Abstract
A new alternative method for the production of biodiesel from rendered fat of all categories of animal by‐products was assessed. The process was compared to the approved biodiesel production process described in Chapter IV Section 2 D of Annex IV of Commission Regulation (EU) 142/2011. Tallow derived from Category 1 material is treated according to Method 1 from the same Regulation (133°C, 20 min, 3 bar) and subsequently mixed with 15% methanol, heated to reaction temperature (220°C) in several heat exchangers and transferred into the continuous conversion reactor by means of a high pressure pump (80 bar) for 30 min. In the conversion phase, there is an exposure to methanol in the absence of alkaline or acidic conditions. The impact of this procedure on the thermostability of transmissible spongiform encephalopathy (TSE) has not been assessed in the literature. After the reaction, the biodiesel/glycerol mixture is distilled under vacuum at a minimum temperature of 150°C and a maximum pressure of 10 mbar, which is equivalent to the distillation step in the approved biodiesel production process, for which a 3 log10 reduction factor in PrP27–30 was obtained. Therefore, a similar level of TSE infectivity reduction could be expected for that phase of the method. A previous EFSA Opinion established that a reduction of 6 log10 in TSE infectivity should be achieved by any proposed alternative method in order to be equivalent to the approved processing method. This level of reduction has not been shown with experimental trials run under conditions equivalent to the ones described for the RepCat process. It was not possible to conclude whether or not the level of TSE infectivity reduction in the RepCat process is at least of 6 log10. Therefore, it was also not possible to conclude about the equivalence with the approved biodiesel production process.
Keywords: animal by‐product, category 1, TSE, risk reduction, biodiesel, rendered fat, conversion
Summary
Following a request from the Federal Ministry of Health and Women's Affairs of Austria (Competent Authority) on behalf of the company BDI – BioEnergy International AG, the European Food Safety Authority (EFSA) Scientific Panel on Biological Hazards (BIOHAZ Panel) was asked to deliver a scientific opinion on an alternative method for the production of biodiesel from rendered fat of all categories of animal by‐products.
Under point 5 of Article 20 of Regulation 1069/2009, it is specified that EFSA shall assess whether the alternative method submitted ensures that risks to public or animal health are: ‘(a) controlled in a manner which prevents their proliferation before disposal in accordance with this Regulation or the implementing measures thereof; or (b) reduced to a degree which is at least equivalent, for the relevant category of animal by‐products, to the processing methods laid down pursuant to point (b) of the first subparagraph of Article 15(1)’.
The process consists of esterification and transesterification with methanol in a conversion unit, followed by water/methanol recovery and distillation of the products: biodiesel and glycerine. As fat/tallow derived from Category 1 material is used in the new process, a prion reduction target is presented by the Applicant fulfilling the requested limits according to a previous opinion of EFSA. The process was compared to the approved biodiesel production process described in Chapter IV Section 2 D of Annex IV of Regulation (EU) No 142/2011. The feedstock is mixed with 15% methanol, heated to reaction temperature (minimum 220°C) in several heat exchangers and transferred into the continuous conversion reactor by means of a high pressure pump (pressure higher than 80 bar). The reaction conditions are maintained for a minimum of 30 min at 220°C and 80 bar pressure. After the conversion phase, the pressure is reduced to ambient pressure; methanol and water are evaporated and recovered from the steam as a distillate. Methanol is reused for the process and the distilled water is discharged into a wastewater treatment plant. After the reaction, the biodiesel/glycerol mixture is distilled under vacuum at a minimum temperature of 150°C and a maximum pressure of 10 mbar.
The data used in the assessment were provided by the Applicant in a dossier and in response to an ad hoc request for missing information. Three scientific papers and a previous EFSA Scientific Opinion which were cited in support of the application were also provided and were evaluated together with other relevant scientific articles dealing with prion inactivation.
The references provided by the Applicant which were relevant for this evaluation have been thoroughly reviewed in terms of the test conditions in which experiments were performed, validity of the titration method, the strain and animal model used, the confirmation of disease and the interpretation of the results. Although a certain level of similarity can be found between the proposed method and the approved biodiesel production process, there is an exposure to methanol in the absence of alkaline or acidic conditions. The impact of this procedure on the thermostability of transmissible spongiform encephalopathy (TSE) has not been assessed in the literature. It was, therefore, concluded that there are no available data on the level of TSE infectivity reduction achieved in conditions equivalent to those used in the proposed process.
A previous EFSA Opinion established that a reduction of 6 log10 in the TSE infectivity should be achieved for Category 1 of animal by‐products, by any proposed alternative method in order to be equivalent to the approved processing method. This level of reduction has not been shown with experimental trials run under conditions equivalent to the ones described for the RepCat process.
The distillation phase of the RepCat process is equivalent to the distillation step of the approved biodiesel production process and similar to the one described in one of the articles provided by the Applicant, where a 3 log10 risk reduction factor in PrP27–30 was obtained. Therefore, a similar level of risk reduction could be expected for this phase of the proposed process.
It cannot be assumed that the overall risk reduction corresponds to the sum of the reduction of TSE infectivity in each of the process phases. This has not been confirmed by experimental data. This is the rationale behind requesting a 6 log10 reduction by the process itself.
It was not possible to conclude whether or not the level of TSE infectivity reduction in the RepCat process is at least of 6 log10. Therefore, it was also not possible to conclude about the equivalence with the approved biodiesel production process.
1. Introduction
1.1. Background and Terms of Reference as provided by the Austrian Competent Authority
On 22 February 2017, the European Food Safety Authority (EFSA) received from the Federal Ministry of Health and Women's Affairs of Austria (Competent Authority) an application (mandate and technical dossier) under Regulation (EC) No 1069/20091 and Regulation (EU) No 142/20112, for the evaluation of a new alternative process, known as the RepCat process, for production of biodiesel from rendered fat of all categories of animal by‐products, submitted on behalf of the company BDI ‐ BioEnergy International AG (hereinafter referred to as the Applicant).
The application dossier includes a number of supporting documents including an evaluation of the application by the Competent Authority. The supporting documents are listed in the enclosed Index.
1.2. Interpretation of the Terms of Reference
According to what is described in Chapter IV Section 2 D of Annex IV of Regulation (EU) No 142/2011 for the biodiesel production process as an alternative processing method, the Competent Authority may authorise a process using the following process parameters:
‘D. Biodiesel production process
-
Starting material
For this process, a fat fraction derived from animal by‐products of all categories may be used.
-
Processing method
Biodiesel production shall be carried out according to the following processing standards:
-
Unless fish oil or rendered fat are used which have been produced in accordance with Sections VIII or XII of Annex III to Regulation (EC) No 853/2004, respectively, the fat fraction derived from animal by‐products must be first processed using:
in the case of Category 1 or 2 materials, processing method 1 (pressure sterilisation) as set out in Chapter III; and
in the case of Category 3 materials, any of the processing methods 1 to 5 or processing method 7 or, in the case of material derived from fish, processing methods 1 to 7 as set out in Chapter III; EN L 54/34 Official Journal of the European Union 26.2.2011.
-
The processed fat must then be processed further using one of the following methods:
-
a process whereby the processed fat must be separated from the protein and in the case of fat from ruminant origin, insoluble impurities in excess of 0.15% by weight must be removed, and the processed fat must be subsequently submitted to esterification and transesterification.
However, esterification is not required for processed fat derived from Category 3 material. For esterification the pH must be reduced to less than 1 by adding sulphuric acid (H2SO4) or an equivalent acid and the mixture must be heated to 72°C for at least 2 h during which it must be intensely mixed.
Transesterification must be carried out by increasing the pH to about 14 with potassium hydroxide or with an equivalent base at 35–50°C for at least 15 min. Transesterification shall be carried out twice under the conditions described in this point using a new base solution. This process must be followed by refinement of the products including vacuum distillation at 150°C, leading to biodiesel;
a process using equivalent process parameters authorised by the Competent Authority’.
-
-
The Applicant requested recognition that the conversion process (minimum 220°C, minimum 80 bar, minimum 30 min) to be equivalent in terms of the transmissible spongiform encephalopathy (TSE) reduction as described for several other applications with milder conditions as described in the literature. Furthermore, the Applicant highlighted the fact that the conditions of the RepCat process are at least equivalent to a process set out in Annex XIII of Commission Regulation EU No 142/2011.
After discussion, the EFSA Panel on Biological Hazards (BIOHAZ) acknowledged that the proposal was to be considered as a proposal for a new alternative method, and not a proposal for applying alternative parameters for an approved alternative processing method (biodiesel production process) as provided for in Chapter IV Section 2 D of Annex IV of Regulation (EU) No 142/2011, as there were too many fundamental differences between the methods. Therefore, after receiving the request, EFSA evaluated the method proposed by the Applicant under the frame of Art. 20 of Regulation (EU) No 1069/2009. The Panel decided that the process set out in Annex XIII of Commission Regulation EU No 142/2011 was not directly relevant to an assessment under Art. 20 of Regulation (EC) No 1069/2009.
1.3. Additional information
During the assessment for the authorisation of an alternative method as set out in Article 20 of Regulation (EU) No 1069/2009, it was deemed necessary to request additional information and data from the Applicant on certain technical aspects of the dossier (letter sent on 20 July 2017). The Applicant was asked to describe better the specifications of the procedure, mainly in relation to the conversion phase, and if the process could be considered a closed system and how the conditions were monitored and maintained. Clarification was also sought on how the minimum retention time for the conversion phase is monitored and how well the conditions for the vacuum distillation phase, mainly in relation to pressure, are maintained and controlled.
2. Data and methodologies
2.1. Data
The data used in the assessment were provided by the Applicant as requested in Annex VII of Commission Regulation (EU) No 142/2011 and its amendment by Commission Regulation (EU) No 749/20113. A process flow diagram and a Hazards Analysis and Critical Control Point (HACCP) plan were attached to the application dossier. Additional data provided by the Applicant were added to the application and reviewed accordingly.
Three scientific papers which were cited in support of the application were provided by the Applicant and evaluated (Müller et al., 2006, 2008; Mittelbach et al., 2007) together with a previous EFSA Scientific Opinion (EFSA BIOHAZ Panel, 2011). A fourth paper (not provided by the Applicant) that includes additional data on the thermal degradation of prions in the presence of fats (Müller and Riesner, 2005) was also reviewed. In addition, relevant scientific papers provided by experts of the Working Group (WG) were considered during the assessment.
A report submitted by the Competent Authority, in this case the Federal Ministry of Health and Women's Affairs of Austria, related to the application, was also considered.
2.2. Methodology
The methodology followed by the BIOHAZ Panel to evaluate the application is described below.
As set out in Article 20 of European Union Regulation (EC) No 1069/2009, EFSA is required to assess whether the method submitted ensures that the risks to public or animal health are:
‘controlled in a manner which prevents their proliferation before disposal in accordance with this Regulation or the implementing measures thereof; or
reduced to a degree which is at least equivalent, for the relevant categories of animal by‐products, to the processing methods laid down pursuant to point (b) of the first subparagraph of Article 15(1)’.
In essence, point (b) above means that the proposed processing method must reduce the risk to a degree which is at least equivalent to that achieved by the processing methods that have already been approved for the same category of animal by‐products (ABPs).
This requirement for applications is elaborated in the EU Regulation (EC) No 142/2011 implementing Regulation (EC) No 1069/2009 and amended by Commission Regulation (EU) No 749/2011. According to point 2(d), Chapter II, Annex VII of Regulation 142/2011, any application for the evaluation of alternative methods shall ‘show that the most resistant biological hazards associated with the category of materials to be processed are reduced in any products generated during the process, including the wastewater, at least to the degree achieved by the processing standards laid down in this Regulation for the same category of animal by‐products. The degree of risk reduction must be determined with validated direct measurements, unless modelling or comparisons with other processes are acceptable’.
The approved alternative processing methods are described in Chapter IV, Annex IV of Regulation (EU) No 142/2011. The approved alternative method for biodiesel production must be carried out following prior processing of rendered animal fat by pressure sterilisation (processing Method 1 – particle size of no greater than 50 mm must be heated to a core temperature of more than 133°C for at least 20 min without interruption at a pressure (absolute) of at least 3 bar), as set in paragraph A of Chapter III of Annex IV of Commission Regulation (EU) 142/2011.
No definitive standards have been set down in relation to risk reduction for alternative methods dealing with Category 1 material. The BIOHAZ Panel decided that a reduction of 6 log10 in TSE infectivity by the alternative method is required to consider it at least equivalent, for Category 1 ABPs, to the processing methods laid down in the legislation. This issue is further discussed in Section 3.1.
In the proposal, the Applicant did not completely follow the standard format for applications for alternative methods, defined by Art. 16 of Regulation (EU) No 142/2011 and set out in its Annex VII, amended by the Regulation (EU) No 749/2011.
The main points requested by the EFSA Statement on the format for applications for new alternative methods for ABPs (EFSA BIOHAZ Panel, 2010a) are:
Full description of the process
Full description of the material to be treated
Hazard identification
Level of risk reduction
HACCP plan
Risks associated with interdependent processes
Risks associated with the intended end use of the products.
The general EFSA Guidelines on ‘the general principles of transparency in the scientific aspects of risk assessments carried out by EFSA’ (EFSA, 2009) and ‘uncertainty in EFSA scientific assessment’ (EFSA Scientific Committee, 2016) from EFSA's Scientific Committee have been taken into account in answering the Terms of Reference (TOR) of this Scientific opinion document.
The Applicant should document as fully as possible the different aspects of each of these steps. In this proposal, only limited risk assessment elements were given for the following steps: hazard identification (Section 3.4), risk associated with interdependent processes (Section 3.7) and risk associated with the intended end use (Section 3.8). So, although the Applicant provided a dossier which followed, in general terms, the standard format, it was not structured completely as established by EFSA and some sections were not completely addressed. In any case, the experts/Panel agreed to go ahead with the assessment on the basis that the information provided with the clarifications received in response to the request sent to the Applicant and to the CA was sufficient.
3. Assessment
3.1. Introduction
The BIOHAZ Panel has undertaken the assessment of different applications involving the use of Category 1 material proposed for the production of fuels and other oleochemical processes (EFSA, 2003, 2004; EFSA BIOHAZ Panel, 2010b, 2011). More recently, the BIOHAZ Panel published a scientific opinion on the application by Neste Oil for an alternative method for the treatment of Category 1 rendered animal fat intended for the production of renewable fuels by a continuous multiple‐step catalytic hydro‐treatment process (EFSA BIOHAZ Panel, 2015) where all the previous opinions were extensively discussed and taken into consideration. The alternative method evaluated in the 2015 opinion was based on a pretreatment by degumming and bleaching followed by a hydro‐treatment comprising three main processes: catalytic hydro‐treatment, stripping and isomerisation. The minimum processing conditions were pressure 30 bar, temperature 265°C and retention time 20 min. In a Scientific Opinion on the capacity of oleochemical processes to limit risk of TSEs (EFSA BIOHAZ Panel, 2011), a 5 log10 reduction was considered sufficient for the whole process, based on estimation of the percentage of insoluble impurities and probability of TSE infectivity present. However, based on the outcome of previous EFSA Opinions and expert evaluation, the BIOHAZ Panel considered that a reduction of 6 log10 in the TSE agent by the alternative method was necessary to consider the process at least equivalent, for Category 1 of ABPs, to the processing methods previously approved. As stated in previous opinions, there is uncertainty on the adding effect of consecutive processes on risk reduction. In the process evaluated (EFSA BIOHAZ Panel, 2015), experimental data in the reviewed literature showed that similar processes at temperatures between 160°C and 200°C deliver at least a 6 log10 reduction in TSE infectivity in 20 min. Thus, at least a similar level of reduction would be expected to be produced by the alternative method proposed. This was in addition to the inactivation achieved by the pressure sterilisation method prior to the application of the alternative method. The treatment specified by Neste Oil and subsequently assessed by EFSA was considered to achieve the required level of TSE inactivation, as long as the critical limits of key parameters were achieved as specified by the Applicant.
3.2. Full description of the RepCat process
As stated by the Applicant, the RepCat process involves pressure sterilisation (Method 1) applied prior to the application of the alternative conversion method in a pressurised conversion unit at high temperature with the addition of methanol under the following conditions: temperature higher than 220°C, pressure higher than 80 bar with a retention time of at least 30 min and a methanol concentration of at least 15% to achieve steam. After removal of the methanol and water by distillation, the biodiesel and glycerol are distilled under vacuum at temperatures higher than 150°C.
In Table 1, the process presented by the Applicant is compared to the approved biodiesel production process.
Table 1.
Comparison between the process presented by the applicant and the approved biodiesel production process
| Commission Regulation (EU) No 142/2011 ANNEX IV – processing/CHAPTER IV – alternative processing methods/D. Biodiesel production process | RepCat process | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Starting material |
Fat fraction derived from animal by‐products – Category 1 Maximum insoluble impurities – 0.15% by weight |
Starting material |
Fat fraction derived from animal by‐products – Category 1 Maximum insoluble impurities – 0,15% by weight |
||||||||
| Processing method | pH | Temperature | Pressure | Time | Final product | Processing method | Other parameter | Temperature | Pressure | Time | Final product |
| Processing Method 1 (pressure sterilisation)a | Not applicable | 133°C | 3 bar | 20 min | Glycerine and esters (biodiesel) | Processing Method 1 (pressure sterilisation)a | Not applicable | 133°C | 3 bar | 20 min | FAME (fatty acid methyl esters) and glycerol |
| Esterification | Reduced to less than 1 by adding sulphuric acid (H2SO4) or an equivalent acid | 72°C | Not applicable |
2 h Mixed intensely |
Conversion (esterification and transesterification) | Feedstock mixed with 15% methanol | 220°C | 80 bar | 30 min | ||
| Transesterification | Increasing the pH to about 14 with potassium hydroxide or with an equivalent base | 35–50°C | Not applicable |
15 min To be done twice using a new base solution |
|||||||
| Vacuum distillation | Not applicable | 150°C | Not specified | Not specified | Vacuum distillation | Not applicable | 150°C | Not specified | Not specified | ||
Set in paragraph A of Chapter III in Annex IV of Commission Regulation (EU) 142/2011.
The following description has been extracted verbatim from the application with minor editorial changes for clarity purposes (Sections 3.2.1, 3.2.2 and 3.2.3):
3.2.1. Feedstock treatment (Method 1)
Category 1 ABP tallow, pretreated using Method 1 (133°C for at least 20 min without interruption at a pressure (absolute) of at least 3 bar (as described in Chapter III of Annex IV of Commission Regulation (EU) 142/2011) is used as feedstock with a maximum permitted level of insoluble impurities of 0.15%, which represents a relatively low level of possible TSE infectivity compared to untreated material.
3.2.2. Conversion phase
In the conversion phase, the feedstock (i.e. triacylglycerides, free fatty acids and its salts) is mixed with 15% methanol and the feed is heated to the reaction temperature (minimum 220°C) in several heat exchangers and transferred into the continuous conversion reactor by means of a high pressure pump (pressure higher than 80 bar). The reaction conditions are maintained for a minimum of 30 min at 220°C and 80 bar pressure, which are monitored by temperature and pressure indicators. The retention time is controlled by the feedstock flow into the conversion system as further described in CCP 5 of the HACCP Plan (see Section 3.6).
As only fluids are handled, no dead areas filled with air will occur in the conversion reactor.
After the conversion, the pressure is released to ambient pressure; methanol and water are evaporated and recovered from the steam as a distillate. Methanol is reused for the process, while distilled water (which is free of any solids according to the Regulation (EU) No 142/2011 Annex IV Chapter 1 Section 2) is discharged into a wastewater treatment plant (WWTP).
The Applicant confirmed that in the new RepCat process, sulphuric acid and potassium hydroxide are not used as in the approved method for biodiesel production and that the mandatory TSE reduction is provided by the conversion (transesterification) phase by temperature and pressure as well as by the vacuum distillation at 150°C of the final products.
3.2.3. Distillation phase
After the conversion phase, the biodiesel/glycerol mixture is purified as described in Commission Regulation (EU) No 142/2011, Annex IV, Chapter IV, Section 2 D 2. (b) (i) by vacuum distillation at 150°C.
All critical process parameters (temperature, pressure, feedstock flow) are visualised in a process control system and also recorded and stored electronically.
The RepCat process is a fully automated and closed system. In contrast to the approved biodiesel production process according to Commission Regulation (EU) No 142/2011 Annex IV Chapter IV Section 2 D, no salt, i.e. potassium sulphate, is produced. In the case of glycerine production, this by‐product also undergoes the same process steps followed for biodiesel production, i.e. equivalent temperature, pressure and final vacuum distillation conditions.
The by‐product distillation residue is equivalent to that generated by the approved biodiesel production process and will be reused in the process as much as possible. The wastewater is a distilled product without any solids and will be discharged into a WWTP.
The Applicant claims that, as it is a fully automated and closed process, the biological risks can be considered identical to the ones in the approved biodiesel production process.
3.3. Full description of the material to be treated
Category 1 ABP tallow, pretreated according to Method 1, is used as feedstock with a maximum permitted level of insoluble impurities of 0.15%.
3.4. Hazard identification
Although the Applicant did not present the complete hazard identification, taking into consideration the type of process, TSEs must be considered the most relevant hazard. This application is specifically aimed at using Category 1 animal fat, a high‐risk material due to the potential presence of TSE agents. The method proposed by the Applicant is suitable for all kinds of animal fats as a feedstock. Besides TSE agents, Category 1 material can contain other biological hazards (including some highly heat‐resistant bacterial spores and viruses). However, given the high resistance to destruction, and, in particular, the high thermostability of the infectious agents causing TSEs (Somerville et al., 2009), it is assumed that if the alternative method ensures the inactivation of the TSE agent, then all microorganisms, including spore‐forming bacteria and thermoresistant viruses, will be completely inactivated. Therefore, the focus will be on the risk reduction in relation to TSE agents.
3.5. Level of risk reduction
3.5.1. Level of risk reduction according to the RepCat process application
The description presented in the current section has been extracted verbatim from the application with minor editorial changes for clarity purposes.
Category 1 ABP tallow (i.e. triacylglycerides, free fatty acids and their salts), pretreated according to Method 1, will be used as feedstock with a maximum permitted level of insoluble impurities of 0.15%, which represents a relatively low level of possible TSE infectivity compared to untreated material.
Inactivation of the TSE agent depends on the combined effects of pressure, temperature and retention time during the process within the different sections of the treatment unit.
The whole process covers the following reductions:
Sterilised ABP tallow (Method 1)
The sterilisation of tallow, according to Method 1, as set in paragraph A of Chapter III of Annex IV of Commission Regulation (EU) 142/2011, contributes to a reduction of at least 3 log10 in TSE infectivity.
Conversion process (conversion phase)
The RepCat biodiesel process uses high temperature and high pressure for the conversion, and includes methanol, which is present partly as steam during the reaction.
The following reduction factors were published in the literature for processes involving high temperature and high pressure (Table 2).
Table 2.
Level of TSE reduction provided by similar processes described in the literature – based on the table provided in the application in section risk reduction
| Source | Temperature | Pressure | Time | Reduction of TSE |
|---|---|---|---|---|
| Müller et al. (2008) | 160°C | 12 bar | 20 min | > 4.7 log10 |
| Müller et al. (2006) | 200°C | 15 | 20 min | > 6 log10 |
| Müller and Riesner (2005) | 200°C | 15.5 | 20 min | 7 log10 |
| BDI RepCat process | 220°C | 80 bar | 30 min | – |
TSE: transmissible spongiform encephalopathy.
The Applicant claims that all conditions (temperature, pressure and time) during the conversion step in the proposed biodiesel (RepCat) production process exceed the conditions from the trials described in the referenced literature, and therefore, the process achieves at least the same level of risk reduction as the one reported in the published articles.
Vacuum distillation (distillation phase)
The distillation of biodiesel under vacuum at 150°C is already part of the approved biodiesel production process according to Commission Regulation (EU) No 142/2011 Chapter IV Section 2.
According to the Applicant, Mittelbach et al. (2007) showed that such distillation has a reduction factor of at least 103 (3 log10) for TSE infectivity. Since proteins are not volatile under the conditions of distillation, it was assumed that they remained in the distillation residue. Mittelbach et al. found no traces of prion protein in either the distilled samples or in the distillation residue. Therefore, the reported effect on the level of risk reduction was considered realistic.
According to the Applicant, the total reduction in TSE infectivity during the new RepCat process is at least 9 log10 (see Table 3).
Table 3.
Level of TSE reduction achieved with by the RepCat process, as provided by the applicant
| Step | Reduction of TSE |
|---|---|
| Sterilisation of feedstock | 3 log10 |
| Conversion process | 3 log10 |
| Distillation | 3 log10 |
| In total | 9 log 10 |
3.5.2. Review of the relevant literature
According to the EFSA Statement on the format for applications for new alternative methods for ABPs (EFSA BIOHAZ Panel, 2010a), the degree of risk reduction must be determined with validated direct measurements, unless modelling or comparisons with other processes are acceptable. In this case, the Applicant did not provide their own experimental data, but provided a number of references which refer to presumptively similar methods performed at laboratory level.
After analysing the data described in Section 2.1 including the information provided by the Applicant in support of the RepCat process (Müller et al., 2006, 2008; Mittelbach et al., 2007; EFSA BIOHAZ Panel, 2011) and other relevant information, the following general observations can be made:
Animal bioassays are considered to be the gold standard for assessing TSE infectivity. Ideally, the natural host for a specific TSE strain should be the animal of choice. These bioassays may be impractical and mouse or other rodent bioassay models are used as proxy.
Data from rodent bioassays may not be a good indicator for the susceptibility of the natural host to TSE strains. This is because, at first passage, susceptibility may be low and incubation periods will be prolonged, a property designated as the species barrier. Therefore, rodent bioassays are used in conjunction with TSE strains that have been adapted in the rodent host after serial passaging, which increases susceptibility of the host to that strain.
Bioassays using Syrian gold hamsters challenged with the 263K hamster adapted strain are accepted as a good and well‐validated model for demonstration of infectivity. This is because of the short incubation periods and the finding that prion titres can be ˜ 10 times higher compared to mice. The short incubation periods mean that the time required for completion of endpoint bioassay experiments is decreased significantly. Further reduction is achieved by the incubation time interval assay which, in addition, employs fewer animals allowing experiments to be more cost effective.
Adaptation to the rodent host may alter some TSE agent strain properties, including thermostability (Giles et al., 2008) and it has been shown that different strains can have different thermostability properties (Somerville and Gentles, 2011, Somerville et al., 2002). It has been suggested that bovine spongiform encephalopathy (BSE) is 104‐ to 106‐fold more resistant to inactivation compared to 263K prions and 103‐fold more resistant than 301V prions, which are a mouse‐adapted BSE strain (Giles et al., 2008).
The introduction of transgenic mice can minimise or abolish the species barrier. As a result, transgenic mouse bioassays can be used to directly assess infectivity of animal or human TSEs without altering the original prion properties, provided that the appropriate mouse line is selected.
Biochemical methods that directly assess PrPSc levels are not always good indicators of TSE infectivity as it has been reported that they can be less sensitive compared to bioassays (Bruederle et al., 2008; Gonzalez et al., 2012).
It is believed that protein misfolding cyclic amplification (PMCA), which allows PrPSc amplification in vitro, may offer a powerful alternative to mouse bioassays (Murayama et al., 2006; Matsuura et al., 2013; Yoshioka et al., 2013), although additional validation data are required before PMCA can replace mouse bioassays as TSE detection methods.
After reviewing information provided by the Applicant relevant to the RepCat method, it was concluded that:
Two of the publications (Müller et al., 2006, 2008) describe inactivation of TSE prions in tallow after it has been subjected to oleochemical manufacturing processes of hydrolytic fat splitting, not production of Biodiesel. Therefore, they are not considered to be directly relevant to the RepCat process.
The paper of Mittelbach et al. (2007) assessed the reduction of infectivity or PrP27–30 degradation by Western blot and this was used as proxy to infectivity. In that paper, biodiesel production involved exposure of tallow to highly acidic and alkaline solutions to achieve pre‐esterification and transesterification of fatty acids. The RepCat method does not involve exposure of tallow to strong acids or bases, and therefore, it is not equivalent to that described by Mittelbach et al. (2007). However, the distillation phase that follows biodiesel production as described in the same article is comparable to the one described for the RepCat process.
The effectiveness of other tallow treatments to reduce TSE infectivity have been assessed using the hamster‐adapted scrapie strain 263K in conjunction with a Syrian gold hamster model; no other data are available (Müller et al., 2006, 2008).
Considering other relevant references, the following observations can be made:
The comparison of the risk reduction achieved by different processes indicated that there is a limited number of studies describing risk reduction in alternative processes. The effect of methanol, in the absence of acid or alkaline conditions, on the thermostability of TSEs has not been assessed in literature.
The real reduction of infectivity, even with 6 log10 measured decrease during the biodiesel production process, has to be placed into perspective (Bruederle et al., 2008). It was stated that ‘biochemical analysis alone is insufficient for detection of prion infectivity after a biodiesel process’ and ‘The biodiesel reaction cannot be considered a viable prion decontamination method for meat and bone meal (MBM), although we observed increased survival time of hamsters and reduced infectivity greater than 6 log10 orders in the solid MBM residue’.
3.5.3. Assessment of the level of risk reduction
In the references indicated by the Applicant, which are the closest to the process under evaluation, (Müller et al., 2006, 2008; Mittelbach et al., 2007), a minimum reduction factor in prion inactivation of 103 (3 log10) (depending on the conditions) at temperatures and pressures lower than those of each of the conversion phase of the RepCat process was demonstrated. In principle, since higher temperature, time and pressures are used, at least a similar level of risk reduction could be expected. However, no experimental trials have been run under equivalent conditions, and therefore, the following limitations associated to the process evaluation need to be considered:
With respect to the inactivation of TSE agents, it is important to note that the efficacy of different methods is dependent on the TSE strain. Additionally, the results of different experiments are sometimes contradictory. In some cases, prior exposure to high temperatures or ethanol may lead to increased thermostability (Taylor, 1999; Giles et al., 2017), although these data cannot be considered conclusive, as there are contradictory observations and they do not cover all the temperature ranges used in the process under evaluation.
In the proposed process, there is an exposure to methanol in the conversion phase in the absence of alkaline or acidic conditions. The impact of this procedure on the thermostability of TSEs has not been assessed in the literature. TSE infectivity is reduced by NaOH and this has been removed from the process. In addition, methanol could have protein‐fixation potential, and this may impact on the level of TSE reduction attained (Giles et al., 2017). Further experiments showed resistance to inactivation by various chemicals including glutaraldehyde, peracetic acid or ethanol, and to extended heating, including at 160°C for 24 h (Dickinson and Taylor, 1978).
In the available literature, reduction of TSE infectivity has been assessed in spiked tallow which has been treated chemically or physically after addition of aqueous solutions which may also contain methanol. In the proposed method prior to heat treatment, methanol is the only chemical added to tallow; no aqueous solutions, either acidic or alkaline, are incorporated. Therefore, the proposed process is not directly comparable to the ones described in published data.
It should be mentioned that the total risk reduction may not be simply the sum of the risk reductions attained by the three process phases, and could be less, since a pretreatment (heat or methanol) could increase thermostability of the TSE agent during the subsequent steps. However, there are no conclusive data from studies with experimental conditions similar to those used in the proposed process.
As the Applicant mentions in the ‘Detailed description’ Section, the reaction temperature will be held for a minimum of 30 min at minimum 220°C and 80 bar. These 30 min should be the confirmed minimum retention time (to be specified under 3.6.2 CCP5 in the application).
Moreover, a 9 log10 reduction, as stated by BDI, is rather an optimistic scenario. Category 1 BSE‐infected material treated with Method 1 gives a logarithmic reduction of about 2.8 log10. Although, as claimed by BDI, under optimal conditions the other steps (conversion and distillation phase) could give a reduction of around 6 log10, this was not demonstrated by the studies supplied.
The distillation phase of the RepCat process is equivalent to the distillation step of the approved biodiesel production process and similar to the one described by Mittelbach et al. (2007). A 3 log10 reduction factor in PrP27–30 was obtained by these authors, and therefore, a similar level of PrP27–30 reduction could be expected for the distillation phase of the proposed process.
In the submission, the Applicant highlighted the fact that the conditions of the BDI RepCat conversion process (without its distillation) fulfil the conditions for obtaining fat derivatives as described in the Commission Regulation (EU) No 142/2011, Annex XIII, Chapter XI 1(a):
1 (a) transesterification or hydrolysis at least 200°C, under corresponding appropriate pressure, for 20 minutes (glycerol, fatty acids and esters).
The Applicant stated that the RepCat process (200°C, 80 bar for 30 min) met these requirements as the corresponding vapour pressure of methanol in a transesterification process at 200°C would be 40.3 bar, which is much lower than the 80 bar in the RepCat process.
The Panel acknowledges that the parameters of the conversion phase of the RepCat process are similar to those described in that Commission Regulation (EU) although this was not the purpose of the application and this annex XIII is not relevant to the assessment under Art. 20 of Regulation (EU) No 1069/2009.
3.6. HACCP plan
3.6.1. HACCP plan, as provided by the Applicant
The Applicant states that critical parameters (pressure, temperature and retention time) are controlled by process measuring and control technology devices. Visualisations and recording of data are achieved by the process control system. If specific process values are not met, the system automatically switches into a stand‐by mode where unprocessed product cannot leave the closed system.
The conditions during the vacuum distillation are fully monitored by several temperature and pressure indicators.
The Applicant claims that since the Category 1 animal fat is already processed by a steam sterilisation process (Method 1), and as the content of solids must be below 0.15%, minimal risk can be expected from the feedstock for the RepCat process.
The Applicant also claims that since the process involves a high pressure unit and a distillation step, any remaining risk after the RepCat process will be reduced to the accepted value demanded from EFSA as described in Chapter 5.
The whole process takes place in a closed industrial system without any human or animal exposure, except for the sample taking necessary to control the reaction conditions.
It is claimed that deviation from any critical control point (CCP) conditions will automatically trigger a stand‐by operation within the process where the incorrectly processed material will be reprocessed. According to the Applicant, if there is significant exposure, the potential risk would be negligible as the feedstock itself must already be treated according to the Method 1 of Commission Regulation (EU) No 142/2011.
The effectiveness of the production system is monitored through several measuring points for flow of liquids, temperature and pressure. These values are visualised in a process control system as well as recorded and saved automatically. In case of deviations from critical process conditions, the system automatically switches to a stand‐by mode until all conditions are within the specified parameters again. This stand‐by process cannot be manually overridden. However, even if these conditions are fulfilled, the return to operation mode must be acknowledged by an operator.
The application also suggests that, for additional safety, the conversion of the feedstock is monitored by laboratory analyses of remaining fat in terms of triacylglycerol (maximum limit 0,05% m/m) and solids (maximum limit 24 mg/kg), measured by approved analytical methods EN 14105 and EN 12662, respectively. This is interpreted as an index that the process has been applied effectively and not related to TSE reduction.
As the production of biodiesel from ABP Category 1 is already approved in Commission Regulation (EU) 142/2011, no indirect impacts from transport, storage and disposal of end‐ and by‐products are foreseen in the RepCat process.
Any wastewater from cleaning processes will be collected, any fat will be separated and reused as a feedstock; remaining wastewater will be treated in a WWTP.
Unauthorised persons and animals do not have access to the processing plant (Figure 1).
Figure 1.
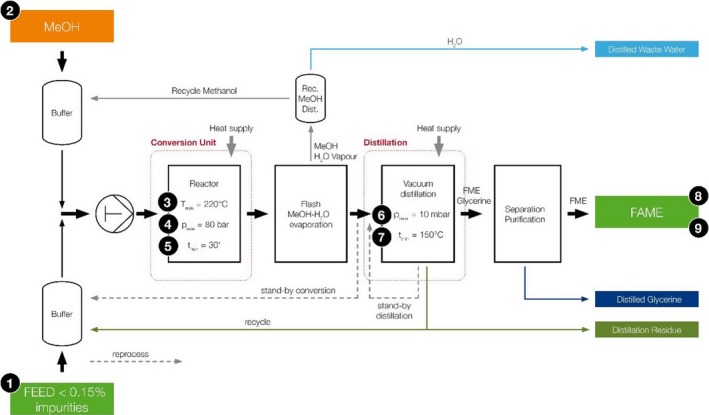
Flow diagram of the RepCat process as presented by the Applicant
3.6.1.1. Critical Control Points
The Applicant described within the application the critical control points (CCP) which were identified and assessed for the new biodiesel process as presented in Table 4.
Table 4.
Description of the critical control points (CCP) in the proposed biodiesel production process (as described by the Applicant)
| CCP no. | Critical point | Parameter | If not fulfilleda |
|---|---|---|---|
| 1 | Delivery of ABP tallow | Solids < 0.15% | Return to supplier |
| 2 | Methanol dosing | Dosing min. 15% | Automatic stand‐by operation of process |
| 3 | Temperature during conversion | T min. 220°C | Automatic stand‐by operation of process |
| 4 | Pressure during conversion | p min. 80 bar | Automatic stand‐by operation of process |
| 5 | Retention time | t min. 30 min., by flow rate at given volume | Automatic stand‐by operation of process |
| 6 | Vacuum in distillation column | p max. 10 mbar | Automatic stand‐by operation of distillation |
| 7 | Temperature and time for distillation | T min. 150°C | Automatic stand‐by operation of distillation |
| 8 | Remaining fat content | Triglycerides max. 0.05%b | Reprocess |
| 9 | Solids | Max. 24 mg/kg | Reprocess |
Interpreted as ‘corrected actions’, although they are incomplete.
Remaining fat component triacylglycerol < 0.05% m/m should be measured by EN 14105 (lower values are expected, but cannot be determined by the measuring method due to the low reproducibility at this low value (R = 0.069% m/m at a mean value of 0.0725% m/m)).
3.6.1.2. Detailed description
Besides the quality of the feedstock (CCP 1, animal fat sterilised according to Method 1, Regulation (EC) No 142/2011, with a solid content < 0.15%), the process parameters (flow rate, temperature and pressure (CCP 2–5)) are essential to guarantee the TSE reduction as described in Chapter 5.
To guarantee a 3 log10 reduction in the distillation step, the conditions (pressure and temperature) are controlled (CCP 6–7) during the distillation.
As a final quality control of the process, the conversion of the fat is monitored by measuring the remaining triglyceride content (CCP 8) in the biodiesel. Additionally, the content of solids is measured (CCP 9) and must be below 24 mg/kg in the biodiesel. If CCP 8 and CCP 9 are fulfilled, the product is released for sale.
CCP No 1
The first parameter that must be checked is the content of solids in the fat, which must be below 0.15%. Every production plant is equipped with a laboratory for feedstock analyses, production control and determination of the final product quality. So, the determination of solids can easily be performed on site.
The solid content must be below 0.15%, so, before usage, this content must be measured regularly, e.g. once a day or each unloading. If the value is higher than 0.15%, the feedstock must be rejected for biodiesel production and reprocessed until solid content is below the limit (returned to the supplier).
CCP No 2
It is claimed that highly sophisticated measuring devices monitor all critical process parameters; their signals are visualised and processed as well as stored in a process control system. Several risk assessments (e.g. according to EN ISO 12100) are performed.
Methanol will be dosed automatically. If the dosing amount is below the limit, the system will automatically switch to stand‐by. In this mode, the material is not further processed to the distillation unit and is automatically transferred back to the buffer vessel (stand‐by conversion).
CCP No 3
The temperature for the conversion is provided by steam or thermal oil via a heat exchanger.
The temperature is measured and automatically controlled as it is also needed for the conversion reaction. If the temperature is below the limit, the system will automatically switch to stand‐by. The material is not further processed to the distillation unit and is automatically transferred back to the buffer vessel.
CCP No 4
The pressure is created by means of a high‐pressure pump. The pressure is automatically controlled and needed for the reaction. If the pressure is below the limit, the material is not further processed to the distillation unit and automatically transferred back to the buffer vessel (stand‐by conversion).
CCP No 5
The reaction time is defined by the retention time during the conversion process. The high‐pressure pump flow is controlled (refer to CCP No 4) by a flow indicator which is connected to the process control system for data monitoring and visualisation.
The 30‐min dwell time is considered a minimum retention time and is calculated by the process control system as the volume of the high pressure reactor (m3) divided by the flow rate (m3/h), measured by the flow indicator. If the flow is above the limit, the system will automatically operate in stand‐by mode. In this mode, the material is not further processed to the distillation unit and automatically transferred back to the buffer vessel (stand‐by conversion).
CCP No 6
The following two CCPs are related to the distillation step, which is fully controlled by the process control system by means of a pressure and a temperature indicator. The distillation is performed under vacuum by means of several vacuum pumps. The vacuum is monitored by pressure indicators. If the pressure in the column is too high, the distillation will automatically operate in a stand‐by mode (stand‐by distillation). Furthermore, due to the nature of this distillation, no unprocessed product will leave the distillation column, as the biodiesel (methyl ester) does not evaporate at higher pressures.
CCP No 7
Heat is required for the evaporation of the biodiesel. The temperature is monitored by temperature indicators. If the temperature is too low, the distillation will automatically operate in stand‐by mode (stand‐by distillation). Furthermore, due to the nature of this distillation, no unprocessed product will leave the distillation column, as the methyl ester does not evaporate at lower temperatures.
CCP No 8
The quality of the biodiesel has to be analysed for the parameter triglycerides, determined according EN 14105 for maximum 0.05%.
If the results are higher than the limits, the distillation step must be repeated.
CCP No 9
As a final control measure, the quality of the biodiesel has to be analysed for the parameters ‘solids’, determined according to EN 12662 to be below 24 mg/kg (limit according to EN 14214).
If the result is higher than the limit, the distillation step must be repeated.
3.6.2. Assessment of the HACCP plan
The Applicant provided the details of the HACCP plan, which considered all the main control points associated with the process. However, a hazard analysis was not carried out. With respect to the HACCP plan, information related to corrective actions in case of failure of one of the CCPs are pointed out to some extent in Table 1, but these should be more detailed. Particularly, in the case of the closed system being opened by accident/explosion, a plan should exist on how to protect the workers and the environment.
Given the dependence on the system to cease operation if critical conditions are not met, Good Manufacturing Practices must be followed by the Applicant to ensure that the system is functioning as per design.
3.7. Risk associated with interdependent processes
As claimed by the Applicant, since a process for production of biodiesel from Category 1 tallow is already approved in the Regulation (EU) No 142/2011, the same risk associated with interdependent processes can be expected.
Point 5(a), Chapter II, Annex VII of Regulation (EU) No 142/2011, amended by Commission Regulation (EU) No 749/2011, requires the Applicant to provide information on the risks associated with interdependent processes. In particular, the Applicant is required to provide information:
on the outcome of an evaluation of possible indirect impacts, which may: (i) influence the level of risk reduction of a particular process; or (ii) arise from transport or storage of any products generated during the process and from the safe disposal of such products, including wastewater.
All solid by‐products of the process will be disposed of as waste using the methods described in Chapter IV of Annex IV of Commission Regulation (EU) No 142/2011.
Point 1(a), Section 3, Chapter IV, Annex IV of Regulation (EU) No 142/2011, amended by Commission Regulation (EU) No 294/20134, states that products derived from the processing of Category 1 material shall be:
disposed in accordance with Article 12(a) or (b) of Regulation (EC) No 1069/2009;
disposed of by burial in an authorised landfill;
transformed into biogas. In such case the digestion residues must be disposed of in accordance with point (i) or (ii), except where the material results from processing in accordance with point 2(a) or (b) where the residues can be used in accordance with the conditions set out in point 2(a) or point 2(b)(iii) as appropriate; or;
further processed into fat derivatives for uses other than feeding.
The Applicant did not provide a detailed description of the risk associated with interdependent processes, only on wastewater processing and failure of the process.
3.7.1. Wastewater handling
According to Section 2, Chapter I, Annex IV, of Regulation (EU) No 142/2011, ABP processing plants processing Category 1 material shall have a pretreatment process for the retention and collection of animal material as an initial step in the treatment of wastewater. The equipment used in the pretreatment process shall consist of drain traps or screens with apertures with a filter pore or a mesh size of no more than 6 mm in the downstream end of the process or equivalent systems that ensure that the solid particles in the wastewater passing through them are no larger than 6 mm. No grinding, maceration or other processing or application of pressure that could facilitate the passage of solid animal material through the pretreatment process shall be carried out. ‘All animal material retained in the pre‐treatment process in premises as referred to above shall be collected and transported as Category 1 (…) material, as appropriate, and disposed of in accordance with Regulation (EC) No 1069/2009’, which includes in Article 12 the obligation to dispose of Category 1 material by incineration or co‐incineration with or without prior processing, use as fuel for combustion and, in certain circumstances, by burial in an authorised landfill.
According to the Applicant, wastewater from the process is distilled, hence free of solids as required in Regulation (EU) No 142/2011 Annex IV Chapter 1 Section 2. As the feedstock is free of solids from ABP (< 0.15%), so too will be the wastewater from the cleaning processes. This wastewater is collected and any fat will be separated in a grease trap and reused as feedstock. All wastewater streams will be treated in a WWTP.
The procedures for dealing with wastewater meet the requirements set out in Section 2, Chapter I, Annex IV, of Regulation (EU) No 142/2011.
3.8. Risk associated with the intended end use of the products from the process
According to Point 5(a), Chapter II, Annex VII, of Regulation (EU) No 142/2011: (i) the risks associated with the intended end use of any products generated during the process must be specified; (ii) the likely risks for human health and animal health and possible impacts on the environment must be assessed on the basis of the risk reduction estimated in accordance with point 2(d).
In this process, the end products are biodiesel and glycerine. Considering the nature of the final product, a very low level of risk for the humans and animals associated with the intended end use is expected.
4. Uncertainty evaluation
4.1. Background
Basic principles for addressing uncertainty in risk analysis are stated in the Working Principles for Risk Analysis for Application in the Framework of the Codex Alimentarius. These state that ‘constraints, uncertainties and assumptions having an impact on the risk assessment should be explicitly considered at each step in the risk assessment and documented in a transparent manner’ and that ‘responsibility for resolving the impact of uncertainty on the risk management decision lies with the risk manager, not the risk assessors’ (CAC, 2015).
The Scientific Committee of EFSA has already explicitly endorsed this principle in its guidance on transparency in risk assessment (EFSA, 2009). Therefore, it is recognised that in the risk assessment process, it is important to characterise, document and explain all types of uncertainty arising in the process. In the EFSA context, the term ‘uncertainty’ is intended to cover all types of limitations in available knowledge that affects the range and probability of possible answers to an assessment question (EFSA Scientific Committee, 2016). Further guidance in relation to different tools to assess uncertainty can be found in EFSA draft Guidance on uncertainty in EFSA scientific assessments (EFSA Scientific Committee, 2016). According to this draft guidance, the analysis of the uncertainty in a risk assessment would require the following steps:
Initial plan for assessment strategy
Identify and list uncertainties affecting the assessment
Select which uncertainties to assess individually
Assess individual sources of uncertainty
Quantify combined uncertainty
Investigate influence
Describe unquantified uncertainties
Document and report the assessment, including the uncertainty analysis
A summary of the main sources of uncertainty identified and their possible impact for this assessment is provided in Appendix A.
4.2. Identification and description of the sources of uncertainty
The most common type of uncertainty identified for assessment components is ‘Ambiguity’, mainly because of scarcity of data on the extent of the loss of infectivity of TSE agents in response to heat and limited data on the impact of either substrate properties or agent strain on the reduction in TSE infectivity attained. There is very limited information on the reduction of infectivity of TSE agents under the conditions for biodiesel or biofuels production, and the data on the loss of prion infectivity and the effect of media composition and processing under the conditions of the proposed alternative method.
This is followed by ‘Extrapolation uncertainties’, due to uncertainties relating to extrapolation from data generated under different test conditions and ‘Sampling and Measurement uncertainty’, due to the small‐scale nature and lack of replication of the existing published experimental work. ‘Distribution uncertainty’ is also an issue as the loss of infectivity is very variable depending on the experimental conditions (prions used, laboratory animal species, etc.) used. Such experiments take years to perform and are both financially and ethically expensive, so some extrapolation is generally necessary/unavoidable.
There is a lack of quantitative data on the loss of prion infectivity and the effect of media composition and processing under the conditions of the proposed alternative method. Nevertheless, it is unlikely that there will be the possibility to look specifically at TSE reduction under industrial scale process conditions, so there will always be uncertainties.
5. Conclusions
In the proposed method, there is an exposure to methanol in the conversion phase in the absence of alkaline or acidic conditions. The impact of this procedure on the thermostability of TSEs has not been assessed in the literature.
The distillation phase of the RepCat process is equivalent to the distillation phase of the approved biodiesel production process and similar to the one described in Mittelbach et al. (2007), where a 3 log10 reduction factor in PrP27–30 was obtained. Therefore, a similar level of TSE infectivity reduction could be expected for that phase of the proposed process.
It cannot be assumed that the overall risk reduction corresponds to the sum of the reduction of TSE infectivity in each of the process phases. This has not been confirmed by experimental data.
A previous EFSA Opinion established that a reduction of 6 log10 in the TSE infectivity should be achieved for Category 1 of ABPs, by any proposed alternative method in order to be equivalent to the approved processing method. This level of reduction has not been shown with experimental trials run under conditions equivalent to the ones described for the RepCat process.
The minimum retention time of 30 min in a continuous flow, proposed by the Applicant, where only indirect measurement will be applied to guarantee the monitoring of the process may increase the uncertainty associated with the efficacy of the heat treatment.
It was not possible to conclude whether or not the level of TSE infectivity reduction in the RepCat process is at least of 6 log10. Therefore, it was also not possible to conclude about the equivalence with the approved biodiesel production process.
Documentation provided to EFSA
Application on production of biodiesel from rendered fat of all categories of animal by‐products. Submitted by the company BDI – BioEnergy International AG to the Austrian Competent Authority and than submitted to EFSA on 22 February 2017 and revised on 30 July 2017.
Report of the Competent Authority related to the application of production of biodiesel from rendered fat of all categories of animal by‐products. Submitted by the Austrian Federal Ministry of Health on 22 February 2017 and revised on 30 July 2017.
List of references provided by the applicant
Müller H, Lothar S and Riesner D, 2006. Risk assessment for fat derivatives in case of contamination with BSE. European Journal of Lipid Science and Technology, 108, 812–826.
Müller H, Lothar S and Riesner D, 2008. Prion decontamination during the oleochemical process of fat hydrogenation. European Journal of Lipid Science and Technology, 110, 392–399.
Mittelbach M, Pokits B, Müller H, Müller M and Riesner D, 2007 Risk assessment for prion protein reduction under the conditions of the biodiesel production process, European Journal of Lipid Science and Technology, 109, 79–90.
EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Scientific Opinion on the capacity of oleochemical processes to minimise possible risks linked to TSE in Category 1 animal by‐products. EFSA Journal 2011;9(2):1976, 26 pp. https://doi.org/10.2903/j.efsa.2011.1976
Glossary and Abbreviations
- ABP
Animal by‐product
- BIOHAZ
EFSA Panel on Biological Hazards
- BSE
bovine spongiform encephalopathy
- CCP
Critical Control Point
- CAC
Codex Alimentarius Commission
- FAME
fatty acid methyl esters
- HACCP
Hazard Analysis and Critical Control Points
- MBM
meat and bone meal
- PMCA
protein misfolding cyclic amplification
- PrP
The only macromolecule demonstrated to be specifically associated with TSE diseases is a host‐encoded, hydrophobic glycoprotein called prion protein (PrP).
- PrPSc
Abnormal isoform of prion proteins (PrP) resulting from a post‐translational modification of the cellular prion protein (PrPC)
- PrP27–30
Upon purification using detergents and limited digestion with proteinase K (PK), PrPSc is transformed into an N‐terminally truncated but still infectious form of 27–30 kDa, designated PrP27–30, which is highly resistant to further PK digestion and forms rod‐shaped fibrils, so‐called prion rods
- TSE
Transmissible spongiform encephalopathy
- TOR
Terms of Reference
- WG
Working group
- WWTP
wastewater treatment plant
Appendix A – Main sources of uncertainty identified and potential impact
1.
| Assessment components | Sources of uncertainty | Types of uncertainty | Potential impact of the uncertainty | |
|---|---|---|---|---|
| Assessment/subassessment | Assessment inputs | |||
| Loss of infectivity | Process parameters approved for biodiesel and fat derivatives according to Regulation (EU) No 142/2011 |
Very limited set of experimental data published in the scientific literature are available on biodiesel processes Laboratory thermostability studies have only been undertaken with a subset of experimental TSE strains |
Ambiguity (incomplete information) |
The impact may lead to an unrealistic assumption concerning the effectiveness of the RepCat process, because each treatment step could influence the thermoresistance of the BSE prion, if present Inactivation experiments have been done on laboratory scale and under different processing conditions. As the kinetics of prion reduction are not understood, at present it is therefore questionable whether the RepCat process can be considered equivalent to those published |
| No validated evidence that replacement with methanol vapour pressure and less extreme pH is at least equivalent to the approved biodiesel method. The new parameters may have different abilities to inactivate BSE, which can only be determined by an experimental validation. No experimental data are available showing whether the BSE would be inactivated to the required level | Extrapolation uncertainty |
There is the possibility of increased heat resistance occurring as documented for methanol or other substance with tissue fixation potential on treated BSE‐infected tissues. It is unclear if there is a similar effect on the thermoresistance by mixing the feedstock with 15% methanol, but it cannot be excluded as this, like ethanol or formalin, leads to protein fixation Impact of heat and presence of alcohols (ethanol, methanol) on the stability of TSEs and their subsequent inactivation by additional treatments |
||
| A simple adding of 3 log10 decrease of BSE infectivity in each of the three major reaction steps is not validated by experimental data. The references to similar data from the literature with laboratory scale experiments and titrations using logarithmic dilutions to measure the degradation of PrP and inoculations with hamster‐adapted 263K strain of scrapie agent cannot be extrapolated as such to the BSE agent | Extrapolation uncertainty | Since there is no experimental evidence, the total risk reduction may not just be the sum of the risk reduction obtained for each step of a process | ||
| Release of unprocessed material | Efficacy of control measures | Uncertainty regarding the ability of the system to trigger reprocessing of product when it does not meet the processing specifications | Ambiguity uncertainty | If the system does not work correctly, contaminated material may be released |
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Alvarez Ordoñez A, Griffin J, Spiropoulos J, Vanopdenbosch E, Correia S and Fernández Escámez PS, 2017. Scientific Opinion on the evaluation of the Application for new alternative biodiesel production process for rendered fat of Cat 1 (BDI‐RepCat process, AT). EFSA Journal 2017;15(11):5053, 22 pp. 10.2903/j.efsa.2017.5053
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00136
Panel members: Ana Allende, Declan Bolton, Marianne Chemaly, Robert Davies, Pablo Salvador Fernández Escámez, Rosina Girones, Lieve Herman, Konstantinos Koutsoumanis, Roland Lindqvist, Birgit Nørrung, Antonia Ricci, Lucy Robertson, Giuseppe Ru, Moez Sanaa, Marion Simmons, Panagiotis Skandamis, Emma Snary, Niko Speybroeck, Benno Ter Kuile, John Threlfall and Helene Wahlström.
Amendment: To improve the clarify of the output, the following corrections have been applied: on page 14 the text in the second bullet point: ‘Moreover, a 9 log10 reduction is rather an optimistic scenario. Category 1 BSE‐infected material treated with Method 1 gives a logarithmic reduction of about 2.8 log10, and under optimal conditions, the other steps can give a reduction of around 6 log10.’ has been revised as ‘Moreover, a 9 log10 reduction, as stated by BDI, is rather an optimistic scenario. Category 1 BSE‐infected material treated with Method 1 gives a logarithmic reduction of about 2.8 log10. Although, as claimed by BDI, under optimal conditions the other steps (conversion and distillation phase) could give a reduction of around 6 log10, this was not demonstrated by the studies supplied.’; also on page 14 in the last paragraph of Section 3.5.3 the word 'equivalent' has been replaced with 'similar'; on page 20 the text of the reference EFSA BIOHAZ Panel, 2015 has been updated. The overall conclusions of the output are unchanged. To avoid confusion, the older version has been removed from the EFSA Journal, but is available on request as is a version showing all the changes made.
Adopted: 19 October 2017
Updated: 25 May 2018
Notes
Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by‐products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by‐products Regulation). OJ L 300, 14.11.2009, p. 1–33.
Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 54, 26.2.2011, p. 1–254.
Commission Regulation (EU) No 749/2011 of 29 July 2011 amending Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 198, 30.7.2011, p. 3–22.
Commission Regulation (EU) No 294/2013 of 14 March 2013 amending and correcting Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 98, 6.4.2013, p. 1–57.
References
- Bruederle CE, Hnasko RM, Kraemer T, Garcia RA, Haas MJ, Marmer WN and Carter JM, 2008. Prion infected meat‐and‐bone meal is still infectious after biodiesel production. PLoS ONE 3, e2969 10.1371/journal.pone.0002969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAC (Codex Alimentarius Commission), 2015. Joint FAO/WHO Food Standards Programme. Procedural manual. 24th Edition. p. 116 of 241. Available online: http://www.fao.org/3/a-i5079e.pdf
- Dickinson AG and Taylor DM, 1978. Resistance of scrapie agent to decontamination. The New England Journal of Medicine 299, 1413–1414. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2003. Opinion of the Scientific Panel on Biological Hazards on a request from the Commission related on the process of High Pressure Hydrolysis Biogas (HPHB) as method for safe disposal of category 1 Animal by‐Products (ABP) not intended for human consumption. EFSA Journal 2004;2(3):11, 4 pp. 10.2903/j.efsa.2004.11 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Biodiesel Process as a method for safe disposal of category 1 animal by‐products. EFSA Journal 2004;2(6):23, 3 pp. 10.2903/j.efsa.2004.23 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(5):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010a. Statement on technical assistance on the format for applications for new alternative methods for animal by‐products. EFSA Journal 2010;8(7):1680, 12 pp. 10.2903/j.efsa.2010.1680 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010b. Scientific Opinion on the Neste Oil Application for a new alternative method of disposal or use of Animal By‐Products. EFSA Journal 2010;8(10):1825, 9 pp. 10.2903/j.efsa.2010.1825 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Scientific Opinion on the capacity of oleochemical processes to minimise possible risks linked to TSE in Category 1 animal by‐products. EFSA Journal 2011;9(2):1976, 26 pp. 10.2903/j.efsa.2011.1976 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2015. Scientific Opinion on a continuous multiple‐step catalytic hydro-treatment for the processing of rendered animal fat (Category 1). EFSA Journal 2015;13(11):4307, 23 pp. 10.2903/j.efsa.2015.4307 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2016. Revised draft Guidance on uncertainty in EFSA scientific assessment. DRAFT. Available online: https://www.efsa.europa.eu/sites/default/files/160321DraftGDUncertaintyInScientificAssessment.pdf
- Giles K, Glidden DV, Beckwith R, Seoanes R, Peretz D, DeArmond SJ and Prusiner SB, 2008. Resistance of bovine spongiform encephalopathy (BSE) prions to inactivation. PLoS Pathogens, 4, e1000206 10.1371/journal.ppat.1000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles K, Woerman AL, Berry DB and Prusiner SB, 2017. Bioassays and inactivation of prions. Cold Spring Harbor Perspectives in Biology. 9, pii: a023499 10.1101/cshperspect.a023499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez L, Thorne L, Jeffrey M, Martin S, Spiropoulos J, Beck KE, Lockey RW, Vickery CM, Holder T and Terry L, 2012. Infectious titres of sheep scrapie and bovine spongiform encephalopathy agents cannot be accurately predicted from quantitative laboratory test results. Journal of General Virology, 93, 2518–2527. [DOI] [PubMed] [Google Scholar]
- Matsuura Y, Ishikawa Y, Bo X, Murayama Y, Yokoyama T, Somerville RA, Kitamoto T and Mohri S, 2013. Quantitative analysis of wet‐heat inactivation in bovine spongiform encephalopathy. Biochemical and Biophysical Research Communications, 432, 86–91. [DOI] [PubMed] [Google Scholar]
- Mittelbach M, Pokits B, Müller H, Müller M and Riesner D, 2007. Risk assessment for prion protein reduction under the conditions of the biodiesel production process. European Journal of Lipid Science and Technology, 109, 79–90. [Google Scholar]
- Müller H and Riesner D, 2005. Thermal degradation of prions in the presence of fats: implication for oleochemical processes. European Journal of Lipid Science and Technology, 107, 833–839. [Google Scholar]
- Müller H, Lothar S and Riesner D, 2006. Risk assessment for fat derivatives in case of contamination with BSE. European Journal of Lipid Science and Technology, 108, 812–826. [Google Scholar]
- Müller H, Lothar S and Riesner D, 2008. Prion decontamination during the oleochemical process of fat hydrogenation. European Journal of Lipid Science and Technology, 110, 392–399. [Google Scholar]
- Murayama Y, Yoshioka M, Horii H, Takata M, Yokoyama T, Sudo T, Sato K, Shinagawa M and Mohri S, 2006. Protein misfolding cyclic amplification as a rapid test for assessment of prion inactivation. Biochemical and Biophysical Research Communications, 348, 758–762. [DOI] [PubMed] [Google Scholar]
- Somerville RA, Oberthür RC, Havekost U, MacDonald F, Taylor DM and Dickinson AG, 2002. Characterization of thermodynamic diversity between transmissible spongiform encephalopathy agent strains and its theoretical implications. Journal of Biological Chemistry, 277, 11084–11089. 10.1074/jbc.M111766200 [DOI] [PubMed] [Google Scholar]
- Somerville RA and Gentles N, 2011. Characterization of the effect of heat on agent strains of the transmissible spongiform encephalopathies. Journal of General Virology, 92(Pt. 7), 1738–1748. 10.1099/vir.0.030452-0 [DOI] [PubMed] [Google Scholar]
- Somerville RA, Fernie K, Smith A, Andrews R, Schmidt E and Taylor DM, 2009. Inactivation of a TSE agent by a novel biorefinement system. Process Biochemistry, 44, 1060–1062. [Google Scholar]
- Taylor DM, 1999. Inactivation of prions by physical and chemical means. Journal of Hospital Infection, 43(Suppl), S69–S76. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Matsuura Y, Okada H, Shimozaki N, Yamamura T, Murayama Y, Yokoyama T and Mohri S, 2013. Rapid assessment of bovine spongiform encephalopathy prion inactivation by heat treatment in yellow grease produced in the industrial manufacturing process of meat and bone meals. BMC Veterinary Research, 9, 134 10.1186/1746-6148-9-134 [DOI] [PMC free article] [PubMed] [Google Scholar]


