Abstract
During 2012–2014, the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) issued three opinions on the safety and efficacy of vitamin D3 for all animal species and concluded that no safety concern was identified for the use of vitamin D3 for fish at the maximum authorised content of 0.075 mg/kg feed. The Norwegian Food Safety Authority made available to the Commission some studies on the safety of vitamin D3 for fish and consumers at substantially higher levels (1.5 mg/kg feed) than those proposed by EFSA. The European Commission asked EFSA to review the information provided to estimate if it would be possible to increase the current levels of vitamin D3 in feed for fish. The increasing use of plant‐based feed materials in aquaculture feeds could induce a decrease in vitamin D3 content in feedingstuffs. However, there is no evidence that the current total (background + supplemented) maximum EU content of vitamin D3 may cause any appreciable risk of deficiency in salmonids. The possible contribution of vitamin D2 in plant‐based ingredients to the total vitamin D intake is considered to be low, although it cannot be reliably estimated. The FEEDAP Panel concludes that a total level of 1.5 mg vitamin D3/kg compound feed is safe for salmonids with a margin of safety of at least 10. For other fish, insufficient data are available to conclude on the safety of a total level of 1.5 mg vitamin D3/kg feed. Although the assessment of safety for the consumer is impaired by uncertainties concerning the transfer of vitamin D3 from feed to fish flesh, it was concluded that an increase of total vitamin D content in fish feeds up to 1.5 mg/kg feed would not lead the tolerable upper intake level to be exceeded even in high consumers.
Keywords: nutritional additive, vitamins and pro‐vitamins, vitamin D3, cholecalciferol, fish, safety
Summary
During 2012, 2013 and 2014, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) issued three opinions on the safety and efficacy of vitamin D3 (cholecalciferol) as an additive to feed and water for drinking for all animal species. The Panel concluded that the current maximum authorised contents were temporarily acceptable for the safety of target animals and considered a complete review of the most recent literature necessary to maintain or revise these maximum contents. Particularly, no safety concern was identified for the use of vitamin D3 for fish, for which the maximum authorised content is 3,000 IU/kg feed (corresponding to 0.075 mg/kg feed). The FEEDAP Panel also concluded that the use of vitamin D in animal nutrition at the currently authorised maximum dietary content has not and will not cause the tolerable upper intake level for consumers to be exceeded.
The Standing Committee on Plants, Animals, Food and Feed discussed and agreed to support the revision of the maximum content of vitamin D3 in feed for farmed fish. The Norwegian Food Safety Authority (NFSA) made available to the European Commission (EC) some studies on the safety of vitamin D3 for fish and consumers at substantially higher levels (1.5 mg/kg feed corresponding to 60,000 IU/kg feed) than those proposed by EFSA. The EC asked EFSA to review the information provided by the NFSA and to take into account any other relevant information to estimate if it would be possible to increase the current levels of vitamin D3 in feed for fish and the impact it may have on the safety for the consumer.
It was considered possible that the increasing use of plant‐based feed materials in aquaculture feeds could induce an appreciable decrease in vitamin D3 content in compound feedingstuffs. However, the available evidence evaluated by the FEEDAP Panel does not indicate that an appreciable decrease in total vitamin D3 has occurred in the EU. Furthermore, the estimated levels of vitamin D3 in the novel fish feed formulations (with fish meal/oil replaced by plant protein sources) and the current upper legal level of vitamin D3 in the EU do not indicate any risk of vitamin D3 deficiency in farmed fish. The possible contribution of vitamin D2 in plant‐based ingredients to the total vitamin D intake is likely to be low but it cannot be reliably estimated to date.
The FEEDAP Panel reiterates its previous conclusion that the currently authorised level for vitamin D3 is sufficient to meet the requirements for salmonids. Therefore, it does not see a need to increase the current maximum authorised content based on considerations of animal nutrition.
The FEEDAP Panel concludes that a total level of 1.5 mg vitamin D3/kg compound feed is safe for salmonids with a margin of safety of at least 10. For other fish, insufficient data are available to conclude on the safety of a total level of 1.5 mg vitamin D3/kg feed.
The assessment of safety for the consumer is impaired by uncertainties concerning the transfer of vitamin D3 from feed to fish flesh, including the influence of factors such as fish species, the background content and the supplementation level of vitamin D3, and farming conditions. Even taking into account the identified uncertainties, it was concluded that an increase in total vitamin D content in fish feeds up to 1.5 mg/kg feed would not lead the upper level to be exceeded even in high consumers.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EC) No 1831/20031 establishes rules governing the Community authorisation of additives for use in animal nutrition and, in particular, Article 9 thereof defines the terms of such authorisation by the Commission.
The applications for authorisation of the additive pursuant Article 10(2) and 4 (use in water for drinking for terrestrial animals) were submitted by Lohmann Animal Health GmbH, DSM and Fermenta Biotech Ltd. Three opinions were issued by the FEEDAP Panel on 13 November 2012, 20 June 2013 and 30 January 2014, respectively.2
All applications requested the additive to be classified in the category ‘nutritional additives’ (Table 1).
Table 1.
Description of the additive as referred to in the applications for authorisation
| Category of additive | Nutritional additive |
|---|---|
| Functional group of additive | Vitamins, provitamins and chemically well‐defined substances having a similar effect |
| Description |
DSM – Cholecalciferol + precholecalciferol (67.2%) Fermenta Biotech Ltd – Vitamin D3 40 MIU/g powder and Vitamin D3 resin 22 MIU/g Lohmann Animal Health GmbH – Vitamin D3 84% |
| Target animal category | All species and categories. Fish 3,000 IU maximum content (equivalent to 0.075 mg/kg) |
| Applicant | DSM, Lohmann Animal Health GmbH and Fermenta Biotech Ltd |
| Type of request | Generic opinion |
During the discussions at the Standing Committee on Plants, Animals, Food and Feed it was agreed to request the Authority to revise the proposed levels (3,000 IU −0.075 mg/kg) for fish feed, taking into account the current feeding practices that replace the ingredients from animal origin rich in vitamin D by ingredients from crop origin with negligible content of this vitamin.
The proposed levels seemed to be insufficient to satisfy the dietary requirements for fish.
The Commission has now received supplementary information concerning the safety of vitamin D, containing, in particular, the following items:
Changes in the composition of feeds: animal protein/versus crop protein
Real contents of vitamin D in feed for fish
Correlation between vitamin D intake and deposition in edible parts
Consequences of a low upper limit in fish production
Tolerance of fish to higher levels of vitamin D
Effects of an increase in vitamin D in fish on consumer health
The supplementary information has been submitted by the Norwegian Food Safety Authority (NFSA). The NFSA concluded that levels of 1.5 mg/kg vitamin D3 supplementation are safe for fish and for the consumer.
The Commission asks EFSA to review the information provided by the NFSA and to take into account any other relevant information. The opinion should estimate if it is possible to increase the current levels of vitamin D3 in feed for fish.
1.2. Interpretation of the Terms of Reference
The Commission asked EFSA to estimate if it is possible to increase the current levels of vitamin D3 in feedingstuffs for fish up to 1.5 mg/kg complete feed.
The supplementary information provided by the NFSA and the scope of their assessment were mainly referring to safety for target animals and consumer: as a consequence, the FEEDAP Panel considers that the present assessment should be limited to the assessment of the safety for target animals and consumers. In addition, since the dataset did not include any new information concerning the safety for the user and the environment, this opinion will not address the potential effects of the proposed increase in the maximum authorised levels of vitamin D3 on the safety for the user and the environment.
1.3. Additional information
In 2012, 2013 and 2014, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) issued three opinions on the safety and efficacy of vitamin D3 (cholecalciferol) as an additive to feed and water for drinking for all animal species (EFSA FEEDAP Panel, 2012a, 2014, 2015). The FEEDAP Panel noted that whereas for many terrestrial species (turkeys for fattening, equines, bovines, ovine and pigs) the current maximum authorised contents of vitamin D3 in feeds do not provide any margin of safety, no safety concern was identified for the use of vitamin D3 in fish. Additionally, notwithstanding the long history of supplementing compound feed with vitamin D and the absence of publicly reported intolerances, the FEEDAP Panel could not draw final conclusions on the safety of vitamin D in animal nutrition based on the NRC data collection, which has not been revised for 25 years. The FEEDAP Panel considered the current maximum authorised contents to be temporarily acceptable for the target animals. Nutritional surveys in 14 European countries showed that vitamin D intake by consumers is sufficiently below the upper level (UL) (EFSA NDA Panel, 2012). The FEEDAP Panel assumes that foodstuffs of animal origin monitored in these studies were produced following current production practices, including vitamin D3 supplementation of feed. Therefore, the FEEDAP Panel concluded that the use of vitamin D in animal nutrition at the currently authorised maximum dietary content has not and will not cause the UL to be exceeded.
Vitamin D in the form of vitamin D3 (E 671) is included in the European Union Register of Feed Additives. Maximum limits have been established for inclusion in complete feeds of different target species3: in fish the current maximum limit is 3,000 IU/kg equal to 0.075 mg/kg.4 The simultaneous use of vitamin D2 (ergocalciferol) and vitamin D3 is prohibited for all species.
Vitamin D in the form of cholecalciferol (D3) and ergocalciferol (D2) is authorised for use in food,5 and in food supplements,6 for addition for specific nutritional purposes in foods for particular nutritional uses,7 to processed cereal‐based foods for infants and young children8 and to infant formulae and follow‐on formulae when reconstituted as instructed by the manufacturer.9 Vitamin D is also listed as a pharmacologically active substance in veterinary medicinal products and is not subject to maximum residue levels when used in medicinal products for food‐producing animals.10
Cholecalciferol is described in the European Pharmacopoeia (PhEur), Monograph (MG) 0072 (European Pharmacopoeia, 2010a). Cholecalciferol concentrated, oily form, powder form and water‐dispersible forms are described in MG 0575, MG 0574 and MG 0598, respectively (European Pharmacopoeia, 2010b,c,d).
One milligram of vitamin D3 is equal to 40,000 international units (IU). In the present opinion, the amounts of vitamin D in feed are expressed as mg/kg feed, and in food as μg/kg food.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the NFSA11 in support of the review of the use of vitamin D3 as a feed additive, particularly to estimate if it is possible to increase its current levels in feed for fish.
The FEEDAP Panel used the data provided by the NFSA together with data from other sources, including previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports, experts' knowledge and information received through a call for data from EU Member states and EFSA Focal Point network,12 to deliver the present output.
Following a call for data on 29 April 2016, a total of seven European countries submitted data consisting on scientific papers and grey literature (technical reports, outputs from databases, non‐published data).13 A summary of the data received is available in Appendix A.
2.2. Methodologies
The approach followed by the FEEDAP Panel to review the safety of vitamin D3 is in line with the principles laid down in Regulation (EC) No 429/200814 and the relevant guidance documents: Guidance on nutritional additives (EFSA FEEDAP Panel, 2012a), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012b).
3. Assessment
The two major natural sources of vitamin D are cholecalciferol (vitamin D3, which occurs in animals) and ergocalciferol (vitamin D2, which occurs predominantly in plants). Vitamin D3 is metabolised to the active steroid hormone 1,25‐dihydroxyvitamin D3 (1,25(OH)D3) by successive hydroxylation in the liver and kidney (in fish both hydroxylation steps occur in the liver only). Vitamin D2 is metabolised to 1,25‐dihydroxyvitamin D2 by the same enzyme systems. Vitamin D3 is also produced by endogenous synthesis in mammalian species and birds; it is also recognised that trout may synthetize vitamin D3 in the skin when exposed to blue light (Pierens and Fraser, 2015). 7‐Dehydrocholesterol in the skin is converted by exposure to ultraviolet light and then enzymatically into vitamin D3. Vitamin D2 is formed by photochemical reaction from ergosterol in plants, fungi and lower life forms. Moreover, a few plants (e.g. Solanum glaucophyllum and Trisetum flavescens) are known to produce vitamin D3‐related metabolites (EFSA FEEDAP Panel, 2015). Both forms of the vitamin can also be produced by chemical synthesis. Details of the vitamin D‐metabolism in fish have been reviewed by Lock et al. (2010).
The principal physiological role of vitamin D in all vertebrates (including teleost fish, reviewed by Lock et al., 2010) is in calcium and phosphorus homeostasis. Vitamin D is a key regulator of transcellular calcium uptake. Furthermore, vitamin D modulates the expression of Na+‐dependent inorganic phosphate (Pi) transporters (i.e. in the intestine and the kidneys). Bone represents the largest store of calcium phosphate, and vitamin D directly affects osteoblast activity and osteoclast formation. The vitamin D‐regulated metabolic pathways also play important roles in other biological processes not related to calcium and phosphorus homeostasis, i.e. in muscle function, immunological regulation and cardiovascular physiology. Vitamin D has been shown to affect cell proliferation and differentiation. Like other steroids and transcription factors, vitamin D is a specific ligand of a nuclear receptor, the vitamin D receptor (VDR) (EFSA NDA Panel, 2012, EFSA FEEDAP Panel 2012a, EFSA NDA Panel 2016 and references herein). However, a full vitamin D endocrine function, characterised by a specific VDR, specific vitamin D metabolizing CYP450 enzymes regulated by calciotropic hormones and a dedicated plasma transport protein, is only found in terrestrial vertebrates. The morphology of fish bone is very different from that of terrestrial vertebrates and the calcium reservoir of bone in fish does not represent a major factor in calcium homeostasis even in situations of calcium stress. The evolutionary biology of vitamin D in vertebrates is reviewed by Bouillon and Suda (2014).
Excess of vitamin D disrupts the Ca‐P homeostasis with consequences, e.g. on bone health in vertebrates. In humans, an upper tolerable intake level (UL) of 100 and 50 μg/day for adults and children, respectively, has been assessed (EFSA NDA Panel, 2012).
Vitamin D3 (IUPAC name: (3β,5Z,7E)‐9,10‐secocholesta‐5,7,10(19)‐trien‐3‐ol; synonyms: 9,10‐ secocholesta‐5,7,10(19)‐trien‐3‐ol, cholecalciferol, calciol) is identified with the Chemical Abstracts Service (CAS) number 67‐97‐0 and the European Inventory of Existing Chemical Substances (EINECS) number 200‐673‐2. The molecular weight is 384.64 g/mol. The structural formula of cholecalciferol is shown in Figure 1.
Figure 1.
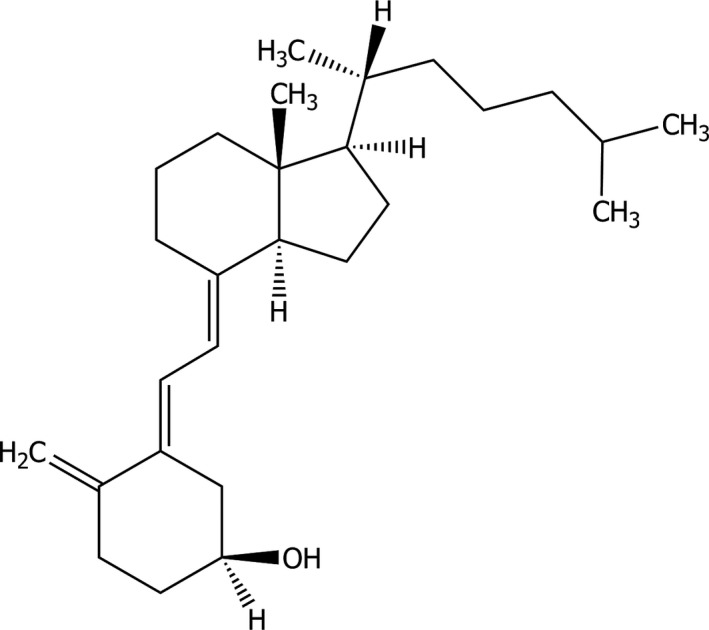
Structural formula of cholecalciferol
The FEEDAP Panel adopted three scientific opinions on different vitamin D3 products (EFSA FEEDAP Panel 2012a, 2013, 2014). The Panel concluded that the current maximum authorised contents were temporarily acceptable for the safety of all animal species but recommended a complete review of the most recent literature. The currently maximum authorised level of vitamin D in feed for fish is 0.075 mg/kg feed (corresponding to 3,000 IU/kg feed). In the meantime, the FEEDAP Panel did not foresee any risk of animal deficiency at the current authorised levels in the EU, noting that data on requirements and allowances are easily available.
The aim of the present assessment is to evaluate the consequences for fish health and consumer safety of levels of vitamin D3 substantially higher than the one currently authorised, as proposed by Norway (maximum total level of 1.5 mg vitamin D3/kg feed). To this purpose, data on the tolerance of salmon to vitamin D3 supplementation and on the correlation between vitamin D3 in feed and in edible fish products were submitted by the NFSA.
3.1. Safety
3.1.1. Safety for the target species
The information submitted by the NFSA suggests that the increasing use of plant‐based feed materials could induce a decrease in vitamin D3 content in fish feed. Consequently, the NFSA proposed to increase the total level of vitamin D3 up to a maximum of 1.5 mg/kg complete fish feed.
The following sections aim (i) to assess the potential impact of the change in the composition of feed on fish nutrition and (ii) to establish whether an increase in the current levels of supplemental vitamin D3 in feed for fish up to 1.5 mg/kg feed is safe for fish.
3.1.1.1. Changes in the composition of feeds for salmonids and the impact on fish nutrition
The composition of fish feed has changed considerably in the last 20 years as a result of a continuing trend to lower the content of fish‐derived feed materials, especially for Atlantic salmon. Previously, feed for Atlantic salmon comprised mainly fish‐derived materials, about 45% fish meal, 30% fish oil, plus wheat, vitamins and minerals; currently, the Atlantic salmon feed contains only about 20% of marine ingredients (10% fish meal and 10% fish oil).15 The rapidly growing farming industry and the limited global marine resources to be used for fish feed have increased the use of alternative feed ingredients, mainly of plant origin; moreover, the shift toward plant‐based ingredients can effectively reduce the burden of bioaccumulating contaminants in farmed fish, such as methylmercury (fish meals) and dioxins and polychlorinated biphenyls (PCB; fish oil) (reviewed by Mantovani et al., 2015). A 2011 report of Norden Nordic Innovation (an inter‐government foundation to boost innovation and competitiveness in the Nordic Countries) lists a number of main plant‐based ingredients of the new aquaculture feeds: soy (meal, protein concentrate, cake), peas and pea protein, horse beans, grains (wheat, corn gluten) as well as rapeseed oil (Norden Nordic Innovation, 2011).
Fish meals and oils can provide a good, albeit highly variable, source of vitamin D3. According to the NFSA, fish meal and fish oil show highly variable content of vitamin D3: according to the available data (Table 2), fish meal may contain from 0.01 to 0.18 mg/kg and fish oil from 0.03 to 3.9 mg/kg, depending on the species and the season.16 Therefore, based on these figures, in traditional complete feeds for farmed fish, fish meal (45%) and fish oil (30%) are estimated to provide vitamin D3 in the range of 0.01–1.25 mg/kg. Nowadays, with the new feed formulation containing more vegetable based ingredients, vitamin D3 provided by fish meal (10%) and fish oil (10%) is estimated to range from 0.004 to 0.408 mg/kg complete feed.17
Table 2.
Available data on vitamin D3 content in fish meal and fish oil
| Fish meal | Fish oil |
|---|---|
| 0.01–0.18 mg/kg (Horvli and Lie, 1994) | 0.2–3.9 mg/kg (Opstvedt et al., 1997) |
| 0.02–0.15 mg/kg (Biomar database)a | 0.03–2.4 mg/kg (Biomar database) |
In‐house database cited by the applicant in the technical dossier Annex II Vitamin D supplementing note to the Commission.
The FEEDAP also considered the available information on vitamin D2 content in feed materials. Vitamin D2 has a markedly lower efficiency in fishes compared to vitamin D3 (NRC, 2011). The USDA nutrients database18 indicates that cereals and soybeans do not contain vitamin D. Rao and Raghuramulu (1996) studied the concentration of provitamin D and vitamin D in fresh water plankton. Vitamin D2 was markedly more abundant in zooplankton than in phytoplankton, but concentrations were lower than vitamin D3 in both commodities: Vitamin D2 concentrations in phytoplankton and in zooplankton were 0.053 and 0.724 mg/kg dry matter (DM), whereas concentrations of vitamin D3 were 0.804 and 2.717 mg/kg DM in phytoplankton and in zooplankton, respectively. To the best knowledge of the FEEDAP Panel, no studies have yet measured vitamin D2 in currently used aquaculture feeds. Data on vitamin D2 content in plant‐derived ingredients normally used in fish feed were requested in the call for data launched by EFSA, but no information was received. Therefore, the FEEDAP Panel cannot assess the contribution of plant‐based materials to vitamin D intake of farmed fish. However, considering the available data on vitamin D2 in a range of feed materials, including phytoplankton, and the lower efficiency of vitamin D2, its contribution to the overall vitamin D content of aquaculture feeds is expected to be a minor one.
Although the data obtained in the Norwegian fish feed surveillance program appear to indicate that total vitamin D3 content in commercial feed samples has decreased from 2002 to 2008, a clear trend was not apparent (Sissener et al., 2012; see Appendix B). Across all years, the mean value of vitamin D3 was always equal to or higher than 0.2 mg/kg complete feed. However, the data provided do not clarify whether and to what extent these fish feeds were supplemented with vitamin D3 (from either fish meal/oil or chemically synthesised). The FEEDAP Panel notes that the average values are always at least fivefold above the vitamin D requirements for salmonids of 0.04 mg/kg (rainbow trout; NRC, 2011) as well as always at least 2.5‐fold above the maximum authorised content in the EU of vitamin D in complete feedingstuffs for fish of 0.075 mg/kg.
Following the call for data launched by EFSA, more recent data on the content of vitamin D3 in fish feed were available. Data obtained from the Norwegian feed surveillance program of NIFES showed average contents of vitamin D3 in commercial samples of 0.14 mg/kg (range 0.05–0.46, n = 73) in 2014 (Sanden et al., 2015) and 0.11 mg/kg (range 0.05–0.21, n = 63) in 2015 (Sanden et al., 2016), still approximately threefold the requirements in salmonids and above the maximum vitamin D content authorised in the EU. Experiments with feeds based on formulations with low level of fish products (replaced by plant protein sources) indicate that vitamin D levels from the feed ingredients can be reduced to 0.07 mg/kg (Waagbø et al., 2013, unpublished data).19 Data from the Danish control of nutrients in fish feed of 2015 (unpublished) showed an average vitamin D3 content of 0.03 mg/kg (0.02–0.04, n = 8).20 Feed monitoring data collected by federal inspection agencies of the German States from 2006 to 2016 showed no appreciable decrease in vitamin D3 (Appendix C).21 In these two last cases, the vitamin D3 contents in feeds were compliant with the maximum level authorised in the EU. However, uncertainties remain on how representative are these two data sets for the whole EU.
The vitamin D requirements of fish are in a broad range, 0.006–0.06 mg/kg feed, depending on species as well as farming conditions: salmonids are close to the upper range, i.e. 0.04–0.06 mg/kg (NRC, 2011; Woodward, 1994). There is no risk of deficiency at levels of vitamin D compliant with the upper limit in the EU of 0.075 mg/kg feed.
In conventional fish feed (containing fish meal/oil) the requirements are met, at least to a large extent, by the background concentration of vitamin D naturally present in the marine resources used (Ostermeyer and Schmidt, 2006). The new feed formulations that largely use plant‐based ingredients are expected to contain only 25–33% of the vitamin D3 content in the conventional formulations, owing to the greatly reduced content of fish meal and fish oil (see above for calculations). Although the contribution by vitamin D2 cannot be reliably estimated, it is likely to be a minor one.
Conclusions on changing feed composition
The increasing use of plant‐based feed materials could result in a decrease in vitamin D3 content in feedingstuffs. However, there is no evidence that the current total (background + supplemented) maximum EU content of vitamin D3 may cause any appreciable risk of deficiency in salmonids.
3.1.1.2. Tolerance of fish to vitamin D
In its previous opinions on vitamin D3, the FEEDAP Panel did not identify any safety concern for the use of vitamin D3 in fish (EFSA FEEDAP Panel, 2012a, 2013, 2014). The current maximum authorised feed content of vitamin D3 (0.075 mg/kg) is well below (about 300‐fold) the upper safe level estimated for fish (rainbow trout) for a period of more than 60 days (NRC, 1987).
The existing literature on elevated feed levels of vitamin D has been reviewed by Lock et al. (2010), Darias et al. (2011) and NRC (2011). The NFSA submitted some publications which show that carnivorous species, such as Atlantic salmon, have evolved having a normal high intake of vitamin D and may tolerate overdosing of two magnitude orders (Horvli and Lie, 1998; Graff et al., 2002a,b). When Atlantic salmon were given diets containing 0.04, 2.21 and 28.68 mg vitamin D3/kg feed, no effects were observed on weight gain, survival, plasma level of calcium, red blood cell count or haematocrit (Horvli and Lie, 1998). The effects on growth, mortality, calcium content and bone formation were investigated in Atlantic salmon fry fed diets containing 0.2, 5 and 57 mg vitamin D3/kg feed from first feeding for 14 weeks. No differences were observed on growth parameters, kidney calcium concentration, skeletal malformations or histopathological changes between the different feeding groups, suggesting that salmon can tolerate up to 57 mg/kg feed (Graff et al., 2002a). Comparable data are not available for other fish species.
Conclusions on the tolerance of fish to vitamin D
As regards tolerance to high levels of vitamin D3, the FEEDAP Panel reiterates its previous conclusion that salmonid fish are highly tolerant (EFSA FEEDAP Panel, 2012a, 2013, 2014). Based on the available literature, the FEEDAP Panel estimated a safe upper dietary level of about 25 mg/kg in fish feed. A total level of 1.5 mg/kg as proposed by the NFSA will remain safe for salmonids with a more than 10‐fold margin of safety.
For other fish species no sufficient data are available. An interspecies uncertainty factor of 10 would not suffice to protect some fish species as e.g. channel cat fish (NRC, 1987). Therefore, the FEEDAP Panel cannot conclude on the safety of a total level of 1.5 mg vitamin D3/kg feed in fish species other than salmonids.
3.1.1.3. Conclusions on safety for the target species
The FEEDAP Panel reiterates its previous conclusion that (i) the currently authorised levels for vitamin D3 are sufficient to meet the requirements for salmonids. (ii) Vitamin D3 is highly tolerated by salmonids. Consequently, a total level of 1.5 mg vitamin D3/kg compound feed as proposed by the NFSA would still afford a more than 10‐fold margin of safety in salmonids.
For other fish, no sufficient data are available to conclude on the safety of a total level of 1.5 mg vitamin D3/kg feed.
3.1.2. Safety for the consumer
3.1.2.1. Deposition of vitamin D and vitamin D3 in fish
Limited studies are available on the feed‐fillet transfer ratio for vitamin D3 in Atlantic salmon. The available data indicate that the feed‐fillet transfer ratio for vitamin D3 at high supplementation levels, i.e. above 2 mg/kg feed, can range from 0.1 (Horvli et al., 1998) to 0.13 (Graff et al., 2016); when supplementation levels are lower by one order of magnitude (i.e. in the 0.2 mg/kg feed range), the transfer ratio may be higher, i.e. 0.4 (Graff et al., 2016). However, an experimental study in rainbow trout (450 g body weight, fed for 4 months) performed by Mattila et al. (1999) showed no correlation between increasing dietary concentrations of vitamin D3 (89, 174 and 539 μg/kg feed) and vitamin D3 concentration in fillet muscle (which ranged from 5.7 to 15.6 μg/100 g fillet). Upon a request of data to EFSA focal points, two sets of unpublished experimental data in finfish species were received on the transfer of vitamin D3 from fish feed to fish flesh following high‐dose feed supplementation with vitamin D3 (e.g. 10, 100 or 1,000 times the maximum authorised content of 0.075 mg/kg feed). According to unpublished data from Denmark, a supplementation of 1.4 mg vitamin D3/kg feed results in 100 μg/kg fillet in salmon, suggesting a feed‐flesh transfer ratio of 0.07. Unpublished data from Portugal indicate that 0.75 mg/kg feed in rainbow trout results in approximately 120 μg/kg in fillet, with a feed‐flesh transfer ratio of 0.16. The FEEDAP Panel notes that in most of the above studies, no information was provided on the background content of vitamin D3 in feed; considering this uncertainty, the feed‐flesh transfer is expected to be somewhat lower than the estimates given. However, taking into account the highly variable vitamin D3 background in aquaculture feeds, no reliable assumption can be made to account for the uncertainty.
Overall, the transfer of vitamin D3 from feed to salmonid flesh seems variable, possibly in relation to the species and the farming conditions. Even taking into account the uncertainty outlined above, for high supplementation levels the transfer ratio is estimated to be in the range of 0.1–0.15. Accordingly, the proposed increase in the total authorised content of vitamin D3 in feed to 1.5 mg/kg would lead to vitamin D3 content in fish flesh ranging from 150 up to 220 μg/kg.
3.1.2.2. Assessment of consumer safety
A UL of 100 and 50 μg/day for adults and children, respectively, has been indicated by the EFSA NDA Panel (2012).
According to the EFSA NDA Panel, data from European populations indicate that vitamin D intakes from all sources in high consumers are about 25%, 75%, 30% and 8% of the UL for adults, infants (up to 1 year), children (1–10 years) and adolescents (11–17 years), respectively. This assessment is consistent with that of the FEEDAP Panel, where the highest intake of vitamin D3 by adults would result from the consumption of fish (salmon) and milk amounting to 23% of the UL. In particular, the consumption of salmon flesh containing 160 μg/kg of vitamin D3 (figures from Mattila et al., 1999; Souci et al., 2008 and upper range of NIFES data in 2006) gives the following intakes: high‐consumer toddlers (65 g/day) 10.4 μg/day, high‐consumer adults (125 g/day) 20.0 μg/day, both figures corresponding to approximately 20% of the respective UL (EFSA FEEDAP Panel, 2014, see also Table 3).
Table 3.
Daily exposure of consumers to vitamin D3 resulting from the consumption of food of animal origin (milk and fish fillet) calculated at the current authorised vitamin D levels (EFSA FEEDAP Panel, 2014) and at the proposed supplementation levels
| Toddlersa | Adultsb | ||||
|---|---|---|---|---|---|
| Concentration in food (μg/kg) | Chronic intakec (g) | Exposure (μg) | Chronic intake (g) | Exposure (μg) | |
| Currently authorised vitamin D levels (0.075 mg/kg) | |||||
| Milkd | 2.1 | 1,050 | 2.21 | 1,500 | 3.15 |
| Fish (salmon) | 160 | 65 | 10.4 | 125 | 20.0 |
| Proposed supplementation levels (1.5 mg/kg, transfer rate 0.15) | |||||
| Milkd | 2.1 | 1,050 | 2.21 | 1,500 | 3.15 |
| Fish (salmon) | 220 | 65 | 14.3 | 125 | 27.5 |
Toddlers: 1–3 years of age, 12 kg body weight.
Adults: 18–65 years of age, 60 kg body weight.
Chronic intake is the 95th percentile of the distribution of average individual consumption levels (over the survey period) for consumers only from all available EU national surveys.
Milk including dairy products.
The FEEDAP Panel notes that a concentration of 220 μg/kg in salmon flesh, resulting from the highest estimate of feed‐flesh transfer with 1.5 mg/kg feed, will increase the intake of vitamin D from salmon to 28% of the UL in high consumers (Table 3). Whereas uncertainties exist regarding the influence of factors such as fish species, the background content of vitamin D3 and the farming conditions, as well as the limitations of the available data set, the FEEDAP Panel considers unlikely that the proposed increase in vitamin D3 supplementation in aquaculture feeds would pose any concern that the UL might be exceeded. In addition, considering that, based on FEEDAP Panel calculations (EFSA FEEDAP Panel, 2014), milk and other foods of animal origin would provide no more than 5 μg/day (5% of the UL) in high‐consumer adults, the increase in vitamin D3 in fish flesh would not pose safety concerns for the consumer. This is consistent with the conclusion of the EFSA NDA Panel that the UL for vitamin D is unlikely to be exceeded in the EU even for high consumers (EFSA NDA Panel, 2012).
3.1.2.3. Conclusions on consumer safety
The FEEDAP Panel recognizes several uncertainties associated with the conditions influencing the transfer of vitamin D3 from feed to fish flesh, including (but not limited to) the fish species, the background content and the supplementation level of vitamin D3, and environmental conditions.
Even taking into account the identified uncertainties, an increase in total vitamin D content in fish feeds up to 1.5 mg/kg feed would not lead the UL to be exceeded even in high consumers. Therefore, the proposed increase is not of concern for consumer safety.
4. Conclusions
The FEEDAP Panel reiterates its previous conclusion that the currently authorised level for vitamin D3 is sufficient to meet the requirements for salmonids. Therefore, it does not see a need to increase the current maximum authorised content based on considerations of animal nutrition.
The FEEDAP Panel concludes that a total level of 1.5 mg vitamin D3/kg compound feed is safe for salmonids with a margin of safety of at least 10. For other fish, insufficient data are available to conclude on the safety of a total level of 1.5 mg vitamin D3/kg feed.
The proposed increase is not of concern for consumer safety.
5. General remarks
The FEEDAP Panel recommends that targeted studies are carried out in order to reduce the identified uncertainties concerning nutritional and safety aspects of vitamin D in feeds of farmed fish species other than salmonids. Particular consideration should be given to the tolerance and the influence on vitamin deposition in fish flesh of such factors as: fish species, the capacity for endogenous synthesis, the background content of vitamin D3 and D2 of different feed formulations, the supplementation level of vitamin D3 and farming conditions.
If the total maximum level of vitamin D3 in fish feed is increased as proposed by the NFSA, this may have implications for the safety for the user and for the aquatic compartment of the environment, not considered here.
Documentation provided to EFSA
Vitamin D3 addition to feedingstuffs for fish. August 2014. Submitted by Norwegian Food Safety Authority.
Vitamin D3 addition to feedingstuffs for fish. September 2016. Submitted by EU Member states and EFSA Focal Points network.
Abbreviations
- ANS
EFSA Scientific Panel on Additives and Nutrient Sources added to Food
- CAS
Chemical Abstracts Service
- DM
dry matter
- EC
European Commission
- FEEDAP
EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed
- IU
International Unit
- IUPAC
International Union of Pure and Applied Chemistry
- IENECS
European Inventory of Existing Chemical Substances
- NFSA
Norwegian Food Safety Authority
- NIFES
Norwegian National Institute of Nutrition and Seafood Research
- NRC
National Research Council
- PCB
polyclhorinated biphenyl
- PhEur
European pharmacopoeia
- UL
upper level
- VDR
vitamin D receptor
- Vitamin D
vitamin D2 and vitamin D3
- VKM
Norwegian Scientific Committee for Food Safety
Appendix A – Data obtained from Member states and EFSA focal points through a call for data
1.
Denmark:
Data from Danish food composition database (Technical University of Denmark): vitamin D3 content in raw Atlantic salmon from aquaculture was 6.74 μg/kg (n = 2, http://www.fooddata.dk).
Unpublished data of part of EU project F 7 ODIN. Trial in salmon fed up to 57,000 IU (1.425 mg) vitamin D3/kg. Salmon contain up to 10 μg vitamin D3/100 g flesh.
Unpublished data of Danish Veterinary and Food Administration. Results from Danish control of nutrients in fish feed sampled and analysed in 2015. Eight samples had an average of 1,152 IU/kg, ranging from 889 to 1,692 IU/kg (average 0.029 mg/kg, range 0.022–0.042 mg/kg).
United Kingdom:
Department of Health. Institute of Food Research. Nutrient Analysis of Fish and Fish Products. Sampling Report. 2013. http://www.dh.gov.uk/documents. Vitamin D content of canned salmon (red or pink) and smoked salmon (hot or cold smoked).
Germany:
Federal Office of Consumer Protection and Food Safety (BVL) provided data on analytical results of vitamin D in food and feed from the inspection agencies of the 16 Federal States in Germany. Analytical values of 44 samples (2006–2016) for vitamin D3 in compound feedingstuffs of fish were provided.
Federal Research Institute of Nutrition and Food (Max Rubner‐Institute, MRI) provided information on vitamin D content in different fish species from a database of nutritional values of about 15,000 foods (Bundeslebensmittelschlussel, BLS): 87 Analytical values of vitamin D‐cholecalciferol (μg/100 g) of different fish species (period 1989–2010). Salmon raw 3.8 μg/100 g.
-
MRI, department of safety and quality of milk and fish products. List of publications on vitamin D contents in fishery products including fish meal and on analytical methods (68 references). In addition, data of:
-
–
European nutrition values databases
-
–
USDA nutrients database
-
–
Souci Fachmann Kraut online database
-
–
Swedish food composition database
-
–
McCance and Widdowson's composition of foods integrated dataset (Public Health England)
-
–
Extract from literature, vitamin D3 content in rainbow trout, raw:
-
–
Ostermeyer and Schmidt (2006) Vitamin D and provitamin D in fish, n = 110, 8.14 μg/100 g
-
–
Health D.o. 2013. Nutrient analysis of fish and fish products, n = 9, 6.99 μg/100 g
-
–
-
–
Greece:
Greek Ministry of Rural Development and Food: two literature references and vitamin D3 content in feed for sea bass and sea bream.
Iceland:
Icelandic Food and Veterinary Authority: published report of the Matís laboratory – Nutrient value of seafoods (http://www.matis.is/media/matis/utgafa/33-11-Naeringargildi-sjavarafurda.pdf), containing data on Vitamin D3 in farmed salmon (pooled sample containing up to 10 subsamples).
Portugal:
Data from a Portuguese feed producer on vitamin D3 content in fish feed during initiation and pre‐fattening (all species). Additional data on vitamin D3 content in fish feed during fattening of trout 1,250 IU/kg (0.031 mg/kg) but not of salmon.
Data (Poster of Ramallho‐Ribeiro et al., year not provided) on transference of vitamin D3 from feed to trout flesh originating from the European Project FP 7 ODIN. Feeding rainbow trout with a control diet (0.031 mg vitamin D3/kg feed) or an enriched diet (maximum legal limit of 0.075 mg vitamin D3/kg feed), the content of vitamin D3 in trout flesh is 18% higher in the trout fed enriched diet (10 μg/100 g fillet in the control vs 11.8 μg/100 g fillet in the enriched).
Norway:
Data from the Norwegian feed surveillance program, years 2014 (n = 73, average 0.14 mg vitamin D3/kg feed, range 0.05–0.46) and 2015 (n = 63, average 0.11 mg vitamin D3/kg feed, range 0.05–0.21). Sanden et al. (2015, 2016)
Unpublished data on declining levels of vitamin D3 in fish (Lock E.J. et al., in preparation)
Unpublished data on vitamin D3 content in feed for rainbow trout (Waagbo et al., 2013)
Experimental data of transference of vitamin D3 from fish feed to fish flesh (Graff et al., 2016), including an exposure assessment for the consumer.
Appendix B – Data submitted by the Norwegian Food Safety Authority on the changes of vitamin D3 in commercial fish feed
1.
Figure B.1.
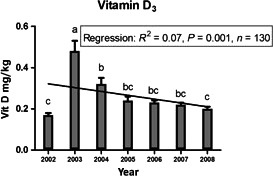
Available data on vitamin D in commercial Norwegian fish feed in the period 2002–2008. All data are given as mg/kg, represented by mean ± standard error. The line shows the linear regression analysis and the letters above the bars that do not share the same letters are significantly different (p < 0.05) (Reprinted from Sissener et al., 2012. Copyright John Wiley and Sons)
Figure B.2.
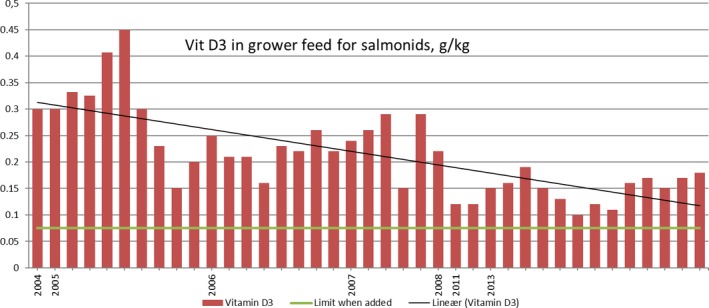
Vitamin D3 analyses fish feed, grower feed size (fish feed company: EWOS) (copyright Norwegian Food Safety Authority)
Appendix C – Raw data on the content of vitamin D3 in feedingstuffs for fish provided by the German Federal Office of Consumer Protection and Food Safety (BVL)
1.
The German Federal Office of Consumer Protection and Food Safety (BVL) provided the following raw data on vitamin D3 content in compound feedingstuffs for fish. All the analyses were performed following the VDLUFA Bd. III, Chapter 13.8.1 method. It was obtained through the Federal Inspection Agencies of German States within the period 2006–2016.
| No | Year of sample collection | Year of analysis | Water content (%) | Vitamin D3 (mg/kg) on the base of 88% DM of mixed feed |
|---|---|---|---|---|
| 1 | 2013 | 2013 | 4.7 | 0.03 |
| 2 | 2014 | 2014 | 7 | 0.10 |
| 3 | 2013 | 2013 | 8.5 | 0.08 |
| 4 | 2013 | 2013 | 6.8 | 0.06 |
| 5 | 2011 | 2011 | 6.4 | 0.06 |
| 6 | 2012 | 2012 | 6.7 | 0.07 |
| 7 | 2013 | 2013 | 4.7 | 0.36 |
| 8 | 2013 | 2013 | 6.1 | 0.13 |
| 9 | 2013 | 2013 | 7.9 | 0.04 |
| 10 | 2013 | 2013 | 6.6 | 0.16 |
| 11 | 2013 | 2014 | 7.6 | 0.06 |
| 12 | 2013 | 2014 | 7.1 | 0.06 |
| 13 | 2013 | 2013 | 9.19 | 0.07 |
| 14 | 2013 | 2013 | 7.9 | 0.15 |
| 15 | 2013 | 2013 | 4.08 | 0.04 |
| 16 | 2013 | 2013 | 4.08 | 0.05 |
| 17 | 2013 | 2014 | 7.27 | 0.05 |
| 18 | 2013 | 2013 | 8.74 | n.b. |
| 19 | 2013 | 2013 | 8.5 | 0.02 |
| 20 | 2013 | 2014 | 5.8 | 0.03 |
| 21 | 2014 | 2014 | 8.5 | 0.06 |
| 22 | 2014 | 2014 | 6 | 0.10 |
| 23 | 2014 | 2014 | 8.4 | 0.16 |
| 24 | 2014 | 2014 | 9.3 | 0.06 |
| 25 | 2014 | 2015 | 8.28 | 0.04 |
| 26 | 2014 | 2015 | 7.49 | 0.03 |
| 27 | 2009 | 2009 | 9.2 | 0.04 |
| 28 | 2013 | 2013 | 1.7 | 0.08 |
| 29 | 2013 | 2013 | 6.4 | 0.04 |
| 30 | 2013 | 2013 | 5.9 | 0.06 |
| 31 | 2014 | 2014 | 10.7 | 0.06 |
| 32 | 2016 | 2016 | 8.1 | 0.08 |
| 33 | 2014 | 2014 | 7.8 | 0.05 |
| 34 | 2011 | 2011 | 8.1 | 0.05 |
| 35 | 2010 | 2010 | 7.9 | 0.06 |
| 36 | 2010 | 2010 | 7.8 | 0.04 |
| 37 | 2009 | 2010 | 8 | 0.03 |
| 38 | 2006 | 2006 | 7.9 | 0.07 |
| 39 | 2006 | 2007 | 7.8 | 0.09 |
| 40 | 2013 | 2013 | 6.8 | 0.04 |
| 41 | 2015 | 2015 | 6.2 | 0.06 |
| 42 | 2014 | 2014 | 7 | 0.01 |
| 43 | 2015 | 2015 | 6.75 | 0.02 |
| 44 | 2013 | 2013 | 5.4 | 0.05 |
Figure C.1.
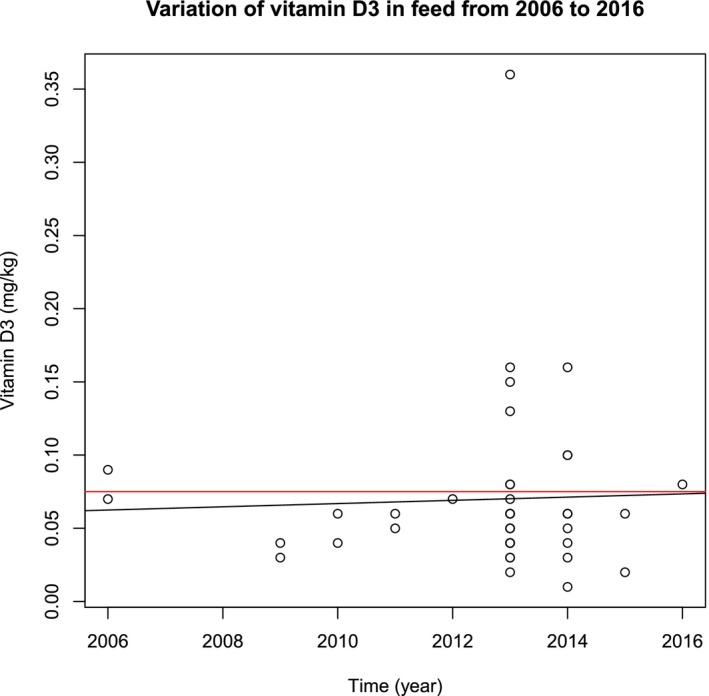
Graphic representation (scattered plot) of the data obtained by the German Federal Office of Consumer Protection and Food Safety (BVL) through the Federal Inspection Agencies of German States on vitamin D3 content in compound feedingstuffs for fish in the period 2006–2016. The red line indicates the maximum level of vitamin D3 allowed in fish feed. The black line indicates absence of a lineal trend (copyright BVL)
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wester P, Costa LG, Dierick N, Manini P, Tarrés‐Call J and Wallace RJ, 2017. Scientific opinion on the safety of vitamin D3 addition to feedingstuffs for fish. EFSA Journal 2017;15(3):4713, 19 pp. doi: 10.2903/j.efsa.2017.4713
Requestor: European Commission
Question number: EFSA‐Q‐2014‐00604
Panel members: Gabriele Aquilina, Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Georges Bories, Andrew Chesson, Pier Sandro Cocconcelli, Gerhard Flachowsky, Jürgen Gropp, Boris Kolar, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Alberto Mantovani, Baltasar Mayo, Fernando Ramos, Guido Rychen, Maria Saarela, Roberto Edoardo Villa, Robert John Wallace and Pieter Wester.
Acknowledgements: The FEEDAP Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 25 January 2017
Reproduction of the following images is prohibited and permission must be sought directly from the individual copyright holders: Figures B.1, B.2 and C.1.
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
EFSA Journal 2012;10(12):2968; EFSA Journal 2013;11(7):3289; EFSA Journal 2014;12(2):3568.
Commission Directive 91/248/ECC of 12 April 1991 amending the Annexes to Council Directive 70/524/ECC concerning additives in feeding‐stuffs. OJ L 124, 18.5.1991, p. 1.
Commission List of the authorised additives in feedingstuffs published in application of Article 9t (b) of Council Directive 70/524/EEC concerning additives in feedingstuffs (2004/C 50/01). OJ C 50, 25.2.2004., p. 144. Available at http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52004XC0225(03)&from=EN
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJ L 404, 30.12.2006, p. 26. Last amended by Commission Regulation (EC) No 1170/2009 of 30 November 2009 amending Directive 2002/46/EC of the European Parliament and of Council and Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards the lists of vitamin and minerals and their forms that can be added to foods, including food supplements. OJ L 314, 1.12.2009, p. 36.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. OJ L 183, 12.7.2002, p. 51.
Commission Regulation (EC) No 953/2009 of 13 October 2009 on substances that may added for specific nutritional purposes in foods for particular nutritional uses. OJ L 269, 14.10.2009, p. 9.
Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby‐foods for infants and young children. OJ L 339, 6.12.2006, p. 16.
Commission Directive 2006/141 EC of 22 December 2006 on infant formulae and follow‐on formulae and amending Directive 1999/21/EC. OJ L 401, 30.12.2006, p. 1.
Commission Regulation (EU) 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1.
FEED dossier reference: FAD‐2014‐0035.
The Focal Point network (EU food safety interfaces) comprises members from all 28 EU Member States, Iceland and Norway, as well as observers from Switzerland and EU candidate countries.
Technical dossier/call for data.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Annex II Vitamin D supplementing. Data from the NFSA and from feed producing company Biomar.
Technical dossier/Annex II Vitamin D supplementing note to Commission/Annexes I and IV Vitamin D in Feed to Salmon, BioMar, unpublished data.
Technical dossier/Annex II Vitamin D supplementing note/Annex IV Vitamin D in Feed to Salmon, BioMar, unpublished data and Sissener et al., 2012.
USDA nutrients database available online: https://ndb.nal.usda.gov/ndb/search/list
Technical dossier/Annex II Vitamin D supplementing note to the commission/Annex I.
Technical dossier/Call for data/Data provided by Denmark.
Technical dossier/Call for data/Data provided by Germany/Annex E2.
References
- Bouillon R and Suda T, 2014. Vitamin D: calcium and bone homeostasis during evolution. BoneKEy Reports 3, 10 pp. Article number: 480. doi: 10.1038/bonekey.2013.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darias MJ, Mazurais D, Koumoundouros G, Cahu CI and Zambonino‐Infante JL, 2011. Overview of vitamin D and C requirements in fish and their influence on the skeletal system. Aquaculture, 315, 49–60. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Scientific Opinion on the safety and efficacy of vitamin D3 (cholecalciferol) as a feed additive for chickens for fattening, turkeys, other poultry, pigs, piglets (suckling), calves for rearing, calves for fattening, bovines, ovines, equines, fish and other animal species or categories, based on a dossier submitted by DSM. EFSA Journal 2012;10(12):2968, 26 pp. doi: 10.2903/j.efsa.2012.2968 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance for the preparation of dossiers for nutritional additives. EFSA Journal 2012;10(1):2535, 14 pp. doi: 10.2903/j.efsa.2012.2535 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. doi: 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2013. Scientific Opinion on the safety and efficacy of vitamin D3 (cholecalciferol) as a feed additive for pigs, piglets, bovines, ovines, calves, equines, chickens for fattening, turkeys, other poultry, fish and other animal species or categories, based on a dossier submitted by Fermenta Biotech Ltd. EFSA Journal 2013;11(7):3289, 26 pp. doi: 10.2903/j.efsa.2013.3289 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014. Scientific Opinion on the safety and efficacy of vitamin D3 (cholecalciferol) as a feed additive for all animal species or categories based on a dossier submitted by Lohmann Animal Health GmbH. EFSA Journal 2014;12(2):3568, 24 pp. doi: 10.2903/j.efsa.2014.3568 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015. Scientific Opinion on the safety of Solanum glaucophyllum standardised leaves as feed material. EFSA Journal 2015;13(1):3967, 43 pp. doi: 10.2903/j.efsa.2015.3967 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2012. Scientific Opinion on the tolerable upper intake level of vitamin D. EFSA Journal 2012;10(7):2813, 45 pp. doi: 10.2903/j.efsa.2012.2813 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016. Scientific opinion on dietary reference values for vitamin D. EFSA Journal 2016;14(10):4547, 145 pp. doi: 10.2903/j.efsa.2016.4547 [DOI] [Google Scholar]
- European Pharmacopoeia (PhEur), 2010a. Cholecalciferol, Monograph (MG) 0072, 7th Edition Council of Europe (COE)—European Directorate for the Quality of Medicines, Strasbourg, France. [Google Scholar]
- European Pharmacopoeia (PhEur), 2010b. Cholecalciferol concentrated, oily form, Monograph (MG) 0575, 7th Edition Council of Europe (COE)—European Directorate for the Quality of Medicines, Strasbourg, France. [Google Scholar]
- European Pharmacopoeia (PhEur), 2010c. Cholecalciferol concentrated, powder form, Monograph (MG) 0574, 7th Edition Council of Europe (COE)—European Directorate for the Quality of Medicines, Strasbourg, France. [Google Scholar]
- European Pharmacopoeia (PhEur), 2010d. Cholecalciferol concentrated, water‐dispersible form, Monograph (MG) 0598, 7th Edition Council of Europe (COE)—European Directorate for the Quality of Medicines, Strasbourg, France. [Google Scholar]
- Graff IE, Høie S, Totland GK and Lie Ø, 2002a. Three different levels of dietary vitamin D3 fed to first‐feeding fry of Atlantic salmon (Salmo salar L.): effect on growth, mortality, calcium content and bone formation. Aquaculture Nutrition, 8, 103–111. [Google Scholar]
- Graff IE, Waagbø R, Fivelstad S, Vermeer C, Lie Ø and Lundebye AK, 2002b. A multivariate study on the effects of dietary vitamin K, vitamin D3 and calcium, and dissolved carbon dioxide on growth, bone minerals, vitamin status and health performance in smolting Atlantic salmon Salmo salar L. Journal of Fish Diseases, 25, 599–614. [Google Scholar]
- Graff IE, Øyen J, Kjellevold M, Frøyland L, Gjesdal CJ, Almås B, Rosenlund G and Lie Ø, 2016. Reduced bone resorption by intake of dietary vitamin D and K from tailor‐made Atlantic salmon: a randomized intervention trial. Oncotarget, 7, 69200–69215. Available online: 10.18632/oncotarget.10171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvli O and Lie Ø, 1994. Determination of vitamin D3 in fish meals by HPLC. Fisk. Dir. Skr., Ser. Ernæring, 6, 163–175. [Google Scholar]
- Horvli O and Lie Ø, 1998. Tissue distribution of vitamin D3 in Atlantic salmon Salmo salar: effect of dietary level. Aquaculture Nutrition, 4, 127–131. [Google Scholar]
- Horvli O, Lie O and Aksnes L, 1998. Tissue distribution of vitamin D3 in Atlantic salmon Salmo salar: effect of dietary level. Aquaculture Nutrition, 4, 127–131. http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2095.1998.00062.x/abstract [Google Scholar]
- Lock E‐J, Waagbø R, Wendelaar Bonga S and Flik G, 2010. The significance of vitamin D for fish: a review. Aquaculture Nutrition, 16, 100–116. [Google Scholar]
- Mantovani A, Ferrari D and Frazzoli C, 2015. Sustainability, security and safety in the feed‐to‐fish chain: focus on toxic contamination. International Journal of Nutrition and Food Sciences, 4, 6–24. [Google Scholar]
- Mattila P, Piironen V, Hakkarainen T, Hirvi T, Uusi‐Raurva E and Päivi Eskelinen, 1999. Possibilities to raise vitamin D content of rainbow trout (Oncorhynchus mykiss) by elevated feed cholecalciferol contents. Journal of the Science of Food and Agriculture, 79, 195–198. [Google Scholar]
- NRC (National Research Council) , 1987. Vitamin Tolerance of Animals. National Academy Press, Washington, DC. 108 p. ISBN: 0‐309‐59567‐3 [Google Scholar]
- Norden Nordic Innovation , 2011. Local raw materials for production of fish feed for aquaculture. Project Number 10102. June 2011 Available online: http://www.nordicinnovation.org/Global/_Publications/Reports/2011/2011_lokal_raw_material_fish_feed_rep.pdf
- NRC (National Research Council), 2011. Nutrient Requirements of Fish and Shrimp. National Research Council of the National Academies, The National Academies Press, Washington, DC. [Google Scholar]
- Opstvedt J, Knudsen G and Asbjørnsen B, 1997. Innhold av fettløselige vitaminer I fiskemel og fiskeolje produsert av sild, lodde, tobis, kolmule og brisling. SSF Report C‐295, 10.10.97, 10 pp (in Norwegian).
- Ostermeyer U and Schmidt T, 2006. Vitamin D and provitamin D in fish. European Food Research and Technology, 222, 403–413. [Google Scholar]
- Pierens SL and Fraser DR, 2015. The origin and metabolism of vitamin D in rainbow trout. The Journal of Steroid Biochemistry and Molecular Biology, 145, 58–64. [DOI] [PubMed] [Google Scholar]
- Rao DS and Raghuramulu N, 1996. Food chain as origin of vitamin D in fish. Comparative Biochemistry and Physiology, A‐Physiology, 114, 15–19. [Google Scholar]
- Sanden M, Hemre G‐I, Måge A, Lunestad BT, Espe M, Lundebye AK, Amlund H, Torstensen B and Ørnsrud R, 2015. Program for overvåking av fiskefôr Årsrapport for prøver innsamlet i 2014. Nasjonalt institutt for ernærings‐ og sjømatforskning (NIFES). 49 pp. ISBN 978‐82‐91065‐27‐4.
- Sanden M, Hemre G‐I, Måge A, Lunestad BT, Espe M, Lundebye AK, Amlund H, Torstensen B and Ørnsrud R, 2016. Program for overvåking av fiskefôr Årsrapport for prøver innsamlet i 2015. Nasjonalt institutt for ernærings‐ og sjømatforskning (NIFES). 50 pp. ISBN 978‐82‐91065‐38‐0.
- Sissener NH, Julshamn K, Espe M, Lunestad BT, Hemre GI, Waagbø R and Måge A, 2012. Surveillance of selected nutrients, additives and undesirables in commercial Norwegian fish feeds in the years 2000–2010. Aquaculture Nutrition, 19, 555–572. [Google Scholar]
- Souci SW, Fachmann W and Kraut H, 2008. Food Composition and Nutrition Tables. 7th Edition, MedPharm Scientific Publ. and Taylor Francis, Stuttgart, Germany: pp. 473–558. [Google Scholar]
- Woodward B, 1994. Dietary vitamin requirements of cultured young fish, with emphasis on quantitative estimates for salmonids. Aquaculture, 124, 133–168. [Google Scholar]


