Abstract
According to Article 12 of Regulation (EC) No 396/2005, the European Food Safety Authority (EFSA) has reviewed the maximum residue levels (MRLs) currently established at European level for the pesticide active substance paclobutrazol. To assess the occurrence of paclobutrazol residues in plants, processed commodities, rotational crops and livestock, EFSA considered the conclusions derived in the framework of Directive 91/414/EEC as well as the European authorisations reported by Member States (including the supporting residues data). Based on the assessment of the available data, MRL proposals were derived and a consumer risk assessment was carried out. Although no apparent risk to consumers was identified, some information required by the regulatory framework was missing. Hence, the consumer risk assessment is considered indicative only and some MRL proposals derived by EFSA still require further consideration by risk managers.
Keywords: paclobutrazol, MRL review, Regulation (EC) No 396/2005, consumer risk assessment, triazole, plant growth regulator
Summary
Paclobutrazol was included in Annex I to Directive 91/414/EEC on 1 June 2011 by Commission Directive 2011/55/EU, and has been deemed to be approved under Regulation (EC) No 1107/2009, in accordance with Commission Implementing Regulation (EU) No 540/2011, as amended by Commission Implementing Regulation (EU) No 541/2011. As the active substance was approved after the entry into force of Regulation (EC) No 396/2005 on 2 September 2008, the European Food Safety Authority (EFSA) is required to provide a reasoned opinion on the review of the existing maximum residue levels (MRLs) for that active substance in compliance with Article 12(1) of the aforementioned regulation. To collect the relevant pesticide residues data, EFSA asked the United Kingdom, the designated rapporteur Member State (RMS), to complete the Pesticide Residues Overview File (PROFile) and to prepare a supporting evaluation report. The PROFile and evaluation report provided by the RMS were made available to the Member States. A request for additional information was addressed to the Member States in the framework of a completeness check period, which was initiated by EFSA on 16 December 2016 and finalised on 16 February 2017. After having considered all the information provided, EFSA prepared a completeness check report which was made available to Member States on 9 March 2017.
Based on the conclusions derived by EFSA in the framework of Directive 91/414/EEC and the additional information provided by the RMS and Member States, EFSA prepared in May 2017 a draft reasoned opinion, which was circulated to Member States for consultation via a written procedure. Comments received by 19 June 2017 were considered during the finalisation of this reasoned opinion. The following conclusions are derived.
The primary crop metabolism of paclobutrazol was investigated in rapeseed. For pulses and oilseeds, the following residue definition for monitoring and risk assessment is proposed: paclobutrazol (sum of constituent isomers). Pending submission of metabolism studies on fruit crops the same residue definition is tentatively applied also to this crop group. The residue definition as paclobutrazol (sum of constituent isomers) is also proposed to rotational crops.
A validated analytical method for enforcement of the proposed residue definition in the four main analytical matrices is available.
Studies investigating the effect of processing on the nature of residues of paclobutrazol were not necessary since the chronic exposure is below 10% of the acceptable daily intake (ADI).
The available data are considered sufficient to derive MRL proposals as well as risk assessment values for oilseeds, pome fruits, apricots and peaches. Nevertheless, considering the lack of a metabolism study on fruit crops, the derived MRLs for pome fruits, peaches and apricots should be considered tentative only. For table olives/olives for oil production, table and wine grapes and plums, the available data were insufficient to derive MRL proposals.
Only the dietary burden calculated for cattle (all diets) was found to exceed the trigger value of 0.1 mg/kg dry matter (DM). The metabolism of paclobutrazol in ruminants was not investigated and no feeding studies were available for this MRL review. Therefore, it was not possible to derive a residue definition and MRLs for animal commodities.
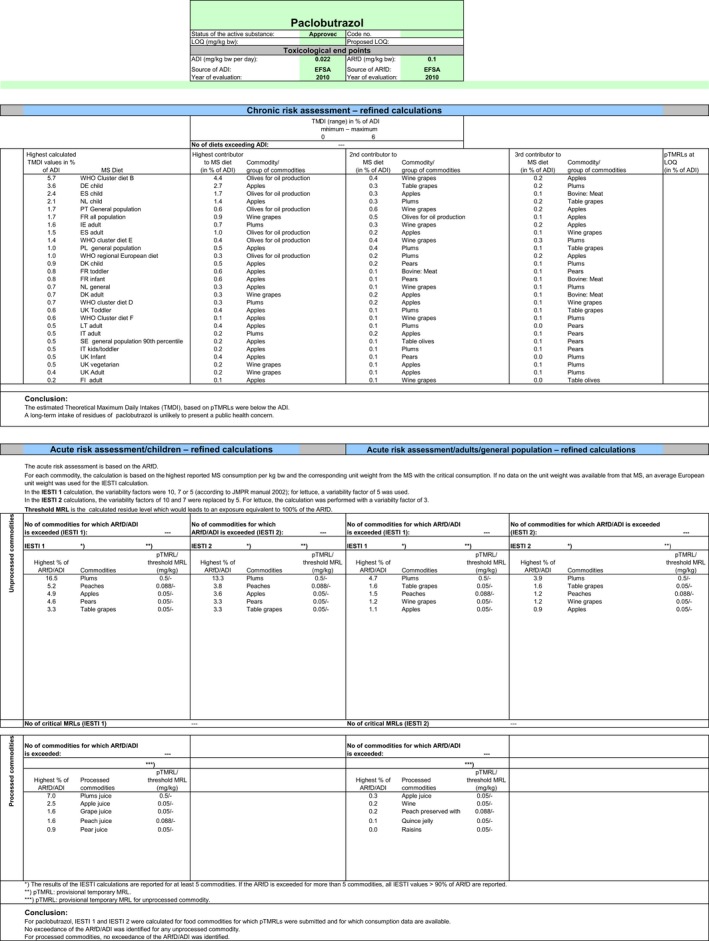
Chronic consumer exposure resulting from the authorised uses reported in the framework of this review accounts for 5.7% of the ADI (WHO, Cluster diet B). The highest acute exposure was calculated for plums, representing 16.5% of the acute reference dose (ARfD).
EFSA emphasises that the above assessment does not consider the possible impact of plant and livestock metabolism on the isomer ratio of the active substance and further investigation on this matter would in principle be required. Since guidance on the consideration of isomer ratios in the consumer risk assessment is not yet available, EFSA recommends that this issue is reconsidered when such guidance is available.
EFSA also emphasises that the above assessment does not yet take into consideration the triazole derivative metabolites (TDMs). Since these metabolites may be generated by several pesticides belonging to the group of triazole fungicides, EFSA recommends that a separate risk assessment should be performed for TDMs as soon as the confirmatory data requested for triazole compounds in the framework of Directive 91/414/EEC and Regulation (EC) No 1107/2009 have been evaluated and a general methodology on the risk assessment of triazole compounds and their TDMs is available.
Background
Regulation (EC) No 396/20051 (hereinafter referred to as ‘the Regulation’) establishes the rules governing the setting and the review of pesticide maximum residue levels (MRLs) at European level. Article 12(1) of that Regulation stipulates that the European Food Safety Authority (EFSA) shall provide within 12 months from the date of the inclusion or non‐inclusion of an active substance in Annex I to Directive 91/414/EEC2 a reasoned opinion on the review of the existing MRLs for that active substance. As paclobutrazol was included in Annex I to Council Directive 91/414/EEC on 1 June 2011 by means of Commission Directive 2011/55/EU,3 and has been deemed to be approved under Regulation (EC) No 1107/20094, in accordance with Commission Implementing Regulation (EU) No 540/20115, as amended by Commission Implementing Regulation (EU) No 541/20116, EFSA initiated the review of all existing MRLs for that active substance.
According to the legal provisions, EFSA shall base its reasoned opinion in particular on the relevant assessment report prepared under Directive 91/414/EEC. It should be noted, however, that, in the framework of Directive 91/414/EEC, only a few representative uses are evaluated, whereas MRLs set out in Regulation (EC) No 396/2005 should accommodate all uses authorised within the EU, and uses authorised in third countries that have a significant impact on international trade. The information included in the assessment report prepared under Directive 91/414/EEC is therefore insufficient for the assessment of all existing MRLs for a given active substance.
To gain an overview of the pesticide residues data that have been considered for the setting of the existing MRLs, EFSA developed the Pesticide Residues Overview File (PROFile). The PROFile is an inventory of all pesticide residues data relevant to the risk assessment and MRL setting for a given active substance. This includes data on:
the nature and magnitude of residues in primary crops;
the nature and magnitude of residues in processed commodities;
the nature and magnitude of residues in rotational crops;
the nature and magnitude of residues in livestock commodities;
the analytical methods for enforcement of the proposed MRLs.
The United Kingdom, the designated rapporteur Member State (RMS) in the framework of Directive 91/414/EEC, was asked to complete the PROFile for paclobutrazol and to prepare a supporting evaluation report (United Kingdom, 2012). The PROFile and the supporting evaluation report were submitted to EFSA on 29 March 2012 and made available to the Member States. A request for additional information was addressed to the Member States in the framework of a completeness check period which was initiated by EFSA on 16 December 2016 and finalised on 16 February 2017. Additional evaluation reports were submitted by France, Germany, Hungary, Italy, Spain, the United Kingdom (Hungary, 2016; France, 2017; Germany, 2017; Italy, 2017a,b; Spain, 2017; United Kingdom, 2017) and the European Union Reference Laboratories for Pesticide Residues (EURLs) (EURL, 2017) and, after having considered all the information provided by RMS and Member States, EFSA prepared a completeness check report which was made available to all Member States on 9 March 2017. Further clarifications were sought from Member States via a written procedure in March 2017.
Based on the conclusions derived by EFSA in the framework of Directive 91/414/EEC and the additional information provided by the Member States, EFSA prepared in May 2017 a draft reasoned opinion, which was submitted to Member States for commenting via a written procedure. All comments received by 19 June 2017 were considered by EFSA during the finalisation of the reasoned opinion.
The evaluation report submitted by the RMS (United Kingdom, 2012) and the evaluation reports submitted by France, Germany, Hungary, Italy, Spain, the United Kingdom (Hungary, 2016; France, 2017; Germany, 2017; Italy, 2017a,b; Spain, 2017; United Kingdom, 2017) and the EURLs (EURL, 2017) are considered as supporting documents to this reasoned opinion, and thus are made publicly available.
In addition, key supporting documents to this reasoned opinion are the completeness check report (EFSA, 2017a) and the Member States consultation report (EFSA, 2017b). These reports are developed to address all issues raised in the course of the review, from the initial completeness check to the reasoned opinion. Also, the chronic and acute exposure calculations for all crops reported in the framework of this review performed using the EFSA Pesticide Residues Intake Model (PRIMo) (excel file) and the PROFile are key supporting documents and made publicly available as background documents to this reasoned opinion. Furthermore, a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
Terms of Reference
According to Article 12 of Regulation (EC) No 396/2005, EFSA shall provide a reasoned opinion on:
the inclusion of the active substance in Annex IV to the Regulation, when appropriate;
the necessity of setting new MRLs for the active substance or deleting/modifying existing MRLs set out in Annex II or III of the Regulation;
the inclusion of the recommended MRLs in Annex II or III to the Regulation;
the setting of specific processing factors as referred to in Article 20(2) of the Regulation.
The active substance and its use pattern
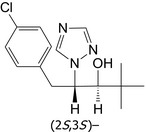
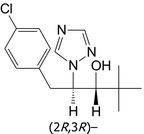
Paclobutrazol is the ISO common name for (2RS,3RS)‐1‐(4‐chlorophenyl)‐4,4‐dimethyl‐2‐(1H‐1,2,4‐triazol‐1‐yl)pentan‐3‐ol (IUPAC), in a 1:1 ratio of (2S,3S)‐ and (2R,3R)‐enantiomers.
Paclobutrazol belongs to the group of triazole chemical class compounds which are used as plant growth regulators. Paclobutrazol inhibits gibberllin biosynthesis by inhibition of the conversion of ent‐kaurene to ent‐kaurenoic acid, and inhibits sterol biosynthesis by inhibition of demethylation; hence inhibits the rate of cell division.
The chemical structure of the active substance and its main metabolites are reported in Appendix F.
Paclobutrazol was evaluated in the framework of Directive 91/414/EEC with the United Kingdom designated as rapporteur Member State (RMS). The representative use supported for the peer review process was outdoor foliar spray, north/south application on winter oilseed rape. Initially, paclobutrazol was not included in Annex I to Council Directive 91/414/EEC by Decision 2008/934.7 Following the first decision on non‐inclusion of the active substance in Annex I to Directive 91/414/EEC, the applicant submitted a new application within the framework of Commission Regulation (EC) No 33/20088, for the inclusion of the active substance in Annex I of Directive 91/414/EEC. Following the peer review, which was carried out by EFSA, a decision on inclusion of the active substance in Annex I to Directive 91/414/EEC was published by means of Commission Directive 2011/55/EU, which entered into force on 1 June 2011. According to Regulation (EU) No 540/2011, as amended by Commission Implementing Regulation (EU) No 541/2011, paclobutrazol is deemed to have been approved under Regulation (EC) No 1107/2009. This approval is restricted to uses as plant growth regulator only.
The EU MRLs for paclobutrazol are established in Annex IIIA of Regulation (EC) No 396/2005 and codex maximum residue limits (CXLs) for paclobutrazol are not available. No MRL changes occurred since the entry into force of the Regulation mentioned above.
For the purpose of this MRL review, the critical uses of paclobutrazol currently authorised within the EU have been collected by the RMS and reported in the PROFile. The additional good agricultural practices (GAPs) reported by Member States during the completeness check were also considered. The details of the authorised GAPs for paclobutrazol are given in Appendix A. Member States did not report any use authorised in third countries that might have a significant impact on international trade.
Assessment
EFSA has based its assessment on the PROFile submitted by the RMS, the evaluation report accompanying the PROFile (United Kingdom, 2012), the draft assessment report (DAR), the additional report to the draft assessment report and the final addendum to the additional report prepared under Council Directive 91/414/EEC and in the framework of Commission Regulation (EC) No 33/2008 (United Kingdom 2006, 2010a,b), the conclusion on the peer review of the pesticide risk assessment of the active substance paclobutrazol (EFSA, 2010) as well as the evaluation reports submitted during the completeness check (Hungary, 2016; EURL, 2017; France, 2017; Germany, 2017, Italy, 2017a,b; Spain, 2017; United Kingdom, 2017). The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/20119 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1997a,b,c,d,e,f,g, 2000, 2010a, b, 2016 and OECD, 2011, 2013).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved from the list of end points reported in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of paclobutrazol, labelled on the phenyl and triazole moieties, was investigated in rapeseed (United Kingdom, 2006). After foliar application of 62.5 g a.s./ha or 187.5 g a.s./ha, the parent compound was extensively metabolised and was found in seed at only 0.03% of the total radioactive residues (TRR), corresponding to 0.0001 mg/kg. The major metabolite in the seed was triazole alanine (31.1% of TRR, 0.06 mg/kg). Other unknown metabolites detected did not exceed 0.01 mg/kg.
A metabolism study on apples was reported during the completeness check (Italy, 2017a) and it is considered in this review. After foliar application on apples of 250 g a.s./ha and preharvest interval (PHI) of 56 days, the majority of the TRR was detected in the peel. Only three apples were examined per radiolabel (triazole and ‘backbone’) and the technique used for identification and characterisation (thin‐layer chromatography (TLC)) was not considered sufficiently specific and did not allow for structural identification of metabolites. Aqueous solubles (20.5–41% TRR) were not characterised although contained free triazole amongst other compounds. Due to these deficiencies, this metabolism study was deemed not appropriate to support the GAPs on fruit crops.
1.1.2. Nature of residues in rotational crops
Paclobutrazol is authorised on crops that may be grown in rotation. According to the soil degradation studies evaluated in the framework of the peer review, there was no field DT90 reported, but the DT90 for paclobutrazol obtained from laboratory studies was higher than 100 days, indicating that paclobutrazol is persistent (EFSA, 2010).
One confined rotational crop study with paclobutrazol labelled on the phenyl and triazole rings was assessed during the peer review (EFSA, 2010). After one application on bare soil (100 g a.s/ha), radish, mustard and wheat were planted at three different plant back intervals (30, 120 and 365 days after treatment (DAT)). As for the primary crop metabolism, the parent compound was not detected and the residues in rotational crops were mainly composed of triazole derivative metabolites (TDMs): triazole alanine (up to 78% TRR in radish roots), triazole lactic acid (up to 20% TRR in wheat grain, radish tubers) and triazole acetic acid (up to 52% TRR in wheat straw). The levels for triazole alanine were higher than 0.01 mg/kg in all crops and at all sampling intervals, whereas the levels of triazole acetic acid and triazole lactic acid were higher than 0.01 mg/kg in all sampling intervals for wheat forage, wheat straw and wheat grain, but were below 0.01 mg/kg in all sampling intervals for mustard leaves, radish leaves and radish tubers. Therefore, it can be concluded that significant levels of TDMs can be observed in cereals even 365 DAT.
1.1.3. Nature of residues in processed commodities
Studies investigating the effect of processing on the nature of residues of paclobutrazol were not available. Nevertheless, they are not necessary since the total theoretical maximum daily intake is below 10% of the acceptable daily intake (ADI).
1.1.4. Methods of analysis in plants
During the peer review, a multiresidue analytical method using liquid chromatography with tandem mass spectrometry (LC–MS/MS) was validated for the determination of paclobutrazol in high oil content commodities with a limit of quantification (LOQ) of 0.01 mg/kg (EFSA, 2010). A multiresidue analytical method using high‐performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) was validated for high acid content and high water content matrices with a LOQ of 0.01 mg/kg (Italy, 2017a). Furthermore, the EURLs reported validation data for the four main plant matrices, with a LOQ of 0.01 mg/kg (EURL, 2017). Hence, it is concluded that paclobutrazol can be enforced with a LOQ of 0.01 mg/kg in high water content, high acid content, high oil content and dry commodities.
1.1.5. Stability of residues in plants
In the framework of the peer review, storage stability of paclobutrazol was demonstrated for a period of 27 months at −18°C in high oil content matrices (EFSA, 2010). Furthermore, the storage stability of paclobutrazol was demonstrated for a period of 12 months at −18°C in high water content and high acid content matrices (Italy, 2017a).
1.1.6. Proposed residue definitions
In the framework of the peer review, the residue definition for monitoring was defined as the parent compound paclobutrazol only; however, two separate residue definitions were proposed for risk assessment: (1) paclobutrazol and (2) triazole derivative metabolites (provisional).
A comprehensive risk assessment for TDMs is being currently carried out by EFSA (United Kingdom, 2016) and (EFSA, 2016). However, at this stage of the assessment, issues on the toxicological reference values for the TDMs need to be further discussed and it is not yet possible to conclude whether the TDMs should be summed with the parent levels or whether they should be considered separately. Therefore, in the present review, EFSA is proposing that the residue definition for enforcement and risk assessment is paclobutrazol only. Considering that the active substance is a racemic mixture of two enantiomers, EFSA also proposes to modify the wording of the residue definition as following: paclobutrazol (sum of constituent isomers). For the future, a second residue definition for risk assessment including TDMs should be considered. This will be assessed pending upon the overall assessment of the confirmatory data on the TDMs. The above residue definition applies to pulses and oilseeds and to rotational crops. Pending submission of metabolisms studies on fruit crops the same residue definition is tentatively applied also to this crop group. There was no need to investigate the nature of residues in processed commodities.
An analytical method for the enforcement of the proposed residue definition at the LOQ of 0.01 mg/kg in all matrices is available.
In addition, EFSA emphasises that the above studies do not investigate the possible impact of plant metabolism on the isomer ratio of paclobutrazol and further investigation on this matter would in principle be required. Since guidance on the consideration of isomer ratios in the consumer risk assessment is not yet available, EFSA recommends that this issue is reconsidered when such guidance is available.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
To assess the magnitude of paclobutrazol residues resulting from the reported GAPs, EFSA considered all residue trials reported by the RMS in its evaluation report (United Kingdom, 2012), including residue trials evaluated in the framework of the peer review (United Kingdom, 2006; EFSA, 2010) and additional data submitted during the completeness check (Italy, 2017b; Spain, 2017). All residue trial samples considered in this framework were stored in compliance with the demonstrated storage conditions. Decline of residues during storage of the trial samples is therefore not expected.
The number of residue trials and extrapolations were evaluated in accordance with the European guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs (European Commission, 2016).
For some crops, the number of residue trials reported is not compliant with the data requirements, therefore MRL and risk assessment values could not be derived by EFSA and the following data gaps were identified:
Table olives/olives for oil production: eight trials compliant with the southern outdoor GAP are required;
Table/wine grapes: six additional trials on table/wine grapes compliant with the southern outdoor GAP are required;
Plums: six additional trials on plums compliant with the southern outdoor GAP are required.
For all other crops, the available residue trials are sufficient to derive MRL and risk assessment values, taking note of the following considerations:
Sesame seeds, rapeseeds, borage seeds, gold of pleasure, hempseeds: the number of residue trials supporting the southern outdoor GAPs is not compliant with the data requirements for these crops (seven trials instead of eight). However, the reduced number of residue trials is considered acceptable in this case because all results were below the LOQ and a no‐residue situation is expected. Further residue trials are therefore not required.
It is noted that different southern outdoor GAPs not supported by data are authorised in Spain for pome fruits, apricots, peaches and plums. Full data sets supporting these GAPS are therefore still required.
1.2.2. Magnitude of residues in rotational crops
According to the results from the confined rotational crop studies, it can be concluded that, with the possible exception of the triazole metabolites, no significant residues are expected to occur in rotational crops provided that paclobutrazol is applied according to the GAPs considered in this review.
1.2.3. Magnitude of residues in processed commodities
There were no studies on the magnitude of residues in processed commodities available for this MRL review.
1.2.4. Proposed MRLs
The available data are considered sufficient to derive MRL proposals as well as risk assessment values for oilseeds, pome fruits, apricots and peaches. Nevertheless, considering the lack of a metabolism study on fruit crops, the derived MRLs for pome fruits, peaches and apricots should be considered tentative only. For table olives/olives for oil production, table and wine grapes and plums the available data were insufficient to derive MRL proposals.
2. Residues in livestock
Paclobutrazol is authorised for use on oilseeds and pome fruits that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance (OECD, 2013), which has now also been agreed upon at European level. The input values for all relevant commodities are summarised in Appendix D. The dietary burden calculated for cattle (all diets) was found to exceed the trigger value of 0.1 mg/kg dry matter (DM). However, since no studies investigating the behaviour of residues in livestock or feeding studies were available, it was not possible to derive residue definition and MRL proposals for animal commodities in this MRL review.
3. Consumer risk assessment
Chronic and acute exposure calculations for all crops reported in the framework of this review were performed using revision 2 of the EFSA PRIMo (EFSA, 2007). Input values for the exposure calculations were derived in compliance with the decision tree reported in Appendix E. Hence, for those commodities where a (tentative) MRL could be derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies (FAO, 2009). For those commodities where data were insufficient to derive a MRL in Section 1, EFSA considered the existing EU MRL for an indicative calculation. All input values included in the exposure calculations are summarised in Appendix D.
The exposure values calculated were compared with the toxicological reference values for paclobutrazol, derived by EFSA (2010) in the framework of Commission Regulation (EC) No 33/2008. The highest chronic exposure was calculated for WHO Cluster diet B, representing 5.7% of the ADI, and the highest acute exposure was calculated for plums, representing 16.5% of the acute reference dose (ARfD). Although major uncertainties remain due to the data gaps identified in the previous sections, this indicative exposure calculation did not indicate a risk to consumers.
EFSA emphasises that the above assessment does not consider the possible impact of plant and livestock metabolism on the isomer ratio of the active substance and further investigation on this matter would in principle be required. Since guidance on the consideration of isomer ratios in the consumer risk assessment is not yet available, EFSA recommends that this issue is reconsidered when such guidance is available.
EFSA also emphasises that the above assessment does not yet take into consideration TDMs. Since these metabolites may be generated by several pesticides belonging to the group of triazole fungicides, EFSA recommends that a separate risk assessment should be performed for TDMs as soon as the confirmatory data requested for triazole compounds in the framework of Directive 91/414/EEC and Regulation (EC) No 1107/2009 have been evaluated and a general methodology on the risk assessment of triazole compounds and their TDMs is available (United Kingdom, 2016) and (EFSA, 2016).
Conclusions
The primary crop metabolism of paclobutrazol was investigated in rapeseed. For pulses and oilseeds, the following residue definition for monitoring and risk assessment is proposed: paclobutrazol (sum of constituent isomers). Pending submission of metabolism studies on fruit crops the same residue definition is tentatively applied also to this crop group. The residue definition as paclobutrazol (sum of constituent isomers) is also proposed to rotational crops.
A validated analytical method for enforcement of the proposed residue definition in the four main analytical matrices is available.
Studies investigating the effect of processing on the nature of residues of paclobutrazol were not necessary since the chronic exposure is below 10% of the ADI.
The available data are considered sufficient to derive MRL proposals as well as risk assessment values for oilseeds, pome fruits, apricots and peaches. Nevertheless, considering the lack of a metabolism study on fruit crops, the derived MRLs for pome fruits, peaches and apricots should be considered tentative only. For table olives/olives for oil production, table and wine grapes and plums, the available data were insufficient to derive MRL proposals.
Only the dietary burden calculated for cattle (all diets) was found to exceed the trigger value of 0.1 mg/kg DM. The metabolism of paclobutrazol in ruminants was not investigated and no feeding studies were available for this MRL review. Therefore, it was not possible to derive a residue definition and MRLs for animal commodities.
Chronic consumer exposure resulting from the authorised uses reported in the framework of this review represents 5.7% of the ADI (WHO, Cluster diet B). The highest acute exposure was calculated for plums, representing 16.5% of the ARfD.
EFSA emphasises that the above assessment does not consider the possible impact of plant and livestock metabolism on the isomer ratio of the active substance and further investigation on this matter would in principle be required. Since guidance on the consideration of isomer ratios in the consumer risk assessment is not yet available, EFSA recommends that this issue is reconsidered when such guidance is available.
EFSA also emphasises that the above assessment does not yet take into consideration TDMs. Since these metabolites may be generated by several pesticides belonging to the group of triazole fungicides, EFSA recommends that a separate risk assessment should be performed for TDMs as soon as the confirmatory data requested for triazole compounds in the framework of Directive 91/414/EEC and Regulation (EC) No 1107/2009 have been evaluated and a general methodology on the risk assessment of triazole compounds and their TDMs is available (United Kingdom, 2016) and (EFSA, 2016).
Recommendations
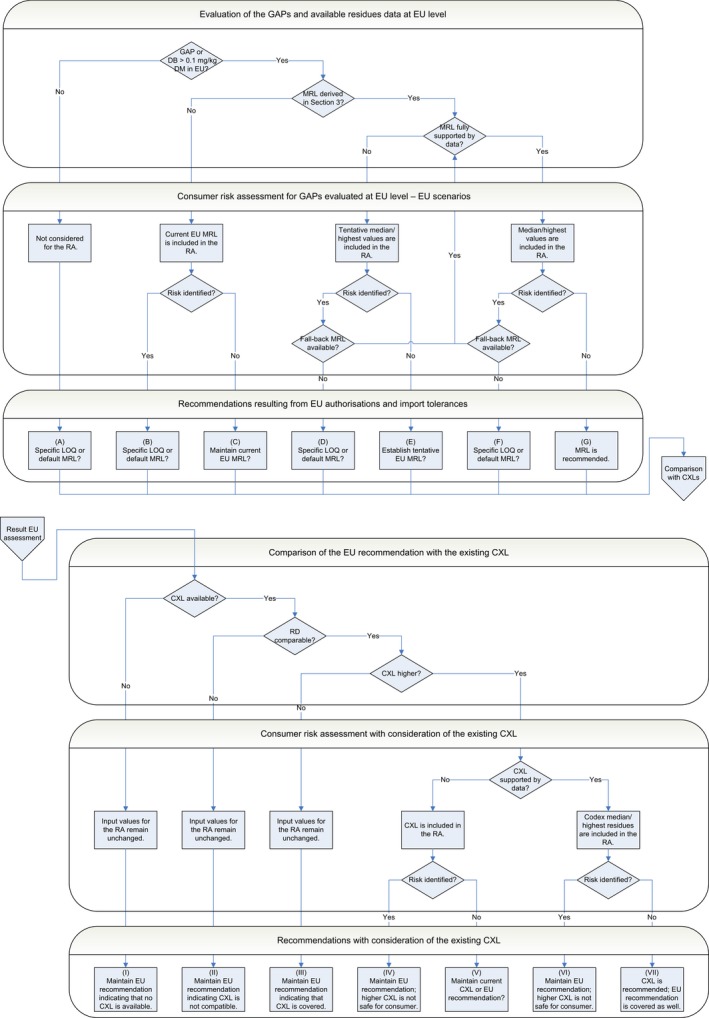
MRL recommendations were derived in compliance with the decision tree reported in Appendix E of the reasoned opinion (see Table 1). MRL values listed as ‘Recommended’ in the table are sufficiently supported by data and are therefore proposed for inclusion in Annex II to the Regulation. The remaining MRL values listed in the table are not recommended for inclusion in Annex II because they require further consideration by risk managers (see Table 1 footnotes for details). In particular, some tentative MRLs and existing EU MRLs need to be confirmed by the following data:
a representative study investigating primary crop metabolism in fruit crops;
residue trials supporting the southern outdoor GAP on table olives/olives for oil production, table/wine grapes and plums;
a representative study investigating the metabolism in ruminants and, eventually, livestock feeding studies (data gap relevant also for the authorisations on apples).
Table 1.
Summary table
| Code numbera | Commodity | Existing EU MRL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|
| MRL (mg/kg) | Comment | |||
|
Enforcement residue definition (existing): paclobutrazol (sum of constituent isomers) Enforcement residue definition (proposed): paclobutrazol (sum of constituent isomers) | ||||
| 130010 | Apples | 0.5 | 0.05* | Further consideration neededb |
| 130020 | Pears | 0.5 | 0.05* | Further consideration neededb |
| 130030 | Quinces | 0.5 | 0.05* | Further consideration neededb |
| 130040 | Medlars | 0.5 | 0.05* | Further consideration neededb |
| 130050 | Loquats/Japanese medlars | 0.5 | 0.05* | Further consideration neededb |
| 140010 | Apricots | 0.5 | 0.15 | Further consideration neededb |
| 140030 | Peaches | 0.5 | 0.15 | Further consideration neededb |
| 140040 | Plums | 0.5 | 0.5 | Further consideration neededd |
| 151010 | Table grapes | 0.05 | 0.05 | Further consideration neededd |
| 151020 | Wine grapes | 0.05 | 0.05 | Further consideration neededd |
| 161030 | Table olives | 0.5 | 0.5 | Further consideration neededd |
| 401010 | Linseeds | 0.02* | 0.01* | Recommendedc |
| 401040 | Sesame seeds | 0.02* | 0.01* | Recommendedc |
| 401060 | Rapeseeds/canola seeds | 0.02* | 0.01* | Recommendedc |
| 401080 | Mustard seeds | 0.02* | 0.01* | Recommendedc |
| 401120 | Borage seeds | 0.02* | 0.01* | Recommendedc |
| 401130 | Gold of pleasure seeds | 0.02* | 0.01* | Recommendedc |
| 401140 | Hemp seeds | 0.02* | 0.01* | Recommendedc |
| 402010 | Olives for oil production | 0.5 | 0.5 | Further consideration neededd |
| 1012010 | Bovine muscle | 0.02* | 0.02 | Further consideration neededd |
| 1012020 | Bovine fat tissue | 0.02* | 0.02 | Further consideration neededd |
| 1012030 | Bovine liver | 0.02* | 0.02 | Further consideration neededd |
| 1012040 | Bovine kidney | 0.02* | 0.02 | Further consideration neededd |
| 1012010 | Bovine muscle | 0.02* | 0.02 | Further consideration neededd |
| 1015010 | Equine muscle | 0.02* | 0.02 | Further consideration neededd |
| 1015020 | Equine fat tissue | 0.02* | 0.02 | Further consideration neededd |
| 1015030 | Equine liver | 0.02* | 0.02 | Further consideration neededd |
| 1015040 | Equine kidney | 0.02* | 0.02 | Further consideration neededd |
| – | Other commodities of plant and/or animal origin |
See Commission Regulation (EC) No 149/2008f |
– | Further consideration needede |
MRL: maximum residue level; CXL: codex maximum residue limit.
* Indicates that the MRL is set/proposed at the limit of quantification.
Commodity code number, as listed in Annex I of Regulation (EC) No 396/2005.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); no CXL is available (combination E‐I in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination G‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); no CXL is available (combination C‐I in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Commission Regulation (EC) No 149/2008 of 29 January 2008 amending Regulation (EC) No 396/2005 of the European Parliament and of the Council by establishing Annexes II, III and IV setting maximum residue levels for products covered by Annex I thereto. OJ L 58, 1.3.2008, p. 1–398.
It is highlighted, however, that some of the MRLs derived result from GAPs supported by data whereas other GAPs reported by Member States were not supported by data. EFSA therefore identified the following data gaps which are not expected to impact on the validity of the MRLs derived but which might have an impact on national authorisations:
residue trials supporting the GAPs reported by Spain on pome fruits, apricots, peaches and plums for an application of 750 g a.s./ha and PHI 60 days;
If the above reported data gaps are not addressed in the future, Member States are recommended to withdraw or modify the relevant authorisations at national level.
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- BVL
Bundesamt für Verbraucherschutz und Lebensmittelsicherheit, Germany
- bw
body weight
- CAC
Codex Alimentarius Commission
- CAS
Chemical Abstract Service
- CF
conversion factor for enforcement residue definition to risk assessment residue definition
- CXL
codex maximum residue limit
- DAR
draft assessment report
- DAT
days after treatment
- DB
dietary burden
- DM
dry matter
- DT90
period required for 90% dissipation (define method of estimation)
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- HPLC–MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- Mo
monitoring
- MRL
maximum residue level
- MS
mass spectrometry detector
- MS/MS
tandem mass spectrometry detector
- NEU
northern European Union
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- RA
risk assessment
- RD
residue definition
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SEU
southern European Union
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TDMs
triazole derivative metabolites
- TLC
thin‐layer chromatography
- TRR
total radioactive residue
- WHO
World Health Organization
Appendix A – Summary of authorised uses considered for the review of MRLs
1.
| Crop | Region | Outdoor/ Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common name | Scientific name | Type | Content | Growth stage | Number | Interval (days) | Rate | |||||||||||||
| Conc. | Unit | Method | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | |||||||||
| Critical outdoor GAPs for Northern Europe | ||||||||||||||||||||
| Linseeds | Linum usitatissimum | NEU | Outdoor | FR | Growth regulator | SC | 125.0 | g/L | Foliar treatment – spraying | 31 | 53 | 2 | 150 | 0.04 | 0.06 | kg a.i./ha | 90 | First application in autumn (BBCH 31) at 0.3 L product/ha followed by a second application in spring (BBCH 31–53) at 0.5 L product/ha | ||
| Rapeseeds | Brassica napus subsp. napus | NEU | Outdoor | CZ, PL | Growth regulator | Foliar treatment – spraying | 14 | 51 | 2 | 0.04 | 0.06 | kg a.i./ha | ||||||||
| Mustard seeds | Brassica juncea; Brassica nigra; Sinapis alba | NEU | Outdoor | FR | Growth regulator | SC | 125.0 | g/L | Foliar treatment – spraying | 31 | 53 | 1 | 0.06 | kg a.i./ha | 90 | |||||
| Borage seeds | Borago officinalis | NEU | Outdoor | FR | Growth regulator | SC | 125.0 | g/L | Foliar treatment – spraying | 31 | 53 | 1 | 0.06 | kg a.i./ha | 90 | |||||
| Gold of pleasure seeds | Camelina sativa | NEU | Outdoor | FR | Growth regulator | SC | 125.0 | g/L | Foliar treatment – spraying | 31 | 53 | 1 | 0.06 | kg a.i./ha | 90 | |||||
| Hemp seeds | Cannabis sativa subsp. sativa; Cannabis sativa subsp. spontanea | NEU | Outdoor | FR | Growth regulator | SC | 125.0 | g/L | Foliar treatment – spraying | 31 | 53 | 1 | 0.06 | kg a.i./ha | 90 | |||||
| Critical outdoor GAPs for Southern Europe | ||||||||||||||||||||
| Apples | Malus domestica | SEU | Outdoor | ES | Growth regulator | SC | 250.0 | g/L | Foliar treatment – general (see also comment field) | 71 | 1 | 0.38 | kg a.i./ha | 45 | Application one month after petals fallen. More critical GAP authorised in ES (foliar, 1 × 750 g a.i./ha, PHI 60 days) but not supported by data | |||||
| Pears | Pyrus communis | SEU | Outdoor | ES | Growth regulator | SC | 250.0 | g/L | Foliar treatment – general (see also comment field) | 71 | 1 | 0.38 | kg a.i./ha | 45 | Application one month after petals fallen. More critical GAP authorised in ES (foliar, 1 × 750 g a.i./ha, PHI 60 days) but not supported by data | |||||
| Quinces | Cydonia oblonga | SEU | Outdoor | ES | Growth regulator | SC | 250.0 | g/L | Foliar treatment – general (see also comment field) | 71 | 1 | 0.38 | kg a.i./ha | 45 | Application one month after petals fallen. More critical GAP authorised in ES (foliar, 1 × 750 g a.i./ha, PHI 60 days) but not supported by data | |||||
| Medlars | Mespilus germanica | SEU | Outdoor | ES | Growth regulator | SC | 250.0 | g/L | Foliar treatment – general (see also comment field) | 71 | 1 | 0.38 | kg a.i./ha | 45 | Application one month after petals fallen. More critical GAP authorised in ES (foliar, 1 × 750 g a.i./ha, PHI 60 days) but not supported by data | |||||
| Loquats | Eriobotrya japonica | SEU | Outdoor | ES | Growth regulator | SC | 250.0 | g/L | Foliar treatment – general (see also comment field) | 71 | 1 | 0.38 | kg a.i./ha | 45 | Application one month after petals fallen. More critical GAP authorised in ES (foliar, 1 × 750 g a.i./ha, PHI 60 days) but not supported by data | |||||
| Apricots | Armeniaca vulgaris, syn: Prunus armeniaca | SEU | Outdoor | ES | Growth regulator | SC | 250.0 | g/L | Foliar treatment – general (see also comment field) | 71 | 1 | 0.38 | kg a.i./ha | 45 | Application one month after petals fallen. More critical GAP authorised in ES (foliar, 1 × 750 g a.i./ha, PHI 60 days) but not supported by data | |||||
| Peaches | Persica vulgaris, syn: Prunus persica | SEU | Outdoor | ES | Growth regulator | SC | 250.0 | g/L | Foliar treatment – general (see also comment field) | 71 | 1 | 0.38 | kg a.i./ha | 45 | Application one month after petals fallen. More critical GAP authorised in ES (foliar, 1 × 750 g a.i./ha, PHI 60 days) but not supported by data | |||||
| Plums | Prunus domestica | SEU | Outdoor | IT | Growth regulator | SC | 250.0 | g/L | Foliar treatment – spraying | 10 | 57 | 1 | 0.20 | kg a.i./ha | Dissolve the corresponding dose for each tree in 250–500 cc water. PHI covered by period between application and harvest. More critical GAP authorised in ES (foliar, 1 × 750 g a.i./ha, PHI 60 days) but not supported by data | |||||
| Table grapes | Vitis vinifera | SEU | Outdoor | IT | Growth regulator | SC | 250.0 | g/L | Foliar treatment – spraying | 53 | 57 | 1 | 0.06 | kg a.i./ha | Preflowering treatment | |||||
| Wine grapes | Vitis vinifera | SEU | Outdoor | IT | Growth regulator | SC | 250.0 | g/L | Foliar treatment – spraying | 53 | 57 | 1 | 0.06 | kg a.i./ha | Preflowering treatment | |||||
| Table olives | Olea europaea | SEU | Outdoor | ES | Growth regulator | SC | 250.0 | g/L | Foliar treatment – spraying | 1 | 0.13 | 2.00 | kg a.i./ha | 60 | 45 days after flowering. Although in practice the farmer never uses more than 3 L product/ha. | |||||
| Sesame seeds | Sesamum indicum | SEU | Outdoor | FR | Growth regulator | SC | 125.0 | g/L | Foliar treatment – spraying | 31 | 53 | 1 | 0.06 | kg a.i./ha | 90 | |||||
| Rapeseeds | Brassica napus subsp. napus | SEU | Outdoor | FR | Growth regulator | SC | 125.0 | g/L | Foliar treatment – spraying | 31 | 53 | 1 | 0.06 | kg a.i./ha | 90 | |||||
| Borage seeds | Borago officinalis | SEU | Outdoor | FR | Growth regulator | SC | 125.0 | g/L | Foliar treatment – spraying | 31 | 53 | 1 | 0.06 | kg a.i./ha | 90 | |||||
| Gold of pleasure seeds | Camelina sativa | SEU | Outdoor | FR | Growth regulator | SC | 125.0 | g/L | Foliar treatment – spraying | 31 | 53 | 1 | 0.06 | kg a.i./ha | 90 | |||||
| Hemp seeds | Cannabis sativa subsp. sativa; Cannabis sativa subsp. spontanea | SEU | Outdoor | FR | Growth regulator | SC | 125.0 | g/L | Foliar treatment – spraying | 31 | 53 | 1 | 0.06 | kg a.i./ha | 90 | |||||
| Olives for oil production | Olea europaea var. europaea | SEU | Outdoor | ES | Growth regulator | SC | 250.0 | g/L | Foliar treatment – spraying | 1 | 0.13 | 2.00 | kg a.i./ha | 60 | 45 days after flowering. Although in practice the farmer never uses more than 3 L product/ha | |||||
MRL: maximum residue level; GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient; SC: suspension concentrate.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
|
Primary crops (available studies) |
Crop groups | Crop(s) | Application(s) | Sampling (DAT) |
|---|---|---|---|---|
| Pulses/oilseeds | Rapeseed | Foliar, 1 × 62.5 g a.s./ha or 1 × 187.5 g a.s./ha | 90 (whole plant), 117–125 (mature seeds) | |
| Source: United Kingdom, 2006 | ||||
|
Rotational crops (available studies) |
Crop groups | Crop(s) | Application(s) |
PBI (DAT) |
| Root/tuber crops | Radish | Bare soil, 100 g a.s./ha | 30, 120, 365 | |
| Leafy crops | Mustard | Bare soil, 100 g a.s./ha | 30, 120, 365 | |
| Cereal (small grain) | Wheat | Bare soil, 100 g a.s./ha | 30, 120, 365 | |
| Source: United Kingdom, 2006 | ||||
|
Processed commodities (hydrolysis study) |
Conditions | Investigated? |
| Pasteurisation (20 min, 90 °C, pH 4) | No | |
| Baking, brewing and boiling (60 min, 100 °C, pH 5) | No | |
| Sterilisation (20 min, 120 °C, pH 6) | No | |
| Not available and not required. | ||
| Can a general residue definition be proposed for primary crops? | No |
| Rotational crop and primary crop metabolism similar? | Yes (tentative) |
| Residue pattern in processed commodities similar to residue pattern in raw commodities? | Not applicable (chronic exposure is lower than 10% of the ADI) |
| Plant residue definition for monitoring (RD‐Mo) | paclobutrazol (sum of constituent isomers) (limited to oilseeds, tentative for fruit crops) |
| Plant residue definition for risk assessment (RD‐RA) |
RD – risk assessment 1: paclobutrazol (sum of constituent isomers) (limited to oilseeds, tentative for fruit crops) RD – risk assessment 2 (provisional): a separate risk assessment needs to be carried out for the triazole derivative metabolites (TDMs). This is foreseen in the framework of the on‐going assessment of the confirmatory data for triazole compounds and TDMs |
| Conversion factor (monitoring to risk assessment) | Not applicable |
| Methods of analysis for monitoring of residues (analytical technique, crop groups, LOQs) |
LC–MS/MS (EFSA, 2010):
HPLC–MS/MS (Italy, 2017a):
LC–MS/MS (EURL, 2017):
|
a.s.: active substance; DAT: days after treatment; PBI: plant‐back interval; ADI: acceptable daily intake; HPLC–MS/MS: high‐performance liquid chromatography with tandem mass spectrometry; LC–MS/MS: liquid chromatography with tandem mass spectrometry; LOQ: limit of quantification.
B.1.1.2. Stability of residues in plants
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Crop | Region/indoora | Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) | Recommendations/comments (OECD calculations) |
MRL proposals (mg/kg) |
HRMo (mg/kg)b | STMRMo (mg/kg)c |
|---|---|---|---|---|---|---|
| Pome fruits | SEU | 8 × < 0.05 |
Combined data set of trials on apples (4) and pears (4) compliant with GAP (Italy, 2017b; Spain, 2017). Due to the lack of a metabolism study on fruit crops, a no‐residue situation cannot be anticipated. Extrapolation to the whole group of pome fruits is possible MRLOECD = 0.05 |
0.05* (tentative)d | 0.05 | 0.05 |
|
Apricots Peaches |
SEU | 3 × < 0.01; 0.012; 0.019; 0.027; 0.052; 0.088 |
Combined data set of trials on peaches (4) and apricots (4) compliant with GAP (Spain, 2017) MRLOECD = 0.14 |
0.15 (tentative)d | 0.09 | 0.02 |
| Plums | SEU | 2 × < 0.01 | Trials compliant with GAP (Italy, 2017b). Due to the lack of a metabolism study on fruit crops, a no‐residue situation cannot be anticipated. Number of trials is therefore not sufficient to derive a MRL proposal | – | – | – |
|
Wine grapes Table grapes |
SEU | 2 × < 0.01 | Trials compliant with GAP (Italy, 2017b). Due to the lack of a metabolism study on fruit crops, a no‐residue situation cannot be anticipated. Number of trials is therefore not sufficient to derive a MRL proposal | – | – | – |
| Table olives | SEU | – | No data available | – | – | – |
| Olives for oil production | SEU | – | No data available | – | – | – |
|
Linseeds Rapeseeds Mustard seeds Borage seeds Golds of pleasure seeds Hemp seeds |
NEU | 15 × < 0.01 |
Trials on rapeseeds compliant with GAP (United Kingdom, 2006; EFSA, 2010). Extrapolation to linseeds, mustard seeds, borage seeds, golds of pleasure seeds and hemp seeds is applicable MRLOECD = 0.01 |
0.01* | 0.01 | 0.01 |
|
Sesame seeds Rapeseeds Borage seeds Gold of pleasure seeds Hemp seeds |
SEU | 7 × < 0.01 |
Trials on rapeseeds compliant with GAP (United Kingdom, 2006; EFSA 2010). Extrapolation to sesame seeds, borage seeds, golds of pleasure seeds and hemp seeds is applicable MRLOECD = 0.01 |
0.01* | 0.01 | 0.01 |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
* Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue according to the residue definition for monitoring.
Supervised trials median residue according to the residue definition for monitoring.
MRL is tentative because a metabolism study on fruits crops is missing.
B.1.2.2. Residues in succeeding crops
| Confined rotational crop study(quantitative aspect) | According to the results from the confined rotational crop studies, no significant residues (with the exception of the triazole derivative metabolites) are expected to occur in rotational crops, provided that paclobutrazol is applied according to the GAPs considered in this review |
| Field rotational crop study | Not available. Required for the assessment of triazole derivative metabolites |
B.2. Residues in livestock
| Relevant groups | Dietary burden expressed in | Most critical dieta | Most critical commoditya | Trigger exceeded (Y/N) | |||
|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | ||||||
| Med. | Max. | Med. | Max. | ||||
| Cattle (all diets) | 0.0030 | 0.0030 | 0.13 | 0.13 | Cattle (beef) | Apple, pomace, wet | Yes |
| Cattle (dairy only) | 0.0024 | 0.0024 | 0.06 | 0.06 | Cattle (dairy) | Apple, pomace, wet | No |
| Sheep (all diets) | 0.0027 | 0.0027 | 0.06 | 0.06 | Sheep (lamb) | Apple, pomace, wet | No |
| Sheep (ewe only) | 0.0021 | 0.0021 | 0.06 | 0.06 | Sheep (ram/ewe) | Apple, pomace, wet | No |
| Swine (all diets) | 0.0001 | 0.0001 | 0.00 | 0.00 | Swine (finishing) | Canola, meal | No |
| Poultry (all diets) | 0.0002 | 0.0002 | 0.00 | 0.00 | Poultry (turkey) | Canola, meal | No |
| Poultry (layer only) | 0.0002 | 0.0002 | 0.00 | 0.00 | Poultry (layer) | Canola, meal | No |
bw: body weight; DM: dry matter.
Calculated for the maximum dietary burden.
B.2.1. Nature of residues and methods of analysis in livestock
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
| Livestock (available studies) | Animal | Dose (mg/kg bw per day) | Duration (days) | N rate/comment |
|---|---|---|---|---|
| Lactating goat/cow | – | – | – | |
| Not available but required | ||||
| Time needed to reach a plateau concentration in milk and eggs (days) | Not available |
| Metabolism in rat and ruminant similar (Yes/No) | Not available |
| Animal residue definition for monitoring (RD‐Mo) | Not available |
| Animal residue definition for risk assessment (RD‐RA) | Not available |
| Conversion factor (monitoring to risk assessment) | Not available |
| Fat soluble residues (Yes/No) | Not available |
| Methods of analysis for monitoring of residues (analytical technique, crop groups, LOQs) | Not available |
B.2.1.2. Stability of residues in livestock
| Animal products (available studies) | Animal | Commodity | T (°C) | Stability (Months/years) |
|---|---|---|---|---|
| – | Muscle | – | – | |
| – | Liver | – | – | |
| – | Kidney | – | – | |
| Not available but required | ||||
B.2.2. Magnitude of residues in livestock
B.2.2.1. Summary of the residue data from livestock feeding studies
| Animal commodity |
Residues at the closest feeding level (mg/kg) |
Estimated value at 1N |
MRL proposal (mg/kg) |
CF | ||
|---|---|---|---|---|---|---|
| Mean | Highest |
STMR (mg/kg) |
HR (mg/kg) |
|||
|
Cattle (all diets) Not available but required | ||||||
|
Cattle (dairy only) MRLs are not required since the trigger value is not exceeded | ||||||
|
Sheep (all diets) MRLs are not required since the trigger value is not exceeded | ||||||
|
Sheep (dairy only) MRLs are not required since the trigger value is not exceeded | ||||||
|
Swine MRLs are not required since the trigger value is not exceeded | ||||||
|
Poultry (all diets) MRLs are not required since the trigger value is not exceeded | ||||||
|
Poultry (layer only) MRLs are not required since the trigger value is not exceeded | ||||||
B.3. Consumer risk assessment
B.3.1. Consumer risk assessment
| ADI | 0.022 mg/kg bw per day (EFSA, 2010) |
| Highest IEDI, according to EFSA PRIMo | 5.7% ADI (WHO Cluster diet B) |
| Assumptions made for the calculations |
The calculation is based on the median residue levels in the raw agricultural commodities For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation The contributions of commodities where no GAP was reported in the framework of this review were not included in the calculation |
| ARfD | 0.10 mg/kg bw (EFSA, 2010) |
| Highest IESTI, according to EFSA PRIMo | 16.5% ARfD (plums) |
| Assumptions made for the calculations |
The calculation is based on the highest residue levels in the raw agricultural commodities For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation |
ADI: acceptable daily intake; bw: body weight; IEDI: international estimated daily intake; PRIMo: (EFSA) Pesticide Residues Intake Model; WHO: World Health Organization; ARfD: acute reference dose; IESTI: international estimated short‐term intake.
B.4. Proposed MRLs
| Code numbera | Commodity | Existing EU MRL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|
| MRL (mg/kg) | Comment | |||
|
Enforcement residue definition (existing): paclobutrazol (sum of constituent isomers) Enforcement residue definition (proposed): paclobutrazol (sum of constituent isomers) | ||||
| 130010 | Apples | 0.5 | 0.05* | Further consideration neededb |
| 130020 | Pears | 0.5 | 0.05* | Further consideration neededb |
| 130030 | Quinces | 0.5 | 0.05* | Further consideration neededb |
| 130040 | Medlars | 0.5 | 0.05* | Further consideration neededb |
| 130050 | Loquats/Japanese medlars | 0.5 | 0.05* | Further consideration neededb |
| 140010 | Apricots | 0.5 | 0.15 | Further consideration neededb |
| 140030 | Peaches | 0.5 | 0.15 | Further consideration neededb |
| 140040 | Plums | 0.5 | 0.5 | Further consideration neededd |
| 151010 | Table grapes | 0.05 | 0.05 | Further consideration neededd |
| 151020 | Wine grapes | 0.05 | 0.05 | Further consideration neededd |
| 161030 | Table olives | 0.5 | 0.5 | Further consideration neededd |
| 401010 | Linseeds | 0.02* | 0.01* | Recommendedc |
| 401040 | Sesame seeds | 0.02* | 0.01* | Recommendedc |
| 401060 | Rapeseeds/canola seeds | 0.02* | 0.01* | Recommendedc |
| 401080 | Mustard seeds | 0.02* | 0.01* | Recommendedc |
| 401120 | Borage seeds | 0.02* | 0.01* | Recommendedc |
| 401130 | Gold of pleasure seeds | 0.02* | 0.01* | Recommendedc |
| 401140 | Hemp seeds | 0.02* | 0.01* | Recommendedc |
| 402010 | Olives for oil production | 0.5 | 0.5 | Further consideration neededd |
| 1012010 | Bovine muscle | 0.02* | 0.02 | Further consideration neededd |
| 1012020 | Bovine fat tissue | 0.02* | 0.02 | Further consideration neededd |
| 1012030 | Bovine liver | 0.02* | 0.02 | Further consideration neededd |
| 1012040 | Bovine kidney | 0.02* | 0.02 | Further consideration neededd |
| 1012010 | Bovine muscle | 0.02* | 0.02 | Further consideration neededd |
| 1015010 | Equine muscle | 0.02* | 0.02 | Further consideration neededd |
| 1015020 | Equine fat tissue | 0.02* | 0.02 | Further consideration neededd |
| 1015030 | Equine liver | 0.02* | 0.02 | Further consideration neededd |
| 1015040 | Equine kidney | 0.02* | 0.02 | Further consideration neededd |
| – | Other commodities of plant and/or animal origin | See Commission Regulation (EC) No 149/2008 | – | Further consideration needede |
MRL: maximum residue level; CXL: codex maximum residue limit.
* Indicates that the MRL is set/proposed at the limit of quantification.
Commodity code number, as listed in Annex I of Regulation (EC) No 396/2005.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); no CXL is available (combination E‐I in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination G‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); no CXL is available (combination C‐I in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: paclobutrazol (sum of constituent isomers) | ||||
| Apple, pomace, wet | 0.25 |
STMR × 5 (tentative)a |
0.25 |
STMR × 5 (tentative)a |
| Flaxseed/linseed, meal | 0.01* | STMRb | 0.01* | STMRb |
| Canola (Rape seed), meal | 0.01* | STMRb | 0.01* | STMRb |
| Rape, meal | 0.01* | STMRb | 0.01* | STMRb |
STMR: supervised trials median residue.
* Indicates that the input value is proposed at the limit of quantification.
For apple pomace, in the absence of processing factors supported by data, default processing factor of 5 was included in the calculation to consider the potential concentration of residues in these commodities (it is noted that the occurrence of residues between 0.05 mg/kg (LOQ of residue trials) and 0.01 mg/kg (LOQ for enforcement) cannot be excluded).
For oilseed meals, no default processing factor was applied because paclobutrazol is applied early in the growing season and residues are expected to be below the LOQ. Concentration of residues in these commodities is therefore not expected.
D.2. Consumer risk assessment
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: paclobutrazol (sum of constituent isomers) | ||||
| Apples | 0.05 | STMRMo (tentative) | 0.05 | HRMo (tentative) |
| Pears | 0.05 | STMRMo (tentative) | 0.05 | HRMo (tentative) |
| Quinces | 0.05 | STMRMo (tentative) | 0.05 | HRMo (tentative) |
| Medlars | 0.05 | STMRMo (tentative) | 0.05 | HRMo (tentative) |
| Loquats/Japanese medlars | 0.05 | STMRMo (tentative) | 0.05 | HRMo (tentative) |
| Apricots | 0.02 | STMRMo (tentative) | 0.09 | HRMo (tentative) |
| Peaches | 0.02 | STMRMo (tentative) | 0.09 | HRMo (tentative) |
| Plums | 0.50 | EU MRL | 0.50 | EU MRL |
| Table grapes | 0.05 | EU MRL | 0.05 | EU MRL |
| Wine grapes | 0.05 | EU MRL | 0.05 | EU MRL |
| Table olives | 0.50 | EU MRL | 0.50 | EU MRL |
| Linseeds | 0.01* | STMR | 0.01* | HR |
| Sesame seeds | 0.01* | STMR | 0.01* | HR |
| Rapeseeds/canola seeds | 0.01* | STMR | 0.01* | HR |
| Mustard seeds | 0.01* | STMR | 0.01* | HR |
| Borage seeds | 0.01* | STMR | 0.01* | HR |
| Gold of pleasure seeds | 0.01* | STMR | 0.01* | HR |
| Hemp seeds | 0.01* | STMR | 0.01* | HR |
| Olives for oil production | 0.50 | EU MRL | 0.05 | EU MRL |
| Bovine meat | 0.02* | EU MRL | 0.02* | EU MRL |
| Bovine fat | 0.02* | EU MRL | 0.02* | EU MRL |
| Bovine liver | 0.02* | EU MRL | 0.02* | EU MRL |
| Bovine kidney | 0.02* | EU MRL | 0.02* | EU MRL |
| Equine meat | 0.02* | EU MRL | 0.02* | EU MRL |
| Equine fat | 0.02* | EU MRL | 0.02* | EU MRL |
| Equine liver | 0.02* | EU MRL | 0.02* | EU MRL |
| Equine kidney | 0.02* | EU MRL | 0.02* | EU MRL |
STMR: supervised trials median residue; HR: highest residue; Mo: monitoring; MRL: maximum residue level.
* Indicates that the input value is proposed at the limit of quantification.
Appendix E – Decision tree for deriving MRL recommendations
1.
Appendix F – Used compound codes
1.
| Code/trivial name | Chemical name/SMILES notation | Structural formula |
|---|---|---|
| Paclobutrazol | (2RS,3RS)‐1‐(4‐chlorophenyl)‐4,4‐dimethyl‐2‐(1H‐1,2,4‐triazol‐1‐yl)pentan‐3‐ol |
|
| 1,2,4‐Triazole |
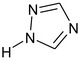
1H‐1,2,4‐triazole (free triazole) (CAS number 288‐88‐0) |
|
| Triazole alanine |
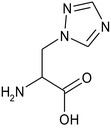
(RS)‐2‐amino‐3‐(1H‐1,2,4 triazol‐1‐yl)propanoic acid or 3‐(1H‐1,2,4‐triazol‐1‐yl)‐d,l‐alanine (CAS number 86362‐20‐1) |
|
| Triazole acetic acid |
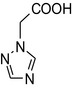
1H‐1,2,4‐triazol‐1‐ylacetic acid (CAS number 28711‐29‐7) |
|
| Triazole lactic acid or triazolehydroxy propionic acid | (R,S)‐2‐hydroxy‐3‐(1H‐1,2,4‐triazol‐1‐yl) propanoic acid |
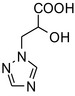
|
SMILES: simplified molecular‐input line‐entry system; CAS: Chemical Abstract Service.
Suggested citation: EFSA (European Food Safety Authority) , Brancato A, Brocca D, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Janossy J, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Nougadere A, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Reasoned opinion on the review of the existing maximum residue levels for paclobutrazol according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2017;15(8):4974, 34 pp. 10.2903/j.efsa.2017.4974
Requestor: European Commission
Question number: EFSA‐Q‐2009‐00067
Amendment: An editorial correction was carried out that does not materially affect the contents or outcome of this scientific output. To avoid confusion, the older version has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made. Values were corrected for existing and proposed MRL for wine and table grapes on page 12 and page 27 of the scientific output.
Acknowledgement: EFSA wishes to thank the rapporteur Member State United Kingdom for the preparatory work on this scientific output.
Approved: 3 August 2017
Amended: 13 September 2017
Notes
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Commission Directive 2011/55/EU of 26 April 2011 Commission Implementing Directive 2011/55/EU of 26 April 2011 amending Council Directive 91/414/EEC to include paclobutrazol as active substance and amending Commission Decision 2008/934/EC. OJ No L 106, 27.4.2011, p. 5–8.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 187–188.
2008/934/EC: Commission Decision of 5 December 2008 concerning the non‐inclusion of certain active substances in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing these substances (notified under document number C(2008) 7637), OJ L 333, 11.12.2008, p. 11–14.
Commission Regulation (EC) No 33/2008 of 17 January 2008 laying down detailed rules for the application of Council Directive 91/414/EEC as regards a regular and an accelerated procedure for the assessment of active substances which were part of the programme of work referred to in Article 8(2) of that Directive but have not been included into its Annex I. OJ L 15, 18.1.2008, p. 5–12.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers' health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Conclusion on the peer review of the pesticide risk assessment of the active substance paclobutrazol. EFSA Journal 2010;8(11):1876, 60 pp. 10.2903/j.efsa.2017.1876 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for triazole derivative metabolites in light of confirmatory data. EFSA Supporting Publication 2016:EN‐1080, 90 pp. 10.2903/j.efsa.2016.1080 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017a. Completeness check report on the review of the existing MRLs of paclobutrazol prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 22 May 2017. Available online: http://www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2017b. Member States consultation report on the review of the existing MRLs of paclobutrazol prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 1 August 2017. Available online: http://www.efsa.europa.eu
- EURL (European Union Reference Laboratories for Pesticide Residues), 2017. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Analytical methods validated by the EURLs and overall capability of official laboratories to be considered for the review of the existing MRLs for paclobutrazol, February 2017. Available online: http://www.efsa.europa.eu
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2016. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.2, September 2016.
- FAO (Food and Agriculture Organization of the United Nations), 2009. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Ed. FAO Plant Production and Protection Paper 197, 264 pp. [Google Scholar]
- France , 2017. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for paclobutrazol, February 2017. Available online: http://www.efsa.europa.eu
- Germany , 2017. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for paclobutrazol, February 2017. Available online: http://www.efsa.europa.eu
- Hungary , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for paclobutrazol, December 2016. Available online: http://www.efsa.europa.eu
- Italy , 2017a. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for paclobutrazol, February 2017. Available online: http://www.efsa.europa.eu
- Italy , 2017b. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Review of the existing MRLs for paclobutrazol, March 2017. Available online: http://www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- Spain , 2017. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for paclobutrazol, February 2017. Available online: http://www.efsa.europa.eu
- United Kingdom , 2006. Draft assessment report on the active substance paclobutrazol prepared by the rapporteur Member State United Kingdom in the framework of Council Directive 91/414/EEC, September 2006. Available online: http://www.efsa.europa.eu
- United Kingdom , 2010a. Additional report to the draft assessment report on the active substance paclobutrazol prepared by the rapporteur Member State United Kingdom in the framework of Commission Regulation (EC) No 33/2008, January 2010. Available online: http://www.efsa.europa.eu
- United Kingdom , 2010b. Final addendum to the additional report on the active substance paclobutrazol prepared by the rapporteur Member State United Kingdom in the framework of Commission Regulation (EC) No 33/2008, compiled by EFSA, September 2010. Available online: http://www.efsa.europa.eu
- United Kingdom , 2012. Evaluation report prepared under Article 12.1 of Regulation (EC) No 396/2005. Review of the existing MRLs for paclobutrazol., March 2012. Available online: http://www.efsa.europa.eu
- United Kingdom , 2016. Triazole Derivative Metabolites – Addendum – Confirmatory Data under Regulation (EC) No. 1107/2009 of the European Council and Parliament, May 2016. Available online: http://www.efsa.europa.eu
- United Kingdom , 2017. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing MRLs for paclobutrazol, February 2017. Available online: http://www.efsa.europa.eu


