Abstract
Following a request from the European Commission, the EFSA Panel on Plant Health (PLH) performed a pest categorisation of Davidsoniella virescens, a well‐defined and distinguishable fungal species of the family Ceratocystidaceae. The species was moved from the genus Ceratocystis to the genus Davidsoniella following a revision of the family. The former species name Ceratocystis virescens is used in the Council Directive 2000/29/EC. The pathogen is regulated in Annex IIAI as a harmful organism whose introduction into the EU is banned on plants (other than fruit and seeds) and wood (including wood which has not kept its natural round surface) of Acer saccharum, originating in the USA and Canada. The fungus is native to eastern North America and causes symptoms mainly on A. saccharum, but also on Liriodendron tulipifera. The fungus is also reported as a saprotroph on various hardwood species. The pest could enter the EU via wood, plants for planting and cut branches. Hosts and favourable climatic conditions are widespread in the EU. The pest would be able to spread following establishment through sap‐feeding insects, root grafts and movement of infected wood and plants for planting. The pest introduction could have impacts on Acer spp. and L. tulipifera trees in the EU, by causing wilting, yellowing and the development of small leaves, as well as dieback of branches and, eventually, the death of trees. Avoiding damaging trees (as wounding facilitates infection of the fungus) and maintaining healthy trees (as tree stress facilitates the disease) are available measures to reduce impacts. The main knowledge gaps concern (i) the biology and epidemiology of the pathogen (including the saprotrophic form), (ii) the role of insect vectors for entry and spread, and (iii) the susceptibility of Acer spp. either native to or more recently established in Europe. The criteria assessed by the Panel for consideration as potential quarantine pest are met. For regulated non‐quarantine pests, the criterion on the pest presence in the EU is not met.
Keywords: European Union, forest pathology, pest risk, plant health, plant pest, quarantine, tree health
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorisations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/2002,3 to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria on to be taken particularly under consideration for these cases is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L.,Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Ceratocystis virescens is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest (RNQP) for the area of the EU.
The recommended valid name for the fungus is currently Davidsoniella virescens (de Beer et al., 2014; Wingfield et al., 2015).
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on D. virescens was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using both the current and the former (C. virescens; including synonyms) scientific names of the pest as search terms. Relevant papers were reviewed, further references and information were obtained from experts, from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO, 2017).
Data about import of commodity types that could potentially provide a pathway for the pest to enter the EU (http://epp.eurostat.ec.europa.eu/newxtweb/) and about the area of hosts grown in the EU were obtained from EUROSTAT (http://ec.europa.eu/eurostat/web/agriculture/data/database).
Information on European Union Member State (EU MS) imports of Acer plants for planting from North America were sought in the ISEFOR database (Eschen et al., 2017).
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Food Safety (DG SANTE), and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the MSs and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for D. virescens, following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was started following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union regulated non‐quarantine pest in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required as per the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to qualify either as a quarantine pest or as a regulated non‐quarantine pest. If one of the criteria is not met, the pest will not qualify. In such a case, the working group should consider the possibility to stop the assessment early and be concise in the sections preceding the question for which the negative answer is reached. Note that a pest that does not qualify as a quarantine pest may still qualify as a regulated non‐quarantine pest which needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone, thus the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32‐35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism. | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pes must be present in the risk assessment area). |
| Regulatory status (Section 3.3) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future. |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone). |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met. |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regards to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with the EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed 2‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes
Davidsoniella virescens (R.W. Davidson) Z.W. de Beer, T.A. Duong & M.J. Wingf. (1996) is a fungus of the family Ceratocystidaceae.
The species was moved from the genus Ceratocystis to the genus Davidsoniella following a major revision of the family Ceratocystidaceae (de Beer et al., 2014). The former species name Ceratocystis virescens is used in the Council Directive 2000/29/EC.
Other species synonyms are: Endoconidiophora virescens and Ophiostoma virescens (Index Fungorum, http://www.indexfungorum.org/names/names.asp).
3.1.2. Biology of the pest
Davidsoniella virescens causes sapstreak disease of sugar maple (Acer saccharum) and was first reported in 1935 (Hepting, 1944).
There appears to be limited knowledge regarding the biology of the fungus. Wounds, especially at or near the ground on the stem or roots, are thought to be important (Mielke and Charette, 1989). Houston (1993) found that infection rarely occurs in trees without wounds. Transmission between trees through root grafts was also observed but was suggested to be less important than aboveground wounds for infection (Houston, 1993). Sap beetles (Coleoptera: Nitidulidae) visiting fresh wounds are thought to act as vectors (Sinclair and Lyon, 2005).
After infection, the fungus spreads rapidly in the sapwood and generally overcomes the defences of the host (Sinclair and Lyon, 2005). Water soaked lesions develop in the sapwood and may eventually extend throughout the cross section of the lower trunk, down into the roots and up into the branches. Necrosis of the sapwood leads to visible external symptoms such as slow growth, chlorosis and small leafs and dieback of branches. Foliar and branch symptoms appear 1–6 years after infection and usually intensify year to year (Sinclair and Lyon, 2005). Elongate cankers develop where the fungus reaches the cambium. Affected trees with crown symptoms may die within two to three years, but some may survive longer before the decline accelerates (Houston, 1993; Sinclair and Lyon, 2005; Bal et al., 2013). Secondary agents, such as Armillaria spp. and Xylaria spp., have been associated with a major part of dying trees (Houston, 1993).
Sporulation is observed on moist wood surfaces, either as a result of wounding or if the trees are cut down. A dark gray mat of mycelium is formed, producing conidiophores and long‐necked black perithecia. Two types of endoconidia are formed; microconidia which are hyaline, cylindrical and vary in length (6–25 × 2–3 μm) and short barrel‐shaped endoconidia (5–9 × 5–6.5 μm) (Davidson, 1944). Ascospores are hyaline, slightly curved and collected in sticky white spherical masses (Davidson, 1944).
The main host A. saccharum, sugar maple, is most susceptible during late spring and summer (Houston, 1993). In diseased sugar maple stands, damage was strongly associated with human activities such as logging, thinning, road building or sap hauling which had injured the trees (Ohman and Spike, 1966; Houston, 1993).
3.1.3. Intraspecific diversity
D. virescens has earlier been thought to be synonymous to Endoconidiophora coerulescens (syn. Ceratocystis coerulescens), which is widespread in Europe causing blue‐staining of conifers (EPPO, 2017). However, this has been contradicted based on morphological criteria (Nag Raj and Kendrick, 1976; Kile and Walker, 1987), isozyme variation (Harrington et al., 1996) and by analysis of DNA‐sequence data (Witthuhn et al., 1998; de Beer et al., 2014).
D. virescens has been reported as a common saprophyte on fresh cut logs of sugar maple and other hardwood species in North America (Shigo, 1962; Bal et al., 2013), but whether the saprophyte species observed on hardwood tree species other than A. saccharum is synonymous with D. virescens causing sapstreak of sugar maple has been questioned (Smith, 1990). Fingerprint nuclear markers detected differences between pathogenic strains causing symptoms on Acer spp. and Liriodendron and saprotrophic strains colonising wood of Fagus and other hardwoods (Harrington et al., 1998).
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, detection and identification methods are available.
Cultures of D. virescens from infected wood tissue may be identified based on morphological descriptions provided by Davidson (1944). The species can also be identified based on sequences of the internal transcribed spacer (ITS) region (DNA sequence data given in Qbank: http://www.q-bank.eu).
3.2. Pest distribution
D. virescens is only reported from limited areas of eastern North America (EPPO, 2017) (Figure 1).
Figure 1.
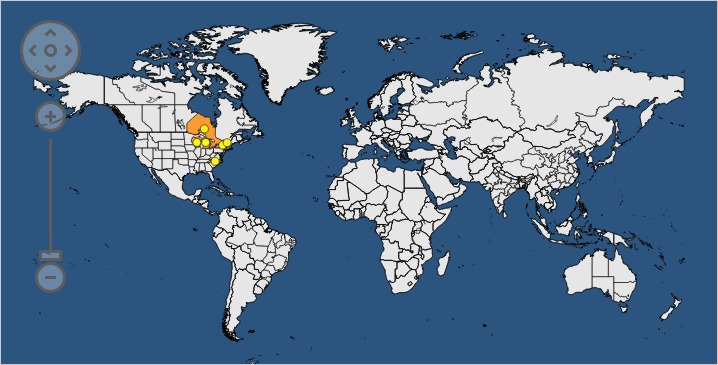
Global distribution map for Davidsoniella virescens (extracted from EPPO Global Database, accessed August 2017). There are no reports of transient populations
3.2.1. Pest distribution outside the EU
The pathogen is reported as present with restricted distribution in the USA and found in the states of Michigan, New York, North Carolina, Vermont and Wisconsin (EPPO, 2017). The pathogen had previously been reported also in Minnesota and California (EPPO, 1997), but these records are absent in EPPO (2017). In Canada, the pathogen is currently reported as ‘Absent, no longer present’ in Manitoba and as ‘Present, no details’ in Ontario (EPPO, 2017).
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
No, the pest is not reported to be present in the EU.
There are no reports of D. virescens from the EU (EPPO, 2017). The Netherlands has reported the pathogen as ‘Absent, confirmed by survey’ based on information dated December 2013. Similarly, Slovenia has reported the pathogen to be absent (no pest record), based on information dated July 2017.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
D. virescens is listed in Council Directive 2000/29/EC as Ceratocystis virescens. Details are presented in Tables 2 and 3 (only the relevant extracts have been included here).
Table 2.
Davidsoniella virescens in Council Directive 2000/29/EC
| Annex II, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned if they are present on certain plants or plant products | |
| Section I | Harmful organisms not known to occur in the community and relevant for the entire community | |
| (c) | Fungi | |
| Species | Subject of contamination | |
| 4. | Ceratocystis virescens (Davidson) Moreau | Plants of Acer saccharum Marsh., other than fruit and seeds, originating in the USA and Canada, wood of Acer saccharum Marsh., including wood which has not kept its natural round surface, originating in the USA and Canada |
3.3.2. Legislation addressing plants and plant parts on which Davidsoniella virescens is regulated
Table 3.
Regulated hosts and commodities that may involve Davidsoniella virescens in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
| Description | Country of origin | |
| 7. Isolated bark of Acer saccharum Marsh | North American countries | |
| Annex IV, Part A | Special requirements which must be laid down by all member states for the introduction and movement of plants, plant products and other objects into and within all member states | |
| Section I | Plants, plant products and other objects originating outside the community | |
| Plants, plant products and other objects | Special requirements | |
|
2.1 Wood of Acer saccharum Marsh., including wood which has not kept its natural round surface, other than in the form of:
|
Official statement that the wood has undergone kiln‐drying to below 20% moisture content, expressed as a percentage of dry matter, achieved through an appropriate time/temperature schedule. There shall be evidence thereof by a mark ‘Kiln‐dried’ or ‘KD’ or another internationally recognised mark, put on the wood or on any wrapping in accordance with current usage | |
| 2.2 Wood of Acer saccharum Marsh., intended for the production of veneer sheets, originating in the USA and Canada | Official statement that the wood originates in areas known to be free from Ceratocystis virescens (Davidson) Moreau and is intended for the production of veneer sheets | |
|
7.1.1. Whether or not listed among the CN‐codes in Annex V, Part B, wood in the form of chips, particles, sawdust, shavings, wood waste and scrap and obtained in whole or in part from: — Acer saccharum Marsh., originating in the USA and Canada |
Official statement that the wood:
|
|
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community—in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community | |
| Part B | Plants, plant products and other objects originating in territories, other than those territories referred to in part A | |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community | |
| 2. Parts of plants, other than fruits and seeds, of: | – Acer saccharum Marsh., originating in the USA and Canada | |
| 5. Isolated bark of | Acer saccharum Marsh | |
|
6. Wood within the meaning of the first subparagraph of Article 2(2), where it:
|
Acer saccharum Marsh., including wood which has not kept its natural round surface, originating in the USA and Canada | |
|
CN code ex 4407 93 |
Description Wood of Acer saccharum Marsh, sawn or chipped lengthwise, sliced or peeled, whether or not planed, sanded or end‐jointed, of a thickness exceeding 6 mm |
| Ex 4407 99 | Non‐coniferous wood (other than tropical wood specified in subheading note 1 to Chapter 44 or other tropical wood, […] maple (Acer spp.), […]), sawn or chipped lengthwise, sliced or peeled, whether or not planed, sanded or end‐jointed, of a thickness exceeding 6 mm | |
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
The fungus is found causing symptoms mainly on A. saccharum (sugar maple), but also on Liriodendron tulipifera (Ohman and Spike, 1966). The DEFRA risk register in the UK also mentions Acer saccharinum (silver maple) as a major host (https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=609&riskId=609), based on the UK Forest Research pest risk assessment on C. virescens, where the report of A. saccharinum as a host for the pathogen appears to be mistaken (Webber, 2008). In the literature, however, there is one record of D. virescens on A. saccharinum from New York State reported in a Master thesis (Langham, 1994). In addition, there is a mention of Acer rubrum as host of D. virescens (Kehr et al., 2004).
The species has also been found as a saprophyte on logs of a number of other hardwood species, but these strains may be a different variety not pathogenic on Acer (Harrington et al., 1998 and pers. comm. therein). Tree species reported to be colonised by the saprotroph include Fagus spp. and Betula spp. (Shigo, 1962).
The pest is regulated in Council Directive 2000/29/EC on A. saccharum only. It is not regulated on other Acer species, L. tulipifera, other hardwood species, or their products.
Some maple species native to Europe (Acer campestre, Acer pseudoplatanus and Acer platanoides) have been introduced in the native range of A. saccharum in North America (United States Department of Agriculture (USDA) Plants Database), but there appear to be no available reports of D. virescens on them.
3.4.2. Entry
Is the pest able to enter into the EU territory?
Yes, the pest could enter the EU on wood, plants for planting and cut branches (see below)
The most likely pathway of entry is wood from diseased Acer trees. Houston (1986) observed that the fungus could be isolated from surface mycelium on infected wood cut into boards, sticker‐piled and air‐dried (to a moisture content of 20%) after 5 months.
The stain in infected wood is initially easily observed as water‐soaked yellow‐green but changes quickly on drying to light‐brown (Houston, 1986) and may thus be difficult to detect on wood from diseased trees (EPPO, 1997).
In addition, plants for planting and cut branches of A. saccharum are considered host commodities providing a pathway for entry (EPPO, 2017).
The main pathways of entry are thus:
wood
plants for planting
cut branches.
As of August 2017, there is one interception of D. virescens in the Europhyt database (reported by Finland and originating in the USA). This interception was reported on A. saccharum in 1996 without any indication of the involved commodity.
In the ISEFOR database of plants for planting, there are some records of shipments of Acer spp. plants for planting imported by the EU from Canada and the USA.
3.4.3. Establishment
Is the pest able to become established in the EU territory?
Yes, the pest could establish in the EU, as hosts and favourable climatic conditions are widespread in the EU.
3.4.3.1. EU distribution of main host plants
The main host A. saccharum is not cultivated in the EU other than as an ornamental tree. In a multi‐country biodiversity experiment, there is a small plantation including A. saccharum in Germany (http://www.treedivnet.ugent.be/ExpIDENT.html). In the UK, the species is reported as having escaped from cultivation (BSBI website: https://database.bsbi.org/maps/?taxonid=2cd4p9h.kzm).
A. saccharinum is reported as established from France, Poland and the UK (DAISIE database, http://www.europe-aliens.org/speciesFactsheet.do?speciesId=17113#).
There are several native species of Acer spp. in the EU, e.g. A. campestre, A. platanoides, A. pseudoplatanus, and Acer monspessulanum, together with numerous introduced ornamental species and hybrids. The distribution of the most widespread Acer species in Europe is reported in Figures 2–5. The susceptibility of these hosts to the fungus, however, is not known. D. virescens is stated to damage or kill seedlings of A. campestre in nurseries but no further details are given (Zecchin et al., 2016).
Figure 2.
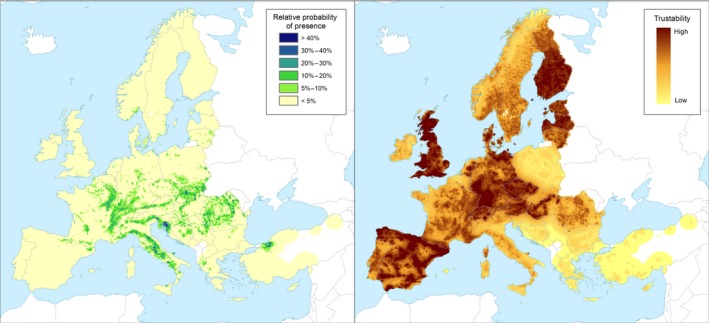
Left‐hand panel: Relative probability of presence (RPP) of the genus Acer (based on data from the species: A. campestre, A. pseudoplatanus, A. platanoides, A. lobelii, A. tataricum, A. opalus, A. monspessulanum, A. negundo, A. saccharinum, A. obtusatum) in Europe, mapped at 100 km2 resolution. The underlying data are from European‐wide forest monitoring data sets and from national forestry inventories based on standard observation plots measuring in the order of hundreds m2. RPP represents the probability of finding at least one individual of the taxon in a standard plot placed randomly within the grid cell. For details, see Appendix A (courtesy of JRC, 2017). Right‐hand panel: Trustability of RPP. This metric expresses the strength of the underlying information in each grid cell and varies according to the spatial variability in forestry inventories. The colour scale of the trustability map is obtained by plotting the cumulative probabilities (0–1) of the underlying index (for details see Appendix A).
Figure 5.
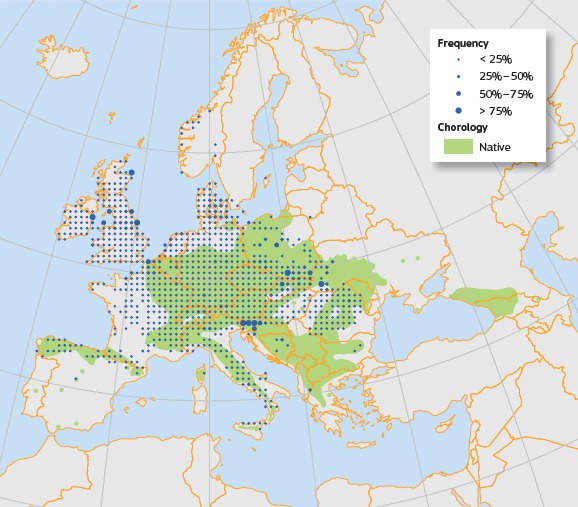
Plot distribution and simplified chorology map for Acer pseudoplatanus. Frequency of A. pseudoplatanus occurrences within the field observations as reported by the National Forest Inventories. The chorology of the native spatial range for A. pseudoplatanus is derived from EUFORGEN (from Pasta et al., 2016)
Liriodendron tulipifera, which is also native to North America, is planted as an ornamental tree in Europe (in the UK, the species is reported as having escaped from cultivation (BSBI website: https://database.bsbi.org/maps/?taxonid=2cd4p9h.der). There are no native species of the genus in Europe.
If the saprotroph on other hardwood species is the same species as D. virescens (see Sections 3.1.3 and 3.4.1), then various additional widespread tree species in the EU could harbour the pathogen.
Figure 3.
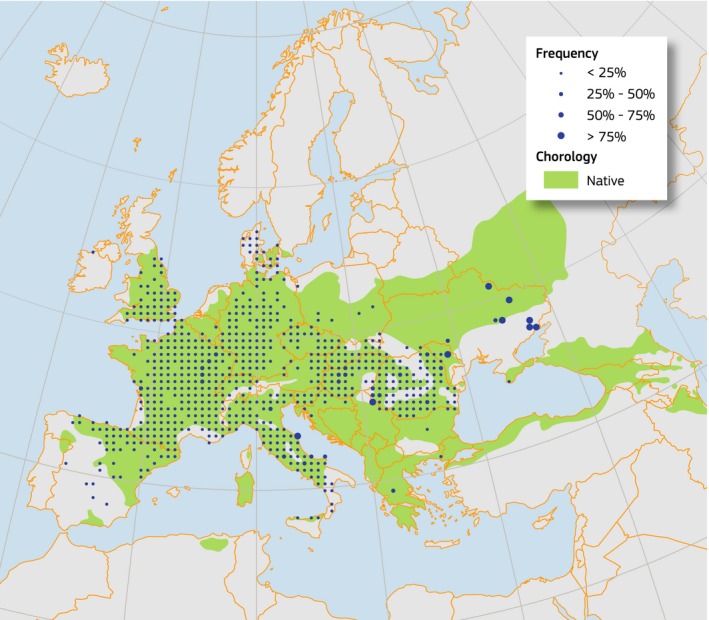
Plot distribution and simplified chorology map for Acer campestre. Frequency of A. campestre occurrences within the field observations as reported by the National Forest Inventories. The chorology of the native spatial range for A. campestre is derived from EUFORGEN (from Zecchin et al., 2016)
Figure 4.
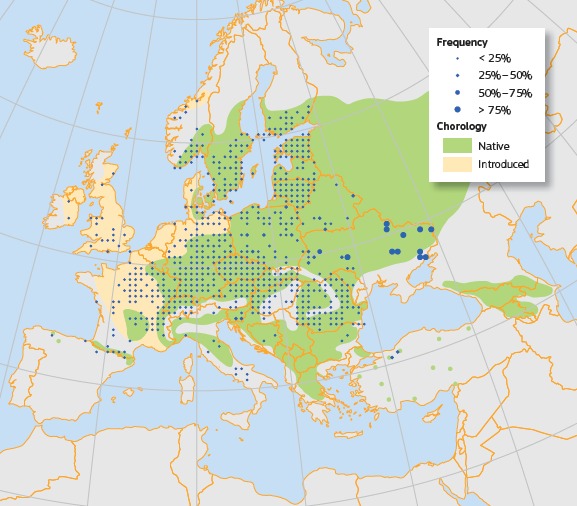
Plot distribution and simplified chorology map for Acer platanoides. Frequency of A. platanoides occurrences within the field observations as reported by the National Forest Inventories. The chorology of the native and introduced spatial range for A. platanoides is derived from several sources (from Caudullo and Rigo, 2016)
3.4.3.2. Climatic conditions affecting establishment
The distribution of D. virescens in North America (Figure 1; Section 3.2.1) covers areas with cold and temperate Köppen–Geiger climate types (Peel et al., 2007). These climate types overlap to a large extent with the distributions of the native Acer species in Europe.
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment?
Yes, by various means, i.e. through sap‐feeding insects, root grafts and movement of infected wood and plants for planting.
Infection in A. saccharum is mainly associated with wounding by human activities, such as logging, thinning, road building or sap hauling (Ohman and Spike, 1966; Houston, 1993).
Sap‐feeding insects have been suggested to play a role in bringing inoculum to wounds in the trees where the infection takes place (Sinclair and Lyon, 2005).
Occasional transmission between trees through root grafts has been observed in North America (Houston, 1993).
Longer distance spread may be due to transport of wood from infected trees as the fungus is able to survive and sporulate in cut and air dried wood (Houston, 1986).
Given that plants for planting and cut branches are considered to be a potential pathway of entry (see section 3.4.2), these commodities could also be a means of spread within the EU.
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, the pest introduction could have impacts on Acer and Liriodendron trees in the EU.
RNQPs: Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting?4
Yes, the introduction of the pest could have an impact on the intended use of plants for planting.
The infection causes wilting, yellowing and the development of small leaves, as well as dieback of branches. This leads to a decline and eventual death of the tree, often within a few years (Mielke and Charette, 1989; Houston, 1993, 1994; Bal et al., 2013). Mortality is however, often associated with secondary attacks by, for example, Armillaria spp. (Houston, 1999).
The fungus further causes a reddish‐brown to blue‐green stain of the wood, generally found at the base of the trunk in the root‐flare region of the tree (Houston, 1993). Wood strength is not affected but the stain lowers the commercial value of the timber significantly (Ohman and Spike, 1966) (Figure 6).
Figure 6.

Stem of Acer saccharum showing sap streak symptoms due to Davidsoniella virescens (courtesy of John Gibbs, Forestry Commission, Bugwood.org, available online: https://www.forestryimages.org/browse/detail.cfm?imgnum=0725094)
In North America, sapstreak disease is stated as being rare in healthy stands of sugar maple (Richter, 2012) and is mainly associated with stand properties that may stress the trees, e.g. root compaction, water‐logging and human disturbance (Houston, 1993, 1994). Nevertheless, sapstreak disease has been described as a serious threat to A. saccharum forests. Although in some cases trees may recover (Houston, 1993), the salvage value of the timber is low.
There is no strong evidence that D. virescens is able to infect Acer spp. other than A. saccharum. The latter is considered as of negligible importance for the EU. As native Acer species in Europe lack adaption to the pathogen, they may be susceptible, but evidence is lacking.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes, please see Section 3.6.3.
3.6.1. Biological or technical factors limiting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
The fungus is able to survive and sporulate on cut pieces of air dried wood for several months (Houston, 1986).
Provided that the saprotroph is the same species as the pathogen, D. virescens is often found as a saprotroph of several different hardwood tree species in its native range.
Kiln‐drying may be effective against D. virescens, though there is no information available (EPPO, 2017).
3.6.2. Biological or technical factors limiting the ability to prevent the presence of the pest on plants for planting
It is uncertain how effective chemical control in nurseries could be and whether it might just mask symptoms, hence allowing the movement of the pathogen via the trade in plants for planting.
3.6.3. Control methods
3.7. Uncertainty
The knowledge regarding the biology of the pathogen is limited and the role of insects as vectors is unclear.
Whether the fungus can infect (and cause symptoms on) species of the genus Acer other than A. saccharum is unclear.
Similarly, it is unclear whether strains of D. virescens infecting and causing disease in A. saccharum and Liriodendron tulipifera can also be a saprotroph of other hardwood species.
4. Conclusions
D. virescens meets the criteria assessed by EFSA for consideration as a potential quarantine pest (Table 4).
Table 4.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the pest as a species is clear | The identity of the pest as a species is clear | None |
| Absence/presence of the pest in the EU territory (Section 3.2) | The pest is not reported to be present in the EU | The pest is not reported to be present in the EU | Confirmation of absence has only been provided by the Netherlands and Slovenia |
| Regulatory status (Section 3.3) | D. virescens is regulated by Council Directive 2000/29/EC (Annex IIA) on plants of Acer saccharum, other than fruit and seeds, originating in the USA and Canada, as well as on wood of A. saccharum, including wood which has not kept its natural round surface, originating in the USA and Canada | D. virescens is regulated by Council Directive 2000/29/EC (Annex IIA) on plants of Acer saccharum, other than fruit and seeds, originating in the USA and Canada, as well as on wood of A. saccharum, including wood which has not kept its natural round surface, originating in the USA and Canada | None |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) |
Entry: the pest could enter the EU via wood, plants for planting, and cut branches Establishment: hosts and favourable climatic conditions are widespread in the risk assessment (RA) area Spread: the pest would be able to spread following establishment by various means, i.e. insects, root grafts and movement of infected wood and plants for planting |
Entry: the pest could enter the EU via wood, plants for planting, and cut branches Establishment: hosts and favourable climatic conditions are widespread in the RA area Spread: the pest would be able to spread following establishment by various means, i.e. insects, root grafts and movement of infected wood and plants for planting |
There is uncertainty about the susceptibility of Acer spp. native to Europe There is uncertainty on whether the saprotrophic form is the same species as the pathogenic D. virescens |
| Potential for consequences in the EU territory (Section 3.5) | The pest introduction could have impacts on Acer and Liriodendron trees | The introduction of the pest could have an impact on the intended use of plants for planting | There is uncertainty about the susceptibility of Acer spp. native to Europe |
| Available measures (Section 3.6) | Avoiding damaging the trees (as wounding facilitates infection of the fungus) and maintaining healthy stands (as tree stress facilitates the disease) are available measures to reduce impacts | There is a lack of information on available measures to reduce the risk of establishment in nurseries | It is uncertain how effective chemical control in nurseries could be and whether it might just mask symptoms, hence allowing the movement of the pathogen via the trade in plants for planting |
| Conclusion on pest categorisation (Section 4) | The criteria assessed by the Panel for consideration as potential quarantine pest are met | The criterion on the pest presence in the EU is not met | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | The main knowledge gaps concern (i) the biology and epidemiology of the pathogen (including whether the saprotrophic form is the same species as the pathogen), (ii) the role of insect vectors for entry and spread, and (iii) the susceptibility of Acer spp. native to Europe | ||
Abbreviations
- CLC
Corine Land Cover
- C‐SMFA
Constrained spatial multi‐scale frequency analysis
- DG SANTE
Directorate General for Health and Food Safety
- EPPO
European and Mediterranean Plant Protection Organization
- EUFGIS
European Information System on Forest Genetic Resources
- EU MS
European Union Member State
- FAO
Food and Agriculture Organization
- GD2
Georeferenced Data on Genetic Diversity
- IPPC
International Plant Protection Convention
- ITS
Internal transcribed spacer
- JRC
Joint Research Centre of the European Commission
- PLH
EFSA Panel on Plant Health
- RA
Risk assessment
- RNQP
Regulated non‐quarantine pest
- RPP
Relative probability of presence
- ToR
Terms of Reference
- USDA
United States Department of Agriculture
Appendix A – Methodological notes on Figure 2
The relative probability of presence (RPP) reported here for Acer spp. in Figure 2 and in the European Atlas of Forest Tree Species (de Rigo et al., 2016; San‐Miguel‐Ayanz et al., 2016) is the probability of that genus to occur in a given spatial unit (de Rigo et al., 2017). In forestry, such a probability for a single taxon is called ‘relative’. The maps of RPP are produced by means of the constrained spatial multi‐scale frequency analysis (C‐SMFA) (de Rigo et al., 2014, 2017) of species presence data reported in geolocated plots by different forest inventories.
A.1. Geolocated plot databases
The RPP models rely on five geodatabases that provide presence/absence data for tree species and genera: four European‐wide forest monitoring data sets and a harmonised collection of records from national forest inventories (de Rigo et al., 2014, 2016, 2017). The databases report observations made inside geolocalised sample plots positioned in a forested area, but do not provide information about the plot size or consistent quantitative information about the recorded species beyond presence/absence.
The harmonisation of these data sets was performed within the research project at the origin of the European Atlas of Forest Tree Species (de Rigo et al., 2016; San‐Miguel‐Ayanz, 2016; San‐Miguel‐Ayanz et al., 2016). Given the heterogeneity of strategies of field sampling design and establishment of sampling plots in the various national forest inventories (Chirici et al., 2011a,b), and also given legal constraints, the information from the original data sources was harmonised to refer to an INSPIRE compliant geospatial grid, with a spatial resolution of 1 km2 pixel size, using the ETRS89 Lambert Azimuthal Equal‐Area as geospatial projection (EPSG: 3035, http://spatialreference.org/ref/epsg/etrs89-etrs-laea/).
A.1.1. European National Forestry Inventories database
This data set was derived from National Forest Inventory data and provides information on the presence/absence of forest tree species in approximately 375,000 sample points with a spatial resolution of 1 km2/pixel, covering 21 European countries (de Rigo et al., 2014, 2016).
A.1.2. Forest Focus/Monitoring data set
This project is a Community scheme for harmonised long‐term monitoring of air pollution effects in European forest ecosystems, normed by EC Regulation No 2152/20035. Under this scheme, the monitoring is carried out by participating countries on the basis of a systematic network of observation points (Level I) and a network of observation plots for intensive and continuous monitoring (Level II). For managing the data, the JRC implemented a Forest Focus Monitoring Database System, from which the data used in this project were taken (Hiederer et al., 2007; Houston Durrant and Hiederer, 2009). The complete Forest Focus data set covers 30 European Countries with more than 8,600 sample points.
A.1.3. BioSoil data set
This data set was produced by one of a number of demonstration studies performed in response to the ‘Forest Focus’ Regulation (EC) No 2152/2003 mentioned above. The aim of the BioSoil project was to provide harmonised soil and forest biodiversity data. It comprised two modules: a Soil Module (Hiederer et al., 2011) and a Biodiversity Module (Houston Durrant et al., 2011). The data set used in the C‐SMFA RPP model came from the Biodiversity module, in which plant species from both the tree layer and the ground vegetation layer were recorded for more than 3,300 sample points in 19 European Countries.
A.1.4. European Information System on Forest Genetic Resources (EUFGIS)
EUFGIS (http://portal.eufgis.org) is a smaller geodatabase providing information on tree species composition in over 3,200 forest plots in 34 European countries. The plots are part of a network of forest stands managed for the genetic conservation of one or more target tree species. Hence, the plots represent the natural environment to which the target tree species are adapted.
A.1.5. Georeferenced Data on Genetic Diversity (GD2)
GD2 (http://gd2.pierroton.inra.fr) provides information about 63 species of interest for genetic conservation. The database covers 6,254 forest plots located in stands of natural populations that are traditionally analysed in genetic surveys. While this database covers fewer species than the others, it covers 66 countries in Europe, North Africa and the Middle East, making it the data set with the largest geographic extent.
A.2. Modelling methodology
For modelling, the data were harmonised in order to have the same spatial resolution (1 km2) and filtered to a study area comprising 36 countries in the European continent. The density of field observations varies greatly throughout the study area and large areas are poorly covered by the plot databases. A low density of field plots is particularly problematic in heterogeneous landscapes, such as mountainous regions and areas with many different land use and cover types, where a plot in one location is not representative of many nearby locations (de Rigo et al., 2014). To account for the spatial variation in plot density, the model used here (C‐SMFA) considers multiple spatial scales when estimating RPP. Furthermore, statistical resampling is systematically applied to mitigate the cumulated data‐driven uncertainty.
The presence or absence of a given forest tree species then refers to an idealised standard field sample of negligible size compared with the 1 km2 pixel size of the harmonised grid. The modelling methodology considered these presence/absence measures as if they were random samples of a binary quantity (the punctual presence/absence, not the pixel one). This binary quantity is a random variable having its own probability distribution which is a function of the unknown average probability of finding the given tree species within a plot of negligible area belonging to the considered 1 km2 pixel (de Rigo et al., 2014). This unknown statistic is denoted hereinafter with the name of ‘probability of presence’.
C‐SMFA performs spatial frequency analysis of the geolocated plot data to create preliminary RPP maps (de Rigo et al., 2014). For each 1 km2 grid cell, the model estimates kernel densities over a range of kernel sizes to estimate the probability that a given species is present in that cell. The entire array of multi‐scale spatial kernels is aggregated with adaptive weights based on the local pattern of data density. Thus, in areas where plot data are scarce or inconsistent, the method tends to put weight on larger kernels. Wherever denser local data are available, they are privileged ensuring a more detailed local RPP estimation. Therefore, a smooth multi‐scale aggregation of the entire arrays of kernels and data sets is applied instead of selecting a local ‘best performing’ one and discarding the remaining information. This array‐based processing, and the entire data harmonisation procedure, are made possible thanks to the semantic modularisation which defines the Semantic Array Programming modelling paradigm (de Rigo, 2012).
The probability to find a single species (e.g. a particular coniferous tree species) in a 1 km2 grid cell cannot be higher than the probability of presence of all the coniferous species combined. The same logical constraints applied to the case of single broadleaved species with respect to the probability of presence of all the broadleaved species combined. Thus, to improve the accuracy of the maps, the preliminary RPP values were constrained so as not to exceed the local forest‐type cover fraction with an iterative refinement (de Rigo et al., 2014). The forest‐type cover fraction was estimated from the classes of the Corine Land Cover (CLC) maps which contain a component of forest trees (Bossard et al., 2000; Büttner et al., 2012).
The resulting probability of presence is relative to the specific tree taxon, irrespective of the potential co‐occurrence of other tree taxa with the measured plots, and should not be confused with the absolute abundance or proportion of each taxon in the plots. RPP represents the probability of finding at least one individual of the taxon in a plot placed randomly within the grid cell, assuming that the plot has negligible area compared with the cell. As a consequence, the sum of the RPP associated with different taxa in the same area is not constrained to be 100%. For example, in a forest with two co‐dominant tree species which are homogeneously mixed, the RPP of both may be 100% (see e.g. the Glossary in San‐Miguel‐Ayanz et al. (2016), http://forest.jrc.ec.europa.eu/media/atlas/Glossary.pdf).
The robustness of RPP maps depends strongly on sample plot density, as areas with few field observations are mapped with greater uncertainty. This uncertainty is shown qualitatively in maps of ‘RPP trustability’. RPP trustability is computed on the basis of the aggregated equivalent number of sample plots in each grid cell (equivalent local density of plot data). The trustability map scale is relative, ranging from 0 to 1, as it is based on the quantiles of the local plot density map obtained using all field observations for the species. Thus, trustability maps may vary among species based on the number of databases that report a particular species (de Rigo et al., 2014, 2016).
The RPP and relative trustability range from 0 to 1 and are mapped at a 1 km spatial resolution. To improve visualisation, these maps can be aggregated to coarser scales (i.e. 10 × 10 pixels or 25 × 25 pixels, respectively, summarising the information for aggregated spatial cells of 100 and 625 km2) by averaging the values in larger grid cells.
Suggested citation: EFSA Panel on Plant Health (PLH) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Boberg J, Gonthier P and Pautasso M, 2017. Scientific Opinion on the pest categorisation of Davidsoniella virescens . EFSA Journal 2017;15(12):5104, 28 pp. 10.2903/j.efsa.2017.5104
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00324
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Gregoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Adopted: 23 November 2017
Reproduction of the images listed below is authorised, provide the source is acknowledged:
Figure 1: © European and Mediterranean Plant Protection Organization (EPPO); Figures 2–5: © European Union; Figure 6: © Bugwood.org
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
See Section 2.1 on what falls outside EFSA's remit.
Council of the European Union, 2003. Regulation (EC) No 2152/2003 of the European Parliament and of the Council of 17 November 2003 concerning monitoring of forests and environmental interactions in the Community (Forest Focus). Official Journal of the European Union 46 (L 324), 1–8.
References
- Bal TL, Richter DL, Storer AJ and Jurgensen MF, 2013. The relationship of the sapstreak fungus, Ceratocystis virescens, to sugar maple dieback and decay in Northern Michigan. American Journal of Plant Sciences, 4, 436–443. [Google Scholar]
- de Beer ZW, Duong TA, Barnes I, Wingfield BD and Wingfield MJ, 2014. Redefining Ceratocystis and allied genera. Studies in Mycology, 79, 187–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossard M, Feranec J and Otahel J, 2000. CORINE land cover technical guide ‐ Addendum 2000. Tech. Rep. 40, European Environment Agency. Available online: https://www.eea.europa.eu/ds_resolveuid/032TFUPGVR
- Büttner G, Kosztra B, Maucha G and Pataki R, 2012. Implementation and achievements of CLC2006. Tech. rep., European Environment Agency. Available online: http://www.eea.europa.eu/ds_resolveuid/GQ4JECM8TB
- Caudullo G and de Rigo D, 2016. Acer platanoides in Europe: distribution, habitat, usage and threats In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A. (eds.). European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg. pp. e019159+. [Google Scholar]
- Chirici G, Bertini R, Travaglini D, Puletti N and Chiavetta U, 2011a. The common NFI database In: Chirici G, Winter S. and McRoberts RE. (eds.). National forest inventories: contributions to forest biodiversity assessments. Springer, Berlin: pp. 99–119. [Google Scholar]
- Chirici G, McRoberts RE, Winter S, Barbati A, Brändli U‐B, Abegg M, Beranova J, Rondeux J, Bertini R, Alberdi Asensio I and Condés S, 2011b. Harmonization tests In: Chirici G, Winter S. and McRoberts RE. (eds.). National Forest Inventories: Contributions to Forest Biodiversity Assessments. Springer, Berlin: pp. 121–190. [Google Scholar]
- Davidson RW, 1944. Two American hardwood species of Endoconidiophora described as new. Mycologia, 36, 300–306. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2903/j.efsa.2010.1495 [DOI] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 1997. Data sheets on quarantine pests: Ceratocystis virescens In: Smith IM, McNamara DG, Scott PR. and Holderness M. (eds.). Quarantine Pests for Europe, 2nd Edition CABI/EPPO, Wallingford, 1425 pp. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2017. EPPO Global Database. Available online: https://gd.eppo.int
- Eschen R, Douma JC, Grégoire JC, Mayer F, Rigaux L and Potting RP, 2017. A risk categorisation and analysis of the geographic and temporal dynamics of the European import of plants for planting. Biological Invasions, 19, 3243–3527. 10.1007/s10530-017-1465-6 [DOI] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- Harrington TC, Steimel JP, Wingfield MJ and Kile GA, 1996. Isozyme variation and species delimitation in the Ceratocystis coerulescens complex. Mycologia, 88, 104–113. [Google Scholar]
- Harrington TC, Steimel J and Kile G, 1998. Genetic variation in three Ceratocystis species with outcrossing, selfing and asexual reproductive strategies. Forest Pathology, 28, 217–226. [Google Scholar]
- Hepting GH, 1944. Sapstreak, a new killing disease of sugar maple. Phytopathology, 34, 2069–2076. [Google Scholar]
- Hiederer R, Houston Durrant T, Granke O, Lambotte M, Lorenz M, Mignon B and Mues V, 2007. Forest focus monitoring database system ‐ validation methodology. Vol. EUR 23020 EN of EUR – Scientific and Technical Research. Office for Official Publications of the European Communities. 10.2788/51364 [DOI]
- Hiederer R, Houston Durrant T and Micheli E, 2011. Evaluation of BioSoil demonstration project ‐ soil data analysis. Vol. 24729 of EUR ‐ Scientific and Technical Research. Publications Office of the European Union. 10.2788/56105 [DOI] [Google Scholar]
- Houston DR, 1986. Sapstreak of sugar maple‐appearance of lumber from diseased trees and longevity of Ceratocystis coerulescens in air‐dried lumber. Phytopathology, 76, 653. [Google Scholar]
- Houston DR, 1993. Recognizing and managing sapstreak disease of sugar maple. USDA, Forest Service. Research Paper NE‐675. North‐eastern Forest Experiment Station, Radnor, Pennsylvania, USA. 15 pp.
- Houston DR, 1994. Sapstreak disease of Sugar maple: development over time and space. USDA, Forest Service. Research Paper NE‐687. North‐eastern Forest Experiment Station, Radnor, Pennsylvania, USA. 24 pp.
- Houston DR, 1999. History of sugar maple decline In: Horsley SB. and Long RP. (eds.). Sugar Maple Ecology and Health: proceedings of an International Symposium; 1998 June 2‐4; Gen. Tech. Rep. NE‐261. Radnor, PA: USDA, Forest Service, North‐eastern Research Station, pp. 19–26. [Google Scholar]
- Houston Durrant T and Hiederer R, 2009. Applying quality assurance procedures to environmental monitoring data: a case study. Journal of Environmental Monitoring, 11, 774–781. [DOI] [PubMed] [Google Scholar]
- Houston Durrant T, San‐Miguel‐Ayanz J, Schulte E and Suarez Meyer A, 2011. Evaluation of BioSoil demonstration project: forest biodiversity ‐ analysis of biodiversity module. Vol. 24777 of EUR – Scientific and Technical Research. Publications Office of the European Union. 10.2788/84823 [DOI] [Google Scholar]
- Kehr R, Pehl L, Wulf A, Schröder T and Kaminski K, 2004. Zur Gefährdung von Bäumen und Waldökosystemen durch eingeschleppte Krankheiten und Schädlinge. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes, 56, 217–238. [Google Scholar]
- Kile GA and Walker J, 1987. Chalara australis, a vascular pathogen of Nothofagus cunninghamii in Australia and its relationship to other Chalara species. Australian Journal of Botany, 35, 1–32. [Google Scholar]
- Langham RJ, 1994. A biosystematics study of three plant pathogenic fungi. Master Thesis, Eastern Illinois University, Charleston, Illinois, USA, 48 pp.
- Mielke ME and Charette DA, 1989. The incidence of sapstreak disease of sugar maple in Menominee County, Wisconsin, and its relationship to wounds and season of logging. Northern Journal of Applied Forestry, 6, 65–67. [Google Scholar]
- Nag Raj TR and Kendrick B, 1976. A monograph of Chalara and allied genera. Wilfrid Laurier University Press, Waterloo, Ontario, Canada: 200 pp. [Google Scholar]
- Ohman JH and Spike AB, 1966. Effect of straining caused by sapstreak disease on sugar maple log and lumber values. Research Paper NC‐12. USDA, Forest Service, North‐central Forest Experiment Station, St. Paul, Minnesota, USA. 4 pp.
- Pasta S, de Rigo D and Caudullo G, 2016. Acer pseudoplatanus in Europe: distribution, habitat, usage and threats In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T. and Mauri A. (eds.). European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg, pp. e01665a+. [Google Scholar]
- Peel MC, Finlayson BL and McMahon TA, 2007. Updated world map of the Köppen‐Geiger climate classification. Hydrology and Earth System Sciences Discussions, 4, 439–473. [Google Scholar]
- Richter DL, 2012. The sugar maple sapstreak fungus (Ceratocystis virescens Davidson) Moreau, Ascomycota) in the Huron Mountains, Marquette County, Michigan. The Michigan Botanist, 51, 73–82. [Google Scholar]
- de Rigo D, 2012. Semantic Array Programming for environmental modelling: application of the Mastrave library In: Seppelt R, Voinov AA, Lange S. and Bankamp D. (eds.). International Environmental Modelling and Software Society (iEMSs) 2012 International Congress on Environmental Modelling and Software ‐ Managing Resources of a Limited Planet: pathways and Visions under Uncertainty, Sixth Biennial Meeting. pp. 1167–1176. [Google Scholar]
- de Rigo D, Caudullo G, Busetto L and San‐Miguel‐Ayanz J, 2014. Supporting EFSA assessment of the EU environmental suitability for exotic forestry pests: final report. EFSA Supporting Publications 2014: 11(3), EN‐434. [Google Scholar]
- de Rigo D, Caudullo G, Houston Durrant T and San‐Miguel‐Ayanz J, 2016. The European Atlas of Forest Tree Species: modelling, data and information on forest tree species In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T. and Mauri A. (eds.). European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg, pp. e01aa69+. [Google Scholar]
- de Rigo D, Caudullo G, San‐Miguel‐Ayanz J and Barredo JI, 2017. Robust modelling of the impacts of climate change on the habitat suitability of forest tree species. Publication Office of the European Union, 58 pp.
- San‐Miguel‐Ayanz J, 2016. The European Union Forest Strategy and the Forest Information System for Europe In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T. and Mauri A. (eds.). European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg, pp. e012228+ [Google Scholar]
- San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T. and Mauri A. (eds.). 2016. European Atlas of Forest Tree Species. Publication Office of the European Union, Luxembourg. [Google Scholar]
- Shigo AL, 1962. Observations on the succession of fungi on hardwood pulpwood bolts. Plant Disease Reporter, 46, 379–380. [Google Scholar]
- Sinclair WA and Lyon HH, 2005. Diseases of Trees and Shrubs, 2nd Edition. Comstock Publishing Associates, a division of Cornell University Press, Ithaca, NY. 660 pp. [Google Scholar]
- Smith KT, 1990. Sapstreak disease and biodeterioration of sugar maple. Biodeterioration Research, 3, 303–310. [Google Scholar]
- Webber J, 2008. Abbreviated Pest Risk Analysis for Ceratocystis virescens. Forest Research, Alice Holt, UK: 8 pp. [Google Scholar]
- Wingfield BD, Barnes I, de Beer ZW, De Vos L, Duong TA, Kanzi AM, Naidoo K, Nguyen HD, Santana QC, Sayari M, Seifert KA, Steenkamp ET, Trollip C, van der Merwe NA, van der Nest MA, Wilken PM and Wingfield MJ, 2015. IMA Genome‐F 5: Draft genome sequences of Ceratocystis eucalypticola, Chrysoporthe cubensis, C. deuterocubensis, Davidsoniella virescens, Fusarium temperatum, Graphilbum fragrans, Penicillium nordicum, and Thielaviopsis musarum . IMA Fungus, 6, 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn RC, Wingfield BD, Wingfield MJ, Wolfaardt M and Harrington TC, 1998. Monophyly of the conifer species in the Ceratocystis coerulescens complex based on DNA sequence data. Mycologia, 90, 96–101. [Google Scholar]
- Zecchin B, Caudullo G and de Rigo D, 2016. Acer campestre in Europe: distribution, habitat, usage and threats In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T. and Mauri A. (eds.). European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg, pp. e012c65+. [Google Scholar]


