Abstract
Objectives:
Mindfulness is effective for reducing anxiety and depression and increasing chronic disease self-management. An accessible, insurance-reimbursable model for implementation in patient-centered medical homes within US healthcare systems has promise for patients with multi-morbid conditions. Clarifying both the dose needed to impact anxiety, depression and self-management, and the design requirements for accessible primary care implementation, is essential.
Methods:
We tested feasibility, acceptability, and effectiveness of Mindfulness Training for Primary Care (MTPC), an 8-week, referral-based, insurance-reimbursable mindfulness program integrated within primary care, compared with a Low-Dose Comparator (LDC), consisting of a 60-minute mindfulness introduction plus referral to community and digital resources. Outcome measures were assessed at baseline and 8 weeks. MTPC is trauma-informed, incorporates mindfulness-oriented behavior change skills, and is designed to target anxiety, depression, stress, and chronic illness selfmanagement. Participants schedule a PCP visit to co-create a self-management action plan during week 6.
Results:
Primary care providers (PCP) referred 344 patients over 14 months. Eighty-one participants with DSM-V anxiety disorders, depressive disorders, trauma- and stress-related disorders participated in this pilot randomized-controlled comparative effectiveness trial [MTPC (n=54); LDC (n=27)]. These data suggest that MTPC was more effective than LDC for reducing anxiety (p=0.01), enhancing mindfulness (p=0.02) and self-compassion (p=0.001), and for catalyzing selfmanagement behavior change through action plan initiation (OR=4.34, p=0.03).
Conclusions:
MTPC was successfully integrated into a health system, was billed to insurance, and was acceptable to a diverse primary care population. Replication with a larger study and further accessibility adaptations are needed to confirm and expand these pilot results.
Keywords: anxiety, primary care, mindfulness, self-regulation, health behavior, self-management
A major aim of the movement to integrate mental health services into primary care(Croghan, 2010; Klein, 2013) is to increase access to these services for patients with co-morbid chronic conditions. The majority of individuals with anxiety and depression seek mental health treatment in primary care(Eisenberg, 1992; Olfson, Kroenke, Wang, & Blanco, 2014; Culpepper, Clayton, Lieberman, & Suskin, 2008), often presenting with pain or other somatic distress(Allen, Gara, Escobar, Waitzkin, & Silver, 2001; Chong, Reinschmidt, & Moreno, 2010; deGruy III, 1996), especially racial and ethnic minorities that may also otherwise not access mental health care because of associated stigmas(Lesser et al., 2008; Miranda & Cooper, 2004; Nadeem, Lange, & Miranda, 2008). Anxiety and depression are frequently co-morbid with chronic illness (e.g., diabetes)(D. P. Chapman, Perry, & Strine, 2005; S. Cohen, Janicki-Deverts, & Miller, 2007; Kessler, DuPont, Berglund, & Wittchen, 1999; Lin et al., 2010), and the combination of mental illness and a chronic medical illness predicts poor health outcomes(Moussavi et al., 2007). Collaborative care interventions in primary care that integrate mental and physical health have improved overall health outcomes(Friis, Johnson, Cutfield, & Consedine, 2016; Katon et al., 2010), suggesting that improvement of mental health symptoms and the self-regulation deficits that often accompany onset of mental illness (e.g., emotion regulation difficulties(Joormann & Stanton, 2016), disrupted attention and cognitive control(Rock, Roiser, Riedel, & Blackwell, 2014), etc.) might be a pre-requisite for successful chronic illness self-management among patients with co-morbid mental and physical illness. Therefore, initiatives are needed that successfully combine increased access to mental health treatment with enhancement of self-management skills for chronic illness.
Mindfulness training programs represent an evidence-based treatment modality that reduces anxiety and depression(Goyal et al., 2014) and increases quality of life for people living with chronic illness(M. M. Demarzo, Montero-Marin, et al., 2015). Mindfulness training also has the potential to enhance self-regulation in a way that could catalyze health behavior change in the context of chronic illness(Benzo, 2013; Loucks et al., 2015; Tang, Holzel, & Posner, 2015). Despite the evidence supporting Mindfulness-Based Programs(Crane et al., 2017) and the spread of mindfulness into mainstream contexts, minimal research exists on healthcare system mindfulness implementation models. Successful implementation of mindfulness training programs into primary care across an entire healthcare system holds promise as a large-scale and sustainable mechanism for enhancing chronic illness self-management, improving patient symptoms(Sundquist, Palmer, Johansson, & Sundquist, 2017), and reducing healthcare service utilization(Kurdyak, Newman, & Segal, 2014; McCubbin et al., 2014; Roth & Stanley, 2002). However, there are implementation barriers for mind-body oriented treatments. Barriers include limited time within a primary care visit, limited coverage by insurance payers(McGuire, Gabison, & Kligler, 2016), need for high-quality mindfulness group leader training for healthcare professionals, culturally-relevant adaptations(Proulx et al., 2018), appropriate group location, creation of an appropriate referral process, and level of onsite administrative support(M. M. Demarzo, Cebolla, & Garcia-Campayo, 2015). The Mindfulness-Based Program (MBP)(Crane et al., 2017) format based on an 8-week training with a recommended 45 minutes of daily home practice(Carmody & Baer, 2009) is associated with the strongest evidence base in clinical settings, and is modeled after Mindfulness-Based Stress Reduction (MBSR)(Kabat-Zinn et al., 1992). In addition, efficacy of mindfulness training administered in a smaller range of doses (e.g., about 1-2 hours total in one time-session, over days or weeks, or 8 hours over 4 weeks(M. Demarzo et al., 2017; Tang et al., 2007; Zeidan et al., 2015)) has been previously demonstrated, yet there is little guidance about which training dose is required in a healthcare setting for impacting health outcomes and health-related behavior change. Furthermore, existing MBPs target symptoms associated with specific diagnoses or stress reduction in general. A mindfulness training program that improved medical regimen adherence and patients’ capacity for chronic illness self-management and health behavior change, through improved self-regulation, would address a central aim of the integration of mental health into primary care and the healthcare reform movement underlying Patient-Centered Medical Homes and the Chronic Care Model(Antonucci, 2008; Bodenheimer, Lorig, Holman, & Grumbach, 2002; Grady & Gough, 2014; Kanaan, 2008; Pearson, 2007).
We developed a primary care-adapted 8-week MBP called Mindfulness Training for Primary Care (MTPC), which is referral-based, insurance-reimbursable, trauma-informed, and fully integrated into a regional healthcare system. We conducted a pilot comparative effectiveness trial(Fiore & Lavori, 2016) integrated into a primary care setting, comparing MTPC with a 60-minute introduction to mindfulness plus a referral to community and digital mindfulness resources (Low-Dose Comparator). The primary aims were to determine (1) feasibility and acceptability of mindfulness training integrated into the health system as a referral-based program with insurance-reimbursement (Feasibility Aim); (2) pilot test the effective dose of mindfulness training for reducing anxiety symptoms, as well as other health outcomes relevant in primary care (depression, stress, self-efficacy) in primary care patients with anxiety, depression, and co-morbid chronic conditions (Health Outcomes Aim); and (3) to pilot test the effectiveness of mindfulness training on medical regimen adherence and health behavior change, through a novel action plan initiation protocol (Medical Regimen Adherence Aim).
METHOD
PARTICIPANTS
Participants were recruited via flyer and information about the study through their primary care teams across six Patient-Centered Medical Homes (PCMHs) within a public healthcare system in the metro-north Boston area. Interested primary care patients were referred by their primary care provider (PCP) through a customized referral order in the electronic health record (EHR) in which primary and secondary referral diagnoses were indicated.
Participant Recruitment and Health System Referral Process.
The implementation of the clinical program in primary care, the referral process, and clinician template scheduling and documentation processes within the electronic health record involved ongoing collaboration and ongoing communication with key stakeholders. This implementation and institutional change initiative, called the MINDFUL-PC project, included discussions with health system referral coordinators, a patient advisory committee, electronic health record specialists, medical billing departments, members of departmental leadership in medicine, family medicine, and psychiatry, the hospital executive team, as well as clinic practice managers and regional medical directors for primary care sites. As part of the institutional change process resulting from integrating mindfulness within this health system(Schuman & Abrahm, 2005), primary care providers were educated about this opportunity for patients, the referral process and inclusion/exclusion criteria through grand rounds presentations, in-person presentations at all-staff meetings, and written materials designed to educate providers. Simultaneously, as part of an employee wellbeing healthcare transformation program, all primary care team staff (>400) were given a 1-hour introduction to mindfulness at staff trainings and MBSR was offered free of cost to all interested primary care providers. Behavioral health providers were educated about clinical and safety issues related to mindfulness(Lindahl, Fisher, Cooper, Rosen, & Britton, 2017), and were trained to apply the inclusion/exclusion criteria during 60-minute screening behavioral health evaluations.
PROCEDURE
Participant Screening.
Eligible individuals were 18 years or older, received primary care within the health system, and had a DSM-V diagnosis expected to potentially benefit from mindfulness (Figure 1). PCPs were encouraged to refer patients who had co-morbid mental and physical conditions who may have interest in mindfulness, mind-body approaches to managing their chronic illness, or who might be interested in a group-based approach to managing their anxiety, stress, or depression. All referrals underwent a preliminary chart review by a study coordinator. To assess clinical appropriateness and confirm diagnosis for insurance billing, patients new to mental health treatment in the health system were required to have a behavioral health evaluation in the health system within the past 6 months. In this way, patients who were new to mental health treatment were connected with a mental health clinician during the initial evaluation. Patients in active treatment with a health system mental health clinician (social worker, psychologist, psychiatrist) were required to have a documented session at least once in the past 3 months. All evaluating mental health clinicians filled out a 9-item checklist on a secure online REDCap(Harris et al., 2009) screening form to confirm single or co-morbid DSM-V diagnoses and inclusion/exclusion criteria prior to patient enrollment. We considered the first-listed diagnosis on the REDCap form to be the primary referring diagnosis for analysis. Exclusion criteria were presence of symptoms of psychosis, thought disorder, and/or severe mental illness including schizophrenia, schizoaffective disorder, bipolar I disorder, current severe episode of major depressive disorder, active moderate-severe substance use disorder, cognitive impairment, high risk of imminent hospitalization (including current suicidal ideation or an inpatient admission or psychiatric emergency department visit in the last 6 months), an insurance payer that did not cover group psychotherapy, English reading proficiency below 7th grade (<4 of 7 on REALM-SF Health Literacy Assessment(Arozullah et al., 2007)), or third-trimester pregnancy. Exclusion criteria were known to referring PCPs and were applied by the study coordinator in collaboration with a board-certified psychiatrist, who provided clarification and reached out to individual’s providers when diagnosis or exclusion criteria required clarification. Presence of the following common chronic medical conditions(Disease Control and Prevention, 2018; Ornstein, Nietert, Jenkins, & Litvin, 2013) for the 6-month period prior to diagnosis was determined through the electronic medical record: hypertension, hypercholesterolemia, arthritis, coronary heart disease/ischemic heart disease, chronic kidney disease, heart failure, chronic obstructive pulmonary disease, and obesity).
FIGURE 1.
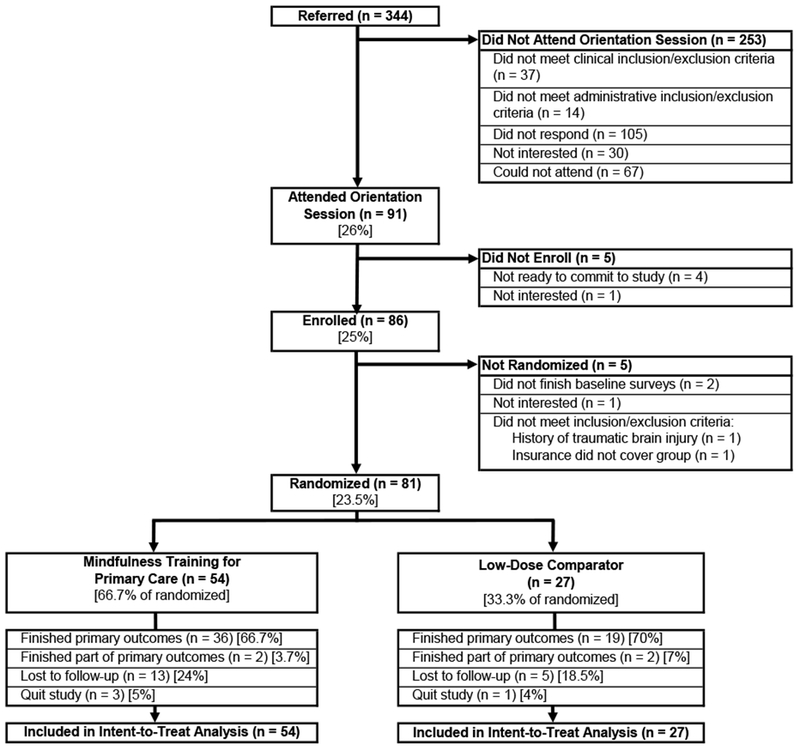
CONSORT DIAGRAM
Informed Consent and 60-Minute Introduction to Mindfulness.
Eligible individuals were invited to an informed consent session in one of two regional hub PCMHs. Participants were informed that they would continue receiving standard mental health treatment in the healthcare system (including access to psychopharmacology and psychotherapy)(Unutzer & Park, 2012) regardless of the outcome of randomization. Participants were enrolled in one of 7 cycles between October 2015 and October 2016. Directly after informed consent and completion of baseline measures, all participants received a 60-minute introduction to mindfulness prior to randomization. The session was led by a MTPC group leader (trained health system mental health provider or primary care provider, see below for details) and included a conceptual/experiential mindfulness introduction, inquiry, and digital(Plaza, Demarzo, Herrera-Mercadal, & Garcia-Campayo, 2013) and community resources (Supplemental Materials). To reduce the impact of variation in motivation for mindfulness, participants were able to decline study continuation after the 60-minute introduction and before randomization. All participants included in this study gave informed consent.
Randomization and Allocation.
Overseen by a blinded study staff member, a computer-based forced block randomization algorithm assigned participants who finished their baseline surveys (N=81) in a 2:1 ratio to MTPC (n=54) or Low-Dose Comparator (LDC) arm (n=27) (Figure 1). The 2:1 ratio was chosen as a response to patient and PCP feedback to make MTPC more accessible. The randomization ensured the 2:1 ratio was maintained across the two PCMH sites where MTPC groups were being offered, and across two PCP mindfulness training levels (as defined by PCP’s previous participation in a prior program at the same health system that included a MBSR training for PCPs) to prevent potential confounding caused by differences in PCP mindfulness expertise. Randomization was conducted by the data manager and assignment for each participant was given to the research coordinator who informed participants by telephone.
Intervention: Mindfulness Training for Primary Care.
MTPC builds upon MBSR’s transdiagnostic approach and combines training in evidence-based targeted mindfulness skills from other MBPs(Brewer et al., 2011; Garland, Schwarz, Kelly, Whitt, & Howard, 2012; Neff & Germer, 2013; Teasdale et al., 2000) with both psychotherapy elements from Mindfulness-Based Cognitive Therapy(Teasdale et al., 2000) and with approaches to behavior change adapted from Cognitive Behavioral Therapy(Fenn & Byrne, 2013), relapse prevention(Marlatt & Donovan, 2007), and motivational enhancement(Miller & Rollnick, 1995). MTPC offered eight weekly 2-hour sessions with a 7-hour all-day session and a recommended 30-45 minutes of daily home practice with guided recordings. MTPC was designed to be trauma-informed(Amaro, Spear, Vallejo, Conron, & Black, 2014; Pollak, 2014) through, for example, availability of choice in guided practices, language during meditations that emphasizes freedom, choice, self-compassion and self-care, explicit modules on trauma during MTPC group leader training, and careful case-by-case discussion with patients and referring providers during the screening phase about whether active trauma symptoms (such as flashbacks, flooding, dissociation) may worsen during practice. Foundational sessions 1-4 fostered awareness of body sensations, breathing, autopilot and stress responses, and skills for relating to discomfort. In sessions 5-8, MTPC incorporated core practices adapted from Mindfulness-Based Cognitive Therapy(Teasdale et al., 2000) and Mindful Self-Compassion(Neff & Germer, 2013). A thread focused on “Living Well” with chronic illness was woven throughout most sessions and included harnessing mindfulness and chronic illness self-management(K. R. Lorig, Sobel, Ritter, Laurent, & Hobbs, 2001) to support health behavior change as a way of living well. Since ambivalence is often rooted in values conflict and behavior change emerges from becoming aware of discrepancy between deeply-held values and current behavior, MTPC incorporates a mindful exploration of core values(W. R. Miller, Rollnick, Stephen 2012). MTPC avoids promoting any specific values, but uses mindfulness practice to provide a safe and illuminating container for identification of one’s own deeply held values through adapted values clarification card sort process(W. R. Miller, C’de Baca J, Matthews D.B., Wilbourne, P.L, 2001), followed by encouragement to identify which important personal values are associated with Living Well. Finally, MTPC includes a short-term action planning process aimed at behavior change related to chronic illness self-management that followed the SMART model(Lazarus, 2004).
Weekly session curricula were designed to be adaptable to different primary care treatment contexts. For instance, groups were adapted to a format that was appropriate to be led by mental health clinicians (6 MTPC groups in this study) and to be billed to insurance as group psychotherapy. Components of this intervention that specifically met insurance billing criteria were as follows: patients had a recent documented DSM-V diagnosis, groups were led by a licensed mental health clinician, session-specific therapeutic aims met medical necessity criteria(American College of Medical Quality, 2010), psychotherapy groups were capped at 10 participants per group due to state Medicaid restrictions on psychotherapy in the hospital-affiliated health center setting, there was a documented referral placed by a primary care provider for each participant, and EHR documentation for each weekly session followed standard group psychotherapy progress note format. We also demonstrated that the curriculum could be adapted to be led and billed as a group medical visit by a primary care physician (1 MTPC group in this study). Condition-specific vital signs were obtained as part of the check-in when MTPC was billed in group medical visit format with similar components that met insurance billing criteria as listed above.
MTPC and LDC introduction were co-led in pairs by 12 licensed health system mental health clinicians (psychologist, clinical social worker, or psychiatrist) and 1 primary care physician. All group leaders completed 1) an 8-week MBSR course (27 hours total); 2) a weekly practicum during this MBSR course (8 hours total) [delivered by UMass Center for Mindfulness (CFM) senior teacher trainers with 44 years of combined teaching experience (Z.V.(Amaro et al., 2014), E.R.(Altschuler, Rosenbaum, Gordon, Canales, & Avins, 2012))]; 3) followed by 40 hours of MTPC-specific training that focused on core goals of MTPC, inquiry, attitudinal components of being a group leader, including a focus on the importance of adapting mindfulness instructions to be accessible to people with history of trauma. MTPC curriculum adherence was tracked through weekly supervision and session-specific fidelity checklists(Chawla et al., 2010) completed by a trained in-session observer to prevent drift(Breitenstein et al., 2010; Waller, 2009). Adherence to each curriculum item was rated as 0-1-2 (absent-partial-complete), with the mean scores for 7 consecutive cycles of MTPC groups delivered in this study as follows: 1.9 (SD=0.10), 2.0 (SD=0.0), 1.98 (SD=0.05), 1.85 (SD=0.17), 1.89 (SD=0.13), 1.94 (SD=1.6), 1.84 (SD=0.23). Sessions were audio recorded and 10% were reviewed for adherence and leader competency.
Low-dose comparator: 60-minute introduction to mindfulness + referral to community resources + 6-month waitlist.
Participants randomized to the LDC arm were encouraged to practice mindfulness and engage with community resources (such as mobile apps, mindfulness center websites, books, local centers; see Appendix 1 for list), while continuing with ongoing standard mental health care (based on the IMPACT model of stepped care(Unutzer et al., 2002) including psychopharmacology and psychotherapy). Interested participants were guaranteed a health system mindfulness group 6 months later, contingent on survey completion. The 6-month waitlist was chosen to determine the feasibility of a larger study which would evaluate outcomes at 6 months. The choice of a low-dose mindfulness comparator with only the 60-minute introduction to mindfulness and regular encouragement to access digital and community resources was intended to represent the current “standard of care” in primary care where a brief experiential introduction to mindfulness and provision of digital and community resources is often offered by providers who encourage mindfulness.
Collaborative Goal Setting Primary Care Visit.
At the end of the informed consent session, study staff scheduled participants who would be randomized to either MTPC or LDC for a health maintenance visit with their referring PCP during week 6 of the intervention. PCPs received a standardized study training email encouraging engagement with patients in shared decision-making(Elwyn et al., 2012) and collaborative SMART goal-setting(Bigi, 2014; Lazarus, 2004; Lenzen SA, 2015). PCPs were encouraged to maintain, refine, or change the EHR-documented chronic illness care plan(Bodenheimer, Wagner, & Grumbach, 2002), aligning with the PCMH’s National Committee for Quality Assurance certification expectations(Gerra et al., 2006). After the visit, participants completed a survey describing their short-term chronic illness self-management action plan.
Participant Outreach.
Participants in both arms received a twice-monthly engagement call (average duration generally lasting about 10 minutes) for the first two months to provide attention-matched study staff support (i.e., logistical, emotional, mindfulness-practice encouragement), reduce attrition(Kinser & Robins, 2013), and facilitate survey completion(Richards et al., 2010).
MEASURES
Study data were collected and managed using REDCap electronic data capture tools(Harris et al., 2009). Participants completed a baseline survey of socio-demographic variables and meditation and mindfulness experience (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of Participants by Study Arm
| Variable |
MTPC (n=54) |
Comparator (n=27) |
Total (n=81) |
|---|---|---|---|
| Female, N (%)++ | 35 (64.8) | 21 (77.8) | 56 (69.1) |
| Age (years), Mean (SD) | 42.5 (12.6) | 48.2 (14.2) | 44.4 (13.4) |
| Race, N (%) | |||
| White | 45 (83.3) | 18 (66.7) | 63 (77.8) |
| Black, Haitian or African American | 2 (3.7) | 4 (14.8) | 6 (7.4) |
| Other+++ | 7 (13.0) | 5 (18.5) | 12 (14.8) |
| Ethnicity Hispanic, N (%) | 8 (14.8) | 3 (11.1) | 11 (13.6) |
| English as Second Language | 9 (16.7) | 3 (11.1) | 12 (14.8) |
| Annual Income < $40,000, N (%) | 30 (56 .0) | 20 (74.0) | 50 (62.0) |
| Marital Status, N (%)+ | |||
| Single | 24 (46.1) | 13 (48.1) | 37 (46.0) |
| Married/Cohabitating | 18 (34.6) | 6 (22.2) | 24 (30.0) |
| Divorced | 9 (17.3) | 7 (25.9) | 16 (20.0) |
| Education (years), Mean (SD)^ | 15.5 (2.9) | 16.1 (2.8) | 15.7 (2.8) |
| Insurance, N (%) | |||
| Medicaid or Medicare | 8 (14.8) | 6 (22.2) | 14 (17.3) |
| Subsidized | 17 (31.5) | 11 (40.7) | 28 (34.6) |
| Private | 27 (50.0) | 10 (37.0) | 37 (45.7) |
| Other+ | 2 (3.7) | 0 (0.0) | 2 (2.5) |
| DSM-V Diagnosis, N (%) | |||
| Single DSM-V Diagnosis | 38 (70.4) | 18 (66.7) | 56 (69.1) |
| 2+ DSM-V Diagnoses | 16 (29.6) | 9 (33.3) | 25 (30.9) |
| Primary DSM-V Diagnosis | |||
| Major Depressive Disorder* | 14 (25.9) | 6 (22.2) | 20 (24.7) |
| Other Anxiety Disorder^ | 12 (22.2) | 6 (22.2) | 18 (22.2) |
| Generalized Anxiety Disorder (300.02) | 10 (18.5) | 3 (11.1) | 13 (16.0) |
| Adjustment Disorder** | 8 (14.8) | 5 (18.5) | 13 (16.0) |
| Other Depressive Disorder^^ | 5 (9.2) | 1 (3.7) | 6 (7.4) |
| PTSD (309.81) | 2 (3.7) | 3 (11.1) | 5 (6.2) |
| Other*** | 3 (5.6) | 3 (11.1) | 6 (7.4) |
| Outcome Measures at Baseline, Mean (SD) | |||
| PROMIS Anxiety | 63.6 (7.2) | 62.7 (8.3) | 63.3 (7.6) |
| PROMIS Depression | 58.8 (8.5) | 58.7 (7.4) | 58.7 (8.1) |
| Perceived Stress (PSS) | 23.9 (6.2) | 24.7 (5.5) | 24.1 (6.0) |
| Self-Efficacy for Chronic Disease (SECD) | 6.3 (2.2) | 5.6 (2.1) | 6.1 (2.2) |
| Perceived Control (PCQ) | 23.7 (4.3) | 22.4 (4.2) | 23.3 (4.3) |
| Self-Compassion (SCS-SF) | 2.5 (0.7) | 2.7 (0.6) | 2.6 (0.7) |
| Mindfulness Total (FFMQ) | 115.6 (18.9) | 120.3 (21.8) | 117.1 (19.9) |
Not shown: Widowed (MTPC n=1; LDC n=1)
Participants were given the option to choose “female”, “male” or “other.” No participants chose “other.”
Participants were given the option to choose “White”, “Asian”, “Black, Haitian, or African American”, “American Indian or Alaska Native”, “Native Hawaiian or Other Pacific Islander”, or “Other (with write-in option).”
includes DSM-V codes: Major depressive disorder, Single episode, Unspecified (296.20), Major depressive disorder, Single episode, Moderate (296.22), Major depressive disorder, Recurrent episode, Unspecified (296.3), Major depressive disorder, Recurrent episode, Mild (296.31), Major depressive disorder, Recurrent episode, Moderate (296.32), Major depressive disorder, Recurrent episode, Severe (296.33), Major depressive disorder, Recurrent episode, In partial remission (296.35), Major depressive disorder, Recurrent episode, In full remission (296.36);
Includes DSM-V codes: Unspecified anxiety disorder (300), Panic disorder (300.01);
Includes DSM-V codes: Adjustment disorder, with depressed mood (309.0), Adjustment disorder, with anxiety (309.24), Adjustment disorder, with mixed anxiety and depressed mood (309.28)
Incudes DSM-V codes: Dysthymia (300.4), Unspecified depression (311)
Includes DSM-V codes: Somatic symptom disorder (300.82), Attention-deficit/hyperactivity disorder, Combined presentation (314.01), Insomnia disorder (780.52) +Self-pay (n = 1) or workers’ compensation (n = 1);
Only reporting on n = 53 total (36 MTPC, 17 LDC) T-test and χ2 test conducted; there were no significant differences between groups. Abbreviations: DSM-V: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; PROMIS: Patient-Reported Outcomes Measures.
Feasibility.
Feasibility of the program was defined a priori as the following categories: (1) Implementation: Implementation of at least eight MTPC intervention groups within the first year in multiple primary care clinics; (2) Insurance Accessibility: Enrollment of a socio-economically diverse population of patients reflecting the underlying insurance distribution of the local health system catchment area (50% with federally subsidized insurance). Additional insurance feasibility outcome included percentage of participants enrolled whose insurance policies successfully covered the 1-hour behavioral health evaluation and weekly MTPC sessions. (3) Ethnic/Racial/Linguistic Representation: Enrollment of participants with at least 30% who identified as Hispanic, non-Caucasian, or speaking a primary language other than English at home, reflecting the underlying ethnic/racial/linguistic distribution of health care system catchment area; (4) PCP Visit: A post-hoc outcome of feasibility of a PCP health behavior change visit was also explored through anonymous referring PCP survey and through a patient focus group about the program in which the PCP visit was one of the topics of discussion.
Acceptability and Satisfaction.
Using a combination of checklist and open-ended questions, acceptability data were collected at 8 weeks for participants randomized to MTPC. Primary acceptability outcome was response to the item, “I would recommend this program to a friend,” rated from 1 (Strongly Disagree) to 5 (Strongly Agree). PCPs who referred a patient during the study period were sent an anonymous survey after the completion of the 7 patient enrollment cycles, inquiring about how clinically helpful the PCP visit was, and the impact of this visit on the relationship with the patient.
Primary Health Outcomes.
Anxiety was assessed at baseline and after 8 weeks using the 8-item Patient Reported Outcomes Measurement Information System (PROMIS) Anxiety Short Form Version 8a (Cronbach’s α=0.92)(Hays, Bjorner, Revicki, Spritzer, & Cella, 2009), scored from 1 (never) to 5 (always)(Schalet, Cook, Choi, & Cella, 2014). PROMIS Scoring Service generated a T-score. PROMIS instruments have measured patient-reported outcomes for clinical research with high reliability and validity (Hays et al., 2009). Depression was assessed at baseline and after 8 weeks using the 8-item PROMIS Depression Short Form Version 8a (α=0.97)(Vilagut et al., 2015). Perception of stress in the past 30 days was assessed at baseline and after 8 weeks by the 10-item Perceived Stress Scale (PSS)(S. Cohen, Kamarck, & Mermelstein, 1983), with items scored from 0 (never) to 4 (very often) (α=0.83)(Cole, 1999). The 6-item Self-Efficacy for Chronic Disease (SECD-6) scale(K. R. Lorig et al., 2001) was assessed at baseline and after 8 weeks, and asked participants to rate their confidence in their ability to do certain activities related to managing their health condition from 1 (not at all confident) to 10 (totally confident) (α=0.90)(Ritter & Lorig, 2014).
Secondary Health Outcomes.
Mindfulness was assessed by the 39-item Five-Facet Mindfulness Questionnaire (FFMQ)(Baer, Smith, Hopkins, Krietemeyer, & Toney, 2006; Baer et al., 2008) with items scored from 1 (never or very rarely true) to 5 (very often or always true). Total score was sum of five facets (α=0.75-0.92)(Baer et al., 2006). Self-compassion was assessed by the 12-item Self-Compassion Scale Short Form (SCS-SF)(Raes, Pommier, Neff, & Van Gucht, 2011), with items scored from 1 (almost never) to 5 (almost always) (α=0.87)(Raes et al., 2011). Mean score was reported. The 5-item Perceived Control Questionnaire (PCQ), adapted from Jerant(Jerant, Moore, Lorig, & Franks, 2008) and Armitage(Armitage, 1999), asked participants to rate their sense of control over chronic illness from 1 (none) to 7 (total) (α=0.74)(Jerant et al., 2008).
Action Plan Initiation.
Participants went through a pilot action planning session in which they 1) were encouraged to meet their PCP between Weeks 6 & 7 of the intervention period; 2) created and documented, in the Action Plan Worksheet, an action plan focused on making a behavior change related to self-management of chronic disease and/or health maintenance; 3) reported on their action plan initiation two weeks after action plan creation in the Action Plan Initiation Survey (APIS-5), adapted from Guck(Guck, 2008). Participants listed their action plan goal and reported the extent to which they met the goal from 1 (not at all met) to 7 (completely met). Evidence of initiation was defined a priori as a self-rating of 5-7.
Adverse Events.
Adverse events during the intervention period (week 0-8) were collected during biweekly engagement calls and the week 8 survey session.
DATA ANALYSES
To evaluate randomization, socio-demographic and baseline variables were compared between groups using t-test (continuous variables) or χ2 test (categorical variables). Feasibility and acceptability were measured by descriptive statistics and qualitative feedback. Average reported home practice and community resource usage were compared between arms using t-test (continuous variables) or χ2 test (categorical variables).
Health Outcomes Aim.
Pre/post within-group effectiveness of MTPC was measured by paired t-test among 8-week survey finishers (defined as study participants who completed or partially completed the 8-week follow-up survey); effect size was reported as Cohen’s d. Pre/post within-group changes are also reported for the LDC arm. The size of this pilot study was powered based on expected pre/post changes in PROMIS Anxiety using within-group paired t-test, expecting an estimated MTPC effect size of 0.38(Goyal et al., 2014). Assuming alpha (type I error) of 0.05, a sample of 54 randomized to MTPC would have 80% power to detect a difference between pre and post means of 0.38. To evaluate comparative effectiveness of MTPC versus LDC, we conducted a between-group Intent-To-Treat (ITT) analysis for primary outcome of PROMIS Anxiety, as well as other main health outcomes of PROMIS Depression, PSS, and SECD using linear mixed effects models(Rabe-Hesketh, 2012) (mixed) to evaluate time × treatment interaction from baseline to 8 weeks. Mixed effects models allowed use of all survey data, accounted for clustering of multiple observations within participants and handled missing data with maximum likelihood estimation. We also conducted mixed effects analyses on secondary outcomes of FFMQ, SCS-SF, and PCQ.
Action Plan Initiation.
To evaluate the pilot chronic disease self-management action planning protocol, we analyzed the self-rating of action plan initiation (score of 1 – 7) in the Action Plan Initiation Survey in a cross-sectional ITT analysis using Fisher’s exact test to assess difference in action plan initiation between study arms, and logistic regression (logit) to determine additional predictors of action plan initiation.
Multiple Comparisons Testing.
Statistical significance of pre/post within- and between-group differences was determined using the Benjamini-Hochberg false discovery rate (FDR) procedure(Benjamini & Hochberg, 1995), which accounts for multiple comparisons. Multiple comparisons correction was performed according to Cao et al(Cao & Zhang, 2014) in which a cutoff p-value is determined for a family of similar variables and analyses (family-wise error rate=0.05)(Glickman, Rao, & Schultz, 2014; Wahbeh, Goodrich, Goy, & Oken, 2016). Analysis families were separated by primary and secondary health outcomes, and within and between-group analyses. To prevent bias during analysis, a statistical consultant (T.C.) external to study implementation oversaw the analysis plan and conduct, and reviewed STATA MP 14.2(“Stata Statistical Software: Release 14.,” 2015) results and syntax. This study had a NIH-approved data safety monitoring plan with an independent monitor, was approved by the Cambridge Health Alliance (CHA) Institutional Review Board, and followed all provisions of the Declaration of Helsinki.
RESULTS
Participant Flow and Characteristics
Over 14 months, 85 PCPs referred 344 patients, 86 (25%) of whom gave informed consent. Reasons for not enrolling at the time included: did not respond, schedule conflict, did not meet inclusion/exclusion criteria, not interested. Participant flow through enrollment, randomization, and outcomes is detailed in Figure 1. Of the 344 referred patients, 159 patients had a 1-hour behavioral health evaluation as part of the screening process, conducted by 43 different behavioral health providers across the health system.
Randomized participants (N=81) were 69% female and 31% male (no participant reported other gender) and were an average of 44 years old (SD=13.4). Nearly half of participants (44%, n=36) had never practiced any form of mindfulness or meditation. Baseline characteristics and baseline outcome scores were similar between intervention and LDC arms. Of participants, 69% had a single DSM-V diagnosis upon behavioral health evaluation for the study, and 31% had two or more DSM-V diagnoses. Participants most commonly had a type of anxiety disorder (37%), which included generalized anxiety disorder, panic disorder, anxiety disorder unspecified, or a type of depressive disorder (32%), which included major depressive disorder, unspecified depressive disorder, and dysthymia. Diagnoses are outlined in Table 1. The ten most common chronic medical conditions affected 48% of participants (see Supplementary Table 1) at, or within 6 months of, the referral to the program. There were no significant differences between groups in any of the demographics or diagnoses categories. Participants randomized to the MTPC arm (n=54) attended a mean of 5.2 (SD 2.5) of eight weekly sessions, 55% (n=30) attended the 7-hour all-day session, and 65% (n=35) attended at least six of nine sessions.
Feasibility
(1) Implementation: MTPC was successfully implemented as an insurance-reimbursable group psychotherapy intervention in two Patient-Centered Medical Home primary care clinics for seven groups. Participants enrolling at these two clinics had referring providers who were based at six out of twelve total clinics in the health system. (2) Insurance Accessibility: Of enrolled participants, 52% had subsidized health insurance or Medicare/Medicaid (Table 1). One participant was excluded from randomization because her insurance policy would not cover MTPC as group psychotherapy (Figure 1). Of the 81 participants who were randomized, two participants (both randomized to MTPC) had a change in insurance status during the study - one of these participants quit the study before the intervention began, and one participant remained in the study but stopped attending the intervention after Week 6 once her insurance status changed. Two participants (both randomized to MTPC) reported having a high bill for the required behavioral health evaluation; one was lost to follow-up after Week 2 and the other after Week 6. One enrolled participant (MTPC arm) reported that his copay was higher than expected. Co-pays ranged from $0 to $50 depending on insurance coverage. (3) Ethnic-Racial-Linguistic Representation: Of participants, 22% (n=18) identified with non-white race (Black: [MTPC n=2; LDC n=4], Other non-white race: [MTPC n=7; LDC n=5]); 13.6% (n=ll) identified with Hispanic ethnicity [MTPC n=8; LDC n=3]; and 14.8% (n=12) spoke English as a second language (MTPC n=9; LDC n=3). Participants who finished their 8-week survey sessions (68%, n=55 [MTPC n=36; LDC n=19]) had similar diagnoses and baseline scores as those who did not finish 8-week surveys. However, 8-week survey finishers were more likely than non-finishers to be male (38% versus 15%, p=0.04) and white (86% versus 62%, p=0.02). Among non-finishers, gender and race characteristics were similarly distributed between MTPC and LDC arms. (4) PCP Visit Feasibility: Of enrolled participants, 70% (n=57) attended a PCP visit as part of their participation in this study. Feedback on the PCP visit is detailed below. MTPC was acceptable to this population, with 92% (n=32 of 36) of survey finishers randomized to MTPC indicating that they would recommend MTPC to a friend, and 94% indicating that they found the program helpful. Qualitative feedback indicated that the program was offered within a supportive and collaborative care environment and helped participants feel more agency over their experience and illness (“Through meditation and ‘aware’ breathing exercises, I was able to address my depression and chronic pain issues, learning tools and techniques to aid in tackling these ongoing health concerns…my team has worked in a cohesive manner to support me” [Male, 64 years old]), more self-acceptance (“[The most important thing I learned was] listening to my body and letting go of judgment about what I should be feeling” [Female, 32 years old]), and common humanity (“[The most helpful part was] sharing in the experience with a group of people who have similar challenges” [Male, 34 years old]). In response to questions about the hardest or least favorite part of the program, participants responded most frequently about time constraints, (“I had to rush from work to be there” [Female, 26 years old ]), amount of content, (“A lot of material in a short time frame”, [Male, 34 years old]), and the challenge of developing a home practice (“Building an actual practice at home” [Female, 33 years old]). While 90% of patients in the focus group had a positive experience with the PCP collaborative action planning visit, a few patients and one PCP in the anonymous PCP survey expressed strong frustration about the required PCP visit (“Don’t like extra doctor visits”, “Was a waste of my and his time”, PCP: “We are struggling with access…during flu/cold season…you add…un-needed and un-helpful visit…patient was forced/asked to follow-up…and had…a co-pay”).
Over the 8 weeks, participants reported an average of 135 (SD=221) minutes formal practice per week [MTPC: 116.5 (SD=134.6); LDC: 171.9(SD=333.7), p =0.29], Percent of participants who reported formal practice on greater than 4 of 8 weeks was 40.1% (MTPC: 48.1%, LDC: 25.9%, p = 0.06). Percent of participants who utilized mindfulness community resources on greater than 4 weeks was 30.1% (MTPC: 24.1%, LDC: 44.4%, p=0.06). Participants reported using these resources on an average of 2.9 weeks (SD=3.0) out of 8 [MTPC: 2.5(2.8); LDC: 3.9(3.0), p=0.04], suggesting that LDC arm participants were more likely to engage community mindfulness resources.
Health Outcomes
Participants randomized to MTPC arm who finished surveys (n=36 of 54, 67%) demonstrated a large reduction in the primary outcome of PROMIS Anxiety (d= −0.72, p<0.001) between baseline and 8 weeks. There was a moderate reduction in depression (PROMIS DSF) (d= −0.40, p<0.005), a moderate increase in self-efficacy for chronic disease self-management (SECD) (d= 0.43, p<0.05), and large reductions in perceived stress (PSS) (d= −0.81, p<0.001). In secondary outcomes, there were large increases in self-compassion (SCS-SF) (d= 1.01, p<0.001), total mindfulness (FFMQ) (d= 0.93, p<0.001), and the associated 5 mindfulness subscales. Individuals randomized to LDC experienced significant reductions in stress (PSS) (d= −0.50, p<0.01), but there were no significant improvements in any of the other outcomes.
In mixed effects ITT analysis comparing pre/post changes in individuals randomized to the MTPC arm versus the LDC arm, reduction in primary outcome of PROMIS Anxiety score was significantly greater in the MTPC arm (B= −4.2, p=0.012) (Table 2). There were also significantly greater increases in total FFMQ mindfulness score (B=11.6, p=0.02) and self-compassion score (B=0.54, p=0.001). There were no significant differences in change in self-efficacy, PROMIS Depression, perceived control (PCQ) or perceived stress (PSS) scores.
Table 2.
Between- and Within-Group Changes from Baseline to 8 Weeks
| Within-Group Paired t-tests |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mixed Effects |
MTPC |
Low Dose Comparator |
||||||||||||
| Outcome | N | β | SE | p | N | Pre-Mean (SD) | Post-Mean (SD) | p | d | N | Pre-Mean (SD) | Post-Mean (SD) | p | d |
| Anxiety | 81 | −4.16 | 1.66 | 0.012* | 36 | 62.7 (7.8) | 57.4 (7.1) | <0.001* | −0.72 | 20 | 63.5 (8.7) | 61.7 (8.9) | 0.21 | −0.20 |
| Depression | 81 | −2.43 | 1.79 | 0.18 | 36 | 57.4 (8.9) | 53.8 (8.0) | 0.003* | −0.43 | 20 | 58.6 (8.2) | 57.1 (10.6) | 0.31 | −0.16 |
| PSS | 81 | −2.00 | 1.57 | 0.20 | 36 | 23.0 (6.4) | 18.0 (5.9) | <0.001* | −0.81 | 21 | 24.9 (6.1) | 21.3 (8.2) | 0.007* | −0.50 |
| SECD | 81 | 0.51 | 0.48 | 0.29 | 36 | 6.4 (2.3) | 7.3 (1.9) | 0.02* | 0.43 | 20 | 5.6 (2.1) | 6.1 (2.3) | 0.08 | 0.20 |
| FFMQ | 81 | 11.56 | 4.99 | 0.021* | 38 | 116.3 (20.5) | 133.3 (15.5) | <0.001* | 0.93 | 20 | 119.1 (24.2) | 125.5 (22.1) | 0.10 | 0.28 |
| SCS-SF | 81 | 0.54 | 0.16 | 0.001* | 36 | 2.5 (0.7) | 3.2 (0.7) | <0.001* | 1.01 | 21 | 2.8 (0.7) | 2.9 (0.8) | 0.11 | 0.22 |
| PCQ | 81 | −0.11 | 1.27 | 0.93 | 36 | 24.1 (4.2) | 24.6 (5.3) | 0.54 | 0.10 | 18 | 22.7 (4.4) | 22.9 (4.9) | 0.80 | 0.06 |
Significant after Hochberg FDR procedure, family-wise p<0.05 Beta coefficient ( ) is expressed on the scale of the outcome measure.
) is expressed on the scale of the outcome measure.
Abbreviations: PSS: Perceived Stress Scale; SECD: Self-Efficacy for Chronic Disease; PCQ: Perceived Control Questionnaire; FFMQ: Five-Facet Mindfulness Questionnaire; SCS-SF: Self-Compassion Scale Short Form.
PCP Visit, Action Plan Creation, and Action Plan Initiation
We hypothesized that participants randomized to MTPC versus LDC would demonstrate greater self-reported action plan initiation within 2 weeks of creating their plan. We found no difference in PCP visit attendance (MTPC: 67%, n=36; LDC: 78%, n=21, p=0.30), and no difference in rates of action plan creation during an identical SMART goal setting session (MTPC: 54%, n=29; LDC: 56%, n=15, p=0.88). Yet, participants in the MTPC arm reported a higher rate of action plan initiation compared with LDC (MTPC: 35%, n=19 versus LDC: 11%, n=3; p=0.03). Action plans were individualized (e.g., “1-2 mile bike or run 2-3 times a week” or “5-10 minutes a day of mindfulness practice”). Baseline perceived control score was positively associated with initiation (OR: 1.14, p=0.03) in bivariate analyses (Table 3), and in a multivariate analysis including baseline perceived control, depression, and stress scores, study randomization arm remained an independent predictor of initiation (Table 3).
Table 3.
Analysis of Action Plan Initiation
| Bivariate Analysis | ||||
|---|---|---|---|---|
| Odds Ratio |
95% CI |
Z |
p |
|
| MTPC Group | 4.34 | (1.2-16.3) | 2.17 | 0.03* |
| Male Gender | 2.44 | (0.9–6.8) | 1.71 | 0.09 |
| Hispanic Ethnicity | 0.23 | (0.3–1.9) | −1.35 | 0.18 |
| White Race | 2.16 | (0.6–8.3) | 1.12 | 0.26 |
| Age (years) | 0.98 | (0.9–1.0) | −0.82 | 0.41 |
| Baseline Scores | ||||
| Anxiety | 1.01 | (0.9–1.1) | 0.34 | 0.73 |
| Depression | 0.94 | (0.9–1.0) | −1.82 | 0.07 |
| PSS | 0.92 | (0.8–1.0) | −1.85 | 0.06 |
| SECD | 1.02 | (0.9–1.5) | 1.57 | 0.12 |
| PCQ | 1.14 | (1.0–1.3) | 2.23 | 0.03* |
| FFMQ | 1.01 | (0.99–1.0) | 0.93 | 0.36 |
| SCS-SF | 1.76 | (0.8–3.7) | 1.47 | 0.14 |
| Multivariate Analysis | ||||
| Odds Ratio |
95% CI |
Z |
p |
|
| MTPC Group | 4.09 | 1.1–16.1 | 2.02 | 0.04* |
| Baseline Depression | 0.97 | 0.88–1.1 | −0.55 | 0.54 |
| Baseline PSS | 0.98 | 0.86–1.1 | −0.28 | 0.78 |
| Baseline PCQ | 1.09 | 0.95–1.3 | 1.20 | 0.21 |
Significance defined by p< 0.05.
Abbreviations: PSS: Perceived Stress Scale; SECD: Self-Efficacy for Chronic Disease; PCQ: Perceived Control Questionnaire; FFMQ: Five-Facet Mindfulness Questionnaire; SCS-SF: Self-Compassion Scale, Short Form
Adverse Events
During the 8-week intervention period, participants reported three serious adverse events, which were unrelated to the study protocol: two emergency department visits (epistaxis – MTPC, pneumonia – MTPC) and one hospitalization (chest pain – LDC). One non-serious event was related to the protocol: a participant had a panic attack during the all-day session, consulted with a group leader, became calmer, and voluntarily continued participation (MTPC).
DISCUSSION
This pilot randomized-controlled trial demonstrated feasibility, acceptability, and effectiveness of an 8-week insurance-reimbursable, referral-based MBP integrated into Patient-Centered Medical Homes within a diverse healthcare system.
Compared with the lower dose of mindfulness, in the intent-to-treat analysis, 8-week MTPC was more effective than LDC for decreasing anxiety and increasing mindfulness and self-compassion between baseline and 8 weeks. Although this pilot study did not have sufficient power to assess for all between-group outcomes, these results suggest that a larger trial should be conducted to evaluate impact of MTPC on these health outcomes. There were significant within-group improvements for all primary outcomes as well as mindfulness and self-compassion for the participants randomized to the MTPC arm, replicating findings reported for other primary care populations(McCubbin et al., 2014; Sundquist et al., 2015). Participants randomized to MTPC demonstrated a significantly higher rate of action plan initiation compared with LDC. The directionality of the association between action plan initiation and relevant known predictors of behavior change (lower baseline depression and stress, higher perceived control) provides some measure of concurrent validity for the relationship of the SMART goal setting session and action planning paradigm as a behavioral measure of medical regimen adherence and health behavior change. Future research should focus on validating self-reported initiation with objective measures of behavior change (e.g., accelerometer-based activity monitoring for health action plans related to physical activity). The results of the pilot action planning protocol suggest that MTPC may have led to an enhanced self-regulation capacity through the program’s emphasis on regular mindfulness meditation practice, facilitation of further readiness for behavior change through values clarification and skillful action modules, and/or group support(Bodenheimer, Lorig, et al., 2002; K. Lorig, Laurent, Plant, Krishnan, & Ritter, 2014; K. R. Lorig & Holman, 2003). To identify the most active components of this complex intervention, further dismantling research and a larger study powered for identifying meditating variables is needed to replicate this finding in a larger study.
The diagnostic heterogeneity of the sample was a strength of this program, indicating ease of implementation and dissemination in a real-life primary care clinical context. However, the diagnostic heterogeneity may have impacted the power for clinical findings related to anxiety, stress, and depression. The between group change in anxiety was significant while depression was not, which could have been due to the larger effect size on anxiety in a small heterogeneous sample that included patients with severe anxiety, but only included patients with mild-moderate depression. Further research on the mediating variables that impacted anxiety could be helpful.
Participants in the LDC arm who completed 8-week surveys also experienced reduced stress after 8 weeks, perhaps due to the combination of a small dose of mindfulness instruction, bimonthly engagement calls from study staff, ongoing standard mental health services, and community resources, though these were not sufficient to reduce anxiety in the LDC arm. The decrease in stress could also be due to a regression to the mean(Morton & Torgerson, 2003) after a period of elevated stress led individuals to seek out the program. Future secondary analyses of mindfulness practice frequency could elucidate the role that the 60-minute introduction may have on inspiring ongoing practice and community resource utilization.
Participants randomized to the MTPC arm who responded to post-study surveys demonstrated high satisfaction with the program. A limitation of the feedback collection was that only survey finishers were represented. In future cycles, outreach to non-finishers will allow us to understand reasons for not finishing the program or for not replying to surveys. Additional limitations of this study include use of a comparator group that did not receive attention-matched group instruction time, although it did receive attention-matched study interaction time through engagement calls, PCP visits, identical SMART goal setting paradigm, and surveys. The comparator group represented the standard of care consisting of a short introduction to mindfulness from a mental health clinician, standard care and referral to external resources. Future studies with an 8-week active group comparator and longer follow-up time could highlight the short-term and long-term benefits specific to MTPC and better control for therapeutic effects from the group setting. Another limitation was the rate of non-completion of 8-week surveys in both arms (~31%), which limited power to detect time × treatment interactions for outcomes associated with smaller effect sizes. The 2:1 randomization ratio, chosen as a response to PCP/patient feedback, also contributed to reduction in power in ITT between-group analyses. In addition, PCP visit rates (67% - 78%) and action plan creation rates (~55%) were lower than expected in both arms. Finally, attendance of the MTPC sessions (average 5.2 sessions), while within the range expected for a real-world setting in a public healthcare system(Amaro et al., 2014; Roth & Robbins, 2004), were lower than expected compared with some mindfulness interventions integrated into other primary care settings(Burnett-Zeigler et al., 2016).
Feasibility of insurance accessibility was overall successful given the high percentage of insurance coverage. However, for a few patients, out-of-pocket costs associated with the behavioral health evaluation and co-pays associated with the weekly sessions were higher than they had expected. These cases led the study team to modify the informed consent form to include more specific language that is repeated multiple times in the recruitment process, encouraging patients to contact their insurance company with the provided billing code for the MTPC group sessions to determine their co-pay ahead of time. The possibility of changing insurance coverage during an insurance-billed mindfulness treatment is a risk and limitation of this model. Patients who are experiencing instability in employment or housing that may lead to unstable health insurance coverage may be at higher risk of losing access to insurance-billed mindfulness treatment, but this risk also applies to a range of mental health treatments including conventional group psychotherapy. In this trial, study staff continued engagement outreach with participants who lost insurance and encouraged the participants to attend free alumni groups and future no-cost all-day sessions in order to stay connected to the program. In addition, our program has supported the development of an alumni-managed co-pay donation fund, which allows patients who completed MTPC to contribute to the co-pays of those patients who may be unable to pay. Despite these solutions, insurance coverage instability may still present a barrier to patients who are seeking mindfulness treatment during especially stressful times.
While the ethnic/racial/linguistic representation in the population of enrolled participants was similar to that of the local community(Bor, 2017), survey non-completion rates were higher in females and participants identifying as non-white, suggesting that improvements in survey and program accessibility are needed, particularly for trial results to accurately reflect the underlying demographics of enrolled participants. In addition, the low representation of enrolled African-American patients is an important limitation and suggests a need to address fundamental underlying issues of racial accessibility for African Americans through active outreach to patients, providers, and communities, cultural adaptation of the program content, and more representation of African Americans among intervention group leaders(Proulx et al., 2018; Woods-Giscombe & Gaylord, 2014). Furthermore, closer analysis should identify whether there are barriers in enrollment according to race, ethnicity, income, education, and other aspects of identity that are systemically marginalized. Of patients referred to the program, 75% did not enroll in the study, largely (80%) due to no response, lack of interest, or scheduling conflicts. Patients with scheduling conflicts were followed-up with in future cycles. As this program was designed with the long-term intention to be more accessible than existing mindfulness training opportunities, patient focus group and PCP survey feedback are sought in an ongoing way to increase accessibility in future cycles. As a result of initial feedback, we made a few changes to increase transparency in the larger trials now underway, including the following: qualitative interviews with 28 participants to gather participant input, added more clear and accessible language during recruitment about study procedures, removed the PCP visit as a requirement of the program in future cycles, refined the engagement and recruitment calls to provide more details about the requirements of the study, and added a monetary completion bonus for survey completion at the end of the trial. A more representative cohort of group leaders and cultural adaptations will be prioritized in future trainings.
This study demonstrated how a primary care-adapted MBP, which was integrated across an entire healthcare system, as opposed to being offered within a controlled research setting or an isolated wellness program, reduces anxiety and increases mindfulness and self-compassion, and facilitates behavior change for primary care patients with chronic illness and mental health multimorbidity(Wallace et al., 2015). Considering the substantial challenge of co-morbid mental and physical illness in primary care, if the intervention reliably enhances capacity for health behavior change among primary care patients with co-morbid mental and physical health problems in future studies, then it could have a significant public health impact similar to models successfully integrating mental health care into primary care that are being widely disseminated in US (TEAMCare) and Europe(Katon et al., 2010; McGregor, Lin, & Katon, 2011). The strengths of this study include development of a primary care-adapted 8-week MBP, which integrates self-compassion with mindful reflection on core values, skillful action and behavior change, that may be more effective for facilitating behavior change than a low dose of mindfulness plus referral to community resources. We developed a training program curriculum facilitated by 13 health system clinicians to deliver an insurance-reimbursable, MTPC intervention, positioning MTPC as a potentially accessible mental health treatment for primary care patients in this health system, which could be a modality for implementation within other health systems. As this trial was optimized for a patient-centered medical home model, further study would be required for non-PCMH primary care environments. This program addresses implementation challenges in insurance coverage, referral workflow, healthcare provider training, and primary care-based administrative and facility support. MTPC has the potential to help treat prevalent clinical conditions and facilitate chronic illness self-management and behavior change in primary care populations with multimorbidity.
Supplementary Material
ACKNOWLEDGMENTS:
Contributors: Susan Pollak, Alexandra Oxnard, Laura Warren, Paula Gardiner, Eric Loucks, Timothy Martin, Fabio Marcovski, My Ngoc To, Thomas Fatkin, Alexandra Brunel, Pedro Barbosa, Jillian Burley, Cristian Onofrei, Barbara Hamm, Janet Yassen, Nicholas Barnes, Chris Carter-Husk, Nayla Khoury, Alaine Fredericksen, David Lovas, Emily Benedetto, Colleen O’Brien, Robert Joseph, Michael Williams, Mark Albanese, Adam Elias, Somava Stout, Elizabeth Gaufberg, Ben Cook, David Bor, Randy Wertheimer, Assaad Sayah, Jay Burke, Patrick Wardell
Funders: This study was supported by the National Institutes of Health (NIH) Science of Behavior Change Common Fund Program through an award administered by the National Center for Complementary and Integrative Health (NCCIH) (UH2AT009145), as well as The Arthur Vining Davis Foundations – Healthcare System Transformation Grant, The Arnold P. Gold Foundation, Cambridge Health Alliance, and Harvard Medical School Department of Psychiatry.
Footnotes
Clinical Trials Registration Number:
Institutional Review Board Number: CHA-IRB-1002/08/14
DATA AVAILABILITY: The authors will make all data available upon request.
Prior Presentations: Partial data from initial analysis was presented to scientific advisory board for NIH Science of Behavior Change Network Meeting, Bethesda, MD (January 10, 2017).
Conflict of Interest: The authors declare no conflict of interest.
Ethics Statement: This study was approved by the Cambridge Health Alliance Institutional Review Board and procedures followed all provisions of the Declaration of Helsinki.
References
- Allen LA, Gara MA, Escobar JI, Waitzkin H, & Silver RC (2001). Somatization: a debilitating syndrome in primary care. Psychosomatics, 42(1), 63–67. [DOI] [PubMed] [Google Scholar]
- Altschuler A, Rosenbaum E, Gordon P, Canales S, & Avins AL (2012). Audio recordings of mindfulness-based stress reduction training to improve cancer patients’ mood and quality of life--a pilot feasibility study. Support Care Cancer, 20(6), 1291–1297. doi: 10.1007/s00520-011-1216-7 [DOI] [PubMed] [Google Scholar]
- Amaro H, Spear S, Vallejo Z, Conron K, & Black DS (2014). Feasibility, acceptability, and preliminary outcomes of a mindfulness-based relapse prevention intervention for culturally-diverse, low-income women in substance use disorder treatment. Subst Use Misuse, 49(5), 547–559. doi: 10.3109/10826084.2013.852587 [DOI] [PubMed] [Google Scholar]
- Antonucci J (2008). A new approach group visits: helping high-need patients make behavioral change. Fam Pract Manag, 15(4), A6–8. [PubMed] [Google Scholar]
- Armitage CCM (1999). Distinguishing perceptions of control from self-efficacy: predicting consumption of a low-fat diet using the theory of planned behavior. J Appl Soc Psychol, 29, 72–90. [Google Scholar]
- Arozullah AM, Yarnold PR, Bennett CL, Soltysik RC, Wolf MS, Ferreira RM, … Davis T (2007). Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care, 45(11), 1026–1033. doi: 10.1097/MLR.0b013e3180616c1b [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, & Toney L (2006). Using self-report assessment methods to explore facets of mindfulness. Assessment, 13(1), 27–45. doi: 10.1177/1073191105283504 [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Lykins E, Button D, Krietemeyer J, Sauer S, … Williams JM (2008). Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment, 15(3), 329–342. doi: 10.1177/1073191107313003 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B, 57, 289–300. [Google Scholar]
- Benzo RP (2013). Mindfulness and motivational interviewing: two candidate methods for promoting self-management. Chron Respir Dis, 10(3), 175–182. doi: 10.1177/1479972313497372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigi S (2014). Key components of effective collaborative goal setting in the chronic care encounter. Commun Med, 11(2), 103–115. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Lorig K, Holman H, & Grumbach K (2002). Patient self-management of chronic disease in primary care. JAMA, 288(19), 2469–2475. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Wagner EH, & Grumbach K (2002). Improving primary care for patients with chronic illness. JAMA, 288(14), 1775–1779. [DOI] [PubMed] [Google Scholar]
- Bor D (2017). Cambridge Health Alliance: 2017 Academic Overview Retrieved from https://static1.squarespace.com/static/59271313e3df289d83dde161/t/59ef5987cd39c3bc41164c8c/1508858257512/2017+Academic+Overview.+Final+10.18.17+%281%29.pdf
- Breitenstein SM, Gross D, Garvey CA, Hill C, Fogg L, & Resnick B (2010). Implementation fidelity in community-based interventions. Res Nurs Health, 33(2), 164–173. doi: 10.1002/nur.20373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, … Rounsaville BJ (2011). Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend, 119(1-2), 72–80. doi: 10.1016/j.drugalcdep.2011.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett-Zeigler IE, Satyshur MD, Hong S, Yang A,J,TM, & Wisner KL (2016). Mindfulness based stress reduction adapted for depressed disadvantaged women in an urban Federally Qualified Health Center. Complement Ther Clin Pract, 25, 59–67. doi: 10.1016/j.ctcp.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Cao J, & Zhang S (2014). Multiple comparison procedures. JAMA, 312(5), 543–544. doi: 10.1001/jama.2014.9440 [DOI] [PubMed] [Google Scholar]
- Carmody J, & Baer RA (2009). How long does a mindfulness-based stress reduction program need to be? A review of class contact hours and effect sizes for psychological distress. J Clin Psychol, 65(6), 627–638. doi: 10.1002/jclp.20555 [DOI] [PubMed] [Google Scholar]
- Chapman DP, Perry GS, & Strine TW (2005). The vital link between chronic disease and depressive disorders. Prev Chronic Dis, 2(1), A14. [PMC free article] [PubMed] [Google Scholar]
- Chawla N, Collin S, Bowen S, Hsu S, Grow J, Douglass A, & Marlatt GA (2010). The mindfulness-based relapse prevention adherence and competence scale: development, interrater reliability, and validity. Psychother Res, 20(4), 388–397. doi: 10.1080/10503300903544257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J, Reinschmidt KM, & Moreno FA (2010). Symptoms of depression in a Hispanic primary care population with and without chronic medical illnesses. Prim Care Companion J Clin Psychiatry, 12(3). doi: 10.4088/PCC.09m00846blu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE (2007). Psychological Stress and Disease. JAMA, 298(14), 1685–1687. doi: 10.1001/jama.298.14.1685 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. J Health Soc Behav, 24(4), 385–396. [PubMed] [Google Scholar]
- Cole SR (1999). Assessment of differential item functioning in the Perceived Stress Scale-10. J Epidemiol Community Health, 53(5), 319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Medical Quality. (2010). Definition and Application of Medical Necessity. Retrieved from http://www.acmq.org/policies/policy8.pdf
- Crane RS, Brewer J, Feldman C, Kabat-Zinn J, Santorelli S, Williams JM, & Kuyken W (2017). What defines mindfulness-based programs? The warp and the weft. Psychol Med, 47(6), 990–999. doi: 10.1017/S0033291716003317 [DOI] [PubMed] [Google Scholar]
- Croghan ITB,JD (2010). Integrating Mental Health Treatment Into the Patient Centered Medical Home Retrieved from https://pcmh.ahrq.gov/page/integrating-mental-health-treatment-patient-centered-medical-home AHRQ Publication No. 10-0084-EF, Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- deGruy F III (1996). Primary Care: America’s Health in a New Era (Chapter: Mental Health Care in the Primary Care Setting) (Donaldson K. D. Y. Molla S., Lohr Kathleen N., and Vanselow Neal A. Ed.). Washington D.C.: National Academies Press; [PubMed] [Google Scholar]
- Demarzo M, Montero-Marin J, Puebla-Guedea M, Navarro-Gil M, Herrera-Mercadal P, Moreno-Gonzalez S, … Garcia-Campayo J (2017). Efficacy of 8- and 4-Session Mindfulness-Based Interventions in a Non-clinical Population: A Controlled Study. Front Psychol, 8, 1343. doi: 10.3389/fpsyg.2017.01343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarzo MM, Cebolla A, & Garcia-Campayo J (2015). The implementation of mindfulness in healthcare systems: a theoretical analysis. Gen Hosp Psychiatry, 37(2), 166–171. doi: 10.1016/j.genhosppsych.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Demarzo MM, Montero-Marin J, Cuijpers P, Zabaleta-del-Olmo E, Mahtani KR, Vellinga A, … Garcia-Campayo J (2015). The Efficacy of Mindfulness-Based Interventions in Primary Care: A Meta-Analytic Review. Ann Fam Med, 13(6), 573–582. doi: 10.1370/afm.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disease Control and Prevention, C. f. (2018). About Chronic Diseases. Retrieved from https://www.cdc.gov/chronicdisease/about/index.htm
- Eisenberg L (1992). Treating depression and anxiety in primary care. Closing the gap between knowledge and practice. N Engl J Med, 326(16), 1080–1084. doi: 10.1056/NEJM199204163261610 [DOI] [PubMed] [Google Scholar]
- Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, … Barry M (2012). Shared decision making: a model for clinical practice. J Gen Intern Med, 27(10), 1361–1367. doi: 10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn K, & Byrne M (2013). The key principles of cognitive behavioural therapy. 6(9), 579–585. doi: 10.1177/1755738012471029 [DOI] [Google Scholar]
- Fiore LD, & Lavori PW (2016). Integrating Randomized Comparative Effectiveness Research with Patient Care. N Engl J Med, 374(22), 2152–2158. doi: 10.1056/NEJMra1510057 [DOI] [PubMed] [Google Scholar]
- Friis AM, Johnson MH, Cutfield RG, & Consedine NS (2016). Kindness Matters: A Randomized Controlled Trial of a Mindful Self-Compassion Intervention Improves Depression, Distress, and HbA1c Among Patients With Diabetes. Diabetes Care, 39(11), 1963–1971. doi: 10.2337/dc16-0416 [DOI] [PubMed] [Google Scholar]
- Garland EL, Schwarz NM, Kelly A, Whitt A, & Howard MO (2012). Mindfulness-Oriented Recovery Enhancement for Alcohol Dependence: Therapeutic Mechanisms and Intervention Acceptability. J Soc Work Pract Addict, 12(3), 242–263. doi: 10.1080/1533256X.2012.702638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Leonardi C, D’Amore A, Strepparola G, Fagetti R, Assi C, … Lucchini A (2006). Buprenorphine treatment outcome in dually diagnosed heroin dependent patients: A retrospective study. Prog Neuropsychopharmacol Biol Psychiatry, 30(2), 265–272. doi: 10.1016/j.pnpbp.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Glickman ME, Rao SR, & Schultz MR (2014). False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol, 67(8), 850–857. doi: 10.1016/j.jclinepi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, … Haythornthwaite JA (2014). Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med, 174(3), 357–368. doi: 10.1001/jamainternmed.2013.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady PA, & Gough LL (2014). Self-management: a comprehensive approach to management of chronic conditions. Am J Public Health, 104(8), e25–31. doi: 10.2105/AJPH.2014.302041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guck TP (2008). Attributions regarding unmet treatment goals after interdisciplinary chronic pain rehabilitation. The Clinical Journal of Pain, 24(5), 415–420. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 42(2), 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Bjorner JB, Revicki DA, Spritzer KL, & Cella D (2009). Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res, 18(7), 873–880. doi: 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerant A, Moore M, Lorig K, & Franks P (2008). Perceived control moderated the self-efficacyenhancing effects of a chronic illness self-management intervention. Chronic Illn, 4(3), 173–182. doi: 10.1177/1742395308089057 [DOI] [PubMed] [Google Scholar]
- Joormann J, & Stanton CH (2016). Examining emotion regulation in depression: A review and future directions. Behav Res Ther, 86, 35–49. doi: 10.1016/j.brat.2016.07.007 [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, … Santorelli SF (1992). Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry, 149(7), 936–943. doi: 10.1176/ajp.149.7.936 [DOI] [PubMed] [Google Scholar]
- Kanaan S (2008). Promoting Effective Self-Management Approaches to Improve Chronic Disease Care: Lessons Learned. Retrieved from http://www.chcf.org/publications/2008/04/promoting-effective-selfmanagement-approaches-to-improve-chronic-disease-care-lessons-learned
- Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, … McCulloch D (2010). Collaborative care for patients with depression and chronic illnesses. N Engl J Med, 363(27), 2611–2620. doi: 10.1056/NEJMoa1003955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, DuPont RL, Berglund P, & Wittchen HU (1999). Impairment in pure and comorbid generalized anxiety disorder and major depression at 12 months in two national surveys. Am J Psychiatry, 156(12), 1915–1923. doi: 10.1176/ajp.156.12.1915 [DOI] [PubMed] [Google Scholar]
- Kinser PA, & Robins JL (2013). Control group design: enhancing rigor in research of mind-body therapies for depression. Evid Based Complement Alternat Med, 2013, 140467. doi: 10.1155/2013/140467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DB (2013). The patient-centered medical home: A future standard for american healthcare? . Public Administration Review. [Google Scholar]
- Kurdyak P, Newman A, & Segal Z (2014). Impact of mindfulness-based cognitive therapy on health care utilization: a population-based controlled comparison. J Psychosom Res, 77(2), 85–89. doi: 10.1016/j.jpsychores.2014.06.009 [DOI] [PubMed] [Google Scholar]
- Lazarus A (2004). Reality check: Is your behavior aligned with organizational goals? . The Physician Executive, 30(5), 50–52. [PubMed] [Google Scholar]
- Lenzen SA, v DJ, Daniëls R, van Bokhoven MA, van der Weijden T, Beurskens A (2015). Setting goals in chronic care: Shared decision making as self-management support by the family physician. Eur J Gen Pract, 21(2), 138–144. [DOI] [PubMed] [Google Scholar]
- Lesser I, Rosales A, Zisook S, Gonzalez C, Flores D, Trivedi M, … Epstein M (2008). Depression outcomes of Spanish- and english-speaking Hispanic outpatients in STAR*D. Psychiatr Serv, 59(11), 1273–1284. doi: 10.1176/appi.ps.59.11.1273 [DOI] [PubMed] [Google Scholar]
- Lin EH, Rutter CM, Katon W, Heckbert SR, Ciechanowski P, Oliver MM, … Von Korff M (2010). Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care, 33(2), 264–269. doi: 10.2337/dc09-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl JR, Fisher NE, Cooper DJ, Rosen RK, & Britton WB (2017). The varieties of contemplative experience: A mixed-methods study of meditation-related challenges in Western Buddhists. PLoS One, 12(5), e0176239. doi: 10.1371/journal.pone.0176239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig K, Laurent DD, Plant K, Krishnan E, & Ritter PL (2014). The components of action planning and their associations with behavior and health outcomes. Chronic llln, 10(1), 50–59. doi: 10.1177/1742395313495572 [DOI] [PubMed] [Google Scholar]
- Lorig KR, & Holman H (2003). Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med, 26(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Lorig KR, Sobel DS, Ritter PL, Laurent D, & Hobbs M (2001). Effect of a self-management program on patients with chronic disease. Eff Clin Pract, 4(6), 256–262. [PubMed] [Google Scholar]
- Loucks EB, Schuman-Olivier Z, Britton WB, Fresco DM, Desbordes G, Brewer JA, & Fulwiler C (2015). Mindfulness and Cardiovascular Disease Risk: State of the Evidence, Plausible Mechanisms, and Theoretical Framework. Curr Cardiol Rep, 17(12), 112. doi: 10.1007/s11886-015-0668-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, & Donovan DM (2007). Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors’. Guilford Publications. [Google Scholar]
- McCubbin T, Dimidjian S, Kempe K, Glassey MS, Ross C, & Beck A (2014). Mindfulness-based stress reduction in an integrated care delivery system: one-year impacts on patient-centered outcomes and health care utilization. Perm J, 18(4), 4–9. doi: 10.7812/TPP/14-014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor M, Lin EH, & Katon WJ (2011). TEAMcare: an integrated multicondition collaborative care program for chronic illnesses and depression. J Ambul Care Manage, 34(2), 152–162. doi: 10.1097/JAC.0b013e31820ef6a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire C, Gabison J, & Kligler B (2016). Facilitators and Barriers to the Integration of Mind-Body Medicine into Primary Care. J Altern Complement Med, 22(6), 437–442. doi: 10.1089/acm.2016.0043 [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S (1995). Motivational Enhancement Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence’. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Miller WR, C’de Baca J, Matthews DB, Wilbourne PL (2001). Personal Values Card Sort [Google Scholar]
- Miller WR, Rollnick Stephen (2012). Motivational Interviewing: Helping People Change Applications of Motivational Interviewing Series. New York: Guilford Press. [Google Scholar]
- Miranda J, & Cooper LA (2004). Disparities in care for depression among primary care patients. J Gen Intern Med, 19(2), 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton V, & Torgerson DJ (2003). Effect of regression to the mean on decision making in health care. BMJ, 326(7398), 1083–1084. doi: 10.1136/bmj.326.7398.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, & Ustun B (2007). Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet, 370(9590), 851–858. doi: 10.1016/S0140-6736(07)61415-9 [DOI] [PubMed] [Google Scholar]
- Nadeem E, Lange JM, & Miranda J (2008). Mental health care preferences among low-income and minority women. Arch Womens Ment Health, 11(2), 93–102. doi: 10.1007/s00737-008-0002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff KD, & Germer CK (2013). A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol, 69(1), 28–44. doi: 10.1002/jclp.21923 [DOI] [PubMed] [Google Scholar]
- Olfson M, Kroenke K, Wang S, & Blanco C (2014). Trends in office-based mental health care provided by psychiatrists and primary care physicians. J Clin Psychiatry, 75(3), 247–253. doi: 10.4088/JCP.13m08834 [DOI] [PubMed] [Google Scholar]
- Ornstein SM, Nietert PJ, Jenkins RG, & Litvin CB (2013). The prevalence of chronic diseases and multimorbidity in primary care practice: a PPRNet report. J Am Board Fam Med, 26(5), 518–524. doi: 10.3122/jabfm.2013.05.130012 [DOI] [PubMed] [Google Scholar]
- Pearson M (2007). Patient Self-Management Support Programs: An Evaluation Retrieved from Santa Monica, CA. [Google Scholar]
- Plaza I, Demarzo MM, Herrera-Mercadal P, & Garcia-Campayo J (2013). Mindfulness-based mobile applications: literature review and analysis of current features. JMIR Mhealth Uhealth, 1(2), e24. doi: 10.2196/mhealth.2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak S, Pedulla T, Siegel R (2014). Sitting Together: Essential Skills for Mindfulness-Based Psychotherapy: Guilford Publications [Google Scholar]
- Proulx J, Croff R, Oken B, Aldwin CM, Fleming C, Bergen-Cico D, … Noorani M (2018). Considerations for Research and Development of Culturally Relevant Mindfulness Interventions in American Minority Communities. Mindfulness (N Y), 9(2), 361–370. doi: 10.1007/s12671-017-0785-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh SS, A. (2012). Multilevel and Longitudinal Modeling Using Stata (3rd ed Vol. 1 & 2). College Station, TX: Stata Press. [Google Scholar]
- Raes F, Pommier E, Neff KD, & Van Gucht D (2011). Construction and factorial validation of a short form of the Self-Compassion Scale. Clin Psychol Psychother, 18(3), 250–255. doi: 10.1002/cpp.702 [DOI] [PubMed] [Google Scholar]
- Richards J, Wiese C, Katon W, Rockhill C, McCarty C, Grossman D, … Richardson LP (2010). Surveying adolescents enrolled in a regional health care delivery organization: mail and phone follow-up--what works at what cost? J Am Board Fam Med, 23(4), 534–541. doi: 10.3122/jabfm.2010.04.100019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter PL, & Lorig K (2014). The English and Spanish Self-Efficacy to Manage Chronic Disease Scale measures were validated using multiple studies. J Clin Epidemiol, 67(11), 1265–1273. doi: 10.1016/j.jclinepi.2014.06.009 [DOI] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, & Blackwell AD (2014). Cognitive impairment in depression: a systematic review and meta-analysis. Psychological Medicine, 44(10), 2029–2040. doi: 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- Roth B, & Robbins D (2004). Mindfulness-based stress reduction and health-related quality of life: findings from a bilingual inner-city patient population. Psychosom Med, 66(1), 113–123. [DOI] [PubMed] [Google Scholar]
- Roth B, & Stanley TW (2002). Mindfulness-based stress reduction and healthcare utilization in the inner city: preliminary findings. Altern Ther Health Med, 8(1), 60–62, 64–66. [PubMed] [Google Scholar]
- Schalet BD, Cook KF, Choi SW, & Cella D (2014). Establishing a common metric for self-reported anxiety: linking the MASQ, PANAS, and GaD-7 to PROMIS Anxiety. J Anxiety Disord, 28(1), 88–96. doi: 10.1016/j.janxdis.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman ZD, & Abrahm JL (2005). Implementing institutional change: an institutional case study of palliative sedation. J Palliat Med, 8(3), 666–676. doi: 10.1089/jpm.2005.8.666 [DOI] [PubMed] [Google Scholar]
- Stata Statistical Software: Release 14. (2015). College Station, TX: StataCorp LP: StataCorp. [Google Scholar]
- Sundquist J, Lilja A, Palmer K, Memon AA, Wang X, Johansson LM, & Sundquist K (2015). Mindfulness group therapy in primary care patients with depression, anxiety and stress and adjustment disorders: randomised controlled trial. Br J Psychiatry, 206(2), 128–135. doi: 10.1192/bjp.bp.114.150243 [DOI] [PubMed] [Google Scholar]
- Sundquist J, Palmer K, Johansson LM, & Sundquist K (2017). The effect of mindfulness group therapy on a broad range of psychiatric symptoms: A randomised controlled trial in primary health care. Eur Psychiatry, 43, 19–27. doi: 10.1016/j.eurpsy.2017.01.328 [DOI] [PubMed] [Google Scholar]
- Tang YY, Holzel BK, & Posner MI (2015). The neuroscience of mindfulness meditation. Nat Rev Neurosci, 16(4), 213–225. doi: 10.1038/nrn3916 [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Posner MI (2007). Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A, 104(43), 17152–17156. doi: 10.1073/pnas.0707678104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, & Lau MA (2000). Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol, 68(4), 615–623. [DOI] [PubMed] [Google Scholar]
- Culpepper L, Clayton AH, Lieberman JA III., & Susman JL (2008). Treating depression and anxiety in primary care. Prim Care Companion J Clin Psychiatry, 10(2), 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Callahan CM, Williams JW Jr., Hunkeler E, Harpole L, … Langston C (2002). Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA, 288(22), 2836–2845. [DOI] [PubMed] [Google Scholar]
- Unutzer J, & Park M (2012). Strategies to improve the management of depression in primary care. Prim Care, 39(2), 415–431. doi: 10.1016/j.pop.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilagut G, Forero CG, Adroher ND, Olariu E, Cella D, Alonso J, & investigators IN (2015). Testing the PROMIS(R) Depression measures for monitoring depression in a clinical sample outside the US. J Psychiatr Res, 68, 140–150. doi: 10.1016/j.jpsychires.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Wahbeh H Goodrich E, Goy E, & Oken BS (2016). Mechanistic Pathways of Mindfulness Meditation in Combat Veterans With Posttraumatic Stress Disorder. J Clin Psychol, 72(4), 365–383. doi: 10.1002/jclp.22255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, & Smith SM (2015). Managing patients with multimorbidity in primary care. BMJ, 350, h176. doi: 10.1136/bmj.h176 [DOI] [PubMed] [Google Scholar]
- Waller G (2009). Evidence-based treatment and therapist drift. Behav Res Ther, 47(2), 119–127. doi: 10.1016/j.brat.2008.10.018 [DOI] [PubMed] [Google Scholar]
- Woods-Giscombe CL, & Gaylord SA (2014). The Cultural Relevance of Mindfulness Meditation as a Health Intervention for African Americans: Implications for Reducing Stress-Related Health Disparities. J Holist Nurs, 32(3), 147–160. doi: 10.1177/0898010113519010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, & Coghill RC (2015). Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia. J Neurosci, 35(46), 15307–15325. doi: 10.1523/JNEUROSCI.2542-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


