Abstract
Apoptosis and autophagy play seminal roles in maintaining organ homeostasis. Apoptosis represents canonical type I programmed cell death. Autophagy is viewed as pro-survival, however, excessive autophagy can promote type II cell death. Defective regulation of these two obligatory cellular pathways is linked to various diseases, including cancer. Biologic or chemotherapeutic agents, which can reprogram cancer cells to undergo apoptosis-or toxic autophagy-mediated cell death, are considered effective tools for treating cancer. Melanoma differentiation associated gene-7 (mda-7) selectively promotes these effects in cancer cells. mda-7 was identified more than two decades ago by subtraction hybridization showing elevated expression during induction of terminal differentiation of metastatic melanoma cells following treatment with recombinant fibroblast interferon and mezerein (a PKC activating agent). MDA-7 was classified as a member of the IL-10 gene family based on its chromosomal location, and the presence of an IL-10 signature motif and a secretory sequence, and re-named interleukin-24 (MDA-7/IL-24). Multiple studies have established MDA-7/IL-24 as a potent anti-cancer agent, which when administered at supra-physiological levels induces growth arrest and cell death through apoptosis and toxic autophagy in a wide variety of tumor cell types, but not in corresponding normal/non-transformed cells. Furthermore, in a phase I/II clinical trial, MDA-7/IL-24 administered by means of a non-replicating adenovirus was well tolerated and displayed significant clinical activity in patients with multiple advanced cancers. This review examines our current comprehension of the role of MDA-7/IL-24 in mediating cancer-specific cell death via apoptosis and toxic autophagy.
Keywords: MDA-7/IL-24, Cytokine, Apoptosis, Toxic autophagy
1. Introduction
Cancer is a leading cause of morbidity and mortality worldwide [1]. This is attributed largely to diagnosis at a late stage, the aggressive nature of the disease, and development of resistance to therapeutics instigating recurrence [2,3]. Nevertheless, considerable progress has been made in developing anti-cancer therapies in the last few decades, which help to promote long-term remission for specific cancers, an increase in longevity and a decrease in disease-associated morbidity. Induction of cell death in cancer cells remains a focus of many cancer therapeutic approaches [4–6]. Approaches used to accomplish this goal include introduction into cancer cells of apoptosis-inducing and/or toxic autophagy-promoting genes delivered using viruses or induction in cancer cells by small molecule drugs.
In 1993, the Fisher laboratory initially discovered and cloned MDA-7/IL-24 using subtraction hybridization from terminally differentiating human metastatic melanoma cells (HO-1) [7]. Specifically, terminal differentiation was induced in melanoma cells by treatment with a combination of recombinant human fibroblast interferon (IFN-beta) and mezerein (a protein kinase C (PKC) activating agent) and subtraction hybridization was employed to identify genes that were differentially expressed between control and treated groups [7,8]. One gene named melanoma differentiation associated gene-7 (mda-7) was identified as an upregulated transcript following induction of terminal differentiation [7,8]. Based on its chromosomal location (chromosome 1 along with IL-10, IL-19 and IL-20), presence of an IL-10 signature sequence and cytokine-like properties, mda-7 was later designated as Interleukin-24 (IL-24) by the Human Gene Organization [9]. Subsequent extensive studies from several different research group around the world confirmed that exogenous expression of MDA-7/IL-24 either by plasmid transfection, adenovirus-mediated delivery or exposure to a purified protein functions as a tumor suppressor in a wide spectrum of human and rodent cancers [10–26]. The tumor suppressive functions of MDA-7/IL-24 are multimodal, involving: induction of apoptosis with toxic autophagy; an inhibition of migration, invasion, angiogenesis and eventually metastasis; induction of differentiation and/or killing of cancer stem cells; sensitization of cancer cells to radiation, chemotherapy, antibody therapy, immunotherapy; and induction of an anti-tumor immune modulating effect [9,13,19,20,22–24,26]. Additionally, MDA-7/IL-24 induces “bystander activity” which enables it to destroy not only the primary tumor but also distant metastases [27,28]. What makes this therapeutic cytokine of significant therapeutic interest is its’ cancer-selective cell death-inducing properties with apparently no harmful toxicities toward normal cells or tissues, which was evident in a diverse spectrum of in vitro studies and in pre-clinical animal models in vivo (summarized in [13,22,23]). Based on its’ selective and profound anticancer activity in pre-clinical studies, MDA-7/IL-24 rapidly translated (within 5 years) from an experimental tool to a potential therapeutic that was evaluated in patients with diverse cancers. A phase I/II clinical trial was conducted on a cohort of patients with melanomas and other advanced cancers that revealed that administration of MDA-7/IL-24 with a recombinant adenovirus (Ad.mda-7 (INGN-241)) was well-tolerated and had appreciable clinical activities in over 40% of patients, which strongly supported its potential application as a therapeutic cytokine for treating human cancers [17,19,29,30].
In this chapter, we will review the recent insights on apoptosis and autophagy as well as the multifaceted relationship between MDA-7/IL-24, apoptosis, and toxic autophagy in cancer.
2. MDA-7/IL-24: structure and function
2.1. Genetic and protein structure of MDA-7/IL-24 (depicted in fig. 1)
Fig. 1. Schematic representation of MDA-7/IL-24 protein with predicted and established domains and protein modification sites indicated.
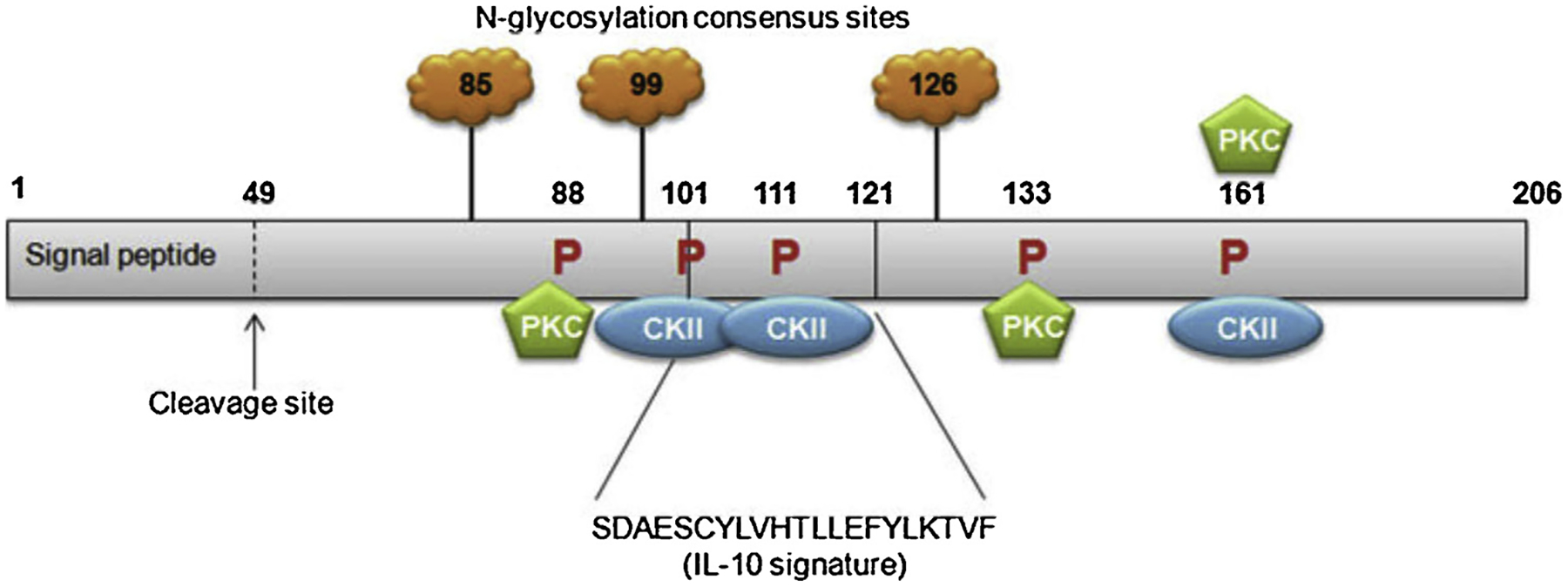
The IL-10 signature sequence is located between amino acid 101 and 121. N-glycosylation can occur at amino acid 85, 99 and 126. Protein kinase C consensus phosphorylation sites are present at amino acid 88, 133 and 161. Casein kinase II (CKII) consensus phosphorylation sites are present at amino acid 101, 111 and 161. Numbers indicate amino acids. Not drawn to scale. Cleavage of the 49-amino acid signal peptide allows for secretion of the MDA-7/IL-24 protein. (Figure reproduced from Menezes et al., 2014).
MDA-7/IL-24 is a secreted protein that is highly conserved throughout evolution with sequence homology between species including yeast, dog, cat, monkey and cow [9,17,24,25,31,32]. mda-7/IL-24 is located on chromosome 1q32–33 in humans along with several other IL-10 cytokine family gene members [31]. mda-7/IL-24 encompasses seven exons and six introns. The cDNA of mda-7/IL-24 is 1,718 base pairs in length and encodes a protein of 206 amino acid with a predicted molecular size of ~24kDa [8,10,24,31]. As previously mentioned mda-7/IL-24 contains an IL-10 signature motif at amino acids 101–121 shared by other IL-10 family member cytokines. Sequence analysis identified a 49-amino acid signal peptide located at the N-terminal of this protein, which allows the molecule to be cleaved and secreted. Three putative N-glycosylation sites [33] at amino acids 85, 99 and 126 were identified while analyzing the peptide sequence using the Prosite database [25]. Additionally, three protein kinase C consensus phosphorylation sites at amino acid 88, 133 and 161 and three casein kinase II consensus phosphorylation sites at amino acid 101, 111 and 161 were identified using this database [25]. MDA-7/IL-24 possibly can form functionally active dimers due to the presence of potential disulfide bonds [34].
Several research groups identified and reported a number of splice variants of MDA-7/IL-24 lacking one or more exons. In 2004, Allen et al. first described a splice variant of MDA-7/IL-24, lacking exons 3 and 5 (mda-7s) [35]. They found that mda-7s was expressed in melanocytes, transformed melanocytes and normal nevi but not in metastatic melanoma. Interestingly, mda-7s can heterodimerize with full length MDA-7/IL-24, without affecting apoptosis-inducing capacities. The same research group identified two other splice variants of mda-7/IL-24 expressed in normal human melanocytes, but not in metastatic melanoma that lacked either exon 3 or exon 5, respectively [36]. Another study described a splice variant of the mouse analog for mda-7/IL-24 (FISP) that lacks 29 nucleotides from the 5’ end of exon 4. This splice variant (FISP-sp) was reported to dimerize with full length FISP and inhibit secretion and apoptotic functions [37]. Filippov et al. compared splicing variants in apoptosis-related genes using splicing microarray technology [38]. Three genes were identified that are sensitive to SRp55 (an ubiquitous splicing factor) activity and mda-7/IL-24 was one of them. Four distinct isoforms of mda-7/IL-24 genes were identified and one isoform that lacks exons 2 and 3 was sensitive to SRp55 activity [38]. In a subsequent study, Whitaker et al. identified and characterized functional properties of five mRNA splice isoforms of mda-7/IL-24, Specifically they found one of the shortest isoforms, which lacks exons 2, 3 and 5 (mda-7/IL-24δ 2,3,5) is the most efficient apoptotic inducer in U2OS osteosarcoma cells being even superior than the full-length variant [39]. Interestingly, none of the splice variants had any effect on the noncancerous NOK cell line. In another study, Yang et al. found that both full length mda-7/IL-24 and an alternatively spliced isoform lacking exon 5 (IL-24 delE5) significantly induced terminal differentiation in a panel of myeloid leukemia cells and blast cells from patients with acute myeloid leukemia-M5, without deleterious effects in normal hematopoietic progenitor cells [40].
Using MDA-7/IL-24 affinity-tagged to the secreted human placental alkaline phosphatase (IL-24-AP) Wang and colleagues showed that MDA-7/IL-24 can interact with two hetero-dimeric receptor complexes including type 1 (IL-20R1/IL-20R2) or type 2 (IL-22R1/IL-20R2) and activate downstream signaling [9,25,33,41]. Two recent studies suggest the existence of a 3rd heterodimeric receptors pair (IL-20R1/IL22-R1) by which MDA-7/IL-24 can exert its cancer selective growth suppressive function [42,43].
Gopalan et al. showed that MDA-7/IL-24 protein gets ubiquitinated and degraded by the 26S proteasome [44]. MDA-7/IL-24 contains 10 lysine sites and to determine the exact site of ubiquitination, Tian and colleagues mutated each of the 10 lysine sites within the protein and converted them to arginine [45]. Their study reveals that lysine 123 is important for ubiquitination of MDA-7/IL-24. They further showed that the mutation of lysine 123 to arginine led to enhanced stability of MDA-7/IL-24 protein and its anti-cancer function [45].
2.2. Physiological functions of MDA-7/IL-24
The tumor suppressive role of MDA-7/IL-24 in cancer has been studied extensively, however, limited information is available about the physiological role of MDA-7/IL-24. Hematopoietic cells such as peripheral blood mononuclear cells (PBMC), T and B lymphocytes, and monocytes produce MDA-7/IL-24 in response to various antigenic/cytokine stimuli and exert diverse immune functions [46–49]. Recent studies have found that MDA-7/IL-24 can be highly induced in Th2 cells along with IL-10 and expression is dependent on IL-4/STAT6 signaling [50,51]. Interestingly, further studies identified MDA-7/IL-24 as one of the top STAT6 target genes in Th2 cells [52]. However, the biological function of MDA-7/IL-24 is not well defined in Th2 cells. One recent study by Dabitao et al. found that IL-4 was not sufficient to induce MDA-7/IL-24 alone, but functioned synergistically with IL-2 or LPS to induce MDA-7/IL-24 in NK cells and macrophages [53]. Physiological levels of MDA-7/IL-24 can also be produced by non-hematopoietic cells such as keratinocytes, melanocytes, human airway epithelial cells and colonic fibroblasts in response to various stimuli [54,55]. As discussed earlier, secreted MDA-7/IL-24 utilizes the cognate receptor pairs (IL-20R1/IL-20R2, IL-22R1/IL-20R2 or IL-20R1/IL22-R1) to exert its cellular functions, however, most immune cells only express IL-20R2 [56,57]. Caudell et al. evaluated the secretion profile of PBMCs treated with MDA-7/IL-24 protein [32], and found increased secretion of immune modulatory cytokines such as IL-1β, TNF-α, IL-6, IL-12, GM-CSF and IFN-γ. IFN- γ secreted by PBMC induced IL-22R1 expression in keratinocytes, and aids in formation of a complete IL-22R1/IL-20R2 receptor pair, which facilitates induction of innate immune responses [58]. MDA-7/IL-24 is also reported to play a role in plasma cell differentiation by impeding B cell maturation to plasma cells by regulating several transcription factors [49]. Apart from immune fnctions, MDA-7/IL-24 also induces several functions in normal skin cells. MDA-7/IL-24 transgenic mice, which overexpress MDA-7/IL-24 specifically in skin are embryonic lethal and display abnormal keratinocyte differentiation and epidermal hyperplasia [59]. However, when human keratinocytes were treated with MDA-7/IL-24 in a wound-healing model this treatment resulted in suppression of keratinocyte proliferation suggesting varied roles of MDA-7/IL-24 in this model [60,61]. MDA-7/IL-24 also plays a protective role in host defense during bacterial and viral infections including Influenza A, Salmonella typhimurium, Pseudomonas aeruginosa and Mycobacterium tuberculosis [62–64]. However, during Staphylococcal aureus infection MDA-7/IL-24 is induced, which in turn inhibits IL-1β and IL-17 production, and aggravates the severity of the infection [65,66]. Thus, MDA-7/IL-24 plays a dual-role in host defense, where it can be either protective or hostile depending on the type of bacterial infection.
Recent studies further highlight the diverse roles of MDA-7/IL-24 in vascular diseases, inflammatory bowel disease, and pro-inflammatory, infectious and autoimmune skin diseases, which were discussed recently in detail in a review by Menezes et al. [23].
2.3. MDA-7/IL-24 as a tumor suppressor
2.3.1. Clinical correlation
To determine the potential clinical relevance of MDA-7/IL-24, studies employing histochemistry with human clinical samples were performed that confirmed MDA-7/IL-24 as a potential tumor suppressor gene. Immunohistochemistry analysis of MDA-7/IL-24 was performed in melanocytes, nevi and different stages of melanoma and the data confirmed that MDA-7/IL-24 protein expression gradually decreased with disease progression from radial growth phase to the metastatic phase and expression was absent in many vertical growth phase and metastatic lesions [19,67,68]. Ishikawa et al. evaluated the clinical significance of MDA-7/IL-24 expression in non-small cell lung cancer (NSCLC) patients, and found that high MDA-7/IL-24 expression was a favorable postoperative prognostic factor in lung adenocarcinoma [69]. Immunohistochemical staining of human breast tissues in a breast cancer cohort of patients revealed a significant enhanced degree of MDA-7/IL-24 positivity in normal mammary epithelial cells, compared to virtually no staining in cancer cells [70]. Additionally, tumors from patients with poor prognosis expressed lower MDA-7/IL-24 transcript levels as compared with their counterparts predicted to have good prognosis. Two recent studies analyzed MDA-7/IL-24 expression and its correlation with clinicopathological parameters and evaluated the prognostic values in hematopoietic tumors including Burkitt lymphoma and diffuse large B cell lymphoma (DLBCL) [71,72]. MDA-7/IL-24 expression was decreased in DLBCL tissues, and low expression of MDA-7/IL-24 was a predictor of poor prognosis [71]. Similar clinical correlations were found in Burkitt lymphoma where low expression of MDA-7/IL-24 was associated with a worse clinical condition [72]. Collectively, all of these studies indicate that loss of MDA-7/IL-24 protein expression correlates with disease progression and further reinforce the concept that MDA-7/IL-24 functions as a tumor suppressor.
2.3.2. Pre-clinical evidence
Jiang et al. first documented that MDA-7/IL-24 functions as a tumor suppressor in human melanoma and other cancer cells [8,10]. Subsequently, several research group both nationally and internationally investigated the functional role of MDA-7/IL-24 as an anti-cancer gene in multiple cancers (summarized in Table 1) including breast cancer [11,73–75], prostate cancer [76–78], lung cancer [79,80], melanoma [81–83], malignant glioma [84–86], neuroblastoma [87], pancreatic cancer [88,89], esophageal squamous cell carcinoma [90], hepatocellular carcinoma [91,92], colorectal cancer [93–95], renal carcinoma [96], cervical cancer [97], ovarian cancer [98,99], osteosarcoma [100], and cancer of blood and lymphatics including leukemia, and lymphoma [101,102]. Many of the above-mentioned studies were initially confirmed using in vitro cells in culture and were subsequently translated into in vivo pre-clinical animal models using human tumor xenografts in immune incompetent mice. This anti-cancer function of MDA-7/IL-24 is a culmination of many diverse biological effects including induction of apoptosis, toxic autophagy, growth arrest, cell cycle perturbation, targeted killing of cancer stem cells, anti-migration/invasion, anti-angiogenesis, “bystander” antitumor effects, synergy with other therapeutic agents (radiation, chemotherapy or monoclonal antibody therapy) and last but not the least immune modulating properties [9,13–15,17–24]. Of direct potential clinical relevance, overexpression of MDA-7/IL-24 in normal cells of different anatomic origin, did not trigger any toxic effects supporting the cancer-selective functional properties of MDA-7/IL-24. How MDA-7/IL-24 differentiates cancer vs. non-transformed/normal cells while eliciting diverse biological effects is not known. Several recent studies demonstrate that cancer cells possess high basal levels of reactive oxygen species (ROS), elevated ER stress indicators and ceramide production as compared to normal cells, which may make them more vulnerable to MDA-7/IL-24-mediated toxic effects [103–106]. Further detailed studies are required to address the mechanism(s) underlying the resistance of normal cells and sensitivity of cancer cells to mda-7/IL-24.
Table 1.
MDA-7/IL-24 as an anti-cancer gene in diverse cancers.
| Cancer indication | Preclinical model | Therapeutic agent | References |
|---|---|---|---|
| Breast cancer | Cells/mouse model | Ad.mda-7 (12), transgenic mouse model (68, 69), Ad.mda-7 and purified MDA-7/IL-24 protein (70) | 11, 73–75 |
| Prostate cancer | Cells/mouse model | MB-Ad.mda-7; MB-CTV :UTMD approach (71), Ad.mda-7 (72), Ad.5/3-mda-7 (73) | 76–78 |
| Lung cancer | Cells/mouse model | VV-IL-24 (74), Ad.mda-7; expression plasmid carrying the mda-7 (75) | 79, 80 |
| Melanoma | Cells/mouse model | Ad.mda-7 (76), purified MDA-7/IL-24 protein (77), purified MDA-7/IL-24 protein (78) | 81–83 |
| Malignant glioma | Cells/mouse model | Ad.mda-7 or GST-MDA-7 as single agent and in combination with radiation (79), GST-MDA-7 (80), Ad.mda-7 as single agent and in combination with radiation (81) | 84–86 |
| Neuroblastoma | Cells/mouse model | Ad.5/3-CTV | 87 |
| Pancreatic Cancer | Cells/mouse model | Ad.mda-7 as single agent and in combination with perillyl alcohol (83, 84) | 88, 89 |
| Esophageal squamous cell carcinoma | Cells/mouse model | Recombinant human IL-24 | 90 |
| Hepatocellular carcinoma | Cells | Ad.mda-7 (86), Ad.mda-7 (87) | 91, 92 |
| Colorectal cancer | Cells/mouse model | Ad.mda-7 (88), Ad.mda-7 (89), small-molecule compound SC144 (90) | 93–95 |
| Renal carcinoma | Cells/mouse model | GST-MDA-7 | 96 |
| Cervical Cancer | Cells/mouse model | Ad.mda-7 | 97 |
| Ovarian Cancer | Cells/mouse model | Ad.mda-7/Ad.RGD.pK7.mda-7/Ad.RGD.mda-7 (93), Ad.mda-7 (94) | 98, 99 |
| Osteosarcoma | Cells/mouse model | OA-IL-24 | 100 |
| Leukemia | Cells/mouse model | GST-MDA-7 | 101 |
| Lymphoma | Cells/mouse model | mda-7/IL-24 gene linked to a lentiviral vector | 102 |
Ad.mda-7: Recombinant non-replicating adenovirus expressing mda-7/IL-24.
Cancer Terminator Virus-CTV: replication competent cancer-selective adenovirus expressing mda-7/IL-24. MB: Microbubbles.
UTMD approach: ultrasound targeted microbubble destruction.
VV-IL-24: cancer-targeted vaccinia virus carrying the IL-24 gene.
Ad.5/3-mda-7: a recombinant Ad.5/3 virus delivering mda-7/IL-24.
GST-MDA-7: GST (glutathione-S-transferase) fusion protein of mda-7.
Ad.5/3-CTV: a recombinant Ad.5/3 virus delivering CTV.
Ad.RGD.pK7.mda-7 and Ad.RGD.mda-7: Infectivity-enhanced adenoviral vectors encoding mda-7.
OA-IL-24: oncolytic adenovirus expressing IL-24.
2.3.3. Clinical trial
Based on its selective and profound anti-cancer effects in vitro and in pre-clinical animal models as discussed above, a phase I/II clinical trial was initiated in a cohort of patients with melanomas and advanced carcinomas. Twenty-eight patients diagnosed with melanoma, lymphoma, hepatoma, breast cancer, non-small cell lung cancer, colorectal cancer, head and neck cancer, and cancers of the parotid gland, lip, kidney, bladder, adrenal gland, and penis, were enrolled in this study. The trial applied a replication incompetent type 5 adenovirus to deliver MDA-7/IL-24 (Ad.mda-7; INGN-241) and used repeated intratumoral injections in a dose-escalating manner [18,19,21,24,29,30,107]. The goal of this phase I trial was to evaluate the safety profile, pharmacokinetics of vector DNA, and MDA-7/IL-24 protein detection both at the tumor site and distally. Ad.mda-7 was mixed with isosulfan blue dye before injection to follow the vector biodistribution with time. Vector-specific DNA and RNA were detected at the site of injection with maximum intensity, which gradually faded in peripheral areas, and faint expression was detected up to 3-cm from the site of injection. High levels of vector DNA and RNA could be detected in tumors at 24–48 hours after injection and the levels went down by 3 logs of magnitude at day 4, but remained above baseline level at day 30 [29,107].
In recent studies Ekmekcioglu et al. showed increased expression of inducible nitric oxide synthase (iNOS) in advanced stages of melanoma [108] and forced expression of mda-7/IL-24 negatively regulated iNOS expression in malignant melanoma cells. Particularly they demonstrate that infection with Ad.mda-7 or treatment with a rhMDA-7 protein decreased iNOS expression in melanoma cell lines in a dose-dependent fashion. Additionally they show that the STAT-3-modulated expression of IFN regulatory factors 1 and 2 is regulated by MDA-7/IL-24, which may alter iNOS gene expression.
Immunohistochemical analysis was done to detect expression of MDA-7/IL-24, beta-catenin, iNOS, CD31 (marker of angiogenesis) and Ki-67 (proliferation marker) [29,30]. With low dose of Ad.mda-7, 20% of tumors displayed positive MDA-7/IL-24 expression and expression went up to 53% when the tumors were treated with high dose. The expression of beta-catenin, iNOS and CD31 decreased in the treated lesions post-treatment with Ad.mda-7. Reduced expression of Ki-67 was also evident in 67% of tumor lesions following Ad.mda-7 injection. Apoptosis was assessed in tumor sections by TUNEL assay, which revealed 80% of tumor cells at the injected site stained positive for TUNEL. Interestingly, MDA-7/IL-24 protein could be detected and increased cancer cell apoptosis was seen many centimeters away from the site of Ad.mda-7 injection in patients, as was seen in pre-clinical animal models, further supporting the “bystander antitumor effects” of MDA-7/IL-24 [27,28].
Results of this Phase I/II trial indicated that repeat injections of Ad.mda-7 into the tumor and tissue surrounding the tumor were safe and well tolerated and an ~ 44% clinical response rate was evident in injected lesions [29,30]. Future clinical trials using improved cancer-selective vectors expressing MDA-7/IL-24 such as the Cancer Terminator Virus (CTV), have potential to further augment clinical efficacy of MDA-7/L-24 [109]. We have developed and tested a CTV that selectively replicates in cancer cells and kills these cells bi-modally through viral replication-dependent cancer cell killing in addition to the synthesis and release of MDA-7/IL-24 that kills and suppresses the growth of uninfected cancer cells [110–112]. We found it to be highly effective in killing primary and distant tumors in multiple pre-clinical animal models including malignant glioma, breast cancer, prostate cancer, melanoma, and neuroblastoma [87,110–116]. The original CTV was engineered in a type 5 adenovirus (Ad.5), which utilizes Coxsackie-Adenovirus receptors (CAR) to infect cells, the expression of which is quite variable among cancer cells. To overcome this issue, we have generated a chimeric CTV that contains components from Ad.5 and Ad.3 permitting entry into cancer cells by CAR as well as other receptors (including CD46 and desmoglein) [116]. Ad.5/3-CTV was a more effective therapeutic for prostate and pancreatic tumors in vivo [113,116]. This improved version of MDA-7/IL-24 virus with targeted delivery methods for systemic administration (ultrasound targeted microbubble destruction (UTMD)/nanoparticle) [76,109,116] either as a single modality or combined with other therapeutic protocols (chemotherapy/radiotherapy/immunotherapy) should elicit profound and more enduring clinical responses.
3. Apoptosis and cancer
Cancer is a heterogeneous disease [117] that can arise in different tissue types and display substantial genetic diversity [118]. Cancer can originate from mutations in genes or epigenetic changes in a single cell, but as the cell proliferates it is at risk of developing further genetic/epigenetic alterations [119]. Cells that have defects in the underlying mechanism(s) promoting cell death or that express increased levels of cell death inhibitors have a survival advantage. These cells may also possess variations in genetic composition, which render them resistant to cell death following radiotherapy or chemotherapy [119–121]. Programmed cell death also known as apoptosis, frequently occurs in development and aging to maintain cellular homeostasis in a tissue [122]. Apoptosis acts as a protective mechanism guarding the organism when cells are harmed by disease or other injurious agents [122]. A major contributing factor in the development and progression of cancer is defective programs of apoptosis [123]. Cancer cells tend to develop the ability to evade induction of apoptosis and this avoidance of apoptosis plays a prominent role in their resistance to therapeutic agents [124]. Noteworthy research performed in the past several years has focused on comprehending the mechanisms employed by tumor cells to elude cell death [124]. This has encouraged the evaluation of novel systems and approaches, as well as new therapeutic agents that can induce cell death pathways to specifically initiate apoptosis in cancer cells.
3.1. Apoptosis pathways
Apoptosis involves multiple inter-related phases that can occur through the extrinsic or intrinsic (mitochondrial) pathways. The tumor necrosis factor (TNF) receptor superfamily of transmembrane proteins controls the extrinsic apoptotic pathway [122]. TNF receptors generally share a region of 80 amino acids, called the death domain and these receptors are referred to as death receptors. The death domain of these receptors plays a decisive role in transmitting apoptotic signals across the cell membrane [125]. Once the ligand-receptor interaction transpires it initiates activation of caspase-8, which in turn results in activation of downstream execution target proteins triggering apoptosis in cells [122,126].
The intrinsic (mitochondrial) apoptotic pathway is triggered by a wide range of stimuli such as chemotherapy/radiotherapy induced DNA damage, genetic damage, hypoxic stress, oxidative stress, imbalance in Ca2+ ions, etc. [122,127,128]. The Bcl-2 family of proteins is a major participant in the regulation of the intrinsic pathway of apoptosis [129]. There are several Bcl-2 family proteins such as Bcl-2, Mcl-1, Bim, Bid, Bcl-xL/xS, etc., that are implicated in the intrinsic pathway of apoptosis [129]. These Bcl-2 family proteins function differently, either they stimulate apoptosis (Pro-apoptotic: Bax, Bid, etc.) or inhibit apoptosis (anti-apoptotic: Bcl-2, Mcl-1, etc.) [130]. The balance between pro and anti-apoptotic molecules determines the fate of cells to instigate or escape cell death. BH3-proteins can detect DNA damage signaling the cell to undergo apoptosis. The pro-apoptotic proteins, Bax or Bak, are activated or anti-apoptotic proteins Bcl-2, Mcl-1, are inhibited when the BH3-proteins move to the mitochondrial membrane [130]. When the pro-apoptotic proteins are activated in the mitochondria, mitochondrial outer membrane permeabilization ensues and this permeabilization promotes the release of important pro-apoptotic factors, such as cytochrome c, into the cytosol [131]. Once cytochrome c is released into the cytosol, it associates with another pro-apoptotic factor, APAF1. This complex is called an “apoptosome” complex. The apoptosome complex then activates a string of caspases which in turn cause cell death [132]. In the intrinsic pathway of apoptosis these cell death proteins are continuously regulated by the tumor suppressor protein p53 [133].
The major apoptotic pathway used by cytotoxic T-lymphocytes is called perforin/granzyme-induced apoptosis [122]. In this pathway, cytotoxic T-lymphocytes secrete a protein called perforin and small particles of particular enzymes. These T-lymphocytes that release perforin create holes in the target cell plasma membrane [122]. These holes are used by additional small particles to enter into the cell and release their enzymes called granzymes A and B [134]. Once these granzymes are released in cells they initiate the apoptotic execution process. Though this pathway is very important in immune response-mediated tumor cell death, the molecular mechanism of perforin-mediated cell death and synergy between perforin- and granzyme-mediated cell death is poorly understood [135].
The execution phase of apoptosis occurs both in the extrinsic and intrinsic pathways of apoptosis. In the execution phase of apoptosis, caspases direct the cell death pathway [123,136]. Mainly caspase 3, 6 and 7 are considered execution caspases, which are present in all cells but are only activated when the initiation process of apoptosis occurs [122]. Among all the caspases, caspase 3 is considered the most essential caspase. Caspase 3 can trigger rearrangement of the cells cytoskeleton, disruption of intracellular transport, interference in cell division and signal transduction and induction of DNA or chromatin damage [122,123,136]. Once the execution caspases are activated, apoptosis is considered irreversible. When the cells are in the final stages of apoptosis cell fragments are created, which can then be identified by surrounding macrophages or epithelial cells, which eventually engulf and digest them [122,136].
As discussed, deletion of pro-apoptotic signals creates a tumor cell having a positive survival advantage over normal cells. Evading apoptosis and continuous proliferation occurs regardless of other abnormalities in the cancer cell [137]. In these situations, loss or inactivation of the tumor suppressor protein, p53, is a common occurrence. When there is inhibition of p53 protein expression/function in cells, it fails to sense DNA damage that triggers apoptosis. In the event of inhibition of p53, anti-apoptotic Bcl-2 family proteins are upregulated and neutralize pro-apoptotic actions of BH3 only proteins [133,138]. Many therapeutic regimens act by forcing the cancer cells to undergo apoptosis by inducing DNA damage or cellular stress [139]. Cancer cells accumulate enough BH3 proteins, but these are not sufficient to provoke a strong apoptotic effect because they cannot overpower the increased Bcl-2 anti-apoptotic protein effect. Genetic agents or drugs that can mimic or enhance the effect of BH3 proteins to induce pro-apoptotic signals or that can inhibit Bcl-2 family proteins can be beneficial in overcoming these problems associated with an imbalance between pro- and anti-apoptotic proteins [140]. All the drugs designed to induce cell death in cancer have the underlying challenge of guaranteeing that they will act only on cancer cells without harming normal cells. A therapeutic agent that induces apoptosis in normal as well as cancer cells poses a significant risk in patients. Much of the previous research has been dedicated to understanding the mechanism(s) acquired by tumor cells to avoid cell death [141]. This has led to the development of novel strategies and therapeutic agents that can modulate cell death pathways in a targeted manner to selectively induce apoptosis in cancer cells [140,141].
3.2. Role of MDA-7/IL-24 in apoptosis
A positive role of MDA-7/IL-24 as a selective anti-cancer agent is now well established. MDA-7/IL-24 exerts its anti-cancer effects when it is overexpressed in cancer cells inducing apoptosis via stimulating stress, toxic autophagy, anti-angiogenesis [13,142,143], and anti-invasion effects in the entire cancer population including cancer stem cells, and sensitizes cancer cells to other therapeutic agents. The apoptotic action of MDA-7/IL-24 selectively toward transformed/cancer cells is the major contributor to tumor growth suppression. Ongoing research efforts by our group and by others continue to shed light on the crucial players that are involved in MDA-7/IL-24-mediated apoptosis (Summarized in Fig. 2). Most of these key proteins that are regulated by MDA-7/IL-24 play critical roles in the regulation of endoplasmic reticulum (ER) stress and mitochondrial function [9,13,20,81,85,96,144–149]. Previous observations from our laboratory suggest that MDA-7/IL-24 can induce cell death by activating PKR-like endoplasmic reticulum kinase (PERK), an unfolded protein response (UPR) sensor. This phosphorylated PERK shuts down the global translation of proteins by activating and phosphorylating its downstream target EIF2a. Shutdown of global translation results in decreased expression of anti-apoptotic proteins like Mcl-1, Bcl-xL, and c-flip. In several other studies, increased levels of pro-apoptotic markers such as Bax and Bak were observed following MDA-7/IL-24 treatment in cancer cells, while the expression of pro-survival markers decreased, i.e., shifting the balance from survival to death [77,101,150,151].
Fig. 2. Signaling pathways associated with MDA-7/IL-24 function in cancer cells: outline of cancer-selective cytotoxic effects of MDA-7/IL-24.
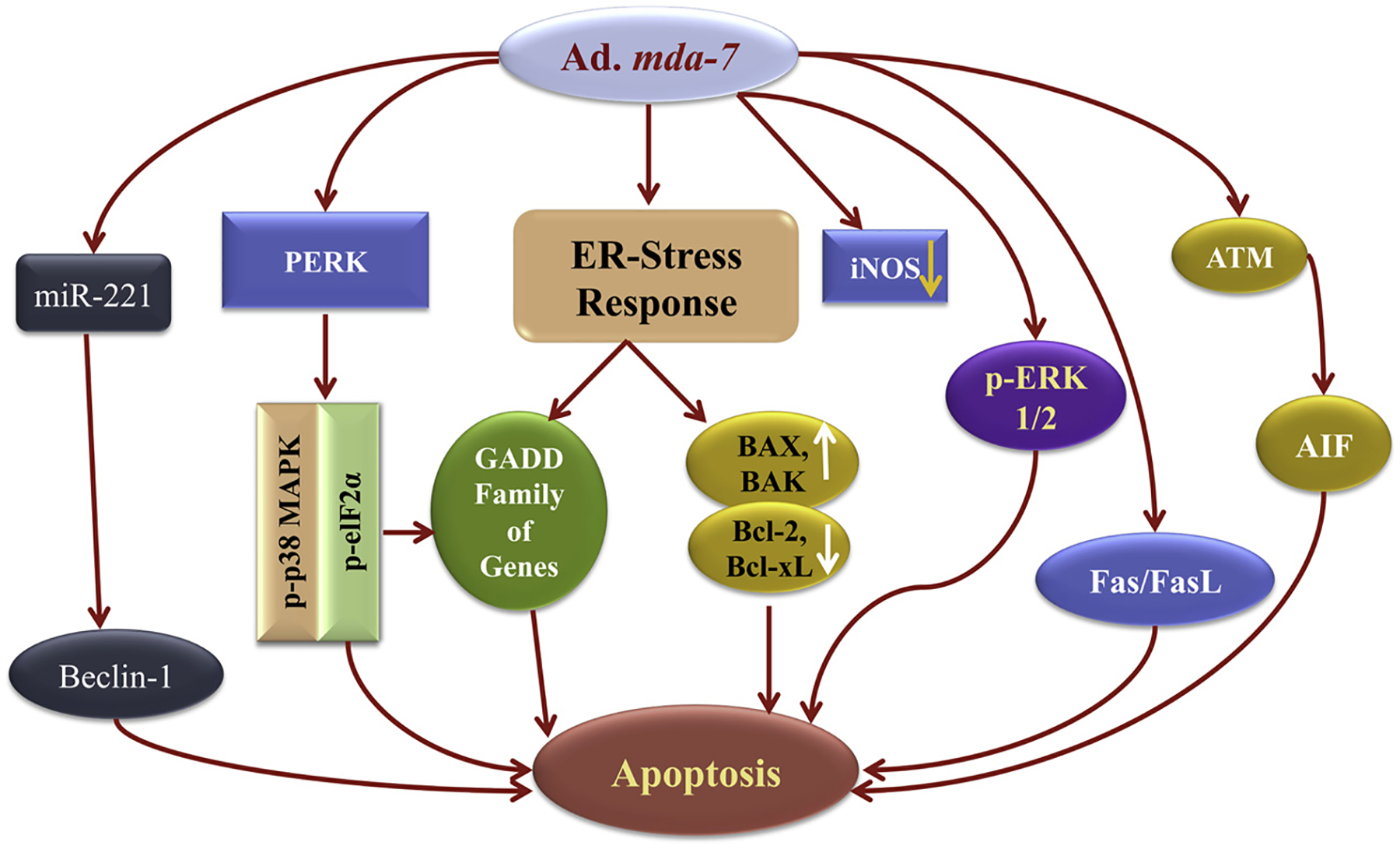
MDA-7/IL-24 when expressed ectopi-cally by Ad.mda-7, it localizes to the ER/Golgi complex. Accumulation of MDA-7/IL-24 protein in transformed/tumor cells in this compartment results in induction of ER stress, and mitochondrial dysfunction eventually culminates to apoptosis. This involves modulation of a series of signaling events including down regulation of Bcl-2 and Bcl-xL, upregulation of Bax and Bak, activation of GADD family of genes, and tumor suppressors i.e. AIF, Fas/FasL and others. A combination of multiple pathways as shown in the figure triggered by MDA-7/IL-24 results in cancer cell apoptosis. Modified from Fisher PB. Cancer Res. 2005 Nov 15;65(22):10128–38.
In another study, we showed that MDA-7/IL-24 can induce ceramide production in prostate cancer cells and induction of ceramide plays pivotal role in MDA-7/IL-24-mediated apoptosis [152]. Treatment with MDA-7/IL-24 resulted in a prominent increase in various ceramides (C16, C24, C24:1) selectively in prostate cancer cells [152]. The role of ceramides in inducing apoptosis upon treatment with MDA-7/IL-24 was confirmed by using the serine palmitoyltransferase (SPT) inhibitor to restrain enzyme activity of SPT. Inhibition of SPT enzyme activity resulted in impaired MDA-7/IL-24-induced apoptosis. SPT inhibitor also reduced the ceramide production when treated with MDA-7/IL-24. MDA-7/IL-24-mediated ceramide production occurs through de novo synthesis of ceramide and MDA-7/IL-24-induced cell death requires ceramide [152]. In the same study, it was also shown that MDA-7/IL-24 treatment enhanced the activity of acid sphingomyelinase (ASMase) and decreased sphingomyelin in cancer cells, activated protein phosphatase 2A (PP2A) and assisted in dephosphorylation of the anti-apoptotic molecule Bcl-2 [152]. Induction of ER stress markers (BiP/GRP78, GADD153 and pospho-eIF2α) triggered by Ad.mda-7 infection were eliminated by treatment of cells with fumonisin B1, an inhibitor of ceramide synthase or ISP-1, an inhibitor of serine palmitoyltransferase thereby supporting the hypothesis that ceramide plays an important role in MDA-7/IL-24-mediated ER stress induction [152]. This study also demonstrated that the production of ceramide following MDA-7/IL-24 treatment occurred explicitly in cancer cells, without producing a parallel effect in normal cells. These studies identified ceramide as an important facilitator of MDA-7/IL-24-mediated ER stress and apoptosis induction in tumor cells [152].
An important role of the p38 mitogen activated protein kinase (MAPK) pathway in MDA-7/IL-24-mediated cell cycle arrest and cell death was first demonstrated by Sarkar et al. [81]. In this study, MDA-7/IL-24 was shown to enhance p38MAPK activity and induction of a subset of growth arrest and DNA damage (GADD) genes [81]. From other studies in some cancer types it was established that treatment with MDA-7/IL-24 resulted in inhibition of phosphorylated ERK1/2 [102,153]. Other studies showed that the c-Jun NH2-terminal kinase (JNK) signaling cascade was triggered by MDA-7/IL-24 treatment [85,86]. Activation of JNK after MDA-7/IL-24 treatment stabilized Bim and activated Bax and Bak proteins, eventually leading to mitochondrial dysfunction and cell death [85,86].
In breast cancer stem cells, it was observed that MDA-7/IL-24 induced apoptosis selectively in cancer stem cells without affecting normal stem cell growth [154]. The differential effect of MDA-7/IL-24 in cancer vs. normal cells may be due to fundamental biochemical differences in these cells, which remain to be defined. Based on published literature, one of the important mechanisms of MDA-7/IL-24-mediated cell death is induction of ER stress. Although ER stress can regulate both pro- and anti-apoptotic pathways, prolonged periods of intense ER stress shifts the balance towards apoptosis [22,155]. Cancer cells have greater ER stress as compared to normal cells [104], and MDA-7/IL-24-mediated ER stress may lead to cell death in cancer cells (Fig. 3).
Fig. 3. A schematic representation of how MDA-7/IL-24 regulates toxic autophagy in cancer cells leading to cell death.
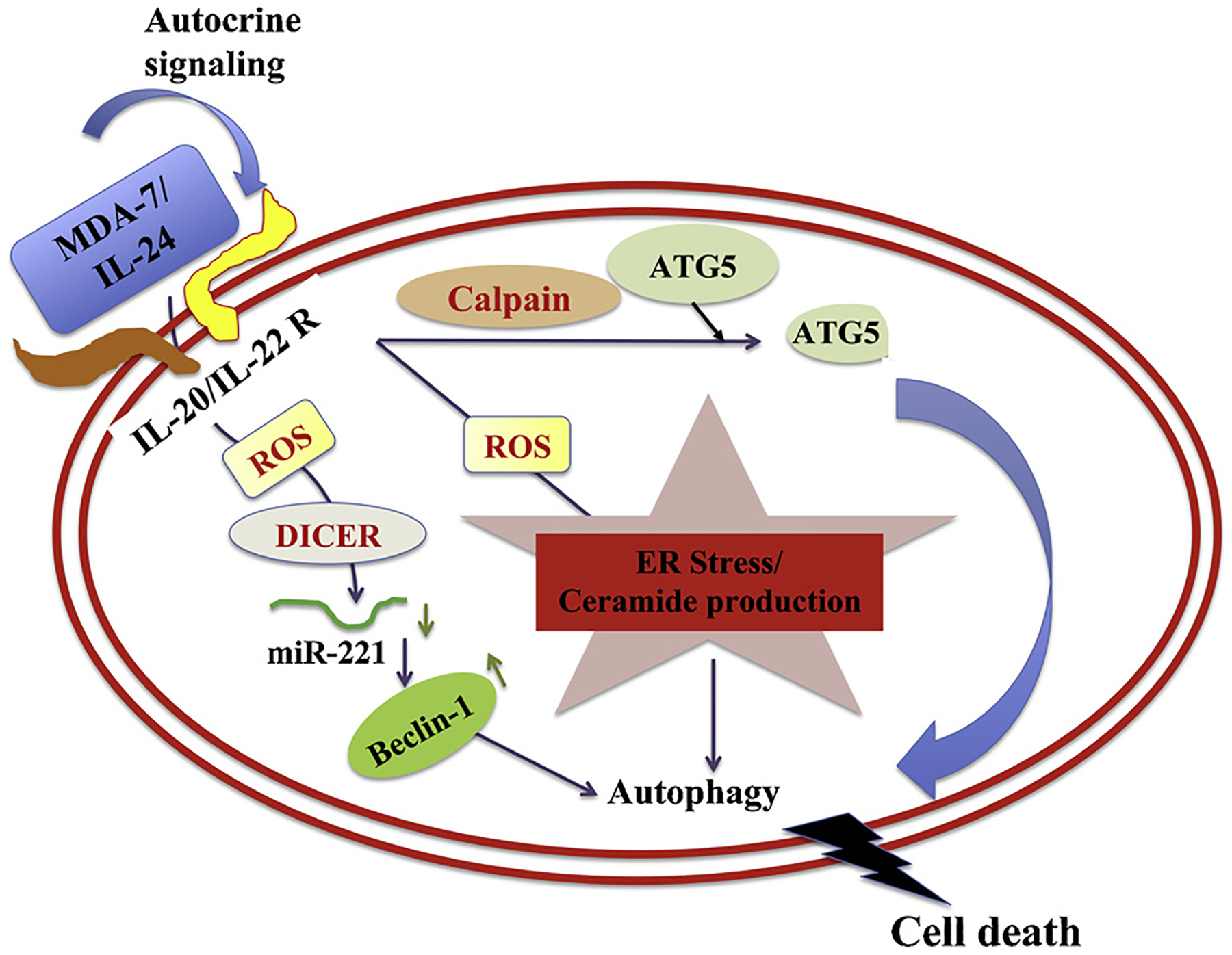
MDA-7/IL-24 regulates autophagy mediated through ER stress and ceramide production. MDA-7/IL-24 first interacts with its receptors which leads to a downstream signaling pathway mediated by reactive oxygen species (ROS). ROS regulates DICER and through this molecule MDA-7/IL-24 downregulates miR-221, which in turn upregulates Beclin-1 to induce toxic autophagy leading to cell death. The transition of protective to toxic autophagy is explained by the cleavage of ATG5 by Calpain, which is also mediated by ROS induced from MDA-7/IL-24 treatment.
Reactive oxygen species (ROS) and mitochondrial dysfunction also play germane roles in mda-7/IL-24-induced apoptosis. Studies from our group and others demonstrated that exogenous expression of mda-7/IL-24 led to the generation of ROS (Fig. 3), and N-acetylcysteine (NAC), a prominent antioxidant nullified both ROS generation and cell death [23,145]. As predicted, increasing ROS production using known ROS inducers, such as arsenic trioxide, perillyl alcohol, 4- hydroxyphenyl-retinamide (4-HPR), and vitamin E facilitated MDA-7/IL-24-induced apoptosis [88,89,146,156]. In GBM cells, Ad.mda-7 infection enhanced expression of superoxide dismutase 2 (SOD2) and thioredoxin (TRX) [148]. Quenching ROS production by over-expressing SOD2 or TRX suppressed autophagy and cell killing, whereas inhibition of SOD2 or TRX enhanced activation of JNK and p38 MAPK, promoting toxic autophagy and cell death. CD95 also played an important role in MDA-7/IL-24-mediated apoptosis. This study specifically showed that inhibiting either ROS generation or ceramide synthesis blocked CD95 activation and apoptosis induction and knockdown of CD95 rescued the cell killing effect. Our group found that pancreatic cancer cells are relatively resistant to MDA-7/IL-24 therapy [88,89,146]. Of potential therapeutic relevance, when perillyl alcohol (ROS inducer) was used in combination with Ad. mda-7, it synergistically enhanced apoptotic cell death in pancreatic cancer in both in vitro cell culture and in vivo xenograft models [88,89,146].
MDA-7/IL-24 also upregulates a tumor suppressor gene SARI (Suppressor of AP-1, Regulated by Interferon) in a cancer-specific manner [11]. Treatment with MDA-7/IL-24 (either by gene delivery or recombinant protein) induces SARI expression in cancer cells but failed to induce this gene product in normal cells. These results suggest that SARI expression is required for MDA-7/IL-24’s apoptotic activity in cancer cells [42].
Other reports suggest that the FasL signaling pathway is activated in cancer cells upon treatment with MDA-7/IL-24. Exogenous administration of MDA-7/IL-24 activates the transcription factor c-Jun, which in turn causes activation of FasL. Further studies confirmed that siRNA-mediated knockdown of FasL rescues MDA-7/IL-24-mediated cell death in ovarian cancer cells [157]. This work suggests that mda-7/IL-24 has a role in regulating the Fas-FasL signaling [157]. Other studies indicate that MDA-7/IL-24 upregulates PKR (serine/threonine protein kinase) in non-small cell lung cancer and induces apoptosis in a p53-independent manner [158,159]. In the context of breast cancer using various cell lines with different p53 status, i.e., MCF7 (p53-wt), MDA-MB-231 (mutant p53), MDA-MB-453 (mutant p53), and T47D (mutant p53), Zheng et al. confirmed that MDA-7/IL-24 also stimulates cell death in breast cancer in a p53-independent manner [75]. The p53-independent activity of mda-7/IL-24 in inducing cell death in human breast cancer cells was initially suggested by Su et al. following delivery of mda-7/IL-24 using a replication incompetent adenovirus (Ad.mda-7) in human breast cancer cells [75]. These results suggest that MDA-7/IL-24 can induce cancer cell apoptosis independent of p53 mutations and function. These studies also revealed that cell death induction is occurring independent of other genetic backgrounds such as ER/PR/HER2 status [75,160]. Another study by Chada et al. showed that MDA-7/IL-24 treatment can induce phosphorylation and nuclear translocation of STAT3 [83]. MDA-7/IL-24’s interaction with its receptor can induce BAX protein leading to cell death independent of STAT3 activation [20]. This phenomena, observed with MDA-7/IL-24, is in agreement with the biochemical actions of other interleukins (IL-10, IL-19, IL-20, and IL-22), which also activate STAT3 without promoting cell death [161]. Interaction of MDA-7/IL-24 with its receptors also results in activation of the JAK/STAT signaling. Other studies showed that MDA-7/IL-24 could induce apoptosis independent of the JAK/STAT pathway [147]. This is supported by using specific inhibitors of the JAK/STAT pathway, which did not inhibit apoptosis in MDA-7/IL-24-treated cells. [147]. These results and that of others show that MDA-7/IL-24 functions independent of tyrosine kinase activation.
Ekmekcioglu et al. observed increased expression of inducible nitric oxide synthase (iNOS) in advanced stages of melanoma [108] and forced expression of MDA-7/IL-24 negatively regulated iNOS expression in malignant melanoma cells [162]. They demonstrated that infection with Ad.mda-7 or treatment with recombinant human MDA-7/IL-24 protein decreased iNOS expression in melanoma cell lines in a dose-dependent manner [162]. Treatment of melanoma cells with MDA-7/IL-24 activated STAT3, which in turn resulted in downregulation of IFN regulatory factors 1 (IRF-1) and upregulated IRF-2 [82,162]. These alterations in IRF balance might be the underlying cause of inhibition of iNOS expression mediated by MDA-7/IL-24. MDA-7/IL-24 may regulate iNOS expression through other indirect pathways, for example secondary cytokine stimulation or interacting with other molecules that co-regulate iNOS expression. Further research is needed to determine if MDA-7/IL-24 and iNOS might interact in a regulatory fashion or iNOS might directly regulate MDA-7/IL-24 expression in melanoma cells.
Our group showed that MDA-7/IL-24 delivered by an Ad.5/3-CTV (a bipartite chimeric adenovirus which express the E1A gene, necessary for viral replication, under control of the PEG-3 promoter [163] and simultaneously expresses mda-7/IL-24 in the E3 region (Ad.PEG-E1A-mda-7); Cancer Terminator Virus (CTV) [111,112]) induces intense anti-proliferative activity and apoptosis in a caspase-independent manner in neuroblastoma, both in vitro and in vivo in a tumor xenograft model [87]. MDA-7/IL-24 promotes apoptosis in neuroblastoma through a unique pathway involving apoptosis-inducing factor [2] translocation into the nucleus. The study further confirmed that inhibiting AIF using a specific AIF inhibitor rescued neuroblastoma cells from MDA-7/IL-24-induced cell death, whereas a pan-caspase inhibitor that is a universal caspase inhibitor of caspases failed to rescue neuroblastoma cells from death. MDA-7/IL-24 treatment of neuroblastoma cells augmented ATM phosphorylation and nuclear translocation resulting in increased γ -H2AX, which in turn triggered nuclear translocation and increased expression of AIF. This was confirmed by using an ATM inhibitor, where the ATM inhibitor resulted in γ-H2AX inhibition and translocation and this inhibited γ -H2AX reduced AIF nuclear translocation resulting in an inhibition in PARP cleavage. Taken together, this study revealed a new pathway in MDA-7/IL-24-mediated apoptosis induction in neuroblastoma cells, independent of caspases and mediated through AIF, ATM, and γ -H2AX alterations [87].
MDA-7/IL-24 regulates a specific subset of miRNAs, including cancer-associated miR-221. MDA-7/IL-24 treatment in cancer cells inhibited miR-221 in a dose-dependent manner. The downregulation of miR-221, results in upregulation of its target proteins PUMA and p27 that facilitate cell death. This is also dependent on increased ROS production, which was rescued by over expression of a miR-221 mimic. This report was the first demonstration that miRNA regulation is directly regulated by mda-7/IL-24 in cancer cells and highlighted the uniqueness of the mda-7/IL-24-miR-221 loop in mediating cancer cell specific death [43].
Recent reports also suggest that MDA-7/IL-24 can induce activation of cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) in a variety of breast cancer cell lines and this event increases ATF4 activity leading to cell death [164]. Treatment with MDA-7/IL-24 can activate PKA, and this activated PKA in turn activates p38 mitogen-activated protein kinase leading to extrinsic pathway-mediated apoptotic cell death. This study also showed that TP53 phosphorylation and nuclear import is downstream of MDA-7/IL-24-mediated PKA activation and cell death [164].
Previous work by our group and others demonstrated that several downstream target genes might be potential mediators of growth suppression by MDA-7/IL-24. A study by Li YJ et al. [74], showed that the PERP, another member of the GAS-3/PMP-22 family of tumor suppressor genes may provide a novel target for MDA-7/IL-24-mediated apoptosis. Here the authors generated a breast cancer transgenic mouse model containing a tet-inducible MDA-7/IL-24 and crossed these mice with Her2/Neu transgenic mice. These transgenic mice treated with doxycycline showed a strong reduction in tumor development validating tumor suppressor activity of MDA-7/IL-24 in immune-competent mice [73,74], as was shown also in the transgenic mice developed by Menezes et al. [73,74] in which breast-specific expression of MDA-7/IL-24 is controlled by the MMTV-promoter. The study by Li et al. [73,74] showed that MDA-7/IL-24 induces apoptosis in HER2+ breast cancer cells in a PERP-dependent manner. Our research and that of others showed that MDA-7/IL-24 could be combined with conditionally replicating adenoviruses (CTV, F5/35-ZD55) or fused with tumor specific peptides (RGD) with unique tumor-penetrating and cell-internalizing properties promoting greater cancer cell death [109,165–168]. Taken together there is now abundant evidence indicating that MDA-7/IL-24 induces apoptosis in a cancer-specific manner, which may occur independent of p53, JAK/Stat, and caspase-dependent pathways [169]. These observations also underline the fact that MDA-7/IL-24 can induce distinct changes in the apoptosis machinery in different cancer cells that culminate in cancer cell death.
4. Toxic autophagy and cancer
Currently the concept of programmed cell death comprises three phenomena: apoptosis, autophagic cell death and necrosis [170]. Autophagy is a critical process among the three. Autophagy (from the Greek word “auto,” meaning self and “phagy,” meaning to eat) is a cellular process by which cytoplasmic constituents are delivered to the lysosome for bulk degradation [171,172]. This term was first used by Nobel laureate Christian de Duve while attending the Ciba Foundation Symposium on Lysosomes, London on February 12–14, 1963. Autophagy can contribute to stability, survival and evasion of stress, where it is often referred to as “protective autophagy” [173]. The autophagic process is initially an adaptive response to stress. During stressful conditions of nutrient limitation, autophagy is utilized as a means of generating amino acids and energy to maintain cell viability through the bulk degradation of cytoplasmic materials. The presence of autophagy in dying cells has also been proposed to be a stress response mechanism to prolong cell viability [174]. But if the cell continues to face continued duress for an extended period of time, the cell eventually reaches a point of no return leading to cell death. Recent studies are questioning this hypothesis, and suggest that specific stages of apoptosis may in fact be reversible [175]. This type of cell death that is caused as a result of autophagy is called autophagic cell death. In such cases, as it contributes to cell death, it is also referred to as toxic autophagy, by many researchers.
4.1. Autophagy pathways
The autophagy pathway starts with the formation of the phagophore and ends with the autolysosome [176]. The formation of the phagophore is initiated by a dedicated unit of proteins which assemble into functional complexes, and are then subsequently activated and recruited to membranes. The major organelles regulating the secretory pathway (ER, Golgi) and endocytosis (sorting endosome, recycling endosome) are heavily involved in membrane contribution to phagophore formation [176]. Formation of the phagophore is initiated by the activation of the ULK kinase complex, which parallels the translocation of the ULK complex at a location on the ER that is marked by ATG9 [176]. This is followed by recruitment of the class III phosphatidylinositol 3-kinase (PtdIns3-kinase) generating the ER domains or structures called the omegasome, containing PtdIns3P. This is followed by PtdIns3P-mediated recruitment of WIPI2B, which recruits the E3-like complex ATG12-ATG5-ATG16L1. The Golgi-endosomal system produces ATG9 vesicles that aid in the formation of omegasome, and directly fuse with the growing phagophore. After closure of the phagophore, the double-membrane autophagosome matures and fuses with lysosomes to degrade its contents. In this fusion process the outer autophagosomal membrane fuses with the single lysosomal membrane. Full fusion is attained by degradation of the inner autophagosomal membrane by lysosomal hydrolases and introduction of the contents of the autophagosome to the lumen of the lysosome [176]. Cytoskeleton molecules and related motor proteins, tethering factors, phospholipids, and specific SNARE complexes, are the key regulators guiding this intricate fusion [176]. After the lysosomes fuse with the outer membrane of an autophagosome, the lysosomal contents begin to occupy the area between the two autophagosome membranes, helping in the degradation of the inner membrane of the autophagosome, in an LC3-dependent manner. After inner membrane degradation, the process of autophagosomal cargo break down begins. The resulting products, including amino acids and sugars, are transported out of the autolysosome through members of a family of lysosome efflux transporters, and the autolysosome continually decreases in size throughout this process. Autolysosomes start to disintegrate as soon as the signal for autophagy termination is received. In this termination process, lysosomal membrane proteins are scavenged from autolysosomes through reformation tubules [176].
Induction of autophagic cell death through overstimulation of autophagy is an important therapeutic strategy for eliminating cancer cells [177]. This therapeutic autophagic response has been observed in several types of cancer, including brain [178], breast [179], prostate [180], and melanoma [181]. Whether the cell will undergo autophagic cell death depends on the intensity and duration of autophagic induction, as well as cellular health prior to induction. According to some researchers, activation of autophagy is necessary, but not sufficient for autophagic cell death, since it requires additional death signals such as c-Jun N-terminal kinase (JNK). When Bax/Bak-deficient cells were exposed to apoptotic stimuli, autophagic cell death was found to occur simultaneously with increased levels of phosphorylated JNK [182,183].
Several studies by different research groups have shown that this autophagic death can contribute to in vitro and in vivo antitumor effects. The onco-suppressive functions of autophagy can also be explained, at least in part, by several other mechanisms [184]. Autophagy is reported to be involved in the degradation of oncogenic proteins, such as mutant (but not wild-type) TP53 [185,186], p62 [174,187,188], PML-RARA [189,190], BCR-ABL1 [177,191], etc. Mutant TP53 is known to accumulate in neoplastic cells. It then acts as a dominant-negative factor, and inhibits the onco-suppressive functions of the wild-type protein [177,192]. Cancer cells lacking the expression of ULK1, BECN1 or ATG5 tend to accumulate increased amounts of mutant TP53, whereas overexpression of BECN1 or ATG5 results in mutant TP53 degradation [186]. This autophagy-dependent depletion of mutant TP53 can restore the ability of wild-type TP53 to inhibit malignant transformation, at least in some situations. Additionally, autophagy may also inhibit on-cogenesis by limiting p62 availability [188].
Autophagic responses in cells may also suppress the accrual of genetic and genomic defects that accompanies malignant transformation, via several different mechanisms. One of these mechanisms could be through ROS, which are highly genotoxic [184]. Autophagy ameliorates overproduction of ROS by removing dysfunctional mitochondria/mitophagy [184,193,194] as well as eliminating redox-active aggregates of ubiquitinated proteins [184,188,195]. In addition, autophagy also aids in the removal of micronuclei that result from disturbances of the cell cycle [196], in retrotransposing RNAs degradation [197], and regulation of ras homolog family member A (RHOA) expression, a small GTPase involved in cytokinesis [184,198]. Additionally, different constituents of the autophagic signaling pathway are responsible for responses to genotoxic stress [184,188,199,200]. Further studies are necessary to elucidate the precise details of how toxic autophagy can be used in cancer therapy.
Therapeutic induction of autophagy-associated cell death can be accomplished by modulation of regulators of autophagy. mTOR is a key regulator of cell growth and an autophagy repressor, and inhibition of this kinase leads to the activation of Atg1/Ulk1 and stimulation of autophagy [201]. Autophagy can also be induced by the protein kinase AMPK. Metformin is an inhibitor of the mitochondrial electron transport chain complex I that leads to decreased ATP production and increased levels of AMP. This causes AMPK activation and autophagy induction, leading to cancer cell death [202]. Chemotherapeutic drugs (such as alkylating agents, actinomycin D, arsenic trioxide), radiation therapy, photodynamic therapy, hormonal therapies (treatment with analogs of tamoxifen and vitamin D), cytokines (IFN-γ), gene therapies (p53, mda-7/IL-24, and p27Kip1), and treatment with certain natural compounds (resveratrol, gossypol and plant lectins) have also been reported to induce autophagic cell death (ACD) in several cancer types in vitro [177,203,204]. ACD can happen in cells independently as a result of a trigger, but it can also occur in synergism with another cellular process and assist in apoptotic cell death [205,206]. Combining two different therapeutic strategies that induce autophagy in cells by targeting different pathways can contribute to increased sensitivity to ACD, resulting in synergistic cancer inhibitory effects [207]. In a majority of cases of malignant tumor progression, autophagy contributes to cell death primarily when the inherent cellular apoptotic machinery is defective. However, use of autophagy inducing drugs can potentially result in unwanted paradoxical effects by essentially protecting tumors against cell death triggered by anticancer therapies or other stressful situations in the tumor environment [203], which should be considered seriously before designing therapeutics.
4.2. MDA-7/IL-24 and regulation of autophagy
As discussed above, autophagy is a process of stepwise degradation of organelles in a cell [208]. This is a variable, intricate, multi-step complex pathway due to its context dependent roles. Autophagy can either be positive or negative for cells, since it has the capacity to either protect or kill cells [209]. If a cell continues to face stress for an extended period of time, the cell eventually crosses the autophagic threshold, resulting in its death. This death as already mentioned, is called autophagic cell death, and is often utilized in therapeutic strategies against cancer. Recently, small molecules that can regulate autophagy are receiving increased attention, because of this property. MDA-7/IL-24 is one of such molecules due to its ability to specifically target cancer cells and induce ACD. MDA-7/IL-24-induced cytotoxic autophagy is well studied, resulting from its ability to induce Beclin-1 [43,210] and activate PERK or PKR-like endoplasmic reticulum kinase [148,211,212]. Activation of PERK is a manifestation of the unfolded protein response (UPR) and ER stress [212]. Several lines of evidence demonstrate PERK’s role in MDA-7/IL-24-induced autophagy [148,211,212].
Recombinant MDA-7/IL-24 or GST tagged MDA-7/IL-24 activate the PERK-mediated pathway to induce mitochondrial dysfunction [212]. GST tagged MDA-7/IL-24 causes cell killing in part through a PERK-dependent mechanism [212]. Cell death is reported to be diminished in PERK-knockout cells [211]. MDA-7/IL-24 localizes to the ER and binds to the chaperone protein BiP/GRP78, preventing its association with PERK [211]. The release of PERK from the BiP/GRP78 complex leads to its oligomerization and subsequent activation of the UPR [211]. MDA-7/IL-24-mediated cell death is dependent on activation of JNK1–3 and this signaling again is PERK-dependent [211]. Also, MDA-7/IL-24-mediated ERK1/2 suppression is PERK-dependent [212].
Adenoviral delivery of mda-7/IL-24 induces ER stress and production of ceramide leading to early autophagy that switches to apoptosis in prostate cancer cells [43,213]. In a different study it was shown that Ad.mda-7 causes cleavage of ATG5, which is mediated by Calpain that can cause a switch from autophagy to apoptosis (Fig. 3) [148,210]. Hence, these studies reveal another aspect of interplay of autophagy and apoptosis that is mediated by MDA-7/IL-24.
Recent studies from our group have shown that MDA-7/IL-24 can regulate a number of microRNAs, including miR-221. miR-221 is one of the most studied oncogenic microRNAs, which is up regulated in many cancers. mda-7/IL-24 down regulated miR-221, which in turn induced beclin-1, that lead to autophagy (Fig. 3) [43,213]. Beclin-1 is characterized as a new transcriptional target of miR-221 [211]. Cleavage of LC3 is also observed, which is a marker protein for autophagy [211]. Taken together, our results suggest that mda-7/IL-24 induces beclin-1 to induce toxic autophagy. miR-221 also targets multiple tumor suppressor genes including p27 [214], PUMA [215], p57 [216], and PTEN [217], besides beclin-1 [43]. This regulatory loop (MDA-7/IL-24/miR-221/beclin-1) defines a novel mechanism of MDA-7/IL-24-mediated cancer-specific toxic autophagy that leads to cancer cell death. In a more recent study [218], we extended our observation on how MDA-7/IL-24 regulates miRNAs. Specifically, we found that MDA-7/IL-24 down-regulates DICER, a critical regulatory enzyme in the miRNA processing machinery in multiple cancer cells, but not in normal cells. MDA-7/IL-24 did not alter the expression of other miRNA processing cofactors, including DROSHA, PASHA, or Argonaute. When DICER was overexpressed in cancer cells, it rescued cells from MDA-7/IL-24-mediated cell death. This study further emphasized that MDA-7/IL-24-mediated DICER regulation was mediated by the transcription factor MITF. This study revealed a distinct function of MDA-7/IL-24 in regulating miRNA biogenesis via the ROS/MITF/DICER pathway in cancer [218].
A most relevant and interesting phenomenon is cancer-specific autophagy induction by MDA-7/IL-24. The differential activity induced by MDA-7/IL-24 in an expansive array of cancer cells compared to normal cells can be explained by the inherent complexities in these cells. ROS is also shown to be involved in the autophagic process. ROS inducers can enhance the activity of mda-7/IL-24 and ROS is a mediator of mda-7/IL-24-mediated autophagic cell death [43]. Blocking ROS by chemical anti-oxidants inhibit the autophagy that is mediated by mda-7/IL-24 [43]. Future studies focused on the clinical use of ROS inducers or autophagy modulators with mda-7/IL-24 could pave the way to achieve enhanced therapeutic effects against cancer.
5. Role of MDA-7/IL-24 in autophagy-apoptosis crosstalk
The involvement of MDA-7/IL-24 in autophagy-apoptosis crosstalk in different cancer models has been studied by several research groups. In GBM and transformed fibroblasts, ER stress induced by this cytokine treatment, causes activation and phosphorylation of PERK, a prolonged activity of which results in cytotoxicity. Park et al. reported regulation of autophagy by PERK upon treatment with MDA-7/IL-24. PERK −/− cells exhibited an increased resistance to MDA-7/IL-24-induced autophagy and cell death [211]. PERK signaling also initiated the vacuolization of LC3 protein, causing increased expression of autophagy markers like ATG5 and Beclin-1 [211]. On a similar note, overexpression of BiP/GRP78 inhibits MDA-7/IL-24-mediated autophagy [212]. Expression of a dominant negative PERK or knockdown of ATG5/Beclin-1 protects GBM cells from MDA-7/IL-24-mediated cell death [211]. 3-methyl adenine (3-MA), an autophagy inhibitor also protects cells from MDA-7/IL-24-mediated cell death. In renal and ovarian cancer cells, MDA-7/IL-24-induced autophagy is regulated predominantly via CD95 signaling [149]. GST-MDA-7 treatment in renal cancer cells resulted in enhanced ER stress response through CD95 signaling by causing toxic autophagy leading to cell death [96]. Studies have shown a direct association between suppression of ATG5 and/or Beclin-1 expression and MDA-7/IL-24-mediated cell death. In prostate cancer cells, unlike GBM cells, blocking autophagy by using 3-MA or by suppressing the expression of Beclin-1 enhanced MDA-7/IL-24 cytotoxicity [210]. Knockdown of ATG5 in these cells resulted in decreased MDA-7/IL-24-mediated toxic autophagy and apoptosis [210]. This variation in signaling could be explained by the differential role of ATG5 in apoptosis. In prostate cancer cells, autophagy aids in the cleavage of ATG5 into a 25-kDa fragment [210]. This small fragment consequently causes mitochondrial dysfunction though activation of pro-apoptotic molecules like Bax and Bak, leading to apoptosis.
6. Summary and future perspectives
MDA-7/IL-24 is an intriguing multidimensional IL-10 gene family member cytokine, which displays profound anti-cancer activities in pre-clinical studies, and in a Phase I/II study when tested in clinical trials with patients with advanced cancers. Within 7 years of discovery, mda-7/IL-24 had a rapid trajectory into the clinic where it was found to be safe and effective when injected intratumorally using a genetically engineered replication incompetent type 5 adenovirus (Ad.mda-7; INGN 241) in a wide-spectrum of cancers that had not responded to prior therapies, including melanomas and advanced carcinomas [43]. Studies performed in the last two decades in many research laboratories around the world are expanding our understanding of the molecular mechanisms of MDA-7/IL-24 functions. New MDA-7/IL-24 targets are being identified in the area of apoptosis and toxic autophagy that will facilitate development of preclinical therapeutic options that together with targeted therapies may engender enhanced anti-cancer outcomes in patients.
Specific areas of focus for current/future research will enhance our appreciation and utility of MDA-7/IL-24 as a broad-based anti-cancer tumor suppressor with potential clinical applications. 1) Determining how MDA-7/IL-24 differentiates cancer cells from normal/non-trans-formed cells when exerting its apoptosis/toxic autophagy functions will afford new leads in therapeutic applications. 2) Identification of new molecules/targets that can enhance or stabilize MDA-7/IL-24 protein will provide potential to enhance clinical outcomes. 3) Designing efficient modes of delivery of MDA-7/IL-24 using newer generation of conditionally replication competent CTVs that selectively replicate in and produce MDA-7/IL-24 in cancer cells will augment further therapeutic outcomes. 4) Development of effective systemic delivery approaches for MDA-7/IL-24 will expand applications for this therapeutic cytokine. The majority of studies performed to date have used intratumoral administration of adenoviruses expressing mda-7/IL-24. However, in the case of metastatic disease, which is the most common cause of patient death from solid cancers, optimum systemic therapies are required. Systemic application of pure cytokine proteins may cause serious adverse effects, such as autoimmunity and nonspecific inflammation, which remain major bottlenecks to therapeutic applications employing these molecules. Utilizing improved drug delivery systems such as UTMD and advanced biomaterials, such as nanoparticles, to deliver MDA-7/IL-24-based therapies, may define ways to more safely and effectively administer this cytokine, as a pure protein or through viral-delivery. Thus, it is mandatory and of clinical relevance to establish strategies for MDA-7IL-24-based systemic therapies (using viruses, cloned genes or recombinant proteins) and test these in preclinical animal models and then patients. 5) Targeted delivery of MDA-7/IL-24 using cell-based vehicles (including T cells, embryonic stem cells, mesenchymal stem cells, etc.) is also an area of intensive research that could reap significant benefits for utilizing MDA-7/IL-24 for cancer therapies. 6) Fusing MDA-7/IL-24 with cell penetrating peptides (CPPs) or tumor homing peptides [219] to target it to neighbor tumor sites to enhance its therapeutic efficiency may also be of value. For a gene therapy approach to be effective, since only a small group of cells are transduced and it is necessary to transduce the neighboring cells, an improved/strong bystander activity is necessary. According to this study the anti-cancer effect of cytokine-mediated gene therapy approaches could be amplified by CPPs/THPs fusion to the secreted protein due to bystander effects. However, caution should be taken to prevent structural miss-folding and abnormal activities while designing tethering of CPPs/THPs to cytokines and further studies (particularly in vivo) are needed. 7) Genetically modifying MDA-7/IL-24 to enhance its biological and biochemical properties may also represent a viable strategy for moving this molecule into the clinical arena. 8) Last but not least, identifying combination therapies with other treatment modalities (including chemotherapy, radiotherapy, antibody-based therapy, immunotherapy) that would augment MDA-7/IL-24-mediated anticancer effects are needed to bring these treatment modalities to the forefront for the therapy of this deadly disease.
Acknowledgements
The present study was supported in part by NCI Cancer Center Support Grant to the VCU Massey Cancer Center P30 CA016059 (to P.B.F. and D.S.), the National Foundation for Cancer Research (P.B.F.), the VCU Institute of Molecular Medicine (VIMM) (P.B.F.) and the Genetics Enhancement Fund (P.B.F., S.K.D. and L.E.). Support was also provided by a Sponsored Research Agreement from InterLeukin Combinatorial Therapies, Inc. (ILCT) (L.E.). P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research at the MCC.
Footnotes
Declaration of Competing Interest
P.B.F. is a co-founder of InterLeukin Combinatorial Therapies, Inc. (ILCT). P.B.F. and Virginia Commonwealth University own stock in ILCT. L.E. is the P.I. of a SRA provided by InterLeukin Combinatorial Therapies, Inc. to Virginia Commonwealth University. No other authors declare any potential conflicts with this research.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, CA Cancer J Clin. 68 (2018) (2018) 7–30, 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- [2].Syrigos KN, Zalonis A, Kotteas E, Saif MW, Targeted therapy for oesophageal cancer: an overview, Cancer Metastasis Rev. 27 (2008) 273–288, 10.1007/s10555-008-9117-z. [DOI] [PubMed] [Google Scholar]
- [3].Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN, Overview of resistance to systemic therapy in patients with breast cancer, Adv Exp Med Biol. 608 (2007) 1–22. [DOI] [PubMed] [Google Scholar]
- [4].Reed JC, Apoptosis-based therapies, Nat Rev Drug Discov. 1 (2002) 111–121, 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- [5].Ashkenazi A, Targeting the extrinsic apoptosis pathway in cancer, Cytokine Growth Factor Rev. 19 (2008) 325–331, 10.1016/j.cytogfr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- [6].Koff JL, Ramachandiran S, Bernal-Mizrachi L, A time to kill: targeting apoptosis in cancer, Int J Mol Sci. 16 (2015) 2942–2955, 10.3390/ijms16022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jiang HF, P.B, Use of a sensitive and efficient subtraction hybridization protocol for the identification of genes differentially regulated during the induction of differentiation in human melanoma cells, Mole. Cellu. Diffe 1 (1993) 285–299. [Google Scholar]
- [8].Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB, Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression, Oncogene 11 (1995) 2477–2486. [PubMed] [Google Scholar]
- [9].Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Dent P, et al. , Mda-7 (IL-24): signaling and functional roles, Biotechniques. Suppl (2002) 30–39. [PubMed] [Google Scholar]
- [10].Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB, The melanoma differentiation associated gene mda-7 suppresses cancer cell growth, Proc Natl Acad Sci U S A. 93 (1996) 9160–9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, et al. , The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice, Proc Natl Acad Sci U S A. 95 (1998) 14400–14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, et al. , MDA-7/IL-24 is a unique cytokine-tumor suppressor in the IL-10 family, Int Immunopharmacol. 4 (2004) 649–667, 10.1016/j.intimp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- [13].Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, et al. , Mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-targeted toxicity, Cytokine Growth Factor Rev. 21 (2010) 381–391, 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dent P, Yacoub A, Hamed HA, Park MA, Dash R, Bhutia SK, et al. , MDA-7/IL-24 as a cancer therapeutic: from bench to bedside, Anticancer Drugs. 21 (2010) 725–731, 10.1097/CAD.0b013e32833cfbe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dent P, Yacoub A, Hamed HA, Park MA, Dash R, Bhutia SK, et al. , The development of MDA-7/IL-24 as a cancer therapeutic, Pharmacol Ther. 128 (2010) 375–384, 10.1016/j.pharmthera.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sauane M, Dash R, et al. , Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24, Cancer Biol Ther. 8 (2009) 391–400. [DOI] [PubMed] [Google Scholar]
- [17].Fisher PB, Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 65 (2005) 10128–10138, 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- [18].Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, et al. , Mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic, Cancer Biol Ther. 2 (2003) S23–37 doi:. [PubMed] [Google Scholar]
- [19].Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, et al. , Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): novel gene therapeutic for metastatic melanoma, Toxicol Appl Pharmacol. 224 (2007) 300–307, 10.1016/j.taap.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, et al. , Mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine, Pharmacol Ther. 111 (2006) 596–628, 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, et al. , Mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (review), Int J Oncol. 31 (2007) 985–1007 doi:.. [PubMed] [Google Scholar]
- [22].Menezes ME, Bhatia S, Bhoopathi P, Das SK, Emdad L, Dasgupta S, et al. , MDA-7/IL-24: multifunctional cancer killing cytokine, Adv Exp Med Biol. 818 (2014) 127–153, 10.1007/978-1-4471-6458-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Menezes ME, Bhoopathi P, Pradhan AK, Emdad L, Das SK, Guo C, et al. , Role of MDA-7/IL-24 a multifunction protein in human diseases, Adv Cancer Res. 138 (2018) 143–182, 10.1016/bs.acr.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, et al. , Melanoma differentiation associated gene-7 (mda-7)/IL-24: a’ magic bullet’ for cancer therapy? Expert Opin Biol Ther. 7 (2007) 577–586, 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- [25].Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Lebedeva IV, Dent P, et al. , MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine, Cytokine Growth Factor Rev. 14 (2003) 35–51 doi:. [DOI] [PubMed] [Google Scholar]
- [26].Whitaker EL, Filippov VA, Duerksen-Hughes PJ, Interleukin 24: mechanisms and therapeutic potential of an anti-cancer gene, Cytokine Growth Factor Rev. 23 (2012) 323–331, 10.1016/j.cytogfr.2012.08.004. [DOI] [PubMed] [Google Scholar]
- [27].Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, et al. , Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells, Oncogene 24 (2005) 7552–7566, 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- [28].Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, et al. , Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis, Proc Natl Acad Sci USA. 105 (2008) 9763–9768, 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, et al. , Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study, Mol Ther. 11 (2005) 149–159, 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- [30].Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, et al. , Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients, Mol Ther. 11 (2005) 160–172, 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- [31].Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, et al. , Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties, Oncogene 20 (2001) 7051–7063, 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- [32].Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, et al. , The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24, J Immunol. 168 (2002) 6041–6046 doi:. [DOI] [PubMed] [Google Scholar]
- [33].Wang M, Tan Z, Zhang R, Kotenko SV, Liang P, Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2, J Biol Chem. 277 (2002) 7341–7347, 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]
- [34].Mumm JB, Ekmekcioglu S, Poindexter NJ, Chada S, Grimm EA, Soluble human MDA-7/IL-24: characterization of the molecular form(s) inhibiting tumor growth and stimulating monocytes, J Interferon Cytokine Res. 26 (2006) 877–886, 10.1089/jir.2006.26.877. [DOI] [PubMed] [Google Scholar]
- [35].Allen M, Pratscher B, Roka F, Krepler C, Wacheck V, Schofer C, et al. , Loss of novel mda-7 splice variant (mda-7s) expression is associated with metastatic melanoma, J Invest Dermatol. 123 (2004) 583–588, 10.1111/j.0022-202X.2004.23321.x. [DOI] [PubMed] [Google Scholar]
- [36].Allen M, Pratscher B, Krepler C, Frei K, Schofer C, Pehamberger H, et al. , Alternative splicing of IL-24 in melanocytes by deletion of exons 3 and 5, Int J Immunogenet. 32 (2005) 375–378, 10.1111/jM744-313X.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- [37].Sahoo A, Jung YM, Kwon HK, Yi HJ, Lee S, Chang S, et al. , A novel splicing variant of mouse interleukin (IL)-24 antagonizes IL-24-induced apoptosis, J Biol Chem. 283 (2008) 28860–28872, 10.1074/jbc.M802510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Filippov V, Schmidt EL, Filippova M, Duerksen-Hughes PJ, Splicing and splice factor SRp55 participate in the response to DNA damage by changing isoform ratios of target genes, Gene. 420 (2008) 34–41, 10.1016/j.gene.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Whitaker EL, Filippov V, Filippova M, Guerrero-Juarez CF, Duerksen-Hughes PJ, Splice variants of mda-7/IL-24 differentially affect survival and induce apoptosis in U2OS cells, Cytokine 56 (2011) 272–281, 10.1016/j.cyto.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang BX, Duan YJ, Dong CY, Zhang F, Gao WF, Cui XY, et al. , Novel functions for mda-7/IL-24 and IL-24 delE5: regulation of differentiation of acute myeloid leukemic cells, Mol Cancer Ther. 10 (2011) 615–625, 10.1158/1535-7163.MCT-10-0863. [DOI] [PubMed] [Google Scholar]
- [41].Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC, Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types, J Immunol. 167 (2001) 3545–3549 doi:. [DOI] [PubMed] [Google Scholar]
- [42].Dash R, Bhoopathi P, Das SK, Sarkar S, Emdad L, Dasgupta S, et al. , Novel mechanism of MDA-7/IL-24 cancer-specific apoptosis through SARI induction, Cancer Res. 74 (2014) 563–574, 10.1158/0008-5472.CAN-13-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pradhan AK, Talukdar S, Bhoopathi P, Shen XN, Emdad L, Das SK, et al. , Mda-7/IL-24 mediates cancer cell-specific death via regulation of miR-221 and the beclin-1 axis, Cancer Res. 77 (2017) 949–959, 10.1158/0008-5472.CAN-16-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gopalan B, Shanker M, Scott A, Branch CD, Chada S, Ramesh R, MDA-7/IL-24, a novel tumor suppressor/cytokine is ubiquitinated and regulated by the ubiquitin-proteasome system, and inhibition of MDA-7/IL-24 degradation enhances the antitumor activity, Cancer Gene Ther. 15 (2008) 1–8, 10.1038/sj.cgt.7701095. [DOI] [PubMed] [Google Scholar]
- [45].Tian H, Li L, Zhang B, Di J, Chen F, Li H, et al. , Critical role of lysine 123 in the ubiquitin-mediated degradation of MDA-7/IL-24, J Interferon Cytokine Res. 32 (2012) 575–582, 10.1089/jir.2012.0055. [DOI] [PubMed] [Google Scholar]
- [46].Poindexter NJ, Walch ET, Chada S, Grimm EA, Cytokine induction of interleukin-24 in human peripheral blood mononuclear cells, J Leukoc Biol. 78 (2005) 745–752, 10.1189/jlb.0205116. [DOI] [PubMed] [Google Scholar]
- [47].Jen EY, Poindexter NJ, Farnsworth ES, Grimm EA, IL-2 regulates the expression of the tumor suppressor IL-24 in melanoma cells, Melanoma Res. 22 (2012) 19–29, 10.1097/CMR.0b013e32834d2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wolk K, Kunz S, Asadullah K, Sabat R, Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 168 (2002) 5397–5402 doi:. [DOI] [PubMed] [Google Scholar]
- [49].Maarof G, Bouchet-Delbos L, Gary-Gouy H, Durand-Gasselin I, Krzysiek R, Dalloul A, Interleukin-24 inhibits the plasma cell differentiation program in human germinal center B cells, Blood. 115 (2010) 1718–1726, 10.1182/blood-2009-05-220251. [DOI] [PubMed] [Google Scholar]
- [50].Sahoo A, Lee CG, Jash A, Son JS, Kim G, Kwon HK, et al. , Stat6 and c-jun mediate Th2 cell-specific IL-24 gene expression, J Immunol. 186 (2011) 4098–4109, 10.4049/jimmunol.1002620. [DOI] [PubMed] [Google Scholar]
- [51].Schaefer G, Venkataraman C, Schindler U, Cutting edge: FISP (IL-4-induced secreted protein), a novel cytokine-like molecule secreted by Th2 cells, J Immunol. 166 (2001) 5859–5863 doi:. [DOI] [PubMed] [Google Scholar]
- [52].Wei L, Vahedi G, Sun HW, Watford WT, Takatori H, Ramos HL, et al. , Discrete roles of STAT4 and STAT6 transcription factors in tuning epigenetic modifications and transcription during T helper cell differentiation, Immunity 32 (2010) 840–851, 10.1016/j.immuni.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dabitao D, Hedrich CM, Wang F, Vacharathit V, Bream JH, Cell-specific requirements for STAT proteins and type I IFN receptor signaling discretely regulate IL-24 and IL-10 expression in NK cells and macrophages, J Immunol. 200 (2018) 2154–2164, 10.4049/jimmunol.1701340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Andoh A, Shioya M, Nishida A, Bamba S, Tsujikawa T, Kim-Mitsuyama S, et al. , Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease, J Immunol. 183 (2009) 687–695, 10.4049/jimmunol.0804169. [DOI] [PubMed] [Google Scholar]
- [55].Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, et al. , Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs, Exp Dermatol. 15 (2006) 991–1004, 10.1111/j.1600-0625.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- [56].Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A, Interleukin-10 and the interleukin-10 receptor, Annu Rev Immunol. 19 (2001) 683–765, 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- [57].Wang M, Liang P, Interleukin-24 and its receptors, Immunology 114 (2005) 166–170, 10.1111/j.1365-2567.2005.02094.xP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R, IL-22 increases the innate immunity of tissues, Immunity 21 (2004) 241–254, 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- [59].He M, Liang P, IL-24 transgenic mice: in vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis, J Immunol. 184 (2010) 1793–1798, 10.4049/jimmunol.0901829. [DOI] [PubMed] [Google Scholar]
- [60].Liang J, Huang RL, Huang Q, Peng Z, Zhang PH, Wu ZX, Adenovirus-mediated human interleukin 24 (MDA-7/IL-24) selectively suppresses proliferation and induces apoptosis in keloid fibroblasts, Ann Plast Surg. 66 (2011) 660–666, 10.1097/SAP.0b013e3181e05039. [DOI] [PubMed] [Google Scholar]
- [61].Poindexter NJ, Williams RR, Powis G, Jen E, Caudle AS, Chada S, et al. , IL-24 is expressed during wound repair and inhibits TGFalpha-induced migration and proliferation of keratinocytes, Exp Dermatol. 19 (2010) 714–722, 10.1111/j.1600-0625.2010.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ma Y, Chen H, Wang Q, Luo F, Yan J, Zhang XL, IL-24 protects against salmonella typhimurium infection by stimulating early neutrophil Th1 cytokine production, which in turn activates CD8+ T cells, Eur J Immunol. 39 (2009) 3357–3368, 10.1002/eji.200939678. [DOI] [PubMed] [Google Scholar]
- [63].Bastonero S, Le Priol Y, Armand M, Bernard CS, Reynaud-Gaubert M, Olive D, et al. , New microbicidal functions of tracheal glands: defective anti-infectious response to pseudomonas aeruginosa in cystic fibrosis, PLoS One. 4 (2009) e5357, , 10.1371/journal.pone.0005357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, et al. , IL-24 modulates IFN-gamma expression in patients with tuberculosis, Immunol Lett. 117 (2008) 57–62, 10.1016/j.imlet.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, et al. , Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus, Nat Immunol. 14 (2013) 804–811, 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Buzas K, Megyeri K, Staphylococci induce the production of melanoma differentiation-associated protein-7/IL-24, Acta Microbiol Immunol Hung. 53 (2006) 431–440, 10.1556/AMicr.53.2006A2. [DOI] [PubMed] [Google Scholar]
- [67].Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, et al. , Loss of MDA-7 expression with progression of melanoma, J Clin Oncol. 20 (2002) 1069–1074, 10.1200/JCQ.2002.20.4.1069. [DOI] [PubMed] [Google Scholar]
- [68].Ekmekcioglu S, Ellerhorst J, Mhashilkar AM, Sahin AA, Read CM, Prieto VG, et al. , Down-regulated melanoma differentiation associated gene (mda-7) expression in human melanomas, Int J Cancer. 94 (2001) 54–59, 10.1002/ijc.1437. [DOI] [PubMed] [Google Scholar]
- [69].Ishikawa S, Nakagawa T, Miyahara R, Kawano Y, Takenaka K, Yanagihara K, et al. , Expression of MDA-7/IL-24 and its clinical significance in resected non-small cell lung cancer, Clin Cancer Res. 11 (2005) 1198–1202 doi:. [PubMed] [Google Scholar]
- [70].Patani N, Douglas-Jones A, Mansel R, Jiang W, Mokbel K, Tumour suppressor function of MDA-7/IL-24 in human breast cancer, Cancer Cell Int. 10 (2010) 29, 10.1186/1475-2867-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ma M, Zhao R, Yang X, Zhao L, Liu L, Zhang C, et al. , The clinical significance of mda-7/IL-24 and C-myb expression in tumor tissues of patients with diffuse large B cell lymphoma, Exp Ther Med. 16 (2018) 649–656, 10.3892/etm.2018.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ma M, Zhao R, Yang X, Zhao L, Liu L, Zhang C, et al. , Low expression of mda-7/IL-24 and high expression of C-myb in tumour tissues are predictors of poor prognosis for burkitt lymphoma patients, Hematology 23 (2018) 448–455, 10.1080/10245332.2018.1435046. [DOI] [PubMed] [Google Scholar]
- [73].Menezes ME, Shen XN, Das SK, Emdad L, Guo C, Yuan F, et al. , MDA-7/IL-24 functions as a tumor suppressor gene in vivo in transgenic mouse models of breast cancer, Oncotarget. 6 (2015) 36928–36942, 10.18632/oncotarget.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li YJ, Liu G, Xia L, Xiao X, Liu JC, Menezes ME, et al. , Suppression of Her2/Neu mammary tumor development in mda-7/IL-24 transgenic mice, Oncotarget 6 (2015) 36943–36954, 10.18632/oncotarget.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zheng M, Bocangel D, Doneske B, Mhashilkar A, Ramesh R, Hunt KK, et al. , Human interleukin 24 (MDA-7/IL-24) protein kills breast cancer cells via the IL-20 receptor and is antagonized by IL-10, Cancer Immunol Immunother. 56 (2007) 205–215, 10.1007/s00262-006-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, et al. , Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach, Mol Ther. 18 (2010) 295–306, 10.1038/mt.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, et al. , bcl-2 and bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24, Oncogene 22 (2003) 8758–8773, 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- [78].Dash R, Dmitriev I, Su ZZ, Bhutia SK, Azab B, Vozhilla N, et al. , Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells, Cancer Gene Ther. 17 (2010) 447–456, 10.1038/cgt.2009.91. [DOI] [PubMed] [Google Scholar]
- [79].Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S, Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells, Mol Ther. 9 (2004) 510–518, 10.1016/j.ymthe.2004.01.019. [DOI] [PubMed] [Google Scholar]
- [80].Lv C, Su Q, Liang Y, Hu J, Yuan S, Oncolytic vaccine virus harbouring the IL-24 gene suppresses the growth of lung cancer by inducing apoptosis, Biochem Biophys Res Commun. 476 (2016) 21–28, 10.1016/j.bbrc.2016.05.088. [DOI] [PubMed] [Google Scholar]
- [81].Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, et al. , Mda-7 (IL-24) mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK, Proc Natl Acad Sci U S A. 99 (2002) 10054–10059, 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ekmekcioglu S, Mumm JB, Udtha M, Chada S, Grimm EA, Killing of human melanoma cells induced by activation of class I interferon-regulated signaling pathways via MDA-7/IL-24, Cytokine. 43 (2008) 34–44, 10.1016/j.cyto.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, et al. , Bystander activity of Ad-mda7: human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism, Mol Ther. 10 (2004) 1085–1095, 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- [84].Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, et al. , Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner, Oncogene. 22 (2003) 1164–1180, 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- [85].Yacoub A, Gupta P, Park MA, Rhamani M, Hamed H, Hanna D, et al. , Regulation of GST-MDA-7 toxicity in human glioblastoma cells by ERBB1, ERK1/2, PI3K, and JNK1–3 pathway signaling, Mol Cancer Ther. 7 (2008) 314–329, 10.1158/1535-7163.MCT-07-2150. [DOI] [PubMed] [Google Scholar]
- [86].Yacoub A, Mitchell C, Lebedeva IV, Sarkar D, Su ZZ, McKinstry R, et al. , Mda-7 (IL-24) inhibits growth and enhances radiosensitivity of glioma cells in vitro via JNK signaling, Cancer Biol Ther. 2 (2003) 347–353 doi:. [DOI] [PubMed] [Google Scholar]
- [87].Bhoopathi P, Lee N, Pradhan AK, Shen XN, Das SK, Sarkar D, et al. , Mda-7/IL-24 induces cell death in neuroblastoma through a novel mechanism involving AIF and ATM, Cancer Res. 76 (2016) 3572–3582, 10.1158/0008-5472.CAN-15-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, et al. , Mechanism of in vitro pancreatic cancer cell growth inhibition by melanoma differentiation-associated gene-7/interleukin-24 and perillyl alcohol, Cancer Res. 68 (2008) 7439–7447, 10.1158/0008-5472.CAN-08-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, et al. , Chemoprevention by perillyl alcohol coupled with viral gene therapy reduces pancreatic cancer pathogenesis, Mol Cancer Ther. 7 (2008) 2042–2050, 10.1158/1535-7163.MCT-08-0245. [DOI] [PubMed] [Google Scholar]
- [90].Ma Q, Jin B, Zhang Y, Shi Y, Zhang C, Luo D, et al. , Secreted recombinant human IL-24 protein inhibits the proliferation of esophageal squamous cell carcinoma eca-109 cells in vitro and in vivo, Oncol Rep. 35 (2016) 2681–2690, 10.3892/or.2016.4633. [DOI] [PubMed] [Google Scholar]
- [91].Wang C, Xue X, Yi J, Wu Z, Chen K, Zheng J, et al. , Replication-incompetent adenovirus vector-mediated MDA-7/IL-24 selectively induces growth suppression and apoptosis of hepatoma cell line SMMC-7721, J Huazhong Univ Sci Technolog Med Sci. 28 (2008) 80–83, 10.1007/s11596-008-0120-y. [DOI] [PubMed] [Google Scholar]
- [92].Wang CJ, Xue XB, Yi JL, Chen K, Zheng JW, Wang J, et al. , Melanoma differentiation-associated gene-7, MDA-7/IL-24, selectively induces growth suppression, apoptosis in human hepatocellular carcinoma cell line HepG2 by replication-incompetent adenovirus vector, World J Gastroenterol. 12 (2006) 1774–1779 doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Emdad L, Lebedeva IV, Su ZZ, Sarkar D, Dent P, Curiel DT, et al. , Melanoma differentiation associated gene-7/interleukin-24 reverses multidrug resistance in human colorectal cancer cells, Mol Cancer Ther. 6 (2007) 2985–2994, 10.1158/1535-7163.MCT-07-0399. [DOI] [PubMed] [Google Scholar]
- [94].Lebedeva IV, Su ZZ, Emdad L, Kolomeyer A, Sarkar D, Kitada S, et al. , Targeting inhibition of K-ras enhances Ad.Mda-7-induced growth suppression and apoptosis in mutant K-ras colorectal cancer cells, Oncogene 26 (2007) 733–744, 10.1038/sj.onc.1209813. [DOI] [PubMed] [Google Scholar]
- [95].Xu S, Oshima T, Imada T, Masuda M, Debnath B, Grande F, et al. , Stabilization of MDA-7/IL-24 for colon cancer therapy, Cancer Lett. 335 (2013) 421–430, 10.1016/j.canlet.2013.02.055. [DOI] [PubMed] [Google Scholar]
- [96].Park MA, Walker T, Martin AP, Allegood J, Vozhilla N, Emdad L, et al. , MDA-7/IL-24-induced cell killing in malignant renal carcinoma cells occurs by a cer-amide/CD95/PERK-dependent mechanism, Mol Cancer Ther. 8 (2009) 1280–1291, 10.1158/1535-7163.MCT-09-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wei L, Wang Z, Cui T, Yi F, Bu Y, Ding S, et al. , Proteomic analysis of cervical cancer cells treated with adenovirus-mediated MDA-7, Cancer Biol Ther. 7 (2008) 510–516 doi:. [DOI] [PubMed] [Google Scholar]
- [98].Mahasreshti PJ, Kataram M, Wu H, Yalavarthy LP, Carey D, Fisher PB, et al. , Ovarian cancer targeted adenoviral-mediated mda-7/IL-24 gene therapy, Gynecol Oncol. 100 (2006) 521–532, https://doi.org/10.1016Zj.ygyno.2005.08.042. [DOI] [PubMed] [Google Scholar]
- [99].Gopalan B, Shanker M, Chada S, Ramesh R, MDA-7/IL-24 suppresses human ovarian carcinoma growth in vitro and in vivo, Mol Cancer. 6 (2007) 11, 10.1186/1476-4598-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liu Z, Xu L, Yuan H, Zhang Y, Zhang X, Zhao D, Oncolytic adenovirusmediated mda7/IL24 expression suppresses osteosarcoma growth and enhances sensitivity to doxorubicin, Mol Med Rep. 12 (2015) 6358–6364, 10.3892/mmr.2015.4180. [DOI] [PubMed] [Google Scholar]
- [101].Rahmani M, Mayo M, Dash R, Sokhi UK, Dmitriev IP, Sarkar D, et al. , Melanoma differentiation associated gene-7/interleukin-24 potently induces apoptosis in human myeloid leukemia cells through a process regulated by endoplasmic reticulum stress, Mol Pharmacol. 78 (2010) 1096–1104, 10.1124/mol.110.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ma M, Zhao L, Sun G, Zhang C, Liu L, Du Y, et al. , Mda-7/IL-24 enhances sensitivity of B cell lymphoma to chemotherapy drugs, Oncol Rep. 35 (2016) 3122–3130, 10.3892/or.2016.4622. [DOI] [PubMed] [Google Scholar]
- [103].Liou GY, Storz P, Reactive oxygen species in cancer, Free Radic Res. 44 (2010) 479–496, 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Riha R, Gupta-Saraf P, Bhanja P, Badkul S, Saha S, stressed Out - therapeutic implications of ER stress related cancer research, Oncomedicine 2 (2017) 156–167, 10.7150/oncm.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wang G, Yang ZQ, Zhang K, Endoplasmic reticulum stress response in cancer: molecular mechanism and therapeutic potential, Am J Transl Res. 2 (2010) 65–74 doi:. [PMC free article] [PubMed] [Google Scholar]
- [106].Schiffmann S, Sandner J, Birod K, Wobst I, Angioni C, Ruckhaberle E, et al. , Ceramide synthases and ceramide levels are increased in breast cancer tissue, Carcinogenesis 30 (2009) 745–752, 10.1093/carcin/bgp061. [DOI] [PubMed] [Google Scholar]
- [107].Eager R, Harle L, Nemunaitis J, Ad-MDA-7; INGN 241: a review of preclinical and clinical experience, Expert Opin Biol Ther. 8 (2008) 1633–1643, 10.1517/14712598.8.10.1633. [DOI] [PubMed] [Google Scholar]
- [108].Ekmekcioglu S, Ellerhorst J, Smid CM, Prieto VG, Munsell M, Buzaid AC, et al. , Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival, Clin Cancer Res. 6 (2000) 4768–4775. [PubMed] [Google Scholar]
- [109].Das SK, Sarkar S, Dash R, Dent P, Wang XY, Sarkar D, et al. , Chapter One—cancer terminator viruses and approaches for enhancing therapeutic outcomes, Adv Cancer Res. 115 (2012) 1–38, 10.1016/B978-0-12-398342-8.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sarkar D, Lebedeva IV, Su ZZ, Park ES, Chatman L, Vozhilla N, et al. , Eradication of therapy-resistant human prostate tumors using a cancer terminator virus, Cancer Res. 67 (2007) 5434–5442, 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- [111].Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, et al. , A cancer terminator virus eradicates both primary and distant human melanomas, Cancer Gene Ther. 15 (2008) 293–302, 10.1038/cgt.2008.14. [DOI] [PubMed] [Google Scholar]
- [112].Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB, Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice, Proc Natl Acad Sci USA. 102 (2005) 14034–14039, 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sarkar S, Azab B, Quinn BA, Shen X, Dent P, Klibanov AL, et al. , Chemoprevention gene therapy (CGT) of pancreatic cancer using perillyl alcohol and a novel chimeric serotype cancer terminator virus, Curr Mol Med. 14 (2014) 125–140 doi:. [DOI] [PubMed] [Google Scholar]
- [114].Sarkar S, Quinn BA, Shen XN, Dash R, Das SK, Emdad L, et al. , Therapy of prostate cancer using a novel cancer terminator virus and a small molecule BH-3 mimetic, Oncotarget. 6 (2015) 10712–10727, 10.18632/oncotarget.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yacoub A, Hamed H, Emdad L, Dos Santos W, Gupta P, Broaddus WC, et al. , MDA-7/IL-24 plus radiation enhance survival in animals with intracranial primary human GBM tumors, Cancer Biol Ther. 7 (2008) 917–933 doi:. [DOI] [PubMed] [Google Scholar]
- [116].Azab BM, Dash R, Das SK, Bhutia SK, Sarkar S, Shen XN, et al. , Enhanced prostate cancer gene transfer and therapy using a novel serotype chimera cancer terminator virus (Ad.5/3-CTV), J Cell Physiol. 229 (2014) 34–43, 10.1002/jcp.24408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Dagogo-Jack I, Shaw AT, Tumour heterogeneity and resistance to cancer therapies, Nat Rev Clin Oncol. 15 (2018) 81–94, 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- [118].Ling S, Hu Z, Yang Z, Yang F, Li Y, Lin P, et al. , Extremely high genetic diversity in a single tumor points to prevalence of non-darwinian cell evolution, Proc Natl Acad Sci USA. 112 (2015) E6496–6505, 10.1073/pnas.1519556112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Loeb LA, Loeb KR, Anderson JP, Multiple mutations and cancer, Proc Natl Acad Sci USA. 100 (2003) 776–781, 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Gerl R, Vaux DL, Apoptosis in the development and treatment of cancer, Carcinogenesis 26 (2005) 263–270, 10.1093/carcin/bgh283. [DOI] [PubMed] [Google Scholar]
- [121].Strasser A, Harris AW, Cory S, bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship, Cell 67 (1991) 889–899 doi:. [DOI] [PubMed] [Google Scholar]
- [122].Elmore S, Apoptosis: a review of programmed cell death, Toxicol Pathol. 35 (2007) 495–516, 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wong RS, Apoptosis in cancer: from pathogenesis to treatment, J Exp Clin Cancer Res. 30 (2011) 87, 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Fernald K, Kurokawa M, Evading apoptosis in cancer, Trends Cell Biol. 23 (2013) 620–633, 10.1016/j.tcb.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Singh A, Ni J, Aggarwal BB, Death domain receptors and their role in cell demise, J Interferon Cytokine Res. 18 (1998) 439–450, 10.1089/jir.1998.18.439. [DOI] [PubMed] [Google Scholar]
- [126].Lavrik IN, Golks A, Krammer PH, Caspases: pharmacological manipulation of cell death, J Clin Invest. 115 (2005) 2665–2672, 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Jin Z, El-Deiry WS, Overview of cell death signaling pathways, Cancer Biol Ther. 4 (2005) 139–163 doi:. [DOI] [PubMed] [Google Scholar]
- [128].Fulda S, Debatin KM, Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy, Oncogene 25 (2006) 4798–4811, 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- [129].Brunelle JK, Letai A, Control of mitochondrial apoptosis by the bcl-2 family, J Cell Sci. 122 (2009) 437–441, 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Shamas-Din A, Kale J, Leber B, Andrews DW, Mechanisms of action of bcl-2 family proteins, Cold Spring Harb Perspect Biol. 5 (2013) a008714, 10.1101/cshperspect.a008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Suhaili SH, Karimian H, Stellato M, Lee TH, Aguilar MI, Mitochondrial outer membrane permeabilization: a focus on the role of mitochondrial membrane structural organization, Biophys Rev. 9 (2017) 443–457, 10.1007/s12551-017-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. , Cytochrome c and dATP-dependent formation of apaf-1/caspase-9 complex initiates an apoptotic protease cascade, Cell 91 (1997) 479–489 doi:. [DOI] [PubMed] [Google Scholar]
- [133].Ozaki T, Nakagawara A, Role of p53 in cell death and human cancers, Cancers (Basel). 3 (2011) 994–1013, 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Chowdhury D, Lieberman J, Death by a thousand cuts: granzyme pathways of programmed cell death, Annu Rev Immunol. 26 (2008) 389–420, 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Martinez-Lostao L, Anel A, Pardo J, How Do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res. 21 (2015) 5047–5056, 10.1158/1078-0432.CCR-15-0685. [DOI] [PubMed] [Google Scholar]
- [136].Yamazaki Y, Tsuruga M, Zhou D, Fujita Y, Shang X, Dang Y, et al. , Cytoskeletal disruption accelerates caspase-3 activation and alters the intracellular membrane reorganization in DNA damage-induced apoptosis, Exp Cell Res. 259 (2000) 64–78, 10.1006/excr.2000.4970. [DOI] [PubMed] [Google Scholar]
- [137].Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G, Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies, Aging (Albany NY). 8 (2016) 603–619, 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Pietsch EC, Sykes SM, McMahon SB, Murphy ME, The p53 family and programmed cell death, Oncogene 27 (2008) 6507–6521, 10.1038/onc.2008.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Ricci MS, Zong WX, Chemotherapeutic approaches for targeting cell death pathways, Oncologist 11 (2006) 342–357, 10.1634/theoncologist.11-4-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Vo TT, Letai A, BH3-only proteins and their effects on cancer, Adv Exp Med Biol. 687 (2010) 49–63 doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Hata AN, Engelman JA, Faber AC, The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics, Cancer Discov. 5 (2015) 475–487, 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE, Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation, Mol Ther. 9 (2004) 818–828, 10.1016/j.ymthe.2004.03.014. [DOI] [PubMed] [Google Scholar]
- [143].Wang Z, Wang Y, Chen Y, Lv J, The IL-24 gene protects human umbilical vein endothelial cells against H(2)O(2)-induced injury and may be useful as a treatment for cardiovascular disease, Int J Mol Med. 37 (2016) 581–592, 10.3892/ijmm.2016.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, et al. , Mechanism by which mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine, Cancer Res. 70 (2010) 5034–5045, 10.1158/0008-5472.CAN-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145.Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, et al. , Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species, Cancer Res. 63 (2003) 8138–8144 doi:. [PubMed] [Google Scholar]
- [146].Lebedeva IV, Washington I, Sarkar D, Clark JA, Fine RL, Dent P, et al. , Strategy for reversing resistance to a single anticancer agent in human prostate and pancreatic carcinomas, Proc Natl Acad Sci U S A. 104 (2007) 3484–3489, 10.1073/pnas.0700042104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Sauane M, Gopalkrishnan RV, Lebedeva I, Mei MX, Sarkar D, Su ZZ, et al. , Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways, J Cell Physiol. 196 (2003) 334–345, 10.1002/jcp.10309. [DOI] [PubMed] [Google Scholar]
- [148].Yacoub A, Hamed HA, Allegood J, Mitchell C, Spiegel S, Lesniak MS, et al. , PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells, Cancer Res. 70 (2010) 1120–1129, 10.1158/0008-5472.CAN-09-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Yacoub A, Liu R, Park MA, Hamed HA, Dash R, Schramm DN, et al. , Cisplatin enhances protein kinase R-like endoplasmic reticulum kinase- and CD95-depen-dent melanoma differentiation-associated gene-7/interleukin-24-induced killing in ovarian carcinoma cells, Mol Pharmacol. 77 (2010) 298–310, 10.1124/mol.109.061820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB, The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells, Oncogene 21 (2002) 708–718, 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- [151].Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, et al. , Apogossypol derivative BI-97C1 (sabutoclax) targeting mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity, Proc Natl Acad Sci U S A. 108 (2011) 8785–8790, 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Sauane M, Su ZZ, Dash R, Liu X, Norris JS, Sarkar D, et al. , Ceramide plays a prominent role in MDA-7/IL-24-induced cancer-specific apoptosis, J Cell Physiol. 222 (2010) 546–555, 10.1002/jcp.21969. [DOI] [PubMed] [Google Scholar]
- [153].Tian H, Zhang D, Gao Z, Li H, Zhang B, Zhang Q, et al. , MDA-7/IL-24 inhibits Nrf2-mediated antioxidant response through activation of p38 pathway and inhibition of ERK pathway involved in cancer cell apoptosis, Cancer Gene Ther. 21 (2014) 416–426, 10.1038/cgt.2014.45. [DOI] [PubMed] [Google Scholar]
- [154].Bhutia SK, Das SK, Azab B, Menezes ME, Dent P, Wang XY, et al. , Targeting breast cancer-initiating/stem cells with melanoma differentiation-associated gene-7/interleukin-24, Int J Cancer. 133 (2013) 2726–2736, 10.1002/ijc.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Pelicano H, Carney D, Huang P, ROS stress in cancer cells and therapeutic implications, Drug Resist Updat. 7 (2004) 97–110, 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- [156].Yacoub A, Mitchell C, Brannon J, Rosenberg E, Qiao L, McKinstry R, et al. , MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity, Mol Cancer Ther. 2 (2003) 623–632 doi:. [PubMed] [Google Scholar]
- [157].Gopalan B, Litvak A, Sharma S, Mhashilkar AM, Chada S, Ramesh R, Activation of the fas-fasL signaling pathway by MDA-7/IL-24 kills human ovarian cancer cells, Cancer Res. 65 (2005) 3017–3024, 10.1158/0008-5472.CAN-04-3758. [DOI] [PubMed] [Google Scholar]
- [158].Pataer A, Vorburger SA, Barber GN, Chada S, Mhashilkar AM, Zou-Yang H, et al. , Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via up-regulation of the double-stranded RNA-dependent protein kinase (PKR), Cancer Res. 62 (2002) 2239–243 doi:. [PubMed] [Google Scholar]
- [159].Pataer A, Vorburger SA, Chada S, Balachandran S, Barber GN, Roth JA, et al. , Melanoma differentiation-associated gene-7 protein physically associates with the double-stranded RNA-activated protein kinase PKR, Mol Ther. 11 (2005) 717–723, 10.1016/j.ymthe.2005.01.018. [DOI] [PubMed] [Google Scholar]
- [160].Bocangel D, Zheng M, Mhashilkar A, Liu Y, Ramesh R, Hunt KK, et al. , Combinatorial synergy induced by adenoviral-mediated mda-7 and herceptin in her-2+ breast cancer cells, Cancer Gene Ther. 13 (2006) 958–968, 10.1038/sj.cgt.7700972. [DOI] [PubMed] [Google Scholar]
- [161].Mosser DM, Zhang X, Interleukin-10: new perspectives on an old cytokine, Immunol Rev. 226 (2008) 205–218, 10.1111/jM600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Ekmekcioglu S, Ellerhorst JA, Mumm JB, Zheng M, Broemeling L, Prieto VG, et al. , Negative association of melanoma differentiation-associated gene (mda-7) and inducible nitric oxide synthase (iNOS) in human melanoma: MDA-7 regulates iNOS expression in melanoma cells, Mol Cancer Ther. 2 (2003) 9–17 doi:. [PubMed] [Google Scholar]
- [163].Su ZZ, Sarkar D, Emdad L, Duigou GJ, Young CS, Ware J, et al. , Targeting gene expression selectively in cancer cells by using the progression-elevated gene-3 promoter, Proc Natl Acad Sci USA. 102 (2005) 1059–1064, 10.1073/pnas.0409141102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Persaud L, Mighty J, Zhong X, Francis A, Mendez M, Muharam H, et al. , IL-24 promotes apoptosis through cAMP-dependent PKA pathways in human breast cancer cells, Int J Mol Sci. 19 (2018), 10.3390/ijms19113561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Yang M, Yang C, Tao Y, Tang J, Huang Q, Guo W, et al. , Combination therapy with F5/35 fiber chimeric conditionally replicative adenoviruses expressing IL-24 enhances the antitumor effect of temozolomide against melanoma, Cancer Med. 7 (2018) 5928–5942, 10.1002/cam4.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Xiao B, Li W, Yang J, Guo G, Mao XH, Zou QM, RGD-IL-24, a novel tumor-targeted fusion cytokine: expression, purification and functional evaluation, Mol Biotechnol. 41 (2009) 138–144, 10.1007/s12033-008-9115-y. [DOI] [PubMed] [Google Scholar]
- [167].Zhang BF, Liu JJ, Pei DS, Yang ZX, Di JH, Chen FF, et al. , Potent antitumor effect elicited by RGD-mda-7, an mda-7/IL-24 mutant, via targeting the integrin receptor of tumor cells, Cancer Biother Radiopharm. 26 (2011) 647–655, 10.1089/cbr.2011.0984. [DOI] [PubMed] [Google Scholar]
- [168].Hosseini E, Hosseini SY, Hashempour T, Fattahi MR, Sadeghizadeh M, Effect of RGD coupled MDA-7/IL-24 on apoptosis induction in a hepatocellular carcinoma cell line, Mol Med Rep. 15 (2017) 495–501, 10.3892/mmr.2016.6009. [DOI] [PubMed] [Google Scholar]
- [169].Puig-Saus C, Rojas LA, Laborda E, Figueras A, Alba R, Fillat C, et al. , iRGD tumor-penetrating peptide-modified oncolytic adenovirus shows enhanced tumor transduction, intratumoral dissemination and antitumor efficacy, Gene Ther. 21 (2014) 767–774, 10.1038/gt.2014.52. [DOI] [PubMed] [Google Scholar]
- [170].Schweichel JU, Merker HJ, The morphology of various types of cell death in prenatal tissues, Teratology 7 (1973) 253–266, 10.1002/tera.1420070306. [DOI] [PubMed] [Google Scholar]
- [171].Mizushima N, Klionsky DJ, Protein turnover via autophagy: implications for metabolism, Annu Rev Nutr. 27 (2007) 19–40, 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- [172].Mizushima N, Ohsumi Y, Yoshimori T, Autophagosome formation in mammalian cells, Cell Struct Funct. 27 (2002) 421–429 doi:. [DOI] [PubMed] [Google Scholar]
- [173].Talukdar S, Pradhan AK, Bhoopathi P, Shen XN, August LA, Windle JJ, et al. , Regulation of protective autophagy in anoikis-resistant glioma stem cells by SDCBP/MDA-9/Syntenin, Autophagy 14 (2018) 1845–1846, 10.1080/15548627.2018.1502564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Lin L, Baehrecke EH, Autophagy, cell death, and cancer, Mol Cell Oncol. 2 (2015) e985913, , 10.4161/23723556.2014.985913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Tang HM, Tang HL, Anastasis: recovery from the brink of cell death, R Soc Open Sci. 5 (2018) 180442, 10.1098/rsos.180442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Yu L, Chen Y, Tooze SA, Autophagy pathway: cellular and molecular mechanisms, Autophagy 14 (2018) 207–215, 10.1080/15548627.2017.1378838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Bhutia SK, Mukhopadhyay S, Sinha N, Das DN, Panda PK, Patra SK, et al. , Autophagy: cancer’s friend or foe? Adv Cancer Res. 118 (2013) 61–95, 10.1016/B978-0-12-407173-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, et al. , Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death, J Natl Cancer Inst. 99 (2007) 1410–1414, 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- [179].Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R, Role of non-canonical beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells, Cell Death Differ. 15 (2008) 1318–1329, 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- [180].Cao C, Subhawong T, Albert JM, Kim KW, Geng L, Sekhar KR, et al. , Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells, Cancer Res. 66 (2006) 10040–10047, 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- [181].Ambrosini G, Musi E, Ho AL, de Stanchina E, Schwartz GK, Inhibition of mutant GNAQ signaling in uveal melanoma induces AMPK-dependent autophagic cell death, Mol Cancer Ther. 12 (2013) 768–776, 10.1158/1535-7163.MCT-12-1020. [DOI] [PubMed] [Google Scholar]
- [182].Shimizu S, Yoshida T, Tsujioka M, Arakawa S, Autophagic cell death and cancer, Int J Mol Sci. 15 (2014) 3145–3153, 10.3390/ijms15023145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Shimizu S, Konishi A, Nishida Y, Mizuta T, Nishina H, Yamamoto A, et al. , Involvement of JNK in the regulation of autophagic cell death, Oncogene 29 (2010) 2070–2082, 10.1038/onc.2009.487. [DOI] [PubMed] [Google Scholar]
- [184].Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, et al. , Autophagy in malignant transformation and cancer progression, EMBO J. 34 (2015) 856–880, 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Rodriguez OC, Choudhury S, Kolukula V, Vietsch EE, Catania J, Preet A, et al. , Dietary downregulation of mutant p53 levels via glucose restriction: mechanisms and implications for tumor therapy, Cell Cycle. 11 (2012) 4436–4446, 10.4161/cc.22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Choudhury S, Kolukula VK, Preet A, Albanese C, Avantaggiati ML, Dissecting the pathways that destabilize mutant p53: the proteasome or autophagy? Cell Cycle. 12 (2013) 1022–1029, 10.4161/cc.24128.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, et al. , The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis, Cancer Cell. 13 (2008) 343–354, 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [188].Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. , Autophagy suppresses tumorigenesis through elimination of p62, Cell 137 (2009) 1062–1075, 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Isakson P, Bjoras M, Boe SO, Simonsen A, Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein, Blood 116 (2010) 2324–2331, 10.1182/blood-2010-01-261040. [DOI] [PubMed] [Google Scholar]
- [190].Wang Z, Cao L, Kang R, Yang M, Liu L, Zhao Y, et al. , Autophagy regulates myeloid cell differentiation by p62/SQSTM1-mediated degradation of PML-RARalpha oncoprotein, Autophagy 7 (2011) 401–411 doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Goussetis DJ, Gounaris E, Wu EJ, Vakana E, Sharma B, Bogyo M, et al. , Autophagic degradation of the BCR-ABL oncoprotein and generation of antileukemic responses by arsenic trioxide, Blood 120 (2012) 3555–3562, 10.1182/blood-2012-01-402578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].de Vries A, Flores ER, Miranda B, Hsieh HM, van Oostrom CT, Sage J, et al. , Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function, Proc Natl Acad Sci U S A. 99 (2002) 2948–2953, 10.1073/pnas.052713099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Green DR, Galluzzi L, Kroemer G, Mitochondria and the autophagy-inflamma-tion-cell death axis in organismal aging, Science 333 (2011) 1109–1112, 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Takahashi Y, Hori T, Cooper TK, Liao J, Desai N, Serfass JM, et al. , Bif-1 haploinsufficiency promotes chromosomal instability and accelerates myc-driven lymphomagenesis via suppression of mitophagy, Blood 121 (2013) 1622–1632, 10.1182/blood-2012-10-459826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, et al. , Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice, Cell 131 (2007) 1149–1163, 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- [196].Rello-Varona S, Lissa D, Shen S, Niso-Santano M, Senovilla L, Marino G, et al. , Autophagic removal of micronuclei, Cell Cycle. 11 (2012) 170–176, 10.4161/cc.11.1.18564. [DOI] [PubMed] [Google Scholar]
- [197].Guo H, Chitiprolu M, Gagnon D, Meng L, Perez-Iratxeta C, Lagace D, et al. , Autophagy supports genomic stability by degrading retrotransposon RNA, Nat Commun. 5 (2014) 5276, 10.1038/ncomms6276. [DOI] [PubMed] [Google Scholar]
- [198].Belaid A, Cerezo M, Chargui A, Corcelle-Termeau E, Pedeutour F, Giuliano S, et al. , Autophagy plays a critical role in the degradation of active RHOA, the control of cell cytokinesis, and genomic stability, Cancer Res. 73 (2013) 4311–4322, 10.1158/0008-5472.CAN-12-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, et al. , Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis, Genes Dev. 21 (2007) 1621–1635, 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Park JM, Tougeron D, Huang S, Okamoto K, Sinicrope FA, Beclin 1 and UVRAG confer protection from radiation-induced DNA damage and maintain centrosome stability in colorectal cancer cells, PLoS One. 9 (2014) e100819, , 10.1371/journal.pone.0100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC, Rapamycin and mTOR-in-dependent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies, Cell Death Differ. 16 (2009) 46–56, 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- [202].Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA, Metformin prevents tobacco carcinogen-induced lung tumorigenesis, Cancer Prev Res (Phila). 3 (2010) 1066–1076, 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Chen N, Karantza V, Autophagy as a therapeutic target in cancer, Cancer Biol Ther. 11 (2011) 157–168 doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Lian J, Wu X, He F, Karnak D, Tang W, Meng Y, et al. , A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating bcl-2-Beclin1 interaction at endoplasmic reticulum, Cell Death Differ. 18 (2011) 60–71, 10.1038/cdd.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Kondo Y, Kanzawa T, Sawaya R, Kondo S, The role of autophagy in cancer development and response to therapy, Nat Rev Cancer. 5 (2005) 726–734, 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- [206].Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, et al. , Control of autophagy by oncogenes and tumor suppressor genes, Cell Death Differ. 16 (2009) 87–93, 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- [207].White E, DiPaola RS, The double-edged sword of autophagy modulation in cancer, Clin Cancer Res. 15 (2009) 5308–5316, 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Eskelinen EL, Saftig P, Autophagy: a lysosomal degradation pathway with a central role in health and disease, Biochim Biophys Acta. 1793 (2009) 664–673, 10.1016/j.bbamcr.2008.07.014. [DOI] [PubMed] [Google Scholar]
- [209].Yonekawa T, Thorburn A, Autophagy and cell death, Essays Biochem. 55 (2013) 105–117, 10.1042/bse0550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Bhutia SK, Das SK, Azab B, Dash R, Su ZZ, Lee SG, et al. , Autophagy switches to apoptosis in prostate cancer cells infected with melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24), Autophagy 7 (2011) 1076–1077 doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Park MA, Yacoub A, Sarkar D, Emdad L, Rahmani M, Spiegel S, et al. , PERK-dependent regulation of MDA-7/IL-24-induced autophagy in primary human glioma cells, Autophagy 4 (2008) 513–515 doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Yacoub A, Park MA, Gupta P, Rahmani M, Zhang G, Hamed H, et al. , Caspase-, cathepsin-, and PERK-dependent regulation of MDA-7/IL-24-induced cell killing in primary human glioma cells, Mol Cancer Ther. 7 (2008) 297–313, 10.1158/1535-7163.MCT-07-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Pradhan AK, Emdad L, Das SK, Sarkar D, Fisher PB, The enigma of miRNA regulation in cancer, Adv Cancer Res. 135 (2017) 25–52, 10.1016/bs.acr.2017.06.001. [DOI] [PubMed] [Google Scholar]
- [214].le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A, et al. , Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation, EMBO J. 26 (2007) 3699–3708, 10.1038/sj.emboj.7601790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Zhang CZ, Zhang JX, Zhang AL, Shi ZD, Han L, Jia ZF, et al. , MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma, Mol Cancer. 9 (2010) 229, 10.1186/1476-4598-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, Calin GA, et al. , MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma, Oncogene 27 (2008) 5651–5661, 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- [217].Li B, Lu Y, Wang H, Han X, Mao J, Li J, et al. , miR-221/222 enhance the tumorigenicity of human breast cancer stem cells via modulation of PTEN/Akt pathway, Biomed Pharmacother. 79 (2016) 93–101, 10.1016/jbiopha.2016.01.045. [DOI] [PubMed] [Google Scholar]
- [218].Pradhan AK, Bhoopathi P, Talukdar S, Scheunemann D, Sarkar D, Cavenee WK, et al. , MDA-7/IL-24 regulates the miRNA processing enzyme DICER through downregulation of MITF, Proc Natl Acad Sci USA. (2019), 10.1073/pnas.1819869116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Rasoolian M, Kheirollahi M, Hosseini SY, MDA-7/interleukin 24 (IL-24) in tumor gene therapy: application of tumor penetrating/homing peptides for improvement of the effects, Expert Opin Biol Ther. 19 (2019) 211–223, 10.1080/14712598.2019.1566453. [DOI] [PubMed] [Google Scholar]


