Abstract
The A(H5N8) highly pathogenic avian influenza (HPAI) epidemic occurred in 29 European countries in 2016/2017 and has been the largest ever recorded in the EU in terms of number of poultry outbreaks, geographical extent and number of dead wild birds. Multiple primary incursions temporally related with all major poultry sectors affected but secondary spread was most commonly associated with domestic waterfowl species. A massive effort of all the affected EU Member States (MSs) allowed a descriptive epidemiological overview of the cases in poultry, captive birds and wild birds, providing also information on measures applied at the individual MS level. Data on poultry population structure are required to facilitate data and risk factor analysis, hence to strengthen science‐based advice to risk managers. It is suggested to promote common understanding and application of definitions related to control activities and their reporting across MSs. Despite a large number of human exposures to infected poultry occurred during the ongoing outbreaks, no transmission to humans has been identified. Monitoring the avian influenza (AI) situation in other continents indicated a potential risk of long‐distance spread of HPAI virus (HPAIV) A(H5N6) from Asia to wintering grounds towards Western Europe, similarly to what happened with HPAIV A(H5N8) and HPAIV A(H5N1) in previous years. Furthermore, the HPAI situation in Africa with A(H5N8) and A(H5N1) is rapidly evolving. Strengthening collaborations at National, EU and Global levels would allow close monitoring of the AI situation, ultimately helping to increase preparedness. No human case was reported in the EU due to AIVs subtypes A(H5N1), A(H5N6), A(H7N9) and A(H9N2). Direct transmission of these viruses to humans has only been reported in areas, mainly in Asia and Egypt, with a substantial involvement of wild bird and/or poultry populations. It is suggested to improve the collection and reporting of exposure events of people to AI.
Keywords: avian influenza, HPAI/LPAI , monitoring, poultry, captive birds, wild birds, humans
Short abstract
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4991/full
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1282/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1283/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1284/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1285/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1286/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1287/full
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Avian influenza (AI) is an infectious viral disease in birds, including domestic poultry. Infections with avian influenza viruses in poultry cause two main forms of that disease that are distinguished by their virulence. The low pathogenic (LPAI) form generally only causes mild symptoms, while the highly pathogenic (HPAI) form results in very high mortality rates in most poultry species. That disease may have a severe impact on the profitability of poultry farming.
Avian influenza is mainly found in birds, but under certain circumstances infections can also occur in humans even though the risk is generally very low.
More than a decade ago, it was discovered that virus acquired the capability to be carried by wild birds over long distances. This occurred for the HP AI of the subtype A(H5N1) from Southeast and Far East Asia to other parts of Asia, Europe and Africa as well as to North America. In the current epidemic, the extent of the wild bird involvement in the epidemiology of the disease is exceptional.
Since late October 2016 up to early February 2017, highly pathogenic avian influenza (HPAI) of the subtype A(H5N8) has been detected in wild migratory birds or captive birds on the territory of 21 Member States (MSs), namely Austria, Belgium, Bulgaria, Croatia, the Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, the Netherlands, Poland, Portugal, Slovakia, Slovenia, Spain, Sweden and the United Kingdom. In 17 MSs, the virus has spilled over to poultry holdings leading also to lateral spread between holdings in a few MSs, in particular in those with a high density of duck and geese holdings where the poultry cannot sufficiently be protected against contacts with wild birds. A second HP AI subtype A(H5N5) has been detected in wild birds and recently also in poultry holdings in Germany.
The number of infected migratory wild birds found dead and the geographical extent of these findings are posing an immense threat for virus introduction into poultry or captive birds holdings as demonstrated by the high number of outbreaks (~ 700 as of 8/2/2017).
In the event of an outbreak of avian influenza, there is a risk that the disease agent might spread to other holdings where poultry or other captive birds are kept. As a result, it may spread from one MS to other MSs or to third countries through trade in live birds or their products.
There is knowledge, legislation,1 technical and financial tools in the European Union (EU) to effectively deal with outbreaks of avian influenza in poultry and captive birds. However, the very wide virus spread by wild birds and the increased risk of direct or indirect virus introduction into poultry or captive bird holdings has led to the largest HP AI epidemic in the EU so far. This situation calls for a reflection and evaluation how preparedness, risk assessment, early detection and control measures could be improved.
The European Food Safety Authority (EFSA) is already carrying out work for an exhaustive scientific opinion on avian influenza with the support of the EU Reference Laboratory (EURL) for avian influenza. That opinion should be finalised by September 2017. This work could benefit from using data from the current epidemic.
The Commission and MSs are therefore in need of an epidemiological analysis based on the data collected from the disease affected Member States. The use of the EFSA Data Collection Framework is encouraged given it promotes the harmonisation of data collection. Any data that is available from neighbouring third countries should be used as well, if relevant.
Therefore, in the context of Article 31 of Regulation (EC) No 178/20022, EFSA should provide the technical and scientific assistance to the Commission based on the following Terms of Reference (TOR):
Analyse the epidemiological data on HPAI and LPAI, where co‐circulating or linked within the same epidemic, from HPAI disease affected MSs.
Analyse the temporal and spatial pattern of HPAI and LPAI as appropriate in poultry, captive birds and wild birds, as well the risk factors involved in the occurrence, spread and persistence of the HPAI virus in and at the interface of these avian populations.
Based on the findings from the points above, describe the effect of prevention and control measures.
Provide for regular quarterly reports updating on the avian influenza situation within the Union and worldwide, in particular with a view to describe the evolution of virus spread from certain regions towards the EU. In case of significant changes in the epidemiology of avian influenza, these reports could be needed more frequently. These reports should in particular closely follow the developments of zoonotic avian influenza viruses (such as HPAI A(H5N6) and LPAI A(H7N9)) in collaboration with the European Centre for Disease Prevention and Control (ECDC).
1.2. Interpretation of the Terms of Reference
In reply to TORs 1 and 2, this scientific report gives an overview of the HPAI and LPAI outbreaks in poultry and captive birds as well as HPAI events in wild birds detected in Europe between 15 October 2016 and 31 August 2017, mainly based on data submitted by MSs and neighbouring countries via the Animal Disease Notification System (ADNS). A phylogenetic characterisation of the circulating viruses is included as well as a brief genetic characterisation to explain how related/distant viruses are. The affected MSs have also submitted additional epidemiological data to EFSA (see Section 2.1.1), which has been used to analyse the characteristics of holdings affected between October 2016 and April 2017. This data collection required a large effort for the Competent Authorities given the short time available and the high numbers of outbreaks. Bulgaria and France also provided information from their epidemiological investigations on the risk factors involved in the spread of HPAI virus (HPAIV) between holdings (Annexes A and B).
The affected MSs made a huge of effort to collect and report data on the outbreaks, which made it possible to provide an overview of the main observations from the 2016/2017 epidemics. On the other hand, it was not possible to collect data for a risk factor analysis on occurrence and persistence of HPAIV within the EU. Risk factor analysis requires also data on the susceptible population (e.g. location of holdings, population structure, etc.), which should be collected in peace time. Limitations in the performed data collection, reporting and analysis are mentioned and recommendations are given on how these activities could be improved. The report provides several examples of definitions that need a common interpretation (e.g. commercial vs non‐commercial). This report will be used as a basis to discuss with MSs how improvements could be achieved in a feasible manner and providing useful outcomes to the MSs and the European Commission.
A description of the applied prevention and control measures (TOR 3) is reported based on case reports provided by MSs’ representatives and attached as Annexes (from Annex C to O) to this report. The main topics covered are increasing awareness, release and repeal of housing order, strengthening biosecurity, preventive culling, implementation of regional stand still, hunting and derogations on restriction zone implementation after a risk assessment.
The monitoring of the avian influenza situation in other continents (TOR 4) focuses on HPAI A(H5N6), HPAI/LPAI A(H7N9), HPAI A(H5N1), HPAI A(H5N8) and LPAI A(H9N2). Background and epidemiology, detections, phenotypic and genetic characterisations are described based on information from confirmed human and poultry cases as well as wild bird events reported in 2016–2017. Possible actions for preparedness in the EU are discussed.
2. Data
2.1. Data on animals
2.1.1. Epidemiological data
The data on the AI outbreaks submitted by MSs between 1 October 2016 and 31 August 2017 to the European ADNS were taken into account for this report. In addition, MSs were asked to provide more detailed epidemiological data (see data dictionary in Table B.1, Appendix B) directly to EFSA on the AI outbreaks that occurred from October 2016 to 30 April 2017. The data model has been discussed with representatives appointed by the MSs during a teleconference. This was carried out via two rounds of exchanging Excel files via email to the representatives appointed by the 19 MSs.3 The slide presentations, which EU MSs affected by AI presented to the Standing Committee on Plants, Animals, Food and Feed (PAFF Committee), were consulted to extract information on the mortality rates and clinical signs of different species of domestic birds from HPAIV A(H5N8) and A(H5N1) infections, both in single species and multiple species holdings. The PDFs of these slide presentations are available on the European Commission website (European Commission, online).
Table B.1.
Additional epidemiological data (complementing ADNS) requested by EFSA (EFSA data collection)
| Variable | Question | Possible answers | Definitions and clarifications |
|---|---|---|---|
| Sampling programme type | In which context the samples were collected? |
Survey; Outbreak‐related surveillance; Surveillance active; Surveillance passive |
Survey’: National poultry survey, mandatory EU; ‘Outbreak‐related surveillance’, as part of outbreak response i.e. control zones, tracings; ‘Surveillance passive’: notifications of disease suspicion ‘Surveillance active’, background screening of apparently healthy populations outside mandatory EU programme but part of early warning mechanisms Early detection of outbreaks can be reported under ‘surveillance passive’, unless it was performed under a survey or an active/outbreak‐related surveillance activity |
| Outbreak detection – mortality | Was the outbreak detected based on increased mortality? | Y/N | Compared to what is expected in the applicable circumstances and on the experience of the farmer |
| Outbreak detection – clinical signs | Was the outbreak detected based on presence of clinical signs? | Y/N | Compared to what is expected in the applicable circumstances and on the experience of the farmer |
| Outbreak detection – drop egg production | Was the outbreak detected based on a drop in egg production? | Y/N | Compared to what is expected in the applicable circumstances and on the experience of the farmer |
| Outbreak detection – drop feed/water intake | Was the outbreak detected based on a drop in feed and/or water intake? | Y/N | Compared to what is expected in the applicable circumstances and on the experience of the farmer |
| Outbreak detection – non‐clinical indicators | Was the outbreak detected based on other non‐clinical indications? | Y/N | Other non‐clinical indications refer for instance to serology, link with another outbreak, etc. In fact, it can be everything that is not covered by ‘increased mortality’, ‘presence of clinical signs’, ‘drop in egg production’ or ‘drop in feed and/or water intake’. Compared to what is expected in the applicable circumstances and on the experience of the farmer |
| Secondary outbreak based on epi data | Are there data available supporting that the outbreak in the affected holding is a secondary outbreak? | Y/N | ‘Primary outbreak’ means an outbreak not epizootiologically linked with another outbreak in domestic birds (including poultry and/or captive birds, commercial and/or non‐commercial holding); ‘secondary outbreak’ means an outbreak epizootiologically linked with another outbreak in domestic birds |
| Holding ID | EFSA will generate a unique holding ID | Text/Number | Dummy identifier. The geo‐coordinates might not be sufficient to identify the same holding. ‘Holding’ means any agricultural or other premises, including hatcheries, circuses, zoos, pet bird shops, bird markets, backyards and aviaries, where poultry or other captive birds are being bred or kept. However, this definition does not include slaughterhouses, means of transport, quarantine facilities and centres, border inspection posts and laboratories authorised by the competent authority to hold avian influenza virus |
| Holding production category | What is the category of affected holding? | Commercial non‐commercial | ‘Commercial poultry holding’ means a holding where poultry are kept for commercial purposes; ‘non‐commercial holding’ means a holding where poultry or other captive birds are kept by their owners: (a) for their own consumption or use; or (b) as pets |
| Total number of production units | Number of production units in the holding? | Number | ‘Production unit’ means a unit on a holding which the official veterinarian is satisfied is independent of any other unit in the same holding for biosecurity measures and hence can be considered another epidemiological unit. If there are 5 sheds on a holding with strict biosecurity separation between them (e.g. Presence of hygiene lock per shed), these can be considered 5 different production units. If there would not be a strict biosecurity separation between them, then they should be seen as one large production unit |
| Production unit ID | Provide a unique identifier for each production unit of the holding | Text/Number | Dummy identifier. Insert as many rows as there are production units on the holding |
| Production unit affected | Is the production unit affected by the virus at the time of outbreak confirmation? | Y/N | It can be that only 2 out of 5 production units on a holding might be affected by the virus at the time of outbreak confirmation |
| Genus of the birds present | What is the Genus of the domestic birds present (Linnaeus) (per production unit)? | Genus of the present birds in the holding | All genus gathered by the 4 orders (Anseriformes, Galliformes, Columbea, Passerea) |
| Species of the birds present | What is the Species of the domestic birds present (Linnaeus) (per production unit)? | Species of the present birds in the holding | All species gathered by the 4 orders (Anseriformes, Galliformes, Columbea, Passerea) |
| Common name of the birds present | What is the common name of the domestic birds present (per production unit)? | ||
| Prod Unit – production type | What is the production type (per production unit)? | Breeding meat/fattening, egg, fois gras mixed other | Breeding’ refers to any breeding programme for the production of poultry, including for restocking of game birds |
| Prod Unit – Susceptible population size | How many susceptible domestic birds were present at the time of the outbreak (per production unit)? | Number | If the holding has 5 production units with 10,000 birds each and two production units are affected, then the number of susceptible birds will be 20,000 |
| Prod Unit – deads | How many dead domestic birds were present at the time of confirmation of the outbreak (per production unit)? | ||
| Prod Unit – Outdoor access | Had the domestic birds outdoor access in the 21 days before the outbreak (per production unit)? | Whole day/Part of the day/No outdoor access/Unknown | Indicate for each production unit if there was outdoor access |
| Most likely source | What is the most likely source of the virus (per production unit)? | Direct wild birds/ Indirect wild birds/ Direct poultry/ Indirect poultry/Not applicable/Unknown |
Direct wild birds: direct contact with wild birds or their secretions Indirect wild birds: transfer of wild bird faeces into the premises by personnel, equipment, vehicles, feed/bedding Direct poultry: movement of infected poultry onto premises Indirect poultry: transfer of poultry faeces/products from another infected premises by personnel, equipment, vehicles, feed/bedding Not applicable: for production units that are not affected Only one answer is possible since the idea is to identify the most likely source. If there are no data supporting a selection of the most likely source, then it is better to select ‘unknown’ If there is indication that wild birds or infected poultry might have access to the production unit, then select the DIRECT route as most likely. Only select the INDIRECT route if wild birds or infected poultry cannot access the production unit |
| Exposed people | How many people were exposed to the virus during culling and destruction? | Number | A rough estimate is fine, if this would be available |
The three MSs (Bulgaria, France and Hungary) that had the highest number of AI secondary outbreaks were also asked to provide more information on these. To collect the information on the dynamics of the AI secondary outbreaks that occurred in the EU between October 2016 and end of April 2017 in a harmonised way, a specific template has been developed, to include:
chronological overview of HPAI secondary spread, also indicating when prevention and control measures were implemented to manage the situation;
production sector(s) that have been affected;
how the secondary outbreaks were detected;
role of surveillance and clinical signs in detecting secondary outbreaks;
risk factor analysis, including assessment of which were the key risk factors for spreading the avian influenza virus (AIV) within a flock/between holdings;
relevant references/evidence (if available).
The provided information to EFSA can be consulted in Annexes A and B.
2.1.2. AI prevention and control measures
All MSs that expressed their interest in supporting the analysis of the 2016–2017 AI outbreaks were asked to submit case reports on the AI prevention and control measures that have been put in countries that experienced at least one case of HPAI either in wild birds or in poultry. A specific template has been developed, to include:
timing of the applied measures, also indicating the event that triggered the measures, and the target audience;
actions implemented to inform stakeholders and general public on the epidemiological situation of AI in the MS, to increase the awareness on the AI‐related risks;
procedures, timing, and territorial extent of any housing order applied in the MS;
biosecurity measures (beside the housing order) implemented to guarantee the bioexclusion and/or biocontainment of AI;
procedures, criteria and extent of preventive culling measures, when applied, to identify its efficacy;
territorial extent and timing of regional standstill, including ban on movements of poultry and eggs, but also game birds release and movements of other captive birds;
criteria considered when allowing derogations on the above mentioned restrictions;
regulation on hunting, to allow inferences on contact between wild birds and humans.
The information provided to EFSA can be consulted in Annexes from C to O.
2.2. Data on humans
The collection of numbers of human cases due to infection with AIVs has been performed by experts at the ECDC. Multiple sources are scanned regularly to collect information about laboratory‐confirmed human cases, e.g. Disease Outbreak Alert pages at the World Health Organization (WHO),4 webpages of WHO's Regional offices, Chinese Center for Disease Control, health authorities in Hong Kong, CDC in Taiwan5 and others (Chinese CDC, online; Centre for Health Protection (CHP, online); TaiwanCDC, online; WHO, online‐f). Data were extracted and collected in line lists. Double entries and validity of data are continuously checked by ECDC duty experts. Line lists have been developed to collect case‐based information on virus type, date of onset of disease, country of reporting, country of exposure, sex, age, exposure, clinical information (hospitalisation, severity.) and outcome. All cases included in the line list and mentioned in the document are laboratory‐confirmed cases.
Literature searches were performed continuously until 8 September 2017 in the PubMed database with the key words: ‘humans’ and ‘A(H5N1)’; ‘A(H5N6)’; ‘A(H5N8)’; ‘A(H7N9)’; A(H9N2)’; and narrowed to the most recent available publications as well as using specific search parameters such as ‘seroprevalence’; ‘risk factors’; ‘transmission’. The literature search was not systematic or comprehensive.
The EU MSs were also asked to provide data on the number of people exposed to AIV during culling and destruction activities in the period October 2016–30 April 2017 (see last row Table B.1, Appendix B). This collection was carried out by EFSA via the same files through which the additional (animal) epidemiological data were requested (see Section 2.1). In addition, ECDC performed a survey about the protection measures recommended by the public health authorities during the outbreaks. Most of the participating 22 EU/EEA countries replied to have identified exposed people via the local veterinary services, food safety or agriculture authorities together with the local public health services. However, it was assessed challenging to retrieve comprehensive data on all events where people were directly exposed to infected birds and from all countries.
3. Results
3.1. Overview of HPAI and LPAI outbreaks in Europe between October 2016 and August 2017 (TOR 1 and TOR 2)
3.1.1. Phenotypic characterisation of AI viruses circulating in the EU
3.1.1.1. HPAI in domestic birds
Information extracted from PAFF Committee presentations
In chickens in single species holdings affected by HPAIV A(H5N8), mortality rates per holding ranged from 3% to 100%,6 but were often greater than 30% (Table A.1, Appendix A). Clinical signs were variable, and consisted of nervous signs including head shaking, ataxia, tremors, diarrhoea and poor general condition. On some holdings, chickens died suddenly without prior clinical signs. At autopsy, haemorrhagic pneumonia and catarrhal or haemorrhagic enteritis were reported. In holdings with multiple species affected by HPAIV A(H5N8), chickens were common (18 of 19 holdings) and usually they had the highest mortality rates (14 of 18 holdings; 6–100%) (Table A.2, Appendix A).
Table A.1.
Morbidity and mortality from HPAIV A(H5N8) and HPAIV A(H5N1) infections in single species holdings of domestic birds from October 2016 to June 2017 (data extracted from PAFF Committee presentations)
| Species | Type of holding | Number in flock | Number dead | Percentage mortality | Comments | Country providing PAFF Committee report |
|---|---|---|---|---|---|---|
| Chicken | Breeder | 36,000 | 4,500 | 13 | Germany | |
| Layer | 37 | 1 | 27 | Germany | ||
| Backyard | 13 | 10 | 77 | Germany | ||
| Head shaking, diarrhoea, rapid death | Hungary | |||||
| Layer | Increased mortality, poor general condition, some animals with ataxia, tremors in agonal animals | Sweden | ||||
| Broiler breeder | Clinical signs, increased mortality | UK | ||||
| Backyard | 30 | 16 | 53 | 7 of 14 dead birds tested A(H5N8)‐positive | UK | |
| Layer | Increased mortality | Italy | ||||
| Backyard | 65 | 64 | 98 | Sudden death without prior clinical signs | Slovakia | |
| Backyard | 25 | 8 | 32 | No typical clinical signs. Pale watery diarrhoea. Pathology: haemorrhagic, pneumonia, catarrhal enteritis | Slovakia | |
| Rooster and laying hen | 6 | 2 | 33 | Mortality in roosters only, 1 rooster with clinical signs | Slovakia | |
| Layer, backyard | 46 | 28 | 61 | Slovakia | ||
| Layer, backyard | 15 | 15 | 100 | Slovakia | ||
| Percentage mortality per holding: 15%, 26%, 36%, 40%, 55%, 74%, 80%, 95% | Belgium | |||||
| Domestic duck | 5 | Austria | ||||
| 35 | 10 | 29 | Most of remaining birds had respiratory signs | Denmark | ||
| Neurological signs are typical | Hungary | |||||
| Increase in dead and sick animals | Netherlands | |||||
| Foie grasa | No clinical signs (in contrast to HP A(H5N8)); detected by analysis: 8‐ and 11‐week‐old ducks seropositive, 1‐day‐old ducks PCR‐positive | France | ||||
| Mallard duck | Found positive based on epidemiological investigation, but no morbidity or mortality observed | Bulgaria | ||||
| Muscovy duck | Zoo | 4 ducks with clinical signs: somnolence, nervous signs including ataxia | Slovakia | |||
| Domestic goose | Bloody diarrhoea, neurological signs after 2–3 days | Hungary | ||||
| Palmipeds | Two‐thirds of outbreaks detected by clinical signs. High sensitivity of palmipeds (ducks, geese) to this subtype. Clinical signs observed: clinical signs 2–3 days before death; water/food consumption decreased; preponderance of neurological signs; prostration; torticollis. At pathology: severe pancreatitis, severe myocarditis (brain congestion, splenomegaly) | France | ||||
| Domestic turkey | 110 | 18 | 16 | Germany | ||
| Fattening | 15,975 | 50 | 0.3 | Germany | ||
| Fattening | Increased mortality | Some birds dead at beginning, followed by high mortality within 24 h | Hungary | |||
| 30 | 30 | 100 | Dead within 2 days | Czech Republic | ||
| > 90% mortality | UK | |||||
| Fattening | Increased mortality | Italy | ||||
| Fattening | Depression, other nervous signs, decreased water consumption, increased mortality | Italy | ||||
| Fattening | Depression, mild respiratory signs, increased mortality | Italy | ||||
| Galliformes | 17 of 19 outbreaks detected by clinical signs | France | ||||
| Peacock | Zoo | 4 | 7 ill | Finland | ||
| Pheasant | Increased mortality | UK |
This holding was affected by HPAIV A(H5N1), not HPAIV A(H5N8).
Table A.2.
Morbidity and mortality from HPAIV A(H5N8) infection in multiple species holdings of domestic birds from October 2016 to June 2017 (data extracted from PAFF Committee presentations)
| Number of dead birds/total number of birds (percentage mortality) of each species per holding | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicken | Turkey | Guinea fowl | Quail | Pheasant | Peacock | Duck | Goose | Waterfowl | Pigeon | Ostrich | Parrot | Zebra finch | Comments | Country providing PAFF Committee report |
| 4/10 (40%) | 0/1 (0%) | 0/3 (0%) | Belgium | |||||||||||
| 63/? (layer) | 0/? | 0/? | 0/? | Croatia | ||||||||||
| 7/? | 3/? | 0/? | 0/? | Czech Republic | ||||||||||
| 1/? | 1/? | 21 birds in flock | Czech Republic | |||||||||||
| 6/95 (6.3%) | 0/50 (0%) | 10 chickens with clinical signs | Czech Republic | |||||||||||
| 0/? | 0/? | 0/? | 0/? | 0/? | 1/? | 0/? | 0/? | 0/? | 1,025 birds in flock | Czech Republic | ||||
| 4/? | 5/? | 0/? | 0/? | 59 birds in flock | Czech Republic | |||||||||
| 9/? | 0/? | 0/? | 0/? | 130 birds in flock | Czech Republic | |||||||||
| 150/350 (43%) | 0/? | 0/? | 0/? | Also no clinical signs in other species | France | |||||||||
| 5/20 (25%) | 0/10 (0%) | 0/40 (0%) | 0/100 (0%) | 0/10 (0%) | 5 broiler chickens and 15 layer chickens. Mortality in broiler chickens only | Italy | ||||||||
| 0/4 (0%; layers) | 0/19 (0%) | 0/3 (0%) | 0/18 (0%) | No clinical signs or mortality, yet positive for HPAIV A(H5N8) | Italy | |||||||||
| 12/12 (100%) | 1/1 (100%) | Italy | ||||||||||||
| 1/20 (5%; layer) | 3/3 (100%) | Pathology: guinea fowl: haemorrhagic pneumonia, chicken, haemorrhagic enteritis | Slovakia | |||||||||||
| 13/35 (37%; layer) | 2/10 (20%) | 0/? | Pathology: catarrhal enteritis | Slovakia | ||||||||||
| 12/30 (40%; layer) | 1/2 (50%) | 1/1 (100%) | Slovakia | |||||||||||
| ?/? | ?/? (Muscovy and call) | Clinical signs in chickens, not in ducks | UK | |||||||||||
| ?/? | ?/? (Indian runner) | Clinical signs in chickens, not in ducks | UK | |||||||||||
| 2/7 (29%) | 1/3 (33%) | UK | ||||||||||||
| 21/24 (88%) | 0/7 (0%) | Geese seropositive for H5 | UK | |||||||||||
In domestic turkeys in single species holdings affected by HPAIV A(H5N8), mortality rates per holding ranged from 0.3% to 100%, but mortality was reported for all affected holdings (Table A.1). In Germany, low mortality was reported at the beginning, followed by high mortality within 24 h. Clinical signs reported were decreased water consumption, nervous signs including depression and mild respiratory signs. In holdings with multiple species affected by HPAIV A(H5N8), turkeys were uncommon (2 out of 19 holdings) and showed mortality in one of these (Table A.2).
In other Galliformes in single species holdings affected by HPAIV A(H5N8), mortality was recorded in pheasants (one holding) and peacocks (one zoo) (Table A.1). In other Galliformes in holdings with multiple species affected by HPAIV A(H5N8), guinea fowl (5 out of 19 holdings), quail (2 out of 19 holdings), pheasants (1 out of 19 holdings) and peacocks (1 out of 19 holdings) were present, but mortality only was observed in guinea fowl (2 out of 5 holdings) (Table A.2). No clinical signs were reported for these species, but at autopsy, haemorrhagic pneumonia was observed in affected guinea fowl.
In domestic ducks in single species holdings affected by HPAIV A(H5N8), mortality occurred7 (Table A.1). Clinical signs reported were decreased water and food consumption, prostration, respiratory signs and neurological signs including torticollis. The latter were considered typical. At autopsy, severe pancreatitis, severe myocarditis, brain congestion and splenomegaly were observed. However, HPAIV A(H5N8) in a holding of mallard ducks only was detected based on epidemiological investigation; these birds showed neither morbidity nor mortality. In holdings with multiple species affected by HPAIV A(H5N8), domestic ducks were commonly present (12 out of 19 holdings), but mortality was uncommon (4 out of 12 holdings) and lower (from 20% to 30%) than commonly observed in chickens (Table A.2). In contrast with ducks infected with HPAIV A(H5N8), no clinical signs or mortality were observed in domestic ducks infected with HPAIV A(H5N1) in the one event reported by France in the past year.
In the one single species holding of domestic geese affected by HPAIV A(H5N8), clinical signs were bloody diarrhoea and neurological signs (Table A.1). In holdings with multiple species affected by HPAIV A(H5N8), domestic geese were commonly present (9 out of 19 holdings), and showed mortality on some of these (4 out of 9 holdings) (Table A.2).
Besides Galliformes and Anseriformes, a few other species were held in holdings with multiple species affected by HPAIV A(H5N8): pigeons (8 out of 19 holdings), parrots (2 out of 19 holdings), ostriches (1 out of 19 holdings) and zebra finches (1 out of 19 holdings) (Table A.2). None of these birds showed clinical signs or mortality from HPAIV A(H5N8) infection.
Information extracted from the scientific literature
There are several publications on the 2016/2017 outbreak of HPAIV A(H5N8) (e.g. El‐Shesheny et al., 2017; Kwon et al., 2017; Lee et al., 2017; Marchenko et al., 2017; Nagarajan et al., 2017; Pohlmann et al., 2017; Selim et al., 2017), but only one (Pohlmann et al., 2017) reports on virulence of the virus infection at the species level. This study, on the outbreak in Germany, reports that macroscopic changes commonly observed in poultry included severe diffuse hepatic necrosis, multifocal petechiae and variably hyperaemic and oedematous lungs. Light microscopy confirmed influenza A virus antigen and variably distinct necrotising lesions in liver, heart, lungs, brain, pancreas, spleen and thymus. Some chickens also displayed severe diffuse catarrhal enterocolitis; influenza A virus antigen was present in the intestinal epithelium. Pohlmann et al. (2017) also determined that the intravenous pathogenicity index for a HPAIV A(H5N8) isolate from 2016 (A/tufted duck/Germany‐SH/AR8444/2016) in chickens was comparable with that for a HPAIV A(H5N8) isolate circulating in 2014 (A/turkey/Germany‐MV/AR2472/2014): 2.93 and 2.81, respectively (Pohlmann et al., 2017). The intravenous pathogenicity index of virus isolated from a turkey (early case in Hungary) was 3.0 (unpublished data of the EURL).
3.1.1.2. LPAI in domestic birds
In the PAFF Committee report of November 2016, Germany reported the detection of LPAI virus (LPAIV) H5 in domestic geese (breeding and fattening). No clinical signs or mortality were reported.
3.1.1.3. HPAI in wild birds
Pathogenicity in the affected species
Information extracted from the World Organisation for Animal Health (OIE) reports
The main HPAIV subtype that was identified in carcasses of wild birds submitted for AIV testing was A(H5N8), followed by A(H5N5) (Table A.3, Appendix A). The subtypes A(H5N1), H5N2 and H5N9 (distinct group of viruses from A(H5N8)/A(H5N5)) were also detected in domestic birds in France in this period, but not in wild birds (Table A.3).
Table A.3.
Mortality of wild birds from HPAIV subtypes H5N5 and A(H5N8), based on cumulative reports to OIE from 1 October 2016 to 5 July 2017. Only virus‐positive dead birds that were identified to species are included. These data show minor differences from those in ADNS and MS reports to the EURL
| Virus | Species | Number of virus‐positive dead birds per species | Number of countries reporting virus‐positive dead birds |
|---|---|---|---|
| A(H5N5) | Mute swan | 28 | 5 |
| Greylag goose | 2 | 2 | |
| Eurasian widgeon | 1 | 1 | |
| Gadwall | 1 | 1 | |
| Spot‐billed pelican | 1 | 1 | |
| Tufted duck | 1 | 1 | |
| Great white pelican | 0 | 1 | |
| A(H5N8) | Mute swan | 1217 | 20 |
| Tufted duck | 190 | 7 | |
| Whooper swan | 149 | 8 | |
| Eurasian widgeon | 89 | 6 | |
| Mallard | 55 | 13 | |
| Herring gull | 41 | 7 | |
| Greylag goose | 31 | 13 | |
| Eurasian buzzard | 29 | 10 | |
| White‐tailed eagle | 28 | 4 | |
| Black‐headed gull | 22 | 8 | |
| Great crested grebe | 17 | 3 | |
| Great black‐backed gull | 16 | 4 | |
| Grey heron | 16 | 9 | |
| Greater white‐fronted goose | 15 | 4 | |
| Common pochard | 10 | 3 | |
| Great cormorant | 10 | 7 | |
| Common teal | 7 | 3 | |
| Peregrine falcon | 7 | 5 | |
| Black swan | 6 | 2 | |
| Common magpie | 5 | 4 | |
| Eurasian collared‐dove | 5 | 1 | |
| Common coot | 4 | 4 | |
| Great egret | 4 | 3 | |
| Great white pelican | 3 | 1 | |
| House sparrow | 3 | 1 | |
| Little grebe | 3 | 1 | |
| Mew gull | 3 | 2 | |
| Rook | 3 | 2 | |
| Common eider | 2 | 1 | |
| Common guinea fowl | 2 | 1 | |
| Common shelduck | 2 | 2 | |
| Eurasian curlew | 2 | 1 | |
| Hooded crow | 2 | 1 | |
| Lesser white‐fronted goose | 2 | 1 | |
| Red‐crested pochard | 2 | 1 | |
| Tundra swan | 2 | 2 | |
| Canada goose | 1 | 1 | |
| Common goldeneye | 1 | 1 | |
| Common kestrel | 1 | 1 | |
| Common moorhen | 1 | 1 | |
| Common raven | 1 | 1 | |
| Common wood‐pigeon | 1 | 1 | |
| Dalmatian pelican | 1 | 1 | |
| Eurasian bittern | 1 | 1 | |
| Eurasian blackbird | 1 | 1 | |
| Eurasian eagle‐owl | 1 | 1 | |
| Eurasian sparrow hawk | 1 | 1 | |
| Fieldfare | 1 | 1 | |
| Green sandpiper | 1 | 1 | |
| Lesser black‐backed gull | 1 | 1 | |
| Little egret | 1 | 1 | |
| Northern goshawk | 1 | 1 | |
| Pink‐footed goose | 1 | 1 | |
| Pygmy cormorant | 1 | 1 | |
| Saker falcon | 1 | 1 | |
| Western cattle egret | 1 | 1 | |
| White stork | 1 | 1 | |
| Common pheasant | 0 | 1 | |
| Indian peafowl | 0 | 1 | |
| Muscovy duck | 0 | 2 | |
| Rhea | 0 | 1 |
The reports to OIE provide the numbers of dead birds per virus‐positive species, but not the average population size of the affected wild bird species from which wild bird carcasses were obtained, let alone the number of animals at risk in these populations. Therefore, it is not possible to make an objective estimate of the mortality rate to assess the pathogenicity of infection with these subtypes of HPAIV in wild bird populations. Some information may be gained using data on the number of carcasses of the different wild bird species that tested positive for HPAIV during surveillance activities. However, these figures need to be interpreted with caution, because they need to take into account multiple factors, including ease of detection of carcasses of different wild bird species, selection of carcasses biased towards certain species, abundance of species and species range.
For HPAIV A(H5N8), five or more dead birds were found to be virus‐positive in the following species: mute swan, tufted duck, whooper swan, Eurasian wigeon, mallard, herring gull, greylag goose, Eurasian buzzard, white‐tailed eagle, black‐headed gull, great crested grebe, great black‐backed gull, grey heron, greater white‐fronted goose, common pochard, great cormorant, common teal (Table A.3). The species for which by far the highest number of virus‐positive carcasses was found was the mute swan, with nearly 10 times more than any other species. Although this number may be biased by the visibility of mute swan carcasses, it probably also reflects the high pathogenicity of HPAIV A(H5N8) for this species. The next three species for which the highest number of virus‐positive carcasses was found are tufted duck, whooper swan, Eurasian wigeon. The assumed susceptibility of these species for HPAIV A(H5N8) appears to be similar to their susceptibility to HPAIV A(H5N1) in the epidemic of 2005–2006 (Olsen et al., 2006). An apparent difference in the pathogenicity of HPAIV A(H5N8) in 2016–2017 compared with HPAIV A(H5N1) in 2005–2006, is the apparently higher pathogenicity of dabbling ducks (Eurasian wigeon, mallard, common teal), gulls (herring gull, black‐headed gull, great black‐backed gull), and geese (greylag goose, greater white‐fronted goose), although other factors, such as differences in exposure, cannot be ruled out.
For HPAIV A(H5N5), the number of dead birds found to be virus‐positive is far lower (Table A.3). However, the number of mute swan carcasses detected as positive for this virus suggests a similar high pathogenicity of HPAIV A(H5N5) for this wild bird species.
Information extracted from the scientific literature
In the 2016, HPAIV A(H5N8) outbreak in Germany (Pohlmann et al., 2017), macroscopic changes that were commonly observed in tufted ducks included severe diffuse hepatic necrosis, multifocal petechiae and variably hyperaemic and oedematous lungs. Light microscopy confirmed influenza A virus antigen and variably distinct necrotising lesions in liver, heart, lungs, brain, pancreas, spleen and thymus. In contrast with the comparable virulence of the 2016 and 2014 HPAIV A(H5N8) viruses for chickens, they suggested that the viruses circulating in 2016 were more virulent for a broad spectrum of wild waterbirds than the viruses circulating in 2014, based on the deaths of wild birds of a variety of species, in particular diving ducks, and extended pathological changes in dead wild birds.
The A(H5N6) HPAI outbreak in poultry in Greece was detected through passive surveillance as a result of severe clinical presentation, consistent with pathogenicity of A(H5N8) viruses. The A(H5N6) virus in Greece, however, differed in that it was derived from an A(H5N8) HPAI (inheriting the H5 haemagglutinin (HA) from A(H5N8)) but following reassortment with local European AI viruses to acquire a different neuraminidase gene (N6).
3.1.2. Genetic characterisation of the circulating viruses
Since 2016, there have been over 2,600 outbreaks of HPAI in wild birds, poultry and captive birds in Europe. In poultry, the virus has commonly caused high mortality in galliform species; however, in other populations, although clinical signs are often severe with marked mortality, they may be more attenuated. For example in ducks, the disease severity has been variable particularly when secondary spread has been detected. In other hosts, such as game birds, the virus could become rapidly attenuated although initial presentation may result in high mortality. In wild birds, there is likely to be species variability in clinical disease severity. The HA gene sequences from October 2016 to the present date are genetically very similar to each other, but distinguishable phylogenetically from the viruses detected in the Russian Federation in June 2016, and also from the A(H5N8) HPAI viruses present in the EU in 2014/2015. Co‐circulation of LPAI and HPAI viruses in wild birds has led to multiple reassortment events, involving all gene segments with the exception of matrix protein (MP) and non‐structural (NS) protein. In particular, the neuraminidase (NA) gene segment derived from currently circulating LPAI viruses in wild birds has reassorted to result in the A(H5N5), A(H5N6) and A(H5N8) subtypes infecting poultry. To date, however, there has not been detection of mammalian adaptation‐associated mutations, with these viruses still being assessed as having predominantly avian affinity. Preliminary analyses have suggested that epizootic strains show significant genotypic variability across the region despite relatively little diversity or evolution in the HA gene. Data used are based on sequences that have been: (a) deposited with the Global Initiative on Sharing All Influenza Data (GISAID); (b) donated by MS's via the EURL (APHA‐Weybridge); (c) produced by the EURL from submitted viruses. The genetic analysis of local H5Nx strains circulating in Germany and Italy suggests multiple introductions of distinct genotypes of the virus (Fusaro et al., 2017; Pohlmann et al., 2017).
3.1.3. Description of the AI‐detections in time and space
3.1.3.1. Domestic and wild birds
The details of detections of H5/H7 LPAI and H5 HPAI in poultry and captive birds reported in ADNS and MS reports between October 2016 and August 2017 are shown in Figures 1 and 2. Maps of the H5 HPAI cases on 5‐week intervals are available in Figures 3–12.
Figure 1.
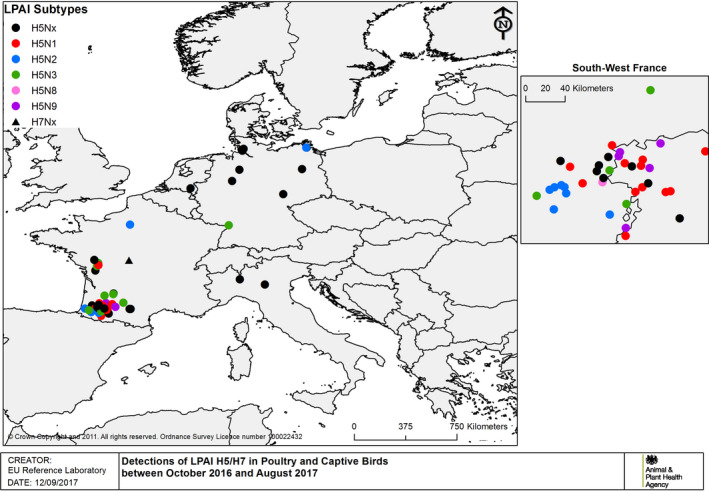
Detections of H5/H7 LPAI in poultry and captive birds between 19 October 2016 and 31 August 2017 (based on ADNS and MS reports)
Figure 2.
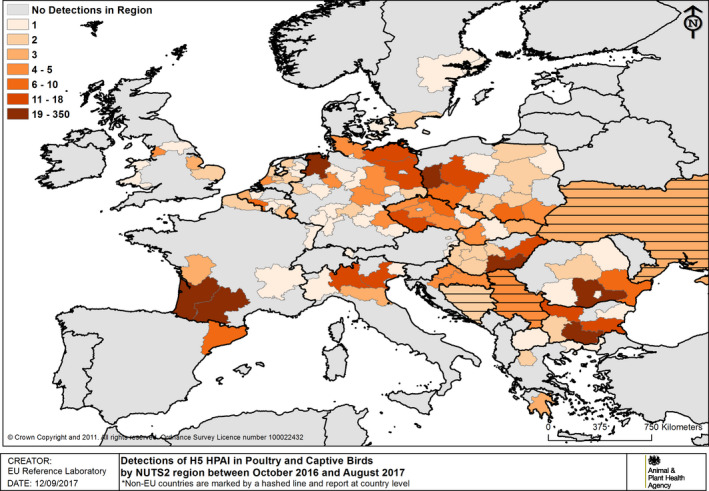
Number of H5 HPAI detections in poultry and captive birds by NUTS2 regions between 19 October 2016 and 31 August 2017 (based on ADNS and MS reports). Non‐EU countries reporting in ADNS (country level) are marked by horizontal lines
Figure 3.
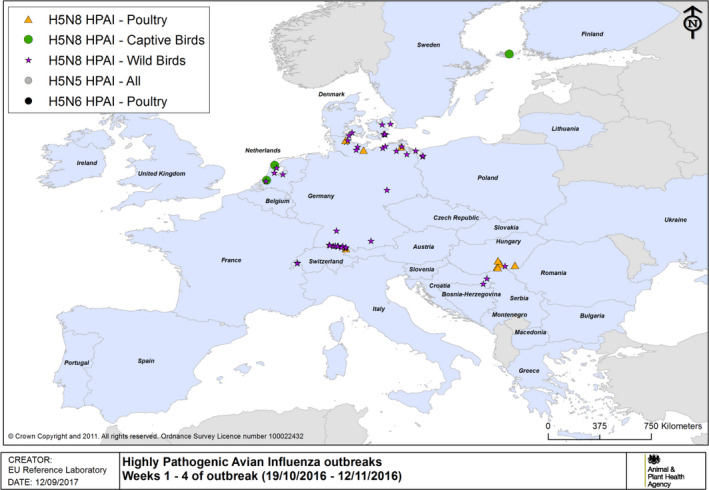
HPAI outbreaks in poultry, captive and wild birds between 19/10/2016 and 12/11/2016 (weeks 1–4 of the 2016/2017 outbreaks) (based on ADNS and MS reports)
Figure 12.
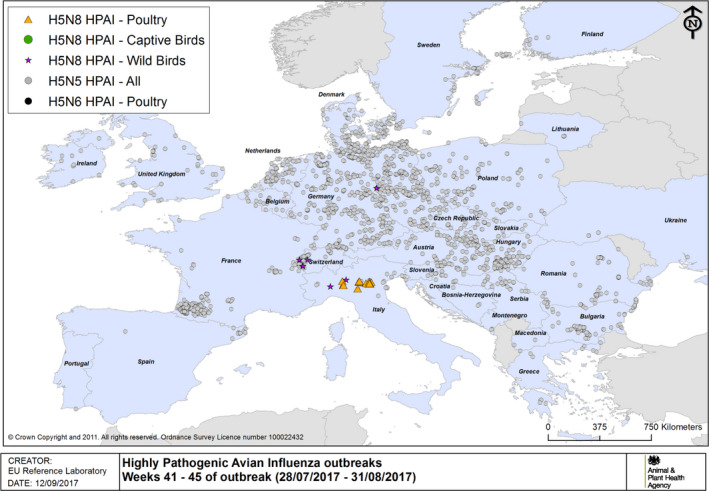
HPAI outbreaks in poultry, captive and wild birds between 28/7/2017 and 31/8/2017 (weeks 41–45 of the 2016/2017 outbreaks) (based on ADNS and MS reports)
Events of H5 HPAI in wild birds reported in the same period are shown in Figure 13. Note that an event in wild birds may relate to single or multiple species from the same location/time; involving a single or multiple mortality event(s) among one or more species; of which one or more birds may have been sampled for laboratory testing. Interpretation of absolute numbers needs to factor in these considerations.
Figure 13.
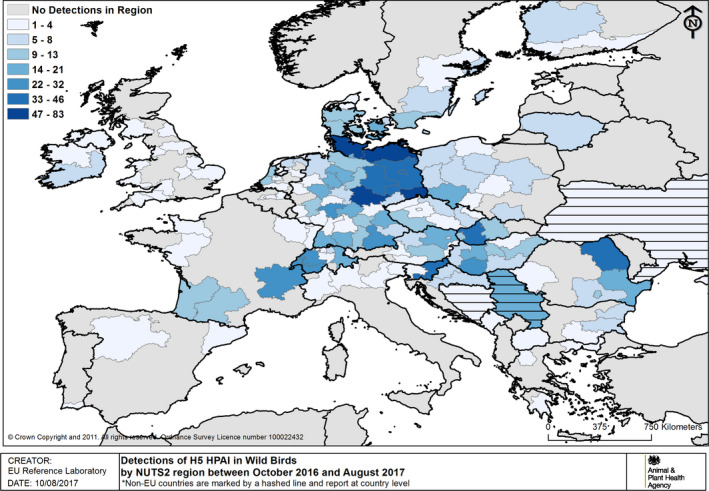
Events of H5 HPAI in wild birds by NUTS2 region between 19 October 2016 and 31 August 2017 (based on ADNS and MS reports). Non‐EU countries reporting in ADNS (country level) are marked by horizontal lines
There are two distinct peaks in the progression of the epizootic (Figure 14). The first peak occurred in early December primarily driven by poultry submissions from Hungary. The second peak occurred in mid‐February as a result of poultry events in France and wild bird submissions from Germany. Following the second peak and a rapid decline, there was a long tail with sporadic cases including a late spike in activity associated with a cluster of cases in predominately in turkeys in northern Italy. Overall, the progression of the epizootic is consistent by initial introduction at multiple points with arriving migratory waterfowl and classically shows clear evidence of systematic introduction in an East–West bias, through both north and central European regions with subsequent lateral spread to the most westerly affected areas such as Ireland and Portugal later in the epidemic. The introduction of HPAI related to multiple events was associated with closely related or identical viruses from the wild bird population. In contrast, the occurrence of LPAI in the review period was sporadic apart from in France in domestic waterfowl, where enhanced surveillance revealed the presence of the virus. In other parts of the EU, these infections were detected most probably through heightened awareness and increased surveillance but were not linked.
Figure 14.
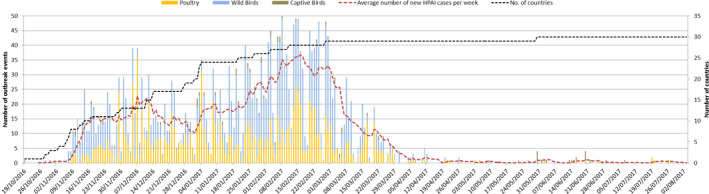
Epidemic curve (number of outbreaks/events in time) H5 HPAI in poultry and wild birds between 19 October 2016 and 31 August 2017 (based on ADNS). The sampling date was used when available to generate this figure; if the sampling date was unavailable, then the confirmation date was used. This approach minimises the impact of delays in submission of results to ADNS
Furthermore, it is possible to look at different wild bird populations affected in different time phases across the epizootic. For example many of the early cases were associated with diving ducks especially tufted duck (Aythya fuligula) in which there were mortality events and a skewed number of detections in this host species associated with the large numbers entering Europe during their autumn migration (Figure 14). In contrast, the mute swan (Cygnus olor), which is a largely indigenous local species across Europe, was more predominately affected later in the epizootic presumably after infection had been established over a wide geographical area and through either direct or indirect contact with migratory waterfowl. These species may also have been infected later in the epizootic as a result of the virus persisting in the environment.
At the time of this report, there were still low level detections in indigenous populations of wild birds, but with no detections in poultry other than the cluster of cases that were sector specific in northern Italy (see Appendix E), and not of continuous maintenance of the virus within wild bird and poultry populations within Europe. This situation would indicate that indigenous populations of wild birds may either: (a) be maintaining infection at a low level and potentially below the sensitivity of detection through passive surveillance or (b) being exposed to virus over an extended time period through environmental contact, in which the virus may have persisted in an infectious form. This situation is different from the repeated incursions in 2006–2008 that were the result of new introductions.
Context for surveillance data is compromised by lack of information on surveillance activity across different MSs and Third countries. It rapidly became apparent that the passive surveillance system for examining dead or moribund wild bird species was a valuable indicator of the presence of virus in a region and location in time. The availability of denominator data provides useful information to assess relative risk of positivity by species and this is applied in reviewing the recommendations for the target species list for future surveillance activity. However, it should be noted that there is still significantly a large volume of data for which accurate speciation of the species affected was not provided, even though the substantive cost in running the surveillance is to actually to facilitate the collection and transport of samples to the laboratory. Furthermore, different definitions have been used across MSs to categorise non‐commercial/commercial poultry and also between captive birds and poultry, as in some cases small numbers of poultry at the same location have been classified as captive birds. This causes potential confusion with captive birds that are not poultry but are part of a zoological collection or kept at private premises for ornamental value.
Figure 4.
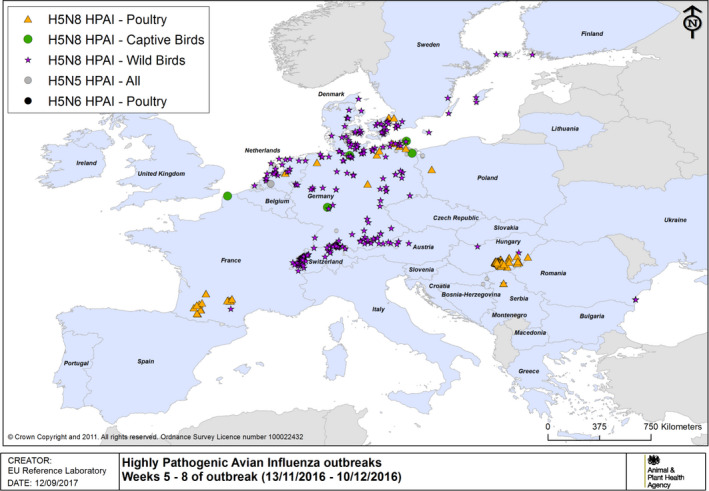
HPAI outbreaks in poultry, captive and wild birds between 13/11/2016 and 10/12/2016 (weeks 5–8 of the 2016/2017 outbreaks) (based on ADNS and MS reports)
Figure 5.
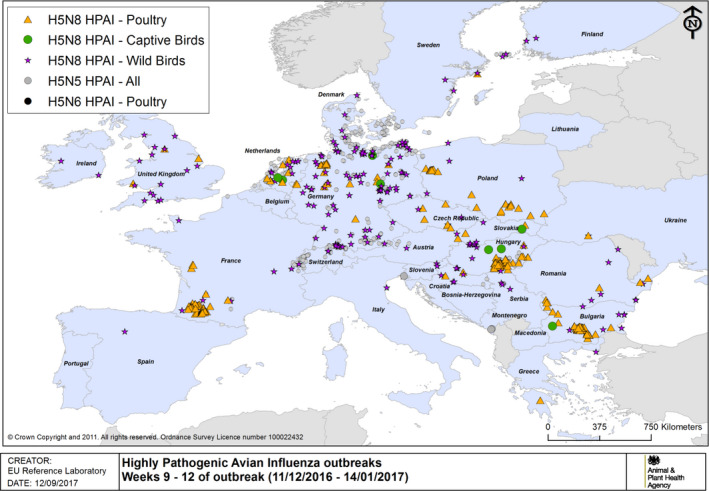
HPAI outbreaks in poultry, captive and wild birds between 11/12/2016 and 14/1/2017 (weeks 9–12 of the 2016/2017 outbreaks) (based on ADNS and MS reports)
Figure 6.
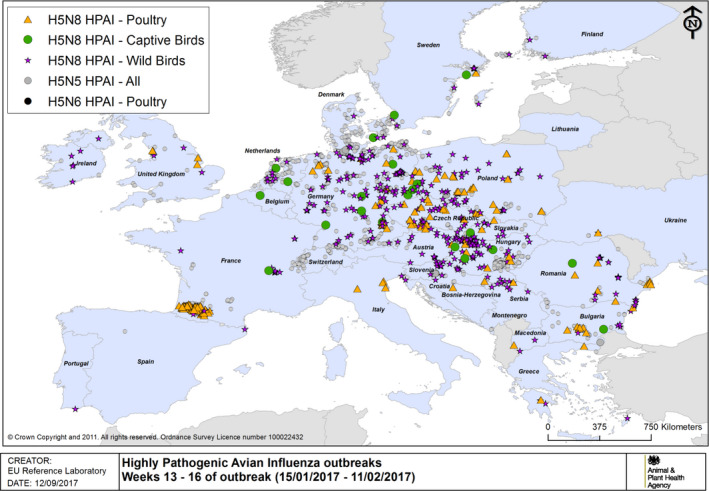
HPAI outbreaks in poultry, captive and wild birds between 15/1/2017 and 11/2/2017 (weeks 13–16 of the 2016/2017 outbreaks) (based on ADNS and MS reports)
Figure 7.
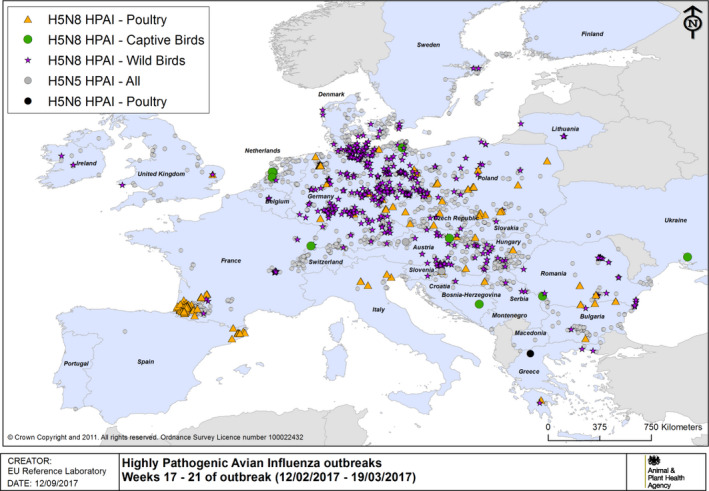
HPAI outbreaks in poultry, captive and wild birds between 12/2/2017 and 19/3/2017 (weeks 17–21 of the 2016/2017 outbreaks) (based on ADNS and MS reports)
Figure 8.
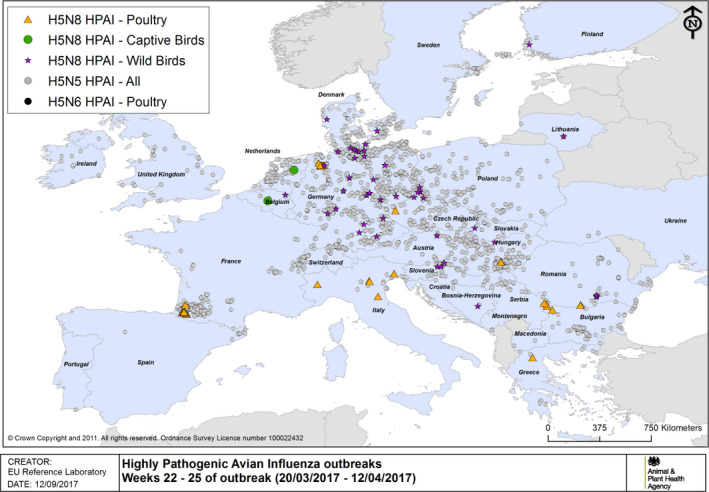
HPAI outbreaks in poultry, captive and wild birds between 20/3/2017 and 12/4/2017 (weeks 22–25 of the 2016/2017 outbreaks) (based on ADNS and MS reports)
Figure 9.
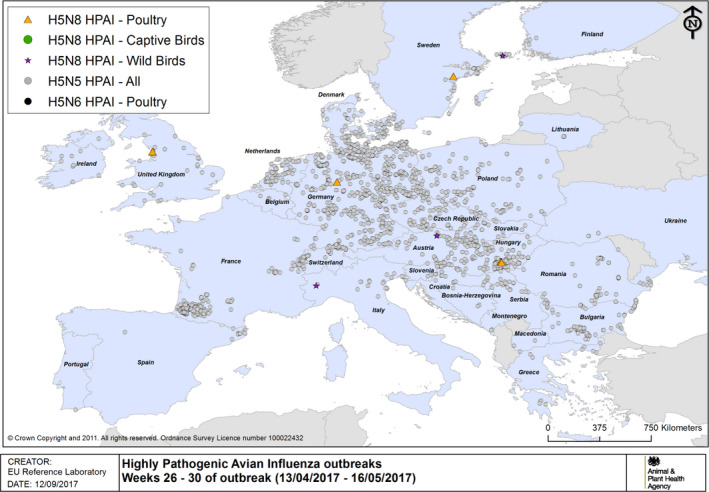
HPAI outbreaks in poultry, captive and wild birds between 13/4/2017 and 16/5/2017 (weeks 26–30 of the 2016/2017 outbreaks) (based on ADNS and MS reports)
Figure 10.
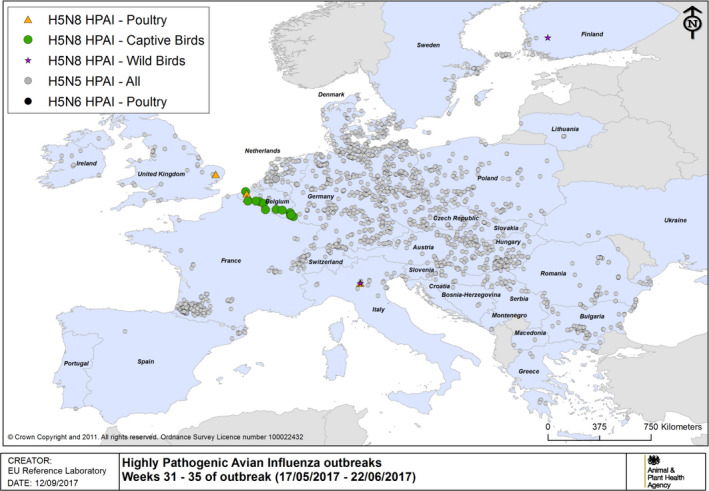
HPAI outbreaks in poultry, captive and wild birds between 17/5/2017 and 22/6/2017 (weeks 31–35 of the 2016/2017 outbreaks) (based on ADNS and MS reports)
Figure 11.
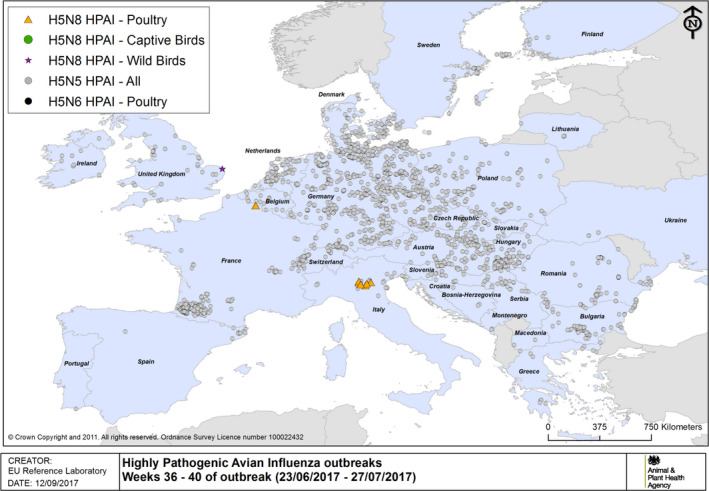
HPAI outbreaks in poultry, captive and wild birds between 23/6/2017 and 27/7/2017 (weeks 36–40 of the 2016/2017 outbreaks) (based on ADNS and MS reports)
Table 2.
Frequency of wild bird events for HPAI A(H5N8) and HPAI A(H5N5) between 19 October 2016 and 31 August 2017 (based on ADNS). Countries have been ranked based on number of wild bird eventsa reported in ADNS
| Country | A(H5N8) | A(H5N5) | Total |
|---|---|---|---|
| Wild birds | Wild birds | ||
| Germany | 741 | 1 | 742 |
| Romania | 93 | 93 | |
| Switzerland | 92 | 92 | |
| Hungary | 86 | 1 | 87 |
| Poland | 66 | 2 | 68 |
| Slovakia | 58 | 58 | |
| Austria | 55 | 1 | 56 |
| France | 51 | 51 | |
| Denmark | 51 | 51 | |
| Netherlands | 47 | 2 | 49 |
| Slovenia | 41 | 3 | 44 |
| Czech Republic | 39 | 39 | |
| Sweden | 30 | 30 | |
| UK | 23 | 23 | |
| Republic of Serbia | 20 | 20 | |
| Finland | 16 | 16 | |
| Bulgaria | 13 | 13 | |
| Croatia | 11 | 1 | 12 |
| Ireland | 10 | 10 | |
| Greece | 8 | 1 | 9 |
| Italy | 8 | 1 | 9 |
| Lithuania | 5 | 5 | |
| Belgium | 3 | 3 | |
| Ukraine | 3 | 3 | |
| Montenegro | 2 | 2 | |
| Spain | 2 | 2 | |
| Bosnia and Herzegovina | 1 | 1 | |
| Portugal | 1 | 1 | |
| FYRO – the former Yugoslav Republic of Macedonia b | 1 | 1 | |
| Grand total | 1,576 | 14 | 1,590 |
Number of events = number of reporting records in ADNS.
Where non‐EU countries have supplied data through the ADNS system this has been included in the data set.
Table 3.
Number of LPAI outbreaks per country for poultry, captive birds between 19 October 2016 and 31 August 2017 (based on ADNS)
| Country | LPAI H5 | LPAI H7 | Total | |
|---|---|---|---|---|
| Poultry | Captive birds | Poultry | ||
| France | 51 | 1 | 52 | |
| Germany | 8 | 2 | 10 | |
| Italy | 2 | 2 | ||
| Netherlands | 1 | 1 | ||
| Grand total | 62 | 2 | 1 | 65 |
Figure 15.
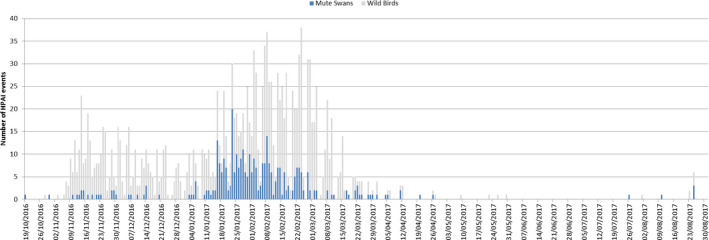
Epidemic curve H5 HPAI in Mute Swans in relation to all reported wild bird events between 19 October 2016 and 31 August 2017 (based on ADNS)
Figure 16.
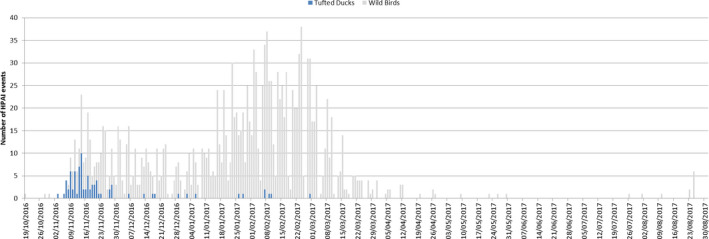
Epidemic curve H5 HPAI in Tufted Ducks in relation to all reported wild bird events between 19 October 2016 and 31 August 2017 (based on ADNS)
3.1.3.2. Wild bird species tested for AIV
An overview of the sampled‐positive wild bird species tested for AIV between January 2016 and April 2017 is reported in Appendix D. These results will be used as a basis to review the list of wild bird target species.
The purpose of the wild bird target species list was firstly: (a) a species that may be known to be highly susceptible to infection with influenza A viruses especially H5 HPAI; and (b) the demographics are such that they enter the EU during their autumn migration in large numbers, and therefore, the probability of being an important factor for the introduction of virus justifies the need to target surveillance activities to such hosts. The target list originally was prepared based on risk in 2005 associated with A(H5N1), by using these data together with new information using the A(H5N8)/A(H5N5) epizootic in Europe, the list can be reviewed with a view to revision to refocus recommendations for targeting of hosts most likely to be carrying risk for introduction of virus.
This future list should be used by MSs applying passive surveillance to wild birds in the autumn/winter periods and by laboratories to select the most relevant species to test when high numbers of samples are submitted.
3.1.3.3. Human cases
No transmission of avian influenza A(H5N8) virus to humans has been reported so far worldwide. No human case was identified during the A(H5N8) outbreaks in Europe in 2014/2015 and 2016/2017.
The newly emerged virus of A(H5N8) clade 2.3.4.4 has been shown to have a low ability to transmit between ferrets, to exhibit low to moderate virulence in mammals and not to be transmissible via airborne infection (Kim et al., 2014; Pulit‐Penaloza et al., 2015; Richard et al., 2015). However, transmission of A(H5N8) virus to an exposed dog on an infected farm in Korea has been observed was documented indicating the ability of A(H5N8) viruses to transmit to dogs which was also confirmed experimentally and discussed to be possible for feline species as well (Kim et al., 2014; Yuk et al., 2017).
The veterinary authorities from nine MSs were able to provide data on the number of exposed people, e.g. involved in control measures with contact to infected poultry or wild birds, during the 2016/2017 AI outbreaks (number of outbreaks with known information about human exposure/number of exposed persons): Austria (2/17), Bulgaria (71/196), Denmark (4/8), Greece (9/67), Poland (100/963), Romania (45/143), Slovakia (9/61), Spain (11/83) and Sweden (7/32). The number of outbreaks where information on human exposure was available is lower than the total number of outbreaks experienced in the respective countries. To better understand the numbers, limitations of the collection and the dataset have to be taken into account:
The numbers provided are an underestimation of the real number of exposed people: many countries did not report exposure data at all, others did not report about all A(H5N8) outbreaks comprehensively and some others provided some figures but the data about exposed people was not available for all outbreaks.
Another limitation of these data is the uncertainty of the number of people that have been exposed in several outbreaks as, e.g. cullers involved in subsequent culling activities, but listed for each outbreak separately. With this, an overestimation of the real number of actually exposed people reported can be assumed.
It is also unclear if the exposure of each person was limited to one day or it was over several days, if the culling operations e.g. in very large holdings took longer.
The veterinary authorities from nine countries reported that overall 1,570 people were exposed during 258 HPAI A(H5N8) outbreaks; 286 of the 1,570 human exposure events occurred in 2016 (from October to December) and 1,284 in 2017 (from January to April). Of those exposure events, 1,030 were related to commercial and 540 to non‐commercial holdings indicating a higher number of exposed people during culling activities when poultry is affected compared to wild bird findings.
The reported bird species related to the exposure of people were: duck (441), turkey (339), goose (245), hen (184), chicken (147), pigeon (39), guinea fowl (22), pheasant (10), peacock (6), ostrich (4), quail (2), mixed bird populations (117) and other birds (10).
No information from the veterinary authorities was reported regarding antiviral prophylaxis or personal protective measures applied; however, public health authorities that participated in an ECDC survey provided information about the national recommendations for occupationally exposed people. Between 19 and 21 of the reporting 22 EU/EEA countries had guidelines for farmers, cullers and veterinarians in place, and 20 countries recommended the use of personal protective equipment (goggles, masks, gloves, body suit) when handling infected birds during poultry outbreaks. Fewer less countries had these strict recommendations when handling wild birds during the outbreaks.
No MS has so far reported any human infection due to A(H5N8). In the ECDC survey, 22 MSs reported to have actively followed‐up more than 250 exposed individuals and more than 70 exposed individuals were monitored passively. Overall, 29 people have been reported to be identified with respiratory symptoms according to the applied national criteria and to have been tested, all with negative results. Information on testing methods has not been provided by the MSs. No data on the number of exposed persons or exposure events were available from the public health side. The identification of exposed persons was conducted together with or via the local veterinary, food safety or agricultural services and public health authorities.
To assess the risk of transmission to humans, it is crucial to identify the number of exposed people, exposure events (number of exposures by time, duration and person), the number of followed‐up people, the number of persons who developed respiratory or other symptoms related to a possible avian influenza infection, and the number of tested people.
The structures and responsibilities for personal protection measures of exposed people e.g. cullers within the countries are different and can be within the mandate of, e.g. occupational health and safety institutes, the ministries of agriculture, the food safety authorities or the public health authorities. Therefore, the applied measures and responses are manifold and different from country to country. These are some existing challenges to retrieve these data timely in a comprehensive way. More and better data are needed to better describe the extent of human exposure to avian influenza particularly during those large outbreaks.
To better assess the real risk of transmission, follow‐up studies with pre‐ and post‐testing of exposed people are needed to identify seroconversion after exposure to the virus. However, quality and standardisation of assays as well as the availability of suitable and large cohorts of exposed people are some of the many challenges for such studies.
3.1.4. Characterisation of the HPAI‐affected poultry holdings (from October 2016 to April 2017)
A more detailed characterisation of the HPAI H5 outbreaks reported in ADNS between 1 October 2016 and 30 April 2017 was undertaken based on additional data provided by the affected MSs.
The additional data (in the following referred to as ‘EFSA data collection’) were obtained by means of a designed data model built on the experience of the HPAI A(H5N8) outbreaks in 2014 (EFSA, 2014). A compromise between rapid action and solidity of the data collection methodology had to be found. Considering the circumstances, it was decided to circulate to each MS an Excel file, prefilled with ADNS information, with the aim of collecting potentially useful epidemiological information (see Appendix B and Section 2.1.1). The data model has been briefly discussed with the MSs’ representatives, but there was no time for proper training of the data providers as MSs were very busy in managing the outbreaks. As known, Microsoft Excel is not a database management system and this affected the final quality of the data, which were not standardised and homogeneous across the different datasets received. In addition, this retrospective collection of data from different MSs, which each has collected its own data from individual suspected and later confirmed outbreaks, have frequently led to incomplete and non‐uniformly recorded data. Moreover, although definitions and descriptions were available both for ADNS and EFSA data collection variables, it came out from this process that the interpretation of those definitions and descriptions is actually diverse in the different countries, making it difficult to interpret the data. As an example, the number of susceptible animals (requested both in the ADNS at holding level and by EFSA at house level), despite the available consolidated definition, has been subject to different interpretations: in some cases, it was the sum of all other categories (diseased, killed and dead), in some others the susceptible animals were interpreted as the non‐affected only (i.e. total number of birds in the holding minus the diseased, the killed and the dead). Non‐consistent numbers were also recorded for the destroyed animals, which in some cases were reported as the total number of birds (susceptible, diseased, dead and killed) and in some others the birds that were destroyed without being diseased, killed or dead.
Furthermore, for EFSA data collection, the original outbreak information had already been recorded in an indigenous form provided for by the respective MS, and retrospective recoding of the already collected observations had to be performed for analyses of the EFSA data collection, which requires standardised classifications.
Last, it has to be pointed out that the data sets do not include any information related to negative samples and this makes it often impossible to draw firm conclusions. In fact, the reference to the entire population and, therefore, to the negative cases is unavoidable to determine if a feature poses a higher risk to a given holding or species. Alternatively, a denominator for all these figures should be available to be able to perform a retrospective case–control study and estimate the odds ratios, as a proxy for the relative risk for possible risk factors. Concretely, looking at Table 4, it appears that there is not a radical difference between commercial and non‐commercial holdings in pre‐disposition to the infection (505 were the commercial holdings affected and 455 the non‐commercial ones), or that the commercial holdings are slightly more exposed to the infection (505/960 = 53% of the infected holdings were commercial holdings). In reality, nothing can be said about this potential risk factor (i.e. the type of commercial activity) as these data refer to the positive holdings only while assessing if a variable is a risk factor or not also the negative data (control population) are needed. Table 4 shows how the data on the holding of the same type of commercial activity that were not found to be infected (should those have been available) could have dramatically changed the conclusions. In the example provided (with fictitious numbers, for illustrative purposes only), only 20% of the total number of commercial holdings are affected, while the non‐commercial holdings are affected at a double rate (43%), making this the most exposed category (twice the risk of being infected compared with a commercial holding).
Table 4.
Fictitious two‐by‐two table for risk‐factor analysis and relative risk estimation
| Commercial | Non‐commercial | Total | |
|---|---|---|---|
| Positive | 505 (20%) | 455 (43%) | 960 |
| Negative | 2,000a | 600a | 2,600a |
| 2,505a | 1,055a | 3,560a |
Fictitious data for illustrative purpose only.
For the above reasons, the following text, Tables 5–9 and A.4, are based on data that are incomplete, not homogeneous and with a lack control population (non‐affected comparative flocks); they should therefore be considered as indicative only.
Table 5.
Reported outbreaks (ADNS) and additional information at house level (EFSA data collection) (October 2016–April 2017)
| HPAI H5 outbreaks in | Reported in ADNS (number of MSs) | Reported to EFSA (number of MSs) | |||
|---|---|---|---|---|---|
| Outbreaks (holdings) | Records | Outbreaks (holdings) | Records (houses/species) | Analysed in this section | |
| Poultry | 1,112 (17 MSs) | 1,112 (17 MSs) | 1,118 (17 MSs) | 1,478 (17 MSs) | Yesa |
| Captive birds – non‐zoo | 47 (13 MSs)b | 47 (13 MSs)b | 34 (9 MSs) | 130 (9 MSs) | |
| Captive birds – zoo | 14 (11 MSs)c | 26 (11 MSs)c | Not analysed | ||
Table 9.
Information on outdoor access of birds in HPAI H5 affected commercial and non‐commercial holdings classified by different holding sizes (from October 2016 to April 2017; EFSA data collection)
| No of susceptible birds per holding | Outdoor access | No of outdoor access | Total | |
|---|---|---|---|---|
| Whole day | Part of the day | |||
| Affected commercial holdings | ||||
| 0–50 | 0/1 (0%) | 1/1 (100%) | 0/1 (0%) | 1 |
| 51–200 | 1/3 (33%) | 0/3 (0%) | 2/3 (67%) | 3 |
| 201–1,000 | 1/9 (11%) | 3/9 (33%) | 5/9 (56%) | 9 |
| 1,001–10,000 | 2/55 (4%) | 38/55 (69%) | 15/55 (27%) | 55 |
| > 10,000 | 1/64 (2%) | 15/64 (23%) | 48/64 (75%) | 64 |
| Total | 5/132 (4%) | 57/132 (43%) | 70/132 (53%) | 132 |
| Affected non‐commercial holdings | ||||
| 0–50 | 12/70 (17%) | 48/70 (69%) | 10/70 (14%) | 70 |
| 51–200 | 11/39 (28%) | 27/39 (69%) | 1/39 (3%) | 39 |
| 201–1,000 | 2/6 (33%) | 3/6 (50%) | 1/6 (17%) | 6 |
| 1,001–10,000 | 0 | 0 | 0 | 0 |
| > 10,000 | 0 | 0 | 0 | 0 |
| Total | 25/115 (22%) | 78/115 (68%) | 12/115 (10%) | 115 |
In each cell, the numerator indicates the count of the holdings with a specific size within the same outdoor access typology; the denominator reports the number of records available for each size category; the percentage is the ratio between numerator and denominator. E.g. 2 holdings out of 55 commercial holdings with a size between 1,001 and 10,000 heads had a ‘whole day access’ typology.
Table A.4.
Most likely source of HPAI H5 introduction into affected only commercial and non‐commercial holdings classified in relation to outdoor access (October 2016–April 2017; EFSA data collection)
| Commercial holdings | ||||
|---|---|---|---|---|
| Most likely virus source | Outdoor access | No outdoor access | Total | |
| Whole day | Part of the day | |||
| Direct poultry | 2/9 (22%) | 7/9 (78%) | 0/9 (0%) | 9 |
| Direct wild birds | 2/60 (3%) | 3/60 (5%) | 55/60 (92%) | 60 |
| Indirect poultry | 0/2 (0%) | 2/2 (100%) | 0/2 (0%) | 2 |
| Indirect wild birds | 0/15 (0%) | 15/15 (100%) | 0/15 (0%) | 15 |
| Total | 4/86 (5%) | 27/86 (31%) | 55/86 (64%) | 86 |
| Non‐commercial holdings | ||||
|---|---|---|---|---|
| Number of susceptible birds | Outdoor access | No outdoor access | Total | |
| Whole day | Part of the day | |||
| Direct poultry | 0/1 (0%) | 0/1 (0%) | 1/1 (100%) | 1 |
| Direct wild birds | 23/56 (41%) | 4/56 (7%) | 29/56 (52%) | 56 |
| Indirect poultry | 0/6 (0%) | 3/6 (50%) | 3/6 (50%) | 6 |
| Indirect wild birds | 1/18 (6%) | 5/18 (28%) | 12/18 (67%) | 18 |
| Total | 24/81 (30%) | 12/81 (15%) | 45/81 (56%) | 81 |
In each cell, the numerator indicates the count of the holdings with a specific size within the same outdoor access typology; the denominator reports the number of records available for each size category; the percentage is the ratio between numerator and denominator. E.g. 2 holdings out of 55 commercial holdings with a size between 1,001 and 10,000 heads had a ‘whole day access’ typology.
Small non‐commercial holdings (backyards) are reported by MSs either as ‘poultry’ or as ‘captive birds’. From an epidemiological outlook, ‘captive birds’ can be split into ‘zoo’ and ‘non‐zoo’. For the analysis, the data from all poultry holdings and non‐zoo captive birds have been considered together, as they have a similar epidemiological context.
MSs have reported to EFSA the species and production type at house level, in particular for holdings where multiple species and/or production types were present.8 The data set used for the analysis described below contained information on 1,608 affected houses (Table 5).
EFSA has asked the MSs to focus on the quality of the submitted data rather than it being complete for all outbreaks. Therefore, the number of data points varies per variable (as described below).
3.1.4.1. Sampling strategy leading to outbreak detection at house level
MSs were asked to report the context of the sampling which resulted in detection of the outbreak by selecting the most relevant category out of the following four options:
‘Survey’: National poultry survey, mandatory EU;
‘Outbreak‐related surveillance’: as part of outbreak response i.e. control zones, tracings;
‘Passive surveillance’: notifications of disease suspicion;
‘Active surveillance’: background screening of apparently healthy populations outwith9 mandatory EU programme but part of early warning mechanisms
Data on both the undertaken type of surveillance linked to the first positive case of the outbreaks and the species bred in the house were received from 1,297 out of 1,608 affected houses. Infection was detected via passive surveillance in around 72% (933/1,297) of the reported houses, whereas outbreak‐related surveillance and active surveillance revealed infection in around 19% (247/1,297) and 9% (117/1,297) of the reported houses, respectively. When analysing the data at species level (Table 6), it can be observed that passive surveillance is the category that records the highest number of detection (positive samples) in basically all the species.
Table 6.
Number of houses (percentage) per species identified to be infected with HPAI H5 per sampling strategy (October 2016–April 2017; EFSA data collection)
| Species | Outbreak‐related surveillance | Active surveillance | Passive surveillance | Missing data on type of surveillance |
|---|---|---|---|---|
| Chickens | 11/115 (10%) | 5/115 (4%) | 99/115 (86%) | 1 |
| Ducks | 220/692 (32%) | 108/692 (16%) | 364/692 (53%) | 62 |
| Geese | 8/133 (6%) | 2/133 (2%) | 123/133 (92%) | 33 |
| Hens | 1/101 (1%) | 0/101 (0%) | 100/101 (99%) | 10 |
| Turkeys | 2/93 (2%) | 0/93 (0%) | 91/93 (98%) | 68 |
| Mixed | 0/69 (0%) | 0/69 (0%) | 69/69 (100%) | 10 |
| Other | 2/22 (9%) | 1/22 (4%) | 19/22 (86%) | 38 |
| Total | 247/1,297 (19%) | 117/1,297 (9%) | 933/1,297 (72%) | 264 |
In each cell, the numerator indicates the count for the specific group obtained by crossing row and column, the denominator indicates the number of records available for that specific species, the percentage is the ration between numerator and denominator. The last column reports the missing data for each species compared with the total records available for that species. E.g. 11 houses of chickens (out of the 115 chicken houses for which the type of surveillance was available) were detected by means of outbreak‐related surveillance.
3.1.4.2. Observations leading to outbreak detection at house level
MSs were asked to report the observation(s) that resulted in detection of the outbreak by indicating ‘yes’ or ‘no’ for increased mortality, clinical signs, drop in feed and/or water intake, drop in egg production and other non‐clinical indicators (which can be any sign that is not covered by the four specific options listed, for instance serology, link with another outbreak, etc.), compared with a normal situation.
Data on at least one observation parameter were received from 1,217 houses out of 1,608 affected houses. Increased mortality was the most reported observation that triggered the detection of HPAI H5 infections for all species (Table 7). The ~20% lower identification of HP H5 by increased mortality in duck houses than in houses of other species fits with lower susceptibility of domestic ducks to severe disease from highly pathogenic H5 infection. Also, clinical signs were observed in many affected houses at the moment of outbreak detection, ranging from 38% to 80% depending on the species. Drops in feed and/or water intake were reported to result in detection of the outbreak mainly in turkey (69%, 64/93), chicken (41%, 18/44) and goose (22%, 26/117) houses, followed at a distance by duck (15%, 43/282) and hen (14%, 12/87) houses.
Table 7.
Number of houses (percentage) per species identified to be infected with HPAI H5 per observation parameter (October 2016‐April 2017; EFSA data collection)
| Species | Increased mortality | Clinical signs | Drop in feed and/or water intake | Other non‐clinical signs |
|---|---|---|---|---|
| Chickens | 50/53 (94%) | 71/90 (79%) | 18/44 (41%) | 2/42 (5%) |
| Ducks | 254/346 (73%) | 279/463 (60%) | 43/282 (15%) | 56/224 (25%) |
| Geese | 133/144 (92%) | 77/149 (52%) | 26/117 (22%) | 1/81 (1%) |
| Hens | 94/97 (97%) | 55/109 (50%) | 12/87 (14%) | 3/85 (4%) |
| Turkeys | 151/159 (95%) | 87/159 (55%) | 64/93 (69%) | 2/90 (2%) |
| Mixed | 70/78 (90%) | 51/77 (66%) | 3/69 (4%) | 3/53 (6%) |
| Other | 155/162 (96%) | 64/167 (38%) | 7/81 (9%) | 2/79 (3%) |
| Total | 907/1,039 (87%) | 684/1,214 (56%) | 173/773 (22%) | 69/654 (11%) |
In each cell, the numerator indicates the count for the specific group obtained by crossing row and column; the denominator indicates the number of records available for that group obtained by crossing row and column; the percentage is the ration between numerator and denominator. E.g. 53 chicken houses (out of the 116 available) reported information whether increased mortality was an important detection method (indicating ‘yes’ or ‘no’); 50 out of 53 reported ‘yes’.
A drop in egg production was reported in 15% (11/73) of affected geese holdings and in 13% (5/38) affected chicken holdings, as an observation leading to identification of the outbreak,10 and to a very limited extent for hens (3%, 3/95), ducks (5%, 10/197) and turkeys (1%, 1/98). The interpretation of the terms ‘chickens’ and ‘hens’ needed to be clarified as the production type ‘egg’ has been reported for both ‘species’.11 However, for the computation of the figures in the tables of this report, all chicken houses indicated as producing eggs were re‐coded into ‘hens’ houses. Although the epidemic overlapped with the period with no fattening geese, there were still some fattening geese flocks around (including positive ones) at the beginning of the epidemic. It cannot be excluded that in some cases the answer ‘no’ to the question about drop in egg production might have been selected by default by the data provider if the flock was a fattening flock, hence skewing the data.
Other non‐clinical signs lead only to detection of 56 out of 224 (25%) infected duck houses with reported information, with 35 out of these 56 without any other signs (18 and 14 derived by active surveillance and outbreak‐related sampling, respectively) (Table 7). The latter suggests that targeted sampling in ducks is important to detect HPAI H5 outbreaks, although interpretation should be undertaken with care since it is mainly based on data from Bulgaria (51/56 reported houses).
3.1.4.3. Size of affected commercial and non‐commercial holdings
The size of the affected holdings was analysed using the number of susceptible birds reported in ADNS. The analysis was performed for both holding production categories, commercial vs non‐commercial, using the definitions used in the EU legislation.12 Table 8 shows that 93% (470/505) of the affected commercial holdings had more than 1,000 susceptible birds on its premises, whereas 92% (420/455) of the affected non‐commercial holdings had less than 200 birds (even with 70% (318/455) having 50 or less birds). These data confirm the difference in size between affected commercial and non‐commercial holdings. However, further harmonisation of the terms such as ‘susceptible birds’ and ‘commercial’ vs ‘non‐commercial’ holdings is still required since a few very small commercial holdings and some large non‐commercial holdings have been reported.
Table 8.
Number of susceptible birds per affected commercial or non‐commercial holding (from October 2016 to April 2017; EFSA data collection)
| Number of susceptible birds per affected holding | Commercial | Non‐commercial |
|---|---|---|
| 0–50 | 6/505 (1%) | 318/455 (70%) |
| 51–200 | 4/505 (1%) | 102/455 (22%) |
| 201–1,000 | 25/505 (5%) | 22/455 (5%) |
| 1,001–10,000 | 201/505 (40%) | 12/455 (3%) |
| > 10,000 | 269/505 (53%) | 1/455 (0%) |
| Total | 505 | 455 |
In each cell, the numerator indicates the count of the holdings with a specific size within the same commercial category; the percentage is the ratio between numerator and denominator. E.g. 6 holdings out of the 505 commercial holdings had a size between 0 and 50 heads.
The number of poultry holdings reported by MSs to EFSA in autumn 2016 is 160,543 (see Appendix C). However, due to the different definitions of a ‘poultry holding’, it is not possible to discriminate commercial and non‐commercial holdings in this data set. As an overall consideration, combining the information from the ADNS and the EFSA data collections of 2016 and 2017, it is possible to estimate the raw prevalence of the total affected holdings out of the total number of holdings in EU, i.e. 960/160,543 (0.6%).
3.1.4.4. Outdoor access of poultry in affected holdings
MSs were asked to report if the domestic birds had outdoor access in the 21 days before the outbreak by selecting whole day, part of the day, no outdoor access or unknown. A time period was specified in the question to take the implementation of housing orders into account as it would change the outdoor access of domestic birds. Analysing outdoor access has been carried out in relation to: (i) the number of susceptible birds present on the affected holding; and (ii) the holding production category (commercial vs non‐commercial). A complete set of information on size, holding production category and outdoor access was received from 250 outbreaks out of the 1,152 reported in Table B.2.
Table B.2.
Number of HPAI H5 outbreaks and number of affected houses reported by MS (poultry and captive birds, excluding zoos) (October 2016–April 2017). The data reported in this table are the result of the descriptive statistics performed on the data submitted to EFSA by the MSs (EFSA data collection)
| Country | Outbreaks | Recordsa |
|---|---|---|
| 02 France | 487 | 526 |
| 24 Hungary | 241 | 247 |
| 01 Germany | 108 | 249 |
| 28 Bulgaria | 76 | 76 |
| 25 Poland | 65 | 176 |
| 32 Romania b | 45 | 45 |
| 22 Czech Republic | 40 | 130 |
| 04 Netherlands | 18 | 18 |
| 03 Italy | 15 | 30 |
| 34 Croatia | 11 | 26 |
| 33 Slovakia | 10 | 18 |
| 11 Spain | 10 | 22 |
| 07 UK | 10 | 19 |
| 10 Greece | 6 | 9 |
| 16 Sweden | 5 | 8 |
| 13 Austria | 2 | 2 |
| 05 Belgium | 2 | 3 |
| 09 Denmark | 1 | 4 |
| Total | 1,152 | 1,608 |
The number of records is the number of rows in the EFSA data collection database. It represents the number of houses and/or of species.
There is a discrepancy between these data and the ones reported in the ADNS (see Table 1).
Only 132 commercial holdings out of the 505 reported information about outdoor access. Around half (53%, 70/132) of the affected commercial holdings had no outdoor access and nearly the other half (43%, 57/132) only part of the day (Table 9). In the database originating from the data submitted by the MSs, which gathers information from affected holdings only, it appears that there is a tendency in commercial holdings to have less outdoor access with increasing holding size (no outdoor access in 27% (15/55) and 75% (48/64) of the commercial holdings with 1,000–10,000 and > 10,000 birds, respectively). Note that it cannot be concluded that commercial holdings with more than 10,000 birds are more at risk if they implement ‘no outdoor access’, not only because of the unusual biological consideration, but also because of the lack of information about the control population (negative samples, see also Table 4 and related text).
Only 115 non‐commercial holdings out of the 455 reported information about outdoor access. Most affected non‐commercial holdings have part of the day (68%, 78/115) or whole day (22%, 25/115) outdoor access, whereas only 10% (12/115) keeps the animals always indoors. Again, the results need to be interpreted with caution and no conclusion can be drawn about the likelihood of being more exposed should a non‐commercial holding practice a ‘part of the day’ outdoor typology.
3.1.4.5. Most likely source of virus introduction
MSs were asked to report the most likely source of the virus based on the epidemiological investigation. One of the following options could be selected:
Direct wild birds: direct contact with wild birds or their secretions.
Indirect wild birds: transfer of wild bird faeces into the premises by personnel, equipment, vehicles, feed/bedding.
Direct poultry: movement of infected poultry onto premises.
Indirect poultry: transfer of poultry faeces/products from another infected premises by personnel, equipment, vehicles, feed/bedding.
Not applicable: for production units that are not affected.
Only one answer is possible as the idea is to identify the most likely source. If there are no data supporting a selection of the most likely source, then it is better to select ‘unknown’.
A complete set of data (outdoor access, production category and most likely infection source) was received for only 166 affected holdings out of the 1,152 reported in Table B.2. Virus introduction via direct contact with wild birds or their secretions was reported as the most likely pathway in around 70% of both commercial and non‐commercial holdings (60/86 and 55/80, respectively) and indirect contact with wild birds accounted for around 20% of the introductions in both production types (see Table A.4). Poultry‐related introduction was reported in around 10% of the holdings, but the direct contacts were more frequently identified as the source in commercial holdings (10%, 9/86), whereas there were more indirect contacts in the non‐commercial holdings (7%, 6/80). It is important to stress that the table describes the affected holdings only, without any knowledge on the control population (negative holdings of the same categories); therefore, no conclusion can be drawn about the relative risk of beings being infected in holdings implementing ‘no outdoor access’ compared with other type of outdoor access strategies. Furthermore, it was not possible to review the evidence underpinning the reported most likely source of virus introduction (see Table A.4).
3.1.4.6. Impact of HPAI H5 outbreaks per production category and species on the affected holdings
From 2016 to 30 April 2017, around 13 millions of domestic birds were reported in ADNS as died or culled due to the HPAI epidemic, a number that is already 10 times greater compared with the 2005–2006 epidemic. Data submitted to EFSA on the number of susceptible animals present in houses were used to describe the impact of HPAI H5 outbreaks on the different production categories and species of affected holdings. Although the number of outbreaks with information from commercial and non‐commercial holdings is in the same range (501 and 459, respectively), the number of birds present on these commercial holdings was 200‐fold higher compared which those in non‐commercial holdings (2,789,464 vs 13,339 birds, respectively). The proportion of birds per species on these commercial holdings was as follows: 36% hens, 30% ducks, 22% turkeys, 7% chickens, 3% pheasants, 2% geese. Further information per species and production type is available in Table B.4 (Appendix B).
Table B.4.
Number of susceptible birds per species and production type on HPAI H5 affected commercial holdings (October 2016–April 2017; EFSA data collection)
| Chickens | Ducks | Geese | Hens | Pheasants | Turkeys | Total | |
|---|---|---|---|---|---|---|---|
| Breeding | 71,357 | 43,120 | 87,477 | 73,700 | 275,654 | ||
| Egg | 1,008,471 | 5,637 | 1,014,108 | ||||
| Foie gras | 474,536 | 474,536 | |||||
| Meat/fattening | 109,796 | 327,151 | 2,839 | 623,048 | 1,062,834 | ||
| Mixed production | 18 | 720 | 738 | ||||
| Other | 6,594 | 6,594 | |||||
| Total | 187,765 | 844,807 | 91,036 | 1,008,471 | 79,337 | 623,048 | 2,834,464 |
3.1.4.7. Secondary HPAI H5 outbreaks (from October 2016 to April 2017)
In total, 316 secondary AI cases were notified by 10 MSs in ADNS, indicating the occurrence of spread between holdings. Of these, 187 (59.17%) were reported from Hungary, 51 (16.13%) from Bulgaria, and 26 (8.22%) from France (Table 10)
Table 10.
ADNS notified occurrence of AI secondary cases per MS (from 1 October 2016 to 30 April 2017)
| Member state | No. secondary outbreaks |
|---|---|
| Hungary | 187 |
| Bulgaria | 51 |
| France | 26 |
| Germany | 18 |
| Romania | 13 |
| Spain | 8 |
| Croatia | 6 |
| Poland | 4 |
| UK | 2 |
| Italy | 1 |
| Grand total | 316 |
A template was sent to the three MSs (Bulgaria, France and Hungary) that reported the highest number of AI secondary outbreaks, to collect the information in a harmonised way on the kinetics of the AI secondary outbreaks that occurred in the EU between October 2016 and the end of April 2017 in a harmonised way. The document was aimed at collecting data including:
chronological overview of HPAI secondary spread, also indicating when prevention and control measures were implemented to manage the situation;
description of the production sector(s) that have been predominantly affected;
information on the detection of the secondary cases, also with the indication on how active and passive surveillance were useful to identify them;
risk factors for spreading the AIV within a flock/between holdings.
Submission of secondary cases reports
Only Bulgaria and France returned the filled template, providing details on the secondary cases. The full documents are included in Annexes A and B. Here, a short summary of the most important characteristics of the secondary cases in the two MS is reported.
Bulgaria indicated that out of a total 72 AI outbreaks occurred, 48 (66.67%) were classified as secondary outbreaks, of which 42 were identified in duck farms (87.50%), 4 in backyard flocks (8.33%) and 2 (4.17%) in industrial laying hens holdings. The 48 secondary outbreaks reported here is lower than the 51 reported in ADNS (Table 1). The objective of ADNS is a quick notification system for disease outbreaks that is mainly designed to inform risk managers at an early stage. This means that the focus is fast reporting above high robustness of the reported information. Therefore, there is a need to develop a complementary data collection platform at EU level where more detailed and high quality epidemiological data are reported for epidemiological analysis and risk assessment purposes. It will be crucial that a unique identifier will be generated for each outbreak to guarantee the linkage between ADNS, epidemiological and preferably also sequence data.
Table 1.
Number of HPAI outbreaks per country for poultry, captive birds for HPAI A(H5N8), HPAI A(H5N5), HPAI A(H5N6) between 19 October 2016 and 31 August 2017 (based on ADNS). Countries have been ranked based on the total number of outbreaks
| Country | A(H5N8) | A(H5N5) | A(H5N6)a | Total | ||
|---|---|---|---|---|---|---|
| Poultry | Captive birds | Poultry | Captive birds | Poultry | ||
| France | 485 | 3 | 488 | |||
| Hungary | 238 | 5 | 243 | |||
| Germany | 89 | 15 | 3 | 107 | ||
| Bulgaria | 71 | 2 | 73 | |||
| Poland | 65 | 65 | ||||
| Romania | 45 | 2 | 47 | |||
| Czech Republic | 38 | 1 | 39 | |||
| Italy | 35 | 35 | ||||
| Netherlands | 8 | 10 | 18 | |||
| Belgium | 2 | 13 | 15 | |||
| United Kingdom (UK) | 13 | 13 | ||||
| Croatia | 7 | 4b | 11 | |||
| Slovakia | 8 | 3 | 11 | |||
| Spain | 10 | 10 | ||||
| Greece | 5 | 1 | 6 | |||
| Sweden | 4 | 2 | 6 | |||
| Luxembourg | 4 | 4 | ||||
| Republic of Serbia | 4 | 4 | ||||
| Austria | 2 | 1 | 3 | |||
| Ukraine | 2 | 1 | 3 | |||
| Bosnia and Herzegovina | 1 | 1 | 2 | |||
| Denmark | 1 | 1 | 2 | |||
| Finland | 1 | 1 | ||||
| FYRO – the former Yugoslav Republic of Macedonia | 1 | 1 | ||||
| Grand total | 1,134 | 64 | 7 | 1 | 1 | 1,207 |
This A(H5N6) virus is not related to the A(H5N6) HPAI contemporaneously circulating in the Far East that has also been associated with infection in humans. This European strain is a secondary reassortant virus between A(H5N8) HPAI and classical Eurasian LPAIs and is therefore of a distinct genotype to the current Far East strains.
The typing of the virus after PCR confirmation of AI was not carried out for two of these outbreaks; however, as they were identified as secondary they were considered as A(H5N5) outbreaks.
Both in Bulgaria and in France, the production sector most affected by secondary cases included duck farms specialised in forced‐feeding (foie gras). This type of farming is characterised by three separated stages:
Demarrage: 1‐day‐old ducklings are reared indoor, within sheds, for 21–25 days.
Pré‐gavage: 3‐week‐old ducks are moved to an open‐air facility, with shelters, up to 6,000 can be housed in the same premise.
Gavage: at about 12 weeks of age, ducks are moved indoor into a closed force‐feeding shed, for 14 days.
During the three stages, ducks are moved frequently between different farms, coincidently with the different rearing periods. This can result in a high amount of movements and an intense flow of vehicles transporting ducks within (and from) infected areas. France indicated that the geographical areas where force‐fed ducks are more present are also characterised by high abundance of free‐range farms (broilers, turkeys and guinea fowls). Furthermore, epidemiological investigations conducted in the outbreaks indicated not only the introduction of infected birds, but also contact through vehicles and staff, among the most likely mean of infection for poultry farms. The hypothesis that the disease in the force‐fed ducks might be transmitted through indirect contacts (mainly vehicles and personnel) is supported by the fact that it was possible to follow the occurrence of cases along the main roads used for transporting poultry.
Secondary cases were mainly detected during routine active surveillance in restriction zones, or through tracing back activities (epidemiological link survey). Clinical signs were more common in galliforms (mortality up to 100%) than in ducks (recorded mortality up to 71%, mainly in juveniles). In fact, ducks were also found to be positive at the slaughterhouse, during preventive culling (France), without showing any clinical signs at the farm during the veterinary visit.
3.1.4.8. Distance/time poultry outbreaks and wild bird events
Assessing spatial clusters of poultry outbreaks and/or wild bird events as well as their correlation would be very interesting but requires data on the susceptible populations or at least a set of controls.
Data on poultry populations are available in the MSs and a discussion is required how these can be used in future epidemiological analysis of outbreaks and risk assessments. Reporting the data in a useful and feasible manner is crucial.
3.2. Applied prevention and control measures (TOR 3)
3.2.1. Case reports submitted by MSs
A template case report was send by EFSA to 25 MSs, with the request of expressing interest to participate to the collection of information, and to return the filled document.
Out of the total 25 MSs contacted, 13 returned the compiled case report. Twenty‐three of the contacted MSs (92%) notified H5 HPAI outbreaks in wild birds, while 19 (76%) and 13 (52%) reported outbreaks in poultry and other captive birds, respectively. For the MSs that returned the case report: 13/13 (100%) had at least one outbreak involving wild birds and 12/13 (92.31%) had outbreaks in poultry (including the hobby flock in Belgium); 7/13 (53.84%) MSs reported cases in other captive birds.
The full case reports for each MS are reported in Annexes C–O. Here, an overview is presented on the applied measures, focusing on differences in the various approaches adopted.
3.2.2. Timing of applied measures
All of the MSs that submitted a case report indicated detailed information on the timing of the applied measures (11). Out of these MSs, 8 (66.67%) applied measures in response of the epidemiological trend of H5 HPAI cases in Europe or in neighbouring countries. The first recorded case of A(H5N8) HPAI in Europe was reported on 26 October 2016 in a wild bird in Hungary; a few days after, on 3 November 2016, the first outbreak in a poultry holding was reported in Hungary in a fattening turkey farm. Romania and Bulgaria resulted being the first MSs issuing provisions to increase the awareness in stakeholders and enhance biosecurity in poultry farms, following the occurrence of HPAI in Hungary.
Austria and Denmark advised increased awareness and surveillance, in response to preliminary tests on suspected wild birds; similarly, the Netherlands increased awareness and enhanced biosecurity measures and surveillance in wild birds following the confirmation of H5 HPAI virus in wild waterfowl.
In 2014/2015, when there was only limited evidence of A(H5N8) circulation in wild birds, five poultry holdings in the Netherlands were infected, and there is anecdotal information that at least in some cases the biosecurity levels on those holdings were low. In contrast, in 2016/2017, the recent experiences provided a strong incentive to maximise biosecurity on poultry holdings in the Netherlands, and it is striking that, despite the much higher infection pressure from the wild bird populations, only a relatively small number of holdings (nine) were infected with A(H5N8). This might suggest that HPAI outbreaks in a MS help to improve awareness and hence the biosecurity level of poultry holdings, leading to higher preparedness and less outbreaks in subsequent epidemics, although not enough data are available to perform a robust analysis. Better monitoring of biosecurity levels across the EU is required.
The first measures provided can be classified into three categories (Table 11):
Increased awareness, mostly addressed to stakeholders and to veterinary authorities.
Enhanced biosecurity, to improve bioexclusion; this included the confinement of reared birds and housing orders, to separate poultry from wild birds, and measures such as the ban on repopulating with waterfowl, and on using living decoy birds.
Increased surveillance/monitoring in wild birds; this measure was mostly applied by the MS that issued the first measures after positive wild birds were detected.
Table 11.
Date of issue of the first measures, with related triggering events and type of measures provided
| Member state | Date of first measures | Trigger event | Measures provided | First case in wild birds | First case in poultry |
|---|---|---|---|---|---|
| Hungary | a | a | a | 2016‐11‐01 | 2016‐11‐04 |
| Romania | Oct 2016 | Epidemiological situation in Europe | Increased awareness; Enhanced biosecurity | 2016‐11‐28 | 2016‐12‐30 (rural farm) |
| Bulgaria | 2016‐11‐01 | Emergency situation in Europe | Increased awareness; Enhanced biosecurity | 2016‐12‐19 | 2016‐12‐19 |
| Austria | 2016‐11‐02 | Suspect in wild birds | Increased awareness; Increased surveillance | 2016‐11‐08 | 2016‐11‐11 |
| Ireland | 2016‐11‐07 | Epidemiological situation in Hungary | Increased awareness; Enhanced biosecurity | 2016‐12‐30 | NA |
| Czech Republic | 2016‐11‐08 | Emergency situation in Europe | Increased awareness | 2017‐01‐03 | 2017‐01‐03 (rural farm) |
| Denmark | 2016‐11‐09 | Suspect in wild birds/Emergency situation in Europe | Increased awareness | 2016‐11‐10 | 2016‐11‐21 (rural farm) |
| Italy | 2016‐11‐09 | Epidemiological situation in Hungary | Increased awareness; Enhanced biosecurity | 2016‐12‐28 | 2017‐01‐21 |
| Netherlands | 2016‐11‐09 | Case in wild birds | Increased awareness; Enhanced biosecurity; Increased surveillance in wild birds | 2016‐11‐09 | 2016‐11‐21 (captive birds) 2016‐11‐26 (poultry) |
| Belgium | 2016‐11‐10 | Introduction of the clade 2.3.4.4. HP A(H5N8) virus into Europe | Enhanced biosecurity | 2017‐02‐21 | 2017‐02‐01(rural farm) |
| France | 2016‐11‐16 | Emergency situation in neighbouring MSs | Increased awareness; Enhanced biosecurity | 2016‐11‐26 | 2016‐12‐02 |
| Greece | 2016‐11‐17 | Emergency situation in Europe | Increased awareness | 2016‐12‐17 | 2017‐01‐19 |
| UK | 2016‐12‐06 | Epidemiological situation in the Netherlands and France | Enhanced biosecurity in Protection Zone | 2017‐01‐12 | 2016‐12‐16 |
Hungary did not provide information on the date of issue of the first measures applied.
3.2.3. Increasing awareness of the stakeholders and the general public
The most of the MSs that provided a case report indicated the presence of websites dedicated to inform the general public on the HPAI epidemiological situation in the MS and in Europe. Those web pages were hosted in governmental websites, on the website of the National Reference Laboratory or on dedicated web pages. Frequently Asked Questions, web forms for requesting specific information and activation of emergency phone numbers were explicitly indicated for three MSs (Bulgaria, the Czech Republic and the Netherlands).
Other media were exploited to communicate with the general public, including press releases, TV and radio speeches and articles in publications in poultry breeder magazines and in professional journals. Publications of letters, flyers and documents made available through the dedicated websites were also used to communicate the need of enhancing biosecurity.
Denmark also largely used web‐based social media, including publication of video and chat sessions. A Smartphone app was created to exploit the increased awareness in the general public that provided data on dead bird findings such as the exact geographical position and images. This permitted the rapid assessment of whether dead birds were of the relevant species, if they were suitable for analysis (e.g. due to decomposition), and enabled the speeding up of collection of carcasses and the notification procedures.
Increased awareness in stakeholders and veterinary authorities, was obtained through meetings/courses (Bulgaria, the Czech Republic, Denmark, Hungary, Italy and the Netherlands), or through other media (e.g. webinars, as for Austria). In Italy, stakeholders were also included in all of the official communications and they were among the recipients of all of the National Provisions.
3.2.4. Housing order
The mandatory keeping of domestic birds within closed sheds was applied in all of the responding MSs. The housing order was mainly applied at an area level, with areas selected on the basis of outbreak occurrence (e.g. Protection and Surveillance Zones around confirmed outbreaks, for Romania; a Further Restriction Zone, which encompassed the most densely populated poultry area, for Italy) and other risk factors, such as proximity to water bodies, and density of poultry farms.
The housing order was applied at the National level (in Denmark, Greece, Ireland and, initially, the UK) or at area level (in Austria, Belgium, Bulgaria, the Czech Republic, France, Italy, Romania and the Netherlands). In the UK, the housing order was issued at the beginning of December 2016, and scheduled to be in force for 30 days. At the expiration date (5 January 2017), due to the epidemiological situation, the housing order was extended to cover a full grace period of 12 weeks. On the 24 February 2017, most poultry keepers in England and all the keepers in Scotland and Wales were permitted to let the birds out provided they had cleaned up any wild bird droppings, made sure the whole area was not attractive to wild birds, and kept the poultry separate from wild birds. Nevertheless, some areas in England were considered at high risk, and poultry farmers had to mandatorily keep poultry housed. These high risk areas were defined in the UK as zones characterised by high density of poultry, high density of wild waterfowl and being located at foraging distance around water bodies. Austria not only used similar criteria to establish where to apply the housing order, but also considered the finding of dead wild birds. On 30 March 2017, Italy defined high risk areas, on the basis of location along migratory pathways, proximity to water bodies, density of waterfowl per water body, density of poultry farms, with a particular focus for laying hens and fattening turkeys. In those risk areas, free‐range farming was banned and a series of strict biosecurity measures were proposed to limit the risk of AI viruses’ introduction.
MSs declared difficulties in applying the housing order, due to the impossibility of guaranteeing the complete separation between wild and domestic birds, or for the impossibility of actually checking that the housing order was correctly applied.
Derogations were to be granted where the housing order was not practicable, e.g. for welfare reasons (Denmark, France, Italy). In that case, free‐range farming was allowed only if defined measures were applied:
clear separation between poultry and wild birds;
the feeding and drinking areas needed to be covered; and
drinking water needed to derive from non‐superficial water reservoirs.
Derogations were also granted to organic farms, to not lose the qualification as ‘organic’ (France). Derogations were given upon a satisfactory visit by a veterinarian to assess the application of suitable biosecurity measures, with a particular focus on keeping drinking and feeding area separated and closed. There is no information available whether outbreaks occurred in holdings to which derogations were granted.
The releasing of the housing order was preceded by a risk assessment (using different approaches within and between MSs), mainly based on the period without HPAI cases occurred within the territory of the MSs, and the epidemiological situation in Europe, and in the neighbouring countries in particular. In the UK, a risk assessment indicated that the risk of AI presence was low for migratory birds and for poultry, and medium for residential wild birds. On this basis, the housing order was lifted on 13 April 2017. Nevertheless, as two cases were observed by 29 April 2017, the definition of high risk areas was expanded to cover the main water ways where wild waterfowl could be found, and the housing order was enforced only in three counties. The housing order was definitely lifted on 1 June 2017, after several weeks with no recorded cases. Information was very limited on the duration and exact geographical areas where housing order was implemented, hampering a more detailed analysis.
3.2.5. Strengthening biosecurity measures
All of the questioned MSs indicated the need to enhance the biosecurity levels, mainly to separate the poultry and captive birds from wild birds. Measures include the physical confinement of the domestic birds, using water from non‐superficial water reservoirs, covering the feed area and the storage area. Although clear information is lacking, it can be expected that the stringency and implementation of biosecurity is very variable across sectors and across MSs.
Official controls were implemented in the Czech Republic, France, Ireland, Romania and Italy to assess an appropriate application of biosecurity measures in commercial poultry farms. In particular, in Ireland, a number of flocks were selected for inspection to assess the correct implementation of biosecurity measures but also of the housing order, and the farm owner awareness towards avian influenza control measures. Farms were selected on the basis of species (priority: ducks and geese), location (priority: high density poultry areas) and size (priority: large commercial farms). Additional flocks were also selected for a phone survey during which questions were asked to assess the level of compliance with the poultry housing order, farm biosecurity and owner awareness. Inspections to evaluate the biosecurity measures applied were mandatorily performed in all commercial and backyard farms in the Surveillance and Protection Zones in Romania. Local veterinary authorities were asked to assess any increase in mortality rates daily. This was undertaken in the whole territory of Romania between 23 November and 31 December 2016, and, starting on 1 January 2017, only in the affected counties. Similarly, the notification of any variations in the production parameters (drop in egg production, decreased consumption of feed or water), and any increase in the mortality rates, were indicated as mandatory in Italy.
Bulgaria, Denmark and Italy explicitly indicated that fairs, bird exhibitions and any other gatherings of poultry or other captive birds were banned, to reduce potential risk contacts. The ban was enforced on a National scale in Denmark, Bulgaria and, initially, also in Italy.
Since 30 March 2017, a series of biosecurity measures were proposed in Italy in high risk‐areas, besides the application of the housing order, including: (i) water supplying from surface water reservoir; (ii) storing fodder and bedding in areas not protected from wild birds and other animals; (iii) gathering of domestic birds for fairs, exhibitions and live bird markets; (iv) using live decoy birds for hunting.
Denmark also implemented aimed controls on vehicles originating from Germany, following an earlier assessment that indicated that empty poultry cages found through the borders were heavily contaminated with poultry faeces. If there was contamination with faeces, vehicles were required to be subject to more deep cleansing and disinfection measures. Particular attention at strengthening biosecurity measures for transports was also paid by Health Authorities in France, which demanded accurate cleaning and disinfections of vehicles. Disinfection of the wheels and the lower parts of the vehicles was to be guaranteed at the entrance and exit from farms.
An enhanced surveillance programme was also ordered in Hungary, for all the vehicles originating in the most affected counties (Bács‐Kiskun, Csongrád and Békés counties and from Kunszentmártno district of Jász‐Nagykun‐Szolnok County).
Guidelines and advices on how to enhance biosecurity at the farm‐level were provided in the Czech Republic, Ireland and the UK, by the means of guides, leaflets, posters but also via the web and social media thorough interviews and chat sessions (the UK).
France and Romania applied a zoning approach at the farm level. In France, the biosecurity regulation was strictly applied from 8 February 2016. This regulation provides that the visitor area is clearly separated from the production area (which hosts the productive units and the storage areas). Furthermore, birds need to be homogenous for age; cleansing and disinfections need to be performed on each shed after flock departure, and a fallowing period should follow. In Romania, commercial farms are divided into three separate areas (the complete list of biosecurity measures is reported in Annex N):
Administrative zone , encompassing offices and administrative spaces; area should have restricted access, and be clearly signalled, personnel needs to wear clean clothes and footwear.
Professional zone , which separates the administrative from the productive zones and contains storage areas; restricted access to personnel and visitors (only after 72 h from their last contact with any domestic/captive birds), storage areas need to be covered and closed, any potential allures for wild birds should be removed (e.g. draining water surfaces and preventing water collections), clothes and footwear need to be changed from the administrative zone, suitable cleansing and disinfection of transports.
Production zone , physically separated from the other two areas, it hosts the poultry sheds; single poultry production type (i.e. only broiler, or only laying hens, etc.), clothing and footwear need to be changed for each shed, and each shed has its own filter zone; further measures for turkeys (need of maintaining dry bedding), and palmipeds (not allowed to any type of water surface).
A functional separation between geographical areas (i.e. Regions) was applied in the most densely populated poultry area in Italy. This decision derived from the highly vertically integrated poultry production, in which the integrating companies work in several regions, owning feed mills, slaughterhouses and processing plants. Therefore, movements of birds (e.g. from hatcheries to farm or from farm to the slaughterhouse), personnel (included technicians and veterinarians) and vehicles (for feed, removal of carcasses, etc.) are quite common between regions. Functional separation was enforced to avoid that these movements of poultry/personnel/vehicles between different regions could take place, hence reducing the potential spread of AI. Following the first two outbreaks in poultry industry in the Veneto region, on 26 January 2017, the functional separation was enforced between the Veneto region and the other regions. On 15 February 2017, the functional separation was extended to all of the Regions with high density of poultry production: Emilia‐Romagna, Lombardy, Piedmont and Veneto.
The enhancement of biosecurity measures indicates also that a strict collaboration between the private sector (stakeholders, farmers) and the public health authorities is paramount. This can be obtained through massive campaign to raise awareness of the risk of AI spreading through the poultry sector, including both animal health/welfare and economical aspects. Shared efforts between the public and private sectors would be needed in time of peace, as it would permit the achievement of a level of preparedness suitable to contrast and prevent the uncontrolled spread of diseases among the poultry farms. Monitoring of biosecurity implementation combined with general and holding‐specific advice is a key element to improve preparedness. Online biosecurity questionnaires are available which provide practical advice to the farmer and allow monitoring the implementation of biosecurity if the questionnaire is performed at regular intervals.
3.2.6. Preventive culling
In this report, preventive culling refers to the killing and disposal of birds in farms considered at risk, due to the close proximity to an outbreak or to evidenced contacts (via sharing of personnel, equipment or vehicles or due to movements of birds). The term ‘preventive’ can be misleading, in particular for communication during a crisis, as there is always suspicion or evidence before a flock is culled. The concept of ‘preventive culling’ is different from ‘pre‐emptive culling’ as in the latter birds are culled holdings located in defined areas around the outbreaks, also in absence of evident contacts, to reduce the risk of uncontrolled spread of the disease.
Eight out of the total 13 responding MSs (61.53%) indicated that preventive culling measures were applied to a various extent, to prevent the risk of virus spread.
In the Netherlands, pre‐emptive culling of commercial poultry holdings in the 1 km zone around the index HPAI holding and the contact holdings was applied to prevent further spread to other holdings. This measure has been included in the National legislation, after the H7N7 HPAI epidemic in 2003. Contact holdings were defined as farms that share personnel and/or equipment for collecting and storing eggs (e.g. same egg collection centres), and vehicles for feed, birds, carcasses or manure transport and disposal. Preventive culling was applied for all commercial poultry holdings in 1 km zones around the nine HPAI positive culled poultry holdings detected in the Netherlands between 8 November 2016 and 12 January 2017 and for the contact commercial poultry holding.
Similarly, in the Czech Republic, farms in the Protection Zone of the first three outbreaks (occurred on 5 and 6 January 2017) were preventively culled (operations lasted between 7 January and 11 January 2017). The criteria and risk factors reported in the Annex 4 of the Council Directive 2005/94/EC were considered when identifying the farms to be subjected to preventive culling, and in particular high density of poultry and wild waterfowl.
In Italy, preventive culling was applied in 10 industrial poultry farms, with an approximate amount of 405,000 culled birds (the precise details on the timeframe of culling and productive types are reported in Annex L). Criteria used to decide which holdings should be depopulated were: (i) proximity to infected farms (within the 3‐km radius); (ii) potential direct contacts (sharing of personnel, farms belonging to the same owner, farms belonging to familiars of the owner).
The Danish Veterinary and Food Administration (DVFA) applied preventive culling measures in two circumstances. After the German Veterinary Authority alerted the DVFA about the import of eggs/day‐old chicks from a HPAI infected farm in Germany all the hatching eggs/day‐old chicks imported from the German breeder within 21 days (considered being the maximum incubation period for HPAI) were preventively destroyed/killed based on Article 15 in Council Directive 2005/94/EC. A backyard poultry farm was subjected to preventive culling in connection to an outbreak detected to 21 November 2016 in a backyard poultry located in close proximity. All samples collected from the contact flock tested negative for HPAI.
A detailed risk assessment was conducted in Romania to assess whether farms could have been exposed to the risk of HPAI spread from the outbreaks (the list of risk factors to be reported in Annex N). Preventive culling was enforced only in farms that had direct contact with the outbreak or where the veterinary authorities found evidence of potential introduction of the disease via personnel movement or through fomites.
In France, preventive culling of contact flocks or farms with strong clinical suspicion was carried out immediately. However, with the rapid spread of HPAI towards the areas with highest density of duck and goose farms (i.e. domestic palmiped farms) by the end of December 2016, in January 2017, a strict culling strategy was enforced. The measure affected the farms within the Protection Zone, although it could be expanded within the Surveillance Zone (if a sufficiently high risk of further spread was identified). While the culling was applied initially only to palmiped farms, in February, it was extended to include also Galliformes flocks within 1 km from the outbreak. The measure could have been expanded to the Surveillance Zone if secondary cases were detected in the initial Protection Zone.
While preventive culling was in most cases conducted on site, in France, the culling was carried out in purposely seized slaughterhouses in the closest proximity to the outbreak. However, flocks were allowed being moved only in the absence of clinical signs, and vehicles needed to be covered and to drive along validated routes. If the flocks were very small, or in areas without slaughterhouses in proximity, preventive culling was applied directly by local authorities or by private vets.
Due to the epidemiological situation, preventive culling measures were also ordered in France for ducks and geese in growing or fattening units that were over the programmed period of rearing and were not allowed to be moved due to restrictions.
In Hungary, preventive culling was applied in densely populated areas and in newly affected zones. Pre‐emptive killing was applied in the densely populated poultry areas on the eastern part of Bács‐Kiskun County, where the whole poultry population was culled as a principle; depopulation of high value flocks was managed on a case‐by‐case basis. Furthermore, at the end of November 2016 also, direct transport of poultry for immediate slaughter was granted to reduce the population in the affected areas.
3.2.7. Regional stand still
Regional standstill was applied in France. A Temporary Controlled Zone (TCZ) was defined around the Surveillance Zone in the areas were the virus was circulating at a high rate. In the TCZ, the introduction of palmipeds was forbidden, palmipeds already in place could move from open runs to finishing units if they tested negative to virological tests, and from finishing units to slaughterhouse following strict clinical surveillance.
3.2.8. Derogations on restriction zone implementation after risk assessment
Nine out of 13 MSs (69.23%) with complete questionnaires allowed derogations to measures applied within the restriction zones. Derogations were granted by the Competent Authorities as for Council Directive 2005/94/EU, following risk assessment that included:
potential contacts with other farms;
farm density in the area where the derogations were requested (in particular presence of commercial farms);
structure of the poultry production;
validations of the route to be followed if movements occur.
Special derogations were applied by Bulgaria, Denmark and France. Bulgaria granted derogations for killing endangered species in captive birds kept within a zoo, where birds were found to be positive for H5 HPAI viruses. As for the Council Directive 2005/94/EU, Article 16 (2), Denmark did not enforce any restriction zones around an outbreak occurred in captive birds within an open‐air museum. In this case, a risk assessment was conducted and no commercial farms were present within 4 km from the outbreak. Similarly, France did not establish Protection and Surveillance Zones around an outbreak in captive wild birds; only a restriction zone was applied in 10 km around the holding. The restriction zone was released after surveillance on all commercial flocks was concluded with no positive results.
Of the MSs that did not grant derogations, Ireland did not experience any outbreak in poultry and derogations were considered not needed. Belgium, Hungary and the Netherlands did not report any derogation granted.
3.2.9. Hunting
Four out of 13 MSs (30.76%) indicated the enforcement of a ban on hunting activities with various extents during the AI epizootic. Only, the Netherlands reported the application of a ban on a national level, starting on 14 November 2016; the ban was subsequently lifted on 9 December 2016, concurrently with the end of the hunting season. While also Bulgaria initially applied the ban on a National scale, the measure was reduced to higher risk areas (defined through risk assessment) at a later time. The UK and France indicated a ban on hunting enforced in the restriction zones around the outbreaks only (i.e. Protection and Surveillance Zones). France in particular banned hunting activities on waterfowl, also prohibiting the release of waterfowl for repopulation.
A risk assessment was carried out in the UK, to inform whether to allow hunting within disease control zones. The assessment considered published evidence on the likelihood of disturbance of birds during shooting, the different types of hunting allowed and the season for hunting. The conclusion was not to ban hunting in areas outside a disease control zone, as any disturbance caused is considered only temporary. Similarly, in Denmark, hunting has been allowed throughout the AI crisis with no particular restrictions. This was based on counselling from the ornithologist in the AI expert group, who referred to investigations documenting that birds in hunting areas only conducted limited movements during the hunt. The DVFA then assessed that the benefit of reducing the wild bird population by hunting, was greater than the risk of spreading AI. Hunting activities were therefore allowed with no particular restrictions. Nevertheless, the DVFA implemented an information campaign aimed at increasing alertness in hunters, providing details on the relevant biosecurity measures in particular for those hunters rearing poultry.
In Italy, the hunting season is scheduled between the third week of September and the end of January. Being the first case in poultry occurred on 21 January 2017, hunting activities were not banned, as the hunting season was ending. Nevertheless, from 30 December 2017, the Italian Competent Authority prohibited the use of live decoy birds, and the release of wild birds for repopulation.
In the Czech Republic, no ban on hunting was enforced; nevertheless, in compliance with Act No 449/2001 (Hunting Act), most waterfowl species were not allowed to be hunted at the time of the HPAI outbreak occurrence in the Czech Republic. Furthermore, repopulation with mallard was not allowed during the AI epizootic.
Also, Ireland decided not to ban hunting activities. Nevertheless, the Irish Competent Authorities provided special guidance to hunters during the AI emergency. Furthermore, no cases in poultry were detected in Ireland.
3.3. AI situation in other continents between October 2016 and August 2017 (TOR 4)
3.3.1. HPAI A(H5N1)
3.3.1.1. Domestic and wild birds
Detections
The Asian lineage HPAI A(H5N1) was initially diagnosed in humans in Hong Kong in 1997. The virus then re‐emerged in 2003 and 2004, and spread from Southeast Asia across Asia to Europe and Africa and has gained an endemic status in poultry populations in several countries including Egypt and Indonesia where spill‐over infections in humans continue to be registered. HPAI A(H5N1) has caused several hundred human cases and deaths, as well as the destruction of hundreds of millions of poultry worldwide. In 2005, HPAIV A(H5N1) caused massive mortalities among several aquatic wild bird species at Lake Qinghai, north‐western China (Chen et al., 2005). During 2005, HPAIV A(H5N1) spread from this location via southern Siberia, Kazakhstan and Russia to reach Europe in autumn 2005, and Africa in 2006 (Wallace et al., 2007). Epidemic outbreaks among wild birds were recorded in spring 2006 in central and south‐eastern Europe and again, in central Europe, during summer 2007 (Globig et al., 2009). Since 2011, a number of countries in Asia have experienced several new virus introductions, particularly of virus clade 2.3.2.1, which in most cases wild birds were implicated (Nagarajan et al., 2012). These include India, Bangladesh, the Republic of Korea, Laos, Malaysia, Japan, Myanmar and Nepal. HPAI A(H5N1) outbreaks in the poultry populations from these countries as well as from China, Vietnam and Indonesia have been continuously reported to date (Figure 17). Bangladesh, India and Nepal informed also about three events in wild birds (crows and whooper swan) in 2016 and 2017. Since the first introduction to Nigeria in 2006 HPAI A(H5N1) has circulated and spread in West African countries (Tassoni et al., 2016; Ekong et al., 2017). The disease was confirmed in Burkina Faso, Cameroon, Côte d'Ivoire, Ghana, Niger, Nigeria and Togo to date. Furthermore, Nigeria reported four wild bird events from the Delta and Kano province in 2016 (Figure 17). The outbreaks in Israel and the Occupied Palestinian Territories have been most frequently related to the endemic situation of clade 2.2.1.2 in Egypt; however, no links could be set up on the outbreaks in Iraq and Iran as clade 2.3.2.1 was identified (Figure 17). Since November 2015, the European avian lineage of HPAI H5 with three different subtypes (A(H5N1), H5N2 and H5N9) emerged in south‐western France and caused severe outbreaks in domestic ducks and domestic geese until March 2017 (Briand et al., 2017).
Figure 17.
Distribution of confirmed HPAI A(H5N1) outbreaks in birds by place of reporting between 1 January 2016 and 14 September 2017 (data source: Food and Agriculture Organization (FAO) EMPRES‐i13; status: 14.9.2017)
Phenotypic characterisation
HPAIV of the A(H5N1) subtype responsible for the ongoing outbreaks worldwide continue to exhibit high pathogenicity for gallinaceous poultry and moderate to low pathogenicity for waterfowl. Comparative assessment of pathogenicity of A(H5N1) HPAIV belonging to clades 2.3.2.1b, 2.3.2.1c, 2.3.4 and 2.3.4.1 for mallards provided evidence for efficient infection, with varied clinical course: relatively mild for 2.3.4 and lethal for 2.3.2.1b (Ducatez et al., 2017). Mandarin ducks (Aix galericulata) inoculated with clade 2.3.2.1 A(H5N1) isolated in South Korea in 2010 remained healthy throughout the experiment and shedding of the virus was minimal (Kang et al., 2017). The clade 7.2 A(H5N1) representatives were subject to animal experiments and showed high pathogenicity in chickens (intravenous pathogenicity index (IVPI) of 2.84–2.97) and no evidence of replication in ducks (Liu et al., 2016). Infection of ferrets with selected avian‐derived A(H5N1) isolates of clade 2.3.2.1 b and 2.3.2.1 c led to severe disease, systemic replication and death (Pearce et al., 2017).
Genetic characterisation
Since the first detection of Gs/Gd/96 ‘Guangdong’ lineage of A(H5N1) HPAIV in 1996, the HA of the virus has undergone an extensive evolution with the continuous emergence (and disappearance) of multiple genetic clades. The following clades were detected between 2013 and 2017 (Smith and Donis, 2015; Liu et al., 2016; Marinova‐Petkova et al., 2016a; Bhat et al., 2017; El Romeh et al., 2017; Ghafouri et al., 2017a; Rashid et al., 2017; Salaheldin et al., 2017; Shittu et al., 2017):
2.1.3.2a (Indonesia)
2.2.1.1 (Egypt)
2.2.1.2 (Egypt)
2.2.1.2a (Egypt, Israel and Occupied Palestinian Territories)
2.3.2.1.a (Bangladesh, India)
2.3.2.1.b (China and Hong Kong SAR)
2.3.2.1.c (Cambodia, China, Laos, Indonesia, India, Vietnam, Iraq, Iran, Lebanon, Nigeria, Burkina Faso, Niger, Ghana, Ivory Coast, Romania and Bulgaria)
7.2 (China).
The A(H5N1) HPAIV virus that was detected in North America in December 2014 should be treated separately as it was a reassortant that contained the HA of the Gs/Gd/96 lineage (clade 2.3.4.4), but four segments (including NA) from the North American lineage LPAIV (Torchetti et al., 2015).
A separate event was also associated with the emergent A(H5N1) virus from France (2015/2016) that turned out to belong to the European avian lineage and was clearly distinguishable genetically from the Gs/Gd/96‐like lineage (Briand et al., 2017).
Intraclade and interclade reassortants of Gs/Gd/96‐like A(H5N1) have been described (Marinova‐Petkova et al., 2016a). There is also evidence of the intersubtype reassortments (including A(H5N1)/A(H9N2)) of clades 2.3.2.1a (Marinova‐Petkova et al., 2016b) or 7.2 (Liu et al., 2016).
A(H5N1) viruses circulating worldwide continue to exhibit markers for increased zoonotic potential (Arafa et al., 2015) but so far no definite mutations have occurred that would enable sustained human‐to‐human transmission. The receptor‐binding site of the HA in the A(H5N1) virus from France (2015/2016) was ‘avian‐like’ and no major host adaptation or transmission markers indicative of the increased affinity to mammalian species were found (Briand et al., 2017).
3.3.1.2. Human infections due to A(H5N1)
Since 2003 and until 31 August 2017, totally 859 confirmed human cases due to A(H5N1), including 453 deaths (case fatality ratio: 53%), have been reported to the WHO from 16 countries (Table 12 and Figure 18) (WHO, 2017d).
Table 12.
Distribution of A(H5N1) cases by country and year, between February 2003 and 25 July 2017 (source: WHO, 2017b)
| Reporting country | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azerbaijan | 8 | 8 | ||||||||||||||
| Bangladesh | 1 | 2 | 3 | 1 | 1 | 8 | ||||||||||
| Cambodia | 4 | 2 | 1 | 1 | 1 | 1 | 8 | 3 | 26 | 9 | 56 | |||||
| Canada | 1 | 1 | ||||||||||||||
| China | 1 | 8 | 13 | 5 | 4 | 7 | 2 | 1 | 2 | 2 | 2 | 6 | 53 | |||
| Djibouti | 1 | 1 | ||||||||||||||
| Egypt | 18 | 25 | 8 | 39 | 29 | 39 | 11 | 4 | 31 | 136 | 10 | 3 | 359 | |||
| Indonesia | 20 | 55 | 42 | 24 | 21 | 9 | 12 | 9 | 3 | 2 | 2 | 199 | ||||
| Iraq | 3 | 3 | ||||||||||||||
| Laos | 2 | 2 | ||||||||||||||
| Myanmar | 1 | 1 | ||||||||||||||
| Nigeria | 1 | 1 | ||||||||||||||
| Pakistan | 3 | 3 | ||||||||||||||
| Thailand | 17 | 5 | 3 | 25 | ||||||||||||
| Turkey | 12 | 12 | ||||||||||||||
| Vietnam | 3 | 29 | 61 | 8 | 6 | 5 | 7 | 4 | 2 | 2 | 127 | |||||
| Total | 4 | 46 | 98 | 115 | 88 | 44 | 73 | 48 | 62 | 32 | 39 | 46 | 145 | 10 | 3 | 859 |
Figure 18.
Distribution of confirmed cases of A(H5N1) by reporting country 2003–2017 (n = 859) (source: WHO, 2017f)
No human cases related to HPAI A(H5N1) have ever been reported from EU/EEA countries.
In 2017, as of 8 September 2017, only Egypt has reported three cases including one fatality.
The most commonly identified risk factors associated with A(H5N1) virus infection include direct contact with infected blood or bodily fluids of infected poultry via food preparation practices or touching and caring for infected poultry (Abdel‐Ghafar et al., 2008). Exposure to avian influenza at live bird markets also represents a risk factor for transmission to humans ((Van Kerkhove et al., 2011a,b). However, other factors like housing poultry within the house and in general touching birds, so having a direct contact with infected birds, are also risk factors for virus transmission from sick or dead birds to humans (Fournie et al., 2017).
Human cases have been described based on infection with A(H5N1) viruses of clade: (a) 2.2.1.2 (Egypt) (WHO, 2017b), (b) 2.3.2.1a (Bangladesh) (WHO, 2016); (c) 2.1.3.2 (Indonesia).
Studies on seroreactivity in people occupationally exposed to poultry showed a high proportion of seroreactive people, indicating frequent transmission events with mild or asymptomatic symptoms in those frequently in contact with infected birds (Wang et al., 2014). This finding was confirmed in another study, which showed that healthy Chinese blood donors were also seroreactive, although the percentage was much lower than for occupationally exposed people (de Bruin et al., 2017).
3.3.2. HPAI A(H5N6)
3.3.2.1. Domestic and wild birds
Detections
A novel reassortant A(H5N6) HPAIV was detected for the first time from sewage in a live poultry market in China in December 2013 (Qi et al., 2014). In subsequent years, a few countries of Southeast Asia had notified infections with this virus. Initially, the detections were largely confined to China, Laos and Vietnam (Wong et al., 2015; Chu et al., 2016). During the autumn/winter season 2016/2017, the virus caused large epidemics in Republic of Korea (poultry) and Japan (wild birds) (Figures 19 and 20, Table 13). In the Republic of Korea, more than 300 outbreaks were confirmed, mostly in ducks and chickens. The virus was also detected in more than 160 cases in dead wild birds (predominantly swans, but also ducks, geese, gulls, cranes, grebes, birds of prey, owls and others), including birds kept at zoos. The affected wild bird species were similar to those found positive for A(H5N8) virus in Europe in 2016/2017. Single outbreaks were recently also reported from Hong Kong SAR, Chinese Taipei, Myanmar and Vietnam (Table 13).
Figure 19.
Outbreaks of A(H5N6) HPAIV in domestic birds between 1 January 2016 and 14 September 2017 (data source: FAO EMPRES‐i; status: 14.9.2017)
Figure 20.
Outbreaks of A(H5N6) HPAIV in wild birds between 1 January 2016 and 14 September 2017 (data source: FAO EMPRES‐i; status: 14.9.2017)
Table 13.
A(H5N6) HPAIV subtype notifications to the OIE between 1 January 2016 and 28 June 2017 (species in bold are included on the current list of species to be targeted for sampling and testing for avian influenza based on Commission Decision 2010/367/ECc)
| Country (period) | Number of outbreaks reported to OIE | Species affected |
|---|---|---|
| China (People's Republic of) (2016) | 2 | Poultry |
| Chinese Taipei (2017) | 14 | Ducks, turkeys, chickens, native chickens |
| Hong Kong SAR (2016/2017) | 3 | Chicken, Northern pintail a (Anas acuta), Red‐whiskered Bulbul (Pycnonotus jocosus) |
| Japan (2016/2017) | 168 | Black Swanb (Cygnus atratus, n = 19), Tundra Swan (Cygnus columbianus, n = 19), Whooper swan (Cygnus cygnus, n = 33), Mute swan (Cygnus olor, n = 53), Greater white‐fronted goose (Anser albifrons, n = 2), Bean goose (Anser fabalis, n = 2), Cackling gooseb (Branta hutchinsii, n = 4), Mallard b (Anas platyrhynchos, n = 3), Northern pintail (Anas acuta, n = 3), Eurasian wigeon (Anas penelope, n = 5), Tufted duck (Aythya fuligula, n = 1), Common pochard (Aythya ferina, n = 4), Greater scaup (Aythya marila, n = 2), Mandarin duck (Aix galericulata, n = 1), Anatidae (unidentified, n = 1), Black‐headed gull (Chroicocephalus ridibundus, n = 11), Hooded crane (Grus monacha, n = 22), White‐naped crane (Grus vipio, n = 1), Eurasian coot (Fulica atra, n = 2), Great crested grebe (Podiceps cristatus, n = 3), Northern goshawk (Accipiter gentilis, n = 4), Accipitridae (unidentified, n = 1), Peregrine falcon (Falco peregrinus, n = 6), Strigidae (unidentified, n = 1), Snowy owl a (Bubo scandiacus, n = 3), Corvidae (unidentified, n = 1) |
| Myanmar (2017) | 1 | Layer chickens |
| Republic of Korea (2016/2017) | 343 | Breeding ducks, Fattening ducks, Layer chickens, Broiler chickens |
| Vietnam | 6 | Backyard poultry |
Apparently healthy.
Including zoo birds.
Commission Decision (EC) No 367/2010 of 25 June 2010 on the implementation by Member States of surveillance programmes for avian influenza in poultry and wild birds. OJ L 166, 1.7.2010, pp. 22–32.
Phenotypic characterisation
The phenotype of the recent A(H5N6) viruses from Asia seems similar to that of the 2016–2017 A(H5N8) viruses: highly virulent for chickens, less virulent for domestic ducks, and variable virulence for wild birds. Increased mortality in galliforms as well as increased mortality and neurological signs (torticollis, ataxia) in domestic ducks were reported from the Republic of Korea (OIE reports). However, virus isolation from apparently healthy ducks and geese in live bird markets in China was also described (Jiao et al., 2016; Sun et al., 2016). A Korean A(H5N6) isolate was tested in 6‐week‐old SPF chickens inoculated intravenously and the IVPI value was 2.66 (Si et al., 2017). Another South Korean A(H5N6) isolate, detected in 2016, had the IVPI value of 2.94 and the mean death time (MDT) for embryos was 36 h (Kim et al., 2017). The IVPI value of two avian‐origin A(H5N6) viruses isolated from swine in China was 2.8 and 2.99 (Li et al., 2015). All IVPI values meet the criteria for high virulence according to OIE Diagnostic Manual. Experimental infection of 6‐week‐old chickens infected via the intranasal route (106 median egg infectious dose EID50) with A(H5N6) HPAIV isolated from apparently healthy ducks in southern China resulted in the quick progression of symptoms, including depression, anorexia and death of 100% birds within 6–7 days post‐infection. The virus replicated in a wide range of organs and was successfully transmitted to naïve chickens that also died (Jiao et al., 2016).
Field observations of clinical manifestation in wild birds are rare. There was detection of A(H5N6) HPAIV in South Korea in three whooper swans (Cygnus cygnus) in 2016, one with neurological signs and two found dead. It is possible that whooper swans brought HPAIV A(H5N6) into Korea. However, it is also possible that whooper swans, which are highly susceptible to HPAI A(H5N1) infection, may have been exposed locally through direct or indirect contact with other A(H5N6)‐infected but asymptomatic migratory species, such as mallards or spot‐billed ducks, or perhaps through exposure to infected poultry (Jeong et al., 2017). In winter 2016–2017 in Japan, 230 cases of HPAI caused by A(H5N6) viruses were reported from wild birds, captive birds and poultry farms throughout the country. The Japanese A(H5N6) isolate differed slightly from that of HPAIVs isolated previously in Japan and China. The virus exhibited high pathogenicity and a high replication capacity in chickens, whereas virus growth was slightly lower in ducks compared with an A(H5N8) HPAIV isolate collected in Japan in 2014 (Hiono et al., in press).
Conversely, the A(H5N6) virus was detected in apparently healthy Northern pintails (Anas acuta) sampled during active surveillance in Hong Kong SAR.
Genetic characterisation
-
i
Phylogenetic analysis
Phylogenetic studies showed that the A(H5N6) viruses have been generated through multiple reassortment events. The primary A(H5N6) virus (detected at the end of 2013 in China) contained the HA gene of the A(H5N1) HPAIV clade 2.3.4.4, the internal genes of A(H5N1) clade 2.3.2.1 and the NA gene from the H6N6 LPAIV (Qi et al., 2014). Since that time, subsequent reports have provided evidence about growing genetic diversity caused by multiple reassortment events, mostly with Eurasian‐origin AIV, including local HPAIV and LPAIV strains circulating in wild birds and poultry. However, the vast majority of HA genes still belong to the 2.3.4.4 lineage, although there are reports of an A(H5N6) virus with the HA derived from the 2.3.2 clade (Du et al., 2017).
In a recently published paper by Yang et al. (2017), three events have been suggested to explain the generation of novel A(H5N6) reassortants. In the first event, the ‘reassortant A‐type’ acquired HA gene segment from H5N2 clade 2.3.4.4 virus, NA gene segment (non‐truncated) from H6N6 virus and internal gene segments from A(H5N1) clade 2.3.2.1.c. In the second event, the ‘reassortant B‐type’ was generated by the acquisition of HA gene segment from A(H5N8) clade 2.3.4.4 virus, NA gene segment (truncated) from H6N6 virus and internal gene segments from A(H5N1) clade 2.3.2.1.c. The ‘reassortant C‐type’ was generated as a result of the reassortment between reassortant B (HA and NA genes) and poultry‐adapted A(H9N2) virus (internal genes). Notably, A(H5N6) viruses with an insert of internal A(H9N2)‐like genes seemed to prevail in live poultry markets (LPMs) in different regions of China (Chen et al., 2017).
Studies carried out in China on 175 A(H5N6) AIVs isolated between 2014 and 2015 in LPMs in Hunan Province provided evidence for the existence of at least six genotypes arising from segment reassortment, including a variant that possessed an HA from A(H5N1) clade 2.3.2 (Du et al., 2017). In the surveillance in LPM in eastern China in 2016, a novel subtype H7N6 was described in chicken. The virus possessed gene segments derived from A(H5N6), A(H9N2) and A(H7N9) viruses (Wu et al., 2017).
The A(H5N6) AIVs detected in Japan in November 2016 were classified into the genetic clade 2.3.4.4 c and were genetically closely related to A(H5N6) HPAIVs that had been recently isolated in South Korea and China (Okamatsu et al., 2017). The A(H5N6) viruses found in wild birds and poultry in Korea in 2016 seem to be closely related A(H5N6) viruses circulating in Guangdong province in China. Reassortment events with Eurasian LPAIV were also detected (Kwon et al., 2017; Lee et al., 2017). In another study, the A(H5N6) from faecal samples was proved to contain genes derived from H4N2 and H1N1 (Si et al., 2017). (Jeong et al., 2017) characterised genetically two novel reassortants A(H5N6) AIVs detected in November 2016 in whopper swans in South Korea and found them to be distinguishable from the A(H5N8) and A(H5N1) HPAIVs previously isolated in Korea. Kim et al. (2017) analysed five A(H5N6) isolates from faecal wild bird samples in South Korea and found that they were reassortants generated from numerous Eurasian AI virus subtypes, including A(H5N8) highly pathogenic viruses.
-
ii
Molecular marker analysis
Two predominant HA cleavage site amino acid motifs found in A(H5N6) viruses from Asia are: PLREKRRKRGLF, PLRERRRKRGLF, occasionally also PLKEKRRKRGLF, PQRERRRKRGLF and PLREKRRRRGLF (Influenza Virus Database, NCBI), all consistent with high virulence.
The available studies on the genetic markers of virulence and host adaptation show that although most of A(H5N6) viruses exhibit preferential binding to sialic acid receptors joined to sugar through an α‐2,3 sialic acid linkage (Kim et al., 2017), a feature typical of avian influenza viruses, a change towards human receptor‐binding preference (α‐2,6 sialic acid linkage) has also been described (Sun et al., 2016; Guo et al., 2017). For example, the A(H5N6) Chinese isolates from poultry had lost the glycosylation site at residue 158 of HA, bound both α2,6‐resialylated and α2,3‐resialylated chicken red blood cells (cRBCs), showed extensive binding to human tracheal epithelial and alveolar cells, replicated in the lungs of mice, and were transmissible through direct contact between ferrets (Sun et al., 2016).
Two types of A(H5N6) can be distinguished based on the length of NA: with‐ and without truncated NA stems (deletions at amino acid positions 59–69), a signature of adaptation to terrestrial poultry (Bi et al., 2016; Sun et al., 2016; Yang et al., 2017).
The A(H5N6) viruses that acquired the internal gene cassette from A(H9N2) viruses have been shown to carry mutations related to transmissibility and virulence in mammals or Adamantine resistance in PB1, PA, M1 and M2. The A(H5N6) reassortant viruses that derived NS1 from A(H5N1) viruses possess mutations indicating increased virulence in mice (Yang et al., 2017).
3.3.2.2. A(H5N6) in mammals (excluding humans)
A fatal infection of a domestic cat with an A(H5N6) virus was reported in China in 2014 (Yu et al., 2015). The virus was phylogenetically similar to the A(H5N6) virus circulating in wild birds at that time and possessed molecular signatures associated with increased virulence and transmissibility in mammals. Another A(H5N6) virus of feline origin (A/feline/Guangdong/1/2015) was characterised in vitro and was demonstrated to suppress the feline interferon response (Wang et al., 2017).
In 2014, two A(H5N6) HPAI viruses were isolated from nasal swabs collected from healthy pigs in a slaughterhouse in Guangdong province, China. The viruses were genetically homologous to A(H5N6) strains circulating at the time in the region in ducks and humans, and were highly pathogenic to chickens; even though the virus induced inflammatory lesions in the lungs of infected mice, it did not cause mortality (Li et al., 2015).
3.3.2.3. Human infections due to A(H5N6)
Since 2014, China has reported 17 human cases, including 12 deaths, due to A(H5N6) in different provinces in China (Figure 21) (CHP, 2017a; WHO, 2017a). Ten of the cases occurred in 2016 (WHO, 2017c). All cases occurred in mainland China, no other country has reported a case due to A(H5N6). The latest case was reported in December 2016. The individuals had an age range of 5–65 years, mean age of 37 years and median of 40 years of age and all reported exposure to poultry previously to the onset of symptoms (Jiang et al., 2017).
Figure 21.
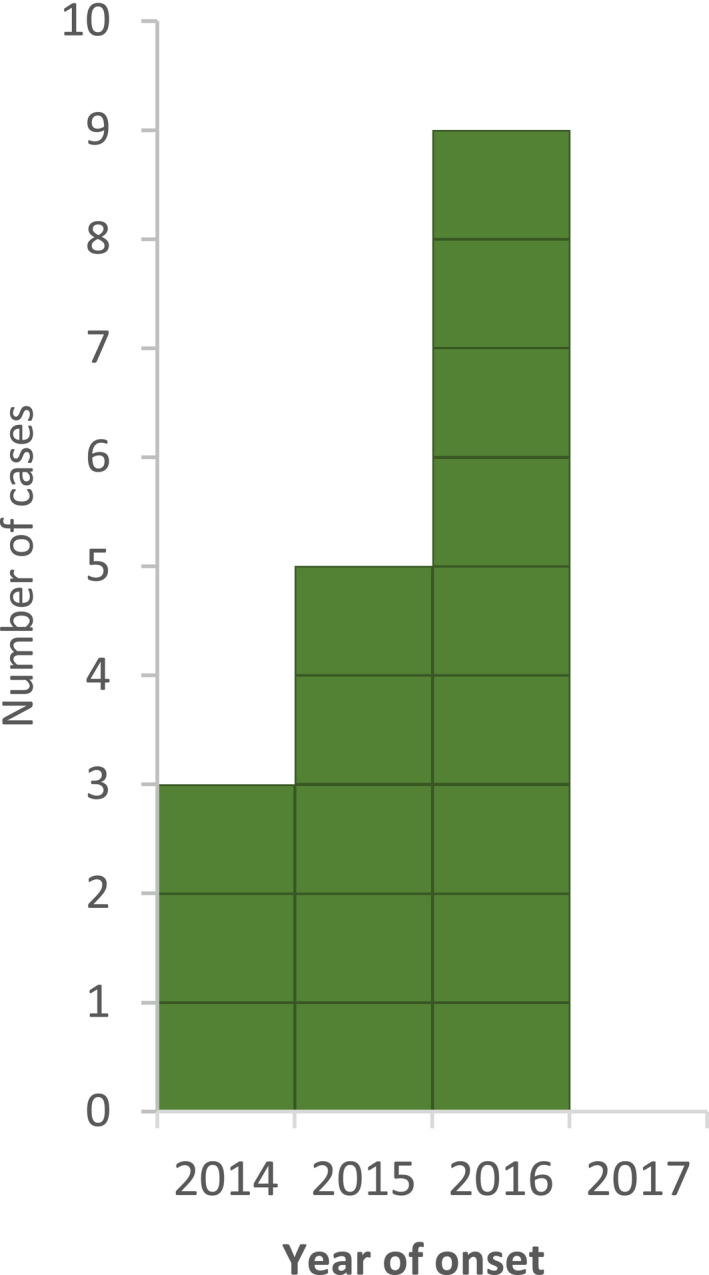
Distribution of laboratory‐confirmed cases of A(H5N6) by month and year of reporting, 2014–2017 (n = 17)
All viruses analysed from human individuals so far belonged to clade 2.3.4.4 (WHO, 2017c).
3.3.3. HPAI A(H5N8)
3.3.3.1. Domestic and wild birds
Detections
Since the first cases of HPAI A(H5N8) were detected in November 2016 in common coots (Fulica atra) and Eurasian wigeons (Anas penelope) in Egypt and Tunisia as well as on a poultry farm in Nigeria in December 2016, A(H5N8) has been recorded also from Cameroon, the Democratic Republic of the Congo, Niger, South Africa, Uganda and Zimbabwe. In Egypt, Cameroon and Nigeria several outbreaks of subtype A(H5N8) were detected in domestic birds in 2017 and currently HPAI A(H5N1) and A(H5N8) viruses are co‐circulating in the poultry population of these countries (see also Section 3.3.3.1). Infections of wild birds (e.g. white‐winged black tern, Chlidonias leucopterus) were reported in January 2017 from Lake Victoria in Uganda and these spread to poultry farms in the Democratic Republic of Congo and Uganda in the following months. Over the last 3 months, further outbreaks of HPAI A(H5N8) have been reported from South Africa and Zimbabwe. In addition to poultry farms the virus was also detected in several wild bird species e.g. Egyptian goose (Alopochen aegyptiaca), southern masked weaver (Ploceus velatus), yellow‐billed duck (Anas undulata), spur‐winged goose (Plectropterus gambensis), sacred ibis (Threskiornis aethiopicus), house sparrow (Passer domesticus) and African rock pigeon (Columba guinea) in South Africa (see Figure 22).
Figure 22.
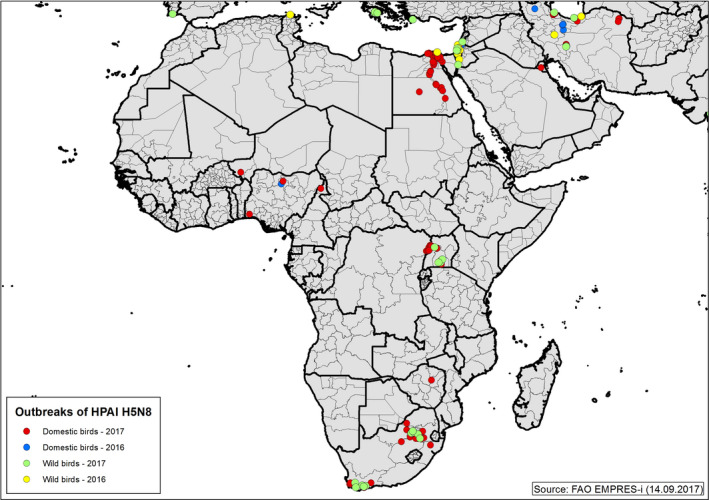
Distribution of confirmed HPAI A(H5N8) outbreaks in birds by place of reporting in Africa and the Middle East between 1 January 2016 and 14 September 2017 (data source: FAO EMPRES‐i; status: 14.9.2017)
HPAI A(H5N8) has also been reported in Asia (China, India, Kazakhstan, Republic of Korea, Nepal, Russia) and the Middle East (Iran, Israel, Kuwait). An overview is provided by FAO via the A(H5N8) HPAI global situation updates.14
Phenotypic characterisation
Data on the phenotypic characteristics of the current A(H5N8) HPAIV clade 2.3.4.4 (2016/2017) virus from outside Europe are scarce. Conversely, a large amount of data has been published recently on the pathogenicity of the A(H5N8) clade 2.3.4.4 (2014/2015) virus detected in Asia and North America. The major conclusions are briefly summarised below. However, caution is recommended when extrapolating these results on the properties of the recent A(H5N8) viruses, as despite some similarities, the recent A(H5N8) virus seems to evoke higher mortality for certain species, especially wild birds.
In general, the A(H5N8) clade 2.3.4.4 (2014/2015) virus seemed to be less virulent for domestic waterfowl than gallinaceous poultry. Despite its high lethality for chickens, its apparent virulence and transmissibility for this species was lower in comparison with A(H5N1) HPAIV (Bertran et al., 2016; Lee et al., 2016a). Intraclade‐dependent differences in virulence were also observed (Tanikawa et al., 2016). Higher resistance of some local breeds/lineages of chickens has been reported from South Korea (Lee et al., 2016a,c).
Experimental inoculation of Pekin ducks with an A(H5N8) virus from North America resulted in no mortality, lack or only mild clinical signs (conjunctivitis, diarrhoea) but virus shedding and transmission to contact‐exposed ducks was observed (Pantin‐Jackwood et al., 2017). Experimentally infected Muscovy ducks survived infection and seroconverted (Lee et al., 2016a). Mild clinical signs but occasionally nervous symptoms were observed following experimental inoculation of Chinese geese (Agnes cygnoides) (Pantin‐Jackwood et al., 2017). The absence of clinical signs but replication of A(H5N8) strains isolated in Korea in 2014 was reported in pigeons. Transmission to contact birds was not observed (Kwon et al., 2017).
The A(H5N8) clade 2.3.4.4 (2014/2015) was detected from a variety of wild bird species in Asia/North America but few studies have addressed the pathogenicity of the isolates for wild birds in the experimental setting. In one of these, the absence of clinical signs but efficient replication and transmission to co‐housed birds was reported after experimental infection of Mandarin ducks (Aix galericulata) with a South Korean A(H5N8) isolate (Kwon et al., 2017). Mallards (Anas platyrhynchos) inoculated with an A(H5N8) HPAIV from North America exhibited fever, decreased body weight, shed low titres of the virus to contact ducks and had moderate lesions at necropsy (Pantin‐Jackwood et al., 2016).
Genetic characterisation
-
i
Phylogenetic analysis
In May, 2016, a novel reassortant A(H5N8) HPAIV belonging to clade 2.3.4.4 was identified in Tyva Republic in Russia near the border with Mongolia and in Qinghai Lake in China. Phylogenetic analysis showed that three genes (HA, NA and NS) of novel Russian and Chinese isolates were derived from clade 2.3.4.4 B, whereas the remaining segments (PB2, PB1, PA, NP and M) clustered with LPAIV detected in wild birds in Mongolia, China and Vietnam (Lee et al., 2017; Li et al., 2017). Since then, further A(H5N8) clade 2.3.4.4 B reassortants have been identified across Europe and Asia.
In October 2016, two slightly different genotypes of A(H5N8) viruses were detected at two zoos in India, with most of the gene segments closely related to the A(H5N8) sequences from Tyva Republic, Qinghai Lake and Uvs‐Nuur Lake. However, the NP and PA genes (first genotype) or only NP gene (second genotype) showed the highest similarities to the Eurasian LPAIV sequences (Nagarajan et al., 2017). Similar observations were also made for the Korean A(H5N8) isolates obtained in December 2016, which were proved to be reassortants generated from A(H5N8) clade 2.3.4.4B and Eurasian LPAI viruses (Kim et al., 2017). Novel reassortants were also detected in December 2016 in Egypt (Kandeil et al., 2017a) The analysis of partial sequences of HA genes from outbreaks in Iran in November 2016 also showed that they belonged to clade 2.3.4.4B (Ghafouri et al., 2017b). The data suggests that multiple genotypes were generated in the summer of 2016 in central Asia, and then were disseminated to the Far East, Middle East, Africa and Europe.
-
ii
Molecular analysis
Most of A(H5N8) isolates identified in Asia and Africa show the avian‐like receptor specificity as indicated by presence of glutamine (Q) at amino acid position 226 of HA protein. However, Marchenko et al. (2017) reported N94S and T123P substitutions in the HA protein, associated with increased interactions with human‐type sialic acid receptors. All available studies indicate that analysed isolates are susceptible to amantadine and neuraminidase inhibitors. The most prominent mutations responsible for increased pathogenicity for mammals such as PB2 E627K and D701N were not detected. However, markers of mammalian host specificity were observed in other genes, e.g. PB1 L13P in isolates from India and Egypt (Kandeil et al., 2017a; Nagarajan et al., 2017). Variability in PB1‐F2 protein length was also observed, isolates from India possessed truncated PB1‐F2 protein (11 amino acids), whereas in isolates from South Korea this protein was of full length.
3.3.3.2. A(H5N8) in mammals (excluding humans)
During the HPAI A(H5N8) outbreak in South Korea, a dog was reported to be seropositive for HPAI A(H5N8); recent experimental infection of dogs led to weak viral replication in the nasal cavity and seroconversion in infected and contact dogs (Yuk et al., 2017).
3.3.4. HPAI‐LPAI A(H7N9)
3.3.4.1. Virological data
Two separate lineages of A(H7N9) viruses have evolved in China – the Yangtze River Delta (YRD) lineage and the Pearl River Delta (PRD) lineage, with a different spatial and temporal distribution in China. Within the YRD lineage highly pathogenic strains as well as two distinct sub‐lineages, YRD‐1 and YRD‐2 have emerged, with the latter being currently the dominant circulating virus (de Bruin et al., 2017). Viral evolution and adaptation processes involved sites relevant for receptor‐recognition and/or antigenicity with YRD‐2 viruses showing low cross‐reactivity to current candidate vaccine virus (CVV) strains under development for pandemic preparedness and being recommended from WHO (Zhu et al., 2017).
Following the OIE notification, the Ministry of Agriculture in China published an emergency notice to strengthen National A(H7N9) surveillance, prevention and control on 18 February 2017 (FAO, online‐a). On 19 February 2017, China's Centre for Disease Control and Prevention reported the first description of a human case with a highly pathogenic A(H7N9) avian influenza virus in Guangdong showing insertion of basic amino acids in the cleavage site of the HA gene known to confer to increased pathogenicity in chickens (Webster and Rott, 1987; Klenk and Garten, 1994). The conversion of LPAIV A(H7N9) into HPAIV has been already described in a previously published ECDC Public Health Development and Rapid Risk Assessment (ECDC, 2017a,b).
A new real‐time assay has been developed for the rapid detection of this highly pathogenic variant of A(H7N9) (Jia et al., 2017).
3.3.4.2. Domestic and wild birds
Avian influenza viruses of the subtype A(H7N9) have been detected in poultry and wild birds with outbreaks reported from the Czech Republic, Guatemala, Japan, Mongolia, the Netherlands, South Korea, Spain, Sweden and the United States between 2000 and 2016 (Olson et al., 2013). But in March 2013, the WHO reported human infections with an Asian lineage of A(H7N9) in three patients from Eastern China (Gao et al., 2013). The novel A(H7N9) virus evolved as a multiple recombination reassortant of several parent viruses observed in chicken, ducks and wild birds from at least four origins in Asia (Liu et al., 2013). These viruses continued to evolve and reassort, particularly with subtype A(H9N2) viruses.
Genetic analysis and animal experiments showed that the new influenza A(H7N9) virus exhibits low pathogenicity in poultry and wild birds (Pantin‐Jackwood et al., 2014; Bui et al., 2016). In the following years, the virus spread in the chicken, duck and captive‐bred pigeon populations of China were noticed although no clear‐cut reservoir hosts or regions have been identified so far. However, five epidemic waves in humans with a high case‐fatality rate were observed (Wang et al., 2017). In January 2017 genetic analysis of avian samples from live bird markets (LBMs) in Guangdong confirmed the occurrence of HPAI A(H7N9) infections and subsequently the same virus was also identified in isolates from two human cases in the same province Guangdong. Currently, HPAI and LPAI A(H7N9) viruses co‐circulate in the poultry population and are the sources of human infections of both subtypes (Figure 23). There is some evidence of antigenic differences between the LP and HP A(H7N9) viruses and hence, LPAI A(H7N9) seropositive birds might mask the spread of HPAI infections and influence the surveillance results.
Figure 23.
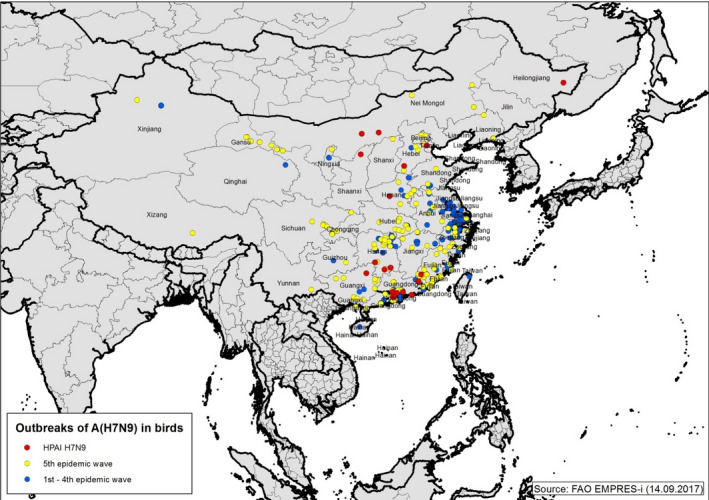
Distribution of confirmed LPAIV and HPAIV A(H7N9) outbreaks in birds and environmental samples by place of reporting in China since March 2013 (source: FAO EMPRES‐I; status: 14.9.2017)
Reports of outbreak investigation teams and animal experiments with LPAIV A(H7N9) indicate that none of the birds testing positive showed clinical signs of infection (Bui et al., 2016). Infection of poultry does not produce any clinical signs. A paper by Pantin‐Jackwood et al. (2014) showed that quail and chickens are susceptible to infection and shed large quantities of virus. Other poultry species including ducks were successfully infected but the quantity of virus shed suggested no significant role in the spread of the disease to humans (Pantin‐Jackwood et al., 2016). Field samples from pigeons were positively tested for A(H7N9), but experimental studies demonstrated the resistance of A(H7N9) and suggested that they had a minor role, if any, as a source of infection (Liu et al., 2015). Unfortunately, no published field or experimental data are available for the HPAI variant of A(H7N9) in China to date.
Besides the virus circulation in the poultry population by direct and indirect contacts between farms, LBMs all over China play the upmost important role in the disease transmission and as the sources of human infection. Particularly during the fourth and fifth epidemic waves, the Chinese authorities strengthened the National and Regional prevention and control measures by temporary closure and disinfection of LBMs, suspension of all live poultry trade, emergency monitoring of poultry and the culling of flocks in the case of HPAI A(H7N9) (Wang et al., 2017).
Knowledge on the role of wild birds is still limited, but a recent study shows that songbirds and parakeets shed the virus in the environment and tree sparrows tested positive during surveillance campaigns (Jones et al., 2014). Nevertheless, the surveillance results and the way of spread demonstrated that the role of migrating wild birds seems to be minor due to the adaptation of the virus to gallinaceous poultry but not to duck and geese species at the moment (Bui et al., 2016, 2017; Pantin‐Jackwood et al., 2016). Despite intensified surveillance activities in several neighbouring countries, A(H7N9) virus has not been detected in avian hosts outside China to date. Nevertheless, the lack of syndromic symptoms hampers the detection of the LPAI A(H7N9) variant by routine surveillance measures.
Chinese authorities have conducted active surveillance in animals since the first occurrence. Each year a surveillance plan, as part of a National prevention and control programme is published classifying provinces into key surveillance provinces and general surveillance provinces based on the presence or absence of the A(H7N9) virus. Virological and serological samples are taken mainly from chicken, ducks and pigeons on commercial fattening and breeding farms as well as backyard farms, LBMs, traders, the environment, and wild birds focusing on species intimately associated with farms and LBMs (e.g. house and tree sparrows). Per month, between 10,000 and 165,000 oropharyngeal and cloacal swab samples have been tested using real‐time polymerase chain reaction (RT‐PCR) or fluorescence RT‐PCR in the years 2016 and 2017 (ECDC, 2017a; Figures 24 and 25).
Figure 24.
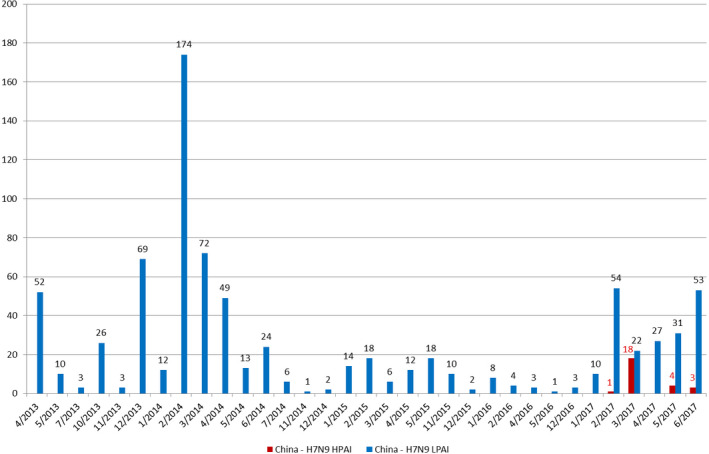
Number of confirmed LPAIV and HPAIV A(H7N9) outbreaks in birds and environmental samples in China since March 2013 (status: 14.9.2017)
Figure 25.
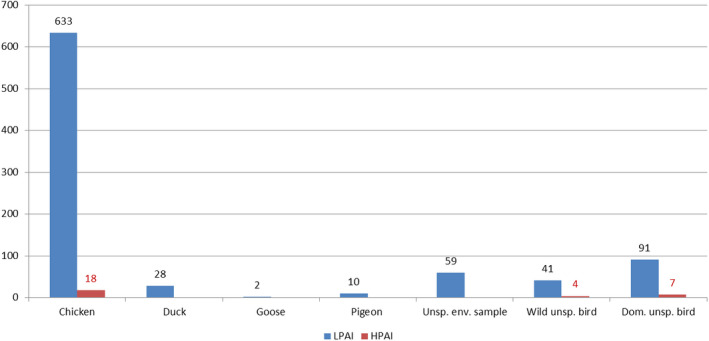
Number of confirmed LPAIV and HPAIV A(H7N9) outbreaks by species and environmental sample type in China since March 2013 (status: 14.9.2017)
Furthermore, the Chinese laboratories have tested per month between 84,000 and 316,000 serum samples by haemagglutination inhibition test in the years 2016 and 2017 (ECDC, 2017a). In this time, period the monthly seroprevalence was regularly below 1% (between 0.02 and 0.53; ECDC, 2017a). The results of the surveillance campaigns are published monthly by the Chinese Ministry of Agriculture (MoA, online) and are also available on the EMPRES‐i website of FAO (FAO, online‐b).
MoA started a H7 vaccination programme for poultry in early July 2017 in Guangdong and Guangxi provinces. A recombinant bivalent (H5 + H7) inactivated vaccine was used. But, in July 2017, the Chinese government decided to implement nationwide A(H7N9) vaccination campaigns starting in September 2017. With exception of poultry in AI‐free zones and export farms all domestic birds will be vaccinated (FAO, online‐b).
3.3.4.3. Human infections due to A(H7N9)
In March 2013, Chinese authorities announced the identification of a reassortant A(H7N9) influenza virus in patients in Eastern China. As of 08 September 2017, 1 562 laboratory‐confirmed cases were reported as infected in China, including two cases reported from Canada in 2015 and one case from Malaysia in 2014 (Figure 26, Table 14) (CHP, 2017c).
Figure 26.
Distribution of confirmed A(H7N9) human cases by place of reporting in China or with recent travel history to China, week 7/2013 to week 35/2017 (n = 1,562) (data source: CHP, 2017d)
Table 14.
Number of reported cases due to A(H7N9) infection by place and time of reporting, week 1/2013 to week 35/2017 (n = 1,562) (source: CHP, 2017c)
| Place of reporting | First wave (weeks 7/2013–40/2013) | Second wave (weeks 41/2013–40/2014) | Third wave (weeks 41/2014–40/2015) | Fourth wave (weeks 41/2015–40/2016) | Fifth wave (weeks 41/2016–35/2017) | Total |
|---|---|---|---|---|---|---|
| Zhejiang | 46 | 93 | 47 | 33 | 91 | 310 |
| Guangdong | 1 | 108 | 72 | 14 | 63 | 258 |
| Jiangsu | 27 | 29 | 22 | 26 | 148 | 252 |
| Fujian | 5 | 17 | 41 | 11 | 34 | 108 |
| Anhui | 4 | 14 | 14 | 6 | 63 | 99 |
| Hunan | 3 | 21 | 2 | 8 | 60 | 94 |
| Shanghai | 34 | 8 | 6 | 3 | 6 | 57 |
| Jiangxi | 5 | 1 | 3 | 3 | 38 | 52 |
| Sichuan | 0 | 0 | 0 | 0 | 38 | 38 |
| Beijing | 2 | 3 | 1 | 3 | 26 | 35 |
| Guangxi | 0 | 4 | 0 | 0 | 28 | 31 |
| Hubei | 0 | 0 | 1 | 1 | 29 | 31 |
| Hebei | 1 | 0 | 0 | 3 | 25 | 29 |
| Henan | 4 | 0 | 0 | 0 | 24 | 28 |
| Shandong | 2 | 2 | 2 | 2 | 19 | 28 |
| Hong Kong | 0 | 10 | 3 | 3 | 5 | 21 |
| Guizhou | 0 | 1 | 1 | 0 | 18 | 20 |
| Xinjiang | 0 | 3 | 7 | 0 | 3 | 13 |
| Chongqing | 0 | 0 | 0 | 0 | 9 | 9 |
| Shaanxi | 0 | 0 | 0 | 0 | 7 | 7 |
| Gansu | 0 | 0 | 0 | 0 | 5 | 5 |
| Taiwan | 1 | 3 | 0 | 0 | 1 | 5 |
| Liaoning | 0 | 0 | 0 | 1 | 3 | 4 |
| Tianjin | 0 | 0 | 0 | 2 | 3 | 5 |
| Jilin | 0 | 2 | 0 | 0 | 1 | 3 |
| Tibet | 0 | 0 | 0 | 0 | 3 | 3 |
| Macau | 0 | 0 | 0 | 0 | 2 | 2 |
| Shanxi | 0 | 0 | 0 | 0 | 3 | 3 |
| Yunnan | 0 | 0 | 0 | 0 | 7 | 7 |
| Inner Mongolia | 0 | 0 | 0 | 0 | 2 | 2 |
| Canada | 0 | 0 | 2 | 0 | 0 | 2 |
| Malaysia | 0 | 1 | 0 | 0 | 0 | 1 |
| Total | 135 | 320 | 223 | 120 | 764 | 1,562 |
An interactive ECDC map that shows the number of human cases due to A(H7N9) over time is available at: http://gis.ecdc.europa.eu/influenza/A(H7N9)/
The outbreak shows a seasonal pattern. Five epidemic waves have been recorded since 2013; a wave is defined as the period between week 41 of a year until week 40 of the subsequent year. The first wave in spring 2013 (weeks 7/2013 to 40/2013) included 135 cases, the second wave (weeks 41/2013 to 40/2014) 320 cases, the third wave (weeks 41/2014 to 40/2015) 223 cases, and the fourth wave (weeks 41/2015 to 40/2016) 120 cases.
The fifth wave started in October 2016 (CHP, 2017b,c), with 764 cases reported from 27 provinces or municipalities in China, Hong Kong and Taiwan as of 8 September 2017 (CHP, 2017c) (Table 14). The A(H7N9) virus has affected so far more men than women and most of the cases were between 40 and 60 years old or more than 60 years old (Su et al., 2017). During the fifth wave, more semi‐urban and rural areas were affected than previously reported, indicating a geographical spread back to the country side where poultry are present.
The current wave has seen the highest number of cases ever reported and shows also the largest spread of the disease across China (Figures 26 and 27, Table 15). A seasonal pattern of the epidemic curve was observed during the first four waves with a clear peak in January, whereas only a few sporadic cases were reported between weeks 20 and 40. In 2017, however, more human cases have been reported also during the summer months and the decrease from cases each week to sporadic cases was slower and later than in previous waves. A change in the epidemiologic situation in poultry and/or adaptation processes in the virus has contributed to a continued transmission beyond week 20 during the fifth wave. The lower number of human cases during the summer period was previously considered to be related to higher temperatures (> 18°C) (Zhang et al., 2015) and solar radiation (Charland et al., 2009).
Figure 27.
Distribution of reported human influenza A(H7N9) cases in China by week, week 1/2013 to week 35/2017 (n = 1,562) (data source: CHP, 2017c). Please note: if week of onset is not available week of reporting has been used.
Table 15.
Distribution of reported A(H7N9) cases and deaths by epidemic wave, weeks 7/2013 to 35/2017 (source: CHP, 2017c)
| First wave (weeks 7/2013–40/2013) | Second wave (weeks 41/2013–40/2014) | Third wave (weeks 41/2014–40/2015) | Fourth wave (weeks 41/2015–40/2016) | Fifth wave (weeks 41/2016–32/2017) | Cumulative number of cases (weeks 7/2013–25/2017) | |
|---|---|---|---|---|---|---|
| Cases | 135 | 320 | 223 | 120 | 760 | 1,558 |
| Deaths | 43 | 134 | 98 | 45 | 246 | 566 |
| CFR (%) | 32% | 42% | 44% | 38% | 32% | 36% |
Risk factors for transmission have been identified as having direct contact to infected birds (touching, handling) and related to live bird markets (Fournie et al., 2017). Closures of live bird markets have previously shown an immediate decreasing effect on the transmission events to humans (Yu et al., 2014). However, market closures could have contributed to the spread of the disease via transport of infected birds to other not yet affected regions as well as transport back to the farms affecting more rural areas during the recent fifths wave (Zhou et al., 2017b).
Several human clusters, including those in common exposure to poultry could not be ruled out, have been reported during all waves. So far, no indications have been observed of sustained chains of human‐to‐human transmission (WHO, [Link], [Link], [Link], [Link], [Link]).
Overall, a case fatality ration of 36%, ranging between 32% and 44%, has been observed and remained at the same high level during all waves (Table 15). There is no indication that this ratio has changed during the current epidemic wave.
Twenty‐seven human cases caused by HPAI A(H7N9) virus were reported with onset of illness before April 2017 from Guangxi, Guangdong, Hunan, Shaanxi and Hebei provinces and one case reported from Taiwan with travel history to Guangdong (CNIC, 2017). The clinical disease was as severe as with LPAI A(H7N9), and 13 of the cases died (CDCP, 2017). So far, detailed clinical and epidemiological data are available for eight patients infected with HPAI viruses. These cases are associated with exposure to sick and dead poultry in rural areas. These eight patients were hospitalised earlier than those patients who were infected with LPAIV A(H7N9) and showed similar epidemiological characteristics and disease severity (Zhou et al., 2017a). Although no increased transmissibility or virulence was reported to be associated with the highly pathogenic variant of the virus, different evolutionary processes with mutations relevant for mammal adaptation processes have been identified as well as mutations related to antiviral resistance (Su et al., 2017). To further assess the impact of HPAI viruses on, for example, severity and transmissibility to humans, better data are needed.
Studies showed a slightly increased binding preference for human airway receptors when comparing the highly pathogenic with the low‐pathogenic form, this may facilitate circulation in poultry and possible transmission among humans (Zhu et al., 2017). Other work has shown that three additional mutations are needed to better adapt the receptor specificity for human‐type receptors and promote binding to human trachea epithelial cells (de Vries et al., 2017). However, further adaptations in addition, relevant for the stability of the virus, might be necessary for enhanced transmission to or between mammals.
3.3.5. LPAI A(H9N2)
3.3.5.1. Domestic and wild birds
Detections
A(H9N2) viruses were first detected in China in 1992 and spread in the early 2000s eastward via southern Asia to Israel, Saudi Arabia, United Arab Emirates and Sultanate of Oman (El Houadfi et al., 2016). LPAI A(H9N2) is now the most common subtype of influenza viruses in poultry in Asia, Middle East and North Africa and causes great economic losses for the poultry industry. Even long‐term vaccination programmes did not prevent the spread and endemic establishment of this disease (Sun and Liu, 2015). With exception of a long‐lasting epidemic in the turkey population of Germany, A(H9N2) infections in poultry have been only sporadically reported from Europe (Smietanka et al., 2014). In European, Asian and North American wild bird populations, influenza viruses of the subtype A(H9N2) have been repeatedly detected and intercontinental reassortants of the different lineages have been recently described (Lee et al., 2016b). The role of wild birds for the transmission cycle and persistence of the infection in the affected countries seems to be limited (Lee et al., 2016b). Despite the potential zoonotic risk of certain A(H9N2) lineages (e.g. G1) and their economic impact, these viruses are not on the list of notifiable or reportable diseases. Therefore, knowledge on the distribution and prevalence of the disease in domestic and wild bird populations is limited.
Phenotypic characterisation
Gallinaceous poultry is the major host for A(H9N2) AIVs worldwide, although sporadic infections in other species, such as ducks or pigeons, have also been reported recently (Kandeil et al., 2017b). Based on the adopted virulence criteria, A(H9N2) virus is low pathogenic but it induces respiratory signs and reproductive disorders (Qi et al., 2016), especially in the presence of concomitant infections with bacterial or viral respiratory pathogens. Co‐infection of poultry with A(H9N2) and other pathogens may exacerbate the infection and lead to high morbidity and increased mortality (Smietanka et al., 2014; Hassan et al., 2017).
Serial passage of A(H9N2) avian‐origin virus in pigs enhances its replication and transmissibility among pigs (Mancera Gracia et al., 2017). The A(H9N2) viruses of the G1‐like and Y280‐like lineages (prevalent in terrestrial poultry) but not Y439‐like lineage (associated with the wild bird reservoir) are able to infect pigs under natural conditions, evoking the assumption that adaptation of the A(H9N2) in poultry may increase infectivity of the virus in mammals (Wang et al., 2016). The A(H9N2) viruses of both G1‐like and Y280‐like lineages induce infection and different levels of shedding in ferrets (Gao et al., 2016).
Genetic characterisation
The A(H9N2) viruses circulating in terrestrial poultry in Northern Africa, the Middle East and part of South Asia belong to the G1‐like group, derived from a progenitor detected for the first time in a quail in Hong Kong in 1997. Genetic characterisation of the G1‐like viruses detected in recent years in Morocco, Libya, Egypt, Israel, Iraq, Iran, the United Arab Emirates, Oman, Afghanistan, Pakistan, India and Bangladesh provided evidence for a high degree of within‐group variation, a preference for both avian‐like and human‐like receptors and the absence of multiple basic amino acids at the HA cleavage site, suggesting low pathogenicity for poultry (Davidson et al., 2013; Shanmuganatham et al., 2014; Bahari et al., 2015; Body et al., 2015; Kammon et al., 2015; El Houadfi et al., 2016; Gowthaman et al., 2016; Lau et al., 2016; Lee et al., 2016b; Hosseini et al., 2017; Kandeil et al., 2017b). Evidence of the circulation of an G1‐like A(H9N2) antigenic variant highly adapted to quail was recently provided in Egypt (Adel et al., 2017; Kandeil et al., 2017b).
The A(H9N2)/Y280‐like lineage is represented by viruses predominant in the recent past in China but also in some countries of Southeast Asia such as Myanmar and Vietnam ((Thuy et al., 2016; Lin et al., 2017; Xia et al., 2017). The human‐like HA receptor specificity seems to be prevalent in the A(H9N2)/Y280‐like lineage viruses.
The A(H9N2)/Y439‐like lineage viruses are mostly associated with wild aquatic birds but in recent years, viruses of this group have been detected in poultry in some European countries. The European viruses were most likely derived from a wild bird reservoir and, following adaptation, they spread in gallinaceous poultry, mostly turkeys. Genetic characterisation has revealed no markers of increased virulence for poultry or mammals but some adaptive features for gallinaceous poultry, such as the NA‐stalk region deletion, have been found (Smietanka et al., 2014; Reid et al., 2016).
Reassortment events with the involvement of A(H9N2) and several other subtypes present in the endemic regions, such as A(H5N1), A(H5N6), H7N2, H7N3, H7N6, A(H7N9) or H10N8 have been frequently described (Iqbal et al., 2009; Sun and Liu, 2015; Marinova‐Petkova et al., 2016a; Tosh et al., 2016; Zhao et al., 2016; Wu et al., 2017). The A(H9N2) virus has served as both the ‘donor’ and the ‘receiver’ of internal genes. Intrasubtypic reassortment between different lineages of A(H9N2) has also been reported (Shanmuganatham et al., 2014; Liu et al., 2016). A reassortant A(H9N2) virus with five internal genes derived from the none A(H9N2) avian gene pool has been recently described in a pigeon in Egypt (Kandeil et al., 2017b). It was shown that the reassortment is one of the factors that increases virus fitness and shapes epidemiological and pathobiological dynamics of A(H9N2) infections (Pu et al., 2017).
3.3.5.2. Human infections due to A(H9N2)
Since 1998 and as of 8 September 2017, overall 41 laboratory‐confirmed human cases including one death due to A(H9N2) have been reported from Bangladesh (3), China (34) and Egypt (4) (WHO, 2017c). Three of these cases were reported from China in 2017 with the latest case identified in April 2017 (Figure 28) (CHP, 2017a; WHO, 2017e,f). Infections in humans caused only mild illnesses so far (WHO, 2017c). People were exposure to backyard or other live poultry as well as to contaminated environments (WHO, 2017f).
Figure 28.
Number of reported cases due to A(H9N2) infection by country and year of reporting, 1998–2017 (n = 41)
In 2017, the virus sequence of the HA gene analysed from the human case was similar to that of the Y280‐lineage A(H9N2) viruses that also circulated in the bird population in China (WHO, 2017c). Sequences from birds were similar to that of previous periods.
Results from a recent study on seroreactivity against H9 viruses in China, showed high seroreactivity in poultry workers (de Bruin et al., 2017) and confirmed previous findings in which 8.5% of the workers with occupationally poultry exposure were seroreactive (Li et al., 2016). A broader range of seroprevalence data has been determined by a systematic review due to the application of different methods to estimate seropositivity (Khan et al., 2015).
3.3.6. Scientific analysis AI spread from Third countries to poultry in the EU
Sections 3.3.1–3.3.5 underpin the dynamically evolving epidemiological situation of AI in Asia and Africa as a result of the acquisition of new biological features by the viruses, including an increase in virulence or altered adaptation to various species of birds and mammals. The multiple pathways through which AIV can be brought to the EU include trade and illegal movements of poultry and poultry products, contaminated fomites and wild birds.
Wild birds play the primary role as potential carriers of H5Nx as seen for A(H5N1) in 2005 and 2010 and A(H5N8) in 2014 and 2016 (EFSA AHAW Panel, 2017). Interestingly, despite the endemic presence of A(H5N1) virus in Egypt and some countries of sub‐Saharan Africa, no introduction from Africa to Europe has been observed in the recent decade, this fact that can be linked to the unique ecology of migratory birds at the European‐African interface. Close collaboration with ornithologists is recommended to provide scientific ground for the explanation of this phenomenon. The Asia‐origin A(H5N6) HPAI virus has not been detected in the EU so far, but it seems to share many pathobiological features with A(H5N8) HPAI virus: it is prone to reassortment, highly lethal to gallinaceous poultry, less virulent in domestic waterfowl and shows varied virulence in wild birds. It has recently occurred on a large scale in wild birds, affecting a great variety of species (mostly Anatidae), most detections have been in wild birds that were found dead. However, it cannot be ruled out (and, given the degree of spread of the virus, is likely) that a percentage of these wild bird populations are infected without showing clinical signs. Therefore, it is likely that at some point it can follow the pattern of intercontinental spread similar to that observed for A(H5N1) or A(H5N8) HPAIV.
The A(H7N9) virus has not been detected on a large scale in wild waterfowl, which can be explained by the high degree of adaptation to gallinaceous poultry. The risk associated with the incursion of this subtype by means of wild birds is currently low but constant monitoring is warranted.
The A(H9N2) virus of the G1‐like group or Y280‐like group is highly adapted to poultry and is also unlikely to be brought to Europe by wild birds. Other risk pathways (including legal and illegal introduction) do not seem to constitute a significant risk as there have been no outbreaks in Europe caused by representatives of these lineages in the past. The introduction of the A(H9N2)/Y439 lineage from wild birds to terrestrial poultry with the subsequent acquisition of adaptive features enabled the virus to circulate for several years in Europe in the recent past. Infections caused by A(H9N2) are not notifiable but, taking into account the zoonotic potential, significant economic losses and propensity for reassortment, there is a need for increased vigilance and regular monitoring of the situation.
The risk of avian influenza viruses being transported to Europe in poultry through trade is negligible as live poultry, day‐old chicks and semen have been identified as the only non‐wild bird pathways via which AIV introduction is non‐negligible and suitable risk management measures are in place, such as testing and quarantine (EFSA AHAW Panel, 2017). EU legislation (Regulation EC/798/200815) prohibits the importation of live poultry, day‐old chicks and hatching eggs, semen and other birds (captive birds such as parrots, finches and ornamental birds for trade) from countries which cannot provide suitable health guarantees to comply with the certification. The list of approved countries is therefore limited (for reference see Table F5 of Appendix F, EFSA AHAW Panel, 2017).
Illegal movement of captive birds (in particular passerines) is a viable pathway for spread of the viruses, in particular H5Nx, but the risk is difficult to assess due to the paucity of data.
3.3.7. Surveillance and diagnosis of human infections and public health measures for prevention and control
3.3.7.1. Surveillance in the EU
Human infections with A(H5N1) are notifiable according to EU Decision 1082/2013/EU16, Commission Implementing Decision 2012/506/EU17 and the International Health Regulations (IHR)18 notification system.
Human infections with A(H7N9), A(H5N6), A(H5N8) and other novel influenza subtypes are notifiable under EU legislation (Decision 1082/2013/EU, Commission Decision 2008/426/EC19), and the IHR through the Early Warning and Response System (EWRS) and the IHR notification system.
ECDC has developed an interim case‐finding algorithm and a case definition for disease surveillance. Patients who are infected with avian influenza A(H7N9) virus in EU/EEA MSs are reported in accordance with this case definition (ECDC, 2013). WHO has published a recommendation for surveillance of human infections with avian influenza A(H7N9) virus (WHO, 2013a).
Study protocols for investigations of human cases are available from the Consortium for the Standardization of Influenza Sero‐Epidemiology (CONSISE, online) and national authorities. Agreed protocols for clinical investigations have been prepared by the International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC, online). Contacts of confirmed cases should be followed‐up and tested. International recommendations for the use of post‐exposure prophylaxis differ according to local risk assessments and the pathogenicity and transmissibility of the virus in question.
Evidence of the effectiveness of contact tracing on aircraft in limiting spread of infection is limited and should only be considered upon a risk assessment on a case‐by‐case basis (ECDC, 2014).
Diagnosis
With routine diagnostic laboratory assays, the novel A(H5Nx) or A(H7Nx) viruses should be detected as positive for influenza A virus, and negative for influenza B, A(H1), A(H1)pdm09 and A(H3) viruses.
Influenza A(H5Nx) or A(H7N9) viruses are expected to be classified as unsubtypeable influenza A if no‐specific A(H5) or A(H7) diagnostic test is performed. It is standard procedure in diagnostic laboratories to send influenza A virus isolates or clinical samples that cannot be subtyped to the national reference laboratory (National Influenza Centres (NICs)), and further to a WHO Collaborating Centre for Reference and Research on Influenza for characterisation, as was undertaken in China for the first influenza A(H7N9) isolates.
The European Reference Laboratory Network for Human Influenza (ERLI‐Net) laboratories have rapidly developed and verified their capabilities for detecting the novel influenza A(H7N9) influenza virus (Broberg et al., 2014). In an external quality assessment RT‐PCR detection panel influenza A(H7N9) samples were included (ECDC, 2017a). To assist European laboratories in verifying and ensuring their diagnostic capabilities for avian influenza A(H7N9) virus, ECDC, ERLI‐Net and the WHO Regional Office for Europe have released a technical briefing note on diagnostic preparedness in Europe for the detection of avian influenza A(H7N9) viruses (CNRL/ECDC/WHO Europe, 2013).
3.3.7.2. Public health control measures (in relation to the EU)
Stamping‐out and disease control measures including closures of LBMs in country of origin should minimise the circulation and prevalence in poultry as well as the contamination in the environment and subsequently lower the risk of transmission to humans.
Occupationally and otherwise exposed people should be identified and monitored for development of influenza‐like symptoms. People at risk are mainly those in direct contact/handling diseased birds or poultry, or their carcasses (e.g. farmers, veterinarians and labourers involved in culling and rendering). Workers should wear personal protective equipment (face mask, goggles/face shield/protective glasses, gloves and gown/overall) and avoid unprotected direct contact with sick or dead birds, carcasses, faeces as well as potentially contaminated environments (see ECDC guidelines20 and tool kit21). A tutorial on the safe use of personal protective equipment that could be used to train people directly exposed to infected poultry has previously been published by ECDC.22
National and regional contingency plans for the control of avian influenza in poultry and birds should be reviewed or further developed in collaboration with public health authorities.
For further information, FAO guidance is available for consultation on the sampling of infected birds and the protection of workers (FAO, online‐a). Poultry workers exposed at the affected holding should be monitored for a minimum of 10 days to document possible related symptoms, including influenza‐like illness (ILI) with fever and cough or conjunctivitis. Local health authorities may consider active monitoring of these groups and offering the groups vaccination against seasonal influenza, if they are not already vaccinated. Antiviral prophylaxis, as recommended for people exposed to avian influenza viruses, could also be considered depending on the local risk assessment (i.e. intensity of exposure).
WHO has produced guidance on laboratory biorisk management for A(H7N9) (WHO, 2013b,c,d,e,f, 2014). These guidelines are broadly applicable to management of all human cases of avian influenza and related samples. Healthcare workers often come into contact with patients with infectious diseases. Therefore, WHO recommends that basic appropriate infection prevention and control measures (standard precautions) be consistently applied in all healthcare settings at all times, and that the health status of healthcare workers be closely monitored. Together with standard precautions, healthcare workers caring for those suspected or confirmed to have avian influenza infection should use additional precautions.
Considering the severity of the disease, the fact that limited human‐to‐human transmission cannot be excluded in some clusters, that no vaccine is available on the market against A(H5Nx) and A(H7N9), and the favourable safety profile of the antiviral drugs of choice, it is likely that the benefits of post‐exposure chemoprophylaxis of close contacts with neuraminidase inhibitors outweigh the risks. Evidence of the benefits and effectiveness of prophylaxis and treatment remain limited.
3.3.7.3. Vaccines
The most important intervention in preparing for the pandemic potential of influenza viruses is the development and use of human vaccines, therefore the viral situation is constantly monitored and assessed by WHO. Following the vaccine composition meeting in March 2017, WHO published an updated overview of recommended CVVs and status of development (WHO, 2017c). Currently, 32 different CVVs for different A(H5) clades are listed as available, for five CVVs with the most recently circulating viruses including A(H5N1) clade 2.3.2.1c and A(H5N6) clade 2.3.4.4 availability is pending.
Eight different CVVs related to A(H7N9) viruses isolated in 2013 are assigned as available. Data showed that the recent emerging highly and low pathogenic viruses are genetically and antigenically distinct from the current recommended A(H7N9) CCVs. Therefore, due to the antigenic and genetic evolution of A(H7N9) viruses, three different CVVs for currently circulating A(H7N9) viruses were proposed for development but are not yet available (WHO, 2017c).
Recommended CVVs for other viruses include A(H7N1), A(H7N2), A(H7N3) and A(H7N7) as well as six different CVVs for A(H9N2) viruses (WHO, 2017c).
Sequences were shared through the GISAID EpiFlu sequence database, and a few viruses containing these new mutations were shared with all WHO Collaborating Centres and other partners in order to improve diagnostics and the development of candidate vaccines (GISAID, 2013). The timely characterisation of viruses and the sharing of sequence information remain crucial for virus vaccine development.
In EU MSs, surveillance for HPAIVs in poultry and wild birds is strong and is carried out on a continuous basis. ECDC is monitoring the situation closely, both in the EU and worldwide, and will regularly reassess the risk that viruses of H5‐ and H7‐type pose to humans.
3.3.8. ECDC risk assessment for the general public in the EU
To date, no human infections with highly pathogenic or related low pathogenic viruses of the H5Nx or A(H7N9) (viruses related to the strains circulating in China) type have ever been reported in the EU. The risk of zoonotic transmission to the general public in the EU/EEA countries is considered to be extremely low.
However, these viruses remain poorly adapted to humans:
Transmission from birds to humans is infrequent.
Only limited clusters of human cases have been reported.
No sustained human‐to‐human transmission has been observed.
The risk of a food‐borne transmission (e.g. eggs and meat) is considered extremely low according to EFSA BIOHAZ Panel (2010).
Zoonotic transmission to humans from infected birds occurs either directly or through environmental contamination. Hence, almost all human infections have been related to close contact with infected sick birds or their faecal products in domestic settings – for example at live bird markets in Asia.
Small clusters of human cases were identified but all cases had been exposed to birds before disease onset. No sustained human‐to‐human transmission has been documented so far. Almost all human cases reported direct exposure to live or sick or dead poultry – mostly backyard poultry or live bird markets – before onset of disease.
HPAI viruses are still circulating in poultry holdings in Asia, the Middle East and Africa, with repeated spill‐over to wild bird populations, as well as spill‐back from wild bird populations into poultry holdings, occasionally as far away as Western Europe and North America. The introduction of HPAI A(H5N8) virus and the reintroduction of HPAI A(H5N1) virus in many African and Asian countries with low biosafety and biosecurity standards lead to a continued circulation among poultry. In addition, silent virus spread by vaccinated but infected gallinaceous poultry must be considered as a risk factor of spread and exposure. In addition, the broad geographic spread, the increase of human cases and the emergence of HPAI A(H7N9) in China contributes to an increasing risk of human exposure and continuing transmission to humans, especially in live bird market settings, backyard facilities or small industrial holdings worldwide.
The recent upsurge of human cases particularly with A(H7N9) virus infections in China is most likely due to a higher percentage of virus‐positive birds, a larger geographical spread and a higher contamination of the environment that results in an increased risk of exposure. The possibility of sporadic importation of cases to Europe cannot be ruled out. Caution should be taken by people travelling to China to avoid direct exposure to poultry, live poultry markets or backyard farms to minimise contact with birds potentially infected with any avian influenza virus. Travellers that have visited affected areas and develop respiratory symptoms and fever after their return should consult a physician and inform about their recent travel history to initiate early diagnosis and treatment. The possibility of humans infected with avian influenza viruses returning to the EU/EEA cannot be excluded. However, the risk of the disease spreading within Europe via humans is still considered low, as the virus does not transmit easily among people.
People in the EU presenting with severe respiratory or influenza‐like infection and a history of travel to the affected areas in China with potential exposure to poultry or live bird markets will require careful investigation, management and infection control. Appropriate samples for influenza tests should be rapidly taken and processed from patients with relevant exposure history within 10 days preceding symptom onset. Early or presumptive treatment with neuraminidase inhibitors should always be considered for suspect or confirmed cases, in line with relevant national and international recommendations. Contacts of confirmed cases should be followed‐up, tested and offered post‐exposure prophylaxis as recommended by relevant national / international guidelines.
4. Conclusions
HPAI and LPAI outbreaks in Europe between 19 October 2016 and 31 August 2017 (TOR 1 and TOR 2)
Main observations:
To date, no human infections with highly pathogenic or related low pathogenic viruses of the A(H5N8), A(H5N6) and A(H5N5) type causing outbreaks in Europe during 2016/2017 have been reported in the EU.
-
Between 19 October 2016 and 31 August 2017 (based on ADNS):
-
1
– 1,197 H5 HPAI outbreaks were reported in poultry or captive birds in 20 MSs 1,188 A(H5N8), 8 A(H5N5) and 1 A(H5N6);
-
2
– 1,470 H5 HPAI events were reported in wild birds in 23 MSs: 1,458 A(H5N8) HPAI and 12 A(H5N5);
-
3
– 65 H5 LPAI outbreaks were reported in poultry and/or captive birds in 4 MSs, and 1 H7 LPAI outbreak was reported in poultry in France
-
1
The first peak of the epizootic occurred in early December 2016 primarily driven by poultry submissions from Hungary, a second peak occurred mid‐February 2017 as a result of poultry events in France and wild bird submissions from Germany, but sporadic outbreaks and wild bird events still occur.
HPAI A(H5N8) induced high mortality in chickens and turkeys but variable (low to high) mortality in ducks, geese and game birds.
Secondary spread has been observed, mainly in duck production in which husbandry practices required a lot of bird movements.
Conclusions:
The risk of zoonotic transmission to the general public in the EU/EEA countries is considered to be very low.
Despite the high number of human exposures to infected poultry during the ongoing outbreaks, no transmission to humans has been identified.
There is no clear information on the number of people and exposure events during the 2016/2017 epizootic.
The 2016/2017 HPAI epidemic was the largest ever recorded in the EU in terms of high number of outbreaks, wide geographic distribution and the high number of dead wild birds.
The HPAI A(H5N8) virus persisted during winter into the late summer at least and still results in sporadic outbreaks in poultry and wild bird infections.
Different interpretations of definitions among MSs, lack of denominator data and lack of zero reporting hamper retrospective data collection, reporting and/or analysis.
Applied prevention and control measures (TOR 3)
Main observations:
Case reports were submitted by 13 MSs to share information on AI applied prevention and control measures.
There is limited information on stringency, enforcement and implementation of biosecurity across the EU.
Awareness campaigns were generally given high relevance also through social media and novel technologies.
Conclusions:
Communication among MSs is paramount to increase the level of preparedness, and to promptly apply control measures before the disease spreads to non‐affected MS, preventing and/or limiting the spread of the disease.
AI situation in other continents between October 2016 and August 2017 (TOR 4)
Conclusions:
The current epidemiology of HPAIV A(H5N6) in Asia, with widespread occurrence in migratory birds of the order Anseriformes, and detection in apparently healthy northern pintails, indicates a risk of long‐distance spread of this virus to wintering grounds westwards to western Europe, Africa, and the Middle East and eastwards to western North America, similar to that of HPAIV A(H5N8) and HPAIV A(H5N1) in previous years.
The HPAI situation in Africa of the subtypes A(H5N1) and A(H5N8) is evolving rapidly and requires close monitoring.
No human case has ever been reported in the EU due to avian influenza viruses subtypes A(H5N1), A(H5N6), A(H7N9) and A(H9N2). Human infections due to transmission of these viruses have only been observed in areas where these viruses circulate in the wild bird and/or poultry populations mainly in Asia and Egypt. Severe diseases and deaths have been reported related to these infections in humans in Asia and Egypt. Small human clusters have been identified, but no sustained human‐to‐human transmission has been observed.
5. Suggestions
Further efforts are required to improve a standardised data collection with a revision of the interpretation of the definitions by the MSs, in particular for ADNS.
Data on poultry population structure are required to facilitate data and risk factor analysis, hence to strengthen science‐based advice to risk managers.
There is a need to promote a common understanding and application of definitions related to control activities and their reporting across MSs (e.g. captive birds vs non‐commercial poultry).
Continued surveillance for avian influenza virus in wild birds and poultry combined with timely data sharing among MSs as well as between animal and human health sectors is crucial to detect and respond early to threats relevant for animal and public health.
An updated of the list of wild birds target species should be considered based on the reported AI‐infected wild birds since 2006.
There is a need for strengthening collaboration at national, EU and global levels to monitor the AI situation helps to increase preparedness. Targeted wild bird surveillance programmes at a few priority locations within the EU should be considered, modulated and pro‐active in relation to risk factors and uncertainties at a global scale (e.g. taking into account temporal and geospatial events).
There is a need to improve the collection and reporting of information on exposure events of people to AI.
strengthening the consistency of the approach to evaluating zoonotic risks based on molecular data would be beneficial.
Abbreviations
- ADNS
Animal Disease Notification System
- AHAW
EFSA Panel on Animal Health and Welfare
- AI
avian influenza
- AIV
avian influenza virus
- CDC
Center for Disease Control
- CHP
Centre for Health Protection
- CNIC
Chinese National Influenza Center
- CONSISE
Consortium for the Standardization of Influenza Sero‐Epidemiology
- CRBCs
chicken red blood cells
- CVO(s)
Chief Veterinary Officer(s)
- CVV
Candidate vaccine virus
- DVFA
Danish Veterinary and Food Administration
- ECDC
European Centre for Disease Prevention and Control
- EID50
median egg infectious dose
- ERLI‐Net
The European Reference Laboratory Network for Human Influenza
- EURL
European Union Reference Laboratory for Avian Influenza
- EWRS
Early Warning and Response System
- FAO
Food and Agriculture Organization
- GISAID
Global Initiative on Sharing All Influenza Data
- HA
haemagglutinin
- HPAI
highly pathogenic avian influenza
- HPAIV
highly pathogenic avian influenza virus
- IHR
International Health Regulations
- ILI
influenza‐like illness
- ISARIC
International Severe Acute Respiratory and Emerging Infections Consortium
- IVPI
intravenous pathogenicity index
- LBM(s)
live bird market(s)
- LPAI
low pathogenic avian influenza
- LPM(s)
live poultry market(s)
- MDT
mean death time
- MP
matrix protein
- MS(s)
Member State(s)
- NA
neuraminidase
- NIC(s)
National Influenza Centre(s)
- NRL
National Reference Laboratory
- NS
non‐structural protein
- OIE
World Organisation for Animal Health
- PAFF Committee
The Standing Committee on Plants, Animals, Food and Feed
- PRD
Pearl River Delta
- Q
glutamine
- RT‐PCR
real‐time polymerase chain reaction
- TCZ
Temporary Controlled Zone
- TRZ
Temporary Restriction Zone
- WHO
World Health Organization
- YRD
Yangtze River Delta
Appendix A – Overview tables morbidity and mortality
1.
Appendix B – Additional data on characterisation of affected holdings
1.
Table B.3.
Number of outbreaks in zoos reported per MS (October 2016–April 2017; EFSA data collection)
| Country | Outbreaks | Recordsa |
|---|---|---|
| 24 Hungary | 4 | 5 |
| 22 Czech Republic | 1 | 5 |
| 14 Finland | 1 | 4 |
| 16 Sweden | 1 | 3 |
| 28 Bulgaria | 1 | 2 |
| 09 Denmark | 1 | 2 |
| 13 Austria | 1 | 1 |
| 01 Germany | 1 | 1 |
| 04 Netherlands | 1 | 1 |
| 25 Poland | 1 | 1 |
| 33 Slovakia | 1 | 1 |
| Total | 14 | 26 |
The number of records is the number of rows in the EFSA data collection database. In the case of zoos, it represents the number of species.
Appendix C – Number of poultry holdings
1.
In autumn 2016, EFSA asked the Chief Veterinary Officers (CVOs) of all MSs to report data on the number of poultry holdings at NUTS 2 level. EFSA extracted the 2014 or 2016 data submitted by the MSs for the annual AI serosurveillance programmes (http://ec.europa.eu/dgs/health_food-safety/funding/cff/animal_health/vet_progs_en.htm) and asked the MSs to review, update and complete the number of poultry holdings. The categories were used as they are reported for the AI serosurveillance: chicken breeders, chicken laying hens (covering both conventional and free‐range), turkeys (covering both fattening and breeders), ducks and geese (covering both fattening and breeders), farmed game birds (covering both gallinaceous and waterfowl). As the definition of ‘poultry holding’ varies between the MSs, it was requested to indicate the minimal number of laying hens on a reported holding (see Table C.1). All MSs provided data on the number of holdings, which was used to generate the maps below (see Figures 24–28). Comparison of the data between the MSs is very difficult given the different definitions of ‘poultry holding’ and the different sizes of the presented regions.
Table C.1.
Minimal number of laying hens on a reported holding per MS
| MS | Minimal number of laying hens on a reported holding |
|---|---|
| Austria | 1 |
| Belgium | 200 |
| Czech Republic | 1 |
| Bulgaria | 250 |
| Cyprus | 100 |
| Germany | 100 |
| Denmark | 100 |
| Estonia | 1 |
| Finland | 500 |
| Croatia | 10 |
| Greece | 50 |
| France | 15 |
| Hungary | Not reported |
| Ireland | 1 |
| Italy | 250 |
| Latvia | 1 |
| Lithuania | 100 |
| Luxembourg | Not reported |
| Malta | 50 |
| Netherlands | 250 |
| Poland | 300 |
| Portugal | Not reported |
| Romania | 10 |
| Slovakia | 1 |
| Slovenia | 50 |
| Spain | 1 |
| Sweden | 350 |
| UK | 500 |
At the same time, MSs were asked to provide information on the proportion of conventional (indoor, standard and organic) and free‐range (outdoor, standard and organic) laying hens. This was carried out asking MSs to update the values reported to EFSA in the context of the baseline surveys for Salmonella (see EFSA, 2007). The updated data EFSA received are shown in Figure C.6
Figure C.6.
Proportion of conventional vs free‐range laying hen flocks per MS reported to EFSA (autumn 2016)
Figure C.1.
Number of turkey holdings at NUTS2 level reported by MSs to EFSA (autumn 2016)
Figure C.2.
Number of farmed game bird holdings at NUTS2 level reported by MSs to EFSA (autumn 2016); NA = not available
Figure C.3.
Number of ducks and geese holdings at NUTS2 level reported by MSs to EFSA (autumn 2016)
Figure C.4.
Number of chicken‐laying hen holdings at NUTS2 level reported by MSs to EFSA (autumn 2016)
Figure C.5.
Number of chicken‐breeder holdings at NUTS2 level reported by MSs to EFSA (autumn 2016)
Appendix D – Wild bird species tested for AIV
1.
Table D.1.
Overview of the sampled‐positive wild bird species tested for AIV between January 2005 and April 2017, excluding the entirety of 2011, 2012 and 2013
| Species | Total birds sampled | Positive HPAI | % of positives | In the existing target list |
|---|---|---|---|---|
| Podiceps nigricollis | 329 | 246 | 74.8 | Yes |
| Pelecanus crispus | 58 | 11 | 19 | No |
| Aythya marila | 83 | 9 | 10.8 | No |
| Aythya fuligula | 3,570 | 339 | 9.5 | Yes |
| Podiceps cristatus | 773 | 51 | 6.6 | Yes |
| Cygnus atratus | 91 | 6 | 6.6 | No |
| Tachybaptus ruficollis | 108 | 6 | 5.6 | Yes |
| Anser erythropus | 55 | 3 | 5.5 | Yes |
| Haliaeetus albicilla | 658 | 35 | 5.3 | No |
| Larus marinus | 509 | 22 | 4.3 | No |
| Mergus albellus | 23 | 1 | 4.3 | Yes |
| Cygnus olor | 23,696 | 971 | 4.1 | Yes |
| Mergus merganser | 206 | 8 | 3.9 | No |
| Larus argentatus argentatus | 1,562 | 58 | 3.7 | No |
| Pelecanus onocrotalus | 59 | 2 | 3.4 | No |
| Buteo spp. | 1,954 | 54 | 2.8 | No |
| Cygnus cygnus | 6,393 | 168 | 2.63 | Yes |
| Buteo lagopus | 42 | 1 | 2.4 | Yes |
| Botaurus stellaris | 47 | 1 | 2.1 | No |
| Tringa ochropus | 52 | 1 | 1.9 | No |
| Porphyrio porphyrio | 68 | 1 | 1.5 | Yes |
| Bucephala clangula | 218 | 3 | 1.4 | No |
| Troglodytes troglodytes | 88 | 1 | 1.1 | No |
| Aythya ferina | 2,490 | 26 | 1 | Yes |
| Buteo buteo | 8,213 | 75 | 0.9 | Yes |
| Falco peregrinus | 1,064 | 10 | 0.9 | Yes |
| Egretta garzetta | 223 | 2 | 0.9 | No |
| Aix sponsa | 126 | 1 | 0.8 | No |
| Ardea cinerea | 6,137 | 40 | 0.7 | Yes |
| Egretta alba | 569 | 4 | 0.7 | No |
| Oxyura jamaicensis | 140 | 1 | 0.7 | No |
| Accipiter gentilis | 1,602 | 8 | 0.5 | Yes |
| Corvus corone | 822 | 4 | 0.5 | No |
| Strigidae | 398 | 2 | 0.5 | No |
| Cairina moschata | 514 | 2 | 0.4 | Yes |
| Turdus pilaris | 278 | 1 | 0.4 | No |
| Anser anser | 23,434 | 68 | 0.3 | Yes |
| Phalacrocorax carbo | 3,874 | 12 | 0.3 | Yes |
| Bubo bubo | 974 | 3 | 0.3 | Yes |
| Netta rufina | 385 | 1 | 0.3 | Yes |
| Larus ridibundus | 19,621 | 31 | 0.2 | Yes |
| Branta canadensis | 9,360 | 19 | 0.2 | Yes |
| Larus argentatus | 4,860 | 11 | 0.2 | No |
| Pica pica | 3,186 | 7 | 0.2 | Yes |
| Ciconia ciconia | 2,015 | 5 | 0.2 | Yes |
| Streptopelia decaocto | 2,029 | 4 | 0.2 | No |
| Corvus sp. | 917 | 2 | 0.2 | No |
| Asio otus | 553 | 1 | 0.2 | No |
| Aythya nyroca | 508 | 1 | 0.2 | No |
| Fulica atra | 9,897 | 9 | 0.1 | Yes |
| Anas penelope | 9,649 | 8 | 0.1 | Yes |
| Anas crecca | 10,271 | 6 | 0.1 | Yes |
| Larus canus | 4,353 | 5 | 0.1 | Yes |
| Anser fabalis | 3,958 | 4 | 0.1 | Yes |
| Branta leucopsis | 5,182 | 3 | 0.1 | Yes |
| Anas acuta | 2,150 | 3 | 0.1 | Yes |
| Anas strepera | 1,985 | 3 | 0.1 | Yes |
| Somateria mollissima | 528 | 3 | 0.1 | No |
| Anser albifrons | 10,865 | 2 | 0.1 | Yes |
| Columba livia | 3,498 | 2 | 0.1 | No |
| Falco tinnunculus | 2,958 | 2 | 0.1 | Yes |
| Accipiter nisus | 2,599 | 2 | 0.1 | Yes |
| Corvus corone corone | 2,081 | 2 | 0.1 | No |
| Corvus frugilegus | 1,629 | 2 | 0.1 | No |
| Accipiter sp. | 1,048 | 2 | 0.1 | No |
| Alopochen aegyptiacus | 3,847 | 1 | 0.1 | No |
| Tadorna tadorna | 3,708 | 1 | 0.1 | No |
| Turdus merula | 3,117 | 1 | 0.1 | No |
| Sturnus vulgaris | 1,886 | 1 | 0.1 | No |
| Larus argentatus cachinnans | 1,584 | 1 | 0.1 | No |
| Larus fuscus | 1,518 | 1 | 0.1 | No |
| Strix aluco | 1,184 | 1 | 0.1 | No |
| Branta bernicla | 941 | 1 | 0.1 | Yes |
| Turdus philomelos | 704 | 1 | 0.1 | No |
| Anser brachyrhynchus | 664 | 1 | 0.1 | Yes |
| Anas platyrhynchos | 13,0658 | 116 | 0.09 | Yes |
| Phasianus colchicus | 10,074 | 0 | 0 | No |
| Phoenicopterus ruber | 3,912 | 0 | 0 | No |
| Columba sp. | 3,508 | 0 | 0 | No |
| Anas clypeata | 2,214 | 0 | 0 | Yes |
| Sterna hirundo | 2,145 | 0 | 0 | No |
| Pluvialis apricaria | 2,096 | 0 | 0 | Yes |
| Gallinula chloropus | 2,014 | 0 | 0 | No |
| Anser cygnoides | 1,992 | 0 | 0 | No |
| Alectoris rufa | 1,752 | 0 | 0 | No |
| Passer domesticus | 1,748 | 0 | 0 | No |
| Coturnix coturnix | 1,667 | 0 | 0 | No |
| Grus grus | 1,572 | 0 | 0 | No |
| Calidris alpina | 1,514 | 0 | 0 | No |
| Branta canadensis canadensis | 1,312 | 0 | 0 | No |
| Columba palumbus | 1,276 | 0 | 0 | No |
| Plegadis falcinellus | 1,157 | 0 | 0 | No |
| Carduelis chloris | 1,137 | 0 | 0 | No |
| Tyto alba | 1,130 | 0 | 0 | No |
| Vanellus vanellus | 1,110 | 0 | 0 | Yes |
| Corvus monedula | 1,074 | 0 | 0 | No |
| Larus melanocephalus | 1,050 | 0 | 0 | No |
| Corvus corone cornix | 1,031 | 0 | 0 | No |
| Scolopax rusticola | 959 | 0 | 0 | No |
| Streptopelia turtur | 947 | 0 | 0 | No |
| Larus argentatus michahellis | 913 | 0 | 0 | No |
| Haematopus ostralegus | 907 | 0 | 0 | No |
| Parus major | 881 | 0 | 0 | No |
| Anas querquedula | 851 | 0 | 0 | Yes |
| Garrulus glandarius | 836 | 0 | 0 | No |
| Anser albifrons albifrons | 740 | 0 | 0 | Yes |
| Arenaria interpres | 697 | 0 | 0 | No |
| Perdix perdix | 696 | 0 | 0 | No |
| Sylvia atricapilla | 662 | 0 | 0 | No |
| Gallinago gallinago | 653 | 0 | 0 | No |
| Erithacus rubecula | 641 | 0 | 0 | No |
| Columba livia livia | 557 | 0 | 0 | No |
| Athene noctua | 544 | 0 | 0 | No |
| Sturnus sp. | 544 | 0 | 0 | No |
| Sterna sandvicensis | 542 | 0 | 0 | No |
| Turdus sp. | 524 | 0 | 0 | No |
| Acrocephalus scirpaceus | 517 | 0 | 0 | No |
| Fringilla coelebs | 511 | 0 | 0 | No |
| Uria aalge | 488 | 0 | 0 | No |
| Tringa glareola | 459 | 0 | 0 | No |
| Milvus milvus | 456 | 0 | 0 | Yes |
| Unknown | 455 | 0 | 0 | No |
| Phoenicopterus ruber roseus | 440 | 0 | 0 | No |
| Corvus corax | 439 | 0 | 0 | No |
| Apus apus | 433 | 0 | 0 | No |
| Rallus aquaticus | 429 | 0 | 0 | No |
| Hirundo rustica | 399 | 0 | 0 | No |
| Passer montanus | 385 | 0 | 0 | No |
| Columba oenas | 381 | 0 | 0 | No |
| Anatidae | 370 | 0 | 0 | No |
| Platalea leucorodia | 364 | 0 | 0 | No |
| Alca torda | 359 | 0 | 0 | No |
| Calonectris diomedea | 356 | 0 | 0 | No |
| Philomachus pugnax | 336 | 0 | 0 | Yes |
| Tringa totanus | 335 | 0 | 0 | No |
| Bombycilla garrulus | 328 | 0 | 0 | No |
| Passer sp. | 328 | 0 | 0 | No |
| Numenius arquata | 320 | 0 | 0 | No |
| Fulica cristata | 310 | 0 | 0 | No |
| Limosa limosa | 297 | 0 | 0 | Yes |
| Parus caeruleus | 292 | 0 | 0 | No |
| Columbidae | 286 | 0 | 0 | No |
| Psittacula krameri | 279 | 0 | 0 | No |
| Sula bassana | 269 | 0 | 0 | No |
| Milvus migrans | 265 | 0 | 0 | Yes |
| Cygnus columbianus | 262 | 0 | 0 | Yes |
| Gyps fulvus | 260 | 0 | 0 | No |
| Ixobrychus minutus | 258 | 0 | 0 | No |
| Pandion haliaetus | 258 | 0 | 0 | No |
| Streptopelia sp. | 255 | 0 | 0 | No |
| Charadrius hiaticula | 245 | 0 | 0 | No |
| Archilochus alexandri | 242 | 0 | 0 | No |
| Carduelis carduelis | 241 | 0 | 0 | No |
| Crex crex | 237 | 0 | 0 | No |
| Carduelis spinus | 235 | 0 | 0 | No |
| Cage & exotic birds | 232 | 0 | 0 | No |
| Sterna paradisaea | 232 | 0 | 0 | No |
| Pyrrhula pyrrhula | 231 | 0 | 0 | No |
| Passer domesticus italiae | 229 | 0 | 0 | No |
| Himantopus himantopus | 224 | 0 | 0 | No |
| Sterna sp. | 220 | 0 | 0 | No |
| Aquila chrysaetos | 212 | 0 | 0 | No |
| Phylloscopus collybita | 209 | 0 | 0 | No |
| Branta bernicla bernicla | 202 | 0 | 0 | No |
| Milvus sp. | 196 | 0 | 0 | No |
| Delichon urbica | 195 | 0 | 0 | No |
| Otus scops | 195 | 0 | 0 | No |
| Somateria sp. | 195 | 0 | 0 | No |
| Bubulcus ibis | 191 | 0 | 0 | No |
| Picus viridis | 188 | 0 | 0 | No |
| Actitis hypoleucos | 187 | 0 | 0 | No |
| Sylvia borin | 182 | 0 | 0 | No |
| Aix galericulata | 180 | 0 | 0 | No |
| Coccothraustes coccothraustes | 176 | 0 | 0 | No |
| Puffinus puffinus | 175 | 0 | 0 | No |
| Dendrocopos major | 172 | 0 | 0 | No |
| Calidris ferruginea | 171 | 0 | 0 | No |
| Strix uralensis | 167 | 0 | 0 | No |
| Branta bernicla hrota | 166 | 0 | 0 | No |
| Alectoris chukar | 161 | 0 | 0 | No |
| Falco naumanni | 160 | 0 | 0 | No |
| Calidris minuta | 157 | 0 | 0 | No |
| Cygnus columbianus bewickii | 157 | 0 | 0 | No |
| Cettia cetti | 154 | 0 | 0 | No |
| Circus aeruginosus | 149 | 0 | 0 | Yes |
| Anser indicus | 147 | 0 | 0 | No |
| Parus sp. | 143 | 0 | 0 | No |
| Branta ruficollis | 140 | 0 | 0 | Yes |
| Luscinia megarhynchos | 140 | 0 | 0 | No |
| Pernis apivorus | 138 | 0 | 0 | No |
| Rallidae | 137 | 0 | 0 | No |
| Emberiza citrinella | 136 | 0 | 0 | No |
| Passer hispaniolensis | 136 | 0 | 0 | No |
| Tetrao urogallus | 135 | 0 | 0 | No |
| Ardea purpurea | 133 | 0 | 0 | No |
| Charadrius dubius | 133 | 0 | 0 | No |
| Nycticorax nycticorax | 128 | 0 | 0 | No |
| Prunella modularis | 128 | 0 | 0 | No |
| Aphriza virgata | 123 | 0 | 0 | No |
| Larus genei | 123 | 0 | 0 | No |
| Aegypius monachus | 116 | 0 | 0 | No |
| Emberiza schoeniclus | 116 | 0 | 0 | No |
| Porzana porzana | 116 | 0 | 0 | No |
| Acrocephalus schoenobaenus | 115 | 0 | 0 | No |
| Sterna albifrons | 114 | 0 | 0 | No |
| Hirundinidae | 113 | 0 | 0 | No |
| Acrocephalus arundinaceus | 102 | 0 | 0 | No |
| Motacilla alba | 101 | 0 | 0 | No |
| Alcedo atthis | 100 | 0 | 0 | No |
| Phylloscopus trochilus | 99 | 0 | 0 | No |
| Gelochelidon nilotica | 97 | 0 | 0 | No |
| Recurvirostra avosetta | 97 | 0 | 0 | No |
| Strix sp. | 95 | 0 | 0 | No |
| Falco eleonorae | 90 | 0 | 0 | No |
| Ciconia nigra | 89 | 0 | 0 | No |
| Hieraaetus pennatus | 88 | 0 | 0 | No |
| Numenius tenuirostris | 87 | 0 | 0 | No |
| Fringilla sp. | 85 | 0 | 0 | No |
| Riparia riparia | 85 | 0 | 0 | No |
| Limosa lapponica | 83 | 0 | 0 | No |
| Sylvia communis | 83 | 0 | 0 | No |
| Calidris alba | 82 | 0 | 0 | No |
| Larus canus canus | 82 | 0 | 0 | No |
| Scolopacidae | 79 | 0 | 0 | No |
| Turdus iliacus | 79 | 0 | 0 | No |
| Serinus serinus | 77 | 0 | 0 | No |
| Charadrius sp. | 72 | 0 | 0 | No |
| Gallus gallus | 70 | 0 | 0 | No |
| Charadrius alexandrinus | 69 | 0 | 0 | No |
| Falco subbuteo | 68 | 0 | 0 | No |
| Upupa epops | 68 | 0 | 0 | No |
| Regulus regulus | 67 | 0 | 0 | No |
| Phalacrocorax aristotelis | 66 | 0 | 0 | No |
| Falco vespertinus | 65 | 0 | 0 | No |
| Alectoris graeca | 64 | 0 | 0 | No |
| Tetrao tetrix | 64 | 0 | 0 | No |
| Mergus serrator | 63 | 0 | 0 | No |
| Oriolus oriolus | 63 | 0 | 0 | No |
| Serinus canaria | 63 | 0 | 0 | No |
| Aegithalos caudatus | 62 | 0 | 0 | No |
| Alauda arvensis | 62 | 0 | 0 | No |
| Fringilla montifringilla | 62 | 0 | 0 | No |
| Phoenicurus phoenicurus | 62 | 0 | 0 | No |
| Burhinus oedicnemus | 61 | 0 | 0 | No |
| Chlidonias niger | 61 | 0 | 0 | No |
| Pluvialis squatarola | 60 | 0 | 0 | No |
| Marmaronetta angustirostris | 59 | 0 | 0 | Yes |
| Circaetus gallicus | 58 | 0 | 0 | No |
| Melanitta nigra | 58 | 0 | 0 | No |
| Strix nebulosa | 58 | 0 | 0 | No |
| Morus capensis | 57 | 0 | 0 | No |
| Fulmarus glacialis | 55 | 0 | 0 | No |
| Gypaetus barbatus | 54 | 0 | 0 | No |
| Ring destroyed or lost | 54 | 0 | 0 | No |
| Rissa tridactyla | 53 | 0 | 0 | No |
| Buteo rufinus | 52 | 0 | 0 | No |
| Caprimulgus europaeus | 51 | 0 | 0 | No |
| Circus pygargus | 51 | 0 | 0 | No |
| Aquila sp. | 49 | 0 | 0 | No |
| Calidris canutus | 49 | 0 | 0 | No |
| Clangula hyemalis | 49 | 0 | 0 | No |
| Larus audouinii | 49 | 0 | 0 | No |
| Melanitta fusca | 49 | 0 | 0 | No |
| Sylvia curruca | 49 | 0 | 0 | No |
| Acrocephalus palustris | 48 | 0 | 0 | No |
| Aegolius funereus | 47 | 0 | 0 | No |
| Falco rusticolus | 44 | 0 | 0 | No |
| Gavia arctica | 44 | 0 | 0 | No |
| Panurus biarmicus | 44 | 0 | 0 | No |
| Parus ater | 44 | 0 | 0 | No |
| Tadorna ferruginea | 44 | 0 | 0 | No |
| Tringa erythropus | 44 | 0 | 0 | No |
| Asio flammeus | 43 | 0 | 0 | No |
| Circus cyaneus | 43 | 0 | 0 | No |
| Locustella luscinioides | 42 | 0 | 0 | No |
| Locustella naevia | 42 | 0 | 0 | No |
| Phoenicurus ochruros | 42 | 0 | 0 | No |
| Phylloscopus sp. | 42 | 0 | 0 | No |
| Anser caerulescens | 41 | 0 | 0 | No |
| Aquila heliaca adalberti | 41 | 0 | 0 | No |
| Gavia stellata | 41 | 0 | 0 | No |
| Cuculus canorus | 40 | 0 | 0 | No |
| Fratercula arctica | 40 | 0 | 0 | No |
| Neophron percnopterus | 40 | 0 | 0 | No |
| Phalacrocorax pygmeus | 40 | 0 | 0 | No |
| Sylvia melanocephala | 40 | 0 | 0 | No |
| Uria sp. | 40 | 0 | 0 | No |
| Lagopus mutus | 39 | 0 | 0 | No |
| Spizaetus nipalensis | 37 | 0 | 0 | No |
| Anthus trivialis | 36 | 0 | 0 | No |
| Bonasa bonasia | 36 | 0 | 0 | No |
| Geronticus eremita | 36 | 0 | 0 | No |
| Hieraaetus fasciatus | 36 | 0 | 0 | No |
| Remiz pendulinus | 36 | 0 | 0 | No |
| Luscinia svecica | 35 | 0 | 0 | No |
| Agapornis pullarius | 34 | 0 | 0 | No |
| Carduelis flammea | 34 | 0 | 0 | No |
| Lymnocryptes minimus | 34 | 0 | 0 | No |
| Merops apiaster | 34 | 0 | 0 | No |
| Sitta europaea | 34 | 0 | 0 | No |
| Surnia ulula | 33 | 0 | 0 | No |
| Anas formosa | 32 | 0 | 0 | No |
| Ficedula hypoleuca | 32 | 0 | 0 | No |
| Podiceps sp. | 32 | 0 | 0 | No |
| Threskiornis aethiopicus | 32 | 0 | 0 | No |
| Branta sp. | 31 | 0 | 0 | No |
| Falco columbarius | 31 | 0 | 0 | No |
| Falconidae | 31 | 0 | 0 | No |
| Lanius collurio | 30 | 0 | 0 | No |
| Saxicola rubetra | 30 | 0 | 0 | No |
| Otis tarda | 29 | 0 | 0 | No |
| Anthus pratensis | 28 | 0 | 0 | No |
| Larus minutus | 28 | 0 | 0 | No |
| Meleagris gallopavo | 28 | 0 | 0 | No |
| Puffinus puffinus yelkouan | 28 | 0 | 0 | No |
| Saxicola torquata | 28 | 0 | 0 | No |
| Turtur afer | 28 | 0 | 0 | No |
| Circus sp. | 27 | 0 | 0 | No |
| Dendrocopos sp. | 27 | 0 | 0 | No |
| Fratercula corniculata | 27 | 0 | 0 | No |
| Sturnus unicolor | 27 | 0 | 0 | No |
| Pyrrhocorax pyrrhocorax | 26 | 0 | 0 | No |
| Muscicapa striata | 25 | 0 | 0 | No |
| Picidae | 25 | 0 | 0 | No |
| Turdus obscurus | 25 | 0 | 0 | No |
| Glareola maldivarum | 24 | 0 | 0 | No |
| Oenanthe oenanthe | 24 | 0 | 0 | No |
| Emberiza rustica | 23 | 0 | 0 | No |
| Nyctea scandiaca | 23 | 0 | 0 | No |
| Oxyura leucocephala | 23 | 0 | 0 | No |
| Lagopus lagopus | 22 | 0 | 0 | No |
| Tringa nebularia | 22 | 0 | 0 | No |
| Falco cherrug | 21 | 0 | 0 | No |
| Numida meleagris | 21 | 0 | 0 | No |
| Streptopelia chinensis | 21 | 0 | 0 | No |
| Dendrocygna bicolor | 20 | 0 | 0 | No |
| Hippolais polyglotta | 20 | 0 | 0 | No |
| Sylvia sp. | 20 | 0 | 0 | No |
| Turdus viscivorus | 20 | 0 | 0 | No |
| Acrocephalus sp. | 19 | 0 | 0 | No |
| Calidris spp. | 19 | 0 | 0 | No |
| Turdus ruficollis ruficollis | 19 | 0 | 0 | No |
| Dryocopus martius | 17 | 0 | 0 | No |
| Laridae | 17 | 0 | 0 | No |
| Parus palustris | 17 | 0 | 0 | No |
| Phoebetria palpebrata | 17 | 0 | 0 | No |
| Psittacus erithacus | 17 | 0 | 0 | No |
| Clamator glandarius | 16 | 0 | 0 | No |
| Coracias garrulus | 16 | 0 | 0 | No |
| Hippolais icterina | 16 | 0 | 0 | No |
| Lanius senator | 16 | 0 | 0 | No |
| Francolinus francolinus | 15 | 0 | 0 | No |
| Histrionicus histrionicus | 15 | 0 | 0 | No |
| Sterna caspia | 15 | 0 | 0 | No |
| Struthio camelus | 15 | 0 | 0 | No |
| Accipitridae | 14 | 0 | 0 | No |
| Acrocephalus melanopogon | 14 | 0 | 0 | No |
| Anser canagicus | 14 | 0 | 0 | No |
| Ardeola ralloides | 14 | 0 | 0 | No |
| Glaucidium passerinum | 14 | 0 | 0 | No |
| Lanius excubitor | 14 | 0 | 0 | No |
| Regulus ignicapillus | 14 | 0 | 0 | No |
| Alle alle | 13 | 0 | 0 | No |
| Amandava amandava | 12 | 0 | 0 | No |
| Anas platyrhynchos platyrhynchos | 12 | 0 | 0 | No |
| Carduelis spp. | 12 | 0 | 0 | No |
| Hippolais pallida (E.O.W.) | 12 | 0 | 0 | No |
| Grus sp. | 11 | 0 | 0 | No |
| Hydrobates pelagicus | 11 | 0 | 0 | No |
| Jynx torquilla | 11 | 0 | 0 | No |
| Loxia curvirostra | 11 | 0 | 0 | No |
| Motacilla cinerea | 11 | 0 | 0 | No |
| Phylloscopus sibilatrix | 11 | 0 | 0 | No |
| Stercorarius skua antarcticus | 11 | 0 | 0 | No |
| Tadorna variegata | 11 | 0 | 0 | No |
| Apus melba | 10 | 0 | 0 | No |
| Aquila rapax nipalensis | 10 | 0 | 0 | No |
| Calidris maritima | 10 | 0 | 0 | No |
| Calidris temminckii | 10 | 0 | 0 | No |
| Cisticola juncidis | 10 | 0 | 0 | No |
| Dendrocopos minor | 10 | 0 | 0 | No |
| Emberiza cirlus | 10 | 0 | 0 | No |
| Larus hyperboreus | 10 | 0 | 0 | No |
| Leiothrix lutea | 10 | 0 | 0 | No |
| Parus cristatus | 10 | 0 | 0 | No |
| Pica pica mauritanica | 10 | 0 | 0 | No |
| Amazona oratrix | 9 | 0 | 0 | No |
| Anas crecca carolinensis | 9 | 0 | 0 | No |
| Anas falcata | 9 | 0 | 0 | No |
| Aquila heliaca | 9 | 0 | 0 | No |
| Coturnix japonica | 9 | 0 | 0 | No |
| Domestic Chicken | 9 | 0 | 0 | No |
| Limicola falcinellus | 9 | 0 | 0 | No |
| Nucifraga caryocatactes | 9 | 0 | 0 | No |
| Phalacrocorax aristotelis desmarestii | 9 | 0 | 0 | No |
| Phylloscopus herberti | 9 | 0 | 0 | No |
| Certhia brachydactyla | 8 | 0 | 0 | No |
| Emberiza sp. | 8 | 0 | 0 | No |
| Ficedula parva | 8 | 0 | 0 | No |
| Gavia sp. | 8 | 0 | 0 | No |
| Pagophila eburnea | 8 | 0 | 0 | No |
| Callonetta leucophrys | 7 | 0 | 0 | No |
| Carduelis cannabina | 7 | 0 | 0 | No |
| Cathartes aura | 7 | 0 | 0 | No |
| Chlidonias leucopterus | 7 | 0 | 0 | No |
| Chrysolophus pictus | 7 | 0 | 0 | No |
| Ficedula hodgsonii | 7 | 0 | 0 | No |
| Galerida cristata | 7 | 0 | 0 | No |
| Perdix sp. | 7 | 0 | 0 | No |
| Picus canus | 7 | 0 | 0 | No |
| Stercorarius sp. | 7 | 0 | 0 | No |
| Tetraonidae | 7 | 0 | 0 | No |
| Accipiter brevipes | 6 | 0 | 0 | No |
| Apus pallidus | 6 | 0 | 0 | No |
| Aquila pomarina | 6 | 0 | 0 | No |
| Aratinga holochlora | 6 | 0 | 0 | No |
| Dendrocopos leucotos | 6 | 0 | 0 | No |
| Gavia immer | 6 | 0 | 0 | No |
| Luscinia luscinia | 6 | 0 | 0 | No |
| Miliaria calandra | 6 | 0 | 0 | No |
| Motacilla alba yarrellii | 6 | 0 | 0 | No |
| Motacilla flava | 6 | 0 | 0 | No |
| Muscivora tyrannus | 6 | 0 | 0 | No |
| Pernis ptilorhyncus | 6 | 0 | 0 | No |
| Phasianus versicolor | 6 | 0 | 0 | No |
| Porzana pusilla | 6 | 0 | 0 | No |
| Puffinus puffinus mauretanicus | 6 | 0 | 0 | No |
| Sylvia melanocephala melanocephala | 6 | 0 | 0 | No |
| Alauda gulgula | 5 | 0 | 0 | No |
| Alauda sp. | 5 | 0 | 0 | No |
| Alectoris barbara | 5 | 0 | 0 | No |
| Balearica pavonina | 5 | 0 | 0 | No |
| Bulweria bulwerii | 5 | 0 | 0 | No |
| Buteo buteo buteo | 5 | 0 | 0 | No |
| Cinclus cinclus | 5 | 0 | 0 | No |
| Cyanopica cyana | 5 | 0 | 0 | No |
| Dendrocopos medius | 5 | 0 | 0 | No |
| Egretta intermedia | 5 | 0 | 0 | No |
| Lullula arborea | 5 | 0 | 0 | No |
| Myiopsitta monachus | 5 | 0 | 0 | No |
| Numenius phaeopus | 5 | 0 | 0 | No |
| Parabuteo unicinctus | 5 | 0 | 0 | No |
| Parus rubidiventris | 5 | 0 | 0 | No |
| Pelecanus rufescens | 5 | 0 | 0 | No |
| Podiceps grisegena | 5 | 0 | 0 | No |
| Porzana parva | 5 | 0 | 0 | No |
| Tetrax tetrax | 5 | 0 | 0 | No |
| Tyto alba alba | 5 | 0 | 0 | No |
| Upupa epops epops | 5 | 0 | 0 | No |
| Anthropoides virgo | 4 | 0 | 0 | No |
| Cepphus grylle | 4 | 0 | 0 | No |
| Certhia familiaris | 4 | 0 | 0 | No |
| Corvus dauuricus | 4 | 0 | 0 | No |
| Dendrocygna autumnalis | 4 | 0 | 0 | No |
| Elanus caeruleus | 4 | 0 | 0 | No |
| Falco biarmicus | 4 | 0 | 0 | No |
| Larus argentatus argenteus | 4 | 0 | 0 | No |
| Passer rutilans | 4 | 0 | 0 | No |
| Petronia petronia | 4 | 0 | 0 | No |
| Phoenicurus sp. | 4 | 0 | 0 | No |
| Pinicola enucleator | 4 | 0 | 0 | No |
| Puffinus assimilis | 4 | 0 | 0 | No |
| Puffinus griseus | 4 | 0 | 0 | No |
| Streptopelia orientalis | 4 | 0 | 0 | No |
| Zenaida aurita | 4 | 0 | 0 | No |
| Ardea herodias | 3 | 0 | 0 | No |
| Burhinus capensis | 3 | 0 | 0 | No |
| Calandrella acutirostris | 3 | 0 | 0 | No |
| Chlidonias hybridus | 3 | 0 | 0 | No |
| Coragyps atratus | 3 | 0 | 0 | No |
| Cyanoliseus patagonus | 3 | 0 | 0 | No |
| Dendrocopos syriacus | 3 | 0 | 0 | No |
| Ficedula albicollis | 3 | 0 | 0 | No |
| Grus virgo (=Ø441Ø A.v.) | 3 | 0 | 0 | No |
| Gyps africanus | 3 | 0 | 0 | No |
| Hirundo daurica | 3 | 0 | 0 | No |
| Lanius sp. | 3 | 0 | 0 | No |
| Larus delawarensis | 3 | 0 | 0 | No |
| Leucosticte brandti | 3 | 0 | 0 | No |
| Loxia sp. | 3 | 0 | 0 | No |
| Monticola solitarius | 3 | 0 | 0 | No |
| Parus montanus | 3 | 0 | 0 | No |
| Phoenicopteridae | 3 | 0 | 0 | No |
| Podilymbus podiceps | 3 | 0 | 0 | No |
| Stercorarius parasiticus | 3 | 0 | 0 | No |
| Sterna dougallii | 3 | 0 | 0 | No |
| Sylvia cantillans | 3 | 0 | 0 | No |
| Sylvia nisoria | 3 | 0 | 0 | No |
| Tchagra senegala | 3 | 0 | 0 | No |
| Upupa africana | 3 | 0 | 0 | No |
| Anas bahamensis | 2 | 0 | 0 | No |
| Anser fabalis jonanseni | 2 | 0 | 0 | No |
| Aquila nipalensis | 2 | 0 | 0 | No |
| Buteo hemilasius | 2 | 0 | 0 | No |
| Caprimulgus ruficollis | 2 | 0 | 0 | No |
| Caprimulgus spp. | 2 | 0 | 0 | No |
| Chlidonias sp. | 2 | 0 | 0 | No |
| Chloephaga picta | 2 | 0 | 0 | No |
| Circus macrourus | 2 | 0 | 0 | No |
| Crossoptilon auritum | 2 | 0 | 0 | No |
| Dendrocygna arborea | 2 | 0 | 0 | No |
| Diomedea albatrus | 2 | 0 | 0 | No |
| Emberiza cioides | 2 | 0 | 0 | No |
| Erithacus rubecula rubecula | 2 | 0 | 0 | No |
| Gyps indicus | 2 | 0 | 0 | No |
| Ixobrychus sturmii | 2 | 0 | 0 | No |
| Lanius minor | 2 | 0 | 0 | No |
| Larus canus heinei | 2 | 0 | 0 | No |
| Locustella fluviatilis | 2 | 0 | 0 | No |
| Lophophorus sclateri | 2 | 0 | 0 | No |
| Luscinia sp. | 2 | 0 | 0 | No |
| Motacilla alba alba | 2 | 0 | 0 | No |
| Oceanodroma castro | 2 | 0 | 0 | No |
| Passer domesticus domesticus | 2 | 0 | 0 | No |
| Phalacrocorax carbo sinensis | 2 | 0 | 0 | No |
| Phalaropus fulicaria | 2 | 0 | 0 | No |
| Phylloscopus collybita ibericus | 2 | 0 | 0 | No |
| Poicephalus senegalus | 2 | 0 | 0 | No |
| Porzana sp. | 2 | 0 | 0 | No |
| Pyrrhocorax graculus | 2 | 0 | 0 | No |
| Saxicola sp. | 2 | 0 | 0 | No |
| Species not accepted | 2 | 0 | 0 | No |
| Tetraogallus himalayensis | 2 | 0 | 0 | No |
| Tragopan temminckii | 2 | 0 | 0 | No |
| Tringa stagnatilis | 2 | 0 | 0 | No |
| Tringa totanus totanus | 2 | 0 | 0 | No |
| Tyto alba guttata | 2 | 0 | 0 | No |
| Aethia pusilla | 1 | 0 | 0 | No |
| Agapornis canus | 1 | 0 | 0 | No |
| Alcidae | 1 | 0 | 0 | No |
| Anas cyanoptera | 1 | 0 | 0 | No |
| Anas discors | 1 | 0 | 0 | No |
| Anas rubripes | 1 | 0 | 0 | No |
| Anhinga melanogaster | 1 | 0 | 0 | No |
| Anser fabalis rossicus | 1 | 0 | 0 | No |
| Anser rossii | 1 | 0 | 0 | No |
| Anthus cervinus | 1 | 0 | 0 | No |
| Apus spp. | 1 | 0 | 0 | No |
| Aratinga leucophthalmus | 1 | 0 | 0 | No |
| Aythya americana | 1 | 0 | 0 | No |
| Botaurus lentiginosus | 1 | 0 | 0 | No |
| Bubo africanus | 1 | 0 | 0 | No |
| Bubo poensis | 1 | 0 | 0 | No |
| Bubo virginianus | 1 | 0 | 0 | No |
| Buteo jamaicensis | 1 | 0 | 0 | No |
| Buteo lineatus | 1 | 0 | 0 | No |
| Buteo regalis | 1 | 0 | 0 | No |
| Calcarius lapponicus | 1 | 0 | 0 | No |
| Carduelis flammea cabaret | 1 | 0 | 0 | No |
| Carduelis hornemanni | 1 | 0 | 0 | No |
| Ceryle lugubris | 1 | 0 | 0 | No |
| Ceryle torquata | 1 | 0 | 0 | No |
| Charadrius leschenaultii | 1 | 0 | 0 | No |
| Chettusia leucura | 1 | 0 | 0 | No |
| Chlamydotis undulata | 1 | 0 | 0 | No |
| Chrysolophus amherstiae | 1 | 0 | 0 | No |
| Colaptes chrysoides | 1 | 0 | 0 | No |
| Columba flavirostris | 1 | 0 | 0 | No |
| Corvidae | 1 | 0 | 0 | No |
| Corvus monedula monedula | 1 | 0 | 0 | No |
| Corvus splendens | 1 | 0 | 0 | No |
| Corvus torquatus | 1 | 0 | 0 | No |
| Crotophaga ani | 1 | 0 | 0 | No |
| Cuculus saturatus | 1 | 0 | 0 | No |
| Dendrocopos himalayensis | 1 | 0 | 0 | No |
| Diomedea melanophris | 1 | 0 | 0 | No |
| Ectopistes migratorius | 1 | 0 | 0 | No |
| Emberiza cia | 1 | 0 | 0 | No |
| Emberiza pusilla | 1 | 0 | 0 | No |
| Ergaticus ruber | 1 | 0 | 0 | No |
| Eudocimus ruber | 1 | 0 | 0 | No |
| Gallinago hardwickii | 1 | 0 | 0 | No |
| Gallinula angulata | 1 | 0 | 0 | No |
| Gypohierax angolensis | 1 | 0 | 0 | No |
| Gyps bengalensis | 1 | 0 | 0 | No |
| Gyps rueppellii | 1 | 0 | 0 | No |
| Hieraaetus ayresii | 1 | 0 | 0 | No |
| Himantornis haematopus | 1 | 0 | 0 | No |
| Hippolais pallida pallida | 1 | 0 | 0 | No |
| Hoplopterus spinosus | 1 | 0 | 0 | No |
| Ixobrychus exilis | 1 | 0 | 0 | No |
| Junco hyemalis | 1 | 0 | 0 | No |
| Lanius meridionalis | 1 | 0 | 0 | No |
| Larus argentatus mongolicus | 1 | 0 | 0 | No |
| Larus brunnicephalus | 1 | 0 | 0 | No |
| Larus californicus | 1 | 0 | 0 | No |
| Larus glaucescens | 1 | 0 | 0 | No |
| Larus glaucoides kumlieni | 1 | 0 | 0 | No |
| Larus maculipennis | 1 | 0 | 0 | No |
| Larus occidentalis | 1 | 0 | 0 | No |
| Limnodromus semipalmatus | 1 | 0 | 0 | No |
| Loxia pytyopsittacus | 1 | 0 | 0 | No |
| Melaniparus leucomelas | 1 | 0 | 0 | No |
| Melanocorypha calandra | 1 | 0 | 0 | No |
| Mitrephanes phaeocercus | 1 | 0 | 0 | No |
| Molothrus aeneus | 1 | 0 | 0 | No |
| Montifringilla nivalis | 1 | 0 | 0 | No |
| Motacilla sp. | 1 | 0 | 0 | No |
| Muscicapa gambagae | 1 | 0 | 0 | No |
| Muscicapa sp. | 1 | 0 | 0 | No |
| Mycteria ibis | 1 | 0 | 0 | No |
| Nettapus coromandelianus | 1 | 0 | 0 | No |
| Nucifraga caryocatactes caryocatactes | 1 | 0 | 0 | No |
| Nycticorax violaceus | 1 | 0 | 0 | No |
| Oceanodroma sp. | 1 | 0 | 0 | No |
| Otus asio | 1 | 0 | 0 | No |
| Otus leucotis | 1 | 0 | 0 | No |
| Parus lugubris | 1 | 0 | 0 | No |
| Passer ammodendri | 1 | 0 | 0 | No |
| Passer pyrrhonotus | 1 | 0 | 0 | No |
| Pelecanus occidentalis | 1 | 0 | 0 | No |
| Phalacrocorax capensis | 1 | 0 | 0 | No |
| Phalaropus lobatus | 1 | 0 | 0 | No |
| Pheucticus ludovicianus | 1 | 0 | 0 | No |
| Phoenicopterus chilensis | 1 | 0 | 0 | No |
| Phylloscopus bonelli | 1 | 0 | 0 | No |
| Phylloscopus collybita abietinus | 1 | 0 | 0 | No |
| Phylloscopus collybita brehmii DELETED | 1 | 0 | 0 | No |
| Phylloscopus collybita canariensis | 1 | 0 | 0 | No |
| Phylloscopus collybita collybita | 1 | 0 | 0 | No |
| Phylloscopus collybita fulvescens | 1 | 0 | 0 | No |
| Phylloscopus collybita tristis | 1 | 0 | 0 | No |
| Phylloscopus proregulus | 1 | 0 | 0 | No |
| Phylloscopus tenellipes | 1 | 0 | 0 | No |
| Phylloscopus trochilus acredula | 1 | 0 | 0 | No |
| Phylloscopus trochilus trochilus | 1 | 0 | 0 | No |
| Picumnus innominatus | 1 | 0 | 0 | No |
| Pinguinus impennis | 1 | 0 | 0 | No |
| Plectropterus gambensis | 1 | 0 | 0 | No |
| Ploceus cucullatus | 1 | 0 | 0 | No |
| Pluvialis dominica fulva | 1 | 0 | 0 | No |
| Podoces biddulphi | 1 | 0 | 0 | No |
| Podoces pleskei | 1 | 0 | 0 | No |
| Polyborus plancus | 1 | 0 | 0 | No |
| Porphyrio porphyrio porphyrio | 1 | 0 | 0 | No |
| Prunella collaris | 1 | 0 | 0 | No |
| Prunella sp. | 1 | 0 | 0 | No |
| Pterodroma hypoleuca | 1 | 0 | 0 | No |
| Ptyonoprogne rupestris | 1 | 0 | 0 | No |
| Puffinus sp. | 1 | 0 | 0 | No |
| Riparia paludicola | 1 | 0 | 0 | No |
| Serinus citrinella | 1 | 0 | 0 | No |
| Sitta canadensis | 1 | 0 | 0 | No |
| Stercorarius pomarinus | 1 | 0 | 0 | No |
| Stercorarius skua | 1 | 0 | 0 | No |
| Sterna fuscata | 1 | 0 | 0 | No |
| Streptopelia roseogrisea | 1 | 0 | 0 | No |
| Sturnus philippensis | 1 | 0 | 0 | No |
| Sturnus roseus | 1 | 0 | 0 | No |
| Sulidae | 1 | 0 | 0 | No |
| Sylvia hortensis | 1 | 0 | 0 | No |
| Sylvia mystacea | 1 | 0 | 0 | No |
| Sylvia sarda | 1 | 0 | 0 | No |
| Sylvietta virens | 1 | 0 | 0 | No |
| Synthliboramphus antiquus | 1 | 0 | 0 | No |
| Tringa totanus robusta | 1 | 0 | 0 | No |
| Turdus albicollis | 1 | 0 | 0 | No |
| Turdus iliacus coburni | 1 | 0 | 0 | No |
| Turdus merula merula | 1 | 0 | 0 | No |
| Turdus migratorius | 1 | 0 | 0 | No |
| Turdus ruficollis | 1 | 0 | 0 | No |
| Vidua macroura | 1 | 0 | 0 | No |
| Xenus cinereus | 1 | 0 | 0 | No |
| Zonotrichia albicollis | 1 | 0 | 0 | No |
| Zonotrichia iliaca | 1 | 0 | 0 | No |
| Cygnus sp. | 6,959 | 252 | Yes | |
| Species unknown | 14,825 | 77 | No | |
| Anas sp. | 12,551 | 72 | No | |
| Larus sp. | 4,310 | 72 | No | |
| Anser spp. | 5,531 | 40 | No | |
| Ardea sp. | 1,386 | 6 | No | |
| Aythya sp. | 187 | 3 | No | |
| Falco sp. | 434 | 2 | No | |
| Egretta sp. | 46 | 2 | No | |
| Numenius sp. | 24 | 2 | No | |
| Phalacrocorax sp. | 370 | 1 | No | |
| Ciconia sp. | 334 | 1 | No | |
| Pelecanus sp. | 50 | 1 | No | |
| Mergus sp. | 47 | 1 | No |
Appendix E – Overview HPAI outbreaks in Italy – July to August 2017
1.
After about 50 days from the last A(H5N8) HPAI outbreak observed in northern Italy, on 20 July 2017, the National Reference Laboratory (NRL) for Avian Influenza and Newcastle Disease confirmed the presence of a A(H5N8) HPAI virus in a fattening turkey farm located in Mantua province, Roncoferraro Municipality. The farm was located at about 30 km away from the last outbreak recorded on May 2017 in Mantua, in close proximity to wetlands. On the same day, a backyard farm situated in the same province also tested positive to HPAI A(H5N8) virus.
In the following 5 weeks, 15 additional cases of HPAI A(H5N8) were confirmed by the NRL, in farms located in north‐eastern Italy: six in Mantua province (Lombardy), six in the adjoining province of Verona (Veneto region), one in the provinces of Pavia and Lodi (Lombardy), and in Parma (Emilia‐Romagna) each.
Epidemiological investigations have been conducted in all of the outbreaks occurred in domestic poultry. The enquiries did not detect any risk contact between the infected farms. In fact, different production types were involved (mainly fattening turkeys and laying hens), belonging to separated Production Companies, which have distinct personnel (veterinarians, technicians, etc.) and exploit different services for feed, birds and egg transportation. Nevertheless, contact tracing activities enabled the identification of other potential contact farms, which underwent enhanced surveillance activities and strict control measures.
The cases occurred in the laying hen farm in Mantua (confirmed on 21 July 2017), and the four fattening turkey farms in province of Mantua (three confirmed on 5 August 2017, and one confirmed on), are acknowledged and correlated. In fact, the three fattening turkey farms were located in the Protection Zone at a distance between 1.8 and 3.4 km from the infected farm. Furthermore, due to the massive number of laying hens to be culled (approximately 460,000 laying hens housed in modified cages), the depopulation activities took about 15 days to be concluded (see Table E.1).
Table E.1.
Overview HPAI outbreaks in Italy from January 2017 in poultry holdings; the outbreaks that occurred between July and August 2017 are highlighted with a black rectangular
The large majority of affected farms was located in areas close to water bodies, and substantial populations of wild water birds were reported in proximity to most of the detected outbreaks (see Table E.2).
Table E.2.
Overview HPAI events in Italy from December 2016 in wild birds; the events that occurred between July and August 2017 are highlighted with a black rectangular
Supporting information
Secondary spread of HPAI during 2016/2017 epidemic crisis in Bulgaria
Secondary spread of HPAI during 2016/17 epidemics in France
Applied prevention and control measures on avian influenza Austria
Applied prevention and control measures on avian influenza Belgium
Applied prevention and control measures on avian influenza Bulgaria
Applied prevention and control measures on avian influenza in the Czech Republic
Applied prevention and control measures on avian influenza Denmark
Applied prevention and control measures on avian influenza France
Applied prevention and control measures on avian influenza Greece
Applied prevention and control measures on avian influenza Hungary
Applied prevention and control measures on avian influenza Ireland
Applied prevention and control measures on avian influenza Italy
Applied prevention and control measures on avian influenza The Netherlands
Applied prevention and control measures on avian influenza in Romania
Applied prevention and control measures on avian influenza United Kingdom
Suggested citation: European Food Safety Authority , European Centre for Disease Prevention and Control , European Union Reference Laboratory for Avian influenza , Brown I, Mulatti P, Smietanka K, Staubach C, Willeberg P, Adlhoch C, Candiani D, Fabris C, Zancanaro G, Morgado J and Verdonck F, 2017. Scientific report on the avian influenza overview October 2016–August 2017. EFSA Journal 2017;15(10):5018, 101 pp. 10.2903/j.efsa.2017.5018
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00229
Competing interests: In line with EFSA's policy on declarations of interest, the following working group (WG) experts: Ian Brown, Paolo Mulatti, Krzysztof Smietanka and Christoph Staubach, have declared that they have current involvement in risk assessment activities at national level related to avian influenza, which constitutes a conflict of interest (with the mandate of the EFSA WG in hand. The CoIs have been waived and the waivers were adopted in accordance with Article 16(5) of the Decision of the Executive Director on Declarations of Interest of 31 July 2017 EFSA/LRA/DEC/02/2014, available at http://www.efsa.europa.eu/sites/default/files/corporate_publications/files/independencerules2014.pdf. Pursuant to Article 16(7) of the above mentioned Decision, the concerned experts were allowed to take part in the discussions and in the drafting phase of the EFSA Scientific report on Avian influenza monitoring (Art. 31) ‐ overview October 2016 ‐ August 2017, and have not been allowed to be, or act as, a chairman, a vice‐chairman or rapporteur of the WG.
Acknowledgements: In addition to the listed authors, EFSA, ECDC and the EURL wish to thank the following: the hearing expert Thijs Kuiken for his support provided to this scientific output; Kaja Kaasik Aaslav, Epidemic Intelligence team at ECDC and Pasi Penttinen, Head of the Disease Programme Influenza and other Respiratory Viruses for the support provided to this scientific output; Members States representatives that provided the data on AI outbreaks and/or animal population for this scientific output: Austria (Andrea Hoeflechner‐Poeltl, Eveline Wodak), Belgium (Philippe Houdart, Katie Vermeersch), Bulgaria (Aleksandra Miteva, Anna Zdravkova), Croatia (Zlatko Krovina, Tihana Miškić) Cyprus (Giorgos Krasias), the Czech Republic (Marie Vágnerová), Denmark (Thorkild Bastholm, Torben Grubbe), Estonia (Kärt Jaarma, Helen Prommik), Finland (Tiia Tuupanen), France (Loïc Evain, Isabelle Guerry, Alexandra Troyano‐Groux), Germany (Franz Conraths, Andrea Coßmann), Greece (Sokratis Perdikaris), Hungary (Zsófia Szepesiné Kókány, Zsolt Terjék, Gabor Wyszoczky), Ireland (Stephanie Ronan, Eoin Ryan), Italy (Anna Sorgente), Latvia (Rudīte Vārna), Lithuania (Paulius Busauskas), Luxembourg (Roger Gindt), Malta (Joseph Caruana), the Netherlands (Nina Berendsen, Dennis Bol, Geert Eleveld, Marcel Spierenburg), Poland (Edyta Swieton), Portugal (Yolanda Vaz), Romania (Ioana Neghirla, Alexandru Supeanu, Claudiu Stroe), Slovakia (Vilem Kopriva, Barbora Pavlikova), Slovenia (Aleksandra Hari), Spain (Ana Fernandez Martín, Inés Moreno), Sweden (Annica Wallén Norell), the United Kingdom (Adam Brouwer, Helen Roberts); Members States representatives that wrote the case reports on the AI secondary outbreaks and on the AI applied prevention and control measures, as reported in the Annex: Austria (Andrea Hoeflechner‐Poeltl, Eveline Wodak), Belgium (Philippe Houdart, Bénédicte Lambrecht, Marjorie Piret, Mieke Steensels), Bulgaria (Aleksandra Miteva, Anna Zdravkova), the Czech Republic (Milada Dubská, Petr Šatrán, Marie Vágnerová), Denmark (Pernille Dahl Nielsen, Stig Mellergaard), France (Mohamed Boukottaya, Anne Bronner, Alexandre Fediaevsky, Claire Guinat, Adeline Huneau‐Salaün, Mathilde Paul), Greece (Sokratis Perdikaris), Hungary (Zsófia Szepesiné Kókány, Gerda Pállai, Anna Luca Vecsei, Gabor Wyszoczky), Ireland (Eoin Ryan), Italy (Tiziano Dorotea, Lebana Bonfanti, Stefano Marangon, Paolo Mulatti), the Netherlands (Marcel Spierenburg), Romania (Nicolae Drăgan, Ioana Neghirlă, Alexandru Supeanu, Claudiu Stroe), the United Kingdom (Adam Brouwer, Ian Brown, Helen Roberts); Dominique Bicout and Arjan Stegeman for reviewing the document.
Approved: 29 September 2017
Amended: 12 December 2017
Figures from 1 to 16 © EURL; Figures 17, 19, 20, 22, 23 © Friedrich‐Loeffler‐Institut (FLI); Figures 18, 21, 26, 27, 28 © ECDC; Figures from C1 to C6 © EFSA
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4991/full
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1282/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1283/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1284/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1285/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1286/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1287/full
Notes
Council Directive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC. OJ L 10, 14.1.2006, p. 16.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Austria, Belgium, Bulgaria, Croatia, the Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Italy, the Netherlands, Poland, Romania, Slovakia, Spain, Sweden and the United Kingdom.
http://www.who.int/influenza/human_animal_interface/HAI_Risk_Assessment/en/ Disease Outbreak News (WHO): http://www.who.int/influenza/human_animal_interface/avian_influenza/archive/en/
Centre for Health Protection Hong Kong: http://www.chp.gov.hk/en/index.html
Time of detection will have an influence on the mortality rate.
On one affected holding, reported in a report of Denmark from November 2016, 29% mortality of domestic ducks was reported. The other reports (Austria, Hungary and the Netherlands) reported no percentage of mortality.
Therefore, analysis at outbreak (holding) level could only be done when species or production type were not considered.
Meaning ‘excluding’.
Experts noted that if the question had been reworded to ‘how many laying flocks experienced affected egg production (including drop in egg production, decreased quality or misshapen eggs, etc.)’, the proportion of positive answers might have been higher.
‘Chickens’ and ‘hens’ were reported as ‘species’ in the data model.
‘Commercial poultry holding’ means a holding where poultry are kept for commercial purposes; ‘non‐commercial holding’ means a holding where poultry or other captive birds are kept by their owners: (a) for their own consumption or use; or (b) as pets (Council Directive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC (OJ L 10, 14.1.2006, p. 16)).
Commission Regulation (EC) No 798/2008 of 8 August 2008 laying down a list of third countries, territories, zones or compartments from which poultry and poultry products may be imported into and transit through the Community and the veterinary certification requirements. OJ L 226, 23.8.2008, pp. 1–94.
Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross‐border threats to health and repealing Decision No 2119/98/EC. OJ: JOL_2013_293_R_0001_01.
Commission Implementing Decision 2012/506/EU of 8 August 2012 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. OJ L 262, 27.9.2012, p. 1–57
Case definitions for the four diseases that require notification in all circumstances under the International Health Regulations (2005) are available at http://www.who.int/ihr/Case_Definitions.pdf.
Commission Decision 2008/426/EC of 28 April 2008 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. OJ L 159, 18.6.2008, p. 46–90
References
- Abdel‐Ghafar A‐N, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, Naghdaliyev A, Peiris JSM, Shindo N, Soeroso S and Uyeki TM and Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza AV , 2008. Update on avian influenza A (H5N1) virus infection in humans. New England Journal of Medicine, 358, 261–273. [DOI] [PubMed] [Google Scholar]
- Adel A, Arafa A, Hussein HA and El‐Sanousi AA, 2017. Molecular and antigenic traits on hemagglutinin gene of avian influenza H9N2 viruses: evidence of a new escape mutant in Egypt adapted in quails. Research in Veterinary Science, 112, 132–140. [DOI] [PubMed] [Google Scholar]
- Arafa AS, Naguib MM, Luttermann C, Selim AA, Kilany WH, Hagag N, Samy A, Abdelhalim A, Hassan MK, Abdelwhab EM, Makonnen Y, Dauphin G, Lubroth J, Mettenleiter TC, Beer M, Grund C and Harder TC, 2015. Emergence of a novel cluster of influenza A(H5N1) virus clade 2.2.1.2 with putative human health impact in Egypt, 2014/15. Eurosurveillance, 20, 2–8. [DOI] [PubMed] [Google Scholar]
- Bahari P, Pourbakhsh SA, Shoushtari H and Bahmaninejad MA, 2015. Molecular characterization of H9N2 avian influenza viruses isolated from vaccinated broiler chickens in northeast Iran. Tropical Animal Health and Production, 47, 1195–1201. [DOI] [PubMed] [Google Scholar]
- Bertran K, Swayne DE, Pantin‐Jackwood MJ, Kapczynski DR, Spackman E and Suarez DL, 2016. Lack of chicken adaptation of newly emergent Eurasian H5N8 and reassortant H5N2 high pathogenicity avian influenza viruses in the U.S. is consistent with restricted poultry outbreaks in the Pacific flyway during 2014–2015. Virology, 494, 190–197. 10.1016/j.virol.2016.04.019 [DOI] [PubMed] [Google Scholar]
- Bhat S, Nagarajan S, Kumar M, Murugkar HV, Kalaiyarasu S, Venkatesh G and Tosh C, 2017. Antigenic characterization of H5N1 highly pathogenic avian influenza viruses isolated from poultry in India, 2006–2015. Archives of Virology, 162, 487–494. [DOI] [PubMed] [Google Scholar]
- Bi YH, Liu HZ, Xiong CC, Liu D, Shi WF, Li MX, Liu SL, Chen J, Chen G, Li Y, Yang GX, Lei YS, Xiong YP, Lei FM, Wang HZ, Chen QJ, Chen JJ and Gao GF, 2016. Novel avian influenza A (H5N6) viruses isolated in migratory waterfowl before the first human case reported in China, 2014. Scientific Reports, 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body MH, Alrarawahi AH, Alhubsy SS, Saravanan N, Rajmony S and Mansoor MK, 2015. Characterization of low pathogenic avian influenza virus subtype H9N2 Isolated from free‐living mynah birds (Acridotheres tristis) in the sultanate of Oman. Avian Diseases, 59, 329–334. [DOI] [PubMed] [Google Scholar]
- Briand FX, Schmitz A, Ogor K, Le Prioux A, Guillou‐Cloarec C, Guillemoto C, Allée C, Le Bras M, Hirchaud E, Quenault H, Touzain F, Cherbonnel‐Pansart M, Lemaitre E, Courtillon C, Gares H, Daniel P, Fediaevsky A, Massin P, Blanchard Y, Eterradossi N, Van Der Werf S, Jestin V and Niqueux E, 2017. Emerging highly pathogenic H5 avian influenza viruses in France during winter 2015/16: phylogenetic analyses and markers for zoonotic potential. Eurosurveillance, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg E, Pereyaslov D, Struelens M, Palm D, Meijer A, Ellis J, Zambon M, McCauley J and Daniels R, 2014. Laboratory preparedness in EU/EEA countries for detection of novel avian influenza A(H7N9) virus, May 2013. Euro Surveillance, 19, 20682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin E, Zhang X, Ke C, Sikkema R and Koopmans M, 2017. Serological evidence for exposure to avian influenza viruses within poultry workers in southern China. Zoonoses and Public Health, 1–9. [DOI] [PubMed] [Google Scholar]
- Bui C, Bethmont A, Chughtai AA, Gardner L, Sarkar S, Hassan S, Seale H and MacIntyre CR, 2016. A systematic review of the comparative epidemiology of avian and human influenza A H5N1 and H7N9‐lessons and unanswered questions. Transboundary and Emerging Diseases, 63, 602–620. [DOI] [PubMed] [Google Scholar]
- Bui CM, Gardner L, Macintyre R and Sarkar S, 2017. Influenza A H5N1 and H7N9 in China: a spatial risk analysis. PLoS ONE, 12, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDCP (Guangdong Provincial Center for Disease Control and Prevention), 2017. Chinese scientists join forces to lead to human infection and death of highly pathogenic H7N9 avian influenza mutation virus. Available online: http://www.cdcp.org.cn/gdsjbyfkzzx/gnwxx001/201706/121a2acb67e747d080fe172d903d3ccb.shtml [Accessed: 28/6/2017].
- Charland KM, Buckeridge DL, Sturtevant JL, Melton F, Reis BY, Mandl KD and Brownstein JS, 2009. Effect of environmental factors on the spatio‐temporal patterns of influenza spread. Epidemiology and Infection, 137, 1377–1387. [DOI] [PubMed] [Google Scholar]
- Chen H, Smith GJ, Zhang SY, Qin K, Wang J, Li KS, Webster RG, Peiris JS and Guan Y, 2005. Avian flu: H5N1 virus outbreak in migratory waterfowl. Nature, 436, 191–192. [DOI] [PubMed] [Google Scholar]
- Chen LJ, Lin XD, Tian JH, Liao Y, Ying XH, Shao JW, Yu B, Guo JJ, Wang MR, Peng Y, Shi M, Holmes EC, Yang ZQ and Zhang YZ, 2017. Diversity, evolution and population dynamics of avian influenza viruses circulating in the live poultry markets in China. Virology, 505, 33–41. [DOI] [PubMed] [Google Scholar]
- Chinese CDC (Chinese Center for Disease Control and Prevention), online. Avian Influenza ‐ disease outbreaks. Available online: http://www.chinacdc.cn/en/
- CNIC (Chinese National Influenza Center), 2017. Chinese Influenza Weekly Report week 33 2017 [8/9/2017]. Available online: http://www.chinaivdc.cn/cnic/en/Surveillance/WeeklyReport/201708/t20170828_151326.htm
- CHP (Centre for Health Protection of the Department of Health Hong Kong), 2017a. Avian Influenza Report, Volume 13, Number 32. Available online: http://www.chp.gov.hk/files/pdf/2017_avian_influenza_report_vol13_wk32.pdf
- CHP (Centre for Health Protection of the Department of Health Hong Kong), 2017b. Avian Influenza Report, Volume 13, Number 23. Available online: http://www.chp.gov.hk/files/pdf/2017_avian_influenza_report_vol13_wk23.pdf
- CHP (Centre for Health Protection of the Department of Health Hong Kong), 2017c. Avian Influenza Report, Volume 13, Number 35. Available online: http://www.chp.gov.hk/files/pdf/2017_avian_influenza_report_vol13_wk35.pdf
- CHP (Centre for Health Protection ‐ Department of Health ‐ The Government of the Hong Kong Special Administrative Region), online. Avian influenza. Available online: http://www.chp.gov.hk/search.asp?search=Hong+Kong&lang=en&page=1
- Chu DH, Okamatsu M, Matsuno K, Hiono T, Ogasawara K, Nguyen LT, Nguyen LV, Nguyen TN, Nguyen TT, Pham DV, Nguyen DH, Nguyen TD, To TL, Nguyen HV, Kida H and Sakoda Y, 2016. Genetic and antigenic characterization of H5, H6 and H9 avian influenza viruses circulating in live bird markets with intervention in the center part of Vietnam. Veterinary Microbiology, 192, 194–203. [DOI] [PubMed] [Google Scholar]
- CNRL/ECDC/WHO Europe , 2013. Diagnostic preparedness in Europe for detection of avian influenza A(H7N9) viruses. Available online: http://ecdc.europa.eu/en/publications/Publications/Forms/ECDC_DispForm.aspx?ID=1103 [Accessed: 2 May 2013]
- CONSISE (Consortium for the Standardization of Influenza Seroepidemiology), online. CONSISE and avian influenza H7N9. Available online: http://consise.tghn.org/
- Davidson I, Shkoda I, Golender N, Perk S, Lapin K, Khinich Y and Panshin A, 2013. Genetic characterization of HA gene of low pathogenic H9N2 influenza viruses isolated in Israel during 2006–2012 periods. Virus Genes, 46, 255–263. [DOI] [PubMed] [Google Scholar]
- Du Y, Chen M, Yang J, Jia Y, Han S, Holmes EC and Cui J, 2017. Molecular evolution and emergence of H5N6 avian influenza virus in central China. Journal of Virology, 91, e00143‐00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatez M, Sonnberg S, Crumpton JC, Rubrum A, Phommachanh P, Douangngeun B, Peiris M, Guan Y, Webster R and Webby R, 2017. Highly pathogenic avian influenza H5N1 clade 2.3.2.1 and clade 2.3.4 viruses do not induce a clade‐specific phenotype in mallard ducks. Journal of General Virology, 98, 1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2013. Proposed interim case definition and case finding algorithm for reporting patients infected by the avian influenza A(H7N9) virus in EU/EEA Member States. Available online at: http://www.ecdc.europa.eu/en/publications/Publications/H7N9-interim-case-definition-april-2013.pdf
- ECDC (European Centre for Disease Prevention and Control), 2014. Risk assessment guidelines for infectious diseases transmitted on aircraft (RAGIDA) – Influenza. Available online at: http://www.ecdc.europa.eu/en/publications/Publications/influenza-RAGIDA-2014.pdf
- ECDC (European Centre for Disease prevention and Control), 2017a. External quality assessment scheme for influenza virus detection and culture for the European Reference Laboratory Network for Human Influenza 2013 Stockholm: ECDC. Available online: http://www.ecdc.europa.eu/en/publications/Publications/influenza-virus-detection-isolation-culture-EQA-2013.pdf
- ECDC (European Centre for Disease Prevention and Control), 2017b. Human infection with avian influenza A(H7N9) virus ‐ Fifth update. Available online: http://ecdc.europa.eu/en/publications/Publications/rra-influenza-a-h7n9-update-five.pdf
- EFSA (European Food Safety Authority), 2007. Report of the task force on zoonoses data collection on the analysis of the baseline study on the prevalence of Salmonella in holdings of laying hen flocks of Gallus gallus . EFSA Journal 2007;97, 85 pp. 10.2903/j.efsa.2007.97r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Scientific report: highly pathogenic avian influenza A subtype H5N8. EFSA Journal 2014;12(12):3941, 32 pp. 10.2903/j.efsa.2014.3941 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2017. Scientific opinion on avian influenza. EFSA Journal 2017;15(10):4991, 233 pp. 10.2903/j.efsa.2017.4991 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Statement on: food safety considerations of novel H1N1 influenza virus infections in humans. EFSA Journal 2010;8(6):1629, 10 pp. 10.2903/j.efsa.2010.1629 [DOI] [Google Scholar]
- Ekong PS, Fountain‐Jones NM and Alkhamis MA, 2017. Spatiotemporal evolutionary epidemiology of H5N1 highly pathogenic avian influenza in West Africa and Nigeria, 2006–2015. Transboundary and Emerging Diseases 2017 Jul 14. 10.1111/tbed.12680. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- El Houadfi M, Fellahi S, Nassik S, Guerin JL and Ducatez MF, 2016. First outbreaks and phylogenetic analyses of avian influenza H9N2 viruses isolated from poultry flocks in Morocco. Virology Journal, 13, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Romeh A, Zecchin B, Fusaro A, Ibrahim E, El Bazzal B, El Hage J, Milani A, Zamperin G and Monne I, 2017. Highly pathogenic avian influenza H5N1 Clade 2.3.2.1c Virus in Lebanon, 2016. Avian Diseases, 61, 271–273. [DOI] [PubMed] [Google Scholar]
- El‐Shesheny R, Barman S, Feeroz MM, Hasan MK, Jones‐Engel L, Franks J, Turner J, Seiler P, Walker D, Friedman K, Kercher L, Begum S, Akhtar S, Datta AK, Krauss S, Kayali G, McKenzie P, Webby RJ and Webster RG, 2017. Genesis of influenza A(H5N8) viruses. Emerging Infectious Diseases, 23, 1368–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , online. Animal health ‐ regulatory committee presentations. Available online: https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en
- FAO (Food and Agriculture Organization), online‐a. Wild birds highly pathogenic avian influenza surveillance ‐ sample collection from healthy, sick and dead birds. Available online: http://www.fao.org/docrep/010/a0960e/a0960e00.HTM
- FAO (Food and Agriculture Organization), online‐b. H7N9 situation update [12 July 2017]. Available online: http://www.fao.org/ag/againfo/programmes/en/empres/h7n9/
- Fournie G, Hog E, Barnett T, Pfeiffer DU and Mangtani P, 2017. A systematic review and meta‐analysis of practices exposing humans to avian influenza viruses, their prevalence, and rationale. The American Journal of Tropical Medicine and Hygiene, 97, 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaro A, Monne I, Mulatti P, Zecchin B, Bonfanti L, Ormelli S, Milani A, Cecchettin K, Lemey P, Moreno A, Massi P, Dorotea T, Marangon S and Terregino C, 2017. Genetic diversity of highly pathogenic avian influenza A(H5N8/H5N5) viruses in Italy, 2016–17. Emerging Infect Diseases, 23, 1543–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, Lu SH, Yang YD, Fang Q, Shen YZ, Xi XM, Gu Q, Zhou XM, Qu HP, Yan Z, Li FM, Zhao W, Gao ZC, Wang GF, Ruan LX, Wang WH, Ye J, Cao HF, Li XW, Zhang WH, Fang XC, He J, Liang WF, Xie J, Zeng M, Wu XZ, Li J, Xia Q, Jin ZC, Chen Q, Tang C, Zhang ZY, Hou BM, Feng ZX, Sheng JF, Zhong NS and Li LJ, 2013. Clinical findings in 111 cases of influenza A (H7N9) virus infection. New England Journal of Medicine, 368, 2277–2285. [DOI] [PubMed] [Google Scholar]
- Gao RB, Bai T, Li XD, Xiong Y, Huang YW, Pan M, Zhang Y, Bo H, Zou SM and Shu YL, 2016. The comparison of pathology in ferrets infected by H9N2 avian influenza viruses with different genomic features. Virology, 488, 149–155. [DOI] [PubMed] [Google Scholar]
- Ghafouri SA, Langeroudi AG, Maghsoudloo H, Tehrani F, Khaltabadifarahani R, Abdollahi H and Fallah MH, 2017a. Phylogenetic study‐based hemagglutinin (HA) gene of highly pathogenic avian influenza virus (H5N1) detected from backyard chickens in Iran, 2015. Virus Genes, 53, 117–120. [DOI] [PubMed] [Google Scholar]
- Ghafouri SA, GhalyanchiLangeroudi A, Maghsoudloo H, Kh Farahani R, Abdollahi H, Tehrani F and Fallah MH, 2017b. Clade 2.3.4.4 avian influenza A (H5N8) outbreak in commercial poultry, Iran, 2016: the first report and update data. Trop Anim Health Production, 49, 1089–1093. 10.1007/s11250-017-1302-z [DOI] [PubMed] [Google Scholar]
- GISAID (The Global Initiative on Sharing All Influenza data), 2013. Genetic sequence data from the human and poultry isolates of A(H7N9) viruses. Available online: http://platform.gisaid.org/epi3/frontend#c6798 [Accessed: 10/4/2013]
- Globig A, Staubach C, Beer M, Köppen U, Fiedler W, Nieburg M, Wilking H, Starick E, Teifke JP, Werner O, Unger F, Grund C, Wolf C, Roost H, Feldhusen F, Conraths FJ, Mettenleiter TC and Harder TC, 2009. Epidemiological and ornithological aspects of outbreaks of highly pathogenic avian influenza virus H5N1 of Asian lineage in wild birds in Germany, 2006 and 2007. Transbound Emerging Diseases, 56, 57–72. [DOI] [PubMed] [Google Scholar]
- Gowthaman V, Singh SD, Dhama K, Srinivasan P, Saravanan S, Murthy TRGK, Sukumar K, Mathapati B, Lebarbenchon C, Malik YS and Ramakrishnan MA, 2016. Isolation and phylogenetic characterization of haemagglutinin and neuraminidase genes of H9N2 low pathogenicity avian influenza virus isolated from commercial layers in India. Virus Disease, 27, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HB, de Vries E, McBride R, Dekkers J, Peng WJ, Bouwman KM, Nycholat C, Verheije MH, Paulson JC, van Kuppeveld FJM and de Haan CAM, 2017. Highly pathogenic influenza A(H5Nx) viruses with altered H5 receptor‐binding specificity. Emerging Infectious Diseases, 23, 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan KE, Ali A, Shany SAS and El‐Kady MF, 2017. Experimental co‐infection of infectious bronchitis and low pathogenic avian influenza H9N2 viruses in commercial broiler chickens. Research in Veterinary Science, 115, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiono T, Okamatsu M, Matsuno K, Haga A, Iwata R, Nguyen LT, Suzuki M, Kikutani Y, Kida H, Onuma M and Sakoda Y, 2017. Characterization of H5N6 highly pathogenic avian influenza viruses isolated from wild and captive birds in the winter season of 2016–2017 in northern Japan Microbiology and Immunology, 61, 387–397. [DOI] [PubMed] [Google Scholar]
- Hosseini H, Ghalyanchilangeroudi A, Fallah Mehrabadi MH, Sediqian MS, Shayeganmehr A, Ghafouri SA, Maghsoudloo H, Abdollahi H and Farahani RK, 2017. Phylogenetic analysis of H9N2 avian influenza viruses in Afghanistan (2016–2017). Archives of Virology, 1–5. [DOI] [PubMed] [Google Scholar]
- ISARIC (International Severe Acute Respiratory and Emerging Infection Consortium), online. ISARIC and WHO SARI and Natural History Protocols. Available online at: https://consise.tghn.org/
- Iqbal M, Yaqub T, Reddy K and McCauley JW, 2009. Novel genotypes of H9N2 influenza A viruses isolated from poultry in Pakistan containing NS genes similar to highly pathogenic H7N3 and H5N1 viruses. PLoS ONE, 4, e5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Woo C, Ip HS, An I, Kim Y, Lee K, Jo S‐D, Son K, Lee S, Oem J‐K, Wang S‐J, Kim Y, Shin J, Sleeman J and Jheong W, 2017. Identification of two novel reassortant avian influenza a (H5N6) viruses in whooper swans in Korea, 2016. Virology Journal, 14, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W, Cao C, Lin Y, Zhong L, Xie S, Wang X, Yin S, Xu Z, Dai Y, Li Z, Niu X, Qi W, Lu T and Liao M, 2017. Detection of a novel highly pathogenic H7 influenza virus by duplex real‐time reverse transcription polymerase chain reaction. Journal of Virological Methods, 246, 100–103. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wu P, Uyeki TM, He J, Deng Z, Xu W, Lv Q, Zhang J, Wu Y, Tsang TK, Kang M, Zheng J, Wang L, Yang B, Qin Y, Feng L, Fang VJ, Gao GF, Leung GM, Yu H and Cowling BJ, 2017. Preliminary epidemiologic assessment of human infections with highly pathogenic avian influenza A(H5N6) virus, China. Clinical Infectious Diseases, 65, 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao PR, Cui J, Song YF, Song H, Zhao ZS, Wu SY, Qu NN, Wang NC, Ouyang GW and Liao M, 2016. New reassortant H5N6 highly pathogenic avian influenza viruses in Southern China, 2014. Frontiers in Microbiology, 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JC, Sonnberg S, Kocer ZA, Shanmuganatham K, Seiler P, Shu YL, Zhu HC, Guan Y, Peiris M, Webby RJ and Webster RG, 2014. Possible role of songbirds and parakeets in transmission of influenza A(H7N9) virus to humans. Emerging Infectious Diseases, 20, 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammon A, Heidari A, Dayhum A, Eldaghayes I, Sharif M, Monne I, Cattoli G, Asheg A, Farhat M and Kraim E, 2015. Characterization of avian influenza and newcastle disease viruses from poultry in libya. Avian Diseases, 59, 422–430. [DOI] [PubMed] [Google Scholar]
- Kandeil A, Kayed A, Moatasim Y, Webby RJ, McKenzie PP, Kayali G and Ali MA, 2017a. Genetic characterization of highly pathogenic avian influenza A H5N8 viruses isolated from wild birds in Egypt. Journal of General Virology, 98, 1573–1586. 10.1099/jgv.0.000847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeil A, El‐Shesheny R, Maatouq A, Moatasim Y, Cai ZP, McKenzie P, Webby R, Kayali G and Ali MA, 2017b. Novel reassortant H9N2 viruses in pigeons and evidence for antigenic diversity of H9N2 viruses isolated from quails in Egypt. Journal of General Virology, 98, 548–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HM, Lee EK, Song BM, Heo GB, Jung J, Jang I, Bae YC, Jung SC and Lee YJ, 2017. Experimental infection of mandarin duck with highly pathogenic avian influenza A (H5N8 and H5N1) viruses. Veterinary Microbiology, 198, 59–63. [DOI] [PubMed] [Google Scholar]
- Khan SU, Anderson BD, Heil GL, Liang S and Gray GC, 2015. A systematic review and meta‐analysis of the seroprevalence of influenza A(H9N2) infection among humans. Journal of Infectious Diseases, 212, 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Pascua PNQ, Kwon HI, Lim GJ, Kim EH, Yoon SW, Park SJ, Kim SM, Choi EJ, Si YJ, Lee OJ, Shim WS, Kim SW, Mo IP, Bae Y, Lim YT, Sung MH, Kim CJ, Webby RJ, Webster RG and Choi YK, 2014. Pathobiological features of a novel, highly pathogenic avian influenza A(H5N8) virus. Emerging Microbes and Infections, 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Park SJ, Kwon HI, Kim EH, Si YJ, Jeong JH, Lee IW, Nguyen HD, Kwon JJ, Choi WS, Song MS, Kim CJ and Choi YK, 2017. Genetic and phylogenetic characterizations of a novel genotype of highly pathogenic avian influenza (HPAI) H5N8 viruses in 2016/2017 in South Korea. Infection Genetics and Evolution, 53, 56–67. [DOI] [PubMed] [Google Scholar]
- Klenk HD and Garten W, 1994. Host cell proteases controlling virus pathogenicity. Trends in Microbiology, 2,39–43. [DOI] [PubMed] [Google Scholar]
- Kwon JH, Noh YK, Lee DH, Yuk SS, Erdene‐Ochir TO, Noh JY, Hong WT, Jeong JH, Jeong S, Gwon GB, Song CS and Nahm SS, 2017. Experimental infection with highly pathogenic H5N8 avian influenza viruses in the Mandarin duck (Aix galericulata) and domestic pigeon (Columba livia domestica). Veterinary Microbiology, 203, 95–102. [DOI] [PubMed] [Google Scholar]
- Lau S, Joseph S, Chan K, Chen H, Patteril NAG, Elizabeth SK, Muhammed R, Vijay B, Lau SKP, Kinne J, Wernery U and Woo PCY, 2016. Complete genome sequence of influenza virus H9N2 associated with a fatal outbreak among chickens in Dubai. Genome Announcements, 4, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Kwon JH, Noh JY, Park JK, Yuk SS, Erdene‐Ochir TO, Lee JB, Park SY, Choi IS, Lee SW and Song CS, 2016a. Pathogenicity of the korean H5N8 highly pathogenic avian influenza virus in commercial domestic poultry species. Avian Pathology, 45, 208–211. 10.1080/03079457.2016.1142502 [DOI] [PubMed] [Google Scholar]
- Lee DH, Kwon JH, Park JK, Yuk SS, Tseren‐Ochir EO, Noh JY, Lee JB, Park SY, Choi IS and Song CS, 2016b. Experimental infection of chickens with intercontinental reassortant H9N2 influenza viruses from wild birds. Avian Diseases, 60, 493–495. [DOI] [PubMed] [Google Scholar]
- Lee EK, Song BM, Kang HM, Woo SH, Heo GB, Jung SC, Park YH, Lee YJ and Kim JH, 2016c. Experimental infection of SPF and Korean native chickens with highly pathogenic avian influenza virus (H5N8). Poultry Science, 95, 1015–1019. 10.3382/ps/pew028 [DOI] [PubMed] [Google Scholar]
- Lee DH, Sharshov K, Swayne DE, Kurskaya O, Sobolev I, Kabilov M, Alekseev A, Irza V and Shestopalov A, 2017. Novel reassortant clade 2.3.4.4 avian influenza A(H5N8) virus in wild aquatic birds, Russia, 2016. Emerging Infectious Diseases, 23, 359–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Fu YG, Yang JY, Guo J, He JJ, Guo JH, Weng ST, Jia YN, Liu B, Li XY, Zhu QY and Chen HL, 2015. Genetic and biological characterization of two novel reassortant H5N6 swine influenza viruses in mice and chickens. Infection Genetics and Evolution, 36, 462–466. [DOI] [PubMed] [Google Scholar]
- Li S, Zhou Y, Song W, Pang Q and Miao Z, 2016. Avian influenza virus H9N2 seroprevalence and risk factors for infection in occupational poultry‐exposed workers in Tai'an of China. Journal of Medicine Virology, 88, 1453–1456. [DOI] [PubMed] [Google Scholar]
- Li M, Liu H, Bi Y, Sun J, Wong G, Liu D, Li L, Liu J, Chen Q, Wang H, He Y, Shi W, Gao GF and Chen J, 2017. Highly pathogenic avian influenza A(H5N8) virus in wild migratory birds, qinghai lake, China. Emerging Infectious Diseases, 23, 637–641. 10.3201/eid2304.161866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TN, Nonthabenjawan N, Chaiyawong S, Bunpapong N, Boonyapisitsopa S, Janetanakit T, Mon PP, Mon HH, Oo KN, Oo SM, Mar Win M and Amonsin A, 2017. Influenza A(H9N2) Virus, Myanmar, 2014–2015. Emerging Infectious Diseases, 23, 1041–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Shi WF, Shi Y, Wang DY, Xiao HX, Li W, Bi YH, Wu Y, Li XB, Yan JH, Liu WJ, Zhao GP, Yang WZ, Wang Y, Ma JC, Shu YL, Lei FM and Gao GF, 2013. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: phylogenetic, structural, and coalescent analyses. Lancet, 381, 1926–1932. [DOI] [PubMed] [Google Scholar]
- Liu YH, Yang ZY, Wang XQ, Chen JM, Yao JZ, Song YJ, Lin J, Han CH, Duan HJ, Zhao JC, Pan J and Xie J, 2015. Pigeons are resistant to experimental infection with H7N9 avian influenza virus. Avian Pathology, 44, 342–346. [DOI] [PubMed] [Google Scholar]
- Liu L, Zeng X, Chen P, Deng G, Li Y, Shi J, Gu C, Kong H, Suzuki Y, Jiang Y, Tian G and Chen H, 2016. Characterization of clade 7.2 H5 avian influenza viruses that continue to circulate in chickens in China. Journal of Virology, 90, 9797–9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancera Gracia JC, Van den Hoecke S, Saelens X and Van Reeth K, 2017. Effect of serial pig passages on the adaptation of an avian H9N2 influenza virus to swine. PLoS ONE, 12, e0175267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko VY, Susloparov IM, Komissarov AB, Fadeev A, Goncharova NI, Shipovalov AV, Svyatchenko SV, Durymanov AG, Ilyicheva TN, Salchak LK, Svintitskaya EP, Mikheev VN and Ryzhikov AB, 2017. Reintroduction of highly pathogenic avian influenza A/H5N8 virus of clade 2.3.4.4. in Russia. Archives of Virology, 162, 1381–1385. [DOI] [PubMed] [Google Scholar]
- Marinova‐Petkova A, Shanmuganatham K, Feeroz MM, Jones‐Engel L, Hasan MK, Akhtar S, Turner J, Walker D, Seiler P, Franks J, McKenzie P, Krauss S, Webby RJ and Webster RG, 2016a. The continuing evolution of H5N1 and H9N2 influenza viruses in Bangladesh between 2013 and 2014. Avian Diseases, 60, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova‐Petkova A, Franks J, Tenzin S, Dahal N, Dukpa K, Dorjee J, Feeroz MM, Rehg JE, Barman S, Krauss S, McKenzie P, Webby RJ and Webster RG, 2016b. Highly pathogenic reassortant avian influenza A(H5N1) virus clade 2.3.2.1a in Poultry, Bhutan. Emerging Infectious Diseases, 22, 2137–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoA (Ministry of Agriculture of the People's republic of China), online. H7N9 situation update. Available online: http://www.moa.gov.cn/govpublic/
- Nagarajan S, Tosh C, Smith DK, Peiris JSM, Murugkar HV, Sridevi R, Kumar M, Katare M, Jain R, Syed Z, Behera P, Cheung CL, Khandia R, Tripathi S, Guan Y and Dubey SC, 2012. Avian influenza (H5N1) virus of clade 2.3.2 in domestic poultry in India. PLoS ONE, 7, e31844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan S, Kumar M, Murugkar HV, Tripathi S, Shukla S, Agarwal S, Dubey G, Nagi RS, Singh VP and Tosh C, 2017. Novel reassortant highly pathogenic avian influenza (H5N8) virus in zoos, India. Emerging Infectious Diseases, 23, 717–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamatsu M, Ozawa M, Soda K, Takakuwa H, Haga A, Hiono T, Matsuu A, Uchida Y, Iwata R, Matsuno K, Kuwahara M, Yabuta T, Usui T, Ito H, Onuma M, Sakoda Y, Saito T, Otsuki K, Ito T and Kida H, 2017. Characterization of highly pathogenic avian influenza virus A(H5N6), Japan, november 2016. Emerging Infectious Diseases, 23, 691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD and Fouchier RA, 2006. Global patterns of influenza a virus in wild birds. Science, 312, 384–388. [DOI] [PubMed] [Google Scholar]
- Olson SH, Gilbert M, Cheng MC, Maget JAK and Joly DO, 2013. Historical prevalence and distribution of avian influenza virus A(H7N9) among wild birds. Emerging Infectious Diseases, 19, 2031–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ, Miller PJ, Spackman E, Swayne DE, Susta L, Costa‐Hurtado M and Suarez DL, 2014. Role of poultry in the spread of novel H7N9 influenza virus in China. Journal of Virology, 88, 5381–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ, Costa‐Hurtado M, Shepherd E, DeJesus E, Smith D, Spackman E, Kapczynski DR, Suarez DL, Stallknecht DE and Swayne DE, 2016. Pathogenicity and transmission of H5 and H7 highly pathogenic avian influenza viruses in mallards. Journal of Virology, 90, 9967–9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ, Costa‐Hurtado M, Bertran K, DeJesus E, Smith D and Swayne DE, 2017. Infectivity, transmission and pathogenicity of H5 highly pathogenic avian influenza clade 2.3.4.4 (H5N8 and H5N2) United States index viruses in Pekin ducks and Chinese geese. Veterinary Research, 48, 33. 10.1186/s13567-017-0435-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce MB, Pappas C, Gustin KM, Davis CT, Pantin‐Jackwood MJ, Swayne DE, Maines TR, Belser JA and Tumpey TM, 2017. Enhanced virulence of clade 2.3.2.1 highly pathogenic avian influenza A H5N1 viruses in ferrets. Virology, 502, 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann A, Starick E, Harder T, Grund C, Hoper D, Globig A, Staubach C, Dietze K, Strebelow G, Ulrich RG, Schinkothe J, Teifke JP, Conraths FJ, Mettenleiter TC and Beer M, 2017. Outbreaks among wild birds and domestic poultry caused by reassorted influenza A(H5N8) clade 2.3.4.4 viruses, Germany, 2016. Emerging Infectious Diseases, 23, 633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu J, Sun H, Qu Y, Wang C, Gao W, Zhu J, Sun Y, Bi Y, Huang Y, Chang KC, Cui J and Liu J, 2017. M Gene reassortment in H9N2 influenza virus promotes early infection and replication: contribution to rising virus prevalence in chickens in China. Journal of Virology, 91, e02055‐16. 10.1128/jvi.02055-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulit‐Penaloza JA, Sun XJ, Creager HM, Zeng H, Belser JA, Maines TR and Tumpey TM, 2015. Pathogenesis and transmission of novel highly pathogenic avian influenza H5N2 and H5N8 viruses in ferrets and mice. Journal of Virology, 89, 10286–10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Cui L, Yu H, Ge Y and Tang F, 2014. Whole‐genome sequence of a reassortant H5N6 avian influenza virus isolated from a live poultry market in China, 2013. Genome Announcements, 2, e00706‐e00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XF, Tan D, Wu CQ, Tang C, Li T, Han XY, Wang J, Liu CH, Li RQ and Wang JY, 2016. Deterioration of eggshell quality in laying hens experimentally infected with H9N2 avian influenza virus. Veterinary Research, 47, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid PMA, Saeed NM and Dyary HO, 2017. Genetic characterization and phylogenic analysis of H5N1 avian influenza virus detected in peafowl in Kirkuk province, Iraq. Journal of Medical Virology, 89, 1179–1185. [DOI] [PubMed] [Google Scholar]
- Reid SM, Banks J, Ceeraz V, Seekings A, Howard WA, Puranik A, Collins S, Manvell R, Irvine RM and Brown IH, 2016. The detection of a low pathogenicity avian influenza virus subtype H9 infection in a turkey breeder flock in the United Kingdom. Avian Diseases, 60, 126–131. [DOI] [PubMed] [Google Scholar]
- Richard M, Herfst S, Van Den Brand JMA, Lexmond P, Bestebroer TM, Rimmelzwaan GF, Koopmans M, Kuiken T and Fouchier RAM, 2015. Low virulence and lack of airborne transmission of the Dutch highly pathogenic avian influenza virus H5N8 in ferrets. PLoS ONE, 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaheldin AH, Veits J, El‐Hamid HSA, Harder TC, Devrishov D, Mettenleiter TC, Hafez HM and Abdelwhab EM, 2017. Isolation and genetic characterization of a novel 2.2.1.2a H5N1 virus from a vaccinated meat‐turkeys flock in Egypt. Virology Journal, 14, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim AA, Erfan AM, Hagag N, Zanaty A, Samir A‐H, Samy M, Abdelhalim A, Arafa A‐SA, Soliman MA, Shaheen M, Ibraheem EM, Mahrous I, Hassan MK and Naguib MM, 2017. Highly pathogenic avian influenza virus (H5N8) clade 2.3.4.4 infection in migratory birds, Egypt. Emerging Infectious Diseases, 23, 1048–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmuganatham K, Feeroz MM, Jones‐Engel L, Walker D, Alam SMR, Hasan MK, McKenzie P, Krauss S, Webby RJ and Webster RG, 2014. Genesis of avian influenza H9N2 in Bangladesh. Emerging Microbes and Infections, 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shittu I, Meseko CA, Gado DA, Olawuyi AK, Chinyere CN, Anefu E, Solomon P, Okewole PA, Shamaki D and Joannis TM, 2017. Highly pathogenic avian influenza (H5N1) in Nigeria in 2015: evidence of widespread circulation of WA2 clade 2.3.2.1c. Archives of Virology, 162, 841–847. [DOI] [PubMed] [Google Scholar]
- Si Y, Lee I, Kim E, Kim Y, Kwon H, Park S, Nguyen HD, Kim SM, Kwon J, Choi W, Beak YH, Song M, Kim C, Webby RJ and Choi Y, 2017. Genetic characterisation of novel, highly pathogenic avian influenza (HPAI) H5N6 viruses isolated in birds, South Korea, November 2016. Eurosurveillance, 22, 2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smietanka K, Minta Z, Swieton E, Olszewska M, Jozwiak M, Domanska‐Blicharz K, Wyrostek K, Tomczyk G and Pikula A, 2014. Avian influenza H9N2 subtype in Poland ‐ characterization of the isolates and evidence of concomitant infections. Avian Pathology, 43, 427–436. [DOI] [PubMed] [Google Scholar]
- Smith GJD and Donis RO and World Health Organization W , 2015. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza and Other Respiratory Viruses, 9, 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Gu M, Liu D, Cui J, Gao GF, Zhou J and Liu X, 2017. Epidemiology, evolution, and pathogenesis of H7N9 influenza viruses in five epidemic waves since 2013 in China. Trends in Microbiology, 25, 713–728. [DOI] [PubMed] [Google Scholar]
- Sun YP and Liu JH, 2015. H9N2 influenza virus in China: a cause of concern. Protein and Cell, 6, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HL, Pu J, Wei YD, Sun YP, Hu J, Liu LT, Xu GL, Gao WH, Li C, Zhang XX, Huang YH, Chang KC, Liu XF and Liu JH, 2016. Highly pathogenic avian influenza H5N6 viruses exhibit enhanced affinity for human type sialic acid receptor and in‐contact transmission in model ferrets. Journal of Virology, 90, 6235–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TaiwanCDC (Centers for Disease Control ROC, ‐Taiwan), online. Avian influenza ‐ disease outbreaks. Available online: http://www.cdc.gov.tw/rwd/english
- Tanikawa T, Kanehira K, Tsunekuni R, Uchida Y, Takemae N and Saito T, 2016. Pathogenicity of H5N8 highly pathogenic avian influenza viruses isolated from a wild bird fecal specimen and a chicken in Japan in 2014. Microbiol Immunology, 60, 243–252. [DOI] [PubMed] [Google Scholar]
- Tassoni L, Fusaro A, Milani A, Lemey P, Awuni JA, Sedor VB, Dogbey O, Commey AN, Meseko C, Joannis T, Minoungou GL, Ouattara L, Haido AM, Cisse‐Aman D, Couacy‐Hymann E, Dauphin G, Cattoli G and Monne I, 2016. Emerging Infection and Diseases, 22, 2132–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuy DM, Peacock TP, Bich VT, Fabrizio T, Hoang DN, Tho ND, Diep NT, Nguyen M, le Hoa NM, Trang HT, Choisy M, Inui K, Newman S, Trung NV, van Doorn R, To TL, Iqbal M and Bryant JE, 2016. Prevalence and diversity of H9N2 avian influenza in chickens of Northern Vietnam, 2014. Infection, Genetics and Evolution, 44, 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchetti MK, Killian ML, Dusek RJ, Pedersen JC, Hines N, Bodenstein B, White CL and Ip HS, 2015. Novel H5 clade 2.3.4.4 reassortant (H5N1) virus from a green‐winged teal in Washington, USA. Genome Announcements, 3, e00195‐00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh C, Nagarajan S, Kumar M, Murugkar HV, Venkatesh G, Shukla S, Mishra A, Mishra P, Agarwal S, Singh B, Dubey P, Tripathi S and Kulkarni DD, 2016. Multiple introductions of a reassortant H5N1 avian influenza virus of clade 2.3.2.1c with PB2 gene of H9N2 subtype into Indian poultry. Infection, Genetics and Evolution, 43, 173–178. [DOI] [PubMed] [Google Scholar]
- Van Kerkhove MD, Mumford E, Mounts AW, Bresee J, Ly S, Bridges CB and Otte J, 2011a. Highly pathogenic avian influenza (H5N1): pathways of exposure at the animal‐human interface, a systematic review. PLoS ONE, 6, e14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerkhove MD, Mumford E, Mounts AW, Bresee J, Ly S, Bridges CB and Otte J, 2011b. Highly pathogenic avian influenza (H5N1): pathways of exposure at the animal‐human interface, a systematic review. PLoS ONE, 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries RP, Peng W, Grant OC, Thompson AJ, Zhu X, Bouwman KM, de la Pena ATT, van Breemen MJ, Ambepitiya Wickramasinghe IN, de Haan CAM, Yu W, McBride R, Sanders RW, Woods RJ, Verheije MH, Wilson IA and Paulson JC, 2017. Three mutations switch H7N9 influenza to human‐type receptor specificity. PLoS Pathogens, 13, e1006390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RG, HoDac H, Lathrop RH and Fitch WM, 2007. A statistical phylogeography of influenza A H5N1. Proceedings of the National Academy of Sciences of the United States of America, 104, 4473–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fang S, Lu X, Xu C, Cowling BJ, Tang X, Peng B, Wu W, He J, Tang Y, Xie X, Mei S, Kong D, Zhang R, Ma H and Cheng J, 2014. Seroprevalence to avian influenza A(H7N9) virus among poultry workers and the general population in southern China: a longitudinal study. Clinical Infectious Diseases, 59, e76–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu MC, Hong WS, Fan XH, Chen RR, Zheng ZY, Zeng Y, Huang R, Zhang Y, Lam TTY, Smith DK, Zhu HC and Guan Y, 2016. Infectivity and transmissibility of avian H9N2 influenza viruses in pigs. Journal of Virology, 90, 3506–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Fu X, Zheng Y, Zhou P, Fang B, Huang S, Zhang X, Chen J, Cao Z, Tian J and Li S, 2017. The NS1 protein of H5N6 feline influenza virus inhibits feline beta interferon response by preventing NF‐κB and IRF3 activation. Developmental and Comparative Immunology, 74, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R and Rott R, 1987. Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell, 50, 665–656. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2013a. Interim WHO surveillance recommendations for human infection with avian influenza A(H7N9) virus. Available online: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/InterimSurveillanceRecH7N9_10May13.pdf?ua=1
- WHO (World Health Organization), 2013b. Real‐time RT‐PCR Protocol for the Detection of Avian Influenza A(H7N9) Virus. Available online at: http://www.who.int/influenza/gisrs_laboratory/cnic_realtime_rt_pcr_protocol_a_h7n9.pdf?ua=1
- WHO (World Health Organization), 2013c. Laboratory biorisk management for laboratories handling human specimens suspected or confirmed to contain avian influenza A(H7N9) virus causing human disease Interim recommendations. Available online at: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/InterimRecLaboratoryBioriskManagementH7N9_10May13.pdf?ua=1
- WHO (World Health Organization), 2013d. Laboratory Procedures Serological detection of avian influenza A(H7N9) infections by microneutralization assay. Available online at: http://www.who.int/influenza/gisrs_laboratory/cnic_serological_diagnosis_microneutralization_a_h7n9.pdf?ua=1
- WHO (World Health Organization), 2013e. Laboratory Procedures Serological detection of avian influenza A(H7N9) virus infections by turkey haemagglutination‐inhibition assay Available online at: http://www.who.int/influenza/gisrs_laboratory/cnic_serological_diagnosis_hai_a_h7n9.pdf?ua=1
- WHO (World Health Organization), 2013f. Laboratory Procedures Serological detection of avian influenza A(H7N9) virus infections by modified horse red blood cells haemagglutination‐inhibition assay. Available online at: http://www.who.int/influenza/gisrs_laboratory/cnic_serological_diagnosis_hai_a_h7n9_20131220.pdf?ua=1
- WHO (World Health Organization), 2014. Avian influenza A(H7N9) virus: Post‐exposure antiviral chemoprophylaxis of close contacts of a patient with confirmed H7N9 virus infection and/or high risk poultry/environmental exposures. Jan 2014. Available online at: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/13_January_2013_PEP_recs.pdf?ua=1
- WHO (World Health Organization), 2016. Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness, February 2016. Available online: http://www.who.int/influenza/vaccines/virus/201602_zoonotic_vaccinevirusupdate.pdf
- WHO (World Health Organization: Western pacific Region), 2017a. Avian influenza weekly update number 590 (23 June 2017). Available online: http://www.wpro.who.int/emerging_diseases/ai_weekly_590_wpro_20170623.pdf?ua=1
- WHO (World Health Organization), 2017b. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2017; as of 25 July 2017. Available online: http://www.who.int/influenza/human_animal_interface/2017_07_25_tableH5N1.pdf?ua=1 [Accessed: 15/8/2017]
- WHO (World Health Organization), 2017c. Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness, March 2017. Available online: http://www.who.int/influenza/vaccines/virus/201703_zoonotic_vaccinevirusupdate.pdf?ua=1
- WHO (World Health Organization), 2017d. Western Pacific Region. Avian Influenza Weekly Update Number 600. Available online: http://www.wpro.who.int/emerging_diseases/ai_weekly_600_wpro_20170901.pdf?ua=1
- WHO (World Health Organization), 2017e. Influenza at the human‐animal interface. Summary and assessment, 16 March to 20 April 2017. Available online: http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_04_20_2017_corrected_23_05_2017.pdf?ua=1 [Accessed: 3/7/2017]
- WHO (World Health Organization), 2017f. Influenza at the human‐animal interface. Summary and assessment, 16 June 2017 to 25 July 2017. Available online: http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_07_25_2017.pdf?ua=1
- WHO (World Health Organization), online‐a. Human infection with avian influenza A(H7N9) virus – China. Available online: http://www.who.int/csr/don/17-january-2017-ah7n9-china/en/ [Accessed: 17 January 2017]
- WHO (World Health Organization), online‐b. Human infection with avian influenza A(H7N9) virus – China. Available online: http://www.who.int/csr/don/18-january-2017-ah7n9-china/en/ [Accessed: 18 January 2017]
- WHO (World Health Organization), online‐c. Human infection with avian influenza A(H7N9) virus – China [15 March]. Available online: http://www.who.int/csr/don/15-march-2017-ah7n9-china/en/
- WHO (World Health Organization), online‐d. Human infection with avian influenza A(H7N9) virus – China. Available online: http://www.who.int/csr/don/20-april-2017-ah7n9-china/en/ [Accessed: 20 April 2017]
- WHO (World Health Organization), online‐e. Human infection with avian influenza A(H7N9) virus – China [23 May]. Available online: http://www.who.int/csr/don/23-may-2017-ah7n9-china/en/
- WHO (World Health Organization), online‐f. Situation updates ‐ avian influenza Available online: http://www.who.int/influenza/human_animal_interface/avian_influenza/archive/en/
- Wong FY, Phommachanh P, Kalpravidh W, Chanthavisouk C, Gilbert J, Bingham J, Davies KR, Cooke J, Eagles D, Phiphakhavong S, Shan S, Stevens V, Williams DT, Bounma P, Khambounheuang B, Morrissy C, Douangngeun B and Morzaria S, 2015. Reassortant highly pathogenic infuenza a(H5N6) virus in laos. Emerging Infectious Diseases, 21, 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HB, Lu RF, Peng XM, Peng XR, Chen B, Cheng LF and Wu NP, 2017. Molecular characterization of a novel reassortant H7N6 subtype avian influenza virus from poultry in Eastern China, in 2016. Archives of Virology, 162, 1341–1347. [DOI] [PubMed] [Google Scholar]
- Xia J, Cui JQ, He X, Liu YY, Yao KC, Cao SJ, Han XF and Huang Y, 2017. Genetic and antigenic evolution of H9N2 subtype avian influenza virus in domestic chickens in southwestern China, 2013–2016. PLoS ONE, 12, e0171564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhu WF, Li XD, Bo H, Zhang Y, Zou SM, Gao RB, Dong J, Zhao X, Chen WB, Dong LB, Zou XH, Xing YC, Wang DY and Shu YL, 2017. Genesis and dissemination of highly pathogenic H5N6 avian influenza viruses. Journal of Virology, 91, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wu JT, Cowling BJ, Liao Q, Fang VJ, Zhou S, Wu P, Zhou H, Lau EH, Guo D, Ni MY, Peng Z, Feng L, Jiang H, Luo H, Li Q, Feng Z, Wang Y, Yang W and Leung GM, 2014. Effect of closure of live poultry markets on poultry‐to‐person transmission of avian influenza A H7N9 virus: an ecological study. Lancet, 383, 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZJ, Gao XL, Wang TC, Li YB, Li YC, Xu Y, Chu D, Sun HT, Wu CJ, Li SN, Wang HJ, Li YG, Xia ZP, Lin WS, Qian J, Chen HL, Xia XZ and Gao YW, 2015. Fatal H5N6 avian influenza virus infection in a domestic cat and wild birds in China. Scientific Reports, 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk S‐S, Lee D‐H, Park J‐K, To E‐O, Kwon J‐H, Noh J‐Y and Song C‐S, 2017. Experimental infection of dogs with highly pathogenic avian influenza virus (H5N8). Journal of Veterinary Science, 18, 381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng C, Ma C, Yang P, Tang S, Lau A, Sun W and Wang Q, 2015. The impact of temperature and humidity measures on influenza A (H7N9) outbreaks‐evidence from China. International Journal of Infectious Diseases, 30, 122–124. [DOI] [PubMed] [Google Scholar]
- Zhao T, Qian YH, Chen SH, Wang GL, Wu MN, Huang Y, Ma GY, Fang LQ, Gray GC, Lu B, Tong YG, Ma MJ and Cao WC, 2016. Novel H7N2 and H5N6 avian influenza A viruses in sentinel chickens: a sentinel chicken surveillance study. Frontiers in Microbiology, 7, 1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ren R, Yang L, Bao C, Wu J, Wang D, Li C, Xiang N, Wang Y, Li D, Sui H, Shu Y, Feng Z, Li Q and Ni D, 2017a. Sudden increase in human infection with avian influenza A(H7N9) virus in China, September‐December 2016. Western Pacific Surveillance and Response Journal, 8, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Tan Y, Kang M, Liu F, Ren R, Wang Y, Chen T, Yang Y, Li C, Wu J, Zhang H, Li D, Greene CM, Zhou S, Iuliano AD, Havers F, Ni D, Wang D, Feng Z, Uyeki TM and Li Q, 2017b. Preliminary epidemiology of human infections with highly pathogenic avian influenza A(H7N9) Virus, China, 2017. Emerg Infect Diseases, 23, 1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zhou J, Li Z, Yang L, Li X, Huang W, Zou S, Chen W, Wei H, Tang J, Liu L, Dong J, Wang D and Shu Y, 2017. Biological characterisation of the emerged highly pathogenic avian influenza (HPAI) A(H7N9) viruses in humans, in mainland China, 2016 to 2017. Euro Surveillance, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Secondary spread of HPAI during 2016/2017 epidemic crisis in Bulgaria
Secondary spread of HPAI during 2016/17 epidemics in France
Applied prevention and control measures on avian influenza Austria
Applied prevention and control measures on avian influenza Belgium
Applied prevention and control measures on avian influenza Bulgaria
Applied prevention and control measures on avian influenza in the Czech Republic
Applied prevention and control measures on avian influenza Denmark
Applied prevention and control measures on avian influenza France
Applied prevention and control measures on avian influenza Greece
Applied prevention and control measures on avian influenza Hungary
Applied prevention and control measures on avian influenza Ireland
Applied prevention and control measures on avian influenza Italy
Applied prevention and control measures on avian influenza The Netherlands
Applied prevention and control measures on avian influenza in Romania
Applied prevention and control measures on avian influenza United Kingdom


