Abstract
Bovine tuberculosis has been assessed according to the criteria of the Animal Health Law (AHL), in particular criteria of Article 7 on disease profile and impacts, Article 5 on the eligibility of bovine tuberculosis to be listed, Article 9 for the categorisation of bovine tuberculosis according to disease prevention and control rules as in Annex IV and Article 8 on the list of animal species related to bovine tuberculosis. The assessment has been performed following a methodology composed of information collection and compilation, expert judgement on each criterion at individual and, if no consensus was reached before, also at collective level. The output is composed of the categorical answer, and for the questions where no consensus was reached, the different supporting views are reported. Details on the methodology used for this assessment are explained in a separate opinion. According to the assessment performed, bovine tuberculosis can be considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL. The disease would comply with the criteria as in Sections 2, 3, 4 and 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in points (b), (c), (d) and (e) of Article 9(1). The main animal species to be listed for bovine tuberculosis according to Article 8(3) criteria are several mammal species, as indicated in the present opinion.
Keywords: Bovine tuberculosis, TB, Mycobacterium bovis, M. bovis, Animal Health Law, Listing, Categorisation, impact
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The background and Terms of Reference (ToR) as provided by the European Commission for the present document are reported in Section 1.2 of the scientific opinion on the ad hoc methodology followed for the assessment of the disease to be listed and categorised according to the criteria of Article 5, Annex IV according to Article 9, and 8 within the Animal Health Law (AHL) framework (EFSA AHAW Panel, 2017).
1.2. Interpretation of the Terms of Reference
The interpretation of the ToR is as in Section 1.2 of the scientific opinion on the ad hoc methodology followed for the assessment of the disease to be listed and categorised according to the criteria of Article 5, Annex IV according to Article 9, and 8 within the AHL framework (EFSA AHAW Panel, 2017).
The present document reports the results of assessment on bovine tuberculosis (bTB) according to the criteria of the AHL articles as follows:
Article 7: bovine tuberculosis profile and impacts
Article 5: eligibility of bovine tuberculosis to be listed
Article 9: categorisation of bovine tuberculosis according to disease prevention and control rules as in Annex IV
Article 8: list of animal species related to bovine tuberculosis.
2. Data and methodologies
The methodology applied in this opinion is described in detail in a dedicated document about the ad hoc method developed for assessing any animal disease for the listing and categorisation of diseases within the AHL framework (EFSA AHAW Panel, 2017).
3. Assessment
3.1. Assessment according to Article 7 criteria
This section presents the assessment of bTB according to the Article 7 criteria of the AHL and related parameters (see Table 2 of the opinion on methodology (EFSA AHAW Panel, 2017)), based on the information contained in the fact‐sheet as drafted by the selected disease scientist (see Section 2.1 of the scientific opinion on the ad hoc methodology) and amended by the AHAW Panel.
3.1.1. Article 7(a) Disease Profile
The EFSA/ECDC Zoonoses report for 2015–2016 (EFSA and ECDC, 2016) states that ‘Bovine tuberculosis is a zoonotic animal disease regulated by Council Directive 64/432/EEC1. According to Directive 2003/99/EC2 on the monitoring of zoonoses and zoonotic agents, the zoonosis and zoonotic agent to be included in monitoring of bovine tuberculosis is tuberculosis due to Mycobacterium bovis (Annex I, A). Since 2003 in addition to M. bovis, Mycobacterium caprae has been recognised as a distinct bacterial species and causative agent of bovine tuberculosis and of tuberculosis in humans and animals other than cattle (Aranaz et al., 2003). Disease caused by M. caprae is not considered to be substantially different from that caused by M. bovis, and the same tests can be used for its diagnosis (OIE, 2015). In the meeting of 7 May 2013 of the Committee on the Food Chain and Animal Health (Section Animal Health and Welfare), the European Commission circulated and presented a working document (SANCO/7059/2013) on the subject matter (European Commission, 2013). Without prejudice to the exclusive competence of the European Court of Justice to authoritatively interpret Union legislation, the document concluded that all provisions explicitly referring to M. bovis in Council Directive 64/432/EEC should be understood as also applicable to M. caprae. Based on the above, a distinction is descriptively made for the first time in the EU annual summary reports on trends and sources of zoonoses, of reporting by MS on Mycobacterium tuberculosis complex, M. bovis and M. caprae’.
Therefore, for the purposes of this fact‐sheet, the definition of bTB is ‘Infection in cattle with any of the disease‐causing mycobacterial species within the M. tuberculosis complex (MTC)’. This is consistent with the EU Working Document on the Eradication of Bovine Tuberculosis in the European Union (EU) that was accepted by the bTB subgroup of the Task Force on monitoring animal disease eradication, i.e. it was drawn up by the Commission's own tuberculosis (TB) task force which comprised experts on bTB eradication from a variety of countries and a number of epidemiologists (European Commission, 2006).
Mycobacterium bovis is by far the most important causative agent of tuberculosis in livestock and wildlife and in bovines is commonly referred to as bTB. However, tuberculosis in mammals, including humans, can be caused by most of the members of the Mycobacterium tuberculosis complex (MTBC), including M. bovis, M. tuberculosis, M. microti, M. africanum, M. canettii, M. caprae, M. pinnipedii, M. orygis, M. mungi and the dassie bacillus (Brosch et al., 2002; Michel et al., 2006). Still, unexplained differences in susceptibility of different species to different members of the MTBC have been observed. Aerosol exposure of cattle to M. bovis is considered the most frequent route of infection; gross lesions usually involve the lungs and thoracic lymph nodes (Francis, 1958). Cattle exposed by ingestion of food and water contaminated with M. bovis often develop primary foci in lymph tissue associated with the intestinal tract (Thoen and Williams, 1994). Pathogenic mycobacteria produce granulomatous lesions in tissues of a wide range of domestic and wild animal species and man causing a general state of illness, coughing and eventual death. Bovine tuberculosis usually has a prolonged course, and clinical signs take months or years to appear. The usual clinical signs include weakness, loss of appetite, weight loss, fluctuating fever, intermittent hacking cough, diarrhoea and large prominent lymph nodes.
3.1.1.1. Article 7(a)(i) Animal species concerned by the disease
The use of the term bTB to refer to an M. bovis infection in species other than bovines creates the impression that bovines are the only species responsible for the spread of this organism, whereas multiple species (domestic and wild) can maintain and spread the infection with M. bovis without an ongoing association with cattle.
Susceptible animal species
Parameter 1 – Naturally susceptible wildlife species (or family/orders)
Most mammalian species are susceptible to infection with M. bovis and other related members of the MTBC that cause tuberculosis (O'Reilly and Daborn, 1995; Brosch et al., 2002; Delahay et al., 2002, 2007; Aranaz et al., 2004). In Europe, major susceptible species include badgers (Murhead and Burns, 1974; Barrow and Gallagher, 1981; Little et al., 1982a,b; Caffrey, 1994) red, roe and fallow deer (Clifton‐Hadley and Wilesmith, 1991; Bode, 1995; Delahay et al., 2002, 2007; Parra et al., 2006; OIE, 2007), wild boar (Sus scrofa) and feral pig (Di Marco et al., 2008). Isolation of M. bovis from wild boar is commonplace in Europe (Biolatti et al., 1992; Schultz et al., 1992; Aranaz et al., 1996; Serraino et al., 1999; Pavlik et al., 2002; Machackova et al., 2003; Duarte et al., 2008; Gortázar et al., 2008; Wilson et al., 2009). Also susceptible are red fox (Martín‐Atance et al., 2005; Delahay et al., 2007), mink (Delahay et al., 2002), ferret (Delahay et al., 2002), stoats (Delahay et al., 2007), rats (Delahay et al., 2002), field vole (Delahay et al., 2002), moles (Delahay et al., 2007), squirrels (Delahay et al., 2007), otters (Stephens, 1957) and Iberian lynx (Briones et al., 2000). M. bovis or other members of the M. tuberculosis‐complex have been also isolated from camels, antelope, primates, llamas, kudu, eland, tapir, elk, elephants, sitatunga, oryx, addax, rhinoceros, possums, seals, hares, rabbits, raccoons, coyotes and several predatory felines including lions, tigers, leopards and lynx (O'Reilly and Daborn, 1995; de Lisle et al., 2001; Michel et al., 2006).
Parameter 2 – Naturally susceptible domestic species (or family/orders)
Although cattle are thought to be the true host of M. bovis, a wide range of domestic species can be infected (Cousins, 2001). Isolations have been made from buffalo, bison, goats, camelids (including alpaca, llama, camels), pigs, deer, antelopes, dogs and cats (EFSA and ECDC, 2013). It rarely affects equids or sheep. However, in Spain and elsewhere, several outbreaks in sheep have been reported recently, all of which had epidemiological links with TB‐infected cattle herds or have occurred in endemic areas (Malone et al., 2003; van der Burgt et al., 2013; Muñoz‐Mendoza et al., 2016).
Parameter 3 – Experimentally susceptible wildlife species (or family/orders)
Badgers (Corner et al., 2007, 2008; Lesellier et al., 2009), deer (Griffin et al., 1999; Palmer et al., 2002), wild boar (Garrido et al., 2011), rabbits (Arrazuria et al., 2017), ferrets (Qureshi et al., 1999; Cross et al., 2000) and possums (Ramsey et al., 2009; Tompkins et al., 2009). Little is known about the susceptibility of birds to M. bovis although they are generally thought to be resistant. Experimental infections have recently been reported in pigeons after oral and intratracheal inoculation (Fitzgerald et al., 2003).
Parameter 4 – Experimentally susceptible domestic species (or family/orders)
Most domestic mammalian species are susceptible to experimental infection. The most developed experimental models of infection of livestock are: cattle (Dean et al., 2005; Buddle et al., 2016), camelids (Stevens et al., 1998), pigs (Bolin et al., 1997), goats (de Val Pérez et al., 2011; Gonzalez‐Juarrero et al., 2013), and deer (Palmer et al., 1999).
Reservoir animal species
Parameter 5 – Wild reservoir species (or family/orders)
Badgers (Meles meles) (Clifton‐Hadley et al., 1995; Griffin et al., 2005; Donnelly et al., 2006; McDonald et al., 2016).
Wild boar (Sus scrofa) (Gortázar et al., 2005; Parra et al., 2006; Naranjo et al., 2008).
Deer (Aranaz et al., 2004; Delahay et al., 2007; Zanella et al., 2008).
Parameter 6 – Domestic reservoir species (or family/orders)
Cattle, sheep, goats, camelids (especially alpaca and llama), buffalo and bison.
3.1.1.2. Article 7(a)(ii) The morbidity and mortality rates of the disease in animal populations
Morbidity
Parameter 1 – Prevalence/incidence
Livestock: EFSA and ECDC (2016) presented an overview of the M. bovis infection in Europe as below.
The 2015 list of countries and regions officially tuberculosis free3 (OTF) was: Austria, Belgium, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, seven regions and 14 provinces in Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, all administrative regions within the superior administrative unit of the Algarve in Portugal, Poland, Slovakia, Slovenia, Sweden, Scotland and the Isle of Man in the United Kingdom, Norway and Switzerland, in accordance with EU legislation (Commission Implementing Decision 2014/91/EU4). Liechtenstein has the same status (OTF) as Switzerland. In Iceland, which has no special agreement concerning animal health status with the EU, the last outbreak of bTB was reported in 1959.
Bulgaria, Croatia, Cyprus, Greece, Ireland, Italy, Portugal, Romania, Spain and the United Kingdom had not yet achieved the country‐level OTF status in 2015. The Umbria Region in Italy has been added to the OTF Regions in 2017 (Commission Implementing Decision (EU) 2017/8885.)
The overall proportion of cattle herds infected with, or positive for, bTB, considering all the EU regions, is 0.7% of the cattle herds in the EU, although there is a heterogeneous distribution of bTB in Europe with a pronounced spatial clustering. The prevalence ranges from absence of infected animals in most OTF regions to a regional prevalence in non‐OTF regions of 15.8% in Andalusia, Spain, considering all herds, or a reported regional prevalence of test‐positive cattle herds of 17.7% within the United Kingdom in Wales and England (Figure 1).
Figure 1.
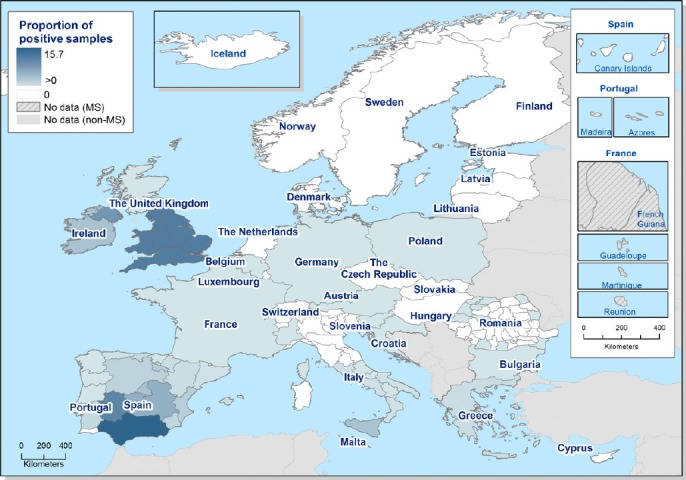
Proportion of cattle herds infected with or positive for bovine tuberculosis in 2015 (from EFSA and ECDC (2016))
In the 18 OTF, Member States (MSs) and in the regions declared OTF in the three non‐OTF MSs, Italy, Portugal and the United Kingdom, which represent a total population of 1,314,645 cattle herds, annual surveillance programmes are carried out to confirm freedom from bTB. Their reporting requirements are, among other indicators, the number of infected herds and the total number of herds existing. Eight of these MSs reported a total of 155 bTB‐infected herds from these areas; six MSs reported infection with M. bovis (Belgium, 3 herds; Germany, 12; Italy, 3; Poland, 28; Slovenia, 1; and the United Kingdom, 2), whereas Austria reported four herds infected with M. caprae and France declared 102 herds infected with M. tuberculosis‐complex. The prevalence of bTB‐infected herds in these OTF regions of 21 MSs in 2015 was 0.012%, compared to 0.011% during 2014 in the OTF regions of 19 MSs. From 2010 to 2015, the number of cattle herds reported infected in the OTF regions of the EU per year was 227, 200, 209, 197, 139 and 155, respectively.
Bovine TB was not detected in 2015 in the non‐MSs Iceland, Norway, Switzerland and Liechtenstein.
In 2015, there were a total of 1,167,945 cattle herds in the non‐OTF regions of the 10 MSs with non‐OTF status. The number of infected herds reported was 21 in Croatia (53 in 2014), 4,002 in Ireland (4,293 in 2014), 433 in Italy (380 in 2014), 94 in Portugal (108 in 2014), 3,070 in Spain (1,867 during 2014) and 9,628 in the United Kingdom (10,172 in 2014). The reports concerned M. bovis (Ireland, Italy, Portugal and the United Kingdom) or Mycobacterium tuberculosis complex (Croatia and Spain). As in 2014, Cyprus did not report any infected herds for the year 2015, whereas Bulgaria reported six (10 in 2014) and Greece 187 (203 in 2014) M. bovis‐infected herds. Romania reported 36 M. caprae‐infected herds (36 also in 2014).
From 2010 to 2015, the total number of test‐positive cattle herds in these EU non‐OTF regions remained at the same level and 17,814; 17,102; 18,208; 18,059; 17,122; and 17,441 were reported from 2010 to 2015, respectively. The overall prevalence of that period is increasing from 1.05% to 1.49% in 2015. Concomitantly, the total number of cattle herds decreased importantly from 1,638,694 in 2010 to 1,167,945 in 2015.
A herd prevalence > 10–20% is reported by the United Kingdom in Wales and England, with a reported highest regional prevalence in the EU of 17.7%. When considering all herds, the regional prevalence was 12.0%. For Northern Ireland, a low prevalence (> 1–10%) of test‐positive herds was reported, but with a slightly increasing trend. Also, Ireland and Spain reported a low prevalence with a decreasing (Ireland) and a slightly increasing trend (Spain) in recent years. When considering all herds, Spain reported a regional prevalence of 15.8% in Andalusia. Italy and Portugal reported prevalences > 0.1–1% in their remaining non‐OTF regions.
Greece reported a very low prevalence of infected cattle herds (M. bovis) with a slightly increasing trend from 0.44% in 2006 to 0.85% in 2015.
In 2015, 12 MSs and two non‐MSs investigated M. bovis in other animals and it was reported in 2,375 animals: water buffalos (1,343), wild boar (398), badgers (311), deer (195), alpacas (35), cats (34), zoo animals (21), pigs (20), foxes (8), sheep (8) and lamas (2). Thirteen MSs and two non‐MSs investigated animals for Mycobacterium species other than M. bovis. M. tuberculosis‐complex was reported not only in cattle, but also from wild alpacas (4), goats (4,022), cat (1), sheep (2), wild boars (31 slaughter batches), wild deer (45) and zoo animals (4), among which elephants. M. caprae was reported, in addition to the M. caprae‐positive herds in Austria and in Romania (see above), in 177 animals by three MSs (Germany, Hungary and Spain): cattle (169), wild boar (5), wild deer (2), and a fox.
Wildlife Badgers: The prevalence of infection in road‐killed or culled badgers is of the order of 10–15% in GB, but locally it can vary between a few percent and 50%. The UK government conducted the Randomised Badger Culling Trial (RBCT), a large‐scale field experiment to assess the effects of badger culling on bTB incidence in cattle. As part of this study, badgers were removed from ten 100 km2 bTB hotspot areas. The prevalence of bTB in badgers in these areas, as determined by microbiological culture of tissue following post‐mortem examination (PME), showed considerable variation among areas, with values ranging from 2% to 37% (Bourne et al., 2007; Jenkins et al., 2008). These are likely to be underestimates of true prevalence given the limited sensitivity (55%) of standard post mortem and culture detection relative to extended post‐mortem and culture (Crawshaw et al., 2008). In a similar study in the RoI, the prevalence of bTB in badgers in four large removal areas was 19.5% (Griffin et al., 2005). During a 22‐year period of a long‐term study of a wild badger population in a bTB hotspot area in south west England, annual bTB prevalence ranged from 1% to 11%, although this was based on the less sensitive approach of microbiological culture of clinical samples (i.e. faeces, urine, sputum, wound and abscess swabs) from live badgers (Delahay et al., 2000; Vicente et al., 2007).
Wild boar: M. bovis infection in Eurasian wild boar is widespread in Europe, being reported in both OTF and non‐OTF countries. In the last 10 years, reports of confirmed infection based on more than 20 animals have originated from Croatia, France, Italy, Portugal, Slovakia and Spain. Prevalence figures range from 1% to 63%. Most reports came from Mediterranean countries and the highest prevalence was recorded in the southern part of the Iberian Peninsula (Wilson et al., 2009; Vicente et al., 2013).
Deer: M. bovis infection in wild deer (mostly fallow and red) is widespread in Europe, and has been reported in both OTF and non‐OTF countries. In the last 10 years, reports of confirmed infection based on more than 20 animals have originated from the United Kingdom, Spain and Ireland. From the limited number of reports, it is clear that infection is highly clustered within certain localities. In red deer, prevalence estimates, again based on 20 or more animals, range from 1% to 27%; in roe deer, from 0% to 3%; in fallow deer, from 3% to 21%; in muntjac there was a single estimate of 5%; and in sika and sika crosses another single estimate of 4% (Wilson et al., 2009).
In carnivores, such as the fox, domestic cat and Iberian lynx, prevalence estimates based on 20 or more animals range from 1% to 17%; similarly, in mustelids (excluding badgers) from 1% to 4%; in rodents, from 1% to 3%; in insectivores (the mole), 1%; in herbivores (the chamois), less than 1% (Wilson et al., 2009).
Parameter 2 – Case‐morbidity rate (% clinically diseased animals out of infected ones)
Figures on TB morbidity in cattle are rarely presented as such and therefore must be inferred or extrapolated from the data available from countries where reactor animals are not culled. Where eradication programmes are in place, animals that fail to thrive are eventually culled from herds. Some of these animals will be found to be infected, exhibiting TB lesions at slaughter. Exceptions to this practice include Nepal and India where for cultural and religious reasons even reactor cattle are not culled. A mycobacteriological examination of milk and faeces from cattle and water buffalo positive on the cervical single intradermal test in Nepal isolated 13 M. bovis strains from 17% of buffaloes and 16% of cattle and in the Kerala state in India 60% of cattle were partially or completely affected by the disease and all crossbred cattle were suffering from tuberculosis. In Ireland, for example in the early 1950s before the bovine TB eradication programme commenced, there were approximately 4.5 million cattle of which some 17% were tuberculous (cows 22%, other cattle 8%) (Good, 2006). In Great Britain, prior to and in the early stage of TB eradication in bovines, Francis (1948) quotes the frequency of TB present in the udder and milk as 0.5–19.5% of tuberculous cows and 5–31% in cows with generalised tuberculosis (Francis, 1948). Analysis of milk in countries with no bTB eradication programme continues to show similar levels of M. bovis detection (Jha et al., 2007; Srivastava et al., 2008). In Argentina in the period 1969–2011, the percentage of animals that suffered total or partial condemnation at slaughter caused by TB lesions decreased from 6.7% to 0.6% as tuberculin testing of live cattle reduced TB incidence; in Chile the detection of TB in slaughtered cattle increased from 33 to 190 cases per 10,000 slaughtered animals between 1996 and 2005 and in 2011 was estimated to be 100 cases per 10,000 with 31.4% of tuberculous carcases condemned for generalised TB (Thoen et al., 2014).
In countries with control programmes, TB in cattle is often confined to one or two animals in a herd, although explosive outbreaks may occur especially if calves have been fed with contaminated milk. Nowadays in EU countries M. bovis infection rarely presents a clinical disease and it normally appears as apparently healthy animals reacting to the diagnostic tests. The severity of the disease varies with the dose of infectious organisms and individual immunity. Infected animals may remain asymptomatic, become ill only after stress or in old age, or develop a chronic, debilitating fatal disease. In developed countries, most reactors are detected during routine testing and mortality from tuberculosis is rare. In developing countries where there are no test and cull control programmes in place, overall prevalence and herd prevalence may be much higher (e.g. ca. 50% herd prevalence has been reported for Ethiopia (Firdessa et al., 2012; Dejene et al., 2016). This is akin to the situation described in GB in the 1940s where incidence was reported to increase 7% for each year of life reaching 40% after 5–6 years in dairy cattle (Francis, 1948).
In maintenance hosts, other than cattle, the prevalence of infection and the severity of the disease vary with species (Wilson et al., 2009).
Mortality
Parameter 3 – Case‐fatality rate
Cattle: Nowadays in EU countries, M. bovis infection rarely presents as clinical disease and it normally appears as apparently healthy animals reacting to the diagnostic tests, or they become ill only after stress or in old age, or develop a chronic, debilitating fatal disease, whose rate is not available. Moreover animals that fail to thrive are eventually culled from herds and they could be found to be affected and exhibiting TB lesions at slaughter. Therefore, case‐fatality rates due to TB in cattle are unavailable.
Wildlife: It is difficult to assess mortality due to M. bovis infection in wildlife populations. However, the following estimations have been reported.
Badgers: There is evidence for early onset of disease‐induced mortality in male but not female badgers. Males also have higher risk of infection and more rapid disease progression (Graham et al., 2013). In a long‐term study of a natural population of badgers to the nearest month, male average life expectancies were estimated to be 32 months for uninfected badgers and 22 months for infected badgers. Female average life expectancies were found to be 40 months for uninfected badgers and 35 months for infected badgers. When they are uninfected, this equates to males having on average a 20% lower life expectancy compared with females, with the acquisition of infection increasing this difference to 37% (McDonald et al., 2014).
Wild boar: A recent study of two different populations of wild boar with endemic tuberculosis in Spain suggested that tuberculosis accounted for 30% of total deaths (Barasona et al., 2016).
3.1.1.3. Article 7(a)(iii) The zoonotic character of the disease
Presence
Parameter 1 – Report of zoonotic human cases (anywhere)
The confirmed cases of tuberculosis due to M. bovis in humans were 157 in 2011 reported by 12 EU MSs, Norway and Switzerland (EFSA and ECDC, 2013), 136 in 2012 by 9 EU MSs, Norway and Switzerland (EFSA and ECDC, 2014), 141 in 2013 by 9 EU MSs and Switzerland (EFSA and ECDC, 2015a), 145 in 2014 by 9 EU MSs, Norway and Switzerland (EFSA and ECDC, 2015b), and 170 in 2015, reported by 26 EU MSs, Norway and Switzerland (EFSA and ECDC, 2016). The EU notification rate was stable between 2011 and 2015 (0.03 cases per 100,000 population), and there was no association between a country's status as OTF and notification rates in humans for the years 2013, 2014 and 2015. M. bovis infection and probably also M. caprae may also be transmitted human to human and thus each M. bovis/M. caprae case may not definitively be zoonotic in origin (Evans et al., 2007; Sunder et al., 2009; Buss et al., 2016).
As tuberculosis is a chronic disease with a long incubation period, it is not possible to assess travel‐associated cases in the same way as diseases with acute onset. Instead, the distinction is made between individuals with the disease born in the reporting country (native infection) and those moving there at a later stage (foreign infection). In a few cases, the distinction is also made based on the nationality of the cases. From 2011 to 2015, among cases with known origin, there were a larger proportion of native cases in countries non‐OTF than in countries that were OTF. However, for the years 2013, 2014 and 2015, there was no clear association between a country's status as OTF and notification rates in humans.
3.1.1.4. Article 7(a)(iv) The resistance to treatments, including antimicrobial resistance
Parameter 1 – Resistant strain to any treatment even at laboratory level
The mycobacteria are susceptible to antibiotics, although M. bovis is intrinsically resistant to pyrazinamide. Infected animals are generally slaughtered and are not treated. Treatment requires prolonged use of multiple drugs, the administration of which can be very difficult to achieve. This risks dosage not being met and a risk of developing antibiotic resistant strains of bacteria, which is a concern for both animal and human health. Inadequate dosage may result in the animal remaining infected often without signs of disease. Thus, antibiotic treatment is rarely attempted because of the high cost, lengthy treatment time, risk of antibiotic resistance and the larger goal of eliminating the disease. In some countries it may be illegal. Few cases of acquired antimicrobial resistance are described in the literature on M. bovis or M. caprae isolated from human cases (Armstrong and Christie, 1998; McLaughlin et al., 2012) and these may have been a result of selection of resistance due to treatment of humans infected with M. bovis (Guerrero et al., 1997).
3.1.1.5. The persistence of the disease in an animal population or the environment
Animal population
Parameter 1 – Duration of infectious period in animals
Bovine TB, caused by M. bovis, is a chronic, primarily respiratory disease that can take a variable amount of time (from a few weeks to a lifetime) to develop from infection to clinical disease and to become infectious to other animals. The transmission rate between animals is low and the epidemic only progresses slowly. Animals infected with MTC tend to shed M. bovis intermittently in relatively low numbers of organisms (compared to acutely infectious and contagious viral diseases such as foot and mouth disease).
Parameter 2 – Presence and duration of latent infection period
After initial infection, the mycobacteria may lie dormant in the body, sometimes without multiplication or producing any visible lesions in tissues for variable amounts of time. Recent modelling has estimated that in a European setting the latent period is between 2 and 8 months (Bekara et al., 2014); however, latent infection may last a lifetime and latency of variable duration is only detected at slaughter (Clegg et al., 2016).
Parameter 3 – Presence and duration of the pathogen in healthy carriers
The symptoms of bTB usually take months to develop. Infections can also remain dormant for years and reactivate during periods of stress or in old age. Thus, the pathogen may persist for a lifetime in latently infected animals.
Environment
Parameter 4 – Length of survival (dpi) of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment (scenarios: high and low T)
M. bovis can survive for several months in the environment, particularly in cold dark and moist conditions (Williams and Hoy, 1930). M. bovis bacilli can survive for up to approximately 400 days in running water (Briscoe, 1912) and water dilution will reduce the population of M. bovis organisms. In a recent study, M. bovis persisted up to 88 days in soil, 58 days in water and hay, and 43 days on corn under experimental conditions (Fine et al., 2011). Persistence of M. bovis in the environment was significantly shorter in the spring/summer season, characterised by the highest average daily temperatures and increased exposure to sunlight over the 12‐month period.
3.1.1.6. Article 7(a)(vi) The routes and speed of transmission of the disease between animals, and, when relevant, between animals and humans
Routes of transmission
Parameter 1 – Types of routes of transmission from animal to animal (horizontal, vertical)
Natural transmission can occur between domestic and wild animals. Cattle‐to‐cattle transmission of organisms of the MTBC occurs mainly by the respiratory route through the inhalation of aerosols containing the bacteria when the animals are in close contact. Oral route of infection due to the ingestion of mycobacteria from the environment cannot be discounted in some cases since it has been also described and vertical transmission is possible. Sources of M. bovis for animals include feed or water contaminated with urine, faeces or exudates from an infected animal (Sheffield et al., 1997; Hirsh and Zee, 1999; Thoen et al., 2008). Contaminated running water was recognised in the first half of the twentieth century as a mean of transmitting the disease (Garner, 1946) as M. bovis survives in water and may enter the respiratory tract during drinking (Phillips et al., 2003). Common use of natural water sources by cattle and wildlife could also transmit the disease (Phillips et al., 2002). M. bovis may survive for some time in the environment (Kukielka et al., 2013; Santos et al., 2015). This observation is key to understanding the indirect transmission and maintenance of infection in complex environments, involving multiple host species. In Mediterranean areas with a high prevalence of TB, it is common to detect MTC DNA in mud and water samples (Santos et al., 2015; Barasona et al., 2016). Direct contact inter species is rare (0.38% of all interactions (Cowie et al., 2016)). Therefore, transmission between species of MTC depends mainly on indirect contacts. Especially in Mediterranean environments, there is a high risk of these contacts at waterholes and other water points during the dry season (Barasona et al., 2014a,b). The evaluation of the MTBC hazard posed to cattle from wildlife across Europe demonstrated that cattle pose the greatest current and potential MTBC hazard for the majority of countries. The most common host communities for MTBC hazard in Europe are cattle‐deer‐wild boar systems, which were found in 15 countries across Europe. Wild boar poses the greatest hazard of all the wildlife species, indicating that wild boar have the greatest ability to transmit disease to cattle (Hardstaff et al., 2014). This is of particular concern given current population and distribution increases of some susceptible wildlife species, especially wild boar and deer which can help to spread pathogens to new areas and the paucity of wildlife MTBC surveillance programmes. In countries and regions with high wild boar populations in particular, there are likely to be significant advantages to MTBC control from developing integrated MTBC surveillance schemes incorporating both cattle and wild boar. In the UK and Ireland, the Eurasian badger (Meles meles) acts as a significant reservoir of infection for cattle. In areas such as south‐west England, where bTB breakdowns are common in the cattle population and the disease is endemic in local badgers, molecular studies show that both cattle and badgers are infected with identical strains of M. bovis, consistent with transmission of this infection between the two species (Hardstaff et al., 2014).
Parameter 2 – Types of routes of transmission between animals and humans (direct, indirect, including food‐borne)
The most important way that people can become infected is by the consumption of unpasteurised milk or dairy products from infected cows and where bTB is poorly controlled in livestock and consumption of raw milk or unpasteurised dairy products is frequent, bTB may represent an important human health risk. This was an important cause of childhood tuberculosis until pasteurisation became widespread. As mycobacteria survive readily in water, this has been identified as one of the major sources of human non‐tuberculous mycobacterioses (Dailloux et al., 1999). Occasional cases may occur through the consumption of other products accidentally contaminated with the organism. Infection through consumption of contaminated meat is thought to be very rare or non‐existent in Europe. Inhalation of the organism is significant for people working with infected animals but is not usually important for the general public. Human to human transmission is rare but has been seen in exceptional circumstances especially in immunocompromised individuals (de la Rua‐Domenech, 2006; Evans et al., 2007). However, while immunocompromised individuals are particularly susceptible to all types of MTBC, human to human spread of M. bovis is not always confined to immune‐compromised individuals. When humans are actively infected with pulmonary MTBC due to M. bovis transmission may be as common as it is with other causes of MTBC since the majority of MTBC infected persons are not actually infectious at all stages of infection (Evans et al., 2007; Sunder et al., 2009; Buss et al., 2016).
Speed of transmission
Parameter 3 – Incidence between animals and, when relevant, between animals and humans
M. bovis infection is spread to cattle primarily through the inhalation of infectious aerosols, either from a coughing or sneezing animal with open tuberculosis or from infected dust particles. Given the predominance of aerosol transmission, infection is spread more rapidly in intensive animal husbandry situations than in extensive or rangeland conditions. Aerosol transmission is effective only over short distances (1–2 m) and hence cattle density is a significant factor in the rate of transmission. Consequently, on dairy farms with a high density of cattle, or in production systems which house animals together for extended periods, the transmission rate between susceptible animals may be very high. In stables and barns used to house tuberculous cattle, infected droplets and particles may be constantly present in the air, presenting a hazard to susceptible animals and farm workers (Cousins, 2001).
Bekara et al. (2014) reported that the median within‐herd transmission coefficient for housed animals in France was five times as high as in the pasture: 0.43 month−1 (0.16–0.84) and 0.08 month−1 (0.01–0.32), respectively. These estimated medians were consistent with the estimates of within‐herd transmission of bTB in Spain proposed by Alvarez et al. (2012b), with higher transmission coefficients in dairy herds (median 0.39 month−1) than in beef herds (0.19 month−1). These estimated values of the transmission coefficients are also in the same range as values obtained by other studies: 0.22 month−1 in the study of Barlow et al. (1997) in New Zealand and 0.18 months−1.
Parameter 4 – Transmission rate (beta) (from R 0 and infectious period) between animals and, when relevant, between animals and humans
Estimates for Great Britain suggest that R0 in High‐Risk Areas for bTB range from 1.3 to 1.9 and in Low‐Risk Areas for bTB the R0 may range from 0.6 to 1.4 (O'Hare et al., 2014). Estimates for between‐herd R0 for bovine tuberculosis in Great Britain have been derived from a strategic model of the interaction between M. bovis, cattle and badgers, and lie in the interval 1.02–1.11 (Cox et al., 2005; Godfray et al., 2013). However, herd size may also affect rate of spread (the larger the herd the higher the potential R0) as will the presence of ‘super‐shedder’ animals. In the absence of a control program and once bTB has become enzootic within a herd, the median effective reproductive ratio was estimated to be 2.2 in beef herds and 1.7 in dairy herds. This difference was due to a lower age at culling for dairy cows than for beef cows. These low estimates were consistent with field observations of a low prevalence level in French bTB‐infected herds (Bekara et al., 2014).
3.1.1.7. Article 7(a)(vii) The absence or presence and distribution of the disease in the Union, and, where the disease is not present in the Union, the risk of its introduction into the Union
Presence and distribution
Parameter 1 – Map where the disease is present in EU
See Figure 1.
Parameter 2 – Type of epidemiological occurrence (sporadic, epidemic, endemic) at MS level
For the OTF, MSs and the regions declared OTF in the non‐OTF MSs, from 2010 to 2015, the number of cattle herds per year, the number of cattle herds reported infected per year and the prevalence of bTB‐infected herds per year are displayed in Figure 2.
Figure 2.
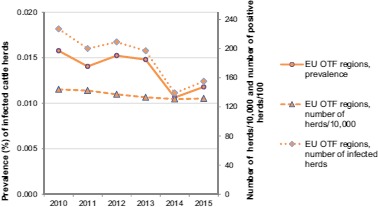
Proportion of cattle herds infected with or positive for bovine tuberculosis, in OTF regions, EU, 2010–2015 (EFSA and ECDC, 2016)
- OTF: Officially bovine tuberculosis free in cattle.
Figure 3 displays the MS‐specific trends of the prevalence of bTB test‐positive cattle herds in the non‐OTF regions of six MSs with the EU co‐financed eradication programmes, during 2004–2015. Greece, a non‐OTF MS without an EU co‐financed eradication programme, reported a very low prevalence of infected cattle herds (M. bovis) with a slightly increasing trend from 0.44% in 2006 to 0.85% in 2015.
Figure 3.
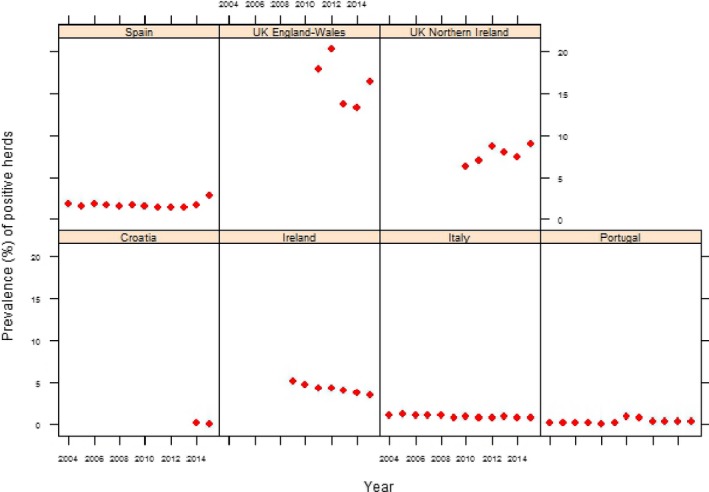
Prevalence of bovine tuberculosis test‐positive cattle herds, in non‐OTF regions of six non‐OTF cofinanced Member States, 2004–2015 (EFSA and ECDC, 2016)
Risk of introduction
Parameter 3 – Routes of possible introduction
Bovine tuberculosis is already present in a number of MSs.
Parameter 4 – Number of animal moving and/or shipment size
Bovine tuberculosis is already present in a number of MSs.
Parameter 5 – Duration of infectious period in animal and/or commodity
Due to the chronic nature of infection and associated variable latency period, the infectious period can last for the lifetime of an infected animal.
Parameter 6 – List of control measures at border (testing, quarantine, etc.)
Import controls – The rules governing control measures at borders are laid down in Council Directive 91/496/EEC6, consolidated version, which foresees veterinary checks on all live animals entering the Community from third countries. Import conditions for bovine animals are regulated in Council Directive 2004/68/EC7 and more specifically in Commission Regulation (EU) No 206/20108, where it is specified that ungulates can be introduced into the Union if they come from a third countries or territories for which there is a model veterinary certificate (Annex I) and if they are accompanied by the veterinary certificate and they comply with the requirements set out in the certificate.
Parameter 7 – Presence and duration of latent infection and/or carrier status
After initial infection, the mycobacteria may lie dormant in the body, sometimes without multiplication or producing any visible lesions in tissues for variable amounts of time. Recent modelling has estimated that in a European setting the latent period is between 2 and 8 months (Bekara et al., 2014). However, the latent period may last for the lifetime of an infected animal.
3.1.1.8. Article 7(a)(viii) The existence of diagnostic and disease control tools
Diagnostic tools
According to Council Directive 64/432/ECC, the available diagnostic tools for bTB are:
The in vivo single and comparative intradermal tuberculin (SIT and SCIT, respectively) tests as the official diagnostic assays for bTB in the MSs. The test details are described in the Directive.
The in vitro interferon‐gamma (IFN‐γ), referred to in the OIE (2015). This test is considered an ancillary assay to the tuberculin test (Council Directive 64/432/EEC, amended by Commission Regulation (EC) No 1226/20029), and may be authorised by MSs to maximise detection of infected and diseased cattle in a herd or in a region. When used in combination with skin tests, overall test sensitivity is increased.
Although the IDEXX serological test is OIE certified, it is not approved by the EU as a test eligible for co‐funding under currently co‐funding rules because it is not specifically listed in the Directive. Further, the performance of this test has not been examined by the EU.
In animals slaughtered as test negative and showing lesions suggestive of TB, tissues are taken and confirmation of infection is by culture or through microscopic histological examination of post mortem material. Polymerase chain reaction (PCR) is also being used increasingly because it allows a more rapid diagnosis.
Monitoring for lesions consistent with tuberculosis by post‐mortem examination of cattle carcasses at abattoirs is also a critical element of tuberculosis eradication. However, meat inspection procedures are estimated to detect only 50% of cattle with tuberculous lesions, compared with a detailed necropsy procedure that included slicing lymph nodes in a laboratory at 2 mm intervals and visual examination of the cut surfaces (85%) (Corner et al., 1990).
The available diagnostic tests are described in the OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (OIE, 2015).
Control tools
Parameter 2 – Existence of control tools
The EU has a set of specific legislation for a number of animal diseases depending on their potential social and economic impact. This includes notification obligations, diagnostic methods, measures to be applied in case of suspicion and confirmation of disease and, where applicable, regionalisation measures.
The most relevant legislation regarding eradication of bovine TB is summarised in Council Directive 77/391/EEC10 which introduced Community measures for the eradication of TB in cattle. In this Directive, MSs are obliged to draw up programmes for accelerated eradication (financial contribution Community), amended and completed by Council Directives 78/52/EEC, 82/400/EEC11 and Council Decision 87/58/EEC12. The legislation is augmented by Annex 1 of European Commission (2006) outlining necessary inputs for bTB eradication. Control tools include:
Compulsory regular periodic testing of cattle at a risk‐based testing frequency by intradermal skin testing together with slaughter of reactor cattle and other cattle likely to have been infected by contact.
Strategic use of the IFN‐γ test.
Prohibition of movements of cattle out of herds that are not OTF and in which infection is suspected or confirmed.
Inspection of the carcasses of all cattle slaughtered for human consumption, with laboratory testing of any lesions, from test negative cattle, suspected of being tuberculous.
Import controls on live animals to reduce risk of importing infected animals.
Pasteurisation of milk and milk products except for specific premises whose products are subject to periodic monitoring and from OTF farms particularly in OTF counties.
On farm biosecurity measures to reduce transmission of M. bovis between cattle, other domestic livestock and wildlife.
Disease should be addressed in all susceptible and infected species.
In principle, the risk‐mitigating measures should be effective and proportionate; the disease has been eradicated in several countries and the prevalence at EU level has decreased following implementation of the specific legislation above and the so called ‘trading directive’ Council Directive 64/432/EEC as amended and the conditions laid down by the OIE and WHO. However, in some settings, the risk‐mitigating measures are neither effective nor proportionate, in particular, the measures laid down in the legislation apply solely to bovine animals, but M. bovis is not a single host pathogen. As a fundamental epidemiological principle, a disease which is shared and maintained independently by a range of species in the same environment cannot be effectively controlled only by addressing the problem in one of the affected species. If the control measures are not applied to all epidemiologically relevant species ‐ either farmed (goats, alpaca, deer, pigs, sheep) or wildlife (badger, wild boar, deer) then eradication of tuberculosis in bovines will be highly improbable.
3.1.2. Article 7(b) The impact of diseases
3.1.2.1. Article 7(b)(i) The impact of the disease on agricultural and aquaculture production and other parts of the economy
The level of presence of the disease in the Union
Parameter 1 – Number of MSs where the disease is present
Bulgaria, Croatia, Cyprus, Greece, Ireland, Italy, Portugal, Romania, Spain and the United Kingdom did not achieve the country‐level OTF status in 2015 (EFSA and ECDC, 2016).
The loss of production due to the disease
Parameter 2 – Proportion of production losses (%) by epidemic/endemic situation
Production losses due to clinical disease are low in Europe due to statutory surveillance in affected MSs. Economic loss is generally due to the implementation of the test and slaughter control strategies and movement restrictions. Examples of such losses are given in Section 3.1.5.1 Parameter 1. Production losses will vary, both between infected animals and infected herds. Studies have found that bTB was associated with a 18% decrease in milk production in cattle in Bangladesh (Rahman and Samad, 2008) and 4% in USA (Hernandez and Baca, 1998). Bovine tuberculosis has been reported to decrease meat production by 6–12% (± 2.5%), depending on the percentage of cows (30–70%) in a herd in which more than 80% of all animals or more than 95% of cows are infected (Zinsstag et al., 2006).
3.1.2.2. Article 7(b)(ii) The impact of the disease on human health
Transmissibility between animals and humans
Parameter 1 – Types of routes of transmission between animals and humans
The routes of transmission of TB from animals to humans are well known and include direct exposure to infected animals or consumption of contaminated animal products (Francis, 1948). Application of fingerprinting tools facilitates analysis of the molecular epidemiology of M. bovis in animal‐to‐human and human‐to‐human transmission. Nevertheless, other members within the MTBC can be zoonotic (Olea‐Popelka et al., 2017). Improvements in diagnostic techniques, application of more advanced discriminatory genotyping tools, and collaboration between veterinary and human health care researchers are key to our understanding of this zoonosis. Infected humans are a potential risk for animals and other humans. All forms of TB may occur as a latent infection in many humans and extra‐pulmonary TB as a consequence of oral infection tends to have limited transmission pathways in humans. When infection is pulmonary, the risk is not only to immunocompromised individuals. The development of genotyping techniques has allowed the demonstration of person‐to‐person transmission events involving M. bovis (Gibson et al., 2004). The few reports that have been able to identify such events investigate clusters comprising a small number of cases with close epidemiological relationships (Evans et al., 2007).
Parameter 2 – Incidence of zoonotic cases
The overall consumption risk in Europe is currently negligible because of wide scale milk pasteurisation and compulsory regular screening of cattle by the tuberculin skin test in non‐OTF regions and inspection of carcasses at slaughter. Inhalation of the organism and risks of cutaneous wounds in those dressing carcasses is significant for people working with infected animals but is not usually important for the general public.
In 2015, 170 confirmed cases of tuberculosis due to M. bovis in humans were reported by 26 EU MSs (EFSA and ECDC, 2016). However, this is probably an underestimate as media designed to culture M. bovis is not always used as a routine. Eleven MSs reported at least one confirmed case and 15 MSs reported zero cases. The EU notification rate was 0.03 cases per 100,000 population, the same as in previous years. Most cases were reported in Germany, the United Kingdom and Spain, while the highest notification rate (0.11 cases per 100,000 population) was observed in Ireland.
Transmissibility between humans
Parameter 3 – Human to human transmission is sufficient to sustain sporadic cases or community‐level outbreak
Infected humans are a potential risk for other humans especially when infection is pulmonary. In 2006, DNA investigation techniques showed that a cluster of 6 cases in the UK were linked by human to human transmission (Evans et al., 2007). In a second reported outbreak, 19 cases in HIV‐1‐infected patients of primary multidrug‐resistant (MDR) tuberculosis were caused by M. bovis resistant to 11 antituberculosis drugs. Both phenotypic and genotypic similarities in the strains of M. bovis were reported (Guerrero et al., 1997).
Parameter 4 – Sporadic, endemic, epidemic, or pandemic potential
There is a sporadic potential in humans.
The severity of human forms of the disease
Parameter 5 – Disability‐adjusted life year (DALY)
In common with all causes of TB in humans, infection with M. bovis is potentially fatal, although not everyone infected with the bacterium develops disease. The bacteria may remain inactive in the body for many years and, in some individuals, reactivation occurs later. Once disease develops, about half the patients will die within 5 years if untreated, the majority of these within the first 18 months. Treatment with a course of antituberculosis drugs usually results in cure. Infection by M. bovis is thought to be clinically indistinguishable from infection with M. tuberculosis. However, discrimination between M. bovis and M. tuberculosis is not routinely performed and reported in many countries (de la Rua‐Domenech, 2006). TB halts work in the formal and informal economies, as well as within households. Country studies document between 3‐ and 4‐month work time lost annually to the disease, and lost earnings of 20–30% of household income. Families of persons who die from the disease lose about 15 years of income. As economic difficulties put pressure on state health budgets, the social cost of this lost productivity is compounded.
The availability of effective prevention or medical treatment in humans
Parameter 6 – Availability of medical treatment and their effectiveness (therapeutic effect and any resistance)
M. bovis is naturally resistant to pyrazinamide, one of the four medications used in the standard first‐line anti‐tuberculosis treatment regimen. Thus, humans with M. bovis infection are treated with antimicrobial drugs including isoniazid, rifampicin and ethambutol. A long intensive course of treatment is required (6–9 months).
Parameter 7 – Availability of vaccines and their effectiveness (reduced morbidity)
At present the only available vaccine for humans is Bacillus Calmette‐Guérin (BCG), which is a live attenuated strain of M. bovis. Reported efficacy ranges from 0% to 80% (Fine, 1995).
3.1.2.3. Article 7(b)(iii) The impact of the disease on animal welfare
Parameter 1 – Severity of clinical signs at case level and related level and duration of impairment
Infection with M. bovis can cause welfare issues once clinical signs are expressed, although farmed animals in the EU are managed and therefore kept under observation; any clinical signs are likely to be investigated and action taken before serious welfare issues arise. Therefore, the modal presentation in cattle is a clinically inapparent infection disclosed as a result of routine TB testing. Some animals will become diseased, with clinical signs such as coughing, difficulty in breathing, enlarged lymph nodes of head and neck, intermittent diarrhoea, extreme emaciation, weakness and acute respiratory distress. In addition, a drop in milk production may be observed in clinically affected high yielding dairy cows. Even in chronically infected animals, characteristic signs of disease may not be readily apparent.
Although M. bovis affects a wide range of animal hosts, particularly mammals, there is wide variation in levels of natural susceptibility and propensity to develop severe disease between species (Francis, 1958; Une and Mori, 2007). However, all species, including those that usually develop mild forms of disease, have the capacity to develop generalised or advanced tuberculosis. Such progressive disease may typically be associated with emaciation, old age, concomitant infections and other factors that may negatively impact on the immune system of the host. Wild animals with end stage tuberculosis typically show emaciation, lethargy and abnormal behaviour (Wilson et al., 2009).
3.1.2.4. Article 7(b)(iv) The impact of the disease on biodiversity and the environment
Biodiversity
Parameter 1 – Endangered wild species affected: listed species as in CITES and/or IUCN list
In the EU, Iberian Lynx, bear, European bison.
Parameter 2 – Mortality in wild species
EU wildlife species at risk – all mammals including badgers, red deer, roe deer, wild boar, feral pigs, red fox, mink, ferret, Iberian lynx. Of this list, only the Iberian Lynx is endangered (Wilson et al., 2009).
Environment
Parameter 3 – Capacity of the pathogen to persist in the environment and cause mortality in wildlife
M. bovis can survive for some weeks in the environment. Duffield and Young (1985) report that M. bovis survived for 4 weeks in non‐sterile dry and moist soils held under 80% shade, in darkness and in the laboratory. It was also isolated from sterile moist soil kept in the shade and in darkness.
Maintenance hosts include badger, deer and wild boar ‐ level of mortality caused by M. bovis infection is largely unknown. Models have been used to estimate differential survival, force of infection and progression in disease states in a population of Eurasian badgers (Meles meles), naturally infected with bTB. These state‐dependent models found evidence for early onset of disease‐induced mortality in male but not female badgers. Males also had higher risk of infection and more rapid disease progression (Graham et al., 2013).
A recent study of two different populations of wild boar with endemic TB in Spain suggested that TB accounted for 30% of total deaths (Barasona et al., 2016).
3.1.3. Article 7(c) Its potential to generate a crisis situation and its potential use in bioterrorism
Parameter 1 – Listed in OIE/CFSPH classification of pathogens
Bovine TB is listed in the OIE list of notifiable terrestrial and aquatic animal diseases (http://www.oie.int/animal-health-in-the-world/oie-listed-diseases-2017/), but not classified as potential bioterrorism agent by CDC.
Parameter 2 – Listed in the Encyclopaedia of Bioterrorism Defence of Australia Group
It is not listed.
Parameter 3 – Included in any other list of potential bio‐agro‐terrorism agents
It is not listed.
Infection with MTBC may have the potential to generate some conflict situations between different stakeholders, like farmers, hunters and conservationists. This is due to the multihost system of MTBC, which can generate a disease gradient between livestock and wildlife, leading to conflict between different parties with different interests. In wild ungulates high MTBC prevalence has been recorded, in a system where the livestock population is also involved. The presence of MTBC at the interface between domestic animals and wildlife also generates conflict around protected areas (Gortázar et al., 2010).
3.1.4. Article 7(d) The feasibility, availability and effectiveness of the following disease prevention and control measures
3.1.4.1. Article 7(d)(i) Diagnostic tools and capacities
Availability
Parameter 1 – Officially/internationally recognised diagnostic tool, OIE certified
Council Directive 64/432/ECC recognises the in vivo SIT and SCIT, respectively, tests as the official diagnostic assays for bovine TB in the Member States. To maximise detection of infected and diseased cattle in a herd or in a region, MS may authorise the employ of the in vitro IFN‐γ referred in the OIE Manual of Standards for Diagnostic Tests and Vaccines (OIE, 2015) as an ancillary assay to the tuberculin test. The Bovigam® M. bovis gamma interferon test is OIE certified. Both in vivo and in vitro assays are performed using purified protein derivatives (PPDs) obtained from the heat‐treated products of growth and lysis of M. bovis (bovine PPD) and M. avium (avian PPD) as described in Annex B of the Directive. IDEXX serological test may be used as an ancillary test in infected herds. The IDEXX test is OIE certified but not listed in the EU as a recognised test.
Tissues are taken from immune positive animals and confirmation of infection is by bacterial culture. Confirmation through microscopic histological examination of PME material is also regularly performed. PCR is becoming increasingly used because it allows a faster diagnosis than conventional culture.
Effectiveness
Parameter 2 – Se and Sp of diagnostic test
Sensitivity and specificity estimates for diagnostic tests can be dependent upon the animal population studied and the disease characteristics within the population. Caution should be exercised in extrapolating sensitivity and specificity estimates made from populations of animals different from those in which the test is being performed (Strain et al., 2011). Biological potency of the tuberculins is critical for the outcome of the intradermal test and a significant difference in the numbers of reactors and infected bovines detected using high and low potency tuberculins has been reported (Good et al., 2011).
A summary of the test performance characteristics can be found on the website of the European Reference Laboratory for Bovine Tuberculosis (https://www.visavet.es/bovinetuberculosis/bovine-tb/diagnosis.php) and are given below.
Reported sensitivity of the SIT test in cattle ranged between 80.2% and 100% although it was evaluated under specific epidemiological circumstances (de la Rua‐Domenech, 2006). More recently and using a Bayesian approach, the estimate sensitivity of the SIT test ranged between 53% (27.3–81.5, 95% CI) and 69.4% (40.1–92.2, 95% CI) depending on the interpretation criteria used (Alvarez et al., 2012a,b). The results from this study highlighted the low sensitivity of the intradermal test after the disclosure test. Reported Sp of the SIT test ranged between 55.1% and more than 99% showing a median value over 95% (de la Rua‐Domenech et al., 2006; Schiller et al., 2010; Downs et al., 2011; Alvarez et al., 2012a).
Sensitivity of the SCIT test is lower than that achieved using the SIT test. Reported values ranged between 52% and 100% (de la Rua‐Domenech et al., 2006; Duarte et al., 2008; Schiller et al., 2010). In the presence of interference factors (e.g. infection by environmental mycobacteria), comparative interpretation increases the Sp (median of 99.5%) (de la Rua‐Domenech et al., 2006; Schiller et al., 2010).
Field studies carried out in the last two decades with the aim of comparing the diagnostic performance of the tuberculin intradermal test and the IFN‐γ assay, demonstrated the higher sensitivity of the IFN‐γ test. Nevertheless, the specificity achieved was similar or slightly lower than that of the SIT, and lower than the Sp of the SCIT test. A meta‐analysis of 15 field studies conducted between 1991 and 2006, showed an estimated median Se of 87.6% with a range between 73% and 100% and a Sp of 96.6% with a range of 85% and 99.6% for the IFN‐γ assay (de la Rua‐Domenech et al., 2006). The higher Se of the IFN‐γ test compared to the skin test is likely due to the fact that the IFN‐γ test detects TB‐infected animals as early as 14 days following infection (Buddle et al., 1995) and 60–120 days earlier than the SCIT test (Lilenbaum et al., 1999). The EFSA Panel on Animal Health and Welfare has published a comprehensive review of the data for the gamma interferon test and their scientific opinion on the use of gamma interferon test for the diagnosis of bovine tuberculosis (EFSA AHAW Panel, 2012).
The OIE accredited IDEXX serological test, recommended by OIE as the most suitable of the antibody‐detection tests which can be a complement, rather than an alternative, to tests based on cellular immunity and helpful in detecting anergic cattle and deer has a reported Sp 97.4–98.8% and herd sensitivity of 77.8%.
Culture is considered to be highly specific but the test procedure requires 6 weeks. Bayesian models have been developed to compare PCR, bacteriology and histopathology, allowing for dependence between bacteriology and PCR, while assuming independence from histopathology (Courcoul et al., 2014). The sensitivity of PCR was higher than that of bacteriology (on average 87.7% [82.5–92.3%] vs 78.1% [72.9–82.8%]) while specificity of both tests was very good (on average 97.0% for PCR [94.3–99.0%] and 99.1% for bacteriology [97.1–100.0%]). Histopathology was at least as sensitive as PCR (on average 93.6% [89.9–96.9%]) but less specific than the two other tests (on average 83.3% [78.7–87.6%]).
Feasibility
Parameter 3 – Type of sample matrix to be tested (blood, tissue, etc.)
Blood (IFN‐γ, serology), milk, urine, faeces, biopsy, post mortem examination of tissues.
3.1.4.2. Article 7(d)(ii) Vaccination
Availability
Parameter 1 – Types of vaccines available on the market (live, inactivated, DIVA, etc.)
Vaccination of cattle against bovine tuberculosis is explicitly forbidden in the EU legislation on disease control (Council Directive 78/52/EEC13) and implicitly also in intra‐Union trade legislation, as vaccination is not compatible with the provisions for testing and herd qualification (Council Directive 64/432/EEC). EU legislation is fully in line with OIE standards on international trade and can be changed only by the European Parliament and the Council. Recently, the EFSA AHAW Panel published a scientific opinion on the requirements for field trials for bovine tuberculosis vaccination (EFSA AHAW Panel, 2013).
A live attenuated vaccine BadgerBCG vaccine has been granted a unique Limited Marketing Authorisation (LMA) for use in badgers only in the UK.
Effectiveness
Parameter 3 – Field protection as reduced morbidity (as reduced susceptibility to infection and/or to disease)
Vaccination with BCG has been shown to reduce the severity and progression of experimentally induced TB in captive badgers. Analysis of data from a 4‐year clinical field study, conducted at the social group level, suggested a similar, direct protective effect of BCG in a wild badger population and both a direct beneficial effect of vaccination in individual badgers and an indirect protective effect in unvaccinated cubs. Intramuscular injection of BCG reduced by 54% (odds ratio = 0.46, 95% CI 0.26–0.88) the risk of free‐living vaccinated individuals testing positive to a diagnostic test combination to detect M. bovis infection following vaccination. In addition, the risk of unvaccinated badger cubs, but not adults, testing positive decreased significantly as the proportion of vaccinated individuals in their social group increased (Odds ratio = 0.08, 95% CI 0.01–0.76; p = 0.03). When more than a third of their social group had been vaccinated, the risk to unvaccinated cubs was reduced by 79% (Odds ratio = 0.21, 95% CI 0.05–0.81; p = 0.02) (Chambers et al., 2011; Carter et al., 2012).
The results of an oral BCG vaccination field trial conducted in Ireland on wild free‐living badgers has also recently been published which has established that oral vaccination of free‐living badgers can reduce the incidence of tuberculosis in free‐ranging badger populations (Gormley et al., 2017).
Parameter 4 – Duration of protection
Duration of immunity for BadgerBCG is currently unknown but annual vaccination is recommended.
Feasibility
Parameter 5 – Way of administration
BadgerBCG has a limited licence in the UK only for intramuscular injection. Trapping of animals is required to carry out this procedure (Brown et al., 2013).
3.1.4.3. Article 7(d)(iii) Medical treatments
The mycobacteria are susceptible to antibiotics, although M. bovis is intrinsically resistant to pyrazinamide. Humans infected with M. bovis are treated with antimicrobial drugs including isoniazid, rifampicin and ethambutol. A long intensive course of treatment (6–9 months) is required. Infected animals are generally slaughtered and are not treated except where the value of the animal is great, e.g. zoo animals or domestic pets.
Antibiotic therapy of bovine tuberculosis is not recommended.
3.1.4.4. Article 7(d)(iv) Biosecurity measures
Availability
Parameter 1 – Available biosecurity measures
RESTRICT contact between wildlife and cattle.
MANAGE cattle feed and water.
STOP infected cattle entering the herd.
REDUCE risk from neighbouring herds.
MINIMISE infection from cattle manure.
The evidence‐base behind these measures can be found at http://www.tbhub.co.uk/biosecurity/protect-herd-tb-review-science/
In addition risk of infection can be reduced by reducing the population of other infected species (farmed or wild) that share the same environment.
Effectiveness
Parameter 2 – Effectiveness of biosecurity measures in preventing the pathogen introduction
The relative importance of various routes of transmission is poorly known and no quantitative estimates of any of these transmission rates are available. Thus, a strong evidence base to evaluate different strategies is currently lacking (Godfray et al., 2013).
Feasibility
Parameter 3 – Feasibility of biosecurity measures
This will vary from farm to farm and will depend upon the most important risk factors for transmission of M. bovis to livestock on the farm.
TB eradication has been achieved by a number of MSs using a test and slaughter control programme laid down by Council Directive 64/432/EEC that includes prohibition of movements of animals from infected herds.
There is a lack of a strong evidence base with which to assess the feasibility of biosecurity measures (Godfray et al., 2013). Simple exclusion measures have been reported to be 100% effective in preventing badger entry into farm buildings, as long as they were appropriately deployed. The installation of exclusion measures also reduced the level of badger visits to the rest of the farmyard thereby reducing some of the potential for contact and disease transmission between badgers and cattle (Judge et al., 2011). No effective measures to prevent badgers utilising cattle pasture for forage have been reported and such practices could conceivably have a negative welfare impact on badgers by limiting food source/supply. However, other studies have demonstrated that there is little direct contact between badgers and cattle and also that badgers do not regularly frequent farm yards/buildings. In such circumstances, biosecurity measures preventing badger ingress to farm buildings are unlikely to have an effect in reducing bTB risk in cograzing cattle (Mullen et al., 2013; O'Mahony, 2014; Mullen et al., 2015; O'Mahony, 2015a,b). There is also evidence of cattle exposure to environmental contamination from wildlife sources (King et al., 2015; Barbier et al., 2016).
3.1.4.5. Article 7(d)(v) Restrictions on the movement of animals and products
Availability
Parameter 1 – Available movement restriction measures
These are laid out in Council Directive 64/432/EEC. Detection of positive animals results in restrictions on the movement of cattle from the herd: only reactor animals going directly to slaughter can be removed and farmers receive compensation for reactor animals. Movement restrictions are only lifted after a minimum of two clear tests, the second of which must be carried out a minimum of 4 months from the date of removal of the last positive animal and only once the premises have been cleaned and disinfected.
Effectiveness
Parameter 2 – Effectiveness of restriction of animal movement in preventing the between farm spread
TB eradication has been achieved by a number of MSs using a test and slaughter control programme laid down by Council Directive 64/432/EEC that includes prohibition of movements of animals from infected herds.
Feasibility
Parameter 3 – Feasibility of restriction of animal movement
This is a requirement of any TB control programme in Europe (Council Directive 64/432/ECC).
3.1.4.6. Article 7(d)(vi) Killing of animals
Availability
Parameter 1 – Available methods for killing animal
Compulsory slaughter of cattle that react to the skin test at licensed abattoirs.
Culling of wildlife reservoirs.
Effectiveness
Parameter 2 – Effectiveness of killing animals (at farm level or within the farm) for reducing/stopping spread of the disease
The test and slaughter programme for the control of bTB in cattle is laid down in Council Directive 64/432/EEC and has proved effective at eradicating bovine tuberculosis from many MSs in Europe. However, the strategy has proved less successful in MSs with wildlife reservoirs of M. bovis or M. caprae.
In Europe, widespread indiscriminate culling of the important wildlife hosts of bovine tuberculosis is unlikely to offer an effective solution on its own. However, targeted culling may have a role under certain circumstances (Griffin et al., 2005; Donnelly et al., 2007) especially if employed together with other measures such as vaccination and improved biosecurity of domestic animals alongside cattle testing and controls. The potential for targeted culling to be used successfully may be enhanced as a result of on‐going developments in diagnostic testing and improved understanding of bTB dynamics in wildlife (Wilson et al., 2009; Smith et al., 2012; Gortazar et al., 2015; Abdou et al., 2016).
Feasibility
Parameter 3 – Feasibility of killing animals
Livestock are regularly disposed of using well‐established methodologies in licensed abattoirs as required by Council Directive 64/432/EEC.
For wildlife, a number of factors must be carefully considered in order to determine if culling is appropriate. Resource availability, the size of the infected area, the ecology of the wildlife host, and the period over which culling is required will all influence whether this is a cost‐effective approach. Culling can have both positive and potentially negative and sometimes unpredictable consequences. Culled populations may respond by increasing productivity, so that culling may have to be repeated at regular intervals, with cost and logistical implications. Such compensatory reproduction may also have counterproductive effects such as increasing the proportion of young, susceptible individuals in the population. Culling may also promote increased dispersal by surviving animals, and increased immigration into the culled area (Wilson et al., 2009; Gortazar et al., 2015).
3.1.4.7. Article 7(d)(vii) Disposal of carcasses and other relevant animal by‐products
Availability
Parameter 1 – Available disposal option
Regulation (EC) No 1069/200914 lays down animal and public health rules for animal by‐products and products derived thereof. It determines the circumstances under which animal by‐products are to be disposed of, in order to prevent the spreading of risks for public and animal health. In addition, that Regulation specifies under which conditions animal by‐products may be used for applications in animal feed and for various purposes, such as in cosmetics, medicinal products and technical applications. It also lays down obligations for operators to handle animal by‐products within establishments and plants that are subject to official controls. Positive animals are normally slaughtered in licensed slaughterhouses, but are kept separate from other cattle. The carcasses are then inspected for ‘MTBC lesions’ and samples are sent to a diagnostic laboratory.
Regulation (EC) No 1069/2009 requires, among other things, that wild animals suspected of being infected with diseases communicable to humans and animals, such as tuberculosis, are disposed of at an approved plant using one of the following methods: (1) incineration; (2) rendering followed by incineration; or (3) pressure rendering followed by landfill. In addition, the regulation also allows for burial and burning of dead animals in specific situations.
Effectiveness
Parameter 2 – Effectiveness of disposal option
Incineration and rendering are closed systems that produce an effective inactivation of M. bovis.
Feasibility
Parameter 3 – Feasibility of disposal option
Livestock are regularly disposed of by the methods described. Strict biosecurity during carcass transport is also required.
3.1.5. Article 7(e) The impact of disease prevention and control measures
3.1.5.1. Article 7(e)(i) The direct and indirect costs for the affected sectors and the economy as a whole
Parameter 1 – Cost of control (e.g. treatment/vaccine, biosecurity)
This is very difficult to quantify since it depends on the size of the premise, type of production and OTF status of the MS. Figures are available for two of the MSs with the highest prevalence of bovine tuberculosis.
UK Government expenditure on bTB control (including testing, compensation and research) in GB during 2010/2011 was over £100 million. The estimated cost of a confirmed herd breakdown in areas of high risk for bovine tuberculosis in England was £14,000 to farmers and £20,000 to government.
Bovine tuberculosis control in Ireland costs approximately €80 million annually: €50 million from government and € 30 million from producers (Duignan et al., 2012).
Parameter 2 – Cost of eradication (culling, compensation)
Over the period of 2005–2011, 15.5% of EU co‐funding covered the eradication of bTB. Across Europe, €62 million has been allocated to support eradication, control and surveillance programmes that aim to eliminate bTB in 2016.
Between 2007 and 2011, the total cost incurred by the Health Service of Lazio Region (Italy) for the eradication of bTB was estimated in 6 788 294 EUR, of those about 6 million were the cost of the veterinarians labour, 35,454 for the transport, 234,314 for disposal and compensation for culled animals (Caminiti et al., 2016).
The expected overall cost of national veterinary programme for the eradication of bTB submitted for obtaining EU co‐financing from non‐OTF MSs to European Commission in 2015 and 2016 is reported in Table 1 (https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en).
Table 1.
Total expected amount of the cost of the eradication programme for bovine tuberculosis in some non‐OTF MSs in 2015 and 2016 (https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en)
| Member State | Total expected cost in € in 2015 | Total expected cost in € in 2016 |
|---|---|---|
| Austria | 542,309 | 213,339 |
| Croatia | 3,008,980 | – |
| Ireland | 62,320,389 | 62,881,246 |
| Italy | 10,954,624 | 10,700,583 |
| Portugal | 3,222,491 | 1,998,849 |
| Spain | 43,130,259 | 29,899,297 |
| UK | 74,868,739 | 107,354,817 |
Parameter 3 – Cost of surveillance and monitoring
UK Spend for surveillance in GB was approximately £64 million for 2010/2011.
Parameter 4 – Trade loss (bans, embargoes, sanctions) by animal product
Assurances on tuberculosis free status are required for EU trade in bovine animals and products.
Parameter 5 – Importance of the disease for the affected sector (% loss or € lost compared to business amount of the sector)
There has been no published systematic assessment of this. It will vary from MS to MS.
3.1.5.2. Article 7(e)(ii) The societal acceptance of disease prevention and control measures
Disease prevention and control methods for bTB in cattle as laid down by Council Directive 64/432/EEC is well accepted across MSs in Europe.
Disease control measures for wildlife are more contentious and societal acceptance is likely to vary according to MS and to different stakeholders within MSs (Carstensen et al., 2011; Gross, 2013; Cowie et al., 2015; More and Good, 2015).
3.1.5.3. Article 7(e)(iii) The welfare of affected subpopulations of kept and wild animals
Parameter 1 – Welfare impact of control measures on domestic animals
The OIE developed international standards to provide advice on humane killing for various species and situations (OIE, 2012). In the situation of emergency killing for diseases control purposes, preventing or minimising risks to human health and safety often take priority over animal welfare concerns. Council Regulation (EC) No 1099/200915 lays down rules for the killing of animals at slaughter and in case of emergency killing outside of a slaughterhouse. The Regulation provides for appropriate planning, supervision and reporting of emergency killing cases. The use of killing methods that pose risks to animal welfare is not allowed except under exceptional circumstances (e.g. risks posed to human health or uncontainable animal diseases). The application of humane slaughterhouse practices has recently been reviewed (Gavinelli et al., 2014; Thornber et al., 2014).
Parameter 2 – Wildlife depopulation as a control measure
Wild boar, deer and badgers are hunted species in most parts of the EU, so welfare is unlikely to be a major issue for these species.
Badger culling has been carried out in at least two MSs as part of a TB eradication strategy. Until recently, these both required animals to be trapped. The two main trapping methods have been stopped cable restraints and cage traps (Woodroffe et al., 2005b; Murphy et al., 2009). Both methods result in low prevalence of serious injury (Woodroffe et al., 2005a; Murphy et al., 2009). However, both trapping methods could cause other welfare concerns related to stress or environmental exposure (Talling and Inglis, 2009; Sharp and Saunders, 2011). A practical adaptive management system has been proposed by Bryne et al. (2015) to monitor trap‐related injuries as a component of broader attempts to maintain welfare standards.
Recently, free‐shooting of non‐trapped badgers has been trialled in GB. An Independent Expert Panel report on these pilot trials found that 7.4–22.8% (95% CI) of badgers were still alive after 5 min, i.e. that the Panel were 95% confident that the number of badgers estimated as taking more than 5 min to die exceeded 5%. Consequently, the Panel recommended that to improve standards of humaneness, only shooters who have demonstrated a high standard of marksmanship in the field, and who have a good knowledge of badger behaviour, should be licensed (Munro, 2014).
Data suggest that a closed season is effective in reducing the suffering of unweaned cubs in badger populations subject to culling (Woodroffe et al., 2005a).
3.1.5.4. Article 7(e)(iv) The environment and biodiversity
Environment
Parameter 1 – Use and potential residuals of biocides or medical drugs in environmental compartments (soil, water, feed, manure)
Manufacture and use of disinfectants in the EU must comply with Regulation (EU) No 528/201216.
Biodiversity
Parameter 2 – Mortality in wild species
It is difficult to assess mortality due to M. bovis infection in wildlife populations. However, as above, the following estimations have been reported.
Badgers: There is evidence for early onset of disease‐induced mortality in male but not female badgers. In a long‐term study of a natural population of badgers to the nearest month, male average life expectancies were estimated to be 32 months for uninfected badgers and 22 months for infected badgers. Female average life expectancies were found to be 40 months for uninfected badgers and 35 months for infected badgers (McDonald et al., 2014).
Wild boar: A recent study of two different populations of wild boar with endemic tuberculosis in Spain suggested that tuberculosis accounted for 30% of total deaths (Barasona et al., 2016).
In a randomised badger culling trial in GB, culling of badgers led to an associated increase in populations of red foxes and hedgehogs. In both cases, the responses were highly variable amongst areas and are likely to be mediated by other complex ecological interactions (Trewby et al., 2008, 2014).
3.2. Assessment according to Article 5 criteria
This section presents the results of the expert judgement on the criteria of Article 5 of the AHL about bTB (Table 2). The expert judgement was based on Individual and Collective Behavioural Aggregation (ICBA) approach described in detail in the opinion on the methodology (EFSA AHAW Panel, 2017). Experts have been provided with information of the disease fact‐sheet mapped into Article 5 criteria (see supporting information, Annex A), based on that the experts indicate their Y/N or ‘na’ judgement on each criterion of Article 5, and the reasoning supporting their judgement.
Table 2.
Outcome of the expert judgement on the Article 5 criteria for bovine tuberculosis
| Criteria to be met by the disease: According to AHL, a disease shall be included in the list referred to in point (b) of paragraph 1 of Article 5 if it has been assessed in accordance with Article 7 and meets all of the following criteria | Final outcome | |
|---|---|---|
| A(i) | The disease is transmissible | Y |
| A(ii) | Animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union | Y |
| A(iii) | The disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character | Y |
| A(iv) | Diagnostic tools are available for the disease | Y |
| A(v) | Risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union | Y |
| At least one criterion to be met by the disease:In addition to the criteria set out above at points A(i)–A(v), the disease needs to fulfil at least one of the following criteria | ||
| B(i) | The disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character | Y |
| B(ii) | The disease agent has developed resistance to treatments and poses a significant danger to public and/or animal health in the Union | NC |
| B(iii) | The disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union | Y |
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | NC |
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | NC |
Colour code: green = consensus (Yes/No), yellow = no consensus (NC).
The minimum number of judges in the judgement was 11. The expert judgement was conducted as described in the methodological opinion (EFSA AHAW Panel, 2017). For details on the interpretation of the questions, see Appendix B of the methodological opinion (EFSA AHAW Panel, 2017).
3.2.1. Non‐consensus questions
This section displays the assessment related to each criterion of Article 5 where no consensus was achieved in form of tables (Tables 3, 4 and 5). The proportion of Y, N or na answers are reported, followed by the list of different supporting views for each answer.
Table 3.
Outcome of the expert judgement related to criterion 5 B(ii)
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| B(ii) | The disease agent has developed resistance to treatments and poses a significant danger to public and/or animal health in the Union | NC | 9 | 91 | 0 |
NC non‐consensus; number of judges: 11.
Table 4.
Outcome of the expert judgement related to criterion 5 B(iv)
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | NC | 27 | 73 | 0 |
NC non‐consensus; number of judges: 11.
Table 5.
Outcome of the expert judgement related to criterion 5 B(v)
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | NC | 91 | 9 | 0 |
NC non‐consensus; number of judges: 11.
Reasoning supporting the judgement
Supporting Yes:
General treatment of animals with antibiotics for concurrent infections may lead, inadvertently, to antibiotic resistant strains of M. bovis.
Antimicrobial resistance in humans has been observed for all bacteria belonging to MTBC, which includes M. bovis. However, M. bovis in humans is underdiagnosed and no differentiation among strains is carried out, so M. bovis is treated more often than realised and the treatment will include the antimicrobials to which M. bovis is naturally resistant; it could survive longer and that increases the likelihood of resistance to the other antimicrobials in the suite developing.
Supporting No:
There are few cases of antimicrobial resistance of M. bovis in humans, but this is not significantly important for public health as these strains were not related to infections in animals. Moreover, infection in animals is not treated with antimicrobials, and all infected animals are killed.
M. bovis has been found naturally resistant to pyrazinamide, although currently other resistant strains are infrequent.
Reasoning supporting the judgement
Supporting Yes:
If the disease was uncontrolled, there is the potential for massive spread of infection, with raw meat or raw milk posing a potential threat to public health. This may lead to a loss of confidence in consumers (similar to the crisis due to BSE). Moreover, if left uncontrolled, slaughtering of flocks would be applied to control the disease, and this may generate an impact on the public opinion.
The disease, since it can affect multiple host species, both domestic and wild, that often share the same environment, can currently lead to critical situations due to the conflict between farmers, hunters and authorities.
Supporting No:
The disease is not listed as a bio‐ or agro‐terrorism agent.
The disease has a slow natural evolution in the host population, and therefore, it seems unlikely to be used for bioterrorism purposes.
A crisis between hunters and breeders would not be significantly relevant for the whole society.
Contamination of food can be prevented by pasteurisation and recall of contaminated milk would not cause a crisis at national level.
It would be of concern if new outbreak(s) occur(s) in a previously free area. However, due to the slow spread of the disease and the efficacy of mitigation measures, there is a very low probability of generating a crisis.
Reasoning supporting the judgement
Supporting Yes:
The disease seriously affects endangered wild species such as Iberian lynx, bear, and bison; the presence of the disease in national parks raises conservation concerns.
In Africa, the disease causes deaths in carnivores, contributing to the endangered status of several wild species.
Supporting No:
Considering the difficulties in assessing the effect of a disease on the ecosystem as a whole, if only one endangered species is affected (Iberian lynx), biodiversity will not be reduced, except in very small meta‐population.
3.2.2. Outcome of the assessment of bovine tuberculosis according to criteria of Article 5(3) of the AHL on its eligibility to be listed
As from the legal text of the AHL, a disease is considered eligible to be listed as laid down in Article 5 if it fulfils all criteria of the first set from A(i) to A(v) and at least one of the second set of criteria from B(i) to B(v). According to the assessment methodology (EFSA AHAW Panel, 2017), a criterion is considered fulfilled when the outcome is ‘Yes’. According to the results shown in Table 2, bovine tuberculosis complies with all criteria of the first set and two criteria of the second set, therefore it is considered eligible to be listed as laid down in Article 5 of the AHL.
3.3. Assessment according to Article 9 criteria
This section presents the results of the expert judgement on the criteria of Annex IV referring to categories as in Article 9 of the AHL about bovine tuberculosis (Tables 6, 7, 8, 9 and 10). The expert judgement was based on ICBA approach described in detail in the opinion on the methodology. Experts have been provided with information of the disease fact‐sheet mapped into Article 9 criteria (see supporting information, Annex A), based on that the experts indicate their Y/N or ‘na’ judgement on each criterion of Article 9, and the reasoning supporting their judgement.
Table 6.
Outcome of the expert judgement related to the criteria of Section 1 of Annex IV (category A of Article 9) for bovine tuberculosis (CI = current impact; PI = potential impact)
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
|---|---|---|
| 1 | The disease is not present in the territory of the Union OR present only in exceptional cases (irregular introductions) OR present in only in a very limited part of the territory of the Union | N |
| 2.1 | The disease is highly transmissible | N |
| 2.2 | There be possibilities of airborne or waterborne or vector‐borne spread | Y |
| 2.3 | The disease affects multiple species of kept and wild animals OR single species of kept animals of economic importance | Y |
| 2.4 | The disease may result in high morbidity and significant mortality rates | N |
| At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, including epidemic or pandemic potential OR possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 4(PI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | Y |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | Y |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | Y |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | Y |
Colour code: green = consensus (Yes/No).
Table 7.
Outcome of the expert judgement related to the criteria of Section 2 of Annex IV (category B of Article 9) for bovine tuberculosis (CI = current impact; PI = potential impact)
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
|---|---|---|
| 1 | The disease is present in the whole OR part of the Union territory with an endemic character AND (at the same time) several Member States or zones of the Union are free of the disease | Y |
| 2.1 | The disease is moderately to highly transmissible | Y |
| 2.2 | There be possibilities of airborne or waterborne or vector‐borne spread | Y |
| 2.3 | The disease affects single or multiple species | Y |
| 2.4 | The disease may result in high morbidity with in general low mortality | Y |
| At least one criterion to be met by the disease:In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, including epidemic potential OR possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 4(PI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | Y |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | Y |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | Y |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | Y |
Colour code: green = consensus (Yes/No).
Table 8.
Outcome of the expert judgement related to the criteria of Section 3 of Annex IV (category C of Article 9) for bovine tuberculosis (CI = current impact; PI = potential impact)
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
|---|---|---|
| 1 | The disease is present in the whole OR part of the Union territory with an endemic character | Y |
| 2.1 | The disease is moderately to highly transmissible | Y |
| 2.2 | The disease is transmitted mainly by direct or indirect transmission | Y |
| 2.3 | The disease affects single or multiple species | Y |
| 2.4 | The disease usually does not result in high morbidity and has negligible or no mortality AND often the most observed effect of the disease is production loss | Y |
| At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, or possible significant threats to food safety | Y |
| 4(CI) | The disease has a significant impact on the economy of parts of the Union, mainly related to its direct impact on certain types of animal production systems | N |
| 4(PI) | The disease has a significant impact on the economy of parts of the Union, mainly related to its direct impact on certain types of animal production systems | Y |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | Y |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | Y |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | Y |
Colour code: green = consensus (Yes/No).
Table 9.
Outcome of the expert judgement related to the criteria of Section 4 of Annex IV (category D of Article 9) for bovine tuberculosis
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
|---|---|---|
| D | The risk posed by the disease in question can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread | Y |
| the disease fulfils criteria of Section 1, 2, 3 or 5 of Annex IV of AHL | Y | |
Colour code: green = consensus (Yes/No).
Table 10.
Outcome of the expert judgement related to the criteria of Section 5 of Annex IV (category E of Article 9) for bovine tuberculosis
| Diseases in category E need to fulfil criteria of Section 1, 2 or 3 of Annex IV of AHL and/or the following: | Final outcome | |
|---|---|---|
| E | Surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment (If a disease fulfils the criteria as in Article 5, thus being eligible to be listed, consequently category E would apply.) | Y |
Colour code: green = consensus (Yes/No).
The minimum number of judges in the judgement was 8. The expert judgement was conducted as described in the methodological opinion (EFSA AHAW Panel, 2017). For details on the interpretation of the questions, see Appendix B of the methodological opinion (EFSA AHAW Panel, 2017).
3.3.1. Outcome of the assessment of criteria in Annex IV for bovine tuberculosis for the purpose of categorisation as in Article 9 of the AHL
As from the legal text of the AHL, a disease is considered fitting in a certain category (A, B, C, D or E corresponding to point (a) to point (e) of Article 9(1) of the AHL) if it is eligible to be listed for Union intervention as laid down in Article 5(3) and fulfils all criteria of the first set from 1 to 2.4 and at least one of the second set of criteria from 3 to 5(d) as shown in Tables 6, 7, 8, 9–10. According to the assessment methodology (EFSA AHAW Panel, 2017), a criterion is considered fulfilled when the outcome is ‘Yes’. With respect to the different type of impact where the assessment is divided into current and potential impact, a criterion will be considered fulfilled if at least one of the two outcomes is ‘Y’ and, in case of no ‘Y’, the assessment is inconclusive if at least one outcome is ‘NC’.
A description of the outcome of the assessment of criteria in Annex IV for bTB for the purpose of categorisation as in Article 9 of the AHL is presented in Table 11.
Table 11.
Outcome of the assessment of criteria in Annex IV for bovine tuberculosis for the purpose of categorisation as in Article 9 of the AHL (CI = current impact; PI = potential impact)
| Category | Article 9 criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° set of criteria | 2° set of criteria | ||||||||||
| 1 | 2.1 | 2.2 | 2.3 | 2.4 | 3 | 4 | 5a | 5b | 5c | 5d | |
| Geographical distribution | Transmissibility | Routes of transmission | Multiple species | Morbidity and mortality | Zoonotic potential | Impact on economy | Impact on society | Impact on animal welfare | Impact on environment | Impact on biodiversity | |
| A | N | N | Y | Y | N | N |
CI: N PI: Y |
N |
CI: N PI: Y |
CI: N PI: Y |
CI: N PI: Y |
| B | Y | Y | Y | Y | Y | N |
CI: N PI: Y |
N |
CI: N PI: Y |
CI: N PI: Y |
CI: N PI: Y |
| C | Y | Y | Y | Y | Y | Y |
CI: N PI: Y |
N |
CI: N PI: Y |
CI: N PI: Y |
CI: N PI: Y |
| D | Y | ||||||||||
| E | Y | ||||||||||
According to the assessment here performed, bTB complies with the following criteria of the Sections 1 to 5 of Annex IV of the AHL for the application of the disease prevention and control rules referred to in points (a) to (e) of Article 9(1):
To be assigned to category A, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment bTB complies with criteria 2.2 and 2.3, but not with criteria 1, 2.1 and 2.4. To be eligible for category A, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and bTB complies with criteria 4, 5b, 5c and 5d, but not with criteria 3 and 5a.
To be assigned to category B, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment bTB complies with all of them. To be eligible for category B, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and bTB complies with criteria 4, 5b, 5c and 5d, but not with criteria 3 and 5a.
To be assigned to category C, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment bTB complies with all of them. To be eligible for category C, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and bTB complies with criteria 3, 4, 5b, 5c and 5d, but not with criterion 5a.
To be assigned to category D, a disease needs to comply with criteria of Section 1, 2, 3 or 5 of Annex IV of the AHL, with which bTB complies.
To be assigned to category E, a disease needs to comply with criteria of Section 1, 2 or 3 of Annex IV of the AHL and/or the surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment. The latter is applicable if a disease fulfils the criteria as in Article 5, with which bTB complies.
3.4. Assessment of Article 8
This section presents the results of the assessment on the criteria of Article 8(3) of the AHL about bTB. The Article 8(3) criteria are about animal species to be listed, as it reads below:
‘3. Animal species or groups of animal species shall be added to this list if they are affected or if they pose a risk for the spread of a specific listed disease because:
they are susceptible for a specific listed disease or scientific evidence indicates that such susceptibility is likely; or
they are vector species or reservoirs for that disease, or scientific evidence indicates that such role is likely’.
For this reason the assessment on Article 8 criteria is based on the evidence as extrapolated from the relevant criteria of Article 7, i.e. the ones related to susceptible and reservoir species or routes of transmission, which cover also possible role of biological or mechanical vectors.17 According to the mapping, as presented in Table 5, Section 3.2 of the scientific opinion on the ad hoc methodology (EFSA AHAW Panel, 2017), the main animal species to be listed for bTB according to the criteria of Article 8(3) of the AHL are as displayed in Table 12.
Table 12.
Main animal species to be listed for bovine tuberculosis according to criteria of Article 8 (source: data reported in Section 3.1.1.1)
| Class | Order | Family | Genus/Species | |
|---|---|---|---|---|
| Susceptible* | Mammalia | Artiodactyla | Bovidae | Bos spp., Bubalus spp., Syncerus spp., Bison spp., Capra spp., antelopes, Tragelaphus spp., Taurotragus spp., Oryx spp., Addax spp., Ovis spp. |
| Cervidae | Cervus elaphus, Capreolus capreolus, Dama dama, Cervus canadensis | |||
| Suidae | Sus spp. | |||
| Camelidae | Camelus spp., Lama glama, Vicugna pacos | |||
| Carnivora | Mustelidae | Badgers (not specified), Neovison spp., Mustela spp., Mustela putorius furo, Lutra spp. | ||
| Canidae | Vulpes vulpes, Canis latrans, Canis lupus familiaris | |||
| Felidae | Lynx spp., Panthera leo, Panthera tigris, Panthera pardus, Felis catus | |||
| Procyonidae | Procyon lotor | |||
| seals (different families) | Not specified | |||
| Rodentia | Muridae | Rattus spp. | ||
| Cricetidae | Microtus agrestis | |||
| Sciuridae | Squirrels | |||
| Eulipotyphla | Talpidae | Not specified | ||
| Primates | not specified | Not specified | ||
| Perissodactyla | Tapiridae | Not specified | ||
| Rhinocerotoidea | Not specified | |||
| Equidae+ | Not specified | |||
| Proboscidea | Elephantidae | Not specified | ||
| Didelphimorphia | Didelphidae | Not specified | ||
| Lagomorpha | Leporidae | Lepus spp., rabbits | ||
| Aves | Columbiformes | Columbidae | Not specified | |
| Reservoir | Mammalia | Carnivora | Mustelidae | Meles meles |
| Artiodactyla | Bovidae | Bos spp., Capra spp., Bubalus spp., Syncerus spp., Bison spp., Ovis spp. | ||
| Suidae | Sus scrofa | |||
| Cervidae | Subfamily Cervinae | |||
| Camelidae | South American camelids, Old World camels | |||
| Vectors | None | |||
* most mammals can be infected; + rarely.
4. Conclusions
TOR 1: for each of those seven diseases an assessment, following the criteria laid down in Article 7 of the AHL, on its eligibility of being listed for Union intervention as laid down in Article 5(3) of the AHL;
According to the assessment here performed, bTB complies with all criteria of the first set and with two criteria of the second set and therefore can be considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL.
TOR 2a: for each of the seven diseases which was found eligible to be listed for Union intervention, an assessment of its compliance with each of the criteria in Annex IV to the AHL for the purpose of categorisation of diseases in accordance with Article 9 of the AHL;
According to the assessment here performed, bTB meets the criteria as in Sections 2, 3, 4 and 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in points (b), (c), (d) and (e) of Article 9(1) of the AHL.
TOR 2b: for each of the seven diseases which was found eligible to be listed for Union intervention, a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL.
According to the assessment here performed, the animal species that can be considered to be listed for bTB according to Article 8(3) of the AHL are several mammals as susceptible and several species of Artiodactyla and Carnivora as reservoirs, as reported in Table 12 in Section 3.4 of the present document.
Abbreviations
- AHAW
EFSA Panel on Animal Health and Welfare
- AHL
Animal Health Law
- BCG
Bacillus Calmette‐Guérin
- bTB
bovine tuberculosis
- CDC
Centers for Disease Control and Prevention
- CITES
Convention on International Trade in Endangered Species of Wild Fauna and Flora
- CFSPH
Centre for Food Security and Public Health
- CI
confidence intervals
- DALY
Disability‐adjusted life year
- ECDC
European Centre for Disease Prevention and Control
- ELISA
enzyme‐linked immunosorbent assay
- ICBA
Individual and Collective Behavioural Aggregation
- IFN‐γ
interferon‐gamma
- IUCN
International Union for Conservation of Nature
- LMA
Limited Marketing Authorisation
- MDR
multidrug‐resistant
- MS
Member State
- MTBC
Mycobacterium tuberculosis complex
- MTC
Mycobacterium tuberculosis complex
- OIE
World Organisation for Animal Health
- OTF
officially tuberculosis free
- PCR
polymerase chain reaction
- PME
post‐mortem examination
- PPD
purified protein derivative
- RBCT
Randomised Badger Culling Trial
- SCIT
comparative intradermal tuberculin
- SIT
single intradermal tuberculin
- TB
tuberculosis
- ToR
Terms of Reference
Supporting information
Mapped fact‐sheet used in the individual judgement on bovine tuberculosis
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Beltrán‐Beck B, Kohnle L and Bicout D, 2017. Scientific Opinion on the assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): bovine tuberculosis. EFSA Journal 2017;15(8):4959, 42 pp. 10.2903/j.efsa.2017.4959
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00598
Panel members: Dominique Bicout, Anette Bøtner, Andrew Butterworth, Paolo Calistri, Klaus Depner, Sandra Edwards, Bruno Garin‐Bastuji, Margaret Good, Christian Gortázar Schmidt, Virginie Michel, Miguel Angel Miranda, Simon More, Søren Saxmose Nielsen, Mohan Raj, Liisa Sihvonen, Hans Spoolder, Jan Arend Stegeman, Hans‐Hermann Thulke, Antonio Velarde, Preben Willeberg and Christoph Winckler.
Acknowledgements: The Panel wishes to thank Glyn Hewinson for the support provided to this scientific output.
Adopted: 30 June 2017
Notes
Council Directive 64/432/EEC of 26 June 1964 on animal health problems affecting intra‐Community trade in bovine animals and swine. OJ 121, 29.7.1964, p. 1977–2012.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003, p. 31–40.
As laid down in Annex A.I of Directive 64/432/EEC, a Member State or region of a Member State may be declared officially tuberculosis‐free if the percentage of bovine herds confirmed as infected with tuberculosis has not exceeded 0.1% per year of all herds for six consecutive years and at least 99.9% of herds have achieved OTF status each year for six consecutive years; each bovine animal is identified in accordance with Community legislation; all bovine animals slaughtered are subjected to an official post‐mortem examination; the procedures for suspension and withdrawal of officially tuberculosis‐free status are complied with.
2014/91/EU: Commission Implementing Decision of 14 February 2014 amending Annex II to Decision 93/52/EEC as regards the recognition of certain regions of Italy and Spain as officially free of brucellosis (B. melitensis) and amending Annexes I, II and III to Decision 2003/467/EC as regards the declaration of Hungary as officially tuberculosis‐free, Romania and certain regions of Italy as officially brucellosis‐free, and certain regions of Italy as officially enzootic‐bovine‐leukosis‐free. OJ L 46, 18.2.2014, p. 12–17.
Commission Implementing Decision (EU) 2017/888 of 22 May 2017 amending Decision 2003/467/EC as regards the official tuberculosis‐free status of the region of Umbria of Italy and of the enzootic‐bovine‐leukosis‐free status of Poland, amending Decision 2004/558/EC as regards the infectious bovine rhinotracheitis‐free status of Germany, and amending Decision 2008/185/EC as regards the Aujeszky's disease‐free status of certain regions of Poland and the approval of the eradication programme for Aujeszky's disease for the region of Veneto of Italy. OJ L 135, 24.5.2017, p. 27–34.
Council Directive 91/496/EEC of 15 July 1991 laying down the principles governing the organization of veterinary checks on animals entering the Community from third countries and amending Directives 89/662/EEC, 90/425/EEC and 90/675/EEC. OJ L 268, 24.9.1991, p. 56–68.
Council Directive 2004/68/EC of 26 April 2004 laying down animal health rules for the importation into and transit through the Community of certain live ungulate animals, amending Directives 90/426/EEC and 92/65/EEC and repealing Directive 72/462/EEC. OJ L 139, 30.4.2004, p. 321–360.
Commission Regulation (EU) No 206/2010 of 12 March 2010 laying down lists of third countries, territories or parts thereof authorised for the introduction into the European Union of certain animals and fresh meat and the veterinary certification requirements. OJ L 73, 20.3.2010, p. 1–121.
Commission Regulation (EC) No 1226/2002 of 8 July 2002 amending Annex B to Council Directive 64/432/EEC. OJ L 179, 9.7.2002, p. 13–18.
Council Directive 77/391/EEC of 17 May 1977 introducing Community measures for the eradication of brucellosis, tuberculosis and leucosis in cattle. OJ L 145, 13.6.1977, p. 44–47.
Council Directive 82/400/EEC of 14 June 1982 amending Directive 77/391/EEC and introducing a supplementary Community measure for the eradication of brucellosis, tuberculosis and leucosis in cattle. OJ L 173, 19.6.1982, p. 18–19.
87/58/EEC: Council Decision of 22 December 1986 introducing a supplementary Community measure for the eradication of brucellosis, tuberculosis and leucosis in cattle. OJ L 24, 27.1.1987, p. 51–53.
Council Directive 78/52/EEC of 13 December 1977 establishing the Community criteria for national plans for the accelerated eradication of brucellosis, tuberculosis and enzootic leukosis in cattle. OJ L 15, 19.1.1978, p. 34–41.
Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by‐products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by‐products Regulation). OJ L 300, 14.11.2009, p. 1–33.
Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. OJ L 303, 18.11.2009, p. 1–30.
Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. OJ L 167, 27.6.2012, p. 1–123.
A vector is a living organism that transmits an infectious agent from an infected animal to a human or another animal. Vectors are frequently arthropods. Biological vectors may carry pathogens that can multiply within their bodies and be delivered to new hosts, usually by biting. In mechanical vectors, the pathogens do not multiply within the vector, which usually remains infected for shorter time than in biological vectors.
References
- Abdou M, Frankena K, O'Keeffe J and Byrne AW, 2016. Effect of culling and vaccination on bovine tuberculosis infection in a European badger (Meles meles) population by spatial simulation modelling. Preventive Veterinary Medicine, 125, 19–30. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Perez A, Bezos J, Marqués S, Grau A, Saez JL, Mínguez O, de Juan L and Domínguez L, 2012a. Evaluation of the sensitivity and specificity of bovine tuberculosis diagnostic tests in naturally infected cattle herds using a Bayesian approach. Veterinary Microbiology, 155, 38–43. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Perez AM, Bezos J, Casal C, Romero B, Rodriguez‐Campos S, Saez‐Llorente JL, Diaz R, Carpintero J, de Juan L and Domínguez L, 2012b. Eradication of bovine tuberculosis at a herd‐level in Madrid, Spain: study of within‐herd transmission dynamics over a 12 year period. BMC Veterinary Research, 8, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranaz A, Liébana E, Mateos A, Dominguez L, Vidal D, Domingo M, Gonzolez O, Rodriguez‐Ferri EF, Bunschoten AE, Van Embden JD and Cousins D, 1996. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. Journal of Clinical Microbiology, 34, 2734–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranaz A, Cousins D, Mateos A and Domínguez L, 2003. Elevation of Mycobacterium tuberculosis subsp. caprae to species rank as Mycobacterium caprae comb. nov., sp. nov. International Journal of Systematic and Evolutionary Microbiology, 53, 1785–1789. [DOI] [PubMed] [Google Scholar]
- Aranaz A, De Juan L, Montero N, Sánchez C, Galka M, Delso C, Alvarez J, Romero B, Bezos J, Vela AI, Briones V, Mateos A and Domínguez L, 2004. Bovine tuberculosis (Mycobacterium bovis) in wildlife in Spain. Journal of Clinical Microbiology, 42, 2602–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J and Christie P, 1998. Two cases of multidrug resistant Mycobacterium bovis infection in Scotland. Euro Surveillance, 2, pii=1159. [Google Scholar]
- Arrazuria R, Juste RA and Elguezabal N, 2017. Mycobacterial infections in rabbits: from the wild to the laboratory. Transboundary and Emerging Diseases, 64, 1045–1058. [DOI] [PubMed] [Google Scholar]
- Barasona JA, Latham MC, Acevedo P, Armenteros JA, Latham ADM, Gortazar C, Carro F, Soriguer RC and Vicente J, 2014a. Spatiotemporal interactions between wild boar and cattle: implications for cross‐species disease transmission. Veterinary Research, 45, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasona JA, Mulero‐Pazmany M, Acevedo P, Negro JJ, Torres MJ, Gortazar C and Vicente J, 2014b. Unmanned aircraft systems for studying spatial abundance of ungulates: relevance to spatial epidemiology. PLoS ONE, 9, e115608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasona JA, Acevedo P, Diez‐Delgado I, Queiros J, Carrasco‐Garcia R, Gortazar C and Vicente J, 2016. Tuberculosis‐associated death among adult wild boars, Spain, 2009‐2014. Emerging Infectious Diseases, 22, 2178–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Boschiroli ML, Gueneau E, Rochelet M, Payne A, de Cruz K, Blieux AL, Fossot C and Hartmann A, 2016. First molecular detection of Mycobacterium bovis in environmental samples from a French region with endemic bovine tuberculosis. Journal of Applied Microbiology, 120, 1193–1207. [DOI] [PubMed] [Google Scholar]
- Barlow ND, Kean JM, Hickling G, Livingstone PG and Robson AB, 1997. A simulation model for the spread of bovine tuberculosis within New Zealand cattle herds. Preventive Veterinary Medicine, 32, 57–75. [DOI] [PubMed] [Google Scholar]
- Barrow PA and Gallagher J, 1981. Aspects of the epidemiology of bovine tuberculosis in badgers and cattle. I. The prevalence of infection in two wild animal populations in south‐west England. The Journal of Hygiene, 86, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekara ME, Courcoul A, Benet JJ and Durand B, 2014. Modeling tuberculosis dynamics, detection and control in cattle herds. PLoS ONE, 9, e108584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolatti B, Bollo E, Mignone M, Caramelli M and derSchrö C , 1992. Tuberculosis in wild boar (Sus scrofa) in Liguria (Italy). Proceedings of the 34th International Symposium on Diseases of Zoo and Wild Animals, Santander, Spain, 55–59.
- Bode R, 1995. Tuberculosis (TB) in deer in Great Britain. State Veterinary Journal, 5, 13–17. [Google Scholar]
- Bolin CA, Whipple DL, Khanna KV, Risdahl JM, Peterson PK and Molitor TW, 1997. Infection of swine with Mycobacterium bovis as a model of human tuberculosis. Journal of Infectious Diseases, 176, 1559–1566. [DOI] [PubMed] [Google Scholar]
- Bourne FJ, Donnelly C, Cox D, Gettinby G, McInerney J, Morrison I and Woodroffe R (Secretary of State for Environment, Food and Rural Affairs), 2007. Bovine TB: The Scientific Evidence. A Science Base for a Sustainable Policy to Control TB in Cattle. An Epidemiological Investigation into Bovine Tuberculosis. Final Report of the Independent Scientific Group on Cattle TB.
- Briones V, de Juan L, Sánchez C, Vela AI, Galka M, Montero Goyache J, Aranaz A and Domínguez L, 2000. Bovine tuberculosis and the endangered Iberian lynx. Emerging Infectious Diseases, 6, 189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe CF, 1912. Fate of tubercle bacilli outside the animal body. Report Number 161, Illinois Agricultural Experimental Station Bulletin, Illinois, USA.
- Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, Garnier T, Gutierrez C, Hewinson G, Kremer K, Parsons LM, Pym AS, Samper S, van Soolingen D and Cole ST, 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proceedings of the National Academy of Sciences of the United States of America, 99, 3684–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E, Cooney R and Rogers F, 2013. Veterinary guidance on the practical use of the BadgerBCG tuberculosis vaccine. In Practice, 35, 143–146. [Google Scholar]
- Bryne AL, Marais BJ, Mitnick CD, Lecca L and Marks GB, 2015. Tuberculosis and chronic respiratory disease: a systematic review. International Journal of Infectious Diseases, 32, 138–146. [DOI] [PubMed] [Google Scholar]
- Buddle BM, Nolan A, McCarthy AR, Heslop J, Aldwell FE, Jackson R and Pfeiffer DU, 1995. Evaluation of three serological assays for the diagnosis of Mycobacterium bovis infection in brushtail possums. The New Zealand Veterinary Journal, 43, 91–95. [DOI] [PubMed] [Google Scholar]
- Buddle BM, Vordermeier HM and Hewinson RG, 2016. Experimental infection models of tuberculosis in domestic livestock. Microbiology Spectrum, 4. [DOI] [PubMed] [Google Scholar]
- van der Burgt GM, Drummond F, Crawshaw T and Morris S, 2013. An outbreak of tuberculosis in Lleyn sheep in the UK associated with clinical signs. Veterinary Record, 172, 69. [DOI] [PubMed] [Google Scholar]
- Buss BF, Keyser‐Metobo A, Rother J, Holtz L, Gall K, Jereb J, Murphy CN, Iwen PC, Robbe‐Austerman S, Holcomb MA and Infield P, 2016. Possible airborne person‐to‐person transmission of Mycobacterium bovis – Nebraska 2014‐2015. Morbidity and Mortality Weekly Report (MMWR), 65, 197–201. [DOI] [PubMed] [Google Scholar]
- Caffrey JP, 1994. Status of bovine tuberculosis eradication programmes in Europe. Veterinary Microbiology, 40, 1–4. [DOI] [PubMed] [Google Scholar]
- Caminiti A, Pelone F, Battisti S, Gamberale F, Colafrancesco R, Sala M, La Torre G, Della Marta U and Scaramozzino P, 2016. Tuberculosis, brucellosis and leucosis in cattle: a cost description of eradication programmes in the Region of Lazio, Italy. Transboundery Emerging Diseases. [DOI] [PubMed] [Google Scholar]
- Carstensen M, O'Brien DJ and Schmitt SM, 2011. Public acceptance as a determinant of management strategies for bovine tuberculosis in free‐ranging US wildlife. Veterinary Microbiology, 151, 200–204. [DOI] [PubMed] [Google Scholar]
- Carter SP, Chambers MA, Rushton SP, Shirley MD, Schuchert P, Pietravalle S, Murray A, Rogers F, Gettinby G, Smith GC, Delahay RJ, Hewinson RG and McDonald RA, 2012. BCG vaccination reduces risk of tuberculosis infection in vaccinated badgers and unvaccinated badger cubs. PLoS ONE, 7, e49833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MA, Rogers F, Delahay RJ, Lesellier S, Ashford R, Dalley D, Gowtage S, Dave D, Palmer S, Brewer J, Crawshaw T, Clifton‐Hadley R, Carter S, Cheeseman C, Hanks C, Murray A, Palphramand K, Pietravalle S, Smith GC, Tomlinson A, Walker NJ, Wilson GJ, Corner LA, Rushton SP, Shirley MD, Gettinby G, McDonald RA and Hewinson RG, 2011. Bacillus calmette‐guerin vaccination reduces the severity and progression of tuberculosis in badgers. Proceedings of the Royal Society B: Biological Sciences, 278, 1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg TA, Good M and More SJ, 2016. Risk factors for cattle presenting with a confirmed bTB lesion at slaughter, from herds with no evidence of within‐herd transmission. Preventive Veterinary Medicine, 126, 111–120. [DOI] [PubMed] [Google Scholar]
- Clifton‐Hadley RS and Wilesmith JW, 1991. Tuberculosis in deer: a review. Veterinary Record, 129, 5–12. [DOI] [PubMed] [Google Scholar]
- Clifton‐Hadley RS, Wilesmith JW, Richards MS, Upton P and Johnston S, 1995. The occurrence of Mycobacterium bovis infection in cattle in and around an area subject to extensive badger (Meles meles) control. Epidemiology & Infection, 114, 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner L, Melville L, McCubbin K, Small KJ, McCormick BS, Wood PR and Rothel JS, 1990. Efficiency of inspection procedures for the detection of tuberculous lesions in cattle. Australian Veterinary Journal, 67, 389–392. [DOI] [PubMed] [Google Scholar]
- Corner LA, Costello E, Lesellier S, O'Meara D, Sleeman DP and Gormley E, 2007. Experimental tuberculosis in the European badger (Meles meles) after endobronchial inoculation of Mycobacterium bovis: I. Pathology and bacteriology. Research in Veterinary Science, 83, 53–62. [DOI] [PubMed] [Google Scholar]
- Corner LA, Costello E, Lesellier S, O'Meara D and Gormley E, 2008. Experimental tuberculosis in the European badger (Meles meles) after endobronchial inoculation with Mycobacterium bovis: II. Progression of infection. Research in Veterinary Science, 85, 481–490. [DOI] [PubMed] [Google Scholar]
- Courcoul A, Moyen JL, Brugere L, Faye S, Henault S, Gares H and Boschiroli ML, 2014. Estimation of sensitivity and specificity of bacteriology, histopathology and PCR for the confirmatory diagnosis of bovine tuberculosis using latent class analysis. PLoS ONE, 9, e90334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins DV, 2001. Mycobacterium bovis infection and control in domestic livestock. Revue scientifique et technique (International Office of Epizootics), 20, 71–85. [DOI] [PubMed] [Google Scholar]
- Cowie CE, Gortazar C, White PC, Hutchings MR and Vicente J, 2015. Stakeholder opinions on the practicality of management interventions to control bovine tuberculosis. Veterinary Journal, 204, 179–185. [DOI] [PubMed] [Google Scholar]
- Cowie CE, Hutchings MR, Barasona JA, Gortazar C, Vicente J and White PCL, 2016. Interactions between four species in a complex wildlife: livestock disease community: implications for Mycobacterium bovis maintenance and transmission. European Journal of Wildlife Research, 62, 51–64. [Google Scholar]
- Cox DR, Donnelly CA, Bourne FJ, Gettinby G, McInerney JP, Morrison WI and Woodroffe R, 2005. Simple model for tuberculosis in cattle and badgers. Proceedings of the National Academy of Sciences of the United States of America, 102, 17588–17593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawshaw TR, Griffiths IB and Clifton‐Hadley RS, 2008. Comparison of a standard and a detailed postmortem protocol for detecting Mycobacterium bovis in badgers. Veterinary Record, 163, 473–477. [DOI] [PubMed] [Google Scholar]
- Cross ML, Labes RE, Griffin JFT and Mackintosh CG, 2000. Systemic but not intra‐intestinal vaccination with BCG reduces the severity of tuberculosis infection in ferrets (Mustela furo). The International Journal of Tuberculosis and Lung Disease, 4, 473–480. [PubMed] [Google Scholar]
- Dailloux M, Laurain C, Weber M and Hartemann P, 1999. Water and nontuberculous mycobacteria. Water Research, 33, 2219–2228. [Google Scholar]
- Dean GS, Rhodes SG, Coad M, Whelan AO, Cockle PJ, Clifford DJ, Hewinson RG and Vordermeier HM, 2005. Minimum infective dose of Mycobacterium bovis in cattle. Infection and Immunity, 73, 6467–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejene SW, Heitkonig IM, Prins HH, Lemma FA, Mekonnen DA, Alemu ZE, Kelkay TZ and de Boer WF, 2016. Risk factors for bovine tuberculosis (bTB) in cattle in Ethiopia. PLoS ONE, 11, e0159083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahay RJ, Langton S, Smith GC, Clifton‐Hadley RS and Cheeseman CL, 2000. The spatio‐temporal distribution of Mycobacterium bovis (bovine tuberculosis) infection in a high‐density badger population. Journal of Animal Ecology, 69, 428–441. [Google Scholar]
- Delahay RJ, De Leeuw AN, Barlow AM, Clifton‐hadley RS and Cheeseman CL, 2002. The status of Mycobacterium bovis infection in UK wild mammals: a review. Veterinary Journal, 164, 90–105. [DOI] [PubMed] [Google Scholar]
- Delahay RJ, Smith GC, Barlow AM, Walker N, Harris A, Clifton‐Hadley RS and Cheeseman CL, 2007. Bovine tuberculosis infection in wild mammals in the South‐West region of England: a survey of prevalence and a semi‐quantitative assessment of the relative risks to cattle. Veterinary Journal, 173, 287–301. [DOI] [PubMed] [Google Scholar]
- Di Marco VMC, Russo M, Aronica V, Bollo E, Amedeo S, Valenza F and Capucchio MT, 2008. Black wild pigs of Nebrodi Park in Sicily: evidence of their possible role as reservoir of Mycobacterium tuberculosis complex infection. Proceedings of the 26th meeting of European Society of Veterinary Pathology, Dubrovnik, Croatia.
- Donnelly CA, Woodroffe R, Cox DR, Bourne FJ, Cheeseman CL, Clifton‐Hadley RS, Wei G, Gettinby G, Gilks P, Jenkins H, Johnston WT, Le Fevre AM, McInerney JP and Morrison WI, 2006. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature, 439, 843–846. [DOI] [PubMed] [Google Scholar]
- Donnelly CA, Wei G, Johnston WT, Cox DR, Woodroffe R, Bourne FJ, Cheeseman CL, Clifton‐Hadley RS, Gettinby G, Gilks P, Jenkins HE, Le Fevre AM, McInerney JP and Morrison WI, 2007. Impacts of widespread badger culling on cattle tuberculosis: concluding analyses from a large‐scale field trial. International Journal of Infectious Diseases, 11, 300–308. [DOI] [PubMed] [Google Scholar]
- Downs S, Parry J, Nuñez‐García J, Abernethy DA, Broughan JM, Cameron AR, Cook A, de la Rua‐Domenech R, Goodchild AV, Greiner M, Gunn J, More SJ, Rhodes S, Rolfe S, Sharp M, Upton HM, Vordermeier HM, Watson E, Welsh M and Whelan AO, 2011. Meta‐analysis of diagnostic test performance and modelling of testing strategies for control of bovine tuberculosis in GB. Proceedings of the Society for Veterinary Epidemiology and Preventive Medicine, Leipzig, Germany, 139–197.
- Duarte EL, Domingos M, Amado A and Botelho A, 2008. Spoligotype diversity of Mycobacterium bovis and Mycobacterium caprae animal isolates. Veterinary Microbiology, 130, 415–421. [DOI] [PubMed] [Google Scholar]
- Duffield BJ and Young DA, 1985. Survival of Mycobacterium bovis in defined environmental conditions. Veterinary Microbiology, 10, 193–197. [DOI] [PubMed] [Google Scholar]
- Duignan A, Good M and More SJ, 2012. Quality control in the national bovine tuberculosis eradication programme in Ireland. Revue scientifique et technique (International Office of Epizootics), 31, 845–860. [DOI] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2012. Scientific Opinion on the use of a gamma interferon test for the diagnosis of bovine tuberculosis. EFSA Journal 2012;10(12):2975, 63 pp. 10.2903/j.efsa.2012.2975 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2013. Scientific Opinion on field trials for bovine tuberculosis vaccination. EFSA Journal 2013;11(12):3475, 35 pp. 10.2903/j.efsa.2013.3475 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke HH, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Gervelmeyer A, Zancanaro G, Kohnle L, Morgado J and Bicout D, 2017. Scientific opinion on an ad hoc method for the assessment on listing and categorisation of animal diseases within the framework of the Animal Health Law. EFSA Journal 2017;15(5):4783, 42 pp. 10.2903/j.efsa.2017.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2013. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2011. EFSA Journal 2013;11(4):3129, 250 pp. 10.2903/j.efsa.2013.3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2014. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2012. EFSA Journal 2014;12(2):3547, 312 pp. 10.2903/j.efsa.2014.3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2015a. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2013. EFSA Journal 2015;13(1):3991, 165 pp. 10.2903/j.efsa.2015.3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2015b. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2014. EFSA Journal 2015;13(12):4329, 190 pp. 10.2903/j.efsa.2015.4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2015. EFSA Journal 2016;14(12):4634, 231 pp. 10.2903/j.efsa.2016.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2006. Working Document on Eradication of Bovine Tuberculosis in the EU accepted by the Bovine tuberculosis subgroup of the Task Force on monitoring animal disease eradication. SANCO/10200/2006 final, 10 August 2006.
- European Commission , 2013. SUMMARY REPORT OF THE STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH HELD IN BRUSSELS ON 07 MAY 2013 (Section Animal Health & Welfare). SANCO G (2013)1681649.
- Evans JT, Smith EG, Banerjee A, Smith RM, Dale J, Innes JA, Hunt D, Tweddell A, Wood A, Anderson C, Hewinson RG, Smith NH, Hawkey PM and Sonnenberg P, 2007. Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person‐to‐person transmission in the UK. Lancet, 369, 1270–1276. [DOI] [PubMed] [Google Scholar]
- Fine PE, 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet, 346, 1339–1345. [DOI] [PubMed] [Google Scholar]
- Fine AE, Bolin CA, Gardiner JC and Kaneene JB, 2011. A Study of the Persistence of Mycobacterium bovis in the Environment under Natural Weather Conditions in Michigan. USA. Veterinary Medicine International, 2011, 765430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firdessa R, Tschopp R, Wubete A, Sombo M, Hailu E, Erenso G, Kiros T, Yamuah L, Vordermeier M, Hewinson RG, Young D, Gordon SV, Sahile M, Aseffa A and Berg S, 2012. High prevalence of bovine tuberculosis in dairy cattle in central Ethiopia: implications for the dairy industry and public health. PLoS ONE, 7, e52851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald SD, Zwick LS, Berry DE, Church SV, Kaneene JB and Reed WM, 2003. Experimental inoculation of pigeons (Columba livia) with Mycobacterium bovis . Avian Diseases, 47, 470–475. [DOI] [PubMed] [Google Scholar]
- Francis J, 1948. Bovine tuberculosis including a contrast with human tuberculosis. Australian Veterinary Journal, 24, 47–48. [Google Scholar]
- Francis J, 1958. Tuberculosis in animals and man. A study in comparative pathology. Tuberculosis, 39, 124. [Google Scholar]
- Garner FH, 1946. British Dairying. Longmans, Green and Co, London. pp. 126, 237. [Google Scholar]
- Garrido JM, Sevilla IA, Beltran‐Beck B, Minguijon E, Ballesteros C, Galindo RC, Boadella M, Lyashchenko KP, Romero B, Geijo MV, Ruiz‐Fons F, Aranaz A, Juste RA, Vicente J, de la Fuente J and Gortazar C, 2011. Protection against tuberculosis in Eurasian wild boar vaccinated with heat‐inactivated Mycobacterium bovis . PLoS ONE, 6, e24905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavinelli A, Kennedy T and Simonin D, 2014. The application of humane slaughterhouse practices to large‐scale culling. Revue scientifique et technique (International Office of Epizootics), 33, 291–301. [DOI] [PubMed] [Google Scholar]
- Gibson AL, Hewinson G, Goodchild T, Watt B, Story A, Inwald J and Drobniewski FA, 2004. Molecular epidemiology of disease due to Mycobacterium bovis in humans in the United Kingdom. Journal of Clinical Microbiology, 42, 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfray HCJ, Donnelly CA, Kao RR, Macdonald DW, McDonald RA, Petrokofsky G, Wood JLN, Woodroffe R, Young DB and McLean AR, 2013. A restatement of the natural science evidence base relevant to the control of bovine tuberculosis in Great Britain. Proceedings of the Proceedings of the Royal Society B: Biological Sciences, 280, 20131634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Juarrero M, Bosco‐Lauth A, Podell B, Soffler C, Brooks E, Izzo A, Sanchez‐Campillo J and Bowen R, 2013. Experimental aerosol Mycobacterium bovis model of infection in goats. Tuberculosis (Edinb), 93, 558–564. [DOI] [PubMed] [Google Scholar]
- Good M, 2006. Bovine tuberculosis eradication in Ireland. Irish Veterinary Journal, 59, 154–162. [Google Scholar]
- Good M, Clegg TA, Costello E and More SJ, 2011. The comparative performance of the single intradermal test and the single intradermal comparative tuberculin test in Irish cattle, using tuberculin PPD combinations of differing potencies. Veterinary journal, 190, e60–e65. 10.1016/j.tvjl.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Gormley E, Ni Bhuachalla D, O'Keeffe J, Murphy D, Aldwell FE, Fitzsimons T, Stanley P, Tratalos JA, McGrath G, Fogarty N, Kenny K, More SJ, Messam LL and Corner LA, 2017. Oral Vaccination of Free‐Living Badgers (Meles meles) with bacille calmette guerin (BCG) vaccine confers protection against tuberculosis. PLoS ONE, 12, e0168851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gortazar C, Diez‐Delgado I, Barasona JA, Vicente J, De La Fuente J and Boadella M, 2015. The Wild Side of Disease Control at the Wildlife‐Livestock‐Human Interface: a Review. Frontiers in Veterinary Science, 1, 10.3389/fvets.2014.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gortázar C, Vicente J, Samper S, Garrido JM, Fernández‐De‐Mera IG, Gavín P, Juste RA, Martin C, Acevedo P, De La Puente M and Höfle U, 2005. Molecular characterization of Mycobacterium tuberculosis complex isolates from wild ungulates in south‐central Spain. Veterinary Research, 36, 43–52. [DOI] [PubMed] [Google Scholar]
- Gortázar C, Torres MJ, Vicente J, Acevedo P, Reglero M, de la Fuente J, Negro JJ and Aznar‐Martin J, 2008. Bovine Tuberculosis in Donana Biosphere Reserve: the Role of Wild Ungulates as Disease Reservoirs in the Last Iberian Lynx Strongholds. PLoS ONE, 3, e2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gortázar C, Ferroglio E, Lutton CE and Acevedo P, 2010. Disease‐related conflicts in mammal conservation. Wildlife research, 37, 668–675. [Google Scholar]
- Graham J, Smith GC, Delahay RJ, Bailey T, McDonald RA and Hodgson D, 2013. Multi‐state modelling reveals sex‐dependent transmission, progression and severity of tuberculosis in wild badgers. Epidemiology & Infection, 141, 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JF, Mackintosh CG, Slobbe L, Thomson AJ and Buchan GS, 1999. Vaccine protocols to optimise the protective efficacy of BCG. Tubercle and Lung disease, 79, 135–143. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Williams DH, Kelly GE, Clegg TA, O'Boyle I, Collins JD and More SJ, 2005. The impact of badger removal on the control of tuberculosis in cattle herds in Ireland. Preventive Veterinary Medicine, 67, 237–266. [DOI] [PubMed] [Google Scholar]
- Gross M, 2013. Where wildlife and tame life collide. Current Biology, 23, 545–548. [DOI] [PubMed] [Google Scholar]
- Guerrero A, Cobo J, Fortun J, Navas E, Quereda C, Asensio A, Canon J, Blazquez J and Gomez‐Mampaso E, 1997. Nosocomial transmission of Mycobacterium bovis resistant to 11 drugs in people with advanced HIV‐1 infection. Lancet, 350, 1738–1742. [DOI] [PubMed] [Google Scholar]
- Hardstaff JL, Marion G, Hutchings MR and White PC, 2014. Evaluating the tuberculosis hazard posed to cattle from wildlife across Europe. Research in Veterinary Science, 97(Suppl), 86–93. [DOI] [PubMed] [Google Scholar]
- Hernandez J and Baca D, 1998. Effect of tuberculosis on milk production in dairy cows. Journal of the American Veterinary Medical Association, 213, 851–854. [PubMed] [Google Scholar]
- Hirsh DC and Zee YC, 1999. Veterinary Microbiology and Immunology. 2nd edition, Wiley, 496 pp. [Google Scholar]
- Jenkins HE, Morrison WI, Cox DR, Donnelly CA, Johnston WT, Bourne FJ, Clifton‐Hadley RS, Gettinby G, McInerney JP, Watkins GH and Woodroffe R, 2008. The prevalence, distribution and severity of detectable pathological lesions in badgers naturally infected with Mycobacterium bovis . Epidemiology & Infection, 136, 1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha VC, Morita Y, Dhakal M, Besnet B, Sato T, Nagai A, Kato M, Kozawa K, Yamamoto S and Kimura H, 2007. Isolation of Mycobacterium spp. from milking buffaloes and cattle in Nepal. The Journal of Veterinary Medical Science, 69, 819–825. [DOI] [PubMed] [Google Scholar]
- Judge J, McDonald RA, Walker N and Delahay RJ, 2011. Effectiveness of biosecurity measures in preventing badger visits to farm buildings. PLoS ONE, 6, e28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HC, Murphy A, James P, Travis E, Porter D, Hung YJ, Sawyer J, Cork J, Delahay RJ, Gaze W, Courtenay O and Wellington EM, 2015. The variability and seasonality of the environmental reservoir of Mycobacterium bovis shed by wild European badgers. Scientific Reports, 5, 12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukielka E, Barasona JA, Cowie CE, Drewe JA, Gortázar C, Cotarelo I and Vicente J, 2013. Spatial and temporal interactions between livestock and wildlife in South Central Spain assessed by camera traps. Preventive Veterinary Medicine, 112, 213–221. [DOI] [PubMed] [Google Scholar]
- Lesellier S, Corner L, Costello E, Lyashchenko K, Greenwald R, Esfandiari J, Singh M, Hewinson RG, Chambers M and Gormley E, 2009. Immunological responses and protective immunity in BCG vaccinated badgers following endobronchial infection with Mycobacterium bovis . Vaccine, 27, 402–409. [DOI] [PubMed] [Google Scholar]
- Lilenbaum W, Schettini JC, Souza GN, Ribeiro ER, Moreira EC and Fonseca LS, 1999. Comparison between a gamma‐IFN assay and intradermal tuberculin test for the diagnosis of bovine tuberculosis in field trials in Brazil. Zentralblatt für Veterinärmedizin. Reihe B, 46, 353–358. [DOI] [PubMed] [Google Scholar]
- de Lisle GW, Mackintosh CG and Bengis RG, 2001. Mycobacterium bovis in free‐living and captive wildlife, including farmed deer. Revue scientifique et technique (International Office of Epizootics), 20, 86–111. [DOI] [PubMed] [Google Scholar]
- Little TW, Swan C, Thompson HV and Wilesmith JW, 1982a. Bovine tuberculosis in domestic and wild mammals in an area of Dorset. II. The badger population, its ecology and tuberculosis status. Journal of Hygiene, 89, 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TWA, Swan C, Thompson HV and Wilesmith JW, 1982b. Mycobacterium bovis infection in cattle, badgers and other mammals in an area of Dorset. Veterinary Record, 110, 318–320. [DOI] [PubMed] [Google Scholar]
- Machackova M, Matlova L, Lamka J, Smolik J, Melicharek I, Hanzlikova M, Docekal J, Cvetnic Z, Nagy G, Lipiec M, Ocepek M and Pavlik I, 2003. Wild boar (Sus scrofa) as a possible vector of mycobacterial infections: review of literature and critical analysis of data from Central Europe between 1983 to 2001. Veterinarni Medicina, 48, 51–65. [Google Scholar]
- Malone FE, Wilson EC, Pollock JM and Skuce RA, 2003. Investigations into an outbreak of tuberculosis in a flock of sheep in contact with tuberculous cattle. Journal of Veterinary Medicine Series B‐Infectious Diseases and Veterinary Public Health, 50, 500–504. [DOI] [PubMed] [Google Scholar]
- Martín‐Atance P, Palomares F, González‐Candela M, Revilla E, Cubero MJ, Calzada J and León‐Vizcaíno L, 2005. Bovine tuberculosis in a free ranging red fox (Vulpes vulpes) from Donana National Park (Spain). Journal of Wildlife Diseases, 41, 435–436. [DOI] [PubMed] [Google Scholar]
- McDonald JL, Smith GC, McDonald RA, Delahay RJ and Hodgson D, 2014. Mortality trajectory analysis reveals the drivers of sex‐specific epidemiology in natural wildlife–disease interactions. Proceedings of the Proceedings of the Royal Society B: Biological Sciences, 281, pii: 20140526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JL, Bailey T, Delahay RJ, McDonald RA, Smith GC and Hodgson DJ, 2016. Demographic buffering and compensatory recruitment promotes the persistence of disease in a wildlife population. Ecology Letters, 19, 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin AM, Gibbons N, Fitzgibbon M, Power JT, Foley SC, Hayes JP, Rogers T and Keane J, 2012. Primary isoniazid resistance in Mycobacterium bovis disease: a prospect of concern. American Journal of Respiratory and Critical Care Medicine, 186, 110–111. [DOI] [PubMed] [Google Scholar]
- Michel AL, Bengis RG, Keet DF, Hofmeyr M, Klerk LM, Cross PC, Jolles AE, Cooper D, Whyte IJ, Buss P and Godfroid J, 2006. Wildlife tuberculosis in South African conservation areas: implications and challenges. Veterinary Microbiology, 112, 91–100. [DOI] [PubMed] [Google Scholar]
- More SJ and Good M, 2015. Understanding and managing bTB risk: perspectives from Ireland. Veterinary Microbiology, 176, 209–218. [DOI] [PubMed] [Google Scholar]
- Mullen EM, MacWhite T, Maher PK, Kelly DJ, Marples NM and Good M, 2013. Foraging Eurasian badgers Meles meles and the presence of cattle in pastures. Do badgers avoid cattle? Applied Animal Behaviour Science, 144, 130–137. [Google Scholar]
- Mullen EM, MacWhite T, Maher PK, Kelly DJ, Marples NM and Good M, 2015. The avoidance of farmyards by European badgers Meles meles in a medium density population. Applied Animal Behaviour Science, 171, 170–176. [Google Scholar]
- Muñoz‐Mendoza M, Romero B, del Cerro A, Gortázar C, García‐Marín JF, Menéndez S, Mourelo J, de Juan L, Sáez JL, Delahay RJ and Balseiro A, 2016. Sheep as a Potential Source of Bovine TB: epidemiology, Pathology and Evaluation of Diagnostic Techniques. Transboundary and Emerging Diseases, 63, 635–646. [DOI] [PubMed] [Google Scholar]
- Munro R (Secretary of State for Environment, Food and Rural Affairs), 2014. Pilot Badger Culls in Somerset and Gloucestershire. Report by the Independent Expert Panel. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/300382/independent-expert-panel-report.pdf
- Murhead RH and Burns KJ, 1974. Tuberculosis in wild badgers in Gloucestershire: epidemiology. Veterinary Record, 95, 552–555. [DOI] [PubMed] [Google Scholar]
- Murphy D, O'Keeffe JJ, Martin SW, Gormley E and Corner LAL, 2009. An Assessment of Injury to European Badgers (Meles Meles) Due to Capture in Stopped Restraints. Journal of Wildlife Diseases, 45, 481–490. [DOI] [PubMed] [Google Scholar]
- Naranjo V, Gortázar C, Vicente J and de la Fuente J, 2008. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Veterinary Microbiology, 127, 1–9. [DOI] [PubMed] [Google Scholar]
- O'Hare A, Orton RJ, Bessell PR and Kao RR, 2014. Estimating epidemiological parameters for bovine tuberculosis in British cattle using a Bayesian partial‐likelihood approach. Proceedings of the Royal Society B‐Biological Sciences, 281, 20140248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE , 2007. Report of the meeting of the OIE working group on wildlife diseases. Proceedings of the 75th General Session International Committee, Paris, France
- OIE , 2012. Killing of animals for disease control purposes. Article 7.6.1. In: Terrestrial Animal Health Code
- OIE , 2015. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2015. Chapter 2.4.7. Bovine Tuberculosis. Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.04.06_BOVINE_TB.pdf
- Olea‐Popelka F, Muwonge A, Perera A, Dean AS, Mumford E, Erlacher‐Vindel E, Forcella S, Silk BJ, Ditiu L, El Idrissi A, Raviglione M, Cosivi O, LoBue P and Fujiwara PI, 2017. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis‐a call for action. The Lancet. Infectious Diseases, 17, 21–25. [DOI] [PubMed] [Google Scholar]
- O'Mahony DT(Agri‐Food and Biosciences Institute), 2014. Badger‐cattle interactions in the rural environment – implications for Bovine Tuberculosis transmission. Report to TB & Brucellosis Policy Branch, Department of Agriculture and Rural Development. Available online: https://www.daera-ni.gov.uk/sites/default/files/publications/dard/badger-cattle-proximity-report.pdf
- O'Mahony DT, 2015a. Badger (Meles meles) contact metrics in a medium‐density population. Mammalian Biology, 80, 484–490. [Google Scholar]
- O'Mahony DT, 2015b. Multi‐species visit rates to farmyards: implications for biosecurity. Veterinary Journal, 203, 126–128. [DOI] [PubMed] [Google Scholar]
- O'Reilly LM and Daborn CJ, 1995. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tubercle and Lung disease, 76(Suppl 1), 1–46. [DOI] [PubMed] [Google Scholar]
- Palmer MV, Whipple DL and Olsen SC, 1999. Development of a model of natural infection with Mycobacterium bovis in white‐tailed deer. Journal of Wildlife Diseases, 35, 450–457. [DOI] [PubMed] [Google Scholar]
- Palmer MV, Waters WR and Whipple DL, 2002. Lesion development in white‐tailed deer (Odocoileus virginianus) experimentally infected with Mycobacterium bovis . Veterinary Pathology, 39, 334–340. [DOI] [PubMed] [Google Scholar]
- Parra A, Garcia A, Inglis NF, Tato A, Alonso JM, Hermoso de Mendoza M, Hermoso de Mendoza J and Larrasa J, 2006. An epidemiological evaluation of Mycobacterium bovis infections in wild game animals of the Spanish Mediterranean ecosystem. Research in Veterinary Science, 80, 140–146. [DOI] [PubMed] [Google Scholar]
- Pavlik I, Machackova M, Ayele WY, Lamka J, Parmova I, Melicharek I, Hanzlikova M, Kormendy B, Nagy G, Cvetnic Z, Ocepek M and Lipiec M, 2002. Incidence of bovine tuberculosis in wild and domestic animals other than cattle in six Central European countries during 1990‐1999. Veterinarni Medicina, 47, 122–131. [Google Scholar]
- Phillips CJ, Foster CR, Morris PA and Teverson R, 2002. Genetic and management factors that influence the susceptibility of cattle to Mycobacterium bovis infection. Animal Health Research Reviews, 3, 3–13. [DOI] [PubMed] [Google Scholar]
- Phillips CJ, Foster CR, Morris PA and Teverson R, 2003. The transmission of Mycobacterium bovis infection to cattle. Research in Veterinary Science, 74, 1–15. [DOI] [PubMed] [Google Scholar]
- Qureshi T, Labes RE, Cross ML, Griffin JF and Mackintosh CG, 1999. Partial protection against oral challenge with Mycobacterium bovis in ferrets (Mustela furo) following oral vaccination with BCG. International Journal of Tuberculosis and Lung Disease (IJTLD), 3, 1025–1033. [PubMed] [Google Scholar]
- Rahman MA and Samad MA, 2008. Prevalence of bovine tuberculosis and its effects on milk production in Red Chittagong cattle. Bangladesh Journal of Veterinary Medicine, 6, 175–178. [Google Scholar]
- Ramsey DS, Aldwell FE, Cross ML, de Lisle GW and Buddle BM, 2009. Protection of free‐living and captive possums against pulmonary challenge with Mycobacterium bovis following oral BCG vaccination. Tuberculosis (Edinb), 89, 163–168. [DOI] [PubMed] [Google Scholar]
- de la Rua‐Domenech R, 2006. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis (Edinb), 86, 77–109. [DOI] [PubMed] [Google Scholar]
- de la Rua‐Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH and Clifton‐Hadley RS, 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, gamma‐interferon assay and other ancillary diagnostic techniques. Research in Veterinary Science, 81, 190–210. [DOI] [PubMed] [Google Scholar]
- Santos N, Santos C, Valente T, Gortazar C, Almeida V and Correia‐Neves M, 2015. Widespread Environmental Contamination with Mycobacterium tuberculosis Complex Revealed by a Molecular Detection Protocol. PLoS ONE, 10, e0142079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, Buddle BM, Thacker TC, Lyashchenko KP and Waters WR, 2010. Bovine Tuberculosis: a Review of Current and Emerging Diagnostic Techniques in View of their Relevance for Disease Control and Eradication. Transboundary and Emerging Diseases, 57, 205–220. [DOI] [PubMed] [Google Scholar]
- Schultz G, Deuter H and Dedek J, 1992. Zum Vorkommen von Mycobacterium bovis‐Infektionen beim freilebenden Schwarzwild. Proceedings of the 34th International Symposium on Diseases of Zoo and Wild Animals, Santander, Spain, 51‐53.
- Serraino A, Marchetti G, Sanguinetti V, Rossi MC, Zanoni RC, Catozzi L, Bandera A, Dini W, Mignone W, Franzetti F and Gori A, 1999. Monitoring of transmission of tuberculosis between wild boars and cattle: genotypical analysis of strains by molecular epidemiology techniques. Journal of Clinical Microbiology, 37, 2766–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T and Saunders G, 2011. A model for assessing the relative humaneness of pest animal control methods. second edition Australian Government Department of Agriculture, Fisheries and Forestry, Canberra, ACT. 126 pp. [Google Scholar]
- Sheffield RMS, Vaughan DH, Collins ER and Allen VG, 1997. Off‐stream water sources for grazing cattle as a stream bank stabilization and water quality. Transactions of the American Society of Agricultural Engineers, 40, 595–604. [Google Scholar]
- Smith GC, McDonald RA and Wilkinson D, 2012. Comparing badger (Meles meles) management strategies for reducing tuberculosis incidence in cattle. PLoS ONE, 7, e39250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K, Chauhan DS, Gupta P, Singh HB, Sharma VD, Yadav VS, Thakral SS, Dharamdheeran JS, Nigam P and Prasad HK, 2008. Isolation of Mycobacterium bovis & M. tuberculosis from cattle of some farms in north India‐possible relevance in human health. Indian Journal of Medical Research, 128, 26. [PubMed] [Google Scholar]
- Stephens M, 1957. The otter report. Universities Federation for Animal Welfare, Potters Bar, London, UK. [Google Scholar]
- Stevens JB, Thoen CO, Rohonczy EB, Tessaro S, Kelly HA and Duncan JR, 1998. The immunological response of llamas (Lama glama) following experimental infection with Mycobacterium bovis . Canadian Journal of Veterinary Research, 62, 102–109. [PMC free article] [PubMed] [Google Scholar]
- Strain SAJ, McNair J and McDowell SWJ (Bacteriology Branch, Veterinary Sciences Division, Agri‐Food and Biosciences Institute), 2011. Bovine tuberculosis: a review of diagnostic tests for M. bovis infection in badgers. Available online: http://www.bovinetb.info/docs/bovine-tuberculosis-a-review-of-diagnostic-tests-for-m-bovis-infection-in-badgers.pdf
- Sunder S, Lanotte P, Godreuil S, Martin C, Boschiroli ML and Besnier JM, 2009. Human‐to‐human transmission of tuberculosis caused by Mycobacterium bovis in immunocompetent patients. Journal of Clinical Microbiology, 47, 1249–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talling JC and Inglis IR, 2009. Improvements to trapping standards. DG ENV, 361 p. [Google Scholar]
- Thoen CO and Williams DE, 1994. Tuberculosis, tuberculoidoses, and other mycobacterial infections In: Beran GW.(ed.). Handbook on zoonosis. 2, CRC Press, Boca Raton, Florida, 560 pp. [Google Scholar]
- Thoen CO, Steele JH and Gilsdorf MJ, 2008. Mycobacterium bovis infection in animals and humans. 2nd edition, Blackwell Publishing, Oxford, UK, 329 pp. [Google Scholar]
- Thoen CO, Steele JH and Kaneene JB, 2014Zoonotic Tuberculosis: Mycobacterium bovis and Other Pathogenic Mycobacteria. 3rd edition. John Wiley & Sons, Inc., 432 pp. [Google Scholar]
- Thornber PM, Rubira RJ and Styles DK, 2014. Humane killing of animals for disease control purposes. Revue scientifique et technique (International Office of Epizootics), 33, 303–310. [DOI] [PubMed] [Google Scholar]
- Tompkins DM, Ramsey DS, Cross ML, Aldwell FE, de Lisle GW and Buddle BM, 2009. Oral vaccination reduces the incidence of tuberculosis in free‐living brushtail possums. Proceedings of the Royal Society B: Biological Sciences, 276, 2987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewby ID, Wilson GJ, Delahay RJ, Walker N, Young R, Davison J, Cheeseman C, Robertson PA, Gorman ML and McDonald RA, 2008. Experimental evidence of competitive release in sympatric carnivores. Biology Letters, 4, 170–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewby ID, Young R, McDonald RA, Wilson GJ, Davison J, Walker N, Robertson A, Doncaster CP and Delahay RJ, 2014. Impacts of removing badgers on localised counts of hedgehogs. PLoS ONE, 9, e95477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une Y and Mori T, 2007. Tuberculosis as a zoonosis from a veterinary perspective. Comparative Immunology Microbiology and Infectious Diseases, 30, 415–425. [DOI] [PubMed] [Google Scholar]
- de Val Pérez B, López‐Soria S, Nofrarías M, Martín M, Vordermeier HM, Villarreal‐Ramos B, Romera N, Escobar M, Solanes D, Cardona PJ and Domingo M, 2011. Experimental model of tuberculosis in the domestic goat after endobronchial infection with Mycobacterium caprae . Clinical and Vaccine Immunology, 18, 1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente J, Delahay RJ, Walker NJ and Cheeseman CL, 2007. Social organization and movement influence the incidence of bovine tuberculosis in an undisturbed high‐density badger Meles meles population. Journal of Animal Ecology, 76, 348–360. [DOI] [PubMed] [Google Scholar]
- Vicente J, Barasona JA, Acevedo P, Ruiz‐Fons JF, Boadella M, Diez‐Delgado I, Beltran‐Beck B, Gonzalez‐Barrio D, Queiros J, Montoro V, de la Fuente J and Gortazar C, 2013. Temporal trend of tuberculosis in wild ungulates from Mediterranean Spain. Transboundery Emerging Diseases, 60(Suppl 1), 92–103. [DOI] [PubMed] [Google Scholar]
- Williams RS and Hoy WA, 1930. The Viability of B. tuberculosis (bovinus) on Pasture Land, in Stored Faeces and in Liquid Manure. Journal of Hygiene, 30, 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G, Jennifer B, Mark C, Richard C‐H, Tim C, de la Josù F, Richard D, Dolores G‐W, Christian G, Glyn H, Vicky J, Martín‐Hernando, Paz M, Aleksija N, Salguero FJ, Joaquin V, Alastair W and Robbie M, 2009. Scientific review on Tuberculosis in wildlife in the EU. EFSA Supporting Publication 2009 2009;6:EN‐12, 117 pp. 10.2903/sp.efsa.2009.en-12 [DOI] [Google Scholar]
- Woodroffe R, Bourne FJ, Cheeseman CL, Cox DR, Donnelly CA, Gettinby G, McInerney JP and Morrison WI, 2005a. Welfare of badgers (Meles meles) subjected to culling: development and evaluation of a closed season. Animal Welfare, 14, 19–25. [Google Scholar]
- Woodroffe R, Bourne FJ, Cox DR, Donnelly CA, Gettinby G, McInerney JP and Morrison WI, 2005b. Welfare of badgers (Meles meles) subjected to culling: patterns of trap‐related injury. Animal Welfare, 14, 11–17. [Google Scholar]
- Zanella G, Duvauchelle A, Hars J, Moutou F, Boschiroli ML and Durand B, 2008. Patterns of lesions of bovine tuberculosis in wild red deer and wild boar. Veterinary Record, 163, 43–47. [DOI] [PubMed] [Google Scholar]
- Zinsstag J, Schelling E, Roth F and Kazwala R, 2006. Economics of bovine tuberculosis In: Thoen CO, Steele JH. and Gilsdorf MJ. (eds.). Mycobacterium bovis infection in animals and humans. Blackwell Publishing, Oxford, UK: pp. 68–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mapped fact‐sheet used in the individual judgement on bovine tuberculosis


