Abstract
This scientific opinion of the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF Panel) deals with the safety evaluation of [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane as a component for sizing glass fibres used for manufacturing glass‐fibre‐reinforced plastics. The substance is typically applied at up to around 0.2% related to the final plastic. The resulting food contact materials are intended for various scenarios of use, i.e. long‐term contact at ambient temperature (e.g. storage tanks) or short‐term contact at elevated temperatures (e.g. kitchen utensils). In extracts of treated fibres, neither the substance was detectable at 10 μg/kg fibre nor its hydrolysis product and oligomers at 60 μg/kg fibre. Based on the detection limits, modelling for the plastics and scenarios of intended use resulted in maximum migrations of 0.05 μg/kg food for the substance and 0.15 μg/kg food for the sum of the reaction products. The Panel concludes that the substance has a genotoxic potential. This may also apply to some of its reaction products which contain the epoxy function. However, due to the very low exposure, if any, [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane does not raise safety concern if used as a component of sizing agents to treat glass fibres imbedded into low diffusivity plastics (polyethylene terephthalate, polycarbonate, polybutylene terephthalate, thermoset polyesters and epoxy bisphenol vinylester) in contact with all foodstuffs. In addition, the residues in the treated glass fibres must not be detectable at 10 μg/kg for the substance and 60 μg/kg for each of the reaction products (hydrolysed monomers and epoxy‐containing cyclic dimer, trimer and tetramer).
Keywords: [3‐(23‐epoxypropoxy)propyl]trimethoxy silane, CAS No 2530‐83‐8, FCM substance No 01068, glass‐fibre‐reinforced plastics, food contact materials, safety assessment
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Before a substance is authorised to be used in food contact materials (FCM) and is included in a positive list EFSA's opinion on its safety is required. This procedure has been established in Articles 8, 9 and 10 of Regulation (EC) No 1935/20041 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food.
According to this procedure, the industry submits applications to the Member States competent authorities which transmit the applications to the European Food Safety Authority (EFSA) for their evaluation.
In this case, EFSA received an application from the Bundesamt für Verbraucherschutz und Lebensmittelsicherheit, Germany, requesting the evaluation of the substance [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane, with the CAS number 2530‐83‐8, and the FCM substance No 01068. The dossier was submitted by a Project Consortium of Silane and Glass Fibre Producers comprising eight companies (Evonik Industries AG; Shin‐Etsu Silicones Europe bv; Momentive Performance Materials GmbH; 3B‐the fibreglass company; European Owens Corning Fibreglas sprl.; PPG Industries Fibre Glass bv; Johns Manville Slovakia a.s.; P‐D Glasseiden GmbH).
According to Regulation (EC) No 1935/2004 of the European Parliament and of the Council on materials and articles intended to come into contact with food, EFSA is asked to carry out an assessment of the risks related to the intended use of the substance and to deliver a scientific opinion.
2. Data and methodologies
2.1. Data
The applicant has submitted a dossier in support of their application for the authorisation of [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane (3‐glycidyloxypropyltrimethoxysilane (GLYMO)) to be applied as a sizing ingredient (coupling agent) for continuous filament glass fibres used for reinforcement of plastic FCM, such as storage tanks or kitchen utensils.
Data submitted and used for the evaluation are:
Non‐toxicological data and information
Chemical identity
Description of manufacturing process of substance/FCM
Physical and chemical properties
Intended use
Existing authorisation(s)
Migration of the substance
Residual content of the substance
Oligomers
Identification, quantification and migration of reaction products and impurities
Toxicological data
Bacterial gene mutation test
In vitro mammalian cell gene mutation test
In vitro mammalian chromosome aberration test
Mammalian erythrocyte micronucleus test
In vivo mouse bone marrow micronucleus test
2.2. Methodologies
The assessment was conducted in line with the principles laid down in Regulation (EC) No 1935/2004 on materials and articles intended to come into contact with food. This Regulation underlines that applicants may consult the Guidelines of the Scientific Committee on Food (SCF) for the presentation of an application for safety assessment of a substance to be used in FCM prior to its authorisation (European Commission, 2001), including the corresponding data requirements. The dossier that the applicant submitted for evaluation was in line with the SCF guidelines (European Commission, 2001).
The methodology is based on the characterisation of the substance that is the subject of the request for safety assessment prior to authorisation, its impurities and reaction and degradation products, the evaluation of the exposure to those substances through migration and the definition of minimum sets of toxicity data required for safety assessment.
To establish the safety from ingestion of migrating substances, the toxicological data indicating the potential hazard and the likely human exposure data need to be combined. Exposure is estimated from studies on migration into food or food simulants and considering that a person may consume daily up to 1 kg of food in contact with the relevant FCM.
As a general rule, the greater the exposure through migration, the more toxicological data is required for the safety assessment of a substance. Currently, there are three tiers with different thresholds triggering the need for more toxicological information as follows:
In case of high migration (i.e. 5–60 mg/kg food), an extensive data set is needed.
In case of migration between 0.05 and 5 mg/kg food, a reduced data set may suffice.
In case of low migration (i.e. < 0.05 mg/kg food), only a limited data set is needed.
More detailed information on the required data is available in the SCF guidelines (European Commission, 2001).
The assessment was conducted in line with the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and considering the relevant guidance from the EFSA Scientific Committee.
3. Assessment
According to the applicant, the substance [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane (or 3‐glycidyloxypropyltrimethoxysilane) is intended to be applied as a component for sizing continuous filament glass fibres used for manufacturing of glass‐fibre‐reinforced plastics. It acts as a coupling agent between the surface of glass fibres and the film former as well as the polymer matrix. The polymers are the thermosets epoxy bisphenol vinylester, orthophthalic and isophthalic polyesters, as well as the thermoplastics polyethylene terephthalate (PET), polybutylene terephthalate (PBT) and polycarbonate (PC). The resulting FCMs are used, e.g. for storage tanks, processing equipment, table ware, chop sticks or kitchen utensils.
According to the applicant, the substance is typically applied at up to 4% in aqueous solutions also containing a film former, such as a bisphenol A epoxy resin. Up to 2% sizing (solid weight) is retained on the fibres (determined by weight loss after burning off the sizing), of which 23% is related to the substance (0.47% related to the glass fibres).
The related FCMs are intended for all foods, except those with an ethanol content higher than 20%, under the following conditions of use:
single and repeated use with long‐term storage at room temperature for epoxy bisphenol vinylester, orthophthalic and isophthalic polyesters, PET and PC (simulated at 10 days/60°C),
short‐term repeated contact at high temperature for PET and PC (0.5 h at 175°C)
short‐term repeated contact at increased temperature or up to a few days at room temperature for PBT (2 h at 70°C or 10 days/40°C).
For all intended applications, the area to volume ratio is foreseen to be significantly lower than 6 dm²/kg.
The substance fits into a group listed by the German Federal Institute for Risk Assessment (BfR) (Recommendation LII) and the US Food and Drug Administration (FDA) (CFR 177.1390), but was not evaluated by the SCF or EFSA.
Being a consortium of producers, three versions of the substance were described in the dossier with their impurities.
3.1. Non‐toxicological data
Chemical formula: C9H20O5Si, 236.34 Da
Chemical structure: 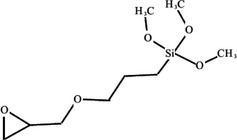
At ambient temperature, the substance, [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane, is a liquid. The melting point is expected to be below −70°C, the calculated boiling point is 233°C. The substance has a log Po/w of 0.5 at 20°C and is soluble in alcohols. Upon addition to water, it hydrolyses (with a half‐life of 6.5 h at pH 7 and 24.5°C) and polymerises. The hydrolysis product, [3‐(2,3‐epoxypropoxy)propyl] silanetriol, is soluble in water with a log Po/w −2.6 at 20°C.
Thermostability on glass fibres treated with the substance was tested under inert atmosphere in a pyrolyser coupled to gas chromatography/mass spectrometry (GC/MS). At 300°C (5 min), the maximum temperature during manufacture of glass‐fibre‐reinforced plastics, no degradation products were detected with a detection limit of around 10 mg/kg treated glass fibres.
The silane function of the substance was found to be hydrolysed already in the sizing solution. The intact substance was not detectable in either the aqueous sizing solutions or in extracts from sized glass fibres (detection limit, 10 μg/kg fibre).
The potentially migrating reaction products, including oligomers, were identified in aqueous solutions of the substance (after hydrolysis and polymerisation), in extracts from non‐cured glass fibres only treated with the substance as well as treated with the substance and epoxy resin as film former, using flow injection analysis (FIA)‐MS, liquid chromatography/mass spectrometry (LC/MS) and liquid chromatography with tandem mass spectrometry (LC–MS/MS).
Quantitative data on the migration of these reaction products were based on LC–MS(/MS) analysis of the identified compounds, mainly linear, branched or cyclic oligomers n = 2–5 with hydrolysed silanes and intact or hydrolysed glycidols, in extracts from treated glass fibres, assuming a response equal to that of the hydrolysed substance, i.e. [3‐(2,3‐epoxypropoxy)propyl]silanetriol. None of the substances were detected with a detection limit of 60 μg/kg fibre.
Using this detection limit, maximum migrations were estimated from the plastics, scenarios of use and the time/temperature conditions of intended uses by modelling, assuming articles of 5 mm thickness and high solubility in foods:
single long‐term use (10 days/60°C) with 70% glass fibres in thermoset and thermoplastic polymers
repeated use scenarios at up to 175°C with 50% glass fibres in thermoplastic polymers.
The results for the substance [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane (third use for repeated use articles) were calculated to be between 2 and 8 ng/kg food, except for PC and PET in the third use simulated with 0.5 h/175°C (11 and 50 ng/kg, respectively). These calculations are based on the detection limits for the treated glass fibres. Also taking into account that the substance was already undetectable in the applied sizing solution because of hydrolysis, reactions during incorporation into the plastics, application for repeated use articles with a long service life and that, if residues persisted and migrated, they could be hydrolysed in food, no exposure for consumers to the substance is expected.
The results of modelling for the summed migration of the individual reaction products (third use for repeated use articles) did not exceed 0.15 μg/kg food for any plastic, scenario and conditions of use intended by the petitioner. This is again based on worst‐case assumptions, such as that each reaction product was present at the detection limit, that the epoxy group remained intact and that a conservative surface area to volume ratio of 6:1 is applicable. A conservative assumption of 1 kg/person per day of food containing the reaction products at the detection limit being consumed would result in a potential intake of the sum of the reaction products below 0.15 μg/person per day.
The data provided on impurities of the products manufactured by the three producers varied. ■■■■■ For the impurities including the silane moiety, the Panel considered that they would bond in the sizing process to a similar extent as the substance. Propene glycidyl ethers, non‐reacted starting substances, were not detectable in the glass fibres sized with the substance with a detection limit of 4 μg/kg fibre.
3.2. Toxicological data
The substance (purity not reported) dissolved in ethylene glycol dimethyl ether was tested for bacterial mutations in three strains of Salmonella Typhimurium TA97, TA98 and TA100 in triplicate plates with and without metabolic activation at concentrations up to 5,000 μg/plate following the OECD guideline TG 471. In TA100 strain, both in the absence or presence of S9 fraction, the test substance induced a statistically significant dose‐related increase in the number of revertants. A statistically significant increase of the revertants was also observed in TA97 strain only at the highest concentration tested. No increase of revertants was detected in TA98 strain.
The substance (purity not specified) was tested for gene mutation in mouse lymphoma L5178Y cells up to 3,000 ng/mL in the absence and up to 8,000 ng/mL in the presence of metabolic activation system. In the absence of metabolic activation, a dose‐dependent increase in mutation frequency, exceeding the Global Evaluation Factor at 3,000 ng/mL was observed. In the presence of metabolic activation, a dose‐dependent increase in mutation frequency, exceeding the Global Evaluation Factor was detected at all the concentrations tested. Considering that the two criteria for evaluation and interpretation of results as positive on the basis of the OECD TG 490 were fulfilled: (i) an increase in mutation frequency above concurrent negative control, exceeding the Global Evaluation Factor, and (ii) a concentration‐related effect, the test chemical is considered able to induce mutations in this test system.
Three in vivo studies on chromosomal damage were reported.
In a first study, the substance (purity not reported) dissolved in water, was tested in vivo with the mammalian erythrocyte micronucleus test using intragastric administration, according to the OECD guideline. The substance was administered by gavage to male and female CD1 mice at time 0 and 24 h. Three different doses were tested: 0.5, 1.67 and 5 g/kg body weight (bw). No significant increase of micronucleated polychromatic erythrocytes (MNPCE) was observed in any treated group compared with the negative controls. Because no indication of exposure of the bone marrow was reported, the Panel considered the negative outcome of this study of limited relevance.
In a second study the substance (purity 98.4%) dissolved in corn oil, was administered intraperitoneally as a single dose (1,600 mg/kg bw) to male and female NMRI mice. Animals were sacrificed at 24 and 48 h after the treatment. A statistically significant increase of MNPCE was observed in the group of male mice sampled at 24 h. No increase was reported for the other group. Considering that only a single dose was tested, these results were considered of limited relevance by the Panel.
In a third study, the substance (100% pure) dissolved in water was administered intraperitoneally as a single dose to male and female mice (ICR) at 500, 1,000 and 2,000 mg/kg bw. Animals were sacrificed at 24 and 48 h after the treatment. A statistically significant dose‐related increase of MNPCE was observed in male and female mice 24 h after treatment at all dose levels with respect to the concurrent control values. A statistically significant increase of MNPCE was also observed in animals treated at 2,000 mg/kg bw and sampled at 48 h. Moderate reductions in the ratio PCE/total erythrocytes were observed in male and female mice at 24 h, while severe reductions were detected 48 h after treatment. Although the study does not fulfil all the criteria requested by the OECD TG 474 due to the lack of historical controls, the results meet two of the criteria (statistically significant increase dose‐related at one sampling time) and are considered positive.
In summary, in in vitro experiments, the substance is positive for gene mutation in Ames test and mouse lymphoma assay, both with and without metabolic activation. In vivo, the compound induces a significant dose‐related increase of micronuclei frequency in bone marrow. Overall, the Panel concludes that the applied substance has a genotoxic potential.
No residue of the substance [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane was detected on treated glass fibres. Considering, furthermore, the reactivity of the substance, it can be concluded that there would be essentially no exposure for the consumer when used in the intended applications.
Concerning the reaction products, it cannot be ruled out that they contain the epoxy function that may contribute to the genotoxic potential of the substance. However the potential intake of the sum of these compounds is below 0.15 μg/person per day. The Panel notes that this is below the Threshold of Toxicological Concern (TTC) value for genotoxic compounds (EFSA Scientific Committee, 2012).
4. Conclusions
Based on the above‐mentioned data, the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF Panel) concludes that the substance [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane has a genotoxic potential. This may also apply to some of the reaction products which contain the epoxy function. However, due to the very low exposure, if any, it does not rise safety concern if used as a component of sizing agents to treat glass fibres imbedded into low diffusivity plastics (PET, PC, PBT, thermoset polyesters and epoxy bisphenol vinylester) in contact with all foodstuffs. In addition, the residues in the treated glass fibres must not be detectable at 10 μg/kg for the substance and 60 μg/kg for each of the reaction products (hydrolysed monomers and epoxy‐containing cyclic dimer, trimer and tetramer).
Documentation provided to EFSA
Initial dossier. January 2016. Submitted by Project Consortium of Silane and Glass Fibre Producers.
Additional data. April 2017. Submitted by Project Consortium of Silane and Glass Fibre Producers.
Abbreviations
- BfR
German Federal Institute for Risk Assessment
- bw
body weight
- CAS
Chemical Abstracts Service
- CEF Panel
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- FDA
US Food and Drug Administration
- FIA
flow injection analysis
- FCM
food contact materials
- GC/MS
gas chromatography/mass spectrometry
- GLYMO
[3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane
- LC/MS
liquid chromatography/mass spectrometry
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- MNPCE
micronucleated polychromatic erythrocyte
- OECD
Organisation for Economic Co‐operation and Development
- PET
polyethylene terephthalate
- PBT
polybutylene terephthalate
- PC
polycarbonate
- Po/w
octanol/water partition coefficient
- SCF
Scientific Committee on Food
- TTC
Threshold of Toxicological Concern
Suggested citation: EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , Silano V, Bolognesi C, Chipman K, Cravedi J‐P, Engel K‐H, Fowler P, Grob K, Gürtler R, Husøy T, Kärenlampi S, Mennes W, Milana MR, Pfaff K, Riviere G, Srinivasan J, Tavares Poças MF, Tlustos C, Wölfle D, Zorn H, Kolf‐Clauw M, Lampi E, Svensson K, Van Haver E and Castle L, 2017. Scientific Opinion on the safety assessment of the substance [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane, for use in food contact materials. EFSA Journal 2017;15(10):5014, 8 pp. 10.2903/j.efsa.2017.5014
Requestor: Bundesamt für Verbraucherschutz und Lebensmittelsicherheit, Germany
Question number: EFSA‐Q‐2016‐00001
Panel members: Claudia Bolognesi, Laurence Castle, Kevin Chipman, Jean‐Pierre Cravedi, Karl‐Heinz Engel, Paul Fowler, Roland Franz, Konrad Grob, Rainer Gürtler, Trine Husøy, Sirpa Kärenlampi, Wim Mennes, Maria Rosaria Milana, Karla Pfaff, Gilles Riviere, Vittorio Silano, Jannavi Srinivasan, Maria de Fátima Tavares Poças, Christina Tlustos, Detlef Wölfle and Holger Zorn.
Note: The full opinion will be published in accordance with Article 10(6) of Regulation (EC) No 1935/2004 once the decision on confidentiality, in line with Article 20(3) of the Regulation, will be received from the European Commission. The following information has been provided under the confidentiality framework and has been redacted awaiting the decision of the Commission: the data provided on the impurities of the products manufactured by three producers of the project consortium of silane and glass fibre producers.
Competing interests: In line with EFSA's policy on declarations of interest, Panel member Roland Franz did not participate in the development and adoption of this scientific output
Adopted: 21 September 2017
Note
Regulation (EC) No 1935/2004 of the European parliament and of the council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. OJ L 338, 13.11.2004, p. 4–17.
References
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(5):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Scientific Opinion on Exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC). EFSA Journal 2012;10(7):2750, 103 pp. 10.2903/j.efsa.2012.2750 [DOI] [Google Scholar]
- European Commission , 2001. Guidelines of the Scientific Committee on Food for the presentation of an application for safety assessment of a substance to be used in food contact materials prior its authorisation. Available online: http://ec.europa.eu/food/fs/sc/scf/out82_en.pdf


