Abstract
The Panel on Food Additives and Nutrient Sources added to Food (ANS) provides a scientific opinion re‐evaluating the safety of tara gum (E 417) as a food additive. Tara gum (E 417) has been evaluated by the EU Scientific Committee for Food (SCF, 1992) and by the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 1987), who both allocated an acceptable daily intake (ADI) ‘not specified’ for this gum. Following the conceptual framework for the risk assessment of certain food additives, re‐evaluated under Commission Regulation (EU) No 257/2010, the Panel considered that adequate exposure and toxicity data were available for tara gum (E 417). Tara gum (E 417) is unlikely to be absorbed intact and is expected to be fermented by intestinal microbiota. No adverse effects were reported at the highest doses tested in subchronic, chronic and carcinogenicity studies and there is no concern with respect to the genotoxicity. The Panel concluded that there is no need for a numerical ADI for tara gum (E 417) and that there is no safety concern for the general population at the refined exposure assessment of tara gum (E 417) as a food additive at the reported uses and use levels.
Keywords: tara gum (E 417), CAS Registry Number 39300‐88‐4, food additive
Summary
Following a request from the European Commission, the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to re‐evaluate the safety of tara gum (E 417) when used as a food additive.
Tara gum (E 417) is authorised as a food additive in the European Union (EU) according to Annex II and III to Regulation (EC) No 1333/2008 on food additives and it was previously evaluated by the EU Scientific Committee for Food (SCF) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA), who both allocated an acceptable daily intake (ADI) ‘not specified’ for this gum.
The Panel was not provided with a newly submitted dossier and based its evaluation on previous evaluations and reviews, additional literature that became available since then and the data provided following public calls for data. Not all original studies on which previous evaluations were based were available for re‐evaluation by the Panel.
Tara gum is a galactomannan isolated from the endosperm of the seeds of the tara tree, Caesalpinia spinosa L. Tara gum is commonly defined as a high‐viscosity polysaccharide composed mainly of a linear chain of (1‐4)‐β‐d‐mannopyranose units with α‐d‐galactopyranose units attached by (1‐6) linkages. The ratio of mannose to galactose in tara gum is 3:1.
Specifications for tara gum have been defined in Commission Regulation (EU) 231/2012 and by JECFA (2006).
Because of both the botanical origin and the polysaccharidic nature of gums, they can be a substrate of microbiological contamination and of field and storage fungal development. The latter has been recently demonstrated by the mycotoxin contaminations of gums (Zhang et al., 2014). The Panel noted that the microbiological specifications for polysaccharidic thickening agents, such as gums, should be harmonised and that for tara gum criteria for total aerobic microbial count (TAMC) and total combined yeasts and moulds count (TYMC) should be included into the EU specifications.
Data on in vitro degradation by human gastrointestinal fluids and on in vivo digestibility of tara gum in animals demonstrated that this compound would not be absorbed intact or hydrolysed by digestive enzymes. Based on the fermentation of other galactomannans, the Panel considered that tara gum would be fermented with production of short‐chain fatty acids (SCFAs) such as acetic, propionic and butyric acids, during its passage through the large intestine by strains of bacteria found in the human colon. Based on the available knowledge on the role of SCFA as end products of the fermentation of dietary fibres by the anaerobic intestinal microbiota (den Besten et al., 2014; Topping and Clifton, 2001), the Panel considered that their potential formation as fermentation products from tara gum does not raise any concern. Despite the absence of convincing in vivo study in humans, the Panel considered that tara gum would most probably not be absorbed intact but could be fermented by intestinal microbiota in humans.
Acute oral toxicity studies with tara gum on rats and mice showed no toxic effects up to a dose of 630 mg/kg body weight (bw) tara gum.
Short‐term and subchronic animal toxicity studies on tara gum have not shown biologically significant adverse effects associated to the treatment under the conditions of the tests. From studies in rats fed high doses of tara gum, ≥ 5% dietary level, decrease of mean body weight and other findings, e.g. increased blood urea nitrogen have been reported. The Panel noted that these findings could be attributed to a nutritional imbalance induced by the high percentage of the compound added in the diet. No‐observed‐adverse effect levels (NOAELs) identified in short‐term and subchronic studies corresponded to the highest dose tested of 12,000, 4,500 and 2,250 mg/kg bw per day for rats (Til et al., 1974; NTP, 1982), of approximately 20,000 and 10,000 mg/kg bw per day for mice (NTP, 1982) and of approximately 1,250 mg/kg bw per day in dogs (Oshita et al., 1975; as reported in JECFA, 1987; Borzelleca et al., 1993).
Based on the data available, the Panel concluded that there is no concern with respect to the genotoxicity of tara gum (E 417).
Tara gum was tested in chronic toxicity and carcinogenicity assays in rats (two strains) and mice (one strain) receiving diets containing 25,000 and 50,000 ppm tara gum for 103 weeks (equivalent to 3,750 and 7,500 mg/kg bw per day in mice and, 1,250 and 2,500 mg/kg bw per day in rats). No major differences (2–9%) were observed in body weight gain either in males or females, contrary to the sub‐chronic studies.
In rats, 2,500 mg/kg bw per day and in mice, 7,500 mg/kg bw per day (both the highest dose tested) was identified as the NOAEL by the Panel from chronic toxicity assays. Further, the Panel considered tara gum as not carcinogenic in mice and rats.
Tara gum did not show reproductive or developmental toxicity effects and a NOAEL of 2,500 mg/kg bw per day, the highest dose tested, could be identified by the Panel.
To assess the dietary exposure to tara gum (E 417) from its use as a food additive, the exposure was calculated based on (1) maximum levels of data provided to EFSA (defined as the maximum level exposure assessment scenario) and (2) reported use levels (defined as the refined exposure assessment scenario, brand‐loyal and non‐brand‐loyal consumer scenario).
Tara gum (E 417) is authorised as a Group I food additive in a wide range of foods. The Panel did not identify a brand‐loyalty to specific food categories, and therefore, the Panel considered that the non‐brand‐loyal scenario was the more appropriate and realistic scenario for risk characterisation; thus, it was assumed that the population would probably be exposed long term to the food additive present at the mean reported use levels in processed food.
The refined estimates were based on 11 out of 67 food categories in which tara gum (E 417) is authorised. Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the real exposure to tara gum (E 417) as a food additive in European countries for the refined scenarios when considering only food additive uses for which data have been provided.
However, the Panel noted that given the information from the Mintel's GNPD, it may be assumed that tara gum (E 417) is not used in food categories in which it is authorised.
A specific exposure assessment scenario taking into account the consumption of food supplements for consumers only was also performed to estimate exposure for children, adolescents, adults and the elderly as exposure via food supplements may deviate largely from that via food, and the number of food supplement consumers may be low depending on populations and surveys.
The Panel noted that the exposure to tara gum (E 417) from its use according the Annex III (Parts 2, 3, 4 and 5A) was not considered in the exposure assessment.
The Panel also noted that the refined exposure estimates are based on information provided on the reported level of use of tara gum (E 417). If actual practice changes, this refined estimates may no longer be representative and should be updated.
Due to the discrepancies observed between the data reported from industry and the Mintel database, where tara gum are labelled in more products than in food categories for which data were reported from industry, the Panel noted that the collection of data on usage and use levels of tara gum (E 417) would allow for a more realistic exposure assessment.
Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014), and given that
the data received for the 11 food categories were adequate for a refined exposure assessment for these categories;
based on the reported use levels a refined exposure of up to 70 mg/kg bw per day in children (3–9 years) in these categories (non‐brand‐loyal scenario) was estimated;
highest refined exposure assessments on consumers only of food supplements was also calculated and ranged from 10 mg/kg bw per day for adolescents to 51 mg/kg bw per day for children;
tara gum is unlikely to be absorbed intact and is expected to be fermented by intestinal microbiota;
adequate toxicity data were available;
no adverse effects were reported in subchronic studies at the highest doses tested in rats (4,500 mg tara gum/kg bw per day), mice (10,000 mg tara gum/kg bw per day) and dogs (1,250 mg tara gum/kg bw per day);
no adverse effects were reported in chronic toxicity studies at the highest doses tested in rats (2,500 mg tara gum/kg bw per day) and mice (7,500 mg tara gum/kg bw per day);
no adverse effects were reported in reproductive and developmental toxicity studies at the highest doses tested in rats (2,500 mg tara gum/kg bw pe day);
there is no concern with respect to the genotoxicity of tara gum;
there is no concern with respect to carcinogenicity of tara gum;
the Panel concluded that there is no need for a numerical ADI for tara gum (E 417), and that there is no safety concern for the general population at the refined exposure assessment for the reported uses of tara gum (E 417) as a food additive.
The Panel recommended that the European Commission considers
revising the current limits for the toxic elements (lead, cadmium, mercury and arsenic) in the EU specification for tara gum (E 417) in order to ensure that tara gum (E 417) as a food additive will not be a significant source of exposure to those toxic elements in food.
harmonising the microbiological specifications for polysaccharidic thickening agents, such as gums, and to include criteria for the absence of Salmonella spp. and Escherichia coli, for TAMC and for TYMC into the EU specifications of tara gum (E 417).
investigating whether polycyclic aromatic hydrocarbons are generated during the roasting process and if so establishing maximum levels.
1. Introduction
The present opinion deals with the re‐evaluation of the safety of tara gum (E 417) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background as provided by the European Commission
Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union (EU). In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/20101. This Regulation also foresees that food additives are re‐evaluated whenever necessary in light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU of 2001. The report ‘Food additives in Europe 2000’ submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference as provided by the European Commission
1.1.2.1. Re‐evaluation of tara gum (E 417) as a food additive
The Commission asks EFSA to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.2. Information on existing evaluations and authorisations
Tara gum (E 417) is an authorised food additive in the EU according to Annex II and Annex III of Regulation (EC) No 1333/20082.
In the EU, tara gum was evaluated by the SCF in 1990 (SCF, 1992). The SCF was supplied with new information on digestibility of tara gum and confirmed the acceptable daily intake (ADI) ‘not specified’ allocated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) based on the use level assumptions presented at that time.
Tara gum was evaluated by JECFA in 1986 (JECFA, 1987). Based on the lack of adverse effects in the available toxicity studies, an ADI ‘not specified’ was allocated.
The Food Standards Australia New Zealand (FSANZ, 2006) approved tara gum as a new food additive. The Final Assessment Report concludes that approval of tara gum as a food additive does not raise any public health and safety concerns and is technologically justified. No other studies than those in JECFA (1987) and SCF (1992) reports were used for the assessment.
Tara gum is one of the food additives that composed jelly mini‐cups which were suspended in 2004 by the European Commission to be placed on the market and import (Commission Decision 2004/37/EC), following the measures taken and information provided by different Member States. Jelly mini‐cups are defined as ‘jelly confectionery of a firm consistence, contained in semi rigid mini‐cups or mini‐capsules, intended to be ingested in a single bite by exerting pressure on the mini‐cups or mini‐capsule to project the confectionery into the mouth’.
In 2004, the EFSA Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) prepared a scientific opinion on a request from the European Commission related to the use of certain food additives derived from seaweed or non‐seaweed origin, including tara gum (E 417) in jelly mini‐cups (EFSA AFC Panel, 2004). The AFC Panel concluded that any of these gel‐forming additives or of any other type that gave rise to a confectionery product of a similar size, with similar physical and/or physicochemical properties and that could be ingested in the same way as the jelly mini‐cups, would give rise to a risk for choking (EFSA AFC Panel, 2004). The use of these additives in jelly mini‐cups is not authorised in the EU. The use of these additives in jelly mini‐cups is not authorised in the EU.3
2. Data and methodologies
2.1. Data
The Panel on Food Additives and Nutrient Sources added to Food (ANS) was not provided with a newly submitted dossier. EFSA launched public calls for data4 , 5 to collect information from interested parties and, if relevant, contacted risk assessment bodies.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to 8 February 2017. Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based; however not always these were these available to the Panel.
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database6) was used to estimate the dietary exposure.
The Mintel's Global New Products Database (GNPD) is an online resource listing food products and compulsory ingredient information that should be included in labelling. This database was used to verify the use of tara gum (E 417) in food products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The ANS Panel assessed the safety of tara gum (E 417) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the SCF (2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
In the context of this re‐evaluation, the Panel followed the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EC) No 257/2010 (EFSA ANS Panel, 2014).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg body weight (bw) per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as equivalent. When in human studies in adults (aged above 18 years), the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012).
Dietary exposure to tara gum (E 417) from its use as a food additive was estimated combining food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum levels according to Annex II to Regulation (EC) No 1333/20087 and/or reported use levels and analytical data submitted to EFSA following a call for data. Different scenarios were used to calculate exposure (see Section 3.3.1). Uncertainties on the exposure assessment were identified and discussed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
Tara gum (E 417) has the CAS Registry No. 39300‐88‐4, EINECS No. 254‐409‐6. Tara gum is also known by the synonyms peruvian carob, Tara‐Kern‐Mehl among others.
Tara gum is a galactomannan, which is a plant reserve carbohydrate like starch, isolated from the endosperm of the seeds of the tara tree, Caesalpinia spinosa L (Ullmann, 2007).
No molecular weight is reported for tara gum in the EU legislation [Regulation (EU) 231/2012]. The molecular weight of tara gum reported in the literature, determined by capillary viscometry technique, depended on the temperature, pressure and time. It ranged between 106 and 2.33 × 106 g/mol (Picout et al., 2002).
Tara gum is a high‐viscosity polysaccharide composed mainly of a linear chain of (1‐4)‐β‐d‐mannopyranose units with α‐d‐galactopyranose units attached by (1‐6) linkages. The ratio of mannose to galactose in tara gum is 3:1. According to the literature, tara gum consists of 77–78% galactomannan, 14–15% moisture, 2.5% fibres (acid insoluble), 3–4% nitrogen compounds, 1.5% minerals (ash) and 1% fatty compounds (ether soluble) (Benk, 1977);. The composition of tara gum, when the endosperm was separated manually, was reported to be 97.2% galactomannan, 0.5% acid insoluble material and 0.2% ash (Anderson, 1949; cited from Borzelleca et al., 1993). The carbohydrate composition was determined to be 61.3–64.9% mannose and 21.4–22.3% galactose (Daas et al., 2000). The galactomannan composition, is similar to that of other gums, the ratio of mannose to galactose in tara gum (3:1) is intermediate between that of locust bean gum (about 4:1) and guar gum (about 2:1) (Merck index 2006; Benk, 1977; Tapie et al., 2008).
The major amino acids of the proteinaceous fraction of tara gum were identified to be aspartic acid (13.4%), glutamic acid (13.3%) and glycine (8.8%) (Anderson et al., 1986).
The structural formula of the carbohydrate content of tara gum is presented in Figure 1.
Figure 1.
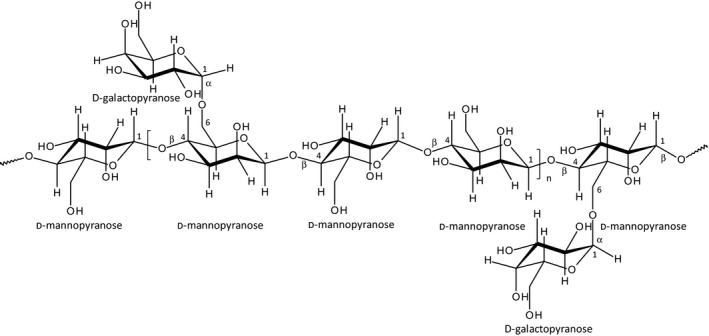
Structural formula of the carbohydrate content of tara gum.
Tara gum is a white to white‐yellow odourless powder [Regulation (EU) No 231/2012; Benk, 1977; Borzelleca et al., 1993; JECFA 2006]. Tara gum is reported to form colloidal solutions in water and to be insoluble in ethanol [Regulation (EU) No 231/2012; JECFA, 2006]. A 1% aqueous dispersion of tara gum has a viscosity of 2000–3600 centipoise (cP). Its viscosity does not change at pH values between 3.0 and 7.5 (Benk, 1977). Tara gum has synergistic gel‐strength enhancing effects when combined with carrageenan, xanthan or agar (Borzelleca et al., 1993)
3.1.2. Specifications
The specifications for tara gum (E 417) as defined in the Commission Regulation (EU) No 231/2012 and by JECFA (2006) are listed in Table 1.
Table 1.
Specifications for tara gum (E 417) according to Commission Regulation (EU) No 231/2012 and JECFA (2006)
| Commission Regulation (EU) No 231/2012 | JECFA (2006) | |
|---|---|---|
| Definition | Tara gum is obtained by grinding the endosperm of the seeds of natural strains of Caesalpinia spinosa (family Leguminosae). It consists chiefly of polysaccharides of high molecular weight composed mainly of galactomannans. The principal component consists of a linear chain of (1‐4)‐β‐d‐mannopyranose units with α‐d‐galactopyranose units attached by (1‐6) linkages. The ratio of mannose to galactose in tara gum is 3:1. (In locust bean gum, this ratio is 4:1 and in guar gum 2:1) | Obtained by grinding the endosperm of the seeds of Caesalpinia spinosa (family Leguminosae); consists chiefly of polysaccharides of high molecular weight composed mainly of galactomannans. The principal component consists of a linear chain of (1,4)‐beta‐d‐mannopyranose units with alpha‐d‐galactopyranose units attached by (1‐6) linkages; the ratio of mannose to galactose in tara gum is 3:1. (In carob bean gum, this ratio is 4:1 and in guar gum 2:1.) The article of commerce may be further specified as to viscosity and loss on drying |
| Description | A white to white‐yellow odourless powder | White to white‐yellow, nearly odourless powder |
| Functional uses | Thickening agent, stabiliser | |
| Identification | ||
| Solubility | Soluble in water, insoluble in ethanol | Soluble in water; insoluble in ethanol |
| Gel formation | To an aqueous solution of the sample add small amounts of sodium borate. A gel is formed | To an aqueous solution of the sample add small amounts of sodium borate. A gel is formed |
| Viscosity | – | Transfer 2 g of the sample into a 400‐mL beaker and moisten it thoroughly with about 4 mL of isopropanol. Add, with vigorous stirring, 200 mL of water and continue stirring until the gum is completely and uniformly dispersed. An opalescent, moderately viscous solution is formed. (This solution is less viscous than a tara gum solution, but more viscous than a carob bean gum solution when prepared and tested as indicated in the above described test.) Transfer 100 mL of this solution into another 400‐mL beaker, heat the mixture in a boiling water‐bath for about 10 min and cool to room temperature. The solution shows a marked increase in viscosity |
| Gum constituents | – | Proceed as directed under Gum Constituents Identification, using galactose and mannose as standards. Galactose and mannose should be present |
| Microscopic examination | – | Place some ground sample in an aqueous solution containing 0.5% iodine and 1% potassium iodide on a glass slide and examine under a microscope. Tara gum contains groups of round to pear‐shaped cells; their contents are yellow to brown.(Tara gum cells are similar in form but markedly larger in size. Carob bean gum shows long, stretched tubiform cells, separate or slightly interspaced and can be easily distinguished from tara gum.) |
| Purity | ||
| Loss on drying | Not more than 15% | Not more than 15% |
| Ash | Not more than 1.5% | Not more than 1.5% |
| Acid insoluble matter | Not more than 2% | Not more than 2% |
| Protein | Not more than 3.5% (factor N × 5.7) | Not more than 3.5%. Proceed as directed under Nitrogen Determination (Kjeldahl method) (see Volume 4). The percentage of nitrogen determined multiplied by 5.7 gives the percentage of protein in the sample |
| Starch | Not detectable | Not detectable |
| Arsenic | Not more than 3 mg/kg | – |
| Lead | Not more than 2 mg/kg | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg | – |
| Cadmium | Not more than 1 mg/kg | – |
Regarding possible allergies by proteins, the Panel noted that according to Borzelleca et al. (1993) and the current literature research no reports on the allergenicity of tara gum were found.
According to the information from the interested party (Documentation provided to EFSA n. 3), protein content (N × 6.25) is below 3.5%. The proteins in the final product originate from the germ which is removed during the processing of tara seeds. INEC proposes using a conversion factor of 6.25 instead of 5.7 for the protein content calculation in the EC specifications. The Panel was not provided with analytical data on the content of nitrogen in tara germ proteins and therefore could not conclude to use a different conversion factor for protein calculation in the EC specifications for tara gum (E 417).
The Panel noted that, according to the Annex 1 of the Regulation (EU) 1169/2011, for the purpose of food labelling the content of protein is calculated using the formula: protein = total Kjeldahl nitrogen × 6.25, where the conversion factor 6.25 corresponds to the average content of around 16% of nitrogen in proteins. This use of a single factor 6.25 is confounded by two considerations. First, not all nitrogen in foods is found in proteins, and second, the nitrogen content of specific amino acids varies according to the molecular weight of the amino acid and the number of nitrogen atoms it contains. Based on these facts, and the different amino acid compositions of various proteins, the nitrogen content of proteins actually varies from around 13% to 19% which would equate to nitrogen conversion factors (also known as Jones factors) ranging from 5.26 to 7.69 (FAO, 2003). In response to these considerations, for more accurate calculations for specific food proteins, more specific conversion factors are used.
Some residual enzymatic activity (amylase, galactosidase, mannosidase) may be present in tara gum (Documentation provided to EFSA n.3), depending on the manufacturing process. The Panel noted that specifications on the enzymatic activity are not included in the tara gum specifications from the EU and JECFA.
Because of both the botanical origin and the polysaccharidic nature of gums, they can be a substrate of microbiological contamination and of field and storage fungal development. The latter has been recently demonstrated by the mycotoxin contaminations of gums (Zhang et al., 2014). The Panel noted that, different from other gums, no microbiological criteria were defined for tara gum by the EU Regulation. The Panel also noted that the microbiological specifications for polysaccharidic thickening agents, such as gums, should be harmonised and that for tara gum criteria for the absence of Salmonella spp. and Escherichia coli, for total aerobic microbial count (TAMC) and for total combined yeasts moulds count (TYMC) should be included into the EU specifications, as it is the case for other polysaccharidic thickening agents [e.g. alginic acids and its salts (E 400–E 404), agar (E 406), carrageenan (E 407), processed eucheuma sea weed (E 407a), xanthan gum (E 415), gellan gum (E 418)].
No information on the particle size distribution of tara gum (E 417) has been provided.
In view of the botanical origin of tara gum, limitations of possible contamination with pesticides should be considered.
The Panel noted that, according to the EU specifications for tara gum (E 417), impurities of the toxic elements lead, mercury, cadmium and arsenic are accepted up concentrations of 2, 1, 1, and 3 mg/kg, respectively. Contamination at those levels could have a significant impact on the exposure to these metals, for which the intake is already close to the health‐based guidance values or benchmark doses (lower confidence limits) established by the EFSA (EFSA CONTAM Panel, 2009a,b, 2010, 2012a,b,c, 2014). No information on the levels of heavy metals on batches of tara gum has been provided.
3.1.3. Manufacturing process
Tara gum is extracted from seeds of the tara tree (C. spinosa L.), which grows mainly in Peru.
‘For removal of the hulls, two different processes exist. In the acid process the hulls are carbonised by treating the kernels with moderately dilute sulfuric acid at elevated temperature. Similar to the guar gum process, the remaining hull fragments are then removed by washing and brushing operations. After a drying step the dehulled kernels are cracked and the germs sifted off from the endosperm. Commercial products are obtained by milling and screening. In the vanishing roasting process the kernels are roasted in a rotating furnace, where the hulls pop. The endosperm is then obtained as described above’. This process in which the use of sulfuric acid is avoided yields products of somewhat darker colour than those from the acid process (Ullmann, 2007). The Panel noted that this roasting process could generate polycyclic aromatic hydrocarbons. The Panel noted that limits for polycyclic aromatic hydrocarbons are not addressed in the specifications.
Separation of the endosperm of tara seeds can also be done manually. The endosperm obtained under these conditions can be up to 27% of the total weight of the seed (Anderson, 1949; cited from Borzelleca et al., 1993). Depending on the manufacturing process, some residual enzymatic activity (amylase, galactosidase, mannosidase) may be present in tara gum. The Panel noted that there are no specifications for the enzymatic activity. The article of commerce may be further specified as to viscosity and loss on drying (Documentation provided to EFSA n.3).
3.1.4. Methods of analysis in food
No specific methods of analysis were identified in the literature for the analysis of tara gum in food.
General methods for the qualitative test of gums in mayonnaise and French dressing the AOAC Official Method 937.12 are reported by the Association of Official Agricultural Chemists (AOAC, 2002). The gums are precipitated from the food sample, hydrolysed to monosaccharides which are qualitatively identified. This method is not applicable in the presence of starch. A similar method (AOAC Official Method 935.61) for qualitative determination of gums in salad dressing based on a precipitation reaction is applicable in presence of starch (AOAC, 2002). Both methods are usable for determination of the sum of different gums used in foodstuff.
Methods for quantitative measurements of monosaccharides after hydrolysis of the polymers are described by Scherz and Mergentaler (1980). The monosaccharides are analysed using thin‐layer chromatography (TLC) or, after derivatisation, by gas chromatography. An anion‐exchange liquid chromatography method coupled to pulse amperometric detection is described to determine quantitatively complex carbohydrates mixtures (neutral saccharides, aminosacharides, glucuronic acids and disaccharides) in nutritional products (Eberendu et al. 2005).
A Fourier transform infrared spectroscopy (FTIR) method was developed for quality control of selected gums and gum mixtures used in the food industry (Prado et al., 2005). This method allows differentiation of tara, locust bean, tara and fenugreek gums. Quantification of individual gums in gum mixtures (0.5–15%, w/w) was possible.
A method establishing protein profiles of several gums including tara gum, by capillary electrophoresis was applied to predict the content of tara gum in carob (locust) bean‐tara gum mixture in samples (Ramis‐Ramos et al., 2003).
Identification of the gum constituents by thin layer chromatography is described in JECFA (2006).
A reverse phase high‐performance liquid chromatography (HPLC)/ultraviolet (UV) detection method was described for quantitative measurements of oligosides, mannose and galactose obtained by combined acid hydrolysis and enzymatic degradation of galactomannans (Tapie et al., 2008).
3.1.5. Stability of the substance, and reaction and fate in food
No specific information on reaction and fate of tara gum in food was identified.
Galactomannans are rapidly degraded in acidic aqueous solutions at elevated temperatures, resulting in a rapid loss of viscosity. They can also be degraded by oxidants and by microbial enzymes (mannanases). Galactomannans are rather stable under alkaline conditions (Ullmann, 2007). When heated to decomposition acrid smoke and irritating fumes are emitted (Sax, 1984).
3.2. Authorised uses and use levels
Maximum levels of tara gum (E 417) have been defined in Annex II to Regulation (EC) No 1333/2008 on food additives, as amended. In this document, these levels are named maximum permitted levels (MPLs).
Currently, tara gum (E 417) is an authorised food additive in the EU at quantum satis (QS) in all food categories listed in Table 2. Tara gum (E 417) is included in the Group I of food additives authorised at QS.
Table 2.
MPLs of tara gum (E 417) in foods according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | E‐number/Group | Restrictions/exception | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 01.3 | Unflavoured fermented milk products, heat‐treated after fermentation | Group I | QS | |
| 01.4 | Flavoured fermented milk products including heat treated products | Group I | QS | |
| 01.6.3 | Other creams | Group I | QS | |
| 01.7.1 | Unripened cheese excluding products falling in category 16 | Group I | Except mozzarella | QS |
| 01.7.5 | Processed cheese | Group I | QS | |
| 01.7.6 | Cheese products (excluding products falling in category 16) | Group I | QS | |
| 01.8 | Dairy analogues, including beverage whiteners | Group I | QS | |
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | Group I | QS | |
| 02.3 | Vegetable oil pan spray | Group I | QS | |
| 03 | Edible ices | Group I | QS | |
| 04.2.1 | Dried fruit and vegetables | Group I | E 417 may not be used to produce dehydrated foods intended to rehydrate on ingestion | QS |
| 04.2.2 | Fruit and vegetables in vinegar, oil, or brine | Group I | QS | |
| 04.2.4.1 | Fruit and vegetable preparations excluding compote | Group I | QS | |
| 04.2.5.4 | Nut butters and nut spreads | Group I | QS | |
| 04.2.6 | Processed potato products | Group I | QS | |
| 05.1 | Cocoa and Chocolate products as covered by Directive 2000/36/EC | Group I | Only energy‐reduced or with no added sugar | QS |
| 05.2 | Other confectionery including breath refreshening microsweets | Group I | E 417 may not be used in jelly mini‐cups, defined, for the purpose of this Regulation, as jelly confectionery of a firm consistence, contained in semi rigid mini‐cups or mini‐capsules, intended to be ingested in a single bite by exerting pressure on the mini‐cups or mini‐capsule to project the confectionery into the mouth; E 417 may not be used to produce dehydrated foods intended to rehydrate on ingestion | QS |
| 05.3 | Chewing gum | Group I | QS | |
| 05.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | Group I | QS | |
| 06.2.2 | Starches | Group I | QS | |
| 06.3 | Breakfast cereals | Group I | QS | |
| 06.4.2 | Dry pasta | Group I | Only gluten free and/or pasta intended for hypoproteic diets in accordance with Directive 2009/39/EC | QS |
| 06.4.4 | Potato Gnocchi | Group I | Except fresh refrigerated potato gnocchi | QS |
| 06.4.5 | Fillings of stuffed pasta (ravioli and similar) | Group I | QS | |
| 06.5 | Noodles | Group I | QS | |
| 06.6 | Batters | Group I | QS | |
| 06.7 | Pre‐cooked or processed cereals | Group I | QS | |
| 07.1 | Bread and rolls | Group I | Except products in 7.1.1 and 7.1.2 | QS |
| 07.2 | Fine bakery wares | Group I | QS | |
| 08.3.1 | Non‐heat‐treated meat products | Group I | QS | |
| 08.3.2 | Heat‐treated meat products | Group I | Except foie gras, foie gras entier, blocs de foie gras, Libamáj, libamáj egészben, libamáj tömbben | QS |
| 08.3.3 | Casings and coatings and decorations for meat | Group I | QS | |
| 09.2 | Processed fish and fishery products including molluscs and crustaceans | Group I | QS | |
| 09.3 | Fish roe | Group I | Only processed fish roe | QS |
| 10.2 | Processed eggs and egg products | Group I | QS | |
| 11.2 | Other sugars and syrups | Group I | QS | |
| 12.1.2 | Salt substitutes | Group I | QS | |
| 12.2.2 | Seasonings and condiments | Group I | QS | |
| 12.3 | Vinegars | Group I | QS | |
| 12.4 | Mustard | Group I | QS | |
| 12.5 | Soups and broths | Group I | QS | |
| 12.6 | Sauces | Group I | QS | |
| 12.7 | Salads and savoury‐based sandwich spreads | Group I | QS | |
| 12.8 | Yeast and yeast products | Group I | QS | |
| 12.9 | Protein products, excluding products covered in category 1.8 | Group I | QS | |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | Group I | QS | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | Group I | QS | |
| 13.4 | Foods suitable for people intolerant to gluten as defined by Regulation (EC) No 41/2009 | Group I | Including dry pasta | QS |
| 14.1.2 | Fruit juices as defined by Directive 2001/112/EC and vegetable juices | Group I | Only vegetable juices | QS |
| 14.1.3 | Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products | Group I | Only vegetable nectars | QS |
| 14.1.4 | Flavoured drinks | Group I | QS | |
| 14.1.5.2 | Other | Group I | Excluding unflavoured leaf tea; including flavoured instant coffee | QS |
| 14.2.3 | Cider and perry | Group I | QS | |
| 14.2.4 | Fruit wine and made wine | Group I | QS | |
| 14.2.5 | Mead | Group I | QS | |
| 14.2.6 | Spirit drinks as defined in Regulation (EC) No 110/2008 | Group I | Except whisky or whiskey | QS |
| 14.2.7.1 | Aromatised wines | Group I | QS | |
| 14.2.7.2 | Aromatised wine‐based drinks | Group I | QS | |
| 14.2.7.3 | Aromatised wine‐product cocktails | Group I | QS | |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol | Group I | QS | |
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | Group I | QS | |
| 15.2 | Processed nuts | Group I | QS | |
| 16 | Desserts excluding products covered in category 1, 3 and 4 | Group I | QS | |
| 17.1a | Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms | Group I | E 417 may not be used to produce dehydrated foods intended to rehydrate on ingestion | QS |
| 17.2a | Food supplements supplied in a liquid form | Group I | QS | |
| 17.3a | Food supplements supplied in a syrup‐type or chewable form | Group I | QS | |
| 18 | Processed foods not covered by categories 1–17, excluding foods for infants and young children | Group I | QS |
MPL: maximum permitted level.
FCS 17 refers to food supplements as defined in Directive 2002/46/EC of the European Parliament and of the Council excluding food supplements for infants and young children
Table 2 summarises foods that are permitted to contain tara gum (E 417) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008.
According to Annex III, Part 2 of Regulation (EC) No 1333/2008, tara gum (E 417) is authorised as a food additive other than carriers in all food additive preparations at QS.
According to Annex III, Part 3 of Regulation (EC) No 1333/2008, tara gum (E 417) is also authorised as a food additive in food enzymes with a maximum level in the products (beverages or not) at QS.
In addition, according to Annex III, Part 4 of Regulation (EC) No 1333/2008, tara gum (E 417) is authorised as a food additive including carriers in all food flavourings at QS.
Finally, according to Annex III, Part 5, Section A of Regulation (EC) No 1333/2008, tara gum (E 417) is also authorised as a food additive in all nutrients except nutrients intended to be used in foodstuffs for infants and young children listed in point 13.1 of Part E of Annex II.
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of tara gum (E 417)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels was required for performing a more realistic exposure assessment, especially for those food additives for which no MPL is set and which were authorised according to QS.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued public calls8 , 9 for occurrence data (usage level and/or concentration data) on tara gum (E 417). In response to this public call, updated information on the actual use levels of tara gum (E 417) in foods was made available to EFSA by industry. No analytical data on the concentration of tara gum (E 417) in foods were made available by the Member States.
3.3.1.1. Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 28) of tara gum (E 417) in foods for 15 out of the 67 food categories in which tara gum (E 417) is authorised.
Updated information on the actual use levels of tara gum (E 417) in foods was made available to EFSA by FoodDrinkEurope (FDE), BABBI Confectionary Industry, EUROGUM A/S, EMCESA Fabricante Embutidos del centro SA.
The Panel noted that EUROGUM A/S is not a food industry using gums in its food products but food additive producer. Usage levels reported by food additive producers were not considered at the same level as those provided by food industry. Food additive producers might recommend usage levels to the food industry but the final levels might, ultimately, be different. Therefore, unless food additive producers confirmed that the recommended levels are used by food industry, they were not considered in the refined exposure scenario. Data from food additive producers will only be used in the maximum level exposure assessment scenario in case of QS authorisation when no data are available from food industry. In this way, the most complete exposure estimates are calculated.
In the case of EUROGUM A/S, all submitted data were theoretical amounts suggested or recommended; they were ‘based on [their] own technical know‐how regarding adequate/recommended levels of use in different food applications’.
Appendix A provides data on the use levels of tara gum (E 417) in foods as reported by industry (food industry and food additive producer).
3.3.2. Summarised data extracted from the Mintel GNPD database
The Mintel's GNPD is an online database which monitors product introductions in consumer packaged goods markets worldwide. It contains information of over 2 million food and beverage products of which more than 900,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the GNPD.10
For the purpose of this Scientific Opinion, GNPD11 was used for checking the labelling of products containing tara gum (E 417) within the EU's food products as GNPD shows the compulsory ingredient information presented in the labelling of products.
According to Mintel, tara gum (E 417) is labelled on less than 1,000 products since 1996. Between 2012 and 2017, tara gum (E 417) is labelled mainly on chilled desserts [i.e. dairy‐based desserts, chilled custard, fruit compote, mousse, panna cotta and pudding (prepared)] and dairy‐based frozen products (i.e. ice‐cream).
Appendix B presents the percentage of the food products labelled with tara gum (E 417) between 2012 and 2017, out of the total number of food products per food subcategories according to Mintel food classification.
3.3.3. Food consumption data used for exposure assessment
3.3.3.1. EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country [cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a)]. New consumption surveys recently12 added in the Comprehensive database were also taken into account in this assessment.6
The food consumption data gathered by EFSA were collected by different methodologies, and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database represents the best available source of food consumption data across Europe at present.
Food consumption data from the following population groups: infants, toddlers, children, adolescents, adults and the elderly were used for the exposure assessment. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 3).
Table 3.
Population groups considered for the exposure estimates of tara gum (E 417)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlers | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrena | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlya | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Sweden, UK |
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, FoodEx food codes were matched to the FCS food categories.
3.3.3.2. Food categories selected for the exposure assessment of tara gum (E 417)
The food categories in which the use of tara gum (E 417) is authorised were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Some food categories are not referenced in the EFSA Comprehensive Database and could therefore not be taken into account in the present estimate. This was the case for 10 food categories and may result in an underestimation of the exposure. The food categories which were not taken into account are described below (in ascending order of the FCS codes):
01.7.6 Cheese products (excluding products falling in category 16);
02.3 Vegetable oil pan spray;
06.6 Batters;
06.7 Pre‐cooked or processed cereals;
08.3.3 Casings and coatings and decorations for meat;
12.1.2 Salt substitutes;
14.1.3 Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products, only vegetable nectars;
14.2.5 Mead;
14.2.7.2 Aromatised wine‐based drinks;
14.2.7.3 Aromatised wine‐product cocktails.
For the following food categories, the restrictions/exceptions which apply to the use of tara gum (E 417) could not be taken into account, and therefore the whole food category was considered in the exposure assessment. This may result in an overestimation of the exposure:
07.1 Bread and rolls, except products in 7.1.1 and 7.1.2;
08.3.2 Heat‐treated processed meat, except foie gras, foie gras entier, blocs de foie gras, Libamáj, libamáj egészben, libamáj tömbben;
In addition, for the following three food categories, FC 17.1, FC 17.2 and FC 17.3 Food supplements, in solid, liquid and syrup‐type or chewable form, which were used only in the specific exposure scenario including food supplements, the restrictions which apply to the use of tara gum (E 417) could not be taken into account, and therefore, the whole food categories were considered in the exposure assessment (use reported for food category 17 were used for the three FC 17.1, 17.2 and 17.3).
Consumption records belonging to food categories 13.2, 13.3, 13.4 and 18 were treated differently and recoded.
Indeed considering that the food category 18 (Processed foods not covered by categories 1–17, excluding foods for infants and young children) is extremely unspecific (e.g. composite foods), processed foods, prepared or composite dishes belonging to the food category 18 were reclassified under food categories in accordance to their main component. Therefore, food category 18 is not taken into account as contributor to the total exposure estimates.
Food categories 13.2, 13.3 and 13.4 are food for special medical purposes (FSMP) consumed in population groups of children, adolescents, adults and the elderly. They may be very diverse and cannot be considered because of very limited information on consumption. Eating occasions belonging to the food categories 13.2, 13.3 and 13.4 were therefore reclassified under food categories in accordance to their main component.
As mentioned in Section 3.3.1 above, reported use levels were made available to EFSA for few food categories. Thus, 39 additional food categories were not taken into account because no concentration data were provided to EFSA. For the remaining food categories, the refinements considering the restrictions/exceptions as set in Annex II to Regulation No 1333/2008 were applied. Overall, for all exposure scenarios, only 14 food categories were included in the present exposure assessment to tara gum (E 417) (Appendix C). Use levels reported for the food categories 13.2, 13.3 and 18 were not included.
3.4. Exposure estimate
3.4.1. Exposure to tara gum (E 417) from its use as a food additive
The Panel estimated chronic exposure to tara gum (E 417) for the following population groups: infants; toddlers, children, adolescents, adults and the elderly. Dietary exposure to tara gum (E 417) was calculated by multiplying tara gum (E 417) concentrations for each food category (Appendix C) with their respective consumption amount per kilogram of body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only 1 day per subject were excluded as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 3). On the basis of these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. 95th percentile of exposure was only calculated for those population groups where the sample size was sufficiently large to allow this calculation (EFSA, 2011a). Therefore, in the present assessment, 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not included.
Exposure assessment to tara gum (E 417) was carried out by the ANS Panel based on two different sets of concentration data (1) maximum levels of data provided to EFSA (defined as the maximum level exposure assessment scenario) and (2) reported use levels (defined as the refined exposure assessment scenario) as provided by industry. These two scenarios are discussed in detail below and can consider only food categories for which the reported use levels were available to the Panel.
These scenarios do not consider the consumption of food supplements (FC 17.1, FC 17.2 and FC 17.3) which was covered in an additional scenario detailed below (food supplements consumers only scenario).
A possible additional exposure from the use of tara gum (E 417) as a food additive in food enzymes and nutrients in accordance with Annex III to Regulation (EC) No 1333/2008 (Part 3 and 5) was not considered in any of the exposure assessment scenarios.
3.4.1.1. Maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008 and listed in Table 2. As tara gum (E 417) is authorised according to QS in all food categories, a ‘maximum level exposure assessment’ scenario was estimated based on the maximum reported use levels provided by industry (food industry and food additive producers), excluding exposure via food supplements, as described in the EFSA Conceptual framework (EFSA ANS Panel, 2014). This exposure scenario could consider only food categories for which the above data were available to the Panel (11/67 categories).
The Panel considered the exposure estimates derived following this scenario as the most conservative since it was assumed that the population will be exposed to tara gum (E 417) present in food at maximum reported levels over a longer period of time.
3.4.1.2. Refined exposure assessment scenario
The refined exposure assessment scenario was based on use levels reported by food industry. This exposure scenario could consider only food categories for which the above data were available to the Panel (11/67 categories).
Appendix C summarises the concentration levels of tara gum (E 417) used in the refined exposure assessment scenario. Based on the available data set, the Panel calculated two refined exposure estimates based on different model populations excluding exposure via food supplements.
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to tara gum (E 417) present at the maximum reported use level for one food category. This exposure estimate was calculated as follows:
-
1
— Combining food consumption with the maximum of the reported use levels for the main contributing food category at the individual level.
-
2
— Using the mean of the typical reported use levels for the remaining food categories.
-
1
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to tara gum (E 417) present at the mean reported use levels in food. This exposure estimate is calculated using the mean of the typical reported use levels for all food categories.
Appendix C summarised the concentration levels of tara gum (E 417) used in the refined exposure scenarios.
3.4.1.3. Specific scenario: Consumers of food supplements
One additional scenario based on consumers only of food supplements (food supplements consumers only scenario) was also calculated. Exposure via food supplements was addressed in an additional exposure scenario because the exposure via this source may deviate largely from the exposure via food and the number of food supplement consumers may be low. Due to these two factors, the potentially higher exposure to tara gum (E 417) in food supplement users may not become evident in a whole population approach.
This scenario was estimated as follows:
Consumers only of food supplements (three food categories: 17.1, 17.2 and 17.3) were assumed to be exposed to tara gum (E 417) present at the maximum reported use level on a daily basis via consumption of food supplements.
For the remaining food categories (11/67 categories), the mean of the typical reported use levels was used.
As food category 17 does not include food supplements for infants and toddlers [Regulation (EC) No 1333/2008], exposure to tara gum (E 417) from food supplements was not estimated for these two population groups.
3.4.1.4. Dietary exposure to tara gum (E 417)
Table 4 summarised the estimated exposure to tara gum (E 417) from their use as a food additive in six population groups (Table 3) according to the different exposure scenarios (Section 3.4.1). Detailed results per population group and survey were presented in Appendix E.
Table 4.
Summary of dietary exposure to tara gum (E 417) from its use as a food additive in the maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Maximum level exposure assessment scenario | ||||||
|
3.5–42.8 11.5–109.6 |
8.0–131.1 20.9–160.4 |
16.2–97.3 34.7–173.9 |
7.7–60.5 19.2–106.2 |
21.6–38.4 46.0–74.8 |
21.3–33.6 36.5–65.5 |
| Refined estimated exposure assessment scenario | ||||||
| Brand‐loyal scenario | ||||||
|
3.1–41.5 10.3–106.9 |
6.7–110.2 16.8–142.3 |
12.6–81.6 27.8–162.3 |
6.0–47.8 16.3–94.0 |
19.7–32.7 39.7–70.3 |
18.3–31.4 33.6–63.7 |
| Non‐brand‐loyal scenario | ||||||
|
1.4–14.5 5.4–38.2 |
4.3–54.7 12.6–58.2 |
8.6–40.6 18.2–69.9 |
4.0–22.7 10.2–39.9 |
6.6–14.4 13.9–27.8 |
6.3–11.8 11.9–22.6 |
In the maximum level exposure assessment scenario, mean exposure to tara gum (E 417) from its use as a food additive ranged from 3 mg/kg bw per day in infants to 131 mg/kg bw per day in toddlers. The 95th percentile of exposure to tara gum (E 417) ranged from 12 mg/kg bw per day in infants to 174 mg/kg bw per day in children.
In the refined estimated exposure scenario, in the brand‐loyal scenario, mean exposure to tara gum (E 417) from its use as a food additive ranged from 3 mg/kg bw per day in infants to 110 mg/kg bw per day in toddlers. The 95th percentile of exposure to tara gum (E 417) ranged from 10 mg/kg bw per day in infants to 162 mg/kg bw per day in children. In the non‐brand‐loyal scenario, mean exposure to tara gum (E 417) from its use as a food additive ranged from 1 mg/kg bw per day in infants to 55 mg/kg bw per day in toddlers. The 95th percentile of exposure tara gum (E 417) ranged from 5 mg/kg bw per day in infants to 70 mg/kg bw per day in children.
In the food supplements consumers only exposure scenario, mean exposure to tara gum (E 417) from its use as a food additive ranged between 4 mg/kg bw per day for adolescents and 45 mg/kg bw per day for children. The 95th percentile of exposure to tara gum (E 417) ranged between 10 mg/kg bw per day for adolescents and 51 mg/kg bw per day for children.
3.4.1.5. Main food categories contributing to exposure to tara gum (E 417) using the maximum level exposure assessment scenario
In the maximum level exposure assessment scenario, the main contributing food category to the total mean exposure estimates were bread and rolls for all population groups.
The main food categories contributing to the exposure to tara gum (E 417) were presented in Appendix D.
3.4.1.6. Main food categories contributing to exposure to tara gum (E 417) using the refined exposure assessment scenario
The main contributing food categories in the refined estimated exposure scenario, in the brand‐loyal scenario were bread and rolls for all population groups. In the non‐brand‐loyal scenario, the main contributing food categories were bread and rolls and meat products for infants, toddlers and the elderly, and bread and rolls, meat products and flavoured drinks for children, adolescents and adults.
The main food categories contributing to the exposure to tara gum (E 417) were presented in Appendix D.
All appendixes can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4863
3.4.1.7. Uncertainty analysis
Uncertainties in the exposure assessment of tara gum (E 417) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 5.
Table 5.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/− |
| Use of data from food consumption survey of a few days to estimate long‐term (chronic) exposure for high percentiles (95th percentile) | + |
| Correspondence of reported use levels and analytical data to the food items in the EFSA Comprehensive Food Consumption Database: uncertainties to which types of food the levels refer to | +/− |
| Uncertainty in possible national differences in use levels of food categories | +/– |
Concentration data:
|
+ +/− + |
| Food categories selected for the exposure assessment: exclusion of food categories due to missing FoodEx linkage (n = 10/67 food categories) | − |
| Food categories selected for the exposure assessment: inclusion of food categories without considering the restriction/exception (n = 2/67 food categories) | + |
| Food categories included in the exposure assessment: no data available for authorised food categories (n = 39/67 food categories) | − |
| The 14 food categories which were taken into account in the refined exposure assessment scenarios out of all authorised foods (n = 67), corresponded to only 11–72% of the amount (g of foods by body weight) of food consumption documented in the EFSA Consumption Database. | − |
Maximum level exposure assessment scenario:
|
+ − |
Refined exposure assessment scenarios:
|
+/− − |
+, uncertainty with potential to cause over‐estimation of exposure; −, uncertainty with potential to cause underestimation of exposure.
Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the real exposure to tara gum (E 417) as a food additive in European countries considered in the EFSA European database for the maximum level exposure scenario and the refined scenario, assuming that the food additive is not used in the food categories for which no use levels were reported.
This assumption of non‐use was supported by the observation that tara gum (E 417) is only authorised in food categories as a Group I food additive (Table 2). Thus tara gum (E 417) may not necessarily be used in some of these food categories, and it may explain why reported use levels of tara gum (E 417) were available only for few food categories. The Panel noted that the information from the Mintel GNPD supported the observation that due to its Group I authorisation, tara gum (E 417) may not be used in all food categories in which it is authorised (Section 3.3.2). For instance, concerning beverages, very few flavoured drinks and soups and broths were reported in Mintel GNPD as labelled with tara gum (E 417).
The main food categories not taken into account were cheeses, breakfast cereals, fine bakery wares, alcoholic beverages (e.g. cider and perry, spirit drinks) and snacks because no data from industry were provided. Among them, different information was obtained from the Mintel's GNPD indicating that a small number of products (e.g. snacks, cheeses or fine bakery wares) do contain tara gum and therefore this will induce a certain level of underestimation of exposure assessments. For breakfast cereals and alcoholic beverages (e.g. cider and perry, spirit drinks), the Mintel GNPD confirms that there is no use of tara gum (E 417) and therefore no underestimation is expected from this.
Regarding food supplements consumers only scenario, the Panel considered that the uncertainties would result in an overestimation of the exposure to tara gum (E 417) as a food additive, given that the calculations were based on consumers only of food supplements using maximum reported use levels and assuming a long‐term brand loyalty consumption of these food products on a daily basis.
In none of the exposure scenarios, the use of tara gum (E 417) according to Annex III to Regulation No 1333/2008 was considered. Neglecting this source of exposure may have resulted in an underestimation of exposure to tara gum (E 417) in all scenarios.
3.5. Biological and Toxicological data
3.5.1. Absorption, distribution, metabolism and excretion
There is evidence that certain high‐molecular‐weight dietary polysaccharides, such as gums, could be partially broken down in the large intestine of man. In addition to intermediate metabolites such as lactate, acrylate or fumarate, the main end products of this colonic anaerobic digestive process are short‐chain fatty acids (SCFA) such as acetic, propionic and butyric acids, which are absorbed from the colon (Cummings and Englyst, 1987).
3.5.1.1. In vitro studies
Solutions and suspensions of tara gum were incubated with human gastric juice, duodenal juice plus bile, pancreatic juice or intestinal juice with or without added rabbit small intestine membrane enzymes. No evidence of hydrolysis was found (Semenza, 1975; as reported in JECFA, 1987).
Rat large intestine microbiota partially hydrolysed tara gum in vitro after conditioning the animals to 1% tara gum in the diet for 3 weeks (Towle and Schranz, 1975; as reported in JECFA, 1987).
3.5.1.2. In vivo studies
In a bioavailability calorie assay, groups of 10 male weanling Sprague–Dawley rats were given 5 g of tara gum for 10 days. Weight‐gain comparisons showed that tara gum was not a source of bioavailable calories (Robaislek, 1974, as reported in JECFA, 1987).
Five male and five female Purdue rats on a mannose‐free diet were fed 1% tara gum in diet for 18 hours in order to test the digestibility of the test substance. Eighty‐eight to 100% of the mannose from tara gum was excreted in the faeces over a total of 30 h. The report states that some decrease observed in the chain length of galactomannan may have occurred through the action of intestinal microbiota of the rats, since mammals are not known to possess mannosidases. Liberation of galactose was not determined (Tsai and Whistler, 1975; as reported in JECFA, 1987).
The galactomannans of tara gum were nearly completely digested by Wistar rats, when tara gum was fed at a dietary level of 2% or 5%. Diets were given for 21 days and stool samples of treated rats were analysed for galactose and mannose. In this study, digestibility has been defined as ‘the part of the ingested material, which was not excreted in the stool’. Digestibility of galactomannan was 97% in the 2% group and 98% in the 5% group (Barry et al., 1990; as reported in Borzelleca et al., 1993).
Overall, data on in vitro degradation by human gastrointestinal fluids and on in vivo digestibility of tara gum in animals demonstrated that this compound would not be absorbed intact or hydrolysed by digestive enzymes. However, there is evidence that certain high‐molecular‐weight dietary polysaccharides, such as gums, could be partially broken down in the large intestine of man. In addition to intermediate metabolites such as lactate, acrylate or fumarate, the main end products of this colonic anaerobic digestive process are SCFAs, such as acetic, propionic and butyric acids, which are absorbed from the colon (Cummings and Englyst, 1987). Therefore, it is expected that tara gum would be fermented with production of SCFAs such as acetic, propionic and butyric acids, during its passage through the large intestine by strains of bacteria found in the human colon. Based on the available knowledge on the role of SCFA as end products of the fermentation of dietary fibres by the anaerobic intestinal microbiota (den Besten et al., 2014; Topping and Clifton, 2001), the Panel considered that their potential formation as fermentation products from tara gum does not raise any concern. Despite the absence of convincing in vivo study in humans, the Panel considered that these data indicate that tara gum, like other galactomannans (e.g. guar gum, locust bean gum) would most probably not be absorbed intact but could be fermented by intestinal microbiota in humans.
3.5.2. Acute toxicity
In single‐dose toxicity test with tara gum to be used in subchronic studies groups of five male and five female F344 rats and B6C3F1 mice were given a single oral dose of 630 mg/kg bw tara gum in distilled water by gavage. All animals were sacrificed on day 15. In both species, no compound‐related toxic effects were observed (NTP, 1982).
3.5.3. Short‐term and subchronic toxicity
3.5.3.1.
Mice
Groups of five male and five female B6C3F1 mice were fed diets containing 0, 6,300, 12,500, 25,000, 50,000 and 100,000 mg tara gum/kg diet (equivalent to 0, 1,260, 2,500, 5,000, 10,000 and 20,000 mg/kg bw per day) for 2 weeks (NTP, 1982). On day 15, animals were fed control diets and necropsied on day 16. No deaths were reported during the study. Male mice loss body weight (more than 20%) at all doses tested compared to control animals, not gaining any weight when fed diets containing 100,000 mg tara gum/kg diet. On the contrary, female mice gained body weight at all doses excepted at the dose of 10,000 mg/kg bw per day. No other compound‐related effects were observed during clinical observation or at necropsy (NTP, 1982)
Groups of B6C3F1 mice fed diets containing 0, 3,100, 6,300, 12,500, 25,000 and 50,000 mg tara gum/kg diet (equivalent to 0, 620, 1,260, 2,500, 5,000 and 10,000 mg/kg bw per day) for 13 weeks (NTP, 1982). Ten animals of each sex per dose were used and separate control groups of 10 animals for each sex were included. The investigations included clinical signs, body weights, feed consumption and histopathology of all major organs were conducted on all animals from control and highest dose groups. Haematology, clinical chemistry and urine were not investigated. Mortality was checked twice daily and animals were weighed weekly. At day 91, all animals were sacrificed and subjected to a gross examination and various tissues were examined microscopically of all controls and animals of the highest dose group. Contrary to the 2‐week study, no notable detrimental effect was observed on mean body weight gain of male mice. Only the highest dose group showed decreased body weight gain of 8% at the end of the study. Female mice showed a decrease of mean body weight gain at all doses (from approximately 6% to 23%) especially in animals from the two highest doses. During the experiment, none of the mice died and upon histopathological examination no tara gum‐related effects were observed (NTP, 1982). The Panel could not derive a no‐observed‐adverse effect level (NOAEL) from this study given the decreased body weight gain of female mice relative to controls at all doses tested.
Rats
Fifty male and female weanling Wistar origin rats (10 males and 10 females/group) were given doses of 0%, 1%, 2% and 5% tara gum in diet (equivalent to 0, 900, 1,800 and 4,500 mg/kg bw per day) for 90 days (Til et al., 1974). Food intake and individual body weights were recorded weekly. Haematological investigations (haemoglobin, packed cell volume, erythrocytes and leucocytes counts) were carried out at weeks 4 and 12 from five animals of each sex and group. Urine examinations were conducted from three males and three females of each group in weeks 5 and 13. Determinations of serum enzymes (serum glutamic‐oxaloacetic transaminase (SGOT), serum glutamic‐pyruvic transaminase (SGPT) and serum alkaline phosphatase (SAP)) were conducted from five males and five females of each group in week 13. Detailed microscopic examinations (~ 17 organs and tissues) were conducted on 10 male and 10 female rats. No abnormalities were observed in general appearance, behaviour, or survival in any of the groups. Growth, food intake, and food efficiency were slightly decreased at the 2% and 5% dietary level in males. The mean body‐weights of males were statistically significantly decreased relative to controls at the 5% dietary level starting at week 4 through week 13, but the decrease was slight, around 10% body weight loss. In females, the mean body weight decrease was observed in a non‐dose‐related manner. Food intake during 12 weeks and food efficiency during 4 weeks were unchanged in both sexes as compared to controls. Haematology and urinalysis showed no treatment‐related differences. There were no significant differences in serum enzyme activities measured or blood sugar. Blood urea nitrogen levels were significantly increased at 5% in males only. The relative weights of the caecum, thyroids, kidneys and testes increased slightly in males at the 2% and 5% levels. The caecum weights were only increased in males. No relevant lesions were found on gross or histopathological examination that could be attributable to the ingestion of tara gum. The authors considered that the effects on the weights of some organs were not toxicological significant since there was no evidence of a dose‐related response and since they were not accompanied by histological abnormalities (Til et al., 1974). The authors concluded that it ‘seems justified that tara gum at dietary levels up to 2% did not induce any distinct adverse effects’. The Panel noted that the decrease in mean body weight and other findings such as increases in blood urea nitrogen might be attributed to a nutritional imbalance induced by the high percentage of the compound added in the diet.
As during previous evaluation of other gums, the Panel noted that an increased caecum weight in animals fed high amounts of carbohydrates is considered a physiological response to an increased fermentation due to a carbohydrate‐induced modification on the composition of the intestinal microbiota. Increased caecum weight has been observed in rats fed carbohydrates other that tara gum (Leegwater et al., 1974; Licht et al., 2006). Animals fed diets containing potato starch, inulin or oligofructose had significantly higher caecum weights and lower pH values than the reference animal group (Licht et al., 2006). Different groups of animals fed modified diets containing increased concentration of potato starch, hydroxypropyl starch and hydroxypropyl distarch glycerol showed increases in the relative caecal weights, filled and emptied, with increasing concentrations of the various hydroxypropyl starches. These increases were accompanied by increased severities of diarrhoea that was related to an increased osmotic activity of the caecal fluid in the animals (Leegwater et al., 1974). The authors hypothesised that dietary components not completely digested and/or absorbed in the small intestine, and further fermented by the gut microbiota, enhance the amounts of osmotically active material resulting in an increase in water retention and the animals drinking more water leading to the caecum distention to a size larger than normal.
Groups of five male and five female F344 rats were fed 0, 6,300, 12,500, 25,000, 50,000 and 100,000 mg tara gum/kg diet (equivalent to 0, 756, 3,000, 6,000 and 12.000 mg/kg bw per day) for 2 weeks (NTP, 1982). On day 15, the animals received control diets. All animals were observed daily throughout the study and were sacrificed on day 16. No increased death rate was reported. Compared with controls the mean body weight gain was depressed by 12–18% in male rats and by 8% in female rats receiving the highest dose. No other compound‐related effects were reported during clinical observation or at necropsy.
Groups of F344 rats fed diets containing 0, 3,100, 6,300, 12,500, 25,000 and 50,000 mg/kg tara gum (equivalent to 0, 279, 567, 1,125 and 2,250 mg/kg bw per day) for 13 weeks (NTP, 1982). Ten animals of each sex and each species per dose were used and separate control groups of 10 animals for each sex were included. The investigations included clinical signs, body weights, feed consumption and histopathology of all major organs. Haematology, clinical chemistry and urine were not investigated. Mortality was checked twice daily and animals were weighed weekly. At day 91, all animals were sacrificed and subjected to a gross examination and various tissues were examined microscopically of all controls and animals of the highest dose group. No deaths occurred among the rats. Male rats slightly loss body weight (between 4% and 0,2%) starting at 1,260 mg/kg bw per day and onwards compared to controls. As reported by the authors, in 4 out of 10 male rats of the highest dose group, fewer mature spermatozoa were found in comparison to controls during histopathological examination. No other compound‐related effects were reported (NTP, 1982).
Dogs
Three groups of three male and three female beagle dogs received 0%, 1% or 5% tara gum (equivalent to 0, 250 or 1,250 mg/kg bw per day) in their diet for 90 days. No abnormalities were noted with regard to behaviour, mortality, haematology, urinalysis, clinical chemistry, organ weights or gross histopathology examination (Oshita et al., 1975; as reported in JECFA, 1987). Borzelleca et al. (1993) in their comments to the study by Oshita et al. (1975) wrote that the feed intake values for the male dogs fed the highest tara gum diet were 14% below those of the group receiving the control diet and gross and microscopic examination of the tissues did not show any changes that could be attributed to the tara gum diets (Borzelleca et al., 1993).
Overall, repeated oral administration studies in rats, mice and dogs revealed no significant adverse effects attributable to tara gum at doses equivalent up to 12,000 mg/kg bw per day in rats, of up to 20,000 mg/kg bw per day in mice and up to 1,250 mg/kg bw per day in dogs.
3.5.4. Genotoxicity
3.5.4.1. In vitro
Tara gum was tested in Salmonella Typhimurium strains TA98, TA100, TA1535, TA1537 and TA1538 with and without metabolic activation in concentrations of 10–1,000 μg/plate. Three tests were made and tara gum was negative in the first and third assay. In the second assay, increases in revertants were noted but the increments were not dose‐related (Boeuf et al., 1988; as reported in Borzelleca et al., 1993). No further information available. The Panel could not evaluate this study because no original report was available and based on the available information considered this study of limited relevance for risk assessment.
Tara gum was tested for induction of gene mutation in S. Typhimurium strains TA97, TA98, TA100, TA1535 and TA1537 using the pre‐incubation method with and without metabolic activation (liver S‐9 fractions from Aroclor‐induced male Sprague–Dawley rats or Syrian hamsters) (Zeiger et al., 1992). In this study, 311 test compounds were evaluated at concentrations which ranged from 100–10,000 μg/plate if not limited by solubility and/or toxicity. Dose levels of tara gum were selected based on solubility and/or toxicity, but are not reported. Tara gum was shown to be negative with all strains both in the absence and presence of metabolic activation. The study meets the criteria stated in the relevant OECD guideline no. 471 with the exception that the use of TA102 tester strain is not clearly indicated. However, the Panel noted that no oxidising or cross‐linking activities are expected to occur and considered the negative results observed as reliable.
3.5.4.2. In vivo
Five male and five female Swiss Crl mice were given by gavage a single dose of 350 mg/kg bw tara gum in distilled water. Control mice were treated with distilled water only. Treated and control animals were sacrificed 24, 48 and 72 h later and the bone marrow was examined for the presence of micronuclei in polychromatic erythrocytes. At each sampling time, the number of micronuclei in the mice treated with tara gum were not statistically different from the corresponding control values. Tara gum did not induce micronuclei in polychromatic bone marrow cells thus not showing a clastogenic potential in this assay (Vanrel et al., 1988; as reported in Borzelleca et al., 1993). The Panel could not evaluate this study because no original report was available and based on the information reported considered it of limited relevance for risk assessment due to limitations. These included i) the appropriateness of the dose level used since no indication of target tissue cytotoxicity or exposure and ii) no indication of the numbers of polychromatic erythrocytes scored for induction of micronuclei. The Panel further noted that tara gum is not absorbed intact but significantly fermented by the intestinal microbiota in the intestine to short chain fatty acids.
Overall, based on the data available, the Panel concluded that there is no concern with respect to the genotoxicity of tara gum (E 417).
3.5.5. Chronic toxicity and carcinogenicity
Mice
Groups of B6C3F1 mice were fed diets containing 0, 25,000 and 50,000 mg tara gum/kg diet (equivalent to 0, 3,750 and 7,500 mg/kg bw per day) for 103 weeks (NTP, 1982; Melnick et al., 1983). Fifty animals of each sex per dose were used and separate control groups of each sex were included. The investigations included clinical signs, body weights, feed consumption and histopathology of all major organs (more than 26 tissues). Histopathology was also done on the animals found dead unless precluded by autolysis or cannibalism. Haematology, clinical chemistry and urinalysis have not been performed. In males from the 25,000 mg/kg diet group, the mean body weight of all animals was comparable to the controls throughout the duration of the study, no effects on feed consumption were observed. Female animals from the same group loss body weight (between 5% and 7%) up to week 84 but gained weight (+11%) at the end of the study. All male and female animals from the 50,000 mg/kg diet group showed lower mean body weight throughout the study compared to controls. No other major significant differences were observed in body weight gain both in males or females. The survival of treated and control groups of both sexes was not statistically significantly different. Histopathological examination demonstrated lower incidences of hepatocellular adenomas or carcinomas and in alveolar bronchiolar adenomas or carcinomas in female mice treated with tara gum. No other statistically significant differences in tumour incidences were observed in tara gum treated male and female mice as compared to controls.
Rats
JECFA reports a long‐term study in rats in which a single dose of tara gum was tested (JECFA, 1987). The description of the study is quoted here:
Groups of 50 male and 50 female Charles River albino rats were fed diets containing 5% alpha‐cellulose (control) or 5% tara gum) for 2 years (Carlson and Domanski, 1980; as reported in JECFA, 1987). An interim sacrifice of 10 animals/sex/group was carried out after 12 months. Statistically‐significant lower body weight and body‐weight changes were noted at a number of weeks in both male and female animals in the tara gum group. There were also statistically‐significant reductions in food consumption in the tara gum group of both males and females‐ at a number of weeks. This may have been due to the physical characteristics of the control diet (alpha cellulose added) which may have accounted for greater spillage and therefore greater apparent food consumption in the control animals. Some changes in haematological measurements were noted in rats in the tara gum group. These included statistically significant decrease in haematocrit values at 12 months in male rats, in total erythrocyte and leukocyte counts in male rats at 99 weeks, in monocyte counts in female rats at 12 months and in reticulocyte counts in female rats at 18 months. Statistically significant increases were reported in the haemoglobin concentration at 99 weeks, in the monocytes at 12 months and reticulocyte count in female rats given tara gum. With respect to clinical chemistry, statistically‐significant increases in animals given tara gum were noted for the following measurements: SGPT13 activity in males at 12 months, fasting serum glucose, BUN14 at 12 months in females, and SGOT15 activity in females at 3 months. A significant decrease was noted in total cholesterol levels at 6 and 12 months in females given tara gum. At the 12‐month interim sacrifice the following statistically significant changes were noted in males fed 5% tara gum: significantly greater brain to body‐weight, testes to body‐weight, and heart to body‐weight ratios and significantly lower liver to brain‐weight ratio. At the final sacrifice (2 years under treatment) the following statistically‐significant changes were noted in animals given tara gum: higher adrenal gland to body‐weight ratio in males and lower absolute brain weight in females. No significant differences were reported between the tara gum and control groups with respect to gross or microscopic pathology (Carlson and Domanski, 1980; as reported in JECFA, 1987).
Borzelleca et al. (1993) reported apparently the same rat study as the one reviewed by JECFA (1987), in the publication referred as from Industrial Bio‐Test Laboratories Inc. (1980). Borzelleca et al. (1993) reported that the parameters that differed significantly (not further specified, but according to the text assumed to be the same as stated above) were within the normal range of historical values in the performing laboratory for rats of the same strain and age. Borzelleca et al. (1993) added that none of the neoplasms found were considered to be attributable to the effects of the test material. The final sacrifice, which included post‐mortem and moribund sacrificed animals, indicated that tara gum powder had no effect on the occurrence or type of histopathological lesions observed in the study. The neoplasms observed were not considered to be related to treatment with the test material (tara gum).
Chronic effects of tara gum were determined in F344 rats fed diets containing 0, 25,000 and 50,000 mg/kg (equivalent to 0, 1,250, and 2,500 mg/kg bw per day) tara gum for 103 weeks (NTP, 1982; Melnick et al., 1983). Fifty animals of each sex per dose were used and separate control groups of each sex were included. The investigations included clinical signs, body weights, feed consumption and histopathology of all major organs (more than 26 tissues). Histopathology was also done on animals which were found dead unless precluded by autolysis or cannibalism. Haematology, clinical chemistry and urinalysis have not been performed. In the 25,000 mg/kg diet animals, the mean body weight of all animals was slightly decreased in females (between 1% and 3%) but was comparable to controls at the end of the study, no effects on feed consumption were observed. In the 50,000 mg/kg diet animals, the mean body weight was slightly decreased (between 2% and 9%) throughout the study compared to controls. No major differences were observed in body weight gain either in males or females, contrary to the subchronic studies. No substance‐related effects on survival were reported.
Upon histopathological examination, a statistically significant increase in Leydig cell tumours of the testis in F344 male rats was observed. However, when data from incidences of Leydig cell tumour of the testis underwent time‐adjusted analysis,16 statistical significance was no longer established. The authors noted that the Leydig cell tumours of the testis occurred spontaneously in F344 rats at high rates as shown by the 83% incidences in controls animals in this study. Furthermore, those tumours had shown a high historical incidence in control male F344 rats (74–98%, Haseman et al., 1998), and overall, the authors concluded that the association between the increased incidences of Leydig cell tumours and the administration of tara gum was not established. The Panel agreed with this interpretation.
Overall, tara gum was investigated in chronic toxicity and carcinogenicity assays in rats (two strains) and mice (one strain) receiving diets containing 25,000 and 50,000 mg tara gum/kg of diet for 103 weeks (equivalent to 3,750 and 7,500 mg/kg bw per day in mice and 1,250 and 2,500 mg/kg bw per day in rats). The Panel considered tara gum not carcinogenic in mice and rats (NTP, 1982; Melnick et al., 1983).
3.5.6. Reproductive and developmental toxicity
No new studies from those evaluated by JECFA (1987) were identified for this evaluation. Borzelleca et al. (1993) and Borzelleca and Egle (1993) describe the same studies as in JECFA but with some more detail.
3.5.6.1. Reproductive toxicity studies
‘A 3‐generation reproduction study was carried out in CD strain Charles River albino rats. Groups of 10 male and 20 female animals were fed a diet containing 5% alpha‐cellulose (control) or 5% tara gum’ (commercial supplier, analysis confirmed that tara gum met the FAO/WHO specifications) (equivalent to 2,500 mg/kg bw per day) (Domanski et al., 1980, as referred to in JECFA, 1987 and described in more detail by Borzelleca and Egle, 1993 and Borzelleca et al., 1993). The ‘same dose and animal numbers were employed for successive generations throughout the study’ (F0, F1, F2, F3). ‘In each generation the parental animals received the test diets for 11 weeks prior to mating and then through mating, gestation, and weaning. The females of the F0 and F2 generations were mated to produce 2 litters. Females of the F1 generation produced 3 litters. Ten males and 20 females were retained at weaning from the second litter of each dietary group for use as parental animals for the next generation. Ten weanlings per sex per dietary group from the F3b litters were selected for histopathological examination of 12 tissues and organs; organ‐weight values were also recorded. All other animals were subjected to gross necropsy’.
There were statistically significant premating lower body weights and body weight gains in the F0 parental female animals, whereas parental F1 generation females only showed final mean body weights statistically significant lower than controls. In contrast, the premating body weights of parental F1 generation female animals were not affected by the treatment. In the F2b litters, a statistically significant reduction in the number of pups viable at lactation days 12 and 21 in the tara gum group was reported. Survival rates of controls and F0 litters were low in all three F2 litters, whereas survival rate was reported as generally good for the F1 and F3 litters. Borzelleca et al., 1993 mentioned that the authors of the study stated that respiratory infection may have contributed to reduction in survival of the F2 litters, since respiratory symptoms were noted among the parental animals. Pup weights were significantly lower than those of the controls in the tara gum groups at days 4, 12, and 21 of the F1a litter, at days 1, 4, 12 and 21 of the F3a litter, and at lactation days 1, 4, 12 and 21 of the F3b litter. There were no significant differences in reproductive performance. No significant differences were noted in parental premating feed consumption, mortality, or gross and microscopic pathology. Borzelleca et al. (1993) reported that necropsy examination of the parental animals and histopathological examination of the tissues of selected F3b progeny did not show any abnormalities attributable to the tara gum diet. The following statistically significant differences in organ weights and organ‐weight ratios were reported by JECFA (1987) from the unpublished report of Domanski et al. 1980 for the tara gum group compared to the control group: lower absolute liver and brain weights and higher kidney, testes, heart and brain‐to‐body‐weight ratios. These differences were attributed to the lower body weights of the tara gum F2 weanlings, from whom the values were obtained.
According to Borzelleca and Egle (1993), there were no consistent, statistically significant, and compound‐related effect on mortality, feed consumption, body weight, general health, and behaviour in the above mentioned study. No statistically significant differences were obtained for the mating and reproductive performance indices. According to Borzelleca and Egle (1993), the data suggest that tara gum has no adverse effect on reproductive performance and in utero development. The offspring survival indices and mean body weights were lower on days 12 and 21 in the tara gum group. However, the reproductive indices showed no statistically significant differences. Data from those progeny selected as parental animals for subsequent generations (F1 and F2 parents) indicated that these animals exhibited normal growth patterns and reproductive performance. Animal behaviour was not affected. Gross examination of the parental animals and offspring and histopathological examination of the tissues from selected F3b progeny did not identify any tara gum‐related abnormalities. The Panel had no access to the original data for evaluation of this study.
3.5.6.2. Developmental toxicity studies
Developmental effects of tara gum were investigated in four groups of 25 female rats Wistar rats fed diets containing 0%, 1.25%, 2.5% or 5.0% tara gum (commercial supplier, analysis confirmed that tara gum met the FAO/WHO specifications) (equivalent to 0, 625, 1,250 and 2,500 mg/kg bw per day) from gestational day (GD) 6 to 16 (Becker, 1986, as referred to in JECFA, 1987, and described in more detail by Borzelleca and Egle, 1993 and Borzelleca et al., 1993). On GD 21, all females were sacrificed and the fetuses removed by caesarean section. Post‐mortem examination included gross examination of all organs, particularly the uterus and the number of corpora lutea. The investigation of the fetuses included weight and gross examination of abnormalities and of viscera, brain and skeleton. No major differences were observed among the groups concerning maternal toxicity, the number of corpora lutea, implantations or pre‐implantation losses. The total number of fetuses alive or death was not affected by the treatment nor was the number of total resorptions, embryonic resorptions, fetal resorptions and post‐implantation losses. Neither were the weights of live fetuses or the sex ratio affected by the treatment with tara gum. Borzelleca and Egle (1993) reported that the external, visceral and skeletal examinations of the fetuses did not reveal any compound‐related abnormalities. The Panel had no access to the original data for evaluation of this study.
Overall, the Panel noted that based on the available data from the dietary three‐generation toxicity study and a developmental study in rats, tara gum did not induce parental, reproductive or developmental toxicity up to the highest dose tested, 2,500 mg tara gum/kg bw per day.
3.5.7. Hypersensitivity, allergenicity and food intolerance
According to Borzelleca et al. (1993) and recent literature searches, no reports on the allergenicity of tara gum were found.
3.6. Discussion
Tara gum is a galactomannan isolated from the endosperm of the seeds of the tara tree, C. spinosa L. Tara gum is commonly defined as a high‐viscosity polysaccharide composed mainly of a linear chain of (1‐4)‐β‐d‐mannopyranose units with α‐d‐galactopyranose units attached by (1‐6) linkages. The ratio of mannose to galactose in tara gum is 3:1.
Specifications for tara gum have been defined in Commission Regulation (EU) 231/2012 and by JECFA (2006).
Because of both the botanical origin and the polysaccharidic nature of gums, they can be a substrate of microbiological contamination and of field and storage fungal development. The latter has been recently demonstrated by the mycotoxin contaminations of gums (Zhang et al., 2014). The Panel noted that the microbiological specifications for polysaccharidic thickening agents, such as gums, should be harmonised and that for tara gum criteria for TAMC and TYMC should be included into the EU specifications.
Data on in vitro degradation by human gastrointestinal fluids and on in vivo digestibility of tara gum in animals demonstrated that this compound would not be absorbed intact or hydrolysed by digestive enzymes. Based on the fermentation of other galactomannans, the Panel considered that tara gum would be fermented with production of SCFAs, such as acetic, propionic and butyric acids, during its passage through the large intestine by strains of bacteria found in the human colon. Based on the available knowledge on the role of SCFA as end products of the fermentation of dietary fibres by the anaerobic intestinal microbiota (den Besten et al., 2014; Topping and Clifton, 2001), the Panel considered that their potential formation as fermentation products from tara gum does not raise any concern. Despite the absence of convincing in vivo study in humans, the Panel considered that tara gum would most probably not be absorbed intact but could be fermented by intestinal microbiota in humans.
Acute oral toxicity studies with tara gum on rats and mice showed no toxic effects up to a dose of 630 mg/kg bw tara gum.
Short‐term and subchronic animal toxicity studies on tara gum have not shown biologically significant adverse effects associated to the treatment under the conditions of the tests. From studies in rats fed high doses of tara gum, ≥ 5% dietary level, decrease of mean body weight and other findings, e.g. increased blood urea nitrogen have been reported. The Panel noted that these findings could be attributed to a nutritional imbalance induced by the high percentage of the compound added in the diet. NOAELs identified in short‐term and subchronic studies corresponded to the highest dose tested of 12,000, 4,500 and 2,250 mg/kg bw per day for rats (Til et al., 1974; NTP, 1982), of approximately 20,000 and 10,000 mg/kg bw per day for mice (NTP, 1982) and of approximately 1250 mg/kg bw per day in dogs (Oshita et al., 1975; as reported in JECFA, 1987; Borzelleca et al., 1993).
Based on the data available, the Panel concluded that there is no concern with respect to the genotoxicity of tara gum (E 417).
Tara gum was tested in chronic toxicity and carcinogenicity assays in rats (two strains) and mice (one strain) receiving diets containing 25,000 and 50,000 ppm tara gum for 103 weeks (equivalent to 3,750 and 7,500 mg/kg bw per day in mice, and 1,250 and 2,500 mg/kg bw per day in rats). No major differences (2–9%) were observed in body weight gain either in males or females, contrary to the subchronic studies.
In rats, 2,500 mg/kg bw per day, and in mice, 7,500 mg/kg bw per day (both the highest dose tested) was identified as the NOAEL by the Panel. Further, the Panel considered tara gum as not carcinogenic in mice and rats.
Tara gum did not show reproductive or developmental toxicity effects, and a NOAEL of 2,500 mg/kg bw per day, the highest dose tested, could be identified by the Panel.
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014), the Panel considered that sufficient toxicity data were available in animals showing no adverse effects at highest doses tested. Therefore, the Panel considered that there is no need to allocate a numerical ADI for tara gum (E 417).
To assess the dietary exposure to tara gum (E 417) from its use as a food additive, the exposure was calculated based on (1) maximum levels of data provided to EFSA (defined as the maximum level exposure assessment scenario) and (2) reported use levels (defined as the refined exposure assessment scenario, brand‐loyal and non‐brand‐loyal consumer scenario).
Tara gum (E 417) is authorised as Group I food additive in a wide range of foods. The Panel did not identify a brand‐loyalty to specific food categories, and therefore, the Panel considered that the non‐brand‐loyal scenario was the more appropriate and realistic scenario for risk characterisation thus it was assumed that the population would probably be exposed long term to the food additive present at the mean reported use levels in processed food.
The refined estimates were based on 11 out of 67 food categories in which tara gum (E 417) is authorised. Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the real exposure to tara gum (E 417) as a food additive in European countries for the refined scenarios when considering only food additive uses for which data have been provided.
However, the Panel noted that given the information from the Mintel's GNPD, it may be assumed that tara gum (E 417) is not used in food categories in which it is authorised.
A specific exposure assessment scenario taking into account the consumption of food supplements for consumers only was also performed to estimate exposure for children, adolescents, adults and the elderly as exposure via food supplements may deviate largely from that via food, and the number of food supplement consumers may be low depending on populations and surveys.
The Panel noted that the exposure to tara gum (E 417) from its use according the Annex III (Parts 2, 3, 4 and 5A) was not considered in the exposure assessment.
The Panel also noted that the refined exposure estimates are based on information provided on the reported level of use of tara gum (E 417). If actual practice changes, this refined estimates may no longer be representative and should be updated.
Due to the discrepancies observed between the data reported from industry and the Mintel database, where tara gum are labelled in more products than in food categories for which data were reported from industry, the Panel noted that the collection of data on usage and use levels of tara gum (E 417) would allow for a more realistic exposure assessment.
4. Conclusions
Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014), and given that
the data received for the 11 food categories were adequate for a refined exposure assessment for these categories;
based on the reported use levels a refined exposure of up to 70 mg/kg bw per day in children (3–9 years) in these categories (non‐brand‐loyal scenario) was estimated;
highest refined exposure assessments on consumers only of food supplements was also calculated and ranged from 10 mg/kg bw per day for adolescents to 51 mg/kg bw per day for children;
tara gum is unlikely to be absorbed intact and is expected to be fermented by intestinal microbiota;
adequate toxicity data were available;
no adverse effects were reported in sub‐chronic studies at the highest doses tested in rats (4,500 mg tara gum/kg bw per day), mice (10,000 mg tara gum/kg bw per day) and dogs (1,250 mg tara gum/kg bw per day);
no adverse effects were reported in chronic toxicity studies at the highest doses tested in rats (2,500 mg tara gum/kg bw per day) and mice (7,500 mg tara gum/kg bw per day);
no adverse effects were reported in reproductive and developmental toxicity studies at the highest doses tested in rats (2,500 mg tara gum/kg bw per day);
there is no concern with respect to the genotoxicity of tara gum;
there is no concern with respect to carcinogenicity of tara gum;
the Panel concluded that there is no need for a numerical ADI for tara gum (E 417), and that there is no safety concern for the general population at the refined exposure assessment for the reported uses of tara gum (E 417) as a food additive.
Recommendations
The Panel recommended that the European Commission considers
revising the current limits for the toxic elements (lead, cadmium, mercury and arsenic) in the EU specification for tara gum (E 417) in order to ensure that tara gum (E 417) as a food additive will not be a significant source of exposure to those toxic elements in food.
harmonising the microbiological specifications for polysaccharidic thickening agents, such as gums, and to include criteria for the absence of Salmonella spp. and Escherichia coli, for TAMC and for TYMC into the EU specifications of tara gum (E 417).
investigating whether polycyclic aromatic hydrocarbons are generated during the roasting process and if so establishing maximum levels.
Documentation provided to EFSA
Pre‐evaluation document on tara gum (E 417). Fraunhofer ITEM. February 2012.
INEC (Association of Producers of Carob Bean Bum), 2015. Data in response to the call for technical data on certain thickening agents permitted as food additives in the EU (EFSA‐Q‐2014‐00928). Submitted on 13 January 2016.
INEC (Association of Producers of Carob Bean Bum), 2015. Data in response to the call for technical data on certain thickening agents permitted as food additives in the EU (EFSA‐Q‐2014‐00928). Submitted on 15 December 2015.
FDE (FoodDrinkEurope), 2014. Data on usage levels of tara gum (E 417) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 20 September 2014.
Babbi (BABBI Confectionery Industry), 2014. Data on usage levels of tara gum (E 417) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 12 August 2014.
EMCESA (Fabricante Embutidos del centro SA (España)), 2014. Data on usage levels of tara gum (E 417) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 29 August 2014.
EUROGUM A/S, 2013. Data on usage levels of tara gum (E 417) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 30 September 2014.
Abbreviations
- ADI
acceptable daily intake
- AFC
EFSA Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- ANS
Panel on Food Additives and Nutrient Sources added to Food
- AOAC
Association of Analytical Communities
- bw
body weight
- CAS
Chemical Abstracts Service
- EFSA
European Food Safety Authority
- EFSA NDA
EFSA Panel on Dietetic Products, Nutrition and Allergies
- EINECS
European Inventory of Existing Commercial Chemical Substances
- FAO/WHO
Food and Agriculture Organization/World Health Organisation
- FCS
food categorisation system
- FDE
Food Drink Europe
- FSANZ
Food Standards Australia New Zealand
- FSMP
foods for special medical purposes
- FTIR
Fourier transform infrared spectroscopy
- GD
gestational day
- GNPD
Global New Products Database
- HPLC
high‐performance liquid chromatography
- INS
International Numbering System for Food Additives
- IOM
Institute of Medicine
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- MPL
maximum permitted level
- NOAEL
no‐observed‐adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- QS
quantum satis
- SAP
serum alkaline phosphatase
- SCF
Scientific Committee on Food
- SCFA
short‐chain fatty acid
- SGOT
serum glutamic‐oxaloacetic transaminase
- SGPT
serum glutamic‐pyruvic transaminase
- TAMC
total aerobic microbial count
- TLC
thin‐layer chromatography
- TYMC
total combined yeasts and moulds count
- UV
ultraviolet
Appendix A – Summary of the reported use levels (mg/kg or mg/L as appropriate) of tara gum (E 417) provided by industry
Appendix B – Number and percentage of food products labelled with tara gum (E 417) out of the total number of food products present in Mintel GNPD per food sub‐category between 2012 and 2017
Appendix C – Concentration levels of tara gum (E 417) used in the maximum level and refined exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Appendix D – Main food categories contributing to exposure to tara gum (E 417) using the maximum level and refined exposure assessment scenarios
Appendix E – Summary of total estimated exposure of tara gum (E 417) from its use as a food additive for the maximum level exposure assessment scenario and the refined exposure assessment scenarios per population group and survey: mean and high level (mg/kg bw per day)
1.
Appendices A–E can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4863
Supporting information
Summary of the reported use levels (mg/kg or mg/L as appropriate) of tara gum (E 417) provided by industry
Number and percentage of food products labelled with tara gum (E 417) out of the total number of food products present in Mintel GNPD per food sub‐category between 2012 and 2017
Concentration levels of tara gum (E 417) used in the maximum level and refined exposure assessment scenarios (mg/kg or mL/ kg as appropriate)
Main food categories contributing to exposure to tara gum (E 417) using the maximum level and refined exposure assessment scenarios
Summary of total estimated exposure of tara gum (E 417) from its use as a food additive for the maximum level exposure assessment scenario and the refined exposure assessment scenarios per population group and survey: mean and high level (mg/kg bw per day)
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Lambré C, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Oskarsson A, Parent‐Massin D, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Brimer L, Christodoulidou A, Lodi F, Tard A and Dusemund B, 2017. Scientific Opinion on the re‐evaluation of tara gum (E 417) as a food additive. EFSA Journal 2017;15(6):4863, 37 pp. 10.2903/j.efsa.2017.4863
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00516
Panel members: Fernando Aguilar, Riccardo Crebelli, Alessandro Di Domenico, Birgit Dusemund, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Claude Lambré, Jean‐Charles Leblanc, Oliver Lindtner, Peter Moldeus, Alicja Mortensen, Pasquale Mosesso, Agneta Oskarsson, Dominique Parent‐Massin, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific opinion: Jacqueline Wiesner, Dario Battacchi and Lemmetyinen Juho. The ANS Panel wishes to acknowledge all the European competent institutions, the Member State bodies and other organisations that provided data for this scientific opinion.
Adopted: 17 May 2017
Notes
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Annex II to Regulation (EC) No 1333/2008.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Published: 22 November 2009. Available from: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for technical data on certain thickening agents permitted as food additives in the EU – Extended Deadline: 31 December 2015. Available online: http://www.efsa.europa.eu/it/data/call/141219
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Commission Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 14/3/2017.
Available online: http://www.efsa.europa.eu/en/press/news/150428.htm
Alanine aminotransferase.
Blood urea nitrogen.
Aspartate aminotransferase.
In a time‐adjusted analysis, early deaths are excluded by basing the statistical tests on animals that survived until the appearance of the first tumour. Once this data set is obtained, the standard statistic protocols are applied to the tumour incidences.
References
- Anderson E, 1949. Endosperm mucilages of legumes‐occurrence and composition. Industrial and Engineering Chemistry, 41(lt), 2887–2890. As referred to by Borzelleca, 1993. [Google Scholar]
- Anderson DMW, Howlett JF and McNab CGA, 1986. The amino acid composition of the proteinaceous components of konjac mannan, seed endosperm galactomannans and xanthan gum. Food Hydrocolloids, 1, 95–99. [Google Scholar]
- AOAC (Association of Official Agricultural Chemists, now AOAC International), 2002. 43 Spices and other Condiments. In: Official Methods of Analysis of AOAC International, 17th Edition. Vol.II Food Composition, Additives, Natural Contaminants. Ed. Horwitz W. p 10.
- Barry JL, Bonnet C, David A and Koslowski F, 1990. Measurement of the digestibility of tara galactomannans in rats. Unpublished report, cited in: Borzelleca et al. 1993.
- Becker B, Schafroth P, Terrier C and Sachsse K, 1986. Embryo‐toxicity (including teratogenicity) study with tara gum in the rat. Unpublished report No. 53335 from Research & Consulting Company AG, Itingen, Switzerland. Submitted to WHO by Unipektin AG, Zurich, Switzerland as referred by JECFA, 1987.
- Benk E, 1977. Tara‐Kern‐Mehl. Riechstoffe, Aromen, Kosmetika, 27, 275–276. [Google Scholar]
- Boeuf C, Bonafous J, Vanrell B, Glomot R and LeBigot JF, 1988. Tara gum. Reverse mutation assay in vitro. Ames test. Unpublished report, cited in Borzelleca et al. 1993.
- Borzelleca JF and Egle JR, 1993. An evaluation of the reproductive and developmental effects of tara gum in rats. Journal of the American College of Toxicology, 12, 91–97. [Google Scholar]
- Borzelleca JF, Ladu BN, Senti FR and Egle JR, 1993. Evaluation of the safety of tara gum as a food ingredient: a review of literature. Journal of the American College of Toxicology, 12, 81–89. [Google Scholar]
- Carlson WA and Domanski J 1980. Two‐year chronic oral toxicity study with tara gum in albino rats. Unpublished report from Industrial Bio‐Test Laboratories, Inc., Northbrook, IL, USA, cited in JECFA 1986.
- Cummings JH and Englyst HN, 1987. Fermentation in the human large intestine and the available substrates. American Journal of Clinical Nutrition, 45, 1243–1255. [DOI] [PubMed] [Google Scholar]
- Daas PJ, Schols HA and de Jongh HH, 2000. On the galactosyl distribution of commercial galactomannans. Carbohydrate Research, 329(3), 609–619. [DOI] [PubMed] [Google Scholar]
- den Besten G, van Eunen K, Groen A, Venema K, Reijngoud DJ and Bakker M, 2013. The role of short‐chainfattyacids in the interplay between diet, gut microbiota, and host energy metabolism. The Journal ofLipidResearch, 54, 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanski J, Carlson W and Frawley J, 1980. Three generation reproduction study with tara gum in albino rats. Unpublished report from Industrial Bio‐Test Laboratories, Inc., Northbrook, IL, USA as referred by JECFA 1987.
- Eberendu AR, Booth C, Luta G, Edwards JA and McAnalley BH, 2005. Quantitative determination of saccharides in dietary glyconutritional products by anion‐exchange liquid chromatography with integrated pulsed amperometric detection. Journal of AOAC International, 88, 998–1007. [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal, 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9 (3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA AFC Panel (EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food), 2004. Opinion on a request from the Commission related to the use of certain food additives in jelly mini cups. EFSA Journal 2004;82(2):8, 11 pp. 10.2903/j.efsa.2004.82 [DOI] [Google Scholar]
- EFSA ANS Panel (European Food Safety Authority Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760. Available online: http://www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA ANS Panel on Food Additives and Nutrient Sources added to Food), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. 10.2903/j.efsa.2014.3697 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009a. Scientific Opinion on cadmium in food. EFSA Journal 2009;7(10):980, 139 pp. 10.2903/j.efsa.2009.980 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009b. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012a. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012b. Scientific Opinion on lead dietary exposure in the European population. EFSA Journal 2012;10(7):2831, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012c. Scientific Opinion on cadmium dietary exposure in the European population. EFSA Journal 2012;10(1):2551, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on dietary exposure to inorganic arsenic in the European population. EFSA Journal 2014;12(3):3597, 68 pp. 10.2903/j.efsa.2014.3597 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: General Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579. Available online: http://www.efsa.europa.eu [DOI] [Google Scholar]
- FAO , 2003. Methods of food analysis in Food energy – methods of analysis and conversion factors. FAO Food and nutrition paper 77. Food and Agriculture Organization of the United Nations, Rome, pp. 7–9. [Google Scholar]
- FSANZ , 2006. Final Assessment Report, Tara gum as a food additive. Food Standards Australia New Zealand, Application, A546, 1–44. [Google Scholar]
- Haseman JK, Hailey JR and Morris RW, 1998. Spontaneous neoplasm incidences in Fischer 344 rats and B6C3F1 mice in two‐year carcinogenicity studies: A National Toxicology Program Update. Toxicologic Pathology, 26, 428–441. [DOI] [PubMed] [Google Scholar]
- INDUSTRIAL BIO‐TEST LABORATORIES, INC ., 1980. Two‐year chronic toxicity study with tara gum in albino rats. Unpublished report of study conducted under contract with the Tara Development Group and monitored (and found acceptable) 96 Downloaded from http://ijt.sagepub.com at Fraunhofer‐Gesellschaft ‐ FhG on October 17, 2011 REPRODUCTIVE EFFECTS OF TARA GUM by W.A. Carlson (scientific consultant), J.J. Domanski, Jr., (Hercules, Inc., Wilmington, DE); P.H. Errico, M.A. Hoover, and C. Rose, (Tracor Jitco, Inc., Rockville, MD) as referred to Borzelleca, 1993.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1987. Evaluation of certain food additives and contaminants. Thirtieth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 751, 27 pp. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Sodium nitrite. Joint FAO/WHOExpert Committee on Food Additives. Available online: http://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-455.pdf
- Joint FAO/WHO Expert Committee on Food Additives , 1987. JECFA Monographs 1.
- Leegwater DC, de Groot P and van Kalmthout‐Kuyper M, 1974. The aetiology of caecal enlargement in the rat. Food and Cosmetics Toxicology, 12, 687–697. [DOI] [PubMed] [Google Scholar]
- Licht TR, Hansen M, Poulsen M and Dragsted LO, 2006. Dietary carbohydrate source influences molecular fingerprints of the rat faecal microbiota. BMC Microbiology, 6, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick RL, Huff J, Haseman JK, Dieter MP, Grieshaber CK, Wyand DS, Russfield AB, Murthy ASK, Fleischmann RW and Lilja HS, 1983. Chronic effects of agar, tara gum, gum arabic, locust bean gum, or tara gum in F344 rats and B6C3F1 mice. Food and Chemical Toxicology, 21, 305–311. [DOI] [PubMed] [Google Scholar]
- Merck Index , 2006. Guaran In: The Merck Index, an Encyclopedia of Chemicals, Drugs, and Biologicals. Available online: http://themerckindex.cambridgesoft.com/themerckindex/Forms/Search/ContentArea/C [Google Scholar]
- NTP , 1982. Carcinogenesis bioassay of tara gum in F344 rats and B6C3F1 mice (feed study). National Toxicology Program Technical Report Series No. 224. Available from National Technical Information Service (Publication No. PB82‐195546), Springfield, VA, USA. [PubMed]
- Oshita G, Burtner BR, Kennedy GLjr, Kinoshita FK and Keplinger MLML, 1975. 90 day subchronic oral toxicity study with tara gum in beagle dogs. Unpublished report from Industrial Bio‐Test Laboratories, Inc., Northbrook, IL, USA. Submitted to WHO by Hercules Incorporated, cited in JECFA 1986.
- Picout DR, Ross‐Murphy SB, Jumel K and Harding SE, 2002. Pressure cell assisted solution characterization of polysaccharides. 2. Locust bean gum and tara gum. Biomacromolecules, 3, 761–767. [DOI] [PubMed] [Google Scholar]
- Prado BM, Kim S, Özen BF and Mauer LJ, 2005. Differentiation of carbohydrate gums and mixtures using Fourier transform infrared spectroscopy and chemometrics. Journal of agricultural and food chemistry, 53, 2823–2829. [DOI] [PubMed] [Google Scholar]
- Ramis‐Ramos G, Simo‐Alfonso EF, Mongay‐Fernandez C and Ruiz‐Angel MJ, 2003. Leguminosae gum labeling and guar evaluation in carob gum by capillary electrophoresis. GIT laboratory journal Europe, 7, 66–68. [Google Scholar]
- Robaislek E, 1974. Bioavailable calorie assay of guar gum. Unpublished report from WARF Institute, Inc. Submitted to WHO by Institut Européen des Industries de la Gomme de Caroube. as referred by JECFA, 1987.
- Sax NI, 1984. Gum tara. Dangerous Properties of Industrial Materials, 6th Edition Van Nostrand Reinhold, New York, NY, 1490 pp. [Google Scholar]
- SCF (Scientific Committee for Food), 1992. Opinion expressed 1990 Annex 1: Evaluation of the additives. Food science and techniques. Reports of the Scientific Committee for Food (Twenty‐sixth series).
- SCF (Scientific Committee on Food), 2001. Guidance on submissions for food additive evaluations by the scientific committee on food. Opinion expressed on 11 July 2001. Available online: http://ec.europa.eu/food/fs/sc/scf/out98_en.pdf
- Scherz H and Mergenthaler E, 1980. Analytical determination of polysaccharide thickening agents for foods–a review (author's transl). Zeitschrift fur Lebensmittel‐Untersuchung und‐Forschung, 170, 280–286. [DOI] [PubMed] [Google Scholar]
- Semenza G, 1975. The possible digestion of tara gum in the stomach and/or in the small intestine. An in vitro study. Unpublished report, cited in JECFA 1986.
- Tapie N, Malhiac C, Hucher N and Grisel M, 2008. Determination of galactose and mannose residues in natural galactomannans using a fast and efficient high‐performance liquid chromatography/UV detection. Journal of Chromatography A, 1181, 45–50. [DOI] [PubMed] [Google Scholar]
- Til HP, Spanjers MT and DeGroot AP, 1974. Subchronic study with tara gum. Unpublished report.
- Topping DL and Clifton PM, 2001. Short‐chain fatty acids and human colonic function: roles and resistant starchand nonstarch polysaccharides. Physiological Reviews, 81, 1031–1064. [DOI] [PubMed] [Google Scholar]
- Towle GA and Schranz RE, 1975. The action of rat microbiota on tara gum solutions in vitro. Unpublished report, cited in JECFA 1986.
- Tsai LB and Whistler LR, 1975. Digestibility of galactomannans. Unpublished report, cited in JECFA 1986.
- Ullmann , 2007. Polysaccharides. Ullmann's Encyclopedia of Industrial Chemistry, Online version. Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim. [Google Scholar]
- Vanrel B, Glomot R and LeBigot JF, 1988. Tara gum. Micronucleus test in mice. Unpublished report, cited in Borzelleca et al. 1993.
- Zeiger E, Anderson B, Haworth S, Lawlor T and Mortelmans K, 1992. Salmonella mutagenicity tests: V. Results from testing of 311 chemicals. Environmental and Molecular Mutagenesis, 19, 2–141. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wong JW, Jia Z, Vaclavikova M, Trucksess MW and Begley TH, 2014. Screening multimycotoxins in food‐grade gums by stable isotope dilution and liquid chromatography/tandem mass spectrometry. Journal of AOAC International, 97, 889–895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the reported use levels (mg/kg or mg/L as appropriate) of tara gum (E 417) provided by industry
Number and percentage of food products labelled with tara gum (E 417) out of the total number of food products present in Mintel GNPD per food sub‐category between 2012 and 2017
Concentration levels of tara gum (E 417) used in the maximum level and refined exposure assessment scenarios (mg/kg or mL/ kg as appropriate)
Main food categories contributing to exposure to tara gum (E 417) using the maximum level and refined exposure assessment scenarios
Summary of total estimated exposure of tara gum (E 417) from its use as a food additive for the maximum level exposure assessment scenario and the refined exposure assessment scenarios per population group and survey: mean and high level (mg/kg bw per day)


