Abstract
The European Commission requested EFSA to conduct a pest categorisation of Spodoptera frugiperda (Lepidoptera: Noctuidae) a pest with hosts in 27 plant families. Favoured hosts include maize, rice and sorghum (Poaceae). Hosts also include crops within the Brassicaceae, Cucurbitaceae, Solanaceae, Rutaceae and other families. S. frugiperda is a taxonomic entity with reliable methods for identification. It is regulated in the EU as a harmful organism whose introduction into the EU is banned. It is native to tropical and subtropical regions of the Americas and migrates to temperate regions in North and South America during the summer. Establishment in temperate areas is prevented by its inability to overwinter. S. frugiperda has been intercepted on plant produce entering the EU. Phytosanitary measures are available to impede entry via traded commodities. In 2016, S. frugiperda was reported damaging maize in Africa. Subsequent reports indicate that it continues to spread severely damaging maize and other crops. If S. frugiperda spreads into north Africa, the likelihood of adults migrating into the temperate EU increases. Within the scope and level of analysis appropriate for pest categorisation, the EFSA Plant Health Panel concludes that S. frugiperda could establish in a small area of the southern EU from where it is likely to enter more northern regions forming transient summer populations, particularly in maize growing regions where impacts on yield could occur. However, uncertainties regarding establishment remain. Considering the criteria within the remit of EFSA to assess as regards status as a potential Union quarantine pest (QP) or as a potential regulated non‐quarantine pest (RNQP), S. frugiperda satisfies the criteria to be regarded a Union QP but does not meet the criteria of (i) occurring in the EU territory, and (ii) plants for planting being the principal means of spread, criteria required for RNQP status.
Keywords: European Union, fall armyworm, migration, pest risk, plant pest, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/2031 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L.. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by the end of 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches' broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L.,Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches' broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | |
| Ralstonia solanacearum (Smith) Yabuuchi et al. | |
| (c) Fungi | |
| Melampsora medusae Thümen | |
| Synchytrium endobioticum (Schilbersky) Percival | |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | |
| Liriomyza bryoniae (Kaltenbach) | |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Spodoptera frugiperda is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest (QP) or those of a regulated non‐quarantine pest (RNQP) for the area of the European Union (EU) excluding Ceuta, Melilla and the outermost regions of Member States (MSs) referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores. The pest categorisation of S. frugiperda was initially requested to be delivered by December 2020. However, following its introduction into Africa, where its rapid spread was reported in news media, the reports of which were detected by EFSA's media monitoring activities, European Commission concerns were increased and the Commission therefore requested that the pest categorisation be brought forwards to be delivered in June 2017.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on S. frugiperda was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as a search term. Relevant papers focusing on geographic distribution, general biology and life cycle, host plants and the damage it causes were reviewed. Further references and information were obtained from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO, 2017a,b) and the CABI Crop Protection Compendium (CABI, 2017) and further updated with reports compiled in Abrahams et al. (2017).
Data about import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT.
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Consumers (DG SANCO), and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the MSs and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for S. frugiperda following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union QP and for a Union RNPQ in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required as per the specific ToR received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to qualify either as a QP or as a RNPQ. If one of the criteria is not met, the pest will not qualify. In such a case, the working group should consider the possibility to terminate the assessment early and be concise in the sections preceding the question for which the negative answer is reached. Note that a pest that does not qualify as a quarantine pest may still qualify as a RNQP which needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone, thus the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests' introduction have an economic or environmental impact on the EU territory? | Would the pests' introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6 ) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regards to the principle of separation between risk assessment and risk management (EFSA founding regulation1); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? (Yes or No)
Yes, the identity of the pest is established. Conventional taxonomic keys based on morphology can be used to identify S. frugiperda. However, see notes below.
S. frugiperda (J.E. Smith, 1797) is an insect in the order Lepidoptera and the family Noctuidae. It is known in English as fall armyworm, corn leafworm, and southern grassworm; in French as légionnaire d'automne; in German as Heerwurm, and in Spanish as cogollero del maíz.
There was a revision of the world Spodoptera by Pogue (2011) but the taxonomy of species within the genus is still an area of ongoing research, e.g. Juárez et al. (2014), Dumas et al. (2015a,b), Hanniger et al. (2017). Two morphologically identical strains of S. frugiperda are recognised, commonly referred to as the corn strain and the rice strain, due to host preferences. There is a high level of genetic differentiation between the strains (Pashley, 1986; Kergoat et al., 2012; Juárez et al., 2014) as well as differences in diurnal mating pattern (Schöfl et al., 2009; Hanniger et al., 2017) and differences in female sex pheromones (Lima and McNeil, 2009). Drès & Mallet (2002) considered the rice‐ and corn‐associated S. frugiperda as separate species. Prowell et al. (2004) suggested that they were recently evolved species that are not completely reproductively isolated. Other authors suggest that S. frugiperda is still in the process of speciation (e.g. Groot et al., 2008; Juárez et al., 2014).
This pest categorisation follows the taxonomy of Pogue (2011) and considers S. frugiperda as a single species with two strains.
3.1.2. Biology of the pest
Newly emerged adults appear from pupae in the soil shortly after dusk to feed and complete maturation. They do not mate on the first night. On subsequent nights, females sit at the top of a host plant and release a sex pheromone to attract a mate. Females mate once per night. Virgin females mate earlier in the night than females that have already mated (Sparks, 1979). Most oviposition occurs within 4 or 5 days although some females have been reported to oviposit for up to 17 days (Johnson, 1987). Adult females are relatively short‐lived (13–19 days at 26.8°C) but highly fecund, with around 1,000 eggs being laid per female (Johnson, 1987).
Eggs are typically laid on the underside of leaves although at high population densities, almost any surface can be used. Eggs are laid in clusters of 100–300, sometimes in two layers. Clusters are protected with a covering of abdominal scales (CABI, 2017).
Depending on temperature, eggs usually take between 2 and 10 days to hatch. At mean temperatures of 21–27°C, eggs hatch in 2–4 days (Sparks, 1979).
After hatching, the first instars move to find a suitable feeding site on the plant where eggs were laid (Pannuti et al., 2015). The first and second instars feed together on young leaves and on the tender growing tips of hosts. Larvae can become cannibalistic at high larval densities when there is a shortage of host plant material to feed on (Andow et al., 2015). After about a week of development, third instar larvae disperse away from each other and continue to feed. In a trial examining larval dispersal of S. frugiperda, Pannuti et al. (2016) reported finding over 90% of recovered larvae within a 1.1 m radius of a maize plant 14 days after being infested with an egg mass. However, if larvae develop at high densities and when host resources are depleted, they will ‘march’ in search of food and move further and faster. There are five or six larval instars.
From studies examining larval development on different hosts, da Silva et al. (2017) found larvae developed 6–8 days faster feeding on maize, oats and wheat than on cotton. Development on soybean was about 5 days slower than on maize. Mature larvae burrow into the soil and create a pupal chamber 2–8 cm below the surface.
At 29°C, the pupal stage lasts around 7 days while at 15°C pupal development takes approximately 37 days (Sparks, 1979). Pupae developing during the winter in Florida did not complete development during a month where the minimum soil temperature was below 10°C for two or more days (Wood et al., 1979).
Overall, egg to adult development takes around 66 days at 18.3°C and 18 days at 35.0°C (Barfield et al., 1978). There is no significant difference between the development rates of males and females (Barfield et al., 1978). A threshold temperature of 10.9°C and 559 day‐degrees above the threshold is required for development of the complete life cycle. The optimum temperature for development from egg to adult is 28°C (Ramirez Garcia et al., 1987).
All stages are usually killed by freezing temperatures (CABI, 2017).
In southern Florida, S. frugiperda can breed year round (Abrahams et al., 2017). There are continuous generations in Central and South America (Johnson, 1987) where there can be four to six generations per year (CABI, 2017).
3.1.3. Intraspecific diversity
As noted in Section 3.1.1, S. frugiperda is composed of two morphologically indistinguishable strains referred to as the maize and the rice strains. The two strains can be distinguished by strain‐specific genetic markers (Lu et al., 1992; McMichael and Prowell, 1999). There are physiological and behavioural differences between strains, such as host plant preferences (Quisenberry and Whitford, 1988; Veenstra et al., 1995) and mating behaviour (Pashley et al., 1992). However, it is now recognised that host preference is not a reliable indicator of a strain (Juárez et al., 2012a, 2012b).
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
YES. All stages of the pest can be detected visually, light traps and pheromone lures are available for monitoring; diagnostic keys are available.
3.1.4.1. Detection
All stages of the pest can be detected visually; use of a hand lens will help detect early stages (eggs and early larval instars). Eggs and larvae can be found on all above ground plant parts, mostly on the underside of leaves. Occasionally, larvae may bore into plant parts. Pupation usually takes place in the soil, which may hinder detection of that stage. Light traps, capturing males and females, and pheromone traps, capturing only males, can be used to detect adults in the field and in production‐, storage‐ and handling facilities (EPPO, 2015).
The female sex pheromone of S. frugiperda can be used for monitoring purposes. It was identified by Tumlinson et al. (1986). Unbehend et al. (2014) recommend a monitoring blend which can be used to equally attract the rice and the corn strains.
3.1.4.2. Symptoms
Symptoms caused by larvae are not specific to Spodoptera but generic for most primarily foliage feeding Lepidoptera species (Smith et al., 1997). These consist of holes in fruits or leaves along with the presence of frass. Early stages can be found scraping the epidermis of the underside of the leaves. Larvae never tie leaves together. Young plants of maize (up to an age of 30 days) can be cut through at the base, similar to symptoms caused by cutworms. At high densities, larvae feed gregariously and disperse in swarms, usually moving to grasses when available (Smith et al., 1997).
3.1.4.3. Identification
Conventional taxonomic keys based on morphology can be used to identify S. frugiperda. For example, Todd and Poole (1980) provide a key to 14 species of adult New World Spodoptera and Pogue (2011) provides keys for adults and larvae to the 30 species in the genus. EPPO (2015) provides a key for adult Spodoptera spp. identification based on morphological characteristics, as well as a protocol for real‐time polymerase chain reaction (PCR) molecular identification (Cano‐Calle et al., 2015). The latter identification is recommended for earlier stages, especially when experience is lacking and when the origin of the larvae is unknown.
3.1.4.4. Morphology
EPPO (2015) provides a full description (including stage‐specific photographs of all necessary details) of the different development stages of S. frugiperda.
The eggs are dome‐shaped (0.45 × 0.35 mm) and are usually deposited in groups of 100–300 together with greyish scales covering the whole egg mass, which results in a furry appearance (Capinera, 2014b).
Mature larvae (LVI) are 35–40 mm in length and are characterised by an inverted Y‐shape in yellow on the head, black dorsal pinaculae with long primary setae and four black spots arranged in a square on the last abdominal segment. They eventually spin a loose cocoon, oval in shape and 20–30 mm in length (Capinera, 2014b), which contains a typical brown shiny noctuid pupa 18–20 mm in length.
Adults (32–28 mm wing span) might be confused with other Spodoptera spp. However, in S. frugiperda the veins of the hindwing are brown and distinct, and in the male forewing the pale orbicular stigma has a pronounced pale ‘tail’ distally. In the male genitalia, the valve is almost rectangular and there is no marginal notch at the position of the tip of the harpe; the female bursa lacks a signum.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
S. frugiperda is native to tropical and subtropical regions of the Americas. It is established year round in northern Bolivia and from some parts of southern Brazil northwards through Central America and Mexico, the Caribbean and subtropical areas of southern Florida and Texas in the southern United States where winter temperatures rarely fall below 10°C (Sparks, 1979; Ashley et al., 1989; Nagoshi and Meagher, 2008). In mild winters, it can survive in Louisiana and Arizona (Wood et al., 1979). In summer, populations migrate into southern and northern temperate regions where it can become abundant in late summer and autumn.
In 2016, S. frugiperda was reported for the first time in Africa with outbreaks in Benin, Nigeria, Sao Tomé and Principe and Togo (Goergen et al., 2016; IITA, 2016) (Figure 1 and Table 2). How S. frugiperda arrived in Africa is uncertain, it may have entered via trade, or via weather systems associated with El Nino events of 2014‐2016 (Wild, 2017). Subsequent reports confirm S. frugiperda has spread to several countries in west, central, east and southern Africa. News reports and media coverage indicate that S. frugiperda continues to spread in sub‐Saharan Africa.
Figure 1.

Global distribution of Spodoptera frugiperda (as at April, 2017)
Table 2.
Global distribution of Spodoptera frugiperda with sub‐national distribution for large countries. Distribution primarily based on information from the CABI Crop Protection Compendium, EPPO GD, IITA and Abrahams et al. (2017) (r = resident; m = migrant)
| Region | Country (EPPO, 2017a, 2017b; CABI 2017 unless shown otherwise) | Sub‐national distribution (e.g. States/Provinces) |
|---|---|---|
| North America | Bermuda, Canada, Mexico, USA |
Canada (only in summer as a migrant): Manitoba, New Brunswick, Nova Scotia, Ontario, Prince Edward Island, Quebec USA (resident in Texas and Florida; elsewhere migrant): Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Virginia, West Virginia, Wisconsin, Wyoming |
| Central America & Caribbean | Assumed to be resident (r) in Anguilla, Antigua and Barbuda, Bahamas, Barbados, Belize, British Virgin Islands, Cayman Islands, Costa Rica, Cuba, Dominica, Dominican Republic, El Salvador, Grenada, Guadeloupe, Guatemala, Haiti, Honduras, Jamaica, Martinique, Montserrat, Nicaragua, Panama, Puerto Rico, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Trinidad and Tobago, US Virgin Islands | |
| South America | Argentina (m), Bolivia, Brazil, Chile (m), Colombia, Ecuador, French Guiana, Guyana, Paraguay (m), Peru, Suriname, Uruguay (m), Venezuela | |
| Europe | An occurrence in Germany in 1999 is assumed not to have persisted (see Section 3.2.2). There are interception records for the UK and the NL but no establishment (see Section 3.4) | |
| Africa | Benin (IITA, 2016), Democratic Republic of Congo (FAO, 2017), Ghana, Kenya (Republic of Kenya Ministry of Agriculture, Livestock & Fisheries, 2017), Nigeria (IITA, 2016), Sao Tome and Principe (FAO, 2017), South Africa (Abrahams et al., 2017; Daff, 2017), Swaziland (IPPCa, 2017), Togo (IITA, 2016), Zambia, (IPPCb, 2017), Zimbabwe (FAO, 2017) | |
| Asia | An occurrence in Israel in 1967 is assumed not to have persisted (see below) | |
| Oceania | Not known to occur |
Wiltshire (1977) reports receiving a sample of male and female S. frugiperda from a colleague who collected specimens from the Jordan Valley in Israel in 1967. However, no other literature provided supporting evidence that S. frugiperda was established in Israel. We assume that any S. frugiperda found in Israel in 1967 were from a transient population that is no longer present.
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? (Yes or No) If present, is the pest widely distributed within the EU?
No. S. frugiperda is not known to be present in the EU.
S. frugiperda was found on maize plants in Germany in August 1999. A total of 40 infected plants were found. Larvae were collected and destroyed (EPPO, 2000). Based on this incident, the EPPO Global Database and CABI (2017) currently records S. frugiperda as present in Germany based on information from the German NPPO in 2000. However, due to the eradication efforts at the time and the lack of subsequent reports, the Panel assumes that S. frugiperda in no longer present in Germany.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
S. frugiperda is listed in Council Directive 2000/29/EC. Details are presented in Tables 3 and 4.
Table 3.
The listing of Spodoptera frugiperda within Council Directive 2000/29/EC
| Annex I, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned |
|---|---|
| Section I | Harmful organisms not known to occur in the community and relevant for the entire community |
| (a) | Insects, mites and nematodes, at all stages of their development |
| Species | |
| 22. | Spodoptera frugiperda (Smith) |
3.3.2. Legislation addressing plants and plant parts on which Spodoptera frugiperda is regulated
Table 4.
Regulated hosts and commodities that may involve Spodoptera frugiperda in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
|---|---|---|
| 9 | Plants of Chaenomeles Ldl., Cydonia Mill., Crateagus L., Malus Mill., Prunus L., Pyrus L., and Rosa L., intended for planting, other than dormant plants free from leaves, flowers and fruit | Non‐European countries |
| 11 | Plants of stolon‐ or tuber‐forming species of Solanum L. or their hybrids, intended for planting, other than those tubers of Solanum tuberosum L. as specified under Annex III A (10) | Third countries |
| 13 | Plants of Solanaceae intended for planting, other than seeds and those items covered by Annex III A (10), (11) or (12) | Third countries, other than European and Mediterranean countries |
| 14 | Soil and growing medium as such, which consists in whole or in part of soil or solid organic substances such as parts of plants, humus including peat or bark, other than that composed entirely of peat | Turkey, Belarus, Moldavia, Russia, Ukraine and third countries not belonging to continental Europe, other than the following: Egypt, Israel, Libya, Morocco, Tunisia |
| 15 | Plants of Vitis L., other than fruits | Third countries other than Switzerland |
| 16 | Plants of Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids, other than fruit and seeds | Third countries |
| 18 | Plants of Cydonia Mill., Malus Mill., Prunus L. and Pyrus L. and their hybrids, and Fragaria L., intended for planting, other than seeds … | Without prejudice to the prohibitions applicable to the plants listed in Annex III A (9), where appropriate, non‐European countries, other than Mediterranean countries, Australia, New Zealand, Canada, the continental states of the USA |
| 19 | Plants of the family Graminacae, other than plants of ornamental perennial grasses of the subfamilies Bambusoideae and Panicoideae and of the genera Buchloe, Bouteloua Lag., Calamagrostis, Cortaderia Stapf., Glyceria R. Br., Hakonechloa Mak. ex Honda, Hystrix, Molinia, Phalaris L., Shibataea, Spartina Schreb., Stipa L. and Uniola L., intended for planting, other than seeds | Third countries, other than European and Mediterranean countries |
| Annex IV, Part A | Special requirements which must be laid down by all member states for the introduction and movement of plants, plant products and other objects into and within all member states | |
| Section I | Plants, plant products and other objects originating outside the community | |
| Plants, plant products and other objects | Special requirements | |
| 27.2 | Plants of Dendranthema (DC.) Des Moul., Dianthus L. and Pelargonium l'Hérit. ex Ait., other than seeds |
Without prejudice to the requirements applicable to the plants listed in Annex IV(A) (I)(27.1), official statement that: (aa) the plants originate in an area free from Spodoptera eridania (Cramer), Spodoptera frugiperda Smith and Spodoptera litura (Fabricius), established by the national plant protection organisation in accordance with relevant International Standards for Phytosanitary Measures, or (a) no signs of Spodoptera eridania (Cramer), Spodoptera frugiperda Smith, or Spodoptera litura (Fabricius) have been observed at the place of production since the beginning of the last complete cycle of vegetation, or (b) the plants have undergone appropriate treatment to protect them from the said organisms |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community – in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community | |
| Part B | PLANTS, PLANT PRODUCTS AND OTHER OBJECTS ORIGINATING IN TERRITORIES, OTHER THAN THOSE TERRITORIES REFERRED TO IN PART A | |
| 2 | Parts of plants, other than fruits and seeds, of:— Castanea Mill., Dendranthema (DC.) Des Moul., Dianthus L., Gypsophila L., Pelargonium l'Herit. ex Ait, Phoenix spp., Populus L., Quercus L., Solidago L. and cut flowers of Orchidaceae | |
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
S. frugiperda is a polyphagous pest reported to infest 186 host plant species in North and Central America (Casmuz et al., 2010). It has a preference for wild and cultivated grasses, maize, rice, sorghum, millet and sugarcane (Poaceae). Other hosts from 27 families include Allium (Liliaceae), Brassica spp. (Brassicaceae), Capsicum and other Solanaceae including aubergines, potatoes and tomatoes, Cucumis (Cucurbitaceae), Gossypium (Malvaceae), Phaseolus (Fabaceae) and Ipomoea (Convolvulaceae) as well as various ornamental plants (chrysanthemums, carnations and Pelargonium) (Smith et al., 1997; CABI, 2017). In laboratory host preference studies examining larval feeding choices, maize and wheat were preferred above soybean and cotton (da Silva et al., 2017).
3.4.2. Entry
S. frugiperda is also a strong migrant flier. Having recently been introduced into Africa where it continues to spread, in future it may be able to enter the EU via summer migratory flight were it to establish in north Africa.
Is the pest able to enter into the EU territory? Yes
Due to the polyphagy of S. frugiperda and the number of countries where S. frugiperda occurs, there are many combinations of hosts and country of origin that could potentially provide a pathway to facilitate entry into the EU. See Tables 5, 6–7 below.
Table 5.
Trade pathway combinations (plants for planting) imported into EU from countries where S. frugiperda occurs (Source: Isefor database, Dutch plant imports database)
| Country of origin\Host | Dianthus spp. | Dendranthema spp. | Solanum spp. | Capsicum spp. | Cucurbitaceae | Solanum lycopersicon | Momordica spp. | Solanum melongena |
|---|---|---|---|---|---|---|---|---|
| Costa Rica | x | x | x | x | x | x | ||
| United States of America | x | x | x | x | x | x | ||
| Chile | x | x | x | x | x | |||
| Guatemala | x | x | x | x | x | |||
| Brazil | x | x | x | |||||
| Dominican Republic | x | x | x | |||||
| Mexico | x | x | x | |||||
| Ghana | x | x | ||||||
| South Africa | x | x | ||||||
| Zimbabwe | x | x | ||||||
| Canada | x | |||||||
| Colombia | x | |||||||
| Ecuador | x | |||||||
| El Salvador | x | |||||||
| Kenya | x | |||||||
| Nicaragua | x | |||||||
| Panama | x | |||||||
| Uruguay | x | |||||||
| Virgin Islands | x |
Table 6.
Host and country of origin for Europhyt notifications of Spodoptera frugiperda 1995–2017
| Country of origin\Host | Capsicum | Solanum melongena | Solanum macrocarpon | Momordica | Asparagus | Solanum aculeatissimum | Solanum sp. | Vigna | Rosa (cut flowers) | Eryngium (cut flowers) | Tillandsia (p4P cuttings) | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suriname | 14 | 6 | 6 | 4 | 1 | 1 | 1 | 33 | ||||
| Dominican Republic | 5 | 5 | ||||||||||
| Peru | 3 | 3 | ||||||||||
| Mexico | 2 | 2 | ||||||||||
| Brazil | 1 | 1 | ||||||||||
| Ecuador | 1 | 1 | ||||||||||
| Guatemala | 1 | 1 | ||||||||||
| Sum | 21 | 6 | 6 | 4 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 46 |
Table 7.
EU area of some S. frugiperda host crops 2012–2016. Area (cultivation/harvested/production) (1,000 ha)
| 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|
| Grain maize | 9,847 | 9,775 | 9,610 | 9,259 | 8,570 |
| Forage maize | 5,866 | 6,023 | 6,072 | 6,186 | 5,139 |
| Rice | 454 | 433 | 432 | 443 | 440 |
| Cotton | 366 | 318 | 355 | 349 | 351 |
| Sorghum | 119 | 145 | 158 | 141 | 126 |
| Sum | 16,652 | 16,694 | 16,627 | 16,378 | 14,626 |
3.4.2.1. Plants for planting pathways
There is a lack of comprehensive detailed trade information describing the imports of plants for planting at an EU level. Nevertheless, using what data is available in the Isefor and national Dutch plant import databases, import activity for plants for planting which could host S. frugiperda, from countries where S. frugiperda, occurs is indicated by an ‘x’ in Table 5. The table is not necessarily comprehensive and additional hosts might be traded from third countries into EU MSs.
3.4.2.2. Interceptions
S. frugiperda has been intercepted on a range of produce and cut flowers from the Americas. A search of Europhyt notifications of interceptions between January 1995 and May 2017 revealed that there were 46 records of interceptions of S. frugiperda, the earliest being May 2005 (EUROPHYT, 2017). All interceptions were notified by the Netherlands with the majority from Suriname, primarily on Capsicum, Solanum melongena and Solanum macrocarpon.
Table 6 indicates the hosts and country of origin for interceptions up to May 2017.
All notifications of S. frugiperda interceptions on Europhyt have been made by the Netherlands. Nevertheless, based on long term surveys in the Netherlands in 2007, 2009, 2011, 2012, 2014 and 2015 EPPO evaluated the status of S. frugiperda in the Netherlands as ‘Absent, confirmed by survey’ (EPPO, 2017a,b).
UK records, prior to the establishment of Europhyt, indicate occasional interceptions of S. frugiperda on produce from South America (Seymour et al., 1985).
3.4.2.3. Fresh produce pathways
The majority (almost 60%) of Europhyt notifications from the Netherlands of non‐compliance due to presence of S. frugiperda have occurred on Capsicum and Solanum melongena. Between January 2011 and December 2015, over 2,200 tonnes of sweet peppers (Capsicum) and 14,000 tonnes of aubergines (Solanum melongena) were imported into the EU from countries where S. frugiperda is now known to occur (Appendix A).
3.4.2.4. Migration from Africa
If S. frugiperda continues to spread and becomes established in Africa north of the Sahara, then summer migration to more temperate northern regions, as occurs in North America, could result in S. frugiperda entering the EU on a regular annual basis. Abrahams et al. (2017) report results of preliminary modelling that suggests that seasonal temperatures and precipitation patterns are suitable for the establishment of S. frugiperda in North Africa. Whether or not the Sahara will provide a barrier to establishment in North Africa is unknown.
In addition, adults are capable of surviving being carried at altitude with weather fronts and travelling hundreds or occasionally thousands of miles in a few days (Rose et al., 1975). Given the appropriate weather conditions, it may be possible for S. frugiperda to be carried into the EU from further south in Africa, beyond the range of usual migration (assumed to be approximately 300 miles per generation, as in the USA – see Section 3.4.4).
3.4.3. Establishment
Is the pest able to become established in the EU territory? (Yes or No)
Yes. Preliminary analysis, appropriate for pest categorisation, suggests areas of southern Europe provide conditions for establishment.
Following the recent introduction of S. frugiperda into Africa, a preliminary examination of potential future distribution indicates that small parts of southern Europe provide suitable abiotic conditions for establishment (Abrahams et al., 2017). As a highly polyphagous pest, some of its hosts will be available, including maize and rice, in such regions. Biotic factors (host availability) and abiotic factors (summer climate) indicate that transient populations of S. frugiperda could occur in the summer further north in central and northern EU where maize is widely available.
3.4.3.1. EU distribution of main host plants
As noted above, S. frugiperda is a polyphagous pest and many hosts grow in the EU. Table 7 shows the EU harvested area for five host crops (maize (grain & forage), rice, cotton and sorghum) for the years 2012–2016, for which S. frugiperda is reported to be a major pest elsewhere.
3.4.3.2. Climatic conditions affecting establishment
S. frugiperda is native to tropical and subtropical regions of the Americas and has recently established in tropical and subtropical regions of Africa. It has no capacity to overwinter and all life stages are killed by freezing temperatures. In the USA, it is established in southern Texas and southern Florida where winter temperatures rarely fall below 10°C. Ramirez Garcia et al. (1987) report a life cycle is completed with 559 day‐degrees above a threshold of 10.9°C. Based simply on this threshold and accumulated temperature, Figure 2 indicates that regions of the EU could be suitable for the development of S. frugiperda. Southern regions of Portugal, Spain, Italy, Greece and Cyprus are most suitable. More sophisticated modelling and mapping, e.g. taking into account factors such as consecutive days below 10°C or precipitation (amount and frequency), is beyond the scope of pest categorisation but would better inform decision makers as to the areas of the EU where abiotic factors most favour establishment.
Figure 2.
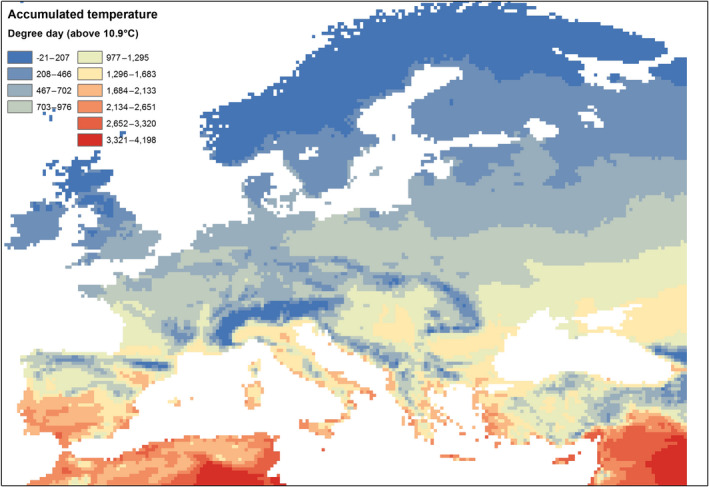
Accumulated temperature (degree day) above 10.9°C
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment?
Yes, this is a migratory species which is able to expand annually from its endemic area in the tropical and sub‐tropical regions of the Americas to cover more than 2,000 km across the entire US up to Canada in the North and reaching Argentina and Chile in the South (Pair et al., 1986)
As found in other species within the same genus, S. frugiperda has a notable dispersal capacity (Johnson, 1987). Adult annual migrations occurring in the summer result in the pest expanding from its endemic area in the warmer parts of the New World over more than 2,000 km across the entire US up to Canada in the north and reaching Argentina and Chile in the south (Luginbill, 1928; Sparks, 1979; Pair et al., 1986). Sparks (1979) and Johnson (1987) reproduce a map showing the typical northwards progression through time in the USA. Starting from southern Florida and Texas, the spring generation flies north, generally spreading up to approximately 300 miles before settling to reproduce the next generation. For example, in April and May, populations can be expected in Alabama and Georgia; in June and July later generations can be expected from South Carolina to Colorado and by late August subsequent generations can reach between North Dakota and Maine and into Canada. Prevailing winds and host availability influence the rate and direction of migrations (Hogg et al., 1982; Johnson, 1987).
More locally, larvae also disperse frequently from the original host plant, in part to compensate for the negative effects of overcrowding (Sparks, 1979; Pitre et al., 1983; Chapman et al., 1999). However, the contribution of such activities to overall spread is negligible compared to the movement by winged adults.
If S. frugiperda does establish in the Mediterranean region of the EU, there is potential for it to undertake spring and summer migrations, similar to those reported in the USA, so that there could be seasonal spread to more temperate areas of the EU. However, such populations would be transient and establishment limited by winter temperatures.
Given the natural dispersal ability of S. frugiperda, spread via plants for planting is not the main pathway for spread. S. frugiperda therefore fails to satisfy the important criterion that to be a RNQP, the main means of spread should be via plants for planting (Table 1).
3.5. Potential impacts in the EU
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, the introduction of S. frugiperda could cause yield losses to host crops; preferred hosts such as maize, rice and sorghum could primarily be affected.
3.5.1. Potential pest impacts
3.5.1.1. Direct impacts of the pest
S. frugiperda is a pest of several major crops in the Americas, particularly maize, sorghum, rice and sugar cane although many more crops and wild plants can be attacked.
In field trials, there was a significant loss in yield (17%), as measured by grain weight, when 20% of maize plants were infested with an egg mass (Cruz and Turpin, 1983). In trials in Mexico over a number of years, yield losses averaged 13%, maximum yield loss was 30% (Andrews, 1998). Pantoja et al. (1986) found yield reduction in rice was linearly related to larval density. Larval densities greater than 35 m−2 resulted in yields significantly lower than in control plots.
Yield losses are due to larval feeding reducing photosynthetic capacity, destroying the growing tip of crops or if later instar larvae cut through the stems of young plants. In a laboratory trial, the first three instars were reported to eat less than 2% of all the foliage consumed during development, while the final and largest instar consumed over 75% of the total (Sparks, 1979).
In the USA, outbreaks occur in warmer years when adults spread north earlier than normal and populations build up faster and affect less mature plants, causing greater yield losses and increasing expenditure on control. Outbreaks occurred irregularly in the USA during the 19th and 20th Centuries. In 1977 costs were estimated to be almost $300 million (Johnson, 1987) with losses in Georgia estimated at around $138 million (Sparks, 1979).
S. frugiperda is described by the USDA as ‘economically important’ (Ellis, 2005) and is an important pest in nine south‐eastern states of the USA where annual average yield loss between 1975 and 1983 was $ 60 million.
S. frugiperda is considered the most important pest of maize in Brazil, estimated to cause more than US $400 million damage annually (IITA, 2016). The FAO estimates Brazil spends US $600 million annually controlling infestations of S. frugiperda (Wild, 2017).
Following its introduction into Africa, S. frugiperda has been reported damaging maize (Goergen et al., 2016). In Uganda, S. frugiperda is also attacking cotton, sugar cane, banana and vegetables (Tajuba, 2017). Across the infested area, rice and sorghum are important food crops that are also threatened (Abrahams et al., 2017). At an FAO organised meeting to discuss plans to respond to S. frugiperda in Africa, it was reported that 290,000 ha of crops had been destroyed by larvae in four countries although this was probably an underestimate (Wild, 2017).
Were S. frugiperda to establish in the EU, it could reduce yields on crops particularly maize, rice and sorghum during outbreak years. However, the areas most suitable for the pest to establish occur in the far south of the EU where these crops, other than rice, are less intensively planted. Figure 3 shows a preliminary estimate of the annual number of generations that might develop in the EU. Four generations might be possible in the far south of Spain, Portugal, Italy and Greece and in Cyprus.
Figure 3.
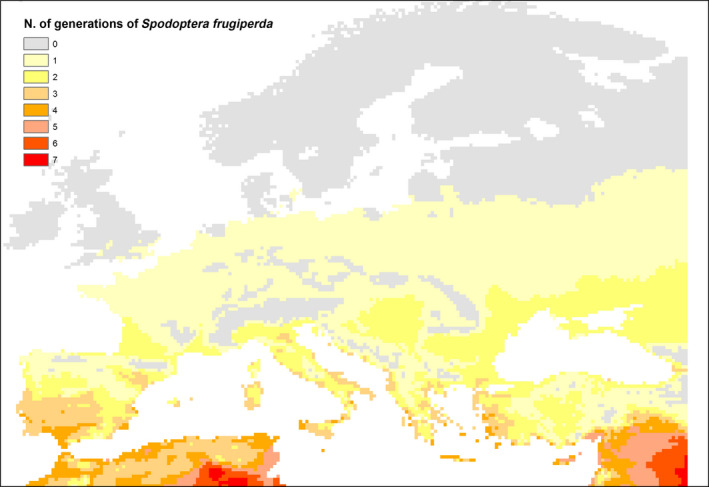
Preliminary estimate mapping the potential number of generations of S. frugiperda possible per year
Other major crops grown in the EU such as wheat, onions, potatoes, strawberries, sugarbeet and Citrus are also hosts to S. frugiperda although no yield loss reports were obtained during the pest categorisation. There is therefore uncertainty as to the likely consequences to these crops were S. frugiperda to establish in the EU.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
However, the efficacy of these measures remains doubtful because of the long distance migration capacity of adult moths.
Host commodities liable to provide a pathway can be visually inspected and infested consignments can be detected (see evidence of interceptions, Section 3.4.2).
As with the situation in North America, described by Meagher & Nagoshi (2004), if resident populations in future overwintering sites can be controlled (Africa, Mediterranean countries), it should be possible to substantially reduce or delay their northward annual migrations, mitigating agricultural damage. Such area‐wide management requires knowledge of the population distribution in the major habitats (both agricultural and non‐agricultural) within the overwintering region and in the case of the Old World it would most likely require international co‐operation with non EU‐countries.
3.6.1. Biological or technical factors limiting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
Possible seasonal natural migration of adult moths northwards from Africa into the EU; spread can exceed 2,000 km during the summer.
Cultivated preferred hosts such as maize, rice and cotton occur in Mediterranean EU countries where the climate could support establishment.
Polyphagy, 186 hosts, including non‐cultivated species, have been described for S. frugiperda (Casmuz et al., 2010)
In the Americas, transgenic maize expressing Bacillus thuringiensis insecticidal proteins is widely used to control a range of pests, including S. frugiperda. However, although the use of Bt maize is allowed in the EU, there is a reluctance and only five EU MS (Spain, Portugal, the Czech Republic, Slovakia and Romania, in decreasing order of ha of Bt‐maize cultivated) actually grew this type of crop in 2015 (http://isaaa.org/resources/publications/briefs/51/executivesummary/default.asp).
S. frugiperda has developed resistance against at least 24 different active substances (University of Michigan, 2017) including some B. thuringiensis insecticidal proteins used in transgenic maize crops (Aguirre et al., 2016; Abrahams et al., 2017).
3.6.2. Biological or technical factors limiting the ability to prevent the presence of the pest on plants for planting
Resistance to insecticides (as above).
3.6.3. Control methods
Chemical control (Dequech et al., 2013; Abrahams et al., 2017)
-
Biological control:
-
–
Effective natural enemies occur in the Americas and could be considered as candidates for Classical Biological Control (Abrahams et al., 2017)
-
–
Different entomopathogens have been screened and could be effectively used as bioinsecticides to control this pest (Barrera et al., 2011; Behle and Popham, 2012)
-
–
Biotechnological control: GMOs,, use of semiochemicals (pheromone)
Area‐wide IPM to control resident populations in overwintering sites (Meagher & Nagoshi, 2004)
Cultural control: could the push‐and‐pull method currently practiced in Africa against maize borers by small farmers be effective against S. frugiperda?
It should be noted that European maize growers currently manage lepidopteran pests related to S. frugiperda; they are Sesamia nonagrioides (the Mediterranean corn borer) and Ostrinia nubilalis (European corn borer). What effect the management practices used against these pests may have on S. frugiperda is unknown.
3.7. Uncertainty
Rapid and long distance dispersal in Africa similar to what has been reported from the Americas could lead to the occurrence of transient populations in the EU, especially in southern EU countries, even if the pest is not able to establish north of the Sahara Desert. Establishment north of the Sahara would increase the likelihood that transient populations could occur naturally in parts of the EU.
Most of the literature examined for this pest categorisation referred to impacts on maize, sorghum or rice. There is uncertainty as to potential impacts on the range of hosts grown in the EU.
4. Conclusions
The conclusions of the pest categorisation are summarised in Table 8.
Table 8.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the pest is established. Conventional taxonomic keys based on morphology can be used to identify S. frugiperda. Molecular methods are also available | The identity of the pest is established. Conventional taxonomic keys based on morphology can be used to identify S. frugiperda. Molecular methods are also available | The existence of two genetically distinct strains of S. frugiperda indicates that the organism may be in the process of speciation |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Spodoptera frugiperda is not known to be established in the EU. There are interception records for UK and NL but no establishment |
Spodoptera frugiperda is not known to be established in the EU. (A criterion to satisfy the definition of a regulated non‐quarantine pest is that the pest must be present in the risk assessment area ‐ this criterion is not met by S. frugiperda) | None |
| Regulatory status (Section 3.3 ) | Spodoptera frugiperda is currently regulated by Council Directive 2000/29/EC within which it is listed as a harmful organisms whose introduction into, and spread within, all member states shall be banned (i.e. it is aI/AI pest) | Spodoptera frugiperda is currently regulated by Council Directive 2000/29/EC within which it is listed as a harmful organisms whose introduction into, and spread within, all member states shall be banned (i.e. it is aI/AI pest) | |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Spodoptera frugiperda can enter and potentially establish in the EU. It can be carried into the EU on several host commodities such as Capsicum, Solanum melongena and Momordicaand on cut flowers such as Rosa and Dianthus. If it were to establish it is likely to spread in summer months and form transient populations, particularly in maize growing regions of the EU. If S. frugiperda spreads into north Africa, the likelihood of adults entering the EU during summer migration from Africa increases | Plants for planting are not the main pathway for entry or spread | Whether or not S. frugiperda can establish, as opposed to form transient summer populations, in the EU is uncertain. More detailed and sophisticated modelling and mapping would better inform this judgment. If establishment is not possible then S. frugiperda would not meet a key criterion for it to be classified as a Union quarantine pest |
| Potential for consequences in the EU territory (Section 3.5 ) | Establishment in the EU could cause yield and quality losses in crops such as maize and rice | Larval feeding damage to hosts would impact on the quality of plants for planting and hence affect the value of hosts regarding their intended use | There is uncertainty about impacts on other hosts |
| Available measures (Section 3.6 ) | Measures are available to inhibit entry via traded commodities but these will have no affect against potential entry via natural migration | Plants for planting are not the main pathway for entry or spread | If S. frugiperda becomes able to reach the EU from Africa through annual migration then measures against traded commodities are undermined |
| Conclusion on pest categorisation (Section 4 ) | S. frugiperda does satisfy the criteria, that are within the remit of EFSA to assess, to be regarded as a Union quarantine pest | S. frugiperda does not meet the criteria of (a) occurring in the EU territory, and (b) plants for planting being the principal means of spread | None |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | Spodoptera frugiperda is currently spreading in Africa. Any future more detailed assessment should consider focussing on adult entry from Africa via migratory flights and examine more of the factors affecting establishment so as to better identify any endangered area within the EU | ||
5. Recommendations
As the pest is at present spreading in Africa, it is important to monitor the situation and especially whether it eventually becomes established in northern African countries.
The EU should be alert and look out for potential seasonal migration of adult moths from Africa.
A key uncertainty is whether the Sahara Desert is a barrier for northwards spread and migration.
More sophisticated modelling as to whether EU environmental conditions are suitable for establishment would better inform future phytosanitary decision making.
European growers currently manage related pests such as the Mediterranean corn borer (S. nonagriodes) and European corn borer (O. nubilalis). It would be useful to find out what effect the management practices against these pests could have against S. frugiperda. Such information would help inform phytosanitary decision making.
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization of the United Nations
- IPPC
International Plant Protection Convention
- ISEFOR
Increasing Sustainability of European Forests
- MS
Member State
- PCR
polymerase chain reaction
- PLH
EFSA Panel on Plant Health
- QP
quarantine pest
- RNQP
Regulated Non‐Quarantine Pest
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Appendix A – EU imports of sweet peppers and aubergines 2011‐2015 from countries where Spodoptera frugiperda is now known to occur
Countries marked (*) indicate sources of S. frugiperda interceptions on Capsicum.
Tables A.1 and A.2 report the total amount of sweet peppers (Capsicum) and aubergines (Solanum melongena) imported into the EU between January 2011 and December 2015, from countries where S. frugiperda is now known to occur. Imports from African nations occurred before the arrival of S. frugiperda in Africa and are included to indicate the relative importance of these countries as a source of such produce.
Table A.1.
EU import of fresh or chilled sweet pepper (CN 0709 6010) 2011–2015 from countries where S. frugiperda is now known to occur (100 kg)
| TOTAL | 22,531 | % of total |
|---|---|---|
| Mexico* | 12,375 | 54.92 |
| Dominican Republic* | 4,024 | 17.86 |
| Peru | 3,788 | 16.81 |
| South Africa | 1,156 | 5.13 |
| Ghana | 849 | 3.77 |
| Mozambique | 101 | 0.45 |
| Argentina | 78 | 0.35 |
| Brazil | 46 | 0.20 |
| Zimbabwe | 30 | 0.13 |
| Bolivia | 26 | 0.12 |
| United States | 21 | 0.09 |
| St Lucia | 11 | 0.05 |
| Suriname* | 10 | 0.04 |
| Zambia | 10 | 0.04 |
| Chile | 4 | 0.02 |
| Togo | 1 | 0.00 |
| Uruguay | 1 | 0.00 |
Countries marked (*) indicate sources of S. frugiperda interceptions on Capsicum.
Table A.2.
EU import of fresh or chilled aubergines (CN 0709 3000) 2011–2015 from countries where S. frugiperda is now known to occur (100 kg)
| TOTAL | 140,066 | % of total |
|---|---|---|
| Dominican Republic | 67,223 | 47.99 |
| Kenya | 66,703 | 47.62 |
| Ghana | 3,170 | 2.26 |
| Dominica | 2,122 | 1.52 |
| South Africa | 665 | 0.47 |
| United States | 102 | 0.07 |
| Honduras | 33 | 0.02 |
| Peru | 28 | 0.02 |
| D R Congo | 15 | 0.01 |
| Colombia | 2 | 0.00 |
| Nicaragua | 2 | 0.00 |
| Nigeria | 1 | 0.00 |
Appendix B – Area of Spodoptera frugiperda selected host crops harvested in EU member states, 2012‐2016.
Tables B.1, B.2, B.3, B.4–B.5 report the area of key Spodoptera frugiperda hosts grown in EU member states.
Table B.1.
Grain maize and corn‐cob‐mix Area (cultivation/harvested/production) (1,000 ha)
| 2012 | 2013 | 2014 | 2015 | 2016 | Mean annual % of EU | |
|---|---|---|---|---|---|---|
| Romania | 2,731 | 2,519 | 2,514 | 2,607 | 2,552 | 27.5 |
| France | 1,719 | 1,840 | 1,848 | 1,639 | 1,489 | 18.1 |
| Hungary | 1,191 | 1,243 | 1,191 | 1,146 | 1,023 | 12.3 |
| Italy | 977 | 908 | 870 | 727 | 661 | 8.8 |
| Poland | 544 | 614 | 678 | 670 | 582 | 6.6 |
| Germany | 526 | 497 | 481 | 456 | 416 | 5.0 |
| Bulgaria | 467 | 428 | 408 | 499 | 407 | 4.7 |
| Spain | 390 | 442 | 419 | 398 | 357 | 4.3 |
| Croatia | 299 | 288 | 253 | 264 | 250 | 2.9 |
| other EU MS | 1,003 | 995 | 948 | 852 | 833 | 9.8 |
| Sum | 9,847 | 9,775 | 9,610 | 9,259 | 8,570 | 100.0 |
Table B.2.
Forage maize area (cultivation/harvested/production) (1,000 ha)
| EU MS | 2012 | 2013 | 2014 | 2015 | 2016 | Mean annual % of EU |
|---|---|---|---|---|---|---|
| Germany | 2,038 | 2,003 | 2,093 | 2,100 | 2,145 | 37.4 |
| France | 1,388 | 1,487 | 1,412 | 1,475 | 1,507 | 26.2 |
| Poland | 508 | 462 | 541 | 555 | – | 9.3 |
| Italy | 296 | 327 | 343 | 337 | 321 | 5.8 |
| Czech Republic | 205 | 234 | 237 | 245 | 234 | 4.2 |
| Netherlands | 232 | 230 | 226 | 224 | 202 | 4.0 |
| Denmark | 185 | 181 | 178 | 182 | 182 | 3.3 |
| United Kingdom | 148 | 183 | 171 | 179 | 186 | 3.1 |
| Belgium | 171 | 178 | 178 | 173 | 169 | 3.1 |
| Spain | 107 | 107 | 113 | 108 | 108 | 2.0 |
| Austria | 82 | 111 | 84 | 92 | 85 | 1.6 |
| Sum | 5,866 | 6,023 | 6,077 | 6,186 | > 5,139 | 100.0 |
Table B.3.
Rice area in the EU 2012–2016 (1,000 ha)
| 2012 | 2013 | 2014 | 2015 | 2016 | Mean annual % of EU | |
|---|---|---|---|---|---|---|
| Italy | 235 | 216 | 220 | 227 | 227 | 51.1 |
| Spain | 113 | 112 | 110 | 109 | 109 | 25.2 |
| Greece | 30 | 29 | 31 | 35 | 35 | 7.3 |
| Portugal | 31 | 30 | 29 | 29 | 29 | 6.7 |
| France | 21 | 21 | 17 | 16 | 15 | 4.1 |
| Bulgaria | 10 | 10 | 11 | 12 | 12 | 2.5 |
| Romania | 11 | 12 | 13 | 11 | 9 | 2.5 |
| Other EU | 3 | 3 | 2 | 3 | 3 | 0.6 |
| Sum | 454 | 433 | 432 | 443 | 440 | 100.0 |
Table B.4.
Cotton Area in the EU, 2012–2016 (cultivation/harvested/production) (1,000 ha)
| 2012 | 2013 | 2014 | 2015 | 2016 | Mean annual % of EU | |
|---|---|---|---|---|---|---|
| Greece | 296 | 254 | 280 | 283 | 286 | 80.5 |
| Spain | 70 | 64 | 74 | 63 | 61 | 19.1 |
| Bulgaria | 0 | 0 | 0 | 2 | 4 | 0.4 |
| Sum | 366 | 318 | 355 | 349 | 351 | 100.0 |
Table B.5.
Sorghum area in the EU 2012–2016 (1,000 ha)
| 2012 | 2013 | 2014 | 2015 | 2016 | Mean annual % of EU | |
|---|---|---|---|---|---|---|
| France | 42 | 51 | 63 | 54 | 46 | 37.0 |
| Italy | 37 | 51 | 52 | 45 | 43 | 33.2 |
| Romania | 20 | 22 | 19 | 13 | 13 | 12.6 |
| Spain | 8 | 9 | 7 | 8 | 9 | 6.0 |
| Hungary | 4 | 5 | 5 | 5 | 5 | 3.5 |
| Bulgaria | 5 | 4 | 7 | 7 | 3 | 3.8 |
| Greece | 0 | 0 | 2 | 3 | 3 | 1.1 |
| Austria | 1 | 2 | 3 | 3 | 2 | 1.6 |
| Slovakia | 1 | 0 | 1 | 1 | 1 | 0.6 |
| Slovenia | 0 | 0 | 0 | 0 | 0 | 0.0 |
| Other EU | No data | No data | 0 | 2 | 0 | 0.6 |
| Sum | >=119 | >=145 | 158 | 141 | 126 | 100.0 |
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Jaques Miret JA, Navarro MN, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Gardi C, Aukhojee M and MacLeod A, 2017. Scientific Opinion on the pest categorisation of Spodoptera frugiperda . EFSA Journal 2017;15(7):4927, 32 pp. 10.2903/j.efsa.2017.4927
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00425
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Gregoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Note: This version replaces the early version which was published on 6 July in line with EFSA's scientific procedures for urgent scientific advice. Table 4 was amended and editorial changes were made.
Adopted: 28 June 2017
Amended: 31 January 2018
Note
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
References
- Abrahams P, Beale T, Cock M, Corniani N, Day R, Godwin J, Murphy S, Rochard G and Vos J, 2017. Fall armyworm status. Impacts and control options in Africa: Preliminary Evidence Note (April 2017)_Report by Department for International Development and CABI http://www.cabi.org/Uploads/isc/Dfid%20Faw%20Inception%20Report28apr2017final.pdf [Accessed 7th June 2017]
- Aguirre LA, Hernández‐Juàrez A, Flores M, Cerna E, Landeros J, Frías GA and Harris MK, 2016. Evaluation of foliar damage by Spodoptera frugiperda (Lepidoptera: Noctuidae) to genetically modified corn (Poales: Poaceae) in Mexico. Florida Entomologist, 99, 276–280. [Google Scholar]
- Andow DA, Farias JR, Horikoshi RJ, Bernardi D, Nascimento ARB and Omoto C, 2015. Dynamics of cannibalism in equal‐aged cohorts of Spodoptera frugiperda . Ecological Entomology, 40, 229–236. 10.1111/een.12178 [DOI] [Google Scholar]
- Andrews KL, 1998. Latin American Research on Spodeoptera frugiperda (Lepidoptera: Noctuidae). Florida Entomologist, 71, 630–653. [Google Scholar]
- Ashley TR, Wiseman BR, Davis FM and Andrews KL, 1989. The fall armyworm: a bibliography. Florida Entomologist, 72, 152e202. 10.2307/3494982 [DOI] [Google Scholar]
- Barfield CS, Mitchell ER and Poeb SL, 1978. A temperature‐dependent model for fall armyworm development 1,2. Annals of the Entomological Society of America, 71, 70–74. 10.1093/aesa/71.1.70 [DOI] [Google Scholar]
- Barrera G, Simón O, Villamizar L, Williams T and Caballero P, 2011. Spodoptera frugiperda multiple nucleopolyhedrovirus as a potential biological insecticide: Genetic and phenotypic comparison of field isolates from Colombia. Biological Control, 58, 113–120. [Google Scholar]
- Behle RW and Popham HJ, 2012. Laboratory and field evaluations of the efficacy of a fast‐killing baculovirus isolate from Spodoptera frugiperda . Journal of invertebrate pathology, 109, 194–200. [DOI] [PubMed] [Google Scholar]
- CABI , (2017). Spodoptera frugiperda (fall armyworm). Cabi Crop Protection Compendium Datasheet, CABI Wallingford, http://www.cabi.org/cpc/datasheet/5731 Last modified 2nd May, 2017; [Accessed: 23rd May 2017].
- Cano‐Calle D, Arango‐Isaza RE and Saldamando‐Benjumea CI, 2015. Molecular identification of Spodoptera frugiperda (Lepidoptera: Noctuidae) corn and rice strains in Colombia by using a PCR‐RFLP of the mitochondrial gene cytochrome oxidase I (COI) and a PCR of the gene FR (for rice). Annals of the Entomological Society of America, 108, 172–180. [Google Scholar]
- Capinera JL, (2014b). Fall army worm, Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae). University of Florida/IFAS Extension. Publication EENY‐98. Available at: http://entnemdept.ufl.edu/creatures/field/fall_armyworm.htm (last access 24 March 2016)
- Casmuz A, Juárez ML, Socías MG, Murúa MG, Prieto S, Medina S, Willink E and Gastaminza G, 2010. Revisión de los hospederos del gusano cogollero del maíz, Spodoptera frugiperda (Lepidoptera: Noctuidae). Revista de la Socie‐ dad Entomológica Argentina, 69, 209–231. [Google Scholar]
- Chapman JW, Williams T, Escribano A, Caballero P, Cave RD and Goulson D, (1999). Fitness consequences of cannibalism in the fall armyworm, Spodoptera frugiperda . Behavioral Ecology, 10, 298–303. [Google Scholar]
- Cruz I. and Turpin FT, 1983. Yield impact of larval infestations of fall armyworm (Lepidoptera: Noctuidae) to midwhorl growth stage of corn. Journal of Economic Entomology, 76, 1052–1054. [Google Scholar]
- DAFF (Dep. of Agriculture, Forestry and Fisheries, Republic of South Africa), 2017. Pest alert: detection of Spodoptera frugiperda (fall army worm) for the first time in South Africa. http://www.nda.agric.za/docs/media/Pest%20Alert%20Media%20Release%20FAW%20confirmed%20.pdf [Accessed: 13 February 2017]
- Dequech STB, Camera C, Sturza VS, Ribeiro LD, Querino RB and Poncio S, 2013. Population fluctuation of Spodoptera frugiperda eggs and natural parasitism by Trichogramma in maize. Acta Scientiarum‐Agronomy, 35, 295–300. 10.4025/actasciagron.v35i3.16769 [DOI] [Google Scholar]
- Drès M and Mallet J, 2002. Host races in plant–feeding insects and their importance in sympatric speciation. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 357, 471–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas P, Barbut J, Le Ru B, Silvain JF, Clamens AL, d'Alencon E and Kergoat GJ, 2015a. Phylogenetic molecular species delimitations unravel potential new species in the pest genus Spodoptera Guenée, 1852 (Lepidoptera, Noctuidae). PLoS ONE, 10, e0122407. 10.1371/journal.pone.0122407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas P, Legeai F, Lemaitre C, Scaon E, Orsucci M, Labadie K, Gimenez S, Clamens AL, Henri H, Vavre F, Aury JM, Fournier P, Kergoat GJ and d'Alencon E, 2015b. Spodoptera frugiperda (Lepidoptera: Noctuidae) host‐plant variants: two host strains or two distinct species? Genetica, 143, 305–316. 10.1007/s10709-015-9829-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2093/j.efsa.2010.1495 [DOI] [Google Scholar]
- Ellis SE, 2005. New Pest Response Guidelines: Spodoptera. USDA/APHIS/PPQ/PDMP. https://www.aphis.usda.gov/import_export/plants/manuals/emergency/downloads/nprg_spodoptera.pdf [Accessed: 15 June 2017]
- EPPO , 2000. Situation of several quarantine pests in Germany in 1999 and 2000. EPPO Reporting Service, 2000, 171. [Google Scholar]
- EPPO , 2015. PM 7/124 (1) Spodoptera littoralis, Spodoptera litura, Spodoptera frugiperda, Spodoptera eridania . Bulletin OEPP/EPPO Bulletin, 45, 410–444. DoI/ 10.1111/epp.12258/epdf [DOI] [Google Scholar]
- EPPO , 2017a. EPPO Global Database (available online). https://gd.eppo.int
- EPPO , 2017b. EPPO Global database. Spodoptera frugiperda. Notification from the NPPO of the Netherlands to EPPO: Pest status of harmful organisms in the Netherlands (Fytosignalering covering 2007, 2009, 2011, 2012, 2014, 2015). https://gd.eppo.int/taxon/LAPHFR/distribution/NL
- EUROPHYT , 2017. Interceptions of harmful organisms in imported plants and other objects, annual Interception. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/interceptions/index_en.htm Accessed 6th June 2017
- EUROSTAT , online. European Commission, Statistical Office of the European Communities. Available online: http://ec.europa.eu/eurostat/data/database
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- FAO , 2017. FAO Advisory Note on Fall Armyworm (FAW) in Africa. http://www.fao.org/3/a-bs914e.pdf
- Goergen G, Kumar PL, Sankung SB, Togola A and Tamò M, 2016. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE, 11, 1–9. 10.1371/journal.pone.0165632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot AT, Marr M, Schöfl G, Lorenz S, Svatos A and Heckel DG, 2008. Host strain specific sex pheromone variation in Spodoptera frugiperda . Frontiers in Zoology, 5, 20. 10.1186/1742-9994-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanniger S, Dumas P, Schofl G, Gebauer‐Jung S, Vogel H, Unbehend M, Heckel DG and Groot AT, 2017. Genetic basis of allochronic differentiation in the fall armyworm. BMC Evolutionary Biology, 17, 68. 10.1186/s12862-017-0911-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg DB, Pitre HN and Anderson RE, 1982. Assessment of early‐season phenology of the fall armyworm (Lepidoptera: Noctuidae in Mississippi. Environmental Entomology, 11, 705–710. [Google Scholar]
- IPPCa , 2017. Detection of Fall Army Worm Spodoptera frugiperda in Swaziland. https://www.ippc.int/en/countries/swaziland/pestreports/2017/02/detection-of-fall-army-worm-spodoptera-frugiperda-in-swaziland/
- IPPCb , 2017. Preliminary Report on Fall Armyworm in Zambia. https://www.ippc.int/en/countries/zambia/pestreports/2017/02/preliminary-report-on-fall-armyworm-in-zambia/
- IITA , 2016. First report of outbreaks of the “Fall Armyworm” on the African continent. IITA Bulletin, No. 2330. http://bulletin.iita.org/index.php/2016/06/18/first-report-of-outbreaks-of-the-fall-armyworm-on-the-african-continent/
- Johnson SJ, 1987. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the Western Hemisphere. Insect Science Application, 8 (4/5/6), 543–549. [Google Scholar]
- Juárez ML, Murua MG, Garcia MG, Ontivero M, Vera MT, Vilardi JC, Groot AT, Castagnaro AP, Gastaminza G and Willink E, 2012a. Host association of Spodoptera frugiperda (Lepidoptera: Noctuidae) corn and rice strains in Argentina, Brazil, and Paraguay. Journal of Economic Entomology, 105, 573–582. [DOI] [PubMed] [Google Scholar]
- Juárez ML, Schöfl G, Vera MT, Vilardi JC, Murúa MG, Willink E, Hanninger S, Heckel DG and Groot AT, 2014. Population structure of Spodoptera frugiperda maize and rice host forms in South America: are they host strains?. Entomologia Experimentalis et Applicata, 152, 182–199. [Google Scholar]
- Kergoat GJ, Prowell DP, Le Ru BP, Mitchell A, Dumas P and Clamens A‐L, 2012. Disentangling dispersal, vicariance and adaptive radiation patterns: a case study using armyworms in the pest genus Spodoptera (Lepidoptera: Noctuidae). Molecular Phylogenetic Evol, 65, 855–870. [DOI] [PubMed] [Google Scholar]
- Lima E and McNeil J, 2009. Female sex pheromones in the host races and hybrids of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Chemoecology, 19, 29–36. [Google Scholar]
- Lu Y, Adang MJ, Eisenhour DJ and Kochert GD, 1992. Restriction fragment length polymorphism analysis of genetic variation in North American populations of the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). Molecular Ecology, 1, 199–208. [Google Scholar]
- Luginbill P, 1928. The fall armyworm. USDA Technical. Bulletin, 34, 1–91. [Google Scholar]
- McMichael M and Prowell DP, 1999. Differences in amplified fragment‐length polymorphisms in fall armyworm (Lepidoptera: Noctuidae) host strains. Annals of the Entomological Society of America, 92, 175–181. [Google Scholar]
- Meagher RL and Nagoshi RN, 2004. Population dynamics and occurrence of Spodoptera frugiperda host strains in southern Florida. Ecological entomology, 29, 614–620. [Google Scholar]
- Nagoshi RN and Meagher RL, 2008. Review of fall armyworm (Lepidoptera: Noctuidae) genetic complexity and migration. Florida Entomologist, 91, 546e554. [Google Scholar]
- Pair SD, Raulston JR, Sparks AN, Westbrook JK and Douce GK, 1986. Fall armyworm distribution and population dynamics in the southeastern states. Florida Entomologist, 69, 468–487. [Google Scholar]
- Pannuti LER, Baldin ELL, Hunt TE and Paula‐Moraes SV, 2015. On‐plant larval movement and feeding behavior of fall armyworm (Lepidoptera: Noctuidae) on reproductive corn stages. Environmental Entomology, 2015, 1–9. 10.1093/ee/nvv159 [DOI] [PubMed] [Google Scholar]
- Pannuti LER, Paula‐Moraes SV, Hunt TE, Baldin ELL, Dana L and Malaquias JV, 2016. Plant‐to‐plant movement of striacosta albicosta (Lepidoptera: Noctuidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae) in Maize (Zea mays). Journal of Economic Entomology, 109, 1125–1131. 10.1093/jee/tow042. [DOI] [PubMed] [Google Scholar]
- Pantoja A, Smith CM and Robinson JF, 1986. Effects of the fall armyworm (Lepidoptera: Noctuidae) on rice yields. Journal of economic entomology, 79, 1324–1329. [Google Scholar]
- Pashley DP, 1986. Host‐associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): a sibling species complex? Ann Entomol Soc America, 79, 898–904. 10.1093/aesa/79.6.898 [DOI] [Google Scholar]
- Pashley DP, Hammond AM and Hardy TN, 1992. Reproductive isolating mechanisms in fall armyworm host strains (Lepidoptera: Noctuidae). Annals of the Entomological Society of America, 85, 400–405. [Google Scholar]
- Pitre HN, Mulrooney JE and Hogg DB, 1983. Fall armyworm (Lepidoptera: Noctuidae) oviposition: Crop preferences and egg distribution on plants. Journal of Economic Entomology, 76, 463–466. [Google Scholar]
- Pogue MG, 2011. Using genitalia characters and mitochondrial COI sequences to place “Leucochlaena” hipparis (Druce) in Spodoptera Guenée (Lepidoptera: Noctuidae). Proceedings of the Entomological Society Washington, 113, 497–507. [Google Scholar]
- Prowell DP, McMichael M and Silvain J‐F, 2004. Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae). Annals of the Entomological Society of America, 97, 1034–1044. 10.1098/rstb.2002.1059 [DOI] [Google Scholar]
- Quisenberry SS and Whitford F, 1988. Evaluation of bermudagrass resistance to fall armyworm (Lepidoptera: Noctuidae): influence of host strain and dietaryconditioning. Journal of Economic Entomology, 81, 1463–1468. [Google Scholar]
- Ramirez Garcia L, Bravo Mojica H and Llanderal Cazares C, 1987. Development of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) under different conditions of temperature and humidity. Agrociencia, Mexico, 67, 16l–171. [Google Scholar]
- Republic of Kenya Ministry of Agriculture, Livestock & Fisheries , 2017. http://www.kalro.org/arlri/sites/default/files/STATUS_OF_FALL_ARMY_WORM_IN_WESTERN_KENYA_March_2017.pdf
- Rose AH, Silversides RH and Lindquist OH, 1975. Migration flight by an aphid, Rhopalosiphum maidis (Hemiptera: Aphididae) and a noctuid, Spodoptera frugiperda (Lepidoptera: Noctuidae). Canadian Entomologist, 107, 567–576. [Google Scholar]
- Schöfl G, Heckel DG and Groot AT, 2009. Time‐shifted reproductive behaviours among fall armyworm (Noctuidae: Spodoptera frugiperda) host strains: evidence for differing modes of inheritance. Journal of Evolutionary Biology, 22, 1447–1459. [DOI] [PubMed] [Google Scholar]
- Seymour PR, Roberts H and Davis ME (Compilers), 1985. Insects and other invertebrates found in plant material imported into England and Wales, 1984. Reference Book, Ministry of Agriculture, Fisheries and Food, UK, 442/84.
- da Silva DM, Bueno AD, Andrade K, Stecca CD, Neves PMOJ and de Oliveira MCN, 2017. Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Scientia Agricola, 74, 18–31. 10.1590/1678-992X-2015-0160 [DOI] [Google Scholar]
- Smith IM, McNamara DG, Scott PR and Holderness M, 1997. Spodoptera frugiperda . In: (eds.). Quarantine Pests for Europe, 2nd Edn., CABI/EPPO, Wallingford, 1425pp. [Google Scholar]
- Sparks AN, 1979. A review of the biology of the fall armyworm. Florida Entomologist, 62, 82e87. 10.2307/3494083 [DOI] [Google Scholar]
- Tajuba A, 2017. Army worm now turns to vegetables, bananas as government mulls aerial spray, Daily Monitor, POSTED 25/4/2017. http://mobile.monitor.co.ug/News/Army-worm-turns-vegetables-government-mulls-aerialspray/2466686-3902296-format-xhtml-39kst2z/index.html
- Todd EL and Poole RW, 1980. Keys and illustrations for the armyworm moths of the noctuid genus Spodoptera Guenée from the Western Hemisphere. Annals of the Entomological Society of America, 73, 722–738. [Google Scholar]
- Tumlinson JH, Mitchell ER, Teal PE, Heath RR and Mengelkoch LJ, 1986. Sex pheromone of fall armyworm,Spodoptera frugiperda (J.E. Smith) : Identification of components critical to attraction in the field. J Chem Ecol, 12, 1909–1926. 10.1007/BF01041855 [DOI] [PubMed] [Google Scholar]
- Unbehend M, Hänniger S, Vásquez GM, Juárez ML, Reisig D, McNeil JN … & Groot AT, 2014. Geographic variation in sexual attraction of Spodoptera frugiperda corn‐and rice‐strain males to pheromone lures. PLoS One, 9, e89255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Michigan , 2017. Arthropod Pesticide Resistance Database. https://www.pesticideresistance.org/index.php (search performed on June 14 2017)
- Veenstra KH, Pashley DP and Ottea JA, 1995. Host‐plant adaptation in fall armyworm host strains: comparison of food consumption, utilisation, and detoxication enzyme activities. Annals of the Entomological Society of America, 88, 80–91. [Google Scholar]
- Wild S, 2017. African countries mobilize to battle invasive caterpillar. Nature, 543, 13–14. [DOI] [PubMed] [Google Scholar]
- Wiltshire EP, 1977. Middle East Lepidoptera. XXXVII: notes on the Spodoptera li tura (F.) group (Noctuidae Trifinae). Proceedings and transactions.
- Wood JR, Poe SL and Leppla NC, 1979. Winter survival of fall armyworm 1 pupae in Florida. Environmental Entomology, 8, 249–252. 10.1093/ee/8.2.249 [DOI] [Google Scholar]


