Abstract
West Nile fever (WNF) has been assessed according to the criteria of the Animal Health Law (AHL), in particular criteria of Article 7 on disease profile and impacts, Article 5 on the eligibility of WNF to be listed, Article 9 for the categorisation of WNF according to disease prevention and control rules as in Annex IV and Article 8 on the list of animal species related to WNF. The assessment has been performed following a methodology composed of information collection and compilation, expert judgement on each criterion at individual and, if no consensus was reached before, also at collective level. The output is composed of the categorical answer, and for the questions where no consensus was reached, the different supporting views are reported. Details on the methodology used for this assessment are explained in a separate opinion. According to the assessment performed, WNF can be considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL. The disease would comply with the criteria as in Sections 2 and 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in points (b) and (e) of Article 9(1). The animal species to be listed for WNF according to Article 8(3) criteria are several orders of birds and mammals as susceptible species and several families of birds as reservoir. Different mosquito species can serve as vectors.
Keywords: West Nile fever, WNF, West Nile virus, WNV, Animal Health Law, listing, categorisation, impact
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The background and Terms of Reference (ToR) as provided by the European Commission for the present document are reported in Section 1.2 of the scientific opinion on the ad hoc methodology followed for the assessment of the disease to be listed and categorised according to the criteria of Article 5, Annex IV according to Article 9, and 8 within the Animal Health Law (AHL) framework (EFSA AHAW Panel, 2017a).
1.2. Interpretation of the Terms of Reference
The interpretation of the ToR is as in Section 1.2 of the scientific opinion on the ad hoc methodology followed for the assessment of the disease to be listed and categorised according to the criteria of Article 5, Annex IV according to Article 9, and 8 within the AHL framework (EFSA AHAW Panel, 2017a).
The present document reports the results of assessment on West Nile fever (WNF) according to the criteria of the AHL articles as follows:
Article 7: West Nile fever profile and impacts
Article 5: eligibility of West Nile fever to be listed
Article 9: categorisation of West Nile fever according to disease prevention and control rules as in Annex IV
Article 8: list of animal species related to West Nile fever.
2. Data and methodologies
The methodology applied in this opinion is described in detail in a dedicated document about the ad hoc method developed for assessing any animal disease for the listing and categorisation of diseases within the AHL framework (EFSA AHAW Panel, 2017a).
3. Assessment
3.1. Assessment according to Article 7 criteria
This section presents the assessment of WNF according to the Article 7 criteria of the AHL and related parameters (see Table 2 of the opinion on methodology (EFSA AHAW Panel, 2017a)), based on the information contained in the fact sheet as drafted by the selected disease scientist (see Section 2.1 of the scientific opinion on the ad hoc methodology) and amended by the AHAW Panel.
Table 2.
Duration of infection period in experimentally infected birds
| Species | Viraemia duration | Cloacal and oropharyngeal WNV shedding | Inoculum (WNV isolate, dose and inoculation route) | Challenge dose | Reference |
|---|---|---|---|---|---|
| Rock pigeons (Columbia livia) | 2 days (viraemia) | 15 dpi | 3 WNV Italian isolates (L1*) (IT/2009‐IT/2011‐IT/2012) 1 mL subcutaneously | 106 TCID50/mL | Spedicato et al. (2016) |
| Red‐legged partridge (Alectoris rufa) | 4 days (viraemia) | 7 dpi | 1 WNV Morocco isolate (Mo/03) (L1) 1 WNV Spanish isolate (SP/07)(L1) 0.1 mL subcutaneously | 104 PFU/bird | Sotelo et al. (2011b) |
| House sparrows (Passer domesticus) | 3 days (viraemia) | 12 dpi | 2 WNV Italian isolates (IT/2008 and IT/2009)(L1) 1 WNV Spanish isolate (SP/07)(L1) 1 WNV US isolate (NY99)(L1) 0.1 mL subcutaneously | 104 PFU/bird | Del Amo et al. (2014) |
| Gyrfalcons (Falco rusticolus) | 4–6 days (viraemia) | 21 dpi | 1 WNV US isolate (NY99)(L1) 1WNV Austrian isolate (Aus/09)(L2*) 1 mL subcutaneously | Low dose: 500 TCID50/mL Medium dose: 104 TCID50/mL High dose: 106 TCID50/mL | Ziegler et al. (2013) |
WNV: West Nile virus; TCID50: tissue culture infective dose, median; PFU: plaque‐forming unit.
3.1.1. Article 7(a) Disease Profile
West Nile virus (WNV) belongs to the Flaviviridae family, genus Flavivirus, and is included in the serocomplex of Japanese Encephalitis virus together with Murray Valley encephalitis (MVE), St. Louis encephalitis (SLE), Kunjin (KUN), Usutu (USU), Koutango (KOU), Cacipacore (CPC), Alfuy (ALF) and Yaounde (YAO) viruses. Apart from Usutu virus, the other viruses in the serocomplex are not present in Europe. The virus was isolated for the first time in 1937 in Uganda, from the blood of a woman with febrile symptoms who came from the West Nile district (hence the name West Nile fever).
Different genetic lineages have been identified worldwide but the strains responsible for serious epidemics are attributable to Lineage 1 and, more recently, also to Lineage 2. Phylogenetic analyses revealed that all European WNV lineage 1 and 2 strains are derived from a limited number of independent introductions, most likely from Africa, followed by local spread and evolution. Other lineages have been identified but not associated so far with human or animal diseases.
WNV is transmitted by different genera and species of mosquitoes. The main vectors are some of the species of ornithophilic mosquitoes belonging to the genus Culex, which is always closely associated with the transmission of WNV during outbreaks. The mosquitoes cease their activity during the colder months, but it has been demonstrated that the virus is able to survive during this period in the infected mosquitoes, which overwinter indoors.
3.1.1.1. Article 7(a)(i) Animal species concerned by the disease
Susceptible animal species
Parameter 1 – Naturally susceptible wildlife species (or family/orders)
Several orders of birds can be naturally susceptible to WNV infections, i.e. Anseriformes, Apodiformes, Caprimulgiformes, Casuariiformes, Charadriiformes, Ciconiformes, Columbiformes, Coraciiformes, Cuculiformes, Falconiformes, Galliformes, Gaviformes, Gruiformes, Musophagiformes, Passeriformes, Pelecaniformes, Piciformes, Podicipediformes, Psittaciformes, Spheniscformes, Strigiformes and Struthioniformes.
Also several orders of mammals can be naturally susceptible to WNV infections, i.e. Artiodactyla, Carnivora, Chiroptera, Perissodactyla, Primates, Proboscidea and Rodentia.
Two orders of reptiles can be naturally susceptible to WNV infections: Crocodylia and Squamata.
Details concerning the susceptible families and species of the above mentioned orders are listed in Table A.1 in Appendix A.
Table A.1.
Naturally susceptible wildlife species (or family/orders)
| Class | Order | Family | Species |
|---|---|---|---|
| Aves | Anseriformes | Anatidae | Wood duck‐Aix sponsa, Eurasian wigeon‐Anas penelope (c), bronze‐winged duck (spectacled duck)‐Anas specularis (c), canvasback‐Aythya valisineria, Canada goose‐Branta Canadensis, barnacle goose‐Branta leucopsis (c)(a), emperor goose‐Chen canagica (c), greater Magellan goose (Andean goose)‐Chloephagapicta leucoptera (c)(a), Abyssinian blue‐winged goose‐Cyanochen cyanopterus (c)(a), tundra swan‐Cygnus columbianus (c), trumpeter swan‐Cygnus Cygnus buccinator (c)(a), mute swan‐Cygnus olor, Rosy‐billed pichard‐Netta peposaca (c)(a), ruddy duck‐Oxyura jamaicensis |
| Apodiformes | Apodidae | Chimney swift‐Chaetura pelagica | |
| Trochilidae | Ruby‐throated hummingbird‐Archilochus colubris | ||
| Caprimulgiformes | Caprimulgidae | Common nighthawk‐Chordeiles minor | |
| Casuariiformes | Dromaiidae | Emu‐Dromaius novaehollandiae (c) | |
| Charadriiformes | Haradriidae | Ruddy turnstone‐Arenaria interpres, killdeer‐Charadrius vociferous, piping plover‐Charadrius melodus | |
| Laridae | European herring gull‐Larus argentatus, laughing gull‐Larus atricilla, ring‐billed gull‐Larus delawarensis, great black‐backed gull‐Larus marinus, black skimmer‐Rhynchops niger, grey gull‐Larus modestus (c)(a), Inca tern‐Larosterna inca (c)(a) | ||
| Ciconiformes | Ardeidae | Yellow‐crowned night‐heron‐Nyctanassa violacea (c), black‐crowned night‐heron‐Nycticorax nycticorax (c), great blue heron‐Ardea Herodias, green heron‐Butorides virescens, least bittern‐Ixobrychus exilis | |
| Cathartidae | Turkey vulture‐Cathartes aura, black vulture‐Coragyps atratus, king vulture‐Sarcoramphus papa (c)(a) | ||
| Ciconiidae | Saddle‐billed stork‐Ephippiorhynchos senegalensis (c)(a), marabou stork‐Leptopilos crumeniferus (c)(a), lesser adjutant‐Leptoptilos javanicus (c)(a) | ||
| Phoenicopteridae | Chilean flamingo‐Phoenicopterus chilensis (c), greater flamingo‐Phoenicopterus ruber ruber (c) | ||
| Threskiornithidae | Scarlet ibis‐Eudocimus ruber (c), northern bald ibis‐Geronticus eremita (c)(a) | ||
| Columbiformes | Columbidae | White‐crowned pigeon‐Columba leucocephala, rock dove‐Columba livia, Mauritius pink pigeon‐Columba mayeri (c)(a), common ground‐dove‐Columbina passerine, Eurasian collared‐dove‐Streptopelia decaocto, white‐winged dove‐Zenaida asiatica, mourning dove‐Zenaida macroura, Luzon bleeding‐heart‐Gallicolumba luzonica (c)(a), Inca dove‐Columbina inca | |
| Coraciiformes | Alcedinidae | Belted kingfisher‐Ceryle alcyon | |
| Cuculiformes | Cuculidae | Yellow‐billed cuckoo‐Coccyzus americanus | |
| Falconiformes | Accipitridae | Cooper's hawk‐Accipiter cooperii, Northern goshawk‐Accipiter gentilis, sharp‐shinned hawk‐Accipiter striatus, golden eagle‐Aquila chrysaetos, red‐tailed hawk‐Buteo jamaicensis, rough‐legged hawk‐Buteo lagopus (c), red‐shouldered hawk‐Buteo lineatus, broad‐winged hawk‐Buteo platypterus, Swainson's hawk‐Buteo swainsoni, Northern harrier‐Circus cyaneus, swallow‐tailed kite‐Elanoides forficatus, bald eagle‐Haliaeetus leucocephalus, Mississippi kite‐Ictinia mississippiensis, Osprey‐Pandion haliaetus, Harris's hawk‐Parabuteo unicinctus (c) | |
| Falconidae | Merlin‐Falco columbarius, prairie falcon‐Falco mexicanus, peregrine falcon‐Falco peregrinus, American kestrel‐Falco sparverius | ||
| Galliformes | Numididae | Crested guineafowl‐Guttera pucherani (c)(a) | |
| Odontophoridae | Northern bobwhite‐Colinus virginianus | ||
| Phasianidae | Chukar‐Alectoris chukar (c)(a), ruffed grouse‐Bonasa umbellus, green junglefowl‐Gallus varius (c)(a), Himalayan monal‐Lophophorus impeyanus (c), Bulwer's pheasant‐Lophura bulweri (c)(a), ring‐necked pheasant‐Phasianus colchicus, mountain peacock‐pheasant‐Polypectron inopinatum (c)(a), crested partridge‐Rollulus roulroul (c)(a), Blyth's tragopan‐Tragopan blythii (c), argus pheasant (unspecified)‐various (c)(a), greater sage grouse‐Centrocerus urophasianus | ||
| Gaviiformes | Gaviidae | Common loon‐Gavia immer | |
| Gruiformes | Gruidae | Demoiselle crane‐Anthropoides virgo (c)(a), West African crowned crane‐Balearica pavonina pavonina (a), wattled crane‐Bugeranus carunculatus (c)(a), whooping crane‐Grus americana (c)(a), Mississippi sandhill crane‐Grus canadensis pulla (c), red‐crowned crane‐Grus japonensis (c)(a), Siberian crane‐Grus leucogeranus (c)(a), hooded crane‐Grus monacha (c)(a), white‐naped crane‐Grus vipio (c)(a) black‐necked crane‐Grus nigricollis (c)(a) | |
| Rallidae | Virginia rail‐Rallus limicola | ||
| Musophagiformes | Musophagidae | Lady Ross's turaco‐Musophaga rossae (c)(a) | |
| Passeriformes | Bombycillidae | Cedar waxwing‐Bombycilla cedrorum | |
| Cardinalidae | Northern cardinal‐Cardinalis cardinalis, blue grosbeak‐Guiraca caerulea (a), rose‐breasted grosbeak‐Pheucticus ludovicianus, dickcissel‐Spiza americana | ||
| Corvidae | Western scrub‐jay‐Aphelocoma californica, American crow‐Corvus brachyrhynchos, common raven‐Corvus corax, fish crow‐Corvus ossifragus, blue jay‐Cyanocitta cristata, Steller's jay‐Cyanocitta stelleri, black‐billed magpie‐Pica hudsonia (c) | ||
| Emberizidae | Song sparrow‐Melospiza melodia, savannah sparrow‐Passerculus sandwichensis, fox sparrow‐Passerella iliaca, Eastern towhee‐Pipilo erythrophthalmus, field sparrow‐Spizella pusilla | ||
| Estrildidae | Zebra finch‐Taeniophygia guttata (c) | ||
| Fringillidae | American goldfinch‐Carduelis tristis, house finch‐Carpodacus mexicanus, purple finch‐Carpodacus purpureus, evening grosbeak‐Coccothraustes vespertinus, European goldfinch‐Carduelis carduelis (c) | ||
| Hirundinidae | Barn swallow‐Hirundo rustica, purple martin‐Progne subis, tree swallow‐Tachycineta bicolor | ||
| Icteridae | Red‐winged blackbird‐Agelaius phoeniceus, rusty blackbird‐Euphagus carolinus, Brewer's blackbird‐Euphagus cyanocephalus, Baltimore oriole‐Icterus galbula, brown‐headed cowbird‐Molothrus ater, boat‐tailed grackle‐Quiscalus major, great‐tailed grackle‐Quiscalus mexicanus, common grackle‐Quiscalus quiscula | ||
| Laniidae | Loggerhead shrike‐Lanius ludovicianus | ||
| Mimidae | Gray catbird‐Dumetella carolinensis, Northern mockingbird‐Mimus polyglottos, brown thrasher‐Toxostoma rufum | ||
| Paridae | Tufted titmouse‐Baeolophus bicolor, varied tit‐Parus varius (c), black‐capped chickadee‐Poecile atricapilla, Carolina chickadee‐Poecile carolinensis | ||
| Parulidae | Black‐throated blue warbler‐Dendroica caerulescens, yellow‐rumped warbler‐Dendroica coronate, yellow warbler‐Dendroica petechial, blackpoll warbler‐Dendroica striata, common yellowthroat‐Geothlypis trichas, Kentucky warbler‐Oporornis formosus, Northern parula‐Parula Americana, ovenbird‐Seiurus aurocapillus, Northern waterthrush‐Seiurus noveboracensis, Nashville warbler‐Vermivora ruficapilla, Canada warbler‐Wilsonia Canadensis, hooded warbler‐Wilsonia citrina | ||
| Passeridae | House sparrow‐Passer domesticus | ||
| Sylviidae | White‐crested laughingthrush‐Garrulax leucolophus (c)(a) | ||
| Sittadae | White‐breasted nuthatch‐Sitta carolinensis | ||
| Sturnidae | European starling‐Sturnus vulgaris | ||
| Thraupidae | Palm tanager‐Thraupis palmarum (c) | ||
| Troglodytidae | Carolina wren‐Thryothaurus ludovicianus, winter wren‐Troglodytes troglodytes | ||
| Turdidae | Veery‐Catharus fuscescens, hermit thrush‐Catharus guttatus, gray‐cheeked thrush‐Catharus minimus, Swainson's thrush‐Catharus ustulatus, wood thrush‐Hylocichla mustelina, Eastern bluebird‐Sialia sialis, American robin‐Turdus igratorius | ||
| Tyrannidae | Traill's flycatcher‐Empidonax traillii/alnorum, Eastern phoebe‐Sayornis phoebe, scissor‐tailed flycatcher‐Tyrannus forficatus, Eastern kingbird‐Tyrannus tyrannus | ||
| Vireonidae | Black‐whiskered vireo‐Vireo altiloquus, warbling vireo‐Vireo gilvus, red‐eyed vireo‐Vireo olivaceus | ||
| Pelecaniformes | Pelecanidae | American white pelican‐Pelecanus erythrorhynchos, brown pelican‐Pelicanus occidentalis (c)(a), double‐crested cormorant‐Phalacrocorax auritus, guanay cormorant‐Phalacrocorax bougainvillei (c) | |
| Piciformes | Picidae | Red‐headed woodpecker‐Melanerpes erythrocephalus, downy woodpecker‐Picoides pubescens, yellow‐bellied sapsucker‐Sphyrapicus varius | |
| Podicipediformes | Podicipedidae | Pied‐billed grebe‐Podilymbus podiceps | |
| Psittaciformes | Cacatuidae | Cockatoo (unspecified)‐Cacatua spp. (c), cockatiel‐Nymphicus hollandicus (c) | |
| Psittacidae | Red‐crowned parrot‐Amazona viridigenalis (c), macaw (unspecified)‐Ara spp. (c), budgerigar‐Melopsittacus undulatus (c), lorikeet spp.‐Tricheglossus spp. (c) | ||
| Sphenisciformes | Spheniscidae | African penguin‐Spheniscus demersus (c), Magellan penguin‐Spheniscus humboldti (c)(a) | |
| Strigiformes | Strigidae | Northern saw‐whet owl‐Aegolius acadicus, boreal owl‐Aegolius funereous (c), short‐eared owl‐Asio flammeus, Verreaux's eagle owl (milky eagle owl)‐Bubo lacteus (c)(a), great horned owl‐Bubo virginianus, snowy owl‐Nyctea scandiaca (c), Eastern screech owl‐Otus asio, tawny owl‐Strix aluco (c), great grey owl‐Strix nebulosa (c), spotted owl‐Strix occidentalis (c), barred owl‐Strix varia, Northern hawk owl‐Surnia ulula (c) | |
| Tytonidae | Barn owl‐Tyto alba | ||
| Struthioniformes | Struthionidae | Ostrich‐Struthio camelis (c)(a) | |
| Mammalia | Artiodactyla | Bovidae | Mountain goat‐Oreamnos americanus (c) |
| Camelidae | Llama‐Lama glama (c), alpaca‐Lama pacos (c) | ||
| Cervidae | White‐tailed deer‐Odocoileus virgninianus, reindeer‐Rangifer tarnadus (c), mule deer‐Odocoileus hemionus | ||
| Suidae | Babirusa‐Babyrousa babyrousa (c)(a) | ||
| Carnivora | Canidae | Timber wolf‐Canis lupus (c) | |
| Mustelidae | Striped skunk‐Mephitis mephitis | ||
| Phocidae | Harbor seal‐Phoca vitulina (c) | ||
| Procyonidae | Red panda‐Ailurus fulgens fulgens (c)(a) | ||
| Ursidae | Black bear‐Ursus americanus (a) | ||
| Chiroptera | Vespertilionidae | Big brown bat‐Eptesicus fuscus, little brown bat‐Myotis lucifugus | |
| Perissodactyla | Rhinocerotidae | Great Indian rhinoceros‐Rhinoceros unicornis (c)(a) | |
| Primates | Cercopithecidae | Barbary macaque‐Macaca sylvanus (c) | |
| Lemuridae | Ring‐tailed lemur‐Lemur catta (c) | ||
| Proboscidea | Elephantidae | Indian (Asian) elephant‐Elephas maximus indicus (c)(a) | |
| Rodentia | Sciuridae | Gray squirrel‐Sciurus carolinensis, fox squirrel‐Sciurus niger, Eastern chipmunk‐Tamias striatus | |
| Reptilia | Crocodylia | Alligatoridae | American alligator‐Alligator mississippiensis (c) |
| Squamata | Varanidae | Crocodile monitor‐Varanus salvadorii (c)(a) |
(c) Denotes either a captive or farmed animal(s). Virus or viral RNA was detected in animal tissue unless followed by an (a), which denotes detectable antibodies only have been reported (Source: USGS, National Wildlife Health Center (USGS, online)).
Parameter 2 – Naturally susceptible domestic species (or family/orders)
Several families of domestic animals can be naturally susceptible to WNV infections, i.e. Phasianidae, Anatidae, Bovidae, Canidae, Felidae, Leporidae and Equidae.
Details concerning the susceptible species of the above mentioned families are listed in Table A.2 in Appendix A.
Table A.2.
Naturally susceptible domestic species (or family/orders)
| Class | Order | Family | Species |
|---|---|---|---|
| Aves | Galliformes | Phasianidae |
Domestic chicken (Red junglefowl)‐Gallus gallus Turkey (domestic and wild)‐Meleagris gallopavo |
| Anseriformes | Anatidae |
Mallard‐Anas platyrhynchos Domestic goose‐Anser chinensis (c)(a) |
|
| Mammalia | Artiodactyla | Bovidae |
Domestic cattle‐Bos taurus Domestic (suffolk) sheep‐Ovis aries |
| Carnivora | Canidae | Domestic dog‐Canis familiaris | |
| Felidae | Domestic cat (feral)‐Felis catus | ||
| Lagomorpha | Leporidae | Domestic rabbit‐Oryctolagus cuniculus | |
| Perissodactyla | Equidae |
Domestic horse‐Equus equus przewalski caballus Donkey‐Equus asinus Mule |
(c) Denotes either a captive or farmed animal(s). Virus or viral RNA was detected in animal tissue unless followed by an (a), which denotes detectable antibodies only have been reported (Source: USGS, National Wildlife Health Center (USGS, online)).
Parameter 3 – Experimentally susceptible wildlife species (or family/orders)
Several wild birds of the orders Passeriformes, Falconiformes, Accipitriformes, Strigiformes, Galliformes, Pelecaniformes, Columbiformes, Gruiformes, Anseriformes, Charadriiformes, Psittaciformes and Piciformes were successfully infected (see Table A.3 in Appendix A for the outcomes of experimental infections of WNV performed in wild birds (adapted from Pérez‐Ramírez et al. (2014) (Pérez‐Ramírez et al., 2014)).
Table A.3.
Summary outcomes of experimental infections of West Nile virus performed in wild birds (adapted from Pérez‐Ramírez et al. (2014))
| Order | Family | Species | Strain | Mortality | Viraemia | Distribution | References |
|---|---|---|---|---|---|---|---|
| Passeriformes | Turdidae | American robin (Turdus migratorius) | NY | < 20% | H | AM | Komar et al. (2003), VanDalen et al. (2013) |
| Swainson's thrush (Catharus ustulatus) | NY | < 20% | M | AM | Owen et al. (2006) | ||
| Clay‐coloured thrush (Turdus grayi) | TEC/TAB | 20–50%/< 20% | M | AM | Guerrero‐Sánchez et al. (2011) | ||
| Corvidae | Carrion crow (Corvus corone) | FR/ISR | 20–50%/> 50% | L | EUR/ASIA | Dridi et al. (2013) | |
| American crow (Corvus brachyrhynchos) | NY/TEX/MEX | > 50% | H | AM | McLean et al. (2001), Komar et al. (2003), Brault et al. (2004), Weingartl et al. (2004), Kinney et al. (2006), Kipp et al. (2006), Brault et al. (2007, 2011), Nemeth et al. (2011) | ||
| KEN/KUN | 20–50%/< 20% | M | |||||
| Fish crow (Corvus ossifragus) | NY | > 50% | H | AM | Komar et al. (2003), Kipp et al. (2006), Nemeth et al. (2011) | ||
| Little raven (Corvus mellori) | NY | < 20% | M | OCE | Bingham et al. (2010) | ||
| KUN | < 20% | L | |||||
| Hooded crow (Corvus cornix) | EGY | > 50% | H | EUR/ASIA/AFR | Work et al. (1955) | ||
| Western scrub‐jay (Aphelocoma californica) | NY | > 50% | H | AM | Reisen et al. (2005) | ||
| Blue jay (Cyanocitta cristata) | NY | > 50% | H | AM | Komar et al. (2003), Weingartl et al. (2004) | ||
| Black‐billed magpie (Pica hudsonia) | NY | > 50% | H | AM | Komar et al. (2003) | ||
| Jungle crow (Corvus macrorhynchos) | NY | > 50% | H | ASIA | Shirafuji et al. (2008) | ||
| Passeridae | House sparrow (Passer domesticus) | NY/CA/KEN/EGY/TAB/TEC/SP/IT09 | > 50% | H | WORLDWIDE | Work et al. (1955), Komar et al. (2003, 2005), Langevin et al. (2005), Reisen et al. (2005, 2006), Nemeth et al. (2008), LaPointe et al. (2009); Nemeth et al. (2009a,b), Brault et al. (2011), Guerrero‐Sánchez et al. (2011), Wheeler et al. (2012), Del Amo et al. (2014) | |
| TEX/KUN/IT08 | < 20% | M | |||||
| MEX | < 20% | L | |||||
| Cape sparrow (Passer melanurus) | SA* | Und | L | AFR | McIntosh et al. (1969) | ||
| Icteridae | Red‐winged blackbird (Agelaius phoeniceus) | NY | < 20% | M/L | AM | Komar et al. (2003), Reisen and Hahn (2007), Nemeth et al.(2009b) | |
| Brown‐headed cowbird (Molothrus ater) | NY | < 20% | L | AM | Reisen et al. (2006), Reisen and Hahn (2007) | ||
| Brewer's blackbird (Euphagus cyanocephalus) | NY | < 20% | H | AM | Reisen et al. (2006), Reisen and Hahn (2007) | ||
| Tricolored blackbird (Agelaius tricolor) | NY | < 20% | H | AM | Reisen and Hahn (2007) | ||
| Common grackle (Quiscalus quiscula) | NY | 20–50% | H | AM | Komar et al. (2003) | ||
| Great‐tailed grackle (Quiscalus mexicanus) | TAB/TEC | > 50%/20–50% | H | AM | Guerrero‐Sánchez et al. (2011) | ||
| Bay‐winged cowbird (Agelaioides badius) | ARG | < 20% | L | AM | Diaz et al. (2011) | ||
| Shiny cowbird (Molothrus bonariensis) | ARG | < 20% | L | AM | Diaz et al. (2011) | ||
| Emberizidae | Song sparrow (Melospiza melodia) | NY | < 20% | M | AM | Reisen and Fang (2007) | |
| White‐crowned sparrow (Zonotrichia leucophrys) | NY | Und | na | AM | Reisen et al. (2006) | ||
| Fringillidae | Hawai'i ‘amakihi (Hemignathus virens) | NY | 20–50% | H | AM | LaPointe et al. (2009) | |
| House finch (Haemorhous mexicanus) | NY | > 50% | H | AM | Komar et al. (2003), Reisen et al. (2005), Fang and Reisen (2006), Reisen et al. (2006) | ||
| Ploceidae | African masked weaver (Ploceus velatus) | SA* | Und | M | AFR | McIntosh et al. (1969) | |
| Red‐billed quelea (Quelea quelea) | SA* | Und | L | AFR | McIntosh et al. (1969) | ||
| Red bishop (Euplectes orix) | SA* | Und | M | AFR | McIntosh et al. (1969) | ||
| Hirundinidae | Cliff swallow (Petrochelidon pyrrhonota) | NY | < 20% | M | AM | Oesterle et al. (2009, 2010) | |
| Mimidae | Gray catbird (Dumetella carolinensis) | NY | < 20% | M | AM | Owen et al. (2006) | |
| Northern mockingbird (Mimus polyglottos) | NY | < 20% | H | AM | Komar et al. (2005) | ||
| Sturnidae | European starling (Sturnus vulgaris) | NY | < 20% | M | WORLDWIDE | Komar et al. (2003); Reisen et al. (2006) | |
| Cardinalidae | Northern cardinal (Cardinalis cardinalis) | NY | < 20% | H | AM | Komar et al. (2005); Owen et al. (2012) | |
| Paridae | Tufted titmouse (Baeolophus bicolor) | NY | > 50% | H | AM | Kilpatrick et al. (2013) | |
| Troglodytidae | Carolina wren (Thryothorus ludovicianus) | NY | 20–50% | H | AM | Kilpatrick et al. (2013) | |
| Falconiformes | Falconidae | Gyrfalcon (Falco rusticolus) | AUS* | 20–50% | H | AM/EUR/AS | Ziegler et al. (2013) |
| NY | 20–50% | M | |||||
| Hybrid falcon (Falco rusticolus x Falco cherrug) | NY | < 20% | L | WORLDWIDE | Busquets et al. (2012) | ||
| American kestrel (Falco sparverius) | NY | < 20% | H | AM | Komar et al. (2003), Nemeth et al. (2006a) | ||
| Common kestrel (Falco tinnunculus) | EGY | < 20% | L | EUR/AS/AFR | Work et al. (1955) | ||
| Accipitriformes | Accipitridae | Red‐tailed hawk (Buteo jamaicensis) | NY | < 20% | H | AM | Nemeth et al. (2006a) |
| Strigiformes | Tytonidae | Barn owl (Tyto alba) | NY | < 20% | L | WORLDWIDE | Nemeth et al. (2006a) |
| Strigidae | Great horned owl (Bubo virginianus) | NY | < 20% | H | AM | Komar et al. (2003), Nemeth et al. (2006a) | |
| Eastern screech‐owl (Megascops asio) | NY | > 50% | H | AM | Nemeth et al. (2006a) | ||
| Galliformes | Odontophoridae | California quail (Callipepla californica) | NY | < 20% | L | AM | Reisen et al. (2005, 2006) |
| Gambel's quail (Callipepla gambelii) | NY | < 20% | L | AM | Reisen et al. (2006) | ||
| Northern bobwhite (Colinus virginianus) | NY | < 20% | L | AM | Komar et al. (2003) | ||
| Phasianidae | Red‐legged partridge (Alectoris rufa) | SP/MO | 20–50%/> 50% | H | EUR | Sotelo et al. (2011b) | |
| NY | > 50% | L | Escribano‐Romero et al. (2013) | ||||
| Japanese quail (Coturnix japonica) | NY | < 20% | L | WORLDWIDE | Komar et al. (2003) | ||
| Ring‐necked pheasant (Phasianus colchicus) | NY | < 20% | L | WORLDWIDE | Komar et al.(2003) | ||
| Greater sage‐grouse (Centrocercus urophasianus) | NY | > 50% | M | AM | Clark et al. (2006) | ||
| Pelecaniformes | Ardeidae | Rufous night‐heron (Nycticorax caledonicus) | KUN | < 20% | L | OCE | Boyle et al. (1983b,a) |
| Little egret (Egretta garzetta) | KUN | < 20% | L | EUR/AS/AFR/OCE | Boyle et al. (1983a,b) | ||
| Intermediate heron (Mesophoyx intermedia) | KUN | < 20% | L | AFR/AS | Boyle et al. (1983a,b) | ||
| Cattle egret (Bubulcus ibis) | SA*/EGY | Und/< 20% | L | WORLDWIDE | Work et al. (1955); McIntosh et al. (1969) | ||
| Threskiornithidae | African sacred ibis (Threskiornis aethiopicus) | SA* | Und | L | AFR/AS | McIntosh et al. (1969) | |
| Columbiformes | Columbidae | Rock pigeon (Columba livia) | SA*/NY/TEC/TAB | Und/< 20% | L | WORLDWIDE | McIntosh et al. (1969); Guerrero‐Sánchez et al. (2011) |
| Ring‐necked dove (Streptopelia capicola) | SA* | Und | L | AFR | McIntosh et al. (1969) | ||
| Eurasian collared‐dove (Streptopelia decaocto) | NY/CO | < 20%/< 20% | M | AM/EUR/AS/AFR | Panella et al. (2013) | ||
| Laughing dove (Spilopelia senegalensis) | SA*/EGY | Und/< 20% | L | AFR/AS | Work et al. (1955), McIntosh et al. (1969) | ||
| Common ground‐dove (Columbina passerina) | NY | Und | na | AM | Reisen et al. (2006, 2008) | ||
| Mourning dove (Zenaida macroura) | NY | < 20% | M | AM | Komar et al. (2003), Reisen et al. (2005, 2006) | ||
| Picui ground‐dove (Columbina picui) | ARG | < 20% | M | AM | Diaz et al. (2011) | ||
| Gruiformes | Rallidae | American coot (Fulica americana) | NY | < 20% | L | AM | Komar et al. (2003) |
| Crested coot (Fulica cristata) | SA* | Und | L | AFR/EUR | McIntosh et al. (1969) | ||
| Gruidae | Sandhill crane (Grus canadensis) | NY | < 20% | L | AM | Olsen et al. (2009) | |
| Anseriformes | Anatidae | Common goose (Anser anser) | SA* | > 50% | M | WORLDWIDE | Banet‐Noach et al. (2003) |
| Canada goose (Branta canadensis) | NY | < 20% | M | AM/EUR | Komar et al. (2003) | ||
| Mallard (Anas platyrhynchos) | NY | < 20% | H | WORLDWIDE | Komar et al. (2003) | ||
| Yellow‐billed duck (Anas undulata) | SA* | Und | L | AFR | McIntosh et al. (1969) | ||
| Red‐billed teal (Anas erythrorhyncha) | SA* | Und | L | AFR | McIntosh et al. (1969) | ||
| Southern pochard (Netta erythrophthalma) | SA* | Und | L | AFR | McIntosh et al. (1969) | ||
| Charadriiformes | Charadriidae | Killdeer (Charadrius vociferus) | NY | < 20% | H | AM | Komar et al. (2003) |
| Laridae | Ring‐billed gull (Larus delawarensis) | NY | > 50% | H | AM | Komar et al. (2003) | |
| Psittaciformes | Psittacidae | Monk parakeet (Myiopsitta monachus) | NY | < 20% | L | AM | Komar et al. (2003) |
| Budgerigar (Melopsittacus undulatus) | NY | < 20% | L | OCE | Komar et al. (2003) | ||
| Piciformes | Picidae | Northern flicker (Colaptes auratus) | NY | < 20% | M | AM | Komar et al. (2003) |
CA: California 04; NY: New York 99; CO: Colorado 08; SA: South Africa; ARG: Argentina 06; EGY: Egypt; KUN: Kunjin; SP: Spain 07; MO: Morocco 03; AUS: Austria 09; MEX: Mexico 03; TEX: Texas 03; KEN: Kenya 3829; FR: France 00; ISR: Israel 98; TEC: Tecato (Mexico); TAB: Tabasco (Mexico); IT08: Italy 08; IT09: Italy 09.* Lineage 2.
L: Low viraemia (mean peak viraemia ≤ 104 PFU/mL); M: Medium viraemia (mean peak viraemia 104–106 PFU/mL); H: High viraemia (mean peak viraemia > 106 PFU/mL); na: Data not available.
AFR: Africa; AM: America; AS: Asia; EUR: Europe; OCE: Oceania.
Und: Undetermined.
Parameter 4 – Experimentally susceptible domestic species (or family/orders)
Table A.4 in Appendix A lists the outcomes of experimental infections of WNV performed in domestic animal species. Infections have been successfully established in cats, dogs, horses, pigs, rabbits and sheep.
Table A.4.
Summary outcomes of systematic review of experimental infections of domestic animals with WNV (papers published up to January 2016)
| Species | References | Number of animal groupsa | Agent detectionb | Observation of clinical signsc | Clinical signs (and number of groups in which were reported) | ||
|---|---|---|---|---|---|---|---|
| Min day | Max day | Min day | Max day | ||||
| Cats | Austgen et al. (2004) | 3 (19 animals) | Virus isolation from blood: 1 (0.5–3) | Virus isolation from blood: 7 (4.5–8) | 1 | 6 | No clinical signs observed (2), fever (1), depression/apathy (1) |
| 0 dead animals | |||||||
| Dogs | Austgen et al. (2004), Karaca et al. (2005) | 2 (19 animals) | Virus isolation from blood: 1.3 (0.5–2) | Virus isolation from blood: 5.3 (4.5–6) | 1 | 1 | No clinical signs observed (1), fever (1) |
| 0 dead animals | |||||||
| Horses | Bunning et al. (2002), Shirafuji et al. (2009), Castillo‐Olivares et al. (2011) | 4 (17 animals) | Virus isolation from blood (3 groups): 3(1–4) | Virus isolation from blood (3 groups): 6 (6–7) | 6.5 (3–8) | 10 (9–11) | No clinical signs observed (1), twitching/tremors (1), neurological signs (2), fever (1) |
| PCR from blood (1 group): 3 | PCR from blood (1 group): 7 | 1 dead animal in 1 group | |||||
| Pigs | Teehee et al. (2005) | 2 (12 animals) | Virus isolation from blood: 1.5 (1.5–4.5) | Virus isolation from blood: 5 (4.5–5) | Not reported | No clinical signs observed (1), not reported (1) | |
| 0 dead animals | |||||||
| Rabbits | Suen et al. (2015) | 2 (27 animals) | Not reported | 1 | Not reported | No clinical signs observed (1), fever (1) | |
| 0 dead animals | |||||||
| Sheep | Barnard and Voges (1986) | 1 (2 animals) | Virus isolation from blood: 3 | Virus isolation from blood: 11 | 3 | 3 | Fever |
ll data were analysed at animal group level, reflecting the animal groups followed and reported in the individual references. Some references reported more than one animal group.
Min = first day (in dpi) that pathogen/RNA was detected in a sample for each reported animal group; Max = last day (in dpi) that virus/RNA was detected in a sample for each reported animal group. Min and Max were recorded individually for each animal group, and median (min‐max) for each of those values were calculated from all group data (each group representing one observation, with no weighting based on the size of the animal groups). Contact transmission groups were not included in the summary.
Min = first day (in dpi) in which clinical signs were observed in each whole animal group reported; Max = last day (in dpi) in which clinical signs were observed in each whole animal group reported. Min and Max were recorded individually for each animal group, and median (min‐max) for each of those values were calculated from all group data (each group representing one observation, with no weighting based on the size of the animal groups). Contact transmission groups were not included in the summary.
Reservoir animal species
Parameter 5 – Wild reservoir species (or family/orders)
Several bird species, particularly passerine species (jays, finches, sparrows, and crows) can be potential reservoirs of WNV. House finches (Carpodacus mexicanus) and house sparrows (Passer domesticus) experimentally inoculated showed persistent infection in spleen and kidney 28 weeks p.i. (post infection). The virus was still detected by real time reverse transcription polymerase chain reaction (RT‐PCR) in the spleen of two house sparrows at 36 weeks p.i. However, viral isolation attempts were unsuccessful (Wheeler et al., 2012). In a previous work (Nemeth et al., 2009a), a higher number of organs were analysed in WNV‐infected house sparrows, and viral RNA was detected in juvenile sparrows up to 65 days p.i in kidney and spleen, although infectious virus could be isolated at low titres only in one sparrow at 43 days p.i. Reisen et al. (2006) confirmed the persistent infection in five species of Passeriformes and in common ground‐dove (Columbina passerina) detecting the virus in spleen and kidney, but also in lungs at > 6 weeks p.i. (Reisen et al., 2006).
Outside the United States of America (USA), clinical symptoms signs due to WNV infection have been reported in few cases and limited to scarce number of avian species in course of outbreaks: domestic geese (Anser anser domesticus) and white storks (Ciconia ciconia) during the WNV epidemic in Israel (Malkinson et al., 2002), goshawks (Accipiter gentilis) in Hungary (Bakonyi et al., 2006), Eurasian jays (Garrulus glandarius), little owl (Athene noctua), mallard (Anas plathyrynchos), and common buzzard (Buteo buteo) in Italy (Monaco et al., 2015). However, mass mortality of highly susceptible species (such as corvids or other species) is less frequently observed in the Old than in the New World although some species, as the jackdaws (Corvus monedula) could potentially function as sentinel (Lim et al., 2014). Surveillance activities carried out in Italy where WNV is endemic since 2008, pointed out the high susceptibility to the viral infection of three species of synantropic resident wild birds, namely carrion crow (Corvus corone), magpie (Pica pica) and Eurasian jay (Garrulus glandarius) which justifies their use as sentinel in endemic areas (Italian Ministry of Health, 2016).
Some species of mammals including squirrels (Sciurus sp.), eastern chipmunks (Tamias striatus) and eastern cottontail rabbits (Sylvilagus floridanus) may be capable of transmitting WNV to mosquitoes, although their epidemiological role importance as reservoir hosts is still uncertain.
Among reptiles, clinical signs were mainly reported during outbreaks in alligators, although there is also a report on neurological signs associated with WNV infection in a crocodile monitor (Varanus salvadori) lizard. Some infections in garter snakes (Thamnophis sirtalis) experimentally inoculated with WNV were also fatal. Green iguanas (Iguana iguana) can be infected.
Amphibians including lake frogs (Rana ridibunda) and North American bullfrogs (Rana catesbeiana) can also be infected with WNV. Some alligators (e.g. American alligators, Alligator mississippiensis) and frogs (e.g. Rana ridibunda in Russia) may develop viraemia sufficient to infect mosquitoes. As with mammals, their epidemiological importance as reservoir hosts is still uncertain.
Based on preliminary research carried out in Italy and Spain, only a few bird species seem to play a major role in the transmission of infection to the mosquitoes (Hamer et al., 2009; Munoz et al., 2012; Roiz et al., 2012; Spedicato et al., 2016). Unfortunately, the reservoir competence for many European bird species is still unknown even though the persistence of WNV in infected birds has been assessed in some species through experimental trials. Table B.1 in Appendix B provides an overview of wild and domestic WNV reservoir/sentinel animal species.
Table B.1.
List of wild and domestic WNV reservoir/sentinel animal species
| Family | Reservoir | Sentinel | Notes |
|---|---|---|---|
| Turdidae | ND | Y | Intense viraemia and clinical signs developed by infected birds |
| Corvidae | Potential | Y | Intense viraemia and clinical signs developed by the infected birds with high mortality |
| Passeridae | Y | Y | Intense and long viraemia and clinical signs developed by infected birds |
| Anatidae | – | Y | Intense viraemia and clinical signs developed by infected birds |
| Columbidae | Y | – | Common ground‐dove (Columbina passerina): WNV detection in spleen and kidney and lung at > 6 weeks p.i |
| Fringillidae | Y | – | Persistent infection in house finches (Haemorhous mexicanus) |
| Falconidae | – | Y | Intense viraemia and clinical signs developed by infected birds |
| Phasianidae | – | Y | Viraemia short and scarce, asymptomatic infection, detectable serological response |
| Laridae | – | Y | Intense viraemia and clinical signs developed by infected birds |
| Strigidae | Y | Intense viraemia and clinical signs developed by infected birds | |
| Equidae | – | Y | Viraemia short and scarce, development of clinical symptoms, detectable serological response |
| Canidae | – | Potential | Viraemia short and scarce, rare development of clinical symptoms, detectable serological response. Potential use as sentinel in urban areas |
| Felidae | – | Potential | Viraemia short and scarce, rare development of clinical symptoms, detectable serological response. Potential use as sentinel in urban areas |
Parameter 6 – Domestic reservoir species (or family/orders)
WNV has been associated with sporadic disease infection in small numbers of domestic animal species (see above in parameter 2 and Table A.2 in Appendix A); however, these species do not play a role in the further transmission of WNV to mosquitoes and are thus considered as dead‐end hosts. See also Table B.1 in Appendix B which lists wild and domestic WNV reservoir/sentinel animal species.
3.1.1.2. Article 7(a)(ii) The morbidity and mortality rates of the disease in animal populations
Parameter 1 – Prevalence/incidence
WNV has been found in all the continents from tropical to north temperate latitudes (Reisen, 2013). Table 1 lists the number of horses positive for WNV detections (either by immunoglobulin M enzyme‐linked immunosorbent assay (IgM‐ELISA) or PCR), reported to the Animal Diseases Notification System since between (ADNS) 2013 and 2016.
Table 1.
Number of horses positive for WNV reported to the ADNS
| Country | Year | Number of positive horses |
|---|---|---|
| France | 2015 | 46 |
| Italy | 2013 | 43 |
| Italy | 2014 | 19 |
| Italy | 2015 | 31 |
| Italy | 2016 | 53 |
| Greece | 2013 | 15 |
| Greece | 2014 | 4 |
| Spain | 2013 | 37 |
| Spain | 2014 | 12 |
| Spain | 2015 | 17 |
| Spain | 2016 | 79 |
| Portugal | 2015 | 9 |
| Portugal | 2016 | 6 |
| Austria | 2016 | 1 |
| Hungary | 2013 | 1 |
| Hungary | 2014 | 1 |
| Hungary | 2015 | 7 |
| Hungary | 2016 | 49 |
| Bulgaria | 2015 | 1 |
Source: Animal Diseases Notification System (2013–2016).
Table C.1 in Appendix C summarises the prevalence of cases reported to the OIE in Europe, namely in Portugal, Spain, France, Croatia, Greece, Romania, Former Yugoslav Republic of Macedonia and Bulgaria. Also, the cases in Italy reported to the Italian authorities are summarised in Table C.1.
Table C.1.
WNV morbidity and mortality rates in horses (2010–2016 EU outbreaks)
| Outbreaks in equids | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | Year | No outbreaks | No outbreaks with clinical symptoms | No horses present | No total cases | No horses with symptoms | Died/culled | Prevalence of infection | Case‐morbidity rate | Case‐fatality rate |
| Italy | 2008 | 273 | 18 | 1,941 | 563 | 32 | 5 | 29% | 2% | 1% |
| 2009 | 137 | 32 | 1,398 | 223 | 37 | 9 | 16% | 3% | 24% | |
| 2010 | 67 | 11 | 415 | 128 | 11 | 5 | 31% | 3% | 45% | |
| 2011 | 91 | 41 | 881 | 197 | 58 | 14 | 22% | 7% | 24% | |
| 2012 | 30 | 13 | 313 | 63 | 15 | 3 | 20% | 24% | 20% | |
| 2013 | 35 | 11 | 308 | 50 | 12 | 1 | 16% | 24% | 8% | |
| 2014 | 17 | 6 | 257 | 27 | 6 | 2 | 11% | 22% | 33% | |
| 2015 | 26 | 6 | 302 | 30 | 6 | 5 | 10% | 20% | 17% | |
| 2016a | 33 | 13 | 310 | 37 | 13 | 4 | 7% | 35% | 11% | |
| Portugal | 2016 | 1 | 1 | 2 | 1 | 1 | 0 | 50% | 50% | 0% |
| 2015 | 3 | 3 | 82 | 4 | 4 | 0 | 5% | 5% | 0% | |
| 2010 | 2 | 2 | 71 | 2 | 2 | 1 | 3% | 3% | 1% | |
| Spain | 2011 | 5 | Unknown | 44 | 11 | Unknown | 1 | 25% | Unknown | 9% |
| 2010 | 31 | 2 | 845 | 39 | 2 | 2 | 4% | 0% | 5% | |
| France | 2015 | 35 | 26 | 262 | 49 | 34 | 5 | 19% | 13% | 0–5,26% |
| 2006 | 4 | 1 | 63 | 4 | 1 | 1 | 6% | 2% | 25% | |
| Croatia | 2014 | 1 | 0 | 2 | 1 | 0 | 0 | 50% | 0% | 0% |
| 2012 | 11 | 0 | 87 | 12 | 0 | 0 | 14% | 0% | 0% | |
| Greece | 2014 | 4 | 0 | 51 | 4 | 0 | 0 | 8% | 0% | 0% |
| 2013 | 10 | 2 | 559 | 15 | 2 | 1 | 3% | 0% | 7% | |
| 2012 | 14 | 3 | 100 | 15 | 3 | 0 | 15% | 3% | 0% | |
| 2011 | 17 | 0 | 374 | 23 | 0 | 1b | 6% | 0% | 0% | |
| 2010 | 27 | 3 | 559 | 30 | 3 | 3 | 5% | 1% | 10% | |
| Romania | 2010 | 3 | Unknown | 9 | 6 | Unknown | 0 | 67% | Unknown | Unknown |
| Former Yugoslav Republic of Macedonia | 2011 | 4 | 0 | 51 | 10 | 0 | 0 | 20% | 0% | 0% |
| Bulgaria | 2010 | 2 | 0 | 118 | 8 | 0 | 0 | 7% | 0% | 0% |
2016 Italian data: updated to 14 October 2016.
Death may have been the result of conditions other than West Nile virus infection (possible snake bite reported).
Source: Italian National information system and (OIE, online).
Parameter 2 – Case‐morbidity rate (% clinically diseased animals out of infected ones)
WNF can cause disease in horses, several species of birds and rarely in other species such as camels, dogs, cats, sheep, squirrels and alligators (Go et al., 2014; Hubalek et al., 2014). In horses, the majority of the infections are asymptomatic, but some individuals (about 10%) can develop severe neurological illness (ataxia, weakness, recumbency and muscle fasciculation). Experimental infections have shown that the clinical picture of the disease can be quite divergent depending on the species. High susceptible species (e.g. corvids) can develop an hyperacute phase resulting in death without exhibiting symptoms, whereas other species (e.g. raptors, owls, Passeriformes) can develop only mild lesions with low mortality rates or chronic disease (Pérez‐Ramírez et al., 2014). The case‐morbidity rate in the outbreaks reported to the OIE and the Italian National Authorities are shown in Table C.1 in Appendix C.
Parameter 3 – Case‐fatality rate
The case‐fatality rate in the outbreaks in equids reported to the OIE and the Italian National Authorities are shown in Table C.1 in Appendix C.
LaDeau et al. (2007) demonstrated that the American crow population declined by up to 45% since WNV arrival in 1999 and only two of the seven species with documented impact recovered to pre‐WNV levels by 2005 (LaDeau et al., 2007).
3.1.1.3. Article 7(a)(iii) The zoonotic character of the disease
Presence
Parameter 1 – Report of zoonotic human cases (anywhere)
WNV zoonotic transmission is known to be present in Europe for many years: in the 1960s, the virus emerged in southern France in the Camargue. Yet, the first large outbreak in humans was reported from Bucharest, Romania in 1996–1997. Up to 2010, infection in humans and/or horses have been reported in the Czech Republic (1997), France (2000, 2003, 2004, 2006), Italy (1998, 2008, 2009), Hungary (2000–2009), Romania (1997–2001, 2003–2009), Spain (2004) and Portugal (2004). In 2010, a human outbreak was reported in northern Greece, and human cases were reported in Romania, Hungary, Italy, Spain and in Volgograd (Russian Federation). The number of human cases notified in Europe and in the Mediterranean Basin since 2010 is reported in Table 5.
3.1.1.4. Article 7(a)(iv) The resistance to treatments, including antimicrobial resistance
Parameter 1 – Resistant strain to any treatment even at laboratory level
This is not applicable to WNV since there is no specific antiviral therapy.
3.1.1.5. Article 7(a)(v) The persistence of the disease in an animal population or the environment
Animal population
Parameter 1 – Duration of infectious period in animals
Viral titres in blood equal or greater than 105 TCID50/mL have been considered able to infect competent mosquito species. In relation to viraemia duration, the following results of experimental infections in European bird species are reported:
Parameter 2 – Presence and duration of latent infection period
Evidence of persistent WNV infection has been demonstrated in experimentally infected monkeys (Pogodina et al., 1983) and hamsters (Tesh et al., 2005). WNV is also capable of long‐term persistence in human patients, particularly in the presence of chronic clinical symptoms (Murray et al., 2010). The importance of these persistent infections, however, needs still to be elucidated, as virus titres are low and these hosts are considered to be dead‐end hosts.
Parameter 3 – Presence and duration of the pathogen in healthy carriers
Refer to the data reported in Section 3.1.1.1 parameter 5.
Environment
Parameter 4 – Length of survival (dpi) of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment (scenarios: high and low T)
WNV is rapidly inactivated in the environment outside hosts. Low temperatures preserve infectivity, with stability being greatest below −60°C. It is inactivated by heat (50–60°C for at least 30 min), ultraviolet light, and gamma irradiation (Burke and Monath, 2001). The virus is also susceptible to disinfectants such as 3–8% formaldehyde, 2% glutaraldehyde, 2–3% hydrogen peroxide, 500–5,000 ppm available chlorine, alcohol, 1% iodine and phenol iodophors.
Data related to the persistence of the virus in the vectors are provided in Table 3.
Table 3.
Detailed outcomes of systematic review on survival time of WNV in mosquitoes at different temperatures (data extracted from (Turell et al., 2002))
| Matrix | Target | Test | Temperatures | Maximum detection |
|---|---|---|---|---|
| Mosquito | Nucleic acid | RT‐PCR | 4°, 20°, 70°C | 14 days |
| Mosquito | Virus | Culture | 4°, 20°, 70°C | 2 days |
RT‐PCR: reverse transcription polymerase chain reaction.
3.1.1.6. Article 7(a)(vi) The routes and speed of transmission of the disease between animals, and, when relevant, between animals and humans
Routes of transmission
WNV is maintained in nature by a primary cycle of transmission mosquito–bird–mosquito (endemic cycle): adult ornithophilic mosquitoes (vectors) become infected by biting viraemic birds (amplifying hosts). A secondary cycle (epidemic cycle) is characterised by the involvement in the transmission cycle of accidental hosts such as horses and humans due to particular ecological conditions. In this case, arthropod vectors, called bridge vectors, are able to transmit the virus to hosts other than birds, such as horses and humans. Humans, equids and other mammals are considered to be dead‐end accidental hosts. In these hosts, the virus does not reach a concentration in the bloodstream high enough to infect vectors, so the transmission cycle is not perpetuated. In Europe, the transmission cycle of WNV can be restricted to two main ecosystems: the rural (sylvatic) cycle, which occurs near wet/marshy areas between wild birds and ornithophilic mosquitoes, and the synanthropic/urban cycle, which arises between synanthropic or domestic birds and mosquitoes which can feed on the blood of birds and humans.
WNF vectors are mosquitos belonging to the Culex, Aedes and Coquillettidia genera (family Culicidae) (link to storymap VBD: https://efsa.maps.arcgis.com/apps/MapJournal/index.html?appid=512a03aa8df84d54a51bcb69d1b62735) (EFSA AHAW Panel, 2017b).
Parameter 1 – Types of routes of transmission from animal to animal (horizontal, vertical)
Results of experimental trials on WNV transmission routes in wild birds are summarised in Table 4 and Table A.3 in Appendix A.
Table 4.
Experimental data on WNV transmission in wild birds
| Direct | Indirecta | Horizontal | Vertical | Species | Reference |
|---|---|---|---|---|---|
| C | Y | Y | NT | American crow (Corvus brachyrhynchos) | Komar et al. (2003) |
| C | Y | Y | NT | Blue jay (Cyanocitta cristata) | Komar et al. (2003) |
| C | Y | Y | NT | Black‐billed magpie (Pica hudsonia) | Komar et al. (2003) |
| C | Y | Y | NT | Ring‐billed gull (Larus delawarensis) | Komar et al. (2003) |
| C | Y | Y | N | Chicken (Gallus gallus domesticus)b | Langevin et al. (2001) |
| C | NT | Y | N | Domestic geese (Anser anser domesticus) | Swayne et al. (2001) |
| C | NT | NT | Common goose (Anser anser domesticus) | Banet‐Noach et al. (2003) | |
| C | NT | Y | NT | Red‐legged partridge (Alectoris rufa) | Sotelo et al. (2011b) |
| NT | Y | NT | NT | Canada goose (Branta canadensis) | Komar et al. (2003) |
| N | Y | N | NT | Mallard (Anas platyrhynchos) | Komar et al. (2003) |
| O | Y | Y | NT | American kestrel (Falco sparverius) | Komar et al. (2003) (C); Nemeth et al. (2006a) (O) |
| N | Y | N | NT | Northern bobwhite (Colinus virginianus) | Komar et al. (2003) |
| N | Y | N | NT | Japanese quail (Coturnix japonicus) | Komar et al. (2003) |
| NT | Y | NT | NT | Ring‐necked pheasant (Phasianus colchicus) | Komar et al. (2003) |
| N | Y | N | NT | American coot (Fulica americana) | Komar et al. (2003) |
| NT | Y | NT | NT | Killdeer (Charadrius vociferus) | Komar et al. (2003) |
| N | Y | N | NT | Mourning dove (Zenaida macroura) | Komar et al. (2003) |
| N | Y | N | NT | Rock dove (Columba livia) | Komar et al. (2003) |
| N | Y | N | NT | Monk parakeet (Myiopsitta monachus) | Komar et al. (2003) |
| N | Y | N | NT | Budgerigar (Melopsittacus undulatus) | Komar et al. (2003) |
| O | Y | Y | NT | Great horned owl (Bubo virginianus) | Komar et al. (2003) (C); Nemeth et al. (2006a) (O) |
| NT | Y | NT | NT | Northern flicker (Colaptes auratus) | Komar et al. (2003) |
| N | Y | N | NT | Fish crow (Corvus ossifragus) | Komar et al. (2003) |
| N | Y | N | NT | American robin (Turdus migratorius) | Komar et al. (2003) |
| N | Y | N | NT | European starling (Sturnus vulgaris) | Komar et al. (2003) |
| NT | Y | NT | NT | Red‐winged blackbird (Agelaius phoeniceus) | Komar et al. (2003) |
| N | Y | N | NT | Common grackle (Quiscalus quiscula) | Komar et al. (2003) |
| N | Y | N | NT | House finch (Carpodacus mexicanus) | Komar et al. (2003) |
| N | Y | N | NT | House sparrow (Passer domesticus) | Komar et al. (2003) |
| N | NT | N | NT | Red‐tailed hawk (Buteo jamaicensis) | Nemeth et al. (2006a) |
| N | NT | N | NT | Song sparrow (Melopiza melodia) | Reisen and Fang (2007) |
| O | NT | Y | NT | Eastern screech owls (Megascops asio) | Nemeth et al. (2006b) |
C: Contact transmission; O: oral transmission; N: no evidence of direct transmission; NT: not tested.
Mosquitoes‐exposed.
Only 1 animal in 16 in contact hens.
Mosquito bites are the usual source of WNV for mammals, reptiles and amphibians; however, in some animals, there is also evidence for transmission by other routes. Carnivorous mammals and reptiles (e.g. cats and alligators) can be infected by eating contaminated tissues. Direct transmission during close contact has also been reported in alligators, possibly via faecal shedding of virus. Chipmunks, squirrels and raccoons can also shed WNV in faeces, oral secretions and/or urine. WNV has been found in the urine of experimentally infected hamsters, and in very small amounts in the oral and/or cloacal fluids of experimentally infected North American bullfrogs (Rana catesbeiana) and green iguanas (Iguana iguana). Transplacental transmission was reported in experimentally infected sheep and mice, as well as in a horse that was fatally infected with a Lineage 1 virus in Africa, and aborted in the final stage of the disease. The epidemiological significance (if any) of mammalian, reptilian and amphibian hosts in the maintenance or amplification of WNV remains to be established.
Parameter 2 – Types of routes of transmission between animals and humans (direct, indirect, including food‐borne)
There is no evidence of natural direct transmission between vertebrates and humans. However, human infections from the exposure of conjunctival membranes (Fonseca et al., 2005) and/or percutaneous injury to the body fluids or tissues of WNV‐infected birds (CDC, 2002) have been described.
Speed of transmission
Transmission rate of WNV infection between vector (mosquito) and avian population has been expressed through the calculation of the basic reproduction number (R0) by using different mathematical models. In the EU context, Calistri et al. (2016) developed a transitional mathematical model to calculate the R0 values for the various part of the Italian territory from May to September, which resulted in a mean R0 value for the whole Italy varying between 0.4 and 4.8, with values > 1 from the end of May to the beginning of September.
3.1.1.7. Article 7(a)(vii) The absence or presence and distribution of the disease in the Union, and, where the disease is not present in the Union, the risk of its introduction into the Union
Presence and distribution
Parameter 2 – Type of epidemiological occurrence (sporadic, epidemic, endemic) at MS level
WNV introduction and circulation have been demonstrated on multiple occasions in southern Europe and in the Mediterranean basin since the 1960s when seropositive animals or virus isolates were discovered in France, Portugal and Cyprus (Filipe and Pinto, 1969; Joubert et al., 1970). Migratory birds have been associated with the introduction of viral strains from endemic areas (Calistri et al., 2010); however, the mechanism of virus persistence in animal hosts in Europe leading to endemicity of the infection is still unknown.
In Europe, WNV circulation was mainly detected in the Mediterranean and south‐eastern regions, where notifications of human and horses cases of WNV infection have increased in the last 5–7 years, with the involvement of new areas, where the infection was not notified before, such as Bulgaria and Greece in 2010, Albania and Former Yugoslav Republic of Macedonia in 2011, and Croatia, Serbia and Kosovo in 2012. Accordingly, alarming outbreaks were reported in several European countries in 2010; 261 confirmed human cases, including 34 deaths, occurred in Greece, 57 cases and five deaths occurred in Romania, and 480 cases and six deaths occurred in Russia (Papa et al., 2010; Onishchenko et al., 2011; Sirbu et al., 2011).
Sporadic occurrence of the disease has been reported in France since 1962, when it first appeared in Camargue. In the same region, WNV was detected in 2000, 2004 and, after a ten‐year period, in 2015 (Bahuon et al., 2016).
In Italy, WNV annual epidemics have been consistently registered since 2008 (Savini et al., 2008) caused by genetically divergent isolates and, to date, WNV is considered endemic in the north‐eastern regions of the country, in Sardinia and in Sicily (Italian Ministry of Health, 2016).
The geographic distribution of West Nile cases in Europe and in Mediterranean Basin from 2008 to 2016 shown in Figure 1.
Figure 1.
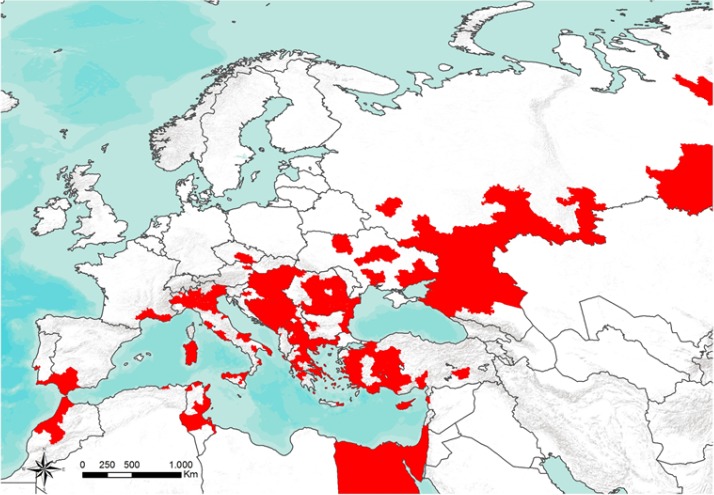
Geographic distribution of cases (confirmed and probable) of West Nile fever in Europe and in Mediterranean Basin (2008–2016) (source Arbozoonet: https://arbozoonet.izs.it/arbozoonet (ArboZoonet, online))
Risk of introduction
Data are not provided since the disease is already present in the Union. It should be noted, however, that a continuous introduction from Africa through migratory birds is suspected.
3.1.1.8. Article 7(a)(viii) The existence of diagnostic and disease control tools
Diagnostic tools
Parameter 1 – Existence of diagnostic tools
Details concerning the different types of diagnostic tools and their accuracy are listed in Table 7 in Section 3.1.4.1.
Table 7.
Diagnostic tests for WNV
| Test | Target | Se | Sp | Matrix | Reference | Notes |
|---|---|---|---|---|---|---|
| NS1‐antigen protein microarray | Antibodies | 95% | 100% | Serum | Cleton et al. (in press) | Differential diagnosis of flavivirus infections in horses |
| Real‐time RT‐PCR | Antigen | From 1.5 to 15 copies per reaction | 100% | Viral strains, human samples (cerebrospinal fluid, biopsies, serum and plasma) and mosquito pools | Vázquez et al. (2016) | Specificity evaluated using viral RNA from a panel of different flaviviruses and other encephalitic viruses belonging to several viral families |
| Real‐time RT‐PCR | Antigen | 80 genome copies | 100% | Viral strains Lineages 1 and 2 | Faggioni et al. (2014) | Specificity evaluated using TBE, Usutu, Dengue 1, Dengue 4, YF, JEV |
| SYBR Green I‐based real‐time RT‐PCR | Antigen | 20 copies | 100% | Human serum/plasma | Kumar et al. (2014) | Specificity evaluated using DEN‐1–4, JEV, YFV, SLEV |
| Antigen capture ELISA | Antigen | 90% | 98% | Human serum | Saxena et al. (2013) | Detection of NS1 antigen |
| Real‐time RT‐PCR | Antigen | 10 copies | 100% | Viral strains | Barros et al. (2013) | Detection and differentiation between WNV and JEV; specificity evaluated using DEN‐1–4, JEV, YFV, ZIKAV, Ntaya, TBEV, USUV, Toscana, CHIKV |
| Real‐time RT‐PCR | Antigen | 1.26 TCID50/ml for WNV‐L1, 6.3 TCID50/ml for WNV‐L2 | 100% | Tissue, feathers, oropharyngeal and cloacal swabs and blood from wild birds, samples from mice infected experimentally | Del Amo et al. (2013) | Detection and differentiation between WNV and USUV; specificity evaluated using SLEV, MVEV, JEV, BAGV, DEN‐1, TBEV, VEEV, VSV, AIV, EIV, NDV, AHS4 |
| Competitive ELISA | Antibodies | 100% | Wild birds: 79.5% compared to VNT | Sera from mammals and wild birds | Sotelo et al. (2011a) | |
| Horses: 96.5% compared to VNT | ||||||
| South african mammals: 79.5% compared to HAI | ||||||
| Giraffes: 67% compared to HAI | ||||||
| IgM capture ELISA | Antibodies | 91.7% | 99.2% | Horse sera | Long et al. (2006) | |
| Real‐time RT‐PCR | Antigen | 2–4 genome copies of WNV | 100% | Viral strains | Eiden et al. (2010) | In OIE manual. For simultaneous detection and differentiation of WNV Lineage 1 and Lineage 2. Specificity evaluated using TBEV, YFV, JEV |
| Nested RT‐PCR | Antigen | 10–8.0/100 μL | ND | Equine brain, blood, and cerebrospinal fluid; avian brain tissues | Johnson et al. (2001) | In OIE manual |
| Real‐time RT‐PCR | Antigen | 0.1 PFU | 100% | Human serum, CSF, brain tissue, mosquito pools, and avian tissues | Lanciotti et al. (2000) | In OIE manual. Specificity evaluated using DEN‐2, JEV, YFV, SLEV, Lacrosse virus, Powassan virus, MVE, WEEV, EEEV |
RT‐PCR: reverse transcriptase polymerase chain reaction; IgM: immunoglobulin M; ELISA: enzyme linked immunosorbent assay; PFU: plaque‐forming unit.
Viral nucleic acid and viral antigens can be demonstrated in tissues of infected animals by RT‐PCR and immuno‐histochemistry, respectively.
Antibodies can be detected in equine serum by IgM capture ELISA, haemagglutination inhibition (HI), IgG ELISA, plaque reduction neutralisation (PRN) or virus neutralisation (VN). In some serological assays, antibody cross‐reactions with related flaviviruses, such as St. Louis encephalitis virus, Usutu virus, Japanese encephalitis virus or tick‐borne encephalitis (TBE) virus may be encountered.
According to the OIE, the following tests are suitable methods for confirmation of clinical cases: Nested RT‐PCR, real time RT‐PCR and IgM capture ELISA. The PRN and serum neutralisation tests are both suitable methods for detecting prevalence of infection, population freedom from infection and immune status in animals post‐vaccination (Table 7).
Equine WNV‐specific IgM antibodies are usually detectable from 7–10 days to 1–2 months post‐infection. Most of horses with WNV encephalitis test positive in the IgM capture ELISA at the time that clinical signs are first observed. WNV neutralising antibodies are detectable in equine serum by 2 weeks post‐infection and can persist for more than 1 year.
Several PCR methods are available as commercial kits. In view of the continued evolution and possible emergence of new WNV strains, it is important that the designs of PCR tests are constantly monitored and updated when necessary.
Control tools
Parameter 2 – Existence of control tools
In areas where the disease is endemic, horses may be protected from the clinical signs by vaccination (Table 7). In the infected areas, however, strategies aiming at reducing the circulation of the virus through the reduction of mosquito density (reduction/treatment of stagnant water, adulticidal and larvicidal targeted treatments) and of contacts between vectors and receptive hosts (application of repellent, mosquito netting, etc.) are the bases of any control policy for mosquito‐borne diseases. Among biocidal products, the use of pyrethrin (6%) and piperonyl butoxide (60%) by aerial spray, indicated that the odds of infection after spraying were around six times higher in the untreated area than in treated areas, and that the treatments successfully disrupted the WNV transmission cycle (Carney et al., 2008).
3.1.2. Article 7(b) The impact of diseases
3.1.2.1. Article 7(b)(i) The impact of the disease on agricultural and aquaculture production and other parts of the economy
The level of presence of the disease in the Union
Parameter 1 – Number of MSs where the disease is present
Since the beginning of the 2016 transmission season, the presence of WNV has been confirmed in MSs and neighbouring countries. As of 27 October 2016, 205 human cases of WNF have been reported in the EU and 261 cases in the neighbouring countries (Austria, Croatia, Cyprus, Egypt, Hungary, Italy, Israel, Portugal, Romania, Russian Federation, Serbia, Spain and Syrian Arab republic, Tunisia, Ukraine) (ECDC, 2016).
The loss of production due to the disease
Parameter 2 – Proportion of production losses (%) by epidemic/endemic situation
In European outbreaks, WNV has not been associated with any mortality in domestic birds but has been connected to a few cases in wild birds (see Section 3.1.1.1).
3.1.2.2. Article 7(b)(ii) The impact of the disease on human health
Transmissibility between animals and humans
Table 5.
Number of cases (confirmed and probable) of West Nile fever in Europe and in Mediterranean Basin (updated to 2 December 2016)
| Country | Year | Species | No. total casesa | No. confirmed casesb | Source |
|---|---|---|---|---|---|
| Albania | 2011 | Human | 2 | ECDC (online) | |
| Algeria | 2012 | Human | 1 | 1 | ECDC (online) |
| Austria | 2016 | Human | 2 | 2 | ECDC (online) |
| 2015 | Human | 3 | 3 | ||
| 2014 | Human | 1 | 1 | ||
| Bosnia and Herzegovina | 2014 | Human | 13 | 0 | ECDC (online) |
| 2013 | Human | 3 | 3 | ||
| Bulgaria | 2016 | Human | 1 | 1 | ECDC (online) |
| 2015 | Human | 2 | 0 | ||
| Croatia | 2016 | Human | 1 | 0 | ECDC (online) |
| 2013 | Human | 16 | 1 | ECDC (online) | |
| 2012 | Human | 5 | 3 | ECDC (online) | |
| 2013 | Horses | – | 12 | OIE (online) | |
| Cyprus | 2016 | Human | 1 | 1 | ECDC (online) |
| Egypt | 2016 | Human | 1 | 1 | ECDC (online) |
| France | 2015 | Human | 1 | 1 | ECDC (online) |
| Former Yugoslav Republic of Macedonia | 2013 | Human | 1 | ECDC (online) | |
| 2012 | Human | 6 | 1 | ||
| Greece | 2014 | Human | 15 | 13 | HCDCP (online) |
| 2014 | Horses | 4 | 4 | OIE (online) | |
| 2013 | Human | 86 | 58 | HCDCP (online) | |
| 2013 | Horses | – | 15 | OIE (online) | |
| 2012 | Human | 161 | 47 | HCDCP (online) | |
| 2012 | Horses | – | 15 | OIE (online) | |
| 2011 | Human | 101 | – | HCDCP (online) | |
| 2011 | Horses | 23 | – | OIE (online) | |
| 2010 | Human | 261 | – | HCDCP (online) | |
| 2010 | Horses | 30 | – | OIE (online) | |
| Hungary | 2016 | Human | 39 | 16 | ECDC (online) |
| 2015 | Human | 18 | 13 | ECDC (online) | |
| 2014 | Human | 11 | 3 | ECDC (online) | |
| 2013 | Human | 31 | 6 | ECDC (online) | |
| 2012 | Human | 12 | 7 | ECDC (online) | |
| 2011 | Human | 3 | – | ECDC (online) | |
| 2010 | Human | 3 | – | ECDC (online) | |
| Israel | 2016 | Human | 80 | 47 | ECDC (online) |
| 2015 | Human | 123 | 89 | ||
| 2014 | Human | 17 | 7 | ||
| 2013 | Human | 63 | 28 | ||
| 2012 | Human | 59 | 31 | ||
| 2011 | Human | 39 | – | ||
| Italy | 2016 | Human | 71 | 71 | ISS (online) |
| 2016 | Horses | 51 | 51 | IZSAM (online) | |
| 2015 | Human | 61 | 61 | ISS (online) | |
| 2015 | Horses | 30 | 30 | IZSAM (online) | |
| 2014 | Human | 24 | 24 | ISS (online) | |
| 2014 | Horses | 27 | 27 | IZSAM (online) | |
| 2013 | Human | 70 | 70 | ISS (online) | |
| 2013 | Horses | – | 50 | IZSAM (online) | |
| 2012 | Human | 50 | 39 | ISS (online) | |
| 2012 | Horses | – | 63 | IZSAM (online) | |
| 2011 | Human | – | 15 | ISS (online) | |
| 2011 | Horses | 197 | – | IZSAM (online) | |
| Kosovo | 2012 | Human | 4 | 0 | ECDC (online) |
| Former Yugoslav Republic of Macedonia | 2011 | Human | 4 | – | ECDC (online) |
| 2011 | Horses | 10 | – | OIE (online) | |
| Montenegro | 2013 | Human | 4 | – | ECDC (online) |
| 2012 | Human | 1 | 1 | ||
| Morocco | 2010 | Horses | 25 | – | OIE (online) |
| Palestine | 2014 | Human | 1 | 1 | ECDC (online) |
| 2012 | Human | 2 | 1 | ||
| Portugal | 2016 | Horses | 1 | 1 | OIE (online) |
| 2015 | Human | 1 | 1 | ECDC (online) | |
| 2015 | Horses | 4 | 4 | OIE (online) | |
| Romania | 2016 | Human | 93 | 80 | ECDC (online) |
| 2015 | Human | 18 | 18 | ECDC (online) | |
| 2014 | Human | 23 | 22 | ECDC (online) | |
| 2013 | Human | 24 | 22 | ECDC (online) | |
| 2012 | Human | 14 | 13 | ECDC (online) | |
| 2011 | Human | 11 | – | ECDC (online) | |
| 2010 | Human | 52 | – | Sirbu et al. (2011) | |
| 2010 | Horses | 6 | – | OIE (online) | |
| Russian Federation | 2016 | Human | 135 | 135 | ECDC (online) |
| 2015 | Human | 39 | 39 | ECDC (online) | |
| 2014 | Human | 29 | – | ECDC (online) | |
| 2013 | Human | 177 | – | ECDC (online) | |
| 2012 | Human | 447 | – | ECDC (online) | |
| 2011 | Human | 153 | – | ECDC (online) | |
| 2010 | Human | 480 | – | Promed (online) | |
| Serbia | 2016 | Human | 41 | 41 | ECDC (online) |
| 2015 | Human | 28 | 28 | ||
| 2014 | Human | 76 | 56 | ||
| 2013 | Human | 302 | 200 | ||
| 2012 | Human | 70 | 41 | ||
| Spain | 2016 | Human | 3 | 3 | Andalucía Ministry of Agriculture (online) |
| 2016 | Horses | 70 | 70 | ||
| 2015 | Horses | 18 | 18 | ||
| 2013 | Horses | 40 | – | ||
| 2011 | Horses | 12 | – | ||
| Syrian Arab Republic | 2016 | Human | 2 | 1 | ECDC (online) |
| Tunisia | 2016 | Human | 1 | 1 | ECDC (online) |
| 2015 | Horses | 1 | 1 | OIE (online) | |
| 2013 | Human | 6 | 6 | ECDC (online) | |
| 2012 | Human | 63 | 33 | ECDC (online) | |
| 2011 | Human | 3 | – | ECDC (online) | |
| Turkey | 2014 | Horses | 1 | 1 | OIE (online) |
| 2011 | Human | 3 | – | ECDC (online) | |
| 2010 | Human | 7 | – | ECDC (online) | |
| Ukraine | 2016 | Human | 1 | 0 | ECDC (online) |
| 2013 | Human | 1 | – | ||
| 2012 | Human | 12 | – | ||
| 2011 | Human | 8 | – |
For EU countries, probable and confirmed cases as per EU case definition (Commission Decision 2008/426/EC1).
For EU countries, confirmed cases as per EU case definition (Commission Decision 2008/426/EC).
Transmissibility between humans
WNV is most commonly transmitted to humans by mosquitoes but additional routes of human‐to‐human transmission have also been documented as blood transfusions, organ transplants, exposure in a laboratory setting or the transmission from the mother to baby during pregnancy, delivery or breastfeeding. It is important to note that these methods of transmission represent a very small proportion of cases thus sufficient to evoke only a sporadic occurrence of the disease.
Humans are dead‐end hosts since are not able to infect mosquitoes during the viraemic phase of the infection. Thus, the above‐mentioned routes of direct transmission represent the main risk of infection dissemination among community. Laboratory acquired infections have also been reported (Campbell et al., 2002).
Parameter 3 – Human to human transmission is sufficient to sustain sporadic cases or community‐level outbreak
WNV transmission through blood transfusion and organ transplantation is able to sustain community‐level outbreak.
Parameter 4 – Sporadic, endemic, epidemic, or pandemic potential
Neuroinvasive human cases are usually sporadic, occurring mainly in immunocompromised persons or elderly.
The severity of human forms of the disease
Parameter 5 – Disability‐adjusted life year (DALY)
Human infections are mostly asymptomatic. However, in some cases, they can exhibit a mild form of the disease (less than 1%) with encephalitis, meningoencephalitis or meningitis mainly among elderly or immunosupressed individuals (Go et al., 2014). As for most arthropod‐borne diseases causing fever syndromes worldwide, the cumulative impact of WNV on global disease burden has not been fully assessed. Evaluations should include both the severe forms of the disease and the milder clinical manifestations which may result in neurological and ophthalmologic complications (Carson et al., 2006). WNV has been recognised able to induce a wide range of post‐infection, long‐term sequelae with the recovery of the affected patients within two years from the infection (Murray et al., 2008). However, a recent paper has emphasised that 40% of WNV‐infected patients continued to experience symptoms related to their WNV infection up to 8 years later demonstrating the health and economic impact of a result of prolonged recovery, continued morbidity, and related disability (Murray et al., 2014).
The availability of effective prevention or medical treatment in humans
Parameter 6 – Availability of medical treatment and their effectiveness (therapeutic effect and any resistance)
There is no specific recommended treatment, other than supportive care, at present. Intensive care and mechanical ventilation may be required in some cases. Various therapies including interferon, antisense nucleotides and intravenous immunoglobulins (passive immunisation) are being tested in clinical trials. While a few case reports suggest that some of these treatments may be promising, larger studies are still lacking. Screening for new drugs that may inhibit WNV is underway.
Parameter 7 – Availability of vaccines and their effectiveness (reduced morbidity)
There are no vaccines available for human use in EU.
3.1.2.3. Article 7(b)(iii) The impact of the disease on animal welfare
Parameter 1 – Severity of clinical signs at case level and related level and duration of impairment
The incubation period for equine WNV encephalitis following mosquito transmission is estimated to be 3–15 days. A fleeting viraemia of low virus titre precedes clinical onset (Bunning et al., 2002). WNV encephalitis occurs in only a small per cent of infected horses; the majority of infected horses do not display clinical signs (Ostlund et al., 2000). The disease in horses is frequently characterised by mild to severe ataxia. Additionally, horses may exhibit weakness, muscle fasciculation and cranial nerve deficits (Cantile et al., 2000; Ostlund et al., 2000, 2001; Snook et al., 2001). Fever is an inconsistently recognised feature. Treatment is supportive and signs may resolve or progress to terminal recumbency. The mortality rate is approximately one out of three neurologically affected horses.
Many species of birds can become infected with WNV; the clinical outcome of infection is variable. Some species appear resistant while others suffer fatal neurologic disease. WNV infection associated with severe clinical signs have been described in several species of European wild birds (Bakonyi et al., 2006; Hofle et al., 2008; Jimenez‐Clavero et al., 2008; Monaco et al., 2015).
3.1.2.4. Article 7(b)(iv) The impact of the disease on biodiversity and the environment
Biodiversity
Parameter 1 – Endangered wild species affected: listed species as in CITES and/or IUCN list
CITES (online)
Phoenicopteridae spp. (App. II)
Falco rusticolus (App. I)
Aquila adalberti (App. I)
Falconiformes spp. (App. II)
Parameter 2 – Mortality in wild species
A number of outbreaks have been reported recently in Europe, Russia and parts of the Middle East. Since 2004, one introduced Lineage 2 virus in Central Europe has affected significant numbers of wild and captive raptors (Erdélyi et al., 2007). Therefore, the potential for WNV to cause illness or deaths in other European birds should be re‐examined. Some virus lineages seem to have become endemic and are spreading (CFSPH, 2013). Species known to be susceptible to this isolate include sparrow hawks (Accipiter nisus), goshawks (Accipiter gentilis) and gyrfalcons (Falco rusticolus). The same virus was isolated from a dead collared dove (Streptopelia decaocto) in Italy, during an outbreak characterised by observed of mortality in collared doves and other species, including blackbirds. Different lineages of the WNV have also been found occasionally in other dead birds including European robins (Erithacus rubecula), raven (Corvus corax), common magpies (Pica pica), Eurasian jay (Garrulus glandarius), house sparrows (Passer domesticus), black redstart (Phoenicurus ochruros), sedge warbler (Acrocephalus schoenobaenus) and Savi's warbler (Locustella luscinioides).
LaDeau et al. (2007) demonstrated a high impact on the abundance of seven species of North American wild birds after the emergence of WNV in 1999. Host susceptibility, spatio‐temporal heterogeneity in pathogen transmission and other environmental impacts on populations were accounted for using Bayesian modelling techniques. These seven species included two members of the family Corvidae (American crow and blue jay), two from Turdidae (American robin and eastern bluebird), two from Paridae (chickadees and tufted titmouse) and one from Troglodytidae (house wren). Also, George et al. (2015) demonstrated significant negative effects on survival of 47–49% bird species in North America, using an extensive capture‐recapture technique study of nearly two decades, combined with recently developed models of WNV risk (George et al., 2015). The authors suggested that WNV in the US has a significant persistent effect on wild bird populations long after initial concerns had stopped.
3.1.3. Article 7(c) Its potential to generate a crisis situation and its potential use in bioterrorism
Parameter 1 – Listed in OIE/CFSPH classification of pathogens
WNV is listed in the CDC list of potential bioterrorism agents.
Parameter 2 – Listed in the Encyclopaedia of Bioterrorism Defence of Australia Group
WNV is not listed in the Encyclopaedia of Bioterrorism Defence of Australia Group.
Parameter 3 – Included in any other list of potential bio‐ agro‐terrorism agents
WNV is not reported in any other list of potential bio‐agro‐terrorism agents.
3.1.4. Article 7(d) The feasibility, availability and effectiveness of the following disease prevention and control measures
3.1.4.1. Article 7(d)(i) Diagnostic tools and capacities
Availability
Parameter 1 – Officially/internationally recognised diagnostic tool, OIE certified
Table 6.
Test methods available for the diagnosis of WNV and their purpose (Source: (OIE, 2013))
| Method | Purpose | ||||
|---|---|---|---|---|---|
| Population freedom from infection | Individual animal freedom from infection | Confirmation of clinical signs | Prevalence of infection | Immune status in individual animals or populations post‐vaccination | |
| Agent identification | |||||
| Nested RT‐PCR | – | ** | ** | – | – |
| Real‐time RT‐PCR | – | ** | ** | – | – |
| Isolation in tissue culture | – | ** | ** | – | – |
| Detection of immune response | |||||
| IGM capture ELISA | – | – | ** | – | |
| Plague reduction neutralisation | ** | – | * | ** | ** |
| Serum neutralisation | ** | – | * | ** | ** |
| Immunohistochemistry | – | – | * | – | |
*** = recommended method; ** = suitable method; * = may be used in some situations, but cost reliability, or other factors severity limits its application; – = not appropriate for this purpose.
Although not all of the rests listed as category *** have undergone formal validation, their routine nature and the fact that they have been used widely without dubious results, makes them acceptable.
RT‐PCR: reverse transcriptase polymerase chain reaction; IgM: immunoglobulin M; ELISA: enzyme linked immunosorbent assay.
Effectiveness
Parameter 2 – Se and Sp of diagnostic test
Diagnostic tests, their type, accuracy and basis for WNF diagnosis are listed in Table 7.
Feasibility
Parameter 3 – Type of sample matrix to be tested (blood, tissue, etc.)
See Table 7.
3.1.4.2. Article 7(d)(ii) Vaccination
WNV vaccines approved by EMA are listed in Table 8.
Table 8.
Vaccines for horses authorised for commercialisation in the EU by the European Medicines Agency (updated in October 2016) and their efficacy as emerged from a systematic review (updated to January 2016)
| Commercial name of vaccine | Type of vaccine | Way of administration | Doses | Species for which authorised | Countries in which authorised | Manufacturer | Efficacy | Field protection | Yearly availability production capacity | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Proteq West Nile | West Nile recombinant canarypox virus, vCP2017 virus | IM | Horses | All EU | Merial | NA | NA | NA | ||
| Equilis West Nile | Inactivated chimaeric flavivirus strain YF‐WN | IM | Horses | All EU | Intervet International BV | NA | NA | NA | ||
| Equip WNV (previously Duvaxyn WNV) | Inactivated West Nile virus, strain VM‐2 | IM | 2 doses (21 days apart) | Horses | All EU | Zoetis Belgium SA | Viruses could be isolated from 8 out of 10 non‐vaccinated animals up to 14 days after challenge, but only 1 vaccinated animals. Sixty per cent of the controls had to be euthanised after challenge compared to none of the vaccinates. From 10 non‐vaccinated animals, all presented, up to 21 days after challenge, pyrexia, head tremors or muscle fasciculations, and anxiety, and 9 showed mild paresis. In controls, these numbers were 2, 2, 6 and 2, respectively | Experimental trial | NA | Bowen et al. (2014) |
NA: data not available; IM: intramuscular.
3.1.4.3. Article 7(d)(iii) Medical treatments
There is no specific recommended treatment, other than supportive care, at present.
3.1.4.4. Article 7(d)(iv) Biosecurity measures
The biosecurity measures aiming at reducing the WNV spread are focused on controlling the vectors primarily responsible for the viral transmission. Farm‐to‐farm movement of infected horses is not effective to spread the disease since they are neither able to transmit the virus to biting mosquitoes nor, directly, to vertebrates including humans.
To minimise the possibilities of contact between the vectors and receptive hosts, it is advisable to use mosquito nets to avoid the vector entrance in the stables as well as the use of repellents on the animals. Data related to the efficacy of these substances has been detailed in Section 3.1.5.4 parameter 1.
To prevent any inter‐human spread, the screening of blood and organs for transplantation in areas with WNV circulation is a common measure.
3.1.4.5. Article 7(d)(v) Restrictions on the movement of animals and products
No specific measures are mentioned in the EU legislation for WNV outbreak control.
3.1.4.6. Article 7(d)(vi) Killing of animals
No specific measures are mentioned in the EU legislation for WNV outbreak control.
3.1.4.7. Article 7(d)(vii) Disposal of carcasses and other relevant animal by‐products
No specific measures are mentioned in the EU legislation for WNV outbreak control.
3.1.5. Article 7(e) The impact of disease prevention and control measures
3.1.5.1. Article 7(e)(i) The direct and indirect costs for the affected sectors and the economy as a whole
The major impact of WNV on animal health in the EU ecosystem is limited to the development of clinical signs in horses and, to date, there are no reports of clinical illness in domestic bird species. Thus, the major costs of WNV control in animals, namely horses, should include:
the cost of vaccination: primary vaccination consists of two doses, the second dose being administered 3–6 weeks later, depending on the vaccine used;
the cost to prevent mosquitoes bites: keeping horses indoor is not be very effective against Culex pipiens if additional measures such as mosquito nets or fans are not installed, as these mosquitoes are also active indoors. The use of insecticides or repellents is also able to reduce the possibilities for contact between the vectors and receptive hosts. Control of vectors can be recommended to individuals and to public health authorities in case of a severe epidemic, but the associated costs are difficult to estimate since emergency aerial spraying, even if proven to be effective in reducing mosquito populations and the number of human cases of WNV infection in the US (Barber et al., 2010), would not be the first option of vector control in MSs, given the substantial environmental risks and not easily accepted by the population (Humblet et al., 2016).
the costs of active surveillance activities, which may vary considerably between MSs. Usually animal surveillance encompasses domestic solipeds (horses and donkeys), birds and other animal species (e.g. cattle and farmed deer), as well as entomological surveillance activities. The main objective of the surveillance in humans during the transmission period is to ensure an immediate response in the implementation of the blood safety measures and the prevention of human cases, and, on an annual basis, to improve and adapt the surveillance and strengthen the preparedness.
WNV RNA screening of all blood donors in areas where the WNV circulation is in place. As an example in Italy during the 2015 epidemics, a total of 316,614 WNV NAT screening tests were conducted in blood donors in the affected provinces and 13 asymptomatic infected donors, were identified. No donor or organ transplant recipients were positive for WNV among the 168 tested.
3.1.5.2. Article 7(e)(ii) The societal acceptance of disease prevention and control measures
The control of the mosquito population through the intensive use of biocidal products, e.g. by aerial spray is not easily accepted by the population (Humblet et al., 2016).
3.1.5.3. Article 7(e)(iii) The welfare of affected subpopulations of kept and wild animals
Parameter 1 – Welfare impact of control measures on domestic animals
Since no specific measures are mentioned in the EU legislation for the WNV outbreak control, there is no impact on the welfare of domestic animals of official control measures.
Parameter 2 – Wildlife depopulation as control measure
Wild bird depopulation is not a control measure applied in course of WNV outbreak and its efficacy, as emerged from epidemiological models, not ascertained since the potential reduction of bird densities could enhance WNV transmission (Wonham et al., 2004).
3.1.5.4. Article 7(e)(iv) The environment and biodiversity
Environment
Parameter 1 – Use and potential residuals of biocides or medical drugs in environmental compartments (soil, water, feed, manure)
In WNV‐infected areas strategies must be implemented to reduce the circulation of the virus through measures that modify the density of the vectors (reduction of stagnant water, performance of adulticidal and larvicidal treatments) and to reduce the possibilities of contact between the vectors and receptive hosts (application of repellent, mosquito netting, etc.). Among biocidal products, the use of pyrethrin (6%) and piperonyl butoxide (60%) by aerial spray indicated that the odds of infection after spraying were around 6 times higher in the untreated area than in treated areas, and that the treatments successfully disrupted the WNV transmission cycle (Carney et al., 2008). Since Cx. pipiens is considered to be the main vector of WNV in Europe a list of biocidal products targeting mosquito control are reported in Table 9.
Table 9.
Biocidal products targeting mosquito control (genus Culex), for which reports were found in a systematic review of available treatments against the vectors of vector‐borne infections (papers published up to January 2016)
| Active substance | Reference | Intended use (route investigated in the study) | Study findings |
|---|---|---|---|
| Studies not targeting any particular host | |||
| Deltamethrin | Marcombe et al. (2011) | Fogging | Efficacy was assessed by monitoring mortality rates of naturally resistant and laboratory susceptible mosquitoes placed in sentinel cages. Results showed high mortality rates of susceptible sentinel mosquitoes (64%) while resistant mosquitoes exhibited very low mortality (10%) |
| Vehicle‐mounted thermal foggers (1 g/ha) | |||
| Studies focused on vector control in housing/environment | |||
| Deltamethrin | Akogbéto et al. (2010) | Indoor spraying | Deterrence ratea: Anopheles gambiae (31.25%, 24.75%, 30 and 60 dpt; Culex sp. and Mansonia sp. 30 dpt 46.15%) |
| Huts were treated with insecticides. The absorption of the walls was 112 mL of insecticide per m2 and that of the ceiling (polyethylene), the entry slits, and the door (painted metal) was in total 53.13 mL/m2 | Exophily rateb: Anopheles gambiae (45.4%, 26.3%, 30 and 60 dpt; Culex sp. and Mansonia sp. 30 dpt 33.3%) | ||
| Blood‐feeding ratec: Anopheles gambiae (18.2%, 23.7%, 30 and 60 dpt; Culex sp. and Mansonia sp. 30 dpt, 14.3%) | |||
| Immediate mortalityd: Anopheles gambiae (32.7%, 15.8%, 30 and 60 dpt; Culex sp. and Mansonia sp. 30 dpt, 8.5%) | |||
| Overall mortalitye: Anopheles gambiae (72.7%, 31.6%, 30 and 60 dpt; Culex sp. and Mansonia sp. 30 dpt, 21%) | |||
| Deltamethrin | Badolo et al. (2014) | Mortality of mosquitoes was 90.5 (86–94)% in unwashed nets (3 min exposure, 24‐h mortality), and remained above 90% after 5 washes. Average mortality after 10, 15 and 20 washes were 81 (75–86)%, 68.7 (63–75)% and 66.3 (60–72)%, respectively | |
| Deltamethrin | Dabiré et al. (2006) | Treated mosquito nets | Mosquito entrance rate was 10‐fold higher in control houses than in houses with long lasting impregnated nets (LLINs) and there was no difference between the two tested net types. Among mosquitoes found in the houses, 36% were dead in LLIN houses compared to 0% in control houses. Blood feeding rate was 80% in control houses compared to 43% in LLIN houses. The type of net did not significantly impact any of these parameters |
| Concentration of 55 mg/m2 | |||
| Deltamethrin | Darriet et al. (2000) | Treated mosquito nets | The 24‐h mortality was 56% for Anopheles gambiae females, and 45% for Culex spp. females (compared to 4 and 6% in controls) |
| Concentration of 25 mg/m2 | |||
| Deltamethrin | Moosa‐Kazemi et al. (2007) | Treated mosquito nets | Recorded 24‐h‐mortality was 100% even after 9 months |
| Concentrations of 25 mg/m2 | |||
| Deltamethrin | Muller et al. (2002) | Treated mosquito nets | Mortality of mosquitoes was 97% in washed nets, and reduced to 84%, 54% and 7% after 6, 12 and 18 months (with respective average of times washed of 1.1, 1.9 and 3) |
| Concentrations from 55 mg/m2 (unwashed) to 1.6 mg/m2 (18 months old and washed 3 times) | |||
| Deltamethrin | Van Roey et al. (2014) | Treated mosquito nets | A positive control (commercial product PermaNet® 2.0, 55 mg a.i./m2) was able to kill over 90% of mosquitoes (3 min exposure, 24‐h‐mortality) for up to 30 months, while the observed mortality with the experimental product (Netprotect®, 68 mg a.i./m2) was 85.7% after 12 months, and remained below 90% |
| Concentrations of 55 and 68 mg/m2 | |||
| Diflubenzuron | Cetin et al. (2006) | Septic tank water treatment | Recorded adult inhibition for Culex pipiens was always 100% in the first 2 weeks, for all concentrations tested, and remained at 100% for up to 4 weeks with 30 g/L, and 2 weeks with 10 g/L |
| 0.01, 0.02, and 0.03 mg (AI)/L, using a 25% wettable powder or a 4% granular formulation in wastewater tank | |||
| Lambda‐cyhalothrin | Okumu et al. (2012) | Indoor spraying | Mortality (24‐h mortality of Anopheles arabiensis) was 90% after 30 days but reduced to 35% after 60 days |
| 0.03 g/m2 sprayed on mud walls | |||
| Lambda‐cyhalothrin | Trout et al. (2007) | Outdoors Spraying | The reduction in Aedes albopictus in sites was of 89.5% compared to controls, and in laboratory bioassays exposing mosquitoes to treated leaves, mortality varies from 80% after 2 weeks, to 35% after 8 weeks. In contrast, Culex spp. were not reduced |
| Mist (concentration of 62.52 mL/L) directly applied to vegetation in the backyard of houses, and other resting sites | |||
| Permethrin | Rozendaal et al. (1989) | Treated mosquito nets | Cotton cloth impregnated with permethrin at a rate of 0.5 g/m2 killed all Anopheles darlingi females exposed for 2 min, but after the material had been washed twice in soapy water the bioassay mortality fell to only 21.4%. Bioassays with Culex quinquefasciatus females showed that sprayed nets were less effective than nets impregnated by soaking (at equivalent dosages of 0.16–1.34 g/m2) |
| Concentrations of 125–1,000 mg/m2 | |||
| Permethrin | Soleimani‐Ahmadi et al. (2012) | Treated mosquito nets | Mortality of mosquitoes was 100% in the first 90 days, 92.4% (88–97) after 5 months, and reduced to 81.6% (75–88) after 9 months, and 72.3% (65–79) after 12 months |
| The nets were blended with 1,000 mg a.i/m2 (2%, w/w), and final concentrations varied from 814 to 937 mg/m2 | |||
| Studies focused on humans as the host species (personal protection) | |||
| DEET | Soonwera and Phasornkusolsill (2015) | External use – topic/spray | DEET was used as control when evaluating other (non‐ECHA approved) substances. The formulation gave protection for up to 182 min, and 98.5% protection from bites of Aedes aegypti and Culex quinquefasciatus |
| DEET 20% (w/w), 0.1 mL applied on a 3 × 10 cm area on the ventral portion of the forearm | |||
| DEET | Gupta et al. (1987) | Treated clothes and topic applications of repellent, in different concentrations and combinations | The field trials were arranged in a four‐way factorial design which compared fabric types, permethrin treatment and repellent treatments over a 14‐h test period. The repellent formulations and the permethrin‐treated clothing used as one system provided better protection (81% mortality) than the repellent formulations or permethrin‐treated clothing used separately |
| DEET + permethrin | Mani et al. (1991) | External use – soap | Percentage repellency (reduction in biting rates) was 96% for Culex vishnui, 89.6% for Culex tritaeniorhynchus and 94.8% for Culex pseudouishnui |
| Containing 20% DEET and 5% permethrin | |||
| Metofluthrin | Dame et al. (2014) | ‘Clip‐on’ spatial repellent device | Efficacy in reduction of Anopheles quadrimaculatus, in 2 study years, compared to control, were 16% and 8%) |
| 31.20% | 19% and 8% for Psorophora columbiae and 69% for Culex erraticus. Total mosquito reduction was 13% | ||
| Metofluthrin | Revay et al. (2013) | External use | Biting on the arms of volunteers was reduced by 96.28% for Ae. albopictus, and by 94.94% for Cx. pipiens |
| ‘Clip‐on’ metofluthrin (31.2%) | |||
Percentage of reduction in the number of mosquitoes caught in treated hut relative to the number caught in the control hut.
Percentage of mosquitoes that have escaped the hut and have taken refuge in the veranda trap divided by the total number of mosquitoes collected in the hut.
Percentage of blood fed mosquitoes collected divided by the total of mosquitoes collected in veranda and hut.
Percentage of dead mosquitoes collected in the morning compared to total mosquitoes collected in the hut.
Immediate mortality plus delayed mortality recorded after 24 h.
Biodiversity
Parameter 2 – Mortality in wild species
The main risk may be represented by the environmental residual of biocides which may interfere with ecology of wild species.
3.2. Assessment according to Article 5 criteria
This section presents the results of the expert judgement on the criteria of Article 5 of the AHL about WNF (Table 10). The expert judgement was based on Individual and Collective Behavioural Aggregation (ICBA) approach described in detail in the opinion on the methodology (EFSA AHAW Panel, 2017a). Experts have been provided with information of the disease fact‐sheet mapped into Article 5 criteria (see supporting information, Annex A), based on that the experts indicate their Y/N or ‘na’ judgement on each criterion of Article 5, and the reasoning supporting their judgement.
Table 10.
Outcome of the expert judgement on the Article 5 criteria for West Nile fever
| Criteria to be met by the disease: According to AHL, a disease shall be included in the list referred to in point (b) of paragraph 1 of Article 5 if it has been assessed in accordance with Article 7 and meets all of the following criteria | Final outcome | |
|---|---|---|
| A(i) | The disease is transmissible | Y |
| A(ii) | Animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union | Y |
| A(iii) | The disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character | Y |
| A(iv) | Diagnostic tools are available for the disease | Y |
| A(v) | Risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union | Y |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at points A(i)‐A(v), the disease needs to fulfil at least one of the following criteria | ||
| B(i) | The disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character | Y |
| B(ii) | The disease agent has developed resistance to treatments and poses a significant danger to public and/or animal health in the Union | na |
| B(iii) | The disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union | N |
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | NC |
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | NC |
Colour code: green = consensus (Yes/No); yellow = no consensus (NC); red = not applicable (na), i.e. insufficient evidence or not relevant to judge.
The minimum number of judges in the judgement was 12. The expert judgement was conducted as described in the methodological opinion (EFSA AHAW Panel, 2017a). For details on the interpretation of the questions, see Appendix B of the methodological opinion (EFSA AHAW Panel, 2017a).
3.2.1. Non‐consensus questions
This section displays the assessment related to each criterion of Article 5 where no consensus was achieved in form of tables (Tables 11 and 12). The proportion of Y, N or na answers are reported, followed by the list of different supporting views for each answer.
Table 11.
Outcome of the expert judgement related to criterion 5 B(iv)
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | NC | 83 | 17 | 0 |
NC: non‐consensus; number of judges: 12.
Table 12.
Outcome of the expert judgement related to criterion 5 B(v)
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | NC | 82 | 18 | 0 |
NC: non‐consensus; number of judges: 11.
Reasoning supporting the judgement
Supporting Yes:
It is listed in OIE/CFSPH, there is public concern on the disease and the potential to create a crisis.
There have been examples of public health crisis in Romania in the 1990s, in Greece in 2010, in Hungary and Russia following outbreaks in humans.
US army indicates that virulent genes could be modified to increase pathogenicity for humans and used as a weapon (since transmitted by mosquitoes).
There were crisis in naïve areas, but not in endemic areas like France.
Supporting No:
Some MSs do not have any WNV monitoring system in place, while others have been operating systems for several years, e.g. Italy and Greece. The main objective of the surveillance in humans during the transmission period is to ensure an immediate response in the implementation of the blood safety measures and the prevention of human cases, and, on an annual basis, to improve and adapt the surveillance and strengthen the preparedness. In Italy, for example, though the repeated and constant WNV circulation, surveillance on blood samples has been put in place and this also has not generated a crisis. Moreover, WNV circulation in France have not generated crisis.
The situation in the US is not related with bioterrorism.
Every virus could be genetically modified and become a threat, a worst‐case scenario exists for every disease and should not be considered here.
Reasoning supporting the judgement
Supporting Yes:
The North American experience shows that there is the potential for significant impact on the biodiversity.
Supporting No:
There is no report of an impact of WNF at population level on endangered species in EU.
3.2.2. Outcome of the assessment of West Nile fever according to criteria of Article 5(3) of the AHL on its eligibility to be listed
As from the legal text of the AHL, a disease is considered eligible to be listed as laid down in Article 5 if it fulfils all criteria of the first set from A(i) to A(v) and at least one of the second set of criteria from B(i) to B(v). According to the assessment methodology (EFSA AHAW Panel, 2017a), a criterion is considered fulfilled when the outcome is ‘Yes’. According to the results shown in Table 10, WNF complies with all criteria of the first set and with one criterion of the second set, therefore it is considered eligible to be listed as laid down in Article 5 of the AHL.
3.3. Assessment according to Article 9 criteria
This section presents the results of the expert judgement on the criteria of Annex IV referring to categories as in Article 9 of the AHL about WNF (Tables 11, 12, 15, 16 and 17). The expert judgement was based on ICBA approach described in detail in the opinion on the methodology. Experts have been provided with information of the disease fact‐sheet mapped into Article 9 criteria (see supporting information, Annex A), based on that the experts indicate their Y/N or ‘na’ judgement on each criterion of Article 9, and the reasoning supporting their judgement.
Table 15.
Outcome of the expert judgement related to the criteria of Section 3 of Annex IV (category C of Article 9) for West Nile fever
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
|---|---|---|
| 1 | The disease is present in the whole OR part of the Union territory with an endemic character | Y |
| 2.1 | The disease is moderately to highly transmissible | Y |
| 2.2 | The disease is transmitted mainly by direct or indirect transmission | Y |
| 2.3 | The disease affects single or multiple species | Y |
| 2.4 | The disease usually does not result in high morbidity and has negligible or no mortality AND often the most observed effect of the disease is production loss | N |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, or possible significant threats to food safety | Y |
| 4(CI) | The disease has a significant impact on the economy of parts of the Union, mainly related to its direct impact on certain types of animal production systems | N |
| 4(PI) | The disease has a significant impact on the economy of parts of the Union, mainly related to its direct impact on certain types of animal production systems | N |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | NC |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | NC |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | NC |
Colour code: green = consensus (Yes/No); yellow = no consensus (NC).
Table 16.
Outcome of the expert judgement related to the criteria of Section 4 of Annex IV (category D of Article 9) for West Nile fever
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
|---|---|---|
| D | The risk posed by the disease in question can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread | N |
| The disease fulfils criteria of Sections 1, 2, 3 or 5 of Annex IV of AHL | Y | |
Colour code: green = consensus (Yes/No).
Table 17.
Outcome of the expert judgement related to the criteria of Section 5 of Annex IV (category E of Article 9) for West Nile fever
| Diseases in category E need to fulfil criteria of Sections 1, 2 or 3 of Annex IV of AHL and/or the following: | Final outcome | |
|---|---|---|
| E | Surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment (If a disease fulfils the criteria as in Article 5, thus being eligible to be listed, consequently category E would apply.) | Y |
Colour code: green = consensus (Yes/No).
The minimum number of judges in the judgement was 12. The expert judgement was conducted as described in the methodological opinion (EFSA AHAW Panel, 2017a). For details on the interpretation of the questions, see Appendix B of the methodological opinion (EFSA AHAW Panel, 2017a).
Table 13.
Outcome of the expert judgement related to the criteria of Section 1 of Annex IV (category A of Article 9) for West Nile fever
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
|---|---|---|
| 1 | The disease is not present in the territory of the Union OR present only in exceptional cases (irregular introductions) OR present in only in a very limited part of the territory of the Union | N |
| 2.1 | The disease is highly transmissible | N |
| 2.2 | There be possibilities of airborne or waterborne or vector‐borne spread | Y |
| 2.3 | The disease affects multiple species of kept and wild animals OR single species of kept animals of economic importance | Y |
| 2.4 | The disease may result in high morbidity and significant mortality rates | N |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at points 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, including epidemic or pandemic potential OR possible significant threats to food safety | N |
| 4 (CI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 4 (PI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | NC |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | NC |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | NC |
Colour code: green = consensus (Yes/No); yellow = no consensus (NC).
Table 14.
Outcome of the expert judgement related to the criteria of Section 2 of Annex IV (category B of Article 9) for West Nile fever
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
|---|---|---|
| 1 | The disease is present in the whole OR part of the Union territory with an endemic character AND (at the same time) several Member States or zones of the Union are free of the disease | Y |
| 2.1 | The disease is moderately to highly transmissible | Y |
| 2.2 | There be possibilities of airborne or waterborne or vector‐borne spread | Y |
| 2.3 | The disease affects single or multiple species | Y |
| 2.4 | The disease may result in high morbidity with in general low mortality | Y |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, including epidemic potential OR possible significant threats to food safety | Y |
| 4 (CI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 4 (PI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | NC |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | NC |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | NC |
Colour code: green = consensus (Yes/No); yellow = no consensus (NC).
3.3.1. Non‐consensus questions
This section displays the assessment related to each criterion of Annex IV referring to the categories of Article 9 of the AHL where no consensus was achieved in form of tables (Tables 18, 19 and 20). The proportion of Y, N or ‘na’ answers are reported, followed by the list of different supporting views for each answer.
Table 18.
Outcome of the expert judgement related to criterion 5(b)(PI) of Article 9
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | NC | 83 | 17 | 0 |
NC: non‐consensus; number of judges: 12.
Table 19.
Outcome of the expert judgement related to criterion 5(c)(PI) of Article 9
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | NC | 58 | 0 | 42 |
NC: non‐consensus; number of judges: 12.
Table 20.
Outcome of the expert judgement related to criterion 5(d)(PI) of Article 9
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| 5(d) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | NC | 67 | 33 | 0 |
NC: non‐consensus; number of judges: 12.
Reasoning supporting the judgement
Supporting Yes:
WNF may have a potential impact on animal health and consequently welfare if introduced in naive populations in the absence of controls.
The percentage of affected horses with severe clinical signs undergoes a significant impact on animal welfare.
Supporting No:
Currently, there is no significant impact on welfare although the virus is circulating in many countries. It could have a potential impact in naïve populations.
Although there has been extensive geographical expansion of the European territories with WNV circulation, the number of clinical cases in horses remains very limited.
Reasoning supporting the judgement
Supporting Yes:
The impact on wild birds could be potentially significant, if there was a substantial increase in outbreaks.
Impacts of controls on the environment may be substantial.
Supporting na:
There are no data on the potential effect of vector control easures on the environment.
Reasoning supporting the judgement
Supporting Yes:
There may be a potential impact, given the range of bird species potentially affected. Some endangered species of, e.g. prey birds could disappear or be seriously threatened.
The emergence of WNV strains more virulent for wild birds can affect larger numbers of animals.
Supporting No:
There is an impact on individual animals, but no long‐term effect on a population scale. Furthermore there are periodic epidemic outbreaks, but the probability of long‐term epidemics in wild fauna is low.
3.3.2. Outcome of the assessment of criteria in Annex IV for West Nile fever for the purpose of categorisation as in Article 9 of the AHL
As from the legal text of the AHL, a disease is considered fitting in a certain category (A, B, C, D or E corresponding to point (a) to point (e) of Article 9(1) of the AHL) if it is eligible to be listed for Union intervention as laid down in Article 5(3) and fulfils all criteria of the first set from 1 to 2.4 and at least one of the second set of criteria from 3 to 5(d) as shown in Tables 11–17. According to the assessment methodology (EFSA AHAW Panel, 2017a), a criterion is considered fulfilled when the outcome is ‘Yes’. With respect to different type of impact where the assessment is divided into current and potential impact, a criterion will be considered fulfilled if at least one of the two outcomes is ‘Y’ and, in case of no ‘Y’, the assessment is inconclusive if at least one outcome is ‘NC’.
A description of the outcome of the assessment of criteria in Annex IV for WNF for the purpose of categorisation as in Article 9 of the AHL is presented in Table 21.
Table 21.
Outcome of the assessment of criteria in Annex IV for WNF for the purpose of categorisation as in Article 9 of the AHL (CI = current impact; PI = potential impact)
| Category | Article 9 criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° set of criteria | 2° set of criteria | ||||||||||
| 1 | 2.1 | 2.2 | 2.3 | 2.4 | 3 | 4 | 5a | 5b | 5c | 5d | |
| Geographical distribution | Transmissibility | Routes of transmission | Multiple species | Morbidity and mortality | Zoonotic potential | Impact on economy | Impact on society | Impact on animal welfare | Impact on environment | Impact on biodiversity | |
| A | N | N | Y | Y | N | N | N | N |
CI: N PI: NC |
CI: N PI: NC |
CI: N PI: NC |
| B | Y | Y | Y | Y | Y | Y | N | N |
CI: N PI: NC |
CI: N PI: NC |
CI: N PI: NC |
| C | Y | Y | Y | Y | N | Y | N | N |
CI: N PI: NC |
CI: N PI: NC |
CI: N PI: NC |
| D | N | ||||||||||
| E | Y | ||||||||||
According to the assessment here performed, WNF complies with the following criteria of the Sections 1–5 of Annex IV of the AHL for the application of the disease prevention and control rules referred to in points (a)–(e) of Article 9(1):
To be assigned to category A, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment WNF complies with criteria 2.2 and 2.3, but not with 1, 2.1 and 2.4. To be eligible for category A, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and WNF does not comply with criteria 3, 4 and 5a and the assessment is inconclusive on compliance with criteria 5b, 5c and 5d.
To be assigned to category B, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment WNF complies with all of them. To be eligible for category B, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and WNF complies with criterion 3, but not with criteria 4 and 5a and the assessment is inconclusive on compliance with criteria 5b, 5c and 5d.
To be assigned to category C, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment WNF complies with criteria 1, 2.1, 2.2 and 2.3, but not with 2.4. To be eligible for category C, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and WNF complies with criterion 3, but not with criteria 4 and 5a and this assessment is inconclusive on compliance with criteria 5b, 5c and 5d.
To be assigned to category D, a disease needs to comply with criteria of Sections 1, 2, 3 or 5 of Annex IV of the AHL and with the specific criterion D of Section 4. WNF does not comply with the latter.
To be assigned to category E, a disease needs to comply with criteria of Sections 1, 2 or 3 of Annex IV of the AHL and/or the surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment. The latter is applicable if a disease fulfils the criteria as in Article 5, with which WNF complies.
3.4. Assessment of Article 8
This section presents the results of the assessment on the criteria of Article 8(3) of the AHL about WNF. The Article 8(3) criteria are about animal species to be listed, as it reads below:
‘3. Animal species or groups of animal species shall be added to this list if they are affected or if they pose a risk for the spread of a specific listed disease because:
they are susceptible for a specific listed disease or scientific evidence indicates that such susceptibility is likely; or
they are vector species or reservoirs for that disease, or scientific evidence indicates that such role is likely’.
For this reason the assessment on Article 8 criteria is based on the evidence as extrapolated from the relevant criteria of Article 7, i.e. the ones related to susceptible and reservoir species or routes of transmission, which cover also possible role of biological or mechanical vectors.2 According to the mapping, as presented in Table 5, Section 3.2 of the scientific opinion on the ad hoc methodology (EFSA AHAW Panel, 2017a), the main animal species to be listed for WNF according to the criteria of Article 8(3) are several species of birds and mammals, displayed in details in Table A.1 in Appendix A, as susceptible species. Several bird species belonging to the families of Corvidae, Passeridae and Fringillidae (order of Passeriformes) and to the family of Columbidae (order Columbiformes) can be considered reservoir species for WNV in Europe, details are shown in Table B.1 in Appendix B. The main vectors are some species of mosquitoes belonging to the genera Culex, Aedes and Coquillettidia (family Culicidae, order Diptera). The vector species are listed in https://efsa.maps.arcgis.com/apps/MapJournal/index.html?appid=512a03aa8df84d54a51bcb69d1b62735 (EFSA AHAW Panel, 2017b).
4. Conclusions
TOR 1: for each of those diseases an assessment, following the criteria laid down in Article 7 of the AHL, on its eligibility of being listed for Union intervention as laid down in Article 5(3) of the AHL;
According to the assessment here performed, WNF complies with all criteria of the first set and with one criterion of the second set and therefore can be considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL.
TOR 2a: for each of the diseases which was found eligible to be listed for Union intervention, an assessment of its compliance with each of the criteria in Annex IV to the AHL for the purpose of categorisation of diseases in accordance with Article 9 of the AHL;
According to the assessment here performed, WNF meets the criteria as in Sections 2 and 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in points (b) and (e) of Article 9(1) of the AHL.
TOR 2b: for each of the diseases which was found eligible to be listed for Union intervention, a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL.
According to the assessment here performed, the animal species that can be considered to be listed for WNF according to Article 8(3) of the AHL are, as susceptible species, several orders of birds and mammals and two orders of reptiles, as reported in Table A.1 in Appendix A of the present document. Reservoirs are several bird species belonging to the families of Corvidae, Passeridae and Fringillidae (order of Passeriformes) and to the family of Columbidae (order Columbiformes). Vectors are some species of mosquitoes belonging to the genera Culex, Aedes and Coquillettidia (family Culicidae, order Diptera).
Abbreviations
- ADNS
Animal Diseases Notification System
- AHAW
EFSA Panel on Animal Health and Welfare
- AHL
Animal Health Law
- ALF
Alfuy
- CDC
Centers for Disease Control and Prevention
- CFSPH
Center for Food Security and Public Health
- CITES
Convention on International Trade in Endangered Species of Wild Fauna and Flora
- CPC
Cacipacore
- DALY
disability‐adjusted life year
- ECDC
European Centre for Disease Prevention and Control
- ELISA
enzyme‐linked immunosorbent assay
- HCDCP
Hellenic Center for Disease Control & Prevention
- HI
haemagglutination inhibition
- ICBA
Individual and Collective Behavioural Aggregation
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- ISS
Istituto Superiore di Sanità
- IZSAM
Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise
- IUCN
International Union for Conservation of Nature
- KOU
Koutango
- KUN
Kunjin
- LLIN
long lasting impregnated net
- MS
Member State
- MVE
Murray Valley encephalitis
- OIE
World Organisation for Animal Health
- PCR
polymerase chain reaction
- p.i.
post infection
- PFU
plaque‐forming unit
- PRN
plaque reduction neutralisation
- RNA
ribonucleic acid
- RT‐PCR
reverse transcription polymerase chain reaction
- SLE
St. Louis encephalitis
- TBE
tick‐borne encephalitis
- TCID50
tissue culture infective dose, median
- ToR
Terms of Reference
- USU
Usutu
- VN
virus neutralisation
- WNF
West Nile fever
- WNV
West Nile virus
- YAO
Yaounde
Appendix A – Animal species infected naturally and experimentally by WNV
1.
Appendix B – List of wild and domestic WNV reservoir/sentinel animal species
1.
Appendix C – WNV morbidity and mortality rates in horses
1.
Supporting information
Mapped fact‐sheet used in the individual judgement on West Nile Fever
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Dhollander S, Beltrán‐Beck B, Kohnle L, Morgado J and Bicout D, 2017. Scientific Opinion on the assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): West Nile fever. EFSA Journal 2017;15(8):4955, 51 pp. 10.2903/j.efsa.2017.4955
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00592
Panel members: Dominique Bicout, Anette Bøtner, Andrew Butterworth, Paolo Calistri, Klaus Depner, Sandra Edwards, Bruno Garin‐Bastuji, Margaret Good, Christian Gortázar Schmidt, Virginie Michel, Miguel Angel Miranda, Simon More, Søren Saxmose Nielsen, Mohan Raj, Liisa Sihvonen, Hans Spoolder, Jan Arend Stegeman, Hans‐Hermann Thulke, Antonio Velarde, Preben Willeberg and Christoph Winckler.
Acknowledgements: The Panel wishes to thank Federica Monaco for the support provided to this scientific output.
Adopted: 30 June 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1 and Figure 3 (Annex): © ICT – Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise ‘G. Caporale’; Table 6: © World Organisation for Animal Health (OIE); Figures 1,2 (Annex): Rizzo et al., 2016 (CC BY 4.0). Eurosurveillance
Amendment: Due to a data extraction error, the wrong data were reported in Table 1 (pp. 6–7) of the original opinion. This has been corrected in the version published on 1 October 2018. The error has no impact on the conclusions, recommendations, scientific understanding or interpretation of the opinion. The original version is available on request.
Amended: 2 October 2018
Notes
Commission Decision of 28 April 2008 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council (notified under document number C(2008) 1589) (Text with EEA relevance). OJ L 159, 18.6.2008, p. 46–90.
A vector is a living organism that transmits an infectious agent from an infected animal to a human or another animal. Vectors are frequently arthropods. Biological vectors may carry pathogens that can multiply within their bodies and be delivered to new hosts, usually by biting. In mechanical vectors, the pathogens do not multiply within the vector, which usually remains infected for shorter time than in biological vectors.
References
- Akogbéto MC, Padonou GG, Gbénou D, Irish S and Yadouleton A, 2010. Bendiocarb, a potential alternative against pyrethroid resistant Anopheles gambiae in Benin, West Africa. Malaria Journal, 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalucía Ministry of Agriculture , online. Available online: http://www.juntadeandalucia.es/organismos/agriculturapescaydesarrollorural/areas/ganaderia/sanidad-animal/paginas/fiebre-nilo-occidental.html [Accessed: 21 July 2017]
- ArboZoonet , online, 2016. Distribution cases (confirmed and probable) of West Nile fever in Europe and in Mediterranean Basin (2008–2016) Available online: https://arbozoonet.izs.it/arbozoonet [Accessed: 21 July 2017]
- Austgen LE, Bowen RA, Bunning ML, Davis BS, Mitchell CJ and Chang GJJ, 2004. Experimental infection of cats and dogs with West Nile Virus. Emerging Infectious Diseases, 10, 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badolo A, Guelbéogo WM, Tiono AB, Traoré A, Sagnon N and Sirima SB, 2014. Laboratory evaluation of Fendona 6SC® treated bednets and Interceptor® long‐lasting nets against Anopheles gambiae s.l. in Burkina Faso. Parasitology Research, 113, 1069–1075. [DOI] [PubMed] [Google Scholar]
- Bahuon C, Marcillaud‐Pitel C, Bournez L, Leblond A, Beck C, Hars J, Leparc‐Goffard I, L'Ambert G, Pat yM, Cavalerie L, Daix C, Tritz P, Durand B, Zientara S and Lecollinet S, 2016. West Nile virus epizootics in Camargue, France, in 2015, and reinforcements of west Nile virus surveillance and control networks. Revue Scientifique Et Technique‐Office International Des Epizooties, 35, 811–824. [DOI] [PubMed] [Google Scholar]
- Bakonyi T, Ivanics E, Erdelyi K, Ursu K, Ferenczi E, Weissenbock H and Nowotny N, 2006. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerging Infectious Diseases, 12, 618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banet‐Noach C, Simanov L and Malkinson M, 2003. Direct (non‐vector) transmission of West Nile virus in geese. Avian Pathology, 32, 489–494. [DOI] [PubMed] [Google Scholar]
- Barber LM, Schleier Iii JJ and Peterson RKD, 2010. Economic cost analysis of West Nile virus outbreak, Sacramento County, California, USA, 2005. Emerging Infectious Diseases, 16, 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard BJ and Voges SF, 1986. Flaviviruses in South Africa: pathogenicity for sheep. Onderstepoort Journal of Veterinary Research, 53, 235–238. [PubMed] [Google Scholar]
- Barros SC, Ramos F, Ze‐Ze L, Alves MJ, Fagulha T, Duarte M, Henriques M, Luis T and Fevereiro M, 2013. Simultaneous detection of West Nile and Japanese encephalitis virus RNA by duplex TaqMan RT‐PCR. Journal of Virological Methods, 193, 554–557. [DOI] [PubMed] [Google Scholar]
- Bingham J, Lunt RA, Green DJ, Davies KR, Stevens V and Wong FYK, 2010. Experimental studies of the role of the little raven (Corvus mellori) in surveillance for West Nile virus in Australia. Australian veterinary journal, 88, 204–210. [DOI] [PubMed] [Google Scholar]
- Bowen RA, Bosco‐Lauth A, Syvrud K, Thomas A, Meinert TR, Ludlow DR, Cook C, Salt J and Ons E, 2014. Protection of horses from West Nile virus Lineage 2 challenge following immunization with a whole, inactivated WNV lineage 1 vaccine. Vaccine, 32, 5455–5459. [DOI] [PubMed] [Google Scholar]
- Boyle DB, Marshall ID and Dickerman RW, 1983a. Primary antibody responses of herons to experimental infection with Murray Valley encephalitis and Kunjin viruses. The Australian journal of experimental biology and medical science, 61, 665–674. [DOI] [PubMed] [Google Scholar]
- Boyle DB, Dickerman RW and Marshall ID, 1983b. Primary viraemia responses of herons to experimental infection with Murray Valley encephalitis, Kunjin and Japanese encephalitis viruses. Australian Journal of Experimental Biology and Medical Science, 61, 655–664. [DOI] [PubMed] [Google Scholar]
- Brault AC, Langevin SA, Bowen RA, Panella NA, Biggerstaff BJ, Miller BR and Komar N, 2004. Differential virulence of West Nile strains for American Crows. Emerging Infectious Diseases, 10, 2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Huang CYH, Langevin SA, Kinney RM, Bowen RA, Ramey WN, Panella NA, Holmes EC, Powers AM and Miller BR, 2007. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nature Genetics, 39, 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Langevin SA, Ramey WN, Fang Y, Beasley DWC, Barker CM, Sanders TA, Reisen WK, Barrett ADT and Bowen RA, 2011. Reduced avian virulence and viremia of West Nile virus isolates from Mexico and Texas. American Journal of Tropical Medicine and Hygiene, 85, 758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunning ML, Bowen RA, Bruce Cropp C, Sullivan KG, Davis BS, Komar N, Godsey MS, Baker D, Hettler DL, Holmes DA, Biggerstaff BJ and Mitchell CJ, 2002. Experimental infection of horses with West Nile virus. Emerging Infectious Diseases, 8, 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DS and Monath TP, 2001. Flaviviruses In: Knipe DM. and Howley PM. (eds.). Fields Virology. Lippincott Williams and Wilkins, Philadelphia, USA: pp. 1043–1126. [Google Scholar]
- Busquets N, Bertran K, Costa TP, Rivas R, De La Fuente JG, Villalba R, Solanes D, Bensaid A, Majó N and Pagès N, 2012. Experimental West Nile virus infection in Gyr‐Saker hybrid falcons. Vector‐Borne and Zoonotic Diseases, 12, 482–489. [DOI] [PubMed] [Google Scholar]
- Calistri P, Giovannini A, Hubalek Z, Ionescu A, Monaco F, Savini G and Lelli R, 2010. Epidemiology of West Nile in Europe and in the Mediterranean basin. Open Virology Journal, 4, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calistri P, Savini L, Candeloro L, Di Sabatino D, Cito F, Bruno R and Danzetta ML, 2016. A transitional model for the evaluation of West Nile Virus Transmission in Italy. Transbound and Emerging Diseases, 63, 485–496. [DOI] [PubMed] [Google Scholar]
- Campbell G, Lanciotti R, Bernard B and Lu H, 2002. Laboratory‐acquired West Nile virus infections ‐ United States, 2002. Morbidity and Mortality Weekly Report, 51, 1133–1135. [PubMed] [Google Scholar]
- Cantile C, Di Guardo G, Eleni C and Arispici M, 2000. Clinical and neuropathological features of West Nile virus equine encephalomyelitis in Italy. Equine Veterinary Journal, 32, 31–35. [DOI] [PubMed] [Google Scholar]
- Carney RM, Husted S, Jean C, Glaser C and Kramer V, 2008. Efficacy of aerial spraying of mosquito adulticide in reducing incidence of West Nile Virus, California, 2005. Emerging Infectious Diseases, 14, 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson PJ, Konewko P, Wold KS, Mariani P, Goli S, Bergloff P and Crosby RD, 2006. Long‐term clinical and neuropsychological outcomes of West Nile virus infection. Clinical Infectious Diseases, 43, 723–730. [DOI] [PubMed] [Google Scholar]
- Castillo‐Olivares J, Mansfield KL, Phipps LP, Johnson N, Tearle J and Fooks AR, 2011. Antibody response in horses following experimental infection with West Nile virus lineages 1 and 2. Transboundary and Emerging Diseases, 58, 206–212. [DOI] [PubMed] [Google Scholar]
- CDC , 2002. Laboratory‐acquired West Nile virus infections–United States, 2002, MMWR. Morbidity and Mortality Weekly Report, 51, 1133–1135. [PubMed] [Google Scholar]
- Cetin H, Yanikoglu A and Cilek JE, 2006. Efficacy of diflubenzuron, a chitin synthesis inhibitor, against Culex pipiens larvae in septic tank water. Journal of the American Mosquito Control Association, 22, 343–345. [DOI] [PubMed] [Google Scholar]
- CFSPH (Center for Food Security and Public Health), 2013. West Nile Virus Infection. Iowa, USA, 19 pp, Available online: http://elearning.unite.it/pluginfile.php/436/mod_resource/content/1/Reading%208%20on%20West%20Nile%20Fever.pdf
- CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora), online, 2016. Appendices I, II and III. Available online: https://cites.org/sites/default/files/eng/app/2016/E-Appendices-2016-03-10.pdf
- Clark L, Hall J, McLean R, Dunbar M, Klenk K, Bowen R and Smeraski CA, 2006. Susceptibility of greater sage‐grouse to experimental infection with West Nile virus. Journal of Wildlife Diseases, 42, 14–22. [DOI] [PubMed] [Google Scholar]
- Cleton NB, van Maanen K, Bergervoet SA, Bon N, Beck C, Godeke GJ, Lecollinet S, Bowen R, Lelli D, Nowotny N, Koopmans MPG and Reusken CBEM, in press. A serological protein microarray for detection of multiple cross‐reactive flavivirus infections in horses for veterinary and public health surveillance. Transboundary and Emerging Diseases. Available online: http://onlinelibrary.wiley.com/doi/10.1111/tbed.12569/epdf1.5 [DOI] [PubMed] [Google Scholar]
- Cruz‐Pacheco G, Esteva L, Montano‐Hirose JA and Vargas C, 2005. Modelling the dynamics of West Nile Virus. Bulletin of Mathematical Biology, 67, 1157–1172. [DOI] [PubMed] [Google Scholar]
- Dabiré RK, Diabaté A, Baldet T, Paré‐Toé L, Guiguemdé RT, Ouédraogo JB and Skovmand O, 2006. Personal protection of long lasting insecticide‐treated nets in areas of Anopheles gambiae s.s. resistance to pyrethroids. Malaria Journal, 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame DA, Meisch MV, Lewis CN, Kline DL and Clark GG, 2014. Field evaluation of four spatial repellent devices against Arkansas rice‐land mosquitoes. Journal of the American Mosquito Control Association, 30, 31–36. [DOI] [PubMed] [Google Scholar]
- Darriet F, N'Guessan R, Koffi AA, Konan L, Doannio JMC, Chandre F and Carnevale P, 2000. Impact of the resistance to pyrethroids on the efficacy of impregnated bednets used as a means of prevention against malaria: results of the evaluation carried out with deltamethrin SC in experimental huts. Bulletin de la Societe de Pathologie Exotique, 93, 131–134. [PubMed] [Google Scholar]
- Del Amo J, Sotelo E, Fernández‐Pinero J, Gallardo C, Llorente F, Agüero M and Jiménez‐Clavero MA, 2013. A novel quantitative multiplex real‐time RT‐PCR for the simultaneous detection and differentiation of West Nile virus lineages 1 and 2, and of Usutu virus. Journal of Virological Methods, 189, 321–327. [DOI] [PubMed] [Google Scholar]
- Del Amo J, Llorente F, Figuerola J, Soriguer RC, Moreno AM, Cordioli P, Weissenbock H and Jimenez‐Clavero MA, 2014. Experimental infection of house sparrows (Passer domesticus) with West Nile virus isolates of Euro‐Mediterranean and North American origins. Veterinary Research, 45, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz LA, Flores FS and Contigiani MS, 2011. Viremia profiles and host competence index for West Nile virus (Flavivirus, Flaviviridae) in three autochthonous birds species from Argentina. Journal of Ornithology, 152, 21–25. [Google Scholar]
- Dridi M, Vangeluwe D, Lecollinet S, van den Berg T and Lambrecht B, 2013. Experimental infection of Carrion crows (Corvus corone) with two European West Nile virus (WNV) strains. Veterinary Microbiology, 165, 160–166. [DOI] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2016. EU Threats: West Nile virus ‐ Multistate (Europe) ‐ Monitoring season 2016. Communicable Disease Threats Report, Stockholm, Sweden, 13, Available online: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/communicable-disease-threats-report-29-oct-2016.pdf
- ECDC (European Centre for Disease Prevention and Control), online. Available online: http://ecdc.europa.eu/en/healthtopics/west_nile_fever/west-nile-fever-maps/pages/index.aspx [Accessed: 21 July 2017]
- EFSA AHAW Panel , More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Gervelmeyer A, Zancanaro G, Kohnle L, Morgado J and Bicout D, 2017a. Ad hoc method for the assessment on listing and categorisation of animal diseases within the framework of the Animal Health Law. EFSA Journal 2017;15(7):4783, 42 pp. 10.2903/j.efsa.2017.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel , More S, Bicout D, Bøtner A, Butterworth A, Calistri P, De Koeijer A, Depner K, Edwards S, Garin‐Bastuji B, Good M, GortazarSchmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Bau A, Beltran‐Beck B, Carnesecchi E, Casier P, Czwienczek E, Dhollander S, Georgiadis M, Gogin A, Pasinato L, Richardson J, Riolo F, Rossi G, Watts M, Lima E and Stegeman JA, 2017b. Vector‐borne diseases. EFSA Journal 2017;15(5):4793, 91 pp. 10.2903/j.efsa.2017.4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden M, Vina‐Rodriguez A, Hoffmann B, Ziegler U and Groschup MH, 2010. Two new real‐time quantitative reverse transcription polymerase chain reaction assays with unique target sites for the specific and sensitive detection of lineages 1 and 2 West Nile virus strains. Journal of Veterinary Diagnostic Investigation, 22, 748–753. [DOI] [PubMed] [Google Scholar]
- Erdélyi K, Ursu K, Ferenczi E, Szeredi L, Rátz F, Skáre J and Bakonyi T, 2007. Clinical and pathologic features of lineage 2 West Nile virus infections in birds of prey in Hungary. Vector‐Borne and Zoonotic Diseases, 7, 181–188. [DOI] [PubMed] [Google Scholar]
- Escribano‐Romero E, Gamino V, Merino‐Ramos T, Blazquez AB, Martin‐Acebes MA, de Oya NJ, Gutierrez‐Guzman AV, Escribano JM, Hofle U and Saiz JC, 2013. Protection of red‐legged partridges (Alectoris rufa) against West Nile virus (WNV) infection after immunization with WNV recombinant envelope protein E (rE). Vaccine, 31, 4523–4527. [DOI] [PubMed] [Google Scholar]
- Faggioni GDSR, Pomponi A, Fantini M, Savini G, Monaco F, Polci A, Bei R and Lista F, 2014. Rapid molecular detection and genotyping of West Nile Virus lineages 1 and 2 by real time PCR and melting curve analysis. Journal of Virological Methods, 207, 54–59. [DOI] [PubMed] [Google Scholar]
- Fang Y and Reisen WK, 2006. Previous infection with West Nile or St. Louis encephalitis viruses provides cross protection during reinfection in house finches. American Journal of Tropical Medicine and Hygiene, 75, 480–485. [PubMed] [Google Scholar]
- Filipe AR and Pinto MR, 1969. Survey for antibodies to arboviruses in serum of animals from southern Portugal. American Journal of Tropical Medicine and Hygiene, 18, 423–426. [DOI] [PubMed] [Google Scholar]
- Fonseca K, Prince GD, Bratvold J, Fox JD, Pybus M, Preksaitis JK and Tilley P, 2005. West Nile virus infection and conjunctival exposure. Emerging Infectious Diseases, 11, 1648–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TL, Harrigan RJ, Lamanna JA, Desante DF, Saracco JF and Smith TB, 2015. Persistent impacts of West Nile virus on North American bird populations. Proceedings of the National Academy of Sciences of the United States of America, 112, 14290–14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YY, Balasuriya UBR and C‐k Lee, 2014. Zoonotic encephalitides caused by arboviruses: transmission and epidemiology of alphaviruses and flaviviruses. Clinical and Experimental Vaccine Research, 3, 58–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero‐Sánchez S, Cuevas‐Romero S, Nemeth NM, Trujillo‐Olivera MTJ, Worwa G, Dupuis A, Brault AC, Kramer LD, Komar N and Estrada‐Franco JG, 2011. West Nile virus infection of birds, Mexico. Emerging Infectious Diseases, 17, 2245–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Sweeney AW, Rutledge LC, Cooper RD, Frances SP and Westrom DR, 1987. Effectiveness of controlled‐release personal‐use arthropod repellents and permethrin‐impregnated clothing in the field. Journal of the American Mosquito Control Association, 3, 556–560. [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB and Walker ED, 2009. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. American Journal of Tropical Medicine and Hygiene, 80, 268–278. [PubMed] [Google Scholar]
- HCDCP (Hellenic Center for Disease Control & Prevention), online. Available online: http://www.keelpno.gr/en-us/home.aspx [Accessed: 21 July 2017]
- Hofle U, Blanco JM, Crespo E, Naranjo V, Jimenez‐Clavero MA, Sanchez A, de la Fuente J and Gortazar C, 2008. West Nile virus in the endangered Spanish imperial eagle. Veterinary Microbiology, 129, 171–178. [DOI] [PubMed] [Google Scholar]
- Hubalek Z, Rudolf I and Nowotny N, 2014. Arboviruses pathogenic for domestic and wild animals In: Maramorosch K. and Murphy FA. (eds.). Advances in Virus Research. Elsevier Academic Press Inc, San Diego: pp. 201–275. [DOI] [PubMed] [Google Scholar]
- Humblet MF, Vandeputte S, Fecher‐Bourgeois F, Léonard P, Gosset C, Balenghien T, Durand B and Saegerman C, 2016. Estimating the economic impact of a possible equine and human epidemic of West Nile virus infection in Belgium. Eurosurveillance Weekly, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISS (Istituto Superiore di Sanità), online. Available online: http://www.epicentro.iss.it/problemi/westNile/bollettino.asp [Accessed: 21 July 2017]
- Italian Ministry of Health , 2016. Piano Nazionale integrato di sorveglianza e risposta al virus della West Nile. Circolare 10/08/2016, n. 23689. 39. Available online: http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2016&codLeg=55662&parte=1%20&serie=null
- IZSAM (Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise), online. Available online: http://sorveglianza.izs.it/emergenze/west_nile/emergenze.html [Accessed: 21 July 2017]
- Jimenez‐Clavero MA, Sotelo E, Fernandez‐Pinero J, Llorente F, Blanco JM, Rodriguez‐Ramos J, Perez‐Ramirez E and Hofle U, 2008. West Nile virus in golden eagles, Spain, 2007. Emerging Infectious Diseases, 14, 1489–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DJ, Ostlund EN, Pedersen DD and Schmitt BJ, 2001. Detection of North American West Nile virus in animal tissue by a reverse transcription‐nested polymerase chain reaction assay. Emerging Infectious Diseases, 7, 739–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert L, Oudar J, Hannoun C, Beytout D, Corniou B, Guillon JC and Panthier R, 1970. Epidemiology of the West Nile virus: study of a focus in Camargue. IV. Meningo‐encephalomyelitis of the horse. Annales de l'Institut Pasteur, 118, 239–247. [PubMed] [Google Scholar]
- Karaca K, Bowen R, Austgen LE, Teehee M, Siger L, Grosenbaugh D, Loosemore L, Audonnet JC, Nordgren R and Minke JM, 2005. Recombinant canarypox vectored West Nile virus (WNV) vaccine protects dogs and cats against a mosquito WNV challenge. Vaccine, 23, 3808–3813. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Peters RJ, Dupuis AP, Jones MJ, Daszak P, Marra PP and Kramer LD, 2013. Predicted and observed mortality from vector‐borne disease in wildlife: West Nile virus and small songbirds. Biological Conservation, 165, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney RM, Huang CYH, Whiteman MC, Bowen RA, Langevin SA, Miller BR and Brault AC, 2006. Avian virulence and thermostable replication of the North American strain of West Nile virus. Journal of General Virology, 87, 3611–3622. [DOI] [PubMed] [Google Scholar]
- Kipp AM, Lehman JA, Bowen RA, Fox PE, Stephens MR, Klenk K, Komar N and Bunning ML, 2006. West Nile virus quantification in feces of experimentally infected American and fish crows. American Journal of Tropical Medicine and Hygiene, 75, 688–690. [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R and Bunning M, 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerging Infectious Diseases, 9, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Panella NA, Langevin SA, Brault AC, Amador M, Edwards E and Owen JC, 2005. Avian hosts for West Nile Virus in St. Tammany Parish, Louisiana, 2002. American Journal of Tropical Medicine and Hygiene, 73, 1031–1037. [PubMed] [Google Scholar]
- Kumar JS, Saxena D and Parida M, 2014. Development and comparative evaluation of SYBR Green I‐based one‐step real‐time RT‐PCR assay for detection and quantification of West Nile virus in human patients. Molecular and Cellular Probes, 28, 221–227. [DOI] [PubMed] [Google Scholar]
- LaDeau SL, Kilpatrick AM and Marra PP, 2007. West Nile virus emergence and large‐scale declines of North American bird populations. Nature, 447, 710‐U713. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS and Roehrig JT, 2000. Rapid detection of West Nile virus from human clinical specimens, field‐collected mosquitoes, and avian samples by a TaqMan reverse transcriptase‐PCR assay. Journal of Clinical Microbiology, 38, 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin SA, Bunning M, Davis B and Komar N, 2001. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerging Infectious Diseases, 7, 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin SA, Brault AC, Panella NA, Bowen RA and Komar N, 2005. Variation in virulence of West Nile virus strains for house sparrows (Passer domesticus). American Journal of Tropical Medicine and Hygiene, 72, 99–102. [PubMed] [Google Scholar]
- Laperriere V, Brugger K and Rubel F, 2011. Simulation of the seasonal cycles of bird, equine and human West Nile virus cases. Preventive Veterinary Medicine, 98, 99–100. [DOI] [PubMed] [Google Scholar]
- LaPointe DA, Hotmeister EK, Atkinson CT, Porter RE and Dusek RJ, 2009. Experimental infection of Hawai'i ‘amakihi (Hemignathus virens) with west nile virus and competence of a co‐occurring vector, Culex quinquefasciatus: potential impacts on endemic Hawaiian avifauna. Journal of Wildlife Diseases, 45, 257–271. [DOI] [PubMed] [Google Scholar]
- Lim SM, Brault AC, van Amerongen G, Sewbalaksing VD, Osterhaus AD, Martina BE and Koraka P, 2014. Susceptibility of European jackdaws (Corvus monedula) to experimental infection with lineage 1 and 2 West Nile viruses. Journal of General Virology, 95, 1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MT, Jeter W, Hernandez J, Sellon DC, Gosche D, Gillis K, Bille E and Gibbs EP, 2006. Diagnostic performance of the equine IgM capture ELISA for serodiagnosis of West Nile virus infection. Journal of Veterinary Internal Medicine, 20, 608–613. [DOI] [PubMed] [Google Scholar]
- Malkinson M, Banet C, Weisman Y, Pokamunski S, King R, Drouet MT and Deubel V, 2002. Introduction of West Nile virus in the Middle East by migrating white storks. Emerging Infectious Diseases, 8, 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani TR, Reuben R and Akiyama J, 1991. Field efficacy of “Mosbar” mosquito repellent soap against vectors of bancroftian filariasis and Japanese encephalitis in Southern India. Journal of the American Mosquito Control Association, 7, 565–568. [PubMed] [Google Scholar]
- Marcombe S, Darriet F, Tolosa M, Agnew P, Duchon S, Etienne M, Tcha MMY, Chandre F, Corbel V and Yebakima A, 2011. Pyrethroid resistance reduces the efficacy of space sprays for dengue control on the Island of Martinique (Caribbean). PLoS Neglected Tropical Diseases, 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh BM, Dickinson DB and McGillivray GM, 1969. Ecological studies on Sindbis and West Nile viruses in South Africa. V. The response of birds to inoculation of virus. South African Journal of Medical Sciences, 34, 77–82. [PubMed] [Google Scholar]
- McLean RG, Ubico SR, Docherty DE, Hansen WR, Sileo L and McNamara TS, 2001. West Nile virus transmission and ecology in birds. Conference Paper, 951, 54–57. [DOI] [PubMed] [Google Scholar]
- Monaco F, Goffredo M, Briguglio P, Pinoni C, Polci A, Iannetti S, Marruchella G, Di Francesco G, Di Gennaro AP, Pais M, Teodori L, Bruno R, Catalani M, Ruiu A, Lelli R and Savini G, 2015. The 2011 West Nile disease outbreak in Sardinia region, Italy. Veterinaria Italiana, 51, 5–16. [DOI] [PubMed] [Google Scholar]
- Moosa‐Kazemi SH, Vatandoost H, Raeisi A and Akbarzadeh K, 2007. Deltamethrin impregnated bed nets in a malaria control program in chabahar, Southeast Baluchistan, IR Iran. Iranian Journal of Arthropod‐Borne Diseases, 1, 43–51. [Google Scholar]
- Muller O, Ido K and Traore C, 2002. Evaluation of a prototype long‐lasting insecticide‐treated mosquito net under field conditions in rural Burkina Faso. Transactions of the Royal Society of Tropical Medicine and Hygiene, 96, 483–484. [DOI] [PubMed] [Google Scholar]
- Munoz J, Ruiz S, Soriguer R, Alcaide M, Viana DS, Roiz D, Vazquez A and Figuerola J, 2012. Feeding patterns of potential West Nile virus vectors in south‐west Spain. PLoS ONE, 7, e39549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KO, Baraniuk S, Resnick M, Arafat R, Kilborn C, Shallenberger R, York TL, Martinez D, Malkoff M, Elgawley N, McNeely W and Khuwaja SA, 2008. Clinical investigation of hospitalized human cases of West Nile virus infection in Houston, Texas, 2002–2004. Vector Borne and Zoonotic Diseases, 8, 167–174. [DOI] [PubMed] [Google Scholar]
- Murray K, Walker C, Herrington E, Lewis JA, McCormick J, Beasley DW, Tesh RB and Fisher‐Hoch S, 2010. Persistent infection with West Nile virus years after initial infection. Journal of Infectious Diseases, 201, 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KO, Garcia MN, Rahbar MH, Martinez D, Khuwaja SA, Arafat RR and Rossmann S, 2014. Survival analysis, long‐term outcomes, and percentage of recovery up to 8 years post‐infection among the Houston West Nile virus cohort. PLoS ONE, 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasci RS, Savage HM, White DJ, Miller JR, Cropp BC, Godsey MS, Kerst AJ, Bennett P, Gottfried K and Lanciotti RS, 2001. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerging Infectious Diseases, 7, 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth N, Gould D, Bowen R and Komar N, 2006a. Natural and experimental West Nile virus infection in five raptor species. Journal of Wildlife Diseases, 42, 1–13. [DOI] [PubMed] [Google Scholar]
- Nemeth NM, Hahn DC, Gould DH and Bowen RA, 2006b. Experimental West Nile virus infection in Eastern Screech Owls (Megascops asio). Avian Diseases, 50, 252–258. [DOI] [PubMed] [Google Scholar]
- Nemeth NM, Oesterle PT and Bowen RA, 2008. Passive immunity to West Nile virus provides limited protection in a common passerine species. American Journal of Tropical Medicine and Hygiene, 79, 283–290. [PubMed] [Google Scholar]
- Nemeth N, Young G, Ndaluka C, Bielefeldt‐Ohmann H, Komar N and Bowen R, 2009a. Persistent West Nile virus infection in the house sparrow (Passer domesticus). Archives of Virology, 154, 783–789. [DOI] [PubMed] [Google Scholar]
- Nemeth NM, Bosco‐Lauth AM and Bowen RA, 2009b. Cross‐protection between West Nile and Japanese encephalitis viruses in red‐winged blackbirds (Agelaius phoeniceus). Avian Diseases, 53, 421–425. [DOI] [PubMed] [Google Scholar]
- Nemeth NM, Thomsen BV, Spraker TR, Benson JM, Bosco‐Lauth AM, Oesterle PT, Bright JM, Muth JP, Campbell TW, Gidlewski TL and Bowen RA, 2011. Clinical and pathologic responses of american crows (Corvus brachyrhynchos) and Fish Crows (C. ossifragus) to experimental West Nile Virus Infection. Veterinary Pathology, 48, 1061–1074. [DOI] [PubMed] [Google Scholar]
- Oesterle PT, Nemeth NM, VanDalen K, Sullivan H, Bentler KT, Young GR, McLean RG, Clark L, Smeraski C and Hall JS, 2009. Experimental infection of cliff swallows (Petrochelidon pyrrhonota) with varying doses of West Nile virus. American Journal of Tropical Medicine and Hygiene, 81, 1159–1164. [DOI] [PubMed] [Google Scholar]
- Oesterle P, Nemeth N, Young G, Mooers N, Elmore S, Bowen R, Doherty P, Hall J, McLean R and Clark L, 2010. Cliff Swallows, Swallow Bugs, and West Nile Virus: an unlikely transmission mechanism. Vector‐Borne and Zoonotic Diseases, 10, 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE (World Organization for Animal Health), 2013. West Nile Fever In: OIE (ed.). Terrestrial Animal Health Code OIE. OIE, Paris, France: pp. 1–13. [Google Scholar]
- OIE (World Organization for Animal Health), online. World Animal Health Information System. Available online: http://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/Immsummary [Accessed: 21 July 2017]
- Okumu FO, Chipwaza B, Madumla EP, Mbeyela E, Lingamba G, Moore J, Ntamatungro AJ, Kavishe DR and Moore SJ, 2012. Implications of bio‐efficacy and persistence of insecticides when indoor residual spraying and long‐lasting insecticide nets are combined for malaria prevention. Malaria Journal, 11, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GH, Miller KJ, Docherty DE, Bochsler VS and Sileo L, 2009. Pathogenicity of west nile virus and response to vaccination in sandhill cranes (Grus Canadensis) using a killed vaccine. Journal of Zoo and Wildlife Medicine, 40, 263–271. [DOI] [PubMed] [Google Scholar]
- Onishchenko GG, Lipnitskii AV, Alekseev VV, Antonov VA, Kriuchkova TP and Krutogolovova TA, 2011. [Epidemiologic situation of West Nile fever in Russia in 2010]. Zhurnal Mikrobiologii Epidemiologii i Immunobiologii, 115–120. [PubMed] [Google Scholar]
- Ostlund EN, Andresen JE and Andresen M, 2000. West Nile encephalitis. Veterinary Clinics of North America. Equine Practice, 16, 427–441. [DOI] [PubMed] [Google Scholar]
- Ostlund EN, Crom RL, Pedersen DD, Johnson DJ, Williams WO and Schmitt BJ, 2001. Equine West Nile encephalitis, United States. Emerging Infectious Diseases, 7, 665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J, Moore F, Panella N, Edwards E, Bru R, Hughes M and Komar N, 2006. Migrating birds as dispersal vehicles for West Nile virus. EcoHealth, 3, 79–85. [Google Scholar]
- Owen JC, Nakamura A, Coon CA and Martin LB, 2012. The effect of exogenous corticosterone on West Nile virus infection in Northern Cardinals (Cardinalis cardinalis). Veterinary Research, 43, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panella NA, Young G and Komar N, 2013. Experimental infection of Eurasian collared‐dove (Streptopelia decaocto) with West Nile virus. Journal of Vector Ecology, 38, 210–214. [DOI] [PubMed] [Google Scholar]
- Papa A, Danis K, Baka A, Bakas A, Dougas G, Lytras T, Theocharopoulos G, Chrysagis D, Vassiliadou E, Kamaria F, Liona A, Mellou K, Saroglou G and Panagiotopoulos T, 2010. Ongoing outbreak of West Nile virus infections in humans in Greece, July‐August 2010. Eurosurveillance, 15, 20–29. [DOI] [PubMed] [Google Scholar]
- Pérez‐Ramírez E, Llorente F and Jiménez‐Clavero MÁ, 2014. Experimental infections of wild birds with West Nile virus. Viruses, 6, 752–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogodina VV, Frolova MP, Malenko GV, Fokina GI, Koreshkova GV, Kiseleva LL, Bochkova NG and Ralph NM, 1983. Study on West Nile virus persistence in monkeys. Archives of Virology, 75, 71–86. [DOI] [PubMed] [Google Scholar]
- Promed (Program for Monitoring Emerging Diseases), online. Available online: http://www.promedmail.org/pls/apex/f?p=2400:1202:2540120449811172::NO::F2400_P1202_CHECK_DISPLAY,F2400_P1202_PUB_MAIL_ID:X,85131 [Accessed: 21 July 2017]
- Reisen WK, 2013. Ecology of West Nile Virus in North America. Viruses, 5, 2079–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK and Fang Y, 2007. Does feeding on infected mosquitoes (Diptera: Culicidae) enhance the role of song sparrows in the transmission of arboviruses in California? Journal of Medical Entomology, 44, 316–319. [DOI] [PubMed] [Google Scholar]
- Reisen WK and Hahn DC, 2007. Comparison of immune responses of Brown‐headed Cowbird and related blackbirds to West Nile and other mosquito‐borne encephalitis viruses. Journal of Wildlife Diseases, 43, 439–449. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y and Martinez VM, 2005. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis Encephalitis virus transmission. Journal of Medical Entomology, 42, 367–375. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Lothrop HD, Martinez VM, Wilson J, O'Connor P, Carney R, Cahoon‐Young B, Shafii M and Brault AC, 2006. Overwintering of West Nile virus in Southern California. Journal of Medical Entomology, 43, 344–355. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Wheeler SS, Kennsington M, Gutierrez A, Fang Y, Garcia S and Lothrop B, 2008. Persistent West Nile virus transmission and the apparent displacement St. Louis encephalitis virus in Southeastern California, 2003‐2006. Journal of Medical Entomology, 45, 494–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revay EE, Junnila A, Xue RD, Kline DL, Bernier UR, Kravchenko VD, Qualls WA, Ghattas N and Muller GC, 2013. Evaluation of commercial products for personal protection against mosquitoes. Acta Tropica, 125, 226–230. [DOI] [PubMed] [Google Scholar]
- Rizzo C, Napoli C, Venturi G, Pupella S, Lombardini L, Calistri P, Monaco F, Cagarelli R, Angelini P, Bellini R, Tamba M, Piatti A, Russo F, Palù G, Chiari M, Lavazza A and Bella A, and the Italian WNV surveillance working group , 2016. West Nile virus transmission from 2008 to 2015: results from the integrated surveillance system in Eurosurveillance. Eurosurveillance, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiz DVA, Rosà R, Muñoz J, Arnoldi D, Rosso F, Figuerola J, Tenorio A and Rizzoli A, 2012. Blood meal analysis, flavivirus screening, and influence of meteorological variables on the dynamics of potential mosquito vectors of West Nile virus in northern Italy. Journal of Vector Ecology, 37, 20–28. [DOI] [PubMed] [Google Scholar]
- Rozendaal JA, Voorham J, Vanhoof JPM and Oostburg BFJ, 1989. Efficacy of mosquito nets treated with permethrin in Suriname. Medical and Veterinary Entomology, 3, 353–365. [DOI] [PubMed] [Google Scholar]
- Rubel F, Brugger K, Hantel M, Chvala‐Mannsberger S, Bakonyi T, Weissenböck H and Nowotny N, 2008. Explaining Usutu virus dynamics in Austria: model development and calibration. Preventive Veteterinary Medicine, 85, 166–186. [DOI] [PubMed] [Google Scholar]
- Savini G, Monaco F, Calistri P and Lelli R, 2008. Phylogenetic analysis of West Nile virus isolated in Italy in 2008. Eurosurveillance Weekly, 13, pii: 19048. [PubMed] [Google Scholar]
- Saxena D, Kumar JS, Parida M, Sivakumar RR and Patro IK, 2013. Development and evaluation of NS1 specific monoclonal antibody based antigen capture ELISA and its implications in clinical diagnosis of West Nile virus infection. Journal of Clinical Virology, 58, 528–534. [DOI] [PubMed] [Google Scholar]
- Shirafuji H, Kanehira K, Kubo M, Shibahara T and Kamio T, 2008. Experimental West Nile virus infection in jungle crows (Corvus macrorhynchos). American Journal of Tropical Medicine and Hygiene, 78, 838–842. [PubMed] [Google Scholar]
- Shirafuji H, Kanehira K, Kamio T, Kubo M, Shibahara T, Konishi M, Murakami K, Nakamura Y, Yamanaka T, Kondo T, Matsumura T, Muranaka M and Katayama Y, 2009. Antibody responses induced by experimental West Nile virus infection with or without previous immunization with inactivated Japanese encephalitis vaccine in horses. Journal of Veterinary Medical Science, 71, 969–974. [DOI] [PubMed] [Google Scholar]
- Sirbu A, Ceianu CS, Panculescu‐Gatej RI, Vazquez A, Tenorio A, Rebreanu R, Niedrig M, Nicolescu G and Pistol A, 2011. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Eurosurveillance, 16, pii: 19672. [PubMed] [Google Scholar]
- Snook CS, Hyman SS, Del Piero F, Palmer JE, Ostlund EN, Barr BS, Desrochers AM and Reilly LK, 2001. West Nile virus encephalomyelitis in eight horses. Journal of the American Veterinary Medical Association, 218, 1576–1579. [DOI] [PubMed] [Google Scholar]
- Soleimani‐Ahmadi M, Vatandoost H, Shaeghi M, Raeisi A, Abedi F, Eshraghian MR, Madani A, Safari R, Oshaghi MA, Abtahi M and Hajjaran H, 2012. Field evaluation of permethrin long‐lasting insecticide treated nets (Olyset(R)) for malaria control in an endemic area, southeast of Iran. Acta Tropica, 123, 146–153. [DOI] [PubMed] [Google Scholar]
- Soonwera M and Phasornkusolsill S, 2015. Efficacy of Thai herbal essential oils as green repellent against mosquito vectors. Acta Tropica, 142, 127–130. [DOI] [PubMed] [Google Scholar]
- Sotelo E, Llorente F, Rebollo B, Camuñas A, Venteo A, Gallardo C, Lubisi A, Rodríguez MJ, Sanz AJ, Figuerola J and Jiménez‐Clavero MÁ, 2011a. Development and evaluation of a new epitope‐blocking ELISA for universal detection of antibodies to West Nile virus. Journal of Virological Methods, 174, 35–41. [DOI] [PubMed] [Google Scholar]
- Sotelo E, Gutierrez‐Guzman AV, del Amo J, Llorente F, El‐Harrak M, Perez‐Ramirez E, Blanco JM, Hofle U and Jimenez‐Clavero MA, 2011b. Pathogenicity of two recent Western Mediterranean West Nile virus isolates in a wild bird species indigenous to Southern Europe: the red‐legged partridge. Veterinary Research, 42, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedicato MCI, Marruchella G, Bellacicco AL, Marini V, Pisciella M, Di Francesco G, Lorusso A, Monaco F and Savini G, 2016. Experimental infection of rock pigeons (Columba livia) with three West Nile virus lineage 1 strains isolated in Italy between 2009 and 2012. Epidemiology and Infection, 144, 1301–1311. [DOI] [PubMed] [Google Scholar]
- Steele KE, Linn MJ, Schoepp RJ, Komar N, Geisbert TW, Manduca RM, Calle PP, Raphael BL, Clippinger TL, Larsen T, Smith J, Lanciotti RS, Panella NA and McNamara TS, 2000. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Veterinary Pathology, 37, 208–224. [DOI] [PubMed] [Google Scholar]
- Suen WW, Uddin MJ, Wang WQ, Brown V, Adney DR, Broad N, Prow NA, Bowen RA, Hall RA and Bielefeldt‐Ohmann H, 2015. Experimental West Nile virus infection in rabbits: an alternative model for studying induction of disease and virus control. Pathogens, 4, 529–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne DE, Beck JR, Smith CS, Shieh WJ and Zaki SR, 2001. Fatal encephalitis and myocarditis in young domestic geese (Anser anser domesticus) caused by West Nile virus. Emerging Infectious Diseases, 7, 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teehee ML, Bunning ML, Stevens S and Bowen RA, 2005. Experimental infection of pigs with West Nile virus, Brief Report. Archives of Virology, 150, 1249–1256. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Siirin M, Guzman H, Travassos da Rosa AP, Wu X, Duan T, Lei H, Nunes MR and Xiao SY, 2005. Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. Journal of Infectious Diseases, 192, 287–295. [DOI] [PubMed] [Google Scholar]
- Thomas D and Urena B, 2001. A model describing the evolution of West Nile‐like encephalitis in New York City. Mathematical and Computer Modelling, 34, 771–781. [Google Scholar]
- Trout RT, Brown GC, Potter MF and Hubbard JL, 2007. Efficacy of two pyrethroid insecticides applied as barrier treatments for managing mosquito (Diptera: Culicidae) populations in suburban residential properties. Journal of Medical Entomology, 44, 470–477. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Spring AR, Miller MK and Cannon CE, 2002. Effect of holding conditions on the detection of west nile viral RNA by reverse transcriptase‐polymerase chain reaction from mosquito (Diptera: Culicidae) pools. Journal of Medical Entomology, 39, 1–3. [DOI] [PubMed] [Google Scholar]
- USGS (US Geological Survey ‐ National Wildlife Health Center), online. Wildlife species affected by West Nile virus. Available online: https://www.nwhc.usgs.gov/disease_information/west_nile_virus/affected_species.jsp
- Van Roey K, Sovannaroth S, Sochantha T, Touch MS, Pigeon O, Sluydts V, Durnez L and Coosemans M, 2014. A phase III trial to evaluate the efficacy, fabric integrity and community acceptance of Netprotect® using a recommended long‐lasting insecticidal net as positive control. Malaria Journal, 13, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDalen KK, Hall JS, Clark L, McLean RG and Smeraski C, 2013. West Nile Virus Infection in American Robins: new insights on dose response. PLoS ONE, 8, e68537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez A, Herrero L, Negredo A, Hernández L, Sánchez‐Seco MP and Tenorio A, 2016. Real time PCR assay for detection of all known lineages of West Nile virus. Journal of Virological Methods, 236, 266–270. [DOI] [PubMed] [Google Scholar]
- Weingartl HM, Neufeld JL, Copps J and Marszal P, 2004. Experimental west nile virus infection in blue jays (Cyanocitta cristata) and crows (Corvus brachyrhynchos). Veterinary Pathology, 41, 362–370. [DOI] [PubMed] [Google Scholar]
- Wheeler SS, Vineyard MP, Woods LW and Reisen WK, 2012. Dynamics of West Nile virus persistence in house sparrows (Passer domesticus). PLoS Neglected Tropical Diseases, 6, e1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonham MJ, De‐Camino‐Beck T and Lewis MA, 2004. An epidemiological model for West Nile virus: invasion analysis and control applications. Proceedings of the Royal Society B: Biological Sciences, 271, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work TH, Hurlbut HS and Taylor RM, 1955. Indigenous wild birds of the Nile Delta as potential West Nile virus circulating reservoirs. American Journal of Tropical Medicine and Hygiene, 4, 872–888. [DOI] [PubMed] [Google Scholar]
- Ziegler U, Angenvoort J, Fischer D, Fast C, Eiden M, Rodriguez AV, Revilla‐Fernandez S, Nowotny N, de la Fuente JG, Lierz M and Groschup MH, 2013. Pathogenesis of West Nile virus lineage 1 and 2 in experimentally infected large falcons. Veterinary Microbiology, 161, 263–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mapped fact‐sheet used in the individual judgement on West Nile Fever


