Abstract
Low pathogenic avian influenza (LPAI) has been assessed according to the criteria of the Animal Health Law (AHL), in particular criteria of Article 7 on disease profile and impacts, Article 5 on the eligibility of LPAI to be listed, Article 9 for the categorisation of LPAI according to disease prevention and control rules as in Annex IV and Article 8 on the list of animal species related to LPAI. The assessment has been performed following a methodology composed of information collection and compilation, expert judgement on each criterion at individual and, if no consensus was reached before, also at collective levels. The output is composed of the categorical answer, and for the questions where no consensus was reached, the different supporting views are reported. Details on the methodology used for this assessment are explained in a separate opinion. According to the assessment performed, LPAI can be considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL. The disease would comply with the criteria as in Sections 3 and 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in points (c) and (e) of Article 9(1). The animal species to be listed for LPAI according to Article 8(3) criteria are all species of domestic poultry and wild species of mainly Anseriformes and Charadriiformes, as indicated in the present opinion.
Keywords: avian influenza, LPAI, Animal Health Law, listing, categorisation, impact
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The background and Terms of Reference (ToR) as provided by the European Commission for the present document are reported in section 1.2 of the scientific opinion on the ad hoc methodology followed for the assessment of the disease to be listed and categorised according to the criteria of Article 5, Annex IV according to Article 9 and Article 8 within the Animal Health Law (AHL) framework (EFSA AHAW Panel, 2017b).
1.2. Interpretation of the Terms of Reference
The interpretation of the ToR is as in section 1.2 of the scientific opinion on the ad hoc methodology followed for the assessment of the disease to be listed and categorised according to the criteria of Article 5, Annex IV according to Article 9 and Article 8 within the AHL framework (EFSA AHAW Panel, 2017b).
The present document reports the results of assessment on low pathogenic avian influenza (LPAI) according to the criteria of the AHL articles as follows:
Article 7: Low Pathogenic Avian Influenza profile and impacts
Article 5: eligibility of Low Pathogenic Avian Influenza to be listed
Article 9: categorisation of Low Pathogenic Avian Influenza according to disease prevention and control rules as in Annex IV
Article 8: list of animal species related to Low Pathogenic Avian Influenza
2. Data and Methodologies
The methodology applied in this opinion is described in detail in a dedicated document about the ad hoc method developed for assessing any animal disease for the listing and categorisation of diseases within the AHL framework (EFSA AHAW Panel, 2017b).
3. Assessment
3.1. Assessment according to Article 7 criteria
This section presents the assessment of LPAI according to the Article 7 criteria of the AHL and related parameters [see Table 2 of the opinion on methodology (EFSA AHAW Panel, 2017b)], based on the information contained in the factsheet as drafted by the selected disease scientist (see section 2.1 of the scientific opinion on the ad hoc methodology) and amended by the AHAW Panel.
3.1.1. Article 7(a) disease profile
The assessment focuses on H5 and H7 LPAI viruses (LPAIV) since they are internationally notifiable and subject to legislative control (Directive 94/2005/EC1). The rationale for legislative control and surveillance of H5/H7 LPAIV is to ensure effective control before they may mutate to highly pathogenic avian influenza (HPAI) virus (HPAIV), which could have more severe consequences for veterinary public health. In gathering evidence for the factsheet, some supporting material has been utilised, based on biological findings of other LPAIV (subtypes other than H5/H7) which have common ecology and biology.
3.1.1.1. Article 7(a)(i) Animal species concerned by the disease
Susceptible animal species
Parameter 1 – Naturally susceptible wildlife species (or family/orders)
Mainly Anseriformes and Charadriiformes (Daoust et al., 2011; Kuiken, 2013) have been reported as LPAI‐infected wild bird species (see Table 1). HPAIV have been detected in over a hundred species of wild birds from at least 13 different orders (EFSA, 2006; EFSA AHAW Panel, 2017a). The LPAI susceptibility of many wild bird species has not been assessed.
Table 1.
LPAIV‐infected wild birds (based on Kuiken (2013) and Duncan et al. (2017); EMPRES‐I, online 2007–2017 data)
| Common name (Latin name) | Family | Order |
|---|---|---|
| Ruddy turnstone (Arenaria interpres), common snipe (Gallinago gallinago) | Scolopacidae | Charadriiformes |
| Laughing gull (Leucophaeus atricilla), ring‐billed gull (Larus delawarensis), black‐headed gull (Larus ridibundus), herring gull (Larus argentatus), great black‐backed gull (Larus marinus) | Laridae | Charadriiformes |
| Pied avocet (Recurvirostra avosetta) | Recurvirostridae | Charadriiformes |
| mute swan (Cygnus olor), mallard (Anas platyrhynchos), northern pintail (Anas acuta), northern shoveler (Anas clypeata), Canada goose (Branta canadensis), tundra swan (Cygnus columbianus), Redhead (Anas americana), wood duck (Anas sponsa), greater white‐fronted goose (Anas albifrons), common teal (Anas crecca), Eurasian wigeon (Anas penelope), garganey (Anas querquedula), gadwall (Anas strepera), greylag goose (Anser anser), lesser white‐fronted goose (Anser erythropus), bean goose (Anser fabalis), common pochard (Aythya ferina), tufted duck (Aythya fuligula), ashy‐headed goose (Chloephaga poliocephala), whooper swan (Cygnus cygnus), harlequin duck (Histrionicus histrionicus), ruddy duck (Oxyura jamaicensis), common shelduck (Tadorna tadorna) | Anatidae | Anseriformes |
| Common coot (Fulica atra) | Rallidae | Gruiformes |
| Black‐crowned night heron (Nycticorax nycticorax) | Ardeidae | Pelecaniformes |
| Great cormorant (Phalacrocorax carbo) | Phalacrocoracidae | Suliformes |
LPAIV: low pathogenic avian influenza virus; EMPRES‐I: Global Animal Diseases Information System, Food Agriculture Organisation.
Parameter 2 – Naturally susceptible domestic species (or family/orders)
All species of domestic poultry, to include the family Phasianidae (chickens, turkeys and related poultry such as quail and pheasant), Odontophoridae (quail), Numidae (guinea fowl) and Struthionidae (ostriches) and also other birds native and introduced such as farmed Anseriformes, particularly including ducks, geese and ratites are susceptible (Table 2). In general, viruses from birds rarely infect mammals (reviewed in Swayne, 2016).
Table 2.
Main domestic bird families susceptible to LPAIV
| Common name | Family | Order |
|---|---|---|
| Chicken, turkey, quail (Old World species), pheasant | Phasanidae | Galliformes |
| Quail (New World species) | Odontophoridae | Galliformes |
| Guinea fowl | Numididae | Galliformes |
| Duck, geese | Anatidae | Anseriformes |
| Ostrich, ratitesa | Struthionidae | Struthioniformes |
LPAIV: low pathogenic avian influenza virus.
Sometimes classified as a diverse group of large, flightless birds of the infraclass Palaeognathae.
Parameter 3 – Experimentally susceptible wildlife species (or family/orders)
There is no additional information (see Table 1).
Parameter 4 – Experimentally susceptible domestic species (or family/orders)
Pigs, mustelid, horses, domestic carnivores (cats and dogs), seals and other sea mammals, rats, rabbits, guinea pigs, mice and non‐human primates have been infected experimentally (Short et al., 2015) (Table 3).
Table 3.
Main domestic families/orders susceptible to LPAIV
| Common name | Family | Order |
|---|---|---|
| Pig | Suidae | Artiodactyla |
| Horse | Equidae | Perissodactyla |
| Dog | Canidae | Carnivora |
| Cat | Felidae | Carnivora |
| Mustelid | Mustelidae | Carnivora |
| Seals and other sea mammals | Enaliarctidae | Carnivora |
| Rat, mouse | Muridae | Rodentia |
| Guinea pig | Caviidae | Rodentia |
| Non‐human primates | Several | Primates |
LPAIV: low pathogenic avian influenza viruses.
Reservoir animal species
Parameter 5 – Wild reservoir species (or family/orders)
Wild water birds, in particular Anseriformes and Charadriiformes (Daoust et al., 2011; Kuiken, 2013; Verhagen et al., 2014), are considered the main reservoir of LPAIV.
Parameter 6 – Domestic reservoir species (or family/orders)
No domestic species are reported as natural reservoirs and these become infected normally via spillover from wild bird reservoir species (Swayne, 2016). In some ecosystems or situations (e.g. wild bird markets, duck paddy field systems), domestic ducks (Anseriformes) may maintain virus for long periods. Also, domestic water bird populations (Anseriformes) could become reservoir.
3.1.1.2. Article 7(a)(ii) The morbidity and mortality rates of the disease in animal populations
Morbidity
Parameter 1 – Prevalence/Incidence
EU Member States (MS) perform annual surveillance programmes in poultry, mainly using a risk‐based sampling approach. Table 4 indicates that the proportion of seropositive holdings was between 0.15% and 0.25% in the period 2010–2014. Positive samples were reported in 8–11 MS. Comparing MS or poultry categories is not possible due to large variations in the number of samples per country and per poultry category.
Table 4.
LPAI serosurveillance data in number of holdings (%) (based on Annual EU surveillance reports)
| Category | Year | ||||
|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | |
| Holdings sampled | 29,484 | 29,806 | 29,404 | 25,220 | 19,813 |
| H5 positive holdings | 50 | 50 | 40 | 57 | 38 |
| H7 positive holdings | 14 | 15 | 4 | 6 | 5 |
| Number Member States positive | 8 | 10 | 9 | 11 | 8 |
| Poultry category | |||||
| Chicken breeders | 1 | 2 | |||
| Laying hens | 4 | 4 | 4 | 1 | 4 |
| Free range laying hens | 1 | 1 | 3 | 7 | 4 |
| Broilers | |||||
| Fattening turkeys | 1 | ||||
| Turkey breeders | |||||
| Fattening ducks | 6 | 7 | 8 | 11 | |
| Breeder ducks | 4 | 25 | 21 | 27 | 7 |
| Fattening geese | 22 | 2 | 1 | 1 | 2 |
| Breeder geese | 8 | 8 | 4 | 5 | 10 |
| Backyard | 4 | 8 | 1 | 3 | |
| Farmed game birds | 15 | 3 | 1 | 2 | |
| Ratites | 1 | ||||
| Other | 5 | 6 | 7 | 2 | |
LPAI: low pathogenic avian influenza.
LPAI has been detected in wild birds across the year and in several MS (Barral et al., 2008; Busquets et al., 2010; Perez‐Ramirez et al., 2010, 2012; Jurado‐Tarifa et al., 2014; Latorre‐Margalef et al., 2014; Swieton et al., 2017). Furthermore, the maps in Figure 1 show that some regions had seropositive holdings in 2014 and 2015. This was, for instance, the case in south‐west France, where new highly pathogenic H5 viruses emerged during winter 2015/16, which were never identified in wild birds before (Briand et al., 2017).
Figure 1.
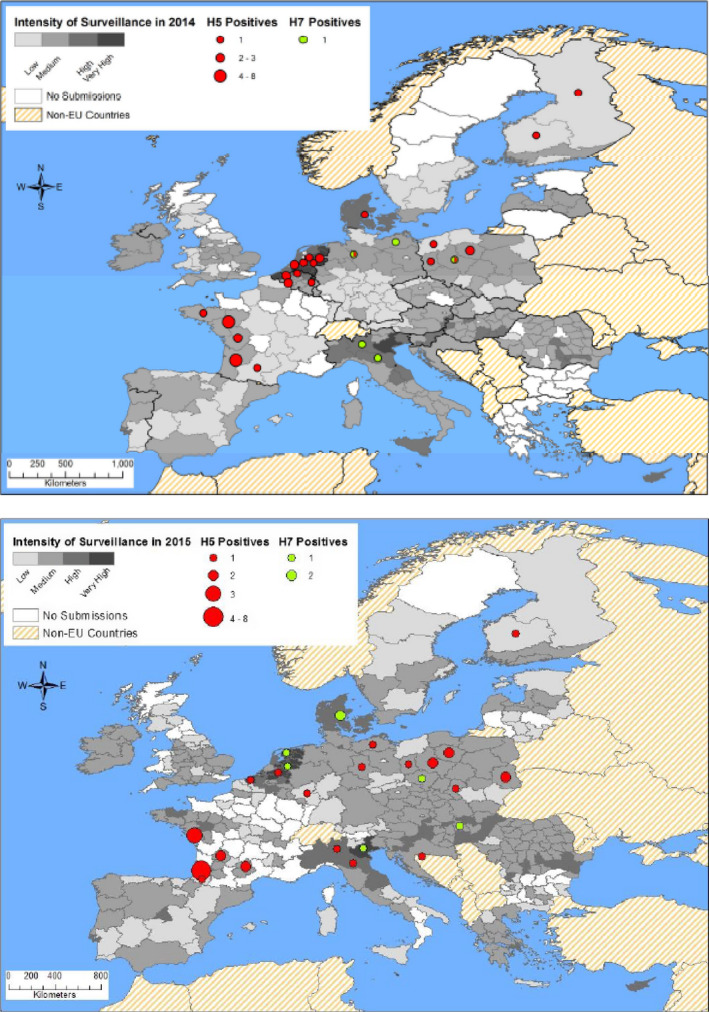
- The classification of the intensity of surveillance is grouped by holdings sampled per 100 km2 Low: up to 10, medium: 11–100, high: 101–500, very high: > 500
In the period 2007–2017, LPAI outbreaks were reported in 321 holdings located in 14 MS (Table 5). The seroprevalence data and the outbreak data clearly show that LPAIV circulate every year in multiple MS and mainly in ducks, geese and game birds.
Table 5.
LPAI outbreaks within the EU (reported in ADNS between 1/1/ 2007 and 31/12/2016)
| Country | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | Grand total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Germany | 0 | 32 | 6 | 3 | 23 | 3 | 10 | 2 | 3 | 10 | 92 |
| France | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 14 | 28 | 46 |
| Italy | 18 | 3 | 37 | 9 | 23 | 16 | 9 | 5 | 8 | 4 | 132 |
| Netherlands | 0 | 0 | 0 | 1 | 5 | 2 | 6 | 2 | 3 | 2 | 21 |
| Belgium | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| United Kingdom | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 4 |
| Ireland | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Denmark | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 2 | 6 |
| Spain | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Portugal | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Norway | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Czech Republic | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Bulgaria | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 6 |
| Romania | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Grand total | 24 | 39 | 50 | 17 | 57 | 22 | 27 | 9 | 29 | 47 | 321 |
LPAI: low pathogenic avian influenza; ADNS: Animal Disease Notification System.
Parameter 2 – Case morbidity rate (% clinically diseased animals out of infected ones)
Morbidity of LPAI can be variable depending on the virus strain and poultry species. In ducks, morbidity can be very low with subclinical infection due to localised enteric infection, whereas in chicken layers it may be higher but influenced, for instance, by flock size and density (Gonzales et al., 2012a).
Mortality
Parameter 3 – Case fatality rate
In general, mortality due to LPAI is usually less than 5% in most avian species unless exacerbated by secondary pathogens. Localised infection is normal, it remains enteric and respiratory (Swayne et al., 2013).
3.1.1.3. Article 7(a)(iii) The zoonotic character of the disease
Presence
Parameter 1 – Report of zoonotic human cases (anywhere)
Humans can be infected with LPAIV such as H7N9. Rare mild zoonotic cases have been reported principally with conjunctivitis and mild respiratory infection in humans occupationally exposed. The number of reported H7N9 human cases worldwide in the period 2007–2016 is 1,157 (based on EMPRES‐I, online), of which two cases in the America and all the others in Asia. The majority of the H7N9 human cases have been associated with direct or indirect contact with infected live or dead poultry. There is no scientific evidence suggesting efficient transmission of these viruses between persons.
3.1.1.4. Article 7(a)(iv) The resistance to treatments, including antimicrobial resistance
Parameter 1 – Resistant strain to any treatment even at laboratory level
Amantadine resistance has been described in poultry in China and Egypt (Cheng et al., 2009; Zaraket et al., 2010; CDC, 2017). A H7N9 virus isolated from a human case has been reported (ECDC, online) to contain a mutation in the neuraminidase protein relevant for antiviral resistance against oseltamivir and zanamivir.
3.1.1.5. Article 7(a)(v) The persistence of the disease in an animal population or the environment
Animal population
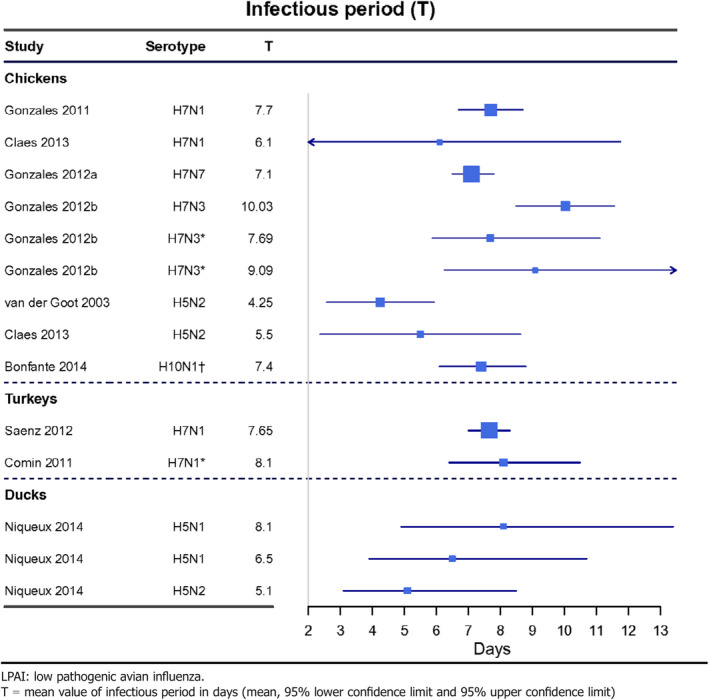
Parameter 1 – Duration of infectious period in animals
The mean infectious period in individuals is 4–10, 7–8 and 5–8 days in chickens, turkeys and ducks, respectively (Table 6).
Table 6.
LPAI infectious period in poultry (in days) (data received as per procurement, Erasmus University Medical Centre, OC/EFSA/ALPHA2015/01, unpublished)
Parameter 2 – Presence and duration of latent infection period
Animal latent period up to 3 days depending on virus strain, infecting dose and species have been reported; flock level latency ranges 14 days (Swayne and Halvorson, 2008).
Parameter 3 – Presence and duration of the pathogen in healthy carriers
LPAIV infections may go unreported for some time (Marche et al., 2014). By definition most populations are mildly affected (Daoust et al., 2013). In extreme circumstances, shedding in individual birds has been recorded for up to 3–4 weeks in chickens, ducks and turkeys (Brown et al., 2012).
Environment
Parameter 4 – Length of survival (dpi) of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment (scenarios: high and low T)
Survivability will depend on the virus strain and the substrate together with temperature, humidity and pH. For example, at 4°C, the maximum resistance time varied between 18 and 176 days for 12 LPAIV of different H types (Brown et al., 2009). In general, viruses were most stable at slightly basic pH (7.4–8.2), low temperatures (< 17°C) and fresh to brackish salinities. In faeces, 35 days at 4°C and 7 days at 20°C; in water, up to 158 days at 17°C, whereas in manure and litter, 2–7 days; rapidly inactivated in air less than 1 day due to desiccation (Keeler et al., 2012), low pH (< 5.0) or salinity (EFSA, 2008; Swayne and Halvorson, 2008; Reis et al., 2012).
3.1.1.6. Article 7(a)(vi) The routes and speed of transmission of the disease between animals, and, when relevant, between animals and humans
Routes of transmission
Parameter 1 – Types of routes of transmission from animal to animal (horizontal, vertical)
A comparison of the different transmission routes is provided in Table 7. Although differences between isolates have been observed, results from Pillai et al. (2010) indicate that a poultry derived isolate transmits better between chickens than a wild bird derived isolate. Wild bird isolates transmit better among turkeys and ducks than among chickens. Once the virus is adapted to a species, it can spread easily within flocks. Infectious excretions are considered the most important source of virus transmission between animals. Therefore, contaminated fomites are an important entry route for the virus into poultry houses. Transmission of the virus via direct contact is considered less important than via indirect contacts, both for domestic and wild birds. Vertical transmission has not been observed.
Table 7.
| Mode | Chicken | Turkey | Duck |
|---|---|---|---|
| Horizontal intraspecies | +++ | +++ | +++ |
| Vertical | _ | _ | _ |
| Fomite | ++ | ++ | ++ |
| Wild bird origin | + | ++ | +++ |
| Horizontal interspecies | + | ++ | ++ |
+++: highly efficient; ++: effective; +: can occur, but low efficiency; –: not reported.
Parameter 2 – Types of routes of transmission between animals and humans (direct, indirect, including foodborne)
Transmission is by direct exposure to aerosolised droplet materials in poultry environment through the conjunctiva or the upper respiratory tract. Food is not considered a route of avian influenza virus transmission to humans since acidic pH in the stomach and bile salts in the duodenum reduce the virus infectivity (EFSA BIOHAZ Panel, 2010).
Speed of transmission
Parameter 3 – Incidence between animals and, when relevant, between animals and humans
Interspecies transmission can occur with high probability between animals of the same taxonomic family such as chickens and turkeys (Pillai et al., 2010; Mughini‐Gras et al., 2014); interspecies transmission across different orders, such as duck to turkey is less likely (Mughini‐Gras et al., 2014; Claes et al., 2015) with even greater reduced efficiency between species of different classes, i.e. avian to human.
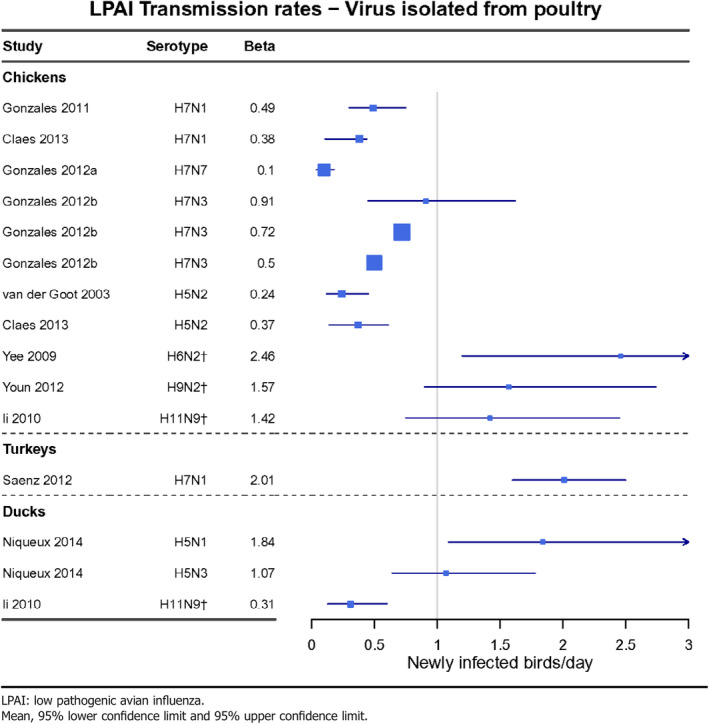
Parameter 4 – Transmission rate (beta) (from R 0 and infectious period) between animals and, when relevant, between animals and humans
In chicken, the basic reproduction ratio, R0 (the average number of secondary infections caused by one infectious bird introduced in a fully susceptible population), for LPAI ranges 0.8–9.1 (data received as per procurement, Erasmus8University Medical Centre, OC/EFSA/ALPHA2015/01, unpublished). The average infectious period of LPAI (Table 6) is considerably longer than that of HPAI, resulting in an transmission rate parameter, β (the average number of secondary cases caused by one infectious individual per day) at lower ranges for LPAI than for HPAI (0.1–2.46 and 0.76–4.5 respectively).
For turkeys, less information is available than for chicken, however, the trend appears similar to that of chicken. Also, for ducks the information is limited, although it points towards higher transmission parameters of LPAIV in ducks than in chickens (Table 8).
Table 8.
LPAI transmission rates (beta) between animals for viruses isolated from poultry (data received as per procurement, Erasmus University Medical Centre, OC/EFSA/ALPHA2015/01, unpublished)
3.1.1.7. Article 7(a)(vii) The absence or presence and distribution of the disease in the Union, and, where the disease is not present in the Union, the risk of its introduction into the Union
Presence and distribution
Parameter 1 – Map where the disease is present in the European Union (EU) (Figure 2)
Figure 2.
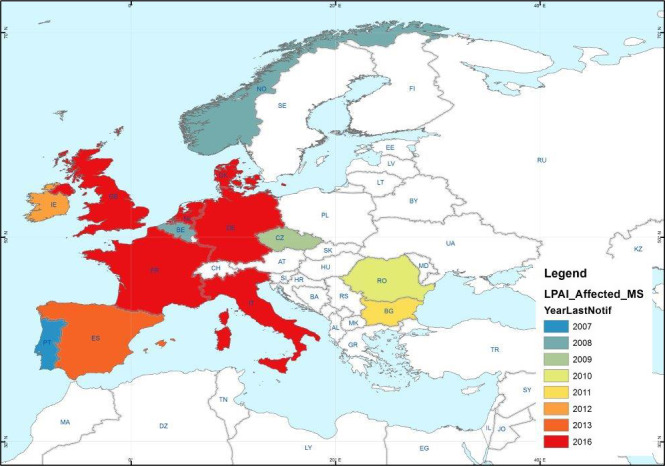
H5 and H7 LPAI outbreaks in poultry holdings and captive birds reported in ADNS between 2007 and 2016
Parameter 2 – Type of epidemiological occurrence (sporadic, epidemic, endemic) at MS level
In the MS that reported LPAI outbreaks in the last 10 years, some had very few outbreaks (e.g. only in a period of one or two years), whereas others reported outbreaks every year (see Table 5). In some cases, the sporadic outbreaks lead to wider epidemics, e.g. H7N7 Germany 2011 (Probst et al., 2012), H7N1 Italy 1999–2000 (Capua et al., 2002).
Risk of introduction
Parameter 3 – Routes of possible introduction
The disease could be introduced by several routes (EFSA, 2008):
-
1
Introduced by migration of wild birds. Infected migratory and indigenous wild birds are the most likely source of the virus for poultry in the EU. Direct contact with secretions and excretions, especially faeces, and possibly aerosol transmission from infected birds over short (few metres) distances (Jonges et al., 2015). At‐risk matrices are contaminated feed, water, premises, human clothing, etc.
Usually by the introduction of infective faeces, e.g. on boots, clothing, vehicles, egg trays, birds cages or other fomites (feed and water contaminated by infective faeces have been shown to be responsible for introduction).
-
2
Outbreak of disease in a territory of a trading partner and infected birds or contaminated products imported that then may infect local birds:
There may be a risk if live birds are imported from affected countries where infection has been acquired after certification on premises of origin. Although this is considered a low probability event, for example the mean annual probability for HPAI introduction into Poland by legal trade was considered to be one outbreak in every 326 years (Gierak et al., 2016).
-
3
Illegal importation of birds from areas where disease is present.
Parameter 4 – Number of animal moving and/or shipment size
It was not possible to source reliable data within the time frame of this mandate. An overview will be provided in the scientific opinion on avian influenza that is scheduled for adoption in September 2017.
Parameter 5 – Duration of infectious period in animal and/or commodity
The duration of the infectious period in animals is described under parameter 1 of Article 7(a)(v) (see Section 3.1.1.5). The risk related to commodities is mainly due to contamination with (faecal) excretions. Survival of LPAIV will depend on temperature, humidity and pH as described under parameter 4 of Article 7(a)(v) (see Section 3.1.1.5).
Parameter 6 – List of control measures at border (testing, quarantine, etc.)
Legislation on imports of live poultry, day‐old chicks and hatching eggs, table eggs, egg products and the meat of game birds and poultry is laid down in Regulation (EC) No 798/20082. This includes a list of approved countries for which there are certificates to allow the import of such consignments. Some countries are regionalised, some have special additional measures.
Once the consignments are imported into the EU, the Regulation requires that the birds be kept at the destination for a minimum of 3 weeks with sampling and (negative) testing for AI, during which time there is also monitoring for clinical signs.
The OIE Terrestrial Code chapter 10.4 (2010) describes the recommendations for importation of live birds other than poultry. In essence, regardless of the avian influenza status of the country of origin, an international veterinary certificate is required, attesting: (i) that the birds showed no clinical sign of avian influenza infection at the day of shipment, (ii) that the birds were kept in isolation since they were hatched or for at least 21 days prior to shipment and showed no clinical signs of avian influenza, (iii) that a statistically valid sample of the birds was tested to demonstrate freedom from avian influenza infection, (iv) that the birds are transported in new or appropriately sanitised containers.
EU rules are in place for the movement of pet birds (i.e. accompanied by their owner) or birds which are destined for special breeding programmes or for Approved Bodies (Council Directive 92/65/EEC3) into the Union.
Import into the EU of poultry meat is according to Regulation (EC) 798/2011. It is allowed to trade poultry meat from a country, zone or compartment affected by LPAI if the animals did not show any sign suggestive of AI infection during ante and post mortem inspection.
For the importation of egg products of poultry, regardless of the AI status of the country of origin, the commodity fulfils the requirements for importation of eggs for human consumption or the commodity has been processed to ensure the destruction of AI (heat inactivation achieving a 7‐log reduction of AI, see OIE Code Article 10.4.25 for technical information) and contact with any source of AIV is prevented.
For imports into the Union of feathers and down, Regulation (EC) No 142/20114 applies, requiring a prohibition on the import of untreated feather or down, while treated feathers and down (hot steam at 100°C for 30 min) may be placed on the market with no restrictions.
A list of control measures implemented in third countries or at the EU border related to import of live birds and commodities is provided in Table 9.
Table 9.
List of control measures at the border related to import of live birds and commodities
| Item | Measure |
|---|---|
| Live birds | Pre‐inspection at origin, certification, veterinary inspection |
| Hatching eggs | Pre‐inspection at origin, certification, veterinary inspection |
| Day‐old chicks | Pre‐inspection at origin, certification, veterinary inspection |
| Animal products | Certification, third country inspections for assurance (as required) |
| Captive birds | Certification, quarantine, testing |
Parameter 7 – Presence and duration of latent infection and/or carrier status
See Section 3.1.1.5.
3.1.1.8. Article 7(a)(viii) The existence of diagnostic and disease control tools
Diagnostic tools
Parameter 1 – Existence of diagnostic tools
The avian influenza diagnostic tools are described in the Commission Decision 2006/437/EC5. In addition, there are specified laboratory manuals in the EU, Regulation (EC) No 2006/4376 applies, and at global level (OIE, 2017). Polymerase chain reaction (PCR) is used to determine the presence of LPAIV in suspected samples of live animals, commodities or environmental samples. The sensitivity and specificity varies depending on the virus subtype and the analysed matrix. Virus isolation is required for detailed analysis of a virus and is done with one or a few isolates per outbreak or epidemic. Sequencing is used to analyse the relation with other circulating viruses. The intravenous pathogenicity test (IVPI) is used to determine the pathogenicity level of a virus. Haemagglutination inhibition (HI) and enzyme‐linked immunosorbent assay (ELISA) are used in seroprevalence studies. An overview of the available tools is provided in Table 10.
Table 10.
Overview of AI diagnostic tools
| Method | Test performance | Purpose | Sample type | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Dse | Dsp | Population freedom | Animal freedom | Disease confirmation | Surveillance inc post vacc | |||
|
Detection/culture Virus isolation a |
+ | +++ | +++ | + |
Tissues C&O swabs |
(Commission Decision 2006/437/EC) | ||
| PCR (real‐time) b | NK | NK | NK | NK | NK | NK |
Tissues C&O swabs |
(Commission Decision 2006/437/EC) |
| Influenza A | NK | NK | ++ | +++ | +++ | ++ |
Tissues C&O swabs |
(Commission Decision 2006/437/EC; Slomka et al., 2010) |
| H5 | 100% | – | ++ | +++ | +++ | ++ |
Tissues C&O swabs |
(Commission Decision 2006/437/EC; Slomka et al., 2007) |
| H7 | 100% | 90% | ++ | +++ | +++ | ++ |
Tissues C&O swabs |
(Commission Decision 2006/437/EC; Slomka et al., 2009) |
|
Immune response HI |
– | – | +++ | ++ |
++ convalescent |
+++ | Serum | (Commission Decision 2006/437/EC) |
| ELISA | NK | NK | + | + |
+ convalescent |
++ | Serum | (Marche and van den Berg, 2010; OIE, 2017) |
|
Virus characterisation gene sequencing |
NK | 100% | – | – | +++ | – | Tissue /swab or virus | (OIE, 2017) |
| IVPI | – | – | – | – | +++ | – | Virus | (OIE, 2017) |
C&O swabs: cloacal and oropharyngeal swabs.
NK: not known; HI, haemagglutination inhibition test; IVPI, intravenous pathogenicity index.
Gold standard but proven to be less sensitive and furthermore, real‐time PCR reactors specific from known population.
Senstivity (Dse) and specificity (Dsp) values measured against virus isolation.
Control tools
Parameter 2 – Existence of control tools
The control measures for avian influenza mainly aim to prevent virus spread to other poultry holdings. The measures are described in Council Directive 2005/94/EC7 and an overview is presented in Table 11. In case of a LPAI outbreak, the competent authority shall immediately establish a restricted zone with a radius of at least 1 km around the holding. An inventory is made of the animals present on the affected holding and the animals should be brought and kept inside. No carcasses, meat, animal by‐products, manure, slurry used litter or anything likely to transmit LPAIV may leave the holding without authorisation. Furthermore, an epidemiological investigation is performed. Holdings in which infection with LPAIV has been confirmed are depopulated, followed by cleaning and disinfection of the houses and equipment. Poultry and eggs cannot be moved until the risk of LPAI spread is determined as minimal. Hatching eggs are put under surveillance. Persons entering or leaving the holding and transport vehicles have to follow specific biosecurity requirements aiming to prevent the spread of the virus. Preventive or emergency vaccination is possible although only used in few cases8 (e.g. in Italy between 2000 and 2008 and in Portugal between 2007 and 2011) (Capua and Marangon, 2006). Repopulation is done in a controlled manner.
Table 11.
Available control measures for avian influenza
| Measure | Application |
|---|---|
| Farm restrictions | Live birds, hatching eggs, meat, animal by‐products, table eggs, equipment, vehicles, people, slurry/manure, litter |
| Zoning/quarantine/surveillance | 1 km protection zone, farm census/inspection, laboratory testing of farms |
| Movement restrictions | Live birds, hatching eggs, meat, animal by‐products, table eggs |
| Culling and disposal | Infected farm |
| Biosecurity | Specified requirements |
| Transport | Specified requirements |
| Epidemiological enquiry | Determine spread, identify source |
| Cleansing and disinfection | Specified requirements |
| Vaccination | Exceptional use |
| Repopulation | Controlled |
3.1.2. Article 7(b) The impact of diseases
3.1.2.1. Article 7(b)(i) The impact of the disease on agricultural and aquaculture production and other parts of the economy
The level of presence of the disease in the Union
Parameter 1 – Number of MSs where the disease is present
In the period 2007–2016, LPAI outbreaks were reported in 14 MS through ADNS (see Table 5). The number of outbreaks per year ranged from 9 to 57 and reached a total of 321 in these 10 years.
The loss of production due to the disease
Parameter 2 – Proportion of production losses (%) by epidemic/endemic situation
Morbidity and mortality of LPAI in poultry is limited, as described in Section 3.1.1.2. The main loss due to LPAI is the depopulation of affected holdings. In the period 2007–2016, 1.7 × 106 poultry were culled within the EU (see Table 12). Additional losses are related to movement restrictions, which lead to economic loss of table eggs, hatching eggs and live animals. Indirect costs are for instance trade bans to third countries, treatment and removal of manure and wash water, closing of processing plants. The estimated total economic cost of the LPAI epidemic in Italy in 1999 was about 507 million euro (112 and 395 million euro direct and indirect costs respectively) (Sartore et al., 2010). The total cost for five subsequent LPAI outbreaks in Italy was estimated around 143 million euro.
Table 12.
Overview of diseased, dead and culled poultry due to LPAI outbreaks reported in ADNS between 2007 and 2016
| Diseased poultry | Dead poultry | Culled poultry | Grand total | |
|---|---|---|---|---|
| 2007 | 31,776 | 3,491 | 126,398 | 161,665 |
| 2008 | 452 | 8,199 | 358,724 | 367,375 |
| 2009 | 355 | 572 | 176,327 | 177,254 |
| 2010 | 2,093 | 1 | 55,527 | 57,621 |
| 2011 | 51,955 | 201 | 308,457 | 360,613 |
| 2012 | 103,390 | 148 | 221,970 | 325,508 |
| 2013 | 11,973 | 135 | 135,689 | 147,797 |
| 2014 | 186 | 50 | 50,300 | 50,536 |
| 2015 | 11,282 | 152 | 69,767 | 81,201 |
| 2016 | 236 | 5 | 21,568 | 21,809 |
| Grand total | 213,698 | 12,954 | 1,524,727 | 1,751,379 |
LPAI: low pathogenic avian influenza.
3.1.2.2. Article 7(b)(ii) The impact of the disease on human health
Transmissibility between animals and humans
Parameter 1 – Types of routes of transmission between animals and humans
Avian influenza viruses do not readily infect people, but can do so when people have close contact with infected birds or exposure to high viral loads in untreated products. The illness caused by avian influenza viruses can present itself as a flu‐like respiratory illness or conjunctivitis (Kim et al., 2016).
Parameter 2 ‐ Incidence of zoonotic cases
With increased awareness that AI viruses can infect humans, investigations of human contacts during poultry outbreaks with LPAI have detected a small number of human cases. Poor transmissibility to humans with most LPAIV has been reported with only four human cases reported in Europe (Kim et al., 2016; Table 4). One exception is an H7N9 LPAIV confined to China that has caused 1,157 human cases since 2013 with approximately 30% mortality (WHO, 2015). The composition of this virus is entirely distinct from viruses circulating in European poultry and wild birds.
Transmissibility between humans
Parameter 3 – Human to human transmission is sufficient to sustain sporadic cases or community‐level outbreak
LPAIV lacks human to human transmissibility.
Parameter 4 – Sporadic, endemic, epidemic or pandemic potential
Only four human cases have been reported in Europe, nonetheless, there is theoretical pandemic potential.
The availability of effective prevention or medical treatment in humans
Parameter 6 – Availability of medical treatment and their effectiveness (therapeutic effect and any resistance)
Antiviral drugs are available but some levels of resistance are reported. These drugs are effective only if taken early in the infection course (Loregian et al., 2014).
Parameter 7 – Availability of vaccines and their effectiveness (reduced morbidity)
Vaccines are not routinely available for human application. WHO has pre‐pandemic vaccine stock containing some LPAIV strains for rapid production, if a pandemic were to emerge from an LPAI virus (WHO, 2015).
3.1.2.3. Article 7(b)(iii) The impact of the disease on animal welfare
Parameter 1 – Severity of clinical signs at case level and related level and duration of impairment
Clinical signs, as defined above, are usually very mild, i.e. laying birds going out of lay so the impact is very low unless exacerbated by secondary infections. Low levels of mortality can occur in susceptible species.
3.1.2.4. Article 7(b)(iv) The impact of the disease on biodiversity and the environment
Biodiversity
Parameter 1 – Endangered wild species affected: listed species as in CITES and/or IUCN list
Not applicable as LPAI in wild species is usually asymptomatic.
Environment
Parameter 3 – Capacity of the pathogen to persist in the environment and cause mortality in wildlife
While the pathogen can survive in the environment, it presents no risk to wildlife in terms of mortality.
3.1.3. Article 7(c) Its potential to generate a crisis situation and its potential use in bioterrorism
Parameter 1 – Listed in OIE/CFSPH classification of pathogens
It is listed.
Parameter 2 – Listed in the Encyclopaedia of Bioterrorism Defence of Australia Group
It is not listed.
Parameter 3 – Included in any other list of potential bio‐agroterrorism agents
It is not listed.
3.1.4. Article 7(d) The feasibility, availability and effectiveness of the following disease prevention and control measures
3.1.4.1. Article 7(d)(i) Diagnostic tools and capacities
See Section 3.1.1.8 and Table 10 which provides summary data for all three aspects.
3.1.4.2. Article 7(d)(ii) Vaccination
There are five vaccines available for LPAI in poultry, which reduce viral shedding. The duration of protection varies from 14 weeks to 12 months in chicken and 14 weeks in ducks. All vaccines comply to the DIVA principle, as required by the EU legislation. Table 13 (FAO, 2009; EMA, 2010; Discontools, 2016) provides an overview of the vaccine characteristics (Marangon et al., 2003; Busani et al., 2007; Capua et al., 2009; Beato et al., 2014).
Table 13.
LPAI vaccine availability and effectivenessa
| Vaccine | Type/administration | Effectiveness | Field protection | Duration of protection | Diva | Availability/production capacityd |
|---|---|---|---|---|---|---|
|
Nobilis flu H5N2b |
Inactivated IM and SC |
Reduced clinical signs and viral shedding | n/a | 12 months in chicken | Yc |
Y Capacity unknown |
|
Nobilis flu H7N1/H7N7 |
Inactivated IM and SC |
Reduced clinical signs and viral shedding |
Italy 2002–2003 R0 2.9 pre‐vaccination to 0.6 post‐vaccination |
45 weeks | Yc |
Y Capacity unknown |
|
Poulvac Flu‐Fend H5N3 |
Inactivated IM and SC |
Reduced clinical signs and viral shedding | n/a | 14 weeks in ducks | Yc |
Y Capacity unknown |
|
Merial Trovac H5 |
Vectored HA insert SC |
Reduced clinical signs and viral shedding | n/a | 20 weeks in chicken | Y |
Y Capacity unknown |
|
Gallimune H5N9 or H7N1 |
Inactivated IM and SC |
Reduced clinical signs and viral shedding | n/a | Not known | Yc |
Y Capacity unknown |
SC: subcutaneous; IM: intramuscular.
Note this is not an exhaustive list of vaccines, but it indicates some vaccines historically used in the EU.
EMA authorisation.
Companion diagnostics not on the shelf.
Commercial suppliers tailor supply to demand.
Feasibility
Parameter 5 – Way of administration
Vaccinations by commercially available vaccines are carried out by injection (IM or SC).
3.1.4.3. Article 7(d)(iii) Medical treatments
Antiviral drugs are prohibited for application in the veterinary sector and therefore this section is not applicable.
3.1.4.4. Article 7(d)(iv) Biosecurity measures
Availability
Parameter 1 – Available biosecurity measures
Procedures or practices that prevent or limit exposure to LPAIV include farm hygiene, environmental control, medication to prevent secondary challenge and effective sanitation. Key elements are farm location to mitigate risk for introduction, farm design, access control of people and vehicles, sanitation of materials entering and leaving the site including equipment, litter, vermin control, limiting access to wild birds or their faeces when birds are kept outdoors, water sanitation, physical barriers to poultry house, including boot changes and protective clothing, quarantine new stock, exclude access of wild birds to feed and litter disposal (Lister, 2008; EFSA AHAW Panel, 2017a,b).
Effectiveness
Parameter 2 – Effectiveness of biosecurity measures in preventing the pathogen introduction
A combination of factors will provide effective biosecurity, but key elements include limiting access for wild birds and reducing fomite transmission risk through effective sanitisation when entering into poultry houses. In some systems, mitigation of access to wild bird or their faeces, e.g. in outdoor system can be achieved by ensuring that feed is not readily accessible or by limiting the access to outdoor range by wild birds (by nets or fences). Appropriate management to monitor changes in production and flock health are critical for prompt awareness of early indicators of infection, i.e. more than 20% reduction in feed or water intake or more than 5% of egg drop for more than 2 days in laying birds (Annex II to Decision 2005/734/EC). Implementation of biosecurity measures is key to disease prevention/control (Gonzales et al., 2014).
Feasibility
Parameter 3 – Feasibility of biosecurity measure
In large‐scale integrated commercial operations, biosecurity programmes are a usual part of business. However, in other production systems, such as outdoor rearing, implementation of biosecurity measures is more challenging and only limited elements in practice can be applied. The introduction of LPAIV almost always can be attributed to failures in application of biosecurity measures or direct contact with wild birds or their faeces. In reality, when the disease threat is perceived to increase, measures are strengthened but may be relaxed in lower risk periods.
3.1.4.5. Article 7(d)(v) Restrictions on the movement of animals and products
Availability
Parameter 1 – Available movement restriction measures
Effectiveness
Parameter 2 – Effectiveness of restriction of animal movement in preventing the between farm spread
Proven in EU; when applied promptly and coupled with early reporting, disease spread is mitigated. Between‐farm spread of LPAI is generally low in the absence of control measures, dependent on the species, e.g. duck sector.
Feasibility
Parameter 3 – Feasibility of restriction of animal movement
Appropriate veterinary infrastructure is in place in the MSs to comply. The implementation of movement restrictions is feasible because risk‐based derogations are permitted.
3.1.4.6. Article 7(d)(vi) Killing of animals
Availability
Parameter 1 – Available methods for killing animals
Controlled through Regulation (EC) No 1099/20099 under which the Competent Authority can derogate the method of killing ‘where it considers that compliance is likely to affect human health or significantly slow down the process of eradication of a disease’. Multiple methods are available principally on site to include gassing in containers and use of anoxic foam on site or through safe transport to slaughterhouse where a veterinary risk assessment deems the risk of spreading the virus to be very low. LPAI killed birds, provided that they are properly labelled, are fit for entering the food chain.
Effectiveness
Parameter 2 – Effectiveness of killing animals (at farm level or within the farm) for reducing/stopping spread of the disease
It is highly effective to kill animals on site, and mitigating the risk for transport of animals away from infected premise contributes to reducing or stopping disease spread, which needs to be done in a controlled environment to avoid aerosols and local windborne spread of virus. Attention has to be paid to secure the transportation of dead animals.
Feasibility
Parameter 3 – Feasibility of killing animals
Logistical challenges and R0 values will depend on speed of slaughter; to reduce risk for a fast‐spreading outbreak‐infected flocks must be culled quickly, ideally within 48 hours of diagnosis. Logistical challenges in some operational settings, such as outdoor production, where for moderately small (juvenile) populations manual neck dislocation is an option. Contingency plans are required under Regulation (EC) No 1099/2009. These plans need to factor in animal welfare (humane culling methods), public health risk (by not killing out fast and use methods to reduce exposure) and personnel safety, practicality of measures applied and speed and all should be under official veterinary supervision.
3.1.4.7. Article 7(d)(vii) Disposal of carcasses and other relevant animal by‐products
Availability
Parameter 1 – Available disposal option
Disposal options for poultry carcases and associated wastes are: commercial fixed plant incineration, rendering (category 1 and 2 Animal By‐Product Regulation approved), permitted commercial landfill sites.
Effectiveness
Parameter 2 – Effectiveness of disposal option
Incineration and rendering are closed systems that produce an effective inactivation of LPAIV. Landfill may not inactivate all pathogens but could be used only for non‐infected carcases.
Feasibility
Parameter 3 – Feasibility of disposal option
Operational protocols for use of incineration, rendering and permitted landfill have been successfully utilised in a number of exotic avian disease outbreaks.
3.1.5. Article 7(e) The impact of disease prevention and control measures
3.1.5.1. Article 7(e)(i) The direct and indirect costs for the affected sectors and the economy as a whole
Parameter 1 – Cost of control (e.g. treatment/vaccine, biosecurity)
This is difficult to quantify, but significant costs are linked with the implementation of biosecurity. Nevertheless, the industry would need to deploy for good practice regardless of LPAI threat.
Parameter 2 – Cost of eradication (culling, compensation)
The economic burden for five successive LPAI epidemics in Italy was estimated €143 million (€105 million in direct losses and €38 million in consequential losses) (Sartore et al., 2010). There were no other data identified regarding LPAI culling and/or compensation. Eradication of LPAI is not an objective.
Parameter 3 – Cost of surveillance and monitoring
Since 2003, MS have the obligation (Council Directive 2005/94/EC) to carry out surveillance programmes in poultry aiming to detect LPAI H5 and H7 viruses which have the potential to mutate to HPAI. Based on MS' programmes submitted for EU co‐financing, a cost of approximately €4.3 million per annum is estimated (based on last 3 years). The cost of a surveillance programme is also dependent on its design (Rutten et al., 2012). LPAI surveillance in wild birds is not performed.
Parameter 4 – Trade loss (bans, embargoes, sanctions) by animal product
Information not logged with Commission services. No relevant data could be identified.
Parameter 5 – Importance of the disease for the affected sector (% loss or euro lost compared to business amount of the sector
It is difficult to estimate the loss for the sector in the EU since the loss is different between MS and one needs to differentiate between the trade in hatching eggs and day‐old chicks that may be differentiated too as there are chicks at different stages from great grandparent, parent and broiler.
3.1.5.2. Article 7(e)(ii) The societal acceptance of disease prevention and control measures
There is little information on case studies relating to LPAI more conversely with HPAI, however, culling of affected flocks raises societal issues and a perceived lack of vaccination to control. In an EU context, there are very limited episodes of extensive LPAI spread and these primarily occurred before formal notification and control tools were implemented. Some pressure has come from welfare sectors to quarantine‐infected birds until infection is resolved. Possibilities do exist, but the rationale for control of LPAI by culling is to reduce risk of virus mutation to the more impactful HPAI.
3.1.5.3. Article 7(e)(iii) The welfare of affected subpopulations of kept and wild animals
Parameter 1 – Welfare impact of control measures on domestic animals
For every outbreak, an assessment will be made when putting restrictions in place that will consider animal welfare implications; where animal welfare is compromised, and there is no alternative, culling will be considered as a mechanism to prevent animal suffering. Movement restriction can lead to overcrowding, i.e. broilers but derogations are available to alleviate this on welfare grounds without overt disease risk. As part of the mechanism to set up biosecurity measures EU MS's factor animal welfare considerations, i.e. confining some ducks indoors is unacceptable and options exist to permit some outdoor access by use of netting (OIE, 2017).
Parameter 2 – Wildlife depopulation as control measure
It is not practised.
3.1.5.4. Article 7(e)(iv) The environment and biodiversity
Environment
Parameter 1 – Use and potential residuals of biocides or medical drugs in environmental compartments (soil, water, feed, manure)
They are not used.
Biodiversity
Parameter 2 – Mortality in wild species
LPAI infections do not generally cause major clinical signs, nor do they cause mortality in wild birds (Ferreira et al., 2010; Bertran et al., 2012; Kuiken, 2013).
3.2. Assessment according to Article 5 criteria
This section presents the results of the expert judgement on the criteria of Article 5 of the AHL about LPAI (Table 14). The expert judgement was based on Individual and Collective Behavioural Aggregation (ICBA) approach described in detail in the opinion on the methodology (EFSA AHAW Panel, 2017a,b). Experts have been provided with information of the disease factsheet mapped into Article 5 criteria (see supporting information, Annex A), based on that the experts indicate their Y/N or ‘na’ judgement on each criterion of Article 5, and the reasoning supporting their judgement. As from the legal text of the AHL, a disease is considered eligible to be listed as laid down in Article 5 if it fulfils all criteria of the first set from A(i) to A(v) and at least one of the second set of criteria from B(i) to B(v). According to the assessment methodology (EFSA AHAW Panel, 2017a,b), a criterion is considered fulfilled when the outcome is ‘Yes’.
Table 14.
Outcome of the expert judgement on the Article 5 criteria for LPAI
|
Criteria to be met by the disease: According to AHL, a disease shall be included in the list referred to in point (b) of paragraph 1 of Article 5 if it has been assessed in accordance with Article 7 and meets all of the following criteria |
Final outcome | |
| A(i) | The disease is transmissible | Y |
| A(ii) | Animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union | Y |
| A(iii) | The disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character | Y |
| A(iv) | Diagnostic tools are available for the disease | Y |
| A(v) | Risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union | Y |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point A(i)–A(v), the disease needs to fulfil at least one of the following criteria | ||
| B(i) | The disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character | Y |
| B(ii) | The disease agent has developed resistance to treatments and poses a significant danger to public and/or animal health in the Union | NC |
| B(iii) | The disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union | Y |
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | N |
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | N |
Colour code: green = consensus (Yes/No); yellow = no consensus (NC)
The minimum number of judges in the judgement was 10. The expert judgement was conducted as described in the methodological opinion (EFSA AHAW Panel, 2017a,b). For details on the interpretation of the questions, see Appendix B of the methodological opinion (EFSA AHAW Panel, 2017a,b).
3.2.1. Non‐consensus questions
This section displays the assessment related to each criterion of Article 5 where no consensus was achieved in form of tables (Table 15). The proportion of Y, N or na answers are reported, followed by the list of different supporting views for each answer.
Table 15.
Outcome of the expert judgement related to criterion 5 B(ii)
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| B(ii) | The disease agent has developed resistance to treatments and poses a significant danger to public and/or animal health in the Union | NC | 30 | 70 | 0 |
NC: non‐consensus; number of judges: 10.
Reasoning supporting the judgement
supporting yes:
It is entirely plausible that resistance will increase, posing increasing dangers to public and/or animal health, extrapolating from broader AMR experiences, problems of resistance will increase.
Resistance has been demonstrated in humans.
The illegal use of antivirals could possibly cause resistance.
Resistance has been demonstrated in Egypt and China.
supporting no:
Resistance occurrence has been reported on two occasions with probable re‐assortment, but this seems not relevant for the EU.
Possible resistance does not pose a significant danger to public health because symptoms in humans are usually mild and often not treated.
There is no treatment in poultry in the EU. It is not indicated and too expensive.
Resistance has only been described in human strains.
3.2.2. Outcome of the assessment of LPAI according to criteria of Article 5(3) of the AHL on its eligibility to be listed
As from the legal text of the AHL, a disease is considered eligible to be listed as laid down in Article 5 if it fulfils all criteria of the first set from A(i) to A(v) and at least one of the second set of criteria from B(i) to B(v). According to the assessment methodology (EFSA AHAW Panel, 2017a,b), a criterion is considered fulfilled when the outcome is ‘yes’. According to the results shown in Table 14, LPAI complies with all criteria of the first set and with two criteria of the second set, therefore it is considered eligible to be listed as laid down in Article 5 of the AHL.
3.3. Assessment according to Article 9 criteria
This section presents the results of the expert judgement on the criteria of Annex IV referring to categories as in Article 9 of the AHL about LPAI (Tables 16–20). The expert judgement was based on ICBA approach described in detail in the opinion on the methodology. Experts have been provided with information of the disease factsheet mapped into Article 9 criteria (see supporting information, Annex A), based on that the experts indicate their Y/N or ‘na’ judgement on each criterion of Article 9, and the reasoning supporting their judgement. The minimum number of judges in the judgement was 10. The expert judgement was conducted as described in the methodological opinion (EFSA AHAW Panel, 2017a,b). For details on the interpretation of the questions, see Appendix B of the methodological opinion (EFSA AHAW Panel, 2017a,b).
Table 16.
Outcome of the expert judgement related to the criteria of section 1 of Annex IV (category A of Article 9) for LPAI (CI = current impact; PI = potential impact)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Final outcome | |
| 1 | The disease is not present in the territory of the Union OR present only in exceptional cases (irregular introductions) OR present in only in a very limited part of the territory of the Union | N |
| 2.1 | The disease is highly transmissible | N |
| 2.2 | There be possibilities of airborne or waterborne or vector‐borne spread | Y |
| 2.3 | The disease affects multiple species of kept and wild animals OR single species of kept animals of economic importance | Y |
| 2.4 | The disease may result in high morbidity and significant mortality rates | N |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, including epidemic or pandemic potential OR possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 4(PI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(d) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
Colour code: green = consensus (Yes/No)
Table 20.
Outcome of the expert judgement related to the criteria of section 5 of Annex IV (category E of Article 9) for LPAI
| Diseases in category E need to fulfil criteria of section 1, 2 or 3 of Annex IV of AHL and/or the following: | Final outcome | |
| E |
Surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment (If a disease fulfils the criteria as in Article 5, thus being eligible to be listed, consequently category E would apply.) |
Y |
Colour code: green = consensus (Yes/No)
Table 17.
Outcome of the expert judgement related to the criteria of section 2 of Annex IV (category B of Article 9) for LPAI (CI = current impact; PI = potential impact)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Final outcome | |
| 1 | The disease is present in the whole OR part of the Union territory with an endemic character AND (at the same time) several Member States or zones of the Union are free of the disease | N |
| 2.1 | The disease is moderately to highly transmissible | Y |
| 2.2 | There be possibilities of airborne or waterborne or vector‐borne spread | Y |
| 2.3 | The disease affects single or multiple species | Y |
| 2.4 | The disease may result in high morbidity with in general low mortality | Y |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, including epidemic potential OR possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 4(PI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(d) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
Colour code: green = consensus (Yes/No)
Table 18.
Outcome of the expert judgement related to the criteria of section 3 of Annex IV (category C of Article 9) for LPAI (CI = current impact; PI = potential impact)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Final outcome | |
| 1 | The disease is present in the whole OR part of the Union territory with an endemic character | Y |
| 2.1 | The disease is moderately to highly transmissible | Y |
| 2.2 | The disease is transmitted mainly by direct or indirect transmission | Y |
| 2.3 | The disease affects single or multiple species | Y |
| 2.4 | The disease usually does not result in high morbidity and has negligible or no mortality AND often the most observed effect of the disease is production loss | Y |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, or possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems | N |
| 4(PI) | The disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems | Y |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(d) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
Colour code: green = consensus (Yes/No)
Table 19.
Outcome of the expert judgement related to the criteria of section 4 of Annex IV (category D of Article 9) for LPAI
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Final outcome | |
| D | The risk posed by the disease in question can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread | N |
| Criteria of section 1, 2, 3 or 5 of Annex IV of AHL | Y | |
Colour code: green = consensus (Yes/No)
3.3.1. Outcome of the assessment of criteria in Annex IV for LPAI for the purpose of categorisation as in Article 9 of the AHL
As from the legal text of the AHL, a disease is considered fitting in a certain category (A, B, C, D or E corresponding to point (a) to point (e) of Article 9(1) of the AHL) if it is eligible to be listed for Union intervention as laid down in Article 5(3) and fulfils all criteria of the first set from 1 to 2.4 and at least one of the second set of criteria from 3 to 5(d) as shown in Tables 16–20. According to the assessment methodology (EFSA AHAW Panel, 2017a,b), a criterion is considered fulfilled when the outcome is ‘yes’. With respect to different type of impact where the assessment is divided into current and potential impact, a criterion will be considered fulfilled if at least one of the two outcomes is ‘Y’ and, in case of no ‘Y’, the assessment is inconclusive if at least one outcome is ‘NC’.
A description of the outcome of the assessment of criteria in Annex IV for LPAI for the purpose of categorisation as in Article 9 of the AHL is presented in Table 21.
Table 21.
Outcome of the assessment of criteria in Annex IV for LPAI for the purpose of categorisation as in Article 9 of the AHL (CI = current impact; PI = potential impact)
| Category | Article 9 criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° set of criteria | 2° set of criteria | ||||||||||
| 1 | 2.1 | 2.2 | 2.3 | 2.4 | 3 | 4 | 5a | 5b | 5c | 5d | |
| Geographical distribution | Transmissibility | Routes of transmission | Multiple species | Morbidity and mortality | Zoonotic potential | Impact on economy | Impact on society | Impact on animal welfare | Impact on environment | Impact on biodiversity | |
| A | N | N | Y | Y | N | N | N | N | N | N | N |
| B | N | Y | Y | Y | Y | N | N | N | N | N | N |
| C | Y | Y | Y | Y | Y | N |
CI: N PI: Y |
N | N | N | N |
| D | N | ||||||||||
| E | Y | ||||||||||
According to the assessment here performed, LPAI complies with the following criteria of the sections 1 to 5 of Annex IV of the AHL for the application of the disease prevention and control rules referred to in points (a) to (e) of Article 9(1):
To be assigned to category A, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment LPAI complies with criteria 2.2 and 2.3 but does not comply with criteria 1, 2.1 and 2.4. To be eligible for category A, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and LPAI does not comply with any of them.
To be assigned to category B, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment LPAI complies with criteria 2.1, 2.2, 2.3 and 2.4 but does not comply with criterion 1. To be eligible for category A, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and LPAI does not comply with any of them.
To be assigned to category C, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment LPAI complies with all. To be eligible for category A, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and LPAI complies with criterion 4 and does not comply with criteria 3, 5a–5d.
To be assigned to category D, a disease needs to comply with criteria of section 1, 2, 3 or 5 of Annex IV of the AHL, which LPAI complies with, and with the specific criterion D of section 4, which LPAI does not comply with.
To be assigned to category E, a disease needs to comply with criteria of section 1, 2 or 3 of Annex IV of the AHL and/or the surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment. The latter is applicable if a disease fulfils the criteria as in Article 5, which LPAI complies with.
3.4. Assessment of Article 8
This section presents the results of the assessment on the criteria of Article 8(3) of the AHL about LPAI. The Article 8(3) criteria are about animal species to be listed, as it reads below:
‘3. Animal species or groups of animal species shall be added to this list if they are affected or if they pose a risk for the spread of a specific listed disease because:
they are susceptible for a specific listed disease or scientific evidence indicates that such susceptibility is likely; or
they are vector species or reservoirs for that disease, or scientific evidence indicates that such role is likely.'
For this reason, the assessment on Article 8 criteria is based on the evidence as extrapolated from the relevant criteria of Article 7, i.e. the ones related to susceptible and reservoir species or routes of transmission, which cover also possible role of biological or mechanical vectors.10 According to the mapping, as presented in Table 5, section 3.2 of the scientific opinion on the ad hoc methodology (EFSA AHAW Panel, 2017b), the main animal species to be listed for LPAI according to the criteria of Article 8(3) of the AHL are as displayed in Table 22.
Table 22.
Animal species to be listed for LPAI according to criteria of Article 8 (source: data reported in Section 3.1.1.1)
| Class | Order | Family | Genus/Species | |
|---|---|---|---|---|
| Susceptible | Aves | Anseriformes | Anatidae |
Cygnus olor, Anas platyrhynchos, Anas acuta, Anas clypeata, Branta canadensis, Cygnus columbianus, Anas americana, Anas sponsa, Anas albifrons, Anas crecca, Anas penelope, Anas querquedula, Anas strepera, Anser anser, Anser erythropus, Anser fabalis, Aythya ferina, Aythya fuligula, Chloephaga poliocephala, Cygnus cygnus, Histrionicus histrionicus, Oxyura jamaicensis, Tadorna tadorna |
| Charadriiformes | Scolopacidae |
Arenaria interpres, Gallinago gallinago |
||
| Laridae |
Leucophaeus atricilla, Larus delawarensis, Larus ridibundus, Larus argentatus, Larus marinus |
|||
| Recurvirostridae | Recurvirostra avosetta | |||
| Gruiformes | Rallidae | Fulica atra | ||
| Pelecaniformes | Ardeidae | Nycticorax nycticorax | ||
| Suliformes | Phalacrocoracidae | Phalacrocorax carbo | ||
| Galliformes | Phasianidae |
Gallus spp., Meleagris spp., Phasianinae, Coturnix spp., Anurophasis spp., Perdicula spp., Ophrysia spp. |
||
| Odontophoridae | not specified | |||
| Numididae | not specified | |||
| Struthioniformes | Struthionidae | Struthio spp. and other ratitesa | ||
| Mammalia | Artiodactyla | Suidae | Sus spp. | |
| Perissodactyla | Equidae | Equus spp. | ||
| Carnivora | Canidae | Canis spp. | ||
| Felidae | Felis catus | |||
| Mustelidae | Not specified | |||
| Enaliarctidae | Not specified | |||
| Rodentia | Muridae |
Mus spp., Rattus spp. |
||
| Caviidae | Cavia porcellus | |||
| Primates | Not specified | |||
| Reservoir | Aves | Anseriformes | Not specified | |
| Charadriiformes | Not specified | |||
| Vectors | None | |||
Sometimes classified as a diverse group of large, flightless birds of the infraclass Palaeognathae.
4. Conclusions
TOR 1: for each of those diseases an assessment, following the criteria laid down in Article 7 of the AHL, on its eligibility of being listed for Union intervention as laid down in Article 5(3) of the AHL;
According to the assessment here performed, LPAI complies with all criteria of the first set and with two criteria of the second set and therefore can be considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL.
TOR 2a: for each of the diseases which was found eligible to be listed for Union intervention, an assessment of its compliance with each of the criteria in Annex IV to the AHL for the purpose of categorisation of diseases in accordance with Article 9 of the AHL;
According to the assessment here performed, LPAI meets the criteria as in Sections 3 and 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in points (c) and (e) of Article 9(1) of the AHL.
TOR 2b: for each of the diseases which was found eligible to be listed for Union intervention, a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL.
According to the assessment here performed, the animal species that can be considered to be listed for LPAI according to Article 8(3) of the AHL are Anseriformes and Charadriiformes as well as all domestic poultry species (chicken, turkeys and related poultry such as quail, guinea fowl and pheasant, and ostriches) as susceptible species, and mainly Anseriformes and Charadriiformes as reservoir species, as reported in Table 22 in Section 3.4 of the present document.
Abbreviations
- AI
avian influenza
- AHL
Animal Health Law
- ELISA
enzyme‐linked immunosorbent assay
- HI
Haemagglutination inhibition
- HPAI
highly pathogenic avian influenza
- HPAIV
highly pathogenic avian influenza virus
- ICBA
individual and Collective Behavioural Aggregation
- IM
intramuscular
- IVPI
intravenous pathogenicity test
- LPAI
low pathogenic avian influenza
- LPAIV
low pathogenic avian influenza virus
- PCR
polymerase chain reaction
- ToR
Terms of Reference
- C&O swabs
Cloacal and oropharyngeal swabs
- SC
Subcutaneous
Supporting information
Mapped fact‐sheet used in the individual judgement on Low Pathogenic Avian Influenza
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Verdonck F, Beltrán Beck B, Kohnle L, Morgado J and Bicout D, 2017. Scientific Opinion on the assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): low pathogenic avian influenza. EFSA Journal 2017;15(7):4891, 34 pp. 10.2903/j.efsa.2017.4891
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00599
Panel members: Dominique Bicout, Anette Bøtner, Andrew Butterworth, Paolo Calistri, Klaus Depner, Sandra Edwards, Bruno Garin‐Bastuji, Margaret Good, Christian Gortázar Schmidt, Virginie Michel, Miguel Angel Miranda, Simon More, Søren Saxmose Nielsen, Mohan Raj, Liisa Sihvonen, Hans Spoolder, Jan Arend Stegeman, Hans‐Hermann Thulke, Antonio Velarde, Preben Willeberg and Christoph Winckler.
Acknowledgements: The Panel wishes to thank Ian Brown for the support provided to this scientific output.
Adopted: 9 June 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1 (Annex): © World Organisation for Animal Health (OIE)
Notes
Directive 2005/94/EC of the European Parliament and of the Council of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC. OJ L 10, 14.1.2006, p. 16–65.
Regulation (EC) No 798/2008 of the European Parliament and of the Council of 8 August 2008 laying down a list of third countries, territories, zones or compartments from which poultry and poultry products may be imported into and transit through the Community and the veterinary certification requirements. OJ L 226, 23.8.2008, p. 1–94.
Council Directive 92/65/EEC of 13 July 1992 laying down animal health requirements governing trade in and imports into the Community of animals, semen, ova and embryos not subject to animal health requirements laid down in specific Community rules referred to in Annex A (I) to Directive 90/425/EEC. OJ L 268, 14.9.1992, p. 54–72.
Regulation (EC) No 142/2011 of the European Parliament and of the Council of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 54, 26.2.2011, p. 1–254.
Commission Decision of 4 August 2006 approving a Diagnostic Manual for avian influenza as provided for in Council Directive 2005/94/EC. OJ L 237, 31.8.2006, p. 1–27.
Regulation (EC) No 2006/437 of the European Commission of 4 August 2006 approving a diagnostic manual for avian influenza as provided for in Council Directive 2005/94/EC. OJ L 237, 31.8.2006, p. 1–27.
Council Directive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC. OJ L 10, 14.1.2006, p. 16‐65.
Regulation (EC) No 1099/2009 of the European Parliament and of the Council of 24 September 2009 on the protection of animals at the time of killing. OJ L 303, 18.11.2009, p. 1–30.
A vector is a living organism that transmits an infectious agent from an infected animal to a human or another animal. Vectors are frequently arthropods. Biological vectors may carry pathogens that can multiply within their bodies and be delivered to new hosts, usually by biting. In mechanical vectors the pathogens do not multiply within the vector, which usually remains infected for shorter time than in biological vectors.
References
- APHA (Animal & Plant Health Agency), 2015. Annual report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2014. APHA, Weybridge, United kingdom. 131 pp. Available online: http://www.academia.edu/24284623/Annual_Report_on_surveillance_for_avian_influenza_in_poultry_and_in_wild_birds_in_Member_States_of_the_European_Union_in_2014
- APHA (Animal & Plant Health Agency), 2016. Annual report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2015. APHA, Weybridge, United kingdom. 134 pp. Available online: http://ec.europa.eu/food/sites/food/files/ad_control-measures_ai_surv-rslt_pltry-wld-brds_2015.pdf
- Barral M, Alvarez V, Juste RA, Agirre I and Inchausti I, 2008. First case of highly pathogenic H5N1 avian influenza virus in Spain. Bmc Veterinary Research, 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato MS, Xu YF, Long LP, Capua I and Wan XF, 2014. Antigenic and genetic evolution of low‐pathogenicity avian influenza viruses of subtype H7N3 following heterologous vaccination. Clinical and Vaccine Immunology, 21, 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran K, Busquets N, Abad FX, García de la Fuente J, Solanes D, Cordón I, Costa T, Dolz R and Majó N, 2012. Highly (H5N1) and low (H7N2) pathogenic avian influenza virus infection in falcons via nasochoanal route and ingestion of experimentally infected prey. PLoS ONE, 7, e32107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante F, Fusaro A, Zanardello C, Patrono LV, De Nardi R, Maniero S and Terreqino C, 2014. Lethal nephrotropism of an H10N1 avian influenza virus stands out as an atypical pathotype. Veterinary Microbiology, 173, 189–200. [DOI] [PubMed] [Google Scholar]
- Briand F, Schmitz A, Ogor K, Le Prioux A, Guillou‐Cloarec C, Guillemoto C, Allee C, Le Bras M, Hirchaud E, Quenault H, Touzain F, Cherbonnel‐Pansart M, Lemaitre E, Courtillon C, Gares H, Daniel P, Fediaevsky A, Massin P, Blanchard Y, Eterradossi N, van der Werf S, Jestin V and Niqueux E, 2017. Emerging highly pathogenic H5 avian influenza viruses in France during winter 2015/16: phylogenetic analyses and markers for zoonotic potential. Eurosurveillance, 22, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Goekjian G, Poulson R, Valeika S and Stallknecht DE, 2009. Avian influenza virus in water: Infectivity is dependent on pH, salinity and temperature. Veterinary Microbiology, 136, 20–26. [DOI] [PubMed] [Google Scholar]
- Brown JD, Berghaus RD, Costa TP, Poulson R, Carter DL, Lebarbenchon C and Stallknecht DE, 2012. Intestinal excretion of a wild bird‐origin H3N8 low pathogenic avian influenza virus in mallards (Anas platyrhynchos). Journal of Wildlife Diseases, 48, 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busani L, Pozza MD, Bonfanti L, Toson M, Ferre N and Marangon S, 2007. Intervention strategies for low‐pathogenic avian influenza control in Italy. Avian Diseases, 51, 470–473. [DOI] [PubMed] [Google Scholar]
- Busquets N, Alba A, Napp S, Sanchez A, Serrano E, Rivas R, Nunez JI and Majo N, 2010. Influenza A virus subtypes in wild birds in North‐Eastern Spain (Catalonia). Virus Research, 149, 10–18. [DOI] [PubMed] [Google Scholar]
- Capua I and Marangon S, 2006. Control of avian influenza in poultry. Emerging Infectious Diseases, 12, 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capua I, Mutinelli F, Dalla Pozza M, Donatelli I, Puzelli S and Cancellotti FM, 2002. The 1999‐2000 avian influenza (H7N1) epidemic in Italy: veterinary and human health implications. Acta Tropica, 83, 7–11. [DOI] [PubMed] [Google Scholar]
- Capua I, Schmitz A, Jestin V, Koch G and Marangon S, 2009. Vaccination as a tool to combat introductions of notifiable avian influenza viruses in Europe, 2000 to 2006. Revue Scientifique Et Technique‐Office International Des Epizooties, 28, 245–259. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention), 2017. Influenza Antiviral Drug Resistance – Questions & Answers. Available online: https://www.cdc.gov/flu/about/qa/antiviralresistance.htm [Accessed: 30 May 2017]
- Cheng PKC, Leung TWC, Ho ECM, Leung PCK, Ng AYY, Lai MYY and Lim WWL, 2009. Oseltamivir‐ and amantadine‐resistant influenza viruses A (H1N1). Emerging Infectious Diseases, 15, 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes G, Welby S, Van Den Berg T, Van Der Stede Y, Dewulf J, Lambrecht B and Marché S, 2013. The impact of viral tropism and housing conditions on the transmission of three H5/H7 low pathogenic avian influenza viruses in chickens. Epidemiology & Infection, 141, 2428–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes G, Marché S, Dewulf J, van den Berg T and Lambrecht B, 2014. An experimental model to analyse the risk of introduction of a duck‐originated H5 low‐pathogenic avian influenza virus in poultry through close contact and contaminative transmission. Epidemiology and Infection, 142, 1836–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes G, Lambrecht B, Dewulf J, van den Berg T and Marché S, 2015. Extended transmission of two H5/H7 low pathogenic avian influenza viruses in chickens. Epidemiology and Infection, 143, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SP, 2011. The spread of pathogens through trade in poultry meat: overview and recent developments. Revue Scientifique Et Technique‐Office International Des Epizooties, 30, 149–164. [DOI] [PubMed] [Google Scholar]
- Comin A, Klinkenberg D, Marangon S, Toffan A and Stegeman A, 2011. Transmission dynamics of low pathogenicity avian influenza infections in Turkey flocks. PLoS ONE, 6, e26935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoust PY, Kibenge FSB, Fouchier RAM, van de Bildt MWG, van Riel D and Kuiken T, 2011. Replication of low pathogenic avian influenza virus in naturally infected mallard ducks (Anas platyrhynchos) causes no morphologic lesions. Journal of Wildlife Diseases, 47, 401–409. [DOI] [PubMed] [Google Scholar]
- Daoust PY, van de Bildt M, van Riel D, van Amerongen G, Bestebroer T, Vanderstichel R, Fouchier RAM and Kuiken T, 2013. Replication of 2 subtypes of low‐pathogenicity avian influenza virus of duck and gull origins in experimentally infected mallard ducks. Veterinary Pathology, 50, 548–559. [DOI] [PubMed] [Google Scholar]
- Discontools , 2016. Avian Influenza. Available online: http://www.discontools.eu/Diseases/Detail/37 [Accessed: 30 May 2017]
- Duncan D, Harris K, Poen M, Kowalezyk S, Fouchier R, Staubach C and Kuiken T, 2017. LPAI detection in wild birds and LPAI spread between European holdings in the period 2005‐2015. EFSA external scientific report, publication in September 2017.
- ECDC (European Centre for Disease Prevention and Control), online. Mutation of avian influenza A(H7N9): now highly pathogenic for poultry but risk of human‐to‐human transmission remains low. Available online: http://ecdc.europa.eu/en/activities/sciadvice/_layouts/forms/Review_DispForm.aspx?List=a3216f4c-f040-4f51-9f77-a96046dbfd72&ID=808 [Accessed: 30 May 2017]
- EFSA (European Food Safety Authority), 2006. Opinion of the Scientific Panel Animal Health and Welfare (AHAW) related with the Migratory Birds and their Possible Role in the Spread of Highly Pathogenic Avian Influenza. EFSA Journal 2006;4(5):357, 46 pp. 10.2903/j.efsa.2006.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Animal health and welfare aspects of avian influenza and the risk of its introduction into the EU poultry holdings ‐ Scientific opinion of the Panel on Animal Health and Welfare. EFSA Journal 2008;6(6):715, 162 pp. 10.2903/j.efsa.2008.715 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), More S, Bicout D, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Saxmose Nielsen S, Raj M, Sihvonen L, Spoolder H, Thulke HH, Velarde A, Willeberg P, Winckler C, Adlhoch C, Baldinelli F, Breed A, Brouwer A, Guillemain M, Harder T, Monne I, Roberts H, Cortinas Abrahantes J, Mosbach‐Schulz O, Verdonck F, Morgado J and Stegeman A, 2017a. Urgent request on avian influenza. EFSA Journal 2017;15(1):4687, 32 pp. 10.2903/j.efsa.2016.4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke HH, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Gervelmeyer A, Zancanaro G, Kohnle L, Morgado J and Bicout D, 2017b. Scientific opinion on an ad hoc method for the assessment on listing and categorisation of animal diseases within the framework of the Animal Health Law. EFSA Journal 2017;15(5):4783, 42 pp. 10.2903/j.efsa.2017.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Statement on Food safety considerations of novel H1N1 influenza virus infections in humans. EFSA Journal 2010;8(6):1629, 43 pp. 10.2903/j.efsa.2010.1629 [DOI] [Google Scholar]
- EMA (European Medicines Agency), 2010. Guideline on data requirements for multi‐strain dossiers for inactivated vaccines against avian influenza (AI), Bluetongue (BT) and Foot‐and‐Mouth disease (FMD). EMA, London, United Kingdom. 8 pp. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/03/WC500077950.pdf. [Google Scholar]
- EMPRES‐I (Global Animal Diseases Information System), online. LPAIV‐infected wild birds data from 2007‐2017. Available online: http://empres-i.fao.org/eipws3g/ [Accessed: 30 May 2017]
- European Commission , 2007a. Avian influenza. Available online: http://ec.europa.eu/food/animals/animal-diseases/control-measures/avian-influenza/index_en.htm. [Accessed: 30 May 2017]
- European Commission , 2007b. Final Report – Customs Working Group on Avian Influenza. Available online: http://ec.europa.eu/food/animals/docs/bips_customs_working_group_final_report.pdf [Accessed: 30 May 2017]
- FAO (Food and Agriculture Organization of the United Nations), 2009. Avian influenza vaccine producers and suppliers for poultry. FAO, Rome. 4 pp. Available online: http://www.fao.org/3/a-ai326e.pdf [Google Scholar]
- Ferreira HL, Pirlot JF, Kaspers B, Kothlow S, vanden Berg T and Lambrecht B, 2010. Development of specific enzyme‐linked immunosorbent assays to evaluate the duck immune response after experimental infection with H5N1 and H7N1 low pathogenic avian influenza viruses. Avian Diseases, 54, 660–667. [DOI] [PubMed] [Google Scholar]
- Gierak A, Bocian L and Smietanka K, 2016. Risk assessment of high pathogenicity avian influenza virus introduction into Poland via legal importation of live poultry. Avian Diseases, 60, 178–182. [DOI] [PubMed] [Google Scholar]
- Gonzales JL, van der Goot JA, Stegeman JA, Elbers AR and Koch G, 2011. Transmission between chickens of an H7N1 low pathogenic avian influenza virus isolated during the epidemic of 1999 in Italy. Veterinary Microbiology, 152, 187–190. [DOI] [PubMed] [Google Scholar]
- Gonzales JL, Elbers ARW, van der Goot JA, Bontje D, Koch G, de Wit JJ and Stegeman JA, 2012a. Using egg production data to quantify within‐flock transmission of low pathogenic avian influenza virus in commercial layer chickens. Preventive Veterinary Medicine, 107, 253–259. [DOI] [PubMed] [Google Scholar]
- Gonzales JL, Elbers AR, Bouma A, Koch G, de Wit JJ and Stegeman JA, 2012b. Transmission characteristics of low pathogenic avian influenza virus of H7N7 and H5N7 subtypes in layer chickens. Veterinary Microbiology, 155, 207–213. [DOI] [PubMed] [Google Scholar]
- Gonzales JL, Boender GJ, Elbers ARW, Stegeman JA and de Koeijer AA, 2014. Risk based surveillance for early detection of low pathogenic avian influenza outbreaks in layer chickens. Preventive Veterinary Medicine, 117, 251–259. [DOI] [PubMed] [Google Scholar]
- Jonges M, van Leuken J, Wouters I, Koch G, Meijer A and Koopmans M, 2015. Wind‐mediated spread of low‐pathogenic avian influenza virus into the environment during outbreaks at commercial poultry farms. PLoS ONE, 10, e0125401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado‐Tarifa E, Napp S, Gomez‐Pacheco JM, Fernandez‐Morente M, Jaen‐Tellez JA, Arenas A and Garcia‐Bocanegra I, 2014. Surveillance of influenza viruses in waterfowl used as decoys in Andalusia, Spain. PLoS ONE, 9(6), e98890. 10.1371/journal.pone.0098890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler SP, Berghaus RD and Stallknecht DE, 2012. Persistence of low pathogenic avian influenza viruses in filtered surface water from waterfowl habitats in Georgia, USA. Journal of Wildlife Diseases, 48, 999–1009. [DOI] [PubMed] [Google Scholar]
- Kim SM, Kim YI, Pascua PNQ and Choi YK, 2016. Avian influenza a viruses: evolution and zoonotic infection. Seminars in Respiratory and Critical Care Medicine, 37, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, 2013. Is low pathogenic avian influenza virus virulent for wild waterbirds? Proceedings of the Royal Society B: Biological Sciences, 280, 20130990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre‐Margalef N, Tolf C, Grosbois V, Avril A, Bengtsson D, Wille M, Osterhaus A, Fouchier RAM, Olsen B and Waldenstrom J, 2014. Long‐term variation in influenza A virus prevalence and subtype diversity in migratory mallards in northern Europe. Proceedings of the Royal Society B‐Biological Sciences, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Kwon JH, Park JK, Lee YN, Yuk SS, Lee JB, Park SY, Choi IS and Song CS, 2012. Characterization of low‐pathogenicity H5 and H7 Korean avian influenza viruses in chickens. Poultry Science, 91, 3086–3090. [DOI] [PubMed] [Google Scholar]
- Li J, Zu Dohna H, Anchell NL, Adams SC, Dao NT, Xing Z and Cardona CJ, 2010. Adaptation and transmission of a duck‐origin avian influenza virus in poultry species. Virus Research, 147, 40–46. [DOI] [PubMed] [Google Scholar]
- Lister SA, 2008. Chapter 4 ‐ Biosecurity in poultry management A2 ‐ Pattison, Mark. In: McMullin PF, Bradbury JM and Alexander DJ (eds). Poultry Diseases, 6th Edition. W.B. Saunders, Edinburgh. pp. 48–65. [Google Scholar]
- Loregian A, Mercorelli B, Nannetti G, Compagnin C and Palu G, 2014. Antiviral strategies against influenza virus: towards new therapeutic approaches. Cellular and Molecular Life Sciences, 71, 3659–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangon S, Bortolotti L, Capua I, Bettio M and Dalla Pozza M, 2003. Low‐pathogenicity avian influenza (LPAI) in Italy (2000‐01): Epidemiology and control. Avian Diseases, 47, 1006–1009. [DOI] [PubMed] [Google Scholar]
- Marche S and van den Berg T, 2010. Evaluation of different strategies for the use of ELISA tests as first screening tools for serologic surveillance of low pathogenic avian influenza in the Belgian poultry sector. Avian Diseases, 54, 627–631. [DOI] [PubMed] [Google Scholar]
- Marche S, Van Borm S, Lambrecht B, Houdart P and van den Berg T, 2014. Chasing notifiable avian influenza in domestic poultry: a case report of low‐pathogenic avian influenza H5 viruses in two Belgian holdings. Transboundary and Emerging Diseases, 61, 526–536. [DOI] [PubMed] [Google Scholar]
- Mondal S, Xing Z and Cardona C, 2013. A comparison of virulence of influenza A virus isolates from mallards in experimentally inoculated turkeys. Avian Diseases, 57, 790–796. [DOI] [PubMed] [Google Scholar]
- Mughini‐Gras L, Bonfanti L, Mulatti P, Monne I, Guberti V, Cordioli P and Marangon S, 2014. Environmental correlates of H5N2 low pathogenicity avian influenza outbreak heterogeneity in domestic poultry in Italy. PLoS ONE, 9, e86788. 10.1371/journal.pone.0086788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niqueux É, Picault JP, Amelot M, Allée C, Lamandé J, Guillemoto C, Pierre I, Massin P, Blot G, Briand FX, Rose N and Jestin V, 2014. Quantitative transmission characteristics of different H5 low pathogenic avian influenza viruses in Muscovy ducks. Veterinary Microbiology, 168, 78–87. [DOI] [PubMed] [Google Scholar]
- OIE (World Organization for Animal Health), 2017. OIE Terrestrial Animal Health Code (Chapter 10.4. Infection with Avian Influenza Viruses). Available online: http://www.oie.int/index.php?id=169&L=0&htmfile=chapitre_avian_influenza_viruses.htm [Accessed: 30 May 2017]
- Pantin‐Jackwood MJ, Smith DM, Wasilenko JL and Spackman E, 2012. Low Pathogenicity Avian Influenza Viruses Infect Chicken Layers by Different Routes of Inoculation. Avian Diseases, 56, 276–281. [DOI] [PubMed] [Google Scholar]
- Parker CD, Reid SM, Ball A, Cox WJ, Essen SC, Hanna A, Mahmood S, Slomka MJ, Irvine RM and Brown IH, 2012. First reported detection of a low pathogenicity avian influenza virus subtype H9 infection in domestic fowl in England. Veterinary Record, 171, 372. [DOI] [PubMed] [Google Scholar]
- Perez‐Ramirez E, Gerrikagoitia X, Barral M and Hofle U, 2010. Detection of low pathogenic avian influenza viruses in wild birds in Castilla‐La Mancha (south central Spain). Veterinary Microbiology, 146, 200–208. [DOI] [PubMed] [Google Scholar]
- Perez‐Ramirez E, Acevedo P, Allepuz A, Gerrikagoitia X, Alba A, Busquets N, Diaz‐Sanchez S, Alvarez V, Abad FX, Barral M, Majo N and Hofle U, 2012. Ecological factors driving avian influenza virus dynamics in Spanish wetland ecosystems. PLoS ONE, 7, e46418. 10.1371/journal.pone.0046418. Epub 2012 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai SPS, Pantin‐Jackwood M, Yassine HM, Saif YM and Lee CW, 2010. The high susceptibility of turkeys to influenza viruses of different origins implies their importance as potential intermediate hosts. Avian Diseases, 54, 522–526. [DOI] [PubMed] [Google Scholar]
- Post J, de Geus ED, Vervelde L, Cornelissen J and Rebel JMJ, 2013. Systemic distribution of different low pathogenic avian influenza (LPAI) viruses in chicken. Virology Journal, 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst C, Gethmann JM, Petermann HJ, Neudecker J, Jacobsen K and Conraths FJ, 2012. Low pathogenic avian influenza H7N7 in domestic poultry in Germany in 2011. Veterinary Record, 171, 624. [DOI] [PubMed] [Google Scholar]
- Reis A, Stallknecht D, Ritz C and Garcia M, 2012. Tenacity of low‐pathogenic avian influenza viruses in different types of poultry litter. Poultry Science, 91, 1745–1750. [DOI] [PubMed] [Google Scholar]
- Rutten N, Gonzales JL, Elbers ARW and Velthuis AGJ, 2012. Cost analysis of various low pathogenic avian influenza surveillance systems in the Dutch egg layer sector. PLoS ONE, 7, e33930. 10.1371/journal.pone.0033930. Epub 2012 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz RA, Essen SC, Brookes SM, Igbal M, Wood JL, Grenfell BT, McCauley JW, Brown IH and Gog JR, 2012. Quantifying transmission of highly pathogenic and low pathogenicity H7N1 avian influenza in turkeys. PLoS ONE, 7, e45059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore S, Bonfanti L, Lorenzetto M, Cecchinato M and Marangon S, 2010. The effects of control measures on the economic burden associated with epidemics of avian influenza in Italy. Poultry Science, 89, 1115–1121. [DOI] [PubMed] [Google Scholar]
- Short KR, Richard M, Verhagen JH, van Riel D, Schrauwen EJA, van den Brand JMA, Mänz B, Bodewes R and Herfst S, 2015. One health, multiple challenges: The inter‐species transmission of influenza A virus. One Health, 1, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomka MJ, Coward VJ, Banks J, Londt BZ, Brown IH, Voermans J, Koch G, Handberg KJ, Jorgensen PH, Cherbonnel‐Pansart M, Jestin V, Cattoli G, Capua I, Ejdersund A, Thoren P and Czifra G, 2007. Identification of sensitive and specific avian influenza polymerase chain reaction methods through blind ring trials organized in the European Union. Avian Diseases, 51, 227–234. [DOI] [PubMed] [Google Scholar]
- Slomka MJ, Pavlidis T, Coward VJ, Voermans J, Koch G, Hanna A, Banks J and Brown IH, 2009. Validated RealTime reverse transcriptase PCR methods for the diagnosis and pathotyping of Eurasian H7 avian influenza viruses. Influenza and Other Respiratory Viruses, 3, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomka MJ, Irvine RM, Pavlidis T, Banks J and Brown IH, 2010. Role of real‐time RT‐PCR platform technology in the diagnosis and management of notifiable avian influenza outbreaks: experiences in Great Britain. Avian Diseases, 54, 591–596. [DOI] [PubMed] [Google Scholar]
- Swayne D, 2016. Animal Influenza, 2nd Edition. Wiley‐Blackwell, New Jersey, USA. 656 pp. [Google Scholar]
- Swayne DE and Halvorson DA, 2008. Influenza. In: Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK and Swayne DE (eds). Diseases of Poultry, 12th edition. Blackwell Publishing, Iowa, USA. pp. 153–184. [Google Scholar]
- Swayne DE, Suarez DL and Sims LD, 2013. Influenza. In: Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL and Nair VL (eds). Diseases of Poultry, 13th Edition. Blackwell Publishing, Iowa, USA. pp. 181–218. [Google Scholar]
- Swieton E, Wyrostek K, Jozwiak M, Olszewska‐Tomczyk M, Domnska‐Blicharz K, Meissner W, Wlodarczyk R, Minias P, Janiszewski T, Minta Z and Smietanka K, 2017. Surveillance for avian influenza virus in wild birds in Poland, 2008‐15. Journal of Wildlife Diseases, 53, 330–338. [DOI] [PubMed] [Google Scholar]
- van der Goot JA, de Jong MC, Koch G and Van Boven M, 2003. Comparison of the transmission characteristics of low and high pathogenicity avian influenza A virus (H5N2). Epidemiology & Infection, 131, 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JH, van Dijk JGB, Vuong O, Bestebroer T, Lexmond P, Klaassen M and Fouchier RAM, 2014. Migratory birds reinforce local circulation of avian influenza viruses. PLoS ONE, 9, e112366. 10.1371/journal.pone.0112366. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2015. WHO RISK ASSESSMENT of Human infections with avian influenza A (H7N9) virus. Available online: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/RiskAssessment_H7N9_23Feb20115.pdf?ua=1 [Accessed: 30 May 2017]
- Yee KS, Cardona CJ and Carpenter TE, 2009. Transmission of low‐pathogenicity avian influenza virus of subtype H6N2 from chickens to Pekin ducks and Japanese quail (Coturnix coturnix japonica). Avian Pathology: Journal of the W.V.P.A, 38, 59–64. [DOI] [PubMed] [Google Scholar]
- Youn HN, Lee YN, Lee DH, Park JY, Yuk SS, Lee HJ, Yeo JM, Yang SY, Lee JB, Park SY, Choi IS and Song CS, 2012. Effect of intranasal administration of Lactobacillus fermentum CJL‐112 on horizontal transmission of influenza virus in chickens. Poultry Science, 91, 2517–2522. [DOI] [PubMed] [Google Scholar]
- Zaraket H, Saito R, Suzuki Y, Baranovich T, Dapat C, Caperig‐Dapat I and Suzuki H, 2010. Genetic makeup of amantadine‐resistant and oseltamivir‐resistant human influenza A/H1N1 viruses. Journal of Clinical Microbiology, 48, 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepeda C and Salman MD, 2007. Assessing the probability of the presence of low pathogenicity avian influenza virus in exported chicken meat. Avian Diseases, 51, 344–351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mapped fact‐sheet used in the individual judgement on Low Pathogenic Avian Influenza


