Abstract
According to Article 12 of Regulation (EC) No 396/2005, EFSA has reviewed the maximum residue levels (MRLs) currently established at European level for the pesticide active substance imazalil. To assess the occurrence of imazalil residues in plants, processed commodities, rotational crops and livestock, EFSA considered the conclusions derived in the framework of Directive 91/414/EEC and under Regulation (EC) No 1107/2009, the MRLs established by the Codex Alimentarius Commission as well as the European authorisations reported by Member States (including the supporting residues data). Based on the assessment of the available data, MRL proposals were derived and a consumer risk assessment was carried out. Some information required by the regulatory framework was missing and a possible chronic and acute risk to consumers was identified. Hence, the consumer risk assessment is considered indicative only, some MRL proposals derived by EFSA still require further consideration by risk managers and measures for reduction of the consumer exposure should also be considered.
Keywords: imazalil, MRL review, Regulation (EC) No 396/2005, consumer risk assessment, imidazole, pesticide, fungicide
Summary
Imazalil was included in Annex I to Directive 91/414/EEC on 1 January 1999 by Commission Directive 97/73/EC. The active substance has been approved under Regulation (EC) No 1107/2009, by Commission Implementing Regulation (EU) No 705/2011, which entered into force on 1 January 2012 amending the Annex to Commission Implementing Regulation (EU) No 540/2011, as amended by Commission Implementing Regulation (EU) No 541/2011. As imazalil was approved before the entry into force of Regulation (EC) No 396/2005 on 2 September 2008, the European Food Safety Authority (EFSA) is required to provide a reasoned opinion on the review of the existing maximum residue levels (MRLs) for that active substance in compliance with Article 12(2) of the aforementioned regulation. To collect the relevant pesticide residues data, EFSA asked the Netherlands, the designated rapporteur Member State (RMS), to complete the Pesticide Residues Overview File (PROFile) and to prepare a supporting evaluation report. The PROFile and evaluation report provided by the RMS were made available to the Member States. A request for additional information was addressed to the Member States in the framework of a completeness check period, which was initiated by EFSA on 10 March 2016 and finalised on 10 May 2016. After having considered all the information provided, EFSA prepared a completeness check report which was made available to Member States on 4 July 2016.
Based on the conclusions derived by EFSA under Regulation (EC) No 1107/2009, the MRLs established by the Codex Alimentarius Commission and the additional information provided by the RMS and Member States, EFSA prepared in April 2017 a draft reasoned opinion, which was circulated to Member States for consultation via a written procedure. Comments received by 18 May 2017 were considered during the finalisation of this reasoned opinion. The following conclusions are derived.
The metabolism of imazalil was investigated for three different modes of applications (foliar, post‐harvest and seed treatment) in three different crop groups (cereals, fruit crops and root crops), hereby covering all uses under assessment. Based on the available studies, the residue definition for enforcement was proposed as imazalil (any ratio of constituent isomers). Imazalil can be enforced with a limit of quantification (LOQ) of 0.01 mg/kg in the four main plant matrices. For risk assessment purpose, it was proposed to consider the sum of imazalil and R014821, expressed as imazalil in order to cover the possible occurrence of metabolite R014821 which may be formed after post‐harvest applications. It is noted that the toxicity of metabolite R014821 still needs to be fully addressed. The proposed residue definitions apply to all plant commodities as well as to processed commodities since the nature of residues is unchanged through standard hydrolysis. However, no conclusion could be proposed regarding the nature of residues in rotational crops as no metabolism studies are available.
The available residue trials data allowed deriving MRL proposals as well as risk assessment values for all commodities under evaluation, except for peppers (for which no data were available) and melons (for which the number of data was insufficient to derive a MRL). For citrus fruits, bananas, potatoes, cucumber, gherkins and courgettes, the MRLs derived from the most critical good agricultural practices (GAPs) are only tentative. Lower MRLs could also be derived based on less critical GAPs reported for oranges/grapefruits (tentative), apples/pears (tentative) and potatoes (fully supported by data).
Robust processing factors were derived for peeled fruits (citrus fruits, bananas and melons) as well as for many processed commodities of oranges (juice, dry pomace, wet pomace and marmalade), apples (juice and wet pomace) and potatoes (unpeeled/boiled, peeled/boiled and fried). For the other processed commodities assessed in this review, the processing factors are considered tentative because of the limited number of data.
The metabolism of imazalil was investigated in lactating goats and laying hens. However, as the metabolism study performed on hens provides very limited information, only tentative conclusion could be derived for poultry. Imazalil was not identified as a sufficient marker in livestock commodities. Therefore, it was necessary to consider an additional compound (metabolite FK‐772) for enforcement purpose. The residue definition for enforcement in livestock commodities was proposed as the sum of imazalil and its metabolite FK‐772 (any ratio of constituent isomers), expressed as imazalil. This residue definition is not fat soluble. A multiresidue analytical method is available and validated in all relevant matrices for the determination of imazalil and FK‐772, with a LOQ of 0.01 mg/kg for each compound. Considering that the toxicity of metabolite FK‐772 is not yet fully addressed, the proposed residue definition is tentative. For risk assessment purpose, it was proposed to consider the sum of imazalil and all identified/characterised metabolites observed in the goat metabolism study. Conversion factors from enforcement to risk assessment were therefore tentatively derived on the basis of the metabolism studies.
Based on the feeding study performed on lactating cows, MRL and risk assessment values were derived for the sum of imazalil and its metabolite FK‐772 expressed as imazalil, in all ruminants and swine commodities. However, since the storage stability of imazalil and its metabolite FK‐772 in animal commodities still needs to be addressed and since the storage conditions of the samples of the feeding study were not reported, these MRLs are proposed on a tentative basis only. For poultry commodities, the available feeding study did not allow deriving MRL and risk assessment values. It is noted that MRLs and risk assessment values for livestock commodities were derived under two different scenarios, considering all critical GAPs (EU1) and considering the fall‐back GAPs for oranges/grapefruits and potatoes (EU2). In the scenario EU2, no MRLs were considered needed for poultry commodities.
Chronic and acute consumer exposure resulting from the authorised uses reported in the framework of this review was calculated using revision 2 of the EFSA Pesticide Residues Intake Model (PRIMo). For those commodities where data were insufficient to derive a MRL, EFSA considered the existing European Union (EU) MRL for an indicative calculation. For potatoes, apples, pears, bovine liver, oranges and grapefruits, an exceedance of the acute reference dose (ARfD) was identified representing 1415%, 686%, 638%, 221%, 183% and 123% of the ARfD, respectively. Considering fall‐back MRLs for potatoes, oranges and grapefruits and disregarding the GAPs on apples and pears (for which no safe fall‐back option could be identified), the highest chronic exposure represented 40.7% of the acceptable daily intake (ADI) (French toddler) and the highest acute exposure amounted to 76.8% of the ARfD (mandarins).
Apart from the MRLs evaluated in the framework of this review, internationally recommended codex maximum residue limits (CXLs) have also been established for imazalil. Additional calculations of the consumer exposure, considering these CXLs, were therefore carried out and exceedances of the ARfD were identified for the existing CXLs in potatoes (1538%), oranges (1326%), apples (980%), pears (911%), grapefruits (892%), quinces (147%), persimmon (131%) and medlar (121%). Excluding these CXLs from the calculation, the highest chronic exposure represented 45.7% of the ADI (French toddler) and the highest acute exposure amounted to 76.8% of the ARfD (mandarins).
Background
Regulation (EC) No 396/20051 (hereinafter referred to as ‘the Regulation’) establishes the rules governing the setting and the review of pesticide maximum residue levels (MRLs) at European level. Article 12(2) of that Regulation stipulates that EFSA shall provide by 1 September 2009 a reasoned opinion on the review of the existing MRLs for all active substances included in Annex I to Directive 91/414/EEC2 before 2 September 2008. As imazalil was included in Annex I to Council Directive 91/414/EEC on 1 January 1999 by means of Commission Directive 97/73/EC,3 and has been approved under Regulation (EC) No 1107/20094, amending the Annex to Commission Implementing Regulation (EU) No 540/20115, as amended by Commission Implementing Regulation (EU) No 541/20116, EFSA initiated the review of all existing MRLs for that active substance.
According to the legal provisions, EFSA shall base its reasoned opinion in particular on the relevant assessment report prepared under Directive 91/414/EEC. It should be noted, however, that, in the framework of Directive 91/414/EEC, only a few representative uses are evaluated, whereas MRLs set out in Regulation (EC) No 396/2005 should accommodate all uses authorised within the European Union (EU), and uses authorised in third countries that have a significant impact on international trade. The information included in the assessment report prepared under Directive 91/414/EEC is therefore insufficient for the assessment of all existing MRLs for a given active substance.
To gain an overview of the pesticide residues data that have been considered for the setting of the existing MRLs, EFSA developed the Pesticide Residues Overview File (PROFile). The PROFile is an inventory of all pesticide residues data relevant to the risk assessment and MRL setting for a given active substance. This includes data on:
the nature and magnitude of residues in primary crops;
the nature and magnitude of residues in processed commodities;
the nature and magnitude of residues in rotational crops;
the nature and magnitude of residues in livestock commodities;
the analytical methods for enforcement of the proposed MRLs.
The Netherlands, the designated rapporteur Member State (RMS) under Regulation (EC) No 1107/2009, was asked to complete the PROFile for imazalil and to prepare a supporting evaluation report (Netherlands, 2015). The PROFile and the supporting evaluation report were submitted to EFSA in March 2015 and made available to the Member States. A request for additional information was addressed to the Member States in the framework of a completeness check period which was initiated by EFSA on 10 March 2016 and finalised on 10 May 2016. Additional evaluation reports were submitted by Belgium, France, Germany, Greece, Italy, Portugal, Spain, the Netherlands and the European Union Reference Laboratories for Pesticide Residues (Belgium, 2016; EURL, 2016; France, 2016; Germany, 2016; Greece, 2016; Italy, 2016; Netherlands, 2016; Portugal, 2016 and Spain, 2016) and, after having considered all the information provided by RMS and Member States, EFSA prepared a completeness check report which was made available to all Member States on 4 July 2016. Further clarifications were sought from Member States via a written procedure in July 2016.
Based on the conclusions derived by EFSA under Regulation (EC) No 1107/2009, the MRLs established by the Codex Alimentarius Commission (codex maximum residue limit; CXLs) and the additional information provided by the Member States, EFSA prepared in April 2017 a draft reasoned opinion, which was submitted to Member States for commenting via a written procedure. All comments received by 18 May 2017 were considered by EFSA during the finalisation of the reasoned opinion.
The evaluation report submitted by the RMS (Netherlands, 2015) and the evaluation reports submitted by Belgium, France, Germany, Greece, Italy, Portugal, Spain, the Netherlands and the EU Reference Laboratories for Pesticide Residues (Belgium, 2016; EURL, 2016; France, 2016; Germany, 2016; Greece, 2016; Italy, 2016; Netherlands, 2016; Portugal, 2016; Spain, 2016) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available.
In addition, key supporting documents to this reasoned opinion are the completeness check report (EFSA, 2017a) and the Member States consultation report (EFSA, 2017b). These reports are developed to address all issues raised in the course of the review, from the initial completeness check to the reasoned opinion. Also, the chronic and acute exposure calculations for all crops reported in the framework of this review performed using the EFSA Pesticide Residues Intake Model (PRIMo) (excel file) and the PROFile are key supporting documents and made publicly available as background documents to this reasoned opinion. Furthermore, a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
Terms of Reference
According to Article 12 of Regulation (EC) No 396/2005, EFSA shall provide a reasoned opinion on:
the inclusion of the active substance in Annex IV to the Regulation, when appropriate;
the necessity of setting new MRLs for the active substance or deleting/modifying existing MRLs set out in Annex II or III of the Regulation;
the inclusion of the recommended MRLs in Annex II or III to the Regulation;
the setting of specific processing factors as referred to in Article 20(2) of the Regulation.
The active substance and its use pattern
Imazalil is the ISO common name for (RS)‐1‐(β‐allyloxy‐2,4‐dichlorophenethyl)imidazole or allyl (RS)‐1‐(2,4‐dichlorophenyl)‐2‐imidazol‐1‐ylethyl ether (IUPAC). Imazalil is a racemic mixture.
Imazalil belongs to the group of imidazole compounds which are used as fungicides.
The chemical structure of the active substance and its main metabolites are reported in Appendix F.
Imazalil was evaluated in the framework of Directive 91/414/EEC with Belgium designated as rapporteur Member State (RMS). The representative uses supported for the peer review process were dipping/drenching or spraying waxing for citrus (post‐harvest), foliar spray applications for tomatoes grown on artificial substrate, and seed treatment for winter and spring barley and wheat.
Following the first peer review, in which EFSA was not yet involved, a decision on inclusion of the active substance in Annex I to Directive 91/414/EEC was published by means of Commission Directive 97/73/EC, which entered into force on 1 January 1999.
Imazalil has been approved under Regulation (EC) No 1107/2009 by means of Commission Implementing Regulation (EU) No 705/20117 which entered into force on 1 January 2012. EFSA carried out the peer review of the pesticide risk assessment for imazalil for its renewal in the framework of Commission Regulation (EC) No 737/20078, with the Netherlands designated as rapporteur Member state (RMS) and Spain as co‐Rapporteur Member State (co‐RMS).
The EU MRLs for imazalil are established in Annexes II and IIIB of Regulation (EC) No 396/2005, as amended by Commission Regulation (EU) No 750/20109, and codex maximum residue limits (CXLs) for imazalil were also established by the Codex Alimentarius Commission (CAC).
For the purpose of this MRL review, the critical uses of imazalil currently authorised within the EU have been collected by the RMS and reported in the PROFile. The additional good agricultural practices (GAPs) reported by Member States during the completeness check were also considered. The details of the authorised GAPs for imazalil are given in Appendix A. The RMS did not report any use authorised in third countries that might have a significant impact on international trade.
Assessment
EFSA has based its assessment on the PROFile submitted by the RMS, the evaluation report accompanying the PROFile (Netherlands, 2015), the assessment reports and their addenda prepared under the first peer review (Belgium, 1996) and under Regulation (EC) No 1107/2009 (Netherlands, 2009a,b, 2014), the EFSA conclusion on the peer review of the pesticide risk assessment of the active substance imazalil in the context of the renewal procedure under Commission Regulation (EC) No 737/2007 (EFSA, 2010), the Joint Meeting on Pesticide residues (JMPR) Evaluation report (FAO, 1977, 1984, 1985, 1994) as well as the evaluation reports submitted during the completeness check (Belgium, 2016; EURL, 2016; France, 2016; Germany, 2016; Greece, 2016; Italy, 2016; Netherlands, 2016, Portugal, 2016 and Spain, 2016). The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/201110 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1997a, b, c, d, e, f, g, 2000, 2010a, b, 2016; OECD, 2011, 2013).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved from the list of end points reported in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of imazalil was investigated for different modes of applications in cereals and fruit crops (Belgium, 1996; Netherlands, 2009a) as well as in root crops (Netherlands, 2015). Foliar application (tomatoes), post‐harvest application (oranges, apples, potatoes) and seed treatment (wheat and potatoes) were investigated, hereby covering all uses under assessment.
After foliar or post‐harvest applications, imazalil is a major constituent of the residues in fruit crops and potatoes (80% of the total radioactive residues (TRR) in tomato, 89–99% TRR in orange/apples and 69–94% TRR in potatoes). No other compounds above 10% of the TRR were found in tomatoes (foliar treatment only). However, after post‐harvest applications, the metabolite R01482111 was observed in significant proportions in apple and potatoes (11% TRR and 9% TRR, respectively after a withholding period (WHP) of 6–7 months). Another degradation product (metabolite R04417712) was found in potatoes at a level remaining below 4% TRR. These studies indicate that imazalil is likely to degrade into metabolite R014821 during the storage of post‐harvest treated commodities (fruit and root crops). This degradation was not observed in fruit crops sampled early after foliar application (with a short preharvest interval (PHI)).
Studies performed with seed treatment showed much lower residue levels in cereal grains and potato tubers where the TRR always remain below 0.01 mg eq/kg. Therefore, no further attempt was carried out to characterise the residues in these matrices. However, the residue levels measured in wheat forage (1.36 mg eq/kg) and straw (0.15 mg eq/kg) indicate that, following the exaggerated application rate of 49 g a.s./100 kg seeds (> 6N compared to GAP), transfer of residues from seed to other parts of the crop may occur. The parent compound was extensively degraded in wheat forage and straw, only representing 17–24% of the TRR. The degradation products were not identified as the highest peak observed in straw does only represent 11% TRR (< 0.02 mg eq/kg). Also considering that this study is highly overdosed compared to GAP, further identification of the residue in these matrices is not deemed necessary.
Chiral analyses were performed in the study performed on potato tubers. These analyses indicate that the S/R ratios of imazalil enantiomers remain unchanged during the storage period after post‐harvest application (Netherlands, 2015).
1.1.2. Nature of residue in rotational crops
Imazalil is authorised on crops that may be grown in rotation such as cereals and potatoes (where seed treatments are authorised) as well as tomatoes, sweet peppers and cucurbits with edible peel (where foliar treatments are authorised). It is noted that although foliar treatments are only authorised for ‘indoor’ uses, there is no evidence that these GAPs are restricted to artificial substrates. Therefore, these GAPs are also relevant for rotational crops. According to the soil degradation studies evaluated in the framework of the peer review under the renewal process, the geometric mean of the DT50 value is 93.2 days (EFSA, 2010). Therefore, the DT90 value of imazalil is expected to be much higher than the trigger value of 100 days. According to the European guidelines on rotational crops (European Commission, 1997b), further investigation of residues in rotational crops is relevant.
Studies investigating the nature of residues in rotational crops are not available. The RMS made an attempt to theoretically estimate the residue levels that would occur if succeeding crops would be sown after a crop failure on potatoes previously subject to seed treatment (Netherlands, 2015). The RMS considered the case of a normal rotation (i.e. rotational crop harvested 15 months/450 days after planting of the treated seed potatoes) and a more critical scenario where the rotational crop is sown immediately after a crop failure. For this latter scenario, the RMS considered a period of 150 days between planting of the treated seed potatoes and harvest of the following crop. The theoretical estimation of the RMS took into account an ‘application rate’ of 31.5 g a.s./ha13 and an average breakdown kinetic in soil corresponding to a DT50 of 93.2 days.14 With these assumptions, residue levels in rotational crops would always remain below 0.004 mg/kg assuming a transition factor of 1.15 However, there is still an uncertainty regarding this potential transition factor and considering higher transition factors of 5 or 10, residues above 0.01 mg/kg may occur under the scenario of a crop failure. Furthermore, the RMS and EFSA have still reservations regarding the highest residue (HR) value of 4.6 mg/kg observed on potatoes (see also Section 1.2.1), which is an important assumption in this theoretical calculation.
In addition, the above calculation does not address the rotational crops sown after a crop failure on cereals (for which the application rate is five times higher than for potatoes16) or on the fruiting vegetables crops where foliar applications are authorised and for which a period lower that 150 days between planting of the treated seed potatoes and harvest of the following crop may need to be considered. Therefore, EFSA is of the opinion that the theoretical calculation reported by the RMS does not sufficiently address the data gap already identified during the peer review under the renewal procedure (EFSA, 2010). Therefore, studies investigating the nature of residues in rotational crops are still required.
1.1.3. Nature of residues in processed commodities
The effect of processing on the nature of residues was investigated after the peer review, in the framework of the confirmatory data process (Netherlands, 2014). Studies were conducted with imazalil, simulating representative hydrolytic conditions for pasteurisation (20 min at 90°C, pH 4), boiling/brewing/baking (60 min at 100°C, pH 5) and sterilisation (20 min at 120°C, pH 6). Although this study was not conducted with radiolabelled material, the test compound was found at an amount of 94–99% after any kind of hydrolysis. Therefore, it was concluded that processing by pasteurisation, baking/brewing/boiling and sterilisation is not expected to have a significant impact on the composition of residues in matrices of plant origin.
1.1.4. Methods of analysis in plants
During the peer review under the renewal procedure, an analytical method using high‐performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) was validated for the determination of imazalil in high water content, high oil content and dry commodities with a limit of quantification (LOQ) of 0.01 mg/kg (EFSA, 2010). In the framework of the present review, the RMS provided validation data on high acid content commodities for a new analytical method also using HPLC–MS/MS (Netherlands, 2015). Independent laboratory validation (ILV) data for these methods are available for high water content commodities (EFSA, 2010), high acid content commodities (Netherlands, 2015) and dry commodities (Germany, 2016).
Hence, it is concluded that imazalil can be enforced with a LOQ of 0.01 mg/kg in high water content, high acid content, high oil content and dry commodities. This conclusion was also confirmed by the EURLs during the completeness check (EURL, 2016).
1.1.5. Stability of residues in plants
In the framework of the peer review under the renewal procedure, storage stability of imazalil was demonstrated for a period of 6 months at −18°C in commodities with high acid, dry commodities and in cereal straw (EFSA, 2010). However, the storage stability of the metabolite R014821 was not investigated in these matrices. As the metabolite R014821 is relevant for post‐harvest treatments of citrus fruits, additional data demonstrating its storage stability in high acid content commodities are still required.
As regards to high water content commodities, the storage stability was separately demonstrated for each compound (imazalil and its metabolite R014821) for a period of 12 months at −20°C (Netherlands, 2015).
1.1.6. Proposed residue definitions
Based on the available metabolism studies performed on three different crop categories and with different modes of application, the parent compound is considered as a sufficient marker for enforcement purpose. Therefore, the residue definition for enforcement (imazalil only) proposed during the peer review is still valid and now applicable to all crop categories and for any kind of treatment. As imazalil is a mixture of two enantiomers, it is proposed to modify the wording of the residue definition as follows: imazalil (any ratio of constituent isomers). This residue definition also applies to processed commodities as the nature of residues is unchanged through standard hydrolysis. However, no conclusion could be proposed regarding the nature of residues in rotational crops since studies are still missing.
Imazalil is still present after long WHP and is therefore a valid marker for post‐harvest uses. Nevertheless, after a post‐harvest treatment, a slow degradation (O‐dealkylation) of the parent compound may occur during the storage and metabolite R014821 can be formed. Many residue trials performed with simultaneous analysis of imazalil and R014821 showed that significant levels of metabolite R014821 are only expected after a long WHP (i.e. more than 3 months). The RMS made an attempt to address the toxicity of the metabolite R014821 and concluded that this compound does not have a higher toxic potential than the parent compound (Netherlands, 2015). However, as the available data are not fully conclusive and were not assessed at EU level, a full conclusion on this point is still required. In the meantime, comparable toxicity with the parent imazalil is assumed for the metabolite R014821. Considering that it was not possible to conclude on its toxicological relevance and having regard to the significant proportion of R014821 that can occur when the length of storage is increasing, EFSA is still of the opinion that the residue for risk assessment should tentatively be defined as the sum of imazalil and R014821, expressed as imazalil. It is highlighted that this residue definition is only relevant when commodities are subject to post‐harvest uses (with longer WHP), but since plant commodities from different crop groups may be treated with post‐harvest applications, the residue definition applies to all plant commodities and is therefore considered as a general residue definition.
In addition, EFSA emphasises that, except the metabolism study performed with ware potatoes, the available metabolism studies do not investigate the possible impact of plant metabolism on the isomer ratio of imazalil and further investigation on this matter would in principle be required. Since guidance on the consideration of isomer ratios in the consumer risk assessment is not yet available, EFSA recommends that this issue is reconsidered when such guidance becomes available.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
To assess the magnitude of imazalil residues resulting from the GAPs reported in this review, EFSA considered all residue trials made available by the RMS in its evaluation reports (Netherlands, 2015, 2016), including residue trials evaluated in the framework of the peer reviews (Belgium, 1996; Netherlands, 2009a,b; EFSA, 2010) and additional data submitted during the completeness check (France, 2016; Greece, 2016; Spain, 2016). All residue trial samples considered in this framework were stored in compliance with the storage conditions for which storage stability of the parent compound was demonstrated. Decline of imazalil levels during storage of the trial samples is therefore not expected. Regarding the trials performed with post‐harvest application on commodities with high water content (apples, pears, bananas and potatoes), the storage stability of the metabolite R014821 is also covered by the available study. However, the storage stability of metabolite R014821 was not investigated in high acid content commodities. Therefore, degradation of metabolite R014821 residues in samples from residue trials on citrus fruits cannot be excluded. A study investigating the storage stability of this metabolite in high acid content commodities is required to confirm the results of these residue trials.
The number of residue trials and extrapolations were evaluated in accordance with the European guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs (European Commission, 2016).
GAPs with seed treatment and foliar treatment:
Seed treatments are authorised on potatoes and cereals while foliar treatments are authorised on tomatoes, peppers, cucumbers, courgettes and gherkins. For all these GAPs, the metabolite R014821 is not expected to occur (see also Section 1.1.6). Therefore, residue trials analysing only for the parent compound are sufficient to derive MRL and risk assessment values. For all these GAPs, a default conversion factor (CF) of 1 can be set. However, the following data gaps were identified for peppers, cucumbers, courgettes and gherkins:
Sweet peppers/bell peppers: Residue trials are not available to support the reported GAP and MRL or risk assessment values cannot be derived for this crop. Therefore, eight trials on peppers compliant with the indoor GAP are required.
Cucurbits with edible peel: Only four GAP‐compliant trials performed on cucumber are available. Tentative MRL and risk assessment values can be derived from these data but four additional trials performed on cucumber and/or courgettes and compliant with the indoor GAP are still required.
GAPs with post‐harvest treatment:
Post‐harvest treatment are authorised on citrus fruits, apples, pears, bananas, potatoes and melons. Since all these crops are worldwide major crops according to the above mentioned European guidelines (European Commission, 2016), eight residue trials compliant with GAP and analysing for parent and metabolite R014821 are necessary to derive MRLs, risk assessment values and CFs from enforcement to risk assessment (see also Section 1.1.6).
A sufficient number of trials including simultaneous analysis of imazalil and metabolite R014821 was available to support the GAPs on citrus fruits (drenching) and apples/pears (drenching: 25–38 g a.i./hL). In these trials, levels of metabolite R014821 were found to be insignificant, and thus, a CF of 1 was derived for these GAPs. For the other GAPs reported in this review however, the following data gaps and/or concerns were identified:
Bananas (dipping): Four trials including analysis of parent and metabolite R014821 are available (Greece, 2016). Although these trials were performed on the same site and on the same day, they can be considered independent as they were performed on different varieties. Tentative MRL and risk assessment values can be derived for this crop and four additional trials (including simultaneous analysis of imazalil and metabolite R014821) are still required;
Melons (drenching): Only three trials are available to support this GAP. Furthermore, these trials do not include analysis of the metabolite R014821. MRL and risk assessment values cannot be derived for this crop and eight trials including simultaneous analysis of imazalil and metabolite R014821 are required;
Apples/pears (smoke can): Only four trials are available to support this GAP. Therefore, only tentative MRL and risk assessment values can be derived from this GAP. Furthermore, these trials do not include analysis of the metabolite R014821. Based on the results observed in other commodities and considering that this GAP is defined with a very short WHP (≤ 1 day), a CF of 1 can reasonably be assumed for this GAP. Nevertheless, four additional trials including simultaneous analysis of imazalil and metabolite R014821 should be required to fully support this GAP. As a clear difference was observed between apples (0.36–0.38 mg/kg) and pears (0.74–0.79 mg/kg), these trials should equitably be performed on apples and pears;
Oranges/grapefruits (waxing): Eight trials are available to support this GAP. However, these trials do not include analysis of the metabolite R014821. Based on the results observed in other commodities and considering that this GAP is defined with a very short WHP (≤ 1 day), a CF of 1 can reasonably be assumed for this GAP. Four trials including simultaneous analysis of imazalil and metabolite R014821 are desirable (minor deficiency).
Potatoes (post‐harvest GAP): In four of the nine available residue trials, it was indicated by the RMS that samples were washed before analysis (Netherlands, 2015). However, these four trials do also show the highest residue levels (from 3.4 to 4.6 mg/kg) of the data set. Therefore, these results are rather questionable and should be considered on a tentative basis only. The RMS is invited to provide further clarifications with regard to these data. In the meantime, MRL and risk assessment values derived from the post‐harvest treatment on ware potatoes are deemed tentative.
1.2.2. Magnitude of residues in rotational crops
Studies investigating the nature and/or magnitude of residues in rotational crops are not available and are still required (see Section 1.1.2).
1.2.3. Magnitude of residues in processed commodities
Studies investigating the magnitude of residues in several processed commodities of citrus fruits, apples, potatoes and melons were evaluated in the framework of the peer reviews (Belgium, 1996; Netherlands, 2009a,b). In the framework of the present MRL review, the RMS has evaluated and reported additional processing studies performed on these crops (Netherlands, 2015) and peeling factors for bananas were made available during the completeness check (Greece, 2016).
An overview of all available processing studies is available in Appendix B.1.2.3.
Residue distribution in peel/pulp:
The transfer of residues from peel to pulp has been investigated in citrus fruits, bananas and melons. Overall, it is demonstrated that residue levels observed in pulp are generally lower compared to residue levels observed in whole fruits. Based on the available data, processing factors can be derived for citrus fruits (0.07), bananas (0.11) and melons (0.12), taking into account the following considerations:
Citrus fruits: More than 50 residue trials performed with different GAPs on several citrus fruits are available. In order to derive a peeling factor for all citrus fruits, EFSA considered only the sampling performed after a WHP of 0 day (compliant with the critical GAP assessed in this review) and disregarded the data where residues were below LOQ in pulp. Based on these criteria, 36 peeling factors are available, as reported in Appendix B.1.2.3. No significant difference was observed between the peeling factors derived from oranges, mandarins, lemons and grapefruits; thus a general peeling factor of 0.07 was derived from the median value of the overall data set. However, in each crop, the available data show a wide distribution of the peeling factors, ranging from 0.01 to 0.28.
Bananas: Residue data in pulp were not available at WHP 35 days (i.e. compliant with GAP) but only at WHP 28 days (Greece, 2016). Considering the slight difference between WHP 28 and 35 days, this is considered acceptable to derive a robust peeling factor.
Melons: Residue data in pulp were available in the three trials compliant with GAP. In addition, as the other available residue trials performed with a higher application rate provide similar peel/pulp ratios, they were all considered to derive a more robust peeling factor (Belgium, 1996).
Other processing factors assessed in this review:
Robust processing factors were derived for processed commodities of oranges (juice, dry pomace, wet pomace and marmalade), apples (juice and wet pomace) and potatoes (unpeeled/boiled, peeled/boiled and fried). For other processed commodities where the data set was limited, the processing factors are considered tentative: apples (dry pomace and sauce), potatoes (unpeeled/microwaved, crisps and granules/flakes).
Further processing studies are not required as they are not expected to affect the outcome of the risk assessment. However, if more robust processing factors were to be required by risk managers, in particular for enforcement purposes, additional processing studies would be needed.
1.2.4. Proposed MRLs
The available data are considered sufficient to derive (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for peppers (where no data were available) and melons (for which the number of data was insufficient to derive a MRL). For citrus fruits, bananas, potatoes, cucumber, gherkins and courgettes, the MRLs derived from the most critical GAPs are only tentative. Tentative MRLs were also derived for cereal straw in view of the future need to set MRLs in feed items.
It is noted that fall‐back MRLs and risk assessment values could also be derived from less critical GAPs reported for oranges/grapefruits (post‐harvest waxing; tentative), apples/pears (post‐harvest smoke can; tentative) and potatoes (seed treatment; fully supported by data).
2. Residues in livestock
Imazalil is authorised for use on citrus fruits, apples, potatoes and small grain cereals that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance (OECD, 2013), which has now also been agreed upon at European level. The input values for all relevant commodities are summarised in Appendix D.1. Considering all critical GAPs reported in this review (scenario EU1), the dietary burdens calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg dry matter (DM). Behaviour of residues was therefore assessed in all commodities of animal origin.
It is highlighted that no conclusion was achieved regarding the potential residue uptakes in rotational crops. The animal intake of imazalil residues via rotational crops has therefore not been assessed.
2.1. Nature of residues and methods of analysis in livestock
The metabolism of imazalil was investigated in lactating goats and laying hens (Belgium, 1996). The summary of the study with laying hens initially provided during the two peer reviews was not sufficient to conclude on a metabolic pathway in poultry as the identification of the metabolites was limited. Although further detailed results were provided by the RMS in its evaluation report (Netherlands, 2015), EFSA is of the opinion that an additional study would still be needed to fully depict the metabolic pathway in poultry.
There are no studies investigating the nature of the residues for metabolite R014821 in livestock. However, this compound is only relevant for post‐harvest uses (see also Section 1.1.1), and thus, the only feed items where it could be retrieved are apple pomaces, citrus fruits pomaces and potatoes. For these commodities, residue levels of metabolite R014821 were found to be insignificant and a CF of 1 from enforcement to risk assessment was derived. Therefore, the metabolite R014821 is not expected to be present in feed items treated in accordance with the critical GAP reported in this review. Consequently, there is no need to further investigate the metabolism of this compound in livestock.
The available metabolism studies showed imazalil to be extensively metabolised in animals. In goat tissues, the parent compound represents less than 6% of the TRR and is not detected at all in milk. Two metabolites, FK‐772 (goat kidney and muscle) and FK‐284 (goat muscle), were found in higher proportions than the parent compound. These metabolites are the only degradation products representing more than 10% of the TRR in goat tissues (15–21% TRR) and they were also present in low proportion in milk (3–6% TRR). In poultry, imazalil was only detected in eggs and fat, representing 8% and 11% of TRR, respectively. The metabolite FK‐772 was only retrieved in liver, where it accounted for less than 9% of the TRR. One metabolite (FK‐858) was found in proportion higher than 10% of the TRR in eggs and hen muscle (11–15% TRR) but corresponding to quite low levels in these matrices (0.02–0.09 mg eq/kg). In both ruminants and poultry, the remaining radioactivity consists of several minor metabolites, all remaining in very low proportions.
Based on the above results, imazalil may not be a sufficient marker in livestock commodities. The peer review under the renewal procedure already concluded that metabolites FK‐772 and/or FK‐284 should be taken into account for enforcement purpose in ruminant matrices (EFSA, 2010). According to the RMS, metabolites FK‐772 and FK‐284 are not of higher toxic potential than the parent compound and comparable toxicity to the parent imazalil can be assumed (Netherlands, 2015). However, as the available data are not fully conclusive and since they were not assessed at EU level, a full conclusion on this point is still required. The metabolite FK‐772 is more predominant than metabolite FK‐284 in the metabolism studies and the feeding study performed on cows showed that both compounds could be sufficient markers in tissues and milk. Also, considering that metabolite FK‐772 was found in both ruminant and poultry matrices, EFSA is of the opinion that metabolite FK‐772 is the best marker for enforcement purpose. Therefore, the residue definition for enforcement in livestock commodities is proposed as the sum of imazalil and its metabolite FK‐772 (any ratio of constituent isomers17), expressed as imazalil. This residue definition is not fat soluble. This is also in line with the proposal of the RMS (Netherlands, 2015). However, considering the deficiencies spotted in the metabolism study performed on laying hens, the proposed residue definition should still be confirmed for poultry. In addition, considering that the toxicity of metabolite FK‐772 is not yet fully addressed, the proposed residue definition remains tentative.
Although imazalil and its metabolite FK‐772 are expected to be sufficient residue markers in animal matrices, it is highlighted that the remaining identified and characterised radioactivity still represents an important part of the TRR (30–60% TRR in ruminants; information not available for poultry). For risk assessment purpose, it was therefore proposed to consider the sum of imazalil and all identified/characterised metabolites observed in the goat metabolism study (EFSA, 2010). CFs from enforcement to risk assessment were therefore tentatively derived on the basis of the goat metabolism study: 3 in muscle, 11 in fat, 4 in liver, 3 in kidney and 12 in milk. As the metabolism study performed on poultry does not allow to derive CFs, the CFs derived above for muscle, fat and liver are tentatively extrapolated to poultry tissues. For eggs, an indicative and conservative CF of 10 should be used considering that the sum of imazalil and its metabolite FK‐772 represents at least 10% of the TRR in this matrix.
EFSA already emphasised that the above studies do not investigate the possible impact of the livestock metabolism on the isomer ratio of imazalil. Further investigation on this matter would in principle be required. Since guidance on the consideration of isomer ratios in the consumer risk assessment is not yet available, EFSA recommends that this issue is reconsidered when such guidance becomes available.
EURLs informed EFSA that no validation data were available for the metabolite FK‐772. However, the RMS has reported a multiresidue analytical method using HPLC–MS/MS, which was validated for the determination of imazalil, FK‐772 and FK‐284, with a LOQ of 0.01 mg/kg for each compound. This method was validated in all relevant tissues, milk and eggs and is supported by an ILV for each compound (Netherlands, 2015). Hence, it is concluded that the sum of imazalil and its metabolite FK‐772 can be enforced in all animal matrices with the combined LOQ of 0.02 mg/kg.
The storage stability of imazalil and its metabolites in animal commodities has not been studied at European level, neither during the peer reviews nor in the framework of this MRL review. As no information on the storage conditions of the samples of the feeding studies was reported, a study properly investigating the storage stability of imazalil and its metabolite FK‐772 in animal matrices is still required. It is noted that the RMS stated that the stability of imazalil in animal commodities was covered for a period of 15 months under frozen storage. However, as this statement is not supported by a detailed report and since data on the storage stability of FK‐772 are anyhow not available, the information provided by the RMS is not deemed sufficient to address this data gap.
2.2. Magnitude of residues in livestock
During the peer review, the magnitude of imazalil residues in livestock was investigated in one study performed with lactating cows and one study performed with laying hens (Netherlands, 2009a).
In the ruminant study, lactating cows were fed with imazalil at three different dose levels, adequately covering the different estimated dietary intakes of ruminants. As the metabolite R014821 is not expected to be present in feed commodities (see also Section 2.1), imazalil is the only driver of the dietary burden and this study is therefore suitable to derive MRLs in ruminant commodities. Residue levels of imazalil and its metabolite FK‐772 were measured in all relevant tissues as well as in milk. The raw results expressed for FK‐772 were converted to imazalil equivalents to fit with the enforcement residue definition (sum of imazalil and its metabolite FK‐772, expressed as imazalil), considering a molecular weight conversion of 0.82.18 At the closest dose level of 4.95 mg/kg body weight (bw), imazalil residue levels expressed according to the residue definition for enforcement were quantified as follows: 0.03 mg/kg in muscle, 0.04 mg/kg in fat, 3.1 mg/kg in liver, 0.46 mg/kg in kidney and 0.02 mg/kg in milk. By scaling these figures according to the respective dietary burdens calculated under the two different scenarios (EU1 considering all authorised GAPs and EU2 considering fall‐back options, see also Section 3.1), it is possible to derive MRLs and risk assessment values for the sum of imazalil and its metabolite FK‐772 expressed as imazalil, in all ruminant commodities under each scenario (see Appendix B.2.2.1). As the metabolic pathways are expected to be similar in ruminants and pigs, the same approach was followed for swine commodities. However, since the storage stability of imazalil and its metabolite FK‐772 in animal commodities still needs to be addressed and since the storage conditions of the samples of the feeding study were not reported, these MRLs are proposed on a tentative basis only.
For poultry commodities, the available feeding study does not allow deriving MRL and risk assessment values due to the following reasons:
The maximal investigated feeding level (0.13 mg/kg bw19) is more than 10 times lower than the calculated dietary burden under scenario EU1 (1.78 mg/kg bw per day).
The metabolite FK‐772 was not measured; only imazalil and three non‐relevant metabolites were analysed.
For all analysed compounds (including imazalil), residue levels are < 0.01 mg/kg in all commodities.
Therefore, also considering that the metabolism study performed on hens only provide limited information (see Section 2.1), no MRL proposal could be derived for poultry products. It is however noted that MRL and risk assessment values in poultry commodities would only be required under the scenario EU1 since under the scenario EU2 (i.e. excluding GAPs on apples/pears and considering fall‐back GAPs on potatoes, oranges and grapefruits – see also Section 3.1), the dietary burden calculated for poultry is below the trigger value (see Appendix B.2). Hence, if MRLs for plant commodities are derived in compliance with scenario EU2, additional data on the nature and magnitude of residues in poultry would not be required.
Furthermore, it is noted that the conclusions presented under this section may need to be reconsidered in the future depending on the final outcome of the assessment of residues in rotational crops.
3. Consumer risk assessment
In the framework of this review, only the uses of imazalil reported by the RMS in Appendix A were considered; however, the use of imazalil was previously also assessed by the JMPR (FAO, 1977, 1984, 1985, 1994). The CXLs, resulting from these assessments by JMPR and adopted by the CAC, are now international recommendations that need to be considered by European risk managers when establishing MRLs. To facilitate consideration of these CXLs by risk managers, the consumer exposure was calculated both with and without consideration of the existing CXLs.
3.1. Consumer risk assessment without consideration of the existing CXLs
Chronic and acute exposure calculations for all crops reported in the framework of this review were performed using revision 2 of the EFSA PRIMo (EFSA, 2007). Input values for the exposure calculations were derived in compliance with the decision tree reported in Appendix E. Hence, for those commodities where a (tentative) MRL could be derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies (FAO, 2009). For all commodities of plant origin, input values should refer to the raw agricultural commodities, except for bananas, citrus fruits and melons where the residue concentration in pulp should be taken into account. For bananas, the residue levels in pulp were estimated by using the peeling factor derived under Section 1.2.3. For citrus fruits, although the peeling factor (0.07) can be considered relevant according to the guidance (36 data available), its reliability is affected by the wide distribution of the results, ranging from 0.01 to 0.28 (see Section 1.2.3). Therefore, the use of the median peeling factor in the consumer exposure may drive too much uncertainty. In addition, the residue levels in pulp fractions are available in a large set of residue trials compliant with GAP (see also Appendix B.1.2.1) and the highest residue value (HR) observed in orange pulp is much higher than the value that would be derived from a theoretical calculation considering the HR and STMR (supervised trials median residue) in the whole fruit and the median peeling factor.20 Therefore, in accordance with the recommendation of the JMPR (FAO, 2009), and in order not to underestimate the possible exposure to consumer, it was preferred to use in the risk assessment the HR and STMR values directly derived from residue levels measured in pulp in the available supervised trials. This approach is particularly relevant because adequately conservative for orange and grapefruits where the margin of safety is very low (see assessment below). For melons, where data were insufficient to derive MRLs, EFSA considered the existing EU MRLs for an indicative calculation and the residue levels in pulp were estimated by applying the peeling factor derived in Section 1.2.3 to the EU MRL. For sweet peppers/bell peppers as well as for commodities of poultry origin, where data were also insufficient to derive MRLs in Sections 1 and 2, EFSA also considered the existing EU MRLs for an indicative calculation. For all plant commodities, the CF from enforcement to risk assessment of 1, as proposed in Section 1.2.1, was considered. For animal commodities, the indicative CFs proposed in Section 2.1 were considered on a tentative basis.
All input values included in the exposure calculations are summarised in Appendix D.2.
The exposure values calculated were compared with the toxicological reference values for imazalil, derived by EFSA (2010) under Commission Regulation (EC) No 737/2007. The highest chronic exposure was calculated for Dutch children, representing 130.1% of the acceptable daily intake (ADI). With regard to the acute exposure, an exceedance of the acute reference dose (ARfD) was identified for potatoes, apples, pears, bovine liver, oranges and grapefruits, representing 1415%, 686%, 638%, 221%, 183% and 123% of the ARfD, respectively.
It is noted that the RMS made an attempt to refine the acute exposure assessment by proposing lower variability factors (VFs) for apples/pears (VF = 1.8) and for potatoes (VF = 2) (Netherlands, 2015). However, these VFs were derived on the basis of one single study for each commodity, which is rather limited. Furthermore, the available study summaries do not contain the individual values from which the calculations of the VF are derived and some discrepancies have been observed regarding the results reported on apples.21 Considering the high exposures observed in potatoes, apples and pears, EFSA is of the opinion that any attempt to refine the consumer exposure to these commodities should be more robust and transparent. In any case, it is highlighted that even using these lower variability factors in the assessment, acute exposure concerns would remain for the more critical GAPs assessed on apples, pears and potatoes.
A second exposure calculation was therefore performed, considering a fall‐back GAP for potatoes (seed treatment instead of post‐harvest treatment) as well as for orange and grapefruits (post‐harvest waxing instead of drenching). For apples and pears, however, no fall‐back option could be identified by EFSA because all other GAPs received in this review do also lead to exceedances of the ARfD.22 Apples and pears were therefore excluded from this second calculation. It is highlighted that considering a less critical GAP on potatoes and excluding the use of imazalil on apples and pears would considerably decrease the residue intake to livestock (see dietary burden calculated under scenario EU2 in Appendix B.2). Therefore, the residue levels in livestock commodities were recalculated in accordance with this scenario (see scenario EU2 in Appendix B.2.2.1). A detailed overview of the values included in the exposure calculations under scenario EU2 is available Appendix D.2.2. According to the results of the second calculation, the highest chronic exposure declined to 40.7% of the ADI (French toddler); the highest acute exposure is then calculated for mandarins, representing 76.8% of the ARfD.
Based on these calculations, a potential risk to consumers was identified for the most critical GAPs reported on oranges, grapefruits and potatoes as well as for all GAPs reported on apples and pears. However, fall‐back GAPs were identified for oranges, grapefruits and potatoes, for which a second risk assessment did not result in an exceedance of the toxicological reference values. For apples and pears however, no fall‐back GAPs could be identified. For the remaining commodities, although uncertainties remain due to the data gaps identified in Sections 1 and 2, the indicative exposure calculation did not indicate a risk to consumers.
EFSA emphasises that the above assessment does not consider the possible impact of plant and livestock metabolism on the isomer ratio of imazalil and further investigation on this matter would in principle be required. Since guidance on the consideration of isomer ratios in the consumer risk assessment is not yet available, EFSA recommends that this issue is reconsidered when such guidance becomes available.
Furthermore, it is noted that the conclusions presented under this section may need to be reconsidered in future depending on the final outcome of the assessment of residues in rotational crops.
3.2. Consumer risk assessment with consideration of the existing CXLs
CXLs are defined for imazalil. To include these CXLs in the calculations of the consumer exposure, CXLs were compared with the EU MRL proposals in compliance with Appendix E and all data relevant to the consumer exposure assessment have been collected from JMPR evaluations. It is noted that no data on metabolite R014821 are available in the JMPR evaluations as this compound was not considered for risk assessment at JMPR level. In order to consider the residue definition for risk assessment derived in this review (sum of imazalil and R014821, expressed as imazalil), the CF derived in Section 1.2.1 can be applied to CXLs if sufficient information is available to demonstrate that they are applicable. For strawberries, blackberries, raspberries, persimmon, cucumbers, gherkins and wheat, where the CXLs are not derived from post‐harvest GAPs, the CF of 1 can apply. For citrus fruits, pome fruits, bananas, potatoes and melons, the CXLs are linked to post‐harvest treatments. For bananas and melons, there was no need to include the CXLs in this calculation as they are covered by the EU MRLs assessed in Section 3.1. For the remaining commodities (citrus fruits, pome fruits and potatoes), CXLs are higher than the EU MRLs. However, as the detailed parameters of the post‐harvest GAP (mode of application and WHP) were not available, these CXLs were assessed on a tentative basis, assuming CF of 1 from enforcement to risk assessment. For citrus fruits, as the degree of peel/pulp transfer is expected to depend on the mode of post‐harvest application (drenching, waxing, etc.) and on the WHP, it was not considered appropriate to apply the peeling factor derived under Section 1.2.3 to the CXL for citrus fruits; this is also consistent with the approach followed in the consumer risk assessment of the European uses (see also Section 3.1). Furthermore, it is noted that no risk assessment values are available for the CXLs currently in place for citrus fruits, pome fruits, strawberries, potatoes, cucumbers and gherkins. For all these commodities, an indicative calculation was therefore performed considering directly the CXL values (and the CF) in the risk assessment. It is noted that the data gap identified for all high acid commodities in Section 1.1.5.23 should also apply to CXLs; therefore the CXLs reported for citrus fruits, strawberries, blackberries and raspberries are also considered tentative. It is noted that no CXLs are currently in place for livestock commodities; therefore, for livestock commodities, the MRL and risk assessment values derived under scenario EU2 were reported in this calculation. An overview of the input values used for this exposure calculation is provided in Appendix D.3.
Chronic and acute exposure calculations were also performed using revision 2 of the EFSA PRIMo and the exposure values calculated were compared with the toxicological reference values derived for imazalil. The highest chronic exposure was calculated for German children, representing 408% of the ADI. With regard to the acute exposure, an exceedance of the ARfD was identified for potatoes, oranges, apples, pears, grapefruits, quinces, persimmon and medlar representing 1538%, 1326%, 980%, 911%, 892%, 147%, 131% and 121% of the ARfD, respectively. A second exposure calculation was therefore performed, excluding the CXLs for these crops. As acute concerns were identified for the four main pome fruits (apples, pears, quinces and medlar) and considering the uncertainty related to the CXL for these commodities, it was also deemed reasonable to exclude the CXL for loquat from this second calculation. According to the results of this second calculation, the highest chronic exposure declined to 45.7% of the ADI (French toddler); the highest acute exposure is then calculated for mandarins, representing 76.8% of the ARfD.
Based on these calculations, a potential risk to consumers was identified for the CXLs of imazalil on potatoes, oranges, pome fruits, grapefruits and persimmon and no further refinements of the risk assessment were possible. For the remaining CXLs, although uncertainties remain due to the data gaps identified for some of them, the indicative exposure calculation did not indicate a risk to consumers.
Conclusions
The metabolism of imazalil was investigated for three different modes of applications (foliar, post‐harvest and seed treatment) in three different crop groups (cereals, fruit crops and root crops), hereby covering all uses under assessment. Based on the available studies, the residue definition for enforcement was proposed as imazalil (any ratio of constituent isomers). Imazalil can be enforced with a LOQ of 0.01 mg/kg in the four main plant matrices. For risk assessment purpose, it was proposed to consider the sum of imazalil and R014821, expressed as imazalil in order to cover the possible occurrence of metabolite R014821 which may be formed after post‐harvest applications. It is noted that the toxicity of metabolite R014821 still needs to be fully addressed. The proposed residue definitions apply to all plant commodities as well as to processed commodities since the nature of residues is unchanged through standard hydrolysis. However, no conclusion could be proposed regarding the nature of residues in rotational crops as no metabolism studies are available.
The available residue trials data allowed deriving MRL proposals as well as risk assessment values for all commodities under evaluation, except for peppers (for which no data were available) and melons (for which the number of data was insufficient to derive a MRL). For citrus fruits, bananas, potatoes, cucumber, gherkins and courgettes, the MRLs derived from the most critical GAPs are only tentative. Lower MRLs could also be derived based on less critical GAPs reported for oranges/grapefruits (tentative), apples/pears (tentative) and potatoes (fully supported by data).
Robust processing factors were derived for peeled fruits (citrus fruits, bananas and melons) as well as for many processed commodities of oranges (juice, dry pomace, wet pomace and marmalade), apples (juice and wet pomace) and potatoes (unpeeled/boiled, peeled/boiled and fried). For the other processed commodities assessed in this review, the processing factors are considered tentative because of the limited number of data.
The metabolism of imazalil was investigated in lactating goats and laying hens. However, as the metabolism study performed on hens provides very limited information, only tentative conclusion could be derived for poultry. Imazalil was not identified as a sufficient marker in livestock commodities. Therefore, it was necessary to consider an additional compound (metabolite FK‐772) for enforcement purpose. The residue definition for enforcement in livestock commodities was proposed as the sum of imazalil and its metabolite FK‐772 (any ratio of constituent isomers), expressed as imazalil. This residue definition is not fat soluble. A multiresidue analytical method is available and validated in all relevant matrices for the determination of imazalil and FK‐772, with a LOQ of 0.01 mg/kg for each compound. Considering that the toxicity of metabolite FK‐772 is not yet fully addressed, the proposed residue definition is tentative. For risk assessment purpose, it was proposed to consider the sum of imazalil and all identified/characterised metabolites observed in the goat metabolism study. CFs from enforcement to risk assessment were therefore tentatively derived on the basis of the metabolism studies.
Based on the feeding study performed on lactating cows, MRL and risk assessment values were derived for the sum of imazalil and its metabolite FK‐772 expressed as imazalil, in all ruminants and swine commodities. However, since the storage stability of imazalil and its metabolite FK‐772 in animal commodities still needs to be addressed and since the storage conditions of the samples of the feeding study were not reported, these MRLs are proposed on a tentative basis only. For poultry commodities, the available feeding study did not allow deriving MRL and risk assessment values. It is noted that MRLs and risk assessment values for livestock commodities were derived under two different scenarios, considering all critical GAPs (EU1) and considering the fall‐back GAPs for oranges/grapefruits and potatoes (EU2). In the scenario EU2, no MRLs were considered needed for poultry commodities.
Chronic and acute consumer exposure resulting from the authorised uses reported in the framework of this review was calculated using revision 2 of the EFSA PRIMo. For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation. For potatoes, apples, pears, bovine liver, oranges and grapefruits, an exceedance of the ARfD was identified representing 1415%, 686%, 638%, 221%, 183% and 123% of the ARfD, respectively. Considering fall‐back MRLs for potatoes, oranges and grapefruits and disregarding the GAPs on apples and pears (for which no safe fall‐back option could be identified), the highest chronic exposure represented 40.7% of the ADI (French toddler) and the highest acute exposure amounted to 76.8% of the ARfD (mandarins).
Apart from the MRLs evaluated in the framework of this review, internationally recommended CXLs have also been established for imazalil. Additional calculations of the consumer exposure, considering these CXLs, were therefore carried out and exceedances of the ARfD were identified for the existing CXLs in potatoes (1538%), oranges (1326%), apples (980%), pears (911%), grapefruits (892%), quinces (147%), persimmon (131%) and medlar (121%). Excluding these CXLs from the calculation, the highest chronic exposure represented 45.7% of the ADI (French toddler) and the highest acute exposure amounted to 76.8% of the ARfD (mandarins).
Recommendations
MRL recommendations were derived in compliance with the decision tree reported in Appendix E of the reasoned opinion (see Table 1). All MRL values listed as ‘Recommended’ in the table are sufficiently supported by data and are therefore proposed for inclusion in Annex II to the Regulation. The remaining MRL values listed in the table are not recommended for inclusion in Annex II because they require further consideration by risk managers (see Table 1 footnotes for details). In particular, some tentative MRLs and existing EU MRLs need to be confirmed by the following data:
full toxicological assessment of metabolite R014821;
a study demonstrating the storage stability of metabolite R014821 in high acid content commodities;
additional residue trials supporting the GAPs as appropriate on bananas, peppers, courgettes and melons;
full toxicological assessment of metabolites FK‐772 and FK‐284;
a study demonstrating the storage stability of imazalil and its metabolite FK‐772 in commodities of animal origin;
information on the storage conditions of the samples of the feeding studies performed with ruminants.
Table 1.
Summary table
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
|
Enforcement residue definition (existing): imazalil Enforcement residue definition 1 (proposed): imazalil (any ratio of constituent isomers) | |||||
| 110010 | Grapefruits | 5 | 5 | 4 | Further consideration neededa |
| 110020 | Oranges | 5 | 5 | 4 | Further consideration neededa |
| 110030 | Lemons | 5 | 5 | 6 | Further consideration neededb |
| 110040 | Limes | 5 | 5 | 6 | Further consideration neededb |
| 110050 | Mandarins | 5 | 5 | 6 | Further consideration neededb |
| 130010 | Apples | 2 | 5 | – | Further consideration neededc |
| 130020 | Pears | 2 | 5 | – | Further consideration neededc |
| 130030 | Quinces | 2 | 5 | – | Further consideration neededd |
| 130040 | Medlar | 5 | 5 | – | Further consideration neededd |
| 130050 | Loquat | 5 | 5 | – | Further consideration neededd |
| 152000 | Strawberries | 0.05* | 2 | 2 | Further consideration needede |
| 153010 | Blackberries | 0.05* | 2 | 2 | Further consideration needede |
| 153030 | Raspberries | 0.05* | 2 | 2 | Further consideration needede |
| 161060 | Persimmon | 0.05* | 2 | – | Further consideration neededf |
| 163020 | Bananas | 2 | 2 | 5 | Further consideration neededb |
| 211000 | Potatoes | 3 | 5 | 0.01* | Recommendedg |
| 231010 | Tomatoes | 0.5 | – | 0.3 | Recommendedh |
| 231020 | Sweet peppers/bell peppers | 0.05* | – | 0.05 | Further consideration neededi |
| 232010 | Cucumbers | 0.2 | 0.5 | 0.5 | Recommendedj |
| 232020 | Gherkins | 0.2 | 0.5 | 0.5 | Recommendedj |
| 232030 | Courgettes | 0.2 | – | 0.1 | Further consideration neededk |
| 233010 | Melons | 2 | 2 | 2 | Further consideration neededl |
| 500010 | Barley grains | 0.05* | – | 0.01* | Recommendedh |
| 500050 | Oat grains | 0.05* | – | 0.01* | Recommendedh |
| 500070 | Rye grains | 0.05* | – | 0.01* | Recommendedh |
| 500090 | Wheat grains | 0.05* | 0.01* | 0.01* | Recommendedm |
| – | Other commodities of plant origin | See Reg. 750/2010 | – | – | Further consideration neededn |
|
Enforcement residue definition (existing): imazalil Enforcement residue definition 2 (proposed): sum of imazalil and metabolite FK‐772 (any ratio of constituent isomers), expressed as imazalil | |||||
| 1011010 | Swine muscle | 0.05* | – | 0.02* | Further consideration neededk |
| 1011020 | Swine fat tissue | 0.05* | – | 0.02* | Further consideration neededk |
| 1011030 | Swine liver | 0.05* | – | 0.02* | Further consideration neededk |
| 1011040 | Swine kidney | 0.05* | – | 0.02* | Further consideration neededk |
| 1012010 | Bovine muscle | 0.05* | – | 0.02* | Further consideration neededk |
| 1012020 | Bovine fat tissue | 0.05* | – | 0.02* | Further consideration neededk |
| 1012030 | Bovine liver | 0.05* | – | 0.03 | Further consideration neededk |
| 1012040 | Bovine kidney | 0.05* | – | 0.02* | Further consideration neededk |
| 1015010 | Equine muscle | 0.05* | – | 0.02* | Further consideration neededk |
| 1015020 | Equine fat tissue | 0.05* | – | 0.02* | Further consideration neededk |
| 1015030 | Equine liver | 0.05* | – | 0.03 | Further consideration neededk |
| 1015040 | Equine kidney | 0.05* | – | 0.02* | Further consideration neededk |
| 1020010 | Cattle milk | 0.05* | – | 0.02* | Further consideration neededk |
| 1020040 | Horse milk | 0.05* | – | 0.02* | Further consideration neededk |
| – | Other commodities of animal origin | See Reg. 750/2010 | – | – | Further consideration neededn |
MRL: maximum residue level; CXL: codex maximum residue limit.
* Indicates that the MRL is set at the limit of quantification.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; CXL is higher but it is also not sufficiently supported by data and a risk to consumers cannot be excluded (combination E‐IV in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; existing CXL is covered by the tentative MRL (combination E‐III in Appendix E).
GAP evaluated at EU level is fully supported by data but a risk to consumers cannot be excluded; CXL is not sufficiently supported by data and a risk to consumers can also not be excluded. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination F‐IV in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; CXL is not sufficiently supported by data and a risk to consumers cannot be excluded. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐IV in Appendix E).
MRL is derived from the existing CXL, which is not sufficiently supported by data but for which no risk to consumers is identified; there are no relevant authorisations or import tolerances reported at EU level (combination A‐V in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; CXL is supported by data but a risk to consumers cannot be excluded. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐VI in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; CXL is higher but it is not sufficiently supported by data and a risk to consumers cannot be excluded (combination G‐IV in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination G‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL; no CXL is available (combination C‐I in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is not fully supported by data, leads to a lower tentative MRL (combination E‐VII in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; no CXL is available (combination E‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL; existing CXL is covered by the existing EU MRL (combination C‐III in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; existing CXL is covered by the recommended MRL (combination G‐III in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
It is highlighted, however, that some of the MRLs derived result from a CXL, whereas some of the GAPs reported by the RMS were not fully supported by data. EFSA therefore identified the following data gaps which are not expected to impact on the validity of the MRLs derived but which might have an impact on national authorisations:
additional residue trials supporting the GAPs on cucumbers and gherkins (it is noted that this data gap can be covered by the major data gap identified for courgettes);
a representative study investigating metabolism in rotational crops.
If the above‐reported data gaps are not addressed in the future, Member States are recommended to withdraw or modify the relevant authorisations at national level.
It is also highlighted that the critical GAPs reported for potatoes (post‐harvest treatment on ware potatoes) as well as on oranges and grapefruits (post‐harvest drenching) lead to an exceedance of the ARfD. Consequently, the MRLs derived in these crops are based on fall‐back GAPs: seed treatment on potatoes and post‐harvest waxing on oranges and grapefruits. Member States are therefore recommended to reconsider or withdraw their national authorisations on potatoes, oranges and grapefruits in order to ensure that the fall‐back MRLs derived for these crops are not exceeded. An exceedance of the ARfD was also identified for all the GAPs reported for apples and pears (post‐harvest drenching from 25 to 37.5 g a.s/hL as well as post‐harvest smoke can at 6 g a.s./tonnes). Therefore, either a specific LOQ or the default MRL of 0.01* mg/kg may be considered by risk managers for these crops. Member States are therefore recommended to reconsider or withdraw their national authorisations on apples and pears consequently. It is noted that MRL proposals in commodities of animal origin were derived in accordance with the above considerations, i.e. taking into account the fall‐back GAPs on potatoes, oranges and grapefruits and disregarding GAPs on apples and pears.
Minor deficiencies were also identified in the assessment but these deficiencies are not expected to impact either on the validity of the MRLs derived or on the national authorisations. The following data are therefore considered desirable but not essential:
residue trials including simultaneous analysis of imazalil and metabolite R014821 in oranges/grapefruits (waxing).
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CAS
Chemical Abstract Service
- CF
conversion factor for enforcement residue definition to risk assessment residue definition
- CXL
codex maximum residue limit
- DAR
draft assessment report
- DAT
days after treatment
- DB
dietary burden
- DM
dry matter
- DS
powder for dry seed treatment
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EC
emulsifiable concentrate
- EMS
evaluating Member State
- eq
residue expressed as a.s. equivalent
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- FID
flame ionisation detector
- GAP
Good Agricultural Practice
- HPLC–MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- Mo
monitoring
- MRL
maximum residue level
- MS
Member States
- MS
mass spectrometry detector
- MS/MS
tandem mass spectrometry detector
- NEU
northern European Union
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- Rber
statistical calculation of the MRL by using a non‐parametric method
- Rmax
statistical calculation of the MRL by using a parametric method
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern European Union
- SL
soluble concentrate
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TRR
total radioactive residue
- UV
ultraviolet (detector)
- VF
variability factor
- WHO
World Health Organization
- WHP
withholding period
Appendix A – Summary of authorised uses considered for the review of MRLs
A.1. Critical uses considered in the review of MRLs
| Crop | Region | Outdoor/Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments (max. 250 characters) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Critical outdoor GAPs for Northern Europe | ||||||||||||||||||||
| Potatoes | Solanum tuberosum subsp. tuberosum | NEU | Outdoor | DE, FR | Silver scurf (Helminthosporium solani), skin spot of potato (Polyscytalum pustulans), Fusarium species, dry rot (Phoma exigua) | SL | 100.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 1 | 15 | g a.i./tonnes | 0 | Spraying immediately after harvest. Use restricted to seed potatoes only | |||
| Barley | Hordeum vulgare | NEU | Outdoor | AT, BE, DK, FI, IE, LU, SE, UK, CZ | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | Other conc. for LS formulation are also authorised: 6 g/L and 50 g/L | |||
| Oat | Avena sativa | NEU | Outdoor | AT, BE, DK, FI, IE, LU, SE, UK | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Rye | Secale cereale | NEU | Outdoor | AT, BE, DK, FI, IE, LU, SE, UK | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Wheat | Triticum aestivum | NEU | Outdoor | AT, BE, DK, FI, IE, LU, SE, UK | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Critical outdoor GAPs for Southern Europe | ||||||||||||||||||||
| Potatoes | Solanum tuberosum subsp. tuberosum | SEU | Outdoor | FR | Silver scurf (Helminthosporium solani) Skin spot (Polyscytalum pustulans) | SL | 100.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 1 | 15 | g a.i./tonnes | 0 | Spraying immediately after harvest. Use restricted to seed potatoes only | |||
| Barley | Hordeum vulgare | SEU | Outdoor | EL, ES, IT | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | Other conc. for LS formulation are also authorised: 6 g/L and 50 g/L | |||
| Oat | Avena sativa | SEU | Outdoor | EL, ES, IT | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Rye | Secale cereale | SEU | Outdoor | EL, ES, IT | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Wheat | Triticum aestivum | SEU | Outdoor | EL, ES, IT | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Critical indoor GAPs for Northern and Southern Europe (including post‐harvest treatments) | ||||||||||||||||||||
| Grapefruits | Citrus paradisi | NEU/SEU | Indoor | ES | Penicillium digitatum, Penicillium italicum, Penicillium expansum, Diaporthe citri, Diplodia natalensis, Alternaria citri, Botrytis spp., Alternaria spp., Phomopsis spp. | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 50 | 50 | g a.i./hL | 0 | Drenching/dipping. Critical waiting period: 0 day. Other less critical post‐harvest treatments are also authorised as waxing and low volumes spraying (PT, EL, ES, IT) | |||
| Oranges | Citrus sinensis | NEU/SEU | Indoor | ES | See grapefruits | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 50 | 50 | g a.i./hL | 0 | See comment on grapefruits | |||
| Lemons | Citrus limon | NEU/SEU | Indoor | ES | See grapefruits | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 50 | 50 | g a.i./hL | 0 | See comment on grapefruits | |||
| Limes | Citrus aurantiifolia | NEU/SEU | Indoor | ES | See grapefruits | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 50 | 50 | g a.i./hL | 0 | See comment on grapefruits | |||
| Mandarins | Citrus reticulata, syn: Citrus deliciosa | NEU/SEU | Indoor | ES | See grapefruits. | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 50 | 50 | g a.i./hL | 0 | See comment on grapefruits | |||
| Apples | Malus domestica | NEU/SEU | Indoor | EL | Penicillium expansum, Gloeosporium sp. | SL | 75.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 25 | 30 | g a.i./hL | 0 | Drenching/dipping. Critical waiting period: 0 day | |||
| Pears | Pyrus communis | NEU/SEU | Indoor | BE, NL | Penicillium expansum, Gloeosporium sp. | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 25 | 25 | g a.i./hL | 0 | Drenching/dipping. Critical waiting period: 0 day (1 day in NL) | |||
| Bananas | Musa acuminata; Musa balbisiana; Musa acuminata x Musa balbisiana | NEU/SEU | Indoor | EL | Crown rot pathogens: Colletotrichum musae, Fusarium moniliforme, Fusarium pusillum, Fusarium semitectum, Verticillium theobromae, Verticillium sp. | SG | 750.0 | g/kg | Post‐harvest treatment – dipping | n.a. | n.a. | 1 | 60 | g a.i./hL | 35 | Spray overhead on transport belt. (application rate equivalent to 1.2 g a.s./tonnes) | ||||
| Potatoes | Solanum tuberosum subsp. tuberosum | NEU/SEU | Indoor | DE | Polyscytalum pustulans, Phoma exigua var. foveata, Helminthosporium solani, Fusarium sulphureum, Fusarium culmorum, Fusarium roseum var. sambucinum | EC | 500.0 | g/L | Post‐harvest – spraying | n.a. | n.a. | 1 | 1 | 15 | g a.i./tonnes | 0 | Spraying immediately after harvest during gathering the harvest in the storage room. No restriction of the WHP (i.e. 0 day possible) | |||
| Tomatoes | Lycopersicon esculentum | NEU/SEU | Indoor | BE, NL | Powdery mildew: Oidiopsis (Leveillula) taurica, Botrytis cinerea, Oidium lycopersici | EC | 100.0 | g/L | Foliar treatment – spraying | 51 | 89 | 1 | 3 | 7 | 14 | 300 | g a.i./ha | 1 | Based on a GAP authorised for 20 g ai/hL and assuming 1,500 L water/ha is applied | |
| Sweet peppers | Capsicum annuum | NEU/SEU | Indoor | BE, NL | Powdery mildew: Oidiopsis (Leveillula) taurica, Botrytis cinerea, Oidium lycopersici | EC | 100.0 | g/L | Foliar treatment – spraying | 51 | 89 | 1 | 3 | 7 | 14 | 300 | g a.i./ha | 3 | Based on a GAP authorised for 20 g ai/hL and assuming 1,500 L water/ha is applied | |
| Cucumbers | Cucumis sativus | NEU/SEU | Indoor | NL, BE, EL, IE, NL, ES, UK | Powdery mildew: Sphaerotheca fuliginea, Mycosphaerella citrullina | EC | 100.0 | g/L | Foliar treatment – spraying | 51 | 89 | 1 | 4 | 7 | 10 | 75 | g a.i./ha | 1 |
Based on a rate of 5 g ai/hL and assuming 1,500 L water/ha). 4 applications authorised in NL; only 3 applications authorised in the other countries |
|
| Gherkins | Cucumis sativus | NEU/SEU | Indoor | NL, BE, EL, IE, NL, ES, UK | Powdery mildew: Sphaerotheca fuliginea, Mycosphaerella citrullina | EC | 100.0 | g/L | Foliar treatment – spraying | 51 | 89 | 1 | 4 | 7 | 10 | 75 | g a.i./ha | 1 | See comment on cucumbers | |
| Courgettes | Cucurbita pepo zucchini group | NEU/SEU | Indoor | NL, BE, EL, IE, NL, ES, UK | Powdery mildew: Sphaerotheca fuliginea, Mycosphaerella citrullina | EC | 100.0 | g/L | Foliar treatment – spraying | 51 | 89 | 1 | 4 | 7 | 10 | 75 | g a.i./ha | 1 | See comment on cucumbers | |
| Melons | Cucumis melo | NEU/SEU | Indoor | ES | Penicillium sp., Fusarium sp., Alternaria sp. | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 45 | g a.i./hL | 0 | Also authorised for foliar spraying in BE (3× 70 g a.s./ha; PHI 3 days) but no supporting trials available | ||||
MRL: maximum residue level; GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient; a.s.: active substance; WHP: withholding period.
A.2. GAPs for which an acute concern was identified
| Crop | Region | Outdoor/Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments (max. 250 characters) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Grapefruits | Citrus paradisi | NEU/SEU | Indoor | ES | Penicillium digitatum, Penicillium italicum, Penicillium expansum, Diaporthe citri, Diplodia natalensis, Alternaria citri, Botrytis spp., Alternaria spp., Phomopsis spp. | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 50 | 50 | g a.i./hL | 0 | Drenching/dipping. Critical waiting period: 0 day. Other less critical post‐harvest treatments are also authorised as waxing and low volumes spraying (PT, EL, ES, IT) | |||
| Oranges | Citrus sinensis | NEU/SEU | Indoor | ES | See grapefruits | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 50 | 50 | g a.i./hL | 0 | See comment on grapefruits | |||
| Apples | Malus domestica | NEU/SEU | Indoor | EL | Penicillium expansum, Gloeosporium sp. | SL | 75.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 25 | 30 | g a.i./hL | 0 | Drenching/dipping. Critical waiting period: 0 day | |||
| Apples | Malus domestica | NEU/SEU | Indoor | ES, PT | Penicillium spp. | SL | 75.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 37.5 | g a.i./hL | 60 | Drenching/dipping. Critical waiting period: 60 days | ||||
| Apples | Malus domestica | NEU/SEU | Indoor | EL, ES | Post‐harvest fungi | FU | 250.0 | g/L | Post‐harvest treatment – smoke can | n.a. | n.a. | 1 | 6.0 | g a.i./tonnes | 30 | – | ||||
| Pears | Pyrus communis | NEU/SEU | Indoor | BE, NL | Penicillium expansum, Gloeosporium sp. | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 25 | 25 | g a.i./hL | 0 | Drenching/dipping. Critical waiting period: 0 day (1 day in NL) | |||
| Pears | Pyrus communis | NEU/SEU | Indoor | ES, PT | Penicillium spp. | SL | 75.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 37.5 | g a.i./hL | 60 | Drenching/dipping. Critical waiting period: 60 days | ||||
| Pears | Pyrus communis | NEU/SEU | Indoor | EL, ES | Post‐harvest fungi | FU | 250.0 | g/L | Post‐harvest treatment – smoke can | n.a. | n.a. | 1 | 6.0 | g a.i./tonnes | 30 | – | ||||
| Potatoes | Solanum tuberosum subsp. tuberosum | NEU/SEU | Indoor | DE | Polyscytalum pustulans, Phoma exigua var. foveata, Helminthosporium solani, Fusarium sulphureum, Fusarium culmorum, Fusarium roseum var. sambucinum | EC | 500.0 | g/L | Post‐harvest – spraying | n.a. | n.a. | 1 | 1 | 15 | g a.i./tonnes | 0 | Spraying immediately after harvest during gathering the harvest in the storage room. No restriction of the WHP (i.e. 0 day possible) | |||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: pre‐harvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient; WHP: withholding period.
A.3. Fall‐back GAPs identified
| Crop | Region | Outdoor/Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments (max. 250 characters) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Grapefruits | Citrus paradisi | NEU/SEU | Indoor | ES, EL | Penicillium digitatum, Penicillium italicum, Penicillium expansum, Diaporthe citri, Diplodia natalensis, Alternaria citri, Botrytis spp., Alternaria spp., Phomopsis spp. | EW | 3.0 | g/L | Post‐harvest – spraying | n.a. | n.a. | 1 | 300 | g a.i./hL | 0 | Pulverisation with wax (waxing). Volume of 1 L/tonnes fruits (equivalent to 3 g a.i.: tonnes fruits) | ||||
| Oranges | Citrus sinensis | NEU/SEU | Indoor | ES, EL | Penicillium digitatum, Penicillium italicum, Penicillium expansum, Diaporthe citri, Diplodia natalensis, Alternaria citri, Botrytis spp., Alternaria spp., Phomopsis spp. | EW | 3.0 | g/L | Post‐harvest – spraying | n.a. | n.a. | 1 | 300 | g a.i./hL | 0 | See comment on grapefruits | ||||
| Potatoes | Solanum tuberosum subsp. tuberosum | NEU | Outdoor | DE, FR | Silver scurf (Helminthosporium solani), skin spot of potato (Polyscytalum pustulans), Fusarium species, dry rot (Phoma exigua) | SL | 100.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 1 | 15 | g a.i./tonnes | 0 | Spraying immediately after harvest. Use restricted to seed potatoes only | |||
| Potatoes | Solanum tuberosum subsp. tuberosum | SEU | Outdoor | FR | Silver scurf (Helminthosporium solani) Skin spot (Polyscytalum pustulans) | SL | 100.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 1 | 15 | g a.i./tonnes | 0 | Spraying immediately after harvest. Use restricted to seed potatoes only | |||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops(available studies) | Crop groups | Crop(s) | Application(s) | Sampling |
|---|---|---|---|---|
| Fruit crops | Tomatoes | Foliar, 3 × 300 g a.s./haFoliar, 3 × 1500 g a.s./ha | 1 DAT 1 DAT | |
| Oranges, apples | Post‐harvest dipping, 0.05 kg/hl | From 2 h to 7 months | ||
| Root crops | Potatoes | Post‐harvest (ware potatoes):15 g a.s./tonnes | 0, 14, 29, 91, 188 DAT | |
| Potatoes | Seed treatment (seed potatoes):15 g a.s./tonnes and 75 g a.s./tonnes | After growing under normal conditions | ||
| Cereals/grass crops | Spring wheat | Seed‐treatment, 0.49 kg a.s./tonnes | After growing under normal conditions.Forage: 42 DAT Grain: 150 DAT | |
| Sources: For tomatoes (Netherlands, 2009a); for oranges, apples and spring wheat (Belgium, 1996); for potatoes (Netherlands, 2015) | ||||
| Rotational crops(available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) |
|---|---|---|---|---|
| Root/tuber crops | – | – | – | |
| Leafy crops | – | – | – | |
| Cereal (small grain) | – | – | – | |
| Studies not available but still required. A theoretical calculation was presented by the RMS (Netherlands, 2015) but does not allow concluding on the residues in rotational crops | ||||
| Processed commodities(hydrolysis study) | Conditions | Investigated? |
|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | |
| Sterilisation (20 min, 120°C, pH 6) | Yes | |
| Source: Netherlands, 2014 | ||
| Can a general residue definition be proposed for primary crops? | Yes |
| Rotational crop and primary crop metabolism similar? | Inconclusive |
| Residue pattern in processed commodities similar to residue pattern in raw commodities? | Yes |
| Plant residue definition for monitoring (RD‐Mo) | Imazalil (any ratio of constituent isomers) |
| Plant residue definition for risk assessment (RD‐RA) | Sum of imazalil and R014821, expressed as imazalil (tentative)a |
| Conversion factor (monitoring to risk assessment) | See B.1.2.1 |
| Methods of analysis for monitoring of residues (analytical technique, crop groups, LOQs) | HPLC–MS/MS: |
a.s.: active substance; DAT: days after treatment; PBI: plant‐back interval; HPLC–MS/MS: high performance liquid chromatography with tandem mass spectrometry; ILV: independent laboratory validation; LOQ: limit of quantification.
Metabolite R014821 is only relevant for commodities subject to post‐harvest treatment.
B.1.1.2. Stability of residues in plants
|
Plant products (available studies) |
Category | Commodity | T (°C) | Stability (months/years) |
|---|---|---|---|---|
| High water content | Apple (raw and processed) | −20 | 12 months | |
| High acid content | Citrus fruits | −18 | 6 months | |
| High oil content | – | – | – | |
| Dry | Cereal grain | −18 | 6 months | |
| Specific matrices | Cereal straw | −18 | 6 months | |
|
For high water content commodities: storage stability was separately demonstrated for imazalil and R014821 (Netherlands, 2015) For other matrices: storage stability only demonstrated for imazalil; no data available for the metabolite R014821 (EFSA, 2010) | ||||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Crop | Region/indoora | Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) |
Recommendations/comments (OECD calculations) |
MRL proposals (mg/kg) |
HRMo (mg/kg)b |
STMRMo (mg/kg)c |
CFd |
|---|---|---|---|---|---|---|---|
| Citrus fruits | EU (drenching: 50 g a.i./hL; WHP: 0 day) |
Mo [whole fruit] – Oranges: 0.57; 0.59; 0.6; 0.66; 0.66; 0.84; 1.36; 1.4; 2.06; 2.09; 2.2; 2.2; 2.46; 2.58; 2.59; 2.81; 2.89; 3.12; 4.95 Mo [whole fruit] – Mandarins: 0.49; 0.6; 1.3; 1.51; 1.6; 1.8; 1.8; 2.19; 2.26; 2.4; 2.66; 2.72; 3.34; 4.84 RA [whole fruit] – Oranges: ‐; ‐; ‐; ‐; ‐; ‐; ‐; ‐; ‐; 2.09; ‐; ‐; 2.46; 2.58;‐; 2.81; ‐; ‐; ‐ RA [whole fruit] – Mandarins: ‐; ‐; ‐; ‐; ‐; ‐; ‐; ‐; 2.27; ‐; 2.68; 2.75; 3.36; ‐ |
Combined data set on oranges and mandarins, compliant with GAP (EFSA, 2010; Netherlands, 2015; Spain, 2016). Residue levels of R014821 may slightly increase with the waiting period. Rber = 5.25 Rmax = 4.46 MRLOECD: not relevante |
6 (tentative)f |
4.95 | 2.09 | 1 |
|
Mo [Pulp] – Oranges: 5 × < 0.01; 0.02; 0.03; 0.04; 0.09; 0.12; 0.21; 0.69 Mo [Pulp] – Mandarins: 0.02; 0.02; 0.05; 0.08; 0.12; 0.18; 0.43; 0.48 |
Residue levels directly measured in pulp and coming from the same set of residue trials as above were reported for a refined risk assessment | – | 0.69 | 0.05 | 1g | ||
|
Oranges Grapefruits (fall‐back option) |
EU (waxing: 300 g a.i./hl Volume of 1 L/ton fruits equivalent to 3 g a.i./ton; WHP: 0 day) |
Mo [whole fruit] – Oranges: 0.43; 0.47; 1.1; 1.3; 1.4; 1.5; 1.7; 2.1 Mo [whole fruit] – Mandarins: 1.2; 1.5; 1.6; 1.7; 2.1; 2.3 RA : ‐ |
Combined data set on oranges and mandarins, compliant with GAP (Spain, 2016). Rber = 3.60 Rmax = 2.89 MRLOECD: not relevante |
4 (tentative)f |
2.3 | 1.5 | 1g |
|
Mo [Pulp] – Oranges: 4 × < 0.01 Mo [Pulp] – Mandarins: < 0.01; 0.02 |
Residue levels directly measured in pulp and coming from the same set of residue trials as above were reported for a refined risk assessment | – | 0.02 | 0.01 | 1g | ||
|
Apples Pears |
EU (drenching: 25–30 g a.i./hL; WHP: 0 day) |
Mo Apples: 0.3; 0.39; 0.42; 0.55; 0.57; 0.59; 0.63; 0.65; 0.76; 0.84; 0.89; 0.9; 1; 1.33 Mo Pears: 0.34; 0.35; 0.38; 0.45; 0.50; 0.57; 0.66; 0.7; 0.82; 1.02; 1.15; 1.23; 1.24; 1.45; 2.0; 2.1; 2.28; 3.5 RA Apples: ‐ RA Pears: ‐; ‐; ‐; ‐; ‐; ‐; ‐; ‐; ‐; 0.82; 1.02; 1.15; 1.23; 1.24; 1.45; ‐; 2.1; 2.28; ‐ |
Combined data set on apples and pears, compliant with GAP (Belgium, 1996; Netherlands, 2016, Spain, 2016). Rber = 2.42 Rmax = 2.47 MRLOECD: not relevante |
4 | 3.50 | 0.73 | 1 |
| EU (drenching: 38 g a.i./hL; WHP: 60 days) |
Mo Apples: 0.4; 0.53; 0.66; 0.75; 1.55; 1.71; 1.81; 2.11; 2.38; 2.77 Mo Pears: 1.2; 1.4 RA Apples: ‐; ‐; ‐; ‐; 1.69; ‐; 1.95; 2.19; ‐; 2.82 RA Pears: ‐; ‐ |
Combined data set on apples and pears, compliant with GAP (Netherlands, 2015; Spain, 2016). Rber = 3.57 Rmax = 4.07 MRLOECD: not relevante |
4 | 2.77 | 1.48 | 1 | |
| EU (smoke can: 6 g a.i./tonnes; WHP: 30 days) |
Mo Apples: 0.36, 0.38 Mo Pears: 0.74, 0.79 RA: ‐ |
Combined data set on apples and pears, compliant with GAP (Spain, 2016). Rber = 1.56 Rmax = 1.75 MRLOECD: not relevante |
2 (tentative)h |
0.79 | 0.56 | 1g | |
| Bananas | EU (post‐harvest dipping) |
Mo [whole fruit]: 2.12; 2.61; 2.71; 2.8 RA [whole fruit]: 2.76; 2.64; 2.76; 2.83 |
Trials compliant with GAP (Greece, 2016). No data available on pulp at the relevant PHI (35 days). Rber = 5.56 Rmax = 4.12 MRLOECD: not relevante |
5 (tentative)h |
2.80 | 2.66 | 1 |
| Potatoes | EU (ware potatoes, post‐harvest spraying) |
Trials compliant with GAP (Netherlands, 2015). Rber = 8.6 Rmax = 7.43 MRLOECD: not relevante |
9 (tentative)i | 4.60 | 2.65 | 1 | |
| NEU (seed potatoes, post‐harvest spraying) |
Mo: 10 × < 0.01 RA: ‐ |
Trials compliant with GAP (France, 2016). Metabolite R014821 not expected to occur after seed treatment | 0.01* | 0.01 | 0.01 | 1j | |
| SEU (seed potatoes, post‐harvest spraying) |
Mo: 10 × < 0.01 RA: ‐ |
Trials compliant with GAP (France, 2016). Metabolite R014821 not expected to occur after seed treatment | 0.01* | 0.01 | 0.01 | 1j | |
| Tomatoes | EU |
Mo: 0.03; 0.03; 0.08; 0.08; 0.09; 0.16; 0.15; 0.14 RA: ‐ |
Trials on tomatoes compliant with GAP. Metabolite R014821 not expected to occur (EFSA, 2010). MRLOECD = 0.3 |
0.3 | 0.16 | 0.09 | 1j |
| Sweet peppers/bell peppers | EU | – | No data available | – | – | – | – |
|
Cucumbers Gherkins Courgettes |
EU |
Mo: 0.02; 0.05; 0.01; 0.02 RA: 0.02; 0.05; 0.01; 0.02 |
Trials performed on cucumbers. Two first trials compliant with GAP. Two additional trials performed with 3 applications instead of 4 (Netherlands, 2016). MRLOECD = 0.09 |
0.1 (tentative)h | 0.05 | 0.02 | 1 |
| Melons | EU (post‐harvest drenching |
Mo: 0.47; 0.47; 0.47 RA: ‐ |
Trials performed with drench application (25% deviation on the application rate) (Belgium, 1996) | – | – | – | – |
|
Barley grains Oats grains Rye grains Wheat grains |
NEU |
Mo: 4 × < 0.05; 4 × < 0.01 RA: ‐ |
Trials performed on barley and compliant with GAP (Belgium, 1996; EFSA, 2010). Based on NEU and SEU trials, an MRL of 0.01* mg/kg can be proposed. Metabolite R014821 not expected to occur (EFSA, 2010) | 0.01* | 0.01 | 0.01 | 1j |
| SEU |
Mo: 4 × < 0.01 RA: ‐ |
||||||
|
Barley straw Oat straw Rye straw Wheat straw |
NEU |
Mo: 4 × < 0.01 RA: ‐ |
Trials on barley compliant with GAP (EFSA, 2010). Based on NEU and SEU trials, an MRL of 0.01* mg/kg can be proposed. Metabolite R014821 not expected to occur (EFSA, 2010) | 0.01* | 0.01 | 0.01 | 1j |
| SEU |
Mo: 4 × < 0.01 RA: ‐ |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level; WHP: withholding period; PHI: preharvest interval; Mo: monitoring; RA: risk assessment.
* Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue according to the residue definition for monitoring.
Supervised trials median residue according to the residue definition for monitoring.
Conversion factor for risk assessment; median of the individual conversion factors at the supported PHI for each residues trial (if trials analysing for both residue definition are available).
MRLOECD calculator is not relevant for data obtained by post‐harvest treatment as it may artificially overestimate the MRL calculation derived from homogeneous data set.
MRL is tentative because the storage stability of the metabolite R014821 is not covered in commodities with high acid content.
In the absence of trials analysing for metabolite R014821, a CF of 1 is assumed based on the results observed on the other fruit commodities following post‐harvest treatment (with short WHP).
MRL is tentative because additional residue data trials are still required.
Highest residue levels were observed after a DAT longer than 0 day in samples that were washed before analysis; therefore the whole results are deemed questionable and are used on a tentative basis to derive MRL and risk assessment values.
Although data for metabolite R014821 are not available (or limited), a conversion factor (CF) of 1 can be considered as the metabolite R014821 is not expected to occur for this GAP.
B.1.2.2. Residues in succeeding crops
|
Confined rotational crop study (quantitative aspect) |
Not available but still required. A theoretical calculation was presented by the RMS (Netherlands, 2015) but does not allow concluding on the potential residues uptake in rotational crops |
| Field rotational crop study | Not available |
B.1.2.3. Processing factors
| Processed commodity | Number of studiesa | Processing factor (PF) | ||
|---|---|---|---|---|
| Individual values | Median PF | CF | ||
| Robust processing factors (sufficiently supported by data) | ||||
| Citrus, peeled | 36b | 0.01; 0.01; 0.01; 0.02; 0.03; 0.04; 0.04; 0.04; 0.04; 0.04; 0.04; 0.04; 0.05; 0.05; 0.05; 0.05; 0.06; 0.07; 0.07; 0.07; 0.08; 0.08; 0.08; 0.08; 0.10; 0.11; 0.12; 0.13; 0.14; 0.15; 0.15; 0.16; 0.20; 0.21; 0.25; 0.28 | 0.07 | 1 |
|
Oranges, juice (→ extrapolated to other citrus) |
10 | 0.01; 0.02; 0.03; 0.05; 0.05; 0.10; 0.11; 0.14; 0.33; 0.35 | 0.08 | 1 |
|
Oranges, dry pomace (→ extrapolated to other citrus) |
10 | 1.0; 1.1; 2.3; 4.03; 4.05; 4.39; 4.48; 4.86; 6.7; 9.6 | 4.2 | 1 |
|
Oranges, wet pomace (→ extrapolated to other citrus) |
7 | 1.74; 1.98; 2.03; 2.04; 2.2; 2.29; 2.7 | 2 | 1 |
|
Oranges, marmalade (→ extrapolated to other citrus) |
7 | 0.15; 0.25; 0.25; 0.27; 0.28; 0.56; 0.68 | 0.27 | 1 |
|
Apples, juice (→ extrapolated to pears) |
3 | < 0.01; 0.03; 0.2 | 0.03 | 1 |
|
Apples, wet pomace (→ extrapolated to pears) |
3 | 1.36; 1.52; 1.9 | 1.5 | 1 |
| Bananas, peeled | 4c | 0.05; 0.09; 0.14; 0.16 | 0.11 | 1 |
| Potatoes, unpeeled and boiled | 4 | 0.12; 0.15; 0.28; 0.50 | 0.22 | 1 |
| Potatoes, peeled and boiled | 2d | < 0.01; < 0.01 | 0.01 | 1 |
| Potatoes, fried | 4 | < 0.01; < 0.01; < 0.01; 0.02 | 0.01 | 1 |
| Melons, peeled | 6 | 0.06e; 0.07e; 0.11; 0.13; 0.18e; 0.20 | 0.12 | 1 |
| Indicative processing factors (limited data set) | ||||
|
Apples, dry pomace (→ extrapolated to pears) |
2 | 3.39; 3.94 | 3.7 | 1 |
| Apples, sauce | 2 | 0.07; 0.31 | 0.19 | 1 |
| Potatoes, unpeeled and microwaved | 2 | 1.09; 1.58 | 1.3 | 1 |
| Potatoes, crisps | 2 | 0.02; 0.02 | 0.02 | 1 |
| Potatoes, granules or flakes | 2 | 0.01; 0.01 | 0.01 | 1 |
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
Based on residue trials compliant with the critical GAP (drenching 50 g/hl; WHP 0 day, including replicates) performed on oranges (n = 15 ranging from 0.01 to 0.28) and mandarins (n = 16 ranging from 0.01 to 0.25) (Netherlands, 2015; Spain, 2016) and considering the processing factors derived during the peer review on oranges (0.08), lemons (0.04; 0.04; 0.05) and grapefruits (0.13) (EFSA, 2010).
Based on sampling performed at PHI 28 days (Greece, 2016).
Although only two studies are available, this PF is considered robust because the two available data show that significant residues are not expected in boiled potatoes (unpeeled and peeled).
Based on residue trials performed with a higher application rate compared to GAP (Belgium, 1996).
B.2. Residues in livestock
| Relevant groups | Dietary burden expressed in | Most critical dieta | Most critical commoditya |
Trigger exceeded (Y/N) |
|||
|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | ||||||
| Med. | Max. | Med. | Max. | ||||
| Scenario EU1: considering all authorised GAPs | |||||||
|
Cattle (all diets) |
5.25 | 5.36 | 180.6b | 183.6b | Cattle (dairy) | Potato, process waste | Yes |
|
Cattle (dairy only) |
5.25 | 5.36 | 136.5 | 139.4 | Cattle (dairy) | Potato, process waste | Yes |
|
Sheep (all diets) |
6.02 | 6.12 | 180.6 | 183.6 | Sheep (ram/ewe) | Potato, process waste | Yes |
|
Sheep (ewe only) |
6.02 | 6.12 | 180.6 | 183.6 | Sheep (ram/ewe) | Potato, process waste | Yes |
|
Swine (all diets) |
2.19 | 2.30 | 95.0 | 99.8 | Swine (breeding) | Potato, process waste | Yes |
|
Poultry (all diets) |
1.71 | 1.78 | 24.2 | 25.2 | Poultry (broiler) | Potato, dried pulp | Yes |
|
Poultry (layer only) |
1.27 | 1.33 | 18.5 | 19.5 | Poultry (layer) | Potato, dried pulp | Yes |
| Scenario EU2: excluding GAPs on apples/pears and considering fall‐back GAPs on potatoes, oranges and grapefruits | |||||||
|
Cattle (all diets) |
0.075 | 0.075 | 1.96c | 1.96c | Cattle (dairy) | Lemons, dried pulp | Yes |
|
Cattle (dairy only) |
0.075 | 0.075 | 1.96 | 1.96 | Cattle (dairy) | Lemons, dried pulp | Yes |
|
Sheep (all diets) |
0.002 | 0.002 | 0.05 | 0.05 | Sheep (ram/ewe) | Potato, process waste | No |
|
Sheep (ewe only) |
0.002 | 0.002 | 0.05 | 0.05 | Sheep (ram/ewe) | Potato, process waste | No |
|
Swine (all diets) |
0.034 | 0.034 | 1.48 | 1.48 | Swine (breeding) | Lemons, dried pulp | Yes |
|
Poultry (all diets) |
0.001 | 0.001 | 0.02 | 0.02 | Poultry (turkey) | Potato, culls | No |
|
Poultry (layer only) |
0.001 | 0.001 | 0.02 | 0.02 | Poultry (layer) | Potato, culls | No |
bw: body weight; DM: dry matter; GAP: good agricultural practice.
Calculated for the maximum dietary burden.
The highest dietary burdens expressed in mg/kg DM result from beef cattle.
The highest dietary burdens expressed in mg/kg DM result from dairy cattle.
B.2.1. Nature of residues and methods of analysis in livestock
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
|
Livestock (available studies) |
Animal |
Dose (mg/kg bw per day) |
Duration (days) |
N rate/comment |
|---|---|---|---|---|
| Laying hen | 4.6 | 10 | 2.6N | |
| Lactating goat | 10 | 3 | 1.6N/compared to sheep | |
|
Sources: Belgium, 1996; Netherlands, 2015 For laying hens, the study reported in Belgium, 1996 was not deemed sufficient; additional analyses were provided by the RMS in Netherlands, 2015. Even though further detailed results were provided by the RMS in its evaluation report (Netherlands, 2015), EFSA is of the opinion that an additional study would still be needed to fully depict the metabolic pathway in poultry | ||||
| Time needed to reach a plateau concentration in milk and eggs (days) | Not reported |
| Metabolism in rat and ruminant similar (Yes/No) | Yes |
| Animal residue definition for monitoring (RD‐Mo) | Sum of imazalil and metabolite FK‐772 (any ratio of constituent isomers)a, expressed as imazalil (tentative) |
| Animal residue definition for risk assessment (RD‐RA) | Sum of imazalil and all identified/characterised metabolites (tentative) |
| Conversion factor (monitoring to risk assessment) |
Muscle:3b Fat:11b Liver:4b Kidney:3b Milk:12b Eggs:10c |
| Fat soluble residues (Yes/No) | No |
| Methods of analysis for monitoring of residues (analytical technique, crop groups, LOQs) |
HPLC–MS/MS (Netherlands, 2015):
|
bw: body weight; HPLC–MS/MS: high performance liquid chromatography with tandem mass spectrometry; ILV: independent laboratory validation; LOQ: limit of quantification.
It is highlighted that both parent and metabolite FK‐772 consist of two isomers. Therefore, the mention “any ratio of constituent isomers” applies to both compounds.
Tentative CF based on the metabolism study performed on goat; also applicable to poultry muscle, fat and liver.
Tentative CF considering that the sum of imazalil and its metabolite FK‐772 represents at least 10% of the TRR in eggs.
B.2.1.2. Stability of residues in livestock
| Animal products (available studies) | Animal | Commodity | T (°C) | Stability (Months/years) |
|---|---|---|---|---|
| – | Tissues | – | – | |
| – | Milk | – | – | |
| – | Egg | – | – | |
| No studies available but required | ||||
B.2.2. Magnitude of residues in livestock
B.2.2.1. Summary of the residue data from livestock feeding studies
Scenario EU1: considering all authorised GAPs
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | MRL proposal (mg/kg) | CFc | ||
|---|---|---|---|---|---|---|
| Mean | Highest | STMRa (mg/kg) | HRb (mg/kg) | |||
|
Cattle (all diets) Closest feeding level (4.95 mg/kg bw; 0.9N rate)d |
||||||
| Muscle | 0.02 | 0.03 | < 0.02 | 0.03 | 0.03e (tentative) | 3 |
| Fat | 0.02 | 0.04 | 0.04 | 0.06 | 0.07e (tentative) | 11 |
| Liver | 2.4 | 3.1 | 2.7 | 3.4 | 4e (tentative) | 4 |
| Kidney | 0.39 | 0.46 | 0.46 | 0.59 | 0.6e (tentative) | 3 |
|
Cattle (dairy only) Closest feeding level (4.95 mg/kg bw; 0.9N rate)d |
||||||
| Milkf | 0.02 | n.a. | 0.02 | 0.03 | 0.03e (tentative) | 12 |
|
Sheep (all diets) g Closest feeding level (4.95 mg/kg bw; 0.81 N rate)d |
||||||
| Muscle | 0.02 | 0.03 | 0.02 | 0.03 | 0.04e (tentative) | 3 |
| Fat | 0.02 | 0.04 | 0.05 | 0.07 | 0.08e (tentative) | 11 |
| Liver | 2.4 | 3.1 | 3.2 | 4.0 | 5e (tentative) | 4 |
| Kidney | 0.39 | 0.46 | 0.54 | 0.70 | 0.8e (tentative) | 3 |
|
Sheep (dairy only) g Closest feeding level (4.95 mg/kg bw; 0.81 N rate)d |
||||||
| Milkf | 0.02 | n.a. | 0.03 | 0.03 | 0.03e (tentative) | 12 |
|
Swine g Closest feeding level (4.95 mg/kg bw; 2 N rate)d |
||||||
| Muscle | 0.02 | 0.03 | < 0.02 | 0.02 | 0.03e (tentative) | 3 |
| Fat | 0.02 | 0.04 | < 0.02 | < 0.02 | 0.02* e (tentative) | 11 |
| Liver | 2.4 | 3.1 | 0.77 | 1.0 | 1.5e (tentative) | 4 |
| kidney | 0.39 | 0.46 | 0.13 | 0.16 | 0.2e (tentative) | 3 |
|
Poultry (all diets) Closest feeding level (0.13 mg/kg bw; 0.07 N rate)d |
||||||
| Muscle | n.r. | < 0.001 | – | – | No proposalh | 3 |
| Fat | n.r. | < 0.003 | – | – | No proposalh | 11 |
| Liver | n.r. | 0.005 | – | – | No proposalh | 4 |
|
Poultry (layer only) Closest feeding level (0.13 mg/kg bw; 0.10 N rate)d |
||||||
| Egg | n.r. | < 0.002 | – | – | No proposalh | 10 |
GAP: good agricultural practice; bw: body weight; MRL: maximum residue level; n.a.: not applicable; n.r.: not reported.
* Indicates that the MRL is proposed at the limit of quantification.
Mean residue levels, recalculated at the 1N rate for the median dietary burden.
Highest residue levels, recalculated at the 1N rate for the maximum dietary burden.
Conversion factor for risk assessment derived from the metabolism studies.
Closest feeding level and N dose rate related to the maximum dietary burden.
MRL proposal is tentative because several data gaps were identified (the toxicological properties of metabolite FK‐772, the storage conditions of the samples of the livestock feeding study, storage stability of imazalil and metabolite FK‐772 in livestock matrices, tentative CF).
Highest residue level from day 1 to day 28 (daily mean of 3 cows).
Since extrapolation from cattle to other ruminants and swine is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in sheep and swine.
The available feeding study does not allow deriving MRLs and risk assessment values because the maximal investigated feeding level (0.13 mg/kg bw) is more than 10 times lower than the maximum dietary burden calculated under scenario EU1 (1.78 mg/kg bw per day). Moreover, the metabolite FK‐772 was not analysed in the study.
Scenario EU2: excluding GAPs on apples/pears and considering fall‐back GAPs on potatoes, oranges and grapefruits.
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | MRL proposal (mg/kg) | CF c | ||
|---|---|---|---|---|---|---|
| Mean | Highest | STMRa (mg/kg) | HRb (mg/kg) | |||
|
Cattle (all diets) Closest feeding level (1.65 mg/kg bw; 20N rate)d |
||||||
| Muscle | 0.01 | 0.02 | < 0.02 | < 0.02 | 0.02* e (tentative) | 3 |
| Fat | 0.01 | 0.02 | < 0.02 | < 0.02 | 0.02* e (tentative) | 11 |
| Liver | 0.44 | 0.55 | 0.02 | 0.03 | 0.03e (tentative) | 4 |
| Kidney | 0.08 | 0.09 | < 0.02 | <0.02 | 0.02* e (tentative) | 3 |
|
Cattle (dairy only) Closest feeding level (1.65 mg/kg bw; 20N rate)d |
||||||
| Milkf | 0.01 | n.a. | < 0.02 | < 0.02 | 0.02* e (tentative) | 12 |
| Sheep (all diets) – MRLs are not required since the dietary burden is below the trigger value | ||||||
| Muscle | – | – | – | – | – | – |
| Fat | – | – | – | – | – | – |
| Liver | – | – | – | – | – | – |
| Kidney | – | – | – | – | – | – |
| Sheep (dairy only) – MRLs are not required since the dietary burden is below the trigger value | ||||||
| Milk | – | – | – | – | – | – |
|
Swine g Closest feeding level (1.65 mg/kg bw; 46 N rate)d |
||||||
| Muscle | 0.01 | 0.02 | < 0.02 | < 0.02 | 0.02* e (tentative) | 3 |
| Fat | 0.01 | 0.02 | < 0.02 | < 0.02 | 0.02* e (tentative) | 11 |
| Liver | 0.44 | 0.55 | < 0.02 | < 0.02 | 0.02* e (tentative) | 4 |
| kidney | 0.08 | 0.09 | < 0.02 | < 0.02 | 0.02* e (tentative) | 3 |
| Poultry (all diets) ‐ MRLs are not required since the dietary burden is below the trigger value | ||||||
| Muscle | – | – | – | – | – | – |
| Fat | – | – | – | – | – | – |
| Liver | – | – | – | – | – | – |
| Poultry (layer only) ‐ MRLs are not required since the dietary burden is below the trigger value | ||||||
| Egg | – | – | – | – | – | – |
GAP: good agricultural practice; bw: body weight; MRL: maximum residue level; n.a.: not applicable; n.r.: not reported.
* Indicates that the MRL is proposed at the limit of quantification.
Mean residue levels, recalculated at the 1N rate for the median dietary burden
Highest residue levels, recalculated at the 1N rate for the maximum dietary burden.
Conversion factor for risk assessment derived from the metabolism studies.
Closest feeding level and N dose rate related to the maximum dietary burden.
MRL proposal is tentative because several data gaps were identified (the toxicological properties of metabolite FK‐772, the storage conditions of the samples of the livestock feeding study, storage stability of imazalil and metabolite FK‐772 in livestock matrices, tentative CF).
Highest residue level from day 1 to day 28 (daily mean of 3 cows).
Since extrapolation from cattle to other ruminants and swine is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in swine.
B.3. Consumer risk assessment
B.3.1. Consumer risk assessment without consideration of the existing CXLs
| ADI | 0.025 mg/kg bw per day (EFSA, 2010) |
| Highest IEDI, according to EFSA PRIMo |
Scenario EU1: 130.1% ADI (NL, child) Scenario EU2: 40.7% ADI (FR, toddler) |
| Assumptions made for the calculations |
Scenario EU1: The calculation is based on the median residue levels in the raw agricultural commodities, except for bananas, citrus fruits and melons. For bananas, the relevant peeling factor was applied. For citrus fruits, the median value is directly derived from residue levels measured in pulp. For melons, sweet peppers/bell peppers and for commodities of poultry origin, where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation. For melons, the relevant peeling factor was applied to the EU MRL For all plant commodities, the conversion factor from enforcement to risk assessment (CF) of 1 is considered. For animal commodities, the indicative CF derived from the metabolism studies are taken into account The contributions of commodities where no GAP was reported in the framework of this review were not included in the calculation Scenario EU2: Fall‐back GAPs were considered for oranges and grapefruits (post‐harvest waxing) and potatoes (seed treatment). For apples and pears however, no fall‐back option was identified (all other GAPs do also lead to exceedances of the ARfD). Residue levels in livestock commodities were recalculated accordingly. All other input values remain unchanged |
| ARfD | 0.05 mg/kg bw (EFSA, 2010) |
| Highest IESTI, according to EFSA PRIMo |
Scenario EU1: 1414.6% ARfD (potatoes) 685.8% ARfD (apples) 637.5% ARfD (pears) 221.2% ARfD (bovine liver) 183.0% ARfD (oranges) 123.1% ARfD (grapefruits) Scenario EU2: 76.8% ARfD (mandarins) |
| Assumptions made for the calculations |
Scenario EU1: The calculation is based on the highest residue levels in the raw agricultural commodities, except for bananas, melons and citrus fruits. For bananas, the relevant peeling factor was applied. For citrus fruits, the highest value is directly derived from residue levels measured in pulp. For melons, sweet peppers/bell peppers and for commodities of poultry origin, where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation. For melons, the relevant peeling factor was applied to the EU MRL. For all plant commodities, the conversion factor from enforcement to risk assessment (CF) of 1 is considered. For animal commodities, the indicative CF derived from the metabolism studies are taken into account The contributions of commodities where no GAP was reported in the framework of this review were not included in the calculation Scenario EU2: Fall‐back GAPs were considered for oranges and grapefruits (post‐harvest waxing) and potatoes (seed treatment). For apples and pears however, no fall‐back option was identified (all other GAPs do also lead to exceedances of the ARfD). Residue levels in livestock commodities were recalculated accordingly. All other input values remain unchanged |
CXL: codex maximum residue limit; ADI: acceptable daily intake; bw: body weight; IEDI: international estimated daily intake; PRIMo: Pesticide Residues Intake Model; MRL: maximum residue level; GAP: good agricultural practice; ARfD: acute reference dose; IESTI: international estimated short‐term intake.
B.3.2. Consumer risk assessment with consideration of the existing CXLs
| ADI | 0.025 mg/kg bw per day (EFSA, 2010) |
| Highest IEDI, according to EFSA PRIMo |
Scenario CX1: 408% ADI (DE, child) Scenario CX2: 45.7% ADI (FR, toddler) |
| Assumptions made for the calculations |
Scenario CX1: For those commodities having a CXL higher than the EU MRL proposal, median residue levels applied in the EU2 scenario were replaced by the median residue levels derived by JMPR. When the median residue level derived by JMPR was not available, the CXL was considered for an indicative calculation. The peeling factor was not considered for citrus fruits and bananas. The same conversion factors from enforcement to risk assessment (CF) as applied in the EU scenario were considered (CF of 1 for plant commodities and indicative CF derived from the metabolism studies for animal commodities) Scenario CX2: The CXLs for potatoes, oranges, grapefruit, pome fruits and persimmon were excluded from the calculation. All other input values remain unchanged. |
| ARfD | 0.05 mg/kg bw (EFSA, 2010) |
| Highest IESTI, according to EFSA PRIMo |
Scenario CX1: 1538% ARfD (potatoes) 1326% ARfD (oranges) 980% ARfD (apples) 911% ARfD (pears) 892% ARfD (grapefruits) 147% ARfD (quinces) 131% ARfD (persimmon) 121% ARfD (medlar) Scenario CX2: 76.8% ARfD (mandarins) |
| Assumptions made for the calculations |
Scenario CX1: For those commodities having a CXL higher than the EU MRL proposal, highest residue levels applied in the EU2 scenario were replaced by the median residue levels derived by JMPR. When the highest residue level derived by JMPR was not available, the CXL was considered for an indicative calculation. The peeling factor was not considered for citrus fruits and bananas. The same conversion factors from enforcement to risk assessment (CF) as applied in the EU scenario were considered (CF of 1 for plant commodities and indicative CF derived from the metabolism studies for animal commodities) Scenario CX2: The CXLs for potatoes, oranges, grapefruits, pome fruits and persimmon were excluded from the calculation. All other input values remain unchanged. |
CXL: codex maximum residue limit; ADI: acceptable daily intake; bw: body weight; IEDI: international estimated daily intake; PRIMo: Pesticide Residues Intake Model; MRL: maximum residue level; GAP: good agricultural practice; ARfD: acute reference dose; IESTI: international estimated short‐term intake.
B.4. Proposed MRLs
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
|
Enforcement residue definition (existing): imazalil Enforcement residue definition 1 (proposed): imazalil (any ratio of constituent isomers) | |||||
| 110010 | Grapefruits | 5 | 5 | 4 | Further consideration neededa |
| 110020 | Oranges | 5 | 5 | 4 | Further consideration neededa |
| 110030 | Lemons | 5 | 5 | 6 | Further consideration neededb |
| 110040 | Limes | 5 | 5 | 6 | Further consideration neededb |
| 110050 | Mandarins | 5 | 5 | 6 | Further consideration neededb |
| 130010 | Apples | 2 | 5 | – | Further consideration neededc |
| 130020 | Pears | 2 | 5 | – | Further consideration neededc |
| 130030 | Quinces | 2 | 5 | – | Further consideration neededd |
| 130040 | Medlar | 5 | 5 | – | Further consideration neededd |
| 130050 | Loquat | 5 | 5 | – | Further consideration neededd |
| 152000 | Strawberries | 0.05* | 2 | 2 | Further consideration needede |
| 153010 | Blackberries | 0.05* | 2 | 2 | Further consideration needede |
| 153030 | Raspberries | 0.05* | 2 | 2 | Further consideration needede |
| 161060 | Persimmon | 0.05* | 2 | – | Further consideration neededf |
| 163020 | Bananas | 2 | 2 | 5 | Further consideration neededb |
| 211000 | Potatoes | 3 | 5 | 0.01* | Recommendedg |
| 231010 | Tomatoes | 0.5 | – | 0.3 | Recommendedh |
| 231020 | Sweet peppers/bell peppers | 0.05* | – | 0.05 | Further consideration neededi |
| 232010 | Cucumbers | 0.2 | 0.5 | 0.5 | Recommendedj |
| 232020 | Gherkins | 0.2 | 0.5 | 0.5 | Recommendedj |
| 232030 | Courgettes | 0.2 | – | 0.1 | Further consideration neededk |
| 233010 | Melons | 2 | 2 | 2 | Further consideration neededl |
| 500010 | Barley grains | 0.05* | – | 0.01* | Recommendedh |
| 500050 | Oat grains | 0.05* | – | 0.01* | Recommendedh |
| 500070 | Rye grains | 0.05* | – | 0.01* | Recommendedh |
| 500090 | Wheat grains | 0.05* | 0.01* | 0.01* | Recommendedm |
| – | Other commodities of plant origin | See Reg. 750/2010 | – | – | Further consideration neededn |
|
Enforcement residue definition (existing): imazalil Enforcement residue definition 2 (proposed): sum of imazalil and metabolite FK‐772 (any ratio of constituent isomers), expressed as imazalil | |||||
| 1011010 | Swine muscle | 0.05* | – | 0.02* | Further consideration neededk |
| 1011020 | Swine fat tissue | 0.05* | – | 0.02* | Further consideration neededk |
| 1011030 | Swine liver | 0.05* | – | 0.02* | Further consideration neededk |
| 1011040 | Swine kidney | 0.05* | – | 0.02* | Further consideration neededk |
| 1012010 | Bovine muscle | 0.05* | – | 0.02* | Further consideration neededk |
| 1012020 | Bovine fat tissue | 0.05* | – | 0.02* | Further consideration neededk |
| 1012030 | Bovine liver | 0.05* | – | 0.03 | Further consideration neededk |
| 1012040 | Bovine kidney | 0.05* | – | 0.02* | Further consideration neededk |
| 1015010 | Equine muscle | 0.05* | – | 0.02* | Further consideration neededk |
| 1015020 | Equine fat tissue | 0.05* | – | 0.02* | Further consideration neededk |
| 1015030 | Equine liver | 0.05* | – | 0.03 | Further consideration neededk |
| 1015040 | Equine kidney | 0.05* | – | 0.02* | Further consideration neededk |
| 1020010 | Cattle milk | 0.05* | – | 0.02* | Further consideration neededk |
| 1020040 | Horse milk | 0.05* | – | 0.02* | Further consideration neededk |
| – | Other commodities of animal origin | See Reg. 750/2010 | – | – | Further consideration neededn |
MRL: maximum residue level; CXL: codex maximum residue limit.
* Indicates that the MRL is set/proposed at the limit of quantification.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; CXL is higher but it is also not sufficiently supported by data and a risk to consumers cannot be excluded (combination E‐IV in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; existing CXL is covered by the tentative MRL (combination E‐III in Appendix E).
GAP evaluated at EU level is fully supported by data but a risk to consumers cannot be excluded; CXL is not sufficiently supported by data and a risk to consumers can also not be excluded. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination F‐IV in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; CXL is not sufficiently supported by data and a risk to consumers cannot be excluded. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐IV in Appendix E).
MRL is derived from the existing CXL, which is not sufficiently supported by data but for which no risk to consumers is identified; there are no relevant authorisations or import tolerances reported at EU level (combination A‐V in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; CXL is supported by data but a risk to consumers cannot be excluded. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐VI in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; CXL is higher but it is not sufficiently supported by data and a risk to consumers cannot be excluded (combination G‐IV in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination G‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL; no CXL is available (combination C‐I in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is not fully supported by data, leads to a lower tentative MRL (combination E‐VII in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; no CXL is available (combination E‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL; existing CXL is covered by the existing EU MRL (combination C‐III in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; existing CXL is covered by the recommended MRL (combination G‐III in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
PRIMo(EU1)
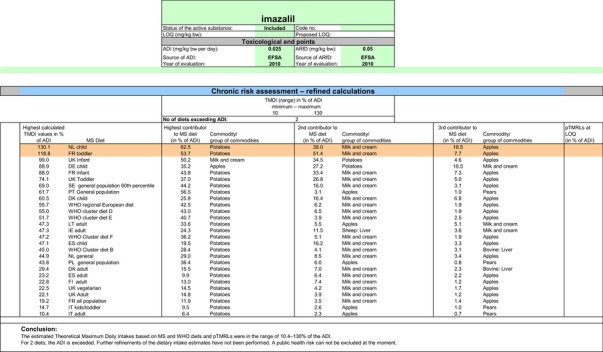
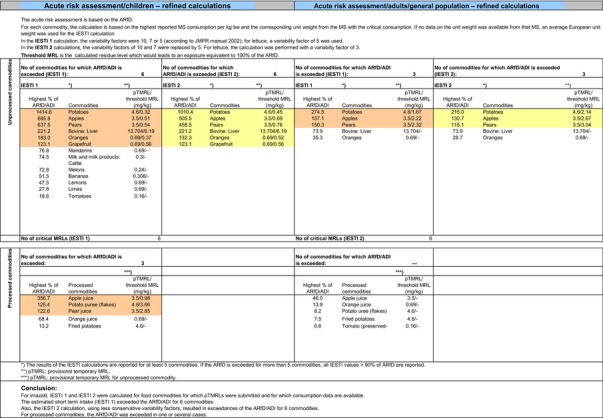
PRIMo(EU2)
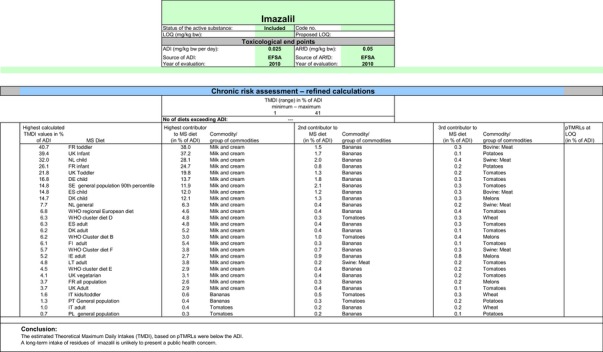
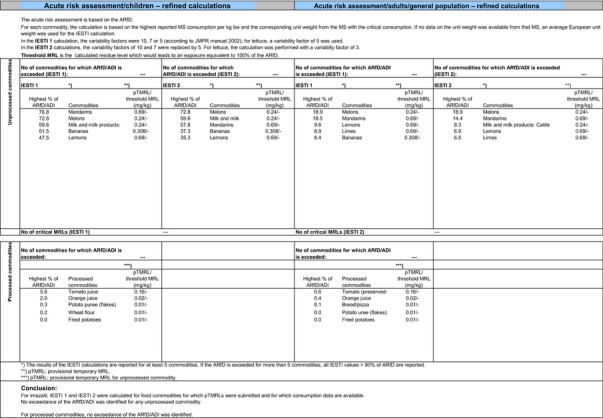
PRIMo(CXL1)
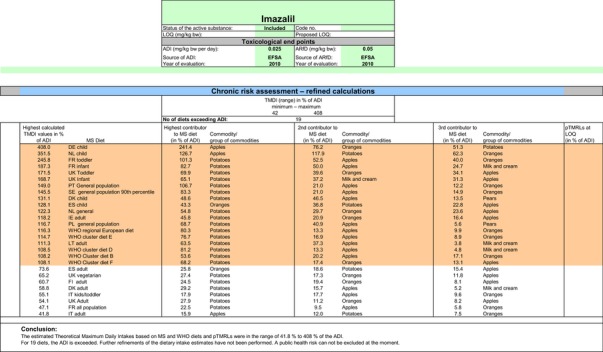
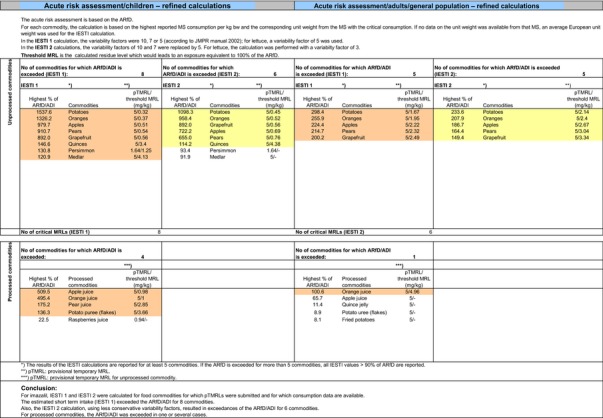
PRIMo(CXL2)
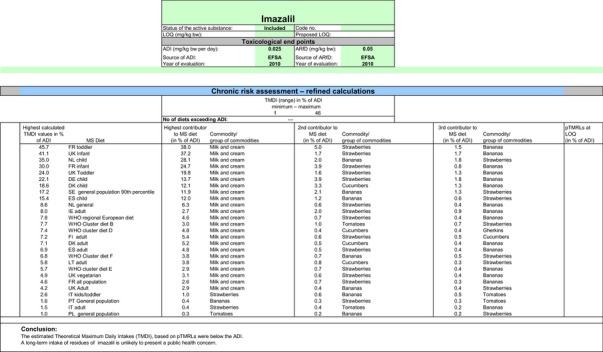
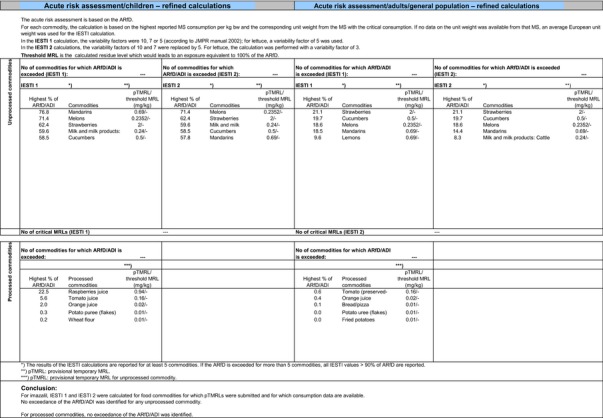
Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Scenario EU1: considering all authorised GAPs | ||||
| Citrus fruits, dried pulp | 8.8 | STMR × PF (4.2) | 8.8 | STMR × PF (4.2) |
| Apple, wet pomace | 1.1 | STMR × PF (1.5) | 1.1 | STMR × PF (1.5) |
| Potato, culls | 2.7 | STMR | 4.6 | HR |
| Potato, process waste | 53 | STMR × 20a | 53 | STMR × 20a |
| Potato, dried pulp | 101 | STMR × 38a | 101 | STMR × 38a |
| Brewer's grain, dried | 0.01* | STMRb | 0.01* | STMRb |
| Wheat, distiller's grain (dry) | 0.01* | STMRb | 0.01* | STMRb |
| Wheat gluten, meal | 0.01* | STMRb | 0.01* | STMRb |
| Wheat, milled by‐products | 0.01* | STMRb | 0.01* | STMRb |
| Small grain cereals, grain | 0.01* | STMR | 0.01* | STMR |
| Small grain cereals, straw | 0.01* | STMR | 0.01* | HR |
| Scenario EU2: excluding GAPs on apples/pears and considering fall‐back GAPs on potatoes (and oranges/grapefruits) | ||||
| Grapefruits, dried pulp | 6.3 | STMR × PF (4.2) | 6.3 | STMR × PF (4.2) |
| Oranges, dried pulp | 6.3 | STMR × PF (4.2) | 6.3 | STMR × PF (4.2) |
| Lemons, dried pulp | 8.8 | STMR × PF (4.2) | 8.8 | STMR × PF (4.2) |
| Lime, dried pulp | 8.8 | STMR × PF (4.2) | 8.8 | STMR × PF (4.2) |
| Mandarin, dried pulp | 8.8 | STMR × PF (4.2) | 8.8 | STMR × PF (4.2) |
| Potato, culls | 0.01* | STMR | 0.01* | HR |
| Potato, process waste | 0.01* | STMRc | 0.01* | HRc |
| Potato, dried pulp | 0.01* | STMRc | 0.01* | HRc |
| Brewer's grain, dried | 0.01* | STMRb | 0.01* | STMRb |
| Wheat, distiller's grain (dry) | 0.01* | STMRb | 0.01* | STMRb |
| Wheat gluten, meal | 0.01* | STMRb | 0.01* | STMRb |
| Wheat, milled by‐products | 0.01* | STMRb | 0.01* | STMRb |
| Small grain cereals, grain | 0.01* | STMR | 0.01* | STMR |
| Small grain cereals, straw | 0.01* | STMR | 0.01* | HR |
GAP: good agricultural practice; STMR: supervised trials median residue; HR: highest residue; PF: processing factor.
* Indicates that the input value is proposed at the limit of quantification.
In scenario EU1 (with critical GAP: ware potatoes), in the absence of processing factors supported by data, default processing factors of 20 (process waste of potatoes) and 38 (potatoes, dried pulp) were, respectively, included in the calculation to consider the potential concentration of residues in these commodities.
For processed commodities of cereals (brewer's and distiller's grain, gluten and milled by‐products), no default processing factor was applied because imazalil is applied as a seed treatment and residues are expected to be below the LOQ. Concentration of residues in these commodities is therefore not expected.
In scenario EU2 (with fall‐back GAP: seed potatoes), default processing factors were not applied to processed commodities of potatoes (process waste and dried pulp) because imazalil is applied as a seed treatment and residues are expected to be below the LOQ. Concentration of residues in these commodities is therefore not expected.
D.2. Consumer risk assessment without consideration of the existing CXLs
D.2.1. Scenario EU1: considering all authorised GAPs
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition 1: sum of imazalil and R014821, expressed as imazalil | ||||
|
Grapefruits Oranges Lemons Limes Mandarins |
0.05 | STMRMo (pulp) × CF (tentative)a | 0.69 | HRMo (pulp) × CF (tentative)a |
|
Apples Pears |
0.73 | STMRMo × CF | 3.5 | HRMo × CF |
| Bananas | 0.29 | STMRMo × PF × CF (tentative) | 0.31 | HRMo × PF × CF (tentative) |
| Potatoes | 2.65 | STMRMo × CF (tentative) | 4.6 | HRMo × CF (tentative) |
| Tomatoes | 0.09 | STMRMo × CF | 0.16 | HRMo × CF |
| Sweet peppers/bell peppers | 0.05 | EU MRL × CFb | 0.05 | EU MRL × CFb |
|
Cucumbers Gherkins Courgettes |
0.02 | STMRMo × CF (tentative) | 0.05 | HRMo × CF (tentative) |
| Melons | 0.24 | EU MRL × CF × PFb | 0.24 | EU MRL × CF × PFb |
| Small grain cereals | 0.01* | STMRMo × CF | 0.01* | HRMo × CF |
| Risk assessment residue definition 2: sum of imazalil and all identified/characterised metabolites | ||||
| Swine meat | 0.06 | STMRMo muscle × CF (tentative) | 0.07 | HRMo muscle × CF (tentative) |
| Swine fat tissue | 0.22 | STMRMo × CF (tentative) | 0.22 | HRMo × CF (tentative) |
| Swine liver | 3.1 | STMRMo × CF (tentative) | 4.2 | HRMo × CF (tentative) |
| Swine kidney | 0.38 | STMRMo × CF (tentative) | 0.49 | HRMo × CF (tentative) |
| Bovine meat | 0.06 | STMRMo muscle × CF (tentative) | 0.09 | HRMo muscle × CF (tentative) |
| Bovine fat tissue | 0.43 | STMRMo × CF (tentative) | 0.67 | HRMo × CF (tentative) |
| Bovine liver | 10.7 | STMRMo × CF (tentative) | 13.7 | HRMo × CF (tentative) |
| Bovine kidney | 1.4 | STMRMo × CF (tentative) | 1.8 | HRMo × CF (tentative) |
| Sheep meat | 0.06 | STMRMo muscle × CF (tentative) | 0.09 | HRMo muscle × CF (tentative) |
| Sheep fat tissue | 0.52 | STMRMo × CF (tentative) | 0.80 | HRMo × CF (tentative) |
| Sheep liver | 12.7 | STMRMo × CF (tentative) | 16.1 | HRMo × CF (tentative) |
| Sheep kidney | 1.6 | STMRMo × CF (tentative) | 2.1 | HRMo × CF (tentative) |
| Goat meat | 0.06 | STMRMo muscle × CF (tentative) | 0.09 | HRMo muscle × CF (tentative) |
| Goat fat tissue | 0.52 | STMRMo × CF (tentative) | 0.80 | HRMo × CF (tentative) |
| Goat liver | 12.7 | STMRMo × CF (tentative) | 16.1 | HRMo × CF (tentative) |
| Goat kidney | 1.6 | STMRMo × CF (tentative) | 2.1 | HRMo × CF (tentative) |
| Equine meat | 0.06 | STMRMo muscle × CF (tentative) | 0.09 | HRMo muscle × CF (tentative) |
| Equine fat tissue | 0.43 | STMRMo × CF (tentative) | 0.67 | HRMo × CF (tentative) |
| Equine liver | 10.7 | STMRMo × CF (tentative) | 13.7 | HRMo × CF (tentative) |
| Equine kidney | 1.4 | STMRMo × CF (tentative) | 1.8 | HRMo × CF (tentative) |
| Poultry meat | 0.15 | EU MRL × CFc | 0.15 | EU MRL × CFc |
| Poultry fat tissue | 0.55 | EU MRL × CFc | 0.55 | EU MRL × CFc |
| Poultry liver | 0.20 | EU MRL × CFc | 0.20 | EU MRL × CFc |
| Cattle milk | 0.29 | STMRMo × CF (tentative) | 0.30 | HRMo × CF (tentative) |
| Sheep milk | 0.32 | STMRMo × CF (tentative) | 0.32 | HRMo × CF (tentative) |
| Goat milk | 0.32 | STMRMo × CF (tentative) | 0.32 | HRMo × CF (tentative) |
| Horse milk | 0.29 | STMRMo × CF (tentative) | 0.30 | HRMo × CF (tentative) |
| Birds eggs | 0.50 | EU MRL × CFc | 0.50 | EU MRL × CFc |
GAP: good agricultural practice; STMR: supervised trials median residue; CF: conversion factor for enforcement residue definition to risk assessment residue definition; HR: highest residue; PF: processing factor; MRL: maximum residue level; Mo: monitoring.
* Indicates that the input value is proposed at the limit of quantification.
For citrus fruits, the median and highest residue levels measured in pulp are directly considered for the risk assessment. The theoretical calculation considering the peeling factor is not considered reliable in this case.
In the absence of supporting data, the existing EU MRL multiplied by the conversion factor for risk assessment of 1 (and by PF if relevant) is used for indicative exposure calculations.
In the absence of supporting data, the existing EU MRL multiplied by the conversion factors for risk assessment derived from the metabolism study performed on laying hens is used for indicative exposure calculations.
D.2.2. Scenario EU2: excluding GAPs on apples/pears and considering fall‐back GAPs on potatoes, oranges and grapefruits
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of imazalil and R014821, expressed as imazalil | ||||
|
Grapefruits Oranges |
0.01* | STMRMo (pulp) × CF (fall‐back; tentative)a , b | 0.02 | HRMo (pulp) × CF (fall‐back; tentative)a , b |
|
Lemons Limes Mandarins |
0.05 | STMRMo (pulp) × CF (tentative)b | 0.69 | HRMo (pulp) × CF (tentative)b |
|
Apples Pears |
– | No fall‐back option availablec | – | No fall‐back option availablec |
| Bananas | 0.29 | STMRMo × PF × CF (tentative) | 0.31 | HRMo × PF × CF (tentative) |
| Potatoes | 0.01* | STMRMo × CF (fall‐back)a | 0.01* | HRMo × CF (fall‐back)a |
| Tomatoes | 0.09 | STMRMo × CF | 0.16 | HRMo × CF |
| Sweet peppers/bell peppers | 0.05 | EU MRL × CFd | 0.05 | EU MRL × CFd |
|
Cucumbers Gherkins Courgettes |
0.02 | STMRMo × CF (tentative) | 0.05 | HRMo × CF (tentative) |
| Melons | 0.24 | EU MRL × CF × PFd | 0.24 | EU MRL × CF × PFd |
| Small grain cereals | 0.01* | STMRMo × CF | 0.01* | HRMo × CF |
| Risk assessment residue definition 2: sum of imazalil and all identified/characterised metabolites | ||||
| Swine meat | 0.06 | STMRMo muscle × CF (tentative)e | 0.06 | HRMo muscle × CF (tentative)e |
| Swine fat tissue | 0.22 | STMRMo × CF (tentative)e | 0.22 | HRMo × CF (tentative)e |
| Swine liver | 0.08 | STMRMo × CF (tentative)e | 0.08 | HRMo × CF (tentative)e |
| Swine kidney | 0.06 | STMRMo × CF (tentative)e | 0.06 | HRMo × CF (tentative)e |
| Bovine meat | 0.06 | STMRMo muscle × CF (tentative)e | 0.06 | HRMo muscle × CF (tentative)e ) |
| Bovine fat tissue | 0.22 | STMRMo × CF (tentative)e | 0.22 | HRMo × CF (tentative)e |
| Bovine liver | 0.08 | STMRMo × CF (tentative)e | 0.10 | HRMo × CF (tentative)e |
| Bovine kidney | 0.06 | STMRMo × CF (tentative)e | 0.06 | HRMo × CF (tentative)e |
| Sheep meat | – | No MRL needede | – | No MRL needede |
| Sheep fat tissue | – | No MRL needede | – | No MRL needede |
| Sheep liver | – | No MRL needede | – | No MRL needede |
| Sheep kidney | – | No MRL needede | – | No MRL needede |
| Goat meat | – | No MRL needede | – | No MRL needede |
| Goat fat tissue | – | No MRL needede | – | No MRL needede |
| Goat liver | – | No MRL needede | – | No MRL needede |
| Goat kidney | – | No MRL needede | – | No MRL needede |
| Equine meat | 0.06 | STMRMo muscle × CF (tentative)e | 0.06 | (0.8 × HRMo muscle + 0.2 × HRMo fat) × CF (tentative)e |
| Equine fat tissue | 0.22 | STMRMo × CF (tentative)e | 0.22 | HRMo × CF (tentative)e |
| Equine liver | 0.08 | STMRMo × CF (tentative)e | 0.10 | HRMo × CF (tentative)e |
| Equine kidney | 0.06 | STMRMo × CF (tentative)e | 0.06 | HRMo × CF (tentative)e |
| Poultry meat | – | No MRL needede | – | No MRL needede |
| Poultry fat tissue | – | No MRL needede | – | No MRL needede |
| Poultry liver | – | No MRL needede | – | No MRL needede |
| Cattle milk | 0.24 | STMRMo × CF (tentative)e | 0.24 | HRMo × CF (tentative)e |
| Sheep milk | – | No MRL needede | – | No MRL needede |
| Goat milk | – | No MRL needede | – | No MRL needede |
| Horse milk | 0.24 | STMRMo × CF (tentative)e | 0.24 | HRMo × CF (tentative)e |
| Birds eggs | – | No MRL needede | – | No MRL needede |
GAP: good agricultural practice; STMR: supervised trials median residue; CF: conversion factor for enforcement residue definition to risk assessment residue definition; HR: highest residue; PF: processing factor; MRL: maximum residue level; Mo: monitoring.
* Indicates that the input value is proposed at the limit of quantification.
For oranges, grapefruits and potatoes, the input values are derived from the fall‐back GAPs identified in this review.
For citrus fruits, the median and highest residue levels measured in pulp are directly considered for the risk assessment. The theoretical calculation considering the peeling factor is not considered reliable in this case.
For apples and pears, no fall‐back option was identified (all other GAPs do also lead to exceedances of the ARfD); therefore, these GAPs are disregarded from the EU2 scenario.
In the absence of supporting data, the existing EU MRL multiplied by the conversion factor for risk assessment of 1 (and by PF if relevant) is used for indicative exposure calculations.
For livestock commodities, all input values were recalculated considering the dietary burden recalculated under scenario EU2 (see also Appendix C.1).
D.3. Consumer risk assessment with consideration of the existing CXLs
D.3.1. Scenario CX1: considering fall‐back GAPs of scenario EU2 and all CXLs
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of imazalil and R014821, expressed as imazalil | ||||
|
Grapefruits Oranges |
5 | CXL × CF (tentative)a | 5 | CXL × CF (tentative)a |
|
Lemons Limes Mandarins |
0.05 | STMRMo (pulp) × CF (tentative)b | 0.69 | HRMo (pulp) × CF (tentative)b |
|
Apples Pears Quinces Medlar Loquat |
5 | CXL × CF (tentative)a | 5 | CXL × CF (tentative)a |
| Strawberries | 2 | CXL (tentative) × CFa | 2 | CXL (tentative) × CFa |
| Blackberries | 0.94 | HRMo × CF (CXL, tentative)c | 0.94 | HRMo × CF (CXL, tentative) |
| Raspberries | 0.94 | HRMo × CF (CXL, tentative)c | 0.94 | HRMo × CF (CXL, tentative) |
| Persimmon | 1.6 | HRMo × CF (CXL)c | 1.6 | HRMo × CF (CXL) |
| Bananas | 0.29 | STMRMo × PF × CF (tentative) | 0.31 | HRMo × PF × CF (tentative) |
| Potatoes | 5 | CXL × CF (tentative)a | 5 | CXL × CF (tentative)a |
| Tomatoes | 0.09 | STMRMo × CF | 0.16 | HRMo × CF |
| Sweet peppers/bell peppers | 0.05 | EU MRL × CFd | 0.05 | EU MRL × CFd |
|
Cucumbers Gherkins |
0.5 | CXL × CFa | 0.5 | CXL × CFa |
| Courgettes | 0.02 | STMRMo × CF (tentative) | 0.05 | HRMo × CF (tentative) |
| Melons | 0.24 | EU MRL × CF × PFd | 0.24 | EU MRL × CF × PFd |
| Small grain cereals | 0.01* | STMRMo × CF | 0.01* | HRMo × CF |
| Risk assessment residue definition 2: sum of imazalil and all identified/characterised metabolites | ||||
| Swine meat | 0.06 | STMRMo muscle × CF (tentative) | 0.06 | HRMo muscle × CF (tentative) |
| Swine fat tissue | 0.22 | STMRMo × CF (tentative) | 0.22 | HRMo × CF (tentative) |
| Swine liver | 0.08 | STMRMo × CF (tentative) | 0.08 | HRMo × CF (tentative) |
| Swine kidney | 0.06 | STMRMo × CF (tentative) | 0.06 | HRMo × CF (tentative) |
| Bovine meat | 0.06 | STMRMo muscle × CF (tentative) | 0.06 | HRMo muscle × CF (tentative) |
| Bovine fat tissue | 0.22 | STMRMo × CF (tentative) | 0.22 | HRMo × CF (tentative) |
| Bovine liver | 0.08 | STMRMo × CF (tentative) | 0.10 | HRMo × CF (tentative) |
| Bovine kidney | 0.06 | STMRMo × CF (tentative) | 0.06 | HRMo × CF (tentative) |
| Equine meat | 0.06 | STMRMo muscle × CF (tentative) | 0.06 | HRMo muscle × CF (tentative) |
| Equine fat tissue | 0.22 | STMRMo × CF (tentative) | 0.22 | HRMo × CF (tentative) |
| Equine liver | 0.08 | STMRMo × CF (tentative) | 0.10 | HRMo × CF (tentative) |
| Equine kidney | 0.06 | STMRMo × CF (tentative) | 0.06 | HRMo × CF (tentative) |
| Cattle milk | 0.24 | STMRMo × CF (tentative) | 0.24 | HRMo × CF (tentative) |
| Horse milk | 0.24 | STMRMo × CF (tentative) | 0.24 | HRMo × CF (tentative) |
GAP: good agricultural practice; CXL: codex maximum residue limit; STMR: supervised trials median residue; CF: conversion factor for enforcement residue definition to risk assessment residue definition; HR: highest residue; MRL: maximum residue level; Mo: monitoring.
* Indicates that the input value is proposed at the limit of quantification.
In the absence of risk assessment values for this CXL, the CXL value multiplied by the conversion factor for risk assessment of 1 is used for indicative exposure calculations.
For citrus fruits, the median and highest residue levels measured in pulp are directly considered for the risk assessment. The theoretical calculation considering the peeling factor is not considered reliable in this case.
As the median residue value is not available, the highest residue (instead of median) is used for an indicative chronic calculation.
In the absence of supporting data, the existing EU MRL multiplied by the conversion factor for risk assessment of 1 (and by PF if relevant) is used for indicative exposure calculations.
D.3.2. Scenario CX2: considering fall‐back GAPs of scenario EU2 and excluding CXLs for oranges, grapefruits, pome fruits, potatoes and persimmon
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of imazalil and R014821, expressed as imazalil | ||||
|
Grapefruits Oranges |
0.01* | STMRMo (pulp) × CF (fall‐back; tentative)a | 0.02 | HRMo (pulp)x CF (fall‐back; tentative)a |
|
Lemons Limes Mandarins |
0.05 | STMRMo (pulp) × CF (tentative)a | 0.69 | HRMo (pulp) × CF (tentative)a |
| Strawberries | 2 | CXL (tentative) × CFb | 2 | CXL (tentative) × CFb |
| Blackberries | 0.94 | HRMo × CF (CXL, tentative)c | 0.94 | HRMo × CF (CXL, tentative) |
| Raspberries | 0.94 | HRMo × CF (CXL, tentative)c | 0.94 | HRMo × CF (CXL, tentative) |
| Bananas | 0.29 | STMRMo × PF × CF (tentative) | 0.31 | HRMo × PF × CF (tentative) |
| Potatoes | 0.01* | STMRMo × CF (fall‐back) | 0.01* | HRMo × CF (fall‐back) |
| Tomatoes | 0.09 | STMRMo × CF | 0.16 | HRMo × CF |
| Sweet peppers/bell peppers | 0.05 | EU MRL × CFd | 0.05 | EU MRL × CFd |
|
Cucumbers Gherkins |
0.5 | CXL × CFb | 0.5 | CXL × CFb |
| Courgettes | 0.02 | STMRMo × CF (tentative) | 0.05 | HRMo × CF (tentative) |
| Melons | 0.24 | EU MRL × CF × PFd | 0.24 | EU MRL × CF × PFd |
| Small grain cereals | 0.01* | STMRMo × CF | 0.01* | HRMo × CF |
| Risk assessment residue definition 2: sum of imazalil and all identified/characterised metabolites | ||||
| Swine meat | 0.06 | STMRMo muscle × CF (tentative) | 0.06 | HRMo muscle × CF (tentative) |
| Swine fat tissue | 0.22 | STMRMo × CF (tentative) | 0.22 | HRMo × CF (tentative) |
| Swine liver | 0.08 | STMRMo × CF (tentative) | 0.08 | HRMo × CF (tentative) |
| Swine kidney | 0.06 | STMRMo × CF (tentative) | 0.06 | HRMo × CF (tentative) |
| Bovine meat | 0.06 | STMRMo muscle × CF (tentative) | 0.06 | HRMo muscle × CF (tentative) |
| Bovine fat tissue | 0.22 | STMRMo × CF (tentative) | 0.22 | HRMo × CF (tentative) |
| Bovine liver | 0.08 | STMRMo × CF (tentative) | 0.10 | HRMo × CF (tentative) |
| Bovine kidney | 0.06 | STMRMo × CF (tentative) | 0.06 | HRMo × CF (tentative) |
| Equine meat | 0.06 | STMRMo muscle × CF (tentative) | 0.06 | HRMo muscle × CF (tentative) |
| Equine fat tissue | 0.22 | STMRMo × CF (tentative) | 0.22 | HRMo × CF (tentative) |
| Equine liver | 0.08 | STMRMo × CF (tentative) | 0.10 | HRMo × CF (tentative) |
| Equine kidney | 0.06 | STMRMo × CF (tentative) | 0.06 | HRMo × CF (tentative) |
| Cattle milk | 0.24 | STMRMo × CF (tentative) | 0.24 | HRMo × CF (tentative) |
| Horse milk | 0.24 | STMRMo × CF (tentative) | 0.24 | HRMo × CF (tentative) |
GAP: good agricultural practice; STMR: supervised trials median residue; CF: conversion factor for enforcement residue definition to risk assessment residue definition; HR: highest residue; MRL: maximum residue level; Mo: monitoring.
* Indicates that the input value is proposed at the limit of quantification.
For citrus fruits, the median and highest residue levels measured in pulp are directly considered for the risk assessment. The theoretical calculation considering the peeling factor is not considered reliable in this case.
In the absence of risk assessment values for this CXL, the CXL value multiplied by the conversion factor for risk assessment of 1 is used for indicative exposure calculations.
As the median residue value is not available, the highest residue (instead of median) is used for an indicative chronic calculation.
In the absence of supporting data, the existing EU MRL multiplied by the conversion factor for risk assessment of 1 (and by PF if relevant) is used for indicative exposure calculations.
Appendix E – Decision tree for deriving MRL recommendations
1.
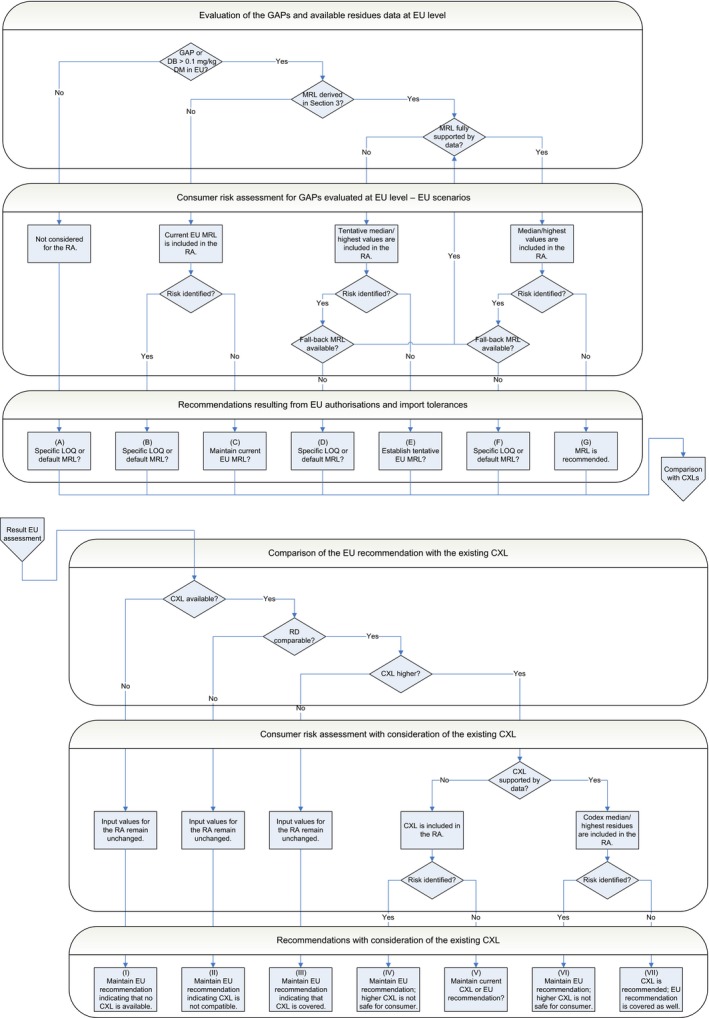
Appendix F – Used compound codes
1.
| Code/trivial name | Chemical name/SMILES notation | Structural formula |
|---|---|---|
| Imazalil |
(RS‐1‐(β‐Allyloxy‐2,4‐dichlorophenethyl)imidazole Clc2ccc(C(OCC=C)Cn1 ccnc1)c(Cl)c2 |
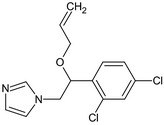
|
| R014821 |
(1RS)‐1‐(2,4‐Dichlorophenyl)‐2‐(1H‐imidazol‐1‐yl)ethanol OC(Cn1ccnc1)c2ccc(Cl)cc2Cl |
|
| R044177 |
(2RS)‐2‐(Allyloxy)‐2‐(2,4‐dichlorophenyl)ethanamine hydrochloride (1:1) Cl.Clc1cc(Cl)ccc1C(OCC=C)CN |
|
| FK‐772 |
3‐{(2RS)‐2‐(2,4‐Dichlorophenyl)‐2‐[(2RS)‐2,3‐dihydroxypropoxy]ethyl}‐2,4‐imidazolidinedione Clc2ccc(C(OCC(O)CO)CN1C(=O)CNC1 = O)c(Cl)c2 |
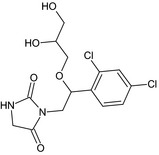
|
| FK‐284 |
3‐[(2RS)‐2‐(2,4‐Dichlorophenyl)‐2‐hydroxyethyl]‐2,4‐imidazolidinedione O=C2NCC(=O)N2CC(O)c1ccc(Cl)cc1Cl |
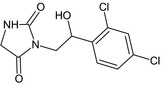
|
| FK‐858 |
(2RS)‐3‐[(1RS)‐1‐(2,4‐Dichlorophenyl)‐2‐(1H‐imidazol‐1‐yl)ethoxy]‐1,2‐propanediol Clc2ccc(C(OCC(O)CO)Cn1ccnc1)c(Cl)c2 |
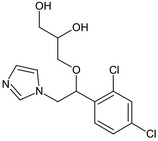
|
SMILES: simplified molecular‐input line‐entry system.
Suggested citation: EFSA (European Food Safety Authority) , Brancato A, Brocca D, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Janossy J, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Nougadere A, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Reasoned opinion on the review of the existing maximum residue levels for imazalil according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2017;15(9):4977, 66 pp. 10.2903/j.efsa.2017.4977
Requestor: European Commission
Question number: EFSA‐Q‐2008‐00562
Acknowledgement: EFSA wishes to thank the rapporteur Member State Netherlands for the preparatory work on this scientific output.
Amendment: An editorial correction was carried out that does not materially affect the contents or outcome of this scientific output. To avoid confusion, the older version has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made.A correction of the application rate value expressed in g a.i/hl has been made for the post‐harvest waxing uses on grapefruits and oranges in the Appendix A3 (fall‐back GAPs) to align with the correct dose rate expressed in terms of g a.i./ton (300 g a.i./hl is now reported, equivalent to 3 g a.i./ton, cf pages 32–33 and 36). The error only occurred in the GAP table while the overall assessment or MRL recommendations remained unchanged.
Approved: 9 August 2017
Amended: 22 October 2018
Notes
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market, OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Commission Directive 97/73/EC of 15 December 1997 including an active substance (imazalil) in Annex I to Council Directive 91/414/EEC concerning the placing of plant protection products on the market, OJ L 353, 24.12.1997, p. 26–28.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 187–188.
Commission Implementing Regulation (EU) No 705/2011 of 20 July 2011 approving the active substance imazalil, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 190, 21.7.2011, p. 43–49.
Commission Regulation (EC) No 737/2007 of 27 June 2007 on laying down the procedure for the renewal of the inclusion of a first group of active substances in Annex I to Council Directive 91/414/EEC and establishing the list of those substances. OJ L 169, 29.6.2007, p. 10–18.
Commission Regulation (EU) No 750/2010 of 7 July 2010 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for certain pesticides in or on certain products. OJ L 220, 21.8.2010, p. 1–56.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Metabolite R014821 ((1RS)‐1‐(2,4‐dichlorophenyl)‐2‐(1H‐imidazol‐1‐yl)ethanol): formed from the dealkylation of the parent compound (see Appendix F).
Metabolite R044177 ((2RS)‐2‐(allyloxy)‐2‐(2,4‐dichlorophenyl)ethanamine hydrochloride (1:1)): formed from the loss of the imidazole ring and carboxylation (see Appendix F).
Considering the highest residues observed in potatoes after post‐harvest treatment of 4.6 mg/kg (see Appendix B.1.2) and assuming a planting density of 7 tonnes potatoes per hectare.
Average derived from different DT50 values calculated in laboratory (EFSA, 2010).
Considering a transition factor of 1 is assuming that the residue levels in rotational crop is equal to the soil concentration (i.e. disregarding accumulation effect).
Application rate for cereals is 75 g a.s./tonnes tubers while application rate for potatoes is 15 g a.s./ton seeds.
It is highlighted that both parent and metabolite FK‐772 consist of two isomers. Therefore, the mention ‘any ratio of constituent isomers’ applies to both compounds.
Molecular weight of imazalil (297.2 g/mol)/molecular weight of FK‐772 (363.2 g/mol).
Reported as 0.25 mg/hen in the study summary, and calculated considering the standard weight for hen (1.9 kg).
HR pulp = 0.69 mg/kg while the theoretical calculation (HR whole fruit (4.95) × 0.07) would give 0.35 mg/kg.
In the unit‐to‐unit study on apples, the composite samples result was reported as 1.52 mg/kg in the summary table while the range of individual units is presented as 0.40–1.28 mg/kg in the calculation.
Based on the available data, the less critical GAP received for apples/pears (smoke can: 6 g a.i./tonnes; WHP: 30 days) would lead to 155% ARfD for apples and 144% of ARfD for pears. It is noted that the use of an alternative VF (derived from a study performed with drenching) is not applicable to refine this calculation since applications with smoke are expected to induce higher variability than drenching applications.
Study demonstrating storage stability of metabolite R014821 in high acid content commodities is required.
References
- Belgium , 1996. Draft assessment report on the active substance imazalil prepared by the rapporteur Member State Belgium in the framework of Council Directive 91/414/EEC, July 1996.
- Belgium , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers’ health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Conclusion on the peer review of the pesticide risk assessment of the active substance imazalil. EFSA Journal 2010;8(2):1526, 69 pp. 10.2903/j.efsa.2010.1526 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017a. Completeness check report on the review of the existing MRLs of imazalil prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 10 April 2017. Available online: http://www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2017b. Member States consultation report on the review of the existing MRLs of imazalil prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 31 July 2017. Available online: http://www.efsa.europa.eu
- EURL (European Union Reference Laboratories for Pesticide Residues), 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Analytical methods validated by the EURLs and overall capability of official laboratories to be considered for the review of the existing MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2016. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.2, September 2016.
- FAO (Food and Agriculture Organization of the United Nations), 1977. Imazalil. 1977 Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues, December 1977. FAO Plant Production and Protection Paper 10.
- FAO (Food and Agriculture Organization of the United Nations), 1984. Imazalil. 1984 Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues, September‐October 1984. FAO Plant Production and Protection Paper 62.
- FAO (Food and Agriculture Organization of the United Nations), 1985. Imazalil. 1985 Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues, September‐October 1985. FAO Plant Production and Protection Paper 72/2, 226 pp.
- FAO (Food and Agriculture Organization of the United Nations), 1994. Imazalil. 1994 Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues, September 1994. Available online: http://www.fao.org
- FAO (Food and Agriculture Organization of the United Nations), 2009. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Ed. FAO Plant Production and Protection Paper 197, 264 pp.
- France , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- Germany , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- Greece , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- Italy , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- Netherlands , 2009a. Assessment Report on the active substance imazalil prepared by the rapporteur Member State Netherlands in consultation with Spain in the framework of Commission Regulation (EC) No 737/2007, May 2009. Available online: http://www.efsa.europa.eu
- Netherlands , 2009b. Final Addendum to Assessment Report on imazalil, prepared by the rapporteur Member State Netherlands in consultation with Spain in the framework of Commission Regulation (EC) No 737/2007, compiled by EFSA, November 2009. Available online: http://www.efsa.europa.eu
- Netherlands , 2014. Post inclusion addendum to the draft assessment report on imazalil on confirmatory data. Vol 3 B.7 Residues, May 2014. Available online: http://www.efsa.europa.eu
- Netherlands , 2015. Evaluation report prepared under Article 8 of Regulation (EC) No 396/2005. Modification of MRLs for imazalil in various crops and products of animal origin, March 2015. Available online: http://www.efsa.europa.eu
- Netherlands , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- Portugal , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- Spain , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu


