Abstract
The EFSA Panel on Plant Health performed a pest categorisation of naturally‐spreading psorosis of citrus for the European Union. Naturally‐spreading psorosis is poorly defined, because the status of both the disease and its causal agent(s) is uncertain. However, Citrus psorosis virus (CPsV) is a well‐ characterised Ophiovirus that is systematically associated with the psorosis disease and therefore considered to be its causal agent. Efficient diagnostics are available for CPsV. It is present in at least three EU MS. Naturally‐spreading psorosis is currently regulated by Directive 2000/29/EC, while CPsV is not explicitly mentioned in this Directive. CPsV has the potential to enter, establish and spread in the EU territory. However, the main pathway for entry is closed by the existing legislation so that entry is only possible through minor alternative pathways. Plants for planting are the major means of spread while there are uncertainties on the existence and efficiency of a natural spread mechanism. CPsV introduction and spread in the EU would have negative consequences on the EU citrus industry. Of the criteria evaluated by EFSA to qualify as a Union quarantine pest or as a Union regulated non‐quarantine pest (RNQP), Naturally‐spreading psorosis does not meet the criterion of being a well characterised pest or disease. As it is not explicitly mentioned in the legislation, it is unclear whether CPsV meets the criterion of being currently regulated or under official control. It meets, however, all the RNQP criteria. The key uncertainties of this categorisation concern: (1) the causal role of CPsV in the psorosis disease as well as elements of its biology and epidemiology, (2) the exact nature of the Naturally‐spreading psorosis syndrome and the identity of its causal agent and, consequently, (3) whether CPsV should be considered as being covered by the current legislation.
Keywords: Citrus psorosis virus, citrus ringspot, psorosis, naturally‐spreading psorosis, European Union, pest risk, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/2002,3 to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L.. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | |
| Cephalcia lariciphila (Klug) | Ips cembrae Heer |
| Dendroctonus micans Kugelan | Ips duplicatus Sahlberg |
| Gilphinia hercyniae (Hartig) | Ips sexdentatus Börner |
| Gonipterus scutellatus Gyll. | Ips typographus Heer |
| Ips amitinus Eichhof | Sternochetus mangiferae Fabricius |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L.,Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Naturally‐spreading psorosis is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation, to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest (RNQP) for the area of the European Union (EU) excluding Ceuta, Melilla and the outermost regions of Member States (MSs) referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
Psorosis is a bark scaling disorder in citrus that may have various causes. Over the years, a number of psorosis or psorosis‐like syndromes have been described, generating a lot of confusion in the literature. It is presently not known with certainty to which syndrome the term ‘naturally‐spreading psorosis’ refers. However, the Ophiovirus Citrus psorosis virus (CPsV) has been characterised starting from the 1980s. CPsV is now assumed to be the causal agent of the psorosis disease of citrus because of its constant association with plants showing typical psorosis symptoms. This pest categorisation therefore focuses on CPsV, taking into account the various names and synonyms given in the past to the postulated causal agent and to the citrus psorosis disease, including the naturally‐spreading psorosis one.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on CPsV was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific and synonymous names of the virus as well as the commonly used disease names as search term. Relevant papers were reviewed, and further references and information were obtained from experts, from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO, 2017).
Data about import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT.
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Consumers (DG SANCO), and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the MSs and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for naturally‐spreading psorosis following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union RNQP in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required as per the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a RNQP . If one of the criteria is not met, the pest will not qualify. Note that a pest that does not qualify as a quarantine pest may still qualify as a RNQP which needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone, thus the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism. | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area). |
| Regulatory status (Section 3.3) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future. |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone). |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met. |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regards to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? (Yes or No)
YES, if ‘naturally‐spreading psorosis’ is interpreted as Citrus psorosis virus (CPsV)
There are doubts about the nature of the specific citrus syndrome and pathogen(s) covered by the term ‘naturally‐spreading psorosis’. However, CPsV is a well characterised virus that is systematically associated with the psorosis disease and therefore assumed to be its causal agent.
As recently reviewed in Achachi et al. (2014) and Moreno et al. (2015), the psorosis disease of citrus was first reported by Swingle and Webber (1896) as a bark scaling disorder of citrus trees and it was the first citrus disease proven to be graft transmissible (Fawcett, 1933, 1934). For many years, it was one of the citrus diseases considered of recalcitrant aetiology (Derrick and Timmer, 2000) and only in 1986 virus‐like particles were found in tissues of diseased plants (Derrick et al., 1988; da Graça et al., 1991).
The most reliable diagnostic symptom of psorosis is the bark scaling that gave its name to the disease. In addition, foliar symptoms are also frequently observed (Roistacher, 1981). The bark scaling symptoms are observed in the trunk and branches with gum production and wood discoloration below the bark lesions. Sometimes, young leaves show chlorotic patterns (flecking, blotching, or ring spots) and some new shoots may show a shock reaction. The fruits may have depressed spots or rings in the rind with discoloured tissue (Achachi et al., 2014; Moreno et al., 2015).
Because the bark scaling or leaf symptoms associated with psorosis may also have other causes4 psorosis was often confused with other diseases (Roistacher, 1981; Malaguti and Knorr, 1961; Knorr, 1981), which were therefore collectively referred to as the ‘psorosis group’. In addition, two versions of the psorosis disease differing in severity have been described, psorosis A (PsA) and psorosis B (PsB) (Fawcett and Klotz, 1938), further adding to confusion in the literature. PsA and PsB were later considered as caused by strains of the same agent because PsA isolates cross protect plants against inoculation with PsB (Wallace, 1957; Velázquez et al., 2012). This cross protection also allowed to differentiate PsB and its causal agent from other diseases with seemingly similar symptoms (Folimonova et al., 2010).
In PsA, bark scaling first appears in some areas of the stem and main branches. Old leaves are usually symptomless but the young ones may show chlorotic flecks. There may be sparse foliage, dieback and reduced yield (Moore and Nauer, 1957). In the more aggressive PsB, bark‐scaling affects even thin branches, sloughing large strips of bark. Chlorotic patterns may appear in the young leaves, while some new shoots may show a necrotic reaction. The old leaves often show chlorotic blotches in the upper side with gum impregnated brownish eruptions in the underside. The PsB‐affected trees may have depressed spots or rings in fruit rind with discoloured tissue or grooves (Moreno et al., 2015). The frequency and severity of symptoms may depend on the variety and the temperature (Roistacher, 1981, 1991, 1993).5
In the EPPO global database (accessed in October 2017), this very complex situation is reflected in two entries, PsA (or citrus scaly bark or psorosis of citrus, associated with Citrus psorosis ophiovirus) and PsB (or naturally‐spreading psorosis or necrotic ringspot, associated with a so‐called Citrus ringspot ophiovirus).
However, partial virus purification and serological assays have provided evidence that citrus ringspot virus and the virus isolates associated with PsA and PsB symptoms are likely strains/isolates of the same viral entity (Navas‐Castillo and Moreno, 1995). Complete genomes of several viral isolates from Florida and Spain associated with citrus psorosis disease were later obtained (Sánchez de la Torre et al., 1998; Sanches de la Torre et al., 2002; Naum‐Onganía et al., 2003; Martín et al., 2005), providing evidence that a single virus with a segmented genome comprising three RNA molecules of negative polarity was implicated in these various syndromes.
Thus, citrus ringspot and citrus psorosis are names given to different syndromes that are associated with the same virus, which is today the type member of the Ophiovirus genus in the family Ophioviridae and was given the name CPsV.6 PsA and PsB have been associated with particular RNA2 sequence variants of CPsV (Velazquez et al., 2015), revealing that ‘subisolate’ sequence variants can be present in the same host and may induce more or less severe symptoms depending on their prevalence/predominance.
Overall, CPsV is the name of a well‐characterised virus that is constantly associated with various syndromes of the psorosis disease, a disease characterised by bark scaling in trunk and branches (with gum production and wood discoloration below the bark lesions) and, frequently, with foliar ringspot or discolouration symptoms. Because infection of citrus plants with purified preparations of CPsV has not been accomplished to fulfil Koch's postulates (Moreno et al., 2015), the assumption that CPsV is the causal agent of the citrus psorosis disease still retains some level of uncertainty. This uncertainty is, however, mitigated by the constant association of CPsV with the disease.
Uncertainty, however, prevails on (the) exact syndrome(s) covered by the term ‘naturally‐spreading psorosis’, which likely was used to discriminate a particular etiological situation corresponding to a progressing psorosis disease, as was for example reported in Argentina (Beñatena and Portillo, 1984) and Texas (Timmer and Garnsey, 1980). The finding that variants of CPsV are associated with most if not all versions of the citrus psorosis disease suggests that CPsV was likely also involved with naturally‐spreading psorosis, but with high uncertainty.
3.1.2. Biology of the pest
CPsV infections are systemic in citrus hosts and phloem‐associated cells as well as parenchymatic tissues are invaded. The main and probably the only pathway of virus dissemination and spread is by vegetative propagation (Moreno et al., 2015). Because of the long period needed for bark symptoms to develop, with scaling appearing only after 10–15 years (Roistacher, 1981), psorosis‐infected trees could be inadvertently selected in the past as budwood sources. This resulted in a high incidence of the disease and its gradual development may have been confused with its spread, possibly leading to the concept of naturally‐spreading psorosis (Bridges et al., 1965; Pujol and Benatena, 1965; Childs and Johnson, 1966; Pujol, 1966; Campiglia et al., 1976).
There are a few reports of seed transmission of CPsV, particularly in trifoliate orange and Carrizo or Troyer citrange. However, there are still uncertainties about the validity of these reports (reviewed in Moreno et al., 2015). Some observations suggested natural spread of psorosis by a vector in Texas and in Argentina but all attempts to identify insects or fungi as potential vectors have been unsuccessful (Timmer, 1974; Timmer and Garnsey, 1980). An apparent natural spread of the psorosis disease in citrus orchards (Timmer and Garnsey, 1980; Beñatena and Portillo, 1984) lead to the isolation of Olpidium sp. zoospores from roots of psorosis‐infected trees (Palle et al., 2005). CPsV presence in or on these zoospores was tentatively detected using polymerase chain reaction (PCR). Since other ophioviruses have been demonstrated to be transmitted by soil Olpidium species (Rochon et al., 2004), transmission of CPsV by this soil‐borne vector is possible. However, it is still unclear whether Olpidium is a vector of CPsV.
3.1.3. Intraspecific diversity
There is evidence that specific CPsV isolates, often in mixed infection, may be associated with more or less severe psorosis symptoms (Velázquez et al., 2012), but the inability to separate these isolates and evaluate independently their pathogenicity adds uncertainty to this assessment.
Sequence comparison based on CP genes (encoded on genomic RNA3) of CPsV isolates from diverse geographical origin showed that different virus phylogroups exist; one including isolates from Spain, Italy, California and Florida, another comprising isolates from Argentina. A CPsV isolate (CPV‐4) included in the analysis clustered separately, suggesting the existence of a third phylogroup (Alioto et al., 2003; Martín et al., 2006).
In addition, the use of monoclonal antibodies has revealed significant epitopic variation in CPsV (Alioto et al., 1999; Djelouah et al., 2000; Martín et al., 2002, 2004).
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
YES, detection methods are available for Citrus psorosis virus (CPsV)
Biological indexing on young sweet orange seedlings was initially used to detect citrus psorosis. This was complemented by a cross protection assay to differentiate PsA and PsB (Roistacher, 1993). With the identification and characterisation of CPsV, other more reliable and less time‐consuming detection techniques became available. Specific antisera and monoclonal antibodies are available for virus detection by ELISA (García et al., 1997; Alioto et al., 1999; D'Onghia et al., 2001; Loconsole et al., 2006).
Complete genome sequences of a number of CPsV isolates are available for comparison and a number of molecular tests, hybridisation assays and reverse transcription polymerase chain reaction (RT‐PCR) protocols allow a reliable detection of CPsV (García et al., 1997; Barthe et al., 1998; Rosa et al., 2007; Osman et al., 2015). A real‐time RT‐PCR‐based assay for simultaneous detection of several citrus viruses, including CPsV, has been developed (Loconsole et al., 2010).
Reliable detection methods are available for CPsV. On the other hand, due to the uncertainties associated with its nature, there are no reliable detection techniques for naturally‐spreading psorosis.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
CPsV has been reported from several countries in the Americas, Asia and Africa (Table 2, Figure 1).
Table 2.
Global distribution of Psorosis Ba (indicated as citrus ringspot virus in the EPPO Global Database) (accessed on the 3 October 2017)
| Continent | Country | Status |
|---|---|---|
| Africa | Algeria | Present, no details |
| Africa | South Africa | Present, no details |
| America | Argentina | Present, no details |
| America | United States of America | Present, restricted distribution/no details |
| America | Uruguay | Present, no details |
| America | Venezuela | Present, no details |
| Asia | India | Present, widespread |
| Asia | Iran | Present, no details |
| Asia | Pakistan | Present, no details |
| Europe | France | Present, restricted distribution/no details (Corse) |
| Europe | Greece | Present, no details |
| Europe | Italy | Present, no details |
| Europe | Netherlands | Absent, confirmed by survey |
| Europe | Slovenia | Absent, no pest record |
| Europe | Spain | Absent, pest no longer present |
| Europe | Turkey | Present, no details |
: Of the two Psorosis entries in the EPPO Global Database, Psorosis B is the only one for which distribution data is available.
Figure 1.
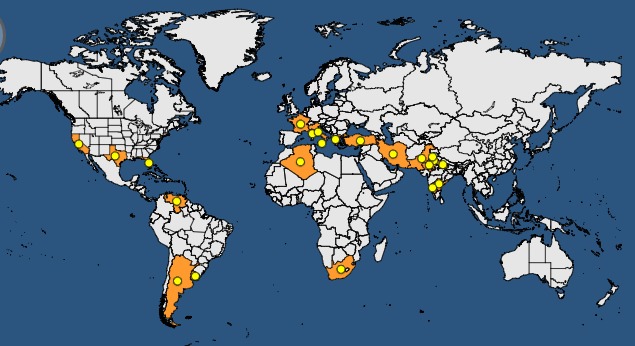
Global distribution of psorosis B (indicated as citrus ringspot virus in the EPPO Global Database) (accessed on the 3 October 2017)
Last updated: 2017‐9‐13
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory?If present, is the pest widely distributed within the EU?
YES
CPsV is reported to be present in Italy (present, no details), in France (present, restricted distribution, except for Corsica: present, no details) and Greece (present, no details). It is reported as ‘Absent, pest no longer present’ in Spain, likely as a consequence of the broad scale certification program in that country. The CPsV status in other citrus‐growing countries is uncertain.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
Naturally‐spreading psorosis is currently regulated in Directive 2000/29 EC. CPsV not formally listed as such in Directive 2000/29. Given the uncertainty on the exact nature of the naturally‐spreading psorosis syndrome, it is unclear whether CPsV should be considered as being covered by the current legislation.
Naturally‐spreading psorosis is listed in Council Directive 2000/29/EC. Details are presented in Tables 3 and 4.
Table 3.
Naturally‐spreading psorosis in Council Directive 2000/29/EC
| Annex II, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned if they are present on certain plants or plant products | |
|---|---|---|
| Section I | Harmful organisms not known to occur in the community and relevant for the entire community | |
| (d) | Virus and virus‐like organisms | |
| Species | Subject of contamination | |
| 10. | Naturally‐spreading psorosis | Plants of Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids, other than fruit and seeds |
3.3.2. Legislation addressing plants and plant parts on which naturally‐spreading psorosis is regulated
Table 4.
Regulated hosts and commodities that may involve naturally‐spreading psorosis in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all member states |
| Description | Country of origin |
| 16. Plants of Citrus L., Fortunella Swinlge, Poncirus Raf., and their hybrids, other than fruit and seeds | Third countries |
| Annex IV, Part A | Special requirements which must be laid down by all member states for the introduction and movement of plants, plant products and other objects into and within all member states |
| Section I | Plants, plant products and other objects originating outside the community |
| Plants, plant products and other objects | Special requirements |
| 16.1 Fruits of Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids, originating in third countries | The fruits shall be free from peduncles and leaves and the packaging shall bear an appropriate origin mark. |
| Section II | Plants, plant products and other objects originating in the community |
| Plants, plant products and other objects | Special requirements |
| 30.1 Fruits of Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids | The packaging shall bear an appropriate origin mark |
| Annex V Part B |
Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the community, before being moved within the community — in the country of origin or the consignor country, if originating outside the community) before being permitted to enter the community Plants, plant products and other objects originating in territories, other than those territories referred to in part A. I. Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community |
|
1. Plants, intended for planting, other than seeds but including seeds of …. Citrus L., Fortunella Swingle and Poncirus Raf., and their hybrids …. 3. Fruits of: ‐ Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids….. |
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
The main natural hosts of CPsV are sweet orange, mandarin and grapefruit which may show symptoms of bark scaling and decline. Many citrus species may show only leaf symptoms (not distinctive of psorosis, being associated to many other syndromes) and may harbour psorosis virus without scaly bark (Roistacher, 1981). Poncirus and Fortunella are also hosts (Moreno et al., 2015). Different varieties and species react either with strong symptoms or remain symptomless (e.g. Fortunella hindsii, Velazquez et al., 2015). Others show resistance to virus inoculation. However, when those tolerant or apparently resistant varieties or species are grafted on CPsV‐infected sweet orange (an indicator host for CPsV), a severe bud union disorder was observed (Velazquez et al., 2016).
Experimental inoculations demonstrated that some citrus relatives, such as Microcitrus, Atalantia, Afraegle, Clausena, Eremocitrus, Pleiospermium, Severinia, Swinglea, are also symptomatic hosts of CPsV (Velazquez et al., 2016). Among those, Microcitrus inodora show asymptomatic infection. There are, however, uncertainties about whether these hosts may be infected under natural conditions.
Aside from citrus and their relatives, the known host range of CPsV is limited and Chenopodium quinoa and Gomphrena globosa, which are used as indicators, are among the few known experimental non‐rutaceous host. Transmission to herbaceous hosts was achieved either by dodder (Price, 1965; Desjardins et al., 1969) or mechanically (Timmer et al., 1978; Garnsey and Timmer, 1980; Roistacher, 1981; Sarachu et al., 1988; Navas‐Castillo et al., 1991).
3.4.2. Entry
Is the pest able to enter into the EU territory? (Yes or No) If yes, identify and list the pathways!
YES
The most important pathway for entry of CPsV is the trade of plants for planting of Citrus, Fortunella and Poncirus and their hybrids, which is closed by the existing Annex III legislation (see Section 3.3.2 and Table 4 above). As a consequence, entry is only considered to be possible through minor alternative pathways.
Trade of plants of Rutaceae species which are not known to be natural hosts of CPsV but have been shown to be experimental hosts (see Section 3.4.1).
Illegal entry of infected plants for planting of susceptible host species for commercial or for personal use.
Between 1995 and the 5 September 2017, there are no interception records for CPsV in the Europhyt database.
3.4.3. Establishment
Is the pest able to become established in the EU territory? (Yes or No)
YES
There are no ecoclimatic constraints for CPsV, except for those affecting its host plants. Therefore, CPsV is expected to be able to establish in areas where its hosts are able to develop and ecoclimatic conditions are not limiting. Indeed CPsV is already present in three EU MS. Citrus cultivation occurs widely in the Mediterranean part of Europe (see EFSA PLH Panel, 2014), while ornamental rutaceous hosts may also grow in protected cultivation in more northern regions of the EU.
3.4.3.1. EU distribution of main host plants
Citrus hosts of CPsV are widely grown for fruit production (oranges, mandarins, lemons, etc.) in eight MS in the Mediterranean part of the EU. In order of decreasing production, they are: Spain, Italy, Greece, Portugal, Cyprus, Croatia, Malta and France (Table 5). In addition, plants of Citrus, Fortunella and Poncirus are grown as ornamentals, either in the open or under protected cultivation in a number of MS.
Table 5.
Area of citrus production (in 1,000 ha) in Europe according to the Eurostat database (Crop statistics apro_acs_a, extracted on 31 August 2017)
| GEO/TIME | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|
| Spain | 310.50 | 306.31 | 302.46 | 298.72 | 295.33 |
| Italy | 146.79 | 163.59 | 140.16 | 149.10 | 141.22 |
| Greece | 50.61 | 49.88 | 49.54 | 46.92 | 44.72 |
| Portugal | 19.85 | 19.82 | 19.80 | 20.21 | 20.21 |
| France | 3.89 | 4.34 | 4.16 | 4.21 | 4.70 |
| Cyprus | 3.21 | 2.63 | 2.69 | 2.84 | 3.29 |
| Croatia | 1.88 | 2.17 | 2.17 | 2.21 | 2.18 |
| Malta | 0.00(n) | 0.00(n) | 0.00(n) | 0.00(n) | 0.00(n) |
Last update 25.8.17.
n: not significant.
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment? (Yes or No) How?
YES. CPsV is able to spread through plants for planting. Natural transmission by Olpidium is an unconfirmed possibility
RNQPs: Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects?
YES
3.4.4.1. Vectors and their distribution in the EU
Plants for planting constitute the main pathway for virus spread and dissemination (Moreno et al., 2015). There are a few reports of seed transmission in Poncirus trifoliata (Pujol, 1966), but there are still uncertainties about the validity of these reports (Moreno et al., 2015). There is no confirmed vector transmission for CPsV (Moreno et al., 2015).
Given CPsV detection in zoospores isolated from roots of an infected citrus (Palle et al., 2005), there is, however the possibility that soil‐borne Olpidium species may be possible vectors. Although Olpidium transmission has not been demonstrated for CPsV, it would probably only account for slow field spread over relatively short distances.7 There are however important uncertainties on this point.
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
YES
RNQPs: Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? 8
YES
Citrus psorosis affects most, if not all, commercial varieties of sweet orange, mandarin (Citrus reticulata), and grapefruit (Citrus paradisi), which in some cases respond with severe growth reduction and decline. In the field, a characteristic bark scaling may be observed on the trunk and branches of CPsV‐infected trees, with gum accumulation and wood staining below the bark scales (Roistacher, 1991, 1993). Other species like sour orange (Citrus aurantium), lemon (Citrus limon) or rough lemon (Citrus jambhiri) do not show bark scaling, but infected plants display psorosis‐like young leaf symptoms (Roistacher, 1981).
Sensitive infected plants have a long latency period (10–12 years) before exhibiting the characteristic bark scaling symptoms (Martín et al., 2002).
Isolate‐dependent resistance has been only confirmed in Cleopatra mandarin (Citrus reshni), trifoliate orange (Poncirus trifoliata), and Carrizo citrange (Citrus sinensis x P. trifoliata). However, when these genotypes are propagated on a CPsV‐inoculated sweet orange a hypersensitive‐like reaction occurs with bark necrosis at the bud union line between the scion and the rootstock (Velazquez et al., 2015). The disease has been a (not relevant) problem in Europe in the past. Since certification systems have started mother trees showing bark scaling have been progressively discarded based on visual check and indexing. Currently only old orchards or some clones may show scaling.
The impact of CPsV appears, however, to be limited in the affected EU MS, possibly as a consequence of the existing voluntary certification schemes. Given that Koch's postulates have not been fulfilled, there are some uncertainties attached to this assessment of the potential impact of CPsV. These uncertainties are, however seen by the Panel as being limited given the constant association of CPsV with psorosis.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
No. Closing the potential minor pathway associated with unregulated rutaceous hosts would likely have limited effects given that CPsV is already present in at least three EU MS
RNQPs: Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated?
YES: existing citrus certification systems constitute a strong limitation to CPsV spread through plants for planting as shown in Spain
3.6.1. Biological or technical factors limiting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
Long latency period for bark symptoms development, transient or absent leaf symptoms at elevated temperatures and uneven distribution of the virus in plants limit visual inspection efficiency.
Existence of asymptomatic CPsV infections in some hosts.
Other factors may induce bark scaling or ringspot symptoms in old leaves or fruits of citrus in the field, possibly resulting in false diagnosis.
3.6.2. Biological or technical factors limiting the ability to prevent the presence of the pest on plants for planting
Long latency period for bark symptoms development, transient or absent leaf symptoms at elevated temperatures and uneven distribution of the virus in plants limit visual inspection efficiency.
Existence of asymptomatic CPsV infections in some hosts.
Other factors may induce bark scaling or ringspot symptoms in old leaves or fruits of citrus in the field, possibly resulting in false diagnosis.
3.6.3. Control methods
Certification programmes are the most efficient control method.
Eradication of infected plants.
3.7. Uncertainty
The major sources of uncertainty concern are as follows:
The causal role of CPsV in the psorosis disease. Koch's postulates have not been fulfilled, and a co‐infection with another, still undetected agent cannot be absolutely excluded.
The existence and efficiency of a natural spread mechanism of CPsV.
The seed transmissibility of CPsV in some citrus species or varieties.
The existence of CPsV natural infection in unregulated rutaceous hosts.
The exact nature of the naturally‐spreading psorosis syndrome and the identity of its causal agent. Consequently, uncertainty on whether CPsV should be considered as being covered by the current legislation.
The precise distribution of CPsV in the EU.
4. Conclusions
Of the criteria evaluated by EFSA to qualify as a Union quarantine pest or as a Union RNQP, naturally‐spreading psorosis does not meet the criterion of being a well characterised pest or disease.
Concerning CPsV, it is unclear whether it meets the quarantine pest criterion of being currently regulated or under official control, as it is not explicitly mentioned in Directive 2000/29/EC. It meets, however, all the RNQP criteria (Table 6).
Table 6.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1) |
There are doubts about the nature of the specific citrus syndrome covered by the term ‘naturally‐spreading psorosis’. However, Citrus psorosis virus (CPsV) is a well characterised virus that is systematically associated with the psorosis disease and therefore considered to be its causal agent |
There are doubts about the nature of the specific citrus syndrome covered by the term ‘naturally‐spreading psorosis’. However, Citrus psorosis virus (CPsV) is a well characterised virus that is systematically associated with the psorosis disease and therefore considered to be its causal agent |
Exact nature of the ‘naturally‐spreading psorosis’ syndrome and identity of its causal agent not clearly established Uncertainty on the causal role of CPsV in the psorosis disease not unambiguously established |
| Absence/presence of the pest in the EU territory (Section 3.2) | CPsV is present in the EU Territory | CPsV is present in the EU Territory | Uncertainty on the precise distribution of CPsV in the EU |
| Regulatory status (Section 3.3) |
Naturally‐spreading psorosis is currently regulated in Directive 2000/29/EC CPsV not listed as such in Directive 2000/29/EC |
Naturally‐spreading psorosis is currently regulated in Directive 2000/29/EC CPsV not listed as such in Directive 2000/29/EC |
Uncertainty on the exact nature of the naturally‐spreading psorosis syndrome and consequently on whether CPsV should be considered as being covered by the current legislation |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | CPsV has the potential to enter, establish and spread in the EU territory. However, the main pathway for entry is closed by the existing legislation so that entry is only possible through minor alternative pathways | Plants for planting are the major mechanism of spread |
Uncertainty on the existence of CPsV natural infection in unregulated rutaceous hosts Uncertainty on the existence and efficiency of a natural spread mechanism of CPsV Uncertainty on the seed‐transmissibility of CPsV in some citrus species or varieties |
| Potential for consequences in the EU territory (Section 3.5) | CPsV introduction and spread in the EU would have negative consequences on the EU citrus industry because CPsV is very likely to be the causal agent of the psorosis disease | Because of its pathogenicity, presence of CPsV on plants for planting would have a negative impact on their intended use | Causal role of CPsV in the psorosis disease not absolutely established |
| Available measures (Section 3.6) | Closing the potential pathway associated with unregulated rutaceous hosts is perceived as having limited relevance given the presence of CPsV in several EU MS | Existing citrus certification systems constitute a strong limitation to CPsV spread |
Uncertainty on the existence of CPsV natural infection in unregulated rutaceous hosts Uncertainty on the seed‐transmissibility of CPsV in some citrus species or varieties |
| Conclusion on pest categorisation (Section 4) |
Of the criteria evaluated by EFSA to qualify as a Union quarantine pest, naturally‐spreading psorosis does not meet the criterion of being a well characterised pest or disease. In parallel, it is unclear whether CPsV meets the criterion of being currently regulated or under official control |
Of the criteria evaluated by EFSA to qualify as a Union RNQP, naturally‐spreading psorosis does not meet the criterion of being a well characterised pest or disease. In parallel, CPsV meets all the criteria |
|
| Aspects of assessment to focus on/scenarios to address in future if appropriate |
The key uncertainties of this categorisation concern:
|
||
Abbreviations
- CPsV
Citrus psorosis virus
- EPPO
European and Mediterranean Plant Protection Organization
- EU MS
European Union Member State
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- PsA
Psorosis A
- PsB
Psorosis B
- PCR
polymerase chain reaction
- RNQP
Regulated non‐quarantine pest
- RT‐PCR
reverse transcription polymerase chain reaction
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Suggested citation: EFSA Panel on Plant Health (PLH) , Jeger M, Bragard C, Caffier D, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Chatzivassiliou E, Winter S, Catara A, Duran‐Vila N, Hollo G and Candresse T, 2017. Scientific Opinion on the pest categorisation of naturally‐spreading psorosis. EFSA Journal 2017;15(11):5076, 23 pp. 10.2903/j.efsa.2017.5076
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00312
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Gregoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Adopted: 15 November 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
Visual observation may at times confuse psorosis bark scaling with scaling around the edge of healing induced by frost or sunburn, or with bark shelling and gumming associated to Rio Grande gummosis or with shell bark reported on lemon (Roistacher 1991). A ‘false psorosis’ disclosing bark scaling was described by Malaguti and Knorr in Venezuela (1961). Leprosis scaling may at times be confused with psorosis (Knorr, 1981).
In Europe, psorosis‐associated bark scaling has been reported mostly on ‘navel’ oranges and very rarely on local varieties. In Sicily, only on few trees of Sanguinello (Salerno and Majorana, 1960) and Tarocco sweet orange (Tessitori et al., 2002) and some clones of ‘navel group’ and Clementine are affected by psorosis. However, psorosis‐like foliar symptoms observed in some regions are not associated with bark scaling symptoms and represent another disease.
Rate of spread by Olpidium may be increased by irrigation.
See Section 2.1 on what falls outside EFSA's remit.
References
- Achachi A, Ait Barka E and Ibriz M, 2014. Recent advances in Citrus psorosis virus. Virus disease, 25, 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioto D, Gangemi M, Deaglio S, Sposato S, Noris E, Luisoni E and Milne RG, 1999. Improved detection of Citrus psorosis virus using polyclonal and monoclonal antibodies. Plant Pathology, 48, 735–741. [Google Scholar]
- Alioto D, Malfitano M, Troisi A, Peluso A, Martín S, Milne RG, Guerri J and Moreno P, 2003. Variability of the coat protein gene of Citrus psorosis virus in Campania, southern Italy. Archives of Virology, 148, 2155–2166. [DOI] [PubMed] [Google Scholar]
- Barthe GA, Ceccardi GL, Manjunath KL and Derrick KS, 1998. Citrus psorosis virus: nucleotide sequencing of the coat protein gene and detection by hybridization and RT‐PCR. Journal of General Virology, 79, 1531–1537. [DOI] [PubMed] [Google Scholar]
- Beñatena HN and Portillo MM, 1984. Natural spread of psorosis in sweet orange seedlings. In: Proceedings 9th Conf. IOCV. IOCV, Riverside, CA, p. 159–164.
- Bridges GD, Youtsey CO and Nixon RR, 1965. Observations indicating psorosis transmission by seed of Carrizo citrange. Proceedings of the Florida State Horticultural Society, 78, 48–50. [Google Scholar]
- Campiglia HC, Silveira CM and Salibe AA, 1976. Psorosis transmission through seeds of trifoliate orange. In: Calavan EC (ed.). Proceedings of the 7th Conference of the International Organization of Citrus Virologists. IOCV, Riverside, CA. pp. 132–134. [Google Scholar]
- Childs JFL and Johnson RE, 1966. Preliminary report of seed transmission of psorosis virus. Plant Disease Reporter, 50, 81–83. [Google Scholar]
- Derrick KS and Timmer LW, 2000. Citrus blight and other diseases of recalcitrant etiology. Annual Review of Phytopathology, 38, 181–205. [DOI] [PubMed] [Google Scholar]
- Derrick KS, Brlansky RH, da Graça JV, Lee RF, Timmer LW and Nguyen TK, 1988. Partial characterization of a virus associated with citrus ringspot. Phytopathology, 78, 1298–1301. [Google Scholar]
- Desjardins PR, Drake RJ and French JV, 1969. Transmission of citrus ringspot virus to citrus and non‐citrus hosts by dodder (Campestris subinclusa). Plant disease reporter. [Google Scholar]
- Djelouah K, Potere O, Boscia D, D'Onghia AM and Savino V, 2000. Production of monoclonal antibodies to citrus psorosis associated virus. In: da Graca JV, Lee RF and Yokomi RH (eds.). Proceedings of the 14th International Organization of Citrus Virologists (IOCV) Conference, Riverside. pp. 152–158.
- D'Onghia AM, Djelouah K, Frasheri D and Potere O, 2001. Detection of Citrus psorosis virus by direct tissue blot immunoassay. Journal of Plant Pathology, 83, 139–142. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2903/j.efsa.2010.1495 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2014. Scientific Opinion on the pest categorisation of Citrus tristeza virus. EFSA Journal 2014;12(12):3923, 32 pp. 10.2903/j.efsa.2014.3923 [DOI] [Google Scholar]
- EPPO , 2017. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 3 of October 2017]
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- Fawcett HS, 1933. New symptoms of psorosis, indicating a virus disease of citrus. Phytopathology, 23, 930. [Google Scholar]
- Fawcett HS, 1934. Is psorosis of citrus a virus disease? Phytopathology, 24, 659–668. [Google Scholar]
- Fawcett HS and Klotz LJ, 1938. Types and symptoms of psorosis and psorosis‐like diseases of citrus. Phytopathology, 28, 670. [Google Scholar]
- Folimonova SY, Robertson CJ, Shilts T, Folimonov AS, Hilf ME, Garnsey SM and Dawson WO, 2010. Infection with strains of Citrus tristeza virus does not exclude superinfection by other strains of the virus. Journal of virology, 84, 1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García ML, Sánchez de la Torre ME, Dal Bó E, Djelouah K, Luisoni E, Milne RG and Grau O, 1997. Detection of citrus psorosis ringspot using RT‐PCR and ELISA. Plant Pathology, 46, 830–836. [Google Scholar]
- Garnsey SM and Timmer LW, 1980. Mechanical transmissibility of citrus ringspot virus isolates from Florida, Texas, and California In Proceedings of the conference International Organization of Citrus Virologists. [Google Scholar]
- da Graça JV, Lee RF, Moreno P, Civerolo EL and Derrick KS, 1991. Comparison of isolates of citrus ringspot, psorosis, and other virus‐like agents of citrus. Plant Disease, 75, 613–616. [Google Scholar]
- Knorr LC, 1981. Leprosis In: Bové JM, Vogel R. (eds.). Virus and virus‐like diseases of citrus: A collection of colour slides. SETCOIRFA, Paris (France). [Google Scholar]
- Loconsole G, Castellano MA, Dell'Orco M, Boscia D and Savino V, 2006. Serological detection of Citrus psorosis virus using a polyclonal antiserum to recombinant virus coat protein. Journal of Plant Pathology, 88, 171–173. [Google Scholar]
- Loconsole G, Saponari M and Savino V, 2010. Development of real‐time PCR based assays for simultaneous and improved detection of citrus viruses. European Journal of Plant Pathology, 128, 251–259. [Google Scholar]
- Malaguti G and Knorr LC, 1961. Psorosis in Venezuela‐an emendation In: Price WC. (eds.). Proceedings of the 2nd Conference of the International Organization of Citrus Virologists. University of Florida Press, Gainesville (FL). pp. 57–59. [Google Scholar]
- Martín S, Alioto D, Milne RG, Guerri J and Moreno P, 2002. Detection of Citrus psorosis virus in field trees by direct tissue immunoassay in comparison with ELISA, symptomatology, biological indexing and cross‐protection tests. Plant Pathology, 51, 134–141. [Google Scholar]
- Martín S, Alioto D, Milne RG, Garnsey SM, García ML and Grau O, 2004. Detection of Citrus psorosis virus by ELISA, molecular hybridization, RT‐PCR and immunosorbent electron microscopy and its association with citrus psorosis disease. European Journal of Plant Pathology, 110, 747–757. [Google Scholar]
- Martín S, López C, García ML, Naum‐Onganía G, Grau O and Flores R, 2005. The complete nucleotide sequence of a Spanish isolate of Citrus psorosis virus: comparative analysis with other ophioviruses. Archives of Virology, 150, 167–176. [DOI] [PubMed] [Google Scholar]
- Martín S, García ML, Troisi A, Rubio L, Legarreta G, Grau O, Alioto D, Moreno P and Guerri J, 2006. Genetic variation of populations of Citrus psorosis virus. Journal of General Virology, 87, 3097–3102. [DOI] [PubMed] [Google Scholar]
- Moore P and Nauer E, 1957. Scaly bark disease of citrus: nine‐year study of seven older orange orchards indicates advance of psorosis may be faster than is generally believed. California Agriculture, 11, 11–15. [Google Scholar]
- Moreno P, Guerri J and García ML, 2015. The psorosis disease of citrus: a pale light at the end of the tunnel. Journal of Citrus Pathology, 2, 1–18. [Google Scholar]
- Naum‐Onganía G, Gago‐Zachert S, Peña E, Grau O and García ML, 2003. Citrus psorosis virus RNA 1 is of negative polarity and potentially encodes in its complementary strand a 24K protein of unknown function and 280K putative RNA dependent RNA polymerase. Virus Research, 96, 49–61. [DOI] [PubMed] [Google Scholar]
- Navas‐Castillo J and Moreno P, 1995. Filamentous flexous particles and serologically related proteins of variable size associated with citrus psorosis and ringspot diseases. European Journal of Plant Pathology, 101, 343–348. [Google Scholar]
- Navas‐Castillo J, Moreno P, Ballester‐Olmos JF, Pina JA and Hermoso de Mendoza A, 1991. Detection of a necrotic strain of citrus ringspot in Star Ruby grapefruit in Spain In: Brlansky RH, Lee RF. and Timmer LW. (eds.). Proceedings of the 11th Conference of the International Organization of Citrus Virologists. IOCV, Riverside, CA. pp. 345–351. [Google Scholar]
- Osman F, Hodzic E, Sun‐Jung K, Jinbo W and Vidalakis G, 2015. Development and validation of a multiplex reverse transcription quantitative PCR (RT‐qPCR) assay for the rapid detection of Citrus tristeza virus, Citrus psorosis virus, and Citrus leaf blotch virus. Journal of Virological Methods, 220, 64–75. [DOI] [PubMed] [Google Scholar]
- Palle SR, Miao H, Seyran M, Louzada ES, da Grac¸a JV and Skaria M, 2005. Evidence for association of Citrus psorosis virus with symptomatic trees and an Olpidium‐like fungus. Proceedings of the 14th International Organisation of Citrus Virologists (IOCV) Conference, Riverside. pp. 423–426.
- Price WC, 1965. Transmission of Psorosis Virus by Dodder. In Proceedings of the Third Conference of the International Organization of Citrus Virologists, Held in Brazil, September 16–25, 1963 (p. 162). University of Florida Press.
- Pujol AR, 1966. Diffusion natural de psorosis en plantas citricas. INTA Est. Exp. Agrop. Concordia. Ser. TBc. No. 8, 15 p.
- Pujol AR and Benatena HN, 1965. A study of psorosis in Concordia, Argentina. In Proc. 3rd Conf IOCV, University of Florida Press, Gainesville, pp. 170–179.
- Rochon D, Kakani K, Robbins M and Reade R, 2004. Molecular aspects of plant virus transmission by Olpidium and Plasmodiophorid vectors. Annual Review of Phytopathology, 42, 211–241. [DOI] [PubMed] [Google Scholar]
- Roistacher CN, 1981. Psorosis A In: Bové JM. and Vogel R. (eds.). Virus and virus‐like diseases of citrus: a collection of colour slides, 2nd Edition Vol 2 SETCOIRFA, Paris, France. [Google Scholar]
- Roistacher CN, 1991. Graft‐transmissible diseases of citrus. Handbook for detection and diagnosis. Food and Agriculture Organization of the United Nations (FAO), Rome, pp. 115–126. [Google Scholar]
- Roistacher CN, 1993. Psorosis‐A review In: Moreno P, da Graca JV. and Timmer LW. (eds.). Proceedings of the 12th International Organisation of Citrus Virologists (IOCV) Conference, Riverside. pp. 139–154. [Google Scholar]
- Rosa C, Polek M, Falk BW and Rowhani A, 2007. Improved efficiency for quantitative and qualitative indexing for Citrus tristeza virus and Citrus psorosis virus. Plant Disease, 91, 1089–1095. [DOI] [PubMed] [Google Scholar]
- Salerno M and Majorana G, 1960. Infezioni di Psorosi B (Citrivir psorosis var. anulatum Faw) accertate sperimentalmente su piante di arancio dolce (Citrus sinensis Linn) in Sicilia. Tecnica Agricola 12, 629–639. [Google Scholar]
- Sanches de la Torre ME, López C, Grau O and García ML, 2002. RNA 2 of Citrus psorosis virus is of negative polarity and has a single open reading frame in its complementary strand. Journal of General Virology, 83, 1777–1781. [DOI] [PubMed] [Google Scholar]
- Sánchez de la Torre E, Riva O, Zandomeni R, Grau O and García ML, 1998. The top component of citrus psorosis virus contains two ssRNAs, the smaller encodes the coat protein. Molecular Plant Pathology, Available online: http://www.bspp.org.uk/mppol/1998/1019sanchez [Google Scholar]
- Sarachu AN, Arrese EL, Casafús C, Costa NB, García ML, Grau O, Marcó GM and Robles A, 1988. Biological assay and preliminary isolation of citrus psorosis disease agent from Argentina In: Timmer LW, Garnsey SM. and Navarro L. (eds.). Proceedings of the 10th Conference of the International Organization of Citrus Virologists. IOCV, Riverside, CA: pp. 343–347. [Google Scholar]
- Swingle WT and Webber HJ, 1896. The principal diseases of citrous fruits in Florida. US Department of agriculture; Division of vegetable pathology. Bulletin 8, 1–42. [Google Scholar]
- Tessitori M, Reina A, La Rosa R and Catara A, 2002. Psorosis bark scaling on Tarocco sweet orange, Riverside, CA Fifteenth IOCV Conference, Short Communications. [DOI] [PubMed]
- Timmer LW, 1974. Necrotic strain of citrus ringspot virus and its relationship to citrus psorosis virus. Phytopathology, 64, 389–394. [Google Scholar]
- Timmer LW and Garnsey SM, 1980. Natural spread of citrus ringspot psorosis‐like diseases in Florida and Texas. In Proceedings of the conference International Organization of Citrus Virologists.
- Timmer LW, Garnsey SM and McRitchie JJ, 1978. Comparative symptomatology of Florida and Texas isolates of citrus ringspot virus on citrus and herbaceous hosts. Plant Disease Reporter, 62, 1054–1058. [Google Scholar]
- Velazquez K, Alba L, Pina JA, Navarro L, Moreno P and Guerri J, 2015. The psorosis B syndrome in citrus is associated with a sequence variant of the citrus psorosis virus RNA2. Xii International Citrus Congress ‐ International Society of Citriculture, 1065, 837–845. [Google Scholar]
- Velazquez K, Alba L, Zarza O, Vives MC, Pina JA, Juarez J, Navarro L, Moreno P and Guerri J, 2016. The response of different genotypes of citrus and relatives to Citrus psorosis virus inoculation. European Journal of Plant Pathology, 144, 73–81. [Google Scholar]
- Velázquez K, Pina JA, Navarro L, Moreno P and Guerri J, 2012. Association of citrus psorosis B symptoms with a sequence variant of the Citrus psorosis virus RNA2. Plant Pathology, 61, 448–456. [Google Scholar]
- Wallace JM, 1957. Virus‐strain interference in relation to symptoms of psorosis disease of citrus. Hilgardia, 27, 223–246. [Google Scholar]


