Abstract
The conclusions of EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State, Sweden, and co‐rapporteur Member State, Latvia, for the pesticide active substance tribenuron‐methyl are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012. The conclusions were reached on the basis of the evaluation of the representative uses of tribenuron‐methyl as a herbicide on winter and spring cereals (wheat, barley, oat, rye, triticale, durum, spelt), pasture, sunflower (tribenuron‐methyl‐tolerant varieties) and olive. The reliable end points, appropriate for use in regulatory risk assessment are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: tribenuron‐methyl, peer review, risk assessment, pesticide, herbicide
Summary
Commission Implementing Regulation (EU) No 844/2012 (hereinafter referred to as ‘the Regulation’) lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012. Tribenuron‐methyl is one of the active substances listed in Regulation (EU) No 686/2012.
In accordance with Article 1 of the Regulation, the rapporteur Member State (RMS), Sweden, and co‐rapporteur Member State (co‐RMS), Latvia, received an application from DuPont de Nemours GmbH and the EU Tribenuron AIR 3 Task Force comprised of Cheminova A/S and Helm AG for the renewal of approval of the active substance tribenuron‐methyl. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicants, the co‐RMS (Latvia), the European Commission and the European Food Safety Authority (EFSA) about the admissibility.
The RMS provided its initial evaluation of the dossier on tribenuron‐methyl in the renewal assessment report (RAR), which was received by EFSA on 23 June 2016. In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicants, DuPont de Nemours GmbH and the EU Tribenuron AIR 3 Task Force comprised of Cheminova A/S and Helm AG, for comments on 8 August 2016. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 10 October 2016.
Following consideration of the comments received on the RAR, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
In accordance with Article 13(1) of the Regulation, EFSA should adopt a conclusion on whether tribenuron‐methyl can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 of the European Parliament and of the Council.
The conclusions laid down in this report were reached on the basis of the evaluation of the representative uses of tribenuron‐methyl as a herbicide on winter and spring cereals (wheat, barley, oat, rye, triticale, durum, spelt), pasture, sunflower (tribenuron‐methyl‐tolerant varieties) and olive, as proposed by the applicants. Full details of the representative uses can be found in Appendix A of this report.
The use of tribenuron‐methyl according to the representative uses proposed at European Union (EU) level results in a sufficient herbicidal efficacy against the target weeds.
In the section identity, physical chemical properties, analytical methods a data gap was identified for formal reasons for additional validation data for the monitoring method for the determination of tribenuron‐methyl residues in air, relevant for the Task Force.
In the section mammalian toxicology, several data gaps were identified. Further assessment of the toxicological relevance of impurities needs to be provided (for DuPont and Cheminova), as well as further evidence that the batches used in the toxicological studies are sufficiently representative of the technical specification (for DuPont). Additional data on comparative in vitro metabolism should also be provided (including human cells), and further assessment of the toxicological profile of some metabolites has to be performed.
Several data gaps were identified in the residue section and the consumer risk assessment cannot be finalised pending upon the toxicological assessment and the magnitude of all relevant compounds to be included in the residue definition for risk assessment for plants and a comprehensive livestock exposure assessment. A data gap was also identified to address the residues of parent tribenuron‐methyl and its relevant metabolites in pollen and bee products.
The data available on environmental fate and behaviour are sufficient to carry out the required environmental exposure assessments at EU level for the representative uses, with the notable exception that satisfactory groundwater exposure assessments for metabolite M2 and the anaerobic soil metabolite IN‐GK521 are not available for the representative use assessed on winter cereals with autumn application. For the representative uses on olives, the majority or all the Forum for the Co‐ordination of Pesticide Fate Models and their Use (FOCUS) groundwater scenarios were predicted to have 80th percentile annual average recharge concentrations moving below 1 m for metabolites IN‐L5296, IN‐A4098, IN‐00581 and IN‐R9805 above the 0.1 μg/L parametric drinking water limit. For the representative uses on sunflower, all the relevant FOCUS scenarios were predicted to be above the limit of 0.1 μg/L for metabolite IN‐A4098 (max. 0.66 μg/L). For the representative uses of tribenuron‐methyl CHA 6310 on winter cereals with spring application, at least half of the relevant FOCUS scenarios were predicted to be above the limit of 0.1 μg/L for metabolite IN‐A4098 (9 out of 9 scenarios in both acidic and alkaline soils, max. 1.07 μg/L), IN‐L5296 (7 out of 9 scenarios with alkaline soils, max. 0.44 μg/L; 5 out of 9 scenarios with acidic soils, max. 0.18 μg/L), IN‐R9805 (7 out of 9 scenarios with alkaline soils, max. 0.18 μg/L) and IN‐00581 (7 out of 9 scenarios in both acidic and alkaline soils, max. 0.76 μg/L with alkaline soils). For the representative uses of tribenuron‐methyl CHA 6310 on winter cereals with autumn application, at least half of the relevant FOCUS scenarios were predicted to be above the limit of 0.1 μg/L for metabolite IN‐A4098 (9 out of 9 scenarios in both acidic and alkaline soils, max. 0.67 μg/L), IN‐L5296 (8 out of 9 scenarios with alkaline soils, max. 0.44 μg/L) and IN‐00581 (7 out of 9 scenarios in acidic soils, max. 0.51 μg/L, and 8 out of 9 scenarios with alkaline soils, max 0.88 μg/L). For the representative uses of tribenuron‐methyl CHA 6310 on spring cereals, at least half of the relevant FOCUS scenarios were predicted to be above the limit of 0.1 μg/L for metabolite IN‐A4098 (6 out of 6 scenarios for both acidic and alkaline soils, max. 1.2 μg/L), IN‐L5296 (6 out of 6 scenarios with alkaline soils, max. 0.41 μg/L), IN‐R9805 (5 out of 6 scenarios with alkaline soils, max. 0.19 μg/L) and IN‐00581 (4 out of 6 scenarios in acidic soils, max. 0.54 μg/L, and 6 out of 6 scenarios with alkaline soils, max 0.89 μg/L). On the basis of the available data in the mammalian toxicology section, the genotoxic potential of metabolites IN‐A4098 and IN‐L5296 cannot be excluded.
In the area of ecotoxicology, data gaps were identified for further information to address the long term risk to wild mammals for tribenuron‐methyl and the plant metabolites IN‐37739, IN‐QHP91 and IN‐GN815 (use on olives and pasture). In addition, further information is needed to address the risk to aquatic organisms and honeybees.
Background
Commission Implementing Regulation (EU) No 844/20121 (hereinafter referred to as ‘the Regulation’) lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/20092. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3).
In accordance with Article 1 of the Regulation, the RMS Sweden and co‐RMS Latvia received an application from DuPont de Nemours GmbH and the EU Tribenuron AIR 3 Task Force comprised of Cheminova A/S and Helm AG for the renewal of approval of the active substance tribenuron‐methyl. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicants, the co‐RMS (Latvia), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on tribenuron‐methyl in the RAR, which was received by EFSA on 23 June 2016 (Sweden, 2016).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicants, DuPont de Nemours GmbH and the EU Tribenuron AIR 3 Task Force comprised of Cheminova A/S and Helm AG, for consultation and comments on 8 August 2016. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 10 October 2016. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicants were invited to respond to the comments in column 3 of the reporting table. The comments and the applicants’ response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicants in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA, the RMS on 18 November 2016. On the basis of the comments received, the applicants’ response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicants, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in June 2017.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative uses of tribenuron‐methyl as a herbicide on winter and spring cereals (wheat, barley, oat, rye, triticale, durum, spelt), pasture, sunflower (tribenuron‐methyl‐tolerant varieties) and olive, as proposed by the applicants. A list of the relevant end points for the active substance and the formulation is provided in Appendix A.
In addition, a key supporting document to this conclusion is the peer review report (EFSA, 2017), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (18 November 2016);
the evaluation table (21 June 2017);
the reports of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Sweden, 2017), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the European Union (EU) for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Tribenuron‐methyl is the ISO common name for methyl 2‐[4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐yl(methyl)carbamoylsulfamoyl]benzoate (IUPAC). This substance is a derivative of tribenuron, 2‐[4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐yl(methyl)carbamoylsulfamoyl]benzoic acid (IUPAC)
The representative formulated products for the evaluation were ‘Tribenuron‐methyl 50SG (DPX‐L5300 50SG)’, a water‐soluble granule (SG) containing 500 g/kg tribenuron‐methyl and ‘Tribenuron‐methyl 750 g/kg WG (CHA 6310)’, a water‐dispersible granule containing 750 g/kg tribenuron‐methyl.
The representative uses evaluated were foliar spray applications in winter and spring cereals (wheat, barley, oat, rye, triticale, durum, spelt), pasture, sunflower (tribenuron‐methyl‐tolerant varieties), and olive to control broadleaved weeds. Full details of the Good Agricultural Practices (GAPs) can be found in the list of end points in Appendix A.
Data were submitted to conclude that the use of tribenuron‐methyl according to the representative uses proposed at EU level results in a sufficient herbicidal efficacy against the target weeds following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014b).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: SANCO/3029/99‐rev. 4 (European Commission, 2000a), SANCO/3030/99‐rev. 4 (European Commission, 2000b) and SANCO/825/00‐rev. 8.1 (European Commission, 2010).
The new proposed specifications for tribenuron‐methyl are based on batch data from industrial scale production and on quality control data. The proposed minimum purity of the technical material is 965 g/kg for DuPont and 960 g/kg for the Task Force. The minimum purities are meeting the requirements of the FAO specification 546/TC (January 2011) available for tribenuron‐methyl of not less than 950 g/kg tribenuron‐methyl, developed under the new procedure. It is proposed to update the original reference specification from min. 950 g/kg to min. 960 g/kg, supported by data from all applicants and taking into consideration that only limited information was available on the impurity profile of the batches used in the toxicological studies.
The assessment of the data package revealed no issues that need to be included as critical areas of concern with respect to the identity, physical, chemical and technical properties of tribenuron‐methyl or the representative formulations. The main data regarding the identity of tribenuron‐methyl and its physical and chemical properties are given in Appendix A.
Adequate methods are available for the generation of pre‐approval data required for the risk assessment. Methods of analysis are available for the determination of the active substance in the technical material and in the representative formulations.
The residue definition for monitoring for food and feed of plant and animal origin was set to tribenuron‐methyl. Monitoring residues of tribenuron‐methyl in plant and animal matrices can be done by liquid chromatography with tandem mass spectrometry (LC–MS/MS) with limit of quantifications (LOQs) of 0.01 mg/kg, in all commodity groups/matrices. The residue definition for monitoring in soil was defined as tribenuron‐methyl and metabolite IN‐L5296. Appropriate LC–MS/MS method exists for monitoring the compounds of the residue definition with LOQs of 1 μg/kg for each compound. Adequate LC–MS/MS methods were available for the determination of residues of tribenuron‐methyl in water with LOQs of 0.01 μg/L and 0.05 μg/L. Tribenuron‐methyl residues in air can be determined by high‐performance liquid chromatography‐ultraviolet (HPLC‐UV) with a LOQ of 1.5 μg/m3. The LC–MS/MS method submitted had a LOQ not sufficient for monitoring, as a consequence a data gap was identified for formal reasons for additional validation data for this method.
LC–MS/MS enforcement methods exist for the determination of tribenuron‐methyl residues in body fluids and tissues, with LOQ of 0.01 mg/kg in body tissues, 1 μg/kg in plasma and 3 μg/kg in urine, respectively.
2. Mammalian toxicity
The following guidance documents were followed in the production of this conclusion: SANCO/221/2000‐rev. 10‐final (European Commission, 2003), SANCO/10597/2003‐rev. 10.1 (European Commission, 2012) and Guidance on dermal absorption (EFSA PPR Panel, 2012).
Tribenuron‐methyl was discussed during the Pesticides Peer Review Meeting 155 in March 2017.
For the DuPont source, three impurities in the technical specification could not be considered as covered by their levels in the batches used for the toxicological studies, and four impurities could not be concluded for their toxicological relevance (data gap). For the Cheminova source, the toxicological relevance of two impurities could not be concluded. For the Helm source, no impurity was considered of toxicological relevance. For these two sources, the technical specifications can be considered as covered by the batches used in the toxicological studies (it is noted that the majority of the toxicological studies were provided by DuPont).
With an oral absorption value of 67%, tribenuron‐methyl has a low acute toxicity, and did not show indications of adverse effects other than skin sensitising properties (harmonised classification as Skin Sens 1, H317). A data gap has been identified for further investigations of comparative in vitro metabolism data (including human cells).
In short‐term toxicity studies, the no observed adverse effect level (NOAEL) in rats is 7 mg/kg body weight (bw) per day (90‐day study), based on clinical signs, reduced body weight (gain), changes in biochemical parameters and organ weight changes, whereas in mice, the NOAEL is 70 mg/kg bw per day based on reduced body weight gain and increased liver weight. For the studies with dogs, the NOAEL is 8 mg/kg bw per day (1‐year study) based on reduced body weight gain and changes in clinical chemistry (kidney parameters).
In the 2‐year rat study, the systemic NOAEL is 1 mg/kg bw per day based on reduced body weight gain in females, organ weight changes and histopathological findings, whereas the carcinogenic NOAEL is 10 mg/kg bw per day based on an increased incidence of mammary gland tumours (at a dose exceeding the maximum tolerated dose). In the 18‐month mouse study, the systemic NOAEL is 2.5 mg/kg bw per day based on effects in males (amyloidosis in testes and oligospermia in epididymides). It was agreed that tribenuron‐methyl is unlikely to be carcinogenic in humans.
In the multigeneration study, the reproductive parameters were not affected and the NOAEL for parental and offspring toxicity was 2 mg/kg bw per day based on reduced body weight (gain) and organ weight changes. Additional studies (one‐generation and reproductive effects in male rats) could not be relied upon due to severe limitations. For the rat developmental toxicity, the maternal NOAEL is 20 mg/kg bw per day based on reduced body weight (gain) and increased relative liver weight; and the developmental NOAEL is 20 mg/kg bw per day based on reduced bodyweight and skeletal alterations in pups. For the rabbit developmental toxicity study, the maternal NOAEL is 20 mg/kg bw per day based on body weight loss, mortalities and abortions; the developmental NOAEL is 20 mg/kg bw per day based on reduced pup bodyweight, decreased live fetuses, decreased nidations and increased number of malformations. On the basis of severe effects observed in rabbit studies (maternal deaths in the developmental toxicity study and histopathological changes in the kidneys in the 28‐day dermal study), the classification STOT RE cat 2 H373 is proposed3 for tribenuron‐methyl (and has been submitted to ECHA by the RMS).
Tribenuron‐methyl is not classified or proposed to be classified3 as toxic for reproduction category 2 or carcinogenic category 2, in accordance with the provisions of Regulation (EC) No 1272/20084, and therefore the conditions of the interim provisions of Annex II, Point 3.6.5 of Regulation (EC) No 1107/2009 concerning human health for the consideration of endocrine disrupting properties are not met. On the basis of the available scientific data, tribenuron‐methyl was concluded unlikely to have endocrine disrupting properties.
In the neurotoxicity studies with rats, no specific neurotoxic effects were observed, leading to an acute neurotoxic NOAEL of 300 mg/kg bw and a short term neurotoxic NOAEL of 40 mg/kg bw per day. In the 28‐day immunotoxicity study, no adverse effect related to immunotoxicity was observed.
Several metabolites of tribenuron‐methyl were discussed by the experts. With regard to saccharin (IN‐00581), it was agreed to apply an acceptable daily intake (ADI) of 3.8 mg/kg bw per day, and no acute reference dose (ARfD) was considered necessary (European Commission, 1997). For the metabolite metsulfuron‐methyl, the ADI is 0.22 mg/kg bw per day and the ARfD is 0.25 mg/kg bw (EFSA, 2015a). For the metabolite IN‐R9805, being a major rat metabolite, it was agreed that the reference values of tribenuron‐methyl are applicable. For the metabolites IN‐A4098, IN‐L5296 and IN‐B5685, the absence of genotoxic potential could not be concluded on the basis of the available data (data gap), and therefore, no reference values could be proposed. IN‐L2596 and IN‐A4098 are considered relevant groundwater metabolites. For the other metabolites [IN‐37739, IN‐D5803, IN‐G7462 (see Section 3), M2 and IN‐GK521 (see Section 4)], insufficient data were available to conclude on their toxicological profile or on appropriate reference values.
The following reference values were agreed for tribenuron‐methyl: an ADI of 0.01 mg/kg bw per day based on the 2‐year rat study and an ARfD of 0.2 mg/kg bw based on the rabbit developmental toxicity study; both were derived with an uncertainty factor (UF) of 100 [and are the same as those derived during the first peer review (European Commission, 2005)]. The acceptable operator exposure level (AOEL) is 0.05 mg/kg bw per day based on the combined 90‐day rat studies [whereas during the first peer review (European Commission, 2005), a value of 0.07 mg/kg bw per day had been derived]; and the acute acceptable operator exposure level (AAOEL) is 0.13 mg/kg bw based on the rabbit developmental toxicity study; both were derived with an UF of 100 and a correction for an oral absorption value of 67%. The default values for dermal absorption are applicable to the products CHA 6310 and DPX‐L5300 (25% for the concentrate and 75% for the dilution). For the representative uses, the non‐dietary exposure estimates were below the AOEL for the operators (German model, 75th percentile, with use of gloves during mixing/loading and application), workers, bystanders and residents.
3. Residues
The assessment in the residue section is based on the OECD guidance document on overview of the residue chemistry studies (OECD, 2009), the OECD publication on the maximum residue level (MRL) calculations (OECD, 2011) and the European Commission guideline document on the MRL setting (European Commission, 2011).
Tribenuron‐methyl was discussed at the Pesticides Peer Review Meeting 156 in March 2017.
Metabolism of tribenuron‐methyl in primary crops was investigated upon foliar application in cereals/grass (wheat), pulses/oilseed (canola, genetically modified (GM) soyabean, cotton) and miscellaneous fruits (olives) using 14C tribenuron‐methyl labelled, respectively, on the phenyl and triazine rings (0.8–3.5 N rate). Most of the radioactivity was recovered in leafy crop parts while the total residues in wheat grain and canola seeds ranged between 0.01 and 0.05 mg eq/kg and accounted for up to 0.13 mg eq/kg in GM soyabean seeds. Tribenuron‐methyl was mainly identified in the immature green parts of all crops at an early stage (i.e. 25% total radioactive residue (TRR) canola) and was hardly detected in the edible parts at maturity (0.3% TRR in grain only). Degradation of tribenuron‐methyl takes place mainly through the cleavage of the sulfonylurea linkage of the parent molecule with the formation of triazine amine and sulfonamide moiety‐related metabolites. Prior to cleavage, N‐demethylation of tribenuron‐methyl was also observed, hereby forming metsulfuron‐methyl which is an active substance itself. Triazine amine metabolites (IN‐R9805, IN‐37739, IN‐L5296 and IN‐A4098) were recovered at significant proportions in all wheat matrices with up to 26% TRR in straw, 36% TRR in forage, 46% TRR in hay, 8% TRR in grain and also in canola seeds (17% TRR). The sulfonamide‐related compounds (IN‐D5803, IN‐G7462, IN‐B5685 and IN‐D5119) were also detected in significant proportions in all wheat plant parts with up to 31% TRR in forage, 15% TRR in straw, 11%TRR in hay, 44.6% TRR in grain and 26% TRR in canola pods. In the treated GM soyabean and besides the presence of the triazine amine and sulfonamide‐related compounds in pods and seeds the metabolic pattern of the parent compound showed also the presence of IN‐QKQ78 (25% of TRR), L9622 glucoside (12% TRR), IN‐QHM63 (10% TRR), IN‐QLQ76 (12% TRRs) in the seeds. It is, however, highlighted that this metabolism study was conducted on a GM crop with an application at a later growth stage (BBCH 60‐63) and cannot therefore be considered as representative of the use on sunflower. It should be highlighted, however that in case of any import tolerance request on GM pulses and oilseeds crops, the investigation of the toxicological profile of these compounds might be required. In olives, following foliar application within the tree rows, the total residues were very low (0.01 mg/kg). Hence, for the specific representative use in olives, residues are not expected and no further metabolites’ identification is requested. There was also no identification in cotton because of the very low total residues in seeds (0.01–0.03 mg/kg).
A confined rotational crops metabolism study was conducted on leafy crops (cabbage), root crops (beet), pulses/oilseed (soya bean) and in cereals (sorghum/wheat) using 14C‐phenyl and 14C‐triazine labelled tribenuron‐methyl at 30 and 120 days plant back intervals (PBIs). The experts agreed that further metabolism data are not needed at a longer preharvest interval (PHI) (365 days) considering the low concentrations of radioactive residues observed in soil at 120 days PBI (0.001 and 0.01 mg eq/kg for the phenyl and triazine labellings, respectively). From the rotational crops studies conducted at 1.2 N, metabolites such as saccharin (IN‐00581), IN‐L5296, IN‐A4098, IN‐37739 were detected in plant tissues. The predominant metabolite in rotational crops was identified as IN‐A4098 being recovered in beet foliage up to 0.019 mg/kg at 30 days PBI. The metabolic pattern of tribenuron–methyl in rotational crops is deemed similar to the one depicted in primary crops, thus the same residue definitions are applicable.
Based on these metabolism studies in primary and rotational crops and in the absence of specific valid marker of the residues in cereal grain, canola/cotton seed and olive fruit, the residue definition for monitoring is proposed by default as tribenuron‐methyl. For risk assessment, besides tribenuron‐methyl, it is proposed to include IN‐D5803, IN‐G7462, IN‐B5685 (sulfonamide‐related compounds) and IN‐L5296, IN‐37739 (free and conjugated), IN‐R9805, IN‐A4098 (triazine amine related compounds) in the residue definition. This proposal will be reconsidered pending upon the toxicity of these compounds (see data gap in Section 2) and their magnitude in all relevant crops. Since the metabolism study in olives covers only the specific representative use on olives and cannot be extrapolated to all fruits group, the residue definitions cover only cereals and pulses/oilseeds crop groups.
Residue trials are available, analysed for tribenuron‐methyl on cereal grains and olives and covered by the analytical method, to derive an MRL of 0.01* mg/kg (LOQ). However, the trials were not analysed for all the metabolites provisionally included in the risk assessment residue definition. A data gap is therefore identified for sufficient trials analysing for all the compounds included in the residue definition for risk assessment (data gap). For sunflower, residue trials compliant with the critical GAP with regard to the PHI were not submitted and are requested (data gap). Two rotational crops field trials conducted on lettuce, radish and barley with a bare soil application of 30 g a.s./ha were also submitted. Considering the genotoxic potential of the metabolites IN‐L5296 and IN‐A4098, sufficient rotational crop field trials conducted on cereals, leafy vegetables and root vegetables and at a dose rate covering the plateau concentration in the soil for the relevant metabolites IN‐L5296 and IN‐A4098 are required (data gap).
Storage stability data demonstrates tribenuron‐methyl residues to be stable up to 37 months in high starch‐ content matrices, up to 14 months in high oil‐content matrices and up to 18 months in high water‐, protein‐ and acid‐content matrices. The stability of all relevant metabolites included in the residue definition for risk assessment was also demonstrated up to 18 months in all commodity categories.
It is noted that hydrolysis studies were not provided considering only tribenuron‐methyl residues in cereals grain and olives (< 0.01 mg/kg). However, the need for hydrolysis studies should be reconsidered pending upon the finalisation of the risk assessment residue definition for plants.
Tribenuron‐methyl metabolism in livestock was investigated in laying hens and lactating goats with both triazine‐ and phenyl‐labelled tribenuron methyl. In goat, the major compound was IN‐A4098, accounting from 35% up to 81% TRRs in all animal matrices. IN‐QKK48 (hydroxyl tribenuron‐methyl) was recovered in whole milk (0.6–10% TRR), kidney (14.5–18% TRR) and fat (12% TRR) for both labellings as well as saccharin that occurred in significant levels in all matrices (13–71% TRR). For poultry, IN‐A4098 was also recovered at significant levels from 40% up to 62% of TRR in all commodities, in addition IN‐L5296 accounted up to 17% of TRRs.
Based on these studies, the agreed animal residue definition for monitoring is tribenuron‐methyl for all matrices while for risk assessment separate residue definitions are proposed as following:
Ruminant matrices: tribenuron methyl and IN‐A4098
Poultries matrices: tribenuron‐methyl, IN‐L5296, IN‐A4098, and IN‐D5803. The way the risk assessment residue definitions will be expressed is pending upon the requested toxicological profile of these compounds (see data gap in Section 2). The potential inclusion of IN‐QKK48 and saccharin in the risk assessment residue definition for ruminants was also discussed during the expert's meeting and the majority opinion was not to include these compounds in the residue definition considering the highly overdosed metabolism studies and the lower toxicity of saccharin compared to the parent compound (ADI: 3.8 mg/kg bw per day; Section 2).
The finalisation of the livestock exposure assessment is however pending the assessment of the relevant residue in food and feed commodities. Therefore, pending upon the outcome of the outstanding data on the magnitude of the pertinent compounds identified in primary and rotational crops and their toxicity, the livestock dietary burden calculation should be reconsidered (data gap). Whether the compounds provisionally included in the risk assessment residue definition for plant, significantly contribute to the livestock dietary burden, their potential transfer in animal matrices may need to be further investigated.
Fish metabolism studies are not triggered with regard to the residue levels in cereals and olives (< 0.01 mg/kg); however, pending the finalisation of the risk assessment residue definition in plants and the GAP‐compliant residue trials in sunflower, fish metabolism and feeding studies may be required.
Results from the EFSA PRIMo rev.2 for chronic dietary risk assessment for consumers for tribenuron‐methyl indicated the highest estimate of chronic dietary intake is 1% of the ADI (WHO cluster diet B). The results of the acute dietary risk assessment (IESTI) do not identify any exceedances of the proposed ARfD of 0.2 mg/kg bw, (max. IESTI 0.1 mg/kg bw). However, in the absence of an agreed residue definition for risk assessment and considering further the potential genotoxicity of metabolite IN‐A4098, IN‐L5296 and IN‐B5685 the consumer risk assessment is not finalised for the representative uses.
It is further noted that IN‐A4098 and IN‐L5296 are common to a number of sulfonylurea compounds and their genotoxic effect cannot be ruled out (see Section 2); therefore, tribenuron‐ methyl may be an additional contributor to the overall exposure of consumers in regard to these compounds, for which an assessment has not yet been performed.
Moreover the level of metabolites IN‐A4098 and IN‐00581 in ground water exceeds 0.75 μg/L for a number of scenarios (see Section 4). The additional intake through drinking water with regard to IN‐00581 residues is negligible (< 0.1% of the ADI) for all considered consumer groups while the consumer exposure through drinking water will be reconsidered with regard IN‐A4098 pending upon the outcome of its toxicological profile (see Section 2).
The overall consumer exposure assessment is regarded as not finalised in view of the outstanding residues data in plant and livestock exposure.
It is noted that in the framework of the peer review for the renewal of the approval of tribenuron‐methyl, different residue definitions for risk assessment for plant and animal commodities were proposed; therefore, the MRLs derived under Article 12 of the Regulation (EC) No 396/2005 may need to be revised (EFSA, 2013b).
The data requirement for the determination of the residues in pollen and bee products for human consumption resulting from residues taken up by honeybees from crops at blossom is not addressed with regard to tribenuron‐methyl and relevant metabolites for risk assessment (data gap).
4. Environmental fate and behaviour
Tribenuron‐methyl was discussed at the Pesticides Peer Review Meeting TC 139 on environmental fate and behaviour in March 2017.
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, tribenuron‐methyl exhibited low to moderate persistence, forming the major (> 10% applied radioactivity (AR)) metabolites saccharin (IN‐00581) (max. 33.9% AR) which exhibited low to high persistence, IN‐L5296 (max. 85.7% AR) which exhibited high to very high persistence, IN‐A4098 (max. 12.6% AR) which exhibited moderate to high persistence, and M2 (max. 16.2% AR) which exhibited moderate to medium persistence. A metabolite which in at least two sequential measurements account for more than 5% of the amount of active substance added, was IN‐R9805 (max. 20.8% AR) which exhibited moderate to very high persistence. The rate of degradation of tribenuron‐methyl was concluded to be pH dependent with degradation being slower as soil pH increases. In these aerobic laboratory incubations, mineralisation of the phenyl and triazine ring 14C radiolabel to carbon dioxide accounted for 33.8–64.8% AR after 60–270 days and 1.5–16.8% AR after 60–365 days, respectively. The formation of unextractable residues (not extracted by acetone/aq. ammonium carbonate) for these radiolabels accounted for 27.9–35.8% AR after 60–270 days and 1.5–16.8% AR after 60–365 days, respectively. In anaerobic soil incubations, tribenuron‐methyl transformed more slowly than under aerobic conditions, forming new metabolites compared to those formed in aerobic incubations. The peer review concluded that the exclusive major anaerobic metabolite that required further assessment is IN‐GK521 (max. 32.1% AR), which exhibited low to moderate persistence in soil under aerobic conditions. It should be noted, however, that anaerobic conditions favourable to the formation of metabolite IN‐GK521 is relevant only for autumn/winter applications followed by transient flooding (i.e. winter cereals at BBCH 12‐19 in the representative GAP). Soil photolysis is not considered a significant degradation mechanism for tribenuron‐methyl.
In satisfactory field soil dissipation studies carried out at seven sites in Europe, tribenuron‐methyl exhibited very low to moderate persistence. The persistence and modelling endpoints for tribenuron‐methyl and its metabolites IN‐00581 (low to moderate persistence in soil) and IN‐L5296 (persistence endpoints only: moderate to very high persistence in soil) were derived. Based on the available data, no dissipation rates could be derived for metabolite IN‐A4098, which when dosed in some laboratory soil incubations, had single first‐order DT50 greater than 60 days. No clear pH dependency was observed for tribenuron‐methyl under field conditions.
The mobility in soil of tribenuron‐methyl and its metabolites relevant for assessment was studied by batch equilibrium tests on a variety of different soils. Tribenuron‐methyl and metabolite M2 exhibited high to very high mobility in soil. It was concluded that the adsorption of tribenuron‐methyl was pH dependent. Metabolites saccharin (IN‐00581), IN‐D5119, IN‐GK521, IN‐GN815 exhibited very high soil mobility, metabolite IN‐L5296 exhibited high mobility, and IN‐R9805 exhibited low to very high mobility. Metabolite IN‐A4098 exhibited medium to very high mobility in soil. It was concluded that the adsorption of these metabolites was not pH dependent.
In laboratory incubations in dark aerobic natural sediment water systems, tribenuron‐methyl exhibited moderate persistence, forming the major metabolites IN‐L5296 (max. 88.9% AR in the total system after 56 days), IN‐D5119 (max. 26.5% AR in the total system after 56 days), IN‐GN815 (max. 13% AR in the total system after 29 days), IN‐R9805 (max. 14.7% AR in the total system after 71 days). The unextractable sediment fraction (not extracted by acetone/aqueous ammonium carbonate) accounted for 16–26% AR after 105 days for phenyl ring 14C radiolabel, and it accounted for 9.3–16% AR after 105 days for triazine ring 14C radiolabel. Mineralisation accounted for 60–65% AR for phenyl ring 14C radiolabel at study end (135 days), and it accounted for 1.4–18% AR for triazine ring 14C radiolabel after 105–135 days. In a laboratory sterile aqueous photolysis experiment, tribenuron‐methyl was photolytically stable, with no new metabolites formed in the irradiated samples as compared to the dark control samples.
The necessary surface water and sediment exposure assessments (predicted environmental concentrations (PEC) calculations) were carried out for tribenuron‐methyl and its metabolites saccharin (IN‐00581), IN‐L5296, M2, IN‐A4098, IN‐R9805, IN‐D5803 (initially included in the list of residues requiring further assessment), IN‐D5119, IN‐GN815 and IN‐GK521, using the FOCUS (2001) step 1 and step 2 approach (version 3.2 of the Steps 1‐2 in FOCUS calculator). For tribenuron‐methyl, step 4 calculations were available. Run‐off scenarios considering a 10 m buffer a combined 10 m spray drift buffer and vegetative strip was simulated (incorporating 60% reduction in run‐off water and mass of pesticide in aqueous phase and 85% reduction in mass of eroded sediment). In step 4, simulations for run‐off scenarios considering a 20 m buffer a combined 20 m spray drift buffer and vegetative strip was simulated (incorporating 80% reduction in run‐off water and mass of pesticide in aqueous phase and 95% reduction in mass of eroded sediment).The SWAN tool (version 3.0.0) was appropriately used to implement these mitigation measures in the simulations. However, risk managers and others may wish to note that while run‐off mitigation is included in the step 4 calculations available, the FOCUS (2007) report acknowledges that for substances with KFoc < 2000 mL/g (i.e. tribenuron‐methyl), the general applicability and effectiveness of run‐off mitigation measures had been less clearly demonstrated in the available scientific literature, than for more strongly adsorbed compounds. It should be noted that no PECsw/PECsed are available for the representative uses 1 × 7.5 or 5.5 g a.s./ha at BBCH 30–39 in winter and spring cereals with underlay crop (grass 7.5 g a.s./ha and red clover 5.5 g a.s/ha) as these representative uses were considered covered by the higher application rates to winter and spring cereals (1 × 24 and 1 × 22.5 g a.s./ha, respectively).
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (European Commission, 2014a) scenarios and the models PEARL 4.4.4 and PELMO 5.5.3 for the active substance tribenuron‐methyl and its metabolites IN‐L5296, IN‐00581, M2, IN‐A4098, IN‐R9805, and IN‐GK521 (for tribenuron‐methyl in CHA 6310). Two sets of calculations were performed due to pH‐dependence of the degradation rate and of adsorption of tribenuron‐methyl: one for acidic soils (pH < 7) and one for alkaline soils (pH > 7). Additionally, higher tier PECgw calculations were conducted for the active substance in alkaline soil using the normalised field DT50 for tribenuron‐methyl and for metabolite IN‐R9805 using a formation fraction of 0.5 (Tier 2 calculations). After the experts’ meeting TC 139, new PECgw were calculated by the RMS for the parent compound and the metabolites reflecting the results of the data requirements set during the commenting phase and the open points identified at the experts’ meeting. It should be noted that a risk envelope approach was used for the representative uses with the application rate of 1 × 7.5 or 5.5 g a.s./ha at BBCH 30–39 in winter and spring cereals with underlay crop. Therefore, in the absence of specific PECgw calculations for these representative uses, the same conclusion on the groundwater exposure available for the higher application rates to winter and spring cereals (1 × 24 and 1 × 22.5 g a.s./ha) is valid also for these representative uses.
PECgw for tribenuron‐methyl DPX‐L5300:
For tribenuron‐methyl DPX‐L5300, although EFSA identified some deficiencies in the input parameters, the information is considered sufficient to conclude on the groundwater exposure assessment for tribenuron‐methyl and its metabolites. The potential for groundwater exposure from the representative uses by tribenuron‐methyl DPX‐L5300 above the parametric drinking water limit of 0.1 μg/L was concluded to be low in all geoclimatic situations that are represented by all FOCUS groundwater scenarios, except for the representative uses on winter cereals with autumn application where PECgw were > 0.1 μg/L in three out of nine scenarios for alkaline soils at Tier 2 (max 0.22 μg/L).
For the representative use on olives, the 80th percentile annual average recharge concentrations leaving the top 1 m soil layer were estimated to be > 0.1 μg/L at three or four out of four scenarios for metabolites IN‐L5296, IN‐00581, IN‐A4098 (exceeding also 0.75 μg/L) and IN‐R9805 in both acid alkaline soils.
For the representative use on pasture, concentrations expressed on this basis were estimated to be < 0.1 μg/L at all scenarios for all metabolites.
For the representative use on spring cereals, concentrations expressed on this basis were estimated to be > 0.1 μg/L at one out of six scenarios in alkaline soils for IN‐A4098 and in two out of six scenarios in alkaline soils for metabolite IN‐00581. For the representative use on spring cereals, concentrations expressed on this basis were estimated to be < 0.1 μg/L for acidic soils at all scenarios for IN‐00581 and in both acid and alkaline soils for IN‐R9805 and IN‐L5296.
For the representative use on winter cereals, concentrations expressed on this basis were estimated to be > 0.1 μg/L at one and three out of nine scenarios in acidic and alkaline soils, respectively, for IN‐A4098 and in one out of nine scenarios in alkaline soils for metabolite IN‐00581. For the representative use on winter cereals, concentrations expressed on this basis were estimated to be < 0.1 μg/L for acidic soils at all scenarios for IN‐00581 and in both acid and alkaline soils for IN‐R9805 and IN‐L5296.
For the representative use on sunflower, concentrations expressed on this basis were estimated to be > 0.1 μg/L in all scenarios for IN‐A4098 in both acid and alkaline soils, and in one out of two scenarios for both alkaline and acidic soils for IN‐00581, IN‐R9805 and IN‐L5296.
PECgw for tribenuron‐methyl CHA 6310:
For tribenuron‐methyl CHA 6310, although EFSA identified some deficiencies in the input parameters, the information is considered sufficient to conclude on the groundwater exposure assessment for tribenuron‐methyl and its metabolites, except for metabolite M2 for the representative use on winter cereals (autumn application) and the anaerobic metabolite IN‐GK521. In these cases, it is difficult to predict a priori the impact that the differences between the correct input parameters that should have been used and the input parameters currently used in the available FOCUS GW modelling, would have on the final risk assessment. Therefore, a data gap has been identified for new FOCUS GW modelling for metabolite M2 and the anaerobic metabolite IN‐GK521 for the representative use on winter cereals (autumn application). Insufficient data were available to conclude on the toxicological profile of these two metabolites (see Section 2).
The potential for groundwater exposure from the representative uses by tribenuron‐methyl CHA 6310 and metabolite M2 above the parametric drinking water limit of 0.1 μg/L was concluded to be low in all geoclimatic situations that are represented by all FOCUS groundwater scenarios, except for the representative use on winter cereals (autumn application) where PECgw were > 0.1 μg/L in three out of nine scenarios at Tier 2.
For the representative use on winter cereals (autumn application), concentrations expressed on this basis were estimated to be > 0.1 μg/L in all scenarios for IN‐A4098 in both acidic and alkaline soils, in one out of nine scenarios in acidic soils for IN‐L5296, and in seven and eight out of nine scenarios for IN‐00581 in acidic and alkaline (also exceeding > 0.75 μg/L) soils, respectively. For the representative use on winter cereals, concentrations expressed on this basis were estimated to be < 0.1 μg/L for acidic soils at all scenarios for IN‐R9805.
For the representative use on winter cereals (spring application), concentrations expressed on this basis were estimated to be > 0.75 μg/L in four and five out of nine scenarios for IN‐A4098 in acidic and alkaline soils, respectively, and > 0.1 μg/L in three and six out of six scenarios for metabolite IN‐L5296 in acidic and alkaline soils, respectively, and in four and seven out of nine scenarios for IN‐R9805 in acidic and alkaline soils, respectively, and in seven out of nine scenarios for IN‐00581 for both acidic and alkaline soils.
For the representative use on spring cereals, concentrations expressed on this basis were estimated to be ≥ 0.75 μg/L at one and four out of six scenarios for IN‐A4098 in acidic and alkaline soils, respectively; in three and five out of six scenarios for IN‐R9805 in acidic and alkaline soils, respectively, and in four and six out of six scenarios for IN‐00581 in acidic and alkaline (also exceeding > 0.75 μg/L) soils, respectively.
In conclusion, PECgw exceeding 0.1 μg/L in more than half of the relevant FOCUS scenarios by metabolites are calculated for the following uses:
Olives: IN‐L5296, IN‐A4098, IN‐00581 and IN‐R9805
Sunflowers: IN‐A4098 and IN‐00581
Pasture: none
Winter cereals (CHA 6310, autumn application): IN‐L5296, IN‐A4098, IN‐00581, data gap for M2 and IN‐GK521 (anaerobic metabolite)
Winter cereals (CHA 6310, spring application): IN‐L5296, IN‐A4098, IN‐00581 and IN‐R9805
Spring cereals (CHA 6310): IN‐L5296, IN‐A4098, IN‐00581 and IN‐R9805.
It should be noted that PECgw were > 0.75 μg/L for IN‐A4098 (representative uses on olives and winter cereals with autumn application in alkaline soils), IN‐00581 (sunflower in acidic soils, spring cereals and both autumn and spring applications to winter cereals in acidic soils for CHA 6310). None of the metabolites exceed 10 μg/L for all uses. Based on the available information in the mammalian toxicology section, the absence of genotoxic potential could not be concluded for metabolites IN‐A4098 and IN‐L5296 (see Section 2).
The PEC in soil, surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix A of this conclusion.
5. Ecotoxicology
The risk assessment was based on the following documents: European Commission (2002a,b), SETAC (2001), EFSA (2009), EFSA PPR Panel (2013) and EFSA (2013a). According to Regulation (EU) No. 283/20135, data should be provided regarding the acute and chronic toxicity to honeybees and data to address the development of honeybee brood and larvae. As the European Commission (2002a) does not provide a risk assessment scheme which is able to use the chronic toxicity data for adult honeybees and the honeybee brood, when performing the risk assessment according to European Commission (2002a), the risk to adult honeybees from chronic toxicity and the risk to bee brood, could not be finalised due to the lack of a risk assessment scheme. Therefore, the EFSA (2013a) was used for risk assessment in order to reach a conclusion for the representative uses.
Some aspects of the risk assessment of tribenuron‐methyl were discussed at the Pesticides Peer Review Meeting 157.
A low acute and long‐term risk to birds was concluded for tribenuron‐methyl and its pertinent metabolites for all the routes of exposures and representative uses. A low acute risk to wild mammals for tribenuron‐methyl and its pertinent metabolites was concluded for all routes of exposure and for all the representative uses. A low long‐term risk to wild mammals for tribenuron‐methyl was concluded for all representative uses with the exception of the use on olives for which a high long term risk to small herbivorous mammals was identified at Tier 1. The available refinements to the risk assessment for this use were discussed at the Pesticides Peer Review Meeting 157, however, the available refinement (selection of common vole as focal species) was not considered acceptable (data gap). In addition, a high long term risk to small herbivorous mammals (vole) could not be excluded at Tier 1 for the uses on olives and pasture for metabolites IN‐37739, IN‐QHP91 and IN‐GN815. The available refinement to the risk for the plants metabolites (RUD refinement) was discussed at the Pesticides Peer Review Meeting 157, however, the available refinement was not considered acceptable (data gap).
Concerning the aquatic organisms, a low acute and chronic risk was concluded for the uses on pasture, olives, sunflower (provided that mitigation measures are implemented, see Section 8) and on spring cereals (provided that mitigation measures are implemented, see Section 8). A low acute risk was concluded for the uses on winter cereals while a high chronic risk (aquatic plants) was identified for the uses on winter cereals. It is noted that for the lowest application rates exposure estimates were not available, therefore, a risk assessment was not performed, and the same conclusion for the higher application rates to winter and spring cereals (1 × 24 and 1 × 22.5 g a.s./ha) is valid also for these representative uses. The possibility to refine the risk assessment was discussed at the Pesticides Peer Review Meeting 157, however, the available refinement was not considered acceptable (data gap). It is noted that the chronic risk assessment was performed with the most sensitive organism (aquatic plants); however, further information to refine the risk on additional taxonomic groups (e.g. algae) might be needed. A low acute and chronic risk to aquatic organisms was concluded for all the pertinent surface water metabolites for all the representative uses.
Suitable acute (oral and contact) and chronic toxicity studies (including the assessment of effects on the hypopharyngeal gland) on honeybees were available for the active substance and the representative formulations. By using this data in a risk assessment, a low risk was concluded for all the representative uses and exposure routes with the exception of exposure to guttation water (data gap). A low risk via exposure to contaminated surface water was concluded while data were not sufficient to assess the risk via exposure to water in puddles (data gap). It is noted that the RMS disagreed with this data gap. A suitable assessment for accumulative effects was not available. Information regarding metabolites occurring in pollen and nectar was not available (data gap). In the case of honeybees larvae, single exposure studies and a repeated dose study (5 days) were available. These studies were not considered as sufficient to address the risk to honeybees’ larvae (data gap).
No data were available for bumblebees and solitary bees.
A low risk to non‐target arthropods was concluded for all the representative uses. A low risk to earthworms and other soil macroorganisms was concluded for tribenuron‐methyl and its pertinent metabolites for all the representative uses. It is noted that in the case of Hypoaspis aculeifer an increase in reproduction was observed in the studies available with ‘Tribenuron methyl 50SG + DPX‐KG691’ and IN‐00581; these effects were not considered as biological relevant and therefore were not considered while setting the ecotoxicological endpoints, the RMS disagreed with this approach. A low risk to soil microorganisms was concluded for tribenuron‐methyl and its pertinent metabolites.
The deterministic and probabilistic approaches for the non‐target terrestrial plants risk assessment were discussed by the experts at the Pesticides Peer Review Meeting. A low risk to non‐target plants was concluded when the endpoint derived in the probabilistic approach was used and when mitigation measures are considered (see Section 8).
A low risk for tribenuron‐methyl for biological methods of sewage treatment was concluded.
With regard to the endocrine disruption potential, as discussed in Section 2, it is unlikely that tribenuron‐methyl is an endocrine disruptor in mammals; however, no firm conclusion can be drawn regarding fish and birds.
6. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1–4)
Table 1.
Soil
| Compound (name and/or code) | Persistence | Ecotoxicology |
|---|---|---|
| Tribenuron‐methyl |
Low to moderate persistence Single first‐order and biphasic kinetics DT50 1.7–18.3 days (DT90 9.7–91.3 days, 20°C pF 2 or 40–60% MWHC soil moisture) Very low to moderate persistence European field dissipation studies single first‐order and biphasic kinetics DT50 0.5–34.7 days (DT90 5.1–115 days) |
Low risk |
| IN‐L5296 |
high to very high persistence Single first‐order DT50 105–505 days (20°C pF 2 or 40–60% MWHC soil moisture) |
Low risk |
| IN‐A4098 |
moderate to high persistence Single first‐order and biphasic kinetics DT50 44.7–333 days (DT90 97–> 1,000 days, 20–25°C pF 2 or 40–55% MWHC soil moisture) |
Low risk |
| IN‐00581 |
Low to high persistence Single first‐order and Hockey Stick kinetics DT50 9.1–237.4 days (DT90 32.2–788.6 days, 20°C pF 2 or 40–45% MWHC soil moisture) Low to moderate persistence European field dissipation studies single first‐order DT50 4.5–26.4 days |
Low risk |
| IN‐R9805 |
Moderate to very high persistence Single first‐order and biphasic kinetics DT50 17.6–380 days (DT90 58.3–> 1,000 days, 20°C pF 2 or 37–50% MWHC soil moisture) |
Low risk |
| M2 |
Moderate to medium persistence Biphasic and Hockey Stick kinetics DT50 11.0–73.2 days (DT90 36.5–244 days, 20°C pF 2 or 37–60% MWHC soil moisture) |
Low risk |
| IN‐GK521 (anaerobic) |
Low to moderate persistence Single first‐order and biphasic kinetics DT50 4.8–51.9 days (DT90 15.9–>172 days, 20°C pF 2 or 36–50% MWHC soil moisture) |
Low risk |
DT50: period required for 50% dissipation; DT90: period required for 90% dissipation; MWHC: maximum water‐holding capacity.
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Tribenuron‐methyl | LC50 > 6.0 mg/L air (4 h exposure, nose‐only) |
LC50: lethal concentration, median.
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| Tribenuron‐methyl |
Very high to high mobility KFoc 4.6–73.7 mL/g |
Yes Winter cereals (autumn application): 3/9 FOCUS scenarios (0.01–0.22 μg/L, alkaline conditions) at Tier 2 |
Yes | Yes |
| IN‐L5296 |
High mobility KFoc 52.7–138 mL/g |
Yes Olives: 3/4 FOCUS scenarios (0.09–0.17 μg/L, acidic conditions); 4/4 FOCUS scenarios (0.20–0.57 μg/L, alkaline conditions) Sunflower: 1/2 FOCUS scenarios (0.004–0.15 μg/L, acidic conditions); 1/2 FOCUS scenarios (0.02–0.33 μg/L, alkaline conditions) Winter cereals, autumn application (tribenuron‐methyl CHA 6310): 1/9 FOCUS scenarios [(<0.001–0.12 μg/L, acidic conditions); 8/9 FOCUS scenarios 0.01–0.37 μg/L, alkaline conditions] Winter cereals, spring application (tribenuron‐methyl CHA 6310): 5/9 FOCUS scenarios [(< 0.001–0.18 μg/L, acidic conditions); 7/9 FOCUS scenarios (< 0.001–0.44 μg/L, alkaline conditions)] Spring cereals (tribenuron‐methyl CHA 6310): 3/6 FOCUS scenarios (0.02–0.16 μg/L, acidic conditions); 6/6 FOCUS scenarios (0.13–0.41 μg/L, alkaline conditions) |
No |
Yes Genotoxic potential cannot be excluded on the basis of the available data |
| IN‐A4098 |
Medium to very high mobility KFoc 2.9–225.5 mL/g |
Yes Olives: 4/4 FOCUS scenarios (0.24–0.77 μg/L, acidic conditions); 4/4 FOCUS scenarios (0.29–0.99 μg/L, alkaline conditions) Sunflower: 2/2 FOCUS scenarios (0.14–0.49 μg/L, acidic conditions); 2/2 FOCUS scenarios (0.21–0.66 μg/L, alkaline conditions) Winter cereals (tribenuron‐methyl DPX‐L5300): 1/9 FOCUS scenarios (0.02–0.10 μg/L, acidic conditions); 3/9 FOCUS scenarios 0.003–0.12 μg/L, alkaline conditions) Winter cereals, autumn application (tribenuron‐methyl CHA 6310): 9/9 FOCUS scenarios (0.11–0.51 μg/L, acidic conditions); 9/9 FOCUS scenarios 0.11–0.67 μg/L, alkaline conditions) Winter cereals, spring application (tribenuron‐methyl CHA 6310): 9/9 FOCUS scenarios (0.23–0.99 μg/L, acidic conditions); 9/9 FOCUS scenarios 0.26–1.07 μg/L, alkaline conditions) Spring cereals (tribenuron‐methyl DPX‐L5300): no for acidic conditions); 1/6 FOCUS scenarios (0.05–0.12 μg/L, alkaline conditions) Spring cereals (tribenuron‐methyl CHA 6310): 6/6 FOCUS scenarios (0.45–1.18 μg/L, acidic conditions); 6/6 FOCUS scenarios (0.49–1.20 μg/L, alkaline conditions) |
No |
Yes Genotoxic potential cannot be excluded on the basis of the available data |
| IN‐00581 |
Very high mobility Kfoc 1.8–20.2 mL/g |
Yes Olives: 3/4 FOCUS scenarios (0.07–0.20 μg/L, acidic conditions); 4/4 FOCUS scenarios (0.23–0.35 μg/L, alkaline conditions) Sunflower: 1/2 FOCUS scenarios (0.01–0.15 μg/L, acidic conditions); 1/2 FOCUS scenarios (0.04–0.31 μg/L, alkaline conditions) Winter cereals (tribenuron‐methyl DPX‐L5300): 1/9 FOCUS scenarios 0.001–0.10 μg/L, alkaline conditions) Winter cereals, autumn application (tribenuron‐methyl CHA 6310): 7/9 FOCUS scenarios (<0.001–0.51 μg/L, acidic conditions); 8/9 FOCUS scenarios 0.02–0.88 μg/L, alkaline conditions) Winter cereals, spring application (tribenuron‐methyl CHA 6310): 7/9 FOCUS scenarios (< 0.001–0.48 μg/L, acidic conditions); 7/9 FOCUS scenarios (< 0.001–0.76 μg/L, alkaline conditions) Spring cereals (tribenuron‐methyl DPX‐L5300): 2/6 FOCUS scenarios (0.03–0.14 μg/L, alkaline conditions) Spring cereals (tribenuron‐methyl CHA 6310): 4/6 FOCUS scenarios (0.05–0.54 μg/L, acidic conditions); 6/6 FOCUS scenarios (0.12–0.89 μg/L, alkaline conditions) |
No |
No ADI 3.8 mg/kg bw per day ARfD not necessary |
| IN‐R9805 |
Low to very high mobility KFoc 16.3–1509 mL/g |
Yes Olives: 4/4 FOCUS scenarios (0.15–0.34 μg/L, acidic conditions); 4/4 FOCUS scenarios (0.18–0.52 μg/L, alkaline conditions) Sunflower: 1/2 FOCUS scenarios (0.04–0.23 μg/L, acidic conditions); 1/2 FOCUS scenarios (0.07–0.32 μg/L, alkaline conditions) Winter cereals, spring application (tribenuron‐methyl CHA 6310): 4/9 FOCUS scenarios (< 0.001–0.15 μg/L, acidic conditions); 7/9 FOCUS scenarios (< 0.001–0.18 μg/L, alkaline conditions) at Tier 2 Spring cereals (tribenuron‐methyl CHA 6310): 3/6 FOCUS scenarios (0.05–0.15 μg/L, acidic conditions); 5/6 FOCUS scenarios (0.09–0.19 μg/L, alkaline conditions) at Tier 2 |
No |
No Major rat metabolite of tribenuron‐methyl |
| M2 |
High to very high mobility KFoc 39.5–98.9 mL/g |
No Data gap for winter cereals with winter application |
Assessment not triggered – pending on confirmation of GW levels | No data – data gap pending on confirmation of groundwater levels |
| IN‐GK521 (anaerobic) |
Very high mobility KFoc 12.5–27.7 mL/g |
Data gap for winter cereals with winter application, only | Assessment not triggered – pending on confirmation of GW levels | No data – data gap pending on confirmation of groundwater levels |
KFoc: Freundlich organic carbon adsorption coefficient; FOCUS: Forum for the Co‐ordination of Pesticide Fate Models and their Use; ADI: acceptable daily intake; bw: body weight; ARfD: acute reference dose.
At least one FOCUS scenario or a relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Tribenuron‐methyl | High risk (winter cereals) |
| IN‐L5296 | Low risk |
| IN‐A4098 | Low risk |
| IN‐00581 | Low risk |
| IN‐R9805 | Low risk |
| M2 | Low risk |
| IN‐GK521 (from soil, anaerobic) | Low risk |
| IN‐GN815 | Low risk |
| IN‐D5119 | Low risk |
7. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of Regulation (EC) No 1107/2009 concerning information on potentially harmful effects).
Additional validation data to prove that the LC–MS/MS monitoring method in air can have a LOQ of 15 μg/m3 (relevant for the Task Force; submission date proposed by the applicant: unknown; see Section 1).
Further assessment of the toxicological relevance of some impurities in the technical specification (relevant for DuPont and Cheminova sources), and of the representativeness of the batches used in the toxicity studies (relevant for DuPont source) (submission date proposed by the applicants: unknown; see Section 2).
Further investigations of comparative in vitro metabolism data (including human cells) (relevant for all representative uses, submission date proposed by the applicant: unknown; see Section 2).
Further toxicological assessment of the metabolites IN‐A4098, IN‐L5296 and IN‐B5685, for which a genotoxic potential could not be excluded, has to be provided (relevant for all representative uses, submission date proposed by the applicant: unknown; see Section 2).
Further toxicological assessment of the metabolites IN‐37739, IN‐D5803 and IN‐G7462 should be provided (relevant for all representative uses, submission date proposed by the applicant: unknown; see Section 2).
Sufficient residue trials analysing for the magnitude of residues for all compounds included in the plant risk assessment residue definition (relevant for all representative uses; submission date proposed by the applicants: unknown; see Section 3)
Residue trials on sunflower compliant with the representative GAP and analysing for tribenuron‐methyl and its metabolites (IN‐D5803, IN‐G7462, IN‐B5685, IN‐L5296 and IN‐37739 (free and conjugated), IN‐R9805 and IN‐A4098) relevant for the risk assessment residue definition (relevant for sunflower; submission date proposed by the applicants: unknown; see Section 3)
Sufficient rotational field trials conducted on cereals, leafy vegetables and root vegetables at a dose rate representative of the maximum plateau concentration in the soil for the relevant metabolites IN‐L5296 and IN‐A4098 are required (relevant for the representative uses on spring & winter cereals (with and without underlay), and sunflower; submission date proposed by the applicants: unknown; see Section 3).
The livestock dietary burden calculation to be reconsidered pending upon the final decision on the risk assessment residue definition in plants and their potential transfer to livestock, (relevant for the representative uses on spring and winter cereals (with and without underlay), sunflower, and pasture; submission date proposed by the applicants: unknown; see Section 3).
Potential residue levels in pollen and bee products (relevant for all representative uses evaluated; submission date proposed by the applicants: unknown; see Section 3).
Satisfactory predicted environmental concentrations in groundwater are not available for metabolite M2 and the anaerobic metabolite IN‐GK521 (relevant for the representative use on winter cereals with autumn application only; submission date proposed by the applicant: unknown; see Section 4).
Further information to address the long term risk to small herbivorous mammals (vole) for tribenuron‐methyl (relevant for the use on olives; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the long term risk to small herbivorous mammals (vole) for metabolites IN‐37739, IN‐QHP91 and IN‐GN815 (relevant for the uses on olives and pasture; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the chronic risk to aquatic organisms. It is noted that the chronic risk assessment was performed with the most sensitive organism (aquatic plants); however, further information to refine the risk on additional taxonomic groups (e.g. algae) might be needed (relevant for the uses on winter cereals; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk to honeybees via exposure to puddle and guttation water (relevant for all the representative uses; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk to honeybees’ larvae (relevant for all the representative uses; submission date proposed by the applicant (DuPont): end of 2017; see Section 5).
Further information regarding metabolites occurring in pollen and nectar (relevant for all the representative uses; submission date proposed by the applicant: unknown; see Section 5).
8. Particular conditions proposed to be taken into account to manage the risk(s) identified
Use of personal protective equipment (gloves) is recommended for operators during mixing/loading and application of the plant protection product (see Section 2).
The risk to aquatic organisms for some scenarios should be mitigated by implementing the following mitigation measures (see Section 5): 20 m drift buffer and a vegetative filter strip (relevant for FOCUS scenario R4, use on sunflower and for FOCUS scenario R3, D3, D5, D6 use on winter cereals – autumn application at 15 g a.s/ha; FOCUS scenario R1 and R3 use on winter cereals – spring application; FOCUS scenario R4 use on spring cereals at 30 g a.s./ha); 10 m drift buffer and a vegetative filter strip (relevant for FOCUS scenario R4, use on winter cereals at 24 g a.s./ha and 30 g a.s./ha and on spring cereals at 22.5 g a.s./ha) (see Section 5).
The risk to non‐target terrestrial plants should be mitigated by implementing mitigation measures such as 50% reduction by nozzles and 10 m buffer zone (see Section 5).
9. Concerns
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/20116 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The overall consumer risk assessment is regarded as not finalised in view of the outstanding residues data needed in order to finalise the risk assessment residue definitions in plants and animals (see Section 3).
The groundwater exposure assessment for metabolite M2 and the anaerobic soil metaboliteIN‐GK521 for the representative use on winter cereals with autumn application (see Section 4).
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at a lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009. None identified for the representative uses assessed
9.3. Overview of the concerns identified for each representative use considered
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
Table 5.
Overview of concerns
| Representative use | Spring cereals 22.5 g a.s./ha | Winter cereals 24 g a.s./ha | Spring/winter cereals 7.5 g a.s./ha | Spring/winter cereals 5.5 g a.s./ha | Pasture 7.5 g a.s./ha | |
|---|---|---|---|---|---|---|
| Operator risk | Risk identified | |||||
| Assessment not finalised | ||||||
| Worker risk | Risk identified | |||||
| Assessment not finalised | ||||||
| Resident/bystander risk | Risk identified | |||||
| Assessment not finalised | ||||||
| Consumer risk | Risk identified | |||||
| Assessment not finalised | X1 | X1 | X1 | X1 | X1 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | |||||
| Assessment not finalised | ||||||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | |||||
| Assessment not finalised | ||||||
| Risk to aquatic organisms | Risk identified |
1/9 FOCUS scenario |
Xb | Xb | ||
| Assessment not finalised | ||||||
| Groundwater exposure to active substance | Legal parametric value breached | |||||
| Assessment not finalised | ||||||
| Groundwater exposure to metabolites | Legal parametric value breached | |||||
| Parametric value of 10 μg/La breached | ||||||
| Assessment not finalised | ||||||
| Representative use |
Sunflower 30 g a.s./ha |
Olive 20 g a.s./ha |
Winter cereals 15 g a.s./ha |
Winter cereals 30 g a.s./ha |
Spring cereals 30 g a.s./ha |
|
|---|---|---|---|---|---|---|
| Operator risk | Risk identified | |||||
| Assessment not finalised | ||||||
| Worker risk | Risk identified | |||||
| Assessment not finalised | ||||||
| Resident/bystander risk | Risk identified | |||||
| Assessment not finalised | ||||||
| Consumer risk | Risk identified | |||||
| Assessment not finalised | X1 | X1 | X1 | X1 | X1 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X | ||||
| Assessment not finalised | ||||||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | |||||
| Assessment not finalised | ||||||
| Risk to aquatic organisms | Risk identified |
3/9 FOCUS scenarios |
2/9 FOCUS scenarios |
|||
| Assessment not finalised | ||||||
| Groundwater exposure to active substance | Legal parametric value breached | |||||
| Assessment not finalised | ||||||
| Groundwater exposure to metabolites | Legal parametric value breached | X | X | X | X | X |
| Parametric value of 10 μg/La breached | ||||||
| Assessment not finalised | X2 | |||||
a.s.: active substance; FOCUS: Forum for the Co‐ordination of Pesticide Fate Models and their Use.
Columns are grey if no safe use can be identified. The superscript numbers relate to the numbered points indicated in Sections 9.1 and 9.2. Where there is no superscript number, see Sections 2, 3, 4, 5–6 for further information.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission, 2003.
PEC calculations were not available for the GAP on spring/winter cereals (7.5 g a.s./ha and 5.5 g a.s./ha)
Abbreviations
- a.s.
active substance
- AAOEL
acute acceptable operator exposure level
- ADI
acceptable daily intake
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GM
genetically modified
- HPLC
high‐pressure liquid chromatography or high‐performance liquid chromatography
- IESTI
international estimated short‐term intake
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- LC
liquid chromatography
- LC50
lethal concentration, median
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- MRL
maximum residue level
- MWHC
maximum water‐holding capacity
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PEC
predicted environmental concentration
- PECair
predicted environmental concentration in air
- PECgw
predicted environmental concentration in groundwater
- PECsed
predicted environmental concentration in sediment
- PECsoil
predicted environmental concentration in soil
- PECsw
predicted environmental concentration in surface water
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RAR
Renewal Assessment Report
- RMS
rapporteur Member State
- SG
water‐soluble granule
- SMILES
simplified molecular‐input line‐entry system
- TRR
total radioactive residue
- UF
uncertainty factor
- UV
ultraviolet
- WG
water‐dispersible granule
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4912
Appendix B – Used compound codes
| Code/trivial namea | Chemical name/SMILES notation | Structural formula |
|---|---|---|
| IN‐L5296 |
4‐methoxy‐N,6‐dimethyl‐1,3,5‐triazin‐2‐amine Cc1nc(NC)nc(OC)n1 |
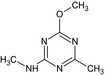
|
|
IN‐00581 saccharin |
1,2‐benzothiazol‐3(2H)‐one 1,1‐dioxide O=C2NS(=O)(=O)c1ccccc12 |
|
| IN‐37739 |
[(4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐yl)amino]methanol Cc1nc(NCO)nc(OC)n1 |
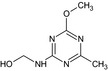
|
| IN‐A4098 |
4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐amine Cc1nc(N)nc(OC)n1 |
|
| IN‐B5528 |
4‐amino‐6‐methyl‐1,3,5‐triazin‐2(1H)‐one Nc1nc(C)nc(O)n1 |
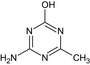
|
| IN‐B5685 |
methyl 2‐(carbamoylsulfamoyl)benzoate O=S(=O)(NC(N)=O)c1ccccc1C(=O)OC |
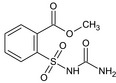
|
| IN‐B9700 |
methyl 4‐(d‐glucopyranosyloxy)‐2‐{[(4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐yl)carbamoyl]sulfamoyl}benzoate Cc1nc(nc(OC)n1)NC(=O)NS(=O)(=O)c3 cc(OC2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)ccc3C(=O)OC |
|
| IN‐D5119 |
2‐sulfamoylbenzoic acid O=S(N)(=O)c1ccccc1C(=O)O |
|
| IN‐D5803 |
methyl 2‐sulfamoylbenzoate O=S(N)(=O)c1ccccc1C(=O)OC |
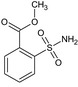
|
| IN‐G7460 |
methyl 4‐hydroxy‐2‐{[(4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐yl)carbamoyl]sulfamoyl}benzoate Cc2nc(NC(=O)NS(=O)(=O)c1cc(O)ccc1C(=O)OC)nc(OC)n2 |
|
| IN‐G7462 |
methyl 4‐hydroxy‐2‐sulfamoylbenzoate O=S(N)(=O)c1cc(O)ccc1C(=O)OC |
|
| IN‐GK521 |
methyl 2‐{[methyl(4‐methyl‐6‐oxo‐1,6‐dihydro‐1,3,5‐triazin‐2‐yl)carbamoyl]sulfamoyl}benzoate Cc1nc(nc(O)n1)N(C)C(=O)NS(=O)(=O)c2ccccc2C(=O)OC |
|
| IN‐GN815 |
2‐{[methyl(4‐methyl‐6‐oxo‐1,6‐dihydro‐1,3,5‐triazin‐2‐yl)carbamoyl]sulfamoyl}benzoic acid Cc1nc(nc(O)n1)N(C)C(=O)NS(=O)(=O)c2ccccc2C(=O)O |
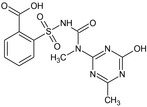
|
| IN‐QHM63 |
3‐(1,1‐dioxido‐3‐oxo‐1,2‐benzothiazol‐2(3H)‐yl)‐DL‐alanine O=C(O)C(N)CN2C(=O)c1ccccc1S2(=O)=O |
|
| IN‐QHP91 |
[4‐methoxy‐6‐(methylamino)‐1,3,5‐triazin‐2‐yl]methanol COc1nc(CO)nc(NC)n1 |

|
| IN‐QKK48 |
methyl 2‐({[4‐(hydroxymethyl)‐6‐methoxy‐1,3,5‐triazin‐2‐yl](methyl)carbamoyl}sulfamoyl)benzoate COc1nc(CO)nc(n1)N(C)C(=O)NS(=O)(=O)c2ccccc2C(=O)OC |
|
| IN‐QKQ78 |
2‐{[(2RS)‐2‐amino‐2‐carboxyethyl]sulfamoyl}benzoic acid O=S(=O)(NCC(N)C(=O)O)c1ccccc1C(=O)O |
|
| IN‐QLQ76 |
methyl 2‐{[carbamimidoyl(methyl)carbamoyl]sulfamoyl}benzoate O=S(=O)(NC(=O)N(C)C(=N)N)c1ccccc1C(=O)OC |
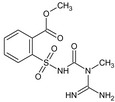
|
| IN‐R9803 |
2‐{[(4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐yl)(methyl)carbamoyl]sulfamoyl}benzoic acid Cc1nc(nc(OC)n1)N(C)C(=O)NS(=O)(=O)c2ccccc2C(=O)O |
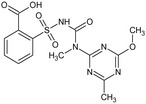
|
| IN‐R9805 |
4‐methyl‐6‐(methylamino)‐1,3,5‐triazin‐2(1H)‐one Cc1nc(NC)nc(O)n1 |
|
| M2 |
1‐(4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐yl)‐1‐methylurea Cc1nc(nc(OC)n1)N(C)C(N)=O |
|
| L9622 glucoside |
(4‐amino‐6‐methoxy‐1,3,5‐triazin‐2‐yl)methyl d‐glucopyranoside Nc2nc(COC1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)nc(OC)n2 |
|
SMILES: simplified molecular‐input line‐entry system.
The compound name in bold is the name used in the conclusion.
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Arena M, Auteri D, Barmaz S, Bellisai G, Brancato A, Brocca D, Bura L, Byers H, Chiusolo A, Court Marques D, Crivellente F, De Lentdecker C, De Maglie M, Egsmose M, Erdos Z, Fait G, Ferreira L, Goumenou M, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Magrans JO, Medina P, Miron I, Molnar T, Nougadere A, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Tarazona J, Terron A, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Conclusion on the peer review of the pesticide risk assessment of the active substance tribenuron‐methyl. EFSA Journal 2017;15(7):4912, 32 pp. 10.2903/j.efsa.2017.4912
Requestor: European Commission
Question number: EFSA‐Q‐2015‐00817
This scientific output, approved on 23 June 2017, supersedes the previous output published on 31 March 2005 (EFSA, 2005)
Amendment 1: Corrections were carried out on page 4 and in Sections 9.2 and 9.3 to provide consistency in interpretation. These corrections do not materially affect the contents or outcome of this scientific output. To avoid confusion, the older version has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made.
Amendment 2: Editorial corrections were carried out in the Appendix A (as regards the geometric and arithmetic mean KFoc values and arithmetic mean 1/n value) that do not affect the contents or outcome of this scientific output. To avoid confusion, the original version of the output has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made.
Approved: 23 June 2017
Amended: 25 May 2018
Amended: 25 November 2019
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
It should be noted that harmonised classification and labelling is formally proposed and decided in accordance with Regulation (EC) No 1272/2008.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- EFSA (European Food Safety Authority), 2005. Conclusion regarding the peer review of the pesticide risk assessment of the active substance tribenuron. EFSA Journal 2005;3(3):15r, 52 pp. 10.2903/j.efsa.2005.15r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Conclusion regarding the peer review of the pesticide risk assessment of the active substance chlorsulfuron. EFSA Journal 2009;7(3):201r, 107 pp. 10.2903/j.efsa.2009.201r [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013a. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2013b. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for tribenuron according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2013;11(11):3457, 32 pp. 10.2903/j.efsa.2013.3457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. Conclusion on the peer review of the pesticide risk assessment of the active substance ethametsulfuron (evaluated variant ethametsulfuron‐methyl). EFSA Journal 2014;12(1):3508, 94 pp. 10.2903/j.efsa.2014.3508 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Conclusion on the peer review of the pesticide risk assessment of the active substance prosulfuron. EFSA Journal 2014;12(9):3815, 94 pp. 10.2903/j.efsa.2014.3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2015a. Conclusion on the peer review of the pesticide risk assessment of the active substance metsulfuron‐methyl. EFSA Journal 2015;13(1):3936, 106 pp. 10.2903/j.efsa.2015.3936 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015b. Conclusion on the peer review of the pesticide risk assessment of the active substance triasulfuron. EFSA Journal 2015;13(1):3958, 78 pp. 10.2903/j.efsa.2015.3958 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015c. Conclusion on the peer review of the pesticide risk assessment of the active substance thifensulfuron‐methyl. EFSA Journal 2015;13(7):4201, 144 pp. 10.2903/j.efsa.2015.4201 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016a. Conclusion on the peer review of the pesticide risk assessment of the active substance iodosulfuron‐methyl‐sodium (approved as iodosulfuron). EFSA Journal 2016;14(4):4453, 111 pp. 10.2903/j.efsa.2016.4453 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016b. Conclusion on the peer review of the pesticide risk assessment of the active substance propoxycarbazone (variant evaluated propoxycarbazone‐sodium). EFSA Journal 2016;14(10):4612, 25 pp. 10.2903/j.efsa.2016.4612 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance tribenuron‐methyl. EFSA Journal 2005;3(3):RN‐15, 52 pp. 10.2903/j.efsa.2005.15r [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- European Commission , 1997. Scientific Committee for Food, European Commission, Directorate‐General III. Opinion on Saccharin and its Sodium, Potassium, and Calcium Salts. Annex III to Document III/5157/97, CS/ADD/EDuL/148‐Final.
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002
- European Commission , 2002b. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003
- European Commission , 2005. Review report for the active substance tribenuron‐methyl. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 15 February 2005 in view of the inclusion of tribenuron‐methyl in Annex I of Council Directive 91/414/EEC. SANCO/10671/04 ‐Final, 15 February 2005, 8 pp.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9. March 2011. pp. 1–46
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2014a. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 3, 613 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2, May 2014.
- European Commission , 2014b. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.1, March 2012.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005‐v. 2.0, 434 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2007. Landscape and mitigation factors in aquatic risk assessment. Volume 1. Extended summary and recommendations. Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v. 2.0, 169 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: www.oecd.org
- SETAC (Society of Environmental Toxicology and Chemistry), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2.
- Sweden , 2016. Renewal Assessment Report (RAR) on the active substance tribenuron‐methyl prepared by the rapporteur Member State Sweden, in the framework of Commission Implementing Regulation (EU) No 844/2012, June 2016. Available online: www.efsa.europa.eu
- Sweden , 2017. Revised Renewal Assessment Report (RAR) on tribenuron‐methyl prepared by the rapporteur Member State Sweden in the framework of Regulation (EC) No 1107/2009, May 2017. Available online: www.efsa.europa.eu
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


