Abstract
The EFSA Panel on Contaminants in the Food Chain (CONTAM) reviewed new studies on nivalenol since the previous opinion on nivalenol published in 2013, but as no new relevant data were identified the tolerable daily intake (TDI) for nivalenol (NIV) of 1.2 μg/kg body weight (bw) established on bases of immuno‐ and haematotoxicity in rats was retained. An acute reference dose (ARfD) of 14 μg/kg bw was established based on acute emetic events in mink. The only phase I metabolite of NIV identified is de‐epoxy‐nivalenol (DE‐NIV) and the only phase II metabolite is nivalenol‐3‐glucoside (NIV3Glc). DE‐NIV is devoid of toxic activity and was thus not further considered. NIV3Glc can occur in cereals amounting up to about 50% of NIV. There are no toxicity data on NIV3Glc, but as it can be assumed that it is hydrolysed to NIV in the intestinal tract it should be included in a group TDI and in a group ARfD with NIV. The uncertainty associated with the present assessment is considered as high and it would rather overestimate than underestimate any risk.
Keywords: nivalenol, modified forms, group health based guidance value
Summary
Following a request from the European Commission, the EFSA Panel on Contaminants in the Food Chain (CONTAM) assessed whether it is appropriate and feasible to set a group health based guidance value (group HBGV) for nivalenol (NIV) and its modified forms related to their presence in food and feed, and to consider, whether it would be appropriate to use the parent compound as a marker for toxicity.
Modified forms of mycotoxins comprise all metabolites of the parent molecule, which are formed in the fungus, infested plant and animals. It is increasingly realised that not only the parent mycotoxins but also their modified forms may contribute to the overall toxicity. Modified forms include phase I metabolites formed through oxidation, reduction or hydrolysis of the parent toxin, as well as phase II metabolites arising from conjugation with endogenous molecules.
Previous risk assessments from EFSA on NIV and on modified mycotoxins have been used as a starting point for the present assessment. In addition, a systematic literature search has been carried out to obtain up‐to‐date and comprehensive information on NIV and its modified forms. In this opinion, the general principles for risk assessment were followed. Before assessing whether modified forms of NIV can be included in a group HBGV for NIV, the CONTAM Panel decided to review new relevant data on NIV and its modified forms published after the European Food Safety Authority (EFSA) assessment on NIV in 2013 to evaluate whether the established tolerable daily intake (TDI) for NIV needed to be revised and if in addition there was a need also to set an acute reference dose (ARfD) for NIV.
NIV is a member of the type B group trichothecenes, which are tetracyclic sesquiterpenoids produced by many species of Fusarium infesting crop plants. To date, the only phase I metabolite of NIV identified is de‐epoxy‐nivalenol (DE‐NIV) and the only phase II metabolite identified is nivalenol‐3‐glucoside (NIV3Glc). It can be expected, however, that other conjugated metabolites may be formed in plants and fungi. Because of a lack of significant toxicity, DE‐NIV was not further considered in the assessment.
Analytical methods (mainly based on liquid chromatography–tandem mass spectrometry (LC–MS/MS)) for NIV and NIV3Glc are available, but due to high polarity of both compounds recovery rates are often affected by matrix effects. This may lead to poor recovery. Standards and reference materials are not commercially available for modified forms of NIV. The higher polarity of NIV compared to other trichothecenes makes analytical detection more difficult under the same chromatographic conditions. Further phase II metabolism would increase the polarity and thus conjugates would be even more difficult to analyse. It is, therefore, likely that there are other phase II metabolites of NIV not yet identified.
NIV3Glc may occur in cereals amounting up to about 50% of its parent form. The transfer of NIV from feed to food products of animal origin is expected to contribute only marginally to human exposure.
NIV is rapidly absorbed, distributed and eliminated without accumulation. No NIV metabolites, except DE‐NIV, which is generated by biota in the rumen and also to some extent in the gut of monogastric animals, have been identified in mammals. There are no in vivo data on the absorption of NIV3Glc, but in vitro data indicate that it is not absorbed. In vitro data suggest that it is hydrolysed by the gut microbiota and it may then be absorbed as NIV.
In vivo acute toxicity studies show that NIV has anorectic effects upon short‐term exposure. Emetic events observed upon single oral exposure to NIV in mink were identified as the critical acute effects in the present assessment. In the previous opinion on NIV, it was concluded that there is no evidence that other toxic effects occur at doses lower than those inducing immunotoxicity and haematotoxicity and that there is no evidence for genotoxicity or carcinogenicity of NIV. De‐epoxidation strongly reduces toxicity of NIV. Albeit there is no toxicity information on NIV3Glc, it can be assumed that it is hydrolysed to and absorbed in the lower intestinal tract as NIV.
It can be assumed that NIV induces anorexia by interfering with gut satiety peptide cholecystokinin. NIV targets the ribosome leading to an inhibition of protein, RNA and DNA synthesis. Rapidly proliferating tissues such as haematopoietic tissue are targets of NIV leading to leucopenia and thrombocytopenia possibly due to induction of apoptosis.
The CONTAM Panel selected the emetic effects of NIV for the acute hazard characterisation. Benchmark dose (BMD) analysis was performed on the incidence of emetic events in mink exposed to NIV. The BMD modelling using a benchmark response (BMR) of 10% resulted in a benchmark dose 95% lower and upper confidence interval (BMDL10–BMDU10) of 0.14–0.23 mg NIV/kg body weight (bw) per day. Using the acute BMDL10 of 0.14 mg/kg bw for NIV and an uncertainty factor of 10 for intraspecies differences, an ARfD of 14 μg NIV/kg bw was established. No interspecies variability factor was applied because humans were not considered more sensitive than mink to the acute emetic effect of NIV, an assumption supported by studies on emesis with emetine showing that similar doses of the compound are effective both in humans and mink. There are no data on acute emetic effects of NIV3Glc but it is assumed that it will be hydrolysed in the lower intestine releasing NIV. Therefore, NIV3Glc should be included in a group ARfD with NIV with the same molar potency factor (i.e. 1).
No new studies relevant for chronic hazard characterisation were identified since publication of the previous opinion on NIV in 2013. Therefore, the BMDL05 of 0.35 mg NIV/kg bw per day based on a reduction in white blood cell counts in a 90‐day rat study as set in the previous opinion on NIV has been retained for the present assessment and the TDI of 1.2 μg NIV/kg bw established in 2013 has been retained. Because NIV3Glc can be hydrolysed to NIV after ingestion, it can be included in a group TDI with NIV with the same molar potency factor (i.e. 1).
The currently established analytical methods might not be adequate for detection of polar conjugates of NIV. Therefore, methods should be developed for accurate detection of NIV and its modified forms to identify potential NIV metabolites not yet detected. Certified reference materials and standards for the modified forms of NIV are also needed. Because there is no information available on the toxicokinetics of modified forms of NIV, toxicokinetic studies, in particular with NIV3Glc should be conducted.
Fusarenon‐X (FUS‐X) is not only a mycotoxin on its own, but also a precursor of NIV, and it frequently co‐occurs with NIV. Therefore, it might be appropriate to perform a hazard characterisation of FUS‐X and to evaluate whether or not it should be included in group HGBVs with NIV.
1. Introduction
1.1. Background Terms of Reference as provided by the requestor
Following a request from the European Commission, the risks to human and animal health related to modified forms of the Fusarium toxins zearalenone, nivalenol, T‐2 and HT‐2 toxins, and fumonisins were evaluated in the scientific opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed,1 adopted by the EFSA Panel on Contaminants in the Food Chain (CONTAM) on 25 November 2014.
The CONTAM Panel considered it appropriate to assess human exposure to modified forms of the various toxins in addition to the parent compounds, because many modified forms are hydrolysed into the parent compounds or released from the matrix during digestion. In the absence of specific toxicity data, toxicity equal to the parent compounds was assumed for modified mycotoxins. Risk characterisation was done by comparing exposure scenarios with reference doses of the parent compounds.
The regulatory follow‐up to this scientific opinion was discussed at the Expert Committee ‘Agricultural contaminants’ on 15 January 2015. The Standing Committee on Plants, Animals, Food and Feed has been informed thereof at its meeting on 11 February 2015.2
Before taking regulatory measures as regards the modified mycotoxins, it was agreed that it is appropriate to request EFSA to assess whether it is appropriate and feasible to set a group health based guidance value (group HBGV) for the parent compound and its modified forms and to consider, if relevant, the appropriateness to use the parent compound as a marker for the presence and toxicity of the parent compound and its modified forms.
1.2. Terms of Reference as provided by the requestor
In accordance with Art. 29 (1) (a) of Regulation (EC) No 178/2002, the Commission asks European Food Safety Authority (EFSA) for scientific opinions to assess whether it is appropriate and feasible to set a group HBGV for the parent compound and its modified forms for zearalenone, fumonisins, nivalenol, and T‐2 and HT‐2 toxins and to consider, if relevant, the appropriateness to use the parent compound as a marker for the presence and toxicity of the parent compound and its modified forms for these mycotoxins.
The four requested scientific opinions are:
assessment whether it is appropriate and feasible to set a group HBGV for zearalenone and its modified forms identified in the CONTAM opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed, and to consider, if relevant, the appropriateness to use the parent compound as a marker for the presence and toxicity of zearalenone and its modified forms.
assessment whether it is appropriate and feasible to set a group HBGV for fumonisin B1 and B2 and their modified forms identified in the CONTAM opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed and to consider, if relevant, the appropriateness to use the parent compounds as a marker for the presence and toxicity of fumonisin B1 and B2 and their modified forms.
assessment whether it is appropriate and feasible to set a group HBGV for nivalenol and its modified forms identified in the CONTAM opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed and to consider, if relevant, the appropriateness to use the parent compound as a marker for the presence and toxicity of nivalenol and its modified forms.
assessment whether it is appropriate and feasible to set a group HBGV for T‐2 and HT‐2 toxins and their modified forms identified in the CONTAM opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed and to consider, if relevant, the appropriateness to use the parent compound as a marker for the presence and toxicity of T‐2 and HT‐2 toxins and their modified forms.
1.3. Introduction to modified mycotoxins
Mycotoxins are secondary metabolites of filamentous fungi. They are usually low molecular weight compounds and serve no function in the intermediary metabolism of the fungus, but provide advantages with respect to its competition for nutrients and habitat. Consequently, many mycotoxins are toxic for bacteria and other microorganisms. As mycotoxins are also toxic for humans and animals, their presence in food and feed may pose a health risk.
Numerous mycotoxins have been characterised. Even though some of these may be metabolites of the other, they are recognised as separate mycotoxins. However, it is increasingly realised that also modified forms of these mycotoxins occur in food and feed and that these modified forms should be taken into account for risk assessment, because they may contribute to the toxicity of the parent toxin.
According to a recent definition, modified forms of mycotoxins comprise all biologically, chemically and physically modified derivatives of the parent molecule, which are formed in the fungus, infested plant and mammalian organism (Rychlik et al., 2014). This includes inter alia phase I metabolites formed through oxidation, reduction or hydrolysis of the parent toxin, as well as phase II metabolites arising from conjugation with endogenous molecules. Phase II metabolites formed in the plant through conjugation with polar low molecular weight molecules such as glucose or sulfate have also been called ‘masked’ mycotoxins because they were hard to capture by routine analysis (Rychlik et al., 2014). However, after intake with the food or feed such conjugates may be hydrolysed in the digestive tract, thereby releasing the parent toxin which may add to the total uptake of toxin. Therefore, phase II metabolism in the plant or fungi is of paramount importance for the risk assessment of mycotoxins.
In the context of risk assessment of mycotoxins in food and feed, modified mycotoxins comprise all metabolites of a given mycotoxin that occur in food or feed. These include phase I and II metabolites formed in the fungus, infested plant used for food and feed or food (and feed) products of animal origin. It does not include metabolites formed in humans, even if these may be similar.
1.4. Legislation
Article 2 of Council Regulation (EEC) No 315/933 stipulates that food containing a contaminant in an amount unacceptable for public health shall not be placed on the market, that contaminant levels should be kept as low as can reasonably be achieved and that, if necessary, the European Commission may establish maximum levels for specific contaminants. These maximum levels are laid down in the Annex of Commission Regulation (EC) No 1881/20064 and may include maximum levels (MLs) for the same contaminants in different foods, analytical detection limits and reference to the sampling and analysis methods to be used. Neither for nivalenol nor for nivalenol metabolites have MLs have been set in the regulation.
1.5. Interpretation of Terms of Reference
The CONTAM Panel took the assumption that the previous risk assessment of nivalenol in food and feed (EFSA CONTAM Panel, 2013) is comprehensively covering all relevant aspects of the compound and therefore used it together with the recent opinion on modified mycotoxins (EFSA CONTAM Panel, 2014) as a starting point for the present assessment.
The CONTAM Panel decided to review the new relevant data on nivalenol (i.e. that published after 2012) to evaluate whether the tolerable daily intake (TDI) established for nivalenol in 2013 needs to be revised and if in addition there is a need also to set an acute reference dose for nivalenol.
The Panel decided to address the modified forms of nivalenol identified to date and reviewed the appropriateness of the methods currently available for their analysis.
In line with the previous EFSA opinion on modified mycotoxins (EFSA CONTAM Panel, 2014), modified nivalenol occurring in plants (arising from both plant and fungal metabolism), formed as a consequence of food processing and transfer from feed to livestock were considered for possible inclusion in group HBGVs.
In order to assess whether it was appropriate to include the modified forms of nivalenol in group HBGVs with nivalenol, all data available and relevant for that task were evaluated.
2. Data and methodologies
2.1. Methodology for data collection and study appraisal
The CONTAM Panel considered the previous assessments on nivalenol (EFSA CONTAM Panel, 2013) and on modified mycotoxins that included modified nivalenol (EFSA CONTAM Panel, 2014) as comprehensive, covering all relevant publications on nivalenol and its modified forms, respectively, until those dates. All publications referenced therein have been considered, wherever appropriate, also for the present evaluation.
In order to cover also new publications not considered in these previous assessments, a systematic and comprehensive search for literature was conducted for peer‐reviewed original research pertaining to nivalenol and its modified forms published after 2012 including scientific literature dealing with analytical determination, chemistry, occurrence, toxicokinetics and toxicity of nivalenol and/or its modified forms. Studies on analytical methods, chemistry and occurrence of the parent compound nivalenol only, however, were excluded because not considered of relevance for the present assessment.
Date, search strings, databases used and numbers of publications retrieved and used for assessment are presented in detail in Appendix A. In total, 371 citations/abstracts were obtained. Those considered relevant by expert judgement were included in the present assessment. Only papers in English language were considered for inclusion in the assessment.
During the development of the opinion, additional relevant studies published until January 2017 and not retrieved in the above‐mentioned literature evaluation have been identified and considered for the assessment when relevant.
2.2. Methodology applied for hazard assessment
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by WHO/IPCS (2009), which include hazard identification and characterisation, exposure assessment and risk characterisation. In addition to the principles described by WHO/IPCS (2009), any EFSA guidance pertaining to risk assessment and relevant for the present assessment has been duly considered for the present assessment.
3. Previous assessments
A comprehensive risk assessment of NIV in food and feed was published in 2013 (EFSA CONTAM Panel, 2013). In this opinion, immunotoxicity and haematotoxicity were identified as the critical effects of NIV for the allocation of a TDI. In an oral 90‐day rat study, effects such as neutropenia, leucopenia, erythropenia and thrombocytopenia were observed. The decrease in white blood cell (WBC) count was identified as the most appropriate endpoint for benchmark dose (BMD) modelling. The 95% lower confidence limit for the benchmark dose response of 5% extra risk (BMDL05) was calculated based on combined data for male and female animals and was 0.35 mg NIV/kg body weight (bw) per day. Applying a default uncertainty factor (UF) of 100 for inter‐ and intraspecies differences and an additional UF of 3 for gaps in the database on NIV to the BMDL05, a TDI of 1.2 μg/kg bw was established for NIV.
In 2014, a risk assessment on human and animal health related to the presence of modified forms of certain mycotoxins in food and feed was carried out (EFSA CONTAM Panel, 2014). In this opinion, no specific information on the toxic effects of the modified forms of NIV could be identified. However, the chemistry and toxicokinetics of NIV as well as general considerations of biotransformation suggested that NIV conjugates may be cleaved in the gastrointestinal tract releasing NIV. Taking a pragmatic approach until more information became available, the CONTAM Panel assumed that modified forms of NIV have the same toxicological profile and potency as their parent compounds. Based on occurrence data available at that time (2014), it was then assumed that modified forms of NIV add another 30% to the exposure to NIV.
4. Chemistry
NIV (Figure 1) is a member of the trichothecene family, which represents the largest group of Fusarium mycotoxins and comprises more than 150 compounds. The common structure of all trichothecenes is a tetracyclic sesquiterpene with a spiro‐epoxide group at C‐12 and C‐13, and an olefinic double bond between C‐9 and C‐10. According to the substituents of the tetracyclic ring system, trichothecenes are grouped into four different types (A–D). Type A and B compounds constitute the majority of trichothecene contaminants in food and feed. Typical type A trichothecenes are characterised by an unsubstituted C‐8 position or an esterified or free hydroxyl group at C‐8, e.g. diacetoxyscirpenol, T‐2 toxin or neosolaniol, respectively. Nivalenol belongs to the type B trichothecenes, all of which carry a keto group at C‐8. Other typical type B trichothecenes are deoxynivalenol and fusarenon‐X (Figure 1). Fusarenon‐X (FUS‐X, 4‐acetylnivalenol) is the biosynthetic precursor of nivalenol (see below). Deoxynivalenol (DON), which is not a nivalenol metabolite but a mycotoxin in its own right, must not be confused with de‐epoxy‐nivalenol (DE‐NIV), which is a phase I metabolite of NIV as it is also a deoxy derivative (see below).
Figure 1.
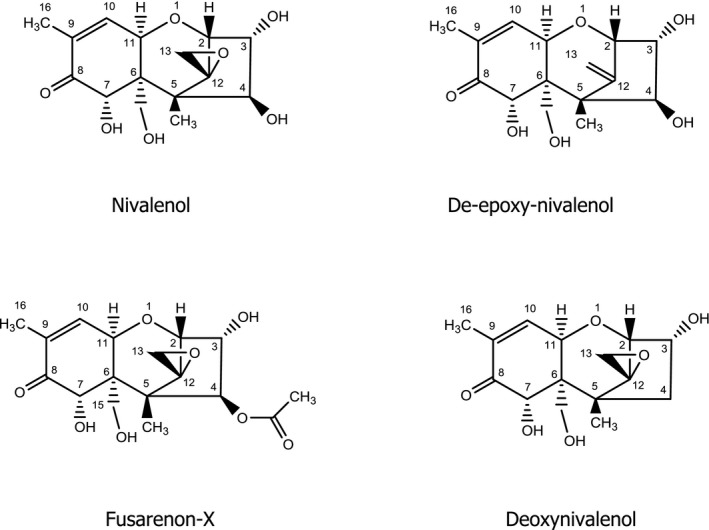
Chemical structures of nivalenol and related compounds
The biosynthesis of trichothecenes involves the initial cyclisation of farnesyl phosphate to the bicyclic sesquiterpene trichodiene, which does not contain oxygen, followed by a complex sequence of hydroxylation, epoxidation, further cyclisation, and acylation reactions (for review, see McCormick et al., 2011). NIV arises through hydrolysis of the 4‐acetoxy group of its biosynthetic precursor FUS‐X.
In general, microorganisms, plants, and animals may biotransform mycotoxins by phase I metabolic pathways, which include oxidation, reduction and hydrolysis of the parent (fungal) compounds, and by phase II metabolism, which comprises all conjugation reactions. Although phase II metabolites formed in the mammalian organism are usually excreted via urine and/or bile, plants store conjugated metabolites in vacuoles and/or attach them to structures of the cell wall. The latter processes are sometimes referred to as phase III metabolism or compartmentation.
4.1. Parent compound and metabolites
NIV has the IUPAC name 3α,4β,7α,15‐tetrahydroxy‐12,13‐epoxytrichothec‐9‐en‐8‐one (CAS No. 23282‐20‐4, C15H20O7, MW 312). It is a colourless crystalline powder with a melting point (m.p.) of 222–223°C, and was first isolated from cultures of Fusarium nivale strain Fn2B and given the name nivalenol by Tatsuno et al. (1968).
The solubility of NIV is low in water and petroleum ether, but good in organic solvents of medium to high polarity (EFSA CONTAM Panel, 2013). Solutions of NIV in acetonitrile are stable for at least 24 months at temperatures up to 25°C, whereas solutions in ethyl acetate are only stable for up to one year if kept at 0°C (Widestrand and Pettersson, 2001). The maximum UV absorption of NIV in acetonitrile is at 220 nm with a molar absorptivity coefficient of 6,955 ± 205 L/mol (Sydenham et al., 1996; Krska et al., 2007).
Although NIV has a short trivial name, it is commonly abbreviated as NIV in the scientific literature. This abbreviation is also used in this Opinion, mainly because it is of advantage for abbreviating the more complex names of the phase II metabolites of NIV.
As discussed in more detail in Sections 6 and 7.3, only one phase I metabolite of NIV has been identified. It is listed in Table 1 and its chemical structure is depicted in Figure 1. As NIV and its metabolite lack a strong chromophore, the analytical method of choice is often liquid chromatography–mass spectrometry (LC–MS) analysis, which has the advantage of an efficient separation from complex matrices and high sensitivity (see Section 5). Therefore, the element formulas and molecular weights of NIV and its metabolites are given in Table 1.
Table 1.
Nivalenol and its metabolites
| Compounda | Element formula | Molecular weight |
|---|---|---|
| NIV | C15H20O7 | 312 |
| DE‐NIV | C15H20O6 | 296 |
| NIV‐3‐β‐Glc | C21H30O12 | 474 |
DE: de‐epoxy; Glc: glucose; NIV: nivalenol.
De‐epoxy‐nivalenol (DE‐NIV) has first been isolated from the faeces of rats after oral administration of NIV (Onji et al., 1989). The chemical structure has been elucidated by the same authors using mass spectrometry (MS) as well as 1H and 13C nuclear magnetic resonance (NMR).
In general, most phase II metabolic pathways lead to the formation of water‐soluble conjugates through covalent binding of the parent compound or a phase I metabolite to a polar molecule, e.g. a carbohydrate or sulfate, from the metabolising organism. In most cases, activation of the conjugating moiety and a transferase enzyme are needed to form the covalent bond. Sulfate is used as conjugate group by fungi, plants and animals. The major carbohydrate employed for conjugation by plants and fungi is glucose, whereas animals use glucuronic acid. The glucose can be further modified in plants, e.g. by esterification with another hexose or with malonic acid. Moreover, plants can use acetate and ferulate for conjugation. The major conjugating moieties in plants are depicted in Figure 2.
Figure 2.
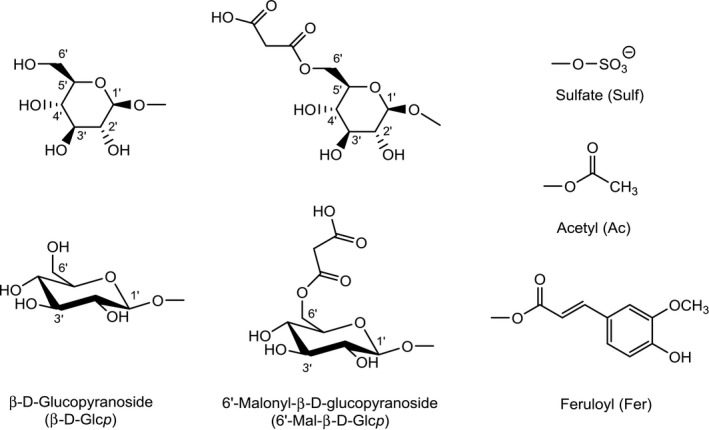
Glucose (depicted in two different stereochemical representations), modified glucose, and sulfate, feruloyl and acetyl groups used for the conjugation of mycotoxins in plants
For the abbreviation of the carbohydrates and their modified forms used in phase II metabolism, the same nomenclature as in the Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed (EFSA CONTAM Panel, 2014) in the Scientific Opinion on the appropriateness to set a group‐based guidance value for zearalenone and its modified forms (EFSA CONTAM Panel, 2016), and in the Scientific Opinion on the appropriateness to set a group‐based guidance value for T‐2 and HT‐2 toxins and their modified forms (EFSA CONTAM Panel, 2017) will be used. These abbreviations, which are common in carbohydrate chemistry, clearly designate the specific carbohydrate (e.g. Glc for glucose, Man for mannose, Xyl for xylose) and its oxidation state (e.g. GlcA for glucuronic acid). For a complete designation, also the type of ring (p for pyranose or f for furanose), the configuration (d or l), and the type of glycosidic bond (α or β) could be given, if known. For further details, see Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed (EFSA CONTAM Panel, 2014).
To date, the only reported phase II metabolite of NIV is its 3‐O‐β‐d‐glucopyranoside (NIV‐3‐β‐Glc, short form NIV3Glc, which is used in this opinion for the compound, Figure 3), which was isolated by Yoshinari et al. (2014) from NIV‐contaminated wheat and structurally identified by LC–MS and 1H and 13C NMR.
Figure 3.
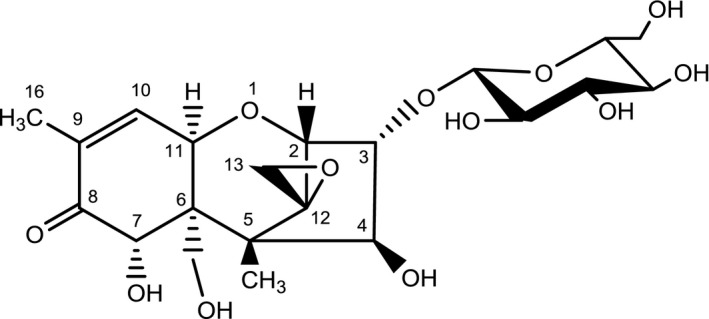
Chemical structure of NIV‐3‐β‐Glc
The fact that other modified forms of NIV have not yet been observed is probably due to the scarcity of studies aiming at their disclosure in fungi, plants and animals. Because NIV has four hydroxyl groups, it must be expected to undergo conjugation with sulfate, acetate and glucuronic acid, as has been described for other trichothecenes such as DON and T‐2 toxin (Wu et al., 2012). The detection of such conjugates of NIV must be expected in future studies.
4.1.1.
Concluding remarks
The only phase I metabolite of NIV identified is de‐epoxy‐NIV (DE‐NIV). The only phase II metabolite identified is NIV‐3‐β‐Glc (NIV3Glc). It is expected that other conjugated metabolites of NIV are formed in plants and fungi.
5. Analytical methods
There is a wide range of methods for analysis of NIV in the literature, which can be applied for both food and feed, among them mainly grains. The CONTAM Panel has recently extensively reviewed the analytical methods developed for NIV (EFSA CONTAM Panel, 2013); thus, this opinion will consider only those methods published since 2013. The accurate quantification of NIV is usually performed together with DON and other major trichothecenes, using liquid chromatography–tandem mass spectrometry (LC–MS/MS) with a multitoxin approach. Methods can be applied to food, feed and other biological samples. As for other Fusarium mycotoxins, sample extraction is based on mixtures of acetonitrile/water or methanol/water acidified with acetic or formic acid (Malachová et al., 2014; Nathanail et al., 2015). Due to the higher polarity of NIV, it is strongly affected by matrix effects. Therefore, although the sensitivity is often higher compared with other trichothecenes, recovery rates are usually lower (Rubert et al., 2012; Capriotti et al., 2014; Malachová et al., 2014). Albeit the common sample preparation is based on ‘dilute & shoot’ approach, cross‐reactive immunoaffinity columns made for DON are still in use for sample clean‐up (Capriotti et al., 2014; Yang et al., 2015). Similarly, dispersive solid‐phase extraction (DSPE) and QuEChERS (quick, effective, cheap, rugged and save) have been proposed as sample clean‐up (Rubert et al., 2012; Zhao et al., 2014). Even if the matrix effect was lowered, recovery rate for NIV was only about 60%.
Methods based on liquid chromatography photodiode array detection (LC‐PDA), liquid chromatography fluorescence detection (LC‐FLD) or gas chromatography–mass spectroscopy (GC–MS) are still in use in control laboratories, although such protocols require extensive sample purification and/or a derivatisation step prior to the analysis. This affects significantly the overall recovery and sensitivity (Nardiello et al., 2014; Pascale et al., 2014; Rodríguez‐Carrasco et al., 2014; Trombete et al., 2016).
In consideration of the little knowledge on modified forms of NIV, their detection is mainly based on screening or semiquantitative LC–MS/MS methods, without properly optimised analytical parameters (Nakagawa et al., 2011; Yoshinari et al., 2014). Such methods might be inadequate with regard to recovery and sensitivity.
None of the currently available analytical methods for NIV and its modified forms has been fully validated in interlaboratory studies. Calibrants are available on the market but not reference materials.
The higher polarity of NIV compared to other trichothecenes leads to a more difficult analytical detection under the same chromatographic conditions. Phase II metabolism would increase the polarity and thus conjugates would be even more difficult to analyse. Therefore fit‐for‐purpose methods should be designed for an accurate detection of NIV and its modified forms.
Due to the lack of antibodies specifically designed for NIV, no rapid immunochemical methods are available. Cross‐reactive antibodies designed for DON have been exploited for combined detection of DON and NIV (Veršilovskis et al., 2011; Polakowski et al., 2015), but their potential use for modified forms has not been investigated.
6. Occurrence of nivalenol and its modified forms
In 2013, the CONTAM Panel extensively evaluated the occurrence of NIV in food commodities (EFSA CONTAM Panel, 2013). As previously described, NIV occurs mainly in grains, in particular in maize, oats and wheat. Most of the reports indicate the co‐occurrence of NIV with DON and minor amounts of other trichothecenes. Since then several publications have been identified by the CONTAM Panel which generally confirm the previous ones regarding the occurrence of NIV (Lindblad et al., 2013; Rodríguez‐Carrasco et al., 2013, 2015; Alkadri et al., 2014; Covarelli et al., 2015; Beccari et al., 2016; Gromadzka et al., 2016).
The CONTAM Panel did not identify any reports on occurrence of phase I metabolites of NIV, and only few studies have been identified reporting occurrence of phase II conjugates.
A monoglucoside of NIV has been reported for the first time in wheat grain that was artificially infected with Fusarium fungi (Nakagawa et al., 2011). LC–MS was used for detection and did not allow to elucidate the exact structure, i.e. position of the glucose moiety. According to the authors, more than 15% of NIV was present as glucoside.
More recently, a glucoside of NIV has also been detected in wheat by Yoshinari et al. (2014) and was identified as NIV3Glc using NMR. It accounted for 12–27% of the total NIV present in the wheat samples.
The co‐occurrence of NIV and NIV3Glc was reported in Finnish cereals by Nathanail et al. (2015). The authors analysed barley (n = 34), oats (n = 31) and wheat (n = 30) harvested in 2015. In barley, 73% of the samples contained NIV and 62% contained NIV3Glc. Corresponding figures for oats where 71% and 16%, and those for wheat 43% and 10%, respectively. For NIV, the mean and maximum concentrations were 96 and 302 μg/kg in barley, 635 and 4,940 μg/kg in oats, and 49 and 74 μg/kg in wheat, respectively. Corresponding figures for NIV3Glc were 25 μg/kg (24% as compared with NIV) and 65 μg/kg (22% as compared with NIV) in barley, 36 μg/kg (6% as compared with NIV) and 58 μg/kg (1.2% as compared with NIV) in oats, and 23 μg/kg (47% as compared with NIV) and 33 μg/kg (45% as compared with NIV) in wheat, respectively.
The CONTAM Panel noted that FUS‐X, which is reported in the literature as a mycotoxin on its own, is also a precursor in the fungal biosynthesis of NIV and frequently co‐occurs with NIV.
The transfer of NIV from feed to food products of animal origin is expected to contribute only marginally to human exposure.
7. Toxicokinetics of modified forms of nivalenol
Toxicokinetics of NIV have been reviewed in the previous opinion on NIV and rapid absorption, distribution and excretion via faeces without accumulation was reported in a few studies using experimental or farm animals (EFSA CONTAM Panel, 2013).
7.1. Absorption
Generally, NIV was rapidly absorbed in all vertebrates investigated, i.e. rodents, pigs and poultry (EFSA, CONTAM Panel, 2013) although new data (not considered in the previous opinion) show a low bioavailability (4%) with a peak plasma concentration at 2.4 h after administration of a single oral dose of 0.8 mg/kg bw to young chicken (Kongkapan et al., 2016a). No in vivo data are available on the absorption of NIV3Glc but it has been shown in vitro that it is not efficiently transported through intestinal epithelial cell monolayers (Gratz et al., 2017). However, based on in vitro data with bacteria (Gratz et al., 2017), it can be assumed that NIV3Glc is hydrolysed in the large intestine, releasing its aglycone, which can then be absorbed.
7.2. Distribution
The very limited studies regarding the tissue distribution of NIV (described in EFSA CONTAM Panel, 2013) show that NIV is distributed into tissues without accumulation and can also be transported into fetal or suckling mice. In a recent publication, Kongkapan et al. (2016a) report that NIV reaches peak concentrations in the liver, kidney, muscle and heart of broiler chicken 3 h after a single oral dose of 0.8 mg/kg bw, followed by a marked decline after 6 and 12 h; the plasma elimination half‐life was 2.5 h.
7.3. Metabolism
De‐epoxidation is the only phase I metabolic pathway of NIV known to date. DE‐NIV has been demonstrated in the faeces of rats, pigs and poultry, and it is assumed to be generated by the gastrointestinal microflora (Wu et al., 2010; EFSA CONTAM Panel, 2013; Kongkapan et al., 2016b). De‐epoxidation is considered to be a detoxification reaction as DE‐NIV is much less toxic in vitro compared with NIV (Eriksen et al., 2004).
7.4. Excretion
Upon multiple oral exposure of male Wistar rats to NIV, 80% of the total administered dose were recovered from the faeces as DE‐NIV and 7% as parent NIV; in urine, DE‐NIV and NIV each accounted for only 1% of the dose (Onji et al., 1989).
8. Toxicity
8.1. In vivo toxicity data on nivalenol
8.1.1. Study used for establishing the TDI by EFSA 2013
In its previous opinion (EFSA CONTAM Panel, 2013), the CONTAM Panel concluded that immunotoxicity and haematotoxicity are the critical endpoints of NIV and that there is no evidence for genotoxicity and carcinogenicity for NIV. The CONTAM Panel based its evaluation on a 90‐day rat dietary study where NIV was given at doses equivalent to 0.4, 1.5 and 6.9 mg/kg bw per day to male rats and 0.4, 1.6 and 6.4 mg/kg bw per day to female rats (Sugita‐Konishi et al., 2008; Takahashi et al., 2008). A significant decrease in the white blood cell (WBC) count was found at the highest dose of 6.9 mg/kg bw per day in males and at all doses in females. In addition, at the highest dose, decreased platelet counts in both sexes, decreased red blood cell counts in males and decreased haemoglobin concentration in females were observed. Hypocellularity and decrease in haematopoietic cell number in the bone marrow of femur and sternum at the highest dose was observed in most male and female rats. The CONTAM Panel concluded in 2013 that the lowest dose of 0.4 mg/kg bw per day tested in this study should be considered as the lowest‐observed‐adverse‐effect‐level (LOAEL) for NIV.
8.1.2. In vivo toxicity studies with NIV published after 2013
8.1.2.1. Acute toxicity
Mice
In order to evaluate acute anorectic effects groups of eight female mice were administered orally 0, 0.01, 0.1, 1 and 5 mg/kg bw NIV. After fasting, mice were given toxin and then immediately provided with pre‐weighed food and food intake was measured after 2, 16, 24, 36, 48, 60 72 and 96 h. Only after the first 2 h was significantly reduced feed uptake observed corresponding to 28% (1 mg/kg bw NIV) and 44% (5 mg/kg bw) less food as compared with the negative control. Doses of 0.01 mg/kg bw NIV had no effect and a slight decrease at 0.1 mg/kg bw NIV was not significantly different. The no‐observed‐adverse‐effect‐levels (NOAELs) and LOAELs identified by the authors for NIV were 0.1 and 1 mg/kg bw, respectively (Wu et al., 2012).
Mink
Female mink (six animals per group), were administered 0, 0.05, 0.1, 0.25 and 0.5 mg NIV/kg bw by gavage (Wu et al., 2013). Animals were then monitored for emesis for 3 h during which the incidence of emesis, latency to emesis, emesis duration and number of emetic events were recorded. No emesis was observed in controls and the two lowest dose group, whereas doses of 0.25 and 0.5 mg/kg bw induced emesis in four out of six and in six out of six animals, respectively. According to the authors, the NOAEL for emesis was 0.1 mg/kg bw per day and the corresponding LOAEL 0.25 mg/kg bw per day. In the same study, minks were given NIV intraperitoneally (i.p.) at similar doses and likewise emesis was observed, albeit already at slightly lower doses.
8.1.2.2. Subacute toxicity
Mice
Three‐week‐old ICR (an outbred mouse strain)‐derived glomerulonephritis (ICGN) mice, a strain that is sensitive to kidney effects, were not more susceptible to NIV than 3‐week‐old ICR mice or adult animals when given oral doses of NIV equivalent to 0, 0.8, 1.6 and 3.2 mg/kg bw per day for 4 weeks, suggesting, according to the authors that kidneys of infant mice are not sensitive to NIV under the treatment conditions (Inoue et al., 2014).
8.1.3. In vitro toxicity studies with NIV published after 2013
New data on in vitro toxicity of NIV published since the last evaluation (EFSA CONTAM Panel, 2013) confirm previously known effects such as induction of apoptosis, cytotoxicity, increased secretion of anti‐haematopoietic macrophage inflammatory proteins, reduction of mitochondrial activity and reduced cell proliferation (Wan et al., 2013a,b,c; Nagashima and Nakagawa, 2014; Park et al., 2014; Cheat et al., 2015; Del Regno et al., 2015; Vejdovszky et al., 2016).
8.1.4. Genotoxicity studies with NIV published after 2013
Le Hégarat et al. (2014) carried out a battery of in vivo assays (Comet assay, micronucleus assay, Pig‐a assay) with NIV in a series of organs (duodenum, colon, blood, liver, spleen, kidney, bone marrow). In addition in vitro Comet assays with TK6 (thymidine kinase heterozygous cell line) cells were performed. The authors report consistently negative results from all investigations.
8.2. Toxicity data on modified forms of nivalenol
8.2.1. In vivo toxicity data
No relevant in vivo data have been identified by the CONTAM Panel.
8.2.2. In vitro toxicity data
Eriksen et al. (2004, cited also in EFSA CONTAM Panel, 2013) studied the cytotoxicity of NIV and DE‐NIV in 3T3 mouse fibroblasts using the incorporation of bromodeoxyuridine (BrdU) into DNA. The inhibitory concentration 50% (IC50) value for DE‐NIV for DNA synthesis was 55 times higher than the IC50 for NIV. The low in vitro toxicity of DE‐NIV as compared with NIV suggests that de‐epoxidation is a strong detoxification reaction.
9. Mode of action for toxicity of nivalenol and its modified forms
The possible mode of action of NIV toxicity has been discussed in the previous opinion on NIV (EFSA CONTAM Panel, 2013) where only a few publications concerning its mode of action have been presented. Similar to other trichothecenes, NIV targets the ribosome leading to an inhibition of protein‐, RNA‐ and DNA synthesis with inhibition of protein synthesis as the primary event. The ribosome binding activates mitogen‐activated protein kinase (MAPK) via a mechanism known as the ‘ribotoxic stress response’. MAPKs are important transducers of downstream signalling events related to immune response and apoptosis.
As the removal of the epoxy group strongly reduces toxicity of NIV, it can be assumed that the epoxy group is central for the binding to the ribosomes, the initial key event in NIV toxicity. The key role of the epoxide group for the binding to the A site of the ribosome peptidyl transferase centre is generally assumed for type A and B trichothecenes and has recently been shown by molecular modelling for DON (Pierron et al., 2016).
Rapidly proliferating tissues such as haematopoietic tissue are targets of NIV leading to leucopenia and thrombocytopenia possibly due to induction of apoptosis (EFSA CONTAM Panel, 2013).
Using both pig jejunum explants and pig intestinal loops, Cheat et al. (2015) observed a 30% decrease of proliferation after 4 h NIV exposure. NIV also increased apoptosis at the top of villi and reduced by almost half the proliferative/apoptotic cell ratio. Lamina propria cells (mainly immune cells) were more sensitive than enterocytes (epithelial cells) to apoptosis induced by NIV.
Recent data have also confirmed the effects of NIV on inflammation. In vitro, NIV increased mRNA expression of pro‐inflammatory cytokines (IL1α, IL1β, IL8, TNFα and MCP‐1) in the porcine jejunal epithelial cell line IPEC‐J2 (Wan et al., 2013b). In vivo, an acute oral exposure of mice to NIV induced a transient splenic inflammatory response as measured by the mRNA expression of cytokines IL‐1β, CXCL‐2, CCL‐2 and CCL‐7 (Wu et al., 2014a). This response was lower than the one observed with a similar dose of DON.
As other trichothecene mycotoxins, NIV induced anorexia/emesis and growth suppression. The mechanism underlying these effects is not fully understood. However, it was demonstrated that oral increasing doses of NIV, administered by oral gavage, correlate with increased plasma concentrations of the gut satiety peptides cholecystokinin (Wu et al., 2014b). This suggests a mechanism of action similar to that of other trichothecenes such as DON and T2 (EFSA CONTAM Panel, 2017).
10. Dose–response analysis
10.1. Acute effects
As described in Section 8.1.2, Wu et al. (2013) dosed mink dosed orally with 0, 0.05, 0.1, 0.25 and 0.5 mg NIV/kg bw. Emesis was observed at doses of 0.25 mg/kg bw (in four out of six animals) and at the highest dose of 0.5 mg/kg bw (in six out of six animals). According to the authors, the NOAEL for emesis was 0.1 mg/kg bw per day and the LOAEL 0.25 mg/kg bw per day. The CONTAM Panel decided that this study was the most appropriate for setting and acute HBGV for NIV.
Using a benchmark response (BMR) of 10% (BMD10) resulted in a benchmark dose 10% (BMD10) total confidence interval (BMDL10–BMDU10) of 0.14–0.23 mg NIV/kg bw, averaging the accepted models. The CONTAM Panel used the BMDL10 value of 0.14 mg NIV/kg bw as a reference point for characterising the acute toxicity of NIV (for details on the BMD modelling, see Appendix B).
10.2. Chronic effects
No new dose–response analysis was performed as there were no new relevant in vivo studies identified.
11. Establishment of health based guidance values
11.1. Establishment of a group ARfD
The CONTAM Panel decided to use the BMDL10 of 0.14 mg NIV/kg bw derived for emetic response in mink as a reference point for establishing a group ARfD for NIV. An UF of 10 for intraspecies variability was applied. No interspecies variability factor was applied because humans were not considered more sensitive than mink to the acute emetic effect of NIV, an assumption supported by studies on emesis with emetine showing that similar doses of the compound are effective both in humans and mink (Gordon, 1985; Zhang et al., 2006; Percie du Sert et al., 2012). Based on the above data and considerations, an ARfD of 14 μg NIV/kg bw was established by the CONTAM Panel.
Because the phase II metabolite NIV3Glc is assumed to be hydrolysed to NIV after ingestion, it is included in a group ARfD with the same molar potency as NIV.
11.2. Establishment of a group TDI
The CONTAM Panel confirmed the previously established TDI of 1.2 μg/kg bw based on a BMDL05 of 0.35 mg/kg bw per day and an UF of 300 (EFSA CONTAM Panel, 2013). Because the phase II metabolite NIV3Glc is assumed to be hydrolysed to NIV after ingestion, it is included a group TDI with the same molar potency as NIV.
12. Uncertainties
The CONTAM Panel identified several uncertainties in their evaluation of the appropriateness to set a group HBGVs for NIV and its modified forms.
Upon reviewing new data, the TDI set for NIV in 2013 (EFSA CONTAM Panel, 2013) has been retained because no new studies relevant for setting a chronic health based guidance value have been identified in the period since then. Consequently, all uncertainties associated with establishment of the TDI as described in the previous opinion (EFSA CONTAM Panel, 2013) are also valid for the present opinion.
An ARfD for NIV was established based on observations of emesis in an acute study with NIV in mink. A total confidence interval (BMDL10–BMDU10) of 0.14–0.23 mg NIV/kg bw was obtained using model averaging in the dose–response analysis. The large dose spacing at the lower doses in these studies and the small number of animals used resulted in a BMDL10 calculation associated with considerable uncertainty. In addition, the steepness of the dose–response curves between two dose groups add to the uncertainty.
In the absence of in vivo toxicokinetic and toxicity data on NIV3Glc, the CONTAM Panel assumed the release of NIV upon ingestion and that therefore NIV3Glc should be included in the group HBGVs with the same molar potency as NIV (i.e. 1).
The higher polarity of NIV compared with that of other trichothecenes leads to a more difficult analytical detection under the same chromatographic conditions. Further phase II metabolism would increase the polarity and thus conjugates would be even more difficult to analyse. It is, therefore, likely that there are other phase II metabolites of NIV not yet identified.
12.1. Summary of uncertainties
In Table 2, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the source of uncertainty leads to over/underestimation of the resulting risk.
Table 2.
Summary of the qualitative evaluation of the impact of uncertainties on the assessment
| Sources of uncertainty | Directiona |
|---|---|
| All uncertainties associated with setting the TDI for NIV in 2013 | See EFSA CONTAM Panel (2013) |
| Assumption of complete cleavage of NIV3Glc to NIV | + |
| Derivation of an ARfD based on a single study with mink | +/− |
TDI: tolerable daily intake; NIV: nivalenol; NIV3Glc: nivalenol‐3‐glucoside.
+ = uncertainty with potential to cause overestimation of exposure/risk; − = uncertainty with potential to cause under‐estimation of exposure/risk, +/− = extent of potential over/underestimation might differ in direction.
The overall uncertainty associated with the inclusion of NIV3Glc into a group ARfD and a group TDI in the present assessment is considered as high and it would rather overestimate than underestimate any risk.
13. Conclusions
13.1. Introduction
NIV is a member of the type B group trichothecenes, which are tetracyclic sesquiterpenoids produced by many fungal species infesting crop plants. In addition to NIV, produced by Fusarium species, plants and fungi generate phase I and phase II metabolites. To date, the only phase I metabolite of NIV identified is DE‐NIV and the only phase II metabolite is NIV3Glc. It is expected that other conjugated metabolites of NIV are formed in plants and fungi. Because of a lack of significant toxicity DE‐NIV was not further considered in the assessment. Analytical methods (mainly based on LC–MS/MS) for NIV and NIV3Glc are available. Due to high polarity of both compounds, recovery rates are often affected by matrix effects which may lead to poor recovery. Standards and reference materials are not commercially available for modified forms of NIV. The higher polarity of NIV compared to other trichothecenes leads to a more difficult analytical detection under the same chromatographic conditions. As further phase II metabolism would increase the polarity, conjugates would be even more difficult to analyse. It is therefore likely that there are other phase II metabolites not yet identified.
13.2. Occurrence of modified forms of nivalenol
NIV3Glc may occur in cereals amounting up to 50% of its parent form.
The transfer of NIV from feed to food products of animal origin is expected to contribute only marginally to human exposure.
13.3. Toxicokinetics of NIV and its modified forms
Based on few studies available, the CONTAM Panel concluded that NIV is rapidly absorbed, distributed and eliminated without accumulation.
With the exception of DE‐NIV, which is generated by microbiota in the rumen and also in the gut of monogastric animals, no other NIV metabolites have been identified in mammals.
No in vivo data are available on the absorption of NIV3Glc, but in vitro data indicate that it is not absorbed. In vitro data suggest, however, that it is hydrolysed by bacteria in the gastrointestinal tract and then absorbed as NIV.
13.4. Toxicity of NIV and its modified forms
In vivo acute toxicity studies show that NIV has anorectic effects upon short term exposure in mice and mink. Emetic events observed upon single oral and i.p. exposure to NIV in mink were identified as the critical acute effect.
In the previous opinion on NIV, it was concluded that there is no evidence that other toxic effects occur at doses lower than those inducing immunotoxicity and haematotoxicity and that there is no evidence for genotoxicity or carcinogenicity of NIV.
De‐epoxidation has been shown to markedly reduce the toxicity of trichothecenes such as DON and T2. This mechanism of detoxification also operates for NIV which has been shown in vitro.
There is no toxicity information on NIV3Glc but it can be assumed that it is hydrolysed to and absorbed in the lower intestinal tract as NIV.
13.5. Mode of action for toxicity
NIV‐induced anorexia correlates with increased plasma concentrations of the gut satiety peptide cholecystokinin, suggesting a similar mode of action to that of other trichothecenes such as DON.
NIV targets the ribosome leading to an inhibition of protein, RNA and DNA synthesis. The ribosome‐binding activates MAPKs, which are important transducers of downstream signalling events related to immune response and apoptosis.
Rapidly proliferating tissues such as haematopoietic tissue are targets of NIV leading to leukopenia and thrombocytopenia possibly due to induction of apoptosis (EFSA CONTAM Panel, 2013).
13.6. Acute dose‐response analysis
The CONTAM Panel selected the emetic effects of NIV for the acute hazard characterisation. BMD analysis was performed on the incidence of emetic events in mink exposed to NIV.
The BMD modelling using a BMR of 10% resulted in 90% benchmark dose confidence interval for the average model (BMDL10–BMDU10) of 0.14–0.23 mg NIV/kg bw NIV/kg bw per day.
13.7. Chronic dose–response analysis
Because no new relevant data for derivation of a chronic HBGV were available, no dose–response analysis was conducted and the BMDL05 of 0.35 mg NIV/kg bw per day based on a reduction in white blood cell counts in a 90‐day rat study as set previously (EFSA CONTAM Panel, 2013) has been retained for the present assessment.
13.7.1. Establishment of health based guidance values
13.7.2. Group Acute Reference dose for NIV and its modified forms
Using the acute BMDL10 of 0.14 mg/kg bw per day for NIV and an UF of 10, for intraspecies differences, an ARfD of 14 μg NIV/kg bw was established. No interspecies uncertainty factor was applied since it was assumed that humans are not more sensitive towards the effect than mink.
There are no data on acute emetic effects of NIV3Glc. It is assumed that it will be hydrolysed in the lower intestine releasing NIV. Therefore, the Panel found it appropriate to include it in a group ARfD with NIV with the same molar potency factor (i.e. 1)
13.7.3. Group Tolerable Daily Intake for NIV and its modified forms
The CONTAM Panel noted that no new studies relevant for chronic hazard characterisation were identified. Therefore, the current TDI of 1.2 μg NIV/kg bw has been retained.
NIV3Glc can be hydrolysed to NIV after ingestion. Therefore, it can be included in a group TDI with NIV.
14. Recommendations
Because of the higher polarity of NIV and its conjugates compared with that of other trichothecenes, the conditions used in the currently established analytical methods might not be adequate for polar conjugates. Therefore, fit‐for‐purpose methods should be designed for an accurate detection of NIV and its modified forms to identify potential NIV metabolites not yet detected. In addition, certified reference materials and standards for the modified forms of NIV are needed.
Studies on the toxicokinetics of modified forms of NIV, in particular NIV3Glc occurring in plant‐derived food items, should be conducted.
The CONTAM Panel noted that FUS‐X, which is reported in the literature as a mycotoxin on its own, is also a precursor in the fungal biosynthesis of NIV and frequently co‐occurs with NIV. Therefore, it might be appropriate to perform a hazard characterisation of FUS‐X and to evaluate whether it should be included in group HGBVs with NIV.
Abbreviations
- AIC
Akaike's information criterion
- ARfD
acute reference dose
- BMD
benchmark dose
- BMD10
the benchmark response of 10% resulted in a benchmark dose 10%
- BMDL5
the 95th benchmark dose lower confidence limit
- BMDL10
the 90th benchmark dose lower confidence limit
- BMDU5
the 95th benchmark dose upper confidence limit
- BMDU10
the 95th benchmark dose upper confidence limit
- BMR
benchmark response
- BrdU
bromodeoxyuridine
- bw
body weight
- CAS
Chemical Abstracts Service
- CONTAM Panel
EFSA Panel on Contaminants in the Food Chain
- DE
de‐epoxy
- DE‐NIV
de‐epoxy‐nivalenol
- DON
deoxynivalenol
- DSPE
dispersive solid‐phase extraction
- FAO
Food and Agriculture Organization of the United Nations
- FUS‐X
fusarenon‐X
- GC
gas chromatography
- Glc
glucoside, glucose
- GlcA
glucuronic acid
- HBGV
health based guidance value
- HO
hydroxyl
- HT2
HT2‐toxin
- LC–MS/MS
liquid chromatography–tandem mass spectrometry
- i.p.
intraperitoneal
- IC50
inhibitory concentration 50%
- ICGN
ICR‐derived glomerulonephritis
- ICR
outbred mouse strain
- IL
interleukin
- IUPAC
International Union of Pure and Applied Chemistry
- LC
liquid chromatography
- LC‐FLD
liquid chromatography‐fluorescence detection
- LC–MS/MS
liquid chromatography–tandem mass spectrometry
- LC‐PDA
liquid chromatography photodiode array detection
- LOAEL
lowest‐observed‐adverse‐effect‐level
- NIV
nivalenol
- NIV3Glc
nivalenol‐3‐glucoside
- Man
mannose
- MAPK
mitogen‐activated protein synthesis kinase
- ML
maximum level
- m.p.
melting point
- mRNA
messenger RNA
- MS
mass spectrometry, mass spectrum
- MS/MS
tandem mass spectrometry
- MW
molecular weight
- NMR
nuclear magnetic resonance
- NOAEL
no‐observed‐adverse‐effect‐level
- QuEChERS
Quick, easy, cheap, effective, rugged and safe
- RNA
ribonucleic acid
- T2
T2‐toxin
- TDI
tolerable daily intake
- TK6
thymidine kinase heterozygous cell line
- UF
uncertainty factor
- WBC
white blood cell
- WHO
World Health Organization
- Xyl
xylose
Appendix A – Search for scientific literature on nivalenol
1.
Table A.1.
Search terms for web of science literature search
| EFSA extensive review | |
| Chemistry and analysis | |
| Search terms | TOPIC: (nivalenol) AND TOPIC: (chemistry OR analysis OR determination OR detection OR identification OR formation OR GC OR GC‐MS OR HPLC OR LC‐MS OR ICP‐MS) |
| Metabolism, Kinetics | |
| Search terms | TOPIC: (nivalenol) AND TOPIC (toxicokinetic* OR metabolism OR distribution OR excretion OR absorption OR distribution OR biomarker OR mode of action OR biotransformation OR elimination OR reduction OR detoxification OR extraction) |
| Toxicity | |
| Search terms | TOPIC: (nivalenol) AND TOPIC: (toxicity OR toxic* OR acute OR subacute OR subchronic OR chronic OR mutagen* OR carcinogen* OR genotox* OR reprotox* OR nephrotox* OR neurotox* OR hepatotox* OR immunotox* OR haemotox* OR hemotox* OR cytotox* OR develop* toxicity OR thyroid OR endocri* OR poisoning OR incidental poisoning OR rat OR mouse OR lab animal OR animal*) |
| Epidemiology | |
| Search terms | TOPIC: (nivalenol) AND TOPIC: (biomarker OR biological marker OR case study OR poisoning OR human poisoning OR human OR epidemiol*) |
| Date accessed | 14 October 2016 |
| Total Number after Removal of duplicates | 371 |
| Number considered relevant | 72 |
Appendix B – Derivation of a benchmark dose for acute effects of nivalenol
B.1. Data description
The dose‐dependent incidences of emesis upon administration of nivalenol (NIV) in mink in the study from Wu et al. (2013) have been selected for derivation of a benchmark dose (BMD) for NIV following the EFSA guidance on the use of the BMD (EFSA Scientific Committee, 2017). The findings selected for deriving a BMD are described in Section 8.2.1 of this opinion.
| Substance | Dose (mg/kg bw) | Animals showing emesis | N | Sex |
|---|---|---|---|---|
| NIV | 0 | 0 | 6 | F |
| 0.05 | 0 | 6 | F | |
| 0.10 | 0 | 6 | F | |
| 0.25 | 4 | 6 | F | |
| 0.5 | 6 | 6 | F |
bw: body weight; N: number of animals.
B.2. Selection of the BMR
The BMD is defined as the dose that corresponds with an extra risk of 10% compared with the background risk. The benchmark response (BMR) is the estimated risk corresponding with the BMD of interest. A 90% confidence interval around the BMD will be estimated, the lower bound is reported by BMDL and the upper bound by BMDU.
B.3. Software used
Results are obtained using the R‐package ‘bmdModeling’. Fitting BMD models is based on the R‐package Proast 61.3. Averaging results from multiple fitted BMD models is based on the methodology in Wheeler and Bailer (2008).
B.4. Results
| Model | Number of parameters | Log‐likelihood | AIC | BMD | BMDL | BMDU | Converged | Accepted AIC |
|---|---|---|---|---|---|---|---|---|
| Null | 1 | −19.10 | 40.20 | NA | NA | NA | Yes | – |
| Full | 5 | −3.82 | 17.64 | NA | NA | NA | Yes | – |
| Logistic | 2 | −3.82 | 11.64 | 0.23 | 0.14 | 0.25 | Yes | Yes |
| Probit | 2 | −16.48 | 36.96 | 0.14 | NA | NA | No | No |
| Log‐logistic | 3 | −3.82 | 13.64 | 0.22 | 0.09 | 0.25 | Yes | Yes |
| Log‐probit | 3 | −3.82 | 13.64 | 0.20 | 0.09 | 0.25 | Yes | Yes |
| Weibull | 3 | −3.82 | 13.64 | 0.22 | NA | NA | Yes | Yes |
| Gamma | 3 | −3.82 | 13.64 | 0.20 | 0.08 | 0.23 | Yes | Yes |
| Two‐stage | 3 | −5.13 | 16.26 | 0.08 | 0.06 | 0.12 | No | No |
B.5. Estimated model weights
| Logistic | Log‐logistic | Log‐probit | Weibull | Gamma |
|---|---|---|---|---|
| 0.4 | 0.15 | 0.15 | 0.15 | 0.15 |
Figure B.1.
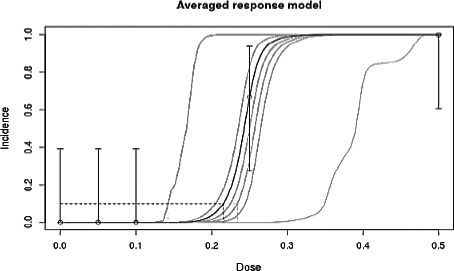
Averaged response model
B.6. Conclusion
Given 1,000 generated data sets, the BMDL is the 5th percentile of all parametric bootstrap BMD values and the BMDU is the 95th percentile.
The BMD is estimated based on the averaged response model which is a weighted average of the accepted models' response values. BMD = 0.22 mg/kg body weight (bw); BMDL = 0.14 mg/kg bw; BMDU = 0.23 mg/kg bw.
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Knutsen HK, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot A‐C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, Dall'Asta C, Gutleb AC, Metzler M, Parent‐Massin D, Binaglia M, Steinkellner H and Alexander J, 2017. Scientific Opinion on the appropriateness to set a group health based guidance value for nivalenol and its modified forms. EFSA Journal 2017;15(4):4751, 25 pp. doi: 10.2903/j.efsa.2017.4751
Requestor: European Commission
Question number: EFSA‐Q‐2015‐00228
Panel members: Jan Alexander, Lars Bårregard, Margherita Bignami, Beat Bruschweiler (from 23 June 2016), Sandra Ceccatelli, Bruce Cottrill, Michael Dinovi, Lutz Edler, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Helle Katrine Knutsen, Carlo Stefano Nebbia, Isabelle P. Oswald, Annette Petersen, Vera Maria Rogiers (until 9 May 2016), Martin Rose, Alain‐Claude Roudot, Tanja Schwerdtle, Christiane Vleminckx, Günter Vollmer and Heather Wallace.
Acknowledgements: The Panel wishes to thank the members of the Working Group on HBGV for mycotoxins and their modified forms: Jan Alexander, Chiara Dall'Asta, Arno Gutleb, Manfred Metzler, Isabelle P Oswald and Dominique Parent‐Massin for the preparatory work on this scientific output, and the EFSA staff members: Marco Binaglia and Hans Steinkellner for the support provided to this scientific output.
Adopted: 15 March 2017
Notes
EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal 2014;12(12):3916, 107 pp. doi:10.2903/j.efsa.2014.3916 Available online: http://www.efsa.europa.eu/efsajournal
Summary report of the Standing Committee on Plants, Animals, Food and Feed, held in Brussels on 11 February 2015 (Section Toxicological Safety of the Food Chain), agenda item A.06. Report available at: http://ec.europa.eu/food/committees/regulatory/scfcah/toxic/docs/sum_20150211_en.pdf
Council Regulation (EEC) No 315/93 of February 1993 laying down Community procedures for contaminants in food. OJ L 37, 13.2.1993, p. 1–5.
Regulation (EC) No 1881/2006 of the European Parliament and the Council of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OJ L 364, 20.12.2006, p. 5–24.
References
- Alkadri D, Rubert J, Prodi A, Pisi A, Manes J and Soler C, 2014. Natural co‐occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chemistry, 157, 111–118. [DOI] [PubMed] [Google Scholar]
- Beccari G, Caproni L, Tini F, Uhlig S and Covarelli L, 2016. Presence of fusarium species and other toxigenic fungi in malting barley and multi‐mycotoxin analysis by liquid chromatography‐high‐resolution mass spectrometry. Journal of Agriculture and Food Chemistry, 64, 4390–4399. [DOI] [PubMed] [Google Scholar]
- Capriotti AL, Cavaliere C, Foglia P, Samperi R, Stampachiacchiere S, Ventura S and Laganà A, 2014. Multiclass analysis of mycotoxins in biscuits by high performance liquid chromatography tandem mass spectrometry. Comparison of different extraction procedures (2014). Journal of Chromatography A, 1343, 69–78. [DOI] [PubMed] [Google Scholar]
- Cheat S, Gerez JR, Cognié J, Alassane‐Kpembi I, Bracarense AP, Raymond‐Letron I, Oswald IP and Kolf‐Clauw M, 2015. Nivalenol has a greater impact than deoxynivalenol on pig jejunum mucosa in vitro on explants and in vivo on intestinal loops. Toxins (Basel), 7, 1945–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarelli L, Beccari G, Prodi A, Generotti S, Etruschi F, Juan C, Ferrerc E and Mañesc J, 2015. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in an area of central Italy. Journal of the Science of Food and Agriculture, 95, 540–551. [DOI] [PubMed] [Google Scholar]
- Del Regno M, Adesso S, Popolo A, Quaroni A, Autore G, Severino L and Marzocco S, 2015. Nivalenol induces oxidative stress and increases deoxynivalenol pro‐oxidant effect in intestinal epithelial cells. Toxicology and Applied Pharmacology, 285, 118–127. [DOI] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2013. Scientific Opinion on risks for animal and public health related to the presence of nivalenol in food and feed. EFSA Journal 2013;11(6):3262, 119 pp. doi: 10.2903/j.efsa.2013.3262 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal 2014;12(12):3916, 107 pp. doi: 10.2903/j.efsa.2014.3916 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2016. Scientific opinion on the appropriateness to set a group health‐based guidance value for zearalenone and its modified forms. EFSA Journal 2016;14(4):4425, 46 pp. doi: 10.2903/j.efsa.2016.4425 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2017. Scientific opinion on the appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA Journal 2017;15(1):4655, 53 pp. doi: 10.2903/j.efsa.2017.4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2017. Update: guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. doi: 10.2903/j.efsa.2017.4658 [DOI] [Google Scholar]
- Eriksen GS, Pettersson H and Lundh T, 2004. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de‐epoxy metabolites. Food and Chemical Toxicology, 42, 619–624. [DOI] [PubMed] [Google Scholar]
- Gordon G, 1985. Ipecacuanha induced emesis in the treatment of self‐poisoned adults. Archives of Emergency Medicine, 2, 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz SW, Dinesh R, Yoshinari T, Holtrop G, Richardson AJ, Duncan G, MacDonald S, Lloyd A and Tarbin J, 2017. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro . Molecular Nutrition and Food Research, 1600680, 10 pp. doi: 10.1002/mnfr.201600680 [DOI] [PubMed] [Google Scholar]
- Gromadzka K, Górna K, Chełkowski J and Waśkiewicz A, 2016. Mycotoxins and related Fusarium species in preharvest maize ear rot in Poland. Plant Soil Environ, 62, 348–354. doi: 10.17221/119/2016-PSE [DOI] [Google Scholar]
- Inoue K, Takahashi M, Kodama Y, Nishikawa A, Sugita‐Konishi Y and Yoshida M, 2014. The kidneys of infant mice are not sensitive to the food mycotoxin contaminant nivalenol. Journal of Toxicology Pathology, 27, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongkapan J, Giorgi M, Poapolathep S, Isariyodom S and Poapolathep A, 2016a. Toxicokinetics and tissue distribution of nivalenol in broiler chickens. Toxicon, 111, 31–36. [DOI] [PubMed] [Google Scholar]
- Kongkapan J, Polapothep A, Owen H and Giorgi M, 2016b. A brief overview of our current understanding of nivalenol: a growing potential danger yet to be fully investigated. Israel Journal of Veterinary Medicine, 71, 3–9. [Google Scholar]
- Krska R, Schubert‐Ullrich P, Josephs RD, Emteborg H, Buttinger G, Schothorst RC, van Egmond HP, Pettersson H, Chan D and MacDonald S, 2007. Determination of molar absorptivity coefficients for major type‐B trichothecenes and certification of calibrators for deoxynivalenol and nivalenol. Analytical and Bioanalytical Chemistry, 388, 1215–1226. [DOI] [PubMed] [Google Scholar]
- Le Hégarat L, Takakura N, Simar S, Nesslany F and Fessard V, 2014. The in vivo genotoxicity studies on nivalenol and deoxynivalenol. EFSA supporting publication 2014:EN‐697, 33 pp. doi: 10.2903/sp.efsa.2014.EN-697 [DOI]
- Lindblad M, Gidlund A, Sulyok M, Börjesson T, Krska R, Olsen M and Fredlund E, 2013. Deoxynivalenol and other selected Fusarium toxins in Swedish wheat – occurrence and correlation to specific Fusarium species. International Journal of Food Microbiology, 167, 284–291. [DOI] [PubMed] [Google Scholar]
- Malachová A, Sulyok M, Beltrán E, Berthiller F and Krska R, 2014. Optimization and validation of a quantitative liquid chromatography‐tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices (2014). Journal of Chromatography A, 1362, 145–156. [DOI] [PubMed] [Google Scholar]
- McCormick SP, Stanley AM, Stover NA and Alexander NJ, 2011. Trichothecenes: from simple to complex mycotoxins. Toxins, 3, 802–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima H and Nakagawa H, 2014. Review: differences in the toxicities of trichothecene mycotoxins, deoxynivalenol and nivalenol, in cultured cells. Japan Agricultural Research Quarterly, 48, 393–397. [Google Scholar]
- Nakagawa H, Ohmichi K, Sakamoto S, Sago Y, Kushiro M, Nagashima H, Yoshida M and Nakajima T, 2011. Detection of a new Fusarium masked mycotoxin in wheat grain by high‐resolution LC‐Orbitrap™ MS (2011) Food Additives and Contaminants ‐ Part A Chemistry. Analysis, Control, Exposure and Risk Assessment, 28, 1447–1456. [DOI] [PubMed] [Google Scholar]
- Nardiello D, Lo Magro S, Iammarino M, Palermo C, Muscarella M and Centonze D, 2014. Recent advances in the post‐column derivatization for the determination of mycotoxins in food products and feed materials by liquid chromatography and fluorescence detection. Current Analytical Chemistry, 10, 355–365. [Google Scholar]
- Nathanail AV, Syvähuoko J, Malachová A, Jestoi M, Varga E, Michlmayr H, Adam G, Sieviläinen E, Berthiller F and Peltonen K, 2015. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography‐tandem mass spectrometric method. Analytical and Bioanalytical Chemistry, 407, 4745–4755. article no. 8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onji Y, Dohi Y, Aoki Y, Moriyama T, Nagami H, Uno M, Tanaka T and Yamazoe Y, 1989. Deepoxynivalenol ‐ a new metabolite of nivalenol found in the excreta of orally‐administered rats. Journal of Agricultural and Food Chemistry, 37, 478–481. [Google Scholar]
- Park J, Lee HH, Youn K, Kim S, Jung B, Lee J and Seo YS, 2014. Transcriptome analyses to understand effects of the Fusarium deoxynivalenol and nivalenol mycotoxins on Escherichia coli . Journal of Biotechnology, 192, 231–239. [DOI] [PubMed] [Google Scholar]
- Pascale M, Panzarini G, Powers S and Visconti A, 2014. Determination of deoxynivalenol and nivalenol in wheat by ultra‐performance liquid chromatography/photodiode‐array detector and immunoaffinity column cleanup. Food Analytical Methods, 7, 555–562. [Google Scholar]
- Percie du Sert N, Holmes AM, Wallis R and Andrews PLR, 2012. Predicting the emetic liability of novel chemical entities: a comparative study. British Journal of Pharmacology, 165, 1848–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierron A, Mimoun S, Murate LS, Loiseau N, Lippi Y, Bracarense AP, Liaubet L, Schatzmayr G, Berthiller F, Moll WD and Oswald IP, 2016. Intestinal toxicity of the masked mycotoxin deoxynivalenol‐3‐β‐D‐glucoside. Archives of Toxicology, 90, 2037–2046. doi: 10.1007/s00204-015-1592-8 Epub 2015 Sep 24. [DOI] [PubMed] [Google Scholar]
- Polakowski S, Davis AH, Albert AL, Gow B, Lombaert G and Ziemer W, 2015. QuickTox™ Kit for QuickScan DON3 (Vomitoxin) method modification (2015). Journal of AOAC International, 98, 85–93. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Carrasco Y, Ruiz MJ, Font G and Berrada H, 2013. Exposure estimates to Fusarium mycotoxins through cereals intake (2013). Chemosphere, 93, 2297–2303. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Carrasco Y, Font G, Moltó JC, and Berrada H, 2014. Quantitative determination of trichothecenes in breadsticks by gas chromatography‐triple quadrupole tandem mass spectrometry. Food Additives and Contaminants ‐ Part A Chemistry Analysis, Control, Exposure and Risk Assessment, 31, 1422–1430. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Carrasco Y, Fattore M, Albrizio S, Berrada H and Mañes J, 2015. Occurrence of Fusarium mycotoxins and their dietary intake through beer consumption by the European population. Food Chemistry, 178, 149–155. [DOI] [PubMed] [Google Scholar]
- Rubert J, Dzuman Z, Vaclavikova M, Zachariasova M, Soler C and Hajslova J, 2012. Analysis of mycotoxins in barley using ultra high liquid chromatography high resolution mass spectrometry: comparison of efficiency and efficacy of different extraction procedures. Talanta, 99, 712–719. [DOI] [PubMed] [Google Scholar]
- Rychlik M, Humpf HU, Marko D, Dänicke S, Mally A, Berthiller F, Klaffke H and Lorenz N, 2014. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Research, 30, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita‐Konishi Y, Kubosaki A, Takahashi M, Park BJ, Tanaka T, Takatori K, Hirose M and Shibutani M, 2008. Nivalenol and the targeting of the female reproductive system as well as haematopoietic and immune systems in rats after 90‐day exposure through the diet. Food Additives and Contaminants. Part A, 25, 1118–1127. [DOI] [PubMed] [Google Scholar]
- Sydenham EW, Thiel PG and Vleggaar R, 1996. Physicochemical data for some selected Fusarium toxins. Journal of AOAC International, 79, 1365–1379. [PubMed] [Google Scholar]
- Takahashi M, Shibutani M, Sugita‐Konishi Y, Aihara M, Inoue K, Woo G, Fujimoto H and Hirose M, 2008. A 90‐day subchronic toxicity study of nivalenol, a trichothecene mycotoxin, in F344 rats. Food and Chemical Toxicology, 46, 125–135. [DOI] [PubMed] [Google Scholar]
- Tatsuno T, Saito M, Enomoto M and Tsunoda H, 1968. Nivalenol, a toxic principle of Fusarium nivale . Chemical and Pharmaceutical Bulletin, 16, 2519–2520. [DOI] [PubMed] [Google Scholar]
- Trombete F, Barros A, Vieira M, Saldanha T, Venâncio A and Fraga M, 2016. Simultaneous determination of deoxynivalenol, deoxynivalenol‐3‐glucoside and nivalenol in wheat grains by HPLC‐PDA with immunoaffinity column cleanup. Food Analytical Methods, 9, 2579–2586. [Google Scholar]
- Vejdovszky K, Warth B, Sulyok M and Marko D, 2016. Non‐synergistic cytotoxic effects of Fusarium and Alternaria toxin combinations in Caco‐2 cells. Toxicology Letters, 241, 1–8. [DOI] [PubMed] [Google Scholar]
- Veršilovskis A, Huybrecht B, Tangni EK, Pussemier L, de Saeger S and Callebaut A, 2011. Cross‐reactivity of some commercially available deoxynivalenol (DON) and zearalenone (ZEN) immunoaffinity columns to DON‐ and ZEN‐conjugated forms and metabolites. Food Additives and Contaminants ‐ Part A Chemistry Analysis, Control, Exposure and Risk Assessment, 28, 1687–1693. [DOI] [PubMed] [Google Scholar]
- Wan LY, Turner PC and El‐Nezami H, 2013a. Individual and combined cytotoxic effects of Fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins B1) on swine jejunal epithelial cells. Food and Chemical Toxicology, 57, 276–283. [DOI] [PubMed] [Google Scholar]
- Wan LY, Woo CS, Turner PC, Wan JM and El‐Nezami H, 2013b. Individual and combined effects of Fusarium toxins on the mRNA expression of pro‐inflammatory cytokines in swine jejunal epithelial cells. Toxicology Letters, 220, 238–246. [DOI] [PubMed] [Google Scholar]
- Wan ML, Woo CS, Allen KJ, Turner PC and El‐Nezami H, 2013c. Modulation of porcine ß‐defensins 1 and 2 upon individual and combined fusarium toxin exposure in a swine jejunal epithelial cell line. Applied and Environmental Microbiology, 79, 2225–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MW and Bailer AJ, 2008. Model averaging software for dichotomous dose response risk estimation. Journal of Statistical Software, 26, 1–15.19777145 [Google Scholar]
- WHO/IPCS (World Health Organization/ International Programme on Chemical Safety), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. International Programme on Chemical Safety, Environmental Health Criteria 240. Available online: http://whqlibdoc.who.int/ehc/WHO_EHC_240_5_eng_Chapter2.pdf
- Widestrand J and Pettersson H, 2001. Effect of time, temperature and solvent on the stability of T‐2 toxin, HT‐2 toxin, deoxynivalenol and nivalenol calibrants. Food Additives and Contaminants, 18, 987–992. [DOI] [PubMed] [Google Scholar]
- Wu Q, Dohnal V, Huang L, Kuca K and Yuan Z, 2010. Metabolic pathways of trichothecenes. Drug Metabolism Reviews, 42, 250–267. [DOI] [PubMed] [Google Scholar]
- Wu W, Flannery BM, Sugita‐Konishi Y, Watanabe M, Zhang H and Pestka JJ, 2012. Comparison of murine anorectic responses to the 8‐ketotrichothecenes 3‐acetyldeoxynivalenol, 15‐acetyldeoxynivalenol, fusarenon X and Nivalenol. Food and Chemical Toxicology, 50, 2056–2061. [DOI] [PubMed] [Google Scholar]
- Wu W, Bates MA, Bursian SJ, Link JE, Flannery BM, Sugita‐Konishi Y, Watanabe M, Zhang H and Pestka JJ, 2013. Comparison of emetic potencies of the 8‐ketotrichothecenes deoxynivalenol, 15‐acetyldeoxynivalenol, 3‐acetyldeoxynivalenol, fusarenon x, and nivalenol. Toxicological Sciences 131, 279–291. doi: 10.1093/toxsci/kfs286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, He K, Zhou HR, Berthiller F, Adam G, Sugita‐Konishi Y, Watanabe M, Krantis A, Durst T, Zhang H and Pestka JJ, 2014a. Effects of oral exposure to naturally‐occurring and synthetic deoxynivalenol congeners on proinflammatory cytokine and chemokine mRNA expression in the mouse. Toxicology and Applied Pharmacology, 278, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Zhou HR, He K, Pan X, Sugita‐Konishi Y, Watanabe M, Zhang H and Pestka JJ, 2014b. Role of cholecystokinin in anorexia induction following oral exposure to the 8‐ketotrichothecenes deoxynivalenol, 15‐acetyldeoxynivalenol, 3‐acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicological Sciences, 138, 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang Y, Beier RC, Zhang H, Ruyck KD, Sun F, Cao X, Shen J, Zhang S and Wang Z, 2015. Simultaneous determination of type A and B trichothecenes and their main metabolites in food animal tissues by ultraperformance liquid chromatography coupled with triple‐quadrupole mass spectrometry. Journal of Agricultural and Food Chemistry, 63, 8592–8600. [DOI] [PubMed] [Google Scholar]
- Yoshinari T, Sakuda S, Furihata K, Furusawa H, Ohnishi T, Sugita‐Konishi Y, Ishizaki N and Terajima J, 2014. Structural determination of a nivalenol glucoside and development of an analytical method for the simultaneous determination of nivalenol and deoxynivalenol, and their glucosides, in wheat. Journal of Agricultural and Food Chemistry, 62, 1174–1180. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang L, Yang ZH, Liu ZT and Yue W, 2006. Wang Yue value of mink vomit model in study of anti‐emetic drugs. World Journal of Gastroenterology, 12, 1300–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Rao Q, Song S, Liu N, Han Z, Hou J and Wu A, 2014. Simultaneous determination of major type B trichothecenes and deoxynivalenol‐3‐glucoside in animal feed and raw materials using improved DSPE combined with LC‐MS/MS Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 963, 75–82. [DOI] [PubMed] [Google Scholar]


