Abstract
The conclusions of EFSA following the peer review of the initial risk assessments carried out by the competent authority of the rapporteur Member State, the Netherlands, for the pesticide active substance Mild Pepino mosaic virus isolate VX1 are reported. The context of the peer review was that required by Regulation (EC) No 1107/2009 of the European Parliament and of the Council. The conclusions were reached on the basis of the evaluation of the representative uses of Mild Pepino mosaic virus isolate VX1 as an elicitor on tomatoes. The reliable end points, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed.
Keywords: Mild Pepino mosaic virus isolate VX1, peer review, risk assessment, pesticide, elicitor
Summary
Mild Pepino mosaic virus isolate VX1 is a new active substance for which, in accordance with Article 7 of Regulation (EC) No 1107/2009 of the European Parliament and of the Council (hereinafter referred to as ‘the Regulation’), the rapporteur Member State (RMS), the Netherlands, received an application from Valto B.V. on 2 December 2013 for approval. Complying with Article 9 of the Regulation, the completeness of the dossier was checked by the RMS and the date of admissibility of the application was recognised as being 30 June 2014.
The RMS provided its initial evaluation of the dossier on Mild Pepino mosaic virus isolate VX1 in the draft assessment report (DAR), which was received by the European Food Safety Authority (EFSA) on 10 November 2015. The peer review was initiated on 15 December 2015 by dispatching the DAR for consultation to the Member States and the applicant, Valto B.V.
Following consideration of the comments received on the DAR, it was concluded that additional information should be requested from the applicant and that there was no need to conduct an expert consultation.
In accordance with Article 12 of the Regulation, EFSA should adopt a conclusion on whether Mild Pepino mosaic virus isolate VX1 can be expected to meet the approval criteria provided for in Article 4 of the Regulation taking into consideration recital (10) of the Regulation. Furthermore, this conclusion also addresses the assessment required from EFSA under Article 12 of Regulation (EC) No 396/2005, provided the active substance will be approved under Regulation (EC) No 1107/2009 without restrictions affecting the residue assessment.
The conclusions laid down in this report were reached on the basis of the evaluation of the representative uses of Mild Pepino mosaic virus isolate VX1 as an elicitor on tomatoes as proposed by the applicant. Full details of the representative uses can be found in Appendix A of this report.
In the section identity, physical and technical properties of the formulation and analytical methods, data gaps were identified for validation data of the methods for the determination of the relevant impurity nicotine in the active agent of the microbial pest control product (MPCA) and microbial pest control product (MPCP).
In the area of toxicology, a data gap was identified for an in vitro cell culture to confirm the lack of infectiveness and pathogenicity of Mild Pepino mosaic virus.
Mild Pepino mosaic virus isolate VX1 is a candidate for inclusion in Annex IV to Regulation (EC) 396/2005 and no maximum residue level (MRL) is needed. Since the product contains nicotine as an impurity, consumers may be exposed to non‐viable residues. The consumer risk assessment for estimated nicotine residues related to the representative use alone did not lead to an exceedance of the toxicological reference values for nicotine. The validity of the assessment should be confirmed by a confirmatory measurement (data gap). A risk assessment considering exposure to nicotine from other dietary sources was not conducted in the remit of the peer review.
No further data is required to address the fate and behaviour of Mild Pepino mosaic virus isolate VX1 in relation to the representative uses. Information presented by the applicant also addresses the exposure assessment of nicotine, a relevant impurity, resulting from the production method of Mild Pepino mosaic virus isolate VX1, and no further data is required in relation to this impurity with respect to fate and behaviour into the environment.
No data gaps or concerns were raised in the area of ecotoxicology.
Background
Regulation (EC) No 1107/2009 of the European Parliament and of the Council1 (hereinafter referred to as ‘the Regulation’) lays down, inter alia, the detailed rules as regards the procedure and conditions for approval of active substances. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of the Member States and the applicant(s) for comments on the initial evaluation in the draft assessment report (DAR), provided by the rapporteur Member State (RMS), and the organisation of an expert consultation, where appropriate.
In accordance with Article 12 of the Regulation, EFSA is required to adopt a conclusion on whether an active substance can be expected to meet the approval criteria provided for in Article 4 of the Regulation (also taking into consideration recital (10) of the Regulation) within 120 days from the end of the period provided for the submission of written comments, subject to an extension of 30 days where an expert consultation is necessary, and a further extension of up to 150 days where additional information is required to be submitted by the applicant(s) in accordance with Article 12(3).
Mild Pepino mosaic virus isolate VX1 is a new active substance for which, in accordance with Article 7 of the Regulation, the RMS, the Netherlands (hereinafter referred to as the ‘RMS’), received an application from Valto B.V. on 2 December 2013 for approval of the active substance Mild Pepino mosaic virus isolate VX1. Complying with Article 9 of the Regulation, the completeness of the dossier was checked by the RMS and the date of admissibility of the application was recognised as being 30 June 2014.
The RMS provided its initial evaluation of the dossier on Mild Pepino mosaic virus isolate VX1 in the DAR, which was received by EFSA on 10 November 2015 (Netherlands, 2015). The peer review was initiated on 15 December 2015 by dispatching the DAR for consultation of the Member States and the applicant, Valto B.V., for consultation and comments. EFSA also provided comments. In addition, EFSA conducted a public consultation on the DAR. The comments received were collated by EFSA and forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant's response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 12(3) of the Regulation were considered in a telephone conference between EFSA and the RMS on 30 March 2016. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant and that there was no need to conduct an expert consultation. The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, were reported in the final column of the evaluation table.
In accordance with Article 12 of the Regulation, EFSA should adopt a conclusion on whether Mild Pepino mosaic virus isolate VX1 can be expected to meet the approval criteria provided for in Article 4 of the Regulation, taking into consideration recital (10) of the Regulation. A final consultation on the conclusions arising from the peer review of the risk assessment took place with the Member States via a written procedure in October/November 2016.
This conclusion report summarises the outcome of the peer review of the risk assessment on the active substance and the representative formulation evaluated on the basis of the representative uses of Mild Pepino mosaic virus isolate VX1 as an elicitor on tomatoes as proposed by the applicant. Furthermore, this conclusion also addresses the assessment required from EFSA under Article 12 of Regulation (EC) No 396/2005, provided the active substance will be approved under Regulation (EC) No 1107/2009. In the event of a non‐approval of the active substance or an approval with restrictions that have an impact on the residue assessment, this conclusion might no longer be relevant and a new assessment under Article 12 of Regulation (EC) No 396/2005 will be required. A list of the relevant end points for the active substance and the formulation is provided in Appendix A.
In addition, a key supporting document to this conclusion is the peer review report (EFSA, 2017), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views where applicable, can be found:
the comments received on the DAR;
the reporting table (30 March 2016);
the evaluation table (18 November 2016);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the DAR including its revisions (Netherlands, 2016) and the peer review report, both documents are considered as background documents to this conclusion.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the European Union (EU) for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Mild Pepino mosaic virus isolate VX1 is a variant of Pepino mosaic virus Peruvian strain deposited at the culture collection of the German Collection of Microorganisms and Cell Cultures (DSMZ), Germany, under the accession number DSM 26974. Pepino mosaic virus was first found in a field of pepino plants (Solanum muricatum) in Peru. The isolate VX1 originates from a natural, indigenous wild type (LP‐genotype), isolated from a tomato plant in a greenhouse in the Westland (the NL) in 2004, and is not genetically modified.
The representative formulated product for the evaluation was ‘V10’, a suspension concentrate (SC) containing 5–25 mg/L Mild Pepino mosaic virus isolate VX1 and 5–25 mg/L Mild Pepino mosaic virus isolate VC1, which corresponds to approximately 1.5 × 1011 and 7.5 × 1011 virus particles/mL, in an approximate ratio of 1:1.
The representative uses evaluated comprise applications on tomatoes by spraying or rubbing individual plants in glasshouses to prevent infections by aggressive strains of the virulent Chilean isolate genotype of Pepino mosaic virus. Full details of the good agricultural practices (GAPs) can be found in the list of end points in Appendix A.
Data were submitted to conclude that the representative uses of Mild Pepino mosaic virus isolate VX1 proposed at the EU level are sufficiently effective, following the guidance document SANCO/10054/2013‐rev. 3 (European Commission, 2013).
Conclusions of the evaluation
1. Identity of the microorganism/biological properties/physical and technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: SANCO/3029/99‐rev. 4 (European Commission, 2000a), SANCO/3030/99‐rev. 4 (European Commission, 2000b), SANCO/825/00‐rev. 8.1 (European Commission, 2010) and SANCO/12116/2012‐rev.0 (European Commission, 2012).
The material used for manufacturing of the microbial pest control agent contains 1.5 × 1011–7.5 ×1011 virus particles/mL (10–50 mg/L). Nicotine is considered a relevant impurity with a maximum content of 0.1 mg/L.
The identity of the Mild Pepino mosaic virus isolate VX1 in the active agent of the microbial pest control product (MPCA) can be determined using a semiquantitative reverse‐transcription PCR (qRT‐PCR) assay using specific primers for Pepino mosaic virus and for variants of the virus. A suitable double‐antibody sandwich enzyme linked immunosorbent assay (DAS‐ELISA) method with specific antibodies for the EU and CH variants of Pepino mosaic virus is available for the concentration determination of VX1 in batch material with a limit of quantification (LOQ) of 0.02 mg/kg. The nicotine concentration in the MPCA of Mild Pepino mosaic virus isolate VX1 can be determined using a gas chromatography–mass spectrometry (GC–MS) method; however, a data gap was identified for validation data of the method.
In all batches of Mild Pepino mosaic virus isolate VX1 tested for human pathogens, these were either absent or below the limits set in the Working Document on Microbial Contaminant Limits for Microbial Pest Control Products (European Commission, 2012)
Plant pathogenic viruses are generally considered to be pathogenic towards plant species only and not towards other organisms, like humans. As with all RNA viruses, genetic mutation is expected to occur with Pepino mosaic virus. Recombinants may arise, however, the few such cases described in the literature were not more aggressive than the original isolates. Viruses are not able to produce metabolites.
The assessment of the data package revealed no issues that need to be included as critical areas of concern with respect to the identity, physical, biological and technical properties of the MPCA or the representative formulation; however, a data gap was identified for additional validation data for the method of determination of the nicotine content in the microbial pest control product (MPCP) to meet the LOQ of 0.1 mg/kg. It should be noted that the product cannot be stored at room temperature and should be stored refrigerated until use. The product is stable for 6 months in its commercial packaging when stored refrigerated at < −15°C. Appropriate methods are available for the determination of the content of contaminating microorganisms.
2. Mammalian toxicity
With regard to the potential microbial contaminants that could be pathogenic for humans, the batch analysis revealed no microbial contaminants exceeding the limits (see Section 1).
As all other viruses, also Mild Pepino mosaic virus does not produce antimicrobial substances, toxins or secondary metabolites and cannot become resistant to antibiotics or spread resistance.
As a default for the microorganisms, the following warning phrase is applicable for reactions by inhalation as well as by dermal exposure: ‘Microorganisms may have the potential to provoke sensitising reactions’.
All the Tier I studies indicated that Mild Pepino mosaic virus is unlikely to have a potential of toxicity, infectivity and pathogenicity. It is considered highly unlikely that intracellular replication of Mild Pepino mosaic virus VX1 will occur. The ongoing in vitro cell culture study is expected to support this assumption (data gap).
Considering all the available information, it would not be necessary to derive reference values for Mild Pepino mosaic virus isolate VX1, as well as no quantitative operator, worker, resident and bystander risk assessment would be needed. Furthermore, nicotine is considered a relevant impurity. A maximum content of 0.1 mg/L as proposed in the new specification is supported from the toxicological point of view.
3. Residues
Since it was not considered necessary to set reference values for Mild Pepino mosaic virus isolate VX1 (see Section 2), it is concluded that Mild Pepino mosaic virus isolate VX1 is a candidate for inclusion in Annex IV to Regulation (EC) 396/2005 and that no maximum residue level (MRL) is needed.
As the product contains nicotine as an impurity, consumers may be exposed to non‐viable residues.
The data submission did not address the requirement to provide confirmatory measurements of the nicotine content on tomatoes upon application of the product. The maximum nicotine content in the formulation has been significantly lowered during the peer review process as response to issues identified during the commenting phase. Following clarification on the new nicotine content, the RMS provided a worst‐case estimate on the basis of the minimum tomato harvest yield in EU countries and assuming that all nicotine will systemically transfer to the growing tomato fruits and will not degrade until harvest. These considerations resulted in an estimated maximum nicotine concentration of 0.0012 mg nicotine/kg tomatoes. This concentration is about 100‐fold higher than the reported natural nicotine contents in tomato (Domino, 1993) but is below the current MRL of 0.01* mg/kg for nicotine in tomato. Since it is considered that nicotine is (bio)degradable, the actual residue on tomatoes is expected to be lower considering early application of the product at growth stages of mono‐ and dicotyledonous plants (BBCH) 13–51, i.e. before flowering. Yet, in view of the hazardous potential of nicotine, a confirmatory measurement of nicotine residues in tomatoes should be submitted upon use of the product (containing Mild Pepino mosaic virus isolates VX1 and VC1) to support the theoretical assessment.
A consumer risk assessment conducted with the estimated maximum nicotine concentration in tomatoes resulting from the representative use and the toxicological reference values established for nicotine (acceptable daily intake (ADI) of 0.0008 mg/kg body weight (bw) per day and acute reference dose (ARfD) of 0.0008 mg/kg bw) using EFSA Pesticide Residues Intake Model (PRIMo)2 resulted in the following: the theoretical maximum daily intake (TMDI) is less than 1% of the ADI for WHO cluster diet B and the highest international estimate of short‐term intake (IESTI) is 9% of the ARfD for Belgian children. The TMDI estimation does only consider the use under assessment in the peer review. A risk assessment considering exposure to nicotine from other dietary sources was not conducted in the remit of the peer review.
4. Environmental fate and behaviour
No information has been provided in relation to potential interference of Pepino mosaic virus isolate VX1 with the analytical systems for the control of the quality of drinking water provided for in Directive 98/83/EC.3 This is a specific decision‐making criterion for the authorisation of plant protection products containing microorganisms (see uniform principles in Commission Regulation (EU) No 546/20114). However, as a virus, Pepino mosaic virus isolate VX1, is related to other plant viruses commonly found in surface water and unlikely to interfere with the analytical systems intended for bacteria. Therefore, no further information or data have been requested.
No information has been provided on the potential transfer of genetic material from Pepino mosaic virus isolate VX1 to other organisms. However, for Pepino mosaic virus isolate VX1 infection and replication is known to be very specific to plants and has not been reported to occur in other organisms, including humans or animals (EFSA BIOHAZ Panel, 2013).
4.1. Fate and behaviour in the environment of the microorganism
Information on persistence and multiplication of Mild Pepino mosaic virus isolate VX1 in soil is not needed for those situations when the product is used in high‐technology greenhouses with hydroponic culture (EFSA PPR Panel, 2010). However, investigation of the fate and behaviour in soil is relevant for greenhouses with cultivation on soil. No investigations of the fate of Mild Pepino mosaic virus isolate VX1 in soil have been provided. Scientific peer‐reviewed literature data indicate that Pepino mosaic virus is persistent to a certain extent outside its host and infection of some weed species has been reported for wild strains of Pepino mosaic virus in the vicinity of greenhouses. No further data is deemed necessary as long the use is restricted to high‐technology permanent greenhouses or if it is demonstrated that no adverse effects are expected to be produced on soil organisms (including non‐target plants, see Section 5). Worst‐case initial predicted initial environmental concentration (PIEC) soil for Mild Pepino mosaic virus isolate VX1 was calculated and reported in the DAR and in Appendix A.
Contamination of surface water through drainage water by Mild Pepino mosaic Virus isolate VX1 can be excluded if the product is used in high‐technology greenhouses with hydroponic culture and water recirculation with disinfection systems in place (e.g. ultraviolet (UV) treatment). However, contamination through aerosols or drainage in low‐technology greenhouses cannot be excluded. A recent scientific publication confirms stability and transmission of Pepino mosaic virus in water. According to this investigation, Mild Pepino mosaic virus may remain infectious in water for up to 3 weeks. This implies that Mild Pepino mosaic virus is persistent to a certain extent outside its host and can be transmitted from injured roots to nutrient solution. Studies specific to investigate the fate and behaviour (persistence and mobility) of Pepino mosaic Virus isolate VX1 in surface water are not available. No further data is deemed necessary as long the use is restricted to high‐technology permanent greenhouses or if it is demonstrated that no adverse effects are expected to occur in aquatic organisms, including algae and aquatic plants (see Section 5).
4.2. Fate and behaviour in the environment of any relevant metabolite formed by the microorganism under relevant environmental conditions
Viruses do not produce metabolites, they can only modify host cell metabolism, as they self‐replicate within host organisms. It is considered that no further information is required at EU level since a qualified presumption of safety has been found to be applicable to Alphaflexiviridae viruses such as Pepino mosaic virus isolate VX1 (EFSA BIOHAZ Panel, 2013).
Mild Pepino mosaic Virus isolate VX1 contains the relevant impurity nicotine at levels up to 50 mg/L impurity resulting from the manufacturing process. During the peer review the nicotine was considered a relevant impurity of Pepino mosaic virus isolate VX1 and further information was requested from the applicant.
The applicant has presented new specifications supported by batch analysis where levels of nicotine were reduced to maximum of 0.1 mg/L. The applicant provided information on the fate and behaviour of nicotine based on end points found in the public domain (mostly peer‐reviewed scientific literature and some quantitative structure–activity relationship (QSAR)‐derived parameters) or derived from worst case assumptions based on that information following European Chemicals Agency (ECHA) recommendations (ECHA, 2016). These end points were used to perform an exposure assessment of nicotine as result of the proposed uses of Pepino mosaic Virus isolate VX1. Both the end points and the exposure assessment are considered acceptable for the purpose of assessing the fate and behaviour of nicotine as an impurity of Pepino mosaic virus isolate VX1 (See Appendix A). No contamination of ground water with nicotine at levels above 0.1 μg/L are expected as a result of the proposed uses of Mild Pepino mosaic virus isolate VX1 (See Appendix A).
5. Ecotoxicology
In the risk assessment, the European Commission, 2002 guidance document was taken into account.
5.1. Infectiveness and pathogenicity of Mild Pepino mosaic virus isolate VX1
No specific data on infectiveness or pathogenicity were available for the ecotoxicological assessments.
However, some data on potential transmission of the virus were available in the dossier.
Mild Pepino mosaic virus isolate VX1 is a naturally occurring plant virus strain. Its host plants are mainly from the Solanaceae family. Pepino mosaic virus infection and replication is known to be very specific to plants and has not been reported to occur in other organisms, including humans or animals. Therefore, even if the virus is released outside the greenhouse, it will unlikely infect the wild fauna or communities of microorganisms. On the basis of this information and considering that the representative use is a greenhouse use, the risk to birds, fish, aquatic invertebrates, bees and other non‐target arthropods, earthworms and soil microorganisms was concluded as low. Some information was available on bumble bees from literature studies, where bumble bees were used as vectoring agent. These studies indicated no visible effects on bumble bee colonies after foraging on tomato plantations infected by some Pepino mosaic virus strains.
Considering that the representative use is a greenhouse, the exposure of non‐target terrestrial plants is generally considered as not relevant. However, some information was available from the scientific literature, including a data set on the host range of Pepino mosaic viruses collected by the European and Mediterranean Plant Protection Organization (EPPO). This information indicated the presence of certain Pepino mosaic viruses in different plant species, including weed species mainly from the vicinity of tomato growing greenhouse areas. However, only mild symptoms were observed on those species. Similar observations were noted during the peer review of Pepino mosaic virus strain CH2, isolate 1906 (EFSA, 2015). Overall, it was concluded that the risk from the representative uses of Mild Pepino mosaic virus isolate VX1 to non‐target terrestrial plants, is low.
Considering the information available for terrestrial plants, the RMS has concluded that it is unlikely that algae and aquatic plants will be infected. However, the virus is persistent in surface water to some extent (see Section 4) and no specific information was available on the virulence or transmission of the virus to algae or aquatic plants. Therefore, the extrapolation from the data available on terrestrial plants includes some uncertainties. Nevertheless, EFSA agrees that the available information indicates rather low probability for pathogenicity or infectiveness towards algae and aquatic plants. On balance, it was concluded that the risk from the representative use of Mild Pepino mosaic virus isolate VX1 to algae and aquatic plants is low.
5.2. Risk from impurity nicotine
The exposure to birds, bees, non‐target arthropods, earthworms, soil microorganisms and non‐target plants to the impurity nicotine was not considered relevant for the representative uses in permanent greenhouses. Therefore, a low risk was concluded for these non‐target organisms. However, some information for both the toxicity and exposure of bees and other non‐target arthropods was available from the open literature. Using this information the RMS has concluded a low risk to bees and other non‐target arthropods for the representative uses, including bumble bees introduced into the greenhouse.
As regards effect of the impurity nicotine to aquatic organisms, some information and some studies on fish, aquatic invertebrates and algae were available from the open literature. Some studies followed testing methodologies comparable to pertinent internationally recognised protocols, although some shortcomings were noted. Nevertheless, end points (mainly acute or short‐term) for fish, aquatic invertebrates and algae were derived from this data set and these end points were considered suitable for a quantitative risk assessment. The risk assessment using these end points indicated a low risk to these groups of organisms with a large margin of safety. No data were available for aquatic plants, except an endpoint of cotinine (molecule from the same chemical group as nicotine) from the open literature. This endpoint indicated a very low toxicity of cotinine to Lemna gibba. Therefore, the risk to aquatic plants is also considered low. Overall, considering all the data and assessments that were available, a low risk from nicotine to aquatic organism was concluded for the representative uses in greenhouse.
6. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1, 2, 3, 4)
Table 1.
Soil
| Compound (name and/or code) | Persistence | Ecotoxicology |
|---|---|---|
| Pepino mosaic virus isolate VX1 | n.a. | The risk for infectiveness and pathogenicity to soil organisms was assessed as low |
| Nicotine (as a relevant impurity) | Moderate (estimated worst‐case DTsoil50 = 30 days) | The risk to soil organisms was assessed as low |
DTsoil50: period required for 50% dissipation in soil.
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| Nicotine (as a relevant impurity) |
High K oc = 48.23 mL/g (worst‐case estimation from log K ow) |
FOCUS GW: No | Yes | Assessment not triggered. Relevant impurity |
FOCUS: Forum for the Co‐ordination of Pesticide Fate Models and their Use; K oc: Soil Organic Carbon‐Water partition coefficient; K ow: octanol/water partition coefficient.
At least one FOCUS scenario or a relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Pepino mosaic virus isolate VX1 | The risk for infectiveness and pathogenicity to aquatic organisms was assessed as low |
| Nicotine (as a relevant impurity) | The risk to aquatic organisms was assessed as low |
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Nicotine (as a relevant impurity) | Regarding the new specification, the maximum content of 0.1 mg/L is acceptable from toxicological point of view |
7. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of the Regulation concerning information on potentially harmful effects).
Validation of the GC–MS method for the determination of the nicotine content in the MPCA to meet the LOQ of 0.1 mg/kg (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown see Section 1).
Additional validation data for the method for the determination of the nicotine content in the MPCP to meet the LOQ of 0.1 mg/kg (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown see Section 1).
In vitro cell culture to confirm the lack of infectiveness and pathogenicity of Mild Pepino mosaic Virus (relevant for all representative uses evaluated; submission date proposed by the applicant: ongoing, see Section 2).
Two trials with the product containing Mild Pepino mosaic Virus isolates VX1 and VC1 should be provided as confirmatory measurements of nicotine residues in treated tomato (and untreated controls) to support the theoretical assessment (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown see Section 3).
8. Particular conditions proposed to be taken into account to manage the risk(s) identified
No particular conditions are proposed for the representative uses evaluated.
9. Concerns
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of the Regulation and as set out in Commission Regulation (EU) No 546/20114 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of the Regulation.
None proposed for the representative uses assessed.
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of the Regulation and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at a lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of the Regulation.
None proposed for the representative uses assessed.
9.3. Overview of the concerns identified for each representative use considered
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
Table 5.
Overview of concerns
| Representative use | Tomatoes spray (glasshouse) | Tomatoes rubbing (glasshouse) | |
|---|---|---|---|
| Operator risk | Risk identified | ||
| Assessment not finalised | |||
| Worker risk | Risk identified | ||
| Assessment not finalised | |||
| Resident/bystander risk | Risk identified | ||
| Assessment not finalised | |||
| Consumer risk | Risk identified | ||
| Assessment not finalised | |||
| Risk to wild non‐target terrestrial vertebrates | Risk identified | ||
| Assessment not finalised | |||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | ||
| Assessment not finalised | |||
| Risk to aquatic organisms | Risk identified | ||
| Assessment not finalised | |||
| Groundwater exposure to active substance | Legal parametric value breached | ||
| Assessment not finalised | |||
| Groundwater exposure to metabolites | Legal parametric value breached | ||
| Parametric value of 10 μg/L breached | |||
| Assessment not finalised | |||
Abbreviations
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- DAR
draft assessment report
- DAS‐ELISA
double‐antibody sandwich enzyme linked immunosorbent assay
- DSMZ
Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH
- DT50
period required for 50 % dissipation (define method of estimation)
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
good agricultural practice
- GC–MS
gas chromatography–mass spectrometry
- IESTI
international estimated short‐term intake
- ISO
International Organization for Standardization
- Koc
Soil Organic Carbon‐Water partition coefficient
- Kow
octanol/water partition coefficient
- LOQ
limit of quantification (determination)
- MPCA
active agent of the microbial pest control product
- MPCP
microbial pest control product
- MRL
maximum residue level
- PIEC
predicted initial environmental concentration
- PRIMo
(EFSA) Pesticide Residues Intake Model
- qRT‐PCR
quantitative reverse‐transcription polymerase chain reaction
- QSAR
quantitative structure–activity relationship
- RNA
ribonucleic acid
- SC
suspension concentrate
- SMILES
simplified molecular‐input line‐entry system
- TMDI
theoretical maximum daily intake
- UV
ultraviolet
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4650.
Appendix B – Used compound codes
1.
| Code/trivial namea | Chemical name/SMILES notation | Structural formula |
|---|---|---|
| Nicotine |
3‐[(2S)‐1‐Methylpyrrolidin‐2‐yl]pyridine CN2CCC[C@H]2c1cnccc1 |
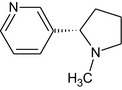
|
SMILES: simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , 2017. Conclusion on the peer review of the pesticide risk assessment of the active substance Mild Pepino mosaic virus isolate VX1. EFSA Journal 2017;15(1):4650, 16 pp. doi: 10.2903/j.efsa.2017.4650
Requestor:European Commission
Question number: EFSA‐Q‐2014‐00472
Approved: 18 November 2016
Notes
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.1998, p. 32.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for the evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- Domino EF, 1993. The nicotine content in common vegetables. The New England Journal of Medicine, 329, 437. doi: 10.1056/NEJM199308053290619 [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency), 2016. Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.16: Environmental exposure assessment. ECHA‐16‐G‐03‐EN.
- EFSA (European Food Safety Authority), 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance Pepino mosaic virus strain CH2 isolate 1906. EFSA Journal 2015;13(1):3977, 25 pp. doi: 10.2903/j.efsa.2015.3977 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance Mild Pepino mosaic virus isolate VX1. Available online: http://www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013. Scientific Opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update). EFSA Journal 2013;11(11):3449, 108 pp. doi: 10.2903/j.efsa.2013.3449 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2010. Scientific Opinion on emissions of plant protection products from green houses and crops grown under cover: outline of a new guidance. EFSA Journal 2015;13(1):3977, 44 pp. doi: 10.2903/j.efsa.2015.3977. Available online: http://www.efsa.europa [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2012. Working Document on Microbial Contaminant Limits for Microbial Pest Control Products. SANCO/12116/2012‐rev.0, September 2012.
- European Commission , 2013. Guidance document on data requirements on efficacy for the dossier to be submitted for the approval of new active substances contained in plant protection products. SANCO/10054/2013‐rev. 3, 11 July 2013
- Netherlands , 2015. Draft Assessment Report (DAR) on Mild Pepino mosaic virus isolate VX1 prepared by the rapporteur Member State the Netherlands in the framework of Regulation (EC) No 1107/2009, November 2015. Available online: http://www.efsa.europa.eu
- Netherlands , 2016. Revised Draft Assessment Report (DAR) on Mild Pepino mosaic virus isolate VX1 prepared by the rapporteur Member State the Netherlands in the framework of Regulation (EC) No 1107/2009, September 2016. Available online: http://www.efsa.europa.eu
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


