;55(5):315–27. doi: 10.1007/s00005-007-0039-1. [DOI] [PubMed] [Google Scholar]
- 9.Dorman HJ, Deans SG. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J App Microbiol. 2000;88(2):308–16. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 10.Douglas NM, Anstey NM, Angus BJ, et al. Artemisinin combination therapy for vivax malaria. Lancet Infect Dis. 2010;10(6):405–16. doi: 10.1016/S1473-3099(10)70079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang M, Lu JJ, Huang MQ, et al. Terpenoids: Natural products for cancer therapy. Expert Opin Investig Drugs. 2012;21(12):1801–18. doi: 10.1517/13543784.2012.727395. [DOI] [PubMed] [Google Scholar]
- 12.Jennewein S, Croteau R. Taxol: Biosynthesis, molecular genetics, and biotechnological applications. Appl Microbiol Biotechnol. 2001;57(1–2):13–19. doi: 10.1007/s002530100757. [DOI] [PubMed] [Google Scholar]
- 13.Rascon-Valenzuela LA, Velazquez-Contreras C, Garibay-Escobar A, Robles-Zepeda RE. Triterpenoids: Synthesis, use in cancer treatment and other biological activities. Vol. 106. Nova Science; Pulishers, New York: 2017. [Google Scholar]
- 14.Jiang ZD, inventor. Millennium Pharmaceuticals Inc. Assignee, Assignee. Antifungal agents. US 5,917,084. United States Patent. 1999 Jun 29;
- 15.Didkowska J, Wojciechowska U, Mańczuk M, Łobaszewski J. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med. 2016;4(8):150. doi: 10.21037/atm.2016.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams MD, Sandler AB. Thoracic Oncology. Springer; Boston, MA, USA: 2001. The epidemiology of lung cancer; pp. 31–52. [DOI] [PubMed] [Google Scholar]
- 17.Tarro G, Paolini M, Rossi A. Mass spectrometry – future perceptions and applications. IntechOpen; London, UK: 2019. Molecular biology of lung cancer and future perspectives for screening; pp. 1–16. [Google Scholar]
- 18.Singh V, Kumar D, Chowdhary S, et al. Bioactive natural products for the management of cancer: From bench to bedside. Springer; Singapore: 2019. Mechanistic insight into cancer aetiology and therapeutic management by natural metabolites; pp. 61–70. [Google Scholar]
- 19.Essafi Rhouma H, Trabelsi N, Chimento A, et al. Olea europaea L. Flowers as a new promising anticancer natural product: phenolic composition, antiproliferative activity and apoptosis induction. Nat Prod Res. 2019;5:1–4. doi: 10.1080/14786419.2019.1637867. [DOI] [PubMed] [Google Scholar]
- 20.Karadas O, Mese G, Ozcivici E. Cytotoxic tolerance of healthy and cancerous bone cells to anti-microbial phenolic compounds depend on culture conditions. App Biochem Biotechnol. 2019;188(2):514–26. doi: 10.1007/s12010-018-02934-7. [DOI] [PubMed] [Google Scholar]
- 21.Rao S, Chinkwo K, Santhakumar A, et al. Apoptosis induction pathway in human colorectal cancer cell line SW480 exposed to cereal phenolic extracts. Molecules. 2019;24(13):2465. doi: 10.3390/molecules24132465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 23.Raisova M, Hossini AM, Eberle J, et al. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Investig Dermatol. 2001;117(2):333–40. doi: 10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- 24.Herman-Antosiewicz A, Singh SV. Signal transduction pathways leading to cell cycle arrest and apoptosis induction in cancer cells by Allium vegetable-derived organosulfur compounds: A review. Mutat Res. 2004;555(1–2):121–31. doi: 10.1016/j.mrfmmm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Gamet-Payrastre L, Li P, Lumeau S, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60(5):1426–33. [PubMed] [Google Scholar]
- 26.Srivastava S, Somasagara RR, Hegde M, et al. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. 2016;6:24049. doi: 10.1038/srep24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vara JÁ, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Sun L, Ma K, Wang H, et al. JAK1-STAT1-STAT3, a key pathway promoting proliferation and preventing premature differentiation of myoblasts. J Cell Biol. 2007;179(1):129–38. doi: 10.1083/jcb.200703184. [DOI] [PMC free article] [PubMed] [Google Scholar]