Abstract
Platinum therapy represents first line of treatment in many malignancies but its high systemic toxicity limits the therapeutic dosage. Herein, we report the synthesis of carboplatin-like complexes with azide and alkyne functional groups and the formation of a platinum (II) - nuclear localization sequence peptide (Pt-NLS) hybrid to improve the import of platinum (II) complexes directly into the cell’s nucleus. The Pt-NLS hybrid successfully enters cells and their nuclei, forming Pt-induced nuclear lesions. The in vitro efficacy of Pt-NLS is high, superior to native carboplatin at the same concentration. The methodology used is simple and cost-effective and most importantly can easily be extended to load the Pt (II) onto other supports, opening new possibilities for enhanced delivery of Pt (II) therapy.
Keywords: platinum (II) complexes, click chemistry, chemoresistant, NLS peptides
Graphical Abstract
Cisplatin, cis-diamminedichloroplatinum (II) is a DNA-damaging chemotherapy agent used to treat various types of malignancies.1–3 The efficacy of cisplatin is afforded by the ability to form covalent DNA adducts, including intrastrand 1,2d (GpG) and 1,3d (GpXpG) adducts and interstrand G-G cross-links. These complexes distort the DNA structure, thereby inhibiting replication and transcription, and, if not repaired, lead to cell death via the apoptotic pathway.4 The driving mechanism of cisplatin activation is the difference in the chloride ion concentration in the bloodstream (~100 mM) and in the cell (~20 mM).5 Once inside the cell, cisplatin activation involves the exchange of chloride ligands to water with the formation of cis-[Pt(NH3)2Cl(H2O)]+ moiety that subsequently enters the nucleus via electrostatic attraction to nucleophilic DNA.6 The high aquation rate of cisplatin leads to high systemic toxicity and reduced clinical dosing of the drug.7 In addition, the deactivation of cisplatin through interactions with plasma proteins or thiol groups in metallothioenin, glutathione, and methionine results in subtherapeutic concentrations of cisplatin reaching the cell’s nucleus. Both of these factors contribute to diminished DNA damage with concomitant activation of DNA repair pathways, contributing to eventual tumor chemoresistance.8 Thus, the low drug levels that reach DNA when using conventional treatments result in negligible effect and failed therapy.9
Second-generation Pt (II) complexes have lower nephro- and neurotoxicities due to their pharmacodynamics, but often do not possess equivalent activity to cisplatin in all platinum-sensitive tumors. For example, cisplatin is still the first treatment option in testicular germ-cell cancers, squamous cell carcinoma of the head and neck and bladder cancer.6 Carboplatin or oxaliplatin, with the similar mechanism of action to cisplatin, are Pt-based agents with cis-amine non-leaving group ligands and chelating carboxylate ligands instead of chlorines.6,10–12 The aquation rate is much slower in these complexes with only 3% hydrolysed species after 150 days.12 This stability can substantially slow down the nuclear entry of the complexes and eventually lead to lower efficacy of the drugs. Thus, new platinum complexes that would address these issues are of great interest, including new Pt (II) complexes, Pt (IV) prodrugs that undergo intracellular reduction to produce active Pt (II) form, or Pt (II) complexes with non-classical mechanisms of action.6,13,14 Although thousands have already been reported, very few new platinum agents advance to clinical trials.
Another strategy being explored is to increase the intracellular concentration of platinum via formation of platinum conjugates with targeting ligands that would direct the therapy directly to cancer cells. Some of such systems include Pt (II) – ligand conjugates that target estrogen receptors,15 folate receptors,16 angiogenic vessels,17 liver,18 or that take the advantage of high glucose uptake by cancer cells.19 Another recently emerging technology involves the use of small peptides as targeting ligands.20 Peptides are considered to be highly selective and well-tolerated, which results in an increased interest for their pharmaceutical applications. Currently, more than a hundred small peptide therapeutics are undergoing clinical trials.21 The peptide-platinum conjugates have appeared but mostly with Pt (IV) prodrugs and only a few with Pt (II)22–26 or PEG-Pt (II).26 The platinum-peptide ligand approach is very promising but yet challenging to produce highly efficacious therapy.
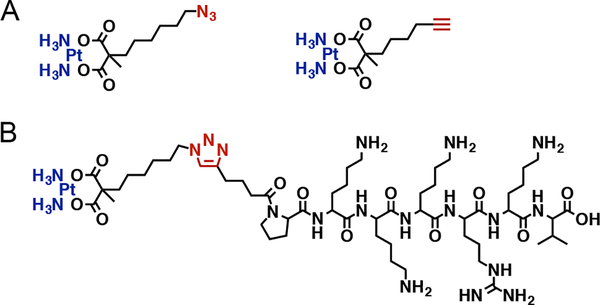
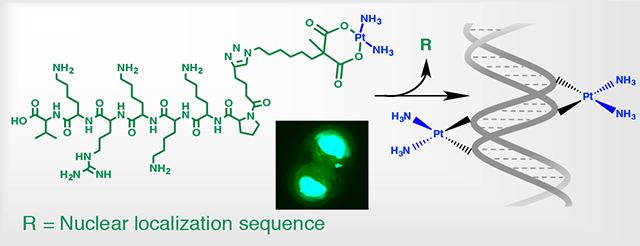
Herein, we report the synthesis of new carboplatin-like Pt (II) complexes; Pt(II)-N3 (C10H21N5O4Pt) and Pt(II)-CCH (C10H18N2O4Pt), and Pt (II)-peptide hybrid with SV40 large T antigen-derived PKKKRKV peptide. Our complexes feature carboxylate ligands coordinated to the Pt metal opposite the amine groups and the azide or the alkyne functional group available for click chemistry. The peptide used represents a canonical nuclear localization signal (NLS), a protein motif recognized by protein carriers called importins, which are characterized by the presence of basic residues lysine and arginines. These motifs direct proteins into the nucleus. This peptide was selected because it is a binding domain to importin a that is necessary to cross the tight nuclear membrane and accumulate into the nucleus in ample amounts.27,28 The low aqueous solubility of both Pt(II)-N3 and Pt(II)-CCH complexes hampered the direct conjugation of the complexes to the NLS peptide. Thus, the Pt-NLS hybrid was formed in two steps at the N- terminus of the peptide, at the end of solid-phase peptide synthesis. The schematic of the synthetic strategy and full description are provided in SI. Shortly, the NLS peptide was reacted with the azide-terminated complex precursor to form intermediate peptide 1. This was followed by the reaction of peptide 1 with activated Pt (II) to form peptide 2, described as the Pt-NLS hybrid. The chemical structure of the complexes and the Pt-NLS hybrid structure are shown in Figure 1, frames A and B respectively.
Figure 1.
Chemical structure of Platinum (II) complexes Pt(II)-N3 and Pt(II)-CCH. (A), and the structure of the Pt-NLS hybrid (B).
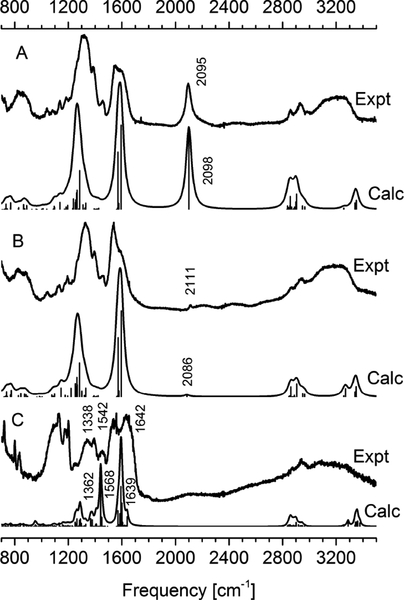
The FT-IR spectra were measured for both Pt(II)-N3 and Pt(II)-CCH complexes, and also for Pt-NLS hybrid. Several characteristic vibrations were detected and their assignment confirmed by density functional theory (DFT) calculations.29 Comparison of experimental with computed spectra is presented in Figure 2. The characteristic N=N=N and C≡C stretching vibrations unique to Pt(II)-N3 and Pt(II)-CCH complexes are observed as an intense signal at 2095 cm−1 (panel A) and weak signal at 2111 cm−1 (panel B), respectively. These complexes also show strong IR bands around 1320 cm−1 and 1600 cm−1, both are assigned to C=O stretchings. The spectrum of Pt-NLS peptide hybrid (panel C) is dominated by the peptide characteristic amide I, II, and III bands observed at 1642 cm−1, 1542 cm−1 and 1338 cm−1, respectively. The positions of IR bands are in a good agreement with those in DFT spectrum. However, the intensities for C=O stretchings at around 1600 cm−1 and 1450 cm−1 are overemphasized by DFT. This is likely due to C=O proximity and coordination to Pt(II) that overestimates electron polarizability in the model and thus over promotes the computed intensities. The IR spectra are also recorded at the lower frequency range of 900–700 cm−1 (supplementary information) which show characteristic deformations for the Pt(II) coordinated six-member ring.
Figure 2.
Experimental and calculated IR spectra of Pt(II) complexes; Pt(II)-N3 complex (panel A), Pt(II)-CCH complex (panel B) and Pt-NLS hybrid (panel C).
To confirm the molecular formulas of the complexes and Pt-NLS hybrid we used HRMS and the spectra obtained are presented in supporting figures S12, S13, and S16. The exact mass of the Pt(II)-N3 complex is 470.1241 au, and of the Pt(II)-CCH complex is 425.0914 au; the Pt-NLS hybrid is 1447.7865 au. All masses are identified in the corresponding mass spectra, and the isotopic distribution pattern is consistent with the proposed composition of the molecule.
The Pt(II)-N3 and Pt(II)-CCH complexes have low aqueous solubility but after tethering to the NLS peptide the hybrid is water soluble at concentrations higher than 35mM; greatly facilitating its application to biological systems.
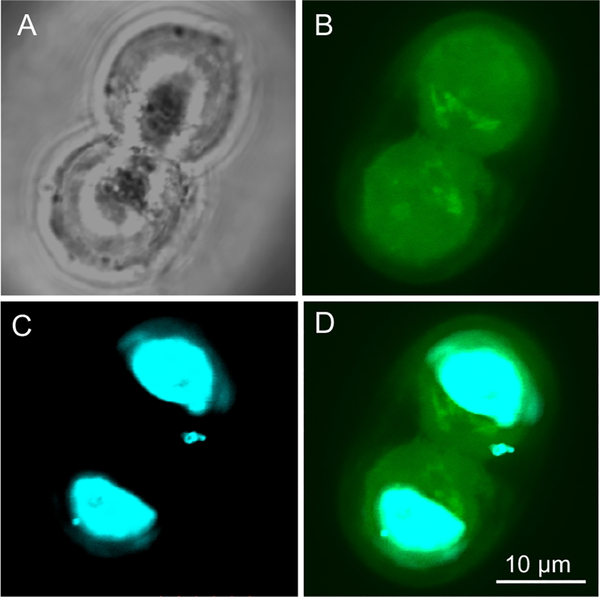
The rationale for delivery of Pt-NLS therapy is twofold. First, the hybrid has to efficiently translocate through the cellular membrane into the cytoplasm. Finally, it has to cross the nuclear membrane to localize into the nucleus, the site of Pt(II) action. The class of NLS peptides, which are rich in basic amino acids, is cationic in nature and some sequences, including PKKKRKV, have cell penetrating properties and effectively carry a cargo such as DNA or small proteins into the cytoplasm.30,31 We first evaluated the membrane-translocating property of the PKKKRKV peptide in the CP70 platinum resistant cell line using confocal microscopy. We used a fluorescein labeled NLS, where the dye was conjugated to the N-terminus of the peptide at the end of solid-state peptide synthesis. Figure 3 demonstrates representative confocal images of the CP70 cells after 72 h of incubation with the NLS peptide, the time point at which the cytotoxicity assay for Pt-NLS hybrid was performed. The NLS peptide (green) effectively penetrated the cellular and nuclear membranes and was distributed evenly in both the cytosol and nucleus (blue). This result confirms that the NLS peptide enters both subcellular compartments and thus is suitable to be used for delivery of small drug molecules.
Figure 3.
Confocal images of CP70 cells incubated with NLS peptide: (A) phase contrast image, (B) fluorescence image of the same cells, (C) nuclei of the cells, (D) overlay of C and D.
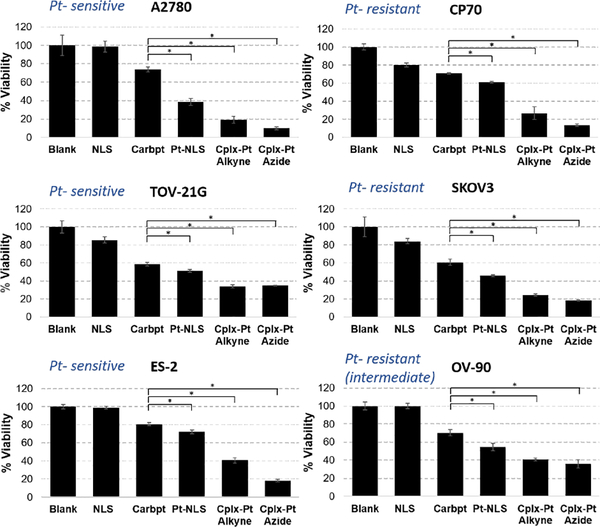
As already noted, platinum therapy represents first-line therapy in a number of different human malignancies and resistance represents a major cause of treatment failure. Therefore, the effect of the Pt-NLS hybrid on cell viability was analyzed using the isogenic cell line models, A2780 and CP70, in which CP70 was derived to be platinum resistant.32 The mechanism by which platinum resistance is gained is unknown but, if defined, could ultimately provide insight into platinum sensitivity. Some authors have suggested the acquisition of a p53 mutation in the CP70 cells. However, we demonstrated by Sanger sequencing that both A2780 and CP70 cell lines are p53 WT and thus a change in p53 status cannot explain differences in platinum response.33 Our selection of these cell lines for this study was specifically based on the fact of the cell lines’ shared genetic background but different responses to platinum. We also tested the effectiveness of the Pt-NLS hybrid on cell viability across a number of other ovarian cancer-derived cell lines with differential platinum resistance, genomic features and histotypes.34 The results were compared to controls of carboplatin, NLS without Pt, and free Pt complexes (added as suspensions) and a summary of the results is presented in Figure 4. The concentrations used correspond to IC50 values of Pt-NLS hybrid obtained for each cell line (supporting information, figures S25–S30). The Pt-NLS hybrid demonstrated high cytotoxicity, significantly superior to carboplatin, regardless of cell line tested. The most pronounced effect of Pt-NLS was observed in A2780 cells, where the viability was decreased by 60% whereas treatment with carboplatin at the same concentration decreased the viability by 25% only. The CP 70 cell viability decreased by 40% following incubation with the Pt-NLS hybrid while carboplatin treated cells had only a 30% decrease. In other cell types of different Pt resistance, Pt-NLS hybrid consistently had a significantly greater effect on cell viability than carboplatin. In the Pt-sensitive cell lines, TOV- 21G and ES-2, the Pt-NLS decreased viability by ~50% and ~30%, respectively, whereas carboplatin reduced viability by 40% (TOV-21G) and 20% (ES-2). The viabilities of the platinum resistant cell lines SKOV3 and OV-90, were decreased by 50% and 45%, respectively, following incubation with Pt-NLS, but only about 40% and 30% respectively by carboplatin. The complexes alone are highly active in vitro in all cell lines but their aqueous solubility is very low, thus they were added as a suspension. The biological application of Pt (II) therapy necessitates aqueous solubility of therapeutics. The formation of Pt-NLS hybrid addresses the solubility issue and at the same time heightens the therapy outcomes by targeting the Pt (II) directly into the cell’s nucleus. Taken together, these results demonstrated an increased effectiveness of the Pt (II) anchored NLS over conventional carboplatin.
Figure 4.
Viability of platinum sensitive (left) and resistant (right) cells after 72h incubation with Pt-NLS hybrid and controls. The purity of Pt-NLS hybrid was 87%. Each column represents the mean and standard deviation of N=3 and p<0.005. The concentrations are constant in each cell line, and are as follows: 24.6 μM (A2780), 52.8 μM (CP70), 56.6 μM (TOV-21G), 56.6 μM (SKOV3), 18.4 μM (ES-2), 59.0 μM (OV-90). Abbreviations: Carboplatin (Carbpt), Complex-Pt (Cplx-Pt).
Our complexes feature carboxylate ligands and the expected mechanism of action should follow that of carboplatin. Carboplatin binds DNA to form intrastrand crosslinks and adducts that change the conformation of DNA and inhibit its replication. Although this mechanism is similar to that of cisplatin, carboplatin is less cytotoxic. This is attributed to higher stability of the leaving group on and delayed formation of active Pt (II) form. This may contribute to slow nuclear entry of Pt (II) and insufficient number of DNA adducts formed, resulting in quick DNA repair. We evaluated the concentration of Pt (II) inside the nuclei of the cells after 72 h incubation with Pt-NLS. We isolated total genomic DNA from the isogenic A2780 and CP70 cell cultures and quantified Pt (II) using AAS. The platinum to DNA base pair ratio was found to be approximately 1:114 for CP70 and 1:244 for A2780 cell lines. Since there are ~10 base pairs per turn in a helix, this gives one Pt (II) attached approximately every 10th in CP70 and every 20th in A2780. The quantification is based on all cellular DNA, including organellar as well, resulting in slight underestimation of Pt (II) inside the nucleus. This indicates that the Pt-NLS is efficiently transported into the cell’s nucleus and Pt (II) effectively binds to nuclear DNA.
In summary, we report on the generation and use of Pt (II) complexes featuring carboxylate ligands and azide or alkyne functionalities. We also synthesized the Pt-NLS peptide hybrid that efficiently translocates through the cellular membranes and delivers Pt (II) therapy directly into the cell’s nucleus. In the cell culture models of differential platinum sensitivity, we demonstrated that Pt-NLS has superior effects on decreasing viability when compared to carboplatin in both platinum sensitive and resistant cancer cell lines. With regard to future potential uses, importantly, the design of the complexes allows for immobilization of active Pt (II) onto other supports. Efforts to extend this approach to incorporate high payloads of Pt (II) into biodegradable nanoparticles to increase the therapeutic dosage are currently underway.
Supplementary Material
Acknowledgements.
We thank Dr. Guillermo Gerona-Navarro for technical support with NLS peptide and Pt-NLS hybrid purification.
Funding Sources.
This work was supported by the National Cancer Institute under grant SC2CA206194. No competing financial interests are declared.
Footnotes
Supporting Information.
The detailed synthesis of complexes and the hybrid, in vitro methods, HRMS, 1H NMR, 13C NMR, IR spectra, HPLC analysis (PDF). The Supporting Information is available free of charge on the ACS publication website.
REFERENCES
- (1).Galanski M; Jakupec MA; Keppler BK Update of the preclinical situation of anticancer platinum complexes: novel design strategies and innovative analytical approaches. Curr. Med. Chem 2005, 12, 2075–2094. [DOI] [PubMed] [Google Scholar]
- (2).Wheate NJ; Walker S; Craig GE; Oun R The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127, DOI: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]
- (3).Dasari S; Tchounwou PB Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol 2014, 740, 364–378, DOI: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Fichtinger-Schepman AM; van Oosterom AT; Lohman PH; Berends F cis-Diamminedichloroplatinum(II)-induced DNA adducts in peripheral leukocytes from seven cancer patients: quantitative immunochemical detection of the adduct induction and removal after a single dose of cis-diamminedichloroplatinum(II). Cancer Res. 1987, 47, 3000–3004. [PubMed] [Google Scholar]
- (5).Reishus JW; Martin DS Jr. cis-Dichlorodiammineplatinum(II). Acid hydrolysis and isotopic exchange of the chloride ligands. J. Am. Chem. Soc 1961, 83, 2457–2462, DOI: 10.1021/ja01472a009. [DOI] [Google Scholar]
- (6).Dilruba S; Kalayda GV Platinum-based drugs: past, present and future. Cancer Chemother. Pharmacol 2016, 77, 1103–1124, DOI: 10.1007/s00280-016-2976-z. [DOI] [PubMed] [Google Scholar]
- (7).Englander EW DNA damage response in peripheral nervous system: coping with cancer therapy-induced DNA lesions. DNA Repair 2013, 12, 685–690, DOI: 10.1016/jj.dnarep.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Galluzzi L; Senovilla L; Vitale I; Michels J; Martins I; Kepp O; Castedo M; Kroemer G Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883, DOI: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- (9).Boeckman HJ; Trego KS; Turchi JJ Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol. Cancer. Res 2005, 3, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Jerremalm E; Videhult P; Alvelius G; Griffiths WJ; Bergman T; Eksborg S; Ehrsson H Alkaline hydrolysis of oxaliplatin--isolation and identification of the oxalato monodentate intermediate. J. Pharm. Sci 2002, 91, 2116–2121, DOI: 10.1002/jps.10201. [DOI] [PubMed] [Google Scholar]
- (11).Di Pasqua AJ; Goodisman J; Kerwood DJ; Toms BB; Dubowy RL; Dabrowiak JC Activation of carboplatin by carbonate. Chem. Res. Toxicol 2006, 19, 139–149, DOI: 10.1021/tx050261s. [DOI] [PubMed] [Google Scholar]
- (12).Di Pasqua AJ; Kerwood DJ; Shi Y; Goodisman J; Dabrowiak JC Stability of carboplatin and oxaliplatin in their infusion solutions is due to self-association. Dalton Trans. 2011, 40, 4821–4825, DOI: 10.1039/c0dt01758b. [DOI] [PubMed] [Google Scholar]
- (13).Johnstone TC; Suntharalingam K; Lippard SJ The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chemical Rev. 2016, 116, 3436–3486, DOI: 10.1021/acs.chemrev.5b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Basu U; Banik B; Wen R; Pathak RK; Dhar S The Platin-X series: activation, targeting, and delivery. Dalton Trans. 2016, 45, 12992–13004, DOI: 10.1039/c6dt01738j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Descoteaux C; Provencher-Mandeville J; Mathieu I; Perron V; Mandal SK; Asselin E; Berube G Synthesis of 17beta-estradiol platinum(II) complexes: biological evaluation on breast cancer cell lines. Bioorg. Med. Chem. Lett 2003, 13, 3927–3931. [DOI] [PubMed] [Google Scholar]
- (16).Aronov O; Horowitz AT; Gabizon A; Gibson D Folate-targeted PEG as a potential carrier for carboplatin analogs. Synthesis and in vitro studies. Bioconjugate Chem. 2003, 14, 563–574, DOI: 10.1021/bc025642l. [DOI] [PubMed] [Google Scholar]
- (17).Abu-Lila A; Suzuki T; Doi Y; Ishida T; Kiwada H Oxaliplatin targeting to angiogenic vessels by PEGylated cationic liposomes suppresses the angiogenesis in a dorsal air sac mouse model. J. Controlled Release 2009, 134, 18–25, DOI: 10.1016/j.jconrel.2008.10.018. [DOI] [PubMed] [Google Scholar]
- (18).Larena MG; Martinez-Diez MC; Macias RI; Dominguez MF; Serrano MA; Marin JJ Relationship between tumor cell load and sensitivity to the cytostatic effect of two novel platinum-bile acid complexes, Bamet-D3 and Bamet-UD2. J. Drug Targeting 2002, 10, 397–404, DOI: 10.1080/1061186021000001841. [DOI] [PubMed] [Google Scholar]
- (19).Mikata Y; Shinohara Y; Yoneda K; Nakamura Y; Brudzinska I; Tanase T; Kitayama T; Takagi R; Okamoto T; Kinoshita I; Doe M; Orvig C; Yano S Unprecedented sugar-dependent in vivo antitumor activity of carbohydrate-pendant cis- diamminedichloroplatinum(II) complexes. Bioorg. Med. Chem. Lett 2001, 11, 3045–3047. [DOI] [PubMed] [Google Scholar]
- (20).Ndinguri MW; Solipuram R; Gambrell RP; Aggarwal S; Hammer RP Peptide targeting of platinum anti-cancer drugs. Bioconjugate Chem. 2009, 20, 1869–1878, DOI: 10.1021/bc900065r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cheetham AG; Keith D; Zhang P; Lin R; Su H; Cui H Targeting Tumors with Small Molecule Peptides. Curr. Cancer Drug Targets 2016, 16, 489–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wisnovsky SP; Wilson JJ; Radford RJ; Pereira MP; Chan MR; Laposa RR; Lippard SJ; Kelley SO Targeting mitochondrial DNA with a platinum-based anticancer agent. Chem. Biol 2013, 20, 1323–1328, DOI: 10.1016/j.chembiol.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Gandioso A; Shaili E; Massaguer A; Artigas G; Gonzalez-Canto A; Woods JA; Sadler PJ; Marchan V An integrin-targeted photoactivatable Pt(IV) complex as a selective anticancer pro-drug: synthesis and photoactivation studies. Chem. Commun 2015, 51, 9169–9172, DOI: 10.1039/c5cc03180j. [DOI] [PubMed] [Google Scholar]
- (24).Massaguer A; Gonzalez-Canto A; Escribano E; Barrabes S; Artigas G; Moreno V; Marchan V Integrin-targeted delivery into cancer cells of a Pt(IV) pro-drug through conjugation to RGD-containing peptides. Dalton Trans. 2015, 44, 202–212, DOI: 10.1039/c4dt02710h. [DOI] [PubMed] [Google Scholar]
- (25).Abramkin S; Valiahdi SM; Jakupec MA; Galanski M; Metzler-Nolte N; Keppler BK Solid-phase synthesis of oxaliplatin-TAT peptide bioconjugates. Dalton Trans. 2012, 41, 3001–3005, DOI: 10.1039/c2dt12024k. [DOI] [PubMed] [Google Scholar]
- (26).Aronov O; Horowitz AT; Gabizon A; Fuertes MA; Perez JM; Gibson D Nuclear localization signal-targeted poly(ethylene glycol) conjugates as potential carriers and nuclear localizing agents for carboplatin analogues. Bioconjugate Chem. 2004, 15, 814–823, DOI: 10.1021/bc0499331. [DOI] [PubMed] [Google Scholar]
- (27).Cartier R; Reszka R Utilization of synthetic peptides containing nuclear localization signals for nonviral gene transfer systems. Gene Ther. 2002, 9, 157–167, DOI: 10.1038/sj.gt.3301635. [DOI] [PubMed] [Google Scholar]
- (28).Zanta MA; Belguise-Valladier P; Behr JP Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc. Natl. Acad. Sci. U.S.A 1999, 96, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Frisch M. J. e. a. Gaussian 09, Revision B.01. Gaussian, Inc.: Wallingford, CT: 2009. [Google Scholar]
- (30).Costantini DL; Hu M; Reilly RM Peptide motifs for insertion of radiolabeled biomolecules into cells and routing to the nucleus for cancer imaging or radiotherapeutic applications. Cancer Biother. Radiopharm 2008, 23, 3–24, DOI: 10.1089/cbr.2007.0430. [DOI] [PubMed] [Google Scholar]
- (31).Ragin AD; Morgan RA; Chmielewski J Cellular import mediated by nuclear localization signal Peptide sequences. Chem. Biol 2002, 9, 943–948. [DOI] [PubMed] [Google Scholar]
- (32).Parker RJ; Eastman A; Bostick-Bruton F; Reed E Acquired cisplatin resistance in human ovarian cancer cells is associated with enhanced repair of cisplatin-DNA lesions and reduced drug accumulation. J. Clin. Invest 1991, 87, 772–777, DOI: 10.1172/JCI115080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Chen Y; Camacho SC; Silvers TR; Razak AR; Gabrail NY; Gerecitano JF; Kalir E; Pereira E; Evans BR; Ramus SJ; Huang F; Priedigkeit N; Rodriguez E; Donovan M; Khan F; Kalir T; Sebra R; Uzilov A; Chen R; Sinha R; Halpert R; Billaud JN; Shacham S; McCauley D; Landesman Y; Rashal T; Kauffman M; Mirza MR; Mau-Sorensen M; Dottino P; Martignetti JA Inhibition of the Nuclear Export Receptor XPO1 as a Therapeutic Target for Platinum-Resistant Ovarian Cancer. Clin. Cancer Res 2017, 23, 1552–1563, DOI: 10.1158/1078-0432.CCR-16-1333. [DOI] [PubMed] [Google Scholar]
- (34).Domcke S; Sinha R; Levine DA; Sander C; Schultz N Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun 2013, 4, 2126, DOI: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.