Abstract
The present opinion deals with the re‐evaluation of konjac (E 425), comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii) when used as food additives. Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010, the Panel considered that current use of konjac (E 425) was limited in all food categories to maximum permitted level (MPL) of 10 g/kg, and that the calculated indicative refined exposure assessment for all population groups was below 0.1 mg/kg body weight (bw) per day for the general population (mean and high level). Konjac gum and konjac glucomannan were unlikely to be absorbed intact and were significantly fermented by intestinal microbiota. The available database on toxicological studies was considered limited, however, no relevant adverse effects were seen in rats and dogs in 90‐day feeding studies according to the SCF, the no‐observed‐effect level (NOEL) in rats being 1,250 mg konjac glucomannan/kg bw per day. Konjac gum and konjac glucomannan were of no concern with respect to the genotoxicity. After a daily dosage of 3,000 mg in adults for 12 weeks, several individuals experienced abdominal discomfort including diarrhoea or constipation. The Panel concluded that there was no need for a numerical acceptable daily intake (ADI) and that there was no safety concern for the general population at the refined exposure assessment for the reported uses of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as food additives under the current conditions of use of 10 g/kg. The Panel agreed with the conclusions of the SCF (1997) that the uses of konjac (E 425) as an additive at the levels up to 10 g/kg in food are acceptable, provided that the total intake from all sources stays below 3 g/day.
Keywords: konjac gum (E 425 i), konjac glucomannan (E 425 ii), konjac mannan, CAS Registry number 37220‐17‐0, food additives
Summary
The present opinion deals with the re‐evaluation of konjac (E 425), comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii) when used as food additives.
In the European Union (EU), konjac gum (E 425 i) and konjac glucomannan (E 425 ii) have been evaluated by the Scientific Committee for Food in 1996 (SCF, 1997), who could not establish an acceptable daily intake (ADI) for both of the substances. The SCF considered that ‘the uses of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as additives at the intended levels up to 10 g/kg in food are acceptable, provided that the total intake from all sources did not exceed 3 g/day'. Konjac flour (INS 425) was evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1993 and 1996 (JECFA, 1993, 1996). In 1993, the Committee allocated a temporary ADI ‘not specified’ for konjac flour. In 1996, an ADI ‘not specified’ was allocated.
The Panel noted that toxicological studies with an alginate‐konjac‐xanthan polysaccharide complex, called PGX, were available for its evaluation as novel food by the EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA NDA Panel, 2017). The EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel) did not consider results of these studies in its re‐evaluation of the individual substance konjac glucomannan (E 425 ii). It is not possible to conclude to what extent are the reported effects attributable to one of the individual components of the complex. The physicochemical properties of the individual components might also have changed during the manufacturing process of PGX.
Konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are authorised as food additives in the EU in accordance with Annex II and Annex III to Regulation (EU) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012. According to this Regulation, there are distinct specifications for konjac gum (E 425 i) and konjac glucomannan (E 425 ii). JECFA has one specification for konjac flour (INS 425). Konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are distinguished by their grade of purity. The JECFA specification for konjac flour (INS 425) covers both EU specifications.
According to Commission Regulation (EU) No 231/2012, both konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are defined as water soluble hydrocolloid obtained from konjac flour. Konjac gum is obtained by aqueous extraction, while konjac glucomannan is obtained by washing with water‐containing ethanol. In the Regulation, konjac flour is defined as the unpurified raw product from the tuber of the perennial plant Amorphophallus konjac.
The in vitro degradation and the in vivo digestibility of konjac glucomannan in animals demonstrated that this compound would not be absorbed intact or hydrolysed by digestive enzymes.
Konjac glucomannan and konjac flour can be regarded as non‐toxic based on the results of acute oral toxicity studies.
No relevant studies on short‐term and subchronic toxicity for konjac gum and konjac glucomannan are available. However, additional studies on nutritional effects are described; no relevant substance‐induced adverse effects were observed.
Based on the data available, the Panel noted that there is no concern with respect to the genotoxicity of konjac flour.
No relevant studies on chronic toxicity and carcinogenicity for konjac gum (E 425 i) and konjac glucomannan (E 425 ii) were available. The Panel noted that no adverse effects were observed in rats in a long‐term feeding trial over 18 months with 1% refined konjac meal in diet, and in mice receiving 10% of konjac glucomannan with the diet for 10 months.
No reproductive toxicity studies were available. The Panel considered that the developmental toxicity studies of Burger et al. (1992) as referred to by JECFA (1993) in cats and of Sun Tan et al. (2015) in sows were both limited and not sufficient for the evaluation of the developmental toxicity of konjac gum (E 425 i) and konjac glucomannan (E 425 ii).
From both human and animal data, the Panel considered that there was no indication for concern for immunotoxicity or allergenicity with konjac gum (E 425 i) and konjac glucomannan (E 425 ii) used as food additives.
In human studies, gastrointestinal discomfort (i.e. laxative effects, flatulence, full stomach, feeling of hungry and abdominal distension) has been reported in several clinical human studies included in two meta‐analyses. In a relevant study, a dosage of 3 g konjac glucomannan (divided in three times 1 g)/person per day corresponding to 33 mg/kg body weight (bw) per day based on mean body weight of approximately 90 kg, for 12 weeks, was associated with gastrointestinal effects (diarrhoea or constipation).
Konjac (E 425) is authorised in a wide range of foods. The Panel did not identify brand loyalty to a specific food category and therefore the Panel considered that the non‐brand‐loyal scenario covering the general population was the most appropriate and realistic scenario for risk characterisation because it is assumed that the population would probably be exposed long‐term to the food additive present at the mean reported use in processed food.
Very few reported use levels were made available to the European Food Safety Authority (EFSA). Only three food categories out of 67 were taken into account in the refined scenario. Thus the two refined exposure estimates (brand‐loyal consumer scenario and non‐brand‐loyal scenario) are similarly low (below 0.1 mg/kg bw per day in any scenario and population).
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014) and given that:
current use of konjac (E 425) was limited in all food categories to maximum permitted level (MPL) of 10 g/kg;
an indicative refined exposure assessment has been calculated: for all population groups, it was below 0.1 mg/kg bw per day for the general population (mean and high level);
konjac gum and konjac glucomannan were unlikely to be absorbed intact and were significantly fermented by intestinal microbiota;
the available database on toxicological studies was considered limited, however no relevant adverse effects were seen in rats and dogs in 90‐day feeding studies according to the SCF, and the no‐observed‐effect level (NOEL) in rats was 1,250 mg konjac glucomannan/kg bw per day;
konjac gum and konjac glucomannan would be of no concern with respect to the genotoxicity;
after a daily dosage of 3,000 mg in adults (corresponding to 33 mg/kg bw based on mean body weight of approximately 90 kg) for 12 weeks, several individuals experienced abdominal discomfort including diarrhoea or constipation,
the Panel concluded that there was no need for a numerical ADI and that there was no safety concern for the general population at the refined exposure assessment for the reported uses of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as food additives under the current conditions of use at level of 10 g/kg.
The Panel agreed with the conclusions of the SCF (1997) that the uses of konjac (E 425), comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii), as an additive at the levels up to 10 g/kg in food are acceptable, provided that the total intake from all sources does stay below 3 g/day.
The Panel recommended that the European Commission considers harmonising the microbiological specifications for polysaccharidic thickening agents, such as gums, and to include criteria for total aerobic microbial count (TAMC) and total combined yeasts and moulds count (TYMC) into the EU specifications of konjac gum (E 425 i) and konjac glucomannan (E 425 ii).
Although the Panel realised that the exposure to these additives is rather low, the Panel recommended that the European Commission considers revising the current limits for the toxic elements (lead and arsenic) in the EU specification for konjac gum (E 425 i) and konjac glucomannan (E 425 ii).
1. Introduction
The present opinion deals with the re‐evaluation of konjac (E 425), comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii) when used as food additives.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/20081 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union (EU). In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the EU before 20 January 2009 has been set up under the Regulation (EU) No 257/20102. This Regulation also foresees that food additives are re‐evaluated whenever necessary in light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU3 of 2001. The report ‘Food additives in Europe 20004’ submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010, the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks EFSA to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.1.3. Interpretation of the Terms of Reference
This re‐evaluation refers exclusively to the uses of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as food additives in food, including food supplements, and does not include a safety assessment of other uses of konjac gum and konjac glucomannan (E 425 ii) as described in Section 3.4.3.
1.2. Information on existing evaluations and authorisations
Konjac gum (E 425 i) and konjac glucomannan (E 425 ii)5 are authorised as food additives in the EU in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20126.
In the EU, konjac gum (E 425 i) and konjac glucomannan (E 425 ii) have been evaluated by the SCF in 1996 (SCF, 1997), who could not establish an acceptable daily intake (ADI) for both the substances. For konjac gum (E 425 i), the SCF (1997) noted ‘Adequate subchronic and long‐term feeding studies with this material are lacking and a no‐observed‐effect level cannot be derived. In addition, it has not been clarified to what extent the main component glucomannan is digested in the human intestine'. For konjac glucomannan (425 ii), the SCF noted that ‘it was tested adequately in 90‐day feeding studies with rats and beagle dogs. These studies did not reveal any relevant toxic effects and a no‐observed‐effect level of 2.5% glucomannan in the diet can be derived, corresponding to 1.25 g/kg body weight per day. However, a long‐term toxicity/carcinogenicity study is lacking and only gene mutation tests in bacteria were performed with a negative result. In addition, it has not been clarified to what extent the glucomannan is digested in the human intestine’. On the other hand, the SCF concluded, that ‘the existing data (including genotoxicity studies with konjac glucomannan (E 425 ii) as well as human experience did not give reason for concern. Konjac materials have a long history as traditional food in Far East countries. Apart from diarrhoea, abdominal pain and an effect on vitamin absorption after ingestion of high doses, no adverse effects of oral ingestion have been reported in humans’. The SCF considered therefore that ‘the uses of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as additives at the intended levels up to 1% in food are acceptable, provided that the total intake from all sources did not exceed 3 g/day. This upper limit should be taken into account when setting the conditions of use. The SCF noted that directive 95/2/EC included a footnote in relation to similar products which points out that these substances should not be used to produce dehydrated foodstuffs intended to rehydrate on ingestion’. The SCF considered that a similar remark would be applicable to konjac gum (E 425 i) and konjac glucomannan (E 425 ii).
Konjac flour (INS 425) was evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1993 and 1996 (JECFA, 1993, 1996). In 1993, the Committee allocated a temporary ADI ‘not specified’ for konjac flour.7 In 1996, an ADI ‘not specified’ was allocated. The evaluation was based on laboratory animal data the ‘available toxicological data from human studies, the long history of use of konjac as a food in China and Japan, and estimates of konjac flour consumption from traditional and anticipated food additive uses. The Committee stressed that its evaluation applies only to the use of konjac flour as a food additive’.
Konjac gum and konjac glucomannan have been also reviewed by the Nordic Council of Ministers (TemaNord, 2002), who concluded that for both substances there is no evidence of any toxic effect, taking into account the long history of safe use in Far Eastern countries. In the review by TemaNord, however, attention was drawn to the effects elicited by these substances on the gastrointestinal tract (diarrhoea, flatulence and slight abdominal pain) and to the fact that it would be prudent to restrict their total daily intake. For konjac gum and konjac glucomannan, the present restrictions in use should ensure that. For konjac glucomannan, the use is restricted to 10 g/kg, which should ensure compliance.
Against the background, that there have been fatal accidents, mainly in children and a few in elderly persons, in various countries inside and outside the EU, resulting from asphyxiation following the ingestion of jelly mini‐cups confectionery containing the additive E 425, the use of this additive in jelly confectionery was prohibited in the EU, by the Directive 2003/52/EC of 18 June 2003 amending Directive 95/2/EC as regards the conditions of use for a food additive E 425 konjac, because of the choking hazard.
In 2009, the EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA NDA Panel, 2009) prepared a scientific opinion on the scientific substantiation of health claims in relation to glucomannan (konjac mannan) and the maintenance of normal blood cholesterol concentrations. On the basis of the data available, the Panel concluded that ‘a cause and effect relationship has been established between the consumption of glucomannan and the reduction of blood cholesterol concentrations. In order to bear the claim, a food should provide at least 4 g/day of glucomannan in one or more servings. The target population is the general population’.
In 2010, the EFSA NDA Panel (2010) prepared another scientific opinion on the scientific substantiation of health claims in relation to glucomannan (konjac mannan) and reduction of body weight, reduction of post‐prandial glycaemic responses, maintenance of normal blood glucose concentrations, maintenance of normal (fasting) blood concentrations of triglycerides, maintenance of normal blood cholesterol concentrations, maintenance of normal bowel function and decreasing potentially pathogenic gastrointestinal microorganisms. The EFSA NDA Panel concluded that a cause and effect relationship has been established between the consumption of glucomannan and the maintenance of normal blood cholesterol concentrations and the reduction of body weight. In order to obtain the claimed effect of reduction of body weight, ‘at least 3 g of glucomannan should be consumed daily in three doses of at least 1 g each, together with 1–2 glasses of water before meals, in the context of an energy‐restricted diet. The target population is overweight adults’. A cause and effect relationship has not been established between the consumption of glucomannan and the other claimed effects.
The conditions and restrictions of use for the health claims for glucomannan (konjac mannan) to contribute to weight loss and to the maintenance of normal blood cholesterol concentrations are authorised by the Commission Regulation (EU) No 432/2012.
In 2017, the EFSA NDA Panel published a scientific opinion on an alginate‐konjac‐xanthan polysaccharide complex (PGX) in the framework of Regulation (EC) No 258/97 (EFSA NDA Panel, 2017). PGX is produced by mixing konjac glucomannan, xanthan gum and sodium alginate in a specific ratio, claimed proprietary and confidential, and then processing them by a proprietary process involving heat. Based on studies comparing different physicochemical parameters for PGX and the three individual substances, the applicant claimed that PGX is a ‘novel complex’ rather than a mixture of the three substances. The maximum daily intake of PGX from fortified foods and food supplements recommended by the applicant was 15 g/person. From a 13‐week study in Sprague–Dawley rats, which received a diet containing 0%, 1.25%, 2.5% or 5% of PGX, the EFSA NDA Panel derived a no‐observed‐adverse effect level (NOAEL) of 2.5% PGX in the diet equivalent to 1.8 g/kg body weight (bw) per day. This was based on statistically significant increases in serum activities of alanine transaminase (ALT) and aspartate transaminase (AST) in females in the high‐dose group. Considering the highest mean and 95th percentile anticipated daily intake of PGX from fortified foods, the EFSA NDA Panel derived margins of exposure (MoE) of 12 and 6. The MoE derived by the EFSA NDA Panel for PGX consumed as food supplements was 9. The EFSA NDA Panel concluded that the safety of PGX as novel food for the intended uses and use levels as proposed by the applicant has not been established.
2. Data and methodologies
2.1. Data
The EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel) was not provided with a newly submitted dossier. EFSA launched public calls for data,8 , 9 , 10 and, if relevant, contacted other scientific risk assessment bodies to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to the last Working Group meeting before the adoption of the opinion.11 Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based, however not always these were available to the Panel.
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database12) was used to estimate the dietary exposure.
The Mintel's Global New Products Database (GNPD) is an online resource listing food products and compulsory ingredient information that should be included in labelling. This database was used to verify the use of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) in food products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing Guidances from the EFSA Scientific Committee.
The ANS Panel assessed the safety of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as food additives in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the SCF (2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
When the test substances were administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee (2012) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as ‘equivalent to’. When in human studies in adults (aged above 18 years) the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012).
Dietary exposure to konjac gum (E 425 i) and konjac glucomannan (E 425 ii) from their use as food additives was estimated combining the food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum permitted levels (MPLs) and/or reported use levels and analytical data submitted to EFSA following a call for data. Different exposure scenarios were calculated (see Section 3.4.1). Uncertainties on the exposure assessment were identified and discussed with regard to their impact on the final exposure calculation.
In the context of this re‐evaluation, the Panel followed the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EC) No 257/2010 (EFSA ANS Panel, 2014).
3. Assessment
3.1. Technical data
3.1.1. Identity of the substances
According to Commission Regulation (EU) No 231/201213, both konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are defined as water‐soluble hydrocolloid obtained from konjac flour. Konjac gum is obtained by aqueous extraction, while konjac glucomannan is obtained by washing with water‐containing ethanol. In the Regulation, konjac flour is defined as the unpurified raw product from the root of the perennial plant Amorphophallus konjac. Its main component is a high‐molecular‐weight polysaccharide glucomannan which consists of d‐mannose and d‐glucose units at a molar ratio of 1.6:1.0, connected by β(1‐4)‐glycosidic bonds. Further in the definition of glucomannan from konjac gum is stated that shorter side chains are attached through β(1‐3)‐glycosidic bonds, and acetyl groups occur at random at a ratio of about 1 group per 9–19 sugar units, while in the definition of konjac glucomannan it is said that branching occur at about each 50th or 60th unit and that about each 19th sugar residue is acetylated.
The CAS registry number 37220‐17‐0 and the EINECS No 253‐404‐6 correspond to konjac mannan, a term that covers for both konjac gum and konjac glucomannan. Konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are distinguished in the definition regarding the main components (≥ 75% carbohydrate vs ≥ 95% total dietary fibre on a dry weight basis) and in molecular weight.
According to Commission Regulation (EU) No 231/2012, the average molecular weight of the polysaccharide in konjac gum is in the range of 200,000–2,000,000 and in konjac glucomannan 500,000–2,000,000. The Panel noted that the indication of the molecular weight is covering a larger range down to 200,000 for konjac gum as compared to konjac glucomannan.
Concerning impurities, konjac gum and konjac glucomannan differ in the maximum permitted content of proteins (N × 5.7) (≤ 3% vs ≤ 1.5%), starch (≤ 3% vs ≤ 1.5%), inorganic substances (total ash) (≤ 5% vs ≤ 2%) and contaminants such as lead (≤ 2% vs ≤ 1%), as well as in physical properties such as solubility and viscosity in 1% solution.
The glucomannan from konjac gum and from konjac glucomannan is composed of linear chains of (1→4)‐linked mannopyranose and glucopyranose units with varying amounts of acetyl groups. The degree of acetyl groups distribution in the konjac gum is reported to be at a ratio of about 1 group per 9–19 sugar units (Takigami, 2000; Nishinari and Gao, 2007; Parry, 2010; Commission Regulation (EU) No 231/1012). The degree of acetyl groups distribution in the konjac glucomannan is reported to be lower at about 19th sugar residue (Commission Regulation (EU) No 231/1012). Regarding the konjac gum, according to Katsuraya et al. (2003) and Nishinari and Gao (2007), shorter polysaccharide side‐chains are attached to the C6 of glucosyl units of the backbone, while according to Takigami (2000) and Commission Regulation (EU) No 231/2012, branching point is at the position 3.
The structural formula of the repeating unit of konjac glucomannan is presented in Figure 1.
Figure 1.
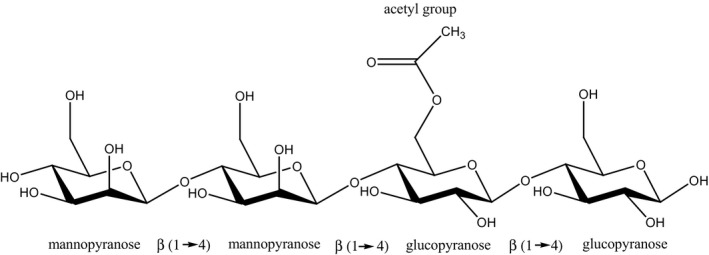
Structural formula of the repeating unit of konjac glucomannan
According to Commission Regulation (EU) No 231/2012, konjac gum (E 425 i) is a white to cream to light tan powder, while konjac glucomannan (E 425 ii) is a white to slightly brownish fine particle size, free flowing and odourless powder. Both konjac gum and konjac glucomannan are dispersible in hot or cold water forming a highly viscous solution. Solubility increases with heat and speed of agitation. pH values of the solutions of konjac gum and glucomannan are in the ranges of 4.0–7.0 and 5.0–7.0, respectively. Depending on purity, they absorb water up to 200 times of their weight. The glucomannan forms viscous, pseudoplastic dispersions. Viscosity of 1% solutions at 35°C were estimated to be in the range between 13.5 and 18.0 mPa·s (Takigami, 2000; BeMiller, 2008; Parry, 2010). An important property of konjac gum, konjac glucomannan and konjac flour in water is the physicochemical interaction with xanthan gum, k‐carrageenan and agar causing a synergistic increase in viscosity (Tako, 1992; Akesowan, 2002; Liang et al., 2011).
The Panel noted that in cases, where konjac gum (E 425 i) and konjac glucomannan (E 425 ii) is added in combination with other gums, such as xanthan gum (E 415), agar (E 406) or carrageenan (E 407), the synergistic increase in viscosity has to be taken into consideration.
Infrared (IR), 1H‐ and 13C‐nuclear magnetic resonance (NMR) spectra of konjac glucomannan are available (Takigami, 2000; Crescenzi et al., 2002; Katsuraya et al., 2003). Ultraviolet/visual (UV/VIS) and mass spectra (MS) were not identified in the literature searches in Toxline, Medline, and SciFinder.
Synonyms for konjac glucomannan are konjac mannan or glucomannan (Merck Index, 2006). However, according to JECFA (2006), konjac flour is known under the synonyms konjac mannan, konjac, konnyaku and INS No 425 (JECFA, 2006), whereas Martindale gives konjac flour and konjak mannan as synonyms for glucomannan (Martindale, 2017). The Panel noted that the use of the terms ‘konjac glucomannan’, ‘konjac mannan’ and ‘konjac flour’ in literature is not always specific and it is ambiguous. According to the EU Regulation, konjac flour is not a synonym for the food additive konjac (E 425) comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii). In this opinion, the term used by the authors of the cited studies is used.5
The Panel noted that according to the EU specifications, konjac gum (E 425 i) and konjac glucomannan (E 425 ii) originated from the plant A. konjac, while according to the JECFA specification konjac flour may also derived from other species from Amorphophallus (see Section 3.1.2).
Upon request of the Panel for information on the particle size distribution, data were provided by industry (Documentation provided to EFSA n.9) regarding the particle size of konjac gum when used as food additive. According to the submitted results of batch analysis by laser diffraction of 100 mesh commercial konjac gum (E 425 i), samples showed a mean particle size of 102.3 μm with a standard deviation of 0.08 μm. The smallest particles detected were 6.61 μm meaning no particles were found in the 0–100 nm range. According to IFAC (Documentation provided to EFSA n.9), ‘It should be noted that when used in food applications, all konjac powder particles are dissolved into solution, making mean particle size powder data meaningless with respect to the nanoscale’.
The Panel noted that the differences between the konjac gum (E 425 i) and the konjac glucomannan (E 425 ii) are based on molecular weight distributions, different degrees of acetylation and different contents of impurity in the konjac gum, leading to different physicochemical properties. This might be due to the use of different solvents to prepare the food additives.
3.1.2. Specifications
Specifications have been defined in Commission Regulation (EU) No 231/1012 and by JECFA (2006). The available specifications are listed in Table 1.
Table 1.
Commission Regulation (EU) No 231/1012 and JECFA (2006) specifications for konjac gum (E 425 i), konjac Glucomannan (E 425 ii) and konjac Flour (INS 425)
| Commission Regulation (EU) No 231/2012 konjac gum (E 425 i) | Commission Regulation (EU) No 231/2012 konjac glucomannan (E 425 ii) | JECFA (2006) konjac Flour (INS 425) | |
|---|---|---|---|
| Definition | Konjac gum is a water‐soluble hydrocolloid obtained from the konjac flour by aqueous extraction. Konjac flour is the unpurified raw product from the root of the perennial plant Amorphophallus konjac. The main component of konjac gum is the water‐soluble high‐molecular‐weight polysaccharide glucomannan, which consists of d‐mannose and d‐glucose units at a molar ratio of 1,6:1,0, connected by β(1‐4)‐glycosidic bonds. Shorter side chains are attached through β(1‐3)‐glycosidic bonds, and acetyl groups occur at random at a ratio of about 1 group per 9–19 sugar units | Konjac glucomannan is a water‐soluble hydrocolloid obtained from konjac flour by washing with water‐containing ethanol. Konjac flour is the unpurified raw product from the tuber of the perennial plant Amorphophallus konjac. The main component is the water‐soluble high‐molecular‐weight polysaccharide glucomannan, which consists of d‐mannose and d‐glucose units at a molar ratio of 1,6:1,0, connected by β(1‐4)‐glycosidic bonds with a branch at about each 50th or 60th unit. About each 19th sugar residue is acetylated | The hydrocolloidal polysaccharide obtained from the tubers of various species of Amorphophallus; principal component is a high‐molecular‐weight, slightly branched, non‐ionic glucomannan consisting of mannose and glucose, connected by β‐1,4 linkages, at a respective molar ratio of approximately 1.6‐4:1; acetyl groups along the glucomannan back‐bone contribute to solubility properties and are located, on average, every 9–19 sugar units |
| Molecular weight | The main component, glucomannan, has an average molecular weight of 200,000–2,000,000 | 500,000–2,000,000 | The main component, glucomannan, has an average molecular weight of 200,000–2,000,000 |
| Assay | Not less than 75% carbohydrate | Total dietary fibre: not less than 95% on a dry weight basis |
Not less than 75% carbohydrate The remainder, after subtracting from 100% the sum of the percentages of total ash, loss on drying and protein, represents the percentage of carbohydrate (glucomannans) in the sample |
| Description | A white to cream to light tan powder | White to slightly brownish fine particle size, free flowing and odourless powder | White or cream to light tan powder |
| Identification | |||
| Solubility | Dispersible in hot or cold water forming a highly viscous solution with a pH between 4.0 and 7.0 | Dispersible in hot or cold water forming a highly viscous solution with a pH between 5.0 and 7.0. Solubility is increased by heat and mechanical agitation |
Dispersible in hot or cold water forming a highly viscous solution with a pH between 4.0 and 7.0 Solubility is increased by heat and mechanical agitation. Addition of mild alkali to the solution results in the formation of a heat‐stable gel that resists melting, even under extended heating conditions |
| Gel formation | Add 5 mL of a 4% sodium borate solution to a 1% solution of the sample in a test tube, and shake vigorously. A gel forms | – | Add 5 mL of a 4% sodium borate solution to a 1% solution of the sample in a test tube, and shake vigorously. A gel forms |
| Formation of heat‐stable gel | Prepare a 2% solution of the sample by heating it in a boiling water bath for 30 min, with continuous agitation and then cooling the solution to room temperature. For each gram of the sample used to prepare 30 g of the 2% solution, add 1 mL of 10% potassium carbonate solution to the fully hydrated sample at ambient temperature. Heat the mixture in a water bath to 85°C, and maintain for 2 h without agitation. Under these conditions, a thermally stable gel is formed | Prepare a 2% solution of the sample by heating it in a boiling water bath for 30 min, with continuous agitation and then cooling the solution to room temperature. For each gram of the sample used to prepare 30 g of the 2% solution, add 1 mL of 10% potassium carbonate solution to the fully hydrated sample at ambient temperature. Heat the mixture in a water bath to 85°C, and maintain for 2 h without agitation. Under these conditions a thermally stable gel is formed | Prepare a 2% solution of the sample by heating it in a boiling water bath for 30 min, with continuous agitation and then cooling the solution to room temperature. For each gram of the sample used to prepare the 2% solution, add 1 mL of 10% potassium carbonate solution to the fully hydrated sample at ambient temperature. Heat the mixture in a water bath to 85°, and maintain for 2 h without agitation. Under these conditions, a thermally stable gel is formed. Related hydrocolloids such as guar gum and locust bean gum do not form thermally stable gels and are negative by this test |
| Purity | |||
| Loss on drying | Not more than 12 % (105°C, 5 h) | Not more than 8% (105°C, 3 h) | Not more than 15% (105°, 5 h) |
| Starch | Not more than 3% | Not more than 1% | – |
| Protein | Not more than 3% (N × 5.7) |
Not more than 1.5% (N × 5.7) Determine nitrogen by the Kjeldahl method. The percentage of nitrogen in the sample multiplied by 5.7 gives the percent of protein in the sample |
Not more than 8% Proceed as directed under Nitrogen Determination (Kjeldahl method). The percentage of nitrogen in the sample multiplied by 5.7 gives the percent of protein in the sample |
| Viscosity (1% solution) | Not less than 3 kg/m·s at 25°C | Not less than 20 kg/m s at 25°C | – |
| Ether‐soluble material | Not more than 0.1% | Not more than 0.5% | – |
| Sulphite (as SO2) | – | Not more than 4 mg/kg | – |
| Chloride | – | Not more than 0.02% | – |
| 50% Alcohol‐soluble material | – | Not more than 2.0% | – |
| Total ash | Not more than 5.0% (800°C, 3–4 h) | Not more than 2.0% (800°C, 3–4 h) | Not more than 5% (800°, 3–4 h) |
| Arsenic | Not more than 3 mg/kg | – | – |
| Lead | Not more than 2 mg/kg | Not more than 1 mg/kg |
Not more than 2 mg/kg Determine using an atomic absorption technique appropriate to the specified level. The selection of sample size and method of sample preparation may be based on the principles of the method described in Volume 4, ‘Instrumental Methods’ |
| Microbiological criteria | |||
| Salmonella spp. | Absent in 12.5 g | Absent in 12.5 g | – |
| Escherichia coli | Absent in 5 g | Absent in 5 g | – |
In Commission Regulation (EU) No 231/1012, there are distinct specifications for konjac gum (E 425 i) and konjac glucomannan (E 425 ii). JECFA has one specification for konjac flour (INS 425). Konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are distinguished by their grade of purity. The JECFA specification for konjac flour (INS 425) covers both EU specifications.
The Panel noted that in the definition of konjac gum the organ of origin should be named tuber instead of root.14
Because of both the botanical origin and the polysaccharidic nature of gums, they can be a substrate of microbiological contamination and of field and storage fungal development. The latter has been recently demonstrated by the mycotoxin contaminations of gums (Zhang et al., 2014). The Panel noted that the microbiological specifications for polysaccharidic thickening agents, such as gums, should be harmonised and that for konjac gum (E 425 i) and konjac glucomannan (E 425 ii) criteria for total aerobic microbial count (TAMC) and total combined yeasts and moulds count (TYMC) should be included into the EU specifications as it is the case for other polysaccharidic thickening agents (e.g. alginic acids and its salts (E 400–E 404), agar (E 406), carrageenan (E 407), processed eucheuma seaweed (E 407a), xanthan gum (E 415), gellan gum (E 418)).
In view of the botanical origin of konjac gum and konjac glucomannan, furthermore limitations of possible contamination with pesticides should be considered.
According to the EU and JECFA specifications, konjac flour and konjac gum should contain not less than 75% carbohydrate and according to EU specifications konjac glucomannan should contain not less than 95% total dietary fibre on a dry weight basis. The Panel noted that, while JECFA expressed the carbohydrate content (glucomannans) by subtracting the sum of the percentages of total ash, loss on drying and protein from 100%, no information on the method of assay for carbohydrate and total dietary fibre are provided in the EU specifications.
The Panel noted that, according to the EU specifications for konjac gum (E 425 i) and konjac glucomannan (E 425 ii), impurities of the toxic elements arsenic and lead are accepted up concentrations of 3 and 2 mg/kg, respectively. Contamination at such levels could have a significant impact on the exposure to these metals, for which the exposures already are close to the health‐based guidance values or benchmark doses (lower confidence limits) established by the EFSA (EFSA CONTAM Panel, 2009, 2010, 2012, 2014). The Panel noted that no limits for cadmium and mercury are defined in the EU specifications.
3.1.3. Manufacturing process
According to Commission Regulation (EU) No 231/1012, konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are prepared from konjac flour. Konjac flour is the unpurified raw product from the root of the perennial plant A. konjac.15 In contrast, according to the JECFA (2006) specification, konjac flour (INS 425) may be produced from various species of Amorphophallus, which is a genus comprising around 150 species (Parry, 2010).
Amorphophallus is cultivated mainly in Japan and China. Two‐year‐old konjac tubers are washed, sliced into thin chips which are dried and then pulverised by dry or wet milling. The glucomannan content in commercial konjac flours is about 70–90% of the dry matter (Takigami, 2000; Parry, 2010).
According to Commission Regulation (EU) No 231/1012, konjac gum (E 425 i) is obtained from konjac flour by aqueous extraction. According to the information provided by one of the manufacturers, the flour is washed with alcohol (isopropanol or ethanol) in the manufacturing process (Documentation provided to EFSA n.2).
Konjac glucomannan (E 425 ii) is obtained from konjac flour by washing with water‐containing ethanol. By this washing procedure, microfine powders remaining on the surface and impurities trapped inside the konjac particles are removed (Takigami, 2000).
3.1.4. Methods of analysis in food
Only one analytical method for the quantification of konjac glucomannan in foods was identified in the literature search in Toxline, Medline and SciFinder. Hurley et al. (2010) developed an enzyme‐linked immunosorbent assay (ELISA) for quantification of the additive in gum mixtures and in confectionary. For the test, a polyclonal antibody obtained from sheep was used. The ELISA was found to be specific for konjac glucomannan and sensitive, with a detection limit of 0.1 mg/L.
Methods for the determination of other polysaccharide gums in food were published. Although these methods were not validated for konjac, they should also be applicable for konjac glucomannan.
Different polysaccharides (locust bean gum, guar gum, gum arabic, tragacanth, arabinogalactan, carrageenan, furcellaran, agar, xanthan) were analysed quantitatively in dairy products (Glueck and Thier, 1980). The polysaccharides are extracted from foodstuff, and then fat, starch, milk proteins and carbohydrates are removed by extraction or degradation. The resulting polysaccharide fraction is analysed by gas chromatography after hydrolysis with trifluoroacetic acid, derivatisation of the resulting monosaccharides with hydroxylamine hydrochloride and acetic acid anhydride to form the aldonitrilacetate derivatives. The polysaccharides can be qualitatively identified by their characteristic monosaccharide pattern, and quantified via the single monosaccharide peaks. In the case of konjac glucomannan, glucose and mannose should be identified as hydrolysis products. For the polysaccharides, recoveries of 80–90% were obtained when adding 0.05% of the thickeners to skim milk or 1–2% to mixtures of ice cream or pudding constituents (Glueck and Thier, 1980). In a later investigation, the analytical procedure was improved by Preuss and Thier (1983). Changes in the separation of interfering substances (fats, proteins and starch) allowed the quantitative determination of polysaccharide gums in a variety of foods like blancmange powder, glaze, fruit ice and cream cheese. Recoveries for most of the thickeners and gums are about 60–85% with a coefficient of variation of 2–8%.
For the qualitative test of gums in mayonnaise and French dressing, the AOAC Official Method 937.12 is reported by the Association of Official Agricultural Chemists (AOAC, now AOAC International) (AOAC, 2002). The gums are precipitated from the food sample, hydrolysed to monosaccharides which are qualitatively identified. This method is not applicable in presence of starch. A similar method (AOAC Official Method 935.61) for qualitative determination of gums in salad dressing based on a precipitation reaction is applicable in presence of starch (AOAC, 2002). Both methods are usable for determination of the sum of different gums used in foodstuff.
3.1.5. Stability of the substance, and reaction and fate in food
Glucomannan starts to decompose around 250°C, decomposition is complete at 350°C. Viscosity is affected at temperatures above 80°C, especially in acidic media. Konjac glucomannan is degraded by enzymes such as β‐d‐glucanase and β‐d‐mannanase; therefore, food preservative should be used to prevent fermentation by air‐borne microorganisms (Parry, 2010).
3.2. Authorised uses and use levels
Maximum levels of konjac (E 425), comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii), have been defined in Annex II to Regulation (EC) No 1333/200815 on food additives, as amended. In this document, these levels are named MPLs.
Currently, konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are authorised food additives in the EU included in Group I at the MPL of 10 g/kg individually or in combination. Table 2 summarises foods that are permitted to contain konjac (E 425), comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008.
Table 2.
MPLs of konjac (E 425), comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii) in foods according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | Restrictions/exception | MPL (g/L or g/kg) |
|---|---|---|---|
| 01.3 | Unflavoured fermented milk products, heat‐treated after fermentation | 10 g/kg, individually or in combination | |
| 01.4 | Flavoured fermented milk products including heat treated products | 10 g/kg, individually or in combination | |
| 01.6.3 | Other creams | 10 g/kg, individually or in combination | |
| 01.7.1 | Unripened cheese excluding products falling in category 16 | Except mozzarella | 10 g/kg, individually or in combination |
| 01.7.5 | Processed cheese | 10 g/kg, individually or in combination | |
| 01.7.6 | Cheese products excluding products falling in category 16 | 10 g/kg, individually or in combination | |
| 01.8 | Dairy analogues including beverage whiteners | 10 g/kg, individually or in combination | |
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | 10 g/kg, individually or in combination | |
| 02.3 | Vegetable oil pan spray | 10 g/kg, individually or in combination | |
| 03 | Edible ices | 10 g/kg, individually or in combination | |
| 04.2.1 | Dried fruit and vegetables | 10 g/kg, individually or in combination | |
| 04.2.2 | Fruit and vegetables in vinegar, oil, or brine | 10 g/kg, individually or in combination | |
| 04.2.4.1 | Fruit and vegetable preparations excluding compote | 10 g/kg, individually or in combination | |
| 04.2.5.4 | Nut butters and nut spreads | 10 g/kg, individually or in combination | |
| 04.2.6 | Processed potato products | 10 g/kg, individually or in combination | |
| 05.1 | Cocoa and Chocolate products as covered by Directive 2000/36/EC | Only energy‐reduced or with no added sugars | 10 g/kg, individually or in combination |
| 05.2 | Other confectionery including breath freshening microsweets |
May not be used in jelly mini‐cups, defined, for the purpose of this Regulation, as jelly confectionery of a firm consistence, contained in semi rigid mini‐cups or mini‐ capsules, intended to be ingested in a single bite by exerting pressure on the mini‐ cups or mini‐capsule to project the confectionery into the mouth E 425 may not be used in jelly confectionery |
10 g/kg, individually or in combination |
| 05.3 | Chewing gum | 10 g/kg, individually or in combination | |
| 05.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | 10 g/kg, individually or in combination | |
| 06.2.2 | Starches | 10 g/kg, individually or in combination | |
| 06.3 | Breakfast cereals | 10 g/kg, individually or in combination | |
| 06.4.2 | Dry pasta | Only gluten free and/or pasta intended for hypoproteic diets in accordence with Directive 2009/39/EC | 10 g/kg, individually or in combination |
| 06.4.4 | Potato gnocchi | Except fresh refrigerated potato gnocchi | 10 g/kg, individually or in combination |
| 06.4.5 | Fillings of stuffed pasta (ravioli and similar) | 10 g/kg, individually or in combination | |
| 06.5 | Noodles | 10 g/kg, individually or in combination | |
| 06.6 | Batters | 10 g/kg, individually or in combination | |
| 06.7 | Pre‐cooked or processed cereals | 10 g/kg, individually or in combination | |
| 07.1 | Bread and rolls | except products in 7.1.1 and 7.1.2 | 10 g/kg, individually or in combination |
| 07.2 | Fine bakery wares | 10 g/kg, individually or in combination | |
| 08.3.1 | Non‐heat treated processed meat | 10 g/kg, individually or in combination | |
| 08.3.2 | Heat‐treated processed meat | Except foie gras, foie gras entier, blocs de foie gras, Libamáj, libamáj egészben, libamáj tömbben | 10 g/kg, individually or in combination |
| 08.3.3 | Casings and coatings and decorations for meat | 10 g/kg, individually or in combination | |
| 09.2 | Processed fish and fishery products including molluscs and crustaceans | 10 g/kg, individually or in combination | |
| 09.3 | Fish roe | Only processed fish roe | 10 g/kg, individually or in combination |
| 10.2 | Processed eggs and egg products | 10 g/kg, individually or in combination | |
| 11.2 | Other sugars and syrups | 10 g/kg, individually or in combination | |
| 12.1.2 | Salt substitutes | 10 g/kg, individually or in combination | |
| 12.2.2 | Seasonings and condiments | 10 g/kg, individually or in combination | |
| 12.3 | Vinegars | 10 g/kg, individually or in combination | |
| 12.4 | Mustard | 10 g/kg, individually or in combination | |
| 12.5 | Soups and broths | 10 g/kg, individually or in combination | |
| 12.6 | Sauces | 10 g/kg, individually or in combination | |
| 12.7 | Salads and savoury‐based sandwich spreads | 10 g/kg, individually or in combination | |
| 12.8 | Yeast and yeast products | 10 g/kg, individually or in combination | |
| 12.9 | Protein products excluding products covered in category 1.8 | 10 g/kg, individually or in combination | |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | 10 g/kg, individually or in combination | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | 10 g/kg, individually or in combination | |
| 13.4 | Foods suitable for people intolerant to gluten as defined by Regulation (EC) No 41/2009 | Including dry pasta | 10 g/kg, individually or in combination |
| 14.1.2 | Fruit juices as defined by Directive 2001/112/EC and vegetable juices | Only vegetable juices | 10 g/kg, individually or in combination |
| 14.1.3 | Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products | Only vegetable nectars | 10 g/kg, individually or in combination |
| 14.1.4 | Flavoured drinks | 10 g/kg, individually or in combination | |
| 14.1.5.2 | Other | Excluding unflavoured leaf tea; including flavoured instant coffee | 10 g/kg, individually or in combination |
| 14.2.3 | Cider and perry | 10 g/kg, individually or in combination | |
| 14.2.4 | Fruit wine and made wine | 10 g/kg, individually or in combination | |
| 14.2.5 | Mead | 10 g/kg, individually or in combination | |
| 14.2.6 | Spirit drinks as defined in Regulation (EC) No 110/2008 | Except whisky or whiskey | 10 g/kg, individually or in combination |
| 14.2.7.1 | Aromatised wines | 10 g/kg, individually or in combination | |
| 14.2.7.2 | Aromatised wine‐based drinks | 10 g/kg, individually or in combination | |
| 14.2.7.3 | Aromatised wine‐product cocktails | 10 g/kg, individually or in combination | |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol and | 10 g/kg, individually or in combination | |
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | 10 g/kg, individually or in combination | |
| 15.2 | Processed nuts | 10 g/kg, individually or in combination | |
| 16 | Desserts excluding products covered in category 1, 3 and 4 | 10 g/kg, individually or in combination | |
| 17.1a | Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms | 10 g/kg, individually or in combination | |
| 17.2a | Food supplements supplied in a liquid form | 10 g/kg, individually or in combination | |
| 17.3a | Food supplements supplied in a syrup‐type or chewable form | 10 g/kg, individually or in combination | |
| 18 | Processed foods not covered by categories 1–17 excluding foods for infants and young children | 10 g/kg, individually or in combination |
MPL: maximum permitted level.
FCS 17 refers to food supplements as defined in Directive 2002/46/EC of the European Parliament and of the Council excluding food supplements for infants and young children.
According to Annex III, Part 1 of Regulation (EC) No 1333/2008, konjac (E 425) is authorised as a carrier in all food additives at quantum satis (QS).
According to Annex III, Part 4 of Regulation (EC) No 1333/2008, konjac (E 425) is authorised as a food additive including as a carrier in all flavourings at QS.
The Regulation (EC) No 1333/2008 stipulates that konjac (E 425), comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii), as a food additive, belonging to group I, is not authorised for the uses to produce dehydrated foods intended to rehydrate on ingestion, in jelly mini‐cups and in jelly confectionery.
The Panel noted that these restrictions have to be seen against the background of human cases of fatal accidents, resulting from asphyxiation following the ingestion of jelly mini‐cups confectionery containing the additive (EFSA, 2004) and of severe adverse effects, such as oesophageal obstruction after oral intake of the additive in the form of tablets without enough liquid (Fung, 1984; Henry et al., 1986; Vanderbeek et al., 2007).
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of konjac gum (E 425 i) and konjac glucomannan (E 425 ii)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment, especially for those food additives for which no MPL is set and which are authorised according to QS.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued public calls,16 , 17 for occurrence data (usage level and/or concentration data) on konjac gum (E 425 i) and konjac glucomannan (E 425 ii). In response to this public call, updated information on the actual use levels of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) in foods was made available to EFSA by industry. No analytical data on the concentration of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) in foods were made available by the Member States.
3.3.1.1. Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 85) of konjac (E 425) in foods for 44 out of the 67 food categories in which konjac (E 425) is authorised.
Updated information on the actual use levels of konjac (E 425) in foods was made available to EFSA by Association for International Promotion of Gums (AIPG, Documentation provided to EFSA n.4), EUROGUM A/S (Documentation provided to EFSA n.6), Fabricante Embutidos del centro SA (España) (EMCESA, Documentation provided to EFSA n.7), FoodDrinkEurope (FDE, Documentation provided to EFSA n.3) and International Food Additives Council (IFAC, Documentation provided to EFSA n.5).
The Panel noted that some data providers (e.g. AIPG, Eurogums A/S, IFAC) are not food industry using gums in their food products but food additive producers. Usage levels reported by food additive producers are not considered at the same level as those provided by food industry. The ANS Panel considered that food additive producers might recommend usage levels to the food industry but the final levels might, ultimately, be different. Therefore, unless food additive producers confirm that the recommended levels are used by food industry, they are not considered in the refined exposure scenario.
For instance, for Eurogum A/S (Documentation provided to EFSA n.6), ‘all the submitted data are theoretical amounts suggested or recommended’; they are ‘based on their own technical know‐how regarding adequate/recommended levels of use in different food applications’. Eurogums A/S provided three identical levels on meat products. These levels were not considered in the current estimates (Documentation provided to EFSA n.6).
Appendix A provides data on the use levels of konjac (E 425) in foods as reported by industry.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors product introductions in consumer packaged goods markets worldwide. It contains information of over 2 million food and beverage products of which more than 900,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the Mintel GNPD.18
For the purpose of this Scientific Opinion, the Mintel GNPD19 was used for checking the labelling of products containing konjac gum (E 425 i) and konjac glucomannan (E 425 ii) within the EU's food products as the Mintel GNPD shows the compulsory ingredient information presented in the labelling of products.
According to Mintel, konjac gum (E 425 i) and konjac glucomannan (E 425 ii) is labelled on around 300 products of pastas, noodles, prepared meals, meat products. Around 250 products were found to be published in this database between 2012 and 201721 out of which few drinks (n = 11). Some of the foods ingredients mention ‘konjac flour’ without referring to any food additive characteristics (e.g. E‐number or function in food such as a gelling agent).
Appendix B presents the percentage of the food products labelled with konjac (E 425) between 2012 and 2017, out of the total number of food products per food subcategories according to the Mintel food classification.
3.3.3. Food consumption data used for exposure assessment
3.3.3.1. EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). New consumption surveys recently20 added in the Comprehensive database were also taken into account in this assessment.12
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database represents the best available source of food consumption data across Europe at present.
Food consumption data from the following population groups: infants, toddlers, children, adolescents, adults and the elderly were used for the exposure assessment. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 3).
Table 3.
Population groups considered for the exposure estimates of konjac gum (E 425 i) and konjac glucomannan (E 425 ii)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlers | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrena | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlya | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Sweden, UK |
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the Food Classification System (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, FoodEx food codes were matched to the FCS food categories.
3.3.3.2. Food categories selected for the exposure assessment of konjac gum (E 425 i) and konjac glucomannan (E 425 ii)
The food categories in which the use of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) is authorised were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Some food categories are not referenced in the EFSA Comprehensive Database and could therefore not be taken into account in the present estimate. This was the case for nine food categories and may result in an underestimation of the exposure. The food categories which were not taken into account are described below (in ascending order of the FCS codes):
06.4.4 Gnocchi;
06.6 Noodles;
06.7 Pre‐cooked or processed cereals;
08.3.3 Casings and coatings and decorations for meat;
12.1.1 Salt substitutes;
13.2 Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5);
13.3 Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet);
13.4 Foods suitable for people intolerant to gluten as defined by Regulation (EC) No 41/2009;
14.1.3 Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products, only vegetable nectars.
The following food subcategories cannot be differentiated from the whole food category and therefore the whole food category was considered in the exposure assessment. This applies to two food categories (Appendix D) and may result in an overestimation of the exposure:
01.6.3 Other creams
17.1/17.2/17.3 Food supplements, in solid, liquid, syrup‐type or chewable form.
For the remaining food categories, the refinements considering the restrictions/exceptions as set in Annex II to Regulation No 1333/2008 were applied. Overall, for the regulatory maximum level exposure scenario, nine food categories were not taken into account because no consumption data are available, thus 58 food categories were included. For the refined exposure assessment scenario, only three food categories were taken into account because either no concentration data were provided to EFSA either the data provided were from food additives producers. (Appendix B).
3.4. Exposure estimates
3.4.1. Exposure to konjac gum (E 425 i) and konjac glucomannan (E 425 ii) from their use as food additives
The Panel estimated chronic exposure for the following population groups: infants; toddlers, children, adolescents, adults and the elderly. Dietary exposure to konjac (E 425) was calculated by multiplying konjac (E 425) concentrations for each food category (Appendix C) with their respective consumption amount per kilogram of body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only one day per subject were excluded as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 3). Based on these distributions, the mean and 95th percentile of exposure were calculated per survey for the total population and per population group. High percentile exposure was only calculated for those population groups where the sample size was sufficiently large to allow calculation of the 95th percentile of exposure (EFSA, 2011a). Therefore, in the present assessment, high levels of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not included.
Exposure assessment to konjac (E 425) was carried out by the ANS Panel based on: (1) MPLs as set down in the EU legislation (defined as the regulatory maximum level exposure assessment scenario); and (2) reported use levels (defined as the refined exposure assessment scenario). These two scenarios are discussed in detail below.
These scenarios do not consider the consumption of food supplements (FC 17.1, FC 17.2 and FC 17.3) which are covered in an additional specific exposure scenario detailed below (food supplements consumers only scenario).
Certain foods for special medical purposes (FSMP) consumed in population groups of children, adolescents, adults and the elderly may be very diverse; they cannot be considered because of very limited information on consumption. Eating occasions belonging to the food categories 13.2, 13.3 and 13.4 were therefore reclassified under food categories in accordance to their main component.
Considering that the food category 18 (Processed foods not covered by categories 1 to 17, excluding foods for infants and young children) is extremely unspecific (e.g. composite foods), processed foods, prepared or composite dishes belonging to the food category 18 were reclassified under food categories in accordance to their main component. Therefore, FC 18 is not taken into account as contributor to the total exposure estimates.
3.4.1.1. Regulatory maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008 and listed in Table 2.
A possible additional exposure from the use of konjac (E 425) as a food additive in food flavourings in accordance with Annex III to Regulation (EC) No 1333/2008 (Part 4) was not considered in the regulatory maximum level exposure assessment scenario. Despite this, the Panel considers the exposure estimates derived following this scenario as the most conservative as it is assumed that the population group will be exposed to konjac (E 425) present in food at MPL over a longer period of time.
3.4.1.2. Refined exposure assessment scenario
The refined exposure assessment scenario is based on use levels reported by industry. This exposure scenario can consider only food categories for which the above data were available to the Panel.
Appendix C summarises the concentration levels of konjac (E 425) used in the refined exposure assessment scenario. Based on the available data set, the Panel calculated two refined exposure estimates based on different model populations:
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long term to konjac (E 425) present at the maximum reported use level for one food category. This exposure estimate is calculated as follows:
-
1
— Combining food consumption with the maximum of the reported use levels for the main contributing food category at the individual level.
-
2
— Using the mean of the typical reported use levels for the remaining food categories.
-
1
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long term to konjac (E 425) present at the mean reported use level in food. This exposure estimate is calculated using the mean of the typical reported use levels for all food categories.
3.4.1.3. Specific exposure assessment scenario
-
‘Food supplement consumers only’ scenario: Konjac (E 425) is authorised in the food categories 17.1, 17.2 and 17.3 Food supplements as defined in Directive 2002/46/EC excluding food supplements for infants and young children. As exposure via food supplements may deviate largely from the one via food, and that the number of food supplement consumers may be low depending on populations and surveys, an additional scenario was calculated in order to reflect additional exposure to food additives from food supplements compared to exposure to food additives excluding these sources. This scenario will be estimated as follow:
-
1
— Consumers only of food supplements will be assumed to be exposed to a food additive present at the MPL on a daily basis via consumption of food supplements (as no data from food industry are available for food supplements).
-
2
— For the remaining food categories (3/67 categories), the mean of the typical reported use levels is used.
-
1
As food categories 17.1, 17.2 and 17.3 do not consider food supplements for infants and toddlers as defined in the legislation, exposure to konjac (E 425) from food supplements are not estimated for these two population groups.
3.4.1.4. Dietary exposure to konjac (E 425)
Table 4 summarises the estimated exposure to konjac (E 425) from their use as food additives in six population groups (Table 3) according to the different exposure scenario's (Section 3.4.1). Detailed results per population group and survey are presented in Appendix D.
Table 4.
Summary of anticipated exposure to konjac (E 425) from their use as food additives in the maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Regulatory maximum level exposure assessment scenario | ||||||
| Mean | 40.7–244.6 | 122.6–521.6 | 116.8–412.5 | 76.2–280.8 | 47.8–193.6 | 42.1–170.5 |
| 95th percentile | 95.3–793.3 | 299.7–842.1 | 241.7–705.5 | 153.0–477.9 | 105.2–375.9 | 90.9–299.5 |
| Refined estimated exposure assessment scenario | ||||||
| Brand‐loyal scenario | ||||||
| Mean | 0.001–0.007 | 0.006–0.030 | 0.009–0.034 | 0.004–0.022 | 0.004–0.014 | 0.003–0.011 |
| 95th percentile | 0.006–0.028 | 0.024–0.059 | 0.025–0.064 | 0.013–0.048 | 0.011–0.031 | 0.009–0.023 |
| Non‐brand‐loyal scenario | ||||||
| Mean | < 0.001–0.004 | 0.003–0.015 | 0.005–0.033 | 0.002–0.022 | 0.002–0.008 | 0.002–0.006 |
| 95th percentile | 0.003–0.015 | 0.013–0.044 | 0.013–0.063 | 0.007–0.048 | 0.006–0.024 | 0.005–0.016 |
From the regulatory maximum level exposure assessment scenario, mean exposure konjac (E 425) from its use as a food additive ranged from 40.7 mg/kg bw per day in infants to 521.6 mg/kg bw per day in toddlers. The 95th percentile of exposure to konjac (E 425) ranged from 90.9 mg/kg bw per day for the elderly to 842.1 mg/kg bw per day in toddlers.
From the refined estimated exposure, in both the brand‐loyal and non‐brand‐loyal scenario, mean and high exposure to konjac (E 425) from its use as a food additive was below 0.1 mg/kg bw per day.
For the food supplements consumers only, in the regulatory maximum level exposure assessment scenario, mean exposure to konjac (E 425) from its use as a food additive ranged from 0.002 mg/kg bw per day for the elderly to 0.033 mg/kg bw per day for children. The 95th percentile of exposure ranged from 0.007 for the elderly mg/kg bw/day to 0.064 mg/kg bw per day for children.
3.4.1.5. Main food categories contributing to exposure to konjac (E 425) using the maximum level exposure assessment scenario
From the regulatory maximum level exposure assessment scenario, the main contributing food categories to the total mean exposure estimates for infants and toddlers were flavoured fermented milk products. For children and adolescents, the main contributing food categories were flavoured drinks, while, for adults, the main contributing food categories were bread and rolls and flavoured drinks and for the elderly, coffee, tea, herbal and fruit infusions, chicory and flavoured drinks (see Appendix E for more details).
3.4.1.6. Main food categories contributing to exposure to konjac (E 425) using the refined exposure assessment scenario
The main contributing food category from the refined estimated exposure scenario, brand‐loyal and non‐brand‐loyal scenario were meat products, this is due to the low number of data available for the food categories (see Appendix E for more details). The second food category was yeast and yeast products.
Appendix E can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4864.
3.4.1.7. Uncertainty analysis
Uncertainties in the exposure assessment of konjac (E 425) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 5.
Table 5.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Use of data from food consumption survey of a few days to estimate long‐term (chronic) exposure for high percentiles (95th percentile) | + |
| Correspondence of reported use levels and analytical data to the food items in the EFSA Comprehensive Food Consumption Database: uncertainties to which types of food the levels refer to | +/– |
| Uncertainty in possible national differences in use levels of food categories | +/– |
| Food categories selected for the exposure assessment: exclusion of food categories due to missing FoodEx linkage (n = 9/67 food categories) | – |
| Food categories selected for the exposure assessment: inclusion of food categories without considering the restriction/exception (n = 2/67 food categories) | + |
| The 3 food categories which were taken into account in the refined exposure assessment scenarios out of all authorised food categories (n = 67), corresponded to only 0.5 and 17% of the amount (g of foods by body weight) of food consumption documented in the EFSA Consumption Database | – |
| Food categories included in the exposure assessment: data not available for certain food categories which were excluded from the exposure estimates (only 3 food categories with available data) | – |
Regulatory maximum level exposure assessment scenario:
|
+ – – |
Refined exposure assessment scenarios:
|
+/– – – |
+, uncertainty with potential to cause over‐estimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the real exposure to konjac (E 425) as a food additive in European countries considered in the EFSA European database for the regulatory maximum level exposure scenario. In the refined scenario, very few food categories authorised were taken into account as usage levels were made available only for three food categories. Konjac (E 425) is a food additive belonging to Group I category and as such is allowed in 67 food categories. According to the Mintel GNPD, konjac gum does not seem to be widely used (Appendix B) as the food category, which contains the most this food additive is noodles for which approx. 6% of all noodles products contain this food additive. In all the other food categories, less than 1% of the products contain konjac gum. Besides, the Panel noted that some of these food products were labelled with ‘konjac flour’. It is not clear whether this refers to the food additive.
For some of the food categories not taken into account in the refined exposure assessment (because no data from food industry were provided), a use according to the Mintel GNPD was recorded, e.g. noodles, pasta and rice. Therefore, this would lead to underestimation in exposure.
If it is considered that the food additive may not be used in food categories for which no usage data have been provided, the refined scenario would result in an overestimation of the real exposure to konjac (E 425) as a food additive in European countries considered in the EFSA European database.
3.4.2. Exposure via the regular diet
The tubers of the plant A. konjac (synonyms for the plant and its tubers: konjac, Konnyaku) are stamped or ground to produce a flour (Dongowski, 2005; Merck Index, 2006). Konnyaku, konjak flour, konjac gum and konjac glucomannan have a long history of human intake as traditional food in Far East countries including China and Japan (SCF, 1997; Chua et al., 2010; and references therein; Merck Index, 2006; JECFA, 1996).
3.4.3. Exposure via other uses
Exposure to konjac gum or konjac glucomannan due to the following uses was not considered in this opinion.
3.4.3.1. Konjac glucomannan as an ingredient in slimming products and other foods
According to the EFSA NDA Panel, cause and effect relationships have been established between the consumption of konjac mannan (glucomannan) and the reduction of body weight. To obtain this effect, at least 3 g of glucomannan should be consumed daily in three doses of at least 1 g each, together with 1–2 glasses of water before meals, in the context of an energy‐restricted diet. The target population is overweight adults (EFSA NDA Panel, 2010).
According to the EFSA NDA Panel, cause and effect relationships have also been established between the consumption of glucomannan (konjac mannan) and maintenance of normal blood cholesterol concentrations. To obtain this effect, a food should provide at least 4 g/day of glucomannan in one or more servings. The target population is the general population (EFSA NDA Panel, 2009).
3.4.3.2. Pharmaceutical uses
Information on pharmaceutical uses was obtained by searches of the literature, the websites of national competent authorities for medicinal products and publicly available SmPC (Summary of product characteristics) on the nationally available authorised products indicated to EFSA by EMA communication (Documentation provided to EFSA n. 8).
Konjac gum is neither used as an active ingredient nor as an excipient in medicinal products authorised in centralised or national procedures as seen from the answer of EMA.
3.5. Biological and Toxicological data
Besides the data available for konjac gum and konjac glucomannan, the Panel also considered study results with konjac flour as partially relevant since konjac flour is the not purified raw product from the tubers of A. konjac. However, the Panel noted that according to the definition in the JECFA specifications konjac flour may be obtained also from the tubers of other species of Amorphophallus than A. konjac (JECFA, 2006).
3.5.1. Absorption, distribution, metabolism and excretion
There is evidence that certain high‐molecular‐weight dietary polysaccharides, such as gums, could be partially broken down in the human large intestine. In addition to intermediate metabolites such as lactate, acrylate or fumarate, the main end products of this colonic anaerobic digestive process are short‐chain fatty acids (SCFA) such as acetic, propionic and butyric acids, which are absorbed from the colon (Cummings and Englyst, 1987).
3.5.1.1. In vitro studies
Konjac flour (83.4% dietary fibre) was incubated in a bacteriological medium inoculated with a dilute suspension of bacteria prepared from pooled human faeces. The pH of the cultures and the volume of gas produced during incubation were measured at 24, 48 and 96 h. In comparison to the basal medium controls, there was a significant drop of pH within 48 h and significant amounts of gas were produced at all time points, indicating fermentation of konjac flour (FMC Corporation, 1995a, Documentation provided to EFSA n.2).
Konjac glucomannan was examined in vitro for its degradation into disaccharides by soluble enzymes in human faeces (from three different subjects), their further conversion to monosaccharides by the use of cell‐associated enzymes of microflora, and the production of SCFAs under anaerobic fermentation conditions (Matsuura, 1998). The test substance was degraded almost 100% to three disaccharides, which were further degraded to glucose and/or mannose. These monosaccharides served as a carbon source for the proliferation of intestinal bacteria which ferment them into acetic acid, propionic acid, 1‐butyric acid and formic acid. These fatty acids were different in their proportions among test subjects, their total amounts ranging from 17.1% to 48.8% of the initial konjac glucomannan.
Chiu and Stewart (2012) compared the in vitro digestibility and fermentability of two preparations of konjac glucomannan to better understand how this gum would improve human health. Konnyaku (yam cake made of A. konjac), isolated konjac glucomannan, inulin and guar gum were subjected to in vitro digestion and in vitro fermentation. Fermentation samples were removed at 0, 4, 8, 12 and 24 h for gas volume, pH and SCFA measurements. This study confirmed that konjac glucomannan and konnyaku are resistant to degradation by salivary and pancreatic enzymes. Gas production in fermentation vessels containing konnyaku and konjac glucomannan was lower than for inulin from 8 to 24 h. Both samples produced SCFA concentrations similar to konjac gum, which favoured acetate and propionate over butyrate production. This study characterised SCFA production by konjac glucomannan in its isolated form and in food form.
3.5.1.2. In vivo studies
Tokunaga et al. (1986) investigated the feeding of konjac glucomannan in diet in a concentration of 20% (equivalent to 24 g/kg bw per day) for 6–8 weeks in rats. Data demonstrated increased faecal excretions of acetate (165%), propionate (900%), isobutyrate (300%) and isovalerate (100%). According to the authors, these volatile SCFAs are the end‐products of microbial degradation of the indigestible polysaccharide.
Fermentation of konjac glucomannan was also documented in mice receiving 5% of konjac glucomannan (equivalent to 10 g/kg bw per day) in diet for 3 weeks (Chen et al., 2010). At the end of the treatment period, the faecal concentration of SCFA was measured. When compared to controls given fibre‐free diet, increased faecal concentrations of the SCFAs acetate, propionate and butyrate (by 160%, 80% and 150%, respectively) were found in mice given konjac glucomannan.
The aim of the study of Wu and Chen (2011a) was to investigate the effects of konjac glucomannan on the colon of rats fed a high‐fat fibre‐free diet. Male Sprague–Dawley rats (n = 8 animals per group) were fed a high‐fat (25% corn oil, w/w) fibre‐free diet or that supplemented with konjac glucomannan fibre (5%, w/w) for 4 weeks. In this study, konjac glucomannan was found to be well‐fermented in rats and increased the concentration and daily excretion of faecal SCFAs, especially acetate and butyrate.
The Panel noted that in an additional study, Nakajima and Matsuura (1997) purified an enzyme which is able to degrade konjac glucomannan. According to the authors, ‘this enzyme was purified from the culture broth of an anaerobic human intestinal bacterium, Clostridium butyricum‐Clostridium beijerinckii group. The enzyme was composed of a single polypeptide chain with a molecular weight of 50,000–53,000. The enzyme was an endo‐β‐mannanase that acted specifically on the polysaccharides such as konjac glucomannan and coffee mannan, producing exclusively their smaller oligosaccharides and the monosaccharides. The optimal pH of the enzyme for the hydrolysis of konjac glucomannan was around 7–8 and the enzyme was stable in rather alkaline pH range of 8–10’. It was suggested that ‘the enzyme might contribute to the decomposition of konjac glucomannan in human digestive tract’.
Overall, data on in vitro degradation by human gastrointestinal fluids and on in vivo digestibility of konjac glucomannan in animals demonstrated that this compound would not be absorbed intact or hydrolysed by digestive enzymes. However, konjac glucomannan would be significantly fermented with production of SCFA such as acetic, propionic and butyric acids, during its passage through the large intestine by strains of bacteria found in the human colon. Based on the available knowledge on the role of SCFA as end products of the fermentation of dietary fibres by the anaerobic intestinal microbiota (Topping and Clifton, 2001; Den Besten et al., 2013), the Panel considered that their potential formation as fermentation products from konjac gum and konjac glucomannan does not raise any safety concern. Despite the absence of convincing in vivo studies in humans, the Panel considered that these data indicated that konjac gum and konjac glucomannan would be most probably not absorbed intact but significantly fermented by intestinal microbiota in humans.
3.5.2. Acute toxicity
Studies on acute oral toxicity of konjac flour and konjac glucomannan were presented by JECFA (1993), the SCF (1997) and one report of FMC Corporation (1993, Documentation provided to EFSA n.2). The oral LD50 were higher than 2,800 mg/kg bw in mice and rats for konjac glucomannan (Oketani et al., 1984), and higher than 5,000 mg/kg bw in rats for konjac flour (Kotkoskie et al., 1992; FMC Corporation, 1993, Documentation provided to EFSA n.2).
The Panel considered that konjac glucomannan and konjac flour can be regarded to be of low acute toxicity.
3.5.3. Short‐term and subchronic toxicity
No studies on short‐term and subchronic toxicity for konjac gum (E 425 i) and konjac glucomannan (E 425 ii) were available to the Panel. However, the following information on short‐term and subchronic toxicity studies from evaluations by the SCF is cited. Additional studies on nutritional effects are described in Section 3.5.8 ‘Other studies’.
3.5.3.1. Konjac gum
According to the SCF (1997), the available toxicity data on konjac gum/flour (specifications not given in most cases) include data from ‘subacute and subchronic feeding studies in rats addressing specific questions e.g. effects on intestine, colonic microflora and protein absorption, an 18‐month study with rats performed to examine the effect on cell‐ageing. None of these studies indicated any relevant toxic effects. The observed reduction in body weight, and caecum/colon enlargement are typical of the administration of high doses of non‐digestible, non‐absorbed, high‐molecular‐weight materials, e.g. cellulose, pectin or guar gum. The studies, however, cannot be regarded as adequate in terms of modern standards. They do not allow to derive a no‐effect level’.
3.5.3.2. Konjac glucomannan
According to the SCF (1997), the available toxicity data on konjac glucomannan include data from ‘a subacute feeding study (28 days) in rats and subchronic feeding studies (90 days) in beagle dogs and rats, the latter combined with a study on reproduction toxicity. The 90‐day studies did not reveal relevant toxic effects. Reduction in food consumption and body weight and caecum/colon enlargement are commonly observed in feeding studies with non‐digestible dietary fibres and were found as well as some changes in hematological and clinical chemical parameters only at high doses. The no‐observed‐effect level (NOEL) was 2.5% konjac glucomannan in the diet, corresponding to 1.25 g/kg body weight/day’. According to the SCF, konjac glucomannan (E 425 ii) ‘was tested adequately in 90‐day feeding studies with rats and beagle dogs’.
The Panel noted that a 12‐week study investigating lipotropic effects on the level of tissue lipids and the absorption of four minerals was available (Hou et al., 1990) which is described in Section 3.5.8. The Panel considered that this study does not comply with the OECD Guidelines for subchronic toxicity studies.
3.5.4. Genotoxicity
3.5.4.1. In vitro
Studies on genotoxicity of konjac flour but not konjac glucomannan were considered by JECFA (1993, 1996) and the SCF (1997).
Konjac flour was tested in the Ames test in five tester strains of Salmonella Typhimurium with and without metabolic activation and was negative (no more information available, report only available as abstract) (Kotokoskie et al., 1992).
Konjac flour was tested in a Salmonella/microsome assay (Ames test) at doses ranging from 50 to 5,000 μg/plate (the dose levels were based on preliminary toxicity and solubility tests) (FMC Corporation, 1994, Documentation provided to EFSA n.2). The tester strains S. Typhimurium TA98, TA100, TA1535, TA1537 and TA1538 were used and the assay was conducted in the absence and presence of metabolic activation. Konjac flour was not mutagenic in any test system with or without metabolic activation. The Panel noted that konjac flour was not tested in TA102 or Escherichia coli WP2 tester strains and therefore considered the negative results reliable with limitations.
Konjac flour was tested in the mouse lymphoma assay (L5178Y cells) at the TK locus (FMC Corporation, 1995b, Documentation provided to EFSA n.2). The mutation assays were performed using the soft agar method. The test item was assayed at the maximum level of 1,000 μg/mL (not cytotoxic in preliminary cytotoxicity tests), limited by solubility in the final treatment medium. Two trials were conducted both with and without metabolic activation. Six dose‐levels ranging from 15.6 to 1,000 μg/mL were used in both experiments. No increase in mutation frequency over the concurrent negative control was observed at any dose level in the absence or presence of S9 metabolic activation, indicating that konjac flour is not mutagenic or clastogenic in mammalian cells. The Panel noted that the study was performed in compliance with the OECD test guideline no 490, with the exception that only the short‐term treatment was performed in the absence of S9 metabolic activation.
3.5.4.2. In vivo
An in vivo mouse micronucleus test was made to evaluate the ability of konjac flour to induce micronuclei in the bone marrow polychromatic erythrocytes of CD‐1 mice (FMC Corporation, 1995c, Documentation provided to EFSA n.2). In a dose limit study with three males and three females, the maximum tolerated dose was estimated to be higher than 5,000 mg/kg bw. Therefore, in the main assay, five males and five females were dosed orally by gavage with 5,000 mg/kg bw konjac flour, and five males and five females served as the vehicle control (deionised water). Test and control animals were euthanatised after approximately 24, 48 and 72 h after application and the bone marrow was extracted. Konjac flour did not induce any increase in micronuclei in bone marrow polychromatic erythrocytes over the vehicle control group. However, no shift in the polychromatic erythrocytes to normochromatic erythrocytes (PCE/NCE) ratio in treated animals compared to the negative control animals was observed at any treatment time; hence, there was no indication of target tissue exposure. This is in line with the evidence that konjac flour is not absorbed intact but appears significantly fermented in the intestine to SCFA. On these bases, the Panel considered the results of this study of limited relevance for risk assessment.
Overall, konjac flour was not mutagenic in S. Typhimurium strains TA1535, TA1537, TA1538, TA98 and TA100 and showed negative results for the induction of gene mutation and clastogenicity in mammalian cells in vitro (mouse lymphoma assay), a condition judged to be sufficient by the Panel to rule out any concern for genotoxicity. Furthermore, the Panel noted that both konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are covered by this conclusion since konjac flour is the not purified raw product from the root of the perennial plant A. konjac,21 and konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are distinguished only in their higher grade of purity compared to konjac flour.
3.5.5. Chronic toxicity and carcinogenicity
In a limited (low number of animals involved and one dose only) long‐term study in male C3H/He mice (specific strain spontaneously developing a high incidence of liver tumours) after feeding 10% konjac glucomannan (equivalent to 13,000 mg/kg bw per day) with the diet for 10 months, slight inhibitory effects of konjac glucomannan on the development of liver nodules but no effects on liver tumour incidences were observed (Mizutani and Mitsuoka, 1982).
The Panel noted that only two 18 months studies investigating the effects of konjac meal on calcium and phosphorous metabolism in rats (Zhang et al., 1995) and its effects on the ageing of the brain, liver and cardiovascular tissues (Peng et al., 1995) were available. The Panel considered that these studies are of limited validity as only one dose (equivalent to 720 mg glucomannan/kg bw per day) was tested and they did not comply with the OECD Guidelines for chronic toxicity and carcinogenicity studies (TG 451 and 453). The objective and limitations are further described in Section 3.5.8.
3.5.6. Reproductive and developmental toxicity
3.5.6.1. Reproductive toxicity studies
No reproductive studies were available.
3.5.6.2. Developmental toxicity studies
Pregnant short‐hair domestic cats were given diets with either 2% konjac flour (KF) (6 cats) or 2% carob gum (9 control cats) during gestation (Burger et al., 1992; as referred to in JECFA, 1993). Actual intake of konjac flour during the week prior to parturition ranged from 0.98 to 3.08 mg/kg bw per day. All pregnant animals completed a normal gestation period and no differences in body weight changes were observed in control and test cats. Mean birth weight (control 104 ± 7 g vs KF 95 ± 22 g) was higher in control cats than in konjac flour treated cats. This was related to the higher mean litter size in the konjac flour‐treated rats (control 3.5 ± 1.6 vs KF 5.1 ± 1.2). All cats completed lactation and reared their progeny successfully. The biochemical and haematological parameters tested were within normal ranges throughout the study (no data provided). The Panel considered this study too limited due to the species tested, number of animals and the very low dose levels, lack of examination of abnormalities (developmental abnormalities) and the details in the description provided in JECFA (1993).
A study was conducted to investigate the effects of different amounts of konjac flour inclusion in the gestation diet (during both reproductive cycles) on the physiochemical properties of diets, post‐prandial satiety in pregnant sows, lactation feed intake of sows and piglet performance during two successive reproductive cycles (Sun Tan et al., 2015). Multiparous Landrace sows (n = 140) were assigned randomly to one of four experimental diets, containing 0%, 0.6%, 1.2% or 2.2% KF (equivalent to 0, 240, 480 or 880 mg KF/kg bw per day, JECFA 2000). During lactation all groups were fed the same lactation diet. As reported, ‘during the second reproductive cycle, increasing dietary KF linearly increased plasma concentrations of SCFA 4 h post‐prandial (p < 0.05) and glucagon‐like peptide (GLP‐1) 2 h post‐prandial (p < 0.05), but decreased the plasma concentration of cortisol (linearly, p < 0.05) 1 h post‐prandial. In addition, there was a linear decrease of the non‐feeding oral behavior of gestating sows (p < 0.01) when dietary KF increased. There were linear increases in lactation feed intake of sows during the entire lactation period (p < 0.01) with increasing amounts of KF in the gestation diet. In addition, the number of piglets weaned (linearly, p < 0.01; quadratic, p = 0.01), average piglet weights and litter weights on day 21 of lactation (linearly, p < 0.01) increased with increasing inclusion amounts of KF’. The design of the study was intended to prove the beneficial effects for breeding and not designed as for the detection of developmental effects.
Overall, the Panel noted that no reproductive toxicity studies were available. The Panel considered that the developmental toxicity studies of Burger et al. (1992) as referred to by JECFA (1993) in cats and of Sun Tan et al. (2015) in sows were both limited and not sufficient for the evaluation of the developmental toxicity of konjac gum (E 425 i) and konjac glucomannan (E 425 ii).
3.5.7. Hypersensitivity, allergenicity and food intolerance
3.5.7.1. Animal studies
NC/Nga mice were used as a murine model for human atopic dermatitis (Onishi et al., 2005). These mice develop spontaneously atopic dermatitis‐like skin lesions accompanied by typical itching behaviour and hyperimmunoglobulin E production. Male NC/Nga mice (5/group) received diets containing 5% of two high‐viscous and two low‐viscous konjac glucomannan powders (equivalent to 10 g/kg bw per day) for 8 weeks. The oral intake of the pulverised test substance with low viscosity inhibited the development of dermatitis and plasma immunoglobulin E (IgE) elevation in the animals through downregulation of interferon gamma (IFN‐γ), a positive cytokine for atopic inflammation production.
In a consecutive publication, the authors (Onishi et al., 2007) reported that the oral administration of pulverised konjac glucomannan (0.2%, 1% and 5%, equivalent to 0.4, 2 and 10 g/kg bw per day) to male NC/Nga mice for 8 or 9 weeks, markedly suppressed the development of scratching behaviour dose dependently, also eczematous skin lesions including hyperkeratosis, dermal mastocytosis and eosinophilia were significantly inhibited. Plasma concentrations of immunoglobulin G1 (IgG1), IgG2a and IgE cutaneous overproductions of substance P, interleukin (IL) 10 and tumour necrosis factor alpha (TNF‐α) were markedly suppressed.
In a study by Suzuki et al. (2010), splenic cells were isolated from 8‐week‐old BALB/c mice, and B and T cells and monocytes were further isolated and incubated with various concentration of hydrolysed konjac glucomannan (prepared by acid hydrolysis at 75°C for 60 min) at an optimal size (between 10 and 500 kDa). The secretion of IgE and various cytokines including IFN‐γ, IL 10 and IL 12 was then followed after stimulation of the cells with IL 4 and anti‐CD 40 antibodies. In addition, in a model of hypersensitisation with ovalbumin, BALB/C mice that had received an injection of hydrolysed konjac glucomannan (1 mg intraperitoneally) showed reduced plasma concentrations of IgE, IgG1 and IgG2b but not IgG2a. The authors concluded that solubilised konjac glucomannan diminished the production of IgE via the suppression of IgE class switching in vitro and in vivo.
The Panel noted that according to these studies, hydrolysed konjac glucomannan suppressed IgE production in vitro and in vivo whereas pulverised konjac glucomannan inhibited spontaneously occurring dermatitis in a murine model of atopic dermatitis. However, to express such activity, konjac glucomannan must be pulverised then hydrolysed under conditions (temperature, pH) which are not possible in the human gastrointestinal tract. Therefore, the Panel considered that these observations do not apply to the food additive E 425 ii.
3.5.7.2. Human data
A case of hypersensitivity pneumonitis in a konnyaku manufacturer was reported (Tajima et al., 2003). A 56‐year‐old man had worked as a konnyaku manufacturer for 38 years, and suffered from dyspnoea on exertion. The diagnosis of hypersensitivity pneumonitis was confirmed immunologically by the detection of serum precipitins to powdered Hijikia fusiforme, and by the positive result of in vitro lymphocytic proliferative response for konjac flour using peripheral blood lymphocytes.
Bernstein et al. (2007) reported a case of respiratory sensitisation of a food manufacturing worker to konjac glucomannan. This index case of occupational respiratory sensitisation to konjac glucomannan was supported by a specific prick skin test and a specific provocation test to konjac glucomannan. The patient also exhibited a positive prick skin test to a guar gum glucomannan, suggesting either a separate sensitisation process or cross‐reactivity with konjac glucomannan.
The Panel noted that there are a few cases of sensitisation via working place exposure by inhalation but this was not regarded to be relevant for oral exposure for food additives use. No case reports showing hypersensitivity after oral intake could be found in the literature.
Overall, considering both human and animal data, the Panel considered that there is no indication for concern for immunotoxicity or allergenicity with konjac gum (E 425 i) and konjac gum glucomannan (E 425 ii) used as food additives.
3.5.8. Other studies
3.5.8.1. Animal studies
In a study to investigate the digestion and absorption of protein, four male Sprague–Dawley rats were fed 10% konjac (no further information on flour or glucomannan) in diet (equivalent to 11,800 mg/kg bw per day) for 28 days. In comparison to controls, the administration of konjac decreased the digestion and absorption of protein in the large intestine, resulting in a reduction of body weight gain (Miyoshi et al., 1987; cited in JECFA, 1996).
The effect of indigestible carbohydrates on the intestinal absorption of calcium was studied in male Wistar rats (Oku et al., 1982). Six rats per group were fed a diet containing 10% and 20% konjac glucomannan (equivalent to 11,800 and 23,600 mg/kg bw per day) for 7 or 8 weeks. In comparison to the untreated control group, the body weight was decreased in treated animals and an inhibitory effect on intestinal calcium absorption was observed, partially due to the loss of calcium binding protein caused by gastrointestinal transit of large amounts of undigested substances.
The hypertrophic effect of indigestible carbohydrate on caecum and colon was investigated in male Wistar rats (6/group) fed 20% konjac glucomannan (equivalent to 23,600 mg/kg bw per day) for 8 weeks (Konishi et al., 1984). The konjac glucomannan ingestion caused an increase of caecum and colon weights. The caecal enlargement was dependent on the increases in both the number and size of mucosal cells and the colonic enlargement resulted from an increase in the number of mucosal cells, i.e. hyperplasia.
The influence of repeated intake of a fructooligosaccharide in diet was investigated on growth and gastrointestinal function of male Wistar rats (Tokunaga et al., 1986). The application of 20% konjac glucomannan in diet (equivalent to 23,600 mg/kg bw per day) served as a positive control. In comparison to negative controls, after feeding of konjac glucomannan for 6 weeks, body weight gain was decreased, faecal weight was increased and gastrointestinal transit time was shortened. A significant reduction in serum triacylglycerol levels was reported in animals receiving konjac glucomannan, but no effects on serum cholesterol were observed. The concentration of volatile fatty acids per gram of wet faeces increased in the konjac glucomannan group in comparison to the negative control group. Also, the faecal excretion of neutral sterol was increased by konjac glucomannan feeding.
The influence of refined konjac meal (containing about 80% glucomannan prepared from the tubers of A. konjac K. Koch) on the levels of tissue lipids and the absorption of four minerals was tested in male and female Sprague–Dawley rats (12/sex per group) (Hou et al., 1990). The test substance was applied in diet at levels of 2.5%, 5% and 10% (equivalent to 2,025, 4,050 and 8,100 mg/kg bw per day and 2,275, 4,550 and 9,100 mg/kg bw per day for male and female, respectively) for 12 weeks. Body weight, feed intake and serum lipids were measured while liver was examined for histopathology. At the end of the 12th week, the serum cholesterol levels of all treated groups were lowered to a normal level as was the liver cholesterol level of the 10% konjac meal group. The lipotropic effect of konjac meal was also confirmed by histopathologic examination of the livers. In addition to the hypocholesterolaemic effects, konjac meal diets also increased stool bulk. Minor effects were found on the absorption and utilisation of Ca, Fe, Zn and Cu.
Venter et al. (1990) compared the effects of a soluble dietary fibre concentrate, konjac glucomannan, and of propionate on plasma fibrinogen, serum and liver lipid, glucose tolerance, insulin response and liver glycogen in baboons. Twelve male baboons were fed a ‘Western’ diet with or without konjac glucomannan (5%, equivalent to 2,500 mg/kg bw per day) or sodium propionate (2%) supplements for periods of 9 weeks in a crossover, randomised order, with stabilisation periods in between. Measurements were taken at baseline and after 4 and 9 weeks of each study period. After 9 week, total serum cholesterol levels were significantly higher than pretest values when baboons consumed the unsupplemented Western diet (25%, p < 0.05) or the propionate diet (17%, p < 0.05). Konjac glucomannan prevented this increase. The high‐density lipoprotein cholesterol (HDL‐C) concentration increased with all experimental diets (p < 0.05). The percentage of total cholesterol (TC) as HDL‐C was significantly higher with konjac glucomannan supplementation than with the other diets. Konjac glucomannan supplementation also resulted in lower than baseline values for triglycerides (p < 0.01) and circulating free fatty acids (p < 0.05) after 9 week. Only the propionate diet raised serum triglycerides significantly (by 6%) above baseline. Liver cholesterol concentration was 31–34% lower, and the area under the glucose tolerance curve was smaller with konjac glucomannan and propionate diets (p < 0.05) than with the unsupplemented diet.
The aim of the study of Wu and Chen (2011a) was to investigate the effects of konjac glucomannan and inulin on the balance between pro‐oxidative status and antioxidative defence systems in the colon, liver and plasma of rats fed a high‐fat fibre‐free diet. Male Sprague–Dawley rats (n = 8 animals per group) were fed a high‐fat (25% corn oil, w/w) fibre‐free diet or that supplemented with konjac glucomannan or inulin fibre (5%, w/w) for 4 weeks. The index of pro‐oxidative status, malondialdehyde (MDA), and blood lymphocyte DNA damage, the antioxidative defence, that is, antioxidant enzymes (glutathione peroxidase, superoxide dismutase and catalase) in the colonic mucosa and liver, and the plasma antioxidant levels were determined. The fermentation of fibre was shown in faecal SCFA. Incorporation of konjac glucomannan and inulin into the high‐fat fibre‐free diet beneficially reduced the MDA levels of the colon and liver and DNA damage in blood lymphocytes. On the other hand, both fibres enhanced the antioxidative defence systems by upregulating the gene expressions of glutathione peroxidase and catalase in the colonic mucosa and of superoxide dismutase and catalase in the liver. Furthermore, konjac glucomannan and inulin promoted antioxidative status in the blood by elevating the α‐tocopherol level. Konjac glucomannan and inulin were well‐fermented in rats and increased the concentration and daily excretion of faecal SCFA, especially acetate and butyrate. These results suggest that in vivo utilisation of konjac glucomannan and inulin stimulated both local and systemic antioxidative defence systems in rats.
Wu and Chen (2011b) determined the effects of konjac glucomannan in a high‐fat corn oil diet on risk factors of colon carcinogenesis (faecal β‐glucuronidase, mucinase and bile acids) and on preventive factors (faecal microflora and caecal SCFA). Sprague–Dawley rats (n = 8 animals per group) were fed a normal‐fat fibre‐free (5% corn oil, w/w) or high‐fat (25% corn oil, w/w) diet containing no fibre, konjac glucomannan (5%, w/w, equivalent to 6 g/kg bw per day), or inulin (5%, w/w, as a prebiotic control) for 4 weeks. Results indicated that the high‐fat fibre‐free diet significantly elevated the faecal β‐glucuronidase and mucinase activities and total bile acid concentration and decreased caecal SCFA contents, as compared with its normal‐fat counterpart. The incorporation of konjac glucomannan, as well as inulin, into the high‐fat fibre‐free diet beneficially reduced the faecal β‐glucuronidase and mucinase activities and lithocholic acid (secondary bile acid) concentration. Although konjac glucomannan elevated the daily faecal total bile acid excretion, the change was due to the primary, instead of the secondary bile acids. In addition, konjac glucomannan beneficially promoted the daily faecal excretion of bifidobacteria and lactobacilli and caecal SCFA contents, as compared with the high‐fat fibre‐free diet. Therefore, the present study suggests that konjac glucomannan potentially attenuated the high‐fat‐induced risk in colon carcinogenesis.
Refined konjac meal (glucomannan content: 79.4%) was tested in a long‐term feeding trial in order to evaluate its effects on the calcium and phosphorus metabolism and the bone in rats (Zhang et al., 1995) and its effects on the ageing of the brain, liver and cardiovascular tissue in cells (Peng et al., 1995). Sprague–Dawley rats of both sexes (15/sex per group) received 1% refined konjac meal (equivalent to 720 mg konjac glucomannan/kg bw per day) for 18 months, the control group was fed basal diet only. In the study of Peng et al. (1995), body weights were measured every week in the first 3 months, and thereafter once a month. Blood was sampled at 3rd and 9th months and at the end of the study. Plasma was investigated for cholesterol and triglycerides. At the end of the study, rats were killed and brain, liver, kidneys, heart and aorta were examined by light microscopy, and the brain and myocardium by transmission electron microscopy. No substance‐induced signs of toxicity were observed. The long‐term ingestion of refined konjac meal had no adverse effect either on the calcium and phosphorus metabolism or on the bone (Zhang et al., 1995). The results of the study performed by Peng et al. (1995) suggest that the test substance may delay cell senescence in the brain, liver and cardiovascular system. The long‐term ingestion of refined konjac meal also induced lowered blood lipid levels. The Panel noted that no substance‐induced toxicity was observed in rats in this long‐term feeding trial over 18 months with 1% refined konjac meal in diet. However, the Panel considered this study of limited validity as only 1 dose was tested and the groups consisted of 15 animals of each sex and only a very limited number of organs were examined histopathologically.
Overall, the Panel noted that the available subacute studies with konjac flour and konjac glucomannan investigated specific questions, e.g. effects on intestine, colon microflora, effects on serum and liver cholesterol levels and absorption of protein and minerals. No relevant substance induced toxic effects were observed in any of these experiments. The observed effects like reduction of body weight, caecum and colon enlargement, influence on cholesterolaemia, increased stool bulk and decreased gastrointestinal transit time are typical of the administration of non‐digestible, non‐absorbed, high‐molecular‐weight materials. The Panel considered that an increased caecum weight in animals fed high amounts of carbohydrates is considered as a physiological response to an increased fermentation due to a carbohydrate‐induced modification on the composition of the intestinal microbiota.
3.5.8.2. Human data
EFSA NDA Panel (2009 and 2010) reviewed the health claims and concluded that cause and effect relationships have been established between the consumption of konjac glucomannan and the reduction of body weight and maintenance of normal blood cholesterol concentrations (see Section 1.2).
Konjac gum (glucomannan) has been described as having anorexiant properties. It was claimed that it absorbs liquid in the gastrointestinal tract and reduces the appetite that way. It is claimed that it also helps to treat hyperlipidaemia (Martindale, 2014). Risk concerning intestinal or oesophageal obstruction and faecal impaction were described, especially if taken with not sufficient amount of fluid (Martindale, 2014; FDA, 2015).
In the study of Kaats et al. (2015), a supplement containing 3 g of konjac glucomannan each day for 60 days reduced body weight, body fat and circulatory cholesterol levels in compliant overweight adults. No significant adverse effects were reported by subjects in either of the study groups. No adverse changes in blood chemistries, fat‐free mass or bone density were found.
3.5.8.3. Case reports
Cases of oesophageal obstruction are reported after ingestion of tablets containing dry non‐hydrated konjac glucomannan as an aid to dieting, the claim being that the appetite is inhibited by swelling of the glucomannan in the stomach, producing a feeling of fullness (Fung, 1984; Henry et al., 1986; Vanderbeek et al., 2007). The obstruction is associated with the great capacity of the konjac glucomannan to absorb water. During 1984–1985, seven cases of oesophageal obstruction caused by swelling of tablets containing glucomannan were reported to the Australian Adverse Drug Reactions Committee (Henry et al., 1986).
In addition, fatal accidents, mainly in children and a few in elderly persons, have been reported in various countries, resulting from asphyxiation following the ingestion of jelly mini‐cups confectionery containing the additive E 425 (EFSA, 2004).
3.5.8.4. Clinical studies
The available studies in humans showed that after ingestion of high doses of konjac flour (about 5 g/day) side effects were reported such as diarrhoea, flatulence and slight abdominal pain or distension (Huang et al., 1990).
Doi et al. (1990) investigated the metabolic and nutritional effects of long‐term use of konjac glucomannan in the treatment of obese or non‐obese diabetics. In this study, 195 patients received 7.8 g glucomannan/day (corresponding to about 110 mg/kg bw per day) for 16 weeks. At the end of the treatment, mean plasma cholesterol levels were reduced in all groups whilst side effects consisted of flatus observed in 13.4% of patients.
In addition, konjac glucomannan reduced the absorption of fat‐soluble vitamin E, but the absorption of water‐soluble vitamin B12 and minerals was not influenced (Doi et al., 1983; Doi, 1995). Pharmacokinetics of co‐administered drugs, for example glibenclamide, can be influenced by consumption of konjac flour (Shima et al., 1983).
The effects of konjac glucomannan on serum cholesterol concentrations were investigated in 63 healthy men in a double‐blind crossover, placebo‐controlled study (Arvill and Bodin, 1995). As reported, ‘after a 2‐week baseline period, the subjects were given 3.9 g konjac glucomannan or placebo daily for 4 weeks. After a washout period of 2 weeks, crossover took place, followed by another 4 weeks of treatment. The subjects were encouraged not to change their ordinary diets or general lifestyle during the investigation. Konjac glucomannan fibres reduced total cholesterol (TC) concentrations by 10% (p < 0.0001), low‐density‐lipoprotein cholesterol (LDL‐C) concentrations by 7.2% (p < 0.007), triglycerides by 23% (p < 0.03), and systolic blood pressure by 2.5% (p < 0.02). High‐density‐lipoprotein cholesterol (HDL‐C) and the ratio of LDL‐C to HDL‐C did not change significantly. No change in diastolic blood pressure or body weight was observed. No adverse effects were observed. The results of this study show that konjac glucomannan is an effective cholesterol‐lowering dietary adjunct’.
Vido et al. (1993) conducted a double‐blinded study in 60 obese children (under 15 years of age) receiving a glucomannan (konjac origin not mentioned) treatment of 1 g twice a day for 2 months or a placebo. The children under glucomannan treatment manifested a decrease in α‐lipoprotein and an increase in pre‐β‐lipoprotein and triglycerides. No side effects were found on intestinal absorption, on thyroid and adrenocortical functions or for clinical symptoms.
Chen et al. (2003) evaluated the effects of konjac glucomannan supplement (3.6 g/day) for 28 days on blood lipid and glucose levels in hyperlipidaemic type‐2 diabetic patients and the possible mechanism for the reductions in blood lipid levels. As reported, ‘the konjac glucomannan supplement improved blood lipid levels by enhancing faecal excretion of neutral sterol and bile acid and alleviated the elevated glucose levels in diabetic subjects. Konjac glucomannan could be an adjunct for the treatment of hyperlipidemic diabetic subjects’.
Loening‐Baucke et al. (2004) evaluated in a double‐blind study, the effect of konjac glucomannan in children (16 boys and 15 girls, from 4.5 to 11.7 years of age) with chronic functional constipation. Konjac glucomannan and placebo were given as 100 mg/kg bw daily (maximal 5 g/day) for 4 weeks. Glucomannan was found to be beneficial in the treatment of constipation in children whereas no significant side effects were reported during each 4‐week treatment period.
A meta‐analysis of randomised clinical trials on konjac glucomannan used in body weight reduction preparations found undesirable effects reported such as flatulence, soft stools, diarrhoea and abdominal discomfort in trials where the average daily dose of glucomannan ranged between 1.2 g and 10 g for 3–12 weeks (17–143 mg/kg bw per day) (Onakpoya et al., 2014). Eighteen trials were identified, and nine with a total of 273 participants, were included in the review. There was a variation in the reporting quality of the included trials. In all trials, the adult or children patients were described as overweight or obese whereas five trials involved participants with comorbid conditions such as osteoarthritis, hyperlipidaemia, diabetes and post‐cardiac treatment. The meta‐analysis (random effect model) revealed a non‐statistically significant difference in weight loss between glucomannan and placebo. Adverse events were mostly related to the gastrointestinal system and included diarrhoea, constipation, abdominal discomfort and mild meteorism. No adverse events were reported in one trial and another one mentioned no significant difference in adverse events between glucomannan and placebo. One trial reported a significant difference in the frequency of adverse events in the glucomannan group compared to placebo. According to the authors, the short duration of the trials precludes them from drawing any conclusions about the long‐term effects of glucomannan on human body weight.
The objective of the meta‐analysis of randomised trials by Zalewski et al. (2015) was to update evidence on the effect of konjac glucomannan supplementation on body weight and body mass index compared with administration of a placebo. Twenty‐four full‐text articles were identified and six eligible trials were included with a total of 291 participants. Only one trial was performed in obese children (from 8 to 14 years of age). The average daily dose of glucomannan consumed ranged between 1.2 and 3.99 g for 4–12 weeks. In adults, three trials reported a significant reduction in body weight in the glucomannan group compared with the control group. None of the trials reported a favourable effect of glucomannan on body mass index. All studies monitored participants for adverse events and in four of them no adverse events were observed. In one trial, patients in the glucomannan group more frequently reported belching, bloating and stomach fullness. In another trial, participants had decreased appetite and increased thirst with similar frequency in both study groups. Diarrhoea was reported significantly more often in the glucomannan group than in the placebo group. According to the authors, in healthy overweight or obese adults, there is some evidence that in the short‐term konjac glucomannan may help to reduce body weight but not body mass index; data in children are too limited to draw any conclusions.
From these meta‐analyses, the study of Wood et al. (2007) was identified by the Panel as the most appropriate for description of unwanted effects. In a parallel‐arm, double‐blind, placebo‐controlled study, the effects of adding 3 g konjac glucomannan (divided in three times 1 g)/person per day (corresponding to 33 mg/kg bw per day based on mean body weight of approximately 90 kg), for 12 weeks, to a carbohydrate‐restricted diet (CRD) were determined in 30 obese men (15/group). Plasma lipids, anthropometrics, body composition, blood pressure and nutrient intake were evaluated. The authors concluded that although clearly effective at lowering LDL‐C, adding konjac glucomannan to a CRD during active and significant weight loss provided no additional benefits to the diet alone. Gastrointestinal side effects reported included diarrhoea (konjac glucomannan = 7, placebo = 2; p < 0.05) and constipation (konjac glucomannan = 5, placebo = 1; p > 0.05). When total gastrointestinal side effects were combined (konjac glucomannan, 12, vs placebo, 3), there were more side effects present in the konjac glucomannan compared with the placebo group (p < 0.001).
4. Discussion
According to Commission Regulation (EU) No 231/2012,8 both konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are defined as water‐soluble hydrocolloid obtained from konjac flour. Konjac gum is obtained by aqueous extraction, while konjac glucomannan is obtained by washing with water‐containing ethanol. In the Regulation, konjac flour is defined as the unpurified raw product from the tuber of the perennial plant A. konjac.
Because of both the botanical origin and the polysaccharidic nature of gums, they can be a substrate of microbiological contamination and of field and storage fungal development. The latter has been recently demonstrated by the mycotoxin contaminations of gums (Zhang et al., 2014). The Panel noted that the microbiological specifications for polysaccharidic thickening agents, such as gums, should be harmonised and that for konjac gum and konjac glucomannan criteria for TAMC and TYMC should be included into the EU specifications.
In view of the botanical origin of konjac gum, possible contamination with pesticides should be considered. The Panel considered it necessary to pay attention to the compliance of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) raw material to existing EU regulation on pesticides.
The Panel noted that toxicological studies with an alginate‐konjac‐xanthan polysaccharide complex (PGX) were available for its evaluation as novel food by the EFSA NDA Panel. The EFSA ANS Panel did not consider results of these studies in its re‐evaluation of the individual substance konjac glucomannan (E 425 ii). It is not possible to conclude to what extent are the reported effects attributable to one of the individual components of the complex. The physicochemical properties of the individual components might also have changed during the manufacturing process of PGX (see Section 1.2).
The in vitro degradation and the in vivo digestibility of konjac glucomannan in animals demonstrated that this compound would not be absorbed intact or hydrolysed by digestive enzymes. However, konjac glucomannan would be significantly fermented to SCFA during its passage through the large intestine by the action of the intestinal microbiota. The rate of hydrolysis in the gastrointestinal tract in humans is unknown, however, it is expected that fermentation of konjac glucomannan would lead to the production of products such as SCFA which were considered of no safety concern by the Panel.
Konjac glucomannan and konjac flour can be regarded as non‐toxic based on the results of acute oral toxicity studies (Oketani et al., 1984; Kotokoskie et al., 1992; FMC Corporation, 1993, Documentation provided to EFSA n.2).
No relevant studies on short‐term and subchronic toxicity for konjac gum and konjac glucomannan are available. However, additional studies on nutritional effects are described. The available subacute and subchronic studies with konjac flour and konjac glucomannan investigate specific questions, e.g. effects on intestine, colon microflora, effects on serum and liver cholesterol levels and absorption of protein and minerals. No relevant substance‐induced adverse effects were observed in any experiment. The observed effects like reduction of body weight, caecum and colon enlargement, hypercholesterolaemia, increased stool bulk and decreased gastrointestinal transit time are typical of the administration of non‐digestible, non‐absorbed, high‐molecular‐weight materials.
Based on the data available, the Panel noted that there is no concern with respect to the genotoxicity of konjac flour. The Panel noted that both konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are covered by this conclusion since konjac flour is defined as the not purified raw product from the tuber of the perennial plant A. konjac.
No relevant studies on chronic toxicity and carcinogenicity for konjac gum (E 425 i) and konjac glucomannan (E 425 ii) were available. The Panel noted that no adverse effects were observed in rats in a long‐term feeding trial over 18 months with 1% refined konjac meal in diet (equivalent to 720 mg glucomannan/kg bw per day), and in mice receiving 10% of konjac glucomannan with the diet (equivalent to 13,000 mg/kg bw per day) for 10 months. However, the Panel considered both studies of limited validity as only one dose was tested and the groups consisted of low number of animals.
No reproductive toxicity studies were available. The Panel considered that the developmental toxicity studies of Burger et al. (1992) as referred to by JECFA (1993) in cats and of Sun Tan et al. (2015) in sows were both limited and not sufficient for the evaluation of the developmental toxicity of konjac gum (E 425 i) and konjac glucomannan (E 425 ii).
From both human and animal data, the Panel considered that there was no indication for concern for immunotoxicity or allergenicity with konjac gum (E 425 i) and konjac glucomannan (E 425 ii) used as food additives.
Traditional consumption of food based on konjac flour and konjac glucomannan in the Far East is generally considered as safe. However, gastrointestinal discomfort (i.e. laxative effects, flatulence, full stomach, feeling of hungry and abdominal distension) has been reported in several clinical human studies included in two meta‐analyses. In a relevant study, a dosage of 3 g konjac glucomannan (divided in three times 1 g)/person per day corresponding to 33 mg/kg bw per day based on mean body weight of approximately 90 kg, for 12 weeks, was associated with gastrointestinal effects (diarrhoea or constipation).
To assess the dietary exposure to konjac (E 425) from its use as food additives, the exposure was calculated based on (1) MPLs from the Regulation (defined as the regulatory maximum level exposure assessment scenario) and (2) reported use levels (defined as the refined exposure assessment scenario).
Konjac (E 425) is authorised in a wide range of foods. The Panel did not identify brand loyalty to a specific food category, and therefore the Panel considered that the non‐brand‐loyal scenario covering the general population was the most appropriate and realistic scenario for risk characterisation because it is assumed that the population would probably be exposed long‐term to the food additive present at the mean reported use in processed food.
A refined estimated exposure assessment scenario taking into account the consumption of food supplements for consumers only was also performed to estimate exposure for children, adolescents, adults and the elderly as exposure via food supplements may deviate largely from that via food, and the number of food supplement consumers may be low depending on populations and surveys.
Very few reported use levels were made available to EFSA. Only three food categories out of 67 were taken into account in the refined scenario. Thus the two refined exposure estimates (brand‐loyal consumer scenario and non‐brand‐loyal scenario) are similarly low (below 0.1 mg/kg bw per day in any scenario and population). In the case of konjac (E 425), despite the low number of data available, the Panel considered that the refined exposure assessment approach should result in more realistic long‐term exposure estimates compared to the regulatory maximum level exposure assessment scenario considering the few uses reported in the Mintel GNPD. Nevertheless, the Panel noted that no data were made available for the food category for which konjac seems the more used (noodles).
The Panel further noted that the exposure to konjac (E 425) from its use according the Annex III (Parts 1 and 4) was not considered in the exposure assessment.
The Panel also noted that the refined exposure estimates are based on information provided on the reported level of use of konjac (E 425). If actual practice changes, this refined estimates may no longer be representative and should be updated.
5. Conclusions
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel), 2014 and given that:
current use of konjac (E 425) was limited in all food categories to MPL of 10 g/kg;
an indicative refined exposure assessment has been calculated: for all population groups, it was below 0.1 mg/kg bw per day for the general population (mean and high level);
konjac gum and konjac glucomannan were unlikely to be absorbed intact and were significantly fermented by intestinal microbiota;
the available database on toxicological studies was considered limited; however, no relevant adverse effects were seen in rats and dogs in 90‐day feeding studies according to the SCF, and the no‐observed‐effect level (NOEL) in rats was 1,250 mg konjac glucomannan/kg bw per day;
konjac gum and konjac glucomannan would be of no concern with respect to the genotoxicity;
after a daily dosage of 3,000 mg in adults (corresponding to 33 mg/kg bw based on mean body weight of approximately 90 kg) for 12 weeks, several individuals experienced abdominal discomfort including diarrhoea or constipation,
the Panel concluded that there was no need for a numerical ADI and that there was no safety concern for the general population at the refined exposure assessment for the reported uses of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as food additives under the current conditions of use at level of 10 g/kg.
The Panel agreed with the conclusions of the SCF (1997) that the uses of konjac (E 425), comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii), as an additive at the levels up to 10 g/kg in food are acceptable, provided that the total intake from all sources does stay below 3 g/day.
6. Recommendations
The Panel recommended that the European Commission considers harmonising the microbiological specifications for polysaccharidic thickening agents, such as gums, and to include criteria for total aerobic microbial count (TAMC) and total combined yeasts and moulds count (TYMC) into the EU specifications of konjac gum (E 425 i) and konjac glucomannan (E 425 ii).
Although the Panel realised that the exposure to these additives is rather low, the Panel recommended that the European Commission considers revising the current limits for the toxic elements (lead and arsenic) in the EU specification for konjac gum (E 425 i) and konjac glucomannan (E 425 ii).
Documentation provided to EFSA
Pre‐evaluation documents on konjac gum (E 425 i) and konjac glucomannan (E 425 ii). Frauenhofer ITEM. December 2012.
IFAC (International Food Additives Council), 2010. Reply to EFSA: Call for data on emulsifiers, stabilisers and gelling agents. Information on “Reaction and fate in food; ADME (Metabolism and Toxicokinetics); Genotoxicity; Other studies”. Submitted on 4 November 2010.
FDE (Food Drink Europe), 2014. Data on usage levels of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 29 November 2013.
AIPG (Association for international Promotion of Gums), 2014. Data on usage levels of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 17 September 2014.
IFAC (International Food Additives Council), 2014. Data on usage levels of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 10 October 2014.
EUROGUM A/S, 2014. Data on usage levels of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 30 September 2014.
EMCESA (Fabricante Embutidos del centro SA, España), 2014. Data on usage levels of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 29 August 2014.
EMA (European Medicines Agency): communication to EFSA request for information on a certain group of substances used as food additives, May 2015 and Jan 2016.
IFAC (International Food Additives Council), 2016. Reply to EFSA. Information on particle size data of certain thickening agents permitted as food additives in the EU. Submitted on 19 January 2016.
Glossary [and/or] Abbreviations
- ADI
acceptable daily intake
- AIPG
Association for International Promotion of Gums
- ALT
alanine transaminase
- ANS
Panel on Food Additives and Nutrient Sources added to Food
- AOAC
Association of Analytical Communities
- AST
aspartate transaminase
- bw
body weight
- CAS
Chemical Abstracts Service
- CRD
carbohydrate‐restricted diet
- EFSA NDA
EFSA Panel on Dietetic Products, Nutrition and Allergies
- EINECS
European Inventory of Existing Commercial Chemical Substances
- ELISA
enzyme‐linked immunosorbent assay
- EMA
European Medicines Agency
- FAO/WHO
Food and Agriculture Organization/World Health Organisation
- FCS
Food Classification System
- FDA
Food and Drug Administration
- FDE
Food Drink Europe
- FSMP
foods for special medical purposes
- GLP‐1
glucagon‐like peptide
- GNPD
Global New Products Database
- HDL‐C
high‐density‐lipoprotein cholesterol
- HPLC
high‐performance liquid chromatography
- IFAC
International Food Additives Council
- IFN‐γ
interferon gamma
- IgE
immunoglobulin E
- IgG
immunoglobulin G
- IL 10
interleukin 10
- INS
International Numbering System for Food Additives
- IOM
Institute of Medicine
- IR
infrared
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- KF
konjac flour
- LD50
lethal dose, 50% i.e. dose that causes death among 50% of treated animals
- LDL‐C
low‐density‐lipoprotein cholesterol
- OECD
Organisation for Economic Co‐operation and Development
- MDA
malondialdehyde
- MoE
margins of exposure
- MPL
maximum permitted level
- MS
mass spectra
- NCE
normochromatic erythrocytes
- NMR
nuclear magnetic resonance
- NOAEL
no‐observed‐adverse effect level
- NOEL
no‐observed‐effect level
- PCE
polychromatic erythrocytes
- PGX
alginate‐konjac‐xanthan polysaccharide complex
- QS
quantum satis
- SCF
Scientific Committee on Food
- SCFA
short‐chain fatty acids
- SmPC
Summary of product characteristics
- TAMC
total aerobic microbial count
- TC
total cholesterol
- TNF‐α
tumour necrosis factor alpha
- TYMC
total combined yeasts and moulds count
- UV/VIS
ultraviolet/visual (UV/VIS)
Appendix A – Summary of the reported use levels (mg/kg or mg/L as appropriate) of konjac (E 425) provided by industry
Appendix B – Number and percentage of food products labelled with konjac (E 425) out of the total number of food products present in Mintel GNPD per food sub‐category between 2012 and 2017
Appendix C – Concentration levels of konjac (E 425) used in the refined exposure scenarios (mg/kg or mL/kg as appropriate)
Appendix D – Summary of total estimated exposure of konjac (E 425) from their use as food additives for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and high level (mg/kg bw per day)
Appendix E – Main food categories contributing to exposure to konjac (E 425) using the regulatory maximum level exposure scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
1.
Appendices A–E can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4864
Supporting information
Summary of the reported use levels (mg/kg or mg/L as appropriate) of konjac (E 425) provided by industry
Number and percentage of food products labelled with konjac (E 425) out of the total number of food products present in Mintel GNPD per food sub‐category between 2012 and 2017
Concentration levels of konjac (E 425) used in the refined exposure scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of konjac (E 425) from their use as food additives for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and high level (mg/kg bw per day)
Main food categories contributing to exposure to konjac (E 425) using the regulatory maximum level exposure scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Lambré C, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Oskarsson A, Parent‐Massin D, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Brimer L, Christodoulidou A, Lodi F, Tard A and Dusemund B, 2017. Scientific Opinion on the re‐evaluation of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as food additives. EFSA Journal 2017;15(6):4864, 43 pp. 10.2903/j.efsa.2017.4864
Requestor: European Commission
Question numbers: EFSA‐Q‐2011‐00520, EFSA‐Q‐2011‐00759
Panel members: Fernando Aguilar, Riccardo Crebelli, Alessandro Di Domenico, Birgit Dusemund, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Claude Lambré, Jean‐Charles Leblanc, Oliver Lindtner, Peter Moldeus, Alicja Mortensen, Pasquale Mosesso, Agneta Oskarsson, Dominique Parent‐Massin, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Dario Battacchi, Juho Lemmetyinen and Jacqueline Wiesner. The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 17 May 2017
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM(2001) 542 final.
Food Additives in Europe 2000, Status of safety assessments of food additives presently permitted in the EU, Nordic Council of Ministers, TemaNord 2002, 560.
According to Commission Regulation (EU) No 231/2012, both konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are defined as water‐soluble hydrocolloid obtained from konjac flour. Konjac flour is defined as the unpurified raw product from the root of the perennial plant Amorphophallus konjac. Konjac gum is obtained by aqueous extraction, while konjac glucomannan is obtained by washing with water‐containing ethanol.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1.
Synonyms for konjac flour from the JECFA specification (2006): konjac mannan, Konjac, konnyaku, INS No 425.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents Published: 23 May 2010. Available online: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption ‐ Extended deadline: 30 September 2014. Available online: http://www.efsa.europa.eu/sites/default/files/consultation/140310.pdf
Call for technical data on certain thickening agents permitted as food additives in the EU – Extended Deadline: 31 December 2015. Available online: http://www.efsa.europa.eu/it/data/call/141219
22–23/3.2017.
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295.
The Panel noted that while the EU specification for konjac gum defines the raw material for the flour (and hence for the gum) as the root of Amorphophallus konjac, the specification for konjac glucomannan mentions the tuber. The JECFA specification for konjac flour mentions the tubers from various species of Amorphophallus. In all cases, the plant organ used (no matter species) is the corm, which is the vertical, swollen underground stem.
Commission Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 16/1/2017.
Available online: http://www.efsa.europa.eu/en/press/news/150428.htm
The Panel noted that while the EU specification for konjac gum defines the raw material for the flour (and hence for the gum) as the root of Amorphophallus konjac the specification for konjac glucomannan mentions the tuber. The JECFA specification for konjac flour mentions the tubers from various species of Amorphophallus. In all cases, the plant organ used (no matter species) is the corm, which is the vertical, swollen underground stem.
References
- Akesowan A, 2002. Viscosity and gel formation of a konjac flour from Amorphophallus oncophyllus . AU Journal of Technology, 5, 1–8. [Google Scholar]
- AOAC (Association of Official Agricultural Chemists, now AOAC International), 2002. 43 Spices and other Condiments. In: Official Methods of Analysis of AOAC International, 17th Edition. Vol.II Food Composition, Additives, Natural Contaminants. Ed. Horwitz W.
- Arvill A and Bodin L, 1995. Effect of short‐term ingestion of konjac glucomannan on serum cholesterol in healthy men. American Journal of Clinical Nutrition, 61, 585–589. [DOI] [PubMed] [Google Scholar]
- BeMiller JN, 2008. Hydrocolloids. Ed. Arendt EKDB, F. Elsevier Inc., Amsterdam. [Google Scholar]
- Bernstein JA, Crandall MS and Floyd R, 2007. Respiratory sensitization of a food manufacturing worker to konjac glucomannan. Journal of Asthma, 44, 675–680. [DOI] [PubMed] [Google Scholar]
- Burger IH, Earle KE and Bailie H, 1992. Evaluation of konjac flor for use in a commercially prepared cat food. Cited in: JECFA, 1993. Konjac flour, WHO Food Additives Series 32.
- Chen HL, Sheu WH, Tai TS, Liaw YP and Chen YC, 2003. Konjac supplement alleviated hypercholesterolemia and hyperglycemia in type 2 diabetic subjects–a randomized double‐blind trial. Journal of the American College of Nutrition, 22, 36–42. [DOI] [PubMed] [Google Scholar]
- Chen H‐L, Cheng H‐C, Lin Y‐M and Wang Y‐C, 2010. Comparative effects of cellulose and soluble fibers (pectin, konjac glucomannan, inulin) on fecal water toxicity toward caco‐2 cells, fecal bacterial enzymes, bile acid, and short‐chain fatty acids. Journal of Agriculture and Food Chemistry, 58, 10277–10281. [DOI] [PubMed] [Google Scholar]
- Chiu Y‐T and Stewart M, 2012. Comparison of konjac glucomannan digestibility and fermentability with other dietary fibers in vitro . Journal of Medicinial Food, 15, 120–125. [DOI] [PubMed] [Google Scholar]
- Chua M, Baldwin TC, Hocking TJ and Chan K, 2010. Traditional uses and potential Health benefits of Amorphophallus konjac K. Koch ex N.E.Br. Journal of Ethnopharmacology, 128, 268–278. [DOI] [PubMed] [Google Scholar]
- Crescenzi V, Skjak‐Braek G, Dentini M, Masci G, Bernalda MS, Risica D, Capitani D, Mannina L and Segre AL, 2002. A high field NMR study of the products ensuing from konjak glucomannan C(6)‐oxidation followed by enzymatic C(5)‐epimerization. Biomacromolecules, 3, 1343–1352. [DOI] [PubMed] [Google Scholar]
- Cummings JH and Englyst HN, 1987. Fermentation in the human large intestine and the available substrates. American Journal of Clinical Nutrition, 45, 1243–1255. [DOI] [PubMed] [Google Scholar]
- Den Besten G, van Eunen K, Groen A, Venema K, Reijngoud DJ and Bakker M, 2013. The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. The Journal of Lipid Research, 54, 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K, 1995. Effect of konjac fibre (glucomannan) on glucose and lipids. European Journal of Clinical Nutrition, 49, Suppl 3, S190–S197. [PubMed] [Google Scholar]
- Doi K, Matsuura M, Kawara A, Tanaka T and Baba S, 1983. Influence of dietary fiber (konjac mannan) on absorption of vitamin B12 and vitamin E. Tohoku Journal of Experimental Medicine 141, 677–681. [DOI] [PubMed] [Google Scholar]
- Doi K, Nakamura T, Aoyama N, Matsuura M and Kawara A, 1990. Metabolic and nutritional effects of long‐term use of konjac glucomannan in the treatment of obese or non‐obese diabetics In: Y Oomura, S Tarui, S Inoue, T Shimazu. (eds). Progress in Obesity Research, Proceedings of the sixth international congress on obesity. John Libbey; (as cited in Doi 1195), London, pp. 507–514 [Google Scholar]
- Dongowski Gerhard. 2005. Lexikon der Lebensmittel und der Lebensmittelchemie. Ed. Waldemar Ternes. Wiss. Verlag‐Ges., 2005.
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760. Available online: http://www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. 10.2903/j.efsa.2014.3697 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012. Scientific Opinion on lead dietary exposure in the European population. EFSA Journal 2012;10(7):2831, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on dietary exposure to inorganic arsenic in the European population. EFSA Journal 2014;12(3):3597, 68 pp. 10.2903/j.efsa.2014.3597 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2009. Scientific Opinion on the substantiation of health claims related to glucomannan and maintenance of normal blood cholesterol concentrations (ID 836, 1560) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 on request from the European Commission. EFSA Journal 2009;7(9):1258, 14 pp. 10.2903/j.efsa.2009.1258 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) 2010. Scientific Opinion on the substantiation of health claims related to konjac mannan (glucomannan) and reduction of body weight (ID 854, 1556, 3725), reduction of post‐prandial glycaemic responses (ID 1559), maintenance of normal blood glucose concentrations (ID 835, 3724), maintenance of normal (fasting) blood concentrations of triglycerides (ID 3217), maintenance of normal blood cholesterol concentrations (ID 3100, 3217), maintenance of normal bowel function (ID 834, 1557, 3901) and decreasing potentially pathogenic intestinal microorganisms (ID 1558) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2010;8(10):1798, 27 pp. 10.2903/j.efsa.2010.1798 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) 2017. Scientific Opinion on the safety of alginate‐konjac‐xanthan polysaccharide complex (PGX) as a novel food pursuant to Regulation (EC) No 258/97. EFSA Journal 2017;15(5):4776, 24 pp. 10.2903/j.efsa.2017.4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579. Available online: http://www.efsa.europa.eu [DOI] [Google Scholar]
- FDA , 2015. CFR ‐ Code of Federal Regulations Title 21 (The information on this page is current as of April 1 2015). FMC Corporation, 1993. Konjac flour FW‐KON Acute oral toxicity study in rats, Study No.: I93‐1775.
- FMC Corporation , 1993. Konjac flour FW‐KON Acute oral toxicity study in rats, Study No.: I93‐1775.
- FMC Corporation , 1994. FMC 12205 Konjac flour FW‐KON Salmonella/mammalian‐microsome mutagenicity assay (Ames test), Lab. Proj. Study No.: I93‐1780.
- FMC Corporation , 1995a. In vitro fermentation of konjac flour FW‐KON by human fecal flora. FMC Study No.: I94‐1976, Protocol CR 010‐RJC‐001.
- FMC Corporation , 1995b. Mutagenicity test on konjac flour FW‐KON in the L5178Y TK+/‐ mouse lymphoma forward mutation assay, CHV Study No.: 166682‐0‐431R, FMC Study No.: I95‐1979.
- FMC Corporation , 1995c. Mutagenicity test on konjac flour FW‐KON in an in vivo mouse micronucleus assay, CHV Study No.: 166682‐0‐455C0, FMC Study No.: I95‐1980.
- Fung M, 1984. Glucomannan diet tablets. The Medical Journal of Australia, 17, 350. [DOI] [PubMed] [Google Scholar]
- Glueck U and Thier HP, 1980. Quantitative determination of some thickeners in dairy products. Zeitschrift fuer Lebensmittel‐Untersuchung und ‐Forschung, 170, 272–279. [DOI] [PubMed] [Google Scholar]
- Henry DA, Mitchell AS, Aykward J, Fung MT, McEwen J and Rohan A, 1986. Glucomannan and risk of oesophageal obstruction. British Journal of Medicien, 292, 591–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y‐H, Zhang S‐S, Zhou H‐M, Wang R‐S and Zhang Y‐Z, 1990. Influences of refined konjac meal on the levels of tissue lipids and the absorption of four minerals in rats. Biomedical and Environmental Sciences, 3, 306–314. [PubMed] [Google Scholar]
- Huang C‐Y, Zhang M‐Y, Peng S‐S, Hong J‐R, Wang X, Jiang H, Zhang F, Bai J, Liang J, Yu Y, Luo Z, Zhang X and Zhou Z, 1990. Effect of konjac food on blood glucose levels in patients with diabetes. Biomedical and Environmental Sciences, 3, 123–131. [PubMed] [Google Scholar]
- Hurley IP, Pickles NA, Qin H, Ireland HE, Coleman RC, Tosun BN, Buyuktuncer Z and Williams JHH, 2010. Detection of Konjac glucomannan by immunoassay. International Journal of Food Science and Technology, 45, 1410–1416. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1993. Konjac flour, WHO Food Additives Series 32.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996. Konjac flour, WHO Food Additives Series 37.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. 976. Lead. WHO Food Additives Series 44.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Konjac flour. Combined Compendium of Food Additive Specifications ‐ all Specifications Monographs from the 1st to the 65th Meeting (1956‐2005).
- Kaats GR, Bagchi D and Preuss HG, 2015. Konjac glucomannan dietary supplementation causes significant fat loss in compliant overweight adults. Journal of the American College of Nutrition, 22, 1–7. [DOI] [PubMed] [Google Scholar]
- Katsuraya K, Okuyama K, Hatanaka K, Oshima R, Sato T and Matsuzaki K, 2003. Constitution of konjac glucomannan: chemical analysis and 13C NMR spectroscopy. Carbohydrate Polymers, 53, 183–189. [Google Scholar]
- Konishi F, Oku T and Hosoya N, 1984. Hypertrophic effect of unavailable carbohydrate on cecum and colon in rats. Journal of Nutritional Science and Vitaminology, 30, 373–379. [DOI] [PubMed] [Google Scholar]
- Kotkoskie LA, Weiner ML, Freeman C, Batt KJ, Jackson GC, Hardy GJ and Fletcher MJ, 1992. Acute toxicity studies with konjac flour. Toxicol, Letters Suppl, 281 pp. [Google Scholar]
- Liang S, Li B, Ding Y, Xu BL, Chen J, Zhu B ··· Knill CJ, 2011. Comparative investigation of the molecular interactions in konjac gum/hydrocolloid blends: concentration addition method (CAM) versus viscosity addition method (VAM). Carbohydrate Polymers, 83, 1062–1067. [Google Scholar]
- Loening‐Baucke V, Miele E and Staiano A, 2004. Fiber (Glucomannan) is beneficial in the treatment of childhood constipation. Pediatrics, 113, 259–264. 10.1542/peds.113.3.e259 [DOI] [PubMed] [Google Scholar]
- Martindale , 2014. The Complete Drug Reference, 38th Edition Pharmaceutical Press London, UK. [Google Scholar]
- Martindale , 2017. The Complete Drug Reference, The Pharmaceutical Press. MedicinesComplete online version. 2017 Royal Pharmaceutical Society.
- Matsuura Y, 1998. Degradation of konjac glucomannan by enzymes in human feces and formation of short‐chain fatty acids by intestinal anaerobic bacteria. Journal of Nutritional Science and Vitaminology, 44, 423–436. [DOI] [PubMed] [Google Scholar]
- Merck Index , 2006. Konjac gum and konjac glucomannan. 14th Edition, Merck & Co., Inc., Whitehouse Station, USA. [Google Scholar]
- Miyoshi H, Okuda T, Ishi K, Oshuga Y, Tomeda M and Koishi H, 1987. The effects of cellulose, pectin and konjac intake on growth and digestibility in rats. Osaka‐Shiritsu Daigaku Seikatsulkaoakubu Kiyo 35, 23‐28, cited in: JECFA, 1996. Konjac flour, WHO Food Additives Series 37.
- Mizutani T and Mitsuoka T, 1982. Effect of Konjac mannan on spontaneous liver tumorigenesis and fecal flora in C3H/He male mice. Caner Letters, 17, 27–32. [DOI] [PubMed] [Google Scholar]
- Nakajima N and Matsuura Y, 1997. Purification and characterization of konjac glucomannan degrading enzyme from anaerobic human intestinal bacterium, Clostridium butyricum‐Clostridium beijerinckii group. Bioscience, Biotechnology, and Biochemistry, 61, 1739–1742. [DOI] [PubMed] [Google Scholar]
- Nishinari K and Gao S, 2007. In: Biliaderis CG, Izydorczyck MS. (eds.). Konjac glucomannan. CRC Press LLC, Boca Raton, FL ‐ USA. [Google Scholar]
- Oketani Y, Ichikawa K, Ono C, Gofuko M, Kiwaki S and Kiriyama S, 1984. Toxicity studies on glucomannan (1) Acute toxicity studies in mice and rats. Appled Pharmacology, 27, 127–131. [Google Scholar]
- Oku T, Konishi F and Hosoya N, 1982. Mechanism of inhibitory effect of unavailable carbohydrate on intestinal calcium absorption. Journal of Nutrition, 112, 410–415. [DOI] [PubMed] [Google Scholar]
- Onakpoya I, Posadski P and Edzard E, 2014. The efficacy of glucomannan supplementation in overweight and obesity: a systematic review and meta‐analysis of randomized clinical trials. Journal of the American College of Nutrition, 33, 70–78. [DOI] [PubMed] [Google Scholar]
- Onishi N, Kawamoto S, Nishimura M, Nakano T, Aki T, Shigeta S, Shimizu H, Hashimoto K and Ono K, 2005. A mew immunomodulatory function of low‐viscous konjac glucomannan with a small particle size: Its oral intake suppresses spontaneous occurring dermatitis in NC/Nga mice. International Archives of Allergy and Immunology, 136, 258–265. [DOI] [PubMed] [Google Scholar]
- Onishi N, Kawamoto S, Suzuki H, Santo H, Aki T, Shigeta S, Hahimoto K, Hide M and Ono K, 2007. Dietary pulverized konjac glucomannan suppresses scratching behaviour and skin inflammatory immune responses in NC/Nga mice. International Archives of Allergy and Immunology, 144, 95–104. [DOI] [PubMed] [Google Scholar]
- Parry J‐M, 2010. Konjac, glucomannan edition. Imeson A. John Wiley & Sons Ltd., Hoboken. [Google Scholar]
- Peng S‐S, Zhang M‐Y, Zhang Y‐Z and Wu Z‐H, 1995. Long term animal feeding trial of the refined konjac meal.II. Effects of the refined konjac meal on the aging of the brain, liver and cardiovascular tissue cells in rats. Biomedical and Environmental Sciences, 8, 80–87. [PubMed] [Google Scholar]
- Preuss A and Thier HP, 1983. Isolation of natural thickeners from food by capillary gas chromatographic determination. Zeitschrift fuer Lebensmittel‐Untersuchung und ‐Forschung, 176, 5–11. [Google Scholar]
- SCF (Scientific Committee for Food), 1997. Food Science and Techniques, Reports of the Scientific Committee for Food, 41st Series.
- SCF (Scientific Committee on Food), 2001. Guidance on submissions for food additive evaluations by the Scientific Committee on Food. Opinion expressed on 11 July 2001.
- Shima K, Tanaka A, Ikegami H, Tabata M, Sawazaki N and Kumahara y, 1983. Effect of dietary fiber, glucomannan, on absorption of sulfonylurea in man. Horm. Metabol. Res. 15, 1–3, cited in: JECFA, 1996. Konjac flour, WHO Food Additives Series 37. [DOI] [PubMed] [Google Scholar]
- Sun Tan CQ, Wei HK, Zou Y, Long G, Ao JT, Xue HX, Jiang SW and Peng J, 2015. Effects of different amounts of konjac flour inclusion in gestation diets on physio‐chemical properties of diets, postprandial satiety in pregnant sows, lactation feed intake of sows and piglet performance. Animal Reproduction Science, 152, 55–64. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Oomizu S, Yanasa Y, Onishi N, Uchida K, Mihara S, Ono K, Kameyoshi Y and Hide M, 2010. Hydrolyzed konjac glucomannan suppresses IgE production in mice B cells. International Archives Immunology, 152, 122–130. [DOI] [PubMed] [Google Scholar]
- Tajima S, Kon H, Oshikawa K, Bando M, Ohno S and Sugiyama Y, 2003. Hypersensitivity pneumonitis induced by konjac flour and powdered hijikia fusiforme. Internal Medicine, 9, 846–849. [DOI] [PubMed] [Google Scholar]
- Takigami S, 2000. Konjac mannan In: Phillips GO. and Williams PA. (eds.). Handbook of hydrocolloids. CRC Press, Boca Raton, FL ‐USA, pp. 413–424. [Google Scholar]
- Tako M, 1992. Synergistic Interaction between Xanthan and Konjac Glucomannan in Aqueous Media. Bioscience, Biotechnology, and Biochemistry, 56, 1188–1192. [Google Scholar]
- TemaNord, 2002. E 425i Konjac Gum and E 425ii Konjac Glucomannan. In: Food Additives in Europe 2000 ‐ Status of safety assessments of food additives presently permitted in the EU, 417–421. [Google Scholar]
- Tokunaga T, Oku T and Hosoya N, 1986. Influence of chronic intake of a new sweetener fructooligosaccharide (Neosugar) on growth and gastrointestinal function of the rat. Journal of Nutritional Science and Vitaminology, 32, 111–121. [DOI] [PubMed] [Google Scholar]
- Topping DL and Clifton PM, 2001. Short‐chain fatty acids and human colonic function: roles and resistant starch and nonstarch polysaccharides. Physiological Reviews, 81, 1031–1064. [DOI] [PubMed] [Google Scholar]
- Vanderbeek PB, Fasano C, O'Malley G and Hornstein J, 2007. Esophageal obstruction from a hygroscopic pharmacobezoar containing glucomannan. Clinical Toxicology, 45, 80–82. [DOI] [PubMed] [Google Scholar]
- Venter CS, Vorster HH and Van der Nest DG, 1990. Comparison between physiological effects of konjac‐glucomannan and propionate in baboons fed “Western” diets. Journal of Nutrition, 120, 1046–1053. [DOI] [PubMed] [Google Scholar]
- Vido L, Facchin P, Gobber D and Rigon F, 1993. Childhood obesity treatment: double blinded trial on dietary fibres (glucomannans versus placebo. Paediatrica‐Paedologica, 28, 133–136. [PubMed] [Google Scholar]
- Wood RJ, Fernandez ML, Sharman MJ, Silvestre R, Greene CM, Zern TL, Shrestha S, Judelson DA, Gomez AL, Kraemer WJ and Volek JS, 2007. Effects of a carbohydrate‐restricted diet with and without supplemental soluble fiber on plasma low‐density lipoprotein cholesterol and other clinical markers of cardiovascular risk. Metabolism Clinical and Experimental, 56, 58–67. 10.1016/j.metabol.2006.08.021 [DOI] [PubMed] [Google Scholar]
- Wu WT and Chen HL, 2011a. Konjac glucomannan and inulin systematically modulate antioxidant defense in rats fed a high‐fat fiber‐free diet. Journal of Agriculture and Food Chemistry, 59, 9194–9200. [DOI] [PubMed] [Google Scholar]
- Wu WT and Chen HL, 2011b. Effects of konjac glucomannan on putative risk factors for colon carcinogenesis in rats fed a high‐fat diet. Journal of Agriculture and Food Chemistry, 59, 989–994. [DOI] [PubMed] [Google Scholar]
- Zalewski BM, Chmielewska A and Szajewska H, 2015. The effects of glucomannan on body weight in overweight or obese children and adults: a systematic review of randomized controlled trials. Nutrition, 31, 437–442. [DOI] [PubMed] [Google Scholar]
- Zhang M‐Y, Peng S‐S, Zhang Y‐Z and Wu Z‐H, 1995. Long term animal feeding trial of the refined konjac meal.I. Effects of the refined konjac meal on the calcium and phosphorus metabolism and the bone in rat. Biomedical and Environmental Sciences, 8, 74–79. [PubMed] [Google Scholar]
- Zhang K, Wong JW, Zhengwei J, Vaclavikova M, Trucksess MW and Begley TH, 2014. Screening multimycotoxins in food‐grade gums by stable isotope dilution and liquid chromatography/tandem mass spectrometry. Journal of the Association of Analytical Chemists International, 97, 889–895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the reported use levels (mg/kg or mg/L as appropriate) of konjac (E 425) provided by industry
Number and percentage of food products labelled with konjac (E 425) out of the total number of food products present in Mintel GNPD per food sub‐category between 2012 and 2017
Concentration levels of konjac (E 425) used in the refined exposure scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of konjac (E 425) from their use as food additives for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and high level (mg/kg bw per day)
Main food categories contributing to exposure to konjac (E 425) using the regulatory maximum level exposure scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure


