Abstract
The performance of different bluetongue control measures related to both vaccination and protection from bluetongue virus (BTV) vectors was assessed. By means of a mathematical model, it was concluded that when vaccination is applied on 95% of animals even for 3 years, bluetongue cannot be eradicated and is able to re‐emerge. Only after 5 years of vaccination, the infection may be close to the eradication levels. In the absence of vaccination, the disease can persist for several years, reaching an endemic condition with low level of prevalence of infection. Among the mechanisms for bluetongue persistence, the persistence in the wildlife, the transplacental transmission in the host, the duration of viraemia and the possible vertical transmission in vectors were assessed. The criteria of the current surveillance scheme in place in the EU for demonstration of the virus absence need revision, because it was highlighted that under the current surveillance policy bluetongue circulation might occur undetected. For the safe movement of animals, newborn ruminants from vaccinated mothers with neutralising antibodies can be considered protected against infection, although a protective titre threshold cannot be identified. The presence of colostral antibodies interferes with the vaccine immunisation in the newborn for more than 3 months after birth, whereas the minimum time after vaccination of animal to be considered immune can be up to 48 days. The knowledge about vectors ecology, mechanisms of over‐wintering and criteria for the seasonally vector‐free period was updated. Some Culicoides species are active throughout the year and an absolute vector‐free period may not exist at least in some areas in Europe. To date, there is no evidence that the use of insecticides and repellents reduce the transmission of BTV in the field, although this may reduce host/vector contact. By only using pour‐on insecticides, protection of animals is lower than the one provided by vector‐proof establishments.
Keywords: bluetongue, vaccination, surveillance, vector, Culicoides, insecticides
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1182/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1171/full
Summary
The European Commission has requested the European Food Safety Authority (EFSA) to provide an updated scientific advice on bluetongue (BT), due to the recent disease evolution in the European Union (EU), the experience gained from the BT control policies and possible alternative methods to ensure safe trade of live animals from BT restricted zones. The scientific advice asked from EFSA should serve to review the overall BT policy at the EU level. The terms of reference of this request covered different topic areas, in particular related to (1) BT control policy through vaccination and surveillance; safe trade of animals moved from BT virus (BTV) infected to BTV‐free country or zone, both (2) about animal immunity and (3) protection from BTV vectors; (4) classification of BT serotypes and (5) BT listing and categorisation in the framework of the Animal Health law. The present opinion covered the first three categories, the fourth and fifth topic area will be covered in a separate scientific opinion.
As regards the first topic area about vaccination, eradication and surveillance, it was requested to assess the most suitable duration of a BT vaccination campaign intended to achieve disease freedom in a country or region. For that purpose, a mathematical model was developed to analyse the disease spread according to different duration of vaccination campaigns in different areas in Europe, selected for their specific patterns of disease and vector ecology (the UK, France, southern Spain and Sardinia in Italy). It was concluded that even when the vaccination of 95% of the susceptible cattle and sheep is constantly applied for three consecutive years, BTV is not eradicated and may re‐emerge after a couple of years. Only after 5 years of vaccination of 95% of susceptible cattle and sheep, the prevalence of infection is close to eradication levels, although reaching zero values for sheep only in the scenario of UK, France and Sardinia, but still not reaching zero for the Spanish scenario. These findings suggest that specific conditions related to animal density, meteorological conditions, etc., should be considered when planning a vaccination strategy against BT.
Secondly it was requested to assess the probability of BT recurrence in affected areas that have regained BT freedom, in particular due to BT low level circulation. Possible persistence in livestock was explored by the above‐mentioned model, by inferring what level of virus circulation could be achieved in a host population on long term without any intervention. It was found out that without any vaccination the disease can persist for several years, reaching an endemic condition with low level of prevalence of infection (1.5% in cattle, 0.6% in sheep) and greater seroprevalence levels (45% in cattle, 14% in sheep).
Further mechanisms for BT persistence were assessed through literature review in particular in relation to the possible persistence in the wildlife, to the transplacental transmission in the host, to the length of BTV viraemia or persistence in other host tissues, and to the vertical transmission in the vectors. The studies carried out on wildlife suggest that among wild ruminant populations, red deer (Cervus elaphus) is the wild ruminant species most likely to be involved in BTV circulation in Europe, and it may be possible that BTV infection persist locally in red deer population or in other wild ruminants in areas of high density of these animals, and where there are a low number of competing domestic animals and favourable vector conditions. Nevertheless, since this evidence is not confirmed, annual cross‐sectional surveys with a focus on yearlings may need to be conducted to ascertain the role of wild ruminant population in the BTV circulation and persistence in specific geographical areas.
Concerning the other persistence mechanisms, there is evidence that transplacental transmission (TPT) occurs in cattle, sheep and goats, under field conditions, for BTV‐8. The incidence varies by animal species and gestational stage of infection. For BTV serotypes other than BTV‐8, TPT was experimentally demonstrated only for BTV‐2 in sheep and BTV‐11 in cattle and North American elks. The overall contributions of TPT to the over‐wintering mechanism and the epidemiological significance of the presence of BTV RNA in the blood of newborn animals, and whether the level of viraemia is sufficiently high to infect Culicoides are not clear and remain to be investigated.
Concerning the other mechanisms for BT persistence and overwintering, about the length of viraemia it was concluded that BTV nucleic acid can be detected by reverse transcription polymerase chain reaction (RT‐PCR) in the blood of infected cattle and sheep till 4–5 months after the infection, and up to 2 months in goats, while infectious virus in the blood can only be detected for up to 50 days in cattle and up to 30 days in small ruminants in the majority of the cases. BTV presence has been demonstrated in other organs, including organs containing lymphoid tissue, skin and reproductive organs. The maximum duration of the presence of BTV is registered in the spleen up to 40 days for infectious virus and up to 3 months for its nucleic acid. The hypothesis of skin and dermal tissue potentially playing a role in virus transmission through midge bite needs to be demonstrated. Other organs with BTV presence, such as tongue, tonsils, nasal mucosa, may potentially play a role in direct virus transmission, but the evidence supporting direct BTV transmission is very limited and for the 24 historical serotypes is likely to be infrequent, with limited contribution to BTV spread during epidemics, in comparison to vector transmission. Concerning vertical transmission of BTV in vectors, to date, there is no scientific evidence in support of vertical transmission of BTV in its biological vectors in Europe; therefore, further studies on virus detection on larvae are recommended, where endemic situations allows it, particularly with European vector species.
The third question in this topic area regarded the revision of the criteria on surveillance laid down in Regulation (EC) No 1266/2007 for demonstration of the absence of virus transmission. For this assessment, reference was made to data of both virus and serological prevalence collected in previous EFSA work, to the levels of virus circulation estimated with the mathematical model described above, as well as an analysis of the performance of the surveillance system in place in France both in time of BT freedom and during the last outbreaks occurred in 2015. The assessment concluded that when surveillance is being undertaken in a zone or country after the cessation of the vaccination, very low levels of infection prevalence (virus circulation) are to be expected. In particular, values below 1% can be observed from the literature review and from the mathematical model developed in this opinion, which are lower than the values foreseen by the Regulation (EC) 1266/2007. Furthermore, based on the surveillance in France from 2013 to 2015 with associated detected prevalences, and considering the reoccurrence of BTV in France in 2015, circulation of BTV might have occurred without being detected. Therefore, when the objective of the surveillance is to demonstrate freedom (BTV‐free status) following application of a successful vaccination campaign, a design prevalence lower than 5% as currently set in the Regulation (EC) 1266/2007, i.e. at least equal to 1%, should be taken into consideration. Furthermore, the evidence suggests that the design prevalence for the surveillance of BTV cannot be generalised, but should be set on a case‐by‐case approach after considering the type of target prevalence (infection or serological prevalence), the geographical unit of concern and the epidemiological phase appropriate to the area concerned.
As regards the options for safe trade of animals moved from BTV‐infected to BTV‐free country or zone, different assessment questions were posed about protection conferred by colostral immunity and vaccination as guarantee for BT susceptible animals to be moved safely from a BTV‐infected to a BTV‐free country or zone. These questions were addressed by systematic literature review. Considering the duration of protection from BT conferred by the colostral immunity in newborn ruminants from vaccinated mothers, the literature review highlighted that in general neutralising antibodies can be considered protective against infection, although a clear and specific threshold of a protective titre of BTV‐specific neutralising antibodies cannot be identified. Still some animals born from vaccinated dams and without detectable neutralising colostral antibodies have also been shown to be protected. In term of duration of protection, based on the limited number of studies available, a marked variation in the level and longevity of neutralising colostral antibodies in lambs and calves (no specific evidence is available for goats) from vaccinated dams have been demonstrated, ranging from 16 up to 270 days in lambs (mean value 210 days) and from 70 to 113 days in calves (mean value 84 days).
The second point to be considered was to assess the minimum age of newborn ruminants where residual colostral antibodies against BTV do not interfere with vaccine immunisation. Results of the experimental studies demonstrated that the presence of colostral antibodies interferes with the induction of the immune response to homologous vaccine in calves and lambs for more than 3 months after birth (no specific evidence is available for goats), although further detailed studies are recommended. Considering this conclusion, during the period of vector activity and potential virus circulation or when an immediate threat for animal health exists, it would be advisable to vaccinate1 calves and lambs born from vaccinated mothers twice, once before 3 months and then again at about 6 months of age so to ensure maximal protection. Outside these periods, in the absence of BTV circulation, a single vaccination at about 5–6 months can be adequate.
When assessing the minimum time after vaccination of an animal as immune, it was concluded that this can be variable ranging from 3 to 48 days depending on the vaccine, the experimental design, diagnostic tests, animal‐related factors and other variables. When commercially available inactivated vaccines and neutralising antibodies are considered, the majority of animals are positive within 21 days after vaccination; an increasing proportion of protected animals can be observed at 28 days after vaccination.
A specific situation was asked to be assessed, i.e. whether 14 days of vector protection for ruminants below the age of 70 days, combined with a negative PCR test at the end of the 14 days or more, qualify them for a safe movement from a BT‐restricted to a BT‐free area. It was stated that these measures are all able to reduce the risk of introducing one or more viraemic animals, both considered singularly or in combination. Nevertheless, a quantitative estimation of the final risk of introducing a viraemic animal following the above described procedure would be of limited utility, given the high levels of uncertainties affecting all variables and the large range of epidemiological conditions influencing the final risk. Given the current uncertainty level, the development of any quantitative model based on a series of assumptions (e.g. the level of infection in the population of origin, the period of the year, the vaccination policy in the country of origin, the specific protocols used for vector protection and the number of animals to be introduced) would make the outcomes rather unrealistic and scarcely applicable in practice.
As regards the provisions for safe movement of animals linked to protection from BTV vectors, an update of the scientific knowledge about vectors ecology and possible mechanisms of over‐wintering of vectors was conducted through literature review, and the conclusions and recommendations from previous EFSA opinions were updated. This was completed by an analysis of field data on seroconversion of sentinel animals and entomological surveillance during winter in the same areas from Italy, as a case study, and by mapping predicted vector activity according to a temperature threshold of 10°C over Europe, selected according to results from laboratory experiments on Culicoides development and to the estimation of temperature‐dependent R0 threshold values for disease transmission. All these components of the assessment served for better definition of the criteria for the determination of the seasonally vector‐free period (SVFP). Regarding these aspects, available data demonstrate that some Culicoides species, in some geographical areas in Europe (e.g. in Mediterranean areas and in mild‐winter areas), are active throughout the year and that an absolute SVFP does not exist. In these areas, the continuous Culicoides activity and long‐lived infected female could collectively contribute to the BTV overwintering. On the other hand, in northern Europe, low winter temperatures mainly inhibit Culicoides life cycle over a period of at least 3 months, and would not allow continuous transmission or survival of females infected during the prior transmission season. This is in agreement with field data were adult populations of Culicoides are in general absent from January to April in most of northern European countries. Long‐standing practical experience demonstrates that transmission of BTV is substantially reduced or halted during these periods.
The criteria considered by the Regulation (EC) 1266/20072 for the definition of the SVFP include the complete absence of adult Culicoides imicola and less than five parous females captured in light traps for the other Culicoides species. Temperature conditions that impact on the behaviour of the vectors activity and related temperature thresholds are considered as possible additional criteria for the definition of the SVFP. Although the available data do not allow the identification of more accurate and applicable criteria for the definition of the SVFP, the analysis of the data produced by the Italian entomological surveillance programme agrees with the current provisions of the Regulation (EC) 1266/2007, as no seroconverted sentinels were observed in absence of C. imicola or with less than 5 captured Culicoides recorded.
In relation to the possible definition of a temperature threshold, the results of the available studies and analysis of the risk of BT transmission through the calculation of the R0 indicated a possible temperature threshold for BT transmission between 9.0 and 12.0°C. This temperature values cannot be taken in absolute way, without considering the different Culicoides species involved and the eco‐climatic conditions of the territory of concern. An in‐field validation of the criteria currently used for the SVFP definition is still needed, the availability of long‐term entomological data, coupled with serological or virological surveillance results in the same locations on animal host and vectors, would be necessary for the main European ecoclimatic zones and different Culicoides species involved.
Considering these knowledge gaps, a series of investigations and products concerning BT vectors are recommended to be produced:
seasonal maps for the presence/absence of the major vector species in Europe;
validated models based on long‐term field data of seasonal captures for predicting the vector seasonality;
survival rates of adult Culicoides at low temperatures under laboratory conditions;
insights on influence of temperature on BTV replication in Culicoides;
BTV presence in vector females collected during winter months coupled with new age‐grading methods to detect the infection season.
The third aspect to be assessed in this topic area was the efficacy of insecticides and repellents against BT insect vectors, and the comparison of that to the protection efficacy provided by vector‐proof establishments. Regarding this aspect, it was concluded that, to date, there is no conclusive evidence that the use of insecticides or repellents singularly reduce the transmission of BTV in the field. In specific scenarios, however, they have been shown to either kill Culicoides or reduce host/vector contact and hence are used as mitigation where vaccines are unavailable. Their use is modified by both logistics and cost. One of the main limitations of these treatments is related to their transient effect which necessitates frequent reapplication, and this is unlikely to be feasible except for very high value stock. According to scientific literature reviewed in this opinion, a high level of efficacy (up to 86%) of pour‐on insecticides is difficult to achieve under field conditions, and little information is available about the effect of reduction on the numbers of engorged Culicoides females in relation to BTV transmission. By only using pour‐on insecticides, protection of animals is lower than the one provided by the vector‐proof establishment which is at least 10% higher.
Among other control methods for reducing host/vector contact, it was concluded that stabling is effective where a high level of containment can be attained. Also, insecticide‐treated meshes applied over windows in stables were demonstrated to substantially reduce vector populations inside stables. The evidence was derived primarily from studies addressing horses and the logistics and reduced coverage provided to ruminants may lessen this effect. Application of insecticides in the environment to kill either adult or larval Culicoides has not been studied since the last EFSA scientific opinion from 2008 and is unlikely to be effective due to the ubiquitous nature of Culicoides larval development sites in Europe.
On the light of those conclusions, further studies would be needed to estimate the risk reduction provided by application of insecticide treatment under field conditions. Protocols of usage of insecticides and repellents on animals should be harmonised in the EU and supported by field evidence.
1. Introduction
1.1. Background and Terms of Reference as provided by the EC
Over the past 15 years, BT incursions of a variety of serotypes occurred and on several occasions became widespread across many parts of Europe with affected countries sometimes adopting diverse control policies, particularly as regards vaccination against the disease in order to cope with both the short as well as the long‐term consequences in animal health, animal production and trade on live animals or their products. Incidences of BT during this period have not only included unexpected epidemics in areas where it had not appeared for more than 10 years (e.g. BTV‐4 in the mainland of the Balkan Peninsula in 2014) but also low‐impact circulation of certain serotypes, some of them of unclear origin, incursions of new serotypes, vaccine incidents and disease resurgence (BTV‐8 in France in 2015) raising concerns and evidencing new challenges.
The European Commission has repeatedly sought scientific advice on bluetongue (BT) from EFSA in the last decade and in response the European Food Safety Authority (EFSA) has produced a number of scientific opinions dealing with various aspects of BT epidemiology, surveillance and control which provided valuable conclusions and recommendations that helped shape the current disease strategy at the European Union (EU) level. Nevertheless, an update appears necessary in the light of the recent disease evolution, the current epidemiological situation, the experience gained so far from the implementation of the various BT control policies and possible alternative methods to ensure safe trade of live animals from BT restricted zones and the latest scientific information available. The need to review the overall BT policy at the EU level is an issue that has been repeatedly emphasised by national authorities of many Member States and the IV International Conference on Bluetongue and related Orbiviruses (Rome, 5–7 November 2014) represents a major milestone for taking stock of the latest state of the art science on BT.
In order to streamline the way forward, the Commission with the Member States have identified a series of issues for which concrete elements of science may provide a good basis for reformulating policies and/or adapting current rules. These are as follows:
-
1
Safe trade provisions
As regards provisions for safe trade, in particular from BT restricted areas, the European Commission, on top of those already in place in Commission Regulation (EC) 1266/2007, is keen to explore other options used by the competent authorities of some EU Member Countries in the framework of bilateral trade agreements drafted in accordance with Article 8 of the same Regulation. Article 8 of Commission Regulation (EC) No 1266/2007 foresees that exemptions from the exit ban are to be based on risk mitigating measures presented in Annex III to the Regulation or on any other appropriate animal health guarantees based on a positive outcome of a risk assessment agreed between the competent authority of the place of origin and the competent authority of the place of destination. Currently, there are such agreements on the movement of live animals concluded between France and Italy of 2015, France and Spain of 2013 and 2015, Italy and Spain of 2012, Spain and Portugal of 2014, France and Luxembourg of 2015, and Italy and Austria of 2016.
-
2
Classification of different BT serotypes
There are indications that more than 25/26 different serotypes of the BT virus have been identified to date. Each of these serotypes, apart from its specific genetic and antigenic features, may also be connected with specific epidemiological and pathogenicity properties. It is necessary to understand whether it is possible to use these properties as a set of standard criteria to divide known BT serotypes in groups, each deserving a distinct treatment as regards surveillance, protection and control measures.
-
3
BT listing and categorisation in the framework of the AHL
In addition to the classification of the different serotypes, BT merits an assessment as part of the listing and categorisation exercise of animal diseases in the framework of the Animal Health Law (AHL) in the same manner as it was requested previously for another seven diseases (Ref. SANTE G2/BL/lp (2015) 4940871).
In the light of the above mentioned ongoing procedure, the Commission is in need of scientific advice on the assessment of the significance of BT (as an integral disease, or separately for each serotype or group of serotypes, depending on the outcome of the grouping exercise) also within the framework of the listing and categorisation according to the AHL. The criteria, provided for ease of reference in Annex II and Attachments I to IV thereof, shall be used as a basis for this analytical assessment. The risk manager needs an updated scientific advice in order to:
assess if the various serotypes or groups of serotypes of BTV cause diseases for which control measures at the EU level are justified;
proceed with the profiling of the diseases caused by the serotypes or groups of serotypes of BTV as above in view to their categorisation; and
assign listed species to the various serotypes or groups of serotypes of BTV identified as eligible for EU intervention.
1.1.1. Terms of Reference (ToR)
In view of the above, and in accordance with Article 29 of Regulation (EC) No 178/2002, the Commission asks EFSA for a scientific opinion under the following headings:
ToR 1. As regards vaccination, eradication and surveillance
ToR 1.1 Assess the most suitable duration of a BT vaccination campaign intended to achieve disease freedom in a country or region considering any relevant factors that may affect and influence disease spread and persistence.
ToR 1.2 Assess the probability of BT recurrence in BT‐affected areas that have regained BT freedom, in particular due to BT virus becoming endemic with low level circulation in these areas and reoccurring ‘spontaneously’ (low‐noise circulation in livestock or wildlife, maintenance in vectors or other possible mechanism to be considered).
ToR 1.3 Revise and assess the suitability of the provisions on surveillance laid down in Regulation (EC) No 1266/2007 to ensure reliable and robust demonstration of absence of virus transmission in a Member State or epidemiologically relevant area, considering point 1.2 above.
ToR 2. As regards specific options for safe trade that could be used for exemptions from the exit ban applicable to movements of live animals from a restricted zone
ToR 2.1 Assess whether maternal immunity against BT of calves, lambs and kids born to and colostrum fed from vaccinated mothers, constitutes a sufficient guarantee for animals of the above species to be moved safely from a BTV‐infected to a BTV‐free country or zone, without a risk for disease spread, with or without the need for any additional premovement testing regime and indicate the main parameters that could be used (minimum/maximum age of calves, testing of dams, etc.).
ToR 2.2 Assess the minimum age of calves, lambs and kids after which residual colostral antibodies against BTV do not interfere any longer with vaccine immunisation of these animals (in an example of BT bilateral agreement this age limit is set at 90 days).
ToR 2.3 Assess the minimum time after completion of the primary vaccination (1–2 doses as indicated by the vaccine manufacturer) for the vaccinated animals to be considered immune to be safely moved from a BT‐infected to a BT‐free country or zone (currently set at 60 days in paragraph 5 of Annex III to Regulation (EC) No 1266/2007).
ToR 2.4 Assess whether vector protection for 14 days of ruminants below the age of 70 days, combined with a negative PCR test at the end of the 14 days or more, qualify them for a safe movement from a BT‐restricted to a BT‐free area.
ToR 3. As regards protection from BTV vectors and vector based provisions for exemption from the exit ban applicable to movements of live animals from a restricted zone
ToR 3.1 Review and update previous opinions as regards vectors ecology (models for distribution/density), in order to have more accurate and applicable criteria for the determination of the seasonally vector‐free period.
ToR 3.2 Review and update previous opinions as regards over‐wintering mechanisms and the duration of the BT viraemia.
ToR 3.3 Review and update previous opinions and provide a scientific assessment of the appropriateness of the use of insecticides and repellents against Culicoides as BT competent vectors, including an assessment of their efficacy and recommendations of adequate protocols for their uses, in particular as regards their suitability to protect animals against attacks by vectors performing at least equal to the protection provided by vector‐proof establishments – without the need to keep animals in a vector‐protected facility.
ToR 4. As regards classification and grouping of different BTV serotypes according to their potential impact on animal health
ToR 4.1 Review and update previous opinions providing a short description of existing serotypes in the EU and elsewhere.
ToR 4.2 Assess, by using appropriate criteria, the feasibility of grouping the currently known BTV serotypes in appropriately defined groups of serotypes sharing similar properties thus creating a number of ‘BTV serotype groups’ separated by significant different levels of impact on animal health (e.g. most serious clinical symptoms in many individuals in large areas, mild symptoms to few individuals within small areas or no symptoms at all in one or more BT susceptible species, etc.).
ToR 4.3 Review and classify the existing serotypes according to the outcome of the assessment in point 4.2 above and assess whether any of the above serotypes/groups of serotype could be candidates for a partial or total exclusion from the overall BT policy currently in place in the EU, in particular, due to their low level of virulence or pathogenicity.
ToR 5. Listing and categorisation of BT in the framework of the Animal Health Law
ToR 5.1 Considering the outcome of the assessments and reviews referred to in paragraph 4 above, for each of the aforementioned groups of serotypes, or BT in general as appropriate, assess, following the criteria laid down in Article 7 of the AHL, its eligibility of being listed for Union intervention as laid down in Article 5(3) of the AHL;
ToR 5.2 Considering the outcome of the assessments and reviews referred to in paragraph 4 above, for each of the aforementioned groups of serotypes, or for BT in general, if found eligible to be listed for Union intervention, provide:
an assessment of its compliance with each of the criteria in Annex IV to the AHL for the purpose of categorisation of diseases in accordance with Article 9 of the AHL;
a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL.
1.2. Interpretation of the Terms of Reference
1.2.1. ToR1
This ToR is addressed in Sections 3.1 and 3.2. The questions are answered basically by using a modelling approach, where the difficulties related to the models, due to the necessity of accounting for different ecosystems, including wildlife, climatic and host composition/densities in Europe do not impede that approach. A choice of the most relevant model compartments balanced with data availability is performed.
The mechanisms for BT persistence useful to answer ToR 1.2 are addressed in Section 2.2 including role of wild animals species, transplacental transmission, duration of viraemia (which covers in this way part of what requested in ToR 3.2), presence of BTV in other tissues and vertical transmission in vectors.
The ToR 1.3 about surveillance performance is addressed in Section 3.3. It was answered by comparing the sensitivity of the prescribed surveillance systems with the prevalence on the long term obtained by the model in domestic animals. A case study of the surveillance in place in France at the time of BT recurrence and how the surveillance was adapted afterwards is also considered.
1.2.2. ToR2
This ToR is addressed in Section 3.4. The approach is to address the first three subquestions of the ToR by a systematic literature review and discussing the results by expert knowledge, taking into account what is observed at experimental level and on the field. The assessment to sub‐ToR 2.3 about the lag time after vaccination for an animal being protected is done at level of individual animal. This cannot be answered at population level, where if a big number of (correctly) vaccinated animals is moved, the probability of having at least one ‘not safe’ animal could be as high as 100%.
The ToR 2.4 is addressed in Section 3.4.1, and cannot be answered quantitatively due to the high level of uncertainty of the many variables involved. About that, a series of considerations are provided about the level of infection in the population of origin, the period of the year, the vaccination policy, the specific protocols used for vector protection and the number of animals to be introduced, which lead to many different scenarios that should be assessed by a case‐by‐case approach. Most important is the high level of uncertainty that would affect the final risk estimation when these variables are combined.
1.2.3. ToR3
The questions posed by this ToR are addressed in Sections 3.5 (vector ecology, overwintering mechanisms and seasonal vector free period) and 3.6 (vector control).
The ToR 3.1 about reviewing the knowledge of vector ecology is addressed by keeping the last EFSA opinion from 2008 as basis of knowledge and updating that with the evidence from the new studies produces since then. An assessment of the validity of conclusions and recommendation from that opinion is also provided.
It seems that the concept of an absolute seasonal vector‐free period (SVFP) is unrealistic for defining the role of the different vector species during winter when transmission is supposed to be absent or very low. Nevertheless, the seasonal occurrence of BT in Europe is clearly related to the seasonal pattern of the vectors throughout the year. The criteria for the vector‐free period are assessed comparing those against field entomological and serological data of sentinel animals obtained from Italy.
The sub‐ToR 3.3 is addressed by considering the efficacy of repellents and insecticides and comparing it with the level of protection achievable with vector‐proof establishment. Currently, different approaches are followed in the use of insecticides and repellents in the context of the animal movement and some clarifications on pros, cons and limits of the different approaches could be of benefit.
1.2.4. ToR 4 and ToR 5
These two ToR will be addressed in a separate scientific opinion that will be published separately.
2. Data and methodologies
This opinion has been selected as a pilot opinion to adopt the PROMETHEUS approach. PROmoting METHods for Evidence Use in Scientific assessments (PROMETHEUS)3 is an EFSA initiative designed to foster these principles. It involves a four‐step approach that can be tailored to the different circumstances and requirements of each scientific assessment:
upfront planning of the assessment strategy, defining the relevant data and the approach for collecting, appraising and integrating them;
conducting the scientific assessment in line with the plan, and independently of prior knowledge of the results of the available studies;
verifying the process to ensure alignment with the plan and the guiding principles;
documenting and reporting of all steps, including deviations from the original plan.
This approach foresees to develop a protocol that illustrates the WG/Panel's strategy for the scientific assessment on bluetongue. The protocol was developed following the principles and process illustrated in the EFSA PROMETHEUS project.4 The PROMETHEUS protocol (including considerations regarding uncertainties) is available as an Annex to this opinion (Annex A).
The methodological approach used in each section is explained as follows.
2.1. ToR 1.1: Assessment of the duration of BT vaccination campaign intended to achieve disease freedom
A model for simulating the transmission of bluetongue virus within and between farms has been used to answer ToR 1.1 and ToR 1.2. The model was originally developed to describe the spread of BTV within and between farms in Great Britain during a single season. However, the model has been extended to include: (i) vaccination; (ii) host births and deaths; (iii) overwintering of BTV; and (iv) application of the model to countries other than Great Britain (GB), in particular France, Italy and Spain, and including parameters related to alternative Culicoides vector species. The full details of the model structure are provided in Appendix A.
2.2. ToR 1.2: Mechanisms for bluetongue persistence and recurrence
In September 2015 the reoccurrence of serotype 8 of BTV was confirmed in continental France, in the département of Allier. The surveillance put in place by the French veterinary authorities allowed the detection of additional cases of infection in the central départements of France, close to the Massif Central area.
The analysis on the sequences of the viral genome confirmed a close match between the BTV‐8 strain currently circulating in France and that causing the vast epidemic in 2006–2008. This similarity with the BTV‐8 previously circulating in France and the results of the epidemiological investigations made by the French Authorities, not revealing any introduction of potentially infected animals, semen or embryos, suggested a possible re‐insurgence of the BTV‐8 infection due to the maintenance of the viral circulation at low level in the host and vector populations since 2009, finally re‐emerging in 2015 as a consequence of the reduction of the immunity in the population of domestic ruminant. This ‘low level circulation’ mechanism, however, has never been taken into consideration before in Europe to explain the re‐emergence of the infection after a long period of time.
In addition, the results of the French entomological surveillance programme from 2009 to 2012 were analysed for the départements of Allier and Puy‐de‐Dôme, where the resurgence of BTV‐8 was firstly observed (Sailleau et al., 2015; Bournez et al., 2016), and all départements (46) classified in the same Culicoides diversity groups, based on the abundance of the different Culicoides species (Figure 1).
Figure 1.
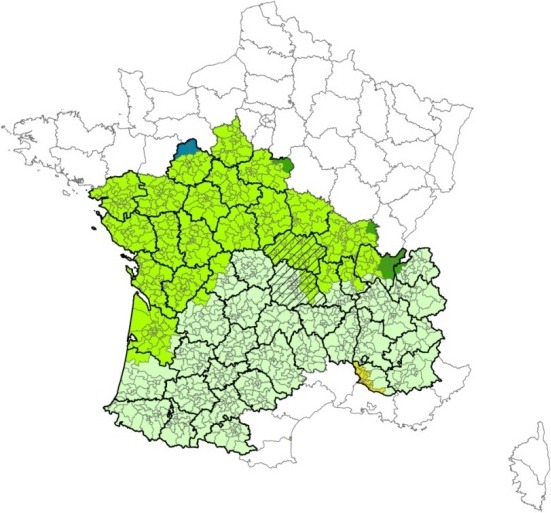
Map of France, with the départements (Allier and Puy‐de‐Dôme) where bluetongue re‐emerged in 2015 (area with diagonal lines), and départements selected for comparison (coloured and highlighted départements)
- Départements were selected by comparison as they belongs to the same Culicoides diversity groups (brilliant and pale green). Both these diversity groups are dominated by the morphologically close C. obsoletus/C. scoticus species, with a rarefaction of C. chiopterus and C. dewulfi southwards. Data were obtained from the French surveillance system of Culicoides populations, funded by the French Ministry of Agriculture.
The analysis of the data of the French entomological surveillance programme from 2009 to 2012 refutes the existence of possible peculiar entomological conditions in the départements of Allier and Puy‐de‐Dôme, where the resurgence of BTV‐8 was observed in 2015. In fact, the Culicoides species diversity in the départements of Allier and Puy‐de‐Dôme is dominated by the closely related species Culicoides obsoletus and Culicoides scoticus, for which the females are difficult to identify by morphology. When the maximum abundance per trap and per month for Allier and Puy‐de‐Dôme are compared with the same parameters observed in the rest of the selected départements, it is evident that the Culicoides population in these two départements was not particularly high compared to the other selected territories (Figure 2).
Figure 2.
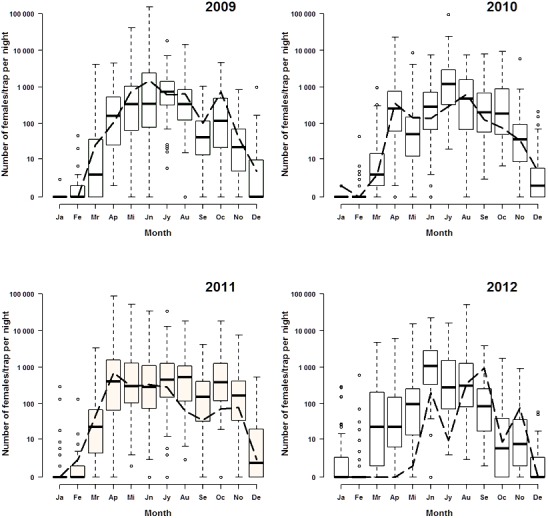
Maximum number of Culicoides per trap and per month caught in Allier and Puy‐de‐Dôme départements (dashed line) and in the other 46 départements classified in the same Culicoides diversity groups (boxplots) in 2009, 2010, 2011 and 2012.
As far as the climatic conditions are concerned, the Massif Central region did not experience any particular climatic events from 2010 to 2014. In 2015, temperatures were 2–4°C higher than normal seasonal temperatures in July in France and +4°C higher than normal in Massif Central. A severe dryness was observed from May to July in a large part of France including the Massif Central.5 It is difficult to state that this warm and dry 2015 summer could have led to a more intense transmission and thus to the resurgence of BTV‐8 in France. The hypothesis of the maintenance of the infection in host and/or vector population, not detected by the surveillance systems in place in the EU, would in theory imply the occurrence of one or more of the following mechanisms:
persistence of the infection in the host populations (domestic or wild) due to a ‘low level circulation’ of the virus, not detectable by the surveillance systems in place;
persistence of the infection in the host populations (domestic or wild) due to additional mechanisms, such as transplacental transmission or the permanence of live virus in organs or tissues of animal hosts (i.e. the establishment of a long lasting carrier state);
persistence of the infection in the vector population through vertical transmission.
2.2.1. Persistence of the infection in the host populations (domestic or wild) due to a ‘low level circulation’ of the virus
A disease spread model has been developed to assess the probability of maintenance of infection in the domestic host population, considering a scenario of ‘low level’ endemic circulation of the virus persisting for several years (see Section 2.1). The full details of the model structure are provided in Appendix A.
In relation to the probability of ‘low level circulation’ in wild ruminants, the lack of comprehensive data on animal density and spatial distribution is hampering the development of any reliable transmission model. In particular, the limited availability of spatial distribution maps on wild ungulates in some European regions6 do not provide enough detailed quantitative data to be used as input values for the transmission model.
The existing knowledge on the possible contribution of the wild ruminant species to the BTV circulation has been retrieved from the scientific published literature, with particular reference to the European situation. The results of a multiannual surveillance carried out in France have been also analysed (Rossi et al., 2010, 2014a).
2.2.2. Persistence of the infection in the host populations due to transplacental transmission or the permanence of live virus in organs or tissues of animal hosts
The evidence already collected in the previous EFSA opinion (EFSA AHAW Panel, 2011b) has been updated by considering the most recent literature published on the topic and the conclusions and recommendations assessed for their validity. For that purpose, the data collected in the systematic literature review conducted in the framework of the EFSA mandate on vector‐borne disease (Dórea et al., 2017) were used.
2.2.3. Persistence of the infection in the vector population through vertical transmission
To date, there is no scientific evidence supporting the existence of this mechanism of transmission in the case of BTV and its vectors, some considerations are addressed based on proxy studies, i.e. preliminary laboratory trials and/or targeting other viruses different from BTV.
2.3. ToR 1.3 – Suitability of the provisions on surveillance laid down in Regulation (EC) No 1266/2007
The Regulation (EC) 1266/2007 lays down implementing rules for the control, monitoring, surveillance of BT. As regulated, the BT monitoring and surveillance programmes shall be aimed at (a) detecting any possible incursions of the bluetongue virus and (b) where appropriate, demonstrating the absence of certain serotypes of that virus in a Member State or epidemiologically relevant geographical area; or (c) determining the seasonal vector free period (entomological surveillance).
The second objective is the relevant one for the question posed by the ToR. The surveillance shall consist of at least passive clinical surveillance for the detection of suspected cases and active laboratory‐based surveillance based on annual survey based on serological/virological monitoring with sentinel animals, or targeted monitoring and surveillance based on a risk assessment. Moreover, the sample size used for the active laboratory‐based surveillance must be calculated to detect a prevalence of at least 5% with 95% confidence. The approach is to assess the possible lowest and persistent levels of BTV circulation in livestock both by using the model as presented in Section 3.1.1 and cross‐checking values of infection prevalence from the literature and to compare these values with what prescribed by the Regulation.
2.4. ToR 2: Immunity and vaccines
The ToR 2.1, 2.2 and 2.3 was addressed by systematic literature review on the following risk questions:
Q1: ‘What is threshold of BTV‐specific maternal antibody titre considered to provide protection to an offspring born from vaccinated mother to one/several BTV serotypes?’
Q2: ‘What is the minimum age of calves, lambs and kids after which residual colostral antibodies against BTV do not interfere any longer with vaccine immunisation of these animals?’
Q3: ‘What is the minimum time after completion of vaccination against BTV and the threshold BTV‐specific antibody titre considered to provide a protective immune response after vaccination?’
The systematic literature review has been performed to support the assessment. The full protocol of the systematic review and the critical appraisal of the studies are provided in the Prometheus protocol published as supplementary information to the present opinion.
The ToR 2.4 about assessing whether vector protection for 14 days of ruminants below the age of 70 days, combined with a negative reverse transcription polymerase chain reaction (RT‐PCR) test at the end of the 14 days or more, qualify them for a safe movement from a BT restricted to a BT‐free area, is addressed based on the findings of the literature.
2.5. ToR 3: Vector ecology and control
The evidence already collected in the previous EFSA opinion (EFSA, 2008) about vector ecology has been updated by considering the most recent limited literature published on the topic and the conclusions and recommendations have been assessed for their validity.
The criteria for the establishment of a SVFP foreseen by the Regulation (EC) 1266/2007 have been tested through the analysis of a subset of entomological and serological surveillance data provided by the Italian national veterinary authority. Further insights on the validity of criteria for SVFP are provided by analysing the basic reproductive number according to different temperatures and numbers of Culicoides caught.
Finally, the efficacy of vector control tools and protocols are reviewed and compared with the requirements and efficacy of vector‐proof establishments (VPE).
3. Assessment
3.1. Modelling the long‐term dynamics of bluetongue virus
3.1.1. Long‐term dynamics of bluetongue virus in the absence of control measures
To explore the long‐term dynamics of BTV and, in particular, the possibility of the virus becoming endemic with low‐level circulation in livestock, the model was used to simulate epidemics in south‐east England (specifically Kent, Surrey and East and West Sussex). This region comprises 5,073 cattle and/or sheep farms with 212,742 cattle and 825,985 sheep. The model was run for 25 years following the initial incursion, which was to a randomly selected farm in the region. Spread between farms occurred via dispersal of infected vectors only; spread via movement of infected animals was not included in the simulations because, although animal movements can significantly alter the spatial dynamics of an epidemic, the main purpose of the model was to explore the probability of long‐term persistence of the infection in a given population and not to simulate the spread of the disease in south‐east England.
Simulated epidemics persisted for 25 years (i.e. the end‐point of the simulations) in a majority of replicates (Figure 3). After around 5 years, the dynamics of BTV infection settled to a stable pattern of seasonal outbreaks (Figure 3), with a peak proportion of infected farms of around 50%. The proportion of animals infected or seropositive in the population was higher for cattle compared with sheep (prevalence of infection: 1.5% in cattle, 0.6% in sheep; seroprevalence: 45% in cattle, 14% in sheep). The mean within‐herd prevalence of infected animals was similar for both cattle and sheep (5% in cattle; 5% in sheep), but mean within‐herd seroprevalence was higher for cattle compared with sheep (64% in cattle; 45% in sheep). The differences between sheep and cattle results are determined by the different parameters describing the population demography and disease‐associated mortality.
Figure 3.
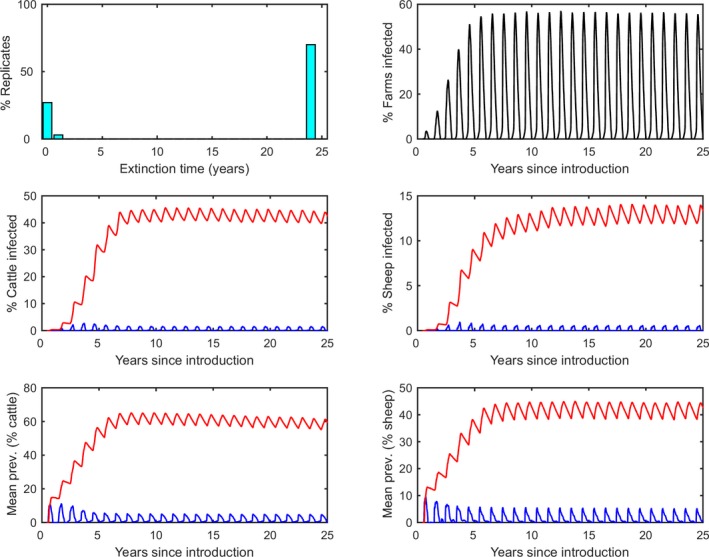
Simulated dynamics of bluetongue virus in south‐east England over a 25‐year period
- Top left: Extinction time (in years). Top right: Time course for the proportion (%) of farms with BTV circulating. Middle: Time course for the proportion (%) of cattle (left) and sheep (right) that are infected (blue lines) or seropositive (red lines). Bottom: Time course for the mean within‐herd prevalence (%) of cattle (left) and sheep (right) that are infected (blue lines) or seropositive (red lines). Each figure shows the mean of one hundred replicates of the model.
3.1.2. Dynamics of bluetongue virus in a vaccinated population
To assess the impact of vaccination and, in particular, the duration of a vaccination campaign on the dynamics of BTV, a range of scenarios were simulated which differed in the level of farm‐level vaccine coverage (i.e. the proportion of farms vaccinated: none, 80% or 95%) and the number of years for which vaccine was used (1, 2, 3 or 5 years). In addition, the dynamics of BTV were simulated for different countries (Great Britain, France, Italy and Spain) to explore the sensitivity of any conclusions to the effects of host density, population structure, temperature and principal vector species (C. obsoletus in Great Britain and France compared with C. imicola in Italy and Spain).
3.1.2.1. Great Britain
When applying the model to Great Britain (GB), the full model (i.e. including spread between farms via animal movements) was used for the simulations. The model was run for 5 years following the initial incursion, which was to a randomly selected farm in south‐east England.
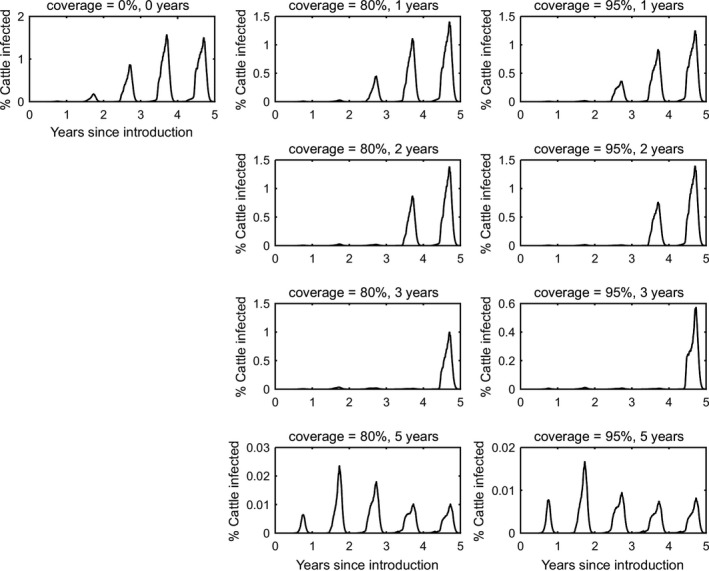
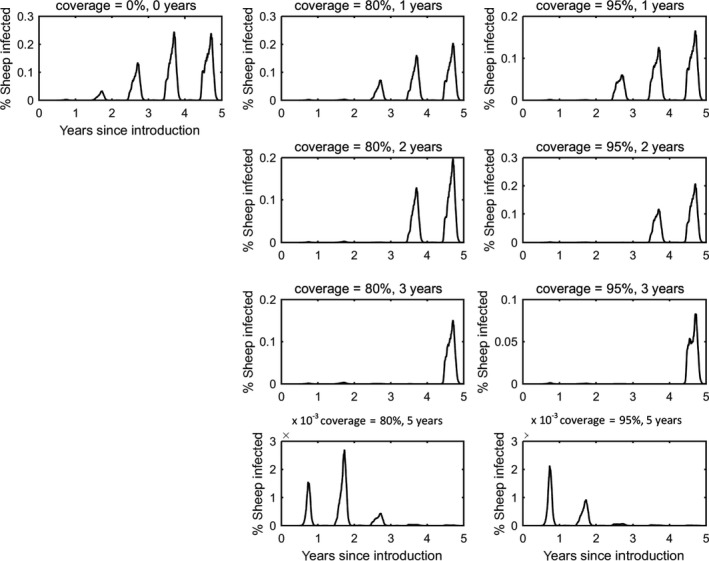
In the absence of vaccination, BTV persisted in most epidemics for the period of the simulations. The prevalence of infected farms increased over time, reaching around 30% in the fifth year (Figure 3). Similarly, the prevalence of infected cattle and sheep increased from 1 year to the next (Figures 4 and 5), although the prevalence was higher in cattle (1.5% after 5 years) compared with sheep (0.2% after 5 years).
Figure 4.
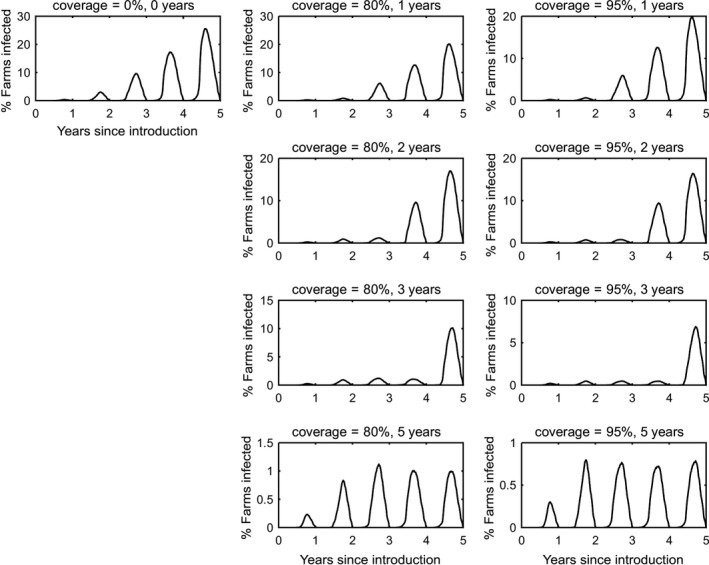
Prevalence of infected farms in simulated epidemics of bluetongue in Great Britain and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Figure 5.
Prevalence of infected cattle in simulated epidemics of bluetongue in Great Britain and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Vaccination contributed to a great decrease in the prevalence of infected farms (Figure 4), cattle (Figure 5) and sheep (Figure 6), with higher levels of coverage resulting in a greater reduction. However, even when vaccination was used for 5 years at 95% coverage, BTV was not eradicated from the population, but persisted at very low levels, although the number of infected farms and infected cattle was very low (< 100 farms or cattle), and infection was eliminated from sheep.
Figure 6.
Prevalence of infected sheep in simulated epidemics of bluetongue in Great Britain and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
When vaccination was stopped after 1, 2 or 3 years, BTV re‐emerged in subsequent years (typically in the second year after vaccination ceased) and did not reach the same prevalence as was observed in the absence of vaccination.
3.1.2.2. Other EU Member States
When applying the model to other EU member states (specifically, France, Italy and Spain), spread between farms was via dispersal of infected vectors only and spread via movement of infected animals was not included in the simulations. The model was run for 5 years following the initial incursion. For France, the model was applied to the whole of the country, with an incursion into a randomly selected farm in Nord‐Pas‐de‐Calais, Lorraine or Champagne‐Ardenne (chosen to reflect the previous incursion of BTV‐8). For Italy, the model was applied to Sardinia alone, with an incursion into a randomly selected farm in the region. Finally, for Spain, the model was applied to Andalusia alone, with an incursion into a randomly selected farm in the region. The simulations performed for the scenarios in these three MSs are shown in Appendix B.
France
The impact of vaccination on the prevalence of infected farms, cattle and sheep in France was similar to that observed for GB. In particular, vaccination leads to a decrease in the prevalence to low levels, but without eliminating infection (at least within the 5‐year period simulated). Moreover, stopping vaccination allows the virus to re‐emerge, typically in the second year after vaccination ceased (Figures in Appendix B).
Italy (Sardinia)
Without vaccination BTV spread rapidly in Sardinia, reaching its long‐term level after 2 years and persists at this level for the remaining 3 years of the simulation. In this case, the prevalence of infected farms was around 30%, the prevalence of infected cattle was around 1% and the prevalence of infected sheep around 0.5%. Although the prevalence varied seasonally, the amplitude of the variation was much smaller than that for GB and France. Vaccination reduced the prevalence of infected farms, cattle and sheep, with the greater reduction seen for higher levels of coverage. However, BTV was not predicted to be eliminated even after 5 years of vaccination at 95% coverage. Indeed, the rapid increase in the prevalence of infected farms during the year following the incursion (i.e. prior to vaccination) means that the peak prevalence of infected herds remains above 5% in all scenarios, although the prevalence of infected cattle and sheep is suppressed to low levels (< 0.1%). Again, stopping vaccination resulted in re‐emergence of BTV, with the prevalence quickly reaching levels similar to those in the scenario for which there was no vaccination (Figures in Appendix B).
Spain (Andalusia)
The simulated dynamics of BTV in Andalusia were similar to those for Sardinia, including the impact of vaccination on the prevalence of infected farms, cattle and sheep. In particular, vaccination reduced the prevalence to very low levels in cattle and sheep, but did not eliminate infection even after 5 years of vaccination at 95% coverage. Consequently, BTV was able to re‐emerge if vaccination was stopped in the simulations (Figures in Appendix B).
The results of the model simulations for C. imicola areas (Italy, Spain) are quite consistent with the field epidemiological data. In Sardinia, for example, even after more than 3 years of vaccination of all susceptible cattle, sheep and goats, the complete eradication of certain BTV serotypes was never achieved, although the level of infection decreased to low levels and the direct impacts in animal health almost eliminated (Paolo Calistri, personal communication).
When interpreting the modelling results, it should to be taken into account that the model considers the same climatic and environmental conditions every year, whereas in the real world, the natural variability of climatic conditions among years can influence the vector's density and behaviour, thus influencing the probability of BTV transmission. The presence of less favourable climatic conditions during one or more years, for example, could facilitate the achievement of the eradication under one of the vaccination scenarios considered by the model.
3.2. Mechanisms for bluetongue persistence and recurrence
In this section, persistence of the infection in the host populations (domestic or wild) due to a ‘low level circulation’ of the virus, or due to additional mechanisms, such as transplacental transmission or the permanence of live virus in organs or tissues of animal hosts (i.e. the establishment of a long lasting carrier state), and the persistence of the infection in the vector population through vertical transmission are assessed as possible mechanisms for the maintenance of the infection.
3.2.1. Persistence of the infection in the wild populations
Several species of wild ruminants are susceptible to BTV infection, but few show clinical signs of disease (Vosdingh et al., 1968; Niedbalski, 2015). Similar to domestic sheep, wild sheep such as bighorn sheep (Ovis canadensis) and mouflon (Ovis aries musimon) can develop fatal clinical disease (Fernandez‐Pacheco et al., 2008). The clinical signs of BT have also been observed after experimental infection in pronghorn antelope (Antilocapra americana), American bison (Bison bison) and African buffalo (Syncerus caffer) (Tessaro and Clavijo, 2001). After experimental infection, clinical disease has been reported in some North American deer species, such as: white‐tailed deer (Odocoileus virginianus), black‐tailed deer (Odocoileus hemionus columbianus) and mule deer (Odocoileus hemionus) (Vosdingh et al., 1968; Work et al., 1992). Camelids have also been reported to be susceptible to BTV infection. Clinical signs of disease, with fatal aftermaths, were reported in naturally infected llamas (Lama glama) (Meyer et al., 2009), whereas alpacas (Vicugna pacos) displayed very mild clinical signs after experimental infection with BTV‐8 (Schulz et al., 2012).
In Europe, BTV infection has been detected in red deer (Cervus elaphus), fallow deer (Dama dama), Alpine chamois (Rupicapra rupicapra rupicapra), Pyrenean chamois (Rupicapra pyrenaica pyrenaica) and Alpine ibex (Capra ibex ibex), among other wild ruminants (Rodriguez‐Sanchez et al., 2010; Rossi et al., 2010, 2014b). In particular, high levels of serological positive and RNA‐positive animals were observed in red deer (C. elaphus) (Linden et al., 2008; Rodriguez‐Sanchez et al., 2010; Corbiere et al., 2012; Grego et al., 2014; Rossi et al., 2014a,b), which may be the wild species that most substantially contributes to BTV circulation in Europe (Table 1).
Table 1.
Summary of the results of studies on the prevalence of bluetongue infection in wild ruminants in Europe
| Species | Year | Country | BTV serotype(s) | Serological results (c‐ELISA) | RNA detection (RT‐PCR) | References | ||
|---|---|---|---|---|---|---|---|---|
| Positives/tested | % | Positives/tested | % | |||||
| Alpine Chamois (Rupicapra rupicapra rupicapra) | 2008 | France | 0/299 | 0.0 | Rossi et al. (2014b) | |||
| 2009 | France | 1/298 | 0.3 | 0/1 | 0.0 | Rossi et al. (2014b) | ||
| 2008–2011 | Italy | BTV‐8 | 4/55 | 7.3 | Grego et al. (2014) | |||
| Alpine ibex (Capra ibex ibex) | 2008 | France | 0/83 | 0.0 | Rossi et al. (2014b) | |||
| 2009 | France | 0/45 | 0.0 | Rossi et al. (2014b) | ||||
| Aoudad (Ammotragus lervia) | 2005–2007 | Spain | BTV‐1 | 1/4 | 25.0 | Ruiz‐Fons et al. (2008) | ||
| Fallow deer (Dama dama) | 2006–2007 | Spain | BTV‐1 | 10/20 | 50.0 | García et al. (2008) | ||
| 2006–2010 | Spain | BTV‐1/BTV‐8/BTV‐4 | 61/188 | 32.4 | Garcia‐Bocanegra et al. (2011) | |||
| 2005–2007 | Spain | BTV‐1 | 34/96 | 35.4 | Ruiz‐Fons et al. (2008) | |||
| Mouflon (Ovis aries musimon) | 2008 | France | BTV‐8 | 3/173 | 1.7 | 3/3 | 100.0 | Rossi et al. (2014b) |
| 2006–2010 | Spain | BTV‐1/BTV‐8/BTV‐4 | 28/101 | 27.7 | Garcia‐Bocanegra et al. (2011) | |||
| 2009 | France | BTV‐9 | 1/133 | 0.8 | 1/1 | 100.0 | Rossi et al. (2014b) | |
| 2006–2007 | Spain | BTV‐1 | 3/9 | 33.3 | García et al. (2008) | |||
| 2005–2007 | Spain | BTV‐1 | 9/68 | 13.2 | Ruiz‐Fons et al. (2008) | |||
| 2011–2013 | France | BTV‐1 | 1/21 | 4.8 | 1/21 | 4.8 | Rossi et al. (2014a) | |
| 2008–2009 | France | BTV‐1 | 0/44 | 0.0 | 0/43 | 0.0 | Corbiere et al. (2012) | |
| 2009–2010 | France | BTV‐1 | 0/20 | 0.0 | 0/27 | 0.0 | Corbiere et al. (2012) | |
| Pyrenean Chamois (Rupicapra pyrenaica pyrenaica) | 2008 | France | 1/108 | 0.9 | 0/1 | 0.0 | Rossi et al. (2014b) | |
| 2009 | France | 0/117 | 0.0 | Rossi et al. (2014b) | ||||
| 2008–2009 | France | BTV‐1 | 1/98 | 1.0 | 2/89 | 2.2 | Corbiere et al. (2012) | |
| 2009–2010 | France | BTV‐1 | 0/179 | 0.0 | 0/176 | 0.0 | Corbiere et al. (2012) | |
| Red deer (Cervus elaphus) | 2008 | France | BTV‐1/BTV‐8 | 145/352 | 41.2 | 112/145 | 77.8 | Rossi et al. (2014b) |
| 2009 | France | BTV‐1/BTV‐9 | 109/485 | 22.5 | 40/109 | 37.0 | Rossi et al. (2014b) | |
| 2006–2008 | Spain | BTV‐1/BTV‐8/BTV‐4 | 5/9 | 55.6 | Arenas‐Montes et al. (2016) | |||
| 2009–2011 | Spain | BTV‐1/BTV‐8/BTV‐4 | 41/60 | 68.3 | Arenas‐Montes et al. (2016) | |||
| 2012–2014 | Spain | BTV‐1/BTV‐8/BTV‐4 | 14/29 | 48.3 | Arenas‐Montes et al. (2016) | |||
| 2008–2011 | Italy | BTV‐8 | 21/102 | 20.6 | Grego et al. (2014) | |||
| 2007 | Spain | BTV‐1/BTV‐4 | 115/200 | 57.5 | 127/510 | 24.9 | Rodriguez‐Sanchez et al. (2010) | |
| 2006–2007 | Spain | BTV‐1 | 65/98 | 66.3 | García et al. (2008) | |||
| 2007 | Belgium | BTV‐8 | 207/513 | 40.4 | Linden et al. (2008) | |||
| 2006 | Belgium | BTV‐8 | 4/221 | 1.8 | Linden et al. (2010) | |||
| 2007 | Belgium | BTV‐8 | 142/216 | 65.7 | Linden et al. (2010) | |||
| 2008 | Belgium | BTV‐8 | 111/185 | 60.0 | Linden et al. (2010) | |||
| 2005–2007 | Spain | BTV‐1 | 309/1409 | 21.9 | Ruiz‐Fons et al. (2008) | |||
| 2007–2010 | Spain | BTV‐4 | 371/2885 | 12.9 | 0/140 | 0.0 | Falconi et al. (2012) | |
| 2006–2010 | Spain | BTV‐1/BTV‐8/BTV‐4 | 381/900 | 42.3 | Garcia‐Bocanegra et al. (2011) | |||
| 2010–2011 | France | BTV‐1/BTV‐8 | 72/252 | 28.6 | 0/311 | 0.0 | Rossi et al. (2014a) | |
| 2011–2012 | France | BTV‐1/BTV‐8 | 95/584 | 16.3 | 0/656 | 0.0 | Rossi et al. (2014a) | |
| 2012–2013 | France | BTV‐1/BTV‐8 | 40/433 | 9.2 | 0/464 | 0.0 | Rossi et al. (2014a) | |
| 2008–2009 | France | BTV‐1 | 83/163 | 50.9 | 92/183 | 50.3 | Corbiere et al. (2012) | |
| 2009–2010 | France | BTV‐1 | 57/115 | 49.6 | 13/120 | 10.8 | Corbiere et al. (2012) | |
| Roe deer (Capreolus capreolus) | 2008 | France | BTV‐1 | 4/431 | 0.9 | 1/3 | 33.3 | Rossi et al. (2014b) |
| 2006–2010 | Spain | BTV‐1/BTV‐8/BTV‐4 | 3/150 | 2.0 | Garcia‐Bocanegra et al. (2011) | |||
| 2009 | France | BTV‐2 | 0/206 | 0.0 | Rossi et al. (2014b) | |||
| 2008–2011 | Italy | BTV‐8 | 12/78 | 15.4 | Grego et al. (2014) | |||
| 2006 | Belgium | BTV‐8 | 5/197 | 2.5 | Linden et al. (2010) | |||
| 2007 | Belgium | BTV‐8 | 8/295 | 2.7 | Linden et al. (2010) | |||
| 2008 | Belgium | BTV‐8 | 4/245 | 1.6 | Linden et al. (2010) | |||
| 2005–2007 | Spain | BTV‐1 | 2/39 | 5.1 | Ruiz‐Fons et al. (2008) | |||
| 2008–2009 | France | BTV‐1 | 0/129 | 0.0 | 2/173 | 1.2 | Corbiere et al. (2012) | |
| Spanish ibex (Capra pyrenaica) | 2006–2007 | Spain | BTV‐1 | 9/83 | 10.8 | García et al. (2008) | ||
BTV: bluetongue virus; c‐ELISA: competitive‐enzyme‐linked immunosorbent assay; RT‐PCR: reverse transcription polymerase chain reaction.
Few experimental studies on the duration of viraemia in wild ruminants have been published (Table 2). A single paper reports the estimation of the viraemia length in red deer (Lopez‐Olvera et al., 2010): two groups of four animals each were experimentally infected with BTV‐1 and BTV‐8, respectively. The attempts to isolate the virus from the blood of the BTV‐8 infected animals were unsuccessful, but a positive response was observed by RT‐PCR until 98 days post‐infection. The BTV‐1 infected animals showed a prolonged RT‐PCR positive response, till 112 days post‐infection, although the virus was isolated only after 12 days post‐infection (dpi).
Table 2.
Summary of the main results of experimental infection studies on wild ruminants
| Species | Year | Country | BTV serotype(s) | Number of animals inoculated | Viraemia onset | Viraemia duration | Duration of RT‐PCR positivity | Laboratory tests used | Reference |
|---|---|---|---|---|---|---|---|---|---|
| White‐tailed deer (Odocoileus virginianus) | Not reported | USA | BTV‐8 | 10 | 2 dpi | 10 days | Virus isolation | Vosdingh et al. (1968) | |
| North American elk (Cervus elaphus canadensis) | Not reported | USA | BTV‐8 | 5 | 2 dpi | 10 days (107 daysa) | Virus isolation | Murray and Trainer (1970) | |
| Black‐tailed deer (Odocoileus hemionus columbianus) | 1989 | USA | BTV‐17/BTV‐10 | 9 | 2–9 dpi | 1–10 days | Virus isolation | Work et al. (1992) | |
| Camel (Camelus dromedarius) | 2008 | Morocco | BTV‐1 | 3 | 7–8 dpi | 27–68 dpi | Virus isolation and RT‐PCR | Batten et al. (2011) | |
| Red deer (Cervus elaphus) | 2009 | Spain | BTV‐1 | 4 | 1 dpi | 12 days | 105–112 dpi | Virus isolation and RT‐PCR | Lopez‐Olvera et al. (2010) |
| Red deer (Cervus elaphus) | 2009 | Spain | BTV‐8 | 4 | 1 dpi | Not determined | 14–98 dpi | Virus isolation and RT‐PCR | Lopez‐Olvera et al. (2010) |
| White‐tailed deer (Odocoileus virginianus) | Not reported | USA | BTV‐8 | 8 | 3–6 dpi | Not determined | 12–28 dpi | RT‐PCR | Drolet et al. (2013) |
| American bison (Bison bison bison) | 1998 | Canada | BTV‐11 | 6 | 4–7 dpi | 21–28 days | Virus isolation | Tessaro and Clavijo (2001) | |
| Alpacas (Vicugna pacos) and llamas (Lama glama) | 2010 | Germany | BTV‐8 | 6 | 2–6 dpi | Not determined | 16–35 dpi | Virus isolation and RT‐PCR | Schulz et al. (2012) |
BTV: bluetongue virus; c‐ELISA: competitive‐enzyme‐linked immunosorbent assay; RT‐PCR: reverse transcription polymerase chain reaction.
In this study, 2 out of 5 experimental inoculated animals have been treated at 105 dpi with 5 mg of Flumethasone intramuscularly injected.
A particularly extreme outcome was reported by Murray and Trainer (1970), who were able to isolate the BTV‐8 on 107 dpi from two experimentally inoculated North American elk (Cervus elaphus canadensis) after the intramuscular injection of 5 mg of Flumethasone on 105 dpi. Although the results of this study may suggest the existence of mechanisms able to promote the potential reservoir role of North American elk, it is not easy to interpret and extrapolate these findings in a more general context. Furthermore, the virus strain used in the study performed by Murray and Trainer (1970), and designed as CA‐8, was subsequently identified as serotype 10 (Maclachlan and Osburn, 2008). These considerations each suggest that these results need to be interpreted with care.
Divergent opinions exist on the possible epidemiological role of wild ruminants, particularly red deer, in the maintenance of BTV infection in the absence of apparent virus circulation in domestic livestock.
In a study performed in France (Rossi et al., 2014b) in 2008 and 2009, high proportions of seropositive and RT‐PCR positive red deer were observed in 2008, suggesting that this species has been widely infected by BTV‐1 and BTV‐8 at the peak of domestic outbreaks. However, the observation of RT‐PCR positive results in six of seven red deer populations in 2009 (i.e. with low domestic incidence) would be consistent with the hypothesis of a role played by red deer in the maintenance of BTV infection in spite of a very low incidence in the livestock. By contrast, the low prevalence observed in other wild ungulate species suggests that exposure of these species to BTV in 2008 was uncommon (Rossi et al., 2014b). In a following study, the same authors reported a low level of seropositivity in young red deer (less than 2 years old) in 2011–2012, limited to some French regions (Rossi et al., 2014a). This result suggested a possible maintenance, albeit limited, of virus circulation within the red deer population. All serological positive animals resulted negative by RT‐PCR, reinforcing the hypothesis of a limited contribution of this animal species on the spread of the infection.
Some authors (Lopez‐Olvera et al., 2010; Rodriguez‐Sanchez et al., 2010) considered red deer able to maintain the BTV for long periods, thus acting as a possible reservoir of the infection. In these studies, however, the estimation of the viraemia duration was mainly based on the detection of viral RNA in blood through RT‐PCR, which can give positive results far beyond the presence of the live virus in the blood. On the contrary, other authors (Grego et al., 2014), analysing the spleen samples from hunted red deer (C. elaphus), roe deer (Capreolus capreolus) and Alpine chamois (Rupicapra rupicapra) by quantitative RT‐PCR in two provinces of Piedmont region, Italy, concluded that the infection in wild ungulates may be considered only as an epiphenomenon, with no importance for the maintenance and spread of the infection in this geographical area.
The presence of a vector species is a prerequisite for disease transmission. Therefore, knowledge is needed of the Culicoides species that inhabit areas where wild ruminants are present. As yet, however, few studies on this aspect have been conducted. In the study by Talavera et al. (2015) samplings were conducted in Spain in areas inhabited by different wild ruminant species. The most abundant vector species were C. imicola and species of the Obsoletus assemblage,7 which represented 15% and 11% of total numbers of specimens collected, over 100,000, respectively. The data suggest that such species do not exhibit strong host specificity towards either domestic or wild ruminants and they could consequently play a prominent role as bridge vectors for different pathogens between both types of ruminants.
In relation to the French situation, in its opinion issued on 22 December 2015,8 the French Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES, 2015) concluded that the BTV‐8 resurgence in France in 2015 was unlikely linked to infection in red deer or other wild ruminant populations, considering the relative short duration of viraemia in red deer, the lower level of serological prevalence detected in this animal species in France after the cessation of virus circulation in domestic populations and the low density of red deer in the Massif Central area.
To date, the information available on the length of viraemia in red deer and in other wild ruminants as well as the results of field surveys do not suggest a substantial role for these animal species in the maintenance of the virus during interepizootic periods. The results of the serological surveys performed in France confirm a strict association between the infection in domestic ruminants and the levels of serological prevalence in red deer (Rossi et al., 2014b). In addition, assuming an equivalent abundance and composition of vector population, it is reasonable to consider a lower probability of contact (and bite) between Culicoides vectors and wild hosts, which can be sparsely distributed in a large geographical scale, than between local Culicoides populations and domestic hosts concentrated in a farm environment.
Nevertheless, the detection of antibodies in young animals of 1–2 years of age may suggest the possible persistence of the infection in the red deer population, albeit limited to few circumscribed areas. The possibility of local maintenance of BTV infection in red deer population, therefore, cannot be excluded, particularly in those areas where a high density of red deer population, a low number of competing domestic animals and favourable vector conditions are present (Garcia‐Bocanegra et al., 2011; Falconi et al., 2012).
3.2.2. Persistence of the infection in the host populations due to transplacental transmission or the permanence of live virus in organs or tissues of animal hosts
3.2.2.1. Transplacental transmission
Transplacental transmission (TPT) of BTV in cattle and sheep has been extensively investigated throughout the years, as this mechanism has been indicated to have an impact on the reproductive performance of infected ewes and cattle causing early embryonic loss, abortion and the birth of offspring with severe malformations. Furthermore, TPT has been suspected to play a role in the overwintering of the infection, through the birth of offspring clinically healthy but viraemic, therefore contributing to maintain the BTV in the host population during the whole winter period in the absence of an active vector population (Zientara and Ponsart, 2015). Since 1955, TPT has been demonstrated for several BTV serotypes (BTV‐2, BTV‐4, BTV‐8, BTV‐9, BTV‐10, BTV‐11, BTV‐13, BTV‐16, BTV‐23) in cattle, sheep, goat, and elk (Cervus elaphus canadensis) (van der Sluijs et al., 2016). However, prior to the BTV‐8 outbreak in northern and central Europe, TPT had generally been associated with strains adapted to cell cultures, with multiple in vitro passages, or linked to the in‐field use of live vaccines (EFSA AHAW Panel, 2011b), although in two studies TPT was observed for BTV‐11 field strains (Stott et al., 1982; Castro and Rodgers, 1984). Other strains, attenuated with limited number of in vitro passages, failed to induce TPT, leading to the hypothesis that the capacity of crossing the placental barrier was a peculiar property of the live‐attenuated vaccine strains only (van der Sluijs et al., 2016).
During the winter 2007–2008, an unprecedented number of cases characterised by lesions in central nervous system in new‐borns, and an increase in abortion and stillbirth of calves and lambs were observed in Belgium, France and more in general in central Europe (De Clercq et al., 2008; Desmecht et al., 2008; Saegerman et al., 2011). The incidence was considered to be associated with the BTV‐8 infection and, therefore, studies were conducted to ascertain the capacity of the BTV‐8 strain to cross the placental barrier and the possible role of this mechanism for BTV spreading across central and north Europe under field conditions.
BTV‐8 in cattle
In Belgium, pairs of dam/calf serum samples were collected from clinically healthy animals and examined for the presence of antibodies against BTV by competitive ELISA (c‐ELISA), resulting in the detection of 38 serologically positive calves out of 102 c‐ELISA positive dams (37%) (Desmecht et al., 2008).
De Clercq et al. (2008) performed a study including 300 aborted fetuses, 68 from dams with suspected clinical signs of BT and 232 from dams which aborted without any suspicion of BT. The authors found evidence of the presence of BTV‐8 field strain in 41% (CI 95%: 30–53) and 18% (CI 95%: 14–24) of bovine aborted fetuses, with and without BT suspicion, respectively, that were examined for the presence of BTV RNA in the spleen. The same authors also took blood samples before colostrum uptake from 123 dam/calf pairs and 50 ewe/lamb pairs and examined them by c‐ELISA and RT‐PCR. Three dams were serologically and RT‐PCR negative and the same result was observed in their offspring. Five dams were positive to both c‐ELISA and RT‐PCR and from them two out of five calves were also positive to both tests (the other two were negative to both tests). Among the 115 calves born from the other dams, which were only serologically positive, six were RT‐PCR positive whereas four resulted positive only for c‐ELISA. In total, therefore, twelve calves were positive (9.8%, CI 95%: 5.7–16.2) by at least one test, and eight were RT‐PCR positive. BTV was isolated from one calf positive to both tests born from dam positive in both test. The same authors also demonstrated that TPT occurred in the 2% (CI 95%: 1.2–3.1) of calves without clinical signs of infection and born before the end of April 2008 (N = 733) during a period of the year where no Culicoides were caught in or out the holdings (De Clercq et al., 2008). In the context of the application of diagnostic tests to allow the animal movement from restricted areas, it is noteworthy that six RT‐PCR negative pregnant dams gave birth to RT‐PCR positive calves (De Clercq et al., 2008; Zanella et al., 2012).
Menzies et al. (2008) described an outbreak of BT in imported cows and their offspring in Northern Ireland during the vector‐free period, as a consequence of TPT of a BTV‐8 field strain. Of the 21 heifers tested negative by RT‐PCR before introduction, eight were c‐ELISA positive after their arrival to Northern Ireland, and two of them gave birth to a total three RT‐PCR positive calves, one of which was also demonstrated viraemic by virus isolation.
In France, the investigation of 780 cases of abortion in cattle occurring from November 2008 to April 2009 in the Nièvre département revealed that 128 fetuses were BTV positive (16%, CI 95%: 14–19), either by RT‐PCR or c‐ELISA (Zanella et al., 2012). Out of 97 RT‐PCR positive dams, 49 (50%) had BT positive fetuses. The authors did not estimate the TPT rate, since dams were sampled only once (at the time of abortion) and the RT‐PCR status of the dams during the first months of gestation was unknown (Zanella et al., 2012).
In the United Kingdom, 61 calves born during the vector‐free period (December 2007–March 2008) from naturally infected dams were tested by RT‐PCR and 21 of them had detectable levels of BTV RNA in their blood or organs (33%, CI 95%: 22–47) (Darpel et al., 2009).
BTV‐8 in sheep
The study of De Clercq et al. (2008) conducted on 50 ewe/lamb pairs did not observed any TPT in these animals. Saegerman et al. (2011) demonstrated for the first time the occurrence of BTV‐8 TPT in the field in sheep analysing lambs and aborted fetuses in a sheep flock in Belgium that experienced severe BTV outbreak. TPT was demonstrated in aborted fetuses from 20 serologically positive ewes by the finding of 4 RT‐PCR positive samples from the spleen Desmecht et al. (2008). In lambs, the presence of antibodies at birth, before the colostrum intake, was demonstrated in nine lambs out of 476 (1.9%, CI 95%: 0.9–3.6). After 14 days, seven out of these nine animals were also RT‐PCR positive (Saegerman et al., 2011).
BTV‐2 in sheep
For the first time, TPT of BTV‐2 strain has been showed in experimental studies suggesting that such transmission might be more frequent than previously thought (Rasmussen et al., 2013). The authors inoculated 24 pregnant ewes (four groups of six animals each) with BTV‐2 and BTV‐8 wild‐type (wt) isolates with minimal passages on cell cultures (passaged once in Culicoides KC cells and once in mammalian cells), and BTV‐2 and BTV‐8 strains obtained by reverse genetics (rg), both isolated during outbreaks in Sardinia in 2001 and 2000, respectively. No major or significant differences were noted among results from wt and rg BTV by BTV‐8 and BTV‐2, suggesting that findings of experimentally challenged infected sheep with cell‐adapted BTV may be valid also for wild‐type strains of the virus. BTV‐2 demonstrated high TPT efficiency as six lambs born from 13 BTV‐2 infected ewes had BTV RNA detectable in their blood at birth. All the six lambs were viraemic, five at birth before colostrum intake and one at 3 days of age. Considering only BTV‐2 wt, two infected and infectious lambs (RNA detectable and infectious virus recovered at birth) out of six challenged ewes were found (Rasmussen et al., 2013).
BTV‐8 in goats
Evidence of TPT of BTV‐8 in goats has recently been reported (Belbis et al., 2013; Coetzee et al., 2013). Coetzee et al. (2013) inoculated four Saanen goats with the European strain of BTV‐8 at 62 days of gestation. Viral RNA was detected by RT‐PCR in blood and tissue samples from three fetuses harvested from two goats at 43 days post‐infection. Belbis et al. (2013) performed two studies. In the first, they inoculated nine goats with BTV‐8 strain at the 61st day of pregnancy, and fetuses were collected 21 dpi. BTV‐8 was evidenced by RT‐PCR and by viral isolation using blood from the umbilical cord and the spleens of 3 out of the 13 fetuses. The observed TPT transmission rate was equal to 33% (3/9). In the second experiment, 10 goats were infected with BTV‐8 at 135 days of pregnancy. Kids were born by caesarean section at the programmed day of birth (15 dpi). BTV‐8 could not be detected by RT‐PCR in blood or spleen samples from the kids.
Chauhan et al. (2014) reported seven abortions and six stillbirths in 25 pregnant goats (about 3 months of pregnancy) on a farm in the Gujarat region, India, in July 2007, due to infection with BTV‐1 field strain. Two viruses were isolated from the spleen of aborted fetuses, and a closely related virus was isolated from Culicoides captured on the same farm 1 month later. As in that Region and anywhere else in India, attenuated or laboratory‐adapted BTV‐1 strains were never used, that finding has been interpreted as evidence of TPT of the wild‐type strain in goats (Chauhan et al., 2014).
Genetic determinant
The genetic determinant responsible for the transplacental transmission of BTV has not been identified for either BTV‐8 modified live vaccine or laboratory‐adapted strains (Zientara and Ponsart, 2015), and more research should be necessary for better understanding the mechanism underlying the genotypic changes that drive the ability to cross the placenta in order to avoid unnecessary stringent control measures causing damage to the livestock industry (van der Sluijs et al., 2016).
3.2.2.2. Duration of BT viraemia
For the purposes of this Opinion, the same definition of viraemia already set in previous EFSA documents on bluetongue is considered: ‘circulation and replication of competent virus in the blood of the mammalian host as detected by virus isolation (EFSA, 2007a)’. It is relevant to clarify that BTV nucleic acid can be detected by RT‐PCR assay in the absence of infectious virus in the blood of ruminants following infection, especially as the time interval, subsequent to infection, increases. Therefore, results of studies on viraemia duration based on RT‐PCR methods must be carefully interpreted, since a positive result in blood samples to viral genomic detection techniques does not automatically prove the presence of live virus in the blood. On the other hand, the presence of live virus in the blood is always associated to a positive result to RT‐PCR, given the sensitivity of the test.
From the systematic literature review conducted in the framework of EFSA mandate on vector‐borne disease (Dórea et al., 2017), data about duration of BT viraemia in animals (cattle, sheep and goats) experimentally infected by intravenous or subcutaneous virus inoculation were extracted from selected papers according to the animal species and the test used for detecting the virus or its nucleic acid. The distribution of the minimum and maximum day of the detection is shown in the Figure 7. For graphical reasons, outliers are indicated out of the graph, i.e. two values of maximum detection in cattle by RT‐PCR of 156 (Barros et al., 2009) and 167 (Bonneau et al., 2002) days, respectively, and in sheep by RT‐PCR of 140 (Worwa et al., 2010) and 222 days.2
Figure 7.
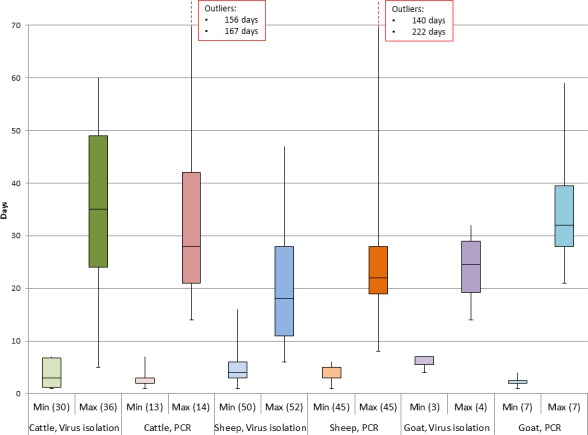
Minimum and maximum day of detection of BTV or nucleic acid in blood of cattle, sheep and goats after experimental infection (in brackets the number of samples)
The systematic literature review confirms the conclusions made in previous EFSA Opinions. It is clear that BTV infection of ruminants is prolonged but not persistent and that the duration of viraemia in BTV‐infected ruminants reflects in part the lifespan of circulating red blood cells carrying the virus (MacLachlan, 2004; Melville et al., 2004; White and Mecham, 2004; Lunt et al., 2006).
The OIE considers an infective period of 60 days for BTV‐infected ruminants on the basis of an analysis of data which indicated a > 99% probability of detectable BTV viraemia ceasing before 9 weeks in adult cattle (OIE, 2014).
This approximately 60‐day infective period is considerably shorter than the interval (up to 7 months or even longer) during which BTV nucleic acid may be detected in ruminant blood by RT‐PCR assays (MacLachlan et al., 1994; Bonneau et al., 2002). Thus, the RT‐PCR assay is overly sensitive in identifying BTV virus‐positive animals.
3.2.2.3. Persistence of BTV in other organs or tissues
From the systematic literature review conducted in the framework of EFSA mandate on vector‐borne disease, data about the presence of BTV in different tissues other than blood, in cattle, sheep and goats experimentally infected by intravenous or subcutaneous virus inoculation were extracted from 25 papers (Dórea et al., 2017), and grouped according to the test used for detecting the virus or its nucleic acid. The distribution of the values of the maximum day of the BTV or nucleic acid detection in these tissues is shown in Figure 8. The data are not stratified by animal species because of the limited number of data. For graphical reasons, outliers are indicated out of the graph scale, i.e. two values of maximum detection by RT‐PCR in spleen and tonsils of 151 and 88 days, respectively.
Figure 8.
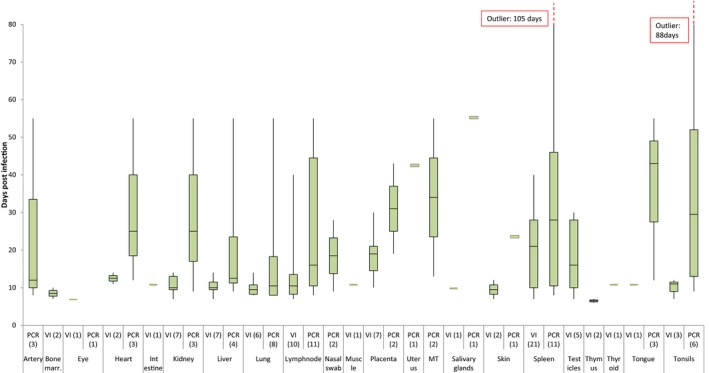
Maximum duration of detection of BTV or nucleic acid in different tissues of cattle, sheep and goat
- VI: virus isolation; MT: mammary tissue; bone marr.: bone marrow. In brackets, the number of samples.
The values of the duration of BTV presence range from 6 to 40 days in thymus and spleen, respectively, detected by virus isolation, and from 8 to 151 days in lymph nodes and spleen by RT‐PCR (Figure 8).
Some limitations remain in clarifying the distinction between ‘virus replication’ and ‘virus presence’. Indeed, this difference is important for the implication in term of BTV persistence in certain organs. In the study by Darpel et al. (2012), BTV replication and organ tropism were studied in a wide range of infected sheep tissues by immunofluorescence‐labelling of non‐structural or structural proteins using confocal microscopy to distinguish between virus presence and replication. Replication was demonstrated in vascular endothelial cells and agranular leucocytes, thus in blood and lymphatic vessels and lymphoid tissue (lymph nodes, spleen, thymus, etc.), respectively. Skin and tonsils were shown to support relatively high levels of BTV replication, although they have not previously been proposed as important replication sites during BTV infection.
Few of the tissues that support BTV presence or persistence could potentially play a role for virus transmission through vectors, apart from blood. For example, the BTV replication in the skin or dermal tissue is thought to be of some significance for the transmission of BTV. A mechanism that supports the role of skin in favouring BTV transmission was also observed in the study by Takamatsu et al. (2003). Skin fibroblasts interact with BTV‐infected T‐cells, inducing lytic reaction and increased virus release. Since Culicoides midges induces skin inflammation and thus the recruitment of activated T‐cells in the biting site, the interaction of persistently infected T‐cells with skin fibroblasts would result in increased virus production at the biting site, favouring transmission to the insect vector. This hypothesis still needs to be confirmed by further studies.
Limited evidence is available for other infected tissues implied in vector‐free horizontal transmission. van der Sluijs et al. (2016) recently review this aspect. BTV infection of cattle through direct contact was observed, both due to ingestion of BTV contaminated placentas (Menzies et al., 2008), or, in an experimental setting, by colostrum spiked with BTV‐8 infected blood (Backx et al., 2009). Direct contact transmission of BTV‐26 was observed in goats under experimental conditions and BTV‐26 RNA was detected in nasal swabs. BTV‐8 and BTV‐1 were observed to be transmitted horizontally between sheep in a vector‐free environment (van der Sluijs et al., 2011, 2013b), most likely orally through contamination of feed and drinking water with saliva or nasal discharge from infected animals. The same authors, however, considered the vector‐free horizontal transmission mechanism infrequent and requiring the close contact of animals and able to influence the morbidity rates only within farm and not supporting between herds spread (van der Sluijs et al., 2016).
3.2.3. Persistence of the infection in the vector population through vertical transmission
Vertical transmission (VT) in vectors is defined as the transmission of an arbovirus from adult females to immature stages being therefore the offspring infected with the virus and leading to adults of next generations capable of virus transmission. Transovarial transmission (TOT), that is infection of the germinal tissue of the female vector, is recognised as the most efficient mechanism of VT. This mechanism would provide a way of interseasonal transmission for the virus and has been described for some arboviruses transmitted by mosquitoes such as La Crosse, Dengue and West Nile viruses (Lequime and Lambrechts, 2014). However, up to date, there are no scientific systematic evidences to support such mechanism of transmission in the case of BTV and its biological vectors. Previous studies about transovarial transmission of BTV in North America were conducted by White et al. (2005) finding BTV nucleic acid in field‐collected larvae of Culicoides sonorensis and Culicoides crepuscularis. However, these findings have not been confirmed by a recent work conducted by Osborne et al. (2015), where colony‐reared adult females of the North America vector C. sonorensis were fed with BTV serotype 17 spiked blood and posteriorly qRT‐PCR analysed for virus detection. BTV was detected very scarcely in eggs, but neither larvae nor pupae nor adults showed any presence of the virus (Ct values > 40). In addition to the same experiment, virus particles were not detected in C. sonorensis larvae (2,171 specimens) collected in wastewater ponds in different farms in California.
Transovarial transmission could be also found by detecting virus particles in nulliparous females. This was evidenced for Schmallenberg virus (Orthobunyaviridae) in Poland since field‐collected nulliparous females of the ‘Obsoletus complex’ (C. obsoletus and C. scoticus) and Culicoides punctatus gave Ct values < 40. However, their role in transmission was not determined since the obtained Ct values corresponded to subtransmissible infection (Larska et al., 2013). Up to date, there are no similar works conducted for BTV; therefore, the role of BTV‐infected nulliparous females could not be determined. In addition, despite the most used technique to identify nulliparous and parous females is based on the examination of abdomen pigmentation developed by Dyce (1969), there is a series of limitations of this technique as demonstrated by Braverman and Mumcuoglu (2009) that found that some old nulliparous females of C. imicola also show pigmented abdomen. This fact was later on also observed by Harrup et al. (2013) in C. obsoletus females obtained by emergence traps.
3.3. Review of the suitability of the provisions on surveillance laid down in Regulation (EC) No 1266/2007
Due to the recurrence of BTV in apparently BT‐free areas, a critical review of the performance of the surveillance in place in the EU as laid down by the Reg. (EC) 1266/2007 is needed. In the Reg. (EC) 1266/2007, the monitoring and surveillance programme that aims at demonstrating the absence of BTV circulation must include a passive clinically based and an active laboratory‐based surveillance. The latter must consist of an annual programme of at least one, or a combination of, serological/virological monitoring with sentinel animals, serological/virological surveys or targeted monitoring and surveillance based on a risk assessment. The sample size used for the active laboratory‐based surveillance must be calculated to detect an infection prevalence (prevalence of the virus infection) of 5% with 95% confidence. The geographical unit of reference for the purposes of BT monitoring and surveillance is defined by a grid of around 45 × 45 km or by subnational administrative units. For the purpose of regaining the free status, the results of the surveillance programme must demonstrate the absence of BTV circulation during a period of at least 2 years, including two seasons of vector activity.
In order to assess the suitability of the provisions for surveillance as in the Reg. (EC) 1266/2007, the possible lowest and persistent levels of BTV circulation in livestock were explored both by using the model as presented in Section 3.1.1, and by cross‐checking values of infection prevalence from the literature.
3.3.1. Review of EFSA opinion on BTV surveillance and monitoring
In a scientific opinion delivered in 2011 (EFSA AHAW Panel, 2011a), the Panel on Animal Health and Welfare of EFSA was asked to provide a scientific advice on the expected prevalence (design prevalence) under different circumstances and on the size of the relevant geographical area for the purpose of monitoring and surveillance programmes for bluetongue.
Five epidemiological phases of a BTV infection in a population were distinguished, each with a specific goal for monitoring and surveillance:
Phase 1 is a BTV‐free population without a history of infection (i.e. fully susceptible). The objective of the surveillance in this phase is the early detection of outbreaks.
Phase 2: upon introduction of BTV, virus transmission will result in a rise in the prevalence of BTV positive animals. The objective of the surveillance in this phase is establishing the extent of the infected area identifying the potentially useful interventions.
Phase 3: a rise in the prevalence of seropositive animals until a plateau. The objective of the surveillance in this phase is the same as in phase 2 and in addition to monitor the impact of the interventions on the prevalence of infected animals.
Phase 4: the prevalence drops again to an endemic equilibrium or to zero. The objective of the surveillance in this phase is the same as in phase 3.
Phase 5: if the prevalence has dropped to zero, the area is again free from BTV infection, but at this point there is a history of infection. The objective of the surveillance in this phase is demonstrating freedom from the disease.
To obtain estimates of the expected prevalence, a systematic literature review (SLR) and a review of monitoring and surveillance data from the EU Member States (MSs) were performed in order to obtain the prevalence observed in the MSs. The infection and serological prevalence values (virus and antibody prevalence) at herd and animal level obtained from the SLR and from the MSs are reported in Tables 3 and 4 (EFSA AHAW Panel, 2011a).
Table 3.
Observed prevalence extracted from the systematic literature review (only those data with a prevalence > 0 are included)
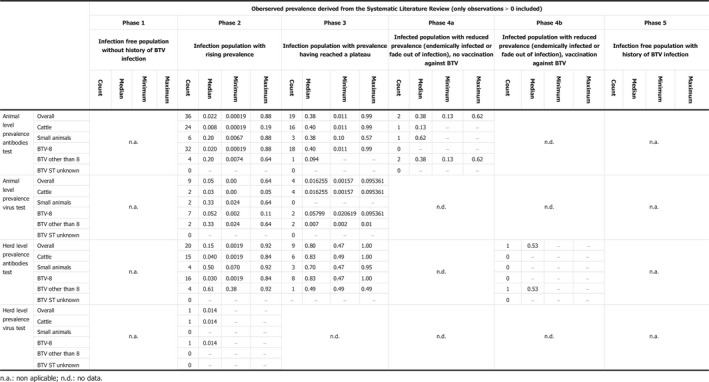
Table 4.
Observed prevalence derived from the EU Member States’ monitoring and surveillance data (only those data with a prevalence > 0 are included)
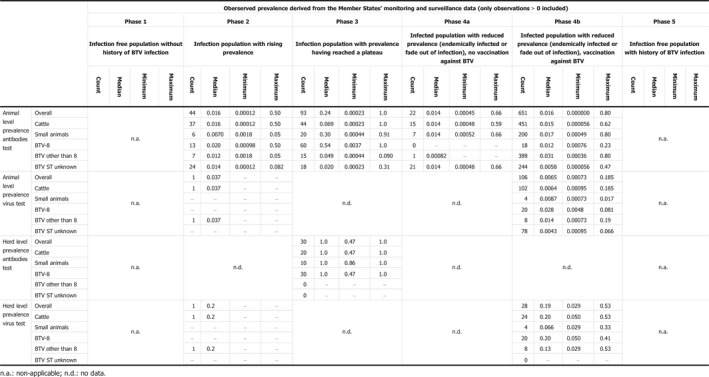
On average, the median of the overall observed seroprevalence at animal level in epidemic phase 2 was 2% (Table 3) and 1.6% (Table 4) as deriving from SLR and MSs data, respectively. In phase 3, the median of the observed prevalence was 30% (38% in the SLR and 24% in MSs data; Table 3 and Table 4). The observed seroprevalence of BTV‐8 infected ruminants in North‐western Europe was markedly higher than that of other serotypes in Southern Europe. In phase 4, the median of the observed prevalence was 1.4% and 1.6% in vaccinated and unvaccinated populations, respectively (Table 4).
3.3.2. Modelled BTV serological and infection prevalence
The model presented in Section 3.1.1 was used to estimate the levels of infection and the serological prevalence of BTV in a pooled (perfectly mixed) population of cattle and small ruminants after a certain number of years from the infection introduction and without the application of any control measure (Figure 9).
Figure 9.
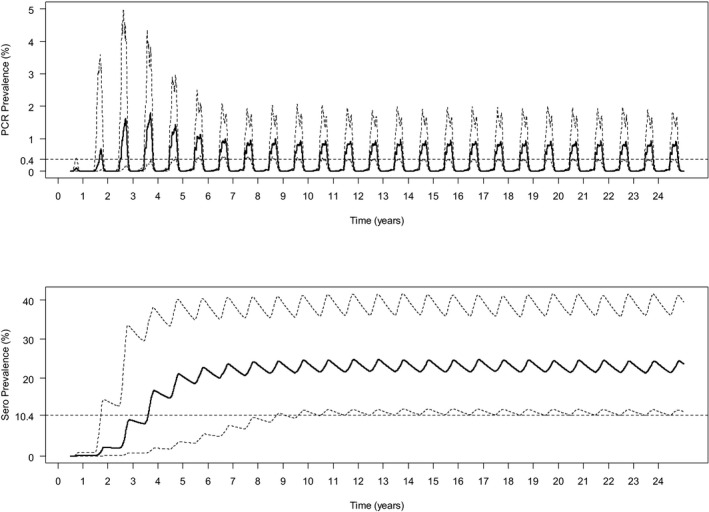
Serological and infection prevalence in cattle and small ruminants after a certain number of years from the infection and without the application of any control measure
- The bold line is the median, the dot lines are the 97.5th and the 2.5th percentiles. The latter can be considered as the lowest possible level of both infection and serological prevalence on the long term.
In relation to the levels of serological prevalence, the results of the model indicate a value of 10.4% as the lowest possible level (2.5th percentiles as worst‐case scenario) of BTV serological prevalence in the long term. Conversely, when the levels of infection prevalence are considered, a value of 0.4% is the lowest level of infection that can be observed each year. These two levels can be considered the design prevalence values for surveillance programmes based on serology or RT‐PCR, respectively.
When the results of the model are compared with the values obtained from the SLR and the review of monitoring and surveillance data from EU MSs (Phases 4a and 4b of Tables 3 and 4), the values of serological prevalence estimated by the model are in agreement with those reported by the SLR (median: 38%), but higher than those reported by the EU MSs (median: 1.4–1.6%). On the other hand, the infection prevalence estimated by the model is quite in line with the values observed in the EU MSs (median: 0.65%, Phase 4b of Table 4).
It is important to clarify that the scenario considered by the model is related to the persistence of BTV infection during years after its introduction in a previously free area and without the application of any control measures. The estimated seroprevalence, therefore, is the effect of the BTV infection only. In case of low‐level BTV circulation following the vaccination of susceptible host populations, similar values of infection prevalence as estimated by the model can be considered under the endemic scenario (i.e. 0.4%), but completely different values should be taken into account for the serological prevalence.
In fact, in the case of a vaccinated population, after the cessation of the vaccination campaign the great majority of the animals are supposed to be already serologically positive. Under the hypothesis of a low‐level circulation, only new‐born replacing animals are exposed to the infection with an infection rate similar to the one estimated by the model (i.e. 0.4%). Therefore, a seroprevalence close to the infection rate is expected during the first year after the cessation of vaccination in non‐vaccinated animals born after the end of the vaccination campaign. After 2 years from the end of the vaccination campaign, a seroprevalence close to the double of the infection rate can be roughly expected in animals born after the cessation of vaccination. And so on for the following years, till reaching the seroprevalence values close to those predicted by the model when no more vaccinated animals will remain in the population. These considerations imply that, whereas a 0.4% threshold can be considered for the infection rate (e.g. detected by RT‐PCR), the design prevalence for the serological surveillance in non‐vaccinated animals varies with the time since the end of the vaccination campaign. The closer to the end of the vaccination activities, the lower is the design prevalence to be considered.
3.3.3. Case study: the surveillance system in France in 2013–2016
3.3.3.1. Surveillance in the period of freedom 2013–2015
Bluetongue appeared in northern Europe for the first time in 2006. In that year, an epidemic of BT caused by serotype 8 (BTV‐8) affected five countries: Germany, Belgium, France, Luxembourg and the Netherlands. Alongside the health measures implemented, campaigns of mandatory immunisation, using inactivated virus vaccines against serotypes 1 and 8, were implemented on the French mainland in spring 2008, until autumn 2010. In France, the last outbreak of BT was identified in June 2010, in the département of Alpes‐Maritimes (serotype 1). France was recognised BTV‐free in December 2012 (Sailleau et al., 2015).
Following the recognition of the free status at the end of 2012, from the year 2013, the French authorities applied a surveillance system in compliance with the provisions of the Reg. (EC) 1266/2007, which for the purpose of detecting any possible incursions of BTV, requires the establishment of a monitoring and surveillance programme, based on passive clinical surveillance and active laboratory‐based surveillance. The latter must be designed in such a way that the samples are taken from susceptible animals (that is animals which have not been vaccinated and which have been exposed to the competent vector), which are representative of the structure of the susceptible species population in the epidemiologically relevant geographical area and the sample size must be calculated to detect the appropriate design prevalence based on the known risk of the target population with 95% confidence in the susceptible species population of that epidemiologically relevant geographical area. In the absence of scientific information on the expected prevalence for the target population, the sample size must be calculated to detect a prevalence of 20%.
Therefore, the French surveillance system in place from 2013 to 2015 was based on the annual random selection and serological testing of 15 animals (5 animals selected in 3 different farms) for each département. Target animals were bovines less than 2 years old, not vaccinated against BTV and exposed to Culicoides vectors (ANSES, 2015).
In order to calculate the effective sample size when considering other type of sampling schemes than the simple random sampling has been extensively studied. Several authors have studied how to adjust the sample size calculation according to the type of design used (Kish, 1965, 1990, 1992; Spencer, 2000; Valliant et al., 2013, 2015). For the calculation of the effective sample size (n eff), we have used the proposed approached by Gabler et al. (1999) in which the actual sample size is divided by the design effect (d eff). The d eff is considered to be the combination of two separate design effects, one due to unequal selection probabilities, d effp, and one due to clustering of samples, d effc. Gabler et al. (1999) proposed to use these two quantities in a multiplicative way to define the overall design effect (d eff). The definition of each of the design effects can be found in Gabler et al. (1999). In order to calculate the d effp, the total number of farms and animals in the regions were extracted from EUROSTAT, to calculate the selection probability of a farm and an animal within a farm for each region (department) considering the sampling scheme followed in France. Once the totals were known, using the actual number of farms and animals within a farm sampled per region the d effp was calculated. Function d effK from Package PracTools in R was used to calculate d effp (Valliant et al., 2015). In order to calculate the design effect due to clustering, the within‐herd correlation reported in (Meroc et al., 2008)9 was used (0.41, CI: 0.36–0.47), since no available published intraclass correlation values is available for France up to date. The cluster size used was the maximum number of animals sampled in a farm within a region, following precautionary principles. Considering the actual number of samples taken in each department and how samples were collected (design used), the effective sample sizes were calculated and the design prevalence that would be able to detect such sampling schemes was computed (Figure 10).
Figure 10.
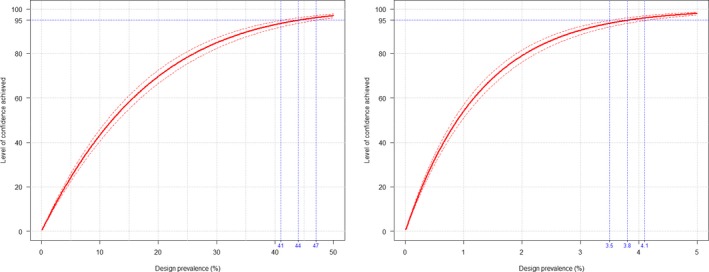
Detectable prevalence at département (left) and country (right) levels considering the sampling procedure used in France from 2013 to 2015
Considering a 95% confidence level, the detectable serological prevalence by the French surveillance system in place from 2013 to 2015 varied between 41–47% and 3.5–4.1% at département and country level, respectively. If the intraherd correlation coefficient would be lower e.g. three times smaller than the value as previously reported in Belgium, the detectable design prevalence for the best case scenario at NUTS3 and at country level would vary between 20–22% and 1.57–1.72%, respectively.
3.3.3.2. Surveillance in the period 2015–2016
At the end of August 2015, a ram located in central France (département of Allier) showed clinical signs suggestive of BTV infection, but none of the other animals located in the herd showed any signs of bluetongue disease. Laboratory analyses identified the virus as BTV serotype 8 and the intraherd virological and serological prevalences were 2.4% and 8.6% in sheep and 18.3% and 42.9% in cattle, respectively. Phylogenetic studies showed that the sequences of this strain were closely related to another BTV‐8 strain that has circulated in France in 2006–2008 (Sailleau et al., 2015).
The re‐occurrence of BTV‐8 in France in 2015 posed new questions about the possible source of this BTV re‐emergence. The French agency ANSES, conducted a thorough risk assessment about the origin of the re‐occurrence of BTV‐8 in continental France and concluded that the likely source was a continual low level of circulation since the previous epizootic in 2007–2008 (ANSES, 2015). According to ANSES experts, apparently the surveillance system was not sensitive enough to detect BTV circulation at low level.
After the re‐occurrence in 2015 of the BTV‐8, a national cross‐sectional survey was conducted in October 2015 to assess the epidemiological situation with more precision than the surveillance previously implemented (Bournez et al., 2016). Sixty herds per administrative Region (NUTS 3) were selected and 30 animals per herd were tested by PCR in order to detect an animal prevalence at the region level of 5% (with 95% confidence), based on a 5% intra herd prevalence. Thus, 1,338 herds and 39,513 animals were sampled. The map in Figure 11 shows the results of this surveillance.
Figure 11.
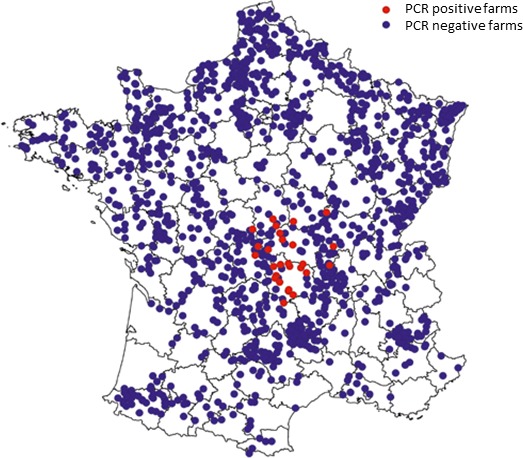
Detection of PCR positive herds as from the cross‐sectional survey carried out in France in October 2015
- Source: (Bournez et al., 2016).
After the winter 2015–2016, a new surveillance protocol was introduced in France in the summer 2016. In the départements without BTV outbreaks, the system aims at demonstrating the absence of seroconversion on sentinel animals at risk, thus ensuring the absence of virus circulation in the département of the free zone and in those of the restricted zone which have not been affected (Figure 12).
Figure 12.
Zoning map of BT serotype 8 in France up to 29 September 2016 and surveillance objectives set for 2016
- Source: (French Ministry of Agriculture, 2016).
The sample in each département consists in 180 seronegative at risk animals randomly selected. This number of animals can detect a minimum prevalence of 2%, with the 95% of confidence. This sample size is a compromise between the 5% design prevalence required by the Regulation (EC) 1266/2007 and the département level selected that is larger than the 45 km x 45 km grid considered in the legislation, which is indeed rather closer to the size of a district (arrondissement). This protocol also considers the complementarity of different types of surveillance (passive + serological surveillance + strengthened programme on output movements from the restricted zones). In practice, this corresponds to select at least nine herds and follow about 20 seronegative cattle per herd. The sampling frequency is monthly. To ensure a good spatial distribution, about three herds per district should be selected with a minimum of nine herds and target of 180 seronegative animals minimum by département. The objective of nine herds is proposed by considering what seems feasible in practice for the département.
The recruitment criteria are the following:
unvaccinated livestock against BT (otherwise the risk of exposure is reduced; some vaccinated animals may of course be present without exceeding a threshold of 10% of animals);
no preference on the type of production;
focus on cattle rather than other species;
farms that are not more than 1,000 m above sea level (Culicoides are less present in altitude)
farms with pasture access;
priority of herds which are surrounded by farms of susceptible species;
focus on big size farms (the number of Culicoides is more important and it seems to promote circulation).
Guided by these criteria, a random selection was made where possible. The serological test used is the c‐ELISA, with a sensitivity of 99.7% and specificity of 98.2%.
According to the above‐mentioned surveillance scheme, data about sampling of herds in each département and the number of animals sampled in each herd were obtained for 33 French départements, where there is sentinel surveillance. These data were used to explore the achievable design prevalence with 95% confidence interval according to Valliant et al. (2013). A curve was drawn for the worst‐ and best‐case scenario at NUTS 3 level (corresponding to the French département) considering the amount of farms and animal sampled within a farm in each département (Figures 13–14) and for the scenario at national level (Figure 15).
Figure 13.
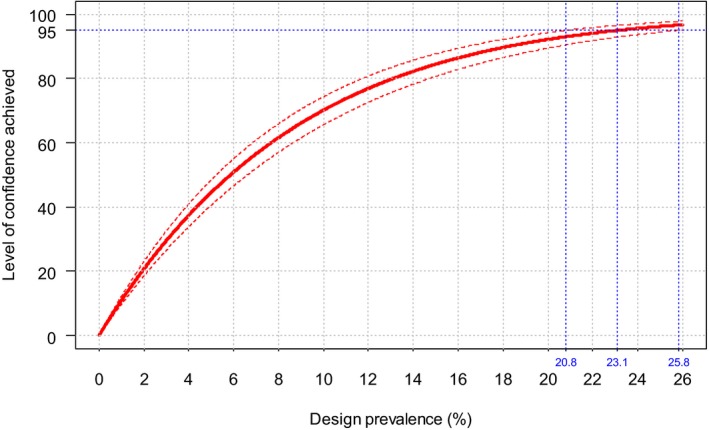
Worst‐case scenario for detectable prevalence given the sample survey at NUTS3 level (the French département)
- The bold line is the median, the dot lines are the 2.5th and 97.5th percentiles, the related values of design prevalence are indicated.
Figure 14.
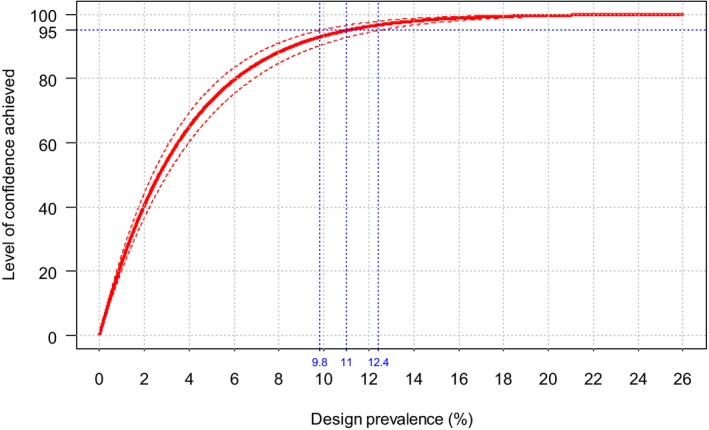
Best‐case scenario for detectable prevalence given the sample survey at NUTS3 level (the French department)
- The bold line is the median, the dot lines are the 2.5th and 97.5th percentiles, the related values of design prevalence are indicated.
Figure 15.
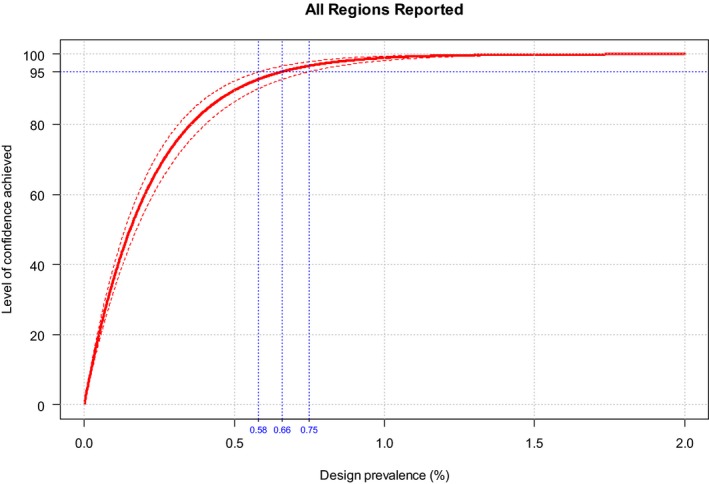
Scenario for detectable prevalence given the sample survey at national level for France
- The bold line is the median, the dot lines are the 2.5th and 97.5th percentiles, the related values of design prevalence are indicated.
This high values of detectable prevalence, thus leading to relatively low performing surveillance within department, are linked to the small sample size in term of farms inside each department and the relatively high correlation of samples within farm considered in the present estimation – the value as observed in Belgium (0.47%) is applied here, since no available published intraclass correlation values are available for France up to date, although these values could be different. The lower this value, more independent would be the samples within farm, thus even a low number of farms would be representative of the department. For example, if the correlation would be three times smaller than the reported ones, the detectable design prevalence for the best and worst case scenario at NUTS3 level would vary between 3.8–4.8% and 8.8–10.8%, respectively.
The same kind of assessment was done for the scenario at national level, thus considering all farms and animal sampled within a farm for all sampled department (Figure 15).
3.4. Maternal immunity and vaccines
The first three ToRs related to ToR 2 about (i) protection of maternal immunity, (ii) its interference with vaccines and (iii) the time lag after vaccination to consider animals as protected were addressed by a systematic literature review, while the fourth ToR of the ToR 2 is addressed in Section 3.4.1. The literature search identified a total of 287 articles. Title and abstract screening led to the exclusion of 184 articles, 103 articles were considered eligible for full‐text screening, 17 did not report data suitable for the data extraction phase and 52 were finally considered eligible for inclusion in the systematic review. In Figure 16 the workflow of search and selection of the studies is displayed.
Figure 16.
Workflow of search and selection of the studies in the SLR
Q1: ‘What is threshold of BTV‐specific maternal antibody titre considered to provide protection to an offspring born from vaccinated mother to one/several BTV serotypes?’
For the first review question about the threshold of BTV‐specific maternal antibody titre considered to provide protection to offspring born from vaccinated mothers to one/several BTV serotypes, the systematic review led to the identification of four studies (Savini et al., 2004a; Oura et al., 2010; Vitour et al., 2011; Leemans et al., 2013).
In the study by Vitour et al. (2011), 22 dams/calves pairs was considered, and cows were vaccinated 5 months before giving birth. The 22 calves were followed until 118 days, when 13 of them were vaccinated with an inactivated BTV‐8 vaccine. Kaplan–Meier survival curves showed that the apparent interval after birth required for loss of passively acquired antibodies depended upon the serological test used, and was found to be earlier by virus‐neutralisation test (VNT). The median time after birth when calves become seronegative was 112 days by c‐ELISA (range 70–173) and 84 days by VNT (range 70–113 days). The time of pregnancy when mothers were vaccinated was not indicated. The critical appraisal of this study about study design, methodology used, statistics and reporting quality was assessed as high, but the appropriateness of the controls was considered not satisfactory.
However, in the field study by Savini et al. (2004a,b),1,005 cows of various breeds and ages were randomly selected from 10 herds in Sardinia island. The cows were vaccinated against BTV‐2 with a live‐attenuated vaccine and blood samples were taken monthly for 3 months after vaccination and tested for the presence of antibodies by c‐ELISA and VNT. To assess the duration of colostral antibodies in calves born from these vaccinated dams, the sera of 47 calves divided in three age groups were screened using c‐ELISA and VNT. Antibodies were detected in 68.2% calves at 1–25 days of age and in 46.1% of the calves at 26–39 days old. The older calves (40–60 days) were all serologically negative. Due to the small number of animals tested, the probability curves were very wide and, for the oldest group of animals, the lower and upper 95% confidence levels for the observed serological prevalence were 0.2% and 21%. No details were provided about titres or whether animals were positive to the c‐ELISA alone or VNT too. The time of pregnancy when mothers were vaccinated was not indicated. It must be also taken into account that this was a field study, and therefore, the effects of the not correct vaccine conservation and administration, and variability in the colostrum uptake by calves could not be excluded. The critical appraisal of this study about study design, methodology used and statistics was assessed as high, but the reporting quality and the appropriateness of the controls were considered not sufficient.
In the study by Oura et al. (2010), the extent and length of colostral antibody protection as well as the degree of colostral antibody induced interference of the immune response to BTV‐8 in sheep were investigated. Lower titres of neutralising antibodies were detected in colostrum‐fed lambs born from sheep vaccinated once than from those vaccinated twice (single vaccine in the first year and a booster vaccine in the second year of BTV‐8 inactivated vaccine). Of the 16–36‐day‐old lambs born from these single vaccinated ewes, none had c‐ELISA antibodies detectable, 31% of the lambs had antibodies detected by double‐antigen sandwich ELISA (s‐ELISA) and only 6% of the lambs had detectable neutralising antibodies.
On the contrary, the 22 lambs born from the double vaccinated ewes were all positive to s‐ELISA and VNT, and 19 out of 22 lambs were also positive to c‐ELISA when they were 6–10 weeks old. When the lambs were 13–14 weeks old, all were still positive to s‐ELISA, 9 out of 22 were positive to c‐ELISA, and 14/22 tested positive by VNT, although at low titre.
Considering the protection from infection, the lambs born from sheep vaccinated twice, with the second booster dose given approximately 1 month prior to lambing, were challenged with BTV‐8. Neutralising antibodies were found until 22 dpi. No lamb showed any clinical sign when challenged by BTV‐8 subcutaneous inoculation at 13–14 weeks old. Fourteen of these lambs had circulating neutralising antibodies at the time of challenge and were fully protected both clinically and virologically from BTV‐8 challenge. Three of the seronegative lambs were also protected both clinically and virologically, and the remaining five lambs with no detectable neutralising antibodies at challenge became BTV RNA positive by RT‐PCR, the virus was isolated from the blood of these animals and a significant increase of neutralising antibody titres was observed after challenge. The time of pregnancy when mothers were vaccinated was not indicated. The critical appraisal of this study about study design, controls, methodology used, statistics and reporting quality was assessed as high. The results from this study show that neutralising antibodies in lambs protects against homologous challenge (no viral RNA detected in blood). This is consistent with previous studies in which vaccinated animals with neutralising antibodies are generally protected against infection, although also some of the vaccinated animals not showing detectable neutralising antibodies may be protected.
Finally, in the study by Leemans et al. (2013), 35 lambs born from naturally infected and yearly vaccinated ewes were followed for 10 months, vaccinated at different times, and then challenged. At 36–48 h after birth, all lambs were positive for BTV‐8 (median titre of neutralising antibody: 2.16) with values ranging from 1.68 to 3.12 log10 PD50.10 Neutralising antibody titres gradually decreased until 7 months old. A Kaplan–Meier survival analysis pooling data from different groups estimated that the median time after birth required for complete loss of passively acquired neutralising antibody was 7 months (range: 5–9 months). The critical appraisal of this study about study design, controls, methodology used, statistics and reporting quality was assessed as high.
The Table 5 shows the main results of the selected studies in relation to the duration of maternal antibodies in calves and lambs.
Table 5.
Range of duration of colostral Abs as reported in the selected studies
| Species | Mother immunity | Sample size | Study setting | Test | % positive | Duration (days) mean | Duration (days) min | Duration (days) max | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination dose/type | Time of vaccination | |||||||||
| Calves | One dose/inactivated | 5 months prepartum | 22 | Experimental | c‐ELISA | 100 | 112 | 70 | 173 | Vitour et al. (2011) |
| One dose/inactivated | 22 | Experimental | VNT | 100 | 84 | 70 | 113 | |||
| Lamb | One dose/inactivated | 20–40 days prepartum | 45 | Experimental | c‐ELISA | 0 | – | 16 | 36 | Oura et al. (2010) |
| VNT | 6 | – | 16 | 36 | ||||||
| s‐ELISA | 31 | – | 16 | 36 | ||||||
| Double dose/inactivated | 25–34 days prepartum | 22 | Experimental | c‐ELISA | 86 | – | 24 | 40 | ||
| VNT | 100 | – | 24 | 40 | ||||||
| s‐ELISA | 100 | – | 24 | 40 | ||||||
| c‐ELISA | 40 | – | 52 | 56 | ||||||
| VNT | 63 | – | 52 | 56 | ||||||
| s‐ELISA | 100 | – | 52 | 56 | ||||||
| Lamb | Triple dose | 3 months prepartum | 35 | Experimental | VNT | 100 | 210 (median) | 150 | 270 | Leemans et al. (2013) |
| Calves | One dose | na | 47 | Field study | VNT | 68.2 | – | 1 | 25 | Savini et al. (2004a) |
| 46.1 | – | 26 | 39 | |||||||
| 0 | 40 | 60 | ||||||||
VNT: virus‐neutralisation test; c‐ELISA: complement‐enzyme linked immunosorbent assay.
Q2: ‘What is the minimum age of calves, lambs and kids after which residual colostral antibodies against BTV do not interfere any longer with vaccine immunisation of these animals?’
For the second question about the age until when residual colostral antibodies may interfere with vaccine immunisation, only two papers were considered eligible (Vitour et al., 2011; Leemans et al., 2013). The critical appraisal of these studies related to this review question about study design, controls, methodology used, statistics and reporting quality was assessed as high.
Currently, the recommended age for vaccination of calves with inactivated BTV‐8 vaccines varies from 1 to 3 months, depending on the vaccine manufacturer. However, these recommendations have not been based on extensive study, especially considering the impact of colostral antibodies on the vaccinal response. In the study by Vitour et al. (2011), a group of 22 pregnant cows were vaccinated against BTV‐8 with an inactivated vaccine 5 months before giving birth. The 22 calves were followed until around 118 days when 13 of those were vaccinated with the same inactivated vaccine. In most calves, vaccination elicited a weak immune response, with c‐ELISA seroconversion in only 3 out of 13 calves. The amplitude of the humoral response to vaccination was inversely proportional to the maternal antibody level prior to vaccination.
Leemans et al. (2013) assessed the interference of colostral antibodies in the immunological response to a BTV‐8 inactivated vaccine in lambs born from immune ewes. Lambs born from naturally infected and yearly vaccinated mothers were followed for 10 months, vaccinated at different times, and then challenged. They were allocated to five groups (7 lambs each group), four vaccinated at different ages (3, 5, 7 and 9 months) and one left unvaccinated. Among lambs vaccinated at 3 months, three (3/7) did not respond to vaccination and were not protected from challenge infection. The others animals (4/7) were fully protected from clinical disease and viraemia in accordance with their seropositive status at time of challenge. BTV‐8 vaccination performed at 5 months of age or later led to seroconversion and full clinical and virological protection in 100% of vaccinated lambs. It can be concluded that maternally derived antibodies interfere with the immune response to BTV‐8 vaccination in lambs for at least 3 months after birth.
Q3: ‘What is the minimum time after completion of vaccination against BTV and the threshold BTV‐specific antibody titre considered to provide a protective immune response after vaccination?’
Concerning the third question on ‘what is the minimum time after completion of vaccination against BTV and the threshold BTV‐specific antibody titre considered to provide a protective immune response after vaccination’, 51 papers were considered eligible to answer this question (see data extraction table in Appendix C). When the results of these studies are compared, high variability and uncertainty seem affecting the results. Various different vaccines, study designs, methods for vaccination, challenge and testing were used. In particular, the time/level of protection seems difficult to assess partly due to the variety of the laboratory methods used (e.g. different ELISAs, VNT titre, RT‐PCR, etc.). Consequently, the studies are very difficult to compare. Nevertheless, some considerations could be formulated. In relation to the minimum time after vaccination (‘minimum protection day’ (MPD)) Figure 17 reports the earliest point in time (day) after vaccination when a positive immune response was observed. Some papers included more than one study, e.g. papers that included experimental data from more than one animal species or more than one vaccine type.
Figure 17.

Plot of values of minimum days after vaccination when a seroconversion was observed by c‐ELISA or VNT in cattle, sheep and goats
- Each point represents the minimum value of days for each study (considering ‘one study’ that performed in a single animal species, with a specific diagnostic test and one type of vaccine: CIN: Commercial Inactivated Vaccine; CLA: Commercial live‐attenuated Vaccine; EX: Experimental vaccine).
When the MPD following the vaccination of commercial inactivated vaccines and detected by VNT only are considered, the following results can observed:
Cattle: In the 40.4% of animals, the MPD was within 14 days, in 61.1% within 21 days and in 96.7% within 28 days.
Sheep and goats: In the 52.9% of animals, the MPD was within 14 days, in 78.7% within 21 days and in 84.7% within 28 days.
When the results to c‐ELISA are considered in animals vaccinated with commercial inactivated vaccines, the MPD was within 21 days post‐vaccination in 94.0% and 98.0% of cattle and small ruminants, respectively.
Sheep. The results obtained from 35 studies were variable, and indicated a lower value of MPD when ELISA is used (in the majority of cases between 6 and 10 days after vaccination) in comparison with VNT (mainly between 14 and 21 days).
Cattle. The results from a total of 15 studies indicated that the MPD varied from 3 to 42 days post‐vaccination depending on the method used to assess the antibody response, bearing in mind that the results are also influenced by the vaccine used and other factors such as the study design and sample size. One study reported a MPD equal to 3 days when using a commercial c‐ELISA. Four studies indicated a MPD of 14 days post‐vaccination when VNT is used.
Goats. The results from one study suggested that the MPD were 10 and 21 days after vaccination when ELISA test and VNT are used, respectively.
3.4.1. Guarantee of safe movement of animals under a specific conditions
The ToR 2.4 pose a very specific question in relation to the risk of introducing the infection (i.e. introducing viraemic animals) into BT‐free areas through the introduction of not vaccinated animals from BT‐infected areas, when a series of risk mitigation measures are applied. In particular, it is asked to:
‘Assess whether vector protection for 14 days of ruminants below the age of 70 days, combined with a negative PCR test at the end of the 14 days or more, qualify them for a safe movement from a BT restricted to a BT‐free area’.
In particular, the following risk reduction measures are considered:
The animals are up to 70 days old and born from vaccinated mothers.
The animals are kept for 14 days under vector protection conditions.
They are tested by RT‐PCR at the end of the 14 days period, before being moved to the final destination.
Any quantitative estimation of the final risk of introducing a viraemic animal following the above described procedure would be affected by high levels of uncertainties in the components of this scenario, thus making the risk estimation relatively useless for the purpose of the risk management.
In fact, the following aspects must be taken into consideration:
The initial risk of having a viraemic animal in the lot of animals to be moved depends from the incidence of disease transmission in the place of animal's origin. In Section 3.1.1, the simulation model identified level of infections between 0.6% and 1.5% in sheep and cattle, respectively. These values, however, have to be applied for the 70 days period of animal life and considering the specific epidemiological conditions of that place and period of the year. The resulting probability of having a viraemic animal, therefore, can vary significantly in relation to the above mentioned factors.
The probability that the animal has maternal antibodies and is protected at 70 days of age is influenced by the amount of colostrum uptake, the type of vaccine used, the number of doses administered and the stage of pregnancy at which the mother has been vaccinated. In Section 3.4, some studies on the level of passive immunity induced by vaccination against BTV in cattle and sheep are reported. All 22 lambs born from 19 ewes vaccinated with two doses of an inactivated vaccine against BTV‐8 had neutralising antibodies at 6–10 weeks of age, but five of them (22.7%), when challenged at 12–13 weeks of age, developed a detectable viraemia (Oura et al., 2010). Similarly, 22 calves born from cows vaccinated with one dose of an inactivated vaccine against BTV‐8, became seronegative at 84 days by VNT (range 70–113 days) (Vitour et al., 2011).
The level of vector protection can vary significantly according to the methods applied. An almost perfect (i.e. close to 100%) vector protection can theoretically be achieved when animals are kept in well implemented vector‐proof establishments (see Section 3.6), but this approach is commonly recognised applicable for small numbers of animals, under specific market conditions which make economically advantageous the application of such an expensive solution. In case of more common use of pour‐on insecticides, the efficacy of these substances in term of risk reduction can vary greatly in relation to the application protocols used, the frequency of administration and the environmental conditions (e.g. animals kept outdoor, exposed to rainfalls, etc.). As reported in Section 3.6.2, some in‐field studies assessed the efficacy of pour‐on insecticides, with dissimilar results. Good results were obtained in Spain, where Mullens et al. (2010) showed good protection of sheep against feeding activity of Culicoides spp. by applying 7.5% deltamethrin (Butox 7.5) directly on exposed skin (face, ears and belly) and a reduction up to 50% of the number of midges associated to animals was obtained in sheep by (Griffioen et al., 2011) using a 3.6% permethrin pour‐on solution (1 mL/10 kg bodyweight; Virbac Animal Health) in the Netherlands. In Germany, Weiher et al. (2014) assessed a dosage of 10 mL of Butox® pour‐on (Intervet, France) on Merino sheep and obtained an efficacy that ranged from 0% (2 weeks post‐treatment) to 71.0% (3 weeks post‐treatment).
The diagnostic sensitivity of RT‐PCR is close to but not 100%, and therefore, a certain number of false negative animals must be expected. In this context, the objective of RT‐PCR testing is to detect infected and possibly viraemic animals, which can transmit the infection. Therefore, false negative results are of particular concern, whereas false positive cases are of no interest. Vandenbussche et al. (2008) estimated the specificity and sensitivity of c‐ELISA and RT‐PCR under field conditions during the epidemic in Belgium in 2006. The estimated sensitivity values for RT‐PCR were 99.55% (95% CI: 99.03–99.98%) in sheep and 99.50 (95% CI: 99.02–99.97) in cattle.
In addition to the above reported considerations, a critical aspect influencing the final risk of introducing viraemic animals into a BT‐free area is related to the number of animals introduced. In fact, even considering to test all animals by RT‐PCR (probability of having false negative results = 1 − sensitivity = 0.45%), depending on the levels of infection in the population of origin, when more than 10,000 animals are introduced, one or more viraemic heads are expected among the introduced animals (Table 6). This simple consideration is also confirmed by past experiences, when, for example, the BTV‐8 infection was introduced in Italy in 2007 with animals entered from France, despite the application of animal testing by RT‐PCR (Giovannini et al., 2008).
Table 6.
Number of expected infected animals in 10,000–25,000–50,000–100,000 introduced animals, according to different levels of infection in the population of origin and considering to test all animals by RT‐PCR (sensitivity = 99.55%)
| Level of infection in the population (%) | Number of introduced animals | |||
|---|---|---|---|---|
| 10,000 | 25,000 | 50,000 | 100,000 | |
| 2.0 | 0.9 | 2.25 | 4.5 | 9 |
| 1.0 | 0.45 | 1.125 | 2.25 | 4.5 |
| 0.5 | 0.225 | 0.5625 | 1.125 | 2.25 |
| 0.1 | 0.045 | 0.1125 | 0.225 | 0.45 |
3.5. Review of vector ecology
In this section, updated knowledge of Culicoides ecology is provided, including distribution, host preference, vector competence and seasonality. A specific section is dedicated to reviewing overwintering mechanisms and an assessment of the criteria for the determination of the SVFP. Further information are also provided in the story map on bluetongue developed in the framework of the EFSA project on development of infographics on vector‐borne diseases (EFSA‐Q‐2016‐00433).11
3.5.1. Geographical distribution in Europe
3.5.1.1. Culicoides (Avaritia) imicola Kieffer, 1913
The last update of the distribution of the Afro‐tropical species C. imicola shows that is present in at least seven EU countries (Portugal, Spain, France, Italy, Greece, Malta, Cyprus).12 The northernmost detection was up to the 43.6°N parallel, considering that records, such as the one in the southern Switzerland (Cagienard et al., 2006), remains anecdotic since there have been no further captures of this species at so northern latitudes. C. imicola has been considered often as an expanding species, particularly related to climate change (Purse et al., 2005; Calvo et al., 2009). However, new records attributed to ‘recent invasion’ or ‘recent colonization’ should be interpreted with caution since based on phylogeographical and population genetic studies (Jacquet et al., 2015). C. imicola has been present in the Mediterranean basin since late Pleistocene or early Holocene (10,000 years ago), and has colonised the southern Europe at least one hundred years ago with recurrent migrations since then. Changes in the northern distribution limits are relatively limited. The expansion range of C. imicola over the 2004–2010 period is estimated in the Var département at 14.5 km/year, and limited by topography and vegetation cover (Venail et al., 2012). In Italy, 8 years of entomological surveillance showed no evidence of C. imicola geographical range expansion (Conte et al., 2009). Genetic analysis of the populations are important to determine the origin of recent records, for example, C. imicola was detected in 2008 in Pyréneés‐Orientales in France and after a 5 years surveillance and analysis of samples, it has been recently demonstrated to have been originated in Corsica and not from the most neighbouring population (< 80 km) of the northeast Spain (Jacquet et al., 2016).
3.5.1.2. Other species of the Avaritia subgenus
The main abundant and widespread species of the Avaritia subgenus in non‐Mediterranean areas are C. obsoletus (Meigen), 1818, C. scoticus Downes and Kettle, 1952, Culicoides dewulfi Goetghebuer, 1936 and Culicoides chiopterus, (Meigen), 1830. This Obsoletus assemblage is therefore widely distributed in the entire EU territory. Probably, there are no countries in the EU that could report absence of any of the species included in this assemblage.
The species C. obsoletus and C. scoticus have a large Palaearctic distribution and females are highly morphologically close. Microscope mounting is needed and therefore differentiation based on morphology is difficult, requires crossing multiple criteria to be reliable, and then is time consuming (Garros et al., 2014). Due to this, all European national entomological surveillance programs include data on the both species grouped together.
For example, in France, in the non‐Mediterranean temperate areas, C. obsoletus/C. scoticus are largely dominant, associated with C. dewulfi and C. chiopterus on the Channel coast, and almost solely with C. chiopterus in north‐east. These two latter species breed on animal dung, thus their spatial distribution is influenced by also by livestock densities and presence, although this is not sufficient condition. Elsewhere, C. obsoletus/C. scoticus are mostly found alone, except locally. Reversely in Corsica, C. obsoletus/C. scoticus are secondary to C. imicola, and on the Mediterranean coast, Culicoides newsteadi is dominant (Balenghien et al., 2012).
Modelling the spatial distribution of C. obsoletus/C. scoticus suggests impact of forest cover and vegetation activity on distribution, as well as shaded breeding site requirements (Kluiters et al., 2013). Previous field observations are consistent with this, suggesting as breeding sites forest leaf litter, stagnant water and marshy areas (EFSA, 2008).
The main abundant and widespread species of the Culicoides subgenus in Europe are C. newsteadi Austen, 1921, C. punctatus (Meigen), 1804, Culicoides pulicaris (Linnaeus), 1758 and Culicoides lupicaris Downes and Kettle, 1952. The specific status of this latter species is still controversial and needs to be resolved (Harrup et al., 2014).
Although widely distributed in Europe, C. newsteadi is abundant in Mediterranean areas, where it could be the dominant species. C. pulicaris is widely distributed, including northern European areas. C. lupicaris seems to be less abundant, but difficulty to distinguish C. pulicaris and C. lupicaris and the doubts of the specific status of C. lupicaris lead many authors and national surveillance systems to group this species together. C. punctatus seems to be widespread in non‐Mediterranean areas and could reach important abundances either in Denmark or in Portugal.
3.5.2. Breeding habitats
General habitats for the European BTV vector species were described in EFSA previous opinions (EFSA, 2007b, 2008). Works conducted in Italy have confirmed the presence of C. imicola associated to farm environment such as in mud 20 cm around a pond shoreline (Foxi and Delrio, 2010). This species is considered to be farm associated to moist soil enriched with organic matter located nearby farms were drippings, sewage leakages and drainage channels are common. In consequence, changes in the environment due to farm practices (i.e. irrigation) and/or climate change, may create new favourable breeding sites and increase the spread of this species in Europe (Guichard et al., 2014).
Dung pats as breeding habitats have been confirmed for C. chiopterus and C. dewulfi in studies conducted in Germany (Steinke et al., 2014; Luhken et al., 2015).
In the case of C. obsoletus, this species breeds in a wide range of habitats (EFSA, 2007b, 2008). Recent works have described breeding in broadleaved wood‐land leaf litter, broadleaved woodland vegetation, marginal vegetation surrounding open water, muck heaps and organically enriched substrates in the UK (Harrup et al., 2013), different types of manure (old and composted manure, manure mixed with organic matter, and fresh manure) in Spain (Gonzalez et al., 2013), silage residues associated to farms (maize, grass, sugar beet pulp and their combinations) (Zimmer et al., 2013), as well as components of a chicken coop, leftover feed along the feed bunk and a compost pile of sugar beet residues and soil of a livestock trampling area (Zimmer et al., 2014) in Belgium. Alternative substrates could be also used as breeding sites, as for example slurry in the case of C. obsoletus, as demonstrated by Thompson et al. (2013) in Northern Ireland. Indoor breeding sites for C. obsoletus has been identified in Belgium in dung adhering to walls inside cowsheds (Zimmer et al., 2010) and in France in old litter left inside dairy cow buildings (Ninio et al., 2011).
Breeding habitats of other species such as C. pulicaris has been found in soil samples from grazed field with manure in Denmark (Kirkeby et al., 2009).
3.5.3. Adult feeding habits/host preferences
Since the last EFSA opinion (EFSA, 2008), there have been several works updating host preferences. In France in 2009, host preferences were checked by collecting Culicoides on different hosts (horse, cattle, sheep, goat and poultry) using sticky covers (Viennet et al., 2012a). Attraction was much higher on horse compared to other species. In Germany in 2012, host preferences were checked by collecting Culicoides by different methods (direct aspiration and drop trap) on sheep and cattle. The species C. obsoletus/C. scoticus correspond to 79.6% of the collected individuals on cattle and to 44.8% on sheep, whereas C. chiopterus correspond to 3.5% on cattle and to 15.1% on sheep (Ayllón et al., 2014). In the Netherlands in 2013, host preferences were checked by collecting Culicoides by two different methods, black‐light suction trap and aspiration (Elbers and Meiswinkel, 2014). Using comparable collection periods, 9.3 times more Culicoides were caught on the cow than on the sheep and 25.4 times less in the black‐light suction trap compared to the sheep. Mean Culicoides biting rates on the cow across the 7‐h collection period were 4.6, 3.5, 1.0, 1.0 and 0.5/min for C. dewulfi, C. obsoletus/C. scoticus, C. chiopterus, C. punctatus and C. pulicaris, respectively; for the sheep, they were 0.6, 0.4 and 0.1/min for C. obsoletus/C. scoticus, C. dewulfi and C. punctatus, respectively. The presence of a vector species is a prerequisite for disease transmission thus the knowledge of the composition of the Culicoides species communities that inhabit areas where there are wild ruminants is important, although few studies on this aspect have been conducted. In the study by Talavera et al. (2015), samplings were conducted in Spain in areas inhabited by different wild ruminant species. The most abundant vector species were C. imicola and C. obsoletus/C. scoticus, which represented 15% and 11% of total numbers of specimens collected, over 100,000, respectively. The data suggest that such species do not exhibit strong host specificity towards either domestic or wild ruminants and that they could consequently play a prominent role as bridge vectors for different pathogens between both types of ruminants.
Molecular techniques, such as analysis of cytochrome b gene (Calvo et al., 2012), and barcoding techniques (Martinez‐de la Puente et al., 2012, 2015) conducted in Spain showed that some species feed in a opportunistic manner from different types of hosts and could not be considered purely mammophilic or ornitophilic.
3.5.4. Hours of attack and dispersal
In the Netherlands, efficacy of light traps was compared with aerial sweeping, and correlated against light intensity (Meiswinkel and Elbers, 2016). C. chiopterus and C. obsoletus/C. scoticus differed critically in their hours of peak activity, being largely crepuscular and nocturnal, respectively. This difference may explain why, routinely, the C. obsoletus/C. scoticus dominates light trap collections and C. chiopterus does not. This discrepancy between UV light trap and animal‐bait collections was already described by Carpenter et al. (2008)). However, Viennet et al. (2012a,b) showed that UV light trap collections were linearly correlated to attack rates on animals for C. obsoletus (overestimation by light trap), C. dewulfi (underestimation by light trap), C. brunnicans (no bias), but not for C. scoticus. Moreover, using a vehicle‐mounted trap during 52 collections (2 years), Sanders et al. (2011b) confirmed that Culicoides of the Obsoletus assemblage (C. obsoletus 50%, C. scoticus 15%, C. dewulfi 4% and C. chiopterus 4%) were mostly crepuscular – C. chiopterus having a greater range of activity recorded across solar incidence and was less confined to sunset, even if some activity could be recorded thought the diel especially when light suddenly decreases for instance due to a cloud passage (Viennet et al., 2012b). Thus, temperature and sunlight causes a swift on crepuscular to diurnal activity as was recorded also for C. sonorensis in California (Mayo et al., 2014) and for Obsoletus assemblage in France by Balenghien et al. (2008).
Moreover, Meiswinkel and Elbers (2016) suggested that at latitudes beyond 45°N, the progressive northward lengthening of the twilight period may have an increasingly adverse impact upon the efficacy of the light trap as a vector surveillance tool. However, up to 10,000 Culicoides could be collected in two consecutive collection nights at 68.7°N using an Onderstepoort‐type light traps in August, including about 5,000 C. punctatus, about 5,000 Grisescens group females, about 760 C. obsoletus/C. scoticus and about 100 C. chiopterus (VectorNet, unpublished data).
During blood‐meal identification studies, Garros et al. (2011) found engorged females of C. chiopterus positive for cattle whereas only sheep were present in the collection farms. The closer cattle were present in a surrounding 2 km buffer zone in pasture areas, suggesting dispersion of blood‐fed females over 1 or 2 km.
Dispersal studies using mark‐release‐recapture technique suggested possible dispersal distance of 1.75 km per 24 h in Denmark and of 1.50 km per 24 h in the UK (Kluiters et al., 2008; Kirkeby et al., 2009). The flight altitude was assessed by Sanders et al. (2011a) in the UK recording adult biting midges at 200 m above land using a tethered balloon.
3.5.5. Vector status
3.5.5.1. Culicoides species implicated in BTV transmission
The genus Culicoides (Diptera: Ceratopogonidae) has nearly 1,350 worldwide distributed species (at least 117 in Europe) and it is characterised by a diversity of biting midges whose haematophagous females can transmit a variety of filarial worms, protozoans and arthropod‐borne viruses to man and wild or domestic animals (Foxi et al., 2016). Only around 30 species have been associated with BTV transmission.
In Europe, Culicoides species that have been implicated as potential vectors of BTV generally belong to the subgenera Avaritia and Culicoides. Potential BT vector species were identified from studies based on virus isolation or detection by RT‐qPCR in field‐collected parous females, detection of virus dissemination in field individuals, and arboviral infection in laboratory assays. C. (Avaritia) imicola, C. (Avaritia) obsoletus and C. (Avaritia) scoticus are presently considered confirmed BTV vectors, while C. (Avaritia) chiopterus, C. (Avaritia) dewulfi, C. (Culicoides) pulicaris and C. (Culicoides) punctatus as probable vectors (Purse et al., 2015; Foxi et al., 2016).
The implication of C. imicola as a vector of BTV in Europe is based on its distribution and abundance on farms, in outbreak areas and historical evidence of its role in transmission elsewhere. Few direct vector competence experiments with European C. imicola have been undertaken and results remained limited (Biteau‐Coroller, 2006), due to the difficulties in feeding and maintenance in the laboratory and limited number of specimens captured on the field. However, an extensive work have been conducted with this species in South Africa where its role for transmission of BTV, African horse sickness virus (AHSV) and epizootic haemorrhagic disease virus (EHDV) has been widely demonstrated by vector competence studies (Venter et al., 2009, 2010, 2011b; Del Rio et al., 2012).
Large‐scale entomological surveillance programmes have been carried out in many countries affected by BTV using standardised sampling methods to investigate the role of several species in the distribution of BTV and quantification of the seasonal activities of the vectors. To maximise the efficiency of the trap and sampling of populations, protocols recommend sampling within the farms or animal shelters. By contrast, very few studies have reported sampling performed outside the farms, in the surrounding landscape. In pastures, decreasing numbers of Culicoides females as a function of the distance to the farm was observed. In woodlands, higher abundance of Culicoides than expected considering the distance of the sampling sites to the farm, was observed, although this varied according to species (Rigot et al., 2013). Talavera et al. (2015) showed that C. imicola and C. obsoletus/C. scoticus are found either in farms and in natural areas were wild ruminants species are the main hosts.
The Obsoletus and the Pulicaris assemblages have been clearly implicated in the outbreak of bluetongue in north‐western Europe in August 2006, since surveillance from Germany during winter 2007–2008 showed that 11 pools of biting midges were RT‐PCR positive to BTV‐8 including pools of non‐engorged midges (Clausen et al., 2009).
During the BT outbreak in Italy in 2012–2014, almost 3,000 pools with over 83,000 midges were sorted and tested for BTV (Goffredo et al., 2015). They were composed by C. obsoletus/C. scoticus (43.2%), C. imicola (23.4%), C. newsteadi (10%), Pulicaris assemblage (9.4%), C. pulicaris (6.8%), C. punctatus (5%), C. dewulfi (1.9%) and Nubeculosus assemblage (0.3%). In total, 1,107 pools of no blood‐engorged parous females were positive for BTV resulting in a minimum infection rate of over 1%. All the taxa tested resulted positive to BTV, at least once. In particular, C. imicola, C. newsteadi, C. pulicaris and C. obsoletus/C. scoticus were found positive during the three epidemics 2012–2014. Based on these findings, C. newsteadi appears as a new potential vector for BTV in southern Europe.
The relationship between temperature and the extrinsic incubation period (EIP) was estimated by a statistical methodology and applied to both published and novel data on virus replication for three orbiviruses (AHSV, BTV and EHDV) in their Culicoides vectors (Carpenter et al., 2011). Differences in vector competence for different orbiviruses in the same vector species and for the same orbivirus in different vector species were detected. Both the rate of virus replication (approximately 0.017–0.021 per degree‐day) and the minimum temperature required for replication (11–13°C), however, were generally consistent for different orbiviruses and across different Culicoides vector species. According to this finding, the replication rate and threshold temperature were previously underestimated because the statistical methods they used included an implicit assumption that all negative vectors were infected.
3.5.6. Vector seasonality
The seasonal annual pattern of the biological vectors of BTV determines the occurrence of the disease in a given year. The seasonality of the vectors is influenced by climate and specific factors, such as conditions related to breeding sites. Since immature stages of Culicoides require humid conditions for developments, humidity and temperature appear to be the main regulating factors.
The adult Culicoides seasonal annual pattern is species‐dependent and even considering the same species the seasonality could vary depending on the climatological conditions. In general, it is considered that cold temperature climate (i.e. those occurring in the northern Europe) correspond to short seasonal activity, meanwhile warmer ones (i.e. those taking place in the southern Europe) allows a longer seasonal activity of adults. In addition, fauna of Culicoides from southern and northern Europe differs in some of the major species, such as C. imicola which is not present in northern Europe (Versteirt et al., 2017).
For practicality, we have divided two major regions in terms of seasonality, South Europe and North Europe.
3.5.6.1. South Europe
Adult annual activity was described for southern Europe in the opinion published by EFSA (EFSA, 2008). In general, the major species C. imicola shows a maximum peak of activity in September–October. On the contrary, other species of the Avaritia subgenus, such as C. obsoletus/C. scoticus and species of the Pulicaris assemblage, generally show the maximum peak of activity during the spring, from April to June, with a potential second and lower peak after summer.
In Sardinia, populations of C. imicola appeared only from April to December, meanwhile C. obsoletus, C. newsteadi and C. pulicaris were captured all year around (Foxi and Delrio, 2010; Foxi et al., 2011). Pili et al. (2010) confirmed that C. scoticus was most abundant from late winter to early spring, whereas C. obsoletus was prevalent in early summer. A similar pattern was also found in Corsica (Venail et al. 2012). All year around activity of C. obsoletus/C. scoticus was also detected in Spain, with a peak in spring and summer (Romon et al., 2012; González et al., 2013).
3.5.6.2. North Europe
Information from North Europe has been extensively produced since the last EFSA opinion published in 2008 (EFSA, 2008; Foxi et al., 2011; Romon et al., 2012; Gonzalez et al., 2013).
Several examples of vector seasonal pattern are available from the Netherlands, Sweden, the UK, Germany, Austria and France.
In the Netherlands, Takken et al. (2008) and Meiswinkel et al. (2014) found C. obsoletus/C. scoticus, C. dewulfi and C. chiopterus (Obsoletus assemblage) from May (when temperatures raised above 10°C) to July. Adult captures were null during the winter, with the exception of capturing few females (46) that were newly hatched nulliparous (Meiswinkel et al., 2014).
In Sweden, the Obsoletus assemblage and C. punctatus were recorded from March to November, and as in the above studies, no biting midges were collected during winter (Ander et al., 2012).
This pattern of seasonality is also similar to the one found in the UK by (Searle et al., 2014). Species of the Obsoletus assemblage started on average in late May and lasted until the end of October. Therefore, the overwintering period was estimated on average in 185 days for the Obsoletus assemblage.
On the contrary, in Germany, outdoor adults of the Obsoletus assemblage were captured during winter months and the peak of abundance was located generally in August (in less frequency September), while low captures (< 30 individuals) were recorded from January to April. Other species, such as C. pulicaris, was mainly captured in May (Balczun et al., 2009; Clausen et al., 2009; Kiel et al., 2009; Vorsprach et al., 2009; Santiago‐Alarcon et al., 2013).
Similar results were also obtained in Austria, where the peak for species of the Obsoletus assemblage species peak located in July or August depending on the year (Brugger and Rubel, 2013).
Seasonality in France was summarised by Venail et al. (2012). In mainland France, the dominant species, C. obsoletus/C. scoticus, highlighted bimodal patterns of population abundance in southern regions of France, meanwhile unimodal patterns were frequent in the north of the country. Culicoides activity was detected in most French continental areas from April and declined in November (Balenghien et al., 2010). Nevertheless, annual activity and overwinter period depended on the region and the Culicoides species in each region. Indeed, adults could be detected continuously all year around in some locations with mild winter (Venail et al., 2012).
Modelling has also contributed to understand the seasonality of Culicoides in different parts of Europe.
The effects of relevant ecological factors and meteorological parameters on Culicoides vector abundances during the BTV‐8 epidemic in the Netherlands in 2007 and 2008 were quantified within a hurdle modelling framework (Scolamacchia et al., 2014). Vector abundance was found to be influenced by edaphic factors, likely related to species‐specific breeding habitat preferences that differed markedly among some species. Smoothing techniques and generalised linear mixed models have been used to relate environmental drivers to key phenological patterns of Culicoides spp., as in the study by (Searle et al., 2013), for the species C. pulicaris and C. impunctatus. The importance of land‐cover and climatic variables in determining the seasonal abundance of these two vector species was demonstrated, as well as the need for more empirical data on the effects of temperature and precipitation on the life history traits of Palaearctic Culicoides spp. in Europe.
A dynamic model describing the effect of ecoclimatic indicators on the monthly abundances of C. imicola in Sardinia was developed (Rigot et al., 2012). A first‐order autoregressive cofactor, a digital elevation model and MODIS Land Surface Temperature (LST) or temperatures acquired from weather stations explained around 77% of the variability encountered in the samplings during 6 years. On average, dynamics simulations showed good accuracy. Although the model did not always reproduce the absolute levels of monthly abundances peaks, it succeeded in reproducing the seasonality in population level and allowed identifying the periods of low abundances and with no apparent activity. On that basis, the C. imicola monthly distribution over the entire Sardinian region was mapped. Such a model could be used to predict monthly population abundances on the basis of environmental conditions, and hence can potentially reduce the amount of entomological surveillance.
3.5.7. Adult vector overwintering and role of transmission
The capacity for diapause, which could be defined as the arrest in development accompanied by suppressed metabolism, is widespread among insects allowing them to bridge harsh winters, dry seasons, or other seasonally inimical conditions. Most commonly, short day lengths of late summer signal the advent of winter to temperate zone species (facultative diapause), and thus winter is anticipated long before the onset of low temperatures. For instance, short day lengths change the behaviour of newly emerged Culex pipiens females which will feed with plant sugar to store energy reserves, seek a protected site for overwintering and show no host‐seeking behaviour.
Some diapause mechanisms have been evidenced in Culicoides, allowing avoiding the risk of adverse climatic conditions after summer (Rieb, 1987), but it is not yet clearly established if the apparent absence of Culicoides adults in winter months is due to ‘true’ diapause mechanisms or to the increase in larval development duration due to the decrease in temperature. Indeed, in Palaearctic zone, adult Culicoides activity could be continuously recorded at least some favourable years in the Mediterranean areas, whereas larval development could take up to 2 years in arctic conditions (Downes, 1962). Rieb (1987) established that diapause mechanisms exist at least in shoreline river Culicoides species. On contrary, Meiswinkel et al. (2008) reported that low numbers of adult Culicoides principally C. obsoletus/C. scoticus, including freshly blood‐fed individuals, and quasi‐exclusively nulliparous females were occasionally captured in light traps operated throughout the winter in Belgium, northern France and the Netherlands (Meiswinkel et al., 2008). This could be explained by recent emergences due to transient increase of temperature or by sporadic diapause endings in some larvae as reported by Rieb (1987). In France, the duration of the apparent Culicoides adult absence seem to be spatially structured by climate, with for instance quite continuous activity throughout the year along the Atlantic coast, suggesting that predominance of direct temperature effect on larval development, rather than ‘true’ diapause mechanisms at least in C. obsoletus/C. scoticus species (Thomas Balenghien, personal communication).
In Mediterranean area, Foxi et al. (2016) recently conducted a retrospective analysis of ethanol preserved Culicoides collected in Sardinia in 2001 for BTV detection by RT‐qPCR. BTV RNA was detected in C. obsoletus and in C. newsteadi species. Foxi et al. (2016) considered the presence of two cryptic species A and B in the C. newsteadi taxon from January to May, meanwhile BTV positive C. imicola were only detected from September to November. The authors concluded that the presence of Culicoides with high viral load in winter–spring in conjunction with low seroconversion rate in animals in Sardinia would support a continuous cycle of infection and transmission between ruminants and midge vectors. Similar results were found by Mayo et al. (2014) in California where BTV‐positive parous females of the vector C. sonorensis were found during winter time, whereas authors suggest, in the absence of animal seroconversion, that long‐lived females, infected with BTV during the prior transmission season, could be the main mechanism in the area for bridging the interseasonal period.
In non‐Mediterranean areas, however, where the winter is cold, the temperatures do not allow to collect parous females. For instance, Meiswinkel et al. (2014) recorded more than 100 days without collecting any parous females. Authors suggested that this evidences that long‐live females, potentially BTV infected during prior season could not survive during winter months, probably because they are decimated once temperatures remain below approximately 5°C. Moreover, in these areas, newly emerged nulliparous females, which would feed on viraemic animals, would not be able to replicate the virus as temperatures would be under 11–13°C considered as the replication threshold for Orbivirus (Carpenter et al., 2011). However, if these females could survive, it is possible that these infected females, with a load under the detection threshold, would be able to replicate the virus later when temperatures would increase, as it is demonstrated for West Nile virus and mosquitoes (Reisen et al., 2006).
3.5.7.1. Mapping threshold temperature for Culicoides development during winter
European climate is under the influence of two gradients, one South/North gradient delimiting mainly Mediterranean and non‐Mediterranean areas, and one West/East gradient reflecting the transition from oceanic to continental climate. Some non‐Mediterranean areas could present a relatively mild winter mostly along the Atlantic coast. In Figure 18, maps of Europe are shown to illustrate temperatures upper a threshold of 10°C during winter months since 2009 until 2014 (see Appendix D for the maps about all the months between 2009 and 2014). This threshold is close to the Orbivirus replication threshold and to the larval development threshold of C. sonorensis under laboratory conditions (Mullens and Rutz, 1983). In some winter months, such as January 2010 and 2011, large areas in the northern Europe, e.g. continental France, showed favourable conditions for Culicoides activity and potential BTV replication (Figure 18).
Figure 18.
Opportunity map for vector activity in Europe considering minimum temperature above 10°C, where the blue zones represent the areas in which Culicoides spp. inability to complete the life cycle (from egg/larvae to adult) according to temperature and the shades of green indicate conditions are favourable for completing the life cycle (from egg/larvae to adult) (expressed in number of days in the month, darker colours indicating longer periods in the month with favourable conditions), black represents regions with no information on temperature for that year
3.5.8. Vector‐free period and criteria for its determination
In the Regulation (EC) 1266/2007, the definition of the seasonal vector‐free period (SVFP) is mainly based on the complete absence of adult Culicoides (specifically C. imicola) captured in light traps and the determination of a certain threshold of Culicoides abundance for the other non‐imicola species. When the cited threshold was not possible to be determined, then a general criterion of less than five captured parous females of those suspected vector species has been recommended to be adopted (EFSA, 2007b, 2008). SVFP is determined until now on the basis of the results of light trap collections of Culicoides spp.
The complete cessation of vector activity measured by means of light traps seems to be restricted during winter to Afro‐tropical species such as C. imicola and only in specific areas of southern Europe, meanwhile in other areas such cessation is too short (< 15 days) or never reached (Ortega et al., 1997; Miranda et al., 2004; Calvete et al., 2006). In addition, it has been demonstrated that other species of the Avaritia subgenus, including C. obsoletus/C. scoticus, can be captured throughout the year both in southern (Foxi and Delrio, 2010; Foxi et al., 2011) and northern Europe (Balczun et al., 2009; Clausen et al., 2009; Kiel et al., 2009; Vorsprach et al., 2009; Santiago‐Alarcon et al., 2013), indicating that in certain areas, their activity does not stop during the winter time. However, during winter, quite only nulliparous females are collected in northern Europe, and in extremely low numbers (Takken et al., 2008; Ander et al., 2012; Meiswinkel et al., 2014; Searle et al., 2014).
It seems that the concept of an absolute SVFP is unrealistic for defining a period of the year during winter when transmission is supposed to be absent. Nevertheless, the seasonal occurrence of BT in Europe is clearly related to the seasonal pattern of the vectors throughout the year.
The validity of five parous females as a threshold for declaring SVFP has been tested through the analysis of a subset of entomological and serological surveillance data provided by the Italian veterinary authority. Since 2002, a robust surveillance system for BT is in place in Italy. It comprises a network of more than 30,000 sentinel animals monthly tested all over the country and around 300 Culicoides Ondersterpoort black‐light suction traps, operating weekly all over the year (Giovannini et al., 2004). Three‐year data (2013–2015) on seroconverted animals during winter months (December–February) were considered. For each seroconverted farms, all results of the entomological catches performed in a radius of less than 5 km during the probable exposure time interval (from the previous negative to the positive serological result in the same animal) were analysed. The total numbers of Culicoides caught were considered. Only for four seroconversions out of 99 (two in Sardinia and two in Sicily regions), matching the given conditions during the period of time considered (Table 7), data on Culicoides catches performed within a 5 km radius were available. In all cases a number of more than five Culicoides was observed, all belonging to the C. imicola species.
Table 7.
Data of presence and peak of abundance based on trapping studies (x: presence; xx: peak of abundance)
| Predominant species | Winter | Spring | Summer | Autumn | Location | Reference |
|---|---|---|---|---|---|---|
| South Europe | ||||||
| C. imicola | x | x | xx | Sardinia (Italy) | Foxi and Delrio (2010) | |
| C. newsteadi | x | |||||
| C. imicola | x | x | x | Foxi et al. (2011) | ||
| C. obsoletus, C. newsteadi and C. pulicaris | x | x | x | x | ||
| C. scoticus | x | x | Pili et al. (2010) | |||
| C. obsoletus | x | x | ||||
| North Europe | ||||||
| C. obsoletus/C. scoticus | x | xx | xx | x | Basque country | Romon et al. (2012) |
| C. obsoletus/C. scoticus | x | xx | xx | xx | González et al. (2013) | |
| C. obsoletus/C. scoticus, C. dewulfi and C. chiopterus | x | x | Netherlands | Takken et al. (2008) | ||
| C. obsoletus/C. scoticus, C. dewulfi, C. chiopterus and C. pulicaris | xx | x | Meiswinkel et al. (2014) | |||
| C. obsoletus and C. scoticus | Dominant species | Sweden | (Nielsen et al., 2010) | |||
| C. obsoletus, C. chiopterus, C. pulicaris, C. scoticus and C. punctatus | x | x | x | Ander et al. (2012) | ||
| C. obsoletus/C. scoticus | x | x | xx | x | Northwest Germany | Kiel et al. (2009) |
| C. pulicaris | xx | |||||
| C. obsoletus | x | xx | Germany | Balczun et al. (2009) | ||
| C. scoticus | x | xx | xx | |||
| Culicoides spp. outdoor | x | x | xx | xx | Clausen et al. (2009) | |
| Culicoides spp. indoor | x | x | xx | xx | ||
| C. obsoletus | x | xx | x | Vorsprach et al. (2009) | ||
| C. obsoletus/C. scoticus | x | xx | Austria | Brugger and Rubel (2013) | ||
| C. obsoletus | xx | x | Southwestern Germany | Santiago‐Alarcon et al. (2013) | ||
| C. obsoletus, C. scoticus, C. dewulfi and C. chiopterus | x | x | x | UK | Searle et al. (2014) | |
| C. obsoletus/C. scoticus | x | x | xx | x | Mainland France | Venail et al. (2012) |
| Culicoides spp. | x | xx | x | (Balenghien et al. (2010, 2012) | ||
Table 8.
Results of the analysis of Italian bluetongue surveillance data
| Farm | Region | Probable BTV exposure time interval (date of negative – date of positive serological result on the same sentinel animal) | Total no. of Culicoides caught during the exposure time interval in a 5 km radius from the seroconverted farm |
|---|---|---|---|
| #1 | Sicily | 2/10/2013–2/12/2013 | 6 |
| #2 | Sardinia | 28/11/2012–28/12/2012 | 418 |
| #3 | Sardinia | 14/12/2012–15/1/2013 | 7 |
| #4 | Sicily | 27/11/2013–13/1/2014 | 68 |
Although related to four cases only, the analysis of the data produced by the Italian entomological surveillance programme is in agreement with the current provisions set by the Regulation (EC) 1266/2007, and no seroconverted sentinels were observed in the absence of C. imicola or with less than five captured Culicoides (EFSA, 2007b).
Among the criteria considered by the Regulation (EC) 1266/2007 for the definition of the SVFP, temperature conditions that impact on the behaviour of the vectors activity could also be considered. When temperatures thresholds are used, values around 10°C are frequently considered on the basis of the results of studies performed in northern America and in Europe (EFSA, 2008).
For the definition of possible temperature thresholds, an approach that could be followed is based on the estimation of the basic reproduction number (R 0) under different temperature conditions. A simplified formula for the calculation of R 0 for vector‐borne diseases is the following (Thomas et al., 2008):
where, a = vector biting rate; β = transmission rate; M = vector population density; p = vector survival (per day); n = number of days needed to be infective (vector) [extrinsic incubation period]; N = host population density; r = recovery rate.
Vector biting rate, vector survival and the extrinsic incubation period are influenced by temperatures. The parameter functions as applied for the calculation of the basic reproduction number in Brugger et al. (2016) were used, and very conservative values for the probability of transmission (equal to 1) and host population density (equal to 1) were chosen in order to possibly overestimate the values of R 0. The values of R 0 were calculated for a range of temperatures from 0 to 30°C and varying the number of Culicoides caught by the traps (the vector density was estimated by assuming that trap catches reflect 1% of the local vector population) (Hartemink et al., 2009).
The Figure 19 shows that R 0 exceed the value of 1 for temperatures ranging from 9.1 (in case of 20 Culicoides caught) to 11.5°C (in relation to 5 Culicoides caught), thus roughly confirming possible thresholds around 10°C for disease transmission.
Figure 19.
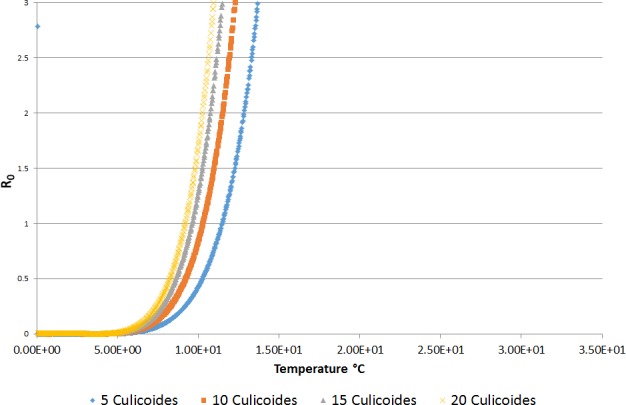
Estimation of R 0 values considering different temperatures and numbers of Culicoides caught in the traps
3.6. Vector control: insecticides and repellents and vector‐proof establishments
The ToR 3.3 request for an assessment of the appropriateness of the use of insecticides and repellents against Culicoides, including an assessment of their efficacy and recommendations of adequate protocols for their uses, in particular, as regards their suitability to protect animals against attacks by vectors performing at least equal to the protection provided by vector‐proof establishments (VPE) – without the need to keep animals in a vector protected facility.
At the beginning of this chapter, an analysis of the current available vector control strategies and tools is presented (Sections 3.6.1–3.6.6), then in Section 3.6.6.1 a comparison of the effectiveness of those measures with the VPE is discussed.
In theory, vector control of Culicoides both larvae and adults appears as a method to reduce BTV transmission in scenarios when vaccine for a particular serotype is not available, there are several serotypes circulating, the serotype/strain is low pathogenic and/or movement restrictions and protection of animals from vector bites are the only way to reduce transmission. Vector control could be also appropriate under emergency outbreak situations or where vaccines are not economically affordable (Harrup et al., 2015; Purse et al., 2015). In practice, the control of Culicoides and its impact on virus transmission at farm level is still poorly implemented and in most of the cases impractical from an environmental and technical point of view.
Here, the general classification of control methods are considered in (i) mechanical, (ii) chemical, (iii) biological (iv) genetic and (v) biotechnological. These methods have been reviewed in several works (Carpenter et al., 2008; Maclachlan and Mayo, 2013; Harrup et al., 2015; Mullens et al., 2015; Pfannenstiel et al., 2015). Here the focus is mainly on updates published after the last EFSA opinion in 2008 (EFSA, 2008).
3.6.1. Mechanical control
3.6.1.1. Habitat modification and source reduction
Larval habitats of Culicoides spp. include a wide variety of humid substrates, from organic matter enriched soils, litter, rotten vegetables, to dung pats, manure heap and farm‐associated wastewater lagoons and were reviewed in previous EFSA opinions (EFSA, 2008). Breeding sites for some of the major vector species in Southern Europe, such as C. imicola, has been recorded around farm premises favoured by organic matter and water losses produced during farm practices (Braverman and Galun, 1973). Species included in the Obsoletus complex (C. obsoletus/C. scoticus) present a wide variety of breeding sites, including farms and deciduous forest. Other species such as C. pulicaris breed near swamp vegetation and C. dewulfi and C. chiopterus in dung pats.
Reducing breeding sites a priori seems feasible only for those species that are located in particular substrates as dung pats or manure heaps. For the other species, breeding in soil and litter surface, larvae control seems to be very impractical. There are only few experiences on source reduction for Culicoides spp. and none of them have demonstrated to have an impact on vector population. Mayo et al. (2014) compared two farms in California where the main breeding site for C. sonorensis (wastewater lagoon) was removed in one of the farms. They found no difference in the population abundance between the two farms, and therefore, it was assumed that breeding site removal would have no effect on BTV transmission in the area. In the UK, Harrup et al. (2014) showed that muck heaps covered with tarpaulins had no effect on the overall vector population when compared to controls. In the same sense, Lühken et al. (2014a) found that mechanical disturbance of cow‐pats had no effect on the populations of C. chiopterus, C. dewulfi and C. scoticus. Interestingly, in a different study demonstrated that flooded cow‐pats had an effect on Culicoides larvae survival, meanwhile dry cow‐pats showed no effect when compared to control (Lühken et al., 2014b).
Stabling and screens
Stabling animals seems to be a reasonable way to protect animals from the bites of Culicoides spp. Meiswinkel et al. (2000) demonstrated that gauzing windows and closing doors in horse stables in South Africa reduced by 14‐fold the presence of C. imicola and Culicoides bolitinos inside the stable. From the last EFSA opinion (EFSA, 2008), several trials have been conducted to test protection of animals either by housing or by using screens in stables. In the UK, Baylis et al. (2010) showed by comparing trap catches of Onderstepoort traps inside and outside stables, that cattle housed at night were less exposed to biting midges activity, particularly during periods of maximum activity of exophilic species (i.e. C. obsoletus) when blood‐fed females were 3–4 times more frequent outside stables than inside. Lincoln et al. (2015) tested different protection systems in horse stables in Switzerland. For that, polypropylene nets (Ultravent® Bemisia TIP 250. Micrometric mesh of 0.1825 mm2, Texinov, France) alone and in combination with fans (ZOO No. 1400, BM Haus Agrotech, Switzerland) were used to protect animals against Culicoides in three stabling systems. Efficacy was measured by assessing the blood‐fed rate of females captured in Onderstepoort light traps. Nets provided significant reduction of blood‐engorged females rate (from 98% to 65% reduction) when compared to control stables, but fans alone and combined with nets did not significantly differed from control.
3.6.2. Chemical control
3.6.2.1. Repellents
Up to date, the only repellent registered in Europe that could be used to protect livestock against midges bites is N,N‐diethyl‐meta‐toluamide (Appendix E). Approved topical repellents are commonly used on horses and for protection of humans (Carpenter et al., 2008; Harrup et al., 2015). Repellents for protecting humans have been further explored in comparison to those for application on animals. Examples of current works conducted on repellents for humans include species which are not major vectors of BTV, such those conducted in Scotland against C. impunctatus (Logan et al., 2009), in Australia against C. ornatus and Culicoides immaculatus (Greive et al., 2010) and in Colombia against Culicoides pachymerus (Santamaría et al., 2012). In regards to research of repellents for animals, most of the studies have been conducted in laboratory testing the repellence of several organic acids compounds on Y‐tubes or using light traps as a proxy to animals attraction to Culicoides (Venter et al., 2011a; Gonzalez et al., 2014). Results showed that light traps equipped with a mesh impregnated with a mixture of octanoic, decanoic and nonanoic fatty acids collected less Culicoides spp. when compared with control traps. In South Africa, Venter et al. (2014) also tested commercial peel‐stick patches with a combination of citronella and lemon eucalyptus oil using light traps. Results showed no repellent effect against C. imicola and no differences were found between treated and control traps in regards to the different gonotrophic stages of females. However, up to date, no field trials on animals have been conducted using those compounds. In the UK, Robin et al. (2015) tested the possible repellent effect of pour‐on 1% solution of the insecticide deltamethrin (‘Spot on’, Zoetis; Zoetis UK, London, UK) when applied on horses. They found no differences between treated and untreated horses in regards to the number of blood‐engorged females captured by Ondersterpoort traps placed nearby the animals. Lincoln et al. (2015) also tried a similar approach to protect horses by applying a spray solution containing permethrin insecticide and DEET (Flymax, 6 mg/mL permethrin and 20 mg/mL DEET, Audevard Ltd., France). The product was applied to the neck, abdomen, flank, back and croup, and efficacy was measured by estimating the rate of blood‐engorged females captured in Ondersterpoort traps. Obtained results indicated that no significant differences were found between captures of blood‐fed females between the treated and control horses. Also, directly on animals, Reeves et al. (2010) tested the repellent effect of ear‐tags (Python: 10% zeta‐cypermethrin 9.8 g/tag and 20% piperonyl butoxide) and a low‐volume‐spray ready‐to‐use sheep insecticide (Y‐TEX: 2.5% permethrin and 2.5% piperonyl butoxide, 12 mL/sheep) separately and in combination on C. sonorensis laboratory‐reared adults. Insects were exposed to treated sheep using feeding tubes in the axillary area. All tested compounds, either individually or in combination, were effective for decreasing feeding rates of C. sonorensis up to 4 weeks; however, no data on mortality was provided, therefore, results in terms of efficacy are difficult to interpret. Insecticide spraying showed to give protection to animals immediately and resulted to be cheaper when compared to ear‐tags, nevertheless, those showed long‐tasting repellence.
Compounds derived from the Neem tree (Azadirachta indica A. Juss; Meliaceae) have shown antilanding and antifeeding effect on adults of a colony of Culicoides nubeculosus and field‐collected C. impunctatus (Blackwell et al., 2004). Leaves of neem tree are usually burned for protecting cattle in India; however, no references are available about its efficacy (Harrup et al., 2015).
3.6.2.2. Insecticides
In vitro assays
Unlike repellents, insecticide usage against biting midges has experienced an important development in the last decade. Several products are commercially available in Europe and broadly used in the MS. The effect of insecticides of Culicoides spp. adults has been demonstrated in vitro adapting WHO testing protocols for mosquitoes to Culicoides. The protocol basically consists on exposing adult Culicoides to insecticides‐impregnated papers of different concentrations and therefore LD50 and LD90 could be estimated. In France,Venail et al. (2011, 2015) obtained high mortality (i.e. 100% after 1 h exposure 0.0025% deltamethrin) for either colony‐reared adults of C. nubeculosus and field‐collected adults of C. obsoletus and C. imicola to different concentrations of deltamethrin‐impregnated papers at 24 h after 1 h exposure. In Spain, Del Rio et al. (2014b) performed a similar test on field‐collected Culicoides spp., results showed that individuals of the C. obsoletus were highly susceptible to doses above 0.001% of deltamethrin, being 3.5 more sensitive to all deltamethrin tested concentrations than the one tested by Venail et al. (2011). In the UK, Onuike et al. (2015) carried out also a WHO protocol trial using deltamethrin‐impregnated papers at different concentrations and adults of a C. nubeculosus colony in the same way as Venail et al. (2011),but testing also effect of treatment and post‐treatment at different temperatures, as well as blood‐feeding behaviour and oviposition after being exposed to deltamethrin. The highest mortality (up to 90%) was observed at 24 h post‐treatment with a concentration of 0.05%. Knock‐down effect was observed after 1 h of exposure, but posterior recovery of midges at 24 h post‐treatment was recorded at low concentrations of deltamethrin. Also, exposure to impregnated papers at different temperatures (25, 20, 15 and 10°C) had no significant effect on mortality, however, higher mortality was observed at 10°C but no clear explanation was provided in the study about the role of lower temperatures on Culicoides mortality. The effect of temperature (25, 20, 15 and 10°C) during the post‐treatment period was only significant at 10 vs 25°C. Interestingly, there was no significant effect of deltamethrin in the feeding behaviour, but numbers of eggs laid by insecticide‐exposed insects were significantly less than the control ones.
From the last EFSA opinion (EFSA, 2008), several works have tested insecticides in vitro by exposing colony‐reared or field‐collected adults to hair or wool clippings from insecticide‐treated animals. In Germany, Schmahl et al. (2008) tested Oxyfly™ (lambda‐cyhalothrin, Novartis), a microencapsulated insecticide that is applied to surfaces of walls where insects rest. In this work, field‐collected C. obsoletus and C. pulicaris were killed in 9–20 s when exposed to plates that were insecticide impregnated 2 weeks before. In the UK Carpenter et al. (2007) tested the pour‐on insecticides Coopers’ Spot On™ (1.25% w/v high‐cis cypermethrin based pour‐on at 450 mg/m2, Schering‐Plough Animal Health, UK) and deltamethrin (1% at 60 mg/m2) in sheep and cattle. Both products showed high mortality for adults exposed in the lab, but low mortality to hair clipped from belly and legs of treated sheep. Interestingly, Coopers’ Spot On showed significant mortalities when applied to cattle for up to 7 days after treatment. In a study conducted in Germany (Liebisch and Liebisch, 2008) tested in vitro efficacy of placing 1 and 2 ear‐tags (Flectron® Flytags, 1,067 g cypermethrin per ear‐tag. Fort Dodge Animal health) per animal in heifers and dairy cows. The in vitro assay using hair clippings from the dorsal line and the ventral abdomen from animals confirmed field observation on the toxic efficacy for 14 days with 1 ear‐tag and up to 21 days with 2 ear‐tags. In similar trials, Schmahl et al. (2009a) treated cattle and sheep with Butox® 7.5 (7.5 g deltamethrin per litre of ready‐to‐use solution, Intervet, Netherlands) and Versatrine® (1 g deltamethrin per 100 mL excipient, Schering‐Plough Vétérinaire, France). Hair clippers were collected from feet of animals at intervals of 7, 14, 21, 28 and 35 days after treatment and were put in contact with unidentified field‐collected Culicoides spp. Both products showed to kill adult Culicoides even 35 days after treatment. In a further study, cattle and sheep were treated with Flypor® (4% w/v of permethrin ready‐to‐use solution, Novartis, UK), Arkofly® (6 g fenvalerate per 100 mL of the ready‐to–use spray, Novartis, France) and Acadrex® 60 (6 g fenvalerate within 100 mL excipient solution, Novartis, France). Results were similar to those obtained in the previous trial and all products showed a killing effect on adult Culicoides 35 days after treatment (Schmahl et al., 2009b), however, neither in this work nor in the previous ones, the percentage of mortality was estimated and therefore comparison with further studies are difficult to assess. Papadopoulos et al. (2009) also tested the killing effect on colony‐reared adults of C. nubeculosus of hair clippers obtained from leg, belly and back of sheep and calves treated with Dysect Cattle Pour‐On (15 g/L alphacypermethrin) and Dysect Sheep Pour‐On (12.5 g/L alphacypermethrin), respectively. Adult Culicoides showed high mortality (near to 100%) up to 21 days post‐treatment. A similar trial (Papadopoulos et al., 2010) was performed applying cypermethrin (Deosect Spray, 5.0% w/v, Fort Dodge Animal Health) to face, legs, back and hindquarters of horses. Percentage of mortality ranged from near 80% at day 7 to 50% at day 35 post‐treatment, being hair clippers from the back those that showed highest killing effect.
Semifield testing
Semifield testing includes the use of animals for testing the efficacy of pour‐on insecticides in enclosed conditions; therefore, data resulted from direct contact of biting midges to the body of animals and not to hair or fleece clippers. Venail et al. (2011) tested the killing effect of Butox® 7.5 pour‐on (Intervet International B.V., The Netherlands, 7.5% w/v deltamethrin) on nulliparous females of a colony of C. nubeculosus directly exposed to shorn sheep at 1, 4, 6 and 13 days after treatment. In this work, the maximum mortality reached the 45% on day 4 after treatment and the persistence of the lethal effect was estimated to be less than 10 days. This study, as others conducted elsewhere (Carpenter et al., 2007; Bauer et al., 2009; Papadopoulos et al., 2009, 2010), concluded that diffusion of active ingredients on the hair/fleece of target livestock species is a key issue for reaching success in deterring Culicoides midges feeding activity.
Field testing
In regards to field trials, several studies have been conducted with commercialised pour‐on insecticides and impregnated ear‐tags to protect horses, cattle and sheep. Concerning systemic biocides, no updates have been available since EFSA (2008).
3.6.2.3. Pour‐on insecticides:
In the Netherlands, De Raat et al. (2008) tested permethrin pour‐on insecticide (Tectonik ® 36 g/L; doses 10 mL/100 kg) on horses assessing field Culicoides feeding rate by aspirating adults from a mosquito net tent trap. The most abundant species showed to be C. obsoletus and C. pulicaris and the pour‐on treatment decreased by 82% the number of individuals collected from treated horses; however, there was no statistical difference when compared to the control. In consequence, the permethrin pour‐on treatment showed poor efficacy to avoid feeding midges on horses. Limited killing effect from different parts of the body of the animal (i.e. back, belly, legs) may explain the poor efficacy of pour‐on treatments. Better results were obtained in Spain, where Mullens et al. (2010) showed good protection of sheep against feeding activity of Culicoides spp. by applying 7.5% deltamethrin (Butox 7.5) directly on exposed skin (face, ears and belly) and therefore strictly not being a pour‐on treatment. In this case, authors attribute the relative success in preventing midges to feed on sheep to the ad‐hoc way of applying the product on animals. Also on sheep, Griffioen et al. (2011) tested the efficacy of Tectonik 3.6% permethrin pour‐on solution (1 mL/10 kg bodyweight. Virbac Animal Health) in the Netherlands, on a mixed group of sheep breeds by using tent traps from where biting midges were aspirated. The most common species were C. chiopterus and C. obsoletus and according to the results, the pour‐on treatment reduced up to 50% the number of midges associated to animals as well as the number of engorged females that fed on animals. In a more robust trial, Weiher et al. (2014) performed a study in Germany assessing a dosage of 10 mL of Butox® pour on (7.5 mg deltamethrin/mL, Intervet, France) on Merino sheep. Engorged females and rate of feeding efficacy was measured by using a drop trap and direct aspiration of midges. Pour‐on treatment showed an efficacy that ranged from 0% (2 weeks post‐treatment) to 71.0% (3 weeks post‐treatment). Overall efficacy reduced 86.4% of engorged females in the pour‐on treated animals from 24 h to 5 weeks of treatment and maximum efficacy on reducing feeding rate was obtained in day 21 (94.6%). According to the authors, low efficacy of the product during the first 24 h after treatment and insufficient spread to different parts of the animal body (i.e. legs, feet, belly, face) are considered main limitation on pour‐on insecticide treatments.
3.6.2.4. Ear‐tags
Insecticide impregnated ear‐tags are commercially available in Europe and represent an alternative to pour‐on treatments. In North Germany, Liebisch and Liebisch (2008) tested the efficacy of placing 1 and 2 ear‐tags (Flectron® Flytags, 1,067 g cypermethrin per ear tag, Fort Dodge Animal health) per animal in heifers and dairy cows. Midge abundance was measured by suction light traps and direct aspiration from the skin of the animal. According to authors, C. obsoletus, C. pulicaris and C. dewulfi were the dominant species. The results of the study are limited since no specific result is given about the decrease of feeding activity of midges on tested animals. Apparently, the toxic efficacy duration was estimated at 14 days when using 1 ear‐tag and up at 21 days for 2 ear‐tags. This result was also confirmed in vitro by exposing field‐collected Culicoides to hair clippers (see in vitro section). In another trial conducted in Germany by Bauer et al. (2009), protection of bulls inside a pen by using insecticide‐treated ear‐tags (Auriplak®) containing 1.2 g of permethrin as well as five pour‐on treatments with deltamethrin (Butox 7.5; 750 mg/100 mL) failed to reduce the number of engorged midges captured by BG‐Sentinel traps placed inside the bull pens.
3.6.2.5. Insecticide‐treated materials
Insecticide‐treated materials are generally nets (insecticide‐treated nets (ITN)) which are recommended to be placed on windows and doors to avoid biting midges to contact with animals. Their efficacy generally depends on the species endo‐ and exophilic behaviour (indoor/outdoor), as well as the characteristics of the ITN to kill adults or to preclude its movement into stables.
Since the last EFSA opinion (EFSA, 2008), there have been trials using ITNs for assessing its effects on Culicoides adults basically by placing them on doors and windows of stables or around light traps.
In regards to trials performed using ITNs to protect animals inside stables, Bauer et al., 2009 tested insecticide‐treated mosquito fences (100 mg/m2 deltamethrin – 180 cm height – 1 × 2 mm mesh) around bull pens in Germany aiming to reduce numbers of engorged females. Midge population measured inside the pens by using BG‐Sentinel traps showed no significant difference with control; consequently, exhibiting poor action in reducing attack of Culicoides. Since there is little information about the flying behaviour of Culicoides, it was assumed that adults were flying over the fence to avoid its contact. In Spain, Calvete et al. (2010) tested cypermethrin manually impregnated on canvas barriers (2.6 m height; 0.5 g/L cypermethrin) aimed to protect yearly ewes. Efficacy was measured by comparing Culicoides captures in CDC mini‐UV light traps inside and outside the pens. The canvas barriers showed only partial (50–78.8%) or no protection against C. imicola, the most abundant species in the area and the major BTV vector in Southern Europe. According to the authors, this species seemed to be able to fly above the barrier avoiding contact with the insecticide impregnated canvas.
Regarding trials assessing the efficacy of ITNs by using light traps as a proxy to the attraction to animals, Del Río et al. (2014) tested blue shading nets, made from inert polyethylene fibres (fibre wideness: 1 mm, gap between fibres: 2 mm), manually impregnated with 1 L of cypermethrin solution 1% and placed in a cylinder (1.5 m high and 1 m wide) with an Onderstepoort light trap inside. There were no statistical differences between ITN and control net in regards to C. imicola populations. In the same sense, no significant difference in mortality after 24 h was found between ITN and control net, indicating that midges were able to pass through the net without any virtual contact with the insecticide impregnated fibres. Interestingly, significant differences were obtained for non‐targeted species, demonstrating the knock‐down effect of the ITN. The same authors (Del Rio et al., 2014a) tested commercial polyethylene nets (ZeroVector® Durable Lining; Dart Association, Lausanne, Switzerland) impregnated with 4.4 g/kg ± 15% of deltamethrin in vitro and in field using the same method as mentioned above. Results showed that 100% mortality was reached after 17 min. of exposure to the net in vitro. However, not significant difference was found between the ITN trap and control. As in the previous trial, although ITN did not prevent midges to pass through the net, the mortality rate of Culicoides collected in the ITN trap (84.9 ± 10.5%) was significantly higher than that of midges collected in the control trap (72.3 ± 5.9%). In a trial conducted in South Africa (Page et al., 2014), high‐density polyethylene nets (HDPE) manually impregnated with alphacypermethrin (20–40 mg/m2) and tested using Onderstepoort black‐light traps, failed to show any repellent effect on Culicoides spp. when compared to untreated one but reducing Culicoides captures in 7.2 times when compared to control. The same nets were tested in a contact bioassay using C. imicola nulliparous females and after 1 and 3 min of exposure, 100% of mortality was reached. Baker et al. (2015) tested seven different commercially available insecticides on a black polyvinyl‐coated polyester mesh (PetMesh. Fine Mesh Metals, Telford, UK; 1.6 mm aperture; 1.6 mm thickness) using WHO cone test and adults of a colony of C. nubeculosus at 1, 7 and 14 days after treatment. Insecticides included were Agropharm's Dairy Fly Spray (Pyrethrins including cinerins 0.25% w/w, Agropharm Ltd, Penn, UK); Degrain Insectaclear C (Cypermethrin 0.1% w/w, Lodi UK, Kingswinform, UK); Fly Free Zone (Permethrin 0.1% w/w; Tetramethrin 0.04% w/w; Fly Away Ltd, Stourbridge, UK); Protector C (Cypermethrin 0.09% w/w, Agropharm Ltd, Penn, UK); Strikeback Insect Killing Spray (Cypermethrin 0.01% w/w, Group 55, Preston, UK); Tri‐Tec 14® (Cypermethrin 0.15% w/w; Pyrethrins 0.2% w/w, LS Sales (Farnham) Ltd, Bloxham, UK) and Insecticide Ultrashield EX (Permethrin 0.5% w/w; Pyrethrins 0.1% w/w, W.F. Young, Inc, East Longmeadow, MA, USA). Insecticide Tri‐Tec 14®A demonstrated to be the most effective on the WHO cone test (100% mortality) and therefore was applied in two field trials where efficacy was assessed by comparing captures from a CDC mini‐UV light trap placed into wooden frame covered with the insecticide‐treated mesh, as well as covering entrance of stables with the treated mesh and measuring Culicoides abundance by the same type of traps. Results obtained either from the traps placed into the wooden frames or the stables showed that there was no significant difference on the number of females Culicoides between the treated and non‐treated mesh. Interestingly, the mesh either treated or non‐treated, decreased the number of Culicoides when compared with the control uncovered wooden frames or stables. In addition, a mean coefficient of protection from intrusion (CPI; % comparing inside captures with no‐mesh and mesh) of 88% was obtained for the untreated mesh and 100% for the treated one in wooden frames. When the mesh was placed in stables, CPI of 71% and 96% were obtained for untreated and treated mesh, respectively. These results indicate that either untreated‐mesh or mesh treated with the insecticide Tri‐Tec 14® significantly reduced the entry of Culicoides into stables.
3.6.3. Biological control
Biological control in entomology has been defined as ‘The use of living organisms to suppress the population density or impact of a specific pest organism, making it less abundant or less damaging than it would otherwise be’ (Eilenberg et al., 2001).
In regards to entomopathogenic fungi a recent review conducted by de Souza et al. (2014) summarises fungal and Oomycete infecting Culicomorpha (Simuliidae, Ceratopogonidae and Chironomidae). Mortality of Culicoides larvae caused by entomopathogenic fungi has been reported by Wright and Easton (1996) which found 31% mortality when exposing Culicoides molestus Skuse to Lagenidium giganteum Couch. Using colony‐reared larvae of Culicoides nubeculosus, Unkles et al. (2004) observed killing effect of the mosquito pathogen fungus Culicinomyces clavisporus only after 72–96 h post‐treatment. Among all entomopathogenic fungi tested, Metarhizium anisopliae has showed to be the most effective against several species of biting midges. Ansari et al. (2010) proved on C. nubeculosus colony that M. anisopliae (Metchnikoff) killed larvae from 81% to 100% of mortality and adults (Ansari et al., 2011) were killed at lethal time 90 (LT90) of 3.26 days when exposed to dry conidia at dose of 1.5 × 108/m2. Similarly, Nicholas and McCorkell (2014) showed significant mortality of Culicoides brevitarsis adults reared from dung to M. anisopliae at day 8, also conidia applied to dung significantly decreased the emergence rate of adults. Narladkar et al. (2015) also tested M. anisopliae and Beauveria bassiana against adults and larvae of Culicoides peregrinus in India. Killing effect on larvae was found to last only 7 days while adults were killed in 24 h according to authors; however, no details were provided about the measure of the efficacy.
The Sporulaceae bacteria Bacillus thuringiensis var. israelensis (Bti) is a lethal bacterium for mosquito larvae that has been widely used for mosquito control programmes during the last decades. However, Culicoides are poorly affected by the same bacteria, which make its use completely inefficacious for biting midge control. Several authors have demonstrated the low efficacy of Bti on C. sonorensis, Culicoides occidentalis, Culicoides mississippiensis and Culicoides guttipennis (Kelson et al., 1980 1980; Lacey and Kline, 1983) as well as on C. impunctatus (Blackwell and King, 1997).
Entomopathogenic viruses to Culicoides has been occasionally isolated but never applied for control. The Iridescent viruses (IIVs) (family Iridoviridae; genus Iridovirus) are the most common viruses infecting biting midges (Williams, 2008). Rates of infection has been described up to 28% on C. sonorensis (Mullens et al., 1999), 1% on Culicoides odibilis, Culicoides cubitalis and Culicoides clastrieri (Rieb et al., 1982) and 4.7% on C. barbosai (Fukuda et al., 2002).
Nematodes of the family Mermithidae are commonly found parasiting biting midges and its use as agent of control has been reviewed by Mullens et al. (2008). The species Heleidomermis magnapapula (Poinar and Mullens, 1987) appears as an adequate candidate for biological control, since it has been described to reach up to 69% of parasitism on C. sonorensis (up to), Culicoides lahontan, Culicoides boydi and Culicoides cacticola (Poinar and Mullens, 1987; Paine and Mullens, 1994; Mullens et al., 1997). Other species such as Heleidomermis cataloniensis was found on adults of Culicoides circumscriptus (Poinar and Sarto i Monteys, 2008). Basic studies on the biology of the species H. magnapapula for improving its use has been conducted by Mullens and Velten (1994), Luhring and Mullens (1997), Mullens et al. (1995) and Mullens and Luhring (1996). Mullens et al. (2008) conducted an inundative semifield trial in California using H. magnapapula on C. sonorensis breeding sites. A reduction of 84% of adults emerged was found; however, those adults that succeeded in emerge showed a low level of parasitism, as in previous trials, it may result up to a 17% (Mullens and Velten, 1994; Paine and Mullens, 1994).
Other organisms that may be used for vector control are heritable endosymbiotic bacteria such as Wolbachia. There has been an increase of interest of this type of bacteria due to the current research for applying in mosquito control programs. Heritable bacteria cause changes in host longevity, virus–host interaction and reproduction compatibility that may lead to control insect populations. Detection of endosymbiotic bacteria in biting midges has been reported by Nakamura et al. (2009) in Culicoides paraflavescens in Japan and in Culicoides wadai, C. brevitarsis and C. imicola in Australia by Mee et al. (2015). Up to date, Wolbachia has not been detected in European Culicoides species, alternatively, the genus Cardinium was detected in C. punctatus and C. pulicaris in the UK (Lewis et al., 2014). Despite the recent interest on endosymbiotic bacteria, its current application for Culicoides control is far away of being a reality on the field.
3.6.4. Genetic control
The current development of genomic and transcriptomic techniques has allowed to better understand genes expression and biology of processes such as blood feeding and vector competence. The study of the transcriptome of C. sonorensis has allowed to better know the functionality of the genome, identifying mid‐gut transcripts in EHDV‐infected adults (Campbell and Wilson, 2002), midgut transcripts associated to antihaemostatic and immunomodulatory functions (Campbell et al., 2005) and the genetic bases of sugar/blood feeding and vitellogenesis (Nayduch et al., 2014b). Despite no current control technique of biting midges is based on genetic methods, analysis of the transcriptome may provide new methodologies for vector control (Nayduch et al., 2014a).
Other genetic control tools include the use of RNA interference (RNAi) that avoids arbovirus replication. This type of RNA has been described in C. sonorensis‐derived KC cells (Schnettler et al., 2013) and artificially induced by intrathoracically injecting double‐stranded RNA (dsRNA) (Mills et al., 2015). As in the case of other genetic control tools, such as Release of insects carrying a dominant lethal genetic system (RIDL), Incompatible Insect Technique (IIT) and Sterile Insect Technique (SIT) (Alphey, 2014), are still of very limited application on Culicoides control due to the lack of basic studies.
3.6.5. Biotechnological control: pheromones and semiochemicals, traps and attractants
Chemical ecology is of great importance for insects, since mating, oviposition and feeding among others processes, are based on the detection of volatile compounds in the environment. Commercially available compounds, such as kairomones, are used for vector control and monitoring, as in the case of mosquitoes. However, in the case of Culicoides, most of the products remain under experimental framework and those available for mosquitoes have limited action on Culicoides.
Several compounds have been tested either in lab or filed conditions (see Harrup et al. (2016) for review), including 1‐octen‐3‐ol, l‐(+)‐lactic acid, butanone, acetone among others. None of these compounds has showed a strong attractant effect on Culicoides spp. and therefore its use for control remains limited.
Commercial traps combining several types of stimuli (i.e. CO2, octenol, heat) are available for mosquito control, and in some cases, they have been tested against Culicoides. The ABC Pro insect suction traps and the Mosquito Magnet® (MM) trap were not effective for controlling biting midge population in Florida (Cilek et al., 2003; Cilek and Hallmon, 2005), but more promising results were obtained by Lloyd et al. (2008) by continuous trapping using Mosquito Magnet®, MM‐FreedomH and MM‐Liberty PlusH against Culicoides furens, Culicoides barbosai and Culicoides mississippiensis.
3.6.6. Comparison of efficacy between vector‐proof establishment and repellents/insecticides
The establishment of VPE requires several interventions and activities, including13:
the implementation of physical barriers to reduce the probability of Culicoides entry;
the application of insecticides‐impregnated screens;
the elimination or limitation of Culicoides‐breeding sites in the proximity of the farms;
the implementation of a constant Culicoides surveillance inside and outside the stables.
Although a 100% vector‐proof level is very hard or even impossible to obtain, a correct application of all measures can significantly reduce the exposure of animals to Culicoides bites, thus providing substantial assurances for the trade of animals and animal products. However, usually the costs related to the implementation of a VPE are quite high and such solution may be cost‐effective for highly value animals only.
Stabling of animals, usually without screening openings, and the use of repellents/insecticides have been general measures recommended in BTV and AHSV outbreak scenarios to protect animals from Culicoides bites (Meiswinkel et al., 2000; Baylis et al., 2010). From the previous EFSA opinion (EFSA, 2008), it is known that stabling of animals may lead to a decrease of exposure of animals to Culicoides populations. However, there is little information about the general use of these measures in the current farm practices at European level.
A further step was achieved when VPE were defined at the EU level to decrease the risk of BTV transmission in animals moved from one to another MS.
The criteria for the VPE are laid down in Annex II of the Regulation (EC) 1266/2007 as amended by Commission Regulation (EC) No 456/2012, and are based on those in the OIE Terrestrial Animal Health Code (OIE, 2014).
A VPE shall at least comply with the following:
it must have appropriate physical barriers at entry and exit points;
openings must be vector‐screened with mesh of appropriate gauge which must be impregnated regularly with an approved insecticide according to the manufacturers’ instructions;
vector surveillance and control must be carried out within and around the establishment;
measures must be taken to limit or eliminate breeding sites for vectors in the vicinity of the establishment;
standard operating procedures must be in place, including descriptions of back‐up and alarm systems, for operation of the VPE and transport of animals to the place of loading.
The competent authority shall approve an establishment as vector protected, if the criteria in point 1 are met. It shall verify at the appropriate frequency, but at least three times during the required protection period (at the beginning, during and at the end of the period) the effectiveness of the measures carried out by means of a vector trap inside the VPE.
It should be pointed out that the Commission Regulation (EC) No 456/2012 considers VPE ‘to be worthwhile for high value livestock or artificial insemination centres for which other means of exiting the restriction zone (i.e. vaccination, natural immunity or movement during a vector‐free period) are not an option’. Therefore, its application for major movements of commercial animals seems not to be feasible. Italy is one of the EU MS which has more experience in the implementation of VPE. A very detailed and specific procedure is in place for the approval of the establishment and its registration into the national list (EC, 2012). The current approved establishment includes mainly bovine genetic centres, interested in the international trade of semen (Calistri, personal communication).
3.6.6.1. Efficacy of VPE and ITNs‐screened stables compared to repellent/insecticide efficacy
Vector protection of the establishment is achieved by combining different methods, such as treating walls and surfaces of VPE with residual insecticides, treating animals with authorised insecticides prior to entrance to the facility, and more importantly, by use of nets of appropriate size (no greater than 1.6 mm2) and preferably insecticide impregnated, to avoid Culicoides to enter the premises.
Efficacy of VPE is exclusively measured by using UV light traps (CDC and Onderstepoort types) inside and outside the VPE. The aim is to demonstrate no presence of adult Culicoides inside the VPE despite the abundance outside.
According to Commission Regulation (EC) No 456/2012, the frequency of operating vector traps should be conducted at least three times: at the beginning, during and at the end of the required protection period. However, each MS could propose its own regime of sampling. For example, in Italy, weekly collections for at least 10 consecutive days during the period of vector activity of the inside and outside trap is considered sufficient to demonstrate no presence of the vector in the VPE; in Spain, it is advised to operate the traps for two consecutive nights/fortnightly.
There is no scientific literature about the efficacy of VPE implemented in different MS. However, data on Culicoides trapping from 2009 to 2012 in a VPE of central Italy provide clear evidence that VPE achieved a mean reduction of 99.7% of Culicoides population when captures from the inside trap are compared to those obtained outside. The maximum number of adult Culicoides collected inside was 14 compared to 23,492 adults collected outside the VPE premises (Calistri, personal communication).
The use of nets of appropriate size to avoid Culicoides to enter the premises is the basis of VPE. In general, it is recommended to have all openings protected by filters or mesh impregnated with insecticide (ITNs) and with a maximum mesh size no greater than 1.6 mm2. There is more information available about the efficacy of the ITNs than that for VPE. As mentioned in Section 3.6.2, in general, ITNs used elsewhere have shown reduction of the Culicoides population but not total protection of animals. Deltamethrin ITN for protecting confined bulls used by Bauer et al., 2009 showed no efficacy, meanwhile the study from Calvete et al. (2010) showed partial protection of sheep (50–78.8%) when using ITN's impregnated with cypermethrin. Del Rio et al. (2014a) showed increased mortality (84.9 ± 10.5%) of Culicoides when using deltamethrin ITN tested on traps, while alphacypermethrin nets used by Page et al. (2014) showed 7.2 times reduction of Culicoides UV light trap captures when compared to control trap. Protection provided by a cypermethrin + pyrethrin ITN reached a range between 78 and 96% in the trial conducted by Baker et al. (2015) on horse stables. We could not consider the above mentioned works equal to the requirements that must be fulfilled by an official VPE, but they give an estimation of the potential protection (up to 96%) that could be provided by different ITN when installed in VPE.
As mentioned in Section 3.6.2, there are no currently authorised repellents in the EU to be used on livestock and only the repellent effect of some approved insecticides could be considered in this category (see Section 3.6.2 for details). Efficacy of repellents used experimentally to protect animals has shown to be very low elsewhere for different active ingredients (octanoic, decanoic and nonanoic fatty acids, citronella and lemon eucalyptus oil, deltamethrin, permethrin and DEET). In consequence, the use of repellents to protect animals against Culicoides biting could not be recommended and therefore reduction of Culicoides bites could not be achieved by using only repellents.
Several types of insecticides have been broadly tested in laboratory conditions against Culicoides in the last decade (EFSA (2008) and Table 9). In general, the great majority of works have shown a high killing effect of insecticides (usually 90–100% mortality after 1 h exposure) such as deltamethrin, cypermethrin, alphacypermethrin, lambda‐cyhalothrin, fenvalerate, zeta‐cypermethrin and permethrin when tested either on hair clippers (Liebisch and Liebisch, 2008; Schmahl et al., 2009a); Papadopoulos et al., 2009, 2010), WHO testing tubes contacting directly animal hair (Reeves et al., 2010; Venail et al., 2011, 2015; Del Rio et al., 2014b; Onuike et al., 2015); or material substrates (see Section 3.6.2 for details) such as plates and nets (Schmahl et al., 2008). However, in some cases, interpretation and comparison among studies are difficult due to the lack of information about the efficacy, times and mode of exposure to insecticides of Culicoides in testing cages or tubes (Harrup et al., 2016). Unfortunately, the promising results on vector mortality obtained in laboratory conditions, are usually not reproduced at the same level of efficacy when insecticides are applied in field conditions.
Table 9.
Summary table of insecticides, type of application and the reported efficacy in the different studies
| Active substance | Commercial name | Type of application | Culicoides sp. | Country | Host | Efficacy (in vitro semifield, field trials) | Reference |
|---|---|---|---|---|---|---|---|
| Field testing | |||||||
| Permethrin Y | Tectonik® 36 g/L; doses 10 mL/100 kg | Pour‐on | C. obsoletus, C. pulicaris | Netherlands | Horses | 82% | De Raat et al. (2008) |
| Tectonik 3.6% (1 ml/10 kg bodyweight, Virbac Animal Health) | Pour‐on | C. chiopterus and C. obsoletus | Netherlands | Sheep | 50% | Griffioen et al. (2011) | |
| Deltamethrin Y | 7.5% deltamethrin (Butox 7.5) | Whole body | Culicoides spp. | Spain | Sheep | 100% | Mullens et al. (2010) |
| 10 mL of Butox® pour on (7.5 mg deltamethrin/mL, Intervet, France) | Pour‐on | Culicoides Avaritia subgenus | Germany | Sheep | Reduced 86.4% of engorged females | Weiher et al. (2014) | |
| In vitro | |||||||
| 0.0025% deltamethrin | Exposure on impregnated paper | Colony of C. nubeculosus, field‐collected C. obsoletus and C. imicola | France | – | 100% after 1 h exposure | Venail et al. (2011, 2015) | |
| 0.001% of deltamethrin | Exposure on impregnated paper | Field‐collected C. obsoletus | Spain | – | 100% | Del Rio et al. (2014a) | |
| 0.05% of deltamethrin | C. nubeculosus colony | UK | 90% mortality | Onuike et al. (2015) | |||
| Lambda‐cyhalothrin | Oxyfly™ (Novartis) | Application on walls where insects rest | Field‐collected C. obsoletus and C. pulicaris | Germany | 100% | Schmahl et al. (2008) | |
| 1.25% w/v high‐cis cypermethrin | Coopers’ Spot On™, Schering‐Plough Animal Health, UK | Pour on | Culicoides spp. | UK | Sheep and cattle | High mortality | Carpenter et al. (2007) |
| 1,067 g cypermethrin per ear tag | Flectron® Flytags, Fort Dodge Animal Health | Ear tags | Culicoides spp. | Heifers and dairy cows | Efficacy for 14 days with 1 ear tag and up to 21 days with 2 ear tags | Liebisch and Liebisch (2008) | |
| 12.5–15 g/L alphacypermethrin | Dysect Cattle Pour‐On and Dysect Sheep Pour‐On (Zoetis) | Hair clippers obtained from leg, belly and back | Colony‐reared adults of C. nubeculosus | GR | Sheep and cattle | Near to 100%) up to 21 days post‐treatment (in vitro) | Papadopoulos et al. (2009) |
| Cypermethrin 5.0% | Deosect Spray, Fort Dodge Animal Health | Hair clippers | Culicoides spp. | GR | Horses | 80% at day 7–50% at day 35 post‐treatment | Papadopoulos et al., 2010) |
| Semi field testing | |||||||
| 7.5% w/v deltamethrin | Butox® 7.5 Pour On (Intervet International B.V., The Netherlands) | Pour on | Nulliparous females of a colony of C. nubeculosus exposed to shorn sheep | France | Sheep | 45% on day 4th after treatment. persistence of the lethal effect was estimated to be less than 10 days | Venail et al., 2011 |
Several pour‐on insecticides are approved at the EU level for field use (see Appendix E). Permethrin and deltamethrin are the most common used insecticides in pour‐on formulations. Results on efficacy of pour‐on insecticides obtained in experimental trials show a wide variety of results depending on the host species and particularities on the application of the topical insecticides. Mullens et al. (2000) obtained up to 80% of protection of calves that were treated with permethrin on the belly but null protection on those treated on the backline with permethrin (5%) and pirimiphosmethyl (27%). Also, in later studies, Mullens et al. (2001) showed that protecting ventral line of heifers by using permethrin (0.2%) was not sufficient to avoid seroconversion of animals. In general, pour‐on application shows less efficacy when compared to whole body application, as obtained by Mullens et al. (2010) when 100% protection was achieved when applying deltamethrin (7.5%) to different parts of the body of sheep (face, legs, belly) and not the backline only. The work by De Raat et al. (2008) using permethrin (3.6%) pour‐on insecticide showed 82% reduction of Culicoides trapped around treated horses only 48 h after the treatment, while Bauer et al. (2009) failed to protect bulls after five pour‐on treatments with deltamethrin (7.5%) on confined bulls, Griffioen et al. (2011) showed 50% protection of sheep using permethrin and Weiher et al. (2014) obtained overall efficacy of 86.4% (from 24 h to 5 weeks) of deltamethrin (7.5%) applied on sheep. Therefore, the range of protection of insecticides is substantially variable according to the active ingredient and the animal species aimed to be protected. A general range of protection of pour‐on insecticides from 50 to 86% could be extracted from scientific literature mentioned above. Problems of the fully spread of the insecticide over the entire body of animals has been described by several authors either in field and in vitro testing and different diffusion of the pour‐on insecticides is known for hair and fleece (Bauer, 1995; Carpenter et al., 2007; Papadopoulos et al. (2009, 2010)). This should be taken into account when using pour‐on insecticides to protect animals against Culicoides bites, particularly when there are differences among species on the preferred feeding region on animals (i.e. belly, legs, face). Viennet et al. (2012a) showed differences on the preferential landing sites of Culicoides on sheep, thus C. dewulfi preferred upper parts of animal while C. obsoletus preferred lower parts.
When comparing protection provided by VPE to that provided by insecticides only, we should consider that the criteria for VPE also includes the use of insecticide/repellents, that means that the protection provided by confinement of animals and use of ITNs is added to the partial protection provided by applying insecticides. In consequence, even if Culicoides are found inside the VPE premises, we should add the efficacy of pour‐on insecticides (50–86%) to that of VPE (96%). That decreases the probability of contact between animals and Culicoides inside VPE. Therefore, by only using pour‐on insecticides, protection of animals could not be considered as equal as the protection provided by the VPE which is at least 10% superior on efficacy compared to pour‐on insecticides. One open question is to know if a protection up to 86% provided by pour‐on insecticides would be sufficient to avoid BTV transmission or biting of BTV‐infected Culicoides. According to scientific literature reviewed in this opinion, high level of efficacy of pour‐on insecticides is difficult to achieve and little information is available about the effect of reduction on the numbers of engorged Culicoides females in relation to BTV transmission. In fact, different methods for assessing insecticide performance may lead to different results when considering the effect on engorged females (Harrup et al., 2016). For example, it is well known that UV light traps underestimate the number of blood‐engorged females when compared to other methods, such as drop traps (Carpenter et al., 2008; Mullens et al., 2010).
Increasing the protection of animals, by applying insecticides to different parts of the body and not only the backline (pour‐on), may increase the level of protection against bites of Culicoides. In fact, these measures are already included in, e.g. the bilateral agreement between France and Spain14 and application of certain doses of insecticide is recommended according to the animal species to be carried out on the backline and legs of animals. Specific commercial products for this purpose should be approved at the EU level since the currently available products have a pour‐on mode of application only. Impact of increasing use of insecticides on animals, including animal health, residues in meat and milk, withdrawal periods, impact on biodiversity and the environment, as well as risk of Culicoides increasing resistance to insecticides, should be addressed before recommending this type of measure.
4. Conclusions and recommendations
TOR 1. As regards vaccination, eradication and surveillance
ToR 1.1. Assess the most suitable duration of a BT vaccination campaign intended to achieve disease freedom in a country or region considering any relevant factors that may affect and influence disease spread and persistence.
Conclusions
The results of the model simulations clearly indicate that:
without any vaccination, the disease can persist for a long time, reaching an endemic condition with low level of prevalence of infection (1.5% in cattle, 0.6% in sheep) and greater seroprevalence levels (45% in cattle, 14% in sheep);
even when the vaccination of 95% of the susceptible cattle and sheep is constantly applied for three consecutive years, BTV is not eradicated and may re‐emerge after a couple of years;
only after 5 years of vaccination of 95% of susceptible cattle and sheep, the prevalence of infection is close to eradication levels, although reaching zero values for sheep only in the scenario of France, Sardinia and the UK, but still not reaching zero for the Spanish scenario.
Recommendations
Specific conditions (e.g. animal density, meteorological conditions, etc.) should be considered when planning vaccination strategy for eradication purposes. The results from one case to another cannot be generalised, but a case‐by‐case approach should be used.
ToR 1.2. Assess the probability of BT recurrence in BT affected areas that have regained BT freedom, in particular due to BT virus becoming endemic with low level circulation in these areas and reoccurring ‘spontaneously’ (low‐noise circulation in livestock or wildlife, maintenance in vectors or other possible mechanisms to be considered).
Role of wildlife
Conclusions
The results of available studies on European wild ruminant populations suggest that red deer (C. elaphus) is the wild ruminant species most likely to be involved in BTV circulation in comparison to the other European wild ruminants.
Considering the divergent and sometimes contrasting results of the currently available information, it is possible that BTV infection may persist locally in red deer population or in other wild ruminants in areas with high density of these animals, and where there are a low number of competing domestic animals and favourable vector conditions.
Recommendations
Annual cross‐sectional surveys with a focus on yearlings may need to be conducted to ascertain the role of wild ruminant population in the BTV circulation and persistence in specific geographical areas.
Role of transplacental transmission
Conclusions
Strong evidence exists that TPT occurs in cattle, sheep and goats, under field conditions, for BTV‐8. The incidence varies by animal species and gestational stage of infection.
For BTV serotypes other than BTV‐8, TPT was experimentally demonstrated only for BTV‐2 in sheep and BTV‐11 in cattle and North American elks.
The overall and relative contributions of TPT to the over‐wintering mechanism are not clear and remain to be investigated.
The epidemiological significance of the presence of BTV RNA in the blood of newborn animals, and whether the level of viraemia is sufficiently high to infect Culicoides are not clear and remain to be investigated.
Role of length of BTV viraemia
Conclusions
BTV nucleic acid can be detected by RT‐PCR in the blood of infected cattle and sheep till 4–5 months after the infection, and up to 2 months in goats, while infectious virus in the blood can only be detected for up to 50 days in cattle and up to 30 days in small ruminants in the majority of the cases (75% cases, upper quartile).
Persistence of BTV in other tissues
Conclusions
BTV presence has been demonstrated in different organs, including lymphoid tissue, skin and reproductive organs. The maximum duration of the presence of BTV is registered in the spleen up to 40 days for infectious virus and up to 3 months for its nucleic acid.
Skin and dermal tissue could, in addition to blood, may potentially play a role in virus transmission through midge bite. However, this hypothesis still needs to be demonstrated.
Other organs with BTV presence may potentially play a role in direct virus transmission, such as tongue, tonsils, nasal mucosa. Nevertheless, the evidence in support of direct BTV transmission is very limited, and as regards the 24 historical serotypes, it is likely that direct transmission is infrequent, with a limited contribution to BTV spread during epidemics, in comparison to vector transmission.
Role of vertical transmissions in vectors
Conclusions
To date, there is no scientific evidence in support of vertical transmission of BTV in its biological vectors.
Recommendations
Further studies on virus detection on larvae are recommended, where endemic situations allow it, particularly with European vector species.
ToR 1.3. Revise and assess the suitability of the provisions on surveillance laid down in Regulation (EC) No 1266/2007 to ensure reliable and robust demonstration of the absence of virus transmission in a Member State or epidemiologically relevant area, considering point 1.2 above.
Conclusions
The design infection prevalence for surveillance aiming at demonstrating the absence of BTV circulation should be defined after considering the type of target prevalence (for example, infection prevalence detected by RT‐PCR, serological prevalence by c‐ELISA), the geographical unit of concern (in case of low‐level circulation the BTV may circulate in small geographical foci and not randomly distributed in large areas), and the epidemiological phase of concern, as defined in a previous EFSA opinion (EFSA AHAW Panel, 2011a).
When surveillance is being undertaken in a zone or country following the cessation of the vaccination, very low levels of infection prevalence are expected. In particular, infection prevalences below 1% can be observed from the literature and from the mathematical model developed for this opinion. These are much lower than the value foreseen by the Regulation (EC) 1266/2007.
Furthermore, based on the surveillance in France from 2013 to 2015 with associated detected prevalences, and considering the reoccurrence of BTV in France in 2015, circulation of BTV might have occurred without being detected.
Recommendations
The low level of prevalence at least equal to 1% should be taken into consideration when surveillance is designed to demonstrate freedom (BTV‐free status) especially during the years immediately after the application of a successful vaccination campaign.
The design prevalence for the surveillance of BTV cannot be generalised, but must be set on a case‐by‐case approach after considering the type of target prevalence (infection or serological prevalence), the geographical unit of concern and the epidemiological phase appropriate to the area concerned.
TOR 2. As regards specific options for safe trade that could be used for exemptions from the exit ban applicable to movements of live animals from a restricted zone
ToR 2.1. Assess whether maternal immunity against BT of calves, lambs and kids born to and colostrum fed from vaccinated mothers, constitutes a sufficient guarantee for animals of the above species to be moved safely from a BTV‐infected to a BTV‐free country or zone, without a risk for disease spread, with or without the need for any additional premovement testing regime and indicate the main parameters that could be used (minimum/maximum age of calves, testing of dams, etc.).
Conclusions
In general, neutralising antibodies can be considered protective against infection, although a clear and specific threshold of a protective titre of BTV‐specific neutralising antibody cannot be identified.
Some animals born from vaccinated dams and not showing detectable neutralising colostral antibodies have also been shown to be protected.
Given the limited number of studies available, a marked variation in the level and longevity of neutralising colostral antibodies in lambs and calves (no specific evidence is available for goats) from vaccinated dams have been demonstrated, ranging from 16 up to 270 days in lambs (mean value 210 days) and from 70 to 113 days in calves (mean value 84 days).
ToR 2.2. Assess the minimum age of calves, lambs and kids after which residual colostral antibodies against BTV do not interfere any longer with vaccine immunisation of these animals (in an example of BT bilateral agreement this age limit is set at 90 days).
Conclusions
The results of the currently available experimental studies demonstrated that the presence of colostral antibodies interferes with the induction of the immune response to homologous vaccine in calves and lambs at least during 3 months after birth (no specific evidence is available for goats).
Recommendations
During the period of vector activity and potential virus circulation, or when an immediate threat for animal health exists, calves and lambs (no specific evidence is available for goats) born from vaccinated mothers may be vaccinated1 twice, once before 3 months and then again at about 6 months of age, to ensure maximal protection. Outside these periods, in the absence of BTV circulation, a single vaccination at about 5–6 months can be adequate.
Due to the limited experimental evidence on interference between colostral and vaccine immunity, further detailed studies are recommended.
ToR 2.3. Assess the minimum time after completion of the primary vaccination (1–2 doses as indicated by the vaccine manufacturer) for the vaccinated animals to be considered immune to be safely moved from a BT‐infected to a BT‐free country or zone (currently set at 60 days in paragraph 5 of Annex III to Regulation (EC) No 1266/2007).
Conclusions
Based on the literature review conducted in order to answer this subquestion, the minimum time after completion of vaccination against BTV considered to provide a protective immune response can be variable ranging from 3 to 48 days depending on the vaccine, the experimental design, the diagnostic tests, the animal related factors and other variables.
When commercially available inactivated vaccines and neutralising antibodies are considered, the majority of animals are positive within 21 days after vaccination, an increasing proportion of protected animals can be observed at 28 days after vaccination.
ToR 2.4. Assess whether vector protection for 14 days of ruminants below the age of 70 days, combined with a negative PCR test at the end of the 14 days or more, qualify them for a safe movement from a BT restricted to a BT‐free area.
Conclusions
The measures considered (animals less than 70 days of age and born from vaccinated dams, kept under vector protection conditions for 14 days and tested with negative result by RT‐PCR at the end of the vector protection period) to allow the movement of animals from a BTV‐infected to a BT‐free area, are all able to reduce the risk of introducing one or more viraemic animals, both considered singularly or in combination.
A quantitative estimation of the final risk of introducing a viraemic animal following the above described procedure would be of limited utility, given the high levels of uncertainties affecting all variables and the large range of epidemiological conditions influencing the final risk. Given the current uncertainty level, the development of any quantitative model based on a series of assumptions (e.g. the level of infection in the population of origin, the period of the year, the vaccination policy in the country of origin, the specific protocols used for vector protection and the number of animals to be introduced) would make the outcomes rather unrealistic and scarcely applicable in practice.
TOR 3. As regards protection from BTV vectors and vector‐based provisions for exemption from the exit ban applicable to movements of live animals from a restricted zone
ToR 3.1. Review and update previous opinions as regards vectors ecology (models for distribution/density), in order to have more accurate and applicable criteria for the determination of the seasonally vector‐free period.
Vector ecology
Conclusions
Table 10 below reports the assessment of the validity of the conclusions of the EFSA opinion on bluetongue vectors and insecticides (EFSA, 2008).
Table 10.
Assessment of the conclusions from the EFSA opinion on bluetongue vectors and insecticides (EFSA, 2008), (left column), and their endorsement or the new modified version (right column)
| Conclusions from EFSA opinion (2008) | Endorsement or new version of the conclusions |
|---|---|
| The distribution of the main vector species is well known in each of the BTV affected countries | No precise distribution maps are yet available, but maps will be available in the framework of Vectornet projecta. However, there is still a need to know detailed distribution of the species included in the Obsoletus assemblage (C. obsoletus, C. scoticus, C. chiopterus and C. dewulfi) |
| The distribution of C. imicola is well documented in the southern European countries. | According to phylogeographical studies, this species has been present in the Mediterranean basin for 10,000 years and in southern Europe for at least 100 years. Therefore, the concept of recent invasion seems to be not valid for the European Mediterranean countries. The northward expansion of this species seems to be very limited in France, and inexistent in Italy |
| The distribution of C. obsoletus/C. scoticus includes all the countries in Europe, although it is relatively more abundant in the northern regions. In the southern regions of Europe, the distributions of C. imicola and C. obsoletus/C. scoticus overlap | This conclusion is still valid |
| C. dewulfi, C. chiopterus and C. pulicaris (sometimes not differentiated from C. lupicaris) are also widespread in Europe, especially in the northern countries. Nevertheless, their abundance as estimated on the basis of light traps has been always reported as being lower than C. obsoletus/C. scoticus, except in Mediterranean areas where C. imicola and/or C. newsteadi is usually dominant | This conclusion is still valid |
| Present data indicate that the biting activity of the majority of the vector species in Europe primarily occurs during the crepuscular and nocturnal hours | This conclusion is still valid |
| There are indications, however, that under suitable meteorological conditions and particularly during the latter part of the season, diurnal feeding activity of potential vector species of Culicoides may occur | This conclusion is still valid |
| The implications for BTV transmission of vector daylight activity are at present unclear as trapping programmes based only on light traps are not adequate to provide information regarding the daylight biting activity | Recent studies suggest that daylight biting activity is limited and not relevant compared to the crepuscular activity. However, in some scenarios day activity may underestimate BTV‐infected females which will be not captured by UV traps |
| Dispersion of vector species of Culicoides at the farm level is still very poorly understood, but assumed to be short distances from the breeding sites | The dispersion of Culicoides may be higher than originally thought |
| Long distance dispersion of vector Culicoides on winds over scores or even hundreds of km has been reported by several workers but the proportion of a population that are involved is thought to be very small. | This conclusion is still valid |
| Distribution and abundance data almost solely obtained by only using UV light traps may underestimate some species important for the transmission of BTV | This conclusion is still valid |
| Northern Palaearctic species of Culicoides are able to transmit BTV | This conclusion is still valid |
| To date, the specific vector(s) of BTV in these areas have not been identified, although strong circumstantial evidence implicates C. obsoletus, C. scoticus, C. dewulfi, C. chiopterus and species of the Pulicaris assemblage as the likeliest candidates. This list is probably not exhaustive and the identification of additional vector species is likely | Further studies (PCR detection) have given more evidence of the role as vectors of the Obsoletus and Pulicaris assemblages |
| To date, standardised and appropriate testing protocols to determine the vector competence levels of Culicoides species for BTV in Europe have not been applied. In northern Europe this has led to the use of pool‐based real‐time RT‐PCR investigations on field‐caught parous female midges to imply vector competence levels | This conclusion is still valid for all EU (north and south) |
| These methods have several technical drawbacks and do not provide a measure of vector transmission in the field. Similarly, studies from southern Europe, based around cell‐based isolation of virus, while superior to those using real‐time RT‐PCR, are still difficult to interpret due to the use of pool‐based isolation methods and an inability to accurately assess viral dissemination levels | This conclusion is still valid |
| Recent publications that allow high‐throughput processing of Culicoides for virus isolation may allow some of these issues to be addressed and also enable standardisation between laboratories | This conclusion is still valid |
| Laboratory‐based studies on vector competence remain time consuming and difficult to perform outside the areas of BTV transmission as they require specialist laboratory accommodation | This conclusion is still valid |
| Vector competence is just one element of the vector capacity of a species for BTV transmission. Other elements some of which have been assessed in southern Europe include host preferences, biting rates, vector survival, location of breeding sites, temporal and spatial distribution, and abundance | Host preferences, biting rates, location of breeding sites, temporal distribution and abundance are currently known for the major vector species in whole Europe. More updated spatial distribution of vectors will be available from the work done by the Vectornet project |
| An integrated assessment of all of these elements is required to gain a realistic idea of the importance of each potential vector species | This conclusion is still valid |
Recommendations
Table 11 below reports the assessment of the validity of the recommendations of the EFSA opinion on bluetongue vectors and insecticides (EFSA, 2008).
Table 11.
Assessment of the recommendations from the EFSA opinion on bluetongue vectors and insecticides (EFSA, 2008) (left column), and their endorsement or the new modified version (right column)
| Recommendations from EFSA opinion (2008) | Endorsement or new version of the recommendations |
|---|---|
| In order to better understand the current distribution of the species included in the ‘Obsoletus assemblages’, it is recommended to perform co‐ordinated European surveys using the molecular identification of C. obsoletus/C. scoticus females to species level. In addition, the routine identification of males from these species is also advisable to have a better picture of each species distribution. These data should be made available in the EU centralised database (BT‐Net) |
This recommendation is still valid for most of the European countries The current VectorNet initiative covers this issue Bt‐Net is no longer available, and current updating of vector distribution is covered by Vectornet consortium |
| An increased number of sampling sites around the known northern limits of the range of C. imicola is recommended to improve understand of the role of this species in the northward spread of BTV | This recommendation is no longer relevant, considering the role of transmission of Northern European species and considering that C. imicola is not experiencing a quick and relevant spread to northern countries |
| Molecular techniques should be used for the differentiation of species from the Obsoletus and Pulicaris assemblages, especially in epidemiologically relevant areas. Where possible these techniques should be integrated into surveillance schemes | This recommendation is still valid, particularly to apply molecular techniques in a routine basis to national surveillance programs |
| Training on dipteran taxonomy is additionally also recommended to be conducted at a European level. Varying levels of circumstantial evidence has linked C. imicola, C. obsoletus, C. scoticus, C. dewulfi, C. chiopterus and species of the Pulicaris assemblage with BTV transmission in Europe. Consequently, targeted surveys should be undertaken for defining their temporal and spatial distributions across Europe | Information has been improved in most European countries, and specific actions on BTV vector taxonomy should be carried out where there are still knowledge gaps |
| A standardisation of techniques to implicate field‐caught Culicoides vectors in BTV transmission is required. It is recommended that this be used to harmonise studies across Europe as, to date, testing methods, in northern Europe particularly, have been inadequate | Common protocols for analysis of potential BTV vector species are recommended across Europe. Ring trials among national reference laboratories are also recommended |
| Laboratory testing for vector competence should be carried out, where possible, in parallel with field‐based testing (EFSA, 2008) | This recommendation is still needed. Efforts on establish a Palaearctic species colony different of the current available of C. nubeculosus is strongly recommended to progress on the understanding of vector competence at the European level |
| Analyses of the vector competence of particular species should be made with reference not only to the ability of the vector to become infected by, replicate and transmit the virus but also to its wider ecological requirements (i.e. its vector capacity), which may vary with region and season | This recommendation is still valid |
Vector‐free period
Conclusions
Available data demonstrate that some Culicoides species, in some geographical areas in Europe, are active throughout the year and that an absolute SVFP does not exist. However, there are periods of the year when the abundance of the Culicoides vector species is extremely low, mainly coinciding with winter time. Long‐standing practical experience demonstrates that transmission of BTV is substantially reduced or halted during these periods.
The criteria considered by the Regulation (EC) 1266/2007 for the definition of the SVFP include the complete absence of adult C. imicola and less than five parous females captured in light traps for the other Culicoides species. Temperature conditions that impact on the behaviour of the vectors activity and related temperature thresholds are considered possible additional criteria for the definition of the SVFP.
Although the available data do not allow the identification of more accurate and applicable criteria for the definition of the SVFP, the analysis of the data produced by the Italian entomological surveillance programme agrees with the current provisions of the Regulation (EC) 1266/2007, as no seroconverted sentinels were observed in the absence of C. imicola or with less than 5 captured Culicoides.
In relation to the possible definition of a temperature threshold, the results of the available studies and analysis of the risk of BT transmission through the calculation of the R0 indicate a possible temperature threshold value for BT transmission between 9.0 and 12.0°C. This temperature values cannot be taken in absolute way, without considering the different Culicoides species involved and the eco‐climatic conditions of the territory of concern.
Recommendations
Since an in‐field validation of the criteria currently used for the SVFP definition is needed, the availability of long‐term entomological data, coupled with serological or virological surveillance results in the same locations on animal host and vectors, would be necessary for the main European eco‐climatic zones and different Culicoides species involved.
ToR 3.2. Review and update previous opinions as regards over‐wintering mechanisms and the duration of the BT viraemia.15
Conclusions
Continuous Culicoides activity could occur almost throughout the year, at least during years when temperatures allow that, in Mediterranean areas and in mild‐winter areas. The occurrence of a probable continuous BTV transmission was established in Sardinia (Foxi et al., 2016), and could thus theoretically occur in other European areas during years with mild‐winter temperatures.
Continuous Culicoides activity and long‐lived infected female could collectively contribute to the BTV overwintering in the European areas characterised by mild‐winter temperatures.
According to the opportunity maps as shown, in northern Europe, low winter temperatures mainly inhibit Culicoides life cycle over a period of at least 3 months, and would not allow continuous transmission or survival of females infected during the prior transmission season. This is in agreement with field data were adult populations of Culicoides are in general absent from January to April in most of North European countries.
Recommendations
Seasonal maps of presence/absence of the major vector species in Europe are recommended to be developed.
Validated models based on long‐term field data of seasonal captures for predicting the vector seasonality, particularly periods of absence and/or low abundance of the major BTV vector species across Europe in relation with environmental variables is also recommended.
The survival rates of adult Culicoides at low temperatures is recommended to be further investigated under laboratory conditions.
The influence of temperature on BTV replication in Culicoides should be investigated to establish if BTV could infect females under the detection threshold and replicate later when temperatures increase.
Systematic analysis of BTV presence in vector females collected during winter months is recommended to elucidate if those vector females suppose an interseasonal bridge for BTV in periodically infected areas in Europe. Further, the development of new age‐grading methods is recommended to assess if females collected during winter could have been infected during the prior transmission season.
ToR 3.3. Review and update previous opinions and provide a scientific assessment of the appropriateness of the use of insecticides and repellents against Culicoides as BT competent vectors, including an assessment of their efficacy and recommendations of adequate protocols for their uses, in particular, as regards their suitability to protect animals against attacks by vectors performing at least equal to the protection provided by vector‐proof establishments – without the need to keep animals in a vector protected facility.
Conclusions
To date, there is no conclusive evidence that the use of insecticides or repellents when applied singularly reduce the transmission of BTV in the field. In specific scenarios, however, they have been shown to either kill Culicoides or reduce host/vector contact and hence are used as a risk mitigation measure where vaccines are unavailable. Their use is modified by both logistics and cost.
Treatment of animals with pour‐on insecticides causes mortality in a proportion of feeding Culicoides but the effect is transient and necessitates frequent application.
Treatment of animals with true repellent products (e.g. DEET) has been less investigated, largely due to the logistics of reapplication every few hours. This is unlikely to be feasible except for very high value stock.
Stabling is effective in reducing host/vector contact where a high level of containment can be attained. Insecticide‐treated meshes applied over windows in stables were found to kill Culicoides quickly enough to inhibit entry and field trials demonstrated substantial reductions in populations found in stables. These studies primarily addressed horses, however, and the logistics and reduced coverage provided to ruminants may lessen this effect.
Treatment of the environment with insecticides to kill either adult or larval Culicoides has not been studied since the previous EFSA scientific opinion from 2008 and is unlikely to be effective due to the ubiquitous nature of Culicoides larval development sites in Europe.
Habitat modification techniques have been trialled for dung heaps and the impact of covering on the emerging adult Culicoides population was limited.
According to scientific literature reviewed in this opinion, high level of efficacy (up to 86%) of pour‐on insecticides is difficult to achieve, particularly under field conditions, and little information is available about the effect of reduction on the numbers of engorged Culicoides females in relation to BTV transmission.
By only using pour‐on insecticides, protection of animals is lower than the one provided by the VPE which is at least 10% higher.
Recommendations
Further studies would be needed to estimate the risk reduction provided by application of insecticide treatment under field conditions.
Protocols of usage of insecticides and repellents on animals should be harmonised in the EU and supported by field evidence.
Glossary
- Herd prevalence
The number of test positive herds of the total number of tested herds
- Prevalence at the animal level
The number of test positive animals of the total number of animals.
- Infection prevalence (or virus prevalence)
Prevalence of positive animals with detectable virus or its nucleic acid
- Serological prevalence
Prevalence of seropositive animals (with detectable antibodies against BTV)
- Epidemiological phase
In the course of a BTV infection in a region, three fundamental steps can be distinguished: introduction, establishment and spread in a geographical sense (EFSA AHAW Panel, 2011a). During these steps, the prevalence of infected animals in a region changes, since, upon introduction into a BTV‐free region, the prevalence in a geographical unit rises from zero to a maximum (plateau prevalence) and subsequently drops again either to zero, in case the infection fades out, or to a level determined by endemic infection in the region. In relation to the expected prevalence, different epidemiological phases can be distinguished, each with a specific goal for monitoring and surveillance.
- Design prevalence
Minimal detectable prevalence specified for detection of infection at a specified level of confidence
- Endemic occurrence
Constant presence of an infection in a population
- Epidemic
Series of outbreaks in a region
- Outbreak
The holding or place situated in the territory of the European Community where animals are assembled and where one or more cases of BTV has or have been officially confirmed tested in a region
- Restricted zone
Demarcated zone considered BTV infected
Abbreviations
- AHL
Animal Health Law
- AHSV
African horse sickness virus
- AMLS
Animal Movements Licensing System
- BT
bluetongue
- BTV
bluetongue virus
- c‐ELISA
competitive ‐enzyme linked immunosorbent assay
- CFU
colony‐forming unit
- CIN
Commercial Inactivated Vaccine
- CLA
Commercial live‐attenuated Vaccine
- CPI
coefficient of protection
- CTS
Cattle Tracing System
- ED50
median effective dose
- EHDV
epizootic haemorrhagic disease virus
- EIP
extrinsic incubation period
- ELISA
enzyme‐linked immunosorbent assay
- EX
Experimental vaccine
- HDPE
high‐density polyethylene nets
- IIT
Incompatible Insect Technique
- ITN
insecticide‐treated nets
- IIV
Iridescent virus
- LD50
median lethal dose
- LST
Land Surface Temperature
- LT90
lethal time 90
- MPD
minimum protection day
- MS
Member State
- NUTS
Nomenclature of Units for Territorial Statistics
- PD50
50% protective dose
- PROMETHEUS
PROmoting METHods for Evidence Use in Scientific assessments
- RNAi
RNA interference
- rg
reverse genetics
- RIDL
Release of insects carrying a dominant lethal genetic system
- RT‐PCR
reverse transcription polymerase chain reaction
- S/P
sample‐to‐positive ratio
- SAMS
Scottish Animal Movements System
- SIT
Sterile Insect Technique
- SLR
systematic literature review
- SNT
serum neutralization test
- SVFP
SVFP seasonally vector‐free period
- TCID50
50% tissue culture infective doses
- TOT
transovarial transmission
- TPT
transplacental transmission
- VNT
virus‐neutralisation test
- VPE
vector proof establishments
- VT
vertical transmission
- WHO
World Health Organization
- wt
wild‐type
Appendix A – Modelling the transmission of bluetongue virus within and between farms
A.1. Data
A.1.1. Demographic data
Data for farms in Great Britain (GB). The location and number of cattle and sheep on each farm were obtained from June agricultural survey data for 2006. Animal movement data for 2006 were extracted from the Cattle Tracing System (CTS) for cattle, from the Animal Movements Licensing System (AMLS) for sheep in England and Wales and from the Scottish Animal Movements System (SAMS) for sheep in Scotland. These represent a normal year for animal movements (i.e. there were no major disease outbreaks).
Data for farms in other EU member states. Farm‐level data could not be obtained for other EU member states. Accordingly, regional‐level data were used to generate synthetic farm‐level data sets for each country of interest (i.e. France, Spain and Italy). More specifically, the number of holdings with cattle, the number of cattle, the number of holdings with sheep and the number of sheep for each NUTS (Nomenclature of Units for Territorial Statistics) level 2 (NUTS2) region in Europe for 2010 were extracted from Eurostat. A location for each farm in a region was generated by sampling a point uniformly at random from within the boundary of that region, while a herd or flock size was generated by sampling from an exponential distribution with mean equal to the mean holding size for the region (EFSA, 2012). Although this could, in principle, generate herds or flocks of unlimited size, in practice, the largest herd comprised 1,434 cattle and the largest flock comprised 3,295 sheep (both in the synthetic data set for France). The synthetic data sets were generated using the maptools (Bivand and Lewin‐Koh, 2013) and spatstat (Baddeley and Turner, 2005) packages in R (Team RC, 2014).
Animal movement data could not be obtained for any of the countries of interest (i.e. France, Spain and Italy).
Seasonal calving and lambing patterns. Seasonal patterns of calving and lambing for each country (France, GB, Italy, Spain) were extracted from those reported by EU member states to EFSA (EFSA, 2012).
A.1.2. Climate data
Data for GB. Daily mean temperatures were obtained from the UK Climate Projections (UKCP09) gridded observation data sets for 2007. These cover the UK at 5 km by 5 km resolution, with farms using the temperature data for the grid square in which they are located.
Data for other EU member states. Temperature data were obtained from the European Commission Joint Research Centre MARS Meteorological Database, which provides daily meteorological data spatially interpolated on a 50 km by 50 km grid. Specifically, we extracted the daily minimum and daily maximum temperatures for 2011 and computed the midpoint of these for each grid square. Farms used the temperature data for the grid square in which they are located.
A.2. Within‐farm transmission of bluetongue virus
The dynamics of BTV within a farm are described using a stochastic compartmental model that includes two ruminant host species (cattle and sheep) and a single Culicoides vector (Szmaragd et al., 2009).
The cattle and sheep populations are subdivided into the number of susceptible (i.e. uninfected), infected and recovered animals, denoted by X (i), Y (i) and Z (i), respectively, where the superscript i indicates cattle (C) or sheep (S). To allow for a more general gamma distribution for the duration of viraemia, the infected host population, Y (i), is subdivided into a number of stages, with newly infected hosts entering the first stage and then passing through each successive stage. If the time spent in each stage follows an exponential distribution with mean 1/n i r i, the total length of time spent in the n i stages follows a gamma distribution, with mean 1/r i and variance 1/n i r i 2 (Anderson and Watson, 1980).
The vector population (N) is subdivided into the number of adult female midges that are susceptible (i.e. uninfected), latent (i.e. infected, but not infectious) and infectious, denoted by S, L and I, respectively. To allow for a more general gamma distribution for the extrinsic incubation (i.e. latent) period (EIP) (Carpenter et al., 2011), the latent class is subdivided into a number of stages in a similar approach to that described above for the duration of host viraemia. Vector mortality occurs at the same rate in all classes and is balanced by the recruitment of susceptible vectors, so that the total vector population (N) remains constant during the vector season.
The force of infection for host species i, λi, is given by,
| (A1) |
where b is the probability of transmission from an infected vector to a host, a is the reciprocal of the time interval between blood meals for the vector (assumed to be equal to the biting rate), m i(= N/H i) is the vector‐to‐host ratio and I/N is the proportion of bites which are from infectious vectors. The proportion of bites on cattle and sheep is given by
| (A2) |
respectively, where σ is the vector preference for sheep relative to cattle. The seasonal vector activity (Sanders et al., 2011b) on day t is given by
| (A3) |
normalised so the maximum value is one. The force of infection for vectors, λV, is
| (A4) |
where β is the probability of transmission from an infected host to a vector and Y (C) and Y (S) are the total number of infected cattle and sheep, respectively.
Parameters in the model are summarised in Table A.1. Most parameters (see Table A.1) were estimated by fitting the BTV model to the summary outbreak data for Great Britain in 2007 (DEFRA, 2008) using approximate Bayesian computation (ABC) sequential Monte Carlo (SMC) sampling (McKinley et al., 2009; Toni et al., 2009). The reciprocal of the time interval between blood meals (a), the vector mortality rate (μ) and the reciprocal of the mean EIP (v) were assumed to vary with the local temperature (see Table A.1 for details).
Table A.1.
Parameters in the model for the transmission of bluetongue virus within a farm in Great Britain
| Description | Symbol | Estimatea or function | Comments and references | |
|---|---|---|---|---|
| Probability of transmission from vector to host | b | 0.82 (0.69, 0.95) | – | |
| Probability of transmission from host to vector | β | 0.02 (0.006, 0.05) | – | |
| Vectors to host ratio for species i | m i | γ(s V, μV/s V) | Sample drawn from gamma distribution for each farm | |
| Mean vector to host ratio | μV | 2,058 (763, 3683) | – | |
| Shape parameter for vector to host ratio | s V | 1.65 (0.47, 3.04) | – | |
| Number of animals of species i on farm | H i | – | Obtained from agricultural survey data | |
| Proportion of bites on species i | ϕi | – | For cattle ϕC = H C/(H C + σH S), while for sheep ϕS = σH S/(H C + σH S) | |
| Vector preference for sheep relative to cattle | σ | 0.15 (0.004, 0.65) | – | |
| Reciprocal of the time interval between blood meals | α | a(T) = 0.0002T(T − 3.7) (41.9 − T)1/2 . 7 | Depends on temperature (Mullens et al., 2004) | |
| Duration of viraemia (cattle) | Mean | 1/r C | 20.6 | Parameters estimated by fitting a gamma distribution to data on naturally infected cattle (Melville et al., 1996) |
| No. stages | n C | 5 | ||
| Disease‐associated mortality rate (cattle) | d C | 0.0015 (0.0001, 0.0037) | – | |
| Duration of viraemia (sheep) | Mean | 1/r S | 16.4 | Parameters estimated by fitting a gamma distribution to data on experimentally infected sheep (Goldsmit et al., 1975; Veronesi et al., 2005) |
| No. stages | n S | 14 | ||
| Disease‐associated mortality rate (sheep) | d S | 0.0078 (0.0006, 0.0020) | – | |
| Extrinsic incubation period (EIP) | Mean | 1/ν | ν(T) = α(T − T min) | Reciprocal of mean EIP depends on temperature (cf. Carpenter et al., 2011) |
| No. stages | k | 10 (2, 25) | ||
| Virus replication rate | α | 0.020 (0.016, 0.024) | ||
| Threshold temperature for virus replication | T min | 13.24 (12.75, 13.72) | ||
| Vector mortality rate | μ | μ(T) = 0.009 exp(0.16T) | Depends on temperature (Gerry and Mullens, 2000) | |
| Vector recruitment rate | ρ | – | For simplicity, assumed to be equal to equal to vector mortality rate | |
| Vector population size | N | – | For simplicity, assumed to be constant; given by N = m i H i | |
| Vector activity | sin, 12 month | b 11 | −1.59 (−1.80, −1.37) | – |
| cos, 12 month | b 21 | −3.81 (−4.40, −3.20) | ||
| sin, 6 month | b 12 | −1.46 (−1.59, −1.33) | ||
| cos, 6 month | b 22 | −0.99 (−1.41, −0.57) | ||
For those parameters estimated as part of the approximate Bayesian computation scheme, the mean and 95% credible interval (in brackets) for the marginal posterior distributions are reported.
Population sizes in the model take integer values, while transitions between compartments are stochastic processes (Table A.2). The number of transitions of each type during a small time interval δt was drawn from a binomial distribution with population size n and transition probability q (the appropriate per capita rate multiplied by δt) (Table A.2). However, binomial random variables are computationally expensive to simulate and an approximating distribution was used wherever possible. If: (i) nq(1 − q) > 25; (ii) nq(1 − q) > 5 and 0.1 < q < 0.9; or (iii) min(nq, n(1 − q)) > 10, an approximating normal variate with mean nq and variance nq(1 − q) was used, while if q < 0.1 and nq < 10, an approximating Poisson variate with mean nq was used (Forbes et al., 2011).
Table A.2.
Transitions, probabilities and population sizes in the model for the transmission of bluetongue virus within a farm
| Description | Transition | Probability | Population size | ||
|---|---|---|---|---|---|
| Hosts | |||||
| Infection |
|
λiδt | X (i) | ||
| Completion of infection stage j (j = 1,…, n i−1) |
|
n i r iδt |
|
||
| Mortality during infection stage j (j = 1,…, n i) |
|
d iδt |
|
||
| Recovery |
|
n i r iδt |
|
||
| Vectors | |||||
| Infection |
|
λVδt | S | ||
| Completion of extrinsic incubation period (EIP), stage j (j = 1,…,k−1) |
|
kvδt | L j | ||
| Vector mortality during EIP (j = 1,…, k) (and compensatory recruitment) |
|
μδt | L j | ||
| Completion of EIP |
|
kvδt | L k | ||
| Mortality of infectious vectors (and compensatory recruitment) |
|
μδt | I |
A.3. Transmission of bluetongue virus between farms
To describe the spread of BTV between farms, a stochastic, spatially explicit model with a daily time step was used. Transmission between farms was assumed to occur via two routes: movement of infected animals or dispersal of infected vectors.
A.3.1. Movement of infected livestock
Movement of infected livestock was modelled by the following sequence of steps. For each farm with infected cattle or sheep:
determine the number of batches of animals moved off the farm that day, which depends on the number of animals on the farm and on the month;
for each batch, determine the batch size (i.e. number of animals moved) and then determine the number of infected animals in the batch (sampling without replacement);
-
if there is at least one infected animal in the batch, determine where it is moved to:
-
1
– select the county to which the batch is moved based on the relative frequency of movements from the county in which the farm is located to all counties (including that in which the farm is located);
-
2
– select a herd or flock at random from the county and test if it buys‐in animals that day (repeating as necessary until a farm does buy‐in animals), where the probability depends on the number of animals on the recipient farm and on the month;
-
3
– if the herd or flock buying‐in animals is uninfected, it acquires infection (i.e. the number of infected animals in the batch).
-
1
The distributions and parameters required for each step are described in detail bellow. Parameters were estimated using data on cattle and sheep movements for GB (Tables A.3 and A.4).
Table A.3.
Parameters in the logistic regression models for the probability of a farm moving (off‐move) or receiving (on‐move) cattle and sheep
| Parameter | Moving | Receiving | ||
|---|---|---|---|---|
| Cattle | Sheep | Cattle | Sheep | |
| Intercept | −4.77 | −4.71 | −4.95 | −6.33 |
| No. animals | 1.96 × 10−3 | 4.48 × 10−4 | 2.13 × 10−3 | 3.38 × 10−4 |
| Month | ||||
| January | 0 | 0 | 0 | 0 |
| February | 0.15 | −0.15 | 0.20 | −8.21 × 10−3 |
| March | 0.27 | −0.19 | 0.31 | 0.17 |
| April | 0.46 | −0.13 | 0.54 | 0.11 |
| May | 0.46 | 4.44 × 10−3 | 0.58 | 9.42 × 10−2 |
| June | 0.25 | 4.96 × 10−3 | 0.36 | −0.15 |
| July | 0.13 | 6.30 × 10−2 | 0.21 | 0.18 |
| August | 0.19 | 0.48 | 0.22 | 0.11 |
| September | 0.29 | 0.77 | 0.35 | 0.19 |
| October | 0.42 | 0.70 | 0.53 | 0.16 |
| November | 0.48 | 0.51 | 0.50 | 0.78 |
| December | 1.31 × 10−2 | 4.52 × 10−2 | −1.99 × 10−3 | 0.11 |
Table A.4.
Probability distribution for the number of batches of cattle or sheep moved off a farm which moves any livestock on a given day
| No. batches | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Cattle | 0.62 | 0.18 | 0.09 | 0.05 | 0.03 | 0.02 | 0.01 |
| Sheep | 0.97 | 0.03 | 0 | 0 | 0 | 0 | 0 |
Step 1: Number of off‐moves. The probability that a farm moves a batch of animals off the farm (i.e. makes an off‐move), p OFF , is given by,
| (A5) |
where α0 and α1 are constants, H is the herd or flock size and α2(m) is the log odds ratio for selling animals in month m (reflecting seasonality in animal movements) (Table A.3). If a farm does make an off‐move, the number of off‐moves made that day is drawn from a multinomial distribution, which was computed empirically from the observed number of off‐moves (Table A.4).
Step 2: Batch size distribution. For each off‐move the batch size (B) was determined by sampling from a negative binomial distribution, that is,
| (A6) |
where μ and k are the mean and dispersion parameter, respectively. These were estimated by fitting the distribution to the observed batch sizes (cattle: μ = 2.02, k = 0.33; sheep: μ = 38.2, k = 0.66). The number of infected animals in the batch (J) are drawn from a hypergeometric distribution, so that,
| (A7) |
where H is the herd or flock size and Y is the number of infected animals in the herd or flock (determined from the simulated within‐farm outbreak).
Step 3a: Selecting a county for an on‐move. The county for each on‐move was selected based on the relative frequency of movements from the county in which the affected flock is located to all counties.
Step 3b: Probability of an on‐move. The probability that a farm makes an on‐move (i.e. buys in animals), pON, was given by,
| (A8) |
where β0 and β1 are constants, N is the herd or flock size and β2(m) is the log odds ratio for buying‐in animals in month m (reflecting seasonality in animal movements) (Table A.3).
A.3.2. Dispersal of infected vectors
Dispersal of infected vectors between farms was modelled as a diffusion process (Backer and Nodelijk, 2011). As such, the dependence of the probability of transmission by this route on distance between farms reflects the diffusive movement of vectors. In addition, the probability of transmission allows for seasonal variation in vector activity and, importantly, incorporates the probability that a dispersing midge will survive for long enough to reach an at‐risk farm. In this case, the force of infection of farm j infected onfarm k on day t was given by
| (A9) |
where γ is the transmission parameter, τ j is the day on which infectious vectors were first present on farm j, θ(t) is seasonal vector activity (given by equation (A3)), I(t) is the number of infectious vectors on the farm, μ(T(t)) is the (temperature‐dependent) vector mortality rate (see Table A.1), D is the diffusion coefficient and x jk is the distance between the farms.
The vector dispersal parameters (γ and D) were estimated by fitting the BTV model to summary outbreak data for GB in 2007 (Defra, 2008) using ABC SMC sampling (McKinley et al., 2009; Toni et al., 2009). The posterior mean (95% credible interval) for γ was 0.57 (0.25, 0.92) while for D it was 2.57 (0.57, 4.65) km2/day.
A.4. Host demography
Cattle. Natural (i.e. non‐BTV‐associated) mortality was assumed to occur at a constant rate in a herd (equal to the reciprocal of the mean life expectancy, assumed to be 5 years). Disease‐associated (i.e. BTV‐related) mortality was assumed to occur at a constant rate while an animal was infected. Host reproduction in cattle was assumed to be continuous, with the number of replacements born each day chosen to restore the herd size to its initial level.
Sheep. Natural mortality was assumed to occur at a constant rate in a flock (equal to the reciprocal of the mean life expectancy, assumed to be 4 years). Disease‐associated mortality was assumed to occur at a constant rate while an animal was infected. Host reproduction in sheep was assumed to be seasonal with a single period of births each year. For simplicity, this was represented in the model as a single pulse on a particular day each year, with the number of replacements chosen to restore the flock size to its initial level. The timing of the pulse for each flock was generated by sampling a month of birth based on reported lambing patterns for each country (see EFSA, 2012, their appendix H) and then sampling a day uniformly from that month.
A.5. Overwintering of BTV
Overwintering of BTV was assumed to occur only through vertical transmission in the ruminant host. It was assumed not to occur through long‐lived adult infected vectors. For Culicoides obsoletus, this was reflected in the model by assuming all adult vectors die at the end of each vector season. For Culicoides imicola, this was not applied, but temperatures are such the vector mean life‐span is typically less than 40 days in winter and much shorter during summer.
Vertical transmission of BTV in the ruminant host was modelled as follows. The number of animals of species i infected via vertical transmission on day t was assumed to depend on the number of infected animals at the time of conception, the probability of vertical transmission and the probability of the dam surviving the gestation period, so that,
where Y (i)(t) is the number of infected hosts at time t, is the initial herd or flock size, H i(t) is the total number of animals at time t, p i is the probability of vertical transmission in an infected host, ψi is the natural host mortality rate and is the duration of the gestation period (assumed to be 280 days for cattle and 150 days for sheep). For cattle, vertical transmission occurred throughout the year, while for sheep it occurred only on the day on which seasonal reproduction occurred. The probability of vertical transmission in cattle and sheep was assumed to be 10% (De Clercq et al., 2008).
A.6. Vaccination
If vaccination was implemented, an additional vaccinated class (V (i)) was included for each species, with animals in this class assumed to be immune to infection with BTV.
Vaccination was assumed to be implemented for all farms on the 1 May in the year following the initial incursion and on the 1 May for a number of years subsequently (up to a further 4 years). We assume that vaccinated animals will be fully protected before virus circulation resumes in a region. In the first year of the vaccination campaign, each farm is vaccinated with probability given by the farm‐level coverage. If the farm is vaccinated, an animal of species i is moved into the vaccinated (and protected) class with probability given by the vaccine effectiveness for the species (εi). In subsequent years, a farm which has vaccinated previously was assumed to revaccinate all animals, with the probability that an animal is protected given by 1 − (1 − εi)y (where y is the number of years for which vaccine has been used). This increase in vaccine effectiveness over time is used to allow for an increase in effectiveness following repeat vaccination. For farms which did not vaccinated previously, they do so in the next year with probability given by the farm‐level coverage and with vaccine effectiveness as described above.
Farm‐level coverage was assumed to be 80% or 95%. Vaccine effectiveness for each species (εi) was sampled uniformly from ranges based on the outcome of challenge experiments (Gubbins et al., 2012). For cattle, the range was 0.60–0.85, while for sheep it was 0.89–1.0.
A.7. Applying the model to other EU member states
When the model was applied to EU member states other than GB, it was modified in two ways. First, transmission between farms was via dispersal of infected vectors alone. Transmission by movement of infected animals was excluded (Section 3.1), because the necessary data to parameterise this part of the model could not be obtained. However, this route accounts for only a small proportion of spread (around 10%) and the focus of the modelling in this opinion was on the temporal rather than spatial dynamics of BTV infection. Second, the vector parameters were adapted to reflect the principal Culicoides vectors in the country. The principal vector species in France are the same as in GB and, accordingly, the same parameter values were used (Table A.1). For Spain and Italy, however, the principal vector species is Culicoides imicola and, hence, parameter values appropriate to this species were used (Table A.5).
Table A.5.
Parameters for the transmission of bluetongue virus by Culicoides imicola, where different from those in Table A.1
| Description | Symbol | Estimate, distribution or function | Comments and references | |
|---|---|---|---|---|
| Probability of transmission from host to vector | β | β(1.02, 232.1) | Distribution derived from data on experimental infection of field‐caught C. imicola with BTV‐1, 2, 4 or 8 (Del Rio et al., 2012) | |
| Reciprocal of the time interval between blood meals | a | a(T) = 0.00014T(T − 3.7) (41.9 − T)1/2 . 7 | Rate for C. sonorensis (Mullens et al., 2004) adjusted to reflect data for C. imicola (Veronesi et al. 2009) | |
| Extrinsic incubation period (EIP) | Mean | 1/v | v(T) = α(T − T min) | Reciprocal of mean EIP Depends on temperature (Carpenter et al., 2011); distributions derived from data on experimental infection of field‐caught C. imicola with BTV‐1 (Carpenter et al., 2011); k is constrained to take integer values |
| No. stages | k | γ(1.12, 83.33) | ||
| Virus replication rate | α | N(0.016, 0.0026) | ||
| Threshold temperature for virus replication | T min | N(12.60, 1.17) | ||
| Vector activity | b 11 | −1.5 | Selected so that there is a single peak of activity in September–October | |
| b 21 | −0.1 | |||
| b 12 | 0 | |||
| b 22 | 0 | |||
Appendix B – Scenarios of bluetongue dynamics in vaccinated population in France, Italy and Spain
1.
As indicated in Section 3.1.2 of this opinion, in this Appendix the figures of the simulations performed for the scenarios in France, Italy and Spain are reported (Figures B.1, B.2, B.3, B.4, B.5, B.6, B.7, B.8–B.9). The spread between farms was via dispersal of infected vectors only and that via movement of infected animals was not included in the simulations. The model was run for 5 years following the initial incursion. For France, the model was applied to the whole of the country, with an incursion into a randomly selected farm in Nord‐Pas‐de‐Calais, Lorraine or Champagne‐Ardenne (chosen to reflect the previous incursion of BTV‐8). For Italy, the model was applied to Sardinia alone, with an incursion into a randomly selected farm in the region. Finally, for Spain, the model was applied to Andalusia alone, with an incursion into a randomly selected farm in the region.
Figure B.1.
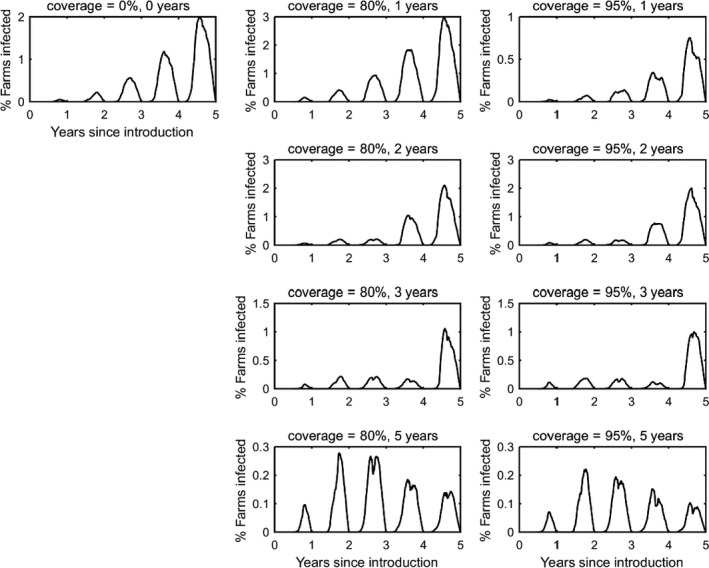
Prevalence of infected farms in simulated epidemics of bluetongue in France (following an incursion into the north‐east of the country) and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Figure B.2.
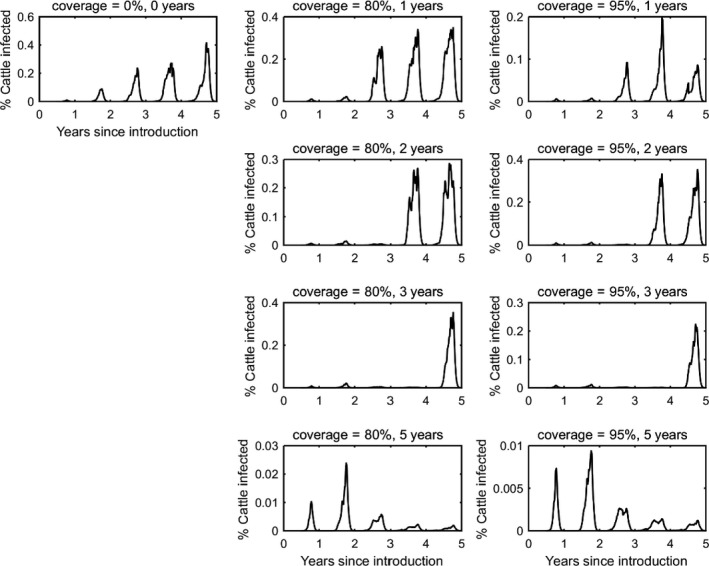
Prevalence of infected cattle in simulated epidemics of bluetongue in France (following an incursion into the north‐east of the country) and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Figure B.3.
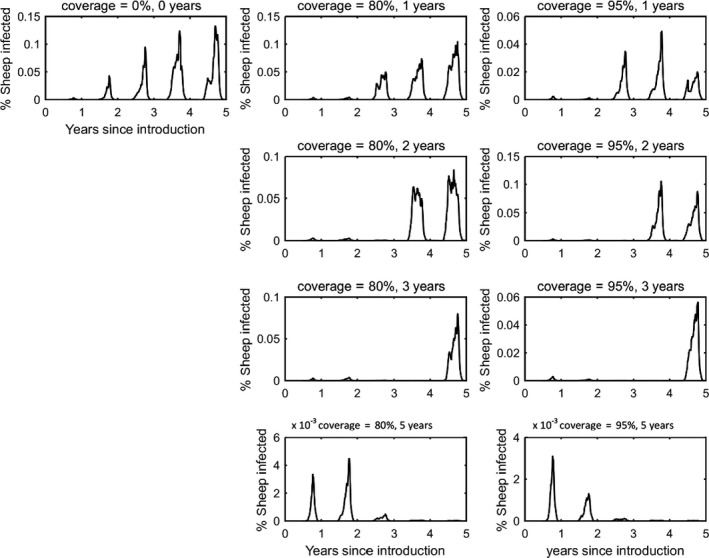
Prevalence of infected sheep in simulated epidemics of bluetongue in France (following an incursion into the north‐east of the country) and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Figure B.4.
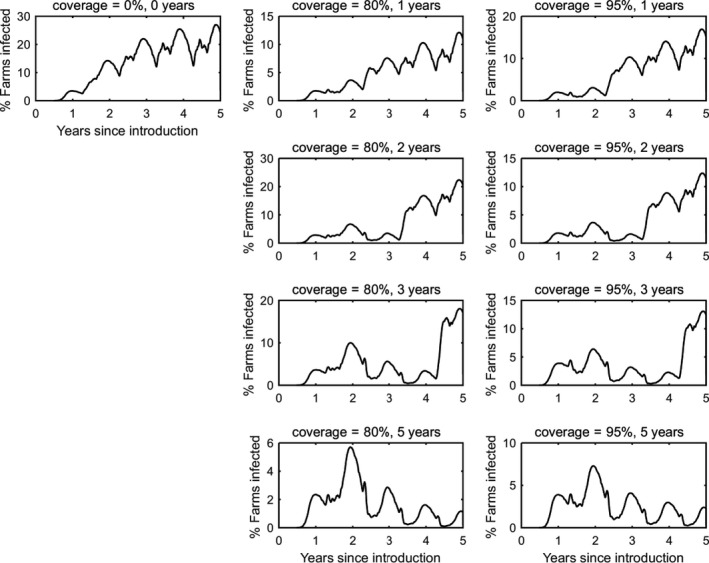
Prevalence of infected farms in simulated epidemics of bluetongue in Andalusia and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Figure B.5.
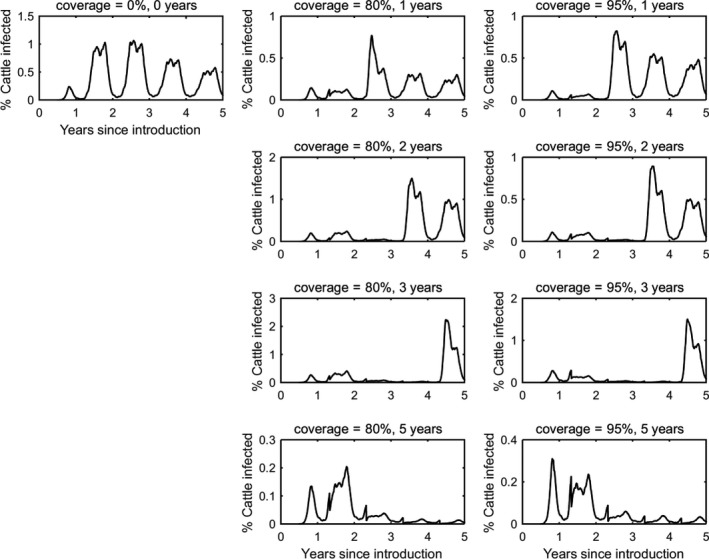
Prevalence of infected cattle in simulated epidemics of bluetongue in Andalusia and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Figure B.6.

Prevalence of infected sheep in simulated epidemics of bluetongue in Andalusia and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Figure B.7.
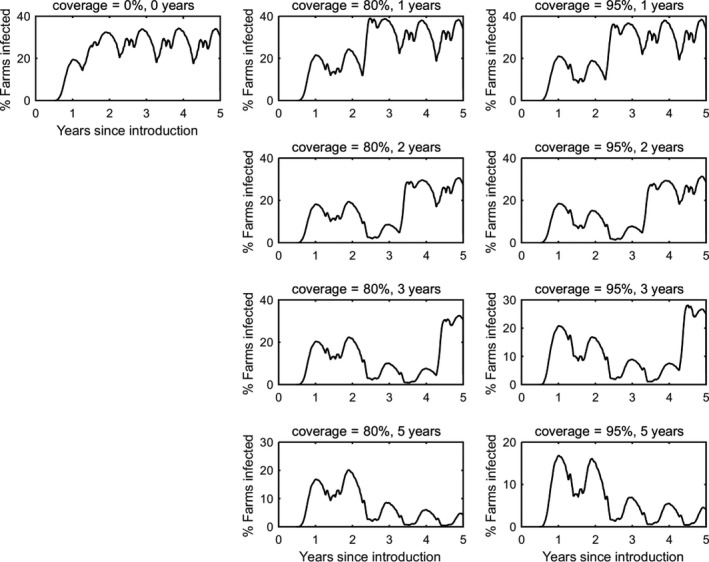
Prevalence of infected farms in simulated epidemics of bluetongue in Sardinia and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Figure B.8.

Prevalence of infected cattle in simulated epidemics of bluetongue in Sardinia and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Figure B.9.
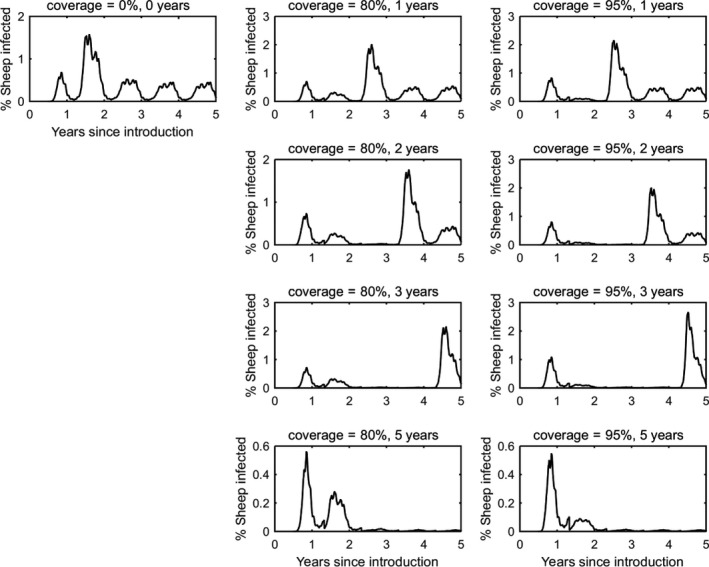
Prevalence of infected sheep in simulated epidemics of bluetongue in Sardinia and the impact of vaccination
- The vaccination strategy simulated is indicated in the title for each panel (farm‐level coverage and number of years for which vaccine is used). Each plot shows the mean prevalence (%) based on 100 replicates of the model.
Appendix C – Data about min protection day extracted from the studies selected as eligible for review question 3 related to ToR 2.3
1.
| Animal species | Study ID and references | Vaccine | Challenge day (post‐vac) | Min protection day | Max protection day | Titre | Comments |
|---|---|---|---|---|---|---|---|
| Spanish Ibex (Capra pyrenaica) | 15173 (Lorca‐Oro et al., 2012) |
Commercial inactivated vaccine Syvazul 1 & Syvazul 8 BTV‐1/BTV‐8 |
Yes, 32 days | 27 days using ELISA and SNT | 60 days |
From 6 to 11.5 SN titre (log2) |
|
| Spanish Ibex (Capra pyrenaica) | 15234 (Lorca‐Oró et al., 2014) |
Commercial inactivated vaccines Syvazul 1 BTV‐1 and Syvazul 8 BTV‐1/BTV‐8 |
No | 120 days | 840 days |
From 3 to 8 log2 SNT |
|
| Goats | 15013 (Di Emidio et al., 2004) | Experimental inactivated BTV‐2 vaccine | No | 14 days | 365 days |
From 1.5 to 2.5 SNT log10 |
|
| Goats | 15048 (Perrin et al., 2007) | Experimental BTV‐2 Vaccine‐ Recombinant BTV‐Cpox NS3 vaccine | Yes, 21 days | No info | No info | No info | |
| Goats | 15128 (Breard et al., 2011) |
Commercial vaccines BTVPUR ALSAP 8 & BOVILIS BTV‐8 |
Yes, 49 days | 10 days when using Antibodies – IP and 21 days when using SNT | No info |
From 0.5 to 3 SNT log10 |
|
| Sheep | 15009 (Hammoumi et al., 2003) | Vaccine produced by Onderstepoort Biological Products, live‐attenuated virus Vryheid prototype strain | No | 14 days after vaccination | No info | Varied from 5 IP (7 days post‐vaccination) to 75 IP (28 days post‐vaccination) | |
| Sheep | 15013 (Di Emidio et al., 2004) |
Live‐attenuated experimental To summarise the results achieved when an experimental inactivated BTV‐2 vaccine is administered to sheep, goats and cattle |
Yes, one group only, 137 days | 14 days | No info | 1.5 SNT titre (log10) (at 14, 28, 60 and 137 days after vaccination) | |
| Sheep | 15030 (Savini et al., 2004b) |
Vaccine produced by Onderstepoort Biological Products BTV‐2 BTV‐9 |
No | 6 days after vaccination when using Antib‐IP | No info | Not clear | |
| Sheep | 15032 (Stelletta et al., 2004) | Vaccine produced by Onderstepoort Biological Products (live‐attenuated virus BTV‐2) | No | 21 days ELISA | No info |
NOT CLEAR (Results in OD) ELISA |
The vaccine did not seem to work |
| Sheep | 15042 (Ramakrishnan et al., 2006) | Experimental BTV‐18 vaccine | Yes, 42 days | No further info | No info | No further info | |
| Sheep | 15044 (Boone et al., 2007) | Experimental infection BTV‐17, Synthetic genes encoding the VP2 and VP5 proteins of BTV‐17 were used in the construction of a recombinant canarypox virus vector vaccine (BTV‐CP) | Yes, 56 days | 14 days post‐vac based on SNT | No info | From 3 to 107 (expressed as reciprocal of the highest dilution that provided > 50% protection of the BHK‐21 cell monolayer) | |
| Sheep | 15045 (Breard et al., 2007) |
Commercial live‐attenuated vaccine BTV‐2 Vaccine produced by Onderstepoort Biological Products |
No | 14 days post‐vac based on ELISA | No info | No further info | |
| Sheep | 15048 (Perrin et al., 2007) | Experimental BTV‐2 vaccine – recombinant BTV‐Cpox NS3 vaccine | Yes, 21 days | 14 days post‐vac based on ELISA | No info | Results were expressed as OD measured at 492 nm. Values were considered significant when ODs were higher than the mean value plus two standard deviations of ODs at day 0 | |
| Sheep | 15050 (Savini et al., 2007) | BTV‐16 Experimental killed vaccine | Yes, 78 days | 14 days post‐vac | No info |
From 7 logs at day 14 post‐vac to 8.5 logs at day 47 post‐vac log(ED50%/50 mL) |
|
| Sheep | 15058 (Dungu et al., 2008) | Live‐attenuated BTV‐8 | Yes, at days 28 and 56 | 14 days post‐vac based on SNT | No info | From 42 to 1,280 (serial dilutions) | |
| Sheep | 15073 (Eschbaumer et al., 2009) |
To determine the level of protection conveyed by the three vaccines employed in Germany when applied under field conditions against an experimental challenge infection with BTV‐8: BTVPUR ALSAP 8 Zulvac 8 Ovis BLUEVAC 8 |
Yes, 90 days | No info | No info |
In one group – From 3.2 to 30.4 ND50 Other groups – No results, it looks like most animals were positive after challenge |
It seems the vaccines did not work |
| Sheep | 15075 (Gethmann et al., 2009) |
To provide information on the safety of three different commercial vaccines: BTVPUR ALSAP 8 Zulvac 8 Ovis BLUEVAC 8 |
No | 21 days based on ELISA | 70 days | ||
| Sheep | 15076 (Hamers et al., 2009a) | Commercial vaccine BTV‐8BTVPUR ALSAP 8 | Yes, 31 days | 14 days | No info | From 0.6 log10 PD50 (14 days) to 3.3 log10 PD50 at 45 days post‐vac | |
| Sheep | 15077 (Hamers et al., 2009b) | Merial vaccine killed BTV‐2 | Yes, 364 days | 14 days | No info |
It varies between 1.4 and 1.8 log10 PD50 at different times between 14 and 378 days With 2 doses, it varies between 1.8 and 2.6 log10 PD50 at different times between 14 and 378 days |
|
| Sheep | 15082 (Oura et al., 2009) |
Commercial killed vaccine BTV‐8 Bovilis BTV‐8 |
Yes but no further information on the paper | No info | No info | No information regarding protection | Some animals presented clinical signs of infection |
| Sheep | 15118 (Stewart et al., 2010) |
Experimental A baculovirus genome was produced which contained genes encoding the inner capsid proteins of BTV and a selectable marker. The genome was used as a basis to insert the VP2 and VP5 genes for several European serotypes of BTV. VLPs representing BTV‐2 were purified and used to elicit protective immunity. BTV‐2Sar = VLP. |
Yes, 49 days post‐vac | No info | No info | No information regarding protection | Some animals recorded as infected |
| Sheep | 15130 (Perez de Diego et al., 2011) |
Experimental, 2 vaccines: One monovalent BTV‐1 VLP vaccine and another vaccine bivalent BTV‐1 and BTV‐4 VLP |
Yes, 48 days | 35 days with ELISA and 48 days with SNT |
Different results depending on the test, some not reported… Neutralisation titre Reported varied from 3 to 118 |
||
| Sheep | 15142 (Matsuo et al., 2011) |
Experimental, 2 vaccines: BTVE1 Disabled Infectious Single Cycle virus BTVD2 Disabled Infectious Single Cycle virus |
Yes, 42 days | 7 days based on ELISA/21 days based SNT | 70 days | From 1.5 to 7.9 | |
| Sheep | 15167 (van Gennip et al., 2012) |
Experimental, 4 vaccines: BTVac‐1 BTVac‐6 BTVac‐8 Combivac |
Challenge with virulent BVT8, 21 days post‐vac |
Depending on the method: when using PCR positive animals detected at days 2, 3 post‐vaccination When using ID vet Antibodies – IP were detected at days 6, 8 post‐vac and when using Antibodies – SNT MIN day was at day 21 post‐vac |
42 days post‐vac | Very variable titre values (from 3 to 1,745) | |
| Sheep | 15177 (Modumo and Venter, 2012) |
Different experimental vaccines BTV‐2 BTV‐8 |
Yes, 120 days | When using ELISA, Min 9 days and when using seroneutralisation test – 28 days | When using ELISA, Max 21 days and when using seroneutralisation test – 120 days | No further info | |
| Sheep | 15179 (Moulin et al., 2012) |
Commercial killed vaccine ‘Bovilis® BTV8’ |
Yes, 21 days | 21 days | No info | The SN titres of the test samples were given as log2 of the reciprocal of the highest dilution where all virus particles were neutralised (no CPE). Titres varied from 1 to 4 | |
| Sheep | 15184 (Stewart et al., 2012) |
Experimental vaccines To compare the protective efficacy afforded by BTV‐1 (RSA strain) VLPs and CLPs derived from a western lineage and challenged with eastern lineage virulent BTV‐1 (Greece strain). |
Yes, Day 53 post‐vac | 21 days when using ELISA 26 days when using SNT | 83 days post‐vac | One of the vaccines worked better from 84 to 1,024 Neutralisation titre | |
| Sheep | 15186 (Top et al., 2012) |
Experimental To evaluate the immune response and protection provided by two MYXV vectors, one that expressed VP2 alone and one that expressed a combination of the VP2 and VP5 proteins of BTV‐8, after homologous challenge with a highly virulent BTV‐8 strain |
Yes, 45 days | 45 days (SNT) | 65 days | No data | |
| Sheep | 15199 (Leemans et al., 2013) |
Commercial vaccine BTVPUR ALSAP® 8 given at 9 months of age |
At different days, 30, 90, 150, 210 days post‐vac | 30 days post‐vac with SNT | 224 days | No info | |
| Sheep | 15208 (van der Sluijs et al., 2013a) | Commercial killed vaccine Bovilis® BTV‐8 | 21 days | 21 days | 43 days | From 0.7 to 7.6 antibody titre (log2) | |
| Sheep | 15210 (Stewart et al., 2013) |
Experimental vaccines To develop BTV‐8 VLP and assess its protective efficacy in BTV‐susceptible sheep either singularly, or in a cocktail with VLPs, with two other serotypes (BTV‐1 and ‐2) |
Yes, 42 days | 10 days when using Antib‐IP and 42 days when using SNT | 65 days |
From 95 to 130 Neutralisation titre |
|
| Sheep | 15212 (Thuenemann et al., 2013) |
Experimental To describe the plant‐based production and assembly of Bluetongue virus‐like particles (VLPs) and their efficacy when used as a vaccine in sheep |
Yes, 63 days | 7 days when using (BTV)‐specific neutralising antibodies were measured according to the procedure of the serum neutralisation test as described in the Office International des Epizooties (OIE) Manual of diagnostic tests and vaccines for terrestrial animals. Antibody titres are expressed as the reciprocal of the serum dilution causing a 50% reduction in cytopathic effect and are calculated using the Spearman–Karber method | 91 days |
Very variable from 0.8 to 2969.6 Neutralising antibody titre |
|
| Sheep | 15229 (Feenstra et al., 2014a) |
Experimental To demonstrate that exchange of only VP2 induces serotype‐specific protection at 9 weeks post‐vaccination in sheep, and to show the ability of DIVA with an experimentally developed NS3 ELISA |
Yes, 84 days | 7 days when using ELISA and 21 days when using SNT | 105 days | No info | |
| Sheep | 15230 (Feenstra et al., 2014b) |
Experimental To generate and test next‐generation vaccines for bluetongue based on the backbone of a laboratory‐adapted strain of BTV‐1, avirulent BTV‐6 or virulent BTV‐ 8 |
21 days | 7 days when using ELISA and 21 days when using SNT | 42 days | No info | |
| Sheep | 15233 (Kochinger et al., 2014) |
Experimental To determine the efficacy of propagation – incompetent VSVΔG vectors Against BTV‐8 |
Yes, day 42 | 21 days when using SNT | 42 days | No info | |
| Sheep | 15239 (Nunes et al., 2014) |
Experimental To describe the development of a strategy for the design and production of inactivated BTV vaccines that can significantly reduce the time taken from the identification of a new BTV emerging strain to the development and production of a new vaccine Against BTV‐8 |
21 days |
21 days The 50% protective dose (PD50) for each serum sample, defined as the serum dilution that inhibits BTV infection in 50% of Vero cell cultures, was determined by using a linear regression after angular transformation. Samples below the detection limit of 0.48 log10 PD50 were considered negative |
35 days |
From 0.53 to 1.84 log10 PD50 |
|
| Sheep | 15254 (Breard et al., 2015) |
Experimental To evaluate the potential protector effect (or not) of three inactivated vaccines in a BTV‐8 spread context (no inactivated vaccine against this serotype was available) and also against a BTV‐16 emergence |
Yes, day 42 | Day 21 | 56 days |
From 0.75 to 1.68 SNT |
|
| Sheep | 15267 (Li et al., 2015) |
Experimental, to determine whether the strategy combining the DNA vaccine pCAG‐(VP2 + VP5) prime and the rFPV‐(VP2 + VP5)boost induced an effective immune response to BTV‐1 in sheep |
No |
21 days – Antib‐IP 28 days when using SNT |
42 days |
2.4–2.5 SNT |
|
| Sheep | 15270 (Martin et al., 2015) |
Experimental To determine the efficiency of vaccination with recombinant adenoviruses in sheep |
Yes, 30 days | 15 days | 45 days |
From 1 to 4 SNT |
|
| Sheep | 15278 (Zhugunissov et al., 2015) |
Experimental To develop and test an attenuated bivalent vaccine against BTV, and examine the protection it confers after a single immunisation BTV‐4/BTV‐16 |
Yes, at 7, 90, 270, 360 days |
7 days when using cELISA, ID Screen Bluetongue Not reported when using SNT |
No info |
From 1 to 4.5 SNT log2, VNA titre (only reported as a figure – estimated from the figure) |
|
| Cattle | 15013 (Di Emidio et al., 2004) |
Live‐attenuated experimental To summarise the results achieved when an experimental inactivated BTV‐2 vaccine is administered to sheep, goats and cattle |
No | 14 days | No info | From 1 to 2.5 log10 at days 14, 28 and 60 post‐vac | |
| Cattle | 15021 (Monaco et al., 2004a) |
Commercial live‐attenuated vaccine produced by Onderstepoort Biological Products To evaluate the immunogenicity, innocuity, efficacy and possible teratogenic effect of monovalent BTV‐2 modified live vaccine in cattle |
Some groups challenged at 420 days | 21 days based on SNT | No info |
From 20 to 1,300 Mean logarithmic titre |
|
| Cattle | 15023 F (Monaco et al., 2004b) |
Commercial live‐attenuated vaccine produced by Onderstepoort Biological Products BTV‐2/BTV‐9 To evaluate duration and levels of viraemia and antibody kinetics in cattle after immunisation with a bivalent BTV vaccine and to determine whether vaccinated cattle serve a s a source of BT vaccine virus to blood‐sucking arthropods |
No | 4 days when using this method: IV egg inoculation followed by 2 blind passages in Vero cells was used to isolate BTV from blood samples 9 days when using SNT | No info |
From 10 to 190 SNT |
|
| Cattle | 15030 (Savini et al., 2004b) | Commercial vaccine produced by Onderstepoort Biological Products BTV‐2/BTV‐9 | No | 9 days when using ELISA Antibodies – IP | No info |
From 5 to 275 SNT |
|
| Cattle | 15066 (Barros et al., 2009) |
Commercial inactivated BTVPUR ALSap 2‐4 (Merial) BTV‐2/BTV‐4 |
Yes, 60 days | 14 days BTV‐4 and 21 days BTV‐2 | No info |
From 0.8 to 3.3 SNT log10 |
|
| Cattle | 15075 (Gethmann et al., 2009) |
3 commercial vaccines: BLUEVAC 8 Zulvac 8 Bovis BTVPUR ALSAP 8 |
No | 21 days all vaccines | 70 days | No info | |
| Cattle | 15076 (Hamers et al., 2009a) |
Commercial vaccine BTV‐8 BTVPUR ALSAP 8 |
Yes, 51 days | 14 days | No info | From 0.6 log10 PD50 (14 days) to 2.5 log10 PD50 at 79 days post‐vac | |
| Cattle | 15086 (Savini et al., 2009) |
Commercial vaccine produced by Merial BTV‐2/BTV‐4 |
Yes, 65 days post‐vac | 14 days when using SNT | No info | No info | |
| Cattle | 15124 (Wäckerlin et al., 2010) |
3 commercial vaccines: BLUEVAC 8 Zulvac 8 Bovis BTVPUR ALSAP 8 |
Yes, 365 days | No info | 365 | No info | |
| Cattle | 15191 (Anderson et al., 2013) | To characterise the immunogenicity of an experimental vaccine BTV‐8 and to compare it with that of a commercial inactivated vaccine (CV) in cattle BTV Pur Alsap 8 | No | 21 days when using this method: Specific antibodies to BTV‐8 VP2 were analysed using commercially available competitive ELISA and double‐antigen sandwich ELISA kits (ID Screen Bluetongue serotype 8 competition (ID Vet, France) and ID Screen Bluetongue early detection (ID Vet) kits, respectively), according to the manufacturer's protocols. Results were expressed as per cent inhibition (1 (ODsample/ODnegative control)) (VP2) or as 100% minus the competition percentage (ODsample/ODpositive) (VP7) and 42 days when using this other method: The range of dilutions was 1:4–1:512, and 8,000 Vero cells were added per well, in 100 L minimal essential medium (Gibco, UK) supplemented with 1% minimal essential amino acids (Gibco) and 1% HEPES (Gibco). Sera were tested in duplicate, and the neutralising titre of each serum sample was defined as the highest dilution allowing neutralisation of 100 50% tissue culture infective doses (TCID50) of BTV‐8 | 63 days | No info | |
| Cattle | 15195 (Celma et al., 2013) |
Experimental 3 vaccines: BTV‐2D – defective BTV‐2 virus BTV‐4D – defective BTV4 virus BTV‐8D – defective BTV‐8 virus |
Yes, 42 days | 7 days when using this method: Serum samples were analysed with the ID Screen Bluetongue Early detection kit (ID VET, Montpellier, France) according to the manufacturer's instructions. In addition to the kit controls, a twofold dilution series of an anti‐BTV antibody‐positive reference serum (CIRAD, Montpellier, France) was included for the competition assay as the working standard in each assay to monitor the performance of the enzyme‐linked immunosorbent assay (ELISA) over time. Results are expressed as percentages of negativity (% Negativity) compared to the negative kit control results and converted to a positive (per cent sample‐to‐positive ratio (% S/P) 30), uncertain (% S/P 30 but 25), or negative (% S/P 25) result according to cutoff values previously determined | 63 | No info | |
| Cattle | 15201 (Martinelle et al., 2013) |
Commercial vaccine BTVpur Alsap 8 |
Yes, 78 days | 26 | 293 |
From 360 (at 26 days) to 1900 (at 293 days) SNT |
|
| Cattle | 15220 (Anderson et al., 2014) |
Experimental Recombinant VP2 of BTV‐8 and NS1 and NS2 of BTV‐2 were produced and purified. Each 2.5 mL subunit vaccine (SubV) dose contained 150 g each of purified VP2, NS1, and NS2 and 450 g AbISCO®‐300 (Isconova AB, Sweden), an immunostimulating complex (ISCOM)‐based adjuvant. To evaluate the clinical and virological protective efficacy of the experimental vaccine against virulent BTV‐8 challenge in cattle and to verify its DIVA compliancy using existing diagnostic assays |
Yes, 42 days | 28 days | 63 days | From 3.5 to 6 log2 antibody titre | |
| Cattle | 15266 (Legisa et al., 2015) | Experimental, inactivated BTV‐4 | No | 30 days | 90 days | From 2.7 to 3.8 log neutralising titre | Considered a lower quality paper than the rest |
| Cattle | 15285 (Martinelle et al., 2016) | Commercial, BTVPUR AlSAP 8 – animals vaccinated following manufacturer's directions | Yes, 75 days | 3 days when using a commercial competitive ELISA kit (ID Screen® Bluetongue Competition ELISA kit, ID Vet, France). Results were expressed as % of negativity (PN) compared to the negative kit control and transferred to a positive, doubtful or negative result according to the cut‐off settings provided by the manufacturer. 32 days when using this other method: The neutralising antibody titre was defined as the reciprocal of the serum dilution causing a 50% reduction in cytopathic effect. Serum samples with a titre < 20, = 20 and > 20 were considered negative, doubtful and positive, respectively | 250 days | No info |
Appendix D – Opportunity map for vector activity in Europe
1.
Opportunity map for vector activity in Europe considering minimum temperature above 10°C, where the blue zones represent the areas in which the temperature is considered to be hampering vector activity and the shades of green indicate number of days in the month in which conditions are favourable for vector activity (darker colours indicating longer periods in the month with favourable conditions), black represents regions with no information on temperature for that year.
Figure D.1.
Opportunity maps considering temperature from year 2009
Figure D.2.

Opportunity maps considering temperature from year 2010
Figure D.3.
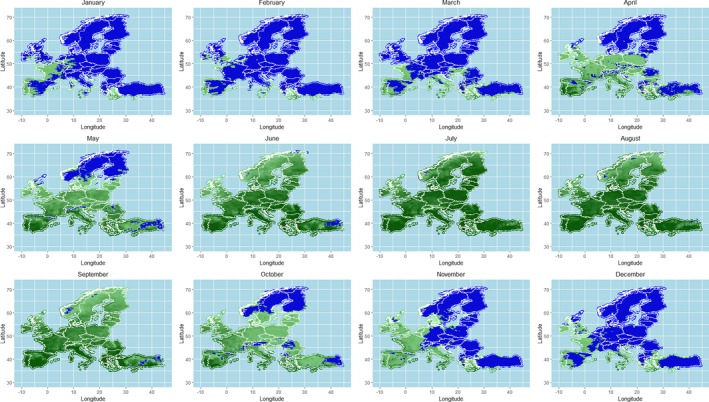
Opportunity maps considering temperature from year 2011
Figure D.4.
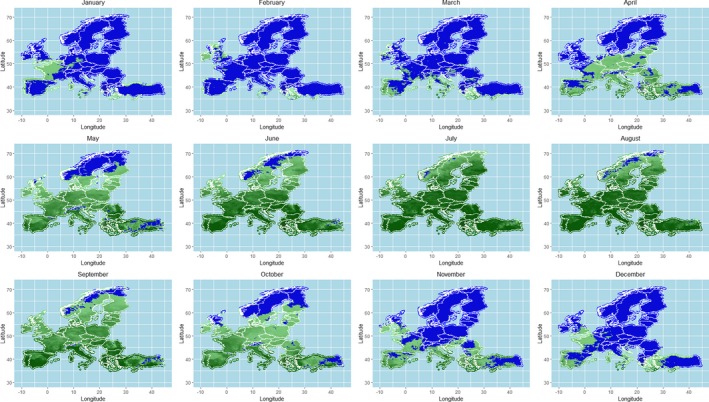
Opportunity maps considering temperature from year 2012
Figure D.5.
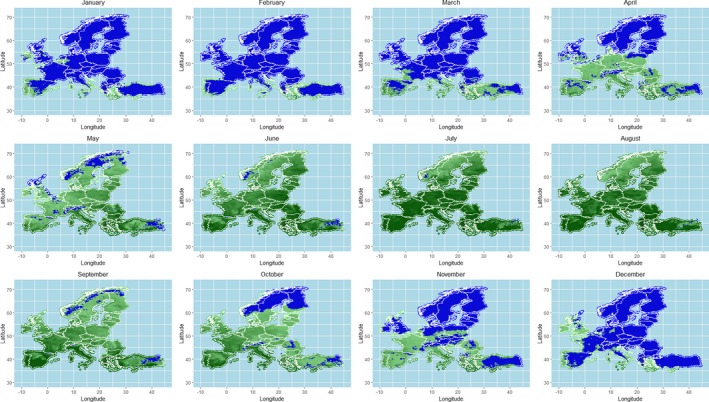
Opportunity maps considering temperature from year 2013
Figure D.6.
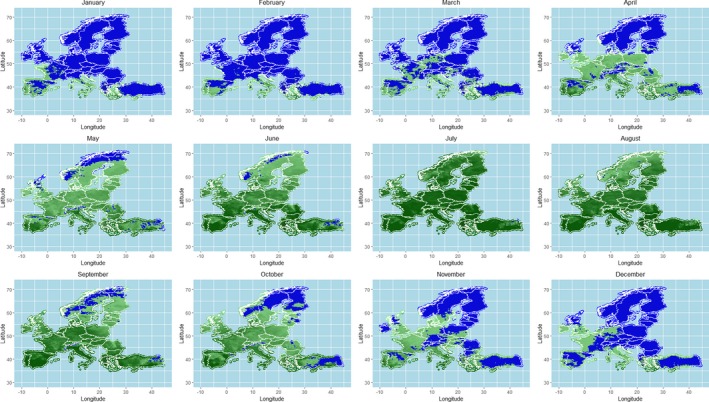
Opportunity maps considering temperature from year 2014
Appendix E – Biocidal products
1.
According to REG (EU) No 528/2012, concerning the making available on the market and use of biocidal products, active substances belonging to product type 18 and 19 for which midges are included under the target species are listed below in the Table E.1. Product type 18 (i.e. insecticides, acaricides and products to control other arthropods) is used for the control of arthropods (e.g. insects, arachnids and crustaceans) by means other than repulsion or attraction. Similarly, product type 19 (i.e. repellents and attractants) is applied to control harmful organisms (invertebrates such as fleas, vertebrates such as birds, fish, rodents), by repelling or attracting, including those that are used for human or veterinary hygiene either directly on the skin or indirectly in the environment of humans or animals.
Table E.1.
Data on EU approved active substances that are to be used for controlling the vectors species, for which midges are included under the target species
| Active substance (product type) | Target species | Intended uses | Application/dose rate (i.e. efficacyb) | Hazard statements (According to Reg. No 1272/2008a) | Risk characterisation ratios (According to REG No 528/2012, Annex VI) | Assessment report (link) |
|---|---|---|---|---|---|---|
| Bacillus thuringiensis subsp. israelensis Serotype H14, Strain AM65‐52 (18) | Larvae of mosquitoes (Aedes spp., Culex spp) and black flies + larvae of filter fly midges in sewage treatment plants |
Ground application: tractor‐mounted or handheld sprayer Aerial application: fixed wing or helicopter Applied during the first to the 4th larval instar |
Rates up to 500 g/ha (9 × 1012°CFU/ha) (mortality greater than 95% of the control was observed after 48 h) |
Limited survival in the environment Limited risk to human health, related only to the possibility to induce sensitisation, based on the results obtained on animal models |
EED/PNED ratio** at local level below 1 indicates NO risk for the environment | http://dissemination.echa.europa.eu/Biocides/ActiveSubstances/0005-18/0005-18_Assessment_Report.pdf |
| Deltamethrin (18) | Indoors: flying insects when at rest (e.g. flies and mosquitoes), black ants, bedbugs, fleas, earwigs, carpet beetles, booklice and cockroaches, as well as spiders and woodlice. Outdoors: ants | Indoors: spray applications, professional users only. Outdoors: directly around the nest entrance, by amateurs |
6.25 mg/a.s per m2 (1 month‐low‐dose rate)** 12.5 mg/a.s per m2 (3 months‐high‐dose rate)** |
H400/410: Aquatic Chronic. H331/H301:Acute tox 3, |
Aquatic compartment:
Terrestrial compartment:
|
http://dissemination.echa.europa.eu/Biocides/ActiveSubstances/0024-18/0024-18_Assessment_Report.pdf |
| Lambda‐cyhalothrin (18) | Flies and other insects in and around animal housing | For fly control, application is as a low‐pressure spray in areas where flies congregate or settle such as floors, walls, ceilings and around doors and windows. For other insects, the product is applied as a low‐pressure spray as a crack and crevice treatment | 25 mg/a.s. per m2 |
H400/410: Aquatic Chronic H312: Harmful in contact with skin H301 or H300: toxic or Fatal/if swallowed H330: Fatal if inhaled |
Aquatic compartment:
|
http://dissemination.echa.europa.eu/Biocides/ActiveSubstances/0041-18/0041-18_Assessment_Report.pdf |
| Permethrin (18) | Flying insects (e.g. flies and mosquitoes) and crawling insects (e.g. roaches, mites, fleas and ticks) | Indoor use (households* and commercial areas), by professional and non‐professional users against flying and crawling insects. Spot treatments | 0.000011 mg/a.s. per m2 | H410 (Acute Cat 1; Chronic Cat 1): Very toxic to aquatic life with long lasting effects. H317: May cause an allergic skin reaction |
|
http://dissemination.echa.europa.eu/Biocides/ActiveSubstances/1342-18/1342-18_Assessment_Report.pdf |
| N,N‐diethyl‐meta‐toluamide (19) | Biting flies, biting midges or black flies (Ceratopogonidae, Simuliidae), chiggers, deer flies, no‐seeums, gnats, horse flies (Tabanidae), mosquitoes (Culicidae), fleas | Aerosol spray, direct dermal application | NA |
H412: Aquatic Chronic 3 H302: Harmful if swallowed H315: Causes skin irritation H319: Causes serious eye irritation |
NO risk to any of the environmental compartments | http://dissemination.echa.europa.eu/Biocides/ActiveSubstances/0023-19/0023-19_Assessment_Report.pdf |
Regulation (EC) No 1272/2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006.
Efficacy of products will be assessed thoroughly at the stage of product authorisation. Moreover the conclusion was reached within the framework of the uses that were proposed and supported by the applicant (see each Assessment Report, Appendix II). Extension of the use pattern beyond those described will require an evaluation at product authorisation level in order to establish whether the proposed extensions of use will satisfy the requirements of Article 5(1) and of the common principles laid down in Annex VI to Directive 98/8/EC.).
Data from the approved active substances that are to be used for controlling the relevant vectors species were extracted, such as information on intended uses and efficacy (e.g. indoor/outdoor, professional/non‐professional use), the target species, the hazard Class Category (i.e. the toxicity, e.g. H400: very toxic to aquatic life).
Supporting information
Prometheus Protocol Bluetongue
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2017. Scientific opinion on bluetongue: control, surveillance and safe movement of animals. EFSA Journal 2017;15(3):4698, 126 pp. doi: 10.2903/j.efsa.2017.4698
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00160
Panel members: Miguel Angel Miranda, Jan Arend Stegeman, Dominique Bicout, Anette Botner, Andrew Butterworth, Paolo Calistri, Klaus Depner, Sandra Edwards, Bruno Garin‐Bastuji, Margaret, Good, Christian Gortazar Schmidt, Virginie Michel, Simon More, Mohan Raj, Søren Saxmose Nielsen, Lisa Sihvonen, Hans Spoolder, Hans H. Thulke, Antonio Velarde, Preben Willeberg and Christoph Winckler.
Acknowledgements: The Panel wishes to thank the members of the Working Group: Anette Bøtner, Miguel Angel Miranda, Paolo Calistri, Simon Carpenter, Simon Gubbins, Stephan Zientara, and the hearing expert Thomas Balenghien for the preparatory work on this scientific opinion and EFSA staff members: Alessandro Broglia, Ana Garcia, Andrey Gogin, Beatriz Beltran, Edoardo Carnesecchi, Ewelina Czwienczek, Francesca Baldinelli, José Cortiñas Abrahantes, Lisa Kohnle, Sofie Dhollander for the support provided to this scientific opinion. A special thanks to Lisa Cavalerie and Stéphanie Desvaux from Animal Health office, Directorate General for Food, French Ministry of Agriculture, Paris, France for the kind provision of data about bluetongue surveillance system in France.
Adopted: 18 January 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 11: Bulletin épidémiologique, santé animale et alimentation; Figure 12: © Ministère de l'agriculture, de l'agroalimentaire et de la forêt
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1182/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1171/full
Notes
Using one single or two doses (first and booster inoculation) according to vaccine's manufacturer instructions.
Commission Regulation (EC) No 1266/2007 of 26 October 2007 on implementing rules for Council Directive 2000/75/EC as regards the control, monitoring, surveillance and restrictions on movements of certain animals of susceptible species in relation to bluetongue. 2007R1266 ‐ EN ‐ 05.06.2012, p. 1–25.
Reports from the French national meteorological services, http://www.meteofrance.fr/climat-passe-et-futur/bilans-climatiques
For example, from Office National de la Chasse e de la Faune Sauvage ‘Le portail cartographique de données’ http://www.oncfs.gouv.fr/Cartographie-ru4/Le-portail-cartographique-de-donnees-ar291, or Lovari S, Herrero J, Conroy J, Maran T, Giannatos G, Stubbe M, Aulagnier S, 2009. Cervus elaphus. In: IUCN 2009, IUCN Red List of Threatened Species. http://www.iucnredlist.org/details/41785/0
By simplicity, several authors use the term Obsoletus group to refer to some species of the Avaritia subgenus, namely C. obsoletus, C. scoticus, C. dewulfi and C. chiopterus, for which females are relatively morphologically close. Moreover, other use the term of Obsoletus complex to refer to species for which females are not reliably possible to distinguished by morphology, namely at least C. obsoletus, C. scoticus and C. montanus. However, phylogenetic studies show that these assemblages are not phylogenetically consistent, and that the taxon C. obsoletus highlights an important cryptic diversity. Thus, we use the term ‘Obsoletus assemblage’ to refer by convenience to species morphologically close to C. obsoletus if they are grouped in the publication.
In order to calculate the design effect due to clustering, the within‐herd correlation reported in Belgium by Meroc et al. (2008) was used.
The titres are expressed as the log10 of the serum dilution causing a 50% reduction in cytopathic effect (log10 PD50).
Vectornet Project report http://ecdc.europa.eu/en/healthtopics/vectors/VectorNet/Pages/VectorNet.aspx
European Commission. Guidance document to assist Member States for the implementation of the criteria for ‘Vector Protected Establishments’ for bluetongue. https://ec.europa.eu/food/sites/food/files/animals/docs/ad_control-measures_bt_guidance_vpe_7068_2012.pdf
Conclusions related to BTV viraemia are addressed under ToR 1.2
References
- Alphey L, 2014. Genetic control of mosquitoes. Annual Review of Entomology, 59, 205–224. [DOI] [PubMed] [Google Scholar]
- Ander M, Meiswinkel R and Chirico J, 2012. Seasonal dynamics of biting midges (Diptera: Ceratopogonidae: Culicoides), the potential vectors of bluetongue virus, in Sweden. Veterinary Parasitology, 184, 59–67. [DOI] [PubMed] [Google Scholar]
- Anderson D and Watson R, 1980. On the spread of a disease with gamma distributed latent and infectious periods. Biometrika, 67, 191–198. [Google Scholar]
- Anderson J, Hagglund S, Breard E, Comtet L, Lovgren Bengtsson K, Pringle J, Zientara S and Valarcher JF, 2013. Evaluation of the immunogenicity of an experimental subunit vaccine that allows differentiation between infected and vaccinated animals against bluetongue virus serotype 8 in cattle. Clinical and Vaccine Immunology, 20, 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J, Hagglund S, Breard E, Riou M, Zohari S, Comtet L, Olofson AS, Gelineau R, Martin G, Elvander M, Blomqvist G, Zientara S and Valarcher JF, 2014. Strong protection induced by an experimental DIVA subunit vaccine against bluetongue virus serotype 8 in cattle. Vaccine, 32, 6614–6621. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Carpenter S and Butt TM, 2010. Susceptibility of Culicoides biting midge larvae to the insect‐pathogenic fungus, Metarhizium anisopliae: prospects for bluetongue vector control. Acta Tropica, 113, 1–6. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Pope EC, Carpenter S, Scholte EJ and Butt TM, 2011. Entomopathogenic fungus as a biological control for an important vector of livestock disease: the Culicoides biting midge. PLoS ONE, 6, e16108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSES (Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail), 2015. Avis relatif à « l’évaluation du risque lié à la réapparition du sérotype 8 de la FCO en France continentale. Saisine n°2015‐SA‐0226.
- Arenas‐Montes A, Paniagua J, Arenas A, Lorca‐Oro C, Carbonero A, Cano‐Terriza D and Garcia‐Bocanegra I, 2016. Spatial‐temporal trends and factors associated with the bluetongue virus seropositivity in large game hunting areas from Southern Spain. Transboundary and Emerging Diseases, 63, 339–346. [DOI] [PubMed] [Google Scholar]
- Ayllón T, Nijhof AM, Weiher W, Bauer B, Allène X and Clausen P‐H, 2014. Feeding behaviour of Culicoides spp.(Diptera: Ceratopogonidae) on cattle and sheep in northeast Germany. Parasites & Vectors, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer JA and Nodelijk G, 2011. Transmission and control of African horse sickness in The Netherlands: a model analysis. PLoS ONE, 6, e23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backx A, Heutink R, van Rooij E and van Rijn P, 2009. Transplacental and oral transmission of wild‐type bluetongue virus serotype 8 in cattle after experimental infection. Veterinary Microbiology, 138, 235–243. [DOI] [PubMed] [Google Scholar]
- Baddeley A and Turner R, 2005. Spatstat: an R package for analyzing spatial point patterns. Journal of Statistical Software, 12, 1–42. [Google Scholar]
- Baker T, Carpenter S, Gubbins S, Newton R, Lo Iacono G, Wood J and Harrup LE, 2015. Can insecticide‐treated netting provide protection for Equids from Culicoides biting midges in the United Kingdom? Parasit Vectors, 8, 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balczun C, Vorsprach B, Meiser CK and Schaub GA, 2009. Changes of the abundance of Culicoides obsoletus s.s. and Culicoides scoticus in Southwest Germany identified by a PCR‐based differentiation. Parasitology Research, 105, 345–349. [DOI] [PubMed] [Google Scholar]
- Balenghien T, Cetre‐Sossah C, Grillet C, Delecolle JC, Mathieu B and Baldet T, 2008. Diurnal activity of potential bluetongue vectors in northern Europe. Veterinary Record, 162, 323–324. [DOI] [PubMed] [Google Scholar]
- Balenghien T, Delécolle J‐C, Setier‐Rio M‐L, Rakotaoarivony I, Allène X, Venail R, Delécolle D, Lhoir J, Gardès L and Chavernac D, 2010. Bluetongue‐report on entomological surveillance in France in 2010. Bulletin épidémiologique, santé animale et alimentation, 46, 26–31. [Google Scholar]
- Balenghien T, Delécolle J‐C, Setier‐Rio M‐L, Rakotoarivony I, Allène X, Venail R, Delécolle D, Lhoir J, Mathieu B and Chavernac D, 2012. Vectors of bluetongue virus: follow‐up of Culicoides populations in 2011 in France. Bull Epidémiologique Santé Anim Aliment, 54, 35–40. [Google Scholar]
- Barros SC, Cruz B, Luis TM, Ramos F, Fagulha T, Duarte M, Henriques M and Fevereiro M, 2009. A DIVA system based on the detection of antibodies to non‐structural protein 3 (NS3) of bluetongue virus. Veterinary Microbiology, 137, 252–259. [DOI] [PubMed] [Google Scholar]
- Batten CA, Harif B, Henstock MR, Ghizlane S, Edwards L, Loutfi C, Oura CA and El Harrak M, 2011. Experimental infection of camels with bluetongue virus. Research in Veterinary Science, 90, 533–535. [DOI] [PubMed] [Google Scholar]
- Bauer B, Amsler‐Delafosse S, Clausen P, Kabore I and Petrich‐Bauer J, 1995. Successful application of deltamethrin pour on to cattle in a campaign against tsetse flies (Glossina spp.) in the pastoral zone of Samorogouan, Burkina Faso. Tropical Medicine and Parasitology, 46, 183–189. [PubMed] [Google Scholar]
- Bauer B, Jandowsky A, Schein E, Mehlitz D and Clausen PH, 2009. An appraisal of current and new techniques intended to protect bulls against Culicoides and other haematophagous nematocera: the case of Schmergow, Brandenburg, Germany. Parasitology Research, 105, 359–365. [DOI] [PubMed] [Google Scholar]
- Baylis M, Parkin H, Kreppel K, Carpenter S, Mellor PS and McIntyre KM, 2010. Evaluation of housing as a means to protect cattle from Culicoides biting midges, the vectors of bluetongue virus. Medical and Veterinary Entomology, 24, 38–45. [DOI] [PubMed] [Google Scholar]
- Belbis G, Breard E, Cordonnier N, Moulin V, Desprat A, Sailleau C, Viarouge C, Doceul V, Zientara S and Millemann Y, 2013. Evidence of transplacental transmission of bluetongue virus serotype 8 in goats. Veterinary Microbiology, 166, 394–404. [DOI] [PubMed] [Google Scholar]
- Biteau‐Coroller F, 2006. Surveillance et évaluation du risque de transmission des maladies vectorielles émergentes: apport de la capacité vectorielle, Exemple de la fièvre catarrhale du mouton. Doctoral Thesis, University of Montpellier II, Montpellier, France, 260 pp. [Google Scholar]
- Bivand R and Lewin‐Koh N, 2013. maptools: Tools for reading and handling spatial objects. R package version 0.8–27.
- Blackwell A and King FC, 1997. The vertical distribution of Culicoides impunctatus larvae. Medical and Veterinary Entomology, 11, 45–48. [DOI] [PubMed] [Google Scholar]
- Blackwell A, Evans KA, Strang RH and Cole M, 2004. Toward development of neem‐based repellents against the Scottish Highland biting midge Culicoides impunctatus. Medical and Veterinary Entomology, 18, 449–452. [DOI] [PubMed] [Google Scholar]
- Bonneau KR, DeMaula CD, Mullens BA and MacLachlan NJ, 2002. Duration of viraemia infectious to Culicoides sonorensis in bluetongue virus‐infected cattle and sheep. Veterinary Microbiology, 88, 115–125. [DOI] [PubMed] [Google Scholar]
- Boone JD, Balasuriya UB, Karaca K, Audonnet JC, Yao J, He L, Nordgren R, Monaco F, Savini G, Gardner IA and Maclachlan NJ, 2007. Recombinant canarypox virus vaccine co‐expressing genes encoding the VP2 and VP5 outer capsid proteins of bluetongue virus induces high level protection in sheep. Vaccine, 25, 672–678. [DOI] [PubMed] [Google Scholar]
- Bournez L, Sailleau C, Breard E, Zientara S, Zanella G, Troyano‐Groux A, Hendrikx P, Fediaevsky A and Cavalerie L, 2016. Ré‐émergence de la fiévre catarrhale ovine BTV‐8 en France: bilan de la situation épidémiolgique entre septembre et décembre 2015. Bulletin Epidémiologique, 74, 1–11. Available online at: http://bulletinepidemiologique.mag.anses.fr/sites/default/files/BEP-mg-BE74-art1.pdf [Google Scholar]
- Braverman Y and Galun R, 1973. The occurrence of Culicoides in Israel with reference to the incidence of bluetongue. Refuah Veterinarith, 30, 121–127, 168–170. [Google Scholar]
- Braverman Y and Mumcuoglu K, 2009. Newly emerged nulliparous Culicoides imicola Kieffer (Diptera: Ceratopogonidae) with pigmented abdomen. Veterinary Parasitology, 160, 356–358. [DOI] [PubMed] [Google Scholar]
- Breard E, Pozzi N, Sailleau C, Durand B, Catinot V, Sellem E, Dumont P, Guerin B and Zientara S, 2007. Transient adverse effects of an attenuated bluetongue virus vaccine on the quality of ram semen. Veterinary Record, 160, 431–435. [DOI] [PubMed] [Google Scholar]
- Breard E, Belbis G, Hamers C, Moulin V, Lilin T, Moreau F, Millemann Y, Montange C, Sailleau C, Durand B, Desprat A, Viarouge C, Hoffmann B, de Smit H, Goutebroze S, Hudelet P and Zientara S, 2011. Evaluation of humoral response and protective efficacy of two inactivated vaccines against bluetongue virus after vaccination of goats. Vaccine, 29, 2495–2502. [DOI] [PubMed] [Google Scholar]
- Breard E, Belbis G, Viarouge C, Nomikou K, Haegeman A, De Clercq K, Hudelet P, Hamers C, Moreau F, Lilin T, Durand B, Mertens P, Vitour D, Sailleau C and Zientara S, 2015. Evaluation of adaptive immune responses and heterologous protection induced by inactivated bluetongue virus vaccines. Vaccine, 33, 512–518. [DOI] [PubMed] [Google Scholar]
- Brugger K and Rubel F, 2013. Bluetongue disease risk assessment based on observed and projected Culicoides obsoletus spp. vector densities. PLoS ONE, 8, e60330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger K, Kofer J and Rubel F, 2016. Outdoor and indoor monitoring of livestock‐associated Culicoides spp. to assess vector‐free periods and disease risks. BMC Veterinary Research, 12, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagienard A, Griot C, Mellor PS, Denison E and Stark KD, 2006. Bluetongue vector species of Culicoides in Switzerland. Medical and Veterinary Entomology, 20, 239–247. [DOI] [PubMed] [Google Scholar]
- Calvete C, Miranda M, Estrada R, Borras D, Monteys VSI, Collantes F, Garcia‐de‐Francisco J, Moreno N and Lucientes J, 2006. Spatial distribution of Culicoides imicola, the main vector of bluetongue virus, in Spain. Veterinary Record, 158, 130–131. [DOI] [PubMed] [Google Scholar]
- Calvete C, Estrada R, Miranda MA, Del Rio R, Borras D, Beldron FJ, Martinez A, Calvo AJ and Lucientes J, 2010. Protection of livestock against bluetongue virus vector Culicoides imicola using insecticide‐treated netting in open areas. Medical and Veterinary Entomology, 24, 169–175. [DOI] [PubMed] [Google Scholar]
- Calvo JH, Calvete C, Martinez‐Royo A, Estrada R, Miranda MA, Borras D, Sarto IMV, Pages N, Delgado JA, Collantes F and Lucientes J, 2009. Variations in the mitochondrial cytochrome c oxidase subunit I gene indicate northward expanding populations of Culicoides imicola in Spain. Bulletin of Entomological Research, 99, 583–591. [DOI] [PubMed] [Google Scholar]
- Calvo JH, Berzal B, Calvete C, Miranda MA, Estrada R and Lucientes J, 2012. Host feeding patterns of Culicoides species (Diptera: Ceratopogonidae) within the Picos de Europa National Park in northern Spain. Bulletin of Entomological Research, 102, 692–697. [DOI] [PubMed] [Google Scholar]
- Campbell CL and Wilson WC, 2002. Differentially expressed midgut transcripts in Culicoides sonorensis (Diptera: ceratopogonidae) following Orbivirus (reoviridae) oral feeding. Insect Molecular Biology, 11, 595–604. [DOI] [PubMed] [Google Scholar]
- Campbell CL, Vandyke KA, Letchworth GJ, Drolet BS, Hanekamp T and Wilson WC, 2005. Midgut and salivary gland transcriptomes of the arbovirus vector Culicoides sonorensis (Diptera: Ceratopogonidae). Insect Molecular Biology, 14, 121–136. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Mellor P and Torr S, 2007. Bluetongue and midge control. Veterinary Record, 161, 633. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Mellor PS and Torr SJ, 2008. Control techniques for Culicoides biting midges and their application in the U.K. and northwestern Palaearctic. Medical and Veterinary Entomology, 22, 175–187. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Wilson A, Barber J, Veronesi E, Mellor P, Venter G and Gubbins S, 2011. Temperature dependence of the extrinsic incubation period of orbiviruses in Culicoides biting midges. PLoS ONE, 6, e27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A and Rodgers S, 1984. Congenital anomalies in cattle associated with an epizootic of bluetongue virus 28 (serotype II). The Bovine Practitioner, 19, 87–91. [Google Scholar]
- Celma CC, Boyce M, van Rijn PA, Eschbaumer M, Wernike K, Hoffmann B, Beer M, Haegeman A, De Clercq K and Roy P, 2013. Rapid generation of replication‐deficient monovalent and multivalent vaccines for bluetongue virus: protection against virulent virus challenge in cattle and sheep. Journal of Virology, 87, 9856–9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan HC, Biswas SK, Chand K, Rehman W, Das B, Dadawala AI, Chandel BS, Kher HN and Mondal B, 2014. Isolation of bluetongue virus serotype 1 from aborted goat fetuses. Revue Scientifique et Technique, 33, 803–812. [DOI] [PubMed] [Google Scholar]
- Cilek JE and Hallmon CF, 2005. The effectiveness of the Mosquito Magnet trap for reducing biting midge (Diptera: Ceratopogonidae) populations in coastal residential backyards. Journal of the American Mosquito Control Association, 21, 218–221. [DOI] [PubMed] [Google Scholar]
- Cilek JE, Kline DL and Hallmon CF, 2003. Evaluation of a novel removal trap system to reduce biting midge (Diptera: Ceratopogonidae) populations in Florida backyards. Journal of Vector Ecology, 28, 23–30. [PubMed] [Google Scholar]
- Clausen PH, Stephan A, Bartsch S, Jandowsky A, Hoffmann‐Kohler P, Schein E, Mehlitz D and Bauer B, 2009. Seasonal dynamics of biting midges (Diptera: Ceratopogonidae, Culicoides spp.) on dairy farms of Central Germany during the 2007/2008 epidemic of bluetongue. Parasitology Research, 105, 381–386. [DOI] [PubMed] [Google Scholar]
- Coetzee P, Stokstad M, Myrmel M, Mutowembwa P, Loken T, Venter EH and Van Vuuren M, 2013. Transplacental infection in goats experimentally infected with a European strain of bluetongue virus serotype 8. Veterinary Journal, 197, 335–341. [DOI] [PubMed] [Google Scholar]
- Conte A, Gilbert M and Goffredo M, 2009. Eight years of entomological surveillance in Italy show no evidence of Culicoides imicola geographical range expansion. Journal of Applied Ecology, 46, 1332–1339. [Google Scholar]
- Corbiere F, Nussbaum S, Alzieu JP, Lemaire M, Meyer G, Foucras G and Schelcher F, 2012. Bluetongue virus serotype 1 in wild ruminants, France, 2008–2010. Journal of Wildlife Diseases, 48, 1047–1051. [DOI] [PubMed] [Google Scholar]
- Darpel KE, Batten CA, Veronesi E, Williamson S, Anderson P, Dennison M, Clifford S, Smith C, Philips L, Bidewell C, Bachanek‐Bankowska K, Sanders A, Bin‐Tarif A, Wilson AJ, Gubbins S, Mertens PPC, Oura CA and Mellor PS, 2009. Transplacental transmission of bluetongue virus 8 in Cattle, UK. Emerging Infectious Diseases, 15, 2025–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darpel KE, Monaghan P, Simpson J, Anthony SJ, Veronesi E, Brooks HW, Elliott H, Brownlie J, Takamatsu HH, Mellor PS and Mertens PP, 2012. Involvement of the skin during bluetongue virus infection and replication in the ruminant host. Veterinary Research, 43, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq K, De Leeuw I, Verheyden B, Vandemeulebroucke E, Vanbinst T, Herr C, Meroc E, Bertels G, Steurbaut N, Miry C, De Bleecker K, Maquet G, Bughin J, Saulmont M, Lebrun M, Sustronck B, De Deken R, Hooyberghs J, Houdart P, Raemaekers M, Mintiens K, Kerkhofs P, Goris N and Vandenbussche F, 2008. Transplacental infection and apparently immunotolerance induced by a wild‐type bluetongue virus serotype 8 natural infection. Transboundary and Emerging Diseases, 55, 352–359. [DOI] [PubMed] [Google Scholar]
- De Raat I, Van Den Boom R, Van Poppel M and van Oldruitenborgh‐Oosterbaan M, 2008. The effect of a topical insecticide containing permethrin on the number of Culicoides midges caught near horses with and without insect bite hypersensitivity in the Netherlands. Tijdschrift voor Diergeneeskunde, 133, 838–842. [PubMed] [Google Scholar]
- DEFRA (Department for Environment, Food & Rural Affairs), 2008. Report on the Distribution of Bluetongue Infection in Great Britain. Department for Environment, Food and Rural Affairs, London. [Google Scholar]
- Del Rio LR, Miranda MA, Paredes‐Esquivel C, Lucientes J, Calvete C, Estrada R and Venter GJ, 2012. Recovery rates of bluetongue virus serotypes 1, 2, 4 and 8 Spanish strains from orally infected Culicoides imicola in South Africa. Medical and Veterinary Entomology, 26, 162–167. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Barcelo C, Paredes‐Esquivel C, Lucientes J and Miranda MA, 2014a. Susceptibility of Culicoides species biting midges to deltamethrin‐treated nets as determined under laboratory and field conditions in the Balearic Islands, Spain. Medical and Veterinary Entomology, 28, 414–420. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Venail R, Calvete C, Barcelo C, Baldet T, Lucientes J and Miranda MA, 2014b. Sensitivity of Culicoides obsoletus (Meigen) (Diptera: Ceratopogonidae) to deltamethrin determined by an adapted WHO standard susceptibility test. Parasitology, 141, 542–546. [DOI] [PubMed] [Google Scholar]
- Del Río R, Barceló C, Lucientes J and Miranda M, 2014. Detrimental effect of cypermethrin treated nets on Culicoides populations (Diptera; Ceratopogonidae) and non‐targeted fauna in livestock farms. Veterinary Parasitology, 199, 230–234. [DOI] [PubMed] [Google Scholar]
- Desmecht D, Bergh RV, Sartelet A, Leclerc M, Mignot C, Misse F, Sudraud C, Berthemin S, Jolly S, Mousset B, Linden A, Coignoul F and Cassart D, 2008. Evidence for transplacental transmission of the current wild‐type strain of bluetongue virus serotype 8 in cattle. Veterinary Record, 163, 50–52. [DOI] [PubMed] [Google Scholar]
- Di Emidio B, Nicolussi P, Patta C, Ronchi GF, Monaco F, Savini G, Ciarelli A and Caporale V, 2004. Efficacy and safety studies on an inactivated vaccine against bluetongue virus serotype 2. Veterinaria Italiana, 40, 640–644. [PubMed] [Google Scholar]
- Dórea FC, Swanenburg M, van Roermund H, Horigan V, de Vos C, Gale P, Lilja T, Comin A, Bahuon C, Zientara S, Young B, Vial F, Kosmider R and Lindberg A, 2017. Data collection for risk assessments on animal health (Acronym: DACRAH): Final Report. EFSA supporting publications, 2017:15(1):EN‐1171, 209 pp. doi: 10.2903/sp.efsa.2017.EN-1171 [DOI] [Google Scholar]
- Downes JA, 1962. What is an Arctic insect? The Canadian Entomologist, 94, 143–162. [Google Scholar]
- Drolet BS, Reister LM, Rigg TD, Nol P, Podell BK, Mecham JO, VerCauteren KC, van Rijn PA, Wilson WC and Bowen RA, 2013. Experimental infection of white‐tailed deer (Odocoileus virginianus) with Northern European bluetongue virus serotype 8. Veterinary Microbiology, 166, 347–355. [DOI] [PubMed] [Google Scholar]
- Dungu BK, Louw I, Potgieter C and von Teichman BF, 2008. Attenuated live bluetongue virus 8 vaccine protects sheep from challenge with the European BTV‐8. The Open Veterinary Science Journal, 2, 130–133. [Google Scholar]
- Dyce AL, 1969. The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. Australian Journal of Entomology, 8, 11–15. [Google Scholar]
- EC (European Commission), 2012. Guidance document to assist Member States for the implementation of the criteria for ‘Vector Protected Establishments’ for bluetongue. SANCO/7068/2012 Rev 7063.
- EFSA (European Food Safety Authority), 2007a. Scientific Opinion of the Scientific Panel on Animal Health and Welfare on the EFSA Selfmandate on bluetongue origin and occurrence. EFSA Journal 2007;5(5):480, 20 pp, doi: 10.2903/j.efsa.2007.480 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007b. Scientific Opinion of the EFSA Panel on Animal Health and Welfare (AHAW) on request from the Commission on bluetongue vectors and vaccines. EFSA Journal 2007;5(5):479, 29 pp, doi: 10.2903/j.efsa.2007.2479 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Scientific Opinion of the EFSA Panel on Animal Health and Welfare on bluetongue vectors and insecticides. EFSA Journal 2008;6(7):735, 70 pp. doi: 10.2903/j.efsa.2008.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. ‘Schmallenberg’ virus: analysis of the epidemiological data and assessment of impact. EFSA Journal 2012;10(6):2768, 89 pp. doi: 10.2903/j.efsa.2012.2768 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2011a. Scientific Opinion of the EFSA Panel on Animal Health and Welfare on bluetongue monitoring and surveillance. EFSA Journal 2011;9(6):2192, 61 pp. doi: 10.2903/j.efsa.2011.2192 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2011b. Scientific Opinion of the EFSA Panel on Animal Health and Welfare on bluetongue serotype 8. EFSA Journal 2011;9(5):2189, 51 pp. doi: 10.2903/j.efsa.2011.2189 [DOI] [Google Scholar]
- Eilenberg J, Hajek A and Lomer C, 2001. Suggestions for unifying the terminology in biological control. BioControl, 46, 387–400. [Google Scholar]
- Elbers A and Meiswinkel R, 2014. Culicoides (Diptera: Ceratopogonidae) host preferences and biting rates in the Netherlands: comparing cattle, sheep and the black‐light suction trap. Veterinary Parasitology, 205, 330–337. [DOI] [PubMed] [Google Scholar]
- Eschbaumer M, Hoffmann B, Konig P, Teifke JP, Gethmann JM, Conraths FJ, Probst C, Mettenleiter TC and Beer M, 2009. Efficacy of three inactivated vaccines against bluetongue virus serotype 8 in sheep. Vaccine, 27, 4169–4175. [DOI] [PubMed] [Google Scholar]
- Falconi C, Lopez‐Olvera JR, Boadella M, Camarena J, Rosell R, Alcaide V, Vicente J, Sanchez‐Vizcaino JM, Pujols J and Gortazar C, 2012. Evidence for BTV‐4 circulation in free‐ranging red deer (Cervus elaphus) in Cabaneros National Park, Spain. Veterinary Microbiology, 159, 40–46. [DOI] [PubMed] [Google Scholar]
- Feenstra F, Maris‐Veldhuis M, Daus FJ, Tacken MG, Moormann RJ, van Gennip RG and van Rijn PA, 2014a. VP2‐serotyped live‐attenuated bluetongue virus without NS3/NS3a expression provides serotype‐specific protection and enables DIVA. Vaccine, 32, 7108–7114. [DOI] [PubMed] [Google Scholar]
- Feenstra F, van Gennip RG, Maris‐Veldhuis M, Verheij E and van Rijn PA, 2014b. Bluetongue virus without NS3/NS3a expression is not virulent and protects against virulent bluetongue virus challenge. Journal of General Virology, 95, 2019–2029. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Pacheco P, Fernandez‐Pinero J, Aguero M and Jimenez‐Clavero MA, 2008. Bluetongue virus serotype 1 in wild mouflons in Spain. Veterinary Record, 162, 659–660. [DOI] [PubMed] [Google Scholar]
- Forbes C, Evans M, Hastings N and Peacock B, 2011. Statistical Distributions. 4th Edition, John Wiley & Sons, Inc., Hoboken, NJ, USA. doi: 10.1002/9780470627242.ch22 [DOI] [Google Scholar]
- Foxi C and Delrio G, 2010. Larval habitats and seasonal abundance of Culicoides biting midges found in association with sheep in northern Sardinia, Italy. Medical and Veterinary Entomology, 24, 199–209. [DOI] [PubMed] [Google Scholar]
- Foxi C, Pinna M, Monteys VSI and Delrio G, 2011. An updated checklist of the Culicoides Latreille (Diptera: Ceratopogonidae) of Sardinia (Italy), and seasonality in proven and potential vectors for bluetongue virus (BTV). Proceedings of the Entomological Society of Washington, 113, 403–416. [Google Scholar]
- Foxi C, Delrio G, Falchi G, Marche MG, Satta G and Ruiu L, 2016. Role of different Culicoides vectors (Diptera: Ceratopogonidae) in bluetongue virus transmission and overwintering in Sardinia (Italy). Parasit Vectors, 9, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French Ministry of Agriculture , 2016. Modality for surveillance of bluetongue (FCO): programmed surveillance for the free zone and part of the departments of the restricted zone. Technical Instruction. DGAL/SDSPA/2016‐594 21/07/2016. Animal Health office, Directorate General for Food, French Ministry of Agriculture, Paris, France. [Google Scholar]
- Fukuda T, Kline DL and Day JE, 2002. An iridescent virus and a microsporidium in the biting midge Culicoides barbosai from Florida. Journal of the American Mosquito Control Association, 18, 128–130. [PubMed] [Google Scholar]
- Gabler S, Häder S and Lahiri P, 1999. A model based justification of Kish's formula for design effects for weighting and clustering. Survey Methodology, 25, 105–106. [Google Scholar]
- García I, Napp S, Casal J, Perea A, Allepuz A, Alba A, Carbonero A and Arenas A, 2008. Bluetongue epidemiology in wild ruminants from Southern Spain. European Journal of Wildlife Research, 55, 173. [Google Scholar]
- Garcia‐Bocanegra I, Arenas‐Montes A, Lorca‐Oro C, Pujols J, Gonzalez MA, Napp S, Gomez‐Guillamon F, Zorrilla I, Miguel ES and Arenas A, 2011. Role of wild ruminants in the epidemiology of bluetongue virus serotypes 1, 4 and 8 in Spain. Veterinary Research, 42, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garros C, Gardes L, Allene X, Rakotoarivony I, Viennet E, Rossi S and Balenghien T, 2011. Adaptation of a species‐specific multiplex PCR assay for the identification of blood meal source in Culicoides (Ceratopogonidae: Diptera): applications on Palaearctic biting midge species, vectors of Orbiviruses. Infection, Genetics and Evolution, 11, 1103–1110. [DOI] [PubMed] [Google Scholar]
- Garros C, Balenghien T, Carpenter S, Delecolle JC, Meiswinkel R, Pedarrieu A, Rakotoarivony I, Gardes L, Golding N, Barber J, Miranda M, Borras DB, Goffredo M, Monaco F, Pages N, Sghaier S, Hammami S, Calvo JH, Lucientes J, Geysen D, De Deken G, Sarto IMV, Schwenkenbecher J, Kampen H, Hoffmann B, Lehmann K, Werner D, Baldet T, Lancelot R and Cetre‐Sossah C, 2014. Towards the PCR‐based identification of Palaearctic Culicoides biting midges (Diptera: Ceratopogonidae): results from an international ring trial targeting four species of the subgenus Avaritia. Parasit Vectors, 7, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gennip RG, van de Water SG, Maris‐Veldhuis M and van Rijn PA, 2012. Bluetongue viruses based on modified‐live vaccine serotype 6 with exchanged outer shell proteins confer full protection in sheep against virulent BTV8. PLoS ONE, 7, e44619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerry AC and Mullens BA, 2000. Seasonal abundance and survivorship of Culicoides sonorensis (Diptera: Ceratopogonidae) at a southern California dairy, with reference to potential bluetongue virus transmission and persistence. Journal of Medical Entomology, 37, 675–688. [DOI] [PubMed] [Google Scholar]
- Gethmann J, Huttner K, Heyne H, Probst C, Ziller M, Beer M, Hoffmann B, Mettenleiter TC and Conraths FJ, 2009. Comparative safety study of three inactivated BTV‐8 vaccines in sheep and cattle under field conditions. Vaccine, 27, 4118–4126. [DOI] [PubMed] [Google Scholar]
- Giovannini A, Paladini C, Calistri P, Conte A, Colangeli P, Santucci U, Nannini D and Caporale V, 2004. Surveillance system of bluetongue in Italy. Veterinaria Italiana, 40, 369–384. [PubMed] [Google Scholar]
- Giovannini A, Calistri P, Conte A, Goffredo M, Paladini C, Lelli R and Caporale V, 2008. Bluetongue virus serotype 8 in Europe in 2007–2008 and the risk of virus introduction and spread in Italy. Symposium on ‘Bluetongue in Europe back to the future’. Brescia, Italy, 7 June 2008.
- Goffredo M, Catalani M, Federici V, Portanti O, Marini V, Mancini G, Quaglia M, Santilli A, Teodori L and Savini G, 2015. Vector species of Culicoides midges implicated in the 2012–2014 bluetongue epidemics in Italy. Veterinaria Italiana, 51, 131–138. [DOI] [PubMed] [Google Scholar]
- Goldsmit L, Barzilai E and Tadmor A, 1975. The comparative sensitivity of sheep and chicken embryos to bluetongue virus and observations on viraemia in experimentally infected sheep. Australian Veterinary Journal, 51, 190–196. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Lopez S, Mullens BA, Baldet T and Goldarazena A, 2013. A survey of Culicoides developmental sites on a farm in northern Spain, with a brief review of immature habitats of European species. Veterinary Parasitology, 191, 81–93. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Venter GJ, Lopez S, Iturrondobeitia JC and Goldarazena A, 2014. Laboratory and field evaluations of chemical and plant‐derived potential repellents against Culicoides biting midges in northern Spain. Medical and Veterinary Entomology, 28, 421–431. [DOI] [PubMed] [Google Scholar]
- González M, Baldet T, Delécolle JC, López S, Romón P and Goldarazena A, 2013. Monitoring of Culicoides Latreille (Diptera: Ceratopogonidae) after Btv outbreaks, in sheep farms and natural habitats from the Basque Country (Northern Spain). Proceedings of the Entomological Society of Washington, 115, 48–69. [Google Scholar]
- Grego E, Sossella M, Bisanzio D, Stella MC, Giordana G, Pignata L and Tomassone L, 2014. Wild ungulates as sentinel of BTV‐8 infection in piedmont areas. Veterinary Microbiology, 174, 93–99. [DOI] [PubMed] [Google Scholar]
- Greive KA, Staton JA, Miller PF, Peters BA and Oppenheim VMJ, 2010. Development of Melaleuca oils as effective natural‐based personal insect repellents. Australian Journal of Entomology, 49, 40–48. [Google Scholar]
- Griffioen K, Van Gemst DB, Pieterse MC, Jacobs F and van Oldruitenborgh‐Oosterbaan MMS, 2011. Culicoides species associated with sheep in the Netherlands and the effect of a permethrin insecticide. The Veterinary Journal, 190, 230–235. [DOI] [PubMed] [Google Scholar]
- Gubbins S, Hartemink NA, Wilson AJ, Moulin V, Noordegraaf CAV, Sluijs MTWvd, Smit AJd, Sumner T and Klinkenberg D, 2012. Scaling from challenge experiments to the field: quantifying the impact of vaccination on the transmission of bluetongue virus serotype 8. Preventive Veterinary Medicine, 105, 297–308. [DOI] [PubMed] [Google Scholar]
- Guichard S, Guis H, Tran A, Garros C, Balenghien T and Kriticos DJ, 2014. Worldwide niche and future potential distribution of Culicoides imicola, a major vector of bluetongue and African horse sickness viruses. PLoS ONE, 9, e112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers C, Galleau S, Chery R, Blanchet M, Besancon L, Cariou C, Werle‐Lapostolle B, Hudelet P and Goutebroze S, 2009a. Use of inactivated bluetongue virus serotype 8 vaccine against virulent challenge in sheep and cattle. Veterinary Record, 165, 369–373. [DOI] [PubMed] [Google Scholar]
- Hamers C, Rehbein S, Hudelet P, Blanchet M, Lapostolle B, Cariou C, Duboeuf M and Goutebroze S, 2009b. Protective duration of immunity of an inactivated bluetongue (BTV) serotype 2 vaccine against a virulent BTV serotype 2 challenge in sheep. Vaccine, 27, 2789–2793. [DOI] [PubMed] [Google Scholar]
- Hammoumi S, Breard E, Sailleau C, Russo P, Grillet C, Cetre‐Sossah C, Albina E, Sanchis R, Pepin M, Guibert JM and Zientara S, 2003. Studies on the safety and immunogenicity of the South African bluetongue virus serotype 2 monovalent vaccine: specific detection of the vaccine strain genome by RT‐PCR. Journal of Veterinary Medicine. B, Infectious Diseases and Veterinary Public Health, 50, 316–321. [DOI] [PubMed] [Google Scholar]
- Harrup LE, Purse BV, Golding N, Mellor PS and Carpenter S, 2013. Larval development and emergence sites of farm‐associated Culicoides in the United Kingdom. Medical and Veterinary Entomology, 27, 441–449. [DOI] [PubMed] [Google Scholar]
- Harrup L, Gubbins S, Barber J, Denison E, Mellor P, Purse B and Carpenter S, 2014. Does covering of farm‐associated Culicoides larval habitat reduce adult populations in the United Kingdom? Veterinary Parasitology, 201, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrup LE, Miranda MA and Carpenter S, 2015. Advances in control techniques for Culicoides and future prospects. Veterinaria Italiana, 52, 247–264. [DOI] [PubMed] [Google Scholar]
- Harrup L, Miranda M and Carpenter S, 2016. Advances in control techniques for Culicoides and future prospects. Veterinaria Italiana, 52, 247–264. [DOI] [PubMed] [Google Scholar]
- Hartemink NA, Purse BV, Meiswinkel R, Brown HE, Koeijer A, Elbers ARW, Boender GJ, Rogers DJ and Heesterbeek JAP, 2009. Mapping the basic reproduction number (R‐0) for vector‐borne diseases: a case study on bluetongue virus. Epidemics, 1, 153–161. [DOI] [PubMed] [Google Scholar]
- Jacquet S, Garros C, Lombaert E, Walton C, Restrepo J, Allene X, Baldet T, Cetre‐Sossah C, Chaskopoulou A, Delecolle JC, Desvars A, Djerbal M, Fall M, Gardes L, De Garine‐Wichatitsky M, Goffredo M, Gottlieb Y, Fall AG, Kasina M, Labuschagne K, Lhor Y, Lucientes J, Martin T, Mathieu B, Miranda M, Pages N, Pereira Da Fonseca I, Ramilo DW, Segard A, Setier‐Rio ML, Stachurski F, Tabbabi A, Seck MT, Venter G, Zimba M, Balenghien T, Guis H, Chevillon C, Bouyer J and Huber K, 2015. Colonization of the Mediterranean basin by the vector biting midge species Culicoides imicola: an old story. Molecular Ecology, 24, 5707–5725. [DOI] [PubMed] [Google Scholar]
- Jacquet S, Huber K, Pages N, Talavera S, Burgin LE, Carpenter S, Sanders C, Dicko AH, Djerbal M, Goffredo M, Lhor Y, Lucientes J, Miranda‐Chueca MA, Pereira Da Fonseca I, Ramilo DW, Setier‐Rio ML, Bouyer J, Chevillon C, Balenghien T, Guis H and Garros C, 2016. Range expansion of the Bluetongue vector, Culicoides imicola, in continental France likely due to rare wind‐transport events. Scientific Reports, 6, 27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelson RV, Colwell AE and McClusky DK, 1980. Studies of Culicoides occidentalis at Borax Lake, California. Proceedings of the Forty‐eighth Annual Conference of the California Mosquito and Vector Control Association, Inc, In: Grant CD. (ed.). 1980. pp. 130–135 ref.13. [Google Scholar]
- Kiel E, Liebisch G, Focke R, Liebisch A and Werner D, 2009. Monitoring of Culicoides at 20 locations in northwest Germany. Parasitology Research, 105, 351–357. [DOI] [PubMed] [Google Scholar]
- Kirkeby C, Bodker R, Stockmarr A and Enoe C, 2009. Association between land cover and Culicoides (Diptera: Ceratopogonidae) breeding sites on four Danish cattle farms. Entomologica Fennica, 20, 228–232. [Google Scholar]
- Kish L, 1965. Survey Sampling. John Wiley & Sons, Inc., New York, London. [Google Scholar]
- Kish L, 1990. Weighting: Why, When, and How. Proceedings of the Section on Survey Research Methods, American Statistical Association, pp. 121–130. [Google Scholar]
- Kish L, 1992. Weighting for unequal Pi. Journal of Official Statistics, 8, 183–200. [Google Scholar]
- Kluiters G, Chaignat V and Schwermer H, 2008. Spatial distribution of Bluetongue surveillance and cases in Switzerland. SAT, Schweizer Archiv fur Tierheilkunde, 150, 543–552. [DOI] [PubMed] [Google Scholar]
- Kluiters G, Sugden D, Guis H, McIntyre KM, Labuschagne K, Vilar MJ and Baylis M, 2013. Modelling the spatial distribution of Culicoides biting midges at the local scale. Journal of Applied Ecology, 50, 232–242. [Google Scholar]
- Kochinger S, Renevey N, Hofmann MA and Zimmer G, 2014. Vesicular stomatitis virus replicon expressing the VP2 outer capsid protein of bluetongue virus serotype 8 induces complete protection of sheep against challenge infection. Veterinary Research, 45, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey LA and Kline DL, 1983. Laboratory bioassay of Bacillus thuringiensis (H‐14) against Culicoides spp. and Leptoconops spp. (Ceratopogonidae). Mosquito News, 43, 502–503. [Google Scholar]
- Larska M, Lechowski L, Grochowska M and Zmudzinski JF, 2013. Detection of the Schmallenberg virus in nulliparous Culicoides obsoletus/scoticus complex and C. punctatus–the possibility of transovarial virus transmission in the midge population and of a new vector. Veterinary Microbiology, 166, 467–473. [DOI] [PubMed] [Google Scholar]
- Leemans J, Hamers C, Chery R, Bibard A, Besancon L, Duboeuf M, Hudelet P, Goutebroze S and Kirschvink N, 2013. Interference of colostral antibodies with response to a Bluetongue serotype 8 inactivated vaccine in lambs born from hyperimmune ewes. Vaccine, 31, 1975–1980. [DOI] [PubMed] [Google Scholar]
- Legisa DM, Perez Aguirreburualde MS, Gonzalez FN, Marin‐Lopez A, Ruiz V, Wigdorovitz A, Martinez‐Escribano JA, Ortego J and Dus Santos MJ, 2015. An experimental subunit vaccine based on Bluetongue virus 4 VP2 protein fused to an antigen‐presenting cells single chain antibody elicits cellular and humoral immune responses in cattle, guinea pigs and IFNAR(−/−) mice. Vaccine, 33, 2614–2619. [DOI] [PubMed] [Google Scholar]
- Lequime S and Lambrechts L, 2014. Vertical transmission of arboviruses in mosquitoes: a historical perspective. Infection, Genetics and Evolution, 28, 681–690. [DOI] [PubMed] [Google Scholar]
- Lewis SE, Rice A, Hurst GD and Baylis M, 2014. First detection of endosymbiotic bacteria in biting midges Culicoides pulicaris and Culicoides punctatus, important Palaearctic vectors of bluetongue virus. Medical and Veterinary Entomology, 28, 453–456. [DOI] [PubMed] [Google Scholar]
- Li J, Yang T, Xu Q, Sun E, Feng Y, Lv S, Zhang Q, Wang H and Wu D, 2015. DNA vaccine prime and recombinant FPV vaccine boost: an important candidate immunization strategy to control bluetongue virus type 1. Applied Microbiology and Biotechnology, 99, 8643–8652. [DOI] [PubMed] [Google Scholar]
- Liebisch G and Liebisch A, 2008. Efficacy of Flectron‐eartags (cypermethrin) for control of midges (Culicoides) as the vectors of bluetongue virus in cattle: field studies and biossays. DTW. Deutsche tierarztliche Wochenschrift, 115, 220–230. [PubMed] [Google Scholar]
- Lincoln VJ, Page PC, Kopp C, Mathis A, von Niederhaeusern R, Burger D and Herholz C, 2015. Protection of horses against Culicoides biting midges in different housing systems in Switzerland. Veterinary Parasitology, 210, 206–214. [DOI] [PubMed] [Google Scholar]
- Linden A, Mousset B, Gregoire F, Hanrez D, Vandenbussche F, Vandemeulebroucke E, Vanbinst T, Verheyden B and de Clerck K, 2008. Bluetongue virus antibodies in wild red deer in southern Belgium. Veterinary Record, 162, 459. [DOI] [PubMed] [Google Scholar]
- Linden A, Gregoire F, Nahayo A, Hanrez D, Mousset B, Massart AL, De Leeuw I, Vandemeulebroucke E, Vandenbussche F and De Clercq K, 2010. Bluetongue virus in wild deer, Belgium, 2005–2008. Emerging Infectious Diseases, 16, 833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd AM, Kline DL, Hogsette JA, Kaufman PE and Allan SA, 2008. Evaluation of two commercial traps for the collection of Culicoides (Diptera: Ceratopogonidae). Journal of the American Mosquito Control Association, 24, 253–262. [DOI] [PubMed] [Google Scholar]
- Logan JG, Seal NJ, Cook JI, Stanczyk NM, Birkett MA, Clark SJ, Gezan SA, Wadhams LJ, Pickett JA and Mordue AJ, 2009. Identification of human‐derived volatile chemicals that interfere with attraction of the Scottish biting midge and their potential use as repellents. Journal of Medical Entomology, 46, 208–219. [DOI] [PubMed] [Google Scholar]
- Lopez‐Olvera JR, Falconi C, Fernandez‐Pacheco P, Fernandez‐Pinero J, Sanchez MA, Palma A, Herruzo I, Vicente J, Jimenez‐Clavero MA, Arias M, Sanchez‐Vizcaino JM and Gortazar C, 2010. Experimental infection of European red deer (Cervus elaphus) with bluetongue virus serotypes 1 and 8. Veterinary Microbiology, 145, 148–152. [DOI] [PubMed] [Google Scholar]
- Lorca‐Oro C, Pujols J, Garcia‐Bocanegra I, Mentaberre G, Granados JE, Solanes D, Fandos P, Galindo I, Domingo M, Lavin S and Lopez‐Olvera JR, 2012. Protection of Spanish Ibex (Capra pyrenaica) against Bluetongue virus serotypes 1 and 8 in a subclinical experimental infection. PLoS ONE, 7, e36380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca‐Oró C, López‐Olvera JR, García‐Bocanegra I, Granados JE, Mentaberre G, Fernández‐Aguilar X, Lavín S, Domingo M and Pujols J, 2014. Vaccination induces long‐lasting neutralising antibodies against bluetongue virus serotypes 1 and 8 in Spanish ibex (Capra pyrenaica). European Journal of Wildlife Research, 60, 297–302. [Google Scholar]
- Luhken R, Kiel E, Steinke S and Fladung R, 2015. Topsoil conditions correlate with the emergence rates of Culicoides chiopterus and Culicoides dewulfi (Diptera: Ceratopogonidae) from cowpats. Parasitology Research, 114, 1113–1117. [DOI] [PubMed] [Google Scholar]
- Lühken R, Kiel E and Steinke S, 2014a. Impact of mechanical disturbance on the emergence of Culicoides from cowpats. Parasitology Research, 113, 1283–1287. [DOI] [PubMed] [Google Scholar]
- Lühken R, Steinke S, Wittmann A and Kiel E, 2014b. Impact of flooding on the immature stages of dung‐breeding Culicoides in Northern Europe. Veterinary Parasitology, 205, 289–294. [DOI] [PubMed] [Google Scholar]
- Luhring KA and Mullens BA, 1997. Improved rearing methods for Heleidomermis magnapapula (Nematoda: Mermithidae), a larval parasite of Culicoides variipennis sonorensis (Diptera: Ceratopogonidae). Journal of Medical Entomology, 34, 704–709. [DOI] [PubMed] [Google Scholar]
- Lunt RA, Melville L, Hunt N, Davis S, Rootes CL, Newberry KM, Pritchard LI, Middleton D, Bingham J, Daniels PW and Eaton BT, 2006. Cultured skin fibroblast cells derived from bluetongue virus‐inoculated sheep and field‐infected cattle are not a source of late and protracted recoverable virus. Journal of General Virology, 87, 3661–3666. [DOI] [PubMed] [Google Scholar]
- MacLachlan NJ, 2004. Bluetongue: pathogenesis and duration of viraemia. Veterinaria Italiana, 40, 462–467. [PubMed] [Google Scholar]
- Maclachlan NJ and Mayo CE, 2013. Potential strategies for control of bluetongue, a globally emerging, Culicoides‐transmitted viral disease of ruminant livestock and wildlife. Antiviral Research, 99, 79–90. [DOI] [PubMed] [Google Scholar]
- Maclachlan NJ and Osburn BI, 2008. Induced brain lesions in calves infected with bluetongue virus. Veterinary Record, 162, 490–491. [DOI] [PubMed] [Google Scholar]
- MacLachlan NJ, Nunamaker RA, Katz JB, Sawyer MM, Akita GY, Osburn BI and Tabachnick WJ, 1994. Detection of bluetongue virus in the blood of inoculated calves: comparison of virus isolation, PCR assay, and in vitro feeding of Culicoides variipennis. Archives of Virology, 136, 1–8. [DOI] [PubMed] [Google Scholar]
- Martin V, Pascual E, Avia M, Pena L, Valcarcel F and Sevilla N, 2015. Protective efficacy in sheep of adenovirus‐vectored vaccines against Bluetongue virus is associated with specific T cell responses. PLoS ONE, 10, e0143273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelle L, Dal Pozzo F, Sarradin P, De Leeuw I, De Clercq K, Thys C, Thiry E and Saegerman C, 2013. Pulmonary artery haemorrhage in newborn calves following bluetongue virus serotype 8 experimental infections of pregnant heifers. Veterinary Microbiology, 167, 250–259. [DOI] [PubMed] [Google Scholar]
- Martinelle L, Dal Pozzo F, Sarradin P, Van Campe W, De Leeuw I, De Clercq K, Thys C, Thiry E and Saegerman C, 2016. Experimental bluetongue virus superinfection in calves previously immunized with bluetongue virus serotype 8. Veterinary Research, 47, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐de la Puente J, Martinez J, Ferraguti M, Morales‐de la Nuez A, Castro N and Figuerola J, 2012. Genetic characterization and molecular identification of the bloodmeal sources of the potential bluetongue vector Culicoides obsoletus in the Canary Islands, Spain. Parasit Vectors, 5, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐de la Puente J, Figuerola J and Soriguer R, 2015. Fur or feather? Feeding preferences of species of Culicoides biting midges in Europe. Trends in Parasitology, 31, 16–22. [DOI] [PubMed] [Google Scholar]
- Matsuo E, Celma CC, Boyce M, Viarouge C, Sailleau C, Dubois E, Breard E, Thiery R, Zientara S and Roy P, 2011. Generation of replication‐defective virus‐based vaccines that confer full protection in sheep against virulent bluetongue virus challenge. Journal of Virology, 85, 10213–10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo CE, Mullens BA, Reisen WK, Osborne CJ, Gibbs EP, Gardner IA and MacLachlan NJ, 2014. Seasonal and interseasonal dynamics of bluetongue virus infection of dairy cattle and Culicoides sonorensis midges in northern California–implications for virus overwintering in temperate zones. PLoS ONE, 9, e106975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley T, Cook AR and Deardon R, 2009. The International Journal of Biostatistics, 5, 1–37. [Google Scholar]
- Mee PT, Weeks AR, Walker PJ, Hoffmann AA and Duchemin JB, 2015. Detection of low‐level Cardinium and Wolbachia infections in Culicoides. Applied and Environment Microbiology, 81, 6177–6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiswinkel R, Baylis M and Labuschagne K, 2000. Stabling and the protection of horses from Culicoides bolitinos (Diptera: Ceratopogonidae), a recently identified vector of African horse sickness. Bulletin of Entomological Research, 90, 509–515. [DOI] [PubMed] [Google Scholar]
- Meiswinkel R, Baldet T, de Deken R, Takken W, Delecolle JC and Mellor PS, 2008. The 2006 outbreak of bluetongue in northern Europe–the entomological perspective. Preventive Veterinary Medicine, 87, 55–63. [DOI] [PubMed] [Google Scholar]
- Meiswinkel R, Scolamacchia F, Dik M, Mudde J, Dijkstra E, Van Der Ven IJ and Elbers AR, 2014. The Mondrian matrix: culicoides biting midge abundance and seasonal incidence during the 2006–2008 epidemic of bluetongue in the Netherlands. Medical and Veterinary Entomology, 28, 10–20. [DOI] [PubMed] [Google Scholar]
- Meiswinkel R and Elbers A, 2016. The dying of the light: crepuscular activity in Culicoides and impact on light trap efficacy at temperate latitudes. Medical and Veterinary Entomology, 30, 53–63. [DOI] [PubMed] [Google Scholar]
- Melville L, Weir R, Harmsen M, Walsh S, Hunt N and Daniels P, 1996. Characteristics of Naturally‐occurring Bluetongue Viral Infections of Cattle. Proceedings of the Australian Centre for International Agricultural Research, 66 pp. 245–250. [Google Scholar]
- Melville LF, Hunt NT, Davis SS and Weir RP, 2004. Bluetongue virus does not persist in naturally infected cattle. Veterinaria Italiana, 40, 502–507. [PubMed] [Google Scholar]
- Menzies FD, McCullough SJ, McKeown IM, Forster JL, Jess S, Batten C, Murchie AK, Gloster J, Fallows JG, Pelgrim W, Mellor PS and Oura CAL, 2008. Evidence for transplacental and contact transmission of bluetongue virus in cattle. Veterinary Record, 163, 203–209. [DOI] [PubMed] [Google Scholar]
- Meroc E, Faes C, Herr C, Staubach C, Verheyden B, Vanbinst T, Vandenbussche F, Hooyberghs J, Aerts A, De Clercq K and Mintiens K, 2008. Establishing the spread of bluetongue virus at the end of the 2006 epidemic in Belgium. Veterinary Microbiology, 131, 133–144. [DOI] [PubMed] [Google Scholar]
- Meyer G, Lacroux C, Leger S, Top S, Goyeau K, Deplanche M and Lemaire M, 2009. Lethal bluetongue virus serotype 1 infection in llamas. Emerging Infectious Diseases, 15, 608–610. [DOI] [PubMed] [Google Scholar]
- Mills MK, Nayduch D and Michel K, 2015. Inducing RNA interference in the arbovirus vector, Culicoides sonorensis. Insect Molecular Biology, 24, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Rincón C and Borràs D, 2004. Seasonal abundance of Culicoides imicola and C. obsoletus. Veterinaria Italiana, 40, 293. [PubMed] [Google Scholar]
- Modumo J and Venter EH, 2012. Determination of the minimum protective dose for bluetongue virus serotype 2 and 8 vaccines in sheep. Journal of the South African Veterinary Association, 83, 17. [DOI] [PubMed] [Google Scholar]
- Monaco F, Bonfini B, Zaghini M, Antonucci D, Pini A and Savini G, 2004a. Vaccination of cattle using monovalent modified‐live vaccine against bluetongue virus serotype 2: innocuity, immunogenicity and effect on pregnancy. Veterinaria Italiana, 40, 671–675. [PubMed] [Google Scholar]
- Monaco F, De Luca N, Spina P, Morelli D, Liberatore I, Citarella R, Conte A and Savini G, 2004b. Virological and serological response of cattle following field vaccination with bivalent modified‐live vaccine against bluetongue virus serotypes 2 and 9. Veterinaria Italiana, 40, 657–660. [PubMed] [Google Scholar]
- Moulin V, Noordegraaf CV, Makoschey B, van der Sluijs M, Veronesi E, Darpel K, Mertens PP and de Smit H, 2012. Clinical disease in sheep caused by bluetongue virus serotype 8, and prevention by an inactivated vaccine. Vaccine, 30, 2228–2235. [DOI] [PubMed] [Google Scholar]
- Mullens BA and Luhring KA, 1996. Salinity and pollution effects oil survival and infectivity of Heleidomermis magnapapula (Stichosomida: Mermithidae) for Culicoides variipennis sonorensis (Diptera: Ceratopogonidae). Environmental Entomology, 25, 1202–1208. [Google Scholar]
- Mullens BA and Rutz DA, 1983. Development of immature Culicoides variipennis (Diptera: Ceratopogonidae) at constant laboratory temperatures. Annals of the Entomological Society of America, 76, 747–751. [Google Scholar]
- Mullens BA and Velten RK, 1994. Laboratory culture and life history of Heleidomermis magnapapula in its host, Culicoides variipennis (Diptera: Ceratopogonidae). Journal of Nematology, 26, 1–10. [PMC free article] [PubMed] [Google Scholar]
- Mullens BA, Paine EO and Velten RK, 1995. Temperature effects on survival and development of Heleidomermis magnapapula in the Laboratory. Journal of Nematology, 27, 29–35. [PMC free article] [PubMed] [Google Scholar]
- Mullens BA, Luhring KA and Breidenbaugh MS, 1997. Experimental host range studies with Heleidomermis magnapapula (Mermithidae), a parasite of Culicoides variipennis (Ceratopogonidae). Journal of the American Mosquito Control Association, 13, 398–401. [PubMed] [Google Scholar]
- Mullens BA, Velten RK and Federici BA, 1999. Iridescent virus infection in Culicoides variipennis sonorensis and interactions with the mermithid parasite Heleidomermis magnapapula. Journal of Invertebrate Pathology, 73, 231–233. [DOI] [PubMed] [Google Scholar]
- Mullens B, Velten R, Gerry A, Braverman Y and Endris R, 2000. Feeding and survival of Culicoides sonorensis on cattle treated with permethrin or pirimiphos‐methyl. Medical and Veterinary Entomology, 14, 313–320. [DOI] [PubMed] [Google Scholar]
- Mullens BA, Gerry AC and Velten RK, 2001. Failure of a permethrin treatment regime to protect cattle against bluetongue virus. Journal of Medical Entomology, 38, 760–762. [DOI] [PubMed] [Google Scholar]
- Mullens B, Gerry A, Lysyk T and Schmidtmann E, 2004. Environmental effects on vector competence and virogenesis of bluetongue virus in Culicoides: interpreting laboratory data in a field context. Veterinaria Italiana, 40, 160–166. [PubMed] [Google Scholar]
- Mullens BA, Sarto IMV and Przhiboro AA, 2008. Mermithid parasitism in Ceratopogonidae: a literature review and critical assessment of host impact and potential for biological control of Culicoides. Russian Entomology Journal, 17, 87–113. [Google Scholar]
- Mullens BA, Gerry AC, Monteys VS, Pinna M and Gonzalez A, 2010. Field studies on Culicoides (Diptera: Ceratopogonidae) activity and response to deltamethrin applications to sheep in northeastern Spain. Journal of Medical Entomology, 47, 106–110. [DOI] [PubMed] [Google Scholar]
- Mullens BA, McDermott EG and Gerry AC, 2015. Progress and knowledge gaps in Culicoides ecology and control. Veterinaria Italiana, 51, 313–323. [DOI] [PubMed] [Google Scholar]
- Murray JO and Trainer DO, 1970. Bluetongue virus in North American elk. Journal of Wildlife Diseases, 6, 144–148. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kawai S, Yukuhiro F, Ito S, Gotoh T, Kisimoto R, Yanase T, Matsumoto Y, Kageyama D and Noda H, 2009. Prevalence of Cardinium bacteria in planthoppers and spider mites and taxonomic revision of ‘Candidatus Cardinium hertigii’ based on detection of a new Cardinium group from biting midges. Applied and Environment Microbiology, 75, 6757–6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narladkar BW, Shivpuje PR and Harke PC, 2015. Fungal biological control agents for integrated management of Culicoides spp. (Diptera: Ceratopogonidae) of livestock. Veterinary World, 8, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayduch D, Cohnstaedt LW, Saski C, Lawson D, Kersey P, Fife M and Carpenter S, 2014a. Studying Culicoides vectors of BTV in the post‐genomic era: resources, bottlenecks to progress and future directions. Virus Research, 182, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayduch D, Lee MB and Saski CA, 2014b. The reference transcriptome of the adult female biting midge (Culicoides sonorensis) and differential gene expression profiling during teneral, blood, and sucrose feeding conditions. PLoS ONE, 9, e98123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas AH and McCorkell B, 2014. Evaluation of Metarhizium anisopliae for the control of Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae), the principal vector of bluetongue virus in Australia. Journal of Vector Ecology, 39, 213–218. [DOI] [PubMed] [Google Scholar]
- Niedbalski W, 2015. Bluetongue in Europe and the role of wildlife in the epidemiology of disease. Polish Journal of Veterinary Sciences, 18, 455–461. [DOI] [PubMed] [Google Scholar]
- Nielsen SA, Nielsen BO and Chirico J, 2010. Monitoring of biting midges (Diptera: Ceratopogonidae: Culicoides Latreille) on farms in Sweden during the emergence of the 2008 epidemic of bluetongue. Parasitology Research, 106, 1197–1203. [DOI] [PubMed] [Google Scholar]
- Ninio C, Augot D, Dufour B and Depaquit J, 2011. Emergence of Culicoides obsoletus from indoor and outdoor breeding sites. Veterinary Parasitology, 183, 125–129. [DOI] [PubMed] [Google Scholar]
- Nunes SF, Hamers C, Ratinier M, Shaw A, Brunet S, Hudelet P and Palmarini M, 2014. A synthetic biology approach for a vaccine platform against known and newly emerging serotypes of bluetongue virus. Journal of Virology, 88, 12222–12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE (Office international des épizooties), 2014. Terrestrial Animal Health Code ‐ Infection with bluetongue virus. Chapter 8.3, pp. 1–10.
- Onuike AL, Ikpeze OO and Ngenegbo UC, 2015. Effects of deltamethrin on mortality, feeding behaviour and oviposition in the UK Culicoides species and at UK environmental temperature. International Journal of Veterinary Science, 4, 175–182. [Google Scholar]
- Ortega MD, Lloyd JE and Holbrook FR, 1997. Seasonal and geographical distribution of Culicoides imicola Kieffer (Diptera: Ceratopogonidae) in southwestern Spain. Journal of the American Mosquito Control Association, 13, 227–232. [PubMed] [Google Scholar]
- Osborne CJ, Mayo CE, Mullens BA, McDermott EG, Gerry AC, Reisen WK and MacLachlan NJ, 2015. Lack of evidence for laboratory and natural vertical transmission of bluetongue virus in Culicoides sonorensis (Diptera: Ceratopogonidae). Journal of Medical Entomology, 52, 274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oura CA, Wood JL, Sanders AJ, Bin‐Tarif A, Henstock M, Edwards L, Floyd T, Simmons H and Batten CA, 2009. Seroconversion, neutralising antibodies and protection in bluetongue serotype 8 vaccinated sheep. Vaccine, 27, 7326–7330. [DOI] [PubMed] [Google Scholar]
- Oura CAL, Wood JLN, Floyd T, Sanders AJ, Bin‐Tarif A, Henstock M, Edwards L, Simmons H and Batten CA, 2010. Colostral antibody protection and interference with immunity in lambs born from sheep vaccinated with an inactivated Bluetongue serotype 8 vaccine. Vaccine, 28, 2749–2753. [DOI] [PubMed] [Google Scholar]
- Page PC, Labuschagne K, Venter GJ, Schoeman JP and Guthrie AJ, 2014. Field and in vitro insecticidal efficacy of alphacypermethrin‐treated high density polyethylene mesh against Culicoides biting midges in South Africa. Veterinary Parasitology, 203, 184–188. [DOI] [PubMed] [Google Scholar]
- Paine EO and Mullens BA, 1994. Distribution, seasonal occurrence, and patterns of parasitism of Heleidomermis magnapapula (Nematoda: Mermithidae), a parasite of Culicoides variipennis (Diptera: Ceratopogonidae) in California. Environmental Entomology, 23, 154–160. [Google Scholar]
- Papadopoulos E, Bartram D, Carpenter S, Mellor P and Wall R, 2009. Efficacy of alphacypermethrin applied to cattle and sheep against the biting midge Culicoides nubeculosus. Veterinary Parasitology, 163, 110–114. [DOI] [PubMed] [Google Scholar]
- Papadopoulos E, Rowlinson M, Bartram D, Carpenter S, Mellor P and Wall R, 2010. Treatment of horses with cypermethrin against the biting flies Culicoides nubeculosus, Aedes aegypti and Culex quinquefasciatus. Veterinary Parasitology, 169, 165–171. [DOI] [PubMed] [Google Scholar]
- Perez de Diego AC, Athmaram TN, Stewart M, Rodriguez‐Sanchez B, Sanchez‐Vizcaino JM, Noad R and Roy P, 2011. Characterization of protection afforded by a bivalent virus‐like particle vaccine against bluetongue virus serotypes 1 and 4 in sheep. PLoS ONE, 6, e26666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin A, Albina E, Breard E, Sailleau C, Prome S, Grillet C, Kwiatek O, Russo P, Thiery R, Zientara S and Cetre‐Sossah C, 2007. Recombinant capripoxviruses expressing proteins of bluetongue virus: evaluation of immune responses and protection in small ruminants. Vaccine, 25, 6774–6783. [DOI] [PubMed] [Google Scholar]
- Pfannenstiel RS, Mullens BA, Ruder MG, Zurek L, Cohnstaedt LW and Nayduch D, 2015. Management of North American Culicoides biting midges: current knowledge and research needs. Vector‐Borne and Zoonotic Diseases, 15, 374–384. [DOI] [PubMed] [Google Scholar]
- Pili E, Carcangiu L, Oppo M and Marchi A, 2010. Genetic structure and population dynamics of the biting midges Culicoides obsoletus and Culicoides scoticus: implications for the transmission and maintenance of bluetongue. Medical and Veterinary Entomology, 24, 441–448. [DOI] [PubMed] [Google Scholar]
- Poinar GO and Mullens BA, 1987. Heleidomermis magnapapula (Mermithidae:Nematoda) parasitizing Culicoides variipennis (Ceratopogonidae:Diptera) in California. Revue Nématology, 10, 387–391. [Google Scholar]
- Poinar G Jr and Sarto i Monteys V, 2008. Mermithids (Nematoda: Mermithidae) of biting midges (Diptera: Ceratopogonidae): Heleidomermis cataloniensis n. sp. from Culicoides circumscriptus Kieffer in Spain and a species of Cretacimermis Poinar, 2001 from a ceratopogonid in Burmese amber. Systematic Parasitology, 69, 13–21. [DOI] [PubMed] [Google Scholar]
- Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PP and Baylis M, 2005. Climate change and the recent emergence of bluetongue in Europe. Nature Reviews Microbiology, 3, 171–181. [DOI] [PubMed] [Google Scholar]
- Purse B, Carpenter S, Venter G, Bellis G and Mullens B, 2015. Bionomics of temperate and tropical Culicoides midges: knowledge gaps and consequences for transmission of Culicoides‐borne viruses. Annual Review of Entomology, 60, 373–392. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan MA, Pandey AB, Singh KP, Singh R, Nandi S and Mehrotra ML, 2006. Immune responses and protective efficacy of binary ethylenimine (BEI)‐inactivated bluetongue virus vaccines in sheep. Veterinary Research Communications, 30, 873–880. [DOI] [PubMed] [Google Scholar]
- Rasmussen LD, Savini G, Lorusso A, Bellacicco A, Palmarini M, Caporale M, Rasmussen TB, Belsham GJ and Botner A, 2013. Transplacental transmission of field and rescued strains of BTV‐2 and BTV‐8 in experimentally infected sheep. Veterinary Research, 44, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W, Lloyd J, Stobart R, Stith C, Miller M, Bennett K and Johnson G, 2010. Control of Culicoides sonorensis (Diptera: Ceratopogonidae) blood feeding on sheep with long‐lasting repellent pesticides. Journal of the American Mosquito Control Association, 26, 302–305. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y and Martinez VM, 2006. Effects of temperature on the transmission of west nile virus by Culex tarsalis (Diptera: Culicidae). Journal of Medical Entomology, 43, 309–317. [DOI] [PubMed] [Google Scholar]
- Rieb JP, 1987. L'estivo‐hibernation et le contrôle de la dynamique du cycle évolutif dans le genre Culicoides: Diptères, Cératopogonidés. Vie et Milieu, 371:23–37. [Google Scholar]
- Rieb JP, Mialhe E and Quiot JM, 1982. Ceratopogonidae larvae infected by an iridovirus. Mosquito News, 42, 529. [Google Scholar]
- Rigot T, Conte A, Goffredo M, Ducheyne E, Hendrickx G and Gilbert M, 2012. Predicting the spatio‐temporal distribution of Culicoides imicola in Sardinia using a discrete‐time population model. Parasites & Vectors, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigot T, Drubbel MV, Delecolle JC and Gilbert M, 2013. Farms, pastures and woodlands: the fine‐scale distribution of Palearctic Culicoides spp. biting midges along an agro‐ecological gradient. Medical and Veterinary Entomology, 27, 29–38. [DOI] [PubMed] [Google Scholar]
- Robin M, Archer D, McGowan C, Garros C, Gardes L and Baylis M, 2015. Paper: repellent effect of topical deltamethrin on blood feeding by Culicoides on horses. Veterinary Record, 176, 574. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Sanchez B, Gortazar C, Ruiz‐Fons F and Sanchez‐Vizcaino JM, 2010. Bluetongue virus serotypes 1 and 4 in red deer, Spain. Emerging Infectious Diseases, 16, 518–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romon P, Higuera M, Delecolle JC, Baldet T, Aduriz G and Goldarazena A, 2012. Phenology and attraction of potential Culicoides vectors of bluetongue virus in Basque Country (northern Spain). Veterinary Parasitology, 186, 415–424. [DOI] [PubMed] [Google Scholar]
- Rossi S, Gibert P, Breard E, Moinet M, Hars J, Maillard D, Wanner M, Klein F, Mastain O, Mathevet P and Bost F, 2010. Circulation et impact des virus de la fievre catarrhale ovine (FCO) chez les ruminants sauvages en France. Bulletin Epidemiologique, 35, 28–32. [Google Scholar]
- Rossi S, Breard E, Viarouge C, Gauthier D, Novella C, Gueneau E, Chenoufi N, Santini J‐M, Reira M, Thion N, Beitia R, Méry J, Simeon D, Barbier S, Odier D, Mondoloni S, Bertaux J‐L, Puthiot G, Tholoniat C, Wanner M, Gibert P, Crampe M, Benedetti P, Hamann J‐L, Brandt S, Klein F, Touratier A, Faure E and Hars J, RFSA , 2014a. Surveillance active de la fièvre catarrhale ovine et de la maladie hémorragique des cervidés chez le cerf elaphe (Cervus elaphus). Rapport du programme 2011‐2013, 21 p. [Google Scholar]
- Rossi S, Pioz M, Beard E, Durand B, Gibert P, Gauthier D, Klein F, Maillard D, Saint‐Andrieux C, Saubusse T and Hars J, 2014b. Bluetongue dynamics in French wildlife: exploring the driving forces. Transboundary and Emerging Diseases, 61, e12–e24. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Fons F, Reyes‐Garcia AR, Alcaide V and Gortazar C, 2008. Spatial and temporal evolution of bluetongue virus in wild ruminants, Spain. Emerging Infectious Diseases, 14, 951–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegerman C, Bolkaerts B, Baricalla C, Raes M, Wiggers L, de Leeuw I, Vandenbussche F, Zimmer J‐Y, Haubruge E, Cassart D, De Clercq K and Kirschvink N, 2011. The impact of naturally‐occurring, trans‐placental bluetongue virus serotype‐8 infection on reproductive performance in sheep. The Veterinary Journal, 187, 72–80. [DOI] [PubMed] [Google Scholar]
- Sailleau C, Bréard E, Viarouge C, Vitour D, Romey A, Garnier A, Fablet A, Lowenski S, Gorna K, Caignard G, Pagneux C and Zientara S, 2015. Re‐emergence of Bluetongue virus serotype 8 in France, 2015. Transboundary and Emerging Diseases, doi: 10.1111/tbed.12453. [DOI] [PubMed] [Google Scholar]
- Sanders CJ, Selby R, Carpenter S and Reynolds DR, 2011a. High‐altitude flight of Culicoides biting midges. Veterinary Record, 169, 208. [DOI] [PubMed] [Google Scholar]
- Sanders CJ, Shortall CR, Gubbins S, Burgin L, Gloster J, Harrington R, Reynolds DR, Mellor PS and Carpenter S, 2011b. Influence of season and meteorological parameters on flight activity of Culicoides biting midges. Journal of Applied Ecology, 48, 1355–1364. [Google Scholar]
- Santamaría E, Cabrera OL, Zipa Y and Pardo RH, 2012. Eficacia en campo de un repelente a base de para‐mentano‐3,8‐diol y aceite de limonaria contra Culicoides pachymerus (Diptera: Ceratopogonidae) en Colombia. Biomédica, 32, 457–460. [DOI] [PubMed] [Google Scholar]
- Santiago‐Alarcon D, Havelka P, Pineda E, Segelbacher G and Schaefer HM, 2013. Urban forests as hubs for novel zoonosis: blood meal analysis, seasonal variation in Culicoides (Diptera: Ceratopogonidae) vectors, and avian haemosporidians. Parasitology, 140, 1799–1810. [DOI] [PubMed] [Google Scholar]
- Savini G, Monaco F, Calistri P, Panichi G, Ruiu A, Leone A and Caporale V, 2004a. Neutralising antibody response in cattle after vaccination with monovalent modified‐live vaccine against bluetongue virus serotype 2. Veterinaria Italiana, 40, 668–670. [PubMed] [Google Scholar]
- Savini G, Tittarelli M, Bonfini B, Zaghini M, Di Ventura M and Monaco F, 2004b. Serological response in cattle and sheep following infection or vaccination with bluetongue virus. Veterinaria Italiana, 40, 645–647. [PubMed] [Google Scholar]
- Savini G, Ronchi GF, Leone A, Ciarelli A, Migliaccio P, Franchi P, Mercante MT and Pini A, 2007. An inactivated vaccine for the control of bluetongue virus serotype 16 infection in sheep in Italy. Veterinary Microbiology, 124, 140–146. [DOI] [PubMed] [Google Scholar]
- Savini G, Hamers C, Conte A, Migliaccio P, Bonfini B, Teodori L, Di Ventura M, Hudelet P, Schumacher C and Caporale V, 2009. Assessment of efficacy of a bivalent BTV‐2 and BTV‐4 inactivated vaccine by vaccination and challenge in cattle. Veterinary Microbiology, 133, 1–8. [DOI] [PubMed] [Google Scholar]
- Schmahl G, Walldorf V, Klimpel S, Al‐Quraishy S and Mehlhorn H, 2008. Efficacy of oxyfly on Culicoides species–the vectors of Bluetongue virus–and other insects. Parasitology Research, 103, 1101–1103. [DOI] [PubMed] [Google Scholar]
- Schmahl G, Klimpel S, Walldorf V, Al‐Quraishy S, Schumacher B, Jatzlau A and Mehlhorn H, 2009a. Pilot study on deltamethrin treatment (Butox 7.5, Versatrine) of cattle and sheep against midges (Culicoides species, Ceratopogonidae). Parasitology Research, 104, 809–813. [DOI] [PubMed] [Google Scholar]
- Schmahl G, Klimpel S, Walldorf V, Schumacher B, Jatzlau A, Al‐Quraishy S and Mehlhorn H, 2009b. Effects of permethrin (Flypor) and fenvalerate (Acadrex60, Arkofly) on Culicoides species‐the vector of Bluetongue virus. Parasitology Research, 104, 815–820. [DOI] [PubMed] [Google Scholar]
- Schnettler E, Ratinier M, Watson M, Shaw AE, McFarlane M, Varela M, Elliott RM, Palmarini M and Kohl A, 2013. RNA interference targets arbovirus replication in Culicoides cells. Journal of Virology, 87, 2441–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Eschbaumer M, Rudolf M, Konig P, Keller M, Bauer C, Gauly M, Grevelding CG, Beer M and Hoffmann B, 2012. Experimental infection of South American camelids with bluetongue virus serotype 8. Veterinary Microbiology, 154, 257–265. [DOI] [PubMed] [Google Scholar]
- Scolamacchia F, Van Den Broek J, Meiswinkel R, Heesterbeek J and Elbers A, 2014. Principal climatic and edaphic determinants of Culicoides biting midge abundance during the 2007–2008 bluetongue epidemic in the Netherlands, based on OVI light trap data. Medical and Veterinary Entomology, 28, 143–156. [DOI] [PubMed] [Google Scholar]
- Searle K, Blackwell A, Falconer D, Sullivan M, Butler A and Purse B, 2013. Identifying environmental drivers of insect phenology across space and time: Culicoides in Scotland as a case study. Bulletin of Entomological Research, 103, 155–170. [DOI] [PubMed] [Google Scholar]
- Searle KR, Barber J, Stubbins F, Labuschagne K, Carpenter S, Butler A, Denison E, Sanders C, Mellor PS and Wilson A, 2014. Environmental drivers of Culicoides phenology: how important is species‐specific variation when determining disease policy? PLoS ONE, 9, e111876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs M, Timmermans M, Moulin V, Noordegraaf CV, Vrijenhoek M, Debyser I, de Smit AJ and Moormann R, 2011. Transplacental transmission of Bluetongue virus serotype 8 in ewes in early and mid gestation. Veterinary Microbiology, 149, 113–125. [DOI] [PubMed] [Google Scholar]
- van der Sluijs MT, Schroer‐Joosten DP, Fid‐Fourkour A, Smit M, Vrijenhoek MP, Moulin V, de Smit AJ and Moormann RJ, 2013a. Transplacental transmission of BTV‐8 in sheep: BTV viraemia, antibody responses and vaccine efficacy in lambs infected in utero. Vaccine, 31, 3726–3731. [DOI] [PubMed] [Google Scholar]
- van der Sluijs MTW, Schroer‐Joosten DPH, Fid‐Fourkour A, Vrijenhoek MP, Debyser I, Moulin V, Moormann RJM and de Smit AJ, 2013b. Transplacental transmission of Bluetongue virus serotype 1 and serotype 8 in Sheep: virological and pathological findings. PLoS ONE, 8, e81429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs MTW, de Smit AJ and Moormann RJM, 2016. Vector independent transmission of the vector‐borne bluetongue virus. Critical Reviews in Microbiology, 42, 57–64. [DOI] [PubMed] [Google Scholar]
- de Souza JI, Gleason FH, Ansari MA, López Lastra CC, Garcia JJ, Pires‐Zottarelli CLA and Marano AV, 2014. Fungal and oomycete parasites of Chironomidae, Ceratopogonidae and Simuliidae (Culicomorpha, Diptera). Fungal Biology Reviews, 28, 13–23. [Google Scholar]
- Spencer BD, 2000. An approximate design effect for unequal weighting when measurements may correlate with selection probabilities. Survey Methodology, 26, 137–138. [Google Scholar]
- Steinke S, Luhken R and Kiel E, 2014. Assessment of the abundance of Culicoides chiopterus and Culicoides dewulfi in bovine dung: a comparison of larvae extraction techniques and emergence traps. Veterinary Parasitology, 205, 255–262. [DOI] [PubMed] [Google Scholar]
- Stelletta C, Cuteri V, Bonizzi L, Frangipane di Regalbono A, Orsi F, Nisoli L, Lulla D and Morgante M, 2004. Effect of levamisole administration on bluetongue vaccination in sheep. Veterinaria Italiana, 40, 635–639. [PubMed] [Google Scholar]
- Stewart M, Bhatia Y, Athmaran TN, Noad R, Gastaldi C, Dubois E, Russo P, Thiery R, Sailleau C, Breard E, Zientara S and Roy P, 2010. Validation of a novel approach for the rapid production of immunogenic virus‐like particles for bluetongue virus. Vaccine, 28, 3047–3054. [DOI] [PubMed] [Google Scholar]
- Stewart M, Dovas CI, Chatzinasiou E, Athmaram TN, Papanastassopoulou M, Papadopoulos O and Roy P, 2012. Protective efficacy of Bluetongue virus‐like and subvirus‐like particles in sheep: presence of the serotype‐specific VP2, independent of its geographic lineage, is essential for protection. Vaccine, 30, 2131–2139. [DOI] [PubMed] [Google Scholar]
- Stewart M, Dubois E, Sailleau C, Breard E, Viarouge C, Desprat A, Thiery R, Zientara S and Roy P, 2013. Bluetongue virus serotype 8 virus‐like particles protect sheep against virulent virus infection as a single or multi‐serotype cocktail immunogen. Vaccine, 31, 553–558. [DOI] [PubMed] [Google Scholar]
- Stott JL, Lauerman L and Luedke A, 1982. Bluetongue virus in pregnant elk and their calves. American Journal of Veterinary Research, 43, 423–428. [PubMed] [Google Scholar]
- Szmaragd C, Wilson AJ, Carpenter S, Wood JL, Mellor PS and Gubbins S, 2009. A modeling framework to describe the transmission of bluetongue virus within and between farms in Great Britain. PLoS ONE, 4, e7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu H, Mellor PS, Mertens PP, Kirkham PA, Burroughs JN and Parkhouse RM, 2003. A possible overwintering mechanism for bluetongue virus in the absence of the insect vector. Journal of General Virology, 84, 227–235. [DOI] [PubMed] [Google Scholar]
- Takken W, Verhulst N, Scholte EJ, Jacobs F, Jongema Y and van Lammeren R, 2008. The phenology and population dynamics of Culicoides spp. in different ecosystems in The Netherlands. Preventive Veterinary Medicine, 87, 41–54. [DOI] [PubMed] [Google Scholar]
- Talavera S, Munoz‐Munoz F, Duran M, Verdun M, Soler‐Membrives A, Oleaga A, Arenas A, Ruiz‐Fons F, Estrada R and Pages N, 2015. Culicoides species communities associated with wild ruminant ecosystems in Spain: tracking the way to determine potential bridge vectors for arboviruses. PLoS ONE, 10, e0141667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC , 2014. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: 2013. [Google Scholar]
- Tessaro SV and Clavijo A, 2001. Duration of bluetongue viremia in experimentally infected American bison. Journal of Wildlife Diseases, 37, 722–729. [DOI] [PubMed] [Google Scholar]
- Thomas F, Guégan JF and Renaud F, 2008. Ecology and Evolution of Parasitism. OUP, Oxford, 375 pp. [Google Scholar]
- Thompson GM, Jess S and Murchie AK, 2013. Differential emergence of Culicoides (Diptera: Ceratopogonidae) from on‐farm breeding substrates in Northern Ireland. Parasitology, 140, 699–708. [DOI] [PubMed] [Google Scholar]
- Thuenemann EC, Meyers AE, Verwey J, Rybicki EP and Lomonossoff GP, 2013. A method for rapid production of heteromultimeric protein complexes in plants: assembly of protective bluetongue virus‐like particles. Plant Biotechnology Journal, 11, 839–846. [DOI] [PubMed] [Google Scholar]
- Toni T, Welch D, Strelkowa N, Ipsen A and Stumpf MP, 2009. Approximate Bayesian computation scheme for parameter inference and model selection in dynamical systems. Journal of the Royal Society Interface, 6, 187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Top S, Foucras G, Deplanche M, Rives G, Calvalido J, Comtet L, Bertagnoli S and Meyer G, 2012. Myxomavirus as a vector for the immunisation of sheep: protection study against challenge with bluetongue virus. Vaccine, 30, 1609–1616. [DOI] [PubMed] [Google Scholar]
- Unkles SE, Marriott C, Kinghorn JR, Panter C and Blackwell A, 2004. Efficacy of the Entomopathogenic Fungus, Culicinomyces clavisporus against Larvae of the Biting Midge, Culicoides nubeculosus (Diptera: Ceratopogonidae). Biocontrol Science and Technology, 14, 397–401. [Google Scholar]
- Valliant R, Dever JA and Kreuter F, 2013. Practical Tools for Designing and Weighting Survey Samples. Springer, 51, pp. 161. [Google Scholar]
- Valliant R, Dever J and Kreuter F, 2015. PracTools: Tools for Designing and Weighting Survey Samples. R package version 0.3 Available at: http://CRAN.R-project.org/package=PracTools.
- Vandenbussche F, Vanbinst T, Verheyden B, Wv D, Demeestere L, Houdart P, Bertels G, Praet N, Berkvens D, Mintiens K, Goris N and Clercq K, 2008. Evaluation of antibody‐ELISA and real‐time RT‐PCR for the diagnosis and profiling of bluetongue virus serotype 8 during the epidemic in Belgium in 2006. Veterinary Microbiology, 129, 15–27. [DOI] [PubMed] [Google Scholar]
- Venail R, Mathieu B, Setier‐Rio ML, Borba C, Alexandre M, Viudes G, Garros C, Allene X, Carpenter S, Baldet T and Balenghien T, 2011. Laboratory and field‐based tests of deltamethrin insecticides against adult Culicoides biting midges. Journal of Medical Entomology, 48, 351–357. [DOI] [PubMed] [Google Scholar]
- Venail R, Balenghien T, Guis H, Tran A, Setier‐Rio M‐L, Delécolle J‐C, Mathieu B, Cêtre‐Sossah C, Martinez D, Languille J, Baldet T and Garros C, 2012. Assessing diversity and abundance of vector populations at a National Scale: example of Culicoides surveillance in France after bluetongue virus emergence In: Mehlhorn H. (ed.). Arthropods as Vectors of Emerging Diseases. Springer, Berlin, Heidelberg: pp. 77–102. [Google Scholar]
- Venail R, Lhoir J, Fall M, del Rio R, Talavera S, Labuschagne K, Miranda M, Pages N, Venter G, Rakotoarivony I, Allene X, Scheid B, Gardes L, Gimonneau G, Lancelot R, Garros C, Cetre‐Sossah C, Balenghien T, Carpenter S and Baldet T, 2015. How do species, population and active ingredient influence insecticide susceptibility in Culicoides biting midges (Diptera: Ceratopogonidae) of veterinary importance? Parasit Vectors, 8, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter GJ, Wright IM, Van Der Linde TC and Paweska JT, 2009. The oral susceptibility of South African field populations of Culicoides to African horse sickness virus. Medical and Veterinary Entomology, 23, 367–378. [DOI] [PubMed] [Google Scholar]
- Venter GJ, Wright IM and Paweska JT, 2010. A comparison of the susceptibility of the biting midge Culicoides imicola to infection with recent and historical isolates of African horse sickness virus. Medical and Veterinary Entomology, 24, 324–328. [DOI] [PubMed] [Google Scholar]
- Venter GJ, Labuschagne K, Boikanyo SN, Morey L and Snyman MG, 2011a. The repellent effect of organic fatty acids on Culicoides midges as determined with suction light traps in South Africa. Veterinary Parasitology, 181, 365–369. [DOI] [PubMed] [Google Scholar]
- Venter GJ, Wright IM, Del Rio R, Lucientes J and Miranda MA, 2011b. The susceptibility of Culicoides imicola and other South African livestock‐associated Culicoides species to infection with bluetongue virus serotype 8. Medical and Veterinary Entomology, 25, 320–326. [DOI] [PubMed] [Google Scholar]
- Venter GJ, Labuschagne K, Boikanyo SN and Morey L, 2014. Assessment of the repellent effect of citronella and lemon eucalyptus oil against South African Culicoides species. Journal of the South African Veterinary Association, 85, 992. [DOI] [PubMed] [Google Scholar]
- Veronesi E, Hamblin C and Mellor P, 2005. Live attenuated bluetongue vaccine viruses in Dorset Poll sheep, before and after passage in vector midges (Diptera: Ceratopogonidae). Vaccine, 23, 5509–5516. [DOI] [PubMed] [Google Scholar]
- Veronesi E, Venter G, Labuschagne K, Mellor P and Carpenter S, 2009. Life‐history parameters of Culicoides (Avaritia) imicola Kieffer in the laboratory at different rearing temperatures. Veterinary Parasitology, 163, 370–373. [DOI] [PubMed] [Google Scholar]
- Versteirt V, Balenghien T, Tack W and Wint W 2017. A first estimation of Culicoides imicola and Culicoides obsoletus/Culicoides scoticus seasonality and abundance in Europe. EFSA supporting publication 2017:15(2), EN‐1182, 35 pp. doi: 10.2903/sp.efsa.2017.EN-1182 [DOI] [Google Scholar]
- Viennet E, Balenghien T, Allene X, Rakotoarivony I, Crochet D, Delecolle JC, Lancelot R, Moulia C, Baldet T and Garros C, 2012a. Assessment of the host/vector contact for Palaearctic Culicoides biting midges (Diptera: Ceratopogonidae). Implications for Orbivirus transmission. In : E‐sove 2012 : from biology to integrated control in a changing world. Abstract book. European Society for Vector Ecology, CIRAD, EID, IRD. Montpellier: European Society for Vector Ecology, Résumé, p. 25. [Google Scholar]
- Viennet E, Garros C, Rakotoarivony I, Allène X, Gardès L, Lhoir J, Fuentes I, Venail R, Crochet D and Lancelot R, 2012b. Host‐seeking activity of Bluetongue virus vectors: endo/exophagy and circadian rhythm of Culicoides in Western Europe. PLoS ONE, 7, e48120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitour D, Guillotin J, Sailleau C, Viarouge C, Desprat A, Wolff F, Belbis G, Durand B, Bakkali‐Kassimi L, Breard E, Zientara S and Zanella G, 2011. Colostral antibody induced interference of inactivated bluetongue serotype‐8 vaccines in calves. Veterinary Research, 42, 18, doi: 10.1186/1297-9716-42-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorsprach B, Meiser CK, Werner D, Balczun C and Schaub GA, 2009. Monitoring of Ceratopogonidae in Southwest Germany. Parasitology Research, 105, 337–344. [DOI] [PubMed] [Google Scholar]
- Vosdingh RA, Trainer DO and Easterday BC, 1968. Experimental bluetongue disease in white‐tailed deer. Canadian Journal of Comparative Medicine and Veterinary Science, 32, 382–387. [PMC free article] [PubMed] [Google Scholar]
- Wäckerlin R, Eschbaumer M, Konig P, Hoffmann B and Beer M, 2010. Evaluation of humoral response and protective efficacy of three inactivated vaccines against bluetongue virus serotype 8 one year after vaccination of sheep and cattle. Vaccine, 28, 4348–4355. [DOI] [PubMed] [Google Scholar]
- Weiher W, Bauer B, Mehlitz D, Nijhof AM and Clausen PH, 2014. Field trials assessing deltamethrin (Butox(R)) treatments of sheep against Culicoides species. Parasitology Research, 113, 2641–2645. [DOI] [PubMed] [Google Scholar]
- White DM and Mecham JO, 2004. Lack of detectable bluetongue virus in skin of seropositive cattle: implications for vertebrate overwintering of bluetongue virus. Veterinaria Italiana, 40, 513–519. [PubMed] [Google Scholar]
- White DM, Wilson WC, Blair CD and Beaty BJ, 2005. Studies on overwintering of bluetongue viruses in insects. Journal of General Virology, 86, 453–462. [DOI] [PubMed] [Google Scholar]
- Williams T, 2008. Natural invertebrate hosts of iridoviruses (Iridoviridae). Neotropical Entomology, 37, 615–632. [DOI] [PubMed] [Google Scholar]
- Work TM, Jessup DA and Sawyer MM, 1992. Experimental bluetongue and epizootic hemorrhagic disease virus infection in California black‐tailed deer. Journal of Wildlife Diseases, 28, 623–628. [DOI] [PubMed] [Google Scholar]
- Worwa G, Hilbe M, Chaignat V, Hofmann MA, Griot C, Ehrensperger F, Doherr MG and Thur B, 2010. Virological and pathological findings in Bluetongue virus serotype 8 infected sheep. Veterinary Microbiology, 144, 264–273. [DOI] [PubMed] [Google Scholar]
- Wright PJ and Easton CS, 1996. Natural Incidence of Lagenidium giganteum Couch (Oomycetes: Lagenidiales) Infecting the Biting Midge Culicoides molestus (Skuse) (Diptera: Ceratopogonidae). Australian Journal of Entomology, 35, 131–134. [Google Scholar]
- Zanella G, Durand B, Sellal E, Breard E, Sailleau C, Zientara S, Batten CA, Mathevet P and Audeval C, 2012. Bluetongue virus serotype 8: abortion and transplacental transmission in cattle in the Burgundy region, France, 2008–2009. Theriogenology, 77, 65–72. [DOI] [PubMed] [Google Scholar]
- Zhugunissov K, Yershebulov Z, Barakbayev K, Bulatov Y, Taranov D, Amanova Z and Abduraimov Y, 2015. Duration of protective immunity after a single vaccination with a live attenuated bivalent bluetongue vaccine. Veterinary Research Communications, 39, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zientara S and Ponsart C, 2015. Viral emergence and consequences for reproductive performance in ruminants: two recent examples (bluetongue and Schmallenberg viruses). Reproduction, Fertility and Development, 27, 63–71. [DOI] [PubMed] [Google Scholar]
- Zimmer JY, Saegerman C, Losson B and Haubruge E, 2010. Breeding sites of bluetongue virus vectors, Belgium. Emerging Infectious Diseases, 16, 575–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer JY, Saegerman C, Losson B, Beckers Y, Haubruge E and Francis F, 2013. Chemical composition of silage residues sustaining the larval development of the Culicoides obsoletus/Culicoides scoticus species (Diptera: Ceratopogonidae). Veterinary Parasitology, 191, 197–201. [DOI] [PubMed] [Google Scholar]
- Zimmer JY, Brostaux Y, Haubruge E and Francis F, 2014. Larval development sites of the main Culicoides species (Diptera: Ceratopogonidae) in northern Europe and distribution of coprophilic species larvae in Belgian pastures. Veterinary Parasitology, 205, 676–686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prometheus Protocol Bluetongue


