Abstract
The Panel on Plant Health performed a pest categorisation of Venturia nashicola, the causal agent of Asian pear scab, for the European Union (EU). The pathogen is a well‐defined, distinguishable fungal species affecting Pyrus pyrifolia var. culta, P. ussuriensis and P. bretschneideri in Asian countries. P. communis (European pear) is not a host of V. nashicola, but the host status of other Pyrus species is unclear. V. nashicola is not known to occur in the EU. It is listed in Annex IIAI of Directive 2000/29/EC. The pathogen could potentially enter the EU on host plants for planting and fruit originated in infested countries. There are no climatic factors limiting the potential establishment and spread of the pathogen in the EU, as its epidemiology is similar to those of Venturia inaequalis (apple scab) and Venturia pyrina (European pear scab), which are well‐established in the EU. The hosts are present in the EU, but no data were found on their abundance and distribution. In the infested areas, V. nashicola causes premature leaf and fruit drop and fruit distortion resulting in considerable yield/quality losses. The introduction of the pathogen into the EU could cause yield/quality losses and environmental consequences because of the additional fungicide sprays for disease control. Cultural practices and chemical measures applied in the infested areas reduce the inoculum sources but they cannot eliminate the pathogen. Phytosanitary measures are available to mitigate the risk of introduction and spread of the pathogen in the EU. All criteria assessed by EFSA for consideration as a potential Union quarantine pest are met. As V. nashicola is not known to occur in the EU, this criterion assessed by EFSA to consider it as a Union regulated non‐quarantine pest is not met.
Keywords: European Union, host distribution, Asian pear scab, plant health, Pyrus spp., quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L.. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Venturia nashicola is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest for the area of the European Union (EU) excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A search of literature (1997–2017) in Web of Science and Scopus was conducted at the beginning of the categorisation. The search focussed on Venturia nashicola and its geographic distribution, life cycle, host plants and the damage it causes. The following search terms (TS) and combinations were used: TS =(“Venturia nashicola” OR “Asian pear scab”) AND TS=(geograph* OR distribution OR “life cycle” OR lifecycle OR host OR hosts OR plant* OR damag*).
Further references and information were obtained from experts, from citations within the references and grey literature
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO, 2017).
Data about import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT.
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Consumers (DG SANCO), and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the MSs and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for V. nashicola, following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union regulated non‐quarantine pest in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required as per the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to qualify either as a quarantine pest or as a regulated non‐quarantine pest. If one of the criteria is not met, the pest will not qualify. In such a case, the working group should consider the possibility to terminate the assessment early and be concise in the sections preceding the question for which the negative answer is reached. Note that a pest that does not qualify as a quarantine pest may still qualify as a regulated non‐quarantine pest which needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone, thus the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism. | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pes must be present in the risk assessment area). |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future. |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone). |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6 ) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met. |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regards to the principle of separation between risk assessment and risk management (EFSA founding regulation3); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
YES, the identity of the pest is well‐established
Venturia nashicola is a well‐established fungus of the family Venturiaceae. The Index Fungorum database (http://www.indexfungorum.org), updated with the works of Hyde et al. (2013) and Zhang et al. (2011), provides the following taxonomical identification:
Accepted name: Venturia nashicola S. Tanaka & S. Yamamoto
Family – Venturiaceae
Genus – Venturia
species – nashicola
Index Fungorum reports Fusicladium nashicola K. Schub. & U. Braun as a synonym for V. nashicola.
V. nashicola is the scab pathogen of Japanese (Pyrus pyrifolia var. culta) and Chinese (P. ussuriensis) pears. The relationship between V. nashicola and Venturia pyrina, the scab pathogen of European pear (P. communis), was re‐examined by Ishii and Yanase (2000). Morphological examination, mating experiments, pathogenicity tests and phylogenetic analyses showed that V. nashicola is a single taxonomic entity distinct from V. pyrina and other genetically related Venturia spp. (Ishii and Yanase, 2000; Zhao et al., 2011).
3.1.2. Biology of the pest
V. nashicola overwinters as immature pseudothecia (sexual form) in diseased leaves on the orchard floor, as dormant mycelia in the inner tissues of the bud scales on pear trees and as conidia (asexual form) on the surface of twigs (Li et al., 2003). Ascospores produced in pseudothecia and conidia are considered to be the primary inoculum in spring in the infested areas (Unemoto, 1990; Lian et al., 2006). The key requirement for production of pseudothecia is the occurrence of rain during winter and early spring. Excess water may lead to accelerated leaf decay and hence to production of fewer pseudothecia (Lian et al., 2006). Discharge of ascospores from pseudothecia occurs mainly during the day and requires free water or 100% relative humidity. In northern China, ascospores begin to mature and be discharged between early April and late June, with most ascospores trapped in May (Lian et al., 2006). The discharge of ascospores and the dispersal of conidia occur mainly in rainy periods (Lian et al., 2007; Eguchi and Yamagishi, 2008). In general, the development of Asian pear scab epidemics is similar to that for apple scab (MacHardy, 1996; Li et al., 2003; Eguchi and Yamagishi, 2008), caused by Venturia inaequalis, a pathogen that is well established and widely distributed in the EU territory. Ascospore and conidial germination occurs under a wide range of temperatures (5–30°C), with optimal temperatures around 20°C, and few hours of wetness (e.g. both conidia and ascospores start germinating after 3 h of wetness at 20°C) (Li et al., 2003; Lian et al., 2007). Environmental requirements for ascospore infection have not been evaluated. For conidia, infection can occur at temperatures between 5 and 30°C (optimum 20°C), with > 6 h of wetness. At 28°C, 2–4 h of dryness reduces the infection by 40–60% (Li et al., 2003, 2005). Under orchard conditions, the minimum time from infection to the appearance of visible lesions on leaves is ca. 3–4 weeks (Li et al., 2007). Infection by V. nashicola can occur at any time throughout the growing season, from early spring until late autumn, if environmental conditions are conducive. Asian pear scab has two peaks in northern China: (i) during the early season until 2–3 months after blossom, and (ii) before harvest (Li et al., 2007). Early infections not only result in a significant amount of fruit infection but also generate secondary inocula for later infections (Li et al., 2007).
3.1.3. Detection and identification of the pest
Are detection and identification methods available for the pest?
YES, V. nashicola can be detected and identified based on symptomatology, cultural/morphological characteristics of its colonies in agar media and molecular methods
V. nashicola can be detected and identified based on host association, symptomatology as well as cultural and morphological characteristics of its colonies in agar media. Nevertheless, pathogenicity tests and/or molecular methods are necessary for confirming the identification based on morphology. Fast, reliable and sensitive molecular methods are available for the identification of the pathogen in cultures (Le Cam et al., 2001; Koh et al., 2013) and on fruit (Yun et al., 2015) as well as for its discrimination from other genetically related Venturia species.
Symptoms
V. nashicola infects fruit, leaves and young shoots causing typical scab symptoms. The first symptoms appear on either side of the leaves as olive green to brown, velvety spots with abundant conidia (Spotts, 2014). Lesions are well defined circular areas (5–10 mm in diameter). Similar but more elongate lesions appear on the main veins of the leaves and on petioles. Lesions on young actively growing shoots appear early in the growing season as black to brown, velvety spots. Later in the season, the twig lesions become corky and canker‐like. Scab lesions on fruit are superficial and occur first on the calyx end adjacent to the sepals and later on the side of fruit. As the lesions expand and coalesce, large, dark brown to back patches are produced. Infections of petioles and peduncles result in premature abscission of leaves and fruit, respectively. Infected fruit often become misshapen (Abe et al., 2008).
Morphology
Mycelium subcuticular, hyphae branched, 2–3 μm wide, septate, subhyaline to pale olivaceous (Schubert et al., 2003). Conidiophores unbranched or rarely branched, 20–70 × 4–6.5 μm, brown, paler towards the apex. Conidia solitary, one‐celled, pale‐brown, ovate, but sometimes irregular in shape, straight or slightly curved, 9–20(–28) × 5.5–10 μm (Schubert et al., 2003). Pseudothecia globose to conical, dark brown to black (Spotts, 2014). Asci oblong, 6–15 x 48–117 μm, with eight ascospores. Ascospores unequally two‐celled, with a septum near the base, pale‐brown, 11.2–12.8 (14.3) × 3.8–6.8 (5.5) μm (Ishii and Yanase, 2000).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
Based on information retrieved from the EPPO Global Database (last updated 30/9/2016; last accessed 27/4/2017), V. nashicola is currently present in Asian countries (Table 2).
Table 2.
Current distribution of Venturia nashicola in Asia based on information from the EPPO Global Database (last updated: 30/9/2016; last accessed: 27/4/2017)
| Country | State | Status |
|---|---|---|
| China | Present, no details | |
| China | Anhui | Present, no details |
| China | Hebei | Present, no details |
| China | Jilin | Present, no details |
| China | Liaoning | Present, no details |
| China | Shaanxi | Present, no details |
| China | Shandong | Present, no details |
| China | Shanxi | Present, no details |
| China | Yunnan | Present, no details |
| Japan | Present, no details | |
| Japan | Honshu | Present, no details |
| Japan | Kyushu | Present, no details |
| Korea, Republic | Present, no details | |
| Taiwan | Present, no details |
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory?
NO, V. nashicola is not known to occur in the EU
In the EPPO PQR, Version 5.3.5 (10‐2‐2015) visited on 1 June 2017, V. nashicola was considered not known to be present in the EU territory.
Nevertheless, in the Le Cam et al. (2001) study, strains of V. nashicola isolated in France were used to develop and validate a set of primers for specific identification of the pathogen. In this paper, the authors listed seven isolates of V. nashicola isolated from P. pyrifolia var. culta during the period 1988–1996 in four localities of France: Balma, Lanxade, Clermont Ferrand and Bergerac. In the text, the authors indicated that ‘The PCR primers we designed with these data were successfully used to identify V. nashicola regardless of the geographic origin (Japan and France)’. No more information regarding the presence of V. nashicola in France is included in the paper of Le Cam et al. (2001).
After having been informed by EFSA (e‐mail sent on 11/5/2017) about the findings in the Le Cam et al. (2001) study, the EPPO updated the information included in its databases (i.e. EPPO Global Database and EPPO PQR) concerning the geographical distribution of V. nashicola and more specifically the status of the pest in France (i.e. present, restricted distribution) and cited as a reference the paper of Le Cam et al. (2001).
EFSA formally requested the French National Plant Protection Organisation (NPPO) about the present status of V. nashicola in France. In an e‐mail received on 4 September 2017, the French NPPO explained that, at the time of the Le Cam et al. (2001) paper, nashi was still considered as a potential fruit crop for France but this crop was finally not further developed due to high production costs. The nashi pears referred in the Le Cam et al. (2001) paper no longer exist and there has been no official detection of Venturia nashicola since then through the French general surveillance system. Therefore, the status of V. nashicola in France should be considered as ‘absent: pest no longer present’.
Based on the above, the Panel considers that V. nashicola is not present in the risk assessment area.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
Venturia nashicola is listed in Council Directive 2000/29/EC. Details are presented in Table 3 and Table 4.
Table 3.
Venturia nashicola in Council Directive 2000/29/EC
| Annex II, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned if they are present on certain plants or plant products | |
| Section I | Harmful organisms not known to occur in the community and relevant for the entire community | |
| (c) | Fungi | |
| Species | Subject of contamination | |
| 15. | Venturia nashicola | Plants of Pyrus L., intended for planting, other than seeds, originating in non‐European countries |
3.3.2. Legislation addressing plants and plant parts on which V. nashicola is regulated
Table 4.
Regulated hosts and commodities that may involve Venturia nashicola in Annexes III and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
| Description | Country of origin | |
| 9. | Plants of Chaenomeles Ldl., Cydonia Mill., Crateagus L., Malus Mill., Prunus L., Pyrus L., and Rosa L., intended for planting, other than dormant plants free from leaves, flowers and fruit | Non‐European countries |
| 18. | Plants of Cydonia Mill., Malus Mill., Prunus L. and Pyrus L. and their hybrids, and Fragaria L., intended for planting, other than seeds | Without prejudice to the prohibitions applicable to the plants listed in Annex III A (9), where appropriate, non‐European countries, other than Mediterranean countries, Australia, New Zealand, Canada, the continental states of the USA |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community—in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community | |
| Part A | Plants, plant products and other objects originating in the Community | |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community and which must be accompanied by a plant passport | |
| 1.1. | Plants, intended for planting, other than seeds, of Amelanchier Med., Chaenomeles Lindl., Cotoneaster Ehrh., Crataegus L., Cydonia Mill., Eriobotrya Lindl., Malus Mill., Mespilus L., Photinia davidiana (Dcne.) Cardot, Prunus L., other than Prunus laurocerasus L. and Prunus lusitanica L., Pyracantha Roem., Pyrus L. and Sorbus L. | |
| Section II | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for certain protected zones, and which must be accompanied by a plant passport valid for the appropriate zone when introduced into or moved within that zone | |
| 1.3. | Plants, other than fruit and seeds, of Amelanchier Med., Castanea Mill., Chaenomeles Lindl., Cotoneaster Ehrh., Crataegus L., Cydonia Mill., Eriobotrya Lindl., Eucalyptus L'Herit., Malus Mill., Mespilus L., Photinia davidiana (Dcne.) Cardot, Pyracantha Roem., Pyrus L., Sorbus L. and Vitis L. | |
| Part B | Plants, plant products and other objects originating in territories, other than those territories referred to in Part A | |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community | |
| 3. | Fruits of:— Annona L., Cydonia Mill., Diospyros L., Malus Mill., Mangifera L., Passiflora L., Prunus L., Psidium L., Pyrus L., Ribes L. Syzygium Gaertn., and Vaccinium L., originating in non‐European countries, | |
| Section II | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for certain protected zones | |
| 4. | Parts of plants, other than fruit and seeds, of Amelanchier Med., Chaenomeles Lindl., Cotoneaster Ehrh., Crataegus L., Cydonia Mill., Eriobotrya Lindl., Malus Mill., Mespilus L., Photinia davidiana (Dcne.) Cardot, Pyracantha Roem., Pyrus L. and Sorbus L. | |
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
The principal hosts of V. nashicola are Pyrus pyrifolia var. culta (Japanese pear), P. ussuriensis (Chinese pear) (Ishii and Yanase, 2000; Abe et al., 2008) and P. bretschneideri (Li et al., 2007). European pear (P. communis) is not a host of V. nashicola, as it has been shown by Ishii and Yanase (2000) and Abe et al. (2008).
With respect to other Pyrus species, Abe et al. (2008) showed that P. aromatica, P. betulaefolia, P. dimorphophylla and P. hondoensis did not develop any scab symptoms when inoculated with V. nashicola conidia. However, for some of the above Pyrus species, there is contradictory information in the literature; more specifically, according to EPPO (OEPP/EPPO, 1977), V. nashicola has been reported on various Pyrus spp., such as P. betulaefolia (manshumamenashi), P. aromatica (iwateyamanashi) and P. vilis. Because of the above contradictory information and the lack of any other host‐related information in the literature, the Panel considers that, except for the host species P. pyrifolia var. culta, P. ussuriensis and P. bretschneideri, and the non‐host species P. communis, the host range of V. nashicola is not fully known.
3.4.2. Entry
Is the pest able to enter into the EU territory?
YES, under the current EU legislation, V. nashicola could potentially enter the risk assessment area via the host plants for planting at dormant stage and the fresh fruit pathways
The PLH Panel identified the following pathways for the entry of V. nashicola into the EU territory:
host plants for planting (with or without leaves), excluding seeds, and
fresh fruit of host plants (with or without leaves)
originated in infested Third countries.
Under the current EU legislation, the host plants for planting at dormant stage (free from leaves, flowers and fruit) and the fresh fruit pathways are relevant for the entry of the pathogen into the risk assessment area.
An average of 9,800 tonnes/year of Pyrus spp. fresh fruit originated in China were imported into the risk assessment area during the period 2011–2015 (source Eurostat, search done on 7/7/2017). However, no specific data exist in Eurostat on imports of P. pyrifolia var. culta and/or P. ussuriensis and/or P. bretschneideri fruit from infested Third countries into the EU. There are no records of interception of V. nashicola in the Europhyt database (search done on 3 May 2017).
3.4.3. Establishment
Is the pest able to become established in the EU territory?
YES, both the biotic (host availability) and abiotic (climate suitability) factors suggest that V. nashicola could potentially establish in the risk assessment area, similarly to other well‐established in the EU Venturia species
3.4.3.1. EU distribution of main host plants
Starting from the 1990s, the cultivation of Asian pears has been promoted for commercial production and for ornamental purposes in the EU, as described by Iglesias (1993), Bassi (2000) and Pontoppidan (1995). There is, however, no data concerning the abundance and distribution of the host plants in the risk assessment area, although, enterprises producing plants for planting and fresh fruit of Asian pears are currently present in the EU territory.
3.4.3.2. Climatic conditions affecting establishment
The environmental requirements of V. nashicola for conidial germination and infection of its hosts are similar to those reported for V. inaequalis (causal agent of apple scab) and V. pyrina (causal agent of European pear scab), both of which are well established and widely distributed in the EU territory.
In Figure 1, the environmental requirements for conidial germination and infection of leaves by the different Venturia spp. affecting fruit crops are summarised. For conidial germination, environmental requirements of V. nashicola are similar to those of V. inaequalis, as both can germinate in a wide range of temperatures and in the absence of free water, although V. nashicola needs less hours of wetness to germinate. For infection of leaves, the requirements of V. nashicola are similar to those of V. inaequalis and V. pyrina: all three can infect under a wide range of temperatures, with an optimal at 20°C; at this temperature, 5–9 h of wetness are enough for triggering the infection process. At very low temperatures, the hours of wetness required for infection are less for V. nashicola than for V. inaequalis and V. pyrina (i.e. 10 h vs 36 and 29 h, respectively at 5°C).
Figure 1.
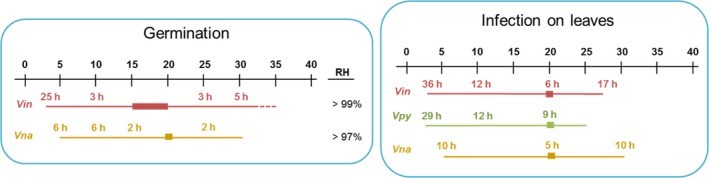
Environmental requirements of Venturia spp. for conidial germination and leaf infection
Based on this comparison and that Le Cam et al. (2001) isolated V. nashicola from Pyrus pyrifolia var. culta during the period 1988–1996 in four localities of France, the Panel concludes that the climatic conditions occurring in the risk assessment area are not a limiting factor for V. nashicola to establish in the EU territory wherever the hosts are present.
A temperature scale from 0 to 40°C is indicated at the top of each panel. Thin lines indicate the temperature at which the different processes occur for each species. Thick lines indicate optimal temperatures. Dotted line indicates temperatures that are known not to support the process based on experimental evidence. Numbers above lines indicate the hours of wetness required at each temperature. For conidial germination, the RH range in which the process can occur is indicated. Vin: Venturia inaequalis (apple scab); Vna: Venturia nashicola (Asian pear scab); Vpy: Venturia pyrina (European pear scab). Adapted from González‐Domínguez et al., 2017.
3.4.4. Spread
3.4.4.1. Vectors and their distribution in the EU (if applicable)
Is the pest able to spread within the EU territory following establishment? YES
How? By natural and human‐assisted means
Following its establishment in the EU territory, the pathogen could potentially spread by both natural and human‐assisted means.
Spread by natural means. The pathogen can spread over relatively short distances by rain‐splashed/washed‐off conidia (asexual spores) and wind‐disseminated ascospores (sexual spores) (Unemoto, 1990; Lian et al., 2006). The conidia are produced on the surface of bud scales in spring and on symptomatic plant tissues during the growing period and are dispersed in raindrops mainly during the day. According to Unemoto (1990) studies, the distance of conidial and ascospore dispersal was at least 8 and 10 m, respectively. The maximum discharged height of ascospores from pseudothecia produced in leaf litter on the orchard floor was approximately 8 mm.
Spread by human assistance. The pathogen could potentially spread over long distances through the movement of infected host plants for planting (rooted or unrooted grafted plants, scions, etc.) and fresh fruit, particularly latently infected.
3.5. Potential or observed impacts in the EU
Would the pest's introduction have an economic or environmental impact on the EU territory?
YES, the introduction of V. nashicola could cause yield and quality losses to Asian pears grown in the risk assessment area.
The Panel considered that the introduction of the pathogen into the EU territory would cause yield and quality losses to Asian pears grown in the risk assessment area. The impact at the EU level is expected to be limited because Asian pears is not a major crop in the EU and European pear (P. communis), which is widely cultivated in the EU territory, is not a host of V. nashicola (see Section 3.4.1.). However, the impacts of the pathogen to individual growers and enterprises could be significant.
3.5.1. Potential pest impacts
3.5.1.1. Direct impacts of the pest
Asian pear scab caused by V. nashicola is the major disease of Japanese pear (Pyrus pyrifolia var. culta) and Chinese pear (P. ussuriensis) in the affected countries. The main commercial cultivars of Japanese pear are susceptible to this pathogen (Park et al., 2000). V. nashicola infects fruit, leaves and young shoots (see Section 3.1.3 Symptoms) resulting in considerable annual yield and quality losses, especially on traditional Chinese pear varieties (Li et al., 2005). Nevertheless, no quantified data on yield/quality losses could be retrieved from the literature.
High resistance to Asian pear scab has been observed in Japanese pear cv. Kinchaku and Chinese pear cvs. Hong‐li, and Mi‐li, and it is related to a single dominant gene (Abe and Kotobuki, 1998; Ishii et al., 2002).
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
YES, the likelihood of pest entry can be mitigated if host plants for planting and fruit are sourced from pest‐free areas or pest‐free places of production and are inspected both at the place of origin and the EU entry point. In infested areas, agricultural practices and fungicide sprays are available for disease management.
The risk of entry of the pathogen into the risk assessment area could be mitigated if the host plants for planting (including plants at dormant stage) and the fresh fruit were imported from pest‐free areas or pest‐free places of production and were inspected both at the place of origin and at the EU entry point.
Sanitation and protectant fungicide sprays combined with routine inspections of host plants would be the only options to prevent the establishment and spread of the pathogen in the risk assessment area.
3.6.1. Biological or technical factors affecting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
-
Factors limiting the feasibility and effectiveness of measures to prevent entry of V. nashicola into the EU:
-
–
Host plants for planting at the dormant stage may carry the pathogen in the form of mycelia in the inner tissues of bud scales without showing any symptoms, thus, escaping visual inspection and detection.
-
–
The incubation period of the pathogen, known to be minimum 3–4 weeks on leaves, but unknown on fruit, may reduce the effectiveness of visual inspection and detection, as latently infected (asymptomatic) plants for planting with leaves and fresh fruit will most likely go undetected.
-
–
Factors limiting the feasibility and effectiveness of measures to prevent establishment and spread of V. nashicola in the EU.
The limited number of fungicides registered in the EU for the control of scab on Asian pears may reduce the effectiveness of chemical control as a measure to mitigate the risk of establishment and spread of V. nashicola in the risk assessment area. For instance, in Greece and Spain, only one fungicide (i.e. fenbuconazole, DMI) is registered for use on Asian pears, while several fungicides, including protectant ones, are registered against V. pyrina on P. communis (MAPAMA, 2017; MRDF, 2017). Moreover, since the number of treatments with DMI fungicides is limited because of the risk of resistance development, the possibility to effectively prevent the establishment and spread of the pathogen in the EU territory may be further reduced.
3.6.2. Control methods
In the affected countries, commercial orchards have been successfully protected by chemical spraying coupled with routine inspections, and removal of diseased plant parts. It is common for growers to spray fungicides more than 15 times a year to control the disease, especially on the most popular but highly susceptible cv. Kosui (Ishii and Yanase, 2000). Strains of V. nashicola resistant to benzimidazole fungicides are widely distributed throughout Japan, making it difficult to control the disease with this group of fungicides (Ishii et al., 1985). Since 1986, DMI fungicides, such as triflumizole, bitertanol and fenarimol, have been introduced into Japan and replaced benzimidazoles for the control of pear scab. However, strains of V. nashicola with significantly lower sensitivities to DMIs have already been identified in the infested areas (Cools et al., 2002).
3.7. Uncertainty
Host range. Except for the host species Pyrus pyrifolia var. culta, P. ussuriensis and P. bretschneideri and the non‐host species P. communis, limited and in some cases contradictory information exists in the literature on the host status of other Pyrus species
Entry: The absence of data regarding the quantity of dormant host plants for planting and fresh fruit imported from affected countries into the EU28. Uncertainty on the latent period of the pathogen on fruit due to lack of knowledge.
The absence of data regarding the abundance and distribution of the host plants in the EU28: this uncertainty affects entry (specifically the transfer of the pathogen from the pathway of entry to the host grown in the risk assessment area), establishment, spread and impact.
Establishment: It is unknown whether currently applied cultural practices and available chemical control methods would be effective in preventing the establishment of V. nashicola in the risk assessment area.
Spread: Uncertainty on the maximum distance the inoculum (conidia, ascospores) of V. nashicola can travel by natural means.
The Panel considers that uncertainties 1–5 do not affect the validity of the conclusions of this pest categorisation.
4. Conclusions
Venturia nashicola meets the criteria assessed by EFSA for consideration as a potential quarantine pest for the EU territory (see Table 5).
Table 5.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the pest is clearly defined and there are reliable methods for its detection and identification | The identity of the pest is clearly defined and there are reliable methods for its detection and identification | None |
| Absence/presence of the pest in the EU territory (Section 3.2 ) | The pest is not known to occur in the EU | The pest is not known to occur in the EU. | None |
| Regulatory status (Section 3.3 ) | The pest is currently officially regulated on Pyrus plants intended for planting, other than seeds, originating in non‐European countries (Dir 2000/29/EC) | The pest is currently officially regulated as a quarantine pest on Pyrus plants intended for planting, other than seeds, originating in non‐European countries (Dir 2000/29/EC). | None |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) |
The pest could potentially enter, establish and spread in the EU. Entry: potential pathways of entry are:
Spread: the pathogen can spread in the risk assessment area by natural means. Moreover, it can spread by human‐assisted means, i.e. movement of host plants for planting and fresh fruit from infested areas. |
The pest could spread in the EU. Potential pathways:
Hosts are present in the risk assessment area and the climatic conditions in the EU territory are compatible for the spread of V. nashicola. The pathogen can spread in the risk assessment area by natural means. Moreover, it can spread by human‐assisted means, i.e. movement of host plants for planting and fresh fruit from infested areas. |
The host range of V. nashicola is not fully known (uncertainty 1) The quantity of dormant host plants for planting and fresh fruit imported from affected countries is unknown (uncertainty 2) The latent period of the pathogen on fruit is unclear. (uncertainty 2) The abundance and distribution of the host plants in the EU28 is unknown (uncertainty 3) It is unknown whether currently applied cultural practices and available chemical control methods would be effective in preventing the establishment of V. nashicola in the risk assessment area. (uncertainty 4) The maximum distance the inoculum of V. nashicola can travel by natural means is unknown (uncertainty 5) |
| Potential for consequences in the EU territory (section 3.5 ) | V. nashicola is known to cause typical scab symptoms on leaves, fruit and shoots, premature leaf and fruit drop as well as fruit distortion. Therefore, the introduction of the pathogen into the EU territory could cause yield and quality losses to Asian pears grown in the risk assessment area. | The spread of the pathogen into the EU territory could cause yield and quality losses to Asian pears grown in the risk assessment area. |
The host range of V. nashicola is not fully known (uncertainty 1) The abundance and distribution of the host plants in the EU28 is unknown (uncertainty 3) |
| Available measures (Section 3.6 ) | Phytosanitary measures are available to mitigate the risk of entry, establishment and spread of the pathogen in the risk assessment area (e.g. importation of host plants for planting and fresh fruit from pest‐free areas or pest‐free places of production, inspection at the place of origin and the EU entry point, fungicide sprays, etc.). | Phytosanitary measures are available to prevent the presence of the pathogen on plants for planting and therefore mitigate the risk of spreading |
The host range of V. nashicola is not fully known (uncertainty 1) The quantity of dormant host plants for planting and fresh fruit imported from affected countries is unknown (uncertainty 2) The latent period of the pathogen on fruit is unclear. (uncertainty 2) The abundance and distribution of the host plants in the EU28 is unknown (uncertainty 3) It is unknown whether currently applied cultural practices and available chemical control methods would be effective in preventing the establishment of V. nashicola in the risk assessment area. (uncertainty 4) The maximum distance the inoculum of V. nashicola can travel by natural means is unknown (uncertainty 5) |
| Conclusion on pest categorisation (Section 4 ) | V. nashicola meets all the criteria assessed by EFSA above for consideration as a potential Union quarantine pest. | V. nashicola is not known to occur in the EU. Therefore, it does not meet at least one of the criteria assessed by EFSA for consideration as a Union regulated non‐quarantine pest. | None |
| Aspects of assessment to focus on/scenarios to address in future if appropriate |
The most important knowledge gaps concern:
In the opinion of the panel, a full PRA can be conducted only if these data gaps are filled in. |
||
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- IPPC
International Plant Protection Convention
- NPPO
National Plant Protection Organisation
- PLH
EFSA Panel on Plant Health
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro Maria, Niere B, Parnell S, Potting R, Rafoss T, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Gonzalez‐Dominguez E, Vicent A, Vloutoglou I, Bottex B and Rossi V, 2017. Scientific Opinion on pest categorisation of Venturia nashicola . EFSA Journal 2017;15(11):5034, 22 pp. 10.2903/j.efsa.2017.5034
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00378
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Gregoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Adopted: 27 September 2017
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
References
- Abe K and Kotobuki K, 1998. Inheritance of high resistance to Venturia nashicola Tanaka et Yamamoto in Japanese pear (Pyrus pyrifolia Nakai) and Chinese pear (P. ussuriensis Maxim.). Journal of the Japanese Society of Horticultural Science, 67, 677–680. [Google Scholar]
- Abe K, Saito T, Terai O, Sato Y and Kotobuki K, 2008. Genotypic difference for the susceptibility of Japanese, Chinese and European pears to Venturia nashicola, the cause of scab on Asian pears. Plant Breeding, 127, 407–412. [Google Scholar]
- Bassi R, 2000. Nashi: messa a dimora, forme di allevamento, cure di coltivazione, raccolta. Vita in Campagna., 07, 25. [Google Scholar]
- Cools HJ, Ishii H, Butters JA, Hollomon DW and Ashton L, 2002. Cloning and Sequence Analysis of the Eburicol 14 a ‐Demethylase Encoding Gene (CYP51) from the Japanese Pear Scab Fungus Venturia nashicola . Journal of Phytopathology, 150, 444–450. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2903/j.efsa.2010.1495 [DOI] [Google Scholar]
- Eguchi N and Yamagishi N, 2008. Ascospores of the Japanese pear scab fungus (Venturia nashicola Tanaka & Yamamoto) are discharged during the day. Journal of General Plant Pathology, 74, 41–45. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , 2017. EPPO Global Database (available online). https://gd.eppo.int
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- González‐Domínguez E, Armengol J and Rossi V, 2017. Biology and epidemiology of Venturia species affecting fruit crops: a review. Front. Plant Sci., 8, 1496 10.3389/fpls.2017.01496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KD, Jones EGB, Liu JK, Ariyawansa HA, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Begoña AH, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukea E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JK, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillip AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY and Zhang M, 2013. Families of Dothideomycetes. Fungal Diversity, 63, 1–313. 10.1007/s13225-013-0263-4 [DOI] [Google Scholar]
- Iglesias I, 1993. El Nashi. Fruticultura Profesional, 54, 15–34. [Google Scholar]
- Ishii H and Yanase H, 2000. Venturia nashicola, the scab fungus of Japanese and Chinese pears: a species distinct from V. pyrina . Mycological Research, 104, 755–759. [Google Scholar]
- Ishii H, Udagawa H, Yanase H and Yamaguchi A, 1985. Resistance of Venturia nashicola to thiophanate‐methyl and benomyl: build‐up and decline of resistance in the field. Plant Pathology, 34, 363–368. [Google Scholar]
- Ishii H, Watanabe H and Tanabe K, 2002. Venturia nashicola: pathological specialization on pears and control trial with resistance inducers. Acta Horticulturae, 587, 613–621. [Google Scholar]
- Koh HS, Sohn SH, Lee YS, Koh YJ, Song JH and Jung JS, 2013. Specific and sensitive detection of Venturia nashicola, the scab fungus of Asian pears, by nested PCR. Plant Pathology Journal, 29, 357–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cam B, Devaux M, Parisi L and Le Cam B, 2001. Specific Polymerase Chain Reaction Identification of Venturia nashicola Using Internally Transcribed Spacer Region in the Ribosomal DNA. Phytopathology, 91, 900–904. [DOI] [PubMed] [Google Scholar]
- Li B, Zhao H and Xu X‐M, 2003. Effects of temperature, relative humidity and duration of wetness period on germination and infection by conidia of the pear scab pathogen (Venturia nashicola). Plant Pathology, 52, 546–552. [Google Scholar]
- Li BHD, Xu XM, Li JT and Li BHD, 2005. Effects of temperature and continuous and interrupted wetness on the infection of pear leaves by conidia of Venturia nashicola . Plant Pathology, 54, 357–363. [Google Scholar]
- Li B‐H, Yang J‐R, Dong X‐L, Li B‐D and Xu X‐M, 2007. A dynamic model forecasting infection of pear leaves by conidia of Venturia nashicola and its evaluation in unsprayed orchards. European Journal of Plant Pathology, 118, 227–238. [Google Scholar]
- Lian S, Li B‐H and Xu X‐M, 2006. Formation and development of pseudothecia of Venturia nashicola . Journal of Phytopathology, 154, 119–124. [Google Scholar]
- Lian S, Li B‐H, Dong X‐L and Xu X‐M, 2007. Effects of environmental factors on discharge and germination of ascospores of Venturia nashicola . Plant Pathology, 56, 402–411. [Google Scholar]
- MacHardy WE, 1996. Apple scab: biology, epidemiology and management. American Phytopathological Society, St. Paul, Minnesota, USA. [Google Scholar]
- MAPAMA , 2017. Registro de Productos Fitosanitarios. Ministerio de Agricultura, Pesca, Alimentación y Medio Ambiente. http://www.mapama.gob.es/es/agricultura/temas/sanidad-vegetal/productos-fitosanitarios/registro/productos/conaplipla.asp [Accessed: 12 May 2017].
- MRDF , 2017. List of authorized plant protection products and biocides. Hellenic Ministry of Rural Development and Food. http://wwww.minagric.gr/syspest/syspest_bfuncs_crops.aspx [Accessed: 10 May 2017].
- Park P, Ishii H, Adachi Y, Kanematsu S, Ieki H and Umemoto S, 2000. Infection behavior of Venturia nashicola, the cause of scab on Asian pears. Phytopathology, 90, 1209–1216. 10.1094/PHYTO.2000.90.11.1209 [DOI] [PubMed] [Google Scholar]
- Pontoppidan A, 1995. Entre pomme et poire: le nashi. Nature and Progress, 147, 34–35. [Google Scholar]
- Schubert KS, Ritschel AR and Braun UB, 2003. A monograph of Fusicladium s. lat (Hyphomycetes). Schlechtendalia, 9, 1–132. [Google Scholar]
- Spotts RA. 2014. Pear scab In: Sutton TB, Aldwinckle HS, Agnello AM, Walgenbach JF. (eds.). Compendium of Apple and Pear Diseases and Pests. Second edition. APS Press, USA: pp. 26–27. [Google Scholar]
- Unemoto S, 1990. Dispersion of ascospores and conidia of causal fungus of Japanese pear scab, Venturia nashicola . Annals of the Phytopathological Society of Japan, 56, 468–473. [Google Scholar]
- Yun YH, Yoon SK, Jung JS and Kim SH, 2015. Specific and sensitive detection of the pear scab fungus Venturia nashicola by SYBR green real‐time PCR. Journal of Microbiology and Biotechnology, 25, 1782–1786. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Bahkali AH, Guo LD and Hyde KD, 2011. A molecular, morphological and ecological re‐appraisal of Venturiales—a new order of Dothideomycetes. Fungal Diversity, 51, 249–277. 10.1007/s13225-011-0141-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Kakishima M, Uzuhashi S and Ishii H, 2011. Multigene phylogenetic analysis of inter‐ and intraspecific relationships in Venturia nashicola and V. pirina . European Journal of Plant Pathology, 132, 245–258. [Google Scholar]


