Abstract
This report of the European Food Safety Authority and the European Centre for Disease Prevention and Control presents the results of the zoonoses monitoring activities carried out in 2016 in 37 European countries (28 Member States (MS) and nine non‐MS). Campylobacteriosis was the most commonly reported zoonosis and the increasing European Union (EU) trend for confirmed human cases since 2008 stabilised during 2012–2016. In food, the occurrence of Campylobacter remained high in broiler meat. The decreasing EU trend for confirmed human salmonellosis cases since 2008 ended during 2012–2016, and the proportion of human Salmonella Enteritidis cases increased. Most MS met their Salmonella reduction targets for poultry, except five MS for laying hens. At primary production level, the EU‐level flock prevalence of target Salmonella serovars in breeding hens, broilers, breeding and fattening turkeys decreased or stabilised compared with previous years but the EU prevalence of S. Enteritidis in laying hens significantly increased. In foodstuffs, the EU‐level Salmonella non‐compliance for minced meat and meat preparations from poultry was low. The number of human listeriosis confirmed cases further increased in 2016, despite the fact that Listeria seldom exceeds the EU food safety limit in ready‐to‐eat foods. The decreasing EU trend for confirmed yersiniosis cases since 2008 stabilised during 2012–2016, and also the number of confirmed Shiga toxin‐producing Escherichia coli (STEC) infections in humans was stable. In total, 4,786 food‐borne outbreaks, including waterborne outbreaks, were reported. Salmonella was the most commonly detected causative agent – with one out of six outbreaks due to S. Enteritidis – followed by other bacteria, bacterial toxins and viruses. Salmonella in eggs continued to represent the highest risk agent/food combination. The report further summarises trends and sources for bovine tuberculosis, brucellosis, trichinellosis, echinococcosis, toxoplasmosis, rabies, Q fever, West Nile fever and tularaemia.
Keywords: zoonoses, monitoring, Salmonella, Campylobacter, Listeria, parasites, food‐borne outbreaks
Introduction
Legal basis of the EU‐coordinated zoonoses monitoring
The EU system for the monitoring and collection of information on zoonoses is based on the Zoonoses Directive 2003/99/EC,1 which obliges European Union (EU) Member States (MS) to collect relevant and, when applicable, comparable data on zoonoses, zoonotic agents, antimicrobial resistance and food‐borne outbreaks. In addition, MS are required to assess trends and sources of these agents, as well as outbreaks in their territory, submitting an annual report each year by the end of May to the European Commission covering the data collected. The European Commission should subsequently forward these reports to the European Food Safety Authority (EFSA). EFSA is assigned the tasks of examining these data and publishing the EU annual Summary Reports. In 2004, the European Commission entrusted EFSA with the task of setting up an electronic reporting system and database on monitoring of zoonoses (EFSA mandate No 2004‐01782).
The data collection on human diseases from MS is conducted in accordance with Decision 1082/2013/EU3 on serious cross‐border threats to health. This Decision replaced Decision 2119/98/EC on setting up a network for the epidemiological surveillance and control of communicable diseases in the EU in October 2013. The case definitions to be followed when reporting data on infectious diseases to the European Centre for Disease Prevention and Control (ECDC) are described in Decision 2012/506/EU.4 ECDC has provided data on zoonotic infections in humans, as well as their analyses, for the EU Summary Reports since 2005. Since 2008, data on human cases have been received via the European Surveillance System (TESSy), maintained by ECDC.
Reporting requirements
According Annex I of the Zoonoses Directive 2003/99/EC data on animals, food and feed must be reported on a mandatory basis (list A of Annex I of the Zoonoses Directive) for the following eight zoonotic agents: Salmonella, Campylobacter, Listeria monocytogenes, Shiga toxin‐producing Escherichia coli (STEC), Mycobacterium bovis, Brucella, Trichinella and Echinococcus. The general rules on monitoring of zoonoses and zoonotic agents in animals, food and feed are laid down in article 4 of Chapter II of the Zoonoses Directive 2003/99/EC, which prescribes that monitoring shall take place at the stage or stages of the food chain most appropriate to the zoonosis or zoonotic agent concerned, that is (a) at the level of primary production; and/or (b) at other stages of the food chain, including in food and feed. For food, monitoring schemes for Salmonella, L. monocytogenes and STEC are implied by EU Regulation 2073/20055 on microbiological criteria that have been in force since 1 January 2006. Specific rules for the coordinated monitoring programmes and for the food business operators are, respectively, laid down in Articles 5 and 6 of Chapter II (‘monitoring of zoonoses and zoonotic agent's) of the Zoonoses Directive 2003/99/EC, while Article 8 of Chapter IV (‘food‐borne outbreaks’) details rules for epidemiological investigation of food‐borne outbreaks. The reporting requirements are described in Annex IV of the Zoonoses Directive 2003/99/EC.
In addition and based on the epidemiological situations in the MS, data must be reported on the following agents and zoonoses (list B of Annex I of the Zoonoses Directive): (i) viral zoonoses: calicivirus, hepatitis A virus, influenza virus, rabies, viruses transmitted by arthropods; (ii) bacterial zoonoses: borreliosis and agents thereof, botulism and agents thereof, leptospirosis and agents thereof, psittacosis and agents thereof, tuberculosis other than in M. bovis, vibriosis and agents thereof, yersiniosis and agents thereof; (iii) Parasitic zoonoses: anisakiasis and agents thereof, cryptosporidiosis and agents thereof, cysticercosis and agents thereof, toxoplasmosis and agents thereof; (iv) Other zoonoses and zoonotic agents (such as Francisella, Cysticercus and Sarcocystis). Furthermore, MS provide data on certain other microbiological contaminants in food – histamine, staphylococcal enterotoxins and Cronobacter spp. (before Enterobacter sakazakii), for which food safety criteria are set down in the EU legislation.
Terms of Reference
In accordance with Article 9 of Directive 2003/99/EC, EFSA shall examine the submitted national reports and data of the MS 2016 zoonoses monitoring activities as described above, and publish an EU Summary Report on the trends and sources of zoonoses, zoonotic agents and antimicrobial resistance in the EU.
The 2016 data on antimicrobial resistance in zoonotic agents submitted and validated by the MS are published in a separate EU Summary Report.
General description of methods
Data sources
This EU Summary Report 2016 on zoonoses, zoonotic agents and food‐borne outbreaks (FBO) was prepared by EFSA in collaboration with ECDC. MS, other reporting countries, the European Commission, members of EFSA's Scientific Panels on Biological Hazards (BIOHAZ) and Animal Health and Welfare (AHAW) and the relevant EU Reference Laboratories (EURLs) were consulted while preparing the report.
The efforts made by the MS, the reporting non‐MS and the European Commission in the reporting of zoonoses data and in the preparation of this report are gratefully acknowledged.
The present EU Summary Report on zoonoses and FBO focus on the most relevant information on zoonoses and FBO within the EU in 2016. If substantial changes compared with the previous year were observed, they have been reported.
1.
1.1.
Human 2016 data collection
The human data analyses in the EU Summary Report for 2016 were prepared by the Food‐ and Waterborne Diseases and Zoonoses programme at ECDC and were based on the data submitted via TESSy, hosted at ECDC. The numbers presented in the report may differ from national reports due to differences in case definitions used at EU and national level or to different dates of data extraction. The latter may also result in some divergence in case numbers presented in different ECDC reports.
TESSy is a software platform that has been operational since April 2008 and in which data on 52 diseases and special health issues are collected. Both aggregated and case‐based data were reported to TESSy. Although aggregated data did not include individual case‐based information, both reporting formats were included when possible to calculate number of cases, country‐specific notification rates and trends in diseases. Human data used in the report were extracted from TESSy as of 1 August, except for human tuberculosis due to M. bovis as of 3 October 2017. The denominators used for the calculation of the notification rates were the human population data from Eurostat 1 January 2017 update.
Data on human zoonoses cases were received from 28 MS and also from two non‐MS: Iceland and Norway. Switzerland sent its data on human cases directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The data should be interpreted with caution and take into account data quality issues and differences between MS surveillance systems. The reader should refrain from making direct comparisons between countries without taking into account the limitations in the data, which may differ between countries depending on the characteristics of their surveillance systems.
Data collection on food, animals, feed, and food‐borne outbreaks
For the year 2016, 28 MS and four non‐MS European Free Trade Association (EFTA) countries (Iceland, Norway, Liechtenstein and Switzerland) submitted data and national zoonoses reports on monitoring results in food, animals, feed and FBO.6 For some food, animal and feed matrices and FBO, EFSA received data and reports from pre‐accession countries Albania, Bosnia and Herzegovina, the Former Yugoslav Republic of Macedonia, Montenegro and Serbia. Data were submitted electronically to the EFSA zoonoses database, through EFSA's Data Collection Framework (DCF). MS could also update data from previous years, before 2016.
The deadline for data submission was 31 May 2017. Two data validation exercises were implemented, by 3 June 2016 and by 1 July 2016. Validated data on food, animals and feed used in the report were extracted from the EFSA zoonoses database on 19 July 2016.
The draft EU Summary Report was sent to MS for consultation on 13 October 2017 and comments were collected by 3 November 2017. The utmost effort was made to incorporate comments and data amendments within the available time frame. The report was finalised by 13 November 2017 and published on‐line by EFSA and ECDC on 7 December 2017.
The detailed description of zoonoses models for data entry and of the terms used in the report is available in the EFSA's manuals for reporting on zoonoses (EFSA, 2017a,b,c,d).
The national zoonoses reports submitted in accordance with Directive 2003/99/EC are published on the EFSA website together with the EU Summary Report. They are available on‐line at http://www.efsa.europa.eu/en/biological-hazards-data/reports.
The Appendix lists all summary tables and figures made for the production of this report. It is an Excel file allowing the user to filter by chapter the corresponding tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
Data analysis
General principles and presentation
The current summary report presenting monitoring data for the year 2016 has a harmonised structure for each chapter, including an abstract with the major findings. Next, a section describes the monitoring and surveillance in the EU for the specific zoonosis or for FBO. A results section summarises the major findings of 2016 as regards trends and sources. Each chapter also contains a discussion and ends with a list of related projects and links with useful information for the specific zoonosis.
A summary table displaying the data of the last 5 years for human cases and for major animal and food matrices is presented. It presents all the MS that reported data during 2012–2016 is made available, with key summary statistics. However, for the summary tables, unless stated otherwise, data from industry own‐control programmes and Hazard Analysis and Critical Control Point (HACCP) sampling as well as data from suspect sampling, selective sampling and outbreak or clinical investigations are excluded. If MS reported only regional data without reporting statistics at the national level, these were not extracted in the summary tables.
When possible, statistical trend analyses were carried out to evaluate the significance of temporal variations in the EU and the specifications of these analyses are explained in each separate chapter.
Spatial trends in food and animals were visualised using R software (http://www.r-project.org); packages ggplot2, lattice and tmap as well as ArcGIS from the Economic and Social Research Institute (ESRI) were used to map the data. Choropleth maps with graduated colours over a continuous scale of values were used to map the proportion of positive sample units across the EU and other reporting countries.
Comparability and quality of the data
Humans
Regarding data on human infections, please note that, as mentioned above, the numbers presented in this report may differ from national zoonoses reports due to differences in case definitions used at EU and national level or because of different dates of data extraction. Results are generally not directly comparable between MS and sometimes not even between different years in one country.
Food, animals, feed, and food‐borne outbreaks
Regarding data on food, animals, feed and food‐borne outbreaks, the numbers presented in this report may differ from national zoonoses reports due to different dates of data extraction.
The zoonoses and food‐borne outbreaks monitoring data obtained in the EFSA DCF, respectively according Chapter II (‘monitoring of zoonoses and zoonotic agent's) and Chapter IV (‘food‐borne outbreaks’) of the Zoonoses Directive 2003/99/EC) vary according to the level of data quality and harmonisation. Therefore, the types of analyses that can be done with these monitoring data and suggested by EFSA, strongly depend on those levels of data quality and harmonisation. These data analyses can either be a descriptive summary, or trend watching, or a full trend analysis. To make this clear for the reader, EFSA proposed throughout the report the types of analyses according to Table 1 and adapted from Boelaert et al. (2016). For each chapter in this report, the applied category according to Table 1 is explained.
Table 1.
Categorisation of zoonoses and food‐borne outbreaks monitoring data used in EUSR 2016 (adapted from Boelaert et al., 2016)
| Category | Type of analyses | Type/comparability between MS | Examples |
|---|---|---|---|
| I |
Descriptive summaries at national level and EU‐level EU trend watching (trend monitoring) Spatial and temporal trends analyses at the EU‐level |
Programmed and harmonised monitoring or surveillance Comparable between MS; results at EU‐level are interpretable |
Salmonella national control programmes in poultry Bovine tuberculosis Bovine and small ruminant brucellosis Trichinella in pigs at the slaughterhouse Echinococcus granulosus at the slaughterhouse |
| II |
Descriptive summaries at national level and EU‐level EU trend watching (trend monitoring) No trend analysis at the EU‐level |
Not fully harmonised monitoring or surveillance Not fully comparable between MS; caution needed when interpreting results at EU‐level |
Food‐borne outbreaks data Monitoring of compliance with process hygiene and food safety criteria for L. monocytogenes, Salmonella and E. coli according Reg No 2073/20055 Monitoring of rabies |
| III |
Descriptive summaries at national level and EU‐level No EU trend watching (trend monitoring) No trend analysis at the EU‐level |
Non‐harmonised monitoring or surveillance data with no (harmonised) reporting requirements Not comparable between MS; extreme caution needed when interpreting results at EU‐level |
Campylobacter Yersinia Q fever Francisella tularensis West Nile virus Taenia spp. other zoonoses Toxoplasma |
Summary human zoonoses data EUSR, 2016
The numbers of the confirmed human cases of 13 zoonoses presented in this report are summarised in Figure 1. In 2016, campylobacteriosis was the most commonly reported zoonoses, as it had been since 2005, representing almost 70% of all the reported cases. Campylobacteriosis was followed by other bacterial diseases: salmonellosis, yersiniosis and STEC infections in being the most frequently reported. The severity of the diseases was analysed based on hospitalisation and outcome of the reported cases (Table 2). Based on data on severity, listeriosis was the most severe zoonoses with the highest hospitalisation and mortality rate followed by West Nile fever. Almost all confirmed cases with data available on hospitalisation for these two diseases were hospitalised. One out of every six and one out of nine confirmed and reported listeriosis and West Nile fever cases, respectively, with known data was fatal.
Figure 1.
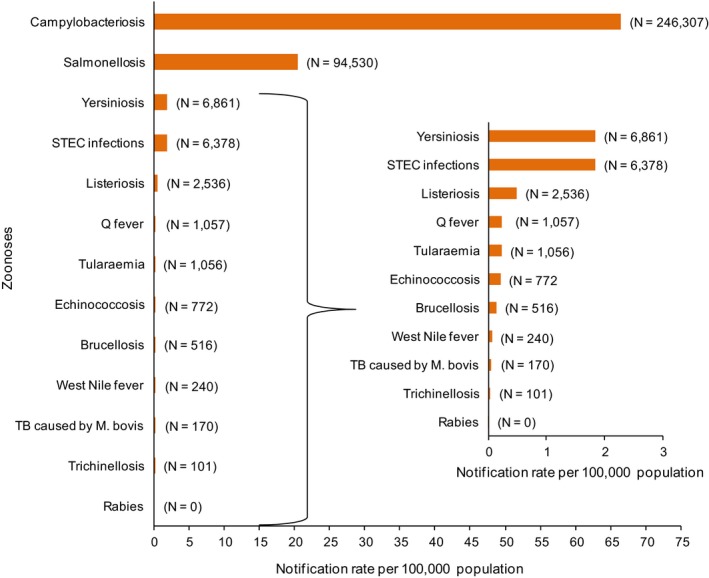
Reported numbers and notification rates of confirmed human zoonoses in the EU, 2016
- Note: Total number of confirmed cases is indicated in parenthesis at the end each bar. Exception: West Nile fever where the total number of cases was used.
Table 2.
Reported hospitalisation and case fatality rates due to zoonoses in confirmed human cases in the EU, 2016
| Disease | Number of confirmeda human cases | Hospitalisation | Deaths | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Status available (%) | Number of reporting MSsb | Reported hospitalised cases | Proportion hospitalised (%) | Outcome available (%) | Number of reporting MSsb | Reported deaths | Case fatality (%) | ||
| Campylobacteriosis | 246,307 | 27.4 | 17 | 19,265 | 28.5 | 72.6 | 16 | 62 | 0.03 |
| Salmonellosis | 94,530 | 33.5 | 14 | 12,182 | 38.4 | 55.2 | 16 | 128 | 0.25 |
| Yersiniosis | 6,861 | 24.1 | 14 | 521 | 31.5 | 63.5 | 15 | 5 | 0.11 |
| STEC infections | 6,378 | 42.6 | 18 | 940 | 34.6 | 58.9 | 20 | 10 | 0.27 |
| Listeriosis | 2,536 | 38.8 | 18 | 962 | 97.7 | 60.1 | 20 | 247 | 16.2 |
| Q‐fever | 1,057 | NAc | NA | NA | NA | 54.3 | 15 | 3 | 0.30 |
| Tularaemia | 1,056 | 12.3 | 11 | 130 | 54.6 | 15.8 | 12 | 0 | 0.0 |
| Echinococcosis | 772 | 26.2 | 14 | 119 | 58.9 | 25.4 | 13 | 1 | 0.51 |
| Brucellosis | 516 | 39.7 | 12 | 146 | 71.2 | 26.0 | 12 | 1 | 0.75 |
| West Nile fevera | 240 | 65.1 | 7 | 147 | 93.6 | 99.2 | 9 | 28 | 11.7 |
| Trichinellosis | 101 | 45.5 | 7 | 30 | 65.2 | 50.5 | 8 | 0 | 0.0 |
| Rabies | 0 | NAc | NA | NA | NA | 0.0 | 0 | 0 | 0.0 |
MS: Member State; STEC: Shiga toxin‐producing Escherichia coli.
Exception: West Nile fever in which the total number of cases was included.
Not all countries observed cases for all diseases.
NA: Not applicable as information is not collected for this disease.
1. Campylobacter
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
1.1. Abstract
In 2016, Campylobacter was the most commonly reported gastrointestinal bacterial pathogen in humans in the European Union (EU) and has been so since 2005. The number of reported confirmed cases of human campylobacteriosis was 246,307, with an EU notification rate of 66.3 per 100,000 population. This represented an increase of 6.1% compared with 2015. There was a significantly increasing trend over the period 2008–2016, however, in the last 5 years (2012–2016) the EU/EEA trend has not shown any statistically significant increase or decrease. Half of the MS reported increasing trends in both in the long term (2008–2016) and in the short term (2012–2016). While the high number of human campylobacteriosis cases, their severity in terms of reported case fatality was low (0.03%), even though this was the third most common cause of mortality amongst the pathogens considered.
Few MS reported 2016 monitoring results of Campylobacter in food, mainly from fresh meat from broilers and turkeys, and from their meat products. In these foods, the occurrence was, respectively, 36.7% and 11% in fresh meat from broilers and fresh meat from turkeys. Campylobacter in milk and milk products (including cheeses) for the year 2016 was reported by nine MS. The occurrence was comparable between milk products and cheeses and was around 1%. Few MS reported 2016 monitoring data on Campylobacter in animals. Sixty‐five per cent of the samples originated from broilers, from 14 MS, and from turkeys, from 5 MS. In addition to the low volumes of food and animal monitoring data reported from investigations on Campylobacter, the sampling and reporting rules are not harmonised, thus precluding trend analyses and trend watching. Together these deficiencies prevent inference being made, beyond the sample statistics, on trends or sources of Campylobacter in foods or animals.
1.2. Surveillance and monitoring of Campylobacter in the EU
1.2.1. Humans
The notification of campylobacteriosis is mandatory in most MS, Iceland, Norway and Switzerland, except for seven MS, where notification is based on a voluntary system (Belgium, France, Italy, Luxembourg and the Netherlands) or other systems (Spain and the United Kingdom). No surveillance system exists in Greece. The surveillance systems for campylobacteriosis cover the whole population in all MS except four (France, Italy, the Netherlands and Spain). The coverage of the surveillance system is estimated to be 20% in France and 52% in the Netherlands. These proportions of populations were used in the calculation of notification rates for these two MS. No estimate of population coverage in Italy and Spain was provided, so notification rates were not calculated for these two MS.
Diagnosis of human infection is generally based on culture from human stool samples and both culture and non‐culture methods (polymerase chain reaction (PCR)) are used for confirmation. Biochemical tests or molecular methods are used for species determination of isolates submitted to the National Reference Laboratory.
1.2.2. Food and animals
Monitoring data on Campylobacter from food and animals and submitted to EFSA (according Chapter II (‘monitoring of zoonoses and zoonotic agent's) of the Zoonoses Directive 2003/99/EC) are collected without harmonised design. These data allow for descriptive summaries at EU‐level to be made. They preclude trend analyses and trend watching at EU‐level (Table 1).
Detection of Campylobacter from food and animals is generally based on culture and both biochemical and molecular methods (PCR and matrix assisted laser desorption ionisation‐time of flight (MALDI‐TOF)) are used for confirmation.
1.2.3. Food‐borne outbreaks of human campylobacteriosis
The reporting of FBO of human campylobacteriosis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
1.3. Results
1.3.1. Overview of key statistics along the food chain, EU, 2012–2016
Table 3 summarises EU‐level statistics related to human campylobacteriosis, and to Campylobacter occurrence and prevalence in food and animals, respectively, in the EU, during 2012–2016. A more detailed description of these statistics is in the results section of this chapter and in the chapter on food‐borne outbreaks.
Table 3.
Summary of Campylobacter statistics related to humans and major food categories, EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 246,307 | 232,134 | 236,818 | 214,710 | 214,300 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 66.3 | 62.9 | 66.5 | 61.4 | 61.7 | ECDC |
| Number of reporting countries | 27 | 27 | 26 | 26 | 26 | ECDC |
| Infection acquired in the EU | 122,806 | 142,536 | 135,822 | 120,521 | 124,070 | ECDC |
| Infection acquired outside the EU | 6,347 | 6,838 | 7,401 | 7,481 | 7,513 | ECDC |
| Unknown travel status or unknown country of infection | 117,154 | 82,760 | 93,595 | 86,708 | 82,717 | ECDC |
| Total number of food‐borne outbreaks (including waterborne outbreaks) | 461 | 399 | 454 | 417 | 503 | EFSA |
| Number of outbreak‐related cases | 4,606 | 1,488 | 2,082 | 1,836 | 1,555 | EFSA |
| Food | ||||||
| Meat and meat products | ||||||
| Number of sampled units | 18,048 | 16,134 | 15,758 | 21,383 | 25,348 | EFSA |
| Number of reporting MS | 19 | 18 | 20 | 20 | 20 | EFSA |
| Milk and milk products | ||||||
| Number of sampled units | 1,896 | 2,126 | 2,708 | 3,324 | 3,313 | EFSA |
| Number of reporting MS | 10 | 10 | 10 | 10 | 9 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
Food data of interest reported were classified into the major categories ‘Meat and meat products’ and ‘Milk and milk products’, and aggregated by year over the period 2012–2016 to get an annual overview of the amount of data submitted. In the summary table, data from suspect and selective sampling and from industry own‐control programmes and Hazard Analysis and Critical Control Point (HACCP) sampling were excluded. The number of sampled units reported tends to generally decrease since 2012, and originated for ‘Meat and meat products’ and ‘Milk and milk products’ from, respectively, two‐thirds and one‐third of the MS.
1.3.2. Human campylobacteriosis
For 2016, campylobacteriosis data were reported by 27 MS. The number of confirmed cases in 2016 of human campylobacteriosis in the EU was 246,307, which represents an increase of 14,173 cases (6.1%) compared with 2015 (Table 4). Twenty MS reported an increase in the number of cases and notification rates compared with 2015. The EU notification rate was 66.3 per 100,000 population in 2016, an increase by 6.1% compared with 2015 (62.9 per 100,000 population).
Table 4.
Reported human cases of campylobacteriosis and notification rates per 100,000 in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 7,086 | 7,083 | 81.5 | 6,258 | 73.0 | 6,514 | 76.6 | 5,731 | 67.8 | 4,710 | 56.0 |
| Belgium | Y | A | 10,055 | 10,055 | 88.9 | 9,066 | 80.7 | 8,098 | 72.4 | 8,148 | 73.0 | 6,607 | 59.6 |
| Bulgaria | Y | A | 202 | 202 | 2.8 | 227 | 3.2 | 144 | 2.0 | 124 | 1.7 | 97 | 1.3 |
| Croatia | Y | A | 1,547 | 1,524 | 36.4 | 1,393 | 33.0 | 1,647 | 38.8 | 0 | 0.0 | 0 | 0.0 |
| Cyprus | Y | C | 21 | 21 | 2.5 | 29 | 3.4 | 40 | 4.7 | 56 | 6.5 | 68 | 7.9 |
| Czech Republic | Y | C | 24,291 | 24,084 | 228.2 | 20,960 | 198.9 | 20,750 | 197.4 | 18,267 | 173.7 | 18,287 | 174.1 |
| Denmark | Y | C | 4,712 | 4,712 | 82.6 | 4,327 | 76.5 | 3,773 | 67.0 | 3,772 | 67.3 | 3,720 | 66.7 |
| Estonia | Y | C | 382 | 298 | 22.6 | 318 | 24.2 | 285 | 21.7 | 382 | 28.9 | 268 | 20.2 |
| Finland | Y | C | 4,637 | 4,637 | 84.5 | 4,588 | 83.8 | 4,889 | 89.7 | 4,066 | 74.9 | 4,251 | 78.7 |
| Franceb | N | C | 6,698 | 6,698 | 50.2 | 6,074 | 45.7 | 5,958 | 45.2 | 5,198 | 39.6 | 5,079 | 38.9 |
| Germany | Y | C | 73,999 | 73,663 | 89.6 | 69,829 | 86.0 | 70,571 | 87.4 | 63,280 | 78.6 | 62,548 | 77.9 |
| Greecec | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Hungary | Y | C | 8,579 | 8,556 | 87.0 | 8,342 | 84.6 | 8,444 | 85.5 | 7,247 | 73.5 | 6,367 | 64.4 |
| Ireland | Y | C | 2,511 | 2,511 | 53.1 | 2,453 | 53.0 | 2,593 | 56.3 | 2,288 | 49.8 | 2,391 | 52.2 |
| Italyd | N | C | 1,057 | 1,057 | – | 1,014 | – | 1,252 | – | 1,178 | – | 774 | – |
| Latvia | Y | C | 93 | 90 | 4.6 | 74 | 3.7 | 37 | 1.8 | 9 | 0.4 | 8 | 0.4 |
| Lithuania | Y | C | 1,225 | 1,225 | 42.4 | 1,186 | 40.6 | 1,184 | 40.2 | 1,139 | 38.3 | 917 | 30.5 |
| Luxembourg | Y | C | 518 | 518 | 89.9 | 254 | 45.1 | 873 | 158.8 | 675 | 125.7 | 581 | 110.7 |
| Malta | Y | C | 212 | 212 | 48.8 | 248 | 57.8 | 288 | 67.7 | 246 | 58.4 | 220 | 52.7 |
| Netherlandse | N | C | 3,383 | 3,383 | 38.3 | 3,778 | 43.0 | 4,159 | 47.5 | 3,702 | 42.4 | 4,248 | 48.8 |
| Poland | Y | C | 787 | 773 | 2.0 | 653 | 1.7 | 650 | 1.7 | 552 | 1.4 | 431 | 1.1 |
| Portugal | Y | C | 366 | 359 | 3.5 | 271 | 2.6 | – | – | – | – | – | – |
| Romania | Y | C | 517 | 517 | 2.6 | 311 | 1.6 | 256 | 1.3 | 218 | 1.1 | 92 | 0.5 |
| Slovakia | Y | C | 7,738 | 7,623 | 140.5 | 6,949 | 128.2 | 6,744 | 124.5 | 5,845 | 108.0 | 5,704 | 105.5 |
| Slovenia | Y | C | 1,642 | 1,642 | 79.5 | 1,328 | 64.4 | 1,184 | 57.4 | 1,027 | 49.9 | 983 | 47.8 |
| Spaind | N | C | 15,556 | 14,856 | – | 13,227 | – | 11,481 | – | 7,064 | – | 5,548 | – |
| Sweden | Y | C | 11,021 | 11,021 | 111.9 | 9,180 | 94.2 | 8,288 | 85.9 | 8,114 | 84.9 | 7,901 | 83.3 |
| United Kingdom | Y | C | 58,987 | 58,987 | 90.2 | 59,797 | 92.2 | 66,716 | 103.7 | 66,382 | 103.9 | 72,500 | 114.2 |
| EU total | – | – | 247,822 | 246,307 | 66.3 | 232,134 | 62.9 | 236,818 | 66.5 | 214,710 | 61.4 | 214,300 | 61.7 |
| Iceland | Y | C | 128 | 128 | 38.5 | 119 | 36.2 | 142 | 43.6 | 101 | 31.4 | 60 | 18.8 |
| Norway | Y | C | 2,317 | 2,317 | 44.5 | 2,318 | 44.9 | 3,386 | 66.3 | 3,291 | 65.2 | 2,933 | 58.8 |
| Switzerlandf | Y | C | 7,688 | 7,688 | 91.9 | 7,055 | 85.3 | 7,565 | 92.9 | 7,481 | 93.1 | 8,432 | 106.0 |
Y: yes; N: no; A: aggregated data; C: case‐based data; −: no report.
Sentinel surveillance; notification rates calculated with an estimated coverage of 20%.
No surveillance system.
Sentinel surveillance; no information on estimated coverage. So notification rate cannot be estimated.
Sentinel surveillance; notification rates calculated with an estimated coverage 52%.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The highest country‐specific notification rates in 2016 were observed, as in previous years, in the Czech Republic (228.2 cases per 100,000), Slovakia (140.5), Sweden (111.9) and the United Kingdom (90.2). The lowest rates in 2016 were reported by Bulgaria, Cyprus, Latvia, Poland, Portugal and Romania (≤ 4.6 per 100,000).
In most MS, campylobacteriosis was mainly a domestically acquired infection with ≥ 90% of cases reported as domestic. Almost half of the cases (46.9%), however, were reported as being of unknown origin (Table 3). The highest proportions of domestic cases (> 99%) were reported in the Czech Republic, Hungary, Latvia, Malta, Poland, Portugal, Romania and Slovakia. The highest proportions of travel‐associated cases were reported by three Nordic countries – Finland (65.4%), Iceland (51.4%) and Norway (53.5%). Sweden, which in previous years reported most of the campylobacteriosis cases as travel associated, experienced an increase in domestic cases by 46.5% compared with 2015. Among 14,257 travel‐associated cases with known probable country of infection, 47.6% of the cases were linked to travel within EU, with most of the cases from Spain, France and Greece (17.8%, 4.3% and 4.2%, respectively). Thailand, Turkey and India were most often reported as the probable country of infection outside EU (10.2%, 5.5% and 3.6%, respectively).
Between 2012 and 2016, there was a clear seasonality in the number of confirmed campylobacteriosis cases reported in the EU/EEA, with sharp peaks in the summer months. Small annual winter peaks were also observed in January starting from 2011. Over the period from 2008 to 2016, a statistically significant increasing trend was observed in EU/EEA (p < 0.05); however, the trend did not show any significant increase or decrease in the period 2012–2016 (Figure 2).
Figure 2.
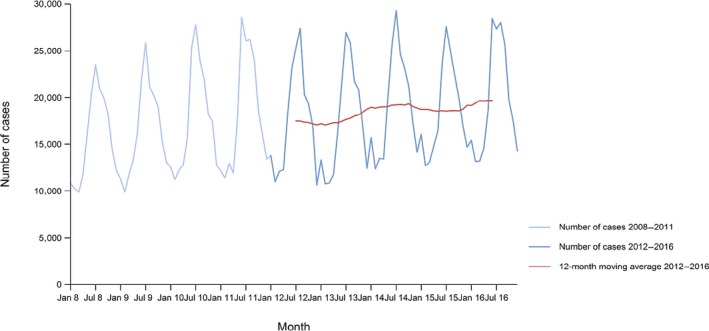
Trend in reported confirmed human cases of campylobacteriosis in the EU/EEA, by month, 2012–2016
- Source(s): Austria, Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom. Belgium, Bulgaria, Croatia and Portugal did not report data at the level of detail required for the analysis. In Greece, campylobacteriosis is not under surveillance.
At country level, 14 MS (Austria, Estonia, France, Hungary, Ireland, Italy, Latvia, Lithuania, Malta, Poland, Slovakia, Slovenia, Spain and Sweden) reported significantly increasing trends between 2008 and 2016 while none of the MS reported a decreasing trend.
In 2012–2016, 12 MS continued to report increasing trends (Austria, the Czech Republic, France, Hungary, Latvia, Lithuania, Poland, Romania, Slovakia, Slovenia, Spain and Sweden). In four MS (Estonia, Ireland, Italy and Malta), no significant change was observed, and for one MS (Cyprus) a decreasing trend was observed.
Information on hospitalisation status was provided for 27.0% of all campylobacteriosis cases in 2016 by 17 MS. Of cases with known hospitalisation status, 28.5% were hospitalised. The highest hospitalisation rates (72.9–90.5%) were reported in Cyprus, Latvia, Lithuania, Poland, Romania and the United Kingdom.
Outcome was reported for 72.6% (178,726 cases) by 16 MS. The number of reported deaths attributed to campylobacteriosis increased from 25 deaths in 2014 to 62 deaths in 2016, resulting in an EU case fatality of 0.03%. This was similar to the average percentage of fatal outcome observed over the last 5 years.
Campylobacter species information was provided for 53.2% of confirmed cases reported in the EU, which was more than in 2015 (43.2%). Of these, 83.6% were Campylobacter jejuni, 8.5% Campylobacter coli, 0.2%, Campylobacter lari 0.06%, Campylobacter fetus 0.05% and 0.04% Campylobacter upsaliensis. ‘Other’ Campylobacter species accounted for 7.6%, but the large majority of those cases was reported at the national level as ‘C. jejuni/C. coli/C. lari not differentiated’.
1.3.3. Campylobacter in foods
Table 5 summarises the reported occurrence, in 2016, of Campylobacter in the most important food categories (fresh meat, ready‐to‐eat (RTE) meat products). Few MS reported data on Campylobacter in food: 14 MS reported data on fresh meat mainly from broilers and turkeys. Highest occurrence was observed in fresh meat from broilers (36.7%) followed by fresh meat from turkeys (11%). Very few MS (1–4) reported on RTE meat products with occurrence between 0% and 2%.
Table 5.
Summary of Campylobacter statistics related to major food categories and animal species, reporting EU MS and non‐MS, 2016
| Number of reporting MS/non‐MS | Number of tested units, EU | Proportion (%) of positive units, EU | ||
|---|---|---|---|---|
| Fresh meat | Broilers | 14/0 | 11,495 | 36.7 |
| Turkey | 7/0 | 1,505 | 11.0 | |
| Pig | 6/0 | 554 | 2.9 | |
| Bovine | 7/0 | 1,220 | 1.0 | |
| Meat products, RTE | Broilers | 1/0 | 54 | 1.9 |
| Turkey | 1/0 | 16 | 0 | |
| Pig | 4/0 | 44 | 0 | |
| Bovine | 2/0 | 64 | 1.6 | |
| Unspecified | 7/0 | 116 | 0.9 | |
| Milk and milk products | Milk | 9/0 | 1,327 | 1.2 |
| Cheese | 5/0 | 289 | 1.0 | |
| Animals | Broilers | 14/0 | 13,558 | 27.3 |
| Turkeys | 5/1 | 2,894 | 65.3 | |
| Pigs | 1/0 | 50 | 0.7 | |
| Bovine animals | 6/0 | 6,469 | 1.1 | |
| Cats and dogs | 5/2 | 1,196 | 5.5 | |
| Other animalsa | 3/0 | 1,031 | 12.4 |
RTE: ready‐to‐eat; MS: Member State.
‘Other animals’ include: sheep, goats, water buffalos, pigeons, magpies, foxes, deer, birds and pet animals.
Campylobacter in milk and milk products (including cheeses) for the year was reported by nine MS. The occurrence was comparable between milk products and cheeses and was around 1%.
1.3.4. Campylobacter in animals
Few MS reported 2016 monitoring data on Campylobacter in animals. More than 60% of the samples originated from broilers, from 14 MS, and from turkeys, from 5 MS. The highest apparent prevalence was in turkeys (Table 5).
As regards food‐borne campylobacteriosis outbreaks in humans, in 2016, the largest food‐borne outbreak was reported by Sweden and involved more than 3,000 domestic cases who infected with Campylobacter after consumption of poultry meat.
1.4. Discussion
Campylobacteriosis has been the most commonly reported zoonosis in humans in the EU since 2005. There was a significantly increasing trend in the number of cases at EU/EEA level and at country level in half of the MS between 2008 and 2016. The EU notification rate did not change significantly over the last 5 years. Half of the MS, however, had statistically significant increasing trends also in the period 2012–2016 and majority of the countries had an increase in the number of confirmed cases in the last 5 years. The increase in reported cases in some of these countries may not only reflect changes in exposure, but also improvements in MS surveillance systems. In Belgium, more laboratories have begun to report campylobacteriosis since 2015, and the number of notified cases increased. In the Czech Republic, testing and diagnostics for campylobacteriosis has improved since 2013.
In Spain, coverage of the surveillance system for campylobacteriosis has improved and the number of reported confirmed cases has almost doubled since 2012. In Sweden, an outbreak of Campylobacter in 2016 resulted in almost a double number of domestic human cases compared to previous years.
Campylobacter has a characteristic seasonality with a sharp increase in the number of cases in the summer and early autumn. A smaller but distinct winter peak has become apparent in the past few years, including 2016. The peak of cases was mainly seen in five MS (Austria, Belgium, Germany, Luxembourg and the Netherlands) covering more than 45% of all cases reported in January. The observed winter peak in Campylobacter infections in Switzerland has been partly attributed to a traditional meal, meat fondue, especially if served with chicken meat (Bless et al., 2014). This meal is also often consumed in several other countries at festive occasions in wintertime such as Christmas and New Year. In Sweden, the winter peaks in 2014 and 2015 were linked to the increased incidence of Campylobacter in domestic chicken (Skarin et al., 2017). Typing of human and chicken isolates with whole genome sequencing and pulsed field gel electrophoresis (PFGE) confirmed the link between the increase in the incidence in humans and domestic chicken.
The proportion of hospitalised campylobacteriosis cases was higher than expected in some MS, which also reported the lowest notification rates. In some countries, the surveillance is known to focus mainly on severe cases. In others, hospitalisation status is ascertained and reported for a higher fraction of cases by hospitals, while for cases reported from other sources, e.g. laboratories, hospitalisation status is often missing. Both of these factors may result in an overestimation of the proportion of hospitalised cases.
As regards the food and animal monitoring data from investigations on Campylobacter; as for the previous years, about two‐thirds to one‐third of MS reported some major food and animal matrix data for the year 2016. According to the Zoonoses Directive 2003/99/EC, MS are obliged to report on Campylobacter occurrence or prevalence in food and animals. In addition to the low volume of data reported, the sampling and reporting rules are not harmonised, precluding trend analyses and trend watching. These deficiencies prevent inference being made, beyond the sample statistics, on trends or sources of Campylobacter in foods or animals. Despite this, documenting reports with the aim of understanding trends and sources of Campylobacter along the food chain is essential to the overall goal of reducing campylobacteriosis, whether food‐borne or sporadic, as Campylobacter is the most commonly reported gastrointestinal bacterial pathogen in humans in the EU, and has been so since 2005.
1.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Fact sheet on Campylobacter | https://www.cdc.gov/foodsafety/diseases/campylobacter/index.html |
| ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx | |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| WHO (World Health Organization) – Campylobacter Fact sheet | http://www.who.int/mediacentre/factsheets/fs255/en/ | |
| Food | European Union Reference Laboratory (EURL) for Campylobacter | http://www.sva.se/en/service-and-products/eurl-campylobacter |
| EFSA Scientific Opinion: Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU | http://www.efsa.europa.eu/en/efsajournal/pub/1437 | |
| EFSA Scientific Opinion: Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain | https://www.efsa.europa.eu/en/efsajournal/pub/2105 | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
2. Salmonella
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
2.1. Abstract
In 2016, 94,530 confirmed salmonellosis cases were reported by all MS. The EU notification rate was at the same level as in the previous 5 years. A statistically significant decreasing trend of salmonellosis has been observed between 2008 and 2016, however during the last 5 years (2012–2016) the trend has not shown any statistically significant increase or decrease. Seven MS reported an increasing trend and four MS a decreasing trend over the period 2012–2016.
The top five most commonly reported serovars in human cases acquired in EU during 2016 were, in decreasing order: S. Enteritidis, S. Typhimurium, monophasic S. Typhimurium, S. Infantis and S. Derby. The proportion of human salmonellosis illnesses due to S. Enteritidis continued to increase in 2016. The data reported on food and animals showed that S. Enteritidis was markedly associated with laying hens, broilers and broiler meat. A similar evolution during 2012–2016 was noticeable between the proportion of S. Enteritidis illnesses in humans acquired in EU and the EU flock prevalence of S. Enteritidis in laying hens that significantly increased during 2015 and 2016. S. Typhimurium cases in humans decreased. S. Typhimurium was reported from pigs and cattle and meats from these species and to a lesser extent from poultry and their meat. Human cases infected in EU due to monophasic S. Typhimurium remained at a stable level compared with previous years and this serovar was mostly reported and associated with (contact with) pigs and (consumption of) pig meat. The proportion of human illnesses due to S. Infantis, the fourth most common serovar in humans, also remained stable. S. Infantis was mostly reported from the broiler and turkey chains and has been able to massively spread along the entire broiler production chain. S. Infantis represents an important public health concern, because of its high levels of multidrug resistance. Serovar Derby, the fifth most frequently reported serovar among cases in infections in humans within EU, was most commonly reported from pigs and pig meat and to a lesser extent from poultry and cattle.
The 2016 monitoring data related to the compliance of foods with Salmonella food safety criteria, showed that, as in the previous years, the highest level of non‐compliance was reported for certain meat categories intended to be eaten cooked (mechanically separated meat, minced meat and meat preparations from poultry to be eaten cooked and meat products from poultry to be eaten cooked). For fresh poultry meat, in contrast, that has exclusively targeted serovars as a food safety criterion, the percentage of non‐compliant samples was negligible. The non‐compliance for RTE products was also rare. The overall percentage of non‐compliance with the Salmonella process hygiene criterion for pig carcass swabs was about 2%.
At primary production level, in the context of the National Control Programmes, the EU‐level flock prevalence of target Salmonella serovars in breeding hens, broilers, breeding and fattening turkeys decreased or stabilised compared with previous years. However, the decreasing EU‐level flock prevalence of target Salmonella serovars in laying hens reported since the implementation in 2008 of National Control Programmes, has been reversed into a statistically significant increasing trend during the last two years. The EU prevalence of S. Enteritidis in laying hens notably increased. This recent increase involved several MS, and it was more pronounced in some of them.
2.2. Surveillance and monitoring of Salmonella in the EU
2.2.1. Humans
The notification of non‐typhoidal salmonellosis in humans is mandatory in most MS, Iceland, Norway and Switzerland, except for six MS where reporting is based on a voluntary system (Belgium, France Luxembourg and the Netherlands) or other systems (Spain and the United Kingdom). The surveillance systems for salmonellosis cover the whole population in all MS except four (Belgium, France, the Netherlands and Spain). The coverage of the surveillance system is estimated to be 48% in France and 64% in the Netherlands. These proportions of populations were used in the calculation of notification rates for these two MS. No estimation for population coverage in Belgium and Spain was provided, so the notification rates were not calculated.
Diagnosis of human Salmonella infections is generally performed by culture from human stool samples. Most countries perform serotyping of isolates.
2.2.2. Food, animals and feed
Salmonella monitoring and surveillance data reported in the framework of EU Regulation 2073/2005 on microbiological criteria
Monitoring of Salmonella in foods is mainly based on data originating from the reporting obligations of MS under EU Regulation 2073/20055 on microbiological criteria that has been in force since 1 January 2006. Generally, data submitted to EFSA for compliance with the Salmonella microbiological criteria allow for descriptive summaries at EU‐level to be made, and also allows EU trends to be monitored but data quality precludes trend analyses at EU‐level (Table 1).
Salmonella food safety criteria at the retail level
Regulation (EC) No 2073/2005 on microbiological criteria in foodstuffs lays down Salmonella food safety criteria and these should be monitored by the individual food business operator in the context of their own HACCP programmes. The Salmonella food safety criteria prescribe that Salmonella monitoring results must be compliant with ‘absence in 25 or 10 grams’, when products are placed on the market, during their shelf life. Absence is defined by testing five or, depending on the food category, 30 sampling units per batch, for specified food categories. Moreover, according to Regulation (EC) No 1086/20117 that has been in force since December 2011, compliance with ‘absence in 25 grams’ is required for the regulated serovars in the context of EU control programmes for poultry populations (S. Enteritidis and S. Typhimurium including monophasic S. Typhimurium strains with the antigenic formula 1,4,[5],12:i:‐) in batches of fresh poultry meat (including fresh meat from breeding flocks of Gallus gallus, laying hens, broilers and breeding and fattening flocks of turkeys).
Salmonella process hygiene criteria at the level of the processing plant
Regulation (EC) No 2073/2005 on microbiological criteria in foodstuffs also lays down process hygiene criteria and monitoring of compliance with these criteria is the legal task of the individual food business operator in the context of their own HACCP programmes. Salmonella process hygiene criteria are regulated for carcasses of pigs, cattle, sheep, goats, horses and broilers and turkeys. Specifically, for Salmonella on pigs’ carcases the process hygiene criteria is met by complying with a maximum 3 positive out of 50 tested carcases, where 3 is a suggested number that should be reviewed according to the MS previous results. The Competent Authority (CA) verifies the correct implementation by the food business operator of this process hygiene criterion for Salmonella on pig carcases and sampling schemes are laid down in point G (a) of Chapter 9 of Annex 1 of Regulation (EC) No 854/2004.
Monitoring data for compliance with the Salmonella National Control Programmes in poultry
According to EU Regulation (EC) No 2160/2003 and its following amendments, MS have to set up Salmonella National Control Programmes (NCP) aimed at reducing the prevalence of Salmonella serovars, which are considered relevant for public health, in certain animal populations. Currently, prevalence targets have been defined for breeding flocks of Gallus gallus, laying hens, broilers and breeding and fattening turkeys and correspond to the maximum annual percentage of flocks remaining positive for relevant serovars (S. Enteritidis and S. Typhimurium, including its monophasic variant, except for breeding flocks of Gallus gallus, where S. Infantis, S. Virchow and S. Hadar are considered to be relevant as well). In particular, the prevalence target is equal to 1% or less for breeding flocks of Gallus gallus, broilers and breeding and fattening turkeys and to 2% or less for laying hens (for this last animal category the prevalence reduction to be obtained annually initially had to be calculated according to the prevalence in the preceding year, as described in Regulation (EU) No 517/20118).
In breeding flocks of Gallus gallus, 2016 was the 10th year in which MS were obliged to implement a Salmonella NCP. These NCPs are based on Regulation (EC) No 200/20109 and the prevalence target (1% or less) was set for all commercial‐scale adult breeding flocks, during the production period, comprising at least 250 birds (however, MS with fewer than 100 breeding flocks would attain the target if only one adult breeding flock remained positive). In laying hens flocks, it was the ninth year in which MS were obliged to implement a Salmonella NCP. According to Regulation (EC) No 517/2011, the prevalence target (which depends on the prevalence of the preceding year and was equal in 2016 to 2% or less for all MS except for Poland where it was 2.5% or less) was set for all commercial‐scale adult laying hen flocks in the production period. However, MS with fewer than 50 flocks of adult laying hens would attain the target if only one adult flock remained positive. In broilers, 2016 was the eighth year of mandatory implementation of Salmonella NCPs, which are based on Regulation (EC) No 200/201210. Salmonella NCPs in turkey flocks have been mandatory since 2010. Regulation (EU) No 1190/201211 defined the reduction targets.
The NCP are set up in individual MS to achieve the EU prevalence targets in these animal populations at the primary production level. NCP have to be approved by the European Commission, which evaluates the compliance of the programmes with the relevant EU legislation. The results of the programmes have to be reported to the European Commission and EFSA as part of the annual EU zoonoses monitoring.
Salmonella monitoring data originating from the Salmonella National Control Programmes in poultry are based on programmed surveillance/monitoring. They are collected in a fully harmonised way, are a census and are reported also according to harmonised rules. Therefore, they allow subsequent data analysis such as assessing spatial and temporal trends at the EU‐level. They also allow for descriptive summaries at EU‐level to be made, and allow EU trends to be monitored (Table 1).
Other Salmonella monitoring data of foods, animals and feed
Other monitoring data on Salmonella from food, animals and feed and submitted to EFSA (according Chapter II (‘monitoring of zoonoses and zoonotic agent's) of the Zoonoses Directive 2003/99/EC) are collected without harmonised design, but monitoring is required if listed in the Annex I of Directive 2003/99/EC on the monitoring of zoonoses, at the most appropriate stage of the food chain, as laid down in Article 4 of that Directive.
Salmonella monitoring data submitted to EFSA and collected without harmonised design allows for descriptive summaries at EU‐level to be made. They however preclude trend analyses and trend watching at EU‐level (Table 1).
Within this category, Salmonella serovar data should also be included. MS are obliged to report the target serovars as part of NCPs in poultry populations, whereas for the remaining production categories serotyping is not mandatory. Also, for the food sector, the food safety criteria consider the absence of Salmonella spp., with the exception of fresh poultry meat, for which the criterion is limited to the absence of the target serovars. Hence, some MS could decide to not report the presence of non‐target serovars. This could lead to a possible bias in the reporting of target serovars for poultry populations and fresh poultry meat. Hence, the mandatory reporting of target serovars in the context of NCP and evaluation of the food safety criterion for fresh poultry meat guarantees the consistency of such data over years and among MS, but could result in an overestimation of these target serovars compared with the other serovars. For the remaining matrices, serovar data collected could be strongly biased by what each MS actually serotyped and notified. Hence, serovar data, especially for non‐target serovars, allow descriptive summaries only since substantial differences in reporting matrices observed over the years and among MS could be simply related to what each MS decides to report. This could lead to the overestimation or underestimation of specific serovars for certain matrices.
Complementary to the mandatory NCP for Salmonella in poultry, MS can have compulsory or voluntary Salmonella control or monitoring programmes in place for a number of farm animal species. These programmes, which are based on national requirements, may fluctuate over time.
2.2.3. Food‐borne outbreaks of human salmonellosis
The reporting of FBO of human salmonellosis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
2.3. Data analyses
2.3.1. Statistical trend analyses (methods) of poultry monitoring data
Statistical trend analyses were carried out with the objectives of evaluating the significance of temporal variations in the EU‐level flock prevalence of Salmonella, and Salmonella target and non‐target serovars in poultry species, since the start of the implementation of NCP.
To take into account the potential correlations among observations in the same MS in subsequent years and the heterogeneity among MS in terms of probability of finding positive flocks, a generalised mixed model for longitudinal binary data was computed (EFSA and ECDC, 2016d). To take into account the different levels (baselines) of risk of MS having positive flocks, but similar patterns over time, a random MS‐specific intercept effect was included in the model. The correlation among repeated observations in the same MS in subsequent years was considered using a first autoregressive correlation matrix structure (AR1) for the residuals.
According to the behaviour of the prevalence in different MS over time and for the species analysed, constant, linear and polynomial models for the logit of the probability (p) of flocks being positive were computed. The logit is defined as the logarithm of p/(1−p), where p/(1−p) is the odds of becoming positive for Salmonella. A constant value was used when no significant change in the behaviour of the logit of the probability over time was evident; linear regression when a straight line could describe the increase or decrease in the logit of the probability over time; second‐degree polynomial regression when the response increased or decreased monotonically over time, but in a curvilinear way, and third‐degree polynomial regression when the logit of the probability had two inflection points (decreasing and then increasing or increasing and then decreasing).
The interpretation of the parameters obtained through these models was conducted at the population level (i.e. the EU‐level), whereas no inference was directly made at the level of individual MS.
The results of the estimated parameters of models, odds ratio, prevalence and graphical analysis are reported in the Appendix for each poultry species (2016_OUTCTRENDANAL).
GLIMMIX and SGPLOT procedures in SAS 9.4 software were used to fit the generalised mixed model and to produce the graphical outputs, respectively.
2.3.2. Descriptive analyses of Salmonella serovars
With the aim to evaluate the distribution of Salmonella serovars along the food chain and identify the potential sources for human infections, descriptive analyses were made from food and animal data of the five most commonly reported Salmonella serovars for 2016 in human illnesses acquired within EU, while dropping human cases who got infected outside the EU. Note that serovars monitoring data, which are not covered by harmonised programmes (non‐target Salmonella serovars from poultry and Salmonella serovars from pigs, cattle and meat from broilers, turkeys, pigs and cattle) are not harmonised as regards sampling schemes and reporting, and so can only be used for descriptive summaries.
Monophasic variants of S. Typhimurium have been reported by MS by using different designations, generally as the generic denomination ‘monophasic S. Typhimurium’ or by using the antigenic formula with different levels of details in terms of antigens investigated and identified. This lack of harmonisation in the nomenclature could lead to an erosion of data, and eventually an underestimation of the real frequency of isolation of this serovar in the different sources. From the epidemiological point of view, all the isolates of the monophasic S. Typhimurium group have the same significance. So, in this report, the isolates belonging to the group of monophasic variants of S. Typhimurium and reported by MS with different designations (S. Typhimurium monophasic, S. 1,4,[5],12:i:‐, S. 1,4,5,12:i:‐, S. 1,4,12:i:‐, S. 4,[5],12:i:‐, S. 4,5,12:i:‐ and S. 4,12:i:‐) were merged into the same group and named ‘monophasic variants of S. Typhimurium’.
The Sankey diagram of the most reported Salmonella serovars from humans in relation to their food and animal sources was produced in HTML format using Google Chart libraries (http://developers.google.com/chart/).
Pyramid plots for the serovars of interest were prepared to show for each source the frequency of notification between animal and food sources using R software (http://www.r-project.org).
2.4. Results
2.4.1. Overview of key statistics along the food chain, EU, 2012–2016
Table 6 summarises EU‐level statistics related to human salmonellosis, and to Salmonella occurrence and prevalence in food and animals, respectively, in the EU, during 2012–2016. More detailed descriptions of these statistics are in the results section of this chapter and in the FBO.
Table 6.
Summary of Salmonella statistics related to humans, major food categories and major animal species, EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 94,530 | 94,597 | 92,012 | 87,753 | 94,278 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 20.4 | 20.9 | 20.7 | 20.3 | 21.9 | ECDC |
| Number of reporting countries | 28 | 28 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 50,400 | 49,672 | 48,451 | 44,706 | 52,550 | ECDC |
| Infection acquired outside the EU | 6,404 | 6,773 | 6,202 | 7,334 | 7,334 | ECDC |
| Unknown travel status or unknown country of infection | 37,726 | 38,152 | 37,359 | 35,713 | 34,394 | ECDC |
| Total number of food‐borne outbreaks (including waterborne outbreaks) | 1,067 | 953 | 1,049 | 1,168 | 1,533 | EFSA |
| Number of outbreak‐related cases | 9,061 | 6,616 | 9,294 | 8,709 | 11,895 | EFSA |
| Food | ||||||
| Meat and meat products | ||||||
| Number of sampled units | 277,346 | 203,683 | 503,647 | 410,529 | 370,752 | EFSA |
| Number of MS | 28 | 27 | 25 | 27 | 25 | EFSA |
| Milk and milk products | ||||||
| Number of sampled units | 24,509 | 29,170 | 70,464 | 59,234 | 49,316 | EFSA |
| Number of MS | 25 | 22 | 24 | 23 | 20 | EFSA |
| Fish and fishery products | ||||||
| Number of sampled units | 5,403 | 5,652 | 9,893 | 10,712 | 12,960 | EFSA |
| Number of MS | 20 | 21 | 20 | 19 | 21 | EFSA |
| Eggs and egg products | ||||||
| Number of sampled units | 11,137 | 9,768 | 23,536 | 30,283 | 26,324 | EFSA |
| Number of MS | 21 | 19 | 20 | 19 | 19 | EFSA |
| Fruits and vegetables (and juices) | ||||||
| Number of sampled units | 8,013 | 7,370 | 10,652 | 10,684 | 28,512 | EFSA |
| Number of MS | 21 | 22 | 23 | 23 | 20 | EFSA |
| Animals | 2016 | 2015 | 2014 | 2013 | 2012 | |
| Fowl | ||||||
| Number of sampled flocks | 703,924 | 528,245 | 509,242 | 479,098 | 466,640 | EFSA |
| Number of MS | 28 | 28 | 27 | 28 | 26 | EFSA |
| Turkeys | ||||||
| Number of sampled flocks | 78,063 | 54,246 | 41,239 | 36,723 | 35,303 | EFSA |
| Number of MS | 25 | 24 | 24 | 24 | 24 | EFSA |
| Ducks and geese | ||||||
| Number of sampled flocks | 2,627 | 2,757 | 3,020 | 2,283 | 3,951 | EFSA |
| Number of MS | 9 | 7 | 8 | 8 | 7 | EFSA |
| Pigs | ||||||
| Number of sampled herds | 8,560 | 12,100 | 11,988 | 9,901 | 207,803 | EFSA |
| Number of MS | 8 | 7 | 7 | 7 | 6 | EFSA |
| Bovine animals | ||||||
| Number of sampled herds | 4,888 | 12,178 | 8,334 | 6,004 | 7,866 | EFSA |
| Number of MS | 4 | 5 | 4 | 5 | 5 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
Humans
The number of human salmonellosis cases infected domestically and through travel within EU tended to increase since 2013 after a decrease from 2012 to 2013. The number of outbreak‐related cases and the total number of food‐borne salmonellosis outbreaks were higher in 2016 compared with 2015 and after having decreased from 2012 to 2015.
Food categories
The numbers of sampled units reported for the general food category ‘meat and meat products’ reported for 2016 were lower compared with the years 2012–2014. This was generally also the case with the other food categories (milk and milk products, fish and fishery products, eggs and egg products, fruits and vegetables including juices). In contrast, the number of reporting MS was fairly stable during these years, within these major food groups.
Animal categories
The number of sampled flocks reported by MS from Gallus gallus fowl and from turkeys progressively increased during 2012–2016. These global statistics are underpinned by data submitted by MS in compliance with the mandatory NCP in poultry. For the category ‘ducks and geese’, the number of sampled flocks decreased over the period 2014–2016, but the number of reporting MS remained stable. For pigs and bovine animals, the numbers of reported sampled herds for 2016 were the lowest compared with the previous years.
2.4.2. Human salmonellosis
In total, 96,039 salmonellosis cases were reported by 28 MS for 2016, with 94,530 confirmed cases resulting in an EU notification rate of 20.4 cases per 100,000 (Table 7). This was at the same level as in 2015 (20.9 cases per 100,000). As in the previous year, the highest notification rates in 2016 were reported by the Czech Republic (110 per 100,000) and Slovakia (97.7 per 100,000), while the lowest rates were reported by Greece, Italy, Ireland and Portugal (≤ 6.8 per 100,000). The increase (212.8%) in notification rate in Estonia was mainly due to two general outbreaks, one of which was not food‐borne (person‐to‐person transmission), with a large number of illnesses; whereas in Poland the increased notification rate (18.0%) was accompanied by an increase in the number of Salmonella outbreaks.
Table 7.
Reported human cases of salmonellosis and notification rates per 100,000 population in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 1,415 | 1,415 | 16.3 | 1,544 | 18.0 | 1,654 | 19.4 | 1,404 | 16.6 | 1,773 | 21.1 |
| Belgiumb | Y | A | 2,806 | 2,806 | 24.8 | 3,170 | 28.2 | 2,698 | 24.1 | 2,528 | 22.7 | 3,101 | 28.0 |
| Bulgaria | Y | A | 719 | 718 | 10.0 | 1,076 | 14.9 | 730 | 10.1 | 766 | 10.5 | 839 | 11.5 |
| Croatia | Y | A | 1,259 | 1,240 | 29.6 | 1,593 | 37.7 | 1,494 | 35.2 | 0 | 0.0 | 0 | 0.0 |
| Cyprus | Y | C | 77 | 77 | 9.1 | 65 | 7.7 | 88 | 10.3 | 79 | 9.1 | 90 | 10.4 |
| Czech Republic | Y | C | 11,809 | 11,610 | 110.0 | 12,408 | 117.7 | 13,255 | 126.1 | 9,790 | 93.1 | 10,056 | 95.7 |
| Denmark | Y | C | 1,081 | 1,081 | 18.9 | 925 | 16.3 | 1,124 | 20.0 | 1,137 | 20.3 | 1,207 | 21.6 |
| Estonia | Y | C | 358 | 351 | 26.7 | 112 | 8.5 | 92 | 7.0 | 183 | 13.9 | 249 | 18.8 |
| Finland | Y | C | 1,512 | 1,512 | 27.6 | 1,650 | 30.2 | 1,622 | 29.8 | 1,984 | 36.6 | 2,210 | 40.9 |
| Francec | N | C | 8,876 | 8,876 | 27.7 | 10,305 | 32.3 | 8,880 | 28.1 | 8,927 | 28.4 | 8,705 | 27.8 |
| Germany | Y | C | 12,963 | 12,858 | 15.6 | 13,667 | 16.8 | 16,000 | 19.8 | 18,696 | 22.8 | 20,493 | 25.1 |
| Greece | Y | C | 756 | 735 | 6.8 | 466 | 4.3 | 349 | 3.2 | 414 | 3.7 | 404 | 3.6 |
| Hungary | Y | C | 5,101 | 4,722 | 48.0 | 4,894 | 49.7 | 5,249 | 53.1 | 4,953 | 50.2 | 5,462 | 55.2 |
| Ireland | Y | C | 301 | 299 | 6.3 | 270 | 5.8 | 259 | 5.6 | 326 | 7.1 | 309 | 6.7 |
| Italy | Y | C | 4,138 | 4,134 | 6.8 | 3,825 | 6.3 | 4,467 | 7.3 | 5,048 | 7.8 | 4,829 | 8.1 |
| Latvia | Y | C | 472 | 454 | 23.1 | 380 | 19.1 | 278 | 13.9 | 385 | 19.0 | 547 | 26.8 |
| Lithuania | Y | C | 1,076 | 1,076 | 37.3 | 1,082 | 37.0 | 1,145 | 38.9 | 1,199 | 40.4 | 1,762 | 58.7 |
| Luxembourg | Y | C | 108 | 108 | 18.7 | 106 | 18.8 | 110 | 20.0 | 120 | 22.3 | 136 | 25.9 |
| Malta | Y | C | 158 | 158 | 36.4 | 126 | 29.3 | 132 | 31.0 | 84 | 19.9 | 88 | 21.1 |
| Netherlandsd | N | C | 1,150 | 1,150 | 10.6 | 974 | 9.0 | 970 | 9.0 | 979 | 9.1 | 2,199 | 20.5 |
| Poland | Y | A | 10,032 | 9,718 | 25.6 | 8,245 | 21.7 | 8,042 | 21.2 | 7,315 | 19.2 | 7,959 | 20.6 |
| Portugal | Y | C | 443 | 376 | 3.6 | 325 | 3.1 | 244 | 2.3 | 167 | 1.6 | 185 | 1.8 |
| Romania | Y | C | 1,499 | 1,479 | 7.5 | 1,330 | 6.7 | 1,512 | 7.6 | 1,302 | 6.5 | 698 | 3.5 |
| Slovakia | Y | C | 5,651 | 5,299 | 97.7 | 4,841 | 89.3 | 4,078 | 75.3 | 3,807 | 70.3 | 4,627 | 85.6 |
| Slovenia | Y | C | 311 | 311 | 15.1 | 401 | 19.4 | 597 | 29.0 | 316 | 15.4 | 392 | 19.1 |
| Spainb | N | C | 9,819 | 9,818 | – | 9,015 | – | 6,633 | – | 4,537 | – | 4,224 | – |
| Sweden | Y | C | 2,247 | 2,247 | 22.8 | 2,312 | 23.7 | 2,211 | 22.9 | 2,842 | 29.7 | 2,922 | 30.8 |
| United Kingdom | Y | C | 9,902 | 9,902 | 15.1 | 9,490 | 14.6 | 8,099 | 12.6 | 8,465 | 13.2 | 8,812 | 13.9 |
| EU total | – | – | 96,039 | 94,530 | 20.4 | 94,597 | 20.9 | 92,012 | 20.7 | 87,753 | 20.3 | 94,278 | 21.9 |
| Iceland | Y | C | 39 | 39 | 11.7 | 44 | 13.4 | 40 | 12.3 | 48 | 15.2 | 38 | 11.9 |
| Norway | Y | C | 865 | 865 | 16.6 | 928 | 18.0 | 1,118 | 21.9 | 1,361 | 26.9 | 1,371 | 27.5 |
| Switzerlande | Y | C | 1,517 | 1,517 | 18.1 | 1,375 | 16.6 | 1,241 | 15.0 | 1,265 | 15.5 | 1,242 | 15.6 |
Y: yes; N: no; A: aggregated data; C: case‐based data; −: no report.
Sentinel surveillance; no information on estimated coverage. So notification rate cannot be estimated.
Sentinel system; notification rates calculated with an estimated population coverage of 48%.
Sentinel system; notification rates calculated with an estimated population coverage of 64%.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The proportion of domestic vs travel‐associated cases varied markedly between countries, but most of the salmonellosis cases were infected in EU (53.3% cases acquired in EU, 6.8% travel outside EU and 39.9% of unknown origin) (Table 6). The highest proportions of domestic cases, ranging from 92.9 to 100% were reported in the Czech Republic, Estonia, Greece, Hungary, Latvia, Lithuania, Malta, the Netherlands, Portugal, Romania, Slovakia and Spain. The highest proportions of travel‐related cases were reported by three Nordic countries – Finland (78.7%), Norway (77.5%) and Sweden (70.6%). Among 8,337 travel‐associated cases with known information on probable country of infection, 79.0% of the cases represented travel outside EU and 21.0% travel within EU. Thailand, Turkey and India were most frequently reported travel destinations (15.5%, 10.3% and 6.3%, respectively), followed by two MS – Spain (5.8%) and Greece (4.3%).
A seasonal trend was observed for confirmed salmonellosis cases in the EU/EEA in 2012–2016, with more cases reported during summer months (Figure 3). There was a statistically significant (p < 0.05) decreasing trend for salmonellosis in the EU/EEA in 2008–2016; however, the trend did not show any significant increase or decrease over the last 5 years (2012–2016) (Figure 3).
Figure 3.
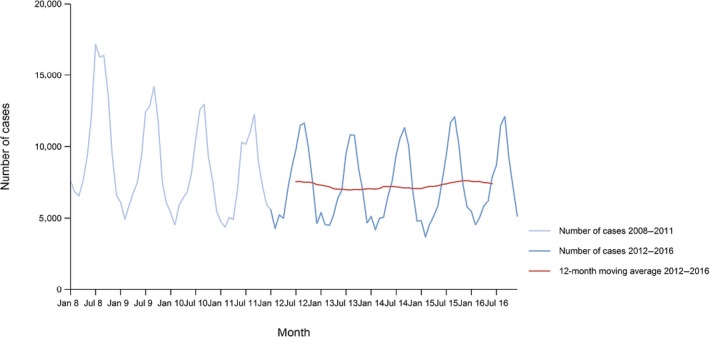
Trend in reported confirmed human cases of non‐typhoidal salmonellosis in the EU/EEA, by month, 2012–2016
- Source(s): Austria, Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Lithuania, Luxembourg, Latvia, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom. Belgium, Bulgaria and Croatia did not report data to the level of detail required for the analysis.
Twelve MS (Austria, Cyprus, Denmark, Estonia, Finland, Germany, Ireland, Italy, Lithuania, Luxembourg, Slovenia and Sweden) reported decreasing trends from 2008 to 2016, whereas four MS (Denmark, Finland, Germany and Sweden) continuously reported decreasing trend also in the last 5 years (2012–2016).
In contrast, a statistically significant increasing trend was observed in seven MS (Greece, Malta, Poland, Portugal, Romania, Slovakia and Spain) in 2012–2016 compared with three MS (the Czech Republic, France and Spain) in 2008–2016.
Fourteen MS provided information on hospitalisation for some or all of their cases of salmonellosis. Of cases with known hospitalisation status (33.6%; 31,728 cases), 38.4% were hospitalised, which was at the same level as in 2015. The highest hospitalisation proportions (77–92%) were reported in Cyprus, Greece, Lithuania, Portugal, Romania and the United Kingdom. Four of these countries (67%) also reported the lowest notification rates of salmonellosis, and indicates that the surveillance systems in these countries primarily capture the more severe cases.
Sixteen MS provided data on the outcome of salmonellosis for 55.2% (52,217 cases) and, among these, 11 MS reported a total of 128 fatal cases. The EU case fatality was 0.25%. Forty per cent of the fatal cases (51 cases) were reported by the United Kingdom.
For a description on human serovar data, we refer to Section 2.4.6.
2.4.3. Salmonella in foods
Monitoring and surveillance data reported in the framework of EU Regulation 2073/2005 on microbiological criteria
As in previous years, the highest levels of non‐compliance with Salmonella food safety criteria generally occurred in foods of meat origin which are intended to be cooked before consumption (Figure 4, Table 2016_SALMCOMPL). Minced meat and meat preparations from poultry intended to be eaten cooked were non‐compliant in 6.45% of single samples and 6.0% of batches, similar to the year 2015. The percentage of positives batches ranged from 3.24% in 2014 to 6.00% in 2016. The proportion of positive single samples in the period 2014–2016 ranged from 6.32% to 8.11%. Among meat products from poultry meat intended to be eaten cooked, 3.4% of single samples were non‐compliant in 2016, which was higher than in the two previous years. No non‐compliance was observed in batch samples of these products from 2014 onwards. In minced meat and meat preparations from other species than poultry intended to be eaten cooked, 1.09% of single samples and 2.32% of batch samples were Salmonella positive in 2016 with small differences compared with the year 2015.
Figure 4.
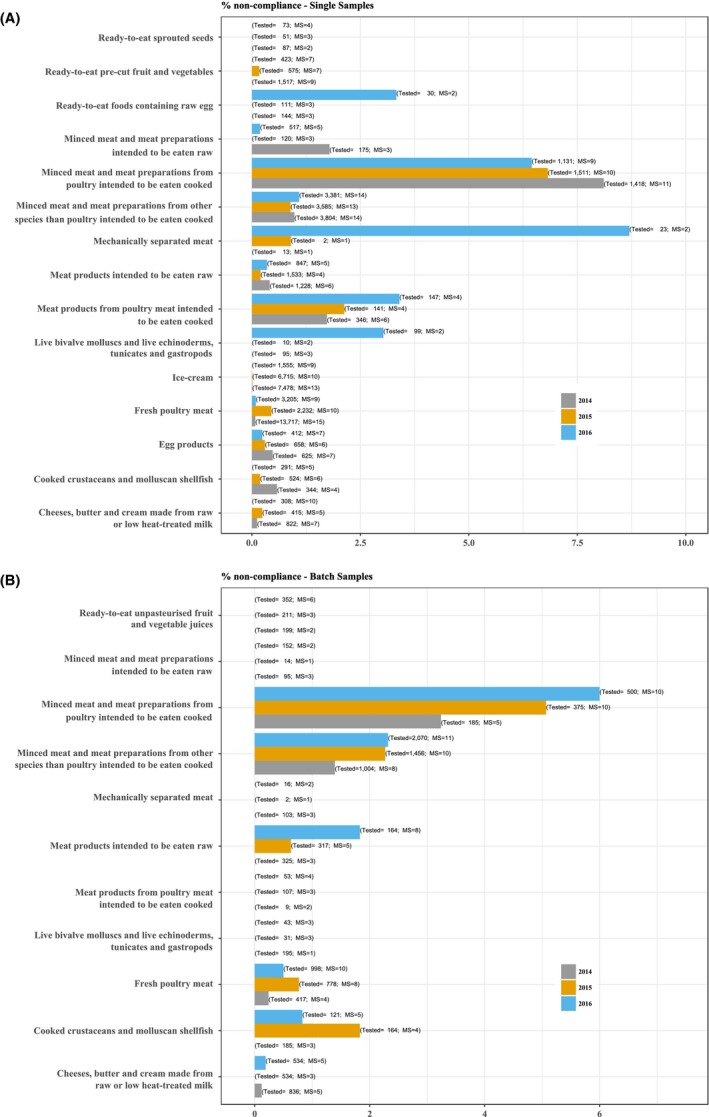
Proportion of units (A – single samples; B – batches) not complying with the EU Salmonella criteria, per food category, MS, 2014–2016
- Total sample size at EU‐level per year is indicated at the top of each bar.
As regards foods of meat origin intended to be eaten raw, in the product category minced meat and meat preparations, 0.2% of single samples were non‐compliant and all batches were compliant, compared with all sampling units (single samples and batches) that were compliant in 2015. In meat products intended to be eaten raw, 0.35% of single samples and 1.83% of batches were Salmonella positive compared with 0.20% and 0.63%, respectively, of these products in 2015. The occurrence of Salmonella in these foods of meat origin intended to be eaten raw is of particular relevance because of the risk such foods pose to human health.
The reported non‐compliance for fresh poultry meat (including fresh meat from breeding flocks of Gallus gallus, laying hens, broilers and breeding and fattening flocks of turkeys) was 0.09% for single samples and 0.50% for batches.
In 2016, ‘mechanically separated meat’ had the highest level of non‐compliance among the single samples. However, this was due to a very small study in one MS, which reported two non‐compliant samples out of an investigation of seven, leading to 2 (8.7%) out of 23 single samples being non‐compliant at EU‐level. In 2015, the non‐compliance of single samples was 0.90% while in 2014 and 2013 there were no non‐compliant samples. No non‐compliant samples were found in batches from 2013 to 2016.
As regards food products of animal origin other than meat, one MS reported a very small study of one non‐compliant single sample out of an investigation of five from RTE foods containing raw eggs, leading to 1 (3.3%) out of 30 single samples being non‐compliant at EU‐level while, before that, no Salmonella‐positive samples had been found since 2011.
Among live bivalve molluscs and live echinoderms, tunicates and gastropods, 2% of 199 single samples contained Salmonella during 2016, but none of the sampled batches was positive, as had also been the case in 2014 and 2015.
All reported samples/batches of dried infant formulas and dried dietary foods for medical purposes, milk and whey powder, unpasteurised fruit and vegetable juices (RTE), were found to be compliant with the Salmonella criteria in 2016. The proportion of non‐compliant samples for the other food categories was very low or rare, as in previous years.
Monitoring data from HACCP on Salmonella on pigs’ carcasses for compliance with the process hygiene criterion were reported by 16 MS and overall 1.9% of the 96,030 units were positive, compared with 5.1% reported by 9 MS in 2015. Monitoring data from sampling by CA showed 2.5% positive units out of a total of 16,456 samples from 9 MS compared with 1.2% in 2015 and reported by 3 MS. Four MS (Bulgaria, Romania, Italy and Slovakia) provided both data collected by the food business operators and by the CA and, in all cases, the occurrence reported in the context of CA programmes was higher than the one reported by the food business operators. For five MS (Ireland, Lithuania, Malta, the Netherland and Poland), no validated data were reported on Salmonella on pig carcasses according to Commission Regulation (EU) No 218/2014. Finland, Sweden and Norway, which are countries with special guarantees in relation to Salmonella on pig carcasses (according to Regulation (EU) No 853/2004), reported two positive carcasses (0.02%) out of 10,354 tested.
Occurrence in food
Meat and meat products
Broiler meat and its products
Monitoring activities and control programmes for Salmonella in fresh broiler meat are based on sampling at the slaughterhouse, where mainly neck skin samples are taken, and/or at processing or cutting plants and at retail, where meat samples are usually collected. Overall, Salmonella was detected in 6.39% of the 25,276 units tested in 2016, and these results were comparable with results in 2015. In 2016, Salmonella was found in 0.27% of the 1,093 units of RTE broiler meat products tested at retail or at processing and including in the large majority of cases cooked and RTE broiler products.
Turkey meat and its products
In total, 4,250 units of fresh turkey meat were sampled and overall, 7.74% were positive for Salmonella. This was higher than in 2015 mainly because two MS, which did not report during 2015, reported high numbers of positive investigations at slaughterhouse level. Salmonella was found in 1 out of 462 (0.22%) RTE turkey meat products and most tested units were from Hungary, which reported about 47% of all units tested in the MS. The overall results for 2016 are comparable with 2014 and 2015.
Pig meat and its products
Within the EU in 2016, 25,049 units of fresh pig meat were tested, of which 2.38% were Salmonella‐positive, which was comparable with 2015. Of this total number of samples tested, approximately 70% were from three MS, Finland, the Netherlands and Sweden. In 2016, 1.93% of the 8,641 tested samples of RTE minced meat, meat preparations and meat products from pig meat were Salmonella positive.
Bovine meat and its products
Data from the testing of fresh bovine meat mainly originate from surveillance programmes, in which samples were mainly collected at slaughterhouses. Among the 23,708 samples of fresh bovine meat tested, 0.21% was Salmonella positive. Only 0.16% of the 1,244 units of RTE minced meat, meat preparations and meat products from bovine meat tested were Salmonella positive.
Eggs and egg products
In 2016 in total, 0.29% of the 5,782 tested table egg units were Salmonella positive. These data are similar with what was observed in 2015.
Other foodstuffs
Altogether, 8.0% of the 525 samples of dried seeds were Salmonella positive in 2016, most of which were collected during border inspection activities (84% of total samples).
Out of the 675 tested units of sprouted seeds, one sample at processing plant was reported Salmonella positive by Hungary.
Of the 2,429 units of vegetables tested, 0.21% was Salmonella positive. Most units were tested at retail (81 %). Among fruits, of the 1,200 tested units, none was positive for Salmonella, and the same was true for the 680 samples reported as ‘Fruit and vegetables’.
Regarding Salmonella in spices and herbs, of 1,390 units examined, 1.51% was Salmonella positive. About 50% of the positive samples were from border inspection activities reported by the Netherlands.
2.4.4. Salmonella in animals
Poultry monitoring data in compliance with the Salmonella National Control Programmes
Achievement of Salmonella reduction targets
Breeding flocks of Gallus gallus
In 2016, 25 MS and 3 non‐MS reported Salmonella NCP data for breeding flocks of Gallus gallus. Luxembourg and Malta do not have such flocks and Lithuania did not report validated data. In 2016, Salmonella was found in 1.47% of breeding flocks in the EU during the production period compared with 1.42% in 2015. The flock prevalence of the five target Salmonella serovars was 0.54% in 2016, compared with 0.34% in 2015. Eleven MS and three non‐MS reported no flocks positive for target serovars. A geographical overview of the country‐specific flock prevalence of the five target Salmonella serovars in breeding flocks of Gallus gallus is in the Appendix. All reporting countries except Greece and Poland met the flock prevalence target of maximum 1% (Figure 5). In Greece, two flocks were positive for S. Enteritidis and one flock for S. Infantis, whereas in Poland 26 flocks were reported positive for S. Enteritidis and two for S. Typhimurium. The most commonly reported target serovar in breeding flocks of Gallus gallus in 2016 was S. Enteritidis (EU flock prevalence 0.32%) (Figure 6), followed by S. Typhimurium (EU flock prevalence 0.16%) (Figure 7) and S. Infantis (EU flock prevalence 0.06%) (Figure 8). Only one flock tested positive for S. Virchow or to S. Hadar.
Figure 5.
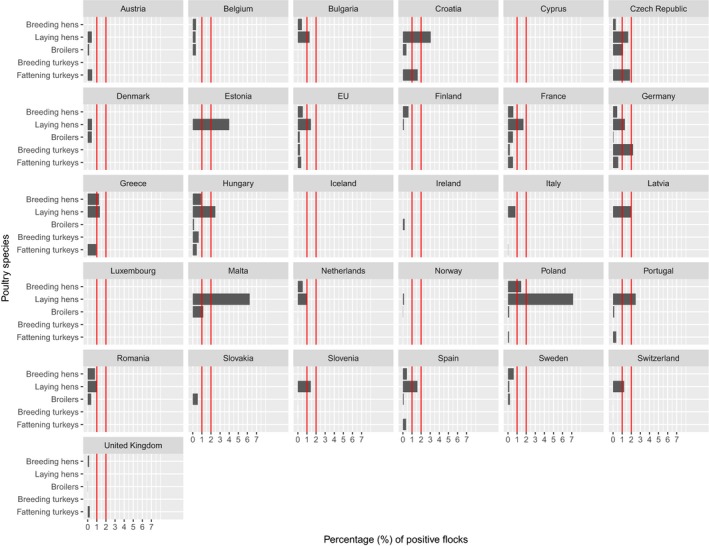
Prevalence of poultry flocks (breeding flocks of Gallus gallus, laying hens, broilers, breeding turkeys and fattening turkeys) positive for target Salmonella serovars in MS, 2016
- Red vertical bars indicate the target to be reached, which was fixed at 1% for all categories with the exception of laying hens, which was 2% for all MS with the exception of Poland, for which it was 2.5%. Malta met the target in laying hens because only 1 flock tested positive for target serovars (S. Enteritidis) but had less than 50 flocks of adult laying hens.
Figure 6.
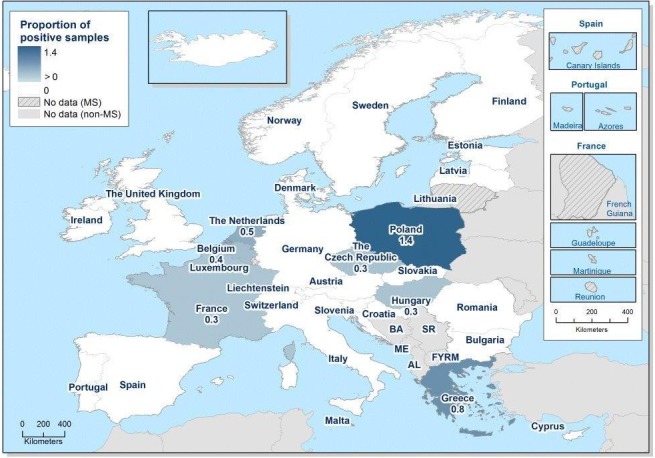
Prevalence of the S. Enteritidis‐positive breeding flocks of Gallus gallus during the production period, 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; and SR: Serbia.
Figure 7.
Prevalence of the S. Typhimurium‐positive breeding flocks of Gallus gallus during the production period, 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; and SR: Serbia.
Figure 8.
Prevalence of the S. Infantis‐positive breeding flocks of Gallus gallus during the production period, 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; and SR: Serbia.
Flocks of laying hens
In 2016, 27 MS and three non‐MS reported Salmonella NCP data for laying hen flocks. Lithuania did not report validated data for laying hen flocks. Salmonella was found in 3.71% of adult laying hen flocks in 2016, compared with 2.67% in 2015. The flock prevalence of the two target Salmonella serovars was 1.44% in 2016, compared with 1.04% in 2015. Five MS and one non‐MS reported no flocks positive for target serovars. A geographical overview of the country‐specific flock prevalence of the two target Salmonella serovars in flocks of laying flocks is in the Appendix. Five MS (Croatia, Estonia, Hungary, Poland and Portugal) did not meet their 2016 reduction target (Figure 5), while in 2015 only one MS, Poland, did not meet it. Malta met the target in laying hens because only 1 flock tested positive for target serovars (S. Enteritidis) but had less than 50 flocks of adult laying hens. The most commonly reported target serovar in laying hen flocks was S. Enteritidis (EU flock prevalence 1.21%) (Figure 9), which was detected by Poland in 169 flocks (7.15%) out of 2,362, while the EU laying hen flock prevalence for S. Typhimurium was 0.24% (Figure 10).
Figure 9.
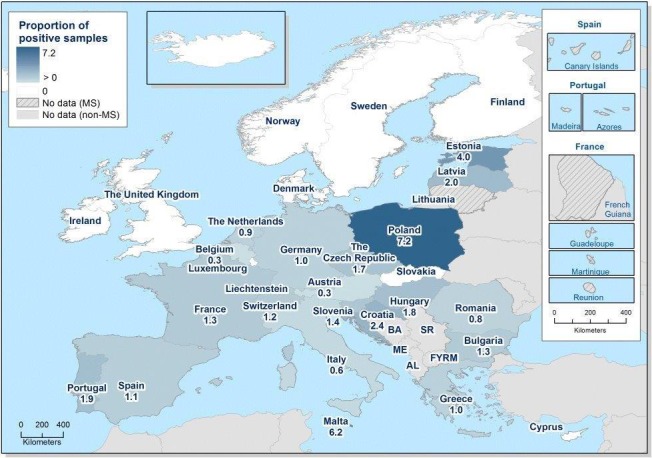
Prevalence of the S. Enteritidis‐positive laying hen flocks of Gallus gallus during the production period, 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; and SR, Serbia.
Figure 10.
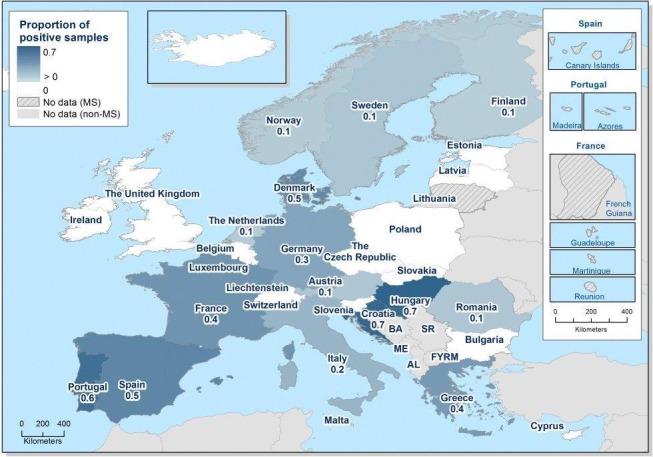
Prevalence of the S. Typhimurium‐positive laying hen flocks of Gallus gallus during the production period, 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; and SR: Serbia.
Broiler flocks
In 2016, 27 MS and three non‐MS reported Salmonella NCP data for broiler flocks. Lithuania did not report validated data for broiler flocks. The EU‐level flock prevalence of Salmonella in broiler flocks was 2.6%, compared with 2.2% in 2015. The flock prevalence of the two target Salmonella serovars was 0.21% in 2016 compared with 0.26% in 2015. Nine MS and two non‐MS reported 0% of flocks infected with S. Enteritidis or S. Typhimurium. A geographical overview of the country‐specific flock prevalence of the two target Salmonella serovars in broiler flocks is in the Appendix. In 2016, all reporting MS except the Czech Republic and Malta met the target of 1% or less of broiler flocks positive for S. Enteritidis and/or S. Typhimurium (Figure 5). No difference was reported between S. Enteritidis and S. Typhimurium in terms of flock prevalence (0.1%) (Figures 11 and 12).
Figure 11.
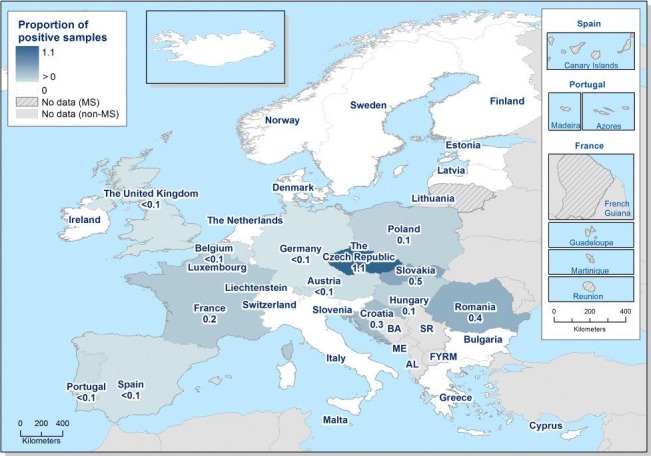
Prevalence of the S. Enteritidis‐positive broiler flocks of Gallus gallus before slaughter, 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia: ME: Montenegro; and SR: Serbia.
Figure 12.
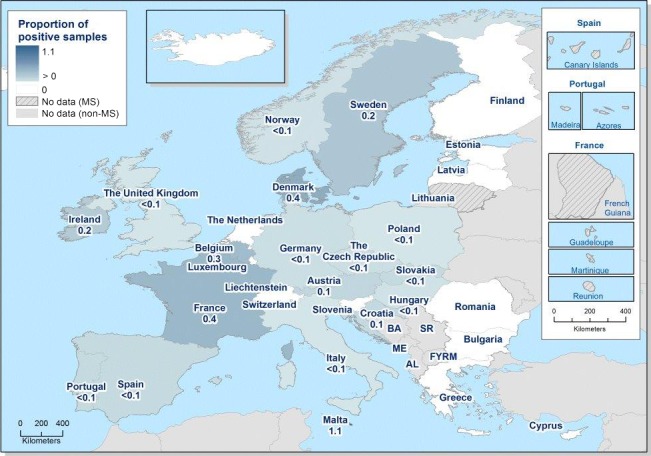
Prevalence of the S. Typhimurium‐positive broiler flocks of Gallus gallus before slaughter 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; and SR: Serbia.
Salmonella NCP monitoring data for broiler flocks must also be reported separately for investigations carried out by CA and by industry sampling. Most MS complied although for three MS no data were available for both CA and industry sampling. Also, some inconsistencies between the reported data for the two systems were noted among data provided by some other MS. Overall, CA tested 8,212 flocks, of which 10.5% were positive for Salmonella and 1.5% were positive for the target serovars. The total number of flocks tested by industry was 241,673 with 2.4% positive for Salmonella and 0.08% positive for the target serovars. Different hypotheses may explain the discrepancies between the data reported by CA and industry. The difference in terms of percentage of positive flocks may partly relate to the fact that sampling by CA may be performed on a risk basis and each time the CA considers it necessary: in this case problematic flocks in terms of Salmonella and target serovars may be over‐represented in CA sampling. However, these discrepancies could also related differences in sampling techniques and in the sensitivity of the laboratory methods used, between CA and industry.
Turkey flocks
For breeding turkeys, 14 MS and two non‐MS reported Salmonella NCP data in 2016. Altogether, 2,098 flocks were tested, of which 1.1% (23) were positive for Salmonella, compared with 1.4% in 2015. The percentage of positive flocks for target serovars was 0.24%, whereas in 2015, 0.4% of the tested flocks were positive for these serovars. S. Enteritidis was not isolated from breeding turkeys in 2016. A geographical overview of the country‐specific flock prevalence of the two target Salmonella serovars in turkey breeding flocks is in the Appendix. Only Germany did not meet the target as two flocks were positive for S. Typhimurium (Figure 5). Salmonella NCP monitoring data for turkey breeding flocks must also be reported separately for investigations performed by CA and by industry sampling. The great majority of the MS did comply with this reporting although, for six MS, data from industry or from CA were not provided and, in some cases, some inconsistencies are present. Overall, CA tested 262 flocks, of which 1.15% was positive for Salmonella and 0.38% was positive for the target serovars. The total number of flocks tested by industry was 414 with 1.69% positive for Salmonella and 0.48% for the target serovars. So, a similar percentage of positive flocks was reported in the context of samplings conducted by CA and the self‐check controls conducted by industry.
For fattening turkeys, in total, 24 MS and 3 non‐MS provided data from flocks before slaughter in 2016. Estonia, Luxembourg and Malta do not have such flocks and Lithuania did not report validated data. The EU‐level Salmonella flock prevalence among turkey fattening flocks was 4.37% compared with 3.6% in 2015. The percentage of positive flocks for target serovars was 0.36%, similar to the value for the year 2015 (0.34%). S. Typhimurium and S. Enteritidis were detected in 0.26% and 0.11% of the tested flocks, respectively; 12 MS and 3 non‐MS reported that none of their flocks tested positive for S. Enteritidis or S. Typhimurium. A geographical overview of the country‐specific flock prevalence of the two target Salmonella serovars in turkey fattening flocks is in the Appendix. Two MS, the Czech Republic and Croatia, did not meet the target of 1% (Figure 5) whereas, in the previous year, both of these countries met the target. Salmonella NCP monitoring data for turkey fattening flocks must also be reported separately for investigations carried out by CA and by industry sampling. However, for two MS no data from industry or CA were available and, in some cases, inconsistencies were present. The total number of flocks tested by CA was 1,145, of which 7.6% were positive for Salmonella and 3.58% were positive for target serovars. Considering sampling conducted by industry, overall the number of flocks tested was 28,191 and the prevalence of those positive for Salmonella and the target serovars were 5.55% and 0.20%, respectively. The number of flocks of fattening turkeys positive for target serovars was comparable between the samplings conducted by CA (41) and industry (57), even though a far greater number of samples were collected by industry in the context of self‐check controls (28,191) than were collected by CA (1,145). As for broiler flocks, the differing sampling regimes CA – industry are based on different approaches, and difference in terms of percentage of positive flocks between data reported by industry and CA may partly relate to the fact that sampling by CA may be carried out on a risk basis and additionally each time the CA considers it necessary: in this case problematic flocks in terms of Salmonella and target serovars may be over‐represented in CA sampling. However, as already mentioned, these discrepancies may relate also to different sensitivity among sampling as well as to controversial performances of the laboratories in charge of testing.
Trends in Salmonella poultry flock prevalence
The trends in the EU flock prevalence of target Salmonella serovars in poultry flocks since the implementation of EU‐wide NCPs 2007–2016 is displayed in Figure 13. Trends at MS level of the prevalence of poultry flocks infected with target Salmonella serovars since the implementation of EU‐wide NCPs are displayed in the figures in the Appendix.
Figure 13.
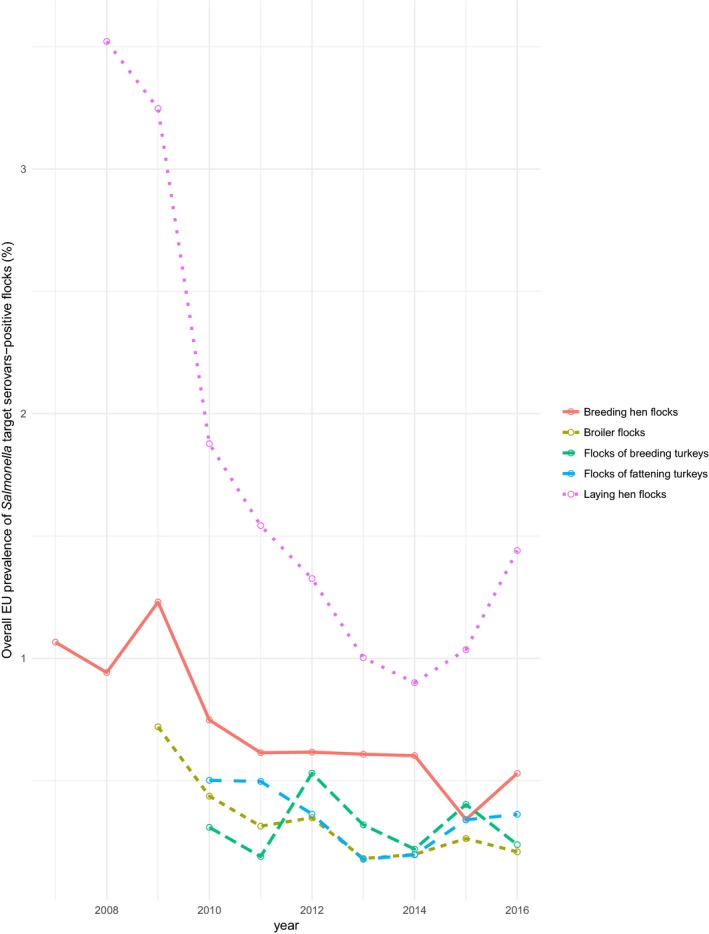
Overall prevalence of poultry flocks positive for Salmonella target serovars relevant for public health in different animal populations, among all reporting MS, 2007–2016
Breeding flocks of Gallus gallus
The data used to model the trend in EU Salmonella flock prevalence for target and non‐target serovars in breeding Gallus gallus for the period 2007–2016 were from 26 MS. Three MS reported a 0% prevalence for target serovars in their flocks all along this period.
The estimated EU Salmonella target serovars flock prevalence in breeding Gallus gallus decreased from 1.12% CI95[0.77; 1.62] in 2007 to 0.32% CI95[0.20; 0.49] in 2016 (Figure 14A). The odds of breeding flocks being positive for target Salmonella serovars decreased significantly by 13.2% for every year of implementation of the NCP. A further analysis was performed focusing on the last 5 years to explore the apparent inversion of tendency that was measured during the last 2 years. The increase of odds of breeding flocks being positive for target Salmonella serovars seen since 2014 was not significant.
Figure 14.
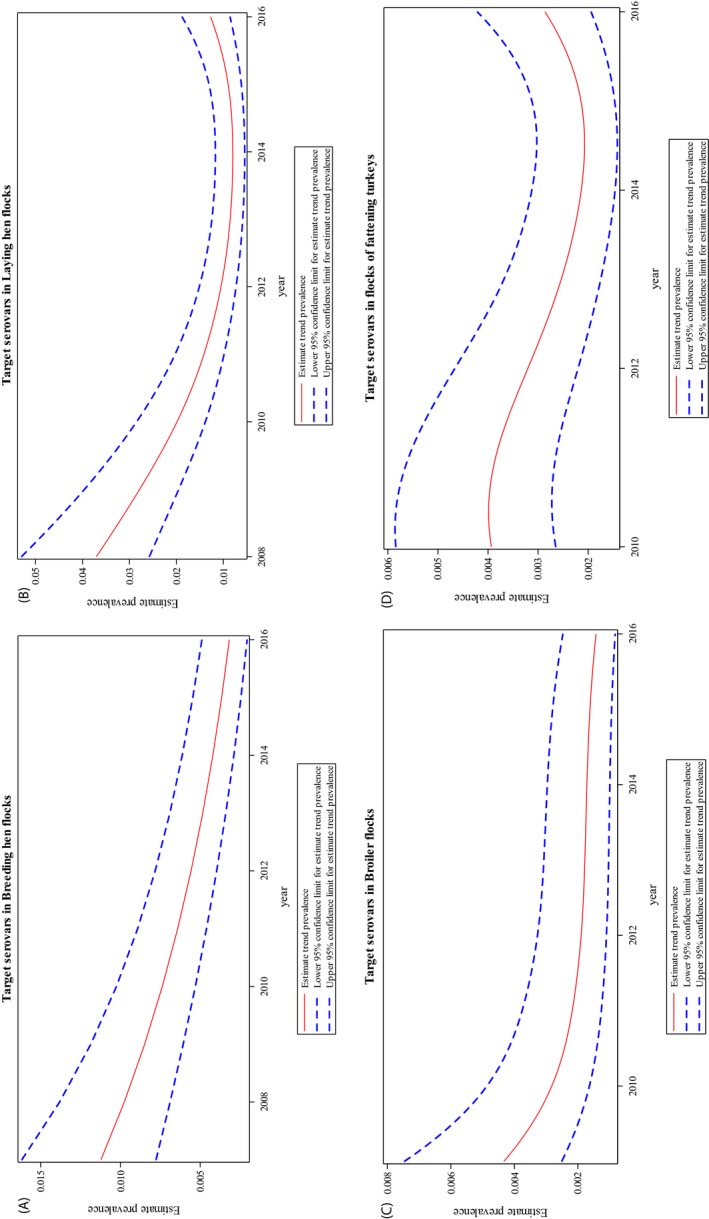
Estimate of the trend prevalence of poultry flocks positive for Salmonella serovars relevant for public health, at EU‐level, in different animal populations
The trend for the estimated EU S. Enteritidis flock prevalence of breeding Gallus gallus during 2007–2016 was very similar to the one described for target serovars. The prevalence decreased from 0.65% CI95[0.40; 1.04] in 2007 to 0.16% CI95[0.09; 0.28] in 2016. The estimated odds of flocks of breeding flocks of Gallus gallus being positive for S. Enteritidis decreased significantly by 14.4% for every year of implementation of the NCP. Also, for S. Enteritidis, an apparent inversion of the decreasing trend was observed during the last 2 years. But also for this outcome, the increase of odds of breeding flocks being positive for S. Enteritidis in 2016 vs both 2015 and 2014 was found not significant after a further analysis. Noteworthy, both for target serovars and S. Enteritidis, almost all MS that reported an increase in flock prevalence for 2016, had experienced decreases in 2015, with 2016 flock prevalence being comparable with 2014.
As regards the estimated EU Salmonella flock prevalence and the EU non‐target Salmonella serovars flock prevalence, the logit of prevalence decreased monotonically over time in a curvilinear way. Focusing on the most recent years, the former prevalence decreased from 2.7% CI95[1.8; 4] in 2012 to 1.3% CI95[0.92; 2.02] in 2014 and then it remained stable over time. The odds of flocks being positive for Salmonella decreased significantly by 35% from 2012 to 2013 and 25% from 2013 to 2014, and remained constant afterwards. The EU non‐target Salmonella serovars flock prevalence decreased from 2.2% CI95[1.33; 3.59] in 2012 to 0.8% CI95[0.49; 1.39] in 2014 and then it stabilised over time. The odds of flocks being positive for non‐target serovars decreased significantly by 46% from 2012 to 2013 and 31% from 2013 to 2014, and then for the following years it remained constant.
Flocks of laying hens
The data used to model the trend in EU Salmonella flock prevalence for target and non‐target serovars in laying hens for the period 2008–2016 were from all MS. No MS reported a 0% prevalence for target serovars in these flocks all along this period.
The estimated EU target Salmonella serovars flock prevalence in laying hens was 3.7% CI95[2.6; 5.3] in 2008 and decreased to 0.8% CI95[0.54; 1.2] in 2014. From 2014 onwards, it increased to 0.9% CI95[0.62; 1.3] in 2015 and to 1.3% CI95[0.86; 1.9] in 2016. The 2016 prevalence was not significantly different compared with the 2015 prevalence (p = 0.111), but it was higher than 2014 at the limits of significance (p = 0.056) (Figure 14B). The estimated odds of laying hen flocks being positive for target serovars decreased significantly by 25–30% from 2009 to 2012, by 20% in 2013 vs 2012 and by 7% in 2014 vs 2013. Next, the odds of flocks being positive for target serovars increased significantly by 10% in 2015 compared with 2014, and by 40% in 2016 compared with 2015.
In the last 2 years, different countries (Croatia, the Czech Republic, Finland, France, Germany, Poland, Estonia) reported an increased target Salmonella serovars flock prevalence in laying hens. The exclusion of Poland did not change this EU trend, even though it led to a reduction of both the estimated odds and prevalence, in a constant way over time. After removing the Polish data, only the estimated odds of flocks being positive for target serovars for the last year significantly increased (by 23% from 2015 to 2016), but the estimated target Salmonella serovars flock prevalence in 2016 (approximately 1% CI95[0.66; 1.5]) was not significantly different from the previous year (p = 0.239).
The trend for estimated EU S. Enteritidis flock prevalence in laying hens during 2008–2016 was similar to the trend described for the target serovars. The prevalence was 3.1% CI95[2.1; 4.6] in 2008, which decreased to 0.57% CI95[0.37; 0.88] in 2014, and subsequently increased to 0.67% CI95[0.44; 1.0] in 2015 and to 1% CI95[0.67; 1.6] in 2016. Despite this increase, the prevalence in 2016 was not significantly different compared with 2015 (p = 0.085), but it was different compared with 2014 (p = 0.0371). The odds of laying hen flocks in EU being positive for S. Enteritidis decreased significantly by 25–30% from 2009 to 2012, by 21% in 2013 vs 2012 and by 7% in 2014 vs 2013. In 2015, the odds of flocks being positive for S. Enteritidis increased significantly by around 17% in 2015 vs 2014 and by 57% in 2016 vs 2015. The analysis, repeated after removing the Polish data, provided similar conclusions as were obtained for target serovars. The odds of laying hen flocks being positive for S. Enteritidis increased significantly during the last 2 years (around 6% in 2015 vs 2014 and 33% in 2016 vs 2015). Despite this increase, the estimated EU S. Enteritidis flock prevalence in 2016 (approximately 0.77% CI95[0.48; 1.2]) was not significantly different from the 2015 and 2014 prevalence (p = 0.195 and p = 0.153, respectively).
Finally, EU estimated Salmonella spp. flock prevalence and the EU non‐target Salmonella serovars flock prevalence in laying hens were very similar. Focusing on the most recent years, the EU Salmonella flock prevalence increased to 3% CI95[1.87; 4.78] in 2016 and it was not significantly different compared with 2015 (p = 0.115), but it was different, at the limit of significance, compared with 2014 (p = 0.068). The odds of laying hen flocks being positive for Salmonella increased significantly by around 11% in 2015 vs 2014 and by 55% in 2016 vs 2015. The EU non‐target Salmonella serovars flock prevalence in laying hens increased to 1.35% CI95[0.68; 2.67] in 2016, but it was not significantly different from the previous 2 years. The odds of laying hen flocks being positive for non‐target serovars increased significantly by around 8% in 2015 vs 2014 and by 53% in 2016 vs 2015.
Figure 15 displays the EU S. Enteritidis flock prevalence in laying hens and the number of human cases due to S. Enteritidis infection acquired in the EU. The EU S. Enteritidis flock prevalence in laying hens decreased from 2012 to 2014, where after it significantly increased during 2015 and 2016. The number of human cases due to S. Enteritidis infection acquired in the EU seemed to follow during 2012–2016 an analogous trend. After a sharp decrease in human cases of S. Enteritidis in 2013 compared with 2012, an increase was observed during the following years. For human data, this increase in the notification rate for some MS was generally accompanied by an increase in the number of Salmonella outbreaks as reported in 2014 and the following years. However, these data could have also been partly affected by an improvement over time in the efficiency and sensitivity of the surveillance system in place in some MS as well as the inclusion of MS reporting data for the first time.
Figure 15.
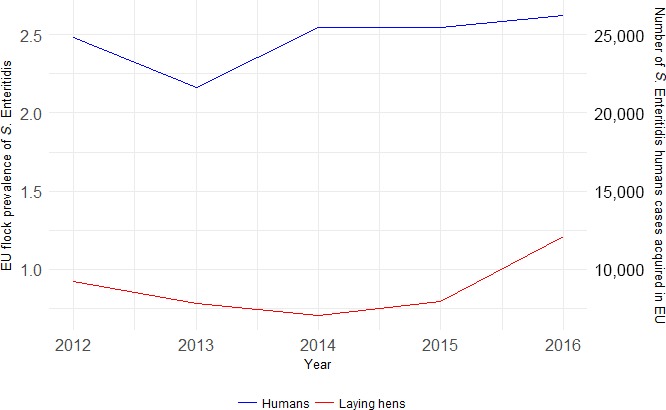
Percentage of positive laying hen flocks for S. Enteritidis and number of EU domestic cases of S. Enteritidis, MS, 2012–2016
Broiler flocks
The data used to model the trend in EU Salmonella flock prevalence for target serovars in broilers for the period 2009–2016 were obtained from all MS. Two MS reported 0% prevalence for target Salmonella serovars in their broiler flocks all along this period.
The estimated EU target Salmonella serovars flock prevalence in broilers was 0.43% CI95[0.25; 0.75] in 2009, decreased to 0.18% CI95[0.10; 0.31] in 2013 and then remained stable up to 2015. In 2016 the prevalence decreased to 0.14% CI95[0.08; 0.25], but it was not significantly different from the prevalence in 2015 (p = 0.374) (Figure 14C). The estimated odds of broiler flocks being positive for target Salmonella serovars decreased significantly by 35% from 2009 to 2010, by 23% from 2010 to 2011 and by 13% from 2011 to 2012. A constant decrease (around 5%) was estimated for the period 2013 to 2015. Then, from 2015 to 2016, there was a further decrease (12%) of the odds.
Turkey flocks
The data used to model the trend in EU Salmonella flock prevalence for target serovars in breeding turkeys for the period 2010–2016 were from 15 MS. Most MS (nine) reported 0% prevalence for target Salmonella serovars in their breeding turkey flocks over this period. The remaining countries had, from time to time, some positive flocks. Hence, no plausible trend can be estimated. The estimated EU target Salmonella serovars flock prevalence in breeding turkey flocks was around 0.2% CI95[0.06; 0.76] for the entire period.
The data used to model the trend in EU Salmonella flock prevalence for target serovars in fattening turkeys for the period 2010–2016 were from 25 MS. Eight MS reported 0% prevalence for target Salmonella serovars in their fattening turkey flocks all along this period.
The estimated EU target Salmonella serovars flock prevalence in fattening turkeys was 0.4% CI95[0.26; 0.58] in 2010, it decreased to 0.21% CI95[0.14; 0.31] in 2014; it remained stable in 2015, and finally in 2016, the estimated prevalence increased to 0.28% CI95[0.19; 0.42], even though this was not significantly different from 2015 (p = 0.150) (Figure 14D). The estimated odds of fattening turkey flocks being positive for Salmonella target serovars decreased significantly by 17% from 2011 to 2012, by 21% from 2012 to 2013 and by 16% from 2013 to 2014. The odds estimated for the years 2015 and 2014 were similar. However, in 2016, the odds of flocks being positive for target serovars increased significantly by around 33% in 2016 vs 2015.
Salmonella monitoring data in other animals
Three MS (Estonia, Latvia and Sweden) reported monitoring data on Salmonella flock prevalence in ducks and geese for the year 2016. Of 44 flocks reported, 4.6% were positive for Salmonella whereas 2.3% were positive for S. Enteritidis and/or S. Typhimurium. Norway reported no positive flocks.
Ten MSs and one non MS (Norway) reported data on Salmonella prevalence in pigs. Overall, the prevalence was 6.7% (ranging from 0% to 63.0%) at herd level and 3.5% (ranging from 0% to 10.6%) at animal level. With regard to samples at animal level, Bulgaria, Finland, Sweden and Norway reported 0% prevalence, whereas the other reporting countries (Denmark, Germany, Slovakia and Italy) reported markedly higher prevalence. Norway reported 0% prevalence also for samples collected at herd level. Pig samples belong to both fattening and breeding animals, and were characterised by a high heterogeneity of analysed matrices and sampling schemes.
In cattle, the overall Salmonella herd prevalence, based on data reported by seven MS and two non‐MS (Iceland and Norway) was 0.2% at herd level (ranging from 0 and 9.1%) and 4.2% (ranging from 0 and 17.1%) at animal level. For Finland, Slovakia, Sweden, Iceland, Norway and Greece the prevalence was 0%. A high level of heterogeneity was found among the reporting countries in terms of matrices sampled, sites of sampling and sample size.
2.4.5. Salmonella in feedingstuffs
The overall prevalence of Salmonella‐positive units in animal‐ and vegetable‐derived feed supplies in 2016 was 3.9% out of 4,750 units reported by MS. In compound feeding stuffs (the finished feed for animals), the prevalence of Salmonella‐positive units in 2016 was low for all animal populations: 1.2% of 2,473 tested samples for poultry, 1.0% of 971 tested samples for cattle and 0.5% of 1,106 tested samples for pigs.
2.4.6. Salmonella serovars in humans, food and animals
Humans
Serovars among all confirmed salmonellosis cases
For humans, information on Salmonella serovars was available at 71.3% (67,418 cases of the total 94,530 confirmed cases) from 24 MS (Belgium, Bulgaria, Croatia and Poland did not report case‐based serovar data), Iceland and Norway. Data includes all cases reported with serovar information regardless the importation/travel status. As in previous years, the three most commonly reported Salmonella serovars in 2016 were S. Enteritidis, S. Typhimurium and monophasic S. Typhimurium (1,4,[5],12:i:‐), representing 70.3% of the 67,418 confirmed human cases with known serovar in 2016 (Table 8). The proportion of S. Enteritidis continued to increase in 2016 compared with 2014 and 2015, the proportion of S. Typhimurium decreased while its monophasic variant strains 1,4,[5],12:i:‐ and S. Infantis were at same level as in 2015 and 2014. Cases of S. Stanley decreased in 2016 to the same level as before the outbreak in 2013. Serovars S. Newport, S. Derby and S. Kentucky replaced S. Stanley as 5–7th most commonly reported Salmonella serovars. Two ‘new’ serovars (S. Bareilly and S. Weltevreden) entered the top 20 list in 2016, and replaced serovars S. Oranienburg and S. Thompson.
Table 8.
Distribution of reported confirmed cases of human salmonellosis in the EU/EEA, 2014–2016, by the 20 most frequent serovars in 2016
| Serovar | 2016 | 2015 | 2014 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MS | % | Cases | MS | % | Cases | MS | % | |
| Enteritidis | 32,685 | 26 | 48.5 | 31,887 | 26 | 45.6 | 32,874 | 27 | 44.4 |
| Typhimurium | 9,012 | 26 | 13.4 | 11,032 | 26 | 15.8 | 12,864 | 27 | 17.4 |
| Monophasic Typhimurium 1.4.[5].12:i:‐ | 5,666 | 15 | 8.4 | 5,786 | 15 | 8.3 | 5,774 | 13 | 7.8 |
| Infantis | 1,596 | 24 | 2.4 | 1,588 | 24 | 2.3 | 1,843 | 26 | 2.5 |
| Newport | 731 | 17 | 1.1 | 730 | 19 | 1.0 | 753 | 20 | 1.0 |
| Derby | 570 | 20 | 0.8 | 648 | 21 | 0.9 | 753 | 23 | 1.0 |
| Kentucky | 531 | 20 | 0.8 | 511 | 19 | 0.7 | 604 | 21 | 0.8 |
| Stanley | 520 | 20 | 0.8 | 767 | 23 | 1.1 | 756 | 23 | 1.0 |
| Virchow | 497 | 20 | 0.7 | 507 | 22 | 0.7 | 509 | 22 | 0.7 |
| Saintpaul | 441 | 20 | 0.7 | 279 | 18 | 0.4 | 374 | 19 | 0.5 |
| Agona | 428 | 16 | 0.6 | 377 | 16 | 0.5 | 378 | 23 | 0.5 |
| Paratyphi B var. Java | 418 | 15 | 0.6 | 436 | 17 | 0.6 | 388 | 17 | 0.5 |
| Braenderup | 377 | 18 | 0.6 | 238 | 15 | 0.3 | 276 | 17 | 0.4 |
| Bovismorbificans | 362 | 20 | 0.5 | 372 | 20 | 0.5 | 441 | 21 | 0.6 |
| Panama | 310 | 15 | 0.5 | 261 | 14 | 0.4 | 244 | 15 | 0.3 |
| Naples | 292 | 15 | 0.4 | 369 | 14 | 0.5 | 333 | 14 | 0.4 |
| Chester | 272 | 17 | 0.4 | 261 | 16 | 0.4 | 294 | 18 | 0.4 |
| Hadar | 262 | 17 | 0.4 | 235 | 19 | 0.3 | 286 | 16 | 0.4 |
| Bareilly | 258 | 15 | 0.4 | 220 | 17 | 0.3 | 183 | 18 | 0.2 |
| Weltevreden | 224 | 14 | 0.3 | 178 | 15 | 0.3 | 163 | 14 | 0.2 |
| Other | 11,966 | – | 17.7 | 13,183 | – | 18.9 | 13,931 | – | 18.8 |
| Total | 67,418 | 26 | 100.0 | 69,865 | 26 | 100.0 | 74,021 | 27 | 100.0 |
MS: Member State.
Source(s): Twenty‐five MS and two non‐MS; Austria, Belgium (2014), Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom.
Serovars acquired in the EU
To estimate the impact of the Salmonella infections acquired at the EU‐level, serovar data were analysed for domestic and travel‐associated cases when the probable country of infection was a MS (Table 6). Information on Salmonella serovars with importation/travel data was available from 22 MS, representing 65.9% of cases with known serovar data in 2016. The majority (96.8%) of the cases were acquired in the reporting country. Among the travel‐related cases (3.2%), the most frequently reported travel destinations in the EU were Spain (24.3%), Greece (14.7%), Italy (9.1%) and Poland (7.1%).
S. Enteritidis dominated amongst human cases and more than half (59.0%) of the reported cases were infected by this serovar. The five most reported serovars were: S. Enteritidis (59.0%; 26,240 cases), S. Typhimurium (13.6%; 6,049 cases), monophasic S. Typhimurium (4.7%; 2,088 cases), S. Infantis (2.3%; 1,040 cases) and S. Derby (0.7%; 325 cases) and other serovars combined (19.6%; 8,720 cases) (Table 9). Together with S. Typhimurium, and monophasic S. Typhimurium 1,4,[5],12:i:‐, these three serovars represented 77.3% of the confirmed human cases with known serovar and importation data in 2016. The proportion of S. Enteritidis continued to increase in 2016 compared with 2014 and 2015, the proportion of S. Typhimurium decreased significantly, while its monophasic variant strains 1,4,[5],12:i:‐ and S. Infantis remained at the same level as in 2015 and 2014. The number of cases acquired in the EU of S. Derby, the fifth in the top five, also remained stable during the last 3 years. By serovar, the cases of the top five were mostly acquired in the EU: S. Enteritidis (93.7%), S. Typhimurium (92.8%), monophasic S. Typhimurium (91.8%), S. Infantis (91.2%) and S. Derby (93.9%), whereas S. Newport, the fifth most commonly reported serovar in all cases, had 36.6% of cases associated to travel outside the EU.
Table 9.
Distribution of reported cases of human salmonellosis acquired in the EU, 2014–2016, by the five most frequent serovars in 2016
| Serovar | 2016 | 2015 | 2014 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MS | % | Cases | MS | % | Cases | MS | % | |
| Enteritidis | 26,240 | 22 | 59.0 | 25,458 | 21 | 56.7 | 25,474 | 20 | 54.6 |
| Typhimurium | 6,049 | 22 | 13.6 | 7,228 | 21 | 16.1 | 8,625 | 19 | 18.5 |
| Monophasic Typhimurium 1,4,[5],12:i:‐ | 2,088 | 15 | 4.7 | 2,303 | 14 | 5.1 | 1,775 | 11 | 3.8 |
| Infantis | 1,040 | 20 | 2.3 | 1,094 | 20 | 2.4 | 1,163 | 18 | 2.5 |
| Derby | 325 | 17 | 0.7 | 300 | 16 | 0.7 | 447 | 16 | 1.0 |
| Other | 8,720 | – | 19.6 | 8,540 | – | 19.0 | 9,214 | – | 19.7 |
| Total | 44,462 | 22 | 100.0 | 44,923 | 21 | 100.0 | 46,698 | 20 | 100.0 |
Source(s): Twenty‐four MS: Austria, Belgium (2014), the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom.
There was a statistically significant (p < 0.01) decreasing trend for S. Enteritidis acquired in the EU in 2008–2016, however the trend stabilised during 2012–2016 and did not show any more a statistically significant increase or decrease (Figure 16).
Figure 16.
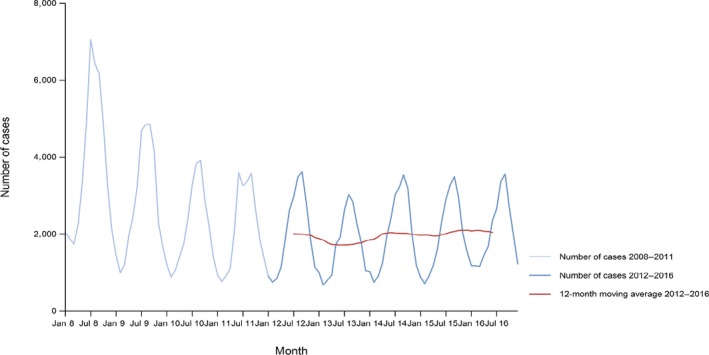
Trend in reported confirmed human cases of S. Enteritidis acquired in the EU, by month, 2012–2016
- Source(s): Austria, Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Lithuania, Luxembourg, Latvia, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom. Belgium, Bulgaria and Croatia did not report data to the level of detail required for the analysis.
At the country level, seven MS (Denmark, Estonia, Finland, Germany, Italy, Malta and the Netherlands) reported a decreasing trend of S. Enteritidis cases acquired within the EU in 2008–2016, whereas only one MS (the Czech Republic) reported an increasing trend over the same period.
In contrast, a significant increasing trend was observed in eight MS (the Czech Republic, Greece, Ireland, Portugal, Slovakia, Spain, Sweden and the United Kingdom) over the last 5 years (2012–2016) compared with two MS (Finland and Germany) with significant decreasing trends of S. Enteritidis cases acquired within the EU for the last 5 years.
Food and animals
Descriptive analyses were made from food and animal 2016 data of the five most commonly reported Salmonella serovars that were reported from domestic human cases in the EU (including cases that travelled within EU) for the year 2016 (Table 9). These five most reported serovars were: S. Enteritidis, S. Typhimurium, monophasic S. Typhimurium, S. Infantis and S. Derby, and other serovars combined (Table 9).
For food and animals, all 2016 serotyping data reported by MS were collated into the following eight matrices: broilers, broiler meat, turkeys, turkey meat, pigs, pig meat, cattle and bovine meat (Table SERALLMATRIX in the Appendix). Considering isolates reported by MS from all monitoring activities – NCP data and all other monitoring data reported – and whether for animals, flocks/herds, single or batches, a total of 11,976 serotyped Salmonella isolates were reported from these matrices. Of these, the proportions reported were, in decreasing order, from broilers (5,938 isolates, 49.6%), turkeys (1,524 isolates, 12.7%), cattle (1,482 isolates, 12.4%), broiler meat (1,464 isolates, 12.2%), pig meat (814 isolates, 6.8%), pigs (528 isolates, 4.4%), turkey meat (162 isolates, 1.4%) and bovine meat (64 isolates, 0.5%).
The most commonly reported serovar was S. Infantis (4,344 isolates; 36.3% of the serotyped isolates), followed by S. Typhimurium (1,551 isolates; 13.0%), S. Enteritidis (807 isolates; 6.7%), S. Dublin (600 isolates; 5.0%) and monophasic variants of S. Typhimurium (589 isolates; 4.9%). All these serovars except S. Dublin were reported amongst the most frequent from human cases. Almost all (97%; 582 out of 600) S. Dublin isolates were reported from cattle by four MS (Germany, Ireland, the Netherlands and the United Kingdom). S. Derby, which was ranked as the fifth most common serovar isolated from humans (acquired in EU), was seventh in the ranking of veterinary isolates (390 isolates; 3.3%), after S. Mbandaka (416 isolates; 3.5%).
The Sankey diagram in Figure 17 illustrates the distribution of the EU top‐5 Salmonella serovars in human salmonellosis acquired in the EU across different food, animal and meat sectors in the EU, in 2016. As the scope of this graph is to show which sources were mainly associated with the top human serovars, Salmonella serovars isolated from the same animal and food source were merged. Hence the category ‘broiler’ refers to serovar data obtained from broilers and broiler meat, ‘cattle’ refers to serovar data obtained from cattle and bovine meat, ‘pig’ refers to serovar data from pigs and pig meat and ‘turkey’ refers to data from turkeys and turkey meat. Even though S. Enteritidis was markedly associated with ‘broiler’ (87.0%) a marginal number of S. Enteritidis isolates were from turkey (7.2%), pig (4.2%) or bovine (1.6%) sources. S. Typhimurium was reported from all matrices, although more from cattle (40.4%), pigs (26.9%) and broilers (25.5%). Monophasic S. Typhimurium was reported, in decreasing order, from the pig source (61.9%), the broiler source (26.9%), the turkey source (6.1%) and the bovine source (5.1%). S. Derby was from, in decreasing order, the pig source (64.4%), the turkey source (21.0%), the broiler source (11.3%) and the bovine source (3.3%). S. Infantis was mostly reported from the poultry chains (both from broiler (90.6%) and turkey (8.1%)) and less from the pig (1.2%) or cattle sources (0.1%).
Figure 17.
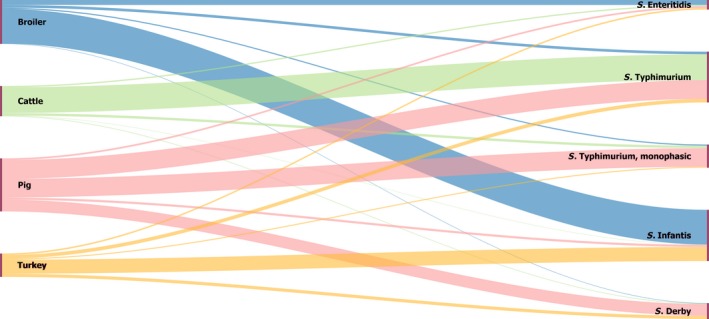
Sankey diagram of the distribution of the EU top‐five Salmonella serovars in human salmonellosis acquired in the EU, across different food, animal and meat sectors (broiler, cattle, pig and turkey), by source, EU, 2016
- The left side of the diagram shows the five most commonly reported serovars from EU domestic cases of human infection: S. Infants (blue), S. Typhimurium (green), S. Enteritidis (pink), monophasic S. Typhimurium (yellow) and S. Derby (violet). Animal and food data from the same source were merged: broiler includes isolates from broilers and broiler meat, cattle includes isolates from bovine animals and bovine meat, pig includes isolates from pigs and pig meat, turkey includes isolates from turkeys and turkey meat. The right side shows the four sources considered (broiler, cattle, pig and turkey). The width of the coloured bands linking sources and serovars is proportional to the percentage of isolation of each serovar in each source.
In the following paragraphs, the description and discussion refer exclusively to the Salmonella isolates serotyped (from sampling units, whether animals or herds, or single or batches of foods) and reported by MS for the different matrices. For categories covered by NCP, only serovar data reported in the context of these programmes are presented, whereas for matrices not sampled in the context of an EU harmonised programme all isolates serotyped and reported by MS (from all monitoring activities) were considered for analyses. For poultry production, serovar data are presented, when relevant, as serovars from Gallus gallus (including aggregated data from breeders, laying hens and broilers) and/or as serovars reported separately from each production category. Minor differences may exist between data underpinning the tables SERALLMATRIX and the ‘SERTARGET’ in the Appendix because of different extraction dates and MS’ data corrections in between these dates.
Salmonella Enteritidis
Salmonella Enteritidis is the most commonly detected serovar in cases of human non‐typhoidal salmonellosis in the EU in the context of NCPs in Gallus gallus (Table SERGALTARG). S. Enteritidis was the second most common serovar reported accounting for 811 out of 6,213 (13.1%) isolates, even though for some MS (the Czech Republic, France, Germany, Hungary, the Netherlands, Poland, Portugal, Spain) it was the most common serovar reported. To interpret these data, it must be kept in mind that for NCP the mandatory reporting is limited to target serovars, and this could lead to a possible bias towards the reporting of these regulated serovars to the detriment of non‐regulated ones. As an example, considering serotyping data reported from domestic fowl (Gallus gallus) in the context of all monitoring activities (without any constraints) (Table SERGAL) some MS, such as Hungary, reported by far a greater number of S. Infantis isolates (1,095) compared with S. Enteritidis (26), providing a picture different from the one obtained looking at NCP data (Table SERGALTARG), in which the same country reported one isolate of S. Infantis and 26 isolates of S. Enteritidis.
Data collected in the context of NCP showed a decrease of S. Enteritidis isolates occurred in 2016 (811) compared with 2015 (875). In 2016 some MS (e.g. the Czech Republic, Germany and the Netherlands) reported a lower number of S. Enteritidis isolates from Gallus gallus compared with 2015, whereas other MS reported more S. Enteritidis isolates. Poland, for instance, reported a higher number in 2016 (247) compared with 2015 (169) from Gallus gallus.
In breeding hens of Gallus gallus, seven of 25 reporting MS detected S. Enteritidis (Figure 6). For laying hen flocks sampled in the context of NCP (Table SERLAYTARG), S. Enteritidis was the most common serovar, accounting for 434 out of the 1,034 isolates reported (42%). Compared with 2015, in 2016, an increased number of S. Enteritidis isolates was notified (297 isolates in 2015 and 434 in 2016). Only five isolates obtained from eggs were reported by two MS (Bulgaria and Romania); S. Enteritidis, S. Typhimurium (two isolates each) and S. Gallinarum (one isolate) (Table SEREGG).
S. Enteritidis, with 328 isolates, was the fourth most common serovar notified from broiler flocks sampled in the context of NCP, accounting for 6.6% of the isolates from this source (Table SERBROTARG). As for Gallus gallus, most of the S. Enteritidis isolates reported for broiler flocks were from a limited number of MS (mostly the Czech Republic, France, Poland and Romania). S. Enteritidis from broiler meat was found in 383 out of the 1,522 (25.2%) Salmonella isolates and was reported from a limited number of MS (mostly Belgium, the Czech Republic, the Netherlands, Poland and Slovakia).
Table SERTURKTARG reports 43 S. Enteritidis isolates notified from turkey flocks in the context of NCP, corresponding to 4.7% of the total isolates and ranked the seventh position among the top‐10 serovars. S. Enteritidis with 17 isolates accounted for 10.5% of Salmonella isolates notified from turkey meat and 15 of those were reported by Poland.
In 2016, S. Enteritidis appeared among the top‐10 serovars from both pigs (Table SERPIG) and pig meat (Table SERPIG MEAT), even though a negligible number of S. Enteritidis isolates was reported from these sources.
Table SERBOV showed the overall marked drop of the number of serotyped Salmonella isolates from cattle in 2016 compared with 2015 (1,500 isolates in 2016 and 3,243 in 2015) and this decrease can be attributed to Germany, which notified about 1/5 of the isolates reported from cattle during the previous year (448 isolates in 2016 and 2,138 in 2015). For S. Enteritidis in cattle, in 2016, five MS reported a total of 13 isolates. Similarly, in bovine meat a negligible number of S. Enteritidis isolates was reported (Table SERBOVMEAT).
A ‘pyramid plot’ was constructed for each serovar of interest to show for each source the frequency of reporting between animal and food (meat) sources. S. Enteritidis accounted for less than 10% of all Salmonella isolates reported from broiler flocks (considering data from the poultry NCPs and all data from other matrices), but more than 20% of all isolates from broiler meat. Similarly, for turkeys, pigs and cattle the specific proportion of reported S. Enteritidis among the isolates was less than 5%, whereas for bovine meat and turkey meat that specific proportion was higher than 10% (Figure 18). For these last types of meat, the number of reported isolates was very low and, as mentioned before, reporting of distinct serovars serotyped is not a legal mandatory reporting requirement, hampering any drawing of conclusions from these data.
Figure 18.
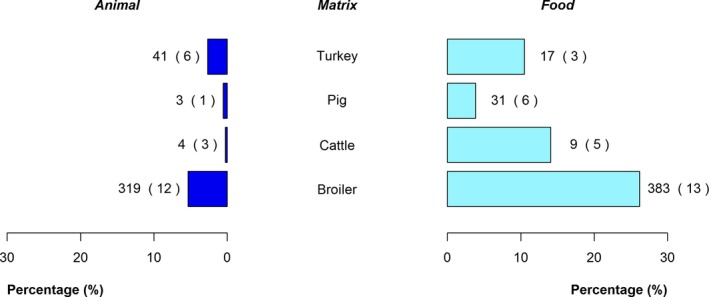
Pyramid plot showing the distribution of S. Enteritidis among food (meat) and animal sources for each species, EU, 2016
- The percentages are calculated on the total number of isolates for each category (animal and food). The values at the side of each bar are the number of S. Enteritidis isolates for each species and category and the number in parentheses indicates the number of reporting Member States.
Salmonella Typhimurium
Salmonella Typhimurium, which ranked as the second most commonly reported serovar from humans infected in the EU, was among the top‐10 serovars reported from all matrices, and was the most commonly reported serovar from cattle, pigs, pig meat and turkey meat.
S. Typhimurium accounted for 5.5% and 5.1% of the isolates serotyped from all flocks of Gallus gallus and broiler flocks, respectively, in the context of NCP in 2016 (Tables SERGALTARG and SERBROTARG).
In breeding hens of Gallus gallus, eight of 25 reporting MS detected S. Typhimurium (Figure 7). S. Typhimurium was the fourth most common serovar in laying hen flocks in the context of NCP, accounting for 7.1% of the isolates (Table SERLAYTARG). As for 2015, when S. Typhimurium ranked second in laying hens, France and Germany reported most of the isolates.
Considering isolates reported from broiler meat (Table SERBROMEAT), S. Typhimurium was the third most common serovar reported numbering 143 (9.4%) out of 1,522 Salmonella isolates. Compared with 2015, when S. Typhimurium ranked fifth from broiler meat, more S. Typhimurium isolates were reported, in particular by Poland which reported about 66% of the isolates.
For turkey flocks, S. Typhimurium was the fifth most common serovar reported, accounting for 81 (8.6%) out of the 915 Salmonella isolates (Table SERTURKTARG) and most isolates were reported by France and Germany. In turkey meat S. Typhimurium was the first‐ranked serovar reported and accounted for 31 isolates (19.1%) of 162 isolates (SERTURKMEAT) and Poland together with France reported most isolates.
In 2016, S. Typhimurium was the second most reported common serovar from pigs and accounted for 215 (29.5%) out of 730 isolates (Table SERPIG). In meat from pigs, S. Typhimurium was the most commonly reported serovar and accounted for 271 (30.7%) out of the 883 Salmonella isolates serotyped (Table SERPIG MEAT). Two‐thirds of 23 reporting MS recorded at least one isolate of S. Typhimurium from pig meat and the United Kingdom contributed 40% of the S. Typhimurium reported.
At the EU‐level in cattle herds, S. Typhimurium was the most common serovar, accounting for 620 out of 1,500 Salmonella isolates (41.3%) in 2016 (Table SERBOV). For bovine meat, S. Typhimurium, with 12 isolates (17.4%) reported out of 69 serotyped, was the second most common serovar, consistent with data reported in 2015.
The ‘pyramid plot’ for S. Typhimurium shows that more than 40% of the isolates reported from cattle herds were S. Typhimurium, whereas for bovine meat about 20% of the isolates from this source were S. Typhimurium. Considering the pig chain, S. Typhimurium accounted for 30% of all isolates notified from pig herds and pig meat, confirming the wide prevalence of this serovar along the entire pig chain (Figure 19).
Figure 19.
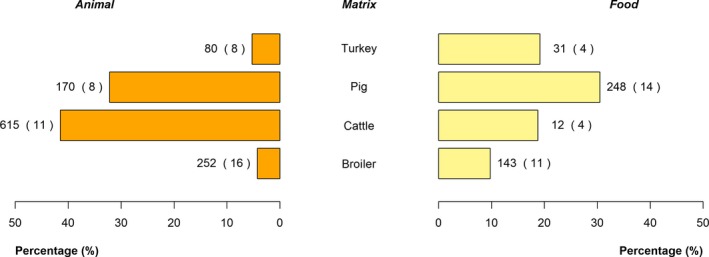
Pyramid plot showing the distribution of S. Typhimurium among food (meat) and animal sources for each species, EU, 2016
- The percentages are calculated based on the total number of isolates for each category (animal and food). The values at the side of each bar are the number of S. Typhimurium isolates for each species and category and the number in parentheses indicates the number of reporting Member States.
Monophasic variants of Salmonella Typhimurium
In poultry flocks, the serovar reported by countries as ‘monophasic variant of S. Typhimurium’ did not appear among the EU‐level top‐10 serovars for laying hens or for broilers. This serovar was reported as the fifth most common serovar (2.5%) from broiler meat (Table SERBROMEAT), even though it was reported from this source by very few MS, and the United Kingdom reported almost all isolates. From meat from turkeys, the monophasic variant of S. Typhimurium ‘1,4,[5],12:i:‐’ was reported as the ninth most reported serovar and isolates were reported by the Czech Republic and France (Table SERTURKMEAT).
In 2016, monophasic variants of S. Typhimurium (including ‘monophasic variant of S. Typhimurium’, ‘4,[5],12:i:‐’ and ‘4,,12:i:‐’) were the most common serovars reported from pigs and accounted for 249 (34.1%) out of 730 isolates (Table SERPIG). In pig meat, these serovars accounting for 215 (24.3%) isolates out of the 883 Salmonella, and it was widespread among the reporting MS (Table SERPIG MEAT). These descriptive results tend to confirm pigs to be the main animal reservoir for monophasic variants of S. Typhimurium.
In cattle, the specific proportion reported for ‘monophasic variant of S. Typhimurium’ was 1.3% out of 1,500 serotyped Salmonella isolates (Table SERBOV), whereas in bovine meat it was 4.4% out of 69 serotyped Salmonella isolates (SERBOVMEAT).
The ‘pyramid plot’ showed that about 25–30% of all isolates notified from pigs and pig meat during 2016 were monophasic variants of S. Typhimurium. As already described for S. Typhimurium, these data confirmed that monophasic variants of S. Typhimurium are prevalent along the entire pig production chain. For the other sources considered, the percentage of isolation of this serovar was lower than 5% (Figure 20).
Figure 20.
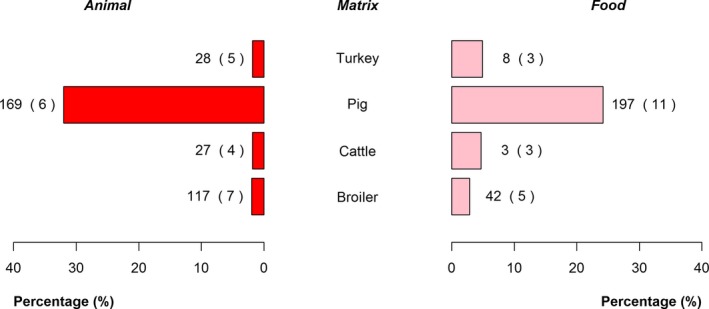
Pyramid plot showing the distribution of monophasic variants of S. Typhimurium, grouped as one serovar, among food (meat) and animal, for each species, EU, 2016
- The percentages are calculated on the total number of isolates for each category (animal and food). The values at the side of each bar are the number of monophasic variant of S. Typhimurium isolates for each species and category and the number in parentheses indicates the number of reporting Member States.
Salmonella Infantis
Salmonella Infantis was the fourth most commonly reported serovar from humans infected in the EU. In the context of NCP in Gallus gallus (Table SERGALTARG), S. Infantis was the most common serovar reported, accounting for 2,399 out of 6,213 (38.6%) isolates. In breeding hens of Gallus gallus, seven of 25 reporting MS detected S. Typhimurium (Figure 8). It is noteworthy that from Gallus gallus this serovar accounted for the great majority of the isolates reported by some MS (e.g. Austria, Croatia, Cyprus, Italy, Romania, Slovakia and Slovenia), while for some other MS that reported significant number of isolates from Gallus gallus (e.g. France and the United Kingdom), this serovar was hardly reported. These data may simply reflect the fact that the reporting of this serovar is mandatory only for breeding flocks of Gallus gallus; hence, the different situation among the MS in terms of presence of S. Infantis in poultry flocks could be due to biases related to the different reporting strategies among MS. However, these data could also reflect different epidemiological situations among MS: different approaches have been followed by MS upon S. Infantis detection in breeding flocks of Gallus gallus, as published in the MS's specific cofinanced national control programmes (European Commission, online). Nine MS (with cofinanced control programmes) apply depopulation measures when this serovar is identified in breeding flocks of Gallus gallus, seven MS do not apply such a measure whereas, for the remaining MS, the measures are applied from time to time or, for some MS, no information was provided. Noteworthy 91.7% and 92.0% of the S. Infantis isolates from breeding flocks of Gallus gallus and broiler flocks were notified by MS that do not apply depopulation measures for breeding flocks positive for S. Infantis.
For Gallus gallus, a significant increase in the number of isolates of S. Infantis was reported in 2016 (2,399 isolates) compared with 2015 (1,859 isolates) and this trend was strongly influenced by the reporting of a few MS.
S. Infantis was the most frequent reported serovar from broiler flocks (Table SERBROTARG) as well as broiler meat (Table SERBROMEAT), accounting for 45.6% and 47.4% of all serotyped Salmonella isolates reported from all MS from these sources. For some MS, there was not an evident link between the number of S. Infantis isolates from broiler flocks and broiler meat.
Among laying hens S. Infantis accounted for 111 isolates (10.7% of the 1,034 isolates reported) (Table SERLAYTARG) and for turkey flocks, it accounted for 124 isolates (13.6% of the 915 isolates reported) (Table SERTURKTARG). A marked increase of S. Infantis was overall reported from turkey flocks (Table SERTURKTARG) (124 in 2016 vs 67 in 2015). However, it should be pointed out that only three MS (Austria, Croatia and Italy) notified S. Infantis from turkey flocks in 2016. From turkey meat (Table SERTURKMEAT), S. Infantis was the third‐ranked serovar notified (11.7% of the 162 isolates reported), but all isolates were reported by Hungary.
S. Infantis did not appear among the top‐10 serovars reported from pigs (Table SERPIG) and it accounted for only 49 out of the 883 (5.6%) isolates reported from pig meat by eight MS (Table SERPIG MEAT).
Similarly, for cattle, S. Infantis did not occur among the top serovars (Table SERBOV), but it was the fifth‐ranked serovar (Table SERBOVMEAT) from bovine meat (5 out of 69 isolates).
Salmonella Derby
Salmonella Derby has been recognised as the fifth most common serovar notified from human cases of salmonellosis acquired in EU.
S. Derby did not appear among the top‐10 serovars for Gallus gallus flocks (Table SERGALTARG). It was however reported in very low numbers by four MS as the eight‐ranked serovar (14 out of 1,522 isolates) from broiler meat (Table SERBROMEAT).
Table SERTURKTARG shows that S. Derby was the fourth most common serovar in turkey flocks accounting for a total of 81 out of 915 isolates (8.9%) reported by two MS (the United Kingdom and Ireland). A significant decrease of S. Derby isolates was recorded in 2016, mostly due to the high number of S. Derby reported for 2015 (217) from United Kingdom. This serovar remained limited to turkey flocks from two MSs (the United Kingdom and Ireland), confirming the situation described in 2015, where S. Derby from turkeys was notified exclusively by the United Kingdom. For turkey meat samples, S. Derby was not reported among the top‐10 serovars.
S. Derby with 140 isolates (19.2% of the 730 serotyped isolates) reported by 11 MS was the third‐ranked serovar from pigs (SERPIG). In pig meat, it was the second most common reported serovar, accounting for 150 isolates (17% of the 883 serotyped isolates) reported by 17 MS (SERPIG MEAT). These descriptive results tend to confirm pigs to be the main animal reservoir for S. Derby.
As in 2015, S. Derby was the fourth most common serovar (SERBOVMEAT) reported from bovine meat (7 out of the 69 isolates).
2.5. Discussion
Salmonellosis remains the second most common zoonosis in humans in the EU despite a significant decreasing long‐term trend in salmonellosis cases since 2008. In recent years (2012–2016), however, the trend has stabilised, and the number of reported cases and EU notification rate has slightly increased. The majority of MS reported a decreasing trend during 2008–2016, but in half of those countries the trend has stabilised, and the number of MS reporting a significantly increasing trend doubled in 2012–2016. This could be partly attributable to more complete reporting and improvements in the surveillance of salmonellosis in few countries and could also partly reflect eventual decreasing focus on Salmonella control.
The increase of salmonellosis has been mainly attributed to S. Enteritidis. Its proportion continued to increase, particularly in human cases acquired within the EU. The number of cases and proportion of the second most common serovar S. Typhimurium continued to decrease in 2016. Together, S. Enteritidis and S. Typhimurium (including monophasic variants) accounted for almost 80% of human cases acquired in the EU. In 2016, a multicountry outbreak of S. Enteritidis that was associated with contaminated eggs from Poland was confirmed in 14 EU/EEA countries. It is likely that this multicountry outbreak has been ongoing since 2012 (EFSA and ECDC 2016b; ECDC 2017a,b,c). S. Infantis has been consistently the fourth most frequently reported serovar both in the domestically‐acquired and travel‐associated cases. Serovars S. Newport, S. Derby and S. Kentucky replaced S. Stanley as 5–7th most commonly reported Salmonella serovars for the time after the S. Stanley outbreak linked to the turkey meat in several MS in 2011. Cases of S. Stanley peaked in 2012 and increased again in 2015, suggesting a continued circulation of the serovar in the food chain until 2016 (ECDC, 2015). S. Newport, the fifth in the top five among all cases, was replaced by S. Derby, among the cases acquired in the EU. The top five serovars were almost exclusively acquired in the EU, whereas more than every third of S. Newport cases were associated to travel outside the EU.
Salmonellosis notification rates for human infections vary between MS, reflecting variations in, for example, quality, coverage and severity focus of the surveillance systems, practices in sampling and testing, disease prevalence in the production animal population, food and animal trade between MS, and the proportion of travel‐associated cases. The variation in national surveillance systems is reflected, for example, by the fact that countries that reported the lowest notification rate for salmonellosis had the highest proportions of hospitalisation, suggesting that the surveillance systems in these countries focused on the most severe cases.
For the analyses of non‐compliance with EU Salmonella criteria, important fluctuations were noted from year to year. Although there is a certain level of harmonisation in terms of matrices sampled and analytical methods, other aspects are interpreted differently between MS. So, the comparability of these data among MS is rather limited and reported findings must be interpreted with extreme caution. In contrast with the previous years, when ice‐cream was the most commonly sampled food, in 2016 minced meat and meat preparations from other species than poultry intended to be eaten cooked and fresh poultry meat were most frequently sampled to verify their compliance. The highest levels of non‐compliance were reported for food categories intended to be eaten cooked. The matrices with the high frequencies of non‐compliant samples were minced meat and meat preparations from poultry to be eaten cooked, and meat products from poultry to be eaten cooked. By contrast, the percentage of non‐compliant samples among fresh poultry meat, for which the food safety criterion considers exclusively S. Enteritidis and S. Typhimurium, was negligible. Some non‐compliant samples were also reported for RTE products (minced meat and meat preparations, meat products, RTE products containing raw eggs).
In 2016, the great majority of MS provided validated data on Salmonella on pig carcasses for compliance with the process hygiene criterion according to Commission Regulation (EU) No 218/2014, whereas five MS did not. Overall Salmonella occurrence data obtained by food business operators in the context of self‐checks and those obtained by CA, the reported proportion of Salmonella‐positive units was, respectively, 1.9% and 2.5%. Four MS provided both data collected by the food business operators and by the CA and, in all cases, the proportion of Salmonella‐positive units reported in the context of CA programmes was higher than the one reported by the food business operators. The Nordic countries Finland, Sweden and Norway, which are countries with special guarantees in relation to Salmonella on pig carcasses, reported two positive carcasses (0.02%) out of 10,354 tested.
As in previous years, in 2016 Salmonella was most frequently isolated in poultry meat that was intended to be cooked before consumption, producing a potential for consumer infection that was linked to cross‐contamination or improper preparation of contaminated meat. The poultry meat that had the highest probability of being contaminated by Salmonella was minced meat and meat preparations from broilers and turkeys. However, in RTE poultry meat, which represents a matrix with more direct risk for consumers as no further mitigation steps are applied before consumption, the prevalence was much lower. In addition, Salmonella was rarely found in table eggs, in products of vegetable origin and in sprouted seeds. Salmonella was, however, found in 1.2% of feed samples for poultry. This makes feed an important source of infection for poultry species and subsequently for the consumer, although the dominant Salmonella serovars found in human cases are rarely identified in animal feed, especially compound feed.
As regards Salmonella in animals, for all poultry categories covered by NCP, in 2016, as in previous years, overall decreases or at least a stable situation in the prevalence of target Salmonella serovars were documented, with the exception of laying hens. In laying hens, the prevalence of positive flocks for target serovars and especially for S. Enteritidis increased, after a long period of documented reduction. This scenario seems to involve different countries, even if in some specific MS it appears to be more marked. In this context, Poland, that by far failed to meet the target for laying hens, played a major role in relation to the S. Enteritidis outbreaks recently documented and involving several MS (ECDC, 2016, 2017a,b,c). Together with the detection of these recent S. Enteritidis outbreaks (see chapter on food‐borne outbreaks), the trends in monitoring data, especially for S. Enteritidis, raise questions as they indicate a reversal of the declining trend in the EU in humans and poultry. Further cross‐sectorial investigations are needed to better understand underlying reasons for the increase.
The data presented here suggest that it is pivotal not to underestimate the potential risk posed by S. Enteritidis especially in laying hens, as deteriorating management of the risk could have a direct negative effect on control of Salmonella cases in humans. Hence, premature relaxation of the effective control measures implemented to date in laying hen farms, in particular related to the implementation of vaccination programmes as well as the application of strict farm hygiene controls, should be avoided. Also, some doubts have been raised about the sensitivity of the statutory sampling implemented in commercial laying hen flocks. It has been demonstrated that the sensitivity of the sampling approaches defined as part of the NCP is influenced by the prevalence of infection within the flocks being sampled and by the type of sampling applied, which depends on the housing system (Arnold et al., 2014). The improvement of the biosecurity status of farms and better vaccination of flocks, as a direct result of the application of control programmes, have probably led to a reduction of the within‐flock prevalence, and this could challenge the identification of positive flocks, which is also restrained because of the limits of the laboratory testing methods available (EFSA, 2014a,b). Under‐detection of positive flocks especially in major exporting MS, relaxation of effective vaccination programmes in some MS once prevalence targets have been achieved, as well as relaxation of farm hygiene controls to reduce costs could have resulted in an increased spread of Salmonella.
Despite the increase in the EU flock prevalence of S. Enteritidis in laying hens as well as the documentation of important multistate human outbreaks due to this serovar and related to eggs (ECDC, 2016, 2017a,b,c), the number of Salmonella‐positive eggs, as well as the number of isolates serotyped from this source, remain very low, confirming the data reported in the previous years. This finding can be attributed to the fact that Salmonella‐positive laying hen flocks produce a small number of contaminated eggs. With a low prevalence of individual egg contamination, large numbers of eggs have to be tested to detect Salmonella and to obtain an accurate measure of the egg contamination rate (Carrique‐Mas and Davies, 2008). Although the prevalence of Salmonella in egg and egg products is normally low, the number of human cases associated with these sources can be large, especially because eggs are frequently used to produce pooled dishes that are not properly heat‐treated or stored (EFSA, 2014a,b) and also because, due to their nutritional and functional properties, eggs are used in different ways to produce and enrich many types of foods.
2.5.1.
2.5.1.1.
Serovars
For S. Enteritidis, which was first‐ranked serovar reported from human cases in the EU, the number of isolates serotyped and reported in the context of the NCP in 2016 was comparable with the number of isolated reported in previous years, thus not showing a decrease. In breeding hens of Gallus gallus, seven of 25 reporting MS detected S. Enteritidis. S. Enteritidis was by far the most prominent serovar reported among the Salmonella isolates from laying hen flocks, accounting for more than 40% of the isolates from this source. The importance of laying hens as a major source of S. Enteritidis is confirmed by the recent multistate outbreaks due to this serovar and related to the egg production chain (ECDC, 2016, 2017a,b,c; Dallman et al., 2016; Inns et al., 2017). S. Enteritidis was also reported to a certain extent from broiler flocks and broiler meat and in both cases most of the isolates were reported by just a few MS.
The second most reported serovar in human cases was S. Typhimurium. In breeding hens of Gallus gallus, eight of 25 reporting MS detected S. Typhimurium. S. Typhimurium was the second most reported serovar from pig herds and the first most common serovar from pig meat. Lastly, it was also the most reported serovar from cattle herds and the second most common serovar from bovine meat. Considering the pig chain, the data showed S. Typhimurium to be widely prevalent along the entire pig chain. It was reported a lesser extent from poultry and their meat.
The pig chain was also the principal source for monophasic variants of S. Typhimurium, which was the most common serovar reported in pig herds and the second‐ranked serovar from pig meat. This observation may be explained by the fact that his serovar has developed some adaptive mechanisms, such as resistance to heavy metals, which allow it to grow and proliferate in pig herds even when some other Salmonella serovars do not normally persist (Petrovska et al., 2016). The persistence of this serovar, as well as of S. Typhimurium is favoured by the immune deficiency of weaned pigs and the continuous contamination of pig holdings.
S. Infantis has emerged as the fourth most common serovar causing human salmonellosis in Europe, representing an important public health concern, because of its high levels of multidrug resistance. S. Infantis was mostly reported from the broiler and turkey chains. Poultry, and especially broilers, are the main animal reservoir for S. Infantis. In particular, considering broiler flocks as well as broiler meat, S. Infantis accounted for almost 50% of all Salmonella isolates reported from all MS in 2016. These data confirm how this serovar has been able to massively spread along the entire broiler production chain and is remarkably persistent on farms once it has become established. In some MS with a significant number of isolates from Gallus gallus, this serovar was hardly reported from this source. This could be due to a lack of notification of this serotype, which is not mandatory either in poultry flocks (with the exception of breeding flocks) nor in poultry meat. Another explanation for this evidence could be the different approaches implemented by MS for breeding flocks positive for S. Infantis. In some countries, depopulation is the measure prescribed for breeding flocks positive for this serovar, in some other MS no specific measures are implemented, and in others, even infected broiler flocks are stamped out in recognition of the serious threat from S. Infantis if it becomes established in the national broiler industry. Indeed, when comparing the calculated proportions of S. Infantis in MS that depopulate positive breeding flocks with those that do not depopulate, it is evident that this serovar is reported at a notably higher rate by some MS not implementing corrective measures against S. Infantis. This evidence strongly suggests that depopulation of positive flocks can be an effective measure to limit the spread of this serovar. For some reporting MS, there was no evident link between the prevalence of S. Infantis isolates from broiler flocks and from broiler meat. These findings could be due to the reporting biases rather than to any real evolution of the epidemiological situation along the chain. So, the data here presented are strongly influenced by the reporting MS and the type and amount of data supplied by those MS concerned. S. Infantis is an important public health concern due to its frequent isolation from humans (it ranks in fourth position among the top‐10 human serovars), its high levels of multidrug resistance (Hindermann et al., 2017), the successful spread of certain clones of the serovar, and finally, its extensive isolation from different poultry sources.
Finally, S. Derby has been recognised as the fifth most common serovar reported from human cases of salmonellosis acquired in EU. On the food‐animal end S. Derby was most commonly reported from pigs and pig meat and to a lesser extent from poultry and cattle.
For the volume (number) of monitoring data reported during recent years 2012–2016 for the great majority of food and animal matrices not considered in harmonised control programmes, a general reduction was observed. An opposite scenario was documented for poultry samples, where, in the last 2 years (2015 and 2016), the number of samples and notifications has doubled compared with those reported in 2010, when the control programmes had just started.
2.6. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| WHO (World Health Organization) – Salmonella (non‐typhoidal) Fact sheet | http://www.who.int/mediacentre/factsheets/fs139/en/ | |
| Food | European Union Reference Laboratory (EURL) for Salmonella | http://www.eurlsalmonella.eu |
| Microbiological criteria | https://ec.europa.eu/food/safety/biosafety/food_hygiene/microbiological_criteria_en | |
| EFSA Scientific Opinion: public health risks of table eggs due to deterioration and development of pathogens | https://www.efsa.europa.eu/en/efsajournal/pub/3782 | |
| EFSA Scientific Opinion: link between Salmonella criteria at different stages of the poultry production chain | https://www.efsa.europa.eu/en/efsajournal/pub/1545 | |
| Animals | Control of Salmonella in animals | https://ec.europa.eu/food/safety/biosafety/food_borne_diseases/salmonella_en |
| EFSA Scientific Opinion: quantitative estimation of the public health impact of setting a new target for the reduction of Salmonella in laying hens | http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2010.1546/abstract | |
| EFSA Scientific Opinion: public health impact of new target for the reduction of Salmonella in turkey flocks | https://www.efsa.europa.eu/en/efsajournal/pub/2616 | |
| EFSA Scientific Opinion: public health impact new target for the reduction of Salmonella in broiler flocks | https://www.efsa.europa.eu/en/efsajournal/pub/2106 | |
| EFSA Scientific Opinion: Salmonella in slaughter and breeder pigs | https://www.efsa.europa.eu/en/efsajournal/pub/1547 |
3. Listeria
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
3.1. Abstract
Twenty‐eight MS reported 2,536 confirmed invasive human cases of listeriosis for the year 2016. The EU notification rate was 0.47 cases per 100,000 population, which was an increase of 9.3% compared with 2015. There has been a statistically significant increasing trend of confirmed listeriosis cases in the EU/EEA during the overall period 2008–2016, as well as during the period from 2012 to 2016. Half of the MS reported a higher number of listeriosis cases in 2016 compared with 2015. Nineteen MS reported 247 deaths due to listeriosis in 2016. The EU case fatality was 16.2% among the 1,524 confirmed cases with known outcome. Listeria monocytogenes infections were most commonly reported in the elderly population in the age group over 64 years and particularly in the age group over 84 years.
Twenty‐six MS reported 2016 data on the compliance of 10 categories of RTE foods with the Listeria food safety criteria listed in the Commission Regulation (EC) No 2073/2005. The number of MS reporting data on the different RTE food categories, however, varied considerably. Non‐compliance estimates in the different RTE food categories were consistently higher at the processing stage (ranging from 0% to 6.3%) compared with retail (ranging from 0% to 1.7%). At processing, the highest overall (batch and single‐unit) level of non‐compliance was observed in the food category ‘fish and fishery products’ (6.2%), followed by ‘meat products other than fermented sausages’ (2.5%), ‘other RTE foods’ (1.0%), ‘unspecified cheeses’ (1.0%), ‘fermented sausages’ (0.8%), ‘milk’ (0.8%), ‘soft and semi‐soft cheeses’ (0.7%), ‘hard cheeses’ (0.5%) and ‘other dairy products’ (0.1%). At retail, the highest non‐compliance was observed in ‘fish and fishery products’ (0.7%) and ‘fermented sausages’ (0.2%), whereas the overall non‐compliance estimates in the remaining food categories at retail were below 0.1%.
In 2016, among the different RTE food categories and across all sampling stages, L. monocytogenes was most frequently detected in ‘fishery products’ (5.6%), ‘fish’ (4.7%), ‘pork meat products other than fermented sausages’ (3.1%) and in ‘soft and semi‐soft cheeses made from raw milk’ (2.5%). Compared with 2015, there was a noticeable decrease (around 15%) in the sample sizes tested for the major RTE food categories.
Fourteen MS reported findings of Listeria spp. (mainly L. monocytogenes) in various animal species and mainly in domestic ruminants (cattle, sheep and goats). As data reported on animals originated primarily from clinical (suspect) investigations, they are not suitable for estimating accurate occurrence estimates or trends over time in the different animal species or animal holdings at the EU‐level.
3.2. Surveillance and monitoring of Listeria monocytogenes in the EU
3.2.1. Humans
Surveillance of human listeriosis is focused on invasive forms of L. monocytogenes infection, mostly manifested as septicaemia, meningitis or spontaneous abortion. The disease is reported by MS and EEA countries according to the Decision No 1082/2013 on serious cross‐border threats to health, repealing Decision No 2119/98/EC.12 Cases are reported annually to TESSy according to the EU case definition for listeriosis.13 Between 2008 and 2015, the national surveillance systems were comprehensive in 27 European countries (28 since 2012). The human data are published annually in the EU Summary Reports14 and are available in the interactive ECDC Surveillance Atlas of Infectious Diseases15 on the ECDC website. In addition, annual epidemiological reports are available on the ECDC website.16
The notification of listeriosis in humans is mandatory in most MS, Iceland, Norway and Switzerland, except for four MS, where notification is based on a voluntary system (Belgium and Luxembourg) or other system (Spain and the United Kingdom). The surveillance systems for listeriosis covers the entire population in all MS except in Spain. No estimate for the population coverage was provided for Spain, so the notification rate was not calculated.
Diagnosis of human L. monocytogenes infections is generally performed by culture from blood, cerebrospinal fluid and vaginal swabs.
3.2.2. Food, animals, and feed
Monitoring of L. monocytogenes in foods is mainly based on data originating from the reporting obligations of MS under the EU Regulation (EC) No 2073/2005 on microbiological criteria,6 which lays down food safety criteria for L. monocytogenes in RTE foods and which has been in force since January 1 of 2006. Generally, data submitted to EFSA for compliance of RTE foods with the L. monocytogenes microbiological criteria allow for making descriptive summaries at the EU‐level, and also allow EU trends to be monitored. However, they preclude trend analyses at the EU‐level (Table 1).
Monitoring of L. monocytogenes in RTE food is conducted along the food chain during preharvest, processing and post‐harvest (at retail and catering). The public health risk of L. monocytogenes in RTE food depends, among other reasons, on the effectiveness of its control and monitoring procedures, which include Good Agricultural Practices (GAP) at the farm stage, the HACCP programme and Good Hygiene Practices (GHP) at the processing and retail stages. It also depends on the sampling and testing procedures to evaluate the compliance of RTE foods with the food safety criteria (FSC) for L. monocytogenes. These are laid down in Commission Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. This Regulation requires the following:
For RTE foods intended for infants and RTE foods for special medical purposes placed on the market during their shelf life, the absence of L. monocytogenes is required in 25 g of sample (n = 10, c = 0).17
For RTE foods able to support the growth of L. monocytogenes,18 other than those intended for infants and for special medical purposes, absence of L. monocytogenes is required in 25 g of sample (n = 5, c = 0) before the RTE foods has left the immediate control of the food business operator, who has produced it. This criterion shall apply to products when the food business operator is not able to demonstrate, to the satisfaction of the competent authority, that the product will not exceed the limit of 100 CFU/g throughout the shelf life. For products placed on the market during their shelf life the limit is 100 CFU/g (n = 5, c = 0) if the food business operator is able to demonstrate, to the satisfaction of the competent authority, that the product will not exceed the limit 100 CFU/g throughout the shelf life.
For RTE foods unable to support the growth of L. monocytogenes 18 (based on their pH, water activity values and/or other intrinsic factors) and for products with a shelf life of less than 5 days, other than those intended for infants and for special medical purposes, the limit is 100 CFU/g (n = 5, c = 0) during their shelf life on the market.
Analogous to the situation in humans, listeriosis in animals is a relatively uncommon disease. Most of the monitoring data on L. monocytogenes in animals provided by the MS to EFSA are generated by non‐harmonised monitoring schemes across MS and for which no mandatory reporting requirements exist. The 2016 data originated primarily from clinical investigations (61.8% of the total number of units tested) and more particularly from suspect animals (95.4% of the total number of units tested). Therefore, this sampling bias may result in an overestimation of the L. monocytogenes occurrence in the different animal species tested. Consequently, the reported data in animals are not comparable among MS and the reported findings must be interpreted with caution. These data preclude subsequent data analyses such as assessing temporal and spatial trends at the EU‐level.
Among several transmission routes, listeriosis in animals can be transmitted via the consumption of contaminated feed. For instance, listeriosis in domestic ruminants is usually caused by the ingestion of poor‐quality silage. Data on L. monocytogenes in feed are however only collected as part of investigations in farm animals in case of listeriosis outbreaks. Hence, monitoring data on L. monocytogenes in animal feeding stuffs are rarely available.
The rationale for the surveillance and monitoring of L. monocytogenes in animals, food and feed is shown in Figure 21.
Figure 21.
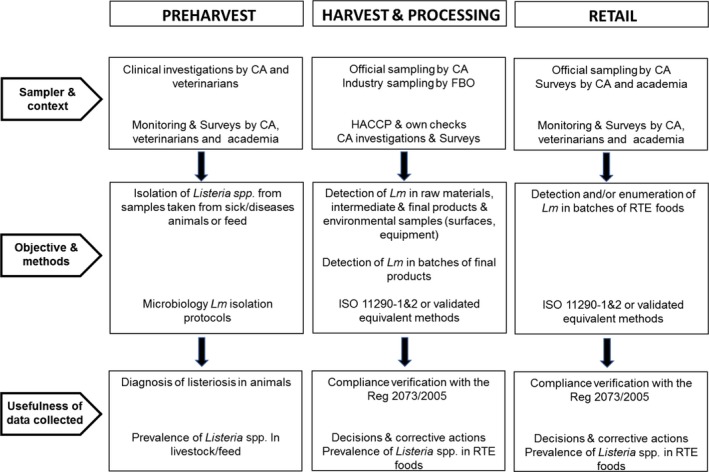
The surveillance and monitoring of L. monocytogenes in food, animals and feed according to the sampling stage, the sampler and the objective of the sampling
- Lm: Listeria monocytogenes; CA: Competent Authorities. HACCP: Hazard Analysis and Critical Control Points; RTE: ready‐to‐eat.
3.2.3. Food‐borne outbreaks of human listeriosis
The reporting of food‐borne outbreaks is mandatory according the EU Zoonoses Directive 2003/99/EC7 and the recorded data represent the most comprehensive set of data available at the EU‐level for assessing the burden of food‐borne outbreaks – including those caused by L. monocytogenes. Further details are provided in the chapter on FBO.
3.3. Data analyses
In this chapter, the data (food, animals and feed) submitted by the MS were extracted from the data warehouse (DWH) of EFSA to produce and analyse the summary tables for compliance and occurrence and prevalence purposes.
In general, the data extracted for compliance and occurrence were obtained from samplings (census, convenience and objective sampling) conducted by industry and/or national competent authorities (official samplings). Data on L. monocytogenes from suspect samplings and selective samplings were only included for the description of the occurrence of L. monocytogenes in animals.
3.3.1. Monitoring of compliance
The results from qualitative examinations using the detection method EN ISO 11290‐1:1996, amended in 2004 (ISO, 1996; ISO, 2004a) were used to assess the compliance with the criterion of ‘absence in 25 grams’, and the results from quantitative analyses using the enumeration method EN ISO 11290‐2:1998 amended in 2004 (ISO, 1998; ISO, 2004b) were used to assess compliance with the criterion of ‘≤ 100 CFU/g’.
In order to categorise the reported data according RTE food categories in combination with the sampling stage, a categorisation was made regarding all reported sampling stages to assess compliance: all sampling units that were obtained from ‘cutting plants’, ‘packing centres’ and ‘processing plants’ were considered as units collected at the processing stage; all sampling units that were obtained from ‘catering’, ‘hospital or medical care facility’, ‘retail’, ‘wholesale’, ‘unspecified’, ‘restaurant or cafe or pub or bar or hotel or catering service’ and ‘automatic distribution system for raw milk’ were considered as units collected at retail. The limited data reported by some MS from investigations of RTE foods during ‘border inspection activities’ were not taken up in the compliance table.
The data presented in the section on L. monocytogenes in food should be considered in the light of certain assumptions and decisions made by EFSA because of some underlying uncertainties and limitations in the reported data. These assumptions/decisions and related data uncertainties/limitations are:
Unknown status of RTE foods in terms of their ability to support the growth of L. monocytogenes . For many of the reported data, it was not evident whether the RTE foods tested were able to support the growth of L. monocytogenes or not, because data on crucial physicochemical parameters such as pH, aw and levels and types or preservatives present on the sampled foods were not reported. For assessing the compliance of samples collected at the processing stage, the criterion of ‘absence in 25 grams’ was applied. However, for the sampling units – taken at the processing stage – but obtained from ‘hard cheeses’, ‘fermented sausages’ and some of the RTE food types reported under the ‘other dairy products’ category (Table 12) the criterion of ‘≤ 100 CFU/g’ was applied because these types of RTE foods are considered to be unable to support the growth of L. monocytogenes. Therefore, it is possible that some of the foods that were considered as able to support the growth of L. monocytogenes (and for which the criterion of ‘absence in 25 grams’ was applied to assess compliance at processing) may actually not be permissive to the pathogen's growth. Such foods could be misclassified as non‐compliant in the event of a detection‐positive result at processing.
Incorporation of the ‘unspecified’ sampling stage into ‘retail’ for purposes of compliance assessment. In cases where the sampling stage for certain of the reported investigations was not specified, EFSA assumed that the investigations were conducted at the retail stage. Consequently, this assumption may allow more samples to be classified as compliant due to the more lenient microbiological criteria applied at the retail stage (up to 100 CFU/g) compared with those applied at the processing stage (‘absence in 25 grams’, i.e. up to 0.04 CFU/g).
Assessment of compliance at the processing stage. As previously mentioned, for the assessment of compliance of RTE foods collected at the processing stage (except for the above‐mentioned three RTE food categories/subcategories that are assumed to be unable to support the growth of L. monocytogenes, and for which the criterion of ‘≤ 100 CFU/g’ was applied), the criterion of ‘absence in 25 grams’ was applied and the results of the detection method were used to classify foods as compliant or non‐compliant. For some investigations on RTE foods sampled at processing, MS reported either only quantitative (enumeration) data, or both quantitative and qualitative (detection) data. However, in such cases, due to the aggregated nature of the reported data and/or the lack of information of the independence status of the qualitative and quantitative data from the same investigation, quantitative data for foods tested at processing were not utilised in assessing compliance. In practice, the vast majority of RTE foods testing positive in enumeration analyses, would have tested positive also if detection methods were used. However, EFSA applies a single and uniform rule for assessing compliance of RTE foods from all reported investigations (except for investigations on the before‐mentioned three RTE food categories/subcategories that are considered as not supporting the growth of L. monocytogenes) by only considering results from the detection method. In addition, given the risk for double‐reporting (overestimating non‐compliance upon usage of non‐independent data), quantitative data from foods sampled at processing (except for investigations on the before‐mentioned three RTE food categories/subcategories) were excluded from the assessment of compliance (Table 12).
Non‐compliance of single samples (units) with the food safety limits. According to Regulation (EC) No 2073/2005, L. monocytogenes food safety criteria are set for batch sampling with 5 or 10 units comprising the sample (n). There is a single microbiological limit (m = M) and the maximum allowable number of sampling units (c) yielding unsatisfactory test results is 0. In addition to assessing the compliance of batches according to the Regulation 2073/2005 criteria, EFSA assessed the compliance of single samples (units) based on the reported test results by the different methods. This ‘classification/assessment’ of single units is based on the rationale that any sampled batch (comprised of 5 or 10 units) containing even one (sampled) unit with counts of L. monocytogenes exceeding the corresponding microbiological limit (at processing or at retail) would automatically render the corresponding batch unsatisfactory/not compliant.
Consideration of RTE foods tested with the enumeration method irrespective of sample weight. In the analysis of the reported data, EFSA did not exclude samples that deviate from the ISO requirements on the minimum weight of the test sample. According to ISO 6887‐1 (ISO, 1999), a minimum of 10 g or 10 mL of food should be used as the test portion for the microbiological examinations of products intended for human consumption. Data from samples that do not meet this minimum weight requirement (e.g. test samples of 1 g) may lead to less accurate enumeration results (EN/ISO 11290‐2).
These assumptions/decisions and related data uncertainties/limitations are:
Table 12.
Non‐compliance (%) with L. monocytogenes food safety criteria laid down by Regulation (EC) No 2073/2005 in main ready‐to‐eat (RTE) food categories in the EU, 2016 according to sampling stage and analytical method and sampling unit (single units vs batch samples)a
| RTE food categoryb | Sampling unit | Processing stage | Retail (including ‘unspecified’) | ||
|---|---|---|---|---|---|
| Analytical methodo | |||||
| Detection | Enumeration | Detection | Enumeration | ||
| Foods intended for infants and foods for special medical purposes | Batch | 0.0 (n = 11; 2 MS) | 0.0 (n = 3; 1 MS) | ||
| Single | 0.0 (n = 21; 2 MS) | 0.0 (n = 318; 9 MS) | |||
| Fish c and fishery products d | Batch | 6.1 (n = 652; 5 MS) | 1.7 (n = 753; 9 MS) | ||
| Single | 6.3 (n = 459; 12 MS) | 0.4 (n = 1,750; 14 MS) | |||
| Cheeses, soft and semi‐soft e | Batch | 0.5 (n = 1,895; 6 MS) | 0.3 (n = 636; 10 MS) | ||
| Single | 0.8 (n = 2,148; 12 MS) | 0.0 (n = 1,479; 10 MS) | |||
| Cheeses, hard f | Batch | Unable to support the growth of L. monocytogenes | 0.0 (n = 105; 1 MS) | 0.0 (n = 361; 5 MS) | |
| Single | 0.6 (n = 310; 8 MS) | 0.0 (n = 314; 8 MS) | |||
| Cheeses, unspecified g | Batch | 0.0 (n = 3; 1 MS) | 0.0 (n = 31; 2 MS) | ||
| Single | 1.0 (n = 2,254; 5 MS) | 0.0 (n = 514; 5 MS) | |||
| Other dairy products (excluding cheeses) – entire category h | Batch | 0.2 (n = 1,256; 5 MS) | 0.0 (n = 295; 7 MS) | ||
| Single | < 0.1 (n = 1,045; 11 MS) | 0.0 (n = 933; 9 MS) | |||
| Other dairy products (excluding cheeses) – supporting the growth of L. monocytogenes i | Batch | 0.2 (n = 548; 3 MS) | 0.0 (n = 73; 4 MS) | ||
| Single | 0.4 (n = 262; 6 MS) | 0.0 (n = 201; 6 MS) | |||
| Other dairy products (excluding cheeses) – NOT supporting the growth of L. monocytogenes j | Batch | Unable to support the growth of L. monocytogenes | 0.0 (n = 227; 3 MS) | 0.0 (n = 222; 7 MS) | |
| Single | 0.0 (n = 408; 8 MS) | 0.0 (n = 732; 9 MS) | |||
| Milk k | Batch | 0.0 (n = 139; 4 MS) | 0.0 (n = 29; 5 MS) | ||
| Single | 1.4 (n = 218; 8 MS) | 0.0 (n = 124; 6 MS) | |||
| Products of meat origin: fermented sausages l | Batch | Unable to support the growth of L. monocytogenes | 0.0 (n = 5; 1 MS) | 0.0 (n = 14; 3 MS) | |
| Single | 0.8 (n = 261; 5 MS) | 0.2 (n = 515; 6 MS) | |||
| Products of meat origin other than fermented sausages m | Batch | 1.2 (n = 1,852; 5 MS) | 0.1 (n = 1,567; 7 MS) | ||
| Single | 3.0 (n = 4,777; 10 MS) | < 0.1 (n = 4,687; 13 MS) | |||
| Other products n | Batch | 1.9 (n = 522; 8 MS) | 0.1 (n = 1,825; 8 MS) | ||
| Single | 0.6 (n = 1,069; 12 MS) | < 0.1 (n = 4,951; 16 MS) | |||
CFU: colony‐forming unit; MS: Member State; n: number of sampling units.
Each cell contains the percentage of non‐compliant samples (the presence of L. monocytogenes in 25‐g of sample for detection analyses or populations of L. monocytogenes > 100 CFU/g for enumeration analyses) and in parenthesis the number of tested samples and the number of reporting MS at the batch‐ and single‐unit levels. Retail includes also data from sampling stage reported as ‘unspecified’.
In the absence of relevant data (pH, aw), EFSA assumes that foods listed under ‘Fish and fishery products’, ‘Soft and semi‐soft cheeses’, ‘Unspecified cheeses’, ‘Milk’, ‘Products of meat origin other than fermented sausages’ and ‘Other products’ belong to the category of foods that are able to support the growth of L. monocytogenes. Foods classified under these categories of RTE products are expected to have near‐neutral or moderately low pH and relatively high water activity (aw) values or can be very heterogeneous in terms of their manufacturing technology and physicochemical characteristics (‘Other products’). EFSA assumes that ‘Fermented sausages’ and ‘Hard cheeses’ belong to the category of foods that are unable to support the growth of L. monocytogenes, because foods classified under these two categories of RTE products undergo ripening/fermentation and are expected to have low pH and moderate aw values. In assessing compliance of ‘other dairy products’, EFSA is presenting the results of two different approaches: (a) a conservative approach, classifying/considering all ‘other dairy products’ as capable of supporting the growth of L. monocytogenes; and (b) a ‘splitting’ of ‘other dairy products’ into two subcategories (one subcategory encompassing products that are likely to support the growth of L. monocytogenes as well as unspecified products, and one subcategory encompassing products that are unlikely to support the growth of L. monocytogenes).
Includes RTE fish which is ‘cooked’, ‘gravad lax/slightly salted’, ‘marinated’ or ‘smoked’ (cold‐ or hot‐smoked).
Includes cooked crustaceans (shrimps, prawns, unspecified) that were ‘chilled’, ‘frozen’ or ‘shelled and shucked’, cooked molluscan shellfish (‘chilled’, ‘frozen’ or ‘shelled, shucked and frozen’), fishery products unspecified (‘cooked’, ‘cooked and chilled’, ‘ready‐to‐eat chilled or frozen’, ‘seafood pâté’, ‘smoked’).
Includes ‘curd’, ‘fresh’ and ‘soft or semi‐soft’, cheeses made from different milk kinds and types (‘pasteurised’ or ‘raw or low‐heat treated’ and from ‘cows’, ‘goats’, ‘sheep’, ‘mixed’, ‘unspecified’ or from other animals’ milk).
Includes ‘hard’ cheeses made from different milk kinds and types (‘pasteurised’ or ‘raw or low‐heat treated’ and from ‘cows’, ‘goats’, ‘sheep’, ‘mixed’, ‘unspecified’ or from other animals’ milk).
Includes ‘unspecified’ cheeses made from different milk kinds (‘cows’, ‘goats’, ‘sheep’, ‘mixed’, ‘unspecified’ or from other animals’ milk).
Includes ‘butter’, ‘buttermilk’, ‘cheese analogue’, ‘cream’, ‘dairy desserts’, ‘fermented dairy products’, ‘ice‐cream’, ‘milk‐based drinks’, ‘milk powder and whey powder’, ‘sour milk’, ‘yoghurt’ and ‘unspecified’ ready‐to‐eat dairy products.
Includes ‘butter’, ‘cheese analogue’, ‘cream’, ‘dairy desserts’, ‘milk‐based drinks’ and ‘unspecified’ ready‐to‐eat dairy products.
‘Buttermilk’, ‘fermented dairy products’, ‘ice‐cream’, ‘milk powder and whey powder’, ‘sour milk’ and ‘yoghurt’.
Includes milk (‘pasteurised’, ‘UHT’, or ‘raw, intended for direct human consumption’) from ‘cows’, ‘goats’, ‘sheep’, ‘unspecified’ or from other animals’ milk. Raw milk and raw milk for the manufacture of raw and low heat‐treated products are not included.
Includes fermented sausages made from meat of different animal species (‘bovine animals’, ‘deer’, ‘horse’, ‘pig’, ‘mixed’, ‘other animal species or unspecified’).
Includes ‘meat products’ (‘intended to be eaten raw’ or ready‐to‐eat), meat preparations (‘pâté’) and ‘minced meat’ (‘intended to be eaten raw’ or ‘ready‐to‐eat’) from different animal species (‘bovine animals’, ‘pigs’, poultry (‘broilers’, ‘geese’, ‘ducks’, ‘turkeys’, ‘other poultry species’ or ‘unspecified poultry’), ‘mixed’, ‘goats’, ‘sheep’, ‘horses’, ‘bison’, ‘donkeys’, ‘water buffalos’, ‘wild boar’, ‘farmed game‐land animals’, or ‘other animal species’).
Includes RTE salads, fruits and vegetables (pre‐cut or not), processed food products and prepared dishes (sandwiches, ices and frozen desserts, sushi and other ready‐to‐eat foods), spices and herbs, bakery products (bread, cakes, desserts, pastry), vegetables (pre‐cut or not, canned, cooked or cooked and chilled), confectionery products and pastes, beverages (non‐alcoholic), chocolate, nuts and nut products, fats and oils (excluding butter), juices (from fruits, vegetables or mixed, pasteurised or unpasteurised), sauces and dressings, cereals and meals, cocoa and cocoa preparations, coffee and tea, sweets, fruits (pre‐cut or not, chilled or frozen, canned, dried or fruit puree), coconut, soups, seeds (sprouted or dried), potato chips, egg products (ready‐to‐eat).
The results from qualitative examinations using the detection method were used to assess compliance with the criterion of ‘absence in 25 grams’, and the results from quantitative analyses using the enumeration method were used to assess compliance with the criterion of ‘≤ 100 CFU/g’.
Given all these assumptions and uncertainties about the data, EFSA categorised the RTE food categories according the ability to support growth (or not) L. monocytogenes and the sampling stage (where the samples were taken). A distinction was made regarding the sampling stages: all sampling units that were obtained from ‘cutting plants’, ‘packing centres’ and ‘processing plants’ were considered as units collected at the processing stage; all sampling units that were obtained from ‘catering’, ‘hospital or medical care facility’, ‘retail’, ‘wholesale’, ‘unspecified’, ‘restaurant or cafe or pub or bar or hotel or catering service’ and ‘automatic distribution system for raw milk’ were considered as units collected at retail. The limited data reported by some MS from investigations of RTE foods during ‘border inspection activities’ were not taken up in the compliance table. For assessing the compliance table (Table 12), compliance of the reported samples collected at retail, the criterion of ‘≤ 100 CFU/g’ was applied, except for ‘RTE products intended for infants and for special medical purposes’, where L. monocytogenes shall not be detected in 25 g of sample, according to the Commission Regulation (EC) No 2073/2005. For the analysis of compliance of samples collected at the processing stage, the criterion of ‘absence in 25 grams’ was applied, except for hard cheeses, other dairy products not supporting the growth of L. monocytogenes and fermented sausages. As described above, the use of singly samples for the assessment of compliance may lead to under‐ or overestimation of the compliance for a specific RTE category.
3.3.2. Monitoring of occurrence
To describe the presence of L. monocytogenes in RTE food only the data from the detection method (qualitative investigations) at different sampling stages (at retail, at the processing level, at farms, during border inspection activities as well as at an unspecified sampling stage) were used to calculate the overall (all sampling stages combined) and sampling‐stage‐specific (retail stage and processing stage) occurrence estimates for each level of sampling unit (single‐unit and batch). Detection methods are considered to be the most sensitive and appropriate methods to describe the presence of L. monocytogenes in foods. Data from quantitative investigations (using the enumeration method) in RTE foods were also submitted to EFSA. However, enumeration data were not used for estimating the occurrence of L. monocytogenes in the different RTE food matrices because, although enumeration‐positive results prove the presence of L. monocytogenes in foods, the opposite is not necessarily true (enumeration‐negative results do not assure the absence of L. monocytogenes).
It should be clear that the simple presence (‘prevalence/occurrence’, via a detection‐positive result) of L. monocytogenes in RTE foods, per se, may not necessarily pose a health risk to consumers, because the theoretical sensitivity of the detection method is as low as 1 CFU of L. monocytogenes per 25 g of food, and the risk of listeriosis in humans depends on several factors including, but not limited to, the ability of the RTE foods to support the growth of L. monocytogenes, the populations of L. monocytogenes in the contaminated foods at the time of consumption and the consumers’ immune status. Hence, for an assessment – from a public health viewpoint – of the reported findings of L. monocytogenes in RTE foods, the reader should refer to the section on compliance, in which data from different RTE food categories at the EU level are assessed according to the food safety criteria laid down in the Commission Regulation 2073/2005.
3.4. Results
3.4.1. Overview of key statistics along the food chain, EU, 2012–2016
Table 10 summarises EU level statistics related to listeriosis in humans, and to the prevalence of L. monocytogenes prevalence (via detection method) in major RTE food categories, respectively, in the EU, during 2012–2016.
Table 10.
Summary statistics of human invasive L. monocytogenes infections and L. monocytogenes occurrence in the major RTE food categories in the EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 2,536 | 2,206 | 2,242 | 1,883 | 1,720 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.47 | 0.43 | 0.46 | 0.39 | 0.36 | ECDC |
| Number of reporting MS | 28 | 28 | 27 | 27 | 27 | ECDC |
| Infection acquired in the EU | 1,437 | 1,461 | 1,509 | 1,298 | 1,278 | ECDC |
| Infection acquired outside the EU | 7 | 9 | 10 | 14 | 12 | ECDC |
| Unknown travel status or unknown country of infection | 1,092 | 736 | 723 | 571 | 430 | ECDC |
| Total number of food‐borne outbreaks (including waterborne outbreaks) | 5 | 15 | 13 | 9 | 8 | EFSA |
| Number of outbreak‐related cases | 25 | 233 | 94 | 56 | 71 | EFSA |
| RTE food | ||||||
| Fish and fishery products | ||||||
| Number of sampled units | 2,918 | 4,658 | 3,436 | 3,479 | 7,235 | EFSA |
| Number of reporting countries | 22 | 22 | 16 | 20 | 19 | EFSA |
| Meat and meat products (beef, pork, broiler and turkey meat) | ||||||
| Number of sampled units | 15,161 | 16,789 | 67,215 | 44,977 | 30,652 | EFSA |
| Number of reporting countries | 23 | 21 | 18 | 21 | 21 | EFSA |
| Soft and semi‐soft cheeses made from raw or low‐heat‐treated milk | ||||||
| Number of sampled units | 853 | 730 | 2,573 | 2,542 | 2,013 | EFSA |
| Number of reporting countries | 15 | 13 | 13 | 13 | 13 | EFSA |
| Hard cheeses made from raw or low‐heat‐treated milk | ||||||
| Number of sampled units | 509 | 858 | 10,175 | 1,609 | 1,940 | EFSA |
| Number of reporting countries | 9 | 11 | 9 | 12 | 10 | EFSA |
| Fruit and vegetables | ||||||
| Number of sampled units | 1,043 | 1,456 | 1,503 | 1,991 | 1,010 | EFSA |
| Number of reporting countries | 16 | 17 | 17 | 15 | 15 | EFSA |
| Salads | ||||||
| Number of sampled units | 1,042 | 1,238 | 1,154 | 1,822 | 2,071 | EFSA |
| Number of reporting countries | 14 | 13 | 15 | 14 | 12 | EFSA |
RTE: ready‐to‐eat; ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority.
Over the years, overall, a noticeable decrease was observed in the sample sizes tested and reported to EFSA for many RTE food categories.
3.4.2. Human listeriosis
Twenty‐eight MS reported 2,536 confirmed human cases of listeriosis for the year 2016 (Table 11). The EU notification rate was 0.47 cases per 100,000 population, which was an increase of 9.3% compared with 2015. The highest notification rates were observed for Finland, Belgium, Germany, Slovenia and Denmark with 1.22, 0.92, 0.85, 0.73 and 0.70 cases per 100,000 population, respectively. Spain further improved their surveillance system for listeriosis in 2016, which resulted in an increase of reported confirmed cases by 75.7%. The lowest notification rates were reported by Bulgaria, Croatia, Cyprus and Romania (≤ 0.1 per 100,000).
Table 11.
Reported cases of human invasive listeriosis and notification rates per 100,000 in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 46 | 46 | 0.53 | 38 | 0.44 | 49 | 0.58 | 36 | 0.43 | 36 | 0.43 |
| Belgium | Y | A | 104 | 104 | 0.92 | 83 | 0.74 | 84 | 0.75 | 66 | 0.59 | 83 | 0.75 |
| Bulgaria | Y | A | 5 | 5 | 0.07 | 5 | 0.07 | 10 | 0.14 | 3 | 0.04 | 10 | 0.14 |
| Croatia | Y | A | 4 | 4 | 0.10 | 2 | 0.05 | 4 | 0.09 | 0 | 0.00 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.12 | 1 | 0.12 |
| Czech Republic | Y | C | 47 | 47 | 0.45 | 36 | 0.34 | 38 | 0.36 | 36 | 0.34 | 32 | 0.30 |
| Denmark | Y | C | 40 | 40 | 0.70 | 44 | 0.78 | 92 | 1.64 | 51 | 0.91 | 50 | 0.90 |
| Estonia | Y | C | 9 | 9 | 0.68 | 11 | 0.84 | 1 | 0.08 | 2 | 0.15 | 3 | 0.23 |
| Finland | Y | C | 67 | 67 | 1.22 | 46 | 0.84 | 65 | 1.19 | 61 | 1.12 | 61 | 1.13 |
| France | Y | C | 375 | 375 | 0.56 | 412 | 0.62 | 373 | 0.57 | 369 | 0.56 | 346 | 0.53 |
| Germany | Y | C | 707 | 697 | 0.85 | 580 | 0.71 | 598 | 0.74 | 463 | 0.57 | 414 | 0.52 |
| Greece | Y | C | 20 | 20 | 0.19 | 31 | 0.29 | 10 | 0.09 | 10 | 0.09 | 11 | 0.10 |
| Hungary | Y | C | 25 | 25 | 0.25 | 37 | 0.38 | 39 | 0.40 | 24 | 0.24 | 13 | 0.13 |
| Ireland | Y | C | 13 | 13 | 0.28 | 19 | 0.41 | 15 | 0.33 | 8 | 0.17 | 11 | 0.24 |
| Italy | Y | C | 179 | 179 | 0.30 | 153 | 0.25 | 132 | 0.22 | 143 | 0.24 | 112 | 0.19 |
| Latvia | Y | C | 6 | 6 | 0.30 | 8 | 0.40 | 3 | 0.15 | 5 | 0.25 | 6 | 0.29 |
| Lithuania | Y | C | 10 | 10 | 0.35 | 5 | 0.17 | 7 | 0.24 | 6 | 0.20 | 8 | 0.27 |
| Luxembourg | Y | C | 5 | 2 | 0.35 | 0 | 0.00 | 5 | 0.91 | 2 | 0.37 | 2 | 0.38 |
| Malta | Y | C | 1 | 1 | 0.23 | 4 | 0.93 | 1 | 0.24 | 1 | 0.24 | 1 | 0.24 |
| Netherlands | Y | C | 89 | 89 | 0.52 | 71 | 0.42 | 90 | 0.54 | 72 | 0.43 | 73 | 0.44 |
| Poland | Y | C | 101 | 101 | 0.27 | 70 | 0.18 | 87 | 0.23 | 58 | 0.15 | 54 | 0.14 |
| Portugal | Y | C | 32 | 31 | 0.30 | 28 | 0.27 | – | – | – | – | – | – |
| Romania | Y | C | 9 | 9 | 0.05 | 12 | 0.06 | 5 | 0.03 | 9 | 0.05 | 11 | 0.05 |
| Slovakia | Y | C | 10 | 10 | 0.18 | 18 | 0.33 | 29 | 0.54 | 16 | 0.30 | 11 | 0.20 |
| Slovenia | Y | C | 15 | 15 | 0.73 | 13 | 0.63 | 18 | 0.87 | 16 | 0.78 | 7 | 0.34 |
| Spainb | N | C | 363 | 362 | – | 206 | – | 161 | – | 140 | – | 109 | – |
| Sweden | Y | C | 68 | 68 | 0.69 | 88 | 0.90 | 125 | 1.30 | 93 | 0.97 | 72 | 0.76 |
| United Kingdom | Y | C | 201 | 201 | 0.31 | 186 | 0.29 | 201 | 0.31 | 192 | 0.30 | 183 | 0.29 |
| EU total | – | – | 2,551 | 2,536 | 0.47 | 2,206 | 0.43 | 2,242 | 0.46 | 1,883 | 0.39 | 1,720 | 0.36 |
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 4 | 1.23 | 1 | 0.31 | 4 | 1.25 |
| Norway | Y | C | 19 | 19 | 0.37 | 18 | 0.35 | 29 | 0.57 | 21 | 0.42 | 30 | 0.60 |
| Switzerlandc | Y | C | 50 | 50 | 0.60 | 54 | 0.65 | 98 | 1.20 | 64 | 0.80 | 39 | 0.49 |
Y: yes; N: no; A: aggregated data; C: case‐based data.
Sentinel surveillance; no information on estimated coverage so notification rate cannot be estimated.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The vast majority (> 99%) of listeriosis cases with known origin of infection were reported to be acquired in the EU (Table 11). Eight MS reported 15 travel‐associated listeriosis cases (six cases outside the EU and nine cases within the EU) in 2016. The proportion of reported listeriosis cases without data on the travel status or unknown country of infection increased from 25.0% to 43.1% of all confirmed cases from 2012 to 2016 (Table 10).
In the period 2008–2016, a seasonal pattern was observed in the listeriosis cases reported in the EU/EEA, with high summer peaks followed by less high winter peaks (Figure 22). Over the same nine‐year period, a statistically significant increasing trend of confirmed listeriosis cases was observed in the EU/EEA (p < 0.01), as well as in the last 5 years (2012–2016) (Figure 22).
Figure 22.
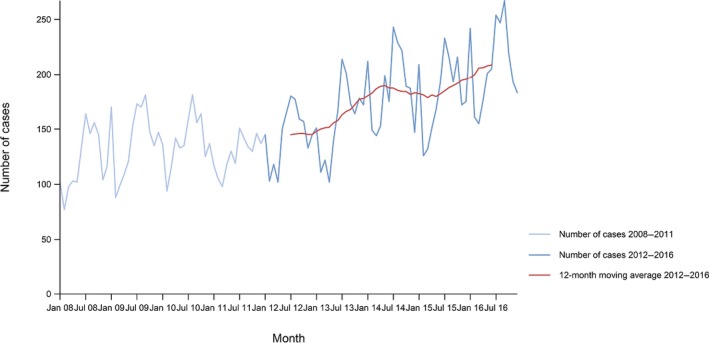
Trend in reported confirmed human cases of listeriosis in the EU/EEA, by month, 2012–2016
- Source(s): Austria, Belgium, Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Malta, the Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom. Bulgaria, Croatia, Luxembourg and Portugal did not report data to the level of detail required for the analysis.
Twelve MS (Finland, France, Germany, Greece, Hungary, Italy, the Netherlands, Poland, Romania, Slovenia, Spain and Sweden) had a significant increasing trend of confirmed listeriosis cases (p < 0.01) since 2008. None of the MS observed decreasing trends between 2008–2016 or 2012–2016.
In 2012–2016, seven MS reported significantly increasing trends (Croatia, Estonia, Germany, Greece, Italy, Poland and Spain). In seven MS (Finland, France, Hungary, the Netherlands, Romania, Slovenia and Sweden), which had an increasing overall trend in 2008–2016, no significant change over the last 5 years (2012–2016) was observed and none of the MS had decreasing trends.
Information on hospitalisation was provided by 17 MS for 38.8% of all confirmed cases in 2016. Among the cases with information on hospitalisation status, 97.7% were hospitalised. Listeriosis had the highest proportion of hospitalised cases of all zoonoses under EU surveillance.
The outcome was reported for 1,524 confirmed cases (60.1%). Nineteen MS reported 247 deaths due to listeriosis in 2016. There was a steady increase in annual number of deaths recorded since 2008 (annual average: 187). The overall EU case fatality among cases with known outcome was 16.2%. France reported the highest number of fatal cases (53) followed by Germany (48).
L. monocytogenes infections were most commonly reported in the age group over 64 years. At the EU level, the proportion of listeriosis cases in this age group has steadily increased from 52.9% in 2008 to 61.9% in 2016, and especially in the age group over 84 years, with an increase from 7.6% to 10.4%. The case fatality was 18.9% and 26.1% in the age group over 64 years and over 84 years, respectively, in 2016. The proportion of fatal cases in the age group over 84 years of age increased from 7.5% in 2008 to 22.0% in 2016.
3.4.3. Listeria monocytogenes in foods
Monitoring and surveillance data reported from RTE foods in the framework of EU Regulation 2073/2005 on microbiological criteria
Compliance was assessed for 10 RTE food categories according to the food safety criteria listed in Regulation (EC) No 2073/2005 (Table 12). The total number of tested samples is reported by analytical method (L. monocytogenes detection method or L. monocytogenes enumeration method) and according to the criterion that should be applied for compliance assessment (‘presence’ or ‘absence’ in 25 g of food based on the detection method results and ‘> 100 CFU/g’ or ‘≤ 100 CFU/g’ based on the enumeration method results) for each sampling stage (‘processing’ vs ‘retail’).
In total, 26 MS reported data that were included in the assessment of compliance with the EU food safety criteria. Malta and Poland did not submit any data on food. At the retail stage, depending on the RTE food category, 0–0.4% of single units and 0–1.7% of batches were found not to be compliant. At the processing stage, higher and varying (depending on the RTE food category) levels of non‐compliance (primarily presence in 25 g) were reported, ranging from 0% to 6.3% in single units and from 0% to 6.1% in batches. It must be emphasised that between one and 16 MS contributed data that were used for the assessment of the compliance of these specified foods. Consequently, as data were mostly reported by a limited number of MS, the findings based on this data may not be representative of the EU‐level.
In ‘fish and fishery products’, a low overall level of non‐compliance was noted at retail (0.7%; batch level 1.7%, 9 MS; single‐unit level 0.4%, 14 MS). At the processing stage, the level of non‐compliance was considerable higher than retail (6.2%) and comparable between single units and batches sampled. More than half (58%) of these non‐compliant samples were reported by Bulgaria.
Among samples of ‘products of meat origin, other than fermented sausages’, a rare overall level of non‐compliance was noted at retail (< 0.1%, 16 MS), and higher at the processing stage (2.5%, 13 MS). Most (63%) of the non‐compliant samples at processing were from pork.
‘Fermented sausages’ are assumed not to support the growth of L. monocytogenes. A limited number of batches (n = 19) were tested and found to be compliant. A low level of non‐compliance (three out of the 776 units tested) was found at the single‐unit level: 0.2% at retail (6 MS) and 0.8% at processing (5 MS). All three non‐compliant units were sampled in Hungary and were manufactured from pork.
All ‘RTE milk’ samples collected at retail by 11 MS were compliant. Only three single units of ‘raw cows’ milk intended for direct human consumption’ sampled at processing were not compliant (1.4% non‐compliance).
In ‘soft and semi‐soft cheeses’ sampled at retail, non‐compliance was only found in batches (0.3%) tested by 10 MS. A slightly higher level of non‐compliance was noted at the processing stage (0.7%). Most (74%) of the non‐compliant soft and semi‐soft samples at processing were manufactured from cows’ milk.
Non‐compliance in ‘hard cheeses’ – which are assumed not to support the growth of L. monocytogenes – was only found in 2 out of the 310 single units tested by eight MS at processing (0.6%). Among samples of ‘unspecified cheeses’ (mainly reported by Italy), a low level of non‐compliance was observed in single units at the processing stage (1.0%), whereas all batches were found to be compliant.
All samples from ‘other dairy products, excluding cheeses’ tested at retail (14 MS) were compliant. At processing, a very low (0.2%, 5 MS) and a rare (< 0.1%, 11 MS) proportion of tested batches and single units tested, respectively, were not compliant. This non‐compliance was due to ‘other dairy products’ assumed to support growth of L. monocytogenes (butter) (Table 12).
As in previous years, all samples of ‘RTE food intended for infants and for special medical purposes’ taken in 2016 by three MS were compliant.
Non‐compliance in the food category ‘other RTE products’ at retail was very low (0.1% of batches and < 0.1% of single samples). At the processing stage, the percentage of non‐compliance at the single‐unit level was 0.6% (12 MS) and 1.9% at batch level (8 MS). Non‐compliant samples at processing were found in the following food categories: ‘bakery products’ (desserts containing heat‐treated cream, pastry), ‘RTE salads’, ‘pre‐cut vegetables’, ‘sandwiches’, ‘fats and oils’ (excluding butter), ‘confectionery products and pastes’ and ‘unpasteurised vegetable juice’. Non‐compliant samples at retail were only noted in bakery products (desserts containing heat‐treated cream, cakes).
Occurrence of Listeria monocytogenes in RTE foods
Fish and fishery products, RTE
Eighteen MS reported 2016 data on RTE fish. When combining all sampling stages (‘retail’, ‘processing’, ‘border inspection activities’ and ‘unspecified’) and all sampling units (‘single’ and ‘batch’), the overall occurrence of L. monocytogenes in RTE fish was 4.7%. Although 12 MS reported positive results, more than the half (56%) of the positive findings originated only from two MS (Germany and the Netherlands). Comparing the data of 2016 of RTE fish (single units at retail and excluding heat‐treated (cooked) fish) with those of the EU baseline survey (EFSA, 2013, 2014a), a much lower proportion was found to be contaminated with L. monocytogenes (4.6%; n = 1,397 vs 10.4% in the EU baseline study).
Twelve MS reported 2016 data on L. monocytogenes in RTE fishery products (‘crustaceans and molluscs’ and ‘other fishery products’). The overall occurrence of L. monocytogenes in RTE fishery products was 5.6%. Five MS (Bulgaria, Estonia, Ireland, Spain and Sweden) reported positive findings from unspecified fishery products.
A summary of the proportion of L. monocytogenes‐positive units in fish and fishery products is presented in Figure 23.
Figure 23.
Proportion of L. monocytogenes‐positive sampling units in ready‐to‐eat fish and fishery‐product categories in the reporting Member States, 2016 across all sampling stages (overall), retail and processing plant levels
-
‘Overall’ and the number of MS correspond to data across all major sampling stages (‘retail’ + ‘processing’ + ‘border inspection activities’ + ‘unspecified’). ‘Retail’ corresponds to data obtained from catering, hospitals or medical care facilities, retail, wholesale and restaurants or cafes or pubs or bars or hotels or catering services. ‘Processing’ corresponds to data obtained from packing centres, cutting plants and processing plants. For each sampling stage (‘overall’, ‘retail’ and ‘processing’), data are pooled across both types of sampling units (‘single’ and ‘batch’). As data were mostly reported by a limited number of MS, the findings presented in this figure may not be representative of the EU‐level.‘Fish, RTE ’ includes detection data on RTE fish from 18 MS (Austria, Belgium, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Estonia, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, the Netherlands, Slovakia, Spain and Sweden) and includes data on ‘Fish’ of the following types: ‘chilled’, ‘cooked’, ‘gravad lax/slightly salted’, ‘marinated’ and ‘smoked (hot‐ and cold‐smoked)’.
- ‘Fishery products, RTE ’ includes detection data from 12 MS (Austria, Belgium, Bulgaria, Croatia, Cyprus, Estonia, Hungary, Ireland, Portugal, Slovakia, Spain and Sweden) and includes data on the following types: ‘prawns, cooked’, ‘prawns‐shelled, shucked and cooked’, ‘shrimps, cooked’, ‘shrimps, shelled, shucked and cooked’, ‘crustaceans, unspecified, cooked’, ‘crustaceans, unspecified, shelled, shucked and cooked’, ‘molluscan shellfish, cooked’, ‘unspecified’ (cooked, ready‐to‐eat, pâté, smoked) and ‘Surimi’.
Meat and meat products, RTE
Nineteen MS reported 2016 data on RTE meat products, mainly meat products from pig meat.
Combining the most important RTE meat‐product categories (‘beef’, ‘broiler’, ‘pork’ and ‘turkey’), for all sampling stages (‘retail’, ‘processing’, ‘border inspection activities’ and ‘unspecified’) and all sampling units (‘single’ and ‘batch’), the overall occurrence of L. monocytogenes in RTE meat products was 2.6% (363 out of 13,826 samples tested were positive). Considering only single units of RTE meat products tested at retail (excluding ‘fermented sausages’, ‘raw ham’ and ‘raw meat products intended to be eaten raw’) (data not shown), 2.1% tested positive which is equal to the proportion reported in the 2010–2011 EU baseline survey (single units of RTE heat‐treated meat products sampled at retail and tested at the end of shelf life) (EFSA, 2013, 2014a).
Pig meat products
Eighteen MS reported 2016 data on RTE pig meat products and, overall, L. monocytogenes was detected in 3.1% of the 10,961 units tested. At retail, more than 75% of the total number of single units tested originated from 3 MS (the Czech Republic, Germany and Hungary), while at processing almost 80% of the data were obtained from 2 MS (the Czech Republic and Italy). At retail, L. monocytogenes was detected in 2.7% of the tested samples, whereas at the processing stage 3.4% the samples tested positive.
Poultry meat products (broilers and turkeys)
Ten MS reported 2016 data on RTE broiler meat. Positive findings were only reported by two MS (the Czech Republic and the United Kingdom). Overall, L. monocytogenes was detected in 0.8% of the 1,098 units tested.
Ten MS reported data from RTE turkey meat products. Overall, L. monocytogenes was detected in 1.6% of the 321 units tested. Positive findings were only observed in single units sampled by Luxembourg and Spain at retail and by Sweden (sampling stage not specified).
Bovine meat products
Twelve MS reported 2016 data on RTE bovine meat products. Overall, L. monocytogenes was detected in 0.7% of the 1,446 units tested. At retail, L. monocytogenes was detected in 0.7% of the single units, whereas at processing, 0.5% of the batches and 1% of the single units tested were positive. The positive findings in RTE bovine meat products were reported by three MS (the Czech Republic, Germany and Ireland).
A summary of the proportion of L. monocytogenes‐positive units in RTE meat products is presented in Figure 24.
Figure 24.
Proportion of L. monocytogenes‐positive sampling units in ready‐to‐eat meat‐product categories in the reporting Member States, 2016 across all sampling stages (overall), retail and processing plant levels
- ‘Overall’ and the number of MS correspond to data across all major sampling stages (‘retail’ + ‘processing’ + ‘border inspection activities’ + ‘unspecified’). ‘Retail’ corresponds to data obtained from catering, hospital or medical care facilities, retail, wholesale and restaurants or cafes or pubs or bars or hotels or catering services. ‘Processing’ corresponds to data obtained from packing centres, cutting plants and processing plants. For each sampling stage (‘overall’, ‘retail’ and ‘processing’) data are pooled across both types of sampling units (‘single’ and ‘batch’). Since data were mostly reported by a limited number of MS, the findings presented in this figure may not be representative of the EU‐level.
- ‘ RTE pork meat’ includes detection data on RTE pig meat products from 18 MS (Austria, Belgium, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Estonia, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, Portugal, Slovakia, Spain and Sweden) and includes data on ‘Meat from pig, meat products’ of the following types: ‘cooked ham (sliced or non‐sliced)’, ‘cooked, RTE’, ‘fermented sausages’, ‘fresh raw sausages’, ‘meat specialities’, ‘pâté’, ‘raw and intended to be eaten raw’, ‘raw ham’, ‘unspecified, ready‐to‐eat’.
- ‘ RTE turkey meat’ includes detection data on RTE turkey meat products from 10 MS (Austria, Cyprus, Estonia, Hungary, Ireland, Italy, Luxembourg, Portugal, Spain and Sweden) and includes data on turkey ‘meat products’ of the following types: ‘cooked, RTE’, ‘preserved’ and ‘raw and intended to be eaten raw’.
- ‘ RTE broiler meat’ includes detection data on RTE broiler meat products from 10 MS (Bulgaria, the Czech Republic, Estonia, Hungary, Ireland, Italy, Portugal, Spain, Sweden and the United Kingdom) and includes data on broiler ‘meat products’ of the following types: ‘cooked, RTE’ and ‘cooked, RTE, chilled’.
- ‘ RTE bovine meat’ includes detection data on RTE bovine meat products from 12 MS (Austria, Bulgaria, the Czech Republic, Denmark, Estonia, Germany, Hungary, Ireland, Italy, Luxembourg, Spain and Sweden) and includes data on ‘Meat from bovine animals, meat products’ of the following types: ‘cooked, RTE’, ‘cooked, RTE, chilled’, ‘fermented sausages’, ‘raw and intended to be eaten raw’, ‘unspecified, RTE’.
Milk and milk products, RTE
Thirteen MS reported 2016 data on RTE milk (‘pasteurised’, ‘UHT’ and ‘raw milk intended for direct human consumption’). Overall, L. monocytogenes was detected in 0.7% of the 968 units tested. Positive findings were only reported by three MS (Croatia, Germany and Estonia) and concerned ‘raw milk intended for direct human consumption’.
Cheeses
Nineteen MS reported 2016 data on cheeses, mainly (61%) cheeses made from pasteurised cows’ milk. Overall, considering all sampling stages, all sampling units and all types of cheeses (irrespective of cheese texture and of the animal origin and heat treatment of milk), L. monocytogenes was detected in 0.7% of the 6,078 cheese samples tested.
A summary of the proportion of units positive for cheeses is presented in Figure 25. A low or very low and comparable frequency of detection of L. monocytogenes across the different cheese subcategories was noted, although the occurrence of the pathogen was higher in soft and semi‐soft cheeses made from raw or low‐heat‐treated milk.
Figure 25.
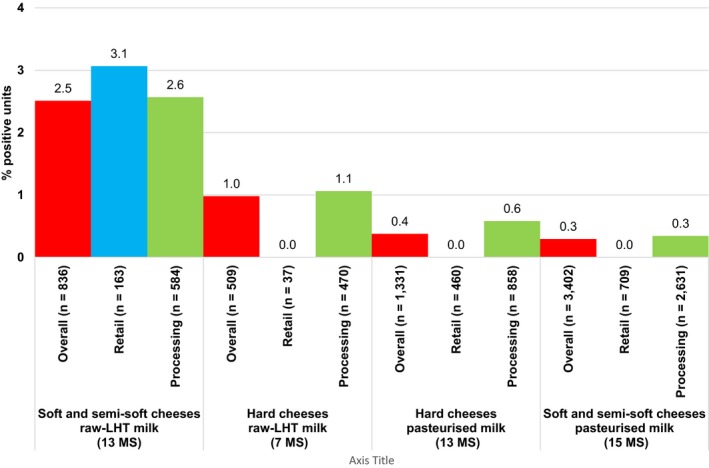
Proportion of L. monocytogenes‐positive sampling units in soft and semi‐soft cheeses, and in hard cheeses made from raw or low‐heat‐treated milk or pasteurised milk in the reporting Member States, 2016 across all sampling stages (overall), retail and processing plant levels
- LHT: low heat treated. ‘Overall’ and the number of MS correspond to data across all major sampling stages (‘retail’ + ‘processing’ + ‘farm’ + ‘border inspection activities’ + ‘unspecified’). ‘Retail’ corresponds to data obtained from catering, hospital or medical care facilities, retail, wholesale and restaurants or cafes or pubs or bars or hotels or catering services. For each sampling stage (‘overall’, ‘retail’ and ‘processing’), data are pooled across both types of sampling units (‘single’ and ‘batch’). ‘Processing’ corresponds to data obtained from packing centres, cutting plants and processing plants. Since data were mostly reported by a limited number of MS, the findings presented in this figure may not be presentative of the EU‐level. Soft and semi‐soft cheeses as well as hard cheeses include all cheeses for which Level 2 at matrix level was specified (‘fresh’ or ‘soft’ or ‘semi‐soft’ or ‘hard’).
- ‘Soft and semi‐soft cheeses, made from raw‐ LHT milk’ includes detection data from 13 MS (Austria, Belgium, Croatia, the Czech Republic, Estonia, France, Hungary, Ireland, Italy, the Netherlands, Portugal, Slovakia and Spain).
- ‘Hard cheeses, made from raw‐ LHT milk’ includes detection data from 7 MS (Austria, Bulgaria, Estonia, France, Ireland, Portugal and Spain).
- ‘Hard cheeses, made from pasteurised milk’ includes detection data from 13 MS (Austria, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Estonia, Finland, Greece, Hungary, Ireland, Portugal and Spain).
- ‘Soft and semi‐soft cheeses, made from pasteurised milk’ includes detection data from 15 MS (Austria, Belgium, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Estonia, Greece, Hungary, Ireland, Luxembourg, Portugal, Slovakia and Spain).
Soft and semi‐soft cheeses
In 2016, 4,238 units of soft and semi‐soft cheeses were tested using the detection method and almost half (48%) were sampled by two MS (Bulgaria and the Czech Republic).
In 2016, the occurrence of L. monocytogenes in soft and semi‐soft cheeses made from raw or low‐heat‐treated milk (2.5% of the 836 units tested) was higher than in soft and semi‐soft cheeses made from pasteurised milk (0.3% of the 3,402 units tested).
When considering only single units of soft and semi‐soft cheeses sampled at retail (and excluding fresh cheeses), none of the samples tested positive in qualitative analyses (n = 657). This estimate is comparable with the very low corresponding estimate (0.5%) obtained from the 2010–2011 EU baseline survey (RTE soft and semi‐soft cheeses sampled at retail and tested at the end of shelf life) (EFSA, 2013, 2014a).
Hard cheeses
In the reporting MS in 2016, 1,840 units of hard cheeses were tested using the detection method and almost two‐thirds of these units (66.4%) were sampled by two MS (Bulgaria and Finland.
In 2016, L. monocytogenes was detected in 1.0% of the 509 tested units made from raw or low‐heat‐treated milk (across all animal‐origin cheese‐milk types) and in 0.4% of the 1,331 tested units of hard cheeses made from pasteurised milk (across all animal‐origin cheese‐milk types). None of the 497 tested samples of hard cheeses at retail (460 single units and 37 batches) contained L. monocytogenes.
Other ready‐to‐eat food products
In 2016, results from other RTE food‐product categories, such as ‘bakery products’, ‘confectionery products and pastes’, ‘egg products’, ‘fruits and vegetables’, ‘salads’, ‘sauces and dressings’, ‘spices and herbs’ and ‘other processed food products and prepared dishes’ were reported.
Regarding ‘bakery products’, most of the data were from single samples collected at retail and were reported by 11 MS. Overall, out of the 1,984 units of bakery products tested, 0.8% were found positive for L. monocytogenes. Bosnia and Herzegovina conducted two large surveys in RTE bakery products in which L. monocytogenes was detected in only two out of the 4,074 single units tested.
In 2016, 13 MS provided data from investigations of L. monocytogenes on 1,772 units of ‘RTE fruit and vegetables’ tested using the detection method and half of these data were reported by Italy. Positive findings with detection analyses were only reported from four MS (Austria, Ireland, Italy and Spain) and, overall, 0.5% of these units were positive for L. monocytogenes.
Regarding ‘RTE salads’, 13 MS reported data on 1,042 units tested using the detection method. Overall, 2.0% of the units tested (mainly at retail) were reported as positive.
Regarding ‘sauces and dressings’, 11 MS reported information on 299 units tested using the detection method and L. monocytogenes was detected only in one batch sampled (at an unspecified stage) by Ireland.
Regarding ‘spices and herbs’, four MS reported information on 48 units tested using the detection method with no positive findings.
In ‘other processed food products and prepared dishes’, 12 MS submitted data and two MS (the Czech Republic and Denmark) reported positive findings. Overall, L. monocytogenes was detected in 0.3% of the 646 units tested. The positive units were related to sandwiches taken at processing plants.
Regarding ‘confectionery products and pastes’, in 2016 only five MS submitted data and only one investigation was positive. Overall, 0.6% of 154 units of confectionery products and pastes tested were found positive.
L. monocytogenes was not detected in any of the reported qualitative investigations (72 units, 6 MS) of ‘egg products’.
Details on occurrence of L. monocytogenes in main RTE food matrices in 2016 can be found in Appendix A at the end of this report.
3.4.4. Listeria spp. in animals
Fourteen MS and one non‐MS reported 2016 data on several animal categories (food‐producing, wild‐, zoo‐ and pet animals, including birds) and animal species tested for Listeria spp. Reported data were mainly (98.5%) from the animal sampling unit level. The majority (96.3%) of the total units tested concerned domestic ruminants (cattle, sheep and goats). Among the reporting countries, Italy reported on the highest variety of animal categories and species. The sample size of the investigations, the sampling strategy and the proportion of positive samples varied considerably among the reporting countries and animal species. Hence, the vast majority of the EU data in animals (90.9% of the total units tested) were reported by two MS (Ireland and the Netherlands); more than half (61.7%) of the total number of units tested were sampled under a clinical investigation context and the vast majority of the data (95.4% of the total units tested) originated from a suspect sampling strategy.
In total, considering all different sampling unit levels (‘animal’, ‘herd/flock’ or ‘holding’) 31,849 units were tested for Listeria spp. and 293 (0.9%) were found positive, much lower compared with 2015 (3.0% of 31,490 units). Most positive findings were reported in domestic ruminants (cattle, sheep and goats) followed by pigs, solipeds, zoo animals and wild rodents. Among the positive units, 209 (71.3%) were reported as being positive for L. monocytogenes. As MS testing for Listeria spp. in animals was expected to concentrate on L. monocytogenes (EFSA, 2017a), only limited positive findings were reported for Listeria ivanovii (7 units, 2.4%) and Listeria innocua (34 units, 11.6%). Interestingly, 47 units (16%) were reported as positive under the ‘unspecified Listeria spp.’ or ‘Listeria spp.’ other than L. ivanovii and L. innocua category. Presumably, in most of these positive units, the Listeria spp. isolates were not identified to the species level, given that listeriosis in animals is known to be almost exclusively caused by L. monocytogenes and L. ivanovii.
3.4.5. Listeria monocytogenes in feed
Only two MS (Bulgaria and Romania) reported 2016 monitoring data on investigations of L. monocytogenes in feed. A total of seven investigations (including data from selective‐ and suspect‐sampling strategies and from HACCP and own checks) were conducted with no positive findings.
3.5. Discussion
While still relatively rare, human listeriosis is one of the most serious food‐borne diseases under EU surveillance, causing hospitalisation, high morbidity and high mortality, particularly among the elderly. EU surveillance of listeriosis focuses on severe, invasive forms of the disease, for which the risk groups are mainly the elderly and immunocompromised people as well as pregnant women and infants. Long‐term human invasive listeriosis has shown a significant increasing trend since EU surveillance was initiated in 2008. In addition, listeriosis is the only food‐borne zoonosis, which continues to show a significantly increasing trend in the EU/EEA in the last 5 years (2012–2016). Seven MS reported increasing trends over the last 5 years. This is partly attributable to more complete reporting and improvements in the surveillance of listeriosis in a few countries. Some countries also reported Listeria outbreaks in 2016. Most listeriosis cases, when this information is known, have been domestically acquired and a few cases have been linked to travel, particularly outside the EU. In the last few years, the number of cases acquired within the EU, however remained unchanged compared with the significant increase of in the overall number of listeriosis cases in the EU since 2008. At the same time, more countries reported cases without information on travel.
Since the beginning of EU‐level surveillance, most listeriosis cases have been reported among persons over 64 years of age. The number and proportion of cases reported for this age group has increased steadily from 2008 to 2016, and almost doubled in the age group over 84 years. As in previous years, almost all (97%) reported listeriosis cases were hospitalised, and caused the highest proportion of fatal cases compared with the other zoonotic agents in this report. The increase of Listeria infections may be partially explained by the ageing population in the EU. As ageing of the populations will continue in most MS (Eurostat, 2016) in the coming years, it is important to raise awareness of listeriosis and the risk, especially to older people, associated with certain types of foods.
The overall trend in the number of confirmed invasive listeriosis cases in the EU/EEA increased significantly between 2008 and 2016 as well as between 2012 and 2016. These observations were the result of analysing by linear regression the significance of the trend in a 12‐month moving average. In the EFSA SO (EFSA BIOHAZ Panel, 2011) a time series analysis was conducted for the time period 2008–2015 of 14,002 confirmed human invasive listeriosis cases in the EU/EEA. This analysis did not show an increasing trend at the overall level but disclosed clear increasing trends by specific age‐gender groups (elderly over 75 years and females 25–44 year old). The difference between both conclusions may be attributed to the difference in the time period considered, the countries and cases included in the analyses, and the different statistical methodology used. The statistical method used in the present report may be more sensitive to detect statistically significant trends compared to the time series analysis used in the EFSA SO.
L. monocytogenes is a food‐borne human and animal pathogen that is widely distributed in the environment (agricultural, aquacultural and food processing). L. monocytogenes can enter the food‐processing environment via incoming raw materials and the movement of personnel and equipment. The pathogen can colonise, in the form of biofilms, food‐processing equipment and food‐contact surfaces and can therefore persist for prolonged time periods in food‐handling environments. Hence, a wide range of foodstuffs can occasionally get contaminated during various steps of food production and distribution, particularly during the food‐processing stage and many different RTE food types (RTE meat‐, dairy‐, fish‐ and fishery products but also vegetables fruits, salads and other RTE products) have been implicated in cases or outbreaks of listeriosis in humans. In general, listeriosis cases/outbreaks in humans have been associated with RTE foods that are permissive to the pathogen's growth and are held for extended periods under refrigeration before consumption. In recent years, however, listeriosis outbreaks were also caused by foods that have not been considered as likely vehicles based on previous experience and risk assessments (Buchanan et al., 2017).
Compliance was assessed for 10 RTE food categories according to the food safety criteria listed in Regulation (EC) No 2073/2005. In 2016, for RTE products at retail the level of non‐compliance was very low (0.1–1%) to rare (< 0.1%), depending on the RTE food category. The RTE food category with the highest overall level of non‐compliance at retail was ‘fish and fishery products’ (0.7%). Non‐compliance in the remaining nine RTE food categories at retail was very low to zero. However, higher and varying (depending on the RTE food‐type) levels of non‐compliance were reported in samples of RTE products at the processing stage. Parallel to the findings at the retail stage, the RTE food category with the highest overall level of non‐compliance at processing was ‘fish and fishery products’ (6.2%); this was followed by ‘meat products other than fermented sausage’ (2.5%). In the remaining eight RTE food categories, the overall level of non‐compliance at processing remained at 1% (‘other RTE foods’, ‘unspecified cheeses’) or below 1% (remaining food categories). The several‐fold higher levels of non‐compliance noted at the processing stage compared with the retail stage ought to be mainly attributed to the stricter food safety criterion applied at processing.
In 2016, when considering RTE food samples originating from all sampling stages and unit levels (single units and batches) L. monocytogenes was most frequently detected in ‘fishery products’ (5.6%), ‘fish’ (4.7%), ‘pork meat products other than fermented sausages’ (3.1%) and in ‘soft and semi‐soft cheeses made from raw or low‐heat‐treated milk’ (2.5%). The higher occurrence of L. monocytogenes in unpasteurised milk (soft or hard) cheeses is to be expected given that pasteurisation effectively controls the pathogen's populations in raw milk. L. monocytogenes was detected in 2.0% of the tested samples of ‘RTE salads’ and at low to very low levels (0–1.6%) in the remaining RTE food categories. Recently, the results of an extensive literature search (covering the years 1990–2015) on the prevalence of L. monocytogenes in different RTE food categories was published (Jofre et al., 2016). The estimated mean (median) prevalence values for the ‘meat’, ‘seafood’, dairy’ and ‘produce’ food groups were 10.3% (4.0%), 13.4% (10.4%), 4.8% (0.4%) and 2.7% (0.0%), respectively. The L. monocytogenes prevalence estimates in the different RTE food categories in 2016 appear to be (much) lower than the corresponding 1990–2015 mean prevalence values and comparable with the corresponding 1990–2015 median prevalence values.
Recently, source‐attribution model analyses suggested that L. monocytogenes isolates of ‘bovine’ origin could act as the main source (32–64%) of human listeriosis, although it was recognised that the genetic distribution of isolates associated with a particular source may change along the food chain and that this could affect the source attribution results (Nielsen et al., 2017). However, other than the exclusive finding of L. monocytogenes in raw cow's milk (among the tested raw‐milk samples of all (cow, goat or sheep) animal species), the 2016 relevant reported data in foods (compliance or occurrence in bovine‐origin foods) or animals do not appear to corroborate this hypothesis.
The annually reported occurrence and compliance estimates for the different RTE food categories may not be adequately robust due to the variation in the number of tested samples and the number of MS reporting data across reporting years. Nonetheless, in 2016, the occurrence of the pathogen in the different RTE food categories in the EU as well as their levels of non‐compliance at retail appear to be at comparable or even at lower levels compared with previous reporting years, with no unexpected findings. Based on this, the increase in the number of human listeriosis cases in the EU may not be attributable to elevated consumer exposure via increased occurrence of the pathogen in RTE foods.
There are many factors influencing the risk of invasive listeriosis in humans which are often interrelated (complex interaction between the pathogen, the foods and the hosts) and not easily quantifiable. Hence, the level of initial contamination, the ability of the contaminated RTE food to support or not the growth of the pathogen, the temperature variations and temperature distribution throughout production and distribution of RTE foods (including domestic storage), the consumer food safety attitudes and practices, the food consumption and the portion size can all influence the level of exposure of consumers to L. monocytogenes. Furthermore, the interstrain variations of L. monocytogenes in terms of virulence potential, the long‐term (e.g. pregnancy, old age, immunodeficiency diseases such as cancer and diabetes) and short‐term (immunosuppressive therapies or medications such as administration of antacids/proton pump inhibitors) host susceptibility or even the treatment choices can determine the outcome of the infection (Evans and Redmond, 2016; Buchanan et al., 2017; Charlier et al., 2017a,b). Recently, using a developed L. monocytogenes generic quantitative microbiological risk assessment model, the EFSA BIOHAZ Panel proposed that among several risk factors that could be potential drivers for L. monocytogenes contamination of RTE foods and listeriosis illness, also the increase in the number of elderly people as well as the susceptible population (except for women in the age group 25–44 years) was considered as probably responsible for the increasing trend in cases of human listeriosis. Regarding the increased incidence rates and cases, the increased proportion of susceptible people in the age groups over 45 years old of both genders was proposed as a likely factor.19 It may be necessary to re‐evaluate the significance of factors such as the frequency of consumption of low doses of L. monocytogenes by different human subpopulations (susceptibility‐groups) and further investigate factors influencing L. monocytogenes virulence and host susceptibility (Buchanan et al., 2017).
Several MS reported findings of Listeria spp. in animals. Findings of Listeria spp. were almost exclusively reported in domestic ruminants (cattle, sheep and goats). As more than half of the total number of units tested were sampled under a clinical investigation context and the vast majority of the data originated from suspect sampling strategies, data on animals are not suitable for estimating meaningful occurrence estimates or trends over time in the different animal species or animal holdings at the EU‐level.
Over the years, a noticeable decrease has been observed in the sample sizes tested and reported to EFSA for many RTE food categories (Table 10). So, MS should be prompted to increase sampling and testing efforts and abide by their regulatory obligations on the monitoring of L. monocytogenes in RTE foods. This may be particularly necessary for RTE food categories that have been incriminated in listeriosis FBO in recent years (RTE salads and RTE fruits and vegetables) and for RTE foods intended for consumption by particularly vulnerable individuals such as foods intended for infants and for special medical purposes as well as foods consumed by the elderly.
3.6. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| Humans and food | European Commission Regulation (EC) No 2073/2005 – Food Safety Criteria for L. monocytogenes in the EU | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20170101&rid=1 |
| EFSA Scientific Report: EU Baseline Survey 2010–2011 – Part A: Listeria monocytogenes prevalence estimates | https://www.efsa.europa.eu/en/efsajournal/pub/3241 | |
| EFSA Scientific Report: EU Baseline Survey 2010–2011 – Part B: analysis of factors related to prevalence and exploring compliance | https://www.efsa.europa.eu/en/efsajournal/pub/3810 | |
| EFSA Scientific Opinion: EU scientific advice on L. monocytogenes risk in RTE foods | http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2008.599/epdf | |
| EFSA Draft Scientific Opinion: L. monocytogenes contamination of RTE foods and the risk for human health in the EU | https://www.efsa.europa.eu/sites/default/files/engage/170724-0.pdf | |
| FDA (Food and Drug Administration, FDA or USFDA) of United States: Quantitative assessment of relative risk to public health from food‐borne Listeria monocytogenes among selected categories of ready‐to‐eat foods | https://www.fda.gov/downloads/Food/FoodScienceResearch/UCM197330.pdf | |
| FAO (Food and Agriculture Organization of the United Nations) Risk assessment of Listeria monocytogenes in ready‐to‐eat foods: Technical report | http://www.fao.org/3/a-y5394e.pdf | |
| FAO (Food and Agriculture Organization of the United Nations) and WHO: Risk assessment of Listeria monocytogenes in ready‐to‐eat foods – Interpretive Summary | http://www.fao.org/fileadmin/templates/agns/pdf/jemra/mra4_en.pdf | |
| FSIS comparative risk assessment for Listeria monocytogenes in ready‐to‐eat meat and poultry deli meats | US FDA/FSIS 2010, https://www.fsis.usda.gov/shared/PDF/ | |
| Interagency risk assessment: Listeria monocytogenes in retail delicatessens technical report | US FDA/FSIS 2013, https://www.fsis.usda.gov/shared/PDF/Comparative_RA_Lm_Report_May2010.pdf | |
| EFSA External Scientific Report: Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 1, an extensive literature search and study selection with data extraction on L. monocytogenes in a wide range of RTE food | https://www.efsa.europa.eu/en/supporting/pub/1141e | |
| EFSA External Scientific Report 2017a: Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 2, a quantitative risk characterisation on L. monocytogenes in RTE foods; starting from the retail stage | https://www.efsa.europa.eu/en/supporting/pub/1252e | |
| EFSA External Scientific Report 2017b: Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 3, the comparison of isolates from different compartments along the food chain, and from humans using whole genome sequencing (WGS) analysis | https://www.efsa.europa.eu/en/supporting/pub/1151e | |
| ECDC Surveillance Atlas of Infectious Diseases in humans including listeriosis – Tool for infectious disease data manipulation and presentation | https://ecdc.europa.eu/en/surveillance-atlas-infectious-diseases | |
| EC Guidance document on Listeria monocytogenes shelf‐life studies for ready‐to‐eat foods, under Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs | https://ec.europa.eu/food/sites/food/files/safety/docs/biosafety_fh_mc_guidance_document_lysteria.pdf | |
| EURL (European Union Reference Laboratory) activities and documents on L. monocytogenes for member laboratories | https://eurl-listeria.anses.fr/ | |
| Technical guidance document for conducting shelf‐life studies on Listeria monocytogenes in ready‐to‐eat foods (challenge testing and durability testing) | https://eurl-listeria.anses.fr/en/minisite/listeria/eurl-lm-technical-guidance-document-conducting-shelf-life-studies-listeria | |
| CAC (Codex Alimentarius Commission) of FAO/WHO: Guidelines on the application of general principles of food hygiene to the control of Listeria monocytogenes in foods | http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCAC%2BGL%2B61-2007%252FCXG_061e.pdf | |
| FDA (Food and Drug Administration, FDA or USFDA) of United States and others: A public database of genome sequences, including L. monocytogenes sequences – GenomeTrakr | https://www.fda.gov/food/foodscienceresearch/wholegenomesequencingprogramwgs/ucm363134.htm | |
| CDC (Centers for Disease Control and Prevention) of United States: General overview and facts on L. monocytogenes and listeriosis | https://www.cdc.gov/listeria/ | |
| A web‐based platform (‘Listeriomics’) integrating different tools for Listeria ‘omics’ data analyses | https://listeriomics.pasteur.fr | |
| Animals | Merck Veterinary Manual: General overview of listeriosis in animals | http://www.merckvetmanual.com/generalized-conditions/listeriosis/overview-of-listeriosis |
| OIE (World Organisation for Animal Health) Overview and diagnosis of listeriosis in animals | http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.09.06_LISTERIA_MONO.pdf |
4. Shiga toxin‐producing Escherichia coli
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
4.1. Abstract
In 2016, 6,378 confirmed cases of Shiga toxin‐producing Escherichia coli (STEC) infections were reported in the EU. The EU notification rate was 1.82 cases per 100,000 population, which was an 8.3% increase compared with 2015. The EU notification rate following the large outbreak in 2011 was higher in 2012–2016 than before the outbreak. Over the last 5‐year‐period from 2012 to 2016, the trend has been stable, but at a higher level than before 2011. In 2016, 10 deaths due to STEC infection were reported, which resulted in an EU case fatality of 0.3%.
As in previous years, the most commonly reported STEC serogroup in 2016 was O157 (38.6%) although its relative proportion compared with other non‐O157 serogroups declined. This is possibly an effect of increased awareness and of more laboratories testing for other serogroups. Serogroup O157 was followed by O26, which has increased in the last 3 years, since 2014. In 2016, for the first time, serogroup O26 was the most frequently reported cause of haemolytic uraemic syndrome (HUS) instead of serogroup O157. These observations were also made for the food and animal testing results: in 2016 MS reported less STEC O157 and more STEC non‐O157 serogroups, with STEC O26 being the most reported serogroup in 2016 for food samples. This may be explained by the more widespread use by laboratories of the international standard ISO TS 13136:2012, which is unbiased in identifying specific STEC serogroups. In 2016, 91.5% of the food samples were reported to have been tested by the international standard ISO TS 13136:2012 or equivalent methods, constituting a major achievement.
During 2016, 18,975 units of food (batches or single samples) and 2,496 units from animals (animals or herds or flocks) were tested for the presence of STEC, by 21 MS and one non‐MS. The amount of data reported on foods was comparable with previous years, whereas only about one‐third of the data on animals was reported as compared with 2015. While two‐thirds of the MS reported on the presence of STEC in foods, only a few MS reported monitoring results for each of the different food categories. This aspect is crucial and should be improved to obtain data suitable for making inference on the existence of specific trends in the geographical distribution of STEC. Although based on much smaller amounts of data as compared with 2015, a higher proportion of STEC‐positive samples was evidenced in sheep and goats (18.5%) as compared to cattle, as also observed in 2015.
4.2. Surveillance and monitoring of Shiga toxin‐producing Escherichia coli in the EU
4.2.1. Humans
The notification of Shiga toxin‐producing E. coli (STEC)20 infections is mandatory in most MS, Iceland, Norway and Switzerland, except for six MS, where notification is based on a voluntary system (Belgium, France, Italy, Luxembourg and Spain) or other system (the United Kingdom). The surveillance systems for STEC infections cover the whole population in all MS except three (France, Italy and Spain). The notification rates were not calculated in these three countries for the following reasons: (a) in France, the STEC surveillance is based on paediatric HUS; (b) in Italy, STEC surveillance is sentinel and primarily based on the HUS cases through the national registry of HUS; and (c) no estimation for population coverage of STEC cases was provided in Spain.
Diagnosis of human STEC infections is generally performed by culture from stool samples and indirect diagnosis by the detection of antibodies against the O‐lipopolysaccharides E. coli in serum in cases of HUS. Diagnosis by direct detection of the toxin or the toxin genes by PCR without strain isolation is increasing.
4.2.2. Food and animals
Monitoring and surveillance data reported from sprouts in the framework of EU Regulation 2073/2005 on microbiological criteria
In the aftermath of the large outbreak of STEC O104:H4 infections that occurred in Germany and France in 2011, associated with the consumption of fenugreek sprouts and involving about 4,000 cases with 800 HUS and 52 deaths (Bielaszewska et al., 2011), the European Commission released an amendment to Regulation (EC) No 2073/2005 issuing a microbiological criterion for sprouted seeds. This food safety criterion prescribes that sprouted seed monitoring results must be compliant with ‘absence in 25 grams’, of STEC O157, O26, O111, O103, O145 and O104:H4, at retail (Regulation (EC) No 209/201321). The STEC monitoring data for sprouted seeds that EFSA receives consist of data originating from the reporting obligations of MS under the EU Regulation on microbiological criteria. These data are not generated by fully harmonised schemes across MS. The reason is that, although the matrices sampled are harmonised and the sampling and analytical methods are indicated in the regulation, the sampling objectives, the place of sampling and the sampling frequency vary or are interpreted differently between MS and according to food types. As such, the STEC monitoring data on sprouted seeds are not fully comparable across MS. Most of these data concerns the food chain control (official monitoring) and are collected by the national competent authorities conducting investigations to verify whether food business operators implement correctly the legal framework of own‐control programmes, as well as the analyses, as part of HACCP (industry monitoring) according to the General Food Law principles. Industry data are seldom reported to EFSA because of data ownership sensitivities. In essence, food chain control data are compliance checks and are collected with the aim of provide an early warning system and initiate control measures. Although they can be used for descriptive summaries to be made at EU‐level and also for EU‐level trend watching, these data are unsuitable for trends analyses, because a reference (study) population is mostly absent and because sampling is risk based and, therefore, non‐representative. In addition, the data sources are not transparently documented, as industry IT‐based traceability solutions are currently not mandatory and companies may store data in arbitrary formats, including non‐digital ones, as evidenced during food‐borne disease outbreaks.
Other STEC monitoring data from foods and animals
The monitoring data on STEC in foods other than sprouted seeds, and in animals, originate from the reporting obligations of MS under Directive 2003/99/EC, which stipulates that MS must monitor STEC at the most appropriate stage or stages of the food chain. The Directive is not explicit about the sampling strategy and the data generated by MS are based on investigation with non‐harmonised sampling methods and obtained with different laboratory analytical tests. The Directive does not indicate strict details of the mandatory reporting requirements. Therefore STEC monitoring data according to Chapter II (‘monitoring of zoonoses and zoonotic agent's) of the Directive 2003/99/EC are not comparable among MS and preclude subsequent data analysis such as assessing temporal and spatial trends at the EU‐level. Sampling biases and imprecision due to limited numbers of specimens examined also preclude extending findings to reflect current prevalence or accurate prevalence estimations. Especially for STEC, the use by MS of laboratory analytical methods testing for one specific STEC serogroup would lead to biased STEC prevalence estimations or biased STEC serogroup frequency distributions when analysing data at the EU‐level. Nonetheless, descriptive summaries of sample statistics at EU‐level may be made.
To improve the quality of the data from STEC monitoring in the EU, EFSA issued technical specifications for the monitoring and reporting of STEC in animals and food in 2009 (EFSA, 2009a). Those guidelines were developed to facilitate the generation of more harmonised data, which would enable more thorough analysis of STEC in food and animals. The specifications encourage MS to monitor and report data on STEC serogroups that are considered by the BIOHAZ Panel as important in terms of human pathogenicity (EFSA, 2013).
4.2.3. Food‐borne outbreaks of human STEC infections
The reporting of FBO of human STEC infections is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
4.3. Data validation and analyses
4.3.1. Food and animals
Data validation
The STEC monitoring data from food and animals reported for the year 2016 to EFSA were verified as regards plausibility and reliability, in line with current domain knowledge. The occurrence of STEC in foods and animals and the frequency distribution of STEC serogroups was then descriptively analysed. The following criteria were used to disclose possible implausible data, which were next reviewed by the MS.
The following plausibility criteria focused on the level of completion and coherence of the information and on the consistency of the laboratory results with the analytical method reported.
Plausibility of reported occurrence values with respect to the specific STEC epidemiology through revision of the updated scientific literature.
Consistency of the reported laboratory results within the objective of the STEC monitoring data collection. An example of data not consistent with the objective of data collection is the reporting of pathogenic E. coli with negative results for stx‐genes testing.
Consistency of the reported laboratory results with the analytical method reported. An example was the reporting of STEC O26 or other non‐O157 STEC serogroups for samples assayed with the standard ISO 16654:2001, or equivalent methods, that can only detect serogroup O157.
A reliability criterion has been used to identify those data that did not match (partly or totally) the current scientific knowledge on STEC epidemiology. A reliability criterion was the consistency between 2016 STEC data reported by MS and their recent historical data. In addition, the reliability of the number of samples reported for STEC was verified. As an example, countries reporting the testing of more than 100,000 samples for STEC have been asked to double‐check their data. Lastly, high numbers and proportions of samples tested for animal species and/or food categories usually not considered ‘at risk’ for STEC have also been considered as eligible for a further check by the MS.
Data ‘recovery’: recoding
Data or information that were erroneously reported in free‐text variables were detected and recoded to augment the information value.
Data analysis
For the description of the proportion of STEC‐positive samples in the different food categories, the following data were excluded: data reported with a sampler ‘industry sampling’ or ‘HACCP and own checks’, or as sampling strategy; ‘selective sampling’ or ‘suspect sampling’, or having ‘clinical investigations’ as sampling context, or as outbreak data. In these instances a subset of all validated monitoring data was used. The full (without excluding any data) data set was used instead for any other descriptive analysis on STEC findings in food and animals, including those on the methods used and the serogroups’ frequency distributions.
The analysis of the data provided by the reporting countries on STEC detected during 2016 in food and animal samples has been carried out, like in the previous year, by dividing the analytical methods used in two main categories:
Methods aiming at detecting any STEC, regardless of the serotype. This category includes method ISO TS 13136:2012 (ISO, 2012) and other PCR‐based methods as well as methods based on the detection of verocytotoxin production by immunoassays.
Methods designed to detect only STEC O157, such as method ISO 16654:2001 (ISO, 2001) and the equivalent methods NMKL 164:2005 (NMKL, 2005) and DIN 1067:2004‐03 (DIN, 2004).
Such distinction was necessary when analysing the frequency of the STEC serogroup to minimise any bias introduced by the use of methods directed towards the isolation of specific serogroups such as O157, and which would not allow the identification of other STEC serogroups possibly present in the samples.
4.4. Results
4.4.1. Overview of key statistics along the food chain, EU, 2012–2016
Table 13 summarises EU‐level statistics related to human STEC infections, and to the occurrence and prevalence of STEC in food and animals, respectively, in the EU, during 2012–2016. A more detailed description of these statistics is presented in the results section of this chapter and in the chapter on FBO.
Table 13.
Summary of STEC statistics related to humans, major food categories and major animal species, EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 6,378 | 5,929 | 5,900 | 6,042 | 5,680 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 1.82 | 1.68 | 1.75 | 1.80 | 1.70 | ECDC |
| Number of reporting MS | 28 | 28 | 27 | 27 | 27 | ECDC |
| Infection acquired in the EU | 3,994 | 3,991 | 3,959 | 3,916 | 3,678 | ECDC |
| Infection acquired outside the EU | 342 | 532 | 474 | 485 | 543 | ECDC |
| Unknown travel status or unknown country of infection | 2,042 | 1,406 | 1,467 | 1,641 | 1,459 | ECDC |
| Total number of food‐borne outbreaks (including waterborne outbreaks) | 42 | 69 | 67 | 74 | 41 | EFSA |
| Number of outbreak‐related cases | 735 | 674 | 957 | 633 | NA | EFSA |
| Food | ||||||
| Meat and meat products | ||||||
| Number of sampled units | 9,242 | 10,385 | 8,576 | 11,024 | 11,876 | EFSA |
| Number of reporting MS | 18 | 16 | 16 | 19 | 18 | EFSA |
| Milk and milk products | ||||||
| Number of sampled units | 4,119 | 4,518 | 6,811 | 4,933 | 4,606 | EFSA |
| Number of reporting MS | 12 | 11 | 12 | 13 | 12 | EFSA |
| Fruits and vegetables (and juices) | ||||||
| Number of sampled units | 1,543 | 2,052 | 2,054 | 3,250 | 2,025 | EFSA |
| Number of reporting MS | 11 | 13 | 13 | 13 | 12 | EFSA |
| Animals | ||||||
| Bovine animals | ||||||
| Number of sampled herds | 62 | 49 | 1,178 | 1,307 | 1,664 | EFSA |
| Number of reporting MS | 2 | 2 | 5 | 4 | 4 | EFSA |
| Small ruminants | ||||||
| Number of sampled herds | 208 | 109 | 44 | 11 | 58 | EFSA |
| Number of reporting MS | 8 | 7 | 7 | 7 | 6 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States; NA: Not available/not reported; STEC: Shiga toxin‐producing Escherichia coli.
Humans
Table 13 summarises EU‐level statistics related to human STEC infections as reported to ECDC and EFSA on the FBOs. More detailed description of statistics are in the results section and also in the FBO chapter. It is noteworthy that the number of human STEC cases infected domestically and through travel within the EU has tended to be at a stable level since 2013, after an increase from 2012 to 2013. The statistics for FBO due to STEC show that the number of outbreak‐related cases fluctuates around 600–700 with a peak during 2014 (957 cases) and the total number of outbreaks tends to decrease since 2012.
Food categories
Data notified by reporting MS over the period 2012–2016 were aggregated in macrocategories to get an overview, by year, of the amount of data sent for each category, the number of reporting MS and the percentage of positive sampled units.
For all food categories, the numbers of sampled units reported for 2016 is lower compared with the previous years 2015, 2014, 2013 and 2012. Only in year 2014 for ‘meat and meat products’ was the number of sampled units reported lower than in 2016. The number of reporting MS is fairly stable for every food group.
For the year 2016, 19 MS plus Switzerland provided results from the analysis of 18,975 food units (batches or single samples). The proportion of food and animal samples reported by MS and non‐MS and tested for STEC by the different analytical methods is in the Appendix.
Animal categories
For animals, the number of reported sampled herds of ‘small ruminants’ progressively increased during 2012–2016 and the number of reporting MS was about seven. For the category ‘cattle’, the number of sampled herds/flocks decreased over the period 2012 to 2016 as did the number of reporting MS.
For the year 2016, 2,496 units from animals (animals or herds or flocks) tested for the presence of STEC were reported by nine MS. This number represents a very noticeable decrease in the numbers of animal samples reported, considering that in the period 2013–2015 an average of 6,200 sample units were reported. Seven reporting MS used the methods indicated in EFSA technical specifications for the monitoring and reporting of STEC (EFSA, 2009a), while the remaining two MS did not specify the methods used to test animal samples. The standard methods ISO TS 13136:2012 (ISO, 2012), ISO 16654:2001 (ISO, 2001), NMKL 164:2005 (NMKL, 2005) and DIN 1067:2004‐03 (DIN, 2004), which are intended for testing food and feed, have been adapted to test animal samples by the reporting countries, following EFSA recommendations (EFSA, 2009a).
4.4.2. STEC infections in humans
In 2016, 6,548 cases of STEC infections, including 6,378 confirmed cases, were reported in the EU (Table 14). Twenty‐five MS reported at least one confirmed STEC case and three MS reported zero cases. The EU notification rate was 1.82 cases per 100,000 population, which was an 8.3% increase compared with 2015 (1.68 cases per 100,000 population). The highest country‐specific notification rates were observed in Ireland, Sweden, the Netherlands and Denmark (15.60, 6.48, 3.92 and 3.68 cases per 100,000 population, respectively). Six countries (Bulgaria, Greece, Latvia, Poland, Portugal and Slovakia) reported ≤ 0.1 cases per 100,000 population.
Table 14.
Reported human cases of STEC infections and notification rates per 100,000 population in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 177 | 177 | 2.04 | 107 | 1.25 | 131 | 1.54 | 130 | 1.54 | 130 | 1.55 |
| Belgium | Y | C | 34 | 34 | 0.30 | 100 | 0.89 | 85 | 0.76 | 117 | 1.05 | 105 | 0.95 |
| Bulgaria | Y | A | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 |
| Croatia | Y | A | 9 | 9 | 0.21 | 0 | 0.00 | 4 | 0.09 | 0 | 0.00 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic | Y | C | 28 | 28 | 0.27 | 26 | 0.25 | 29 | 0.28 | 17 | 0.16 | 9 | 0.09 |
| Denmark | Y | C | 275 | 210 | 3.68 | 201 | 3.55 | 226 | 4.02 | 191 | 3.41 | 199 | 3.57 |
| Estonia | Y | C | 5 | 5 | 0.38 | 8 | 0.61 | 6 | 0.46 | 8 | 0.61 | 3 | 0.23 |
| Finland | Y | C | 144 | 139 | 2.53 | 74 | 1.35 | 64 | 1.17 | 98 | 1.81 | 32 | 0.59 |
| Franceb | N | C | 332 | 302 | – | 262 | – | 221 | – | 218 | – | 208 | – |
| Germany | Y | C | 1,867 | 1,843 | 2.24 | 1,616 | 1.99 | 1,663 | 2.06 | 1,639 | 2.00 | 1,573 | 1.93 |
| Greece | Y | C | 2 | 2 | 0.02 | 1 | 0.01 | 1 | 0.01 | 2 | 0.02 | 0 | 0.00 |
| Hungary | Y | C | 12 | 12 | 0.12 | 15 | 0.15 | 18 | 0.18 | 13 | 0.13 | 3 | 0.03 |
| Ireland | Y | C | 745 | 737 | 15.60 | 598 | 12.92 | 572 | 12.42 | 564 | 12.29 | 412 | 8.99 |
| Italyb | N | C | 91 | 78 | – | 59 | – | 68 | – | 64 | – | 50 | – |
| Latvia | Y | C | 1 | 1 | 0.05 | 4 | 0.20 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lithuania | Y | C | 4 | 4 | 0.14 | 3 | 0.10 | 1 | 0.03 | 6 | 0.20 | 2 | 0.07 |
| Luxembourg | Y | C | 4 | 4 | 0.69 | 4 | 0.71 | 3 | 0.55 | 10 | 1.86 | 21 | 4.00 |
| Malta | Y | C | 4 | 4 | 0.92 | 4 | 0.93 | 5 | 1.18 | 2 | 0.48 | 1 | 0.24 |
| Netherlands | Y | C | 665 | 665 | 3.92 | 858 | 5.08 | 919 | 5.46 | 1,184 | 7.06 | 1,049 | 6.27 |
| Poland | Y | C | 8 | 4 | 0.01 | 0 | 0.00 | 5 | 0.01 | 5 | 0.01 | 3 | 0.01 |
| Portugal | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | – | – | – | – | – | – |
| Romania | Y | C | 49 | 29 | 0.15 | 0 | 0.00 | 2 | 0.01 | 6 | 0.03 | 1 | 0.01 |
| Slovakia | Y | C | 2 | 2 | 0.04 | 1 | 0.02 | 2 | 0.04 | 7 | 0.13 | 9 | 0.17 |
| Slovenia | Y | C | 27 | 27 | 1.31 | 23 | 1.11 | 29 | 1.41 | 17 | 0.83 | 29 | 1.41 |
| Spainc | N | C | 51 | 51 | – | 86 | – | 50 | – | 28 | – | 32 | – |
| Sweden | Y | C | 638 | 638 | 6.48 | 551 | 5.65 | 472 | 4.89 | 551 | 5.77 | 472 | 4.98 |
| United Kingdom | Y | C | 1,374 | 1,373 | 2.10 | 1,328 | 2.05 | 1,324 | 2.06 | 1,164 | 1.82 | 1,337 | 2.11 |
| EU total | – | – | 6,548 | 6,378 | 1.82 | 5,929 | 1.68 | 5,900 | 1.75 | 6,042 | 1.80 | 5,680 | 1.70 |
| Iceland | Y | C | 3 | 3 | 0.90 | 1 | 0.30 | 3 | 0.92 | 3 | 0.93 | 1 | 0.31 |
| Norway | Y | C | 239 | 239 | 4.59 | 221 | 4.28 | 151 | 2.96 | 103 | 2.04 | 75 | 1.50 |
| Switzerlandd | Y | C | 463 | 463 | 5.54 | 308 | 3.72 | 125 | 1.51 | 82 | 1.00 | 66 | 0.82 |
Y: yes; N: no; A: aggregated data; C: case‐based data; −: no report.
Sentinel surveillance; primarily based on HUS cases.
Sentinel surveillance; no information on estimated coverage, so notification rate cannot be estimated.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The majority of the STEC cases reported were from infections in EU (62.6% domestic cases and travel in the EU, 5.4% travel outside EU and 32% of unknown importation or unknown country of infection) (Table 13). Three Nordic countries – Finland, Norway and Sweden – reported the highest proportion of travel‐associated cases (36.8%, 27.5% and 25.0%, respectively). Among 516 travel‐associated cases with known probable country of infection, 67.7% of the cases represented travel outside EU and 32.3% travel within EU. Turkey was the most frequently reported probable country of infection, followed by Spain and Italy (15.9%, 7.4% and 4.3%, respectively).
There was a clear seasonal trend in confirmed STEC cases in the EU/EEA between 2008 and 2016, with more cases reported during the summer months (Figure 26). There was a statistically significant (p < 0.01) increasing trend for STEC in the EU/EEA in 2008–2016, however results of statistical testing of trends for this period should be interpreted with caution due to a large outbreak in 2011. In the years after the outbreak (2012–2016), the overall EU/EEA trend did not show any significant increase or decrease (Figure 26).
Figure 26.
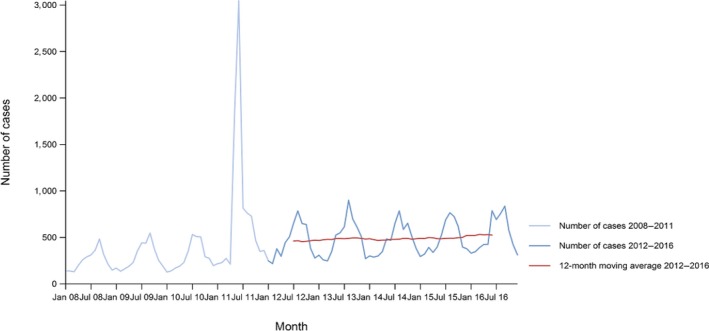
Trend in reported confirmed cases of human STEC infection in the EU/EEA, by month, 2012–2016
- Source(s): Austria, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom. Belgium, Bulgaria, the Czech Republic, Croatia and Portugal did not report data to the level of detail required for analysis.
In 2008–2016, a significant increasing trend (p < 0.05) was observed in nine MS (Finland, France, Hungary, Ireland, Italy, Lithuania, the Netherlands, Slovenia and Sweden). Two MS (Cyprus and Slovakia) observed decreasing trends. Over the 5‐year period in 2012–2106, six MS (Finland, France, Ireland, Malta, Romania and Spain) continued to report significantly increasing trends (p < 0.01), and three MS (Luxembourg, the Netherlands and Slovakia) had decreasing trends.
Eighteen MS provided information on hospitalisation for 42.6% of all confirmed STEC cases in the EU in 2016. This number was four countries more than in 2015. Out of the 2,720 cases with known hospitalisation status, 34.6% were hospitalised. The highest proportions of hospitalised cases (91–100%) were reported in Estonia, Greece, Italy, Latvia, Malta, Poland and Slovakia. Three‐hundred and ninety cases of HUS were reported, with the majority of patients in the youngest age groups from 0–4 years (226 cases; 59%) to 5–14 years (76 cases; 20%). The most common serogroups among HUS cases were O26 (33.0%), O157 (24.8%), O80 (9.6%) and O111 (5.0%); while 7.6% were untypable.
In 2016, 10 deaths due to STEC infection were reported in the EU compared with eight in 2015. Seven MS reported one to three fatal cases each, and 13 MS reported no fatal cases. This reporting resulted in an EU case fatality of 0.3% among the 3,756 confirmed cases with known outcome (58.9% of all reported confirmed cases). The serogroup associated with more fatal cases was O157 (three cases). The serogroups O8, O26, O55 and O111 were linked to one fatal case each. For three fatal cases, the serogroup was not specified.
4.4.3. STEC in foods
Monitoring and surveillance data reported from sprouted seeds in the framework of EU Regulation 2073/2005 on microbiological criteria
In 2016, eight MS reported STEC monitoring data of sprouted seeds at the retail level, for a total of 344 units tested. This amount of data is slightly below the average when compared with previous years (Table 15).
Table 15.
STEC sprouted seeds monitoring results at retail, EU, 2013–2016
| Sprouted seeds | Number of reporting MS | Sample units tested | Sample units positive (%) |
|---|---|---|---|
| 2013 | 6 | 444 | 0 (0.0) |
| 2014 | 6 | 481 | 0 (0.0) |
| 2015 | 7 | 576 | 1 (0.2) |
| 2016 | 8 | 344 | 1 (0.3) |
MS: Member State; STEC: Shiga toxin‐producing Escherichia coli.
For the year 2016, one non‐compliant batch was reported. The isolated strain was reported to be negative for the eae gene and no information on the serogroup was provided.
Occurrence in food
Meat and meat products
Fresh bovine meat
In 2016, 12 MS provided data from 2,055 units of fresh bovine meat tested for STEC with 1.6% of these being positive (0.2% for STEC O157). More than half of the reported data were from two MS (Belgium and the Netherlands). The proportion of positive units sampled was 2.4% at processing plant, 1.8% at slaughterhouse and 0.9% at retail. From all samples, only four single samples, which were reported by the Netherlands, were STEC O157 positive: these samples were from the retail level with no information reported on their country of origin.
For 30 isolates, the information on the serogroup of the STEC strain was provided. The serogroups most frequently reported in bovine meat (including all types of bovine meat) were O157 (11 isolates), O113 (6 isolates), O26 (4) O145 (1) O174 (1) and ‘other’. Most of these serogroups are reported as the causes of human disease (EFSA and ECDC, 2016c), confirming the importance of this food category in the epidemiology of STEC infections.
Fresh sheep and goat meat
Five MS reported investigational results on 354 sample units of fresh sheep meat tested for STEC with 15.5% of them being positive. Almost all units tested were reported by two MS only. Two MS reported on fresh goat meat with four samples STEC positive out of the 28 sample units tested (14.3%).
The analysis of the serogroups, carried out including all types of sheep and goat meat, indicated that the most frequently isolated STEC strains belonged to the O146 serogroup (5 isolates, 21.7% of the 23 isolates with information on the serogroup), followed by O6 (3 strains reported) and O113 (2 strains reported). As in the previous year, STEC O157 was not the most prevalent STEC serogroup in this food category, with only one isolate reported.
Fresh meat from other ruminants
One MS provided information on the presence of STEC in 33 samples from deer meat. Seven samples proved positive for non‐O157 STEC with STEC O146 being the most represented STEC serogroup (four isolates).
Fresh meat from other animal species
Four MS provided information on 307 samples of fresh pig meat tested and 10 (3.3%) were positive for the presence of STEC. No STEC O157 isolates were found in 2016.
Four MS reported on the analyses carried out on 237 samples of food from animal species other than cattle, sheep, goat, pigs and deer. These samples included those taken from horses, poultry, rabbits and wild boar and 8.5% of the 270 sampling units were STEC positive with all isolates belonging to non‐O157 serogroups. Information on the serogroup of the isolated STEC was provided for 23 isolates and included serogroups O26 (two isolates) and O111 (two isolates), which are part of the ‘top five’ STEC serogroups, associated with severe diseases in humans, among others.
Data on the presence of STEC in meat from broilers and turkeys have been reported by three MS. In total, 197 samples from meat from turkeys and 220 from meat from broilers were tested, with only one STEC non‐O157 reported in fresh meat from broilers.
Milk and milk products
In 2016, seven MS reported monitoring results of 863 sample units of raw cows’ milk. STEC have been isolated from 16 units (1.9%), all belonging to non‐O157 serogroups. The detected STEC serogroups in raw cows’ milk were O26 (two samples) followed by O22, O103, O116, O150 and O179 with one isolate each. Information on the serogroup was not reported for 11 out of the 18 isolates present in the full data set.
Three MS reported monitoring results of seven sample units of raw goats’ milk, and one MS reported on one sample of raw sheep’ milk. No isolation of STEC was reported.
Eight MS provided STEC monitoring data on 1,515 sample units of ‘milk and dairy products excluding raw milk’. Most samples were from cheeses (91%) followed by other ‘dairy products other than cheese’ (8.7%) and treated or fermented milk (0.3%). In total, 37 sample units were positive for STEC with 36 of these from cheeses and one from butter made from raw or low‐heat‐treated milk. One sample of cheese from raw cows’ milk cheese was positive for STEC O157. The most prevalent STEC non‐O157 serogroup identified was O26 (seven isolates).
Vegetables
Nine MS reported data on the testing of 925 sample units of vegetables for the presence of STEC, all with negative results.
Fruits
STEC‐negative results were provided by five MS resulting from the analysis of 146 fruit specimens.
Other foodstuffs
This category comprises miscellaneous food commodities, including cereals and meals, bakery products, non‐alcoholic beverages, juices, crustaceans and molluscan shellfish, eggs, fish, RTE salads, sauces and dressing, dried seeds and fresh and dried spices and herbs, infant formulas and foodstuffs intended for special nutritional uses.
For the whole category, 3,353 samples were analysed with 1% of positive specimens reported. The main serogroup identified was O146 (12.9% of the 31 isolates with information on the serogroup reported), whereas no STEC O157 serogroup was identified.
4.4.4. STEC in animals
Overall, the presence of STEC was reported in 12.7% of the 2,496 units from animals (animals or herds or flocks) for 2016.
As in the previous year, the highest proportions of STEC‐positive sample units, calculated on the full data set (all reported data), were reported from ruminants other than cattle (70.8% for goats and sheep and 20.2% for other ruminants) followed by pigs (11.8%), cattle (5%) and other animals (4.7%).
Most relevant results for the animal categories for the epidemiology of STEC infections are presented below.
Cattle
Two MS reported on 1,057 units of cattle tested with positive findings for presence of STEC. In total, 3.5% of the sample units were positive for STEC and 1.2% for STEC O157 serogroup. Interestingly, one MS reported all the STEC O157‐positive samples and used methods aimed at detecting only this STEC serogroup. All the other STEC‐positive samples reported belonged to unspecified serogroups.
When the analyses on the serogroups were carried out considering the full data set for cattle, with no restrictions on the sampling context or the methods used, eight additional STEC O157 isolates, one O26 isolate and one O103 STEC isolate were identified.
Sheep and goats
In 2016, only one MS reported on analysis on STEC in 20 samples of sheep taken at the farm, with no positive results.
By analysing the data regardless their sampling context or the methods used for the tests, 267 samples from sheep and goats were reported from seven MS with 206 of them from one single MS. One hundred and eighty‐nine positive specimens were identified, including four STEC O157 isolates. As for non‐O157 serogroups, O146, O76 and O113 were the most represented (21.7%, 17.2% and 6.7% of the 180 isolates with serogroup reported, respectively).
Pigs and other animal species
Pigs were tested by one MS that reported no positive results from the two samples. When using the full data set, and any analytical method reported for serogroup analyses, two samples (11.8%) from pigs out of a total of 17 were found to be positive for STEC O157; both samples were reported to have been tested with the OIE adaptation from the ISO 16654:2001 (ISO, 2001) method, which only detects this STEC serogroup.
In 2016, one MS reported on the presence of STEC in bats, Cantabrian chamois, deer, dogs, ibex (steinbock) and wild boar. In total, 356 samples have been analysed, with 27 of these positive for the STEC non‐O157 serotype (7.58%).
Additional positive samples were also reported from dogs, cats and from broilers. In total, 14 STEC O157 isolates were reported from dogs, cats and broilers. Twenty‐six non‐O157 STEC isolates were reported with two STEC O103 isolates from a dog and a tiger, STEC O111 (two isolates), STEC O145 (two isolates) and STEC O26 (two isolates) from dogs. Finally, two STEC isolates were reported from dogs, for which only the information ‘non‐O157’ was specified, and 16 STEC were reported with unspecified serogroup isolated from an ibex, broilers and bats.
4.4.5. Serogroups in humans, food and animals
Humans
Data on STEC serogroups (based on O antigens) were reported in 2016 by 22 MS, Iceland and Norway. As in previous years, the most commonly reported serogroup was O157, which accounted for 38.6% of cases with known serogroup, although its proportion continued to decrease (Table 16). The proportion of the second most common serogroup, O26, increased in 2016 compared with 2015 and 2014. Serogroup O157 and O26 were followed by serogroups O103, O146, O91, O145 and O128. One new serogroup (O63) entered and one serogroup (O78) was dropped from, the ‘top 20 list’ in 2016. The proportion of non‐typeable STEC strains declined in 2016 compared with 2015 to the same level as in 2014, representing 8.3% of the reported cases with known serogroup.
Table 16.
Distribution of the 20 most frequent serogroups reported in confirmed cases of human STEC infections in EU/EEA, 2014–2016
| Serogroup | 2016 | 2015 | 2014 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MS | % | Cases | MS | % | Cases | MS | % | |
| O157 | 1,553 | 22 | 38.6 | 1,510 | 21 | 42.1 | 1,692 | 23 | 47.0 |
| O26 | 671 | 19 | 16.7 | 537 | 16 | 15.0 | 444 | 16 | 12.3 |
| NTa | 335 | 12 | 8.3 | 397 | 10 | 11.1 | 265 | 9 | 7.4 |
| O103 | 218 | 18 | 5.4 | 172 | 14 | 4.8 | 192 | 12 | 5.3 |
| O146 | 159 | 11 | 4.0 | 74 | 10 | 2.1 | 82 | 9 | 2.3 |
| O91 | 150 | 11 | 3.7 | 114 | 12 | 3.2 | 105 | 11 | 2.9 |
| O145 | 121 | 12 | 3.0 | 95 | 12 | 2.6 | 105 | 11 | 2.9 |
| O128 | 65 | 13 | 1.6 | 49 | 12 | 1.4 | 47 | 11 | 1.3 |
| O113 | 60 | 11 | 1.5 | 25 | 7 | 0.7 | 31 | 10 | 0.9 |
| O111 | 57 | 14 | 1.4 | 42 | 11 | 1.2 | 54 | 11 | 1.5 |
| O80 | 42 | 8 | 1.0 | 24 | 4 | 0.7 | 15 | 3 | 0.4 |
| O55 | 34 | 10 | 0.8 | 28 | 8 | 0.8 | 37 | 11 | 1.0 |
| O117 | 29 | 7 | 0.7 | 23 | 7 | 0.6 | 21 | 8 | 0.6 |
| O5 | 29 | 7 | 0.7 | 21 | 6 | 0.6 | 16 | 7 | 0.4 |
| O‐roughb | 26 | 4 | 0.6 | 44 | 8 | 1.2 | 54 | 7 | 1.5 |
| O182 | 25 | 6 | 0.6 | 24 | 5 | 0.7 | 13 | 5 | 0.4 |
| O8 | 25 | 10 | 0.6 | 20 | 9 | 0.6 | 15 | 7 | 0.4 |
| O121 | 24 | 5 | 0.6 | 17 | 4 | 0.5 | 31 | 6 | 0.9 |
| O63 | 24 | 4 | 0.6 | 8 | 4 | 0.2 | 24 | 6 | 0.7 |
| O27 | 22 | 3 | 0.5 | 16 | 4 | 0.4 | 9 | 3 | 0.2 |
| O76 | 21 | 7 | 0.5 | 31 | 9 | 0.9 | 21 | 7 | 0.6 |
| O177 | 16 | 6 | 0.4 | 23 | 5 | 0.6 | 14 | 8 | 0.4 |
| Other | 313 | – | 7.8 | 296 | – | 8.2 | 314 | – | 8.7 |
| Total | 4,019 | 24 | 100.0 | 3,590 | 21 | 100.0 | 3,601 | 24 | 100.0 |
MS: Member State; STEC: Shiga toxin‐producing Escherichia coli.
Non‐typeable STEC include strains for which the laboratory tried, but was not able, to define the O serogroup. This depended on the numbers of sera/molecular tools that are included in the typing panel.
O‐rough strains lack the O‐chains in the lipopolysaccharide, leading to autoagglutination in the agglutination tests used to determine serogroup or serotype.
Source(s): 23 MS and 2 non‐MS: Austria, Belgium, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom.
Food
The proportion of food samples that was positive for the so‐called top five STEC serogroups (O157, O26, O103, O111 and O145) was estimated by considering only the reported STEC monitoring results that mentioned the use of laboratory analytical method TS 13136:2012 (ISO, 2012). The scope of this standard is to detect any STEC, and additionally, it allows identification of the ‘top 5’ serogroups. This subset of data can be considered homogeneous and may facilitate a more comparable estimate of the level of contamination of the different food categories with these STEC serogroups. Since the publication of the standard in 2012, there has been an increasing trend in its adoption by the MS for the food testing, with a proportion of food samples tested using the ISO TS 13136:2012 standard (ISO, 2012) in 2016 of 91.5%.
The top five serogroups most commonly reported in foods were: O26 (0.1% of 17,364 samples tested and 5.1% of the positive specimens), O157 (0.07% of 17,364 samples tested and 3.2% of the positive specimens), O103 (0.02% of 17,364 samples tested), O111 (0.01%) and O145 (0.01%) (Table 17).
Table 17.
Proportion of positive samples for any STEC and STEC belonging to the ‘top‐5’ serogroups in food categories in reporting Member States and reporting non‐Member States, 2016a
| Food categoryb | Samples tested by ISO 13136 | Samples positive for | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any STEC | O157 | O26 | O145 | O103 | O111 | ||||||||
| n | n | % | n | % | n | % | n | % | n | % | n | % | |
| Bovine meat | 4,852 | 98 | 2.02 | 6 | 0.12 | 4 | 0.08 | 1 | 0.02 | 0 | 0.00 | 0 | 0.00 |
| Ovine and goat meat | 413 | 62 | 15.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Other ruminants meatc | 48 | 8 | 16.67 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Pig meat | 938 | 24 | 2.56 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Other meatd | 2,263 | 63 | 2.78 | 3 | 0.13 | 2 | 0.09 | 0 | 0.00 | 1 | 0.04 | 2 | 0.09 |
| Mixed meat | 100 | 8 | 8.00 | 0 | 0.00 | 4 | 4.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Milk and dairy productse | 2,399 | 34 | 1.42 | 3 | 0.13 | 7 | 0.29 | 0 | 0.00 | 2 | 0.08 | 0 | 0.00 |
| Raw milkf | 1,188 | 21 | 1.77 | 0 | 0.00 | 2 | 0.17 | 0 | 0.00 | 1 | 0.08 | 0 | 0.00 |
| Fruit and vegetable | 1,359 | 9 | 0.66 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Seedsg | 433 | 1 | 0.23 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Other food | 3,371 | 42 | 1.25 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Total | 17,364 | 370 | 2.13 | 12 | 0.07 | 19 | 0.11 | 1 | 0.01 | 4 | 0.02 | 2 | 0.01 |
n: number of samples; STEC: Shiga toxin‐producing Escherichia coli.
Only samples tested by the ISO/TS 13136 method are included.
n: number of samples; STEC: Shiga toxin‐producing Escherichia coli.
The different meat categories presented in this table include all type of meat (not only fresh).
Includes meat from deer.
Includes meat from other animals (other than ruminants and pigs).
Includes any type of dairy product, cheese and milk other than raw milk.
Includes raw milk from different species, but most of the tested and all the positive samples were from cows.
Includes only sprouted seeds.
In 2016, 18 MS plus Switzerland provided data on the detection of STEC in food obtained using method ISO TS 13136:2012 (ISO, 2012) on 17,364 out of the total 18,975 samples analysed. Three hundred and seventy samples (2.1%) gave positive results for the presence of STEC (Table 17).
The relative frequency of all the STEC serogroups identified in the reported sample units for 2016 was estimated by considering all the reported results regardless of the specified analytical method.
In total, 474 isolates were obtained from the 18,975 samples analysed (2.5%). For 138 isolates, only the information that the strain did not belong to the O157 serogroup was reported, while for 174 no information on the serogroup was provided. The remaining 162 positive samples were reported with information on the serogroup of the isolated strain. Eighteen STEC O157 isolates were reported, mainly from bovine meat (11 isolates), milk and dairy products (3 isolates), other meat (3 isolates) and sheep and goat meat (1 strain).
The relative frequency distribution of the non‐O157 STEC serogroups in the different food categories is shown in Table 17.
As for the remaining 144 STEC non‐O157 detected in 2016 (Tables 17 and 18), the main serogroup identified was O26 (4.0% of the total 474 STEC isolates, 13.2% of the 144 strains with an identified serogroup). This STEC serogroup was mainly detected in meat samples of different origin and from milk and dairy products. STEC O146 was the third serogroup reported (3.2% of the total 474 STEC isolates, 10.4% of the 144 strains with an identified serogroup) and was identified only in meat samples of different origin with the exception of bovine meat. Other STEC serogroups identified included O113 (6.3% of the 144 strains with an identified serogroup), O103 (2.8%), O91 (2.8%), O111 (1.4%) and O145 (0.7%). These STEC serogroups are all among the 15 most commonly reported groups in human infections in the EU in the period 2013–2016 (EFSA and ECDC, 2015).
Table 18.
Frequency distribution of non‐O157 STEC serogroups in food categories in reporting Member States, 2016a
| Food categoryb | STEC isolates with serogroup reported | STEC serogroups | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of total STEC isolates with serogroup reported in the specific food category | ||||||||||||||||
| n | O26 | O103 | O145 | O111 | O146 | O91 | O76 | O113 | O5 | O174 | O87 | O116 | O6 | Other serogroups (list) | ||
| Bovine meat | 30 | 13.3 | 0.0 | 3.3 | 0.0 | 0.0 | 3.3 | 3.3 | 20.0 | 0.0 | 3.3 | 0.0 | 0.0 | 0.0 | 53.3 | (O100, O108, O130, O139, O153, O171, O177, O182, O22, O39, O79, O8) |
| Ovine and goat meat | 23 | 0.0 | 0.0 | 0.0 | 0.0 | 21.7 | 4.3 | 4.3 | 8.7 | 0.0 | 0.0 | 8.7 | 0.0 | 13.0 | 39.1 | (O108, O14, O15, O168, O176, O38, O65) |
| Other ruminants meatc | 7 | 0.0 | 0.0 | 0.0 | 0.0 | 57.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 14.3 | 28.6 | (O153, O8) |
| Pig meat | 6 | 0.0 | 0.0 | 0.0 | 0.0 | 16.7 | 16.7 | 0.0 | 0.0 | 0.0 | 16.7 | 0.0 | 0.0 | 0.0 | 50.0 | (O119, O68, O8) |
| Other meatd | 23 | 8.7 | 4.3 | 0.0 | 8.7 | 4.3 | 0.0 | 4.3 | 4.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 65.2 | (O100, O101, O112, O121, O168, O188, O21, O23, O27, O38, O7, O77, O8) |
| Mixed meat | 4 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Milk and dairy productse | 13 | 53.8 | 15.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 30.8 | (O15, O177, O38, O8) |
| Raw milkf | 7 | 28.6 | 14.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 14.3 | 0.0 | 42.9 | (O150, O179, O22) |
| Fruit and vegetable | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Seeds | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Other food | 31 | 0.0 | 0.0 | 0.0 | 0.0 | 12.9 | 3.2 | 0.0 | 0.0 | 3.2 | 3.2 | 0.0 | 0.0 | 3.2 | 74.2 | (O100, O130, O166, O168, O181, O21, O27, O39, O55, O8, O81) |
| Total | 144 | 13.2 | 2.8 | 0.7 | 1.4 | 10.4 | 2.8 | 2.1 | 6.3 | 0.7 | 2.1 | 1.4 | 0.7 | 3.5 | 52.1 | (O100, O101, O108, O112, O119, O121, O130, O139, O14, O15, O150, O153, O166, O168, O171, O176, O177, O179, O181, O182, O188, O21, O22, O23, O27, O38, O39, O55, O65, O68, O7, O77, O79, O8, O81) |
n: number of isolates; STEC: Shiga toxin‐producing Escherichia coli.
Data originating from any analytical method are included.
Non‐O157 STEC serogroups are listed according to their public health relevance as a cause of human infections in the EU (EFSA, 2009a).
The different meat categories presented in this table include all type of meat (not only fresh).
Includes meat from deer.
Includes meat from animals other than ruminants and pigs.
Includes any type of dairy product, cheese and milk other than raw milk.
Includes raw milk from different species, but most of the tested samples and all the positive samples were from cows.
In 2016, an increasing trend of reporting for non‐O157 STEC serogroups was observed in food, in particular O26, in parallel with the decreasing trend in the reporting of STEC O157. This result confirms the findings for 2015.
STEC serogroup O8 was first reported in 2015 (0.43 per 1,000 samples analysed) and the reporting of this STEC serogroup continued in 2016 at a higher rate (0.58 per 1,000 samples analysed). Interestingly, this serogroup is one of the 20 most frequently isolated groups for human disease and reported to ECDC in 2015 (EFSA and ECDC, 2016c).
This result might be correlated with the increasing adoption of the ISO TS 13136:2012 analytical method, which aims at detecting any STEC, in contrast with the use of those specific for the STEC O157 serogroup, commonly used in previous years.
Animals
In total, 316 positive samples were reported, with information on serogroup provided for 231 isolates. Forty‐one STEC O157 (1.6%) were detected, with 25 of these from ruminants.
As regards the non‐O157 serogroups, 190 isolates were reported with information on the serogroup (Table 19). The most frequently reported groups were O146, O76, O113 and O5, all reported only in goats and sheep. STEC O26 was also commonly reported (3.7% of the total number of isolates with serogroup reported) from all animal categories except pigs and deer.
Table 19.
Frequency distribution of non‐O157 STEC serogroups in animals in reporting Member States, 2016a
| Animal category | STEC isolates with serogroup reported | STEC serogroups (g) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of total STEC isolates with serogroup reported in the specific animal category | ||||||||||||||
| n | O26 | O103 | O145 | O111 | O146 | O91 | O76 | O113 | O5 | O87 | O6 | Other serogroups (list) | ||
| Cattle | 2 | 50.0 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Goat and sheep | 180 | 2.2 | 0.6 | 0.0 | 0.0 | 21.7 | 0.6 | 17.2 | 6.7 | 5.0 | 1.1 | 3.3 | 41.7 | (O1, O104, O112, O12, O128, O134, O141, O148, O15, O153, O166, O175, O176, O178, O182, O21, O38, O43, O65, O77, O92) |
| Other ruminantsb | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Pigsc | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Other animalsd | 8 | 25.0 | 25.0 | 25.0 | 25.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Total | 190 | 3.7 | 2.1 | 1.1 | 1.1 | 20.5 | 0.5 | 16.3 | 6.3 | 4.7 | 1.1 | 3.2 | 39.5 | (O1, O104, O112, O12, O128, O134, O141, O148, O15, O153, O166, O175, O176, O178, O182, O21, O38, O43, O65, O77, O92) |
n: number of isolates; STEC: Shiga toxin‐producing Escherichia coli.
Data originating from any analytical method are included.
Non‐O157 STEC serogroups are listed according to their occurrence in the animal samples tested.
Includes only deer.
Includes also wild boar.
Includes birds, cats, dogs, fowl, solipeds and turkeys.
Atlases of STEC serogroups: food and animals
All data provided by MS and non‐MS was used to generate an atlas of the STEC serogroups’ frequencies in the different food and animal categories for the period 2012–2016 (Figure 27) as well as for the year 2016 separately for food (Figure 28) and animals (Figure 29). Other atlas in the Appendix shows the STEC serogroups’ frequencies in the different food and animal categories for the year 2016 by separate reporting country. It has to be emphasised that the differences in the sampling strategies, and to a lesser extent the analytical methods, applied by reporting countries do not allow confirmation of the existence of specific trends in the geographical distribution of STEC serogroups.
Figure 27.
Frequency distributions of reported STEC serogroups in food and animals, Member States and non‐Member States, during 2012 and 2016
- Note: Presence (red boxes) and absence of STEC serogroups in foods (left) and animals (right). STEC: Shiga toxin‐producing Escherichia coli.
Figure 28.
Relative presence of reported STEC serogroups in foods, Member States and non‐Member States, 2016
- STEC: Shiga toxin‐producing Escherichia coli.
- Proportions of STEC serogroups: red boxes > 1%, orange boxes > 0.1% and ≤ 1%, yellow boxes > 0.0001% and ≤ 0.1% of positive samples. White boxes indicate absence of the serogroup.
- (a) Other ruminants’ meat includes meat from deer.
- (b) Other meat includes meat from animals other than ruminants.
- (c) Milk and dairy products include any type of dairy product, cheese and milk other than raw milk.
- (d) Raw milk includes raw milk from different species but most of the tested, and all the positive, samples were from cows.
- (e) Seeds category includes mostly sprouted seeds, but dry seeds are also included.
- Sources: 19 MS and Switzerland.
Figure 29.
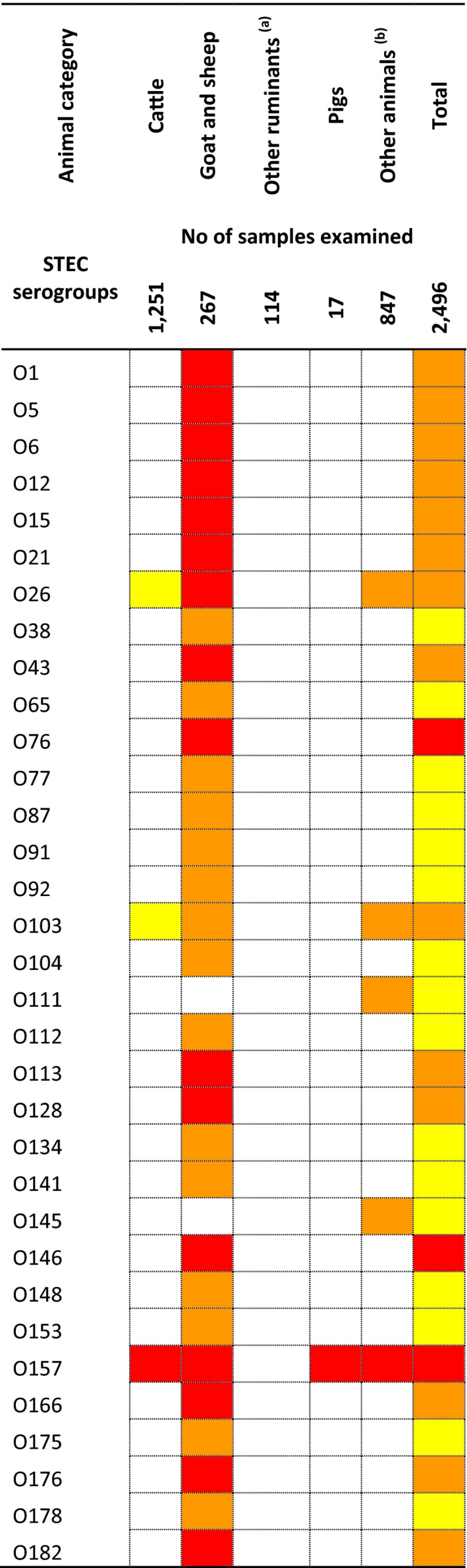
Relative presence of reported STEC serogroups in animals, Member States and non‐Member States, 2016
- STEC: Shiga toxin‐producing Escherichia coli.
- Proportions of STEC serogroups: red boxes > 1%, orange boxes > 0.1% and ≤ 1%, yellow boxes > 0.0001% and ≤ 0.1% of positive samples. White boxes indicate absence of the serogroup.
- (a) The animal category ‘other ruminants’ includes deer.
-
(b) The ‘other animal’ category comprises bats, Cantabrian chamois, deer, dogs, ibex and wild boar.Sources: 9 MS.
4.5. Discussion
STEC was the fourth most commonly reported zoonosis in the EU in 2016, and the trend for STEC infections increased from 2008 to 2016, which was mainly due to the large STEC outbreak in 2011. In the years after the outbreak (2012–2016), the overall trend of reported cases remained stable, and stayed at a markedly higher level than before the outbreak. Part of the increase may be explained by improved general awareness of STEC detection following the reported STEC outbreak. Other contributing factors are probably the increasing number of laboratories that are testing for serogroups other than O157 and the shift in diagnostic methods, with PCR being more commonly used for detection of STEC.
Of the STEC cases with known hospitalisation status, more than one‐third of cases were hospitalised. Some countries reported very high proportions of hospitalised cases, but had notification rates that were among the lowest, indicating that the surveillance systems in these countries primarily capture the most severe cases. As in previous years, the most commonly reported serogroup was O157, followed by O26. The proportion of serogroup O157 continued to decrease, whereas the proportion of serogroup O26 increased in the last 3 years since 2014. In 2016, for the first time, serogroup O26 was the most frequently reported cause of HUS, instead of serogroup O157. This highlights the importance of results showing serogroup O26 as the most common finding in food samples.
In 2016, data on the presence of STEC in food and animals were reported by 19 MS and Switzerland. Nine MS did not provide data and this represents one of the most critical aspects of the data collection. STEC are, in fact, included among pathogens of highest priority, as laid down in EU Directive 99/2003/EC.
Two‐thirds of the reporting MS plus Switzerland reported food monitoring data for STEC contamination in 2016. However, despite the expectation that monitoring activities should lead to a broad data collection, few MS reported on the presence of STEC in each of the different food categories (from one MS reporting monitoring data in meat from other ruminants up to 12 MS testing bovine meat). This observation is crucial and the reporting on at least the more epidemiologically relevant food commodities should be improved in order to obtain data suitable for making inferences on the existence of specific trends in the geographical distribution of STEC and their serogroups.
While for food the amount of data provided in 2016 was comparable to that provided in 2015, the amount of data on animal samples tested was far below that of the previous years, although the number of reporting countries remained approximately stable (EFSA and ECDC, 2015, 2016d).
The major breakthrough during 2016 related to the proportion of food samples tested by the ISO TS 13136:2012 standard, the reference method for the detection of STEC in food, or by equivalent methods ‐ methods detecting all STEC serogroups. In total, 91.5% of the samples tested during 2016 were tested by this reference method. The use of a common method meets the principles of standardisation and the data from 2016 brings the EU very close to the ideal goal of 100% of food samples analysed with an equivalent standard method, which allows a more homogenous analysis of the results and facilitates making inferences on the occurrence or prevalence of STEC. However, a major critical aspect is represented by the number of samples tested by the reporting countries for each food and animal category, which is highly variable; such an unequal distribution is likely to introduce selection bias in the estimates of STEC prevalence or STEC serogroup distribution, hindering spatial and temporal trend analyses.
Overall, the presence of STEC was reported in 2.5% of the 18,975 food samples tested and in 12.7% of the 2,496 animal samples tested. The highest proportion of positive food specimens was reported from meat samples, particularly from small ruminants (sheep and goat) followed by milk and dairy products. Such a finding consolidates the awareness of the importance of these food commodities in the spreading of STEC infections. Importantly, fruits and vegetables were contaminated with STEC at very low levels (below 1%), as observed in the previous 2 years (EFSA and ECDC, 2015, 2016a,b,c,d). For sprouted seeds, the sole food commodity for which a microbiological criterion for STEC has been set up in the EU, for products placed on the market during their shelf‐life (a food safety criterion), one non‐compliant batch was reported by one of the eight reporting MS.
Forty‐nine different STEC serogroups were reported in food samples of different origin for 2016. STEC O26 (5.1% of the positive specimens) was the most commonly reported serogroup in food with a higher specific proportion than that of STEC O157 (3.2% of the positive specimens), when considering the samples tested with the ISO TS 13136:2012 method (ISO, 2012) as the denominator. Finally, among the 13 serogroups detected with a frequency between 0.7% and 13.2%, eight were included among those most commonly found as cause of human infections in the EU/EEA in 2015 and in the preceding years (EFSA and ECDC, 2016c).
Although based on much smaller amounts of data as compared with 2015, a higher proportion of STEC‐positive samples was evidenced in sheep and goats (18.5%) as compared to cattle, as also observed in 2015.
Thirty‐three STEC serogroups were reported in the full data set of animal samples. Also, in this case, the ‘top five’ serogroups and a few others such as O91, O113 and O146, all important STEC serogroups in public health, ranked above 1% of the total samples assayed, with all the others found below the 1% threshold.
4.6. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| WHO (World Health Organization) – E. coli Fact sheet | http://www.who.int/mediacentre/factsheets/fs125/en/ | |
| National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) | https://www.cdc.gov/ncezid/ | |
| Food and animals | EFSA Scientific Opinion: Monitoring of verotoxigenic Escherichia coli (VTEC) and identification of human pathogenic VTEC types | http://www.efsa.europa.eu/en/efsajournal/pub/579 |
| EFSA Scientific Opinion: Monitoring of verotoxigenic Escherichia coli (VTEC) and identification of human pathogenic VTEC types | http://www.efsa.europa.eu/en/efsajournal/pub/579 | |
| Molecular typing of VTEC strains isolated from food, feed and animals | http://www.efsa.europa.eu/en/supporting/pub/en-704 | |
| VTEC‐seropathotype and scientific criteria on pathogenicity assessment | http://www.efsa.europa.eu/en/efsajournal/pub/3138 | |
| Public health advice on prevention of diarrhoeal illness with special focus on Shiga toxin‐producing Escherichia coli (STEC), also called verotoxin‐ producing E. coli (VTEC) or enterohaemorrhagic E. coli (EHEC) | http://www.efsa.europa.eu/en/press/news/110611 | |
| Reg (EC 209/2013) | http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32013R0209 | |
| EURL (EU Reference Laboratory) VTEC webpage: laboratory methods for VTEC/STEC detection and typing | http://www.iss.it/vtec/index.php?lang=2&anno=2017&tipo=3 | |
| EURL (EU Reference Laboratory) VTEC webpage: Focus on STEC facts | http://www.iss.it/vtec/index.php?lang=2&anno=2017&tipo=20# |
5. Yersinia
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
5.1. Abstract
Twenty‐six MS reported 6,861 confirmed cases of yersiniosis in 2016, making it the third most commonly reported zoonosis in the EU. The EU notification rate was 1.82 cases per 100,000 population which was 4.7% lower than in 2015. There was a decreasing trend from 2008 to 2016, the trend has not shown any significant increase or decrease in the past 5 years (2012–2016). The highest country‐specific notification rates were observed in MS in north‐eastern Europe. Yersinia enterocolitica was the most common species reported to be isolated from human cases. The most common serotype was O:3 followed by O:9 and O:8. Five fatal cases were reported among the 4,350 confirmed yersiniosis cases for which this information was available in 2016.
For the year 2016, eight yersiniosis outbreaks caused by Y. enterocolitica (one strong‐evidence outbreak and seven weak‐evidence outbreaks) were reported by five MS and this was comparable with the previous years monitoring results.
As regards the food and animal monitoring data from investigations on Yersinia, as for the previous years, very few MS reported food and animal data for the year 2016, precluding any meaningful observations at the EU‐level. Despite this, documenting findings with the aim of understanding trends and sources of Yersinia along the food chain, including reporting of information on the biotype of each Y. enterocolitica isolate and also serotyping data, is essential to the overall goal of understanding and reducing yersiniosis.
5.2. Surveillance and monitoring of Yersinia in the EU
5.2.1. Humans
Notification of yersiniosis in humans is mandatory in most MS, Iceland and Norway. In Switzerland, it is not notifiable. Belgium, France, Italy and Luxembourg have a voluntary notification system, and Spain and the United Kingdom have another (not specified) system. No surveillance system exists in Greece and the Netherlands. The surveillance systems for Yersinia infections covers the whole population in all MS, except three (France, Italy and Spain). When no estimate for population coverage was provided, notification rates were not calculated.
Diagnosis of human gastrointestinal infections is generally based on culture from human stool samples.
5.2.2. Food and animals
Although the reporting of Yersinia occurrence or prevalence in food and animals is not mandatory, MS can report monitoring data on Yersinia to the EC in accordance with the Zoonoses Directive 2003/99/EC. The Directive specifies that, in addition to the number of zoonoses and zoonotic agents, for which monitoring is mandatory, zoonoses such as yersiniosis and their agents shall also be monitored when the epidemiological situation so warrants. At present, there is no harmonised surveillance of Yersinia in the EU for food or animals and Yersinia food and animal monitoring data submitted by the MS to EFSA are collected without harmonised design. These data allow for descriptive summaries at EU‐level to be made but they preclude trend analyses and trend watching at EU‐level (Table 1). A scientific report of EFSA suggested technical specifications for the harmonised monitoring and reporting of Y. enterocolitica in slaughter pigs in the EU (EFSA, 2009b).
5.2.3. Food‐borne outbreaks of human yersiniosis
The reporting of FBO of human yersiniosis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
5.3. Results
5.3.1. Overview of key statistics along the food chain, EU, 2012–2016
Table 20 summarises EU‐level statistics related to human yersiniosis, and to Yersinia occurrence in food and animals, respectively, in the EU, during 2012–2016. More detailed descriptions of these statistics are in the results section of this chapter and in the FBO.
Table 20.
Summary of Yersinia statistics related to humans, major food categories and major animal species, EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 6,861 | 6,928 | 6,435 | 6,352 | 6,215 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 1.82 | 1.91 | 1.83 | 1.92 | 1.93 | ECDC |
| Number of reporting MS | 26 | 26 | 25 | 25 | 25 | ECDC |
| Infection acquired in the EU | 3,198 | 3,336 | 3,314 | 3,263 | 3,878 | ECDC |
| Infection acquired outside the EU | 82 | 84 | 87 | 87 | 79 | ECDC |
| Unknown travel status or unknown country of infection | 3,581 | 3,508 | 3,034 | 3,002 | 2,258 | ECDC |
| Total number of food‐borne outbreaks (including waterborne outbreaks) | 8 | 13 | 11 | 8 | 12 | EFSA |
| Number of food‐borne outbreak‐related cases | 41 | 54 | 208 | 16 | 90 | EFSA |
| Food | ||||||
| Meat and meat products | ||||||
| Number of sampled units | 961 | 1,234 | 1,505 | 2,213 | 945 | EFSA |
| Number of MS | 5 | 5 | 4 | 7 | 4 | EFSA |
| Milk and milk products | ||||||
| Number of sampled units | 4 | 48 | 121 | 203 | 114 | EFSA |
| Number of MS | 1 | 4 | 2 | 4 | 2 | EFSA |
| Animals | ||||||
| Pigs | ||||||
| Number of sampled units | 100 | 2,050 | 2,447 | 5,892 | 5,071 | EFSA |
| Number of MS | 1 | 3 | 3 | 8 | 4 | EFSA |
| Cattle | ||||||
| Number of sampled units | 47 | 2,707 | 6,482 | 6,646 | 2,891 | EFSA |
| Number of MS | 1 | 2 | 3 | 4 | 2 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
Humans
The number of human yersiniosis cases infected domestically and through travel within EU, about half of the total number of confirmed cases, was stable during 2012–2016. During 2012–2016, the total number of reported food‐borne yersiniosis outbreaks in EU varied annually around 10, and some tens of outbreak‐related illnesses per year were reported with a peak of 208 cases for the year 2014.
Food and animal categories
For the year 2016, as for the previous years, very few MS reported food and animal monitoring data on investigations on Yersinia. Due to the scarcity of the reported data during 2012–2016, no inference can be made, beyond the sample statistics, on trends or sources of Yersinia.
5.3.2. Human yersiniosis
In total, 6,861 confirmed cases of yersiniosis were reported in the EU for 2016 by 26 MS (Table 21). The number of confirmed cases slightly decreased compared with 2015. The EU notification rate was 1.82 cases per 100,000 population, which was 4.7% lower than in 2015. The highest country‐specific notification rates were observed in Finland and the Czech Republic (7.42 and 5.76 cases per 100,000 population, respectively).
Table 21.
Reported human cases of yersiniosis and notification rates in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 86 | 86 | 0.99 | 118 | 1.38 | 107 | 1.26 | 158 | 1.87 | 130 | 1.55 |
| Belgium | Y | A | 355 | 355 | 3.14 | 350 | 3.11 | 309 | 2.76 | 350 | 3.14 | 256 | 2.31 |
| Bulgaria | Y | A | 10 | 10 | 0.14 | 12 | 0.17 | 20 | 0.28 | 22 | 0.30 | 11 | 0.15 |
| Croatia | Y | A | 23 | 22 | 0.52 | 16 | 0.38 | 20 | 0.47 | 0 | 0.00 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.12 | 0 | 0.00 |
| Czech Republic | Y | C | 608 | 608 | 5.76 | 678 | 6.43 | 557 | 5.30 | 526 | 5.00 | 611 | 5.82 |
| Denmark | Y | C | 278 | 278 | 4.87 | 273 | 4.82 | 250 | 4.44 | 225 | 4.02 | 182 | 3.26 |
| Estonia | Y | C | 45 | 45 | 3.42 | 53 | 4.04 | 62 | 4.71 | 72 | 5.45 | 47 | 3.55 |
| Finland | Y | C | 407 | 407 | 7.42 | 582 | 10.64 | 579 | 10.62 | 549 | 10.12 | 565 | 10.46 |
| Franceb | N | A | 735 | 735 | – | 624 | – | 574 | – | 430 | – | 314 | – |
| Germany | Y | C | 2,774 | 2,764 | 3.36 | 2,741 | 3.38 | 2,470 | 3.06 | 2,579 | 3.15 | 2,690 | 3.29 |
| Greecec | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Hungary | Y | C | 72 | 70 | 0.71 | 41 | 0.42 | 43 | 0.44 | 62 | 0.63 | 53 | 0.54 |
| Ireland | Y | C | 3 | 3 | 0.06 | 13 | 0.28 | 5 | 0.11 | 4 | 0.09 | 2 | 0.04 |
| Italyb | N | C | 9 | 9 | – | 7 | – | 18 | – | 25 | – | 14 | – |
| Latvia | Y | C | 51 | 47 | 2.39 | 64 | 3.22 | 28 | 1.40 | 25 | 1.24 | 28 | 1.37 |
| Lithuania | Y | C | 155 | 155 | 5.37 | 165 | 5.65 | 197 | 6.69 | 262 | 8.82 | 276 | 9.19 |
| Luxembourg | Y | C | 12 | 12 | 2.08 | 15 | 2.66 | 19 | 3.46 | 15 | 2.79 | 28 | 5.33 |
| Malta | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlandsc | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Poland | Y | C | 168 | 168 | 0.44 | 172 | 0.45 | 212 | 0.56 | 199 | 0.52 | 201 | 0.52 |
| Portugal | Y | C | 14 | 14 | 0.14 | 24 | 0.23 | – | – | – | – | – | – |
| Romania | Y | C | 40 | 40 | 0.20 | 25 | 0.13 | 32 | 0.16 | 43 | 0.22 | 26 | 0.13 |
| Slovakia | Y | C | 201 | 200 | 3.69 | 224 | 4.13 | 172 | 3.18 | 164 | 3.03 | 181 | 3.35 |
| Slovenia | Y | C | 31 | 31 | 1.50 | 10 | 0.48 | 19 | 0.92 | 26 | 1.26 | 22 | 1.07 |
| Spainb | N | C | 514 | 485 | – | 432 | – | 436 | – | 243 | – | 221 | – |
| Sweden | Y | C | 230 | 230 | 2.33 | 245 | 2.51 | 248 | 2.57 | 313 | 3.28 | 303 | 3.20 |
| United Kingdom | Y | C | 87 | 87 | 0.13 | 44 | 0.07 | 58 | 0.09 | 59 | 0.09 | 54 | 0.09 |
| EU total | – | – | 6,908 | 6,861 | 1.82 | 6,928 | 1.91 | 6,435 | 1.83 | 6,352 | 1.92 | 6,215 | 1.93 |
| Iceland | Y | C | 1 | 1 | 0.30 | 1 | 0.30 | 3 | 0.92 | 0 | 0.00 | – | – |
| Norway | Y | C | 57 | 57 | 1.09 | 76 | 1.47 | 211 | 4.13 | 55 | 1.09 | 43 | 0.86 |
Y: yes; N: no; A: aggregated data; C: case‐based data; −: no report.
Sentinel surveillance; no information on estimated coverage, so notification rate cannot be estimated.
No surveillance system.
Most (97.5%) of the yersiniosis cases acquired from infection within their own country, however 52.2% of the cases at the EU‐level were reported to be of unknown origin (Table 20). The highest proportions of domestic cases (> 98%) were reported in the Czech Republic, Estonia, Hungary, Latvia, Poland, Portugal and Slovakia. The highest proportions of travel‐related cases were reported by three Nordic countries – Finland (45.8%), Denmark (31.5%) and Norway (31.3%). Among the 203 travel‐associated cases with known information on probable country of infection, 42.3% of the cases represented travel within EU. Spain, Croatia and Greece were the most frequently reported travel destinations within EU (15.3%, 5.9% and 3.9%, respectively). Cuba and Thailand were the most common probable countries of infection outside EU representing 7.9% and 5.4% of the travel‐associated cases, respectively.
The case reports showed some seasonality with most of the cases reported between May and August. Despite a decreasing trend from 2008 to 2016 (p < 0.01), the trend did not show any significant increase or decrease in the past 5 years (2012–2016) (Figure 30).
Figure 30.
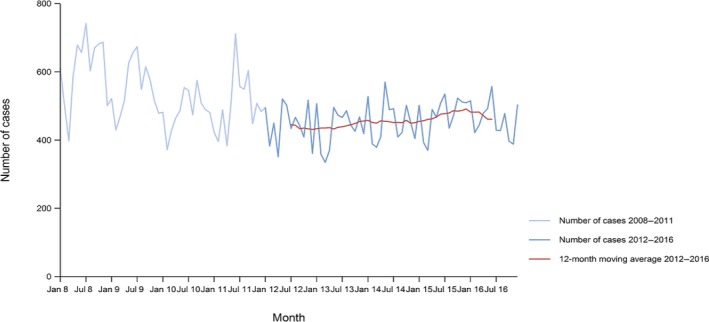
Trend in reported confirmed human cases of yersiniosis in the EU/EEA, by month, 2012–2016
- Source(s): Austria, Cyprus, the Czech Republic, Denmark, Estonia, Finland, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Romania Slovakia, Slovenia, Spain, Sweden and the United Kingdom. Belgium, Bulgaria, Croatia, France, Iceland and Portugal did not report data to the level of detail required for the analysis. Greece and the Netherlands do not have any formal surveillance system for the disease.
Among 17 MS with data available for the whole period 2008–2016, the Czech Republic, Slovakia and Spain reported significantly increasing trends (p < 0.01), while Finland, Germany and Sweden reported decreasing trends (p < 0.01) from 2008 to 2015.
In 2012–2016, Latvia and Spain continued to report increasing trends (p < 0.01), and five MS (Austria, Italy, Lithuania, Luxembourg and Sweden) observed decreasing trends among the 21 MS having data available for the whole period.
Species information was reported by 20 countries for 5,535 (80.2%) of the confirmed yersiniosis cases in the EU/EEA in 2016. Y. enterocolitica was the most common species reported in all countries, with the isolation percentage being 99.1% at the EU‐level. Information about the Y. enterocolitica serotypes was provided for 2,726 (39.5%) of confirmed Y. enterocolitica cases by 14 countries. The most common serotype was O:3 (84.6%), followed by O:9 (11.8%) and O:8 (1.7%). Biotype information was provided for 319 (4.6%) confirmed cases by five countries (Austria, Denmark, Finland, Lithuania and Poland) resulting in a 10.0% decrease of biotyped cases compared with 2015. The most commonly reported biotypes in 2016 were biotype 4 (79.6%) followed by biotype 2 (16.9%) and biotype 3 (2.5%).
Yersinia pseudotuberculosis represented 0.9% of cases reported by eight countries (Austria, the Czech Republic Finland, Poland, Romania, Slovakia, Sweden and the United Kingdom). The United Kingdom and Finland reported the highest proportion of Y. pseudotuberculosis infections, representing 12.2% and 5.7% of all their confirmed yersiniosis cases, respectively.
Fourteen MS provided information on hospitalisation. Of 1,653 cases (24.1%) with known hospitalisation status, 31.5% were hospitalised, about the same level than in 2015 (30.9%). As in previous years, the highest hospitalisation rates (54.8–91.7% of cases) were reported in Lithuania, Poland and Romania. Five fatal cases were reported among 4,358 cases (63.1%) by 15 MS in 2016.
5.3.3. Yersinia in food and animals
As reported in Table 20, for the year 2016, very few MS reported some food and animal monitoring data on investigations on Yersinia. Results of 971 single units of meat and meat products were reported by five MS. The reported occurrence of Y. enterocolitica in meats was low (> 1–10%) to high (> 20–50%). Results on Yersinia in milk and milk products were reported for six single units by two MS in 2016 (with none positive for Yersinia). One MS, Italy reported monitoring data on Yersinia in pigs, cattle, sheep and goats, and other animal species.
Results of monitoring data on food‐borne yersiniosis are in the chapter on FBO and also summarised in Table 20. In 2016, eight outbreaks caused by Y. enterocolitica (one strong‐evidence outbreak and seven weak‐evidence outbreaks) were reported by five MS. In addition, one weak‐evidence outbreak was reported by Norway. With some more detail, France reported three weak‐evidence outbreaks, with ‘broiler meat and its products’ and ‘other foods’ being incriminated; Latvia, the Netherlands and Slovakia each reported one weak‐evidence outbreak of yersiniosis without a food vehicle being suggested as the cause; Finland reported one weak‐evidence outbreak and one strong‐evidence outbreak, with, respectively, an ‘unknown’ food and ‘vegetables and juices and the products’ being reported to be implicated, and lastly, Norway reported one weak‐evidence outbreak without a reported food vehicle being suspected.
5.4. Discussion
Yersiniosis remains the third most commonly reported bacterial food‐borne zoonosis in the EU, despite the significantly decreasing EU/EEA trend between 2008 and 2016. The trend, however, did not show any significant increase or decrease in the last 5 years 2012–2016. The highest notification rates were reported in MS in north‐eastern Europe. This increase was partly due to improvements in surveillance systems (Denmark, Spain). Y. enterocolitica was the dominating species in all countries.
To assess the public health significance and pathogenicity for humans, reporting information on the biotype of each Y. enterocolitica isolate and preferably also serotyping data is recommended. Y. enterocolitica represents six biotypes (1A, 1B, 2–5), which are all considered pathogenic for human, except biotype 1A. Serotype information is provided frequently, but biotype information is only available for a small fraction of the yersiniosis cases reported by few MS. Pathogenicity of the isolates can also be confirmed by using more advanced methods e.g. molecular typing, although currently this information cannot be reported through TESSy. As biotype information is rarely reported, a proportion of the non‐typed isolates might belong to the non‐pathogenic biotype 1A. According to the EU case definition, only human pathogenic Y. enterocolitica or Y. pseudotuberculosis cases should be reported.22
For the year 2016, eight yersiniosis outbreaks caused by Y. enterocolitica (one strong‐evidence outbreak and seven weak‐evidence outbreaks) were reported by five MS and this number was comparable with the previous years monitoring results.
As regards the food and animal monitoring data for investigations on Yersinia, as for the previous years, very few MS reported food and animal data for the year 2016. This may be explained by the fact that the Zoonoses Directive 2003/99/EC does not prescribe mandatory reporting of Yersinia occurrence or prevalence in food and animals; reporting on zoonoses such as yersiniosis and agents thereof only becomes compulsory when the epidemiological situation in the MS warrants it. In addition to the scarcity of the reported data, the sampling and reporting rules are not harmonised, precluding trend analyses and trend watching. A scientific report of EFSA suggested technical specifications for the harmonised monitoring and reporting of Y. enterocolitica in slaughter pigs in the EU (EFSA, 2009b). Data deficiencies have as a consequence that no inference can be made beyond the sample statistics, on trends or sources of Yersinia in foods or animals. Still, documenting with the aim of understanding trends and sources of Yersinia along the food chain, including reporting of information on the biotype of each Y. enterocolitica isolate and preferably also serotyping data, is for the overall goal of understanding and reducing yersiniosis, whether food‐borne or sporadic.
5.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | CDC (Centers for Disease Control and Prevention) of United States: Fact sheet yersiniosis | https://www.cdc.gov/yersinia/faq.html |
| ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx | |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| Food and Animals | EFSA Scientific Opinion: Monitoring and identification of human enteropathogenic Yersinia spp. | https://www.efsa.europa.eu/en/efsajournal/pub/595 |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
6. Tuberculosis due to Mycobacterium bovis
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
6.1. Abstract
Tuberculosis due to M. bovis is a rare infection in humans in the EU, with 170 confirmed human cases reported in 2016 and a notification rate of 0.04 cases per 100,000 population. The notification rates in the EU have been stable in 2012–2016. There was no clear association between a country's status as officially free of bovine tuberculosis (OTF) and notification rates in humans.
The 2016 monitoring data on bovine tuberculosis in EU cattle demonstrate that the current situation in Europe on bovine tuberculosis infection, detection and control is heterogeneous. In the OTF regions of 21 MS, the detection during 2016 of bovine tuberculosis‐infected herds remained a rare event, as in the previous years.
In the non‐OTF regions of the 10 non‐OTF MS, the total number of remaining positive cattle herds decreased only slightly and the prevalence of bovine tuberculosis‐positive cattle herds was 1.56%. All 10 non‐OTF MS, except Cyprus reported having detected bovine tuberculosis. The United Kingdom reported a prevalence of 16.7% test‐positive cattle herds for Wales and England, which ranged in the previous years between 13.1% and 20.3%, and in Northern Ireland a 9.7% prevalence, which slightly increased. Greece, Ireland and Spain reported a prevalence of, respectively, 3.8%, 2.9% and 3.6%, with a stable (Ireland) or an increasing trend (Greece, Spain) in recent years. Italy and Portugal reported very low prevalence (> 0.1–1%) in their remaining non‐OTF regions, and Croatia, Bulgaria and Romania a rare prevalence (< 0.1%).
6.2. Surveillance and monitoring of tuberculosis due to M. bovis in the EU
6.2.1. Humans
The notification of tuberculosis in humans is mandatory in all MS, Iceland, Norway and Switzerland and covers the whole population, with the possible exception of Greece (no information about the population coverage was provided). France did not report tuberculosis cases in case‐based format for 2016 (i.e. do not separate by species). In previous years, France did not report tuberculosis species data within the Mycobacterium tuberculosis complex; therefore, no human M. bovis incidence or trend data are available for France.
As tuberculosis is a chronic disease with a long incubation period, it is not possible to assess travel‐associated cases in the same way as diseases with an acute onset. Instead, the distinction is made between individuals with the disease born or having citizenship in the reporting country (native case), individuals who have moved to the reporting country, but had citizenship of other MS (foreign case of EU origin), and those moving to the reporting country from outside the EU (foreign case outside EU). In a few cases (cases from Austria and Belgium), the distinction is also made based on the nationality of the infected people.
Treatment outcome of tuberculosis cases due to M. bovis are assessed 1 year (12 months) after the case notification, since the shortest duration for treatment completion is 6 months by the international treatment guidelines of tuberculosis (WHO, online).
6.2.2. Animals
Bovine tuberculosis monitoring data from bovine animals originating from the National Control and Eradication Programmes and/or Officially Free status
According to the Zoonoses Directive 2003/99/EC, bovine tuberculosis monitoring data have to be reported annually by the MS. These reports of the MS submitted are based on Council Directive 64/432/EEC and subsequent legislation, and are therefore essential for the assessment of the epidemiological situation in MS and MS’ regions, whether declared officially free (OF) or not yet declared OF. These data sets submitted by MS are comparable across MS because the monitoring schemes are harmonised, and the data collected and reported to EFSA originate from a census sampling. In addition to trend analysis both at the EU‐level and at MS level, and to trend watching and descriptive summaries, these data may also be used to assess the indirect effect of control and eradication programmes (Table 1). Such data originate from national control and surveillance programmes implemented by the MS in accordance with EU legislation.
Mycobacterium monitoring data from animals other than bovine animals
Mycobacterium monitoring data from animals other than bovine animals submitted to EFSA and collected without harmonised design allows for descriptive summaries at EU‐level, but are not suitable for trend analyses and trend watching (Table 1).
Food‐borne outbreaks of human tuberculosis due to M. bovis
The reporting of FBO of human tuberculosis due to M. bovis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
6.3. Results
6.3.1. Overview of key statistics along the food chain, EU, 2012–2016
Table 22 summarises EU‐level statistics related to human tuberculosis due to M. bovis, and to bovine tuberculosis prevalence in animals, respectively, in the EU, during 2012–2016. A more detailed description of these statistics is in the results section of this chapter and in the FBO chapter.
Table 22.
Summary of tuberculosis due to M. bovis statistics related to humans, major food categories and animal species, EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 170 | 181 | 167 | 144 | 132 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.04 | 0.04 | 0.04 | 0.03 | 0.03 | ECDC |
| Number of reporting MS | 27 | 27 | 27 | 27 | 27 | ECDC |
| TB cases in individuals of EU origin | 105 | 113 | 106 | 97 | 88 | ECDC |
| TB cases in individuals originating outside of EU | 56 | 60 | 55 | 41 | 34 | ECDC |
| TB cases in individuals of unknown origin | 9 | 8 | 6 | 6 | 10 | ECDC |
| Total number of food‐borne outbreaks | 0 | 0 | 0 | 0 | 0 | EFSA |
| Number of outbreak‐related cases | 0 | 0 | 0 | 0 | 0 | EFSA |
| Animals | ||||||
| Bovine animals | ||||||
| Number of positive herds in OTF regions in non‐OTF MS and OTF MS | 147 | 155 | 139 | 197 | 209 | EFSA |
| Number of reporting OTF MS | 18 | 18 | 16 | 15 | 15 | EFSA |
| Number of positive herds in non‐OTF regions in non‐OTF MS | 17,421 | 17,441 | 17,122 | 18,059 | 18,208 | EFSA |
| Number of reporting non‐OTF MS | 10 | 10 | 12 | 13 | 12 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States; OTF: Officially bovine tuberculosis free (status on freedom from bovine tuberculosis, in cattle); TB: tuberculosis due to M. bovis.
The statistics displayed in Table 22 are the numbers of OF MS and non‐OF MS, and the cattle herds remaining positive for bovine tuberculosis, during 2012–2016. Further descriptions of findings are in Section 6.3.3.
6.3.2. Tuberculosis due to M. bovis in humans
In 2016, 170 confirmed cases of tuberculosis due to M. bovis in humans were reported by 27 MS (Table 23). Twelve MS reported at least one confirmed case and 15 MS reported zero cases. The EU notification rate was 0.04 cases per 100,000 population, the same as in previous years. The highest notification rates in 2016 were reported by Belgium (0.12 per 100,000) and the Netherlands (0.07 per 100,000), followed by Germany, Ireland, Spain and the United Kingdom (0.06 per 100,000). There was no clear association between a country's OTF status (OTF; Officially bovine tuberculosis free in cattle) and its notification rate in humans. The notification rate of human cases for both country groups (OTF and non‐OTF) was 0.04 per 100,000 population.
Table 23.
Reported human cases of tuberculosis due to M. bovis and notification rates per 100,000 population in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria (OTF)b | Y | C | 1 | 0.01 | 3 | 0.04 | 1 | 0.01 | 1 | 0.01 | 1 | 0.01 |
| Belgium (OTF)c | Y | C | 14 | 0.12 | 9 | 0.08 | 10 | 0.09 | 10 | 0.09 | 4 | 0.04 |
| Bulgaria | Y | C | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Croatia | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic (OTF) | Y | C | 1 | 0.01 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Denmark (OTF) | Y | C | 2 | 0.04 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 | 0 | 0.00 |
| Estonia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 |
| France (OTF)d | – | – | – | – | – | – | – | – | – | – | – | – |
| Germany (OTF) | Y | C | 52 | 0.06 | 51 | 0.06 | 50 | 0.06 | 44 | 0.05 | 45 | 0.05 |
| Greece | – | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Hungary (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Ireland | Y | C | 3 | 0.06 | 5 | 0.11 | 3 | 0.07 | 6 | 0.13 | 4 | 0.08 |
| Italye | Y | C | 13 | 0.02 | 17 | 0.03 | 18 | 0.03 | 14 | 0.02 | 10 | 0.02 |
| Latvia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lithuania (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Luxembourg (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands (OTF) | Y | C | 12 | 0.07 | 9 | 0.05 | 8 | 0.05 | 10 | 0.06 | 8 | 0.05 |
| Poland (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Portugalf | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Romania | Y | C | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Slovakia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Slovenia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Spain | Y | C | 26 | 0.06 | 37 | 0.08 | 33 | 0.07 | 28 | 0.06 | 14 | 0.03 |
| Sweden (OTF) | Y | C | 5 | 0.05 | 6 | 0.06 | 4 | 0.04 | 0 | 0.00 | 5 | 0.05 |
| United Kingdomg | Y | C | 39 | 0.06 | 42 | 0.07 | 39 | 0.06 | 30 | 0.05 | 41 | 0.06 |
| EU total | – | – | 170 | 0.04 | 181 | 0.04 | 167 | 0.04 | 144 | 0.03 | 132 | 0.03 |
| Icelandh | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway (OTF) | Y | C | 5 | 0.10 | 1 | 0.02 | 4 | 0.08 | 0 | 0.00 | 2 | 0.04 |
| Switzerland (OTF)i | Y | C | 5 | 0.06 | 6 | 0.07 | 2 | 0.02 | 2 | 0.02 | 5 | 0.06 |
Y: yes; N: no; A: aggregated data; C: case‐based data; –: no report.
OTF: Officially bovine tuberculosis free (status on freedom from bovine tuberculosis, in cattle).
There is an underestimation of the number of M. bovis in human cases in Belgium because the identification within the M. tuberculosis complex strains is not performed systematically by all the laboratories.
Not reporting TB case‐based data for 2016 and species of the M. tuberculosis complex for previous years.
In Italy, 7 regions and 14 provinces are OTF.
In Portugal, all administrative regions within the superior administrative unit of the Algarve are OTF.
In the United Kingdom, Scotland and the Isle of Man are OTF (in cattle).
In Iceland, which has no special agreement on animal health (status) with the EU, the last outbreak of bovine tuberculosis was in 1959.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein (OTF).
The majority 61.8% (105/170) of the cases reported in 2016 were of EU origin (native cases and/or cases originating from other MS), 32.9% (56/170) were from outside EU, and 5.3% (9/170) were of unknown origin (Table 22). Among cases with known origin, there was a larger proportion (67.5%) of native cases in non‐OTF countries than in OTF countries (47.1%).
Treatment outcome after 12 months of treatment was reported for 128 (90.4%) of 170 human M. bovis cases reported in 2015. Successful treatment was reported for 76 cases (59.4%), while 19 cases (14.8%) died during treatment, 12 cases (9.4%) were still on treatment, one case (0.8%) was lost to follow‐up. The treatment outcome was not evaluated for 20 cases (15.6%).
The drug susceptibility test results reported for rifampicin and isoniazid for 124 M. bovis cases, 120 cases were tested for ethambutol, and 46 cases tested for streptomycin in 2016. Drug resistance was low; there were only three isoniazid‐resistant cases (2.4%) and two streptomycin resistant cases (4.3%). No multidrug‐resistant (MDR; WHO, online) (resistance to at least isoniazid and rifampicin) were detected.
Figure 31 shows, for the year 2016, the number of confirmed tuberculosis cases due to M. bovis in individuals of EU origin (native cases) and country‐level aggregated prevalence of bovine tuberculosis, in the EU. The map indicates that there was no clear association between the two parameters.
Figure 31.
Number of confirmed tuberculosis cases due to M. bovis in individuals of native cases of EU origin and country‐level aggregated prevalence of bovine tuberculosis‐positive cattle herds (due to M. bovis and/or M. caprae), EU, 2016
6.3.3. Bovine tuberculosis in animals
As previously, the present annual report data from cattle of the specific types of bacteria that are part of the M. tuberculosis complex were taken into account to summarise the EU situation on bovine tuberculosis. Previously, the separate reporting of bacterial species of the M. tuberculosis complex in the EFSA disease status data model was not possible. In this chapter a distinction is made descriptively, whenever possible, of reporting by MS of the Mycobacterium tuberculosis complex, M. bovis and Mycobacterium caprae.
The status on freedom from bovine tuberculosis (OTF) and occurrence of the disease at region or national levels for MS and non‐MS in 2016 are presented in Figures 32 and 33, respectively. The 2016 list of countries and regions OTF was Austria, Belgium, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, seven regions and 14 provinces in Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, all administrative regions within the superior administrative unit of the Algarve in Portugal, Poland, Slovakia, Slovenia, Sweden, Scotland and the Isle of Man in the United Kingdom, Norway and Switzerland, in accordance with EU legislation. Liechtenstein has the same status (OTF) as Switzerland. In Iceland, which has no special agreement on animal health status with the EU, the last outbreak of bovine tuberculosis was reported in 1959.
Figure 32.
Status of countries on bovine tuberculosis, EU/EEA, 2016
- OF: Officially bovine tuberculosis free in cattle; MS: Member State.
Figure 33.
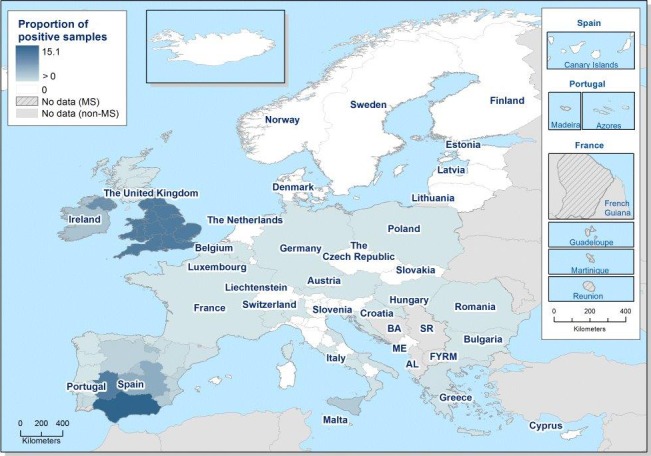
Proportion of cattle herds infected with or positive for bovine tuberculosis, EU/EEA, 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: Former Yugoslav Republic of Macedonia, ME: Montenegro; and SR: Serbia.
Bulgaria, Croatia, Cyprus, Greece, Ireland, Italy, Portugal, Romania, Spain and the United Kingdom had not yet achieved the country‐level OTF status in 2016.
The overall proportion of cattle herds infected with, or positive for, bovine tuberculosis, considering all EU regions, remained very low in the EU (0.7% of the cattle herds in the EU), although there is a heterogeneous distribution of bovine tuberculosis in Europe with a pronounced spatial clustering (Figure 34). The prevalence ranges from the absence of infected/positive animals in most OTF regions to a regional prevalence in non‐OTF regions of 15.1% in Andalusia, Spain, when considering all existing herds or a reported regional prevalence of test‐positive cattle herds of 18.4% within the United Kingdom in Wales and England.
Figure 34.
Proportion of cattle herds infected with or positive for bovine tuberculosis, in OTF regions, EU, 2010–2016
- OTF: Officially bovine tuberculosis free in cattle.
In the 18 OTF MS and in the regions declared OTF in the three non‐OTF MS Italy, Portugal and the United Kingdom, which represent a total a population of 1,249,693 cattle herds, annual surveillance programmes are carried out to confirm freedom from bovine tuberculosis. Their reporting requirements are, among other indicators, the number of infected herds and the total number of herds existing. Eight of these MS reported 147 bovine tuberculosis‐infected herds for the year 2016; seven MS reported infection with M. bovis (Belgium, 2 herds; France, 92 herds; Germany, 3; Hungary, 1; Italy, 5; Poland, 24; and the United Kingdom, 3), whereas Austria23 reported 17 herds infected with M. caprae. The prevalence of bovine tuberculosis‐infected herds in these OTF regions of 21 MS in 2016 was 0.012%, such as during 2015 in the OTF regions of these 21 MS (Figure 34). From 2010 to 2016, the number of cattle herds reported infected in the OTF regions of the EU per year was 227, 200, 209, 197, 139, 155 and 147, respectively (Figure 34).
Bovine tuberculosis was not detected in 2016 in the non‐MS Iceland, Norway, Switzerland and Liechtenstein.
In 2016, there were a total of 1,120,292 cattle herds in the non‐OTF regions of the 10 MS with non‐OTF status. National control and eradication programme for bovine tuberculosis are in place in all these regions. In 2016, five of these MS (Ireland, Italy, Portugal, Spain and the United Kingdom) received EU cofinancing for eradication programmes. These MS reported on the prevalence situation in their non‐OTF regions by the number of positive herds, the number of herds tested under the eradication programme, and the total number of herds existing. The number of positive herds reported was 4,047 in Ireland (4,002 in 2015), 335 in Italy (433 in 2015), 77 in Portugal (94 in 2015), 3,048 in Spain (3,070 during 2015) and 9,694 in the United Kingdom (9,628 in 2015) and all reports concerned M. bovis.
The five other non‐OTF MS did not receive cofinancing by the EU for their eradication programmes during 2016. They reported, among other indicators, the number of infected herds and the total number of herds existing. Of these MS, Cyprus did not report any infected herds for the year 2016 (as in 2015 and 2014). Croatia reported 2 M. tuberculosis complex‐infected herds (21 in 2015), whereas M. bovis‐infected herds were reported by Bulgaria (10 (6 in 2015)), Greece (147 (187 in 2015)) and Romania (61 (36 in 2015)).
From 2010 to 2016, the total number of test‐positive cattle herds in these EU non‐OTF regions remained at the same level and 17,814; 17,102; 18,208; 18,059; 17,122; 17,441 and 17,421 were reported from 2010 to 2016, respectively. The overall prevalence during this period increased from 1.05% in 2010 to 1.56% in 2016. Concomitantly, the total number of cattle herds in these EU non‐OTF regions decreased from 1,638,694 in 2010 to 1,120,292 in 2016 (Figure 35).
Figure 35.
Proportion of cattle herds infected with or positive for bovine tuberculosis, in non‐OTF regions, EU, 2010–2016
- OTF: Officially bovine tuberculosis free in cattle.
Figure 36 displays the MS‐specific trends of the prevalence of bovine tuberculosis test‐positive cattle herds in the non‐OTF regions of five MS with EU cofinanced eradication programmes, and in one non‐OTF not funded MS, Greece, during 2004–2016. A prevalence of ‘> 10–20%’ was reported by the United Kingdom in Wales and England, with a reported highest regional prevalence of bovine tuberculosis test‐positive cattle herds of 16.7%. When considering all existing herds, the regional prevalence was 11.9% (Figure 33). Northern Ireland reported a prevalence between ‘> 1–10%’, yet increasing prevalence of test‐positive herds. Also Ireland and Spain reported a low prevalence with a stable (Ireland) and an increasing trend (Spain) in recent years. When considering all herds, Spain reported a regional prevalence of 15.1% in Andalusia (Figure 33). Italy and Portugal reported very low prevalence (> 0.1–1%) in their remaining non‐OTF regions. Greece reported 147 bovine tuberculosis‐infected cattle herds out of 3,841 tested resulting in a test‐positive prevalence of 3.8%. During 2004–2016, the test‐positive cattle herds reported by Greece increased and ranged from 1.9% in 2008 to 5.2% in 2015.
Figure 36.
Prevalence of bovine tuberculosis test‐positive cattle herds, in non‐OTF regions of five non‐OTF cofinanced Member States and of one non‐OTF not funded Member State, Greece, 2004–2016
In 2016, M. bovis in other animals was reported by various countries in domestic breeding pigs, sheep and goats, and solipeds, farmed water buffalos, farmed wild boar, badgers, wild red and roe deer. M. caprae, recognised to cause bovine tuberculosis, was reported in cattle, wild boar and wild red deer.
6.4. Discussion
Tuberculosis due to M. bovis is a rare disease in humans in the EU because of decades of disease control and elimination in cattle and by routine pasteurisation of cows’ milk. Human M. bovis cases represented only a small proportion (< 0.3%) of all confirmed tuberculosis cases reported in 2016 in the MS (ECDC Surveillance Atlas of Infectious Diseases). The EU notification rate of M. bovis has been stable between 2012 and 2016. There was no clear association between a country's OTF status and its notification rate in humans. No matter what the country status, notified cases who have immigrated might have acquired the infection outside the reporting country. Cases native to the country could have been infected before the disease was eradicated from the animal population, as it may take years before disease symptoms develop.
The 2016 monitoring data on bovine tuberculosis in EU cattle demonstrate that the current situation in Europe on bovine tuberculosis infection, detection and control is heterogeneous, as documented by EFSA (EFSA AHAW Panel, 2014). The prevalence ranges from the absence of infected/positive animals in most OTF regions to a regional prevalence in non‐OTF regions of 15.1% in Andalusia, Spain, when considering all existing herds or a reported regional prevalence of test‐positive cattle herds of 18.4% within the United Kingdom in Wales and England.
In the OTF regions of 21 MS during 2016, 147 infected cattle herds were detected, resulting in a remaining prevalence of bovine tuberculosis‐infected herds of 0.012%, meaning infection of herds in these OTF regions was a rare event, as in previous years. Eight of these MS reported a total of 147 bovine tuberculosis‐infected herds; seven MS (Belgium, France, Germany, Hungary, Italy, Poland and the United Kingdom) reported infection with M. bovis, whereas Austria reported herds infected with M. caprae.
In the non‐OTF regions of the 10 non‐OTF MS, the overall prevalence of bovine tuberculosis‐positive cattle herds increased from 1.1% in 2010 to 1.6% in 2016. This slight increase might be explained by the gradual declaration of few MS as OTF and of regions within non‐OTF MS as OTF, resulting in the total number of remaining cattle herds in non‐OTF decreasing from 2010 to 2016, whereas the total number of remaining positive cattle herds decreased only slightly. All 10 non‐OTF MS, except Cyprus, reported having detected bovine tuberculosis. At MS level, the United Kingdom reported a prevalence of 16.7% test‐positive cattle herds for Wales and England, which ranged in the previous years between 13.1% and 20.3%, and Northern Ireland a 9.7% prevalence, which is a slight increase. Greece, Ireland and Spain reported a prevalence of, respectively, 3.8%, 2.9% and 3.6%, with a stable (Ireland) and an increasing trend (Greece, Spain) in recent years. Italy and Portugal, reported very low prevalence (> 0.1–1%) in their non‐OTF regions, and Croatia, Bulgaria and Romania a rare prevalence (< 0.1%). Reports from non‐OTF MS were on M. bovis (Bulgaria, Greece, Ireland, Italy, Portugal, Romania, Spain and the United Kingdom) or M. tuberculosis complex (Croatia). Stagnating or increasing trends in prevalence of bovine tuberculosis‐positive cattle herds demonstrate that control and eradication of bovine tuberculosis is a challenge, owing to the complex interactions between the pathogen, hosts and the local environments (EFSA AHAW Panel, 2014).
In 2016, M. bovis was reported to be isolated from a wide range of animal species, both domestic and wild, reflecting the broad host range of this causative agent of tuberculosis in cattle. M. caprae, also recognised to cause bovine tuberculosis was reported in cattle, wild boar and wild red deer in some countries.
6.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| European Tuberculosis Surveillance Network | http://ecdc.europa.eu/en/healthtopics/Tuberculosis/european_tuberculosis_surveillance_network/Pages/index.aspx | |
| Animals | European Union Reference Laboratory for Bovine Tuberculosis | https://www.visavet.es/bovinetuberculosis/ |
| Summary Presentations on the situation as regards Bovine Tuberculosis control and eradication programmes in Member States | https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en#20160705 | |
| Bovine Tuberculosis Fact sheet | http://www.cfsph.iastate.edu/Factsheets/pdfs/bovine_tuberculosis.pdf | |
| General information on EU Food Chain Funding | https://ec.europa.eu/food/funding_en | |
| General information on National Veterinary Programmes, in EU | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en | |
| EU approved and cofinanced veterinary programmes for Bovine Tuberculosis carried out by the MS | http://ec.europa.eu/dgs/health_food-safety/funding/cff/animal_health/vet_progs_en.htm | |
| OIE (World Organisation for Animal health), Summary of Information on Bovine tuberculosis | http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Disease_cards/BOVINE-TB-EN.pdf | |
| 2016 National Veterinary Programmes funded (cofinanced) by the EU for bovine tuberculosis (approved programmes and type of measures approved) | https://ec.europa.eu/food/sites/food/files/safety/docs/cff_animal_vet-progs_working_doc_12114_rev2_2016.pdf | |
| EU approved and cofinanced eradication programmes for bovine tuberculosis in cattle carried out by the MS is available on‐line at | http://ec.europa.eu/dgs/health_food-safety/funding/cff/animal_health/vet_progs_en.htm | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| EFSA Scientific Opinion: Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): bovine tuberculosis | https://www.efsa.europa.eu/en/efsajournal/pub/4959 | |
| EU Task Force on the eradication of animal diseases – Bovine tuberculosis is subgroup reports | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en |
7. Brucella
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
7.1. Abstract
Brucellosis is now a rare infection in humans in the EU with a stable trend in the last 5 years, though it is a severe disease with most infected cases hospitalised, and with one death reported in 2016. The highest notification rates and most domestic brucellosis cases were reported from three MS (Greece, Italy and Portugal) that are not officially brucellosis free in cattle, sheep or goats. These three MS reported most Brucella‐positive or Brucella‐infected herds of these ruminant species in the EU. Italy reported Brucella‐positive findings in milk, at the processing plant level, during 2016, but in general very few monitoring data are reported by these three countries on milk and milk products, in particular those destined to be consumed raw, which are the main food sources of brucellosis in human. Non‐food‐borne transmission of brucellosis to humans also happens by direct contact with infected animals.
In animals, bovine brucellosis and ovine and caprine brucellosis have been eradicated by most MS. As a result, food‐borne disease outbreaks due to Brucella have become rare in large areas of the EU and, for the year 2016, no such food‐borne outbreaks were reported in the EU.
The total number of Brucella‐positive or Brucella‐infected cattle, sheep and goat herds, further slightly decreased in the not officially brucellosis‐free regions or countries during 2016. Brucellosis in animals is very much clustered in the EU and Croatia, Greece, Italy, Portugal and Spain continue to report Brucella in domestic ruminants in 2016. These findings underline that brucellosis is still an animal health problem with public health relevance in a few MS.
7.2. Surveillance and monitoring of Brucella in the EU
7.2.1. Humans
The notification of brucellosis in humans is mandatory in all MS, Iceland, Norway and Switzerland. In Belgium, the notification is mandatory in the two main regions out of three. In Denmark, no surveillance system is in place for brucellosis and the disease is not notifiable nor reported at the EU‐level.
7.2.2. Food and animals
Brucella monitoring data from bovine animals, and sheep and goats originating from the National Control and Eradication Programmes and/or Officially Free status
According to the Zoonoses Directive 2003/99/EC, bovine brucellosis and sheep’ and goats’ brucellosis annual monitoring data has to be provided by the MS. These submitted reports from MS are based on Council Directive 64/432/EEC and subsequent legislation, so are essential for the assessment of the epidemiological situation in MS and MS’ regions, whether declared officially free (OF) or not yet declared OF. These data sets submitted by MS are comparable across MS because the monitoring schemes are harmonised, and the data collected and reported to EFSA originate from a census sampling. In addition to trend analysis both at the EU‐level and at MS level, and to trend watching and descriptive summaries, these data may also be used to assess the indirect impact of control and eradication programmes (Table 1). Such data originate from national control and surveillance programmes implemented by the MS in accordance with EU legislation.
Brucella monitoring data from food and animals other than bovine animals, and sheep and goats
Brucella monitoring data from food and animals other than bovine animals, and sheep and goats, submitted to EFSA (according Chapter II (‘monitoring of zoonoses and zoonotic agent's) of the Zoonoses Directive 2003/99/EC) and collected without harmonised design, allow for descriptive summaries at EU‐level to be made. They preclude trend analyses and trend watching at EU‐level (Table 1).
7.3. Results
7.3.1. Overview of key statistics along the food chain, EU, 2012–2016
Table 24 summarises EU‐level statistics related to human brucellosis, and to Brucella occurrence and prevalence in food and animals, respectively, in the EU, during 2012–2016. A more detailed description of these statistics is in the results section of this chapter and in the FBO chapter.
Table 24.
Summary of Brucella statistics related to humans, major food categories and animal species, EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 516 | 437 | 462 | 498 | 503 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.12 | 0.09 | 0.09 | 0.10 | 0.10 | ECDC |
| Number of reporting MS | 26 | 27 | 27 | 27 | 27 | ECDC |
| Infection acquired in the EU | 194 | 281 | 325 | 375 | 401 | ECDC |
| Infection acquired outside the EU | 41 | 40 | 45 | 41 | 43 | ECDC |
| Unknown travel status or unknown country of infection | 281 | 116 | 92 | 82 | 59 | ECDC |
| Total number of food‐borne outbreaks | 0 | 1 | 2 | 4 | 5 | EFSA |
| Number of outbreak‐related cases | 0 | 2 | 7 | 10 | 16 | EFSA |
| Food | ||||||
| Milk and milk products | ||||||
| Number of sampled units | 283 | 282 | 1,030 | 778 | 27,603 | EFSA |
| Number of reporting MS | 2 | 2 | 3 | 2 | 1 | EFSA |
| Animals | ||||||
| Bovine animals | ||||||
| Number of positive herds in OBF regions in OBF or non‐OBF MS | 2 | 4 | 2 | 2 | 9 | EFSA |
| Number of reporting OBF MS | 19 | 19 | 18 | 16 | 16 | EFSA |
| Number of positive herds in non‐OBF regions | 808 | 938 | 879 | 1,019 | 1,181 | EFSA |
| Number of reporting non‐OBF MS | 9 | 9 | 10 | 12 | 11 | EFSA |
| Small ruminants | ||||||
| Number of positive herds in ObmF regions in ObmF or non‐ ObmF MS | 2 | 10 | 3 | 4 | 5 | EFSA |
| Number of reporting ObmF MS | 20 | 20 | 19 | 19 | 19 | EFSA |
| Number of positive herds in non‐ObmF regions | 870 | 1,094 | 1,133 | 1,440 | 1,693 | EFSA |
| Number of reporting non‐ObmF MS | 8 | 8 | 9 | 9 | 8 | EFSA |
OBF/ObmF: Officially brucellosis free in cattle/Officially B. melitensis free in sheep and goats.
Food data of interest reported were categorised in the major category ‘Milk and milk products’, and aggregated by year over the period 2012 to 2016 to get an overview, by year, of the amount of data sent. In the summary table, data from industry own‐control programmes and HACCP sampling were excluded. The number of sampled units reported is extremely low, except for the year 2012, when Belgium reported a screening investigation of cows’ milk. As regards the reporting MS, these are mostly Italy and Portugal, and Spain, which did not yet gain country‐level official freedom from bovine brucellosis and ovine and caprine brucellosis. Animal data statistics displayed in Table 24 are the numbers of OF MS and non‐OF MS, and the herds remaining Brucella‐positive, during 2012–2016.
7.3.2. Human brucellosis
In 2016, 26 MS provided information on brucellosis in humans, which was one country less than in the previous 4 years (no data from the United Kingdom) (Table 25). In total, 531 cases, were reported in the EU. They included 516 confirmed cases, with a notification rate of 0.12 cases per 100,000 population (Table 25). This represents a 35.2% increase compared with 2015 and was the highest notification rate in the last 5 years. This was mainly due to increase in the number of cases in one country (Italy), where cases more than doubled compared with 2015. Eight MS (Bulgaria, Cyprus, Estonia, Finland, Hungary, Latvia, Lithuania and Malta) and Iceland reported no human cases.
Table 25.
Reported human cases of brucellosis and notification rates per 100,000 in the EU/EEA, by country and year, 2012–2016
| Countryb | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria (OBF/ObmF) | Y | C | 4 | 4 | 0.05 | 1 | 0.01 | 1 | 0.01 | 7 | 0.08 | 2 | 0.02 |
| Belgium (OBF/ObmF) | Y | A | 4 | 4 | 0.04 | 9 | 0.08 | 2 | 0.01 | 0 | 0.00 | 4 | 0.04 |
| Bulgaria | Y | A | 0 | 0 | 0.00 | 36 | 0.50 | 2 | 0.03 | 0 | 0.00 | 1 | 0.01 |
| Cyprus (ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 | 0 | 0.00 |
| Croatia | Y | C | 2 | 2 | 0.05 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic (OBF/ObmF) | Y | C | 1 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Denmarkc (OBF/ObmF) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 | 1 | 0.02 |
| Franced (OBF) | Y | C | 22 | 19 | 0.03 | 17 | 0.03 | 14 | 0.02 | 19 | 0.03 | 28 | 0.04 |
| Germany (OBF/ObmF) | Y | C | 36 | 36 | 0.04 | 44 | 0.05 | 45 | 0.06 | 26 | 0.03 | 28 | 0.03 |
| Greece | Y | C | 122 | 119 | 1.10 | 109 | 1.00 | 135 | 1.24 | 159 | 1.44 | 123 | 1.11 |
| Hungary (ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Ireland (OBF/ObmF) | Y | C | 2 | 2 | 0.04 | 0 | 0 | 3 | 0.07 | 1 | 0.02 | 2 | 0.04 |
| Italye | Y | C | 211 | 211 | 0.35 | 105 | 0.17 | 121 | 0.22 | 141 | 0.24 | 184 | 0.31 |
| Latvia (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.05 | 0 | 0.00 |
| Lithuania (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 0.07 | 0 | 0.00 |
| Luxembourg (OBF/ObmF) | Y | C | 1 | 1 | 0.17 | 0 | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta (OBF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.24 | 0 | 0.00 |
| Netherlands (OBF/ObmF) | Y | C | 5 | 5 | 0.03 | 7 | 0.04 | 1 | 0.01 | 5 | 0.03 | 3 | 0.02 |
| Poland (OBF/ObmF) | Y | C | 3 | 3 | 0.01 | 4 | 0.01 | 1 | 0.00 | 1 | 0.00 | 0 | 0.00 |
| Portugalf | Y | C | 50 | 50 | 0.48 | 46 | 0.44 | 50 | 0.48 | 22 | 0.21 | 37 | 0.35 |
| Romania (ObmF) | Y | C | 1 | 1 | 0.01 | 0 | 0.00 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 |
| Slovakia (OBF/ObmF) | Y | C | 1 | 1 | 0.02 | 1 | 0.02 | 0 | 0 | 1 | 0.02 | 1 | 0.02 |
| Slovenia (OBF/ObmF) | Y | C | 1 | 1 | 0.05 | 0 | 0.00 | 0 | 0 | 0 | 0.00 | 0 | 0.00 |
| Spaing | Y | C | 46 | 37 | 0.08 | 33 | 0.07 | 56 | 0.12 | 87 | 0.19 | 62 | 0.13 |
| Sweden (OBF/ObmF) | Y | C | 19 | 19 | 0.19 | 13 | 0.13 | 16 | 0.17 | 10 | 0.11 | 13 | 0.14 |
| United Kingdomh (OBF/ObmF) | Y | C | – | – | – | 12 | 0.02 | 11 | 0.02 | 15 | 0.02 | 14 | 0.02 |
| EU total | – | – | 531 | 516 | 0.12 | 437 | 0.09 | 462 | 0.09 | 498 | 0.10 | 503 | 0.10 |
| Icelandi | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway (OBF/ObmF) | Y | C | 4 | 4 | 0.08 | 2 | 0.04 | 2 | 0.04 | 2 | 0.04 | 4 | 0.08 |
| Switzerlandj (OBF/ObmF) | Y | C | 7 | 7 | 0.08 | 1 | 0.01 | 3 | 0.04 | 4 | 0.05 | 3 | 0.04 |
Y: yes; N: no; A: aggregated data; C: case‐based data; −: no report.
OBF/ObmF: Officially brucellosis free in cattle/Officially B. melitensis free in sheep and goats.
No surveillance system.
In France, all but 1 of the 96 metropolitan departments (due to Rev. 1 vaccination against Brucella ovis) are ObmF and no cases of brucellosis have been reported in small ruminants since 2003.
In Italy, 11 regions and nine provinces are OBF and also 12 regions and 9 provinces are ObmF.
In Portugal, six islands of the Azores and the superior administrative unit of Algarve are OBF whereas all nine Azores islands are ObmF.
In Spain, the Canary Islands, the Balearic Islands, Basque Country, Murcia, Rioja and Navarre are OBF and the Canary Islands, Asturias, Cantabria, Castile and Leon, Galicia, Navarre, Basque Country and the Balearic Islands are ObmF.
In the United Kingdom, Great Britain (England, Scotland and Wales), the Isle of Man and Northern Ireland are OBF, and the whole of the United Kingdom is ObmF.
In Iceland, which has no special agreement on animal health (status) with the EU, brucellosis (B. abortus, B. melitensis, B. suis) has never been reported.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
As in previous years, the highest notification rates of brucellosis were reported in three MS that were not ‘officially free of bovine brucellosis’ (OBF Table 25, Figure 38) and/or not officially free of ovine and caprine brucellosis (Brucella melitensis) (ObmF; Table 25, Figure 38): Greece (1.1 per 100,000 population), Portugal (0.48) and Italy (0.35) together accounting for 73.6% of all confirmed cases reported in 2016 (Table 25). The lowest notification rates were observed in MS with the status ‘officially brucellosis‐free’ in cattle, sheep or goats, in which brucellosis cases were mainly travel‐associated. Sweden, which has the status OBF/ObmF and had a relatively high notification rate (0.19 cases per 100,000 population), reported all confirmed brucellosis cases as travel associated.
Figure 38.
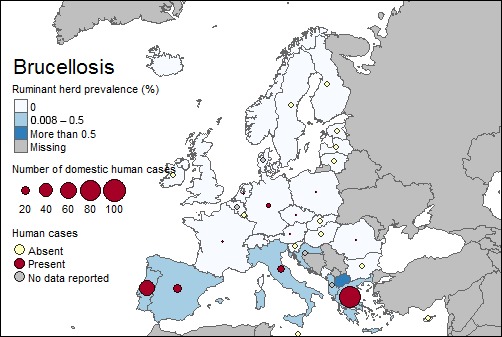
Number of domestically acquired confirmed brucellosis cases in humans, and prevalence of Brucella test‐positive cattle, sheep and goats herds, EU, 2016
The majority (82.6%) of brucellosis cases with known data on importation and travel destination were reported to be acquired in the EU (Table 24). The proportion of infections acquired in the EU has decreased from 79.7% (401 cases) to 37.6% (194 cases) in 2012–2016. During the same time period, cases without data about the importation or travel destination have increased from 11.7% (59 cases) to 54.5 (281 cases).
Among the travel‐associated cases with known probable country of infection, 85.4% (41/48) of the cases represented travel outside EU. Iraq, Syria, Somalia and Turkey were stated as the probable country of infection (27.1%, 10.4%, 10.4% and 8.3% of the imported cases, respectively).
A clear seasonality was observed in the number of confirmed brucellosis cases in the EU/EEA with more cases reported from April to September. There was a significantly (p < 0.01) declining trend from 2008 to 2016. Three MS (Greece, Portugal and Spain) reported decreasing trends (p < 0.05), whereas Germany and Sweden reported an increasing trend (p < 0.01) over the same period. In 2012–2016, the EU/EEA trend was not decreasing or increasing (Figure 37). One MS (Spain) continued to report a decreasing trend (p < 0.01) during 2012–2016. None of the countries observed an increasing trend from 2012 to 2016.
Figure 37.
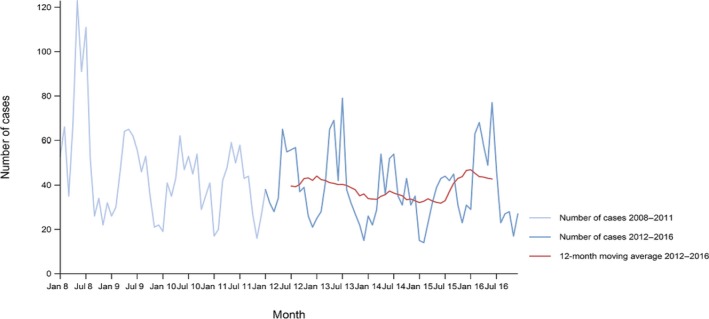
Trend in reported confirmed human cases of brucellosis in the EU/EEA, by month, 2012–2016
- Source(s): Austria, Cyprus, the Czech Republic, Croatia, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia and Spain. Sweden. Belgium, Bulgaria, Luxembourg and the United Kingdom did not report data to the level of detail required for the analysis. Denmark does not have a surveillance system for this disease.
Twelve MS provided data on hospitalisation, accounting for 39.7% of confirmed cases in the EU. On average, 71.2% of the confirmed brucellosis cases with known status were hospitalised. One death due to brucellosis was reported in 2016 among 134 confirmed cases (26.0% of all confirmed cases) by 12 MS.
Brucella species information was missing for 79.8% of the 516 confirmed cases reported in the EU. Of the 97 cases with known species, 84.5% were infected by B. melitensis, 11.3% by Brucella abortus, 2.1% Brucella suis and 2.1% by other Brucella species.
Figure 38 shows, for the year 2016, the number of domestically acquired confirmed brucellosis cases in humans overlaid with the prevalence of Brucella‐positive cattle, sheep and goats herds. The map indicates that Greece, Italy, Portugal and Spain have a higher number of domestically acquired confirmed brucellosis cases in humans and a higher prevalence of Brucella‐positive ruminant herds.
7.3.3. Brucella in foods
Three MS (Italy, Portugal and Spain) provided 2016 Brucella monitoring data from food, in the following categories: single samples from raw milk from cows, sheep and goats, milk from other animal species, cheese and other dairy products excluding cheeses submitted by Italy and Portugal, and fresh meat batch samples from cattle, pigs, sheep and goats, submitted by Spain. In total, 283 samples taken by Italy and Portugal in processing plants, farms and at retail level were tested, and Italy found a total of 24 positive samples at the processing level, of milk from cows (20), milk from sheep (2) and milk from ‘other animal species or unspecified’. Spain did not find any positive meat batch of 1,220,852 tested.
7.3.4. Brucella in animals
Cattle
The status on freedom from bovine brucellosis (OBF) and occurrence of the disease at regional or national levels for MS and non‐MS in 2016 is presented in Figures 39 and 40, respectively. The 2016 list of MS and regions OBF was Austria, Belgium, the Czech Republic, Denmark, Estonia, Finland, France, Germany, 11 regions and 9 provinces in Italy, Ireland, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, all administrative regions within the superior administrative unit of the Algarve as well as six of the nine islands of the Azores in Portugal, Poland, Romania, Slovakia, Slovenia, Sweden, England, Scotland, Wales, Northern Ireland and the Isle of Man in the United Kingdom, the Canary Islands, the Balearic Islands, Basque Country, Murcia, Rioja and Navarre in Spain. The nine MS that did not yet gain country‐level OBF status by the end of 2016 were: Bulgaria, Croatia, Cyprus, Greece, Hungary, Italy, Portugal, Spain and the United Kingdom (Channel Islands Jersey and Guernsey are not yet OBF). Norway and Switzerland were OBF in accordance with EU legislation and Liechtenstein had the same status (OBF) as Switzerland. Iceland, which has no special agreement on animal health (status) with the EU, has never reported brucellosis due to B. abortus, B. melitensis or B. suis.
Figure 39.
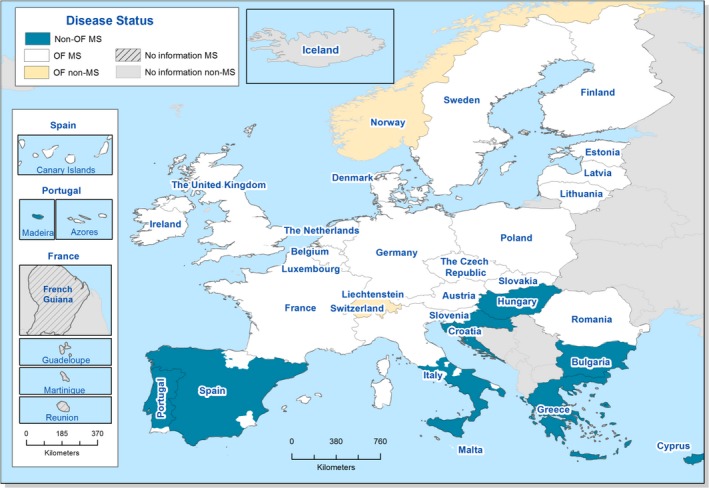
Status of countries on bovine brucellosis, EU/EEA, 2016
- OF: Officially brucellosis free in cattle.
Figure 40.
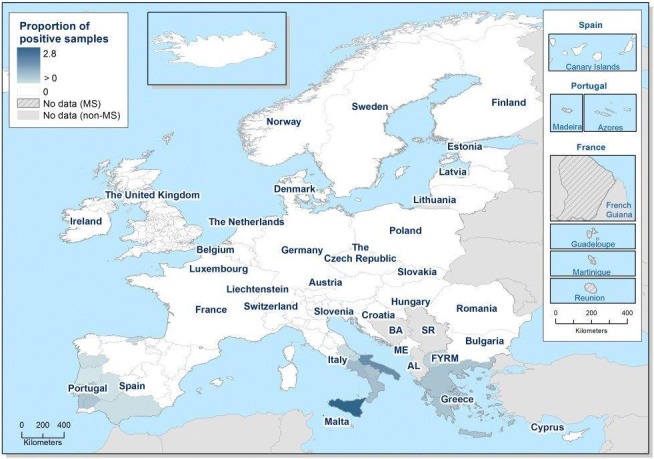
Proportion of cattle herds infected with or positive for Brucella, EU/EEA, 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: Former Yugoslav Republic of Macedonia; ME: Montenegro; and SR, Serbia.
In the 19 MS declared OBF and in the regions declared OBF of the four non‐OBF MS Italy, Portugal, Spain and the United Kingdom, which represents a total population of 2,016,386 cattle herds, annual surveillance programmes are carried out to confirm freedom from bovine brucellosis. In these OBF regions, bovine brucellosis was only detected in two cattle herds in Italy. Bovine brucellosis was not detected in the non‐MS Iceland, Norway, Switzerland and Liechtenstein.
In total, there are 352,893 cattle herds in the non‐OBF regions of the nine non‐OBF MS. Three of the nine non‐OBF MS, namely, Italy, Portugal and Spain had their eradication programmes for bovine brucellosis in their non‐OBF regions approved and cofinanced during 2016 by the EU. These MS reported on the prevalence situation in their non‐OBF regions by the number of positive herds, the number of herds tested under the eradication programme, and the total number of herds existing. The number of positive herds reported in non‐OBF regions was 510 in Italy (598 in 2015), 64 in Portugal (73 in 2015) and 26 in Spain (47 during 2015). The six non‐OBF MS with eradication programmes that were not cofinanced by the EU during 2016 reported the number of infected herds and the total number of herds existing. Of these MS, only Greece reported infected herds, 208 in total compared with 199 in 2015, whereas Bulgaria, Croatia, Cyprus, Hungary and the United Kingdom did not report any cases of infected herds for the year 2016. Therefore, for 2016, 808 positive or infected cattle herds were reported in total in the non‐OBF regions of the non‐OBF MS (938 in 2015).
During the years 2012–2016, there were, respectively, nine, two, two, four and two cattle herds reported infected in the OBF MS or OBF regions of non‐OBF MS, meaning it was an extremely rare event. In the non‐OBF regions of the non‐OBF MS, the overall prevalence of Brucella‐positive or Brucella‐infected cattle herds increased during those years from 0.10% in 2012 to 0.23% in 2016 (Figure 41). Concomitantly, the total number of cattle herds in the non‐OBF regions in the EU decreased from 1,162,978 to 352,893 while the total number of Brucella‐positive or Brucella‐infected cattle herds decreased from 1,181 to 808. The overall increase in the prevalence of Brucella‐positive or Brucella‐infected cattle herds is mainly due to the decrease of number of cattle herds in the non‐OBF regions of the non‐OBF MS.
Figure 41.
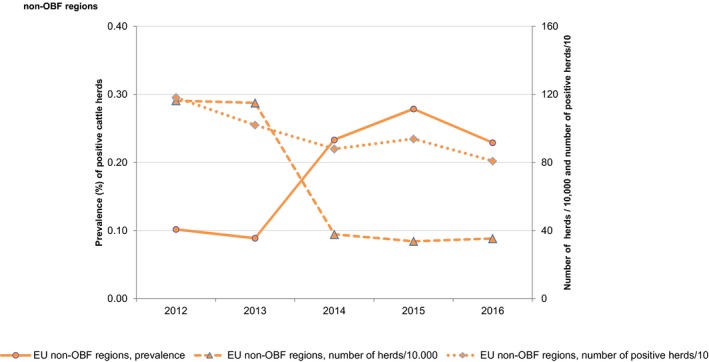
Proportion of Brucella‐positive cattle herds, in non‐OBF regions, EU, 2012–2016
- Non‐OBF: Non‐officially brucellosis free in cattle.
Figure 42 displays the trends in Brucella test‐positive cattle herds in the non‐OBF regions of three MS (Italy, Spain and Portugal) with EU‐cofinanced eradication programmes for bovine brucellosis, during 2004–2016, and in one non‐OBF MS, Greece, that had no EU‐funded eradication programmes for bovine brucellosis. In Portugal and Spain, 64 and 26 test‐positive herds, respectively, remained in their non‐OBF regions in the year 2016 resulting in a very low to rare prevalence, respectively, of 1.59% and 0.02%. In Italy, 510 test‐positive herds remained, leading to a prevalence of 1.59% in its non‐OBF regions, with a reported highest regional prevalence in Sicily (2.8%) (Figure 38). Greece reported 208 Brucella‐infected cattle herds out of 4,026 tested resulting in a Brucella test‐positive prevalence of 5.2%. During 2004–2016, the test‐positive cattle herds reported by Greece ranged from 2.9% in 2006 to 11.5% in 2012.
Figure 42.
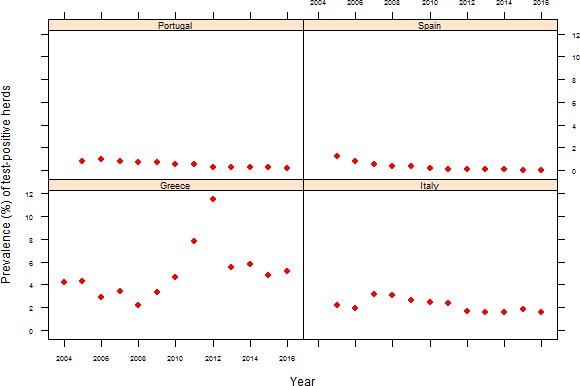
Prevalence of Brucella test‐positive cattle herds, in Greece, Italy, Portugal and Spain, 2004–2016
Three pre‐accession countries, namely Albania, the Former Yugoslav Republic of Macedonia and Montenegro submitted monitoring data on bovine brucellosis for the first time, for the year 2016. Albania reported nine (0.01%) Brucella‐infected cattle herds in their national herd of 119,000 units; the Former Yugoslav Republic of Macedonia 82 (0.31%) out of 26,340 units, whereas Montenegro reported none infected in their national herd of 24,977 units.
Sheep and goats
The status on freedom from ovine and caprine brucellosis caused by B. melitensis (ObmF) and occurrence of the disease at regional or national levels for MS and non‐MS in 2016 is presented in Figures 43 and 44, respectively. The 2016 list of MS and regions ObmF was Austria, Belgium, Cyprus, the Czech Republic, Denmark, Estonia, Finland, all but one of the 96 metropolitan departments in France (Perrin et al., 2015), Germany, Hungary, 13 regions and four provinces in Italy, Ireland, Latvia, Lithuania, Luxembourg, the Netherlands, the Azores in Portugal, Poland, Romania, Slovakia, Slovenia, the Canary Islands, Asturias, Cantabria, Castile and Leon, Galicia, Navarre, Basque Country and the Balearic Islands in Spain, Sweden and the United Kingdom. The eight MS that by the end of 2016 had not yet gained a country‐level ObmF status are Bulgaria, Croatia, France, Greece, Italy, Malta, Portugal and Spain. Norway and Switzerland were ObmF in accordance with EU legislation and Liechtenstein had the same status (ObmF) as Switzerland. Iceland, which has no special agreement on animal health (status) with the EU, has never reported brucellosis due to B. abortus, B. melitensis or B. suis.
Figure 43.
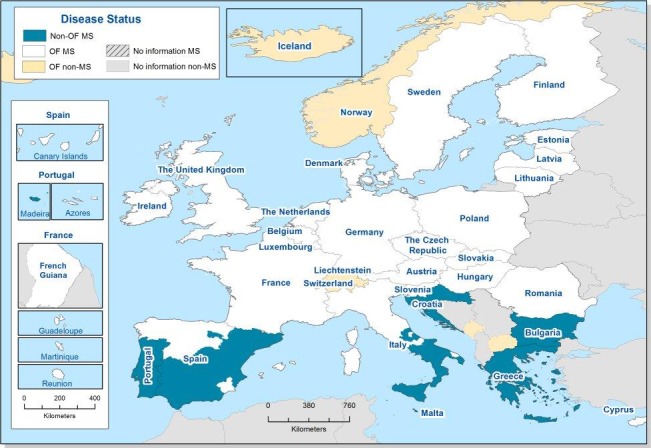
Status of countries and regions on ovine and caprine brucellosis, EU/EEA, 2016
- OF: Officially B. melitensis free in sheep and goats.
- In France, all but one of the 96 metropolitan departments (due to Rev.1 vaccination against Brucella ovis) are ObmF and no cases of brucellosis have been reported in small ruminants since 2003.
Figure 44.
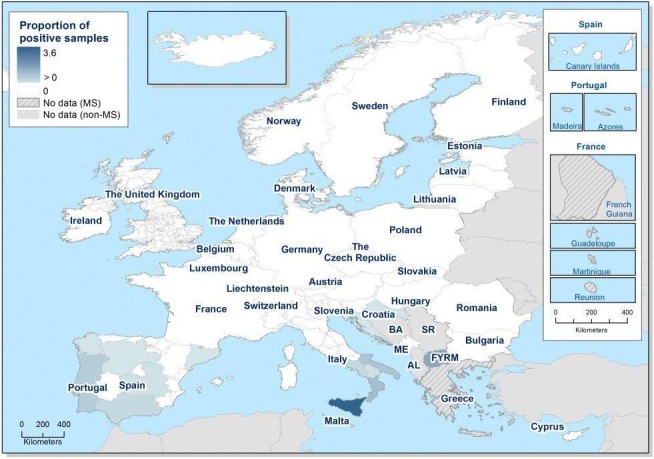
Proportion of sheep and goat herds infected with or positive for brucellosis, EU/EEA, 2016
- Note. AL: Albania; BA: Bosnia and Herzegovina; FYRM: Former Yugoslav Republic of Macedonia; ME: Montenegro; and SR: Serbia.
In the 20 MS declared ObmF and in the regions declared ObmF in the four non‐ObmF MS, France, Italy, Portugal and Spain, which totalled a population of 1,043,677 sheep and goat herds, annual surveillance programmes are carried out to confirm freedom from sheep and goat brucellosis. In these 20 ObmF MS and ObmF regions of the four non‐ObmF MS, brucellosis due to B. melitensis was only detected in two sheep and goats herds in Spain. It was not detected in the non‐MS Iceland, Norway, Switzerland and Liechtenstein.
In 2016, there were 325,989 sheep and goat herds in the non‐ObmF regions of the eight non‐ObmF MS. Four of the eight non‐ObmF MS, namely Croatia, Italy, Portugal and Spain, had their eradication programmes for ovine and caprine brucellosis in their non‐ObmF regions during 2016 approved and cofinanced by the EU. These MS reported on the prevalence situation in their non‐ObmF regions by the number of positive herds, the number of herds tested under the eradication programme, and the total number of herds existing. The number of positive herds reported in non‐ObmF regions was, respectively, 325 in Portugal (482 in 2015), 447 in Italy (465 in 2015), 49 in Spain (77 in 2015) and 8 herds in Croatia (28 in 2015). The four non‐ObmF MS with eradication programmes that were not cofinanced by the EU during 2016 reported the number of infected herds and the total number of herds existing. Of these, Greece reported 41 infected herds (5 in 2015), whereas Bulgaria, France and Malta reported no positive case of infected herds. Therefore, for 2016, 870 positive or infected sheep and goats herds were reported in total in the non‐ObmF regions of the non‐ObmF MS (1,094 in 2015).
During the years 2012–2016, there were, respectively, 5, 4, 3, 10 and 2 sheep and goat herds reported infected in the ObmF MS or ObmF regions of non‐ObmF MS, meaning it was an extremely rare event. In the non‐ObmF regions of the non‐ObmF MS, the overall prevalence of B. melitensis ‐positive sheep and goat herds decreased from 0.49% in 2012 to 0.25% in 2016 (Figure 45). This is due to the decrease in the total number of positive sheep and goat herds from 1,693 in 2012 to 870 in 2016, while the total number of sheep and goat herds remained quite stable in these non‐ObmF regions and was 377,690 in 2012 and 325,989 in 2016.
Figure 45.
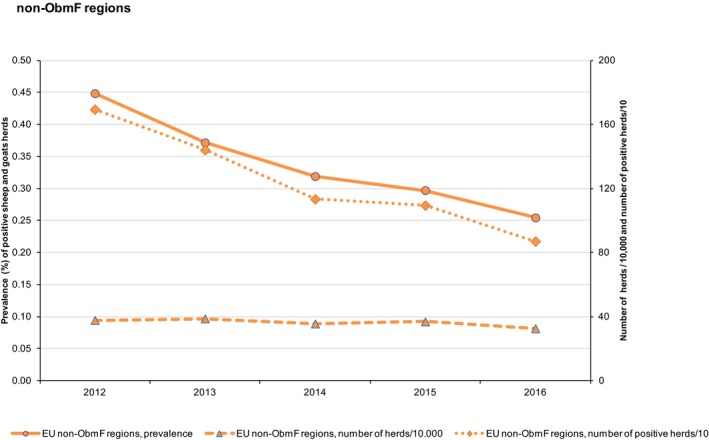
Proportion of sheep and goat herds infected with or positive for B. melitensis, in non‐ObmF regions, EU, 2012–2016
- Non‐ObmF: Non‐officially B. melitensis free in sheep and goats.
Figure 46 displays the trends in B. melitensis test‐positive sheep and goat herds in the non‐ObmF regions of three MS with EU cofinanced eradication programmes for small ruminant brucellosis, during 2005–2016. In Spain, 49 test‐positive sheep and goats herds remained in 2016 resulting in a very low prevalence of 0.09%. Italy and Portugal reported, respectively, 447 and 325 test‐positive herds leading to a low to very low prevalence of 1.21% and 0.57% in their non‐ObmF regions, with a reported highest regional prevalence in Sicily (3.6%) (Figure 44).
Figure 46.
Prevalence of Brucella melitensis test‐positive sheep and goat herds, in three cofinanced MS: Italy, Portugal and Spain, 2004–2016
During 2004–2016, Greece had an approved cofinanced programme in 2007, 2009, 2011, 2012, 2013, 2014 and 2015. During 2016, only vaccination was cofinanced. The monitoring data reported by Greece on brucellosis in sheep and goats pertain exclusively to years of the eradication programme that runs in the Greek islands. The number of reported B. melitensis test‐positive sheep and goats herds vary rather importantly from 0.04% in 2006 to 8.63% in 2012 and was 1.51% during 2016 (Figure 47).
Figure 47.
Prevalence of Brucella melitensis test‐positive sheep and goat herds, in the Greek islands where an eradication programme is implemented, 2004–2016
- Note: During the final production stage of the present report, Greece informed that in mainland Greece where a control programme is implemented; of 20,569 sheep and goats herds tested with blood sampling in the vaccination zone (male animals only), 1,206 (5.9%) were positive, in 2016. The total number of animals tested serologically in the vaccination zone during was 124,770 and 2,294 (1.8%) were positive.
Two pre‐accession countries, namely the Former Yugoslav Republic of Macedonia and Montenegro submitted monitoring data on ovine and caprine brucellosis for the first time, for the year 2016. The Former Yugoslav Republic of Macedonia reported 129 (1.51%) B. melitensis‐infected sheep and goat herds in their national herd of 8,544 units, whereas Montenegro reported none infected in their national herd of 5,676 units.
7.4. Discussion
Brucellosis is a rare, although severe, disease in the EU, with most human cases hospitalised. The highest notification rates and most domestically acquired cases of brucellosis in humans within the EU were reported as in previous years from three MS (Greece, Italy and Portugal) in 2016. These MS, which are not officially brucellosis free in cattle, sheep or goats, consistently report the highest notification rates. These findings might be explained by the Brucella 2016 monitoring and surveillance findings in the cattle and small ruminant populations of these three MS, in which still some hundreds of herds were Brucella‐positive or Brucella‐infected. These data illustrate that brucellosis is still an animal health problem with public health relevance in Greece, Italy and Portugal.
In 2016, the EU notification rate in humans was at the highest level compared with previous years. This was mainly due to increase in the number of cases in Italy, where cases more than doubled compared with 2015. The improvement of surveillance was a possible contributory factor in the increase of reported cases. Most brucellosis cases have been domestically acquired and a few cases have been linked to travel, particularly outside the EU. In the last few years, the number of cases acquired within the EU, however decreased compared with the stable trend of brucellosis in the EU since 2012. At the same time, the majority of the cases were reported without information on travel.
In food, very few monitoring data are reported during these last years by two non‐OF MS Italy and Portugal, on milk and milk products. For 2016, Brucella‐positive findings were reported with 24 samples of cows’ milk and ‘milk from other animal species or unspecified’ at processing plants found positive for Brucella in Italy. The other two MS (Portugal and Spain) that reported surveillance results in food did not report any positive findings.
In livestock, the MS have national surveillance and/or eradication programmes for brucellosis in place. Bovine brucellosis and ovine and caprine brucellosis have been widely eradicated by most MS24 for some years. As a result, outbreaks of these diseases have become rare in large areas of the EU. In the EU OF regions no bovine brucellosis or ovine and caprine brucellosis was reported for the year 2016, except for two Brucella‐infected cattle herds in Italy and two B. melitensis‐infected sheep and goat herds in Spain. In the EU non‐OF regions of non‐OF MS, the overall prevalence of bovine brucellosis and of ovine and caprine brucellosis was very low, 0.23% and 0.25%, respectively, for the year 2016. For bovine brucellosis, the overall prevalence of Brucella‐positive or Brucella‐infected cattle herds has been increasing in the non‐OBF regions of the non‐OBF MS during the years 2012–2016, from 0.10% in 2012 to 0.23% in 2016. This is mainly due to the decrease of number of cattle herds in these non‐OBF regions of the non‐OBF MS while the total number of Brucella‐positive or Brucella‐infected cattle herds only decreased slightly. Italy and Greece reported some hundreds of Brucella‐positive herds, leading to prevalence of test‐positive cattle herds in non‐OBF regions of these MS remaining low (Italy 1.59%, Greece 5.2%). Portugal and Spain reported a very low to rare prevalence. It is noteworthy that in Greece, a National Eradication programme for bovine brucellosis is in place. Vaccination with RB‐51 vaccine is permitted only in dairy cows in three regional units whereas vaccination with REV‐1 vaccine is permitted only in semi‐wild bovines in 17 regional units. Elsewhere, amongst other measures to eradicate bovine brucellosis, a test and cull policy is implemented.
The overall prevalence of B. melitensis‐positive sheep and goat herds in the non‐ObmF regions of the non‐ObmF MS decreased during the years 2012–2016, from 0.49% in 2012 to 0.25% in 2016. Italy and Portugal still reported some hundreds of Brucella test‐positive sheep and goat herds leading to a remaining low to very low prevalence. Spain reported a very low prevalence with some remaining tens of positive herds. The situation of brucellosis in sheep and goats in Croatia and Greece is peculiar. Croatia, which reported eight positive herds, is almost free of the disease but is confronted with rare accidental imports from Bosnia and Herzegovina. However the surveillance coverage has been incomplete until recently, which prevents the country being declared ObmF (Report of the ‘brucellosis’ Task Force subgroup meeting held in Split, Croatia, 17–18 June 2015, EU25). Greece, generally not cofinanced during 2004–2016, reported a 1.51% B. melitensis test‐positive sheep and goat herds. These monitoring results pertain, as for the previous years, exclusively to the eradication programme that runs in the Greek islands, with a test and cull policy. On the Greek mainland, where the prevalence is high, a control programme with, among other measures, mass vaccination is implemented to reduce the prevalence. No data are reported about mainland Greece. Moreover, the numbers of sampled and tested herds from the Greek islands vary considerably from one year to another and is very low in the islands that are considered to be free (Report of the ‘brucellosis’ Task Force subgroup meeting held in Athens, Greece 29–31 March 2017, EU26). These observations may explain importantly why the reported Greek prevalence of Brucella test‐positive sheep and goat herds varied during 2012–2016. As such, these monitoring results may not reliably present the true prevalence situation in Greece, as compared to Italy, Portugal and Spain, because the latter MS apply the same monitoring programme with an exhaustive coverage of the national herd.
Albania, Montenegro and the Former Yugoslav Republic of Macedonia reported data for a first time, as pre‐accession countries. While Montenegro reported no test‐positive cattle herds or sheep and goats herds, Albania and the Former Yugoslav Republic of Macedonia reported 0.01% and 0.31%, respectively, positive cattle herds and the Former Yugoslav Republic of Macedonia reported 1.51% B. melitensis‐infected sheep and goat herds.
No FBO were reported due to Brucella by any MS (see chapter on FBO). Serbia, a pre‐accession country that reported data for a first time, reported one weak‐evidence food‐borne outbreak due B. melitensis causing 15 illnesses of which two patients needed to be hospitalised. The suspected food was a ‘mixed type’ of food.
7.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| Animals | EURL (EU Reference Laboratory) for Brucella | https://sites.anses.fr/en/minisite/lrue-brucellose/brucellosis-home |
| Summary Presentations on the situation as regards Bovine Brucellosis and Brucellosis in Sheep and Goats control and eradication programmes in Member States | https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en#20160705 | |
| General information on EU Food Chain Funding | https://ec.europa.eu/food/funding_en | |
| General information on National Veterinary Programmes, in EU | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en | |
| EU approved and cofinanced veterinary programmes for Bovine Brucellosis and Brucellosis in Sheep and Goats carried out by the MS | http://ec.europa.eu/dgs/health_food-safety/funding/cff/animal_health/vet_progs_en.htm | |
| OIE (World Organisation for Animal health), Summary of Information on Brucellosis | http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Disease_cards/BCLS-EN.pdf | |
| 2016 National Veterinary Programmes funded (cofinanced) by the EU for bovine brucellosis and for ovine and caprine brucellosis (approved programmes and type of measures approved) | https://ec.europa.eu/food/sites/food/files/safety/docs/cff_animal_vet-progs_working_doc_12114_rev2_2016.pdf | |
| EU Task Force on the eradication of animal diseases – Brucellosis subgroup reports | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en |
8. Trichinella
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
8.1. Abstract
In 2016, 101 confirmed trichinellosis cases in humans were reported in the EU. The EU notification was 0.02 cases per 100,000 population, which was a decrease of 26.5% compared with 2015. This was the lowest number of cases and lowest notification rate reported since the beginning of the EU‐level surveillance. Romania reported the highest notification rate, followed by Bulgaria. The EU trend for trichinellosis was greatly influenced by a number of smaller and larger outbreaks with peaks often occurring in January and February. The most commonly reported species in human cases was Trichinella spiralis followed by Trichinella britovi.
In 2016, Trichinella infections have not been reported in samples from 55,563,944 fattening pigs, 1,708,284 breeding pigs and 1,273,947 slaughtered batches from pigs kept under controlled housing conditions, confirming that the farming conditions are the key factor to prevent this zoonosis. In total, 187 (0.0001%) out of 121,232,589 tested fattening pigs and 1 (0.00002%) out of 4,167,862 breeding pigs kept under not controlled housing conditions, were positive. Romania accounted for most positive pigs followed by Poland, Croatia, Bulgaria, France and Spain. In total, 90 (0.29%) farmed wild boar tested positive out of 31,039 tested animals. No Trichinella infection was reported in solipeds. In the last 5 years (2012–2016), the trend for Trichinella infections in domestic animals has been stable, with infections documented in only few hundreds of free‐ranging and backyard pigs and farmed wild boar reared in remote EU regions of Bulgaria, Croatia, France, Poland, Romania and Spain.
In 2016, the Trichinella prevalence in hunted wild boar was 0.02% and 7.23% in brown bears. In red fox, which can be considered as an indicator animal, Trichinella prevalence was 1.1%. During the last 5 years, the reported EU prevalence of Trichinella decreased in the wild boar population (from 0.14% to 0.02%) and in the red fox population (from 3% to 1.1%).
8.2. Surveillance and monitoring of Trichinella in the EU
8.2.1. Humans
The notification of Trichinella infections in humans is mandatory in all MS, Iceland, Norway and Switzerland, except in three MS (Belgium, France and the United Kingdom) having voluntary surveillance systems. No surveillance system for trichinellosis exists in Denmark. The surveillance systems for trichinellosis cover the whole population in all MS except one (Belgium). When no estimate for population coverage was provided, the notification rates were not calculated.
In humans, diagnosis of Trichinella infections is primarily based on clinical symptoms and serology (indirect enzyme‐linked immunosorbent assay (i‐ELISA) and western blot). Histopathology on muscle biopsies is rarely performed.
8.2.2. Animals
Domestic and wild swine, as well as different wildlife species, are the most important potential sources for human infection or may serve as Trichinella spp. reservoirs in the EU.
Trichinella monitoring data from Trichinella‐susceptible animals intended for human consumption in the EU market (domestic pigs, horses, wild boar and other farmed or wild animal species)
According to the Commission Regulation 2015/137527, all Trichinella‐susceptible animals intended for human consumption in the EU market, should be tested for the presence of the parasite larvae in the muscles or carcasses should be appropriately frozen to make the product safe. Therefore, carcasses of domestic pigs, horses, wild boar and other farmed or wild animal species that are susceptible to Trichinella infection should be systematically sampled at slaughter as part of the meat inspection process and tested for Trichinella. It follows that data on Trichinella infections in these animals are comparable across MS because the monitoring schemes are harmonised and the data collected and reported to EFSA originates from a census sampling.
Trichinella monitoring data from Trichinella‐susceptible animals intended for human consumption in the EU market (domestic pigs, horses, wild boar and other farmed or wild animal species), the carcasses of which are intended for human consumption in the EU market, are based on programmed surveillance/monitoring. They are collected in a fully harmonised way and with harmonised reporting rules, and therefore allow subsequent data analyses such as spatial and temporal trends at the EU‐level. They can be used for descriptive summaries at EU‐level, and monitoring of EU trends (Table 1).
Commission Regulation (EU) No 1375/2015 states that the reporting of data from domestic swine shall at least provide specific information on the number of animals tested that were raised under controlled housing conditions and the number of breeding sows, boars and fattening pigs tested. Furthermore, the Regulation states that a negligible risk status for a country or region is no longer recognised. Instead, such recognition is linked to compartments of one or more holdings applying specific controlled housing conditions. Belgium and Denmark have had such a status since 2011, and the holdings and compartments of domestic swine in those two MS complied with the conditions for controlled housing at the date when this Regulation came into force. Taking account of this legislation, detailed information on the data reported by MS and non‐MS on the occurrence of Trichinella in pigs raised under controlled housing conditions, and in pigs and farmed wild boar not raised under controlled housing conditions has been summarised in Table 28 and data for wild animals are presented in Table 29.
Table 28.
Number of Trichinella‐positive/tested (% positive) domestic pigs and farmed wild boar in EU and non‐MS in 2016
| Reporting country | Positive/tested (% positive) | ||||
|---|---|---|---|---|---|
| Farmed wild boar | Fattening pigs | Breeding pigs | Fattening pigs | Breeding pigs | |
| Not controlled housing conditions or not specified | Controlled housing conditions | ||||
| AT | 0/696 | 0/5,109,372 | 0/88,191 | ||
| BE | 0/11,212,479 | ||||
| BG | 3/323 (0.99)a | 0/165,994b | 0/1,935c | ||
| HR | 13/1,359,580d (< 0.01) | ||||
| CY | 0/563,343 | 0/9,947 | |||
| CZ | 0/2,463,053 | ||||
| DK | 0/589 | 0/698,944 | 0/309,942 | 0/17,052,191 | 0/184,579 |
| EE | 0/483,510 | 0/4,972 | |||
| FI | 0/342 | 0/2,007,052 | 0/43,038 | 0/329 | 0/13 |
| FR | 1/2,250e (0.04) | 4/340,942 (< 0.01) | 0/237,784 | ||
| DE | 0/59,611,276 | ||||
| EL | 0/1,272 | 0/3,023 | 0/1,076,871 | 0/21,482 | |
| ES | 0/13,077,478f | 1/3,550,915 (< 0.01) | 0/3,060,989g | 0/1,178,734 | |
| HU | 0/4,275,004 | 0/132,608 | |||
| IE | 0/3,226,075 | 0/93,732 | |||
| IT | 0/14,935 | 0/144,833 | 0/9,249,768 | 0/109,051 | |
| LV | 0/452,533 | ||||
| LT | 0/1,598,881 | ||||
| LU | 0/173,835 | ||||
| MT | 0/55,043 | 0/1,269 | |||
| NL | 0/1,241 | 0/155,573 | |||
| PL | 16/22,438,554 (< 0.01) | ||||
| PT | 0/218,951 | 0/2,216 | 0/4,324,239 | 0/23,874 | |
| RO | 89h/9,714 (0.92) | 151/4,501,501 (< 0.01) | 0/108 | 0/35,237 | |
| SK | 0/543,719 | 0/11,527 | |||
| SI | 0/258,307 | ||||
| SE | 0/416,950 | 0/19,370 | 0/860,609 | 0/27,428 | |
| UK | 0/436,582 | 0/4,936,071 | 0/30,950 | ||
| EU total | 90/31,039 (0.29) | 187/121,232,589 (< 0.01) | 1/4,167,862 (< 0.01) | 0/55,563,944 | 0/1,708,284 |
| IS | 0/77,603 | ||||
| ME | 0/9,995 | ||||
| NO | 0/1,651,000 | ||||
| CH | 0/2,487,220 | 0/32,760 | |||
| Total non‐MS | 0/4,138,220 | 0/32,760 | 0/87,598 | ||
| Total EU + 4 non‐MS | 90/31,039 (0.29) | 187/125,370,809 (< 0.01) | 1/4,200,622 (< 0.01) | 0/55,651,542 | 0/1,708,284 |
Trichinella was also detected in 2 out of 311 (0.64%) pig herds; no Trichinella was detected in 28,912 slaughter batches.
In addition, 185,208 slaughter batches were reported of which none was positive.
In addition, 16 slaughter batches were reported of which none was positive.
Mixed herds (both breeding and fattening pigs).
Imported from Poland and infected with T. spiralis.
In addition, no Trichinella larvae were detected in 79,997 slaughter batches.
In addition, no Trichinella larvae were detected in 979,814 slaughter batches.
19 out of 89 were infected with T. spiralis.
Table 29.
Number of Trichinella‐positive/tested (% positive) hunted wild boar and other wild animals in EU and non‐MS in 2016
| Country | Positive/tested (% positive) | |||
|---|---|---|---|---|
| Hunted or not specified wild boar | Brown bears | Red foxes | Other wild animals | |
| AT | 0/22,468 | 0/9a | ||
| BE | 2/11,507 (0.2) | |||
| BG | 2/976b (1.1) | |||
| HR | 13/25,523 (0.05) | 3/150 (2) | ||
| CY | 0/81 | |||
| CZ | 3/163,550 (< 0.01) | 2/3,015 (0.07) | ||
| DK | 0/28 | 0/15c | ||
| EE | 22/4,342 (0.51) | 8/36 (22.2) | ||
| ES | 132/63,040 (0.2) | |||
| FI | 0/924 | 5/127 (4.0) | 30/90 (33.3) | 139/497 (28)d |
| FR | 1/42,560 (< 0.01) | |||
| DE | 4/477,880 (< 0.01) | |||
| EL | 0/40 | 11/126 (8.7)m | 8/19e | |
| HU | 3/67,029 (< 0.01) | 0/13 | 0/2f | |
| IT | 9/147,930 (< 0.01) | 7/2,270 (0.3) | 25/761 (3.3)g | |
| LV | 47/7,312 (0.6) | 3/4 | 3/11h | |
| LU | 0/3,513 | 0/141 | 0/47i | |
| PT | 0/133 | |||
| RO | 16/89 (18) | |||
| SK | 11/16,818 (0.06) | 0/10 | 19/181 (10.5) | |
| SI | 4/4,625 (0.1)j | 0/16 | ||
| SE | 3/91,289 (< 0.01) | 1/225 (0.4) | 1/55 (1.8) | 10/239 (4.2)k |
| UK | 0/1191 | 0/285 | 0/74a | |
| EU total | 256/1,152,650 (0.02) | 33/507 (6.5) | 73/6,435 (1.1) | 185/1,674 (11.1) |
| ME | 0/133 | |||
| CH | 0/4,142 | 0/1 | 0/2 | 3/26 (11.5)l |
| Total EFTA | 0/4,275 | 0/1 | 0/2 | 3/26 (11.5) |
| Total EU + EFTA | 256/1,156,925 (0.02) | 32/508 (6.5) | 73/6,437 (1.1) | 188/1,700 (11.0) |
Badgers.
In addition, 3 (0.32%) out of 929 tested wild boar batches were positive.
Raccoon dogs.
88/227 (38.76%) raccoon dogs, 27/90 (30%) wolves, 2/2 wolverines, 15/46 (32.60%) lynxes, 1/11 badgers, 3/11 martens, 1/39 (2.6%) otters, 1/1 rats, 0/1 beaver, 0/6 mink and 0/5 seals; interesting to note the detection of T. pseudospiralis in a goshawk (Accipiter gentilis) of Finland out of 58 (1.72%) tested carnivore birds.
6/8 wolves, 2/3 ferrets, 0/3 badgers, 0/2 jackals, 0/2 mink and 0/1 wild cat.
Jackals.
13/98 wolves, 0/275 badgers, 0/121 stone martens, 1/58 birds.
3/3 lynxes, 0/1 badger and 0/7 beavers.
0/29 raccoons and 0/18 wild cats.
Positive animals originated from Hungary.
7/103 (6.79%) lynxes, 3/43 (6.97) wolves, 0/13 badgers, 0/15 beavers, 0/33 birds, 0/1 marten, 0/20 otters, 0/10 seals and 0/1 wolverine.
3/21 lynxes, 0/1 jackal and 0/3 wolves.
Results reported by Greece in 2016 refers to samplings between 2012 and 2015.
Trichinella monitoring data from other animals
MS should monitor the circulation of Trichinella in the main natural reservoir hosts (carnivore and omnivore animals) to acquire information on the risk of transmission to domestic animals and from them to humans, and on the introduction of new Trichinella species from non‐EU countries. Both, domestic and wild animals (e.g. wild boar) slaughtered for own consumption are not covered by Commission Regulation 2015/1375 and are subject to national rules. The national legislation differs between MS and such data reported to EFSA may not be comparable between MS. For the year 2016, only Italy provided such data. Lastly, the reported number of pigs slaughtered for own consumption is probably an underestimation of the true number since most of these animals escape any veterinary control.
Generally, sampling biases and low numbers of specimens examined preclude reliable prevalence estimations. Consequently, these data must be considered monitoring data provided by the MS to EFSA that are generated by non‐harmonised monitoring schemes across MS and for which no mandatory reporting requirements exist. The main reservoir hosts of Trichinella are wild animals and their biology and ecology vary from MS one to another and from one region or habitat to another in the same MS due to the human and environmental impact on the ecosystems, which results in different transmission patterns and prevalence of infection.
Trichinella monitoring data from other animals, submitted to EFSA and collected without harmonised design allows for descriptive summaries at EU‐level. Lack of harmonisation precludes trend analyses and trend watching at EU‐level (Table 1).
8.2.3. Food‐borne outbreaks of human trichinellosis
The reporting of FBO of human trichinellosis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
8.3. Results
8.3.1. Human trichinellosis
In 2016, 166 cases of trichinellosis, including 101 confirmed cases, were reported in 27 MS (Table 26). The EU notification rate in 2016 was 0.02 cases per 100,000 population which represented a decrease of 26.5% compared with 2015 (0.03 per 100,000) and a steady decrease in the last 5 years. In 2016, Bulgaria had the highest notification rate in the EU (0.49 cases per 100,000), followed by Romania and Croatia with 0.13 and 0.12 cases per 100,000, respectively. Together, Bulgaria and Romania accounted for 60.4% of all confirmed cases reported at the EU‐level in 2016. Fourteen MS reported zero confirmed cases in 2016 including four MS (Cyprus, Luxembourg, Malta and Portugal) that have never reported any trichinellosis cases. Three countries (the Czech Republic, Finland and the Netherlands) have reported only one case each since the beginning of EU‐level surveillance in 2007.
Table 26.
Reported human cases of trichinellosis and notification rates per 100,000 population in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 2 | 2 | 0.0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Belgiumb | Y | A | 0 | 0 | – | 0 | – | 16 | – | 1 | – | 0 | – |
| Bulgaria | Y | A | 35 | 35 | 0.49 | 22 | 0.31 | 60 | 0.83 | 36 | 0.49 | 30 | 0.41 |
| Croatia | Y | A | 5 | 5 | 0.12 | 3 | 0.07 | 3 | 0.07 | 0 | 0.00 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic | Y | C | 1 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 |
| Denmarkc | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0.00 | 2 | 0.15 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland | Y | C | 0 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| France | Y | C | 3 | 3 | 0.00 | 3 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Germany | Y | C | 4 | 4 | 0.00 | 3 | 0.00 | 1 | 0.00 | 14 | 0.02 | 2 | 0.00 |
| Greece | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Hungary | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Ireland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Italy | Y | C | 5 | 5 | 0.01 | 36 | 0.06 | 4 | 0.01 | – | – | 33 | 0.06 |
| Latvia | Y | C | 1 | 1 | 0.05 | 4 | 0.20 | 5 | 0.25 | 11 | 0.54 | 41 | 2.01 |
| Lithuania | Y | C | 1 | 1 | 0.03 | 21 | 0.72 | 5 | 0.17 | 6 | 0.20 | 28 | 0.93 |
| Luxembourg | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Poland | Y | C | 4 | 4 | 0.01 | 1 | 0.00 | 6 | 0.02 | 4 | 0.01 | 1 | 0.00 |
| Portugal | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Romania | Y | C | 88 | 26 | 0.13 | 55 | 0.28 | 221 | 1.11 | 116 | 0.58 | 149 | 0.74 |
| Slovakia | Y | C | 1 | 1 | 0.02 | 1 | 0.02 | 0 | 0.00 | 5 | 0.09 | 5 | 0.09 |
| Slovenia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.05 | 1 | 0.05 |
| Spain | Y | C | 14 | 12 | 0.03 | 3 | 0.01 | 1 | 0.00 | 23 | 0.05 | 10 | 0.02 |
| Sweden | Y | C | 2 | 2 | 0.02 | 1 | 0.01 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 |
| United Kingdom | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| EU total | – | – | 166 | 101 | 0.02 | 156 | 0.03 | 324 | 0.06 | 217 | 0.04 | 301 | 0.06 |
| Iceland | Y | C | 0 | 0 | 0.00 | 0.0 | 0.00 | 0 | 0.00 | 0 | 0.00 | – | – |
| Norway | Y | C | 0 | 0 | 0.00 | 0.0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Switzerlandd | Y | C | 0 | 0 | 0.00 | 2 | 0.02 | 0 | 0.00 | 1 | 0.01 | 1 | 0.01 |
Y: yes; N: no; A: aggregated data; C: case‐based data; −: no report.
Disease not under formal surveillance.
No surveillance system.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The vast majority (> 99%) of trichinellosis cases with known data on importation was reported to be domestically acquired (Table 27). Two MS reported one trichinellosis case each as travel associated (one case infected outside EU and one case infected within EU).
Table 27.
Summary of Trichinella statistics related to humans and major animal species, EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 101 | 156 | 324 | 217 | 301 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.02 | 0.03 | 0.06 | 0.04 | 0.06 | ECDC |
| Number of reporting MS | 27 | 27 | 27 | 27 | 27 | ECDC |
| Infection acquired in the EU | 53 | 126 | 40 | 170 | 114 | ECDC |
| Infection acquired outside the EU | 1 | 0 | 0 | 0 | 0 | ECDC |
| Unknown travel status or unknown country of infection | 47 | 30 | 284 | 47 | 187 | ECDC |
| Number of food‐borne outbreaks | 5 | 15 | 17 | 22 | 24 | EFSA |
| Number of outbreak‐related cases | 14 | 119 | 187 | 174 | 148 | EFSA |
| Animals | ||||||
| Domestic fattening pigs NRUCH | ||||||
| Number of tested animals | 121,232,589 | 110,442,115 | 158,823,132 | 127,725,287 | 122,000,276 | EFSA |
| Proportion of positive animals (%) | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | EFSA |
| Number of reporting MS | 25 | 20 | 24 | 24 | 24 | EFSA |
| Farmed wild boar | ||||||
| Number of tested animals | 31,039 | 31,617 | 41,623 | 7,905 | 6,405 | EFSA |
| Proportion of positive animals (%) | 0.29 | 0.0 | 0.24 | 0.025 | 0.14 | EFSA |
| Number of reporting MS | 8 | 8 | 10 | 9 | 9 | EFSA |
| Hunted wild boar | ||||||
| Number of tested animals | 1,152,650 | 957,480 | 891,159 | 872,216 | 859,205 | EFSA |
| Proportion of positive animals (%) | 0.02 | 0.05 | 0.12 | 0.13 | 0.14 | |
| Number of reporting MS | 20 | 19 | 20 | 24 | 22 | EFSA |
| Red foxes | ||||||
| Number of tested animals | 6,435 | 9,762 | 10,447 | 8,708 | 9,073 | EFSA |
| Proportion of positive animals (%) | 1.1 | 1.3 | 1.2 | 2.1 | 3.0 | EFSA |
| Number of reporting MS | 12 | 11 | 13 | 17 | 15 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States; NRUCH: not raised under controlled housing conditions.
The EU/EEA trend from 2009 to 2016 in confirmed cases of trichinellosis was substantially influenced by a number of smaller and larger outbreaks, often with peaks in January and February (Figure 48). No significant increasing or decreasing trends were observed for any country from 2012 to 2016.
Figure 48.
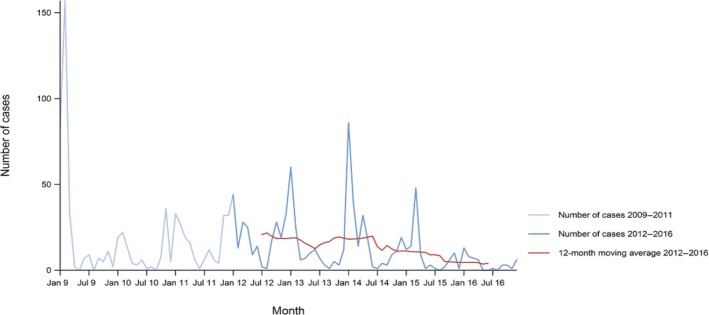
Trend in reported confirmed human cases of trichinellosis in the EU/EEA by month, 2012–2016
- Source(s): Austria, Cyprus, the Czech Republic, Estonia, Finland, France, Greece, Hungary, Ireland, Latvia, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Sweden and the United Kingdom. Bulgaria, Croatia, Germany, Iceland, Italy, Lithuania and Spain did not report data to the level of detail required for the analysis. Belgium and Denmark do not have any formal surveillance system for the disease.
Of the 14 MS reporting confirmed cases for 2016, seven provided information on hospitalisation (45.5% of all confirmed cases reported in the EU) with 65.2% of these cases reported as having been hospitalised. Eight MS provided information on the outcome of their cases (50.5% of all confirmed cases). No deaths due to trichinellosis were reported in 2016 among the 51 confirmed cases for which this information was available.
Species information was available for 42.6% of the reported confirmed cases from 12 MS. The most commonly reported species was T. spiralis (72.1%) followed by T. britovi (27.9%). Bulgaria reported all the cases infected by T. britovi.
Table 27 summarises EU‐level statistics related to human trichinellosis, and to Trichinella occurrence and prevalence in major animal species, respectively, in the EU, during 2012–2016.
Figure 49 shows the number of domestically acquired confirmed human cases of Trichinella overlaid with the proportion of positive animals in wildlife species (wild boar, red foxes, brown bears and other wild animals). The map indicates that Bulgaria, Romania and Spain have a higher number of domestically acquired confirmed trichinellosis cases as well as a higher occurrence of Trichinella‐positive wildlife. Finland, Estonia and Greece, on the contrary, reported no human cases domestically acquired for 2016 while the prevalence of Trichinella is relatively high in their wildlife.
Figure 49.
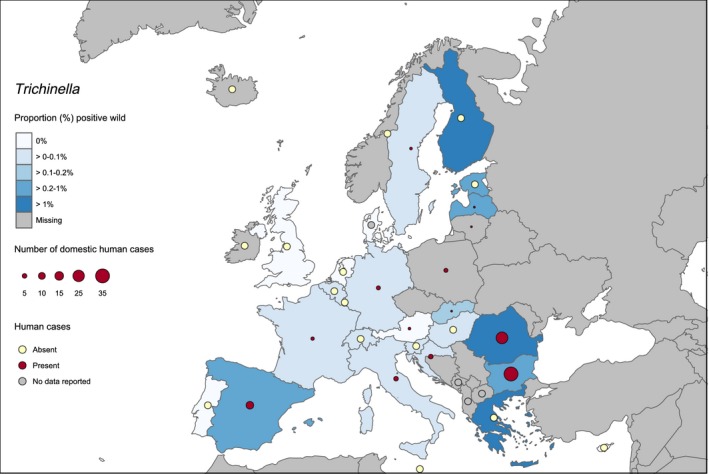
Confirmed domestically acquired Trichinella cases in humans, and prevalence of Trichinella in wild animals (wild boar, red foxes, brown bear and other wild animals), EU, 2016
- The results by Greece, reported in 2016, refer to samples taken between 2012 and 2015.
8.3.2. Trichinella in animals
In 2016, 32 countries (28 MS and four non‐MS) provided information on Trichinella in domestic animals (pigs and/or farmed wild boar) and 6 MS reported positive findings.
Sixteen MS reported data on breeding and fattening pigs raised under controlled housing conditions, no positive findings were reported (Table 28).
In total, 55,563,944 fattening pigs, 1,708,284 breeding pigs and 1,165,038 slaughtered batches from pigs kept under controlled housing conditions were reported to have been tested for Trichinella spp. in 16 MS. None of these animals tested positive. Iceland and Montenegro tested 87,598 fattening pigs kept under controlled housing conditions, and all were negative.
Twenty‐six MS and two non‐MS reported data on breeding and fattening pigs or farmed wild boar that were not raised under controlled housing conditions and six MS reported positive findings among fattening or breeding pigs (Table 28). In total, 187 out of 121,232,589 tested fattening pigs and 1 out of 4,167,862 breeding pigs were positive. Romania accounted for most positive pigs followed by Poland, Croatia, Bulgaria, France and Spain. In total, 90 farmed wild boar tested positive in France and Romania out of 31,039 tested animals from eight MS. Two out of 311 pig herds not kept under controlled housing conditions tested positive for Trichinella in Bulgaria. Norway and Switzerland tested 4,138,220 fattening pigs from not controlled housing conditions and all were negative (Table 28).
As shown in Figure 50 from 1995 to 2016 (21‐year period), Trichinella spp. were not documented in domestic pigs in 13 MS (Austria, Belgium, Cyprus, the Czech Republic, Denmark, Ireland, Luxembourg, Malta, the Netherlands, Portugal, Slovenia, Sweden and the United Kingdom) while this was the case in the other 15 MS (Bulgaria, Croatia, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovak Republic and Spain).
Figure 50.
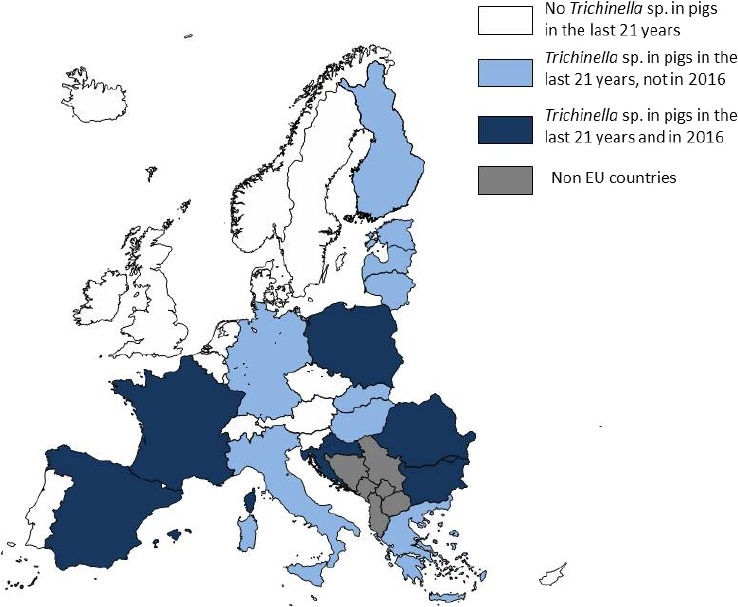
Trichinella spp. in domestic pigs of 28 Member States and 3 non‐Member States (IS, NO and CH) in the last 21 years and reported to EFSA for 2016
- This distribution map has been built based on data from the International Trichinella Reference Centre (ITRC, online) EFSA reports and published papers.
In the EU in total, more than 186 million animals (breeding pigs, fattening pigs and unspecified pigs kept under controlled or non‐controlled housing conditions and farmed wild boar) were tested for Trichinella and 278 were positive (1.6 per million tested). Most (82.5%) of the positive findings were reported by Romania followed by Poland (8.6%), Croatia (7%) and Bulgaria (1.5%). All Trichinella spp.‐infected pigs originated from animals not kept under controlled housing conditions. Most (67%) of Trichinella spp. isolates from swine were not identified at the species level. However, when larvae were identified (33.4% of cases), 80.2% of the isolates were T. spiralis and 19.8% were T. britovi. Information on the number of fattening and breeding pigs reared under controlled housing conditions was reported from 14 and 12 MS, respectively (Table 28).
No positive findings were reported from 139,543 domestic solipeds tested in 21 MS (Austria, Belgium, Bulgaria, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Luxembourg, Malta, the Netherlands, Portugal, Romania, Slovenia, Spain and the United Kingdom) and in two non‐MS (Iceland and Switzerland).
Twenty MS and two non‐MS provided data on hunted wild boar (Table 29). Fourteen MS reported 256 positive findings out of 1,152,650 animals tested (0.02%). Most positive animals were reported by Spain, Latvia, Estonia, Bulgaria, Croatia and Slovakia. Most findings were reported as Trichinella spp. (74%) followed by T. britovi (21%) and T. spiralis (4.7%). A wild boar infected by Trichinella pseudospiralis was identified in Sweden. In addition, three (0.3%) out of 929 tested wild boar batches from Bulgaria were reported positive for Trichinella spp.
Twelve MS and one non‐MS reported data on Trichinella in red foxes (Vulpes vulpes) with a total of 73 (1.1%) positive out of 6,435 tested animals in seven MS (Table 29). Fourteen MS and one non‐MS reported data on Trichinella in other wild animals. Positive findings were detected in 11 species (lynx, brown bear, raccoon dog, wolf, wolverine, badger, marten, ferret, otter, rat and goshawk) of five MS and in one non‐MS (Table 29). The highest prevalence was detected in lynxes, brown bears, raccoon dogs and wolves.
8.4. Discussion
Trichinellosis is a rare but serious human disease, which is still present in the EU. Almost half of the MS reported zero cases including four MS (Cyprus, Luxembourg, Malta and Portugal) that never have reported any trichinellosis cases in humans during the last 50 years.
Most human cases were reported from a few MS mainly in eastern Europe and were domestically acquired. The EU/EEA trend for trichinellosis has been greatly affected by the number and size of disease outbreaks. The number of cases and EU notification rate has, however, been steadily decreasing in the last 5 years since 2012, and in 2016 the lowest rate (0.02) was reported since the beginning of the EU‐level surveillance. The decrease was mainly due to a markedly reduced number of trichinellosis cases over the same period reported from two MS (Bulgaria and Romania), which had experienced most Trichinella outbreaks in previous years. The main reason of this reduction was probably the increasing number of pigs raised under controlled housing conditions and the reduction of pigs not raised under controlled housing conditions, farmer's education and increased control at slaughtering of pigs not raised under controlled housing conditions. These measures strongly reduced the parasite biomass in the domestic habitat and so the risk for humans to get infected. Despite these reduced numbers, Bulgaria and Romania still reported more than half of the confirmed cases and outbreaks in 2016. The recurring peak in trichinellosis cases in January and February may reflect the consumption of various pork products during the Christmas period as well as the wild boar hunting season. On average, one‐third of the confirmed human trichinellosis cases were hospitalised, but with no fatal outcomes.
In 2016, seven Trichinella outbreaks were reported by five MS (reporting rate < 0.01 outbreak per 100.000 population). Five outbreaks were reported by Serbia (a pre‐accession country). In total 27 people were affected of which 11 were hospitalised. Four of the outbreaks were reported with strong evidence by Poland, Romania and Spain, and all were associated with ‘meat and its products’ mainly from pigs.
Generally, Trichinella is considered a medium risk for public heath related to the consumption of pig meat, and integrated preventive measures and controls on farms and at slaughterhouses can ensure effective control of Trichinella (EFSA BIOHAZ, CONTAM and AHAW Panels, 2001). In pigs raised indoors, the risk of infection is mainly related to the lack of compliance with rules on the treatment of animal waste. Pigs raised outdoors are at risk of contact with potentially Trichinella‐infected wildlife (EFSA and ECDC, 2011). In the last decades in the MS, investigations carried out to identify the source of Trichinella infections in domestic pigs, identified direct (free‐ranging pigs) or indirect (e.g. farmers, who were hunters) contacts with wild animals, which are the reservoir of these zoonotic nematodes (Pozio, 2014).
In the EU, most pigs are subject to official meat inspection at slaughter in accordance with Regulation (EC) No 2015/1375; only pigs slaughtered for own consumption are not covered by the regulation. In total, 186 million pigs were tested for Trichinella in MS in 2016, out of about 246 million reared pigs in the EU (Marquer et al., 2014), with only 184 positive animals, i.e. 0.7 per million reared pigs.
There was a peak at the beginning of the 1990s of Trichinella infections in domestic pigs and also in wild animals that were fed on pork scraps and offal dispersed by humans in the environment of some MS. Since then, the prevalence has reduced significantly in the pig population (all cases arising from pigs that were not kept in controlled housing conditions) but has become stable. This reported prevalence is probably an underestimation of the true prevalence, as some free‐ranging and backyard pigs from remote areas of EU are not controlled by veterinary services. There is a vicious circle between uneducated and low‐income people, remote areas, inadequacy of local veterinary services and the occurrence of Trichinella in domestic animals in the EU. Therefore, providing information and training courses to people rearing backyard and free‐ranging pigs and hunters in villages of remote areas on the risk of acquiring trichinellosis and on the pig rearing conditions to prevent the transmission of this zoonotic parasite is of importance.
Only six out of 28 MS reported Trichinella in pigs in 2016, with an overall prevalence of 0.00011%. All the positive findings were from pigs not raised under controlled housing conditions. As in 2015, Romania accounted for most (80.7%) of the reported positive findings in pigs not raised under controlled housing conditions or for which the raising conditions were unknown. Also Poland, Croatia, Bulgaria, France and Spain reported positive pigs. Compared with 2015, there is an increased number of animals reported positive; however, since data reports are inconsistent across years as regards countries and regions, and animal species, this apparent increase may not necessarily mirror a true increase. In fact, most of the pigs considered in the group of those not reared under controlled housing conditions, are backyard or free‐ranging animals from a myriad of herds, which may not undergo veterinary inspection. Consequently, the true underlying prevalence and spread may be underestimated. The herd size of the pig herds and their distribution in the EU is a key factor favouring the circulation of Trichinella parasites among pigs. In the 28 EU countries, the distribution of the pig population by herd size (in numbers of fattening pigs) showed that 1.5% of pig farms have at least 400 fattening pigs and manage 75.7% of these (approximately 120 million animals) (Marquer, 2010). These figures conceal national differences. For example, only 21.6% of fattening pigs in Poland are kept under controlled conditions on farms as compared with 90% or more in nine other EU countries. However, pigs kept in units of less than 10 animals represent a consistent part of the pig population in Bulgaria (34.8%), Lithuania (31.9%) and Romania (66.2%). These small units manage 5.3% of fattening pigs (approximately 8.5 million animals), but account for 85.8% of the pig farms (Marquer, 2010). In fact, the higher the number of small pig herds in a country, the higher the risk of Trichinella infections in pigs (Pozio, 2014). Furthermore, most Trichinella infections are in domestic pigs that are intended for own consumption, i.e. the pigs at higher risk for this infection, that are not registered or are not reported to EFSA. EFSA has identified that non‐controlled housing condition is a main risk factor for Trichinella infections in domestic pigs, and the risk of Trichinella infection in pigs from well managed officially recognised controlled housing conditions is considered negligible (EFSA and ECDC, 2011). Most humans become infected when consuming undercooked meat from pigs or wild boar that have not been tested for Trichinella spp. In 2016, Trichinella spp. have been detected in farmed wild boar in Romania and France (imported from Poland), which are assumed to be reared as pigs not raised under controlled conditions.
No positive findings were reported from solipeds in 2016. In the last decade, only four horses tested positive out of more than one million tested animals in 2008, 2010 and 2012. This extremely low (< 0.001%) prevalence could be related to the reduction of Trichinella infections in domestic pigs, as pig scraps and offal were the main source of infection for horses.
Trichinella circulates among wild animals in large parts of Europe and only Cyprus, Luxembourg and Malta have never reported any positive findings. In 2016, 17 MS and one non‐MS reported positive findings in wild animals. The lack of positive findings or confirmation of previous findings in other MS during 2016 is simply due to the lack of surveys, inadequacy of sample sizes, or investigation in regions where the environmental conditions do not favour the transmission of these zoonotic nematodes among wildlife.
In addition to domestic pigs, hunted wild boar is the second source of trichinellosis infection for humans. However, the prevalence of Trichinella spp. infections in this animal species has declined over the years due to the increased control for these pathogens in the domestic habitat. In fact, the prevalence of Trichinella infections in wild animals is influenced by human behaviour and rearing practices, which favour the transmission of these pathogens from the domestic to the sylvatic habitat by the spread of pork and hunted animal scraps and offal. In the last 5 years (2012–2016), the prevalence of infection decreased in the wild boar population (from 0.14% in 2012 to 0.02% in 2016) and in the red fox population (from 3.0% in 2012 to 1.1% in 2016). This reduction could be only considered ‘apparent’ due to different sampling areas and the lack of data from some MS.
The proportion of positive samples from wildlife, other than wild boar, was higher in bears, lynxes, raccoon dogs and wolves. In 2016, Trichinella spp. was also reported from badgers, ferrets, martens, otters, red foxes and wolverines. Carnivore mammals at the top of the food chain and with a life span longer than that of other animals are more likely to be infected (e.g. lynxes, wolves, bears); however, the population size and the distribution of these animals in Europe are generally limited. Red foxes, with a much larger and widespread population, can be considered as the main natural reservoirs for these pathogens throughout Europe. The lower prevalence of infection in red foxes (1.12%) than that detected in other carnivores is probably related to the spread of this mammal in populated areas where it feeds mainly on garbage resulting from human activities, where Trichinella spp. are not transmitted. It follows that only a percentage of tested foxes originated from regions where Trichinella spp. are circulating. In support of this, the highest prevalence of infection in red foxes has been detected in Finland (33.3%), Slovakia (10.5%) and Greece (8.7%), MS with a low human density in EU. In the next years, it will be important to acquire information on the regions of origin of foxes and other carnivores in the MS to better define the transmission risk areas for pigs not reared under controlled conditions in the MS.
Identification of Trichinella larvae at the species level, carried out in 2016 confirms that T. spiralis is more prevalent than T. britovi in swine and that the opposite occurs in carnivores. However, as T. spiralis is distributed patchily, only T. britovi has been detected in swine in some countries. Trichinella nativa has been documented in wild carnivores. T. pseudospiralis has been documented only in two animals (one raptorial bird in Finland and one wild boar in Sweden) confirming its extremely low frequency in target animals (Pozio, 2016a,b). The increasing number of wild boar and red foxes and the spread of the raccoon dogs from eastern to western Europe and of the jackal from southern‐eastern to northern‐western Europe may increase the prevalence of Trichinella circulating among wild animals (Alban et al., 2011; Szell et al., 2013). Therefore, it is important to continue to educate hunters and others eating wild game about the risk of eating undercooked game meat.
8.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://ecdc.europa.eu/en/data-tools/atlas/Pages/atlas.aspx |
| FAO/WHO/OIE Guidelines for the surveillance, management, prevention and control of trichinellosis | http://www.trichinellosis.org/uploads/FAO-WHO-OIE_Guidelines.pdf | |
| International Trichinella Reference Centre | https://www.iss.it/site/Trichinella/ | |
| International Commission on Trichinellosis | http://www.trichinellosis.org/ | |
| European Union Reference Laboratory for Parasites (humans and animals) | http://www.iss.it/crlp/ | |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| Animals | OIE (World Organisation for Animal health), Summary of Information on Trichinellosis | http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Disease_cards/TRICHI-EN.pdf |
| FAO/WHO/OIE Guidelines for the surveillance, management, prevention and control of trichinellosis | http://www.trichinellosis.org/uploads/FAO-WHO-OIE_Guidelines.pdf | |
| International Trichinella Reference Center | https://www.iss.it/site/Trichinella/ | |
| International Commission on Trichinellosis | http://www.trichinellosis.org/ | |
| EFSA Scientific Report: Development of harmonised schemes for the monitoring and reporting of Trichinella in animals and foodstuffs in the European Union | http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/35e.pdf | |
| OIE Manual Chapter 2.1.16 Trichinellosis | https://web.oie.int/eng/normes/MMANUAL/2008/pdf/2.01.16_TRICHINELLOSIS.pdf | |
| Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat | http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32015R1375 | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| Pig farming in the EU: considerable variations from one Member State to another | http://ec.europa.eu/eurostat/statistics-explained/index.php/Pig_farming_sector_-statistical_portrait_2014 |
9. Echinococcus
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
9.1. Abstract
Alveolar (AE) and cystic echinococcosis (CE) are food‐borne zoonotic parasitic diseases transmitted to humans through the ingestion of eggs shed by the tiny tapeworms Echinococcus multilocularis and Echinococcus granulosus sensu lato (s.l.),28 respectively, in the faeces of canid definitive hosts. Even if human AE and CE are notifiable in some MS, in practice these parasitic diseases are largely underreported in Europe.
In 2016, 772 confirmed human echinococcosis cases were reported in the EU. The EU notification rate was 0.20 cases per 100,000 population, which was the same level as in the previous five years. A high proportion (> 70%) of the human echinococcosis cases were reported without information on travel destination. Species information was provided for 72.8% of the cases and E. granulosus and E. multilocularis accounted, respectively, for 415 cases (58.2%) and 104 cases (14.6%). The proportion of the cases who were hospitalised continued to decrease during the last five years, with higher rates for AE compared with CE. One fatal case (species not specified) was reported in 2016.
Twenty‐five MS provided 2016 monitoring data on Echinococcus in animals. Twelve MS reported data on 4,561 foxes examined for E. multilocularis, and 10 MS reported positive findings with a total prevalence of 19.5%. Data of 2016 from Finland, Ireland, Malta, the United Kingdom and Norway confirmed the status of these countries with regards to E. multilocularis in the context of Regulation (EU) 1152/2011. For E. granulosus, 21 countries reported data from around 95 million animals of which mainly domestic livestock animals. Eleven MS reported positive samples with an overall prevalence of 0.24%.
9.2. Surveillance and monitoring of cystic and alveolar echinococcosis in humans and animals in the EU
9.2.1. Humans
The EU case definition does not differentiate between the two clinical forms of the diseases, but the two species can, however, be reported separately to ECDC. The notification of echinococcosis in humans is mandatory in most MS, Iceland and Norway. In three MS, reporting is based on a voluntary surveillance system (Belgium, the Netherlands and the United Kingdom). In one MS (France) the type of reporting system is not specified. Denmark and Italy have no surveillance system for echinococcosis. In Switzerland, echinococcosis in humans is not notifiable.
E. multilocularis and E. granulosus s.l. are the aetiological agents of human AE and CE, respectively, which cause substantial impact on human and animal public health. AE and CE are zoonotic parasitic diseases transmitted to humans through the ingestion of eggs of the tapeworm prevalence in these two species.
CE and AE are two distinct chronic diseases, with CE considered mainly a disabler, while AE poses a much greater health threat to people and is fatal if left untreated. Clinical manifestations of human CE typically result from the presence of single/multiple cysts in the liver, lungs and/or other organs, which slowly enlarge and often grow unnoticed and neglected for years, and which can eventually produce a mass effect and impair organ function. In contrast, AE acts more like an invasive parasitic tumour, which manifests predominantly in the liver, but can infiltrate adjacent organs and tissues and produce peripheral metastases. Globally, it has been estimated that there would be 188,000 and 18,400 new cases per year attributable to CE and AE, respectively (synthesis in Budke et al., 2017). However, due to the high proportion of asymptomatic infected individuals and of symptomatic patients who do not get medical attention, and the unknown magnitude of underreporting, the true prevalence and incidence and burden of AE and CE are difficult to estimate.
An attempt to collect harmonised clinical data in the EU on a voluntary basis is represented by the European Register of Cystic Echinococcosis (ERCE) (Rossi et al., 2016; http://www.heracles-fp7.eu/erce.html) and the European (Alveolar) Echinococcosis Registry (EurEchinoReg) (Kern et al., 2003).
9.2.2. Animals
Echinococcus multilocularis in Europe is mainly transmitted to humans by a sylvatic cycle that is wildlife based. Intermediate hosts (HIs) for E. multilocularis are wild small rodents (microtine or arvicolid), while definitive hosts (DHs) are mainly red foxes, raccoon dogs and, to a lesser extent, dogs and wolves. E. granulosus s.l. in Europe is mainly transmitted to humans by a pastoral cycle. IHs for E. granulosus s.l. are mainly livestock species (sheep, cattle, goats and secondarily pigs), while DHs are shepherd dogs (rarely wild canids). As mentioned before, people become infected with AE and CE through the ingestion of eggs of the tapeworm prevalent in these definitive hosts.
Surveillance for E. multilocularis in Europe is usually carried out on voluntary basis, with the exception of the five reporting countries claiming to be free from this parasite according to Regulation (EU) No 1152/201329. Surveillance is carried out on the main European DHs, the red fox (Vulpes vulpes), using parasitological (sedimentation and counting technique (SCT)) or molecular PCR‐based methods for the identification of eggs or adult worms such as conventional or real‐time PCRs (Siles‐Lucas et al., 2017). However, there is a lack of standardisation of these diagnostic procedures detecting E. multilocularis that complicates drawing any consistent conclusions from these (EFSA AHAW Panel, 2015). Four MS (Finland, Ireland, Malta and the United Kingdom) have demonstrated the absence of E. multilocularis through the implementation of an annual surveillance programme required in accordance with Regulation (EU) No 1152/2011. One EEA State, mainland Norway (Svalbard excluded), also implements a surveillance programme in line with Regulation (EU) No 1152/2011. In all other MS, data on E. multilocularis rely on whether findings are notifiable and if monitoring is in place or if studies on E. multilocularis are performed. As data on E. multilocularis in animals vary geographically (also within countries) and over time, reported cases of E. multilocularis are difficult to compare within and between countries. According to a recent meta‐analysis, based on studies published between 1900 and 2015, E. multilocularis has been documented in red foxes from 21 countries (Oksanen et al., 2016; Figure 51).
Figure 51.
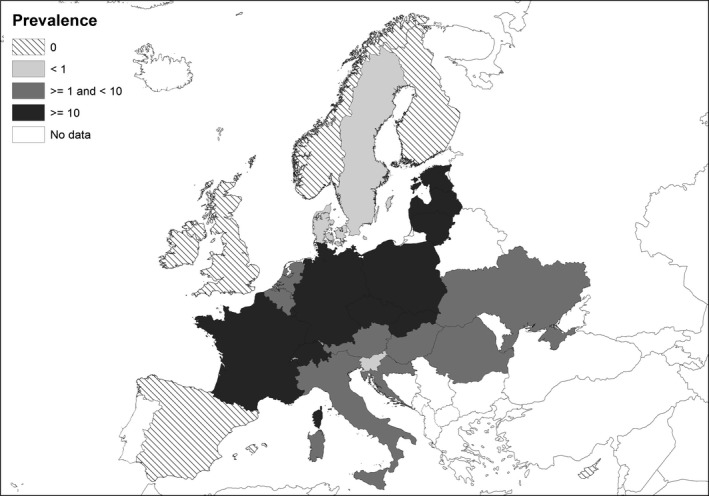
Pooled prevalence of Echinococcus multilocularis in red and Arctic foxes within the European Union and adjacent countries at national level depicting current epidemiological situation in Europe (Oksanen et al., 2016)
- Map adopted from Oksanen et al. (2016) and based on studies performed between 2000 and 2016: the pooled prevalence of Echinococcus multilocularis on the main land of Norway is zero, however the pooled prevalence is 9% on the Svalbard islands due to Artic foxes. Prevalence data from Spain originated from single studies.
Echinococcus multilocularis monitoring data from wild animals (intermediate hosts: wild small rodents (of microtine and arvicolid families, definitive hosts: mainly red foxes and raccoon dogs and, to a lesser extent, dogs and wolves), submitted to EFSA and collected without harmonised design allows for descriptive summaries at EU‐level to be made. Lack of harmonisation precludes trend analyses and trend watching at EU‐level (Table 1).
Surveillance of E. granulosus s.l. is usually carried out on livestock IHs during slaughterhouse inspections. In particular, necroscopy on sheep liver is used to detect the presence of parasitic cysts, while molecular PCR‐based methods are used to confirm and to identify genotype/species belonging to the Echinococcus genus (Siles‐Lucas et al., 2017). As for E. multilocularis, no standardisation has been provided for molecular or parasitological methods to detect E. granulosus s.l. Although, in terms of number of human cases, CE is more frequent than AE in Europe, no specific regulation is in place for detecting this parasite in animals or humans.
Echinococcus granulosus monitoring data from livestock (intermediate hosts, sheep and pigs) are based on programmed surveillance/monitoring. They are collected in a fully harmonised way and with harmonised reporting rules, and therefore allow descriptive summaries at EU‐level to be made, trend watching and moreover subsequent data analysis such as assessing spatial and temporal trends at the EU‐level (Table 1).
9.3. Results
9.3.1. Overview of key statistics, EU, 2012–2016
Table 30 summarises EU‐level statistics related to human echinococcosis, and to occurrence and prevalence in animals, respectively, in the EU, during 2012–2016. A more detailed description of these statistics is in the results section of this chapter.
Table 30.
Summary of Echinococcus granulosus sensu lato and Echinococcus multilocularis/cystic and alveolar echinococcosis in humans and most important animal species
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 772 | 883 | 820 | 805 | 865 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.20 | 0.20 | 0.19 | 0.18 | 0.20 | ECDC |
| Number of reporting MS | 25 | 26 | 26 | 26 | 26 | ECDC |
| Infection acquired in the EU | 127 | 163 | 99 | 195 | 274 | ECDC |
| Infection acquired outside the EU | 33 | 29 | 23 | 14 | 16 | ECDC |
| Unknown travel status or unknown country of infection | 612 | 691 | 698 | 596 | 575 | ECDC |
| Animals | ||||||
| Echinococcus multilocularis in red foxes | ||||||
| Number of samples tested | 4,561 | 7,353 | 8,243 | 5,994 | 7,444 | EFSA |
| Proportion of positive samples (%) | 19.5 | 13 | 8.3 | 10.9 | 10.6 | EFSA |
| Number of reporting MS | 12 | 11 | 14 | 12 | 10 | EFSA |
| Echinococcus multilocularis in raccoon dogs | ||||||
| Number of samples tested | 483 | 477 | 409 | 515 | 576 | EFSA |
| Proportion of positive samples (%) | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | EFSA |
| Number of reporting MS | 2 | 4 | 5 | 3 | 4 | EFSA |
| Echinococcus granulosus s.l. in dogs | ||||||
| Number of samples tested | 2,183 | 3,478 | 2,759 | 1,469 | 1,279 | EFSA |
| Proportion of positive samples (%) | 0.4 | 0.2 | 0.3 | 0.0 | 0.0 | EFSA |
| Number of reporting MS | 5 | 8 | 7 | 5 | 6 | EFSA |
| Echinococcus granulosus s.l. in cattle | ||||||
| Number of samples tested | 6,885,353 | 5,636,424 | 5,263,603 | 7,591,851 | 8,602,633 | EFSA |
| Proportion of positive samples (%) | 0.1 | 0.1 | 0.2 | 0.3 | 0.3 | EFSA |
| Number of reporting MS | 19 | 17 | 15 | 13 | 13 | EFSA |
| Echinococcus granulosus s.l. in small ruminants | ||||||
| Number of samples tested | 9,617,700 | 5,281,192 | 13,335,803 | 29,135,951 | 28,852,777 | EFSA |
| Proportion of positive samples (%) | 1.3 | 1.1 | 1.3 | 0.4 | 0.6 | EFSA |
| Number of reporting MS | 14 | 13 | 11 | 13 | 12 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
9.3.2. Human echinococcosis
In 2016, 772 laboratory‐confirmed echinococcosis cases were reported in the EU by 25 MS (Table 31). Twenty‐two MS reported at least one confirmed case and three MS reported zero cases. The EU notification rate was 0.20 cases per 100,000 population, which was at the same level as in the previous five years. The highest notification rates were, as in previous years, observed in Bulgaria with 3.76 cases per 100,000, followed by Lithuania and Latvia with 0.90 and 0.56 cases per 100,000, respectively. In 2016, Bulgaria reported the lowest number of cases and notification rate compared with the previous 4 years.
Table 31.
Reported human cases of cystic and alveolar echinococcosis and notification rates per 100,000 population in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 26 | 26 | 0.30 | 8 | 0.09 | 14 | 0.17 | 11 | 0.13 | 3 | 0.04 |
| Belgium | Y | A | 17 | 17 | 0.15 | 6 | 0.05 | 15 | 0.13 | 15 | 0.13 | 6 | 0.05 |
| Bulgaria | Y | A | 269 | 269 | 3.76 | 313 | 4.35 | 302 | 4.17 | 278 | 3.82 | 320 | 4.37 |
| Croatia | Y | A | 11 | 9 | 0.21 | 7 | 0.17 | 20 | 0.47 | 0 | 0.00 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 2 | 0.24 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic | Y | C | 4 | 4 | 0.04 | 3 | 0.03 | 6 | 0.06 | 2 | 0.02 | 0 | 0.00 |
| Denmarkb | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.08 | 3 | 0.23 | 3 | 0.23 |
| Finlandc | Y | C | 4 | 4 | 0.07 | 2 | 0.04 | 0 | 0.00 | 4 | 0.07 | 3 | 0.06 |
| France | Y | C | 38 | 38 | 0.06 | 48 | 0.07 | 32 | 0.05 | 34 | 0.05 | 49 | 0.08 |
| Germany | Y | C | 109 | 109 | 0.13 | 156 | 0.19 | 131 | 0.16 | 132 | 0.16 | 119 | 0.15 |
| Greece | Y | C | 18 | 18 | 0.17 | 13 | 0.12 | 13 | 0.12 | 10 | 0.09 | 21 | 0.19 |
| Hungary | Y | C | 5 | 5 | 0.05 | 2 | 0.02 | 2 | 0.02 | 5 | 0.05 | 6 | 0.06 |
| Irelandc | Y | C | 2 | 2 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.00 | 1 | 0.02 |
| Italyb | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Latvia | Y | C | 11 | 11 | 0.56 | 10 | 0.50 | 13 | 0.65 | 7 | 0.35 | 8 | 0.39 |
| Lithuania | Y | C | 26 | 26 | 0.90 | 33 | 1.13 | 22 | 0.75 | 23 | 0.77 | 23 | 0.77 |
| Luxembourg | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Maltac | Y | C | 1 | 1 | 0.23 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands | Y | A | 33 | 33 | 0.19 | 64 | 0.38 | 30 | 0.18 | 33 | 0.20 | 50 | 0.30 |
| Poland | Y | C | 64 | 64 | 0.17 | 47 | 0.12 | 48 | 0.13 | 39 | 0.10 | 28 | 0.07 |
| Portugal | Y | C | 2 | 2 | 0.02 | 4 | 0.04 | 4 | 0.04 | 3 | 0.03 | 2 | 0.02 |
| Romania | Y | C | 13 | 13 | 0.07 | 18 | 0.09 | 31 | 0.16 | 55 | 0.28 | 96 | 0.48 |
| Slovakia | Y | C | 4 | 4 | 0.07 | 5 | 0.09 | 8 | 0.15 | 20 | 0.37 | 3 | 0.06 |
| Slovenia | Y | C | 3 | 3 | 0.15 | 7 | 0.34 | 5 | 0.24 | 6 | 0.29 | 6 | 0.29 |
| Spain | Y | C | 87 | 87 | 0.19 | 83 | 0.18 | 77 | 0.17 | 94 | 0.20 | 96 | 0.21 |
| Sweden | Y | C | 27 | 27 | 0.27 | 26 | 0.27 | 21 | 0.22 | 16 | 0.17 | 16 | 0.17 |
| United Kingdomc | Y | C | – | – | – | 26 | 0.04 | 25 | 0.04 | 14 | 0.02 | 7 | 0.01 |
| EU total | – | – | 774 | 772 | 0.20 | 883 | 0.20 | 820 | 0.19 | 805 | 0.18 | 865 | 0.20 |
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | – | – |
| Norwayc | Y | C | 3 | 3 | 0.06 | 2 | 0.04 | 0 | 0.00 | 2 | 0.04 | 2 | 0.04 |
Y: yes; N: no; A: aggregated data; C: case‐based data; –: no report.
No surveillance system.
Finland, Ireland, Malta, the United Kingdom and mainland Norway have been declared free of E. multilocularis.
A high proportion (> 70%) of echinococcosis cases were reported without data about the travel status or unknown country of infection (Table 30). Seven MS (the Czech Republic, Hungary, Latvia, Lithuania, Portugal, Romania and Slovakia) out of 12 MS reporting importation in 2016, notified all their Echinococcus cases as being domestically acquired. Among 37 travel‐associated cases, the majority was reported to originate from outside the EU. Syria, Iraq and Turkey were the most frequently reported probable countries of infection, representing 64.9% of the imported cases in 2016.
E. multilocularis accounted for 104 cases (14.6%), which was a decrease of 23.0% compared with 2015. This was mainly due to a decrease in reported cases in Germany. There was a significant increasing (p < 0.01) trend of E. multilocularis in 2008–2016, but the trend stabilised in 2012–2016 Figure 52). For 13 MS with available data for the whole period 2008–2016, one country (Poland) reported increasing trends (p < 0.01) since 2008. None of the MS reported decreasing trends, neither long term (2008–2016) nor short term (2012–2016).
Figure 52.
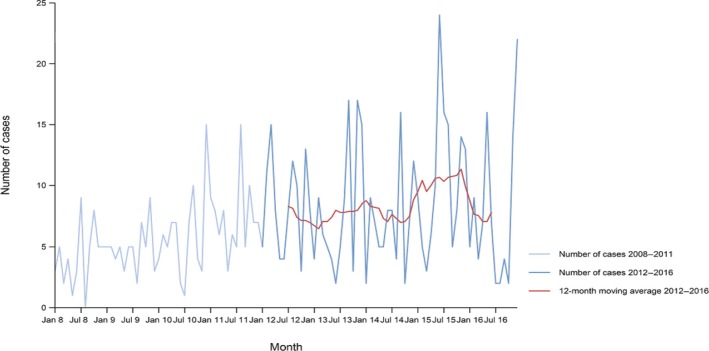
Trend in reported confirmed human cases of E. multilocularis in the EU/EEA, by month, 2012–2016
- Source(s): Austria, Estonia, France, Germany, Hungary, Latvia, Lithuania, the Netherlands, Poland, Romania, Sweden, Slovakia and Slovenia. Belgium, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Finland, Greece, Iceland, Italy, Ireland, Luxembourg, Malta, Norway, Portugal, Spain and the United Kingdom did not report data to the level of detail required for the analysis.
In 2016, species information was provided for 519 confirmed echinococcosis cases (72.8%) by 19 MS. E. granulosus accounted for 80.0% (415 cases) of those with species information available. Majority (64.8%; 269 cases) of the cases were from Bulgaria. E. multilocularis accounted for 20.0% (104 cases).
There was a decreasing trend of E. granulosus (p < 0.01) in the EU/EEA in 2008–2016, but the trend did not show any significant increase or decrease in 2012–2016 (Figure 53). For 19 countries with available data for the whole period 2008–2016, two countries (Latvia and Spain) reported significantly (p < 0.01) decreasing trends. Spain was the only country reporting a decreasing trend in 2008–2016 and 2012–2016. None of the MS reported increasing trends, either in 2008–2016 or 2012–2016. Bulgaria, which reported the majority of the cases in the EU in 2008–2016 (all cases were E. granulosus) was not included in the EU trend calculations since no monthly data were available. Cases from Bulgaria decreased by 30.3% from 2008 to 2016.
Figure 53.
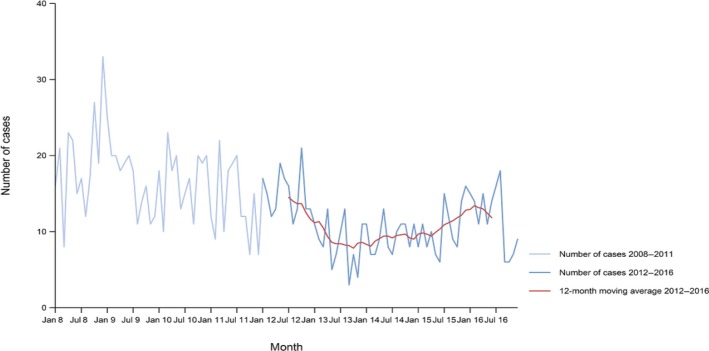
Trend in reported confirmed human cases of E. granulosus in the EU/EEA, by month, 2012–2016
- Source(s): Austria, Estonia, Finland, Germany, Greece, Hungary, Ireland, Latvia, Lithuania, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden. Belgium, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, France, Iceland, Italy, Luxembourg, Malta and the United Kingdom did not report data to the level of detail required for the analysis.
Fourteen MS provided information on hospitalisation, covering 26.2% of all confirmed cases of echinococcosis in the EU in 2016. The overall hospitalisation rate was 58.9%, a continuous decrease during the last 5 years from 80% in 2011. In 2016, the highest proportions of hospitalised cases (80–100%) were reported in Greece, Poland and Romania. The proportion of hospitalised E. multilocularis cases was 71.1% and E. granulosus cases was 60.5%, based on reporting by five and nine MS, respectively.
Information on the outcome of the cases was provided by 13 MS. One fatal case (species not specified) was reported in Latvia. This resulted in an EU case fatality of 0.51% among the 196 cases for which this information was reported (25.4% of all confirmed cases) in 2016.
9.3.3. Echinococcus in animals
Twelve MS and two non‐MS (Norway and Switzerland) reported 2016 monitoring data on 4,561 foxes examined for E. multilocularis, and eight MS reported positive findings with a total prevalence of 19.5%. The Czech Republic (35.7%), Denmark (22.2%), France (24.8%), Germany (23.5%), Luxembourg (28.9%), Slovakia (20.7%) and Switzerland (22.7%) reported the highest proportion of positive samples. Hungary (6.35%) reported lower prevalence in foxes. It is also important to stress that some MS, such as France, did not provide data from the whole country but only from some regions.
Belgium, Croatia, Estonia, Ireland, Malta, the Netherlands, Norway, Slovenia, Sweden and the United Kingdom didn't report any finding of E. granulosus or E. multilocularis.
In addition to foxes, E. multilocularis has been reported in one cat from France and one dog and one beaver and pigs from Switzerland. Poland reported 37,233 positive pigs with Echinococcus spp. out of 22,438,554, while Slovakia reported one positive pig out of 555,229 tested. In these last cases, it was not possible to confirm the Echinococcus species as in these two countries, as in the other countries negative for Echinococcus species in pigs, this IH potentially can harbour both E. multilocularis and E. granulosus s.l. Such uncertainty in species identification in coendemic countries for E. multilocularis and E. granulosus s.l. can be also applied for dogs and wolves.
These findings are similar to those of recent years. Findings from most of the endemic countries fluctuated between years but, in most years, they reported positive findings. Fluctuations in reported numbers of infected animals are probably associated with investigational efforts performed in a particular year, than reflecting a change in true prevalence. Table 32 summarises the most relevant DH and IH species tested for Echinococcus multilocularis, such as foxes, raccoon dogs, dogs, wolves, cats, beaver, voles and pigs by MS and adjacent countries in 2016.
Table 32.
Echinococcus multilocularis positive/tested (%) animals (wild and domestic) in EU/EEA, 2016
| Country | Foxes | Raccoon dogs | Dogsa | Wolvesa | Cats | Beaver | Voles | Pigsa |
|---|---|---|---|---|---|---|---|---|
| Austria | 0/5,197,563 | |||||||
| Czech Republic | 540/1,513 (35.69%) | |||||||
| Denmarka | 2/9 (22.22%) | 0/17 | ||||||
| Estonia | 0/524,227 | |||||||
| Finlanda | 0/230 | 0/466 | 0/1,857 | |||||
| France | 61/246 (24.8%) | 0/6 | 1/38 (2.63%) | |||||
| Germany | 195/830 (23.49%) | |||||||
| Hungarya | 12/189 (6.35%) | |||||||
| Ireland | 0/405 | |||||||
| Italya | 1/3 | 0/2 | ||||||
| Latvia | 0/452,533 | |||||||
| Luxembourga | 37/128 (28.91%) | |||||||
| Poland | 37,233/22,438,554 (0.17%) | |||||||
| Slovakia | 41/198 (20.71%) | 0/1,685 | 0/583 | 1/555,229 (< 0.001%) | ||||
| Swedena | 0/12 | 0/5 | 0/41 | |||||
| United Kingdom | 0/798 | |||||||
| Total EU | 889/4,561 (19.5%) | 0/483 | 0/1,696 | 0/41 | 1/623 (0.2%) | 0/0 | 0/1,875 | 37,234/29,168,106 (0.1%) |
| Norwaya | 0/575 | 0/8 | ||||||
| Switzerland | 20/88 (22.72%) | 1/22 (4.55%) | 0/1 | 1/2 (50%) | 42/58 (72.4%) |
Slaughter batch data and animals from zoo were not included in the table.
Dogs, wolves and pigs for which the species level of Echinococcus was not specified could be allocated in both Tables 32 and 33 if there is circulation of E. multilocularis and E. granulosus s.l. For pigs: Bulgaria (527 positives out of 1,043,004), Croatia (0 positives out of 6), Denmark (0 positives out of 17,843,548), Finland (0 positives out of 2,051,168), Greece (60 positives out of 452,126), Hungary (8 positives out of 45), Italy (407 positives out of 4,837,977), Luxembourg (0 positives out of 176,968), Romania (5 positives out of 17), Slovenia (0 positives out of 258,307), Spain (3,345 positives out of 16,175,576), Sweden (0 positives out of 2,526,500) and Norway (0 positives out of 1,651,000); For dogs: Malta (0 postivies out of 333), Italy (5 positives out of 13), Romania (4 positives out of 147); For Wolves: Finland (15 positives out of 74) and Norway (0 positives out of 8).
In total, 23 countries (21 MS and 2 non‐MS) reported data from around 95 million domestic and wild animals tested for E. granulosus s.l. of which 99.7% were domestic animals (sheep, cattle, goats, pigs, horses, water buffalos and dogs) These data were obtained mainly from the meat inspection performed at the slaughterhouse. Wild animals tested included mouflons, reindeer, deer, wild boar, moose, wolves and foxes (Table 33).
Table 33.
Echinococcus granulosus sensu lato positive/tested (%) animals (domestic and wild) in 2016
| Country | Sheep | Sheep and goats | Goats | Cattle | Pigsa | Mouflons | Reindeer | Solipeds, domestic | Deer | Water buffalos | Wild boars | Moose | Dogsa | Wolvesa | Fox |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 28/130,740 (0.02%) | 0/7,304 | 92/686,525 (0.01%) | 0/5,197,563 | |||||||||||
| Belgium | 0/913,745 | ||||||||||||||
| Bulgaria | 7,566/173,466 (4.36%) | 21/3,408 (0.6%) | 1,882/31,539 (5.96%) | 527/1,043,004 (0.05%) | |||||||||||
| Croatia | 0/5 | 0/6 | |||||||||||||
| Cyprus | 1/18 (5.56%) | ||||||||||||||
| Denmark | 0/539,600 | 0/17,843,548 | |||||||||||||
| Estonia | 0/6,748 | 0/26 | 0/37,701 | 0/524,227 | 0/10 | ||||||||||
| Finland | 0/60,153 | 0/273 | 0/279,402 | 0/2,051,168 | 6/62,464 (< 0.01%) | 0/1,261 | 0/488 | 0/338 | 0/230 | 15/74 (20.27%) | |||||
| Greece | 10,308/1,491,742 (0.69%) | 2,097/313,644 (0.67%) | 862/67,695 (1.27%) | 60/452,126 (0.01%) | 0/110 | ||||||||||
| Hungary | 7/16 (43.75%) | 3/3 (100%) | 8/45 (17.77%) | 0/1 | |||||||||||
| Italy | 42,882/355,357 (12.06%) | 772/37,705 (2.05%) | 4,754/863,409 (0.55%) | 407/4,837,977 (< 0.01%) | 3/5,281 (0.005%) | 0/377 | 26/26,783 (0.09%) | 103/34,177 (0.3%) | 5/13 (38.46%) | 1/3 (33%) | |||||
| Latvia | 0/22,273 | 0/93 | 0/93,496 | 0/452,533 | 0/67 | ||||||||||
| Luxembourg | 0/25,750 | 0/176,968 | |||||||||||||
| Malta | 0/333 | ||||||||||||||
| Netherlands | 0/1 | ||||||||||||||
| Poland | 190/35,527 (0.53%) | 2/1,920,854 (< 0.01%) | 37233/22,438,554 (0.17%) | ||||||||||||
| Romania | 0/25 | 0/17 | 298/360 (82.77%) | 5/17 (29.41%) | 4/147 (2.72%) | ||||||||||
| Slovakia | 0/9,992 | 0/120 | 0/36,587 | 1/555,229 (< 0.01%) | 0/1,685 | ||||||||||
| Slovenia | 0/10,179 | 0/1,252 | 0/111,634 | 0/258,307 | 0/1,424 | ||||||||||
| Spain | 80,478/8,389,626 (0.01%) | 0/1,848 | 24,144/889,029 (0.03%) | 5,239/1,727,190 (0.3%) | 3,345/16,175,576 (0.02%)b | 0/1 | 6/16,956 (0.03%) | 21/80,667 (0.02%) | 8/26995 (0.02%) | ||||||
| Sweden | 0/217,980 | 0/1,218 | 0/411,020 | 0/2,526,500 | 0/54,745 | 0/2,670 | 0/5,774 | 0/15,670 | 0/5 | 0/41 | |||||
| Total EU | 141,269/11,782,042 (1.2%) | 190/35,527 (0.5%) | 27,034/1,254,089 (2.2%) | 13,132/6,832771 (0.2%) | 41,578/74,533,348 (0.06%) | 1/19 (5.3%) | 6/117,209 (< 0.001%) | 9/27,669 (0.03%) | 21/87,306 (0.02%) | 26/26,783 (0.1%) | 111/77,290(0.14%) | 0/230 | 9/2,183 (0.41%) | 15/115 (13.%) | 1/3 (33.3%) |
| Norway | 0/1,285,000 | 0/23,800 | 0/286,000 | 0/1,651,000 | 0/8 |
Slaughter batch data and animals from zoo were not included in the table.
Dogs, wolves and pigs for which the species level of Echinococcus was not specified could be allocated in both Tables 32 and 33 if there is circulation of E. multilocularis and E. granulosus s.l. For dogs: France (0 positives out of 6).
Among pigs, 68 positives out of 46,559 tested were wild.
Twelve MS reported a total of 223,410 positive samples mainly from domestic animals. Positive animals were mainly sheep and goats, having a low (> 1–10%) prevalence. Also cattle and pigs were found positive with a very low prevalence (> 0.1–1%). Cyprus, Finland, Italy and Spain reported findings of E. granulosus s.l. in mouflons, reindeer, deer, wild boar and wolves.
9.4. Discussion
The EU case definition does not differentiate between the two clinical forms of the disease in humans, CE and AE, caused by E. granulosus and E. multilocularis, respectively. These two species can, however, be reported separately to ECDC. The majority of MS reported species information through TESSy from 2007 to 2016. Since the beginning of the surveillance of human echinococcosis in the EU, E. granulosus has been more frequently reported than E. multilocularis. The EU notification rate of confirmed human echinococcosis cases was stable, and the trends for both species did not show any significant increase or decrease in the last 5 years since 2012. In a few countries, the increase in the number of cases in 2016 could be explained by intensified surveillance and improved notification system for echinococcosis. The awareness of the disease among clinicians and the migration (people from endemic countries) may also have influenced the number of diagnosed cases in some countries.
The EFSA Panel on Animal Health and Welfare has stated in a scientific opinion that in many human cases the diagnosis is established only as echinococcosis, and the aetiological agent of the disease, E. multilocularis or E. granulosus, is not determined (EFSA AHAW Panel, 2007). Distinction between infection with E. granulosus and E. multilocularis is needed because the two diseases require different management of prevention and treatment. Furthermore, the detection of CE or AE in EU citizens or immigrants is of great epidemiological importance. In this context, a reconsideration of ‘echinococcosis’ case definition in the current Commission Decision 2012/506/EU, differentiating AE from CE, will be crucial to collect specific epidemiological and clinical data to manage and trace back these infections. Furthermore, it is important that notification of human AE and CE cases be made mandatory in all MS to enable effective and coherent monitoring of trends of AE and EC occurrence in humans.
It should be emphasised that human AE and CE cases notified by country to ECDC do not reflect the real epidemiological situation in Europe. In fact, the true prevalence of these diseases is extremely difficult to estimate due to the long incubation period (AE and CE), the high proportion of asymptomatic or paucisymptomatic carriers who never seek medical attention (CE) and the underreporting/misdiagnosed cases (AE and CE), factors, which contribute to their neglected status. For these reasons, the patchy data on the number of people affected by ‘echinococcosis’ currently reported by MS, represents the tip of the iceberg. The invisible portion includes asymptomatic carriers of CE and misdiagnosed cases of AE especially in recently discovered foci where physicians do not have experience with these diseases.
As an example for this underreporting, data recently published in peer review journals reported around 34,000 hospitalisations of CE from Italy, France and Spain in 12‐, 16‐ and 12‐year period, respectively (Brundu et al., 2015; van Cauteren et al., 2016; Herrador et al., 2016). It should be noted that these three studies showed a negative trend in time in the number of hospitalisations. More recently, an extended study conducted in Italy (which is currently not reporting any human CE cases to the EU annual zoonoses monitoring data collection) identified 21,050 hospital discharge records with CE diagnosis from 2001 to 2014 related to 12,619 patients (Piseddu et al., 2017). The median of CE hospitalisations per year in Italy was 848, which is equal to the total number of CE and AE cases reported by all the MS in the EU annual zoonoses monitoring data collection. The direct costs, estimated on the basis of hospital discharge records for CE during this time period in Italy, were estimated to be around € 53 million. The disability‐adjusted life years (DALYs) of the population from 2001 to 2014 were 223.35 annually and 5.26 DALYs per 105 inhabitants in Italy. Conversely, the Italian Ministry of Health funded for the period 2017–2019, a national project to evaluate the possibility to set up a national surveillance programme for CE (Casulli pers. comm.). Extended ultrasound surveys conducted in Romania during 2014/2015, screening around 0.1% of the rural population, identified double the number of CE cases compared to those notified at national level in Romania during the same time period (Casulli, 2016). These published data and findings give an indication of the true magnitude of human CE as a public health problem and related costs in Europe.
Recently, the European Commission funded extended ultrasound screenings performed in 2014–2015 on 24,693 people that will contribute to partially fill this gap providing the prevalence of abdominal CE and an estimate of the number of infected individuals in the rural areas of Bulgaria, Romania and Turkey (HERACLES: ‘Human cystic Echinococcosis ReseArch in CentraL and Eastern Societies’ http://www.heracles-fp7.eu/index.html).
In animals, in 2016, E. granulosus s.l., aetiological agent of cystic echinococcosis, and E. multilocularis, aetiological agent of alveolar echinococcosis, have been documented in 11 and 9 MS, respectively. The highest number of animals infected with E. granulosus s.l. was reported in Bulgaria, Greece, Italy and Spain. The highest number of animals (mainly foxes) infected with E. multilocularis was noted in Germany, Slovakia, France, Luxembourg, Switzerland and the Czech Republic.
The surveillance of E. multilocularis in foxes is important to assess the prevalence in Europe, as the distribution of E. multilocularis seems to be enlarged in the last decades and the fox population is increasing in Europe (Casulli et al., 2015; Oksanen et al., 2016). Whether the increased range of distribution of E. multilocularis is due to range expansion or reflects an increased surveillance effort is difficult to be disentangled, since there is a general lack of baseline data. Possibly, the parasite had been present, but undetected, in small focuses, which rapidly expanded in the wake of an increasing red fox population (EFSA AHAW Panel, 2015).
In addition, the prevalence data of E. multilocularis identified in 2016 in 10 countries (MS and non‐MS) must be interpreted with caution as many variables such as temperature, rainfall, humidity levels and soil have been identified as relevant factors that partially explain the distribution of the parasite. These factors may vary considerably, leading to local focuses within MS reporting positive cases.
Also, in animals, notification is a requirement for reliable data and information on parasite speciation is very important for risk management efforts as E. granulosus and E. multilocularis have different epidemiology and pose different health risks to humans. For E. granulosus, a notification requirement would ensure that comparable data between MS is obtained from meat inspection of food producing animals. For E. multilocularis, a general notification requirement for all MS can be questioned but it is required in countries free from this parasite, according to EU regulation 1152/2011. In countries where the parasite is endemic, reporting each case gives no additional valuable information. Therefore, repeated surveys, as surveillance for E. multilocularis, can be a basis for follow‐up and monitoring (EFSA AHAW Panel, 2015).
In addition, the debate on the importance of unwashed contaminated fresh fruit, vegetables and mushrooms in the transmission of E. multilocularis and E. granulosus s.l. is still ongoing (Federer et al., 2016; Lass et al., 2016; Robertson et al., 2016). Two recent reviews and meta‐analyses suggested that the chance of AE and CE transmission through the ingestion of food and water contaminated with E. multilocularis and E. granulosus eggs does exist, but potential risk factors associated with food‐borne and waterborne transmission do not significantly increase the risk of infection to humans (Possenti et al., 2016; Conraths et al., 2017). It should be also emphasised that waterborne and food‐borne transmission seems to be more evident for AE compared with CE. This finding is also supported by another systematic review on food‐borne parasitic diseases, in which the percentage of food‐borne CE and AE (reported as food‐borne_DALYs/total_DALYs x100) was estimated to be 21% and 48%, respectively (Torgerson et al., 2015).
Finally, it is noteworthy that, in general, reported data on animals and humans represent a substantial underestimation of the real burden of these two diseases in Europe in which around 200 human cases and in the range of 1,000 human cases are annually expected for AE and CE, respectively (Conraths and Deplazes, 2015; A. Casulli, personal communication, European Multicolloquium on Parasitology, Turku, Finland, 2016).
9.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://ecdc.europa.eu/en/data-tools/atlas/Pages/atlas.aspx |
| EU case definitions (all diseases) | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| CDC (Centers for Disease Control and Prevention) of United States: echinococcosis | https://www.cdc.gov/parasites/echinococcosis/index.html | |
| WHO (World Health Organization) – echinococcosis | http://www.who.int/echinococcosis/en/ | |
| WHO (World Health Organization) – Echinococcosis Fact sheet | http://www.who.int/mediacentre/factsheets/fs377/en/ | |
| Human cystic Echinococcosis ReseArch in CentraL and Eastern Societies (HERACLES project) | http://www.heracles-fp7.eu/index.html | |
| European Register of Cystic Echinococcosis (ERCE) | http://www.heracles-fp7.eu/erce.html | |
| Humans and Animals | WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern | http://apps.who.int/iris/bitstream/10665/42427/1/929044522X.pdf |
| OIE Manual, Chapter 2.1.6. Echinococcosis (infection with Echinococcus granulosus and with E. multilocularis) | http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.06_ECHINOCOCCOSIS.pdf | |
| COMMISSION DELEGATED REGULATION (EU) No 1152/2011 (preventive health measures for the control of Echinococcus multilocularis infection in dogs) | http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1152 | |
| European Union Reference Laboratory for Parasites (humans and animals) | http://www.iss.it/crlp/ | |
| Animals | EFSA Scientific Opinion: Echinococcus multilocularis infection in animals (Panel on Animal Health and Welfare) | http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.4373/pdf |
| EFSA External Scientific Report: Echinococcus multilocularis infection in animals GP/EFSA/AHAW/2012/01 | http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2015.EN-882/pdf | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
10. Toxoplasma
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
10.1. Abstract
Toxoplasma gondii is a protozoan and the aetiological agent of toxoplasmosis. lt is widely prevalent in humans and animals throughout the world. Virtually all warm‐blooded animals can act as intermediate hosts but the life cycle is completed only in cats and other felines (including the lynx in Europe), the definitive hosts. In 2016, in total, 47 cases of congenital toxoplasmosis were reported in EU by 19 MS. The EU notification rate was 1.57 cases per 100,000 live birth. The number of cases reported in 2016 is comparable with the annual number of cases reported between 2012 and 2015, after excluding France, which report their data with a 2‐year delay and represent over 80% of the annual cases in the EU. It is not possible to make a good estimate of the prevalence of congenital toxoplasmosis in the EU, as only three MS have an active surveillance system for this disease.
Thirteen MS and two non‐MS reported 2016 monitoring data on Toxoplasma infections in animals. The highest overall prevalence of Toxoplasma infections in animals was detected in small ruminants (sheep and goats; around 9%) followed by cattle (around 3%) and pigs (around 2%). Higher prevalence was detected using indirect diagnostic tests detecting antibodies (ELISA, complement fixation test (CFT), LAT or immunofluorescence assay (IFA)) compared with direct diagnostic tests (staining, PCR, immunohistochemistry (IHC)). As most samples were obtained from clinical investigations, the epidemiological value of the information provided is low. In addition, the sample size of tested livestock, as well as the number of MS reporting data on Toxoplasma (between 3 (pigs) to 12 (small ruminants)), was low. In general, there was a lack of information on the animals’ age and rearing conditions, which prevented a comparison of the data collected in the MS.
A harmonised protocol for sample collection and parasite detection could improve the epidemiological information on the occurrence of T. gondii among the different livestock species in the EU.
10.2. Surveillance and monitoring of Toxoplasma in the EU
10.2.1. Humans
Only congenital toxoplasmosis is reported to ECDC. National surveillance systems for toxoplasmosis differ between countries. In some countries, surveillance focuses on severe cases in all age groups. Only three MS (the Czech Republic, France and Slovakia) have active surveillance of congenital cases covering the whole population. In 18 MS and Iceland, a compulsory surveillance system is implemented, the United Kingdom has a voluntary system and Spain has another, unspecified system. The surveillance systems for toxoplasmosis covers the whole population in all 19 MS reporting data at the EU‐level, except in one country (Spain). No surveillance system for toxoplasmosis exists in eight MS (Austria, Belgium, Denmark, Greece, Italy, the Netherlands, Portugal and Sweden), and Norway and Switzerland. France reports cases with a 2‐year delay.
10.2.2. Animals
No regulation exists in the EU on the surveillance and monitoring of Toxoplasma in animals. It therefore follows that the available information is strictly determined by national legislation.
The main animal species tested are small ruminants (goat and sheep), cattle, pigs and pet animals (cats and dogs) using samples from aborted animals (ruminants) as well as from clinical investigations.
Mainly blood samples but also tissue and organs are taken and analysed with either indirect methods to detect antibodies (ELISA, LAT, CFT and IFA) or direct methods (PCR and IHC).
As the surveillance of Toxoplasma in animals is not harmonised, data on Toxoplasma only allow descriptive summaries at the EU‐level.
The detection of Toxoplasma in animals across the different reporting countries is variable in relation to the diagnostic methods used as well as to the different matrices analysed. The diagnostic methods reported for the detection of Toxoplasma in animals in 2016 were LAT, ELISA, IFA and CFT as indirect (serological) methods while histology, IHC, PCR and real‐time PCR were used as direct methods. Indirect methods are used for the detection of Toxoplasma‐specific antibodies in serum or meat juice samples while the direct methods are applied to specific organs or tissues of the sampled animals. The results from different countries and from different regions in a country may not be directly comparable due to the use of different tests and analytical methods, as well as different sampling schemes. It should also be noted that both age of animals and production systems at farm level influence the Toxoplasma prevalence and therefore further influence comparability. Furthermore, more than one‐third of the tested sheep samples and more than 90% of the tested goat samples, were obtained from clinical samples and the number of samples collected as part of surveillance and/or monitoring programmes is too small to provide significant epidemiological data.
Monitoring data on Toxoplasma in animals submitted to EFSA are collected without harmonised design. These data allow for descriptive summaries at EU‐level but lack of harmonisation precludes trend analyses and trend watching at EU‐level (Table 1).
10.2.3. Food‐borne outbreaks of human toxoplasmosis
The reporting of FBO of human toxoplasmosis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
10.3. Results
10.3.1. Human toxoplasmosis
In 2016, 47 cases of congenital toxoplasmosis were reported in the EU by 19 MS (Table 34). Seven MS (Finland, Germany, Poland, Slovakia, Slovenia, Spain and the United Kingdom) reported at least one confirmed congenital toxoplasmosis case and 12 MS reported zero cases. The EU notification rate was 1.57 cases per 100,000 population. This is not comparable with notification rates from previous years as France, whose data represented over 80% of the annual cases in the EU in 2012–2015, reports their data with a 2‐year delay. Excluding the French congenital toxoplasmosis data, the number of cases reported by 19 MS in 2016 is comparable with the annual number of cases (an average of 40 cases/year) and EU notification rate of 1.48 cases per 100,000 population in 2012–2015.
Table 34.
Reported human cases of congenital toxoplasmosis and notification rates per 100,000 live birth in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Belgium | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Bulgaria | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Croatia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic | Y | C | 0 | 0 | 0.00 | 1 | 0.90 | 1 | 0.90 | 0 | 0.00 | 1 | 0.90 |
| Denmark | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland | Y | C | 1 | 1 | 1.80 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Franceb | Y | C | – | – | – | 246 | 30.76 | 216 | 26.40 | 179 | 22.00 | 104 | 12.70 |
| Germany | Y | C | 10 | 10 | 1.36 | 15 | 2.03 | 6 | 0.80 | 10 | 1.50 | 20 | 3.00 |
| Greece | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Hungary | Y | C | 0 | 0 | 0.00 | 1 | 1.10 | 3 | 3.20 | 0 | 0.00 | 0 | 0.00 |
| Ireland | Y | C | 0 | 0 | 0.00 | 1 | 1.50 | 0 | 0.00 | 1 | 1.50 | 1 | 1.40 |
| Italy | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Latvia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 5.03 |
| Lithuania | Y | C | 0 | 0 | 0.00 | 1 | 3.30 | 0 | 0.00 | 1 | 3.30 | 1 | 3.30 |
| Luxembourg | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 16.50 |
| Malta | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Poland | Y | C | 20 | 20 | 5.42 | 15 | 4.00 | 20 | 5.30 | 18 | 4.90 | 10 | 2.60 |
| Portugal | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Romania | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.50 | 0 | 0.00 | 0 | 0.00 |
| Slovakia | Y | C | 2 | 2 | 3.60 | 0 | 0.00 | 0 | 0.00 | 2 | 3.60 | 0 | 0.00 |
| Slovenia | Y | C | 1 | 1 | 4.84 | 1 | 4.80 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Spain c | N | C | 5 | 5 | – | 0 | – | 0 | – | 0 | – | 0 | – |
| Sweden | – | – | – | – | – | – | – | – | – | – | – | – | – |
| United Kingdom | Y | C | 8 | 8 | 1.03 | 7 | 0.90 | 11 | 1.40 | 2 | 0.30 | 5 | 0.60 |
| EU total | – | – | 47 | 47 | 1.57 | 288 | 8.27 | 258 | 7.40 | 213 | 6.20 | 144 | 4.20 |
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | – | – |
| Norway | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Switzerlandd | – | – | – | – | – | – | – | – | – | – | – | – | – |
Y: yes; N: no; A: aggregated data; C: case‐based data; −: no report.
France: 2016 data not reported as there is a 2‐year delay in reporting of congenital toxoplasmosis in France.
Sentinel surveillance; no information on estimated coverage. So, notification rate cannot be estimated.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
In 2016, the highest country‐specific notification rates were observed in Poland, Slovenia and Slovakia (5.42, 4.84 and 3.60 cases per 100,000 population, respectively). Data from Poland alone accounted for 54.1% of all confirmed cases reported at the EU‐level in 2016. In 2012–2015, France reported the highest notification rates, showing significant increase of 142.2% from 12.7 to 30.8 cases per 100,000 population over the 4‐year period.
Three MS provided data on outcome, accounting for 70.3% of confirmed cases in the EU (≥ 90% in 2012–2014). No fatal cases due to congenital toxoplasmosis were reported in 2016 among 26 confirmed cases.
Table 35.
Summary of congenital toxoplasmosis related to humans and major animal species, EU 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 47 | 288 | 258 | 213 | 144 | ECDC |
| Total number of confirmed cases/100,000 live birth (notification rates) | 1.57 | 8.27 | 7.40 | 6.20 | 4.20 | ECDC |
| Number of reporting MS | 19 | 20 | 20 | 20 | 20 | ECDC |
| Infection acquired in the EU | 34 | 23 | 28 | 28 | 22 | ECDC |
| Infection acquired outside the EU | 0 | 1 | 1 | 0 | 0 | ECDC |
| Unknown travel status or unknown country of infection | 13 | 264 | 229 | 185 | 122 | ECDC |
| Animals | ||||||
| Small ruminants (animal level) | ||||||
| Number of sampled units | 5,561 | 3,139 | 4,694 | 4,813 | 5,291 | EFSA |
| Proportion of positive units (%)a | 18.7 | 38.8 | 26.8 | 42.4 | 28 | EFSA |
| Number of reporting MS | 12 | 11 | 12 | 12 | 10 | EFSA |
| Cattle (animal level) | ||||||
| Number of sampled units | 451 | 1,177 | 1,000 | 1,078 | 1,348 | EFSA |
| Proportion of positive units (%)a | 3.3 | 4.2 | 6.2 | 13.8 | 9.1 | EFSA |
| Number of reporting MS | 8 | 7 | 9 | 5 | 7 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
For the summary statistics, indirect and direct diagnostic methods were taken together to calculate the proportion of positive units.
10.3.2. Toxoplasma in animals
Thirteen MS (Croatia, Finland, Germany, Greece, Hungary, Ireland, Italy, Latvia, the Netherlands, Romania, Slovakia, Spain and the United Kingdom) and two non‐MS (Norway and Switzerland) provided 2016 data on Toxoplasma in livestock (sheep, goats, cattle, solipeds and pigs).
In small ruminants (sheep and goats), 12 MS and two non‐MS reported data. The overall prevalence detected using indirect tests (ELISA, CFT, LAT or IFA) was around 20%, while using direct tests (staining, PCR and IHC) this prevalence was around 6.1%.
Eight MS (Croatia, Germany, Ireland, Italy, Romania, Slovakia, Spain and the United Kingdom) and one non‐MS reported data on Toxoplasma‐specific antibodies in cattle. A small number of animals was tested (324) with an overall prevalence (indirect and direct diagnostic methods) of 3.3% within the MS.
Three MS (Croatia, Italy and the United Kingdom) reported data on pigs: in total 360 animals were tested and only 8 (2.2%) were detected as positive by two MS (Croatia and Italy) using indirect tests (ELISA).
Eight MS and one non‐MS provided data on pet animals (cats and dogs): 11.5% out of 1,889 tested animals were positive and these were mainly from suspected animals and clinical investigations.
Positive results with direct and/or indirect tests for Toxoplasma were also documented in other species such as rabbits, alpacas and wild animals (deer, dolphin, fox, hares, badgers), but the available information is extremely fragmented for hosts and MS. Furthermore, most data originate from clinical samples.
10.4. Discussion
Toxoplasma gondii is a zoonotic protozoan parasite that can cause serious disease among humans, especially when primary infection is acquired during pregnancy. Based on the reported data for the year 2016, congenital toxoplasmosis in the EU shows a stable number of confirmed cases and notification rates from 2012 to 2016, but remains a rare disease overall. The decrease in notifications of cases in 2016 compared with previous years is a surveillance artefact due to France (reporting > 80% of the cases in EU) not reporting toxoplasmosis data at the time of data collection for this report.
Very few EU countries have active surveillance for congenital toxoplasmosis. A quarter of the EU countries do not have any surveillance for toxoplasmosis and the majority of the countries having surveillance systems reported zero cases. Therefore, a good estimate of the prevalence of this disease in the EU is not possible.
Recently, WHO reported that food‐borne toxoplasmosis, spread through undercooked or raw meat and fresh produce, may cause up to 20% of the total food‐borne disease burden in EU and affects more than 1 million people in the European Region each year (WHO, 2015).
The information reported by MS in 2016 shows that Toxoplasma is present in most livestock species across the EU. However, the sample size is too small to draw any epidemiological conclusions useful for the risk assessment for humans and to support the WHO estimation. For example, the total number of tested sheep and goat samples (both clinical and surveillance/monitoring samples) represents only a very small fraction of the sheep (85.5 million) and goat (12.5 million) populations in EU, respectively (Eurostat, 2016). Therefore, it is extremely difficult to evaluate the prevalence of infection and to compare the data collected in different MS, and there is a lack of information on the animal age and rearing conditions. To collect useful epidemiological information on the circulation of this pathogen among the different livestock species, a protocol for sample collection and parasite detection should be prepared and shared among MS. Important epidemiological variables, which should be considered, when a sampling scheme is established, are: species, age production sector, rearing conditions (e.g. free‐ranging hunted animals, outdoor or indoor bred or reared), contact with cats or rodents if animals reared inside, information on water resource and type of feed, which can be contaminated by cat faeces and consequently by Toxoplasma oocysts, if they are not controlled or appropriately treated.
The detection of Toxoplasma‐specific antibodies as well as the detection of the parasite DNA do not imply a direct risk for the consumers, in fact there is no direct correlation between the presence of antibodies and/or DNA and the parasite infectivity. Furthermore, as the tissue cysts are not uniformly distributed in the edible tissues, a negative result obtained by a direct detection method in a serologically positive animal, cannot exclude the presence of infectious cysts in other edible portions. Literature data show a high incidence of toxoplasmosis as parasite–animal contacts in sheep (Bacci et al., 2016), pigs (Djokic et al., 2016; Herrero et al., 2016; Wallander et al., 2016), goats (Deng et al., 2016), horses (Aroussi et al., 2015), small mammals including rodents (Machacova et al., 2016), pets (Cano‐Terriza et al., 2016) and wild animals (Formenti et al., 2016; Reiterova et al., 2016) suggesting that the control of this parasite is extremely difficult and can be reached only for livestock reared under strict housing conditions. A critical review of data on the prevalence of Toxoplasma in pigs shows a 90% decline in Toxoplasma prevalence in commercially marketed pigs over the last two decades (Dubey, 2009; Davies, 2011; Bayarri et al., 2012). Currently, the risk of acquiring toxoplasmosis from pork and pork products is mainly related to the consumption of raw or undercooked pork from free‐ranging pigs reared outdoors in organic systems (Papini et al., 2017). The high incidence of Toxoplasma in sheep (21%) and goats (9%) could, in part, be explained by their feeding behaviour, which exposes them to Toxoplasma. In addition, Sheep might be more susceptible to Toxoplasma compared to cattle. The high positivity in sheep could also be a result of vaccination since indirect tests have been used to detect Toxoplasma‐specific antibodies, but none of the reporting MS mentioned the vaccinations status of investigated animals in 2016.
Most MS use indirect methods to detect Toxoplasma. Opsteegh et al. (2016) investigated the relationship between indirect and direct detection methods and concluded that MAT‐based detection of antibodies, and possibly serological screening in general, is not recommended as an indicator of the presence of viable T. gondii in cattle and horses and that in these species direct detection methods are preferred. For pigs, poultry and small ruminants serological methods could be useful for the detection of high risk animals/herds but not as an indicator if infection in individual animals since the concordance between direct and indirect methods was estimated as low to moderate as confirmed by other recent studies as well (Aroussi et al., 2015; Djokic et al., 2016). In addition, direct methods should be applied on matrices taken post mortem as it was shown that the parasite load in different skeletal muscles in sheep and pigs does not vary much and that clear predilection sites are the brain, heart and lung tissues.
Certain risk factors are associated with higher risk for transmission from animals to humans such as the presence of cats on farm and outdoor/backyard husbandry practices at farm level (EFSA BIOHAZ Panel, 2013; Opsteegh et al., 2016). It appears that risk factor studies should be based on data obtained by direct methods rather than indirect methods. To manage the risk of Toxoplasma and propose intervention strategies in livestock (e.g. vaccination), it is important to collect and analyse information obtained from epidemiological investigations and surveys that standardise the sample matrix (brain, heart, lungs), the analytical method (direct methods preferentially) and the target population (species and risk categories). Direct methods are expensive and can be applied on a small amount of tissues, and so, they can be used only on a very limited number of samples which cannot be representative of the target population and makes reliable epidemiological investigations difficult. In addition to the direct detection method, strain identification is important since their pathogenicity and virulence to humans are quite different among the three genotypes and the so‐called atypical strains.
Limited information is available on the contamination of vegetables, fruits and drinking water by Toxoplasma oocysts in EU. In 2016, no MS reported on investigations in these matrices; however, it could be worth investigating possible transmission via these matrices.
Finally, the direct and indirect detection tests for Toxoplasma do not provide evidence to distinguish between an infection through ingestion of infected meat with tissue cysts and ingestion of food contaminated by oocysts shed by cats. Understanding the transmission routes would improve risk assessments for this food‐borne parasite and facilitate the identification of control measures.
10.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| Toxoplasmosis in Pregnancy: Prevention, Screening and Treatment | https://sogc.org/wp-content/uploads/2013/02/gui285CPG1301E-Toxoplasmosis.pdf | |
| European Union Reference Laboratory for Parasites | http://www.iss.it/crlp/ | |
| Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV‐Infected Adults and Adolescents | https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/322/toxo | |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| Animals | European Union Reference Laboratory for Parasites | http://www.iss.it/crlp/ |
| OIE Manual Chapter 2.9.9. Toxoplasmosis | http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.09.09_TOXO.pdf | |
| EFSA Scientific Opinion: Surveillance and monitoring of Toxoplasma in humans, food and animals | http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2007.583/epdf | |
| EFSA External Scientific Report: Relationship between seroprevalence in the main livestock species and presence of Toxoplasma gondii in meat (GP/EFSA/BIOHAZ/2013/01) An extensive literature review. |
http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2016.EN-996/pdf http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2016.EN-995/abstract |
|
| EFSA Supporting publication: Experimental studies on Toxoplasma gondii in the main livestock species (GP/EFSA/BIOHAZ/2013/01) Final report. M. Opsteegh, G. Schares, R. Blaga and J. van der Giessen. | http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2016.EN-995/abstract | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
11. Rabies
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
11.1. Abstract
During 2015 and 2016, no human cases of rabies were reported in the EU, while previously, between 2012 and 2014, six human cases of rabies were reported. Most cases reported in the EU have been exposed outside the EU. The risk of infection in eastern Europe however remains and therefore vaccination of people at higher risk of infection should be considered, in line with the relevant national and international recommendations.
The most cost‐effective strategy for preventing rabies in people is eliminating the disease in dogs and wildlife through animal vaccinations and dog and fox population management. In 2016, as compared with 2015, there was a decrease from 0.2% (2015) to 0.04% (2016) in the proportion of rabies‐positive foxes in the EU but also a reduction of 25% in the number of foxes investigated for rabies. One raccoon dog in Poland was found to be positive.
A remarkable finding was that all investigated wild animals, other than foxes and raccoon dogs, and approximately 2,000 samples in total reported by 15 MS tested negative. Since 2013, there has been a decline in the proportion of rabies cases in wildlife species in EU. Surveillance in relation to canine/domestic rabies confirmed that rabies remains endemic in eastern Europe. In Poland and Romania, positive cases were detected in farmed domestic animals (cattle and horses) and in domestic carnivores(cats and dogs).
Nineteen MS reported results of their surveillance on Lyssavirus in bats. Belgium reported its first case in bats (EBLV‐1) and Finland reported its second case (EBLV‐2, Daubenton's bat) since 2009. Six other MS (France, Germany, the Netherlands, Poland, Spain and the United Kingdom) reported positive cases in bats. The proportion of bats reported positive has increased from 1.4% in 2012 to 3.5% in 2016 and may reflect an increased surveillance effort in bats by MS.
Effective surveillance of rabies in humans, wildlife and domestic animals in the endemic areas as well as in rabies‐free areas should be maintained as there is a continuous risk of reintroduction of the virus from endemic areas to free zones in the EU.
11.2. Surveillance and monitoring of rabies in the EU
11.2.1. Humans
The notification of rabies in humans is mandatory in most MS, Iceland, Norway and Switzerland. Greece and Lithuania did not provide information about the nature of their surveillance system. Most countries use the EU case definition apart from Denmark, Finland, France, Germany, Greece and Italy who have other/non‐specified case definitions.
Most countries examine saliva and neck skin biopsies for ante mortem diagnosis of rabies. For post‐mortem examinations, the central nervous system is sampled. Identification is mostly based on antigen detection, viral genome detection by real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) and/or isolation of virus. Serum and spinal fluid are used to test for the presence of antibodies to rabies virus.
11.2.2. Animals
Surveillance data in relation to rabies is mainly used to demonstrate the absence of disease or to identify its presence or distribution in order to allow timely dissemination of information for integrated action among different sectors.
Member States considered free from risk of rabies can be consulted on OIE website.30
According to the Regulation (EU) No 652/201431, multiannual programmes for eradication of rabies may be cofinanced by the EU. In 2016, 13 MS (Bulgaria, Croatia, Estonia, Finland, Greece, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovakia and Slovenia) had approved eradication, control and surveillance programmes for rabies. The eradication programmes involve mainly assessing the prevalence of the disease in animals that are more at risk of being infected. Therefore, rabies is mainly monitored in wildlife using indicator animals that are found dead in their natural habitat and/or suspected animals from target species (foxes, badgers, raccoon dogs, etc.).
In addition, the monitoring of rabies relies on the analysis of routine testing in domestic animals (cattle, sheep, goats, rabbits, etc.) showing neurological clinical signs compatible with rabies and on the evaluation of vaccination (titres) in imported or travel‐related companion animals (mainly dogs and cats) from territories and third countries not included in Annex II to implementing Regulation (EC) No 577/201332.
Assessment of the oral vaccination coverage in foxes is another aim of the surveillance for rabies. Oral vaccination programmes for foxes are currently executed in parts of Finland (south eastern border with Russian Federation), Estonia (border with Russian Federation), Latvia and Lithuania (border with Belarus), Poland (50% of country bordering the Russian Federation), Hungary (15 regions) and Slovakia (whole territory except the areas bordering the Czech Republic, Austria and partially Hungary), Croatia (whole country except Adriatic islands), Greece (24 regional units), Bulgaria (16 administrative districts), Slovenia (whole territory), Romania (whole territory), Italy (Region of Friuli Venezia Giulia). Other neighbouring countries of MS that apply oral vaccination are Serbia, Kosovo, the Former Yugoslav Republic of Macedonia, Albania, Bosnia and Herzegovina, Ukraine, Russian Federation and Moldova.
Nineteen MS (Austria, Belgium, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Luxembourg, the Netherlands, Poland, Romania, Slovakia, Slovenia, Spain and the United Kingdom) reported 2016 monitoring of rabies in bats.
Rabies in animals is a notifiable disease in national health and veterinary systems.
Monitoring of rabies in animals and the data related to and reported to EFSA allows a descriptive summary at EU‐level as well as EU trends to be monitored (Table 1).
11.3. Data analyses
Results of surveillance activities for wildlife rabies were summarised for the major indicator/target species such as foxes, raccoon dogs and raccoons and other wild species (badgers, deer, marten, rodents, jackals, lynx, bears, hares, hedgehogs, mink, wolverine, wild boar, squirrels, ferrets, otters, polecats, etc.). A separate overviewing table was produced for the surveillance activities of the MS in bats.
Lastly, separate tables for dogs, cats and farmed domestic animals (cattle, small ruminants, solipeds, pigs, rabbits, ferrets) were also produced to summarise the data obtained from surveillance activities in the different MS for canine/domestic rabies. All data are summarised (aggregated) at MS level and when MS only reported regional data, the total number of tested animals are not integrated in the summary tables because of residual unclarity whether all regions in the MS were tested or not.
11.4. Results
11.4.1. Overview of key statistics, EU, 2012–2016
Table 36 summarises EU‐level statistics related to human cases of rabies, and to rabies/Lyssavirus occurrence and prevalence in major animal species in the EU, during 2012–2016.
Table 36.
Summary of rabies/Lyssavirus statistics related to humans and major animal species, EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 0 | 0 | 3 | 1 | 2 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ECDC |
| Number of reporting countries | 27 | 28 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | − | − | 0 | 0 | 1 | ECDC |
| Infection acquired outside the EU | − | − | 3 | 1 | 1 | ECDC |
| Unknown travel status or unknown country of infection | − | − | 0 | 0 | 0 | ECDC |
| Animals | ||||||
| Foxes | ||||||
| Number of tested animals | 37,296a | 49,875 | 41,854 | 49,190 | 25,503 | EFSA |
| Proportion of positive animals (%) | 0.04 | 0.20 | 0.25 | 1.11 | 0.05 | EFSA |
| Number of reporting MS | 22 | 21 | 22 | 23 | 20 | EFSA |
| Raccoons and raccoon dogs | ||||||
| Number of tested animals | 1,172 | 725 | 795 | 1,040 | 815 | EFSA |
| Proportion of positive animals (%) | 0.09 | 0.28 | 0.13 | 0.00 | 0.49 | EFSA |
| Number of reporting MS | 7 | 7 | 10 | 12 | 7 | EFSA |
| Wildlife (other than foxes and raccoon dogs) | ||||||
| Number of tested animals | 2,036 | 3,789 | 3,934 | 3,468 | 2,337 | EFSA |
| Proportion of positive animals (%) | 0 | 0.2 | 0.3 | 1.1 | 0 | EFSA |
| Number of reporting MS | 15 | 15 | 16 | 20 | 16 | EFSA |
| Dogs | ||||||
| Number of tested animals | 2,456 | 2,964 | 2,943 | 3,326 | 2,110 | EFSA |
| Proportion of positive animals (%) | 0.1 | 0.5 | 0.3 | 2.2 | 0.4 | EFSA |
| Number of reporting MS | 23 | 21 | 22 | 24 | 21 | EFSA |
| Bats | ||||||
| Number of tested animals | 1,405 | 1,747 | 1,969 | 1,442 | 2,012 | EFSA |
| Proportion of positive animals (%) | 3.5 | 1.9 | 1.7 | 1.3 | 1.4 | EFSA |
| Number of reporting MS | 19 | 17 | 16 | 19 | 17 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
Lithuania (regional data), Slovenia and Greece reported suspect and selective sampled foxes; Greece tested 195 foxes via active monitoring.
11.4.2. Rabies in humans
In 2016, all MS except Malta reported data on rabies in humans. During previous years, all MS had reported data.
Between 2012 and 2014, six human cases of rabies were reported while no cases were reported in 2015 and 2016. Among these six cases, five have been exposed outside the EU (i.e. Haiti, Indonesia, Mali and Morocco). The remaining case was infected in Romania where rabies remains endemic in dogs and wild animals.
11.4.3. Rabies in animals
Wildlife rabies
Twenty‐two MS and four non‐MS reported investigations on red foxes (Vulpes vulpes) carried out during 2016. Within EU, 37,296 foxes were investigated of which 14 were found to be positive (0.04%) in three different MS (Poland (9), Romania (4) and Hungary (1)). Also, in Serbia, four rabies cases in foxes were detected. Lithuania (461 foxes) and Slovenia (1,603 foxes) reported regional data from suspect sampling and selective sampling, respectively. Compared with 2015, 25% fewer foxes were investigated in the EU. The geographical distribution of reported cases in foxes in 2016 is shown in Figure 54.
Figure 54.
The geographical distribution in EU of reported cases in foxes in 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Investigations from raccoons (Procyon lotor) and raccoon dogs (Nyctereutes procyonoides) were provided by seven MS (the Czech Republic, Estonia, Finland, France, Latvia, Poland and Slovakia). Only one raccoon dog from Poland was found to be positive for rabies (1,172 tested in total).
Fifteen MS and two non‐MS reported approximately 2,000 samples (a reduction of more than 40% compared with 2015) in wildlife species other than foxes and raccoons/raccoon dogs. The species tested were badgers, deer, rodents, jackals, lynx, moose, wild mustelids, wolves, bears, hares, hedgehogs, wolverines, wild boar, squirrels, otters, polecats, wild birds, moles, wild cats and other wild carnivores. No positive animals were found. Since 2013, there has been a decline in the EU of the proportion of positive wildlife other than foxes and raccoons/raccoon dogs (Table 36).
Nineteen MS and one non‐MS reported 2016 results of the surveillance for Lyssavirus in bats. In total 1,405 bats were tested and 49 (3.5%) positive cases were found in eight different MS (Germany (23), the Netherlands (9), France (6), Poland (6), the United Kingdom (2), Belgium (1), Finland (1) and Spain (1)). The Lyssavirus species was only reported for 20 out of these 49 positive samples (by Belgium, Finland, France, the Netherlands and Spain). In Finland, the second case of EBLV‐2 in bats (Daubenton's bat) since 2009 was reported while Belgium reported a case in bats (EBLV‐1) for the first time. All cases in France were identified as EBLV‐1. Both cases of in the United Kingdom were EBLV‐2 and were in Daubenton's bats. The geographical distribution of reported cases in bats (EBLV‐1 or EBLV‐2) in 2016 is shown in Figure 55.
Figure 55.
The geographical distribution of reported cases (EBLV‐1 or EBLV‐2) in bats, EU, 2016
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Domestic/canine rabies
Sixteen MS reported 714 samples in domestic farmed animals, mainly cattle, small ruminants and domestic solipeds, which was a reduction of 23% compared with 2015. In total, 10 animals (1.4%) were found to be positive; one horse in Poland and nine bovine animals in Romania. The results reported by 24 MS from monitoring data in domestic carnivores (cats and dogs), approximately 5,000 samples, showed that Poland and Romania were the only two MS reporting positive cases; Poland reported two cases in dogs, and Romania one case in a dog and two in cats. All these cases were obtained from suspected animals (clinical investigations).
11.5. Discussion
The 2016 rabies monitoring results show that rabies remains endemic in dogs and cats and wild animals in Eastern Europe with cases reported by Poland, Romania, Hungary, Serbia. Consequently, people may be exposed and domestic human cases may occur. The most cost‐effective strategy for preventing rabies in people is elimination of the disease in dogs and wildlife through animal vaccination and dog and fox population management.
The results of the Lyssavirus monitoring in bats, with positives in eight MS, indicate that bats are a reservoir of EBLV and that surveillance of bats is gaining more interest in Europe. The recently reported rabies cases among bats in Belgium and Finland support the idea that the public health hazard of bat rabies in Europe should not be underestimated. The proportion of bats found positive for Lyssavirus increased from 1.4% in 2012 to 3.5% in 2016. To prevent rabies transmission from bats, all bat handlers should be informed of the risks of rabies exposure and advised to be vaccinated, in line with relevant national and international recommendations (Van der Poel et al., 2005).
Surveillance for rabies among humans and in domestic animals should be pursued even in countries that have successfully eliminated animal rabies as there is a continuous risk of reintroduction of the virus via illegally imported infected companion animals from endemic areas (Lardon et al., 2010).
11.6. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Global alliance for Rabies control | https://rabiesalliance.org/world-rabies-day |
| Rabies surveillance blueprint | http://rabiessurveillanceblueprint.org/?lang=en | |
| EU case definitions (all diseases, you can choose specific disease, if needed) | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Emerging and Vector‐borne Diseases Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/emerging-and-vector-borne-diseases-programme | |
| Emerging Viral Diseases‐Expert Laboratory Network (EVD‐LabNet) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/evd-labnet | |
| WHO (World Health Organization) – Rabies Fact sheet | http://www.who.int/mediacentre/factsheets/fs099/en/ | |
| Animals | EURL (EU Reference Laboratory) Rabies | https://eurl-rabies.anses.fr |
| Summary Presentations on the situation as regards Rabies veterinary programmes in Member States | https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en#20160705 | |
| General information on EU Food Chain Funding | https://ec.europa.eu/food/funding_en | |
| EU approved and cofinanced veterinary programmes for Rabies carried out by the MS | http://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en | |
| WHO (World Health Organization) – Rabies – Bulletin – Europe | http://www.who-rabies-bulletin.org/ | |
| The Joint FAO–OIE–WHO Global Early Warning System | http://www.glews.net/ | |
| EFSA Scientific Opinion: on the risk of rabies introduction into the UK, Ireland, Sweden and Malta as a consequence of abandoning serological tests measuring protective antibodies to rabies | https://www.efsa.europa.eu/en/efsajournal/pub/436 | |
| OIE (World Organisation for Animal health), Summary of Information on Rabies | http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Disease_cards/RABIES-EN.pdf | |
| OIE (World Organisation for Animal health), Questions & Answers on Rabies | http://www.oie.int/fileadmin/Home/fr/Animal_Health_in_the_World/docs/pdf/Portail_Rage/QA_Rage_EN.pdf | |
| OIE (World Organisation for Animal health), Technical disease card on Rabies | http://www.oie.int/fileadmin/Home/eng/AnimalHealth_in_the_World/docs/pdf/Disease_cards/RABIES_FINAL.pdf | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
12. Q fever
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
12.1. Abstract
An increasing trend in confirmed Q fever cases has been observed over the period 2012–2016 in the EU. Trends per country are very diverse with increases in France, Germany and Spain, and stable or decreasing prevalence in other MS. There is no clearly identified explanation for the increasing trend in humans in the EU between 2012 and 2016. Among other hypotheses, there might be a loosening of the control measures in place, or an increase in exposure or specific climatic conditions that may have favoured the spread of the bacteria. In 2016, 1,057 confirmed cases of Q fever were reported in by 19 MS; 2016 is marked by a large increase in number of cases reported by Spain, which is mostly due to a change in their notification system, from voluntary to mandatory.
The monitoring of Q fever in animals in the EU is not harmonised and therefore the data submitted to EFSA allow only a descriptive summary at EU‐level. The main animal species tested are ruminants (cattle and small ruminants) via passive monitoring strategies using samples from aborted animals, animals suspected of being infected by Coxiella burnetii or from animals tested in connection with trade or travel (export/import/fairs/licensing purposes). There is an active and planned monitoring of sheep and goats by frequently sampling and analysing the presence of C. burnetii‐specific antibodies in bulk milk samples in a small number of MS. In 2016, the highest overall prevalence was observed in sheep and goats (12.8%) and cattle (6.3%), but results in animals differ across the MS according to testing, coverage of the monitoring system and sensitivity of the surveillance for C. burnetii.
12.2. Surveillance and monitoring of Coxiella burnetii in the EU
12.2.1. Humans
Q fever in humans is a mandatory notifiable disease at the EU‐level and cases are reported through TESSy. Twenty‐seven MS, Iceland, Norway and Switzerland provided 2016 information on Q fever in humans. Twenty EU countries used the EU case definition, whereas Belgium, Denmark, France, Germany, Greece, Italy and Romania used another case definition. Finland did not specify its case definition. In 2016, Italy started to report Q fever data.
Reporting is compulsory in 24 EU countries and voluntary in France. Austria, Greece and Spain did not specify the legal basis of their surveillance system. Disease surveillance is mostly passive except in the Czech Republic and Slovakia. Data reporting is case based except in Belgium, Bulgaria and Croatia, and at the national level except in Spain.
12.2.2. Animals
The main pillar of surveillance for Q‐fever in animals implemented by most MS is passive monitoring and there is no EU harmonised active surveillance in place. The main animal species tested are small ruminants (goats and sheep) and cattle using samples from aborted animals, animals suspected of being infected by C. burnetii or from animals in connection with trade or travel (export/import/fairs/licensing purposes). In a few MS (Belgium, Germany, Slovakia and the Netherlands, the United Kingdom) and in Norway there is active and planned monitoring of milksheep and milkgoats by regularly sampling and analysing the presence of C. burnetii‐specific antibodies in bulk milk samples. Systematic surveys are performed occasionally in order to estimate the national (sero)prevalence or to confirm the presence of C. burnetii in bovine or small ruminant livestock regionally or even at herd level.
Mainly milk samples followed by blood samples, tissue samples or placentae are analysed and the diagnostic methods used are ELISA, CFT (for detection of antibodies) and/or fluorescence in situ hybridisation (FISH) or RT‐PCR (for the direct detection of C. burnetii).
As the surveillance in animals is mainly based on case reporting and passive surveillance at national level, and data reported by MS to EFSA are generated by non‐harmonised monitoring schemes across MS with no mandatory reporting requirements, the data on C. burnetii only descriptive summaries at the EU‐level. The data on Q‐fever preclude additional data analysis such as assessing temporal and spatial trends at the EU‐level. This is because the results between the MS differ in relation to testing methods, coverage of the monitoring and sensitivity of the surveillance for Coxiella burnetii.
Q fever food and animal monitoring data allow for descriptive summaries at EU‐level (Table 1). Lack of harmonisation precludes trend analyses and trend watching at EU‐level.
12.3. Results
Table 37 summarises EU‐level statistics related to Q fever in human and to Q fever occurrence and prevalence in major animal species, respectively, in the EU, during 2012–2016.
Table 37.
Summary of Coxiella burnetii statistics related to human and major animal species, EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 1,057 | 822 | 780 | 647 | 518 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.16 | 0.18 | 0.18 | 0.15 | 0.12 | ECDC |
| Number of reporting MS | 27 | 26 | 25 | 25 | 25 | ECDC |
| Infection acquired in the EU | 758 | 550 | 518 | 516 | 363 | ECDC |
| Infection acquired outside the EU | 36 | 8 | 21 | 16 | 11 | ECDC |
| Unknown travel status or unknown country of infection | 263 | 264 | 241 | 115 | 144 | ECDC |
| Animals | ||||||
| Small ruminants (animal level) | ||||||
| Number of sampled units | 7,545 | 15,819 | 9,005 | 9,057 | 15,183 | EFSA |
| Proportion of positive units (%)a | 12.8 | 10.3 | 6 | 1.1 | 10 | EFSA |
| Number of reporting MS | 16 | 14 | 18 | 14 | 15 | EFSA |
| Cattle (animal level) | ||||||
| Number of sampled units | 17,480 | 62,335 | 48,141 | 36,757 | 24,345 | EFSA |
| Proportion of positive units (%)a | 6.3 | 13 | 9.1 | 8.3 | 7.5 | EFSA |
| Number of reporting MS | 16 | 15 | 18 | 16 | 14 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
For the summary statistics indirect and direct diagnostic methods were taken together to calculate the proportion of positive units.
12.3.1. Coxiella burnetii in humans
Overall, 1,057 confirmed cases of Q fever were reported by 19 MS, 2 cases were reported by Norway and 48 cases were reported by Switzerland (Table 38). In 2016, Spain was the country that reported the most cases (n = 331), followed by Germany and France (270 and 251, respectively).
Table 38.
Reported human cases of Q fever and notification rates per 100,000 population in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austriab | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Belgium | Y | A | 22 | 16 | 0.14 | 8 | 0.07 | 3 | 0.03 | 5 | 0.04 | 7 | 0.06 |
| Bulgaria | Y | A | 19 | 17 | 0.24 | 15 | 0.21 | 15 | 0.21 | 23 | 0.32 | 29 | 0.4 |
| Croatia | Y | A | 8 | 8 | 0.19 | 14 | 0.33 | 21 | 0.49 | 0 | 0 | 43 | 1.01 |
| Cyprus | Y | C | 3 | 2 | 0.24 | 4 | 0.47 | 1 | 0.12 | 3 | 0.35 | 4 | 0.46 |
| Czech Republic | Y | C | 2 | 2 | 0.02 | 1 | 0.01 | 0 | 0 | 0 | 0 | 1 | 0.01 |
| Denmark | Y | C | 0 | 0 | 0 | 0 | 0 | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finland | Y | C | 2 | 2 | 0.04 | 3 | 0.05 | 0 | 0 | 5 | 0.09 | 0 | 0 |
| France | Y | C | 251 | 251 | 0.38 | 250 | 0.38 | 209 | 0.32 | 158 | 0.24 | 5 | 0.01 |
| Germany | Y | C | 275 | 270 | 0.33 | 310 | 0.38 | 238 | 0.29 | 114 | 0.14 | 198 | 0.25 |
| Greece | Y | C | 9 | 9 | 0.08 | 10 | 0.09 | 15 | 0.14 | 11 | 0.10 | 11 | 0.10 |
| Hungary | Y | C | 39 | 39 | 0.40 | 35 | 0.36 | 59 | 0.60 | 135 | 1.36 | 36 | 0.36 |
| Ireland | Y | C | 6 | 6 | 0.13 | 4 | 0.09 | 0 | 0 | 0 | 0 | 5 | 0.11 |
| Italy | Y | C | 5 | 3 | 0.00 | – | – | – | – | – | – | – | – |
| Latvia | Y | C | 0 | 0 | 0 | 1 | 0.05 | 3 | 0.15 | 1 | 0.05 | 1 | 0.05 |
| Lithuania | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | Y | C | 0 | 0 | 0 | 1 | 0.18 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.47 | 0 | 0 |
| Netherlands | Y | C | 14 | 14 | 0.08 | 20 | 0.12 | 26 | 0.15 | 20 | 0.12 | 63 | 0.38 |
| Poland | Y | C | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Portugal | Y | C | 17 | 17 | 0.16 | 20 | 0.19 | 25 | 0.24 | 21 | 0.2 | 26 | 0.25 |
| Romania | Y | C | 33 | 32 | 0.16 | 3 | 0.02 | 21 | 0.11 | 24 | 0.12 | 16 | 0.08 |
| Slovakia | Y | C | 0 | 0 | 0 | 0 | 0 | 1 | 0.02 | 0 | 0 | 0 | 0 |
| Slovenia | Y | C | 1 | 1 | 0.05 | 1 | 0.05 | 3 | 0.15 | 1 | 0.05 | 1 | 0.05 |
| Spainc | N | C | 358 | 331 | – | 97 | – | 77 | – | 75 | – | 58 | – |
| Sweden | Y | C | 3 | 3 | 0.03 | 4 | 0.04 | 2 | 0.02 | 3 | 0.03 | 2 | 0.02 |
| United Kingdom | Y | C | 34 | 34 | 0.05 | 21 | 0.03 | 60 | 0.09 | 46 | 0.07 | 12 | 0.02 |
| EU total | – | – | 1,101 | 1,057 | 0.16 | 822 | 0.18 | 780 | 0.18 | 647 | 0.15 | 518 | 0.12 |
| Iceland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norway | Y | C | 2 | 2 | 0.04 | 1 | 0.02 | 1 | 0.02 | 4 | 0.08 | 0 | 0 |
| Switzerlandd | Y | C | 48 | 48 | 0.57 | 40 | 0.48 | 44 | 0.54 | 27 | 0.33 | – | – |
Y: yes; N: no; A: aggregated data; C: case‐based data;‐: no report.
Not notifiable, no surveillance system exists.
No information on estimated coverage; thus, notification rate cannot be estimated.
Switzerland provided data directly to EFSA. The human data for Switzerland also include the ones from Liechtenstein.
The EU notification rate was 0.16 per 100,000 population, which has remained stable since 2012. The highest notification rate (0.40 cases per 100,000 population) was observed in Hungary, followed by France (0.38), Germany (0.33), Bulgaria and Cyprus (0.24), and Portugal and Romania (both 0.16). An increasing trend in confirmed Q fever cases was observed over the period 2012–2016 in the EU (Figure 56).
Figure 56.
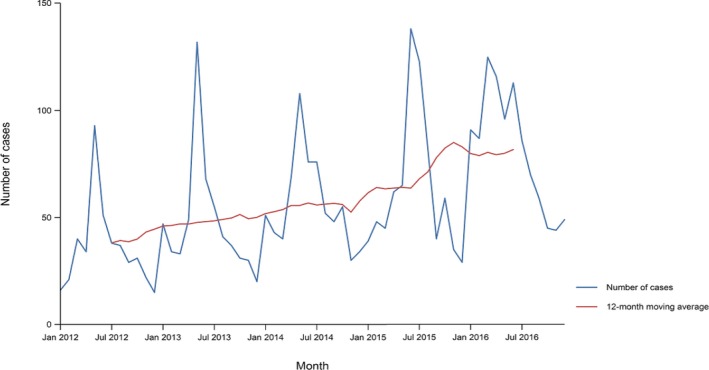
Trend in reported confirmed human cases of Q fever in the EU/EEA by month, 2012–2016
- Source(s): Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden. Austria, Belgium, Bulgaria, Croatia, Italy, Switzerland and the United Kingdom did not report data to the level of detail required for the analysis.
Nine countries (Denmark, Estonia, Iceland, Latvia, Lithuania, Luxembourg, Malta, Poland and Slovakia) reported no human cases. The large majority (71.8%) of Q fever cases in the EU was domestically acquired. In total, 36 travel‐associated cases were reported, of which 10 had travelled to Kosovo33 and six had travelled to Turkey.
Cases occurred during the whole year but with a seasonal increase between March and June when 44% of the cases reported in 2016 occurred.
Three deaths due to Q fever were reported in 2016 in the EU (two cases in Spain and one case in Hungary), resulting in EU case fatality of 0.5% among the 552 confirmed cases with reported outcome.
12.3.2. Coxiella burnetii in animals
Sixteen MS and three non‐MS provided data on sheep and goats for 2016. Most samples were collected in Spain, Poland and Italy. In total, 7,545 individual animals were tested of which around 13% tested positive for C. burnetii. Poland performed a national survey in sheep at holding level and only 1 holding out of 3,217 tested positive using PCR.
Sixteen MS and four non‐MS provided data on cattle for 2016. In total, 202 herds and 17,480 animals were tested of which 10% and 6.3% were positive, respectively. Poland monitored 929 cattle holdings with PCR and 1% of these holdings tested positive. Most sampling was conducted in Belgium, the Czech Republic, Slovakia, Spain and the United Kingdom. Note that 75% of all animals tested were taken from suspected animals and/or clinical investigations.
Five MS and two non‐MS reported on animals other than sheep, goats and cattle. In total, 720 different domestic and wild animal species (alpacas, antelopes, cats, deer, dogs, hedgehogs, solipeds, wild boar, wild birds, wolves) were tested and mainly clinical investigations were found positive.
12.4. Discussion
While France and Germany have reported the majority of the confirmed human cases since 2012, in 2016 Spain accounted for more than a third of the overall number of cases and the number of human cases reported by Spain tripled compared to previous years. This peak in Spain is mostly explained by a change in their reporting system: from voluntary to mandatory.
Between 2007 and 2010, the Netherlands experienced a large outbreak with more than 4,000 human cases (Schneeberger et al., 2014). The number of cases in the Netherlands returned to pre‐outbreak levels in 2013 and has remained low since then. Between 2012 and 2016, the overall number of human cases reported in the EU/EEA has continuously increased. After several consecutive years of increase in France and Germany, the numbers reported in 2016 were, respectively, equivalent and lower than in 2015. The overall increase in 2016 is due to the cases reported by Spain.
Despite the increased number of cases between 2015 and 2016, the EU rate decreased. This is due to the fact that Italy started to report data in 2016, which impacted the overall population considered and therefore the EU notification rate.
Besides the change in reporting systems in some MS, there is no clear and identified explanation for the increasing trend in the EU between 2012 and 2016. Among other hypothesis there might be a loosening of the control measures in place, or an increase in exposure (e.g. increasing farm tourism), or finally specific climatic conditions that may have favoured the spread of the bacteria.
Q fever is associated with the parturition: kidding (goats), lambing (sheep) and calving (cows). This is a time when people are exposed to contaminated birth material and when the bacteria are excreted into the environment. Considering that births are occurring all year round and that the bacteria are resistant in the environment, human cases are observed all year round. A seasonal increase is observed in spring and summer each year, possibly associated with a higher number of animal births and suitable environmental conditions (i.e. dryness, wind) for the spread of the bacteria. In 2016, the peak in numbers of cases occurred a few months earlier than in the previous year. The early peak is mostly due to an increase in the number of cases in Germany and Spain. There is no specific reason to explain this early peak, which is biased by the change in the notification system in Spain.
The results obtained in 2016 from animals – mainly from small ruminants and cattle – do not allow a trend analysis for Q fever in EU. The results of different MS differ with relation to testing, coverage of monitoring and sensitivity of the surveillance for C. burnetii. The regional variability within Europe highlights the importance of understanding risk factors that may operate at a local level and may be subtle (Georgiev et al., 2013).
12.5. Related projects and internet sources
13. West Nile virus
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
13.1. Abstract
After a sharp decrease in West Nile fever (WNF) human cases in 2014, the number of cases became comparable with the situation before 2014. In 2016, 240 cases were reported. Most cases were reported in Romania and Italy, with, respectively, 39% and 34% of the total EU cases. An increase in reported deaths due to WNF compared with previous years was notified.
MS with areas that are typically prone to harbouring mosquitoes were affected by both human illness and also outbreaks in animals. Cyprus reported its first ever human case of WNF. Other MS reporting human illness were Austria, Bulgaria, Croatia, France, the Netherlands and Spain.
In southern Europe, West Nile virus (WNV) outbreaks and positive animals were detected and reported by 13 MS, during recent years; Austria, Bulgaria, Croatia, Cyprus, the Czech Republic, France, Greece, Hungary, Italy and Portugal, Romania, Slovakia, Spain.
Human, animal and entomological WNF surveillance is crucial to allow the early detection of WNV infections in humans and take timely preventive measures.
13.2. Surveillance and monitoring in the EU of West Nile fever
13.2.1. Humans
Human WNF disease data are collected through two complementary processes. During the period of high mosquito activity (June to November), the MS timely report human cases to TESSy at ECDC. Complementary to this real‐time data collection, an annual data collection is carried out. Countries that did not detect any case during the year are asked to report ‘zero cases’; all other countries are encouraged to report complementary data on detected cases if considered relevant.
Twenty‐six EU/EEA MS provided 2016 information on WNF in humans to TESSy. The EU case definition was applied by 23 countries; Finland did not specify which case definition was used and France and the United Kingdom used an alternative case definition. Twenty‐six reporting countries had a comprehensive surveillance system. Reporting is compulsory in 24 countries and voluntary in two (France and the United Kingdom). Denmark and Germany did not specify if reporting is compulsory. Surveillance is passive, except the Czech Republic, Portugal, Slovakia and the United Kingdom. All countries have a national coverage of reporting and case based reporting (except Croatia).
13.2.2. Animals
Although the reporting of WNV infections in animals is not mandatory, MS can report WNV infections in animals to the European Commission in accordance with the Zoonoses Directive 2003/99/EC. The Directive specifies that, in addition to the number of zoonoses and zoonotic agents for which monitoring is mandatory, others shall also be monitored when the epidemiological situation so warrants.
WNV monitoring data from animals submitted to EFSA are collected without harmonised design. Due to heterogeneity in study design and the variety of analytical methods used, the reported WNV prevalence in animals from different countries is not directly comparable. These data allow for descriptive summaries at EU‐level to be made but lack of harmonisation precludes trend analyses and trend watching at EU‐level (Table 1).
Proposals for harmonised schemes for the monitoring and reporting of WNV in animals can be found in an External Scientific Report submitted to EFSA (Mannelli et al., 2012).
13.3. Results
13.3.1. Overview of key statistics along the food chain, EU, 2012–2016
Table 39 summarises EU‐level statistics related to human WNF cases, and to occurrence of WNV in birds and solipeds, respectively, in the EU, during 2012–2016. More detailed descriptions of these statistics are in the results section of this chapter.
Table 39.
Summary of West Nile fever statistics related to humans, and West Nile virus in birds and solipeds, EU, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of cases | 240 | 128 | 78 | 250 | 241 | ECDC |
| Total number of cases/100,000 population (notification rates) | 0.05 | 0.02 | 0.02 | 0.06 | 0.06 | ECDC |
| Number of reporting MS | 26 | 26 | 24 | 25 | 25 | ECDC |
| Infection acquired in the EU | 225 | 119 | 75 | 250 | 241 | ECDC |
| Infection acquired outside the EU | 3 | 0 | 2 | 0 | 0 | ECDC |
| Unknown travel status or unknown country of infection | 12 | 9 | 1 | 0 | 0 | ECDC |
| Animals | ||||||
| Birds | ||||||
| Number of sampled animals | 8,060 | 8,443 | 10,317 | 8,639 | 5,083 | EFSA |
| Number of reporting MS | 4 | 7 | 7 | 6 | 2 | EFSA |
| Solipeds | ||||||
| Number of sampled animals | 9,751 | 12,733 | 14,512 | 11,389 | 8,255 | EFSA |
| Number of reporting MS | 9 | 9 | 12 | 12 | 8 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
13.3.2. West Nile fever in humans
Table 40 presents the locally acquired and travel‐related reported human cases of WNF and notification rates per 100,000 in the EU/EEA, by country and year during 2012–2016.
Table 40.
Locally acquired and travel‐related reported human cases of West Nile Fever and notification rates per 100,000 in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National data | Report typea | Confirmed cases | Total cases & rates | Total cases & rates | Total cases & rates | Total cases & rates | Total cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 5 | 5 | 0.06 | 7 | 0.08 | 2 | 0.02 | 0 | 0 | 3 | 0.04 |
| Belgium | Y | C | 0 | 0 | – | 0 | – | 0 | – | 0 | – | 2 | – |
| Bulgaria | Y | C | 1 | 2 | 0.03 | 3 | 0.04 | 0 | 0 | 0 | 0 | 4 | 0.06 |
| Croatia | Y | A | 2 | 2 | 0.05 | 1 | 0.02 | – | – | 20 | 0.48 | 6 | 0.14 |
| Cyprus | Y | C | 1 | 1 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Czech Republic | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.01 | 0 | 0 |
| Denmarkb | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finland | Y | C | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| France | Y | C | 3 | 3 | 0.004 | 1 | – | 0 | 0 | 1 | – | 3 | – |
| Germanyb | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Greece | Y | C | 0 | 0 | 0 | 0 | 0 | 15 | 0.14 | 86 | 0.78 | 162 | 1.46 |
| Hungary | Y | C | 23 | 48 | 0.5 | 22 | 0.22 | 11 | 0.11 | 36 | 0.37 | 17 | 0.17 |
| Ireland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.02 | 0 | 0 |
| Italyc | N | C | 81 | 81 | – | 61 | – | 24 | – | 79 | 0.13 | 28 | 0.05 |
| Latvia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lithuania | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Netherlands | Y | C | 1 | 1 | 0.006 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Poland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Portugal | Y | C | 0 | 0 | 0 | 1 | 0.01 | – | – | – | – | – | – |
| Romania | Y | C | 85 | 93 | 0.5 | 32 | 0.16 | 24 | 0.12 | 24 | 0.12 | 15 | 0.08 |
| Slovakia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slovenia | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.05 | 0 | 0 |
| Spain | Y | C | 4 | 4 | 0.009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sweden | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.01 | 1 | 0.01 |
| United Kingdom | Y | C | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| EU total | – | – | 206 | 240 | 0.05 | 128 | 0.02 | 78 | 0.02 | 250 | 0.06 | 241 | 0.06 |
| Iceland | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Norway | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Switzerland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.01 | 1 | 0.01 |
Y: yes; N: no; A: aggregated data report; C: case‐based data report;–: no report.
No surveillance system.
No national coverage in 2016, hence notification rate not calculated.
Figure 57.
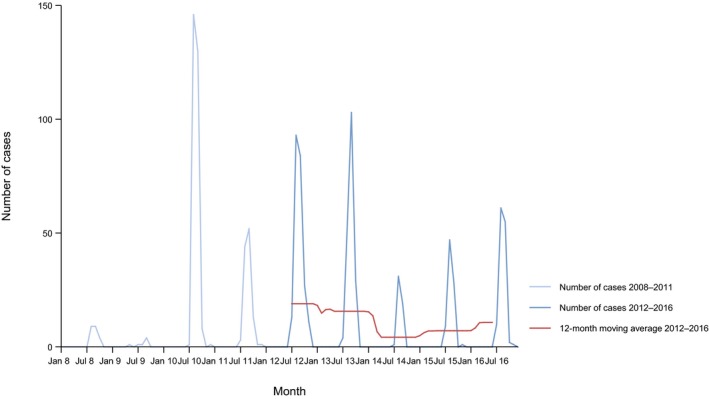
Trend in reported cases of human West Nile fever in the EU/EEA, by month, 2008–2016
- Source(s): Austria, Belgium, Cyprus, the Czech Republic, Estonia, Finland, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden, the United Kingdom. Bulgaria, Croatia, Denmark, France, Germany, Iceland and Portugal did not report data to the level of detail required for the analysis.
WNF is a seasonal disease with most cases occurring in the summer and early autumn. A total number of 240 cases34 were reported by 10 MS. Eighty‐six per cent of those cases were confirmed. Most of the total cases were reported in Romania and Italy, respectively, 39% and 34% of the total EU cases. Hungary, Romania and Italy reported an increase in number of cases compared with 2015. The first human case of WNF ever detected in Cyprus was notified in August 2016 in the district of Larnaca. The overall notification rate per 100,000 population was 0.05 as compared to 0.02 in 2016. This rate is comparable with those in 2012 and 2013, before the sharp decrease in 2014.
In 2016, 93% of the total cases were domestically acquired or acquired during travel within EU. Among cases associated with travel, the travel destination was unknown for 67% of the cases. Travel‐associated cases were reported from Anguilla (a British overseas territory), Egypt, Tunisia and Canada.
Seven MS provided data on the hospitalisation status of their cases. Almost two‐thirds of the total cases in 2016 were hospitalised. Seventy‐four per cent of the total cases were neuroinvasive and 23 cases were asymptomatic blood donors. Data on the outcome of cases was provided by nine MS. Twenty‐eight deaths were reported due to West Nile fever in 2016, compared to two in 2015 and seven in 2014.
13.3.3. West Nile virus in animals
For the year 2016 WNV testing results of 8,504 birds, mostly wild birds but also fowl on farms, have been reported by four MS and one non‐MS; Belgium (85), Hungary (149), Italy (5,070), Spain (2,954) and Switzerland (246). Hungary reported six positive birds, Italy 68 and Spain 274. Italy and Spain reported the bird samples to be positive using the PCR test, which detects viral genetic material, or using an immunoglobulin G (IgG) antibody ELISA (serological) test or seroneutralisation test. Hungary did not report the laboratory analytical method used.
The results from 9,953 solipeds were reported by 10 MS and one non‐MS (Cyprus, the Czech Republic, Greece, Hungary, Ireland, Italy, Portugal, Romania, Slovakia, Spain and Switzerland). All these countries except Greece, Ireland and Portugal, and Switzerland detected positive animals that were unvaccinated or had an unknown vaccination status; Cyprus (11), the Czech Republic (1), Hungary (54), Italy (51), Romania (3), Slovakia (2) and Spain (73). Countries reported the horses (plus one donkey in both Italy and Slovakia) to be confirmatory test‐positive, specifically to the IgM‐capture ELISA (MAC‐ELISA), except for the Czech Republic reporting confirmatory test‐positivity to neutralising antibody testing and for Spain reporting to have used the PCR and/or seroneutralisation test for confirmation testing. Cyprus and Portugal reported (IgG) ELISA screening test‐positives whereas horses positive to the immunofluorescence assay test were reported by Hungary.
Complementary to the reporting to EFSA, Hungary, Italy, Portugal and Spain reported, respectively, 48, 45, 5 and 73 outbreaks to the EU Animal Disease Notification System (ADNS).35 In Portugal, there were five outbreaks with a total of six positive animals. Austria notified one WNV outbreak in animals to ADNS but did not report them to EFSA by the time data for this report was compiled. Previously, outbreaks in animals were also reported by Bulgaria, Croatia, France and Greece to EFSA or to ADNS.
An interactive overview map for both the EU and neighbouring countries, including the regional level, is published on the ECDC website (ECDC, 2017d) with an epidemiological update summarising the WNF season, historical maps and the weekly updates of the ECDC West Nile risk map. As from 6 October 2017 this includes three types of maps: (1) human WNF cases; (2) equine WNF cases; and (3) combined human and equine WNF cases (ECDC website36). In addition, the number of equine cases per area (at NUTS 3 level) is shown in the table in the ECDC Surveillance Atlas of Infectious Diseases as from the transmission season 2017 onwards. The map with combined 2016 human and equine WNF cases is in Figure 58.
Figure 58.
Distribution of human and equine West Nile fever cases by affected areas, EU/EEA region, transmission season 2016 (Source: TESSy and ADNS)
13.4. Discussion
WNV is a mosquito‐borne flavivirus that circulates among mosquitoes and wild birds. Humans and horses are incidental dead‐end hosts. WNV is normally passed on to humans and animals via mosquitoes that feed on infected birds. The virus has been reported in Europe since the 1950s.
The notification rate of WNF in humans in the EU/EEA increased compared with 2015, but was similar to 2012 and 2013 suggesting a fluctuant multiannual pattern of this infection, driven by inter‐annual variations of local climate conditions. The highest number of cases was reported by Romania, followed by Italy and Hungary. Specifically, the notification rates from Romania and Hungary increased considerably compared to previous years. France and Portugal did not report any autochthonous WNF cases in 2016, while they did notify cases in 2015. This is not surprising as WNV is known to circulate in those countries causing only sporadic human cases. Greece did not report any human WNF case for two consecutive years, although virus circulation was frequently detected via serological testing of birds. Cyprus reported its first WNF case in 2016.
Highly populated areas and capital cities were affected by WNF in 2016. The deferral of blood donations from donors leaving affected areas has an impact on national blood supplies, as according to Commission Directive 2014/110/EU, blood donation should be deferred for 28 days after leaving a risk area of locally acquired WNV unless an individual nucleic acid test (NAT) is negative.
An increase of number of deaths has been reported compared to previous years. A hypothesis for this increase could be an introduction of new lineage strains, with different pathogenicity. However, this has not yet been confirmed.
Test‐positive birds and solipeds were reported for the year 2016 by Hungary, Italy and Spain. Additionally positive solipeds, reported as not vaccinated against WNV or as having an unknown vaccination status, were reported by Cyprus, the Czech Republic, Romania and Slovakia. Complementary to the reporting to EFSA, Hungary, Italy, Portugal and Spain reported, respectively, 48, 45, 5 and 73 outbreaks to the ADNS.35 In Portugal, there were five outbreaks involving six positive animals. Austria notified one WNV outbreak in animals to ADNS but did not report them to EFSA in time to be included in this report. Previously, outbreaks in animals were also reported by Bulgaria, Croatia, France and Greece to EFSA or to ADNS.
In southern Europe, WNV in animals has been detected and reported to EFSA by 13 MS during recent years, mostly Austria, Bulgaria, Croatia, Cyprus, the Czech Republic, France, Greece, Hungary, Italy, Portugal, Romania, Slovakia and Spain. This finding is consistent with the OIE's conclusion that the occurrence of WNF in humans and animals along with bird and mosquito surveillance for WNV activity demonstrates that the virus range has dramatically expanded including North, Central and South America as well as Europe and countries facing the Mediterranean Basin (OIE Terrestrial Manual).
The disease in horses is preventable with proper vaccination.
13.5. Related projects and internet sources
14. Tularaemia
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
14.1. Abstract
Tularaemia is a seasonal and cyclical disease with a complex ecological cycle. Between 2008 and 2016, no specific trend in the number of human tularaemia cases has been observed in the EU. Between 2012 and 2015, Sweden reported the highest notification rate while in 2016 Finland reported the highest notification rate. For the year 2016, 1,056 confirmed cases were reported by 18 MS. The number of cases is comparable with 2015 but higher than in previous years.
The high number of human cases in Finland in 2016 follows a peak in the number of voles in 2015 and climatic conditions favouring the abundance of mosquitoes transmitting the bacteria to humans.
Tularaemia is not a reportable disease in animals in EU, and the submission of the data to EFSA is voluntary. For the year 2016, only one MS (Sweden) and one non‐MS (Switzerland) reported data on the occurrence of Francisella tularensis (F. tularensis) in animals. Six brown hares out of 41 tested animals (14.6%) were found positive in Sweden. The number of positive tested animals in 2016 is comparable with previous years with no reported outbreaks. However the detection of F. tularensis in brown hares in Sweden during 2016 suggests that the bacterium is still present and outbreaks may happen in the future, particularly in the Nordic countries.
Greater efforts are needed to assess the extent of true animal reservoirs of F. tularensis and the occurrence of this zoonotic pathogen in the EU animal reservoir populations, as well as in the environment, to help predict outbreaks and to avoid them whenever possible. Moreover, MS are encouraged to submit all available data regarding F. tularensis to EFSA to allow a better understanding of this disease at EU‐level.
14.2. Surveillance and monitoring of tularaemia in the EU
14.2.1. Humans
Twenty‐six MS, Iceland, Norway and Switzerland provided 2016 information on tularaemia in humans.
All reporting EU countries have a comprehensive surveillance system, besides Denmark and Malta where no surveillance system for human tularaemia exists.. Twenty‐one EU countries used the EU case definition. Germany and Italy used an alternative case definition. Finland, France and Greece did not specify their case definition. The reporting is compulsory in 24 countries, voluntary in the United Kingdom and not specified for Greece. The surveillance is mostly passive except in the Czech Republic, Portugal and Slovakia where it is active. Greece did not specify the type of surveillance in place.
Belgium, Bulgaria and Croatia reported aggregated data while all other countries reported case‐based data.
14.2.2. Animals
Tularaemia in animals is not a reportable disease in MS, according to Council Directive 82/894/EEC37 on the notification of animal diseases within the Community; amended and consolidated version of 1 January 2013, but it is reportable to OIE when a new disease event occurs in a country. However, notification is mandatory by national law in the Netherlands, Sweden, Iceland and Switzerland. The monitoring data from animals on F. tularensis are voluntarily submitted by MS and EFTA countries to EFSA. The data are collected without harmonised design at EU‐level and only allow for descriptive summaries at the EU‐level and not for trend analyses and trend watching.
Monitoring data from animals on F. tularensis and submitted to EFSA are collected without harmonised design. These data allow for descriptive summaries at EU‐level to be made but lack of harmonisation precludes trend analyses and trend watching at EU‐level (Table 1).
14.3. Results
Table 41 summarises EU‐level statistics related to human tularaemia, and to tularaemia occurrence and prevalence in major animal species, respectively, in the EU, during 2012–2016.
Table 41.
Summary of tularaemia statistics related to humans and major animal species (brown hares) MS, 2012–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 1,056 | 1,080 | 482 | 280 | 945 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.21 | 0.23 | 0.10 | 0.06 | 0.2 | ECDC |
| Number of reporting MS | 26 | 25 | 26 | 26 | 25 | ECDC |
| Infection acquired in MS | 326 | 902 | 396 | 248 | 683 | ECDC |
| Infection acquired outside MS | 5 | 4 | 6 | 2 | 1 | ECDC |
| Unknown travel status or unknown country of infection | 725 | 174 | 80 | 30 | 261 | ECDC |
| Animals (Brown hares) | ||||||
| Total number of animals tested | 41 | 65 | 31 | 37 | 41 | EFSA |
| Proportion of positive animals (%) | 14.6 | 47.7 | 6.5 | 29.7 | 29.3 | EFSA |
| Number of reporting MS | 1 | 1 | 1 | 1 | 1 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
14.3.1. Tularaemia in humans
Table 42 presents the reported human cases of tularaemia and notification rates per 100,000 population in the EU/EEA, by country and year during 2012–2016.
Table 42.
Reported human cases of tularaemia and notification rates per 100,000 population in the EU/EEA, by country and year, 2012–2016
| Country | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 9 | 9 | 0.1 | 4 | 0.05 | 0 | 0 | 2 | 0.02 | 2 | 0.02 |
| Belgium | Y | A | 1 | 1 | 0.01 | 1 | 0.01 | 2 | 0.02 | 1 | 0.01 | 1 | 0.01 |
| Bulgaria | Y | A | 3 | 2 | 0.03 | 17 | 0.24 | 1 | 0.01 | 1 | 0.01 | 0 | 0.00 |
| Croatia | Y | A | 2 | 2 | 0.05 | 13 | 0.31 | 2 | 0.05 | 2 | 0.05 | 1 | 0.02 |
| Cyprus | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| Czech Republic | Y | C | 59 | 59 | 0.56 | 56 | 0.53 | 48 | 0.46 | 36 | 0.34 | 42 | 0.40 |
| Denmarkb | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 1 | 1 | 0.08 | 0 | 0 | 1 | 0.08 | 1 | 0.08 | 0 | 0.00 |
| Finland | Y | C | 699 | 699 | 12.74 | 104 | 1.90 | 9 | 0.17 | 15 | 0.28 | 233 | 4.31 |
| France | Y | C | 98 | 47 | 0.07 | 28 | 0.04 | 19 | 0.03 | 21 | 0.03 | 5 | 0.01 |
| Germany | Y | C | 41 | 41 | 0.05 | 34 | 0.04 | 21 | 0.03 | 20 | 0.02 | 21 | 0.03 |
| Greece | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| Hungary | Y | C | 22 | 22 | 0.22 | 35 | 0.36 | 140 | 1.42 | 48 | 0.48 | 18 | 0.18 |
| Ireland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| Italy | Y | C | 0 | 0 | 0 | – | – | 0 | 0 | 1 | 0 | 4 | 0.00 |
| Latvia | Y | C | 1 | 1 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0.29 |
| Lithuania | Y | C | 2 | 2 | 0.07 | 4 | 0.14 | 4 | 0.14 | 4 | 0.13 | 3 | 0.10 |
| Luxembourg | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| Maltab | – | C | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | 0.00 |
| Netherlands | Y | C | 5 | 5 | 0.03 | 1 | 0.01 | 5 | 0.03 | 0 | 0 | – | – |
| Poland | Y | C | 18 | 18 | 0.05 | 9 | 0.02 | 11 | 0.03 | 8 | 0.02 | 6 | 0.02 |
| Portugal | Y | – | 0 | 0 | 0 | 0 | 0 | – | – | – | – | – | – |
| Romania | Y | C | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.01 | 0 | 0.00 |
| Slovakia | Y | C | 7 | 7 | 0.13 | 28 | 0.52 | 6 | 0.11 | 9 | 0.17 | 8 | 0.15 |
| Slovenia | Y | C | 3 | 3 | 0.15 | 0 | 0 | 1 | 0.05 | 2 | 0.10 | 4 | 0.19 |
| Spain | Y | C | 3 | 3 | 0.01 | 22 | 0.05 | 62 | 0.13 | 0 | 0 | 1 | 0.00 |
| Sweden | Y | C | 134 | 134 | 1.36 | 722 | 7.41 | 150 | 1.56 | 108 | 1.13 | 590 | 6.22 |
| United Kingdom | Y | C | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| EU total | – | – | 1,108 | 1,056 | 0.21 | 1,080 | 0.23 | 482 | 0.10 | 280 | 0.06 | 945 | 0.20 |
| Iceland | Y | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| Norway | Y | C | 40 | 40 | 0.77 | 42 | 0.81 | 46 | 0.9 | 28 | 0.55 | 50 | 1.00 |
| Switzerlandc | Y | C | 55 | 55 | 0.66 | 48 | 0.57 | 39 | 0.46 | 29 | 0.35 | 40 | 0.50 |
Y: yes; N: no; A: aggregated data; C: case‐based data.
Not notifiable, no surveillance system exists.
Switzerland provided data directly to EFSA. Liechtenstein has no surveillance system.
Figure 59.
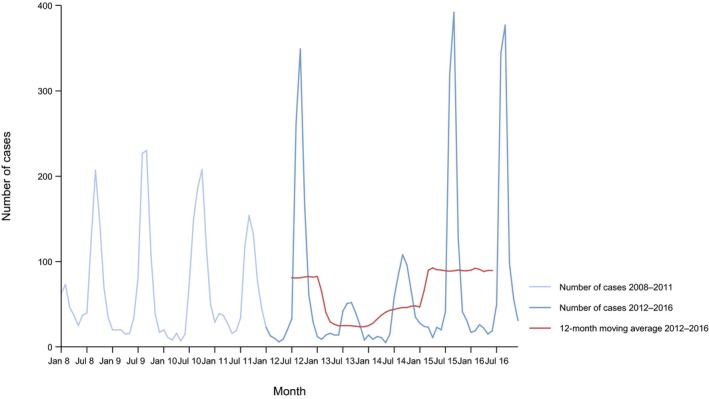
Trend in reported confirmed human cases of tularaemia in the EU/EEA, by month of reporting, 2012–2016
- Source(s): Cyprus, the Czech Republic, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Luxembourg, Latvia, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom. Austria, Belgium, Bulgaria, Croatia, Denmark, Italy, Lithuania, Malta, the Netherlands and Portugal did not report data to the level of detail required for the analysis.
In total, 1,108 cases of tularaemia in humans were reported in 18 MS. Among those cases, 1,056 (95.3%) were confirmed (Table 42). The highest case numbers were reported from Finland and Sweden, 699 and 134 confirmed cases, respectively (Table 42). Eight MS (Cyprus, Greece, Ireland, Italy, Luxembourg, Portugal, Romania and the United Kingdom) did not report any case. The overall notification rate was 0.23 and 0.21 per 100,000 population in 2015 and 2016, respectively, twice the rate reported in 2014 (0.10 per 100,000 population). In the past four years, the notification rate was highest in Sweden (ranging from 1.13 per 100,000 in 2013 to 7.41 per 100,000 in 2015). In 2016, Finland had the highest notification rate with 12.74 per 100,000 followed by Sweden with 1.36 per 100,000 population.
Less than 0.5% of tularaemia cases in the EU were reported to be travel related in 2016. Travel information was only available for 31.3% of the confirmed cases. Five travel‐associated cases were reported: two cases acquired in Norway and one case each associated to travel to Georgia, Russia and Serbia.
Between 2008 and 2016, three peaks in number of cases were observed in 2012, 2015 and 2016. These peaks were due to high numbers of reported cases in Finland and Sweden. Tularaemia shows a seasonal pattern, with most cases occurring between July and October, but some cases also occur during the winter.
Eleven MS provided data on hospitalisation status of their cases, representing 12.3% of the confirmed cases. Fifty‐five per cent of the confirmed cases were hospitalised. Twelve MS provided information on the outcome of their cases, representing 15.8% of the confirmed cases. No deaths due to tularaemia were reported in 2016.
14.3.2. Tularaemia in animals
Only one MS (Sweden) and one non‐MS (Switzerland) reported 2016 data to EFSA on the occurrence of F. tularensis in animals. In total, 41 hares including 36 brown hares, three mountain hares and two hares of unidentified species were submitted and examined. Six out of 41 tested animals (14.6%) were found to be positive. All positive animals were brown hares and had died of sepsis due to acute tularaemia. Also Switzerland reported six out of 10 tested hares positive.
14.4. Discussion
Tularaemia is a zoonotic disease caused by F. tularensis, a Gram‐negative non‐motile, non‐sporing, facultative intracellular coccobacillus. Four different subspecies of F. tularensis are known. F. tularensis subsp. holarctica (type B) is the only one reported to infect humans and animals in the northern hemisphere, including Europe.
Tularaemia is widely distributed throughout most of Europe and has repeatedly shown signs of local emergence and re‐emergence in humans and wildlife (Hestvik et al., 2015). In Europe, the ingestion of contaminated water from streams, ponds, lakes and rivers is the main mode of infection (ECDC, 2017c). However, tularaemia is typically transmitted by mosquito bites in the endemic regions of Sweden and Finland (Eliasson et al., 2002; Rossow et al., 2014).
The disease shows a clear seasonality in humans, which is consistent with greater exposure to contaminated water and mosquito activity during the summer and early autumn months.
For the second consecutive year, the number of human cases observed in the EU was higher than in previous years. Climatic conditions suitable for the propagation of the bacteria among the animal reservoirs are the main factor of this increase (Rossow et al., 2015).
Notification rates of tularaemia vary considerably among MS and over time. In previous years, Sweden had the highest notification rate, while in 2016, Finland had the highest notification rate, which was also the highest notification rate observed among MS in previous years. In Finland, tularaemia outbreaks are preceded 1 year earlier by a peak in vole populations (Rossow et al., 2015). Such an increase in vole population was observed in 2015, and with the 2016 climatic conditions that contributed to an abundant mosquito population, impacted the level of transmission to humans.
Tularaemia has terrestrial and aquatic ecological cycles with an extensive host range among animals, including vertebrates and invertebrates. Lagomorphs of the genus Lepus and small rodents are considered reservoirs, but antibodies against F. tularensis have been detected in other wild animals, such as red foxes and wild boar, and domestic animals such as cats and dogs (Hestvik et al., 2015; Maurin and Gyuranecz, 2016). As for humans, the animal species susceptible to tularaemia may be infected either through the terrestrial or the aquatic cycle. A study performed in the Netherlands during an outbreak in hares in 2015 to assess potential reservoirs and transmission routes of F. tularensis showed the importance of the environmental surveillance of water and its valuable use to monitor this pathogen (Janse et al., 2017). Only one MS, Sweden, reported 2016 data on brown hares and the number of positive hares decreased in Sweden compared to 2015, while it increased in Finland (SVA, 2016; EVIRA, 2017). From the national report of Finland, it could be retrieved that 45 blue hares and 107 brown hares were tested for tularaemia by the Finnish Food Safety Authority Evira in Finland, and 24 brown hares, 11 blue hares and one squirrel were found positive (EVIRA, 2017). According to these data, it can be inferred that this zoonotic pathogen is still present in wildlife in these two MS, and wildlife will continue to play a role in the maintenance of F. tularensis in the ecological cycle and the occurrence of human cases.
14.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://ecdc.europa.eu/en/data-tools/atlas/Pages/atlas.aspx |
| European tularaemia case definition | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012D0506&qid=1428573336660&from=EN#page=36 | |
| ECDC Factsheet on tularaemia in humans | https://ecdc.europa.eu/en/tularaemia/facts | |
| Guidelines on tularaemia by WHO | http://apps.who.int/iris/bitstream/10665/43793/1/9789241547376_eng.pdf | |
| Animals | Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
| List of animal diseases subject to notification in EU | http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01982L0894-20130101 | |
| OIE (World Organisation for Animal Health): Terrestrial Manual 2008, Chapter 2.1.18. Tularaemia | https://web.oie.int/fr/normes/mmanual/2008/pdf/2.01.18_TULAREMIA.pdf | |
| OIE (World Organisation for Animal Health): exceptional epidemiological events by region and year | http://www.oie.int/wahis_2/public/wahid.php/Countryinformation/Countryreports |
15. Other zoonoses and zoonotic agents
Monitoring data for the year 2016 on Anisakis, Bacillus, Chlamydia, Clostridium, calicivirus, Cysticercus, pathogenic and non‐pathogenic Enterococcus spp., Erysipelothrix rhusiopathiae, hepatitis A virus, Klebsiella, Leptospira and Staphylococcus spp. were reported to EFSA.
15.1. Anisakis
One MS (Spain) and Albania reported 2016 data on raw fish taken at retail and processing plant and tested for Anisakis spp. Only 3 out of the 23 samples tested by Albania were positive, whereas in Spain no positive samples were detected out of 53 tested.
15.2. Bacillus and B. cereus enterotoxins in foods
Three MS, Bulgaria, Lithuania and Spain submitted data on the prevalence of Bacillus in food in 2016. In Bulgaria, all the samples tested were obtained from bakery products (n = 2) at processing plant and tested negative. Lithuania reported 27 samples obtained from different food matrices: bakery products (n = 11), processed food products and prepared dishes (n = 11) and RTE salads (n = 5), recovered at processing plant, retail and catering. All were negative.
Only in Spain, Bacillus cereus was detected in 1 (food category ‘processed food product and prepared dishes’) out of the 66 samples tested and taken at catering.
In addition, Spain also reported data on B. cereus enterotoxins in foods. Suspected products (meat preparations and other processed food products and prepared dishes) from retail were tested and none of these were positive.
15.3. Chlamydia spp.
Greece was the only MS which submitted 2016 data on Chlamydia psittaci in sheep and goats and these were obtained from clinical investigations. In sheep (100 tested), 11% and in goats (71 tested) 25% were confirmed being infected with C. psittaci.
15.4. Clostridium spp. and Clostridium botulinum toxin
Three MS (Bulgaria, Slovenia and Spain) provided information on Clostridium, in various food products at retail and processing plant as well as feed collected at processing plant.
In Bulgaria, 12 suspected feed samples taken at processing plant were tested for the presence of Clostridium and none of these was positive.
Slovenia tested 178 samples taken at retail and farm. The presence of Clostridium difficile was confirmed in seven samples (poultry and broilers meat, mixed meat preparation and vegetables). The presence of toxin A/B has been confirmed in one out of two samples tested for presence of toxins.
In Spain, only one out of the 55 (1.8%) tested samples and collected from other processed food products and prepared dishes at processing plant level was positive for the presence of Clostridium perfringens. All the other samples from retail (n = 219; including fruits and vegetables, meat preparations, meat from poultry and cattle, sauce and dressings, bottled water and other processed foods) were negative. One suspect sample (canned vegetables at retail) was reported positive for C. botulinum toxin.
15.5. Calicivirus
Three MS (Romania, Slovenia and Spain) reported information on the occurrence of calicivirus in food (mainly fish and fishery products and fruit and vegetables) in 2016. All samples tested by Romania (fruits) from retail (n = 10) and processing plant (n = 6) were negative.
In Slovenia, 32 samples were reported from retail (n = 32) and four out of the eight samples from live bivalve molluscs were positive while all other samples tested were negative for calicivirus.
In Spain, 1 out of 12 suspected tested samples (bottled water) was positive and taken at conservation facilities as well as 1 out of 7 suspected samples from live bivalve molluscs. None of the remaining tested samples (n = 191, fruit and vegetables, fishery products and sprouted seeds) was positive.
15.6. Staphylococcus spp.
Four MS (Bulgaria, Hungary, Italy and Spain) and one non‐MS (Bosnia and Herzegovina) reported 2016 monitoring data on Staphylococcus spp. (unspecified, Staphylococcus aureus and Staphylococcus intermedius) in various animals and food products.
Bulgaria reported data on the prevalence of Staphylococcus spp. in various food categories such as, bakery products, milk and dairy products, beverages, salads ready to eat, cereals and meals, meat from broilers and pigs and fruits. Only 1 out of the 2,433 samples tested was positive for S. aureus. This positive sample was taken at retail from soft and semi‐soft cheese made from pasteurised cows’ milk.
Hungary provided data from various animals from farms and veterinary clinics. Out of the 311 samples tested in Hungary at farm level, 165 (53%) were positive for S. aureus. Among them there were samples from cattle (27 out of the 75 tested), pigs (16 out of the 22 tested), broilers (85 out of the 154 tested) and turkeys (17 out of the 36 tested). Few samples were taken from ducks (n = 1), geese (n = 2) and goats (n = 1) and all were positive. Italy tested a total of 4,964 samples from different food products taken at retail (n = 2,149), processing plant (n = 2,814) and slaughterhouse (n = 1). From the monitoring of milk from different animal species, 165 (17.2%) out of the 962 samples tested were positive. Of them 110 (23.8%) were positive for S. aureus and 55 (5.7%) were positive for Staphylococcus spp. (unspecified). In addition, Italy carried out a national survey on milk, for which 6,482 samples were tested of these, 451 (7%) were positive for S. aureus, 54 (0.8%) were positive for S. intermedius and 628 (9.7%) were positive for Staphylococcus spp. (unspecified). Another national survey on fruit and different animal species for Staphylococcus spp. was performed. In total, 225 animals were tested and of these 127 (56.4%) were found positive in different species, mainly sheep, followed by horses, goats, cats, cattle and dogs. Only 2 of 51 fruit samples, taken at retail, were positive for Staphylococcus spp.
Spain reported results of their 2016 monitoring on milk, cheeses, meat, bakery products, vegetable products (fruit purée) and other processed food products and prepared dishes. Out of a total of 940 samples tested, 201 (21%) were positive for Staphylococcus spp. (unspecified).
Bosnia and Herzegovina submitted data from bakery products. In total, eight out of 4,074 (0.2%) tested bakery products (cakes) were positive for S. aureus.
15.7. Cysticercus
Six MS (Belgium, Bulgaria, Croatia, Slovenia, Spain and Sweden) submitted 2016 data on Cysticercus mainly based on reports from slaughterhouse surveillance/inspections.
In Belgium, 1,282 (0.14%) out of the 913,745 cattle inspected at the slaughterhouse showed bovine cysticercosis, caused by Taenia saginata. This is the same prevalence as reported in previous years by Belgium (0.14% of bovine cysticercosis in 2015 and 2014).
Bulgaria provided information on the prevalence of Cysticercus in various animals: cattle, pigs, sheep, goats and buffaloes at slaughterhouse. Cysticercus was detected only in small ruminants: 3,429 (2%) out of the 173,466 tested sheep (95% below one year of age) and in 21 (1.4%) out of the 1,469 tested goats (over 1 year of age). Tested cattle (n = 31,539), pigs (n = 1,043,004), buffaloes (n = 542) and goats less than 1 year (n = 1,939) were all negative for Cysticercus.
Croatia, Slovenia and Sweden reported data on both bovine and porcine cysticercosis. All the bovine (n = 42) and porcine (n = 2) samples tested at slaughterhouse in Croatia were negative for the presence of Cysticercus. In Slovenia, none of the 258,307 porcine samples analysed was found positive, whereas 1 out of the 111,634 bovine samples inspected was detected as positive for T. saginata. These results are in concordance with the results from previous years 2015, 2014 and 2013. Sweden tested 2,526,500 and 411,020 porcine and bovine samples, respectively, and all of these were negative, as in previous years 2015 and 2014.
Spain provided data on the prevalence of Cysticercus in various animals (cattle, small ruminants, pigs, deer and wild boar) in 2016: 57 (0.03%) out of the 165,327 cattle, 1,248 (0.034%) – and mainly breeding animals– out of the 2,960,303 pigs, 36,955 (8.8%) out of the 421,062 sheep and 387 (0.41%) out of the 95,071 goats were positive for Cysticercus spp. Finally, 18,785 wild boar and 37,110 deer at hunting stage were tested and 0.016% and 0.1% were positive for Cysticercus spp., respectively. Although in previous years Spain has not reported data on Cysticercus in animals, the positive proportions reported in pigs and cattle at slaughterhouse are similar to the prevalence reported by Laranjo‐Gonzalez et al. (2017).
15.8. Erysipelothrix
For the first time, data on Erysipelothrix were reported to EFSA. Erysipelas is a bacterial disease caused by Erysipelothrix rhusiopathiae, which may infect swine as well as several other species of mammals and birds, including domestic fowl. In 2016, Spain submitted data on the occurrence of Erysipelothrix during a survey at slaughterhouse in the region of Murcia. In total, 3,605,307 pig carcasses were screened and 145 (0.004%) were found with signs of swine Erysipelas.
15.9. Hepatitis A virus
Romania (n = 16, fruit), Slovenia (n = 21, bivalve molluscs and fruit) and Spain (n = 65, fruit (berries), vegetables and molluscan shellfish) provided information on the presence of hepatitis A virus in food products in 2016. No samples were found positive for hepatitis A virus.
15.10. Klebsiella
In 2016, one suspected sample from an infant formula intended for infants below 6 months, was tested in Spain and found positive for Klebsiella pneumoniae.
15.11. Leptospira spp.
Only Bulgaria provided 2016 monitoring data on Leptospira in cattle and pigs. Bulgaria tested 10,433 bovine animals at farm, 2,748 bovine animals from zoos, 758 porcine animals at farm and 14 pigs from a zoo. None of these samples was positive.
15.12. Tick‐borne encephalitis virus (TBE)
One MS, Slovenia, tested raw milk samples (animal species unspecified) that were automatically distributed to customers at farm level via vending machines. None of the 61 tested samples was positive. This is in accordance with their results from milk samples in 2014.
15.13. Related projects and internet sources
16. Food‐borne outbreaks
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
16.1. Abstract
In total, 4,786 food‐borne and waterborne outbreaks have been reported for the year 2016 by 27 MS. Another 108 outbreaks were notified by seven non‐MS. In the EU, compared with 2015, the numbers of reported outbreaks, illnesses and deaths were increased whereas the number of hospitalisations decreased. The EU‐level reporting rate was 1.03 food‐borne outbreaks per 100,000 population, which was a slight increase compared with 2015 but in line or slightly lower than the EU‐level outbreak reporting rates during 2010–2016.
In 2016, most reported food‐borne and waterborne outbreaks for which the causative agent was known were associated with bacterial agents (33.9% of all outbreaks). Bacterial toxins ranked second, among the causative agent group (17.7%), followed by viruses (9.8% of all outbreaks), other causative agents (2.2%) and parasites (0.4%). Salmonella was identified as the most frequently reported causative agent of food‐borne and waterborne outbreaks at the EU‐level (22.3% of all outbreaks). Among bacterial agents Salmonella alone accounted for two‐thirds of the outbreaks (65.8%) and, together with Campylobacter, for the vast majority of outbreaks by bacterial agents (94.1%).
Important differences were observed in the number of outbreaks reported by MS, with more than 70% of food and waterborne disease outbreaks reported by five MS only (France, Germany, the Netherlands, Poland and Slovakia). This reflects a different sensitivity of the passive surveillance systems in place in the MS and suggests that the EU‐level epidemiological trends should be interpreted with caution because they may actually reflect the trends of those MS that contribute more to food‐borne outbreak reporting.
At the MS level, the most commonly reported causative agent of food and waterborne outbreaks was Salmonella in 15 MS, calicivirus (including norovirus) in 5 MS, bacterial toxins other than C. botulinum in 3 MS, Campylobacter in 2 MS, STEC and histamine in 1 MS, each.
Although the number of reported food‐borne outbreaks of salmonellosis significantly decreased during 2010–2016 in most MS, the EU‐level number of reported salmonellosis food‐borne outbreaks for the year 2016 increased compared with 2015. This increase was primarily attributable to a 23.6% increase in reported outbreaks in the EU of S. Enteritidis and one in six (about 15%) of all reported food‐borne and waterborne outbreaks was due to S. Enteritidis. Thirteen MS reported a more outbreaks of S. Enteritidis compared to 2015. This agent was also responsible during 2016 for large multicountry outbreaks that involved many MS.
In 2016, food‐borne outbreaks due to Salmonella had the highest burden in terms of the number of outbreaks, of numbers of hospitalisations (1,766, 45.6% of the total number of hospitalised cases) and of deaths (10 deaths, 50% of the total number of deaths among outbreak cases). Calicivirus (including norovirus) caused on average the highest number of illnesses per outbreak (31.6 cases per outbreak).
At the MS level, during 2010–2016, the number of reported food‐borne outbreaks was quite stable in most MS. In Belgium, France, the Netherlands and Portugal, however, a statistically significant increasing trend was observed that was primarily due to reported outbreaks of calicivirus (including norovirus), bacterial toxins other than C. botulinum, and ‘unknown’ causative agents. Whether this increase was caused by a true rise in the exposure to contaminated food or a higher reporting rate of outbreaks is not known. Conversely, the number of outbreaks reported by Austria, Denmark, Estonia, Hungary during 2010–2016 significantly decreasing.
A major limitation of the food‐borne outbreak surveillance in the EU is that for many outbreaks the causative agent was unknown and in many cases information on the suspected food vehicle was not available. In 2016, the outbreaks with no information on the causative agent accounted for 36% of the total outbreaks. During 2010–2016 these types of reports increased in many MS.
In 2016, strong‐evidence food‐borne outbreaks excluding waterborne outbreaks (n = 521) represented 10.9% of the total food‐borne outbreaks recorded and were mostly (n = 313) associated with foods of animal origin. Of these, 41.5% involved ‘eggs’ and ‘poultry meat’ (23.0% and 18.5%, respectively), 22.4% involved ‘fish and fisheries’ including ‘crustaceans, shellfish, molluscs and its products’, 21.7% involved meat and meat products other than poultry, and 14.4% ‘milk and milk products’. ‘Mixed food’ and ‘buffet meals’, as well as ‘other foods’ including ‘unspecified foods’ were reported in almost one‐third of all strong‐evidence outbreaks. Compared with previous years, no important trends for any of the food items implicated in the strong‐evidence food‐borne outbreaks were observed.
Agent/food combinations more frequently reported in strong‐evidence outbreaks were Salmonella in ‘eggs and egg products’ and ‘bakery products’, calicivirus including norovirus in ‘crustaceans, shellfish, molluscs and their products’ and bacterial toxins other than C. botulinum in ‘mixed food’ and ‘poultry meat’. Some of these pairs were also ranked among the combinations responsible for the highest burden in terms of cases of illness, hospitalisations and deaths.
Figure 60.
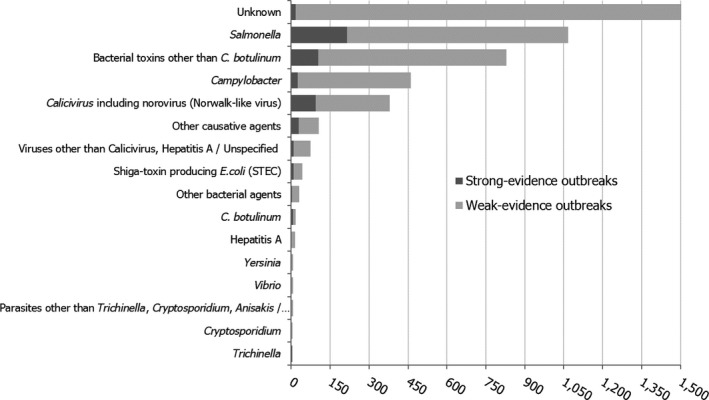
Distribution of strong‐evidence and weak‐evidence food‐borne and waterborne outbreaks, per causative agent, EU, 2016
- Other bacterial agents include Francisella, Enterococcus, enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), Shigella and other unspecified bacteria. Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Viruses other than calicivirus and hepatitis A virus include flavivirus, rotavirus and other unspecified viruses. Other causative agents include chemical agents, histamine, lectin, marine biotoxins, mushroom toxins and scombrotoxin. Parasites other than Trichinella and Cryptosporidium include Giardia and other unspecified parasites.
16.2. Surveillance and monitoring of food‐borne and waterborne outbreaks in the EU
The annual reporting of investigated FBO has been mandatory for MS since 2003, according to Directive 2003/99/EC, with the aim of providing data on the epidemiological profile of the outbreaks, the foodstuffs potentially implicated and the potential causes of the outbreaks. Starting in 2007, harmonised specifications on the reporting of FBO at the EU‐level have been increasingly applied in the EU. The current system for reporting FBO is known as the European Union Food‐borne Reporting System (EU‐FORS) and was implemented for the first time in the reporting of data from 2010 and subsequent years. Since then, the reported outbreaks have been categorised as having ‘strong evidence’ or ‘weak evidence’ based on the strength of evidence implicating a suspected food vehicle as the cause of the outbreak (EFSA, 2011, 2014a,b). The evaluation of the strength of evidence implicating a suspected food vehicle in FBO as being strong or weak, is based on the assessment of all available types of evidence (i.e. microbiological, epidemiological, descriptive, environmental, based on tracing‐back of the investigated foodstuffs) and according to the EU‐FORS guidance and the last published manual for reporting on FBO (EFSA, 2014b, 2017b).
The reporting system for FBO includes any bacterium, virus, parasite, alga, fungus and their products, such as toxins and biological amines (e.g. histamine), not just zoonotic agents. Outbreaks caused by ingestion of drinking water are also deemed food‐borne since drinking water is defined as a food, in Regulation 178/2002/EC. Information reported to EFSA by MS on FBO include data on the causative agents, the numbers of human cases (illnesses), of hospitalisations and of deaths, the type of FBO (i.e. general/household), the implicated type of food and the place of consumption (exposure). Moreover, information on the place of origin of the problem leading to contamination of food and on factors that may have contributed (e.g. cross‐contamination, inadequate heat treatment, etc.) are also collected.
Although food‐borne outbreak reporting rules follow the EFSA harmonised specifications on the reporting of FBO (EFSA, 2014b), the MS collect outbreak data according to their own specific surveillance methods, criteria, sampling schemes and reporting systems and food‐borne outbreak investigation systems, relying basically on passive surveillance/monitoring, are not harmonised amongst MS. Therefore, differences in the numbers and types of reported outbreaks, as well as in the causative agents, may not necessarily reflect the level of food safety among MS; rather they may indicate differences in the sensitivity of the national surveillance systems in identifying and investigating FBO. In addition, some MS have implemented changes in national systems over time, which may have had an impact on the number of outbreaks reported by the same MS in different years. For this reason, EU‐level trends and statistics should be interpreted with caution because they may reflect the trends and statistics of MS that reported more outbreak data. FBO monitoring data at EU‐level allow for trend watching, and for descriptive summaries, but are not suitable for trends analyses.
16.3. Data analyses
All reported FBO including waterborne outbreaks are summarised in tables and figures. Data on reported FBO in the MS and non‐MS are separately and descriptively analysed for ‘strong‐evidence’ and for ‘weak‐evidence’ outbreaks. For the former, it is compulsory for reporting countries to report a detailed data set, while for the latter types of outbreaks this is not mandatory but voluntary. The types of evidence reported for the strong‐evidence outbreaks are presented in Table FBOEVID2016 of Appendix.
In Section 16.4.1, outbreaks are generally summarised according the associated health burden in terms of the total number of cases, hospitalisations and deaths, and also according the causative agent. In Section 16.4.2, the distributions of the incriminated food vehicles and the places of exposure are described based on the reported strong‐evidence outbreaks only. However, as MS are allowed since 2014 to report detailed information on the suspected vehicles also in weak‐evidence outbreaks, trends observed over the last three years as for the implicated vehicle and for the most important agent/food combinations have been also described considering all the outbreaks with available information.
Details on FBO by the causative agent, excluding waterborne outbreaks, are provided in the tables in the Appendix. Waterborne outbreaks are addressed separately.
Causative agents, food vehicles and outbreak settings have been grouped to facilitate the understanding of the epidemiological picture at the EU‐level. Causative agents were categorised according to the prioritisation criteria of Directive 99/2003/EC. Outbreaks by pathogens listed under annex IA of that directive (Brucella, Salmonella, Campylobacter, Listeria, Shiga toxin‐producing E. coli and Trichinella) have been described separately. All the causative agents not explicitly mentioned in the annex of the Directive have been reported as ‘other agents’ except Vibrio, C. botulinum toxin, calicivirus including norovirus, hepatitis A virus and Cryptosporidium, which have also been also described separately, given their importance as causative agents in food‐borne and waterborne outbreaks. Food vehicles have been uniformly grouped following the general criteria adopted by EFSA for presenting data in this report. Places of exposure have been grouped so as to basically represent the different characteristics and level of risk connected to the setting and the process behind food preparation.
In tables and figures, statistics used are sums and proportions (%) of the reported counts (numbers) of reported disease outbreaks. The ‘Reporting rate’ of reported outbreaks per 100,000 population was also calculated to compare reports between MS independently from demographic variations, across years and taking account of the number of reporting MS. For estimations of the ‘Reporting rate’ at supranational or EU‐level, the overall population has been calculated by summing the populations of those MS that provided data on the specific reported FBO. Data on resident population at 1 January 2016 and from Eurostat have been used for this purpose.
At the MS level, statistical trend analyses were carried out to evaluate the significance of temporal variations in the number of reported FBO. Poisson regression and an autoregressive integrated moving average (ARIMA) model was implemented using p ≤ 0.05 as the value to identify a statistical significant trends, beyond chance. The outcome evaluated was the number of outbreaks, assuming that no substantial demographic changes had occurred, at MS level. For countries yielding a significant trend (p < 0.05) using the Poisson model, the ARIMA model was used. Only those countries with a statistically significant increasing or decreasing trend (p < 0.05) in both the analyses were considered in the figures and the narrative text.
16.4. Results
16.4.1. General overview
Health burden
For the year 2016, 28 MS and seven non‐MS provided data on FBO (Table 2016_FBOOVERVIEW in the Appendix). The FBO reported by Spain after the end of the reporting period were not included in this report. A total of 4,786 food‐ and waterborne outbreaks including both strong‐evidence (n = 525) and weak‐evidence outbreaks (n = 4,261) were reported by 27 MS (Table 43 and Figure 61). This is 3% less compared with the mean annual number (n = 4,955) of outbreaks reported for the years 2010–2015. Also, the EU‐level trend in reported number of outbreaks during 2010–2016 was fairly stable, even though there were 424 more food‐ and waterborne outbreaks reported for 2016 compared with 2015 (Figure 61). Also, the EU‐level trends for reported strong‐evidence and weak‐evidence outbreaks were stable during 2010–2016. Another 108 outbreaks were reported by seven non‐MS including Norway, Switzerland and the pre‐accession countries Albania, Bosnia and Herzegovina, the Former Yugoslav Republic of Macedonia, Montenegro and Serbia which for the first time reported data to EFSA.
Table 43.
Number of food‐borne and waterborne outbreaks and human cases, hospitalisations and deaths in reporting Member States and reporting non‐Member States, 2016
| Country | Strong‐evidence outbreaks | Weak‐evidence outbreaks | Total number of outbreaks | Reporting rate per 100,000 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Cases | Hospitalised | Deaths | Number | Cases | Hospitalised | Deaths | 2016 | 2010–2015 (mean) | ||
| Austria | 9 | 144 | 40 | 0 | 71 | 292 | 28 | 0 | 80 | 0.92 | 1.69 |
| Belgium | 14 | 621 | 50 | 0 | 364 | 1,368 | 23 | 0 | 378 | 3.34 | 2.62 |
| Bulgaria | 0 | 0 | 0 | 0 | 17 | 318 | 100 | 0 | 17 | 0.24 | 0.12 |
| Croatia | 5 | 283 | 31 | 1 | 43 | 315 | 39 | 1 | 48 | 1.15 | 1.26 |
| Cyprus | 0 | 0 | 0 | 0 | 4 | 32 | 9 | 0 | 4 | 0.47 | 0.35 |
| Czech Republic | 7 | 279 | 13 | 0 | 23 | 614 | 40 | 0 | 30 | 0.28 | 0.22 |
| Denmark | 23 | 1,324 | 4 | 0 | 26 | 501 | 3 | 0 | 49 | 0.86 | 1.15 |
| Estonia | 0 | 0 | 0 | 0 | 6 | 98 | 12 | 0 | 6 | 0.46 | 1.08 |
| Finland | 19 | 792 | 7 | 0 | 40 | 750 | 4 | 0 | 59 | 1.08 | 0.83 |
| France | 133 | 1,577 | 74 | 1 | 1,319 | 12,410 | 557 | 3 | 1,452 | 2.17 | 1.91 |
| Germany | 41 | 784 | 71 | 1 | 356 | 1,724 | 185 | 3 | 397 | 0.48 | 0.51 |
| Greece | 6 | 261 | 86 | 1 | 1 | 10 | 5 | 0 | 7 | 0.06 | 0.12 |
| Hungary | 37 | 1,636 | 97 | 3 | 39 | 1,047 | 59 | 0 | 76 | 0.77 | 1.33 |
| Ireland | 2 | 24 | 8 | 0 | 25 | 186 | 8 | 0 | 27 | 0.57 | 0.52 |
| Italy | 0 | 0 | 0 | 0 | 91 | 734 | 148 | 1 | 91 | 0.15 | 0.40 |
| Latvia | 0 | 0 | 0 | 0 | 49 | 425 | 97 | 0 | 49 | 2.49 | 18.10 |
| Lithuania | 27 | 216 | 105 | 0 | 0 | 0 | 0 | 0 | 27 | 0.93 | 4.47 |
| Luxembourg | 2 | 23 | 0 | 0 | 1 | 6 | 0 | 0 | 3 | 0.52 | 0.45 |
| Malta | 1 | 34 | 8 | 0 | 38 | 159 | 0 | 0 | 39 | 8.98 | 8.30 |
| Netherlands | 11 | 291 | 21 | 0 | 583 | 2,438 | 6 | 0 | 594 | 3.50 | 1.63 |
| Poland | 126 | 1,433 | 316 | 0 | 346 | 4,900 | 729 | 2 | 472 | 1.24 | 1.18 |
| Portugal | 9 | 213 | 64 | 0 | 15 | 416 | 16 | 0 | 24 | 0.23 | 0.13 |
| Romania | 13 | 244 | 195 | 0 | 6 | 68 | 25 | 0 | 19 | 0.10 | 0.09 |
| Slovakia | 10 | 196 | 70 | 0 | 442 | 2,153 | 386 | 0 | 452 | 8.33 | 9.56 |
| Slovenia | 0 | 0 | 0 | 0 | 8 | 448 | 2 | 0 | 8 | 0.39 | 0.32 |
| Sweden | 12 | 3,641 | 0 | 0 | 317 | 1,895 | 0 | 0 | 329 | 3.34 | 3.01 |
| United Kingdom | 18 | 797 | 94 | 3 | 31 | 1,830 | 34 | 0 | 49 | 0.07 | 0.11 |
| EU total | 525 | 14,813 | 1,354 | 10 | 4,261 | 35,137 | 2,515 | 10 | 4,786 | 1.03 | 1.08 |
| Albania | 0 | 0 | 0 | 0 | 1 | 84 | 7 | 0 | 1 | 0.03 | – |
| Bosnia and Herzegovina | 5 | 343 | 0 | 0 | 1 | 12 | 1 | 0 | 6 | 0.17 | – |
| Former Yugoslav Republic of Macedonia, the | 4 | 55 | 15 | 0 | 1 | 86 | 0 | 0 | 5 | 0.24 | – |
| Montenegro | 5 | 167 | 25 | 0 | 7 | 23 | 11 | 0 | 12 | 1.93 | – |
| Norway | 25 | 411 | 4 | 0 | 4 | 87 | 2 | 0 | 29 | 0.56 | 1.16 |
| Serbia | 41 | 350 | 107 | 0 | 3 | 132 | 27 | 0 | 44 | 0.62 | – |
| Switzerland | 5 | 195 | 2 | 0 | 6 | 111 | 9 | 0 | 11 | 0.13 | 0.11 |
Outbreak reporting rate for 2016 and mean value for the five previous years (2010–2015) is also provided.
Figure 61.
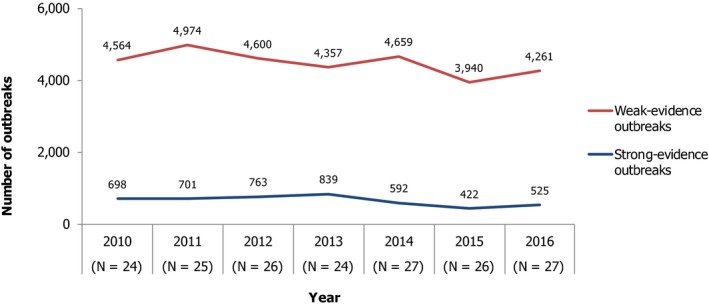
Number of food‐borne and waterborne outbreaks reported in the reporting Member States, 2010–2016
The reported numbers of outbreaks by countries differed importantly and more than 70% of the outbreaks were reported by five MS only. France reported by far the largest number of outbreaks (n = 1,452) accounting for 30.3% of all reported outbreaks, followed by the Netherlands (n = 594; 12.4% of total outbreaks), Poland (n = 472; 9.9%), Slovakia (n = 452; 9.4%), Germany (n = 397; 8.3%), Belgium (n = 378; 7.9%) and Sweden (n = 329; 6.9%). Conversely, the proportion of outbreaks reported by 17 MS (Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Estonia, Finland, Greece, Ireland, Latvia, Lithuania, Luxembourg, Malta, Portugal, Romania, Slovenia and the United Kingdom) did not exceed the 10.0% of all outbreaks reported in the EU in 2016. Compared with 2015, four MS (Luxembourg, Bulgaria, Italy and Slovenia) reported an increase in FBO of over 50%, while a marked decrease was only reported by Latvia (50.0% less than in 2015).
At the MS level, the number of outbreaks reported over the period 2010–2016 had a statistically significant increasing trend in Belgium, France, the Netherlands and Portugal while conversely for Austria, Denmark, Estonia and Hungary the trend was decreasing. No specific trends were observed for the other MS.
Overall in 2016, food‐borne outbreaks (including waterborne outbreaks) caused 49,950 illnesses (4,076 more than in 2015), 3,869 hospitalisations (23 less than in 2015) and 20 deaths (3 more than in 2015). More details on hospitalisation and deaths by causative agents are provided in Table 43.
Overall, in the EU, the reporting rate of food‐ and waterborne outbreaks per 100,000 was 1.03 (Table 43) which represents a slight increase compared with 2015 and a 4.5% reduction compared with the mean reporting rate observed during 2010–2015.
In 2016, outbreak reporting rates per 100,000 population varied importantly among MS, ranging from 0.06 (Greece) to 8.98 (Malta) (median: 0.77 reported outbreaks per 100,000 population). Similarly, the reporting rate of human illnesses due to in food‐borne outbreaks varied importantly among countries and ranged from 1.21 (Italy) to 56.2 (Sweden) cases per 100.000 (median: 8.5 reported outbreaks per 100,000 population). The reporting rates of human cases, hospitalisation and deaths involved in food‐borne and waterborne outbreak did not substantially change during 2010 to 2016. In total, 2,056 cases and 210 hospitalisations were also reported in non‐MS.
In the MS, food‐borne outbreaks involving cases from a single household numbered 472 (9.9% of total outbreaks) in 2016, while those with cases from more than one household, general outbreaks were 715 (14.9% of total outbreaks). However, the level of uncertainty of this estimation is high given that this information was not available for 3,599 outbreaks (75.2% of total outbreaks) and not all MS collect information on outbreaks that affect only a single household.
Causative agents in strong‐evidence and weak‐evidence food‐borne outbreaks
Sixty‐four percent of all 2016 outbreaks were reported with a known causative agent, whereas for 36.0% of these the agent was unknown. In strong‐evidence outbreaks, the proportion with unknown agent was 3.4% compared with 40.0% for weak‐evidence outbreaks. Details on food‐borne and waterborne outbreaks reported, by the causative agent, are shown in Table 44. Figure 62 summarises all reported 2016 food‐borne and waterborne outbreaks, by MS and by causative agent, and their relative weight proportional to the total number of outbreaks. This figure illustrates that the EU‐level trends and statistics should be interpreted with caution because they may reflect the trends and statistics of MS that reported more outbreak data due to a higher sensitivity of the national surveillance system in identifying and investigating food‐borne outbreaks.
Table 44.
Number of food‐borne outbreaks (including waterborne outbreaks), human cases, hospitalisations and deaths per causative agents in the reporting Member States, 2016
| Type of agent | Outbreaks | Cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strong‐evidence outbreaks | Weak‐evidence outbreaks | Total outbreaks | Reporting rate per 100,000 | Human cases | Mean number per outbreak | Hospitalised | Deaths | ||||
| Number | Number | Number | Number | Number | % of cases | Number | % of cases | ||||
| Bacteria | Campylobacter | 24 | 437 | 461 | 0.10 | 4,606 | 10.0 | 140 | 3.0 | 0 | 0 |
| Listeria | 2 | 3 | 5 | 0.00 | 25 | 5.0 | 14 | 56.0 | 2 | 8.0 | |
| Salmonella | 215 | 852 | 1,067 | 0.23 | 9,061 | 8.5 | 1,766 | 19.5 | 10 | 0.1 | |
| Shiga toxin‐producing E. coli (STEC) | 9 | 33 | 42 | 0.01 | 735 | 17.5 | 125 | 17.0 | 3 | 0.4 | |
| Vibrio | 1 | 7 | 8 | 0.00 | 76 | 9.5 | 50 | 65.8 | 0 | 0 | |
| Yersinia | 1 | 7 | 8 | 0.00 | 41 | 5.1 | 3 | 7.3 | 0 | 0 | |
| Other bacterial agents | 4 | 26 | 30 | 0.01 | 279 | 9.3 | 51 | 18.3 | 1 | 0.4 | |
| Subtotal | 256 | 1,365 | 1,621 | 0.35 | 14,823 | 9.1 | 2,149 | 14.5 | 16 | 0.4 | |
| Bacterial toxins | C. botulinum | 8 | 10 | 18 | 0.00 | 49 | 2.7 | 39 | 79.6 | 0 | 0 |
| Other bacterial toxins | 105 | 725 | 830 | 0.18 | 8,918 | 10.7 | 362 | 4.1 | 1 | < 0.1 | |
| Subtotal | 113 | 735 | 848 | 0.18 | 8,967 | 10.6 | 401 | 4.5 | 1 | < 0.1 | |
| Viruses | Calicivirus including norovirus (Norwalk‐like virus) | 94 | 285 | 379 | 0.08 | 11,993 | 31.6 | 404 | 3.4 | 1 | < 0.1 |
| Hepatitis A | 1 | 15 | 16 | 0.00 | 155 | 9.7 | 63 | 40.6 | 0 | 0 | |
| Other viruses/unspecified | 10 | 65 | 75 | 0.02 | 937 | 12.5 | 97 | 10.4 | 0 | 0 | |
| Subtotal | 105 | 365 | 470 | 0.10 | 13,085 | 27.8 | 564 | 4.3 | 1 | < 0.1 | |
| Parasites | Cryptosporidium | 2 | 4 | 6 | 0.00 | 62 | 10.3 | 0 | 0.0 | 0 | 0 |
| Trichinella | 3 | 2 | 5 | 0.00 | 14 | 2.8 | 9 | 64.3 | 0 | 0 | |
| Other parasites/unspecified | 0 | 7 | 7 | 0.00 | 17 | 2.4 | 0 | 0.0 | 0 | 0 | |
| Subtotal | 5 | 13 | 18 | 0.00 | 93 | 5.2 | 9 | 9.7 | 0 | 0 | |
| Other causative agents | 28 | 78 | 106 | 0.02 | 489 | 4.6 | 74 | 15.1 | 0 | 0 | |
| Other causative agents | Subtotal | 28 | 78 | 106 | 0.02 | 489 | 4.6 | 74 | 15.1 | 0 | 0 |
| Unknown | Unknown | 18 | 1,705 | 1,723 | 0.37 | 12,493 | 7.3 | 672 | 5.4 | 2 | < 0.1 |
| Subtotal | 18 | 1,705 | 1,723 | 0.37 | 12,493 | 7.3 | 672 | 5.4 | 2 | < 0.1 | |
| Total (EU) | 525 | 4,261 | 4,786 | 1.03 | 49,950 | 10.4 | 3,869 | 7.7 | 20 | < 0.1 | |
Other bacterial agents include Francisella, Enterococcus, enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), Streptococcus, Shigella and other unspecified bacteria. Other bacterial toxins include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Other viruses include adenovirus, flavivirus, rotavirus and other unspecified viruses. Other causative agents include chemical agents, histamine, lectin, marine biotoxins, mushroom toxins and scombrotoxin. Other parasites include Giardia and other unspecified parasites.
Figure 62.
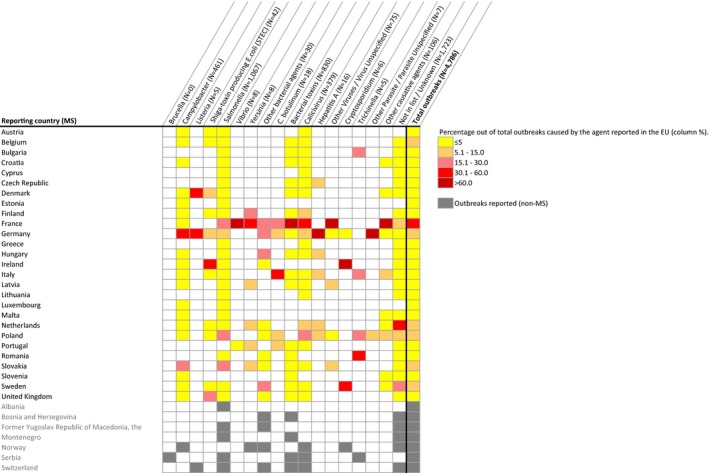
Reporting of food‐borne and waterborne outbreaks, by causative agent and by reporting Member States and reporting non‐Member States, 2016
- Other bacterial agents include Francisella, Enterococcus, Enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), Streptococcus, Shigella and other unspecified bacteria. Other bacterial toxins include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Other viruses include adenovirus, flavivirus, rotavirus and other unspecified viruses. Other causative agents include chemical agents, histamine, lectin, marine biotoxins, mushroom toxins and scombrotoxin. Other parasites include Giardia and other unspecified parasites.Percentage out of total outbreaks caused by the agent reported in the EU (column %): Grey boxes indicate reporting by non‐MS.
Figure 63 shows the EU‐level trends in numbers of reported food‐ and waterborne outbreaks, by the causative agent, during 2010–2016. Figure 64 displays trends for several MS and shows that during 2010–2016 the number of outbreaks reported with an unknown causative agent increased in many MS and with statistical significance for data reported by Belgium, the Netherlands, Poland and Sweden. For Belgium, the Netherlands and Sweden, such outbreaks accounted for more than half of their reported outbreaks for 2016.
Figure 63.
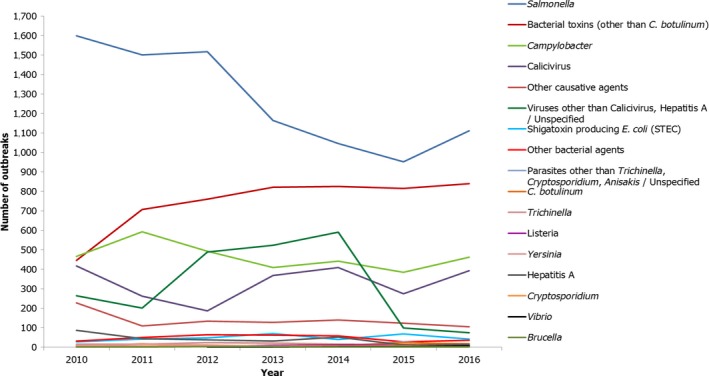
Number of food‐borne and waterborne outbreaks reported by causative agent in reporting Member States, 2010–2016
- Other bacterial agents include Francisella, Enterococcus, enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), Streptococcus, Shigella and other unspecified bacteria. Other bacterial toxins include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Other viruses include adenovirus, flavivirus, rotavirus and other unspecified viruses. Other causative agents include chemical agents, histamine, lectin, marine biotoxins, mushroom toxins and scombrotoxin. Other parasites include Giardia and other unspecified parasites.
Figure 64.
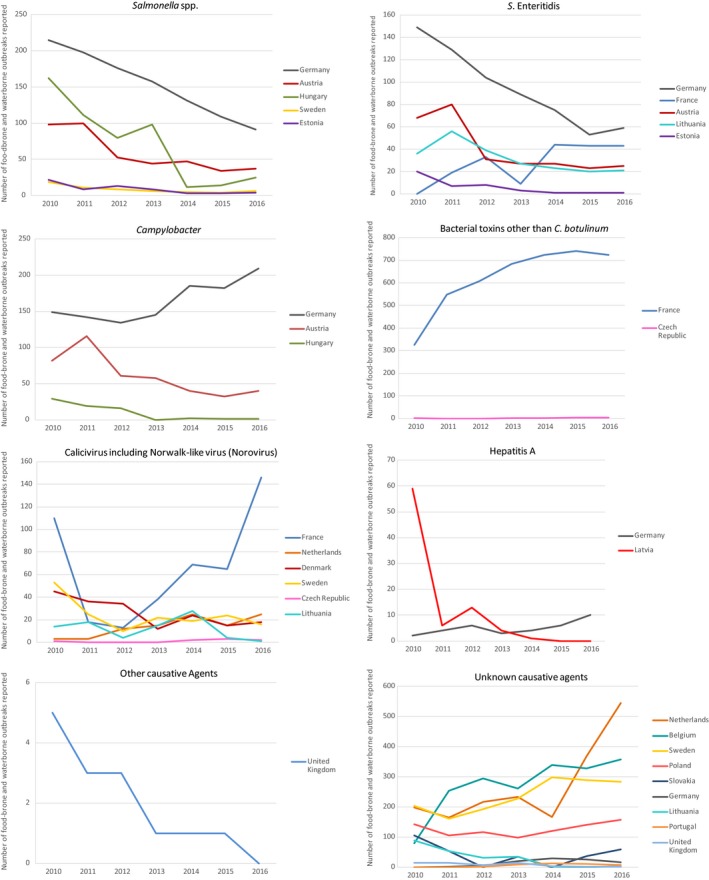
Number of food‐borne and waterborne outbreaks reported from 2010 to 2016, by Member State
- Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Other causative agents include chemical agents, histamine, lectin, marine biotoxins, mushroom toxins and scombrotoxin.
- Only MS with statistically significant trends (either increasing or decreasing) over years are shown.
In 2016, most of the outbreaks for which the causative agent was known were associated with bacterial agents (33.9% of all outbreaks), in particular Salmonella (22.3% of all outbreaks) and Campylobacter (9.6% of all outbreaks). Outbreaks caused by these agents had a moderate increase compared with 2015 (Salmonella 110 more outbreaks, corresponding to an increase of 11.5%; Campylobacter more 74 outbreaks, corresponding to an increase of 19.1%), even if during 2010–2016 the number of outbreaks reported by MS decreased for both the agents. It is notable that for some MS (France, Hungary, Latvia, Poland, Slovakia) the number of food‐borne outbreaks of Salmonella increased in the last three years, although not statistically significant, after years of progressive reduction. S. Enteritidis was reported in 66.6% of the salmonellosis outbreaks, meaning S. Enteritidis was the causative agent in about one in six (14.9%) of all reported outbreaks. Compared with 2015, a 23.6% increase in the number of outbreaks due to S. Enteritidis was reported at EU‐level, with 13 MS (Austria, Belgium, Denmark, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, the Netherlands, Poland and Slovakia) reporting a number of outbreaks higher than in 2015. Slovakia (n = 250) and Poland (n = 203) reported the highest number of outbreaks due to S. Enteritidis and reported an increase of 23.2% and 42.0% compared with 2015, respectively. A marked increase was also reported by Hungary with the 2016 number of outbreaks by S. Enteritidis (n = 21) being almost twice the number reported for 2015. Still, considering the period 2010–2016, the overall trend of number of outbreaks of S. Enteritidis outbreaks decreased, at the EU‐level. At the MS level, during 2010–2016, a statistically significant favourable trend towards a reduction of number of S. Enteritidis outbreaks was observed for four MS (Austria, Estonia, Germany and Lithuania) (Figure 64). In contrast, France was the only country with a statistically significant increasing trend over years 2010–2016 of S. Enteritidis outbreaks. S. Typhimurium, including monophasic variants, accounted for 6.2% of the outbreaks by Salmonella. Details of the serovars reported in food‐borne and waterborne salmonellosis outbreaks are described in Table 2016_FBOSALMSEROVAR in the Appendix. This information was not available for 21.2% of salmonellosis outbreaks. As for Campylobacter, Germany was the only MS with a significant increasing trend in the number of reported outbreaks during 2010–2016 while in Austria and Hungary, outbreaks of campylobacteriosis significantly decreased during that period (Figure 64).
Bacterial toxins ranked second in 2016, among the causative agent group in food‐borne and waterborne outbreaks (17.7% of all outbreaks). As in previous years, the vast majority (86.0%) of the outbreaks associated with these agents (i.e. toxins by Clostridium, Staphylococcus and Bacillus cereus) was reported by a single MS (France). So, the marked increase in the number of food‐borne outbreaks due to bacterial toxins other than C. botulinum reported for the period 2010–2016 in the EU is due to the reporting by France (Figure 64). As for botulism, most of the outbreaks reported in the EU in 2016 were reported by Italy (n = 8).
Viruses accounted overall for the 9.8% of total outbreaks in 2016, which is comparable with 2015. At the EU‐level, no trends in the outbreaks reported were observed for calicivirus (including norovirus) and for viruses other than hepatitis A and calicivirus, while for hepatitis A, a small reduction was observed throughout the 2010–2016 period (Figure 63). At the MS level, during 2010–2016, Denmark, Lithuania and Sweden reported a statistically significant decreasing trend in the number of outbreaks of calicivirus including norovirus, while France and the Netherlands reported a statistically significant increase (Figure 64). Three other MS (Finland, Germany and United Kingdom) reported for 2016 an increase in the number of outbreaks by calicivirus including norovirus of over the 50%, compared with 2015. Trends in hepatitis A outbreaks showed a statistically significant decrease, over the whole 2010–2016 period, for Latvia while a moderately significant increase was observed for Germany in the same period although the overall number of outbreaks reported in the period was small (35 outbreaks).
Food‐borne and waterborne outbreaks during 2016 due to parasites doubled compared to 2015 but only totalled 0.4% of all outbreaks. No trends over recent years could be assessed at the EU and the MS level.
Other causative agents, including histamine, marine biotoxins, chemical agents and lectin were reported in 2.2% of the outbreaks. No significant trends were observed in reported outbreaks due to other causative agents in MS, except for a decreasing trend over the last 7 years in the United Kingdom Figure 64).
At the MS level, the most commonly reported causative agent of food‐borne outbreaks was Salmonella in 15 MS, calicivirus including norovirus in 5 MS, bacterial toxins other than C. botulinum in 3 MS, Campylobacter in 2 MS, STEC and histamine in 1 MS, each.
In terms of the number of hospitalisations, food‐borne and waterborne outbreaks caused by Salmonella and viruses presented the biggest health impact in the MS (Table 44). Calicivirus including norovirus caused the highest number of illnesses, 11,993 cases which was 24.0% of all illnesses caused by all outbreaks and was associated with the highest mean number of cases per outbreak (31.6). In 2016, the largest food‐borne outbreak was reported by Sweden and involved more than 3,000 domestic cases, which became infected with Campylobacter after consumption of contaminated poultry meat. C. botulinum, Vibrio, Listeria and Trichinella were associated with the highest proportions of hospitalisations, with more than 50% of outbreak cases hospitalised. Outbreaks due to Listeria were associated with the highest proportion (8.0%) of deaths among illnesses, while outbreaks of salmonellosis overall caused the highest number of deaths; 10 (Table 44).
16.4.2. Detailed descriptions according to strength of evidence
Food vehicle in strong‐evidence food‐borne outbreaks (excluding waterborne outbreaks)
Twenty‐one MS reported 521 strong‐evidence outbreaks, which were 10.9% of the food‐borne outbreaks reported for the year 2016. France and Poland reported the highest number of strong‐evidence outbreaks and together their outbreaks totalled about half (49.3%) of the all strong‐evidence outbreaks (Table 43). Consequently, at the EU‐level the description of food vehicles implicated in 2016 in strong‐evidence outbreaks and the place of exposure (epidemic setting) should be interpreted with caution because they may mostly reflect the trends and statistics of France and Poland because these MS contributed more data. Food vehicles implicated in strong‐evidence food‐borne outbreaks are described in Table 45.
Table 45.
Frequency distribution of strong‐evidence food‐borne outbreaks, by type of vehicle (excluding waterborne outbreaks), reporting Member States, 2016
| Type of vehicle | Strong‐evidence outbreaks | Reporting rate per 100,000 | |||||
|---|---|---|---|---|---|---|---|
| Number of outbreaks | % of total outbreaks | Number of cases | % of total cases | 2016 | 2010–2015 (mean) | ||
| Meat and meat products (and its products) | Poultry meat | 58 | 11.1 | 4,388 | 33.0 | 0.013 | 0.010 |
| Meat and meat products | 24 | 4.6 | 593 | 4.5 | 0.005 | 0.003 | |
| Pig meat | 18 | 3.5 | 140 | 1.1 | 0.004 | 0.009 | |
| Bovine meat | 16 | 3.1 | 725 | 5.4 | 0.003 | 0.004 | |
| Sheep meat | 1 | 0.2 | 9 | 0.1 | 0.000 | < 0.001 | |
| Other or mixed red meat and their products | 9 | 1.7 | 106 | 0.8 | 0.002 | 0.003 | |
| Subtotal | 126 | 24.2 | 5,961 | 44.8 | 0.027 | 0.029 | |
| Mixed food and buffet meals | Mixed food | 67 | 12.9 | 1,604 | 12.0 | 0.014 | 0.018 |
| Buffet meals | 18 | 3.5 | 557 | 4.2 | 0.004 | 0.004 | |
| Subtotal | 85 | 16.3 | 2,161 | 16.2 | 0.018 | 0.022 | |
| Other foods | Bakery products | 33 | 6.3 | 632 | 4.3 | 0.007 | 0.006 |
| Other foods | 45 | 8.6 | 1,094 | 7.4 | 0.010 | – | |
| Confections | 5 | 1.0 | 59 | 0.4 | 0.001 | 0.009 | |
| Subtotal | 83 | 15.9 | 1,785 | 13.4 | 0.018 | 0.019 | |
| Eggs and egg products | Eggs and egg products | 72 | 13.8 | 1,192 | 9.0 | 0.016 | 0.026 |
| Subtotal | 72 | 13.8 | 1,192 | 9.0 | 0.016 | 0.026 | |
| Fish and Fisheries | Crustaceans, shellfish, molluscs and their products | 42 | 8.1 | 550 | 4.1 | 0.009 | 0.009 |
| Fish and fish products | 28 | 5.4 | 256 | 1.9 | 0.006 | 0.011 | |
| Subtotal | 70 | 13.4 | 806 | 6.1 | 0.015 | 0.020 | |
| Milk and milk products | Cheese | 25 | 4.8 | 243 | 1.8 | 0.005 | 0.004 |
| Milk | 13 | 2.5 | 103 | 0.8 | 0.003 | 0.002 | |
| Dairy products (other than cheeses) | 7 | 1.3 | 177 | 1.3 | 0.002 | 0.001 | |
| Subtotal | 45 | 8.6 | 523 | 3.9 | 0.010 | 0.007 | |
| Vegetables, fruits, cereals, sprouted seeds, herbs and spices (and their products) | Vegetables and juices and their other products | 22 | 4.2 | 1,645 | 12.4 | 0.005 | 0.008 |
| Cereal products including rice and seeds/pulses (nuts, almonds) | 7 | 1.3 | 146 | 1.1 | 0.002 | 0.001 | |
| Fruit, berries and juices and their other products | 5 | 1.0 | 61 | 0.5 | 0.001 | 0.002 | |
| Subtotal | 34 | 6.5 | 1,852 | 13.9 | 0.007 | 0.011 | |
| Unknown | Unknown | 6 | 1.2 | 224 | 1.7 | 0.001 | < 0.001 |
| Subtotal | 6 | 1.2 | 224 | 1.7 | 0.001 | < 0.001 | |
| Total (EU) | 521 | 100.0 | 14,504 | 100.0 | 0.112 | 0.141 | |
Sixty per cent of all strong‐evidence food‐borne outbreaks (n = 521) were associated with food of animal origin; ‘Meat and meat products’, ‘Eggs and egg products’, ‘Fish and Fisheries’ and ‘Milk and milk products’. ‘Mixed food and buffet meals’ and ‘other foods’ including ‘unspecified foods’ were together reported in almost one‐third of all strong‐evidence outbreaks (32.2%). For single reported types of food vehicles, ‘eggs and egg products’ (n = 72) and ‘poultry meat and its products’ (n = 58) were the items most frequently reported. Compared with previous years, no significant trends for any of the food items implicated in the strong‐evidence food‐borne outbreaks was observed even though eleven food groups were reported in 2016 more frequently than in 2015.
Information on factors suspected to have contributed to the contamination of food and to the occurrence of outbreaks was reported in 243 strong‐evidence outbreaks (46.6%). Contamination of the food vehicle by the use of unprocessed contaminated ingredients and by cross‐contamination was reported in 97 (39.9% of strong‐evidence outbreaks with information available) and 36 (14.8%) outbreaks, respectively. For 100 outbreaks (41.1%) storage/time temperature abuse as well as inadequate heat treatment or chilling were reported. Manipulation of the food vehicle by an infected food handler was reported in 56 outbreaks (23%). In one outbreak, ‘failure in the treatment of water or use of untreated water’ was reported. Contributory factors categorised as ‘other’ with no further details were reported for 17 outbreaks (6.9%).
Top‐5 combinations of causative agents and food vehicles associated with the highest health burden in strong‐evidence food‐borne outbreaks (including waterborne outbreaks)
This section aims to provide insight into the combinations of the causative agents and the food vehicles that in 2016 were associated with the highest health burden in the EU, in terms of the total numbers of outbreaks reported (Table 46), of the overall numbers of human cases involved (Table 47), of hospitalisations (Table 48), and of deaths (Table 49). The five causative agent/food combinations with the highest impact are ranked in each table according to their frequency of reporting, in EU. Rank position occupied by the same combination in the 7 previous years, is also reported to provide rapid information on its trend of occurrence, over time. Rank for the period 2010–2015 was estimated based on the mean annual number of strong‐evidence outbreaks, human cases, hospitalisations and deaths reported by MS in this period for the given combination. As mentioned above, the descriptions of the most important agent/food pairs for 2016 should be interpreted with caution because they mainly may reflect the trends and statistics of France and Poland which contributed more data. Salmonella in ‘eggs and egg products’ had the highest impact among agent/food combinations implicated in food‐borne outbreaks in the EU. In 2016, this food‐pathogen pair caused the highest number of outbreaks, hospitalisations and deaths and was also responsible of a large number of outbreak cases. Although its overall rank measure did not change substantially compared with 2015 for any of the items except deaths, the number of outbreaks, cases of illness and hospitalisations in 2016 were more than twice as high as those observed in 2015. The number of deaths reported in 2016 was also markedly higher than in the previous 6 years (one death in 2014). Other agent/food combinations including Salmonella in foodstuffs of animal and non‐animal origin were also associated with a high number of cases and deaths. As in 2015, calicivirus (including norovirus) were reported among the combinations causing the highest number of outbreaks and cases but interestingly the implicated foodstuffs (‘Crustaceans, shellfish, molluscs and products thereof’ and ‘Vegetables and juices and other products thereof’) were not the same as reported last year (‘Tap water, including well water’, ‘Buffet meals’ and ‘Broiler meat and product thereof’). Despite the low number of reported outbreaks, Shiga toxin‐producing E. coli in ‘Vegetables and juices and other products thereof’ and ‘cheese’ and Listeria in ‘meat and meat products’ were both responsible for deadly illnesses among outbreaks cases in 2016. This is not unexpected given the high case‐fatality rates which frequently characterise outbreaks caused by these agents, and the types of food vehicles which were also reported in outbreaks of STEC and Listeria in recent years.
Table 46.
Top‐5 combinations (agent/food vehicle) causing the highest number of strong‐evidence food‐borne outbreaks (including waterborne outbreaks), reporting Member States, 2016
| Causative agent | Food vehicle | 2016 | 2010–2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Number of outbreaks | Cases | Outbreak reporting rate per 100,000 | Reporting Member State | Rank | Number of outbreaks (mean) | Outbreak reporting rate per 100,000 (mean) | ||||
| Number | Hospitalised | Deaths | |||||||||
| Salmonella | Eggs and egg products | 1 | 67 | 1,099 | 222 | 4 | 0.014 | 17 | 1 | 89.0 | 0.022 |
| Calicivirus including norovirus | Crustaceans, shellfish, molluscs and their products | 2 | 36 | 436 | 6 | 0 | 0.008 | 9 | 7 | 18.8 | 0.005 |
| Salmonella | Bakery products | 3 | 28 | 290 | 80 | 0 | 0.006 | 5 | 6 | 20.2 | 0.004 |
| Bacterial toxins other than C. botulinum | Mixed food | 4 | 26 | 697 | 27 | 0 | 0.006 | 8 | 3 | 31.8 | 0.015 |
| Bacterial toxins other than C. botulinum | Poultry meat | 5 | 25 | 813 | 6 | 0 | 0.005 | 4 | 30 | 4.7 | 0.004 |
Bacterial toxins other than C. botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins.
Table 47.
Top‐5 combinations (agent/food vehicle) causing the highest number of cases, in strong‐evidence food‐borne outbreaks (including waterborne outbreaks), reporting Member States, 2016
| Causative agent | Food vehicle | 2016 | 2010–2016 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Number of outbreaks | Cases | Outbreak reporting rate per 100,000 | Reporting MS | Rank | Number of cases (mean) | Outbreak reporting rate per 100,000 (mean) | ||||
| Number | Hospitalised | Deaths | Mean | ||||||||
| Campylobacter | Poultry meat | 1 | 9 | 3,231 | 1 | 0 | 0.002 | 12 | 26 | 116.7 | 0.005 |
| Salmonella | Eggs and egg products | 2 | 67 | 1,099 | 222 | 4 | 0.014 | 17 | 2 | 891.3 | 0.022 |
| Calicivirus including norovirus | Vegetables and juices and their other products | 3 | 6 | 903 | 6 | 0 | 0.001 | 7 | 10 | 283.2 | 0.002 |
| Bacterial toxins other than C. botulinum | Poultry meat | 4 | 25 | 813 | 6 | 0 | 0.005 | 4 | 23 | 154.8 | 0.004 |
| Calicivirus including norovirus | Other foods | 5 | 14 | 741 | 10 | 0 | 0.003 | 4 | 14 | 229.7 | 0.004 |
MS: Member State.
Bacterial toxins other than C. botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins.
Other foods include bakery products; confections; canned food products; other foods (unspecified).
Table 48.
Top‐5 combinations (agent/food vehicle) causing the highest number of hospitalisations, in strong‐evidence food‐borne outbreaks (including waterborne outbreaks), reporting Member States, 2016
| Causative agent | Food vehicle | 2016 | 2010–2016 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Number of outbreaks | Cases | Outbreak reporting rate per 100,000 | Reporting Member State | Rank | Number of hospitalised | Outbreak reporting rate per 100,000 (mean) | ||||
| Number | Hospitalised | Deaths | (mean) | ||||||||
| Salmonella | Eggs and egg products | 1 | 67 | 1,099 | 222 | 4 | 0.014 | 17 | 2 | 246.2 | 0.022 |
| Salmonella | Mixed food | 2 | 23 | 266 | 123 | 1 | 0.005 | 12 | 4 | 87.5 | 0.005 |
| Salmonella | Bakery products | 3 | 28 | 290 | 80 | 0 | 0.006 | 5 | 6 | 71.3 | 0.004 |
| Salmonella | Meat and meat products | 4 | 17 | 307 | 69 | 0 | 0.004 | 8 | 21 | 14.5 | 0.001 |
| Salmonella | Poultry meat | 5 | 23 | 328 | 63 | 0 | 0.005 | 10 | 10 | 40.8 | 0.005 |
Table 49.
Top‐5 combinations (agent/food vehicle) causing the highest number of deaths, in strong‐evidence food‐borne outbreaks (including waterborne outbreaks), reporting Member States and reporting non‐Member States, 2016
| Causative agent | Food vehicle | 2016 | 2010–2016 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Number of outbreaks | Cases | Outbreak reporting rate per 100,000 | Reporting Member State | Rank | Number of deaths (mean) | Outbreak reporting rate per 100,000 (mean) | ||||
| Number | Hospitalised | Deaths | |||||||||
| Salmonella | Eggs and egg products | 1 | 67 | 1,099 | 222 | 4 | 0.014 | 17 | 3 | 0.8 | 0.022 |
| Shiga toxin‐producing E. coli (STEC) | Vegetables and juices and their other products | 2 | 2 | 407 | 63 | 2 | < 0.001 | 2 | 1 | 9.0 | < 0.001 |
| Salmonella | Mixed food | 3 | 23 | 266 | 123 | 1 | 0.005 | 12 | 3 | 0.8 | 0.005 |
| Salmonella | Cheese | 3 | 14 | 97 | 27 | 1 | 0.003 | 3 | 17 | 0.2 | 0.002 |
| Shiga toxin‐producing E. coli (STEC) | Cheese | 3 | 2 | 29 | 18 | 1 | < 0.001 | 3 | na | na | na |
| Listeria | Meat and meat products | 3 | 1 | 11 | 10 | 1 | < 0.001 | 1 | na | na | na |
Causative agent of strong‐evidence food‐borne outbreaks (excluding waterborne outbreaks) by food vehicle
The distribution of causative agents by type of food in strong‐evidence food‐borne outbreaks is shown in Figure 65. Salmonella was the most frequently reported causative agent in outbreaks associated with ‘eggs and eggs products’ (93.0%), ‘other foods’ (46.9%), ‘meat and meat products’ (39.6%), ‘milk and milk products’ (37.7%), ‘mixed food and buffet meals’ (31.7%) and ‘vegetables, fruits, cereals, sprouted seeds, herbs and spices and their products’ (26.4%). Calicivirus including norovirus was ranked first among causative agent in outbreaks with ‘fish and fishery products’ (51.4%) and was also reported in high proportions in outbreaks by ‘other foods’ (22.8%), ‘mixed food and buffet meals’ (22.3%) and ‘vegetables, fruits, cereals, sprouted seeds, herbs and spices and their products’ (26.4%). Bacterial toxins other than Clostridium botulinum were reported mainly in outbreaks linked to ‘mixed food and buffet meals’ (34.1%). Campylobacter was reported in a on‐negligible proportion of outbreaks associated with the consumption of ‘milk, cheese and dairy products’ (22.2%) and reported in this food category with a frequency higher than in any other one. Campylobacter was also implicated in 7.9% of outbreaks by ‘meat and meat products’, in particular poultry meat. Poultry meat was implicated in the largest outbreak reported during 2016 in the EU, which occurred in Sweden, with more than 3,000 people involved. The contamination of poultry meat was connected to infected poultry flocks from one of the major slaughterhouses. A failure in a cleaning facility was one of the causes leading to the increased incidence of infected flocks.
Figure 65.
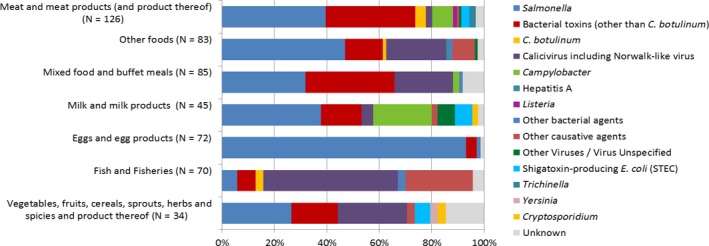
Frequency distribution of causative agents associated with strong‐evidence food‐borne outbreaks (excluding waterborne outbreaks), by food vehicle, reporting Member States, 2016
- Ten strong‐evidence outbreaks with food vehicle ‘unknown’ are not shown in the figure. Other bacterial agents include Shigella and other unspecified bacteria. Bacterial toxins include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Other viruses include flavivirus and other unspecified viruses. Other causative agents include ciguatoxin and other unspecified toxins.
Other causative agents (i.e. histamine and ciguatoxin) accounted for the 27.5% of outbreaks associated with consumption of ‘fish, shellfish, molluscs, crustaceans and product thereof’.
Places of exposure in strong‐evidence food‐borne outbreaks (excluding waterborne outbreaks)
Information on the place of exposure (epidemic setting) was provided in 2016 for 484 strong‐evidence food‐borne outbreaks (excluding waterborne outbreak), which was 92.9% of all reported outbreaks (Figure 66). As mentioned above, these descriptions for 2016 should be interpreted with caution because they mainly may reflect the trends and statistics of France and Poland because these MS contributed with more data. ‘Household’ was the most frequent place of exposure of cases to the implicated food, followed by ‘Restaurants, pubs, street vendors and take away’ and ‘Canteen or Catering to Workplace, school, hospital’ that are settings where food was prepared and/or served by catering services. ‘Other settings’ such as farms, fairs and festivals, and other undefined places were reported less frequently.
Figure 66.
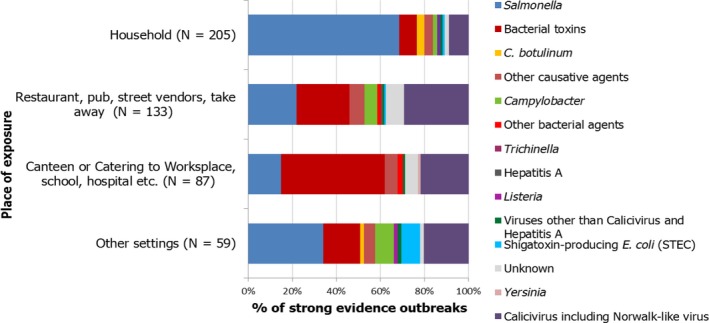
Frequency distribution of causative agents associated with strong‐evidence food‐borne outbreaks (excluding waterborne outbreaks), by place of exposure, reporting Member States, 2016
- Thirty‐seven food‐borne‐outbreaks with setting ‘unknown’ are not shown in the figure. Other setting include: Camp or picnic, farm, multiple places of exposure in more than one country, multiple places of exposure in one country, temporary mass catering (fairs or festivals), other unspecified settings.
Table 50.
Frequency distribution of strong‐evidence food‐borne outbreaks (excluding waterborne outbreaks), by place of exposure (setting), reporting Member States, 2016
| Type of setting | Strong‐evidence outbreaks | Reporting rate per 100,000 | |||||
|---|---|---|---|---|---|---|---|
| Number of outbreaks | % of total | Number of human cases | % of total | 2016 | 2010–2015 (mean) | ||
| Household | Household | 205 | 39.3 | 1,231 | 8.5 | 0.044 | 0.053 |
| Subtotal | 205 | 39.3 | 1,231 | 8.5 | 0.044 | 0.053 | |
| Restaurant, pub, street vendors, take away, etc. | Restaurant or Cafe or Pub or Bar or Hotel or Catering service | 130 | 25.0 | 2,626 | 18.1 | 0.028 | 0.034 |
| Mobile retailer or market/street vendor | 2 | 0.4 | 52 | 0.4 | < 0.001 | 0.001 | |
| Take‐away or fast‐food outlet | 1 | 0.2 | 21 | 0.1 | < 0.001 | 0.001 | |
| Subtotal | 133 | 25.5 | 2,699 | 18.6 | 0.029 | 0.036 | |
| Canteen or Catering to Workplace, school, hospital, etc. | School or kindergarten | 49 | 9.4 | 1,820 | 12.5 | 0.011 | 0.009 |
| Residential institution (nursing home or prison or boarding school) | 18 | 3.5 | 758 | 5.2 | 0.004 | 0.004 | |
| Canteen or workplace catering | 14 | 2.7 | 613 | 4.2 | 0.003 | 0.006 | |
| Hospital or medical care facility | 6 | 1.2 | 349 | 2.4 | 0.001 | 0.002 | |
| Subtotal | 87 | 16.7 | 3,540 | 24.4 | 0.019 | 0.022 | |
| Other settings | Others | 32 | 6.1 | 1,152 | 7.9 | 0.007 | 0.008 |
| Multiple places of exposure in one country | 10 | 1.9 | 1,078 | 7.4 | 0.002 | < 0.001 | |
| Camp or picnic | 6 | 1.2 | 136 | 0.9 | 0.001 | 0.002 | |
| Farm | 6 | 1.2 | 118 | 0.8 | 0.001 | 0.001 | |
| Multiple places f exposure in more than one country | 5 | 1.0 | 323 | 2.2 | 0.001 | < 0.001 | |
| Subtotal | 59 | 11.3 | 2,807 | 19.4 | 0.013 | 0.014 | |
| Unknown | Unknown | 37 | 7.1 | 4,227 | 29.1 | 0.008 | 0.016 |
| Subtotal | 37 | 7.1 | 4,227 | 29.1 | 0.008 | 0.016 | |
| Total (EU) | 521 | 100.0 | 14,504 | 100.0 | 0.112 | 0.124 | |
Causative agents of strong‐evidence food‐borne outbreaks (excluding waterborne outbreaks) by place of exposure
The distribution of causative agents by the place of exposure to the implicated food, in strong‐evidence food‐borne outbreaks is shown in Figure 66. As in 2015, outbreaks due to Salmonella were strongly associated with ‘Household’ (68.3% of outbreaks in this setting), about four‐times higher compared with other settings. Calicivirus including norovirus and bacterial toxins (other than Clostridium botulinum) were more frequently reported in ‘Canteen or Catering to Workplace, school, hospital’ (21.8 and 47.1%, respectively), which are settings where food was prepared and/or served by catering services and in ‘Restaurants, pubs, street vendors and take away’ (29.3% and 24.1%, respectively), compared with ‘Household’ (6.3% and 7.7%, respectively). Outbreaks by Clostridium botulinum toxins were quite exclusively associated with food consumed at home.
Food vehicles implicated in strong‐evidence food‐borne outbreaks by place of exposure
The distribution of implicated food vehicles by the place of exposure (consumption) to the contaminated food, in strong‐evidence food‐borne outbreaks is shown in Table 2016_FBOEXPVEHIC in the Appendix. ‘Eggs and egg products’ were predominantly associated with ‘Household’ (21.7% of the outbreaks in this setting) but were not rarely reported also in outbreaks occurred in ‘Canteen or Catering to Workplace, school, hospital’ (12.6%). The most common food vehicles implicated in outbreaks ‘Restaurants, pubs, street vendors and take away’ (27.8%) were ‘Mixed foods and buffet meals’ which accounted also for an important proportion of outbreaks in ‘Canteen or Catering to Workplace, school, hospital’ (23.0%). ‘Fish, shellfish, molluscs and crustaceans’ were mainly reported in outbreaks in ‘Restaurants, pubs, street vendors and take away’ (22.6%). Strong‐evidence outbreaks by ‘meat and meat products’ were frequently reported in all type of settings, where this food vehicle group accounted for a proportion not less than 18.3%. More specific details on food vehicle implicated in the different outbreak settings are available in Table 2016_FBOEXPVEHIC in the Appendix.
Trends in implicated food vehicle by the causative agent in strong‐evidence and weak‐evidence food‐borne outbreaks
Strong‐evidence outbreaks represent only a small proportion of all food‐borne outbreaks reported by MS for the year 2016, similarly to previous years. In the last 3 years, they accounted for just the 10.7% of all reported outbreaks in the EU (n = 14,403).
Since 2014, MS have the possibility to provide detailed information regarding the suspected food vehicle also for weak‐evidence outbreaks. Therefore, the number of outbreaks reported with information on both the implicated food vehicles and the causative agents has increased since 2014 and provides an opportunity for further descriptive analyses and trend watching. In 2016, these outbreaks were 1,862 (39.1% of all food‐borne outbreaks). For the first time, the trend over years, of the most important combination of causative agents and the food vehicles, based on reporting of all outbreaks with available information reported in 2014, 2015 and 2016 are described. It should be emphasised that much caution is needed in interpreting such trends as the relationship between food items and causative agent are only ‘suspect’ for most of the outbreaks (weak evidence). Moreover, as explained above, these supranational trends may reflect the trends and statistics of the MS that contributed more data.
Overall reporting rates of food‐borne outbreaks per 100,000 population are shown in Figure 67 for the most frequently reported combinations of causative agent and food vehicle from 2014 to 2016. In food‐borne outbreaks due to Salmonella and bacterial toxins other than Clostridium botulinum, the implication of ‘mixed food’ and ‘poultry meat’ increased over time. For outbreaks due to calicivirus including norovirus, ‘buffet meals’ was progressively more reported to be incriminated throughout 2014–2016, while the reporting of ‘crustaceans, shellfish, molluscs and their products’ as food vehicle increased dramatically between 2015 and 2016. As regards the reports on food‐borne outbreaks due to other causative agents, ‘fish and fishery products’ were progressively less reported to be implicated in the last 3 years.
Figure 67.
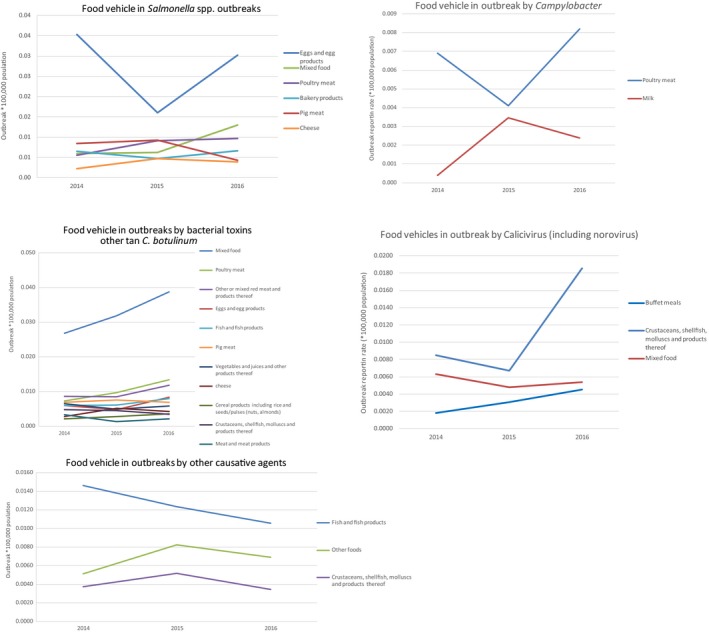
Rate of outbreak reporting (*100,000 population) of strong‐evidence and weak‐evidence food‐borne outbreaks by the causative agent and implicated food vehicle, reporting Member States, 2014–2016
- Only combinations with at least 10 outbreaks reported per year are shown. Food vehicles reported as ‘Unspecified’ and those classified as ‘Other Foods’ are not shown.
16.4.3. Waterborne outbreaks
Nine MS reported 26 strong‐evidence waterborne outbreaks for the year 2016. Four of these were reported as strong‐evidence outbreaks by three MS. These outbreaks involved 309 cases of which 8 were hospitalised. Agents detected in the four strong‐evidence outbreaks were calicivirus including norovirus and Campylobacter. In addition, two non‐MS, the Former Yugoslav Republic of Macedonia and Norway, reported two large strong‐evidence outbreaks (Table 51).
Table 51.
List of reported strong‐evidence waterborne outbreaks, reporting Member States and reporting non‐Member States, 2016
| Causative agent | Country | Setting | Additional information | Strong‐evidence outbreaks | Reporting Rate (per 100,000) | |||
|---|---|---|---|---|---|---|---|---|
| Number | Human cases | Hospitalised | Deaths | |||||
| Calicivirus including norovirus | Belgium | Camp or picnic | 1 | 115 | 5 | 0 | < 0.001 | |
| Czech Republic | Unknown | 1 | 52 | 1 | 0 | < 0.001 | ||
| Finland | Household | 1 | 120 | 0 | 0 | < 0.001 | ||
| Campylobacter | Finland | Household | 1 | 22 | 2 | 0 | < 0.001 | |
| Total (MS) | 4 | 309 | 8 | 0 | 0.001 | |||
| Escherichia coli | The Former Yugoslav Republic of Macedonia | Multiple places of exposure in one country | 1 | 86 | 0 | 0 | < 0.001 | |
| F. tularensis | Norway | Unknown | Untreated water | 1 | 6 | 1 | 0 | < 0.001 |
Six MS reported 22 weak‐evidence waterborne outbreaks caused by calicivirus including norovirus, Campylobacter, Cryptosporidium, hepatitis A virus and STEC. Moreover, four weak‐evidence outbreaks were caused by unknown agents. Among non‐MS, Bosnia and Herzegovina reported two outbreaks caused by E. coli without further specifying the pathotype. Further details on the number of strong‐evidence outbreaks and human cases, including information on the causative agents, and reporting countries are available in the Appendix (Table 2016_FBOWATERWEAK).
16.5. Discussion
16.5.1. Trends
In total, 4,786 food‐borne and waterborne outbreaks have been reported in 2016 by 27 MS. Another 108 outbreaks were notified by seven non‐MS. In the EU, compared with 2015, the numbers of reported outbreaks, illnesses and deaths were increased whereas the number of hospitalisations decreased. The EU‐level reporting rate was 1.03 food‐borne outbreaks per 100,000 population, which was a slight increase compared with 2015 but in line or slightly lower than the EU‐level outbreak reporting rates during 2010–2016.
Large differences were observed among MS in the number of outbreaks reported, with few MS accounting for most of the outbreaks (more than 70% were reported by five MS only). France reported by far the largest number of outbreaks and accounted alone for 30.3% of all reported outbreaks, followed by the Netherlands, Poland, Slovakia and Germany, Belgium and Sweden. This huge variability may be explained by differences in the sensitivity and approach of the passive surveillance systems in place for food‐borne outbreaks detection in the MS. The lack of harmonisation among countries in overall architecture, components (e.g. case definition, diagnostic methods, challenges of food testing, assessment of the implication of specific foods) and in reporting system of the surveillance programmes affect the capability of detecting outbreaks, and of identifying the causative agent, the implicated food vehicle and the contributory factors. The capability of detecting and investigating food‐borne outbreaks is highly dependent on the availability and routine application of laboratory methods. This is of high importance given that recent advances in the routine application of the molecular characterisation methods, including WGS, for outbreak surveillance including the implementation of the Joint ECDC–EFSA Molecular Typing Database (Rizzi et al., 2017) have probably been implemented in MS in different timeframes.
The lack of harmonisation hampers data comparability between MS, and therefore trend analysis at the supranational, EU‐level. Estimates and trends at EU‐level should be interpreted with caution, as they may be biased by the different relative ‘weight’ of each single MS contributing to the overall reported outbreak data. This was particularly evident when analysing data on food‐borne outbreaks by bacterial toxins other than Clostridium botulinum. The marked increase observed in recent years at the EU‐level is almost exclusively due to the trend of a single MS (France), which during 2010–2016 provided the 84.5% (3,630 out of 4,295) of total outbreaks by these causative agents.
In 2016, bacteria, in particular Salmonella, were the most commonly detected causative agents in food‐borne outbreaks (33.9% of all outbreaks; reported by 27 MS), followed by bacterial toxins (17.7% of all outbreaks; reported by 18 MS), viruses (9.8% of all outbreaks; reported by 21 MS), other causative agents (2.2% of all outbreaks; reported by 10 MS) and parasites (0.4% of all outbreaks; reported by 7 MS). Among bacterial agents Salmonella alone accounted for two‐thirds of the outbreaks (65.8%) and, together with Campylobacter, for the vast majority of outbreaks by bacterial agents (94.1%). For a large proportion of the reported outbreaks (n = 1,723; 36% of the all reported outbreaks), the causative agent could not be identified and this represents a major limitation of the overall food‐borne outbreak surveillance at the EU. Many of these outbreaks lacked also of information on the suspected food vehicle and setting.
At the MS level, the leading causative agent of food‐borne outbreaks was Salmonella in 15 MS, calicivirus including norovirus in 5 MS, bacterial toxins other than C. botulinum in 3 MS, Campylobacter in 2 MS, Shiga toxin‐producing E. coli (STEC) in 1 MS and histamine in 1 MS.
Over the period 2010–2016 the trend in reported outbreaks at the EU‐level was quite stable although the number of MS providing information on outbreaks increased from 24 in 2010, up to 27 in 2016. Also for most of the MS, the number of outbreaks reported during 2010–2016 was fairly stable (Finland, Germany, Greece, Poland, Romania, Slovakia, Slovenia, Sweden, the United Kingdom), or only slightly increasing (Ireland, the Czech Republic), or decreasing (Latvia, Lithuania), over years. On the contrary, for a few MS a statistical significant increasing (Belgium, France, the Netherlands, Portugal) or decreasing trend (Austria, Denmark, Estonia, Hungary) was observed. Interestingly, almost all the MS with a significant rise in the number of outbreaks reported over years, include those countries which contributed more (2,424 outbreaks; 50.6% of total outbreaks) to the overall outbreak data reporting at the EU‐level in 2016 (Belgium, France, the Netherlands). In these MS, the increasing trend in numbers of outbreaks reported was mainly due to outbreaks by calicivirus (including norovirus) (France and the Netherlands), with an ‘unknown’ causative agent (Belgium and the Netherlands), by bacterial toxins other than C. botulinum and, to a lesser extent, by Salmonella in France. These findings suggest that during 2010–2016 Salmonella did not represent a driver for outbreaks increase in any MS except France. On the contrary, the decrease in the number of reported Salmonella outbreaks in some MS may have contributed to the overall decrease in the total number of food‐borne outbreaks observed in some MS. This is an important finding, since Salmonella is the only causative agent in the EU subjected to specific NCP, at the primary production level. On the contrary, it is of concern that in those MS where Salmonella outbreaks had the highest burden (Poland, Slovakia) the number of outbreaks of salmonellosis has progressively increased between years 2014 and 2016, despite NCP having been implemented for many years. In the absence of specific changes in the outbreak detection capability (e.g. change in case definition, implementation of molecular based surveillance for Salmonella infection), these findings would suggest that Salmonella control programme in animal reservoirs and food safety interventions are becoming less successful in some MS.
In 2016, Salmonella was associated with the highest burden in terms of overall number of hospitalisations and 1,766 food‐borne salmonellosis cases were hospitalised, which corresponded to 45.6% of all food‐borne outbreak illnesses that needed hospitalisation during 2016. In terms of deaths, 50% of all deaths due to food‐borne disease, being 10 of 20 deaths, were reported due to food‐borne salmonellosis. At the EU‐level, a decreasing trend in the number of reported salmonellosis outbreaks was observed between 2010 and 2016. The increase in the number of outbreaks of salmonellosis reported for 2016 seems to reverse this favourable trend. The increase is primarily attributable to a 23.6% increase in the number of outbreaks due to S. Enteritidis reported at EU‐level leading to the fact that S. Enteritidis was the reported causative agent in about one in six (14.9%) of all reported outbreaks for the year 2016. At the single MS level, an increase above the EU mean rate was observed in 2016, in three MS (Hungary +90.9%; Poland +42.0%; Slovakia +23.2%) compared with 2015. Noteworthy, in this context was the large multicountry outbreak of S. Enteritidis which involved more than 15 EU/EEA countries (ROAs) since mid‐2016. The outbreak was linked to the distribution of contaminated ‘eggs and eggs products’ across EU (ECDC and EFSA, 2014, 2017). These signals indicate the importance of ensuring effective implementation of the NCP for Salmonella in laying hens and the restrictions on sale of fresh eggs from infected flocks. On the other hand significant decreasing trends over years 2010–2016 in the number of outbreaks caused by both Salmonella and S. Enteritidis were reported for many MS (Germany, Austria, Lithuania, Hungary and Estonia). Together with the disclosed increasing trend of S. Enteritidis flock prevalence in poultry and in laying hens in particular (see Salmonella chapter), the above mentioned recently detected S. Enteritidis food‐borne outbreaks raise questions as they indicate a reversal of the declining trend in the EU in humans and poultry. Further cross‐sectorial investigations are needed to better understand underlying reasons for the increase.
Analysis over years did not show any particular trend of reporting of Campylobacter outbreaks over time at the EU‐level. At the single MS level a marked significant increase was observed in Germany, where Campylobacter in 2016 represented the most frequent implicated agent in food‐borne outbreaks.
Calicivirus including norovirus was associated at the EU‐level with the highest burden in terms of total number of illness and mean number of cases per outbreak, with 23 single outbreaks involving more than 100 cases, each. An important contribution to the marked increase in the number of outbreaks of norovirus infection observed for France may probably arise from the circulation of both new and/re‐emergent strains of norovirus in France (Bidalot et al., 2017).
STEC were responsible in 2016 of two large outbreaks occurred in Finland (237 cases) and the United Kingdom (107 cases), both implicating leafy green vegetables as the vehicle of infection. Moreover, a multicounty outbreak of STEC O26 infection with many cases of HUS, hospitalisations and deaths among young children was reported in Romania and Italy. A contaminated cheese was implicated in the transmission of infection to some but not all outbreak cases (EFSA and ECDC, 2016c).
Another multicountry outbreak of Clostridium botulinum toxin type E poisoning was reported in the EU and involved in Germany and Spain cases who had consumed a dried and salted fish product (roach) (EFSA and ECDC, 2016a).
Multicountry outbreaks by Salmonella Enteritidis in the EU, 2016 (EFSA and ECDC, 2016b, 2017).
Two large multicountry outbreaks by Salmonella Enteritidis were reported in the EU in 2016. The first included 218 confirmed cases belonging to two distinct clusters identified by WGS and 252 probable cases sharing the S. Enteritidis MLVA profiles 2‐9‐7‐3‐2 or 2‐9‐6‐3‐2. Cases including fatal illness were continuously reported by 14 EU countries (Belgium, Croatia, Denmark, Finland, France, Greece, Hungary, Italy, Luxembourg, the Netherlands, Norway, Slovenia, Sweden and the United Kingdom), starting from May 2016, and peaking at the end of September 2016. Available evidence from epidemiological, microbiological, environmental and tracing investigations identified eggs originating from three Polish packing centres as the vehicle of infection in this outbreak. The contamination of eggs was suspected to originate at laying hen farms even if a contamination at a higher level in the food chain was not excluded. Control measures were adopted at the farm and distribution level, to limit the spread of the outbreak included banning the placing on the market of table eggs originating from the positive farms and from the concerned Polish packing centres as a precautionary measure. All the specific requirements on flocks of laying hens as laid down in Regulation 2160/2003 were applied as soon as a positive flock was identified by the Polish authorities. New pullets were introduced after the culling of Salmonella‐positive flocks and the cleaning and disinfection of the poultry houses, all under the supervision of the Polish authorities. An enhanced surveillance period was established at the EU‐level by the international outbreak investigation team coordinated by the ECDC, to monitor the public health impact of control measures, based on WGS of any human isolates characterised by MLVA profiles 2‐9‐7‐3‐2 or 2‐9‐6‐3‐2.
The other multicountry outbreak of infection by S. Enteritidis was identified by the detection of a WGS‐defined cluster in the United Kingdom, in autumn 2016. Four MS (France, Ireland, Spain and the United Kingdom) were involved in the outbreak. Molecular characterisation by WGS highlighted the re‐emergence of the S. Enteritidis strain that was first identified in 2014 and caused an outbreak that was investigated from May to October 2015 in Spain and the United Kingdom. The high genetic similarity between the 2015 and 2016 isolates (isolates from 2015 all fell within the same 5 single nucleotide polymorphism (SNP) single linkage cluster as the 2016 outbreak isolates) suggested that the cases in both years were part of a common source outbreak.
16.5.2. Sources
Similarly to previous years, in 2016, food vehicles involved in strong‐evidence outbreaks were mostly of animal origin. ‘Eggs and egg products’ and ‘poultry meat and their products’ were the items most frequently reported. Interestingly, outbreaks implicating some specific types of food were mainly associated with a single pathogen. This is the case for ‘eggs and eggs product’ and Salmonella (75.7% of the outbreaks implicating this food), ‘crustaceans, shellfish, molluscs and their products’ and calicivirus including norovirus (63.7%), ‘poultry meat’ and Campylobacter and Salmonella (52.5% overall), ‘fish and fisheries’ with histamine (46.7%). S. Enteritidis multicountry outbreaks occurred in 2016 in the EU and were traced back to eggs, similarly to previous years (EFSA and ECDC, 2014; Dallman et al., 2016). Poultry meat is a well‐known vehicle of Salmonella infection leading to both sporadic and outbreak cases of food‐borne salmonellosis world‐wide. In recent years (2013–2015) this agent/food combination was also very frequently reported in the notifications of the Rapid Alert System for Food and Feed (RASFF) which ranked Salmonella in poultry meat among the top‐10 RASFF number of notifications, by country of origin in years 2013, 2014, 2015. Similarly, outbreaks of campylobacteriosis are frequently reported as caused by the consumption of poultry meat, in particular undercooked liver pâté, not only in the EU but also in many other industrialised countries (Scott et al., 2015; Moffatt et al., 2016).
Outbreaks with incriminated foods ‘mixed foods’, ‘buffet meals’ and ‘other foods’, which in 2016 represented a third of total strong‐evidence outbreaks, were associated with a large variety of causative agents. A reason for this could be that these are foods whose production cycles comprise many steps of food processing, manipulation and preservation. This implies that the risk for a post‐harvest contamination or for an increase of the bacteria or toxin load is more likely in these types of food, as a consequence of factors such as cross‐contamination, temperature abuse and food handling. These contributory factors are more frequently reported in outbreaks implicating ‘mixed foods’ and ‘other foods’ than in any other food group. This finding reinforces the need to adopt strict hygiene procedures for food processing and suggests that efforts to control outbreaks implicating these foodstuffs should target the post‐harvest steps of food production as well as the primary production level. This is of particular relevance given that ‘mixed foods’ are among the vehicles for which an increasing trend was identified over the last three years, in the EU.
Information on setting highlights the need to continue delivering recommendations to general population to improve hygiene rules for preservation, manipulation and cooking foods at home. In 2016, most of the strong‐evidence outbreaks involved cases that fallen ill after having consumed food at home. It is important to note that the causative agents and the food vehicles identified in household outbreaks differed from those reported in other settings (i.e. canteen, catering services, restaurants, pubs, street vendors and take away). Such findings clearly indicate the need to deliver targeted recommendations for outbreak control policies to the general population and to food business operators.
In 2016, strong‐evidence outbreaks represented only a minor proportion of all the reported food‐borne outbreaks, and in the last 3 years they accounted for 10.7% of all reported outbreaks in the EU (n = 14,403). Since 2014, MS have the possibility to provide detailed information on the suspected vehicle in weak‐evidence outbreaks. This possibility enlarges importantly the number of observations (outbreaks) for which the information on both the implicated causative agents and the food vehicles may be analysed jointly. This allowed analysing for the first time the trend over years, for the most important combination of causative agents and the food vehicles. It should be highlighted however that the estimates presented in this report describe a general tendency but should be carefully interpreted as the relationship between food items and causative agent had a high level of uncertainty, being only ‘suspect’ for most of the outbreaks.
16.6. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Food and Waterborne disease programme in the EU | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme |
| Food‐borne illness and outbreaks – WHO | http://www.who.int/mediacentre/factsheets/fs399/en/ | |
| CDC (Centers for Disease Control and Prevention) of United States: Food‐borne Outbreaks in the USA | https://www.cdc.gov/foodsafety/fdoss/overview/index.html | |
| CDC (Centers for Disease Control and Prevention) of United States: FoodNet USA | https://www.cdc.gov/foodnet/index.html | |
| United States FoodSafety.gov: gateway to food safety information provided by government agencies. Finding the Sources of Food‐borne Disease Outbreaks | https://www.foodsafety.gov/blog/complexmystery.html | |
| Animals | Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
|
ECDC. Expert Opinion on the introduction of next‐generation typing methods for food‐ and waterborne diseases in the EU and EEA. Stockholm: ECDC; 2015 Zoonoses in Finland |
https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/food-and-waterborne-diseases-next-generation-typing-methods.pdf | |
| EFSA Scientific Colloquium: on Whole Genome Sequencing of food‐borne pathogens for public health protection | https://www.efsa.europa.eu/en/supporting/pub/en-743 | |
| Other |
ECDC Expert Opinion: on the introduction of next‐generation typing methods for food‐ and waterborne diseases in the EU and EEA. Stockholm: ECDC; 2015 Zoonoses in Finland |
https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/food-and-waterborne-diseases-next-generation-typing-methods.pdf |
|
ECDC. Expert Opinion on the introduction of next‐generation typing methods for food‐ and waterborne diseases in the EU and EEA. Stockholm: ECDC; 2015 Zoonoses in Finland |
https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/food-and-waterborne-diseases-next-generation-typing-methods.pdf | |
| EFSA Scientific Opinion: on the evaluation of molecular typing methods for major food‐borne microbiological hazards and their use for attribution modelling, outbreak investigation and scanning surveillance: Part 1 (evaluation of methods and applications). Part 2 (Evaluation of molecular typing methods for major food‐borne pathogens |
17. Microbiological contaminants (for which food safety criteria are laid down in EU legislation)
This chapter summarises the information provided on the non‐zoonotic microbiological contaminants histamine, Cronobacter sakazakii and staphylococcal enterotoxins in food, for the year 2016, in the framework of EU Regulation 2073/2005 on microbiological criteria.
The Appendix lists all summary tables and figures made for the production of this section. It is an Excel file allowing the user to filter by chapter the corresponding summary tables and figures with their abbreviated file name and titles. All tables and figures are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1044742
17.1. Histamine
Histamine is an endogenous compound of the human body which can also be introduced from external sources such as contaminated food. If histamine reaches a critical threshold, it can lead to symptoms such as skin flushing, rash, gastrointestinal complaints and throbbing headache. The Commission Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs defines food safety criteria for histamine in food, at retail level, in two major food categories: ‘fish, fishery products from fish species associated with a high amount of histidine’ (food category 1.25: n = 9; c = 2; m = 100 mg/kg; M = 200 mg/kg) and ‘fish, fishery products which have undergone enzyme maturation treatment in brine’ (Food category 1.26: n = 9; c = 2; m = 200 mg/kg; M = 400 mg/kg).
For 2016, the presence of histamine in ‘fish, fishery products from fish species associated with a high amount of histidine’ was reported at retail by 9 MS (Austria, Belgium, Bulgaria, the Czech Republic, Portugal, Romania, Slovakia, Slovenia and Spain) (Table 2016_HISTAMINEFISHALL). In total, 458 units were reported as batches, of which two units had levels of histamine above 200 mg/kg (0.4% of the total units sampled as batches); no samples have been reported with histamine between 100 and 200 mg/kg. In total, 97 units were reported as single units, of which nine units had levels of histamine above 200 mg/kg (9.3% of the total units sampled as singles); no samples have been reported with histamine between 100 and 200 mg/kg. Spain reported 10 out of the 11 histamine‐positive samples in ‘fish, fishery products from fish species associated with a high amount of histidine’ (batch and single samples).
For the food category ‘fish, fishery products which have undergone enzyme maturation treatment in brine’, data at retail were reported by four MS (Austria, France, Romania and Spain). In total, 46 batch samples and 26 single samples were investigated. No histamine was detected above the limit of 200 mg/kg (Table 2016_HISTAMINEFISHALL).
MS reported also data for other ‘fish and fishery products’ without details on the specific food categories, not allowing their classification according to the Commission Regulation (EC) No 2073/2005. In total, 58 batch samples and 1,721 single samples were reported.
An improved quality of the data in conformity to the requirements of the Commission Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs would allow a better assessment of the results. Indeed, despite that the regulation defines limits on histamine for the two food categories mentioned above, some MS reported data without sufficient specification on the food category.
17.2. Staphylococcal enterotoxins
Nine MS (Croatia, the Czech Republic, Hungary, Italy, Portugal, Romania, Slovakia, Slovenia and Spain) provided 2016 data on the presence of staphylococcal enterotoxins in milk and dairy products. In total, 2,061 samples (batch samples and single samples) were analysed and of them seven (one batch and six single samples of soft, semi‐soft and hard cheese) were found to be positive and reported by three MS (Italy, Slovakia and Spain).
Data on staphylococcal enterotoxins in other food were submitted by three MS (the Czech Republic, Italy and Spain). In total, 572 samples (batch samples and single samples) were tested, of which four were found positive. The positive samples (processed food products and prepared dishes, bakery products) were reported by Italy (1) and Spain (3) and obtained at retail and processing plant, respectively.
17.3. Cronobacter sakazakii
The presence of C. sakazakii in infant formula and dietary foods for special medical purposes was reported by 11 MS (Austria, Belgium, Cyprus, the Czech Republic, Estonia, Hungary, Ireland, Romania, Slovakia, Slovenia and Spain). In total, 1,157 samples (84% taken at retail level) were examined. Only one sample was reported positive by Slovakia.
Two MS (the Czech Republic and Ireland) reported 2016 data on C. sakazakii in other foods. In total, 61 samples (cheeses and other dairy products) were tested, and the Czech Republic reported 10 positive batches of milk and whey powder out of 55 tested.
Abbreviations
- ADNS EU
Animal Disease Notification System
- AE
alveolar echinococcosis
- AER
Annual Epidemiological Reports
- AHAW
EFSA Panel on Animal Health and Welfare
- ARIMA
autoregressive integrated moving average
- BIOHAZ
EFSA Panel on Biological Hazards
- CA
Competent Authority
- CE
cystic echinococcosis
- CFT
complement fixation test
- CFU
colony forming unit
- CONTAM
EFSA Panel on Contaminants in the Food Chain
- DALY
disability‐adjusted life years
- DCF
Data Collection Framework
- DH
definitive hosts
- DWH
Data Warehouse
- EBLV
European bat lyssavirus
- EC
European Commission
- ECDC
European Centre for Disease Prevention and Control
- EEA
European Economic Area
- EFSA
European Food Safety Authority
- EFTA
European Free Trade Association
- EIEC
enteroinvasive E. coli
- ELISA
enzyme‐linked immunosorbent assay
- EPEC
enteropathogenic E. coli
- ERCE
European Register of Cystic Echinococcosis
- ESRI
Economic and Social Research Institute
- ETEC
enterotoxigenic E. coli
- EURL
European Union Reference Laboratory
- FAO
Food and Agriculture Organization of the United Nations
- FAT
fluorescent antibody test
- FBO
food‐borne outbreaks
- FDA
Food and Drug Administration
- FISH
fluorescence in situ hybridisation
- FSC
food safety criteria
- FWD‐Net
European Food‐ and Waterborne Diseases and Zoonoses Network
- GAP
Good Agricultural Practices
- GHP
Good Hygiene Practices
- HACCP
hazard analysis and critical control point
- HI
intermediate hosts
- HUS
haemolytic–uraemic syndrome
- i‐ELISA
indirect enzyme‐linked immunosorbent assay
- IFA
immunofluorescence assay
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IHC
immunohistochemistry
- ISO
International Organization for Standardization
- LHT
low heat‐treated
- MALDI‐TOF
matrix assisted laser desorption ionisation‐time of flight
- MDR
multidrug‐resistant
- MS
Member State
- NAT
nucleic acid test
- NCEZID
National Center for Emerging and Zoonotic Infectious Diseases
- NCP
National Control Programmes
- NMKL
Nordic Committee on Food Analysis
- NRUCH
not raised under controlled housing conditions
- NT
not typable
- OBF
official brucellosis‐free in cattle
- ObmF
official Brucella melitensis‐free in sheep and goats
- OF
officially free
- OIE
World Organisation for Animal Health
- OTF
official tuberculosis‐free in cattle
- PCR
polymerase chain reaction
- PFGE
pulsed field gel electrophoresis
- PHC
process hygiene criteria
- RASFF
Rapid Alert System for Food and Feed
- RTE
ready‐to‐eat
- RT‐PCR
reverse transcriptase‐polymerase chain reaction
- SCT
sedimentation and counting technique
- STEC
Shiga toxin‐producing Escherichia coli
- TBE
Tick‐borne encephalitis virus
- TESSy
The European Surveillance System
- VTEC
verocytotoxigenic Escherichia coli
- WAHID
World Animal Health Information Database
- WGS
whole genome sequencing
- WHO
World Health Organization
- WNF
West Nile fever
- WNV
West Nile virus
Country codes
- Austria
AT
- Belgium
BE
- Bulgaria
BG
- Croatia
HR
- Cyprus
CY
- Czech Republic
CZ
- Denmark
DK
- Estonia
EE
- Finland
FI
- France
FR
- Germany
DE
- Greece
GR
- Hungary
HU
- Iceland
IS
- Ireland
IE
- Italy
IT
- Latvia
LV
- Liechtenstein
LI
- Lithuania
LT
- Luxembourg
LU
- Malta
MT
- Netherlands
NL
- Norway
NO
- Poland
PL
- Portugal
PT
- Romania
RO
- Slovakia
SK
- Slovenia
SI
- Spain
ES
- Sweden
SE
- Switzerland
CH
- United Kingdom
UK
Appendix A – Details on prevalence of Listeria monocytogenes in main ready‐to‐eat (RTE) food matrices in 2016
1.
| RTE food category | Food subcategories | Sampling unit | Number of tested samples | % of positive samples |
|---|---|---|---|---|
| Fish and fishery products | Fish | Batch | 373 | 4.0 |
| Single | 1,845 | 4.8 | ||
| Fishery products | Batch | 441 | 7.0 | |
| Single | 288 | 3.5 | ||
| Milk | Pasteurised | Batch | 123 | 0 |
| Single | 568 | 0 | ||
| UHT | Batch | 15 | 0 | |
| Single | 14 | 0 | ||
| Raw, intended for direct human consumption | Batch | 10 | 0 | |
| Single | 238 | 2.9 | ||
| Hard cheeses from pasteurised milk | From cow milk | Batch | 466 | 0 |
| Single | 608 | 0.8 | ||
| From goat milk | Batch | 67 | 0 | |
| Single | 11 | 0 | ||
| From sheep milk | Batch | 114 | 0 | |
| Single | 5 | 0 | ||
| Hard cheeses from raw milk | From cow milk | Batch | 193 | 0 |
| Single | 231 | 2.2 | ||
| From goat milk | Batch | 2 | 0 | |
| Single | 5 | 0 | ||
| From sheep milk | Batch | 1 | 0 | |
| Single | 50 | 0 | ||
| Soft and semi‐soft cheeses from pasteurised milk | From cow milk | Batch | 779 | 0.6 |
| Single | 1,852 | 0.2 | ||
| From goat milk | Batch | 60 | 0 | |
| Single | 88 | 0 | ||
| From sheep milk | Batch | 113 | 0 | |
| Single | 74 | 1.4 | ||
| Soft and semi‐soft cheeses from raw milk | From cow milk | Batch | 43 | 2.3 |
| Single | 416 | 2.9 | ||
| From goat milk | Batch | 30 | 0 | |
| Single | 37 | 0 | ||
| From sheep milk | Batch | 170 | 4.7 | |
| Single | 111 | 0 | ||
| Meat products | From bovine animals | Batch | 379 | 0.5 |
| Single | 1,067 | 0.8 | ||
| From broilers | Batch | 207 | 0 | |
| Single | 891 | 1.0 | ||
| From turkeys | Batch | 27 | 0 | |
| Single | 294 | 1.7 | ||
| From pigs | Batch | 1,214 | 3.6 | |
| Single | 9,747 | 3.0 | ||
| Other RTE products | Salads | Single + Batch | 1,042 | 2.0 |
| Bakery products | Single + Batch | 1,984 | 0.8 | |
| Fruits and vegetables | Single + Batch | 1,772 | 0.5 | |
| Sauces and dressings | Single + Batch | 299 | 0.3 | |
| Egg products | Single + Batch | 72 | 0 | |
| Confectionery products and pastes | Single + Batch | 154 | 0.6 | |
| Spices and herbs | Single + Batch | 48 | 0 | |
| Prepared dishes | Single + Batch | 646 | 0.3 |
For each food category the number of tested samples (using the detection method) and the proportion of positive samples are shown. The total number of tested samples as well as total number of positive samples is calculated over all sampling stages.
Suggested citation: EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2017. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA Journal 2017;15(12):5077, 228 pp. 10.2903/j.efsa.2017.5077
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00572
Acknowledgements: EFSA and the ECDC wish to thank the members of the Scientific Network for Zoonoses Monitoring Data and the Food and Waterborne Diseases and Zoonoses Network, who provided the data and reviewed the report; the members of the Scientific Network for Zoonoses Monitoring Data for their endorsement of this scientific report; the EFSA staff members (Frank Boelaert, Yves Van der Stede, Krisztina Nagy, Valentina Rizzi, Raquel Garcia Fierro, Francesca Latronico, Domenico Deserio, Anca Stoicescu, Francesca Riolo, Michaela Hempen and Frank Verdonck), the ECDC staff members (Taina Niskanen, Celine Gossner, Johanna Young, Herve Zeller, Joana Gomes Dias and Vahur Hollo) and the EFSA contractors: the Istituto Zooprofilattico Sperimentale delle Venezie, Italy (and staff members: Antonia Ricci, Lisa Barco, Marzia Mancin, Patuzzi Ilaria, Lettini Antonia Anna, Losasso Carmen and Belluco Simone), the Istituto Superiore di Sanita, Italy (and staff members: Stefano Morabito, Gaia Scavia, Arnold Knijn, Ida Luzzi, Ilaria Di Bartolo, Antonella Maugliani, Paola Chiani, Fabio Minelli, Claudia Lucarelli, Edoardo Pozio and Adriano Casulli) and Apostolos Angelidis, School of Veterinary Medicine – Aristotle University of Thessaloniki, Greece, for the support provided to this scientific report.
Approved: 13 November 2017
Notes
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003, p. 31–40.
EFSA Registry of Questions: http://raw-app.efsa.eu.int:8080/raw-war/wicket/page?2
Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross‐border threats to health and repealing Decision No 2119/98/EC. OJ L 293, 5.11.2013, p. 1–15.
Commission Decision 2012/506/EU amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the European Union network under Decision No 2119/98/EC of the European Parliament and of the Council. OJ L 262, 27.9.2012, p. 1–57.
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. OJ L 338, 22.12.2005, p. 1–26.
Based on the customs union treaty of the Principality of Liechtenstein with Switzerland, Liechtenstein is part of the Swiss customs territory. Due to the tight connection between the veterinary authorities of Liechtenstein and Switzerland as well as Liechtenstein's integration into the Swiss system in the veterinary field, in principle, all legislation, rules and data on contagious diseases are identical for both Switzerland and Liechtenstein.
Commission Regulation (EU) No 1086/2011 of 27 October 2011 amending Annex II to Regulation (EC) No 2160/2003 of the European Parliament and of the Council and Annex I to Commission Regulation (EC) No 2073/2005 as regards Salmonella in fresh poultry meat Text with EEA relevance. OJ L 281, 28.10.2011, p. 7–11.
Commission Regulation (EU) No 517/2011 of 25 May 2011 implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of certain Salmonella serotypes in laying hens of Gallus gallus and amending Regulation (EC) No 2160/2003 and Commission Regulation (EU) No 200/2010. OJ L 138, 26.5.2011, p. 45–51.
Commission Regulation (EU) No 200/2010 of 10 March 2010 implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of Salmonella serotypes in adult breeding flocks of Gallus gallus. OJ L 61, 11.3.2010, p. 1–9.
Commission Regulation (EC) No 200/2012 of 8 March 2012 on a Union target for the reduction of Salmonella Enteritidis and Salmonella Typhimurium in flocks of broilers, as provided for in Regulation (EC) No 2160/2003 of the European Parliament and of the Council. OJ L 71, 9.3.2012, p. 31–36.
Commission Regulation (EU) No 1190/2012 of 12 December 2012 on a Union target for the reduction of Salmonella Enteritidis and Salmonella Typhimurium in flocks of turkeys, as provided for in Regulation (EC) No 2160/2003 of the European Parliament and of the Council. OJ L 340, 13.12.2012, p. 29–34.
Decision No 1082/2013/EC of the European Parliament and of the Council of 22 October 2013 on serious cross‐border threats to health and repealing Decision No. 2119/98/EC. OJ L 293, 5.11.2013, p. 1–15.
Commission implementing Decision of 8 August 2012 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the European Union network under the Decision No 2119/98/EC of the European Parliament and of the Council (2012/506/EU). OJ L 262, 29.9.2012, p. 1–57.
n = number of units comprising the sample (number of sample units per food batch that are required for testing); c = the maximum allowable number of sample units yielding unsatisfactory test results. In a two‐class attributes sampling plan defined by n = 10, c = 0 and a microbiological limit of'absence in 25 grams’, in order for the food batch to be considered acceptable, L. monocytogenes must not be detected in qualitative (detection) analyses of 25‐g food portions obtained from each one of 10 sample units comprising the batch. If even one of the sample units comprising the batch is found to contain L. monocytogenes (presence in 25 g), then the entire batch is deemed unacceptable.
According to Commission Regulation (EC) No 2073/2005, Products with pH ≤ 4.4 or aw ≤ 0.92, products with pH ≤ 5.0 and aw ≤ 0.94, products with a shelf life of less than 5 days shall be automatically considered to belong to this category. Other categories of products can also belong to this category, subject to scientific evidence. The detailed classification of RTE foods into those that are assumed to support, or not, the growth of L. monocytogenes is presented in Table 12.
Draft scientific opinion proposed for adoption at the 117th BIOHAZ Panel meeting on 6‐7 December 2017 – see http://www.efsa.europa.eu/sites/default/files/event/171206-a.pdf
Also known as verotoxigenic, verocytotoxigenic, verotoxin‐producing, verocytotoxin‐producing E. coli (VTEC).
Commission Regulation (EU) No 209/2013 of 11 March 2013 amending Regulation (EC) No 2073/2005 as regards microbiological criteria for sprouts and the sampling rules for poultry carcases and fresh poultry meat Text with EEA relevance. OJ L 68, 12.3.2013, p. 19–23.
Decision 2012/506/EU. Commission implementing Decision of 8 August 2012 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the European Union network under Decision No. 2119/98/EC of the European Parliament and of the Council. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:262:0001:0057:EN:PDF
During 2015, Austria was an OTF MS for all its regions and also covered by an EU‐cofinanced eradication programme for some single regions.
Commission Implementing Decision of 27 November 2012 amending Annexes I and II to Council Directive 82/894/EEC on the notification of animal diseases within the European Union. OJ L 329, 29.11.2012, p. 19–22.
Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat (Text with EEA relevance). OJ L 212, 11.8.2015, p. 7–34.
Echinococcus granulosus, formerly regarded as a single species, is now recognised as a complex of cryptic species. Based on phenotypic characters and gene sequences, E. granulosus s.l. circulating in Europe has by now been subdivided into E. granulosus sensu stricto (the ‘sheep strain’ and ‘buffalo strain’, genotypes G1 and G3), Echinococcus equinus (the ‘horse strain’, G4), Echinococcus ortleppi (the ‘cattle strain’, G5) and Echinococcus canadensis. (the ‘camel strain’, G6; the ‘pig strain’, G7; two ‘cervid strains’, G8 and G10).
Commission Delegated Regulation (EU) No 1152/2011 of 14 July 2011 supplementing Regulation (EC) No 998/2003 of the European Parliament and of the Council as regards preventive health measures for the control of Echinococcus multilocularis infection in dogs Text with EEA relevance. OJ L 296, 15.11.2011, p. 6–12.
Regulation (EU) No 652/2014 of the European Parliament and of the Council of 15 May 2014 laying down provisions for the management of expenditure relating to the food chain, animal health and animal welfare, and relating to plant health and plant reproductive material, amending Council Directives 98/56/EC, 2000/29/EC and 2008/90/EC, Regulations (EC) No 178/2002, (EC) No 882/2004 and (EC) No 396/2005 of the European Parliament and of the Council, Directive 2009/128/EC of the European Parliament and of the Council and Regulation (EC) No 1107/2009 of the European Parliament and of the Council and repealing Council Decisions 66/399/EEC, 76/894/EEC and 2009/470/EC OJ L 189, 27.6.2014, p. 1–32.
Commission Implementing Regulation (EU) No. 577/2013 of 28 June 2013 on the model identification documents for the non‐commercial movement of dogs, cats and ferrets, the establishment of lists of territories and non‐EU countries and the format, layout and language requirements of the declarations attesting compliance with certain conditions provided for in Regulation (EU) No 576/2013 of the European Parliament and of the Council. OJ L 178, 28.6.2013, p. 109–148.
This designation is without prejudice to positions on status, and is in line with UNSCR 1244/1999 and the ICJ Opinion on the Kosovo declaration of independence.
At present, EUSR reporting on WNF cases strongly differs from the Annual Epidemiological Reports (AER) prepared by ECDC. The AER only includes non‐imported cases, which are considered as illnesses not acquired by the cases in their own country.
ADNS, the EU Animal Disease Notification System, see http://ec.europa.eu/food/animals/animal-diseases/not-system_en
Council Directive of 21 December 1982 on the notification of animal diseases within the Community (82/894/EEC). OJ L 378, 31.12.1982, p. 58.
References
- Alban L, Pozio E, Boes J, Boireau P, Boue F, Claes M, Cook AJC, Dorny P, Enemark HL, van der Giessen J, Hunt KR, Howell M, Kirjusina M, Noeckler K, Rossi P, Smith GC, Snow L, Taylor MA, Theodoropoulos G, Vallee I, Viera‐Pinto MM and Zimmer IA, 2011. Towards a standardised surveillance for Trichinella in the European Union. Preventive Veterinary Medicine, 99, 148–160. [DOI] [PubMed] [Google Scholar]
- Arnold ME, Martelli F, McLaren I and Davies RH, 2014. Estimation of the sensitivity of environmental sampling for detection of Salmonella in commercial layer flocks post‐introduction of national control programmes. Epidemiology and Infection, 142, 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroussi A, Vignoles P, Dalmay F, Wimel L, Darde M‐L, Mercier A and Ajzenberg D, 2015. Detection of Toxoplasma gondii DNA in horse meat from supermarkets in France and performance evaluation of two serological tests. Parasite, 22, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci C, Vismarra A, Passeri B, Sciarrone F, Mangia C, Genchi M, Fabbi M, Vicari N, Bruini I, Brindani F and Kramer L, 2016. Detection of Toxoplasma gondii and Sarcocystis tenella in indigenous Cornigliese sheep in Italy using serological and molecular methods. Small Ruminant Research, 135, 13–16. [Google Scholar]
- Bayarri S, Gracia MJ, Pérez‐Arquillué C, Lázaro R and Herrera A, 2012. Toxoplasma gondii in commercially available pork meat and cured ham: a contribution to risk assessment for consumers. Journal of Food Protection, 75, 597–600. [DOI] [PubMed] [Google Scholar]
- Bidalot M, Thery L, Kaplon J, De Rougemont A and Ambert‐Balay K, 2017. Emergence of new recombinant noroviruses GII.p16‐GII.4 and GII.p16‐GII.2, France, winter 2016 to 2017. Eurosurveillance, 22, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielaszewska M, Mellmann A, Zhang W, Koeck R, Fruth A, Bauwens A, Peters G and Karch H, 2011. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infectious Diseases, 11, 671–676. [DOI] [PubMed] [Google Scholar]
- Bless PJ, Schmutz C, Suter K, Jost M, Hattendorf J, Maeusezahl‐Feuz M and Maeusezahl D, 2014. A tradition and an epidemic: determinants of the campylobacteriosis winter peak in Switzerland. European Journal of Epidemiology, 29, 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert F, Amore G, Van der Stede Y and Hugas M, 2016. EU‐wide monitoring of biological hazards along the food chain: achievements, challenges and EFSA vision for the future. Current Opinion in Food Science, 12, 52–62. [Google Scholar]
- Brundu D, Piseddu T, Stegel G, Masu G, Ledda S and Masala G, 2015. Retrospective study of human cystic Echinococcosis in Italy based on the analysis of hospital discharge records between 2001 and 2012 (vol 140, pg 91, 2014). Acta Tropica, 146, 158. [DOI] [PubMed] [Google Scholar]
- Buchanan RL, Gorris LGM, Hayman MM, Jackson TC and Whiting RC, 2017. A review of Listeria monocytogenes: an update on outbreaks, virulence, dose‐response, ecology, and risk assessments. Food Control, 75, 1–13. [Google Scholar]
- Budke CM, Casulli A, Kern P and Vuitton DA, 2017. Cystic and alveolar echinococcosis: successes and continuing challenges. PLoS Neglected Tropical Diseases, 11, e0005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano‐Terriza D, Puig‐Ribas M, Jiménez‐Ruiz S, Cabezón Ó, Almería S, Galán‐Relaño Á, Dubey JP and García‐Bocanegra I, 2016. Risk factors of Toxoplasma gondii infection in hunting, pet and watchdogs from southern Spain and northern Africa. Parasitology International, 65, 363–366. [DOI] [PubMed] [Google Scholar]
- Carrique‐Mas JJ and Davies RH, 2008. Bacteriological detection of Salmonella Enteritidis in eggs: a review. Revue scientifique et technique‐Office International Des Epizooties, 27, 657–664. [PubMed] [Google Scholar]
- Casulli A, 2016. Preliminary results from extended ultrasound surveys in Eastern Europe, European Multicolloquium on Parasitology, Turku, Finland (oral presentation).
- Casulli A, Possenti A, La Torre G, Boue F, Busani L, Colamesta V, Conraths FJ, D'Aguanno S, De Giusti M, De Vito C, Karamon J, Maas M, Mannocci A, Maffongelli E, Mipatrini D, Oksanen A, Probst C, Saulle R, Siles‐Lucas M, Umhang G, van den End S, van der Giessen J and Villari P, 2015. Echinococcus multilocularis infection in animals GP/EFSA/AHAW/2012/01. EFSA supporting publication 2015:EN‐882, 168 pp. 10.2903/sp.efsa.2015.en-882/pdf [DOI] [Google Scholar]
- van Cauteren D, Millon L, de Valk H and Grenouillet F, 2016. Retrospective study of human cystic echinococcosis over the past decade in France, using a nationwide hospital medical information database. Parasitology Research, 115, 4261–4265. 10.1007/s00436-016-5204-1 [DOI] [PubMed] [Google Scholar]
- Charlier C, Perrodeau E and Leclercq A, 2017a. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infectious Diseases, 17, 897. [DOI] [PubMed] [Google Scholar]
- Charlier C, Perrodeau E, Leclercq A, Cazenave B, Pilmis B, Henry B, Lopes A, Maury MM, Moura A, Goffinet F, Dieye HB, Thouvenot P, Ungeheuer M‐N, Tourdjman M, Goulet V, de Valk H, Lortholary O, Ravaud P, Lecuit M and Grp MS, 2017b. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infectious Diseases, 17, 510–519. [DOI] [PubMed] [Google Scholar]
- Conraths FJ and Deplazes P, 2015. Echinococcus multilocularis: epidemiology, surveillance and state‐of‐the‐art diagnostics from a veterinary public health perspective. Veterinary Parasitology, 213, 149–161. [DOI] [PubMed] [Google Scholar]
- Conraths FJ, Probst C, Possenti A, Boufana B, Saulle R, La Torre G, Busani L and Casulli A, 2017. Potential risk factors associated with human alveolar echinococcosis: systematic review and meta‐analysis. PLoS Neglected Tropical Diseases, 11, e0005801. 10.1371/journal.pntd.0005801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman T, Inns T, Jombart T, Ashton P, Loman N, Chatt C, Messelhaeusser U, Rabsch W, Simon S, Nikisins S, Bernard H, le Hello S, Jourdan da‐Silva N, Kornschober C, Mossong J, Hawkey P, de Pinna E, Grant K and Cleary P, 2016. Phylogenetic structure of European Salmonella Enteritidis outbreak correlates with national and international egg distribution network. Microbial Genomics, 2, e000070 10.1099/mgen.0.000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PR, 2011. Intensive Swine production and pork safety. Foodborne Pathogens and Disease, 8, 189–201. 10.1089/fpd.2010.0717 [DOI] [PubMed] [Google Scholar]
- Deng H, Dam‐Deisz C, Luttikholt S, Maas M, Nielen M, Swart A, Vellema P, van der Giessen J and Opsteegh M, 2016. Risk factors related to Toxoplasma gondii seroprevalence in indoor‐housed Dutch dairy goats. Preventive Veterinary Medicine, 124, 45–51. 10.1016/j.prevetmed.2015.12.014 [DOI] [PubMed] [Google Scholar]
- DIN (Deutsches Institut Fur Normung E.V. ‐ German National Standard‐), 2004. DIN 10167:2004 ‐03. Nachweis von Escherichia coli O157 in Fleisch und Fleischerzeugnissen. Detection of Escherichia coli O157 in meat and meat products.
- Djokic V, Blaga R, Aubert D, Durand B, Perret C, Geers R, Ducry T, Vallee I, Djakovic O, Mzabi A, Villena I and Boireau P, 2016. Toxoplasma gondii infection in pork produced in France. Parasitology, 143, 557–567. 10.1017/s0031182015001870 [DOI] [PubMed] [Google Scholar]
- Dubey JP, 2009. Toxoplasmosis in pigs ‐ the last 20 years. Veterinary Parasitology, 164, 89–103. 10.1016/j.vetpar.2009.05.018 [DOI] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) , 2015. Communicable Disease Threats Report – CDTR. Week 41, 4–10 October, 2015
- ECDC (European Centre for Disease Prevention and Control) , 2016. Multi‐country outbreak of Salmonella Enteritidis phage type 8 MLVA type 2‐9‐7‐3‐2 and 2‐9‐6‐3‐2 infections, 27 October 2016. ECDC, Stockholm. [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2017a. Multi‐country outbreak of Salmonella Enteritidis phage types 56 and 62, MLVA profile 2‐11‐3‐3‐2 and 2‐12‐3‐3‐2 infections 20 July 2017. Multi‐country outbreak of Salmonella Enteritidis phage types 56 and 62, MLVA profile 2‐11‐3‐3‐2 and 2‐12‐3‐3‐2 infections. 20 July 2017, Stockholm, Sweden. 7 pp. Available online: https://ecdc.europa.eu/sites/portal/files/documents/rapid-risk-assessment-multi-country-outbreak-Salmonella-Enteritidis.pdf
- ECDC (European Centre for Disease Prevention and Control), 2017b. Re‐emerging multi‐country WGS‐defined outbreak of Salmonella Enteritidis, MLVA type 2‐12‐7‐3‐2 and 2‐14‐7‐3‐2, 3 February 2017. Stockholm, Sweden, 6 pp. Available online: https://ecdc.europa.eu/en/publications-data/re-emerging-multi-country-wgs-defined-outbreak-salmonella-enteritidis-mlva-type-2
- ECDC (European Centre for Disease Prevention and Control), 2017c. Tularaemia: factsheet for health professionals. Last updated 15 July 2017. Available online: https://ecdc.europa.eu/en/tularaemia/facts
- ECDC (European Centre for Disease Prevention and Control), 2017d. West Nile fever data 2016. Available online: https://ecdc.europa.eu/en/publications-data/west-nile-fever-annual-epidemiological-report-2016-2014-data
- ECDC and EFSA (European Centre for Disease Prevention and Control and European Food Safety Authority), 2014. Multi‐country outbreak of Salmonella Enteritidis infections associated with consumption of eggs from Germany – 25 August 2014. Stockholm and Parma: ECDC/EFSA. EFSA Supporting Publications 2014;11(8):EN‐646, 10 pp. 10.2903/sp.efsa.2014.en-646 [DOI]
- ECDC and EFSA (European Centre for Disease Prevention and Control and European Food Safety Authority), 2017. Multi‐country outbreak of Salmonella Enteritidis phage type 8, MLVA type 2‐9‐7‐3‐2 and 2‐9‐6‐3‐2 infections, 7 March 2017. ECDC and EFSA: Stockholm and Parma; 2017. EFSA Supporting Publications 2017;14(3):EN‐1188, 22 pp. 10.2903/sp.efsa.2017.en-1188 [DOI]
- EFSA (European Food Safety Authority), 2009a. Technical specifications for the monitoring and reporting of verotoxigenic Escherichia coli (VTEC) on animals and food (VTEC surveys on animals and food. EFSA Journal 2009;7(11):1366, 43 pp. 10.2903/j.efsa.2009.1366 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009b. Technical specifications for harmonised national surveys of Yersinia enterocolitica in slaughter pigs on request of EFSA. EFSA Journal 2009;7(11):1374, 23 pp. 10.2903/j.efsa.2009.1374 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Updated technical specifications for harmonised reporting of food‐borne outbreaks through the European Union reporting system in accordance with Directive 2003/99/EC. EFSA Journal 2011;9(4):2101, 24 pp. 10.2903/j.efsa.2011.2101 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready‐to‐eat foods in the EU, 2010–2011. Part A: Listeria monocytogenes prevalence estimates. EFSA Journal 2013;11(6):3241, 75 pp. 10.2903/j.efsa.2013.3241 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready‐to‐eat foods in the EU, 2010–2011. Part B: analysis of factors related to prevalence and exploring compliance. EFSA Journal 2014;12(8):3810, 73 pp. 10.2903/j.efsa.2014.3810 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Update of the technical specifications for harmonised reporting of food‐borne outbreaks through the European Union reporting system in accordance with Directive 2003/99/EC. EFSA Journal 2014;12(3):3598, 25 pp. 10.2903/j.efsa.2014.3598 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017a. Manual for reporting on zoonoses and zoonotic agents, within the framework of Directive 2003/99/EC, and on some other pathogenic microbiological agents for information deriving from the year 2016. EFSA supporting publication 2017:14(1):EN‐1175, 98 pp. 10.2903/sp.efsa.2017.en-1175 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017b. Manual for reporting on food‐borne outbreaks in accordance with Directive 2003/99/EC for information deriving from the year 2016. EFSA supporting publication 2017:14(1):EN‐1174, 44 pp. 10.2903/sp.efsa.2017.en-1174 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017c. Data dictionaries—guidelines for reporting data on zoonoses, antimicrobial resistance and food‐borne outbreaks using the EFSA data models for the Data Collection Framework (DCF) to be used in 2017, for 2016 data. EFSA supporting publication 2017:14(2):EN‐1178, 106 pp. 10.2903/sp.efsa.2017.en-1178 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017d. User manual for data providers for mapping Member State standard terminology to EFSA standard terminology. EFSA supporting publication 2017:14(2):EN‐1181, 26 pp. 10.2903/sp.efsa.2017.en-1181 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2007. Scientific Opinion of the Panel on Animal Health and Welfare (AHAW Panel) regarding the assessment of the risk of Echinococcosis introduction into the UK, Ireland, Sweden, Malta and Finland as a consequence of abandoning national rules. EFSA Journal 2007;7(1):441, 59 pp. 10.2903/j.efsa.2007.441 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2014. Statement on a conceptual framework for bovine tuberculosis. EFSA Journal 2014;12(5):3711, 59 pp. 10.2903/j.efsa.2014.3711 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2015. Echinococcus multilocularis infection in animals. EFSA Journal 2015;13(12):4373, 129 pp. 10.2903/j.efsa.2015.4373 [DOI] [Google Scholar]
- EFSA BIOHAZ, CONTAM and AHAW Panels (EFSA Panel on Biological Hazards, EFSA Panel on Contaminants in the Food Chain, and EFSA Panel on Animal Health and Welfare), 2001. Scientific Opinion on the public health hazards to be covered by inspection of meat (swine). EFSA Journal 2011;9(10):2351, 198 pp. 10.2903/j.efsa.2011.2351 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013. Scientific Opinion on the public health hazards to be covered by inspection of meat from sheep and goats. EFSA Journal 2013;11(6):3265, 186 pp. 10.2903/j.efsa.2013.3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, Girones R, Herman L, Koutsoumanis K, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Takkinen J, Wagner M, Arcella D, Da Silva Felicio MT, Georgiadis M, Messens W and Lindqvist R, 2017. Draft scientific Opinion on Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. Draft Opinion endorsed for public consultation, 189 pp. Available online: https://www.efsa.europa.eu/sites/default/files/engage/170724-0.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2011. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2009. EFSA Journal 2011;9(3):2090, 378 pp. 10.2903/j.efsa.2011.2090 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2014. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2012. EFSA Journal 2014;12(2):3547, 312 pp. 10.2903/j.efsa.2014.3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2015. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2014. EFSA Journal 2015;13(12):4329, 191 pp. 10.2903/j.efsa.2015.4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016a. Type E botulism associated with fish product consumption ‐ Germany and Spain. EFSA supporting publication 2016:13(12):EN‐1157, 7 pp. 10.2903/sp.efsa.2016.en-1157 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016b. Multi‐country outbreak of Salmonella Enteritidis phage type 8, MLVA type 2‐9‐7‐3‐2 and 2‐9‐6‐3‐2 infections. EFSA supporting publication 2016:13(10):EN‐1110, 20 pp. 10.2903/sp.efsa.2016.en-1110 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016c. Technical report on multi‐country outbreak of Shiga toxin‐producing Escherichia coli infection associated with haemolytic uraemic syndrome. EFSA Supporting Publication 2016; 13(4):EN‐1017, 13 pp. 10.2903/sp.efsa.2016.en-1017 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016d. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2015. EFSA Journal 2016;14(12):4634, 231 pp. 10.2903/j.efsa.2016.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2017. Multicountry outbreak of Salmonella Enteritidis phage type 8, MLVA type 2‐9‐7‐3‐2 and 2‐9‐6‐3‐2 infections, 7 March 2017. ECDC and EFSA, Stockholm and Parma. [Google Scholar]
- Eliasson H, Lindback J, Nuorti JP, Arneborn M, Giesecke J and Tegnell A, 2002. The 2000 tularemia outbreak: a case‐control study of risk factors in disease‐endemic and emergent areas, Sweden. Emerging Infectious Diseases, 8, 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , online. Control of Salmonella. Available online: https://ec.europa.eu/food/safety/biosafety/food_borne_diseases/salmonella_en
- Eurostat , 2016. Agriculture, forestry and fishery statistics. 2016 Edition. Theme: agriculture and fisheries collection: statistical books. Table 4.4, 103 pp. Available online: http://ec.europa.eu/eurostat/documents/3217494/7777899/KS-FK-16-001-EN-N.pdf/cae3c56f-53e2-404a-9e9e-fb5f57ab49e3 [Google Scholar]
- Evans EW and Redmond EC, 2016. Older adult consumer knowledge, attitudes, and self‐reported storage practices of ready‐to‐eat food products and risks associated with listeriosis. Journal of Food Protection, 79, 263–272. [DOI] [PubMed] [Google Scholar]
- EVIRA (Finnish Food Safety Authority), 2017. Animal diseases in Finland 2016. Evira publications 4/2017. Available online: https://www.evira.fi/globalassets/tietoa-evirasta/julkaisut/julkaisusarjat/elaimet/evira_publications_4_2017.pdf
- Federer K, Teresa Armua‐Fernandez M, Gori F, Hoby S, Wenker C and Deplazes P, 2016. Detection of taeniid (Taenia spp., Echinococcus spp.) eggs contaminating vegetables and fruits sold in European markets and the risk for metacestode infections in captive primates. International Journal for Parasitology Parasites and Wildlife, 5, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formenti N, Gaffuri A, Trogu T, Vigano R, Ferrari N and Lanfranchi P, 2016. Spread and genotype of Toxoplasma gondii in naturally infected alpine chamois (Rupicapra r. rupicapra). Parasitology Research, 115, 2115–2120. [DOI] [PubMed] [Google Scholar]
- Georgiev M, Afonso A, Neubauer H, Needham H, Thiery R, Rodolakis A, Roest HJ, Staerk KD, Stegeman JA, Vellema P, van der Hoek W and More SJ, 2013. Q fever in humans and farm animals in four European countries, 1982 to 2010. Eurosurveillance, 18, 13–25. [PubMed] [Google Scholar]
- Gossner CM, de Jong B, Hoebe CJ, Coulombier D and European Food Waterborne Disease Study Group , 2015. Event‐based surveillance of food‐ and waterborne diseases in Europe: ‘urgent inquiries’ (outbreak alerts) during 2008 to 2013. Eurosurveillance, 20, 19–28. [DOI] [PubMed] [Google Scholar]
- Herrador Z, Siles‐Lucas M, Aparicio P, Lopez‐Velez R, Gherasim A, Garate T and Benito A, 2016. Cystic Echinococcosis epidemiology in spain based on hospitalization records, 1997–2012. PLoS Neglected Tropical Diseases, 10, e0004942. 10.1371/journal.pntd.0004942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero L, Jesus Gracia M, Perez‐Arquillue C, Lazaro R, Herrera M, Herrera A and Bayarri S, 2016. Toxoplasma gondii: pig seroprevalence, associated risk factors and viability in fresh pork meat. Veterinary Parasitology, 224, 52–59. 10.1016/j.vetpar.2016.05.010 [DOI] [PubMed] [Google Scholar]
- Hestvik G, Warns‐Petit E, Smith LA, Fox NJ, Uhlhorn H, Artois M, Hannant D, Hutchings MR, Mattsson R, Yon L and Gavier‐Widen D, 2015. The status of tularemia in Europe in a one‐health context: a review. Epidemiology and Infection, 143, 2137–2160. 10.1017/s0950268814002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindermann D, Gopinath G, Chase H, Negrete F, Althaus D, Zurfluh K, Tall BD, Stephan R and Nuesch‐Inderbinen M, 2017. Salmonella enterica serovar infantis from food and human infections, Switzerland, 2010–2015: poultry‐related multidrug resistant clones and an emerging esbl producing clonal lineage. Frontiers in Microbiology, 8, art. 1322, 9 pp. 10.3389/fmicb.2017.01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inns T, Ashton PM, Herrera‐Leon S, Lighthill J, Foulkes S, Jombart T, Rehman Y, Fox A, Dallman T, De Pina E, Browning L, Coia JE, Edeghere O and Vivancos R, 2017. Prospective use of whole genome sequencing (WGS) detected a multi‐country outbreak of Salmonella Enteritidis . Epidemiology and Infection, 145, 289–298. 10.1017/S0950268816001941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO (International Organization for Standardization), 1996. 11290‐1:1996. Microbiology of food and animal feeding stuffs – Horizontal method for the detection and enumeration of Listeria monocytogenes – Part 1: Detection method. ISO 11290‐1:1996.
- ISO (International Organization for Standardization), 1998. Microbiology of food and animal feeding stuffs – Horizontal method for the detection and enumeration of Listeria monocytogenes – Part 2: Enumeration method. ISO 11290‐2:1998.
- ISO (International Organization for Standardization), 1999. Microbiology of food and animal feeding stuffs – Preparation of test samples, initial suspension and decimal dilutions for microbiological examination – Part 1: General rules for the preparation of the initial suspension and decimal dilutions. ISO 6887‐1:1999.
- ISO (International Organization for Standardization), 2001. Microbiology of food and animal feeding stuffs – Horizontal method for the detection of Escherichia coli O157. ISO 16654:2001. [DOI] [PubMed]
- ISO (International Organization for Standardization), 2004a. Microbiology of food and animal feeding stuffs — Horizontal method for the detection and enumeration of Listeria monocytogenes — Part 1: Detection method AMENDMENT 1: Modification of the isolation media and the haemolysis test, and inclusion of precision data. ISO 11290‐1:1996/Amd.1:2004(en).
- ISO (International Organization for Standardization), 2004b. Modification of the enumeration medium. ISO 11290‐2:1998/Amd 1:2004.
- ISO (International Organization for Standardization), 2012. Microbiology of food and animal feed. Real‐time polymerase chain reaction (PCR)‐based method for the detection of food‐borne pathogens. Horizontal method for the detection of shiga toxin‐producing Escherichia coli (STEC) and the determination of O157, O111, O26, O103 and O145 serogroups. ISO/TS 13136:2012. 22 pp. [Google Scholar]
- ITRC (International Trichinella Reference Center), online. Available online: https://w3.iss.it/site/Trichinella/ [Accessed: 29 August 2016]
- Janse I, Maas M, Rijks JM, Koene M, van der Plaats RQ, Engelsma M, van der Tas P, Braks M, Stroo A, Notermans DW, de Vries MC, Reubsaet F, Fanoy E, Swaan C, Kik MJ, IJzer J, Jaarsma RI, van Wieren S, de Roda‐Husman AM, van Passel M, Roest HJ and van der Giessen J, 2015. Environmental surveillance during an outbreak of tularaemia in hares, the Netherlands. Euro Surveill, 22, pii: 30607. 10.2807/1560-7917.es.2017.22.35.30607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre A, Garriga M, Aymerich T, Perez‐Rodriguez F, Valero A, Carrasco E and Bover‐Cid S, 2016. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 1, an extensive literature search and study selection with data extraction on L. monocytogenes in a wide range of RTE food. EFSA supporting publication 2016:13(12):EN‐1141, 184 pp. 10.2903/sp.efsa.2016.en-1141 [DOI] [Google Scholar]
- Kern P, Bardonnet K, Renner E, Auer H, Pawlowski Z, Ammann RW, Vuitton DA, Kern P and European Echinococcsis Registry , 2003. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982–2000. Emerging Infectious Diseases, 9, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjo‐Gonzalez M, Devleesschauwer B, Trevisan C, Allepuz A, Sotiraki S, Dorny P, Gabriel S and Dermauw V, 2017. The epidemiology of Taenia Saginata and Taenia Solium in Western Europe: a systematic review. Tropical Medicine and International Health, 22, 189–190. [Google Scholar]
- Lardon Z, Watier L, Brunet A, Bernède C, Goudal M, Dacheux L, Rotivel Y, Guillemot D and Bourhy H, 2010. Imported episodic rabies increases patient demand for and physician delivery of antirabies prophylaxis. PLoS Neglected Tropical Diseases, 4, e723 10.1371/journal.pntd.0000723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Szostakowska B, Myjak P and Korzeniewski K, 2016. Fresh fruits, vegetables and mushrooms as transmission vehicles for Echinococcus multilocularis in highly endemic areas of Poland: reply to concerns. Parasitology Research, 115, 3637–3642. 10.1007/s00436-016-5149-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacova T, Ajzenberg D, Zakovska A, Sedlak K and Bartova E, 2016. Toxoplasma gondii and Neospora caninum in wild small mammals: seroprevalence, DNA detection and genotyping. Veterinary Parasitology, 223, 88–90. 10.1016/j.vetpar.2016.04.018 [DOI] [PubMed] [Google Scholar]
- Mannelli A, Martello E, Tomassone L, Calzolari M, Casalone C, De Meneghi D, Dottori M, Estrada‐Peña A, Fabbi M, Ferreri L, Ferroglio E, Luini M, Nicolau Solano S, Ortega C, Pautasso A, Prati P and Vesco U, 2012. Inventory of available data and data sources and proposal for data collection on vector‐borne zoonoses in animals. EFSA Supporting Publication 2012; 9(3):EN‐234, 189 pp. 10.2903/sp.efsa.2012.en-234 [DOI] [Google Scholar]
- Marquer P, 2010. Pig farming in the EU, a changing sector. In: Eurostat Statistics in Focus 8/2010. pp. 1–11, Eurostat. Available online: http://ec.europa.eu/eurostat/web/hicp/publications/statistics-in-focus/
- Marquer P, Rabade T and Forti R, 2014. Pig farming in the European Union: considerable variations from one Member State to another. Statistics in focus 15/2014. Available online: http://ec.europa.eu/eurostat/web/hicp/publications/statistics-in-focus/
- Maurin M and Gyuranecz M, 2016. Tularaemia: clinical aspects in Europe. Lancet Infectious Diseases, 16, 113–124. [DOI] [PubMed] [Google Scholar]
- Moffatt CRM, Greig A, Valcanis M, Gao W, Seemann T, Howden BP and Kirk MD, 2016. A large outbreak of Campylobacter jejuni infection in a university college caused by chicken liver pate Australia, 2013. Epidemiology and Infection, 144, 2971–2978. 10.1017/s0950268816001187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen EM, Björkman JT, Kiil K, Grant K, Dallman T, Painset A, Amar C, Roussel S, Guillier L, Félix B, Rotariu O, Perez‐Reche F, Forbes K and Strachan N, 2017. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 3, the comparison of isolates from different compartments along the food chain, and from humans using whole genome sequencing (WGS) analysis. EFSA supporting publication 2017:EN‐1151, 170 pp. 10.2903/sp.efsa.2017.en-1151 [DOI] [Google Scholar]
- NMKL (Nordisk Metodikkomité for Næringsmidler – Nordic Committee on Food Analysis), 2005. Escherichia coli O157. Detection in food and feeding stuffs. NMKL No. 164, 2 Edition. 2005. Available online: http://www.nmkl.org/index.php?option=com_zoo&task=item&item_id=337&Itemid=319&lang=en
- Oksanen A, Siles‐Lucas M, Karamon J, Possenti A, Conraths FJ, Romig T, Wysocki P, Mannocci A, Mipatrini D, La Torre G, Boufana B and Casulli A, 2016. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: a systematic review and meta‐analysis. Parasites and Vectors, 9, 519 10.1186/s13071-016-1746-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsteegh M, Maas M, Schares G and Giessena J, 2016. Relationship between seroprevalence in the main livestock species and presence of Toxoplasma gondii in meat (GP/EFSA/BIOHAZ/2013/01) an extensive literature review. Final report. EFSA Supporting Publication 2016; 13(2):EN‐996, 294 pp. 10.2903/sp.efsa.2016.en-996 [DOI] [Google Scholar]
- Papini R, di Ciccio P, Marangi M, Ghidini S, Zanardi E, Vergara A, Giangaspero A, Nardoni S, Rocchigiani G, Mancianti F and Ianieri A, 2017. Occurrence of Toxoplasma gondii in Carcasses of pigs reared in intensive systems in Northern Italy. Journal of Food Protection, 80, 515–522. 10.4315/0362-028X.JFP-16-314 [DOI] [PubMed] [Google Scholar]
- Perrin J‐B, Rautureau S, Bronner A, Hosteing S, Jaÿ M, Garin‐Bastuji B and Dufour B, 2015. Brucellosis in small ruminants in 2014: 95 départements of metropolitan France are now officially disease‐free. 2015. Bull. Epid. Santé Anim. Alim., 71, 17–21. [Google Scholar]
- Petrovska L, Mather AE, AbuOun M, Branchu P, Harris SR, Connor T, Hopkins KL, Underwood A, Lettini AA, Page A, Bagnall M, Wain J, Parkhill J, Dougan G, Davies R and Kingsley RA, 2016. Microevolution of Monophasic Salmonella Typhimurium during Epidemic, United Kingdom, 2005–2010. Emerging Infectious Diseases, 22, 617–624. 10.3201/eid2204.150531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piseddu T, Brundu D, Stegel G, Loi F, Rolesu S, Masu G, Ledda S and Masala G, 2017. The disease burden of human cystic echinococcosis based on HDRs from 2001 to 2014 in Italy. PLoS Neglected Tropical Diseases, 11, e0005771 10.1371/journal.pntd.0005771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possenti A, Manzano‐Roman R, Sanchez‐Ovejero C, Boufana B, La Torre G, Siles‐Lucas M and Casulli A, 2016. Potential risk factors associated with human cystic Echinococcosis: systematic review and meta‐analysis. PLoS Neglected Tropical Diseases, 10, e0005114. 10.1371/journal.pntd.0005114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozio E, 2014. Searching for Trichinella: not all pigs are created equal. Trends in Parasitology, 30, 4–11. 10.1016/j.pt.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Pozio E, 2016a. Trichinella pseudospiralis an elusive nematode. Veterinary Parasitology, 231, 97–101. 10.1016/j.vetpar.2016.03.021 [DOI] [PubMed] [Google Scholar]
- Pozio E, 2016b. Adaptation of Trichinella spp. for survival in cold climates. Food and Waterborne Parasitology, 4, 4–12. 10.1016/j.fawpar.2016.07.001 [DOI] [Google Scholar]
- Reiterova K, Spilovska S, Blanarova L, Derdakova M, Cobadiova A and Hisira V, 2016. Wild boar (Sus scrofa) ‐ reservoir host of Toxoplasma gondii, Neospora caninum and Anaplasma phagocytophilum in Slovakia. Acta Parasitologica, 61, 255–260. 10.1515/ap-2016-0035 [DOI] [PubMed] [Google Scholar]
- Rizzi V, Da Silva Felicio T, Félix B, Gossner CM, Jacobs W, Johansson K, Kotila S, Michelon D, Monguidi M, Mooijman K, Morabito S, Pasinato L, Torgny Björkman J, Torpdahl M, Tozzoli R and Van Walle I, 2017. The ECDC‐EFSA molecular typing database for European Union public health protection Euroreference 2 – March 2017, 2–12.
- Robertson LJ, Troell K, Woolsey ID and Kapel CMO, 2016. Fresh fruit, vegetables, and mushrooms as transmission vehicles for Echinococcus multilocularis in Europe: inferences and concerns from sample analysis data from Poland. Parasitology Research, 115, 2485–2488. 10.1007/s00436-016-5015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P, Tamarozzi F, Galati F, Pozio E, Akhan O, Cretu CM, Vutova K, Siles‐Lucas M, Brunetti E, Casulli A and Network Heracles Extended , 2016. The first meeting of the European Register of Cystic Echinococcosis (ERCE). Parasites and Vectors, 9, 243 10.1186/s13071-016-1532-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow H, Ollgren J, Klemets P, Pietarinen I, Saikku J, Pekkanen E, Nikkari S, Syrjala H, Kuusi M and Nuorti JP, 2014. Risk factors for pneumonic and ulceroglandular tularaemia in Finland: a population‐based case‐control study. Epidemiology and Infection, 142, 2207–2216. 10.1017/s0950268813002999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow H, Ollgren J, Hytonen J, Rissanen H, Huitu O, Henttonen H, Kuusi M and Vapalahti O, 2015. Incidence and seroprevalence of tularaemia in Finland, 1995 to 2013: regional epidemics with cyclic pattern. Eurosurveillance, 20, 13–22. [DOI] [PubMed] [Google Scholar]
- Schneeberger PM, Wintenberger C, van der Hoek W and Stahl JP, 2014. Q fever in the Netherlands‐2007‐2010: what we learned from the largest outbreak ever. Médecine et maladies infectieuses, 44, 339–353. 10.1016/j.medmal.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Scott MK, Geissler A, Poissant T, DeBess E, Melius B, Eckmann K, Salehi E, Cieslak PR and Centers for Disease Control and Prevention (CDC) , 2015. Notes from the field: campylobacteriosis outbreak associated with consuming undercooked chicken liver pâté – Ohio and Oregon, December 2013–January 2014. MMWR Morbidity and Mortality Weekly Report, 64, 399. [PMC free article] [PubMed]
- Siles‐Lucas M, Casulli A, Conraths FJ and Muller N, 2017. Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. Advances in Parasitology, 96, 159–257. 10.1016/bs.apar.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Skarin H, Trönnberg L, Hansson I, Alm E, Ågren J, Pääjärvi A, Lahti E, Olsson Engvall E and Jernberg C, 2017. Swedish winter peaks of Campylobacter in humans and chicken are connected. The 19th International Workshop on Campylobacter, Helicobacter and Related Organisms, Abstract book p. 120.
- SVA (National Veterinary Institute), 2016. Surveillance of infectious diseases in animals and humans in Sweden 2016, National Veterinary Institute (SVA), Uppsala, Sweden. SVA:s rapportserie 45 ISSN 1654‐7098. Available online: http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/surveillance-2016-w.pdf
- Szell Z, Marucci G, Pozio E and Sreter T, 2013. Echinococcus multilocularis and Trichinella spiralis in golden jackals (Canis aureus) of Hungary. Veterinary Parasitology, 197, 393–396. 10.1016/j.vetpar.2013.04.032 [DOI] [PubMed] [Google Scholar]
- Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, Rokni MB, Zhou X‐N, Fevre EM, Sripa B, Gargouri N, Fuerst T, Budke CM, Carabin H, Kirk MD, Angulo FJ, Havelaar A and de Silva N, 2015. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: a data synthesis. PloS Medicine, 12, e1001920. 10.1371/journal.pmed.1001920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Poel WHM, Van der Heide R, Verstraten E, Takumi K, Lina PHC and Kramps JA, 2005. European bat lyssaviruses, the Netherlands. Emerging Infectious Diseases, 11, 1854–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallander C, Frossling J, Dorea FC, Uggla A, Vagsholm I and Lunden A, 2016. Pasture is a risk factor for Toxoplasma gondii infection in fattening pigs. Veterinary Parasitology, 224, 27–32. 10.1016/j.vetpar.2016.05.005 [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), online. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. Available online: http://apps.who.int/iris/bitstream/10665/42427/1/929044522X.pdf
- WHO (World Health Organization), 2015. The World Health Organization Estimates of the Global Burden of Foodborne Dieseases: FERG Project Report. Available online: http://www.who.int/foodsafety/areas_work/foodborne-diseases/ferg/en/


