Abstract
This scientific opinion addresses animal welfare aspects of slaughtering of livestock pregnant animals. Term of Reference (ToR) 1 requested assessment of the prevalence of animals slaughtered in a critical developmental stage of gestation when the livestock fetuses might experience negative affect. Limited data on European prevalence and related uncertainties necessitated a structured expert knowledge elicitation (EKE) exercise. Estimated median percentages of animals slaughtered in the last third of gestation are 3%, 1.5%, 0.5%, 0.8% and 0.2% (dairy cows, beef cattle, pigs, sheep and goats, respectively). Pregnant animals may be sent for slaughter for health, welfare, management and economic reasons (ToR2); there are also reasons for farmers not knowing that animals sent for slaughter are pregnant. Measures to reduce the incidence are listed. ToR3 asked whether livestock fetuses can experience pain and other negative affect. The available literature was reviewed and, at a second multidisciplinary EKE meeting, judgements and uncertainty were elicited. It is concluded that livestock fetuses in the last third of gestation have the anatomical and neurophysiological structures required to experience negative affect (with 90–100% likelihood). However, there are two different possibilities whether they perceive negative affect. It is more probable that the neurophysiological situation does not allow for conscious perception (with 66–99% likelihood) because of brain inhibitory mechanisms. There is also a less probable situation that livestock fetuses can experience negative affect (with 1–33% likelihood) arising from differences in the interpretation of the fetal electroencephalogram, observed responses to external stimuli and the possibility of fetal learning. Regarding methods to stun and kill livestock fetuses at slaughter (ToR4), sets of scenarios and respective actions take account of both the probable and less probable situation regarding fetal ability for conscious perception. Finally, information was collated on methods to establish the dam's gestational stage based on physical features of livestock fetuses (ToR5).
Keywords: Slaughter, pregnant animals, livestock species, animal welfare, conscious perception, pain, fetus
Summary
Observations have indicated that slaughtering of pregnant animals is not an isolated phenomenon and Regulation (EC) No 1099/2009 on the protection of animals at the time of killing does not contain any provisions with regard to the protection of fetuses of livestock species when a pregnant dam is slaughtered. Four European Member States (Germany, the Netherlands, Sweden and Denmark) requested the EFSA Panel on Animal Health and Animal Welfare (AHAW) to deliver a Scientific Opinion concerning the animal welfare aspects in respect of the slaughter or killing of pregnant livestock species (cattle, pigs, sheep, goats, horses). In particular, the European Food Safety Authority (EFSA) was requested to address the following five Terms of Reference (ToRs): (1) to assess the prevalence of pregnant livestock animals slaughtered in a critical developmental stage at which livestock fetuses might experience suffering; (2) to assess the reasons why pregnant animals are slaughtered in the critical phase of gestation and propose recommendations to reduce the number of animals slaughtered while pregnant in this stage; (3) to assess the available scientific evidence on the capacity of livestock fetuses to experience pain; (4) to provide scientific advice on methods suitable for stunning and killing of fetuses or neonates of the main livestock species when a pregnant dam has been delivered to the slaughterhouse at a critical phase of gestation, and (5) to provide scientific advice on methods suitable for estimating the age of fetuses of the main livestock species at the slaughterhouse after the dam has been slaughtered.
This mandate has been chosen as a case study for testing the approaches proposed in the draft Guidance on Uncertainty in EFSA scientific assessment. Following provisions of the uncertainty guidance, uncertainty as regards ToR1 and ToR3 was quantified using expert knowledge elicitation guided by literature evaluation. The uncertainty assessment was addressed differently for ToR1 and ToR3. For ToR1, the prevalence of slaughtered pregnant animals was elicited by an Expert Knowledge Elicitation meeting (referred to as EKE 1). This resulted in ‘median estimates’ for the prevalence (e.g. 3% dairy cows in the last term of gestation) accompanied by a probability distribution range (e.g. from 9% to 27%) that gives the level of uncertainty. For ToR3, expert knowledge was elicited on statements, e.g. that livestock fetuses have the anatomical structures required (and further statements derived from the logical model; see below). The answers were formulated as likelihoods of the statement being true with the distribution of the answers providing the certainty distribution (e.g. from 5% to 40% likelihood that a certain statement is true). From the range of likelihood values and further interpretation of the literature, a qualitative translation of the uncertainty was derived (e.g. very likely).
The remaining ToRs were addressed by the Panel through appraisal of the scientific literature.
For ToR1, several possible sources of information including literature review and reports from the Member States were investigated but the information retrieved was limited. Therefore, a survey in 10 Member States was carried out, addressed to slaughterhouse operators, asking for estimates of the proportion of total pregnancies in slaughtered animals and the proportion of pregnant animals found in the different terms of gestation in 2015. The experts who performed the survey were then invited to an Expert Knowledge Elicitation (referred to as EKE 1) meeting with the aim to generate probability judgements around the prevalence estimates per livestock species in Europe. Outcomes of the EKE 1 meeting are estimates for the median prevalence of pregnant animals slaughtered for each species as well as the 50% and 98% uncertainty range. Estimated median percentages of all mature female animals slaughtered while pregnant in Europe were 16%, 11%, 6%, 10% and 4% (dairy cows, beef cattle, pigs, sheep and goats, respectively). The respective estimated median percentages of all mature female animals slaughtered in the last third of gestation were 3%, 1.5%, 0.5%, 0.8% and 0.2% (dairy cows, beef cattle, pigs, sheep and goats, respectively). Estimates for horses are not given in the opinion due to lack of information.
For ToR2, literature was investigated and a discussion was held at the EKE 1 meeting. It was concluded that the reasons for unknowingly sending pregnant animals for slaughter include (i) lack of supervision of breeding, especially in extensive systems, (ii) absence or failure of pregnancy diagnosis, and (iii) poor record keeping or loss of information in the trading chain. The reasons for knowingly sending pregnant animals for slaughter can be categorised into (i) health and welfare benefits, (ii) management advantages and (iii) economic necessity or benefit. To reduce unplanned slaughtering, it is recommended to improve the health status of animals on farm and to reduce unplanned pregnancies by single sex housing and supervised breeding. In addition, the pregnancy status of all animals should be established before they are sent for slaughter; for this purpose, a decision tree to support farmers has been developed. Information about insemination and pregnancy diagnosis should be required in documentation accompanying animals at the time of sale. Finally, further research should be undertaken to improve pregnancy diagnostic test accuracy and feasibility, especially for the diagnosis of later stages of pregnancy.
For ToR3, the scope was not exclusively on pain, but also on other types of negative affect. The topic was subdivided into (i) anatomical structures required, (ii) the neurophysiological situation (e.g. inhibitory and excitatory systems) and (iii) the response of the livestock fetuses to specified stunning and slaughter conditions. Based on literature review, a logical model was created showing how the above‐mentioned subquestions link together to address the overall question on the capacity of livestock fetuses to experience pain and other negative affect. Due to divergent views in the literature, in a second EKE (referred to as EKE 2) meeting (i) probability distributions for the propositions in the subquestions to be true were elicited, and (ii) the level of uncertainty around each subquestion was expressed through a standardised methodology. The outcomes of the EKE 2 meeting were evaluated by the AHAW Panel in the context of additional findings from the literature and further discussion with some of the EKE 2 experts to generate final conclusions for this opinion. These following conclusions, expressed using an approximate probability scale, are obtained:
It is very likely to extremely likely (i.e. with 90–100% likelihood) that livestock fetuses in the last third of gestation have the anatomical and neurophysiological structures/correlates for experiencing pain and/or other forms of discomfort.
Based on the available scientific evidence and expert opinion, it cannot be determined with certainty whether livestock fetuses are capable of cortically based conscious perception, and therefore, there exist two different situations. It is more probable that ‘the neuro‐physiological situation of the livestock fetuses in the last third of gestation (i.e. inhibitory and excitatory systems) does not allow for cortically based conscious perception’ (this statement is likely to very likely correct, i.e. with 66–99% likelihood). This view is supported by the presence of adenosine‐mediated brain inhibitory (neuroprotective) mechanisms operating in utero, demonstrated by electroencephalogram (EEG) records, the low level of fetal brain oxygen, the predominance of sleep like states in the fetal EEG and the lack of any direct evidence of cortically based conscious perception. There is also a less probable situation that ‘the neuro‐physiological situation of the livestock fetuses in the last third of gestation does allow for cortically based conscious perception’ (this statement is unlikely to very unlikely to be correct, i.e. with 1–33% likelihood). The reasons on which this less probable situation is based relate to the lack of any direct evidence proving that livestock fetuses are incapable of cortically based conscious perception and to differences in the interpretation of indirect evidence relating to fetal EEG (e.g. significance of transitional EEG), observed fetal behavioural and physiological responses to external stimuli and the possibility of fetal learning (conscious learning versus conditioned responses).
There is no direct evidence demonstrating the existence of subcortical awareness in livestock fetuses and the existence of a hypothesised raw basic affect. However, even if this were to exist, it is unlikely to very unlikely (i.e. with 1–33% likelihood) that the neurophysiological situation of the livestock fetuses in the last third of gestation (i.e. inhibitory and excitatory systems) would allow for subcortically based conscious perception.
It is very likely to extremely likely (i.e. with 90–100% likelihood) that livestock fetuses show measurable responses to extreme hypercapnic hypoxia, mechanical stimulation and electrical current. Probabilities are given in the opinion that livestock fetuses are subjected to each of these stimuli during slaughtering of the dam.
However, since all slaughtering procedures involve a maternal circulatory collapse and rapid fetal hypoxia increasing adenosine mediated brain inhibitory mechanisms, it is unlikely to very unlikely (i.e. with 1–33% likelihood) that changes/responses occurring during stunning and bleeding of the dam are associated with pain or other negative affect in the livestock fetuses.
For ToR4, a set of scenarios and respective actions has been developed for both the assumptions that livestock fetuses might or might not perceive pain or other negative affect: (1) if it is accepted that livestock fetuses are not able to experience pain or other negative affect (i.e. the above indicated 66–99% likelihood), the fetus should be left undisturbed in utero for 30 min after the death of the dam by which time it will be dead. If the livestock fetus is exteriorised before this time, it should be stunned and killed using approved methods for neonates in accordance with Reg 1099/2009); (2) if it is accepted that livestock fetuses might experience pain or other negative affect (i.e. the above indicated 1–33% likelihood), the fetus should be killed in situ together with the dam by an overdose of anaesthetic drug if pregnancy is detected at arrival in the slaughterhouse. If the dam is already dead at the time of detection, the fetus should immediately be exteriorised, stunned and killed using approved methods.
Regarding ToR5, the determination of gestational stage after post‐mortem detection can be based on physical/morphological characteristics of the livestock fetuses as suggested in the opinion. Due to variation between breeds, preference should be given to external features such as hair cover over linear morphological measures. For the allocation to a given term, criteria towards the midstage are more reliable indicators than those suggested for the exact boundaries between gestational stages, due to the limitations of available data.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Request for a Scientific Opinion concerning the animal welfare aspects in respect of the slaughter or killing of pregnant livestock species (cattle, pigs, sheep, goats, horses).
Recent scientific studies indicate that the slaughter of pregnant animals in the European Union (EU) is not an isolated phenomenon (Lücker et al., 2003; Di Nicolo, 2006; Riehn et al., 2010; Braunmiller, 2015). These results contradict the statements of the Scientific Committee on Veterinary Measures relating to Public Health (SCVPH) that assumed in its opinion on the potential risks of hormonal residues in beef to human health (1999) that heifers are only slaughtered in exceptional cases.
Scientific studies on the sensation of pain in fetuses come to controversial results: Some of these studies say that, not being in an awake state and lacking appropriate cortex participation, fetuses have no faculty of sensation and perception as long as they have not started breathing following their development from the uterus (Mellor and Gregory, 2003; Mellor and Stafford, 2004; Mellor et al., 2005; Mellor and Diesch, 2006; Mellor, 2010). The Terrestrial Animal Health Code of the OIE (2008) also recommends, among other things, that from an animal welfare point of view, fetuses should remain in the unopened uterus until death. This concept of ‘fetal unconsciousness’ with regard to the sensation and perception of, e.g. pain is rejected by other scientists, however. According to Merker (2007), a conscious perception of sensory stimuli also takes place in the brain stem. Bellieni and Buonocore (2012) indicate that a fetal sensation of pain is present during the last third of the pregnancy at the latest.
Directive 2010/63/EC1 also covers fetal forms of mammals, as there is evidence that they are at an increased risk of experiencing pain, suffering and distress in the last third of the period of their development (cf. recital no 9).
In contrast to this, Regulation (EC) No 1099/20092 does not contain any provisions with regard to the protection of unborn animals. At the same time, Regulation (EC) No 1099/2009 does not provide the Member States with a possibility to adopt stricter national regulations for the killing of animals (here: the fetuses) in slaughterhouses either.
If it can be assumed from an animal welfare point of view that fetuses are able to experience pain and suffering from a certain developmental stage onwards, provisions for the protection of unborn animals should not only regard laboratory animals. Appropriate regulations should rather also be included in the EU legislation on the protection of animals at the time of killing.
It should be clarified at EU level how the slaughter of pregnant animals from a critical phase of gestation onwards (after which the fetus is considered to be able to perceive pain) can be avoided. As it can be assumed that even in the case of a potential ban on the slaughter of pregnant animals a certain part of slaughter animals will be falsely declared as not pregnant it should be clarified how the fetuses should be killed after the killing of the dam. Furthermore, comparable animal welfare problems with regard to dealing with fetuses also exist in the case of emergency slaughters or killings of the dam for animal health reasons.
Above all, we consider it necessary to review the current practice in slaughterhouses to leave developed fetuses in the uterus until death and to determine appropriate stunning and killing methods for fetuses of dams slaughtered in the critical phase of gestation. As it can be assumed from an animal welfare point of view that fetuses in general are able to experience pain and suffering from a critical phase of gestation onwards, the scientific opinion should cover the main livestock species (cattle, pigs, sheep, goats, horses).
Therefore, in view of the above, and in accordance with Article 29 of Regulation (EC) No 178/20023, Denmark, Germany, the Netherlands and Sweden ask the European Food Safety Authority (EFSA) for a scientific opinion on the following aspects:
Assess the prevalence of pregnant livestock animals slaughtered in the critical developmental stage after which the fetus is considered to be able to perceive pain in EU Member States and at EU level; the study should include cattle, pigs, sheep, goats, horses; cattle could be feasible due to data being collected under the Livestock Database;
Assess the reasons why pregnant animals are slaughtered in the critical phase of gestation and propose recommendations to reduce the number of animals slaughtered while pregnant in this stage;
Assess the scientific evidence available on the capacity of fetuses to experience pain;
Provide scientific advice on methods suitable for stunning and killing of fetuses or neonates of the main livestock species when a pregnant dam has been delivered to the slaughterhouse at a critical phase of gestation;
Provide scientific advice on methods suitable for estimating the age of fetuses of the main livestock species at the slaughterhouse after the dam has been slaughtered, in order to identify if the fetus has reached the critical developmental stage after which it is considered to be able to perceive pain.
1.2. Interpretation of the Terms of Reference
ToR1 asks to ‘Assess the prevalence of pregnant livestock animals slaughtered in the critical developmental stage after which the fetus is considered to be able to perceive pain’ within the EU.
Prior to the discussions in ToR3 relating to the existence and timing of the critical period, it was decided for the purpose of ToR1 to use the definition previously presented by Directive 2010/63/EC which states that this critical period is represented by the last third of pregnancy.
ToR2 asks to ‘Assess the reasons why pregnant animals are slaughtered in the critical phase of gestation and propose recommendations to reduce the number of animals slaughtered while pregnant in this stage’. This ToR will be addressed taking into consideration three main reasons for slaughtering pregnant animals, namely related to health and welfare, economic or management reasons, as well as lack of knowledge of pregnancy state. The opinion will relate the above three reasons as much as possible to the actual (or estimated) stage of pregnancy. Based on this analysis, approaches to reduce the number of animals slaughtered in late pregnancy can then be proposed.
ToR3 asks to ‘Assess the scientific evidence available on the capacity of fetuses to experience pain’. For a comprehensive assessment, fetal/neonatal physiology, developmental physiology, embryology including expertise from human medicine/life sciences need to be considered, to assess the developmental stage at which the fetus has the anatomical features required to experience pain and the time at which the fetus is aware of painful stimuli. Regarding the assessment of potential pain in fetuses, the scope will be extended also to other negative affect (such as distress and discomfort), which might be experienced following the stunning and slaughter of the dam.
ToR4 asks to ‘provide methods suitable for stunning and killing of fetuses or neonates of the main livestock species when a pregnant dam has been delivered to the slaughterhouse at a critical phase of gestation’. If the evidence assessed for ToR3 conclusively demonstrates that the fetus has no ability to experience pain or other negative welfare consequences prior to birth, then the fetus can be left in the uterus as suggested in a past EFSA Opinion (EFSA, 2004). If the evidence indicates the certainty or possibility that the fetus can experience pain or other negative welfare consequences in utero, the scientific literature about methods for stunning and killing of fetuses at the defined developmental stage will be reviewed.
ToR5 asks to ‘provide scientific advice on methods suitable for estimating the age of fetuses of the main livestock species at the slaughterhouse after the dam has been slaughtered, in order to identify if the fetus has reached the critical developmental stage after which it is considered to be able to perceive pain’. If the evidence conclusively demonstrates that the fetus has no ability to experience pain or other negative welfare consequences prior to birth, then no further action is required for this ToR. If the evidence indicates the certainty or possibility that the fetus can experience pain or other negative welfare consequences in utero, the scientific literature about practical methods to assess the age of the fetus will be reviewed.
Emergency killing for disease control remains out of the remit of this work, because this scientific opinion is focussed on the slaughterhouse situation. However, such emergency killing is a situation in which many pregnant animals might be slaughtered and the most humane methods for dealing with the unborn fetus in such a situation could be extrapolated from the findings of this Opinion.
2. Data and methodologies
2.1. Data
2.1.1. Data for ToR1 (prevalence of pregnant animals at slaughter in EU)
In the first instance, data were collected from the Eurostat Livestock Database on the numbers of cattle, pigs, sheep, goats slaughtered within the EU and associated countries. This database does not record data on slaughtered horses. While it is possible to collect the denominator data on the total number of livestock animals slaughtered in the EU from official data, there is no database which records state of pregnancy. For cattle and horses, it is possible to know the age at slaughter from the Livestock Database but this is not the case for pigs, sheep and goats.
Following a discussion with governmental representatives of Member States and associated countries at the AHAW Network meeting, on 10–11 November 2015, EFSA requested Member States to indicate whether they have any collected data on:
the number of female adult/post‐puberty animals slaughtered;
the number of pregnant animals killed;
the gestational age at which these animals are slaughtered.
Responses were received from 10 countries (Belgium, Estonia, Latvia, Lithuania, the Netherlands, Norway, Portugal, Spain, Sweden and Switzerland).
Furthermore, as a proxy for the number of pregnant animals killed in late pregnancy, EFSA asked Member States for access to records submitted annually to the Commission under Council Regulation (EC) No 1/20054, since the number of animals unfit for transport should be indicated in such reports and pregnancy beyond 90% of gestation is a condition for animals being unfit for transport. A standard format for these reports has been prescribed either in the Council Regulation (EC) No 1/2005 or in the Commission Implementing Decision 2013/188/EU5. These reports include information on total number of animals checked during any type of transport and whether they were checked during transport, in resting areas or at arrival at slaughter. The regulation requires that no animal shall be transported unless it is fit for the intended journey. Among the various health related issues, an animal is not to be considered fit for transport if it is a pregnant female for whom 90% or more of the expected gestation period has already passed, or females who have given birth in the previous week. However, the reports submitted to the EU Commission include information on total number of non‐compliances out of total number of inspections only, without specifying the reason for non‐compliance but in broad categories (e.g. truck features, missing transport journal, unfit animals).
Due to the fact that reliable information was not retrieved from the above‐mentioned sources (see Section 3.1.1), the feasibility of an ex‐novo data collection was also considered. However, after careful evaluation and discussion, this option was excluded for the lack of representativeness, i.e. to be relevant, a data collection should last at least 1 year, to reflect the variations between different periods (e.g. due to the seasonality of breeding in the sheep sector).
Therefore, it was decided to design a survey, which was outsourced to 10 national scientific contact points, set up by the Regulation 1099/2009, or to research institutions in the following EU countries: Sweden, Italy, France, Spain, Romania, Poland, Greece, Ireland, the UK and Belgium. In each country, the contractor convenience sampled 10 slaughterhouses – four for cattle, three for pigs, two for sheep, one for goats and one for horses, when possible.
The surveys were carried out using a questionnaire based on the on‐going German project S!GN6 investigating the proportion of pregnancies in slaughtered animals and the reasons for sending pregnant animals for slaughter. The questionnaire consisted of six questions for which the slaughterhouse operators were asked to provide either information relating to 2015 from slaughterhouse records or personal judgements according to their experience:
the estimated number of adult/post‐puberty female animals (cattle, pigs, sheep, goats, horses) that were slaughtered in 2015 in their facilities.
the estimated number of female animals (cattle, pigs, sheep, goats, horses) that were found pregnant when they were slaughtered in their facilities in 2015.
any particular action foreseen in the surveyed facility when a pregnant animal has been slaughtered or if there is any particular action applied for managing the fetuses.
the estimated proportion of fetuses per gestational age category after killing of the dam and, if present, the protocol used for establishing the gestational age. To facilitate the respondents’ answer to the first part of this question, the categorisation tool used in the German project S!GN was reproduced in the questionnaire.
The surveys were performed in the period February–April 2016.
The questionnaire was also shared with the other Member States who requested the mandate, so that they could use it for their ongoing national projects on this topic.
2.2. Uncertainty
The EFSA's Scientific Committee is developing a guidance document (EFSA, under development) to offer a toolbox of methodologies – both quantitative and qualitative – for analysing scientific uncertainties in all its scientific assessments. Through the application of these tools EFSA aims to give decision‐makers a clearer picture of the scientific uncertainties affecting each assessment.
This mandate has been chosen as a case study for testing the applicability of the approach proposed in the draft Guidance on Uncertainty in the EFSA scientific assessment. The experience gained with this specific risk assessment and the other case‐studies identified in each Unit in EFSA will be used to fine‐tune the Guidance document.
In this mandate, it was decided to apply approaches from the uncertainty guidance to ToR1 and ToR3. In both cases, an Expert Knowledge Elicitation was performed and uncertainty was quantified by appropriate statistical methodology (see below). The other ToRs were addressed by the Panel through appraisal of the scientific literature.
2.3. Methodologies
2.3.1. Methodology for ToR1 (prevalence of pregnant animals at slaughter)
Several methods have been used to gather scientific publications, reports and official documents relevant for this opinion. A literature search was conducted on Web of Science. Detailed information on the literature search performed is provided in Appendix A. Literature from outside Europe was not considered relevant. The number of papers with information related to the prevalence of pregnant animals slaughtered was very limited. Additional papers and specific reports from Member States were found on the web (Google search) or provided by the experts of the Working Group (WG).
As described under the section on data for ToR1 (see Section 2.1.1), estimates about the prevalence of pregnant livestock animals slaughtered and the developmental stage were collected in 10 EU countries through surveys requesting slaughterhouse operators to give a personal estimate of prevalence of slaughtered pregnant animals and their distribution across terms of gestation. The experts who performed the surveys were then invited to an Expert Knowledge Elicitation (EKE) meeting that was held on 16–17 June 2016 at EFSA premises. In EKE 1,7 following the EFSA guidance on EKE (EFSA, 2014), the experts were asked, based on the estimates collected through the surveys, to give probability judgements per species about the overall prevalence of pregnant animals slaughtered at European level and respective proportions for terms of gestation. Finally, a collective view on the prevalence and phases of gestation of slaughtered pregnant animals per each livestock species in Europe was agreed upon (see Appendix C for EKE 1 report). At the EKE 1 meeting, the prevalence estimates for pregnancies distributed along the three terms of gestation were obtained as prevalence in each term of gestation out of the total number of female animals slaughtered. After the meeting, the probability distribution of animals in the third term of gestation was recalculated, using the original raw data from individual responses, to provide the percentage of animals in the third term of gestation out of the total number of pregnant female animals. In the opinion, only the latter is reported.
Uncertainty involved in estimating the prevalence is represented using a probability distribution which expresses the likelihood of possible estimates. These distributions were obtained from a structured expert knowledge elicitation considering both available evidence (e.g. existing survey results) and judgements on the remaining uncertainties. Judgements on the prevalence of gestation and conditional prevalence on the gestation stage were combined by stochastic simulation to obtain the unconditional prevalence of the gestation stage, whereby values were drawn randomly from the distribution specified for each input parameter. For these calculations, smooth distributions were fitted to the specified percentiles. The stochastic simulations were repeated at least 10,000 times to generate a probability distribution of outcomes. The distributions of the uncertainty components are characterised by different values and ranges. The median is a central value with equal probability of over‐ or underestimating the actual value. The interquartile range which is bounded by the 1st and 3rd quartile (the 25th and 75th percentile) of the distribution is an interval around the median, where it is as likely that the actual value is inside as it is likely that the actual value is outside that range. This range expresses the precision of the estimation of interest. The wider the interquartile range, the greater is the uncertainty on the estimate. In this opinion, we refer to the interquartile range by using the term ‘50% uncertainty range’.
Following the meeting, a summary report of the proceedings was produced and circulated electronically to all participants to check for accuracy. Corrections were incorporated in a final report provided as an Appendix to the opinion.
2.3.2. Methodology for ToR2 (reasons for slaughtering pregnant animals and recommendations to reduce the number of pregnant animals slaughtered)
This opinion suggests reasons for knowingly and unknowingly slaughtering pregnant animals. A literature search was conducted on Web of Science. Detailed information on the literature search performed is provided in Appendix B. However, the number of papers with information related to the reasons for slaughtering of pregnant animals was very limited. Additional papers and specific reports from Member States were found on the web (Google search) or provided by the experts of the WG.
For all species, the results of the assessment reflect the opinion of the experts of the WG, considering the results from literature reviews and the outcomes from the discussion held at the EKE 1 meeting on factors increasing the prevalence.
A list of potential measures to reduce the number of animals which are pregnant when slaughtered is also proposed. This is based on the knowledge of the experts of the WG and the outcomes from the discussion held at the EKE 1 meeting on factors decreasing the prevalence.
2.3.3. Methodology for ToR3 (assessment of the scientific evidence available on the capacity of fetuses to experience pain)
Regarding the assessment of potential suffering in fetuses, the scope was not exclusively on pain, but also on other types of negative affect (e.g. possible discomfort experienced by the fetus during asphyxia in utero). Anatomical/morphological/physiological structures to experience pain/negative affect as well as evidence for capacity to experience pain/negative affect were investigated separately for each species. These were addressed by focussing on new evidence available since the past EFSA opinions of 2004 (EFSA, 2004, 2005). Furthermore, the evidence used in the EU Directive on the protection of animals used for scientific purposes (DIR. 2010/63/EU) was considered.
To answer this ToR of the mandate, the WG agreed to use a step‐wise approach. The topic was subdivided into (i) anatomical structures required for perception of pain and other types of negative affect including discomfort and suffering, (ii) the neurophysiological situation (e.g. inhibitory and excitatory systems) and (iii) the response of the fetus to specified stunning and slaughter conditions. For answering these questions the WG reviewed the available literature. The WG also created a logical model showing how those subquestions relate to each other to address the overall question on the capacity of fetuses to experience pain and other negative affect. In addition, an EKE meeting was performed (c.f. EKE 2) to collect expert judgements related to this ToR through a standardised methodology (EFSA, 2014). The EKE 2 meeting was held on 6–8 July 2016 and nine external hearing experts were selected based on the additional expertise needed for the exercise. Expertise needed related to fetal anaesthesia, fetal consciousness, fetal and neonatal physiology, pain treatment of the prematurely born child from the human field; animal consciousness, animal welfare, fetal consciousness, effect of hypoxia on fetuses and effect of various stunning interventions on fetuses from the veterinary field. Relating to the logical model, the experts were asked to (i) elicit probability distributions for the propositions in the subquestions to be true and (ii) express the level of uncertainty associated to each subquestion.
Following EKE 2, a summary report of the proceedings was produced and circulated electronically to all participants to check for accuracy. Corrections were incorporated in a final report provided as an Appendix to this opinion (Appendix D).
The outcomes of the EKE 2 meeting were evaluated by the AHAW Panel in the context of additional findings from the literature and further discussion with some of the EKE 2 experts to generate final conclusions for this opinion. These conclusions were developed by expert discussion and expressed using the approximate probability scale proposed in the draft Guidance on Uncertainty produced by EFSA (EFSA, 2016 in progress pp. 58 and pp. 96).
Uncertainty judgements on the substatements and final conclusion of the logical model (ToR3) are expressed as the certainty of the correctness of the statement versus its incorrectness. It is referred to as percentage certainty with the interpretation of the strength of belief that the statement is correct.
It is to be noted that the uncertainty assessment is addressed differently in ToR1 and ToR3, particularly. A schematic representation is given in Figure 1. Briefly, for ToR1, the prevalence of slaughtered pregnant animals was elicited by an Expert Knowledge Elicitation meeting (referred to as EKE 1). This resulted in ‘median estimates’ for the prevalence (e.g. 3% dairy cows in the last term of gestation) accompanied by a probability distribution range (e.g. from 9% to 27%) that gives the level of uncertainty. For ToR3, expert knowledge was elicited on statements e.g. that fetuses have the anatomical structures required (and further statements derived from the logical model; see below). The answers were formulated as likelihoods of the statement being true with the distribution of the answers providing the certainty distribution (e.g. from 5% to 40% likelihood that a certain statement is true). From the range of likelihood values and further interpretation of the literature, a qualitative translation of the uncertainty was derived (e.g. very likely).
Figure 1.
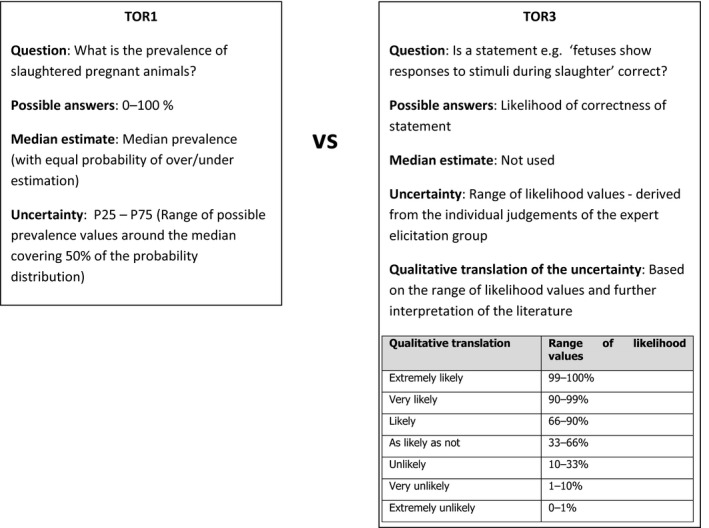
Schematic comparison of the uncertainty assessment of ToR1 and ToR3
2.3.4. Methodology for ToR4 (methods for stunning and killing of fetuses or neonates of the main livestock species)
Literature review was used as a basis to develop scenarios and related actions relevant to this question. The past EFSA opinions (EFSA, 2004, 2005) were also consulted as well as the methods prescribed under Regulation 1099/2009.
2.3.5. Methodology for ToR5 (methods for estimating the age of fetuses of the main livestock species at the slaughterhouse after the dam has been slaughtered)
Literature review was carried out to extract information on potential indicators of fetal age and whether reliable information on sensitivity and specificity of indicators exists or could be generated.
3. Assessment
3.1. Assessment for ToR1(prevalence of pregnant animals slaughtered in EU)
3.1.1. Information on prevalence from Member States
Discussion with Member State representatives of the AHAW network revealed that no official records of the pregnancy state of slaughtered animals were available. Few countries provided some information: in Latvia, the number of animals reported in 2014 to the national authorities as having been killed pregnant was four dairy cattle (between 5 and 8 months of pregnancy) and one sow. In Lithuania, the official inspectors in slaughterhouses estimated that the percentage of pregnant animals slaughtered annually is less than 5%. As explained in Section 2.1.1, further information was collected about possible infringements of regulation 1/2005; however, most of the Member States do not specify the cause of the non‐compliances due to animals unfit for transport. The number of non‐compliances due to animals slaughtered in the last 10% of pregnancy was only reported by Belgium (15 bovines in 2015) and Catalonia (15 bovines, 6 horses and 1 ovine in 2015, and 21 bovines, 11 horses and 4 sheep in 2014).
3.1.2. Information on prevalence from literature
The literature search resulted in 16 papers that were further assessed. The results of the search are reported in Table 1 below divided by species and country. When available, the proportion or number of pregnant animals at slaughter and the stage of pregnancy are reported.
Table 1.
Results of the literature search on the prevalence of pregnant animals slaughtered in the EU and Switzerland divided by species and country (n.a.: data not available)
| Species | Country | Percentage/number of animals found pregnant at slaughter | Percentage per term of gestation | Reference | |
|---|---|---|---|---|---|
| Of total number slaughtered | Of pregnant animals | ||||
| Cattle | United Kingdom | 23.5% (out of 6,670 cows; 1 slaughterhouse) | 6.3% 3rd term | 26.8% | Singleton and Dobson (1995) |
| Cattle | Luxemburg Germany Belgium Italy |
LU: 5.3% (out of 3,619 cows, range 1.3–5.4%; 3 slaughterhouses) DE: 4.9% (out of 1,012 cows; 1 slaughterhouse) BE: 10.1% (out of 965 cows; 1 slaughterhouse) IT: 4.5% (out of 3,071 cows; 1 slaughterhouse) |
LU: 1.3% 3rd term DE: 2.3% 3rd term IT: 0.7% 3rd term |
LU: 25% 3rd term DE: 46% 3rd term IT: 15% 3rd term |
Di Nicolo (2006) |
| Cattle | Germany | 4.4% pregnant (median 2.9%; range 0–10.8%; 10 slaughterhouses) | n.a. | n.a. | Lücker et al. (2003) |
| Cattle | Germany | 7.1% (median; 53 slaughterhouses, questionnaire study) | 6.4% 2nd and 3rd term | 90% 2nd and 3rd term | Riehn et al. (2011) |
| Pig | Sweden | 2.6% (out of 115 tracts examined) | n.a. | n.a. | Dalin et al. (1997) |
| Pig | Finland | 1.5% (out of 1,708 sows from one Finnish abattoir over 1 year period) | No information on stage, but tracts weighed 2.2 kg with large SD | n.a. | Heinonen et al. (1998) |
| Pig | Denmark | 4.2% pregnant sows involuntarily culled | n.a. | n.a. | Jensen et al. (2010) |
| Pig | United Kingdom | 13% (out of 142 tracts of sows and gilts from Scottish herds culled for infertility) |
7.7% early pregnancy (up to 6 weeks gestation), 2.8% mid‐pregnancy (7–13 weeks) 2.8% late pregnancy (14 weeks or more) |
59.2% early pregnancy (up to 6 weeks gestation), 21.4% mid‐pregnancy (7–13 weeks) 21.4% late pregnancy (14 weeks or more) |
Ward et al. (2010) |
| Pig | Belgium | 3%/14% (sows/gilts; out of 502 sows from 7 Belgian commercial pig herds) | n.a. | n.a. | de Jong et al. (2014) |
| Sheep | Ireland | 10% (out of 870 ewes) |
8.5% early stage of gestation 1.4% midgestation 0.1% late gestation |
85.1% early stage of gestation 13.8% midgestation 1.1% late gestation |
Alosta et al. (1998) |
| Sheep | Spain | n.a. |
Number of pregnant females registered over 90% gestation: 1 sheep (2014) 4 sheep (2015) |
Data from Catalan government | |
| Goat | Spain | n.a. |
Number of pregnant females registered over 90% gestation: 6 goats (2014) 11 goats (2015) |
Data from Catalan government | |
The literature search revealed a very scattered picture of prevalence of pregnant animals at slaughter with the limited evidence focussing on German speaking countries in the case of cattle and North‐Western European countries for pigs. Regarding small ruminants and horses, only very little or no information was available. Since reliable conclusions on the European situation could not be drawn from the available evidence, it was decided to perform a survey in 10 EU countries to obtain estimates from slaughterhouse operators of the total prevalence of pregnant animals at slaughter and the distribution across different phases of gestation. This information served as the starting point for an EKE meeting to judge the overall European situation.
3.1.3. Information and weighing of uncertainty on prevalence resulting from Expert Knowledge Elicitation
Tables 2 and 3 summarise the results of the EKE exercise held in June 16, 2016 (see report in the Appendix). In Table 2, the median estimate for the prevalence of pregnant animals slaughtered for each species is indicated. The percentiles (P) which define the 50% uncertainty range (P25–P75) as well as the 98% uncertainty range (P1–P99) are also given. As an indication of the uncertainty, there is a 1 in 2 chance that the actual prevalence lies between the P25 and P75 value, and there is a 98 in 100 chance that the actual prevalence lies between the P1 and P99 value.
Table 2.
Estimated prevalence (median) of mature female animals that are pregnant at the time of slaughter in Europe, including the uncertainties expressed as probability distribution. The latter is described by the percentiles (P) which define the 50% uncertainty range (P25–P75), as well as the 98% uncertainty range (P1–P99)
| All pregnancies | P1 | P25 | Median | P75 | P99 |
|---|---|---|---|---|---|
| Dairy cows | 2% | 9% | 16% | 27% | 60% |
| Beef cattle | 1% | 7% | 11% | 18% | 40% |
| Pigs | 0% | 3% | 6% | 9% | 20% |
| Sheep | 0% | 5% | 10% | 14% | 40% |
| Goats | 0% | 2% | 4% | 6% | 10% |
Table 3.
Estimated prevalence (median) of all mature female animals which are in the last third of gestation at the time of slaughter in Europe, including the uncertainties expressed as probability distribution. The latter is described by the percentiles (P) which define the 50% uncertainty range (P25–P75), as well as the 98% uncertainty range (P1–P99)
| Last 3rd | P1 | P25 | Median | P75 | P99 |
|---|---|---|---|---|---|
| Dairy cows | 0.2% | 1.6% | 3% | 5.2% | 14.4% |
| Beef cattle | 0.1% | 0.8% | 1.5% | 2.5% | 7.2% |
| Pigs | 0.0% | 0.2% | 0.5% | 1.0% | 3.6% |
| Sheep | 0.0% | 0.4% | 0.8% | 1.6% | 5.3% |
| Goats | 0.0% | 0.1% | 0.2% | 0.4% | 1.5% |
Table 3 provides the same information in relation to the percentage of all female animals which are in their last third of gestation when sent to slaughter.
For bovines, the Elicitation group agreed to differentiate between dairy and beef cattle, due to the importance of differences in management systems of the two groups. For pigs, the data were restricted to cull breeding animals and did not include female fattening pigs. The slaughtering of pregnant female fattening pigs can be an issue in farming systems where males for fattening are not routinely castrated, which is the normal situation in the UK and likely to increase across the EU because of concerns about the welfare implications of castration. Anecdotally, the proportion of gilts pregnant at slaughter in the UK is significant. Reports from vets working in abattoirs indicated pregnancy prevalence of at least 10% and up to 40% in extreme cases. These gilts are likely to be in early pregnancy in most cases because age at puberty will typically be 170–180 days and with current UK slaughter weights most gilts will be 160–180 days at slaughter. If entire males are kept in countries where slaughter weight and age is higher, this prevalence could increase, but would be easily avoided by split‐sex rearing.
Due to a limited number of representatives at the EKE meeting from countries where horses are slaughtered, it was not possible to elicit expert judgements about the prevalence of mares which are sent to slaughter when pregnant and the respective proportions across the various terms of gestation. However, the survey data from countries, where slaughtering of horses is practised (Belgium, Ireland, Italy, Poland, Romania, Spain) indicate a rather low prevalence of pregnant mares arriving at the slaughterhouse with a maximum of 6% stated for one slaughterhouse, but the majority indicating a 0% prevalence.
Estimates for the percentage of pregnant animals which are in the first and second term of gestation were also obtained from the EKE, but the AHAW Panel considers these estimates unreliable because of difficulties in detection of early stages of gestation in the course of the slaughter process.
3.1.3.1. Country differences in prevalence of slaughtered pregnant cattle
Major differences in the overall prevalence of slaughtered pregnant dairy cows in the EU are due to the production intensity, i.e. the incidence of production diseases such as lameness resulting in the involuntary culling of pregnant animals (EFSA, 2009), and to the prevalence of on‐farm euthanasia. Major differences regarding proportions of dairy cows in different terms of gestation at EU level are linked to the production system (intensiveness of farming and ability to keep records), and to economic considerations relating to pregnancy anabolism of the dam or the value of the new‐born calf. The EKE participants agreed that the comparatively high prevalence of slaughtered pregnant beef cattle in some countries may be explained by the type of breed slaughtered by the surveyed abattoirs.
3.1.3.2. Country differences in prevalence of slaughtered ewes
In most cases, sheep are raised for dual (e.g. meat and wool or milk and meat) or multiple purposes. Breeding ewes (for reproductive, milk and wool production purposes) and lambs (as breeding replacements or for meat production) are the dominant sheep types present on farm (EFSA AHAW Panel, 2014). In intensive and semi‐intensive systems, ewes are highly selected for milk yield or meat traits and the rate of replacement is high. The EKE participants agreed that in those countries with intensive and semi‐intensive systems, pregnant breeding ewes are sent to the abattoir because of production or health reasons. In extensive and very extensive production systems, where the stockperson does not have frequent and close contact with the sheep, ewes might be transported to the slaughterhouse without prior pregnancy diagnosis.
3.2. Assessment for ToR2 (reasons why pregnant animals are slaughtered and recommendations to reduce the number of animals slaughtered while pregnant)
3.2.1. Summary of known reasons for the slaughter of pregnant animals
The review of the available literature showed that there were very few published articles on reasons for slaughtering pregnant animals. The results are therefore primarily based on a synthesis of expert opinion obtained at the EKE 1 and from the knowledge and discussions of working group members.
In the first part of this section, an overview of known reasons for the slaughter of pregnant animals is reported, while the second part summarises the few publications found for each species.
Pregnant animals may be slaughtered because of lack of awareness of their condition, because of mistaken belief that the animal might be barren combined with lack of pregnancy testing or determination of an incorrect test result. Where females are kept together with mature male animals, particularly in more extensive systems where breeding activity is difficult to monitor, animals may unknowingly become pregnant. It is also possible that breeding by stray or feral males can unknowingly occur. Unobserved breeding may also occur in more intensive systems if large herd size and limited staff time for animal observation pertain. Many farms do not have equipment for pregnancy testing, or staff may be poorly trained in its use, and animals believed not to be pregnant and showing no obvious external signs are consequently sent for slaughter. Where animals are traded through markets or between different farms, information on pregnancy status can be lost during the transaction process. The final owner, knowing the animals could not have become pregnant while in their premises, therefore unknowingly can send to slaughter an animal which became pregnant during a previous ownership.
However, in addition to this accidental slaughtering of pregnant animals, various reasons for knowingly slaughtering pregnant animals were also identified. These can be divided into three main categories: health and welfare reasons, management reasons and economic reasons.
Health and welfare reasons may include the slaughter or killing of animals identified as infected by a disease whose spread is to be controlled by their immediate removal as a source of further spread. In extreme cases, this may require on‐farm killing as part of an EU or national eradication policy (a topic outside the scope of this opinion). In other cases, specific animals identified as carriers of a pathogen, which may spread to susceptible animals, may be slaughtered through an abattoir in the normal way. If a farm is identified as having infected animals, a management decision may be made to depopulate the whole farm as a precursor to establishment of a new disease‐free population. In this circumstance, animals at all stages of pregnancy may be affected by the decision, as such depopulation is usually done in the fastest possible time so as to minimise the risk of possible exposure of other susceptible animals as well as infection of humans in the case of a zoonosis or for cash‐flow reasons. If disease or injury is causing acute or chronic pain to an individual animal, slaughter or killing may be required on welfare grounds. This may take place on farm if the animal is deemed unfit for transport or if the disease or injury makes the meat unfit for human consumption. However, in other circumstances including chronic disease states, loss of condition or old age, in which animals fail to thrive or produce efficiently but have meat fit for human consumption, animals may be sent to an abattoir.
There are also situations in which animals are deliberately made pregnant for management reasons, despite knowing that they will subsequently be slaughtered. This can occur when an excess number of animals in a batch production system are inseminated to ensure that, allowing for possible conception failures, the correct batch size will be achieved to maximise subsequent utilisation of facilities. Once the pregnancy outcome of each animal is known, a selection is made of the best individuals to retain and the surplus are sent for slaughter, usually in early pregnancy. Such an approach is commonly recommended in the case of batch farrowing systems for sows, where it is widely recommended to serve all available sows (see WattAgNet8), and then to cull the excess animals after pregnancy diagnosis has confirmed which ones have successfully conceived. With this practice, slaughter is likely to be in early/mid‐pregnancy as the batch would normally receive a pregnancy diagnosis at 4 weeks after service.
Animals may also be made pregnant, despite the intention for subsequent slaughter, to modify their behavioural characteristics in ways desirable for the duration of their period on the farm. This is most commonly seen in the case of dairy cattle, where non‐pregnant animals show periodic disruptive riding behaviours associated with oestrus.
Economic reasons giving rise to the slaughter of pregnant animals may relate to a conscious decision at the time of insemination to exploit pregnancy anabolism in meat animals (Robinson, 1986), or to improve carcass quality by reducing consequences of preslaughter stress on meat quality (Ferguson and Warner, 2008). These practices were identified as applying particularly to cattle.
In contrast to these planned strategies, unplanned slaughter of pregnant animals may also be dictated by economic circumstances because their unexpectedly poor performance, such as low milk yield in dairy cattle or sheep, makes it uneconomic to retain them until their next parturition. Changes in the market opportunities may also result in pregnant animals being sold. For example, seasonal marketing opportunities give higher values to sheep or goat sales at particular times, such as around Easter or end of Ramadan, and animals may be held back to older ages to exploit these markets or a decision taken that their value for slaughter in these market conditions outweighs the value of keeping them until the offspring are delivered. If the current market value of the offspring is low, as may be the case for progeny of dairy cattle and sheep, the incentive to retain less productive animals until parturition is reduced. Pregnant animals may also be sent for slaughter because of unexpected extreme economic necessity, as in the case of bankruptcy of a business or an urgent cash‐flow requirement.
Table 4 presents a summary of data found in literature on the reasons for slaughter of pregnant animals for the different livestock species. Only data on cattle and pigs could be retrieved. Lack of awareness of pregnancy state was commonly reported, either in association with unplanned matings or an incorrect pregnancy diagnosis.
Table 4.
Summary of the papers found in literature on the reasons for slaughter of pregnant animals for the different livestock species (cattle, pigs)
| Species | Country | Reason why | Reference |
|---|---|---|---|
| Cattle | United Kingdom | Lack of awareness of pregnancy (50% of farmers), natural mating (66% of cows) | Singleton and Dobson (1995) |
| Cattle | Luxemburg, Germany, Belgium, Italy | False negative pregnancy check | Di Nicolo (2006) |
| Cattle | Switzerland | Lack of awareness of pregnancy (70% of farmers), high rate of false negative pregnancy check | BLV (2014) |
| Pig | Denmark | Among the observed sows, 4.2% were involuntarily culled for lameness | Jensen et al. (2010) |
| Pig | United Kingdom | Infertility incorrectly diagnosed | Ward et al. (2010) |
| Pig | Belgium | 4% of the sows culled because they were assumed to be not pregnant were pregnant and sows culled due to leg weakness demonstrated 10% pregnancy | de Jong et al. (2014) |
| Pig | Norway | Out of the 491 gilts selected for mating, 39 died/were culled before farrowing their first litter because of reproductive disorders, lameness and injuries | Thingnes et al. (2015) |
3.2.2. Results of expert opinion on the reasons for slaughtering of pregnant animals
Based on the results from literature review (see Table 4) and the outcomes of the discussion held at the EKE 1 meeting, the reasons for sending pregnant animals to slaughter are as summarised in the following Table 5. The relative importance of these causes cannot be established from the available information.
Table 5.
Summary of reasons divided by species and based on expert opinion, for sending pregnant animals to slaughter
| Dairy cows | Beef Cattle | Pigs | Sheep | Goat | Horses | |
|---|---|---|---|---|---|---|
| Economic reasons |
|
|
|
|
|
|
| Management reasons |
|
|
|
|
|
|
| Health and welfare reasons |
|
|
|
|
|
|
For bovines, results for dairy and beef cattle are presented separately because of differences in the management systems of the two cattle categories resulting in differences in the respective influencing factors.
3.2.2.1. Measures to reduce the number of animals slaughtered while pregnant at a critical stage of gestation
If risk managers consider that the likelihood of welfare problems in fetuses as indicated by ToR3, together with other risk management relevant considerations, warrants action then there are several possible options which might reduce the prevalence of slaughter of pregnant females in the last third of gestation. It is first necessary to ensure that farmers are aware of the reasons why animals pregnant in the last third of gestation should not be sent to slaughter. This information and education process may then need to be supported by incentives or penalties. Options may include actions at the level of market price intervention (e.g. incentives as regards an increase in the value of the new‐born animal relative to other products such as milk or meat) or at the level of risk management (such as a penalty at slaughter for animals presented in the last third of pregnancy except with veterinary derogation) but these would require a detailed macro‐economic analysis which is outside the remit of the scientific assessment included in this scientific opinion.
Therefore, measures that could reduce the number of animals slaughtered when they are in the last third of pregnancy can be categorised as those designed to increase awareness of the nature and extent of the problem (1 and 2) or to improve pregnancy testing (3 and 4):
Avoid unplanned slaughter (reduce prevalence of production disease). A general improvement in health status of the herd or flock will make it less likely that animals will become chronically ill or low producing in later pregnancy, and thus remove the motivation to slaughter these animals prematurely. Measures to improve health will include correct biosecurity, an appropriate vaccination strategy and good maintenance of facilities to minimise the risk of injury and lameness.
Avoid unplanned pregnancies. The separate housing of male and female animals throughout the production period, or the castration of all male animals in mixed sex groups, would preclude unplanned pregnancies by only allowing supervised breeding. Proper maintenance of stock‐proof fencing between groups is necessary to avoid accidental contact. This approach is likely to be unfeasible in extensive production systems where stray or feral males may roam in uncontrolled conditions.
Ensure the pregnancy status of all animals is confirmed before sending to slaughter (see Figure 2 below). Where it is not possible to be certain that unobserved breeding has not occurred, a confirmed negative pregnancy diagnosis is the only method to ensure pregnant animals are not unknowingly sent for slaughter. Methods are available for on‐farm use by farm staff or the veterinarian, but false negative results have been reported and it is essential that staff is trained in correct use and interpretation of these methods.
Ensure that information about insemination and pregnancy diagnosis are required in documentation accompanying animals at the time of sale, so that this information is reliably transmitted along the trading chain. This could be done by requiring all insemination events, co‐housing with males and pregnancy diagnosis results to be recorded on animal passports.
Figure 2.
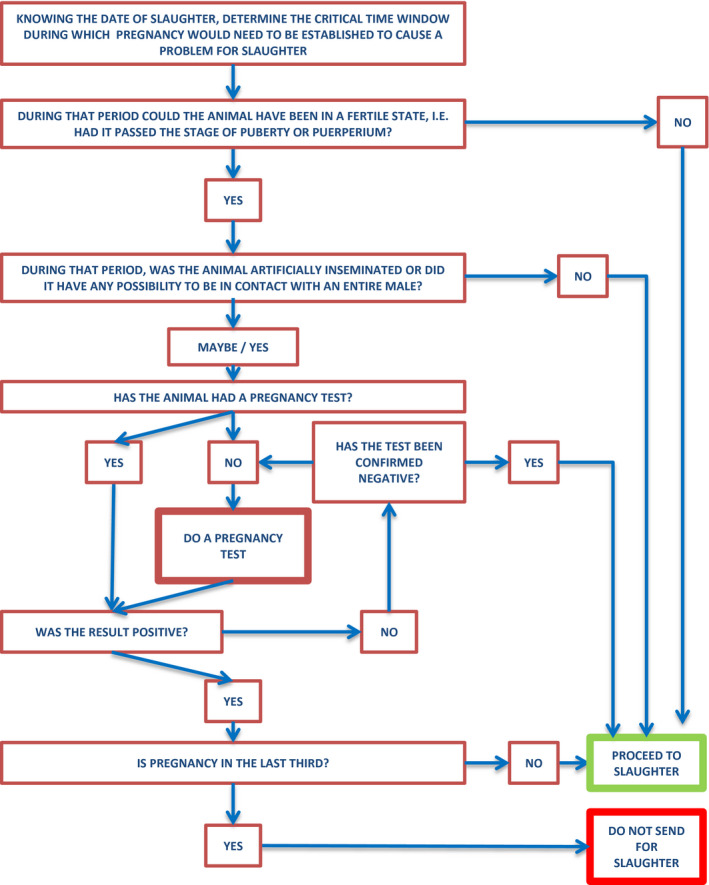
Decision tree to support farmers to reduce the likelihood of pregnant animals sent to slaughter during the last third of gestation
Effectiveness of these measures in reducing the possibility that farmers send animals in the last third of gestation to slaughter will be impaired by failure of education and communication strategies and a pregnancy test efficacy of less than 100%.
3.2.2.2. Decision‐tree to support farmers to reduce the likelihood of pregnant animals sent to slaughter
A decision tree to support farmers to reduce the likelihood of pregnant animals sent to slaughter during the last third of gestation is presented below in Figure 2. The first action, when the planned date for slaughter is known, is to retrospectively calculate the window of time where the pregnancy could have been established. A series of questions is then suggested leading to yes or no answers and thus to the possibility to proceed to slaughter or the need to carry out pregnancy diagnosis beforehand. Information is required on the puberty age of the animals. Indicative period ranges for the timing of puberty and length of pregnancy in females of different livestock species are reported in Table 6 below the figure (taken from the Merck Veterinary Manual9). However, puberty onset may be modified within species by genetic, nutritional and environmental influences, particularly contact with males, and more specific benchmarks for each farm circumstance should be sought. For multiparous animals where the date of previous parturition is known, this can also be used in determination of the possibility that an animal could be in the last third of a subsequent pregnancy. In the case of cattle, it is legally required that this information is recorded in the Livestock Database (Maher et al., 2008).
Table 6.
Indicative period ranges for the timing of puberty and length of pregnancy in females of different livestock species
| Species | |||||
|---|---|---|---|---|---|
| Cattle | Sheep | Goats | Pigs | Horses | |
| Age at puberty (months) | 10–12 | 6–9 | 5–7 | 6–7 | 10–24 |
| Length of pregnancy | 9 months | 5 months | 5 months |
3 months 3 weeks 3 days |
11 months |
Pregnancy diagnosis is suggested at different points in time. Various methods exist to diagnose pregnancy in farmed animals; they are discussed after Figure 1.
3.2.2.3. Methods for pregnancy diagnosis
Methods for pregnancy diagnosis fall into four broad categories, which have different degrees of reliability and on‐farm practicality (Ishwar, 1995; Purohit, 2010). In general, the emphasis in scientific study has been on the reliability of early detection of pregnancy as soon as possible after insemination, as this has important economic effects. The reliability of diagnosis of later stages of pregnancy, as more relevant for this Opinion, has received less study.
External observation
This relies on visual observation of morphological changes in animal size, shape or other features associated with pregnancy. These include growth of the abdomen as uterine volume expands, increase in size of the mammary glands (e.g. from 4 months onwards in dairy heifers), vaginal discharge (e.g. from 4 to 5 months onward in dairy cows) and fetal movements seen by movement of the abdominal wall (6 months onwards in cattle). These changes usually only become apparent in late pregnancy as fetal size becomes significant and as the endocrine changes associated with preparation for parturition and lactation are initiated. They are generally very unreliable for the purposes of pregnancy diagnosis as they may be influenced by the body condition of the dam, the presence of a thick coat or fleece and number or size of fetuses present. The absence of any signs of oestrus behaviour, such as intense activity, mounting or being mounted, is also sometimes taken as a sign of pregnancy, but is again very unreliable because of the sporadic nature and large individual differences in expression.
Physical examination
These methods rely on external palpation of the abdomen or rectal palpation of the uterus. Abdominal palpation can detect the fetal presence in cows from ~ 7 months onwards and in small ruminants from ~ 4 months. However, the method is unreliable and can be influenced by maternal body condition.
Transrectal palpation is the most widely used method in large animals (cattle, horses), but requires facilities for restraint of the animal. It is considered accurate for a trained person from ~ 35 days in cattle and ~ 20 days in horses, by detection of uterine changes, but becomes more reliable as pregnancy progresses and direct palpation of the fetus is possible. In pigs and small ruminants, the method is limited by the size of the pelvis and rectum/anus and therefore less practical.
Ultrasonic methods
This method relies on reflection of high frequency sound waves which occurs differentially according to tissue acoustic impedance. A simple on‐farm equipment uses either A‐mode ultrasonography or Doppler detection. A‐mode detectors generally operate based on detecting the presence of anechoic fluid within the uterine lumen. This has been shown to be reliable from 50–120 days of gestation in small ruminants, with commercial devices showing accuracy (combining both sensitivity and specificity) of positive pregnancy diagnosis exceeding 95% after 50 days (Watt et al., 1984). False negatives may occur in late gestation because of the decrease in ratio of uterine fluid to fetal tissue.
Doppler‐based detection is based on detection of movements associated with increased blood flow in the uterine artery, or later the fetal heartbeat. Accuracy has been reported as 100% in sheep between 66 and 122 days of gestation (Shone and Fricker, 1969). In sows, the test has been reported to have 86% accuracy at 31–35 days, with a similar accuracy for A‐mode ultrasound (Almond and Dial, 1986).
More sophisticated equipment uses B‐mode ultrasonography, which generates a 2‐D picture of tissue structure for diagnostic imaging. In early pregnancy, the presence of anechoic fluid within the uterine lumen is used diagnostically, but later the fetal heart beat and fetal skeleton can be seen (e.g. fetal bone by day 60 in cattle and day 70 in sheep). This technique also allows the possibility of estimating fetal age by morphological characteristics, but the equipment is likely to be too expensive and lacking in robustness for widespread routine on‐farm use.
Ultrasonography can be carried out transabdominally in pigs and small ruminants (after coat clipping), and is often used transrectally in cattle and horses. Transrectal scanning is possible in small ruminants with a specialised probe, and more accurate in the early stage of pregnancy (before 50 days). In goats, 98% sensitivity and 100% specificity have been reported after 26 days (Gonzalez et al., 2004). However, good restraint facilities are necessary to minimise the possibility of injury during the process, making it less suitable for field conditions. To ensure reliable results from ultrasonographic pregnancy diagnosis, staff should have appropriate training to operate equipment and interpret results.
Biochemical markers in body fluids
The most commonly used endocrine marker is progesterone, which shows sustained elevation in pregnant animals. This can be measured in serum or in milk of lactating animals. Measurement by radioimmunoassay requires specialised laboratory facilities but enzyme‐linked immunosorbant assay (ELISA) tests have now been developed as commercial kits which can be used on farm for immediate results. Sensitivity of the test (i.e. correct detection of animals which are pregnant) is generally good, but specificity (i.e. ability to correctly detect animals which are not pregnant) can be low because of false positives occurring during the luteal phase of the oestrus cycle of non‐pregnant animals, ovarian cysts or cases of embryonic death. In small ruminants, different studies have reported accuracy of detecting non‐pregnancy by milk samples or blood samples as 100%, or of correct pregnancy confirmation as 80–90% (Holdsworth and Davies, 1979; Gonzalez et al., 2004). In sows, a serum progesterone test has been reported to have 92% accuracy at 31–35 days (Almond and Dial, 1986).
Other diagnostic markers include the presence of estrone sulfate (ES) in urine, milk, faeces or blood, and commercial kits based on this have been developed for use in horses after 120 days. It is detectable in serum of sheep from ~ 70 days, and goats from ~ 50 days. In goats, a milk ES ELISA had a reported accuracy of 82% for pregnancy confirmation (Murray and Newstead, 1988). In sows, the test on blood samples has been reported to have 98% accuracy at 31–35 days (Almond and Dial, 1986), while a faecal test gave a sensitivity of 96% and specificity of 94% at this stage (Vos et al., 1999). The field measurement of urinary oestrone conjugates in horses by ELISA was reported to give 100% accuracy (Kirkpatrick et al., 1993). Furthermore, the same authors reported a 83% accuracy for a simple urinary dipstick ELISA for detection of equine chorionic gonadotrophin, although this was effective only between ~ 35 and 140 days and not in later pregnancy.
Another widely used diagnostic marker for cattle and small ruminants is the presence of pregnancy‐associated glycoproteins (PAG). These can be used from ~ 30 days post‐breeding. In goats, a PAG plasma test by radioimmunoassay (RIA) has been shown to have sensitivity and specificity of 100% by 26 days (Gonzalez et al., 2004). In cows of > 60 days, a milk PAG ELISA test for confirmation of pregnancy gave 98–99% sensitivity and 92–100% specificity (LeBlanc, 2013; Lawson et al., 2014).
At the present time, reliable and feasible on‐farm detection of animals in the last third of gestation should be achievable by:
Pigs: transabdominal Doppler ultrasound
Dairy cattle (lactating): milk progesterone, PAG or rectal palpation
Beef cattle: rectal palpation
Dairy sheep/goats: milk progesterone, PAG or transabdominal ultrasound
Meat sheep/goats: transabdominal ultrasound
Horses: rectal palpation
3.3. Assessment for ToR3 (assessment of the scientific evidence available on the capacity of fetuses to experience pain and other negative affect)
The topic was subdivided into (i) anatomical structures required for the perception of pain and other negative affect, (ii) the neurophysiological situation (e.g. inhibitory and excitatory systems) and (iii) the response of the fetus to specified stunning and slaughter conditions. The WG created a logical model showing five subquestions that link together to address the overall question on the capacity of fetuses to experience pain and other negative affect. As explained in Section 2.3.3, to answer the subquestions, a literature review was completed and an EKE 2 exercise was held. Finally, the experts of the Panel expressed, based on the outcomes of literature and EKE 2, probabilities for each subquestion by expert discussion. The following chapters present the logical model, a summary of the scientific evidence available for each subquestion of the logical model and the outcomes of the expert discussion.
3.3.1. Working definitions
3.3.1.1. Definition of fetus
In this opinion, the term fetus is used for a developing organism from the time of implantation until the moment it takes its first breath ex utero.
3.3.1.2. Definitions of nociception (sensory perception) and pain (emotional experience)
Nociception can be defined as the physical activation of neuronal pathways by potentially harmful stimuli, with or without the subjective emotional experience of pain. For example, somatic reflex arcs occur at the level of spinal cord or even at the brain stem level in response to a stimulus without involving higher centres in the brain. Knee‐jerk response is a typical example of a spinal reflex.
This opinion uses the definition of pain from the International Association for the Study of Pain (IASP): ‘Pain can be defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’. It therefore requires the presence of consciousness as prerequisite to evaluate a stimulus as unpleasant. In this regard, the sensory and emotional experiences may also include other forms of suffering, i.e. negative affect or mental states.
3.3.1.3. Definition of consciousness
For this scientific opinion, the words ‘consciousness’ and ‘awareness’ are considered synonymous. Awareness is often referred to as one of two aspects or dimensions of consciousness, namely the ‘content’ of consciousness, while the other aspect reflects the ‘level’ of consciousness, which addresses the level of arousal or wakefulness (Laureys et al., 2009). Both aspects are usually positively associated thus not requiring a distinction. Awareness may also be addressed as the ‘raw material’ from which animals develop consciousness, i.e. the knowledge about the internal state or the external environment by way of sensory perception and processing presumably at the brain stem level (‘knowing what is going on’ as opposed to nociception), and therefore regarded a prerequisite for consciousness, but this distinction is again not consistently made. This is also reflected in very similar definitions for both terms in dictionaries (see Le Neindre et al., 2017), who provide an extensive review of the concepts of consciousness in humans and animals) or in the synonymous use of the terms in scientific literature (e.g. Duncan, 2006).
Consciousness of an animal is essentially its ability to cognitively process and subjectively evaluate internal and external sensory inputs and to feel emotions. It also implies decision‐making and voluntary motor responses as opposed to mere reflexes. Sentience, i.e. the (functional) capacity to perceive sensations originating from sensory inputs, which is present from a certain developmental stage onwards (see Table 7 below), is considered fundamental (Mellor and Diesch, 2006). Furthermore, consciousness requires being awake, while nociception can occur during sleep and may induce arousal and awakening.
Table 7.
Estimated timelines for fetal brain developments in the different species
| Species | Gestation period (days) | Formation of thalamocortical projections (Mellor and Diesch, 2006) | Onset of somatosensory evoked potentials (SEPs) (Cook et al., 1987) | Onset of cyclical ECoG activity (Szeto and Hinman, 1985; Szeto, 1990) | |||
|---|---|---|---|---|---|---|---|
| Days | Proportion of gestation | Days | Proportion of gestation | Days | Proportion of gestation | ||
| Cattle | 280–291 | > 140 | 0.5 | 244 | 0.85 | 224 | 0.8 |
| Sheep | 144–150 | > 72 | 0.5 | 125 | 0.85 | > 115 | 0.8 |
| Goat | 148–156 | > 74 | 0.5 | 129 | 0.85 | 118 | 0.8 |
| Horse | 330–345 | > 165 | 0.5 | 287 | 0.85 | 264 | 0.8 |
| Pig | 112–120 | > 56 | 0.5 | n.a. | n.a. | n.a. | n.a. |
3.3.2. Logical model
The logical model is shown in Figure 3 as a flow chart. It shows a series of subquestions in boxes and how they link together to address the overall question. The subquestions (indicated in the Figure as C.1–C.5) are then addressed separately in the following sections considering the available scientific evidence and the expert judgements. For each subquestion, the box reports the paragraphs including the relevant scientific literature and/or the paragraph including the outcomes from the expert judgements.
Figure 3.
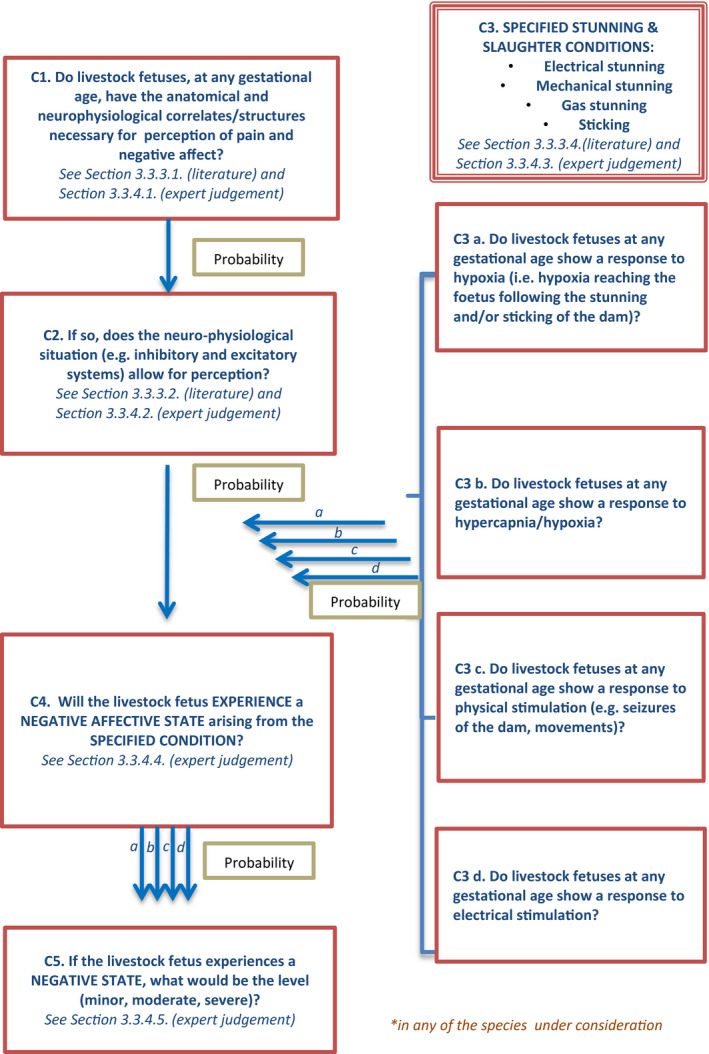
Logical model for assessment of ToR3 (capacity of fetuses to experience pain and other negative affect)
Figure C.1.
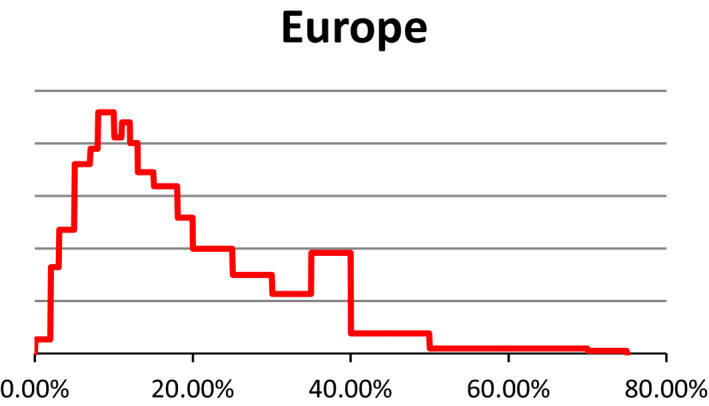
Probability density regarding the overall prevalence of pregnant cattle (dairy cows) slaughtered
Figure C.5.
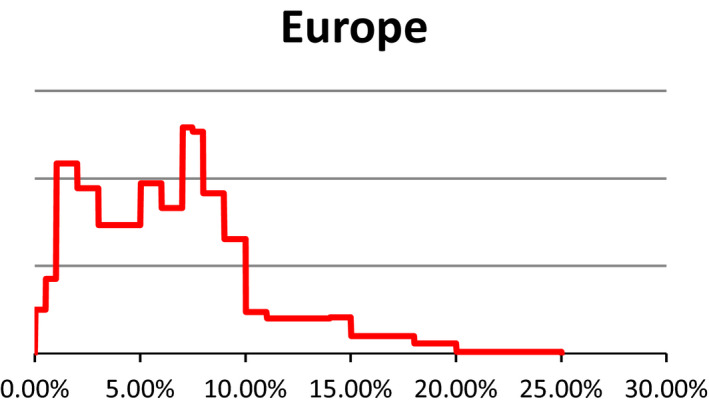
Probability density regarding the overall prevalence of pregnant sows slaughtered
While most the scientific evidence addresses the question of cortically based consciousness, more recently subcortically based consciousness has been hypothesised (Merker, 2007; Campbell et al., 2014). In this context, the logical model can be considered in both the context of cortical and subcortical awareness (see EKE 2 report in Appendix D).
3.3.3. Summary of available scientific evidence for ToR3 (capacity of fetuses to experience pain and other negative affect)
3.3.3.1. Summary of scientific evidence on C1. Anatomical and neurophysiological correlates/structures necessary for perception of pain and other negative affect (at cortical and/or subcortical level)
Fetal brain development
The mandate on killing of pregnant animals involves cattle, sheep, goats, pigs and horses and these species are termed precocial, i.e. their fetal brains are neurologically matured at the time of birth (McIntosh et al., 1979; Morell et al., 1993). It is worth noting that the maturation of fetal pig brain, as in humans, continues until the perinatal period (Conrad and Johnson, 2015). Therefore, fetal brain development data, expressed as a fraction of gestational age, corresponding to sheep, goats, cattle, and horses may be comparable, and these may be different from pigs. In pigs, the most rapid neural development occurs between 50 days prenatal and 40 days postnatal (Dickerson and Dobbing, 1967). Most rodents and carnivores are altricial, i.e. their neonates are born neurologically immature (Dobbing and Sands, 1979; Clancy et al., 2001), so extrapolation from these species is not appropriate.
Campbell et al. (2014) identified three phases of neurological developments to be critical for acquiring capacity for consciousness: (1) development of connectivity of sensory nerves to the spinal cord and the early development of lower brain centres; (2) the connection of peripheral nerves to lower brain centres; and (3) integrated neural processing of sensory inputs involving interactivity between the thalamus and the cerebral cortex via thalamocortical connections.
Fetal brain development involves four key stages: neuronal differentiation, proliferation, migration and synaptogenesis. For example, in domestic pigs by 20–22 days, the brain has divided into five primitive regions: telencephalon, diencephalon, mesencephalon, metencephalon and myelencephalon. Early cortical development begins at this time with the formation of the preplate and subsequent increases in neurogenesis and neuronal migration. A graphical representation of corticogenesis in mice is provided in Figure 4.
Figure 4.
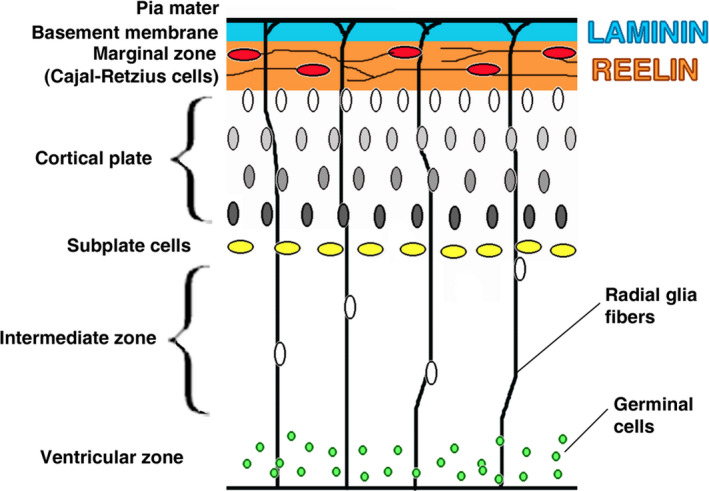
Visualisation of corticogenesis (i.e. migration of neurons formed in the ventricular zones) in mice during neurodevelopment; by Wikipedia, CopperKettle https://commons.wikimedia.org/w/index.php?curid=37111233
It is known that the subplate zone is a transient cytoarchitectonic compartment of the fetal telencephalic wall and contains a population of subplate neurons (SPns), which play a key role in normal development of cerebral cortical structure and connectivity. SPns receive thalamic and neuromodulatory inputs and project into the developing cortical plate. Thus, SPns form one of the first functional cortical circuits and are required to relay early oscillatory activity into the developing cortical plate. In mammals, SPns are well developed by the mid‐pregnancy stage.
Formation of thalamocortical projections
It was suggested that SPns transmit activity patterns to the cortical plate before the thalamic fibres invade the cortex. However, optical recording and current source density analytical studies did not demonstrate any activation of the cortical plate before the substantial invasion of the thalamic fibres (Higashi et al., 2002). Mellor and Diesch (2006) suggested based on these stages of neurological development in mammalian farm animal fetuses that the capacity for conscious perception develops during the second half of pregnancy. Timelines for fetal brain developments in the different species are presented in Table 7.
Onset of somatosensory evoked potentials (SEPs)
According to Tawia (1992), the capacity to consciously perceive somatosensory stimuli is a central event at the level of the cerebral cortex, not the periphery. In this regard, peripheral somatosensory receptors transmit information to second order neurons located in the spinal cord and medulla, and these neurons transmit sensory information to third‐order neurons located in the thalamus which, in turn, relay the sensory input to sensory regions of the cerebral cortex. Sensory information received by the cerebral cortex is transmitted to the motor cortex where stimulus specific voluntary movements are generated, which are then conveyed via efferent neurons to different motor neurons, via the brain stem or spinal cord, resulting in specific muscular movements. In this regard, it is worth nothing that the brain stem neurons, which project axons to the spinal cord in the fetal sheep, are the same as in other mammalian species. More importantly, these neurons responsible for fetal motor/physical behaviour reach the spinal cord by 55 days of gestation (0.4 G) in sheep, an age well before the change in motor behaviour occurs, suggesting that the projections do not become fully functional until later (Stockx et al., 2007).
Tawia (1992) argues that although the basic neuronal substrate for sensory perception in the fetus may be developed by midgestation, the functional capacity of the neuronal circuitry is limited because of its immaturity. In a mature, fully functional nervous system a peripheral somatosensory stimulus is transmitted to, and perceived at the cerebral cortex where an appropriate response is generated. Transmission of information requires appropriate neurotransmitters in adequate concentrations and their receptor systems. Low levels of neurotransmitters within the fetal sensory and motor neurons, and the spinal cord can be seen as evidence of an immature system. On this basis, elicitation and measurement of a somatosensory evoked potential (SEPs) in the brain can be considered as evidence for the existence of a matured nervous system, and hence the capacity for conscious perception. It is worth mentioning that a mature SEP has several primary components: the presence of a first negative (N1) component is indicative of arrival of the somatosensory stimulus at the cortical level and the presence of positive components, especially the second positive component (P2) is indicative of the arrival of the stimulus at the primary somatosensory cortex and information processing (see Tawia, 1992). It is therefore inferred that the elicitation of a mature SEP, especially the late latency potential, can be seen as an indication of the capacity for somatosensory perception, including pain. Cook et al. (1987) successfully recorded SEPs from 97 to 148 days old (0.66–0.99 G) in a chronically instrumented fetal lamb by stimulating the upper lip or upper limb. Stimulation of the upper lip produced SEPs with a pattern including mean peak latencies of 9, 13.2, 17.8, 21.3, 33.8 and 206 ms at a gestation age of 125 days (0.85 G). Similar peaks, but of slightly later mean latencies, were seen following the upper limb stimulation.
Onset of cyclical ECoG activity
Electroencephalograms (EEGs) and electrocorticograms (ECoGs) have been used to record spontaneous and evoked electrical activity in the brain. The ECoGs recorded in fetuses show that it remains predominantly isoelectric during early stages, develops random activity at about 0.6 of gestational age and finally cyclical electrical activity in the brain starts after 0.75 of gestational age (in sheep), i.e. alternating between a high‐voltage, low‐frequency (3–12 Hz) ECoG state known as non‐rapid eye movement sleep (NREM) and a low‐voltage, high‐frequency (10–20 Hz) ECoG state known as rapid eye movement sleep (REM). A so‐called ‘awake state’ (AW), occurs briefly in between the two sleep states and is often referred to as a transition stage (Mellor et al., 2005).
REM sleep becomes established in fetal lambs at 110–125 days of gestational age (0.75–0.85 G) (Berger et al., 1986), and in late gestation fetal sheep (125–140 days, 0.9 G) the percentage of time spent in each state was: NREM sleep 53%, REM sleep 41.4% and AW state 5.6% (Ioffe et al., 1980). Quantitative analysis of the ECoG signals to calculate spectral edge frequency, as a measure of the maturation of electrocortical activity in the fetal lamb with advancing gestation, has been used to determine the frequency below which 90–95% of the power in the spectrum resides (Szeto and Hinman, 1985; Szeto, 1990). The results showed that at gestational ages below 122–125 days (0.85 G), only one spectral edge peak was present representing quiet sleep, whereas above this range of gestational age, two peaks were present representing two sleep states.
Timelines for fetal brain developments in the different species have been estimated by extrapolating the proportion of gestational age from published sheep fetus data and are presented in Table 7. Data are not given for pigs because of the uncertainty of extrapolation from sheep indicated previously.
Development of fetal reflexes and capacity for independent existence
As with brain development, most information on this subject comes from studies on sheep fetuses, although extrapolation to the other precocial species again seems reasonable.
Fetal body movements
Ruckebusch et al. (1977) defined three types of fetal movements in sheep fetuses: (1) simple fetal movements involving activities such as extension of the head and forelimb, without associated changes in fetal heart rate; (2) complex fetal movements involving 2–3 large deflections of the limb, trunk and neck, with associated changes in fetal heart rate; (3) gross fetal movements which occur as bouts of complex movements lasting for 3–10 min, about 15 bouts per day, and which are restricted to the AW state. It is inferred that fetal body movements also occur in both sleep states, but vigorous body movements occur as breathing and swallowing mainly during brief periods of AW state. However, this state is unlikely to represent wakefulness, and these fetal body movements are vital for the development of lungs and musculoskeletal systems (Rurak, 2016).
Rurak and Wittman (2013) measured fetal lamb motility and abdominal diameter at weekly intervals for 30 min from 55 days of gestation (0.37 G) to term. The body movements observed in this study were movements of the limbs and body of the fetus sufficient to cause displacement of the fetal trunk. The results showed that fetal movement counts/min were relatively constant between 55 and about 90 days (0.6 G), and declined progressively thereafter, the breakpoints in the regression curves ranged from 65 to 110 days of gestation and averaged 91.9 + 5.2 days. Fraser and colleagues have studied fetal movements in sheep, cattle and horses (Fraser et al., 1975; Fraser, 1976, 1989; Husa et al., 1988). The types of movements observed include rapid opening and closing of the mouth in the late gestation sheep. In the fetal horse, simple movements were first detected at 90 days of gestation (0.27 G) and the incidence increased until about 180 days (0.53 G), remained at a plateau until 250 days (0.74 G), then decreased until 280 days (0.83 G), and remained at that level until term. There was an increase in the duration of quiescent periods with advancing gestation. In cattle, simple fetal movements increased between 4 and 6.5 months (0.68 G) and then decreased, although the magnitude of the decrease is less than that in fetal cattle and horse. In all the three species, specific ‘righting reflexes’ were observed in late gestation, which are postural and positional changes (foreleg extension and head elevation) manifested by the fetuses of these quadruped species in the prepartum period in preparation for birth. They occurred over the last 2 days of gestation in sheep, 1 day prepartum in the cattle, and in early labour in the horse. These fetal movements in utero appear to be purposeful in preparation for the passage through the birth canal.
Fetal breathing movements
The pattern of breathing seems to change with the gestation age of the fetal lamb, depending upon the method used to record breathing movements. When diaphragmatic activity, recorded using electromyogram (EMG), was used there was continuous breathing prior to 110 days of gestation (0.75 G), breathing becomes episodic after 110 days, and at 120 days (0.82 G), the episodic breathing is largely restricted to REM sleep (Dawes et al., 1972; Clewlow et al., 1983). Between 134 days (0.92 G) and term, the incidence of breathing decreases (Berger et al., 1986). In contrast, when fetal breathing was measured using real time ultrasound, the incidence of breathing has been reported to increase from 6% at 55–64 days (0.4 G) to about 45% between 95 and 134 days of gestation (0.78 G) (Rurak and Wittman, 2013). More importantly, at no stage of fetal development is the breathing continuous.
Nevertheless, two types of breathing movements can be recognised in fetal lamb: (1) single, large amplitude inspiratory effort, and (2) rapid irregular breathing movements (Dawes et al., 1972). Rapid irregular breathing activity occurs mainly during REM sleep. The single inspiratory efforts, often referred to as gasping, occurred 4% of the time. Occasional gasps were not related to any sleep or AW state (Ioffe et al., 1980). Measurement of fluid movement in the sheep fetal airways associated with breathing indicated that the fluid volume rarely exceeded 0.5 mL (Dawes et al., 1972). Further studies revealed that the glottis remained closed when these gasps occurred, leading to the conclusion that these inspiratory efforts mainly occurred during quiet sleep and closely resembled regurgitation associated with rumination after birth (Harding et al., 1980). When observed in fetal pigs, the inspiratory efforts have been termed hiccups (Harding et al., 1991).
Fetal lung development
There are five stages of lung development in sheep (Alcorn et al., 1981; Gnanalingham et al., 2005):
Embryonic – 0 to 40 days
Pseudoglandular – 40 to 80 days
Canalicular – 80 to 120 days
Saccular – 120 to 148 days
Alveolar – 140 days to early postnatal
Studies involving artificial ventilation of lungs of fetal lambs of different gestational ages showed that, inflation of lungs is difficult in fetuses of less than 110 days of gestational age and, consequently, they are unable to oxygenate their blood adequately, and hence have reduced viability (Born et al., 1955). Additionally, functional maturity of lungs is attained over the last few days of gestation after 140 days in fetal sheep (Kitterman et al., 1981).
Based on the available data, it is therefore inferred that only fetuses in the last third of gestation will have the anatomical and functional brain structures associated with the perception of pain or other negative affect. In addition, fetal body and respiratory movements do not represent evidence for capacity to perceive pain or other negative affect. It is also evident that fetuses will require the anatomical development necessary to inflate lungs and the capacity to breathe and oxygenate the brain in order to survive independently.
3.3.3.2. Summary of scientific evidence on C2. Neurophysiological situation (e.g. inhibitory and excitatory systems) and possibility of cortically based perception
Even if a fetus has full development of the peripheral and cortical anatomical features required to feel pain, or other noxious stimulation of sensory mechanisms, this does not mean that it necessarily experiences an unpleasant affective state when nociceptive pathways are stimulated, even if a motor response is observed. To perceive negative affect, it is generally accepted that the fetus must be in a conscious state. Thus, a functional cortex is deemed necessary for pain experience but not sufficient (RCOG, 2010).
To emotionally experience pain or negative affect, the animal has to be cognitively aware of the stimulus (a cortical process), which most scientists believe requires that it is awake. Wakefulness is a state of brainstem and thalamic activity, described by Mellor et al. (2005) as a state of non‐sleep‐arousal, whereas consciousness additionally requires cortical processing. However, they state that it is not possible to be asleep and conscious. Being asleep or awake is not as easy to distinguish in the fetus as it is in adults, but the broad categories can be classified based on EEG recordings.
Most information on cortical states in the fetus comes from studies in the sheep, which is widely used as a model for investigation of pregnancy issues in human medicine. There are good reasons to believe that these findings will extrapolate to the other farm livestock species of interest in this Opinion, since they are all ungulate species.
In late gestation fetal sheep (125–140 days, 0.9 G), NREM sleep accounts for 53% and REM sleep for 41% of time (Ioffe et al., 1980). Therefore, it is inferred that mammalian farm animal fetuses are asleep most of the time during gestation. In the other 6% of time, there is greater cortical activity, with a low‐voltage, irregular, mixed pattern of ECoG and simultaneous appearance of vigorous somatic muscle activity, breathing movements, and the presence of eye activity (Ruckebusch and Gaujoux, 1976; Bissonnette et al., 1995), which some authors refer to as the ‘awake state’ (AW). This gives rise to the question of whether the fetus is truly awake and conscious during these periods, which typically occur as the fetus is transitioning into, or sometimes out of, NREM sleep. Mellor et al. (2005) propose that this AW state represents a transition phase between sleep states, a state which in the newborn is called indeterminate sleep (Mirmiran et al., 2003), and is not indicative of true consciousness. They cite as evidence for this view the study of Rigatto et al. (1986), who directly observed an unanaesthetised sheep fetus, in utero, through a Plexiglas window, for 5,000 h without observing signs of wakefulness such as eyes opening or coordinated movement of the head.
Dawes et al. (1972) observed fetal sheep at 115–147 days (0.9 G) of gestation that were delivered into a warm saline bath (39–40°C) adjacent to the ewes under epidural anaesthesia and with intact umbilical circulation. The authors reported that in sheep fetuses less than 128 days of gestation, it was difficult to identify the two sleep states as the ECoG signal amplitude was consistently low. In addition, the periods of apparent AW state were accompanied with movement of limbs, raising of heads, opening of eyes and the fetuses also responded to tactile and auditory stimuli during this AW period. However, this behavioural state was not accompanied by breathing. In subsequent experiments on mature fetal lambs by Dawes and colleagues (unpublished data, cited on pp 93 by Rurak, 2016), wakefulness was observed when the bath temperature was reduced. In this study, when fetal core temperature reached about 37°C (compared to normal temperature of 39.5°C), wakefulness was observed, which included fetuses raising their heads out of the bath and onset of continuous pulmonary ventilation. More importantly, when the bath and fetal core temperature was raised back to normal, the pulmonary ventilation ceased and the head of fetus sunk back into the bath. These results suggest that surface and core cooling of the fetus following birth could be important for the initiation of arousal, wakefulness and continuous breathing. It is therefore inferred that mammalian farm animal fetuses are asleep most of the time during gestation and any fetal movements observed during studies involving exteriorisation of the uterus from the dam (i.e. the pregnant animal) are probably due to the cooling effect. It has been demonstrated ‘in utero’ that warmth has an inhibitory effect on fetal arousal (Gluckman et al., 1983).
The lack of consciousness in the fetus is attributed to a range of causal factors. As an evolutionary mechanism assumed to protect the comfort of the dam and help maintain pregnancy to full term, it is desirable that the activity of the fetus while in utero should be markedly inhibited. The mechanisms by which consciousness is suppressed in utero have been reviewed by Lyche et al. (2005) and Mellor et al. (2005). This involves control over a number of endogenous factors related to fetal oxygenation (Mellor et al., 2005), supported by physical elements of the uterine environment, which are warmth, buoyancy and cushioning.
There are several chemical suppressors in utero which act to inhibit neural activity in the fetus to a far greater degree than is seen postnatally. This has been attributed to the combined neuroinhibitory actions of adenosine, a powerful EEG suppressor and sleep inducing agent, allopregnanolone and pregnanolone, two neurosteroids which have anaesthetic, sedative and analgesic actions, and prostaglandin D2, a potent sleep‐inducing hormone. There may be additional neuroinhibitory factors produced by the placenta which further support these effects.
Adenosine is a purinergic messenger, with a very short half‐life of under 10 s, which is produced in all tissues but primarily in the placenta and, to a lesser extent, the fetal liver (Slegel et al., 1988; Sawa et al., 1991; Ball et al., 1996). It regulates many physiological processes in excitable tissues by inhibiting metabolic activity and/or by modulating the supply of metabolic substrates via vasodilatation (Dunwiddie and Masino, 2001; Porkka‐Heiskanen et al., 2002), with high circulating and tissue concentrations of adenosine promoting sleep and increasing NREM‐related EEG activity. In the sheep and human, the circulating concentrations of adenosine are two‐ to fourfold higher in the fetus than the dam (Sawa et al., 1991; Yoneyama et al., 1994). Concentrations increase during hypoxaemia (Hunter et al., 2003) and it has been suggested that this is an evolutionary mechanism to reduce metabolic requirement and minimise cerebral neuronal damage when uterine oxygen supply is compromised (Newby et al., 1990). It has been suggested that the first appearance of consciousness after birth occurs only when breathing oxygenates the neonate sufficiently to remove the dominant adenosine inhibition of brain function (Mellor and Diesch, 2006). Allopregnanolone and pregnanolone are metabolites of progesterone which have potent sedative/hypnotic and anaesthetic effects (Majewska, 1992; Paul and Purdy, 1992). They are primarily synthesised within the central nervous system (CNS) where they increase the activity of gamma‐amino‐butyric acid (GABA) inhibitory pathways modulating EEG activity and behavioural state. During later pregnancy, the sheep placenta produces large quantities of progesterone (Bassett et al., 1969) and plasma concentrations and brain concentrations of progesterone and the pregnanes fall markedly after birth (Seamark et al., 1970; Nguyen et al., 2003). Again, in stress situations such as hypoxia, allopregnanolone levels are increased in circulating plasma (Nguyen et al., 2003). Prostaglandin D2 is a sleep‐inducing hormone synthesised in the adult CNS. The required enzyme, prostaglandin D synthase, is present in the fetal sheep at 125 and 135 days of gestation (0.85–0.92 G) but not at 90 days (0.61 G), suggesting a role in inducing sleep in fetal sheep from at least 125 days of gestation (0.85 G), when discrete REM and NREM sleep states are established. There is some evidence for an additional placental inhibitory peptide, since infusion of a placental extract suppresses fetal activity and respiration within 2 min (Alvaro et al., 1993, 1997). A range of other inhibitory factors, including neuropeptide Y (NPY), corticotropin releasing hormone (CRH) and growth hormone (GH) may also contribute to suppression of fetal activity in utero (Mellor et al., 2005).
Extrapolation across species may be problematic. Bellieni and Buonocore (2012) have argued that in the late term human the putative neuroinhibitory substances, adenosine and progesterone, show fetal levels which are similar to those in the mother, who does not experience analgesia. However, the scientific literature is not consistent in this respect. As mentioned previously, in the sheep and human, the circulating concentrations of adenosine have been reported to be two‐ to fourfold higher in the fetus than the dam (Sawa et al., 1991; Yoneyama et al., 1994).
There is a dynamic interaction between oxygen tensions and the concentrations of adenosine and other neuroinhibitory agents. The fetal lamb's arterial oxygen partial pressure (PaO2) is usually < 25% and its arterial carbon dioxide partial pressure (PaCO2) is usually > 135% of the respective values in the conscious ewe because of the concentration gradients required for these gases to diffuse across the placenta. In mature animals, this degree of hypoxaemia causes unconsciousness, and it was suggested that fetal arousal and awareness may be suppressed by low oxygen (O2) status (Mellor and Gregory, 2003). The suggestion that, because of this fetal hypoxia, it is improbable that the fetus could experience consciousness before birth was supported by the observation that artificially increased fetal O2 tensions stimulate continuous breathing and behavioural arousal in fetal lambs (Baier et al., 1990; Hasan and Rigaux, 1991), whereas acute reductions in fetal PaO2 decrease the incidence of behavioural arousal (Bocking and Harding, 1986). It is normally only after birth that a series of changes induce wakefulness: exposure to cold, physical stimulation of delivery and (in some species) maternal licking and nosing, and the reduction in umbilical blood supply act to initiate breathing and increased oxygenation of the blood. It is this change in oxygenation which removes an over‐riding inhibitory effect of adenosine, which when combined with birth‐related neuroactivators allows the neonate to be conscious.
It has been reported that, in mammals born neurologically mature, the withdrawal of neurosuppression, along with concurrent neuroactivation, leads to the onset of consciousness shortly after birth (Mellor et al., 2005; Mellor and Diesch, 2006). However, there are indications that persistence of the neurosuppressive agents after birth may modulate pain responses in the early neonate. For example, in sheep plasma concentrations of the neurosuppressive agents pregnanolone and allopregnanolone were found to be significant up to 3 days after birth (Nguyen et al., 2003), leading to the suggestion that these chemicals may continue to exert some cerebral effects after birth, albeit insufficient to suppress consciousness and concurrent EEG activation (Mellor and Diesch, 2006). A study investigating the effects of postnatal age on EEG responses to castration in lambs over the first 6 weeks of life identified an increase in cerebral responsiveness to noxious stimulation over the period 7–10 days (Johnson et al., 2009), and the authors concluded that the lingering effects of fetal neurosuppressive mechanisms might have been responsible for the lesser responsiveness in younger lambs. Quantitative EEG analysis showed that 1‐day‐old neonatal piglets did not respond to noxious stimulation caused by tail docking, whereas piglets aged between 5 and 15 days showed a characteristic nociceptive response (Kells et al., 2013). It is not known whether significant concentrations of these agents are present in the plasma of premature ungulate fetuses of different gestational ages, and whether they might influence the capacity to attain full consciousness or experience pain when exteriorised and exposed to air.
Despite all the considerations presented above, there is no direct evidence which proves that fetuses remain unaware and unable to experience pain or negative affect throughout pregnancy, and some observations have led to suggestions that this may not be the case. While in utero, the sheep fetus at 0.8 G has been shown to respond to pressure and auditory stimuli/ noise by somatic movements, tachycardia and changes in ECoG (Ruckebusch, 1972). Fetal lambs also show a respiratory response to somatic stimulation which varies according to their sleep state; response was greatest during REM sleep, lowest during NREM sleep, and intermediate during the AW state (Ioffe et al., 1980). Surgery in unaesthesised lambs (ewes given epidural anaesthesia) provokes strong movements, especially after 120 days of gestation (0.82 G); no such reactions are seen in the anaesthesised fetus. Similarly, human fetuses respond to invasive intrahepatic vein transfusion with vigorous body and breathing movements and a rise in cortisol and β‐endorphin levels, which are not seen following non‐invasive umbilical vein transfusion (Giannakoulopoulos et al., 1994). However, such behavioural reactions can be mediated at the subcortical and brainstem level and are not, therefore, conclusive evidence for experience of pain or other negative affect (Mellor et al., 2005). It is known that stimulus processing can be independent of conscious perception, as is demonstrated during surgery in adult animals under general anaesthesia, where nociceptive stimuli can still elicit subcortically mediated physiological stress responses despite unconsciousness (Desborough, 2000; Marana et al., 2000). Electrical activity in the somatosensory cortex can be elicited by sensory input from the forelimb from about 125 days of gestation (0.85 G) in lambs (Cook et al., 1987). However, it is suggested that the ‘exaggerated’ responses to nociceptive stimuli seen in the fetus are a feature of immaturity of the central nervous system rather than enhanced pain perception (Mellor and Gregory, 2003). Despite this view, Mellor and Gregory, 2003 still recommend the use of general anaesthesia of both mother and fetus to minimise fetal movements during surgery and to ensure the fetus remains unaroused and unconscious throughout. Even while it is completely anaesthetised, the fetus will exhibit physiological stress responses due to direct surgical stimulation, to cooling and to increased hypoxaemia/hypercapnia through compromised placental gas exchange (Jones, 1977; Jones and Fox, 1977; Gunn and Bennet, 2009). In human infants and developing mammals, there is an arousal sequence including both subcortical or autonomic and cortical changes that occurs both spontaneously and in response to external stimuli (Lijowska et al., 1997; Dauger et al., 2001; Darnall et al., 2010). Mellor et al. (2005) discuss further evidence that fetal arousal in response to noxious stimuli is suppressed, from studies of the intense and potentially painful or distressing stimuli used in vibroacoustic stimulation (VAS). This is accompanied by variable changes in EEG activity, depending on the fetal sleep state during stimulation. However, they propose that detailed analysis of the EEG indicates similar dynamics to those seen during spontaneous sleep state transitions rather than arousal to an AW state (Schwab et al., 2000).
It is difficult to interpret whether EEG activity is indicative of conscious awareness in the fetus. One indirect line of evidence would be to identify characteristics of the EEG which are associated with conscious experience in the only species able to self‐report – the human. In human literature, conscious awareness has been specifically associated with the presence of late latency potentials following a somatosensory stimulus (Schubert et al., 2006). They demonstrated that the EEG associated with conscious stimulus processing differs significantly from unconscious processing with potentials starting around 100 ms after stimulus presentation when the signal is processed in parietal and frontal cortices, brain regions crucial for stimulus access into conscious perception. In the fetal EEG, such late latency potentials do exist (Cook et al., 1987), but it is uncertain if the same interpretation can be made.
Selective responsiveness of the fetus to external stimuli has been shown in several examples. In human fetuses, an advanced functional magnetic resonance imaging (fMRI) procedure was used to demonstrate selective cortical processing for the maternal voice at 34 weeks of gestational age (0.88 G). At 33 weeks, the left temporal cortex was significantly more activated during exposure to voices than to pure tones, while at 34 weeks a differential response to the maternal voice and an unfamiliar voice was recorded, suggesting the existence of in utero associative learning (Jardri et al., 2010). Furthermore, a single case study suggests the possibility of operant conditioning of a human fetus with an increased frequency of kicking induced in response to a reinforcer of paternal verbalisation (Cautilli and Dziewolska, 2005).
There is also evidence that interventions applied during pregnancy can have long‐term effects on development and behaviour postnatally (Schneider and Suomi, 1992; Janczak et al., 2006). The fetus of various species has been shown to be capable of ‘associative learning’; stimuli with which the human fetus has become familiar in utero are differentiated from novel stimuli postnatally. Human fetuses, and subsequently neonates, have shown differential response to familiar music (Hepper, 1991) and to the maternal voice (Hepper et al., 1993). Using the sucking response, neonates have been shown to differentiate their mother's voices from other female voices, and the maternal voice is a more effective reinforcer when compared to other female voices. Furthermore, neonates were shown to prefer a familiar passage of reading performed during pregnancy over a novel passage of reading, regardless of whether the passage was recited by the infant's mother or an unfamiliar woman (DeCasper and Spence, 1986). There has also been demonstration of postnatal aversion in rodents when presented with stimuli that were associated with an artificially induced episode of hypoxia in utero, or following conditioned taste aversion at the fetal stage (Gruest et al., 2004), and of postnatal attraction to chemical odours and flavours experienced in the uterine environment in a range of species (Hepper, 1988; Schaal et al., 2000). This evidence of memory for classical conditioning, habituation and exposure learning paradigms (James, 2010) is the basis for some to believe in fetal consciousness, but these could occur by passive learning in the absence of any conscious state. Such ‘fetal learning’ responses do not necessarily require a cortex in a state of wakefulness and can be induced in simple circuits in lower organisms (Hawkins and Byrne, 2015). A better test of fetal consciousness could be devised if there were forms of learning that absolutely require a conscious state for successful performance. It has been suggested that the ‘trace conditioning’ paradigm bears on consciousness in a very focused way. It is claimed that trace conditioning does not take place without the learner being focally aware of the conditioning contingency (Clark and Squire, 1998), but testing of such paradigms has not yet been reported for the fetus.
The possibility that the fetus may experience pain or other negative affective states has been considered by previous scientific working groups focussed on both animals used in experiments and on humans. At first sight, their conclusion that legal protection should be given to the fetus and that anaesthesia should be provided during any procedures might be taken to support a view that the fetus is capable of feeling pain or negative affect. However, reading of the justification for their recommendation does not indicate that this is the case. Based on the available evidence, the Scientific Opinion on ‘Aspects of the biology and welfare of animals used for experimental and other scientific purposes’ (EFSA, 2005) concluded that, even though the mammalian fetus can show physical responses to external stimuli, the weight of evidence suggested that consciousness does not occur in the fetus until it is delivered and starts to breathe air. However, since experimental procedures that involve oxygenating a fetus might induce consciousness, and since events in utero can influence the behaviour of the individual once it is born, and some of those effects could be important to its subsequent welfare, they concluded that fetuses in the second half of pregnancy should be given legal protection when used for experimental purposes. This decision that protection is necessary does not have implications for the welfare of the fetus at slaughter of the dam, since such fetuses are not subject to prior experimental intervention and will not survive to experience any possible detrimental effects in later life.
Consideration of the issue of fetal pain by the Royal College of Obstetricians and Gynaecologists (RCOG, 2010), which includes experimental evidence gathered from sheep fetuses, led to similar conclusions regarding the human fetus. While the evidence suggested that the autonomic and endocrine pathways are in place for the fetus to mount a stress response as early as 18 weeks of gestation (0.47 G), with increases in cerebral blood flow, catecholamines and cortisol following invasive procedures, and attenuation of these responses by administration of fetal analgesia at the start of the procedure, the RCOG concluded that these responses cannot be interpreted as evidence that the fetus is feeling pain. This interpretation is disputed by Bellieni and Buonocore (2012), who argue that a dramatic increase in stress hormones during fetal procedures, and its abolition by anaesthesia should be viewed as providing evidence of fetal pain perception. The RCOG report gives credence to the evidence that the fetus never experiences a state of true wakefulness in utero and is kept, by the presence of its chemical environment, in a continuous sleep‐like unconsciousness or sedation which can suppress higher cortical activation in the presence of intrusive external stimuli. However, since fetal exposure to ‘stress’ in utero can modulate the later function of the hypothalamic–pituitary axis, reducing the magnitude of the initial stress response, for example by using fetal analgesia, may have beneficial effects. The degree to which these effects can be observed following fetal exposure to a painful stimulus remains uncertain, as most studies to date are postnatal and refer to intense, repetitive stimuli that are not normally experienced in utero. They concluded that the uncertain benefit of attenuating the fetal stress response to a noxious stimulus in utero by administering analgesia needs to be balanced against the practical difficulties to the administration of effective fetal analgesia, as well as the possibility of adverse effects. While the evidence that analgesia confers any benefit on the fetus at any stage of gestation is lacking, in practice, maternal infusion of opiates has been used to sedate the human fetus, to achieve immobilisation, rather than analgesia.
The balance of evidence therefore indicates that the fetus is subject to overriding brain inhibitory mechanisms which will preclude experience of pain or other negative affect. These inhibitory mechanisms persist until overridden by mechanical and thermal stimuli associated with birth and the increase in oxygenation of the brain when breathing is initiated. However, some lines of evidence give rise to the possibility that the fetus might have periods of conscious experience while in uterus.
3.3.3.3. Summary of scientific evidence on the possibility of ‘sub‐cortical’ awareness
While most research into consciousness assumes (almost without questioning it) that cognitive processes are exclusively performed in the upper cortex of the brain, recent discussions hypothesise that lower regions may also be involved. Merker (2005) triggered this debate by redefining what consciousness means, and describing it as the processes which are inbetween the establishment of a ‘world space’ based on highly complex sensory input, and the intentional and detailed execution of musculoskeletal activities within this space. He argues that with increasing complexity of the organism higher up the phylogenetic ladder, the processes required to link the sensory preliminaries and the motor sequels become equally increasingly complex. He hypothesises that a major category of information for decision‐making is included, and relates to a ‘vast array of emotional/motivational biasing variables experienced as feelings, affects, moods, and sentiments’ (Merker, 2005). To determine the actual location in the brain where these activities take place is not an easy task, but Merker proposes that essential aspects of consciousness are functionally linked to cellular territories which extend from the colliculus to the hypothalamus. Together with the midbrain reticular formation, they provide pivotal structures which determine behavioural states through several endocrine mechanisms including serotonergic, adrenergic and cholinergic systems. They regulate sleep cycles, wakefulness, activity levels, and vigilance, and ‘set the ‘boundary conditions’ for consciousness’ (Merker, 2005). The regions Merker refers to are in direct contact with the upper cortex, but reside in the upper brainstem. In a later paper, Merker (2007) argues that this structure is central in establishing conscious perception, even when cortical input is absent. It may therefore not be necessary to have cortical processing for an organism to be conscious.
Empirical evidence to support this hypothesis comes from work by Whishaw (1990) on decorticated rats, which show no gross abnormalities in behaviour to the casual observer (although some impaired behavioural indicators would be noted by an experienced observer). Furthermore, Merker (2007) presents a medical condition called hydranencephaly (Friede, 1989). It is acquired, for example, by wholesale resorption of the forebrain tissue due to an intrauterine vascular accident (stroke) of the fetal brain. The tissue is replaced by cerebrospinal fluid, filling otherwise empty meninges. The condition is often not diagnosed until several months postnatally when the children start developing a variety of complications that always include motoric ones (e.g. cerebral palsy), and often include seizures. However, this situation can be stabilised and children live for many years or even decades. These fragile patients have no cerebral cortex, but show evidence of not only being awake, but also respond to environmental stimuli in a conscious way (Shewmon et al., 1999). These two examples support the so far unproven theory that some degree of consciousness, including the possibility of suffering, may be present because of these subcortically based structures.
The fetal thalamus develops much earlier than the cortex (Anand, 2007). The brainstem reflexes like breathing are already present before the EEG appears. Therefore, the proposed subcortically based consciousness may even occur before the developmental stage at which the capacity for cortically based consciousness is established. Campbell et al. (2014) therefore postulate that, prior to the stage at which the cortex is sufficiently developed, there may be manifestations of subcortical consciousness that include relatively undifferentiated negative experiences of discomfort. They refer to this as the presence of ‘raw basic affects’, and suggest that ‘although such proposed experiences would be unpleasant, it is not known if their character, intensity and duration would be sufficient to constitute suffering. Thus, the possibility that suffering may occur during this stage of neurological development can, at this point in time, neither be ruled in, nor ruled out’ (Campbell et al., 2014). Data on the exact stage of pregnancy, at which these hypothesised experiences may start, is not available.
There are at least three objections to the notion of subcortically based consciousness according to Campbell et al. (2014). First, Merker made the argument that upper brainstem functions of lower phyla species may be conserved in earlier developmental stages of higher phyla species, but the assumption that neurological development and sequencing of connectivity are uniform across species is not supported in scientific literature. Secondly, the evidence based on decorticated animals or hydranencephalic infants may be misleading due to residual functional cortical tissue. Finally, even if subcortically based consciousness may be present in certain pathologies, it is still uncertain if subcortically based consciousness precedes functional cortically based consciousness in healthy and intact animals or operates if cortical activity is suppressed.
Of course, it is unsure if these awareness ‘states’ (in particular in relation to the hydranencephalic child) are comparable to that of an unborn fetus. It can be argued that, unlike the fetal brain, the brain of the hydranencephalic child is not under inhibitory substance control. This would mean that even if ‘raw basic affects’ in fetuses are anatomically possible, they would not be perceived consciously by the fetus. The subcortical system also requires a sufficient level of oxygen to be active, similar to any other system in the body. It appears the brainstem reflexes are mediated in the same way as cortical reflexes or responses. Although adenosine concentrations are lower in these regions (Bocking, 2003), hypoxia mediated increase will lead to cessation of breathing and fetal movements in utero, which are regulated by brain stem structures.
The hypothesised subcortical consciousness theory is not yet proven. However, in utero raw basic affects are unlikely to cause negative experiences in fetuses in the last third of gestation because the previously described brain inhibitory mechanisms would also suppress subcortical structures.
3.3.3.4. Summary of scientific evidence on C3. Changes occurring during slaughter of the dam and effect on the fetus (e.g. electric current, hypoxia through ceased maternal blood flow, elevated stress hormone levels)
Effect of acute maternal stress through handling in the abattoir on the fetus
In abattoirs, animals are unloaded, held in pens, moved to the stunning area, restrained, stunned and slaughtered. During this period, the dam is exposed simultaneously to a variety of stressors that may result in high levels of fearfulness and pain, inducing psychological and physical stress that might have an indirect effect to the fetus through the maternal hypothalamus–pituitary–adrenal (HPA) axis. In sheep, prenatal maternal stress, during early or late gestation increases fetal cortisol responses (Rakers et al., 2013).
Effect of stunning methods
Captive bolt stunning
During captive bolt stunning, the impact of the bolt on the skull results in brain concussion and immediate loss of consciousness and sensibility. The penetration of the bolt into the brain produces substantial damage of the vital centres in the brain stem rapidly rendering the animal unconscious until death caused by severing the major arteries supplying the brain. Successful induction of brain concussion manifests as immediate collapse of the animal and onset of apnoea (absence of breathing), followed by the onset of a tonic seizure, which can be recognised from the occurrence of arched back and legs flexed under the body, and fixed eyes (EFSA AHAW Panel, 2013). The duration of the tonic seizure is influenced by several factors (e.g. animal category, type of captive bolt gun and ammunition), but usually lasts for seconds and is followed by loss of muscle tone.
Electrical stunning
Electrical stunning is based on passing an electrical current through the head that causes a generalised epileptiform activity in the brain and the immediate loss of consciousness (EFSA, 2004). Successful induction of epileptiform activity is manifested as immediate collapse owing to the onset of tonic seizure. During the tonic phase, the blood pressure increases and the animal shows tetanus (rigidly extended legs), breathing is absent and the eyeballs may be fixed or obscured (cornea not visible due to eyeball rotation into the socket). Tonic seizure is followed by clonic seizures lasting for seconds and terminating in loss of muscle tone (EFSA AHAW Panel, 2013). The seizures are not conducive to prompt and accurate sticking of animals to prevent return of consciousness following stunning.
In head‐to‐body electrical stunning, the current flows from the electrode located on the head to a second one placed on the chest or on the spinal cord, caudal to the position of the heart, thereby causing the current to pass through the heart and the spinal nerves. The inhibition of the spinal nerve function produces a reduction in clonic convulsions. This reduces the intensity of muscle contractions and limits the increase in blood pressure. The current flows through the heart inducing cardiac ventricular fibrillation in the dam, cardiac arrest and death of the animal.
During head‐to‐body electrical stunning, the current might pass through the uterus stimulating muscle convulsions and inducing birth in late pregnant animals. On the other hand, the current passing through the amniotic fluid might cause fetus’ ventricular fibrillation and death.
Peisker et al. (2008) studied the fetal responses in sows killed by electrical current during the second and last third of pregnancy. Treatments implied the application of electrical current either to the head and heart, to the head, heart and the uterus or from the upper body to the vagina. Fetuses were then delivered through caesarean section at intervals of 3–4 min but remained attached to the umbilical cord while the rest of the fetuses remained in the uterus until delivery. No method was found to kill the fetal pigs immediately. However, in the last third of gestation, fetuses from sows that received a current from the upper body to the vagina showed a significantly faster decrease in heart rate and blood pressure as well as a shorter period of time for the absence of fetal body movements and reflexes. Body movements were observed/palpated in both fetuses which had been delivered as well as in fetuses which remained in the uterus. Cardiovascular decompensation occurred after on average 13 and 16 min (second and last third of gestation, respectively) after killing of the sow via head‐to‐heart electrical current and after 15 and 6 min when electrical current was applied from the upper body to the vagina.
Gas stunning
Under the EU legislation, stunning of mammals by exposure to gas mixtures is only permitted in pigs. An atmosphere with high concentrations of carbon dioxide (CO2) (> 80% by volume in air) induces hypercapnic hypoxia in the dam and inhibits neurones through acidosis. During CO2 inhalation, partial pressure of oxygen (pO2) and oxygen saturation (SatO2) in the blood decrease progressively (Rodriguez et al., 2008), and induces respiratory and metabolic acidosis, reduces the pH of cerebrospinal fluid (CSF), which bathes the brain and spinal cord and neurons, thereby exerting its neuronal inhibitory and anaesthetic effects (Woodbury and Karler, 1960). Consequently, the animal loses consciousness (Gregory, 1986). In this regard, normal pH of CSF is 7.4 and unconsciousness begins when the CSF pH falls below 7.1 and reaches a maximum at pH 6.8. The presence of CO2 in the blood is sensed by specific CO2‐sensitive chemoreceptors that stimulate respiration (hyperventilation), heart rate and blood pressure, and sense of breathlessness prior to loss of consciousness.
Piglets exposed to CO2 as an anaesthetic for surgical castration, showed also signs of breathlessness and behavioural excitation (Kohler et al., 1998) as well as an initial stimulation and later depression of breathing and heart rate (Gerritzen et al., 2008).
Hypoxia or anoxia occurring because of the inhalation of argon or nitrogen (< 2% by volume of oxygen) induces unconsciousness by depriving the brain of oxygen. It has been established that cerebral dysfunction occurs in mammals when the partial pressure of oxygen in cerebral venous blood falls below 19 mm Hg. The depletion of O2 causes neuronal depolarisation and intracellular metabolic crisis leading to cellular death in neurons (Rosen and Morris, 1991; Huang et al., 1994). In contrast to hypercapnia, anoxia does not cause aversion prior to loss of consciousness.
The partial pressure of oxygen (pO2) and partial pressure of carbon dioxide (pCO2) in the blood of sheep fetus are 20–27 mm Hg and 40–50 mm Hg that represent 25% and 135% in relation to the maternal pO2 and pCO2, respectively (Mellor and Gregory, 2003). This fetal hypoxaemia contributes to the elevated level of adenosine, being between two‐ and fourfold higher than in the dam.
When exposing the dam to high concentration of CO2, the maternal blood with high pCO2 will flow also to the placenta exhibiting a rapid rise of pCO2 and decrease in pO2 in the fetal circulation. The fetal anoxia accompanying the gas mixture inhalation of the dam triggers adenosine release (Koos and Doany, 1991) that suppresses cerebral metabolism, helping to limit cortical damage during transient hypoxic/anoxic episodes (Hunter et al., 2003). The developing fetal cerebral anoxia, through the agency of adenosine, will shut down the electrocortical activity, reducing cortical oxygen demands, i.e. the EEG becomes isoelectric (Mallard et al., 1992). If the oxygen supply is reinstated within 5–6 min, the EEG and cortical oxygen consumption usually return to normal (Campbell et al., 2014).
Effect of post‐maternal death (by bleeding or killing)
The neck cut of the dam will rapidly stop blood flow to the uterus within about 10 s, preventing oxygen delivery to fetal tissues, including the brain. This severe fetal hypoxaemia and hypercapnia would be equivalent to occluding the umbilical cord in utero and will lead to flattening of the fetal brain electrical activity within 1 min (Mallard et al., 1992). Cortical activity cannot continue in anoxic conditions whether or not adenosine is acting, eliminating any possible behavioural arousal and awareness (Mellor and Gregory, 2003). Once oxygen supply to the fetus has ceased, the isoelectric EEG will continue until death apart from some seizure activity that, according to Mellor and Gregory (2003), indicates disrupted function that precedes neuronal death. If the fetus remains in the uterus, the high PaCO2 will prevent behavioural arousal or awareness.
Acute hypoxia in the sheep fetus induced by reduced uterine blood flow or umbilical cord occlusion decreases fetal breathing movements and this effect is attributed to hypoxia induced elevated levels of adenosine in the brain (Koos et al., 1994, 1997; Watson et al., 2002). When acute fetal hypoxia is associated with hypercapnia, the amplitude of the fetal breathing movement increases (Watson et al., 2002) and is attributed to the effects of hypercapnia and central acidosis (Dawes et al., 1982; Hohimer et al., 1983). Research involving sheep fetal brain sectioning has demonstrated that the fetal midbrain is responsible for the episodic breathing pattern and hypoxic inhibition of breathing (Dawes et al., 1983). The parafascicular complex, located in the rostral part of the reticular activating system, has been identified as responsible for inhibition of fetal breathing during acute hypoxaemia (Koos et al., 1998). The responses to hypoxia and hypercapnia are maintained in the decorticate sheep fetuses (Ioffe et al., 1980), suggesting that the cerebral cortex is not involved. Both central and peripheral chemoreceptors are responsible for these effects.
Both hypoxia and hypercapnia produce bradycardia. However, when the fetal hypoxaemia is maintained in pregnant sheep, there is a gradual return in the fetal heart rate to normal followed by tachycardia which is associated with elevations in fetal catecholamine levels.
Fetal breathing movements (FBMs) are rhythmic contractions of the diaphragm, intercostals and laryngeal muscles presented approximately 30% of the time in the late gestation in human and ovine fetuses (Dawes et al., 1972). In fetal sheep, a reduction to 33% of fetal arterial oxygen saturation from its baseline value did not change frequency and amplitude of breathing movements (Sameshima and Koos, 1986). More acute hypoxia resulted in decline of breathing movements and muscle atonia (Breen et al., 1997), due to the increase of the adenosine levels in the areas of the brain regulating FBMs (Bocking, 2003). Bissonnette (2000) concluded that there is considerable evidence that central mechanisms overcome the excitatory input from peripheral chemoreceptors, resulting in respiratory depression. However, Bocking (2003) suggested that severe hypoxia increases the number and amplitude of deep inspiratory efforts, equivalent to gasps prior to death (Bocking, 2003). When the acute fetal hypoxia is associated with increased PaCO2, the metabolic acidosis increases the amplitude of the remaining FBMs (Bocking, 2003; Darnall, 2010).
In neonates and adult animals, the exposure to high concentration of CO2 induces breathlessness. At least three different qualities of breathlessness are currently recognised: respiratory effort, air hunger and chest tightness (Beausoleil and Mellor, 2015). The form of breathlessness most likely to be experienced by the fetus, if it has any conscious experience, is ‘air hunger’ and is linked to the CO2‐sensitive chemoreceptors activation.
When a pig fetus, still in the amniotic sac, is removed from the uterus of a sow which has been killed by cardiac arrest but not exsanguinated, there is an increased physical activity e.g. gasping and convulsions (Peisker et al., 2008). These movements (convulsions) were not observed in fetuses with a gestational age of 0.3 G but observed in fetuses about 0.6 G (so after midterm) (Peisker et al., 2008). Since the fetus is not in contact with air, it would be interesting to know if, e.g. hypothermia induced by this process acted as an environmental stimulus for these manifestations. These convulsions also occurred when the pig fetuses were left in the uterus, but observed/palpated. Peisker et al. (2008) interpret these movements as ‘hypoxic distress’. These manifestations could be interpreted as a struggle by the fetus to escape a perceived negative situation due to brainstem stimulation associated with raw basic affects. However, there is no evidence for any relation to sub‐cortical structures. To be certain about the interpretation it would be necessary to differentiate between brain stem reflexes and spinal reflexes by abolishing brainstem control.
Therefore, during the last third of gestation, the tonic‐clonic seizures induced by mechanical and electrical stunning of the dam might lead to physical stimulation of the fetus immediately after stunning. However, due to the presence of brain inhibitory mechanisms fetuses may not experience associated pain or other negative affect. The hypercapnic hypoxia occurring in the dam during gas stunning would lead to adenosine mediated inhibitory influences on the fetal brain as previously explained.
3.3.4. Expert judgements on ToR3 (capacity of fetuses to experience pain and other negative affect)
The outcomes from EKE 2 are shown in Appendix D. These outcomes were evaluated in the context of additional findings from the literature and further expert discussion to generate final conclusions for this opinion.
3.3.4.1. Expert judgement for C1. Anatomical and neurophysiological correlates/structures necessary for perception of pain and other negative affect
There was broad agreement among EKE 2 participants that livestock fetuses by the last term of gestation have all structures required to feel pain and other negative affect. The AHAW Panel concludes that it is very likely to extremely likely (i.e. with 90–100% likelihood) that ‘livestock fetuses in the last third of gestation have the anatomical and neurophysiological structures/correlates for experiencing pain and/or other negative affect’. This is based on the expert judgement related to the critical stage of gestation as reported in Appendix D.
3.3.4.2. Expert judgement for C2. Neurophysiological situation and possibility of cortically based conscious perception
There was broad agreement among EKE 2 participants that livestock fetuses by the end of gestation do respond to external stimuli including those that have the possibility to cause pain or negative affect. However, when assessing the possibility of actually experiencing negative states while showing such responses, there was no consensus among EKE 2 participants and no certainty at either end, with two group views: one group (12 experts) with a 5–40% likelihood that such negative experience occurs and another group (2 experts) with a 70–85% likelihood that it does.
Based on the available evidence and expert judgement, the Panel concluded that it is likely to very likely that ‘the neuro‐physiological situation of the livestock fetuses in the last third of gestation (e.g. inhibitory and excitatory systems) does not allow for cortically based conscious perception’ (i.e. with 66–99% likelihood of correctness for this statement according to the qualitative likelihood scale; see Figure 1).
Evidence suggesting lack of conscious perception arises from:
the presence of adenosine mediated brain inhibitory (neuroprotective) mechanisms operating in utero, demonstrated by EEG records;
low level of fetal brain oxygen;
predominance of sleep like states in the fetal EEG;
lack of direct evidence of cortically based conscious perception.
It is therefore possible, but unlikely to very unlikely that ‘livestock fetuses in the last third of gestation can consciously perceive pain and negative affect’ (i.e. with 1–33% likelihood of correctness for this statement). This possibility arises from:
the lack of direct evidence proving that fetuses are incapable of cortically based conscious perception;
-
differences in the interpretation of indirect evidence relating to:
fetal EEG (e.g. significance of transitional EEG);
observed fetal behavioural and physiological responses to external stimuli;
fetal learning (conscious learning versus conditioned responses).
3.3.4.3. Expert judgement for C3. Changes occurring during slaughter of the dam and effect on the fetus
There was broad agreement at EKE 2 that livestock fetuses show responses to specified stunning and slaughter conditions. The Panel concluded that:
It is very likely to extremely likely (i.e. with 90–100% likelihood) that a livestock fetus shows measurable responses to extreme hypercapnic hypoxia, mechanical stimulation and electrical current.
-
The likelihood that ‘the fetus will be subjected to these stimuli during stunning of the dam’ is:
-
–
extremely likely (i.e. with 99–100% likelihood) in the case of CO2 stunning (hypercapnic hypoxia).
-
–
unlikely (i.e. with 10–33% likelihood) in the case of movements or seizures of the dam during handling, stunning and shackling (mechanical stimulation).
-
–
very unlikely (i.e. with 1–10% likelihood) in the case of head‐only electrical stunning (electrical current).
-
–
as likely as not (i.e. with 33–66% likelihood) in the case of head‐to‐body electrical stunning (electrical current).
-
–
it is very likely to extremely likely (i.e. with 90–100% likelihood) that fetuses show measurable responses to hypercapnic hypoxia as induced by maternal circulatory collapse/sticking of the dam.
-
–
3.3.4.4. Expert judgement for C4. Possibility that fetuses experience negative affective state following specified stunning and slaughter conditions
No direct evidence is available on the possibility that livestock fetuses experience negative affective states following the applications of stunning and slaughter conditions to the pregnant animals. This was discussed at the EKE 2 meeting and probability estimates were elicited. The Panel concluded that, since all slaughtering procedures involve a maternal circulatory collapse, it is unlikely to very unlikely (i.e. with 1–33% likelihood) that changes/responses occurring during stunning and bleeding of the dam are associated with pain or other negative affective states in the fetus. This is because of the combination of the probability of exposure to the stimulus and the considerations detailed here below in the conclusions on cortical and subcortical awareness (see Section 3.3.4.6).
3.3.4.5. Expert judgement for C5. Level of negative affective state experienced by fetuses during slaughter of the dam
It was not possible to provide a meaningful answer to this question during the EKE 2 meeting because of the difficulty of objectively categorising intensity of affective state. The AHAW Panel concluded that on the basis of present scientific evidence it is not possible to reach an objective conclusion on the intensity of affective states which would be present if livestock fetuses did have conscious experience.
3.3.4.6. Logical model for subcortically based conscious perception
Discussion at the EKE 2 meeting led to agreement that the presence of a sleep‐like state in livestock fetuses (due to neuroinhibitors in utero) would make the distinction between cortex and subcortex irrelevant. There is no direct evidence demonstrating the existence of subcortical awareness in livestock fetuses and the existence of the hypothesised raw basic affect. However, even if this were to exist it is unlikely to very unlikely (i.e. with 1–33% likelihood of correctness) that ‘the neuro‐physiological situation of the livestock fetuses in the last third of gestation (e.g. inhibitory and excitatory systems) allows for sub‐cortically based conscious perception’. This probability estimate arises from the same considerations as for the cortical situation because the same inhibitory mechanisms will pertain. The combination of the low likelihood of the existence of raw basic affect and the high likelihood of an inhibitory uterine environment makes it very unlikely (i.e. with 1–10% likelihood of correctness) that the livestock fetuses will experience negative affect as a result of subcortical awareness.
3.4. Assessment for ToR4 (methods suitable for stunning and killing of fetuses or neonates of the main livestock species when a pregnant dam has been delivered to the slaughterhouse at a critical phase of gestation)
According to the outcomes of the assessment of ToR3 (see above), it seems appropriate to restrict actions as regards stunning and killing of fetuses to the third term of gestation, since there is no indication of capacity to experience pain and other negative affect at earlier stages. Focusing on the third term, however, two perspectives may be taken when approaching this term of reference. A first set of scenarios and respective actions to be taken was developed under the assumption that it is likely to very likely (i.e. with 66–99% likelihood) that the neurophysiological situation of the fetus in the third term of gestation does not allow for conscious perception (see Section 3.4.1).
Since, however, uncertainty exists regarding the ability of fetuses to experience negative affective states (i.e. with 1–33% likelihood that the neurophysiological situation does allow for consciousness), a set of scenarios which would be applicable to this less likely situation was also developed (see Section 3.4.2).
3.4.1. Scenarios operating under the assumption that the neurophysiological situation of the fetus does not allow for consciousness
Four scenarios are described taking into consideration when the pregnancy is detected and if the fetus has been exposed to air (Figure 5). For each scenario a course of action is proposed. These actions operate under the assumption that the neurophysiological situation of the fetus does not allow for consciousness.
Figure 5.
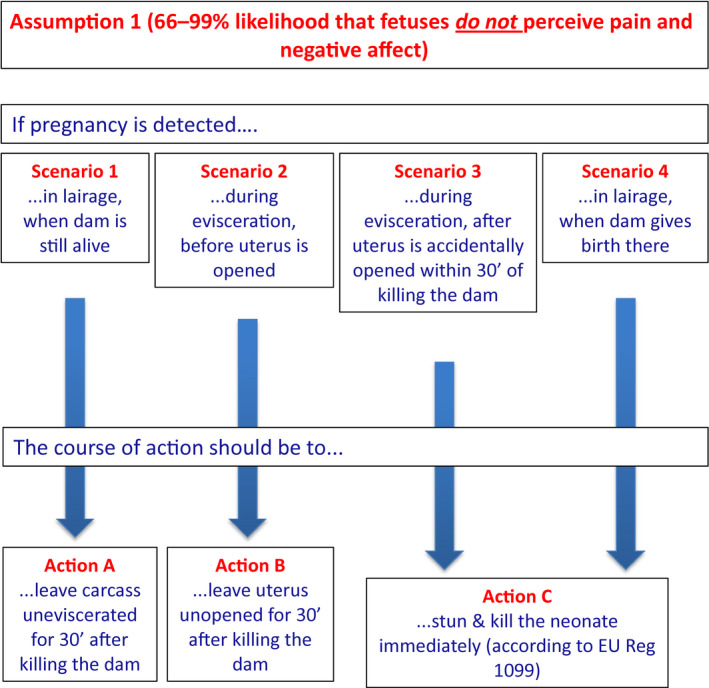
Scenarios and respective courses of action as regards handling of the fetus/neonate under the assumption that the neurophysiological situation of the fetus does not allow for consciousness and taking into consideration when the pregnancy is detected and if the fetus has been exposed to air.
Scenario 1: The pregnancy is detected during unloading or in lairage when the dam is still alive.
Action A: Pregnant animals in the last third of gestation should be stunned and slaughtered without any delay. If the dam is fit, move it to the slaughter area for stunning and sticking. If not, perform the emergency killing on the spot according to the standard operating procedure of the slaughterhouse. In both cases, leaving the fetus in the uterus inside the dam (uneviscerated) for 30 min after slaughter of the dam eliminates the possibility of occurrence of hypothermia and physical stimulation due to evisceration before the death of the fetus from hypoxia and hypercapnia. After that time, the survival risk of the fetus is low. However, if afterwards the uterus is opened and the neonate has a heartbeat or starts to show any breathing movements, it should be stunned and killed immediately, following Action C (see scenario 3 below).
In the slaughter situation, cessation of maternal circulation and its physiological consequences for the fetus, analogous to experimental cord occlusion, would occur rapidly. Independent of the stunning method, cutting of the major blood vessels in the neck of the dam results in a time to loss of 50 and 90% of the total blood volume, respectively, of 38 and 94 s in cattle and 14 and 56 s in sheep (Anil et al., 2004, 2006). There are no published corresponding data for horses, goats and pigs. Nevertheless, cutting both carotid arteries or the vessels from which they arise is mandatory in all animal species slaughtered for human consumption under the EC Slaughter Regulation 1099/2009 and therefore most of the circulating blood volume is expected to be lost within the first minute after slaughter of the dam. It is also worth noting that, according to Regulation 1099/2009, carcass dressing must not begin until the absence of signs of life of the animal has been verified and bleeding rails are normally used as buffer to hold and maintain steady supply of carcasses to the evisceration line, especially in high‐throughput slaughterhouses. Owing to this, the time interval between maternal slaughter and carcass evisceration may exceed 20 min in modern high throughput slaughterhouses.
Physiologically, systemic blood pressure in the dam would be reduced significantly when 50% of the total blood volume is lost during slaughter and uterine blood flow would also be expected to cease (Mellor and Gregory, 2003). As a consequence, placental gas exchange would be expected to stop, leading to rapid onset of fetal hypoxia and hypercapnia (referred to as asphyxia in literature), and marked reduction in oxygen supply to the fetal brain (Jensen et al., 1987; Gunn and Bennet, 2009). Under this situation, fetal brain activity (recorded using electrocorticogram, ECoG) will be severely depressed within 60–90 s, as demonstrated by complete umbilical cord occlusion in utero in sheep (Mallard et al., 1992; Hunter et al., 2003). Although brainstem functions controlling respiration and cardiac activity are preserved in these fetuses under severe hypoxia and hypercapnia (Jensen et al., 1987), in the absence of maternal circulation required to oxygenate the brain and remove metabolic waste, they are unlikely to become aware or conscious. More importantly, these fetuses left in the uterus would exhibit gasping and heartbeat for different durations (Boddy et al., 1974; Jensen et al., 1982; Rigatto et al., 1986), which means that, especially those in the last third of gestation, they have the capacity to attain consciousness if removed from the uterus and allowed to breath air.
The World Organisation for Animal Health (OIE) has proposed some guidelines in its Terrestrial Animal Health Code (Article 7.5.5. Management of fetuses during slaughter of pregnant animals) to deal with fetuses under different scenarios.
According to this Code, first, ‘fetuses should not be removed from the uterus sooner than 5 min after the maternal neck or chest cut to ensure absence of consciousness’. It also states that ‘if a live mature fetus is removed from the uterus, it should be prevented from inflating its lungs and breathing air (e.g. by clamping the trachea)’. These statements may be relevant only to scenarios where access to live, but unconscious, fetuses is warranted. Secondly, the Code states that ‘when uterine, placental or fetal tissues are to be collected, where practical, fetuses should not be removed from the uterus until at least 15–20 min after the maternal neck or chest cut’. Thirdly, the Code states that ‘when uterine, placental or fetal tissues, including blood, are not to be collected as part of the post‐slaughter processing of pregnant animals, all the fetuses should be left inside the unopened uterus until they are dead’, which is considered to be most relevant to this Mandate. The time period of 30 min after the slaughter of the dam suggested by the AHAW Panel experts is based on the consideration that this period would be sufficient to cause death in fetuses, although the time to onset of brain death in fetuses left in utero after slaughter of dams has not been studied in any of the species addressed in this opinion (i.e. all gestational ages of cattle, sheep, goat, pig and horse). Nevertheless, the impact of acute, near‐terminal umbilical cord occlusion (UCO) in sheep fetuses has been studied to some extent to elucidate the neurological consequences of asphyxia (hypoxia plus hypercapnia with metabolic acidosis), which is the expected physiological outcome during slaughter of dams in all these species of animals. The majority of data comes from sheep fetuses and examples are listed in Table 8; it is to be noted that the experimental protocol used in these studies involved re‐establishment of fetal circulation (i.e. removal of UCO), which does not apply to the slaughter situation.
Table 8.
Examples of umbilical cord occlusion (UCO) studies carried out in sheep fetuses and outcomes
| Author(s) | Gestational age – GA (in days) | Number of animals | Duration of UCO (min) | Outcome | EEG | Brain histology |
|---|---|---|---|---|---|---|
| Keunen et al. (1997) | 0.6 (85–90) | 11 | 10 | Survived | Not recorded | No neuronal damage |
| 8 | 15 | Survived | ||||
| 4 | 20 | Survived | ||||
| Mallard et al. (1992) | 0.8 (120–127) | 6 | 10 | Survived | Suppressed EEG during UCO | Neuronal loss in hippocampus |
| Bennet et al. (1999) | 0.6 (89–93) | 10 | 30 | Survived | Not recorded | Not recorded, but cerebral vascular responses studied indicated cerebral injury |
| George et al. (2004) | 0.6 (90–92) | 7 | 20 | Survived |
Suppressed EEG during UCO in both In 30 min group, epileptic activity superimposed on suppressed EEG during reperfusion |
Severe brain stem injury in 30 min group but not in 20 min group |
| 10 | 30 | Survived | ||||
| Drury et al. (2014) | 0.85 (125–129) | 29 | Until mean arterial pressure (MAP) dropped to 8 mm Hg (on average 16 min of UCO) |
3 died during UCO 8 died during recovery 5 ewes entered labour, hence euthanised 13 survived until euthanised for post‐mortem |
Suppressed EEG during UCO and recovery for 3 h 13 fetuses developed status epilepticus |
Neuronal loss in cortical and subcortical regions Neuronal loss was more severe in fetuses showing status epilepticus |
| Wassink et al. (2007) | 0.6 (90–92) | 12 | 30 | Survived | Remained suppressed during occlusion. Magnitude of suppression was greater in 0.85 GA group | Not recorded |
| 0.7 (103–105) | 12 | 25 | Survived | |||
| 0.85 (124–126) | 7 | 15 | 6 out of 9 survived |
In addition, Dawes et al. (1972) investigated UCO in fetal lambs of 40–146 days of gestation age (5 g–6 kg body weight) delivered under maternal epidural or spinal anaesthesia into a warm saline bath (39–40°C). The results showed that the time to the last gasp after tying the umbilical cord, when body temperature was maintained constant, varied with gestational age in fetal lambs. At 80–100 days, it was 7.1 ± 0.38 (mean ± S.E.) min, which is statistically significantly longer than at term (5.1 ± 0.17 min) and at 40–55 days of gestation (2.0 ± 0.13 min). Prolonged survival time in midgestation fetuses is attributed to relatively higher glycogen store, greater anaerobic capacity in vital tissues and lower basal metabolic activity (Dawes et al., 1959). The only other species on which data are found is the pig (Peisker et al., 2008), indicating that at 102 days of gestation all piglets were dead within 20 min after killing of the sow. However, further research on in utero survival time is needed in all species.
It is also reassuring from the welfare point of view to note that Karlsson and Kjellmer (1974) investigated changes occurring in the somatosensory evoked responses (SERs), as a measure of the extent of neuronal disruption, in 25 lamb fetuses of 0.7–1.0 gestational ages (105–145 days) that were removed from their uterus with intact umbilical cords but maintained on a heated table during exposure of artificially ventilated dams (through open circuit) to hypoxic‐normocapnic and hypoxic‐hypercapnic gas mixtures. The results showed that the SERs were abolished in the latter group, typically within 15 min of administration of hypoxic‐hypercapnic gas mixture to the dam. At the moment of abolition of SERs, the fetal arterial partial pressure of oxygen level (PaO2) fell to 15 mm Hg, partial pressure of carbon dioxide (PaCO2) increased to 76 mm Hg, blood pH fell to 6.92 and oxygen saturation was 34% of baseline value indicating a rapid onset of profound brain dysfunction. Peripartum asphyxia has been known to result in epileptic seizures and neuronal loss in foals (Vaala, 1999) and increased perinatal mortality in piglets (Alonso‐Spilsbury et al.,2005).
It is therefore inferred that leaving fetuses in utero for 30 min after the slaughter of the dam would be sufficient to cause death. As a matter of precaution, the uterus should only be opened when fetal movements (including seizures) have ceased. These movements are not to be interpreted as signs of experiencing pain or negative affect but are triggered by reflexes (see Section 3.3.3.1).
Scenario 2: The pregnancy is detected during evisceration, but before the uterus is opened.
Action B: If the fetus has not been exposed to air, it is recommended to leave the uterus unopened and the fetus kept in the uterus for at least 30 min after killing the dam. It has to be ensured that the uterus is intact during this time and the fetus is not exposed to air allowing it to inflate the lungs.
Leaving the fetus in the uterus outside the dam (after evisceration) carries the risk of stimulation of the fetus due to hypothermia and physical stimulation due to evisceration. However, the fetus is not expected to survive 30 min after the collapse of maternal circulation, but to die from hypoxia and hypercapnia as discussed in scenario 1. However, if afterwards the uterus is opened and the neonate has a heartbeat or starts to show any breathing movements, it should be stunned and killed immediately, following Action C (see scenario 3 below).
Scenario 3: The pregnancy is detected during evisceration, but after the uterus has been accidentally opened within 30 min of killing the dam.
Action C: In this case, there is a risk of breathing leading to arousal and perhaps to a conscious state of the fetus. If this occurs, intervention must be applied to stun and kill it humanely.
There are no legal requirements concerning the methods of killing fetuses, and there are no available data in literature. However, as soon as the uterus is opened the fetus effectively becomes a neonate and Regulation 1099/2009 applies. The options available under Regulation 1099/2009 include penetrative captive bolt for all species and weights, percussive blow to the head of neonates up to 5 kg, and non‐penetrative captive bolt stunning of ruminant neonates intended for human consumption and weighing up to 10 kg, followed by bleeding.
Data regarding the efficacy of using penetrating captive bolt on exteriorised fetuses of any species is scarce. This raises the welfare concern about whether firing a penetrative captive bolt on the soft (unossified) skull with pliable bone sutures of a fetus or neonate will lead to brain concussion inducing unconsciousness. However, it has been reported that firing on the forehead of Jersey calves (n = 8) a captive bolt gun (Blitz PTB‐No3‐69; Jopp) with a bolt diameter of 12 mm and extrusion length of 75 mm using a blue cattle cartridge resulted in immediate, extensive trauma, with disintegration of large parts of the cerebrum and vital centres in the brainstem (recorded by macroscopic examination and CT scans), leading to immediate and lasting unconsciousness (Svendsen et al., 2008). Therefore, it is inferred that penetrative captive bolts used for stunning adult animals in slaughterhouses may also be used to stun exteriorised fetuses and neonates before slaughter by severing two carotid arteries.
In the case of non‐penetrative captive bolt and percussive blow, the force and location of the blow are two key parameters that determine the outcome. Regulation 1099/2009 states that, when using non‐penetrative captive bolts, fracture of the skull should be avoided. This consideration may not be applicable to neonates because application of a non‐penetrative captive bolt would cause extensive damage to the skull and the brain, leading to immediate death. However, food business operators should ensure that the size of the non‐penetrative captive bolt is appropriate for size of the skulls of exteriorised fetuses. Non‐penetrative captive bolts have been used effectively to kill neonatal goats up to 48 h of age (Sutherland et al., 2016), 3‐day‐old piglets (Casey‐Trott et al., 2013), 5‐ to 49‐day‐old (3–9 kg) piglets (Casey‐Trott et al., 2014) and neonatal piglets weighing up to 10.9 kg (Grist et al., 2017). Based on the EEG evidence in goat kids and computed tomography scan results in piglets, it is suggested that, when maintained and used correctly according to the manufacturers’ instructions, the magnitude of traumatic brain injury caused by commercially available non‐penetrating captive bolts, would be sufficient to kill ungulate neonates.
Regulation 1099/2009 also allows head‐only electrical stunning followed by bleeding or head‐to‐body electrical stunning for neonates. However, the large size of the electrodes designed for stunning large animals may not be appropriate for neonates. Even in the event of using specially designed and constructed stunning electrodes, application of an electric current to neonates that have been freshly removed from the uterus may lead to shunting of current over the surface of their wet skin (least resistant pathway to current flow), rather than flowing through the skull or body required to achieving desired welfare outcome.
Lethal injection is not permitted for slaughter under Regulation 1099/2009. It could be used for killing of the neonate, which is excluded from the human food chain, but has limitations including availability of suitable drugs (e.g. barbiturates) and requirement for administration by a licensed veterinarian. There are also considerations of food safety – in the event of fetuses entering the food chain e.g. pet food – and environmental impact associated with carcass disposal. It is worth noting that a publication from the USA showed sodium pentobarbital was detectable for 367 days in equine mortality compost piles, which would have environmental consequences (Payne et al., 2015).
Scenario 4: The dam gives birth in the lairage.
Since it is illegal to transport a newly born animal in which the navel is not completely healed (see EC Regulation 1/2005), Action C as described for scenario 3 applies and the neonate should be stunned and killed immediately.
3.4.2. Scenarios operating under the assumption that the neurophysiological situation of the fetus does allow for consciousness
Figure 6 here below summarises four scenarios, taking into consideration when the pregnancy is detected and if the fetus has been exposed to air, and the related courses of action. These actions operate under the assumption that the neurophysiological situation of the fetus does allow for consciousness.
Figure 6.
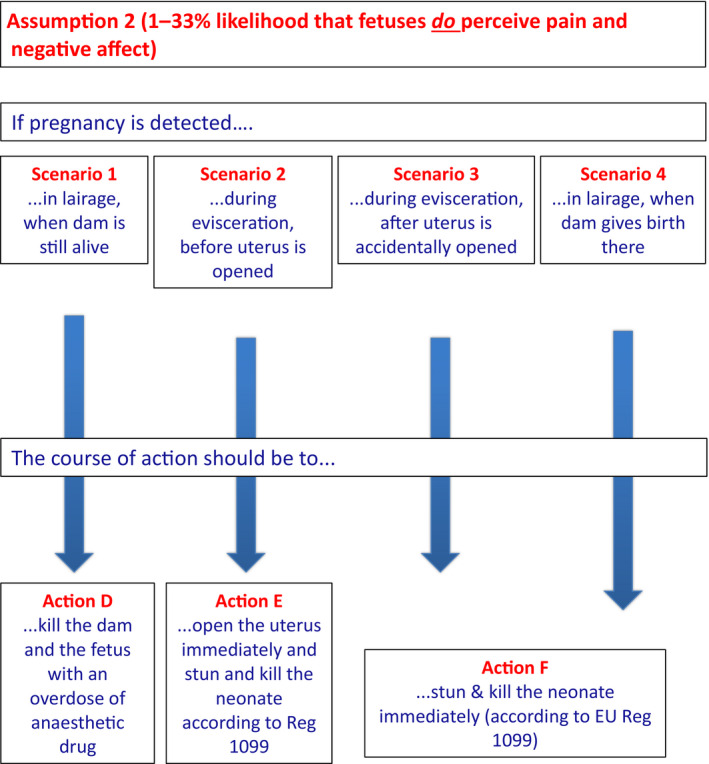
Scenarios and respective courses of action as regards handling of the fetus/neonate under the assumption that the neurophysiological situation of the fetus does allow for consciousness and taking into consideration when the pregnancy is detected and if the fetus has been exposed to air
Scenario 1: The pregnancy is detected during unloading or in lairage when the dam is still alive.
Action D: The dam and the fetus should be euthanised with an overdose of anaesthetic agents. For killing of the fetus, the drug should be able to cross the placental barrier. Barbiturates, when administered as overdose, depress the central nervous system and the respiratory centre, causing apnoea, cardiac arrest and death. These also cross the placental barrier, thus killing the fetus. Intravenous administration is preferred because it is the most rapid and reliable method. Prior sedation should therefore be considered as a means to reduce the stress associated with the administration of the anaesthetic drug. After the injection, the animals should be left without any disturbance until death supervenes. Handling and use of anaesthetic agents is restricted to trained, competent and authorised personnel. Furthermore, this intervention will render the meat of the dam and the fetus unsuitable for consumption and gives rise to other safety risks associated with the presence of hazardous agents in a food plant.
Scenario 2: The pregnancy is detected during evisceration, but before the uterus is opened.
Action E: After killing of the dam, the uterus should be immediately opened and the neonate stunned and killed according to the description given previously for Action C (see Figure 5).
Scenarios 3 and 4: The pregnancy is detected during evisceration, but after the uterus has been accidentally opened within 30 min of killing the dam or the dam gives birth in lairage.
Action F: In both cases, the neonate should be stunned and killed according to the description given previously for Action C (see Figure 5).
3.5. Assessment for ToR5 (methods to determine the gestational phase under practical conditions by examining the fetus after the dam has been killed)
3.5.1. Results from literature review
The available literature was assessed for compiling the following tables (Tables 9–12) presented separately for each species (one common table has been produced for sheep and goats). The tables include criteria, based on physical features of the uterus and the fetus, for estimating the gestational age after the dam has been slaughtered. In order to allow for a first assessment of the gestational age when the reproductive tract has been eviscerated, physical features of the uterus such as width/diameter of the uterus or largest circumference of the uterus are provided. Once a critical gestational age is assumed and the decision taken to examine the fetus (for timelines since killing of the dam see Sections 3.4.1 and 3.4.2), external features of the fetus (such as the presence of hair, teeth) or linear measures such as crown‐rump length, nose‐to‐tail length or length of large bones may be applied.
Table 9.
Gestational stages of cattle based on physical features of the fetuses (n.a.: data not available)
| Cattle | Width/diameter of the uterus in cm | Largest circumference of the uterus (in cm) | External features | Crown‐rump‐ length in cm | Nose‐to‐tail length in cm | Length of metatarsus in mm | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Rüsse and Grunert (1993) | Roberts (1986) | Rüsse and Grunert (1993) | Roberts (1986) | Evans and Sack (1973) | Nielsen and Andersen (2016) | Evans and Sack (1973) | Roberts (1986) | Thomsen (1975) | Habermehl (1975; based on Regli, 1963) | Bünger‐Marek (1972) |
| First third of gestation (day 1–day 90) | |||||||||||
| Day 90 | 12.4 | 10.0–13.0 | 51.4 | Hair on lips, chin and eyelids, scrotum present |
Scrotum present Hoofs becoming firm and opaque (day 100) |
n.a. | 15.0 | 13.0–17.0 | 17.5 | 10.0 | 10.0 |
| Second third of gestation (day 91–day 180) | |||||||||||
| Day 120 | 15.0 | 12.5–18.0 | 74.0 | Fine hair on eyebrows, claws developed and yellow‐coloured | n.a. | n.a. | 24.0 | 22.0–32.0 | 28.2 | 22.0 | 20.0 |
| Day 150 | n.a. | 18.0–23.0 | n.a. | Hair on eyebrows and lips, testes in the scrotum, teats developing | n.a. | n.a. | 36.0 | 30.0–45.0 | 40.0 | 37.0 | 29.0 |
| Day 180 | 22.2 | n.a. | 110.8 | Hair on inside of ear and around horn pits, tip of tail and muzzle | Horn bud covered with hair, tail tip hairs present | n.a. | 50.0 | 40.0–60.0 | 52.7 | 59.0 | 38.0 |
| Last third of gestation (day 181 – day 285) | |||||||||||
| Day 210 | n.a. | n.a. | n.a. | Hair on metatarsal, metacarpal, and phalangeal region of extremities and beginning on back, long hair on tip of tail | n.a. | n.a. | 62.0 | 55.0–75.0 | 66.2 | 90.0 | 47.0 |
| Day 240 | n.a. | n.a. | n.a. | Fine short hair all over body, incisor teeth not erupted | Body fully covered with hair (day 230) | n.a. | 78.0 | 60.0–85.0 | 83.9 | 121.0 | 57.0 |
| Day 255 | n.a. | n.a. | n.a. | n.a. | n.a. | Six incisors erupted: 97% probability to be > 90% of gestation | n.a. | n.a. | n.a. | n.a. | n.a. |
| Day 280 | 31.5 | n.a. | 96.0 | Hair coat complete and long | n.a. | n.a. | 92.0 | n.a. | 106.5 | 148.0 | 69.0 |
| Information on source reliability | No source provided | Multiple sources | No source provided | Multiple sources | 68 embryos from the Cornell Collection (+ data from multiple references) | 18 calves (7 Jersey, 8 Holstein, 3 crossbreeds), convenience sample |
68 embryos from the Cornell Collection (+ data from multiple references) Numbers extrapolated from graph |
Multiple sources | 82 Red Danish and Holstein fetuses + re‐examination of older data |
Gjesdal (1969) High correlations of length of long bones diaphysis with fetal age (but not for width and diameter) Simmental and Fribourg cattle fetuses |
German Black and White Lowland fetuses |
Table 12.
Gestational stages of horses based on physical features of the fetuses
| Horse | Volume of the uterus in L | Width/diameter of the uterus in cm | External features | Crown‐rump‐length in cm | Femur length | Length of metatarsus in mm | Eye length mm (Ultrasonography) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Rüsse and Grunert (1993) | Roberts (1986) | Njaa (2012) | Evans and Sack (1973) | Evans and Sack (1973) | Platt (1978) | Njaa (2012) | Habermehl (1975) | Habermehl (1975) | Guffy et al. (1970) | Murase et al. (2014) | Turner et al. (2006) |
| First third of gestation (day 1–day 112) | ||||||||||||
| Day 112 | 15–3.5 (d90) | 12.5–15 (d90) | Mammary nipples and hooves visible (d90) | Tactile hairs on lips; teats well formed. | ~ 20 | n.a. | 10–14 (d90) | 23 (15 weeks) | 17 (15 weeks) | n.a. | ~ 16 | 14 for horse (for pony) |
| Second third of gestation (day 113–day 226) | ||||||||||||
| Day 120 | n.a. | n.a. | External genitalia formed, scrotum empty, ergots and orbital areas prominent | Fine hair on muzzle, chin and around eyes; orbital area prominent; ergot prominent | ~ 25 | n.a. | 15–20 | 41 (20 weeks) | 32 (20 weeks) | n.a. | ~ 17 | 16 (15) |
| Day 210 | 10–15 (d180) | n.a. | Hair on lips, nose eyebrow, eyelids, edge of ear, tip of tail, back and mane | Eyelashes emerged, mane and tail hair present, mane hair ~ 2.5 mm long | ~ 65 | 70 | 55–70 | 89 (29 weeks) | 80 (29 weeks) | n.a. | ~ 28 | 29 (25) |
| Last third of gestation (day 227–day 340) | ||||||||||||
| Day 240 | n.a. | n.a. | Hair on mane and tail, back and distal portion of extremities | Hair appears on poll, pinnae, throat chin and muzzle; mane hair 5 mm long; hair covers distal half of tail | ~ 80 | 83 | 60–80 | n.a. | n.a. | n.a. | ~ 28 | 30 (28) |
| Day 270 | n.a. | n.a. | Short fine hair over entire body | Body covered with fine hair; mane hair 1.5 cm long; short switch on tail. | ~ 100 | 99 | 80–90 | 140 (37 weeks) | 154 (37 weeks) | n.a. | ~ 31 | 30 (30) |
| 330 | 60–90 | n.a. | Complete hair coat, testes descended | n.a. | n.a. | 118 | n.a. | 212 (44 weeks) | 236 (44 weeks) | n.a. | ~ 34 | 37 (d320) (35) |
| Information on source reliability | No source provided | Multiple resources which can mostly not be retrieved | Data from Bergin et al., (1967; 93 specimens); Roberts, (1971) | Data from Bergin et al., (1967; 93 specimens) Ewart, 1897, review of Zietzschmann and Krolling (1955) |
Bergin et al., (1967; 93 specimens) Ewart, 1897, review of Zietzschmann and Krolling, 1955) Numbers extrapolated from graph |
179 aborted thoroughbred foals | n.a. | Data from Habermehl (1975) on 22 horse fetuses | Data from Habermehl (1975) on 22 horse fetuses | 101 American fetuses |
10 thoroughbred mares Numbers extrapolated from graph |
23 ponies (36 pregnancies) Back calc from parturition assuming 340 days and data from Kahn and Leidl (1987 – n = 77) and Turner et al. (2006 – n = 66) on light horses Concluded houses and ponies had diff equations |
Table 10.
Gestational stages of pigs based on physical features of the fetuses (n.a.: data not available)
| Pigs | Length of the uterine horn in cm | External features | Crown‐rump‐length in mm | Humerus length mm | |||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Wu et al. (1987) | Evans and Sack (1973) | Evans and Sack (1973) | Njaa (2012) | Odlaug (1955) | Ullrey et al. (1965) | Ullrey et al. (1965) | Wenham et al. (1973) | Gjesdal (1972) |
| First third of gestation (day 1–day 38) | |||||||||
| Day 38 | 270 | Palate fused, facial clefts closed, eyelids begin to cover eyes | ~ 45 | 46 (d40) | 17 (d35) | 25 (d30) | n.a. | 2 | n.a. |
| Second third of gestation (day 39–day 76) | |||||||||
| Day 44 | 245 | Prepuce, scrotum, labia and clitoris present | ~ 75 | n.a. | 28 (d49) | 98 (d51) | 15 | 3 | n.a. |
| Day 76 | 264 | Eyelids fused (d50) | ~ 200 | 170 (d70) | n.a. | 163 (d72) | 26 | 20 | 19 |
| Last third of gestation (day 77–day 115) | |||||||||
| Day 90 | 278 | Eyelids separated | ~ 250 | 207 (d85) | 220 (d100) | 228 (d93) | 41 | 29 | 28 |
| Day 115 | 273 | n.a. | ~ 300 | 270 (d110) | 300 | 294 | 53 | 42 | 43 |
| Information on source reliability |
320 pregnant pigs at 3, 5, 7, 9, 11, 13 and 15 weeks of gestation Uterine size affected by number of fetuses but not pregnancy stage |
Data from range of sources 1897–1968 |
Data from range of sources 1897–1968 Numbers extrapolated from graph |
Not given | n.a. | 254 Yorkshire fetuses (30, 51, 72, 93 days) plus 35 full term |
254 Yorkshire fetuses (30, 51, 72, 93 days) plus 35 full term Tuberosity to condyle |
34 large white fetuses Diaphyseal length from radiograph |
519 Norwegian Landrace fetuses Diaphyseal length |
Table 11.
Gestational stages of sheep and goats based on physical features of the fetuses (n.a.: data not available)
| Sheep and goatsa | External features | Crown‐rump‐length (mm) | Total body weight (kg) | Length of major bones (mm)b | ||||
|---|---|---|---|---|---|---|---|---|
| Reference | Njaa (2012) | Evans and Sack (1973) | Alcorn et al. (1981) | Njaa (2012) | Evans and Sack (1973) | Alcorn et al. (1981) | Femur McDonald et al. (1977) | Tibia McDonald et al. (1977) |
| First third of gestation (day 1–day 50) | ||||||||
| 50 | Philtrum present; eyelids fused; pinna covers acoustic meatus; external genitals differentiated; teats present (43) | n.a. | 95 (55) | 90 (42) | 50 (42) | 0.05 (55) | 0.431 | 0.622 |
| Second third of gestation (day 50–day 100) | ||||||||
| 67 | Hair begins to cover body (67) | No hair; rumen development near the end of this gestational period (63) | 125 (60) | 155 (63) | 115 (63) | 0.09 (60) | 1.167 | 1.608 |
| 84 | Testes have descended into scrotum (80) | Large tactile hairs appear on lips and upper eyelids (91) | 230 (80) | 350 (91) | 230 (91) | 0.26 (80) | 2.351 | 3.108 |
| 100 | Colour markings appear (104) | n.a. | 360 (108) | n.a. | n.a. | 1.26 (108) | 3.745 | 4.804 |
| Last third of gestation (day 100 – day 150) | ||||||||
| 116 | Hair covering complete (116) | n.a. | 355 (117) | n.a. | n.a. | 1.85 (117) | 5.253 | 6.545 |
| 132 | Eyelids separated (126) | Eyelashes are well developed, some hair on tail and head (126) | 460 (133) | 400 (126) | 400 (126) | 3.9 (133) | 6.685 | 8.150 |
| 147 | Birth (144) | Fetus becomes fully developed with the body covered with hair; hoofs complete but soft (144) | 480 (141) | 480 (144) | 470 (144) | 3.75 (141) | 7.877 | 9.446 |
| Information on source reliability | No source mentioned | Based on a large number of data sets from between 1936 and 1966,c as well as 26 embryos in the ‘Cornell Collection’ | Based on a data set of 14 fetuses from Merino‐ Corriedale or Border Leicester cross ewes | No source mentioned | Based on a large number of data sets from between 1936 and 1966,c as well as 26 embryos in the ‘Cornell Collection’ | Based on a data set of 14 fetuses from Merino‐ Corriedale or Border Leicester cross ewes | Based on radiography of 215 fetuses, obtained from 80 Finish landrace x Dorset horn ewes | Based on radiography of 215 fetuses, obtained from 80 Finish landrace x Dorset horn ewes |
Data on goats were only found in one case (Singh et al., 2004), but the observations were before day 50 of gestation and therefore not included. The data in this table relate to sheep embryos, in all but one case: Njaa (2012) refers to ‘sheep and goats’ in his Appendix A, without further indication of the source. It is worth noting that Prummel (1988) who uses the bone length data of McDonald et al. (1977) suggests that ‘the identification of foetal bones of sheep and goat will be even more difficult than that of postnatal bones from these’. This would imply that the developmental stages of both species are comparable.
Data based on an equation developed by McDonald et al., 1977: ln (y/P) = (Q/100R) × (1−eR(144−t)) to relate foetal age of sheep in days after conception (t) and the diaphyseal length (y in cm). The underlying constant values are presented in the paper: P is an estimate of length at birth; Q is an estimate of the specific growth rate (%) at birth; R is the rate of exponential decay of the specific growth rate; s is the residual standard deviation of diaphyseal length.
Barcroft (1946), 186 specimens; Batten (1960), 120 specimens; Bogolyubsksiy (1959); Cloete (1939), 49 specimens; Eaton (1952), 18 specimens; Evans et al. (1966); Galpin (1935), 18 specimens; Green and Winters (1945); Harris (1937), 25 specimens; Joubert (1956), 17 specimens; Malon and Curson (1936), 56 specimens; Romanes (1947), 11 specimens; Stephensons (1959), 100 specimens; Winters and Feuffel (1936), 73 specimens; and 26 embryos in the Cornell Collection.
As indicated by Tables 9–12, for some species there are relatively few studies on the characterisation of fetal age. Where these data exist, they seldom give a satisfactory basis for conclusion. In terms of reliability of the measures suggested, the following needs to be considered: (i) most data were collected years/decades ago on older genotypes which makes it difficult to establish reliable benchmarks for modern breeds or selection lines; (ii) there is a lack of sensitivity/specificity studies on the determination of gestational age from the physical features; (iii) criteria for the exact boundaries between gestational stages are therefore less reliable indicators for the allocation to a given term than those towards midstage; (iv) given the differences between breeds in mature body weight and size, and the individual variation within breeds, external morphological features should be better indicators of gestational stage than linear dimensions such as fetus size or bone length.
4. Conclusions
While most evidence used in this opinion regarding anatomical and neurophysiological correlates of consciousness has been obtained from sheep fetuses, it is considered rather similar for precocial ungulate and equid species (ToR3).
Since there are no conclusive objective measures of the capacity of livestock fetuses to experience pain and other negative affect (ToR3), the assessment of this question is based on the available scientific evidence and on expert opinion. Each conclusion is expressed as a statement accompanied by the likelihood – that the statement is correct – in the form of a percentage.
It is very likely to extremely likely (i.e. with 90–100% likelihood) that livestock fetuses in the last third of gestation have the anatomical and neurophysiological correlates for experiencing pain and/or other forms of discomfort (ToR3).
-
It is likely to very likely (i.e. with 66–99% likelihood) that the neurophysiological situation of the livestock fetuses in the last third of gestation (e.g. inhibitory and excitatory systems) does not allow for perception of pain or other negative affect (ToR3). This arises from:
-
–
the presence of brain inhibitory (neuroprotective) mechanisms operating in the fetus,
-
–
low levels of fetal brain oxygen,
-
–
predominance of sleep like states in the fetal EEG,
-
–
lack of any direct evidence of perception of pain and other negative affect.
-
–
-
It is therefore possible, but unlikely to very unlikely (i.e. with 1–33% likelihood) that livestock fetuses in the last third of gestation can perceive pain and other negative affect (ToR3). This possibility arises from:
-
–
the lack of any direct evidence proving that fetuses are incapable of perceiving pain and other negative affect.
-
–
differences in the interpretation of indirect evidence relating to:
-
–
fetal EEG (e.g. significance of transitional EEG);
-
–
fetal behavioural and physiological responses to external stimuli;
-
–
fetal learning (conscious learning versus conditioned responses);
-
–
-
–
There is no direct evidence demonstrating the existence of subcortical awareness in livestock fetuses and the existence of the hypothesised raw basic affect. However, even if this were to exist it is unlikely to very unlikely (i.e. with 1–33% likelihood) that the neurophysiological situation of the livestock fetuses in the last third of gestation (e.g. inhibitory and excitatory systems) allows for sub‐cortically based conscious perception because the same inhibitory mechanisms will pertain. The combination of the low likelihood of the existence of raw basic effect and the high likelihood of an inhibitory uterine environment makes it very unlikely (i.e. with 1–10% likelihood) that the livestock fetuses will experience pain or other negative affect because of subcortically based conscious perception (ToR3).
It is very likely to extremely likely (i.e. with 90–100% likelihood) that livestock fetuses show measurable responses to extreme hypercapnic hypoxia, mechanical stimulation and electrical current. However, since all slaughtering procedures involve a maternal circulatory collapse, it is unlikely to very unlikely (i.e. with 1–33% likelihood) that these responses observed in the fetus during stunning and bleeding of the dam are associated with pain or other negative affect (ToR3).
-
The most appropriate management of livestock fetuses at slaughter (ToR4) depends on the point in the slaughter chain at which it is detected that the dam is in the last third of gestation. Once detected, the recommended course of action depends on the risk management decision regarding the probability that fetuses might experience pain and other negative affect.
Under the more likely assumption (i.e. with 66–99% likelihood) that the neurophysiological situation of the livestock fetus in the last third of gestation does not allow for perception of pain or other negative affect, actions should be taken to prevent onset of breathing by the fetus (ToR4, Section 3.4.1).
However, if the risk management decision is based on the less likely assumption (i.e. with 1–33% likelihood) that the livestock fetus in the last third of gestation can experience pain or other negative affect, alternative actions for immediate stunning and killing of the fetus are required (ToR4, Section 3.4.2).
Determination of fetal age after post‐mortem detection (ToR5) should be possible from morphological characteristics including measures of fetal size. Due to breed differences and variations within breed, external features, e.g. hair growth, are likely to be better indicators of gestational age of the fetus than linear measures. However, the available data do not currently allow reliable benchmarks to be derived.
-
The prevalence of slaughtered pregnant animals (ToR1) is not available in the literature or in existing data sets. The median estimate for the overall percentage of female animals that are in the third term of gestation when sent to slaughter in Europe was obtained by a structured expert knowledge elicitation (EKE exercise) summarising the limited evidence in form of a probability distribution and resulted to be:
-
–
3% for dairy cows (with a 50% uncertainty range from 1.6% to 5.2%)
-
–
1.5% for beef cows (with a 50% uncertainty range from 0.8% to 2.5%)
-
–
0.5% for pigs (sows) (with a 50% uncertainty range from 0.2% to 1%)
-
–
0.8% for sheep (with a 50% uncertainty range from 0.4% to 1.6%)
-
–
0.2% for goats (with a 50% uncertainty range from 0.1% to 0.4%)
-
–
The experts expressed the opinion that the prevalence of pregnant mares slaughtered is lower compared to the other farm animal species.
Estimates from the EKE for the percentage of pregnant animals which are in the first and second term of gestation were considered unreliable because of difficulties in detection of early stages of gestation in the course of the slaughter process.
There is paucity of information on the reasons why pregnant animals are sent to slaughter (ToR2), but they include (i) health and welfare, (ii) management and (iii) economic reasons. The relative importance of these reasons is unknown.
The reasons for farmers not knowing that animals sent for slaughter are pregnant in the last third of gestation include (i) lack of supervision of breeding, especially in extensive systems, (ii) the absence or failure of pregnancy diagnosis, (iii) poor record keeping or loss of information in the trading chain.
Measures to reduce the prevalence of pregnant animals slaughtered in the last third of gestation (ToR2) can be linked to each of these reasons. The effectiveness of the proposed measures in reducing the possibility that farmers send pregnant animals to slaughter will be impaired by (i) failure of education and communication strategies on the welfare implications and preventive measures and (ii) a pregnancy test efficacy of less than 100%.
5. Recommendations
Further research is needed using multidisciplinary approaches to establish the ability of fetuses of different developmental stages to perceive pain or other negative affect (ToR3).
Food business operators should include in their standard operation procedures actions with regard to management of female animals detected to be in the last third of gestation (ToR4).
-
If the more likely conclusion that the livestock fetus is not capable of perceiving pain or other negative affect is accepted, the fetus should be left undisturbed in utero for 30 min after the death of the dam by which time it should be dead. If the fetus is exteriorised before this time or subsequently shows signs of life, it should be stunned and killed using approved methods for neonates in accordance with Regulation 1099/2009 (ToR4).
-
–
If the less likely conclusion that the livestock fetuses can experience pain and other negative affect is accepted, the fetus should be killed in situ together with the dam by an overdose of anaesthetic drug if pregnancy is detected at arrival in the slaughterhouse. If the dam is already dead at the time of detection, the fetus should immediately be exteriorised, stunned and killed using approved methods for neonates in accordance with Regulation 1099/2009 (ToR4). The food safety risks associated with each course of action should be considered.
-
–
-
Research should be carried out to develop and evaluate appropriate methods of stunning and killing exteriorised fetuses (ToR4).
-
–
Further research should be carried out to determine the survival time in utero of fetuses of different livestock species and gestational stages following slaughtering of the dam (ToR4).
-
–
Further research should be carried out to assess the sensitivity and specificity of animal based measures in livestock fetuses to confirm death (ToR4).
Sensitivity and specificity of indicators for establishing gestational age in different species and breeds/selection lines should be determined and models to estimate the gestational age should be developed (ToR5).
Systematic data collection for prevalence estimation is needed on the present situation regarding the slaughter of animals during the last third of gestation (ToR1).
-
To reduce the prevalence of animals slaughtered in the last third of pregnancy (ToR2), it is recommended to:
-
–
implement measures to improve the health status of animals on farm and thus to reduce unplanned slaughtering.
-
–
implement management practices such as single sex housing and supervised breeding and thus to reduce unplanned pregnancies.
-
–
establish the pregnancy status of all animals to ensure that they are not sent for slaughter in the last third of gestation.
-
–
ensure information on insemination and pregnancy diagnosis is present in documentation accompanying animals at the time of sale.
-
–
implement education and communication strategies for farmers.
-
–
undertake further research to improve rapid on‐farm pregnancy diagnostic test accuracy and feasibility, especially for the diagnosis of later stages of pregnancy in small ruminants and pigs.
-
–
Each of these measures can be addressed at different levels by the appropriate stakeholder including farmers, veterinarians and other advisors, supply chain managers, competent authorities and researchers.
Glossary and Abbreviations
- Analgesia
The absence of pain in response to stimulation which would normally be painful.
- Anoxia
The absence of oxygen in the blood.
- Arousal
The state or condition of being alert or stimulated
- Aspiration reflex
Stimulation (chemical, electrical or mechanical) of the pharyngeal branch of glossopharyngeal nerve or trigeminal afferents that evokes a short‐duration spasmodic inspiratory sniff‐ or gasp‐like aspiration.
- Aversion
A tendency to show behaviour to avoid or to withdraw from a situation which is associated with a noxious stimulus.
- Awareness
is linked with wakefulness and implies that responses to stimuli involve higher brain centres and are not merely reflexes.
- Consciousness
is the state in which an animal is able to cognitively process and subjectively evaluate internal and external sensory inputs and to feel emotions
- Death
is the state of an animal where all vital functions have permanently ceased.
- Electroencephalogram (EEG)
Electrical activity of the brain usually recorded from the surface of the skull using non‐invasive techniques.
- Electrocorticogram (ECoG)
Electrical activity of the brain usually recorded on the surface of the brain or dura (a membrane covering the brain).
- Fetus
is an unborn animal from the stage of its development when its main adult features can be recognised
- Gagging or gasping
Rudimentary respiratory activity occurring through mouth (oral breathing).
- Hazard
Any factor with the potential to cause poor welfare.
- Humane killing
A method of killing that causes no avoidable pain, distress or other suffering to the animal(s) concerned.
- Hypercapnia
An increased blood carbon dioxide levels in the blood or atmosphere.
- Hypoxaemia
A decrease in oxygen levels in the blood.
- Killing
Any intentionally induced process which causes the death of an animal
- Nociception
The neural process of encoding noxious stimuli. Consequences of encoding may be autonomic (e.g. elevated blood pressure) or behavioural (motor withdrawal reflex or more complex nocifensive behaviour). Pain sensation is not necessarily implied.
- Pain
An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.
- Seizure
Convulsions that may occur with or without loss of consciousness or pathological electroencephalogram.
- Sensibility
Ability to perceive external stimuli and internal stimuli (e.g. pain).
- Sentience
(Functional) capacity to perceive sensations originating from sensory inputs
- Slaughter
Slaughter means the killing of animals intended for human consumption by the process of bleeding to induce death, usually by severing major blood vessels supplying oxygenated blood to the brain.
- Somatosensory evoked potentials (SEPs) or responses (SERs)
Electrical activity in the brain evoked by somatosensory (painful) stimuli.
- Sticking or bleeding
Act of severing major blood vessels (also see neck cutting).
- Stun or stunning
Any intentionally induced process which causes loss of consciousness and sensibility without pain, prior to killing.
- Suffering
One or more unpleasant feelings (mental state) such as pain, distress and other welfare consequences.
- Vibroacoustic stimulation (VAS)
Application of a vibratory sound stimulus to the abdomen of a pregnant woman to induce response in a fetuses
- Wakefulness
Wakefulness is a state of brainstem and thalamic activity and of non‐sleep‐arousal, not requiring cortical processing
Glossary related to the uncertainty
- Elicitation Group
The elicitation group performs the elicitation protocol and elicits the information from the expert panel. The elicitation group is responsible for all contacts with the expert panel, the documentation of the elicitation phase, the result report, and the feedback to the experts.
- Median estimate
Median prevalence (with equal probability of over/under estimation).
- Expert knowledge elicitation (EKE)
A systematic, documented and reviewable process to retrieve expert judgements from a group of experts, often in the form of a probability distribution.
- Likelihood
The chance or probability of something.
- Probability
Quantification of uncertainty as degree of belief regarding the likelihood of a particular range or category.
- Probability distribution
A probability distribution is a thorough description of uncertainty regarding a quantity. It is built up from a series of expert judgements about ranges of the uncertain quantity containing the true value with a particular probability.
- Subquestion
A question whose answer is useful to address a subsequent question. Assessment of a complex question may be facilitated by dividing it into a series of subquestions.
- Uncertainty
In this document, uncertainty is used as a general term referring to all types of limitations in knowledge.
- Uncertainty analysis
A collective term for the processes used to identify, characterise, explain and account for uncertainties.
- 50% Uncertainty range
Range of possible prevalence values around the median covering 50% of the probability distribution (P25–P75).
- 98% Uncertainty range
Range of possible prevalence values around the median covering 98% of the probability distribution (P1–P99).
Abbreviations
- AHAW
EFSA Panel on Animal Health and Animal Welfare
- AW
awake
- CNS
central nervous system
- CRH
corticotropin releasing hormone
- CSF
cerebrospinal fluid
- ECoG
electrocorticogram
- EEG
electroencephalogram
- ELISA
enzyme‐linked immunosorbant assay
- EKE
Expert Knowledge Elicitation
- EMG
electromyogram
- ES
estrone sulfate
- FBM
fetal breathing movement
- fMRI
functional magnetic resonance imaging
- GABA
gamma‐amino‐butyric acid
- GH
growth hormone
- HPA
hypothalamus–pituitary–adrenal
- IASP
International Association for the Study of Pain
- MAP
mean arterial pressure
- MS
Member State
- NPY
neuropeptide Y
- NREM
non‐rapid eye movement sleep
- OIE
World Organisation for Animal Health
- PAG
pregnancy‐associated glycoproteins
- RIA
radioimmunoassay
- RCOG
Royal College of Obstetricians and Gynaecologists
- REM
rapid eye movement sleep
- SCVPH
Scientific Committee on Veterinary Measures relating to Public Health
- SEP
somatosensory evoked potential
- SER
somatosensory evoked response
- SPn
subplate neuron
- ToR
Term of Reference
- UCO
umbilical cord occlusion
- VAS
vibroacoustic stimulation
- WG
Working Group
Appendix A – Literature search on the prevalence of slaughtered pregnant animals in Europe
As indicated in Sections 2.3.1 (Methodology) and 3.1.2 (Assessment), a literature search was conducted to identify available evidence concerning the prevalence of slaughtered pregnant animals in Europe.
The searches were conducted using combinations of terms covering four main question components: 1) livestock animals, 2) gestation, 3) slaughter and 4) prevalence. The search string used is reported in Table A.1. The search was conducted in the following database: Web of Science. The search was conducted for years 1975–2017 and on the following indexes: SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC.
Table A.1.
Search string used for a literature search on the prevalence of slaughtered pregnant animals in Europe
| Search terms | Field searched |
|---|---|
| (((Cull* OR kill* OR slaughter* OR abattoir*) NEAR (pregnan* OR gestat*))) | Title |
| AND | |
| ((Livestock* OR ((Farm OR farms OR farmed OR farming) NEAR/3 animal*) OR ruminant* OR cattle OR bovin* OR cow OR cows OR calf OR calves OR heifer* OR “Bos Taurus” OR (dairy NEAR/1 (herd* OR breed*)) OR weaner* OR yearling* OR stirk OR stirks OR springer* OR feeder* OR beef* OR swine OR (sus NEAR/1 (scrofa OR domestica OR domesticus)) OR pork OR porks OR porcine OR suidae OR pig OR pigs OR piglet* OR “sow” OR “sows” OR gilt OR gilts OR equidae* OR equus OR horse OR horses OR equine* OR yearling* OR mare OR mares OR pony OR ponies OR filly OR fillies OR ass OR asses OR mule OR mules OR donkey* OR ovis OR ovine* OR ewe OR ewes OR lamb OR lambs OR sheep* OR mouflon* OR hogget* OR ram OR rams OR tup OR tups OR Goat OR goats OR capra OR capras OR caprin* OR dam OR dams)) | Title |
| AND | |
| ((prevalence OR number) OR (per cent* OR percent*)) | Topic |
Titles and abstracts of the references were screened to identify relevant papers to be reviewed in detail. When screening the references, papers were considered relevant if:
they were about pregnant animals during the slaughter situation (and not, e.g. examining gestation on‐farm prior to slaughter);
they were based in Europe;
the paper language was English.
Sixteen references (9 February 2017) were retrieved and screened for studies of interest. After the screening, four papers were evaluated (Singleton and Dobson, 1995; Jensen et al., 2010; Riehn et al., 2011; Maurer et al., 2016)
Appendix B – Literature search on the reasons for slaughtering pregnant animals in Europe
As indicated in Sections 2.3.2 (Methodology) and 3.2.1 (Assessment), a literature search was conducted to identify available evidence concerning the reasons for slaughtering pregnant animals in Europe.
The searches were conducted using combinations of terms covering four main question components: (1) livestock animals, (2) gestation, (3) slaughter and (4) prevalence. The search string used is reported in Table B.1. The search was conducted in the following database: Web of Science. The search was conducted for years 1975–2017 and on the following indexes: SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC.
Table B.1.
Search string used for a literature search on the reasons for slaughtering pregnant animals in Europe
| Search terms | Field searched |
|---|---|
| (((Cull* OR kill* OR slaughter* OR abattoir*) NEAR (pregnan* OR gestat*))) | Title |
| AND | |
| ((Livestock* OR ((Farm OR farms OR farmed OR farming) NEAR/3 animal*) OR ruminant* OR cattle OR bovin* OR cow OR cows OR calf OR calves OR heifer* OR “Bos Taurus” OR (dairy NEAR/1 (herd* OR breed*)) OR weaner* OR yearling* OR stirk OR stirks OR springer* OR feeder* OR beef* OR swine OR (sus NEAR/1 (scrofa OR domestica OR domesticus)) OR pork OR porks OR porcine OR suidae OR pig OR pigs OR piglet* OR “sow” OR “sows” OR gilt OR gilts OR equidae* OR equus OR horse OR horses OR equine* OR yearling* OR mare OR mares OR pony OR ponies OR filly OR fillies OR ass OR asses OR mule OR mules OR donkey* OR ovis OR ovine* OR ewe OR ewes OR lamb OR lambs OR sheep* OR mouflon* OR hogget* OR ram OR rams OR tup OR tups OR Goat OR goats OR capra OR capras OR caprin* OR dam OR dams)) | Title |
| AND | |
| (reason* OR choice* OR decision* OR attitude* OR judgment* OR ethic* OR survey OR surveys OR questionnaire* OR Respondent*) | Topic |
Titles and abstracts of the references were screened to identify relevant papers to be reviewed in detail. When screening the references, papers were considered relevant if:
they were about pregnant animals during the normal slaughter situation (and not, e.g. on‐farm emergency killing);
they were based in Europe;
the paper language was English.
Thirteen references (9 February 2017) were retrieved and screened for studies of interest. After the screening, three papers were evaluated (Singleton and Dobson, 1995; Jensen et al., 2010; Thingnes et al., 2015).
Appendix C – Elicitation Report
‘prevalence of pregnant animals slaughtered in EU’
EFSA premises , parma ( italy ), 16–17 june 2016
This is a summary of the Expert Knowledge Elicitation (EKE) 1 exercise. Country specific information has been removed for confidentiality reasons.
part 1 – expert knowledge elicitation exercise description
Purpose of the elicitation
The purpose of this exercise was to estimate probabilities around the prevalence of pregnant animals slaughtered in the European Union (EU). The exercise follows up from a set of surveys carried out in 10 EU countries about estimates perceived of such prevalence in 2015 and information from three data collections currently on‐going in the Netherlands (NL), Denmark (DK) and Germany (DE).
Attendants, roles and expertise
The elicitation group included an Elicitor and analyst – from the EFSA Assessment and Methodology Unit – experienced in the elicitation of expert knowledge using the Sheffield method, a recorder – from the EFSA scientific secretariat staff – and a member of the Working Group (WG) on ‘Slaughter of pregnant animals’ (EFSA‐Q‐2015‐00477).
The Elicitation experts were 6 experts out of the 10 who conducted the surveys in the EU countries (experts were from Italy, France, Spain, Ireland, the UK and Belgium; experts from Romania, Poland, Sweden and Greece were not available to participate to the workshop); these were mainly researchers on animal welfare at slaughter, official veterinarians and auditors in their national slaughterhouses. The Elicitation group also included representatives of the two EU countries (DE, DK) that are currently working on national projects to collect data about slaughtering of pregnant animals in their countries and two observers from the Dutch and German Governments where similar projects are also being performed.
The participants introduced themselves and explained their background and relation to the elicitation topic. They were invited to indicate possible conflicts of interests.
Participants were made aware that judgements made in the elicitation, and the reasoning used, were going to be recorded, but that they were not going to be attributed to the experts by name (applying the ‘Chatham House rule’).
Introduction
At the start of the workshop, the Elicitor gave a short introduction into the Expert Knowledge Elicitation process. He explained the purpose of the elicitation workshop and the tasks that Elicitation experts would be asked to perform.
Evidence
Under commercial circumstances, the uterus is not opened to specifically look for pregnancies and especially first term of gestation pregnancies are usually undetected at slaughter. Therefore, the starting point for the elicitation were a set of surveys carried out in 10 EU countries about estimates of pregnancy prevalence in 2015 for all species and three data collections currently ongoing in the Netherlands (NL), Denmark (DK) and Germany (DE). The representatives of NL, DE and DK presented the preliminary outcomes of their national projects collecting data on the prevalence of slaughtered pregnant animals.
The project carried out in the Netherlands is a study run in 14 slaughterhouses (5 for cattle, 2 for pigs, 1 for horses, and 6 for sheep and goats) through a questionnaire similar to that outsourced by EFSA in the surveys. The overall prevalence of slaughtered animals detected as pregnant was found to be 1% for cattle, 0.5% for pigs, 1% for horses and 15% for sheep and goats. It was highlighted that no special attention was given to detect pregnancies in the slaughterhouses, one slaughterhouse reported very high (50%) incidence of pregnant sheep; also sheep giving birth in the waiting room was reported. Slaughterhouses usually have no special protocol for managing uterus and fetus: the uterus is not opened, and will be discarded as usual. One slaughterhouse always reports pregnant bovines to veterinary authorities.
The project in Germany has been running from the beginning of 2015 and is collecting data on prevalence, reasons for slaughtering pregnant animals, stage of gestation at slaughter, cost‐benefit calculation and indications on the fate of fetuses after slaughter of the dam. For cattle, a prevalence of 1.05% was found (but the German meat industry and slaughter associations think it is higher) with most pregnancies around the second and third terms. For pigs and small ruminants, a prevalence of 0.06% and 2.03% was found with most pregnancies at the second term. Overall, the project leaders advise that prevalence cannot be averaged because it depends on supply structure, animal species and category and cooperation of stakeholders.
The project in DK has collected data – for bovines and pigs – about the total numbers of slaughtered female animals (227,636 bovines and 306,548 sows) and the number of adult female animals (150,521 for bovines and 298,819 for pigs). The prevalence of those found pregnant at slaughter was investigated and ranged from 25–75% (best estimate), 20% as estimate for reasonable low number and 35–75% for reasonable high number. It was also highlighted that pregnancy is usually not detected until after slaughtering, that the distribution between the pregnancy stages is quite even, that no differences in stunning of pregnant animals are applied and that the uterus is only opened if the gestation period is suspected to be in the last 10th. Regarding the latter, if six teeth (three in each side) have erupted, it is evaluated that the gestation is in the last 10th and a fine will be issued to the farmer (2004 act on prohibition of slaughtering and killing of pregnant production animals and horses in the last 10% of the gestation period; for 2015, in bovines 32 non‐compliances were found out of 440,000 adult females slaughtered).
Prior to the meeting, the Elicitation experts received a background document summarising i) the Eurostat statistics about slaughtering and ii) the outcomes of the 10 surveys performed in Sweden, Italy, France, Spain, Romania, Poland, Greece, Ireland, the UK and Belgium. In each country, 10 slaughterhouses – 4 for cattle, 3 for pigs, 2 for sheep, 1 for goats and 1 for horses, when possible – were visited. During the meeting, a summary overview from all conducted surveys was given by the WG member and the main outcomes were discussed.
Training on Expert Knowledge Elicitation process
At the start of the workshop, the Elicitor gave a short training about the EKE process. Afterwards, the Elicitation experts carried out a practice elicitation in which each expert gave a probability for an example question. The Elicitor explained that for the questions that followed, the group would also be asked to arrive at a consensus probability or range of probabilities expressing their collective judgement.
The elicitation protocol
The same procedure was adopted for questions for each of the slaughtered species of interest, in this order:
-
1A
Slaughtering of dairy cows
-
1B
Slaughtering of female beef cattle
-
2
Slaughtering of breeding sows
-
3
Slaughtering of female sheep
-
4
Slaughtering of female goats
-
5
Slaughtering of female horses.
The protocol comprised a sequence of individual and group elicitations as follows:
-
a
The question was reviewed and any queries were clarified.
-
b
The evidence was reviewed: the relevant section of the background document was examined i.e. for the species under discussion, the Elicitor presented the figures from Eurostat about national and total EU slaughtering and the results from the 10 slaughterhouses visited in each of the 10 EU countries. Any points of clarification or differences were discussed; participants were asked to summarise any additional evidence they considered relevant.
-
c
Discussion on factors increasing or decreasing the prevalence of pregnant animals slaughtered: experts were asked to consider their country survey results and any additional information related to influencing factors they might have collected during the surveys. An overall consensus list of factors increasing and decreasing the prevalence at EU level was then drafted for defining factors.
-
d
Elicitation about prevalence of pregnant animals slaughtered at national level and EU level: The Elicitor first presented the figures from Eurostat about slaughtered animals per each EU country and total EU together with the figures resulting from each survey performed at national level. Any points of clarification and important country differences were discussed.
Elicitation of judgements about the prevalence of pregnant dairy cows slaughtered was then done in three steps: first the experts were individually asked to judge about national prevalence, then about the EU overall prevalence and finally consensus judgements were derived for the EU overall prevalence.
-
1st step:
The group of experts were asked to write down their initial judgements on the prevalence at their national level individually, expressed as lower and upper limit, followed by the best estimate for the prevalence (median), and finally as interquartile range (1st and 3rd quartile) to express their uncertainty about the prevalence. The experts were invited to reflect on the evidence provided in their national surveys, representativeness of the surveys for their whole country, and important factors increasing or decreasing the prevalence of pregnant animals slaughtered to be considered in their country.
The initial probability judgements were collected and immediately visualised as probability densities (using EXCEL) to feedback the individual judgements to the group. A weighted average for the European prevalence was calculated taking into account the different production figures of the countries involved (EUROSTAT). The Elicitor facilitated a discussion of the judgements made, and the reasons for major changes compared to their national survey outcomes, or differences between European regions. In the following step, the experts were asked to consider individually whether to retain their initial judgement or amend it.
-
2nd step:
The group of experts were then asked to give their judgements on the same question for the European level. The experts were invited to reflect on European differences and importance for the elicitation question.
Again, the initial probability judgements were collected and immediately visualised as feedback to the group. An unweighted average was calculated as proposal for a group result. The Elicitor facilitated a discussion of the judgements made, and the reasons for major differences in the group.
-
3rd step:
The Elicitor facilitated a discussion working towards a consensus judgement expressing the collective view of the group. The Elicitor checked for agreement on the result of this discussion before moving on to the next question.
-
e
Elicitation about terms of gestation of pregnant animals slaughtered at EU level: The Elicitor first presented the figures resulting from each survey performed at national level. Any additional points of clarification and important country differences were discussed.
Elicitation of judgements about three terms (1st, 2nd or 3rd term) of gestation of slaughtered animals was then done in three steps, similarly to the elicitation about the prevalence (see point d above): first, the experts were individually asked to judge about national figures divided by term of gestation, then about the EU figures and finally consensus judgements were derived for the EU figures. To simplify the procedure for the proportion of pregnant animals in the first and second term of gestation, only best estimates were asked. Full uncertainty assessment was done for the third term.10
Strengths and weaknesses
Three experts from the surveys conducted in Romania, Poland and Greece were not available to participate to the workshop. The participating experts were asked to take into account these three national survey results when judging probabilities at EU level and when considering factors increasing or decreasing the prevalence. The experts from Romania, Poland and Greece were given the opportunity to comment on this report before publication.
PART 2 – ELICITATION QUESTIONS
It was agreed that beef and dairy cows should be dealt with separately due to the differences in management.
Expert knowledge elicitation on dairy cattle
Discussion on main factors influencing increase and decrease of prevalence in dairy cattle
The experts discussed about the main factors increasing the prevalence of slaughtered pregnant dairy cows and agreed that they are as follows:
Market reasons (end of production period)
Health reasons (lameness, mastitis, claw problems) leading to increased costs for keeping the cows
Lack of information during trading
At regional level (i.e. depending on the EU region): non‐awareness as an effect of natural mating in pasture farming during the summer period. However, in general the issue of non‐awareness of dairy cow pregnancy is limited due to the use of artificial insemination
Economic crises and low milk prices
Lactation period/different breeds/intensity of milk production/exchange time
Pregnancy not recognised at early stage (1st third of gestation)
Avoiding unwanted behaviour and accidents on slippery floor because of cows in heat
False negative pregnancy test
Anabolic effect of pregnancy (more muscle growth)
There was a general agreed view that all reasons could be summarised into economic reasons.
Afterwards, the experts discussed about the main factors decreasing the prevalence in dairy cows and agreed the following:
Good farming practice
Increased prices for calves and milk (when milk price gets lower, the prevalence gets higher – but this is a theoretical factor because dairy calf prices are very low in most EU countries)
Surveillance by authorities or slaughterhouses
Complete awareness along the production chain.
Judgements about prevalence of pregnant dairy cows slaughtered at EU level
Question 1: Please give your judgement on the average occurrence of pregnancy per 100 slaughtered adult dairy cows in Europe in 2015 [in %]
Discussion on the differences between the countries:
Based on the discussion on the Eurostat figures and national survey outcomes, the participants agreed that a high prevalence of slaughtered pregnant dairy cows found in some countries is due to the high prevalence in those regions where cows have 2.5 lactations (meaning that their life is 5 years). This depends on the intensity of farms because the more intensive they are the more likely the dairy cows develop metabolic disorders and other health and welfare deficiencies and are sent to slaughter. In other countries, replacement and peak lactation are (heavy) factors leading to selling dairy cows at the first term of gestation. There are other countries with a high prevalence of slaughtered pregnant dairy cows due again to the intensive production – it is then common to inseminate the dairy cows and then send them to slaughter when most convenient. On the other hand, there are countries where the production is less intensive and allows for better surveillance and more care to the whole production cycle.
Collective view on question 1:
Figure C.1 here below represents the density of probabilities, obtained by consensus judgement, expressing the collective view of the group regarding the overall prevalence of pregnant cattle (dairy cows) slaughtered.
Table C.1 represents the estimated proportion of all dairy cows which are pregnant at the time of slaughter in Europe.
Table C.1.
Estimated proportion of all dairy cows which are pregnant at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| Lower | 1st Q | Median | 3rd Q | Upper |
|---|---|---|---|---|
| 2% | 9% | 16% | 27% | 60% |
Overall, in the EU, it is possible to observe high and low prevalence of slaughtered pregnant dairy cows depending on the production intensity.
Judgements about terms of gestation of slaughtered pregnant dairy cows at EU level
Question 2: Please give your judgement on the EU average distribution of the stages of pregnancy for 100 slaughtered pregnant dairy cows [in %]
Discussion on the differences between the countries:
Participants were asked to discuss particularly low and high figures from the surveys, especially related to the third term of gestation. In some countries, figures for the third term of gestation are low because farmers usually do not sell pregnant animals to slaughter (and there is no intermediate salesman). In case of health reasons, they cull on farm. High prevalence at third term of gestation was given in some countries but it was clarified that this was due to the fact that judgement was given without differentiating beef and dairy cattle. High prevalence in second term can be explained by an underrating of third term due to incapability of distinction. High prevalence in first term is linked to the fact that high performance dairy herds depend on milk yield and not on gestation phase, so the farmer can decide to sell the cow at an early stage. It would not be convenient to sell it at the third term because the feed used to feed that cow is almost already converted into a calf.
Collective view on question 2:
Mostly the differences at EU level are linked to the production system and in general dairy cows are sold to slaughter only at a very early stage of gestation. Table C.2 here below represents the estimated proportion of all dairy cows which are pregnant at any stage of gestation at the time of slaughter in Europe.
Table C.2.
Estimated proportion of all dairy cows which are pregnant at any stage of gestation at the time of slaughter in Europe
| 1st term of gestation | 2nd term of gestation | 3rd term of gestation |
|---|---|---|
| 46% | 35% | 19% |
Full uncertainty assessment was done for the third term of gestation only and is reported here below: Figure C.2 represents the density of probabilities, obtained by consensus judgement, expressing the collective view of the group regarding the overall prevalence of pregnant cattle (dairy cows) slaughtered at the third term of gestation.
Figure C.2.
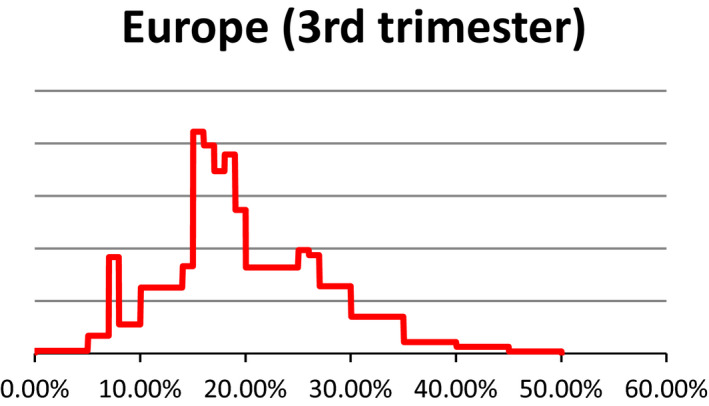
Probability density regarding the overall prevalence of pregnant cattle (dairy cows) slaughtered at the third term of gestation
Table C.3 represents the estimated proportion of all dairy cows which are pregnant at the third term of gestation at the time of slaughter in Europe.
Table C.3.
Estimated proportion of all dairy cows which are pregnant at third term of gestation at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| Lower | 1st Q | Median | 3rd Q | Upper |
|---|---|---|---|---|
| 5% | 15% | 19% | 25% | 45% |
Expert knowledge elicitation on beef cattle
Discussion on main factors influencing increase and decrease of prevalence in beef cattle
The experts discussed about the main factors increasing the prevalence of slaughtered pregnant beef cattle and agreed that they are as follows:
Natural breeding in extensive outdoor farms (note: in beef cattle there is more extensive production than in dairy) due to unexpected mating plus lack of surveillance in outdoor conditions
Longer trading chain leading to reduced/non‐awareness of pregnancy
Anabolic effect of pregnancy (more muscle growth).
Afterword, the experts discussed about the main factors decreasing the prevalence in beef cattle and agreed the following:
Better pregnancy detection diagnosis due to higher prices of calves
Intensive indoor production (less pregnancy, fewer females)
Better human‐animal management, care/accessibility
Retailers and consumer pressure on animal welfare
Penalty at slaughter for pregnant animals.
Judgements about prevalence of pregnant beef cattle slaughtered at EU level
Question 3: Please give your judgement on the average occurrence of pregnancy per 100 slaughtered adult female beef cattle in Europe in 2015 [in %]
Discussion on the differences between the countries:
Based on the discussion on the Eurostat figures and national survey outcomes, for some countries, the average was increased based on the assumption that the first term of gestation was not detected in the surveys and also because the survey revealed that sometimes the percentage is very high because of the anabolic effect. It was clarified that in Italy beef cattle are not grown but the largest amount is bought from France.
Collective view on question 3:
The participants agreed that a high prevalence of slaughtered pregnant beef cattle in some countries is explained by the breed slaughtered by the surveyed abattoirs in which very expensive breeds for meat are kept extensively outdoors so the bull is there and natural mating occurs (but calves are not expensive so the dam may get slaughtered). On the other hand, there are countries where the production is less intensive with more care to the whole chain and it is convenient to keep the calves.
Figure C.3 represents the density of probabilities, obtained by consensus judgement, expressing the collective view of the group regarding the overall prevalence of pregnant beef cattle slaughtered.
Figure C.3.
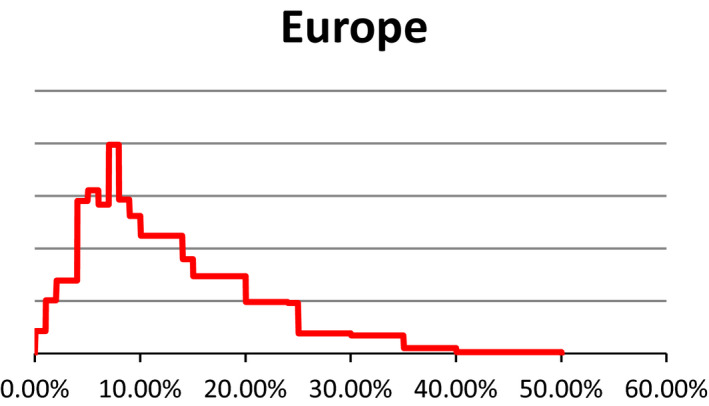
Probability density regarding the overall prevalence of pregnant beef cattle slaughtered
Table C.4 represents the estimated proportion of all beef cattle which are pregnant at the time of slaughter in Europe.
Table C.4.
Estimated proportion of all beef cattle which are pregnant at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| Lower | 1st Q | Median | 3rd Q | Upper |
|---|---|---|---|---|
| 1% | 7% | 11% | 18%% | 40% |
Judgements about stages of gestation of slaughtered pregnant beef cattle at EU level
Question 4: Please give your judgement on the EU average distribution of the stages of pregnancy for 100 slaughtered pregnant female beef cattle [in %]
Discussion on the differences between the countries:
Based on the discussion on national survey outcomes, participants were asked to discuss particularly low and high figures, especially related to the third term of gestation of gestation.
For some countries, the prevalence of beef cattle found at their third term of gestation is low because it is an infringement to the transport regulation since cows should not be transported in the last 10% of gestation. It was, however, noted that the last 10% of gestation can be recognised but not the whole last third of gestation. Some countries have low percentages representing the accidents to the transport regulation.
In some other countries, higher prevalence figures were given and it was explained that often the first is not recognised before cattle are sold to an intermediate salesman. The salesmen buy the live weight but might keep the cow for some time before selling it to slaughter. Therefore, it can occur that the salesmen do not know about the cow pregnancy when they buy it (still they know it when they sell it to slaughter). In other countries, there is also a long trading but the payment is different so the prevalence of pregnancies is lower: the salesmen pay for the live weight and then they sell the carcass on the carcass weight (they do not keep the cow for some time but they sell it almost immediately after buying). In this case, they will not buy and sell a pregnant cow.
Table C.5 represents the estimated proportion of all beef cattle which are pregnant at third term of gestation at the time of slaughter in Europe.
Table C.5.
Estimated proportion of all dairy cows which are pregnant at the third term of gestation at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|
| 47% | 38% | 15% |
Full uncertainty assessment was done for the third term of gestation only and is reported here below: Figure C.4 represents the density of probabilities, obtained by consensus judgement, expressing the collective view of the group regarding the overall prevalence of pregnant beef cattle slaughtered. Overall, the prevalence of beef cattle found at their third term of gestation is quite low explained by lower prevalence of pregnancy in general. Country differences exist especially in terms of the trading chain.
Figure C.4.
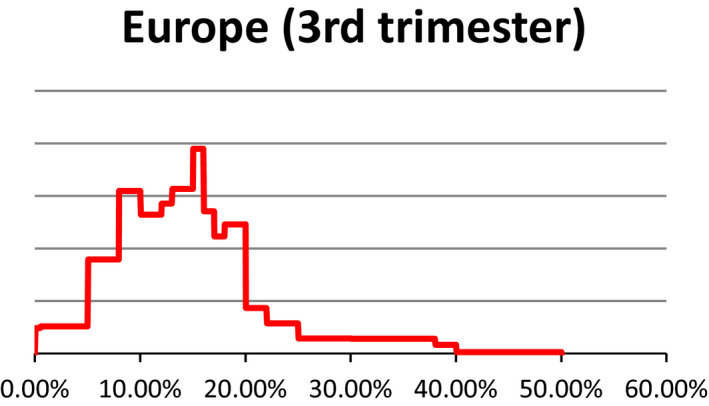
Probability density regarding the overall prevalence of pregnant beef cattle slaughtered at the third term of gestation
Table C.6 represents the estimated proportion of all beef cattle which are pregnant at the third term of gestation at the time of slaughter in Europe.
Table C.6.
Estimated proportion of all beef cattle which are pregnant at the third term of gestation at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| Lower | 1st Q | Median | 3rd Q | Upper |
|---|---|---|---|---|
| 1% | 10% | 14% | 18% | 40% |
Expert knowledge elicitation on pigs (sows)
Discussion on main factors influencing increase and decrease of prevalence in pigs (sows)
The experts agreed the main factors increasing the prevalence of slaughtered pregnant sows are as follows:
Economic situation of breeders (bankruptcy, etc)
Health status of breeding sows (e.g. lameness)
Farm management of pregnant sows (over production): mostly health reasons (weaker sows, unwanted behaviour)
Insemination and high pregnancy rate. In some farms, there is not enough space in the farrowing crates. If pregnant sows are sent to slaughter in a late stage of gestation, it is because the farmer waited to send the sows with lower health status
Difficulty to recognise pregnancy (3 week time before confirmation)
Slaughter pigs (early gestation)
No control for slaughter pigs (mixed groups with non‐castrated males)
Country differences of age for slaughtering (up to 9 months in Italy)
Production system: outdoor farming has less control of mounting in entire animals.
Among the main factors decreasing the prevalence in sows, the experts agreed:
Better prices for piglets
Good farm management (big farms).
Judgements about prevalence of pregnant pigs slaughtered at EU level
Question 5: Please give your judgement on the average occurrence of pregnancy per 100 slaughtered adult breeding sows in Europe in 2015 [in %]
Discussion on the differences between the countries:
In some countries, a high prevalence of pregnancies (all stages) is due to the overproduction leading to a high rate of animals sent to slaughter. Another factor is the over‐insemination: calculation of loss rate due to health problems leads to more insemination. Then, farrowing crates are the limiting factor resulting in more sows sent to slaughter. Other countries instead do not commonly have such overproduction and production of piglets is an added value to the sow production. In other countries, replacement strategy plays an important role: sows that are not needed are not inseminated leading to a low prevalence of pregnant sows. The feed cost of a pregnant animal also represents an influencing factor: if feed cost is low it is not a problem to feed a sow otherwise they try to avoid empty days. Some other countries gave low figures and clarified that, although they have a high level of uncertainty, they think the prevalence is low because farmer does not send sows to slaughter for not losing money.
It was agreed that related to the prevalence of pregnant slaughtered sows there is a quite big variation, associated to high uncertainty, as reflected in the discussion.
Collective view on question 5:
Figure C.5 represents the density of probabilities, obtained by consensus judgement, expressing the collective view of the group regarding the overall prevalence of pregnant sows slaughtered.
Table C.7 represents the estimated proportion of all sows which are pregnant at the time of slaughter in Europe.
Table C.7.
Estimated proportion of all sows which are pregnant at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| Lower | 1st Q | Median | 3rd Q | Upper |
|---|---|---|---|---|
| 0% | 3% | 6% | 9% | 20% |
Judgements about stages of gestation of slaughtered pregnant sows at EU level
Question 6: Please give your judgement on the EU average distribution of the stages of pregnancy for 100 slaughtered pregnant breeding sows [in %]
Discussion on the differences between the countries:
Participants were asked to discuss particularly low and high figures, especially related to the third term of gestation. One country explained that a high average (30%) of sows was found at their third term of gestation. The reason was unknown but it might be due to bankruptcy of two farms.
Table C.8 represents the estimated proportion of all sows which are pregnant at any term of gestation at the time of slaughter in Europe.
Table C.8.
Estimated proportion of all sows which are pregnant at any term of gestation at the time of slaughter in Europe
| 1st term of gestation | 2nd term of gestation | 3rd term of gestation |
|---|---|---|
| 60% | 30% | 10% |
Full uncertainty assessment was done for the third term of gestation only and is reported here below: Figure C.6 represents the density of probabilities, obtained by consensus judgement, expressing the collective view of the group regarding the overall prevalence of pregnant sows slaughtered at the third term of gestation.
Figure C.6.
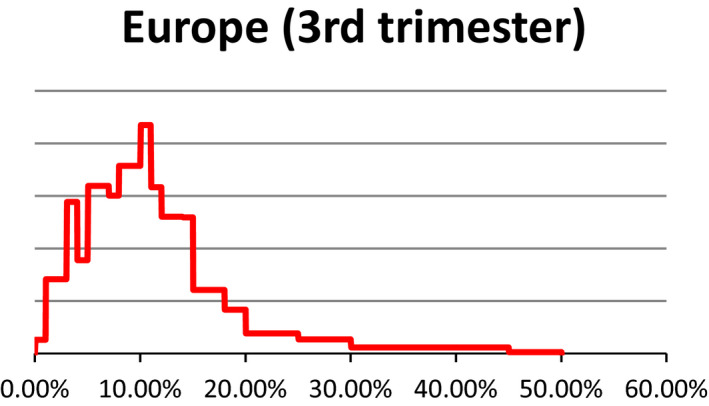
Probability density regarding the overall prevalence of sows slaughtered at the third term of gestation
Table C.9 represents the estimated proportion of all sows which are pregnant at the third term of gestation at the time of slaughter in Europe.
Table C.9.
Estimated proportion of all sows which are pregnant at third term of gestation at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| Lower | 1st Q | Median | 3rd Q | Upper |
|---|---|---|---|---|
| 1% | 6% | 10% | 14% | 40% |
Expert knowledge elicitation on sheep
Discussion on main factors influencing increase and decrease of prevalence in sheep (ewes)
The experts agreed that the main factors increasing the prevalence of slaughtered pregnant ewes are:
High seasonality (holidays): Muslim community concentrates the slaughtering normally around the end of Ramadan period but the period changes every year – the ewes that are not good in lambing are discharged and slaughtered but they might be pregnant. The same issue occurs over the Christmas and Easter period in EU
Mainly outdoor/pasture farming, seasonal life cycle, trade in autumn
Use of sheep for environmental purpose
Less pregnancy diagnosis for outdoor farming (small farms)
Health reasons
Farming during winter.
Among factors decreasing the prevalence:
More attention paid to year periods of heavy lamb consumption
Good price for sheep milk/cheese.
Judgements about prevalence of pregnant sheep slaughtered at EU level
Question 7: Please give your judgement on the average occurrence of pregnancy per 100 slaughtered adult female sheep in your country in 2015 [in %]
Discussion on the differences between the countries:
The participants tackled that in sheep management systems do not influence much the slaughter practices because animals are moved all around the EU. Main differences between Eurostat data and data from the surveys were explained. It was clarified that in the figures from Eurostat, data related to northern countries is based on the total population slaughtered (ewes for milk, meat or wool consumption and also females in extensive condition that might arrive to the adult age but not for breeding purposes) while in southern countries it only relates to adult ewes.
Collective view on question 7:
Figure C.7 represents the density of probabilities, obtained by consensus judgement, expressing the collective view of the group regarding the overall prevalence of pregnant ewes slaughtered.
Figure C.7.
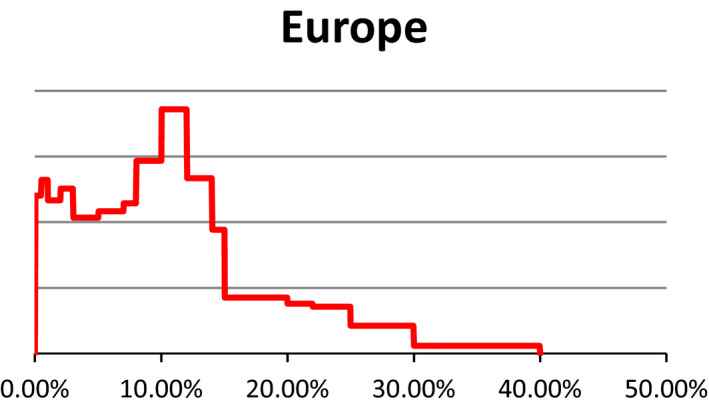
Probability density regarding the overall prevalence of pregnant ewes slaughtered
Table C.10 represents the estimated proportion of all ewes which are pregnant at the time of slaughter in Europe.
Table C.10.
Estimated proportion of all ewes which are pregnant at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| Lower | 1st Q | Median | 3rd Q | Upper |
|---|---|---|---|---|
| 0% | 5% | 10% | 14% | 40% |
Overall, the main difference related to judgements was for some southern EU countries. Breeding pregnant ewes are sent to the abattoir because of the end of production or for health reason (culling) and they are likely to be pregnant. It was noted that in some countries, e.g. Italy and Spain, only lambs are consumed.
Judgements about stages of gestation of slaughtered pregnant ewes at EU level
Question 8: Please give your judgement on the EU average distribution of the stages of pregnancy for 100 slaughtered pregnant female sheep [in %]
Discussion on the differences between the countries:
From the national surveys, it resulted that 10% of slaughtered pregnant ewes fall into the third period of pregnancy. Third stage of pregnancy was found to be high in some countries and was explained as seasonality of the slaughtering. Instead, reasons for slaughtering at the second stage of gestation are linked to health or other reasons for which it may not be worth to wait for birth, or associated to the fact that in the period between 1st and second stage it is difficult to detect pregnancy. In some countries, slaughtering for Muslim consumption occurs during the whole year and most of slaughtered pregnant ewes are found at the first stage of gestation.
Table C.11 represents the estimated proportion of all ewes which are pregnant at third term of gestation at the time of slaughter in Europe.
Table C.11.
Estimated proportion of all ewes which are pregnant at third term of gestation at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| 1st term of gestation | 2nd term of gestation | 3rd term of gestation |
|---|---|---|
| 55% | 35% | 10% |
Full uncertainty assessment was done for the third term of gestation only and is reported here below: Figure C.8 represents the density of probabilities, obtained by consensus judgement, expressing the collective view of the group regarding the overall prevalence of pregnant ewes slaughtered at the third term of gestation.
Figure C.8.
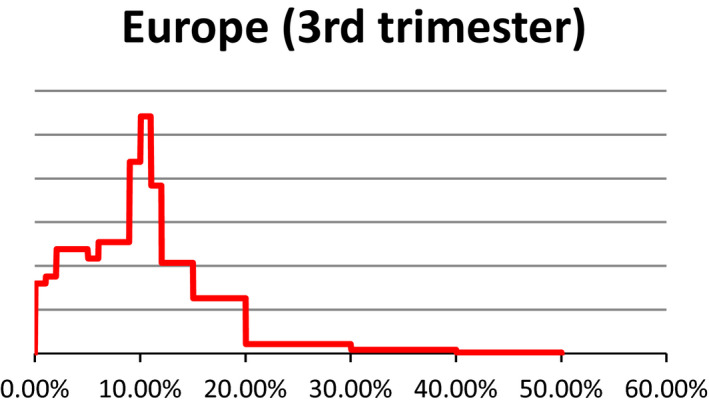
Probability density regarding the overall prevalence of ewes slaughtered at the third term of gestation
Table C.12 represents the estimated proportion of all ewes which are pregnant at the third term of gestation at the time of slaughter in Europe.
Table C.12.
Estimated proportion of all ewes which are pregnant at third term of gestation at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| Lower | 1st Q | Median | 3rd Q | Upper |
|---|---|---|---|---|
| 0% | 6% | 10% | 14% | 40% |
Expert knowledge elicitation on goats
Discussion on main factors influencing increase and decrease of prevalence in goats
The experts discussed about the main factors increasing the prevalence of slaughtered pregnant goats and agreed that they are as follows:
In southern Europe, meat/milk production from goats is similar to sheep production: outdoor farming and poor pregnancy diagnosis
Goat milk production is a growing sector
Seasonality: goat kids are eaten at Christmas time and Eastern in Italy and Spain
Milk production is intensive while meat production is in outdoor farms.
Factors decreasing the prevalence:
Goats kept as pets
Higher level of artificial insemination
Higher prices for kids of goats
Judgements about prevalence of pregnant goats slaughtered at EU level
Question 9: Please give your judgement on the average occurrence of pregnancy per 100 slaughtered adult female goats in Europe in 2015 [in %]
Discussion on the differences between the countries:
From the figures from Eurostat, it was noted that Spain counts for 75% of the total EU goat slaughtering. Differences between Germany, Belgium, Italy (where goat is only produced for milk) and Spain (where it is for meat and milk) are not reflected in the European figures. The UK and Ireland clarified they have no experience with goats. France and Denmark representatives have left the meeting prior to this elicitation.
Collective view on question 9:
Figure C.9 represents the density of probabilities, obtained by consensus judgement, expressing the collective view of the group regarding the overall prevalence of pregnant goats slaughtered.
Figure C.9.
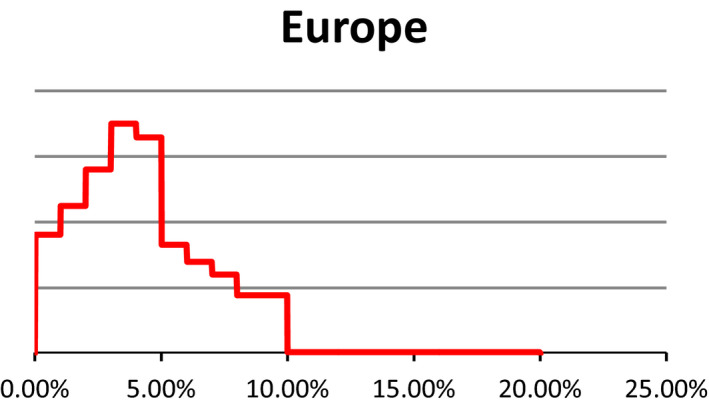
Density of probabilities expressing the collective view of the group regarding the overall prevalence of pregnant goats slaughtered
Table C.13 represents the estimated proportion of all goats which are pregnant at the time of slaughter in Europe.
Table C.13.
Estimated proportion of all goats which are pregnant at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| Lower | 1st Q | Median | 3rd Q | Upper |
|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 10% |
Judgements about stages of gestation of slaughtered pregnant goats at EU level
Question 10: Please give your judgement on the EU average distribution of the stages of pregnancy for 100 slaughtered pregnant female goats [in %]
Discussion on the differences between the countries:
Most figures from the national surveys indicated that most slaughtered pregnant goats fall into the first period. When pregnancy is recognised, the goat is not sent to slaughter for the value of the kids. In Italy, a high percentage was found in the first stage of gestation but the reason is not clear.
Table C.14 represents the estimated proportion of all ewes which are pregnant at any stage of gestation at the time of slaughter in Europe.
Table C.14.
Estimated proportion of all dairy cows which are pregnant at any stage of gestation at the time of slaughter in Europe
| 1st term of gestation | 2nd term of gestation | 3rd term of gestation |
|---|---|---|
| 67% | 27% | 6% |
Full uncertainty assessment was done for the third term of gestation only and is reported here below: Figure C.10 represents the density of probabilities, obtained by consensus judgement, expressing the collective view of the group regarding the overall prevalence of pregnant goats slaughtered at the third term of gestation.
Figure C.10.
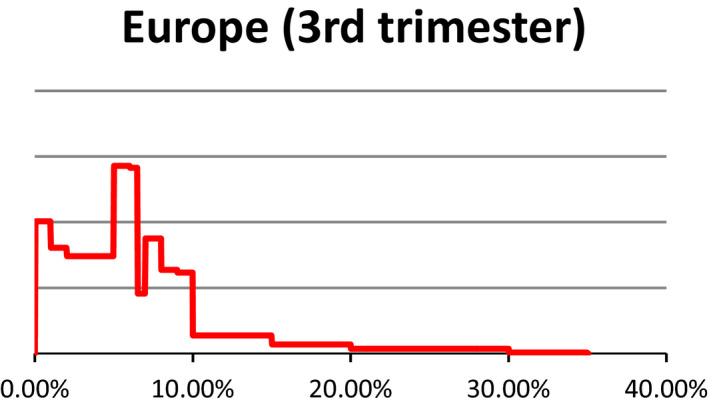
Probability density regarding the overall prevalence of sows slaughtered at the third term of gestation
Table C.15 represents the estimated proportion of all goats which are pregnant at the third term of gestation at the time of slaughter in Europe.
Table C.15.
Estimated proportion of all goats which are pregnant at third term of gestation at the time of slaughter in Europe. Given are the best estimate (median) and the quartiles (Q) which define the 50% uncertainty range (1st Q–3rd Q) as well as the 98% uncertainty range (lower–upper)
| Lower | 1st Q | Median | 3rd Q | Upper |
|---|---|---|---|---|
| 0% | 3% | 6% | 8% | 30% |
Expert knowledge elicitation on horses
Discussion on main factors influencing increase and decrease of prevalence in horses
Due to lack of time, it was only possible to discuss about the factors increasing the prevalence of slaughtered pregnant horses (mares). The production, from Eurostat figures, resulted to be quite important in Italy (more than 70% of total European horse slaughtering). A large number of horses are imported from Romania to Italy. The prevalence of pregnant mares that are slaughtered is quite low (max 6%). Health or economic problems are the factors leading to slaughtering horses.
Among the factors increasing the prevalence:
Declaration for meat consumption to reduce killing costs
Mixing of male and female
Horse traders without knowledge of animal pregnancy, less control
Injuries, accidents.
Among the factors decreasing the prevalence:
Horses are considered pets
Individual slaughtering
Higher health surveillance.
Closing remarks
The Elicitor and Recorder thanked everyone for their valuable contributions to the workshop.
Appendix D – Elicitation Report
SLAUGHTER OF PREGNANT ANIMALS ‘CAPACITY OF FETUSES TO FEEL PAIN AND NEGATIVE AFFECT’
EFSA premises , parma ( italy ), 6–8 july 2016
part 1 – expert knowledge elicitation exercise description
Purpose of the elicitation
The purpose of this exercise was to judge probabilities11 around the possibility that fetuses feel pain – and other negative affects – by expert knowledge elicitation.
Attendants, roles and expertise
The elicitation group included an elicitor – experienced in the elicitation of expert knowledge using the Sheffield method – a recorder – from the EFSA scientific secretariat staff – and an analyst – from the EFSA Assessment and Methodology Unit.
The Elicitation experts were members of the Working Group (WG) on ‘Slaughter of pregnant animals’ (EFSA‐Q‐2015‐00477) together with nine external hearing experts selected based on the additional expertise needed for the exercise. Expertise needed related to fetal anaesthesia, fetal consciousness, fetal and neonatal physiology, pain treatment of the prematurely born child from the human field; animal consciousness, animal welfare, fetal consciousness, effect of hypoxia on fetuses and effect of various stunning interventions on fetuses from the veterinary field.
Participants were made aware that judgements made in the elicitation, and the reasoning used, were going to be recorded, but that they were not going to be attributed to the experts by name.
Training for the elicitation workshop
A week before the workshop, experts were sent a document explaining the purpose of the elicitation workshop and the tasks that Elicitation experts would be asked to perform. This was reinforced by a presentation at the start of the workshop. The Elicitation experts then carried out a practice elicitation in which each expert gave a probability for an example question, using probability wheels as a visual aid. The Elicitor explained that for the questions that followed, the group would also be asked to arrive at a consensus probability or range of probabilities expressing their collective judgement.
Evidence
Prior to the meeting, the Elicitation experts received a background document summarising evidence reviewed by the WG, subdivided by key questions needed to address the overall question on whether fetuses feel pain and negative affect. The background document also specified that the concept of pain was extended, by agreement of the AHAW WG and Panel, to negative affect. Additionally, the experts received the logical model developed by the WG.
The background document included a list of important information gaps identified by the WG. Experts were asked to send in advance, or bring to the workshop, any additional evidence on these points and any other additional evidence they considered relevant.
At the meeting, the mandate on the ‘Slaughter of pregnant animals’ was shortly presented; the logical model was presented in depth, also focusing on the particular slaughter situations.
The logical model is shown as a flow chart in Figure D.1. It shows the series of subquestions (approximately corresponding to the questions of the background document), and how they link together to address the overall question. These subquestions are written in bold and each of them is followed by a question in italics – the latter is a hypothetical experiment expressing the question in a way that is potentially observable: this is good practice for elicitation (EFSA, 2014) to aid consistent interpretation of questions and judgements between experts. The logical model was duplicated and one version of it was made specific to the brain cortex and another version specific to the subcortex (Figures D.1 and D.2). The questions related to the cortex are indicated as C1, C2, etc; the questions related to the subcortex are indicated as SC1, SC2, etc.
Figure D.1.
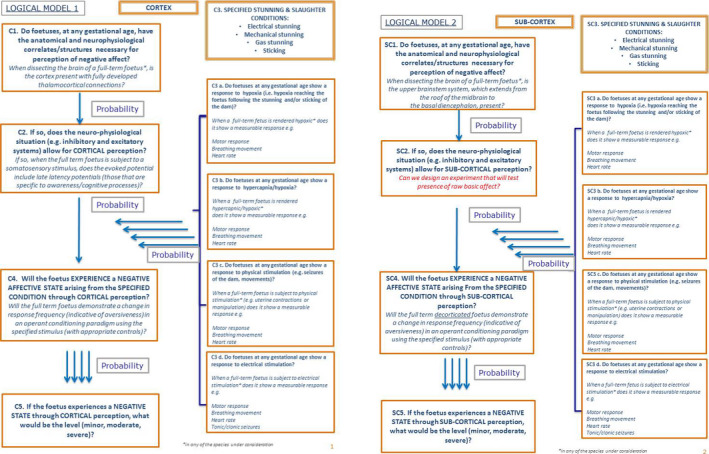
Flow charts of logical models as circulated prior to workshop. For version revised at workshop, with questions as elicited, see Figure D.2 (later)
Figure D.2.
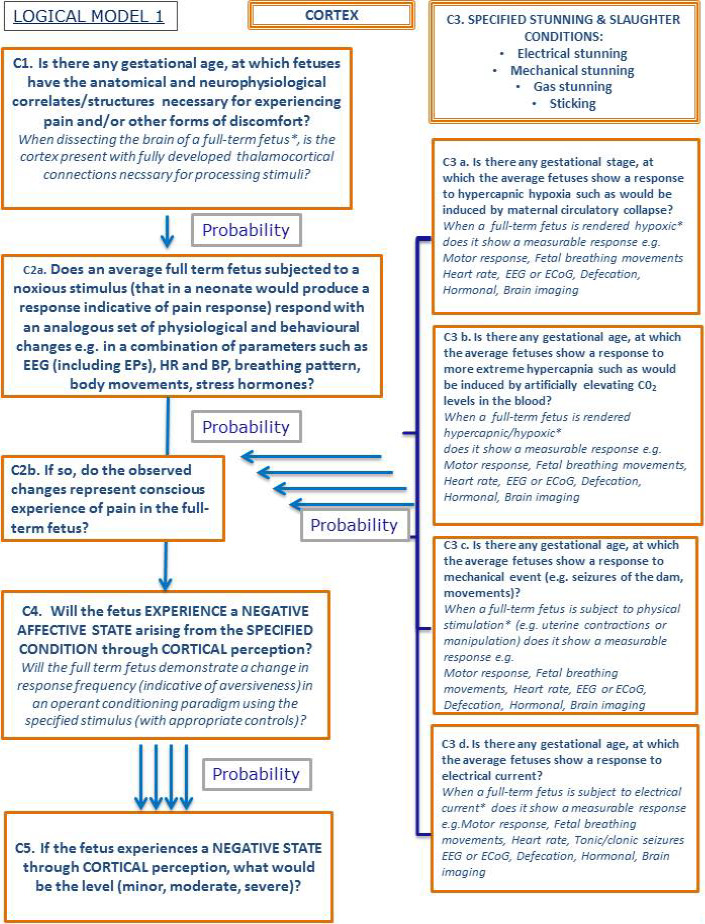
Revision of the logical model
Strengths and weaknesses
Three aspects of the framing of questions in the logical model were discussed before starting the Elicitation Exercise:
Extrapolation from different species:
The framing of the questions assumed that the answers to the questions of the logical model are not species‐specific. A discussion was therefore held whether there are any species‐specific considerations and whether evidence can be meaningfully extrapolated among species. The participants discussed that the neurophysiological development is the same but the timing might slightly change (e.g. precocial animals). From the learning experiments, there are no species‐specific considerations. It was then agreed that the answers are applicable to all species under consideration (pig, sheep, cow, horses) and that if a deviation would be found along the process then the distinction would be made.
Gestational age:
The questions were phrased in such a way that the answer would be positive if the proposition in the question was true at one or more points in the gestational age of the fetuses. A discussion was held on whether the elicitation group should consider the answer in relation to any point in gestation or to a particular stage of fetal development. Clarification was expressed by a WG member that the group will be operating under the precautionary principle, i.e. it is necessary to know if the fetus is conscious at any age. Comments were raised that, since in the slaughterhouse there is usually no way to know the exact stage of pregnancy, then there is no need for considering the precise point in gestation. Other experts suggested that the questions should focus on the stage of neurological development. The Elicitor explained that it would not be possible in the time available to elicit expert knowledge on all these variations. It was finally agreed that the questions would better be re worded as ‘is there any gestational age at which…’ and that at the end of the meeting additional questions referring to different periods of gestational age would be asked.
Any average fetus:
The questions were framed in terms of the average fetus, based on the assumption that the degree of variation in answers for fetuses of the same age would be too small to be of practical importance. Comments were raised that there are important physiological variations, for instance, pathological conditions – e.g. small placenta leading to hypoxia exposure for the fetus – or different levels of development – e.g. for lambs where there might be more than one fetus from the same pregnant dam reaching different levels of physiological development. It was finally agreed that, although there might be variations, these do not need to be quantified but need to be taken into account when considering the average fetus.
Participants additionally requested some clarifications on the process and on the wording of the questions. The need to agree on operational definitions and to include them in the glossary was highlighted. The definition of a fetus was discussed: the WG has defined it as a fetus till the moment it is independent, i.e. the moment it takes its ex‐utero first breath. However, comment was raised that a preterm child or animal cannot breathe air successfully but it becomes a newborn anyway. Also, during the second half of pregnancy, there are several breathing attempts in utero and measurable behavioural changes provoked by external stimuli. The final agreement was that, for the purposes of discussion in this EKE, the fetus is a fetus till removal from the uterus into a non‐fluid (air) environment.
It was clarified that, for questions 3a–3d, all procedures, subsequent to stunning and sticking, that potentially affect the fetus should be considered to define the relevant degree of stimulation.
Structuring of the elicitation
The same procedure was adopted for each question:
The question was reviewed and any queries were clarified.
The evidence was reviewed: the relevant section of the background document was identified and any points of clarification or disagreement were discussed; participants were asked to summarise any additional evidence they considered relevant.
Experts were asked to write down their initial judgements on the question individually, expressed as probabilities (in the form of percentages).
The initial probability judgements were collected verbally and shown together on a flip chart.
The Elicitor facilitated a discussion of the judgements made, and the reasons for major differences.
Experts were asked to consider individually whether to retain their initial judgement or write down an amended judgement.
The revised probability judgements were collected verbally and shown on the flip chart.
The Elicitor facilitated a discussion working towards a consensus judgement expressing the collective view of the group. The Elicitor checked for agreement on the result of this discussion before moving on to the next question.
At several points, the Elicitor made clear that (a) if experts felt they lacked relevant expertise for a question or could not give a judgement, they should not give one, and (b) experts could give either a single probability or a range for each question.
Questions 3a–3d were asked first, then questions 1, 2 and 4. Finally, a set of questions referring to different gestational ages was elicited. Due to shortage of time, the procedure described above was reduced for the later questions (as specified below).
part 2 – elicitation questions
Elicitation for questions 3a–3d
Q3: Is there any gestational age at which the average fetus shows a response to a. hypercapnic hypoxia by maternal circulatory collapse; b. more extreme hypercapnia by artificially elevating CO 2 levels in the blood; c. mechanical event (e.g. seizures of the dam, movements); d. electrical current? When a full‐term fetus is rendered ‘conditions a, b, c, d’ (see logical model revised) does it show a measurable response e.g. Motor responses, breathing movements, heart rate?
Question definition
It was clarified that questions 3a–3d are related to measurable responses irrespective of whether the fetus experiences pain. Any degree of hypoxia should be considered, i.e. the answer would be yes for a high level but also for a low level of hypoxia.
Questions 3a and 3b differentiate a) situations where hypoxia is generated by the fetus itself following cord occlusion or maternal circulation arrest/collapse from b) situations in which the concentration of blood CO2 is increased because the gas‐stunned dam transfers it to the fetus i.e. higher level and quicker level reaching the fetus.
It was agreed that, in terms of the strength of stimulus:
3a is intended as maternal circulation collapse, and 50% of blood of the dam is removed within 20–30 s following sticking.
3b is intended as dam being exposed to 80% CO2 (in pigs).
3c is intended as tonic/clonic seizures of the dam and shackling and hoisting due to inversion of the dam for cutting plus evisceration.
3d is intended as 1.3 Amp head to body current applied to the dam assuming that a high amount also reaches the fetus.
Evidence added in the meeting
Participants were asked if there were other examples of measurable responses and the following were added: EEG, ECoG, defecation, hormone responses, brain imaging changes. These were taken into account for the responses to the question.
Judgements of probability
|
Probability judgements (%) First round judgements in black, second round in red text in brackets, if modified | ||||
|---|---|---|---|---|
| Q3a | Q3b | Q3c | Q3d | |
| 1 | 100 (99) | 100 (99) | 90 | 90 |
| 2 | 90 | 90 | 90 | 50 (90) |
| 3 | 100 (99) | 99 | 75 (85) | 99 |
| 4 | 98 | 98 | 95 | 98 |
| 5 | 95 | 95 | 95 (98) | 95 |
| 6 | 100 | 80 (95) | 80 (95) | 80 (95) |
| 7 | 95 | 60 (95) | 95 | 95 |
| 8 | 100 | 100 | 80 (90) | 80–100 (90) |
| 9 | 100 | 99 (100) | 99 | 95–99 (99) |
| 10 | 100 | 50 | 100 | 90 |
| 11 | 99 | 99 | 95 | 99 |
| 12 | 95 | 95 | 95 | 50 (95) |
| 13 | 100 | 100 | 100 | 100 |
| 14 | 100 | 100 | 100 | 100 |
For the first round of judgements, most experts judged the questions 3a–3d with very high probabilities. The Elicitor first asked explanation about the 100% answers, and secondly to the diverging answers. Expert 14 was asked to explain his/her 100% probability answer and explained that he/she had seen such outcomes in experimental settings. Expert 13 gave 100% probability answer and explained that for question 3d the assumption is that the current passes through the fetus. For the same question, expert 2 gave a 50% probability answer and explained that he/she thought that the electrical application is so brief that there would not be time for any response. Also, expert 12 gave a 50% for the uncertainty related to the positioning of the electrodes, e.g. if electrodes are placed on the thorax there is lower probability that the fetus responds to the electric current. Expert 10 explained that for question 3b, he/she gave a 50% answer for the probability that fetus may somehow become sedated and thus not respond. Expert 5 put all at 95% and not 100% for expressing residual uncertainty reflecting the confidence of experimental studies. Expert 3 put 75% at question 3c because of the scarce evidence about fetal responses to tonic/clonic contractions.
After the discussion on the first round of judgements, the experts made the second round judgements of the same questions. In the above table, amended probabilities, compared to the first round, are indicated in red.
Collective view for questions 3a–3d
Group judgement reasoning was discussed and consensus was reached that the following ranges appropriately represent the collective view of the group:
Question 3a: 95–99.9
Question 3b: 95–99.9
Question 3c: 90–99.9
Question 3d: 90–99.9
Elicitation for question 1
Q1: Is there any gestational age at which fetuses have the anatomical and neurophysiological structures/correlates necessary for cortical perception? When dissecting the brain of a full‐term fetus, is the cortex present with fully developed thalamocortical connections?
Question definition
The question was thoroughly discussed: first, for some experts the question included two questions, the first asking about the presence of the brain ‘equipment’, the second asking whether such equipment would provide for the necessary background for perception of affect. Although the question in italics – i.e. the hypothetical experiment ‐ clarifies this aspect, the question in italics is not necessarily correlated to a positive answer to the question in bold. Therefore, the thalamocortical connections need to be defined as those related to processing stimuli. Related to the cortical (or subcortical) perception, expert 6 expressed his/her view that sensation of pain and perception of affects are two different things. A discussion was held to clarify the meaning of cortical perception. It was agreed to change the word ‘perception’ to ‘experience’ because breathlessness and affect are experienced, not perceived. Experts 5 and 6 argued that perception is a lower process than experience. For instance, perception of pain is an affect but nociception might not be, e.g. nociception can be associated to no pain perception. WG members explained that the concept of pain was extended to other negative affect and an expert explained that by the word ‘affect’ it is meant, in order, breathlessness, hunger and pain. It was finally agreed that the question should be focused on experience of pain and other discomforts.
Agreed question:
Q1 revised: Is there any gestational age at which fetuses have the anatomical and neurophysiological structures/correlates for experiencing pain and/or other forms of discomfort? When dissecting the brain of a full‐term fetus*, is the cortex present with sufficiently developed thalamocortical connections necessary for processing stimuli?
Evidence added in the meeting
Expert 2 suggested adding evidence on EEG of prematurely born child (from 26 weeks of gestation) to the background document.
Judgements of probability
|
Probability judgements (%) First round judgements in black, second round in red (in brackets) if modified | |
|---|---|
| Q1 | |
| 1 | 99.9 |
| 2 | 95 |
| 3 | 99 |
| 4 | 100 |
| 5 | 98 |
| 6 | 85 (95) |
| 7 | 95 |
| 8 | 100 |
| 9 | 95 |
| 10 | 99 |
| 11 | 95 (98) |
| 12 | 98 |
| 13 | 100 |
| 14 | 95 |
The Elicitor asked for explanation about diverging probability values. Expert 13 gave a 100% because in literature it is well documented that, at least at the end of gestation, fetuses have all structures. Expert 6 explained he/she gave 85% as the answer because he/she interpreted processing stimuli as all types of stimuli. Expert 12 explained these were all stimuli relevant to a fetus, while experts 5 and 9 interpreted them as all stimuli relevant to the slaughter conditions. Consensus was achieved in which stimuli were defined as any of those associated with the measurable responses listed in question 3a–3d.
Collective view for question 1
After the second round of judgements, group judgement reasoning was discussed and consensus was reached that the probability for the proposition in this question to be true is in the range from 95% to 99.9%.
Elicitation for question 2
Q2: If so, does the neurophysiological situation allow for cortical perception? If so, when the full term fetus is subject to a somatosensory stimulus, does the evoked potential include late latency potentials (those that are specific to awareness/cognitive processes)?
Due to the intrinsic complexity of the question, the group discussed the appropriateness of the hypothetical experiment used in the second part of this question. Expert 2 suggested that several papers studied magnetoencephalography evoked potentials not only during labour but also during pregnancy (e.g. before and after a mother's meal). Expert 5 suggested continued learning tests, and also suggested that for a better understanding of the question there is a need to clarify the meaning of ‘late’. Expert 13 answered that, in human adults, when stimuli are consciously perceived (as opposed to not perceived) then evoked potentials are observed 100–140 ms after the stimulus application. He/she then asked if somatosensory evoked potentials are identical in anesthetised and non‐anesthetised patients. Expert 8 explained they are not identical but there is no clear measure. In clinics, evoked potentials are used to test the velocity of nerve conductance from peripheral signals, with focus on the early, rather than late signals. The Elicitor asked if the reliable absence of late evoked potentials would imply that there is no awareness. Expert 8 answered that the absence would imply there is brain damage which itself means there is no cortical awareness.
Expert 5 commented that a good question would be if it is possible to arouse a fetus. Expert 12 answered that a fetus cannot be aroused to a semiconscious state by a stimulus because the fetus is maintained in continued unconscious state. Expert 2 did not agree with this view because papers show that four or five behavioural states can be observed in fetuses showing that they have active responses to pain and stimuli, e.g. noises. Expert 8 agreed on the latter, but commented that evoked potentials following arousal of a fetus do not mean that it is conscious and arousal is not only defined as capacity to respond to stimuli. Expert 6 argued that habituation experiments demonstrate cortical involvement in the response not just a spinal reflex.
Expert 2 highlighted that by ‘cortical perception’ it is indeed meant ‘consciously experiencing pain and discomfort’. The elicitation group agreed that common definitions of ‘perception’ and ‘pain’ were needed to respond the question.
Perception might mean ‘state of being aware’. It is characterised by the ability to experience a variety of negative and positive subjective sensations or feelings. However, experts agreed that states of alert awareness or perception may have various levels. Expert 11 highlighted that from the Regulation on Animal Slaughter (EC Reg 1099/2009) awareness is the ability to feel emotions and control voluntary mobility. For expert 2, awareness is a disturbed response to an external stimulus which involves the cortex.
One expert suggested the group consider the IASP definition of pain: An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.
Expert 2 felt that the IASP definition of pain is focused on adult humans, not applicable to fetus and animals.
Expert 7 suggested that ‘pain for an animal is considered as the physiological response coming from any stimulation which is perceived as painful and results in a response to protect against that stimulus’ (Mohony and Kent, 1997).
It was finally agreed that question 2 would better be reworded and split in two:
Question 2a. Does the full term fetus subjected to a noxious stimulus, which in the neonate produces a response indicative of pain, respond with changes e.g. in a combination of parameters such as EEG (including EPs), HR and BP, attempts to breathe, grimaces, body movements, stress hormones?
Question 2b. If so, do the observed changes represent conscious experience of pain for the full term fetus?
Evidence
The Elicitor asked if, in light of the discussion reported above, there was a need to amend the evidence brought in the background document. Main points related to the paragraph on vibroacoustic stimulation (VAS) and observed changes in responses; the reported paper by Jardri and the suggested selective responsiveness, needing to highlight that the evidence is not indicative of associative learning but only of learning. Expert 2 expressed his/her view that the theory of in utero sedation due to adenosine, allopregnanolone and pregnanolone, does not take into account that the levels of these messengers are not different from those in the blood of a mother at the end of pregnancy, thus raising the question why the mother is not also asleep. He/she also pinpointed the difference between analgesia and sedation (the fetus might be sedated but this does not imply analgesia). Expert 12 argued that pregnanolone has analgesic effects. Expert 2 responded that analgesic effect is only observed when injected in cerebral fluid not when naturally produced because the level would not be high enough. Expert 12 said that allopregnanolone and pregnanolone are synthesised in the brain and the fetus is producing the hormone. He/she clarified that in the paper from Gregory, the inhibitory effect was considered to be related to O2 level when in fact it was related to changes in adenosine concentration consequent on this.
Judgements of probability
|
Probability judgements (%) First round judgements in black, second round in red (in brackets) if modified | ||
|---|---|---|
| Q2a. Does the full term fetus subjected to a noxious stimulus, which in the neonates produces a response indicative of pain, respond with changes e.g. in a combination of parameters such as EEG (including EPs), HR and BP, attempts to breathe, grimaces, body movements, stress hormones? | Q2b. If so, do the observed changes represent conscious experience of pain for the full term fetus? | |
| 1 | 99.9 (95.9) | 15 (35) |
| 2 | 95 | 80 (85) |
| 3 | 95 | 20 |
| 4 | 75 (95) | 20 |
| 5 | 33 (85) | 14 (20) |
| 6 | 80 (60) | 10 |
| 7 | 95 (80) | 50 (10) |
| 8 | 50 (90) | 20 (10) |
| 9 | 95 | 40 (20) |
| 10 | 98 | 70 |
| 11 | 85 | 30 |
| 12 | 98 | 5–15 |
| 13 | 90 | 50 (40) |
| 14 | 60 (90) | 50 (15) |
Discussion on judgements for question 2a
The Elicitor asked explanation about diverging probability values for the first round of judgements. Expert 5 gave 33% because he/she thinks fetuses do not show changes because they are in a very specific physiological state. Also, there is confusion in the available data and it is not possible to know if a set of changes observed in some studies derived from responses in neonates exposed to stimuli. Expert 2 and 13 responded that, at pp 8 of the background document, more references are reported of fetuses responding with behavioural changes. Expert 6 rated it 80% because there is evidence of single responses but not of the full set of responses and still thinks 80% is quite high. Expert 12 highlighted that cardiovascular changes are similar in fetus and neonates but the most reliable changes are behavioural and physiological (EEG being the most reliable).
Expert 2 gave 99.9% because he/she interpreted ‘a response’ as any one or more of the set, not requiring the whole set to be present together. The Elicitor confirmed that this interpretation seems consistent with the ‘e.g.’ and ‘such as’ in the question, and asked other group members to use this interpretation when making second round judgements. Other experts rated the question low because they had not observed grimaces. Expert 3 explained grimaces in fetuses have never been observed, they are purely theoretical although in rats facial expressions are described. The group decision was to take out grimaces from the set.
Collective view for question 2a
After the second round judgements, a final range of 80–95% was agreed for the response to question 2a.
Discussion on judgements for question 2b
Expert 2 rated question 2b high (80%) and explained again that he/she does not accept the assumption of ‘sedated’ fetuses, that do not feel pain, because of three main reasons: (a) fetal sleep is not continuous, (b) sedation differs from anaesthesia (the latter is the absence of pain and any movements to any stimulus) and (c) the levels of the supposed sedative substances in fetal blood appear in scientific literature to be similar to those in their mothers, who are neither sedated or anaesthetised during pregnancy. Fetuses respond to external stimuli so they are not anesthetised. Sedation is instead the reduction of responses due to, e.g. benzodiazepine administration and no one does surgery relying on benzodiazepine‐induced sedation. Expert 12 clarified that what he/she refers to is ‘sleep like unconsciousness’ and the EEG pattern is the key observation. When you stimulate arousal there are movements but they are not associated to evoked potentials.
A long discussion was then held on whether this ‘sleep like unconsciousness’ is sedation. Expert 8 said that sedation is a live form of anaesthesia. Expert 2 clarified that with deep sedation there is still a response to external stimuli. Also, all neuroinhibitors can be used as drugs but the difference would be the amount, surely a big amount of pregnanolone injected into the brain can anesthetise.
Expert 13 explained that the key point is the definition of awake and sleep states – and associated interpretation of EEG patterns – but considered that there is not good enough evidence to distinguish those states in the fetus.
Expert 5 said that in surgery the primary aim is to prevent pain: if we imagine removal of the pain system (i.e. receptors) this would be equal to a completely conscious patient without pain. Therefore, anaesthesia is not so relevant for this discussion because pain is not the matter in the slaughter situation, but rather distress and discomfort. The Elicitor reminded that the ToR is about pain, to which Expert 5 responded that there is no pain if you are not conscious.
Expert 12 expressed the view that focusing the discussion on anaesthesia might be incorrect: when you are sleeping you are unconscious, but you can wake up. The fetus in contrast does not wake up.
Expert 2 expressed again the fact that four behavioural states have been described, and observed by obstetricians without EEG, by assessing eye movement, heart and muscle tone but Expert 8 responded that these four behavioural states are observed in humans because it is not possible to do EEG or ECOG. In animals, EEG is possible on fetuses, demonstrating that there are awake states, but wakefulness in fetuses is not comparable to that in neonates (interpreted as transitional stages between sleep states rather than wakefulness seen in neonates).
It was agreed that it is not possible to define a conclusive hypothetical experiment for question 2b.
The Elicitor went around the table inviting every Expert to comment on the probability they had given. Expert 12 rated the probability at 5–15% because there are specific and direct data from sheep – behavioural and EEG states – demonstrating neuroinhibitory evidence, thus indicating that the fetus is in a continuous sleep state of unconsciousness. The probability was not rated 0 because it's difficult to demonstrate conclusively. Similarly, Experts 5 and 6 rating it at 14% and 10%, respectively. Expert 3 also rated it at 20% for the uncertainty and for the findings of Fitzgerald concluding that when the baby is born it is not awake; delivery wakes it up.
Expert 4 rated at 20% after following the discussion and wishing to give a balanced view. Similarly, Expert 9 rated it at 40% to express uncertainty.
Expert 13 rated it 50% because of the uncertainty in the interpretation of changes in EEG observed in fetuses. For the same reason Expert 10 rated it on 70% because those signs still express a higher probability than 50/50.
Since Experts 2 and 12 defended divergent positions, the Elicitor invited each of them to summarise their position in a 1‐min statement:
Expert 2: ‘In the ‘80s and ‘90s of the last century, similar discussions took place about baby's pain and the conclusion was that newborn babies don't feel pain because they are assumed to be always in a sleeping state. Babies then underwent surgery with no anaesthesia and they showed cortisol rises and other signs of pain were observed. There is equivalence between fetuses and newborns. I agree that fetuses spend most of time sleeping but I think studies demonstrate they can be awoken, maybe not in 100% of cases because e.g. sickness or deep sleep. Fetuses can be aroused and birth is an example: when they are just born they have still a certain amount of fetal blood components but they will arouse during birth. Finally, no one would undergo surgery if sleeping only’.
Expert 12: ‘Focusing on distinctions between pharmacologically induced anaesthesia, sedation and analgesia as a refutation of the published evidence, and on circulating concentrations of the different endogenously produced neuroinhibitors misrepresents both the rationale and the nature of the detailed experiments, the results of which support the rationale. Three lines of evidence support specifically the presence of continuous states of “sleep‐like unconsciousness”, not sedation: (i) direct EEG patterns, (ii) evidence showing that the physiological environment of the fetal brain in utero is uniquely neuroinhibitory: there exist at least 8 neuroinhibitory mechanisms with demonstrated neuroinhibitory actions on the fetal cerebral cortex, and (iii) the inability to rouse the fetus to consciousness using noxious stimuli that awaken the sleeping newborns of the same species to consciousness. The question of noxious stimuli and impact on EEG has been assessed several times because all invasive methods in surgery have been followed by EEG: EEG activation is observed as well as cortisol changes. It is clear there is no evidence of arousal during general anaesthesia, so I am convinced the fetuses do not respond to pain’.
Summary of the group view for question 2b
The Elicitor offered a verbal summary of the judgements made by the group: there is a wide range of opinion with one expert going below 10% and the highest at 80% but no one is certain at either end. Most of the judgements are in the range 10–30%, with two at 70% and 80% and three at 50%. The Experts agreed that this would be taken as a summary of their views.
Elicitation for questions 4a–4d
Will the average fetus showing the responses of question 3a EXPERIENCE a NEGATIVE AFFECTIVE STATE arising from the SPECIFIED CONDITIONS through CORTICAL perception? Will the full term fetus demonstrate a change in response frequency (indicative of aversiveness) in an operant conditioning paradigm using the specified stimulus (with appropriate controls)?
Question definition
Expert 13 explained that this qualifying experiment is not a reflex conditioning but an operant paradigm. The Elicitor asked if the hypothetical experiment was possible and Expert 6 confirmed the suggested experiment is possible. Expert 12 agreed. Expert 5 said that a conditioning test is not possible with a conditioned stimulus. Trace conditioning would be the best experiment. Expert 6 said that it would be possible to establish an experiment requiring learning of a contingency between two events, using either classical or operant conditioning paradigms. Expert 1 expressed a view that operant conditioning would help in understanding the outcome while associative learning would be weaker. Experts were asked to consider also the sub‐cortical situation as in question SC4 of the logical model for subcortex: the question is the same but the question in bold asks about sub‐cortical perception and the question in italics asks if the full‐term decorticated fetus demonstrates a change in response frequency.
Calculation based on earlier judgements
The Elicitor explained that the original form of the logical model (Figure D.1) implied that the probabilities given for questions 1–3 could be combined by calculation to give estimated probabilities for question 4, assuming question 2 was conditional on question 1 and both 1 and 2 were independent of question 3, These calculations were carried out by the EFSA analyst during the workshop and results for each expert were printed together in numerical form and as pie charts. These results were distributed to the Experts and briefly explained by the Elicitor. The Elicitor noted that the revision of question 2 had probably removed the conditionality of question 2a on question 1, making the calculated probabilities somewhat lower than they should be. The Elicitor stated that the calculations were presented only to raise awareness of the potential relationships between the questions and encouraged the Experts not to anchor on the calculated results but rather form their own judgements when answering question 4.
Given the limited remaining time, the Elicitor asked the Experts to make their first round judgements on Questions 4a–4d without a preliminary discussion of evidence.
Judgement of probability
|
Probability judgements (%) First round judgements in black, second round in red (in brackets) if modified | ||||
|---|---|---|---|---|
| Q4a | Q4b | Q4c | Q4d | |
| 1 | 25 | 30 | 20 (5) | 30 (40) |
| 2 | 90 | 90 | 80 | 70 |
| 3 | 15–30 (10) | 15–30 (10) | 15–30 (5) | 15–30 |
| 4 | 10 | 10 | 10 | 10 |
| 5 | 5 (20) | 5 (20) | 5 (20) | 5 (20) |
| 6 | 50 | 50 | 0 | 100 |
| 7 | 80 (60) | 80 (60) | 80 (60) | 80 (60) |
| 8 | 10 | 10 | 10 | 10 |
| 9 | 0–15 | 0–15 | 0–15 | 0–15 |
| 10 | 60–70 | 20–30 (50) | 50 | 50 |
| 11 | 5–10 | 15 | 15 | 5–10 |
| 12 | 10 | 10 | 10 | 10 |
| 13 | 10 | 20 | 10 | 30 |
| 14 | 10 | 10 | 10 | 10 |
Discussion on judgements
A big variation was observed from high to low and it was discussed whether the responses were high or low for different reasons than for the previous questions 3a–3d. There was group agreement that these responses would not be possible in a decorticated fetus.
Expert 6 rated the first two questions Q4a and Q4b at 50% due to the uncertainty whether electric and gas stunning would create a response. For the uterine contractions, the answer would be 0 while for the electrical shock it would be 100%.
Expert 10 gave lower probability for the second condition because if the slaughter procedure causes high CO2 to the dam, then the death of mother occurs too quickly for the fetus to go to deep sleep unconsciousness from the CO2.
Expert 12 said that 2% O2 means that any response would be subcortical.
Expert 2 gave different responses for the different conditions because of the uncertainty and lack of familiarity with the slaughter situation.
Summary of the group view for questions 4a–4d
The Elicitor asked whether the Experts would be content for the range of views in the group to be summarised after the workshop in a similar way to what had been agreed for Question 2b (above). This was agreed.
Elicitation on Question C2b in relation to gestational age
The Elicitor noted that insufficient time remained to complete all the planned questions. Related to the questions on the subcortex, there was no time to consider them extensively; yet a relevant aspect of it had been considered during the elicitation for question 4 and the discussion led to the agreement that a decorticated fetus would show no responses to changes. A member of the WG proposed to use the remaining time to consider Question C2b in relation to gestational age, and this was agreed.
Preceding questions had referred either to a fetus of any age or to full‐term fetuses. The Experts were reminded of the wording of Question C2b (‘If so, do the observed changes represent conscious experience of pain the full term fetus?’). The probability judgements they had made for C2b were displayed, and the experts’ own written records of their judgements and reasoning were made available to those who wished to review them.
The question was expressed as:
Given your probability for question C2b what is your probability of conscious experience of pain for any ante‐natals in each of the specified gestational age ranges?
|
Probability judgements (%) First round judgements in black, second round in red (in brackets) if modified | |||||
|---|---|---|---|---|---|
| 100–80% pregnancy | 80–60% pregnancy | 60–40% pregnancy | 40–20% pregnancy | 20–0% pregnancy | |
| 1 | 30–35 | 20–30 | 5 (0–2) | 0 | 0 |
| 2 | 95 | 90 | 50 | 0 | 0 |
| 3 | 20 | 7–11 (14) | 1 | 0 | 0 |
| 4 | No answer | No answer | No answer | No answer | No answer |
| 5 | 20 | 10 | 0 | 0 | 0 |
| 6 | 10 | 2 (8) | 0 | 0 | 0 |
| 7 | 10 | 2 | 0 | 0 | 0 |
| 8 | 10 | 10 | 2 | 0 | 0 |
| 9 | 15–20 | 15 | 0–5 (2) | 0 | 0 |
| 10 | 70 | 50 | 0–2 (1) | 0 | 0 |
| 11 | 30 | 10 | 5 | 0 | 0 |
| 12 | 5–15 (0) | 5–10 (0) | 0 | 0 | 0 |
| 13 | 40 | 40 | 0 | 0 | 0 |
| 14 | 15 | 10 | 5 | 0 | 0 |
Discussion on judgements
Expert 5 had to leave at this point. Before going, the expert said that he/she now considered that the evidence presented in the workshop for a sleep‐like state in fetuses would make the distinction between cortex and subcortex distinction irrelevant.
It was observed that probabilities drop off in the third column (40–20% of pregnancy). Experts 1 and 11 agreed that they drop because it is demonstrated that it is only at the last third of gestation that they may feel pain.
Expert 10 put already a drop in probability at 60–40% of pregnancy but he/she raised such probability value at the second round.
Expert 2 gave high values for the first three periods of pregnancy because there is possibility of subcortical experience and also because from the 26th week of gestation onwards the human doctors usually give opioids.
Expert 2 asked about the interpretation of those studies demonstrating that fetuses are awake from 26th weeks. Expert 12 explained that transitional sleep states occupy 5% of the last period of pregnancy (3 s per minute) but they are sleep transitions and are therefore a continuation of unconsciousness.
Experts were asked to make their second round judgements on the question. These were not displayed or discussed, due to lack of time. The Experts were asked to leave their written judgements and notes on reasoning at the end of the workshop.
Closing remarks
It was not possible to address question C5 within the available time because the difficulty of objectively categorising intensity of affective state would require prolonged discussion.
The Elicitor and Recorder thanked everyone for their valuable contributions to the Workshop. The Recorder confirmed that the draft report would be circulated to all participants for review.
An updated version of the logical model showing the changes agreed during the workshop. The wording of questions 1 and 2 was changed; question 2 was subdivided into two questions. The second logical model relating to the subcortex was not used in the elicitations.
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Animal Welfare) , More S, Bicout D, Botner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortazar Schmidt C, Michel V, Miranda MA, Saxmose Nielsen S, Velarde A, Thulke H‐H, Sihvonen L, Spoolder H, Stegeman JA, Raj M, Willeberg P, Candiani D and Winckler C, 2017. Scientific Opinion on the animal welfare aspects in respect of the slaughter or killing of pregnant livestock animals (cattle, pigs, sheep, goats, horses). EFSA Journal 2017;15(5):4782, 96 pp. 10.2903/j.efsa.2017.4782
Requestor: Denmark, Germany, the Netherlands and Sweden
Question number: EFSA‐Q‐2015‐00477
Panel members: Dominique Bicout, Anette Botner, Andrew Butterworth, Paolo Calistri, Klaus Depner, Sandra Edwards, Bruno Garin‐Bastuji, Margaret Good, Christian Gortazar Schmidt, Virginie Michel, Miguel Angel Miranda, Simon More, Søren Saxmose Nielsen, Antonio Velarde, Hans‐Hermann Thulke, Liisa Sihvonen, Hans Spoolder, Jan Arend Stegeman, Mohan Raj, Preben Willeberg and Christoph Winckler.
Acknowledgements: The Panel wishes to thank the members of the Working group on animal welfare aspects in respect of the slaughter or killing of pregnant livestock animals (cattle, pigs, sheep, goats, horses): Sandra Edwards, Mohan Raj, Hans Spooler, Antonio Velarde, Andy Hart and Christoph Winckler for the preparatory work on this scientific opinion, the following hearing experts: Ioana Andronie, Carlo Valerio Bellieni, Lotta Berg, Cécile Bourguet, Laura Boyle, Véronique Debarge, Richard Donker, Bernadette Doyle, Else Enemark, Nicholas Fisk, Lucy Green, Peter Hepper, Pierre Le Neindre, Michael Marahrens, David Mellor, Bjorn Merker, Joaquim Pallisera, Katharina Riehn, Sara Rota Nodari, Matthias Schwab, Piotr Ślósarz, Evangelia Sossidou, Miguel Stevens, Claudia Terlouw, Anne von Thaden, Noelia Yusta and EFSA staff Francesca Porta and Olaf Mosbach‐Schulz for the support provided to this scientific opinion.
Adopted: 5 April 2017
Notes
Directive 2010/63/EU of the European parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes.
Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety.
Council Regulation (EC) No 1/2005 of 22 December 2004 on the protection of animals during transport and related options and amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97.
Commission Implementing Decision of 18 April 2013 on annual report on non‐discriminatory inspections carried out pursuant to Council regulation (EC) No 1/2005 on the protection of animals during transport and related operations and amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97.
Since two EKE meetings were performed for the development of this opinion, from here onwards this first meeting will be referred to as EKE 1, and the second as EKE 2 (see Section 2.3.3).
After the meeting, the probability distribution of animals in the third term of gestation was recalculated based on animals in the third term of gestation out of the total number of pregnant female animals. In the opinion only the latter is reported.
The term ‘probability’ used in this report equals to the term ‘likelihood’ as used in the body of the scientific opinion.
References
- Alcorn DG, Adamson TM, Maloney JE and Robinson PM, 1981. A morphologic and morphometric analysis of fetal lung development in the sheep. The Anatomical Record, 201, 655–667. [DOI] [PubMed] [Google Scholar]
- Almond GW and Dial GD, 1986. Pregnancy diagnosis in swine: a comparison of the accuracies of mechanical and endocrine tests with return to estrus. Journal of the American Veterinary Medical Association, 189, 1567–1571. [PubMed] [Google Scholar]
- Alonso‐Spilsbury M, Mota‐Rojas D, Villanueva‐Garcıa D, Martinez‐Burnes J, Orozco H, Ramirez‐Necoechea R, Lopez Mayagoitia A and Elena Trujillo ME, 2005. Perinatal asphyxia pathophysiology in pig and human: A review. Animal Reproduction Science 90, 1–30. [DOI] [PubMed] [Google Scholar]
- Alosta RA, Vaugnan L and Collins JD, 1998. An abattoir survey of ovine reproductive tracts in Ireland. Theriogenology, 50, 457–464. [DOI] [PubMed] [Google Scholar]
- Alvaro R, de Almeida V, Al‐Alaiyan S, Robertson M, Nowaczyk B, Cates D and Rigatto H, 1993. A placental extract inhibits breathing induced by umbilical cord occlusion in fetal sheep. Journal of Developmental Physiology, 19, 23–28. [PubMed] [Google Scholar]
- Alvaro RE, Robertson M, Al‐Saedi S, Lemke RP, Cates DB and Rigatto H, 1997. Preliminary characterization of a placental factor inhibiting breathing in fetal sheep. Reproduction, Fertility and Development, 9, 641–649. [DOI] [PubMed] [Google Scholar]
- Anand KJS, 2007. Consciousness, cortical function, and pain perception in nonverbal humans. Behavioral and Brain Sciences, 30, 82–83. [Google Scholar]
- Anil MH, Yesildere T, Aksu H, Matur E, McKinstry JL, Erdogan O, Hughes S and Mason C, 2004. Comparison of religious slaughter of sheep with methods that include pre‐slaughter stunning, and the lack of differences in exsanguination, packed cell volume and meat quality parameters. Animal Welfare, 13, 387–392. [Google Scholar]
- Anil MH, Yesildere T, Aksu H, Matur E, McKinstry JL, Weaver HR, Erdogan O, Hughes S and Mason C, 2006. Comparison of Halal slaughter with captive bolt stunning and neck cutting in cattle: exsanguination and quality parameters. Animal Welfare, 15, 325–330. [Google Scholar]
- Baier RJ, Hasan SU, Cates DB, Hooper D, Nowaczyk B and Rigatto H, 1990. Effects of various concentrations of O2 and umbilical cord occlusion on fetal breathing and behavior. Journal of Applied Physiology, 68, 1597–1604. [DOI] [PubMed] [Google Scholar]
- Ball KT, Gunn TR, Gluckman PD and Power GG, 1996. Suppressive action of endogenous denosine on ovine fetal nonshivering thermogenesis. Journal of Applied Physiology, 81, 2393–2398. [DOI] [PubMed] [Google Scholar]
- Barcroft J, 1946. Growth and development. Chapter 3. In: Barcroft J (ed.). Researches on Prenatal Life, Blackwell, Oxford. [Google Scholar]
- Bassett JM, Oxborrow TJ, Smith ID and Thorburn GD, 1969. The concentration of progesterone in the peripheral plasma of the pregnant ewe. Journal of Endocrinology, 45, 449–457. [DOI] [PubMed] [Google Scholar]
- Batten EH, 1960. The placodal relations of the glossopharyngeal nerve in the sheep: a contribution to the early development of the carotid body. Journal of Comparative Neurology, 114, 11–37. [DOI] [PubMed] [Google Scholar]
- Beausoleil NJ and Mellor DJ, 2015. Introducing breathlessness as a significant animal welfare issue. New Zealand Veterinary Journal, 63, 44–51. 10.1080/00480169.2014.940410 [DOI] [PubMed] [Google Scholar]
- Bellieni CV and Buonocore G, 2012. Is fetal pain a real evidence? The Journal of Maternal‐Fetal & Neonatal Medicine, 25, 1203–1208. [DOI] [PubMed] [Google Scholar]
- Bennet L, Rossenrode S, Gunning MI, Gluckman PD and Gunn AJ, 1999. Cardiovascular and cerebrovasucular responses of the immature fetal sheep to acute umbilical cord occlusion. The Journal of Physiology, 517, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger PJ, Walker AM, Horne R, Brodecky V, Wilkinson MH, Wilson F and Maloney JE, 1986. Phasic respiratory activity in the fetal lamb during last gestation and labour. Respiratory Physiology, 65, 55–68. [DOI] [PubMed] [Google Scholar]
- Bergin WC, Gier HT, Frey RA and Marion GB, 1967. Developmental horizons and measurements useful for age determination of equine embryos and fetuses. pp. 179–196. In: Annual Convnention of American Association of Equine Practitioners. New Orleans.
- Bissonnette J, 2000. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. American Journal of Physiology. Regulatory, Integrative and Compared Physiology, 278, R1391–R1400. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Hohimer AR and Knopp SJ, 1995. GABAergic and glutamatergic effects on behaviour in fetal sheep. The Journal of Physiology, 487, 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLV (Bundesamt für Lebensmittelsicherheit und Veterinärwesen), 2014. Projekt: Schlachtung von trächtigen Rindern – Prävalenz und Gründe der Schlachtung. (Project report: Slaughter of pregnant cattle – prevalence and reasons for slaughtering).
- Bocking AD, 2003. Assessment of fetal heart rate and fetal movements in detecting oxygen deprivation in‐utero. European Journal of Obstetrics & Gynecology and Reproductive Biology, 110, 108–112. [DOI] [PubMed] [Google Scholar]
- Bocking AD and Harding R, 1986. Effects of reduced uterine blood flow on electrocortical activity, breathing, and skeletal muscle activity in fetal sheep. American Journal of Obstetrics and Gynecology, 154, 655–662. [DOI] [PubMed] [Google Scholar]
- Boddy K, Dawes GS, Fisher R and Robinson JS, 1974. Fetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. Journal of Physiology, 243, 599–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogolyubsksiy N, 1959. Intrauterine formation and development of body structure of Merion and Precoce sheep. Academy of Sciences of the U. S. S. R., 23, 277–339. [Google Scholar]
- Born GV, Dawes GS and Mott JC, 1955. The viability of premature lambs. Journal of Physiology, 130, 191–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunmiller K, 2015. Schlachtung von trächtigen Kähen ‐ Erfahrungen der Schlachthoftierärzte. Deutsches Tierärzteblatt, 01, S.6–8. [Google Scholar]
- Breen S, Rees S and Walker D, 1997. Identification of brainstem neurons responding to hypoxia in fetal and newborn sheep. Brain Research, 748, 107–121. [DOI] [PubMed] [Google Scholar]
- Bünger‐Marek I, 1972. Beitrag zur Altersbestimmung von Feten des Deutschen Schwarzbunten Rindes insbesondere auf Grund von Längenmassen. University of Hannover: unpublished Thesis. [Google Scholar]
- Campbell MLH, Mellor DJ and Sandoe P, 2014. How should the welfare of fetal and neurologically immature postnatal animals be protected? Animal Welfare, 23, 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey‐Trott TM, Millman ST, Turner PV, Nykamp SG and Widowski TM, 2013. Effectiveness of a nonpenetrating captive bolt for euthanasia of piglets less than 3 d of age. Journal of Animal Science, 91, 5477–5484. [DOI] [PubMed] [Google Scholar]
- Casey‐Trott TM, Millman ST, Turner PV, Nykamp SG, Lawlis PC and Widowski TM, 2014. Effectiveness of a nonpenetrating captive bolt for euthanasia of 3 kg to 9 kg pigs. Journal of Animal Science, 92, 5166–5174. [DOI] [PubMed] [Google Scholar]
- Cautilli J and Dziewolska H, 2005. Brief report: the reinforcing effects of paternal verbals stimulation and gentle pushing on kicking behavior in a 35 week old in utero. The Behavior Analyst Today, 6, 160–163. [Google Scholar]
- Clancy B, Darlington RB and Finlay BL, 2001. Translating developmental time across mammalian species. Neuroscience, 105, 7–17. [DOI] [PubMed] [Google Scholar]
- Clark RE and Squire LR, 1998. Classical conditioning and brain systems. The role of awareness. Science, 280, 77–81. 10.1126/science.280.5360.77 [DOI] [PubMed] [Google Scholar]
- Clewlow F, Dawes GS, Johnston BM and Walker DW, 1983. Changes in breathing, electrocortical and muscle activity in unanaesthetised fetal lamb with age. Journal of Physiology, 341, 463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloete JHL, 1939. Prenatal growth in the Merino sheep. Onderstepoort Journal of Veterinary Science and Animal Industry, 13, 417–546. [Google Scholar]
- Conrad MS and Johnson RW, 2015. The domestic piglet. An important model for investigating the neurodevelopmental consequences of early life insults. Annual Reviews in Animal Biosciences, 3, 245–264. [DOI] [PubMed] [Google Scholar]
- Cook CJ, Gluckman PD, Johnston BM and Williams C, 1987. The development of the somatosensory evoked potential in the unanaesthetized fetal sheep. Journal of Developmental Physiology, 9, 441–445. [PubMed] [Google Scholar]
- Dalin AM, Gidlund K and Eliasson‐Selling L, 1997. Post‐mortem examination of genital organs from sows with reproductive disturbances in a sow‐pool. Acta Veterinaria Scandinavica, 38, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall R, 2010. The role of CO2 and central chemoreception in the control of breathing in the fetus and the neonate. Respiratory Physiology & Neurobiology 173, 201–212. 10.1016/j.resp.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauger S, Aizenfisz S, Renolleau S, Durand E, Vardon G, Gaultier C and Gallego J, 2001. Arousal response to hypoxia in newborn mice. Respiration Physiology, 128, 235–240. [DOI] [PubMed] [Google Scholar]
- Dawes GS, Mott JC and Shelley HJ, 1959. The importance of cardiac glycogen for the maintenance of life in fetal lambs and new‐born animals during anoxia. The Journal of Physiology, 146, 516–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes GS, Fox HE, Leduc BM, Liggins GC and Richards RT, 1972. Respiratory movements and rapid eye movement sleep in the fetal lamb. Journal of Physiology, 220, 119–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes GS, Gardner WN, Johnston BM and Walker DW, 1982. Effects of hypercapnia on tracheal pressure, diaphragm, and intercostal electromyograms in unanaesthetized fetal lamb. The Journal of Physiology, 326, 461–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes GS, Gardner WN, Johnston BM and Walker DW, 1983. Breathing in fetal lambs. The effect of brain stem section. The Journal of Physiology, 335, 535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCasper AJ and Spence MJ, 1986. Prenatal maternal speech influences newborns’ perception of speech sounds. Infant Behavior and Development, 9, 133–150. [Google Scholar]
- Desborough JP, 2000. The stress response to trauma and surgery. British Journal of Anaesthesia, 85, 109–117. [DOI] [PubMed] [Google Scholar]
- Di Nicolo K, 2006. Studie zum zusätzlichen Eintrag von Hormonen in die menschliche Nahrungskette durch das Schlachten von trächtigen Rindern in der Europäischen Union am Beispiel Luxemburg und Italien. Dissertation med. vet. Universität Leipzig, Veterinärmedizinische Fakultät, Institut für Lebensmittelhygiene, unpublished thesis. [Google Scholar]
- Dickerson JWT and Dobbing J, 1967. Prenatal and postnatal growth and development of the central nervous system of pig. Proceedings of the Royal Society of London, Series B, Biological Sciences, 166, 384–395. [DOI] [PubMed] [Google Scholar]
- Dobbing J and Sands J, 1979. Comparative aspects of the brain growth spurts. Early Human Development, 26, 61–67. [DOI] [PubMed] [Google Scholar]
- Drury PP, Davidson JO, Bennet L, Booth LC, Tan S, Fraser M, van den Heuij LG and Gunn AJ, 2014. Partial neural protection with prophylactic low‐dose melatonin after asphyxia in preterm fetal sheep. Journal of Cerebral Blood Flow and Metabolism 34, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan IJH, 2006. The changing concept of sentience. Applied Animal Behaviour Science, 100, 11–19. [Google Scholar]
- Dunwiddie TV and Masino SA, 2001. The role and regulation of adenosine in the central nervous system. Annual Review of Neuroscience, 24, 31–55. [DOI] [PubMed] [Google Scholar]
- Eaton ON, 1952. Weight and length measurements of fetuses of Karakul sheep and goats. Growth, 16, 175–187. [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the Commission related to welfare aspects of the main systems of stunning and killing the main commercial species of animals. EFSA Journal 2004;2(7):45, 29 pp. 10.2903/j.efsa.2004.45 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Scientific opinion on welfare aspects of the main systems of stunning and killing applied to commercially farmed deer, goats, rabbits, ostriches, ducks, geese and quail. EFSA Journal 2005;3(3):326, 18 pp. 10.2903/j.efsa.2006.326 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009. Scientific Opinion of the Panel on Animal Health and Welfare on a request from the Commission on the risk assessment of the impact of housing, nutrition and feeding, management and genetic selection on leg and locomotion problems in dairy cows. EFSA Journal 2009;7(7):1142, 57 pp. 10.2903/j.efsa.2009.1142 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Guidance on Expert Knowledge Elicitation in Food and Feed Safety Risk Assessment. EFSA Journal 2014;12(6):3734, 278 pp. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2013. Scientific opinion on monitoring procedures at slaughterhouses for bovines. EFSA Journal 2013;11(12):3460, 65 pp. 10.2903/j.efsa.2013.3460 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2014. Scientific Opinion on the welfare risks related to the farming of sheep for wool, meat and milk production. EFSA Journal 2014;12(12):3933, 128 pp. 10.2903/j.efsa.2014.3933 [DOI] [Google Scholar]
- Evans HE and Sack WO, 1973. Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Anatomia, Histologia, Embryologia, 2, 11–45. [DOI] [PubMed] [Google Scholar]
- Evans HE, Ingalls TH and Binns W, 1966. Teratogenesis of craniofacial malformations in animals. 111. Natural and experimental deformities in sheep. Archives of Environmental Health, 13, 706–714. [DOI] [PubMed] [Google Scholar]
- Ewart JC, 1897. A critical period in the development of the horse. London: Adam and Charles Black. [Google Scholar]
- Ferguson DM and Warner RD, 2008. Have we underestimated the impact of pre‐slaughter stress on meat quality in ruminants? Meat Science, 80, 12–19. [DOI] [PubMed] [Google Scholar]
- Fraser AF, 1976. Some features of an ultrasonic study of bovine foetal kinesis. Applied Animal Ethology, 2, 379–383. [Google Scholar]
- Fraser AF, 1989. A monitored study of major physical activities in the perinatal calf. Veterinary Record, 125, 38–40. [DOI] [PubMed] [Google Scholar]
- Fraser AF, Hastie H, Callicot RB and Brownlie S, 1975. An exploratory ultrasonic study of quantitative foetal kinesis in the horse. Applied Animal Ethology, 1, 395–404. [Google Scholar]
- Friede RL, 1989. Developmental Neuropathology. Springer, Berlin, pp. 31–39. [Google Scholar]
- Galpin N, 1935. The prenatal development of the coat of the New Zealand Romney lamb. Journal of Agricultural Science, 25, 344–360. [Google Scholar]
- George S, Gunn AJ, Westgate JA, Brabyn C, Guan J and Bennet L, 2004. Fetal heart rate variability and brainstem injury after asphyxia in preterm fetal sheep. American Journal of Physiology. Regulatory, Intergrative and Comparative Physiology, 287, R925–R933. [DOI] [PubMed] [Google Scholar]
- Gerritzen MA, Kluivers‐Poodt M, Reimert HGM, Hindle V and Lambooij E, 2008. Castration of piglets under CO2‐gas anaesthesia. Animal, 2, 1666–1673. [DOI] [PubMed] [Google Scholar]
- Giannakoulopoulos X, Sepulveda W, Kourtis P, Glover V and Fisk NM, 1994. Fetal plasma cortisol and beta‐endorphin response to intrauterine needling. Lancet, 344, 77–81. [DOI] [PubMed] [Google Scholar]
- Gjesdal F, 1969. Age determination of bovine foetuses. Acta Veterinaria Scandinavica 10, 197–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjesdal F, 1972. Age determination of swine foetuses. Acta veterinaria Scandinavica Supplementum, 40, 1–29. [PubMed] [Google Scholar]
- Gluckman PD, Gunn TR and Johnston BM, 1983. The effect of cooling on breathing and shivering in unaesthetized fetal lambs in utero. Journal of Physiology, 343, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanalingham MG, Mostyn A, Dandrea J, Yakubu DP, Symonds ME and Stephenson T, 2005. Ontogeny and nutritional programming of uncoupling protein‐2 and glucocorticoid receptor mRNA in the ovine lung. Journal of Physiology, 565, 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F, Cabrera F, Batista M, Rodrıguez N, Alamo D, Sulon J, Beckers JF and Gracia A, 2004. A comparison of diagnosis of pregnancy in the goat via transrectal ultrasound scanning, progesterone, and pregnancy‐associated glycoprotein assays by determination of pregnancy‐associated glycoprotein assays. Theriogenology, 62, 1108–1115. [DOI] [PubMed] [Google Scholar]
- Green WW and Winters LM, 1945. Prenatal development of the sheep. University of Minnesota Agri Exp Sta Tech Bull No, 169, pp. 1–36.
- Gregory NG, 1986. The Physiology of Electrical Stunning and Slaughter. Humane Slaughter of Animals for Food, UFAW (Universities Federation of Animal Welfare), St Albans, UK. pp. 3–14. [Google Scholar]
- Grist A, Murrell JC, McKinstry JL, Knowles TG and Wotton SB, 2017. Humane euthanasia of neonates I: Validation of the effectiveness of the Zephyr EXL non‐penetrating captive bolt euthanasia system on neonate piglets up to 10.9 kg live‐weight. Animal Welfare, 26, 111–120. [Google Scholar]
- Gruest N, Richer P and Hars B, 2004. Emergence of long‐term memory for conditioned aversion in the rat fetus. Developmental Psychobiology, 44, 189–198. [DOI] [PubMed] [Google Scholar]
- Guffy MM, Bergin WC and Gier HAT, 1970. Radiographic fetometry of the horse. Cornell Veterinarian, 50, 359–371. [PubMed] [Google Scholar]
- Gunn AJ and Bennet L, 2009. Fetal hypoxia insults and patterns of brain injury. Insights from animal models. Clinics in Perinatology, 36, 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermehl KH, 1975. Die Altersbestimmung bei Haus‐ und Labortieren. Verlag Paul Parey, Berlin. [Google Scholar]
- Harding R, Johnson P and McClelland ME, 1980. Respiratory function of the larynx in developing sheep and the influence of sleep state. Respiratory Physiology, 40, 165–179. [DOI] [PubMed] [Google Scholar]
- Harding R, Fowden AL and Silver M, 1991. Respiratory and non‐respiratory thoracic movements in the fetal pig. Journal of Developmental Physiology, 15, 269–275. [PubMed] [Google Scholar]
- Harris H, 1937. The foetal growth of sheep. Journal of Anatomy, 71, 516–527. [PMC free article] [PubMed] [Google Scholar]
- Hasan SU and Rigaux A, 1991. The effects of lung distension, oxygenation, and gestational age on fetal behavior and breathing movements in sheep. Pediatric Research, 30, 193–201. [DOI] [PubMed] [Google Scholar]
- Hawkins RD and Byrne JH, 2015. Associative learning in invertebrates. Cold Spring Harbor Perspectives in Biology, 7, a021709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen M, Leppavuori A and Pyorala S, 1998. Evaluation of reproductive failure of female pigs based on slaughterhouse material and herd record survey. Animal Reproduction Science, 52, 235–244. [DOI] [PubMed] [Google Scholar]
- Hepper PG, 1988. Adaptive fetal learning. Prenatal exposure to garlic affects postnatal preferences. Animal Behaviour, 36, 935–936. [Google Scholar]
- Hepper PG, 1991. An examination of fetal learning before and after birth. Irish Journal of Psychology, 12, 95–107. [Google Scholar]
- Hepper PG, Scott D and Shahidullah S, 1993. Newborn and fetal response to maternal voice. Journal of Reproductive and Infant Psychology, 11, 147–153. [Google Scholar]
- Higashi H, Molnar Z, Kurotani T and Toyama K, 2002. Prenatal development of neural excitation in rat thalamocortical projections studied by optical recording. Neuroscience, 115, 1231–1246. [DOI] [PubMed] [Google Scholar]
- Hohimer AR, Bissonnette JM, Richardson BS and Machida CM, 1983. Central chemical regulation of breathing movements in fetal lamb. Respiratory Physiology, 52, 99–111. [DOI] [PubMed] [Google Scholar]
- Holdsworth RJ and Davies J, 1979. Measurement of progesterone in goat's milk: an early pregnancy test. Veterinary Record, 105, 535. [PubMed] [Google Scholar]
- Huang QF, Gebrewold A, Zhang A, Altura BT and Altura BM, 1994. Role of excitatory amino acid in regulation of rat pial microvasculature. American Journal of Physiology, 266, R158–R163. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Bennet L, Power GG, Roelfsems V, Blood AB, Quaedackers JS, George S, Guan J and Gunn AJ, 2003. Key neuroprotective role for endogenous adenosine A1 receptor activation during asphyxia in the fetal sheep. Stroke, 34, 2240–2245. [DOI] [PubMed] [Google Scholar]
- Husa L, Bieger D and Fraser AF, 1988. Fluoroscopic study of the birth posture of the sheep fetus. Veterinary Record, 123, 645–648. [PubMed] [Google Scholar]
- Ioffe S, Jansen AH, Russell BJ and Chernick V, 1980. Respiratory response to somatic stimulation in fetal lambs during sleep and wakefulness. Pflügers Archiv European Journal of Physiology, 388, 143–148. [DOI] [PubMed] [Google Scholar]
- Ishwar AK, 1995. Pregnancy diagnosis in sheep and goats: a review. Small Ruminant Research, 17, 37–44. [Google Scholar]
- James DK, 2010. Fetal learning: a critical review. Infant and Child Development, 19, 45–54. [Google Scholar]
- Janczak AM, Braastad BO and Bakken M, 2006. Behavioural effects of embryonic exposure to corticosterone in chickens. Applied Animal Behaviour Science 96, 69–82. [Google Scholar]
- Jardri R, Houfflin‐Debarge V, Delion P, Pruvo JP, Thomas P and Pins D, 2010. Assessing fetal response to maternal speech using a noninvasive functional brain imaging technique. International Journal of Developmental Neuroscience, 30, 159–161. 10.1016/j.ijdevneu.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Jensen AH, Ioffe S, Russell BJ and Chernick V, 1982. Influence of sleep state on the response to hypercapnia in fetal lambs. Respiratory Physiology, 48, 125–142. [DOI] [PubMed] [Google Scholar]
- Jensen A, Hohmann M and Kunzel W, 1987. Dynamic changes in organ blood flow and oxygen consumption during acute asphyxia in fetal sheep. Journal of Developmental Physiology, 9, 41–55. [PubMed] [Google Scholar]
- Jensen TB, Bonde MK, Kongsted AG, Toft N and Sørensen JT, 2010. The interrelationships between clinical signs and their effect on involuntary culling among pregnant sows in group‐housing systems. Animal, 4, 1922–1928. [DOI] [PubMed] [Google Scholar]
- Johnson CB, Kells N, Sutherland MA and Beausoleil NJ, 2015. Validation of EEG measures for pain assessment in piglets aged 0 to 10 days. Final Report NPB C‐13–188.
- Johnson C, Sylvester SP, Stafford KJ, Mitchinson SL, Ward RN and Mellor DJ, 2009. Effects of age on the electroencephalographic response to castration in lambs anaesthetized with halothane in oxygen from birth to 6 weeks old. Veterinary Anaesthesia and Analgesia 36, 273–279. [DOI] [PubMed] [Google Scholar]
- Jones CJ and Fox H, 1977. Syncytial knots and intervillous bridges in the human placenta: an ultrastructural study. Journal of Anatomy, 124, 275–286. [PMC free article] [PubMed] [Google Scholar]
- Jones CT, 1977. The development of some metabolic responses to hypoxia in the foetal sheep. Journal of Physiology, 266, 743–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong E, Appeltant R, Cools A, Beek J, Boyend F, Chiers K and Maes D, 2014. Slaughterhouse examination of culled sows in commercial pig herds. Livestock Science, 167, 362–369. [Google Scholar]
- Joubert DM, 1956. A study of prenatal growth and development in the sheep. Journal of Agricultural Science, 47, 382–428. [Google Scholar]
- Kahn W and Leidl W, 1987. Ultrasonicbiometry of horse fetuses in uteroand sonographic representation of their organs DTW. Deutsche Tierarztliche Wochenschrift 94, 509–515. [PubMed] [Google Scholar]
- Karlsson K and Kjellmer I, 1974. The uterine and maternal placental blood flow during hyperoxia. Journal of Perinatal Medicine 2, 170. [DOI] [PubMed] [Google Scholar]
- Kells N, Beausoleil N, Chambers JP, Sutherland MA, Morrison R and Johnson CB, 2013. EEG assessment of acute pain in pigs during tail docking. In: Pluske JR, Pluske JM (eds.). Manipulating Pig Production XIV: Proceedings of the Fourteenth Biennial Conference of the Australasian Pig Science Association (APSA), 129 pp. [Google Scholar]
- Keunen H, Blanco CE, van Reempts JL and Hasaart TH, 1997. Absence of neuronal damage after umbilical cord occlusion of 10, 15, and 20 minutes in midgestation fetal sheep. American Journal of Obstetrics and Gynecology, 176, 515–520. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick JF, Lasley BL, Shideler SE, Roser JF and Turner JW, 1993. Non‐instrumented immunoassay field tests for pregnancy detection in free‐roaming feral horses. The Journal of Wildlife Management, 57, 168–173. [Google Scholar]
- Kitterman JA, Liggins GC, Campos GA, Clements JA, Forster CS, Lee CH and Creasy RK, 1981. Prepartum maturation of the lung in fetal sheep: relation to cortisol. Journal of Applied Physiology. Respiration, Environment and Exercise Physiology, 51, 384–390. [DOI] [PubMed] [Google Scholar]
- Kohler I, Moens Y, Busato A, Blum J and Schatzman U, 1998. Inhalation anaesthesia for the castration of piglets: CO2 compared to halothane. Journal of Veterinary Medicine, 45, 625–633. [DOI] [PubMed] [Google Scholar]
- Koos BJ and Doany W, 1991. Role of plasma adenosine in responses to hypoxia in fetal sheep. Journal of Developmental Physiology, 16, 81–85. [PubMed] [Google Scholar]
- Koos BJ, Mason BA, Punla O and Adinolfi AM, 1994. Hypoxic inhibition of breathing in fetal sheep: relationship to brain adenosine concentrations. Journal of Applied Physiology, 77, 2734–2739. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Kruger L and Murray TF, 1997. Sources of extracellular brain adenosine during hypoxia in fetal sheep. Brain Research, 778, 439–442. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Chau A, Matsuura M, Punla O and Kruger L, 1998. Thalamic locus mediates hypoxic inhibition of breathing in fetal sheep. Journal of Neurophysiology, 79, 2383–2393. [DOI] [PubMed] [Google Scholar]
- Laureys S, Boly M, Moonen G and Maquet P, 2009. Coma. Encyclopedia of Neuroscience, 2, 1133–1142. [Google Scholar]
- Lawson BC, Shahzad AH, Dolecheck KA, Martel EL, Velek KA, Ray DL, Lawrence JC and Silvia WJ, 2014. A pregnancy detection assay using milk samples: evaluation and considerations. Journal of Dairy Science, 97, 6316–6325. [DOI] [PubMed] [Google Scholar]
- Le Neindre P, Boissy A, Boivin X, Calandreau L, Delon N, Deputte B, Desmoulin‐Canselier S, Faivre N, Giurfa M, Guichet J‐L, Lansade L, Larrere R, Mormede P, Prunet P, Schaal B, Serviere J, Terlouw C, Bernard E and Dunier M, 2017. Animal consciousness. EFSA supporting publication 2017:EN‐NNNN, 169 pp.
- LeBlanc SJ, 2013. Short communication: field evaluation of a pregnancy confirmation test using milk samples in dairy cows. Journal of Dairy Science, 96, 2345–2348. [DOI] [PubMed] [Google Scholar]
- Lijowska AS, Reed NW, Mertins Chiodini BA and Thach BT, 1997. Sequential arousal and airway defensive behavior of infants in asphyxial sleep environments. Journal of Applied Physiology, 83, 219–228. [DOI] [PubMed] [Google Scholar]
- Lücker E, Bittner A and Einspanier A, 2003. Zur toxikologisch‐hygienischen Bewertung der Exposition mit hormonell wirksamen Stoffen bei Schlachtungen trächtiger Rinder unter verschiedenen Produktionsbedingungen. In: Deutsche Veterinärmedizinische Gesellschaft (DVG) (ed.). Proceedings 44. Arbeitstagung DVG Lebensmittelhygiene 2003, Gießen, pp. 628–633. [Google Scholar]
- Lyche JL, Janczak M, Eriksen MS and Braastad BO, 2005. Fosterets evne til å oppleve ubehag, smerte og stress. Rapport Institutt for Produksjonsdyrmedisin Norges veterinærhøgskole, Oslo, Norway, 17 pp.
- Maher P, Good M and More SJ, 2008. Trends in cow numbers and culling rate in the Irish cattle population, 2003 to 2006. Irish Veterinary Journal, 61, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, 1992. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Progress in Neurobiology, 38, 379–395. [DOI] [PubMed] [Google Scholar]
- Mallard ECM, Gunn AJ, Williams CE, Johnston BM and Gluckman PD, 1992. Transient umbilical cord occlusion causes hippocampal damage in the fetal sheep. American Journal of Obstetrics and Gynecology, 167, 1423–1430. [DOI] [PubMed] [Google Scholar]
- Malon AP and Curson HH, 1936. Studies in sex physiology. No. 15. Further observations on the body weight and crown‐rump length of Merino fetuses. Onderstepoort Journal of Veterinary Science and Animal Industry, 7, 239–249. [Google Scholar]
- Marana R, Margutti F, Catalano GF and Marana E, 2000. Stress responses to endoscopic surgery. Current Opinion in Obstetrics and Gynecology, 12, 303–307. [DOI] [PubMed] [Google Scholar]
- Maurer P, Lücker E and Riehn K, 2016. Slaughter of pregnant cattle in German abattoirs – current situation and prevalence: a cross‐sectional study. BMC Veterinary Research, 12, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald I, Wenam Aito G and Robinson JJ, 1977. Studies on reproduction in prolific ewes. 3. The development in size and shape of the foetal skeleton. Journal of Agricultural Sciences, 89, 373–391. [Google Scholar]
- McIntosh GH, Baghurst KI, Potter BJ and Hetzel BS, 1979. Foetal brain development in the sheep. Neuropathology Applied Neurobiology, 5, 103–114. [DOI] [PubMed] [Google Scholar]
- Mellor DJ, 2010. Galloping colts, fetal feelings and reassuring regulations: putting farm animal welfare science into practice. Journal of Veterinary Medical Education, 37, 94–102. [DOI] [PubMed] [Google Scholar]
- Mellor DJ and Diesch TJ, 2006. Onset of sentience: the potential for suffering in fetal and newborn farm animals. Applied Animal Behaviour Science, 100, 48–57. [Google Scholar]
- Mellor DJ and Diesch TJ, 2007. Birth and hatching: key events in the onset of awareness in the lamb and chick. New Zealand Veterinary Journal, 55, 51–60. [DOI] [PubMed] [Google Scholar]
- Mellor DJ and Gregory NG, 2003. Responsiveness, behavioural arousal and awareness in fetal and newborn lambs: experimental, practical and therapeutic implications. New Zealand Veterinary Journal, 51, 2–13. [DOI] [PubMed] [Google Scholar]
- Mellor DJ and Stafford KJ, 2004. Animal welfare implications of neonatal mortality and morbidity in farm animals. The Veterinary Journal, 168, 118–133. [DOI] [PubMed] [Google Scholar]
- Mellor DJ, Diesch TJ, Gunn AJ and Bennet L, 2005. The importance of ‘awareness’ for understanding fetal pain. Brain Research Reviews, 49, 455–471. [DOI] [PubMed] [Google Scholar]
- Merker B, 2005. The liabilities of mobility: a selection pressure for the transition to consciousness in animal evolution. Consciousness and Cognition, 14, 89–114. [DOI] [PubMed] [Google Scholar]
- Merker B, 2007. Consciousness without a cerebral cortex: a challenge for neuroscience and medicine. Behavioral and Brain Sciences, 30, 63–81. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Maas YGH and Ariagno RL, 2003. Development of fetal and neonatal sleep and circadian rhythms. Sleep Medicine Reviews, 7, 321–334. [DOI] [PubMed] [Google Scholar]
- Mohony V and Kent JB, 1997. Assessment of acute pain in farm animals using behavioural and physiological measurement. Journal of Animal Science, 75, 266–272. [DOI] [PubMed] [Google Scholar]
- Morell RH, Quarles RH and Norton WT, 1993. Myelin formation, structure and biochemistry. In: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB (eds.). Basic Neurochemistry. Molecular, Cellular and Medical Aspects. Raven Press, New York, pp. 118–143. [Google Scholar]
- Murase H, Endo Y, Tsuchiya T, Kotoyori Y, Shikichi M, Ito K, Sato F and Nambo Y, 2014. Ultrasonographic evaluation of equine fetal growth throughout gestation in normal mares using a convex transducer. Journal of Veterinary Medical Science, 76, 947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RD and Newstead R, 1988. Determination of steroid hormones in goats’ milk and plasma as an aid to pregnancy diagnosis using an ELISA. Veterinary Record, 122, 158–161. [DOI] [PubMed] [Google Scholar]
- Newby AC, Worku Y, Meghji P, Nakazawa M and Skladanowski AC, 1990. Adenosine: a retaliatory metabolite or not? Physiology, 5, 67–70. [Google Scholar]
- Nguyen PN, Billiards SS, Walker DW and Hirst JJ, 2003. Changes in 5 alpha‐pregnane steroids and neurosteroidogenic enzyme expression in the perinatal sheep. Pediatric Research, 53, 956–964. [DOI] [PubMed] [Google Scholar]
- Nielsen LK and Andersen BD, 2016. Prævalens og årsager til slagtning af drægtige malkekøer i Danmark, samt drægtighedslængde ved slagtning og sammenhængen mellem alder og tandfremdbrud hos kalvefostre. Kandidatspeciale i veterinærmedicin. Københavns Universitet, Det Sundhedsvidenskabelige Fakultet, Copenhagen. [Google Scholar]
- Njaa BL (ed.), 2012. Kirkbride's Diagnosis of Abortion and Neonatal Loss in Animals, 4th Edition. Appendix A. Gestational Age Estimation Based on Fetal Measures and Phenotypic Characteristics. John Wiley & Sons, UK. [Google Scholar]
- Odlaug TO, 1955. Laboratory Anatomy of the Fetal Pig, 2nd Edition. W.C. Brown Co, Dubuque, Iowa. [Google Scholar]
- Paul SM and Purdy RH, 1992. Neuroactive steroids. FASEB Journal, 6, 2311–2322. [PubMed] [Google Scholar]
- Payne J, Farris R, Parker G, Bonhotal J and Schwarz M, 2015. Quantification of sodium pentobarbital residues from equine mortality compost piles. Journal of Animal Science, 93, 1824–1829. [DOI] [PubMed] [Google Scholar]
- Peisker N, Preissel AK, Ritzmann M, Schuster T, Thomes R and Henke J, 2008. Fetal responses to different methods of electrocution of pregnant sows. Berliner und Münchener Tierärztliche Wochenschrift, 121, 317–328. [PubMed] [Google Scholar]
- Platt, H , 1978. Growth of the equine fetus. Equine Veterinary Journal, 16, 247–252. [DOI] [PubMed] [Google Scholar]
- Porkka‐Heiskanen T, Alanko L, Kalinchuk A and Stenberg D, 2002. Adenosine and sleep. Sleep Medicine Reviews, 6, 321–332. [DOI] [PubMed] [Google Scholar]
- Purohit G, 2010. Methods of pregnancy diagnosis in domestic animals: the current status. WebmedCentral, Reproduction, 12, WMC001305. [Google Scholar]
- Rakers F, Frauendorf V, Rupprecht S, Schiffner R, Bischoff SJ, Kiehntopf M, Reinhold P, Witte OW, Schubert H and Schwab M, 2013. Effects of early‐ and late‐gestational maternal stress and synthetic glucocorticoid on development of the fetal hypothalamus‐pituitary‐adrenal axis in sheep. Stress, 16, 122–129. [DOI] [PubMed] [Google Scholar]
- RCOG (Royal College of Obstetricians and Gynecologists), 2010. Fetal Awareness. Review of Research and Recommendations for Practice. Published by Royal College of Obstetricians and Gynaecologists, London. [Google Scholar]
- Regli K, 1963. Beitrag zur Altersbestimmung von Feten des Simmentaler und Freiburger Fleckviehrindes insbesondere auf Grund von Messungen an Gliedmassenknochen. Diss Vet Med Fak Univ Zürich, P.G. Keller, Winterthur, Zürich, pp. 67. [Google Scholar]
- Riehn K, Domel G, Einspanier A, Gottschalk J, Hildebrandt G, Luy J and Lücker UE, 2010. Schlachtung gravider Rinder ‐ ethische und rechtliche Aspekte. Fleischwirtschaft 90, 100–106. [Google Scholar]
- Riehn K, Domel G, Einspanier A and Lücker E, 2011. Schlachtung gravider Rinder ‐ Aspekte der Ethik und des gesundheitlichen Verbraucherschutzes. Tierärztliche Umschau, 66, 391–405. [Google Scholar]
- Rigatto H, Moore M and Cates D, 1986. Fetal breathing and behaviour measured through a double‐wall plexiglass window in sheep. Journal of Applied Physiology, 61, 160–164. [DOI] [PubMed] [Google Scholar]
- Roberts SJ, 1971. Veterinary obstetrics and genital diseases. 2nd Edition. Edwards Brothers, Inc., Ann Arbor, Mich. 542 pp. [Google Scholar]
- Roberts SJ (ed.), 1986. Examinations for pregnancy. In: Veterinary Obstetrics and Genital Diseases (Theriogenology), Published by the author, Woodstock, pp. 14–37. [Google Scholar]
- Robinson JJ, 1986. Changes in body composition during pregnancy and lactation. Proceedings of the Nutrition Society, 45, 71–80. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Dalmau A, Ruiz‐de‐la‐Torre JL, Manteca X, Jensen EW, Rodriguez B, Litvan H and Velarde A, 2008. Assessment of unconsciousness during carbon dioxide stunning in pigs. Animal Welfare, 17, 341–349. [Google Scholar]
- Romanes GJ, 1947. The prenatal medullation of the sheep's nervous system. Journal of Anatomy, 81, 64–81. [PubMed] [Google Scholar]
- Rosen AS and Morris ME, 1991. Depolarising effects of anoxia on pyramidal cells of rat neocortex. Neuroscience Letters, 124, 169–173. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y, 1972. Development of sleep and wakefulness in the foetal lamb. Electroencephalography and Clinical Neurophysiology, 32, 119–128. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y and Gaujoux M, 1976. Evolution perinatale des cycles de sommeil chez l'agneau. Comptes rendus hebdomadaires des séances de l'Academie des sciences, Serie D: Sciences naturelles, 282, 871–874. [PubMed] [Google Scholar]
- Ruckebusch Y, Gaujoux M and Eghbali B, 1977. Sleep cycles and kinesis in the fetal lamb. Electroencephalography Clinical Neurophysiology, 42, 226–237. [DOI] [PubMed] [Google Scholar]
- Rurak DW, 2016. Prenatal and postnatal determinants of development. Neuromethods, 109, 89–146. 10.1007/978-1-4939-3014-2_6. [DOI] [Google Scholar]
- Rurak DW and Wittman B, 2013. Real‐time ultrasound assessment of body and breathing movements and abdominal diameter in fetal lambs from 55 days of gestation to term. Reproduction Science, 20, 414–425. [DOI] [PubMed] [Google Scholar]
- Rüsse I and Grunert E, 1993. Die normale Gravidität. In: Grunert E, Arbeiter K (eds.). Tiergeburtshilfe. Hamburg. [Google Scholar]
- Sameshima H and Koos BJ, 1986. Effects of moderate hypoxia on foetal electrocortical activity, eye movements, and breathing activity in sheep. Journal of Developmental Physiology, 8, 411–419. [PubMed] [Google Scholar]
- Sawa R, Asakura H and Power GG, 1991. Changes in plasma adenosine during simulated birth of fetal sheep. Journal of Applied Physiology, 70, 1524–1528. [DOI] [PubMed] [Google Scholar]
- Schaal B, Marlier L and Soussignan R, 2000. Human fetuses learn odours from their pregnant mother's diet. Chemical Senses, 25, 729–737. [DOI] [PubMed] [Google Scholar]
- Schneider Mdel P, Strandberg E, Emanuelson U, Grandinson K and Roth A, 2007. The effect of veterinary‐treated clinical mastitis and pregnancy status on culling in Swedish dairy cows. Preventive Veterinary Medicine, 80, 179–192. [DOI] [PubMed] [Google Scholar]
- Schubert R, Blankenburg F, Lemm S, Villringer A and Curio G, 2006. Now you feel it – now you don't: ERP correlates of somatosensory awareness. Psychophysiology, 43, 31–40. [DOI] [PubMed] [Google Scholar]
- Schwab M, Schmidt K, Witte H and Abrams RM, 2000. Investigation of nonlinear ECoG changes during spontaneous sleep state changes and cortical arousal in fetal sheep. Cerebral Cortex, 10, 142–148. [DOI] [PubMed] [Google Scholar]
- Seamark RF, Nancarrow CD and Gardner J, 1970. Progesterone metabolism in ovine fetal blood: the formation of 3‐alpha‐hydroxy‐pregn‐4‐en‐20‐one and other substances. Steroids, 15, 589–603. [DOI] [PubMed] [Google Scholar]
- Shewmon DA, Holmes GL and Byrne PA, 1999. Consciousness in congenitally decorticate children: developmental vegetative state as self‐fulfilling prophesy. Developmental Medicine and Child Neurology, 41, 364–374. [DOI] [PubMed] [Google Scholar]
- Shone DK and Fricker JW, 1969. The diagnosis of pregnancy in the ewe with an ultra‐sonic fetal pulse detector. Journal of the South African Veterinary Association, 40, 377–378. [Google Scholar]
- Singh NS, Gawande PG, Mishra OP, Nema RK, Mishra UK and Singh M, 2004. Accuracy of ultrasonography in early pregnancy diagnosis in doe. Asian‐Australasian Journal of Animal Science, 17, 760–768. [Google Scholar]
- Singleton GH and Dobson H, 1995. A survey of the reasons for culling pregnant cows. Veterinary Record, 136, 162–165. [DOI] [PubMed] [Google Scholar]
- Slegel P, Kitagawa H and Maguire MH, 1988. Determination of adenosine in fetal perfusates of human placental cotyledons using fluorescence derivatization and reversed‐phase high‐performance liquid chromatography. Analytical Biochemistry, 171, 124–134. [DOI] [PubMed] [Google Scholar]
- Stephensons K, 1959. Wool follicle development in the New Zealand Romney and N‐type sheep. IV. Prenatal growth and changes in body proportions. Australian Journal of Agricultural Research, 10, 433–452. [Google Scholar]
- Stockx EM, Anderson CR, Murphy SM, Cooke IRC and Berger PJ, 2007. The development of descending projections from the brainstem to the spinal cord in the fetal sheep. BMC Neuroscience, 8, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MA, Watson TJ, Johnson CB and Millman ST, 2016. Evaluation of the efficacy of a non‐penetrating captive bolt to euthanase neonatal goats up to 48 hours of age. Animal Welfare, 25, 471–479. [Google Scholar]
- Svendsen O, Jensen SK, Karlsen LV, Svalastoga E and Jensen HE, 2008. Observations on newborn calves rendered unconscious with a captive bolt gun. Veterinary Record, 162, 90–92. [DOI] [PubMed] [Google Scholar]
- Szeto HH and Hinman DJ, 1985. Prenatal development of sleep‐wake patterns in sheep. Sleep, 8, 347–355. [DOI] [PubMed] [Google Scholar]
- Szeto HH, 1990. Spectral edge frequency as a simple quantitative measure of the maturation of electrocortical activity. Pediatric Research, 27, 289–292. [DOI] [PubMed] [Google Scholar]
- Tawia S, 1992. When is the capacity for sentience acquired during human fetal development? Journal of Maternal‐Fetal Medicine, 1, 153–165. [Google Scholar]
- Thingnes SL, Hallenstvedt E, Sandberg E and Framstad T, 2015. The effect of different dietary energy levels during rearing and mid‐gestation on gilt performance and culling rate. Livestock Science, 172, 33–42. [Google Scholar]
- Thomsen JL, 1975. Body length, head circumference, and weight of bovine fetuses: prediction of gestational age. Journal of Dairy Science, 58, 1370–1373. [DOI] [PubMed] [Google Scholar]
- Turner RM, McDonnell SM, Fit EM, Grogan EH and Foglia R, 2006. Real‐time ultrasound measure of the fetal eye (vitreous body) for prediction of parturition date in small ponies. Theriogenology, 66, 331–337. [DOI] [PubMed] [Google Scholar]
- Ullrey DE, Sprague JI, Becker DE and Miller ER, 1965. Growth of the swine fetus. Journal of Animal Science, 24, 711–717. [DOI] [PubMed] [Google Scholar]
- Vaala WE, 1999. Peripartum asphyxia syndrome in foals. Proceedings of the Annual Convention of the American Association of Equine Practioners, 45, 247–253. [Google Scholar]
- Vos EA, van Oord R, Taverne MAM and Kruip TAM, 1999. Pregnancy diagnosis in sows: direct ELISA for estrone in feces and its prospects for an on‐farm test, in comparison to ultrasonography. Theriogenology, 51, 629–840. [DOI] [PubMed] [Google Scholar]
- Ward A, Nevel M and Thomson JR, 2010. Infertility investigation in Scottish pig herds by examination of reproductive tracts. Proceedings, 21st International Pig Veterinary Society (IPVS) Congress, IPVS 2010, Vancouver, Canada, 788, 1094 pp.
- Wassink G, Bennet L, Booth LC, Jensen EC, Wibbens B, Dean JM and Gunn AJ, 2007. The ontogeny of hemodynamic responses to prolonged umbilical cord occlusion in fetal sheep. Journal of Applied Physiology, 103, 1311–1317. [DOI] [PubMed] [Google Scholar]
- Watson CS, Schaefer R, White SE, Homan JH, Fraher L, Harding R and Bocking AD, 2002. Effect of intermittent umbilical cord occlusion on fetal respiratory activity and brain adenosine in late‐gestation sheep. Reproduction Fertility and Development, 14, 35–42. [DOI] [PubMed] [Google Scholar]
- Watt BR, Anderson GA and Campell LP, 1984. A comparison of six methods used for detecting pregnancy in sheep. Australian Veterinary Journal, 61, 377–381. [DOI] [PubMed] [Google Scholar]
- Wenham G, Fowler VR and McDonald I, 1973. A radiographic study of skeletal growth and development in the pig. Temporal pattern of growth. Journal of Agricultural Science, 80, 125–133. [Google Scholar]
- Whishaw IQ, 1990. The decorticate rat. In: Kolb B and Tees RC (eds.). The Cerebral Cortex of the Rat. The MIT Press, Cambridge, MA. pp. 239–267. [Google Scholar]
- Winters LM and Feuffel G, 1936. Studies on the physiology of reproduction in sheep. IV. Foetal development. Tech. Bull. University of Minnesota No. 118.
- Woodbury DM and Karler R, 1960. The role of carbon dioxide in the nervous system. Journal of American Society of Anaesthesiologists, 21, 686–703. [DOI] [PubMed] [Google Scholar]
- Wu MC, Hentzel MD and Dziuk PJ, 1987. Relationships between uterine length and number of fetuses and prenatal mortality in pigs. Journal of Animal Science, 65, 762–770. [DOI] [PubMed] [Google Scholar]
- Yoneyama Y, Wakatsuki M, Sawa R, Kamoi S, Takahashi H, Shin S, Kawamura T, Power GG and Araki T, 1994. Plasma adenosine concentration in appropriate‐for‐gestational‐age and small‐for‐gestational‐age foetuses. American Journal of Obstetrics and Gynecology, 170, 684–688. [DOI] [PubMed] [Google Scholar]
- Zietzschmann O and Krolling O, 1955. Lehrbuch der Entwicklungsges‐chichte der Haustiere. Parey, Berlin. [Google Scholar]


