Abstract
In 2016, the European Food Safety Authority (EFSA) received a mandate from the European Commission to update its 2009 opinion on the appropriate age for introduction of complementary feeding of infants. In order to retrieve data on health outcomes related to the age of introduction of complementary food, a systematic literature review will be conducted in line with the EFSA guidance on the application of systematic review methodology to food and feed safety assessments to support decision making. These data will be considered together with data on the nutritional adequacy of exclusive breastfeeding or formula feeding at different ages and with data on neuromuscular, gastrointestinal and renal development which may affect the capacity of the infant to introduce non‐milk foods in the diet as the basis for the scientific assessment. This protocol serves as a basis for conducting the systematic literature search aiming to retrieve data on health outcomes related to the age of introduction of complementary food. As EFSA wished to seek advice from stakeholders on the draft protocol for the systematic literature review, the NDA Panel endorsed the draft protocol for public consultation on 1 February 2017. The consultation was open from 16 February to 23 March 2017 (5 weeks).
Keywords: infants, breastfeeding, formula feeding, complementary feeding, age of introduction, protocol, systematic review
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1275/full
1. Introduction
1.1. Background
In 2016, the European Food Safety Authority (EFSA) received a mandate from the European Commission to update its opinion on the appropriate age for introduction of complementary feeding of infants (EFSA NDA Panel, 2009).
In its 2009 opinion (EFSA NDA Panel, 2009), the Panel considered that the appropriate age for starting complementary feeding is determined by the nutritional adequacy of exclusive breastfeeding at different ages, by potential health benefits (or hazards) related to continued exclusive breastfeeding or formula feeding, including effects on development of motor, cognitive and social functions, and by the impact of early feeding on the risk of diseases in later life, particularly obesity, cardiovascular disease, diabetes mellitus, etc.
In the context of that opinion (EFSA NDA Panel, 2009) and of this protocol, ‘complementary feeding’ means the period when complementary foods are given together with either human milk or a breast‐milk substitute. ‘Complementary food’ comprises, therefore, all liquid, semisolid and solid foods other than breast milk and breast‐milk substitutes. Complementary food can be beverages, spoon‐fed food, or finger‐food. ‘Weaning’ means the time period of gradual reduction of breast‐feeding (or formula‐feeding) both with respect to frequency and to volume of milk, which starts with the first introduction of complementary food and which gradually leads to a dietary pattern customary in the infant's family during the second year of life. These definitions are in line with the definitions adopted by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) (Agostoni et al., 2008; Fewtrell et al., 2017), but differ from those of the World Health Organization (WHO), for which complementary feeding means the period when foods are given together with breast‐milk and therefore complementary food means any (liquid, semisolid and solid) food other than breast‐milk, including breast‐milk substitutes.
In order to retrieve data on health outcomes related to the age of introduction of complementary food, a systematic literature review will be conducted in line with the EFSA guidance on the ‘Application of systematic review methodology to food and feed safety assessments to support decision making’ (EFSA, 2010). These data will be considered together with data on the nutritional adequacy of exclusive breastfeeding or formula feeding at different ages and with data on neuromuscular, gastrointestinal and renal development which may affect the capacity of the infant to introduce non‐milk foods in the diet as the basis for the scientific assessment. These aspects will be addressed in a narrative way through comprehensive literature searches.
This document presents the draft protocol developed in relation to this systematic review, which in line with EFSA's Strategic Objective 1 (Prioritise public and stakeholder engagement in the process of scientific assessment), was subject to public consultation.
Section 2 has been developed by an external contractor (i.e. consortium of Pallas health research and consultancy and Wageningen University and Research) in the framework of a procurement procedure, who will also perform the systematic literature search up to (including) the stage of full text screening as part of the outsourcing project. Sections 3–5 have been developed by the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) and its Working Group (WG) on Infant Nutrition who, together with EFSA staff members, will be responsible for carrying out the remaining steps of the systematic literature review.
1.2. Objectives of the systematic literature review
The objectives of the systematic literature review are to identify, select, appraise and synthesise the evidence from human studies in healthy term infants and preterm infants (both intervention and observational studies) which investigated the health effect of the age at which complementary foods (solid or liquid foods consumed in addition to breast milk or breast‐milk substitutes) are introduced into an infant's diet. When possible, the potential impact of the type and the amount of specific foods on the effect of the age at which they are introduced into an infant's diet on the health outcome(s) (e.g. allergic manifestations) will also be addressed.
Pertinent studies are those in which the groups which are compared are alike in terms of the type of initial feeding (breast milk or breast‐milk substitutes), and in which the only important difference is the time at which complementary food is introduced into the diet of the infant. Studies which investigate the effects of breastfeeding vs formula feeding or the timing at which formula is introduced to an infant's diet are not pertinent for the task.
The health outcomes which will be considered, at least, are the following:
Overweight and obesity
Diabetes mellitus types 1 and 2
Risk factors for cardiovascular disease (e.g. blood pressure, blood cholesterol)
Coeliac disease
Allergy
Dental health
Renal function
Gastrointestinal infections (literature search to be limited to countries with infection rates in the target population similar to those in EU Member States)
Respiratory tract infections including otitis media (literature search to be limited to countries with infection rates in the target population similar to those in EU Member States)
Neuromuscular development, cognitive development and cognitive function
Growth
Body composition
Food patterns, food preferences and feeding disorders
Indicators of nutrient status.
In case during the literature search, additional health outcomes are identified which have been investigated in conjunction with the age of introduction of complementary feeding, these will be added to the list.
2. Identification of relevant studies
In order to meet the objectives of the systematic literature review, as outlined in Section 1.2, a systematic and extensive literature search will be performed according to the EFSA Guidance document (EFSA, 2010), which was developed considering the Cochrane Handbook (Higgins and Green, 2011), and will be reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement (Moher et al., 2009).
A consortium of Pallas health research and consultancy and Wageningen University and Research (WUR) developed literature search strategies, and the selection strategy, including criteria for the selection of literature based on title and abstracts, and for the selection of full‐text articles and thus for pertinent references. The contractor will conduct the literature search and the selection of pertinent studies after finalisation of the protocol.
2.1. Performing the literature search
2.1.1. Literature databases
According to the defined scope of the systematic review, the following literature databases are selected to be searched:
PubMed
Web of Science Core Collection
Cochrane Library.
2.1.2. Search strings and limits
Four search strings were composed, which can be combined for a literature database search for the research objectives:
-
A
Terms for infants
-
B
Terms for complementary feeding
-
C
Terms for introduction of complementary feeding
-
D
Terms for age of introduction of complementary feeding.
To exclude studies performed in low‐ and lower middle‐income‐countries (according to the World Bank), animal studies and non‐relevant publication types such as editorials and case‐series, specific search strings for human studies and study designs will be applied:
-
E
Terms for non‐EU low‐income and lower middle‐income countries according to the World Bank (used to exclude these)
-
F
Terms for study types and designs (used to exclude these)
-
G
Terms for animal studies (used to exclude these).
The search strings will be combined as (A AND B AND C AND D NOT (E OR F OR G)), with no additional language limits.
The PubMed limit ‘human studies’ will not be used as previous experiences have indicated that these limits may exclude relevant articles. This has been confirmed by information specialists. Animal studies, studies performed in low‐income countries or non‐pertinent study designs that still result from the database search will be excluded during the literature selection (see Section 2.2).
As living standards in the EU/EEA have changed in the past decades, the focus has shifted from deficiency diseases to chronic diseases and diseases of affluence. The search will be limited to studies published since 1990 (last 27 years). This time limit has been chosen because the majority of studies were performed after this period (Pearce et al., 2013; Qasem et al., 2015), and because relevant studies published before that date will be retrieved by hand searching (see Section 2.3).
The search strings are presented in Appendix A.
Output from the searched databases, including all indexed fields per hit (e.g. title, authors, abstract), will be exported into separate folders of Endnote™ version X7.4, allowing a count of the individual hits per database. Thereafter, duplicate articles will be removed by keeping PubMed as the main database and only adding additional new articles from Web of Science and Cochrane Library to the file. For each reference, the database of origin will be stated. The library without duplicates will be used for the selection procedure in EndNote™ (see Section 2.3).
2.2. Eligibility criteria for study selection
Inclusion and exclusion criteria to be applied during the selection phase are listed in Table 1.
Table 1.
Inclusion and exclusion criteria to be applied
| Inclusion | Exclusion | |
|---|---|---|
| Topic |
|
|
| Study design |
|
|
| Publication type | Peer‐reviewed scientific articles |
|
| Study characteristics |
|
|
| Population |
|
|
| Setting |
|
|
It should be noted that studies that include a large general population of infants might have included some infants with diseases or disorders affecting nutritional status.
Studies conducted in countries outside EU/EEA that have been categorised by the World Bank as low‐ and lower middle‐income countries in 2017 will be excluded. For studies in other countries outside EU/EEA, comparability of the study population with infants in EU/EEA will be assessed studying baseline characteristics such as proportion of children wasted or stunted, nutritional status and living conditions. A full list of countries and incomes can be downloaded from the website of the World Bank: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
2.3. Study selection process
Pertinent references will be selected by a three‐stage selection procedure by two reviewers independently from each other, using EndNote™. Inclusion and exclusion criteria applied in these steps are provided in Table 1.
Selection steps:
-
1
Screening of title and abstract: this will yield the articles that will be assessed in full text.
The EndNote™ library with unique citations (i.e. without overlap from the different databases) will be used for the first selection step. Titles and abstracts will be screened for relevance according to the inclusion criteria. Articles that do not describe information in the title or abstract relevant to the research objectives will not be selected for full text assessment. In addition, if it is obvious that the article does not meet one of the other inclusion criteria, it will be excluded. In case this cannot be determined based on the title or the abstract, the article will be assessed in more detail in full text during the second selection step.
Articles that have been excluded during screening of title and abstract will be stored in an indexed folder in EndNote™. Non‐pertinent articles are sorted in separate folders based on the criterion of exclusion (see Table 1).
-
2
Screening of full article: in this selection step, the full text articles will be assessed.
Articles will be included if the reported information is relevant based on the same inclusion and exclusion criteria as used for selection step 1. In this stage, a checklist will be used to evaluate the relevance of the full articles. The checklist comprises the specific inclusion and exclusion criteria that have been formulated for this review (see Table 1).
When a full article is excluded from the review, the criterion based on which it has been excluded will be documented and presented in an exclusion table. In this way, the selection procedure is transparent, will assure reproducibility, and provides a tool to the EFSA NDA Panel to assess the selection procedure. A separate Endnote™ library will be made containing the excluded articles together with the reasons for exclusion and information from which database/source the article was retrieved.
-
3
Screening of full article: in this selection step, duplicate publications and secondary research papers will be assessed.
Further scrutiny of the article during the screening phase might lead to exclusion. For example:
From articles presenting similar results from identical data sets, only one will be included. Usually this will be the most recently published article, except when this article presents too limited information.
Systematic reviews and meta‐analyses will be indexed in a separate Endnote folder. The reference lists will be checked for relevant articles that may have been missed with our search. If this is the case, these original articles will be included (see also under ‘Hand search’).
The screening and selection of full text articles will also be recorded using EndNote™. In this way, transparency will be maintained at all phases and a tool will be provided for the EFSA NDA Panel to retrospectively assess the selection procedure. First, a preliminary Endnote file will be provided to EFSA for reviewing. After discussing and adapting this where needed, a final Endnote™ file will be delivered.
A final list of exclusion criteria applied in all steps of the selection procedure will be reported in the final report. Furthermore, the contractor will report the exclusion reasons per reference for papers excluded during all selection steps.
The results of the different selection steps will be represented in a flow chart in the final report. In Figure 1, an example of this schematic representation of the selection procedure is shown, with an estimated number of hits and included articles for each selection step.
Figure 1.
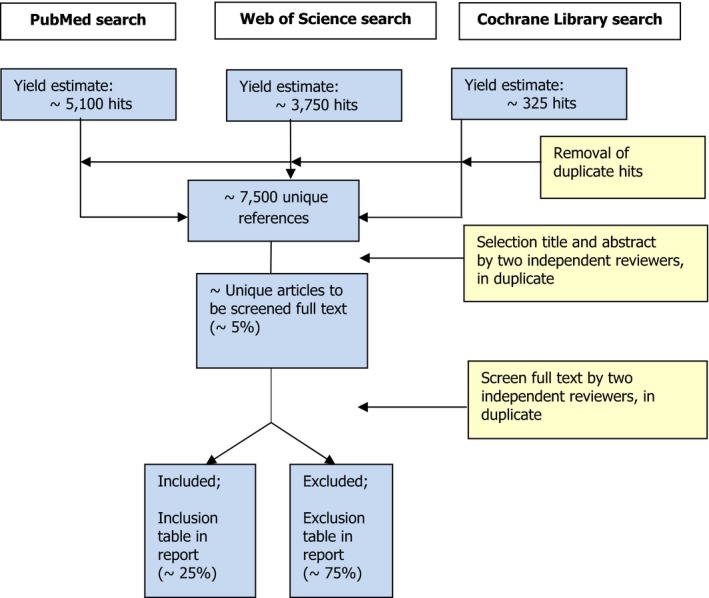
Selection procedure of papers based on number of hits in PubMed, Web of Science and the Cochrane Library
Hand search
During full text selection, the reference lists of relevant review articles and included studies will be checked for potentially relevant primary articles. With this, the contractor will update key references possibly missed by the search in the above‐mentioned bibliographic databases. Articles included via hand search will be added to the Endnote™ file in a separate folder.
2.4. Grey literature
Grey literature is defined as information produced at all levels of government, academia, business and industry in electronic and print formats not controlled by commercial publishing, i.e. ‘where publishing is not the primary activity of the producing body’. A search in grey literature can be useful for example when data from peer‐reviewed articles are inconsistent or lacking. In addition to the peer‐reviewed literature search, the contractor will therefore also perform a grey literature search in data sources that are not peer‐reviewed to find information that is relevant to the research objectives.
The following databases will be searched for grey literature:
http://www.ntis.gov: The National Technical Information Service (NTIS) provides access to the results of both US and non‐US government‐sponsored research and can provide the full text of the technical report for most of the results retrieved.
http://www.opengrey.eu: System for Information on Grey Literature in Europe, open access to 700,000 bibliographical references of grey literature (paper) produced in Europe.
CAB Abstracts: a database containing also non‐journal coverage, such as general reports, books/book chapters, conference proceedings, discussion papers and theses.
Open Access Theses and Dissertations, https://oatd.org/: a database for finding open access graduate theses and dissertations published around the world.
The search will be based on the following key‐words: complementary feeding, infant diet, infant nutrition. Date and time of the search will be recorded.
From the above‐mentioned databases, the following sources relevant for collecting data will be listed:
Scientific reports (e.g. reports from the European Commission, national Health Ministries, UNICEF or WHO)
Conference abstracts or posters or dissertations published since 2011
Non‐peer reviewed reports (e.g. reports from national health institutes, governmental documents and statistics)
Evidence‐based guidelines (see below).
For conference abstracts, posters or dissertations, a time limit will be put into place because good quality and relevant scientific data acquired before 2011 should have been published in peer‐reviewed literature.
In the collected grey literature, references will also be checked for relevant peer‐reviewed articles that may have been missed via the systematic literature search in bibliographic databases.
2.4.1. Evidence‐based guidelines
An additional search will be conducted in Google to find international and national evidence‐based guidelines on infant nutrition. The search for guidelines will at least include the website of the US National Guideline Clearinghouse. This website contains guidelines developed by organisations such as the National Institute for Health and Care Excellence (NICE) and the Scottish Intercollegiate Guidelines Network (SIGN). Also, guidelines may be available from international organisations such as the European Paediatric Association (EPA), ESPGHAN, the American Academy of Pediatrics (AAP), the United Nations Children's Fund (UNICEF) and WHO. The search terms complementary feeding, infant diet, and infant nutrition will be used to find potentially relevant guidelines. Date and time of the search will be recorded.
The AGREE (Appraisal of Guidelines for Research and Evaluation) II tool1 will be used for the quality assessment of guidelines found via the search. Inclusion or exclusion of a guideline will be based on a stepwise approach. In a first step, guidelines will be included or excluded primarily based on three main criteria from the AGREE II tool, i.e.:
The overall objective(s) of the guideline is (are) specifically described.
Systematic methods were used to search for scientific evidence, and clearly described.
The recommendations are specific and unambiguous.
In case of doubt on the quality of a guideline, a guideline will be assessed on all 23 criteria from the AGREE II tool and thereafter included or excluded.
Recommendations presented in the guidelines can be evidence‐based (EB), practice‐based (PB) or a combination of both. EB recommendations are exclusively based on the scientific literature and not on good clinical practices or expert opinions. PB recommendations are not based on scientific evidence and reflect expert opinion or information derived from good clinical practices. For this project, EB guidelines and recommendations that are a combination of EB and PB will be included. If more than one version of a guideline is available, the most comprehensive up‐to‐date version will be included.
A list will be prepared with relevant evidence‐based guidelines, focussing on infants representative of infants living in EU/EEA countries. Guidelines for malnourished or vulnerable infants will not be selected.
2.5. Quality control during selection
The following quality control measures will be put in place:
Selection of title and abstract will be executed by two reviewers independently from each other; all references will be screened in duplicate. After the first 20%, the results will be compared and discussed, and in case of persistent disagreements, references will always be included for full text assessment. In case of doubts on references covering the scope of the review, the contractor will contact EFSA for agreement.
Screening and critical appraisal of full text articles will be executed by two independent reviewers from Pallas; all full texts will be screened in duplicate. After the first 20%, the results will be compared and discussed, and this will be repeated after every 20% of screened articles during the screening process. Any disagreements will be adjudicated by a third (senior) reviewer if necessary. In case of persistent doubts, the eligibility of the article will be discussed with an advisory expert of WUR and EFSA.
3. Assessment of the internal validity (risk of bias) of the studies included
The internal validity or risk of bias (RoB) of each study included in the assessment will be appraised using a customised version of the Office of Health Assessment and Translation (OHAT) RoB tool (NTP, 2015), which is suitable for both randomised controlled trials (RCTs) and observational studies.2 The OHAT RoB tool was developed based on guidance from, for example, the Agency for Healthcare Research and Quality (Viswanathan et al., 2012, 2013), the Cochrane risk‐of‐bias tool for non‐randomised studies of interventions (Sterne et al., 2014), the Cochrane Handbook (Higgins and Green, 2011), and the CLARITY Group at McMaster University (2013). It provides a harmonised approach to the evaluation of the risk of bias in the context of hazard identification for human risk assessment of chemicals, facilitating the consideration of risk of bias across evidence streams (i.e. human, animal and mechanistic studies) with common terms and categories of risk of bias rating.
The OHAT RoB tool is structured as follows:
Risk of bias questions or domains, grouped in six types of bias (selection, confounding, performance, attrition/exclusion, detection, and selective reporting) plus ‘other sources of bias’, help identify the practices that may introduce bias (Table 2).
Each question addresses aspects relevant to specific study designs, i.e. eight questions apply to RCTs and seven questions apply to prospective studies (Table 2).
Judgements are made by applying a four‐level rating scale ((++) definitely low bias, (+) probably low bias, (−) probably high bias, or (−) definitely high bias). If there is no clear rationale for judging the likely direction of bias, the reviewers are invited to simply outline the evidence and not to attempt a guess.
Judgements to the RoB questions are combined into an overall RoB judgment for each individual study (by outcome) by using an algorithm, resulting in each study being allocated to a different ‘risk of bias tier’.
Table 2.
| Bias domains and questions | RCT | Observational |
|---|---|---|
| Selection bias | ||
| 1. Was the administered dose or exposure level adequately randomised? | X | |
| 2. Was allocation to study groups adequately concealed? | X | |
| 3. Did selection of study participants result in appropriate comparison groups? | X | |
| Confounding bias | ||
| 4. Did the study design or analysis account for important confounding and modifying variables? | X | |
| Performance bias | ||
| 5. Were the research personnel and human subjects blinded to the study during the study? | X | |
| Attrition/exclusion bias | ||
| 6. Were outcome data complete without attrition or exclusion from analysis? | X | X |
| Detection bias | ||
| 7. Can we be confident in the exposure characterisation? | X | X |
| 8. Can we be confident in the outcome assessment? | X | X |
| Selective reporting bias | ||
| 9. Were all measured outcomes reported? | X | X |
| Other sources of bias | ||
| 10. Were there no other potential threats to internal validity (e.g. statistical methods were appropriate and researchers adhered to the study protocol)? | X | X |
For this assessment, the use of the tool will be limited to the aspects relevant to RCTs and prospective observational studies in humans, and will be customised to specifically address the needs of the review to be conducted.
For each study, the appraisal will be done at the outcome level, because for the same study the design and conduct may affect RoB differently depending on the outcomes measured.
The assessment of the RoB of the studies included in the review will be done independently by two expert reviewers (i.e. WG members and EFSA senior scientific staff). If a discrepancy in the assessment occurs and cannot be resolved through discussion among the two reviewers, a third expert reviewer will be involved to facilitate decisions. In case no decision can be reached, the more conservative assessment (i.e. the assessment with the highest risk of bias) will be carried over.
Distiller SR will be used to document the ratings for each individual study.
Each study will be reported using a study ‘ID’ form, which will include the key elements of the study and a summary of the results of the RoB tool.
Aspects related to the studies’ external validity (directness, generalisability, applicability) will be addressed when considering the various sources of uncertainty at the level of the body of evidence during the scientific assessment procedure.
The assessment of the precision of a study will not be part of the risk of bias assessment, but will rather be taken into account in meta‐analyses whenever possible, or considered in the weight of evidence process.
4. Data extraction from the studies included
The data extraction will serve the purpose of summarising the results of the studies considered pertinent to the assessment and, where possible, to pool the effect estimates across individual studies, including relevant subgroup and sensitivity analyses. Data for each outcome as well as data on preterm and term infants will be extracted and considered separately.
For studies with multiple arms, only information pertaining to the relevant study arms will be extracted. In case more than two study arms are relevant, the data sheets will be adapted accordingly for the particular study in order to accommodate the information. Data will be extracted in the original units and units will be indicated in the data sheets. In case transformations are necessary in order to make the data from different studies more comparable, a separate line will be included in the data sheets in order to allow the reporting of both the original and the transformed data.
Data extraction from each article will be carried out by two reviewers (members of the WG on Infant Nutrition and EFSA staff members), one extracting the data and the other checking for accuracy and completeness (i.e. sequential review). If non‐resolvable discrepancies occur between the two reviewers, a third reviewer will be asked for an opinion. In order to have a common understanding on the information to be extracted, the data extraction exercise will be piloted on a subset of the data and the outcome will be discussed by all reviewers (NTP, 2015).
It is not planned to gather missing data from the authors of publications on a systematic basis, unless they relate to key information on a pivotal study for the assessment.
A non‐exhaustive list of the information which is proposed to be extracted from each publication is given in Table 3. This list will be adapted to the specific needs following the pilot testing.
Table 3.
Information to be included in the data extraction sheets for each study and outcome (non‐exhaustive; to be adapted)
| Study ID |
|---|
| Endpoint description |
| Age(s) at which the endpoint was assessed |
| Method by which the endpoint was assessed |
| Study design |
| Population |
| Country |
| Setting |
| Sample/group size |
| Inclusion/exclusion criteria of the study |
| Education of the mother |
| Information on feeding breast‐milk exclusively/partially and/or feeding breast‐milk substitutes |
| Duration of feeding breast‐milk exclusively/partially and/or of feeding breast‐milk substitutes |
| Timing of introduction of complementary foods |
| Type of food(s) first introduced |
| Amount of food(s) |
| Tool(s) used for data collection on complementary feeding (time of introduction, type and amount) |
| Length of follow‐up |
| N lost to follow‐up |
| Information used pertaining to the statistical analysis (test/model used, type (e.g. intention to treat, per protocol)) |
| Summary statistics (unadjusted/adjusted) |
5. Data analysis
In the context of this assessment, a high statistical heterogeneity across included studies and relevant outcomes is expected. Based on the availability of data, heterogeneity will be quantified and the relative contributions of methodological heterogeneity and of ‘clinical’ heterogeneity, if any, will be evaluated in meta‐analyses and meta‐regressions applying random‐effects models. If data do not allow for a quantitative synthesis of data, heterogeneity will be taken into account qualitatively while weighing the evidence.
5.1. Summary measures
Summary weighted mean differences (with 95% CI) for continuous variables and weighted risk ratios (with 95% CI) for dichotomous data will be calculated by pooling the study‐specific estimates using a random‐effects model, which considers both within‐study and between‐study variations.
5.2. Dealing with missing data
Since authors of original papers will not be contacted, possible missing summary data will be calculated/estimated by applying suitable approaches (e.g. standard deviations will be derived from standard errors and group size or from confidence intervals). If no indirect calculation is possible, the missing summary data will be imputed according to the approach proposed by Furukawa et al. (2006).
Sensitivity analysis to assess the impact of imputed summary data and of the inclusion of studies with high participant attrition (or with other missing data) on the overall analyses will be performed.
5.3. Assessment of heterogeneity
Heterogeneity will be identified by visual inspection of the forest plots, and by using a standard chi‐squared test and a significance level of α = 0.1, in view of the low power of this test. Heterogeneity will be specifically examined with the I2 statistic, quantifying inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins and Thompson, 2002; Higgins et al., 2003). Where heterogeneity is found, its potential sources will be explored by examining individual studies and sub‐group characteristics.
5.4. Data checking
Various data checks will be performed. For each variable, the proportion of missing observations in relation to the meta‐analysis will be calculated; range checks will be carried out for all included variables to ensure that all values are reasonable; categorical variables will be tabulated and key variables cross‐tabulated to check for consistency.
5.5. Meta‐analyses
Random‐effects meta‐analyses of weighted mean differences and/or risk ratios will be carried out using the DerSimonian and Laird approach (DerSimonian and Laird, 1986) to synthesise the effect of age of introduction of complementary foods on relevant outcomes across studies.
5.6. Sub‐group analyses and meta‐regressions
A number of factors potentially influencing the effect of the age of introduction of complementary feeding on different outcomes have been identified a priori, both from the literature and upon feedback from the experts in the WG on Infant Nutrition.
Examples of variables which could be considered for sub‐group analyses include:
sex
type and amount of food
type of initial feeding
term and preterm infants
method of measurement of health outcome
study design
other
A selection of priority study‐level characteristics will be tested in independent subgroup analyses and/or incorporated in the meta‐regression models one at a time and in the final multivariable model.
5.7. Addressing risk of bias
The rating of individual studies in terms of risk of bias (individual dimensions and overall assessment) will be used to evaluate whether inconsistency of results can be attributed to differences in internal validity, both in meta‐analyses and meta‐regression models, for each of the study types (RCTs and observational studies).
The following approaches will be considered:
To run a sensitivity analysis and see how the response changes if studies at high risk of bias are excluded: this will be considered depending on the number of studies that fall in this category.
To run a subgroup analysis (or meta‐regression) by risk of bias level, reporting separately all level‐specific estimates; lack of a statistically significant difference between studies at high and low risk of bias should be interpreted cautiously, as meta‐regression analyses typically have low power.
In case it is not possible to formally evaluate whether the RoB may be a source of the heterogeneity observed among studies, RoB will be addressed in a qualitative (narrative) way in the evaluation of the body of evidence.
5.8. Sensitivity analyses
A number of sensitivity analyses will be carried out to evaluate whether the findings are robust to the assumptions made in the systematic review protocol and the data analyses.
There are a number of assumptions/decisions/issues provisionally identified that can potentially be tested in sensitivity analyses:
on data cleaning issues: implausible values, missing data;
on quality dimensions: incomplete follow‐up; confounding adjustments;
on analytical approaches: data imputation, cut‐off points, choice of categories.
5.9. Publication bias
Specific tests will be performed to evaluate publication bias. These tests can be one or more of the following: (a) visual inspection of funnel plots to investigate the association between study size and effect size (Light and Pillemer, 1984); (b) Egger's regression test (Egger et al., 1997; Sterne and Egger, 2005); (c) trim‐and‐fill analysis (Duval and Tweedie, 2000) following the approach as suggested by Peters et al. (2007).
6. Plans for updating the literature search
The literature search will be updated once before the release of the Scientific Opinion of the NDA Panel for public consultation, and once before the final adoption of the Opinion. The timeframe will be chosen so that both the WG on Infant Nutrition and the NDA Panel have sufficient time to review and assess the additional evidence. This will be done by EFSA staff. Databases and keywords will be those of the original search. Date limits will be defined based on the cut‐off date of the preceding search. All other steps described in the present protocol will then be applied to the retrieved studies/systematic reviews/meta‐analyses.
Abbreviations
- AGREE
Appraisal of Guidelines for Research and Evaluation
- AAP
American Academy of Pediatrics
- Distiller SR
Distiller software for systematic reviews
- EB
Evidence based
- EEA
European Economic Area
- EPA
European Paediatric Association
- ESPGHAN
European Society for Paediatric Gastroenterology, Hepatology and Nutrition
- IYCF
Infant and Young Child Feeding
- NDA Panel
EFSA's expert panel on Dietetic Products, Nutrition and Allergies
- NICE
National Institute for Health and Care Excellence
- NTIS
National Technical Information Service
- NTP
National Toxicology Program
- OHAT
Office of Health Assessment and Translation
- PB
Practice based
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RCT
Randomised controlled trial
- RoB
Risk of bias
- SIGN
Scottish Intercollegiate Guidelines Network
- UNICEF
United Nations Children's Fund (formerly United Nations International Children's Emergency Fund)
- WG
Working group
- WHO
World Health Organization
- WUR
Wageningen University and Research
Appendix A – Search strings
1.
PubMed search strings
Search strings will be combined as: (#1 AND #2 AND #3 AND #4) NOT (#5 OR #6 OR #7), with time limit 1990‐current
#1 Search string infants
“Infant”[mh:noexp] OR infan*[tiab] OR young child*[tiab] OR baby[tiab] OR babies[tiab] OR early childhood[tiab] OR weanling*[tiab] OR “first year of life”[tiab] OR “early life”[tiab]
AND
#2 Search string complementary feeding
“Infant Nutritional Physiological Phenomena”[Mesh:NoExp] OR “Infant Food”[Mesh:NoExp] OR “Weaning”[Mesh] OR wean*[tiab] OR diet[tiab] OR nutrition*[tiab] OR food*[tiab] OR feeding[tiab] OR beikost[tiab] OR “partial breast‐feeding”[tiab] OR “partial breastfeeding”[tiab] OR “non‐exclusive breast‐feeding”[tiab] OR “non‐exclusive breastfeeding”[tiab] OR “mixed breast‐feeding”[tiab] OR “mixed breastfeeding”[tiab] OR fruit*[tiab] OR vegetable*[tiab] OR cereal*[tiab] OR wheat[tiab] OR gluten[tiab] OR egg*[tiab] OR peanut*[tiab] OR fish[tiab] OR shellfish[tiab] OR porridge[tiab] OR rice[tiab] OR meat[tiab] OR bread[tiab] OR juice[tiab] OR corn[tiab] OR puree*[tiab] OR IYCF[tiab] OR solid*[tiab] OR “spoon‐fed”[tiab] OR “spoonfed”[tiab] OR meal*[tiab]
AND
#3 Search string introduction
introduction[tiab] OR introduce*[tiab] OR introducing[tiab] OR start*[tiab] OR beginning[tiab] OR milestone*[tiab]
AND
#4 Search string age of introduction
time[tiab] OR timing[tiab] OR moment[tiab] OR duration[tiab] OR age[tiab] OR month[tiab] OR months[tiab] OR early[tiab] OR week[tiab] OR weeks[tiab] OR year[tiab] OR years[tiab] OR day[tiab] OR days[tiab]
NOT
#5 Search string excluding letters, editorials, comments, case reports (combine with NOT)
Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Clinical Conference[ptyp] OR pubmed books[filter] OR Comment[sb] OR “Cross‐Sectional Studies”[Mesh] OR “Retrospective studies”[Mesh] OR “retrospective cohort”[tiab] OR “retrospective analysis”[tiab]
#6 Search string animals (combine with NOT)
Animals[Mesh] NOT (Humans[Mesh] AND Animals[Mesh])
#7 Search string to exclude non‐EU low income and lower‐middle income countries according to World Bank (combine with NOT)
(Afghanistan*[tiab] OR Benin*[tiab] OR Burkina Faso[tiab] OR Burund*[tiab] OR Central African Republic[tiab] OR Republique Centrafricaine[tiab] OR Chad*[tiab] OR Comoros[tiab] OR Congo*[tiab] OR Eritrea[tiab] OR Ethiopi*[tiab] OR Gambia[tiab] OR Guinea[tiab] OR Guinean[tiab] OR Guinée[tiab] OR Guinea‐Bissau[tiab] OR Guinea Bissau[tiab] OR Guinée Bissau[tiab] OR Haiti*[tiab] OR Korea*[tiab] OR Liberia*[tiab] OR Madagascar*[tiab] OR Malawi*[tiab] OR Mali[tiab] OR Malian[tiab] OR Mozambiqu*[tiab] OR Nepal[tiab] OR Niger[tiab] OR Rwand*[tiab] OR Senegal*[tiab] OR “Sierra Leone”[tiab] OR Somali*[tiab] OR Sudan*[tiab] OR Tanzani*[tiab] OR Togo[tiab] OR Togolese[tiab] OR Ugand*[tiab] OR Zimbabw*[tiab] OR Armenia*[tiab] OR Banglades*[tiab] OR Bhutan[tiab] OR Bolivia[tiab] OR “Cabo Verde”[tiab] OR “Cape Verde”[tiab] OR Cambodia*[tiab] OR Cameroon*[tiab] OR Congo[tiab] OR “Cote D'Ivoire”[tiab] OR “Ivory Coast”[tiab] OR Djibout*[tiab] OR Egypt*[tiab] OR “El Salvador”[tiab] OR Ghana*[tiab] OR Guatemala[tiab] OR Honduras[tiab] OR India*[tiab] OR Indonesia*[tiab] OR Keny*[tiab] OR Kiribati[tiab] OR Kyrgyzstan*[tiab] OR “Kyrgyz Republic”[tiab] OR Lao*[tiab] OR Lesotho*[tiab] OR Mauritania*[tiab] OR Mauritius[tiab] OR Mauritian*[tiab] OR Micronesi*[tiab] OR Mongolia*[tiab] OR Morocc*[tiab] OR Burma[tiab] OR Myanmar[tiab] OR Nicaragua*[tiab] OR Nigeria*[tiab] OR Pakistan*[tiab] OR “Papua New Guinea”[tiab] OR Philippine*[tiab] OR Samoa[tiab] OR “São Tomé and Principe”[tiab] OR “São Tomé e Príncipe”[tiab] OR Solomon Island*[tiab] OR Sri Lanka[tiab] OR Sudan*[tiab] OR Swazi*[tiab] OR Syria*[tiab] OR Tajikistan*[tiab] OR Timor‐Leste[tiab] OR Tonga[tiab] OR Tunisia*[tiab] OR Uzbekistan*[tiab] OR Vanuatu*[tiab] OR Vietnam*[tiab] OR “West Bank”[tiab] OR Gaza[tiab] OR Yemen*[tiab] OR Zambia*[tiab]) NOT ((Afghanistan*[tiab] OR Benin*[tiab] OR Burkina Faso[tiab] OR Burund*[tiab] OR Central African Republic[tiab] OR Republique Centrafricaine[tiab] OR Chad*[tiab] OR Comoros[tiab] OR Congo*[tiab] OR Eritrea[tiab] OR Ethiopi*[tiab] OR Gambia[tiab] OR Guinea[tiab] OR Guinean[tiab] OR Guinée[tiab] OR Guinea‐Bissau[tiab] OR Guinea Bissau[tiab] OR Guinée Bissau[tiab] OR Haiti*[tiab] OR Korea*[tiab] OR Liberia*[tiab] OR Madagascar*[tiab] OR Malawi*[tiab] OR Mali[tiab] OR Malian[tiab] OR Mozambiqu*[tiab] OR Nepal[tiab] OR Niger[tiab] OR Rwand*[tiab] OR Senegal*[tiab] OR “Sierra Leone”[tiab] OR Somali*[tiab] OR Sudan*[tiab] OR Tanzani*[tiab] OR Togo[tiab] OR Togolese[tiab] OR Ugand*[tiab] OR Zimbabw*[tiab] OR Armenia*[tiab] OR Banglades*[tiab] OR Bhutan[tiab] OR Bolivia[tiab] OR “Cabo Verde”[tiab] OR “Cape Verde”[tiab] OR Cambodia*[tiab] OR Cameroon*[tiab] OR Congo[tiab] OR “Cote D'Ivoire”[tiab] OR “Ivory Coast”[tiab] OR Djibout*[tiab] OR Egypt*[tiab] OR “El Salvador”[tiab] OR Ghana*[tiab] OR Guatemala[tiab] OR Honduras[tiab] OR India*[tiab] OR Indonesia*[tiab] OR Keny*[tiab] OR Kiribati[tiab] OR Kyrgyzstan*[tiab] OR “Kyrgyz Republic”[tiab] OR Lao*[tiab] OR Lesotho*[tiab] OR Mauritania*[tiab] OR Mauritius[tiab] OR Mauritian*[tiab] OR Micronesi*[tiab] OR Mongolia*[tiab] OR Morocc*[tiab] OR Burma[tiab] OR Myanmar[tiab] OR Nicaragua*[tiab] OR Nigeria*[tiab] OR Pakistan*[tiab] OR “Papua New Guinea”[tiab] OR Philippine*[tiab] OR Samoa[tiab] OR “São Tomé and Principe”[tiab] OR “São Tomé e Príncipe”[tiab] OR Solomon Island*[tiab] OR Sri Lanka[tiab] OR Sudan*[tiab] OR Swazi*[tiab] OR Syria*[tiab] OR Tajikistan*[tiab] OR Timor‐Leste[tiab] OR Tonga[tiab] OR Tunisia*[tiab] OR Uzbekistan*[tiab] OR Vanuatu*[tiab] OR Vietnam*[tiab] OR “West Bank”[tiab] OR Gaza[tiab] OR Yemen*[tiab] OR Zambia*[tiab]) AND (Europe[MeSH] OR Europe*[tw] OR Scandinavia* [tw] OR Mediterranean[tw] OR Baltic[tw] OR Andorra*[tw] OR Azerbaijan*[tw] OR Albania*[tw] OR Armenia*[tw] OR Austria*[tw] OR Belarus*[tw] OR Byelarus*[tw] OR Bosni*[tw] OR Herzegovin*[tw] OR Croat*[tw] OR Cyprus[tw] OR Cypriot*[tw] OR Czech[tw] OR Belgi*[tw] OR Bulgaria*[tw] OR Denmark[tw] OR Danish[tw] OR Estonia*[tw] OR Finland[tw] OR Finnish[tw] OR France*[tw] OR French*[tw] OR Georgia*[tw] OR German*[tw] OR Greece[tw] OR Greek[tw] OR Hungar*[tw] OR Iceland*[tw] OR Ital*[tw] OR Sicil*[tw] OR Sardinia*[tw] OR Latvi*[tw] OR Liechtenstein*[tw] OR Lithuania*[tw] OR Luxembourg*[tw] OR Macedonia*[tw] OR Malta[tw] OR Maltese[tw] OR Moldova*[tw] OR Monaco[tw] OR Montenegr*[tw] OR Netherlands[tw] OR Dutch[tw] OR Norway[tw] OR Norwegian*[tw] or Svalbard*[tw] OR Poland*[tw] OR Polish*[tw] OR Portugal[tw] OR Portuguese[tw] OR Romania*[tw] OR Roumania*[tw] OR Rumania*[tw] OR San Marino[tw] OR Serb*[tw] OR Slovak*[tw] OR Slovenia*[tw] OR Spain*[tw] OR Spanish*[tw] OR Sweden[tw] OR Swedish[tw] OR Switzerland[tw] OR Swiss[tw] OR Great Britain*[tw] OR British*[tw] OR Channel Islands*[tw] OR Guerns*[tw] OR England*[tw] OR English*[tw] OR Hebrid*[tw] OR Ireland*[tw] OR Irish*[tw] OR Scotland*[tw] OR Scotch*[tw] OR Scottish*[tw] OR Wales*[tw] OR Welsh*[tw] OR United Kingdom*[tw] OR UK[tw] OR Gibraltar[tw] OR Ukrain*[tw] OR Vatican[tw] OR Yugoslavia*[tw]))
Web of Science search strings
Search strings will be combined as: ((#1 AND #2 AND (#3 NEAR #4)) NOT #5), with limit for document types and time limit 1990‐current
#1 Search string children
TOPIC: (infan* OR “young child*” OR baby OR babies OR “early childhood” OR weanling* OR “first year of life” OR “early life”)
AND
#2 Search string complementary feeding
TOPIC: (diet OR nutrition OR food* OR wean* OR feeding OR beikost OR IYCF OR “partial breast‐feeding” OR “partial breastfeeding” OR “non‐exclusive breast‐feeding” OR “non‐exclusive breastfeeding” OR “mixed breast‐feeding” OR “mixed breastfeeding” OR fruit* OR vegetable* OR cereal* OR wheat OR gluten OR egg* OR peanut* OR fish OR shellfish OR porridge OR rice OR meat OR bread OR juice OR corn OR puree* OR solid* OR “spoon‐fed” OR meal*)
AND
#3 Search string timing complementary feeding
TOPIC: (introduction OR introduce* OR introducing OR start OR beginning OR milestone*)
NEAR
#4 Search string age of introduction
TOPIC: (time OR timing OR moment OR duration OR age OR month OR months OR early OR week OR weeks OR year OR years OR day OR days)
Limits for Document Types (include these Types): Article OR Correction OR Database Review OR Proceedings Paper OR Reprint OR Review
NOT
#5 Search string to exclude non‐EU low income and low‐middle income countries according to World Bank (combine with NOT)
TOPIC: ((Afghanistan* OR Benin* OR Burkina Faso OR Burund* OR Central African Republic OR Republique Centrafricaine OR Chad* OR Comoros OR Congo* OR Eritrea OR Ethiopi* OR Gambia* OR Guinea OR Guinée OR Guinea‐Bissau OR Guinea Bissau OR Guinée Bissau OR Haiti* OR Korea* OR Liberia* OR Madagascar* OR Malawi* OR Mali OR Malian OR Mozambiqu* OR Nepal* OR Niger OR Rwand* OR Senegal* OR “Sierra Leone” OR Somali* OR Sudan* OR Tanzani* OR Togo OR Togolese OR Ugand* OR Zimbabw*) OR (Armenia* OR Banglades* OR Bhutan OR Bolivia OR “Cabo Verde” OR “Cape Verde” OR Cambodia* OR Cameroon* OR Congo OR “Cote D'Ivoire” OR “Ivory Coast” OR Djibout* OR Egypt* OR “El Salvador” OR Ghana* OR Guatemala OR Honduras OR India* OR Indonesia* OR Keny* OR Kiribati OR Kyrgyzstan* OR “Kyrgyz Republic” OR Lao* OR Lesotho* OR Mauritania* OR Mauritius OR Mauritian* OR Micronesi* OR Mongolia* OR Morocc* OR Burma OR Myanmar OR Nicaragua* OR Nigeria* OR Pakistan* OR “Papua New Guinea” OR Philippine* OR Samoa OR “São Tomé and Principe” OR “São Tomé e Príncipe” OR Solomon Island* OR Sri Lanka OR Sudan* OR Swazi* OR Syria* OR Tajikistan* OR Timor‐Leste OR Tonga OR Tunisia* OR Uzbekistan* OR Vanuatu* OR Vietnam* OR “West Bank” OR Gaza OR Yemen* OR Zambia*) NOT ((Afghanistan* OR Benin* OR Burkina Faso OR Burund* OR Central African Republic OR Republique Centrafricaine OR Chad* OR Comoros OR Congo* OR Eritrea OR Ethiopi* OR Gambia* OR Guinea OR Guinée OR Guinea‐Bissau OR Guinea Bissau OR Guinée Bissau OR Haiti* OR Korea* OR Liberia* OR Madagascar* OR Malawi* OR Mali OR Malian OR Mozambiqu* OR Nepal* OR Niger OR Rwand* OR Senegal* OR “Sierra Leone” OR Somali* OR Sudan* OR Tanzani* OR Togo OR Togolese OR Ugand* OR Zimbabw*) OR (Armenia* OR Banglades* OR Bhutan OR Bolivia OR “Cabo Verde” OR “Cape Verde” OR Cambodia* OR Cameroon* OR Congo OR “Cote D'Ivoire” OR “Ivory Coast” OR Djibout* OR Egypt* OR “El Salvador” OR Ghana* OR Guatemala OR Honduras OR India* OR Indonesia* OR Keny* OR Kiribati OR Kyrgyzstan* OR “Kyrgyz Republic” OR Lao* OR Lesotho* OR Mauritania* OR Mauritius OR Mauritian* OR Micronesi* OR Mongolia* OR Morocc* OR Burma OR Myanmar OR Nicaragua* OR Nigeria* OR Pakistan* OR “Papua New Guinea” OR Philippine* OR Samoa OR “São Tomé and Principe” OR “São Tomé e Príncipe” OR Solomon Island* OR Sri Lanka OR Sudan* OR Swazi* OR Syria* OR Tajikistan* OR Timor‐Leste OR Tonga OR Tunisia* OR Uzbekistan* OR Vanuatu* OR Vietnam* OR “West Bank” OR Gaza OR Yemen* OR Zambia*) AND (Europe* OR Scandinavia* OR Mediterranean OR Baltic OR Andorra* OR Azerbaijan* OR Albania* OR Armenia* OR Austria* OR Belarus* OR Byelarus* OR Bosni* OR Herzegovin* OR Croat* OR Cyprus OR Cypriot* OR Czech OR Belgi* OR Bulgaria* OR Denmark OR Danish OR Estonia* OR Finland OR Finnish OR France* OR French* OR Georgia* OR German* OR Greece OR Greek OR Hungar* OR Iceland* OR Ital* OR Sicil* OR Sardinia* OR Latvi* OR Liechtenstein* OR Lithuania* OR Luxembourg* OR Macedonia* OR Malta OR Maltese OR Moldova* OR Monaco OR Montenegr* OR Netherlands OR Dutch OR Norway OR Norwegian* or Svalbard* OR Poland* OR Polish* OR Portugal OR Portuguese OR Romania* OR Roumania* OR Rumania* OR San Marino OR Serb* OR Slovak* OR Slovenia* OR Spain* OR Spanish* OR Sweden OR Swedish OR Switzerland OR Swiss OR Great Britain* OR British* OR Channel Islands* OR Guerns* OR England* OR English* OR Hebrid* OR Ireland* OR Irish* OR Scotland* OR Scotch* OR Scottish* OR Wales* OR Welsh* OR United Kingdom* OR UK OR Gibraltar OR Ukrain* OR Vatican OR Yugoslavia*)))
Cochrane library search strings
Search strings will be combined as: (#1 AND #2 AND (#3 NEAR #4) NOT (#5), with time limit 1990‐current
#1 Search string children
[mh ^Infant] OR infan*:ti,ab OR young child*:ti,ab OR baby:ti,ab OR babies:ti,ab OR early childhood:ti,ab OR weanling*:ti,ab OR “first year of life”:ti,ab OR “early life”:ti,ab
AND
#2 Search string complementary feeding
[mh ^”Infant Nutritional Physiological Phenomena”] OR [mh ^”Infant Food”] OR [mh Weaning] OR diet:ti,ab OR nutrition:ti,ab OR food*:ti,ab OR feeding:ti,ab OR wean*:ti,ab OR beikost:ti,ab OR “partial breast‐feeding”:ti,ab OR “partial breastfeeding”:ti,ab OR “non‐exclusive breast‐feeding”:ti,ab OR “non‐exclusive breastfeeding”:ti,ab OR “mixed breast‐feeding”:ti,ab OR “mixed breastfeeding”:ti,ab OR fruit*:ti,ab OR vegetable*:ti,ab OR cereal*:ti,ab OR wheat:ti,ab OR gluten:ti,ab OR egg*:ti,ab OR peanut*:ti,ab OR fish:ti,ab OR shellfish:ti,ab OR porridge:ti,ab OR rice:ti,ab OR meat:ti,ab OR bread:ti,ab OR juice:ti,ab OR corn:ti,ab OR IYCF:ti,ab OR puree*:ti,ab OR solid*:ti,ab OR “spoon‐fed”:ti,ab OR “spoonfed”:ti,ab OR meal*:ti,ab
AND
#3 Search string timing complementary feeding
introduction:ti,ab OR introduce*:ti,ab OR introducing:ti,ab OR start:ti,ab OR beginning:ti,ab OR milestone*:ti,ab
NEAR
#4 Search string age of introduction
time:ti,ab OR timing:ti,ab OR moment:ti,ab OR duration:ti,ab OR age:ti,ab OR month:ti,ab OR months:ti,ab OR early:ti,ab OR week:ti,ab OR weeks:ti,ab OR year:ti,ab OR years:ti,ab OR day:ti,ab OR days:ti,ab
NOT
#5 Search string to exclude non‐EU low income and low‐middle income countries according to World Bank
(Afghanistan*:ti,ab OR Benin*:ti,ab OR Burkina Faso:ti,ab OR Burund*:ti,ab OR Central African Republic:ti,ab OR Republique Centrafricaine:ti,ab OR Chad*:ti,ab OR Comoros:ti,ab OR Congo*:ti,ab OR Eritrea:ti,ab OR Ethiopi*:ti,ab OR Gambia:ti,ab OR Guinea:ti,ab OR Guinean:ti,ab OR Guinée:ti,ab OR Guinea‐Bissau:ti,ab OR Guinea Bissau:ti,ab OR Guinée Bissau:ti,ab OR Haiti*:ti,ab OR Korea*:ti,ab OR Liberia*:ti,ab OR Madagascar*:ti,ab OR Malawi*:ti,ab OR Mali:ti,ab OR Malian:ti,ab OR Mozambiqu*:ti,ab OR Nepal:ti,ab OR Niger:ti,ab OR Rwand*:ti,ab OR Senegal*:ti,ab OR “Sierra Leone”:ti,ab OR Somali*:ti,ab OR Sudan*:ti,ab OR Tanzani*:ti,ab OR Togo:ti,ab OR Togolese:ti,ab OR Ugand*:ti,ab OR Zimbabw*:ti,ab OR Armenia*:ti,ab OR Banglades*:ti,ab OR Bhutan:ti,ab OR Bolivia:ti,ab OR “Cabo Verde”:ti,ab OR “Cape Verde”:ti,ab OR Cambodia*:ti,ab OR Cameroon*:ti,ab OR Congo:ti,ab OR “Cote D'Ivoire”:ti,ab OR “Ivory Coast”:ti,ab OR Djibout*:ti,ab OR Egypt*:ti,ab OR “El Salvador”:ti,ab OR Ghana*:ti,ab OR Guatemala:ti,ab OR Honduras:ti,ab OR India*:ti,ab OR Indonesia*:ti,ab OR Keny*:ti,ab OR Kiribati:ti,ab OR Kyrgyzstan*:ti,ab OR “Kyrgyz Republic”:ti,ab OR Lao*:ti,ab OR Lesotho*:ti,ab OR Mauritania*:ti,ab OR Mauritius:ti,ab OR Mauritian*:ti,ab OR Micronesi*:ti,ab OR Mongolia*:ti,ab OR Morocc*:ti,ab OR Burma:ti,ab OR Myanmar:ti,ab OR Nicaragua*:ti,ab OR Nigeria*:ti,ab OR Pakistan*:ti,ab OR “Papua New Guinea”:ti,ab OR Philippine*:ti,ab OR Samoa:ti,ab OR “São Tomé and Principe”:ti,ab OR “São Tomé e Príncipe”:ti,ab OR Solomon Island*:ti,ab OR Sri Lanka:ti,ab OR Sudan*:ti,ab OR Swazi*:ti,ab OR Syria*:ti,ab OR Tajikistan*:ti,ab OR Timor‐Leste:ti,ab OR Tonga:ti,ab OR Tunisia*:ti,ab OR Uzbekistan*:ti,ab OR Vanuatu*:ti,ab OR Vietnam*:ti,ab OR “West Bank”:ti,ab OR Gaza:ti,ab OR Yemen*:ti,ab OR Zambia*:ti,ab) NOT ((Afghanistan*:ti,ab OR Benin*:ti,ab OR Burkina Faso:ti,ab OR Burund*:ti,ab OR Central African Republic:ti,ab OR Republique Centrafricaine:ti,ab OR Chad*:ti,ab OR Comoros:ti,ab OR Congo*:ti,ab OR Eritrea:ti,ab OR Ethiopi*:ti,ab OR Gambia:ti,ab OR Guinea:ti,ab OR Guinean:ti,ab OR Guinée:ti,ab OR Guinea‐Bissau:ti,ab OR Guinea Bissau:ti,ab OR Guinée Bissau:ti,ab OR Haiti*:ti,ab OR Korea*:ti,ab OR Liberia*:ti,ab OR Madagascar*:ti,ab OR Malawi*:ti,ab OR Mali:ti,ab OR Malian:ti,ab OR Mozambiqu*:ti,ab OR Nepal:ti,ab OR Niger:ti,ab OR Rwand*:ti,ab OR Senegal*:ti,ab OR “Sierra Leone”:ti,ab OR Somali*:ti,ab OR Sudan*:ti,ab OR Tanzani*:ti,ab OR Togo:ti,ab OR Togolese:ti,ab OR Ugand*:ti,ab OR Zimbabw*:ti,ab OR Armenia*:ti,ab OR Banglades*:ti,ab OR Bhutan:ti,ab OR Bolivia:ti,ab OR “Cabo Verde”:ti,ab OR “Cape Verde”:ti,ab OR Cambodia*:ti,ab OR Cameroon*:ti,ab OR Congo:ti,ab OR “Cote D'Ivoire”:ti,ab OR “Ivory Coast”:ti,ab OR Djibout*:ti,ab OR Egypt*:ti,ab OR “El Salvador”:ti,ab OR Ghana*:ti,ab OR Guatemala:ti,ab OR Honduras:ti,ab OR India*:ti,ab OR Indonesia*:ti,ab OR Keny*:ti,ab OR Kiribati:ti,ab OR Kyrgyzstan*:ti,ab OR “Kyrgyz Republic”:ti,ab OR Lao*:ti,ab OR Lesotho*:ti,ab OR Mauritania*:ti,ab OR Mauritius:ti,ab OR Mauritian*:ti,ab OR Micronesi*:ti,ab OR Mongolia*:ti,ab OR Morocc*:ti,ab OR Burma:ti,ab OR Myanmar:ti,ab OR Nicaragua*:ti,ab OR Nigeria*:ti,ab OR Pakistan*:ti,ab OR “Papua New Guinea”:ti,ab OR Philippine*:ti,ab OR Samoa:ti,ab OR “São Tomé and Principe”:ti,ab OR “São Tomé e Príncipe”:ti,ab OR Solomon Island*:ti,ab OR Sri Lanka:ti,ab OR Sudan*:ti,ab OR Swazi*:ti,ab OR Syria*:ti,ab OR Tajikistan*:ti,ab OR Timor‐Leste:ti,ab OR Tonga:ti,ab OR Tunisia*:ti,ab OR Uzbekistan*:ti,ab OR Vanuatu*:ti,ab OR Vietnam*:ti,ab OR “West Bank”:ti,ab OR Gaza:ti,ab OR Yemen*:ti,ab OR Zambia*:ti,ab) AND (Europe*:ti,ab,kw OR Scandinavia*:ti,ab,kw OR Mediterranean:ti,ab,kw OR Baltic:ti,ab,kw OR Andorra*:ti,ab,kw OR Azerbaijan*:ti,ab,kw OR Albania*:ti,ab,kw OR Armenia*:ti,ab,kw OR Austria*:ti,ab,kw OR Belarus*:ti,ab,kw OR Byelarus*:ti,ab,kw OR Bosni*:ti,ab,kw OR Herzegovin*:ti,ab,kw OR Croat*:ti,ab,kw OR Cyprus:ti,ab,kw OR Cypriot*:ti,ab,kw OR Czech:ti,ab,kw OR Belgi*:ti,ab,kw OR Bulgaria*:ti,ab,kw OR Denmark:ti,ab,kw OR Danish:ti,ab,kw OR Estonia*:ti,ab,kw OR Finland:ti,ab,kw OR Finnish:ti,ab,kw OR France*:ti,ab,kw OR French*:ti,ab,kw OR Georgia*:ti,ab,kw OR German*:ti,ab,kw OR Greece:ti,ab,kw OR Greek:ti,ab,kw OR Hungar*:ti,ab,kw OR Iceland*:ti,ab,kw OR Ital*:ti,ab,kw OR Sicil*:ti,ab,kw OR Sardinia*:ti,ab,kw OR Latvi*:ti,ab,kw OR Liechtenstein*:ti,ab,kw OR Lithuania*:ti,ab,kw OR Luxembourg*:ti,ab,kw OR Macedonia*:ti,ab,kw OR Malta:ti,ab,kw OR Maltese:ti,ab,kw OR Moldova*:ti,ab,kw OR Monaco:ti,ab,kw OR Montenegr*:ti,ab,kw OR Netherlands:ti,ab,kw OR Dutch:ti,ab,kw OR Norway:ti,ab,kw OR Norwegian*:ti,ab,kw or Svalbard*:ti,ab,kw OR Poland*:ti,ab,kw OR Polish*:ti,ab,kw OR Portugal:ti,ab,kw OR Portuguese:ti,ab,kw OR Romania*:ti,ab,kw OR Roumania*:ti,ab,kw OR Rumania*:ti,ab,kw OR San Marino:ti,ab,kw OR Serb*:ti,ab,kw OR Slovak*:ti,ab,kw OR Slovenia*:ti,ab,kw OR Spain*:ti,ab,kw OR Spanish*:ti,ab,kw OR Sweden:ti,ab,kw OR Swedish:ti,ab,kw OR Switzerland:ti,ab,kw OR Swiss:ti,ab,kw OR Great Britain*:ti,ab,kw OR British*:ti,ab,kw OR Channel Islands*:ti,ab,kw OR Guerns*:ti,ab,kw OR England*:ti,ab,kw OR English*:ti,ab,kw OR Hebrid*:ti,ab,kw OR Ireland*:ti,ab,kw OR Irish*:ti,ab,kw OR Scotland*:ti,ab,kw OR Scotch*:ti,ab,kw OR Scottish*:ti,ab,kw OR Wales*:ti,ab,kw OR Welsh*:ti,ab,kw OR United Kingdom*:ti,ab,kw OR UK:ti,ab,kw OR Gibraltar:ti,ab,kw OR Ukrain*:ti,ab,kw OR Vatican:ti,ab,kw OR Yugoslavia*:ti,ab,kw))
Suggested citation: EFSA (European Food Safety Authority) , 2017. Statement on the protocol for a systematic review on health outcomes related to the age of introduction of complementary food for the scientific assessment of the appropriate age of introduction of complementary feeding into an infant's diet. EFSA Journal 2017;15(8):4969, 20 pp. 10.2903/j.efsa.2017.4969
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00864
Acknowledgements: EFSA wishes to thank the members of the Panel on Dietetic Products, Nutrition and Allergies (NDA): Jean‐Louis Bresson, Barbara Burlingame, Tara Dean, Susan Fairweather‐Tait, Marina Heinonen, Karen Ildico Hirsch‐Ernst, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Monika Neuhäuser‐Berthold, Grażyna Nowicka, Kristina Pentieva, Yolanda Sanz, Alfonso Siani, Anders Sjödin, Martin Stern, Daniel Tomé, Dominique Turck, Henk Van Loveren, Marco Vinceti and Peter Willatts, the members of the Working Group on Infant Nutrition: Jean‐Louis Bresson, Mary Fewtrell and Mathilde Kersting, and EFSA staff members: Irene Muñoz Guajardo, Ariane Titz and Silvia Valtueña Martínez for the support provided to this scientific output.
Approved: 19 July 2017
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1275/full
Notes
References
- Agostoni C, Decsi T, Fewtrell M, Goulet O, Kolacek S, Koletzko B, Michaelsen KF, Moreno L, Puntis J, Rigo J, Shamir R, Szajewska H, Turck D, van Goudoever J and the ESPGHAN Committee on Nutrition , 2008. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 46, 99–110. [DOI] [PubMed] [Google Scholar]
- CLARITY Group at McMaster University , 2013. Tools to assess risk of bias in cohort studies, case control studies, randomized controlled trials, and longitudinal symptom research studies aimed at the general population. Available online: https://www.evidencepartners.com/resources/methodological-resources
- DerSimonian R and Laird N, 1986. Meta‐analysis in clinical trials. Controlled Clinical Trials, 7, 177–188. [DOI] [PubMed] [Google Scholar]
- Duval S and Tweedie R, 2000. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics, 56, 455–463. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal 2010;8(6):1637, 90 pp. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2009. Scientific opinion on the appropriate age for introduction of complementary feeding of infants. EFSA Journal 2009;7(12):1423, 38 pp. 10.2903/j.efsa.2009.1423 [DOI] [Google Scholar]
- Egger M, Davey Smith G, Schneider M and Minder C, 1997. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal, 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewtrell M, Bronsky J, Campoy C, Domellöf M, Embleton N, Fidler Mis N, Hojsak I, Hulst JM, Indrio F, Lapillonne A and Molgaard C, 2017. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 64, 119–132. [DOI] [PubMed] [Google Scholar]
- Furukawa TA, Barbui C, Cipriani A, Brambilla P and Watanabe N, 2006. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology, 59, 7–10. [DOI] [PubMed] [Google Scholar]
- Higgins J and Green S, 2011. Cochrane handbook for systematic reviews of interventions – Version 5.1.0. The Cochrane Collaboration, Available online: http://www.cochrane-handbook.org
- Higgins JP and Thompson SG, 2002. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine, 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ and Altman DG, 2003. Measuring inconsistency in meta‐analyses. British Medical Journal, 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light R and Pillemer D, 1984. Summing up. The science of reviewing research. Harvard University Press, Cambridge, MA, USA. 191 pp. [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG and Group P, 2009. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine, 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), 2015. Handbook for conducting a literature‐based health assessment using OHAT approach for systematic review and evidence integration. Office of Health Assessment and Translation (OHAT), 98 pp.
- Pearce J, Taylor MA and Langley‐Evans SC, 2013. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. International Journal of Obesity, 37, 1295–1306. [DOI] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, Abrams KR and Rushton L, 2007. Performance of the trim and fill method in the presence of publication bias and between‐study heterogeneity. Statistics in Medicine, 26, 4544–4562. [DOI] [PubMed] [Google Scholar]
- Qasem W, Fenton T and Friel J, 2015. Age of introduction of first complementary feeding for infants: a systematic review. BMC Pediatrics, 15, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC and Egger M, 2005. Regression methods to detect publication and other bias in meta‐analysis In: Rothstein HR, Sutton AJ. and Borenstein M. (eds.). Publication bias in Meta‐Analysis: Prevention, Assessment, and Adjustments. Wiley, Chichester, England: pp. 99–110. [Google Scholar]
- Sterne J, Higgins J and Reeves B and the development group for ACROBATNRSI , 2014. A Cochrane risk of bias assessment tool: for non‐randomized studies of interventions (ACROBATNRSI). 56 pp.
- Viswanathan M, Ansari M, Berkman N, Chang S, Hartling L, McPheeters L, Santaguida P, Shamliyan T, Singh K, Tsertsvadze A and Treadwell J, 2012. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Agency for Healthcare Research and Quality, Methods Guide for Comparative Effectiveness Reviews. AHRQ Publication No. 12‐EHC047‐EF, 33 pp. [PubMed]
- Viswanathan M, Berkman ND, Dryden DM and Hartling L, 2013. Assessing risk of bias and confounding in observational studies of interventions or exposures: further development of the RTI item bank Agency for Healthcare Research and Quality, Methods Guide for Comparative Effectiveness Reviews. AHRQ Publication No. 13‐EHC106‐EF, 49 pp. [PubMed]


