Abstract
The Panel on Plant Health performed a pest categorisation of the eight‐toothed spruce bark beetle, Ips typographus L. (Coleoptera: Curculionidae, Scolytinae), for the EU. I. typographus is a well‐defined and distinguishable species, recognised mainly as a pest of spruce (Picea spp.) in Eurasia. It also attacks other conifers such as Abies spp., Larix spp., Pinus spp. and Pseudotsuga menziesii. Native to Eurasia, I. typographus has spread from the native range of spruce to new areas in Eurasia where spruce has been planted, and is now widely distributed throughout the EU (22 Member states). It is a quarantine pest listed in Annex IIB of Council Directive 2000/29/EC for Ireland and United Kingdom as protected zones. Coniferous wood, bark and wood packaging material are considered as pathways for the pest, which is also able to disperse by flight over tens of kilometres. The insects normally establish on fallen trees but can also mass‐attack healthy trees, killing millions of spruces. The males produce pheromones that attract conspecifics of both sexes. Each male attracts one to four females; each female produces 2–80 offspring. The insects also inoculate pathogenic fungi to their hosts. There are one to three generations per year. The wide current geographic range of I. typographus suggests that it is able to establish anywhere in the EU where its hosts are present. Sanitary thinning or clear‐felling are the major control methods. Pheromone mass trapping is presently judged unreliable because of the large dispersal capacity of the pest. Quarantine measures are implemented to prevent entry in yet uncolonised areas. All criteria assessed by EFSA for consideration as potential protected zone quarantine pest are met. The criteria for considering I. typographus as a potential regulated non‐quarantine pest are not met since plants for planting are not a pathway.
Keywords: Curculionidae, European Union, pest risk, plant health, plant pest, quarantine, eight‐toothed spruce bark beetle
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips amitinus Eichhof |
| Cephalcia lariciphila (Klug) | Ips cembrae Heer |
| Dendroctonus micans Kugelan | Ips duplicatus Sahlberg |
| Gilphinia hercyniae (Hartig) | Ips sexdentatus Börner |
| Gonipterus scutellatus Gyll. | Ips typographus Heer |
| Sternochetus mangiferae Fabricius | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L.,Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. |
| Gymnosporangium spp. (non‐EU) | malagutii Ciccarone and Boerema |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Thecaphora solani Barrus |
| Melampsora farlowii (Arthur) Davis | Trechispora brinkmannii (Bresad.) Rogers |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex IB | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Ips typographus is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest for the area of the European Union excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
Since I. typographus is regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone (Ireland and the United Kingdom), thus the criteria refers to the protected zone instead of the EU territory.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on I. typographus was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Relevant papers were reviewed, and further references and information were obtained from experts, from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO, online).
Data about the area of hosts grown in the EU and about the import of commodity types that could provide a pathway for the pest to enter the EU from non‐EU European countries were obtained from EUROSTAT.
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Consumers (DG SANCO), and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with the EU legislation, as well as notifications of plant pests detected in the territory of the MSs and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for I. typographus, following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union regulated non‐quarantine pest in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required as per the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a regulated non‐quarantine pest. If one of the criteria is not met, the pest will not qualify. In such a case, the working group should consider the possibility to terminate the assessment early and to be concise in the sections preceding the question for which the negative answer is reached. Note that a pest that does not qualify as a quarantine pest may still qualify as a regulated non‐quarantine pest which needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone, thus the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC) The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regards to the principle of separation between risk assessment and risk management (EFSA founding regulation (EC) 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes, the identity of the pest is established. It can be identified to species using conventional entomological keys.
Ips typographus is an insect of the family Curculionidae, subfamily Scolytinae.4
3.1.2. Biology of the pest
Comprehensive accounts of the biology and ecology of I. typographus are given by Chararas (1962), Christiansen and Bakke (1997), Wermelinger (2004) and Kausrud et al. (2011). The adults overwinter in the litter or in the bark of the trees where they developed, and disperse widely in the spring, flying in search for new hosts. Dispersal can be very wide, sometimes over tens of kilometres (Forsse and Solbreck, 1985) or even longer distances (> 100 km according to Montano et al., 2016). Upon emergence, the males constitute 30–50% of the population (Lobinger, 1996). They start colonising either weakened (e.g. fallen) or healthy trees and attract conspecifics of both sexes with aggregation pheromones (Bakke, 1970, 1976; Birgersson et al., 1984). Each male that has excavated a nuptial chamber in the phloem is joined by one to four females, which bore each a maternal gallery in the phloem, parallel to the phloem fibres and lay one egg at a time at regular intervals (Anderbrant, 1990), each in a small niche created in the lateral wall of the maternal gallery. Up to 80 eggs can be laid by one female but, at usual densities (1–5 females/dm2), 2–10 offspring/female are produced. After egg‐laying, the parent adults often re‐emerge, fly away and establish sister broods on new hosts. Each larva excavates an individual gallery perpendicular to the maternal gallery. Pupation occurs in a small niche in the phloem, at the end of each larval gallery. After metamorphosis, the young adults remain under the bark for maturation feeding before they disperse. An adult diapause is sometimes observed in upper latitudes or elevations (Schopf, 1989). There are one to possibly three generations per year. At low, endemic population levels, the beetles mostly establish on weakened hosts. When populations increase, for example after a storm has provided a large amount of undefended material, the beetles start attacking healthy trees, which they mass‐attack, thus overwhelming their defences. During this process, pathogenic ophiostomatoid fungi are inoculated to the host (Solheim, 1986; Kirisits, 2004; Linnakoski et al., 2016) and contribute to tree death. These massively attacked trees also attract competitors (Schlyter and Anderbrant, 1993) and natural enemies (Mills, 1983, 1985; Kenis et al., 2004). The major triggers for I. typographus outbreaks are the availability of storm‐felled timber, summer rainfall deficits and warm temperatures (Grégoire et al., 2015 and refs. therein; Marini et al., 2016). Climate change is predicted to alter the beetles’ voltinism and the trees’ vulnerability, leading to increased damage in the future (Jönsson et al., 2009, 2011; Bentz and Jönsson, 2015 and references therein; Seidl and Rammer, 2016).
3.1.3. Intraspecific diversity
One subspecies, I. typographus japonicus is known in China and Japan (Stauffer et al., 1999). In Europe, a phylogeographic analysis has revealed the existence of a northern and a southern group of haplotypes within the species I. typographus (Mayer et al., 2015).
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, the organism can be detected by visual searching, often after damage symptoms are seen. The species can be identified by examining morphological features, for which keys exist, e.g. Balachowsky (1949); Grüne (1979); Schedl (1981); Wood (1982).
The standing trees attacked by I. typographus die during the colonisation process. During the attacks, brown sawdust is expelled from the entry holes and, when the broods have metamorphosed and the young adults start feeding on the phloem around the galleries, the bark can flake off, and this phenomenon can be amplified by the action of wood peckers. Within and behind the phloem, vertical maternal galleries and horizontal larval galleries can be seen. The sapwood shows bluestaining due to the fungi introduced by the beetles. The adult insects are dark brown or black in colour, cylindrical, 4.5–5.5 mm long. The larvae are apodous, with a dark amber cephalic capsule.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
I. typographus is present in two continents, continental Europe and Asia. The insect is absent from the other continents. It has been repeatedly intercepted at ports in the United States (period 1985–2000; 286 interceptions out of 6,825 records: Haack, 2001) and in New Zealand (period 1952–2000; 43 interception out of 722 records: Brockerhoff et al., 2003). In non‐EU Europe, the insect has been reported from Bosnia and Herzegovina, Georgia, Norway, Russia, Serbia, Switzerland, Turkey and Ukraine (Figure 1).
Figure 1.
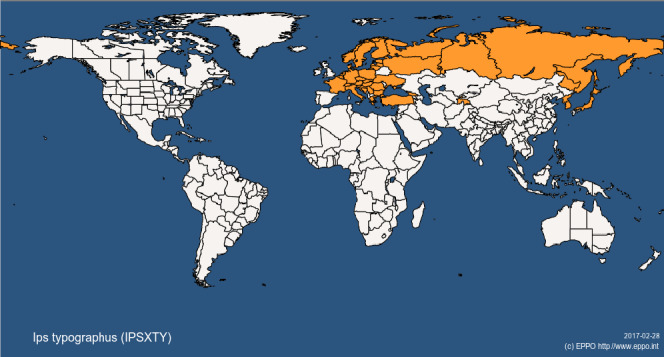
Global distribution map for Ips typographus (extracted from the EPPO Global Database accessed on 28 February 2017)
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
Yes, I. typographus is present and widely distributed in the EU; it has been reported from 22 Member States (MSs). The protected zones, Ireland and the United Kingdom, are free from the pest.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
Ips typographus is listed in Council Directive 2000/29/EC. Details are presented in Tables 3 and 4.
Table 3.
Ips typographus in Council Directive 2000/29/EC
| Annex II, Part B | Harmful organisms whose introduction into, and whose spread within, certain protected zones shall be banned if they are present on certain plants or plant products | ||
|---|---|---|---|
| (a) | Insects, mites and nematodes, at all stages of their development | ||
| Species | Subject of contamination | Protected zones | |
| 6 (e) | Ips typographus | Plants of Abies Mill., Larix Mill., Picea A. Dietr., Pinus L. and Pseudotsuga Carr., over 3 m in height, other than fruit and seeds, wood of conifers (Coniferales) with bark, isolated bark of conifers | IRL, UK |
3.3.2. Legislation addressing plants and plant parts on which Ips typographus is regulated
Table 4.
Regulated hosts and commodities that may involve Ips typographus in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | ||
| Description | Country of origin | ||
| 1 | Plants of Abies Mill., […], Larix Mill., Picea A. Dietr., Pinus L., Pseudotsuga Carr. […], other than fruit and seeds | Non‐European Countries | |
| Annex IV, Part B | Special requirements which shall be laid down by all member states for the introduction and movement of plants, plant products and other objects into and within certain protected zones | ||
| Plants, plant products and other objects | Special requirements | Protected zone(s) | |
| 3. | Wood of conifers (Coniferales) |
Without prejudice to the requirements applicable to the wood listed in Annex IV(A)(I)(1.1), (1.2), (1.3), (1.4), (1.5), (1.6), (1.7), where appropriate, and Annex IV(B)(1) and (2):
|
IRL, UK |
| 9. | Plants of Abies Mill., Larix Mill., Picea A. Dietr., Pinus L. and Pseudotsuga Carr., over 3 m in height, other than fruit and seeds | Without prejudice to the provisions applicable to the plants listed in Annex III(A)(1), Annex IV(A)(I)(8.1), (8.2), (9), (10), Annex IV(A)(II)(4), (5) and Annex IV(B)(7), (8), where appropriate, official statement that the place of production is free from Ips typographus | IRL, UK |
| 14.6 | Isolated bark of conifers (Coniferales) |
Without prejudice to the provisions applicable to the bark listed in Annex IV(B)(14.1), (14.2), (14.3), (14.4), (14.5), official statement that the consignment:
|
IRL, UK |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community – in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community | ||
| Part A | Plants, plant products and other objects originating in the Community | ||
| Section II | Plants, plant products and other objects produced by producers whose production and sale is authorised to persons professionally engaged in plant production, other than those plants, plant products and other objects which are prepared and ready for sale to the final consumer, and for which it is ensured by the responsible official bodies of the Member States, that the production thereof is clearly separate from that of other products | ||
| 2.1 | Plants intended for planting other than seeds of the genera Abies Mill., […] Larix Mill., […], Picea A. Dietr., Pinus L., […], Pseudotsuga Carr., […] | ||
3.3.3. Legislation addressing the organisms vectored by Ips typographus (Directive 2000/29/EC)
Although several phytopathogenic ophiostomatoid fungi are regularly associated with I. typographus, (Solheim, 1986; Kirisits, 2004; Linnakoski et al., 2016), there is currently no legislation addressing this issue.
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
I. typographus attacks mainly spruce (Picea spp.) but has also been observed attacking firs (Abies spp.), larch (Larix spp.), Douglas fir (Pseudotsuga menziesii) and pines (Pinus spp.) (EPPO, online; CABI, online).
The hosts for which I. typographus is regulated are comprehensive of the host range: the pest is regulated on five genera: Abies, Larix, Picea, Pinus and Pseudotsuga.
3.4.2. Entry
Is the pest able to enter into the protected zone areas of the EU territory? If yes, identify and list the pathways!
Yes, the pest is already established in 22 MSs. Since entry by natural dispersal from the EU areas where the pest is present is possible, only isolated areas (e.g. islands) can be long‐term protected zones.
The pest is widely present in 22 MSs of the EU. In addition, it is regularly intercepted in MSs where it is still absent. Between 1994 and 2015, there have been 34 records of interception of I. typographus and 26 interceptions of ‘Ips sp.’ in the Europhyt database, among which 30 I. typographus in the UK and three in Ireland.
The main pathways of entry are:
wood of Abies, Larix, Pinus, Picea and Pseudotsuga from countries where the pest occurs;
wood chips of conifers from countries where the pest occurs;
bark of conifers from countries where the pest occurs;
wood packaging material and dunnage from countries where the pest occurs.
Plants for planting should not be considered as a pathway for I. typographus because small trees are usually not attacked and if attacked, would be killed. Haack (2001) reports that in the US during the period 1985–2000, I. typographus was exclusively intercepted with wood packaging material containing tiles and machinery: crating (230 cases), dunnage (166) and pallets (34). The Europhyt database reports (1994–2015) 29 interceptions on wood and bark, 15 on wood packaging material (pallets, crates and dunnage) and 16 on unclassified plant material.
According to the EUROSTAT database, there are movements of material pertaining to the above pathways from Third countries and EU countries where the pest is present, into the protected zones. For example, concerning the wood pathway, around 41,000 tonnes of coniferous wood including the genera Picea, Pinus and Abies (Eurostat codes 44032011, 44032019, 44022031, 44032039, 44022091, 44032099), has been imported in the period 2011–2015 from EU countries into protected zones. In the same period, around 9,000 tonnes of coniferous wood were imported into the protected zones from third countries where the pest is present (Bosnia and Herzegovina, Norway, Russia, Serbia, Switzerland, Turkey and Ukraine).
3.4.3. Establishment
Is the pest able to become established in the protected zone areas of the EU territory?
Yes, the pest is already established in 22 MSs. The climate of the EU protected zones is similar to that of the MSs where I. typographus is established, and the pest's main host plants are present (Figure 2).
Figure 2.
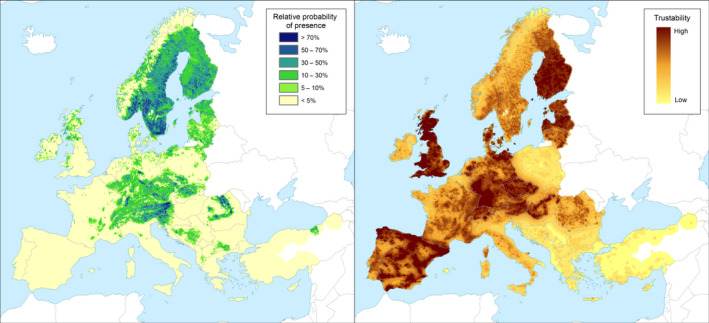
Relative probability of presence of the genus Picea in the European Union territory (based on data from the species: P. abies, P. sitchensis, P. glauca, P. engelmannii, P. pungens, P. omorika, P. orientalis). Left panel: Relative probability of presence (RPP) of species/genera from the European Atlas of Forest Tree Species (de Rigo et al., 2016; San‐Miguel‐Ayanz et al., 2016), aggregated at 100 km² pixel resolution. RPP is defined as the probability of finding species/taxon in a given area, irrespective of the probability of finding other taxa (de Rigo et al., 2017). As a consequence, the sum of all RPPs for different taxa in the same area need not be 100%. The estimates are based on constrained spatial multi‐scale frequency analysis (C‐SMFA) (de Rigo et al., 2014, 2016, 2017): this is a spatial multi‐scale frequency analysis of field observations (de Rigo et al., 2014, 2016), constrained to enhance the estimates’ consistency with the frequency of broadleaved and coniferous taxa derived from Corine Land Cover (Bossard et al., 2000; Büttner et al., 2012). Right panel: Trustability of RPP. This qualitative measure is based on the multi‐scale aggregation of the number of field observations (i.e. the local density of data) for each pixel and taxon. The colour scale of the trustability map is based on the quantiles of this data density (de Rigo et al., 2014, 2016)
3.4.3.1. EU distribution of main host plants
The wide distribution of host trees in the EU territory allowed I. typographus to establish in most MSs (see Table 2). Picea excelsa, Picea omorika and Picea orientalis are native to Europe and are widely planted outside their original range throughout the EU. Other Picea species are widely distributed in the EU territory (Figure 2).
Table 2.
Current distribution of Ips typographus in the 28 EU MS based on information from the EPPO Global Database and other sources if relevant
| Country | EPPO GD Last update: 10/3/2016 Date Accessed: 22/2/17 |
|---|---|
| Austria | Present, no details |
| Belgium | Present, no details |
| Bulgaria | Present, widespread |
| Croatia | Present, restricted distribution |
| Cyprus | No information |
| Czech Republic | Present, widespread |
| Denmark | Present, widespread |
| Estonia | Present, no details |
| Finland | Present, widespread |
| France | Present, restricted distribution |
| Germany | Present, widespread |
| Greece | Present, no details |
| Hungary | Present, restricted distribution |
| Ireland | Absent, confirmed by survey |
| Italy |
Present, restricted distribution Sardegna: Absent, pest no longer present |
| Latvia | Present, no details |
| Lithuania | Present, widespread |
| Luxembourg | Present, no details |
| Malta | No information |
| Poland | Present, restricted distribution |
| Portugal | Absent, confirmed by survey |
| Romania | Present, no details |
| Slovak Republic | Present, widespread |
| Slovenia | Present, no details |
| Spain | Absent, confirmed by survey |
| Sweden | Present, widespread |
| The Netherlands | Present, restricted distribution |
| United Kingdom | Absent, confirmed by survey |
3.4.3.2. Climatic conditions affecting establishment
Given the current distribution of I. typographus, the whole EU area (including protected zones) is suitable for establishment.
3.4.4. Spread
Is the pest able to spread within protected zones areas of the EU territory following establishment? How?
Yes, adults can disperse naturally. They can fly over tens of kilometres or even more. The pest can also spread by human assistance, e.g. with the transportation of wood, wood chips, bark, wood packaging material and dunnage of conifers.
RNQPs: Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects?
No, plants for planting are not a pathway.
As shown in Table 2, I. typographus is present in most of the EU, except in Ireland, Portugal, Spain and the United Kingdom, while there is no information from Cyprus and Malta in the EPPO Global Database. The main pathway for spread is the transportation of infested material, but natural spread by flight regularly occurs over large distances (see Section 3.1.2).
3.5. Potential or observed impacts in the EU
Would the pests’ introduction have an economic or environmental impact on the protected zones of the EU?
Yes, the pest is known to have killed millions of trees, after triggering events such as storms or dry summers.
3.5.1. Potential pest impacts
The species is native to Europe, and hence, it is irrelevant to consider impact outside the EU territory.
3.5.2. Observed pest impacts in the EU
3.5.2.1. Direct impact of the pest
I. typographus mass attacks standing, healthy host trees, which are killed by the combined effect of the beetles and associated pathogenic ophiostomatoid fungi (see Section 3.1.2). The species is considered as the most damaging forest pest in Europe (Grégoire and Evans, 2004; Grégoire et al., 2015). Schelhaas et al. (2003) calculated that 8% of all tree mortality in Europe during the period 1850–2000 was due to bark beetles, and mainly to I. typographus. In Switzerland between 2000 and 2009, the beetles have killed 8 million m3 of spruce (Meier et al., 2013); in Austria, 18 million m3 were killed between 2002 and 2012 (Steyrer and Krehan, 2009; Krehan et al., 2012).
In addition to these silvicultural damage, large outbreaks of I. typographus also have an ecosystemic impact, as tree mortality at this scale negatively influences the ecosystemic services of the forest, as well as the global carbon balance (Kurz et al., 2008; Kautz et al., 2016; Seidl and Rammer, 2016).
3.5.2.2. Indirect pest impact (e.g. by bacteria or viruses transmitted by the pest)
The impact of fungi associated to I. typographus has been analysed by many authors (e.g. Solheim, 1986; Yamaoka et al., 1997; Viiri and Lieutier, 2004; Sallé et al., 2005; Linnakoski et al., 2016). Kirisits (2004) provides a review of the ophiostomatoid fungi associated to I. typographus: At least 23 species were isolated from the galleries of the beetles: Ceratocystiopsis alba; Ceratocystiopsis minuta; Ceratocystis polonica; Graphium fimbriisporum; Graphium pseudormiticum (= G. fimbriisporum?) Graphium (Pesotum?) pycnocephalum; Leptographium euphyes; Leptographium lundbergii; Leptographium spp.; Ophiostoma ainoae; Ophiostoma araucariae; Ophiostoma bicolor; Ophiostoma cainii; Ophiostoma cucullatum; Ophiostoma flexuosum; Ophiostoma floccosum; Ophiostoma japonicum; Ophiostoma penicillatum; [Ophiostoma penicillatum f. chalcographi]; Ophiostoma piceae; Ophiostoma cf. piceae; Ophiostoma piceaperdum; (Ophiostoma pluriannulatum); Ophiostoma serpens; Ophiostoma stenoceras; Ophiostoma tetropii; Ophiostoma spp.; Pesotum fragrans; Pesotum sp.; Pesotum (Graphium?) spp.
Some of these species (e.g. Ceratocystis polonica) are virulent tree pathogens (Christiansen, 1985), others (e.g. Ophiostoma bicolor, O. penicillatum, O. piceaperdum, O. piceae, Pesotum sp.) are more innocuous (Solheim et al., 2001).
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zones of the EU such that the risk becomes mitigated?
Yes, in isolated areas (e.g. islands) that cannot be reached by natural spread, measures can be put in place to prevent the introduction with wood, wood products, wood chips, bark and plants for planting. Debarking wood, heat treatment of wood, bark and chips, and inspection of plants for planting are specified in Annex IVBII of 2000/29/EC. When such geographical barriers do not exist, there is no possibility to prevent the entry, establishment and spread of I. typographus in new areas. This is illustrated by the gradual and sometimes very recent colonisation on continental EU of areas recently planted with spruce, far from the trees’ area of origin (Belgium, Brittany or Normandy in France, etc.).
Is it possible to eradicate the pest in a restricted area within 24 months after the presence of the pest was confirmed in the PZ?
No, when the pest starts killing trees, it has already widely established, inconspicuously living on fallen or broken trees.
3.6.1. Biological or technical factors affecting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
In spite of quarantine regulations bearing on round wood, wood packaging material and wood products other than paper, the pest is regularly intercepted at ports.
It is very difficult, if not impossible, to successfully eradicate the pest from forest areas after an introduction. All infected trees and tree parts (including pieces of fallen or broken material) have to be detected and removed and as a prevention for the possible spread from the affected area all suitable host plants should be removed in a zone of several km.
Despite sylvicultural control, in areas where it is established, the pest continues to develop outbreaks whenever climatic conditions are favourable.
3.6.2. Control methods
Silvicultural methods: sanitation thinning and clearfelling with rapid removal of the infested material (Stadelmann et al., 2013; Fettig and Hilszczannski, 2015; Grégoire et al., 2015);
Pheromone mass‐trapping was largely used at the end of the 20th century (Bakke, 1989, 1991; Raty et al., 1995), but is presently judged unreliable because of the large dispersal capacity of the pest (Duelli et al., 1997);
Log storage under water sprinkling after windstorms, in order to protect them from bark‐beetle attack and reduce bark‐beetle population growth (Björkhem et al., 1977; Flot and Vautherin, 2002). This could involve millions of m3 (Lindelöw and Schroeder, 2008).
3.7. Uncertainty
Ips typographus has been exhaustively studied. Its biology, ecology, relationships to its hosts and to natural enemies are well understood. Uncertainty does not affect most of the categorisation conclusions. However, the apparently very low capacity of the pest to invade new areas across a geographic barrier needs to be investigated further.
4. Conclusions
Ips typographus meets the criteria assessed by EFSA for consideration as a potential protected zone quarantine pest, for the territory of the protected zones: Ireland and the United Kingdom.
Table 5.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Protected Zone quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1) | The identity of the pest is established. It can be identified to species using conventional entomological keys | The identity of the pest is established. It can be identified to species using conventional entomological keys | None |
| Absence/presence of the pest in the EU territory (Section 3.2) | I. typographus is present and widely distributed in 22 EU MSs. The protected zones, Ireland and the United Kingdom, are free from the pest | I. typographus is present and widely distributed in the EU, it has been reported from 22 EU MSs. The protected zones, Ireland and the United Kingdom, are free from the pest | None |
| Regulatory status (Section 3.3) |
The pest is currently officially regulated by 2000/29/EC on plants of Abies, Larix, Picea, Pinus and Pseudotsuga, over 3 m in height, other than fruit and seeds, wood of conifers (Coniferales) with bark, isolated bark of conifers I. typographus is regulated as a quarantine pest in protected zones (Annex IIB): Ireland, United Kingdom |
The pest is currently officially regulated by 2000/29/EC on plants of Abies, Larix, Picea, Pinus and Pseudotsuga, over 3 m in height, other than fruit and seeds, wood of conifers (Coniferales) with bark, isolated bark of conifers I. typographus is regulated as a quarantine pest in protected zones (Annex IIB): Ireland, United Kingdom |
None |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) |
Entry: The pest is already established in 22 MSs. Since entry by natural spread from EU areas where the pest is present is possible, only isolated areas (e.g. islands) can be long‐term protected zones Establishment: The climate of the EU Protected Zones is similar to that of MSs where I. typographus is established, and the pest's main host plants are present Spread: Adults can disperse naturally. They can fly over tens of kilometres or even more. The pest can also spread by human assistance, e.g. with the transportation of wood, wood chips, bark, wood packaging material and dunnage of conifers |
Plants for planting are not a pathway; therefore, other criteria for consideration as regulated non‐quarantine pest do not need to be assessed | There are 16 records of interceptions on ‘unclassified plant material’ in the Europhyt database |
| Potential for consequences in the EU territory (Section 3.5) | The pest is known to have killed millions of trees, after triggering events such as storms or dry summers | Plants for planting are not a pathway; therefore, other criteria for consideration as regulated non‐quarantine pest do not need to be assessed |
None This is illustrated by the pest's past history in the EU |
| Available measures (Section 3.6) |
In isolated areas (e.g. islands) that cannot be reached by natural spread, measures can be put in place to prevent the introduction with wood, wood products, wood chips, bark and plants for planting. Debarking wood and heat treatment of wood, bark and chips, and inspection of plants for planting are effective. If limited entry occurs nevertheless, spread and establishment are unlikely to occur When such geographical barriers do not exist, there is no possibility to prevent the entry, establishment and spread of I. typographus |
Plants for planting are not a pathway; therefore, other criteria for consideration as regulated non‐quarantine pest do not need to be assessed |
Geographic barrier Entry: inspections are difficult Establishment and spread: wide dispersal upon emergence and very high Allee threshold of this mass‐attacking beetle No geographic barrier none |
| Conclusion on pest categorisation | All criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met | The criteria for considering I. typographus as a potential regulated non‐quarantine pest are not met since plants for planting are not a pathway | Listed above |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | Further analysis of the effects of wide dispersal upon emergence and high Allee thresholds could lower the uncertainties regarding the risks of establishment and spread | ||
Abbreviations
- C‐SMFA
constrained spatial multi‐scale frequency analysis
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization of the United Nations
- IPPC
International Plant Protection Convention
- MS
Member State
- PLH
EFSA Panel on Plant Health
- RPP
Relative probability of presence
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Kertész V, Aukhojee M and Grégoire J‐C, 2017. Scientific Opinion on the pest categorisation of Ips typographus . EFSA Journal 2017;15(7):4881, 23 pp. 10.2903/j.efsa.2017.4881
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00195
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Gregoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Adopted: 24 May 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO
Figure 2: © European Union, 2017. Reuse is authorised, provided the source is acknowledged
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
Although the leading taxonomists in the 2000s (Wood, 1982; Bright and Skidmore, 2002) still considered the Scolytidae to be a family distinct from the Curculionidae according to morphological criteria, modern phylogenetics supports the position of scolytine beetles (Scolytinae) within the family Curculionidae (Knížek and Beaver, 2004; Hulcr et al., 2015). This is reflected by the growing number of citations in Scopus (online) referring to Scolytinae (18 in 1990 vs 177 in 2016), as opposed to citations referring to Scolytidae (50 in 1990 vs 15 in 2016). The Scolytinae includes two subcategories, the ‘bark beetles’ which live in the phloem, and the ‘ambrosia beetles’ which live in the sapwood.
References
- Anderbrant O, 1990. Gallery construction and oviposition of the bark beetle Ips typographus (Coleoptera: Scolytidae) at different breeding densities. Ecological Entomology, 15, 1–8. [Google Scholar]
- Bakke A, 1970. Evidence of a population aggregating pheromone in Ips typographus (Coleoptera: Scolytidae). Contributions from Boyce Thompson Institute, 24, 309–310. [Google Scholar]
- Bakke A, 1976. Spruce bark beetle, Ips typographus: pheromone production and field response to synthetic pheromones. Naturwissenschaften, 63, 92–92. [DOI] [PubMed] [Google Scholar]
- Bakke A, 1989. The recent Ips typographus outbreak in Norway ‐ experiences from a control program. Holarctic Ecology, 12, 515–519. [Google Scholar]
- Bakke A, 1991. Socioeconomic aspects of an integrated‐pest‐management program in Norway. Forest Ecology and Management, 39, 299–303. [Google Scholar]
- Balachowsky A, 1949. Faune de France. 50. Coleoptères Scolytides. Lechevalier, Paris. 320 pp. [Google Scholar]
- Bentz BJ and Jönsson AM, 2015. Modeling bark beetle responses to climate change. In: Vega FE and Hofstetter RW (eds.). Bark Beetles. Biology and Ecology of Native and Invasive Species. Elsevier, pp. 333–353. [Google Scholar]
- Birgersson G, Schlyter F, Lfqvist J and Bergström G, 1984. Quantitative variation of pheromone components in the spruce bark beetle Ips typographus from different attack phases. Journal of Chemical Ecology, 10, 1029–1055. [DOI] [PubMed] [Google Scholar]
- Björkhem U, Dehlen R, Lundin L, Nilsson S, Olsson MT and Regnander J, 1977. Storage of Pulpwood Under Water Sprinkling ‐ Effects on Insects and the Surrounding Area. Royal College of Forestry, Department of Operational Efficiency, Research Notes 107. [Google Scholar]
- Bossard M, Feranec J and Otahel J, 2000. CORINE land cover technical guide ‐ Addendum 2000. Technical Report 40, European Environment Agency. INRMM‐MiD:13106045. Available online: https://www.eea.europa.eu/ds_resolveuid/032TFUPGVR
- Bright DE and Skidmore RE, 2002. A catalogue of Scolytidae and Platypodidae (Coleoptera), Supplement 2 (1995–1999). NRC Research Press, Ottawa, Canada, 523 pp. [Google Scholar]
- Brockerhoff EG, Knížek M and Bain J, 2003. Checklist of indigenous and adventive bark and ambrosia beetles (Curculionidae: Scolytinae and Platypodinae) of New Zealand and interceptions of exotic species (1952–2000). New Zealand Entomologist, 26, 29–44. [Google Scholar]
- Büttner G, Kosztra B, Maucha G and Pataki R, 2012. Implementation and achievements of CLC2006. Technical Report European Environment Agency, INRMM‐MiD:14284151. Available online: http://www.eea.europa.eu/ds_resolveuid/GQ4JECM8TB
- CABI Invasive Species Compendium, online. Datasheet report for Ips typographus (eight‐toothed bark beetle). Available online: http://www.cabi.org/isc/datasheetreport?dsid=28843#20127201272 [Accessed: 30 April 2017]
- Chararas C, 1962. Etude biologique des scolytides des conifères. Encyclopedie Entomologique, Ser. A, Nr. 38, Paul Lechevalier, Paris.
- Christiansen E, 1985. Ips/Ceratocystis‐infection of Norway spruce: what is a deadly dosage? 1. Zeitschrift für angewandte Entomologie, 99, 6–11. [Google Scholar]
- Christiansen E and Bakke A, 1997. Does drought really enhance Ips typographus epidemics? A Scandinavian perspective. In: Grégoire JC, Liebhold AM, Stephen FM, Day KR, Salom SM (eds.). Proceedings: Integrating cultural tactics into the management of bark beetle and reforestation pests. USDA Forest Service General Technical Report NE‐236., 163–171. [Google Scholar]
- Duelli P, Zahradnik P, Knizek M and Kalinova B, 1997. Migration in spruce bark beetles (Ips typographus L.) and the efficiency of pheromone traps. Journal of Applied Entomology, 121, 297–303. 10.1111/j.1439-0418.1997.tb01409.x [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2093/j.efsa.2010.1495 [DOI] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 28 February 2017]
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- Fettig CJ and Hilszczannski J, 2015. Management strategies for bark beetles in conifer forests. In: Vega FE and Hofstetter RW (eds.). Bark Beetles. Biology and Ecology of Native and Invasive Species. Elsevier, pp. 555–584. [Google Scholar]
- Flot JL and Vautherin P, 2002. Des stocks de bois a` conserver en forêt ou hors forêt. Revue Forestière Française 136–144, (special issue) “Après les tempêtes”.
- Forsse E and Solbreck C, 1985. Migration in the bark beetle Ips typographus L.: duration, timing and height of flight. Zeitschrift Für Angewandte Entomologie, 100, 47–57. [Google Scholar]
- Grégoire JC and Evans HF, 2004. Damage and control of BAWBILT organisms – an overview. In: Lieutier F, Day K, Battisti A, Grégoire JC and Evans H (eds.). Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis. Kluwer, Dordrecht. pp. 19–37. [Google Scholar]
- Grégoire JC, Raffa KF and Lindgren BS, 2015. Economics and politics of bark beetles. In: Vega FE and Hofstetter RW (eds.). Bark Beetles. Biology and Ecology of Native and Invasive Species. Elsevier, pp. 585–613. [Google Scholar]
- Grüne S, 1979. Brief Illustrated Key to European Bark Beetles. M. & H Schaper, Hannover, 182 pp. [Google Scholar]
- Haack RA, 2001. Intercepted Scolytidae (Coleoptera) at US ports of entry: 1985–2000. Integrated Pest Management Reviews, 6, 253–282. [Google Scholar]
- Hulcr J, Atkinson T, Cognato AI, Jordal BH and McKenna DD, 2015. Morphology, taxonomy and phylogenetics of bark beetles. In: Vega FE and Hofstetter RW (eds.). Bark Beetles. Biology and Ecology of Native and Invasive Species. Elsevier, pp. 41–84. [Google Scholar]
- Jönsson A, Appelberg G, Harding S and Bärring L, 2009. Spatio‐temporal impact of climate change on the activity and voltinism of the spruce bark beetle, Ips typographus. Global Change Biology, 15, 486–499. [Google Scholar]
- Jönsson AM, Harding S, Krokene P, Lange H, Lindelöw Å, Økland B, Ravn HP and Schroeder LM, 2011. Modelling the potential impact of global warming on Ips typographus voltinism and reproductive diapause. Climatic Change, 109, 695–718. 10.1007/s10584-011-0038-4 [DOI] [Google Scholar]
- Kausrud K, Økland B, Skarpaas O, Grégoire JC, Erbilgin N and Stenseth NC, 2011. Population dynamics in changing environments: the case of an eruptive forest pest species. Biological Reviews, 87, 34–51. 10.1111/j.1469-185X.2011.00183 [DOI] [PubMed] [Google Scholar]
- Kautz M, Meddens AJH, Hall RJ and Arneth A, 2016. Biotic disturbances in Northern Hemisphere forests ‐ a synthesis of recent data, uncertainties and implications for forest monitoring and modelling. Global Ecology and Biogeography, 26, 533–552. 10.1111/geb.12558 [DOI] [Google Scholar]
- Kenis M, Wermelinger B and Grégoire JC, 2004. Research on parasitoids and predators of Scolytidae in living trees in Europe – a review. In: Lieutier F, Day K, Battisti A, Grégoire JC, Evans H (eds.). Bark and Wood Boring Insects in Living Trees in Europe, A Synthesis. Kluwer, Dordrecht. pp. 237–290. 10.1007/978-1-4020-2241-8_11 [DOI] [Google Scholar]
- Kirisits T, 2004. Fungal associates of European bark beetles with special emphasis on the Ophiostomatoid fungi. In: Lieutier F, Day K, Battisti A, Grégoire JC, Evans H (eds.). Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis. Kluwer, Dordrecht. pp. 181–235. 10.1007/978-1-4020-2241-8_11 [DOI] [Google Scholar]
- Knížek M and Beaver R, 2004. Taxonomy and systematics of bark and ambrosia beetles. In: Lieutier F, Day K, Battisti A, Grégoire JC, Evans H (eds.). Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis. Kluwer, Dordrecht. pp. 41–54. 10.1007/978-1-4020-2241-8_11 [DOI] [Google Scholar]
- Krehan H, Steyrer G and Hoch G, 2012. Borkenkäfer‐Situation 2011: Schäden deutlich geringer. Forstschutz Aktuell, 56, 11–15. [Google Scholar]
- Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T and Safranyik L, 2008. Mountain pine beetle and forest carbon feedback to climate change. Nature, 452, 987–990. [DOI] [PubMed] [Google Scholar]
- Lindelöw A and Schroeder ML, 2008. The storm “Gudrun” and the spruce bark beetle in Sweden. Forstschutz Aktuell, 44, 5–7. [Google Scholar]
- Linnakoski R, Mahilainen S, Harrington A, Vanhanen H, Eriksson M, Mehtätalo L, Pappinen A and Wingfield MJ, 2016. Seasonal succession of fungi associated with Ips typographus beetles and their phoretic mites in an outbreak region of Finland. PLoS ONE, 11, e0155622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobinger G, 1996. Variations in sex ratio during an outbreak of Ips typographus (Col., Scolytidae) in Southern Bavaria. Anzeiger für Schädlingskunde, Pflanzenschutz, Umweltschutz, 69, 51–53. [Google Scholar]
- Marini L, Økland B, Jönsson AM, Bentz B, Carroll A, Forster B, Grégoire JC, Hurling R, Nageleisen LM, Netherer S, Ravn HP, Weed A and Schroeder M, 2016. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography, 40, 1–10. 10.1111/ecog.02769 [DOI] [Google Scholar]
- Mayer F, Piel FB, Cassel‐Lundhagen A, Kirichenko N, Grumiau L, Økland B, Bertheau C, Grégoire JC and Mardulyn P, 2015. Comparative multilocus phylogeography of two Palaearctic spruce bark beetles: influence of contrasting ecological strategies on genetic variation. Molecular Ecology, 24, 1292–1310. 10.1111/mec.13104 [DOI] [PubMed] [Google Scholar]
- Meier F, Engesser R, Forster B, Odermatt O and Angst A, 2013. Protection des forêts—vue d'ensemble 2012. Institut fédéral de recherches sur la forêt, la neige et le paysage WSL, Birmensdorf.
- Mills NJ, 1983. The natural enemies of scolytids infesting conifer bark in Europe in relation to the biological control of Dendroctonus spp. In Canada. Biocontrol News and Informations, 4, 305–328. [Google Scholar]
- Mills NJ, 1985. Some observations on the role of predation in the natural regulation of Ips typographus populations. Zeitschrift Für Angewandte Entomologie, 99, 209–215. [Google Scholar]
- Montano V, Bertheau C, Dolezal P, Krumböck S, Okrouhlík J, Stauffer C and Moodley Y, 2016. How differential management strategies affect Ips typographus L. dispersal. Forest Ecology and Management, 360, 195–204. 10.1016/j.foreco.2015.10.037 [DOI] [Google Scholar]
- Raty L, Drumont A, De Windt N and Grégoire JC, 1995. Mass trapping of the spruce bark beetle Ips typographus L.: traps or trap trees? Forest Ecology and Management, 78, 191–205. 10.1016/0378-1127(95)03582-1 [DOI] [Google Scholar]
- de Rigo D, Caudullo G, Busetto L and San‐Miguel‐Ayanz J, 2014. Supporting EFSA assessment of the EU environmental suitability for exotic forestry pests: final report. EFSA Supporting Publications 2014:11 (3), EN‐434+, INRMM‐MiD:13114000. 10.2903/sp.efsa.2014.en-434 [DOI]
- de Rigo D, Caudullo G, Houston Durrant T and San‐Miguel‐Ayanz J, 2016. The European Atlas of Forest Tree Species: modelling, data and information on forest tree species. In: San‐Miguel‐Ayanz J, De Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds.). European Atlas of Forest Tree Species. Publication Office of the European Union, Luxembourg. pp. e01aa69+. https://w3id.org/mtv/FISE-Comm/v01/e01aa69 [Google Scholar]
- de Rigo D, Caudullo G, San‐Miguel‐Ayanz J and Barredo JI, 2017. Robust modelling of the impacts of climate change on the habitat suitability of forest tree species. Publication Office of the European Union, Luxembourg, 58 pp. ISBN:978‐92‐79‐66704‐6, 10.2760/296501, INRMM‐MiD:14314400. [DOI]
- Sallé A, Monclus R, Yart A, Garcia J, Romary P and Lieutier F, 2005. Fungal flora associated with Ips typographus: frequency, virulence, and ability to stimulate the host defence reaction in relation to insect population levels. Canadian Journal of Forest Research, 35, 365–373. [Google Scholar]
- San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds.), 2016. European Atlas of Forest Tree Species. Publication Office of the European Union, Luxembourg. ISBN: 978‐92‐79‐36740‐3, 10.2788/4251, Available online: https://w3id.org/mtv/FISE-Comm/v01/ [DOI] [Google Scholar]
- Schedl KE, 1981. 91. Familie: Scolytidae (Borken‐ und Ambrosiakäfer). In: Freude H, Harde KW, Lohse GA (eds.). Die Käfer Mitteleuropas. Goecke & Evers, Krefeld. pp. 34–99. [Google Scholar]
- Schelhaas MJ, Nabuurs GJ and Schuck A, 2003. Natural disturbances in the European forests in the 19th and 20th centuries. Global Change Biology, 9, 1620–1633. [Google Scholar]
- Schlyter F and Anderbrant O, 1993. Competition and niche separation between two bark beetles: existence and mechanisms. Oikos, 68, 437–447. [Google Scholar]
- Schopf A, 1989. The effect of photoperiod on the induction of the imaginal diapause of Ips typographus L. (Coleoptera Scolytidae). Journal of Applied Entomology, 107, 275–288. [Google Scholar]
- Scopus , online. General search: Scolytinae. Available online: https://www.scopus.com/home.uri. [Accessed: 28 March 2017]
- Seidl R and Rammer W, 2016. Climate change amplifies the interactions between wind and bark beetle disturbances in forest landscapes. Landscape Ecology. 10.1007/s10980-016-0396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim H, 1986. Species of Ophiostomataceae isolated from Picea abies infested by the bark beetle Ips typographus . Nordic Journal of Botany, 6, 199–207. 10.1111/j.1756-1051.1986.tb00874.x [DOI] [Google Scholar]
- Solheim H, Krokene P and Långström B, 2001. Effects of growth and virulence of associated blue‐stain fungi on host colonization behaviour of the pine shoot beetles Tomicus minor and T. piniperda. Plant Pathology, 50, 111–116. [Google Scholar]
- Stadelmann G, Bugmann H, Meier F, Wermelinger B and Bigler C, 2013. Effects of salvage logging and sanitation felling on bark beetle (Ips typographus L.) infestations. Forest Ecology and Management, 305, 273–281. 10.1016/j.foreco.2013.06.003 [DOI] [Google Scholar]
- Stauffer C, Lakatos F and Hewitt GM, 1999. Phylogeography and postglacial colonization routes of Ips typographus L. (Coleoptera, Scolytidae). Molecular Ecology, 8, 763–773. [Google Scholar]
- Steyrer G and Krehan H, 2009. Borkenkäfer‐Kalamität 2008: ist ein weiterer Rückgang wahrscheinlich? Forstschutz Aktuell, 46, 9–15. [Google Scholar]
- Viiri H and Lieutier F, 2004. Ophiostomatoid fungi associated with the spruce bark beetle, Ips typographus, in three areas in France. Annals of forest science, 61, 215–219. [Google Scholar]
- Wermelinger B, 2004. Ecology and management of the spruce bark beetle Ips typographus ‐ a review of recent research. Forest Ecology and Management, 202, 67–82. 10.1016/j.foreco.2004.07.018 [DOI] [Google Scholar]
- Wood SL, 1982. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Brigham Young University, Provo, Utah. 1359 pp. [Google Scholar]
- Yamaoka Y, Wingfield MJ, Takahashi I and Solheim H, 1997. Ophiostomatoid fungi associated with the spruce bark beetle Ips typographus f. aponicus [sic] in Japan. Mycological Research, 101, 1215–1227. [Google Scholar]


