Abstract
The additive, ‘Iron dextran 10%’, is a liquid preparation containing iron dextran (25%, of which 10% is total iron), sodium chloride (1.5%), phenol (0.4%) and water (73.1%). Iron dextran 10% is considered safe for suckling piglets when given at an oral dose of 1 mL/kg body weight (bw) once in each of the first 2 weeks of life; this dose corresponds to 100 mg Fe/kg bw. The administration of iron dextran 10% to piglets deficient in vitamin E and/or selenium is considered a risk. The oral use of iron dextran 10% in suckling piglets does not pose any safety concerns to consumers, provided that the conditions identified as safe for the target animal are respected. Iron dextran 10% is considered a respiratory sensitiser and may be harmful if inhaled; however, exposure by inhalation is not expected; therefore, the risk is considered to be negligible. Iron dextran 10% is an irritant to skin and eyes; a risk by skin sensitisation cannot be excluded. The presence of phenol in the additive should be considered to pose a hazard for users. The use of the additive for suckling piglets at the proposed level does not pose a safety concern to the environment. The available studies with orally administered iron dextran indicate that the additive is a bioavailable source of iron for suckling piglets; however, the efficacy of the additive when given to newborn pigs as proposed via water for drinking (voluntary intake) has not been demonstrated. The Panel proposed some recommendations regarding the dextran used in the manufacture of the additive and the procedure for administering the additive to piglets.
Keywords: nutritional additive, compounds of trace elements, iron, iron dextran, safety, efficacy, suckling piglets
Summary
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of iron dextran as a feed additive for all animal species. During the course of the dossier revision and evaluation, the applicant indicated that the target animal of the application was limited to piglets and to a dose of 100 mg Fe from iron dextran per kg body weight (bw) and day.
Iron dextran 10% is considered safe for suckling piglets when given at a daily oral dose of 1 mL/kg bw once in each of the first 2 weeks of life; this dose corresponds to 100 mg Fe/kg bw. The presence of phenol in the additive up to the maximum concentration of 0.4% is considered unlikely to pose a concern for the target animals. The administration of iron dextran 10% to piglets deficient in vitamin E and/or selenium is considered a risk.
The oral use of iron dextran in suckling piglets does not pose any safety concerns to consumers, provided that the conditions identified as safe for the target animal are respected.
Iron dextran 10% is considered a respiratory sensitiser and may be harmful if inhaled. However, exposure by inhalation is not expected; therefore, the risk is considered to be negligible. Iron dextran 10% is an irritant to skin and eyes; a risk by skin sensitisation cannot be excluded. The presence of phenol in the additive should be considered to pose a hazard for skin and eyes of users.
The use of the additive for suckling piglets at the proposed level does not pose a safety concern to the environment.
The available studies with orally administered iron dextran indicate that the additive is a bioavailable source of iron for suckling piglets. However, the efficacy of the additive when given to newborn pigs as proposed via water for drinking (voluntary intake) has not been demonstrated.
The Panel proposed some recommendations on (i) the production conditions and characteristics of the dextran used in the manufacture of the additive and (ii) the procedure for administering the additive to piglets; the additive at the recommended dose could be orally administered directly to the target animals, without prior dilution in water for drinking.
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from PFO VETOS‐FARMA Ltd.2 for authorisation of the product iron dextran, when used as a feed additive for all animal species (category: nutritional additive; functional group: compounds of trace elements). During the course of the assessment, the applicant decided to restrict the application to piglets only.3
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 18 June 2015.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product iron dextran 10%, when used under the proposed conditions of use (see Section 3.1.6).
1.2. Additional information
Iron dextran has not been previously authorised as a feed additive in the European Union (EU). Several other iron compounds are authorised in the EU as nutritional additives (functional group: compounds of trace elements): ferrous carbonate; ferrous chloride, tetrahydrate; ferric chloride, hexahydrate; ferrous citrate, hexahydrate; ferrous fumarate; ferrous lactate, trihydrate; ferric oxide; ferrous sulphate, monohydrate; ferrous sulfate, heptahydrate; ferrous chelate of amino acids, hydrate.4 EFSA (FEEDAP Panel) has delivered several opinions on iron‐based additives: on an iron chelate with synthetic feed grade glycine (EFSA FEEDAP Panel, 2005), on iron chelate of amino acids, hydrate (EFSA FEEDAP Panel, 2013, 2016a), on ferrous sulfate, heptahydrate (EFSA FEEDAP Panel, 2014a, 2016a), on ferrous sulfate, monohydrate (EFSA FEEDAP Panel, 2014b, 2016a), on ferrous carbonate (EFSA FEEDAP Panel, 2015, 2016a), on ferric chloride, hexahydrate, ferrous fumarate and on ferrous chelate of glycine, hydrate (EFSA FEEDAP Panel, 2016a).
Iron dextran for its use in treatment of iron deficiency has been assessed by the Committee for veterinary medicinal products5 of the European Medicines Agency (EMA) and was recommended for inclusion in Annex II of Council Regulation (EEC) No 2377/90. The compound is currently listed as pharmacologically active substance in table 1 of the Annex of Regulation 37/20106 as Allowed substances, no maximum residue level (MRL) required for all food producing species.
More recently, the intravenous use of iron dextran in humans has been assessed by the Committee for Medicinal Products for Human Use (CHMP) of EMA (EMA, 2013).
The Scientific Committee on Food (SCF) of the European Commission delivered an Opinion on a dextran preparation, produced using Leuconostoc mesenteroides, Saccharomyces cerevisiae and Lactobacillus spp., as a novel food ingredient in bakery products (EC, 2000); L. mesenteroides BCCM LMG P‐16878 was considered. Following this Opinion, the dextran preparation produced by L. mesenteroides was authorised as a novel food ingredient in bakery products.7
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier8 in support of the authorisation request for the use of iron dextran as a feed additive. The technical dossier was prepared following the provisions of Article 7 of Regulation (EC) No 1831/2003, Regulation (EC) No 429/20089 and the applicable EFSA guidance documents.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers and other scientific reports to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the iron dextran as an additive for feedingstuffs and water for drinking. The Executive Summary of the EURL report can be found in the Annex A.10
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of iron dextran is in line with the principles laid down in Regulation (EC) No 429/20089 and the relevant guidance documents: Guidance on nutritional additives (EFSA FEEDAP Panel, 2012a), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011), Technical Guidance for assessing the safety of feed additives for the environment (EFSA FEEDAP Panel, 2008), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012b), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012c), Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance (EFSA FEEDAP Panel, 2012d) and Guidance for the preparation of dossiers for additives already authorised for use in food (EFSA FEEDAP Panel, 2012e).
3. Assessment
Iron (Fe) is the most abundant trace element in mammals. It serves as a constituent in proteins (e.g. haemoproteins: haemoglobin (Hb), myoglobin; non‐haemo proteins: ferritin, transferrin) and as a cofactor for many important iron‐dependent enzymes (e.g. cytochromes a, b, c and d; peroxidases, catalases). Aerobic metabolism depends on iron. As a constituent of Hb, it is involved in oxygen and carbon dioxide transport. It plays a central role as cofactor for most of the enzymes of the Krebs cycle and functions as an electron carrier (McDowell, 2003; Suttle, 2010). The intestinal absorption of iron and its retention is highly regulated via homoeostasis (for reviews see Wessling‐Resnick, 2000; Miret et al., 2003).
The first iron dextran complex was discovered in 1953 (Fletcher and London, 1954; cited by London, 2004). Originally, it was used for intravenous infusion as a therapeutic preparation of iron in humans (London and Twigg, 1954). Some months later the first studies supporting the overcoming of anaemia in piglets were published.
Iron dextran is globally used since the 1950s in the prevention of piglet anaemia by intramuscular injection (Barber et al., 1955; Brownlie, 1955; Maner et al., 1955; McDonald et al., 1955; Rydberg et al., 1959; Ullrey et al., 1959). Since intramuscular injection of iron dextran could also result in some adverse effects (on animal welfare by pain from injections, muscle necrosis and arthritis), alternative methods such as oral administration of iron to piglets are considered (Miller and Ullrey, 1997). In the first days of life, larger molecules are absorbed and thus oral iron dextran could meet the iron needs of suckling piglets.
The same iron dextran is the subject of the current application for oral administration. Miller and Ullrey (2007) stated that ‘When the newborn pig receives an oral dose of 100–200 mg of iron from iron dextran, the iron is effectively absorbed. Recent research indicates that this single dose is nearly as effective as an iron injection if dosage occurs within the first six hours of life’.
The oral administration of iron has also some disadvantages (different intake levels of feed or water for drinking), but there are considerable benefits. Intestinal iron absorption is regulated by homoeostatic mechanisms against overload; if necessary, the treatment can be easily repeated. It must be noted that the bioavailability of oral iron is affected by dietary factors such as amino acids, protein sources, other minerals and phytate.
3.1. Characterisation11
3.1.1. Characterisation of the compound
The compound ‘Iron dextran’ is identified with the Chemical Abstracts Service (CAS) No 9004‐66‐4. Its International Union of Pure and Applied Chemistry (IUPAC) name is ferric hydroxide dextran (α1,3‐α1,6 glucan) complex. Its molecular formula is (C6H10O5)n•[Fe(OH)3]m and it has a molecular weight of approximately 4000–6000 Da. The structural formula is given in Figure 1.
Figure 1.
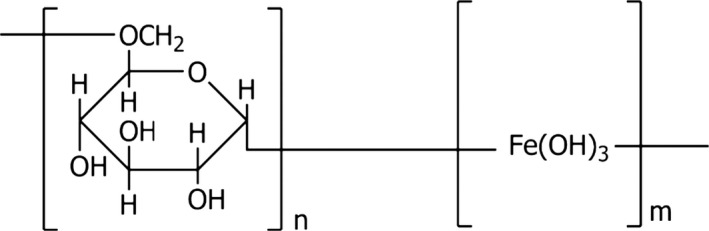
Structural formula of iron dextran
3.1.2. Characterisation of the additive
The additive is ‘Iron dextran 10%’ and it is referred to thereof in this opinion as such or as ‘the additive’. Its typical composition is given as 25% iron dextran (10% total iron, 15% dextran), 1.5% sodium chloride, 0.4% phenol and 73.1% water. The iron content is specified with a range of 9.5–10.5%, sodium chloride 0.5–1.8% (calculated from chloride) and phenol 0.3–0.5% (w/v).
Analytical data from seven batches showed mean values of 10.1% iron (range: 9.9–10.3%), 0.6% chloride (range: 0.4–0.6%) and 0.32% phenol (range: 0.3–0.4%)12; all values complied with the specifications. The mean pH value of the solution (10 g/L) was 5.8 (range 5.4–6.3).
Lead, cadmium, mercury, arsenic and nickel were determined in three batches of the additive.13 , 14 , 15 The values reported comply with the thresholds set in Directive 2002/32/EC16 for compounds of trace elements or, if not mentioned, do not represent a concern (which the exception of the nickel content, which will be considered in Section 3.2.4). No data on microbiological contamination of the additive were submitted.
3.1.2.1. Physical state of the product
The additive is a dark brown slightly viscous colloidal solution of iron dextran in water. The density is 1.12–1.20 g/mL (20°C), and the related viscosity is 10–25 mPa·s. The boiling point is 330°C. Iron dextran is highly soluble in water; however, exact data were not provided.
3.1.3. Manufacturing process
The manufacturing process of the product is fully described in the technical dossier.
3.1.4. Stability and homogeneity
Stability studies are generally not required for compounds of trace elements. The stability of the additive in an aqueous premixture (about 6.6% iron) was tested for 48 h at 25°C; however, only iron was analysed.17 The potential of the additive to promote bacterial growth was not considered.
Since the additive is highly soluble in water and its use is intended in water for drinking only (see Section 3.1.6. Conditions of use), demonstration of homogeneity is not required.
3.1.5. Physicochemical incompatibilities in feed
The applicant recommended that contact of the additive with oxidising agents should be avoided.
3.1.6. Conditions of use
Iron dextran 10% is proposed to be used as a nutritional feed additive (compounds of trace elements) for piglets. The additive is intended to be administered via water for drinking at a dose of 1 mL/kg body weight (100 mg Fe/kg bw) on the second and ninth days of age. Where the piglets do not receive iron‐containing feed in the third week of life, the additive should be administered again on day 16 of age with the same dose.18
The additive should not be given to piglets with diarrhoea, or to vitamin E‐deficient animals; it should not be administered in combination with tetracyclines.
3.2. Safety
Iron dextran is historically known in the prevention of piglets’ anaemia where it is administered by intramuscular injection. The applicant submitted a number of publications describing some pharmacokinetic and pharmacodynamic aspects of iron after parenteral administration; since the application is for oral use of iron dextran, these studies were not considered.
3.2.1. Absorption, distribution, metabolism and Excretion of iron dextran
Some published studies conducted with iron dextran in piglets via gavage have been identified in the scientific literature. A single oral dose of 200 mg Fe from iron dextran to newborn piglets is comparably effective considering Hb values and weight gain as the parenteral administration of the same amount of iron (Blomgren and Lannek, 1970, 1971, 19; Romvary, 1971, 20; Thorén‐Tolling, 1975, 21). Thorén‐Tolling (1975) studied also the absorption of iron after oral administration; the author used 72 newborn piglets from nine litters to determine the retention and distribution of labelled iron given either orally as ferrous fumarate (100 mg Fe2+) or iron dextran (200 mg Fe3+) or by injection as iron dextran (100 mg Fe3+): about 25–30% of the labelled iron from a single oral dose of labelled ferrous fumarate, and about 55–60% from a single oral dose of labelled iron dextran were absorbed. As iron is excreted throughout the experiment, about 20% and 40–50% of the labelled iron from iron fumarate and iron dextran, respectively, were recovered 3 weeks after treatment. In liver, about 15% of the oral dose was present after 5 days, and a rapid decrease occurred in the following 3 weeks. In spleen, the labelled iron level was constant, about 0.3% of the dose.
In previous studies (Cornelius and Harmon, 1973a,b; only abstracts available) with labelled iron dextran22 given orally by gavage to piglets showed a similar magnitude of absorption. An appreciable amount of the iron dose was found in the enterocytes, from where iron was transported to systemic use or eliminated in faeces through natural desquamation of intestinal cells. After systemic absorption, iron was mainly deposited in liver. After 10 days, the liver contents returned to basal levels and no accumulation was found in the muscle and bladder.
There is no indication that iron from iron dextran will behave differently to iron from other authorised iron‐containing additives except the magnitude of absorption. The principles of distribution, metabolism, excretion and tissue deposition of iron administered orally to animals, recently reviewed by the FEEDAP Panel (EFSA FEEDAP Panel, 2016a) can be considered valid also for iron dextran.
3.2.2. Safety for the target species23
The applicant submitted a tolerance study with iron dextran 10% in which iron absorption measured by the increase in serum levels of iron was used as endpoint. The study was conducted with newborn piglets.24 Using this study, it was concluded that the daily dose of 1 mL iron dextran 10% per kg bw is safe for piglets when given orally once in each of the first 2 weeks of life.
Regarding the phenol content of the additive, the FEEDAP Panel notes that phenol is widely used as an excipient in medicines for parenteral use (Pifferi and Restani, 2003; Mehmood and Farooq, 2015). The additive ‘iron dextran 10%’ is identical to the product used as veterinary medicine for parenteral use in piglets, including the phenol concentration. In the context of the oral use of this product in the target species, the FEEDAP Panel notes that a TDI of 0.5 mg phenol/kg bw per day25 has been proposed (EFSA CEF Panel, 2013); taking into account the administration of the additive to piglets (two single events only in the first 2 weeks of life), the FEEDAP Panel considers that the TDI is of limited relevance for the target animals. The Panel further notes that phenol is listed as allowed pharmacologically active substance in all animal species with no MRL required in foodstuffs of animal origin.6 Therefore, the presence of phenol in the additive up to the maximum concentration of 0.4% is considered unlikely to pose a concern for the target animals.
3.2.2.1. Interactions in vivo
Interactions may occur between iron and other divalent cations, such as calcium, copper, manganese and zinc. More details can be found in the FEEDAP opinions on iron (EFSA FEEDAP Panel, 2016a), zinc (EFSA FEEDAP Panel, 2014c) and copper (EFSA FEEDAP Panel, 2016b). However, these interactions are not expected to be of concern considering the conditions of use where only milk from the sow is consumed, except calcium which is known to decrease iron absorption.
It is well known that iron given as iron dextran via intramuscular or subcutaneous injections to piglets from vitamin E‐ and/or selenium‐deficient sows can become toxic, since piglets are also born deficient and the enzymes involved in iron metabolism cannot function (McDowell, 2003; Suttle, 2010).26 The same should be assumed for iron administered orally.
3.2.2.2. Conclusions on the safety for target species
Iron dextran 10% is considered safe for suckling piglets when given at an oral dose of 1 mL/kg body weight once in each of the first 2 weeks of life; this dose corresponds to 100 mg Fe/kg bw. The administration of iron dextran 10% to piglets deficient in vitamin E and/or selenium is considered a risk.
3.2.3. Safety for the consumer
3.2.3.1. Toxicological studies
The applicant did not submit specific toxicity studies on iron dextran under assessment.
Genotoxicity of several iron compounds, including iron dextran, was evaluated in Salmonella Typhimurium (TA97a, TA98, TA 100, TA102 and TA1535) at concentrations up to 10 mg of compound, with and without metabolic activation, both by the incorporation and pre‐incubation methods (Dunkel et al., 1999).27 Iron dextran did not show mutagenic effects. In L5178Y mouse lymphoma cells, iron dextran did not show mutagenic activity at concentrations up to 175 μg/mL without metabolic activation; however, with metabolic activation there was a concentration‐dependent increase in the number of mutants at the two highest tested concentrations (8.75 and 17.5 μg/mL) (Dunkel et al., 1999). This is a common feature of some transition metals, as it is iron, and can be expected to occur in vivo only when high concentration of free ions are present. In normal conditions iron is bound to proteins such as transferrin, ferritin and haemosiderin.
The National Toxicology Program (NTP) has assessed iron dextran complex in its use as injectable human medicine (usual daily dose is 1–5 mL (50–250 mg of iron)). The data available from epidemiological studies were inadequate to evaluate the relationship between human cancer and exposure specifically to iron dextran complex. However, the NTP concluded that iron dextran is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals which developed sarcoma at the injection site after repeated administrations (NTP, 2014).28 This finding is consistent with the effect of high local concentration of iron ions at the site of injection, which is not achieved with the oral administration of iron dextran.
3.2.3.2. Consumer safety assessment
Previous assessments of the FEEDAP Panel on the safety for consumers of iron compounds stated that the evaluated compounds29 would not change the iron concentration in edible tissues and products of animal origin and consequently not the consumer exposure (EFSA FEEDAP Panel 2013, EFSA FEEDAP Panel, 2014a,b, EFSA FEEDAP Panel 2015, 2016a); in contrast, iron dextran 10% would – at least transiently – increase the iron content of piglet body. However, the FEEDAP Panel is of the opinion that the use of the additive in suckling piglets would not result in a measurable increase in the iron intake of consumers since (i) any potential deposition in edible tissues and organs of suckling piglets would disappear within 30 days after administration, (ii) suckling piglets are a very minor food commodity in the EU and, most important, (iii) oral administration of iron dextran will not be used in addition but as a substitute to the current practice of iron injection.
Based on published literature, the SCF concluded that dextran is essentially (90%) hydrolysed in the gut to monosaccharides and that the residual dextran that escapes the digestion is fermented into carboxylic acids (EC, 2000). The FEEDAP Panel adds that, consequently, no exposure of the consumer to dextran would result from consuming food derived from piglets treated with iron dextran 10%.
3.2.3.3. Conclusions on the safety for consumers
The oral use of iron dextran 10% in suckling piglets under the proposed conditions of use does not pose any safety concerns to consumers.
3.2.4. Safety for the users
No specific studies were provided by the applicant regarding the toxicity of the orally administered iron dextran 10% for the users. The applicant submitted some information based on available literature of iron dextran used via parenteral administration.30
3.2.4.1. Effects on the respiratory system
Anaphylactic reactions to iron dextran, administered parenterally, can cause severe bronchoconstriction (Tattersfield and McNicol, 1987; EMA, 2013). However, there is no evidence for such effect by other exposure routes.
Based on data from the MSDS provided by the applicant, iron dextran 10% may sensitise the respiratory system and may be harmful if inhaled.
In addition, the presence of phenol in the additive was considered. It is commonly known that phenol vapours are irritating to the respiratory tract (IPCS, 1999; ATSDR, 2008; PHE, 2016). However, taking into consideration the physical state (Section 3.1.2) of the additive and its conditions of use (Section 3.1.6), no exposure by inhalation should be expected.
3.2.4.2. Effects on skin and eyes
No data on skin and eyes sensitisation/irritation of iron dextran were provided.
Iron compounds are considered irritants to skin and eyes as reported in a previous EFSA Opinion (EFSA FEEDAP Panel, 2016a). Taking into account, the sensitisation potential of parenteral iron dextran (see also EMA, 2013), a risk of skin sensitisation cannot be excluded, also considering the presence of nickel (19.6 mg/kg additive) as an impurity in the additive.
In addition, the presence of phenol in the additive was considered. Repeated skin exposure to phenol may result in yellowing of the skin, skin irritation and skin eruption, as well as dermal inflammation and necrosis in humans (IPCS, 1999; ATSDR, 2008). Ocular exposure to phenol can also cause irritation and corneal opacification (PHE, 2016). The FEEDAP Panel notes that phenol is included in the list of prohibited substances in cosmetics in the EU.31 Therefore, the phenol content of the additive should be considered to pose a hazard for skin and eyes of users.
3.2.4.3. Conclusions on the safety for users
Iron dextran is considered a respiratory sensitiser and may be harmful if inhaled. However, exposure by inhalation is not expected; therefore, the risk is considered to be negligible.
Iron dextran is an irritant to skin and eyes; a risk by skin sensitisation cannot be excluded. The presence of phenol in the additive should be considered to pose a hazard for skin and eyes of users.
3.2.5. Safety for the environment
The applicant did not provide any product‐specific information relevant to support safety for the environment.
The safety for the environment of iron compounds used as feed additives has been previously assessed by the FEEDAP Panel concluding that the supplementation of feed with the evaluated compounds29 was not expected to pose an environmental risk (EFSA FEEDAP Panel, 2013; EFSA FEEDAP Panel, 2014a,b; EFSA FEEDAP Panel, 2015, 2016a).
Considering the maximum amount of iron which could be supplied to a suckling piglet respecting the legal provisions and the proposed levels considered safe for the target animals and the amount of iron which will be fed during the fattening period of one pig, the additional quantity for one piglet (500 mg) would amount to 2.5% of that consumed and calculated as excreted by a fattening pig.32
The additive also contains 15% dextran. It has been demonstrated that both rats and humans are able to digest the orally administered dextran; it is assumed that the polysaccharide is hydrolysed by an intestinal enzyme as well as by bacterial action (Fischer and Stein, 1960); no dextran has been detected in faeces after feeding experiments with dextran containing diets (Dahlqvist, 1961; Dahlqvist, 1963; Jeanes, 1975).
3.2.5.1. Conclusions on the safety for the environment
The oral use of iron dextran 10% as a additive for suckling piglets at the proposed level does not pose a safety concern to the environment.
3.3. Efficacy
The applicant submitted a bioavailability study with suckling piglets comparing the increase in serum iron after oral or intravenous administration of the additive.33 Owing to several weaknesses identified, including the age of the animals, the lack of homogeneity between and within the groups (age, iron status, ratio age to body weight and number of animals) and the different doses applied for oral and intravenous application, the study could not be considered.
The applicant also provided a list of 24 publications describing various reasons to use the iron in the animal's diet, mainly to prevent baby and weaner pigs from the deficiency (anaemia).34 No paper dealt with the oral application of iron dextran in comparison with other application forms. Most studies were done with intramuscular injection of iron dextran to avoid anaemia in piglets.
3.3.1. Experimental studies with orally administered iron dextran
Further to the literature review by the applicant, the FEEDAP Panel identified additional scientific papers for the effects of iron dextran in piglets (e.g. Kirchgessner and Weigand, 1973; Harmon et al., 1974; Glawischnig et al., 1987; Lemacher and Bostedt, 1995; Pechin et al., 1998; Egeli and Framstad, 1999; Gutzwiller, 1999; Chwen et al., 2001; Acda et al., 2002; Svoboda and Drábek, 2007; Jolliff and Mahan, 2011; Maes et al., 2011; Ranjan et al., 2012; Ishaya and Ishaya, 2012). Among those, four publications compared the effects of iron dextran applied via oral or parenteral route in newborn piglets, and are summarised in the paragraphs below. All these studies have in common that the method followed for the oral application is not described in detail; in particular, none of the studies reported that iron dextran was used in water for drinking. The Panel is of the view that in none of the studies the additive was administered in compliance with the conditions of use proposed by the applicant, namely via supplementation in water for drinking.
Harmon et al. (1974) conducted three studies with piglets to evaluate two methods of iron dextran administration.35 Iron dextran was given orally or injected intramuscularly (100 mg Fe/animal) within the first 12 h of life and compared with untreated control animals. A second factor of the studies was the floor type (steel or aluminium in the study 1; uncoated and coated in studies 2 and 3), which did not influence the study results and should not be further considered. Blood samples were collected from the orbital sinus prior treatment and at 14, 21 and 28 days in the experiments 1 (50 piglets per treatment) and 2 (30 piglets per treatment) for Hb and haematocrit, and initially and at 21 days for Hb only in experiment 3 (36 piglets per treatment). Weight gain and welfare of piglets were not significantly influenced by iron treatment. The authors concluded that iron dextran, which has been widely adapted as an injectable iron source, is just as effective when given orally in the first 12 h of live in maintaining Hb and haematocrit values compared with injection through a 28‐day lactation.
Glawischnig et al. (1987) allocated a total of 147 piglets from 16 litters to two groups with approximately equal body weight and gender distribution. One group was treated with 200 mg Fe from iron dextran36/piglet orally at 10 h post‐partum, the other group was treated by intramuscular injection with the same Fe dose from iron dextran36 on the third day of life. The Hb values on day 3, 10, 17 and 24 were in the orally treated group 91, 102, 109 and 117 g/L; those of the group with the iron injection 80, 95, 103 and 115 g/L, respectively. The authors concluded also considering body weight and haematocrit that both routes of iron administration were effective in the prevention of anaemia of piglets caused by iron deficiency.
Lemacher and Bostedt (1995) compared in three studies on groups of 15–24 newborn piglets each the oral administration of iron dextran (225 mg Fe/piglet)37 with the subcutaneous injection of iron dextran (200 mg Fe/piglet),38 both at 4–8 h post‐natum. Blood samples were taken for Hb determination (and of plasma iron in one study) immediately, 3, 8, 14 and 24 days after birth. Both kinds of iron application lead to an increase in plasma iron. Parenteral as well as oral iron administration resulted from the third day of age on to comparable increases of the Hb values. However, it seemed that the effect after parenteral application had a longer persistence than the oral, beginning with the 14th day of age.
In Gutzwiller (1999), groups of 11 piglets each were administered 290 mg Fe as iron dextran orally39 either within 12 h after birth or on the second day of age as well as 100 and 200 mg Fe from iron dextran as subcutaneous injection40 on the second day of life. At 2 weeks of age, the mean Hb values in blood of the orally treated piglets were 127 and 119 g/L, respectively; after subcutaneous injection 138 and 128 g/L blood, respectively. These four values were not significantly different (p > 0.05). The author concluded that 100 (as well as 200) mg Fe from parenterally administered iron dextran would ensure a sufficient iron supply of piglets for the first 2 weeks of life and that the oral administration of 290 mg Fe from iron dextran is bioequivalent. In addition, it was concluded that iron dextran need not to be applied within the first hours of life but positive effects could be obtained when iron dextran is given on the second day of life.
3.3.2. Further considerations
The application is for iron dextran, a liquid additive, to be administered via water for drinking at a dose expressed per kg body weight and applied in not more than three single events (2nd, 9th and, if appropriate, 16th day of life). Concerning the specific administration procedure, the applicant stated that ‘the piglets will drink the water from one source per pen’. The studies submitted and performed by the applicant to demonstrate safety of the additive for target animals do not specify in detail how the additive was administered to the piglets; the only information available is ‘given per os’.
Despite the fact that the applicant indicated in the technical dossier that the animals will ingest the additive when diluted in water for drinking, no further information, including a dilution ratio of the additive in water for drinking, was provided. Moreover, no recommendation of the applicant was available on the concentration of the additive in water for drinking which would ensure the recommended intake of 100 mg Fe/kg bw.
Water for drinking is the only oral administration route to suckling piglets since they do not consume solid feed in the first 2 weeks of life, if enough milk is provided by the sow.
The only data reported by the applicant were combined data on the daily water and milk intake of suckling piglets of 80–120 mL/kg bw up to the 16th day of life and about 60–80 mL water above this period (for which apparently no use of the additive is foreseen). The FEEDAP Panel identified a publication of Nagai et al. (1994) in which the water intake of 199 suckling piglets (day 1–28 of life) was measured. The authors reported that ‘The suckling pigs began to drink water 3 to 5 h after birth. Water consumption per pig increased from 36 mL/day at the age of 1 day to 403 mL/day at the age of 28 days. Water consumption per kg body weight, on the other hand, remained constant at 51 to 62 mL, regardless of age. This result indicates that it may be possible to add drugs to drinking water for the purpose of medication in suckling pigs’. When assuming a constant intake of ~ 50 mL water for drinking/kg bw, 1 mL of the additive should be added to this quantity of water to provide an intake of 100 mg Fe per kg bw and day (about 20 mL iron dextran 10%/L water for drinking).
The FEEDAP Panel notes that (i) no information on the palatability of this iron solution to newborn piglets is available and (ii) an insufficient supply of individual piglets with iron cannot be excluded. The Panel further adds that the above‐mentioned average for the water intake of suckling piglets could considerably vary since it would be influenced by several other factors, e.g. environmental temperature (Nagai et al., 1994) and drinker design (Widowski et al., 2008).
3.3.3. Conclusions on the efficacy for target species
The available studies with orally administered iron dextran indicate that the additive is a bioavailable source of iron for suckling piglets. However, the efficacy of the additive when given to newborn pigs as proposed via water for drinking (voluntary intake) has not been demonstrated.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation41 and Good Manufacturing Practice.
4. Conclusions
Iron dextran 10% is considered safe for suckling piglets when given at an oral dose of 1 mL/kg bw once in each of the first 2 weeks of life; this dose corresponds to 100 mg Fe/kg bw. The administration of iron dextran 10% to piglets deficient in vitamin E and/or selenium is considered a risk.
The oral use of iron dextran 10% in suckling piglets under the proposed conditions of use does not pose any safety concerns to consumers.
Iron dextran 10% is considered a respiratory sensitiser and may be harmful if inhaled. However, exposure by inhalation is not expected, therefore the risk is considered to be negligible. Iron dextran 10% is an irritant to skin and eyes; a risk by skin sensitisation cannot be excluded. The presence of phenol in the additive should be considered to pose a hazard for skin and eyes of users.
The oral use of the additive for suckling piglets at the proposed level does not pose a safety concern to the environment.
The available studies with orally administered iron dextran indicate that the additive is a bioavailable source of iron for suckling piglets. The efficacy of the additive when given to newborn pigs as proposed via water for drinking (voluntary intake) has not been demonstrated.
5. Recommendations
The dextran used in the manufacturing of the additive should meet the production conditions and characteristics described in the European Pharmacopeia for the dextrans for injection.
A direct oral administration of the additive at the recommended dose should be considered. Such a procedure would reduce the uncertainties resulting from different quantities of water intake and, therefore, ensure the intake of the dose intended for prevention of anaemia in piglets.
Documentation provided to EFSA
Iron Dextran. October 2014. Submitted by PFO VETOS‐FARMA Ltd.
Iron Dextran. Supplementary information. July 2016. Submitted by PFO VETOS‐FARMA Ltd.
Evaluation report of the European Union Reference Laboratory for Feed Additives on the Methods(s) of Analysis for iron dextran.
Abbreviations
- AAS
atomic absorption spectrometry
- bw
body weight
- CAS
Chemical Abstracts Service
- CEF
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CHMP
Committee for Medicinal Products for Human Use
- EMA
European Medicines Agency
- EURL
European Union Reference Laboratory
- FEEDAP
Panel on Additives and Products or Substances used in Animal Feed
- Hb
haemoglobin
- IARC
International Agency for Research on Cancer
- ICP‐AES
inductively coupled plasma atomic emission spectroscopy
- IPCS
International Programme on Chemical Safety
- IUPAC
International Union of Pure and Applied Chemistry
- MRL
maximum residue level
- MSDS
Material Safety Data Sheet
- NTP
National Toxicology Program
- SCF
Scientific Committee on Food
Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for iron dextran
In the current application authorisation is sought under article 4(1) for Iron Dextran under the category/functional group 3(b) ‘nutritional additives’/‘compounds of trace elements’ according to the classification system of Regulation (EC) No 1831/2003. Specifically, authorisation is sought for the use of the feed additive for piglets. The feed additive is a dark brown slightly viscous aqueous liquid consisting of 9.5 to 10.5% (w/v) iron; a maximum of 0.9% chloride; and a maximum of 0.5% (w/v) phenol. In addition, the typical content of dextran in the product ranges from 17 to 23% (w/v). According to the Applicant, the feed additive is intended to be added in feed or water for drinking without proposing corresponding minimum or maximum concentration levels of the feed additive and/or iron in feedingstuffs and water.
For the characterisation of the feed additive, the Applicant referred to the Chinese Pharmacopeia monograph where: ‐ specific reactions of iron salts; ‐ the tests for dextran; and ‐ the assays for iron, dextran, chloride and phenol are used. Similar information is provided in the British and US Pharmacopeia monographs for Iron Dextran identified by the EURL. Even though no performance characteristics are provided, the EURL recommends for official control the methods described in the dedicated British and US Pharmacopeia monographs for the characterisation of the feed additive.
For the quantification of total iron in feedingstuffs the Applicant submitted the Community method based on atomic absorption spectrometry (AAS). Furthermore, three additional ringtrial validated CEN methods were previously evaluated and recommended by the EURL in the frame of the Iron group dossiers: EN 15621 and EN 15510 methods based on inductively coupled plasma atomic emission spectroscopy (ICP‐AES) with or without pressure digestion, and EN 6869 method based on atomic absorption spectroscopy (AAS).
For the quantification of total iron in water the Applicant suggested the ISO 6332 method based on spectrophotometry using 1,10‐phenanthroline. In addition, the EURL recommended in the frame of Iron Group dossiers the ring trial validated EN ISO 11885 method based on inductively coupled plasma atomic emission spectrometry (ICP‐AES).
Based on the satisfactory performance characteristics available, the EURL considers the Community, CEN and ISO methods mentioned above fit‐for‐purpose for the quantification of total iron in feedingstuffs and water at the concentration ranges defined by the corresponding scope of the methods. As the conditions of use do not clearly indicate the range of iron content to be monitored in feedingstuffs and water the EURL cannot recommend any methods for the official control for the quantification of total iron in feedingstuffs and water.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López Puente S, López‐Alonso M, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Leng L, López‐Gálvez G and Mantovani A, 2017. Scientific opinion on the safety and efficacy of iron dextran as a feed additive for piglets. EFSA Journal 2017;15(2):4701, 18 pp. doi: 10.2903/j.efsa.2017.4701
Requestor: The European Commission
Question number: EFSA‐Q‐2014‐00592
Panel members: Gabriele Aquilina, Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Georges Bories, Andrew Chesson, Pier Sandro Cocconcelli, Gerhard Flachowsky, Jürgen Gropp, Boris Kolar, Maryline Kouba, Secundino Lopez Puente, Marta Lopez‐Alonso, Alberto Mantovani, Baltasar Mayo, Fernando Ramos, Guido Rychen, Maria Saarela, Roberto Edoardo Villa, Robert John Wallace and Pieter Wester.
Acknowledgements: The FEEDAP Panel wishes to thank the following for the support provided to this scientific opinion (in alphabetical order of the last name): Jaume Galobart, Lucilla Gregoretti, Matteo L. Innocenti, Orsolya Holczknecht, Paola Manini and Manuela Tiramani.
Adopted: 24 January 2017
Amendment: This scientific opinion has been amended following the adoption of the decision of the Commission on confidentiality claims submitted by the applicant, in accordance with Article 8(6) and Article 18 of Regulation (EC) No 1831/2003. The modified sections are indicated in the text.
Updated: 7 April 2017
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
PFO VETOS‐FARMA Ltd, 21 Dzierżoniowska Str., 58‐260, Bielawa, Poland.
An updated Annex I ‘Application Form referred to in Article 2(1) and administrative data (Annex 1 of Regulation 429/2008)’ was submitted to EFSA.
Commission Regulation (EC) No 1334/2003 of 25 July 2003 amending the conditions for authorisation of a number of additives in feedingstuffs belonging to the group of trace elements. OJ L 187, 26.7.2003, p. 11. Commission Regulation (EC) No 479/2006 of 23 March 2006 as regards the authorisation of certain additives belonging to the group compounds of trace elements. OJ L 86, 24.3.2006, p. 4.
EMA confirmed that iron dextran was reviewed by the Committee for Medicinal Products for Veterinary Use in 1995 as part of a group of so‐called ‘old‐substances’ that were already in use. At the time, a summary report was not produced for every substance, and there is no one available in this case.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1.
Commission Decision of 30 January 2001 on authorising the placing on the market of a dextran preparation produced by Leuconostoc mesenteroides as a novel food ingredient in bakery products under Regulation (EC) No 258/97 of the European Parliament and of the Council (2001/122/EC). OJ L 44, 15.2.2001, p. 46.
FEED dossier reference: FAD‐2013‐0020.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/default/files/finrep%20fad-2013-0020%20iron%20dextran.pdf
This section has been edited following the provisions of Article 8(6) and Article 18 of Regulation (EC) No 1831/2003
Technical Dossier/Section II/Annex Certificates of analysis from 2013–2014.PDF/Annex Certificates of analysis from 2015. The test item in the relevant certificates of analysis was FERRODEX® (Iron Dextran 10% bulk solution for filling by sterile filtration of injectable solution for veterinary use).
Technical Dossier/Supplementary Information/Annexes Heavy metals – test from Nobilus ent.pdf Batches manufactured in 2012 ‐ CONFIDENTIAL.
Technical Dossier/Supplementary Information/Annex Heavy metals – test from SGS Lab.pdf Batches manufactured in 2016 ‐ CONFIDENTIAL.
Technical Dossier/Supplementary Information/Annex Heavy metals – test from SGS Lab.pdf ‐ CONFIDENTIAL.
Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. OJ L 140, 30.05.2002, p. 10.
Technical Dossier/Supplementary Information/Stability Study: wyniki_stab._w_wodzie_PSSE. Additional information submitted on 23.11.2016 ‐ CONFIDENTIAL.
Technical Dossier/Supplementary Information/Annex A Description and conditions of use of the additive as proposed by the applicant.
The iron dextran used contained 100 mg Fe/mL (Imposil®, Agrivet, Upsala) and was administered by gavage.
The iron dextran used orally was produced as a by‐product of Chinofer, inj. mixed with sugar and starch and contained about 60 g Fe/kg. The product was very tasteful and was offered in small feed troughs to piglets during the first 3 weeks of life. In addition, during the first week of life of piglets, the udder of sows was dredged with the iron dextran mixture in order to guarantee the intake by piglets.
The iron dextran used contained 100 mg Fe/mL (Imposil®, Agrivet, Upsala) and was administered with bottles equipped with a 1‐ml stroke dosimeter.
Iron dextran containing 100 mg Fe/mL; specific radioactivity 125 µCi/mL.
This section has been edited following the provisions of Article 8(6) and Article 18 of Regulation (EC) No 1831/2003.
Technical Dossier/Supplementary Information/Tolerance study ‐ CONFIDENTIAL.
Based on maternal toxicity in a developmental study in which pregnant rats were exposed to phenol by gavage from gestational day 6–16.
Supplementing the sow's diet with 50 IU of vitamin E/kg and 0.15 mg of selenium/kg will improve the status of the sow and prevent iron toxicity in the piglets. Injections of vitamin E/selenium during late gestation may also help preventing iron toxicity in piglets. (Cromwell, GL. Overview of Iron Toxicity in Newborn Pigs. In Merck Veterinary Manual, 11th print Edition, 2016).
Iron dextran source: Imferon® from Fissons Corporation.
Iron dextran source: Infed® from Watsons Pharma, Inc. containing 50 mg Fe/mL.
Iron chelate of amino acids, hydrate; ferrous sulfate, heptahydrate; ferrous sulfate, monohydrate; ferrous carbonate; ferric chloride, hexahydrate; ferrous fumarate; ferrous chelate of glycine, hydrate.
Technical Dossier/Supplementary Information/Toxicological risk assessment for user_worker safety ‐ CONFIDENTIAL.
Commission Directive 2005/80/EC of 21 November 2005 amending Council Directive 76/768/EEC, concerning cosmetic products, for the purposes of adapting Annexes II and III thereto to technical progress Annex II. List of substances prohibited in cosmetic products (substance no. 1175). Annex II, 2005/80/EC. OJ L 303, 22.11.2005, p. 32.
Growing period 20–100 kg bw; feed consumption 200 kg; iron concentration 100 mg/kg feed; total intake 20 g Fe/pig.
Technical Dossier/Supplementary Information/Bioavailability study ‐ CONFIDENTIAL.
Technical Dossier/Section IV/Annexes Section IV.
Source of iron dextran not described.
Iron dextran used contained 100 mg Fe/mL and 0.5% phenol (Abi‐dex 10%, Fa Schoeller‐Chemie).
Iron dextran used contained 75 mg Fe/mL (source not given).
Myofer® 100, Fa. Hoechst.
Iron dextran used contained 100 mg Fe/mL (Ferrovet®, Chassot AG).
Iron dextran used contained 200 mg Fe/mL (Anämex®, Novartis).
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- Acda SP, Joo JW, Kim WT, Shim YH, Lee SH and Chae BJ, 2002. Influence of a single dose of Fe‐Dextran administration with organic trace mineral supplementation on the performance of piglets. Asian‐Australasian Journal of Animal Science, 15, 1469–1474. [Google Scholar]
- ATSDR (Agency for Toxic Substances & Disease Registry), 2008. Toxicological Profile for Phenol. Availabe online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=148&tid=27
- Barber RG, Braude R and Mitchell KG, 1955. Studies on anaemia in pigs. Veterinary Record,67, 348–349. [Google Scholar]
- Blomgren L and Lannek N, 1970. A new method of treating piglets with iron dextran. Svensk Veterinärtidning, 12, 442–444. [Google Scholar]
- Blomgren L and Lannek N, 1971. Prevention of anaemia in piglets by a single oral dose of iron dextran. Nord Veterinaermed, 23, 529–536. [Google Scholar]
- Brownlie WM, 1955. The treatment of piglet anemia. Veterinary Record, 67, 350–354. [Google Scholar]
- Chwen LT, Heng LK, Lee TH, Kong MC and Yoon CP, 2001. The effects of iron supplementation in preweaning piglets. Malaysian Journal of Nutrition, 7, 41–49. [PubMed] [Google Scholar]
- Cornelius SG and Harmon BG, 1973a. Absorption of oral iron dextran in neonatal pigs. Journal of Animal Science, 37, 277 (Abstract 191). [Google Scholar]
- Cornelius SG and Harmon BG, 1973b. Tissue distribution of oral iron dextran in neonatal pigs. Journal of Animal Science, 37, 277 (Abstract 192). [Google Scholar]
- Dahlqvist A, 1961. The location of carbohydrases in the digestive tract of the pig. Biochemical Journal, 78, 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A, 1963. Rat‐intestinal dextranase. Localisation and relation to the other carbohydrates of the digestive tract. Biochemical Journal, 86, 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel VC, San RHC, Seifried HE and Whittaker P, 1999. Genotoxicity of Iron Compounds in Salmonella typhimurium and L5178Y Mouse Lymphoma Cells. Environmental and Molecular Mutagenesis, 33, 28–41. [DOI] [PubMed] [Google Scholar]
- EC (European Commission), 2000. Opinion of the Scientific Committee on Food on a dextran preparation, produced using Leuconostoc mesenteroides, Saccharomyces cerevisiae and Lactobacillus spp., as a novel food ingredient in bakery products. Report No. CS/NF/DOS/7/ADD3 FINAL, European Commission, Brussels.
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2013. Scientific Opinion on the toxicological evaluation of phenol. EFSA Journal 2013;11(4):3189, 44 pp. doi: 10.2903/j.efsa.2013.3189 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2005. Opinion of the Scientific Panel on Additives and Products or Substances used in Animal Feed on a request from the Commission on the safety of the ‘Chelated forms of iron, copper, manganese and zinc with synthetic feed grade glycine’. The EFSA Journal 2005, 289(12):1–6. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2008. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008,6(10):842, 28 pp. doi: 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011. Technical guidance: tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. doi: 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for nutritional additives. EFSA Journal 2012;10(1):2535, 14 pp. doi: 10.2903/j.efsa.2012.2535 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. doi: 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. doi: 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012d. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal 2012;10(6):2740, 10 pp. doi: 10.2903/j.efsa.2012.2740 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012e. Guidance for the preparation of dossiers for additives already authorised for use in food. EFSA Journal 2012;10(1):2538, 4 pp. doi: 10.2903/j.efsa.2012.2538 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2013. Scientific opinion on the safety and efficacy of iron compounds (E1) as feed additives for all species: iron chelate of amino acids, hydrate, based on a dossier submitted by Zinpro Animal Nutrition Inc. EFSA Journal 2013;11(7):3287, 28 pp. doi: 10.2903/j.efsa.2013.3287 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014b. Scientific opinion on the safety and efficacy of iron compounds (E1) as feed additives for all species: ferrous sulphate monohydrate based on a dossier submitted by Kronos International, Inc. EFSA Journal 2014;12(3):3607, 25 pp. doi: 10.2903/j.efsa.2014.3607 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014c. Scientific Opinion on the potential reduction of the currently authorised maximum zinc content in complete feed. EFSA Journal 2014;12(5):3668, 77 pp. doi: 10.2903/j.efsa.2014.3668 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015. Scientific Opinion on the safety and efficacy of iron compounds (E1) as feed additives for all animal species: ferrous carbonate based on a dossier submitted by Ankerpoort N.V.1. EFSA Journal 2015;13(5):4109, 31 pp. doi: 10.2903/j.efsa.2015.4109 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016a. Scientific opinion on the safety and efficacy of iron compounds (E1) as feed additives for all animal species: ferrous carbonate; ferric chloride, hexahydrate; ferrous fumarate; ferrous sulphate, heptahydrate; ferrous sulphate, monohydrate; ferrous chelate of amino acids, hydrate; ferrous chelate of glycine, hydrate, based on a dossier submitted by FEFANA asbl. EFSA Journal 2016;14(2):4396, 47 pp. doi: 10.2903/j.efsa.2016.4396 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016b. Scientific opinion on the revision of the currently authorised maximum copper content in complete feed. EFSA Journal 2016;14(8):4563, 100 pp. doi: 10.2903/j.efsa.2016.4563 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014a. Scientific opinion on the safety and efficacy of iron compounds (E1) as feed additives for all species: ferrous sulphate heptahydrate based on a dossier submitted by Kronos International, Inc. EFSA Journal 2014;12(2):3566, 24 pp. doi: 10.2903/j.efsa.2014.3566 [DOI] [Google Scholar]
- Egeli AK and Framstad T, 1999. An evaluation of iron‐dextran supplementation in piglets administered by injection on the first, third and fourth day after birth. Research in Veterinary Science, 66, 179–184. [DOI] [PubMed] [Google Scholar]
- EMA (European Medicines Agency), 2013. Assessment report for: Iron containing intravenous (IV) medicinal products. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500150771.pdf
- Fischer EH and Stein EA, 1960. α‐Amylases. In: Boyer P, Lardy H, Myrbäck K (eds.). The Enzymes, 2nd edn, vol. 4, Academic Press, New York, pp. 313–343. [Google Scholar]
- Fletcher F and London E, 1954. Intravenous Iron. British Medical Journal, 1, 84. [Google Scholar]
- Glawischnig Von E, Baumgartner W and Gewessler F, 1987. Anemia prophylaxis in piglets: efficacy of one oral dose of iron‐dextran.Deutsche Tierärztliche Wochenschrift, 94, 237–324. [PubMed] [Google Scholar]
- Gutzwiller A, 1999. Eisenversorgung des Neugeborenen Ferkels. Agrarforschung, 6, 373–375. [Google Scholar]
- Harmon BG, Cornelius SG, Totsch J, Baker DH and Jensen AH, 1974. Oral iron dextran and iron from steel slats as hematinics for swine. Journal of Animal Science, 39, 699–702. [DOI] [PubMed] [Google Scholar]
- IPCS (International Programme on Chemical Safety), 1999. Poisons Information Monograph 412: Phenol. International Programme on Chemical Safety, Geneva, Switzerland. World Health Organisation. [Google Scholar]
- Ishaya V and Ishaya M, 2012. Iron nutrition and anaemia in piglets: a review. Journal of Veterinary Advances, 2, 261–265. [Google Scholar]
- Jeanes A, 1975. Digestibility of food polysaccharides by man: a review. American Chemical Society Symposium Series, 15, 336–347. [Google Scholar]
- Jolliff JS and Mahan DC, 2011. Effect of injected and dietary iron in young pigs on blood haematology and postnatal pig growth performance. Journal of Animal Science, 89, 4068–4080. [DOI] [PubMed] [Google Scholar]
- Kirchgessner Von M and Weigand E, 1973. Zur Wirksamkeit von Eisenkomplexsalzen bei Saugferkeln nach oraler Applikation. Zetschrift tür Tierphysiologie Tierernahrung und Futtermittelkunde, 31, 342–344. [PubMed] [Google Scholar]
- Lemacher S and Bostedt H, 1995. [Development of the iron‐supply in suckling pigs under distinct iron‐substitution in regards to environmental conditions]. Tieriirztl Prax, 23, 457–464. [PubMed] [Google Scholar]
- London E, 2004. The molecular formula and proposed structure of the iron‐dextran complex, IMFERON. Journal of Pharmaceutical Sciences,93, 1838–1846. [DOI] [PubMed] [Google Scholar]
- London E and Twigg GD, 1954. Improved therapeutic preparations of iron. British Patent No 748024.
- Maes D, Steyaert M, Vanderhaeghe C, López Rodríguez A, deJong E , Del Pozo Sacristán R, Vangroenweghe F and Dewulf J, 2011. Comparison of oral versus parenteral iron supplementation on the health and productivity of piglets. Veterinary Record, 168, 188–192. [DOI] [PubMed] [Google Scholar]
- Maner JH, Pond WG and Lowley RS, 1955. Effect of method and level of iron administration on growth, haemoglobin and hematocrit of suckling pigs. Journal of Animal Science, 18, 1373–1377. [Google Scholar]
- McDonald FF, Dunlop D and Bates CM, 1955. An effective treatment for anaemia in pigs. British Veterinary Journal, 3, 3–7. [Google Scholar]
- McDowell LR, 2003. Minerals in Animal and Human Nutrition. 2nd Edition, Elsevier B.V. Amsterdam, The Netherlands, 660 pp. [Google Scholar]
- Mehmood Y and Farooq U, 2015. Excipients use in parenteral and lyophilized formulation development. Open Science Journal of Pharmacy and Pharmacology, 3, 19–27. [Google Scholar]
- Miller ER and Ullrey DE, 1997. Baby pig anemia. In: Pork Industry Handbook, pp. 1 4. Cooperative Extension Service Purdue University West Lafayette Indiana. [Google Scholar]
- Miller ER and Ullrey DE, 2007. Baby Pig Anemia. Pork Information Gateway. Factsheet Number PIG 04‐01‐20. Available online: http://porkgateway.org/wp-content/uploads/2015/07/baby-pig-anemia1.pdf
- Miret S, Simpson R and McKie A, 2003. Physiology and molecular biology of dietary iron absorption. Annual Review of Nutrition, 23, 283–301. [DOI] [PubMed] [Google Scholar]
- Nagai M, Hachimura K and Takahashi K, 1994. Water consumption in suckling pigs. Journal of Veterinary Medical Science, 56, 181–183. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), 2014. Iron Dextran Complex CAS No. 9004‐66‐4. Report on Carcinogens, Fourteenth Edition. Available online: https://ntp.niehs.nih.gov/ntp/roc/content/profiles/irondextrancomplex.pdf
- Pechin GH, Fournier MT, Sanchez EO and Cesan RO, 1998. Effect on iron dextran administration on weight gain and blood parameters related to iron metabolism in suckling piglets raised in contact with soil (in Spanish). Revista de Medicina Veterinaria, 79, 118. [Google Scholar]
- PHE (Public Health English), 2016. Phenol Toxicological Overview. Publications gateway number: 2014790. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/562434/phenol_general_information.pdf
- Pifferi G and Restani P, 2003. The safety of pharmaceutical excipients. Il Farmaco, 58, 541–550. [DOI] [PubMed] [Google Scholar]
- Ranjan R, Prasad CM and Singh SK, 2012. Effect of iron dextran injection on growth performance of crossbred and desi piglets under farm and village conditions. Veterinary World, 5, 599–602. [Google Scholar]
- Romvary A, 1971. Prevention of anemia in suckling piglets using oral and parenteral iron‐dextran. Monatshefte für Veterinärmedizin, 26, 844–847. [PubMed] [Google Scholar]
- Rydberg ME, Self HL, Kowalczyk T and Grummer RH, 1959. The effectiveness of three different methods of iron administration to young pigs. Journal of Animal Science, 18, 410–414. [Google Scholar]
- Suttle NF, 2010. Mineral Nutrition of Livestock. 4th Edition. CABI, Osfordshire, UK. 587 pp. [Google Scholar]
- Svoboda M and Drábek J, 2007. Intramuscular versus subcutaneous administration of iron dextran in suckling piglets. Acta Veterinaria Brno, 76, 11–15. [Google Scholar]
- Tattersfield AE and McNicol MW, 1987. Respiratory disease. Springer‐Verlag, London, ISBN‐13:978‐3‐540‐16209‐4; doi: 10.1007/978-1-4471-3132-8 [DOI] [Google Scholar]
- Thorén‐Tolling K, 1975. Studies on the absorption of iron after oral administration in piglets. Acta Veterinaria Scandinavica, Supplementum 54, 1–121. [PubMed] [Google Scholar]
- Ullrey DE, Miller ER, West DR, Schmidt DA, Seerley RW, Hoefer JA and Luecke RW, 1959. Oral and parenteral administration of iron in the prevention and treatment of baby pig anemia. Journal of Animal Science, 18, 256–263. [Google Scholar]
- Wessling‐Resnick M, 2000. Iron transport. Annual Review of Nutrition, 20, 129–151. [DOI] [PubMed] [Google Scholar]
- Widowski TM, Torrey S, Bench CJ and Gonyou HW, 2008. Development of ingestive behaviour and the relationship to belly nosing in early‐weaned piglets. Applied Animal Behaviour Science, 110, 109–127. [Google Scholar]


