Abstract
EFSA assisted four countries in the analysis of epidemiological data on African swine fever (ASF), collected until September 2017. The temporal analysis demonstrated that the average proportions of PCR and antibody‐ELISA positive samples from the hunted wild boar remained below 3.9 and 6.6, respectively. A peak in the ASF incidence was observed 6 months after the first observed case, followed by a significant reduction of the number of cases and low levels of African swine fever virus (ASFV) circulation at the end of 38 months follow‐up period at different spatial resolutions. The spatial analysis concluded that human‐mediated spread of ASFV continues to play a critical role in the ASF epidemiology, despite all measures currently taken. ‘Wild boar density’, ‘total road length’ (as proxy for human activity) and ‘average suitable wild boar habitat availability’ were identified as predictors for the occurrence of ASF in Estonia by a Bayesian hierarchical model, whereas ‘wild boar density’ and ‘density of pig farms’ were predictors according to a generalised additive model. To evaluate the preventive strategies proposed in EFSA's Scientific Opinion (2015) to stop the spread of ASFV in the wild boar population, a simulation model, building on expert knowledge and literature was used. It was concluded that reduction of wild boar population and carcass removal to stop the spread of ASFV in the wild boar population are more effective when applied preventively in the infected area. Drastic depopulation, targeted hunting of female wild boar and carcass removal solely implemented as measures to control ASF in the wild boar population need to be implemented in a highly effective manner (at or beyond the limit of reported effectivity in wild boar management) to sustainably halt the spread of ASF.
Keywords: African swine fever, epidemiology, risk factors, wild boar, management, prevention
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1312/full
Summary
In February 2016, the European Food Safety Authority (EFSA) was requested to assist the European Commission and the Member States (MSs) by collecting and analysing African swine fever (ASF) epidemiological data from the MS affected by ASF at the eastern border of the European Union (EU) in the context of Article 31 of Regulation (EC) No 178/2002. More in particular, EFSA was requested to analyse the temporal and spatial patterns of ASF; to analyse risk factors involved in the occurrence, spread and persistence of the ASF virus (ASFV) in the wild boar population and in the domestic/wildlife interface. Additionally, EFSA was requested to review the management options for wild boar, identified in the EFSA scientific opinion of June 2015, and indicate whether the conclusions of the latest EFSA scientific opinion are still pertinent.
To harmonise the collection of data from laboratory testing for ASF, the affected MS and EFSA developed a common data model in the EFSA Data Collection Framework (DCF), which collects samples at individual animal level data, from positive and as well as negative test results.
An extensive literature review was carried out to identify potential newly published scientific evidence pertaining the transmission and surveillance of ASFV in wild boar populations as well as the management and the ecology of wild boar in Europe since 2015. Important general conclusions of this review were that there is still a need for a better understanding of the wildlife population dynamics and for good baseline data on wildlife population trends to improve epidemiological analysis of wild boar diseases. Moreover, the currently observed wild boar population growth in the EU is unlikely to stop, unless changes in game management, specifically addressing feeding and baiting as well as an increased hunting harvest, take place at large geographical scales. In order to manage wild boar populations, the social context, the regional diversity and the capacity and willingness of hunters need to be integrated into policy. Pertaining ASF control, a recent study revealed that wild boar represent the highest contribution to scavenging in woodlands. The management implications are that the rapid detection and removal of contaminated carcasses is regarded as an important control measure against ASF in wild boar.
As part of the descriptive epidemiology, a short overview of the ASF situation in the EU MS and the eastern neighbouring countries of the EU were provided. ASFV continues to spread towards unaffected areas in the European territories. In 2017, ASFV has been reported in two new EU Member States, namely the Czech Republic and Romania, bringing the total of affected MS to six. New cases of ASF in wild boar continued to be reported in the Czech Republic at the time of writing this report.
The proportions of the positive samples (either tested by polymerase chain reaction (PCR) or enzyme‐linked immunosorbent assay targeting antibodies (AB‐ELISA)) of wild boar were calculated as the number of positive tested wild boar over the number of tested wild boar (either hunted or found dead). Both the average proportions of PCR and antibody positive samples from the hunted wild boar of Estonia, Latvia and Lithuania remained below 3.9 when tested by PCR and below 6.6 when tested by AB‐ELISA. The data of Poland were not included in the analysis of the PCR and antibody prevalences.
To evaluate the potential seasonality of PCR positive samples of wild boar (hunted and found dead) reported to the DCF by the Baltic States and Poland, the data were pooled and visualised in SAS and Loess smoothing was used in order to describe the global trends. A certain seasonality of the number of notifications in found wild boar was suggested again in the period September 2016–September 2017, however, this trend needs further investigation.
By ranking the minimum distance and time wild boar would need to bridge the closest case reports, it was concluded that human mediated spread of ASFV continues to play a critical role in ASF epidemiology, despite all measures currently taken. Also, a hot‐spot analysis using the hot‐spot analysis tool of ArcMap revealed that some hot spots are formed far beyond the distance that could be explained by spread of the disease through the wild boar population. Several clusters (hot spots) were observed, some of which with mean centres moving towards unaffected wild boar population areas with higher wild boar density over the last year.
The modelled evolution of the ASF incidence at different spatial resolution indicated a peak in the number of ASF cases around 6 months after the first case report in a given region. At the end of the follow up period of 38 months, a significant reduction of the number of cases was predicted, but at the same time the possibility for ASF to circulate at low levels in the spatial resolutions remained.
Two models were used for the risk factor analysis of ASF occurrence in Estonia. With the Bayesian hierarchical model, wild boar density, the total road length (as proxy of human activity) and the average suitable wild boar habitat availability were found to be significant predictors for the occurrence of ASF in the wild boar population in Estonia. According to the generalised additive model, wild boar density and the density of pig farms were predictors associated with the occurrence of ASF in the wild boar population in Estonia.
To evaluate the preventive strategies proposed in the EFSA opinion of 2015 (EFSA AHAW Panel, 2015) to stop the forward spread of an ASF epidemic in the wild boar population, a model building on expert and literature knowledge, including explanatory hypotheses about the ASF epidemiology (transmission, contact infection, role of the carcasses and population dynamics in the affected countries) was used (http://ecoepi.eu/ASFWB). The model has been constructed based on several assumptions, reflecting ad‐hoc expert discussions and preliminary laboratory insights regarding ASF transmission, perpetuation and maintenance by the wild boar host system in central Europe. These include the role of carcasses as a reservoir, late contact with dead animals, maternal antibodies in piglets from seropositive sows, artificial feeding, stringent and consistent application of measures, and exclusion of human‐mediated transmissions. This report, including the model simulations, will need to be updated if new scientific knowledge in contradiction to the assumptions used in the model becomes available.
From the model simulations, it was concluded that measures to reduce wild boar population to finally halt the expansion of ASFV are the most effective when applied in the regions outside or adjacent to already affected areas (treatment zone). Additionally, any carcass should be removed as fast as possible from the infected area as well as its surrounding areas. The width of these surrounding areas should take into account the local epidemiological situation of ASF, the artificial and natural barriers and the ecology of the wild boar.
Drastic depopulation, targeted hunting of female wild boar and carcass removal implemented as only measure to control ASF in the WB population need to be implemented in a highly effective manner (at or beyond the limit of reported effectivity in wild boar management) to sustainably halt the spread of ASF (confirming the scientific opinion of 2015 with the updated data).
There may be delayed contact of wild boar with carcasses from infected wild boar, as indicated by a recent publication, however, this phenomenon needs further investigation and is currently an important area of uncertainty in the model. In the scenario when late contact was applied in the model, the usefulness of carcass removal as measure to halt the spread of ASFV in the wild boar population increased. However, carcass removal only 2–6 weeks after death of the infected wild boar (median 4 weeks) would provide a very limited contribution to the success of control measures, even when the model assumed delayed contact of wild boar with carcasses of infected wild boars.
The model predicted a very limited effect of the simulated measures for a wild boar population density above 1.5/km² prior to reproduction. Early detection of ASFV entry might facilitate the implementation of very intensive, focal emergency measures, which should be differentiated from those applied in large spatio‐temporal scales studied in the model simulations.
Some recommendations were provided in the report, including the need to carry out more detailed modelling efforts using simulations on true landscapes with multiple habitat predictors to improve the understanding of the performance of the management measures. Additionally, it is recommended to develop standardised methods of wild boar density assessment, to facilitate epidemiological analysis on a regional scale.
Human‐mediated spread is still an important constraint that needs to be urgently addressed by intensified awareness building of all persons possibly in contact with infected wild boar or pigs of the different routes of spread of ASF and the economic and ecologic consequences of the disease.
Finally, it was suggested to evaluate the emergency measures, such as drastic depopulation and/or fencing applied in areas with focal ASFV entry in previously free wild boar populations, with existing empirical an epidemiological data.
1. Introduction
During the 12 months following August 2016, African swine fever (ASF) continued to spread in the eastern European region, both within the European Union (EU) and in a number of other European countries third countries. Within the EU, ASF is now present throughout the territory of Estonia, including the Saaremaa Island, new clusters have developed in Latvia and Poland, and there has been limited African swine fever virus (ASFV) circulation in Lithuania. In late June 2017, ASF was confirmed in wild boar in the eastern part of the Czech Republic, 440 km from other known cases, leading to approximately more than 100 confirmed cases over the following three months. In late July, ASF was notified in Romania, near the border with Ukraine. ASFV spread continued in a number of third countries, including Moldova, Georgia (South Ossetia), Russia and Ukraine. The ASF situation in Belarus remained unknown.
Over the last 8 years, the European Food Safety Authority (EFSA) has generated a series of scientific opinions and reports in support of the work of the Commission and affected Member States (MSs) to address the threat posed by ASF, including:
a review of current knowledge on ASF to the end of 2008 (Scientific review on African swine Fever, 2009),
a focus on the risk posed to neighbouring countries and the role of wild boar and vectors in the spread and maintenance of ASF (EFSA AHAW Panel, 2010b),
an update on the role of tick vectors in the epidemiology of ASF (EFSA AHAW Panel, 2010a),
an update on the significance of occurrence and risk of endemicity in neighbouring countries, and possible pathways of introduction (EFSA AHAW Panel, 2014),
a request for urgent scientific and technical assistance of possible mitigation measures to prevent the introduction and spread of ASF virus into the EU (EFSA, 2014),
detailed questions relating to the role of wild boar in the epidemiology and control of ASF (EFSA AHAW Panel, 2015), and
an analysis of the epidemic and of risk factors facilitating its spread (EFSA, 2017).
In this most recent scientific report, from February 2017, EFSA conducts a detailed analysis of epidemiological data from the Baltic countries and Poland during 2014–2016. The current report provides an update following further analysis of available epidemiological data from affected EU MSs.
1.1. Background
ASF is a contagious infectious disease affecting domestic pigs and wild boar. No vaccine exists to combat this virus. It does not affect humans nor does it affect any animal species other than members of the Suidae family.
From the beginning of 2014 up to 1/2/2016, Genotype II of ASF has been notified in the EU MSs Estonia, Latvia, Lithuania and Poland, causing very serious concerns. The disease has also been reported in Russia, Belarus and Ukraine, which creates a constant risk for the MSs bordering these third countries.
There is knowledge, legislation, and technical and financial tools in the EU to properly face ASF. EU legislation primarily targets domestic pig and addresses, when needed, lays down specific aspects related to wild boar. The main pieces of the EU legislation relevant for ASF are:
Council Directive 2002/60/EC1 of 27 June 2002 laying down specific provisions for the control of African swine fever and amending Directive 92/119/EEC as regards Teschen disease and African swine fever: it mainly covers prevention and control measures to be applied where ASF is suspected or confirmed either in holdings or in wild boars to control and eradicate the disease.
Commission Implementing Decision 2014/709/EU2 of 9 October 2014 concerning animal health control measures relating to African swine fever in certain Member States and repealing Implementing Decision 2014/178/EU: it provides the animal health control measures relating to ASF in certain Member States by setting up a regionalisation mechanism in the EU. These measures involve mainly pigs, pig products and wild boar products. A map summarising the current regionalisation applied is https://ec.europa.eu/food/sites/food/files/animals/docs/ad_control-measures_asf_pl-lt-regionalisation.pdf?.
Council Directive No 82/894/EEC3 of 21 December 1982 on the notification of animal diseases within the Community which has the obligation for Member States to notify the Commission of the confirmation of any outbreak or infection of ASF in pigs or wild boar.
The Commission is in need of an updated epidemiological analysis based on the data collected from the Member States affected by ASF at the Eastern border of the EU. The use of the EFSA Data Collection Framework is encouraged given it promotes the harmonisation of data collection.
Any data that is available from neighbouring third countries should be used as well.
1.2. Terms of Reference as provided by the requestor
Analyse the epidemiological data on ASF from Estonia, Latvia, Lithuania, Poland and any other Member States at the Eastern border of the EU that might be affected by ASF. Include an analysis of the temporal and spatial patterns of ASF in wild boar and domestic pigs. Include an analysis of the risk factors involved in the occurrence, spread and persistence of the ASF virus in the wild boar population and in the domestic/wildlife interface.
Based on the findings from the point above, review the management options for wild boar identified in the http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.4163/epdf and indicate whether the conclusions of the latest EFSA scientific opinion are still pertinent.
1.3. Interpretation of the Terms of Reference
This report analyses:
new information on the virus properties and wild boar ecology (extensive literature search);
the temporal and spatial patterns of ASF in wild boar and domestic pigs, in the Baltic States and Poland in the period January 2014–September 2017;
long‐term trends in the proportions of positive samples from wild boar in the Baltic states and Poland tested by polymerase chain reaction (PCR) and enzyme‐linked immunosorbent assay targeting antibodies (AB‐ELISA);
the risk factors involved in the occurrence of the ASF virus in the wild boar population, including the domestic/wildlife interface;
the effectiveness of wild boar management option identified in the http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.4163/epdf (EFSA AHAW Panel, 2015; term of reference 2).
The conclusions and recommendations are based on analyses of epidemiological and laboratory data collected by Estonia, Latvia, Lithuania, Poland, the Czech Republic and Romania in the period January 2014–September 2017.
2. Data
2.1. Extensive literature review
The following databases have been screened (Table 1) for possible publications in 2015–2017, pertaining to the transmission and surveillance of ASFV in wild boar populations (Search 1) as well as the management and ecology of wild boar in Europe (Search 2).
Table 1.
Screened databases for extensive literature review
| Name | Platform | Time span |
|---|---|---|
|
Web of Science Core Collection Science Citation Index Expanded (SCI‐EXPANDED) Social Sciences Citation Index (SSCI) Arts & Humanities Citation Index (A&HCI) Conference Proceedings Citation Index‐Science (CPCI‐S) Conference Proceedings Citation Index‐Social Science & Humanities (CPCI‐SSH) Book Citation Index–Science (BKCI‐S) Book Citation Index–Social Sciences & Humanities (BKCI‐SSH) Emerging Sources Citation Index (ESCI) |
WOSa | 2015–2017 |
| BIOSIS Citation Index | WOS | 2015–2017 |
| CABI: Cab Abstracts | WOS | 2015–2017 |
| Chinese Science Citation Database | WOS | 2015–2017 |
| Current Contents Connect | WOS | 2015–2017 |
| Data Citation Index | WOS | 2015–2017 |
| FSTA | WOS | 2015–2017 |
| KCI‐Korean Journal Database | WOS | 2015–2017 |
| Medline | WOS | 2015–2017 |
| Russian Science Citation Index | WOS | 2015–2017 |
| SciELO Citation Index | WOS | 2015–2017 |
| Zoological Record | WOS | 2015–2017 |
| Pubmed | NLM | 2015–2017 |
| Scopus | Scopus | 2015–2017 |
WOS: Web of Science.
2.2. Descriptive epidemiology and study of the evolution of the incidence
2.2.1. ASF notifications
Data on ASFV detections in wild boar and domestic pigs reported between 24 January 2014 and 30 September 2017 were extracted from the Animal Disease Notification System (ADNS). The numbers of ASF outbreaks in domestic pigs and wild boar cases are presented in Table 2.
Table 2.
Number of African swine fever (ASF) outbreaks in domestic pigs and cases in wild boar notified to the Animal Disease Notification System from 24 January 2014 until 22 September 2017
| Country | Outbreaksa in domestic pigs | Casesb in wild boar | |
|---|---|---|---|
| Found dead | Hunted | ||
| Czech Republic | 0 | 105 | 1 |
| Estonia | 27 | 3,444c | |
| Latvia | 52 | 1,932 | 1,505 |
| Lithuania | 68 | 1,537 | 311 |
| Poland | 98 | 657c | |
| Romania | 2 | 0 | |
An outbreak of ASF in domestic pigs refers to one or more cases detected in a pig holding.
A case of ASF in wild boar refers to any wild boar or wild boar carcass in which clinical symptoms or post‐mortem lesions attributed to ASF have been officially confirmed, or in which the presence of the disease has been officially confirmed as the result of a laboratory examination carried out in accordance with the diagnostic manual.
Found dead/hunted not specified.
2.2.2. Sample‐based data
The data on ASF tests from the Laboratory Information Management System (LIMS) of the national laboratories of the Baltic States and Poland have been collected in the DCF (see the previous report). The data reported to the DCF by the different MSs contained the information on samples tested for ASF in the following periods:
Estonia: January 2014–July 2017
Latvia: January 2014–July 2017
Lithuania: January 2014–September 2017
Poland: January 2014–July 2016
As of July 2017, information about 257,305 tests for ASF, including 85,697 tests of domestic pig samples and 173,594 wild boar samples, have been collated in the DCF (Figure 1).
Figure 1.

Number of tests for African swine fever, January 2014–July 2017, submitted by the Member States to the Data Collection Framework
Samples were tested for ASF using PCR (testing for virus), and AB‐ELISA, immunoblotting (IB), and immunoperoxidase (IPT) tests for antibodies.
The geographical distribution of samples taken from wild boar (January 2014–September 2017) reported to the DCF is shown in Figure 2, illustrating the heterogeneous sampling intensity in the different administrative units.
Figure 2.
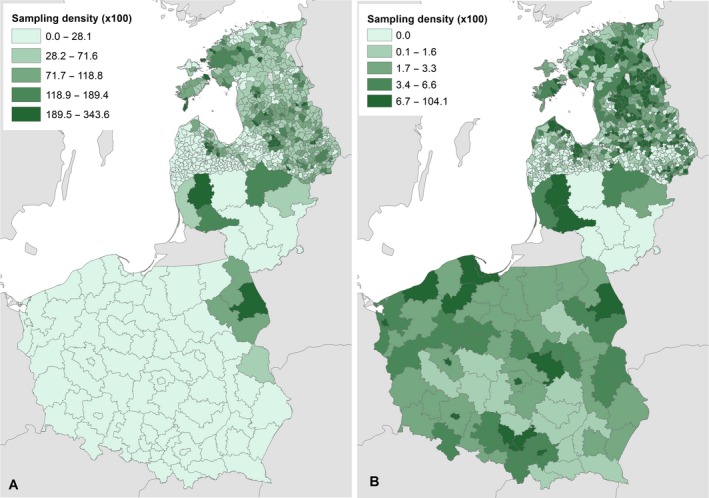
The ratio of number of wild boar tested per square km during 2014–2017 at the different available administrative unit levels in Estonia, Latvia and Lithuania
- A: hunted wild boar; B: wild boar found dead. (Source: Data Collection Framework).
The data contain:
the date and location of samples (LAU*1, LAU 2 or exact location: longitude and latitude)
the age and sex of animals, mostly for the years 2016 and 2017
Decomposition of the carcass
Testing method
* LAU ‐ Local Administrative Level
2.3. Risk factor analysis
In this report, the available datasets from Estonia – because they were the most complete – were used to evaluate potential risk factors for the occurrence of the ASF virus in the wild boar population and at the domestic/wildlife interface. Information on the exact location of samples taken was provided, for both positive and negative results.
The following were considered as potential risk factors: quality of available wild boar habitat (average); wild boar density (estimated number/km2); number of pig farms; number of pigs; number of small pig farms (up to 10 head); number of pigs in small pig farms; human settlement density/km2, total road length (all types, km)/admin unit; and human population density (ind./km2).
2.3.1. Environmental data and regional roads
A raster map of the quality of available habitats (QAHs), developed by CISA‐INIA (Spain), was used. QAH was estimated using a seven‐level scale based on expert opinion and found to correlate closely with the georeferenced presence of wild boar (n = 22,362): the highest wild boar densities (74.47% of the total wild boar population) were found in areas with the two highest QAH levels, while the lowest densities (5.66% of the total wild boar population) were found in areas with the lowest QAH levels. A detailed description of the methodology to establish QAH can be found in Bosch et al. (2016).
The average QAH was calculated based on the raster inputs for each of the spatial regions considered using the zonal statistics tool of the ArcMap software (ESRI). The shape files of the roads were obtained from the website of the GIS‐LAB Project specialising in geographic information systems (GIS) (http://data.nextgis.com/osmshp, last accessed 1/9/2017). The total lengths of all types of roads were measured for each administrative unit and used as an indicator of human activity.
2.3.2. Demographic data and density of settlements
The 2015 data on the human population at district (LAU 2) level were extracted from the official website of the national statistic institution of Estonia: Statistics Estonia (http://www.stat.ee, last accessed 1/8/2017).
The locations of settlements were obtained from the website of the GIS‐LAB Project (available on: http://gis-lab.info/qa/osmshp.html, last accessed 1/9/2017) as shape files.
2.3.3. Susceptible population data
Domestic pig population distribution
Data on the domestic pig population and its distribution were provided by the Estonian Agricultural Registers and Information Board (ARIB) (http://www.pria.ee/en). Table 3 provides a summary of the type of data made available to EFSA for the assessment. The number of small pig farms (< 10 head) has been used as a potential risk factor as it was assumed that these small farms would often implement suboptimal biosecurity measures.
Table 3.
Data items provided by the relevant Member States on pig population and distribution
| MS | DATA | Spatial resolution | Years | Temporal resolution |
|---|---|---|---|---|
| Estonia | Farms/holding | Longitude and latitude | 2014–2017 | Yearly |
| Number of pigs in the holding | ||||
Wild boar population distribution
The size of wild boar populations (based on estimates from the national hunters’ organisations of the population size in the springs of 2014–2017) and the wild boar density (individuals/km2) were provided by Estonia (Estonian Environment Agency), Latvia (State Forest Service) and Poland at ‘hunting ground’ level (Appendix A) and LAU 2 level for Lithuania (see Appendix A).
The data provided by Estonia also include the yearly numbers of hunted wild boar, wild boar road kills and wild boar found dead, as well as the age and sex of the wild boar. It was assumed that the distribution of wild boar was homogeneous within each hunting ground region, and that wild boar locations were randomly generated within each hunting ground that was later used to estimate wild boar density at higher spatial resolution scales.
2.3.4. Aggregation of data
All risk factors considered were aggregated spatially on the basis of the shape file of the administrative units at LAU 2 level. Table 4 lists the risk factors considered.
Table 4.
Potential risk factors based on the available data used in the analysis
| Acronyms | Description | Explanation |
|---|---|---|
| Potential risk factors related to wild boar habitat | ||
| WBEstDens | Wild boar density (estimated number/km2) | Wild boar density could have an effect on the occurrence of the disease |
| QAH | Quality of available habitat of wild boar (average) | Habitat quality could drive wild boar density |
| Potential risk factors related to the pig farming system | ||
| NumPgFrms | Number of pig farms |
Pig density could have an effect on the occurrence of the disease (assuming circulation in domestic pigs) Pig density could have an effect on the occurrence of the disease (assuming circulation in domestic pigs) |
| NumPgs | Number of pigs per admin unit | |
| NmPgFrms1.10 | Number of small pig farms (up to 10 head) | Small pig farms are assumed to have lower biosecurity measures in place, which could have an effect on the occurrence of the disease |
| NumPgs1.10 | Number of pigs in small pig farms | |
| Potential anthropogenic risk factors | ||
| StlmntDens | Human settlements density/km2 | A higher human activity in an area could have an effect on the occurrence of the disease |
| RdLength | Total road length (km)/admin unit | |
| HPDens | Human population density (ind./km2) | |
The proportion of the number of pigs was calculated (NumPgsPrp) as well as the proportion of the human population (HPPrp) for each administrative unit (max num/x) to normalise the numbers to the natural values.
3. Methodologies
3.1. Extensive literature review
An extensive literature review was carried out to identify potential new scientific evidence pertaining both to the transmission and surveillance of ASFV in wild boar populations (Search 1) as well as the management and the ecology of wild boar in Europe (Search 2) that had been published since the previous Scientific Opinion of the AHAW Panel on African swine fever (EFSA AHAW Panel, 2015). Two specific electronic searches were performed in the Web of Science, Scopus and Pubmed 2015–2017 platforms, using the search strings as detailed in Appendix A.3. After removal of duplications between databases and searches, 920 publications were obtained and their relevance checked by two independent reviewers based on screening titles and abstracts. The full text of the remaining 100 potential relevant studies was then classified by the working group according their main topics (virus characteristics, monitoring and control strategies, ASF epidemiology and wild boar ecology), and the working group drafted a narrative section using information identified in 50 of these papers that they considered new and relevant.
3.2. Descriptive Epidemiology
3.2.1. Update of the ASF situation in eastern Europe
A short narrative section is given summarising the information reported to the ADNS and provided by the affected MSs.
3.2.2. Temporal distribution
The temporal analysis was carried out using SAS and R software.
The percentages of the positive samples (either tested by PCR or AB‐ELISA) were calculated as the number of positive tests divided by the total number of tested animals (either hunted or found dead) in a given period of time (i.e. monthly).
LOESS smoothing (Cleveland, 1979) was used to estimate average profiles describing the global trends of the PCR‐ or ELISA‐positive samples, smoothing out potential random fluctuations. Confidence bands are also presented to show uncertainties in the estimation of the smoothing curves.
To evaluate the potential seasonality of PCR‐positive samples from wild boar (hunted and found dead) reported to the DCF by the Baltic States and Poland, the data were pooled and visualised in SAS.
3.2.3. Spatial distribution
The spatial analysis was carried out using ArcMap (ESRI) and Quantum GIS (http://www.qgis.org)
3.2.3.1. Identification of hot spots
To describe the spatial distribution of the disease and potentially identify hot spots, the study area was partitioned into a regular grid of 7,779 hexagons, each with a 5‐km diameter. All wild boar cases reported to the ADNS between 2014 and 2015, 2015 and 2016, and 2016 and 2017 were included in this analysis. Counts of wild boar cases were aggregated to the hexagon level and analysed. The outcome variable was the number of cases reported in the wild boar population in each hexagon during the study periods. A hot‐spot analysis was performed using the Hot Spot Analysis (Getis‐Ord Gi*) tool of ArcMap (Spatial Statistics toolboxes, ESRI). This tool identifies significant spatial clusters of high values (hot spots) and low values (cold spots) using the Getis‐Ord Gi* statistic (http://pro.arcgis.com/en/pro-app/tool-reference/spatial-statistics/h-how-hot-spot-analysis-getis-ord-gi-spatial-stati.htm).
3.2.3.2. Evaluation of potential human‐mediated spread
To evaluate the possible human‐mediated spread of ASF, the cases reported to the ADNS were individually taken and assigned by two measured values: first, the distance to the closest case report older than seven days was determined (distance); second, for each case, the report older than 7 days that required the minimum velocity to bridge the distance between the two cases was identified. Then, the velocity and distance values were ranked. The resulting rank sum was noted for each case report (minimising distance and time between two reported cases). Finally, the geographical maps of all recordings were coloured according to the percentile in which the values fell in the ordered distribution of (i) distances and (ii) rank‐sums. In particular, the upper 1% of values (i.e. 99–100th percentile) were marked on the map.
3.2.3.3. Study of the evolution of ASF incidence at different spatial resolution
To evaluate the evolution of the incidence of ASF after its first detection in a region, the number of cases reported through the DCF was used in the analysis. The data were arranged considering the sampled region, sampling date and test result (a sample was considered positive if any of the tests used were positive). In order to model the number of cases in a region, a starting time was considered as the date on which a positive sample was reported for the first time in that specific region (being defined as LAU1, LAU2 and hunting ground, in increasing order of spatial resolution). Results from any of the tests performed were considered, given that all regions have the same chance to contribute with their seropositive reports and the effects are expected to average out. This is confirmed when the analyses were performed considering only reports from PCR testing, when similar trends were observed. The observed regional profiles and the LOESS smoothing are presented. LOESS smoothing (Cleveland, 1979) was used to estimate smoothing average profiles describing the global trends, smoothing out potential random fluctuations. Confidence bands were also presented in order to show uncertainties in the estimation of the smoothing curves.
The number of positive results was calculated based on different temporal resolution (daily, weekly, monthly and bimonthly). A generalised additive model (GAM) (Wood, 2006) was used to estimate the incidence, including the number of positive cases as a random variable that follows a Poisson distribution, and the expected value was modelled using the logarithmic link. The temporal evolution was modelled using the default basis for the smoothing function in the package mgcv in R (a thin plate regression spline). The model also included additional random effects associated with the regions (LAU1, LAU2 and hunting grounds) to allow for different starting points for each region as well as potential temporal evolution.
3.3. Risk factor analysis
3.3.1. Bayesian Hierarchical Model
Statistical models within the Bayesian paradigm that deal with data collected across space (i.e. different LAU2 regions) and possibly over time (i.e. different years) were used. The analysis of such data types takes into account the spatial and/or temporal dependence of the observations. The linear component of the spatio‐temporal model for the binary data for presence of ASF (ASF status, time, location) can be written including a random effect accommodating temporal dependence, and another one to account for spatial dependence, as well as the possibility of including potential interactions between space and time. Therefore, the Besag, York and Mollié (BYM) model was fitted to the spatial effect. The BYM model takes into account not only the spatial autocorrelation present in the data, but it also assumes that the estimates obtained between areas are independent of each other. The spatial effect of the BYM model assumes that the expected value of each area depends on the values of the neighbouring areas (areas sharing boundaries). Thus, areas close together are considered to be more similar than areas that are far apart. In this application, it was assumed that the structured and unstructured effects are not independent of each other as described by Riebler et al. (2016). Therefore, the model was written considering a mixture formulation in which it reduces to pure over dispersion (spatially unstructured) if the mixture parameter is estimated to be 0, or to the intrinsic conditionally autoregressive (ICAR)/Besag model when the mixture parameter is equal to 1. This means that the proportion of the marginal variance explained by the spatial effect is given by the mixture parameter. Details about the model used can be found in the Zenodo repository: https://doi.org/10.5281/zenodo.889551 (Manual of WEB APP to run spatio‐temporal models, page 14). The backward model‐building procedure was used, where the first model fitted was the one containing all risk factors available and was reduced by excluding non‐significant risk factors (one at a time) until a model containing only significant risk factors is reached.
3.3.2. Generalised additive model
A GAM, which is a linear model that allows for response distributions other than normal and whose linear predictor involves smoothing functions, was also used. The model allows for a rather flexible specification of the dependence between response and non‐responses. The GAM spatio‐temporal model includes a term that models the space as well as time patterns (considered here to be a saturated effect, in which each time point has its own estimate). For each of the measured risk factors, a specific linear effect was added to the model to study its effect on the probability of observing a positive case in a particular LAU2 region. The spatial correlation was approximated using a Markov random field (MRF) smoother. This smoother was defined by the LAU2 regions and a neighbourhood structure for the specific LAU2 region. A full‐rank MRF with a coefficient for each LAU2 region was constructed. The R‐package mgcv was used where estimation was based on quadratically penalised (possibly quasi‐) likelihood maximisation. More information on the GAM can be found in Wood (2006). The backward model selection procedure was also used here, and the model containing the significant risk factors from the Bayesian hierarchical modelling process was fitted to compare both models outputs.
The Bayesian Hierarchical Model and GAMs were fitted on a yearly basis to study the effect of potential risk factors on the probability of reporting ASF‐positive cases in a given region, and how they might change over time.
3.4. Assessment of wild boar management options
The main interest of the assessment concerns the effect of applied measures in halting the spatial spread of an already existing ASF epidemic (e.g. in the Baltic MSs) and the likelihood of eradicating the infection in time once the measures are applied. The situation in which new cases are reported in distant areas (e.g. the Czech Republic) followed by a possible localised emergency measure are not in the scope of this assessment. (See Lange and Thulke (2015) for spatial dimensions of potentially successful approaches using localised emergency depopulation and mobile fences).
The objective of the simulation study was to evaluate the preventive strategies proposed in the EFSA opinion of 2015 (EFSA AHAW Panel, 2015) to stop the forward spread of an ASF epidemic in the wild boar population. The strategies were tested in an expert‐system model building, on expert and literature knowledge, including explanatory hypotheses about ASF epidemiology (transmission, contact infection, role of carcass, population dynamics in the affected countries). The complete model documentation published with the EFSA output in 2015 is still valid (minor adaptions of parameters explicitly documented at http://ecoepi.eu/ASFWB).
Human interferences leading to non‐biological forward transmission of ASFV (i.e. cases without links to past reports of ASF in wild boar, considering biologically plausible movement behaviour of ASFV in the wild boar population in time and space) were not considered in this analysis. Their unpredictable nature can lead to the occurrence of ASF cases in wild boar (e.g. in the Czech Republic) and outbreaks in domestic pigs (e.g. in Romania) that are several hundred kilometres away from previous ASF reports.
The present study addressed the following questions:
Does the newly available knowledge or parameterisation change the simulation outcome of the EFSA opinion of 2015?
Do the measures actually implemented in the MSs meet the proposed target to halt ASF spread and facilitate final eradication over time?
Can the proposed option of intensifying carcass removal, as suggested in the EFSA 2015 opinion, be supported, given new insights about the practical difficulties relating to carcass removal and possible duration of carcass decomposition?
Are the investigated strategies realistic in situations where population capacities in wild boar habitats are similar to those in the region to the west of the affected area?
In order to address the objectives, corresponding simulation scenarios were implemented.
The scenarios are:
Two alternative methods of population management, i.e. short‐term drastic depopulation within 16 weeks [scenario (sc) D1] vs so‐called soft measures targeting females with standard hunting efforts over many years [sc D0];
Combine D1 or D0 with carcass removal [CR] within n weeks of an animal's death [CRnw; n = {1,2,4}]. The scenarios include the MSs’ expert input on carcass removal times of 2–6 weeks, with a median of 4 weeks;
Two alternative expert views regarding time of first carcass contact by wild boar from the surviving cohort, i.e. contacts with the carcass are simulated immediately after death of the individual as in the 2015 Scientific Opinion [sc Default] vs. contacts delayed by two weeks as suggested by recent field experiments (Probst et al., 2017) [sc Delayed]. In 2015, it was assumed that carcasses are immediately contacted by living wild boar. A recent observational study may imply that the fresh cadavers are avoided until they undergo a certain degree of decomposition (Probst et al., 2017). This hypothesis is implemented by discarding contacts with infectious carcasses for two weeks post mortem;
Two alternative scenarios of carcass remediation time, i.e. constant [sc Default] vs. seasonal [sc Seasonal]. Seasonal carcass decomposition rates were used to study the effect of the expert hypothesis about seasonal differences in temperature affecting decomposition rates (Figure 3B);
Three alternative population densities prior to the appearance of ASF using the scaling factors (Sf) 1, 2, 4 relative to the local density proposed by the affected MSs i.e. Baltic expert estimate, doubled, quadrupled. The scenario is also applicable to representing alternative area sizes for wild boar groups (home‐range hypothesis) or landscape‐related differences in average group size (aggregation hypothesis);
-
It was necessary to simulate the above scenarios for a range of parameter values if there was insufficient data or expert knowledge available to address the associated uncertainty. The following parameter variations were systematically performed:
Width of the treatment zone (blue part in Figure 3A) in front of ASF (50 km; 100 km; 200 km). This parameter specifies the dimension of the area in which preventive measures are simulated outside of the area already infected (grey part in Figure 3A).
-
Efficiency of proposed measures in terms of percentage of the targeted endpoint achieved (one short‐term depopulation, continued female reduction, carcass removal). The parameter variations were necessary because it is not fully understood how effectively the measures could be implemented in practice. The outcome of the simulations will therefore provide ranges of success dependent on the value of these parameters.
Depopulation [D1]: Per cent effective (30%, 50%, … 90%) depopulation within a single campaign of 4 months (no repetition);
Targeted Hunting [D0]: Per cent effective (30%, 50%, … 90%) reduction of next‐season reproductive females using unchanged hunting bag size to represent standard hunting pressure;
Carcass removal [CR]4 : Per cent effective (0%, 30%, 50%, … 90%) carcass removal within a certain time after death.
Figure 3.
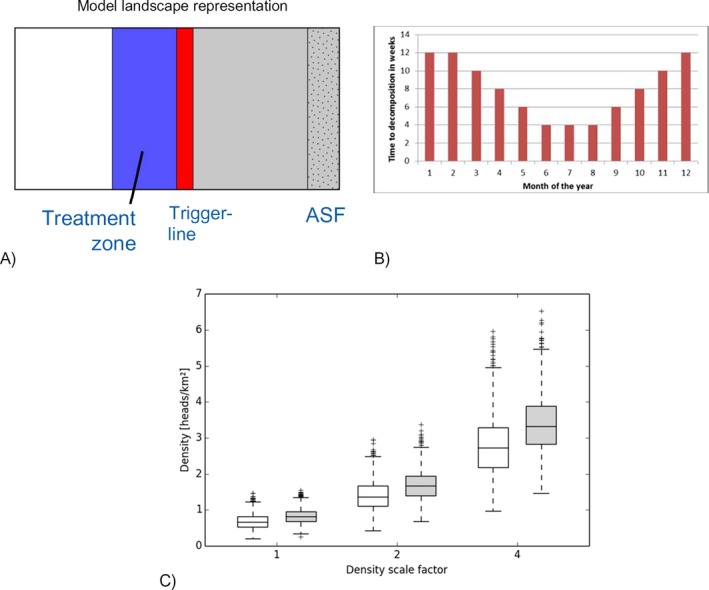
Simulation design and model input distributions
- A: Schematic representation of the spatial landscape of the simulations. African‐swine‐fever‐infected animals enter from the right and continue to spread to the left. Once the simulated spatial spread reaches the Trigger‐line, a prescribed control measure is applied to the blue zone. Width of the Treatment zone (blue) was simulated as 50 km, 100 km and 200 km. Source: EFSA AHAW Panel (2015); B: Seasonal distribution of carcass decomposition varying between 4 and 12 weeks. The function approximates observed decomposition speed and is only indirectly dependent on temperature; C: Population density (y‐axis) in the future control area (blue box in Figure 3A) for the different density scenarios (x‐axis) calculated from the model as emerging from the habitat capacity maps in week 1 (white) and week 26 (shaded) of the calendar year. Variability is mainly due to volatile population dynamics. The graphs show the median (middle line), 26–75% range (box), 1.5 inter‐quartile range (whiskers) and outliers (crosses).
Model simulations are performed on the same landscape as used and described in the 2015 EFSA opinion (Figure 3A). ASF spreads from the right border through the grey area until the infection reaches the trigger line (red). From that moment, the control measures as foreseen by a given scenario are applied according to the associated schedule (once or permanently) within the treatment zone (blue). The recorded output is the time at which the infection is eradicated (success) or breaks out of the treatment zone (failure, 50, 100 or 200 km from the treatment zone, respectively).
4. Assessment
4.1. Extensive literature review
4.1.1. Update on African swine fever virus characteristics
4.1.1.1. Identifying markers for genetic variability
Although the ASF virus is considered a slowly evolving DNA virus, it is known that there are some genetic regions with a level of heterogeneity. Of particular interest are genes related to inhibition or modulation of the infected animal's immune response, including alteration of interferon production by multigene family protein (MGF505‐2R), inhibition of NF‐κB and nuclear activating factor in T cells by the A238L protein, and modulation of host defence by CD2v lectin‐like protein, encoded by the EP402R and EP153R genes (Frączyk et al., 2016). The CD2v transmembrane potential is required for haemadsorption of red blood cells around infected macrophages and for the adhesion of extracellular virions to erythrocytes and consequent viral dissemination. Further, it is known that expression of the CD2v protein enhances virus replication in the tick vector (Sanna et al., 2017).
Several authors have investigated the genetic variability of these genes, seeking a means to discriminate between closely related viruses. Among ASF isolates collected from wild boar and domestic pigs in eastern Poland during 2014/15, Frączyk et al. (2016) observed a slow but consistent evolution of EP402R and MGF505‐2R, and a common origin with highly pathogenic isolates Georgia 2007/1 and Odintsovo 2/2014. Similarly, based on a sequence comparison with the protein encoded by the EP402R gene, Sanna et al. (2017) observed a temporal division of ASFV strains collected in Sardinia from 1978 to 2014, but no differences in isolates recovered from wild boar and domestic pigs. Critically, Frączyk et al. (2016) concluded that the spatio‐temporal analysis of cases aligned with phylogenetic data offered a limited possibility of tracing the molecular evolution of the ASF virus. Similarly, Sanna et al. (2017) proposed the CD2v protein as a new genetic marker that could be used to analyse ASFVs from different locations to track virus spread. They did note that whole‐genome sequence analysis could assist in identifying additional ASFV genetic markers capable of discriminating between circulating viruses, thereby facilitating a better understanding of ASFV evolution and epidemiology worldwide.
In a larger study investigating ASF isolates maintained in a collection at the National Research Institute for Veterinary Virology and Microbiology (VNIIVViM) in Pokrov, Russia, Malogolovkin et al. (2015) described the genetic typing of isolates based on nucleotide sequencing of B646L, the p72 capsid protein gene, and the relationship of these results to the haemadsorption inhibition assay (HAI) serological classification. Using this approach, the authors found that the p72 ASFV phylogenetic analysis does not accurately define ASFV HAI serogroups, and concluded that conventional ASFV genotyping cannot discriminate between viruses of different virulence or predict the efficacy of a specific ASFV vaccine.
4.1.1.2. Clinical presentation and post‐mortem findings
Karalova et al. (2015) described clinical and post‐mortem investigations of ASF infections induced by intramuscular injection of highly pathogenic ASF genotype II virus, the strain currently in Europe. They observed rapid development of clinical signs and post‐mortem findings generally in agreement with changes earlier described for ASF, but concluded that the severity of the disease was likely to have increased as a consequence of the route of administration. Tauscher et al. (2015) similarly emphasised the clinical signs and post‐mortem findings from acute ASF cases, and outlined appropriate sample materials (serum, blood, spleen) that should be collected from domestic pigs with unspecified clinical signs or pathomorphological findings.
4.1.1.3. Stability of ASFV in body excretions
The shedding and stability of ASFV in faeces, urine and oral fluid from pigs infected with the Georgia 2007/1 ASFV isolate has been assessed (Davies et al., 2017). The half‐life of infectious ASFV in faeces was found to range from 0.65 days at 4°C and 0.29 days at 37°C. In urine, the range was from 2.19 days (4°C) to 0.41 days (37°C). Based on these half‐lives and the estimated dose required for infection (10 HAD50), faeces and urine were reported to remain infectious for 8.5 and 15.33 days at 4°C and 3.71 and 2.88 days at 37°C, respectively. The half‐life of ASFV DNA was 8–9 days in faeces and 2–3 days in oral fluid at all temperatures. In urine, the half‐life of ASFV DNA was found to be 32.54 days at 4°C, decreasing to 19.48 days at 37°C. The results indicate that urine is a more stable medium for ASFV than faeces or oral fluids.
The authors concluded that body excretions containing ASFV may be an important route of ASFV transmission. The results of a challenge experiment conducted in Germany, where it was shown that weak or runt animals may need very low virus doses (< 10 HAU) to become infected via oral and nasal routes, support that conclusion (Pietschmann et al., 2015).
4.1.2. Update on monitoring and control strategies
4.1.2.1. Hunting as a means to control wild boar populations in the absence of ASF
Recreational hunting, as currently carried out in Europe, is not controlling wild boar population growth (Keuling et al., 2013; Massei et al., 2015; Quirós‐Fernández et al., 2017). Moreover, the mean age of hunters is growing and their numbers are generally declining (Massei et al., 2015; Quirós‐Fernández et al., 2017). Also, hunters often perceive the hunting of piglets as not sporting, and hunting adult females as unethical, while they generally support feeding. This creates conflicts with other stakeholders, including farmers (Brondum et al., 2017; Storie and Bell, 2017).
However, increased recreational hunting (Quirós‐Fernández et al., 2017) and intense culling (Gürtler et al., 2017) can eventually reduce wild boar abundance to tolerable levels, particularly in the absence of feeding and other human‐driven interferences. The difficulty is convincing the hunters and the hunting organisations to contribute to wild boar control through increased hunting pressure, as most hunters do not feel responsible and see the control of wild boar as ‘somebody else's problem’ (Keuling et al., 2016).
The management implications are that the social context and the regional diversity in situations and in hunters’ willingness and capacity to manage wild boar will have to be incorporated into management policy (Keuling et al., 2016; Storie and Bell, 2017).
4.1.2.2. Hunting as a means to control wild boar populations in the presence of ASF
Currently, no new scientific evidence has been published on the effectiveness of the applied control measures on wild boar populations in the eastern EU MSs listed in Appendix A.2.
4.1.2.3. Other disease control means, considering ASF and wild boar
To enable disease control in the case of shared infections, there is a need for a better understanding of wildlife population dynamics and for good baseline data on wildlife population trends. Interventions regarding wildlife diseases are rarely widespread and not necessarily successful. Three aspects need regulation to improve wildlife health: wildlife feeding, disease control in farmed or translocated wildlife, and hunting offal disposal (Gortázar et al., 2016).
The effectiveness and practicality of control strategies for ASF in wild boar has been preliminarily assessed, with carcass removal rated as the most effective intervention strategy, but also considered the least practicable (Guinat et al., 2017).
The management implications are that there is limited experimental evidence regarding the success of interventions against ASF in wild boar. Trials are currently taking place including changes in hunting or culling, setting up of (temporary) barriers, carcass destruction and improved farm biosafety. Research will be required to identify the best combination of these and other available disease control tools.
4.1.3. Update on ASF epidemiology
4.1.3.1. Transmission experiments and estimation of transmission parameters
Several ASFV transmission studies have been conducted by different research groups in recent years involving various virus strains and experimental conditions.
Loeffen et al. (2015) conducted a study to estimate quantitative parameters for ASFV transmission by carriers (survivors commingled with susceptible pigs) and to elucidate the possible role of indirect virus transmission (repopulating contaminated pens with sentinel pigs). The moderately virulent ASFV strain Netherlands ‘86 was used in this study (Loeffen et al., 2015). Transmission rate parameters (β) were estimated for defined study periods. This experiment demonstrated that the transmission of the virus from surviving pigs to susceptible animals had an estimated overall transmission rate in surviving pigs of 0.015/day. None of the sentinel pigs in the four contaminated pens became infected during the 14‐day observation period.
The authors concluded that the average reproduction ratio (R) of the virus in the carrier phase was approximately 0.6 for any carrier in direct contact with one naïve pig at any time during the infectious period. However, the authors suggested that, while it might be a rare event, the carrier animals may have a role in maintaining the infection in wild boar populations (Loeffen et al., 2015).
During the phase of the epidemic when acutely infected animals are shedding ASFV, the epidemiological role of these infected survivors is likely minor.
As no infections were observed in the sentinel pigs in the contaminated pens, the authors suggested that indirect virus transmission through the environment may be difficult to achieve for ASFV. Indirect transmission between pig herds was therefore likely to play a minor role and the disease may be relatively easy to control and eradicate under those circumstances.
In contrast, in a study conducted by Pietschmann et al. (2015) with the highly virulent ASFV genotype II strain from Armenia, no indication of a prolonged or chronic course of infection was found either in domestic pigs or wild boar. However, moderate contagiousness of the infection was also observed in this experiment (the R0 within pen ranged from 5.0 to 6.1, and between pen was 0.5) (Pietschmann et al., 2015). These estimates coincided with the results of Guinat et al. (2016a) who estimated the pig‐to‐pig R0 for the Georgia 2007/1 ASFV strain using data obtained from another challenge experiment. The models showed that the pig‐to‐pig R0 was 5.0 (95% CI 2.4–9.1) and between pen 2.7 (95% CI 0.7–5.2) (Guinat et al., 2016b). Nielsen et al. (2017) recalculated these transmission parameters taking into account that during the challenge experiment animals were only tested every other day, and obtained similar point estimates for parameters but somewhat different confidence intervals (Nielsen et al., 2017).
4.1.3.2. Survival of infected pigs
In a challenge experiment to study the impact of ASF on blood parameters in pigs of different ages (groups of 12‐week and 18‐week‐old pigs) and infected with different doses of ASF virus of moderate virulence, the survival rate was 30%. Animals that did survive infection were generally older, irrespective of the inoculation dose used (Post et al., 2017).
4.1.3.3. Tick competence studies
A Portuguese study demonstrated that the tick vector Ornithodoros erraticus sensu stricto is highly likely to transmit the two Portuguese ASF viruses of different host origins and that, in field surveys, analysis of tick adults and 5th nymphal stages provides the best chance of detecting virus infection. The results also indicate that infection of pigs with highly virulent ASF viruses will promote higher rates of tick infection and a higher likelihood of virus transmission. There is also a somewhat lower risk that ticks can become infected by pigs that have overcome the acute phase of infection (Ribeiro et al., 2015).
4.1.4. Update on wild boar ecology
4.1.4.1. Wild boar behaviour in relation to wild boar carcasses or wild boar hunting
A recent study in northern Germany, based on camera‐trapping, recorded the behaviour of wild boar towards wild boar carcasses (Probst et al., 2017). The authors state that ‘wild boar seemed to be particularly interested in the soil next to and underneath the carcasses’, while ‘there was no evidence for intra‐species scavenging’. However, about one‐third of the visits of wild boar led to direct contact with dead conspecifics, consisting in sniffing and poking on the carcass. Given the tenacity of ASFV, the authors assume that all these types of contact do represent a risk of ASFV transmission.
A second manuscript is still in evaluation (Carrasco‐Garcia et al., in press). Consumption of big game remains by facultative scavengers reveals potential for disease transmission in south‐central Spain; submitted to Frontiers), also records scavenger behaviour based on camera‐trapping. This study was carried out in central Spain, recording the visit rates and consumption of abandoned animal by‐products from big game (including wild boar), mostly abdominal viscera. The authors found that facultative mammal scavengers such as wild boar presented the highest contribution to scavenging in vegetation‐covered habitats (woodlands). Specifically, the wild boar contributed with almost 40% to the total scavenging.
The management implications are that the rapid detection and removal (or destruction on the spot) of contaminated carcasses are regarded as important control measures against ASF in wild boar (Probst et al., 2017). Similarly, given that percentages of ASFV PCR positive samples in hunter‐harvested wild boar from infected regions have been reported up to 3.9% (see Table 5 in this document), and given that wild boar hunting remains can be consumed by wild boar (Carrasco‐Garcia et al., in press), appropriate hunting waste disposal is equally advisable for ASF control.
Table 5.
Percentages of virus (PCR) and ASFV‐antibody positive samples in the Baltic States and Poland
| Country | Test | Found Dead | Hunted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2014 | 2015 | 2016 | 2017 | ||
| Estonia | PCR | 35.4 (178) | 72.6 (925) | 83.5 (966) | 83.8 (314) | 1.1 (827) | 3.9 (8,586) | 3.2 (14957) | 3.1 (6644) |
| AB‐ELISA | 4.2 (48) | 6.6 (122) | 18.8 (64) | 17.6 (17) | 0.3 (869) | 1.8 (8,561) | 2.7 (14883) | 3.3 (6628) | |
| Latvia | PCR | 53.2 (329) | 73.2 (857) | 73.9 (610) | 75.4 (472) | 0.7 (6,048) | 1.8 (12,478) | 2.1 (13557) | 2.1 (7284) |
| AB‐ELISA | 15.0 (127) | 16.1 (149) | 26.8 (56) | 8.3 (24) | 0.4 (6,016) | 4.8 (12,368) | 6.6 (13094) | 5.9 (7063) | |
| Lithuania | PCR | 0.4 (9,932) | nd | 44.2 (1,637) | 73.5 (1,206) | 0.8 (9,006) | nd | 0.3 (33205) | 0.8 (10781) |
| AB‐ELISA | 0.0 (5,224) | nd | 0.4 (243) | 0.0 (25) | 4.6 (5,559) | nd | 0.6 (28544) | 0.4 (9600) | |
| Poland | PCR | 1.4 (3,454) | 1.4 (4,796) | 0.5 (1793) | nd | 0.0 (11,443) | 0.1 (8,470) | 0.0 (7512) | nd |
| AB‐ELISA | 16.5 (417) | 6.0 (67) | nd | nd | 3.0 (5,419) | 1.8 (6,830) | nd | nd | |
nd: no data submitted to data collection framework.
4.1.4.2. Wild boar as an invasive species
Sales et al. (2017) modelled wild boar (or feral pig) habitat requirements to assess niche conservatism, i.e. the retention of a species’ fundamental niche through evolutionary time, a cornerstone for biological invasion assessments. The invasive potential of wild boar was regarded as high and could lead to the spread of pathogens including ASFV. Although the largest risks of further wild boar expansion were identified for tropical regions, particularly the neotropics, the maps also indicate that suitable habitats are still available for further expansion in northern Europe. In a similar study at European scale, Bosch et al. (2016) used standardised global vegetation land cover and expert opinion to assign land classes to wild boar habitat quality. The resulting values were compared with ASF notifications in wild boar and domestic pigs. In the EU, 95% of ASF notifications occurred in natural landscapes with favourable habitat conditions for wild boar.
It is well established that wild boar populations are growing almost exponentially throughout Europe, partly due to an insufficient hunting harvest (Massei et al., 2015). One of the drivers of wild boar (or feral pig) expansion to new ranges is hunting, specifically, introductions for hunting purposes and escapes from farms (Michel et al., 2017; Sales et al., 2017). Once new populations are established, feeding and insufficient hunting provide opportunities for further population growth. In Sweden, an analysis of hunting‐licence pricing revealed that wild boar are among the game species with higher hunting values (Mensah and Elofsson, 2017). This, along with changes in land use and widespread supplementary feeding, would explain the ongoing northward expansion of wild boar in Europe. In newly colonised habitats in Sweden, wild boar achieve an average litter size of 5.4 (Malmsten et al., 2017). In Estonia, another relatively recently colonised country, the wild boar hunting harvest grew by 31% between 2004 and 2013, while vehicle collisions with wild boar increased by 850% in the same period (Kruuse et al., 2016). However, since the incursion of ASF, the estimated numbers of wild boar declined (see Appendix A).
The management implications are that wild boar are unlikely to stop their current (mostly northward) expansion and ongoing widespread population growth unless there are changes in game management at large geographical scales specifically addressing feeding and baiting as well as increasing the hunting harvest.
4.1.4.3. Wild boar population density
Density assessment (individuals per unit area, for instance wild boar per km2) is costly, especially for largely nocturnal species such as wild boar that use cover‐rich habitats. Tools for density assessment are evolving rapidly and range from direct observation (transects, observation at feeders and others), though camera‐trapping, to several capture–recapture methods, among others. The EnetWild consortium (http://enetwild.tuinbit.com) is currently reviewing the literature to provide a comprehensive overview of wildlife densities, including those of the wild boar. Some relevant recent data on wild boar density can be found in Appendix A. The management implications are that: (1) a homogenisation and standardisation of wild boar density assessment methods is needed; (2) wild boar densities range from relatively low values (around 1/km2) to close to 60/km2. However, ASF outbreaks have taken place even in sites with relatively low density (e.g. Belarus; Sidorovich et al., 2017). This suggests that outbreaks are even more likely at the higher densities often reported for other parts of Europe.
4.1.4.4. Wild boar population dynamics in their historical range
Two key aspects need to be considered for understanding wild boar population dynamics: mortality and reproduction. While the first is relatively easy to study based on the inspection of wild boar carcasses, information on the second is still limited, especially for piglets, since it relies on (expensive) collaring and tracking.
Regarding reproduction, the wild boar is an opportunistic species that is well adapted to thrive on resources, such as acorn mast, which fluctuate over time. Hence, the maximum reproductive output occurs in years when the available resources are plentiful (‘pulsed‐resource consumers’; Gamelon et al., 2017). The litter size of wild boar is variable, depending on factors such as age, body condition and feeding regime. In natural sites, adult (> 1 year old) wild boar sows with mean litter sizes of 3.8–4.4 and 5.2–5.9 were found in central Italy and north‐eastern France, respectively, while juveniles (< 1 year) from north‐eastern France had mean litter sizes of 4–4.2. In both age‐classes, reproductive output depended on mast availability (Gamelon et al., 2017).
However, two human‐driven factors are increasing the availability (and predictability) of food resources for wild boar: the increasing surface devoted to growing crops such as maize (e.g. for Poland, 205 ha (0.9% of arable lands) in 1985 increased to 3,212 ha (14.9%) in 2004; Kopij and Panek, 2016), and feeding (e.g. Miloš et al., 2016). As a consequence, wild boar abundances are increasing throughout Europe in recent decades (Massei et al., 2015). The study by Gamelon et al. (2017) found no effects of feeding on wild boar reproductive performance. Similarly, in central Spain, wild boar sampled in open sites (with no feeding) had larger litter sizes (3.5 and 4.3 for juveniles and adults, respectively) than wild boar sampled in fenced hunting estates with supplementary feeding (3.4 and 4.1, respectively (Ruiz‐Fons et al., 2006). This apparently counter‐intuitive observation (lower litter sizes in sites with higher and more predictable food availability) could perhaps be explained by the balancing effect of diseases, which are more prevalent among spatially aggregated wild boar populations such as those co‐mingling at feeders (Ruiz‐Fons et al., 2006). In northern Germany were the habitat is optimal and feeding is common, a mean litter size of 6.6 is reported (Frauendorf et al., 2016). This value is similar to the one reported for Croatia (6.77 for adults, 6.39 for yearlings and 4 for juveniles; Sprem et al., 2016). In any case, healthy wild boar populations are currently characterised by a net reproduction rate of more than 200% according to literature data, and this is partly due to feeding (Keuling et al., 2013).
Regarding mortality, this key aspect of wild boar dynamics has been assessed only sporadically. The most comprehensive study includes data from 661 radio‐tracked wild boar from eight central‐European countries. It found that the mortality rates of wild boar per annum were low (about 0.53; range 0.34 in adult females to 0.71 in yearling males), and 73% of all observed animals survived at least until the next period of reproduction. Regarding the causes of mortality, 85% of the dead animals were shot, 3% died from disease or starvation, and 3% were killed in traffic accidents (Keuling et al., 2013). Mortality due to hunting is slightly biased towards adult males (Keuling et al., 2013; Merli et al., 2017). In a recent study in central Italy, 97% of the mortality was due to hunting or poaching, and the annual mortality rate was about 50% (re‐calculated). Wild boar with more forest in their home range was less likely to be culled (Merli et al., 2017). Hence, wild boar mortality in central Europe is relatively low and mostly due to hunting. In tuberculosis‐endemic wild boar populations in Spain, hunting accounted for 53% of the total mortality, with tuberculosis causing an additional 30%. In hunted populations of central Spain, the annual wild boar mortality is estimated to be 0.56, i.e. still similar to the central‐European number (Barasona et al., 2016). This range of recorded mortality (between 50 and 56% annually) is below the > 65% that would be needed to maintain stable wild boar populations (reference given by Keuling et al., 2013).
Information on piglet mortality, particularly during the first 6 months of life, is almost completely missing (Keuling et al., 2013).
The management implications are that there is an imbalance between reproductive output and harvest rates that leads to growing wild boar populations. This needs to be counteracted by regulating mainly the reproductive animals and harvesting at least the net reproduction, i.e. 80% of the offspring should be harvested, with additional increased pressure on adult females.
4.1.4.5. Wild boar home‐range size and movements in relation to habitat and hunting
Home‐range size in relation to different harvest regimes and to habitat factors was assessed in a study based on 95 radio‐tracked wild boar from the French–Swiss border. The mean annual home‐range size was 4 km2, and habitat‐related factors such as woodland characteristics and crops were the main explanatory variables. Home‐range sizes were larger in areas with regular hunting than in areas with night culling, but this effect was likely confounded by habitat factors (Fattebert et al., 2017). In Romania, the home‐range size was estimated to be between 1 and 12 km2, and the average daily movement varied between 3.6 and 4.8 km (Fodor et al., 2015).
Effects of hunting, particularly of driven hunts, on wild boar spatial behaviour have been described. These consist of increased movements and (mostly temporary) range shifts (Scillitani et al., 2010; Saïd et al., 2012). However, wild boar movements over large distances (of up to over 100 km, exceptionally even more) are occasionally recorded (e.g. Jerina et al., 2014).
The management implications are that wild boar are flexible in their home‐range selection, with size depending essentially on resource availability (mainly food). Driven hunts and other human interferences may increase movements and home‐range shifts, and should therefore be avoided near an epidemic front.
4.2. Descriptive Epidemiology
4.2.1. Update of the ASF situation in eastern Europe
ASF continued to spread in wild boar and domestic pig farms in Eastern Europe in the period January–September 2017. Figure 4 displays ASF notifications in the Eastern European region in the period 2007–2017.
Figure 4.

Notifications of African swine fever in the Eastern Europe region in 2007–2017 Affected EU countries
- Sources: Animal Disease Notification System; World Animal Health Information System; Official web site of the Federal Service for Veterinary and Phytosanitary Surveillance of Russia; period covered 1 January 2007–31 August 2017.
4.2.1.1. Third countries at the eastern boarder of the EU
In September 2016, ASF was notified for the first time in the Republic of Moldova. Two outbreaks were reported in small backyard farms in the district of Donduseni in the northern Moldova at the border with Ukraine. One outbreak occurred in the village of Mosan in a backyard holding with 10 pigs, while the second was notified in the village Ceornoleuca in a holding with three pigs. In both cases, the source of infection was most probably swill containing leftovers from infected meat and meat products originating in Ukraine. The owners bought cheap pork in Ukraine and pig meat products of unknown source a few days before the animals showed clinical signs. At that time, pig meat products were two‐ to three times cheaper in Ukraine than in Moldova.
ASF is present in neighbouring Ukraine. Of the 140 outbreaks recorded since its first incursion into the country, more than 90 have been in 2017. ASF has continued to spread in the domestic pig sector (mainly in backyard farms) and the wild boar populations, including three cases close to the borders with Hungary Romania, Slovakia and Poland. Based on the unfavourable epidemiological situation in Ukraine and the unknown situation in Transnistria, there is a constant threat of ASF being introduced into Moldova.
The media have reported several outbreaks in South Ossetia, Georgia, but these were not officially notified to the OIE. Also, in Belarus, there is no official information about new ASF outbreaks or cases.
In July 2017, the first ASF outbreak was identified in Romania in a small backyard holding in the Satu Mare District, on the border with Hungary and Ukraine. A secondary outbreak was detected on a contact farm and confirmed and notified on 1 August 2017. A boar used for mating/insemination purposes introduced the virus from the primary outbreak. In Romania, ASF virus isolates belonged to the p72 genotype II, CVR‐1, IGR‐2 and MGF1 variants. These variants are circulating mostly within the EU countries, Moldova (2016), Ukraine (2012, 2015), Belarus (2013) and in certain areas of the Russian Federation.
In the Russian Federation, outbreaks have been reported as far east as in Irkutsk and Omsk (Siberia).
Generally speaking, most of the outbreaks that occurred in domestic pigs were in small backyard holdings.
4.2.1.2. Affected EU countries
From 1 January to 11 September 2017, the total number of ASF notifications to the ADNS (EU countries: EE, LV, LT, PL, CZ and RO) was 6,314 for wild boar and 238 for domestic pigs. The evolution of ASFV spread in domestic pig and wild boar populations in the ASF‐affected regions of EU MSs is shown in Figure 5.
Figure 5.

Evolution of ASF in wild boar in the EU and Ukraine from January 2017 to 11 September 2017 as reported to the Animal Disease Notification System
- Data were reported by Ukraine only from 1/1/2017 onwards.
On 26 June 2017, ASF was reported for the first time in the Czech Republic in three wild boar found dead in the suburban area of the city of Zlín. Consequently, a wild‐boar‐infected area was established on 28 June 2017. By 11/9/2017, there were 97 cases notified in the Czech Republic (ADNS). Considering the distance between the three infected animals and the prereproductive wild boar density in this region, it is likely in the area and region that, respectively, 250–270 and 7,000 wild boar, are directly at risk of ASF. Most probably, ASF was introduced during the first week of June. It can be assumed that at least three cycles of infection had already been completed before the disease was detected (see the CVET MISSION REPORT for more details). Human activities were the most probable route of ASF introduction because the nearest reported ASF cases were about 400–500 km away. Moreover, most of the dead ASF‐positive wild boars were found in inhabited areas.
From the first detection in June until 30/9/2017, a total of 234 dead wild boar has been tested by the State Veterinary Administration in the Zlín Region, and ASFV has been confirmed in 112 of them. In the area with intensive catches, 4,206 wild boar have been shot. Most of these were in the districts of Kroměříž (868), Uherské Hradiště (872) and Vsetín (591). In all cases, the results of ASF examinations were negative. However, new cases of ASF in wild boar continue to be reported in the Czech Republic, including the latest notification of an antibody positive (PCR negative) hunted wild boar a few days before the time of writing this report (on 26 September 2017).
Figure 6 shows the numbers of positive‐testing wild boar found dead in the Zlín Region from the first detection of ASF until 30 September 2017.
Figure 6.

Numbers of wild boar found dead and testing positive for African swine fever virus in the Zlín Region (CZ)
4.2.2. Temporal distribution
In the period January 2014–September 2017, although there was as increase in the proportion of positive samples tested either by PCR or AB‐ELISA from wild boar that were found dead (Figure 7. Proportion of samples positive for African swine fever from wild boar either hunted (blue lines) or found dead (dashed red lines), the proportion of positive samples from hunted wild boar remained low.
Figure 7.
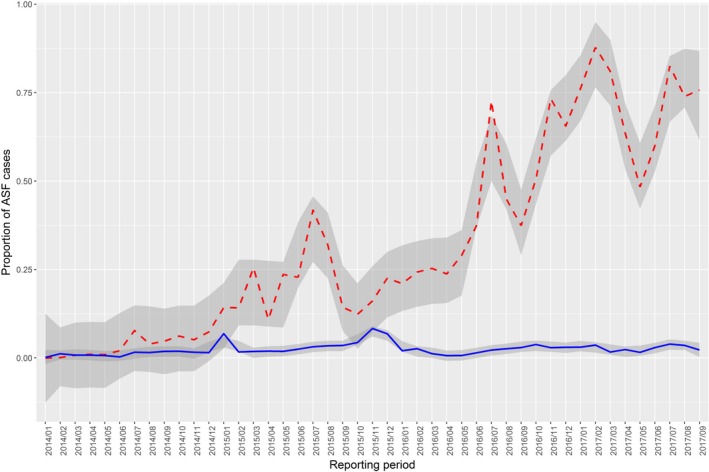
Proportion of samples positive for African swine fever from wild boar either hunted (blue lines) or found dead (dashed red lines)
Table 5 summarises the percentages of ASF‐positive wild boar samples tested either by PCR or AB‐ELISA in the found‐dead and hunted subgroups reported through the DCF since the incursion of ASFV into the eastern countries of the EU in the period January 2014–September 2017. The percentage of ASF‐positive samples reported per year in the hunted population remained below 3.9 when tested by PCR, and below 6.6 when tested by AB‐ELISA.
Figures 8, 9, 10, 11, 12, 13 display the observed proportions of PCR‐ and AB‐ELISA‐positive results for wild boar tested in Estonia, Latvia, Lithuania and Poland, along with their confidence‐interval bands.
Figure 8.
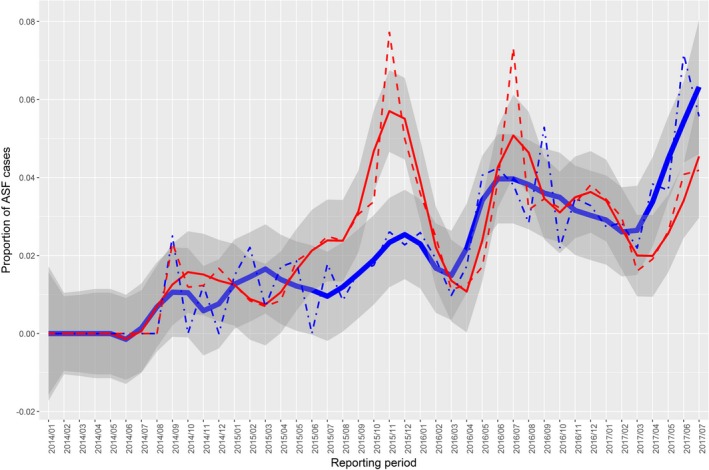
Observed proportion of African‐swine‐fever‐positive results in Estonia
- Samples from the Estonian hunted wild boar population tested by PCR (dashed red line) and AB‐ELISA (dot‐dashed blue line) as well as LOESS‐smoothed data (red and blue lines) and confidence bands (grey regions) for each of the testing methods.
Figure 9.
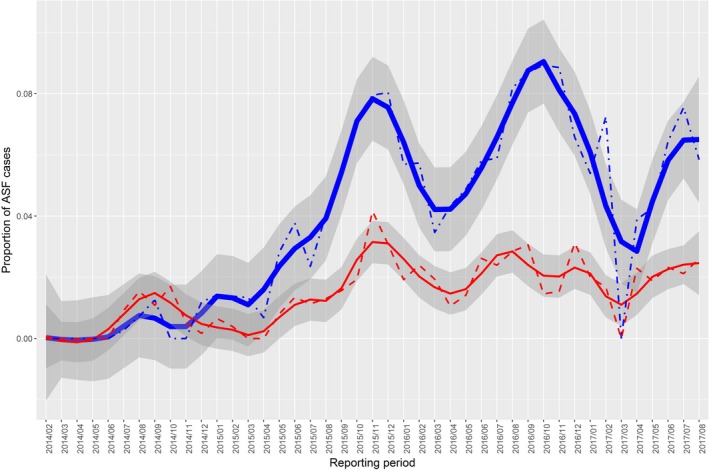
Observed proportion of African‐swine‐fever‐positive results in Latvia
- Samples from the Latvian hunted wild boar population tested by PCR (dashed red line) and AB‐ELISA (dot‐dashed blue line) as well as LOESS‐smoothed data (red and blue lines) and confidence bands (grey regions) for each of the testing methods.
Figure 10.
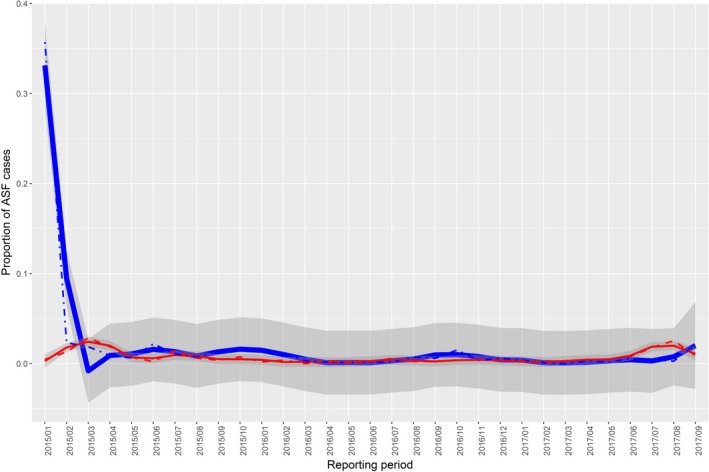
Observed proportion of African‐swine‐fever‐positive results in Lithuania
- Samples from the Lithuanian hunted wild boar population tested by PCR (dashed red line) and AB‐ELISA (dot‐dashed blue line) as well as LOESS‐smoothed data (red and blue lines) and confidence bands (grey regions) for each of the testing methods.
Figure 11.

Observed proportion of African‐swine‐fever‐positive results in Poland
- Samples from the Polish hunted wild boar population tested by PCR (dashed red line) and AB‐ELISA (dot‐dashed blue line) as well as LOESS‐smoothed data (red and blue lines) and confidence bands (grey regions) for each of the testing methods.
Figure 12.
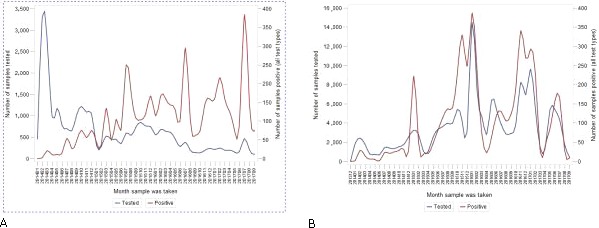
Temporal distribution of PCR‐tested wild boar samples in the Baltic States and Poland
- Total samples tested by PCR for African swine fever virus (blue lines) and positive samples (red lines). Samples were taken from wild boar found dead (A) and from hunted wild boar (B) in the Baltic States and Poland, January 2014–September 2017. Source: Data Collection Framework.
Figure 13.
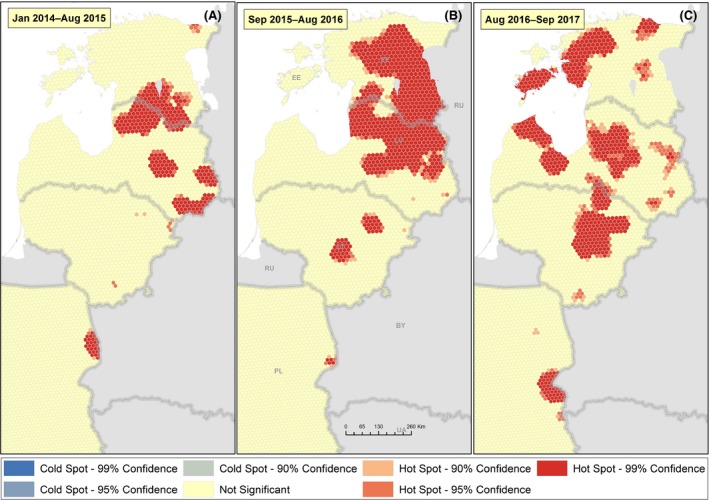
Time course of hot‐spots of notifications to the Animal Disease Notification System (ADNS) in four affected EU Member States
- Reports to the ADNS in the periods July 2014 to August 2016 (A), July 2016 to September 2017 (B), and August 2016 to September 2017 (C).
Figure 14 shows the pooled results reported to the DCF for PCR‐tested samples from wild boar (hunted and found dead) from the Baltic States and Poland. The numbers of ASFV‐positive samples are not randomly distributed throughout the year: more positive samples were observed in summer and winter. The figure illustrates that there is a peak in the number of positive samples in hunted animals in winter that is not obvious in wild boar that were found dead, suggesting the observed winter increase is potentially driven by human activity patterns (i.e. hunting activity over winter). In animals found dead, a peak of positive cases is seen in summer that could be related to the epidemiology of the disease in wild boar and/or its biology; however, this needs further investigation.
Figure 14.

Notifications of African swine fever (ASF) in wild boar in Baltic States, Poland and the Czech Republic
- The graph pinpoints Animal Disease Notification System (ADNS) cases suggestive of human‐mediated spread of ASF. Notifications (cases in wild boar) from the ADNS are coloured according to the possibility of their relationship to older notified cases. The strength of the relationship is evaluated either in terms of direct distance of every new case from any other case recorded at least 7 days earlier (left panel), or by the distance combined with the necessary velocity to cover this distance (right panel). The cases are marked differently in size and colour depending on the value placed on the relationship to other cases. The enlarged purple case notifications refer to values in the upper 99th percentile (the 1% of all cases with longest distance/highest distance‐velocity values); while the red colour refers to values between the 98th and 99th percentiles. Numbers show how many days after 1/2/2014 that individual cases were detected. The insert shows the outcome of the procedure for the Czech Republic using a scale expanded ten‐fold.
Note that the scales of the tested and the positive hunted wild boar in Figure 14A and B are different.
4.2.3. Spatial distribution
4.2.3.1. Identification of hot spots
Different spatio‐temporal behaviour has been observed in areas where hot spots have previously been identified. Some of these showed clear expansion, such as the two clusters that were observed in Lithuania, in the Kaunas, Panev≐žys, Utena and Vilnius counties, which expanded dramatically and merged into a single large hot spot. The centres of other hot spots moved towards a territory with a high density of wild boar, e.g. the hot spot in Podlaskie in Poland (Figure 13).
Several new hot spots formed over the past year (May 2016–July 2017) (Figure 8), and some of these, such as the hot spot in Latvia (region Kurzeme), cannot be explained by wild‐boar‐mediated spread (e.g. spread through nose‐to‐nose, carcass‐to‐nose or environment‐to‐nose contacts).
Another new hot spot formed on the Saaremaa Island of Estonia, which also could not be explained by wild‐boar‐mediated spread, and which now occupies the entire Island. This cluster should further be observed as it could be an excellent example of an epidemic that can be used as a model for explaining the principles of ASF spread and potential persistence.
4.2.3.2. Evaluation of potential human‐mediated spread
The highlighted data points in Figure 14 (purple or red) are those that are the most unlikely to reflect spatial spread as a consequence of natural movements of wild boar. Distance values are provided (see left panel), therefore, it is possible to use literature information about normal wild boar movements to define a different cut‐off. In particular, the purple cases reflect events that were mostly traced to human‐mediated transmission. Clearly, problems such as human non‐compliance (intentional or unintended) are an issue that needs to be addressed urgently, given the damage done to efforts invested in ASF control in wild boar.
4.3. Study of the evolution of ASF incidence at different spatial resolution
The temporal evolution was studied to investigate potential persistence of ASF in different spatial resolutions, LAU1, LAU2 and hunting ground (see Appendix A.1). The results obtained from the model‐fitting showed very similar patterns for all spatial resolutions. Subsequently, therefore, only LAU1 level results are presented. Different temporal scales (daily, weekly, monthly and bimonthly) were considered to investigate regional temporal trends, but similar outputs were obtained in each case (results not shown).
ASF cases have been reported in 14 of 15 LAU1 regions in Estonia. The follow‐up times for each region after the first ASF case was found are presented in Table 6. Summary of number of regions reporting up to 38 months after their first African swine fever case was detected. Of the 14 regions affected, 12 were followed up for 20 months after their first case, which provides sufficient information to estimate the number of cases expected over time. The observed individual region profiles and their averaged smoothing behaviour are shown in Figure 15.
Table 6.
Summary of number of regions reporting up to 38 months after their first African swine fever case was detected
| Time (month) | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | 32 | 34 | 36 | 38 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of regions | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 12 | 12 | 12 | 12 | 11 | 11 | 11 | 9 | 6 | 4 | 4 | 4 | 3 |
Figure 15.
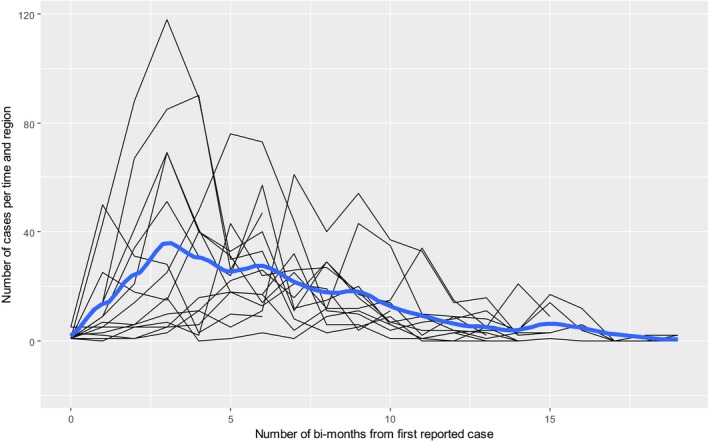
Individual observed profiles with numbers of cases per LAU1 region and LOESS‐smoothed (low span of 0.2) profile defining overall trend
The smoothed temporal trend (Figure 16) indicates a peak (increased number of ASF cases) around 6 months after the first case is reported, and a gradual reduction of the number of cases reported over the years. An increase in the number of ASF cases at around 30 months after the first report can also be seen, but new cases are subsequently limited (see Table 6. Summary of number of regions reporting up to 38 months after their first African swine fever case was detected). The estimated profiles obtained from the GAM described in Section 4.4.2 are presented in Figure 17, showing similar patterns to the observed profiles. Towards the end of the follow‐up period, the estimated time trends predict a significant reduction in the number of cases, but at the same time there is the possibility for ASF to circulate at low levels in the spatial regions considered.
Figure 16.

Individual estimated profile with number of cases per LAU1 region
Figure 17.
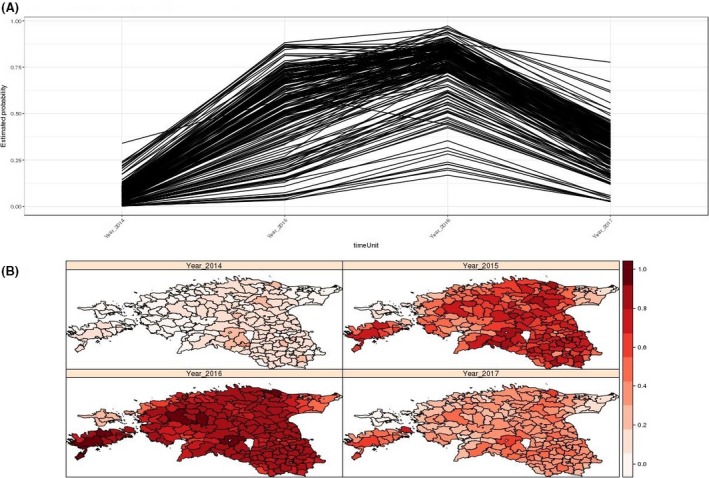
Temporal estimated probabilities for each LAU2 region (panel A) and spatial predictions for each year of the probability of observing African swine fever cases in Estonia (panel B)
Similar results are obtained when using only regions from which samples were taken for more than 20 months after the first reported case; the predicted general trend is the same as when considering all LAU1 regions.
For all spatial resolutions, similar patterns were observed: an initial wave during the first year that gradually fades away but does not die out. Based on the upper bound of the model confidence intervals, by the end of the 38‐month period after the first reported case, the number of cases in the regions is greater than one, indicating potential persistence after 3 years.
4.4. Risk factor analysis
4.4.1. Bayesian Hierarchical Model
After backward elimination of non‐significant (α = 0.05) risk factors, the Bayesian hierarchical model, wild boar density (WBEstDens), road length (RdLength) and habitat suitability (AverQAH) were determined as significant risk factors (Table 7). The results indicate that the odds of observing an ASF‐positive wild boar increase by 2.4 for each unit increase in wild boar density (animals/km2). A similar interpretation can be made for the other two parameters. The estimated temporal trends (Figure 17) were similar to the ones found in Section 4.3, with an increase seen in the first two years followed by a decline in 2017. The spatial predictions clearly indicate an increase over time in the probability of ASF detections for all LAU2 regions up to 2016, and a decrease for 2017.
Table 7.
Parameter estimates and 95% confidence intervals, median and mode of the posterior distributions in the Bayesian hierarchical model
| Parameters | Mean | Standard deviation | Lower 95% CI | Median | Upper 95% CI | Mode |
|---|---|---|---|---|---|---|
| (Intercept) | −8.46 | 1.59 | −11.76 | −8.40 | −5.48 | −8.29 |
| 2015 | 3.15 | 0.41 | 2.39 | 3.14 | 4.64 | 3.11 |
| 2016 | 4.85 | 0.47 | 3.95 | 4.83 | 5.84 | 4.80 |
| 2017 | 2.94 | 0.49 | 2.02 | 2.93 | 3.95 | 2.89 |
| WBEstDens | 2.39 | 0.61 | 1.22 | 2.37 | 3.65 | 2.35 |
| RdLength | 0.10 | 0.04 | 0.02 | 0.10 | 0.19 | 0.10 |
| AverQAH | 0.74 | 0.29 | 0.19 | 0.73 | 1.36 | 0.72 |
4.4.2. Generalised Additive Model
The final GAM included wild boar density (WBEstDens) and number of pig farms (NumPgFrms) as significant risk factors for the detection of ASF‐positive wild boar after backward elimination of non‐significant (α = 0.05) risk factors (Table 8). The results indicate that the odds of observing a positive wild boar increased by 2.5 for each unit increase in wild boar density (animals/km2). Furthermore, for each unit increase in pig farms in a region, the odds of finding a positive wild boar increased by 0.09. The estimated temporal trend (Figure 17) was also similar to the ones found before in Section 4.3. The spatial predictions show clearly the increase in probability for all LAU2 regions over time to 2016, followed by a decrease in 2017.
Table 8.
Outcomes of the risk factor analysis using the generalised additive model
| Parameters | Mean | Standard deviation | Z | p‐value |
|---|---|---|---|---|
| (Intercept) | −4.68 | 0.58 | −8.04 | 8.6E‐16 |
| 2015 | 3.32 | 0.44 | 7.44 | 9.9E‐14 |
| 2016 | 5.17 | 0.52 | 9.83 | 7.9E‐23 |
| 2017 | 3.48 | 0.55 | 6.26 | 3.6E‐10 |
| WBEstDens | 2.52 | 0.59 | 4.21 | 2.5E‐05 |
| NumPgFrms | 0.08 | 0.03 | 2.49 | 1.2E‐03 |
If the risk factors obtained with the Bayesian hierarchical model are used in the GAM, the results are very similar to those obtained in Section 4.3. The results can be found in Table 9 and Figure 18.
Table 9.
Parameter estimates and 95% confidence intervals, median and mode of the posterior distributions in the Bayesian hierarchical model
| Parameters | Mean | Standard deviation | Z | p‐value |
|---|---|---|---|---|
| (Intercept) | −7.75 | 1.67 | −4.62 | 3.7E‐06 |
| 2015 | 3.05 | 0.40 | 7.50 | 6.0E‐14 |
| 2016 | 4.71 | 0.46 | 10.23 | 1.4E‐24 |
| 2017 | 2.89 | 0.48 | 5.94 | 2.8E‐09 |
| WBEstDens | 2.42 | 0.61 | 3.96 | 7.4E‐05 |
| RdLength | 0.10 | 0.00 | 2.37 | 1.7E‐03 |
| AverQAH | 0.64 | 0.31 | 2.04 | 4.1E‐03 |
Figure 18.

Temporal estimated probabilities for each LAU2 region (panel A) and spatial predictions for each year of the probability of observing African swine fever cases in Estonia (panel B)
When the Akaike information criteria are used for model selection, the probability of observing cases in a LAU2 region in Estonia are equally well explained by the GAM containing wild boar density, road network and habitat suitability, and by the GAM containing wild boar density and number of pig farms (difference between two models is less than 2).
Potential risk factors identified by both modelling approaches are wild boar density, road network, habitat suitability and number of pig farms.
4.5. Assessment of wild boar management options
The results of the simulation‐based investigation of ASF spread and control in nature with all parameter variations shown in contour plots are presented in the external scientific report delivered to EFSA (Helmholtz Centre for Environmental Research GmbH, 2017). Model documentation and additional resources are maintained at http://www.ecoepi.eu/ASFWB.
Results are shown below that address these objectives:
– Does the newly available knowledge or parameterisation change the simulation outcome of the EFSA opinion of 2015?
Figure 19 shows the outcome of the simulations equivalent to those provided in 2015 (EFSA AHAW Panel, 2015) for alternative control measures (depopulation vs targeted hunting of females), different starting densities (as suggested for Baltic MSs, doubled and then quadrupled) and varying carcass removal effectiveness within 1 week of the death of the animal (coloured line graphs, 0–90%). Confirming the 2015 output, highly effective measures and rapid removal of the majority of carcasses did halt the spread of the infection at least when assuming a control area size of 200 km with a 70% effective carcass removal within 1 week.
Figure 19.
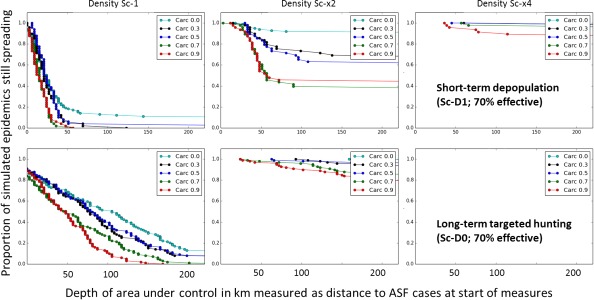
Simulation output
- Output is shown as the proportion of runs (y‐axis) spreading beyond a given distance in km (x‐axis) from the previous location of African swine fever (ASF) at the moment measures were started. The further the control zone stretches from the original ASF recordings, the fewer runs continue to spread. 1 minus the proportion on the y‐axis is the success rate with a given scenario of measures. First row: Short‐term depopulation assuming 70% effectiveness; Second row: Targeted hunting of females over multiple years assuming 70% effectiveness. Alternative density scenarios (left column as reported for the Baltic MSs; middle doubled; right quadrupled). Differently coloured graphs reveal the effects of increasing intensity of carcass removal within 1 week (turquoise: no carcass removal; red: 90% removed within 1 week).
The simulations in Figure 19 assume 70% effectiveness, which is difficult to achieve in field situations (EFSA, 2014). Other, less effective scenarios are summarised in Figure 4 (D and T) of the external report. Assuming lower efficiency for the measures, the success rate dropped sharply below 50%, and this is independent of carcass removal below 80%. Here, it is important to note that, with depopulation, most carcasses are prevented, as the shot wild boar will be removed from the population before they might become infected. Targeted hunting of females showed useful results in the 200 km control area scenario or if measures and carcass removal effectiveness were assumed to be possible at near 100%. Simulations therefore confirm the previous insights that single measures applied with limited effectiveness are not able to halt the spread of infection.
– Are the investigated strategies realistic in situations of population capacities in wild boar habitats similar to those in the area west of the affected area?
It is noticeable that increased host density at the start of the measures weakens the effectiveness of any measure (from left to right in every row of Figure 19; see complete results in the external report). This is reasonable if carcass‐mediated spread is understood to be frequency dependent because this implies reduced effectiveness of removal, depopulation or hunting at the same percentage level compared with the basic density scenario.
– Does the approach implemented in the MSs in principle support the halt of ASF spread and final eradication over time?
Figure 20 shows the outcome of simulations across full parameter ranges of measure efficiencies (x‐axis, which here targeted hunting; see external report for more scenarios on density and depopulation) and efficiency in removing carcasses (y‐axis) according to three different timelines (row‐wise: 1, 2, 4 weeks after death of the animal). The success rate increases with increasing area put under control measures at the start of the simulated programme (i.e. when the trigger line is reached by the infection; red line in Figure 3A). The first row (no contact delay and 1‐week removal period) and the third row (contact delay of 2 weeks and 4‐week removal period) reveal that the recently applied targeted hunting would require a maximised preventive area – here up to 200 km in front of the ASF epidemic – to generate substantial success (efficiency parameter values in orange to red contours) assuming greater than 60% efficiency of the hunting measure.
Figure 20.
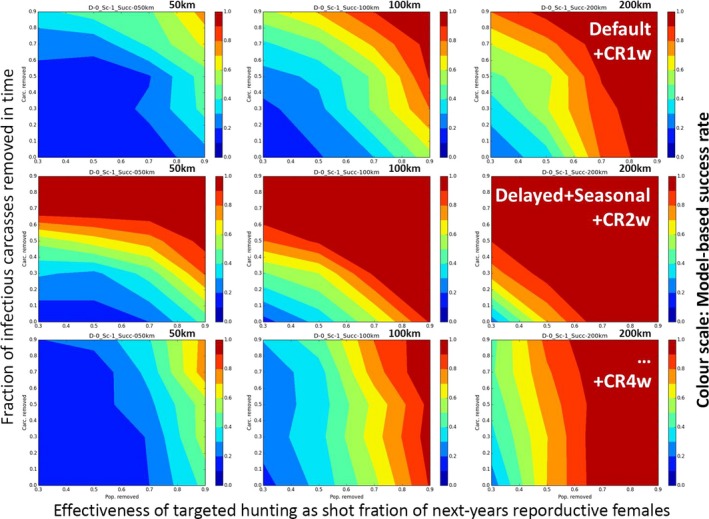
Simulation output shown as proportion of runs (colour coded) successfully eradicated using targeted hunting as control measure combined with carcass removal
- Each row shows a different scenario simulated for three different control zone sizes (i.e. 50, 100 and 200 km distance from the African swine fever cases when the measures start). First row: Default scenario as Figure 19 with immediate contact between the carcass and live animals, no seasonal decomposition and carcass removal within the first week following death of the animal; Second row: Contact between carcass and live animals delayed by 2 weeks (Probst et al., 2017), seasonal decomposition and carcass removal within 2 weeks of death; Third row: As before, but carcass removal only within 4 weeks of death as suggested by the current practice (between 2 and 6 weeks, median 4 weeks).
– Can the proposed option of intensified carcass removal, as suggested in the EFSA 2015 opinion, be supported, given the new insights about the practical difficulties relating to carcass removal and possible duration of carcass decomposition?
The carcass removal in the first and third row showed limited additional effect because it is still late compared with the allowed contact i.e. within the first week using instantaneous contact (first row) or within 4 weeks if contact was delayed by 2 weeks (third row). However, if the contact delay as proposed by Probst et al. (2017) is valid in the control area, a faster removal may dramatically support the effect already seen in smaller control areas (second row). The expert estimation by the MSs proposed 2–6 weeks with a median of 4 weeks as realistic in the field. Hence, the improvement in the carcass removal time could change the situation from row three towards that in row two.
5. Discussion
5.1. Descriptive epidemiological analysis
ASF has continued to spread in the Baltic States and Poland in the period September 2016–September 2017. New incursions into the Czech Republic and Romania occurred and outbreaks continue to be reported at the moment of writing this report.
Prevalences calculations were not performed in the temporal analysis, because sampling of wild boar either found dead or hunted cannot be considered random or representative of the population. However, the temporal analysis of the reported ASF cases showed that there is a seasonal pattern in the total number of cases reported monthly, with peaks observed in winter and summer. Although the winter peak can be related to increased hunting activity, the summer peak remains to be explained. Overall, in the period 2014–2017, there was an increase in the proportion of samples from wild boar found dead that tested positive either by PCR or AB‐ELISA but the proportion of positive samples from the hunted wild boar remained low. In general, the incidence of ASF increases after the first report in a region, with a peak observed after about 6 months and then gradually decreases over years, with a low activity observed around 20 months after the first introduction in an administrative unit.
From the analysis of the spatial distribution of the reported cases, it could be concluded that human‐mediated spread still plays an important role, as the distances between several reported cases in wild boar could not be explained solely by the natural spread in the wild boar populations.
5.2. Risk factor analysis
Pig farm density has been identified as a risk factor for ASF observations in wild boar in Estonia for the period 2014–2017. However, it should be noted that there is no field evidence that there might be any undiscovered circulation of ASF in the Estonian domestic pig population. The surveillance history in domestic pig herds (with frequent checks of all pig farms particularly in infected areas, and a very small number of backyard farms, all of which are visited by official or authorised veterinarians) as well as relatively early detection of all outbreak farms (within 2 weeks of the first observation of clinical signs) give no reason to conclude that this association has direct causal nature (i.e. spill over of the infection from domestic pigs to wild boar). In the beginning of the epidemic, the occurrence of ASF cases in wild boar was geographically associated with high density pig farm areas. This fact may have confounded the analysis.
5.3. Review of the management options for wild boar
The intention of this simulation study was to address the validity of earlier analysis of the impact of certain control measures on the spread of ASF in spatially arranged wild boar populations (EFS AAHAW Panel, 2015). An extension of this earlier work was motivated by new insights and updated data sources since the writing of the 2015 material (EFSA AHAW Panel, 2015; Lange, 2015).
Since early 2015, changes in our understanding of ASF in wild boar particularly related to wild boar carcasses and their role in ASF epidemiology, including their decomposition and time con‐species are scavenging on their carcasses. Both in reality and in the model, only carcasses of individuals dying while affected by the ASF virus are relevant – control measures to address carcass removal as the source of virus perpetuation will not differentiate between infectious and non‐infectious cadavers. We therefore use carcass to describe all cadavers and infectious carcass for those that harbour live virus. Carcass removal in this document is related to the prevention of any possibility of virus transmission by infectious carcasses in the wild (by burning, burying or physical removal).
In 2015, single measures were investigated. In that study, combinations of measures, e.g. focal depopulation and distant application of the preventive population reduction by standard hunting methods were not yet considered. Also in the previous study, the inclusion of carcass removal into combinations of control measures was not subjected to systematic simulations because at that point no information was available about either the time horizon or the efficacy of the implementation of carcass removal (see EFSA AHAW Panel, 2015). Removal was assumed to occur either instantaneously (CR0w) or one week post‐death (CR1w). While the first (0w) led to nearly ideal success rate compared with all other possible options, the latter (1w) failed to improve over doing nothing. In 2015, carcass removal was simulated to improve our understanding of the system rather than offering a control alternative. The insight motivated the recommendation for further study of the role and importance of carcass removal in the context of ASF emergency management in wild boar. At the time of writing, the current report, and based on expert judgement, a time frame for carcass removal of 2–6 weeks with median at four weeks after the death of the animal is considered feasible. This observation is at odds with the assumptions underlying the simulations in 2015 (see EFSA AHAW Panel, 2015; Lange, 2015).
Does new knowledge or parameterisations change the simulation outcome of the EFSA opinion of 2015? Does the simulated control approach in principle support the proposed control target of ASF eradication or halt of spread?
The current simulation study sought to close the gap by integrating the new data about carcass ecology with simulations of control. Epidemiologically and empirically derived data substantiate the understanding that carcass contact in wild boar is rare (Helmholtz Centre for Environmental Research GmbH, Thulke and Lange, 2017; Probst et al., 2017) and scavenging on fresh carcasses is probably limited. Hence, the new model simulations addressing the two main scenarios of 2015 (drastic short term depopulation vs long‐term targeted population reduction) were repeated with all parameters up to the contact frequency per carcass. The impact of this measure on ASF can be determined by estimating the distance the infection spreads forward after the control starts. In Figure 19, individual simulation runs are reported for a selected efficacy of population control measures (e.g. 70%). The turquoise graph (upper line) resembles the outcome with no carcass removal in place, while the other lines represent an increasing efficiency of carcass removal within the first week after death (standard carcass removal scenario in EFSA AHAW Panel, 2015). The main conclusion from these two diagrams is that even with an ambitious level of population reduction measures (70% depopulation in 4 months or multiple years of harvesting 70% of the upcoming years’ reproducing females), final success is likely but not guaranteed, supporting the conclusions in EFSA AHAW Panel (2015). Moreover, the strategy of combining 70% effective targeted hunting with removal of up to 90% of all carcasses within 1 week (red graph, second row in Figure 19) is the first to provide substantial chance of success in the less‐than‐200km control zone. However, both assumptions on hunting efficacy (70%) and carcass elimination (90% in one week) are difficult to implement in the field (EFSA, 2014).
The full picture of simulated combinations is summarised in Figure 20 first row (see external report Figure 4 (T and D) for complete output data). For different targeted hunting efficacies, the contour plots reveal the success rate found in the model simulations using a control area 50 (left), 100 (middle) and 200 km wide (right). Clearly, the largest treatment zones of 200 km would be necessary with the targeted hunting strategy (top right diagram in Figure 20). To reach the part of the diagram indicating success (i.e. orange to red contour surfaces), targeted hunting would require more than 70% efficacy (x‐axis) and carcass removal within one week being nearly perfect (y‐axis). The picture improves slightly using drastic depopulation (population reduction about 70% in only 50 km; Figure 4D in the external report) but is somewhat independent of carcass related efforts. The latter is reasonable, as depopulation will already prevent carcasses from being infectious. Nevertheless, the implied success requires greater depopulation efforts than deemed practical in the field (EFSA, 2014). So far the new insights and altered parameters have not changed the outcome of the model analysis of 2015.
It should be noted that this analysis did not address the scenario of a focal introduction of ASFV in the middle of an ASFV‐free wild boar population (such as the ASFV introduction in the Czech Republic), but simulated the spread of ASFV through a wild boar population adjacent to already infected wild boar populations (i.e. the situation of the Baltic States). However, previous analyses have looked into the performance of temporarily erecting mobile barriers as contingency measures against wild‐boar mediated spread of ASF, compared to local depopulation in the vicinity of detected infected animals in smaller infected areas with focalised introduction of ASFV (http://ecoepi.eu/CSFWB/files/Lange_SVEPM2015_SupplMaterial.pdf).
Are the investigated strategies able to cope with population capacities in more westerly wild boar habitats?
Insight from simulations assuming different habitat quality (i.e. wild boar habitat with greater local density, or more extended home ranges per wild boar group; second and third column in Figure 19; Sc‐x2 and Sc‐x4) are even less optimistic regarding the tested control measures. Here, the simulated measures fail because of the still substantial number of animals left after the proportionate population reduction measures. Hence, assuming carcasses actually play the role generally assigned to them at the time of writing this report, entry of the infection into regions with better wild boar habitat than in the Baltic MSs will likely result the current control measures being negligible effective. If carcasses were not to play a relevant role, an alternative perpetuation mechanism has not been identified. Different situations may possibly originate during the immediate control of a local entry spot (e.g. the Czech Republic) before ASF can develop into an epidemic of the size seen in the Baltic MSs before concerted control measures were implemented. Simulation‐based insights into efficiency of localised depopulation measures following a point entry of the infection were studied by Lange and Thulke (2015).
Can the proposed option of intensified carcass removal suggested by the EFSA AHAW Panel, 2015 opinion be supported given the new insights about effectiveness of carcass removal and possible carcass remediation times?
The next point in the analysis addresses the effect of the hypothesis that carcass contacts do not occur immediately after the animal's death but are delayed for a certain time by, e.g. behavioural avoidance of carcasses as long as they remain fresh. The hypothesis was developed following the first observations of contact behaviour in the field (Probst et al., 2017). A 2‐week refractory period was suggested and simulated in all scenarios named ‘Delayed’ (see Figure 20, second and third row). As one would expect, if carcass‐contact transmission is assumed to occur only when the animal has been dead for at least 2 weeks, any shorter carcass removal period will improve the control's success. In Figure 20, second row, assuming two weeks for both the carcase‐contact delay and the time until carcass removal, there is still a great improvement in the effectiveness of the measures compared with the situation where carcasses are contacted immediately after death (Figure 20, first row), at least with the basic habitat as reported for the north‐eastern MSs. Based on an expert enquiry conducted by EFSA, the most likely time of carcasses removal following the usual ASF‐control practice is 2–6 weeks post‐death, with a median value of 4 weeks. The median value was therefore taken as the carcass removal time in another simulation (Figure 20 third row). As might be expected, when these measures were applied to the model landscape, they had limited effectiveness. Moreover, the level of success was independent of carcass removal (y‐axis in Figure 20). The need for rapid carcass removal does conflict with observations of field measures across the affected MSs (carcass removal in two vs. two‐to‐six weeks). From the model as it reflects the current understanding of the role of carcasses in perpetuating the infection, carcass removal time in the field needs to be enhanced substantially to contribute to the effectiveness of the control measures.
Finally, and in particular with regard to future European areas at risk of ASF entry, it seems reasonable to rethink combinations of ‘soft’ and ‘drastic’ population reduction measures. If the objective is to prevent entry of the virus into territories neighbouring already affected areas, merely applying targeted hunting and as much carcass removal as possible would not be effective in other regions with much larger number of animals present.
The model uses available data and current common sense knowledge of ASF maintenance in wild boar ecology, and of the disease course, transmission pathways and contact behaviour at the individual level. Although many of these aspects are cross‐validated or confirmed in field studies and laboratory experiments, substantial uncertainty remains. One example is the missing proof of the role played by carcasses in ASF transmission. Other uncertainties are more subtle. Even if the more general insights prove to be robust and well‐grounded in the population ecology of infectious diseases, model predictions should be evaluated in terms of general trends between scenarios, rather than a strict interpretation of exact figures.
6. Conclusions
6.1. Extensive literature review
To manage wild boar populations, the social context, the regional diversity and the capacity and willingness of hunters need to be integrated into policy.
There is a need for a better understanding of the wildlife population dynamics and for good baseline data on wildlife population trends.
Interventions regarding wildlife diseases are rarely widespread and not necessarily successful. Three aspects need regulation in order to improve wildlife health: wildlife feeding, disease control in farmed or translocated wildlife and hunting offal disposal.
Wild boar have been reported to make the highest contribution to scavenging in vegetation‐covered habitats (woodlands). The management implications are that the rapid detection and removal (or destruction on the spot) of contaminated carcasses be regarded as an important control measure against ASF in wild boar.
Wild boar are unlikely to stop their current (mostly northward) expansion and on‐going widespread population growth unless changes in game management, specifically addressing feeding and baiting as well as an increased hunting harvest, take place at large geographical scales.
6.2. Descriptive epidemiology
Human‐mediated spread of ASFV continues to play a critical role in ASF epidemiology, despite all measures currently taken.
ASF continues to spread towards unaffected areas in the European territories.
New cases of ASF in wild boar continue to be reported in the Czech Republic, with the latest notification of an ASF‐positive hunted wild boar occurring a few days before the time of writing this report (on 26 September 2017).
A certain seasonality in the number of notifications in found wild boar was again suggested for the period September 2016–September 2017.
The proportions of both PCR and antibody positive samples from hunted wild boar in Estonia, Latvia and Lithuania remained low since the first detection of ASF. (The data from Poland were not included in the analysis of the prevalence of positive samples).
6.3. Study of the evolution of ASF incidence at different spatial resolution
The modelled time trends indicated a peak in the number of ASF cases around 6 months after the first case was reported. At the end of the follow‐up period of 38 months, a significant reduction of the number of cases was predicted, but at the same time there the possibility for ASF to circulate at low levels in the spatial regions considered remained.
6.4. Risk factor analysis
According to both the Bayesian hierarchical model, wild boar density, the total road length (as proxy for human activity) and average availability of suitable wild boar habitat, were significant risk factors for the occurrence of ASF in the wild boar population in Estonia.
According to the GAM, wild boar density and the density of pig farms are significant factors associated with the occurrence of ASF in the wild boar population in Estonia.
6.5. Assessment of wild boar management options
From the model simulations, it was concluded that measures to reduce wild boar population to finally halt the expansion of ASFV are the most effective when applied in the regions outside or adjacent to already affected areas (treatment zone).
Additionally, any carcass should be removed as fast as possible from the infected area as well as its surrounding areas. The width of these surrounding areas should take into account the local epidemiological situation of ASF, the artificial and natural barriers and the ecology of the wild boar.
The model analysis did address the spread of ASFV into wild boar populations adjacent to already infected wild boar populations (i.e. the situation in the Baltic States). The scenario of a focal introduction of ASFV in the middle of an ASFV‐free wild boar population (such as the ASFV introduction in the Czech Republic) would require alternative considerations.
Drastic depopulation, targeted hunting of female wild boar and carcass removal implemented as only the measures to control ASF in the wild boar population need to be implemented in a highly effective manner (at or beyond the limit of reported effectivity in wild boar management) to sustainably halt the spread of ASF (confirming EFSA AHAW Panel, 2015 with updated data).
Contact between wild boar and infected carcasses may be delayed, as indicated in a recent publication (Probst et al., 2017). This phenomenon needs further investigation, however, and is currently an important area of uncertainty in the model. When this delay was incorporated into the model, carcass removal became more useful as measure to halt the spread of ASFV in the wild boar population.
According to the model and assuming delayed contact of wild boar with infected carcasses, carcass removal 2–6 weeks after death of the infected wild boar (median 4 weeks) would provide a very limited contribution to the success of control measures.
The above conclusions apply for measures implemented in wild boar populations mimicking those of the Baltic States, with average densities mostly below 1.5/km² prior to reproduction. When simulating ASF control measures in higher wild boar population densities, the model predicts a very limited effect. Early management would be required to preventively reduce greater population densities.
Early detection of local ASFV entry might facilitate the implementation of intensive focal emergency measures different from those on large spatio‐temporal scales studied in the model simulations.
The GAM concluded that an increased wild boar density increases the probability for the occurrence of ASF, whereas the simulation model concluded that increasing wild boar density decreases the chances of effective control measures solely through hunting or carcass removal.
Wild boar density thresholds for population and carcasses control, and mechanisms how density impacts ASF maintenance are still insufficiently understood.
The model has been constructed based on several assumptions, reflecting ad‐hoc expert discussions and preliminary laboratory insights regarding ASF transmission, perpetuation and maintenance by the wild boar host system in central Europe. These include the role of carcasses as a reservoir, late contact with dead animals, maternal antibodies in piglets from seropositive sows, artificial feeding, stringent and consistent application of measures, and exclusion of human‐mediated transmissions.
7. Recommendations
This report, including the model simulations, will need to be updated if new scientific knowledge becomes available that contradicts the assumptions used in the model.
Detailed analyses using simulations on true landscapes with multiple habitat predictors would improve the understanding of the performance of the control measures.
Standardised methods of wild boar density assessment are needed.
Human‐mediated spread needs to be urgently addressed by intensified awareness‐building in all persons potentially in contact with infected wild boar or pigs about the possible routes for ASFV spread and its economic and ecologic consequences.
Existing local emergency measures using drastic depopulation and/or fencing should be evaluated using current empirical and epidemiological data.
Abbreviations
- AB ELISA
enzyme‐linked immunosorbent assay targeting antibodies
- ADNS
Animal Disease Notification System
- ARIB
Estonian Agricultural Registers and Information Board
- ASF
African swine fever
- ASFV
African swine fever virus
- BYM model
Besag, York and Mollié model
- CR
carcass removal
- DCF
EFSA Data Collection Framework
- GIS
geographic information system
- HAI
haemadsorption inhibition assay
- IB
immunoblotting
- ICAR
intrinsic conditionally autoregressive
- IPT
immunoperoxidase test
- LIMS
Laboratory Information Management System
- MRF
Markov random field
- MS
Member State
- OIE
World Organisation for Animal Health
- PCR
polymerase chain reaction
- QAH
quality of available habitats
- Sf
scaling factor
- VNIIVViM
National Research Institute for Veterinary Virology and Microbiology
Appendix A – Background information about wild boar populations in eastern Europe
A.1. Wild boar populations in Eastern Europe
Maps of wild boar density by region and year have been prepared based on shape files provided by the MSs.
A.1.1. Wild boar population density in Estonia 2014–2017
Figure 21.

- Source: The University of Life Sciences.
A.1.2. Estimated wild boar population density in Lithuania 2014–2017
Figure 22.
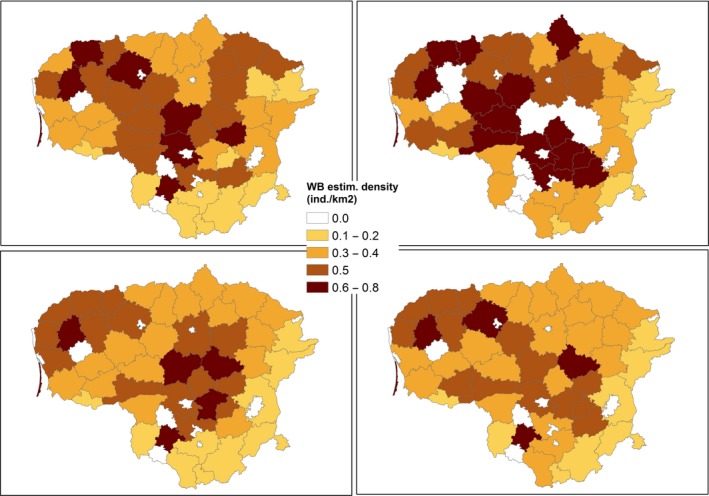
- Source: Ministry of Environment of the Republic of Lithuania.
A.1.3. Estimated wild boar density in hunting management units in Latvia 2014–2017
Figure 23.
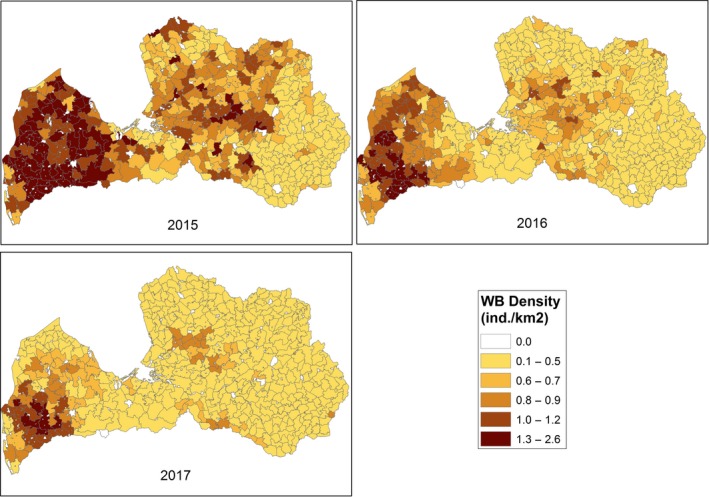
- Source: State Forestry Service.
A.1.4. Estimated wild boar density in hunting grounds in Poland 2014–2016
Figure 24.
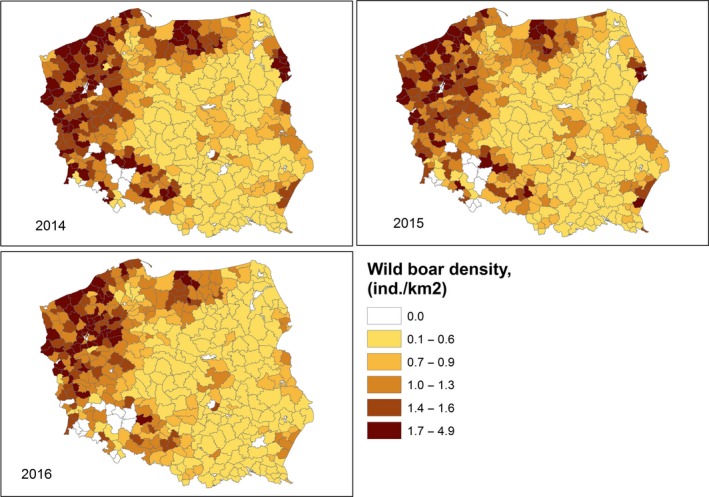
- Source: Łukasz Bocian, personal communication.
A.1.5. Estimated wild boar density in hunting grounds in the Czech Republic 2015
Figure 25.
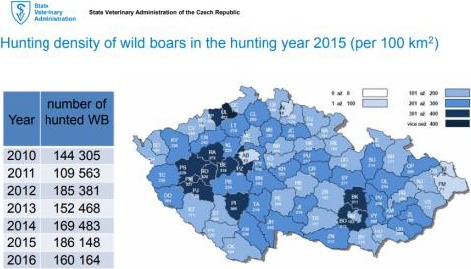
- Source: PAFF presentation.
A.1.6. Estimated wild boar density in hunting grounds in Romania 2017
Figure 26.

A.1.7. Wild boar population size in the Baltic States and Poland according to official records 2014–2017
Figure A.1.
Estimated numbers of wild boar in the Baltic States and Poland (2014–2017)
- *The data is presented only for African‐swine‐fever‐affected forest districts 2014–2017.
A.1.8. Recent information pertaining wild boar density in the European Union, extracted from literature
Table A.1.
Recently available figures on wild boar density in the EU
| Country | Density (per km2) | Method | Reference |
|---|---|---|---|
| Belarus (Naliboki Forest) | 2.34 (declined to 0.27 after ‘a bout of serious disease’ (probably ASF) in summer 2013) | Direct counts on transects | Sidorovich et al. (2017) |
| Czech Republic (south) | 64 | Dung counts | Plhal et al. (2014) |
| France (Arc‐en‐Barrois) | 10.9–13.6 | Not stated | Gamelon et al. (2011) |
| Italy (Tuscany) | 6.91 | Drive census | Ståhlberg et al. (2017) |
| Spain (Ciudad Real) | 10.6–29.6 | Direct counts at feeding sites | Acevedo et al. (2007) |
| Sweden (south and central) | 5–40 | Not stated | Malmsten and Dalin (2016) |
A.2. Wild boar management strategies in the Eastern EU
Table A.2.
Wild boar management strategies in the Eastern EU
| Selective hunting of female wild boars | Removal of dead animals | Additional feeding | Baiting | Driven hunts | Additional measures | |
|---|---|---|---|---|---|---|
| CZ |
The hunting ground owner is required to ensure the presence of the amount of game, specified in the Decree No 491/2002 Coll. (7–16 wild boar per 1,000 ha) Since the first ASF‐positive animal was found (21/6/2017) in accordance with extraordinary veterinary measures (13/7/2017), all users of hunting areas shall perform an intensive year‐round hunting of all wild boar individuals irrespective of their age category and sex by all methods of hunting These measures shall not apply to the infected area All cadastral territories of the District Zlín are considered as the infected area in compliance with Commission Implementing Decision (EU) 2017/1437 of 4/8/2017 concerning certain protective measures relating to African swine fever in the Czech Republic valid until 30/9/2017 |
The surveillance of dead wild boar implemented since 2014 shall continue in the entire territory of the Czech Republic. Wild boar killed in traffic collisions are also examined Active search for dead wild boars was established (in accordance with extraordinary veterinary measures 13/7/2017) Any finding of dead wild boar shall be immediately notified to the RVA |
In cadastral territories of the District Zlín, the ban on feeding of wild boars shall apply (from 13/7/2017) |
Only baiting at baiting places shall be authorised, where not more than 5 kg of feed shall be placed at one baiting place One baiting place shall be placed per 100 ha of a hunting area |
Extraordinary veterinary measures were taken in the entire infected area: a) ban on hunting of wild boar, b) ban on group hunting of all small and cloven‐hoofed game animals (from 13/7/2017) However, there is an exemption from the ban on wild boar hunting since 11/9/2017 Trained hunters designated by the hunt manager are allowed to individually hunt wild boar to reduce their population |
Measures in wild boar population are implemented on four levels according to the risk assessment and results of ASF surveillance: 1) The whole territory of the Czech Republic 2) The area with intensive hunting 3) The infected zone divided into: a) low risk zone b) high risk zone with electrical (installed on 6/8/2017) and odour fences (installed on 22/7/2017) Cereal crops harvested in 2017 in the infected area shall be prohibited from use for feeding pigs for at least 6 weeks after their harvesting (‘quarantine of cereal crops) Users of hunting areas were authorised to use trapping (one per 50 ha of a hunting area) |
| Romania |
June 2017 ‚’Decree no 428/12/5/2017 of the Ministry of Forestry and Waters referring to the approval of hunting quota for some species of fauna where hunting is allowed for the hunting season May 2017–May 2018’ Art. 9 (2) provides that: on the hunting grounds from Satu‐Mare, Maramureș, Bistrița‐Năsăud, Suceava, Botoșani, Iași, Vaslui, Galați and Brăila și Tulcea counties found on the border with Moldova Republic and Ukraine, and also in other zones that might be considered at the highest risk, hunting is permitted of wild boar of both sexes in equal numbers. The hunting of females will be from those adult and sub‐adult age groups Also, measures concerning hunting management, in accordance with the ASF strategy for the eastern part of the EU, are included in the Governmental Decision no/830/2016 amended, at Art. 28, and also in the National Committee for Emergency Situation Decision no. 2, Art. 4, point c, from 1/8/2017 when the first ASF outbreak was confirmed in Romania |
Since 2016, has been provided in the National Committee for Emergency Situations Decision no. 2, Arts. 6 and 7, from 1/8/2017 when the first ASF outbreak confirmed in Romania It is also provided in Governmental Decision no. 830/2016 amended at Art. 31 |
Prohibited since 2016 in Governmental Decision no. 830/2016, amended at Art. 28, point c, and in the National Committee for Emergency Situations Decision no. 2, Art. 4, point b, from 1/8/2017 when the first ASF outbreak was confirmed in Romania Feeding is forbidden during the winter, which is the only time it occurs in Romania |
Allowed, since October 2016 It is provided in Governmental Decision no. 830/2016, amended, at Art. 28, point b |
Prohibited since 2016 It is provided in Governmental Decision no. 830/2016, amended, at Art. 28, point f |
|
| Estonia |
January 2016 Fifty per cent of the sub‐adult and adult wild boar shot must be female Decree of the Environmental Board from 31/8/2016 Contracts with Estonian Hunters’ Society (previously separate contracts with hunting clubs) |
September 2014 |
Forbidden all year around September 2015 |
Max. 100 kg in feeding machine; on‐ground max. 5 kg feed per feeding slot/place (max. 100 kg/month per place). Distance between baiting places must be at least 1 km September 2015 Bating places must be registered 1 baiting place per 1,000 ha of hunting ground October 2016 |
Prohibited October 2014 Allowed September 2015 |
Fifty per cent of hunted wild boar must be piglets Decree of the Environmental Board from 31/8/2016 Trail cameras at baiting places Decree of the Environmental Board from 31/8/2016 |
| Lithuania | November 2015 | February 2014 | Forbidden all year around | Max. 100 kg of the specially designed content per baiting place. Forbidden to put the feed on the ground |
Allowed from 15 October until 1 February Within the areas defined in parts I, II and III of the annex to the Commission Implementing Decision 2014/709/UE, hunt should be organised once a month in one part of the hunting ground, to avoid extensive movement of the wild boar |
|
| Latvia | November 2015 | From June 2014 | Banned since December 2014 | Max. 400 L/1,000 ha only in containers ensuring dosage supply (dosimeter) | Allowed (except in 20 km wide buffer zone in territories of Part 2 bordering Part 1) |
A.3. Extensive literature search methodology
A.3.1. Search 1. Transmission and surveillance of ASF in wild boar
Date of the search: 7/8/2017
Limits: exclude Japanese and Chinese languages, date of publication 2015 onwards
Table A.3.
Search 1 in Web of Science platform
| Set | Query | Results |
|---|---|---|
| # 5 |
#3 Refined by: [excluding] LANGUAGES: (CHINESE OR JAPANESE) Timespan=2015‐2017 Search language=Auto |
464 |
| # 4 |
#3 Timespan=2015‐2017 Search language=Auto |
487 |
| # 3 |
#2 OR #1 Timespan=All years Search language=Auto |
3,550 |
| # 2 |
TS=((ASFV OR ASF) AND (“sus scrofa” OR boar OR boars OR pig OR pigs OR hog OR hogs OR swine)) Timespan=All years Search language=Auto |
1,496 |
| # 1 |
TS=(“African swine fever” OR “African swine virus” OR “African swine plague”) Timespan=All years Search language=Auto |
3,483 |
Results after de‐duplication within Web of Science platform: 422.
Table A.4.
Search 1 in Scopus
| Set | Query | Results |
|---|---|---|
| 22 | (TITLE‐ABS‐KEY (“African swine fever” OR “African swine virus” OR “African swine plague”)) OR (TITLE‐ABS‐KEY ((asfv OR asf) AND (”sus scrofa” OR boar OR pig OR pigs OR hog OR hogs OR swine))) AND PUBYEAR > 2014 | 238 document results |
| 20 | (TITLE‐ABS‐KEY (“African swine fever” OR “African swine virus” OR “African swine plague”)) OR (TITLE‐ABS‐KEY ((asfv OR asf) AND (“sus scrofa” OR boar OR boars OR pig OR pigs OR hog OR hogs OR swine))) | 1,583 document results |
| 15 | TITLE‐ABS‐KEY ((asfv OR asf) AND (“sus scrofa” OR boar OR boars OR pig OR pigs OR hog OR hogs OR swine)) | 866 document results |
| 14 | TITLE‐ABS‐KEY (“African swine fever” OR “African swine virus” OR “African swine plague”) | 1,554 document results |
Results after de‐duplication within Scopus: 233.
Table A.5.
Search 1 in Pubmed
| Search | Query | Items found |
|---|---|---|
| #15 | Search ((“African Swine Fever”[Mesh] OR “African Swine Fever Virus”[Mesh] OR “African swine fever”[tiab] OR “African swine virus”[tiab] OR “African swine plague”[tiab])) OR ((ASFV[tiab] OR ASF[tiab]) AND (“sus scrofa”[tiab] OR boar[tiab] OR boars[tiab] OR pig[tiab] OR pigs[tiab] OR hog[tiab] OR hogs[tiab] OR swine[tiab] OR “Sus scrofa”[Mesh])) Filters: Publication date from 2015/01/01 | 208 |
| #14 | Search ((“African Swine Fever”[Mesh] OR “African Swine Fever Virus”[Mesh] OR “African swine fever”[tiab] OR “African swine virus”[tiab] OR “African swine plague”[tiab])) OR ((ASFV[tiab] OR ASF[tiab]) AND (“sus scrofa”[tiab] OR boar[tiab] OR boars[tiab] OR pig[tiab] OR pigs[tiab] OR hog[tiab] OR hogs[tiab] OR swine[tiab] OR “Sus scrofa”[Mesh])) | 1,338 |
| #13 | Search (ASFV[tiab] OR ASF[tiab]) AND (“sus scrofa”[tiab] OR boar[tiab] OR boars[tiab] OR pig[tiab] OR pigs[tiab] OR hog[tiab] OR hogs[tiab] OR swine[tiab] OR “Sus scrofa”[Mesh]) | 764 |
Final number of results after de‐duplication among databases: 456 results.
A.3.2. Search 2. Management and ecology of wild boar in Europe
Date of the search: 08/08/2017
Limits: exclude Japanese and Chines, Korean languages, date of publication 2015 onwards
Table A.6.
Search 2 in Web of Science platform
| Set | Query | Results |
|---|---|---|
| #9 |
#7 AND #6 Refined by: [excluding] LANGUAGES: (JAPANESE OR KOREAN) Timespan=2015‐2017 Search language=Auto |
888 |
| # 8 |
#7 AND #6 Timespan=2015‐2017 Search language=Auto |
892 |
| # 7 |
TS=(Europe* OR Scandinavi* OR Mediterranean OR Baltic OR Eurasia* OR Iberian OR Andorra* OR Alban OR Austria* OR Belarus* OR Byelarus* OR Bosni* OR Herzegovin* OR Croat* OR Cyprus OR Cypriot* OR Czech OR Belgi* OR Bulgaria* OR Denmark OR Danish OR Estonia* OR Finland OR Finnish OR France* OR French* OR German* OR Greece OR Greek OR Hungar* OR Iceland* OR Ital* OR Sicil* OR Sardinia* OR Latvi* OR Liechtenstein* OR Lithuania* OR Luxembourg* OR Macedonia* OR Malta OR Maltese OR Moldova* OR Monaco OR Montenegr* OR Netherlands OR Dutch OR Norway OR Norwegian* OR Svalbard* OR Poland* OR Polish* OR Portugal OR Portuguese OR Romania* OR Roumania* OR Rumania* OR “San Marino” OR Serb* OR Slovak* OR Slovenia* OR Spain* OR Spanish* OR Sweden OR Swedish OR Switzerland OR Swiss OR “Great Britain* “OR British* OR “Channel Islands*” OR Guerns* OR England* OR English* OR Hebrid* OR Ireland* OR Irish* OR Scotland* OR Scotch* OR Scottish* OR Wales* OR Welsh* OR United Kingdom* OR UK OR Gibraltar OR Ukrain* OR Vatican OR Yugoslavia* OR Russia*) OR AD=(Europe* OR Scandinavi* OR Mediterranean OR Baltic OR Eurasia* OR Iberian OR Andorra* OR Alban OR Austria* OR Belarus* OR Byelarus* OR Bosni* OR Herzegovin* OR Croat* OR Cyprus OR Cypriot* OR Czech OR Belgi* OR Bulgaria* OR Denmark OR Danish OR Estonia* OR Finland OR Finnish OR France* OR French* OR German* OR Greece OR Greek OR Hungar* OR Iceland* OR Ital* OR Sicil* OR Sardinia* OR Latvi* OR Liechtenstein* OR Lithuania* OR Luxembourg* OR Macedonia* OR Malta OR Maltese OR Moldova* OR Monaco OR Montenegr* OR Netherlands OR Dutch OR Norway OR Norwegian* OR Svalbard* OR Poland* OR Polish* OR Portugal OR Portuguese OR Romania* OR Roumania* OR Rumania* OR “San Marino” OR Serb* OR Slovak* OR Slovenia* OR Spain* OR Spanish* OR Sweden OR Swedish OR Switzerland OR Swiss OR “Great Britain* “OR British* OR “Channel Islands*” OR Guerns* OR England* OR English* OR Hebrid* OR Ireland* OR Irish* OR Scotland* OR Scotch* OR Scottish* OR Wales* OR Welsh* OR United Kingdom* OR UK OR Gibraltar OR Ukrain* OR Vatican OR Yugoslavia* OR Russia*) Timespan=2015‐2017 Search language=Auto |
3,272,794 |
| # 6 |
#5 AND #1 Timespan=2015‐2017 Search language=Auto |
1,217 |
| # 5 |
#4 OR #3 OR #2 Timespan=2015‐2017 Search language=Auto |
2,986,094 |
| # 4 |
TS=(depopulat* OR “shot” OR shoot* OR harvest* OR mortalit* OR hunt* OR bait* OR trap* OR “game” OR gaming OR carcass* OR feed* OR fenc* OR barrier* OR trade OR trading OR trade OR feed* OR habitat* OR ecosystem* OR (reproduct* NEAR/3 pattern*) OR diet* OR movement* OR displacement* OR behaviour* OR behavior* OR telemetr* OR collar* OR philopatry OR (space NEAR/3 use) OR (home NEAR/3 range) OR (spatial NEAR/3 (use* OR distribution OR pattern))) Timespan=2015‐2017 Search language=Auto |
2,158,728 |
| # 3 |
TI=(manag* OR limit* OR reduc* OR increas* OR decreas* OR grow* OR abundan* OR regulat* OR control* OR eradicat* OR cull* OR eliminat* OR extermin* OR dynamics* OR distribution OR density OR track*) Timespan=2015‐2017 Search language=Auto |
988,215 |
| # 2 |
TS=(((population OR demograhp*) NEAR/5 (manag* OR limit* OR reduc* OR increas* OR decreas* OR grow* OR abundan* OR regulat* OR control* OR eradicat* OR cull* OR eliminat* OR extermin* OR dynamics* OR distribution OR density OR pattern* OR track* OR model* OR trend* OR size)) OR (number* NEAR/5 (management* OR reduc* OR increase* OR decreas* OR regulat* OR grow* OR control*))) Timespan=2015‐2017 Search language=Auto |
266,059 |
| # 1 |
TS=((pig OR pigs OR boar OR boars OR swine OR hog OR hogs OR scrofa OR sus) NEAR/3 (wild OR feral OR bush OR razorback)) Timespan=2015‐2017 Search language=Auto |
1,855 |
Removing results already obtained in Search 1: 819.
Duplication within database: 719.
Table A.7.
Search 2 in Scopus
| Search | Search Terms | Results |
|---|---|---|
| 15 | #14 AND (EXCLUDE (LANGUAGE, “Chinese”)) | 521 document results |
| 14 | #13 AND #12 AND #7 | 523 document results |
| 13 | TITLE‐ABS‐KEY (europe* OR scandinavi* OR mediterranean OR baltic OR eurasia* OR iberian OR andorra* OR alban OR austria* OR belarus* OR byelarus* OR bosni* OR herzegovin* OR croat* OR cyprus OR cypriot* OR czech OR belgi* OR bulgaria* OR denmark OR danish OR estonia* OR finland OR finnish OR france* OR french* OR german* OR greece OR greek OR hungar* OR iceland* OR ital* OR sicil* OR sardinia* OR latvi* OR liechtenstein* OR lithuania* OR luxembourg* OR macedonia* OR malta OR maltese OR moldova* OR monaco OR montenegr* OR netherlands OR dutch OR norway OR norwegian* OR svalbard* OR poland* OR polish* OR portugal OR portuguese OR romania* OR roumania* OR rumania* OR “San Marino” OR serb* OR slovak* OR slovenia* OR spain* OR spanish* OR sweden OR swedish OR switzerland OR swiss OR “Great Britain*”OR british* OR “Channel Islands*” OR guerns* OR england* OR english* OR hebrid* OR ireland* OR irish* OR scotland* OR scotch* OR scottish* OR wales* OR welsh* OR “united kingdom*” OR uk OR gibraltar OR ukrain* OR vatican OR yugoslavia* OR russia*) OR AFFIL (europe* OR scandinavi* OR mediterranean OR baltic OR eurasia* OR iberian OR andorra* OR alban OR austria* OR belarus* OR byelarus* OR bosni* OR herzegovin* OR croat* OR cyprus OR cypriot* OR czech OR belgi* OR bulgaria* OR denmark OR danish OR estonia* OR finland OR finnish OR france* OR french* OR german* OR greece OR greek OR hungar* OR iceland* OR ital* OR sicil* OR sardinia* OR latvi* OR liechtenstein* OR lithuania* OR luxembourg* OR macedonia* OR malta OR maltese OR moldova* OR monaco OR montenegr* OR netherlands OR dutch OR norway OR norwegian* OR svalbard* OR poland* OR polish* OR portugal OR portuguese OR romania* OR roumania* OR rumania* OR “San Marino” OR serb* OR slovak* OR slovenia* OR spain* OR spanish* OR sweden OR swedish OR switzerland OR swiss OR “Great Britain*” OR british* OR “Channel Islands*” OR guerns* OR england* OR english* OR hebrid* OR ireland* OR irish* OR scotland* OR scotch* OR scottish* OR wales* OR welsh* OR “united kingdom*” OR uk OR gibraltar OR ukrain* OR vatican OR yugoslavia* OR russia*) | 24,992,464 document results |
| 7 | PUBYEAR > 2014 | 7,362,598 document results |
| 6 | #1 AND #5 | 3,250 document results |
| 5 | #2 OR #3 OR #4 | 17,892,862 document results |
| 4 | TITLE‐ABS‐KEY (depopulat* OR “shot” OR shoot* OR harvest* OR mortalit* OR hunt* OR bait* OR trap* OR “game” OR gaming OR carcass* OR feed* OR fenc* OR barrier* OR trade OR trading OR trade OR feed* OR habitat* OR ecosystem* OR (reproduct* W/3 pattern*) OR diet* OR movement* OR displacement* OR behaviour* OR behavior* OR telemetr* OR collar* OR philopatry OR (space W/3 use) OR (home W/3 range) OR (spatial W/3 (use* OR distribution OR pattern))) | 12,472,244 document results |
| 3 | TITLE (manag* OR limit* OR reduc* OR increas* OR decreas* OR grow* OR abundan* OR regulat* OR control* OR eradicat* OR cull* OR eliminat* OR extermin* OR dynamics* OR distribution OR density OR track*) | 6,128,220 document results |
| 2 | TITLE‐ABS‐KEY (((population OR demograhp*) W/5 (manag* OR limit* OR reduc* OR increas* OR decreas* OR grow* OR abundan* OR regulat* OR control* OR eradicat* OR cull* OR eliminat* OR extermin* OR dynamic* OR distribution OR density OR pattern* OR track* OR model* OR trend* OR size)) OR (number* W/5 (management* OR reduc* OR increase* OR decreas* OR regulat* OR grow* OR control*))) | 1,419,372 document results |
| 1 | TITLE‐ABS‐KEY ((pig OR pigs OR boar OR boars OR swine OR hog OR hogs OR scrofa OR sus) W/3 (wild OR feral OR bush OR razorback)) | 5,608 document results |
Removing results already obtained in Search 1: 482.
Duplication within database: 476.
Table A.8.
Search 2 in Pubmed
| Search | Query | Items found |
|---|---|---|
| #54 | Search #52 NOT #53 Filters: Publication date from 2015/01/01 | 382 |
| #53 | Search (“chinese”[Language] OR “japanese”[Language] OR “korean”[language]) Filters: Publication date from 2015/01/01 | 36,569 |
| #52 | Search #51 Filters: Publication date from 2015/01/01 | 383 |
| #51 | Search (#49 AND #50) | 1,447 |
| #50 | Search (Europe[MeSH] OR Europe*[tw] OR Scandinavia* [tw] OR Mediterranean[tw] OR Baltic[tw] OR Iberia*[tw] OR Eurasia*[tw] OR Andorra*[tw] OR Azerbaijan*[tw] OR Albania*[tw] OR Armenia*[tw] OR Austria*[tw] OR Belarus*[tw] OR Byelarus*[tw] OR Bosni*[tw] OR Herzegovin*[tw] OR Croat*[tw] OR Cyprus[tw] OR Cypriot*[tw] OR Czech[tw] OR Belgi*[tw] OR Bulgaria*[tw] OR Denmark[tw] OR Danish[tw] OR Estonia*[tw] OR Finland[tw] OR Finnish[tw] OR France*[tw] OR French*[tw] OR German*[tw] OR Greece[tw] OR Greek[tw] OR Hungar*[tw] OR Iceland*[tw] OR Ital*[tw] OR Sicil*[tw] OR Sardinia*[tw] OR Latvi*[tw] OR Liechtenstein*[tw] OR Lithuania*[tw] OR Luxembourg*[tw] OR Macedonia*[tw] OR Malta[tw] OR Maltese[tw] OR Moldova*[tw] OR Monaco[tw] OR Montenegr*[tw] OR Netherlands[tw] OR Dutch[tw] OR Norway[tw] OR Norwegian*[tw] or Svalbard*[tw] OR Poland*[tw] OR Polish*[tw] OR Portugal[tw] OR Portuguese[tw] OR Romania*[tw] OR Roumania*[tw] OR Rumania*[tw] OR San Marino[tw] OR Serb*[tw] OR Slovak*[tw] OR Slovenia*[tw] OR Spain*[tw] OR Spanish*[tw] OR Sweden[tw] OR Swedish[tw] OR Switzerland[tw] OR Swiss[tw] OR Great Britain*[tw] OR British*[tw] OR Channel Islands*[tw] OR Guerns*[tw] OR England*[tw] OR English*[tw] OR Hebrid*[tw] OR Ireland*[tw] OR Irish*[tw] OR Scotland*[tw] OR Scotch*[tw] OR Scottish*[tw] OR Wales*[tw] OR Welsh*[tw] OR United Kingdom*[tw] OR UK[tw] OR Gibraltar[tw] OR Ukrain*[tw] OR Vatican[tw] OR Yugoslavia*[tw] OR Russia*[tw])OR Europe*[ad] OR Scandinavia* [ad] OR Mediterranean[ad] OR Baltic[ad] OR Iberia*[ad] OR Eurasia*[ad] OR Andorra*[ad] OR Azerbaijan*[ad] OR Albania*[ad] OR Armenia*[ad] OR Austria*[ad] OR Belarus*[ad] OR Byelarus*[ad] OR Bosni*[ad] OR Herzegovin*[ad] OR Croat*[ad] OR Cyprus[ad] OR Cypriot*[ad] OR Czech[ad] OR Belgi*[ad] OR Bulgaria*[ad] OR Denmark[ad] OR Danish[ad] OR Estonia*[ad] OR Finland[ad] OR Finnish[ad] OR France*[ad] OR French*[ad] OR German*[ad] OR Greece[ad] OR Greek[ad] OR Hungar*[ad] OR Iceland*[ad] OR Ital*[ad] OR Sicil*[ad] OR Sardinia*[ad] OR Latvi*[ad] OR Liechtenstein*[ad] OR Lithuania*[ad] OR Luxembourg*[ad] OR Macedonia*[ad] OR Malta[ad] OR Maltese[ad] OR Moldova*[ad] OR Monaco[ad] OR Montenegr*[ad] OR Netherlands[ad] OR Dutch[ad] OR Norway[ad] OR Norwegian*[ad] or Svalbard*[ad] OR Poland*[ad] OR Polish*[ad] OR Portugal[ad] OR Portuguese[ad] OR Romania*[ad] OR Roumania*[ad] OR Rumania*[ad] OR San Marino[ad] OR Serb*[ad] OR Slovak*[ad] OR Slovenia*[ad] OR Spain*[ad] OR Spanish*[ad] OR Sweden[ad] OR Swedish[ad] OR Switzerland[ad] OR Swiss[ad] OR Great Britain*[ad] OR British*[ad] OR Channel Islands*[ad] OR Guerns*[ad] OR England*[ad] OR English*[ad] OR Hebrid*[ad] OR Ireland*[ad] OR Irish*[ad] OR Scotland*[ad] OR Scotch*[ad] OR Scottish*[ad] OR Wales*[ad] OR Welsh*[ad] OR United Kingdom*[ad] OR UK[ad] OR Gibraltar[ad] OR Ukrain*[ad] OR Vatican[ad] OR Yugoslavia*[ad] OR Russia*[ad] | 7,953,884 |
| #49 | Search #43 AND #48 | 2,409 |
| #48 | Search #47 OR #46 OR #45 OR #44 | 6,925,146 |
| #47 | Search “Animal Husbandry”[Mesh] OR “Animal Distribution”[Mesh] OR “Behavior, Animal”[Mesh] OR “Animal Identification Systems”[Mesh] OR “Animal Feed”[Mesh] OR “Locomotion”[Mesh] OR “Movement”[Mesh:NoExp] OR “Population Density”[Mesh] OR “Ecosystem”[Mesh] | 663,431 |
| #46 | Search (shot[tiab] OR shoot*[tiab] OR harvest*[tiab] OR mortalit*[tiab] OR hunt*[tiab] OR bait*[tiab] OR trap*[tiab] OR game[tiab] OR gaming[tiab] OR carcass*[tiab] OR feed*[tiab] OR fenc*[tiab] OR barrier*[tiab] OR trade[tiab] OR tradi*[tiab] OR feed*[tiab] OR habitat*[tiab] OR ecosystem[tiab] OR (reproduct*[tiab] AND pattern*[tiab]) OR diet[tiab] OR dieta*[tiab] OR movement*[tiab] OR displacement*[tiab] OR behaviour*[tiab] OR behavior*[tiab] OR telemetr*[tiab] OR collar*[tiab] OR philopatry[tiab] OR space use[tiab] OR home range*[tiab] OR (spatial[tiab] AND (use*[tiab] OR distribution*[tiab] OR pattern*[tiab]))) | 3,387,654 |
| #45 | Search manag*[ti] OR limit*[ti] OR reduc*[ti] OR increas*[ti] OR decreas*[ti] OR grow*[ti] OR abundan*[ti] OR regulat*[ti] OR control*[ti] OR eradicat*[ti] OR cull*[ti] OR eliminat*[ti] OR extermin* [ti] OR dynamics*[ti] OR distribution[ti] OR density[ti] OR track*[ti] | 2,509,738 |
| #44 | Search ((population[tiab] OR demograhp*[tiab]) AND (manag*[tiab] OR limit*[tiab] OR reduc*[tiab] OR increas*[tiab] OR decreas*[tiab] OR grow*[tiab] OR abundan*[tiab] OR regulat*[tiab] OR control*[tiab] OR eradicat*[tiab] OR cull*[tiab] OR eliminat*[tiab] OR extermin* [tiab] OR dynamics*[tiab] OR distribution[tiab] OR density[tiab] OR pattern*[tiab] OR track*[tiab] OR model*[tiab] OR trend[tiab])) OR population size*[tiab] OR (number*[tiab] AND (manage*[tiab] OR reduc*[tiab] OR increase*[tiab] OR decreas*[tiab] OR grow*[tiab] OR regulat*[tiab] OR control*[tiab])) OR depopulat*[tiab] | 1,885,734 |
| #43 | Search ((pig[tiab] OR pigs[tiab] OR boar[tiab] OR boars[tiab] OR swine[tiab] OR hog[tiab] OR hogs[tiab] OR scrofa[tiab] OR sus[tiab]) AND (wild[tiab] OR feral[tiab] OR bush[tiab] OR razorback[tiab])) | 5,435 |
Removing results already obtained in Search 1: 341.
Suggested citation: EFSA (European Food Safety Authority) , Depner K, Gortazar C, Guberti V, Masiulis M, More S, Oļševskis E, Thulke H‐H, Viltrop A, Woźniakowski G, Cortiñas Abrahantes J, Gogin A, Verdonck F and Dhollander S, 2017. Scientific report on the epidemiological analyses of African swine fever in the Baltic States and Poland. EFSA Journal 2017;15(11):5068, 59 pp. 10.2903/j.efsa.2017.5068
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00154
Acknowledgements: EFSA wishes to thank the following for the support provided to this scientific output: reviewers Irene Iglesias Martín, Liisa Sihvonen, Margaret Good, Søren Saxmose Nielsen, Paolo Calistri, Petr Šatrán. EFSA wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output, especially Anna Hoffman, Daina Pūle, Dina Ivanova, Ilgvars Zihmanis, Jolanta Urbelionyt≐, Katrin Lõhmus, Krystyna Pędrakowska, Łukasz Bocian, Maria Mihaita, Marius Judickas, Mārtiņš Seržants, Maxim Sirbu, Michał Popiołek, Peep Männil, Richard Wallo and Tomasz Podgórski.
Adopted: 27 October 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure A.1.5.: © State Veterinary Administration of the Czech Republic; A.1.6.: © National Sanitary Veterinary and Food Safety Authority of Romania.
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1312/full
Notes
Carcass removal implied the exclusion of carcasses after 1 week (default), 2 weeks (CR2) or 4 weeks (CR4). The carcasses to exclude are randomly withdrawn from all carcasses in the treatment zone using the carcass removal efficiency parameter as individual event probability. Carcasses not removed remain until decomposed.
References
- Acevedo P, Vicente J, Höfle U, Cassinello J, Ruiz‐Fons JF and Gortázar C, 2007. Estimation of European wild boar relative abundance and aggregation: a novel method in epidemiological risk assessment. Epidemiology and Infection, 135, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasona JA, Acevedo P, Diez‐Delgado I, Queiros J, Carrasco‐García R, Gortazar C and Vicente J, 2016. Tuberculosis‐associated death among adult wild boars, Spain, 2009–2014. Emerging Infectious Diseases, 22, 2178–2180. 10.3201/eid2212.160677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J, Iglesias I, Muñoz MJ and de la Torre A, 2016. A cartographic tool for managing African swine fever in Eurasia: mapping wild boar distribution based on the quality of available habitats. Transboundary and Emerging Diseases, 10.1111/tbed.12559 [DOI] [PubMed] [Google Scholar]
- Brondum MC, Collier ZA, Luke CS, Goatcher BL and Linkov I, 2017. Selection of invasive wild pig countermeasures using multicriteria decision analysis. Science of the Total Environment, 574, 1164–1173. 10.1016/j.scitotenv.2016.09.155 [DOI] [PubMed] [Google Scholar]
- Carrasco‐Garcia R, Barroso P, Perez‐Olivares J, Montoro V and Vicente J, 2017. Consumption of big game remains by facultative scavengers: potential risk for diseases transmission in Central Spain. In press. [DOI] [PMC free article] [PubMed]
- Cleveland WS, 1979. Robust locally weighted regression and smoothing scatterplots. Journal of the American Statistical Association, 74, 829–836. 10.2307/2286407 [DOI] [Google Scholar]
- Davies K, Goatley LC, Guinat C, Netherton CL, Gubbins S, Dixon LK and Reis AL, 2017. Survival of African swine fever virus in excretions from pigs experimentally infected with the Georgia 2007/1 isolate. Transboundary and Emerging Diseases, 64, 425–431. 10.1111/tbed.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Evaluation of possible mitigation measures to prevent introduction and spread of African swine fever virus through wild boar. EFSA Journal 2014;12(3):3616, 23 pp. 10.2903/j.efsa.2014.3616 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2010a. Scientific Opinion on African Swine Fever. EFSA Journal 2010;8(3):1556, 149 pp. 10.2903/j.efsa.2010.1556. Available online: http://www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal and Welfare), 2010b. Scientific Opinion on the Role of Tick Vectors in the Epidemiology of Crimean Congo Hemorrhagic Fever and African Swine Fever in Eurasia. EFSA Journal 2010;8(8):1703, 156 pp. 10.2903/j.efsa.2010.1703 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2014. Scientific Opinion on African swine fever. EFSA Journal 2014;12(4):3628, 77 pp. 10.2903/j.efsa.2014.3628 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2015. Scientific opinion on African swine fever. EFSA Journal 2015;13(7):4163, 92 pp. 10.2903/j.efsa.2015.4163 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Cortinas‐Abrahantes J, Gogin A, Richardson J and Gervelmeyer A, 2017. Scientific report on epidemiological analyses on African swine fever in the Baltic countries and Poland. EFSA Journal 2017;15(3):4732, 73 pp. 10.2903/j.efsa.2017.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattebert J, Baubet E, Slotow R and Fischer C, 2017. Landscape effects on wild boar home range size under contrasting harvest regimes in a human‐dominated agro‐ecosystem. European Journal of Wildlife Research, 63, 32. [Google Scholar]
- Fodor JT, Janoska F and Farkas A, 2015. “The comparative analysis of the habitat use of wild boar in different Romanian habitats (partial results).” Proceedings of the Biennial International Symposium. Forest and sustainable development, Brasov, Romania, 24‐25th October 2014: 365–370.
- Frączyk M, Woźniakowski G, Kowalczyk A, Bocian L, Kozak E, Niemczuk K and Pejsak Z, 2016. Evolution of African swine fever virus genes related to evasion of host immune response. Veterinary Microbiology, 193, 133–144. [DOI] [PubMed] [Google Scholar]
- Frauendorf M, Gethöffer F, Siebert U and Keuling O, 2016. The influence of environmental and physiological factors on the litter size of wild boar (Sus scrofa) in an agriculture dominated area in Germany. The Science of the Total Environment, 541, 877–882. 10.1016/j.scitotenv.2015.09.128 [DOI] [PubMed] [Google Scholar]
- Gamelon M, Besnard A, Gaillard J‐M, Servanty S, Baubet E, Brandt S and Giminez O, 2011. High hunting pressure selects for earlier birth date: wild boar as a case study. Evolution, 65, 3100–3112. 10.1111/j.1558-5646.2011.01366.x [DOI] [PubMed] [Google Scholar]
- Gamelon M, Focardi S, Baubet E, Brandt S, Franzetti B, Ronchi F, Venner S, Sæther B‐E and Gaillard J‐M, 2017. Reproductive allocation in pulsed‐resource environments: a comparative study in two populations of wild boar. Oecologia, 183, 1065–1076. 10.1007/s00442-017-3821-8 [DOI] [PubMed] [Google Scholar]
- Gortázar C, Ruiz‐Fonz JF and Höfle U, 2016. Infections shared with wildlife: an updated perspective. European Journal of Wildlife Research, 62, 511–525. 10.1007/s10344-016-1033-x [DOI] [Google Scholar]
- Guinat C, Gubbins S, Vergne T, Gonzales JL, Dixon L and Pfeiffer DU, 2016a. Experimental pig‐to‐pig transmission dynamics for African swine fever virus, Georgia 2007/1 strain. Epidemiology and Infection, 144, 25–34. 10.1017/S0950268815000862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat C, Gubbins S, Vergne T, Gonzales JL, Dixon L and Pfeiffer DU, 2016b. Experimental pig‐to‐pig transmission dynamics for African swine fever virus, Georgia 2007/1 strain‐ CORRIGENDUM. Epidemiology and Infection, 144, 3564–3566. 10.1017/S0950268816001667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat C, Vergne T, Jurado‐Diaz C, Sánchez‐Vizcaíno JM, Dixon L and Pfeiffer DU, 2017. Effectiveness and practicality of control strategies for African swine fever: what do we really know? Veterinary Record, 180, 97 10.1136/vr.103992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Martin Izquierdo V, Gil G, Cavicchia M and Maranta A, 2017. Coping with wild boar in a conservation area: impacts of a 10‐year management control program in north‐eastern Argentina. Biological Invasions, 19, 11–24. 10.1007/s10530-016-1256-5 [DOI] [Google Scholar]
- Helmholtz Centre for Environmental Research GmbH , Thulke H‐H and Lange M, 2017. Simulation‐based investigation of ASF spread and control in wild life without consideration of human non‐compliance to biosecurity. EFSA supporting publication 2017:EN‐1312, 64 pp. 10.2903/sp.efsa.2017.en-1312 [DOI] [Google Scholar]
- Jerina K, Pokorny B and Stergar M, 2014. First evidence of long‐distance dispersal of adult female wild boar (Sus scrofa) with piglets. European Journal of Wildlife Research, 60, 367–370. 10.1007/s10344-014-0796-1 [DOI] [Google Scholar]
- Karalova E, Zakaryan H, Voskanyan H, Arzumanyan H, Hakobyan A, Nersisyan N, Saroyan D, Karalyan N, Tatoyan M, Akopian J, Gazaryantz M, Mkrtchyan Z, Pogosyan L, Nersesova L and Karalyan Z, 2015. Clinical and post‐mortem investigations of genotype II induced African swine fever. Porcine Research, 5, 1–11. [Google Scholar]
- Keuling O, Baubet E, Duscher A, Ebert C, Fischer C, Monaco A, Podgórski T, Prevot C, Ronnenberg K, Sodeikat G, Stier N and Thurfjell H, 2013. Mortality rates of wild boar Sus scrofa L. in central Europe. European Journal of Wildlife Research, 59, 805–814. 10.1007/s10344-013-0733-8 [DOI] [Google Scholar]
- Keuling O, Strauß E and Siebert U, 2016. Regulating wild boar populations is “somebody else's problem”! – human dimension in wild boar management. Science of the Total Environment, 554–555, 311–319. 10.1016/j.scitotenv.2016.02.159 [DOI] [PubMed] [Google Scholar]
- Kopij G and Panek K, 2016. Effect of winter temperature and maize food abundance on long‐term population dynamics of the wild boar Sus scrofa. Polish Journal of Ecology, 64, 436–441. 10.3161/15052249PJE2016.64.3.013 [DOI] [Google Scholar]
- Kruuse M, Enno S‐E and Oja T, 2016. Temporal patterns of wild boar‐vehicle collisions in Estonia, at the northern limit of its range. European Journal of Wildlife Research, 62, 787–791. 10.1007/s10344-016-1042-9 [DOI] [Google Scholar]
- Lange M, 2015. Alternative control strategies against ASF in wild boar populations. EFSA supporting publication 2015:EN‐843, 29 pp. 10.2903/sp.efsa.2015.en-843 [DOI] [Google Scholar]
- Lange M and Thulke H‐H, 2015. Mobile barriers as emergency measure to control outbreaks of African Swine Fever in wild boar. Proceedings of the annual meeting of the Society for Veterinary and Preventive Medicine (SVEPM), Ghent, Belgium, pp. 122–132.
- Loeffen W, Weesendorp E, Moonen‐Leusen B, Hagenaars T and Eble P, 2015. “Quantification of African swine fever virus transmission parameters in carriers and the possible role of indirect virus transmission.” Xth International Congress for Veterinary Virology & 9th Annual Meeting of EPIZONE, Changing Viruses in a Changing World, August 31st ‐ September 3rd 2015, Montpellier, France: 105–106.
- Malmsten A and Dalin AM, 2016. Puberty in female wild boar (Sus scrofa) in Sweden. Acta Veterinaria Scandinavica, 58, 55 10.1186/s13028-016-0236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmsten A, Jansson G, Lundeheim N and Dalin AM, 2017. The reproductive pattern and potential of free ranging female wild boars (Sus scrofa) in Sweden. Acta Veterinaria Scandinavica, 59, 52 10.1186/s13028-017-0321-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malogolovkin A, Burmakina G, Titov A, Sereda A, Gogin A, Baryshnikova E and Kolbasov D, 2015. Comparative analysis of African swine fever virus genotypes and serogroups. Emerging Infectious Diseases, 21, 312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massei G, Kindberg J, Licoppe A, Gačić D, Šprem N, Kamler J, Baubet E, Hohmann U, Monaco A, Ozoliņš J, Cellina S, Podgórski T, Fonseca C, Markov N, Pokorny B, Rosell C and Náhlik A, 2015. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Management Science, 71, 492–500. 10.1002/ps.3965 [DOI] [PubMed] [Google Scholar]
- Mensah JT and Elofsson K, 2017. An empirical analysis of hunting lease pricing and value of game in Sweden. Land Economics, 93, 292–308. [Google Scholar]
- Merli E, Grignolio S, Marcon A and Apollonio M, 2017. Wild boar under fire: the effect of spatial behavior, habitat use and social class on hunting mortality. Journal of Zoology, 303, 155–164. 10.1111/jzo.12471 [DOI] [Google Scholar]
- Michel NL, Laforge MP, Van Beest FM and Brook RK, 2017. Spatiotemporal trends in Canadian domestic wild boar production and habitat predict wild pig distribution. Landscape and Urban Planning, 165, 30–38. 10.1016/j.landurbplan.2017.05.003 [DOI] [Google Scholar]
- Miloš A, Michaela H, Tomáš K and Jaroslav C, 2016. Creeping into a wild boar stomach to find traces of supplementary feeding. Wildlife Research, 43, 590–598. 10.1071/WR16065 [DOI] [Google Scholar]
- Nielsen JP, Larsen TS, Halasa T and Christiansen LE, 2017. Estimation of the transmission dynamics of African swine fever virus within a swine house. Epidemiology and Infection, 145, 2787–2796. 10.1017/S0950268817001613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschmann J, Guinat C, Beer M, Pronin V, Tauscher K, Petrov A, Keil G and Blome S, 2015. Course and transmission characteristics of oral low‐dose infection of domestic pigs and European wild boar with a Caucasian African swine fever virus isolate. Archives of Virology, 160, 1657–1667. 10.1007/s00705-015-2430-2 [DOI] [PubMed] [Google Scholar]
- Plhal R, Kamler J, Homolka M and Drimaj J, 2014. An assessment of the applicability of dung count to estimate the wild boar population density in a forest environment. Journal of Forest Science, 60, 174–180. [Google Scholar]
- Post J, Weesendorp E, Montoya M and Loeffen WL, 2017. Influence of age and dose of African swine fever virus infections on clinical outcome and blood parameters in pigs. Viral Immunology, 30, 58–69. 10.1089/vim.2016.0121 [DOI] [PubMed] [Google Scholar]
- Probst C, Globig A, Knoll B, Conraths FJ and Depner K, 2017. Behaviour of free ranging wild boar towards their dead fellows: potential implications for the transmission of African swine fever. Royal Society Open Science, 4, 170054 10.1098/rsos.170054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirós‐Fernández F, Marcos J, Acevedo P and Gortázar C, 2017. Hunters serving the ecosystem: the contribution of recreational hunting to wild boar population control. European Journal of Wildlife Research, 63, 57 10.1007/s10344-017-1107-4 [DOI] [Google Scholar]
- Ribeiro R, Otte J, Madeira S, Hutchings GH and Boinas F, 2015. Experimental infection of Ornithodoros erraticus sensu stricto with two portuguese African swine fever virus strains. Study of factors involved in the dynamics of infection in ticks. PLoS ONE, 10, e0137718 10.1371/journal.pone.0137718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebler A, Sørbye SH, Simpson D and Rue H, 2016. An intuitive Bayesian spatial model for disease mapping that accounts for scaling. Statistical Methods in Medical Research, 25, 1145–1165. 10.13140/RG.2.1.1899.2080 [DOI] [PubMed] [Google Scholar]
- Ruiz‐Fons JF, Vicente J, Vidal D, Höfle U, Villanúa D, Gauss C, Segalés J, Almería S, Montoro V and Gortazar C, 2006. Seroprevalence of six reproductive pathogens in European wild boar (Sus scrofa) from Spain: the effect of management. Theriogenology, 65, 731–743. [DOI] [PubMed] [Google Scholar]
- Saïd S, Tolon V, Brandt S and Baubet E, 2012. Sex effect on habitat selection in response to hunting disturbance: the study of wild boar. European Journal of Wildlife Research, 58, 107–115. 10.1007/s10344-011-0548-4 [DOI] [Google Scholar]
- Sales LP, Ribeiro BR, Hayward MW, Paglia A, Passamani M and Loyola R, 2017. Niche conservatism and the invasive potential of the wild boar. Journal of Animal Ecology, 86, 12141223 10.1111/1365-2656.12721 [DOI] [PubMed] [Google Scholar]
- Sanna G, Dei Giudici S, Bacciu D, Angioi PP, Giammarioli M, De Mia GM and Oggiano A, 2017. Improved strategy for molecular characterization of African swine fever viruses from sardinia, based on analysis of p30, CD2V and I73R/I329L variable regions. Transboundary and Emerging Diseases, 64, 1280–1286. [DOI] [PubMed] [Google Scholar]
- Scientific review on African swine Fever , 2009. Scientific report submitted to EFSA prepared by Sánchez‐Vizcaíno,J.M., Martínez‐López,B., Martínez‐Avilés, M.,Martins,C., Boinas,F., Vial,L., Michaud,V., Jori,F., Etter,E., Albina,E. and Roger, F. on African Swine Fever. 1–141.
- Scillitani L, Monaco A and Toso S, 2010. Do intensive drive hunts affect wild boar (Sus scrofa) spatial behaviour in Italy? Some evidences and management implications. European Journal of Wildlife Research, 56, 307–318. 10.1007/s10344-009-0314-z [DOI] [Google Scholar]
- Sidorovich V, Schnitzler AE, Schnitzler C, Rotenko I and Holikava Y, 2017. Responses of wolf feeding habits after adverse climatic events in central‐western Belarus. Mammalian Biology, 83, 44–50. 10.1016/j.mambio.2016.11.012 [DOI] [Google Scholar]
- Sprem N, Piria M, Prđun S and Treer T, 2016. Variation of wild boar reproductive performance in different habitat types: implications for management. Russian Journal of Ecology, 47, 96–103. 10.1134/S106741361506017X [DOI] [Google Scholar]
- Ståhlberg S, Bassi E, Viviani V and Apollonio M, 2017. Quantifying prey selection of Northern and Southern European wolves (Canis lupus). Mammalian Biology, 83, 34–43. 10.1016/j.mambio.2016.11.001 [DOI] [Google Scholar]
- Storie JT and Bell S, 2017. Wildlife management conflicts in rural communities: a case‐study of wild boar (Sus scrofa) management in Erglu Novads, Latvia. Sociologica Ruralis, 57, 64–86. 10.1111/soru.12122 [DOI] [Google Scholar]
- Tauscher K, Pietschmann K, Wernike J, Teifke K, Beer Jens P and Blome S, 2015. On the situation of African swine fever and the biological characterization of recent virus isolates. Berliner Und Munchener Tierarztliche Wochenschrift, 128, 169–176. [PubMed] [Google Scholar]
- Wood SN, 2006. Generalized Additive Models: An Introduction with R, 2nd Edition Chapman and Hall/CRC Press, Boca Raton, FL: 419 pp. [Google Scholar]


