Abstract
Zearalenone (ZEN), a mycotoxin primarily produced by Fusarium fungi, occurs predominantly in cereal grains. The European Commission asked EFSA for a scientific opinion on the risk to animal health related to ZEN and its modified forms in feed. Modified forms of ZEN occurring in feed include phase I metabolites α‐zearalenol (α‐ZEL), β‐zearalenol (β‐ZEL), α‐zearalanol (α‐ZAL), β‐zearalanol (β‐ZAL), zearalanone (ZAN) and phase II conjugates. ZEN has oestrogenic activity and the oestrogenic activity of the modified forms of ZEN differs considerably. For ZEN, the EFSA Panel on Contaminants in the Food Chain (CONTAM) established no observed adverse effect levels (NOAELs) for pig (piglets and gilts), poultry (chicken and fattening turkeys), sheep and fish (extrapolated from carp) and lowest observed effect level (LOAEL) for dogs. No reference points could be established for cattle, ducks, goats, horses, rabbits, mink and cats. For modified forms, no reference points could be established for any animal species and relative potency factors previously established from rodents by the CONTAM Panel in 2016 were used. The dietary exposure was estimated on 17,706 analytical results with high proportions of left‐censored data (ZEN about 60%, ZAN about 70%, others close to 100%). Samples for ZEN were collected between 2001 and 2015 in 25 different European countries, whereas samples for the modified forms were collected mostly between 2013 and 2015 from three Member States. Based on exposure estimates, the risk of adverse health effects of feed containing ZEN was considered extremely low for poultry and low for sheep, dog, pig and fish. The same conclusions also apply to the sum of ZEN and its modified forms.
Keywords: zearalenone, modified forms, metabolites, feed, exposure, toxicity, animal health risk assessment
Summary
Following a request from the European Commission, the EFSA Panel on Contaminants in the Food Chain (CONTAM) assessed the risk to animal health related to the presence of zearalenone (ZEN) and its modified forms in feed. The CONTAM Panel was asked to consider all relevant adverse health effects, and in particular to address the co‐occurrence of ZEN and its modified forms, and to estimate the dietary exposure of different animal species.
Previous risk assessments from the European Food Safety Authority (EFSA) on ZEN in feed (2004), ZEN in food (2011), modified forms of certain mycotoxins in food and feed (2014) and on the appropriateness to set a group health‐based guidance value for ZEN and its modified forms (2016) have been used as a starting point for the present assessment.
ZEN is a phenolic resorcylic acid lactone mycotoxin produced by several Fusarium species, particularly Fusarium graminearum. ZEN can be modified in plants, fungi and animals by phase I and phase II metabolism. Modified forms of ZEN occurring in feed include its reduced phase I metabolites, i.e. α‐zearalenol and β‐zearalenol (α‐ZEL and β‐ZEL), α‐zearalanol and β‐zearalanol (α‐ZAL and β‐ZAL), zearalanone (ZAN) and its phase II derivatives, such as those conjugated with glucose, sulfate and glucuronic acid. α‐ZAL, one of the phase I metabolites of ZEN, is used as a growth promoter in non‐European Union (EU) countries under the name of Zeranol. It is banned in Europe, and therefore, it is included in official control plans.
Analytical methods for ZEN and its modified forms in feed are well‐established. However, while methods reported in the scientific literature are widely based on the more sensitive liquid chromatography–tandem mass spectrometry (LC–MS/MS), most of the routine analyses are still performed by liquid chromatography‐fluorescence detector (LC‐FLD) or liquid chromatography‐ultraviolet detector (LC‐UV). Of note, calibrants for ZEN conjugates and reference materials for phase I and phase II modified forms are not commercially available.
Wide interspecies differences in ZEN absorption, distribution, metabolism and excretion (ADME) have been documented. Prehepatic, hepatic end extrahepatic ZEN metabolism has been reported; the nature and the amount of the generated metabolites may affect the species‐sensitivity to the toxin. In farm and companion animals, reductive biotransformations largely prevail; the main ZEN metabolites are α‐ZAL, β‐ZAL, with only very limited amounts of α‐ZEL, β‐ZEL, and other reductive metabolites being produced; reduced metabolites retain or increase the oestrogenic potency of the parent compound. Based on the levels measured in biological fluids (plasma, urine or bile) of ZEN‐treated animals, the α‐derivatives seem to prevail in pigs, dogs, and turkeys while the β‐derivatives appear to be more abundant in cattle, goats, horses, broiler chickens, and laying hens. Cytochrome P450 (CYP)‐mediated aromatic or aliphatic hydroxylation also occurs, but information in farm and companion animals is limited. In orally exposed animals, ZEN and its metabolites are rapidly absorbed, distributed to several organs and quickly excreted mainly via the biliary route as glucuronides; an active enterohepatic circulation has been demonstrated. Excretion in milk and eggs has been documented for ZEN and its metabolites.
There is scant information on the toxicokinetics of ZEN in cattle; rumen microbes extensively metabolise ZEN to α‐ZAL and β‐ZAL, the latter being predominant also in biological fluids. Little is known on the toxicokinetics of ZEN in sheep; ZEN is almost completely biotransformed by ovine rumen fluid to α‐ZAL, β‐ZAL at similar proportions and both metabolites are present in urine of dosed animals as both free and conjugated forms. No studies have been identified on ZEN administration to goats by the oral route.
There is a large data set for ZEN toxicokinetics in pigs, including piglets, gilts, and sows. In all porcine categories, the production of α‐ZEL largely outweighed that of β‐ZEL and other reductive metabolites which were recovered, along with ZEN in blood, urine, and bile mostly in their glucuronidated form. The modified forms ZEN‐14‐O‐β‐glucoside (ZEN14Glc), ZEN‐16‐O‐β‐glucoside (ZEN16Glc) and ZEN‐14‐sulfate (ZEN14Sulf) were not detected in urine and faeces when administered orally, suggesting a complete hydrolysis and thereby contribution to ZEN overall toxicity.
In poultry, ZEN is characterised by a low oral bioavailability and a rapid elimination. β‐ZEL was largely predominant over α‐ZEL in broiler chickens and laying hens, while turkey poults tended to biotransform ZEN more extensively and synthesise a relatively high amount of α‐ZEL; in all cases, glucuronidation was the main conjugation reaction.
In horses, α‐ZEL and β‐ZEL are the main ZEN metabolites, the latter being predominantly formed over α‐ZEL under in vivo conditions.
Little is known about the toxicokinetics of ZEN in rabbits; ZEN, α‐ZEL and β‐ZEL were detected in colonic chyme, faeces, bile and urine of rabbits orally dosed with ZEN.
There is scant information concerning ZEN toxicokinetics in fish. In rainbow trout, there is evidence of a prevalent production of β‐ZEL vs α‐ZEL, while the reverse seems to occur in carp.
No information could be identified for toxicokinetics of cats and farmed mink. In orally exposed dogs, measurable blood levels of ZEN, α‐ and β‐ZEL were detected throughout the experiment, with a clear prevalence of the α‐ over the β‐derivative (up to 100%).
Little is known about the metabolic fate of modified forms, except for α‐ZAL. In all species, α‐ZAL is oxidised to ZAN and isomerised to β‐ZAL; the parent compound and the metabolites are mainly glucuronidated.
The main biological activity of ZEN is its oestrogenicity, i.e. the ability to act like the endogenous steroidal sex hormone 17‐β‐oestradiol. ZEN binds to oestrogenic receptors (ERs) and has a stronger affinity to ER‐α than to ER‐β. ZEN and its modified forms differ considerably in their oestrogenic activity. Based on their ‘uterotrophic activity’ assessed in rodents, ZEN and its modified forms are ranked as follows: α‐ZEL > α‐ZAL > ZEN ≈ ZAN ≈ β‐ZAL > β‐ZEL. ZEN can activate the pregnane X receptor (PXR) and increase the transcription of a number of genes, including several CYPs.
Cattle appear to be more resistant to the adverse effects of ZEN than other farm animals because they biotransform ZEN more into β‐ZEL than α‐ZEL.
The only one available dose–response experiment in dairy cows orally exposed to pure ZEN for which a reduction in the size of corpora lutea was claimed but no effects level could be identified, was too limited to identify adverse effects. Similar limitations were identified in a study on heifers at relatively high exposure level where the conception rate tended to be decreased. No reference point for risk characterisation could be derived in cattle.
Based on ovulation rates and lambing percentages, a lowest observed adverse effect level (LOAEL) of 56 μg ZEN/kg body weight (bw) per day and a no observed adverse effect level (NOAEL) 28 μg ZEN/kg bw per day was established for sheep. No data were available for deriving reference points for risk characterisation for goats.
Pigs are generally regarded as being a very sensitive species to ZEN, the most sensitive being prepubertal female piglets. Based on the appearance of the vulva and the uterus weight, a no observed effect level (NOEL) of 10.4 μg ZEN/kg bw per day was established for piglets by the CONTAM Panel in 2011 and retained in 2016. For sexually mature female pigs, a NOAEL of 40 μg/kg bw per day was identified based on prolonged cycling.
Poultry responds to the presence of ZEN in feed only at rather high dietary concentrations and can generally be regarded as resistant. Based on decreased number of lymphocytes and the vent swelling, NOAELs of 7,500 and 9,100 μg/kg bw per day were identified for chickens and turkeys, respectively. For other poultry species and categories data are scarce. Therefore, LOAELs/NOAELs could not be derived.
The only available study performed in horses with the purified mycotoxin did not allow the derivation of NOAEL or a LOAEL due to poor experimental design and the lack of a control group.
In rabbits, no oestrogenic effects at 100 μg/kg bw per day were observed. However, at 10 μg ZEN/kg bw per day, a transient increase of catecholamine was observed. No NOAEL or LOAEL could be established.
Very limited toxicity data for ZEN are available for fish. The CONTAM Panel estimated a NOAEL for carp of 0.3 mg ZEN/kg feed to correspond to 9 μg ZEN/kg bw per day based on decreased number of monocytes, increased number of granulocytes and increased lipid peroxidation in liver and gill and altered the carbohydrate metabolism. No effect level to characterise the hazard of ZEN could be established for other fish species.
Dogs are considered a sensitive species to ZEN. No NOAEL could be established. Based on myometrium and endometrium lesions, aspect of uterine glands, blood haematology and biochemistry a LOAEL of 25 μg ZEN/kg bw per day has been estimated for dogs (mature bitches).
No data could be identified concerning the effects of ZEN in cats.
For farmed mink, the available studies were not suitable for deriving reference point of hazard characterisation since they were performed at doses hardly occurring in practice and the lowest tested dose (1,000 μg/kg bw per day) already caused overt oestrogenic effects.
Very few experiments investigated the adverse effect of the modified forms of ZEN on livestock species, horses, fish and dogs and none of them were suitable to derive a NOAEL or LOAEL.
The dietary exposure was estimated using a final data set of 17,706 analytical results of ZEN and modified forms occurrence in feed. Data were representing most of the feed commodities but for many commodities only a limited number of samples were analysed. Samples for ZEN were collected between 2001 and 2015 in 25 different European countries, whereas samples on the modified forms were collected mostly between 2013 and 2015 from three Member States. The percentage of left‐censored data (results below limit of detection and/or limit of quantification) was high (ZEN about 60%; ZAN about 70%; α‐ZEL, β‐ZEL, α‐ ZAL and β‐ZAL about 100%). The CONTAM Panel considered it important to estimate the occurrence and the animal exposure to the total concentration of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL, β‐ZEL through feed.
Apart from ‘Cereal grains, their products and by‐products’ and ‘Compound feed’, only a limited number of quantified data were available for other feed groups, i.e. forages, land animal products, legume seeds, minerals, oil and other seeds and tubers. The highest number of reported samples for ZEN corresponded to the feed group ‘Cereal grains, their products and by‐products’ (~ 67%) and particularly for ‘Wheat’ (n = 6,499). Other food groups that were well represented were ‘Complementary/Complete feed’ (n = 2,625), ‘Maize and corn’ (n = 2,048) and ‘Barley’ (n = 1,596). Although an important ingredient in commercial rabbit diets, no data were reported for lucerne meal.
The occurrence assessment for ZEN reported in the literature is consistent with data in the EFSA database. Concerning the occurrence of modified forms, phase II conjugated forms have been often reported in the recent literature, whereas no data have been received by EFSA. The co‐occurrence of ZEN and its phase I and phase II modified forms is mainly described in cereals and products thereof. While milling may lead to a redistribution of ZEN and its modified forms in the final fractions, with a possible enrichment in middlings, there is no evidence of significant degradation by processing.
Molar relative potency factors (RPFs) of the modified forms relative to ZEN were applied to occurrence levels of the respective ZEN metabolites according to the Opinion of the EFSA CONTAM Panel delivered in 2016.
Exposure to ZEN and its modified forms is primarily from consumption of contaminated cereal grains and cereal by‐products. Except for forage maize (and maize silage produced from it) and cereal straw, levels in forages are generally low.
The mean lowest lower bound (LB) to highest upper bound (UB) exposures of dairy cows and beef cattle to ZEN ranged from 0.06 to 5.1 μg/kg bw per day, and the P95 exposures ranged from 0.30 to 32.9 μg/kg bw per day. For sheep and goats, the calculated lowest LB to highest UB mean exposures to ZEN were 0.18 and 1.78 μg/kg bw per day, respectively, while at the 95th percentile the range was from 0.27 (LB) to 10.8 (UB) μg/kg bw per day. The calculated mean LB and UB exposures for pigs were 0.81 and 1.35 μg/kg bw per day, respectively, while the 95th percentile exposures ranged from 2.50 (LB) to 7.88 (UB) μg/kg bw per day, respectively. For poultry, estimates of the mean exposure ranged from 0.74 (LB) to 3.64 (UB) μg/kg bw per day. The equivalent range for the 95th percentile estimates of exposure was 3.46 and 12.7 μg/kg bw per day, respectively. For horses, the LB and UB mean exposure estimates to ZEN were 0.18 and 0.89 μg/kg bw per day, respectively, while for the 95th percentile the range LB to UB was 0.35 to 3.69 μg/kg bw per day. Based on assumed diet compositions, the estimated mean LB and UB exposures for farmed salmonids and carp ranged from 0.08 to 0.50 μg/kg bw per day, respectively. At the 95th percentile, LB and UB estimates of exposure were 0.49 and 1.78 μg/kg bw per day, respectively. The estimated mean exposure for farmed rabbits and mink ranged from 0.31 (LB) to 1.11 (UB) μg/kg bw per day, while the equivalent range for the 95th percentile was from 0.85 to 1.41 μg/kg bw per day. For companion animals (cats and dogs), LB and UB mean exposure to ZEN ranged from 0.22 to 0.33 μg/kg bw per day, respectively, while at the 95th percentile the range was from 0.75 (LB) to 0.80 (UB) μg/kg bw per day.
There was considerable variation between livestock groups in the percentage of the total exposure (ZEN + α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL) that was accounted for by the sum of the modified forms (α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL).
For many species, notably poultry, ZEN accounted for the total exposure (100%) because of lack of data of ZEN modified forms.
At the LB exposure, the modified forms accounted for 0–39% of the total exposure at both the mean and 95th percentile for cattle, goat, sheep, horses, pigs, fish, rabbits, cats, dogs and mink.
At the UB exposure, the modified forms accounted for 50% or more of the total exposure, both at the mean and 95th percentile for dairy cows, beef cattle, lactating sheep, horses, weaned pigs and rabbits. More than half of the UB mean exposure for fattening pigs, lactating sows, cats and dogs was from the modified forms, but was < 50% at the 95th percentile.
The Panel noted that estimating the occurrence and exposure with such a high number of left censored data leads to a very high uncertainty.
Risk characterisation for ZEN, was performed comparing the chronic exposure values in diets at the UB mean and UB 95th percentile concentrations for ZEN with identified reference points. For cattle, horses, rabbit, goat, duck, mink and cats the health risk from the exposure to ZEN could not be assessed as no NOAEL or LOAEL have been identified.
For poultry (chicken and fattening turkeys), the highest estimated chronic exposure of ZEN was less than 0.06% of the NOAEL. The Panel concluded that the estimated risk for chronic adverse health effects from feed containing ZEN was extremely low for poultry.
For sheep, the highest estimated chronic exposure of ZEN was less than 16% of the NOAEL. The Panel concluded that the risk of adverse health effects of feed containing ZEN was low for sheep.
For dogs, only a LOAEL was available. The highest estimated chronic exposure of ZEN was less than 3% of the LOAEL. The Panel concluded that the estimated risk for chronic adverse health effects from feed containing ZEN was low for dog.
For fish, a NOAEL was only available for carp and was extrapolated to all fish species. The highest estimated chronic exposure of ZEN was 24% of the NOAEL. The Panel concluded that the estimated risk for chronic adverse health effects from feed containing ZEN was low for fish.
For piglets and gilts, the highest estimated chronic exposure (P95) of ZEN was 21% and 9% of the NOAEL, respectively. The Panel concluded that the estimated risk for chronic adverse health effects from feed containing ZEN was low for piglets and gilts.
Risk characterisation for the sum of ZEN and its modified forms, was performed comparing the chronic exposure of the sum of ZEN and its modified forms corrected for molar RPFs with identified reference points obtained for ZEN. For cattle, horses, rabbit, goat, duck, mink and cats the health risk from the exposure to ZEN and its modified forms could not be assessed as no NOAEL or LOAEL have been identified. The Panel noted that no data on levels of α‐ZAL, β‐ZAL, ZAN, α‐ZEL or β‐ZEL were provided in species‐specific compound feeds for poultry. However, considering the very high NOAELs for these species, and the composition of their feed, the Panel considered the risk of health effects from ZEN and its modified forms was extremely low for these species.
For sheep, the highest estimated chronic exposure of ZEN and modified forms was less than 25% of the NOAEL. The Panel concluded that the risk of adverse health effects of feed containing ZEN and its modified forms was low for sheep.
For dogs, only a LOAEL was available. For this species, the highest estimated chronic exposure of ZEN and its modified forms were less than 5% of the LOAEL. The Panel concluded that the estimated risk for chronic adverse health effect from consuming feed containing ZEN and its modified forms was low for dog.
For fish, a NOAEL was only available for carp and was extrapolated to all fish species. The highest estimated chronic exposure of ZEN and its modified forms was less than 30% of the NOAEL. The Panel concluded that the estimated risk for chronic adverse health effect from feed containing ZEN and its modified forms was low for fish.
For piglets and gilts, the highest estimated chronic exposure of ZEN and its modified forms were 59% and 12% of the NOAEL, respectively. The Panel concluded that the estimated risk for chronic adverse health effects from feed containing ZEN and its modified forms was low for piglets and gilts.
The CONTAM Panel noted that there is a need for more data on the occurrence of modified forms of ZEN in feed. In addition, there is a need of calibrants and reference materials for the development of properly validated and sensitive routine analytical methods for ZEN modified forms in the feed commodities and especially highly sensitive methods to identify the most potent form α‐ZEL.
There is also a need for toxicological and toxicokinetic data on ZEN modified forms, particularly for cattle, horses, rabbit, poultry, for companion animals and mink to ZEN, to reduce the uncertainties in the animal risk assessment.
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
BACKGROUND
Following a request from the European Commission, the risks to human and animal health related to modified forms of the Fusarium toxins zearalenone, nivalenol, T‐2 and HT‐2 toxins and fumonisins were evaluated in the scientific opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed,1 adopted by the EFSA Panel on Contaminants in the Food Chain (CONTAM) on 25 November 2014.
The CONTAM Panel indicated in the recommendations that the animal health effects of zearalenone need to be re‐assessed in order to possibly set No observed adverse effect levels/lowest observed effect levels (NOAELs/LOAELs) for zearalenone in order to be able to assess the risk for animal health related to the presence of zearalenone and its modified forms in feed.
TERMS OF REFERENCE
In accordance with Art. 29 (1) (a) of Regulation (EC) No 178/2002, the Commission asks the European Food Safety Authority (EFSA) for a scientific opinion on the risks for animal health related to the presence of zearalenone and its modified forms in feed.
1.2. Interpretation of the Terms of Reference
The CONTAM Panel concluded that the terms of reference provided by the Commission were clear.
1.3. Additional information
1.3.1. Previous risk assessments
The Scientific Opinion related to zearalenone (ZEN) as an undesirable substance in animal feed (EFSA, 2004) concluded that zearalenone exerts its toxic action by interacting with oestrogen receptors (ERs) and causing an oestrogenic response in animals. Pigs were considered the most sensitive animal species to zearalenone, with female pigs more sensitive than male pigs, followed by sheep, cattle and poultry. The CONTAM Panel concluded that the calculation of animal exposure levels based on the individual occurrence data of zearalenone in feed materials could not be carried out due to the variability within the European Union (EU) member states feeding regimes for farm animals. No NOAELs/LOAELs for zearalenone in animals were derived since the available data were considered inadequate.
In the human ZEN risk assessment from EFSA in 2011, (EFSA CONTAM Panel, 2011), a tolerable daily intake (TDI) of 0.25 μg/kg body weight (bw) per day was derived from a no observed effect level (NOAEL) of 10 μg/kg bw for oestrogenic effects observed in immature gilts and applying an uncertainty factor of 40 (4 for interspecies differences in toxicokinetics and 10 for inter‐human variability). Lowest‐observed‐effect‐levels (LOELs) for ovary, uterus and vulva in female pigs ranged from 17 to 200 μg/kg bw per day, with an overall no‐observed‐effect‐level (NOEL) of 10.4 μg/kg bw per day.
The CONTAM Panel developed a Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed (EFSA CONTAM Panel, 2014). The toxicity for animals and humans of metabolites and masked or bound forms of fumonisins, zearalenone, T‐2 and HT‐2 and nivalenol was evaluated. The EFSA occurrence database contained no data on modified zearalenone; therefore occurrence was based on limited information reported in the literature. An estimation of the human dietary exposure and animal feed exposure compared with the exposure to the parent mycotoxins and assessments of the human and animal health risks was performed. Risk characterisation was done by comparing exposure scenarios with reference doses for the parent compounds. The CONTAM Panel identified several uncertainties and data gaps for modified mycotoxins and recommended re‐assessing the animal health effects of zearalenone and fumonisins in order to set NOAELs/LOAELs for these compounds.
Recently, the CONTAM Panel assessed the appropriateness to set a group health based guidance value for ZEN and modified forms. Different oestrogenic potencies are observed in vivo for ZEN and modified forms. To account for these differences, molar potency factors relative to ZEN (relative potency factors (RPFs) were calculated and applied to exposure estimates of the respective ZEN metabolites (EFSA CONTAM Panel, 2016). The CONTAM Panel found it appropriate to set a group human TDI of 0.25 μg/kg bw per day expressed as ZEN equivalents for ZEN and its modified forms.
1.3.2. Chemistry
Mycotoxins are secondary metabolites produced by filamentous fungi on food and feed commodities, mainly grains. These low molecular weight compounds may exert a range of toxic activities towards microorganisms, animals and humans.
Recent advances in the field showed that not only parent compounds but also their modified forms may occur simultaneously in raw cereals and products thereof. Definition and formation of modified forms of mycotoxins have been recently addressed by the CONTAM Panel (EFSA CONTAM Panel, 2014, 2016). Although different nomenclature and numbering systems are in use in the literature dealing with ZEN, the CONTAM Panel has followed the IUPAC numbering system in the definition of ZEN and its modified forms as already done in previous opinions (EFSA CONTAM Panel, 2014, 2016).
In living organisms (i.e. plants, microbes, animals), the most relevant modification of ZEN leads to the formation of its main reduced metabolites α‐ and β‐zearalenol (α‐ and β‐ZEL) and, to a lesser extent, to oxidised metabolites (Metzler et al., 2010). In addition, phase II metabolites are formed by conjugation with polar groups, giving rise mainly to glucoside, glucuronide and sulfate conjugates (i.e. ZEN‐14‐Glc, ZEN‐14‐Sulf) (Berthiller et al., 2013). While most of phase I metabolites are shared between different living organisms, phase II conjugates may vary in plants, microbes, and animals.
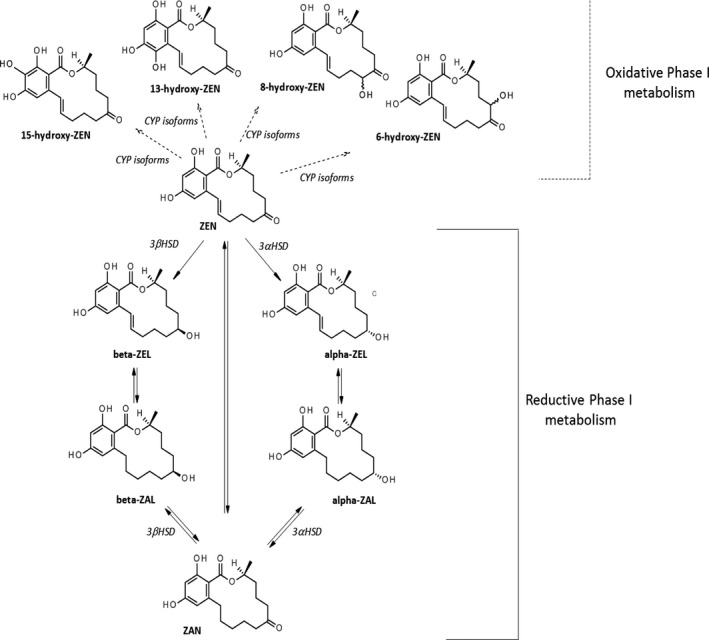
Besides biological modification, chemical modification of ZEN may also occur in food and feed, mainly due to non‐thermal reaction, such as cis‐isomerisation (Köppen et al., 2012). Therefore, modified forms of ZEN are defined as conjugated forms or metabolites originated by in vivo biotransformation (in plants, in fungi or in animals) or process degradation. Modified forms of ZEN potentially occurring in feed of plant or animal origin are summarised in Figure 1.
Figure 1.
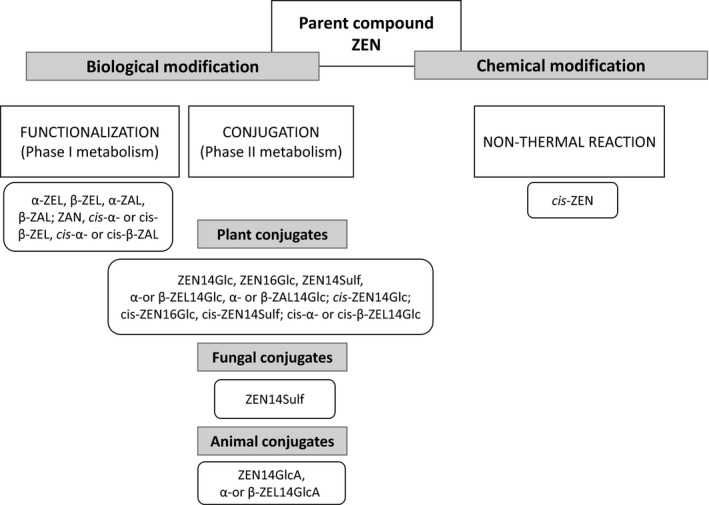
Scheme of mycotoxin modification and metabolism applied to ZEN
Multiple abbreviations and numbering systems are used for zearalenone and its modified forms in the scientific literature. For consistency with previous opinions, the CONTAM Panel has decided to use for this opinion the system defined in the Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed (EFSA CONTAM Panel, 2014), as shown in Appendix A. Appendix A reports the main chemical information on zearalenone and its modified forms potentially occurring in feed.
Parent compound
Zearalenone (ZEN) (C18H22O, CAS No. 17924‐92‐4, m.p. 164–165°C) is a white and crystalline compound, insoluble in water, but soluble in aqueous alkali and various organic solvents such as ethanol, acetone and ethyl acetate. It exhibits a typical fluorescence at 460 nm when excited at 294 nm. ZEN is produced by fungi belonging to Fusarium spp., particularly Fusarium graminearum. ZEN can be chemically classified as a macrocyclic β‐resorcylic acid lactone (RAL), carrying a keto group and an olefinic double bond.
Phase I metabolites
ZEN may undergo phase I metabolism in living organisms (i.e. plants, fungi, animals), mainly leading to the formation of reductive metabolites (Figure 1). The reduction of the keto group leads to the stereoisomeric compounds α‐zearalenol and β‐zearalenol, while the reduction of the olefinic double bond leads to the alkane zearalanone (see Figure 2, Chemical structure and numbering system used for zearalenone). When both the olefinic double bond and the keto groups are reduced, the stereoisomers α‐ and β‐zearalanol are obtained.
Figure 2.
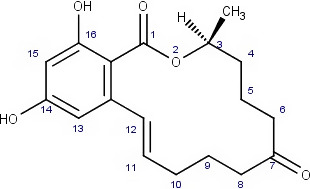
Chemical structure and numbering system used for zearalenone
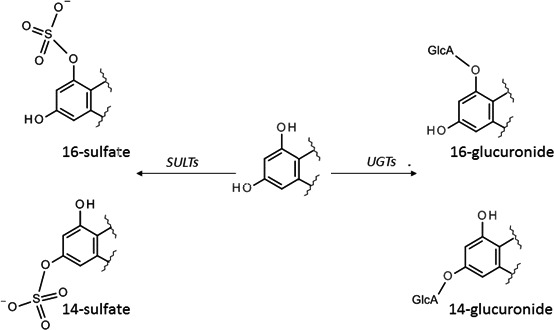
The main conjugated forms of ZEN and its phase I metabolites result from glucosylation and sulfation. Conjugation mainly occurs at position 14 to a minor extent due to sterical hindrance, at position 16.
Phase II metabolites
Conjugation of ZEN and phase I metabolites with more polar low molecular weight molecules such as glucose or sulfate leads to the formation of modified forms that may escape routine mycotoxin analysis. However, the parent compounds may be released upon hydrolysis in the gastrointestinal tract of mammals.
For further details, see Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed (EFSA CONTAM Panel, 2014).
The phase II metabolites of ZEN‐related compounds identified so far in plants and fungi are listed in Table 1. Only metabolites completely characterised by spectroscopic methods [usually nuclear magnetic resonance (NMR)] and/or confirmed by chemical synthesis are included in the Table. The structures of the others, which were tentatively identified, are discussed in the text.
Table 1.
Relative potencies factors (RPFs) given on a molar basis for ZEN and its modified forms as proposed by the CONTAM Panel (EFSA CONTAM Panel, 2016)
| Compound | Proposed RPF |
|---|---|
| ZEN | 1.0 |
| ZEN14Glc, ZEN16Glc, ZEN14Sulf | 1.0 |
| α‐ZEL | 60 |
| α‐ZEL14Glc and other α‐ZELGlcs, α‐ZELSulfs | 60 |
| β‐ZEL | 0.2 |
| β‐ZEL14Glc and other β‐ZELGlcs, β‐ZELSulfs | 0.2 |
| ZAN | 1.5 |
| ZANGlcs and ZANSulfs | 1.5 |
| α‐ZAL | 4.0 |
| α ‐ZALGlcs, α‐ZALSulfs | 4.0 |
| β‐ZAL | 2.0 |
| β‐ZALGlcs, β‐ZALSulfs | 2.0 |
| cis‐ZEN | 1.0 |
| cis‐ZENGlcs and cis‐ZENSulfs | 1.0 |
| cis‐α‐ZEL | 8.0 |
| cis‐α‐ZELGlcs and cis‐α‐ZELSulfs | 8.0 |
| cis‐β‐ZEL | 1.0 |
| cis‐β‐ZELGlcs and cis‐β‐ZELSulfs | 1.0 |
ZEN: zearalenone; ZAN: zearalanone; ZAL: zearalanol; Glc: glucoside; Sulf: sulfate.
Among the two regioisomeric monoglucosides identified for ZEN so far, ZEN‐14‐O‐β‐d‐glucopyranoside (ZEN14Glc) was first identified and characterised as a transformation product of ZEN by Rhizopus spp. (Kamimura, 1986), and later its formation was confirmed in plant cell cultures and in naturally infected cereals (see Section 3.2 Occurrence).
The regioisomeric ZEN‐16‐O‐β‐d‐glucopyranoside (ZEN16Glc) was recently reported in cultures of barley seedlings as well as in Brachypodium, and wheat suspension cultures (Kovalsky Paris et al., 2014). In addition, plants can biotransform ZEN to α‐ZEL and β‐ZEL, which may undergo subsequent glycosylation (Berthiller et al., 2006, 2009).
Fungal conjugation of ZEN with sulfate was first described in cultures of four Fusarium spp. (Plasencia and Mirocha, 1991), and recently confirmed in cultures of Aspergillus oryzae strains and Rhizopus species (Brodehl et al., 2014).
Glucuronidation is the main phase II biotransformation pathway of ZEN in animals. Glucuronides of ZEN, and its phase I metabolites ZAN, α‐ZEL, β‐ZEL, α‐ZAL and β‐ZAL, can be found in urine and tissues of animals fed with contaminated feed.
Chemical information on ZEN and its phase I and II metabolites are reported in Appendix A.
More details are reported in the Scientific Opinion on appropriateness to set a group health based guidance value for zearalenone and modified forms (EFSA CONTAM Panel, 2016)
Formation of modified forms of ZEN in plants
ZEN may accumulate in plants, mainly cereals, upon fungal infection at the plant flowering stage. As a consequence of the plant–pathogen cross‐talk, ZEN, as well as other Fusarium toxins, may undergo modification through the activation of phase I and phase II metabolic pathways.
The biochemical events as well as the genetic factors involved have been studied for deoxynivalenol (DON) modification in wheat and barley (Poppenberger et al., 2003; Berthiller et al., 2013; Kluger et al., 2013), but little is known about the enzymatic pathway affecting ZEN metabolism in grains.
ZEN may undergo directly glucosylation and sulfation (phase II metabolism) to form ZEN14Glc, ZEN16Glc, and ZEN14Sulf. In addition to direct conjugation, reductive phase I metabolism in plants leads mainly to the formation of α‐ and β‐ZEL, and at minor extent of ZAN, α‐ and β‐ZAL. These phase I metabolites may then be further modified to the corresponding phase II conjugated forms (see Figure 3). Besides conjugated forms of the parent compounds, only α‐ and β‐ZEL‐glucosides have been reported to occur in grains from the field so far (Nathanail et al., 2015).
Figure 3.
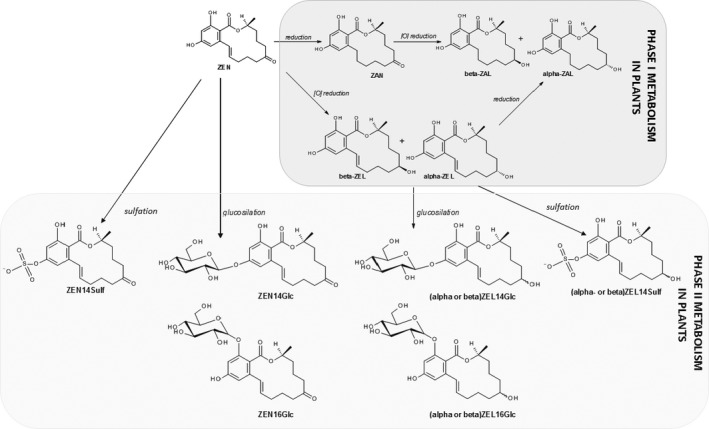
Modification of ZEN in plants through phase I and phase II biotransformations
In addition, ZEN may undergo cis‐isomerisation due to sunlight exposure. The isomeric parent compound, namely cis‐ZEN, may originate the correspondent phase I and phase II cis‐forms (Drzymala et al., 2015).
Although data from the literature indicate that factors such as plant genetic background, the environment, and the agronomic factors may affect the ratio of modification and the spectrum of possible metabolites in plants, further studies are required to better understand the mechanism, and to derive generalisation rules.
According to an in vitro study performed on Arabidopsis thaliana model system, phase II metabolism may lead to the formation of glucosides, malonylglucosides, diglucosides and pentosylhexosides (Berthiller et al., 2007). Therefore, it seems that ZEN follows a similar metabolic pathway as other phenolic compounds, such as flavonoids. However, these forms have never been identified in grains from the field or products thereof, so far.
Formation of modified forms of ZEN in animals and microorganisms
ZEN may be modified from microorganisms growing on food and feed commodities as well. In particular, fungal and bacterial strains used for fermentation may conjugate ZEN, leading to the formation of phase II metabolites. Among them, ZEN‐14‐Sulf has been often described (el‐Sharkaway et al., 1991; Jard et al., 2010). However, little is known about the metabolic processes, and the biological factors affecting the formation of these conjugates.
The metabolic fate of ZEN in animals is described in Section 3.1.1.
1.3.3. Methods of analysis
The extraction of ZEN and its modified forms is mainly based on the same protocols already in use for modified mycotoxins and extensively described in previous opinions (EFSA CONTAM Panel, 2014, 2016).
Concerning the analytical determination, ZEN and its modified forms can be easily detected by fluorescence (λex = 274 nm, λem = 440 nm) after a chromatographic separation. This approach is still in use for feed, usually combined with sample purification on immunoaffinity columns. However, in recent years many protocols based on ultra high‐performance liquid chromatography (HPLC) coupled to mass spectrometry have been reported in literature (Berthiller et al., 2007, 2011; De Boevre et al., 2012a, 2013). Commonly, C18 columns are used for a satisfactory chromatographic separation of the analytes. Gradient elution based on acidified water–methanol or water‐acetonitrile is applied. Although 13C‐ZEN is commercially available, the use of the isotope dilution approach for correction of matrix effects is still not frequently used. More often, liquid chromatography–tandem mass spectrometry (LC–MS/MS) methods for feed may involve a sample clean up step on solid‐phase extraction (SPE) columns to decrease matrix effects. In the recent years, many methods based on multitoxin approaches have been reported in the literature. Although able to detect the co‐occurrence of different mycotoxins, this approach requires a compromise in terms of sensitivity, accuracy, and recovery. For this reason, limits of detection obtained for modified forms are often higher than those obtained for the parent compounds. However, according to the analytical method in use, a broad range of sensitivities is reported in the literature. Those based on mass spectrometry usually have limit of detection (LOD) values in the range 0.05–10 μg/kg for both ZEN and its modified forms.
A different approach involves the enzymatic hydrolysis of the modified forms, and the subsequent quantification of the released parent compound. This indirect method has been often coupled to ELISA‐based techniques to return a total ZEN quantification (as the sum of parent ZEN and ZEN released from modified forms). Among tested enzymes, β‐glucosidase, cellulase and cellobiase have been used to cleave ZEN14Glc, obtaining an almost quantitative release of ZEN (Beloglazova et al., 2013).
Certified reference materials and a certified calibrant for ZEN became available in 2003. They consist of naturally contaminated maize flour and blank maize flour. Certified reference calibrant solutions of ZEN, α‐ZEL and β‐ZEL in acetonitrile are commercially available, while ZAN, α‐ZAL, and β‐ZAL are commercially available only as high purity standards (powder).To date, certified reference materials and calibrants for conjugated forms (i.e. phase II metabolites) are not available on the market.
1.3.4. Legislation
Directive 2002/32/EC on undesirable substances in animal feed stipulates that rules on feedingstuffs are needed to ensure agricultural productivity and sustainability and to make it possible to ensure public and animal health, animal welfare and the environment. Annex I of this Directive contains maximum levels of a number of undesirable substances (chemical contaminants) that may be tolerated in products intended for animal feed. ZEN is not regulated under this Directive.
Guidance values for ZEN have been recommended under Commission Recommendation 2016/1319/EC.2 The guidance values are shown in Table 2. Currently, modified forms of ZEN are not considered in the legislation.
Table 2.
Guidance values for zearalenone in products intended for animal feed in the EU (Commission Recommendation 2016/1319/EC)
| Products intended for animal feed | Guidance value in mg/kg (ppm) relative to a feedingstuff with a moisture content of 12% |
|---|---|
| Feed materials a | |
| – Cereals and cereal productsb with the exception of maize by‐products | 2 |
| – Maize by‐products | 3 |
| Compound feed for: | |
| – Piglets, gilts (young sows), puppies, kittens, dogs and cats for reproduction | 0.1 |
| – Adult dogs and cats other than for reproduction | 0.2 |
| – Sows and fattening pigs | 0.25 |
| – Calves, dairy cattle, sheep (including lamb) and goats (including kids) | 0.5 |
Particular attention has to be paid to cereals and cereals products fed directly to the animals that their use in a daily ration should not lead to the animal being exposed to a higher level of these mycotoxins than the corresponding levels of exposure where only the complete feedingstuffs are used in a daily ration.
The term ‘Cereals and cereal products’ includes not only the feed materials listed under heading 1 ‘Cereal grains and products derived thereof’ of the list of feed materials referred to in part C of the Annex to Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of feed materials (OJ L 29, 30.1.2013, p. 1) but also other feed materials derived from cereals in particular cereal forages and roughages.
2. Data and methodologies
2.1. Feed occurrence data and methodologies
Following an European Commission mandate to EFSA, a call for annual collection of chemical contaminant occurrence data in food and feed, including ZEN, was issued by the former EFSA Dietary and Chemical Monitoring Unit (now DATA Unit)3 in December 2010 with a closing date of 1 October of each year. European national authorities and similar bodies, research institutions, academia, food business operators and other stakeholders were invited to submit analytical data on ZEN in feed. At the time of receiving the request for the scientific opinion from the European Commission, no data on the modified forms of ZEN were available in the EFSA Chemical Occurrence database. The EFSA Evidence Management Unit (DATA Unit) invited European national authorities and similar bodies, research institutions and other stakeholders to submit data on the following ZEN modified forms in feed: ZEN‐14‐glucoside (ZEN14Glc), ZEN‐14‐sulfate (ZEN14Sulf), α‐zearalenol (α‐ZEL), β‐zearalenol (β‐ZEL), zearalanone (ZAN), α‐zearalanol (α‐ZAL), β‐zearalanol (β‐ZAL), α‐ZEL‐14‐glucoside (α‐ZEL14Glc) and β‐ZEL‐14‐glucoside (β‐ZEL14Glc). The data submissions to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a); occurrence data were managed following the EFSA standard operational procedures (SOPs) on ‘Data collection and validation’ and ‘Data analysis and reporting’. By the end of July 2016, a total of 17,706 samples of feed with analytical data on ZEN and its modified forms were available in the EFSA database. Data received after that date were not included in the data set used to estimate dietary exposure.
Following the EFSA SOP on ‘Data analysis and reporting’ to guarantee an appropriate quality of the data used in the exposure assessment the initial data set was carefully evaluated applying several data cleaning and validation steps. Special attention was paid to different parameters such as ‘Sampling strategy’, ‘Sampling year’, ‘Sampling country’, ‘Analytical methods’, and the ‘Reporting unit’. Feeds were classified based on the catalogue of feed materials specified in the Commission Regulation (EU) No 68/20134.
Analytical results were reported either in whole weight basis or 88% dry matter (DM). Before estimating dietary exposure, all results were converted into mg/kg whole weight. For those samples expressed on 88% DM basis, the moisture content was used to convert the analytical result into whole weight; when the moisture content was missing, whenever possible imputation of the moisture content from reported values was done (see Section 3.1.2).
In the analysis of ZEN occurrence data, the left‐censored data, results below the LOD or below the limit of quantification [LOQ5 were treated by the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO/IPCS, 2009). The same method is indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b). The guidance suggests that the lower bound (LB) and upper bound (UB) approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). The LB is obtained by assigning a value of zero (minimum possible value) to all samples reported as lower than the LOD (< LOD)/LOQ (< LOQ). The UB is obtained by assigning the numerical value of LOD to values reported as < LOD and LOQ to values reported as < LOQ (maximum possible value), depending on whether LOD or LOQ is reported by the laboratory.
2.2. Feed consumption data and methodologies
ZEN and its modified forms are predominantly found in cereal crops, cereal grains and by‐products of cereal processing. These are widely used as feed for livestock; in the EU more than 93 million tonnes of cereals and cereal by‐products were used in the manufacture of compound feeds in 2014, accounting for 60% of all feed materials used,6 almost all of which (> 95%) are grown or produced in the EU. In addition, a further 51 million tonnes of cereal grains and by‐products were fed in on‐farm mixes or as single ingredients. However, there are no industry data on the partition of these cereal grains between livestock species (cattle, pigs, poultry, etc.).
Forages are important – and frequently the sole – feeds for ruminant livestock, and ZEN has been widely identified in these feeds. A few studies have reported the presence of ZEN in pasture grasses and grass silage (see Section 3.2.2), although levels are usually lower than have been reported in maize and cereal silages and cereal straws, where ZEN has been regularly observed (See Section 3.2).
Estimating the exposure to ZEN and its modified forms requires estimates of feed intake, and in this opinion two approaches have been adopted. For many livestock in the EU, part or all of the daily ration is commonly provided in the form of manufactured compound feeds, and where data on levels of ZEN and its modified forms in species‐specific compound feeds7 are available, these have been used to estimate exposure. Since compound feeds represent the complete diet for many livestock, this must be the preferred method of calculating exposure. However, for some livestock categories information on levels in compound feeds has not been given, or insufficient data have been provided to allow reliable estimates of exposure to be made, and for these data on individual feed materials have been used, together with example diets (Appendix D) to estimate exposure. It should be stressed that these do not represent ‘average’ diets, nor are the feeding systems ‘typical’ for all of Europe. Instead, they are used to estimate levels of exposure to ZEN and its modified forms that might be indicative. They are based on published guidelines on nutrition and feeding (AFRC, 1993; Carabaño and Piquer, 1998; NRC, 2007a,b; Leeson and Summers, 2008; McDonald et al., 2011; EFSA FEEDAP Panel, 2012; OECD, 2013), and expert knowledge of production systems in Europe. Details of the rations used feed intakes and live weights assumed are given in Appendix D.
2.3. Toxicokinetic and toxicological data
Data were obtained from the scientific literature as described in Section 2.4.
2.4. Methodology for data collection and study appraisal
The CONTAM Panel considered the previous assessments on zearalenone and on modified fusarium toxins (EFSA, 2004; EFSA CONTAM Panel, 2011, 2014, 2016) as comprehensive, covering all relevant publications on ZEN and its modified forms, respectively, until those dates. All publications referenced therein have been considered, wherever appropriate, also for the present evaluation.
In order to cover additional publications not considered in these previous assessments, a search for literature was conducted for peer‐reviewed original research published after the year 2000. Reviews were also considered for the current risk assessment. In addition, when relevant papers were identified during the risk assessment process (e.g. from other studies or reviews) they were also considered for the assessment.
The search strategy was designed to identify scientific literature dealing with methods of analysis, chemistry, occurrence in feed, animal exposure, toxicity, toxicokinetics and adverse effects in the different animal species. An overview of the search terms is given in Appendix B, Section B.1.
Literature search was not restricted to publications in English language; however, literature in other languages was only considered if an English abstract was available. A first general literature search was performed in December 2015 and has since been updated in May and December 2016 for the adverse effects of ZEN and modified forms in farm and companion animals.
Web of Science8 and PubMed9 were identified as databases appropriate for retrieving literature for the present evaluation. The references resulting from the literature search were imported and saved using a software package (EndNote10), which allows effective management of references and citations.
The references obtained were screened using title and abstract to identify the relevant literature and exclusion criteria are shown in Appendix B, Section B.2, and were subsequently reviewed by the CONTAM working group (WG) zearalenone in feed, and has been used for the present assessment based on expert judgement.
2.5. Use of molar RPFs for the ZEN modified forms
Molar RPFs of the modified forms relative to ZEN were applied to feed occurrence levels of the respective ZEN metabolites (see Table 1) according to CONTAM Panel 2016 (EFSA CONTAM Panel, 2016). The CONTAM Panel noted that these molar (RPFs) were based on uterotrophic effects measured for ZEN and its modified forms in rodent experiments and therefore did not take account of other oestrogenic effects and other endpoints. Furthermore, applying these RPFs derived from mice to different farm and companion animal species does not consider the species differences in the toxicokinetics and toxicodynamics.
2.6. Methodologies for dietary exposure assessment in animals
Exposure to ZEN and its modified forms by livestock is a function of their concentration in their diets and the amount of the diet consumed. In the absence of a comprehensive database on the amounts or types of feed consumed by livestock in the EU, estimates of feed consumed for each of the main categories of farmed livestock and companion animals are based on published guidelines on nutrition (e.g. Carabaño and Piquer, 1998; NRC, 2007a,b; Leeson and Summers, 2008; McDonald et al., 2011; EFSA FEEDAP Panel, 2012; OECD, 2013), together with expert knowledge of production systems in Europe.
For many farmed livestock and companion animals, their nutritional requirements are provided in commercially manufactured complete (compound) feeds. Where sufficient (reliable) data on the concentrations of zearalenone and its modified forms in compound feeds have been provided, these have been used to estimate exposure. However, where insufficient compound feed data were available, the CONTAM Panel identified example diets and feed inclusion rates, and used concentrations of ZEN in individual feed materials to estimate P95 and mean exposure both LB and UB. Details of the intakes and composition of diets used in estimating animal exposure to zearalenone and its modified forms are given in Appendix D.
2.7. Methodology applied for risk assessment
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by WHO/IPCS (2009), which include hazard identification and characterisation, exposure assessment and risk characterisation. The principles described by WHO/IPCS (2009), EFSA guidance pertaining to risk assessment have been applied for the present assessment. In addition, the current risk assessment was used for internal testing of the draft Guidance on Uncertainty in EFSA Scientific assessment (EFSA Scientific Committee, 2016). For details on the specific EFSA guidance applied, see Appendix C.
3. Assessment
3.1. Hazard identification and characterisation
3.1.1. Toxicokinetics
The information concerning the toxicokinetics of ZEN, as well as its modified forms, in several animal species has been covered by previous EFSA opinions (EFSA, 2004; EFSA CONTAM Panel, 2011, 2016). The main kinetic pathways in mammalian species are shown in Figure 4.
Figure 4.
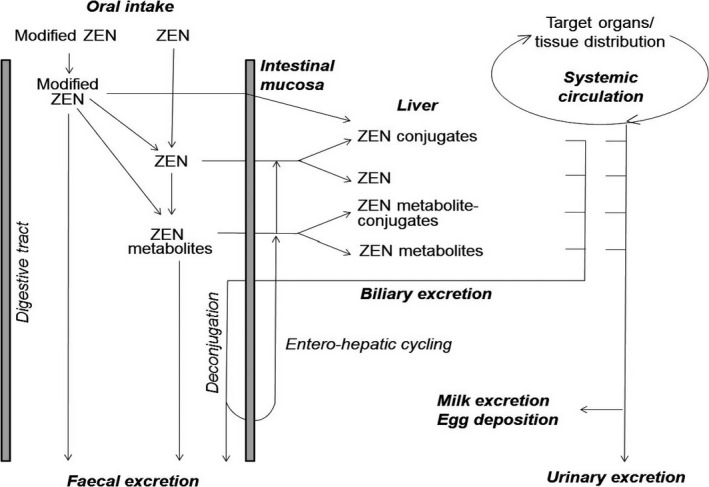
Principal pathways and metabolism of ZEN and its modified forms in some mammalian species (adapted from Dänicke and Winkler, 2015)
The main ZEN metabolites and enzymes involved in their generation in animals are reported in Figures 5 and 6.
Figure 5.
The main metabolites of ZEN formed in animals
- Major phase I reductive metabolism (bold arrow) is mediated by 3α‐ and 3β‐hydroxysteroid dehydrogenases (HSDs); minor phase I oxidative metabolism (dotted arrows) is mediated by CYP isoforms leading to catechol formation.
Figure 6.
Main phase II metabolism involves the phenolic ring, and leads to the formation of sulfate or glucuronic acid conjugates, catalysed by sulfotransferases (SULTs) or uridine diphosphate glucuronosyltransferases (UGTs), respectively
Only new studies published since the above EFSA evaluations or relevant topics not previously covered will be reported below.
3.1.1.1. Absorption
Due to its lipophilicity, which is reflected by a relatively high log partition coefficient between octanol and water (log Kow), ZEN is rapidly absorbed at the enteric level following oral exposure (Kuiper‐Goodman et al., 1987); as an example, bioavailability of approximately 80–85% has been estimated following a single oral dose in piglets of 10 mg/kg bw (Biehl et al., 1993). It is reasonable to assume that also the reduced ZEN metabolites may easily cross biological membranes as it has been demonstrated in the Caco‐2 cell model (Pfeiffer et al., 2011). In an epithelial cell model, both ZEN and the reduced metabolites α‐ZEL and β‐ZEL have been reported to interact with ABCC efflux proteins (Videmann et al., 2009), the expression of which shows wide variation in veterinary species (Schrickx and Fink‐Gremmels, 2008).
ZEN and most of its metabolites will be absorbed and re‐absorbed after being released by enteric hydrolysis.
3.1.1.2. Distribution
Most of the information concerning tissue distribution is related to the parent compound (ZEN) and has been derived from residue studies related to the transfer (carry‐over) (see Section 3.1.1.6). The apparent volume of distribution is large and includes target tissues of ZEN toxic action like uterus, ovarian follicles and testes (Kuiper‐Goodman et al., 1987). In a recent review, Dänicke and Winkler (2015) summarised the available experimental feed studies performed with ZEN in food producing species (lactating cows, fattening cattle, pigs, rabbits and avian species) taking into consideration liver, muscle, kidney and fat. Despite the wide range of dosages and duration of the exposure, in general a very limited amount of ZEN was found in liver followed by kidney, fat and muscle; the few available reports also indicate that the modified forms (ZEN metabolites) are present in lower concentrations than the parent compound.
3.1.1.3. Metabolism
In vitro studies
Prehepatic ZEN biotransformations are mediated by rumen and enteric microorganisms, but may also occur in enteric cells. Microorganisms of the chyme are major players in both extraction from feed and metabolism of ZEN and its modified forms at the gastro‐intestinal level.
The rumen microbiota plays a key role in ZEN biotransformation (Dänicke et al., 2004). Several in vitro studies (reviewed by Dänicke and Winkler, 2015) indicate that reductive reactions largely predominate, with the generation mainly of β‐ZEL and α‐ZEL. Such conclusions have been supported by in vivo studies on fistulated cows (Dänicke et al., 2005b). The reduction of ZEN to α‐ZEL has been demonstrated using porcine chyme collected from the distal part of the enteric tract (Kollarczik et al., 1994). On the other hand, hydrolytic reactions carried out by enteric and presumably rumen microorganisms are involved in the release of ZEN from its modified forms. As detailed in the latest EFSA evaluation (EFSA CONTAM Panel, 2016) few studies have been performed on the hydrolysis of ZEN glucoside (ZEN14G). Gareis et al. (1990) obtained indications for the complete hydrolysis in a pig, with ZEN and α‐ZEL being detected in faeces and urine. In vitro studies with human faeces point to the ability of human enteric microflora to hydrolyse ZEN glucosides (Dall'Erta et al., 2013; Kovalsky Paris et al., 2014).
As mentioned above, metabolism also occurs in enteric cells. Although data concerning target species are seemingly scant, caprine duodenal and jejunal preparations were found able to metabolise ZEN to α‐ZEL and β‐ZEL (Dong et al., 2010a). More recently, in vitro studies performed with Caco‐2 cells indicate that enteric cells are fully competent in carrying out both phase I and phase II RAL biotransformations, generating reduced, glucuronidated, and sulfated metabolites (in various proportions) of ZEN, ZAN, α‐ZEL, β‐ZEL as well as α‐ZAL and β‐ZAL (Pfeiffer et al., 2011).
After prehepatic metabolism, ZEN undergoes extensive liver phase I and phase II biotransformations, which have been described in previous EFSA opinions (EFSA, 2004; EFSA CONTAM Panel, 2011, 2016) and may be summarised as follows: Phase I biotransformations are mainly reductive in nature and involve the NAD(P)H‐mediated sequential reduction of the C6 keto group, yielding α‐ZEL and its stereoisomer β‐ZEL, followed by a further reductive step at C11–C12 double bond with the formation of α‐ZAL and β‐ZAL, respectively. It is generally accepted that α‐ and β‐hydroxysteroid dehydrogenases (HSDs) are the major enzymes performing tissue ZEN reduction in mammalian species; such enzymes play a key role in steroid hormone homeostasis as they participate both in the synthesis of testosterone, oestradiol, and progesterone and in the conversion of the more hormonally active keto‐derivatives (–CO) into the less active reduced (–COH) derivatives (Penning et al., 2004). Wide species‐related quantitative differences in the synthesis of the above metabolites as well as in the subcellular (microsomal or cytosolic) localisation and the cofactor requirement (NADPH vs NADH) of the reductive reactions have been demonstrated. In addition, the amount and the ratio of the in vitro generated metabolites (especially α‐ZAL and β‐ZAL) have been reported to vary according to the ZEN concentrations (Malekinejad et al., 2005a,b). Results from in vitro experiments indicate that pig liver preparations convert ZEN predominantly to α‐ZEL, while β‐ZEL seems to prevail in chicken preparations. Also in primary cultures of equine hepatocytes, ZEN was mostly metabolised to α‐ZEL (Malekinejad, 2013). In addition, the ability of liver microsomes in biotransforming ZEN to α‐ZEL and β‐ZEL seems to be maximal in pig and sheep subfractions compared to cattle, chickens, and rats. As regards poultry, in vitro investigations with liver post‐mitochondrial fractions indicate a general greater synthesis of the α‐ vs the β‐derivative; however, wide interspecies variations also occurred in avian species, with quails being identified as high β‐reducers, guinea‐fowls, ducks and chickens as weak β‐reducers, and geese as weak α‐ and β‐reducers (Kolf‐Clauw et al., 2007).
More recent in vitro studies confirm the occurrence of a CYP‐mediated ZEN hydroxylation, but little is known about the generation of CYP‐mediated ZEN hydroxylated derivatives in domestic animal species. In rat and human liver microsomes, aromatic hydroxylation occurs mainly at C13 and C15, resulting in the formation of 13‐ and 15‐OH catechol ZEN derivatives (Fleck et al., 2012). Similar to catecholoestrogens formed by the CYP1B‐mediated oxidation of 17β‐estradiol (E2), they might be further oxidised to quinones which subsequently enter a futile cycle with the generation of reactive oxygen species and DNA damage. Aliphatic hydroxylation of ZEN and α‐ZEL at various C positions has also been reported in vitro, with dog and rabbit liver microsomes seemingly more active than human, rat and murine preparations. It should be noted that the oestrogenic potency of such hydroxylated derivatives is one order of magnitude lower than ZEN (EFSA CONTAM Panel, 2011, Hildebrand et al., 2012). As far as aromatic hydroxylation is concerned, upon the incubation of 50 μM ZEN with steer, pig, human, mouse and rat liver microsomes, a higher quantity of catechol‐ (mainly C15 hydroxylated) vs. aliphatic hydroxylated metabolites was detected in all species but mice. When expressed per nmoles of CYP, the amount of catechol metabolites was in the order steer< pig < human < mouse < rat, which correlates well with the extent of the oxidative damage measured in the same preparations as the formation of 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine using calf thymus DNA (Fleck et al., 2012). As in the case of E2, catechol derivatives of ZEN may be detoxified by catechol‐O‐methyltransferase (COMT). However, in vitro investigations performed with liver cytosols from mammalian species, including steers and piglets indicate that ZEN catechol‐derivatives are poor substrates of COMT (Fleck et al., 2012). These catechols are actually able to inhibit the COMT activity toward 2‐OH‐E2, the major E2 catechol metabolite (Pfeiffer et al., 2013).
It is worth noting that, in addition to enteric metabolism, further extrahepatic ZEN metabolism has been reported. For example, porcine cumulus oocyte cells and granulosa cells express both 3α‐and 3β‐HSD; similarly, as in liver, α‐ZEL was produced to a higher extent than β‐ZEL after the incubation of GCs with ZEN (Malekinejad et al., 2006), indicating that ZEN‐derived oestrogenic metabolites may be generated also in target tissues. The metabolic conversion of ZEN into α‐ and/or β‐ZEL has been documented in caprine brain, lung, and kidney microsomal and cytosolic subfractions (Dong et al., 2010a). Rat erythrocytes were also found able to convert ZEN, mostly into its α‐reduced derivative (Chang and Lin, 1984).
ZEN itself as well as all its reduced forms are subjected to phase II reactions resulting in the formation of polar derivatives almost devoid of oestrogenic activity. Conjugation is mainly accomplished by uridine diphospho glucuronosyltransferases (UGTs), each hydroxyl group being theoretically prone to form glucuronides which may subsequently undergo biliary or urinary excretion. In the former case, an active enterohepatic cycling is reported to occur. Again, there are well‐known species related differences in the extent of glucuronidation of ZAN and ZEN and its reduced metabolites, as well as in the preferred route of elimination (biliary vs urinary) and in the glucuronidation position (EFSA CONTAM Panel 2011, 2016). Pig liver microsomes exhibited the maximal in vitro glucuronidating capacity towards ZEN, α‐ZEL or β‐ZEL, followed by sheep, cattle, rat and chicken subfractions (Malekinejad et al., 2006). No information is available about the contribution of different UGT families to the glucuronidation of ZEN and its metabolites in farm animal species.
There is general consensus that sulfation presents an additional conjugation route for ZEN and all its reduced metabolites. According to Metzler et al. (2010), however, there is a paucity of data concerning both the structures and the enzymology of such sulfate derivatives.
Overall, due to the well‐ known differences in the oestrogenic activity of ZEN metabolites, the species‐dependent metabolic profile may have a considerable impact on the sensitivity to the mycotoxin.
In vivo studies
The metabolite profile of ZEN in blood, bile or urine of food producing species after oral exposure has recently been reviewed in food producing species (Dänicke and Winkler, 2015). The proportion of the reduced metabolites seems to vary according to both the extent and the duration of the exposure. The majority of the experiments has been carried out in pigs and confirms the results of the vitro studies in that α‐ZEL is largely prevailing over β‐ZEL in all examined biological fluids. In partial contrast with the outcome of the in vitro studies, a much higher proportion of β‐ZEL compared to α‐ZEL was detected in bile and urine samples from ZEN‐exposed bovines, while in broilers α‐ZEL seems to be preferentially formed, with a blood α‐ZEL/β‐ZEL ratio up to 10 and even much higher in turkeys. Evidence of a higher production of α‐ZEL vs β‐ZEL has also been provided for the canine species (Gajęcka et al., 2013). Data are lacking concerning the occurrence of aromatic and/or aliphatic ZEN hydroxylated derivatives in biological fluids of farm and companion animals.
Modified forms
Due to its legal application as a growth‐promoter in certain non‐EU countries, the metabolic fate of Zeranol (α‐ZAL) has been investigated in several species, including rats, humans, pigs, dogs, cattle, and rabbits (Migdalof et al., 1983; Bories and Suarez, 1989; Bories et al., 1991; Kleinova et al., 2002). In all species, the main hepatic phase I biotransformations consist in the oxidation of the C7‐secondary alcoholic group to the corresponding –CO derivative, referred to as ZAN, as well as in the generation of the diastereoisomer β‐ZAL, also known as taleranol, resulting from the aldo‐ketoreductase‐mediated reduction of the same alcoholic group. Phase II biotransformation yields both glucurono‐ and sulfoconjugates, which are produced under in vitro and in vivo conditions (Bories et al., 1991; Pfeiffer et al., 2010; Binder et al., 2017).
The metabolic fate of ZEN14Glc, ZEN16Glc, and ZEN14Sulf has been recently studied in pigs orally dosed with such modified forms; the lack of the recovery of any of such plant metabolites in urine or faeces points to their complete hydrolysis occurring in the gastrointestinal tract (Binder et al., 2017) and hence to a substantial contribution to ZEN overall toxicity.
3.1.1.4. Excretion
In most species, ZEN and its reductive metabolites are predominantly excreted via the faecal route mainly as glucuronides due to an extensive biliary excretion; the occurrence of a remarkable enterohepatic cycling may explain the relatively long persistence of the mycotoxin and its derivatives in the body, particularly in pigs (EFSA CONTAM Panel, 2011, 2016); in rabbits and humans the rate of urinary excretion seems higher than in the other species (Kuiper‐Goodman et al., 1987).
No new information could be identified concerning the placental transfer of zearalenone and its metabolites which has been demonstrated in rats (i.v. administration) and pigs.
Both ZEN and its metabolites are reported to be excreted in ovine, bovine and porcine milk (Dacasto et al., 1995; EFSA, 2004; Dänicke et al., 2014; Flores‐Flores et al., 2015).
Studies with [14C]ZEN or the unlabeled compound in laying hens have demonstrated the progressive accumulation of ZEN and lipophilic metabolites in egg yolk (summarised in EFSA, 2004).
Further details about the carry over rate of ZEN and its metabolites can be found in Section 3.1.1.6.
3.1.1.5. Species related toxicokinetics
The main TK parameters in various species are shown in Table 3. No specific toxicokinetic studies could be retrieved for sheep, rabbit, fish, cats, dogs and farmed mink.
Table 3.
Parameters of toxicokinetics of ZEN in various species
| Species/ category | Dose (mg/kg bw) | Toxin source | Route of administration | tmax (h) | t1/2 el (h) | Bio‐availability (%)a | Analytes considered for evaluation | Experimental disruption of enterohepatic cycling | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Pig | 5 | [3H]ZEN | i.v. | 86.6 | Total ZENs | No | Biehl et al. (1993) | ||
| 10 | [3H]ZEN | p.o. | 2–3 | 86.6 | 80–85b | Total ZENs | No | ||
| 5 | [3H]ZEN | i.v. | 3.34 | Total ZENs | Yes | ||||
| 0.079 | ZEN | p.o. | 0.5 | ZEN | No | Olsen et al. (1985)) | |||
| 4.5 | α‐ZEL | ||||||||
| 1 | ZEN | i.v. | 2.63 | ZEN | No | Dänicke et al. (2005a) | |||
| 2.94 | α‐ZEL | ||||||||
| 1 | ZEN | i.v. | 1.1 | ZEN | Yes | ||||
| 3.04 | α‐ZEL | ||||||||
| 1 | ZEN | p.o. | 0.7 | 5.3 | 78 | ZEN | No | Dänicke and Winkler (2015) | |
| 2.7 | 4.37 | α‐ZEL | |||||||
| 87 | ZEN+ α‐ZEL | ||||||||
| Broiler | 5 | [3H]ZEN | p.o. | 4–8 | 89 | Total ZENs | No | Mirocha et al. (1982) | |
| 0.3 | ZEN | i.v. | 0.52 | ZEN | No | Osselaere et al. (2013) | |||
| 3 | ZEN | i.v. | 0.29 | ZEN | No | Devreese et al. (2015) | |||
| 3 | ZEN | p.o. | 0.35 | 0.34 | 8.34 | ZEN | No | ||
| 3 | ZEN | i.v. | 0.03 | α‐ZEL | No | ||||
| 3 | ZEN | p.o. | 0.63 | α‐ZEL | No | ||||
| 3 | ZEN | i.v. | 0.07 | β‐ZEL | No | ||||
| 3 | ZEN | p.o. | 0.61 | β‐ZEL | No | ||||
| Laying hen | 10 | [14C]ZEN | p.o. | 2–4 | Total ZENs | No | Dailey et al. (1980) | ||
| 3 | ZEN | i.v. | 0.46 | ZEN | No | Devreese et al. (2015) | |||
| 3 | ZEN | p.o. | 0.32 | 0.36 | 10.28 | ZEN | No | ||
| 3 | ZEN | i.v. | 0.03 | α‐ZEL | No | ||||
| 3 | ZEN | p.o. | 0.27 | α‐ZEL | No | ||||
| 3 | ZEN | i.v. | 0.03 | β‐ZEL | No | ||||
| 3 | ZEN | p.o. | 0.42 | β‐ZEL | No | ||||
| Turkey | 3 | ZEN | i.v. | 0.38 | ZEN | No | Devreese et al. (2015) | ||
| 3 | ZEN | p.o. | 0.97 | 0.35 | 6.87 | ZEN | No | ||
| 3 | ZEN | i.v. | α‐ZEL | No | |||||
| 3 | ZEN | p.o. | α‐ZEL | No | |||||
| 3 | ZEN | i.v. | β‐ZEL | No | |||||
| 3 | ZEN | p.o. | β‐ZEL | No | |||||
| Cow | ~ 3.4 | ZEN | p.o. | 12 | ZEN | No | Prelusky et al. (1990) | ||
| ~ 11.3 | ZEN | p.o. | 12 | ZEN | No | ||||
| Goat | 1.2 | ZEN | i.v. | 28.58 | Total ZENs | No | Dong et al. (2010a,b) | ||
| Horse | ~ 0.003 | ZEN | p.o. | 8–12 | ZEN | No | Songsermsakul et al. (2013) | ||
| Rat | 1 | ZEN | i.v. | 0.6 | ZEN | No | Shin et al. (2009) | ||
| 2 | ZEN | i.v. | 1.9 | ZEN | No | ||||
| 4 | ZEN | i.v. | 1.8 | ZEN | No | ||||
| 8 | ZEN | i.v. | 2.8 | ZEN | No | ||||
| 8 | ZEN | p.o. | 16.8 | 2.7 | ZEN | No | |||
| 8 | ZEN | p.o. | 7.0 | 1.1 | ZEN | Yes |
bw: body weight; i.v.: intravenous; p.o.: per os; tmax: time at maximum plasma/serum concentration; t1/2 el: plasma/serum elimination half‐life; ZEN: zearalenone; ZEL: zearalenol.
Note: reported terminal plasma/serum elimination half‐lives (t1/2 el) might depend on models used for evaluation of the kinetics.
Based on area under the curve (AUC) method.
Based on comparisons of cumulative faecal and urinary excretions after i.v. and p.o. ZEN administration.
Ruminants
Cattle
Exposure to ZEN
As detailed in the EFSA 2011 evaluation (EFSA CONTAM Panel, 2011), in vitro studies indicate that ZEN was preferentially converted into β‐ZEL rather than α‐ZEL by bovine liver preparations.
In vivo experiments were carried out to study rumen metabolism, administering ZEN contaminated diets to fistulated cows. In line with the in vitro findings, approximately 89% of the totally ingested ZEN (0.1 mg ZEN/kg diet) were recovered at the proximal duodenum as ZEN (30%), α‐ZEL (30%) and β‐ZEL (40%), respectively, α‐ZAL, β‐ZAL and ZAN being not detectable (Dänicke et al., 2005b). Feed intake level may influence the rate and the extent of ZEN conversion into its metabolites. In fistulated cows fed DM amounts increasing from 5.6 to 20.5 kg with a constant dietary ZEN concentration of 0.062 mg/kg, ZEN reduction to β‐ZEL, the main rumen metabolite, decreased with increasing feed, and consequently ZEN, intake; this was matched by a linear increase in the flow of ZEN at the duodenum (Seeling et al., 2005). These results indicate that high yielding dairy cows, characterised by a high level of feed intake, might be more exposed systemically to ZEN than to β‐ZEL, although the toxicological implications of this finding remains unclear.
There is scant information on the in vivo toxicokinetics and bioavailability of ZEN or its modified forms in cattle. Prelusky et al. (1990) investigated the blood kinetics of ZEN, α‐ZEL and β‐ZEL after a single or repeated oral administration. In a cow fed a contaminated diet for 21 days (545 mg ZEN/days corresponding to ~ 24 mg/kg diet at 88% DM and ~ 1,030 μg/kg bw), a Cmax of 3 ng/mL was observed after a period of 62 h (tmax); after oral administration of a single dose of 1.8 g ZEN (~ 80 mg/kg diet at 88% DM; ~ 3,400 μg/kg bw) or 6 g ZEN (~ 265 mg/kg diet at 88% DM; ~ 11,300 μg/kg bw) per animal, Cmax values of 9 and 13 ng/mL, respectively, were recorded with a unique (tmax) of 12 h. These data indicate that maximum plasma concentrations after oral ZEN administration in cattle are reached later when compared to other species. Tmax in bile is even reached much later, pointing at the role of the enterohepatic cycling of ZEN metabolites.
In another study, a single oral ZEN dose of 10 mg resulted in an approximate peak concentration Cmax = 3 ng ZAL (not further specified which isoform)/mL estimated from the graphic data presentation. The so‐estimated Cmax corresponded to a tmax of 96 h while Cmax of 150 ng α‐ZEL/mL was detected much earlier (tmax = 24 h) (Kennedy et al., 1998).11
Exposure to modified forms
The incubation of α‐ZAL with rumen microflora resulted in the formation of ZAN, while the reverse occurred upon the incubation of ZAN with bovine faecal matter, suggesting a reversible oxidation‐reduction reaction (α‐ZAL  ZAN) carried out by microbial dehydrogenases (Baldwin et al., 1983).
ZAN) carried out by microbial dehydrogenases (Baldwin et al., 1983).
The NAD+‐ and NADP+‐ mediated in vitro biotransformation of α‐ZAL was investigated in bovine liver, muscle, and uterine microsomes. The main metabolite was in all cases ZAN, but the oxidative activity of extrahepatic fractions was negligible (Ingerowski and Stan, 1979).
Five gallbladder cannulated cattle (unspecified breed and gender, live weight ~ 300 kg) were each administered 10 mg α‐ZEL or β‐ZEL and bile samples were collected just before dosing and once daily for the following 14 days, and analyzed for the presence of α‐ZEL and α‐ZAL with a GC/MS method after deconjugation. In α‐ZEL‐treated animals there was a constant rise in biliary α‐ZAL, reaching a plateau at 10 days (~ 30 ng/mL), which was matched by a slow decline in α‐ZEL. By contrast, no α‐ZAL but measurable amounts of α‐ZEL could be detected in bile samples from β‐ZEL‐treated cattle (Kennedy et al., 1998).
The in vivo metabolism of α‐ZAL and of ZEN was investigated in Simmenthal heifers (1.5–2 years of age, average body weight around 400 kg) fed ‘uncontaminated’ oats or ZEN‐contaminated oats for 84 days, corresponding to a daily intake of 158 μg or 2,740 μg ZEN, respectively. Heifers from a third group were implanted with two 25 mg α‐ZAL pellets, one at the beginning of the trial and the other after 42 days. Urine were collected every 5‐days and ZEN and its metabolites were determined by LC–MS/MS. Traces (< 0.5 μg/L) of ZEN and β‐ZEL was found in urine specimens from control animals administered with the ‘uncontaminated’ diet. In ZEN‐treated heifers, there was a predominant excretion of β‐ZEL (range 20–65 μg/L) compared to its α‐epimer (range 3–5 μg/L) in a ratio of approximately 8:1. Urine were also found to contain ZEN (range 5–8 μg/L), as well as both α‐ZAL (Zeranol, range 2–3 μg/L) and β‐ZAL (taleranol, range 2–3 μg/L), the two latter in a ratio 1:1. A completely different picture was found in the urine of Zeranol implanted heifers, with low but measurable levels of both α‐ZAL (Zeranol, range 2–5 μg/L) and β‐ZAL (taleranol, range 2–5 μg/L). Although the ratio between the two epimers was the same as that measured in ZEN exposed animals (1:1), only traces of α‐ZEL and no measurable levels of β‐ZEL could be found (Kleinova et al., 2002).
Sheep
Exposure to ZEN
In vitro experiments revealed that approximately 90% of ZEN was degraded by sheep rumen fluid to α‐ZEL and β‐ZEL at similar proportions, whereby protozoa appeared to be more active than bacteria (Kiessling et al., 1984).
Ovine liver preparations were able to biotransform ZEN to both α‐ and β‐ZEL (EFSA CONTAM Panel, 2011). The α‐derivative seemed to prevail over the β‐one, especially when NADPH instead of NADH was used as the cofactor (Olsen and Kiessling, 1983).
A study was conducted on two adult Romney ewes (live weight 30–35 kg) using radiolabelled ZEN. One ewe received 5 mg 13C‐ZEN via the oral route, the other 1 mg 13C‐ZEN via the intravenous (i.v.) route. Based on the results of analytical investigations (GC–MS) performed on urine samples, dosed sheep were able to biotransform ZEN to α‐ZEL, β‐ZEL, α‐ZAL and β‐ZAL both in their free and conjugated forms (glucuronides and presumably some sulfates); β‐ZEL and α‐ZEL were the predominating forms while ZEN, α‐ZAL and β‐ZAL were less abundant. Interestingly, since the formation of α‐ZAL and β‐ZAL was observed also in the intravenously treated animal, the authors conclude that rumen metabolism did not seem to be a prerequisite for the formation of such metabolites (Miles et al., 1996).
Exposure to modified forms
The incubation of either lamb liver microsomal or cytosolic fractions with α‐ZAL (Zeranol) resulted in the NAD+‐mediated formation of ZAN and of small amounts of β‐ZAL; the latter was almost entirely due to the NADH‐dependent reduction of ZAN (Pompa et al., 1988)
Goats
Exposure to ZEN
After incubating caprine hepatic cell preparations with ZEN, α‐ZEL was proved to be predominantly formed by the cytosolic and postmitochondrial fractions while β‐ZEL turned out to be the main product of the microsomal fraction (Dong et al., 2010a). Corresponding cell preparations of rumen and intestinal tissues were also reported to biotransform ZEN mostly to α‐ZEL. As expressed per mg protein, the amount of α‐ZEL generated by tissues others than liver was approximately 1/8–1/3 compared to liver preparations. Nevertheless, the contribution of extrahepatic tissues should be considered as comparable to that of the liver given the large mass of gastrointestinal tissues in ruminants (Dong et al., 2010a).
There is no study available about administering ZEN via the oral route. Toxicokinetics of ZEN was evaluated in male and female Shiba goats aged 2.5–6 years whereby 1.2 mg/kg bw were administered intravenously. The estimated distribution and terminal elimination half‐lives were 3.15 and 28.58 h, respectively. Analysis of urine revealed the presence of mostly glucuronidated/sulfated ZEN, α‐ZEL and β‐ZEL with the latter being the predominant metabolite. Faecal metabolites were largely in the unconjugated form (Dong et al., 2010b).
Exposure to modified forms
No data available.
Pigs
Exposure to ZEN
Pigs were found to have a ZEN metabolising enteric microbial activity. After incubating ZEN anaerobically with porcine chyme collected from caecum, colon and rectum, α‐ZEL was detected as the only known metabolite, besides another unknown one. However, in incubations with chyme from duodenum and jejunum no ZEN metabolites could be detected (Kollarczik et al., 1994).
Several studies have addressed the in vitro metabolic fate of ZEN in cell subfractions, concluding that the formation of α‐ZEL was largely outweighing that of β‐ZEL and that glucuronidation was by far the most common phase II reaction not only in liver but also in extrahepatic tissues (EFSA, 2004; EFSA CONTAM Panel, 2011, 2016).
In vivo experiments support the in vitro findings and suggest that the formation of α‐ZEL prevails in pigs. ZEN and β‐ZEL are also found in various biological specimens at varying proportions while the other reductive ZEN‐metabolites are of minor importance. The proportions of ZEN, α‐ZEL and β‐ZEL were shown to depend on the age of the pigs and the matrix investigated (for review see Dänicke and Winkler, 2015).
Toxicokinetics of ZEN in pigs was recently reviewed (Dänicke and Winkler, 2015) and is further elaborated in Table 3.
The terminal elimination half‐lives (Table 3) decreased markedly after interrupting the enterohepatic cycling of ZEN metabolites by various techniques; this provides a rationale for their excretion via the bile and subsequent reabsorption in the intestine.
Different methods and kinetic models estimated the bioavailability of ZEN for pigs to vary between 80% and 87%. Bioavailability, however, also depends on consideration of the AUCs of the metabolites generated from ZEN. For example, bioavailability was estimated to be 78% when only ZEN data were used for calculation but was 87% when α‐ZEL was considered additionally (Table 3).
Exposure to modified forms
In vitro studies with radiolabelled α‐ZAL (Zeranol) showed that both glucuronidated and sulfated derivatives were formed by pig hepatic subfractions (Bories et al., 1991). The metabolic fate and disposition of implanted [3H] α‐ZAL were studied in one male and one female pig. Results indicate that α‐ZAL is extensively metabolised to ZAN, which, in turn may be reduced to β‐ZAL or back to α‐ZAL. Both free and conjugated α‐ZAL (Zeranol) and metabolites were excreted via the urinary and the biliary route, mainly as glucurono‐derivatives and were recovered from liver extracts (Bories et al., 1992).
Excretion kinetics of ZEN (10 μg/kg bw per day), equimolar doses of ZEN14Glc, ZEN16Glc, ZEN14Sulf administered as an oral bolus was examined in male piglets in a cross‐over design (Binder et al., 2017). None of the three modified forms was detectable in urine or faeces suggesting a complete hydrolysis. ZEN and its modified forms were excreted in faces as ZEN and α‐ZEL, while in urine additionally ZEN‐14‐glucuronide was detected. The total recoveries with the excreta varied between 19% and 48% of the administered doses of ZEN and its modified forms.
Poultry
Exposure to ZEN and modified forms
A number of in vitro findings indicate that, similar to cattle, β‐ZEL is produced to a much larger extent than α‐ZEL in liver subfractions from laying hens, with no apparent detection of zearalanols (Malekinejad et al., 2006; Zinedine et al., 2007). Further in vitro investigations carried out with liver postmitochondrial fractions from different avian species revealed that at low ZEN concentrations (16 μM) a relatively higher amount of α‐ZEL was generated. A high β‐reducing activity was found in quails, while guinea‐fowls, ducks and chickens were classified as weak β‐reducers and geese as weak α‐ and β‐reducers (Kolf‐Clauw et al., 2008).
The systemic ZEN availability of orally administered ZEN (0.3 mg/kg bw) could not be determined because ZEN and metabolite levels remained undetectable in blood (Osselaere et al., 2013). The low ZEN bioavailability is supported by earlier experiments testing biological effects of increasing ZEN dosages which were administered intramuscularly (i.m.) or orally (p.o.) (Chi et al., 1980a,b). The weight of the oviduct was enhanced dose‐dependently following i.m. administration but inconsistently and less markedly in response to the p.o. route. The relative oestrogenic effect of i.m. administered ZEN was determined at 1.37% compared to oestradiol. Based on the published data by Chi et al. (1980b), the calculated ratio between mean oviduct weights recorded after p.o. or i.m. administration of 800 mg ZEN/kg bw suggests that approximately 86% of p.o. dosed ZEN was without effect. This might be a reflection of an efficient biliary and/or renal elimination processes.
In a recent study (Devreese et al., 2015), six turkey poults, six broiler chickens and six laying hens were administered ZEN (3 mg/kg bw) by oral gavage or by the i.v route. Blood samples were taken at different intervals up to 3 h after dosing and plasma levels of ZEN and its metabolites were determined by LC–MS/MS. ZEN, as well α‐ and β‐ZELs could be measured in plasma samples and only traces of zearalanols and ZAN were detected. In all birds, upon oral administration ZEN was rapidly absorbed, with Tmax and MAT values of ~ 0.30 a.m. and rapidly eliminated as well (T1/2 el of ~ 0.30 a.m.). The bioavailability was low (up to 10% based on comparison with IV treated animals), pointing to a low absorption and/or the occurrence of a remarkable pre‐systemic metabolism. It is noteworthy that, turkey poults exhibited high Vd values (10.65 ± 1.05 L/kg) which were about two‐ and threefold‐higher with respect to broiler chickens and laying hens, respectively, indicating an extensive tissue distribution. The results of the kinetic studies on plasma α‐ZEL/β‐ZEL are in line with in vitro studies indicating the higher production of β‐ZEL vs α‐ZEL in most avian species but the turkey. After p.o. administration, turkey poults were more efficient in biotransforming ZEN to α‐ZEL, with an α‐ZEL/β‐ZEL plasma ratio of 0.749 compared to 0.153 in broiler chickens and 0.027 in laying hens. The lack of significant differences of α‐ZEL/β‐ZEL plasma ratio between IV treated birds further supports the occurrence of a notable pre‐systemic metabolism in poultry (Devreese et al., 2015). The dietary administration of 800 mg ZEN/kg to turkey poults for 2 weeks also resulted in the prevalent presence of α‐ZEL in plasma, β‐ZEL being barely detectable (Olsen et al., 1986).
Horse
Exposure to ZEN and modified forms
In vitro studies performed with primary cultures of equine hepatocytes and liver subfractions indicate that ZEN is extensively biotransformed to its reduced metabolites, α‐ZEL being formed to a greater extent (two‐ to threefold) than β‐ZEL. Both the parent compound and the main metabolite (α‐ZEL) were entirely glucuronidated at low concentrations, while at higher concentrations ZEN conjugation was prevailing (Malekinejad, 2013).
A kinetic study was conducted in six fertile Haflinger mares (463 ± 36 kg) offered a naturally contaminated diet containing 10 mg DON/kg and 1 mg ZEN/kg (approximately 0.003 mg/kg bw) for 18 days, starting from the day of ovulation (Songsermsakul et al., 2013). Blood and urine specimens were collected at days 1 and 10, faecal samples at days 2 and 11. Results revealed a prolonged absorption, Tmax being reached 8–12 h from the beginning of the administration. At day 10, the main plasma metabolite was β‐ZEL with a ratio α‐ZEL/β‐ZEL of about 1:20; both ZEN and its metabolites were nearly 100% present as glucuronides. In the 10‐day urine samples, ZEN as well as α‐ZEL were mainly detected, with a α‐ZEL/β‐ZEL ratio of 1.4:1; lower but measurable amounts of α‐ZAL, β‐ZAL and ZAN were also present. A similar excretion pattern was observed in faeces, in which the α‐ZEL/β‐ZEL ratio rose to 2:1. In plasma and urine, ZEN and its metabolites were detected nearly 100% as glucuronides, while the conjugation degree in faeces amounted to only 4–15%. It is concluded that α‐ZEL and β‐ZEL are the main ZEN metabolites in horse, the latter being predominantly formed over α‐ZEL under in vivo conditions.
In an in vivo screening, 24 blood samples of a total of 49 blood samples collected from patients sent to a Horse Clinic for orthopaedic reasons, were positive for ZEN with a median concentration of 0.11 ng/mL (0.02–0.38 ng/mL), 3 samples were positive for α‐ZEL with a median of 0.19 ng/mL (0.14–0.28 ng/mL), and 37 samples were positive for β‐ZEL with a median of 1.24 ng/mL (0.28–7.65 ng/mL). The concentrations of ZAN, α‐ZAL and β‐ZAL were lower than the corresponding LOD. β‐ZEL was demonstrated as the main ZEN metabolite (Schumann et al., 2016).
Rabbits
Exposure to ZEN
In fattening rabbits which were fed diets with increasing concentrations of supplemented ZEN up to 0.297 mg/kg, a dose‐dependent increase of total (free plus conjugated) ZEN, α‐ZEL and β‐ZEL was detected in colonic chyme, faeces, bile and urine while α‐ZAL concentrations were lower than the LODs of 10–50 ng/g. Neither muscle, liver nor kidneys were detected positive for the analysed ZEN residues in their total forms (free plus conjugated) (Ueberschär, 1999).
Exposure to modified forms
The kinetics of a single oral dose of [3H]zeranol (8 mg/kg) was studied in New Zealand rabbits (Migdalof et al., 1983). The maximum blood radioactivity was reached 8 h after the bolus and decreased thereafter with a terminal elimination half‐life of approximately 26 h. Within 120 h after the bolus, approximately 65% and 17.1% of the administered radioactivity were recovered in urine and faeces, respectively.
Fish
Exposure to ZEN
In vitro studies conducted with rainbow trout liver subfractions point to a predominant production of β‐ZEL vs α‐ZEL (Malekinejad et al., 2012).
ZEN and α‐ZEL were detected in muscles of carp at mean concentrations of 0.13–0.22 and 0.05–0.16 ng/g dry weight, respectively, 12 h after the last feeding for 4 weeks while ZAN, α‐ZAL and β‐ZAL as well as β‐ZEL were lower than the LOQs (Pietsch et al., 2015a). Interestingly, residue concentrations appeared not to be related to the dietary ZEN concentration which increased from the control diet (no added ZEN), to 0.332, 0.621 and 0.797 mg ZEN/kg diet.
Samples of rainbow trout muscle, liver, intestines and ovaries and of the corresponding system water and feed were taken from three commercial Polish fish farms. ZEN was not detected in the muscle but trace ZEN levels were detected in the liver (< LOD of 2 μg/kg). It is worth to note that the highest feed concentration of 81.8 μg/kg was associated to the highest ZEN concentration of 7.1 μg/kg determined in the ovaries (Woźny et al., 2013). However, the HPLC method employed did not include any ZEN metabolite.
Exposure to modified forms
No information on the toxicokinetics of ZEN modified forms in fish was identified.
Farmed mink
No information on the toxicokinetics of ZEN or its modified forms could be identified.
Companion animals
Exposure to ZEN
No studies have been identified concerning the toxicokinetics of ZEN in cats. Very limited information is available for dogs. In an experiment involving prepubertal female Beagle dogs receiving a daily dose 0, 50 or 75 μg ZEN/kg bw for 42 days (Gajęcka et al., 2013), blood samples taken at weekly intervals revealed the presence of ZEN, α‐ and β‐ZEL throughout the experiment, with a clear prevalence of the α‐ over the β‐derivative (up to 100%).
Exposure to modified forms
There are no studies on the toxicokinetics of ZEN modified forms in cats.
The kinetics of a single oral dose of [3H] α‐ZAL (8 mg/kg) was studied in Beagle dogs (Migdalof et al., 1983). Radioactivity rapidly increased after bolus administration and reached a maximum concentration after 4 h from where it declined rapidly and was not detectable after 24 h. Due to a lack of sufficient data, an estimation of the terminal half‐life was not attempted. Approximately 8.2% and 76.1% of the administered radioactivity were recovered with urine and faeces, respectively, within 120 h. Both α‐ZAL and ZAN, the main identified metabolite, underwent glucuronidation. Biliary excretion was largely predominant over urinary excretion.
Conclusions
In most species, ZEN is generally well absorbed, but its bioavailability is relatively low due to an extensive presystemic metabolism which takes place at rumen, enteric, and hepatic level. Reductive reactions largely predominate. They are mediated by α‐ and β‐HSD, yielding α‐ and β‐ZEL and to a much lesser extent α‐ and β‐ZAL; the α‐derivatives display a remarkably higher oestrogenic potency. In vitro and in vivo studies have demonstrated wide interspecies differences in the generation of α‐ and β‐derivatives, which have been correlated with the species‐mediated sensitivity to the mycotoxin. The extensive rumen and/or enteric metabolism underwent by ZEN largely accounts for the discrepancies observed in some species (e.g. poultry, goats, horses) between the results from in vitro investigations and those derived from in vivo studies. Based on the levels measured in biological fluids (plasma, urine, or bile) of ZEN treated animals, the α‐derivatives seem to prevail in pigs, dogs, and turkeys while the β‐derivatives appear to be more abundant in cattle, goats, horses, broiler chickens, and laying hens. Other minor metabolic routes for ZEN include cytochrome P450 (CYP)‐mediated aromatic and aliphatic hydroxylation, although data concerning the occurrence of hydroxylated derivatives in organs or biological fluids from the examined species could not be identified.
Little is known about the metabolic fate of modified forms, except for α‐ZAL, which is used as growth‐promoter in non‐EU countries under the name of Zeranol. In all species, α‐ZAL is oxidised to ZAN and isomerised to β‐ZAL; the parent compound and the metabolites are mainly glucuronidated.
Reduced metabolites but also ZEN and ZAN are subjected to glucuronidation and, to a lesser extent, sulfation. In most species the resulting conjugates are mainly excreted via the biliary route. An active enterohepatic circulation is reported particularly in the pig, thereby prolonging the persistence of ZEN and its metabolites in the body. Scant information is available about ZEN toxicokinetics in fish and dogs and no information is available for cats and farmed mink.
3.1.1.6. Transfer into feed from animal origin
Transfer of ZEN and its modified forms from feed to animal tissues which is subsequently used as animal feed, needs to be considered both from a quantitative and a qualitative (types of metabolites transferred to animal tissue) point of view. A quantitative evaluation is possible by calculating transfer or carry‐over factors as the ratio between the concentrations of ZEN and its modified forms in animal tissue and in feed. A recent review suggested that ZEN, α‐ZEL and β‐ZEL accounted for the majority of total ZEN residues in farm animals (Dänicke and Winkler, 2015). Moreover, it seems that ZEN and α‐ZEL are the main metabolites in pigs and poultry while β‐ZEL contributed significantly to the total residue levels in bovine tissues, including milk. The calculated transfer factors varied from 0.004 to 0.295 in livers from various animal species, from 0.008 to 0.05 in bovine milk, from 0 to 0.021 in porcine muscle and up to 0.0007 in kidneys of turkey. Adipose tissue from various animal species and eggs were virtually free from toxin residues after experimental oral exposure to ZEN (Dänicke and Winkler, 2015). It becomes generally clear from experimental data that the ZEN metabolite pattern in animal tissues is different from that usually found in plant derived feedstuffs (see Section 3.2). Survey data on the occurrence of modified ZEN forms in market‐collected foodstuffs of animal origin principally support the experimental findings.
The occurrence of ZEN was reported up to 12.5 μg/kg in cow's milk from Egypt, USA, UK, and China (Flores‐Flores et al., 2015). Lower amount of ZAN (up to 0.37 μg/L), α‐ZAL (3.03 μg/L), and α‐ZEL (0.073 μg/kg) were reported in cow's milk samples collected in China as well (Xia et al., 2009; Huang et al., 2014).
The co‐occurrence of ZEN and its modified forms α‐ and β‐ZEL in cow's milk production was reported by Meucci et al. (2011). Target compounds were found in a range of 0.01–0.04 μg/L for ZEN, 0.15–0.76 μg/L for α‐ZEL, and 3.5–10.8 μg/L for β‐ZEL. The same authors reported the (co)occurrence of ZEN and α‐ and β‐ZEL in meat‐based products (n = 44) as well. Products based on calf, rabbit, horse, lamb, turkey, ham, and chicken were considered.
Although the food commodities were intended for human consumption the residues reported give indication which patterns of ZEN and modified forms could be expected in the feedstuffs of animal origin. While ZEN, β‐ZEL and β‐ZAL were not detected, α‐ZEL was found in 27% of the samples, with a mean concentration ranging between 1.1 and 8.5 μg/kg and a maximum concentration of 30.5 μg/kg in calf meat. The authors reported the occurrence of α‐ZAL in one sample, at a concentration of 950 μg/kg (Meucci et al., 2011).
In summary, the occurrence of ZEN and its modified forms in food and feed from animal origin is poorly addressed in the literature, with few tissue‐specific information from field studies. However, data obtained from the literature are in agreement with those received by EFSA and used for calculating the exposure. Although distribution would suggest that conjugated forms are present in the tissues, the CONTAM Panel did not identify additional information on the occurrence of conjugated forms of ZEN in products of animal origin.
Conclusions
Taken together, the experimental data on the transfer of ZEN from contaminated feedstuffs into animal tissues suggest that animal derived feedstuffs are unlikely to contribute quantitatively to the exposure of animals to zearalenone and its modified forms.
However, in contrast to feedstuffs of plant origin the pattern of modified ZEN forms in feedstuffs of animal origin includes especially α‐ZEL and β‐ZEL besides ZEN. In evaluating the risk, the higher proportions of the oestrogenic more potent α‐ZEL and the target animal species fed with higher proportions of feedstuffs of animal origin, such as dogs and cats, fish and farmed mink need to be considered.
3.1.2. Mode of action
The mode of action of ZEN has been recently reviewed by the CONTAM Panel (EFSA, 2004; EFSA CONTAM Panel 2011, 2016).
3.1.2.1. Oestrogenic activity
The dominating biological activity of ZEN is its oestrogenic activity, i.e. the ability to act like the endogenous steroidal sex hormone 17 β‐oestradiol (E2). Indeed, numerous in vivo and in vitro studies have indicated that ZEN binds to ERs, generating an oestrogen‐like response (EFSA, 2004; EFSA CONTAM Panel 2011, 2016). Zebrafish was also used as a model fish species to investigate the oestrogenic effect of ZEN and its modified forms (Chen et al., 2010; Johns et al., 2011; Schwartz et al., 2013).
ZEN passively crosses the cell membrane and binds to oestrogenic receptors (ERs). The receptor‐zearalenone complex is rapidly transferred to the nucleus, where it binds to oestrogen‐responsive elements, thereby activating gene transcription (EFSA, 2004). Two subtypes of ERs, i.e. ER‐α and ER‐β, are known to be expressed in mammalian tissues. ER‐ α is commonly assumed to be the dominant ER in the uterus. ER‐α and ER‐β exhibit different binding affinities for oestrogenic compounds (EFSA CONTAM Panel, 2016). Generally, ZEN has a stronger affinity to ER‐α than to ER‐β (EFSA CONTAM Panel, 2011).
Besides its binding to ERs, the endocrine effects of ZEN involve other mechanisms. Indeed, ZEN and its reduced metabolites are competitive substrates for 3α ‐HSD and 3β ‐HSD, two enzymes involved in the synthesis of steroids (Fink‐Gremmels and Malekinejad, 2007).
A landmark effect of oestrogenic compounds is the induction of uterine growth in immature or ovariectomised female animals; the ‘uterotrophic activity’ is a common in vivo assay using mice or rats which measures the increase in wet weight of the uterus (Thigpen et al., 1987). ZEN and its modified forms differ considerably in their oestrogenic activity (EFSA CONTAM Panel, 2016). Based on their ‘uterotrophic activity’ assessed in rodents, ZEN and its modified forms are classified as follows α‐ZEL > α‐ZAL > ZEN ≈ ZAN ≈ β‐ZAL > β‐ZEL (EFSA CONTAM Panel, 2016).
Relative binding affinity of ZEN and its modified form, especially α‐ZEL, to ERs varied for different species (for example, pig > rat > chicken – see Metzler et al., 2010). Moreover, the expression levels of the two ER types differ between species. However, in most ER‐binding assays using cytosolic ER, the proportion of ER‐α and ER‐β is not known.
3.1.2.2. Effects on transcription factors other than ER
ZEN can activate the pregnane X receptor (PXR), a human xenobiotic receptor member of ligand activated nuclear transcription factors (Ding et al., 2006). ZEN activates PXR) by displacing its co‐repressor N‐CoR and by recruiting the co‐activators SRC‐1, PBP and GRIP1. PXR regulates the expression of various genes involved in the metabolism of endobiotics and xenobiotics, such as the CYP3A4, the major human CYP. PXR is also involved in the transcriptional regulation of glutathione‐S‐transferases, sulfotransferases and UDP glucuronosyl transferases as well as organic anion transporters and ATP‐binding cassette (ABC) efflux transporters (reviewed in EFSA CONTAM Panel, 2011). In primary cultures of human hepatocytes, ZEN at concentrations as low as 0.1 μM, activates, in addition to PXR, constitutive androstane receptor (CAR) and aryl hydrocarbon receptor (AhR) mRNA levels. Some of the phase I target genes are also activated, mainly CYP3A4, CYP2B6 and CYP1A1 and to a lesser extent CYP3A5 and CYP2C9 (Fink‐Gremmels and Malekinejad, 2007; EFSA CONTAM Panel, 2011).
The interaction of ZEN and its modified forms, with the human androgen receptor (hAR) was also studied (Molina‐Molina et al., 2014). Their ability to antagonise hAR‐mediated reporter gene expression elicited by a model synthetic androgen (R1881) was examined in androgen‐sensitive PALM cells. The study demonstrates that ZEN and its modified forms possess hAR‐mediated antagonistic activity in PALM cells, in which ZAN, α‐ZAL, and β‐ZAL were the most effective hAR antagonists.
The genotoxicity of ZEN was evaluated by EFSA (EFSA CONTAM Panel, 2011, 2016). ZEN was negative in bacterial mutation studies; it was found clastogenic and aneugenic in a variety of cell culture systems as well as in vivo (EFSA CONTAM Panel, 2011, 2016).
3.1.2.3. Effect of ZEN and modified forms on proliferation and apoptosis
ZEN caused a concentration‐dependent, proliferation‐stimulating effect on T47D and MCF‐7 cell lines similar to that elicited by natural oestrogen compounds. This pro‐proliferative effect was associated with the presence ERs. Of note, while lower concentrations of ZEN induce proliferation, higher concentrations triggered a decrease in cell viability (Kowalska et al., 2016). It has been proposed that α‐ZAL promotes breast cancer cell growth by stimulating aromatase activation and increasing oestrogen biosynthesis in adipose tissue. Indeed, 2–50 nM α‐ZAL (Zeranol) was able to significantly increased aromatase activity, aromatase mRNA expression and oestrogen production in primary cultured human breast preadipocytes (Zhong et al., 2016). The ability of ZEN to stimulate cell proliferation has been documented by its ability to activate cyclin‐dependent kinases in MCF‐7 cells. ZEN increases the cyclin D1 protein levels and activates cyclin‐dependent kinase (Cdk)‐2 resulting in the hyperphosphorylation of retinoblastoma gene product. This activation of Cdk‐2 also leads to an increased rate of G1 to S phase transitions and mitosis (Fink‐Gremmels, 2008b).
ZEN has been shown to induce apoptosis in leukemic cell lines and PBMC via modulation of pro‐ and anti‐apoptotic proteins Bax and Bcl‐2, as well as changes in the mitochondrial potential and induction of cytochrome C release (Banjerdpongchai et al., 2010). In addition ZEN was able to activate both the extrinsic and intrinsic apoptotic pathways in primary rat Sertoli cells (Xu et al., 2016).
3.1.3. Adverse effects in livestock, fish, horses and companion animals
Generally, there are three types of studies which might be used for evaluation of effects of ZEN and modified forms on animals:
controlled experiments using pure ZEN and its modified forms
controlled feeding experiments with naturally contaminated feedstuffs and feed contaminated with fungal material
field studies and epidemiological observations
Controlled experiments using pure ZEN and modified forms added to feed with zero‐ or low levels of natural contamination of ZEN and other oestrogenic compounds are the most appropriate way for toxicity testing. However, adding the toxins to the diet might bear the disadvantage that the toxicokinetics and bioavailability might differ from that of naturally contaminated feedstuffs due to varying forms of binding to the feed matrix.
Controlled feeding experiments with naturally contaminated feedstuffs have the advantage that the effects of ZEN and modified forms can be determined more according to practical conditions, although the influence of co‐occurring mycotoxins (e.g. DON) cannot be excluded.
Field studies are usually poorly controllable but might provide information on a larger number of animals suspected to be exposed to ZEN and modified forms. However, as oestrogenic effects might be induced by other oestrogen‐like substances such as phytoestrogens and xenoestrogens, field studies need to be evaluated carefully, especially with regard to the extent of differential diagnosis. Many of these studies were already reported in the previous EFSA opinion related to the presence of ZEN in feed (EFSA, 2004) and are not addressed in the present section. However, some of these field studies are considered relevant to other sections of the present opinion (e.g. Section 3.1.1 Toxicokinetics).
All types of studies were regarded in earlier evaluations on the effects of ZEN on farm animals by EFSA (EFSA, 2004; EFSA CONTAM Panel 2011, 2014, 2016), FAO/WHO (2000), the SCF (2000) and other reviews (Seeling and Dänicke, 2005; Zinedine et al., 2007; Fink‐Gremmels, 2008a). The current TDI for humans is actually based on oestrogenic effects in pig (EFSA CONTAM Panel, 2011, 2016).
The EFSA (2004) opinion did not set NOAELs or LOAELs for pigs and other livestock due to limitations in the availability, reliability and consistency of the data available at that time. The present assessment takes account of all the evidence available to the CONTAM Panel, and considers the issues of reliability and consistency as part of the uncertainty.
3.1.3.1. Adverse effects in livestock – evaluation of different types of studies
Ruminants
Cattle
Zearalenone
-
a
Controlled experiments using pure ZEN
Only two experiments were performed using pure ZEN in cattle. These studies were evaluated by EFSA in the opinion on ZEN as an undesiderable substance in animal feed (EFSA, 2004). Briefly, the first one involved the oral administration only of a relatively high ZEN dose to virgin heifers (250 mg purified ZEN per head corresponding to a mean exposure of 1,456 μg/kg bw per days for 66 days) and resulted in a slightly decreased conception rate (62% compared to 87% found in the untreated control group) (Weaver et al., 1986a). Another experiment performed by these authors with cows revealed no adverse effects on progesterone levels after oral treatment with daily ZEN doses of 31.25, 62.5, 125.0, 250.0, and 500.0 mg ZEN/cow corresponding to calculated exposures of 48, 96, 192, 385 and 769 μg/kg bw per day and associated dietary concentrations of 1.4, 2.8, 5.7, 11.4 and 22.7 mg/kg for an observation period of two oestrus cycles. However, upon clinical examinations corpora lutea appeared to be smaller (neither actual measurements nor statistics were reported) in all groups treated with ZEN independent of dose (Weaver et al., 1986b). Based on the available information, no NOAEL/LOAEL could be derived.
-
b
Controlled feeding experiments with naturally contaminated feedstuffs and feed contaminated with fungal material
Studies with heifers on the effects of feeding ZEN contaminated oats (1.25 mg ZEN/kg feed) were evaluated by EFSA (2004). These experiments revealed no relevant effects on oestrus cycle or histology of reproductive organs.
Feeding and balance experiments with dairy cows revealed no overt signs of hyperoestrogenism or reproductive disorders when naturally contaminated cereals were used as ZEN vehicles. In these experiments, the diets contained ZEN concentrations of 0.055 mg/kg (Seeling and Dänicke, 2005; Seeling et al., 2005), 0.02–0.58 mg/kg (Winkler et al., 2014), 0.062–0.099 mg/kg (Keese et al., 2008a,b) at a DM content of 88%.
Modified forms
No data on the adverse effects of ZEN modified forms suitable to derive effect levels to characterise the hazard in cattle were identified.
Sheep
Zearalenone
Sensitivity of sheep to ZEN has previously been evaluated by FAO/WHO (2000) and EFSA (2004). Based on the experiments by Smith et al. (1990), significant linear dose–response relationships ‐ covering daily doses of 1.5, 3, 6, 12 and 24 mg/ewe equivalent to calculated exposures of 28, 56, 111, 224 and 444 μg/kg bw per day and dietary concentrations of 0.8, 1.6, 3.2, 6.4 and 12.6 mg/kg ‐ were described for a number of reproductive traits such as cycle length, duration of oestrus, number of ovulations per ewe, as well as ovary and uterus weight. Based on the deviation from the normal duration of the cycle length, a LOAEL of 56 μg ZEN /kg bw per day was identified while the NOAEL corresponded to an exposure of 28 μg ZEN/kg bw per day.
Modified forms
No data on the adverse effects of ZEN modified forms in sheep were identified.
Goat
Zearalenone
No data concerning oral exposure to ZEN were identified. Intravenous application of a single ZEN bolus of 2.4 mg/kg bw resulted in hepatocyte swelling and lymphocytic infiltration of the liver, the kidney pelvic and of the endometrium 48 h post injection (Dong et al., 2010a,b). No lesions were found in the gastrointestinal tract and the ovaries. From this experiment no critical effect levels can be derived.
Modified forms
No data on the adverse effects of ZEN modified forms in goat were identified.
Conclusion on ruminants
In sheep, based on the depressed ovulation rates and lower lambing percentages a LOAEL of 56 μg/kg bw per day was identified while the NOAEL corresponded to an exposure of 28 μg/kg bw per day. These exposure levels corresponded to dietary ZEN concentrations of 3 and 1.5 mg/kg feed, respectively.
Data suitable to derive reference points for risk characterisation for goats could not be identified from the literature.
When exposed to ZEN, cattle appear quite resistant but neither a LOAEL nor a NOAEL could be derived. The only one available dose–response experiment in dairy cows orally exposed to pure ZEN for which a reduction in the size of corpora lutea was claimed but no effects level could be identified, was too limited to identify adverse effects. Similar limitations were identified in a study on heifers at relatively high exposure level where the conception rate tended to be decreased.
No data were available for evaluating dose related effects of ZEN modified forms in ruminant species.
Pigs
Zearalenone
EFSA evaluated ZEN in feed in 2004 and concluded that female pigs were the most sensitive animals, but that the hormonal effects varied with age groups and cycling (EFSA, 2004). Furthermore, EFSA stated that there was a large variation in the outcome from trials with pigs, that seemed to be related to the use of naturally contaminated feed versus pure toxin and that a reference point of hazard characterisation of ZEN in pig feed could not be established.
In 2011, the CONTAM Panel concluded that ovaries, vulva and uterus were the most sensitive tissues, and that the LOAEL in these tissues in mature and immature gilts ranged from 17 to 200 μg/kg bw per day, with the overall NOEL from relevant pig studies being 10.4 μg/kg bw per day (EFSA CONTAM Panel, 2011).
EFSA also reviewed several studies on pigs and gilts exposed to ZEN in 2016 (EFSA CONTAM Panel, 2016), including prepubertal gilts (Teixeira et al., 2011; Gajęcka et al., 2012; Jiang et al., 2012; Oliver et al., 2012; Schoevers et al., 2012; Chen et al., 2015a,b; Gonkowski et al., 2015; Jakimiuk et al., 2015; Obremski and Poniatowska‐Broniek, 2015; Obremski et al., 2015a,b; Pistol et al., 2015). The CONTAM Panel concluded that the observed minor alterations in intestinal lymphoid tissue at doses below the NOEL (10.4 μg/kg bw per day) used to derive the TDI of 0.25 μg/kg bw per day (EFSA CONTAM Panel, 2011) were without evidence of adversity. Furthermore, only one dose was used in these studies and no dose‐effect relations could be established (EFSA CONTAM Panel, 2016).
-
a
Controlled experiments using pure ZEN
The pivotal study in the SCF evaluation (SCF, 2000) a was a study where sexually mature gilts were given 0, 1, 5 or 10 mg ZEN/kg feed on days 5 to 20 of oestrus, corresponding to 0, 40, 200 or 400 μg/kg bw per day (Edwards et al., 1987a). The feeding was performed in summer and winter but, since there were no differences between the seasons, the results were pooled to give 24–25 animals/dose. The inter‐oestrus interval increased from 21.0 ± 0.3 days to 29.2 ± 2.9 days in the medium‐dose group and 32.7 ± 3.3 days in the high‐dose group. Increased plasma concentrations of progesterone and prolonged maintenance of corpora lutea was also observed in pigs with a prolonged interoestrus interval. No effect on any parameter was observed in pigs given the lowest dose of 40 μg /kg bw per day and this was used as a NOAEL in the risk assessment by the SCF.
In their risk assessment of ZEN in food in 2011, EFSA reviewed feeding studies of the effects of ZEN in pigs published since the opinion of ZEN in feed from 2004. The review included several feeding studies where pure ZEN was given to female prepubertal pigs, of which several reported effects on uterus and ovaries at levels similar to what was reported in mature gilts by Edwards et al. (1987a) (Obremski et al., 2003; Wasowicz et al., 2005; Zwierzchowski et al., 2005; Jakimiuk et al., 2010a,b). The pivotal study in this assessment was, however, a study based on naturally infected maize (Döll et al., 2003, see below section b).
Male piglets (n = 3) were given 50 μg pure ZEN/kg bw per day in the diet for 22 days (Jiang et al., 2012). ZEN led to decreased relative weights of testes compared with controls, but the size of the testes was unchanged. Serum levels of progesterone and testosterone were lower in piglets given ZEN compared to controls and the authors concluded that this exposure induced reproductive toxicity in male piglets.
Gajęcka et al. (2016)conducted a controlled experiment where a group of pre‐pubertal female gilts (from 25 kg) were given either a daily oral dose corresponding to 40 μg/kg bw) or a control diet (n = 21) for 42 days in form of water‐soluble capsules every morning. Blood samples were taken weekly during the exposure period. Small, but statistically significant changes were observed in biochemical and haematological parameters, but none of the differences between the exposed and controls animals were consistent over the feeding period. The weight gain was significantly higher in the control group than in the ZEN group after 1 and 4 weeks of exposure, while after 6 weeks, the weight gain was significantly higher in the ZEN group compared to controls. There were no significant differences in weight gain after 2, 3 and 5 weeks of exposure. The authors claim that a stimulating effect of ZEN on the metabolism in the beginning of the exposure period is eliminated due to compensatory and adaptive mechanisms later on.
The same protocol was used for prepubertal gilts (n = 18/group) (Lewczuk et al., 2016). The pigs were euthanised after exposure for 1, 2, 3, 4, 5 or 6 weeks. ZEN did not alter the architecture of the mucosa layer or the ratio between goblet cells and adsorptive cells in the epithelium of the duodenum, but it did increase the quantity of lymphocytes, plasma cells and macrophages with black brown granules in the lamina propria.
Using again this study protocol, the same research group investigated the effects of ZEN on the jejunum (Przybylska‐Gornowicz et al., 2015) in pigs. The microscopic examinations revealed an increased number of goblet cells and lymphocyte number in the villi after one week, but not at other time points. The number of plasma cells in the lamina propria was increased after 1, 3 and 6 weeks of exposure, but not after 2 and 4. No effects were observed in pigs given ZEN in mucosal thickness, crypt depth, ultrastructure of the mucosal epithelium, lamina propria or intestinal barrier permeability.
Piglets weaned at an age of 28 days with an average body weight of 8.74 kg were fed a ZEN supplemented diet (1.04 mg ZEN/kg diet, approximately 70 μg ZEN/kg bw per day) for 35 days (corresponding to approximately 70 μg/kg bw per day) and investigated for protein and mRNA expression of ghrelin and proliferating cell nuclear antigen (PCNA) in the ovaries (Dai et al., 2016).
Results revealed that ZEN exposure could promote the autocrine action or expression of the ghrelin gene and additionally accelerate the development of follicles.
Twelve fistulated barrows with an initial mean body weight of 20.6 kg/bw were given 10 mg ZEN or DON /kg feed in a latin square design with three diets and 2 periods of 5‐day adaptation and a 3‐day sampling period. ZEN reduced the apparent ileal digestibility of tryptophan from 0.840 to 0.771 (p = 0.04), while the digestibility of proteins or other amino acids were not affected (Jo et al., 2016).
An oral dose of 40 μg ZEN/kg bw per day pure ZEN (purity not specified) administered for 6 weeks had no significant effect on the composition of peripheral blood lymphocytes in gilts (mean body weight of 25 kg bw at the start of the experiment. See Dąbrowski et al., 2016).
-
b
Controlled feeding experiments with naturally contaminated feedstuffs and feed contaminated with fungal material
In the human risk assessment of ZEN in food in 2011, EFSA CONTAM Panel reviewed feeding studies of the effects of ZEN in pigs. The review included feeding studies where naturally infected maize containing ZEN was mixed into the feed and given to female pigs (e.g. Alm et al., 2006). The key study in the assessment was the study of Döll et al. (2003) where female piglets were given feed containing 0.01, 0.06, 0.15, 0.22 or 0.42 mg ZEN/kg feed prepared by mixing naturally contaminated maize into the feed, corresponding to 0.5, 3.0, 7.4, 10.4 or 17.6 μg/kg bw per day, for 5 weeks (n = 20/dose). The feed also contained 0.2–3.9 mg DON/kg feed, corresponding to 9.8–163.5 μg/kg bw per day. The ZEN treatment resulted in increased weights of uteri and a dose‐related increase in the number of piglets with a red and swollen vulva and cervix. The increase was statistical significant from controls only at the highest dose. Both the authors and EFSA examined the reported effects and attributed the typical oestrogenic effects to ZEN, based on existing knowledge on clinical effects and mechanisms of action. EFSA also concluded that since feed intake and body weight gain were not affected at these levels, the oestrogenic effects were probably not influenced by the presence of DON in the feed (EFSA CONTAM Panel, 2011). From this study, EFSA established a NOEL of 10.4 μg ZEN/kg bw per day which was used to derive a TDI for human chronic exposure. EFSA also stated that even if this was a NOEL rather than a NOAEL since it is not known whether these alterations would affect the fertility and reproductive performance later in life, the effects are undesirable and are relevant for a risk assessment.
In Kong et al. (2015), gilts were fed with a feed containing graded levels of Fusarium‐infected barley for 2 weeks. The feed contained DON at 0.6 (control) – 14.6 mg DON/kg and below the LOD–33.7 μg ZEN/kg. All effects were typical of DON, like reduced feed intake and weight gain and no typical hormonal effects were observed.
Adverse effects in the male reproductive tract and its functioning have been recorded at concentrations exceeding 20 mg ZEN/kg bw (Review in Diekman and Green, 1992; EFSA CONTAM Panel, 2011, 2016). No effect on the male reproductive system was found when six sexually mature boars were fed a naturally contaminated feed containing 1 mg ZEN/kg of feed (Sutkeviciene et al., 2009). Based on these findings, the CONTAM Panel concluded that ZEN has been found to have adverse effects on male reproduction but at levels far above the levels affecting females (EFSA CONTAM Panel, 2011).
Time‐dependent oestrogenic effects were reported for female rearing piglets fed diets prepared by mixing naturally Fusarium‐contaminated maize into the feed (Rempe et al., 2013b). The final feed contained 0.01, 0.05, 0.08, 0.17 and 0.29 mg ZEN/kg feed, and 0.03, 0.59, 1.27, 2.01 and 4.52 mg DON/kg feed, respectively. Histological examination of the ovaries did not indicate differences in the distribution of follicle stages between the control and highest exposed group.
In a second feeding experiment, two groups of female rearing piglets were given a feed containing 0.03 mg of ZEN and 0.43 mg DON per kg feed or a feed containing 0.32 mg ZEN and 3.67 mg DON per/kg feed for 4 weeks (Rempe et al., 2013a). The uterus weight and vulva width in female rearing piglets increased slightly (p > 0.050 and p = 0.058, respectively). Significant effects on typical DON‐related parameters like feed intake and growth were reported from the study, but there were no statistical significant alterations on oestrogenic effects or other effects that can be attributed to zearalenone.
Modified forms
Zeranol implants (36 mg) applied to peripubertal gilts (171 days of age, 109 kg) did not increase the proportion of gilts available for breeding (treated, 21/39; control, 18/40) as checked daily with a mature boar on days 3–58 of the experiment (Trout et al., 2007). The gilts detected in oestrus were inseminated twice 24 h apart with pooled semen. Gilts were slaughtered on days 58–62 of gestation. Of the gilts inseminated on days 44–58, 16/21 treated gilts and 16/18 control gilts were pregnant. Number of fetuses (7.5 vs 12), fetal weight (83 vs 121 g), fetal length (117 vs 132 mm) and fetal survival (45% vs 78%) were reduced (p < 0.001) by Zeranol implants. The authors concluded that the implant did not increase the proportion of gilts available for breeding but was toxic to the fetuses.
Conclusion on pigs
The pivotal study when EFSA derived a human TDI in 2011 was a feeding study by Döll et al. (2003) where female prepubertal piglets were exposed to 0.01–0.42 mg ZEN/kg feed f (0.5, 3.0, 7.4, 10.4 and 17.6 μg ZEN/kg bw per day) rom naturally contaminated maize. No effects were observed in pigs given 10.4 μg ZEN/kg bw per day (NOEL), while oestrogenic effects like increased uterus weight and reddened and swollen vulva were observed in pigs given 17.6 μg ZEN/kg bw (LOEL). Several other papers, including papers not reviewed in previous EFSA opinions, have reported oestrogenic effects at similar exposure levels of 20 μg/kg bw per day (Gajęcka et al., 2012, 2016; Rempe et al., 2013a,b; Jakimiuk et al., 2015; Przybylska‐Gornowicz et al., 2015; Lewczuk et al., 2016). A review of the reported effect levels and no‐effect levels indicate that young and developing gilts (until first cycling) are most vulnerable to ZEN, as indicated by studies finding effects from 17.6 μg/kg bw per day for female piglets. These effects were not regarded as adverse by EFSA since it was not known whether the observed alterations would have any impact on the reproduction later. However, the alterations were still regarded as undesirable and were the critical effects in the derivation of a human TDI (EFSA CONTAM Panel, 2011). The CONTAM Panel considers these alterations as relevant for the derivation of a reference point for pigs.
For sexually mature female pigs, the prolonged cycling was reported for cycling sows from 200 μg/kg bw per day, with no effect at 40 μg/kg bw per day (Edwards et al., 1987a), which is the lowest reported NOAEL for mature female pigs.
The database for boars is limited. Precocious spermatogenesis, damage to the germinal epithelium and interstitial cell hyperplasia were reported from young boars given feed containing 30 mg ZEN/kg, corresponding to 1.2 mg/kg bw per day in the study, for varying time periods (Vanyi and Szeky, 1980) and reduced serum concentrations of progesterone and testosterone was observed in male piglets given 50 μg ZEN/kg bw per day for 22 days (Jiang et al., 2012).
Poultry
Zearalenone
EFSA (2004) evaluated feeding experiments with poultry and concluded that poultry species can be regarded as quite tolerant because ZEN effects are observed only at exposure levels hardly to occur under practical feeding conditions.
-
a
Controlled experiments using pure ZEN
Dietary concentrations of 800 mg ZEN/kg diet fed to broilers for 6–9 weeks of life failed to evoke negative effects (Allen et al., 1981). JECFA concluded from this experiment the NOEL to be higher than 59 mg/kg bw per day. Weight gain, feed consumption, feed to gain ratio, weights of liver, heart, spleen, testicles, oviduct, comb, and bursa of Fabricius, haematocrit, haemoglobin, erythrocytes, and serum concentrations of calcium, phosphorus, total protein, alkaline phosphatase, and cholesterol remained unaltered in growing broiler chickens fed diets with 10, 25, 50, 100, 200, 400 and 800 mg ZEN/kg diet corresponding to calculated exposures of 1,500, 3,750, 7,500, 15,000, 3,0000, 60,000 and 120,000 μg ZEN/kg bw per day (Chi et al., 1980a), respectively. Lymphocyte count was the only endpoint significantly influenced by increasing dietary ZEN concentrations irrespective of sex. For this endpoint a calculated LOAEL of 30,000 μg/kg bw per day and a NOAEL of 7,500 μg/kg bw per day, corresponding to estimated exposure levels of 200 and 50 mg/kg diet, respectively, have been identified.
Hyperoestrogenism was detected in male and female Leghorn chicks exposed to 10 mg ZEN/kg bw per day via crop intubation over a period of 20 days (Maryamma et al., 1992) and in turkeys fed a diet with a ZEN concentration of 800 mg/kg feed (Olsen et al., 1986). Egg production was decreased in female turkeys fed a diet with 100 mg ZEN/kg (Allen et al., 1983).
Male and female turkeys were fed ZEN contaminated diets from 3.5 to 6.5 weeks of age at 10, 25, 50, 100, 200, 400 and 800 mg/kg feed which corresponded to estimated exposure levels of 940, 2,230, 4,500, 9,100, 19,000, 37,580 and 76,630 μg ZEN/kg bw per day, respectively (Allen et al., 1981). Male turkeys exhibited strutting behaviour when fed diets containing 400 or 800 mg ZEN/kg diet. Moreover, these males showed a more pronounced development of the caruncles and dewlaps compared to their control counterparts. Both males and females demonstrated an increased incidence of swollen vents fed diets containing 200 mg ZEN/kg or more, a condition which was not observed in the control group. Based on vent swelling a LOAEL of 19,000 μg ZEN/kg bw per day and a NOAEL of 9,100 μg ZEN/kg bw per day were calculated, corresponding to a dietary ZEN concentration of 200 mg/kg diet and 100 mg/kg diet, respectively.
-
b
Controlled feeding experiments with naturally contaminated feedstuffs and feed contaminated with fungal material
Studies before 2004 were already evaluated by EFSA (2004). This literature compilation revealed that the broiler performance remained uninfluenced after 7 weeks feeding of a ZEN contaminated diet (30 mg/kg feed) (Bacon and Marks, 1976).
Laying hens fed a diet containing naturally contaminated maize with a ZEN concentration of 1.1 mg ZEN/kg diet did not show signs of hyperoestrogenism (Dänicke et al., 2002). A number of adverse effects were found in this study which could rather be ascribed to the presence of DON in the diet; such as a decreased feed intake, a depressed nutrient digestibility, decreased serum titres to Newcastle disease virus (NDV) and increased yolk titres to the bacterial antigen K88. Since the EFSA evaluation one additional experiment with laying hens was identified. Feeding of laying hens during the period of onset of laying with a diet containing 260.2 μg/kg feed from week 18 to 23 of age did not result in adverse effects on laying performance and egg quality traits (Jia et al., 2016).
Modified forms
No data on the adverse effects of ZEN modified forms in poultry were identified.
Conclusion on poultry
Poultry are very resistant to ZEN as indicated by studies performed in chicken and turkey. In male and female broiler chickens, lymphocyte count was the only endpoint significantly influenced by diets fortified with pure ZEN. A LOAEL of 30,000 μg ZEN/kg bw per day and a NOAEL of 7,500 μg ZEN/kg bw per day were calculated corresponding to exposure levels of 200 mg/kg diet 50 mg/kg diet, respectively.
Based on vent swelling of female and male turkeys the LOAEL of 19,000 μg ZEN/kg bw per day and the NOAEL of 9,100 μg ZEN/kg bw per day were identified, based on dietary ZEN concentrations of 200 mg/kg and 100 mg/kg diet, respectively.
No data on the adverse effects of ZEN modified forms in poultry were identified.
Horses
Zearalenone
-
a
Controlled experiments using pure ZEN
Six cycling trotter mares (age 6–14 years, weight range 530–650 kg) were offered a diet containing 7 mg purified ZEN/day, starting 10 days after ovulation until the subsequent ovulation (approximately 8–10 days). According to the authors, the dose was intended to mimic a ‘natural’ contamination of about 1 mg ZEN/kg feed based on an average consumption of 7 kg feed/day. A control group was not included, but the selected parameters were compared to those recorded in the same animals before treatment. The mycotoxin did not affect the length of the interovulatory intervals and the plasma progesterone profiles as well as the duration of the ovarian follicular and luteal phases or uterine oedema. The authors concluded that the short exposure to toxin concentrations corresponding to 11–13 μg ZEN/kg bw per day did not apparently affect the reproductive parameters of cycling mares (Juhasz et al., 2001). However, the limitations in experimental design did not allow deriving a NOAEL or LOAEL.
-
b
Controlled feeding experiments with naturally contaminated feedstuffs and feed contaminated with fungal material
Three mature mares fed with diets containing naturally contaminated grains averaging 15.0 mg/kg of DON, 0.8 mg/kg of 15‐acetyldeoxynivalenol, 9.7 mg/kg of fusaric acid, and 2.0 mg/kg of ZEN for 21 days. When compared with controls, treated mares exhibited a reduction in feed intake and an increase in serum ɣ‐glutamyltransferase activity at 7 and 14 days but not at 21 days of treatment (Raymond et al., 2003).
Raymond et al. (2005) reported about six mature mares fed with concentrates containing naturally contaminated grains averaging 11.0 mg/kg DON, 0.7 mg/kg 15‐acetyldeoxynivalenol, and 0.8 mg/kg zearalenone for 21 days. Reduced feed intake and weight loss were observed in treated mares in the absence of a reduction in athletic performances. As DON is known to reduce feed intake, it is difficult to decipher the effect of ZEN in this experiment. In a further trial (Aurich et al., 2006), six mature Haflinger mares were fed naturally contaminated oats containing 12 mg DON and 1 mg ZEN per kg. Feeding of the contaminated diet started on the day of ovulation and continued for the whole duration of the oestrous cycle. Previous oestrous cycles in which the experimental mares were offered a non‐contaminated diet served as controls. The treatment did not affect the circulating levels of LH, oestradiol and progesterone, the length of cycle, or the histological picture of uterine biopsies. However, an increased number of growing follicles was noticed during the second part of the cycle. It should be noted that the experimental design suffers from some limitations since it included a recovery period followed by the re‐feeding of the contaminated diet.
Modified forms
No data on the adverse effects of ZEN modified forms in horses were identified.
Conclusion on horses
The only available study performed with the purified mycotoxin (11–13 μg ZEN/kg bw per day for 8–10 days) did not allow to derive a NOAEL or a LOAEL due to limitations of the experimental design.
Rabbits
Zearalenone
In its 2004 opinion, EFSA did not review rabbit studies (EFSA, 2004). A subacute experiment on two groups of 1‐year‐old female rabbits orally exposed for 14 days to 10 or 100 μg purified ZEN/kg bw was reported (Conkova et al., 2001). When compared to control animals (receiving the diluent 1 mL of 8% ethanol per kg bw), the 100 μg/kg bw dose resulted in a significant increase of AST, ALP, GGT and LDH activity. However, no oestrogenic effect was observed in the experimental animals.
In a subsequent study by the same group using the same design, the lowest dose (10 μg/kg bw) was tested for plasma levels of catecholamines at 1, 3, 7 and 14 days (Pastorova et al., 2004). When compared to control animals, ZEN induced an increase in dopamine, noradrenaline and adrenaline which lasted for 3, 3 and 7 days, respectively, until the values returned to the control values. No effect level to characterise the hazard could be derived due to the use of one dose and the transient effect.
Modified forms
Ovariectomised rabbits were fed a high‐cholesterol diet supplemented with 0.01 or 0.05 mg α‐ZAL /kg bw per day for 12 weeks. The aortic intimal atherosclerotic plaque was significantly larger in the cholesterol‐ fed group. α‐ZAL treatments significantly reduced plaque formation and improved serum profile of lipid (TC, TG, HDL‐C and LDL‐C) and lipoprotein (ApoAl and ApoB). α‐ZAL reconciled ovariectomy‐induced uterine atrophy. These findings indicate that α‐ZAL has an important anti‐atherogenic property, analogous to that of oestrogen (Dai et al., 2004).
The CONTAM Panel has been unable to derive any LOAEL or NOAEL for modified forms in rabbits.
Conclusion on rabbits
Very few studies are available for rabbits. One study revealed that the only dose tested of 10 μg/kg bw induced a transient increase of cathecholamines. Another study indicated that 100 μg/kg bw induced no oestrogenic effects. From the data available, the CONTAM Panel has been unable to identify a LOAEL or NOAEL for ZEN or its modified forms in rabbits.
Fish
Zearalenone
Controlled experiments using pure ZEN:
Carp
Juvenile carp (4 tanks, 6 fish/tank for each treatment) were given 0.3, 0.6 or 0.8 mg pure ZEN/kg feed for 4 weeks. Other groups were given the same feed for 4 weeks, followed by a 2‐weeks recovery period where the fish was given uncontaminated feed (Pietsch et al., 2015a). ZEN had no effect on growth. Low concentrations of ZEN and α‐ZEL (reaching 0.22 and 0.16 ng/g dry weight in the medium‐dose group and 0.15 and 0.05 in the high‐dose group) were detected in the fish muscle at the end of the exposure period (see Section 3.1.1.5). The levels decreased to 0.03 and below the LOD after the 2‐weeks recovery period. The relative spleen weight was significantly reduced compared to control only in the low dose. Furthermore, a decrease in monocytes and increase in granulocytes were observed in blood samples from the medium‐ and high‐dose groups compared to control, while no effect was found in blood samples from the low‐dose group. Haemoglobin concentration was significantly reduced at the medium ZEN concentration of 0.6 mg/kg diet. A significant increase in micronuclei occurrence in erythrocytes was found from all groups given ZEN in the feed, indicating a genotoxic potential of ZEN. ZEN did not affect the vitellogenin plasma concentrations in this study. Indications of reactive oxygen species (ROS) formation and DNA breaks were also reported from an in vitro study using cultures of several fish cells lines (Pietsch et al., 2014).
The effects of ZEN on the immune function of carp were studied in carp given the same feed and experimental design as that described above (Pietsch et al., 2015b). Leukocytes from the head kidney and trunk kidney were isolated from the fish at the termination of the experiment and the immune function of the cells was assessed by measuring NO production, the respiratory burst assay, chemiluminescence assay and measurement of arginase activity. Although the results varied between different assays and also between leukocytes from head kidney vs trunk kidney, the authors concluded that the immune response was increased after exposure to low ZEN concentrations in the feed and decreased after exposure to high mycotoxin concentrations. Furthermore, in carp exposed to ZEN through the same experimental design, ZEN affected the lipid peroxidation in liver and gill of exposed fish compared to controls (Pietsch and Junge, 2016). The authors of this study also reported alterations in the carbohydrate metabolism and increased metabolic oxygen demand in carp fed 0.6 or 0.8 mg ZEN/kg feed.
It is known that oestrogens influence the immune system in fish (Iwanowicz and Ottinger, 2009; Burgos‐Aceves et al., 2016), and it cannot be excluded that the effects on the immune system is related to the potential oestrogenic effects of zearalenone.
The Panel does not consider the formation of micronuclei in erythrocytes as a relevant endpoint for derivation of a reference point of hazard characterisation for fish feed. The altered relative weight of the spleen was not dose‐dependent as the effect was only observed at the lowest dose.
The Panel therefore considers 0.3 mg/kg feed as a NOAEL, based on alterations in number of monocytes and granulocytes of immune cells and lipid peroxidation in liver and gill and alteration in metabolism observed at 0.6 and 0.8 mg/kg feed.
Red tilapia
Red tilapia (Oreochromis niloticus × Oreochromis mossambicus) given a feed based on wheat naturally infected by Fusarium containing 0.01–0.98 mg ZEN/kg feed and 0.07–1.15 mg DON/kg feed had a lower feed intake, growth rate and feed efficacy compared to control fish (Tola et al., 2015), while no other effects were observed. The feed was co‐contaminated with DON, and probably other Fusarium toxins, and the observed effects are more likely (at least partly) attributed to DON than ZEN. The study is consequently not suitable for the establishment of a effect level to characterise the hazard of ZEN in fish feed.
Rainbow trout
ZEN (1.81 mg/kg feed) given to premarked size rainbow trout (250 g) for 37 or 71 days did not affect fish growth (Woźny et al., 2015). Histological examinations of liver cross‐sections revealed necrotic areas, disorders of the polygonal hepatocytes, cytoplasmic vacuolisation and macrophage aggregation. ZEN did not alter the plasma biochemical markers. The ovarian development was more advanced in the exposed group compared to controls, but there vitellogenin mRNA was not affected.
The CONTAM Panel concluded that 1.8 mg/kg feed could be considered as a LOAEL in rainbow trout in the latter study as this feed concentrations affected liver histology and ovarian development.
Salmon
No studies with oral administration could be found.
Modified forms
Rainbow trout
Graded levels of Zeranol (0, 2.5, 5, 10 and 20 mg/kg feed) were mixed into the diet given to rainbow trout for 21 days (Keles et al., 2002). Increased length was observed in fish from 5 mg/kg feed, and increased weight from 10 mg/kg feed. Zeranol also increased the phagocytic and bactericidal activities of the leucocytes. The study indicates that zeranol has an anabolic effect in fish and in addition it may stimulate the non‐specific immune system. No adverse effect was observed in the study.
Salmon
Single i.p. injections of 1 and 10 mg/kg of ZEN and α‐ZEL in juvenile salmon induced vitellogenin and zona radiate proteins while β‐ZEL did not cause any significant change in these parameters. The authors concluded that ZEN and α‐ZEL possess oestrogenic potencies that are approximately 50% compared to that of E2, and the order of oestrogenic molar potency is α‐ZEL > ZEN > β‐ZEL (Arukwe et al., 1999).
Only one study with oral exposure to α‐ZAL in fish was available in which no adverse effect was observed. No data on the adverse effects of oral exposure to other ZEN modified forms in fish were identified.
Conclusion on fish
Feeding studies with graded levels applied to fish are only available for carp. In this species, ZEN did not induce vitellogenin synthesis, which is the classical sign of hyperoestrogenism in fish. A NOAEL of 0.3 mg/kg feed to correspond to 9 μg/kg bw per day could be identified based on a decreased the number of monocytes, an increased the number of granulocytes and a increased lipid peroxidation. No NOAEL/LOAEL could be derived for the modified forms of ZEN.
3.1.3.2. Adverse effects in companion animals – Evaluation of different types of studies
Cats
Zearalenone
No information on the adverse effects of ZEN in cats could be identified in the available literature.
Modified forms
No data on the adverse effects of ZEN modified forms in cats were identified.
Dogs
Zearalenone
The effects of the repeated dietary administration of ZEN on reproductive parameters in prepubertal female dogs have been the subject of a recent review (Gajęcka et al., 2015). Most of the available information is derived from a set of studies performed by Gajecka and collaborators in female dogs while no specific reports concerning male dogs could be identified.
ZEN was administered daily at doses equivalent to 0 (control), 25 or 50 μg/kg bw for 100 days corresponding to about 1.7 or 3.5 mg ZEN/kg diet assuming a standard body weight of 25 kg and 0.36 kg daily feed consumption (see Appendix D). Each group consisted of three 1‐ to 3‐year‐old‐bitches of mixed breed (body weight not reported) in the anoestrous phase which underwent ovariohysterectomy at the end of the treatment. Uterine specimens from both treated groups revealed degenerative lesions of myometrium and endometrium, including enlargement and atrophy of the uterine glands with oedema and extravasation. As detected by the number of PCNA‐positive cells, the proliferative activity of the uterine mucosal cells was much lower in treated bitches compared with the control animals (Gajęcka et al., 2007, 2008a). The ultrastructural changes (Gajęcka et al., 2008a) were mostly confined to the 50 μg/kg bw group with an increase in the endoplasmic reticulum in the granulosa cells along with mitochondrial alterations. After 100 days of administration, treated dogs also exhibited a statistically significant decrease in mean cell volume (MCV) (both groups) as well as in mean cell haemoglobin (MCH) and Hb (25 μg/kg bw) along with an unchanged erythrocyte number; WBC number was increased in both treatment groups, but reached the statistical significance only in animals exposed to the lower ZEN dose. Treated animals had also a dose‐related statistically significant rise in bilirubin (up to 66% at 50 μg/kg bw) and a statistically significant decrease in both ALT and AST activities (Gajęcka et al., 2007, 2008a). An increase in progesterone (P4) serum levels was noticed in both treatment groups (25‐ or 50 μg ZEN/kg bw)12 with non consistent effects on 17β‐estradiol (E2) serum levels (Gajęcka et al., 2008b).
A second set of studies of the same group of investigators involved 30 immature female Beagle dogs aged approximately 70 days (average weight 8 ± 0.95 kg). The animals were daily dosed by the oral route with 0 (control), 50 or 75 μg ZEN/kg bw for 42 days corresponding to about 2.7 or 4 mg ZEN/kg diet. One study was aimed at investigating the ZEN metabolite blood profile by LC–MS and the effects on circulating E2 and P4 levels by RIA. Throughout the experiment, plasma ZEN levels were higher13 in the 50 μg/kg bw group than in the 75 μg/kg bw and lower than those of α‐ZEL and β‐ZEL, α‐ZEL concentrations were consistently higher than those of β‐ZEL in both treated groups. A rise (two‐ to threefold over the other groups) in the plasma P4 levels occurred in the 75 μg/kg bw dosed dogs only; statistically significant values (p < 0.01) were reached at week 2, 3 and 4 along with the plasma peaks of α‐ZEL. Both treated groups displayed 3 to 4‐ fold higher E2 plasma levels, particularly at weeks 5 and 6 (Gajęcka, 2013). Histological and ultrastructural lesions related to hyperoestrogenism consisting in degeneration and atrophy of ovarian tissues and granulosa cells and pictures of apoptotic cells along with an increase in mitochondrial calcium storage cells were more pronounced in animals exposed to 75 μg ZEN/kg bw (Gajęcka and Przybylska‐Gornowicz, 2012). A further report was concerned with the effects on uterine enzymes involved in either steroidogenesis (CYPscc) or in the reductive biotransformation of the mycotoxin (3β‐HSD) (Gajęcka et al., 2011b). The treatment did not cause statistically significant changes in CYPscc gene expression but elicited an over four‐fold increase in 3β‐HSD mRNA, although limited to animals receiving the higher ZEN dosage. This finding was consistent with the increase in plasma β‐ZEL reported in another paper (Gajęcka et al., 2013). Immunohistochemical investigations performed on ovarian specimens collected at the end of ZEN administration revealed no positive staining for ERα in all experimental animals; a dose‐related decline in ERβ‐related immunostaining was instead observed in oocyte nuclei, granulosa cells and interstitial cells from both treated groups when compared to control animals (Gajęcka, 2013). Finally, a further report (Stopa et al., 2014) addressed the morphometric and histologic changes in bitches exposed as described above (0, 50 or 75 μg ZEN/kg bw for 42 days). Compared with the untreated bitches, there was a dose‐related increase in both the weight of the uterine horns and the length of the body uterus. Significant dilation and congestion of capillary vessels were present in both the uterine horns and body, irrespective of the ZEN dose. Cellular hyperplasia was found in the uterus body only and tended to be of lower degree in animals exposed to the lower ZEN dosage. The authors interpreted the latter results as a hormetic response. No data on the effects on male fertility were identified.
Based on the histological, histochemical, haematological, and blood biochemical changes, a LOAEL of 25 μg ZEN/kg bw per day could be established, corresponding to about 1.7 mg ZEN/kg diet.
Modified forms
No data on the adverse effects of ZEN modified forms in dogs were identified.
Appendix H presents an overview about the available relevant toxicity studies with ZEN in female dogs.
Conclusion on companion animals
No information could be found among the adverse effects of ZEN or its modified forms on cats. In keeping with the higher formation of α‐ZEL compared to β‐ZEL, the dog appears to be one of the most sensitive species to ZEN. Despite some limitations in the experimental design, the available published studies revealed that already at a dose of 25 μg ZEN/kg bitches displayed uterine and ovarian gross and microscopic changes as well as changes in the haematological and blood biochemical profile. Based on these results, the CONTAM Panel estimated a LOAEL of 25 μg ZEN/kg bw per day for dogs.
3.1.3.3. Adverse effects in other animals ‐ Evaluation of different types of studies
Farmed mink
Zearalenone
In a study performed by Bursian et al. (1992), two experiments were carried out. In the first one, ovariectomised female mink were offered a diet containing 0 (n = 4), 10 (n = 5) or 20 mg ZEN/kg feed (n = 5) for 24 days (corresponding to about 1,000 or 2,000 μg ZEN/kg bw) and then euthanised. Vulvar swelling did not differ between controls and ZEN‐treated animals, whereas ZEN exposure caused a two‐ (10 mg/kg) to threefold (20 mg/kg) increase in uterine weight. In the second one, intact female mink were dietary exposed to 0 (n = 6) or 20 mg ZEN/kg diet (n = 6) for 115–135 days (until whelping). ZEN treatment did not affect the number of individuals that whelped, but caused an increase in the gestation length along with a marked reduction in the number of living pups (3 vs 36 in the control group). Other studies (Yang et al., 1995; Yamini et al., 1997) administering diets containing ZEN at 20 mg/kg point to similar adverse effects on reproduction.
Modified forms
No data on the adverse effects of ZEN modified forms in fur animals were identified.
Conclusion on farmed mink
The available studies concerning the effects of dietary ZEN on reproduction in female mink exhibited overt oestrogenic effects at the lowest tested dose but were performed at very high dosages only 10 or 20 mg/kg feed (corresponding to about 1,000 or 2,000 μg ZEN/kg bw per day) and therefore were not used to derive a reference point for adverse effects for famed mink.
3.1.3.4. Conclusions on toxic effects in farm and companion animals
There are only rather limited data available on oral toxicity in livestock species, horses, fish and dogs, especially studies using purified toxins. Only a few of these are suitable for the derivation of NOAELs and LOAELs. Table 4 summarises the adverse effects observed in ruminants, pigs, poultry, fish and dogs. No suitable data to derive NOAEL or LOAEL were available for cattle, goats, horse, rabbits, cats and fur animals.
Table 4.
Relevant zearalenone toxicity studies with ruminants, pigs, poultry, fish and dogs to possibly set NOAELs/LOAELs for zearalenone
| Species | No observed adverse effect levels (NOAEL) | Lowest observed adverse effect level (LOAEL) | Adverse effects observed (Type of study) | References | Comments |
|---|---|---|---|---|---|
| Sheep |
28 μg/kg bw per day 1.5 mg kg feed |
56 μg/kg bw per day 3 mg/kg feed |
Depressed ovulation rates and lower lambing percentages (Purified ZEN) | Smith et al. (1990) | |
| Piglets |
10.4 μg/kg bw per day (NOEL) 0.22 mg/kg feed |
17.6 μg/kg bw per day (LOEL) 0.42 mg/kg feed |
Swollen and reddened vulva, increased uterus weight (Naturally contaminated feed) | Döll et al. (2003) |
Co‐contamination with DON and other mycotoxins. In the same range of other studies. |
| Gilts |
40 μg/kg bw per day 1 mg/kg feed |
200 μg/kg bw per day 5 mg/kg feed |
Prolonged cycling | Edwards et al. (1987a) | |
| Chickens for fattening |
7,500 μg/kg bw per day 50 mg/kg diet feed |
30,000 μg/kg bw per day 200 mg/kg feed |
Lymphocyte count decreased (Purified ZEN) | Chi et al. (1980a) | Exposure estimated based on published data and assumptions |
| Turkeys |
100 mg/kg feed 9,100 μg/kg bw per day |
200 mg/kg feed 19,000 μg/kg bw per day |
Vent swelling | Allen et al. (1981) | |
| Carp |
0.3 mg kg/feed 9 μg/kg bw per day |
0.6 mg/kg feed 18 μg/kg bw per day |
Decreased number of monocytes, increased number of granulocytes. Lipid peroxidation in liver and gill. Metabolic alterations activation (Purified ZEN) |
Pietsch et al. (2015a) | |
| Dogs | No data | 25 μg/kg bw per day | Degenerative lesions of myometrium and endometrium, enlargement and atrophy of the uterine glands; decrease in MCV, MCH and Hb, increase in WBC, bilirubin and P4 levels, decrease in AST and ALT | Gajęcka et al. (2007, 2008a,b) |
Several reports based on two set of studies. Reduced number of dogs (1 study). Experimental design (e.g. unknown animal weights); dietary concentration for LOAEL based on assumptions. |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; Hb: haemoglobin; LO(A)EL: lowest observed (adverse) effect level; NO(A)EL: no observed (adverse) effect level; MCH: mean cell haemoglobin; MCV: mean cell volume; WBC: white blood cells; ZEN: zearalenone.
The main adverse effects seen in pigs, poultry, fish, dogs, and mink are related to reproductive parameters. They include depressed ovulation rates and lower lambing percentages (sheep), prolonged cycle (pig), increased uterus weight (mink and pig) and red and swollen vulva (pig), vent swelling (turkeys), uterine and ovarian gross and microscopic changes as well as changes in P4 and E2 blood levels (dogs). Of note, in chicken and carp the adverse effect was not hyperoestrogenism. For chicken, it was decreased lymphocyte number and for carp it was decreased number of monocytes, increased number of granulocytes and increased lipid peroxidation in liver and gill and altered the carbohydrate metabolism.
Pigs and dogs were sensitive to ZEN as evidenced by a low LOEL/LOAEL (17.6 and 25 μg/kg bw per day, respectively) and the low NOEL of 10.4 μg/kg bw per day for pigs. Regarding fish, feeding studies with graded level of ZEN were only available for carp for which a NOAEL of 9 μg/kg bw per day was derived.
A NOAEL of 28 μg ZEN/kg bw per day was calculated for sheep. Although neither a LOAEL nor a NOAEL could be derived, cattle appear quite resistant to the adverse effects of ZEN, in line with the prevalent biotransformation to the less oestrogenic metabolite β‐ZEL. Among the examined species, poultry are the least sensitive, with NOAELs for chickens and turkey of 7,500 and 9,100 μg/kg bw per day, respectively.
It was not possible to derive a reference points for horses. However, in view of the only available study performed with the purified mycotoxin and the toxicokinetic data, this animal species can be considered as relatively resistant.
Very limited studies are available for rabbit. No NOEAL/LOAEL could be identified due to the use of one dose and the transient effect.
In the case of mink, only very high concentrations were tested; no LOAEL/NOAEL could be identified.
Very few experiments investigated the adverse effect of the modified form of ZEN on livestock species, horses, fish and dogs and none of them was suitable to derive a NOAEL or LOAEL.
3.2. Feed occurrence data
3.2.1. Feed Occurrence data submitted to EFSA
All the data related to the presence of zearalenone and its modified forms (total ZEN, α‐ZAL, β‐ZAL, α‐ZEL and β‐ZEL and ZAN) in feed were considered.
A total of 17,706 analytical results for ZEN (n = 14,970; 84%), α‐ZAL (n = 882; 5%), and ZAN (n = 664; 4%), β‐ZAL (n = 882; 5%), α‐ZEL (n = 154; 1%) and β‐ZEL (n = 154; 1%) from 25 European countries were available for the assessment.
The major contributing countries were France (24%), the UK (22%) and Bulgaria (12%) (Table 5). Occurrence data on ZEN were provided by all countries, whereas data on modified forms were provided by only three countries, namely Belgium (ZAN), Bulgaria (α‐ZAL, β‐ZAL) and the Netherlands (α‐ZAL, β‐ZAL, ZAN, α‐ZEL, and β‐ZEL). It should be noted that the origin of the samples was not always the European country reporting the data, i.e. the data set also contained samples originating from North and South America, Africa, Asia and Australia.
Table 5.
Distribution of analytical results for ZEN and its modified forms in feed per sampling country (2001–2015)
| Country | ZEN and its modified forms (N) | Total | % of total | |||||
|---|---|---|---|---|---|---|---|---|
| α‐ZAL | β‐ZAL | ZAN | α‐ZEL | β‐ZEL | ZEN | |||
| Austria | . | . | . | . | . | 132 | 132 | < 1 |
| Belgium | . | . | 503 | . | . | 368 | 871 | 5 |
| Bulgaria | 728 | 728 | . | . | . | 739 | 2,195 | 12 |
| Croatia | . | . | . | . | . | 34 | 34 | < 1 |
| Cyprus | . | . | . | . | . | 32 | 32 | < 1 |
| Czech Republic | . | . | . | . | . | 962 | 962 | 5 |
| Denmark | . | . | . | . | . | 5 | 5 | < 1 |
| Estonia | . | . | 4 | . | . | 29 | 33 | < 1 |
| Finland | . | . | . | . | . | 3 | 3 | < 1 |
| France | . | . | . | . | . | 4,327 | 4,327 | 24 |
| Germany | . | . | . | . | . | 1,150 | 1,150 | 7 |
| Hungary | . | . | . | . | . | 308 | 308 | 2 |
| Italy | . | . | . | . | . | 540 | 540 | 3 |
| Lithuania | . | . | . | . | . | 2 | 2 | < 1 |
| Luxembourg | . | . | . | . | . | 241 | 241 | 1 |
| Netherlands | 154 | 154 | 154 | 154 | 154 | 154 | 924 | 5 |
| Norway | . | . | . | . | . | 14 | 14 | < 1 |
| Poland | . | . | . | . | . | 5 | 5 | < 1 |
| Portugal | . | . | . | . | . | 546 | 546 | 3 |
| Romania | . | . | . | . | . | 11 | 11 | < 1 |
| Slovakia | . | . | . | . | . | 488 | 488 | 3 |
| Slovenia | . | . | . | . | . | 225 | 225 | 1 |
| Spain | . | . | 3 | . | . | 332 | 335 | 2 |
| Sweden | . | . | . | . | . | 385 | 385 | 2 |
| United Kingdom | . | . | . | . | . | 3,938 | 3,938 | 22 |
| Total | 882 | 882 | 664 | 154 | 154 | 14,970 | 17,706 | 100 |
N: number of samples; ZAL: zearalanol; ZAN: zearalanone; ZEL: zearalenol; ZEN: zearalenone.
The distribution of the occurrence data over the sampling years is presented in Figure 7. Data on ZEN were sampled throughout 2001–2015, whereas occurrence data on the modified forms were only available from sampling year 2011 onward.
Figure 7.
- ZAL: zearalanol; ZAN: zearalanone; ZEL: zearalenol; ZEN: zearalenone.
Results were reported on whole weight (82% samples) or on 88% DM (18% of samples). For consistency in the assessment, the latter were converted to values expressed on a whole‐weight basis.
Analytical methods
Only occurrence data with information on the analytical method and on LOD/LOQ levels that fulfilled the inclusion criteria for the present analysis were included. The CONTAM Panel considered only quantitative methods able to return a confirmation of the analyte identification and with an adequate sensitivity (Table 6). For this reason, the measurements obtained by ELISA, EIA and TLC methods, as well as missing information were not considered reliable. By this approach, about 37% of feed data were excluded. Mass spectrometry‐based methods (Group 1, 57%) were mostly used, followed by chromatographic methods (Group 2, 23%). Data generated by HPTLC methods have been considered for the occurrence, since this techniques is quantitative and well‐established throughout Europe, especially for the determination of contaminants in feed.
Table 6.
Distribution of analytical results by analytical method
| Analytical method groupa | Abbreviation | N | % | |||||
|---|---|---|---|---|---|---|---|---|
| α‐ZAL | β‐ZAL | ZAN | α‐ZEL | β‐ZEL | ZEN | |||
| Group 1 | 882 | 882 | 158 | 154 | 154 | 7,867 | 10,097 | 57.0 |
| Group 2 | – | – | 506 | – | – | 3,638 | 4,144 | 23.4 |
| Group 3 | – | – | – | – | – | 21 | 21 | 0.1 |
| Group 4 | – | – | – | – | – | 3,444 | 3,444 | 19.5 |
| Total | 882 | 882 | 664 | 154 | 154 | 14,970 | 17,706 | 100 |
ZAL: zearalanol; ZAN: zearalanone; ZEL: zearalenol; ZEN: zearalenone.
GROUP 1: LC–MS/MS, LC‐MS, LC‐MS quadrupole ⟶ mass spectrometry based methods. GROUP 2: HPLC‐FLD, HPLC‐UV, HPLC with standard detection methods, HPLC‐Electrical Conductivity Detector, HPLC‐CF, AAS, ETAAS (GFAAS) ⟶ chromatographic methods with spectroscopic detection. GROUP 3: GC with standard detection methods, GC‐MS, GC‐HRMS, GC‐MS‐MS. GROUP 4: High ‐Performance TLC methods, HPTLC/TLC.
The data set includes 61% of left‐censored data (results below the LOD/LOQ), of which 13% below LOD and 47% between LOD and LOQ. The mean and range LOQs (expressed in μg/kg) varied with the compound: ZEN (11, 0.03–200), α‐ZAL (25, 20–50), β‐ZAL (25, 20–50), ZAN (20, 10–100), α‐ZEL (50, 40–50) and β‐ZEL (10, 10).
Occurrence data on feed by feed group
Table 7 shows the feed samples classified according to the catalogue of feed materials described in Commission Regulation 68/2013. Molar RPFs of the modified forms relative to ZEN were applied to occurrence levels of the respective ZEN metabolites according to EFSA CONTAM Panel (2016) (see section 2.5 1.1).
Table 7.
Statistical description of the concentrations (μg/kg whole‐weight) of zearalenone and its modified forms in feed samples classified according to the Catalogue of feed materials specified in Commission Regulation (EU) No 68/201314 corrected for molar RPFs
| Feed category | Abbreviation | RPF* | N | % Left‐Censored | Mean (μg/kg)a , b | Median (μg/kg)a , b | P75 (μg/kg)a , b | P95 (μg/kg)a , b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | LB | UB | |||||||
| Cereal grains, their products and by‐products | Barley | Barley, unspecified | α‐ZAL | 4 | 26 | 100 | 0 | 167 | 0 | 200 | 0 | 200 | – | – |
| β‐ZAL | 2 | 26 | 100 | 0 | 84 | 0 | 100 | 0 | 100 | – | – | |||
| ZAN | 1.5 | 38 | 82 | 9 | 55 | 0 | 75 | 0 | 75 | – | – | |||
| α‐ZEL | 60 | 19 | 100 | 0 | 3,000 | 0 | 3,000 | 0 | 3,000 | – | – | |||
| β‐ZEL | 0.2 | 19 | 100 | 0 | 2 | 0 | 2 | 0 | 2 | – | – | |||
| ZEN | 1 | 1,586 | 71 | 11 | 15 | 0 | 5 | 5 | 9 | 20 | 35 | |||
| Barley bran | ZEN | 1 | 2 | 0 | 4,391 | 4,391 | 4,391 | 4,391 | 8,750 | 8,750 | – | – | ||
| Barley middlings | α‐ZAL | 4 | 1 | 100 | 0 | 79 | 0 | 79 | 0 | 79 | – | – | ||
| β‐ZAL | 2 | 1 | 100 | 0 | 39 | 0 | 39 | 0 | 39 | – | – | |||
| ZAN | 1.5 | 2 | 100 | 0 | 15 | 0 | 15 | 0 | 15 | – | – | |||
| ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Barley protein feed | α‐ZAL | 4 | 1 | 100 | 0 | 79 | 0 | 79 | 0 | 79 | – | – | ||
| β‐ZAL | 2 | 1 | 100 | 0 | 39 | 0 | 39 | 0 | 39 | – | – | |||
| ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Malt | ZEN | 1 | 2 | 100 | 0 | 24 | 0 | 24 | 0 | 25 | – | – | ||
| Malt rootlets | ZAN | 1.5 | 2 | 50 | 31 | 38 | 31 | 38 | 61 | 61 | – | – | ||
| ZEN | 1 | 4 | 0 | 13 | 13 | 13 | 13 | 20 | 20 | – | – | |||
| Buckwheat | Buckwheat, unspecified | α‐ZAL | 4 | 1 | 100 | 0 | 200 | 0 | 200 | 0 | 200 | – | – | |
| β‐ZAL | 2 | 1 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | – | – | |||
| ZAN | 1.5 | 1 | 100 | 0 | 75 | 0 | 75 | 0 | 75 | – | – | |||
| α‐ZEL | 60 | 1 | 100 | 0 | 3,000 | 0 | 3,000 | 0 | 3,000 | – | – | |||
| β‐ZEL | 0.2 | 1 | 100 | 0 | 2 | 0 | 2 | 0 | 2 | – | – | |||
| ZEN | 1 | 4 | 100 | 0 | 21 | 0 | 15 | 0 | 35 | – | – | |||
| Cereal grains, their products and by‐products | Cereal grains, their products and by‐products | α‐ZAL | 4 | 12 | 100 | 0 | 77 | 0 | 77 | 0 | 77 | – | – | |
| β‐ZAL | 2 | 12 | 92 | 4 | 39 | 0 | 39 | 0 | 39 | – | – | |||
| ZAN | 1.5 | 49 | 76 | 20 | 31 | 0 | 14 | 0 | 15 | – | – | |||
| ZEN | 1 | 164 | 79 | 28 | 103 | 0 | 50 | 0 | 200 | 82 | 200 | |||
| Grains as crops | Other grains | ZEN | 1 | 1 | 100 | 0 | 3 | 0 | 3 | 0 | 3 | – | – | |
| Maize and Corn | Maize screenings | ZEN | 1 | 1 | 0 | 18 | 18 | 18 | 18 | 18 | 18 | – | – | |
| Maize and Corn | α‐ZAL | 4 | 114 | 100 | 0 | 73 | 0 | 73 | 0 | 73 | 0 | 73 | ||
| β‐ZAL | 2 | 114 | 100 | 0 | 37 | 0 | 37 | 0 | 37 | 0 | 37 | |||
| ZAN | 1.5 | 35 | 43 | 101 | 107 | 22 | 22 | 77 | 77 | – | – | |||
| ZEN | 1 | 2,047 | 31 | 102 | 105 | 24 | 25 | 97 | 98 | 481 | 481 | |||
| Millet | Millet | α‐ZAL | 4 | 1 | 100 | 0 | 200 | 0 | 200 | 0 | 200 | – | – | |
| β‐ZAL | 2 | 1 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | – | – | |||
| ZAN | 1.5 | 6 | 83 | 8 | 30 | 0 | 14 | 0 | 48 | – | – | |||
| α‐ZEL | 60 | 1 | 100 | 0 | 3,000 | 0 | 3,000 | 0 | 3,000 | – | – | |||
| β‐ZEL | 0.2 | 1 | 100 | 0 | 2 | 0 | 2 | 0 | 2 | – | – | |||
| ZEN | 1 | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| Mixed grains | Brewers’ grains | ZEN | 1 | 3 | 100 | 0 | 15 | 0 | 10 | 0 | 25 | – | – | |
| Distillers’ dark grains; [Distillers’ dried grains and solubles] | ZAN | 1.5 | 24 | 8 | 96 | 97 | 74 | 74 | 129 | 129 | – | – | ||
| ZEN | 1 | 1 | 0 | 2,400 | 2,400 | 2,400 | 2,400 | 2,400 | 2,400 | – | – | |||
| Distillers’ dried grains | ZEN | 1 | 10 | 30 | 14 | 18 | 19 | 19 | 19 | 20 | – | – | ||
| Grain flour | ZEN | 1 | 1 | 0 | 11 | 11 | 11 | 11 | 11 | 11 | – | – | ||
| Mixed grains, unspecified | ZEN | 1 | 32 | 81 | 4 | 13 | 0 | 10 | 0 | 11 | – | – | ||
| Oats | Oat feed | ZEN | 1 | 72 | 96 | 1 | 6 | 0 | 5 | 0 | 5 | 0 | 7 | |
| Oat flour | ZEN | 1 | 1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | ||
| Oat groats | α‐ZAL | 4 | 1 | 100 | 0 | 80 | 0 | 80 | 0 | 80 | – | – | ||
| β‐ZAL | 2 | 1 | 100 | 0 | 40 | 0 | 40 | 0 | 40 | – | – | |||
| ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Oats, unspecified | α‐ZAL | 4 | 14 | 100 | 0 | 174 | 0 | 200 | 0 | 200 | – | – | ||
| β‐ZAL | 2 | 14 | 100 | 0 | 87 | 0 | 100 | 0 | 100 | – | – | |||
| ZAN | 1.5 | 13 | 92 | 3 | 67 | 0 | 75 | 0 | 75 | – | – | |||
| α‐ZEL | 60 | 11 | 100 | 0 | 3,000 | 0 | 3,000 | 0 | 3,000 | – | – | |||
| β‐ZEL | 0.2 | 11 | 100 | 0 | 2 | 0 | 2 | 0 | 2 | – | – | |||
| ZEN | 1 | 779 | 78 | 10 | 14 | 0 | 3 | 0 | 8 | 48 | 53 | |||
| Rice, broken | Rice bran | ZEN | 1 | 1 | 0 | 128 | 128 | 128 | 128 | 128 | 128 | – | – | |
| Rice middlings | ZAN | 1.5 | 1 | 100 | 0 | 15 | 0 | 15 | 0 | 15 | – | – | ||
| Rice, broken, unspecified | α‐ZAL | 4 | 108 | 100 | 0 | 200 | 0 | 200 | 0 | 200 | 0 | 200 | ||
| β‐ZAL | 2 | 108 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | |||
| ZAN | 1.5 | 108 | 100 | 0 | 75 | 0 | 75 | 0 | 75 | 0 | 75 | |||
| α‐ZEL | 60 | 108 | 100 | 0 | 3,000 | 0 | 3,000 | 0 | 3,000 | 0 | 3,000 | |||
| β‐ZEL | 0.2 | 108 | 100 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | |||
| ZEN | 1 | 152 | 98 | 0 | 37 | 0 | 50 | 0 | 50 | 0 | 50 | |||
| Rice, milled | α‐ZAL | 4 | 1 | 100 | 0 | 82 | 0 | 82 | 0 | 82 | – | – | ||
| β‐ZAL | 2 | 1 | 100 | 0 | 41 | 0 | 41 | 0 | 41 | – | – | |||
| ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Rye | Rye, unspecified | α‐ZAL | 4 | 9 | 100 | 0 | 200 | 0 | 200 | 0 | 200 | – | – | |
| β‐ZAL | 2 | 9 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | – | – | |||
| ZAN | 1.5 | 9 | 100 | 0 | 75 | 0 | 75 | 0 | 75 | – | – | |||
| α‐ZEL | 60 | 9 | 100 | 0 | 3,000 | 0 | 3,000 | 0 | 3,000 | – | – | |||
| β‐ZEL | 0.2 | 9 | 100 | 0 | 2 | 0 | 2 | 0 | 2 | – | – | |||
| ZEN | 1 | 312 | 90 | 2 | 9 | 0 | 5 | 0 | 5 | 7 | 26 | |||
| Rye bran | ZEN | 1 | 1 | 0 | 110 | 110 | 110 | 110 | 110 | 110 | – | – | ||
| Rye middlings | ZAN | 1.5 | 1 | 100 | 0 | 15 | 0 | 15 | 0 | 15 | – | – | ||
| Sorghum; [Milo] | Sorghum; [Milo] | ZAN | 1.5 | 2 | 50 | 24 | 31 | 24 | 31 | 48 | 48 | – | – | |
| ZEN | 1 | 3 | 33 | 151 | 152 | 14 | 14 | 440 | 440 | – | – | |||
| Spelt | Spelt | ZAN | 1.5 | 4 | 75 | 9 | 20 | 0 | 15 | 18 | 26 | – | – | |
| ZEN | 1 | 33 | 85 | 3 | 8 | 0 | 5 | 0 | 10 | – | – | |||
| Triticale | Triticale | α‐ZAL | 4 | 2 | 100 | 0 | 79 | 0 | 79 | 0 | 79 | – | – | |
| β‐ZAL | 2 | 2 | 100 | 0 | 40 | 0 | 40 | 0 | 40 | – | – | |||
| ZEN | 1 | 67 | 72 | 17 | 21 | 0 | 6 | 8 | 13 | 22 | 22 | |||
| Wheat | Malting wheat screenings | ZEN | 1 | 2 | 100 | 0 | 25 | 0 | 25 | 0 | 25 | – | – | |
| Vital wheat gluten | ZEN | 1 | 5 | 60 | 273 | 279 | 0 | 10 | 15 | 15 | – | – | ||
| Wheat, unspecified | α‐ZAL | 4 | 12 | 100 | 0 | 79 | 0 | 79 | 0 | 79 | – | – | ||
| β‐ZAL | 2 | 12 | 100 | 0 | 40 | 0 | 40 | 0 | 40 | – | – | |||
| ZAN | 1.5 | 40 | 53 | 66 | 74 | 0 | 16 | 37 | 37 | – | – | |||
| ZEN | 1 | 6,177 | 55 | 21 | 24 | 0 | 5 | 8 | 12 | 81 | 82 | |||
| Wheat bran | α‐ZAL | 4 | 89 | 100 | 0 | 79 | 0 | 79 | 0 | 79 | 0 | 79 | ||
| β‐ZAL | 2 | 89 | 100 | 0 | 40 | 0 | 40 | 0 | 40 | 0 | 40 | |||
| ZAN | 1.5 | 6 | 67 | 14 | 24 | 0 | 15 | 37 | 37 | – | – | |||
| ZEN | 1 | 111 | 86 | 4 | 12 | 0 | 10 | 0 | 10 | – | – | |||
| Wheat feed | α‐ZAL | 4 | 65 | 98 | 17 | 95 | 0 | 79 | 0 | 79 | 0 | 79 | ||
| β‐ZAL | 2 | 65 | 98 | 4 | 43 | 0 | 40 | 0 | 40 | 0 | 40 | |||
| ZAN | 1.5 | 6 | 50 | 15 | 23 | 8 | 15 | 37 | 37 | – | – | |||
| ZEN | 1 | 99 | 84 | 43 | 51 | 0 | 10 | 0 | 10 | 40 | 40 | |||
| Wheat germ | ZAN | 1 | 100 | 0 | 15 | 0 | 15 | 0 | 15 | – | – | |||
| Wheat gluten feed | ZAN | 1.5 | 2 | 50 | 8 | 15 | 8 | 15 | 16 | 16 | – | – | ||
| ZEN | 1 | 3 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Wheat middlings | ZAN | 3 | 67 | 25 | 35 | 0 | 15 | 74 | 74 | – | – | |||
| ZEN | 1 | 102 | 50 | 18 | 22 | 3 | 10 | 33 | 34 | 65 | 65 | |||
| Compound feed | Complementary/complete feed | Breeding pigs | α‐ZAL | 4 | 2 | 100 | 0 | 44 | 0 | 44 | 0 | 54 | – | – |
| β‐ZAL | 2 | 2 | 100 | 0 | 22 | 0 | 22 | 0 | 27 | – | – | |||
| ZEN | 1 | 97 | 48 | 32 | 37 | 3 | 15 | 25 | 30 | 231 | 231 | |||
| Calves | α‐ZAL | 4 | 4 | 100 | 0 | 54 | 0 | 54 | 0 | 54 | – | – | ||
| β‐ZAL | 2 | 4 | 100 | 0 | 27 | 0 | 27 | 0 | 27 | – | – | |||
| ZEN | 1 | 12 | 42 | 100 | 105 | 17 | 25 | 90 | 90 | – | – | |||
| Complementary feed (incomplete diet) | α‐ZAL | 4 | 1 | 100 | 0 | 34 | 0 | 34 | 0 | 34 | – | – | ||
| β‐ZAL | 2 | 1 | 100 | 0 | 17 | 0 | 17 | 0 | 17 | – | – | |||
| ZAN | 1.5 | 131 | 22 | 29 | 30 | 14 | 14 | 33 | 33 | 113 | 113 | |||
| ZEN | 1 | 248 | 64 | 44 | 66 | 0 | 49 | 1 | 50 | 264 | 264 | |||
| Complete feed | ZAN | 1.5 | 172 | 27 | 39 | 41 | 21 | 21 | 43 | 43 | 126 | 126 | ||
| ZEN | 1 | 224 | 57 | 57 | 81 | 0 | 50 | 3 | 50 | 335 | 335 | |||
| Dairy cows | α‐ZAL | 4 | 53 | 100 | 0 | 53 | 0 | 54 | 0 | 54 | – | – | ||
| β‐ZAL | 2 | 53 | 100 | 0 | 26 | 0 | 27 | 0 | 27 | – | – | |||
| ZEN | 1 | 379 | 48 | 14 | 18 | 0 | 7 | 16 | 20 | 56 | 56 | |||
| Fattening calves | ZEN | 1 | 127 | 39 | 28 | 32 | 15 | 16 | 35 | 35 | 86 | 86 | ||
| Fattening cattle | α‐ZAL | 4 | 15 | 100 | 0 | 51 | 0 | 54 | 0 | 54 | – | – | ||
| β‐ZAL | 2 | 15 | 100 | 0 | 26 | 0 | 27 | 0 | 27 | – | – | |||
| ZEN | 1 | 72 | 69 | 15 | 21 | 0 | 8 | 2 | 13 | 74 | 74 | |||
| Fattening chickens | ZEN | 1 | 129 | 37 | 31 | 36 | 7 | 18 | 31 | 34 | 126 | 126 | ||
| Fattening ducks/Complete feed | ZEN | 1 | 7 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Fattening geese/Complementary feed | ZEN | 1 | 1 | 100 | 0 | 30 | 0 | 30 | 0 | 30 | – | – | ||
| Fattening geese/Complete feed | ZEN | 1 | 5 | 60 | 34 | 40 | 0 | 10 | 50 | 50 | – | – | ||
| Fattening rabbits | ZEN | 1 | 14 | 57 | 1,081 | 1,087 | 0 | 10 | 20 | 21 | – | – | ||
| Fattening sheep | ZEN | 1 | 7 | 57 | 5 | 11 | 0 | 10 | 12 | 12 | – | – | ||
| Fattening turkeys/Complete feed | ZEN | 1 | 27 | 48 | 24 | 37 | 4 | 20 | 20 | 50 | – | – | ||
| Fish/Complete feed | ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Fur animals/Complete feed | ZEN | 1 | 1 | 0 | 140 | 140 | 140 | 140 | 140 | 140 | – | – | ||
| Goat (kids) (weaning diets)/Complementary feed | ZEN | 1 | 2 | 0 | 59 | 59 | 59 | 59 | 108 | 108 | – | – | ||
| Growing/fattening pigs | α‐ZAL | 4 | 4 | 100 | 0 | 44 | 0 | 44 | 0 | 54 | – | – | ||
| β‐ZAL | 2 | 4 | 100 | 0 | 22 | 0 | 22 | 0 | 27 | – | – | |||
| ZEN | 1 | 443 | 41 | 35 | 40 | 7 | 13 | 26 | 27 | 95 | 95 | |||
| Horses | ZEN | 1 | 7 | 43 | 16 | 40 | 10 | 20 | 29 | 54 | – | – | ||
| Lactating/dairy goats/Complete feed | ZEN | 1 | 28 | 64 | 8 | 12 | 0 | 5 | 11 | 11 | – | – | ||
| Lactating/dairy sheep | α‐ZAL | 4 | 4 | 100 | 0 | 49 | 0 | 54 | 0 | 54 | – | – | ||
| β‐ZAL | 2 | 4 | 100 | 0 | 24 | 0 | 27 | 0 | 27 | – | – | |||
| ZEN | 1 | 78 | 59 | 14 | 17 | 0 | 5 | 14 | 14 | 73 | 73 | |||
| Lambs | α‐ZAL | 4 | 1 | 100 | 0 | 34 | 0 | 34 | 0 | 34 | – | – | ||
| β‐ZAL | 2 | 1 | 100 | 0 | 17 | 0 | 17 | 0 | 17 | – | – | |||
| ZEN | 1 | 12 | 67 | 20 | 26 | 0 | 10 | 36 | 36 | – | – | |||
| Laying hens | ZEN | 1 | 98 | 47 | 48 | 53 | 1 | 10 | 34 | 34 | 186 | 186 | ||
| Pet food, birds | α‐ZAL | 4 | 1 | 100 | 0 | 54 | 0 | 54 | 0 | 54 | – | – | ||
| β‐ZAL | 2 | 1 | 100 | 0 | 27 | 0 | 27 | 0 | 27 | – | – | |||
| ZEN | 1 | 15 | 7 | 15 | 15 | 20 | 20 | 20 | 20 | – | – | |||
| Pet food, cats/Complete feed | ZEN | 1 | 4 | 50 | 19 | 24 | 12 | 17 | 38 | 38 | – | – | ||
| Pet food, dogs | ZEN | 1 | 7 | 86 | 4 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Poultry (starter diets) | α‐ZAL | 4 | 52 | 100 | 0 | 53 | 0 | 54 | 0 | 54 | – | – | ||
| β‐ZAL | 2 | 52 | 100 | 0 | 26 | 0 | 27 | 0 | 27 | – | – | |||
| ZEN | 1 | 132 | 46 | 31 | 35 | 10 | 10 | 38 | 38 | 125 | 125 | |||
| Rabbits/Complete feed | ZEN | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | – | – | ||
| Complementary/Complete feed, unspecified | ZEN | 1 | 102 | 34 | 51 | 54 | 15 | 15 | 36 | 36 | 145 | 145 | ||
| Weaning pigs | α‐ZAL | 4 | 42 | 100 | 0 | 53 | 0 | 54 | 0 | 54 | – | – | ||
| β‐ZAL | 2 | 42 | 100 | 0 | 26 | 0 | 27 | 0 | 27 | – | – | |||
| ZEN | 1 | 345 | 63 | 14 | 19 | 0 | 7 | 12 | 15 | 44 | 44 | |||
| Compound feed | Compound feed | α‐ZAL | 4 | 106 | 100 | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 | |
| β‐ZAL | 2 | 106 | 100 | 0 | 40 | 0 | 40 | 0 | 40 | 0 | 40 | |||
| ZAN | 1.5 | 3 | 67 | 8 | 65 | 0 | 23 | 23 | 150 | – | – | |||
| ZEN | 1 | 201 | 69 | 17 | 24 | 0 | 10 | 11 | 12 | 77 | 77 | |||
| Fish, other quatic animals and products derived thereof | Fish | Fish meal | ZEN | 1 | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – |
| Forages and roughage, and products derived thereof | Cereals straw | Cereal straw, treated | α‐ZAL | 4 | 1 | 100 | 0 | 77 | 0 | 77 | 0 | 77 | – | – |
| β‐ZAL | 2 | 1 | 100 | 0 | 39 | 0 | 39 | 0 | 39 | – | – | |||
| ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Cereals straw, unspecified | α‐ZAL | 4 | 40 | 100 | 0 | 77 | 0 | 77 | 0 | 77 | – | – | ||
| β‐ZAL | 2 | 40 | 100 | 0 | 39 | 0 | 39 | 0 | 39 | – | – | |||
| ZEN | 1 | 41 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Forage meal; [Grass meal]; [Green meal] | Forage meal; [Grass meal]; [Green meal] | ZEN | 1 | 4 | 75 | 43 | 80 | 0 | 50 | 85 | 110 | – | – | |
| Forages and roughage, and products derived thereof | Forages and roughage, and products derived thereof | ZEN | 1 | 11 | 73 | 68 | 108 | 0 | 15 | 1 | 200 | – | – | |
| Grass, field dried, [Hay] | Grass, field dried, [Hay] | α‐ZAL | 4 | 5 | 100 | 0 | 62 | 0 | 62 | 0 | 62 | – | – | |
| β‐ZAL | 2 | 5 | 100 | 0 | 31 | 0 | 31 | 0 | 31 | – | – | |||
| ZEN | 1 | 31 | 19 | 57 | 58 | 20 | 20 | 20 | 20 | – | – | |||
| Grass, herbs, legume plants, [green forage] | ZEN | 1 | 16 | 6 | 18 | 19 | 20 | 20 | 20 | 20 | – | – | ||
| Lucerne; [Alfalfa] | Lucerne field dried; [Alfalfa field dried] | ZEN | 1 | 1 | 0 | 16 | 16 | 16 | 16 | 16 | 16 | – | – | |
| Lucerne meal; [Alfalfa meal] | ZEN | 1 | 2 | 50 | 450 | 451 | 450 | 451 | 900 | 900 | – | – | ||
| Lucerne, high temperature dried; [Alfalfa, high temperature dried] | ZEN | 1 | 2 | 50 | 10 | 15 | 10 | 15 | 20 | 20 | – | – | ||
| Maize silage | Maize silage | α‐ZAL | 4 | 1 | 100 | 0 | 41 | 0 | 41 | 0 | 41 | – | – | |
| β‐ZAL | 2 | 1 | 100 | 0 | 21 | 0 | 21 | 0 | 21 | – | – | |||
| ZEN | 1 | 59 | 29 | 121 | 124 | 20 | 20 | 75 | 75 | – | – | |||
| Land animal products and products derived thereof | Animal by‐products | Animal by‐products | ZEN | 1 | 1 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – |
| Processed animal protein | Processed animal protein | ZEN | 1 | 1 | 100 | 0 | 2 | 0 | 2 | 0 | 2 | – | – | |
| Legume seeds and products derived thereof | Carob, dried | Carob pods, dried | ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – |
| Horse beans | Horse beans | ZEN | 1 | 1 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – | |
| Peas | Peas | α‐ZAL | 4 | 1 | 100 | 0 | 77 | 0 | 77 | 0 | 77 | – | – | |
| β‐ZAL | 2 | 1 | 100 | 0 | 38 | 0 | 38 | 0 | 38 | – | – | |||
| ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Sweet lupins | Sweet lupins | ZEN | 1 | 1 | 100 | 0 | 3 | 0 | 3 | 0 | 3 | – | – | |
| Legumes, nuts and oilseeds | Other seeds | Other seeds | α‐ZAL | 4 | 5 | 100 | 0 | 184 | 0 | 200 | 0 | 200 | – | – |
| β‐ZAL | 2 | 5 | 100 | 0 | 92 | 0 | 100 | 0 | 100 | – | – | |||
| ZAN | 1.5 | 5 | 100 | 0 | 57 | 0 | 75 | 0 | 75 | – | – | |||
| α‐ZEL | 60 | 5 | 100 | 0 | 2,760 | 0 | 3,000 | 0 | 3,000 | – | – | |||
| β‐ZEL | 0.2 | 5 | 100 | 0 | 2 | 0 | 2 | 0 | 2 | – | – | |||
| ZEN | 1 | 5 | 100 | 0 | 38 | 0 | 50 | 0 | 50 | – | – | |||
| Minerals and products derived thereof | Calcium carbonate; [Limestone] | Calcium carbonate; [Limestone] | ZEN | 1 | 1 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | – | – |
| Minerals and products derived thereof | Minerals and products derived thereof | ZEN | 1 | 4 | 75 | 1 | 7 | 0 | 8 | 3 | 10 | – | – | |
| Miscellaneous | Miscellaneous | Miscellaneous | ZEN | 1 | 1 | 100 | 0 | 3 | 0 | 3 | 0 | 3 | – | – |
| Products from the bakery and pasta industry | Feed beer | α‐ZAL | 4 | 1 | 100 | 0 | 64 | 0 | 64 | 0 | 64 | – | – | |
| β‐ZAL | 2 | 1 | 100 | 0 | 32 | 0 | 32 | 0 | 32 | – | – | |||
| ZEN | 1 | 1 | 100 | 0 | 8 | 0 | 8 | 0 | 8 | – | – | |||
| Plants by‐products from spirits production | ZEN | 1 | 12 | 8 | 222 | 222 | 105 | 105 | 303 | 303 | – | – | ||
| Products from the bakery and pasta industry, ;unspecified | ZEN | 1 | 4 | 100 | 0 | 9 | 0 | 9 | 0 | 16 | – | – | ||
| Oil seeds, oil fruits, and products derived thereof | Cocoa husks | Cocoa husks | ZEN | 1 | 2 | 0 | 85 | 85 | 85 | 85 | 150 | 150 | – | – |
| Cotton seed | Cotton seed | ZEN | 1 | 5 | 40 | 9 | 89 | 5 | 20 | 20 | 200 | – | – | |
| Niger seed | Niger seed | ZEN | 1 | 1 | 0 | 5 | 5 | 5 | 5 | 5 | 5 | – | – | |
| Oil seeds, oil fruits, and products derived thereof | Oil seeds, oil fruits, and products derived thereof | ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |
| Palm kernel expeller | Palm kernel expeller | ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |
| Rape seed | Rape seed, unspecified | α‐ZAL | 4 | 1 | 100 | 0 | 80 | 0 | 80 | 0 | 80 | – | – | |
| β‐ZAL | 2 | 1 | 100 | 0 | 40 | 0 | 40 | 0 | 40 | – | – | |||
| ZEN | 1 | 4 | 75 | 5 | 12 | 0 | 10 | 10 | 15 | – | – | |||
| Rape seed meal | α‐ZAL | 4 | 1 | 100 | 0 | 80 | 0 | 80 | 0 | 80 | – | – | ||
| β‐ZAL | 2 | 1 | 100 | 0 | 40 | 0 | 40 | 0 | 40 | – | – | |||
| ZEN | 1 | 8 | 25 | 11 | 15 | 13 | 20 | 20 | 20 | – | – | |||
| Rape seed, expeller | α‐ZAL | 4 | 1 | 100 | 0 | 80 | 0 | 80 | 0 | 80 | – | – | ||
| β‐ZAL | 2 | 1 | 100 | 0 | 40 | 0 | 40 | 0 | 40 | – | – | |||
| ZEN | 1 | 5 | 60 | 5 | 35 | 0 | 20 | 5 | 25 | – | – | |||
| Safflower seed | Safflower seed meal, partially decorticated | ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |
| Sunflower seed | Sunflower seed, unspecified | α‐ZAL | 4 | 42 | 100 | 0 | 83 | 0 | 83 | 0 | 83 | – | – | |
| β‐ZAL | 2 | 42 | 100 | 0 | 41 | 0 | 41 | 0 | 41 | – | – | |||
| ZEN | 1 | 47 | 91 | 74 | 83 | 0 | 10 | 0 | 10 | – | – | |||
| Sunflower seed expeller | α‐ZAL | 4 | 29 | 100 | 0 | 83 | 0 | 83 | 0 | 83 | – | – | ||
| β‐ZAL | 2 | 29 | 100 | 0 | 41 | 0 | 41 | 0 | 41 | – | – | |||
| ZEN | 1 | 30 | 97 | 1 | 11 | 0 | 10 | 0 | 10 | – | – | |||
| Sunflower seed meal | α‐ZAL | 4 | 4 | 100 | 0 | 83 | 0 | 83 | 0 | 83 | – | – | ||
| β‐ZAL | 2 | 4 | 100 | 0 | 41 | 0 | 41 | 0 | 41 | – | – | |||
| ZEN | 1 | 6 | 83 | 3 | 14 | 0 | 10 | 0 | 20 | – | – | |||
| Sunflower seed meal, dehulled | ZEN | 1 | 2 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – | ||
| Toasted soya (beans) | Soya (bean) expeller | α‐ZAL | 4 | 5 | 100 | 0 | 80 | 0 | 80 | 0 | 80 | – | – | |
| β‐ZAL | 2 | 5 | 100 | 0 | 40 | 0 | 40 | 0 | 40 | – | – | |||
| ZEN | 1 | 7 | 57 | 36 | 41 | 0 | 10 | 64 | 64 | – | – | |||
| Soya (bean) meal, unspecified | ZEN | 1 | 14 | 79 | 7 | 22 | 0 | 20 | 0 | 20 | – | – | ||
| Soya (bean) meal, dehulled | ZEN | 1 | 5 | 20 | 13 | 17 | 20 | 20 | 20 | 20 | – | – | ||
| Soya (bean) protein concentrate | ZEN | 1 | 1 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – | ||
| Soya beans, extruded | ZEN | 1 | 3 | 100 | 0 | 11 | 0 | 15 | 0 | 15 | – | – | ||
| Toasted soya (beans), unspecified | α‐ZAL | 4 | 3 | 100 | 0 | 80 | 0 | 80 | 0 | 80 | – | – | ||
| β‐ZAL | 2 | 3 | 100 | 0 | 40 | 0 | 40 | 0 | 40 | – | – | |||
| Oil seeds, oil fruits, and products derived thereof | Toasted soya (beans) | Toasted soya (beans) | ZEN | 1 | 8 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – |
| Other seeds and fruits, and products derived thereof | Buckwheat | Buckwheat | ZEN | 1 | 1 | 0 | 65 | 65 | 65 | 65 | 65 | 65 | – | – |
| Fruit kernels | Fruit pulp, dried | ZEN | 1 | 1 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – | |
| Perilla seed | Perilla seed | ZEN | 1 | 1 | 0 | 14 | 14 | 14 | 14 | 14 | 14 | – | – | |
| Tubers, roots, and products derived thereof | Sugar beet | (Sugar) beet molasses | ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – |
| Dried (sugar) beet pulp | ZEN | 1 | 1 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – | ||
| Dried (sugar) beet pulp, molasses | ZEN | 1 | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Sugar beet, unspecified | ZEN | 1 | 1 | 0 | 153 | 153 | 153 | 153 | 153 | 153 | – | – | ||
N: number of samples; LB: lower bound; UB: upper bound; P75/P95: 75th/95th percentile; RPF: relative potency factor; ZAL: zearalanol; ZAN: zearalanone; ZEN: zearalenone; ZEL: zearalenol.
The 95th percentile with less than 60 observations may not be statistically robust (EFSA, 2011b). Those estimates were not included in this table.
Values were rounded to the nearest whole number (0 decimal places).
Occurrence levels corrected for molar RPFs are presented in Table 7. Occurrence levels without the RPFs applied are presented in Appendix G. Overall, 61% of the results were below the LOD or LOQ, accounting for 52% for ZAN, 56% for ZEN, 99.8 % for β‐ZAL, 99.9% for α‐ZAL, 100% for α‐ZEL, and 100% for β‐ZEL.
Apart from ‘Cereal grains, their products and by‐products’ and ‘Compound feed’ only a limited number of quantified data were available for other feed groups, i.e. forages, land animal products, legume seeds, minerals, oil seeds and tubers.
ZEN was quantified mainly in wheat unspecified (n = 6,177, mean LB–UB values of 21–24 μg/kg), maize and corn (n = 2,047, mean LB–UB values of 102–105 μg/kg), barley (n = 1,586, mean LB–UB values of 11–15 μg/kg), and oats (n = 779, mean LB–UB values of 10–14 μg/kg).
ZAN was quantified mainly in barley (n = 38, mean LB–UB values of 9–55 μg/kg), cereal grains (n = 49, mean LB–UB values of 20–31 μg/kg), maize and corn (n = 35, mean LB–UB values of 101–107 μg/kg), distillers’ dark grains (n = 24, mean LB–UB values of 96–97 μg/kg), wheat (n = 40, mean LB–UB values of 66–74 μg/kg), complementary feed (incomplete diet) (n = 131, mean LB–UB values of 29–30 μg/kg), and complete feed (n = 172, mean LB–UB values of 39–41 μg/kg).
α‐ZAL was quantified in one sample of wheat feed (n = 65, mean LB–UB values of 17–95 μg/kg), β‐ZAL in one sample of cereal grains (n = 12, mean LB–UB values of 4–39 μg/kg), and one sample of wheat feed (n = 65, mean LB–UB values of 4–43 μg/kg).
The CONTAM Panel considered it important to estimate the occurrence and the animal exposure to the total concentration of ZEN and the modified forms α‐ZAL, β‐ZAL, ZAN, α‐ZEL, β‐ZEL through feed. However, it is very important to stress that estimating the occurrence and exposure with such a high number of left censored data leads to a very high uncertainty, especially for the more potent compounds. This should be taken into account in the interpretation of the results. This high level of uncertainty is expressed by the very broad interval between the LB and the UB.
Thus, to estimate the total concentration of ZEN and its modified forms, the CONTAM Panel calculated the sum of the concentrations of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL, β‐ZEL by summing up the available analytical concentrations for each analytical sample. However, only 154 samples of the data set (< 1 %) were analysed for all the different forms, 728 (4%) samples were analysed for α‐ZAL, β‐ZAL and ZEN, and 372 (2%) for ZAN and ZEN. Therefore, in order to estimate the concentrations of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL, β‐ZEL in each feed sample, the following approach was used. For samples in which the compound (i.e. ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL, β‐ZEL) was analysed, but not quantified, the substitution method was used to estimate the LB and the UB (see Section 2.1). For samples in which none of the compounds was analysed, the levels were estimated by using the mean concentration of the closest feed group available. For example (see Table 7), for α‐ZAL in ‘Barley, unspecified’ (100% left‐censored) the LB and UB were estimated with the substitution method (i.e. LB = 0 and UB = 167). For ‘barley bran’, since α‐ZAL was not analysed, α‐ZAL level was estimated using the mean measured concentration available of the closest feed group (i.e. in this case the mean of levels quantified in ‘barley, unspecified’ and in ‘malt rootlets’, Table 7).
Appendix F gives the distribution of LODs and LOQs for the different feed categories and compound.
3.2.2. Previously reported feed occurrence data in the open literature
Occurrence of ZEN in feed
ZEN occurs almost exclusively in cereals, particularly maize. As it is produced by Fusarium spp., ZEN frequently co‐occurs with other mycotoxins, mainly trichothecenes. Its occurrence in raw cereals and animal feed has been described in the Scientific Opinion on zearalenone as undesirable substance in animal feed (EFSA, 2004; EFSA CONTAM Panel, 2011). Although analytical methods have been improved over the last decade, recent surveys indicate that the occurrence scenario previously reported is still reliable.
A very comprehensive report on the occurrence of ZEN in animal feed has been published in 2011 by Döll and Dänicke. Among raw materials, wheat, maize and oats were found to be mainly affected by DON and ZEN occurrence (Döll and Dänicke, 2011). According to the 2008 German official monitoring data reported by the authors, more than 60% of the analyzed maize samples were contaminated by DON and ZEN, although at relatively low levels compared to guidance values. Among cereals, ZEN was found in 71 out of 95 maize samples, 120 out of 499 cereal grains, 26 out of 69 feed for piglets, 18 out of 51 feeds for sow, and in 41 out of 126 feeds for fattening pigs.
The occurrence of ZEN and other Fusarium mycotoxins has been described in raw cereals and feed from western Romania (Alexa et al., 2013). The occurrence of ZEN was in the range 69–77%, with an average value of about 188 μg/kg. Although levels of ZEN were commonly low, this mycotoxin was often found in co‐occurrence with DON, as expected in consideration of the agro‐climatic conditions of the harvesting area.
According to a recent survey on the occurrence of mycotoxins in feed from Portugal (Abrunhosa et al., 2016), ZEN was detected in 25% of the considered samples (n = 573), although at generally low concentrations (range: 5.0–356.0 μg/kg). In particular, the highest levels of ZEN were found in feed for pigs and in corn. Concerning fattening pigs, Almeida et al. (2011) reported on the presence of ZEN in 7 out of 21 samples from Portugal. Also in this study, levels of ZEN were generally low but the mycotoxins were found as co‐contaminant with DON. The same authors reported on consistent results found in compound feeds for sows (Almeida et al., 2011) and dairy cows (Almeida et al., 2013). In the latter case, a co‐occurrence of ZEN and aflatoxin B1 was described in 5 feed samples for ewes.
The co‐occurrence of ZEN and DON in feed for dairy cows was investigated by Driehuis et al. (2008). The authors analysed silage (n = 47), compound feed (n = 72), ensile by‐products (n = 29), feed commodities (n = 8), and forage products (n = 13). The highest incidence of ZEN was found in compound feed (28%) together with the highest concentration (363 μg/kg feed). The same authors investigated the occurrence of ZEN in maize, grass, and wheat silage for cattle (Driehuis et al., 2008). The study reported on the significance of maize silage on the total ZEN intake in cattle. In particular, ZEN was detected in 49% of maize and 6% of grass silages, with an average concentration of 174 and 93 μg/kg, respectively, and maximum concentrations of 943 and 308 μg/kg, respectively.
ZEN is most commonly associated with cereal crops, and few studies have examined its presence in pasture grasses. Engels and Krämer (1996) reported that two‐thirds of more than 832 grass samples examined were positive for ZEN using an ELISA method, with concentrations ranging between 40 and 2780 μg/kg dry matter. Golinski et al. (2003) reported the occurrence of ZEN in winter pasture from Poland in 50–65% of pastures sampled in three locations. The concentrations reported were between 2 and 12 μg/kg DM. In an examination of maize (n = 140), grass (n = 120) and cereal silages (n = 30) produced in the Netherlands between 2002 and 2004, ZEN was detected in 49% of maize and 6% of grass silages. Average ZEN concentrations were 174 and 93 μg/kg, respectively, and maximum concentrations 943 and 308 μg/kg, respectively (Driehuis et al., 2008). More recently, Skládanka et al. (2011) have reported levels of up to 122 μg/kg in a multiyear study in the Czech Republic, although there were large within‐ and between‐year differences. In New Zealand, where ZEN‐induced infertility in sheep is common, concentrations of up to 2,600 μg ZEN/kg have been reported in pasture leaves (di Menna et al., 1985). As Driehuis (2013) has noted, ZEN is a field‐derived rather than an ensilage‐derived mycotoxin; since grazed grass and grass for ensiling tend to be consumed or cut when the plant is in an immature growth stage and it may be reasonable to assume that levels of ZEN will be low in these feeds.
ZEN has also been reported in maize and poultry feed (total samples, n = 119) from Brazil (de Lourdes Mendes de Souza et al., 2013). In particular, ZEN was found in 12% of raw maize and 36% of poultry feed.
For horse feed, a recent survey from the Canadian and Irish market showed that all the considered feed categories (total samples, n = 259) contained ZEN with the only exception of oats (Buckley et al., 2007). An incidence of 6–8% of positive samples were found in haylage, Canadian hay, and coarse mix, while higher incidence were recorded for ZEN in Irish hay (21%) and pelleted feed (16%).
Concerning companion animals, a recent survey on the occurrence of mycotoxins in dry dog feed, showed that nearly half (47%) of all samples (n = 76) were contaminated with ZEN, at levels of 10–298 μg/kg (Bohm et al., 2010).
Commercial complete fish feed for cyprinids was characterised by a marked variation of ZEN concentrations ranging from 3 to 511 μg/kg (n = 11, Pietsch et al., 2013). Interestingly, the two diets demonstrating the highest ZEN concentrations (80 and 511 μg/kg) contained corn or corn gluten feed while all other feed mixtures did not contain any corn, pointing at the special importance of corn as a specific ZEN source.
In general, data reported in the literature for ZEN are consistent with those received by EFSA and used for calculating the exposure in terms of the range of concentrations.
Occurrence of modified forms of ZEN in feed from plant origin
Fungi can form phase I metabolites α‐ and β‐ZEL from ZEN, while these can be converted to phase II conjugates by both plants and fungi (Nathanail et al., 2015). Although surveys on feed products are not available, the co‐occurrence of ZEN, α‐ and β‐ZEL and their glucosides has been reported in raw cereals (De Boevre et al., 2013; Nathanail et al., 2015).
Cis‐isomerisation of ZEN has been described to occur in the field as an effect of sunlight (Drzymala et al., 2014). As the current analytical methods do not include cis‐ZEN, occurrence data in feed or raw material are still not available.
Phase II conjugated forms usually co‐occur with the parent compound and other mycotoxins, as reported by Streit et al. (2013). Conjugates such as ZEN14Glc or ZEN14Sulf are mainly localised in outer layers of cereals, and thus can be enriched in bran and fibre fractions exceeding in some cases the parent compound (Schwake‐Anduschus et al., 2015).
Nathanail et al. (2015) reported for the first time the occurrence of ZEN16Glc in naturally infected cereals from Finland, together with ZEN, ZEN14Glc, ZEN14Sulf, α‐ and β‐ZEL, and α‐ and β‐ZEL14Glc. The sum of modified forms (phase I and phase II) exceeded ZEN up to 150% in barley (n = 34), while in oats and wheat they account for up to 50% in respect to the parent form. ZEN14Sulf was the most abundant modified form in Finnish cereals, reaching the highest amount in oats.
Although extensive data on the occurrence of modified forms of ZEN in feed from plant origin are not available, data reported in the literature so far cover a wider range of modified forms compared to those received by EFSA and used for calculating the exposure. In particular, the EFSA database did not receive any data on phase II conjugates (i.e. ZEN14Glc, ZEN16Glc) that are, on the other hand, frequently reported in the literature. The inclusion of these compounds in extensive monitoring plans is probably hampered by the lack of calibrants and reference materials on the market.
Occurrence of modified forms of ZEN in feed from animal origin
The occurrence of ZEN and its modified forms in feed of animal origin results from toxicokinetics. Hereby, metabolism and tissue distribution give rise to occurrence patterns of modified forms of ZEN different from plant derived feedstuffs. The consequences of toxicokinetics for the occurrence of modified forms of ZEN in animal originated feedstuffs are addressed in the paragraph ‘Transfer (carry over)’ within the Section ‘Toxicokinetics’ (see Section 3.1.1.6).
3.2.3. Feed processing
Cereal grains intended for use as animal feed are usually subject to processes such as cleaning, sorting, drying, rolling/grinding and/or extrusion. In addition, by‐products of processing grains for human consumption are widely used as feeds for livestock.
As sorting and cleaning are mechanical processes, no significant thermal degradation of mycotoxins is expected at this stage. However, mechanical steps often result in an overall decrease of feed contamination. Most mycotoxins and their modified forms tend to be mainly concentrated in the bran fractions or outer layers of the grains (Edwards et al., 2011). Consistently, it has been described that ZEN and its modified forms are mainly found in bran or fibre‐enriched products (De Boevre et al., 2012b). Therefore, processes such as dehulling and milling may decrease the contamination in the flour, while enriching by‐products such as bran and shorts.
ZEN is relatively heat stable, and thus thermal degradation is unlikely to occur as a result of heat applied during the manufacturing and pelleting of compound feeds (final temperature: 60–95°C). However, a significant reduction of the parent compound was reported upon extrusion cooking of corn grits (Ryu et al., 1999), while no specific information has been identified on the effect of the thermal treatments on modified forms of ZEN.
Concerning silage, González Pereyra et al. (2014) recently demonstrated that ensilage does not significantly affect the occurrence of ZEN and its modified forms in maize.
As already reported for DON and other mycotoxins, it can be concluded that feed production processes have an impact on the distribution of ZEN and its modified forms between milling fractions, with a possible enrichment of by‐products for feed production (i.e. middlings). On the other side, there is no evidence of degradation of modified forms of ZEN along the feed production chain.
3.3. Exposure assessment
3.3.1. Previously reported exposure assessments in animals
In 2004, the CONTAM Panel (EFSA, 2004) reviewed the presence of ZEN in feed, but concluded that due to the variability in diet composition for the major farm animal species it was not possible to estimate exposure levels based on the occurrence of ZEN in individual feed materials. More recently, EFSA CONTAM Panel (2014) published a Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. For ZEN and its modified forms, the CONTAM Panel concluded that the mean exposure in pigs in general and the 95th percentile exposure in piglets is not of concern. Occurrence data were inadequate to conclude on risks for fattening pigs and sows receiving feed with a higher than average contamination level. Owing to the lack of either occurrence data or NOAELs/LOAELs, the risk for cattle, goats, rabbits and fish could not be fully characterised, while the estimated exposure in sheep, horses, poultry, cats and dogs was not of concern.
3.3.2. Dietary exposure assessment for farm and companion animals
For all species, P95 and mean exposures have been estimated based on the 95th percentile and the mean LB and UB concentrations, respectively. According EFSA (2010b), caution is needed when calculating acute exposure (95th percentile) where data on less than 60 samples are available, since the results may not be statistically robust. Therefore, in this Opinion, estimates of acute exposure (95th percentile) have not been made where data on < 60 samples are available. Furthermore, mean LB and UB data for feeds derived from less than 10 samples have not been used in the estimates of exposure. Exposures by farmed livestock and companion animals are presented below for ZEN (Section 3.3.3) and for the sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL, β‐ZEL, applying the RPFs (Section 3.3.4).
For many livestock in Europe, feeds are supplied in the form of commercially produced species‐specific blends or compound feeds, and for some species data on the ZEN + α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL contents were provided by the European countries (see Section 3.2.1, Table 7). Where these data were available, P95 and mean exposures have been calculated using the concentrations reported and assumed intakes (see Appendix D). For those livestock categories for which insufficient data on species‐specific compound feeds were provided (i.e. for less than 10 samples), the CONTAM Panel identified example diets and feed inclusion rates (see Appendix D for details), and used concentrations of ZEN + α‐ZAL + β‐ZAL+ ZAN + α‐ZEL+ β‐ZEL in individual feed materials to estimate P95 and mean exposure.
As reported in Appendix D, a wide range of feeds and feeding systems are used for livestock in Europe. It must be stressed that the feed intakes or diet compositions used in estimating exposures in this scientific opinion are not ‘average’ diets, nor are they an attempt to describe ‘worst case’ scenarios. Rather, they are intended to provide an indication of likely exposure to ZEN and modified forms across a range of feeding systems in Europe.
For ruminants and horses, forages – fed either fresh or conserved – are important ingredients of the diet. The data submitted to EFSA confirm the presence of ZEN and modified forms in certain forages (Table 7). Although ZEN has been reported in fresh grass or grass silage (see Section 3.2.2), no data for these feeds were available, and therefore it has been assumed that they make no contribution to the exposure. In contrast, data have been provided to EFSA on levels of ZEN and the modified forms in the grass hay, maize silage and cereal straws and these data have been used to estimate exposure in those ruminant feeding systems where these are the main forages.
3.3.3. Estimated exposure by farmed animals and companion animals (cats and dogs) to zearalenone
3.3.3.1. Ruminants and horses
Two scenarios have been considered in estimating exposure by ruminant livestock to ZEN. In the first it has been assumed that species‐specific compound feeds are fed together with fresh grass and/or grass silage, and in the absence of data on the presence of ZEN in these feeds it is assumed that the forages make no contribution to exposure. In the second scenario, grass hay has been assumed to be the sole forage, and levels of ZEN in grass hay have been applied to calculate exposure. Estimated P95 and mean exposures for high yielding dairy cows and fattening beef cattle to ZEN are presented in Table 8.
Table 8.
Estimated P95 and mean exposure to ZEN for ruminants derived from UB concentrations in species‐specific compound feeds
| Species | Diet concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw | ||||
|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | ||
| Cows: high yieldinga | LB | 25.3 | 6.49 | 525 | 134 | 0.81 | 0.21 |
| UB | 25.3 | 8.11 | 525 | 168 | 0.81 | 0.26 | |
| Cows: high yieldingb | LB | 224 | 45.0 | 4,632 | 932 | 7.13 | 1.43 |
| UB | 224 | 47.8 | 4,632 | 990 | 7.13 | 1.52 | |
| Fattening beef cattlea | LB | 12.6 | 2.52 | 121 | 24.2 | 0.30 | 0.06 |
| UB | 12.6 | 3.59 | 121 | 34.5 | 0.30 | 0.09 | |
| Fattening beef cattle b | LB | 294 | 57.1 | 2,820 | 549 | 7.05 | 1.37 |
| UB | 294 | 59.9 | 2,820 | 575 | 7.05 | 1.44 | |
| Sheep: lactatinga | LB | 41.3 | 7.78 | 78.5 | 14.8 | 0.98 | 0.18 |
| UB | 41.3 | 9.48 | 78.5 | 18.0 | 0.98 | 0.23 | |
| Sheep: lactatingb | LB | 207 | 39.9 | 393 | 75.8 | 4.91 | 0.95 |
| UB | 207 | 42.6 | 393 | 80.9 | 4.91 | 1.01 | |
| Goats: lactatinga | LB | – c | 1.44 | – c | 3.03 | – c | 0.05 |
| UB | – c | 1.99 | – c | 4.18 | – c | 0.07 | |
| Goats: lactatingb , c | LB | – c | 56.1 | – c | 118 | – c | 1.96 |
| UB | – c | 58.3 | – c | 122 | – c | 2.04 | |
bw: body weight; LD: lower bound; UB: upper bound; P95: 95th percentile.
Fresh grass‐based diet (i.e. assumes no exposure to ZEN from forages).
Grass hay‐based diets.
Insufficient samples available to estimate P95 exposure.
For other ruminant production systems, insufficient data on species‐specific compound feeds were available, and therefore exposures were estimated using example rations and concentrations in individual feed materials (see Appendix D, Table D.1 for details of rations used). Estimates of exposures for these are given in Table 9.
Table D.1.
Live weights, growth rate/productivity, dry matter intake for cattle, sheep, goats and horses, and the proportions of the diet as non‐forage
| Species | Live weight (kg) | Growth rate or productivity | Dry matter intake (kg/day) | % of diet as non‐forage feed | Reference |
|---|---|---|---|---|---|
| Dairy cows, lactating | 650 | 40 kg milk/day | 20.7 | 40 | OECD (2013) |
| Fattening cattle: beefa | 400 | 1 kg/day | 9.6 | 15 | AFRC (1993) |
| Fattening cattle: maize silage‐based ration | 300 | 1.4 kg/day | 6.6 | 25 | Browne et al. (2004) |
| Fattening cattle: cereal straw‐based diet | 300 | 0.9 kg/day | 8.0 | 68 | EBLEX (2016) |
| Sheep: lactating | 80 | Feeding twin lambs | 2.8 | 50 | OECD (2013) |
| Goats: milking | 60 | 6 kg milk/day | 3.4 | 65 | NRC (2007a) |
| Horses | 450 | Moderate activity | 9.0 | 50 | NRC (2007b) |
Housed castrate cattle, medium maturing breed.
Months 2–3 of lactation.
Table 9.
Estimated P95 and mean exposure to ZEN by ruminants and horses, derived from UB concentrations in individual feed materials and their relative proportions in diets
| Species | Diet concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw | ||||
|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | ||
| Dairy cows: maize silage‐based diet | LB | 777 | 117 | 21,223 | 3,191 | 32.6 | 4.91 |
| UB | 785 | 121 | 21,444 | 3,314 | 32.9 | 5.10 | |
| Beef cattle: cereal‐based diet | LB | 12.75 | 7.81 | 128 | 78 | 0.32 | 0.20 |
| UB | 21.10 | 10.79 | 211 | 108 | 0.53 | 0.27 | |
| Beef cattle: maize silage‐based diet | LB | 795 | 105 | 5,250 | 695 | 17.5 | 2.32 |
| UB | 795 | 108 | 5,250 | 714 | 17.5 | 2.38 | |
| Beef cattle: straw‐based diet | LB | 15.6 | 8.51 | 125 | 68.1 | 0.42 | 0.23 |
| UB | 27.9 | 15.0 | 223 | 120 | 0.74 | 0.40 | |
| Fattening goatsa | LB | 89.4 | 5.60 | 134 | 8.40 | 3.35 | 0.21 |
| UB | 89.4 | 7.83 | 134 | 11.7 | 3.35 | 0.29 | |
| Fattening goats b | LB | 288 | 44.2 | 432 | 66.2 | 10.8 | 1.66 |
| UB | 288 | 47.6 | 432 | 71.3 | 10.8 | 1.78 | |
| Horses a | LB | 17.4 | 9.14 | 156 | 82.3 | 0.35 | 0.18 |
| UB | 18.9 | 11.6 | 170 | 105 | 0.38 | 0.23 | |
| Horses b | LB | 182 | 41.3 | 1,644 | 371 | 3.65 | 0.83 |
| UB | 184 | 44.7 | 1,659 | 403 | 3.69 | 0.89 | |
bw: body weight; LB: lower bound; UB: upper bound; P95: 95th percentile.
Fresh grass‐based diet (i.e. assumes no exposure to ZEN from forages).
Grass hay‐based diets.
Note that the exposures indicated with (b) assume that grass hay is the sole forage. Where fresh grass or grass silage are the main or sole forages, exposures are likely to be lower.
3.3.3.2. Pigs and poultry
Estimates of P95 and mean exposure by pigs and poultry to ZEN were derived from data for species‐specific compound feeds, and are given in Table 10.
Table 10.
Estimates of P95 and mean exposure to ZEN for pigs and poultry derived from UB concentrations in species‐specific compound feeds
| Species | Diet concentration μg/kg dry feed matter | Exposure μg/day | Exposure μg/kg bw per day | ||||
|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | ||
| Pig starter | LB | 50.0 | 16.2 | 50.0 | 16.2 | 2.50 | 0.81 |
| UB | 50.0 | 21.3 | 50.0 | 21.3 | 2.50 | 1.07 | |
| Pig finisher | LB | 108 | 39.9 | 324 | 120 | 3.24 | 1.20 |
| UB | 108 | 45.1 | 324 | 135 | 3.24 | 1.35 | |
| Gilts | LB | 108 | 39.9 | 216 | 79.9 | 3.60 | 1.33 |
| UB | 108 | 45.1 | 216 | 90.2 | 3.60 | 1.50 | |
| Lactating sow | LB | 263 | 36.0 | 1,575 | 216 | 7.88 | 1.08 |
| UB | 263 | 41.8 | 1,575 | 251 | 7.88 | 1.25 | |
| Fattening chickens | LB | 143 | 35.2 | 17.2 | 4.22 | 8.59 | 2.11 |
| UB | 143 | 41.3 | 17.2 | 4.96 | 8.59 | 2.48 | |
| Laying hens | LB | 211 | 54.9 | 25.4 | 6.59 | 12.7 | 3.30 |
| UB | 211 | 60.7 | 25.4 | 7.28 | 12.7 | 3.64 | |
| Fattening turkeysa | LB | 153 | 26.9 | 61.4 | 10.8 | 5.11 | 0.90 |
| UB | 153 | 42.3 | 61.4 | 16.9 | 5.11 | 1.41 | |
| Fattening ducksb | LB | 74.2 | 15.8 | 10.4 | 2.21 | 3.46 | 0.74 |
| UB | 77.4 | 22.9 | 10.8 | 3.20 | 3.61 | 1.07 | |
bw: body weight; LB: lower bound; UB: upper bound; P95: 95th percentile.
Insufficient samples were provided to allow reliable 95th percentile estimates of exposure to be made.
Insufficient species‐specific samples were provided to allow reliable estimates of exposure to be made, and therefore example diets have been used (see Appendix D).
3.3.3.3. Farmed fish (salmonids, carp), rabbits and mink
In the absence of reliable data on concentrations of ZEN in species‐specific compound feeds, estimates of exposure were made by using example rations and concentrations in individual feed materials (see Appendix D, Table D.2 for details of rations used) and are reported in Table 11.
Table D.2.
Live weights and feed intake for pigs, poultry and fish (EFSA FEEDAP Panel, 2012) and ducks (Leeson and Summers, 2008)
| Species | Live weight (kg) | Feed intake (kg dry matter/day) | Reference |
|---|---|---|---|
| Pigs: starter | 20 | 1.0 | EFSA FEEDAP Panel (2012) |
| Pigs: finishing | 100 | 3.0 | EFSA FEEDAP Panel (2012) |
| Pigs: gilts | 50 | 2.0 | NRC (2012) |
| Pigs: lactating sows | 200 | 6.0 | EFSA FEEDAP Panel (2012) |
| Poultry: broilersa | 2 | 0.12 | EFSA FEEDAP Panel (2012) |
| Poultry: laying hens | 2 | 0.12 | EFSA FEEDAP Panel (2012) |
| Turkeys: fattening turkeys | 12 | 0.40 | EFSA FEEDAP Panel (2012) |
| Ducks: fattening ducks | 3 | 0.14 | Leeson and Summers (2008) |
| Salmonids | 2 | 0.04 | EFSA FEEDAP Panel (2012) |
| Carp | 1 | 0.02 | Schultz et al. (2012) |
Fattening chickens.
Table 11.
Estimated P95 and mean exposure to ZEN for rabbits, farmed fish and mink derived from UB concentrations in individual feed materials and their relative proportions in diets
| Species | Diet concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw | ||||
|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | ||
| Salmonids | LB | 24.6 | 4.13 | 0.99 | 0.17 | 0.49 | 0.08 |
| UB | 24.8 | 6.64 | 0.99 | 0.27 | 0.50 | 0.13 | |
| Carp | LB | 112 | 21.5 | 2.48 | 0.47 | 2.48 | 0.47 |
| UB | 113 | 28.7 | 2.48 | 0.63 | 2.48 | 0.63 | |
| Rabbits | LB | 11.4 | 10.1 | 1.70 | 1.51 | 0.85 | 0.76 |
| UB | 17.9 | 14.8 | 2.69 | 2.21 | 1.35 | 1.11 | |
| Mink | LB | 38.5 | 8.50 | 2.89 | 0.64 | 1.40 | 0.31 |
| UB | 38.8 | 8.98 | 2.91 | 0.67 | 1.41 | 0.33 | |
bw: body weight; LB: lower bound; UB: upper bound; P95: 95th percentile.
The higher estimated exposures for rabbits reflect the higher proportions of cereals in the diet of rabbits than for salmonids or mink.
3.3.3.4. Companion animals (dogs and cats)
Few data on levels of ZEN in proprietary feeds for dogs and cats were available, and therefore exposure was estimated using example rations (see Appendix D, Table D.5 for details) and concentrations of these toxins in individual feed materials. The exposures are reported in Table 12.
Table D.5.
Estimated example diet composition (%) for fattening ducks, farmed rabbits, farmed fish, farmed mink and companion animals (cats and dogs)
| Feed materials | Ducks | Rabbits | Farmed fish | Farmed minkb | Companion animals | ||
|---|---|---|---|---|---|---|---|
| Salmonids | Carp | Cats | Dogs | ||||
| Wheat | 45 | ni | 13.2 | 24 | 6 | 10 | 10 |
| Barley | 15 | 18 | ni | 1 | ni | ni | |
| Maize | ni | ni | ni | 10 | 6 | 5 | 6 |
| Oats | ni | ni | ni | ni | ni | 1 | 0.5 |
| Soybean meal | 28 | ni | 12.3 | 32.4 | ni | 8 | 4 |
| Rapeseed meal | ni | ni | ni | 12.5 | ni | ni | ni |
| Maize gluten meal | ni | ni | 11.5 | ni | ni | 17 | 15 |
| Sunflower meal | ni | 20 | ni | ni | ni | ni | ni |
| Lucerne meal | 5 | 19 | ni | ni | ni | ni | ni |
| Beansa | ni | 10 | ni | ni | ni | 1 | 2 |
| Wheat feed | 2 | 16 | ni | ni | ni | 12 | 20 |
| Sugar beet pulpa | ni | 12 | ni | ni | ni | ni | ni |
| Fishmeala | ni | ni | 30.5 | 6.7 | 36 | 6 | 0.5 |
| Meat meala | ni | ni | ni | ni | 40 | 38 | 40 |
| Molassesa | ni | ni | ni | ni | ni | ni | ni |
| Fish and vegetable oilsa | 3 | ni | 31.9 | 2.3 | 8 | ni | Ni |
| Others feeds (unspecified)a | ni | 2 | ni | 1 | ni | ni | ni |
| Minerals, vitamins etca | 2 | 3 | 0.6 | 3.6 | 3 | 2.0 | 2.0 |
ni: not included in the diet formulations.
No data on ZEN concentrations available, and therefore, the contribution to ZEN exposure is assumed to be zero.
Based on data translated from Finnish to English provided by the Finnish Fur Breeders Association in 2015, www.profur.fi
Table 12.
Estimated P95 and mean exposure to ZEN by companion animals (dogs and cats) derived from concentrations in individual feed materials and their relative proportions in diets
| Species | Diet concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw | ||||
|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | ||
| Cats | LB | 50.1 | 14.6 | 3.01 | 0.88 | 0.75 | 0.22 |
| UB | 50.3 | 17.6 | 3.02 | 1.06 | 0.75 | 0.26 | |
| Dogs | LB | 55.4 | 19.4 | 20.0 | 6.99 | 0.80 | 0.28 |
| UB | 55.6 | 22.6 | 20.0 | 8.11 | 0.80 | 0.33 | |
bw: body weight; LB: lower bound; UB: upper bound; P95: 95th percentile.
3.3.4. Estimated exposure by farmed animals and companion animals (cats and dogs) to the sum of ZEN and modified forms
Different oestrogenic potencies are observed in vivo for zearalenone and modified forms. To account for these differences, relative potency factors relative to ZEN (RPFs) were established (EFSA CONTAM Panel, 2016). These RPFs were applied to the occurrence data to calculate exposure estimates of ZEN and its modified forms.
3.3.4.1. Ruminants and horses
Estimates of P95 and exposures for high yielding dairy cows and fattening beef cattle to the sum of ZEN and the modified forms for which data are available α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL (corrected for RPFs as described in Section 1.3.1, Table 1), derived from data on species‐specific compound feed, are presented in Table 13.
Table 13.
Estimated P95 and mean exposure to the sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZELa
| Species | Diet concentration μg/kg dry matter | Exposureμg/day | Exposureμg/kg bw | % of exposure (μg/kg bw) from modified forms | |||||
|---|---|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | P95 | Mean | ||
| Dairy: high yieldinga | LB | 25.3 | 6.49 | 525 | 134 | 0.81 | 0.21 | 0 | 0 |
| UB | 61.5 | 44.2 | 1,272 | 916 | 1.96 | 1.41 | 58.7 | 81.6 | |
| Dairy: high yieldingb | LB | 223 | 45.0 | 4,632 | 932 | 7.13 | 1.43 | 0 | 0 |
| UB | 259 | 94.2 | 5,379 | 1,950 | 8.28 | 3.00 | 13.9 | 49.3 | |
| Fattening beef cattlea | LB | 25.4 | 2.52 | 121 | 24.2 | 0.30 | 0.06 | 0 | 0 |
| UB | 25.7 | 16.7 | 247 | 159 | 0.62 | 0.40 | 51.6 | 77.5 | |
| Fattening beef cattleb | LB | 223 | 57.1 | 2,820 | 548 | 7.05 | 1.37 | 0 | 0 |
| UB | 306 | 87.5 | 2,945 | 839 | 7.36 | 2.10 | 4.2 | 31.4 | |
| Sheep: lactatingb | LB | 41.3 | 7.78 | 78.5 | 14.8 | 1.31 | 0.25 | 25.2 | 28.0 |
| UB | 82.9 | 51.0 | 157 | 97.0 | 2.62 | 1.62 | 62.6 | 85.8 | |
| Sheep: lactatingc | LB | 206 | 39.9 | 392 | 75.8 | 6.54 | 1.26 | 24.9 | 24.6 |
| UB | 248 | 92.7 | 471 | 176 | 7.86 | 2.94 | 37.5 | 65.6 | |
| Goats: lactatingb , d | LB | 1.44 | 3.03 | 0.05 | 0 | ||||
| UB | 1.99 | 4.18 | 0.07 | 0 | |||||
| Goats: lactatingc , d | LB | 56.1 | 117 | 1.96 | 0 | ||||
| UB | 72.8 | 152 | 2.55 | 20.0 | |||||
bw: body weight; LB: lower bound; UB: upper bound; P95: 95th percentile.
The contributions from α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL have been corrected for their potencies relative to ZEN (see Section 1.3.1).
Exposure to fresh grass and species‐specific compound feed only (i.e. assumes no exposure to ZEN from forages).
Exposure to grass hay and species‐specific compound feed.
Insufficient samples available to estimate P95 exposure.
As in the previous section (Section 3.3.4.1) two scenarios have been considered. In the first, it is assumed that the forage is either fresh grass or grass silage, and that these make no contribution to exposure, while in the second grass hay has been assumed to be the forage, and data on levels of ZEN plus the modified forms have been used.
For other ruminant production systems, insufficient data on species‐specific compound feeds were available, and therefore exposures were estimated using example rations and concentrations in individual feed materials (see Appendix D for details of rations used). Estimates of exposures for these (corrected for RPFs as described in Section 1.3.1, Table 1) are given in Table 14.
Table 14.
Estimated P95 and mean exposure to the sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL by ruminants and horses, derived from concentrations in individual feed materials and their relative proportions in dietsa
| Species | Diet concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw | % of exposure (μg/kg bw) from modified forms | |||||
|---|---|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | P95 | Mean | ||
| Dairy cows: maize silage‐based diet | LB | 777 | 117 | 21,223 | 3,191 | 32.6 | 4.91 | 0 | 0 |
| UB | 788 | 124 | 21,505 | 3,397 | 33.1 | 5.23 | 0.6 | 2.5 | |
| Beef cattle: cereal‐based diet | LB | 16.2 | 10.6 | 162 | 106 | 0.41 | 0.26 | 22.0 | 23.1 |
| UB | 46.3 | 55.5 | 463 | 555 | 1.16 | 1.39 | 54.3 | 80.6 | |
| Beef cattle: maize silage‐based diet | LB | 795 | 105 | 5,250 | 695 | 17.5 | 2.32 | 0 | 0 |
| UB | 826 | 140 | 5,454 | 924 | 18.2 | 3.08 | 3.7 | 22.7 | |
| Beef cattle: straw‐based diet | LB | 15.6 | 8.54 | 125 | 68.2 | 0.42 | 0.23 | 0 | 0 |
| UB | 95.2 | 102 | 761 | 820 | 2.54 | 2.73 | 70.9 | 85.3 | |
| Fattening goatsb | LB | 90.3 | 7.23 | 134 | 10.8 | 3.36 | 0.27 | 0.3 | 22.2 |
| UB | 91.2 | 33.0 | 137 | 49.5 | 3.41 | 1.24 | 1.8 | 76.6 | |
| Fattening goatsc | LB | 288 | 45.8 | 432 | 68.7 | 10.8 | 1.72 | 0 | 3.5 |
| UB | 289 | 83.0 | 434 | 124 | 10.9 | 3.11 | 0.6 | 42.8 | |
| Horsesb | LB | 23.0 | 14.8 | 207 | 133 | 0.46 | 0.30 | 23.9 | 40.0 |
| UB | 54.3 | 48.0 | 489 | 432 | 1.09 | 0.96 | 65.1 | 76.0 | |
| Horsesc | LB | 188 | 46.9 | 1,695 | 422 | 3.77 | 0.94 | 3.2 | 11.7 |
| UB | 219 | 89.7 | 1,977 | 807 | 4.39 | 1.79 | 15.9 | 50.3 | |
bw: body weight; LB: lower bound; UB: upper bound; P95: 95th percentile.
The contributions from α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL have been corrected for their potencies relative to ZEN (see Section 1.3.1)
Exposure to species‐specific compound feed only (i.e. assumes no exposure to ZEN from forages)
Assumes that the diet contains 50% grass hay
3.3.4.2. Pigs and poultry
Estimates of P95 and mean exposure by pigs and poultry to the sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL were derived from data for species‐specific compound feeds, and are given in Table 15 (values corrected for RPFs as described in Section 1.3.1). No data on levels of α‐ZAL, β‐ZAL, ZAN, α‐ZEL or β‐ZEL were provided in species‐specific compound feeds for laying hens, fattening chickens and fattening turkeys, and therefore no exposure estimates were made for these species.
Table 15.
Estimated P95 and mean exposure to the sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL for pigs and poultry derived from concentrations in species‐specific compound feedsa
| Species | Diet concentration μg/kg dry feed matter | Exposure μg/day | Exposure μg/kg bw | % of exposure (μg/kg bw) from modified forms | |||||
|---|---|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | P95 | Mean | ||
| Pig starter | LB | 50.0 | 16.2 | 50.0 | 16.2 | 2.50 | 0.81 | 0 | 0 |
| UB | 140 | 111 | 140 | 111 | 7.00 | 5.57 | 64.3 | 80.8 | |
| Pig finisher | LB | 108 | 39.9 | 324 | 120 | 3.24 | 1.20 | 0 | 0 |
| UB | 182 | 119 | 548 | 359 | 5.48 | 3.59 | 40.9 | 62.4 | |
| Gilts | LB | 108 | 39.9 | 216 | 79.9 | 3.60 | 1.33 | 0 | 0 |
| UB | 183 | 120 | 365 | 240 | 6.09 | 3.99 | 40.9 | 66.6 | |
| Lactating sow | LB | 263 | 36.0 | 1575 | 216 | 7.88 | 1.08 | 0 | 0 |
| UB | 337 | 116 | 2023 | 699 | 10.1 | 3.49 | 22.1 | 64.2 | |
| Chickens for fattening | LB | 143 | 35.2 | 17.2 | 4.22 | 8.59 | 2.11 | 0 | 0 |
| UB | 143 | 41.3 | 17.2 | 4.96 | 8.59 | 2.48 | 0 | 0 | |
| Laying hens | LB | 211 | 54.9 | 25.4 | 6.59 | 12.7 | 3.30 | 0 | 0 |
| UB | 211 | 60.7 | 25.4 | 7.28 | 12.7 | 3.64 | 0 | ||
| Turkeys for fattening | LB | 153 | 26.9 | 61.4 | 10.8 | 5.11 | 0.90 | 0 | 0 |
| UB | 153 | 42.3 | 61.4 | 16.9 | 5.11 | 1.41 | 0 | 0 | |
| Fattening ducks b | LB | 75.0 | 16.6 | 10.5 | 2.32 | 3.50 | 0.77 | 1.1 | 3.9 |
| UB | 83.9 | 33.6 | 11.7 | 4.71 | 3.92 | 1.57 | 7.9 | 31.8 | |
bw: body weight; LB: lower bound; UB: upper bound; P95: 95th percentile.
The contributions from α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL have been corrected for their RPFs (see Section 1.3.1).
3.3.4.3. Farmed fish (salmonids, carp), rabbits and mink
In the absence of reliable data on concentrations of the sum of sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL in species‐specific compound feeds, estimates of exposure were made by using example rations and concentrations in individual feed materials (see Appendix D Table D.2 for details of rations used) and are reported in Table 16 (values corrected for RPFs as described in Section 1.3.1).
Table 16.
Estimated P95 and mean exposure to the sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL for rabbits, farmed fish and mink derived from concentrations in individual feed materials and their relative proportions in dietsa
| Species | Diet concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw | % of exposure (μg/kg bw) from modified forms | |||||
|---|---|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | P95 | Mean | ||
| Salmonids | LB | 24.6 | 4.13 | 0.99 | 0.17 | 0.49 | 0.08 | 0 | 0 |
| UB | 25.4 | 6.68 | 1.02 | 0.27 | 0.51 | 0.13 | 2.0 | 0 | |
| Carp | LB | 112 | 21.5 | 2.48 | 0.47 | 2.48 | 0.47 | 0 | 0 |
| UB | 132 | 46.4 | 2.89 | 1.02 | 2.89 | 1.02 | 14.2 | 38.2 | |
| Rabbits | LB | 17.8 | 16.5 | 2.67 | 2.48 | 1.34 | 1.24 | 36.6 | 38.7 |
| UBb | 76.2 | 80.4 | 11.4 | 12.1 | 5.71 | 6.03 | 76.4 | 81.6 | |
| Mink | LB | 38.5 | 8.50 | 2.89 | 0.64 | 1.40 | 0.31 | 0 | 0 |
| UB | 39.5 | 9.88 | 2.96 | 0.74 | 1.43 | 0.36 | 1.4 | 8.3 | |
bw: body weight; LB: lower bound; UB: upper bound; P95: 95th percentile.
The contributions from α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL have been corrected for their RPFs (see Section 1.3.1).
The Estimated mean exposure is higher than the P95 due to the very skewed distribution of the data.
It should be noted that there is considerable uncertainty in the estimate of exposure for rabbits. Two feeds, wheat feed and sunflower meal, accounted for over 85% of the exposure to ZEN and ZEN + α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL, yet for these feeds there were limited numbers of samples (n = 99 and 30, respectively), while 84% and 97% of the data, respectively, were left censored.
3.3.4.4. Companion animals (Dogs and cats)
Few data on levels of ZEN, α‐ZAL and β‐ZAL in proprietary feeds for dogs and cats were available, and therefore exposure was estimated using example rations (see Appendix D, Table D.5 for details) and concentrations of these toxins in individual feed materials. The exposures are reported in Table 17 (values corrected for RPFs as described in Section 1.3.1, Table 1).
Table 17.
Estimated P95 and mean exposure to the sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL by companion animals (dogs and cats) derived from concentrations in individual feed materials and their relative proportions in dietsa
| Species | Diet concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw | % of exposure (μg/kg bw) from modified forms | |||||
|---|---|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | P95 | Mean | ||
| Cats | LB | 55.0 | 19.47 | 3.30 | 1.17 | 0.82 | 0.29 | 8.5 | 24.1 |
| UB | 72.8 | 39.84 | 4.37 | 2.39 | 1.09 | 0.60 | 31.2 | 56.7 | |
| Dogs | LB | 63.5 | 27.46 | 22.9 | 9.9 | 0.91 | 0.40 | 12.1 | 30.0 |
| UB | 93.0 | 59.65 | 33.5 | 21.5 | 1.34 | 0.86 | 40.3 | 61.6 | |
bw: body weight;; LB: lower bound; UB: upper bound; P95: 95th percentile.
The contributions from α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL have been corrected for their RPFs (see Section 1.3.1).
3.3.4.5. Concluding remarks
There was considerable variation in the estimated exposure by farmed livestock and companion animals to ZEN. While the average LB and UB exposures at the mean and 95th percentile for all species were 1.00/1.14 and 5.18/5.24 μg/kg bw per day, respectively, the highest exposure to ZEN, both at the mean and 95th percentile was estimated for dairy cows fed a maize silage‐based diet (Mean LB/UB = 4.91/5.10 μg/kg bw per day; 95th percentile LB/UB = 32.6/32.9 μg/kg bw per day).
Similarly, there was variation between the farmed livestock and companion animals for exposure to the sum of ZEN + α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL (corrected for RPFs). The average LB and UB exposures across all species, at the mean and 95th percentile, were 1.04/2.20 and 5.28/6.23 μg/kg bw per day, respectively. Again, the highest estimated exposures were for dairy cows fed a maize silage‐based ration (P95 LB/UB = 32.36/33.1). These results clearly reflect the markedly higher levels of ZEN and its modified forms in maize silage than in any other forage or feed material reported in this Opinion (see Appendix E).
As indicated in Tables 13–17, there was considerable variation between livestock groups in the percentage of the total exposure (ZEN + α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL) that was accounted for by the sum of the modified forms (α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL). For many species, notably poultry, ZEN accounted for the total exposure (93–100%) because of lack of data for the modified forms of ZEN. For dairy cows, beef cattle, lactating sheep, horses, weaned pigs and rabbits the modified forms accounted for 50% or more of the total UB exposure, both at the mean and 95th percentile. More than half of the UB mean exposure for fattening pigs, lactating sows, cats and dogs was from the modified forms, but was < 50% at the 95th percentile.
The difference between LB and UB exposure to ZEN alone was low with the average UB value for all species approximately 1.1 times the LB value. When modified forms were included in the sum, the difference between LB and UB exposure increased notably, so that the average (across all species) of the UB was 64 and 33 times greater than the LB for the mean and P95 exposures (μg/kg bw).
3.4. Risk characterisation
There is limited knowledge on the effects of ZEN and its modified forms on farm and companion animals. Furthermore, there is no comprehensive database on feed consumption by livestock in the EU. It has therefore not been possible to fully assess the risks of ZEN and its modified forms for farm and companion animal health.
However, for a number of farm livestock and companion animal categories the chronic exposure of ZEN in feed could be estimated at the mean and 95th percentile concentrations in animal diets based on expected feed intakes and example diets. Furthermore, exposure to the sum of ZEN and modified forms was calculated by use of molar RPFs of the modified forms relative to the oestrogenic activity of ZEN derived from information in rodents (EFSA CONTAM Panel, 2016).
These exposures to ZEN and to the sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL have been compared with identified reference points (NOAELs and LOAELs) in farm and companion animals (Table 18 and 19). The identified NOAELs or LOAELs for sheep, pigs, poultry, fish and dogs were used for risk characterisation. For cattle, horses, rabbits, goats, ducks, cats and mink the health risk from the exposure to ZEN could not be assessed as no NOAELs or LOAELs have been identified.
Table 18.
Comparison of estimated ZEN exposure levels and NOAELs/LOAELs for different farm and companion animal species
| Estimated exposure (μg ZEN/kg bw per day)a | Estimated exposure, % of NOAEL or LOAEL | |||||
|---|---|---|---|---|---|---|
| NOAEL (μg ZEN/kg bw) | LOAEL (μg ZEN/kg bw) | P95 (UB) | mean (UB) | P95 (UB) | mean (UB) | |
| Sheep | 28 | 56 | 4.32 | 0.89 | 15.4 | 3.2 |
| Gilts | 40 | 200 | 3.17 | 1.16 | 7.9 | 2.9 |
| Piglets | 10.4 (NOEL) | 17.6 (LOEL) | 2.20 | 0.94 | 21.1 | 9 |
| Chickenb | 7,500 | 30,000 | 16.1 | 4.66 | 0.22 | 0.062 |
| Turkeys (fattening)c | 9,100 | 19,000 | c | 1.24 | c | 0.014 |
| Fish (carp) | 9 | 18 | 2.18 | 0.55 | 24.2 | 6.11 |
| Dogs | – | 25 | 0.70 | 0.25 | 2.8 (LOAEL) | 1 (LOAEL) |
bw: body weight; ZEN: zearalenone; NOAEL: no observed adverse effect level; LOAEL: lowest observed adverse effect level; UB: upper bound.
Exposures have been calculated from dietary concentrations expressed on a fresh weight (88% dry matter) basis to make them comparable with the data from which NOAELS/LOAELS have been derived.
Exposure have been calculated for chicken of the age as reported by Chi et al. (1980a,b) to allow comparison with the NOAEL
Insufficient samples were provided to allow reliable 95th percentile estimates of exposure to be made.
Table 19.
Comparison of estimated ZEN + modified forms (sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL) exposure levels and NOAELs/LOAELs for different farm and companion animal species
| Estimated exposure (μg ZEN /kg bw per day)a | Estimated exposure, % of NOAEL or LOAEL | |||||
|---|---|---|---|---|---|---|
| NOAEL (μg ZEN/kg bw) | LOAEL (μg ZEN/kg bw) | P95 (UB) | mean (UB) | P95 (UB) | mean (UB) | |
| Sheep | 28 | 56 | 6.92 | 2.59 | 24.7 | 9.3 |
| Gilts | 40 | 200 | 4.82 | 3.16 | 12.1 | 7.9 |
| Piglets | 10.4 (NOEL) | 17.6 (LOEL) | 6.16 | 4.90 | 59.2 | 47.1 |
| Fish (carp) | 9 | 18 | 2.54 | 0.90 | 28.2 | 10 |
| Dogs | – | 25 | 1.18 | 0.76 | 4.7 (LOAEL) | 3 (LOAEL) |
bw: body weight; ZEN: zearalenone; NOAEL: no observed adverse effect level; LOAEL: lowest observed adverse effect level; UB: upper bound.
Exposures have been calculated from dietary concentrations expressed on a fresh weight (88% dry matter) basis to make them comparable with the data from which NOAELS/LOAELS have been derived and molar potency factors relative to ZEN were applied for modified ZEN.
In Tables 18 and 19, exposure estimates (UB mean and 95th percentile) are presented together with NOAELs/LOAELs for the different farm and companion animal species. Exposure is expressed as a percentage of the NOAEL in the right‐hand columns. When a NOAEL is lacking, the LOAEL is used instead but provides a less conservative basis for comparison with exposure. Uncertainties affecting the assessment of exposure, toxicity and risk are listed and evaluated in Section 3.5. The estimates of exposure to ZEN and the sum of (ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL) are presented in Section 3.3.
Risk characterisation for ZEN alone (Table 18) was based on estimated UB exposure; however, the difference between UB and LB exposure was small for ZEN alone.
For ZEN alone, for sheep the calculated chronic exposure at the UB mean and UB 95th percentile were 3 and 16% of the identified NOAEL, respectively. This NOAEL was based on ovulation rates and lambing percentages. The Panel concluded that the risk of adverse health effects of feed containing ZEN was low for sheep
For piglets, the estimated exposure of ZEN at the UB mean and 95th percentile were 9 and 21% of the NOAEL, respectively. This NOAEL was based on the appearance of the vulva and the uterus weight. For gilts, the estimated chronic exposure of ZEN at the UB mean and UB 95th percentile were 3 and 8% of the NOAEL (based on prolonged cycling), respectively. The Panel concluded that the estimated risk for chronic adverse health effects from feed containing ZEN was low for piglets and gilts.
For poultry (chickens and fattening turkeys), the estimated exposure of ZEN at the UB mean or the P95th percentile was less than 0.06% of the NOAEL (based on the reduced number of lymphocytes and the vent swelling). The Panel concluded that the estimated risk for chronic adverse health effects from feed containing ZEN was extremely low for poultry.
For fish, a NOAEL was only available for carp and was extrapolated to all fish species. The estimated chronic exposure of ZEN at the UB mean and 95th percentile were 6 and 24% of the NOAEL, respectively. This NOAEL was based on reduced monocytes and granulocytes number, increased lipid peroxidation in liver and gill and altered metabolic activation. The Panel concluded that the estimated risk for chronic adverse health effects from feed containing ZEN was low for fish.
For dogs, only a LOAEL was available. The estimated exposure of ZEN at the UB mean and 95th percentile were 1 and 3%, respectively, of the LOAEL. This LOAEL was based on myometrium and endometrium lesions, enlargement and atrophy of uterine glands, blood haematology and biochemistry. The Panel concluded that the estimated risk for chronic adverse health effects from feed containing ZEN was low for dogs.
Risk characterisation for ZEN and its modified forms (Table 19 ) was based on UB exposures. Because of the high rate of left‐censored data for modified ZEN, the difference between UB and LB exposures is larger than for ZEN alone. The estimated exposures were compared with the NOAELs/LOAELs identified for ZEN, as no reference point could be identified for the modified forms.
The Panel noted that no data on levels of α‐ZAL, β‐ZAL, ZAN, α‐ZEL or β‐ZEL were provided in species‐specific compound feeds for poultry (laying hens, fattening chickens and fattening turkeys). However, considering the very high NOAELs for these species, and the composition of their feed, the Panel considered the risk of health effects from ZEN and its modified forms extremely unlikely for these species.
For sheep, the estimated chronic exposure of ZEN and its modified forms at the UB mean and UB 95th percentile were 9.3 and 25% of the NOAEL, respectively. The Panel concluded that the risk of adverse health effects of feed containing ZEN and its modified forms was low for sheep.
For piglets, the estimated chronic exposure of ZEN and its modified forms at the UB mean and UB 95th percentile were 47 and 59% of the NOAEL, respectively. For gilts, the estimated chronic exposure of ZEN and its modified forms were 8 and 12% of the NOAEL, respectively. The Panel concluded that the estimated risk for chronic adverse health effect from feed containing ZEN and its modified forms was low for piglets and gilts.
For fish, NOAEL was only available for carp and was extrapolated to all fish species. The estimated chronic exposure of ZEN and its modified forms were 10 and 28% of the NOAEL, respectively. The Panel concluded that the estimated risk for chronic adverse health effect from feed containing ZEN and its modified forms was low for fish.
For dogs, only a LOAEL was available. For this species, the estimated chronic exposure of ZEN and its modified forms were 3 and 5% of the LOAEL, respectively. The Panel concluded that the estimated risk for chronic adverse health effect from consuming feed containing ZEN and its modified forms was low for dog.
The CONTAM Panel noted that this risk characterisation of chronic risks for poultry, sheep, pig, fish and dogs should be considered indicative owing to the uncertainties associated to the identified reference points (see Section 3.5) and the dietary exposure to the modified forms of ZEN.
3.5. Uncertainty analysis
Table 20 lists the main sources of uncertainty identified by the Panel. It includes a qualitative assessment of whether each source of uncertainty leads to over/underestimation of the resulting risk. Sections 3.5.1, 3.5.2, 3.5.3 present in more detail the uncertainties affecting different parts of the risk assessment.
Table 20.
Summary of uncertainties
| Source of uncertainty | Parameter affecteda | ZENb | ZEN+modified formsb | Direction and approximate magnitude of uncertainty (+/−)c |
|---|---|---|---|---|
| Limitation in analytical identification and quantification of zearalenone modified forms: lack of calibrants and reference materials for validation | O | N | Y | − |
| Not adequate analytical sensitivity for zearalenone modified forms. | O | N | Y | − |
| Little occurrence data for zearalenone modified forms in EFSA database, especially for feed ingredients of animal origin, no data for some forms, data from 1 or 2 countries for other compounds. | O | N | Y | − |
| No data on ZEN glucosides and sulfates in the EFSA database (in contrast to literature data) | O | N | Y | − |
| Use of substitution method, UB occurrence data in the exposure estimations (applies only to the sum ZEN+modified forms) | O | N | Y | + |
| Imputation of missing occurrence data (using mean of measured data) (applies only to the sum ZEN+modified forms) | O | N | Y | +/− |
| Use of RPFs developed for ZEN modified forms group HBGV based on experiments in rodents | R | N | Y | +/− |
| Applicability of RPFs to different animal species with different metabolism | R | N | Y | + |
| No toxicological/not robust data | T | some species | Y | +/− |
| Toxicity data with naturally contaminated material (usually containing other mycotoxins) | T | some species | Y | +/− |
| Extrapolation from one fish species to another fish species (to check with species there are and not) | T | some species | some species | +/− |
| Differences between ages, sexes and breed | T | Y | Y | +/− |
| Missing information on body weight in toxicity studies | T | Y | Y | +/− |
| The number of samples were not equally distributed across all feed groups | O | Y Grass | Y | +/− |
| Effect of variation between countries, between sampling methods and over time, and uncertainty about moisture content, on extrapolation from occurrence data to 95th percentile for the EU | O | Y | Y | +/− |
| High variability of feedstuffs used and feeding systems for livestock | C | Y | Y | +/− |
| Example animal diets used to calculate animal exposure | C | Y | Y | +/− |
| Body weight assumptions | C | Y | Y | +/− |
RPF: relative potency factor; HBGV: health‐based guidance value.
C = Feed consumption, O = occurrence, R = RPFs, T = Toxicity.
Y = yes, N = No
+ = uncertainty with potential to cause over‐estimation of exposure/risk; − = uncertainty with potential to cause under‐estimation of exposure/risk.
The impact of the uncertainties in the risk assessment of farm and companion animals is large.
3.5.1. Uncertainty associated with occurrence and exposure
Occurrence
A final data set of 17,706 samples was available to estimate dietary exposure to ZEN and its modified forms. ZEN occurrence data were provided by 25 Member States, whereas data on the modified forms were provided by three Member States only. Therefore, there is large uncertainty on the possible country‐based and year based differences in the levels of ZEN modified forms in diverse feed commodities. It is known that mycotoxin accumulation in crops is strongly affected by climate change. The last harvest seasons have been characterised by an increasing trend of contamination, due to exceptional climatic conditions. In particular, in 2014 several EU countries obtained a derogation to MLs for ZEN, DON, and fumonisins in maize. However, data in the EFSA database cover a long period (2001–2015) and have been collected from different geographic area, thus resulting in a decrease in the uncertainty.
The analytical results were performed by different laboratories at varying LOQ/LODs. EFSA replaced left‐censored data with UB values based on the reported LOQ which has an impact on the uncertainty (see Section 2.1). Likewise, the lack of information on the analytical method used to analyse some feed samples (~ 37%) adds some uncertainty to the levels of ZEN and its modified forms reported for some feed commodities.
Feed composition
Representativeness of feeds analysed: As reported in Table 5, there is a wide discrepancy in the geographical spread of samples reported (possible over/underestimation).
Feed data: There were limited or no data available on some key ingredients, e.g. oilseed meals, fresh or conserved pasture grass. The formulations therefore assume no exposure from these feeds (possible underestimation).
Diet formulations: Single diet formulations have been assumed for each species, although as discussed above (Section 2.2) there are large differences in feeding systems and diet formulations for livestock and companion animals in the EU (possible over/underestimation).
Feed intakes
A single level of feed intake has been assumed for each livestock species/companion animal, but in practice this can vary for a given live weight or level of activity/productivity (possible over/underestimation).
Single levels of production or activity have been assumed, but these can vary markedly resulting in greater or lesser amounts of feed required or consumed (possible over/underestimation).
Details of the assumptions made for feed intake and diet composition for the different farmed livestock and companion animals are given in Appendix D.
Exposure assessment
Estimates of exposure to ZEN and its modified forms have been made using their concentrations in feeds and the amounts of those feeds consumed. In making these estimates, a number of assumptions have been made, particularly in respect of the types and amounts of feed consumed which will contribute to the uncertainty associated with the estimates of exposure. The main areas of uncertainty/concern relate to the extent to which the feeds reported are representative of feeds used for livestock and companion animals in the EU, the composition of the diets assumed for each of the livestock species/companion animals, and the estimates of feed consumed.
The Panel noted the wide range between the LB and UB values reported for the estimates of exposure. This is particularly the case for the sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL. The analytical results used for the exposure assessment were reported by different laboratories with varying LOQ/LODs. This, together with the large proportion of samples with left‐censored data (i.e. ZEN about 60%, ZAN about 70%, α‐ZEL and β‐ZEL, and α‐ and β‐ZAL about 100%), is likely to account for these large differences. Furthermore, for certain feeds (particularly for poultry), no data were presented for α‐ZAL, β‐ZAL, ZAN, α‐ZEL or β‐ZEL. Consequently, while the LB values tends to underestimate the chronic dietary exposure to ZEN and its modified forms, the use of UB values tend to overestimate it (see Section 2.1) and this therefore results in a large uncertainty to the overall estimates of dietary exposure.
It should be noted that there is considerable uncertainty in the estimate of exposure for rabbits. Two feeds, wheat feed and sunflower meal, accounted for over 85% of the exposure to ZEN and ZEN +α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL, yet for these feeds there were limited numbers of samples (n = 99 and 30, respectively), while 84% and 97% of the data, respectively, were left censored.
3.5.2. Uncertainties on the studies used for evaluation of adverse effects in farm and companion animals
No toxicological data on ZEN were available for cats. For other animal species such as cattle, goats, horses, rabbit and mink the toxicological data were too limited to allow the establishment of reference points for ZEN.
For fish, the only toxicological data were obtained for carps and therefore have to been extrapolated to all fish species.
Similarly, for poultry, toxicological data were only available for chicken and turkey. This contributed to the overall uncertainty.
For dogs, the experiment was performed on mixed breed animals, only LOAEL was determined and body weight of the animals was not reported.
For pig, the key study was performed with naturally contaminated maize, containing not only ZEN but also other mycotoxins. Assigning observed oestrogenic effects to ZEN might be justified although interactions with other mycotoxins, notably with DON, cannot be excluded (e.g. Tiemann and Dänicke, 2007; Döll and Dänicke, 2011).
Except for pigs no data were available on the effect of sex and age on the toxicity of ZEN. For all the animal species taken into consideration, no data were available on the possible difference of the different breeds. This contributed to the overall uncertainty.
Concerning the modified forms of ZEN, the toxicological data were either lacking or very limited. For the different animal species, it was not possible to identify any reference point for any modified form of ZEN.
3.5.3. Other uncertainties: Use of RPFs of the ZEN modified forms
Potency factors of the modified forms relative to ZEN (RPFs) were applied to occurrence levels of the respective ZEN metabolites according to the CONTAM Panel 2016 (see Table 1) (EFSA CONTAM Panel 2016). The CONTAM Panel noted that these RPFs were based on uterotrophic effects measured for ZEN and its modified forms in a rodent experiment and thereby not considering other oestrogenic effects and other endpoints. Furthermore, applying these RPFs derived from rodents to different farm and companion animal species does not consider the species differences in the toxicokinetics and toxicodynamics (animal species with different pattern of oestrogen receptors and metabolism).
4. Conclusions
Zearalenone is a phenolic resorcylic acid lactone mycotoxin produced by several Fusarium species, particularly F. graminearum. It can be modified in plants, fungi and animals by phase I and phase II metabolism. Modified forms of ZEN occurring in feed include its reduced phase I metabolites (i.e. α‐ and β‐ZEL, α‐ and β‐ZAL, ZAN) and its phase II conjugates, such as those conjugated with glucose, sulfate and glucuronic acid. α‐ZAL, one of the phase I metabolites of ZEN, is used as a growth promoter in non‐EU countries under the name of Zeranol. It is banned in Europe, and therefore it is included in official control plans.
Methods of analysis
Analytical methods for ZEN and its modified forms in feed are well‐established. However, while methods reported in the scientific literature are widely based on the more sensitive LC–MS/MS, most of the routine analyses are still performed by LC‐FLD or LC‐UV.
Calibrants for ZEN conjugates and reference materials for phase I and phase II modified forms are not commercially available.
Hazard identification and characterisation
Toxicokinetics in farm and companion animals
Zearalenone
Wide interspecies differences in ZEN absorption, distribution, metabolism and excretion have been documented.
Rumen, enteric, hepatic end extrahepatic ZEN metabolism has been reported; the nature and the amount of the generated metabolites may affect the species‐sensitivity to the toxin.
Reductive biotransformations largely prevail; the main ZEN metabolites are α‐ZAL, β‐ZAL, with only very limited amounts of α‐ZEL, β‐ZEL, and other reductive metabolites being produced; reduced metabolites retain or increase the oestrogenic potency of the parent compound.
Based on the levels measured in biological fluids of ZEN treated animals, the α‐derivatives seem to prevail in pigs, dogs, and turkeys while the β‐derivatives appear to be more abundant in cattle, goats, horses, broiler chickens and laying hens.
CYP‐mediated aromatic or aliphatic hydroxylation also occurs, but information in farm and companion animals is very limited.
In orally exposed animals, ZEN and its metabolites are rapidly absorbed, distributed to several organs and quickly excreted mainly via the biliary route as glucuronides; an active enterohepatic circulation has been demonstrated.
Excretion in milk and eggs has been documented for ZEN and its metabolites.
Ruminants
There is scant information on the toxicokinetics of ZEN in cattle; rumen microbes extensively metabolise ZEN to α‐ZEL and β‐ZEL, the latter being predominant also in biological fluids
Little is known on the toxicokinetics of ZEN in sheep; ZEN is almost completely biotransformed by ovine rumen fluid to α‐ZEL and β‐ZEL at similar proportions and both metabolites are present in urine as both free and conjugated forms
No studies have been identified on ZEN administration to goats by the oral route.
Pigs
In pigs, the generation of α‐ZEL largely outweigh that of β‐ZEL and other reductive metabolites which are recovered, along with ZEN in blood, urine, and bile mostly in their glucuronidated form.
Poultry
In poultry, ZEN is characterised by a low oral bioavailability and a rapid elimination.
β‐ZEL was largely predominant over α‐ZEL in broiler chickens and laying hens, while turkey poults tended to biotransform ZEN more extensively and synthesise a relatively higher amount of α‐ZEL; in all cases glucuronidation was the main conjugation reaction.
Horse
α‐ZEL and β‐ZEL are the main ZEN metabolites in the horse, the latter being predominantly formed over α‐ZEL under in vivo conditions.
Farmed Rabbit
Little is known about the toxicokinetics of ZEN in rabbits; ZEN, α‐ZEL and β‐ZEL were detected in colonic chyme, faeces, bile and urine of rabbits orally dosed with ZEN.
Fish
There is scant information concerning ZEN toxicokinetics in fish.
In rainbow trout, there is evidence of a prevalent generation of β‐ZEL versus α‐ZEL, while the reverse seems to occur in carp.
Farmed mink
No data on the toxicokinetics of ZEN and modified forms could be identified for farmed mink.
Companion animals
No information could be identified for cats.
In orally exposed dogs, measurable blood levels of ZEN, α‐ and β‐ZEL were detected with a clear prevalence of the α‐ over the β‐derivative (up to 100%).
ZEN modified forms
Little is known about the metabolic fate of modified forms, except for α‐ZAL, which is used legally as a growth promoter in some non‐EU countries under the name of zeranol.
In all species, α‐ZAL is oxidised to ZAN and isomerised to β‐ZAL; the parent compound and the metabolites are mainly glucuronidated.
In pigs, plant modified forms (ZEN14Glc, ZEN16Glc and ZEN14Sulf) are most likely hydrolysed in the gastrointestinal tract and thus contribute to ZEN overall toxicity.
Mode of action
The main biological activity of ZEN is its oestrogenic activity, i.e. the ability to act similar to the endogenous steroidal sex hormone 17‐β‐oestradiol. ZEN binds and activates oestrogenic receptors, and has a stronger affinity to ER‐α than to ER‐β.
ZEN and its modified forms differ considerably in their oestrogenic activity. Based on their uterotrophic activity assessed in rodents, ZEN and its modified forms are ranked as follows: α‐ZEL > α‐ZAL > ZEN ≈ ZAN ≈ β‐ZAL > β‐ZEL.
ZEN can activate the PXR and increase the transcription of a number of genes, including several CYPs.
Adverse effects and identification of reference points for risk characterisation in farm and companion animals for ZEN
Ruminants
Cattle appear to be more resistant to the adverse effects of ZEN than other farm animals because they biotransform ZEN more into β‐ZEL than α‐ZEL.
Limited adverse effects were observed in cattle at exposure levels of 48–1,456 μg/kg bw per day. However, due to limitations in the study, neither a NOAEL nor LOAEL could be established.
Based on ovulation rates and lambing percentages, a LOAEL of 56 μg ZEN/kg bw per day and a NOAEL 28 μg ZEN/kg bw per day was established for sheep.
No data were available for deriving reference points for risk characterisation for goats.
Pigs
Pigs are generally regarded as being a very sensitive species to ZEN, the most sensitive being prepubertal female piglets.
Based on the appearance of the vulva and the uterus weight, a NOEL of 10.4 μg ZEN/kg bw per day was established for piglets.
For sexually mature female pigs, a NOAEL of 40 μg/kg bw per day was identified, based on prolonged cycling.
Poultry
Poultry responds to the presence of ZEN in feed at rather high dietary concentrations and can generally be regarded as resistant.
Based on decreased number of lymphocytes and the vent swelling, NOAELs of 7,500 and 9,100 μg/kg bw per day were identified for chickens and turkeys, respectively.
For other poultry species and categories, data are scarce. Therefore, LOAELs/NOAELs could not be derived.
Horses
The only available study performed with the purified mycotoxin (11–13 μg ZEN kg bw per day for 8–10 days) did not allow to derive a NOAEL or a LOAEL due to poor experimental design and the lack of a control group.
Farmed Rabbit
No oestrogenic effect at 100 μg/kg bw were observed. However, at 10 μg/kg bw per day a transient increase of catecholamine was observed.
No NOAEL or LOAEL could be established for farmed rabbits.
Fish
Very limited toxicity data are available for fish for ZEN.
The CONTAM Panel estimated a NOAEL for carp of 0.3 mg/kg feed to correspond to 9 μg/kg bw per day. It was based on changes in monocytes and granulocytes number, lipid peroxidation in liver and gill and alterations of metabolic activation.
No effect level to characterise the hazard of ZEN could be established for other fish species.
Companion animals
Dogs are considered sensitive to ZEN. No NOAEL could be established for dogs. Based on myometrium and endometrium lesions, enlargement and atrophy of uterine glands, blood haematology and biochemistry, a LOAEL of 25 μg ZEN/kg bw per day has been estimated for mature bitches.
No data could be identified concerning the effects of ZEN in cats.
Farmed Mink
Only studies performed with high ZEN dosages of 10 or 20 mg/kg feed (corresponding to about 1,000 or 2,000 μg ZEN / kg bw per day) are available.
Overt oestrogenic effects were detected already at the lowest tested dose; therefore, the available studies were not suitable for deriving reference point of hazard characterisation.
Adverse effects and identification of reference points for risk characterisation in farm and companion animals for ZEN modified forms
Very few experiments investigated the adverse effect of the modified form of ZEN on livestock species, horses, fish and dogs and none of them were suitable to derive a NOAEL or LOAEL.
Occurrence in Feed
The dietary exposure was estimated using a final data set of 17,706 analytical results. Data were representing most of the feed commodities with potential presence of ZEN. The percentage of left‐censored data reported (results below limit of detection and/or limit of quantification) was high (ZEN about 60%, ZAN about 70%, α‐ZEL and β‐ZEL, and α‐ and β‐ZAL up to 100%).
Samples for ZEN were collected between 2001 and 2015 in 25 different European countries, whereas samples on the modified forms mostly from between 2013 and 2015 in only three Member States.
Apart from ‘Cereal grains, their products and by‐products’ and ‘Compound feed’ only a limited number of quantified data were available for other feed groups, i.e. forages, land animal products, legume seeds, minerals, oil seeds and tubers. The highest number of reported samples for ZEN was available for ‘Cereal grains, their products and by‐products’ (˜67%) and in particular ‘Wheat’ (n = 6,499). Other well represented feed groups that were well represented were ‘Complementary/Complete feed’ (n = 2,625), ‘Maize and corn’ (n = 2,048) and ‘Barley’ (n = 1,596). Although an important ingredient in commercial rabbit diets, no data were reported for Lucerne meal.
The occurrence assessment for ZEN reported in the literature is consistent with data in the EFSA database. Concerning the occurrence of modified forms, phase II conjugated forms have often been reported in the recent literature, whereas no data have been received by EFSA.
The co‐occurrence of ZEN and its phase I and phase II modified is mainly reported in cereals and products thereof.
While milling may lead to a redistribution of ZEN and its modified forms in the final fractions with a possible enrichment in middlings, there is no evidence of significant degradation by processing.
Use of RPFs for the ZEN modified forms
RPFs of the modified forms relative to ZEN, ranging from 0.2 to 60, were applied to occurrence levels of the respective ZEN metabolites according to CONTAM Panel 2016. The CONTAM Panel noted that these RPFs were based on uterotrophic effects measured for ZEN and its modified forms in rodent experiments. However, the application of these RPFs derived from rodents to different farm and companion animal species does not take into account the species differences in the toxicokinetics and toxicodynamics.
Farm and companion animal exposure to ZEN and its modified forms
Exposure to ZEN and its modified forms is primarily from consumption of contaminated cereal grains and cereal by‐products. Except for forage maize (and maize silage produced from it) and cereal straw, levels in forages are generally low.
Exposure to ZEN
The mean lowest LB to highest UB exposures of dairy cows and beef cattle to ZEN ranged from 0.06 to 5.1 μg/kg bw per day, and the P95 exposures ranged from 0.30 to 32.9 μg/kg bw per day.
For sheep and goats, the calculated lowest LB to highest UB mean exposures to ZEN were 0.18 and 1.78 μg/kg bw per day, respectively, while at the 95th percentile the range was from 0.27 (LB) to 10.8 (UB) μg/kg bw per day.
The calculated mean LB and UB exposures for pigs were 0.81 and 1.35 μg/kg bw/day, respectively, while the 95th percentile exposures ranged from 2.50 (LB) to 7.88 (UB) μg/kg bw per day, respectively.
For poultry, the calculated mean exposure ranged from 0.74 (LB) to 3.64 (UB) μg/kg bw per day. The equivalent range for the 95th percentile estimates of exposure was 3.46 and 12.7 μg/kg bw per day, respectively.
For horses, the calculated LB and UB mean exposure estimates to ZEN were 0.18 and 0.89 μg/kg bw per day, respectively, while for the 95th percentile the range LB–UB was 0.35–3.69 μg/kg bw per day.
For farmed salmonids and carp,, the calculated mean LB and UB exposures ranged from 0.08 to 0.50 μg/kg bw per day, respectively. At the 95th percentile, LB and UB estimates of exposure were 0.49 and 1.78 μg/kg bw per day, respectively.
The calculated mean exposure for farmed rabbits ranged from 0.76 (LB) to 1.11 (UB) μg/kg bw per day, while the equivalent range for the 95th percentile was from 0.85 to 1.35 μg/kg bw per day.
The mean calculated daily exposure for farmed mink ranged from 0.31 (LB) to 0.33 (UB) μg/kg bw per day, while the equivalent range for the 95th percentile was from 1.40 to 1.41 μg/kg bw per day.
For companion animals (cats and dogs), the calculated LB and UB mean exposure to ZEN ranged from 0.22 to 0.33 μg/kg bw per day, respectively, while at the 95th percentile the range was from 0.75 (LB) to 0.80 (UB) μg/kg bw per day.
Farm and companion animal exposure to the sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL
There was considerable variation between livestock groups in the percentage of the total exposure (ZEN + α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL) that was accounted for by the sum of the modified forms (α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL).
For many species, notably poultry, ZEN accounted for the total exposure (100%) because of lack of data of ZEN modified forms.
At the LB exposure, the modified forms accounted for 0 to approximately 39% of the total exposure at both the mean and 95th percentile for cattle, goat, sheep, horses, pigs, fish, rabbits, cats, dogs and mink.
At the UB exposure, the modified forms accounted for 50% or more of the total exposure, both at the mean and 95th percentile for dairy cows, beef cattle, lactating sheep, horses, weaned pigs and rabbits. More than half of the UB mean exposure for fattening pigs, lactating sows, cats and dogs was from the modified forms, but was < 50% at the 95th percentile.
The Panel noted that estimating the occurrence and exposure with such a high number of left‐censored data leads to a very high uncertainty.
Farm and companion animal health risk characterisation
The risk characterisation of exposure to ZEN is evaluated taking into consideration the comparison between the exposure of ZEN alone, and the identified NOAELs/LOAELs for chronic adverse effects.
The risk characterisation of exposure to ZEN and its modified forms is evaluated based on the comparison between the exposure of ZEN and its modified forms (sum of ZEN, α‐ZAL, β‐ZAL, ZAN, α‐ZEL and β‐ZEL corrected for molar RPFs, and the identified NOAELs/LOAELs for chronic adverse effects of ZEN as well as the uncertainty analysis.
For cattle, horses, rabbit, duck, goats, mink and cats, the health risk from the exposure to ZEN and to ZEN and its modified forms could not be assessed as no NOAEL or LOAEL have been identified.
For poultry, the risk of adverse health effect of feed containing ZEN was considered extremely low.
For pigs (gilt and piglets), sheep, dog and fish, the risk of adverse health effect of feed containing ZEN was considered low.
The same conclusions apply to the sum of ZEN and its modified forms.
5. Recommendations
More data are needed on the occurrence of modified forms of ZEN in feed.
Calibrants and reference materials are needed for the development of properly validated and sensitive routine analytical methods for ZEN modified forms.
Highly sensitive analytical methods should be designed for the most potent modified form α‐ZEL.
Modified forms of ZEN, especially the more potent α‐ZEL, should be included in official monitoring plans to gather more data on the occurrence on ZEN modified forms in feed.
Well‐designed studies are needed for livestock species, particularly for cattle, horses, rabbits, poultry, companion animals and mink to study toxicokinetics and toxicity of ZEN to reduce the uncertainties in the animal risk assessment.
Information is needed for livestock species, fish, companion animals and mink on toxicokinetics and toxicity of ZEN and its modified forms to perform the animal risk assessment.
Abbreviations
- ADME
absorption, distribution, metabolism and excretion
- AhR
aryl hydrocarbon receptor
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- Apo
apolipoprotein
- AST
aspartate aminotransferase
- bw
body weight
- cmax
peak concentrations
- CAR
constitutive androstane receptor
- CAS
Chemical Abstracts Service
- CONTAM Panel
EFSA Panel on Contaminants in the Food Chain
- CYP
cytochrome P450
- COMT
catechol‐O‐methyltransferase
- DON
deoxynivalenol
- DM
dry matter
- E2
17β‐oestradiol
- EIA
enzyme immunoassay
- ELISA
enzyme‐linked immunosorbent assay
- ERs
oestrogen receptors
- Glc
glucoside
- GC
gas chromatography
- GGT
gamma‐glutamyl transferase
- hAR
human androgen receptor
- Hb
haemoglobin
- HDL‐C
high‐density lipoprotein cholesterol
- HPLC
high‐performance liquid chromatography
- HPLC‐FLD
high‐performance liquid chromatography–fluorescence detection
- HPLC–MS/MS
high‐performance liquid chromatography–tandem mass spectrometry
- HPTLC
high‐performance thin‐layer chromatography
- HSD
hydroxysteroid dehydrogenase
- i.m.
intramuscular
- i.p
intraperitoneal
- i.v.
intravenous
- LB
lower bound
- LC
liquid chromatography
- LC–MS/MS
liquid chromatography–tandem mass spectrometry
- LC‐FLD
liquid chromatography‐fluorescence detector
- LC‐UV
liquid chromatography‐ultraviolet detector
- LDH
lactate dehydrogenase
- LDL‐C
low‐density lipoprotein cholesterol
- log Kow
log partition coefficient between octanol and water
- LOD
limit of detection
- LOQ
limit of quantification
- LOAEL
lowest observed adverse effect level
- LOEL
lowest observed effect level
- MCH
mean cell haemoglobin
- MCV
mean cell volume
- m.p.
melting point
- MS
mass spectrometry, mass spectrum
- NDV
Newcastle disease virus
- NOAEL
no observed adverse effect level
- NOEL
no observed effect level
- NOAEL
no observed adverse effect level
- PCNA
proliferating cell nuclear antigen
- p.o.
per os
- PXR
pregnane X receptor
- RAL
β‐resorcylic acid lactone
- ROS
reactive oxygen species
- RPF
relative potency factor
- P4
progesterone
- SPE
solid‐phase extraction
- Sulf
Sulphate
- SULT
sulfotransferase
- TC
total cholesterol
- TG
thyroglobulin
- TDI
tolerable daily intake
- tmax
peak time
- t1/2
terminal half‐life
- t“ el
terminal half‐life elimination
- UB
upper bound
- UGT
uridine diphosphate glucuronosyltransferase
- WG
working group
- WHO
World Health Organization
- ZAL
zearalanol
- ZAN
zearalanone
- ZEN
zearalenone
- ZEN14Glc
zearalenone‐14‐glucoside
- ZEN16Glc
zearalenone‐16‐glucoside
- ZENSulf
zearalenone sulfate (Position yet to be defined)
- ZEN14Sulf
zen‐14‐sulfate
- α‐ZAL
α‐zearalanol
- α‐ZEL
α‐zearalenol
- β‐ZAL
β‐zearalanol
- β‐ZEL
β‐zearalenol
- β‐ZEL14Glc
β‐zearalenol‐14‐glucoside
Appendix A – Chemical information on zearalenone and modified forms
| Common name | Abbreviation | Formula | MW | Occurrence | Structure | |
|---|---|---|---|---|---|---|
| Parent compound | Zearalenone | ZEN | C18H22O5 | 318 | Microorganisms |
|
| Phase I metabolites | α‐Zearalenol | α‐ZEL | C18H24O5 | 320 | Plants, microorganisms, animals |
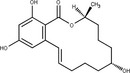
|
| β‐Zearalenol | β‐ZEL | C18H24O5 | 320 | Plants, microorganisms, animals |
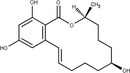
|
|
| Zearalanone | ZAN | C18H24O5 | 320 | Plants, microorganisms, animals |
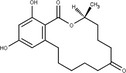
|
|
| α‐Zearalanol* | α‐ZAL | C18H26O5 | 322 | Plants, microorganisms, animals |
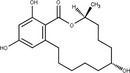
|
|
| β‐Zearalanol | β‐ZAL | C18H26O5 | 322 | Plants, microorganisms, animals |
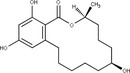
|
|
| Phase II metabolites | Zearalenone‐14‐glucoside | ZEN14Glc | C24H32O10 | 480 | Plants, microorganisms |
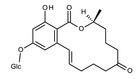
|
| Zearalenone‐16‐glucoside | ZEN16Glc | C24H32O10 | 480 | Plants, microorganisms |
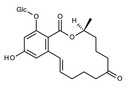
|
|
| α‐Zearalenol‐14‐glucoside | αZEL14Glc | C24H34O10 | 482 | Plants, microorganisms |
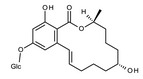
|
|
| β‐Zearalenol‐14‐glucoside | βZEL14Glc | C24H34O10 | 482 | Plants, microorganisms |
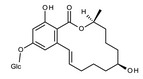
|
|
| Zearalenone‐14‐sulfate | ZEN14Sulf | C18H22O8S | 398 | Plants, microorganisms |
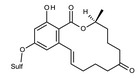
|
|
| α‐Zearalenol‐sulfate | α‐ZELSulf | C18H24O8S | 400 | Plants, microorganisms |
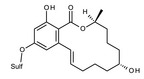
|
MW: molecular weight.
* α‐Zearalanol (e.g. Ralgro®) maybe used as a growth promoter in food producing species in non EU‐countries.
Appendix B – Identification and selection of evidence relevant for the risk assessment of zearalenone and its modified forms in feed
B.1. Search for scientific literature
Table B.1.
Search terms and information source for literature search on zearalenone
| Chemistry and analysis | |
| Search terms | (zearal*) AND (chemistry OR analysis OR determination OR detection OR identification OR formation OR GC OR “GC‐MS” OR HPLC OR” LC‐MS” OR “ICP‐MS”) |
| Metabolism, Kinetics | |
| Search terms | (zearal*) AND (toxicokinetic* OR “absorption” OR distribution OR metabolism OR “excretion OR elimination OR bioconcentration OR biotransformation OR “half‐life” OR “half‐lives” OR modelling OR “carry‐over” OR transfer) |
| Toxicity | |
| Search terms | (zearal*) AND (toxicity OR toxi* OR acute OR subacute OR subchronic OR chronic OR mutagen* OR carcino* OR genotox* OR reprotox* OR nephrotox* OR neurotox* OR hepatotox* OR immunotox* OR haemotox* OR hematotox* OR haematotox* OR cytotox* OR “develop* toxicity” OR thyroid OR endocri* OR uter* OR “endocrine” OR OR estrogen OR oestrogen OR poisoning OR “incidental poisoning” OR rat OR mouse OR “lab animal” OR animal* OR “case studies”) |
| Occurrence and exposure | |
| Search terms | (zearal*) AND (“feed occur”* OR feed OR feedstuffs OR “feed‐stuffs” OR “feed occurrence” OR grains OR barley OR wheat OR maize OR oat OR rice OR rye OR spelt OR “compound feed” OR seeds OR “complete feed” OR “animal exposure” OR “feed intake”) |
| Animal species | |
| Search terms | (zearal*)AND (Livestock OR “farm animals” OR Ruminants OR Cattle OR cows OR heifer OR bovine OR Sheep OR ovine OR goats OR swine OR pigs OR sow OR “lactating sow” OR piglet OR gilts OR poultry OR chickens OR hen OR turkeys OR ducks OR Quail OR “guinea‐fowl” OR Rabbits OR Fishes OR Trout OR Salmon OR zebrafish OR Horses OR herd or equine OR mare OR Pets OR Cats OR Dogs OR bitch OR mink) |
B.2. Exclusion criteria for abstracts
The titles and abstracts of these references were screened to identify the relevant papers. Papers on the following subjects were excluded:
Papers on other mycotoxins, test substance not zearalenone or their modified forms.
Papers in non EU languages, with no English abstract.
Studies on occurrence in food.
Papers on adverse effects in humans.
Papers on human dietary exposure.
Meeting abstracts with no scientific paper published, letter to the editor, extended abstracts and conference proceedings.
Appendix C – EFSA guidance documents applied for the risk assessment
EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA Journal 2005;3(10):282, 31 pp. https://doi.org/10.2903/j.efsa.2005.282
EFSA (European Food Safety Authority), 2006. Guidance of the Scientific Committee on a request from EFSA related to uncertainties in Dietary Exposure Assessment. EFSA Journal 2006;4(5):438, 54 pp. https://doi.org/10.2903/j.efsa.2006.438
EFSA (European Food Safety Authority), 2009a. Guidance of the Scientific Committee on use of the benchmark dose approach in risk assessment. EFSA Journal 2009;7(6):1150, 72 pp. https://doi.org/10.2903/j.efsa.2009.1150
EFSA (European Food Safety Authority), 2009b. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: General principles. EFSA Journal 2009;7(5):1051, 22 pp. https://doi.org/10.2903/j.efsa.2009.1051
EFSA (European Food Safety Authority), 2010. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. https://doi.org/10.2903/j.efsa.2010.1557
EFSA (European Food Safety Authority), 2011a. Use of BMDS and PROAST software packages by EFSA Scientific Panels and Units for applying the Benchmark Dose (BMD) approach risk assessment. Technical Report. EFSA Supporting Publications 2011, EN‐113, 190 pp.
EFSA (European Food Safety Authority), 2011b. Guidance of EFSA on the use of the EFSA Comprehensive European Food Consumption Database in Intakes Assessment. EFSA Journal 2011;9(3):2097, 34 pp. https://doi.org/10.2903/j.efsa.2011.2097
EFSA (European Food Safety Authority), 2011c. Overview of the procedures currently used at EFSA for the assessment of dietary exposure to different chemical substances. EFSA Journal 2011;9(12): 2490, 33 pp. https://doi.org/10.2903/j.efsa.2011.2490
EFSA (EFSA Scientific Committee), 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. https://doi.org/10.2903/j.efsa.2012.2579
EFSA (EFSA Scientific Committee), 2012b. Scientific Opinion on Risk Assessment Terminology. EFSA Journal 2012;10(5):2664, 43 pp. https://doi.org/10.2903/j.efsa.2012.2664
EFSA Scientific Committee 2016. Draft Guidance on Uncertainty in EFSA scientific assessment. EFSA Journal. Draft for Internal Testing. Available online: http://www.efsa.europa.eu/sites/default/files/160321DraftGDUncertaintyInScientificAssessment.pdf
Appendix D – Feed intakes and diet composition (livestock)
To estimate exposure to zearalenone and its modified forms, information on both the amount of feed consumed and the concentration of these compounds in the feed is required. This Appendix gives details of the feed intakes, live weights and diet compositions for different livestock, fish and companion animals used as the basis to estimate exposures. These are based on published guidelines on nutrition and feeding (e.g. Carabaño and Piquer, 1998; NRC 2000, 2007a,b, Leeson and Summers, 2008, McDonald et al., 2011; EFSA FEEDAP Panel, 2012; OECD, 2013) and information provided by European feed manufacturers. They are therefore estimates of the Panel on Contaminants in the Food Chain (CONTAM Panel), but are in agreement with common practice. In Appendix E the concentrations of zearalenone and its modified forms in feeds used to estimate exposure are presented.
D.1. Feed intakes
D.1.1. Cattle, sheep, goats and horses
Dairy cows
The amounts of feed given to lactating dairy cows varies according to the amount and quality of forages and other feeds available, the milk yield and the size of the cow. In this Opinion, it is assumed that non‐forage feeds are fed at the rate of 0.3 kg/kg of milk produced (Nix, 2010). Exposures to zearalenone and the sum of its modified forms have been estimated for a 650‐kg dairy cow, with a milk yield of 40 kg/day, reflecting a relatively high milk yield. Assumptions on the amounts of forages and non‐forage feed are given in Table D.1.
Beef cattle
There are a wide variety of beef production and husbandry systems in Europe. They may be categorised broadly as forage‐based or cereal‐based systems, although combinations of these systems are commonly found. In this opinion, four feeding systems are considered, in which the forages are (1) grass hay (2) maize silage and (3) cereal straw with, in each case, appropriate supplementation with non‐forage feed materials. A fourth system, namely ‘cereal beef’, is also considered. For exposure estimates, live weights of 300 or 400 kg, and feed intakes of between 6.6 and 10 kg dry matter (DM)/day have been assumed, depending on the feeding regime, based on guidelines published by EBLEX (2015, 2016), and details are given in Table D.1.
Sheep and goats
A large number of breeds and systems of management have been developed for sheep and goats to suit the land, climate and husbandry conditions in the EU. As for other ruminants, forages may be the only feeds used after weaning (NRC, 2007a). Common exceptions to this are pregnant and lactating animals, whose feed is usually supplemented with non‐forage feeds or commercial compound (complementary) feeds (AFRC, 1993; NRC, 2007a). In this Opinion, exposure estimates have been made for lactating sheep and goats. The CONTAM Panel has used a daily dry matter intake of 2.8 kg for an 80‐kg lactating sheep feeding twin lambs to estimate the exposures. For lactating goats, the CONTAM Panel has used daily dry matter intakes of 3.3 kg for a 60‐kg goat for milking (4 kg milk/day). No estimates of exposure have been made for fattening sheep and goats. For these livestock, fresh or conserved forages are frequently the sole feed, and levels of zearalenone and modified forms in these feeds are generally low or not reported. Furthermore, no data have been provided for complementary feeds for fattening sheep and goats to allow exposure in those situations where forages are not the sole feed.
Horses
Horses are non‐ruminant herbivores. They generally consume 2–3.5% of their body weight in feed (dry matter) each day, of which a minimum of 50% should be as forage (pasture or hay) (NRC, 2007b). Mature horses with minimal activity can be fed forage alone, but for growing and active horses supplementary feeding with cereal grains, cereal by‐products (e.g. oats, barley, and wheat bran) and vegetable proteins is necessary. The CONTAM Panel has estimated two exposure scenarios for a 450‐kg horse, with a daily intake of 9 kg DM/day; in the first, it is assumed that fresh grass is the forage, and therefore makes no contribution to zearalenone exposure, while in the second scenario grass hay is the forage (Table D.1).
D.1.2. Non‐ruminant animals
Pigs
Although there is a considerable range of pig production systems in Europe, exposure estimates have been made for piglets (pig starter), finishing pigs, lactating sows (using feed intakes proposed by EFSA FEEDAP Panel, 2012) and gilts (Table D.2).
Poultry
The CONTAM Panel applied the live weights and feed intakes reported for fattening chickens (broilers), laying hens and turkeys proposed by EFSA FEEDAP Panel (2012) and for ducks by Leeson and Summers (2008) (Table D.2)
Farmed fish (salmonids and carp)
Commercially reared species include Atlantic salmon, rainbow trout, sea bass, sea bream, cod, halibut, tuna, eel and turbot. In this Scientific Opinion, exposures to zearalenone and its modified forms have been made for farmed salmon and carp. Details of the body weights and feed intakes used are given in Table D.2.
Rabbits
Feed intakes of 65–80 g/kg body weight (bw) per day have been reported (Carabaño and Piquer, 1998). For the exposure estimates, the CONTAM Panel have assumed a live weight of 2 kg, and a daily feed intake of 75 g/kg bw (derived from Carabaño and Piquer, 1998).
Farmed mink
For estimating exposure, the CONTAM Panel have assumed a live weight of 2.07 kg for a male mink at pelting, and with a feed intake of 227 g/day (75 g dry matter) (NRC, 1982).
Companion animals: Dogs and cats
The amount of food consumed is largely a function of the mature weight of the animal, level of activity, physiological status (e.g. pregnancy or lactation) and the energy content of the diet. In this Scientific Opinion, the CONTAM Panel assumed body weights (kg) and feed intakes (g dry matter/day) for dogs and cats were 25/360 and 4/60, respectively (derived from NRC, 2006).
D.2. Diet composition
Many livestock in the European countries are fed proprietary commercial compound feeds. Where sufficient data have been provided on species‐specific compound feeds, estimates of exposure have been made using these data together with estimated intakes given in Appendix D.1. Where data on proprietary compound feeds were not available, or were available but in insufficient numbers, estimates of exposure have been made using dietary inclusion rates of feed materials given in this section.
D.2.1. Cattle, sheep, goats and horses
For most ruminants and horses, forages (either fresh or conserved) are important ingredients in their diet, but they are normally supplemented with non‐forage feeds such as cereals, cereal by‐products, oilseed meals and by‐products of human food production. These may be fed either as individual feeds, mixtures of feed materials or as species‐specific complementary feeds in the form of compound feeds. In some situations, however, forages may represent the total diet.
Fresh (grazed) grass or grass silage are the principal forages for ruminants and horses in the EU. As reported elsewhere in this Opinion (Section 3.3), zearalenone and its modified forms have not been reported in these feeds, and therefore, it has been assumed that where they are fed they make no contribution to exposure. For other forages, however, notably grass hay, maize silage and cereal straw, the presence of zearalenone has been reported. Therefore, two estimates of exposure have been reported for ruminants and horses, the first of which assumes no exposure from forages (i.e. the main forages are fresh grass and/or grass silage). Exposures have also been estimated for diets in which grass hay, maize silage or cereal straw are the forage.
For lactating dairy cows and fattening beef cattle, data for species‐specific compound feeds were provided (Table 7) and therefore these were used to estimate exposure to ZEN in these diets. AFSSA (2009) have provided example intakes of dairy cows fed maize silage supplemented with maize grain and soybean meal, while example diets of beef cattle on maize silage or cereal straw‐based diets are taken from EBLEX (2005, 2015, 2016), and these are given in Table D.3.
Table D.3.
Feed intakes of high yielding lactating dairy cows (40 L/day) and beef cattle fed diets based on different forages with non‐forage feeds adjusted for milk yield, and beef cattle)
| Species | Quantities of feed consumed (kg dry matter/day) | Reference | ||||
|---|---|---|---|---|---|---|
| Forage | Maize grain | Soybean meal | Barley grain | Rapeseed meal | ||
| Lactating dairy cows: maize silage‐based diet | 15.0 | 9.5 | 2.8 | – | – | AFSSA (2009) |
| Fattening beef cattle: maize silage‐based diet | 4.9 | – | – | – | 1.5 | EBLEX (2015) |
| Beef cattle: cereal straw‐based diet | 2.5 | – | – | 4.1 | 1.4 | EBLEX (2016) |
| Beef cattle: “Cereal beef” | 1.5 | – | – | 5.5 | 1.5 | EBLEX (2005) |
For lactating sheep and goats, levels of zearalenone and its modified forms in species‐specific compound feed data were available and therefore these have been used to estimate exposure.
Horses are non‐ruminant herbivores, and consequently their diet should contain a minimum of 50% forages. While mature horses with minimal activity can be fed forage alone (NRC, 2007b), for growing and active horses supplementary feeding with cereal grains, cereal by‐products (e.g. oats, barley, and wheat bran) and vegetable proteins is necessary. Although oats are the preferred cereal for many horse owners, other cereal grains and cereal by‐products are also routinely used. In the absence of sufficient data on levels of zearalenone and an its modified forms in complementary feeds for horses, the CONTAM Panel has The CONTAM Panel has estimated the exposure for a 450‐kg horse, with a daily intake of 9 kg DM/day, of which half is in the form of grass hay and where cereal grains, their products and by‐products represent 82% of the non‐forage component of the daily ration (Table D.5).
Table D.4.
Assumed diet composition (non‐forage feed) for horses
| Feed materialsa | Horses |
|---|---|
| Oats (%) | 40 |
| Beans (%) | 10 |
| Wheat feed (%) | 30 |
| Oat feed (%) | 12 |
| Molasses (%) | 5 |
| Minerals, vitamins etc. (%) | 3 |
See Commission Regulation (EU) No 575/2011 of June 2011 for full description.15
D.2.2. Pigs and poultry
Sufficient data for species‐specific compound feeds or pigs, and for most categories of poultry (fattening chickens and turkeys, and for laying hens), were provided for zearalenone (Appendix D) and have been used to estimate exposure. No data were provided for α‐ZAL, β‐ZAL, ZAN, α‐ZEL or β‐ZEL in compound feeds for laying hens, fattening hens and fattening turkeys, and therefore, no estimates of exposure to ZEN plus the modified forms have been made for these poultry. No compound feed data were available specifically for gilts, and therefore the occurrence data for fattening pigs have been used to estimate exposure.
In common with other poultry, ducks have limited ability to digest fibre,16 and therefore, cereal grains form the major part of their diets. In Europe, wheat, maize and barley are most commonly used, with rye, sorghum triticale and oats used less widely. Other ingredients include cereal by‐products and vegetable proteins, supplemented with minerals, trace elements and vitamins (Leeson and Summers, 2008; McDonald et al., 2011). An example diet used in estimating exposure by ducks to Zearalenone and its modified forms is given in Table D.5.
D.2.3. Rabbits
Rabbits are usually fed a pelleted diet (in the form of complete feedingstuffs) consisting of dried forages, cereals and vegetable proteins supplemented with minerals, vitamins and trace elements. Lebas and Renouf (2009) reviewed diet formulations used in experimental studies: in 58 diets, cereals and cereal by‐products (mostly wheat bran) cereals, cereal by‐products (mostly wheat bran) and oilseed meals (mostly soybean meal and sunflower meal) were 18–20%, 18–20% and 16%, respectively. Dried lucerne is a particularly important ingredient in some diets, and has been included at levels of up to 65% (Lebas and Renof, 2009). No data on levels of zearalenone in lucerne meal were available to EFSA, and therefore, this feed has not been included in the example diet used to estimate exposure. However, since zearalenone occurs predominantly in cereals, the lack of data for lucerne meal is unlikely to result in a major underestimation of exposure, while its inclusion may have resulted in a lower estimate of exposure. In this Scientific Opinion, the feed ingredients used in a typical French commercial rabbit compound, as provided by T. Gidenne (Personal communication, 2011) have been used, details of which are given in Table D.5.
D.2.4. Farmed fish (salmonids and carp)
Traditionally, the principal raw materials used for the manufacture of fish feeds in Europe have been fishmeal and fish oils, and although alternative sources of oil and protein (e.g. soybean meals and vegetable oils) are increasingly being used fish‐derived feeds still remain the major ingredients.
For many fish species, digestion of complex carbohydrates and the metabolic utilisation of the absorbed glucose are low, reflecting the scarcity of carbohydrates in the aquatic environment (Guillaume et al., 2001). Instead, fish obtain much of their energy from protein in the diet. Where carbohydrates are used, they generally require some form of pretreatment (e.g. cooking, flaking or toasting).
Berntssen et al. (2010) provided details of the composition of a diet for growing salmonids, and the CONTAM Panel used this feed formulation to estimate the exposures (Table D.5).
In contrast, studies with the common carp (Cyprinus carpio) have demonstrated greater intestinal amylase activity than in carnivorous fish, which accounts for the better utilisation of carbohydrates by these fish. The optimum level of carbohydrates appears to be 30–40% (FAO, Aquaculture Feed and Fertiliser Resources Information System17), which allows for higher levels of cereals than in diets for salmonids. The CONTAM Panel have adopted the ingredients of commercial compound feeds for carp reported by Schultz et al. (2012) to estimate exposure to ZEN and its modified compounds.
D.2.5. Farmed mink
Mink are carnivorous animals and are fed high protein diets consisting mainly of meat and meat by‐products. Commercially manufactured mink feed consists largely of fish and land animal by‐products, with lesser amounts of cereals and cereal by‐products, and supplemented with mineral/vitamin premixtures. Mink are fed diets high in protein, although their nutritional requirements vary according to the animal's physiological stage (e.g. gestating, lactating, growing) and climatic conditions, particularly temperature. The proportions of cereal grains, their products and by‐products used in estimating the exposure are given in Table D.5.
D.2.6. Companion animals (Cats and dogs)
Most small companion animals derive their nutritional needs from processed food, and in 2010 EU annual sales of pet food products was approximately 8.3 million tonnes.18 Although a wide range of ingredients is used in commercial diets, most dog and cat diets contain at least some animal protein. Other ingredients include cereals (predominantly wheat, rice or maize), cereal by‐products, vegetable proteins and by‐products of human food production.
The European Pet Food Industry Federation (FEDIAF) has provided information on typical inclusion levels of cereals, cereal by‐products and other feed materials in dry cat and dog food.19 In the absence of sufficient data on species‐specific manufactured complete feedingstuffs, the CONTAM Panel has used example diets based on information provided by FEDIAF (details given in Table D.5). However, these should be regarded as indicative only, since actual ingredients will vary depending both on the availability of feed materials and the nutrient requirements of the animals.
Appendix E – Mean and P95 LB and UB concentrations of ZEN and the sum of ZEN + α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL (with molar RPFs applied) in feed materials and species‐specific compound feeds used to estimate exposures for farmed livestock and companion animalsa
| Feed | n | ZEN | Sum of ZEN + α‐ZAL + β‐ZAL + ZAN + α‐ZEL + β‐ZEL (with RPFs applied) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Meanc | P95c , d | Meanc | P95c , d | |||||||
| LB | UB | LB | UB | LB | UB | LB | UB | |||
| Wheat | 6,177 | 24 | 27 | 92 | 94 | 24 | 28 | 92 | 98 | |
| Barley | 1,586 | 12 | 17 | 23 | 40 | 12 | 63 | 23 | 46 | |
| Oats | 779 | 12 | 16 | 55 | 61 | 12 | 70 | 55 | 110 | |
| Maize | 2,046 | 116 | 120 | 547 | 547 | 116 | 127 | 547 | 553 | |
| Soybean meal | 14 | 8 | 25 | – | – | 8 | 25 | – | – | |
| Sunflower meal | 30 | 1 | 12 | – | – | 1 | 153 | – | – | |
| Wheatfeed | 99 | 49 | 58 | 46 | 46 | 89 | 240 | 86 | 227 | |
| Oatfeed | 72 | 1 | 6 | 0 | 8 | 1 | 6 | 0 | 8 | |
| Forages | ||||||||||
| Maize silage | 59 | 138 | 140 | – | – | 138 | 142 | – | – | |
| Grass hay | 31 | 64 | 66 | – | – | 64 | 83 | – | – | |
| Cereal straw | 41 | 0 | 11 | – | – | 0 | 139 | – | – | |
| Dairy: high yielding | 379 | 16 | 20 | 63 | 63 | 16 | 110 | 63 | 154 | |
| Fattening beef cattle | 72 | 17 | 24 | 84 | 84 | 17 | 111 | 84 | 171 | |
| Lactating sheep | 78 | 16 | 19 | 83 | 83 | 16 | 102 | 83 | 166 | |
| Lactating goats | 28 | 10 | 13 | – | – | 10 | 13. | – | – | |
| Pig starter | 345 | 16 | 21 | 50 | 50 | 16 | 111 | 50 | 140 | |
| Growing/fattening pigs | 443 | 40 | 45 | 108 | 108 | 40 | 120 | 108 | 183 | |
| Lactating sow | 97 | 36 | 42 | 263 | 263 | 36 | 116 | 263 | 337 | |
| Fattening chickensb | 129 | 35 | 41 | 143 | 143 | – | – | – | – | |
| Laying hensb | 98 | 55 | 61 | 211 | 211 | – | – | – | – | |
| Turkeys for fatteningb | 27 | 27 | 42 | – | – | – | – | – | – | |
ZEN: zearalenone; ZAL: zearalanol; ZAN: zearalanone; ZEL: zearalenol; N: number of samples; LB: lower bound; UB: upper bound; P95: 95th percentile; RPF: relative potency factor.
Expressed on a dry matter basis.
No data on levels of α‐ZAL, β‐ZAL, ZAN, α‐ZEL or β‐ZEL were provided for compound feeds for these poultry.
Values were rounded to the nearest whole number (0 decimal places).
The 95th percentile with less than 60 observations may not be statistically robust (EFSA, 2011b). Those estimates were not included in this table.
Appendix F – LOD and LOQs of the concentrations (μg/kg whole‐weight) of zearalenone and its modified forms in feed samples classified according to the Catalogue of feed materials specified in Commission Regulation (EU) No 68/201314
| Feed category | Abbreviation | LOD | LOQ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | ||||
| Cereal grains, their products and by‐products | Barley | Barley, unspecified | α‐ZAL | 1.8 | 0 | 6.7 | 41.9 | 20 | 50 |
| β‐ZAL | 1.8 | 0 | 6.7 | 41.9 | 20 | 50 | |||
| ZAN | 2.9 | 0 | 33.3 | 37.4 | 10 | 100 | |||
| α‐ZEL | 0 | 0 | 0 | 50 | 50 | 50 | |||
| β‐ZEL | 0 | 0 | 0 | 10 | 10 | 10 | |||
| ZEN | 2.6 | 0 | 38.3 | 7.0 | 1 | 115 | |||
| Barley middlings | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZAN | 5 | 5 | 5 | 10 | 10 | 10 | |||
| ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | |||
| Barley protein feed | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | |||
| Malt | ZEN | 7.7 | 7.7 | 7.7 | 24 | 23 | 25 | ||
| Malt rootlets | ZAN | 5 | 5 | 5 | 10 | 10 | 10 | ||
| Buckwheat | Buckwheat | α‐ZAL | 0 | 0 | 0 | 50 | 50 | 50 | |
| β‐ZAL | 0 | 0 | 0 | 50 | 50 | 50 | |||
| ZAN | 0 | 0 | 0 | 50 | 50 | 50 | |||
| α‐ZEL | 0 | 0 | 0 | 50 | 50 | 50 | |||
| β‐ZEL | 0 | 0 | 0 | 10 | 10 | 10 | |||
| ZEN | 3.2 | 0 | 6.7 | 21 | 4 | 50 | |||
| Cereal grains, their products and by‐products | Cereal grains, their products and by‐products | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZAN | 5.3 | 5 | 15 | 10.1 | 10 | 15 | |||
| ZEN | 31.6 | 2 | 66.7 | 94.6 | 5 | 200 | |||
| Grains as crops | Other grains | ZEN | 3 | 3 | 3 | 9 | 9 | 9 | |
| Maize and Corn | Maize and Corn | α‐ZAL | 6.67 | 6.67 | 6.67 | 20 | 20 | 20 | |
| β‐ZAL | 6.67 | 6.67 | 6.67 | 20 | 20 | 20 | |||
| ZAN | 5 | 5 | 5 | 10 | 10 | 10 | |||
| ZEN | 7.9 | 0.0 | 38.3 | 13.8 | 0.1 | 115 | |||
| Millet | Millet | α‐ZAL | 0 | 0 | 0 | 50 | 50 | 50 | |
| β‐ZAL | 0 | 0 | 0 | 50 | 50 | 50 | |||
| ZAN | 4 | 0 | 5 | 18 | 10 | 50 | |||
| α‐ZEL | 0 | 0 | 0 | 50 | 50 | 50 | |||
| β‐ZEL | 0 | 0 | 0 | 10 | 10 | 10 | |||
| ZEN | 0 | 0 | 0 | 50 | 50 | 50 | |||
| Mixed grains | Brewers’ grains | ZEN | 10.6 | 3.3 | 25 | 31.7 | 10 | 75 | |
| Distillers’ dark grains; [Distillers’ dried grains and solubles] | ZAN | 5 | 5 | 5 | 10 | 10 | 10 | ||
| Distillers’ dried grains | ZEN | 20 | 20 | 20 | 23.3 | 10 | 50 | ||
| Mixed grains, unspecified | ZEN | 5.3 | 0.0 | 20 | 14.6 | 0.03 | 50 | ||
| Oats | Oat feed | ZEN | 1.7 | 1.67 | 3.33 | 5.1 | 5 | 10 | |
| Oat flour (Feed) | ZEN | 0.3 | 0.3 | 0.3 | 0.9 | 0.9 | 0.9 | ||
| Oat groats (Feed) | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | |||
| Oats, unspecified | α‐ZAL | 1.4 | 0 | 6.7 | 43.6 | 20 | 50 | ||
| β‐ZAL | 1.4 | 0 | 6.7 | 43.6 | 20 | 50 | |||
| ZAN | 0.4 | 0 | 5 | 46.7 | 10 | 50 | |||
| α‐ZEL | 0 | 0 | 0 | 50 | 50 | 50 | |||
| β‐ZEL | 0 | 0 | 0 | 10 | 10 | 10 | |||
| ZEN | 1.9 | 0 | 38.3 | 4.7 | 1 | 115 | |||
| Rice, broken | Rice middlings | ZAN | 5 | 5 | 5 | 10 | 10 | 10 | |
| Rice, broken | α‐ZAL | 0 | 0 | 0 | 50 | 50 | 50 | ||
| β‐ZAL | 0 | 0 | 0 | 50 | 50 | 50 | |||
| ZAN | 0 | 0 | 0 | 50 | 50 | 50 | |||
| α‐ZEL | 0 | 0 | 0 | 50 | 50 | 50 | |||
| β‐ZEL | 0 | 0 | 0 | 10 | 10 | 10 | |||
| ZEN | 1.4 | 0 | 10 | 37.7 | 5 | 50 | |||
| Rice, milled | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | |||
| Rye | Rye | α‐ZAL | 0 | 0 | 0 | 50 | 50 | 50 | |
| β‐ZAL | 0 | 0 | 0 | 50 | 50 | 50 | |||
| ZAN | 0 | 0 | 0 | 50 | 50 | 50 | |||
| α‐ZEL | 0 | 0 | 0 | 50 | 50 | 50 | |||
| β‐ZEL | 0 | 0 | 0 | 10 | 10 | 10 | |||
| ZEN | 4.5 | 0 | 38.3 | 7.6 | 0.1 | 115 | |||
| Rye middlings | ZAN | 5 | 5 | 5 | 10 | 10 | 10 | ||
| Sorghum; [Milo] | Sorghum; [Milo] | ZAN | 5 | 5 | 5 | 10 | 10 | 10 | |
| ZEN | 3 | 3 | 3 | 9 | 9 | 9 | |||
| Spelt | Spelt | ZAN | 5 | 5 | 5 | 10 | 10 | 10 | |
| ZEN | 5.1 | 5 | 6.7 | 6.6 | 5 | 20 | |||
| Triticale | Triticale | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.6 | 2 | 20 | 8.31 | 5 | 50 | |||
| Wheat | Malting wheat screenings | ZEN | . | . | . | 25 | 25 | 25 | |
| Vital wheat gluten | ZEN | . | . | . | 10 | 10 | 10 | ||
| Wheat, unspecified | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZAN | 6.0 | 5 | 15 | 10.5 | 10 | 15 | |||
| ZEN | 3.7 | 0.3 | 50 | 7.3 | 0.1 | 150 | |||
| Wheat bran | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZAN | 5 | 5 | 5 | 10 | 10 | 10 | |||
| ZEN | 3.8 | 3 | 20 | 10.8 | 9 | 50 | |||
| Wheat feed | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZAN | 5 | 5 | 5 | 10 | 10 | 10 | |||
| ZEN | 3.7 | 2 | 20 | 10.7 | 8 | 50 | |||
| Wheat germ | ZAN | 5 | 5 | 5 | 10 | 10 | 10 | ||
| Wheat gluten | ZAN | 5 | 5 | 5 | 10 | 10 | 10 | ||
| ZEN | 7.8 | 3.3 | 10 | 10 | 10 | 10 | |||
| Wheat middlings | ZAN | 5 | 5 | 5 | 10 | 10 | 10 | ||
| ZEN | 9.9 | 3 | 16.7 | 9.5 | 5 | 50 | |||
| Compound feed | Complementary/Complete feed | Breeding pigs | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 |
| β‐ZAL | 6.7 | 6.7 | 6.67 | 20 | 20 | 20 | |||
| ZEN | 6.5 | 2 | 20 | 16.0 | 7.5 | 50 | |||
| Calves | α‐ZAL | 6.7 | 6.67 | 6.67 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.67 | 6.67 | 20 | 20 | 20 | |||
| ZEN | 5.9 | 3 | 10 | 17.8 | 9 | 30 | |||
| Complementary feed (incomplete diet) | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZAN | 5 | 5 | 5 | 10 | 10 | 10 | |||
| ZEN | 14.3 | 3.3 | 38.3 | 41.1 | 10 | 115 | |||
| Complete feed | ZAN | 5 | 5 | 5 | 10 | 10 | 10 | ||
| ZEN | 14.8 | 0.0 | 16.7 | 44.2 | 0.03 | 50 | |||
| Dairy cows | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 5.7 | 0.0 | 20 | 15.4 | 0.03 | 50 | |||
| Fattening calves | ZEN | 8.1 | 3 | 16.7 | 13.4 | 9 | 50 | ||
| Fattening cattle | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 7.1 | 3.3 | 20 | 15.2 | 7.5 | 50 | |||
| Fattening chickens | ZEN | 6.8 | 0.3 | 30 | 18.4 | 1 | 90 | ||
| Fattening ducks/Complete feed | ZEN | 4.3 | 3.3 | 10 | 12.9 | 10 | 30 | ||
| Fattening geese/Complementary feed | ZEN | 10 | 10 | 10 | 30 | 30 | 30 | ||
| Fattening geese/Complete feed | ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | ||
| Fattening rabbits | ZEN | 5.6 | 3.3 | 20 | 15 | 10 | 50 | ||
| Fattening sheep | ZEN | 4 | 4 | 4 | 10 | 10 | 10 | ||
| Fattening turkeys/Complete feed | ZEN | 13.1 | 3.3 | 20 | 34.23 | 10 | 60 | ||
| Fish/Complete feed | ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | ||
| Growing/fattening pigs | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 7.0 | 0.0 | 38.3 | 17.1 | 0.0 | 115 | |||
| Horses | ZEN | 21.1 | 3.3 | 50 | 56.7 | 10 | 150 | ||
| Lactating/dairy goats/Complete feed | ZEN | 5 | 5 | 5 | 15 | 15 | 15 | ||
| Lactating/dairy sheep | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 4.9 | 3.3 | 5 | 14.7 | 10 | 15 | |||
| Lambs | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 5.4 | 3.3 | 10 | 15 | 10 | 30 | |||
| Laying hens | ZEN | 7.4 | 0.3 | 20 | 18.3 | 1 | 50 | ||
| Pet food, birds | α‐ZAL | 6.7 | 6.67 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | |||
| Pet food, cats/Complete feed | ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | ||
| Pet food, dogs | ZEN | 3.1 | 2 | 3.3 | 9.8 | 9 | 10 | ||
| Poultry (starter diets) | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.3 | 2 | 3.3 | 9.9 | 5 | 10 | |||
| Unspecified Complementary/Complete feed | ZEN | 3.0 | 2 | 5 | 8.7 | 5 | 10 | ||
| Weaning pigs | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 4.98 | 3 | 16.7 | 14.1 | 7 | 50 | |||
| Compound feed | Compound feed | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZAN | 24.2 | 15 | 33.3 | 57.5 | 15 | 100 | |||
| ZEN | 5.8 | 3.33 | 50 | 10.3 | 10 | 50 | |||
| Fish, other aquatic animals and products derived thereof | Fish | Fish meal | ZEN | 20 | 20 | 20 | 50 | 50 | 50 |
| Forages and roughage, and products derived thereof | Cereals straw | Cereal straw, treated | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | |||
| Cereals straw, unspecified | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.4 | 3.33 | 6 | 10.1 | 10 | 15 | |||
| Forage meal; [Grass meal]; [Green meal] | Forage meal; [Grass meal]; [Green meal] | ZEN | 50 | 50 | 50 | 50 | 50 | 50 | |
| Forages and roughage, and products derived thereof | Forages and roughage, and products derived thereof | ZEN | 19.3 | 0.3 | 66.7 | 57.3 | 1 | 200 | |
| Grass, field dried, [Hay] | Grass, field dried, [Hay] | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 5.9 | 2 | 20 | 16.7 | 10 | 50 | |||
| Grass, herbs, legume plants, [green forage] | ZEN | 20 | 20 | 20 | 50 | 50 | 50 | ||
| Lucerne; [Alfalfa] | Lucerne meal; [Alfalfa meal] | ZEN | 2 | 2 | 2 | 10 | 10 | 10 | |
| Lucerne, high temperature dried; [Alfalfa, high temperature dried] | ZEN | 10 | 10 | 10 | 30 | 30 | 30 | ||
| Maize silage | Maize silage | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 17.0 | 2 | 20 | 42.7 | 5 | 50 | |||
| Land animal products and products derived thereof | Processed animal protein | Processed animal protein | ZEN | 2 | 2 | 2 | 10 | 10 | 10 |
| Legume seeds and products derived thereof | Carob, dried | Carob pods, dried | ZEN | 10 | 10 | 10 | 10 | 10 | 10 |
| Peas | Peas | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | |||
| Sweet lupins | Sweet lupins | ZEN | 3 | 3 | 3 | 9 | 9 | 9 | |
| Legumes, nuts and oilseeds | Other seeds | Other seeds | α‐ZAL | 0 | 0 | 0 | 46 | 40 | 50 |
| β‐ZAL | 0 | 0 | 0 | 46 | 40 | 50 | |||
| ZAN | 0 | 0 | 0 | 38 | 20 | 50 | |||
| α‐ZEL | 0 | 0 | 0 | 46 | 40 | 50 | |||
| β‐ZEL | 0 | 0 | 0 | 10 | 10 | 10 | |||
| ZEN | 0 | 0 | 0 | 38 | 20 | 50 | |||
| Minerals and products derived thereof | Minerals and products derived thereof | Minerals and products derived thereof | ZEN | 7.3 | 2 | 10 | 16.7 | 10 | 30 |
| Miscellaneous | Miscellaneous | Miscellaneous | ZEN | 1 | 1 | 1 | 3 | 3 | 3 |
| Products from the bakery and pasta industry | Feed beer | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | |||
| Plants by‐products from spirits production | ZEN | 3 | 3 | 3 | 9 | 9 | 9 | ||
| Products from the bakery and pasta industry | ZEN | 11.5 | 3 | 20 | 29.5 | 9 | 50 | ||
| Oil seeds, oil fruits, and products derived thereof | Cotton seed | Cotton seed | ZEN | 66.7 | 66.7 | 66.7 | 200 | 200 | 200 |
| Oil seeds, oil fruits, and products derived thereof | Oil seeds, oil fruits, and products derived thereof | ZEN | 10 | 10 | 10 | 10 | 10 | 10 | |
| Palm kernel expeller | Palm kernel expeller | ZEN | 10 | 10 | 10 | 10 | 10 | 10 | |
| Rape seed | Rape seed, unspecified | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | |||
| Rape seed meal | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 5.7 | 3.3 | 8 | 15 | 10 | 20 | |||
| Rape seed, expeller | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 22.2 | 3.3 | 38.3 | 50 | 10 | 115 | |||
| Safflower seed | Safflower seed meal, partially decorticated | ZEN | 10 | 10 | 10 | 10 | 10 | 10 | |
| Sunflower seed | Sunflower seed, unspecified | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.5 | 3.3 | 10 | 10 | 10 | 10 | |||
| Sunflower seed expeller | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.9 | 3.3 | 20 | 11.4 | 10 | 50 | |||
| Sunflower seed meal | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 6.7 | 3.3 | 20 | 18 | 10 | 50 | |||
| Toasted soya (beans) | Soya (bean) expeller | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 3.3 | 3.3 | 3.3 | 10 | 10 | 10 | |||
| Soya (bean) meal | ZEN | 18.6 | 5 | 20 | 46.8 | 15 | 50 | ||
| Soya (bean) meal, dehulled | ZEN | 20 | 20 | 20 | 50 | 50 | 50 | ||
| Soya beans, extruded | ZEN | 4 | 2 | 5 | 13.3 | 10 | 15 | ||
| Toasted soya (beans) | α‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | ||
| β‐ZAL | 6.7 | 6.7 | 6.7 | 20 | 20 | 20 | |||
| ZEN | 7.5 | 3.3 | 10 | 10 | 10 | 10 | |||
| Tubers, roots, and products derived thereof | Sugar beet | (Sugar) beet molasses | ZEN | 10 | 10 | 10 | 10 | 10 | 10 |
| Dried (sugar) beet pulp, molasses | ZEN | 5 | 5 | 5 | 10 | 10 | 10 | ||
LOD: limit of detection; LOQ: limit of quantification; ZEN: zearalenone; ZAL: zearalanol; ZAN: zearalanone; ZEL: zearalenol.
Appendix G – Mean, median, 75th and 95th percentile concentrations (μg/kg whole‐weight) in feed samples classified according to the Catalogue of feed materials specified in Commission Regulation (EU) No 68/201314 uncorrected for molar RPFs
| Feed category | Abbreviation | N | % Left Censored |
Mean (μg/kg)a |
Median (μg/kg)a |
P75 (μg/kg)a |
P95 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | LB | UB | ||||||
| Cereal grains, their products and by‐products | Barley | Barley, unspecified | α‐ZAL | 26 | 100 | 0 | 42 | 0 | 50 | 0 | 50 | – | – |
| β‐ZAL | 26 | 100 | 0 | 42 | 0 | 50 | 0 | 50 | – | – | |||
| ZAN | 38 | 82 | 6 | 37 | 0 | 50 | 0 | 50 | – | – | |||
| α‐ZEL | 19 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| β‐ZEL | 19 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| ZEN | 1586 | 71 | 11 | 15 | 0 | 5 | 5 | 9 | 20 | 35 | |||
| Barley bran | ZEN | 2 | 0 | 4,391 | 4,391 | 4,391 | 4,391 | 8,750 | 8,750 | – | – | ||
| Barley middlings | α‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | ||
| β‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |||
| ZAN | 2 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Barley protein feed | α‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | ||
| β‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |||
| ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Malt | ZEN | 2 | 100 | 0 | 24 | 0 | 24 | 0 | 25 | – | – | ||
| Malt rootlets | ZAN | 2 | 50 | 20 | 25 | 20 | 25 | 41 | 41 | – | – | ||
| ZEN | 4 | 0 | 13 | 13 | 13 | 13 | 20 | 20 | – | – | |||
| Buckwheat | Buckwheat | α‐ZAL | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |
| β‐ZAL | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| ZAN | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| α‐ZEL | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| β‐ZEL | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| ZEN | 4 | 100 | 0 | 21 | 0 | 15 | 0 | 35 | – | – | |||
| Cereal grains, their products and by‐products | Cereal grains, their products and by‐products | α‐ZAL | 12 | 100 | 0 | 19 | 0 | 19 | 0 | 19 | – | – | |
| β‐ZAL | 12 | 92 | 2 | 19 | 0 | 19 | 0 | 19 | – | – | |||
| ZAN | 49 | 76 | 13 | 21 | 0 | 10 | 0 | 10 | – | – | |||
| ZEN | 164 | 79 | 28 | 103 | 0 | 50 | 0 | 200 | 82 | 200 | |||
| Grains as crops | Other grains | ZEN | 1 | 100 | 0 | 3 | 0 | 3 | 0 | 3 | – | – | |
| Maize_&_Corn | Maize screenings | ZEN | 1 | 0 | 18 | 18 | 18 | 18 | 18 | 18 | – | – | |
| Maize and Corn, unspecified | α‐ZAL | 114 | 100 | 0 | 18 | 0 | 18 | 0 | 18 | 0 | 18 | ||
| β‐ZAL | 114 | 100 | 0 | 18 | 0 | 18 | 0 | 18 | 0 | 18 | |||
| ZAN | 35 | 43 | 68 | 72 | 15 | 15 | 51 | 51 | – | – | |||
| ZEN | 2,046 | 31 | 102 | 105 | 24 | 25 | 97 | 98 | 481 | 481 | |||
| Millet | Millet | α‐ZAL | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |
| β‐ZAL | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| ZAN | 6 | 83 | 5 | 20 | 0 | 10 | 0 | 32 | – | – | |||
| α‐ZEL | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| β‐ZEL | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| ZEN | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| Mixed grains | Brewers’ grains | ZEN | 3 | 100 | 0 | 15 | 0 | 10 | 0 | 25 | – | – | |
| Distillers’ dark grains; [Distillers’ dried grains and solubles] | ZAN | 24 | 8 | 64 | 65 | 49 | 49 | 86 | 86 | – | – | ||
| ZEN | 1 | 0 | 2,400 | 2,400 | 2,400 | 2,400 | 2,400 | 2,400 | – | – | |||
| Distillers’ dried grains | ZEN | 10 | 30 | 14 | 18 | 19 | 19 | 19 | 20 | – | – | ||
| Grain flour | ZEN | 1 | 0 | 11 | 11 | 11 | 11 | 11 | 11 | – | – | ||
| Mixed grains, unspecified | ZEN | 32 | 81 | 4 | 13 | 0 | 10 | 0 | 11 | – | – | ||
| Oats | Oat feed | ZEN | 72 | 96 | 1 | 6 | 0 | 5 | 0 | 5 | 0 | 7 | |
| Oat flour (Feed) | ZEN | 1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | ||
| Oat groats (Feed) | α‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | ||
| β‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |||
| ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Oats, unspecified | α‐ZAL | 14 | 100 | 0 | 44 | 0 | 50 | 0 | 50 | – | – | ||
| β‐ZAL | 14 | 100 | 0 | 44 | 0 | 50 | 0 | 50 | – | – | |||
| ZAN | 13 | 92 | 2 | 45 | 0 | 50 | 0 | 50 | – | – | |||
| α‐ZEL | 11 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| β‐ZEL | 11 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| ZEN | 779 | 78 | 10 | 14 | 0 | 3 | 0 | 8 | 48 | 53 | |||
| Rice, broken | Rice bran | ZEN | 1 | 0 | 128 | 128 | 128 | 128 | 128 | 128 | – | – | |
| Rice middlings | ZAN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Rice, broken, unspecified | α‐ZAL | 108 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | 0 | 50 | ||
| β‐ZAL | 108 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | 0 | 50 | |||
| ZAN | 108 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | 0 | 50 | |||
| α‐ZEL | 108 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | 0 | 50 | |||
| β‐ZEL | 108 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | |||
| ZEN | 152 | 98 | 0 | 37 | 0 | 50 | 0 | 50 | 0 | 50 | |||
| Rice, milled | α‐ZAL | 1 | 100 | 0 | 21 | 0 | 21 | 0 | 21 | – | – | ||
| β‐ZAL | 1 | 100 | 0 | 21 | 0 | 21 | 0 | 21 | – | – | |||
| ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Rye | Rye, unspecified | α‐ZAL | 9 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |
| β‐ZAL | 9 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| ZAN | 9 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| α‐ZEL | 9 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – | |||
| β‐ZEL | 9 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| ZEN | 312 | 90 | 2 | 9 | 0 | 5 | 0 | 5 | 7 | 26 | |||
| Rye bran (Feed) | ZEN | 1 | 0 | 110 | 110 | 110 | 110 | 110 | 110 | – | – | ||
| Rye middlings | ZAN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Sorghum; [Milo] | Sorghum; [Milo] | ZAN | 2 | 50 | 16 | 21 | 16 | 21 | 32 | 32 | – | – | |
| ZEN | 3 | 33 | 151 | 152 | 14 | 14 | 440 | 440 | – | – | |||
| Spelt | Spelt | ZAN | 4 | 75 | 6 | 13 | 0 | 10 | 12 | 17 | – | – | |
| ZEN | 33 | 85 | 3 | 8 | 0 | 5 | 0 | 10 | – | – | |||
| Triticale | Triticale | α‐ZAL | 2 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |
| β‐ZAL | 2 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |||
| ZEN | 67 | 72 | 17 | 21 | 0 | 6 | 8 | 13 | 22 | 22 | |||
| Wheat | Malting wheat screenings | ZEN | 2 | 100 | 0 | 25 | 0 | 25 | 0 | 25 | – | – | |
| Vital wheat gluten | ZEN | 5 | 60 | 273 | 279 | 0 | 10 | 15 | 15 | – | – | ||
| Wheat, unspecified | α‐ZAL | 12 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | ||
| β‐ZAL | 12 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |||
| ZAN | 40 | 53 | 44 | 50 | 0 | 11 | 25 | 25 | – | – | |||
| ZEN | 6177 | 55 | 21 | 24 | 0 | 5 | 8 | 12 | 81 | 82 | |||
| Wheat bran (Feed) | α‐ZAL | 89 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | 0 | 20 | ||
| β‐ZAL | 89 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | 0 | 20 | |||
| ZAN | 6 | 67 | 9 | 16 | 0 | 10 | 25 | 25 | – | – | |||
| ZEN | 111 | 86 | 4 | 12 | 0 | 10 | 0 | 10 | 28 | 30 | |||
| Wheat feed | α‐ZAL | 65 | 98 | 4 | 24 | 0 | 20 | 0 | 20 | 0 | 20 | ||
| β‐ZAL | 65 | 98 | 2 | 21 | 0 | 20 | 0 | 20 | 0 | 20 | |||
| ZAN | 6 | 50 | 10 | 15 | 5 | 10 | 25 | 25 | – | – | |||
| ZEN | 99 | 84 | 43 | 51 | 0 | 10 | 0 | 10 | 40 | 40 | |||
| Wheat germ (Feed) | ZAN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Wheat gluten feed | ZAN | 2 | 50 | 5 | 10 | 5 | 10 | 10 | 10 | – | – | ||
| ZEN | 3 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Wheat middlings | ZAN | 3 | 67 | 17 | 23 | 0 | 10 | 50 | 50 | – | – | ||
| ZEN | 102 | 50 | 18 | 22 | 3 | 10 | 33 | 34 | 65 | 65 | |||
| Compound feed | Complementary/Complete feed | Breeding pigs | α‐ZAL | 2 | 100 | 0 | 11 | 0 | 11 | 0 | 13 | – | – |
| β‐ZAL | 2 | 100 | 0 | 11 | 0 | 11 | 0 | 13 | – | – | |||
| ZEN | 97 | 48 | 32 | 37 | 3 | 15 | 25 | 30 | 231 | 231 | |||
| Calves | α‐ZAL | 4 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | ||
| β‐ZAL | 4 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | |||
| ZEN | 12 | 42 | 100 | 105 | 17 | 25 | 90 | 90 | – | – | |||
| Complementary feed (incomplete diet) | α‐ZAL | 1 | 100 | 0 | 8 | 0 | 8 | 0 | 8 | – | – | ||
| β‐ZAL | 1 | 100 | 0 | 8 | 0 | 8 | 0 | 8 | – | – | |||
| ZAN | 131 | 22 | 19 | 20 | 9 | 9 | 22 | 22 | 75 | 75 | |||
| ZEN | 248 | 64 | 44 | 66 | 0 | 49 | 1 | 50 | 264 | 264 | |||
| Complete feed | ZAN | 172 | 27 | 26 | 28 | 14 | 14 | 29 | 29 | 84 | 84 | ||
| ZEN | 224 | 57 | 57 | 81 | 0 | 50 | 3 | 50 | 335 | 335 | |||
| Dairy cows | α‐ZAL | 53 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | ||
| β‐ZAL | 53 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | |||
| ZEN | 379 | 48 | 14 | 18 | 0 | 7 | 16 | 20 | 56 | 56 | |||
| Fattening calves | ZEN | 127 | 39 | 28 | 32 | 15 | 16 | 35 | 35 | 86 | 86 | ||
| Fattening cattle | α‐ZAL | 15 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | ||
| β‐ZAL | 15 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | |||
| ZEN | 72 | 69 | 15 | 21 | 0 | 8 | 2 | 13 | 74 | 74 | |||
| Fattening chickens | ZEN | 129 | 37 | 31 | 36 | 7 | 18 | 31 | 34 | 126 | 126 | ||
| Fattening ducks/Complete feed | ZEN | 7 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Fattening geese/Complementary feed | ZEN | 1 | 100 | 0 | 30 | 0 | 30 | 0 | 30 | – | – | ||
| Fattening geese/Complete feed | ZEN | 5 | 60 | 34 | 40 | 0 | 10 | 50 | 50 | – | – | ||
| Fattening rabbits | ZEN | 14 | 57 | 1,081 | 1,087 | 0 | 10 | 20 | 21 | – | – | ||
| Fattening sheep | ZEN | 7 | 57 | 5 | 11 | 0 | 10 | 12 | 12 | – | – | ||
| Fattening turkeys/Complete feed | ZEN | 27 | 48 | 24 | 37 | 4 | 20 | 20 | 50 | – | – | ||
| Fish/Complete feed | ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Fur animals/Complete feed | ZEN | 1 | 0 | 140 | 140 | 140 | 140 | 140 | 140 | – | – | ||
| Goat (kids) (weaning diets)/Complementary feed | ZEN | 2 | 0 | 59 | 59 | 59 | 59 | 108 | 108 | – | – | ||
| Growing/fattening pigs | α‐ZAL | 4 | 100 | 0 | 11 | 0 | 11 | 0 | 13 | – | – | ||
| β‐ZAL | 4 | 100 | 0 | 11 | 0 | 11 | 0 | 13 | – | – | |||
| ZEN | 443 | 41 | 35 | 40 | 7 | 13 | 26 | 27 | 95 | 95 | |||
| Horses | ZEN | 7 | 43 | 16 | 40 | 10 | 20 | 29 | 54 | – | – | ||
| Lactating/dairy goats/Complete feed | ZEN | 28 | 64 | 8 | 12 | 0 | 5 | 11 | 11 | – | – | ||
| Lactating/dairy sheep | α‐ZAL | 4 | 100 | 0 | 12 | 0 | 13 | 0 | 13 | – | – | ||
| β‐ZAL | 4 | 100 | 0 | 12 | 0 | 13 | 0 | 13 | – | – | |||
| ZEN | 78 | 59 | 14 | 17 | 0 | 5 | 14 | 14 | 73 | 73 | |||
| Lambs | α‐ZAL | 1 | 100 | 0 | 8 | 0 | 8 | 0 | 8 | – | – | ||
| β‐ZAL | 1 | 100 | 0 | 8 | 0 | 8 | 0 | 8 | – | – | |||
| ZEN | 12 | 67 | 20 | 26 | 0 | 10 | 36 | 36 | – | – | |||
| Laying hens | ZEN | 98 | 47 | 48 | 53 | 1 | 10 | 34 | 34 | 186 | 186 | ||
| Pet food, birds | α‐ZAL | 1 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | ||
| β‐ZAL | 1 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | |||
| ZEN | 15 | 7 | 15 | 15 | 20 | 20 | 20 | 20 | – | – | |||
| Pet food, cats/Complete feed | ZEN | 4 | 50 | 19 | 24 | 12 | 17 | 38 | 38 | – | – | ||
| Pet food, dogs | ZEN | 7 | 86 | 4 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Poultry (starter diets) | α‐ZAL | 52 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | ||
| β‐ZAL | 52 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | |||
| ZEN | 132 | 46 | 31 | 35 | 10 | 10 | 38 | 38 | 125 | 125 | |||
| Rabbits/Complete feed | ZEN | 1 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | – | – | ||
| Unspecified Complementary/Complete feed | ZEN | 102 | 34 | 51 | 54 | 15 | 15 | 36 | 36 | 145 | 145 | ||
| Weaning pigs | α‐ZAL | 42 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | ||
| β‐ZAL | 42 | 100 | 0 | 13 | 0 | 13 | 0 | 13 | – | – | |||
| ZEN | 345 | 63 | 14 | 19 | 0 | 7 | 12 | 15 | 44 | 44 | |||
| Compound feed | Compound feed | α‐ZAL | 106 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | 0 | 20 | |
| β‐ZAL | 106 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | 0 | 20 | |||
| ZAN | 3 | 67 | 5 | 43 | 0 | 15 | 15 | 100 | – | – | |||
| ZEN | 201 | 69 | 17 | 24 | 0 | 10 | 11 | 12 | 77 | 77 | |||
| Fish, other aquatic animals and products derived thereof | Fish | Fish meal | ZEN | 1 | 100 | 0 | 50 | 0 | 50 | 0 | 50 | – | – |
| Forages and roughage, and products derived thereof | Cereals straw | Cereal straw, treated | α‐ZAL | 1 | 100 | 0 | 19 | 0 | 19 | 0 | 19 | – | – |
| β‐ZAL | 1 | 100 | 0 | 19 | 0 | 19 | 0 | 19 | – | – | |||
| ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Cereals straw | α‐ZAL | 40 | 100 | 0 | 19 | 0 | 19 | 0 | 19 | – | – | ||
| β‐ZAL | 40 | 100 | 0 | 19 | 0 | 19 | 0 | 19 | – | – | |||
| ZEN | 41 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Forage meal; [Grass meal]; [Green meal] | Forage meal; [Grass meal]; [Green meal] | ZEN | 4 | 75 | 43 | 80 | 0 | 50 | 85 | 110 | – | – | |
| Forages and roughage, and products derived thereof | Forages and roughage, and products derived thereof | ZEN | 11 | 73 | 68 | 108 | 0 | 15 | 1 | 200 | – | – | |
| Grass, field dried, [Hay] | Grass, field dried, [Hay] | α‐ZAL | 5 | 100 | 0 | 16 | 0 | 16 | 0 | 16 | – | – | |
| β‐ZAL | 5 | 100 | 0 | 16 | 0 | 16 | 0 | 16 | – | – | |||
| ZEN | 31 | 19 | 57 | 58 | 20 | 20 | 20 | 20 | – | – | |||
| Grass, herbs, legume plants, [green forage] | ZEN | 16 | 6 | 18 | 19 | 20 | 20 | 20 | 20 | – | – | ||
| Lucerne; [Alfalfa] | Lucerne field dried; [Alfalfa field dried] | ZEN | 1 | 0 | 16 | 16 | 16 | 16 | 16 | 16 | – | – | |
| Lucerne meal; [Alfalfa meal] | ZEN | 2 | 50 | 450 | 451 | 450 | 451 | 900 | 900 | – | – | ||
| Lucerne, high temperature dried; [Alfalfa, high temperature dried] | ZEN | 2 | 50 | 10 | 15 | 10 | 15 | 20 | 20 | – | – | ||
| Maize silage | Maize silage | α‐ZAL | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |
| β‐ZAL | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| ZEN | 59 | 29 | 121 | 124 | 20 | 20 | 75 | 75 | 937 | 937 | |||
| Land animal products and products derived thereof | Animal by‐products | Animal by‐products | ZEN | 1 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – |
| Processed animal protein | Processed animal protein | ZEN | 1 | 100 | 0 | 2 | 0 | 2 | 0 | 2 | – | – | |
| Legume seeds and products derived thereof | Carob, dried | Carob pods, dried | ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – |
| Horse beans | Horse beans | ZEN | 1 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – | |
| Peas | Peas | α‐ZAL | 1 | 100 | 0 | 19 | 0 | 19 | 0 | 19 | – | – | |
| β‐ZAL | 1 | 100 | 0 | 19 | 0 | 19 | 0 | 19 | – | – | |||
| ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| Sweet lupins | Sweet lupins | ZEN | 1 | 100 | 0 | 3 | 0 | 3 | 0 | 3 | – | – | |
| Legumes, nuts and oilseeds | Other seeds | Other seeds | α‐ZAL | 5 | 100 | 0 | 46 | 0 | 50 | 0 | 50 | – | – |
| β‐ZAL | 5 | 100 | 0 | 46 | 0 | 50 | 0 | 50 | – | – | |||
| ZAN | 5 | 100 | 0 | 38 | 0 | 50 | 0 | 50 | – | – | |||
| α‐ZEL | 5 | 100 | 0 | 46 | 0 | 50 | 0 | 50 | – | – | |||
| β‐ZEL | 5 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |||
| ZEN | 5 | 100 | 0 | 38 | 0 | 50 | 0 | 50 | – | – | |||
| Minerals and products derived thereof | Calcium carbonate; [Limestone] | Calcium carbonate; [Limestone] | ZEN | 1 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | – | – |
| Minerals and products derived thereof | Minerals and products derived thereof | ZEN | 4 | 75 | 1 | 7 | 0 | 8 | 3 | 10 | – | – | |
| Miscellaneous | Miscellaneous | Miscellaneous | ZEN | 1 | 100 | 0 | 3 | 0 | 3 | 0 | 3 | – | – |
| Products from the bakery and pasta industry | Feed beer | α‐ZAL | 1 | 100 | 0 | 16 | 0 | 16 | 0 | 16 | – | – | |
| β‐ZAL | 1 | 100 | 0 | 16 | 0 | 16 | 0 | 16 | – | – | |||
| ZEN | 1 | 100 | 0 | 8 | 0 | 8 | 0 | 8 | – | – | |||
| Plants by‐products from spirits production | ZEN | 12 | 8 | 222 | 222 | 105 | 105 | 303 | 303 | – | – | ||
| Products from the bakery and pasta industry | ZEN | 4 | 100 | 0 | 9 | 0 | 9 | 0 | 16 | – | – | ||
| Oil seeds, oil fruits, and products derived thereof | Cocoa husks | Cocoa husks | ZEN | 2 | 0 | 85 | 85 | 85 | 85 | 150 | 150 | – | – |
| Cotton seed | Cotton seed | ZEN | 5 | 40 | 9 | 89 | 5 | 20 | 20 | 200 | – | – | |
| Niger seed | Niger seed | ZEN | 1 | 0 | 5 | 5 | 5 | 5 | 5 | 5 | – | – | |
| Oil seeds, oil fruits, and products derived thereof | Oil seeds, oil fruits, and products derived thereof | ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |
| Palm kernel expeller | Palm kernel expeller | ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |
| Rape seed | Rape seed | α‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |
| β‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |||
| ZEN | 4 | 75 | 5 | 12 | 0 | 10 | 10 | 15 | – | – | |||
| Rape seed meal | α‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | ||
| β‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |||
| ZEN | 8 | 25 | 11 | 15 | 13 | 20 | 20 | 20 | – | – | |||
| Rape seed, expeller | α‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | ||
| β‐ZAL | 1 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |||
| ZEN | 5 | 60 | 5 | 35 | 0 | 20 | 5 | 25 | – | – | |||
| Safflower seed | Safflower seed meal, partially decorticated | ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | |
| Sunflower seed | Sunflower seed, unspecified | α‐ZAL | 42 | 100 | 0 | 21 | 0 | 21 | 0 | 21 | – | – | |
| β‐ZAL | 42 | 100 | 0 | 21 | 0 | 21 | 0 | 21 | – | – | |||
| ZEN | 47 | 91 | 74 | 83 | 0 | 10 | 0 | 10 | – | – | |||
| Sunflower seed expeller | α‐ZAL | 29 | 100 | 0 | 21 | 0 | 21 | 0 | 21 | – | – | ||
| β‐ZAL | 29 | 100 | 0 | 21 | 0 | 21 | 0 | 21 | – | – | |||
| ZEN | 30 | 97 | 1 | 11 | 0 | 10 | 0 | 10 | – | – | |||
| Sunflower seed meal | α‐ZAL | 4 | 100 | 0 | 21 | 0 | 21 | 0 | 21 | – | – | ||
| β‐ZAL | 4 | 100 | 0 | 21 | 0 | 21 | 0 | 21 | – | – | |||
| ZEN | 6 | 83 | 3 | 14 | 0 | 10 | 0 | 20 | – | – | |||
| Sunflower seed meal, dehulled | ZEN | 2 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – | ||
| Toasted soya (beans) | Soya (bean) expeller | α‐ZAL | 5 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |
| β‐ZAL | 5 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |||
| ZEN | 7 | 57 | 36 | 41 | 0 | 10 | 64 | 64 | – | – | |||
| Soya (bean) meal | ZEN | 14 | 79 | 7 | 22 | 0 | 20 | 0 | 20 | – | – | ||
| Soya (bean) meal, dehulled | ZEN | 5 | 20 | 13 | 17 | 20 | 20 | 20 | 20 | – | – | ||
| Soya (bean) protein concentrate | ZEN | 1 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – | ||
| Soya beans, extruded | ZEN | 3 | 100 | 0 | 11 | 0 | 15 | 0 | 15 | – | – | ||
| Toasted soya (beans), unspecified | α‐ZAL | 3 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | ||
| β‐ZAL | 3 | 100 | 0 | 20 | 0 | 20 | 0 | 20 | – | – | |||
| Oil seeds, oil fruits, and products derived thereof | Toasted soya (beans) | Toasted soya (beans) | ZEN | 8 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – |
| Other seeds and fruits, and products derived thereof | Buckwheat | Buckwheat | ZEN | 1 | 0 | 65 | 65 | 65 | 65 | 65 | 65 | – | – |
| Fruit kernels | Fruit pulp, dried | ZEN | 1 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – | |
| Perilla seed | Perilla seed | ZEN | 1 | 0 | 14 | 14 | 14 | 14 | 14 | 14 | – | – | |
| Tubers, roots, and products derived thereof | Sugar beet | (Sugar) beet molasses | ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – |
| Dried (sugar) beet pulp | ZEN | 1 | 0 | 20 | 20 | 20 | 20 | 20 | 20 | – | – | ||
| Dried (sugar) beet pulp, molasses | ZEN | 1 | 100 | 0 | 10 | 0 | 10 | 0 | 10 | – | – | ||
| Sugar beet, unspecified | ZEN | 1 | 0 | 153 | 153 | 153 | 153 | 153 | 153 | – | – | ||
ZEN: zearalenone; ZAL: zearalanol; ZAN: zearalanone; ZEL: zearalenol; N: number of samples; LB: lower bound; UB: upper bound; P75/P95: 75th/95th percentile; RPF: relative potency factor.
The 95th percentile with less than 60 observations may not be statistically robust (EFSA, 2011b). Those estimates were not included in this table.
Values were rounded to the nearest whole number (0 decimal places).
Appendix H – Effects of the oral zearalenone (ZEN) administration to female dogs
| Effects/parameters |
μg ZEN/kg bw/100 days N/group = 3; age 3 years bw: unknown |
Reference |
μg ZEN/kg bw/42 days N/group = 10; Age 70 days bw 8 Kg |
Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Blood cells and biochemistry | 0 | 25 | 50 | 0 | 50 | 75 | ||
| WBC | 10.7 | 14.1* | 13.9 | Gajęcka et al. (2007) | ND | ND | ND | |
| RBC | 6.5 | 6.5 | 6.4 | Gajęcka et al. (2007) | ND | ND | ND | |
| Total cholesterol | 2.1 | 2.4 | 1.4 | Gajęcka et al. (2007) | ND | ND | ND | |
| MCV | 72.4 | 70.0 | 78.9 | Gajęcka et al. (2007) | ND | ND | ND | |
| MCH | 1.56 | 1.51* | 1.46 | Gajęcka et al. (2007) | ND | ND | ND | |
| Hb | 10.1 | 9.7* | 9.2 | Gajęcka et al. (2007) | ND | ND | ND | |
| Plasma bilirubin | 52.8 | 63.0* | 88.0* | Gajęcka et al. (2007) | ND | ND | ND | |
| Plasma AST | 12.2 | 1.83* | 2.57* | Gajęcka et al. (2007) | ND | ND | ND | |
| Plasma ALT | 30.0 | 24.0* | 25.0* | Gajęcka et al. (2007) | ND | ND | ND | |
| Serum 17β‐estradiol (pg/mL) | NS | NS | Gajęcka et al.(2008b) | 4.6 | 14.66* | 21.58* | Gajęcka (2013)Gajęcka et al. (2013) | |
| Serum progesterone (ng/mL) | < 1 | ↑5–15# | ↑up to 25## | Gajęcka et al.(2008b) | 0.16 | 0.14 | 0.13* | Gajęcka (2013)Gajęcka et al. (2013) |
| Uterus | ||||||||
| Weight (horns); length (body) | ND | ND | ND | +* | +* | Stopa et al. (2014) | ||
| Histological lesions | – | + | + | Gajęcka et al. (2008a) | + | + | Stopa et al. (2014) | |
| PCNA positive cells | + | – – | – – | Gajęcka et al. (2008a) | ND | ND | ND | |
| Ultrastructural changes | – | + | ++ | Gajęcka et al. (2008c) | ND | ND | ND | |
| Ovaries | ||||||||
| Histological lesions | ND | + | ++ | Gajęcka et al. (2008a) | + | ++ | Gajęcka (2013) | |
| Ultrastructural changes | + | ++ | ND | ND | ND | |||
| ER β (immunochemistry) | ND | ND | ND | +++ | ↓+/++ | ↓+ | Gajęcka (2012) | |
| CYPscc (aromatase) mRNA | ND | ND | ND | NS | NS | |||
| 3β‐HSD mRNA | ND | ND | ND | NS | + 400% over control | Gajęcka et al. (2011b) | ||
ALT: alanine aminotransferase; AST: aspartate aminotransferase; ER: oestrogen receptor; Hb: haemoglobin; PCNA: proliferating cell nuclear antigen; MCV: mean cell volume; MCH: mean cell haemoglobin; HSD: hydroxysteroid dehydrogenase; NS: not statistically significant different from the control; ND: not determined; #limited to the first part of the trial; ##limited to or mainly in the second part of the trial; +/− = present/absent.
* p < 0.05 or less vs controls.
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Knutsen H‐K, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Nebbia CS, Petersen A, Rose M, Roudot A‐C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, Dall'Asta C, Dänicke S, Eriksen G‐S, Altieri A, Roldán‐Torres R and Oswald IP, 2017. Scientific opinion on the risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA Journal 2017;15(7):4851, 123 pp. 10.2903/j.efsa.2017.4851
Requestor: European Commission
Question number: EFSA‐Q‐2015‐00247
Panel members: Jan Alexander, Lars Barregard, Margherita Bignami, Beat Brüschweiler (from 23 June 2016), Sandra Ceccatelli, Bruce Cottrill, Michael Dinovi, Lutz Edler, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Helle Katrine Knutsen, Carlo Stefano Nebbia, Isabelle P. Oswald, Annette Petersen, Martin Rose, Alain‐Claude Roudot, Tanja Schwerdtle, Christiane Vleminckx, Günter Vollmer, and Heather Wallace.
Amendment: An editorial amendment was carried out that does not materially affect the contents or outcome of this scientific output. On p. 84 the reference Jiang et al. (2012) has been corrected so as to match the main text and conclusions on adverse effects on pigs. To avoid confusion, the older version has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made.
Acknowledgements: The EFSA CONTAM Panel thanks the hearing expert Andrew David Hart, member of the EFSA WG on Uncertainty in Risk Assessment for the support provided to this scientific output. The Panel acknowledges all European countries and European stakeholder organisations that provided feed consumption data and occurrence data on zearalenone and its modified forms in feed.
Adopted: 10 May 2017
Reproduction of the image listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 4: © Elsevier Ltd.
Amended: 18 July 2018
Notes
EFSA CONTAM Panel (2014). Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal 2014;12(12):3916, 107 pp. https://doi.org/10.2903/j.efsa.2014.3916. Available online: www.efsa.europa.eu/efsajournal
Commission Recommendation (EU) 2016/1319 of 29 July 2016 amending Recommendation 2006/576/EC as regards deoxynivalenol, zearalenone and ochratoxin A in pet food. OJ L 208, 2.8.2016, p. 58–60.
From 1 January 2014 onwards unit, title is Evidence Management Unit (DATA).
Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of feed materials Text with EEA relevance. OJ L 29, 16.1.2013, p. 1.
The LOD can be defined as the lowest concentration level that can be determined to be statistically different from a blank. Similarly, the LOQ is the minimum concentration or mass of the analyte that can be quantified with acceptable accuracy and precision (Keith et al., 1983). Keith LH et al., 1983. Principles of environmental analysis, Analytical Chemistry 55 (14), 2210–2218.
Source: FEFAC Feed and Food Statistical Yearbook 2014 Available online: www.fefac.eu
Complete and complementary feedingstuffs.
Web of Science (WoS), formally ISI Web of Knowledge, Thomson Reuters. Available at: http://thomsonreuters.com/thomson-reuters-web-of-science/
PubMed, Entrez Global Query Cross‐Database Search System, National Center for Biotechnology Information (NCBI), National Library of Medicine (NLM), Department of the National Institutes of Health (NIH), United States Department of Health and Human Services. Available at: http://www.ncbi.nlm.nih.gov/pubmed/
EndNote X5, Thomson Reuters. Available at: http://endnote.com/
Figures extrapolated from published graphs.
Statistical significance not reported.
Mean values not reported and derived from a bar graph.
Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of feed materials Text with EEA relevance(OJ L 29, 16.1.2013, p. 1).
Commission Regulation (EU) No 575/2011 of June 2011 on the Catalogue of feed materials OJ L 159, 17.6.2011, p. 25–65.
An exception to this is geese, which can live entirely on grass and similar forage.
Available online: http://www.fao.org/fishery/affris/en/
Available online: www.Fedif.org
FEDIAF, Personal communication by email, May 2016.
References
- Abrunhosa L, Morales H, Soares C, Calado T, Vila‐Chã AS, Pereira M and Venâncio A, 2016. A review of mycotoxins in food and feed products in Portugal and estimation of probable daily intakes. Critical Reviews in Food Science and Nutrition, 56, 249–265. 10.1080/10408398.2012.720619 [DOI] [PubMed] [Google Scholar]
- AFRC (Agricultural and Food Research Council), 1993. Energy and Protein Requirements of Ruminants. An advisory manual prepared by the AFRC Technical Committee on Responses to Nutrients. Alderman G, Cottrill BR (eds.). CAB International, Wallingford. 159 pp. [Google Scholar]
- AFSSA (French Food Safety Authority/Agence française de sécurité sanitaire des aliments), 2009. Étude Individuelle Nationale des Consommations Alimentaires 2 (INCA 2) 2006‐2007. Available online: http://www.anses.fr/Documents/PASER-Ra-INCA2.pdf
- Alexa E, Dehelean CA, Poiana MA, Radulov I, Cimpean AM, Bordean DM, Tulcan C and Pop G, 2013. The occurrence of mycotoxins in wheat from western Romania and histopathological impact as effect of feed intake. Chemistry Central Journal, 7, 99. 10.1186/1752-153X-7-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NK, Mirocha CJ, Aakhus‐Allen S, Bitgood JJ, Weaver G and Bates F, 1981. Effect of dietary zearalenone on reproduction of chickens. Poultry Science, 60, 1165–1174. [DOI] [PubMed] [Google Scholar]
- Allen NK, Peguri A, Mirocha CJ and Newman JA, 1983. Effects of Fusarium cultures, T‐2‐toxin, and zearalenone on reproduction of turkey females. Poultry Science, 62, 282–289. [DOI] [PubMed] [Google Scholar]
- Alm H, Brussow KP, Torner H, Vanselow J, Tomek W, Danicke S and Tiemann U, 2006. Influence of Fusarium‐toxin contaminated feed on initial quality and meiotic competence of gilt oocytes. Reproductive Toxicology, 22, 44–50. [DOI] [PubMed] [Google Scholar]
- Almeida I, Martins HM, Santos S, Costa JM and Bernardo F, 2011. Co‐occurrence of mycotoxins in swine feed produced in Portugal. Mycotoxin Research, 27, 177–181. [DOI] [PubMed] [Google Scholar]
- Almeida IFM, Guerra MM, Martins HML, Costa JMG and Bernardo FMA, 2013. Aflatoxin B‐1 and zearalenone in dairy feeds in Portugal, 2009‐2011. Mycotoxin Research, 29, 131–133. [DOI] [PubMed] [Google Scholar]
- Arukwe A, Grotmol T, Haugen TB, Knudsen FR and Goksoyr A, 1999. Fish model for assessing the in vivo oestrogenic potency of the mycotoxin zearalenone and its metabolites. Science of the Total Environment, 236, 153–161. [DOI] [PubMed] [Google Scholar]
- Aurich JE, Hoppen HO, Trampler R, Zentek J, Boehm J, Razzazi‐Fazeli E and Aurich C, 2006. Effects of mycotoxins on reproductive function in mares. Animal Reproduction Science, 94, 238–241. [Google Scholar]
- Bacon CW and Marks HL, 1976. Growth of broilers and quail fed Fusarium (Gibberella zeae)‐infected corn and zearalenone (F‐2). Poultry Science, 55, 1531–1535. [DOI] [PubMed] [Google Scholar]
- Baldwin RS, Williams RD and Terry MK, 1983. Zeranol: A review of the metabolism, toxicology, and analytical methods for detection of tissue residues. Regulatory Toxicology and Pharmacology, 3, 9–25. [DOI] [PubMed] [Google Scholar]
- Banjerdpongchai R, Kongtawelert P, Khantamat O, Srisomsap C, Chokchaichamnankit D, Subhasitanont P and Svasti J, 2010. Mitochondrial and endoplasmic reticulum stress pathways cooperate in zearalenone‐induced apoptosis of human leukemic cells. Journal of Hematology & Oncology, 3, 50. 10.1186/1756-8722-3-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloglazova NV, De Boevre M, Goryacheva IY, Werbrouck S, Guo Y and De Saeger S, 2013. Immunochemical approach for zearalenone‐4‐glucoside determination. Talanta, 106, 422–430. [DOI] [PubMed] [Google Scholar]
- Berntssen MHG, Olsvik PA, Torstensen BE, Julshamn K, Midtun T, Goksøyr A, Johansen J, Sigholt T, Joerum N, Jakobsen J‐V, Lundebye A‐K and Lock E‐J, 2010. Reducing persistent organic pollutants while maintaining long chain omega‐3 fatty acid in farmed Atlantic salmon using decontaminated fish oils for an entire production cycle. Chemosphere, 81, 242–252. [DOI] [PubMed] [Google Scholar]
- Berthiller F, Werner U, Sulyok M, Krska R, Hauser MT and Schuhmacher R, 2006. Liquid chromatography coupled to tandem mass spectrometry (LC‐MS/MS) determination of phase II metabolites of the mycotoxin zearalenone in the model plant Arabidopsis thaliana. Food Additives and Contaminants, 23, 1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiller F, Sulyok M, Krska R and Schuhmacher R, 2007. Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. International Journal of Food Microbiology, 119, 33–37. [DOI] [PubMed] [Google Scholar]
- Berthiller F, Hametner C, Krenn P, Schweiger W, Ludwig R, Adam G, Krska R and Schuhmacher R, 2009. Preparation and characterization of the conjugated Fusarium mycotoxins zearalenone‐4O‐beta‐D‐glucopyranoside, alpha‐zearalenol‐4O‐beta‐D‐glucopyranoside and beta‐zearalenol‐4O‐beta‐D‐glucopyranoside by MS/MS and two‐dimensional NMR. Food Additives & Contaminants. Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment, 26, 207–213. [DOI] [PubMed] [Google Scholar]
- Berthiller F, Krska R, Domig KJ, Kneifel W, Juge N, Schuhmacher R and Adam G, 2011. Hydrolytic fate of deoxynivalenol‐3‐glucoside during digestion. Toxicology Letters, 206, 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiller F, Crews C, Dall'Asta C, De Saeger S, Haesaert G, Karlovsky P, Oswald IP, Seefelder W, Speijers G and Stroka J, 2013. Masked mycotoxins: A review. Molecular Nutrition & Food Research, 57, 165–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehl ML, Prelusky DB, Koritz GD, Hartin KE, Buck WB and Trenholm HL, 1993. Biliary excretion and enterohepatic cycling of zearalenone in immature pigs. Toxicology and Applied Pharmacology, 121, 152–159. [DOI] [PubMed] [Google Scholar]
- Binder SB, Schwartz‐Zimmermann HE, Varga E, Bichl G, Michlmayr H, Adam G and Berthiller F, 2017. Metabolism of zearalenone and its major modified forms in pigs. Toxins, 9, 56. 10.3390/toxins9020056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm J, Koinig L, Razzazi‐Fazeli E, Blajet‐Kosicka A, Twaruzek M, Grajewski J and Lang C, 2010. Survey and risk assessment of the mycotoxins deoxynivalenol, zearalenone, fumonisins, ochratoxin A, and aflatoxins in commercial dry dog food. Mycotoxin Research, 26, 147–153. [DOI] [PubMed] [Google Scholar]
- Bories G and Suarez AF, 1989. Profiling of free and conjugated [3H]Zeranol metabolites in pig plasma. Journal of Chromatography B: Biomedical Sciences and Applications, 489, 191–197. [DOI] [PubMed] [Google Scholar]
- Bories GF, Perdudurand EF, Sutra JF and Tulliez JE, 1991. Evidence for glucuronidation and sulfation of Zeranol and metabolites (taleranol and zearalanone) by rat and pig hepatic subfractions. Drug Metabolism and Disposition, 19, 140–143. [PubMed] [Google Scholar]
- Bories GF, Sutra JFP and Tulliez JE, 1992. Metabolism and disposition of 3H Zeranol implanted in the pig. Journal of Agricultural and Food Chemistry, 40, 284–288. [Google Scholar]
- Brodehl A, Moller A, Kunte HJ, Koch M and Maul R, 2014. Biotransformation of the mycotoxin zearalenone by fungi of the genera Rhizopus and Aspergillus . FEMS Microbiology Letters, 359, 124–130. [DOI] [PubMed] [Google Scholar]
- Browne EM, Juniper DT, Bryant MJ, Beever DE and Fisher AV, 2004. Intake, live‐weight gain and carcass characteristics of beef cattle given diets based on forage maize silage harvested at different stages of maturity. Animal Science, 79, 405–413. [Google Scholar]
- Buckley T, Creighton A and Fogarty U, 2007. Analysis of Canadian and Irish forage, oats and commercially available equine concentrate feed for pathogenic fungi and mycotoxins. Irish Veterinary Journal, 60, 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos‐Aceves MA, Cohen A, Smith Y and Faggio C, 2016. Estrogen regulation of gene expression in the teleost fish immune system. Fish & Shellfish Immunology, 58, 42–49. [DOI] [PubMed] [Google Scholar]
- Bursian SJ, Aulerich RJ, Cameron JK, Ames NK and Steficek BA, 1992. Efficacy of hydrated sodium calcium aluminosilicate in reducing the toxicity of dietary zearalenone to mink. Journal of Applied Toxicology, 12, 85–90. [DOI] [PubMed] [Google Scholar]
- Carabaño R and Piquer J, 1998. The digestive system of the rabbit. In: de Blas C, Wiseman J (eds.). The Nutrition of the Rabbit. CABI Publishing, Cambridge, UK. pp. 1–16. [Google Scholar]
- Chang WM and Lin JK, 1984. Transformation of zearalenone and zearalenol by rat erythrocytes. Food and Chemical Toxicology, 22, 887–891. [DOI] [PubMed] [Google Scholar]
- Chen H, Hu J, Yang J, Wang Y, Xu H, Jiang Q, Gong Y, Gu Y and Song H, 2010. Generation of a fluorescent transgenic zebrafish for detection of environmental estrogens. Aquatic Toxicology, 96, 53–61. [DOI] [PubMed] [Google Scholar]
- Chen XX, Yang CW, Huang LB, Niu QS, Jiang SZ and Chi F, 2015a. Zearalenone altered the serum hormones, morphologic and apoptotic measurements of genital organs in post‐weaning gilts. Asian‐Australasian Journal of Animal Sciences, 28, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Lu Z, Hou WX, Shi BM and Shan AS, 2015b. Effects of modified maifanite on zearalenone toxicity in female weaner pigs. Italian Journal of Animal Science, 14, 143–149. [Google Scholar]
- Chi MS, Mirocha CJ, Kurtz HJ, Weaver GA, Bates F, Robison T and Shimoda W, 1980a. Effect of dietary zearalenone on growing broiler chicks. Poultry Science, 59, 531–536. [Google Scholar]
- Chi MS, Mirocha CJ, Weaver GA and Kurtz HJ, 1980b. Effect of zearalenone on female White Leghorn chickens. Applied and Environmental Microbiology, 39, 1026–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkova E, Laciakova A, Pastorova B, Seidel H and Kovac G, 2001. The effect of zearalenone on some enzymatic parameters in rabbits. Toxicology Letters, 121, 145–149. [DOI] [PubMed] [Google Scholar]
- Dąbrowski M, Obremski K, Gajęcka M, Gajęcki MT and Zielonka Ł, 2016. Changes in the Subpopulations of Porcine Peripheral Blood Lymphocytes Induced by Exposure to Low Doses of Zearalenone (ZEN) and Deoxynivalenol (DON). Molecules, 21, 557. 10.3390/molecules21050557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacasto M, Rolando P, Nachtmann C, Ceppa L and Nebbia C, 1995. Zearalenone mycotoxicosis in piglets suckling sows fed contaminated grain. Veterinary and Human Toxicology, 37, 359–361. [PubMed] [Google Scholar]
- Dai SL, Duan JH, Lu Y, Zhang YH, Cheng JX, Ren J, Zhao XY, Wu YQ, Yu Y, Zuo PP, Wu YY and Ge QS, 2004. Phytoestrogen alpha‐zearalanol inhibits atherogenesis and improves lipid profile in ovariectomized cholesterol‐fed rabbits. Endocrine, 25, 121–129. [DOI] [PubMed] [Google Scholar]
- Dai M, Jiang S, Yuan X, Yang W, Yang Z and Huang L, 2016. Effects of zearalenone‐diet on expression of ghrelin and PCNA genes in ovaries of post‐weaning piglets. Animal Reproduction Science, 168, 126–137. [DOI] [PubMed] [Google Scholar]
- Dailey RE, Reese RE and Brouwer EA, 1980. Metabolism of [14C]zearalenone in laying hens. Journal of Agricultural and Food Chemistry, 28, 286–291. [DOI] [PubMed] [Google Scholar]
- Dall'Erta A, Cirlini M, Dall'Asta M, Del Rio D, Galaverna G and Dall'Asta C, 2013. Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chemical Research in Toxicology, 26, 305–312. [DOI] [PubMed] [Google Scholar]
- Dänicke S and Winkler J, 2015. Invited review: Diagnosis of zearalenone (ZEN) exposure of farm animals and transfer of its residues into edible tissues (carry over). Food and Chemical Toxicology, 84, 225–249. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Keese C, Meyer U, Starke A, Kinoshita A and Rehage J, 2014. Zearalenone (ZEN) metabolism and residue concentrations in physiological specimens of dairy cows exposed long‐term to ZEN‐contaminated diets differing in concentrate feed proportions. Archives of Animal Nutrition, 68, 492–506. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Ueberschär KH, Halle I, Matthes S, Valenta H and Flachowsky G, 2002. Effect of addition of a detoxifying agent to laying hen diets containing uncontaminated or Fusarium toxin‐contaminated maize on performance of hens and on carryover of zearalenone. Poultry Science, 81, 1671–1680. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Matthäus K, Valenta H and Halle I, 2004. Effects of Fusarium‐toxin contaminated wheat grains and non‐starch‐polysaccharide (NSP) hydrolyzing enzyme preparation on Pekin duck performance. Archiv für Geflügelkunde, 68, 199–205. [Google Scholar]
- Dänicke S, Swiech E, Buraczewska L and Ueberschär KH, 2005a. Kinetics and metabolism of zearalenone in young female pigs. Journal of Animal Physiology and Animal Nutrition, 89, 268–276. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Matthäus K, Lebzien P, Valenta H, Stemme K, Ueberschär KH, Razzazi‐Fazeli E, Böhm J and Flachowsky G, 2005b. Effects of Fusarium toxin‐contaminated wheat grain on nutrient turnover, microbial protein synthesis and metabolism of deoxynivalenol and zearalenone in the rumen of dairy cows. Journal of Animal Physiology and Animal Nutrition, 89, 303–315. [DOI] [PubMed] [Google Scholar]
- De Boevre M, Di Mavungu JD, Maene P, Audenaert K, Deforce D, Haesaert G, Eeckhout M, Callebaut A, Berthiller F, Van Peteghem C and De Saeger S, 2012a. Development and validation of an LC‐MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T‐2‐toxin and some masked metabolites in different cereals and cereal‐derived food. Food Additives and Contaminants Part A‐Chemistry Analysis Control Exposure & Risk Assessment, 29, 819–835. [DOI] [PubMed] [Google Scholar]
- De Boevre M, Di Mavungu JD, Landschoot S, Audenaert K, Eeckhout M, Maene P, Haesaert G and De Saeger S, 2012b. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin Journal, 5, 207–219. [Google Scholar]
- De Boevre M, Jacxsens L, Lachat C, Eeckhout M, Di Mavungu JD, Audenaert K, Maene P, Haesaert G, Kolsteren P, De Meulenaer B and De Saeger S, 2013. Human exposure to mycotoxins and their masked forms through cereal‐based foods in Belgium. Toxicology Letters, 218, 281–292. [DOI] [PubMed] [Google Scholar]
- Devreese M, Antonissen G, Broekaert N, De Baere S, Vanhaecke L, De Backer P and Croubels S, 2015. Comparative toxicokinetics, absolute oral bioavailability, and biotransformation of zearalenone in different poultry species. Journal of Agricultural and Food Chemistry, 63, 5092–5098. [DOI] [PubMed] [Google Scholar]
- Diekman MA and Green ML, 1992. Mycotoxins and reproduction in domestic livestock. Journal of Animal Science, 70, 1615–1627. [DOI] [PubMed] [Google Scholar]
- Ding X, Lichti K and Staudinger JL, 2006. The Mycoestrogen Zearalenone Induces CYP3A through Activation of the Pregnane X Receptor. Toxicological Sciences: an official journal of the Society of Toxicology, 91, 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döll S and Dänicke S, 2011. The Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Preventive Veterinary Medicine, 102, 132–145. [DOI] [PubMed] [Google Scholar]
- Döll S, Dänicke S, Ueberschär KH, Valenta H, Schnurrbusch U, Ganter M, Klobasa F and Flachowsky G, 2003. Effects of graded levels of Fusarium toxin contaminated maize in diets for female weaned piglets. Archiv für Tierernahrung, 57, 311–334. [DOI] [PubMed] [Google Scholar]
- Dong M, Tulayakul P, Li JY, Dong KS, Manabe N and Kumagai S, 2010a. Metabolic Conversion of Zearalenone to alpha‐Zearalenol by Goat Tissues. Journal of Veterinary Medical Science, 72, 307–312. [DOI] [PubMed] [Google Scholar]
- Dong M, He XJ, Tulayakul P, Li JY, Dong KS, Manabe N, Nakayama H and Kumagai S, 2010b. The toxic effects and fate of intravenously administered zearalenone in goats. Toxicon, 55, 523–530. [DOI] [PubMed] [Google Scholar]
- Driehuis F, 2013. Silage and the safety and quality of dairy foods: a review. Agricultural and Food Science, 22, 16–34. [Google Scholar]
- Driehuis F, Spanjer MC, Scholten JM and Te Giffel MC, 2008. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Addit Contam Part B Surveill, 1, 41–50. [DOI] [PubMed] [Google Scholar]
- Drzymala SS, Herrmann AJ, Maul R, Pfeifer D, Garbe L‐A and Koch M, 2014. In Vitro Phase I Metabolism of cis‐Zearalenone. Chemical Research in Toxicology, 27, 1972–1978. [DOI] [PubMed] [Google Scholar]
- Drzymala SS, Binder J, Brodehl A, Penkert M, Rosowski M, Garbe L‐A and Koch M, 2015. Estrogenicity of novel phase I and phase II metabolites of zearalenone and cis‐zearalenone. Toxicon, 105, 10–12. [DOI] [PubMed] [Google Scholar]
- EBLEX (Division of the Agriculture and Horticulture Development Board, AHDB), 2005. Beef Action for Profit 3: Better Returns from Dairy‐Bred Bulls. EBLEX, Huntingdon. Available online: http://beefandlamb.ahdb.org.uk/wp-content/uploads/2016/01/BRP-Improving-cattle-handling-manualE-3-190116.pdf [Google Scholar]
- EBLEX (Division of the Agriculture and Horticulture Development Board, AHDB), 2015. Growing and feeding maize silage for Better Returns. Beef and sheep BRP Manual 10. Stoneleigh, Kenilworth, UK. Available online: http://beefandlamb.ahdb.org.uk/wp-content/uploads/2016/01/BRP-Growing-and-feeding-maize-silage-Manual-10-180116.pdf
- EBLEX (Division of the Agriculture and Horticulture Development Board, AHDB), 2016. Feeding growing and finishing cattle for Better Returns: Beef BRP Manual 7. EBLEX, Stoneleigh, UK. Available online: http://beefandlamb.ahdb.org.uk/wp/wp-content/uploads/2016/12/BRP-Feeding-growing-and-finishing-manual-7-091216.pdf [Google Scholar]
- Edwards S, Cantley TC, Rottinghaus GE, Osweiler GD and Day BN, 1987a. The effects of zearalenone on reproduction in swine. 1. The relationship between ingested zearalenone dose and anestrus in nonpregnant, sexually mature gilts. Theriogenology, 28, 43–49. [DOI] [PubMed] [Google Scholar]
- Edwards S, Cantley TC and Day BN, 1987b. The effects of zearalenone on reproduction in swine. 2. The effect on puberty attainment and postweaning rebreeding performance. Theriogenology, 28, 51–58. [DOI] [PubMed] [Google Scholar]
- Edwards SG, Dickin ET, MacDonald S, Buttler D, Hazel CM, Patel S and Scudamore KA, 2011. Distribution of Fusarium mycotoxins in UK wheat mill fractions. Food Additives & Contaminants: Part A, 28, 1694–1704. 10.1080/19440049.2011.605770 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Zearalenone as undesirable substance in animal feed. EFSA Journal 2004;2(7):89, 34 pp. 10.2903/j.efsa.2004.89 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Guidance of the Scientific Committee on a request from EFSA related to uncertainties in Dietary Exposure Assessment. EFSA Journal 2006;4(5):438, 54 pp. 10.2903/j.efsa.2006.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2009.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Guidance of EFSA on the use of the EFSA Comprehensive European Food Consumption Database in Intakes Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2011. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA Journal 2011;9(6):2197, 124 pp. 10.2903/j.efsa.2011.2197 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal 2014;12(12):3916, 60 pp. 10.2903/j.efsa.2014.3916 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2016. Scientific Opinion on the appropriateness to set a group health‐based guidance value for zearalenone and its modified forms. EFSA Journal 2016;14(1):4425, 46 pp. 10.2903/j.efsa.2016.4425 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. 10.2903/j.efsa.2012.2534 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2016. Draft Guidance on Uncertainty in EFSA scientific assessment. EFSA Journal, in press. Available online: http://www.efsa.europa.eu/sites/default/files/160321DraftGDUncertaintyInScientificAssessment.pdf [Google Scholar]
- Engels R and Krämer J, 1996. Incidence of Fusaria and occurrence of selected Fusarium mycotoxins on Lolium spp. in Germany. Mycotoxin Res, 12, 31–40. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization ‐ World Health Organization), 2000. Zearalenone. Prepared by the Fifty‐third meeting of the Joint FAO/WHO Expert Committee on Food Additives(JECFA). In: Safety Evaluation of Certain Food Additives and Contaminants, WHO Food Additives Series 44. International Programme on Chemical Safety, World Health Organization, Geneva
- Fink‐Gremmels J, 2008a. The role of mycotoxins in the health and performance of dairy cows. Veterinary Journal, 176, 84–92. [DOI] [PubMed] [Google Scholar]
- Fink‐Gremmels J, 2008b. Biological effect of zearalenone. In: Oswald IP and Taranu I (eds.). Mycotoxins in farm animals. Transworld Research Network, Trivandrum, India. pp. 197–218. [Google Scholar]
- Fink‐Gremmels J and Malekinejad H, 2007. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Animal Feed Science and Technology, 137, 326–341. [Google Scholar]
- Fleck SC, Hildebrand AA, Muller E, Pfeiffer E and Metzler M, 2012. Genotoxicity and inactivation of catechol metabolites of the mycotoxin zearalenone. Mycotoxin Research, 28, 267–273. [DOI] [PubMed] [Google Scholar]
- Flores‐Flores ME, Lizarraga E, de Cerain AL and Gonzalez‐Penas E, 2015. Presence of mycotoxins in animal milk: A review. Food Control, 53, 163–176. [Google Scholar]
- Gajęcka M, 2012. The effect of low‐dose experimental zearalenone intoxication on the immunoexpression of estrogen receptors in the ovaries of pre‐pubertal bitches. Polish Journal of Veterinary Sciences, 15, 685–691. [PubMed] [Google Scholar]
- Gajęcka M, 2013. The effects of experimental administration of low doses of zearalenone on the histology of ovaries in pre‐pubertal bitches. Polish Journal of Veterinary Sciences, 16, 313–322. [DOI] [PubMed] [Google Scholar]
- Gajęcka M and Przybylska‐Gornowicz B, 2012. The low doses effect of experimental zearalenone (ZEN) intoxication on the presence of Ca2 + in selected ovarian cells from pre‐pubertal bitches. Polish Journal of Veterinary Sciences, 15, 711–720. [DOI] [PubMed] [Google Scholar]
- Gajęcka M, Jakimiuk E, Polak M, Zielonka Ł, Obremski K and Gajęcki M, 2007. Metabolic changes in bitches after experimental zearalenone mycotoxicosis. Bulletin of the Veterinary Institute in Puławy, 51, 667–671. [Google Scholar]
- Gajęcka M, Obremski K, Jakimiuk E, Skorska‐Wyszynska E, Zielonka Ł and Gajęcki M, 2008a. Histopathological examination of ovaries in bitches after experimental zearalenone mycotoxicosis. Polish Journal of Veterinary Sciences, 11, 363–366. [PubMed] [Google Scholar]
- Gajęcka M, Janowski T, Jakimiuk E, Zielonka Ł, Podhalicz‐Dziegielewska M, Obremski K and Gajęcki M, 2008b. Influence of long‐term zearalenone intoxication on the concentration of progesterone and 17beta‐oestradiol in blood plasma in bitches. Bulletin of the Veterinary Institute in Puławy, 52, 405–409. [Google Scholar]
- Gajęcka M, Przybylska‐Gornowicz B, Obremski K, Polak M, Jakimiuk E, Skorska‐Wyszynska E, Zielonka Ł and Gajęcki M, 2008c. Ultrastructural changes of ovarian follicle and corpus luteum after experimental zearalenone mycotoxicosis in bitch. Polish Journal of Veterinary Sciences, 11, 327–337. [PubMed] [Google Scholar]
- Gajęcka M, Wozny M, Brzuzan P, Zielonka Ł and Gajęcki M, 2011b. Expression of CYPscc and 3β‐HSD mRNA in bitches ovary after long‐term exposure to zearalenone. Bulletin of the Veterinary Institute of Pulawy, 55, 777–780. [Google Scholar]
- Gajęcka M, Rybarczyk L, Jakimiuk E, Zielonka Ł, Obremski K, Zwierzchowski W and Gajęcki M, 2012. The effect of experimental long‐term exposure to low‐dose zearalenone on uterine histology in sexually immature gilts. Experimental and Toxicologic Pathology, 64, 537–542. [DOI] [PubMed] [Google Scholar]
- Gajęcka M, Zielonka Ł, Dabrowski M, Mroz M and Gajęcki M, 2013. The effect of low doses of zearalenone and its metabolites on progesterone and 17beta‐estradiol concentrations in peripheral blood and body weights of pre‐pubertal female Beagle dogs. Toxicon, 76, 260–269. [DOI] [PubMed] [Google Scholar]
- Gajęcka M, Zielonka Ł and Gajęcki M, 2015. The effect of low monotonic doses of zearalenone on selected reproductive tissues in pre‐pubertal female dogs‐a review. Molecules, 20, 20669–20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajęcka M, Tarasiuk M, Zielonka Ł, Dabrowski M and Gajęcki M, 2016. Risk assessment for changes in the metabolic profile and body weights of pre‐pubertal gilts during long‐term monotonic exposure to low doses of zearalenone (ZEN). Research in Veterinary Science, 109, 169–180. [DOI] [PubMed] [Google Scholar]
- Gareis M, Bauer J, Thiem J, Plank G, Grabley S and Gedek B, 1990. Cleavage of zearalenone‐glycoside, a “masked” mycotoxin, during digestion in swine. Zentralblatt für Veterinärmedizin. Reihe B. Journal of Veterinary Medicine. Series B, 37, 236–240. [DOI] [PubMed] [Google Scholar]
- Golinski P, Kostecki M, Golinska B, Golinska BT and Golinski PK, 2003. Accumulation of mycotoxins in forage of winter pasture during prolonged utilisation of sward. Polish Journal of Veterinary Sciences, 6, 81–86. [PubMed] [Google Scholar]
- Gonkowski S, Obremski K and Calka J, 2015. The Influence of Low Doses of Zearalenone on Distribution of Selected Active Substances in Nerve Fibers Within the Circular Muscle Layer of Porcine Ileum. Journal of Molecular Neuroscience, 56, 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Pereyra ML, Sulyok M, Baralla V, Dalcero AM, Krska R, Chulze S and Cavaglieri LR, 2014. Evaluation of zearalenone, α‐zearalenol, β‐zearalenol, zearalenone 4‐sulfate and β‐zearalenol 4‐glucoside levels during the ensiling process. World Mycotoxin Journal, 7(3), 291–295. [Google Scholar]
- Guillaume J, Kaushik S, Bergot P and Métailler R, 2001. Nutrition and Feeding of Fish and Crustaceans. Praxis‐Springer, Chichester, UK. 408 pp. [Google Scholar]
- Hildebrand AA, Pfeiffer E, Rapp A and Metzler M, 2012. Hydroxylation of the mycotoxin zearalenone at aliphatic positions: novel mammalian metabolites. Mycotoxin Research, 28, 1–8. [DOI] [PubMed] [Google Scholar]
- Huang LC, Zheng N, Zheng BQ, Wen F, Cheng JB, Han RW, Xu XM, Li SL and Wang JQ, 2014. Simultaneous determination of aflatoxin M‐1, ochratoxin A, zearalenone and alpha‐zearalenol in milk by UHPLC‐MS/MS. Food Chemistry, 146, 242–249. [DOI] [PubMed] [Google Scholar]
- Ingerowski GH and Stan HJ, 1979. In vitro metabolism of the anabolic drug Zeranol. Journal of Environmental Pathology and Toxicology, 2, 1173–1182. [PubMed] [Google Scholar]
- Iwanowicz LR and Ottinger CA, 2009. Estrogens, estrogen receptors and their role as immunoregulators in fish. In Zaccone G, Meseguer J, García‐Ayala A, Kapoo BG (eds.). Fish Defenses Vol. 1. Immunology. Science Publishers, Enfield, New Hampshire. pp. 277–322. [Google Scholar]
- Jakimiuk E, Gajęcka M, Jana B, Obremski K and Gajęcki M, 2010a. Effect of zearalenone on steroid secretion by porcine follicular cells in mono‐ and coculture. Bulletin of the Veterinary Institute in Pulawy, 54, 419–423. [Google Scholar]
- Jakimiuk E, Rybarczyk L, Zwierzchowski W, Obremski K, Gajęcka M, Zielonka Ł and Gajęcki M, 2010b. Effect of experimental long‐term exposure to low‐dose zearalenone mycotoxicosis on selected morphometric parameters of the reproductive tract in sexually‐immature gilts. Bulletin of the Veterinary Institute in Pulawy, 54, 25–28. [Google Scholar]
- Jakimiuk E, Radwinska J, Pomianowski A, Wozny M, Obremski K, Gajęcka M, Brzuzan P and Gajęcki M, 2015. Evaluation of selected serum biochemical and haematological parameters in gilts exposed per os to 100 ppb of zearalenone. Polish Journal of Veterinary Sciences, 18, 865–872. [DOI] [PubMed] [Google Scholar]
- Jard G, Liboz T, Mathieu F, Guyonvarc'h A, André F, Delaforge M and Lebrihi A, 2010. Transformation of zearalenone to zearalenone‐sulfate by Aspergillus spp. World Mycotoxin Journal, 3, 183–191. [Google Scholar]
- Jia R, Ma Q, Fan Y, Ji C, Zhang J, Liu T and Zhao L, 2016. The toxic effects of combined aflatoxins and zearalenone in naturally contaminated diets on laying performance, egg quality and mycotoxins residues in eggs of layers and the protective effect of Bacillus subtilis biodegradation product. Food and Chemical Toxicology, 90, 142–150. [DOI] [PubMed] [Google Scholar]
- Jiang SZ, Yang ZB, Yang WR, Wang SJ, Liu FX, Johnston LA, Chi F and Wang Y, 2012. Effect of purified zearalenone with or without modified montmorillonite on nutrient availability, genital organs and serum hormones in post‐weaning piglets. Livestock Science, 144, 110–118. 10.1016/j.livsci.2011.11.004 [DOI] [Google Scholar]
- Jo H, Kong C, Song M and Kim BG, 2016. Effects of dietary deoxynivalenol and zearalenone on apparent ileal digestibility of amino acids in growing pigs. Animal Feed Science and Technology, 219, 77–82. [Google Scholar]
- Johns SM, Denslow ND, Kane MD, Watanabe KH, Orlando EF and Sepulveda MS, 2011. Effects of Estrogens and Antiestrogens on Gene Expression of Fathead Minnow (Pimephales promelas) Early Life Stages. Environmental Toxicology, 26, 195–206. [DOI] [PubMed] [Google Scholar]
- Juhasz J, Nagy P, Kulcsar M, Szigeti G, Reiczigel J and Huszenicza G, 2001. Effect of low‐dose zearalenone exposure on luteal function, follicular activity and uterine oedema in cycling mares. Acta Veterinaria Hungarica, 49, 211–222. [DOI] [PubMed] [Google Scholar]
- Kamimura H, 1986. Conversion of zearalenone to zearalenone glycoside by Rhizopus sp. Applied and Environmental Microbiology, 52, 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keese C, Meyer U, Rehage J, Spilke J, Boguhn J, Breves G and Dänicke S, 2008a. On the effects of the concentrate proportion of dairy cow rations in the presence and absence of a fusarium toxin‐contaminated triticale on cow performance. Archives of Animal Nutrition, 62, 241–262. [DOI] [PubMed] [Google Scholar]
- Keese C, Meyer U, Rehage J, Spilke J, Boguhn J, Breves G and Dänicke S, 2008b. Ruminal fermentation patterns and parameters of the acid base metabolism in the urine as influenced by the proportion of concentrate in the ration of dairy cows with and without Fusarium toxin‐contaminated triticale. Archives of Animal Nutrition, 62, 287–302. [DOI] [PubMed] [Google Scholar]
- Keith LH, Crummett W, Deegan J, Libby RA, Taylor JK and Wentler G, 1983. Principles of environmental analysis. Analytical Chemistry, 55, 2210–2218. [Google Scholar]
- Keles O, Candan A, Bakirel T and Karataş S, 2002. The investigation of the anabolic efficiency and effect on the nonspecific immune system of zeranol in rainbow trout (Oncorhynchus mykiss, Walbaum). Turkish Journal of Veterinary and Animal Sciences, 26, 925–931. [Google Scholar]
- Kennedy DG, Hewitt SA, McEvoy JDG, Currie JW, Cannavan A, Blanchflower WJ and Elliot CT, 1998. Zeranol is formed from Fusarium spp. toxins in cattle in vivo . Food Additives and Contaminants, 15, 393–400. [DOI] [PubMed] [Google Scholar]
- Kiessling KH, Pettersson H, Sandholm K and Olsen M, 1984. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Applied and Environmental Microbiology, 47, 1070–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinova M, Zollner P, Kahlbacher H, Hochsteiner W and Lindner W, 2002. Metabolic profiles of the mycotoxin zearalenone and of the growth promoter Zeranol in urine, liver, and muscle of heifers. Journal of Agricultural and Food Chemistry, 50, 4769–4776. [DOI] [PubMed] [Google Scholar]
- Kluger B, Bueschl C, Lemmens M, Berthiller F, Haubl G, Jaunecker G, Adam G, Krska R and Schuhmacher R, 2013. Stable isotopic labelling‐assisted untargeted metabolic profiling reveals novel conjugates of the mycotoxin deoxynivalenol in wheat. Analytical and Bioanalytical Chemistry, 405, 5031–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolf‐Clauw M, Ayouni F, Tardieu D and Guerre R, 2007. HPLC assay of zearalenone and reduced metabolites in S9 fractions of duck liver. Revue de Médecine Vétérinaire, 158, 504–508. [Google Scholar]
- Kolf‐Clauw M, Ayouni F, Tardieu D and Guerre R, 2008. Variations in zearalenone activation in avian food species. Food and Chemical Toxicology, 46, 1467–1473. [DOI] [PubMed] [Google Scholar]
- Kollarczik B, Gareis M and Hanelt M, 1994. In vitro transformation of the Fusarium mycotoxins deoxynivalenol and zearalenone by the normal gut microflora of pigs. Natural Toxins, 2, 105–110. [DOI] [PubMed] [Google Scholar]
- Kong C, Shin SY, Park CS and Kim BG, 2015. Effects of Feeding Barley Naturally Contaminated with Fusarium Mycotoxins on Growth Performance, Nutrient Digestibility, and Blood Chemistry of Gilts and Growth Recoveries by Feeding a Non‐contaminated Diet. Asian‐Australasian Journal of Animal Sciences, 28, 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppen R, Riedel J, Proske M, Drzymala S, Rasenko T, Durmaz V, Weber M and Koch M, 2012. Photochemical trans‐/cis‐Isomerization and Quantitation of Zearalenone in Edible Oils. Journal of Agricultural and Food Chemistry, 60, 11733–11740. [DOI] [PubMed] [Google Scholar]
- Kovalsky Paris MP, Schweiger W, Hametner C, Stückler R, Muehlbauer GJ, Varga E, Krska R, Berthiller F and Adam G, 2014. Zearalenone‐16‐O‐glucoside: a new masked mycotoxin. Journal of Agricultural and Food Chemistry, 62, 1181–1189. [DOI] [PubMed] [Google Scholar]
- Kowalska K, Habrowska‐Gorczynska DE and Piastowska‐Ciesielska AW, 2016. Zearalenone as an endocrine disruptor in humans. Environmental Toxicology and Pharmacology, 48, 141–149. [DOI] [PubMed] [Google Scholar]
- Kuiper‐Goodman T, Scott PM and Watanabe H, 1987. Risk assessment of the mycotoxin zearalenone. Regulatory Toxicology and Pharmacology, 7, 253–306. [DOI] [PubMed] [Google Scholar]
- Lebas F and Renouf B, 2009. Nutrition: utilisation des matières premières et techniques d'alimentation. Cuniculture Magazine, 36, 12–64. [Google Scholar]
- Leeson S and Summers JD, 2008. Commercial Poultry Nutrition, 3rd Edition. Nottingham University Press, Nottingham, UK. 416 pp. [Google Scholar]
- Lewczuk B, Przybylska‐Gornowicz B, Gajecka M, Targonska K, Ziolkowska N, Prusik M and Gajęcki M, 2016. Histological structure of duodenum in gilts receiving low doses of zearalenone and deoxynivalenol in feed. Experimental and Toxicologic Pathology, 68, 157–166. [DOI] [PubMed] [Google Scholar]
- de Lourdes Mendes de Souza M, Sulyok M, Freitas‐Silva O, Costa SS, Brabet C, Machinski Junior M, Sekiyama BL, Vargas EA, Krska R and Schuhmacher R, 2013. Cooccurrence of mycotoxins in maize and poultry feeds from Brazil by liquid chromatography/tandem mass spectrometry. Scientific World Journal, 2013, 427369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekinejad H, 2013. Zearalenone is converted to a potent oestrogenic metabolite by the equine hepatic subcellular fractions and hepatocytes. Bulgarian Journal of Veterinary Medicine, 16, 260–270. [Google Scholar]
- Malekinejad H, Maas‐Bakker RF and Fink‐Gremmels J, 2005a. Bioactivation of zearalenone by porcine hepatic biotransformation. Veterinary Research, 36, 799–810. [DOI] [PubMed] [Google Scholar]
- Malekinejad H, Maas‐Bakker RF and Fink‐Gremmels J, 2005b. Enzyme kinetics of zearalenone biotransformation: pH and cofactor effects. Archives of Toxicology, 79, 547–553. [DOI] [PubMed] [Google Scholar]
- Malekinejad H, Colenbrander B and Fink‐Gremmels J, 2006. Hydroxysteroid dehydrogenases in bovine and porcine granulosa cells convert zearalenone into its hydroxylated metabolites alpha‐zearalenol and beta‐zearalenol. Veterinary Research Communications, 30, 445–453. [DOI] [PubMed] [Google Scholar]
- Malekinejad H, Agh N, Vahabzadeh Z, Varasteh S and Alavi MH, 2012. In vitro reduction of zearalenone to beta‐zearalenol by rainbow trout (Oncorhynchus mykiss) hepatic microsomal and post‐mitochondrial subfractions. Iranian Journal of Veterinary Research, 13, 28–35. [Google Scholar]
- Maryamma KI, Manomohan CB, Nair MG, Ismail PK, Sreekumaran T and Rajan A, 1992. Pathology of zearalenone toxicosis in chicken and evaluation of zearalenone residues in tissues. Indian Journal of Animal Science, 62, 105–107. [Google Scholar]
- McDonald P, Edwards RA, Greenhalgh JFD, Morgan CA, Sinclair LA and Wilkinson RG, 2011. Animal Nutrition, 7th Edition. Benjamin Cummings, Pearson Canada. 692 pp. [Google Scholar]
- di Menna ME, Lauren DR and Holland PT, 1985. Presence of zearalenone in New Zealand pasture leaves. New Zealand Veterinary Journal, 33, 193–193. 10.1080/00480169.1985.35232 [DOI] [PubMed] [Google Scholar]
- Metzler M, Pfeiffer E and Hildebrand AA, 2010. Zearalenone and its metabolites as endocrine disrupting chemicals. World Mycotoxin Journal, 3, 385–401. [Google Scholar]
- Meucci V, Soldani G, Razzuoli E, Saggese G and Massart F, 2011. Mycoestrogen Pollution of Italian Infant Food. Journal of Pediatrics, 159, 278–U365. [DOI] [PubMed] [Google Scholar]
- Migdalof BH, Dugger HA, Heider JG, Coombs RA and Terry MK, 1983. Biotransformation of Zeranol: disposition and metabolism in the female rat, rabbit, dog, monkey and man. Xenobiotica, 13, 209–221. [DOI] [PubMed] [Google Scholar]
- Miles CO, Erasmuson AF, Wilkins AL, Towers NR, Smith BL, Garthwaite I, Scahill BG and Hansen RP, 1996. Ovine Metabolism of Zearalenone to α‐Zearalanol (Zeranol). Journal of Agricultural and Food Chemistry, 44, 3244–3250. [Google Scholar]
- Mirocha CJ, Robison TS, Pawlosky RJ and Allen NK, 1982. Distribution and residue determination of [3H]zearalenone in broilers. Toxicology and Applied Pharmacology, 66, 77–87. [DOI] [PubMed] [Google Scholar]
- Molina‐Molina JM, Real M, Jimenez‐Diaz I, Belhassen H, Hedhili A, Torne P, Fernandez MF and Olea N, 2014. Assessment of oestrogenic and anti‐androgenic activities of the mycotoxin zearalenone and its metabolites using in vitro receptor‐specific bioassays. Food and Chemical Toxicology, 74, 233–239. [DOI] [PubMed] [Google Scholar]
- Nathanail AV, Syvahuoko J, Malachova A, Jestoi M, Varga E, Michlmayr H, Adam G, Sievilainen E, Berthiller F and Peltonen K, 2015. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography‐tandem mass spectrometric method. Analytical and Bioanalytical Chemistry, 407, 4745–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix JS, 2010. The John Nix Farm Management Pocketbook, 40th Edition. The Anderson Centre. [Google Scholar]
- NRC (National Research Council), 1982. Nutrient requirements of mink and foxes, 2nd Revised Edition. US National Academy of Science. National Academies Press, Washington, DC. Available online: https://www.nap.edu/read/1114/chapter/1111. [Google Scholar]
- NRC (National Research Council), 2000. Nutrient requirements of beef cattle: 7th Revised Edition (Updated 2000). National Academies Press, Washington, DC. Available online: https://www.nap.edu/catalog/9791/nutrient-requirements-of-beef-cattle-seventh-revised-edition-update-2000 [Google Scholar]
- NRC (National Research Council), 2006. Nutrient requirements of dogs and cats. National Academies Press, Washington, DC. Available online: https://www.nap.edu/catalog/10668/nutrient-requirements-of-dogs-and-cats [Google Scholar]
- NRC (National Research Council), 2007a. Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. US National Academy of Science, Washington, DC. Available online: https://www.nap.edu/read/11654/chapter/11651. [Google Scholar]
- NRC (National Research Council), 2007b. Nutrient requirement of horses, 6th Revised Edition. National Academies Press, Washington, DC. Available online: https://www.nap.edu/catalog/11653/nutrient-requirements-of-horses-sixth-revised-edition [Google Scholar]
- NRC (National Research Council), 2012. Nutrient Requirements of Swine, 11th Revised Edition. US National Academy of Science, Washington DC. [Google Scholar]
- Obremski K and Poniatowska‐Broniek G, 2015. Zearalenone induces apoptosis and inhibits proliferation in porcine ileal Peyer's patch lymphocytes. Polish Journal of Veterinary Sciences, 18, 153–161. [DOI] [PubMed] [Google Scholar]
- Obremski K, Gajęcki M, Zwierzchowski W, Bakula T, Apoznaniski J and Wojciechoeski J, 2003. The level of zearalenone and a‐zearalenol in the blood of gilts with clinical symptoms of toxicosis, fed diets with a low zearalenone content. Journal of Animal and Feed Sciences, 12, 529–538. [Google Scholar]
- Obremski K, Gonkowski S and Wojtacha P, 2015a. Zearalenone‐induced changes in the lymphoid tissue and mucosal nerve fibers in the porcine ileum. Polish Journal of Veterinary Sciences, 18, 357–365. [DOI] [PubMed] [Google Scholar]
- Obremski K, Wojtacha P, Podlasz P and Zmigrodzka M, 2015b. The influence of experimental administration of low zearalenone doses on the expression of Th1 and Th2 cytokines and on selected subpopulations of lymphocytes in intestinal lymph nodes. Polish Journal of Veterinary Sciences, 18, 489–497. [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐Operation and Development), 2013. Guidance document on residues in livestock. Series on Pesticides No. 73 ENV/JM/MONO(2013)8. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2013)8&doclanguage=en
- Oliver WT, Miles JR, Diaz DE, Dibner JJ, Rottinghaus GE and Harrell RJ, 2012. Zearalenone enhances reproductive tract development, but does not alter skeletal muscle signaling in prepubertal gilts. Animal Feed Science and Technology, 174, 79–85. [Google Scholar]
- Olsen M and Kiessling KH, 1983. Species‐differences in zearalenone‐reducing activity in subcellular‐fractions of liver from female domestic‐animals. Acta Pharmacologica et Toxicologica, 52, 287–291. [DOI] [PubMed] [Google Scholar]
- Olsen ME, Pettersson HI, Sandholm KA and Kiessling KHC, 1985. Quantitative liquid chromatographic method using fluorescence detection for determining zearalenone and its metabolites in blood plasma and urine. Journal of the Association of Official Analytical Chemists, 68, 632–635. [PubMed] [Google Scholar]
- Olsen M, Mirocha CJ, Abbas HK and Johansson B, 1986. Metabolism of high concentrations of dietary zearalenone by young male turkey poults. Poultry Science, 65, 1905–1910. [DOI] [PubMed] [Google Scholar]
- Osselaere A, Devreese M, Goossens J, Vandenbroucke V, De Baere S, De Backer P and Croubels S, 2013. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T‐2 toxin and zearalenone in broiler chickens. Food and Chemical Toxicology, 51, 350–355. [DOI] [PubMed] [Google Scholar]
- Pastorova B, Laciakova A, Conkova E, Maracek I, Danko J and Pilipcinec E, 2004. Influence of zearalenone on plasma catecholamines in rabbits. Bulletin of the Veterinary Institute in Pulawy, 48, 151–153. [Google Scholar]
- Penning TM, Jin Y, Steckelbroeck S, Lanišnik Rižner T and Lewis M, 2004. Structure–function of human 3α‐hydroxysteroid dehydrogenases: genes and proteins. Molecular and Cellular Endocrinology, 215, 63–72. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E, Hildebrand A, Mikula H and Metzler M, 2010. Glucuronidation of zearalenone, Zeranol and four metabolites in vitro: Formation of glucuronides by various microsomes and human UDP‐glucuronosyltransferase isoforms. Molecular Nutrition & Food Research, 54, 1468–1476. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E, Kommer A, Dempe JS, Hildebrand AA and Metzler M, 2011. Absorption and metabolism of the mycotoxin zearalenone and the growth promotor Zeranol in Caco‐2 cells in vitro . Molecular Nutrition & Food Research, 55, 560–567. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E, Wefers D, Hildebrand AA, Fleck SC and Metzler M, 2013. Catechol metabolites of the mycotoxin zearalenone are poor substrates but potent inhibitors of catechol‐O‐methyltransferase. Mycotoxin Research, 29, 177–183. [DOI] [PubMed] [Google Scholar]
- Pietsch C and Junge R, 2016. Physiological responses of carp (Cyprinus carpio L.) to dietary exposure to zearalenone (ZEN). Comparative Biochemistry and Physiology C‐Toxicology & Pharmacology, 188, 52–59. [DOI] [PubMed] [Google Scholar]
- Pietsch C, Kersten S, Burkhardt‐Holm P, Valenta H and Dänicke S, 2013. Occurrence of deoxynivalenol and zearalenone in commercial fish feed: an initial study. Toxins, 5, 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietsch C, Noser J, Wettstein FE and Burkhardt‐Holm P, 2014. Unraveling the mechanisms involved in zearalenone‐mediated toxicity in permanent fish cell cultures. Toxicon, 88, 44–61. [DOI] [PubMed] [Google Scholar]
- Pietsch C, Kersten S, Valenta H, Dänicke S, Schulz C, Burkhardt‐Holm P and Junge R, 2015a. Effects of Dietary Exposure to Zearalenone (ZEN) on Carp (Cyprinus carpio L.). Toxins, 7, 3465–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietsch C, Junge R and Burkhardt‐Holm P, 2015b. Immunomodulation by Zearalenone in carp (Cyprinus carpio L.). BioMed Research International, 2015, 9 pp. 10.1155/2015/420702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistol GC, Braicu C, Motiu M, Gras MA, Marin DE, Stancu M, Calin L, Israel‐Roming F, Berindan‐Neagoe I and Taranu I, 2015. Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome‐wide gene expression in pig spleen. PLoS ONE, 10, e0127503. 10.1371/journal.pone.0127503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasencia J and Mirocha CJ, 1991. Isolation and characterization of zearalenone sulfate produced by Fusarium spp. Applied and Environmental Microbiology, 57, 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompa G, Montesissa C, Dilauro FM, Fadini L and Capua C, 1988. Zearanol metabolism by subcellular‐fractions from lamb liver. Journal of Veterinary Pharmacology and Therapeutics, 11, 197–203. [DOI] [PubMed] [Google Scholar]
- Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R, Krska R, Kuchler K, Glossl J, Luschnig C and Adam G, 2003. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP‐glucosyltransferase from Arabidopsis thaliana . Journal of Biological Chemistry, 278, 47905–47914. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Scott PM, Trenholm HL and Lawrence GA, 1990. Minimal transmission of zearalenone to milk of dairy cows. Journal of Environmental Science and Health. Part B: Pesticides, Food Contaminants, and Agricultural Wastes, 25, 87–103. [DOI] [PubMed] [Google Scholar]
- Przybylska‐Gornowicz B, Tarasiuk M, Lewczuk B, Prusik M, Ziolkowska N, Zielonka Ł, Gajęcki M and Gajęcka M, 2015. The effects of low doses of two fusarium toxins, zearalenone and deoxynivalenol, on the pig jejunum. A Light and Electron Microscopic Study. Toxins, 7, 4684–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond SL, Smith TK and Swamy H, 2003. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on feed intake, serum chemistry, and hematology of horses, and the efficacy of a polymeric glucomannan mycotoxin adsorbent. Journal of Animal Science, 81, 2123–2130. [DOI] [PubMed] [Google Scholar]
- Raymond SL, Smith TK and Swamy H, 2005. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on feed intake, metabolism, and indices of athletic performance of exercised horses. Journal of Animal Science, 83, 1267–1273. [DOI] [PubMed] [Google Scholar]
- Rempe I, Brezina U, Kersten S and Danicke S, 2013a. Effects of a Fusarium toxin‐contaminated maize treated with sodium metabisulphite, methylamine and calcium hydroxide in diets for female piglets. Archives of Animal Nutrition, 67, 314–329. [DOI] [PubMed] [Google Scholar]
- Rempe I, Kersten S, Brezina U, Hermeyer K, Beineke A and Dänicke S, 2013b. Time dependent effects of graded levels of Fusarium toxin contaminated maize in diets for female piglets. World Mycotoxin Journal, 6, 51–63. [Google Scholar]
- Ryu D, Hanna MA and Bullerman LB, 1999. Stability of zearalenone during extrusion of corn grits. Journal of Food Protection, 62, 1482–1484. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food), 2000. Opinion of the Scientific Committee on Food on Fusarium toxins. Part 2: Zearalenone (ZEA). 12 pp. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_catalogue_out65_en.pdf
- Schoevers EJ, Santos RR, Colenbrander B, Fink‐Gremmels J and Roelen BAJ, 2012. Transgenerational toxicity of Zearalenone in pigs. Reproductive Toxicology, 34, 110–119. [DOI] [PubMed] [Google Scholar]
- Schrickx JA and Fink‐Gremmels J, 2008. Implications of ABC transporters on the disposition of typical veterinary medicinal products. European Journal of Pharmacology, 585, 510–519. [DOI] [PubMed] [Google Scholar]
- Schultz S, Vallant B and Kainz MJ, 2012. Preferential feeding on high quality diets decreases methyl mercury of farm‐raised common carp (Cyprinus carpio L.). Aquaculture, 338–341, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann B, Winkler J, Mickenautsch N, Warnken T and Dänicke S, 2016. Effects of deoxynivalenol (DON), zearalenone (ZEN), and related metabolites on equine peripheral blood mononuclear cells (PBMC) in vitro and background occurrence of these toxins in horses. Mycotoxin Research, 32, 153–161. [DOI] [PubMed] [Google Scholar]
- Schwake‐Anduschus C, Proske M, Sciurba E, Muenzing K, Koch M and Maul R, 2015. Distribution of deoxynivalenol, zearalenone, and their respective modified analogues in milling fractions of naturally contaminated wheat grains. World Mycotoxin J, 8, 433–443. 10.3920/WMJ2014.1818 [DOI] [Google Scholar]
- Schwartz P, Bucheli TD, Wettstein FE and Burkhardt‐Holm P, 2013. Life‐cycle exposure to the estrogenic mycotoxin zearalenone affects zebrafish (Danio rerio) development and reproduction. Environmental Toxicology, 28, 276–289. 10.1002/tox.20718 [DOI] [PubMed] [Google Scholar]
- Seeling K and Dänicke S, 2005. Relevance of the Fusarium toxins deoxynivalenol and zearalenone in ruminant nutrition. A review. Journal of Animal and Feed Sciences, 14, 3–40. [Google Scholar]
- Seeling K, Dänicke S, Ueberschär KH, Lebzien P and Flachowsky G, 2005. On the effects of Fusarium toxin‐contaminated wheat and the feed intake level on the metabolism and carry over of zearalenone in dairy cows. Food Additives and Contaminants, 22, 847–855. [DOI] [PubMed] [Google Scholar]
- el‐Sharkaway SH, Selim MI, Afifi MS and Halaweish FT, 1991. Microbial transformation of zearalenone to a zearalenone sulfate. Applied and Environmental Microbiology, 57, 549–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin BS, Hong SH, Bulitta JB, Hwang SW, Kim HJ, Lee JB, Yang SD, Kim JE, Yoon HS, Kim do J and Yoo SD, 2009. Disposition, oral bioavailability, and tissue distribution of zearalenone in rats at various dose levels. Journal of Toxicology and Environmental Health. Part A, 72, 1406–1411. [DOI] [PubMed] [Google Scholar]
- Skládanka J, Nedelnik J, Adam V, Dolezal P, Moravcova H and Dohnal V, 2011. Forage as a primary source of mycotoxins in animal diets. International Journal of Environmental Research and Public Health, 8, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JF, di Menna ME and McGowan LT, 1990. Reproductive performance of Coopworth ewes following oral doses of zearalenone before and after mating. Journal of Reproduction and Fertility, 89, 99–106. [DOI] [PubMed] [Google Scholar]
- Songsermsakul P, Bohm J, Aurich C, Zentek J and Razzazi‐Fazeli E, 2013. The levels of zearalenone and its metabolites in plasma, urine and faeces of horses fed with naturally, Fusarium toxin‐contaminated oats. Journal of Animal Physiology and Animal Nutrition, 97, 155–161. [DOI] [PubMed] [Google Scholar]
- Stopa E, Gajęcka M, Babinska I, Zielonka Ł and Gajęcki M, 2014. The effect of experimental exposure to low doses of zearalenone on uterine histology and morphometry in prepubertal bitches. Theriogenology, 82, 537–545. [DOI] [PubMed] [Google Scholar]
- Streit E, Naehrer K, Rodrigues I and Schatzmayr G, 2013. Mycotoxin occurrence in feed and feed raw materials worldwide: long‐term analysis with special focus on Europe and Asia. Journal of the Science of Food and Agriculture, 93, 2892–2899. 10.1002/jsfa.6225 [DOI] [PubMed] [Google Scholar]
- Sutkeviciene N, Bakutis B, Banys A, Karveliene B, Rutkauskas A, Sabeckiene J and Zilinskas H, 2009. The effect of the oestrogenic mycotoxin zearalenone on boar reproductive potential and the dynamic of aspartate aminotransferase and alanine aminotransferase levels in the boar blood serum. Veterinarija Ir Zootechnika, 46, 73–77. [Google Scholar]
- Teixeira LC, Montiani‐Ferreira F, Locatelli‐Dittrich R, Santin E and Alberton GC, 2011. Effects of zearalenone in prepubertal gilts. Pesquisa Veterinaria Brasileira, 31, 656–662. [Google Scholar]
- Thigpen JE, Li LA, Richter CB, Lebetkin EH and Jameson CW, 1987. The mouse bioassay for the detection of oestrogenic activity in rodent diets: II. Comparative oestrogenic activity of purified, certified and standard open and closed formula rodent diets. Laboratory Animal Science, 37, 602–605. [PubMed] [Google Scholar]
- Tiemann U and Dänicke S, 2007. In vivo and in vitro effects of the mycotoxins zearalenone and deoxynivalenol on different non‐reproductive and reproductive organs in female pigs: a review. Food Additives and Contaminants, 24, 306–314. [DOI] [PubMed] [Google Scholar]
- Tola S, Bureau DP, Hooft JM, Beamish FW, Sulyok M, Krska R, Encarnacao P and Petkam R, 2015. Effects of Wheat Naturally Contaminated with Fusarium Mycotoxins on Growth Performance and Selected Health Indices of Red Tilapia (Oreochromis niloticus x O. mossambicus). Toxins, 7, 1929–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout WE, Herr CT, Richert BT, Singleton WL, Haglof SA and Diekman MA, 2007. Effects of Zeranol upon luteal maintenance and fetal development in peripubertal gilts. Animal Reproduction Science, 99, 408–412. [DOI] [PubMed] [Google Scholar]
- Ueberschär K‐H, 1999. Einfluß von Zearalenon auf Wachstum und Rückstände in den Geweben von Mastkaninchen. VDLUFA‐Kongreßband 1999, Halle/Saale. VDLUFA‐Schriftenreihe, 52/1999, 425–428. [Google Scholar]
- Vanyi A and Szeky A, 1980. Fusariotoxicoses. 6. The effect of F2 toxin (zearalenone) on the spermatogenesis of male swine. Magyar Allatorvosok Lapja, 35, 242–246. [Google Scholar]
- Videmann B, Mazallon M, Prouillac C, Delaforge M and Lecoeur S, 2009. ABCC1, ABCC2 and ABCC3 are implicated in the transepithelial transport of the myco‐estrogen zearalenone and its major metabolites. Toxicology Letters, 190, 215–223. [DOI] [PubMed] [Google Scholar]
- Wasowicz K, Gajęcka M, Calka J, Jakimiuk E and Gajęcki M, 2005. Influence of chronic administration of zearalenone on the processes of apoptosis in the porcine ovary. Veterinarni Medicina, 50, 531–536. [Google Scholar]
- Weaver GA, Kurtz HJ, Behrens JC, Robison TS, Seguin BE, Bates FY and Mirocha CJ, 1986a. Effect of zearalenone on the fertility of virgin dairy heifers. American Journal of Veterinary Research, 47, 1395–1397. [PubMed] [Google Scholar]
- Weaver GA, Kurtz HJ, Behrens JC, Robison TS, Seguin BE, Bates FY and Mirocha CJ, 1986b. Effect of zearalenone on dairy cows. American Journal of Veterinary Research, 47, 1826–1828. [PubMed] [Google Scholar]
- WHO/IPCS (World Health Organization), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food, International Programme on Chemical Safety, Environmental Health Criteria 240. Chapter 6: Dietary Exposure Assessment of Chemicals in Food. Available online: http://www.inchem.org/documents/ehc/ehc/ehc240_chapter6.pdf
- Winkler J, Kersten S, Meyer U, Engelhardt U and Danicke S, 2014. Residues of zearalenone (ZEN), deoxynivalenol (DON) and their metabolites in plasma of dairy cows fed Fusarium contaminated maize and their relationships to performance parameters. Food and Chemical Toxicology, 65, 196–204. [DOI] [PubMed] [Google Scholar]
- Woźny M, Obremski K, Jakimiuk E, Gusiatin M and Brzuzan P, 2013. Zearalenone contamination in rainbow trout farms in north‐eastern Poland. Aquaculture, 416–417, 209–211. [Google Scholar]
- Woźny M, Dobosz S, Obremski K, Hliwa P, Gomulka P, Lakomiak A, Rozynski R, Zalewski T and Brzuzan P, 2015. Feed‐borne exposure to zearalenone leads to advanced ovarian development and limited histopathological changes in the liver of premarket size rainbow trout. Aquaculture, 448, 71–81. [Google Scholar]
- Xia X, Li XW, Ding SY, Zhang SX, Jiang HY, Li JC and Shen JZ, 2009. Ultra‐high‐pressure liquid chromatography‐tandem mass spectrometry for the analysis of six resorcylic acid lactones in bovine milk. Journal of Chromatography A, 1216, 2587–2591. [DOI] [PubMed] [Google Scholar]
- Xu ML, Hu J, Guo BP, Niu YR, Xiao C and Xu YX, 2016. Exploration of intrinsic and extrinsic apoptotic pathways in zearalenone‐treated rat sertoli cells. Environmental Toxicology, 31, 1731–1739. [DOI] [PubMed] [Google Scholar]
- Yamini B, Bursian SJ and Aulerich RJ, 1997. Pathological effects of dietary zearalenone and/or tamoxifen on female mink reproductive organs. Veterinary and Human Toxicology, 39, 74–78. [PubMed] [Google Scholar]
- Yang HH, Aulerich RJ, Helferich W, Yamini B, Chou KC, Miller ER and Bursian SJ, 1995. Effects of zearalenone and/or tamoxifen on swine and mink reproduction. Journal of Applied Toxicology, 15, 223–232. [DOI] [PubMed] [Google Scholar]
- Zhong S, Liu S, Chen S, Lin H, Wang W and Qin X, 2016. Zeranol stimulates proliferation and aromatase activation in human breast preadipocytes. Molecular Medicine Reports, 14, 1014–1018. [DOI] [PubMed] [Google Scholar]
- Zinedine A, Soriano JM, Molto JC and Manes J, 2007. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food and Chemical Toxicology, 45, 1–18. [DOI] [PubMed] [Google Scholar]
- Zwierzchowski W, Przybylowicz M, Obremski K, Zielonka Ł, Skorska‐Wyszynska E, Gajęcka M, Polak M, Jakimiuk E, Jana B, Rybarczyk L and Gajęcki M, 2005. Level of zearalenone in blood serum and lesions in ovarian follicles of sexually immature gilts in the course of zearalenone micotoxicosis. Polish Journal of Veterinary Sciences, 8, 209–218. [PubMed] [Google Scholar]


