Abstract
The coccidiostat Monimax® (monensin sodium and nicarbazin) is considered safe for turkeys for fattening at the highest use level of 50 mg monensin and 50 mg nicarbazin/kg complete feed. The simultaneous use of Monimax® and certain antibiotic drugs (i.e. tiamulin) is contraindicated. For both active substances, the metabolic pathways in the chicken are similar to those in the turkey and rat. Nicarbazin, when ingested, is rapidly split in its two components dinitrocarbanilide (DNC) and 2‐hydroxy‐4,6‐dimethylpyrimidine (HDP) which behave independently. Monimax® does not represent a genotoxic risk. No safety concerns would arise from the nicarbazin impurities p‐nitroaniline and methyl(4‐nitrophenyl) carbamate. The lowest no observed effect level (NOEL) identified for monensin sodium in a developmental study in rabbits was 0.3 mg monensin sodium/kg body weight (bw) per day for maternal toxicity in rabbits. The lowest no observed adverse effect level (NOAEL) identified in a 52‐week study in rat using DNC + HDP was 20 mg DNC + 8 mg HDP/kg bw per day. No significant interaction between monensin sodium and nicarbazin is expected from toxicological studies. The use of Monimax® at the highest proposed dose will not pose a risk to persons consuming animal products from treated turkeys for fattening. No withdrawal time is required for Monimax® in turkeys for fattening. Residue data comply with the established maximum residue limits for monensin and DNC. Monensin sodium presents a hazard by inhalation and may also be associated with dermal toxicity. Monimax® is not a skin irritant; however, no data are available for the eye irritation potential of monensin. Monimax® is not a skin sensitiser. Based on the available data, the FEEDAP Panel cannot conclude on the safety of Monimax® for the environment. Monimax® has the potential to control coccidiosis in turkeys for fattening at a minimum concentration of 40 mg monensin and 40 mg nicarbazin/kg complete feed.
Keywords: coccidiostat, Monimax, monensin sodium, nicarbazin, safety, efficacy, turkeys for fattening
Summary
Following a request from European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of Monimax® (monensin sodium and nicarbazin), when used as a feed additive for turkeys for fattening.
Monimax® is considered safe for turkeys for fattening at the highest use level of 50 mg monensin and 50 mg nicarbazin/kg complete feed. The margin of safety is about 1.5. The simultaneous use of Monimax® and certain antibiotic drugs (i.e. tiamulin) is contraindicated. Monensin has a selective antimicrobial activity against Gram‐positive bacterial species while many Enterobacteriaceae are naturally resistant. Induction of cross‐resistance with clinically relevant antimicrobials or increased shedding of enteropathogenic bacteria are not reported. Nicarbazin has no antimicrobial activity.
Monensin sodium is absorbed at a limited extent and excreted rapidly, it is extensively metabolised and gives rise to demethylated, oxidised and decarboxylated metabolites. Nicarbazin, when ingested, is rapidly split in its two components 2‐hydroxy‐4,6‐dimethylpyrimidine (HDP) and dinitrocarbanilide (DNC) which behave independently. Liver is the target tissue. DNC residues decline rapidly from tissues following nicarbazin withdrawal. DNC appears as the marker residue. HDP‐related residues are much lower than those derived from DNC. For both compounds of Monimax®, the metabolic pathways in the turkey are similar to those in the chicken and rat.
The FEEDAP Panel concludes that the active substances in Monimax®, monensin sodium and nicarbazin, do not represent a genotoxic risk. No safety concerns would arise from the nicarbazin impurities p‐nitroaniline (PNA) and methyl(4‐nitrophenyl) carbamate (M4NPC). Monensin sodium has no structural alert for carcinogenesis. Monensin sodium is not a reproductive or developmental toxicant. The lowest no observed effect level (NOEL) identified in the developmental study in rabbits is 0.3 mg monensin sodium/kg body weight (bw) per day for maternal toxicity in rabbits. The primary toxicity resulting from the oral use of nicarbazin is renal toxicity. The absence of similar findings after treatment with DNC and HDP confirms that this equimolar association of compounds is better tolerated than nicarbazin at equivalent doses. The lowest no observed adverse effect level (NOAEL) identified in a 52‐week study in rat using DNC + HDP was 20 mg DNC + 8 mg HDP/kg bw per day based on the absence of microcrystals in urine and related microscopic renal observations. No significant interaction between monensin sodium and nicarbazin is expected from toxicological studies.
The use of Monimax® at the highest proposed dose (50 mg monensin and 50 mg nicarbazin/kg complete feed) will not pose a risk to persons consuming animal products from treated turkeys for fattening. No safety concern would arise from the impurity PNA if the maximum content in nicarbazin of 0.1% is respected. The impurity M4NPC is considered safe for the consumer provided that a maximum concentration of 0.4% in nicarbazin is not exceeded. No withdrawal time is required for Monimax® in turkeys for fattening. Residue data comply with the established maximum residue limits (MRLs) for monensin and DNC.
The monensin sodium contained in Monimax® presents a hazard by inhalation. Monimax® is not a skin irritant; however, no data are available for the eye irritation potential of monensin. Monimax® may also act as a dermal toxicant due to its monensin component. Monimax® is not a skin sensitiser.
The use of monensin sodium from Monimax® in complete feed for turkeys for fattening does not pose a risk for the aquatic compartment and sediment, while a risk cannot be excluded for the terrestrial compartment. A final conclusion on the risk resulting from the use of nicarbazin from Monimax® cannot be made because (i) DNC refined predicted environmental concentrations (PECs) show uncertainties linked to the very high persistence of the compound, (ii) DNC might accumulate in the sediment compartment and (iii) DNC can potentially bioaccumulate and may cause secondary poisoning. No concerns would arise for the HDP moiety of nicarbazin excreted from chickens fed Monimax®. Based on the available data, the FEEDAP Panel cannot conclude on the safety of Monimax® for the environment.
The FEEDAP Panel concludes that Monimax® has the potential to control coccidiosis in turkeys for fattening at a minimum concentration of 40 mg monensin and 40 mg nicarbazin/kg complete feed.
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from the company Huvepharma N.V.2 for authorisation of the product Monimax® (monensin sodium and nicarbazin), when used as a feed additive for turkeys for fattening (category: coccidiostats and histomonostats).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). EFSA received directly from the applicant the technical dossier in support of the application. The particulars and documents in support of the application were considered valid by EFSA as of 22 February 2013.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product Monimax® (monensin sodium and nicarbazin) when used under the proposed conditions of use (see Section 3.1.4).
1.2. Additional information
The feed additive Monimax® , containing two active substances, monensin sodium and nicarbazin, has never been assessed by EFSA and it is not authorised in the European Union.
There are two authorised coccidiostats containing monensin sodium: Coxidin® 3 and Elancoban®.4 Both additives are authorised for their use in chickens for fattening, chickens reared for laying and turkeys. The holder of the Coxidin® authorisation is Huvepharma N.V., the same applicant as that of the current submission.
The Scientific Committee on Animal Nutrition (SCAN) issued two opinions in which monensin sodium for its use in poultry (European Commission, 1981) and in turkeys (European Commission, 1983) was assessed. Coxidin® was evaluated by the EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP Panel) for chickens and turkeys for fattening (EFSA, 2005, 2006a), for turkeys (EFSA, 2007a) and for chickens reared for laying (EFSA FEEDAP Panel, 2011a). Elancoban® was evaluated for chickens for fattening, chickens reared for laying and turkeys for fattening (EFSA, 2004a). Both products are currently under re‐evaluation following Article 10(2) of Regulation (EC) No 1831/2003. The maximum residue limits (MRLs) and withdrawal period for monensin sodium in chickens for fattening and turkeys for fattening were evaluated by the FEEDAP Panel in various scientific opinions (EFSA, 2006b, 2008a,b; EFSA FEEDAP Panel, 2013). Provisional MRLs for monensin sodium in chickens for fattening, chickens reared for laying and turkeys for fattening, are in force: 25 μg monensin sodium/kg of wet skin + fat and 8 μg monensin sodium/kg of wet liver, kidney and muscle5; the withdrawal time is one day before slaughter.6
There are two authorised coccidiostats containing nicarbazin: Koffogran7 (nicarbazin) and Maxiban® 8 (nicarbazin and narasin); both products are authorised for chickens for fattening only. Koffogran has been assessed by the FEEDAP Panel (EFSA, 2004b; EFSA FEEDAP Panel, 2010a). Maxiban® has been assessed by the SCAN (European Commission, 1991, 1995) followed by a FEEDAP Panel assessment (EFSA FEEDAP Panel, 2010b). MRLs are in force for nicarbazin (dinitrocarbanilide (DNC) as the marker residue) in chicken tissues: 15,000 μg DNC/kg of fresh liver, 6,000 μg DNC/kg of fresh kidney, 4,000 μg DNC/kg fresh muscle and fresh skin + fat. The withdrawal time before slaughter is one day for nicarbazin from Koffogran and zero day for nicarbazin from Maxiban®.9
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier10 in support of the authorisation request for the use of Monimax® (monensin sodium and nicarbazin) as a feed additive. The technical dossier was prepared following the provisions of Article 7 of Regulation (EC) No 1831/2003 and the applicable EFSA guidance documents.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers and other scientific reports.
The European Union Reference Laboratory (EURL) considered that the conclusions and recommendations reached in another assessment are valid and applicable for the current application.11
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of Monimax® (monensin sodium and nicarbazin) is in line with the principles laid down in Regulation (EC) No 429/200812 and the relevant guidance documents: Guidance for the preparation of dossiers for coccidiostats and histomonostats (EFSA FEEDAP Panel, 2011b), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011c), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008c), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012a), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012b), Technical Guidance: Microbial Studies (EFSA, 2008d), Technical Guidance: Extrapolation of data from major species to minor species regarding the assessment of additives for use in animal nutrition (EFSA 2008e) and Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance (EFSA FEEDAP Panel, 2012c).
3. Assessment
The present opinion assesses safety and efficacy of the coccidiostat Monimax® (monensin sodium and nicarbazin) when used as a feed additive for turkeys for fattening. The recommended inclusion level of Monimax® in complete feed for turkeys up to 16 weeks of age is 40 + 40 to 50 + 50 mg monensin + nicarbazin/kg. MRLs for edible turkey tissues are proposed, (15,000 μg DNC/kg of fresh liver, 6,000 μg DNC/kg of fresh kidney, 4,000 μg DNC/kg fresh muscle and fresh skin + fat); a withdrawal period of zero day is proposed.
3.1. Characterisation
3.1.1. Characterisation of the active substances
3.1.1.1. Monensin sodium
Monensin sodium is a polyether ionophore produced by fermentation from a culture of Streptomyces cinnamonensis 28682.13 The manufacturing process is the same as described in the FEEDAP opinion on Coxidin® (EFSA, 2005). ■■■■■■■■■■
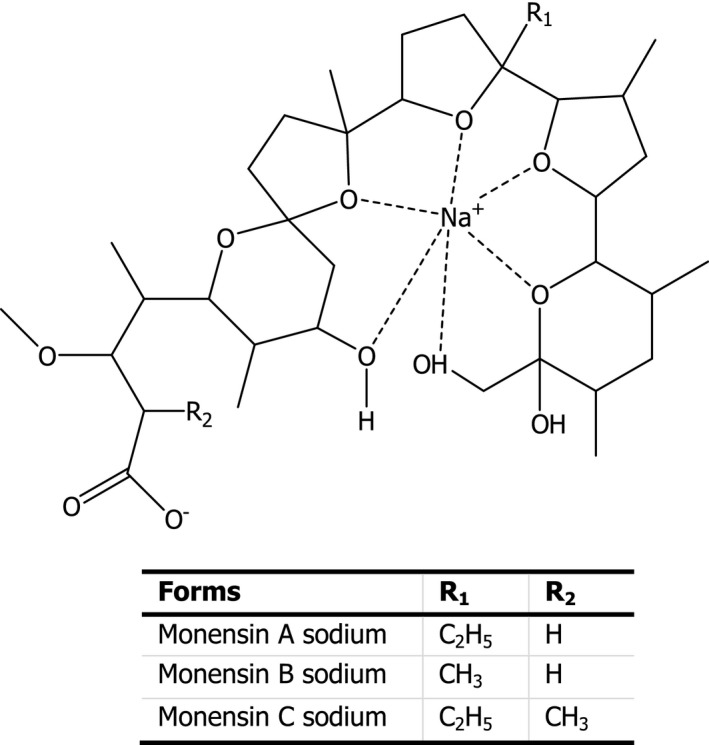
Monensin sodium (CAS No 22373‐78‐0) consists of the main chemical form monensin A sodium (sodium 4‐(2‐(2‐ethyl‐5’‐(6‐hydroxy‐6‐(hydroxymethyl)‐3,5‐dimethyltetrahydro‐2H‐pyran‐2‐yl)‐3’‐methyloctahydro‐[2,2’‐bifuran]‐5‐yl)‐9‐hydroxy‐2,8‐dimethyl‐1,6‐dioxaspiro[4.5]decan‐7‐yl)‐3‐methoxy‐2‐methylpentanoate, C36H61NaO11, molecular weight 692.86), monensin B sodium (sodium 4‐(9‐hydroxy‐2‐(5’‐(6‐hydroxy‐6‐(hydroxymethyl)‐3,5‐dimethyltetrahydro‐2H‐pyran‐2‐yl)‐2,3’‐dimethyloctahydro‐[2,2’‐bifuran]‐5‐yl)‐2,8‐dimethyl‐1,6‐dioxaspiro[4.5]decan‐7‐yl)‐3‐methoxy‐2‐methylpentanoate, C35H59NaO11, molecular weight 678.84) and monensin C sodium (sodium 2‐ethyl‐4‐(2‐(2‐ethyl‐5’‐(6‐hydroxy‐6‐(hydroxymethyl)‐3,5‐dimethyltetrahydro‐2H‐pyran‐2‐yl)‐3’‐methyloctahydro‐[2,2’‐bifuran]‐5‐yl)‐9‐hydroxy‐2,8‐dimethyl‐1,6‐dioxaspiro[4.5]decan‐7‐yl)‐3‐methoxypentanoate, C37H63NaO11, molecular weight 706.89). The structural formula of monensin sodium is given in Figure 1.
Figure 1.
Structural formula of monensin sodium
■■■■■■■■■■■■■■■
Pure monensin sodium is a white crystalline powder having a melting point of 263–283°C, it is sparingly soluble in water (8.78 mg/L) and readily soluble in organic solvents.16
Three batches of monensin sodium technical substance were analysed for the occurrence of the producing microorganism Streptomyces cinnamonensis 28682.17 No live cells of the strain were found.
Characterisation of the production organism
The active substance is produced by fermentation of a strain of Streptomyces. The strain was originally identified as S. cinnamonensis ■■■■■■■■■■
■■■■■■■■■■■■■■■ the identification of strain LMG S‐19095 as S. cinnamonensis is not demonstrated. ■■■■■■■■■■ The production strain is not genetically modified.
Genetic stability was demonstrated ■■■■■■■■■■
The absence of antimicrobial compounds relevant to the use of antibiotics in humans or animals, other than the monensin sodium in the mycelial product, was assessed comparing the minimum inhibitory concentrations (MIC) of three batches of the fermentation product with three batches of pure monensin sodium (88%).22 The batches were tested against 34 strains of aerobic and anaerobic species of both Gram‐positive and Gram‐negative bacteria.23 The MIC values were determined using a twofold broth dilution in appropriate media for the different bacterial species. Monensin sodium shows an antimicrobial activity in a concentration range of 0.5–16 mg/L against all the tested Gram‐positive bacterial species. Differently, all the Gram‐negative species are resistant to this ionophore, with MIC values higher than 128 mg/L with the exception of Fusobacterium necrophorum (MIC = 32 mg/L).
Since no differences in the inhibitory spectrum and in the MIC values were observed between the pure and mycelial form for any of the strain tested, the product is considered to be free of antimicrobial activity, other than monensin sodium.
Since S. cinnamonensis was shown to produce the secondary metabolites endophenazines and furanonaphthoquinones (Seeger et al., 2011), the additive was analysed for the presence of these antimicrobial compounds.24 No presence of endophenazines or furanonaphthoquinones was detected in the three analysed monensin sodium batches.
3.1.1.2. Nicarbazin
Nicarbazin (CAS No: 330‐95‐0) is an equimolar complex of 1,3‐bis(4‐nitrophenyl)urea, also known as N,N’‐bi(4‐nitrophenyl)urea or 4,4’‐dinitrocarbanilide (DNC, molecular formula C13H10N4O5, molecular weight 302.25), and 4,6‐dimethylpyrimidin‐2‐ol, also known as 2‐hydroxy‐4,6‐dimethylpyrimidine (HDP, molecular formula C6H8N2O, molecular weight 124.14). The structural formula of nicarbazin is given in Figure 2.
Figure 2.

Structural formula of nicarbazin
Nicarbazin is a yellow or yellow green powder having a melting point of 260–265°C. It is slightly soluble in dimethyl formamide and insoluble in water.
■■■■■■■■■■
■■■■■■■■■■ The specification for PNA is ≤ 0.1%; values measured were 0.02–0.09%. Another impurity was identified (by IR and NMR spectra, MS analysis) as methyl(4‐nitrophenyl) carbamate and specified with ≤ 1.0%; values measured amounted 0.07–0.34%.
3.1.2. Characterisation of the additive
■■■■■
The approximate composition of Monimax® is summarised in Table 1.
Table 1.
Composition of Monimax®
| Ingredients | g/kg Monimax® |
|---|---|
| Active ingredients | |
| Monensina | 80 |
| Nicarbazinb | 80 |
| Other ingredients | |
| Starch for granulation | 15 |
| Wheat meal | 580 |
| Calcium carbonate | quantum satis 1,000 |
From monensin sodium technical substance containing ≥ 27% monensin activity.
From nicarbazin containing ≥ 95.1% nicarbazin.
Monimax® is specified to contain 76–84 g monensin and 76–84 g nicarbazin per kg product. Batch‐to‐batch consistency was confirmed by analysis of six batches (both monensin and nicarbazin between 80 and 82 g/kg).27
Three batches of Monimax® have been analysed for heavy metals and arsenic, aflatoxins, dioxins and dioxin‐like PCBs and microbial contamination.28 Results showed concentrations of arsenic between 0.216 and 0.390 mg/kg, cadmium between 0.026 and 0.224 mg/kg, lead between 1.77 and 2.75 mg/kg and mercury < 0.005 mg/kg. Levels of aflatoxin B1, B2, G1, G2 were < 1 μg/kg each (total < 1.5 μg/kg. Values for dioxins (polychlorinated dibenzo‐p‐dioxins and dibenzofurans (PCDD/F)) were < 0.14 ng WHO‐PCDD/F‐TEQ/kg, the sum of dioxins and dioxin‐like polychlorinated biphenyls (DL‐PCBs) was < 0.27 ng WHO‐PCDD/F‐DL‐PCB‐TEQ per kg and non‐dioxin‐like PCBs were < 0.005 mg/kg. Salmonella was absent in 25 g samples.29 None of the amounts of these impurities were of concern.
Monimax® is a granulated product of green‐brown colour with an average bulk density of 479 kg/m3 and average tapped density of 560 kg/m3.30 Sieve analysis of three batches of Monimax® showed that about 4% of particles (w/w) were below 100 μm.30 The dusting potential of Monimax® determined in three batches ranged between 0.31 and 0.50 g/m3.31 The dust contained about 32% (v/v) particles of respirable size (< 10 μm).32 The concentration of monensin and nicarbazin in the dust was 16.3–17.6% and 19.0–22.9%, respectively, indicating a considerable enrichment of the active substances compared to the additive (8% each).33
3.1.3. Stability and homogeneity
All the stability studies described below were performed on three batches of Monimax®.
3.1.3.1. Shelf‐life
Monimax® was stored in multiple layer bags with internal polyethylene layer for 36 months at 25°C/60% relative humidity (RH), for 12 months at 30°C/65% RH and for 6 months at 40°C/75% RH. Losses of monensin sodium and nicarbazin after 36 months did not exceed 3% and 5%, respectively. No losses for monensin and nicarbazin were observed at 30°C during 12 months. Storage under accelerated conditions for 6 months resulted in losses of less than 5%.34
3.1.3.2. Stability in premixtures
Monimax® was incorporated in a vitamin/mineral premixture (containing choline chloride) for poultry providing 8 g each of monensin and nicarbazin/kg premixture. Losses after 6 months at 25°C/60% RH amounted to 6.3–7.8% for monensin and to 7.6–7.7% for nicarbazin. The losses at 40°C/75% RH did not exceed 10%.35
3.1.3.3. Stability in feedingstuffs
Monimax® was incorporated in a complete poultry diet based mainly on wheat and soybean (containing about 21% protein and 10% ether extract) providing 40 mg each of monensin and nicarbazin per kg. Samples of the mash feed were stored under 25°C/60% RH for 3 months and under 40°C/75% RH for 4 weeks. Feed processing stability of the active substances in Monimax® was examined after pelleting (85°C). Samples of the pelleted feed were stored under the same conditions as the mash feed. Recoveries were calculated and expressed in % of the initial analysed values.
After 3 months storage of the mash feed at 25°C/65% RH, recoveries were between 94% and 103% for monensin and between 93% and 101% for nicarbazin. The values corresponding to 4 weeks storage at 40°C/75% RH were 92–98% for monensin and 91–95% for nicarbazin. Pelleting did not affect the stability of monensin and nicarbazin. After 3 months storage of the pelleted feed at 25°C/65% RH, recoveries were between 96% and 98% for monensin and between 97% and 109% for nicarbazin. The values corresponding to 4 weeks storage at 40°C/75% RH were 92–95% for monensin and 89–96% for nicarbazin.36
3.1.3.4. Homogeneity
Ten subsamples from each of the three batches of the pelleted feed (described under Section 3.1.3.3) were analysed for monensin and nicarbazin. The coefficients of variation for monensin were between 5.2% and 5.5%, for nicarbazin between 5.4% and 9%.37
3.1.4. Conditions of use
Monimax® is intended to be used to prevent coccidiosis in turkeys for fattening. The recommended inclusion level of Monimax® in complete feed for turkeys up to 16 weeks of age is 40 + 40 to 50 + 50 mg monensin + nicarbazin/kg. The applicant proposes a withdrawal period of 1 day.
3.2. Safety
3.2.1. Safety for the target species
3.2.1.1. Tolerance study in turkeys for fattening
A total of 224 day‐old male and female turkeys for fattening (hybrid Grademaker) was allocated to four groups with four replicates per sex (five birds + two spare birds for the first week/replicate) each fed diets containing 0, 50 + 50 (1x maximum proposed level), 75 + 75 (1.5x) and 100 + 100 (2x) mg monensin + nicarbazin/kg complete feed, respectively, for 56 days.38 The basal diet consisted of dehulled extracted toasted soya, wheat, wheatfeed, barley, wheat gluten meal, full‐fat toasted soya, maize gluten meal and soya oil as feed materials; the starter formulation was calculated to contain 28.2% crude protein (CP), 0.7% methionine (met), and 12.2 MJ metabolisable energy (ME)/kg; the grower formulation 26.5% CP, 0.6% met, and 12.0 MJ ME/kg.39 The starter diet was fed for 14 days, the grower until the end of the study. The birds had ad libitum access to feed and water. The intended concentrations of monensin and nicarbazin in the starter and the grower diet were analytically confirmed.
Clinical observations were made daily; body weight and feed intake were recorded in weekly intervals. On day 56, blood samples were taken from one bird per replicate (four males and four females per treatment) for haematology40 and clinical blood biochemistry.41 On day 57/58, one bird/replicate was killed and subjected to necropsy, organ weights were determined for heart, liver, kidneys and spleen. Histopathology was performed for duodenum, ileum, caeca, colon and for liver, kidneys, spleen, heart and lungs.
Two separate statistical analyses were provided; the first one based on separate datasets for male and female birds, the second one on all birds.42 Both analyses were based on analysis of variance (ANOVA) after verification of the normal distribution and the homogeneity of the data. If the conditions for applying an ANOVA were not proved nonparametric procedures (i.e. Kruskal–Wallis one‐way ANOVA on ranks, Shirley's or Steel's test) were applied. Covariance analysis was applied to organ weights, and Fisher's exact test to clinical pathology. Group comparisons were done by the Tukey test/Dunett's method.
One bird died in the control, in the use level and in the intermediate‐dose groups. No treatment‐related signs of toxicity were observed during the course of the study. The main results are summarised in Table 2.
Table 2.
Main results of an 8‐week tolerance study in turkeys for fattening with Monimax®
| Mo + Ni (mg/kg feed)a | 0 + 0 | 50 + 50 | 75 + 75 | 100 + 100 | ||||
|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | Male | Female | Male | Female |
| Final body weight (g) | 3,736 | 3,376 | 3,773 | 3,324 | 3,570 | 3,230 | 3,528 | 3,389 |
| Feed intake (g/bird and day) | 160 | 131 | 147 | 127 | 156 | 132 | 142 | 134 |
| Feed to gain ratio | 2.15 | 1.99 | 2.06 | 1.99 | 2.23 | 2.09 | 2.06 | 2.02 |
| URAC (μmol/L) | 236 | 266 | 282 | 401 | 319* | 345 | 332* | 374 |
| ALP (U/L) | 2,203 | 2,079 | 2,197 | 1,882 | 1,987 | 1,822 | 1,876* | 1,632 |
| Heterophils (109/L) | 4.83 | 4.55 | 3.59 | 6.63 | 3.66 | 6.13 | 4.49 | 2.36 |
| Liver (g adjusted means) | 83.75 | 67.98 | 71.01* | 62.92 | 68.44* | 66.81 | 76.04* | 68.42 |
* Significant change compared to the control within sex (p < 0.05).
Mo: Monensin; Ni: Nicarbazin.
No significant differences were observed in final body weight, growth rate, feed intake and feed to gain ratio. However, means of the males in the intermediate‐ and high‐dose group appeared lower than in the control and the use level group. Haematological parameters were not different between the treatments. Most clinical biochemical parameters did not reveal relevant significant differences or consistent treatment‐related trends. Significant differences in uric acid concentration were found for the males of the intermediate‐ and the high‐dose group. Alkaline phosphatase (ALP) was significantly lower in the high‐dose group compared to the control group. Considering gender separately, a significant decrease for the adjusted liver weight in males of all treated groups was seen; however, the second analysis did not confirm significance. A macroscopic or microscopic correlate to weight changes was not found. Minimal to slight increased cellularity of the caecal tonsils (part of the gut associated lymphoid tissue) and the spleen (together with an increased number of lymphoid follicles) was seen in the high‐dose group for two of four males and three of four females, respectively, at the high dose.
Conclusions
No toxic signs were found in turkeys administrated the twofold of the highest proposed level of Monimax®. However, slight histological findings in the lymphoid tissue of the caeca and the spleen at the same concentration are considered as adverse signs. No effects were seen at 75 + 75 mg monensin + nicarbazin/kg complete feed.
Monimax® at the highest proposed dose (50 + 50 mg monensin + nicarbazin/kg complete feed) is considered safe for turkeys for fattening with a margin of safety of about 1.5.
3.2.1.2. Interactions
Interactions between the polyether ionophore coccidiostats and the diterpene antibiotic tiamulin as well as other antimicrobials (mainly macrolides) were already described by the FEEDAP Panel in 2004 (EFSA, 2004c). Therefore, the simultaneous use of monensin and certain antibiotic drugs (i.e. tiamulin) is contraindicated. The same contraindications would also apply to Monimax® due to its monensin content. However, since this interaction is dose‐dependent, it could be expected that the lower feed concentration of monensin from Monimax® would result in reduced severity of interactions.
3.2.1.3. Microbiological safety of the additive
The FEEDAP Panel concluded in 2005 on monensin sodium from Coxidin® that ‘The antimicrobial spectrum of monensin is mainly limited to aerobic and anaerobic Gram‐positive bacteria (EFSA, 2005). Monensin did not show activity against Gram‐negative pathogens including Escherichia coli and Salmonella spp. Induction of resistance and cross‐resistance was not observed in experimental conditions. Selection of resistant strains is not reported by antimicrobial resistance programmes. Monensin sodium can therefore be considered safe from a microbiological point of view for the target animal species’. Updated information on the emergence of resistance to monensin, on the cross‐resistance to antimicrobials and to the shedding of enteropathogens was provided by the applicant, which confirmed previous conclusions.43 One of the retrieved papers (Simjee et al., 2012) reports that enterococci and Clostridium perfringens strains, if exposed to monensin in cattle, show an adaptation enabling them to grow at high concentrations of this ionophore (32 times the MICs). This reduced susceptibility is related to the expression of specific proteins and with increased cell wall thickness. The adaptation was shown to be reversible, since the susceptible phenotype was restored when grown in absence of monensin. None of the identified studies reports a significant effect of monensin on the shedding of enteropathogens in chickens and on the insurgence of cross‐resistance with clinically relevant antimicrobials. Danzeisen et al. (2011) studied the effect of monensin on the caecal microbiome of chickens. The monensin supplementation affected the gut microbiota, reducing Roseburia, Lactobacillus and Enterococcus and increasing Coprococcus and Anaerofilum. No effect on E. coli or other enteropathogens was observed. Metagenomic analysis showed that monensin supplementation had no significant effect on the antimicrobial resistance gene counts.
Antibacterial activity of nicarbazin was studied by analysing MIC of a pool of strains isolated from poultry44 and human gut.45 No antimicrobial activity was observed at concentration of 64 mg/L or lower for any of the analysed strains.
3.2.1.4. Conclusions on the safety for the target species
The highest use level, 50 mg monensin and 50 mg nicarbazin/kg complete feed is considered safe for turkeys for fattening with a margin of safety of about 1.5.
The simultaneous use of Monimax® and certain antibiotic drugs (i.e. tiamulin) is contraindicated.
Monensin has a selective antimicrobial activity against Gram‐positive bacterial species while many Enterobacteriaceae are naturally resistant; induction of cross‐resistance with clinically relevant antimicrobials or increased shedding of enteropathogenic bacteria were not reported. Nicarbazin has no antimicrobial activity.
3.2.2. Safety for the consumer
3.2.2.1. Absorption, distribution, metabolism and excretion
Monensin sodium
Data concerning the metabolic fate of monensin sodium in chicken, turkey and rat were submitted in former dossiers of Coxidin® and were assessed by the FEEDAP Panel (EFSA, 2005, 2006a). For the present assessment the same conclusions can be retained which can be summarised as follows: (i) monensin sodium is absorbed to a limited extent and this fraction is eliminated largely through bile, (ii) monensin sodium is metabolised extensively and gives rise to demethylated, oxidised and decarboxylated metabolites, (iii) unchanged monensin represents about 19% of the whole faecal excretion in chicken, up to 40% in turkey (iv) the same metabolites have been found in the excreta and tissues where they represent each less than 10% of the total monensin derivatives, (v) the metabolic pathways in the chicken are similar to those in the turkey and rat.
A recent study of the metabolism of monensin sodium in chickens was submitted.46 Chickens (three males and three females) were administered for eight consecutive days, by gavage and twice a day, 14C‐monensin sodium at a nominal dose corresponding to 125 mg/kg feed (analytically confirmed). The excreta were collected each day and 24 h after the last dose. Tissues were collected from birds slaughtered at 1, 3 and 6 h after the last dose. Monensin and metabolites were analysed by liquid chromatography–mass spectrometry (LC‐MS). Monensin amounted to 28–31% of the radioactivity excreted, all metabolites being below 10%. Metabolites in tissues and excreta were identified as demethylated forms of monensin and (mono‐ and di‐)hydroxylated monensin (hydroxylation positions not established). These findings are in line with the previous conclusions.
Nicarbazin
Nicarbazin is entirely split in the intestinal tract of birds into its two constituents, DNC and HDP. Consequently, nicarbazin cannot appear as residue in tissues and is therefore of no concern for consumer safety; only its two individual components may generate residues.
Absorption, distribution, metabolism and excretion (ADME) studies including total residue determination have been performed with [14C]‐DNC nicarbazin and [14C]‐HDP nicarbazin administered in feed under powder form to avoid splitting of the molecular complex in solution (e.g. water or solvent like dimethyl sulfoxide (DMSO) used for gavage). A comparative in vitro study of the metabolism of nicarbazin in chicken, turkey and rat hepatocytes was also provided.
[ 14 C]‐DNC nicarbazin 47
In a Good Laboratory Practice (GLP) study, chickens (24‐day‐old chickens, three males and three females per group) were administered nicarbazin (both unlabelled and [DNC‐phenyl‐U‐14C]‐nicarbazin) and monensin sodium, each at a nominal level of 55 mg/kg feed (analytically confirmed), for 10 consecutive days to reach steady state. Animals were slaughtered after 0.25‐, 1‐ and 2‐day additive withdrawal. Identity of metabolites in tissues was examined using liquid chromatography with tandem mass spectrometry (LC–MS/MS) analysis and synthesised reference compounds. After 0.25‐day withdrawal time, the metabolic profile was similar in liver, muscle and skin/fat with DNC being the main component detected (68%, 80% and 95% of the total radioactivity, respectively). Monoacetylamino‐4,4′‐dinitrocarbanilide, resulting from the reduction of one nitro group and its subsequent acetylation, represented 18% of the total radioactivity in liver and 10% in muscle. Diacetylamino‐4,4′‐dinitrocarbanilide, resulting from the reduction and acetylation of the two nitro groups, amounted to 12% of the total radioactivity in muscle, monoacetylamino‐4,4′‐dinitrocarbanilide up to 10% and an unknown component 12%. In the kidney, diacetylamino‐4,4′‐dinitrocarbanilide was the main component (54%), followed by N,N’‐1,4‐phenylenebis‐acetamide (27%) resulting from the split of the molecule and DNC (14%).
[ 14 C]‐HDP‐nicarbazin 48
A GLP study following the same experimental design as for the DNC study described above, was performed using [HDP‐pyrimidyl‐2‐14C]‐nicarbazin. Identification of HDP and metabolites in tissues was performed using LC–MS/MS analysis. After 0.25‐day withdrawal time, HDP appeared to be the major compound in the liver of males and females (60% and 53% of total radioactivity, respectively), one major metabolite representing 40% and 37%, and another metabolite 11% (in females only). HDP prevailed in the kidney (92%, average of male and female), muscle (99%) and skin/fat (88%), metabolites being ˂ 10%. None of the metabolites were identified.
In vitro comparison of the metabolic fate of DNC and HDP in chicken, turkey and rat hepatocytes 42
An in vitro comparison of the metabolic fate of DNC in cryopreserved hepatocytes of chicken, turkey and rat was performed, based on the high‐performance liquid chromatography (HPLC) – high resolution mass spectrometry (HRMS) method. Seven to eight metabolites were isolated from hepatocyte incubations of the three species, separated and identified. The main metabolites produced in vivo and already described were identified in vitro. Hydroxylation (position not established) followed by glucuronidation or sulfation and glucuronidation of secondary amine function were also identified. A large overlap but also differences were observed between animal species. Qualitatively the main enzymes involved in DNC metabolism are nitro‐reductase and N‐acetyl transferases in chicken, nitro reductase, N‐acetyl transferases, oxidase and uridine 5′‐diphospho‐glucuronosyltransferases (UGTs) in turkey and oxidase and UGTs in rat hepatocytes. Quantitatively, no significant difference was observed in the amount of DNC metabolised over time between chicken and turkey.
The in vitro metabolism of HDP by chicken, turkey and rat cryopreserved hepatocytes was investigated in the same study using the same experimental design and the analytical approaches used to characterise DNC and metabolites. Only unreacted HDP was detected in any incubation analysed, indicating the absence of significant biotransformation of HDP in the three species.
3.2.2.2. Residues in tissues
Residue studies were performed with the additive Monimax® to investigate the total and marker residue concentrations in tissues of chickens and turkeys for fattening.
Monensin
Monensin residues in poultry tissues were determined by LC–MS/MS analytical method with a limit of quantification (LOQ) of 0.0005 mg/kg for all tissues.
In a monensin residue study in turkeys, three male and three female birds (day‐old) per group were administered feed containing 50 + 50 mg monensin + nicarbazin from Monimax®/kg (analytically confirmed) for 16 weeks.49 Animals were slaughtered after 0, 1, 3, 6, 12 and 24 h withdrawal and tissues sampled. Monensin concentration after 6 h (0.25‐day withdrawal) was 0.0007 mg/kg liver (four samples ˂ LOQ, highest value 0.0008 mg/kg), 0.0009 mg/kg kidney (2 samples ˂ LOQ, highest value 0.001 mg/kg), ˂ LOQ in the muscle and 0.009 ± 0.008 mg/kg skin/fat.
Nicarbazin
DNC
In a GLP study,50 chickens (24‐day‐old, three males and three females per group) were administered nicarbazin (both un‐labelled and [DNC‐phenyl‐U‐14C]‐nicarbazin) and monensin sodium, each at a nominal level of 55 mg/kg feed (analytically confirmed), for 10 consecutive days. Animals were slaughtered after 6, 12 and 48 h (0.25‐, 1‐ and 2‐day withdrawal). Total and marker residue concentrations declined rapidly following nicarbazin withdrawal. The results of total residues and marker residue measured after 0.25‐day withdrawal period are presented in Table 3.
Table 3.
Total residues (expressed as mg equivalent DNC/kg fresh tissue) and marker residue measured in tissues from chickens (3 males and 3 females) administered for 10 days 55 mg 14C‐DNC‐nicarbazin and 55 mg monensin sodium/kg feed in powder form, following a 0.25 day withdrawal period
| Liver | Kidney | Muscle | Skin/fat | |
|---|---|---|---|---|
| TRC a (mg/kg) ± SD | 14.637 ± 2.149 | 9.705 ± 1.733 | 2.154 ± 0.596 | 2.750 ± 0.600 |
| TRC + 2SD | 18.9 | 13.2 | 6.1 | 4.0 |
| MRC b (mg/day) ± SD | 6.857 ± 0.920 | 0.806 ± 0.584 | 0.761 ± 0.207 | 1.269 ± 0.326 |
| MRC + 2SD | 8.7 | 2.0 | 1.2 | 1.9 |
| RMTR c | 0.47 | 0.08 | 0.35 | 0.46 |
Total residue concentration.
Marker residue concentration.
Ratio marker to total residues at 0.25‐day withdrawal time.
SD: standard deviation.
Marker residue studies were provided for chickens51 and turkeys for fattening.49 In both studies, the birds (day‐old, three males and three females per group) were fed a diet containing Monimax® (50 + 50 mg monensin + nicarbazin/kg feed). Duration was 35 days for chickens and 16 weeks for turkeys.
In the chicken study, DNC and HDP residues were analysed by LC–MS/MS analysis with LOQs of 0.1 and 0.11 mg/kg for all tissues, respectively. Mean DNC concentrations plus two times the standard deviation (2SD) measured in the liver, kidney, muscle and skin/fat after 0.25‐day withdrawal were 8.331, 1.514, 1.182 and 1.723 mg DNC/kg tissue, respectively. Average HDP concentrations were 0.117 mg/kg in the liver and below the LOQ in the kidney, muscle and skin/fat.
In the turkey study DNC residues were analysed using a validated and further verified Reversed‐Phase High Performance Liquid Chromatography coupled to a triple quadrupole mass spectrometer (RP‐HPLC‐MS/MS) method with a LOQ of 1 mg/kg for all tissues (the LOD being more than 10 times lower). Animals were slaughtered after 1, 3, 6, 12 and 24 h and tissues sampled. After 6 h (0.25‐day withdrawal), DNC residues in the liver were 0.276 mg/kg (five values < LOQ, one value < LOD), < LOQ in the kidney (not detected in five samples), < LOD in muscle (not detected in five samples) and < LOQ in skin+fat (four samples < LOD). These very low values compared to chickens are likely due to lower absorption since no difference was noted in the quantitative metabolic process in the liver. It can be reasonably assumed that the corresponding total residues would also be lower.
HDP
The quantification of total residues in tissues resulting from the administration of 14C‐HDP‐nicarbazin to chickens was addressed in the study described above.50 The results corresponding to 0.25‐day withdrawal period are presented in Table 4.
Table 4.
Total residues (expressed as mg equivalent HDP/kg fresh tissue) measured in tissues from chickens administered for 10 days 55 mg 14C‐HDP‐nicarbazin and 55 mg monensin sodium/kg feed under powder form, following a 0.25‐day withdrawal period
| Liver | Kidney | Muscle | Skin/fat | |
|---|---|---|---|---|
| TRC (mg/kg) ± SD | 0.065 ± 0.019 | 0.111 ± 0.032 | 0.053 ± 0.032 | 0.060 ± 0.018 |
| TRC + 2SD | 0.103 | 0.175 | 0.085 | 0.096 |
TRC: total residue concentration; SD: standard deviation.
HDP residues were 221, 87, 41 and 46 times lower than the corresponding DNC residues.
3.2.2.3. Toxicological studies
For the current assessment, the applicant provided new studies performed with nicarbazin and with nicarbazin and monensin sodium. Data concerning the toxicity of monensin sodium alone were submitted in former dossiers of Coxidin® and were assessed by the FEEDAP Panel (EFSA, 2005, 2006a).
Nicarbazin
The toxicity of nicarbazin was investigated either administering nicarbazin or the combination of DNC and HDP.
Acute oral toxicity
In an acute oral toxicity study in mice,52 performed according to OECD guideline 432, the oral LD50 of nicarbazin (given by gavage in a 0.5% methyl cellulose vehicle), was found to be > 5,000 mg/kg body weight (bw).
Genotoxicity studies including mutagenicity
The mutagenic potential of nicarbazin was evaluated in Salmonella Typhimurium strains TA1535, TA1537, TA98, TA100 and TA102, with and without metabolic activation (S9 mix from liver of rats induced with Aroclor 1254), in accordance with OECD guideline 471.53 The second experiment with S9 mix was performed according to the pre‐incubation method (60 min, 37°C), all the other experiments with the plate incorporation method. No noteworthy cytotoxicity was observed in a preliminary test, while moderate to strong precipitation was observed in the plates at concentrations ≥ 312.5 μg/plate. Six concentrations of the test item were applied up to a maximum level of 2,500 μg per plate or mL or 1,250 μg/plate for the TA 102 strain in the second experiment, selected on the basis of the level of precipitation. Evident increases in the number of revertants were noted in the TA98 strain in both mutagenicity experiments, both with or without S9 mix. No noteworthy increase in the number of revertants was reported in any other experimental condition.
Nicarbazin was tested for the ability to induce mutations at the thymidine kinase (TK) locus in L5178Y TK+/‐ mouse lymphoma cells with and without metabolic activation (S9 mix from liver of rats induced with Aroclor 1254) according to OECD guideline 476 (revision 1997).54 Two independent experiments were conducted: a 3‐h treatment was used in the first experiment with and without metabolic activation and in the second experiment with metabolic activation; a 24‐h treatment was used in the second experiment without metabolic activation. The test item was poorly soluble in water therefore the concentration ranges were established on the basis of precipitation levels; however slight to marked cytotoxicity (measured as adjusted relative total growth) was observed at the higher concentration levels. Six concentrations of the test item already dissolved in DMSO, were applied up to a maximum level of 125 μg/mL. No increase in the mutation frequency was noted in comparison to the vehicle control in any experiment. The positive controls induced the expected mutagenic effect.
The potential of nicarbazin to induce chromosome aberrations was tested in cultured human lymphocytes from donors, with and without metabolic activation (S9 mix from liver of rats induced with Aroclor 1254), in compliance with OECD guideline 473 (revision 1997).55 Two independent experiments were conducted. The highest concentration of 125 μg/mL was selected on the basis of solubility. At this concentration, a slight precipitate was formed while pH and osmolality were equivalent to those of the vehicle control culture. For each culture, heparinised whole blood was added to culture medium containing the mitogen phytohemagglutinin and incubated at 37°C, for about 48 h. The test item was dissolved in DMSO. In the first experiment, lymphocyte cultures were exposed to the test or control items (with or without S9 mix) for 3 h then rinsed. Cells were harvested 20 h after the beginning of treatment, corresponding to approximately 1.5 normal cell cycles. In the second experiment without S9 mix, cells were exposed continuously to the test or control items until harvest; with S9 mix, cells were exposed to the test or control items for 3 h and then rinsed. Cells were harvested 20 and 44 h after the beginning of treatment. Each culture was treated with a colcemid solution (10 μg/mL) 1.5 h before harvest, to block cells at the metaphase‐stage of mitosis. No cytotoxicity was observed at any of the tested concentrations following the 3‐h treatment while the longer treatment times caused up to a 59% decrease in the mitotic index without any clear evidence of a concentration–response relationship. No significant increase in the frequency of cells with structural chromosomal aberrations was observed in any experimental condition, while the positive controls performed as expected.
An in vivo micronucleus study was conducted to evaluate the potential of nicarbazin to induce structural or numerical chromosome damage in bone marrow cells of Sprague–Dawley rats in compliance with OECD guideline 474 (revision 1997).56 Groups of five males and five females received two oral treatments of the test item at dose levels of 0, 50, 1,000 and 2,000 mg/kg bw per day in 0.5% aqueous methylcellulose, at a 24‐h interval, while the positive control groups received once 15 mg cyclophosphamide/kg bw per day. The animals were killed 24 h after the last treatment and bone marrow smears were then prepared. For each animal, the number of the micronucleated polychromatic erythrocytes (MPE) was counted in 2,000 polychromatic erythrocytes (PE). The PE and normochromatic (NE) erythrocyte ratio was established by scoring a total of 1,000 erythrocytes (PE + NE). In the female group given 500 mg/kg bw per day, the frequency of MPE was statistically significantly decreased; however, in the absence of any dose–response relationship, this decrease was not considered treatment related. No other alteration in the MPE values was reported in any other experimental condition. In the animals treated with the top dosage the following concentration ranges of nicarbazin were measured in the plasma 1 h after the second treatment: 439–682 ng/mL in males and 935–1,088 ng/mL in females. Moreover a clear decrease in the PE/NE ratio was observed in both sexes (statistically significant in females), further confirming the exposure of the target cells to the test item.
Subchronic oral toxicity
A preliminary study of nicarbazin administered to Sprague–Dawley rats of both sexes for 2 weeks at 0, 100, 250 and 625 mg/kg bw per day showed effects on kidney weight (high‐ and mid‐dose male) with effects on body weight and feed consumption at the highest dose. No effect was seen at the lowest dose which was calculated as 93 mg/kg bw per day.57
A study designed to evaluate the potential chronic toxicity of nicarbazin in the feed of Sprague–Dawley rats for 52 weeks was stopped on completion of the first 13 weeks of treatment.58 Rats of both sexes received nicarbazin in the diet at concentrations providing doses of 0, 100, 200, 230 (only male) and 266 (only female) mg/kg bw per day for 13 weeks. There was a dose‐related lower mean body weight gain at all doses and lower feed intake at the highest two doses. Haematological changes (reduced red blood cell count, haemoglobin, white blood cell count and thromboplastin time) were seen in males at the two highest doses and in females at the highest dose. Clinical biochemistry showed many changes in mid‐dose and high‐dose males and mainly in high‐dose females. Higher relative kidney and adrenal weight (highest two doses) and liver effects which differed between sexes at both mid and high dose. Histopathology showed numerous renal effects at mid and high dose, including urothelial hyperplasia; also, lymphoid hyperplasia at mid and high dose, and reduced splenic haematopoiesis at the two highest doses. A no observed effect level (NOEL) could not be established form this study.
The applicant has provided an additional 13‐week study,59 comparing the toxicity of nicarbazin and DNC/HDP, conducted in the same facility as the studies cited above.
Following OECD guideline 408, groups of 10 Sprague–Dawley rats of each sex received either untreated feed (control group) or feed containing a concentration of nicarbazin designed to provide 100 mg/kg bw per day nicarbazin or an equimolar combination of DNC and HDP to provide 71/29 mg kg bw per day (equivalent to 100 mg/kg bw per day nicarbazin) or untreated feed. The concentration (mg/kg diet) of test items in the animals’ feed was modified weekly, according to mean body weight and food consumption data per sex and group, in order to maintain the nominal dose level (mg/kg per day). The actual test item concentrations were determined in samples of each control and test item dietary admixture prepared for use in weeks 1 and 13 using a validated HPLC/UV analytical method.
The animals were checked twice daily for mortality and once a day for clinical signs. Detailed clinical examinations were performed weekly. Body weight and food consumption were recorded three times during the pretreatment period, on the first day of treatment and at least once a week until the end of the study. Ophthalmological examinations were performed on all animals before the beginning of the treatment period and during week 12. Functional observation battery (FOB) was performed on all animals during week 13. Haematology,60 blood biochemistry61 and urinary investigations62 were carried out during week 13. On completion of the treatment period, the animals were sacrificed and submitted to a full macroscopic post‐mortem examination. Kidneys were weighed and selected tissues were preserved. A microscopic examination was performed on selected tissues from designated animals.
The actual concentrations of nicarbazin and DNC/HDP achieved were within ± 10% of nominal throughout the study. No test item‐related clinical signs, ophthalmology or functional observations were observed during the study in animals treated with nicarbazin or DNC/HDP.
A lower mean body weight gain, resulting in lower mean final body weight was seen in males treated with nicarbazin from day 22 to the end of the study, while females of this group were unaffected. There was no effect on body weight of males treated with DNC/HDP, but females had a lower mean body weight gain from day 22 to the end of the study, and had a lower mean final body weight. This was related to a lower mean food consumption in these animals, while no relevant effects on food consumption were noted in all other study animals.
Haematology investigations showed lower mean RBC, haemoglobin level and PCV in males treated with nicarbazin. A shortened activated partial thromboplastin time was also observed in males. This was accompanied by higher mean WBC (due to higher mean neutrophil and eosinophil counts). No similar or relevant haematological effects were observed in males treated with DNC/HDP or in females of both treated groups.
Biochemistry investigations showed higher mean creatinine and urea concentrations and disturbed electrolyte levels (lower chloride level and higher calcium and inorganic phosphorus levels) in males treated with nicarbazin. This was associated with higher mean urine volume with lower specific density and pH. In females of this group, only a mildly higher mean creatinine level was observed. These findings were consistent with the kidney histopathological lesions. In addition, lower mean glucose concentration and higher mean cholesterol level were noted in males given nicarbazin. No similar or relevant blood biochemistry or urinalysis changes were seen in animals treated with DNC/HDP.
Nicarbazin at 100 mg/kg bw per day was associated with a spectrum of pathological changes in the kidneys of both sexes (including birefringent crystals tubular hyaline casts; multifocal tubular basophilia/thickening of tubular basal membrane; tubular dilation, and interstitial chronic active inflammation, frequently associated with fibrosis). These changes were more marked in males. No histological changes were observed in DNC/HDP‐treated male and female rats.
This study confirms that renal toxicity was the primary toxicity resulting from nicarbazin at concentrations in feed providing an intake of 100 mg/kg bw per day. The absence of similar findings after treatment with DNC/HDP at 71/29 mg/kg bw per day confirms that this equimolar association of compounds is better tolerated than nicarbazin at the same equivalent dose.
Chronic oral toxicity
In a study based upon OECD guideline 452, groups of 20 Sprague–Dawley rats of each sex received a control diet or diets containing a combination of DNC/HDP at levels of 20/8, 50/20.5 or 154/63 mg/kg bw per day for 52 weeks.63 Exposure to the test item was confirmed in a blood sample collected from the last 10 surviving animals of each sex from each group at week 52. Animals were caged in groups of four, observed daily and subject to detailed clinical examination once a week throughout the study. Ophthalmological examinations were conducted on all animals at the start of the study and on controls and high‐dose animals during the last week of treatment. Body weight and food intake were recorded weekly during the first 14 weeks and then monthly for the rest of the study. Blood was collected at the end of the study from all animals after 14‐h fasting and assessed for haematology,60 while serum separated from blood was analysed for clinical blood biochemistry.61 Urine collected over a 14‐h period in individual metabolism cages was examined64 and sediment was examined microscopically for presence of crystals and cells. A necropsy was carried out on all animals and included full macroscopic examination and measurement of body weight and organ weights (adrenal, brain, epididymides, heart, kidneys, liver, ovaries, spleen, testes, thymus and uterus) a full range of tissues were preserved for microscopic examination. A microscopic examination was performed on all tissues from all animals.
High‐dose males gained less weight than other groups particularly during the latter part of the study; however, total body weight gain was not significantly different from controls. The only treatment‐related differences in the laboratory investigations of blood, serum and urine were observation of crystals in the urine of both sexes from high and intermediate doses. This was correlated with microscopic renal changes in both sexes including tubular basophilia, interstitial chronic inflammation, mononuclear inflammatory infiltrate, tubular dilation, cysts, intratubular inflammatory cells, hyaline casts, and papillary oedema. Males were more affected than females.
Although the changes seen at the intermediate dose were rather slight, the no observed adverse effect level (NOAEL) derived from this study is DNC/HDP 20/8 mg/kg bw per day.
Reproduction toxicity studies including prenatal development toxicity
In a study65 based upon OECD guideline 416 and 414, groups of 24 Sprague–Dawley rats of each sex received control diet or diets containing DNC/HDP at 100/33, 300/100 or 580/193 mg/kg bw per day over two generations starting 10 weeks before the first paring and continuing through all stages until each animal reached its designated termination. F0 generation offspring were examined at all stages of development. At day 4, litters were culled to approximately 4 per sex per litter. At day 22, post‐partum 1–2 pups of each sex were selected from each litter for the F1 generation. After 10 weeks of treatment, the F0 animals were paired and allowed to produce their litters. One pup per sex per litter from both F0 and F1 generations were brought to necropsy at around 22 days post‐partum. Parents from both generations were brought to necropsy once litters had been weaned. Litters and individual pups were subject to a very wide range of observations at both generations of the study. No treatment‐related effects were observed in F0 or F1 offspring; however, the parental generation at both stages showed evidence of renal crystal deposits, kidney weight changes and nephropathy at all doses. It can be concluded that even at parentally toxic doses there is no impairment of reproductive performance in rats treated with a combination of DNC/HDP at doses up to 580/193 mg/kg bw per day. The parental NOAEL lies below the lowest dose tested of DNC/HDP 100/33 mg/kg bw per day.
A study designed to evaluate the effects of nicarbazin on the pregnant female rabbit and on embryo/fetal development, following daily oral administration (gavage) at doses of 0, 60, 120 and 240 mg/kg bw per day, during organogenesis (from implantation to the day prior to scheduled sacrifice: from day 6 to day 28 post coitum, inclusive), in accordance with OECD guideline 414.66 The results obtained in this study showed no maternal effects at any dose thus the NOAEL was 240 mg/kg bw per day for maternal parameters. Some retardation of fetal development was indicated at the highest dose, by analysis of skeletal ossification thus a NOAEL of 120 mg/kg bw per day is concluded for embryo/fetal development.
Nicarbazin and monensin sodium
Genotoxicity studies including mutagenicity
The mutagenic potential of the combination, crystalline monensin sodium, DNC and HDP, representing a 3.44:2.44:1 ratio on a weight basis, respectively, was evaluated in Salmonella Typhimurium strains TA1535, TA1537, TA98, TA100 and TA102.67 This combination was tested in two independent experiments, with and without a metabolic activation system (S9 mix from liver of rats induced with Aroclor 1254) in compliance with OECD guideline 471. A third confirmatory experiment was undertaken only in the TA98 strain without S9 mix. The second experiment with S9 mix was performed according to the pre‐incubation method (60 min, 37°C), all the other experiments with the plate incorporation method. No cytotoxicity was observed in a preliminary test, while moderate to strong precipitation was observed in the plates at concentrations ≥ 87.5 μg/plate. Six concentrations of the test item were applied up to a maximum level of 700 μg per plate or mL, selected on the basis of the level of precipitation. A 2.7‐fold increase in the number of revertant colonies was observed at the top concentration in the TA98 strain in the first experiment without S9 mix, whereas it was not confirmed in the two following experiments, this increase may reflect the effect of nicarbazin on the strain TA98 reported above. The test item did not induce any other noteworthy increase in the number of revertants, in any other experimental conditions while the positive controls performed as expected.
Subchronic oral toxicity
A study was conducted to investigate a combination of monensin sodium and an equimolar complex of DNC and HDP given simultaneously in feed (dietary admixture) to rats for 13 weeks.68 The study used only a single treatment group given 0.5/300/100 mg/kg bw per day monensin sodium/DNC/HDP, respectively, for the first 14 days then 0.5/150/50 mg/kg bw per day for the remainder of the study and a control group. Reduced erythrocytes (RBC) and mean cell haemoglobin concentration (MCHC) in females and renal histopathology in males were effects which have been associated with nicarbazin and DNC/HDP in other studies but do not allow any conclusion about the monensin/nicarbazin interaction.
In a study based upon OECD guideline, 408 groups of 10 Sprague–Dawley rats of each sex received control diet or diets containing a combination of monensin sodium/DNC/HDP at levels of 0.25/25/10.25 or 0.5/50/20.5 mg/kg bw per day for 13 weeks.69 Animals were caged in groups of two and observed daily and subject to detailed clinical examination once weekly throughout the study. Ophthalmological examinations were conducted on all animals at the start of the study and on controls and high‐dose animals during the last week of treatment. A FOB was conducted on all animals during week 12 of the study. Body weight and food intake was recorded weekly throughout the study. Blood was collected at the end of the study from all animals after 14‐h fasting and assessed for haematology,60 while serum separated from blood was analysed for clinical blood biochemistry.61 Urine collected over a 14‐h period in individual metabolism cages was examined64 and sediment was examined microscopically for the presence of crystals and cells. A necropsy was carried out on all animals and included full macroscopic examination and measurement of body weight and organ weights (adrenal, brain, epididymides, heart, kidneys, liver, ovaries, spleen, testes, thymus and uterus); a full range of tissues were preserved for microscopic examination. A microscopic examination was performed on all tissues from all animals. Body weight, ophthalmology, functional observation tests, food intake, haematology, clinical chemistry, histopathology and organ weight results showed no evidence of treatment‐related effects. In the absence of any treatment‐related findings, the NOAEL is concluded at the highest monensin sodium/DNC/HDP dose of 0.5/50/20.5 mg/kg bw per day.
Monensin sodium
In 2005, the FEEDAP Panel concluded that monensin sodium is not genotoxic in an adequate range of studies and has shown no structural alert for carcinogenesis and not a reproductive or developmental toxin based on adequate current studies in rat and rabbit (EFSA, 2005). The lowest NOEL identified in the developmental study in rabbits is 0.3 mg monensin sodium/kg bw per day for maternal toxicity in rabbits. For the current assessment, the FEEDAP Panel reviewed the former studies and the new studies performed with the combination of monensin sodium with nicarbazin and reiterates its previous conclusions.
3.2.2.4. Impurities in the active substance nicarbazin
p ‐Nitroaniline
The current authorisations for nicarbazin limit the maximum level of PNA to ≤ 0.1% as of 26 October 2013.9 This threshold was introduced based on a review of the Health Council of The Netherlands (GR, 2008) which concluded that PNA has been insufficiently investigated and that there is a cause for concern, and on disposition studies of Chopade and Matthews (1984) in laboratory animals70 allowing the FEEDAP Panel to conclude that consumer exposure would be negligible at a maximum level of 0.1% PNA in nicarbazin (EFSA FEEDAP Panel, 2010a).
The applicant performed a literature search aiming to provide more recent data than those cited above; no new data became available.
Methyl(4‐nitrophenyl) carbamate
In the absence of published data, the applicant performed (i) an in vitro study of the metabolism of methyl(4‐nitrophenyl) carbamate (M4NPC) in the chicken and turkey, (ii) a residue study with M4NPC in chicken and (iii) a battery of mutagenicity tests which are described below.71
In vitro metabolic study
Hepatocytes prepared from chicken and turkey livers were put into contact to M4NPC to investigate the metabolic fate of the substance (concentration of 1 μM M4NPC/million cells) and to isolate and identify eventual metabolites (concentration of 10 μM M4NPC/million cells). A positive control (diclofenac) was used to check the metabolic capability of the cells. Identification of M4NPC and potential metabolites was performed using reverse HPLC with MS/MS detection after ionisation allowing the detection with high sensitivity. Diclofenac was metabolised as expected, whereas no metabolite was produced following incubation with M4NPC.
Residue study
A study of M4NPC residues in tissues of chickens was performed. Day‐old chickens (three males and three females) were administered for 26 days a complete feed supplemented with 50 mg nicarbazin/kg and 1 mg M4NPC/kg. The animals were slaughtered within 3‐h after the last delivery of the diet and tissues sampled. M4NPC was determined in the liver, kidney, muscle and skin/fat using a HPLC‐HRMS analytical method with a LOQ of 0.002 mg/kg. The content of M4NPC after 3 h withdrawal in the liver was 0.009 (± 0.003) mg/kg that in the samples skin/fat 0.013 (± 0.005) mg/kg. Residues in muscle and kidney were below the LOQ.
Genotoxicity including mutagenicity
Bacterial reverse mutation test
A study was performed using Salmonella Typhimurium strains TA98, TA100, TA1535 and TA1537 and E. coli strain WP2 uvrA, in the presence and absence of metabolic activation (S9 prepared from livers of phenobarbital/β‐naphthoflavone‐induced rats) in compliance with OECD guideline 471. The substance was dissolved in DMSO and tested up to a concentration of 5,000 μg/plate. At this concentration, a precipitate interfering with the scoring was observed on the plates, therefore the evaluation was performed by microscope. Slight precipitation was observed also at lower test concentrations. At the top concentration, slight inhibitory effects of the test item were noticed in all examined strains in the presence and absence of S9 mix. This inhibitory effect was shown by a decrease in the revertant colony numbers while the background bacterial growth was not affected. No biologically relevant increases were observed in revertant colony numbers in any experimental condition. The positive controls showed the expected increases in induced revertant colonies.
In vitro micronucleus assays
Two independent studies were performed with and without metabolic activation (S9 from the liver of rats induced with Aroclor 1245). In both studies, the test item was diluted in DMSO and two treatment regimens were applied: (i) a 3‐h pulse treatment in the presence or absence of metabolic activation followed by recovery period (21–24 h); (ii) a continuous treatment (24–27 h) without recovery period only in the absence of metabolic activation. Both studies were conducted in compliance with the OECD guideline 487.
In the first study, TK6 human lymphoblastoid cells were treated up to a maximum test concentration of 62.5 μg/mL; at higher concentrations, precipitate was formed. In the pulse treatment, no cytotoxicity was reported, while in the continuous treatment the test item induced slight cytotoxicity at the highest concentration. No increase in the number of micronucleated cells was observed in any experimental condition, while the positive control performed as expected.
In the second study, the substance was tested in human lymphocyte cultures prepared from the pooled blood of two female donors. Cells were treated 48 h after mitogen stimulation by phytohaemagglutinin. Cytochalasin‐B was added at the end of treatment period (pulse treatment) or at the time of treatment (continuous treatment) to induce the formation of binucleated cells. The top concentrations tested were 200 μg/mL in the in the pulse treatment and 50 μg/mL in the continuous treatment. These concentrations caused evident cytotoxicity, measured as reduction of replication index. Precipitate was observed in the pulse treatment at 200 μg/mL without metabolic activation. The frequencies of micronucleated binucleated cells were comparable to those reported in the concurrent vehicle controls at all the concentrations analysed in both treatment regimens. The positive control substances induced statistically significant increases in the proportion of cells with micronuclei.
Conclusion on genotoxicity
Overall, methyl(4‐nitrophenyl) carbamate was not genotoxic in a bacterial reverse mutation assay and in two independent in vitro micronucleus studies in human cells.
3.2.2.5. Conclusions on toxicology
Monensin sodium is not genotoxic in an adequate range of studies and has shown no structural alert for carcinogenesis. Monensin sodium is not a reproductive or developmental toxin based on adequate current studies in rat and rabbit. The lowest NOEL identified in the developmental study in rabbits is 0.3 mg monensin sodium/kg bw per day for maternal toxicity in rabbits.
Nicarbazin showed mutagenic activity in the Salmonella Typhimurium TA98 strain in the presence and in the absence of metabolic activation, while the substance was negative in the other bacterial strains. Negative results were reported also in a gene mutation assay in L5178Y TK+/− mouse lymphoma cells and in a chromosome aberration test in human lymphocytes in vitro. Moreover nicarbazin did not show any mutagenic activity in an in vivo micronucleus test in rat in conditions warranting the exposure of the target cells to the test substance. The primary toxicity resulting from the oral use of nicarbazin is renal toxicity. The absence of similar findings after treatment with DNC and HDP confirms that this equimolar association of compounds is better tolerated than nicarbazin at equivalent doses. At parentally toxic doses (renal effects), there is no impairment of reproductive performance in rats treated with a combination of DNC/HDP at doses up to 580/193 mg/kg bw per day. The NOAEL for embryo/fetal development is 120 mg nicarbazin/kg bw of rabbits per day. The lowest NOAEL identified in a 52‐week study in rat using DNC+HDP was 20 mg DNC + 8 mg HDP/kg bw per day based on the occurrence of microcrystals in urine and related microscopic renal observations at higher dose level.
When the combination of monensin sodium and DNC + HDP was tested in a bacterial reverse mutation test, the results were consistent with the findings of separate tests with monensin sodium and nicarbazin. The FEEDAP Panel concludes that the active substances in Monimax®, monensin sodium and nicarbazin, do not represent a genotoxic risk. Furthermore, there was no evidence resulting from the toxicological studies for any significant interaction between monensin sodium and nicarbazin.
No safety concerns would arise from the nicarbazin impurities PNA and M4NPC in Monimax®.
3.2.2.6. Assessment of consumer safety
For monensin, the current provisional MRLs in turkeys for fattening (25 μg/kg wet skin + fat and 8 μg/kg wet liver, kidney and muscle) ensure consumer safety. Residue data obtained for monensin after the use of the highest proposed level of Monimax® in feed (50 + 50 mg monensin + nicarbazin/kg) for turkeys for fattening (Section 3.2.2.2) showed that after withdrawal times of 6 h in turkeys all marker residue concentrations were below the respective MRLs in liver, kidney, muscle and skin + fat. The withdrawal times applied are considered equivalent to zero day under practical conditions.
As the consumer will not be exposed to nicarbazin but to DNC and HDP, and DNC residues are orders of magnitude higher than HDP residues (see Tables 3 and 4), DNC is considered the marker residue.
A health‐based guidance value (acceptable daily intake (ADI)) for the nicarbazin moieties (DNC and HDP) can be derived from the NOAEL of 20 mg DNC and 8 mg HDP/kg bw based on the absence of microcrystals in the urine obtained in a 52‐week study in rat using DNC + HDP. The ADI is 0.2 mg DNC and 0.08 mg HDP/kg bw applying an uncertainty factor of 100.
The metabolic similarity between the target animals, chickens and turkeys, and the laboratory animal has been demonstrated (Section 3.2.2.1).
Exposure of the consumer to DNC was calculated applying the food basket of Regulation (EC) No 429/200872 (Table 5). Consumer exposure to total DNC residues from chicken tissues showed compliance with the ADI (35%) after practical zero‐day withdrawal. Similar calculation carried out with HDP total residues (from Table 4) indicates compliance with the corresponding ADI (dietary intake of HDP total residues is 0.047 mg/day corresponding to about 1% of the ADI). Since DNC residues in turkey tissues are considerably lower than in chicken tissues (Section 3.2.2.2), consumer exposure to total residues of DNC in turkey tissues would also comply with the ADI.
Table 5.
Consumer exposure to DNC in tissues of chickens for fattening and compliance with the ADI
| Liver | Kidney | Muscle | Skin/fat | Sum | |
|---|---|---|---|---|---|
| TRC a (mg/kg) + 2SD | 18.9 | 13.2 | 6.1 | 4.0 | |
| DITR b (mg/day) | 1.89 | 0.13 | 1.83 | 0.36 | 4.21 (35% ADI) |
| RMTR c | 0.47 | 0.08 | 0.35 | 0.46 | |
| MRL (mg/kg) | 15 | 6 | 4 | 4 | |
| DITRMRL d (mg/day) | 3.2 | 1 | 3.4 | 0.8 | 8.4 (70% ADI) |
TRC: total residue concentration.
Dietary intake of total residues.
Ratio marker to total residues at 0.25 day withdrawal time.
Dietary intake of total residues calculated from the MRLs.
For nicarbazin, MRLs for DNC of 15 mg/kg liver, 6 mg/kg kidney and 4 mg/kg muscle and skin/fat in chickens for fattening are in force at EU level. Although these MRLs were derived from an ADI of 0.77 mg DNC/kg bw per day, they comply with the newly derived ADI (see Table 5). Calculation of consumer exposure, using the MRLs and applying the ratio marker to total residues (RMTR) of the recent residue study in chickens (Section 3.2.2.2), results in an exposure of about 70% of the new ADI. The MRLs for DNC can equally be applied to tissues of turkeys for fattening since measured values are considerably below the residue levels found in chicken tissues and consequently below the MRLs.
Compliance with the MRLs is given at zero day withdrawal time.
No residues were found in chicken muscle and kidney (< 0.002 mg/kg), when exposed to about the fivefold M4NPC dietary level compared to the highest use level applied for Monimax®; residues in liver and skin/fat were 0.009 and 0.013 mg/kg, respectively. The FEEDAP Panel assumes that a similar range of concentration of M4NPC in turkey tissues would occur. Consequently, M4NPC in Monimax® is considered safe for the consumer provided that a maximum concentration of 0.4% in nicarbazin would not be exceeded. No safety concern would arise from the impurity PNA if the maximum content in nicarbazin of 0.1% is respected.
3.2.2.7. Conclusions on the safety for the consumer
The use of Monimax® at the highest proposed dose (50 + 50 mg monensin + nicarbazin/kg complete feed) will not pose a risk to persons consuming animal products from turkeys for fattening.
No safety concern would arise from the impurity PNA if the maximum content in nicarbazin of 0.1% is respected. The other nicarbazin related impurity, M4NPC, is considered safe for the consumer provided that a maximum concentration of 0.4% in nicarbazin would not be exceeded.
No withdrawal time is required for Monimax® in turkeys for fattening.
3.2.3. Safety for the user
For the current assessment, the applicant provided new studies performed with nicarbazin. Data concerning the user safety of monensin sodium were submitted in former dossiers of Coxidin® and were assessed by the FEEDAP Panel (EFSA, 2005, 2006a). No studies with the additive Monimax® have been provided, other than information on the physical characteristics of the additive (see Section 3.1.2).
Nicarbazin
A study was performed to assess the acute inhalation toxicity of nicarbazin according to OECD guideline 403.73 A group of ten rats (five males and five females) was exposed to a dust atmosphere. The animals were exposed for 4 h using a nose only exposure system, followed by a 14‐day observation period. The mean achieved atmosphere concentration was 5.12 ± 0.20 mg/L. No deaths or major clinical signs were observed, and no macroscopic abnormalities were detected at necropsy. It was therefore considered that the acute inhalation median lethal concentration (4 h LC50) of nicarbazin (atmosphere concentration of 5.12 mg/L for 4 h) in the rat was greater than 5.12 mg/L.
The highest dusting potential of Monimax® was 0.50 g/m3.31 The dust contained about 32% (v/v) particles of respirable size (< 10 μm).32 The concentration of nicarbazin in the dust was 19.0–22.9%.33 Nicarbazin concentration in dust released from Monimax®, could therefore reach a maximum of 100 mg/m3 of which 32 mg are in the respirable fraction.
The potential of nicarbazin for skin and eye irritation was investigated following OECD guideline 404 and 405, respectively.74 Nicarbazin was very slightly irritant when applied dermally to rabbits. The scores for chemosis, conjunctival redness, iris lesions and corneal opacity following a single ocular administration in rabbits after 24, 48 and 72 h were all about zero. Nicarbazin should not be classified as irritating to the eyes.
The skin sensitisation potential of nicarbazin was studied by the murine local lymph node assay (LLNA) (OECD 429).75 Neither mortality nor clinical signs were observed during the study. No cutaneous reaction and no increase in ear thickness were observed. No lymphoproliferation was noted. It is concluded that nicarbazin is not a skin sensitiser.
Monensin sodium
The FEEDAP Panel concluded in its former opinions on Coxidin® (EFSA, 2005; EFSA FEEDAP Panel, 2011a) that pure monensin sodium was shown not to be an irritant to either skin or eyes and not to act as a skin sensitiser. Similarly, the additive was not irritant to skin and not a skin sensitiser. It did, however, cause slight irritation to the eye. No data were provided for the granulated additive. An acute inhalation study considered by the FEEDAP Panel showed that pure monensin sodium was highly toxic by inhalation (LC50 69 mg/m3 for male and 51.9 mg/m3 for female rats). Dermal studies demonstrated that dermal exposure can contribute significantly to toxicity.
The highest dusting potential of Monimax® was 0.50 g/m3.31 The dust contained about 32% (v/v) particles of respirable size (< 10 μm).32 The concentration of monensin in the dust was 16.3–17.6%.33 Monensin concentration in dust released from Monimax® could reach a maximum of 85 mg/m3 of which 27.2 mg are in the respirable fraction.
Conclusions on safety for the user
In the absence of data performed with Monimax®, the FEEDAP concluded on the safety for the user based on the data available on its individual components. The monensin sodium contained in Monimax® presents a hazard by inhalation. Although the dusting potential of Monimax® is low, users will be exposed to monensin by inhalation indicating a risk to persons handling Monimax®.
Monimax® is not a skin irritant; however, no data are available for the eye irritation potential of monensin. Monimax® may also act as a dermal toxicant due to its monensin component. Monimax® is not a skin sensitiser.
3.2.4. Safety for the environment
Active substances are not physiological/natural substances of established safety for the environment. The additive is also not intended for companion animals. Consequently, according to Regulation (EC) No 429/200872, the Phase I assessment has to be continued to determine the predicted environmental concentration.
In Phase I and II initially a total residues approach will be taken, meaning that the predicted environmental concentrations (PECs) will be calculated, based on the assumption that the additives are excreted 100% as parent compound. Nicarbazin is an equimolar complex of DNC and HDP in a 70:30 w/w ratio, which splits during the intestinal passage. Consequently, the environmental risk assessment should not consider nicarbazin but both components separately. Distribution in the environment is based on the properties of the individual components of Monimax® as long as no data on relevant metabolites and on potential interaction are submitted.
MONENSIN SODIUM
3.2.4.1. Phase I
Physicochemical properties
The physical chemical properties of monensin sodium are summarised in Table 6. The dissociation constant pKa was not provided by the applicant but Sun et al. (2016) reported a pKa of 4.5.
Table 6.
Physicochemical properties of monensin sodium
| Property | Value | Unit |
|---|---|---|
| Octanol/water partition coefficient (log Kow)a |
4.48 (pH 5.2–5.7, 25°C) 3.82 (pH 7, 25°C) 3.82 (pH 10, 25°C) |
– |
| Water solubility (20°C)b | 8.78 | mg/L |
| Dissociation constant (pKa) | 4.5 | – |
| Vapour pressurec | 3 × 10−28 | Pa |
Technical dossier/Supplementary information June 2015/Annex 4.
Technical dossier/Supplementary information June 2015/Annex 27.
EPI Suite, 2015.
Fate and behaviour
Fate in soil
Adsorption/desorption in soil
The adsorption of monensin sodium was determined in three studies and a total of 13 soils were analysed. In a GLP‐compliant study, following OECD guideline 106, three soils with differing properties (Table 7) were used to determine the adsorption/desorption behaviour of monensin.76 In another study, eight soils (Table 7) and a batch equilibrium methodology were used to determine the adsorption Freundlich isotherms for monensin (two of the soils had the pH adjusted).77 In a recent study (Doydora et al., 2017), the sorption behaviour of monensin has been investigated. The Kendall test was used to explore pH dependence of adsorption in soil. The test indicated that when adsorption in the 13 soils is considered, it does not demonstrate a simple pH dependency (p > 0.05). Apparently, other factors besides the pH and organic carbon content are also important for the sorption of monensin in soil. Doydora et al. also noted that monensin can form lipophilic neutral complexes with alkali metal ions particularly with sodium at a pH above its pKa of 4.5. During complexation, monensin orients its polar groups around the cation making the inner surface of the complex hydrophilic and the outer surface hydrophobic. Apart from soil pH, also the amount of dissolved organic carbon and cations, like sodium, has a strong influence on the sorption of monensin.
Table 7.
Adsorption of monensin sodium in different soilsa
| Soil | %OC | pH | Kf | 1/n | Koc |
|---|---|---|---|---|---|
| Silty clayb | 2.5 | 6.1 | 4.0448 | 0.9213 | 161.8 |
| Clay loamb | 3.1 | 7.3 | 2.2977 | 0.9191 | 74.1 |
| Sandy loamb | 1.3 | 4.7 | 3.5637 | 0.9395 | 274.1 |
| Drummer‐1c | 2.91 | 7.5 | 23.5 | 0.88 | 1160 |
| Drummer‐30c | 2.2 | 7.3 | 4.01 | 1 | 182 |
| Raub‐12c | 1.35 | 6.2 | 6.06 | 0.97 | 491 |
| Toronto‐4c | 1.34 | 4.2 | 42.7 | 0.92 | 4050 |
| Oakville‐24c | 0.52 | 4.7 | 5.27 | 0.71 | 2423 |
| Oakville‐31c | 0.87 | 7 | 1.09 | 1 | 125 |
| Coloma‐32c | 0.64 | 6.8 | 0.94 | 1.01 | 143 |
| A1A2c | 1.38 | 5.5 | 41.9 | 0.79 | 5700 |
| Unamendedd | 2.4 | 5.2 | 13.1 | 0.98 | 555 |
| BL‐amendedd | 3 | 5.7 | 7.3 | 0.92 | 247 |
%OC: % of organic carbon; Kf: Freundlich constant; Koc: adsorption or desorption coefficient corrected for soil organic carbon content.
Technical dossier/Supplementary information June 2015/Annex 23.
Technical dossier/Supplementary information June 2015/Annex 28.
Doydora et al. (2017).
Considering that the sorption of monensin to soil is dependent on more factors than only the pH and the amount of organic carbon and it is regularly detected in surface water and groundwater (Doydora et al., 2017), the lowest adsorption coefficient normalised to the organic carbon content (Koc) value from the sorption studies (74.1 L/kg organic carbon for the clay loam) was selected as a reasonable worst‐case estimate for further calculations.
The FEEDAP Panel notes that the above selected Koc value is lower than the Koc value used for the environmental risk assessment of monensin in the EFSA opinion on Coxidin® for chickens reared for laying (EFSA FEEDAP Panel, 2011a).
Degradation in soil
A GLP‐compliant study following OECD guideline 307 was performed using 14C radiolabelled monensin sodium.78 The biodegradation rate of monensin was determined in three soils with differing properties (Table 8). In the route of degradation study, the mass balance established in one soil (sandy loam) was > 90% for all time points. The rate of degradation of monensin sodium at 23°C was also determined in another study in two soils using an equivalent destructive sampling incubation approach (Table 8).77 The geometric mean of the soil DT50 (Disappearance Time 50) in five soils was 2.5 days at 20°C,79 which indicates that monensin is not persistent in soils.80
Table 8.
Half‐life (DT50) of monensin sodium in different soils
| Soil | Temperature (°C) | DT50 at 12°C (days) | DT50 at 20°C (days) |
|---|---|---|---|
| S473a | 20 | 2.3 | 2.3 |
| S474a | 20 | 4 | 4 |
| S475a | 20 | 2.5 | 2.5 |
| Drummer‐30b | 23 | 2 | 2.6 |
| Oakville‐31b | 23 | 1.3 | 1.7 |
Technical dossier/Supplementary information June 2015/Annex 24.
Technical dossier/Supplementary information June 2015/Annex 28.
Fate in water
No information on the biodegradation of monensin in surface water or sediment was provided by the applicant.
Conclusion on fate and behaviour
A Koc of 74.1 L/kg and a DT50 of 2.5 days at 20°C will be used for the assessment.
Predicted environmental concentrations (PECs)
The methodology for the calculation of the maximum PECs in soil, groundwater, surface water and sediment are described in the technical guidance for assessing the safety of feed additives for the environment (EFSA, 2008c).
The input values used for monensin sodium were: monensin concentration in turkey feed 50 mg/kg, molecular weight 693.8, vapour pressure 3 × 10−28 Pa, solubility 8.78 mg/L, DT50 2.5 days and Koc 74.1 L/kg. The calculated values are given in Table 9.
Table 9.
Initial predicted environmental concentration (PECs) of monensin sodium, in soil (μg/kg), groundwater (μg/L), surface water (μg/L) and sediment (μg/kg dry weight)
| Compartment | PEC |
|---|---|
| Soil | 232 |
| Ground water | 163 |
| Surface water | 54 |
| Sediment | 287 |
The Phase I PEC trigger values are exceeded, therefore a Phase II assessment is considered necessary.
3.2.4.2. Phase II
Exposure assessment
PECs calculation refined in Phase II
PEC soil refined for metabolism
In a former assessment of the environmental impact of monensin from Coxidin® (EFSA FEEDAP Panel, 2011a), the FEEDAP Panel considered that about 20% of the monensin sodium ingested by chickens was excreted unchanged. No information was given on the mode of administration of labelled monensin (single or multi‐dose). In a newly submitted study (see Section 3.2.2.1),80 14C‐monensin sodium was administered for eight consecutive days together with nicarbazin in order to take account of an eventual interaction of the two compounds. About 30% of the ingested monensin sodium was excreted unchanged, numerous metabolites representing < 10% each. As these experimental conditions mimic the practical use of Monimax®, the FEEDAP Panel considered that about 30% of the monensin sodium ingested by chickens was excreted unchanged. The FEEDAP Panel assumed that also for turkey about 30% of the monensin was excreted unchanged.
Assuming that the ionophoric activity of monensin sodium and its metabolites in turkey excreta will not exceed in total 30% of the orally administered dose, the refined dose used for PEC calculations was 50 × 0.3 = 15 mg/kg feed. The refined PECsoil, PECgroundwater, PECsurfacewater and PECsediment are reported in Table 10.
Table 10.
Refined predicted environmental concentrations (PECs) of monensin sodium, in soil (μg/kg), groundwater (μg/L), surface water (μg/L) and sediment (μg/kg dry weight)
| Input | Value |
|---|---|
| Dose (mg/kg feed) | 15 |
| Molecular weight | 693.8 |
| Vapour pressure (Pa) (at 25°C) | 3 × 10−28 |
| Solubility (mg/L) | 8.78 |
| Koc (L/kg) | 74.1 |
| DT50 in soil at 20°C (days) | 2.5 |
| Output | |
| Application rate kg/ha | 0.21 |
| PECsoil | 70 |
| PECgroundwater | 49 |
| PECsurfacewater | 16 |
| PECsediment | 87 |
When the PECgroundwater is set equal to the concentration in pore water based on a worst‐case assumption (the total residue approach), the concentration exceeds the trigger value of 0.1 μg/L identified by the EU as quality standard.81
PEC groundwater refined with FOCUS
Leaching of monensin to groundwater was simulated using the FOCUS recommended leaching model PEARL (FOCUS Version 4.4.3) (EFSA, 2008c). The calculated groundwater concentrations for the scenarios Jokioinen and Piacenza were below 0.001 μg/L.
PEC surface water and PEC sediment refined with FOCUS
Concentrations in surface waters for monensin were assessed using the FOCUS Step 3 surface water assessment approach. The FOCUS recommended surface water models PRZM_SW, MACRO and TOXSWA were used (EFSA, 2008c). Generally, run‐off was calculated from a soil receiving 0.21 kg monensin/ha which was homogeneously distributed into a 20 cm deep layer. In the sensitive R1‐Stream run‐off scenario from FOCUS 4.4.3 using SPIN 2.2, the global maximum PECsurface water was 0.62 μg/L. The PECsediment value was 0.10 μg/kg dry weight.
Conclusions on PEC used for calculation
The following values are used for the assessment: PECsoil of 70 μg/kg, PECsurface water of 0.62 μg/L and PECsediment 0.10 μg/kg dry weight.
Ecotoxicity studies
Toxicity to soil organisms
Effects on plants
A GLP‐compliant study following OECD guideline 208 was performed to investigate the effect of monensin on terrestrial plants.82 Quartz sand was treated with monensin (as monensin sodium) at five concentrations and seeds from three species were sown (monocotyledon species Triticum aestivum, and dicotyledon species Sinapis alba and Trifolium pratense). Seedlings were allowed to emerge and grow for at least 14 days following 50% emergence of the control plants under glasshouse conditions. The endpoints determined were the effects on emergence/survival, shoot fresh weight and shoot dry weight biomass. Control seedling emergence was ≥ 70% for all species (actual 80–100%). Fresh weight biomass was the most sensitive endpoint for all species (Table 11). Overall S. alba was the most sensitive species with the fresh weight biomass EC50 (median effective concentration) 4.0 mg monensin/kg.
Table 11.
Monensin ecotoxicological effects data (EC50) for terrestrial plants (mg monensin/kg)
| Endpoint | T. aestivum | S. alba | T. pratense |
|---|---|---|---|
| EC50 (emergence) | < 970.9 | 16.5 | 185.4 |
| EC50 (fresh weight) | 28.2 | 4.0 | 7.8 |
| EC50 (dry weight) | 104.9 | 5.8 | 7.8 |
EC50: median effective concentration.
The FEEDAP Panel noted that the data obtained on quartz sand may not be representative for studies with soil types containing organic matter.
Effect on earthworms
A GLP‐compliant study following OECD guideline 207 was performed to investigate the effect of monensin (as monensin sodium) on Eisenia foetida.83 Earthworms were placed in an artificial soil at 62.5, 125, 250, 500 and 1,000 mg monensin sodium/kg soil (dry weight) (equivalent to 60.7, 121.4, 242.7, 485.4 and 970.9 mg monensin/kg soil (dry weight)) and mortality assessed after 7 and 14 days. The 14‐day median lethal concentration (LC50) was determined as 112.1 mg monensin sodium/kg soil (dry weight) (equivalent to 108.8 mg monensin/kg soil (dry weight)).
Effects on soil microorganisms
A GLP‐compliant study following OECD guideline 216 was performed to investigate the effect of monensin (as monensin sodium) on soil microorganisms.84 A sandy loam soil was treated with monensin sodium at a rate of 500 and 5,000 μg monensin sodium/kg soil (dry weight) (equivalent to 485.4 and 4,854.4 μg monensin/kg soil (dry weight)). This study is usually performed at the PECsoil and ten times that value. The treatments used in this study were at least a factor of three greater than the largest PECsoil refined available for monensin. Control and treated soils were incubated for 28 days and subsamples were taken on 0, 7, 14, and 28 days after treatment and analysed for the nitrate concentration. The data analysis performed in the report was performed on the nitrate concentration in soil rather than the nitrate formation rate as indicated in OECD guideline 216 and the Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008c). Therefore, the raw data available in the report appendix were used to calculate the nitrate transformation rate during the study and the extent by which these deviate from the control rates.
The variation in nitrate concentration of control replicates was less than 15% (actual ≤ 1.8%) for all time points (calculated from raw data). Nitrate formation rate deviations from the controls were less than 25% for the 485.4 μg monensin/kg soil treatments, calculated using both the incremental and overall method, for all time points. It can therefore be concluded that monensin present in Monimax® has no long term influence on the nitrogen transformation functionality of soil.
Toxicity to aquatic organisms
Effect on algae
A GLP‐compliant study following OECD guideline 201 was performed to investigate the effect of monensin on green algae (Pseudokirchneriella subcapitata).85 Based on the results of a non‐GLP range finding test, the algae was exposed to range of measured concentrations. To assess the stability of the test item, the concentration of monensin in the test media was determined at the start and end of the exposure period at all concentrations. Mean biomass increase in the control cultures was at least a factor of 16 within the 72 h test period (actual 137), mean coefficient of variation for section‐by‐section specific growth rates in the control cultures was ≤ 35% (actual 13.5%) and the coefficient of variation of average specific growth rates during test period in replicate control cultures were ≤ 7% (actual 2.0%). Some recoveries were more than 20% different from nominal concentration; therefore, the evaluation of test endpoints was performed using geometric mean of 0 and 72 h measured concentrations. Test species was exposed for 72 h to a range of measured concentration of 0.041, 0.150, 0.712, 2.094 and 9.457 mg monensin sodium/L. The 72 h ErC50 (median effective concentration which results in a 50% reduction in growth rate) was established at 3.3 mg monensin sodium/L and the 72 h ErC10 (median effective concentration which results in a 10% reduction in growth rate) at 0.91 mg monensin sodium/L.
Effect on crustaceans
A GLP‐compliant study following OECD guideline 202 was performed to investigate the effect of monensin (as monensin sodium) on aquatic invertebrates.86 Aquatic invertebrate species Daphnia magna were exposed to for up 48 h to nominal concentration of 0.46, 1.0, 2.2, 4.6, 10, 22 and 46 mg monensin sodium/L which corresponded to measured concentrations of 0.35, 0.72, 1.69, 3.74, 8.54, 18.42 and 35.29 mg/L respectively. To assess the stability of the test item, the concentration of monensin in the test media was determined at the start and end of the exposure period. Immobilised daphnids in the control are ≤ 10% (actual 0%) and dissolved oxygen concentration in control and test vessels at the end of the test is ≥ 3 mg/L (actual ≥ 7.4 mg/L (calculated from available air saturation value)). The evaluation of test endpoints was performed using measured monensin concentrations. The 48 h EC50 for immobilisation was determined to be 7.29 mg monensin sodium/L (equivalent to 7.08 mg monensin/L).
Effect on fish
A GLP‐compliant study following OECD guideline 203 was performed to investigate the effect of monensin (as monensin sodium) on fish.87 The fish species Oncorhynchus mykiss was exposed to 0.3125, 0.625, 1.25, 2.5 and 5 mg monensin sodium/L (equivalent to 0.3033, 0.607, 1.21, 2.43 and 4.85 mg monensin/L) for up to 96 h. To assess the stability of the test item, the concentration of monensin in the test media was determined at the start and end of the exposure period. Mortality in the controls was ≤ 10% (actual 0%) and dissolved oxygen in control and test vessels was ≥ 60% of the air saturation value (actual ≥ 80.5–89.4%), the concentrations of monensin determined in the test media were within 90–97% of nominal values and the conditions were within acceptable limits throughout the duration of the test. The 96‐h LC50 was determined to be 1.88 mg monensin sodium/L (equivalent to 1.83 mg monensin/L).
Additional information on aquatic toxicity
A GLP‐compliant study following OECD guideline 201 was performed to investigate the effect of monensin on cyanobacteria.88 Based on the results of a non‐GLP range finding test, the cyanobacterial species (Anabaena flos‐aquae) was exposed to 0.208, 0.685, 2.90, 5.95 and 17.4 mg monensin sodium/L for up to 72 h. To assess the stability of the test item, the concentration of monensin in the test media was determined at the start and end of the exposure period at all concentrations. Mean biomass increase in the control cultures was at least a factor of 16 within the 72 h test period (actual 151.0), mean coefficient of variation for section‐by‐section specific growth rates in the control cultures was ≤ 35% (actual 21.1%) and the coefficient of variation of average specific growth rates during test period in replicate control cultures were ≤ 10% (actual 1.6%). The evaluation of test endpoints was performed using mean measured concentrations. The 72 h ErC50 and the 72 h‐ no observed effect concentration (NOEC) were 6.8 mg/L and 0.2 mg/L, respectively.
A published article on the effect of monensin on zooplankton community from Hills et al. (2007) was made available by the applicant. The NOEL for the community was estimated at 0.05 mg/L. The microcosm study was focused on zooplankton communities and did not provide the information on the effect on different trophic levels. However, the study indicated that the effect on algae resulted in an indirect effect of monensin on zooplankton population's abundance.
Effect on sediment dwelling organisms
A GLP‐compliant study following OECD guideline 218 was performed to investigate the effect of monensin (as monensin sodium) on the sediment‐dwelling larvae of Chironomus riparius.89 The chironomid larvae were exposed to 0.032, 0.1, 0.32, 1.0, 3.2, 10 and 32 mg monensin sodium/kg sediment dry weight basis (dry weight) (equivalent to 0.031, 0.1, 0.31, 0.97, 3.11, 9.71 and 31.07 mg monensin/kg sediment (dry weight)) for up to 28 days. To assess the stability of the test item, the concentration of monensin in the test media was determined at the start and end of the exposure period. The emergence in the controls was > 70% at the end of the test, the emergence of adults in the control vessels occurred between 12 and 28 days, the oxygen concentration was > 60 the air saturation value, the pH of the overlying water was in the range pH 6–9 and the water temperature did not differ by more than 1.0°C. Monensin was not stable during the exposure period with mean measured concentrations 57.5% of nominal start concentrations at the end of the test. Therefore, the evaluation of biological endpoints was performed using mean measured concentrations. Emergence was the most sensitive endpoint and the NOEC was determined as 5.0 mg monensin sodium/kg sediment (dry weight) (equivalent to 4.9 mg monensin/kg sediment (dry weight)).
Conclusions on the ecotoxic effect of monensin sodium on soil, water and sediment
For the terrestrial compartment, data are available for microorganisms, earthworms and plants. The lowest toxicity value for the terrestrial compartment of EC50 4 mg monensin/kg was found for plant species Sinapis alba.
For the aquatic compartment, data are available for algae, aquatic invertebrates, fish and information from public literature on the effect of monensin sodium on zooplankton. The ErC50 and ErC10 used in the assessment are 3.3 mg/L and 0.91 mg/L for algae, respectively. The 48 h EC50 for immobilisation of daphnids was determined to be 7.29 mg monensin sodium/L and the 96 h LC50 for fish was 1.88 mg monensin sodium/L. The NOEL from the microcosmos study on zooplankton of 0.05 mg monensin sodium/L is as well used in the assessment.
Ecotoxicological data for sediment‐dwelling invertebrates are provided for the sediment compartment. The NOEC was determined as 5.0 mg monensin sodium/kg sediment (dry weight).
Risk characterisation (PEC/PNEC ratio)
The risk characterisation ratios for terrestrial, freshwater and sediment compartments are reported in Tables 12, 13 and 14, respectively.
Table 12.
Risk characterisation of monensin (PEC/PNEC ratio) for terrestrial compartment
| Taxa | PECsoil (μg/kg) | EC50/LC50 (mg/kg) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
| Earthworm | 70 | 108.8a | 100 | 1,088 | 0.06 |
| Plants | 4.0b | 100 | 40 | 1.75 |
AF: assessment factor.
LC50.
EC50.
Table 13.
Risk characterisation (PEC/PNEC ratio) for freshwater compartment
| Taxa | PECsurfacewater FOCUS (μg/L) | ErC50/ErC10/NOEL (mg/L) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
|
Algae Selenastrum capricornutum |
0.62 |
3.3a 0.91b |
1,000 1,000 |
3.41 0.91 |
0.2 0.68 |
|
Aquatic invertebrates Daphnia magna |
7.29c | 1,000 | 7.3 | 0.08 | |
|
Fish Brachydanio rerio |
1.83d | 1,000 | 1.83 | 0.34 | |
| Microcosm | 0.62 | 0.05e | 10 | 5 | 0.12 |
AF: assessment factor.
ErC50.
ErC10.
48h EC50.
96h LC50.
NOEL.
Table 14.
Risk characterisation (PEC/PNEC ratio) for sediment
| Taxa | PECsediment (μg/kg dry weight) | NOEC (mg/kg) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
|
Sediment‐dwelling invertebrates Chironomus riparius |
0.10 | 5.0 | 10 | 0.485 | 0.21 |
AF: assessment factor.
The previous risk characterisation for the terrestrial compartment (EFSA, 2005; EFSA FEEDAP Panel, 2011a) for plants was based on a NOEC. A re‐assessment of the original data indicated a large variability between the individual data and showed that the NOEC values would be less reliable than the EC50 values (both EC50 values, 29 and 4 mg/kg for wheat and mustard resulted in the same NOEC of 1 mg/kg). Therefore, the FEEDAP Panel decided to base the risk characterisation of plants on the EC50 values.
Bioaccumulation
No data on bioaccumulation on monensin sodium were submitted. The log Kow at pH 7 is 3.2 and there is evidence that monensin is degraded in the animal body (see Section 3.2.2.1). Therefore, a risk for bioaccumulation is unlikely.
NICARBAZIN (DNC and HDP)
3.2.4.3. Phase I
Physicochemical properties
The physicochemical properties of DNC and HDP are summarised in Tables 15 and 16.
Table 15.
Physicochemical properties of DNC
| Property | Value | Unit |
|---|---|---|
| Octanol/water partition coefficient (log Kow)a |
3.25 (pH 5) 3.21 (pH 7) 3.23 (pH 9) |
– |
| Water solubility (20°C)a | 0.0209 (pH 5–9, 20 ± 0.5°C) | mg/L |
| Dissociation constant (pKa)b | 12.44 ± 0.70c | – |
| Vapour pressurea | 3.1 × 10−10 | Pa |
Technical dossier/Supplementary information June 2015/Annex 9.
Technical dossier/Supplementary information June 2015/Annex 3.
Estimated value since the substance exhibits insufficient water solubility and ultraviolet‐visible absorptivity to enable experimental determination.
Table 16.
Physicochemical properties of HDPa
| Property | Value | Unit |
|---|---|---|
| Octanol/water partition coefficient (log Kow) |
−0.9546 (pH 5) −0.9232 (pH 7) −0.9528 (pH 9) |
– |
| Water solubility (20°C) |
66,740 (pH 5, 20°C) 65,400 (pH 7, 20°C) 70,290 (pH 9, 20°C) |
mg/L |
| Dissociation constant (pKa) | 3.75 (25°C) | – |
| Vapour pressure |
9.084 × 10−6 (20°C) 1.834 × 10−5 (25°C) |
Pa |
Technical dossier/Supplementary information June 2015/Annex 8.
Fate and behaviour
Fate in soil
Adsorption/desorption in soil
A GLP‐compliant soil adsorption/desorption study following the OECD guideline 106 batch equilibrium method was performed with five soils of differing properties using 14C radiolabelled DNC.90 The mass balance requirement of the study (> 90%) was met with recoveries of 92.3% and 98.7% for a loamy sand soil (2.3) and clay soil (6S), respectively. Due to very low water solubility, high adsorption to soil and the limits of analytical method, a determination of adsorption/desorption isotherms could not be performed and this element of the study was performed at two concentrations only. Adsorption coefficients normalised to the organic carbon content (Koc) for DNC are provided in Table 17. The geometric mean Koc value of 74,128.0 mL/g suggests that DNC can be considered non‐mobile.91 Desorption of DNC at twice the equilibrium time was below 75% for all soils, therefore DNC can be considered as irreversibly bound to soil.
Table 17.
Adsorption of DNC in different soilsa
| Soil identification | %OC | pH (CaCl2) | Kd | Koc |
|---|---|---|---|---|
| 2.1 | 0.68 | 5.1 | 279.9 | 41,161.8 |
| 2.2 | 1.93 | 5.5 | 2,192.5 | 113,601 |
| 2.3 | 0.99 | 6.7 | 1,237.7 | 125,020.2 |
| 2.4 | 2.53 | 7.1 | 1,564.8 | 61,849.8 |
| 6S | 1.66 | 7.1 | 1,227.6 | 61,903.6 |
| Geometric mean | 1,040.8 | 74,128 |
%OC: % of organic carbon; Kd: soil adsorption coefficient; Koc: adsorption or desorption coefficient corrected for soil organic carbon content.
A GLP‐compliant soil adsorption/desorption study following the OECD guideline 106 batch equilibrium method was performed with five soils of differing properties using 14C radiolabelled HDP.92 The mass balance requirement of the study (> 90%) was met with recoveries of 94.0% and 102.1% for a loamy sand soil (2.2) and clay soil (6S), respectively. Adsorption/desorption isotherms were performed at five concentrations spanning two orders of magnitude. Freundlich adsorption coefficients (Kf) normalised to the organic carbon content (Kfoc) for HDP are provided Table 18. The geometric mean Kfoc value of 101.8 mL/g suggests that HDP can be considered moderately mobile. Desorption of HDP at twice the equilibrium time was below 75% for all soils, therefore HDP can be considered as irreversibly bound to soil.
Table 18.
Adsorption of HDP in different soilsa
| Soil identification | %OC | pH (CaCl2) | Kf | 1/n | Koc |
|---|---|---|---|---|---|
| 2.1 | 0.68 | 5.1 | 1.014 | 0.8975 | 149.1 |
| 2.2 | 1.93 | 5.5 | 1.259 | 0.9161 | 65.2 |
| 2.3 | 0.99 | 6.7 | 1.345 | 0.8849 | 135.9 |
| 2.4 | 2.53 | 7.1 | 2.082 | 0.931 | 82.3 |
| 6S | 1.66 | 7.1 | 1.666 | 0.9016 | 100.4 |
| Geometric mean | 1.43 | 0.91 | 101.8 |
%OC: % of organic carbon; Kf: Freundlich constant; Koc: adsorption or desorption coefficient corrected for soil organic carbon content.
Degradation in soil
A GLP‐compliant soil biodegradation rate only study following OECD guideline 307 but with an extended incubation period of up to 400 days at 20 ± 2°C was performed with four soils of differing properties using unlabelled DNC.93 The study was performed at an application rate of 500 μg DNC/kg dry weight soil. An extended incubation period was used so that DT50 values could be interpolated rather than extrapolated during the determination of the degradation kinetics. The soil microbial biomass carbon as % of organic carbon for all four soils was > 1% w/w for the first 4 months (~ 120 days) of the study. At the final determination (~ 400 days), two soils, a sand and loamy sand, were at 0.7% and 0.4% w/w respectively. However, during this period, DNC dissipation was minimal and this will therefore not have affected the outcome of the study. The geometric mean of the soil DT50 in four soils was 96.6 days at 20°C. The transformation pattern exhibited a two phase kinetics with a slower phase at longer incubation times. The half‐life of the second phase was 562 days at 20°C. This value is used for the FOCUS calculations. For the simple soil accumulation calculation according to the Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008c), this can be recalculated to a DT50 of 1,191 days at 12°C (EFSA, 2007b). This shows that DNC is very persistent in soils.90 The DT50 values of unlabelled compounds give no information about the mineralisation of DNC hence it must be assumed that DNC is not mineralised.
A GLP‐compliant soil biodegradation rate study following OECD guideline 307 was performed with four soils of differing properties using unlabelled and 14C‐labelled HDP.94 The rate of biodegradation was determined in all four soils and route was determined in a sandy loam soil only. The study was performed at an application rate of 200 μg HDP/kg dry weight soil. The geometric mean of the soil DT50 in four soils was 2.3 days at 20°C. This indicates that HDP is not persistent in soils.90 For the simple soil accumulation calculation according to the Technical Guidance for assessing the safety of feed additives for the environment (EFSA 2008c) this can be recalculated to a DT50 of 4.9 days at 12°C (EFSA, 2007b).
Fate in water
No degradation of DNC or HDP in surface water or sediment was demonstrated.
Conclusion on fate and behaviour
For DNC, a Koc of 74,128 L/kg and a DT50 of 1,191 days will be used for the assessment; for HDP a Koc of 102 and a DT50 of 2.3 days will be used for the assessment.
Predicted environmental concentrations (PECs)
The input values for DNC used were: DNC concentration in turkey feed 35.44 mg/kg, molecular weight 302.24, vapour pressure 3.1 × 10−10 Pa, solubility 0.0209 mg/L, DT50 1,191 days and Koc 74,128 L/kg. The input values for HDP used were: HDP concentration in turkey feed 14.56 mg/kg, molecular weight 124.14, vapour pressure 9.084 × 10−6 Pa, solubility 65,400 mg/L, DT50 4.9 days and Koc 102 L/kg.
The calculated PEC initial values for both DNC and HDP are given in Table 19.
Table 19.
Initial predicted environmental concentration (PECs) of DNC and HDP (μg/kg), in soil (μg/kg), groundwater (μg/L), surface water (μg/L) and sediment (μg/kg dry weight)
| Input | Value | |
|---|---|---|
| DNC | HDP | |
| Dose (mg/kg feed) | 35.44 | 14.56 |
| Molecular weight | 302.24 | 124.14 |
| Vapour pressure (Pa) (at 25°C) | 3E‐10 | 9E‐6 |
| Solubility (mg/L) | 0.0209 | 65,400 |
| Koc (L/kg) | 74,128 | 102 |
| DT50 in soil at 12°C (days) | 1,191 | 4.9 |
| Output | ||
| PECsoil | 164 | 68 |
| PECgroundwater | 0.13 | 35 |
| PECsurfacewater | 0.042 | 12 |
| PECsediment | 155 | 79 |
The Phase I PEC trigger values are exceeded, therefore a Phase II assessment is considered necessary.
3.2.4.4. Phase II
Exposure assessment
PECs calculation refined in Phase II
DNC – refinement of PEC soil for persistent compounds
The DT90 (Disappearance Time 90) for DNC in all four soils was determined to be greater than 1 year, therefore the PECs refined at steady state was calculated (EFSA, 2008c). The results are provided in Table 20.
Table 20.
Refined plateau predicted environmental concentration (PECs) of DNC in soil (μg/kg), groundwater (μg/L), surface water (μg/L), and sediment (μg/kg dry weight)
| Compartment | PECplateau (DNC) |
|---|---|
| Soil | 858 |
| Ground water | 0.66 |
| Surface water | 0.2 |
| Sediment | 811 |
DNC ‐ PEC surface water PEC sediment refined with FOCUS
Concentrations in surface waters for DNC were assessed using the FOCUS Step 3 surface water assessment approach. The FOCUS recommended surface water models PRZM_SW, MACRO and TOXSWA were used.95 A FOCUS PRZM calculation was performed with an application rate of 493 g/ha, a Koc of 74,128 L/kg and a DT50 of 562 days at 20°C. According to the manual the drainage scenarios, D3 and D5 were selected together with the run‐off scenarios R1 and R3. For the run‐off scenarios, a homogeneous distribution of the DNC in 20 cm soil layer was assumed.
After 20 years, 3,212 g/ha was accumulated in the soil. This corresponds to 1,071 μg DNC/kg soil. Due to the high Koc, there was no drainage of DNC to groundwater, surface water or sediment.
The run‐off scenarios revealed a different picture. The R3 stream scenario indicated a maximum time weighted average exposure concentration of DNC in surface water to be 0.02 μg/L in the first year. The corresponding sediment concentration was 48 μg/kg sediment. Since the run‐off will be 3,212/493 = 6.5 times higher after 20 years, the surface water concentration will be 6.5 × 0.02 = 0.16 μg/L. The sediment concentration can exceed 6.5 × 48 = 313 μg/kg since DNC might accumulate over the years in sediment. Nitroaromatic compounds can be reduced in sediment to the corresponding anilines (van Beelen and Burris, 1995). Nevertheless, no data on the transformation of DNC in sediment were provided by the applicant. Especially in aerobic sediments there might be a continuous increase in DNC concentrations over the years.
Conclusions on PEC used for calculation
The following values are used for the assessment: for DNC a PECsoil of 1,071 μg/kg, a PECsurface water of 0.16 μg/L and a PECsediment of > 313 μg/kg; for HDP a PECsoil of 68 μg/kg, PECsurface water of 12 μg/L and PECsediment of 79 μg/kg.
Ecotoxicity studies
Toxicity of nicarbazin (DNC and HDP) to soil organisms
Effects on plants
A GLP‐compliant terrestrial plant seedling emergence and growth study following OECD guideline 208 was performed with six plant species; four dicotyledonous (Phaseolus vulgaris, Raphanus sativus, Cucumis sativus and Solanum lycopersicum) and two monocotyledonous (Hordeum vulgare and Allium cepa) in natural soil (sandy loam).96 DNC and HDP are present in nicarbazin as an equimolar complex. Therefore, they were applied in combination during the study to provide an estimate of the potential risk to terrestrial plants. DNC and HDP were applied at an equimolar ratio equivalent to that present in nicarbazin, 248 mg/kg and 102 mg/kg, respectively. Control seedling emergence for all species was ≥ 70% (lowest 89.3% for A. cepa), the mean survival of emerged control seedlings was ≥ 90% (lowest 96.7% for R. sativus and S. lycopersicum) and the control seedlings did not exhibit and visible phytotoxic effects. Five endpoints were measured during the study; seedling emergence, seedling survival, visual injury, stem length and growth (as dry weight biomass). No negative effects were measured for any of the endpoints at the tested concentrations of HDP and DNC, expect for reductions in shoot dry weight for C. sativus (15.5%) and shoot length for S. lycopersicum (7.4%) on fourteen days after control emergence. EC50 values for the six species tested are provided in Table 21.
Table 21.
DNC and HDP (nicarbazin) ecotoxicological effects data for terrestrial plants (mg/kg)
| Compound | Endpoint | R. sativus | H. vulgare | A. cepa | C. sativus | P. vulgaris | S. lycopersicum |
|---|---|---|---|---|---|---|---|
| DNC | EC50 | > 248 | > 248 | > 248 | > 248 | > 248 | > 248 |
| HDP | EC50 | > 102 | > 102 | > 102 | > 102 | > 102 | 102 |
DNC: dinitrocarbanilide; HDP: 2‐hydroxy‐4,6‐dimethylpyrimidine.
Effect on earthworms
A GLP‐compliant earthworm reproduction study following OECD guideline 222 was performed with the earthworm Eisenia fetida in an artificial soil.97 According to this guideline, the substance is mixed into the soil and the earthworms are fed with clean manure without any toxicants. This is different from the situation in the field where the substance is present in the manure at much higher concentrations than the final concentration in soil. DNC and HDP are present in nicarbazin as an equimolar complex. Therefore, they were applied in combination during the study. DNC and HDP were applied at an equimolar ratio equivalent to that present in nicarbazin, 300 mg/kg and 123.46 mg/kg, respectively. Adult mortality in the controls over the initial 4 weeks of the test was ≤ 10% (actual 0.83%), all control replicates produced > 30 juvenile worms (actual 119) and the coefficient of variation of reproduction was ≤ 30% (actual 23%). No signs of abnormal behaviour were observed in the treated replicates and there was no significant difference in juvenile worm production between the control and treated groups. Therefore, it was concluded that for DNC and HDP, the NOEC was 300 mg/kg and 123.46 mg/kg, respectively.
Effects on soil microorganisms
A GLP‐compliant nitrogen transformation study following OECD guideline 216 was performed in a natural sandy loam soil.98 DNC and HDP are present in nicarbazin as an equimolar complex. Therefore, they were applied in combination during the study to provide an estimate of the potential risk to soil microorganisms. The components of nicarbazin were applied to soil in combination equivalent to their equimolar ratio, at a rate of at 500 μg DNC/kg and 200 μg HDP/kg. This was a factor of ~ 2.5 greater than the estimated maximum initial PECsoil for DNC and HDP. They were also applied at a rate 10 times these levels (5,000 μg DNC/kg and 2,000 μg HDP/kg). The soil nitrogen transformation rate was determined at 7, 14 and 28 days after application. The variation in soil nitrate concentration for replicate control samples was < 15% for all time points (actual ≤ 7.5%); therefore, the study was valid. Nitrogen transformation rates were calculated using two methods: an incremental method calculating rates based on the difference between sequential time‐points and an overall method, calculating rates based on the difference between time‐points and the initial concentration.
According to guidance on the interpretation of results from nitrogen transformation studies (CVMP, 2011), nitrate formation rate deviations from the controls at the PECsoil for time points earlier than 28 days are not considered to be critical. Nitrate formation rate deviations from the controls for the 500 mg DNC/kg and 200 mg HDP/kg application rate during the study using both the incremental and overall methods were greater than 25% on day 28 (48.25% and 74.39%, respectively).
The study was extended to 100 days. Nitrate formation rate deviations from the controls for the all treatments calculated using both the incremental and overall method, were less than 25% on day 100 of the study (all less than 3.7%). Therefore, the combination of DNC and HDP is not expected to pose the risk for nitrogen transforming soil microorganisms.
Toxicity of nicarbazin (DNC and HDP) to aquatic organisms
Effect on algae
A compliant study following OECD guideline 201 was performed to investigate the effect of DNC on green algae.99 Based on the results of a non‐GLP range finding test, the green algal species (Pseudokirchneriella subcapitata) was exposed to nominal concentrations of 0.191, 0.610, 1.953, 6.25 and 20.0 μg/L for up to 72 h. To assess the stability of the test item, the concentration of DNC in the test media was determined at the start and end of the exposure period for the highest and lowest exposure concentrations. Mean biomass increase in the control cultures was at least a factor of 16 within the 72‐h test period (actual 188), mean coefficient of variation for section‐by‐section specific growth rates in the control cultures was ≤ 35% (actual 4.9%) and the coefficient of variation of average specific growth rates during test period in replicate control cultures were ≤ 10% (actual 0.9%). The evaluation of test endpoints was performed using mean measured lowest and highest concentration at the beginning and at the end of the test. The arithmetic mean of the highest exposure concentration at the beginning and at the end of the test was 10.13 μg/L DNC.
A GLP‐compliant study following OECD guideline 201 was performed to investigate the effect of HDP on green algae as a limit test.100 Based on the results of a non‐GLP range finding test, the green algal species (Pseudokirchneriella subcapitata) was exposed to 101.5 mg/L for up to 72 h. To assess the stability of the test item, the concentration of HDP in the test media was determined at the start and end of the exposure period. Mean biomass increase in the control cultures was at least a factor of 16 within the 72 h test period (actual 171), mean coefficient of variation for section‐by‐section specific growth rates in the control cultures was ≤ 35% (actual 10%) and the coefficient of variation of average specific growth rates during test period in replicate control cultures were ≤ 10% (actual 0.7%). The evaluation of test endpoints was performed using nominal concentrations as the measured concentrations were within 20% of nominal values (Table 22).
Table 22.
Effects concentrations for growth rate at 72 h (ErC50) of Pseudokirchneriella subcapitata to DNC and HDP
| ErC50 | NOEC | |
|---|---|---|
| DNC | > 10.13 | 10.13 |
| HDP | > 101.5 | 101.5 |
Effect on crustaceans
A GLP‐compliant study following OECD guideline 202 was performed to investigate the effect of DNC (nicarbazin) on aquatic invertebrates.101 Aquatic invertebrate species Daphnia magna were exposed to filtrate at a loading rate of 100 mg/L for up to 48 h. To assess the stability of the test item, the concentration of DNC in the test media was determined at the start and end of the exposure period. The study was valid, immobilised daphnids in the control are ≤ 10% (actual 0%) and dissolved oxygen concentration in control and test vessels at the end of the test is ≥ 3 mg/L (actual ≥ 8.1 mg/L). The evaluation of test endpoints was performed using measured concentrations. No immobilisation of daphnids were observed at the concentrations of actual substance solubility (1.35 mg/L); thus, the 48 h EC50 for immobilisation was determined to be > 1.35 μg/L.
A GLP‐compliant study following OECD guideline 211 was performed to investigate the chronic effect of DNC (nicarbazin) on aquatic invertebrates.102 Aquatic invertebrate species Daphnia magna were exposed in a limit test to filtrate at a loading rate of 10 mg/L for 21 days. A test with a semi‐static design was used with media renewals every 2–3 days. To assess the stability of the test item, the concentration of DNC in the test media was determined from fresh and aged media for each media renewal. In the controls, mortality of the parent animals (female Daphnia) did not exceed 20% at the end of the test (actual 10%) and the mean number of live offspring produced per parent animal surviving at the end of the text was ≥ 60 (actual 146.7). The evaluation of biological endpoints was performed using the time weighted mean for the exposure concentration. The test was performed at the maximum solubility of DNC in media and no statistical effects were observed, therefore the 21‐day NOEC for reproduction for daphnids was determined to be 4.51 μg/L.
A GLP‐compliant study following OECD guideline 202 was performed to investigate the effect of HDP (nicarbazin) on aquatic invertebrates.103 Aquatic invertebrate species Daphnia magna were exposed 6.25, 12.5, 25, 50 and 100 mg/L for up to 48 h. To assess the stability of the test item, the concentration of HDP in the test media was determined at the start and end of the exposure period. Immobilised daphnids in the control are ≤ 10% (actual 0%) and dissolved oxygen concentration in control and test vessels at the end of the test is ≥ 3 mg/L (actual ≥ 9.4 mg/L). The evaluation of biological endpoints was performed using nominal concentrations since the analytical determinations were less than 20% of nominal values. The 48 h EC50 for immobilisation was determined to be > 100 mg/L.
Effect on fish
A GLP‐compliant study following OECD guideline 203 was performed to investigate the effect of DNC on fish.104 The fish species Danio rerio was exposed to 100 mg/L filtrate for up to 96 h. To assess the stability of the test item, the concentration of DNC in the test media was determined at the start and end of the exposure period. Mortality in the controls was ≤ 10% (actual 0%) and dissolved oxygen in control and test vessels was ≥ 60% of the air saturation value (actual ≥ 95%), the concentrations of the test item determined in the test media were within 73–86% of nominal values and the conditions were within acceptable limits throughout the duration of the test. The 96 h LC50 was determined to be > 5.4 μg/L.
A GLP‐compliant study following OECD guideline 203 was performed to investigate the effect of HDP on fish.105 The fish species Danio rerio were exposed to 6.25, 12.5, 25, 50 and 100 mg/L for up to 96 h. To assess the stability of the test item, the concentration of HDP in the test media was determined at the start and end of the exposure period. Mortality in the controls was ≤ 10% (actual 0%) and dissolved oxygen in control and test vessels was ≥ 60% of the air saturation value (actual ≥ 99%), the concentrations of the test item determined in the test media were within 103–104% of nominal values and the conditions were within acceptable limits throughout the duration of the test. The 96 h LC50 was determined to be > 100 mg/L.
The data on aquatic toxicity endpoints for DNC and HDP considered in the environmental risk assessment are summarised in Table 23.
Table 23.
Data on aquatic toxicity endpoints for DNC and HDP considered in the environmental risk assessment
| Taxonomic group | Tested species | Endpoint | Result DNC (μg/L) | Result HDP (mg/L) |
|---|---|---|---|---|
| Green algae | Pseudokirchneriella subcapitata | ErC50 | > 10.13 | > 101.5 |
| NOEC | 10.13 | 101.5 | ||
| Crustaceans | Daphnia magna | 48‐h EC50 | > 1.35 | > 100 |
| 21‐day NOEC | 4.51 | / | ||
| Fish | Danio rerio | 96‐h LC50 | > 5.4 | > 100 |
Additional information on aquatic toxicity
A GLP‐compliant study following OECD guideline 201 was performed to investigate the effect of DNC on cyanobacteria.106 Based on the results of a non‐GLP range finding test, the cyanobacterial species (Anabaena flos‐aquae) was exposed to 100 mg/L filtrate and 1:22, 1:10, 1:4.6 and 1:2.2 dilutions for up to 72 h. To assess the stability of the test item, the concentration of DNC in the test media was determined at the start and end of the exposure period in all concentrations. Mean biomass increase in the control cultures was at least a factor of 16 within the 72‐h test period (actual 35), mean coefficient of variation for section‐by‐section specific growth rates in the control cultures was ≤ 35% (actual 9.3%) and the coefficient of variation of average specific growth rates during test period in replicate control cultures were ≤ 10% (actual 0.7%). The evaluation of biological endpoints was performed using measured concentrations.
A GLP‐compliant study following OECD guideline 201 was performed to investigate the effect of HDP on cyanobacteria.107 Based on the results of a non‐GLP range finding test, the cyanobacterial species (Anabaena flos‐aquae) was exposed to 1.0, 3.16, 10.0, 31.6 and 100.0 mg/L for up to 72 h. To assess the stability of the test item, the concentration of HDP in the test media was determined at the start and end of the exposure period in all concentrations. Mean biomass increase in the control cultures was at least a factor of 16 within the 72‐h test period (actual 404.0), mean coefficient of variation for section‐by‐section specific growth rates in the control cultures was ≤ 35% (actual 34.9%) and the coefficient of variation of average specific growth rates during test period in replicate control cultures were ≤ 10% (actual 2.7%). The evaluation of biological endpoints was performed using nominal concentrations since the analytical determinations were within 20% of nominal values.
Effect on sediment dwelling organisms
A GLP‐compliant study following OECD guideline 218 was performed to investigate the effect of DNC on the sediment‐dwelling larvae of Chironomus riparius.108 The chironomid larvae were exposed to 15.4, 30.3, 61.2, 120.9, 241.1 and 484.0 mg/kg sediment dry weight basis (dry weight) for up to 28 days. To assess the stability of the test item, the concentration of DNC in the test media was determined at the start and end of the exposure period. The emergence in the controls was > 70% at the end of the test, the emergence of adults in the control vessels occurred between 12 and 24 days, the oxygen concentration was > 60 the air saturation value, the pH of the overlying water was in the range pH 6–9 and the water temperature did not differ by more than 1.0°C. The evaluation of biological endpoints was performed using calculated concentrations. Emergence was the most sensitive endpoint and the NOEC was determined as 241.1 mg/kg sediment (dry weight).
A GLP‐compliant study following OECD guideline 218 was performed to investigate the effect of HDP on the sediment‐dwelling larvae of Chironomus riparius.109 The chironomid larvae were exposed to 35, 70, 140, 280 and 560 mg/kg sediment dry weight basis (dry weight) for up to 28 days. To assess the stability of the test item, the concentration of HDP in the test media was determined at the start and end of the exposure period. The emergence in the controls was > 70% at the end of the test, the emergence of adults in the control vessels occurred between 16 and 28 days, the oxygen concentration was > 60 the air saturation value, the pH of the overlying water was in the range pH 6–9 and the water temperature did not differ by more than 1.0°C. The evaluation of biological endpoints was performed using calculated concentrations. Emergence was the most sensitive endpoint and the NOEC was determined as 556.7 mg/kg sediment (dry weight).
Conclusions on the ecotoxic effect on soil, water and sediment compartment for DNC and HDP from nicarbazin
For the terrestrial compartment, data were provided for microorganisms, earthworms and plants. For the effect of DNC and HDP on the reproduction of earthworms, the NOEC was set on 300 and 123.46 mg/kg (dry weight), respectively. In two of six tested plant species, a statistically significant effect of the equimolar mixture of DNC and HDP was observed, however the effect concentration could not be calculated. The FEEDAP Panel calculated the effect concentration based on information provided in the test report. Assuming the worst case, the EC50 for plants is set to 102 and 248 mg/kg of HDP and DNC, respectively.
For the aquatic compartment, data for DNC are available for acute and chronic effect on algae, aquatic invertebrates and on acute toxic effects on fish. No effect of DNC could be observed in any of performed tests, thus, the applicant proposes the lowest concentration tested as the NOEC value. The DNC NOEC for algae is 10.13 μ/L. The 21‐day NOEC for reproduction of daphnids was determined to be 4.51 μg/L. The 96 h LC50 in fish was determined to be > 5.4 μg/L. Data for the toxic effect of HDP on the aquatic compartment was studied in tests of acute and long term effect on algae and acute effect on daphnids and fish resulting in NOEC value of 101.5 mg/L for algae. The LC50 and EC50 for fish and immobilisation for Daphnia were determined to be > 100 mg/L, respectively. Data on the effect of DNC and HDP on cyanobacteria are considered as supportive information.
Ecotoxicological data for the DNC and HDP for sediment‐dwelling invertebrates are provided for the sediment compartment. The NOEC for DNC and HDP were determined as 241.1 and 556.7 mg/kg sediment (dry weight), respectively.
Risk characterisation (PEC/PNEC ratio) for DNC and HDP
The risk characterisation ratios for terrestrial, freshwater and sediment compartments are reported in the tables below (Tables 24–28).
Table 24.
Risk characterisation (PEC/PNEC ratio) for DNC and for HDP for the terrestrial compartment
| Taxa | PECsoil (μg/kg) | EC50/ NOEC (mg/kg) | AF | PNEC (μg/kg) | PEC/PNEC | |
|---|---|---|---|---|---|---|
| DNC | Earthworm | 1,071 | 300a | 10 | 30,000 | 0.036 |
| Plants | 248b | 100 | 2,480 | 0.4 | ||
| HDP | Earthworm | 68 | 123.46a | 10 | 12,346 | 0.006 |
| Plants | 102b | 100 | 1,020 | 0.07 |
AF: assessment factor.
NOEC.
EC50.
Table 28.
Risk characterisation (PEC/PNEC ratio) for sediment for HDP
| Taxa | PECsediment (μg/kg) | NOEC (mg/kg) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
|
Sediment‐dwelling invertebrates hironomus riparius |
78 | 556.7 | 10 | 55,670 | 0.001 |
AF: assessment factor.
Table 25.
Risk characterisation (PEC/PNEC ratio) for the freshwater compartment for DNC
| Taxa | PECsurfacewater (μg/L) | LC50/ NOEC (μg/L) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
|
Algae Selenastrum subspicatus |
0.16 | 10.13a | 50 | 0.20 | 0.8 |
|
Aquatic invertebrates Daphnia magna |
4.51a | 50 | 0.09 | 2 | |
|
Fish Brachydanio rerio |
> 5.4b | / | / |
AF: assessment factor.
NOEC.
96 h LC50.
According to the FEEDAP guidance on the environmental risk assessment for feed additives (EFSA, 2008c), two chronic tests on the aquatic compartment would allow a risk assessment of this compartment. Chronic tests with DNC were provided for algae and aquatic invertebrates, with HDP only for algae.
Table 26.
Risk characterisation (PEC/PNEC ratio) for the freshwater compartment for HDP
| Taxa | PECsurfacewater (μg/L) | EC50/LC50/NOEC (mg/L) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
|
Algae Selenastrum subspicatus |
12 | 101.5a | 1,000 | 101.5 | 0.12 |
|
Aquatic invertebrates Daphnia magna |
> 100 mg/Lb | / | |||
|
Fish Brachydanio rerio |
> 100 mg/Lc | / |
AF: assessment factor.
NOEC.
EC50.
LC50.
Table 27.
Risk characterisation (PEC/PNEC ratio) for sediment for DNC
| Taxa | PECsediment (μg/kg) | NOEC (mg/kg) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
|
Sediment‐dwelling invertebrates Chironomus riparius |
> 313 | 241 | 10 | 24,100 | / |
AF: assessment factor.
Bioaccumulation
No data on bioaccumulation have been submitted. The log Kow for DNC is > 3 for HDP < 3. The high persistence and hydrophobicity of DNC indicate that there might be a risk for bioaccumulation.
3.2.4.5. Conclusions on safety for the environment
The use of monensin sodium from Monimax® in complete feed for turkeys does not pose a risk for the aquatic compartment and sediment, while a risk cannot be excluded for the terrestrial compartment based on the results of an ecotoxicity test on plants. The bioaccumulation potential of monensin in the environment is low.
A final conclusion on the risk resulting from the use of nicarbazin from Monimax® in turkeys cannot be made for the following reasons: (i) DNC refined PECs showed uncertainties linked to the very high persistence of the compound, (ii) DNC might accumulate in the sediment compartment and (iii) DNC can potentially bioaccumulate and may cause secondary poisoning. The PEC/PNEC ratios indicate a risk for daphnids but no adverse effect were seen at the concentration tested. This adds further uncertainty to the risk assessment of DNC in the aquatic compartment. No concerns would arise for the HDP moiety of nicarbazin excreted from turkeys fed Monimax®.
The potential of DNC to accumulate in soil over the years should be investigated by monitoring in a field study.
In summary, based on the available data, the FEEDAP Panel cannot conclude on the safety of Monimax® for the environment.
3.3. Efficacy
Efficacy data for coccidiostats (following Art. 4 of Reg. (EC) No 1831/2003) should derive from three types of target animal experiments (Reg. (EC) No 429/2008): (a) dose‐titration studies (b) natural/artificial infection to simulate use conditions (e.g. floor pen studies with poultry), at least one of the locations should be in the EU and (c) actual use conditions in field trials, all should be done in the EU within the last five years. Anticoccidial sensitivity tests (AST) could replace field trials provided they follow the criteria mentioned in the guidance document on coccidiostats and histomonostats (EFSA FEEDAP Panel, 2011b).110
The applicant submitted one dose‐titration study, three floor pen studies and three ASTs in turkeys for fattening.
3.3.1. Dose‐titration study
The applicant submitted a dose‐titration study with Monimax® made under controlled conditions in turkeys for fattening artificially infected with mixed Eimeria spp.111
A total of 288 day‐old male turkeys for fattening (Big 9) were distributed to eight treatment groups (six pens per treatment, six turkeys per pen); an uninfected untreated control (UUC) group was compared with an infected untreated control (IUC) and six infected treated (IT) groups. The treatments were 30 + 30 (IT30), 40 + 40 (IT40) and 50 + 50 (IT50) mg/kg monensin + nicarbazin/kg feed; dosage was analytically confirmed. The remaining three groups were treated with nicarbazin but results were not reported. On day 16, birds in the IUC and IT groups were inoculated with 108,000 E. meleagrimitis, 4,000 E. dispersa and 30,000 E. adenoeides. The anticoccidial treatment was provided from day 13 until day 28 (study completion). An ANOVA was performed with the data and group means were compared with least significant difference (LSD) test.
No mortality occurred during the study. Final body weight and daily weight gain were significantly higher in IT30 and IT50 groups than in the IUC group and were similar to the values of the UUC group. Feed intake did not show significant differences between the groups. The feed to gain ratios of the IT groups improved significantly compared to the IUC group and were similar to the UUC group. Oocyst excretion was lower in the IT groups compared to the IUC group; significant difference was found between the IUC and the IT50 group on day 23. No significant differences in lesion scores were seen between the groups.
3.3.2. Floor pen studies in turkeys for fattening
Three floor pen studies in turkeys for fattening, conducted in 2011–2012, were submitted.112 In each study birds were penned and distributed into three treatment groups: an UUC group, an IUC group and an IT group. The IT group received feed containing 40 mg monensin and 40 mg nicarbazin/kg feed, dosage was analytically confirmed (see Table 29). The duration of the studies was 84/85/85 days in trial 1, 2 and 3, respectively. The experimental diets were fed for 83/84/84 days, considering a 1‐day withdrawal period. Trial 1 and 2 were performed with male turkeys while trial 3 with female turkeys. In trial 1, all birds in the pen were inoculated on day 14 with recent field isolates of pathogenic Eimeria species (E. meleagrimitis, E. adenoeides, E. dispersa). In trial 2 and 3 in the infected groups, individually tagged seeder birds (6 out of 34 per pen in trial 2 and 5 out of 11 in trial 3) were inoculated on day 15 and 14, respectively.113 The same number of animals was sham‐inoculated (water only) in the UUC group. Animal health and mortality were monitored daily. Feed intake and body weight of the animals were measured throughout the study, feed to gain ratio was calculated. Samples of excreta were analysed for oocyst excretion. Intestinal lesions were scored in trial 2 and 3 only.
Table 29.
Experimental design of floor pen studies with turkeys for fattening using Monimax®
| Trial | Start date | Replicates per treatment (birds per pen) | Test animal | Dieta | |||
|---|---|---|---|---|---|---|---|
| Type | Period (days) | Feed analysis (mg/kg feed) | |||||
| Monensin | Nicarbazin | ||||||
| 1 | 12/2011 |
10 (12) |
Day‐old male turkeys (BUT 8) |
Starter Grower Finisher |
0–28 28–56 56–84 |
30 36 38 |
36 39 36 |
| 2 | 8/2011 |
9 (34) |
Day‐old male turkeys (Big 7) |
Starter Grower Finisher |
0–21 22–50 51–85 |
28 24 28 |
26 28 34 |
| 3 | 2/2012 |
10 (11) |
Day‐old female turkeys (Hybrid Converter) |
Starter Grower Finisher |
0–28 28–56 56–83 |
33 35 38 |
34 36 39 |
Birds in the IT group were fed a basal diet supplemented with Monimax®. Animals in the control groups UUC and IUC received the same basal diet without inclusion of the coccidiostat.
In all three trials, an ANOVA was applied to the data; the experimental unit was the pen for the zootechnical parameters. Oocyst excretion was statistically analysed only in trials 1 and 3 and the pen was taken as experimental unit. Lesion scoring was not performed in trial 1; in trial 2, the experimental unit for the lesions cores was the bird while in trial 3 lesions were analysed with a non‐parametric test and the pen was taken as experimental unit. Level of significance was set at a p‐value ≤ 0.05. In trial 1 and 3 group means were compared with Tukey test and in trial 2 with LSD test.
Table 30 shows the intestinal lesion scores. In trial 2, no significant reduction of the lesion scores was seen in the IT group compared to the IUC group for E. meleagrimitis and E. adenoeides. In trial 3, for both Eimeria species the lesion scores were significantly lower in the IT group compared to the IUC group on both observation days. Lesions found in UUC birds were comparable to those in IT birds.
Table 30.
Eimeria infection related intestinal lesion scores in seeder birds and contact birds
| Trial | Day 22 (seeder birds) | Day 29 (contact birds) | ||
|---|---|---|---|---|
| E. meleagrimitis | E. adenoeides | E. meleagrimitis | E. adenoeides | |
| Trial 2 a | ||||
| UUC | 0.00 | 0.00a | 0.00 | 0.61 |
| IUC | 0.22 | 0.72b | 0.00 | 0.61 |
| IT | 0.28 | 0.72b | 0.11 | 0.50 |
| Day 20 (seeder birds) | Day 27 (contact birds) | |||
|---|---|---|---|---|
| Trial 3 b | ||||
| UUC | 1.33b | 0.58c | 1.36ab | 0.35b |
| IUC | 3.83a | 2.54a | 1.85a | 1.85a |
| IT | 1.79b | 1.29b | 0.97b | 0.53b |
Lesions scores were determined on two birds per pen according to the method of Johnson and Reid (1970) (0 = no lesion, 1 = very mild, 2 = mild, 3 = moderate and 4 = severe).
Lesions scores were determined on three birds per pen according to the Repérant scoring system.
a,b,c: Means in a column within a study with different superscript are significantly different (p ≤ 0.05).
Results of oocyst excretion are presented in Tables 31 and 32. Total oocyst excretion was not reduced in the IT groups compared to the IUC groups at the different time points. Eimeria strain specific excretion data in trial 1 showed that excretion of E. dispersa was significantly lower in the IT group compared to the IUC group at day 21 as it was for E. adenoeides at day 42. The data also showed oocyst excretion in the UUC groups already at the first measurement (day 21/20) in trials 1 and 3. In all trials, oocyst excretion in the UUC groups near to the end of studies was approximately at the level of the infected groups.
Table 31.
Total number of Eimeria oocysts per gram of excreta (OPG) in floor pen trial 1 and 2
| Trial 1 | Day 21 | Day 28 | Day 35 | Day 42 | Day 49 | Day 56 | Day 77 |
|---|---|---|---|---|---|---|---|
| UUC | 100b | 1,380b | 4,040 | 36,320 | 1,060 | 140 | 160 |
| IUC | 609,000a | 16,620a | 2,960 | 53,040 | 800 | 500 | 220 |
| IT | 602,600a | 4,420ab | 2,580 | 1,720 | 3,020 | 260 | 280 |
| Trial 2 | Day 21 | Day 28 | Day 35 | Day 42 | Day 49 | Day 56 | Day 77 |
| UUC | 0 | 0 | 400 | 24,000 | 24,000 | 200 | 0 |
| IUC | 14,400 | 17,000 | 53,200 | 75,000 | 400 | 0 | 0 |
| IT | 28,200 | 3000 | 34,800 | 52,800 | 1,200 | 0 | 0 |
a,b,c Means in a column within a study with different superscript are significantly different (p ≤ 0.05). In trial 2 the data was not statistically analysed.
Table 32.
LS means of log10 transformed oocyst counts in floor pen trial 3
| Trial 3 | Day 20 | Day 27 | Day 56 | Day 85 |
|---|---|---|---|---|
| UUC | 1.63a | 2.25a | 2.64 | 1.36 |
| IUC | 5.54b | 4.64b | 2.27 | 1.84 |
| IT | 5.67b | 4.29b | 2.75 | 1.61 |
a,b,c Means in a column within a study with different superscript are significantly different (p ≤ 0.05).
Table 33 summarises the results concerning mortality and zootechnical endpoints. Mortality after Eimeria inoculation was lower in the IT group compared to IUC group in all three trials but the difference was significant only in trial 1. In trial 1, body weight gain was significantly higher in the IT group compared to the IUC group. In trial 2, the final body weight and the feed to gain ratio of the IT group was significantly improved compared to IUC group.
Table 33.
Performance data and mortality of turkeys in floor pen trials with Monimax® a
| Feed intakeb (kg) | Body weight (kg) | Weight gainc (kg) | Feed to gain ratio | Mortality n (%) | |
|---|---|---|---|---|---|
| Trial 1 | |||||
| UUC | 22.44b | – | 11.63b | 1.93 | 0 (0)a |
| IUC | 21.84a | – | 11.20a | 1.95 | 13 (10.8)b |
| IT | 22.37ab | – | 11.56b | 1.94 | 1 (0.8)a |
| Trial 2 | |||||
| UUC | 0.290 | 10.72a | 0.125 | 2.30b | 8 (2.6) |
| IUC | 0.284 | 10.46b | 0.122 | 2.31b | 13 (4.3) |
| IT | 0.281 | 10.75a | 0.126 | 2.22a | 6 (2.0) |
| Trial 3 | |||||
| UUC | 0.141 | – | 0.091 | 1.91 | 6 (5.5) |
| IUC | 0.130 | – | 0.088 | 1.92 | 10 (9.1) |
| IT | 0.138 | – | 0.090 | 1.90 | 5 (4.6) |
a,b,c: Means in a column within a study with different superscript are significantly different (p ≤ 0.05).
Results refer to the overall treatment period (84/85/83 days respectively in the three trials).
Results in trial 1 are reported as total feed intake per animal, results in trial 2 and 3 are daily feed intake.
Results in trial 1 are reported as total weight gain, results in trial 2 and 3 are daily weight gain.
3.3.3. Anticoccidial sensitivity tests in turkeys for fattening
Three ASTs with a similar experimental design, performed in 2012, were submitted.114 Each test was made with the groups UUC, IUC and IT, the latter receiving feed supplemented with Monimax® at an intended concentration of 40 mg monensin and 40 mg nicarbazin/kg feed; dosage was analytically confirmed (37 mg monensin, 36 mg nicarbazin). In each test, day‐old male turkeys for fattening (Big 9) were randomly allocated to the groups (6 replicates with 6 birds). Birds received the diets from day 13 to day 28 and were artificially infected on day 15 with sporulated oocysts from field isolates.115 Animal health and mortality were monitored. Feed intake and body weight of the animals were measured, feed to gain ratio was calculated. Samples of excreta were analysed for oocyst excretion. Intestinal lesions were scored following the Repérant scoring system for the species relevant to turkeys.
The data were analysed by ANOVA. Level of significance was set at a p‐value ≤ 0.05 and group means were compared by LSD test.
Table 34 summarises the results of the three ASTs. There was no bird mortality in the three tests in any of the treatment groups. Oocyst excretion 8 days after inoculation indicated successful Eimeria infection on day 23. No oocysts per gram of excreta (OPG) was found in the UUC groups. These findings support the validity of the ASTs. OPG was significantly reduced by the Monimax® treatment (comparison of IT vs IUC) in all ASTs.
Table 34.
Results of anticoccidial sensitivity tests in turkeys
| AST | Tr. group | Daily feed Intake (kg) | Body weight (kg) | Daily weight gain (kg) | Feed to gain ratio | Total OPG | Average lesion scores | ||
|---|---|---|---|---|---|---|---|---|---|
| E. meleagrimitis | E. adenoeides | Total | |||||||
| D13–28 | D28 | D13–28 | D13–28 | D23 | D23 | ||||
| 1 | UUC | 0.065 | 0.793a | 0.039a | 1.67a | 0c | 0.3a | 0b | 0.3b |
| IUC | 0.067 | 0.709b | 0.033b | 2.01b | 118,967b | 0.3a | 0.6a | 0.9a | |
| IT | 0.060 | 0.771a | 0.037a | 1.63a | 69,800a | 0.7b | 0.6a | 1.3a | |
| 2 | UUC | 0.065 | 0.793ab | 0.039ab | 1.67ab | 0c | 0.3b | 0a | 0.3c |
| IUC | 0.068 | 0.756b | 0.036b | 1.89b | 140,667b | 1.2a | 0.5b | 1.7b | |
| IT | 0.068 | 0.850a | 0.042a | 1.64a | 21,800a | 0.9a | 0a | 0.9a | |
| 3 | UUC | 0.065 | 0.793a | 0.039a | 1.67a | 0c | 0.3a | 0a | 0.3a |
| IUC | 0.064 | 0.725b | 0.034b | 1.90b | 90,834b | 1.3b | 0.9b | 2.2b | |
| IT | 0.063 | 0.788a | 0.039a | 1.65a | 26,667a | 0.5a | 0.2a | 0.7a | |
a,b,c: Means in a column within a study with different superscript are significantly different (p ≤ 0.05).
In AST‐3, a reduction of Eimeria spp. specific lesion scores in IT compared to IUC group was observed. The total average lesion score reported in AST‐2 and AST‐3 showed a significant reduction in the IT groups (compared to IUC) and in AST‐3 down to the level of UUC.
The adverse effect of oocyst inoculation was seen by a significant depression (IUC vs UUC) of daily weight gain and final body weight in ASTs 1 and 3. A beneficial effect of the anticoccidial treatment (IT vs IUC) was observed as a significantly higher daily weight gain and significantly improved feed to gain ratio in the three ASTs.
3.3.4. Synopsis of the efficacy studies in turkeys for fattening
The synopsis is based on the three floor pen studies and three anticoccidial sensitivity tests made with the lowest applied concentration of the coccidiostat Monimax® (40 mg monensin and 40 mg nicarbazin/kg feed).
In one floor pen study and two ASTs, total intestinal lesions were significantly reduced.
Treatment with Monimax® did not significantly reduce oocyst excretion in the three floor pen studies measured at different time points. In one trial, Eimeria strain specific excretion data showed some significant improvement for two strains in one time point each. In contrast, the three ASTs showed a significant reduction of oocyst excretion by Monimax®.
The final body weight in one floor pen study and the body weight gain in another study were significantly higher for the Monimax® treated groups compared to the infected non treated birds. In both trials, mortality due to coccidiosis was reduced by the coccidiostatic treatment; however, the reduction was only significant in floor pen study 1.
In the three ASTs, the anticoccidial efficacy of Monimax®, shown by an improvement of coccidiosis‐related specific endpoints.
The efficacy of Monimax® as a coccidiostat for turkeys for fattening was demonstrated in three ASTs showing significant improvements of coccidiosis related endpoints as it was shown for mortality in one floor pen study and for lesion score in another floor pen study. In a third floor pen study, mortality was not significantly reduced; however, significantly higher body weight and better feed to gain ratio indicate together with the reduced mortality a coccidiostatic effect.
3.3.5. Conclusions on efficacy studies
Based on three floor pen studies and three ASTs, the FEEDAP Panel concludes that Monimax® has the potential to control coccidiosis in turkeys for fattening at a minimum concentration of 40 mg monensin and 40 mg nicarbazin/kg complete feed.
3.4. Post‐market monitoring
Field monitoring of Eimeria spp. resistance to monensin sodium/nicarbazin should be undertaken, preferably during the latter part of the period of authorisation.
The potential of DNC to accumulate in soil over the years should be investigated by monitoring and a field study.
4. Conclusions
Monimax® is considered safe for turkeys for fattening at the highest use level of 50 mg monensin and 50 mg nicarbazin/kg complete feed. The margin of safety is about 1.5. The simultaneous use of Monimax® and certain antibiotic drugs (i.e. tiamulin) is contraindicated. Monensin has a selective antimicrobial activity against Gram‐positive bacterial species while many Enterobacteriaceae are naturally resistant. Induction of cross‐resistance with clinically relevant antimicrobials or increased shedding of enteropathogenic bacteria are not reported. Nicarbazin has no antimicrobial activity.
Monensin sodium is absorbed at a limited extent and excreted rapidly, it is extensively metabolised and gives rise to demethylated, oxidised and decarboxylated metabolites. Nicarbazin, when ingested, is rapidly split in its two components HDP and DNC which behave independently. Liver is the target tissue. DNC residues decline rapidly from tissues following nicarbazin withdrawal. DNC appears as the marker residue. HDP‐related residues are much lower than those derived from DNC. For both compounds of Monimax®, the metabolic pathways in the turkey are similar to those in the chicken and rat.
The FEEDAP Panel concludes that the active substances in Monimax®, monensin sodium and nicarbazin, do not represent a genotoxic risk. No safety concerns would arise from the nicarbazin impurities PNA and M4NPC. Monensin sodium has no structural alert for carcinogenesis. Monensin sodium is not a reproductive or developmental toxicant. The lowest NOEL identified in the developmental study in rabbits is 0.3 mg monensin sodium/kg bw per day for maternal toxicity in rabbits. The primary toxicity resulting from the oral use of nicarbazin is renal toxicity. The absence of similar findings after treatment with DNC and HDP confirms that this equimolar association of compounds is better tolerated than nicarbazin at equivalent doses. The lowest NOAEL identified in a 52‐week study in rat using DNC + HDP was 20 mg DNC + 8 mg HDP/kg bw per day based on the absence of microcrystals in urine and related microscopic renal observations. No significant interaction between monensin sodium and nicarbazin is expected from toxicological studies.
The use of Monimax® at the highest proposed dose (50 mg monensin and 50 mg nicarbazin/kg complete feed) will not pose a risk to persons consuming animal products from treated turkeys for fattening. No safety concern would arise from the impurity PNA if the maximum content in nicarbazin of 0.1% is respected. The impurity M4NPC is considered safe for the consumer provided that a maximum concentration of 0.4% in nicarbazin is not exceeded. No withdrawal time is required for Monimax® in turkeys for fattening. Residue data comply with the established MRLs for monensin and DNC.
The monensin sodium contained in Monimax® presents a hazard by inhalation. Monimax® is not a skin irritant; however, no data are available for the eye irritation potential of monensin. Monimax® may also act as a dermal toxicant due to its monensin component. Monimax® is not a skin sensitiser.
The use of monensin sodium from Monimax® in complete feed for turkeys for fattening does not pose a risk for the aquatic compartment and sediment, while a risk cannot be excluded for the terrestrial compartment. A final conclusion on the risk resulting from the use of nicarbazin from Monimax® cannot be made because (i) DNC refined PECs show uncertainties linked to the very high persistence of the compound, (ii) DNC might accumulate in the sediment compartment and (iii) DNC can potentially bioaccumulate and may cause secondary poisoning. No concerns would arise for the HDP moiety of nicarbazin excreted from chickens fed Monimax®. Based on the available data, the FEEDAP Panel cannot conclude on the safety of Monimax® for the environment.
The FEEDAP Panel concludes that Monimax® has the potential to control coccidiosis in turkeys for fattening at a minimum concentration of 40 mg monensin and 40 mg nicarbazin/kg complete feed.
Documentation provided to EFSA
Monimax® for turkeys for fattening. September 2012. Submitted by Huvepharma N.V.
Monimax® for turkeys for fattening. Supplementary information. November 2013. Submitted by Huvepharma N.V.
Monimax® for turkeys for fattening. Supplementary information. June 2015. Submitted by Huvepharma N.V.
Monimax® for turkeys for fattening. Supplementary information. October 2015. Submitted by Huvepharma N.V.
Monimax® for turkeys for fattening. Supplementary information. February 2017. Submitted by Huvepharma N.V.
Monimax® for turkeys for fattening. Supplementary information. October 2017. Submitted by Huvepharma N.V.
Evaluation report of the European Union Reference Laboratory for Feed Additives on the Methods of Analysis for Monimax®.
Comments from Member States.
Abbreviations
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism and excretion
- ANOVA
analysis of variance
- AST
anticoccidial sensitivity tests
- bw
body weight
- CAS
Chemical Abstracts Service
- DITR
Dietary intake of total residues
- DNC
4,4’‐dinitrocarbanilide
- DT50
Disappearance Time 50 (the time within which the concentration of the test substance is reduced by 50%)
- DT90
Disappearance Time 90 (the time within which the concentration of the test substance is reduced by 90%)
- EC50
median effective concentration
- ErC10
median effective concentration which results in a 10% reduction in growth rate
- ErC50
median effective concentration which results in a 50% reduction in growth rate
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FOCUS
FOrum for Co‐ordination of pesticide fate models and their USe
- GLP
Good Laboratory Practice
- HDP
2‐hydroxy‐4,6‐dimethylpyrimidine
- Kf
Freundlich constant
- Koc
adsorption or desorption coefficient corrected for soil organic carbon content
- LC50
median lethal concentration
- LD50
median lethal dose
- LLNA
local lymph node assay
- LOD
limit of detection
- log Kow
octanol/water partition coefficient
- LOQ
limit of quantification
- M4NPC
methyl(4‐nitrophenyl) carbamate
- MIC
minimum inhibitory concentrations
- MRC
marker residue concentration
- MRL
maximum residue limit
- NOAEL
no observed adverse effect level
- NOEC
no observed effect concentration
- NOEL
no observed effect level
- OECD
Organisation for Economic Co‐operation and Development
- OPG
oocysts per gram of excreta
- PEC
predicted environmental concentration
- pKa
dissociation constant
- PNA
p‐nitroaniline
- PNEC
predicted no effect concentration
- RH
relative humidity
- RMTR
ratio marker to total residues
- SCAN
Scientific Committee on Animal Nutrition
- SD
standard deviation
- TRC
total residue concentration
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Brantom P, Halle I, van Beelen P, Holczknecht O, Vettori MV and Gropp J, 2017. Scientific Opinion on the safety and efficacy of Monimax® (monensin sodium and nicarbazin) for turkeys for fattening. EFSA Journal 2017;15(12):5094, 49 pp. 10.2903/j.efsa.2017.5094
Requestor: European Commission
Question number: EFSA‐Q‐2012‐00906
Panel members: Gabriele Aquilina, Vasileios Bampidis, Maria de Lourdes Bastos, Georges Bories, Andrew Chesson, Pier Sandro Cocconcelli, Maria Luisa Fernández‐Cruz, Gerhard Flachowsky, Jürgen Gropp, Boris Kolar, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Alberto Mantovani, Baltasar Mayo, Fernando Ramos, Guido Rychen, Maria Saarela, Roberto Edoardo Villa, Robert John Wallace and Pieter Wester.
Adopted: 29 November 2017
Legal notice: A previous, provisional version of this output containing only the abstract and the summary has been replaced by this version. Relevant information or parts of this scientific output have been blackened in accordance with the European Commission decision on the confidentiality requests formulated by the applicant and further confidentiality requests formulated by the applicant for which a decision by the European Commission is pending. The blackened text will be subject to review once the full decision on the confidentiality requests is adopted by the European Commission. The full output was shared with the European Commission, EU Member States and the applicant.
Amended: 6 August 2018
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Uitbreidingstraat 80, 2600 Antwerp, Belgium.
Commission Regulation (EC) No 109/2007 of 5 February 2007 concerning the authorisation of monensin sodium (Coxidin) as a feed additive. OJ L 31, 6.2.2007, p. 6. amended by Commission Regulation (EC) No 1095/2008 of 6 November 2008 amending Regulation (EC) No 109/2007 as regards the terms of the authorisation of the feed additive monensin sodium (Coxidin). OJ L 298, 7.11.2008, p. 3. and Commission Implementing Regulation (EU) No 140/2012 of 17 February 2012 concerning the authorisation of monensin sodium as a feed additive for chickens reared for laying (holder of authorisation Huvepharma NV Belgium). OJ L 47, 18.2.2012, p. 18.
Commission Regulation (EC) No 1356/2004 of 26 July 2004 concerning the authorisation for 10 years of the additive ‘Elancoban’ in feedingstuffs, belonging to the group of coccidiostats and other medicinal substances. OJ L 251, 27.7.2004, p. 6. amended by Commission Regulation (EC) No 108/2007 of 5 February 2007 amending Regulation (EC) No 1356/2004 as regards the conditions for authorisation of the feed additive Elancoban, belonging to the group of coccidiostats and other medicinal substances. OJ L 31, 6.2.2007, p. 4. and by Commission Regulation (EC) No 1096/2008 of 6 November 2008 amending Regulation (EC) No 1356/2004 as regards the terms of the authorisation of the feed additive ‘Elancoban’, belonging to the group of coccidiostats and other medicinal substances. OJ L 298, 7.11.2008, p. 5.
OJ L 31, 6.2.2007, p. 6. and OJ L 47, 18.2.2012, p. 18.
OJ L 298, 7.11.2008, p. 3. and OJ L 47, 18.2.2012, p. 18.
Commission Regulation (EU) No 875/2010 of 5 October 2010 concerning the authorisation for 10 years of a feed additive in feedingstuffs. OJ L 263, 6.10.2010, p. 4.
Commission Regulation (EU) No 885/2010 of 7 October 2010 concerning the authorisation of the preparation of narasin and nicarbazin as a feed additive for chickens for fattening (holder of the authorisation Eli Lilly and Company Ltd) amending Regulation (EC) No 2430/1999. OJ L 265, 8.10.2010, p. 5.
OJ L 263, 6.10.2010, p. 4. and OJ L 265, 8.10.2010, p. 5.
FEED dossiers reference: FAD‐2012‐0032.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep-fad-2012-0027-monimax.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II/Annex 17.
■■■■■
■■■■■.
Technical dossier/Section II/Annex 13.
Technical dossier/Section II/Annex 5.
■■■■■
■■■■■
■■■■■
■■■■■
Technical dossier/Supplementary information February 2017/Annex 26.
Including the following: Escherichia coli (ATCC 25922), Escherichia coli (UK – human), Pseudomonas aeruginosa (ATCC 27853), Pseudomonas aeruginosa (UK – human), Staphylococcus aureus (ATCC 25923), Staphylococcus aureus (UK – human), Enterococcus faecalis (ATCC 29212), Enterococcus faecalis (UK – human), Bacillus subtilis (ATCC 6633), Bacillus spp. (UK – human), Salmonella Typhimurium (NCIMB 13034), Salmonella Typhimurium (UK – chicken), Campylobacter jejuni (ATCC 33560), Campylobacter jejuni (UK – human), Campylobacter jejuni (German cow isolate), Campylobacter coli (UK – human), Campylobacter coli (German cow isolate), Campylobacter coli (UK cow isolate), Bacteroides spp. (Bacteroides spp.), Bacteroides fragilis (UK – human), Bifidobacterium bifidum (ATCC 29521), Bifidobacterium spp. (UK – human), Clostridium perfringens (NCTC 8237), Clostridium spp. (UK – human), Eubacterium lentum (ATCC 43055), Eubacterium cylindroides (UK – human), Fusobacterium necrophorum (ATCC 25286), Fusobacterium nucleatum (UK – human), Peptostreptococcus anaerobius (ATCC 27337), Peptostreptococcus anaerobius (UK – human), Lactobacillus casei (UK – human), Lactobacillus acidophilus (UK – human), Lactobacillus lactis (DSM 20384).
Technical dossier/Supplementary information February 2017/Annex 09.
■■■■■
■■■■■
Technical dossier/Supplementary information October 2017/Annex 2.
Technical dossier/Supplementary information October 2017/Annex 4.
Technical dossier/Section II/Annex 6.
Technical dossier/Section II/Annex 8.
Technical dossier/Section II/Annex 9.
Technical dossier/Section II/Annex 10.
Technical dossier/Section II/Annex 11.
Technical dossier/Section II/Annex 31 and amendment received via letter dated 13/6/2017.
Technical dossier/Section II/Annex 32.
Technical dossiers/Section II/Annex 33.
Technical dossiers/Section II/Annex 32 and 33.
FAD‐2012‐0032/Technical dossier/Section III/Annex 1.
FAD‐2012‐0032/Technical dossier/Supplementary information February 2017/Annex 11.
RBC (red blood count), haematocrit, haemoglobin, MCH (mean corpuscular haemoglobin), MCV (mean corpuscular volume), MCC (mean corpuscular haemoglobin concentration), thrombocyte check, WBC (white blood cell count: heterophils, eosinophils, basophils, monocytes and lymphocytes).
AST (aspartate aminotransferase), ALT (alanine aminotransferase), ALP (alkaline phosphatase), LDH (lactate dehydrogenase), CK (creatine kinase), total protein, total bilirubin, BIAC (bile acids), total cholesterol, glucose, calcium, inorganic phosphorus, magnesium, sodium, potassium, chloride, URAC (uric acid), creatinine.
Technical dossier/Supplementary information February 2017/Annex 13.
Technical dossier/Supplementary information October 2017/Annex 5.
Technical dossier/Section III/Annex 2. Staphylococcus sp., Pasteurella multocida, Salmonella sp., Escherichia coli (3 serotypes: 078K80, O1K1 and O2K1 strains), Enterococcus sp. and Clostridium perfringens.
Technical dossier/Section III/Annex 3. Bifidobacterium spp., Eubacterium spp., Clostridium spp., Peptostreptococcus spp..
Technical dossier/Supplementary information June 2015/Annex VII_Reference 5.
Cross‐reference to FAD‐2012‐0027 (Monimax® for chickens for fattening and reared for laying) Technical dossier/Supplementary information February 2017/Annex 14.
Cross‐reference to FAD‐2012‐0027 (Monimax® for chickens for fattening and reared for laying) Technical dossier/Supplementary information February 2017/Annex 15.
Technical dossier/Section III/Annex 8.
Technical dossier/Supplementary information February 2017/Annex 14.
Technical dossier/Supplementary information February 2017/Annex 16.
Technical dossier/Section III/Annex 32.
Technical dossier/Section III/Annex 37.
Technical dossier/Section III/Annex 38.
Technical dossier/Section III/Annex 39.
Technical dossier/Section III/Annex 40.
Technical dossier/Section III/Annex 41.
Technical dossier/Section III/Annex 42.
Technical dossier/Supplementary information February 2017/Annex 20.
Erythrocytes (RBC), mean cell volume (MCV), packed cell volume (PCV), haemoglobin (HB), mean cell haemoglobin concentration (MCHC), mean cell haemoglobin (MCH), thrombocytes (PLT), leucocytes (WBC), differential WBC with cell morphology, prothrombin time (PT), fibrinogen, activated partial thromboplastin (APTT) and reticulocytes (RTC).
Na, K, Cl, Ca, P, glucose, urea, creatinine, total bilirubin, total cholesterol, triglycerides, alkaline phosphatase, alkaline aminotransferase, aspartate aminotransferase, total protein, albumin and albumin/globulin ratio.
Volume, pH and specific gravity and semi‐quantitatively for proteins, glucose, ketones, bilirubin, nitrites, blood and urobilinogen. Sediment was analysed microscopically for presence of crystals and cells.
Technical dossier/Supplementary information June 2015/Annex II.
Volume, pH and specific gravity and semi‐quantitatively for proteins, glucose, ketones, bilirubin, nitrites, blood and urobilinogen.
Technical dossier/Supplementary information June 2015/Annex I.
Technical dossier/Section III/Annex 46.
Technical dossier/Supplementary information June 2015/Annex III.
Technical dossier/Section III/Annex 43.
Technical dossier/Supplementary information June 2015/Annex IV.
The exposure of target animals to PNA when using Monimax® at the highest applied concentration would be below 50 μg PNA/kg complete feed for poultry.
Technical dossier/Supplementary information October 2017Annex 5.
OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section III/Annex III.33.
Technical dossier/Section III/Annex III.34 and 35.
Technical dossier/Section III/Annex III.36.
Technical dossier/Supplementary information June 2015/Annex 23.
Technical dossier/Supplementary information June 2015/Annex 28.
Technical dossier/Supplementary information June 2015/Annex 24.
The temperature correction was performed according to the scientific opinion of the Panel on Plant Protection Products and their Residues on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil (EFSA, 2007).
Technical dossier/Supplementary information June 2015/Annex 15.
Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration. OJ L 372, 27.12.2006, p. 19.
Technical dossier/Supplementary information June 2015/Annex 29.
Technical dossier/Supplementary information June 2015/Annex 13.
Technical dossier/Supplementary information June 2015/Annex 6.
Technical dossier/Supplementary information October 2017/Annex 6.
Technical dossier/Supplementary information June 2015/Annex 14.
Technical dossier/Supplementary information June 2015/Annex 19.
Technical dossier/Supplementary information June 2015/Annex 12.
Technical dossier/Supplementary information June 2015/Annex 2.
Technical dossier/Supplementary information June 2015/Annex 25.
Technical dossier/Supplementary information June 2015/Annex 15.
Technical dossier/Supplementary information June 2015/Annex 26.
Technical dossier/Supplementary information June 2015/Annex 30.
Technical dossier/Supplementary information June 2015/Annex 31.
Technical dossier/Supplementary information February 2017/Annex 27.
Technical dossier/Supplementary information June 2015/Annex 16.
Technical dossier/Supplementary information June 2015/Annex 1.
Technical dossier/Supplementary information June 2015/Annex 32.
Technical dossier/Supplementary information February 2017/Annex 29.
Technical dossier/Supplementary information February 2017/Annex 28.
Technical dossier/Supplementary information June 2015/Annex 21.
Technical dossier/Supplementary information October 2015/DNC_Daphnia_chronic.
Technical dossier/Supplementary information June 2015/Annex 7.
Technical dossier/Supplementary information June 2015/Annex 22.
Technical dossier/Supplementary information June 2015/Annex 11.
Technical dossier/Supplementary information June 2015/Annex 20.
Technical dossier/Supplementary information June 2015/Annex 10.
Technical dossier/Supplementary information June 2015/Annex 17.
Technical dossier/Supplementary information June 2015/Annex 18.
The FEEDAP Panel stated in its guidance for the preparation of dossiers for coccidiostats and histomonostats (EFSA FEEDAP Panel, 2011a,b,c) that studies with artificial infection would be preferred over field trials due to their inherent weaknesses. These short term studies should use field strains of Eimeria, recently confirmed as pathogenic/resistant by a sensitivity test or recognised problems in the poultry operation (confirmed by veterinary certificate). The Eimeria field strains should ideally undergo one, but in any case not more than two passage(s) before use in such trials.
Technical dossier/Section IV/Annex IV.1.
Trial 1: Technical dossier/Section IV/Annex IV.2. Trial 2: Technical dossier/Section IV/Annex IV.3. and Trial 3: Technical dossier/Section IV/Annex IV.4.
Trial 1: French field isolate (07/2011); estimated dosage per bird: 110,000 E. meleagrimitis, 22,000 E. adenoeides, 26,000 E. dispersa; Trial 2: German field isolate (04/2011); estimated dose per bird: 105,000 E. meleagrimitis, 19,000 E. adenoeides, 6,000 E. dispersa; Trial 3: Belgium field isolate (07/2011); estimated dose per bird was 98,000 E. meleagrimitis, 30,000 E. adenoeides, 2,000 E. dispersa.
AST 1: Technical dossier/Section IV/Annex IV.5. AST 2: Annex IV.6. AST 3: Annex IV.7.
AST 1: French field isolate (2011), estimated dosage per bird: 108,000 E. meleagrimitis, 4,000 E. dispersa 30,000 E. adenoeides; AST 2: German field isolate (2011), estimated dose per bird: 57,500 E. meleagrimitis, 500 E. dispersa 24,000 E. adenoeides; and AST 3: Belgian field isolate (2011), estimated dosage per bird: 54,000 E. meleagrimitis, 56,000 E. adenoeides.
References
- van Beelen P and Burris DR, 1995. Reduction of the explosive 2,4,6‐trinitrotoluene by enzymes from aquatic sediments. Environmental Toxicology and Chemistry 14, 2115–2123. [Google Scholar]
- Chopade HM and Matthews HB, 1984. Disposition and metabolism of p‐Nitroaniline in the male F‐344 rat. Fundamental and Applied Toxicology, 4, 485–493. [DOI] [PubMed] [Google Scholar]
- CVMP (Committee for Medicinal Products for Veterinary Use), 2011. Questions and answers document on implementation of ERA Guideline in support of VICH guidelines (GL 6 and GL 38), EMEA/CVMP/ERA/172074/2008‐Rev.3.
- Danzeisen JL, Kim HB, Isaacson RE, Tu ZJ and Johnson TJ, 2011. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS ONE 6, e27949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doydora SA, Sun P, Cabrera M, Mantripragada N, Rema J, Pavlostathis SG, Huang C‐H and Thompson A, 2017. Long‐term broiler litter amendments can alter the soil's capacity to sorb monensin. Environmental Science and Pollution Research International, 24, 13466–13473. 10.1007/s11356-017-8727-9 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004a. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the reevaluation of coccidiostat Elancoban in accordance with article 9G of Council Directive 70/524/EEC. EFSA Journal 2004;2(3):42, 61 pp. 10.2903/j.efsa.2004.72 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2004b. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the efficacy and safety of the coccidiostat Koffogran. EFSA Journal 2004;2(3):16, 40 pp. 10.2903/j.efsa.2004.16 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2004c. Opinion of the scientific Panel on Additives and Products or substances used in Animal Feed on a request from the Commission on the re‐evaluation of Sacox 120 microGranulate in accordance with article 9G of Council Directive 70/524/EEC. EFSA Journal 2004;2(7):76, 49 pp. 10.2903/j.efsa.2004.76 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the evaluation of the coccidiostat COXIDIN® (Monensin Sodium). EFSA Journal 2005;3(11):283, 53 pp. 10.2903/j.efsa.2005.283 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006a. Opinion of the Panel on additives and products or substances used in animal feed (FEEDAP) on the safety of Coxidin® (monensin sodium). EFSA Journal 2006;4(7):283, 10 pp. 10.2903/j.efsa.2006.381 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006b. Opinion of the Panel on additives and products or substances used in animal feed (FEEDAP) on the Maximum Residue Limit for monensin sodium for chickens and turkeys for fattening. EFSA Journal 2006;4(11):413, 13 pp. 10.2903/j.efsa.2006.413 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007a. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on efficacy of Coxidin® 25 % (monensin sodium) as a feed additive for turkeys. EFSA Journal 2007;5(9):545, 13 pp. 10.2903/j.efsa.2007.545 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007b. Scientific opinion of the Panel on Plant Protection Products and their Residues on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2007;5(12):622, 32 pp. 10.2903/j.efsa.2007.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on a request from the European Commission on withdrawal period for Elancoban® for chickens for fattening, chickens reared for laying and turkeys for fattening. EFSA Journal 2008;6(7):730, 16 pp. 10.2903/j.efsa.2008.730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008b. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the withdrawal period for Coxidin® for chickens and turkeys for fattening and re‐examination of the provisional Maximum Residue Limit. EFSA Journal 2008;6(7):731, 14 pp. 10.2903/j.efsa.2008.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008c. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008; 6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008d. Technical Guidance: microbial Studies. EFSA Journal 2008;6(10):836, 3 pp. 10.2903/j.efsa.2008.836 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008e. Technical Guidance: extrapolation of data from major species to minor species regarding the assessment of additives for use in animal nutrition. EFSA Journal 2008;6(9):803, 5 pp. 10.2903/j.efsa.2008.803 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products used in Animal Feed), 2010a. Scientific Opinion on the safety and efficacy of Koffogran (nicarbazin) as a feed additive for chickens for fattening. EFSA Journal 2010;8(3):1551, 40 pp. 10.2903/j.efsa.2010.1551 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products used in Animal Feed), 2010b. Scientific Opinion on the safety and efficacy of Maxiban® G160 (narasin and nicarbazin) for chickens for fattening. EFSA Journal 2010;8(4):1574, 45 pp. 10.2903/j.efsa.2010.1574 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products used in Animal Feed), 2011a. Scientific Opinion on the safety and efficacy of Coxidin® (monensin sodium) as feed additive for chickens reared for laying. EFSA Journal 2011;9(12):2442, 15 pp. 10.2903/j.efsa.2011.242 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011b. Guidance for the preparation of dossiers for coccidiostats and histomonostats. EFSA Journal 2011;9(5):2174, 12 pp. 10.2903/j.efsa.2011.2174 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011c. Technical guidance: tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal 2012;10(6):2740, 10 pp. 10.2903/j.efsa.2012.2740 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products used in Animal Feed), 2013. Scientific Opinion on the modification of the withdrawal period for Coxidin® (monensin sodium) for chickens for fattening and chickens reared for laying. EFSA Journal 2013;11(1):3045, 15 pp. 10.2903/j.efsa.2013.3045 [DOI] [Google Scholar]
- European Commission , 1981, online. Report of the Scientific Committee for Animal Nutrition on the use of monensin sodium in feedingstuffs for poultry. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_antibiotics-26.pdf
- European Commission , 1983, online. Report of the Scientific Committee for Animal Nutrition on the use of monensin sodium in feedingstuffs for turkeys. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_antibiotics-27.pdf
- European Commission , 1991, online. Report of the Scientific Committee for Animal Nutrition on the use of Narasin + Nicarbazin in feedingstuffs for chickens. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_antibiotics-31.pdf
- European Commission , 1995, online. Complementary report of the Scientific Committee for Animal Nutrition on Question 52 by the Commission on the of Narasin + Nicarbazin in feedingstuffs for chickens. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_antibiotics-32.pdf
- GR (Gezondheidsraad, Health Council of the Netherlands), 2008. P‐nitroaniline. Evaluation of the carcinogenicity and genotoxicity.
- Hills DG, Lissemore L, Sibley PK and Solomon KR, 2007. Effects of monensin on zooplankton communities in aquatic microcosms. Environmental Science and Technology, 41, 6620–6626. [DOI] [PubMed] [Google Scholar]
- Johnson J and Reid WM, 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor pen experiments with chickens. Experimental Parasitology, 28, 30–36. [DOI] [PubMed] [Google Scholar]
- Seeger K, Flinspach K, Haug‐Schifferdecker E, Kulik A, Gust B, Fiedler H‐P and Heide L, 2011. The biosynthetic genes for prenylated phenazines are located at two different chromosomal loci of Streptomyces cinnamonensis DSM 1042. Microbial Biotechnology 4, 252–262. Published online 2011 Feb 22. 10.1111/j.1751-7915.2010.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simjee S, Heffron AL, Pridmore A and Shryock TR, 2012. Reversible monensin adaptation in Enterococcus faecium, Enterococcus faecalis and Clostridium perfringens of cattle origin: potential impact on human food safety. Journal of Antimicrobial Chemotherapy, 67, 2388–2395. [DOI] [PubMed] [Google Scholar]
- Sun P, Pavlostathis SG and Huang C‐H, 2016. Estimation of environmentally relevant chemical properties of veterinary ionophore antibiotics. Environmental Science and Pollution Research, 23, 18353–18361. 10.1007/s11356-016-7029-y [DOI] [PubMed] [Google Scholar]


