Abstract
Salinomycin sodium (SAL‐Na) is active against certain Gram‐positive bacteria, while Gram‐negative species are resistant. SAL‐Na at the proposed concentration is unlikely to increase shedding of Salmonella, Escherichia coli and Campylobacter and or induce resistance and cross‐resistance to antimicrobials important in human and animal therapy. SAL‐Na is safe for chickens for fattening at 70 mg/kg complete feed, for chickens reared for laying at 50 mg/kg complete feed in the first 12 weeks of life. The simultaneous use of SAL‐Na and certain antibiotic drugs (e.g. tiamulin) is contraindicated. SAL‐Na is absorbed and extensively metabolised. Metabolites have reduced ionophoric activity. SAL is the marker residue (MR). No residues in eggs are expected. SAL‐Na is not genotoxic and not a carcinogen. A NOAEL of 0.5 mg/kg body weight (bw) per day is derived from a cardiovascular study in dogs as well as from a 12‐month dog study. Consumer exposure complies with an acceptable daily intake of 0.005 mg SAL/kg bw after 1 h withdrawal. A withdrawal time and maximum residue limits are not considered necessary. SAL‐Na from Sacox® is not an irritant to skin and eyes; it is a potential sensitiser to skin and the respiratory tract. A toxicological risk by inhalation for persons handling the additive cannot be excluded. SAL‐Na in feed for chickens will not pose a risk for the aquatic environment. A risk for the terrestrial ecosystem is considered unlikely due to metabolisation and the rapid degradation of SAL in the environment. SAL‐Na at a minimum concentration of 50 mg/kg complete feed is an effective coccidiostat for chickens for fattening. This conclusion is extended to chickens reared for laying. SAL‐Na in Sacox® 120 microGranulate and Sacox® 200 microGranulate is considered bioequivalent with respect to its anticoccidial effect.
Keywords: coccidiostat, Sacox, salinomycin sodium, safety, efficacy, chickens for fattening, chickens reared for laying
Summary
Following a request from European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of salinomycin sodium (SAL‐Na) from Sacox® 120 microGranulate and Sacox® 200 microGranulate when fed to chickens for fattening and chickens reared for laying.
SAL‐Na is active against certain Gram‐positive bacteria, while Gram‐negative species are resistant. The use of SAL‐Na as a feed additive at the proposed concentration is unlikely to increase shedding of Salmonella, Escherichia coli and Campylobacter or to induce resistance and cross‐resistance to antimicrobials important in human and animal therapy.
SAL‐Na from Sacox® 120 microGranulate or Sacox® 200 microGranulate is safe for chickens for fattening at a concentration of 70 mg/kg complete feed with a margin of safety of 1.7. For chickens reared for laying, 50 mg SAL‐Na/kg complete feed are considered safe for a feeding period of the first 12 weeks of life; a margin of safety cannot be given. The simultaneous use of Sacox® and certain antibiotic drugs (e.g. tiamulin) is contraindicated.
SAL‐Na is absorbed to a certain extent in the chicken and extensively metabolised. Unchanged SAL represents a very small fraction of the metabolites in tissue and excreta. Many metabolites, predominantly mono‐ and multi‐hydroxylated, have been identified in tissues and excreta. The metabolites in excreta showed a higher degree of hydroxylation than in the liver. SAL‐related metabolites have a reduced ionophoric activity when compared with SAL. SAL is considered the MR; ratios of MR to total residue are available for all relevant tissues for 1 and 6 h withdrawal. No residues in eggs are expected provided that the proposed maximum dose and duration of administration are respected.
The FEEDAP Panel reiterates its conclusion from 2004 that (i) SAL‐Na does not induce gene mutations in vitro and it is not genotoxic in vivo, (ii) SAL‐Na is not a carcinogen and (iii) the findings of the reproduction toxicity studies do not lead to concern. A no observed adverse effect level (NOAEL) of 0.5 mg/kg body weight per day is derived from a cardiovascular study in dogs (pharmacological NOAEL) as well as from a 12‐month dog study (toxicological NOAEL). This value is further supported by the NOAEL from the recent 90‐day study in rats.
Exposure estimates to SAL from products of SAL‐Na treated chickens for fattening at the highest proposed use level indicate compliance with an Acceptable Daily Intake of 0.005 mg SAL/kg body weight after 1 h withdrawal, equivalent to a practical 0‐h withdrawal time. Maximum residue limits (MRLs) are not considered necessary.
SAL‐Na from Sacox® 120 microGranulate is not an irritant to skin and eyes; it is considered a potential dermal sensitiser and a likely respiratory sensitiser. These conclusions are considered valid also for the Sacox® 200 microGranulate. The LC50 for acute inhalation toxicity is > 1.2 mg SAL/L. An 8 h exposure to SAL from inhalation is estimated to be about 0.6 mg from Sacox® 120 microGranulate and 2.1 mg from Sacox® 200 microGranulate (1 mg as alveolar fraction). Since no data on the chronic inhalation toxicity of SAL were available, a risk from inhalation toxicity for persons handling the additive cannot be excluded.
The use of the SAL‐Na in feed for chickens for fattening and chickens reared for laying up to the highest proposed dose will not pose a risk for the aquatic environment. Although the PEC/PNEC ratio for plants slightly exceeds the threshold value, a risk for the terrestrial ecosystem is considered unlikely due to metabolisation and the rapid degradation of SAL in the environment.
SAL‐Na is effective in the control of coccidiosis in chickens for fattening. This conclusion is based on the results of three floor pen studies and three anticoccidial sensitivity tests. The minimum effective concentration is 50 mg SAL‐Na/kg complete feed. The conclusion on efficacy is extended to chickens reared for laying.
SAL‐Na in Sacox® 120 microGranulate and Sacox® 200 microGranulate is considered bioequivalent with respect to its anticoccidial effect.
The FEEDAP Panel recommend to use Streptomyces azureus as the correct name of the fermentation strain and to adjust the impurities by name and content to the recent findings.
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 10(2) of that Regulation also specifies that for existing products within the meaning of Article 10(1), an application shall be submitted in accordance with Article 7, at the latest 1 year before the expiry date of the authorisation given pursuant to Directive 70/524/EEC for additives with a limited authorisation period, and within a maximum of 7 years after the entry into force of this Regulation for additives authorised without a time limit or pursuant to Directive 82/471/EEC. Article 13(3) of that Regulation lays down that if the holder of an authorisation proposes changing the terms of the authorisation by submitting an application to the Commission, accompanied by the relevant data supporting the request for the change, the Authority shall transmit its opinion on the proposal to the Commission and the Member States.
The European Commission received requests from Huvepharma N.V.2 for re‐evaluation and for modification of the terms of authorisation of the product Sacox® 120 microGranulate and Sacox® 200 microGranulate (salinomycin sodium), when used as a feed additive for chickens for fattening and chickens reared for laying (category: coccidiostats and histomonostats).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the applications to the European Food Safety Authority (EFSA) as an application under Article 10(2) (re‐evaluation of an authorised feed additive) and an application under Article 13(3) (modification of the authorisation of a feed additive). EFSA received directly from the applicant the technical dossiers in support of the applications. The particulars and documents in support of the applications were considered valid by EFSA as of 24 February 2014, 6 March 2014 and 27 February 2014.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product Sacox® 120 microGranulate and Sacox® 200 microGranulate (salinomycin sodium), when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
The additive Sacox® 120 microGranulate (salinomycin sodium) has been authorised for 10 years for use in chickens reared for laying (authorisation until 11 November 2013)3 and in chickens for fattening (authorisation until 21 August 2014).4 The authorisation for chickens for fattening has been amended as regards the introduction of a maximum residue limit (MRL) for salinomycin sodium.5
There are two other authorisations of salinomycin sodium for chickens for fattening (Salinomax6 and Kokcisan).7
The Scientific Committee on Animal Nutrition (SCAN) issued a series of opinions on the use of salinomycin sodium in feedingstuffs for chickens for fattening (EC, 1982, 1984), for pigs (EC, 1991), for rabbits (EC, 1992), and for chickens reared for laying (EC, 1997).
The European Food Safety Authority (EFSA) issued an opinion on the re‐evaluation of coccidiostat Sacox® 120 microGranulate for chickens for fattening including the setting of MRLs for salinomycin sodium (EFSA, 2004a). The same active substance but different products were also evaluated by EFSA's Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); three opinions were issued on the safety and efficacy of the product Kokcisan 120G (EFSA, 2004b, 2006, 2007) and two opinions on the safety and efficacy of the product BioCox 120G (authorised as Salinomax 120G) (EFSA, 2004c, 2005).
The applicant is now requesting the re‐evaluation of Sacox® microGranulate (salinomycin sodium) for chickens for fattening and for chickens reared for laying. In a third application the reduction of the withdrawal time from the current one day to zero days and for the change of the current MRL is requested.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of three technical dossiers8 in support of the authorisation request for the use of Sacox® 120 microGranulate and Sacox® 200 microGranulate (salinomycin sodium) as a feed additive. The technical dossiers were prepared following the provisions of Article 7 or Article 13 of Regulation (EC) No 1831/2003, Regulation (EC) No 429/20089 and the applicable EFSA guidance documents.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the active substance in animal feed/MR in tissues. The Executive Summary of the EURL report can be found in Annex A.10
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of Sacox® microGranulate (salinomycin sodium) is in line with the principles laid down in Regulation (EC) No 429/2008 and the relevant guidance documents: Guidance for the preparation of dossiers for coccidiostats and histomonostats (EFSA FEEDAP Panel, 2011a), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011b), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008a), Guidance for the preparation of dossiers for the re‐evaluation of certain additives already authorised under Directive 70/524/EEC (EFSA, 2008b), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012a), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012b), Technical Guidance: Microbial Studies (EFSA, 2008c), Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance (EFSA FEEDAP Panel 2012c).
3. Assessment
The applicant submitted two applications for the re‐evaluation of Sacox® microGranulate (salinomycin sodium) under the category coccidiostats and histomonostats: for chickens for fattening at a dose of 60–70 mg salinomycin sodium/kg complete feed11 and for chickens reared for laying at a dose of 50 mg salinomycin sodium/kg feed.12 The applicant further submitted an application for a reduction of the withdrawal time from the current one day to zero days and for the change of the current MRL of 5 μg/kg (all wet tissues) in chickens for fattening and chickens reared for laying to the following: liver, 140 μg/kg; kidney, 35 μg/kg; muscle, 12 μg/kg; and skin/fat, 145 μg/kg.13
The applications cover two formulations: Sacox®120 microGranulate, already evaluated by the FEEDAP Panel (EFSA, 2004a) and Sacox®200 microGranulate, a more concentrated formulation, not assessed before.
The FEEDAP Panel considers reasonable to conclude on the three applications in one single scientific opinion.
3.1. Characterisation14
3.1.1. Identity of the additive
The additives Sacox® 120 microGranulate and Sacox® 200 microGranulate contain 12% and 20% of salinomycin sodium (SAL‐Na) as active substance, respectively. SAL‐Na is produced by Streptomyces azureus (DSM 32267). The final product Sacox® microGranulate is obtained by mixing the fermentation broth with calcium carbonate (diluent) and silicon dioxide (flowability enhancer) and granulation of the resulting suspension in a fluid‐bed drying equipment (see details below in Section 3.1.3).
Sacox® 120 microGranulate is specified to contain 114–132 g SAL‐Na, 10–100 g silicon dioxide and 500–700 g calcium carbonate per kilogram while the product Sacox® 200 microGranulate contains 190–220 g SAL‐Na, 50–150 g silicon dioxide and 50–150 g calcium carbonate per kilogram. The products also contain materials from the fermentation broth. Derived from the SAL content of the fermentation broth the applicant submitted ranges for the composition of the additives with 28.5–42.6% dried fermentation substrate, 4.8–6.3% silicon dioxide and 51.1–66.8% calcium carbonate for Sacox® 120 microGranulate; 77.3–85.6% dried fermentation substrate, 8.5–11.1% silicon dioxide and 5.3–11.6% calcium carbonate for Sacox® 200 microGranulate.15
Batch to batch consistency was demonstrated by the analysis of 11 batches of each product. The SAL content ranged from 120 to 130 g/kg Sacox® 120 microGranulate and 200–210 g/kg Sacox® 200 microGranulate.16 Loss on drying of Sacox® 120 microGranulate was between 1.5% and 2.5%; that of Sacox® 200 microGranulate between 1.4% and 2.9%.
Three other batches of each product were analysed for SAL‐Na and crude nutrient content.17 Results are shown in Table 1.
Table 1.
SAL‐Na and crude nutrient content of Sacox® products
| Sacox® 120 microGranulate | Sacox® 200 microGranulate | |
|---|---|---|
| SAL‐Na (g/kg) | 123–125 | 204–220 |
| Crude nutrient content (%) | ||
| Moisture (loss on drying) | 1.7–1.8 | 2.3–2.6 |
| Sulphated ash | 60.4–76.0 | 47.6–49.2 |
| Crude protein | 2.2–2.4 | 3.5–4.6 |
| Total sugars (as glucose) | 1.7–2.3 | 2.4–3.8 |
| Crude fat | 2.5–4.0 | 6.2–7.5 |
| Crude fibre | 1.4–2.0 | 3.5–5.4 |
Data on the content of heavy metals, arsenic, mycotoxins, dioxins, and dioxin‐like polychlorinated biphenyls (PCBs) were provided for three batches of each product.18 Mean values for arsenic, lead, cadmium and mercury in Sacox® 120 microGranulate were 2.487, 2.16, 0.275 and 0.007 mg/kg, respectively. Mean values for arsenic, lead, cadmium and mercury in Sacox® 200 microGranulate were 0.559, 2.147, 0.186 and < 0.005 mg/kg, respectively. Values for dioxins (polychlorinated dibenzo‐p‐dioxins and dibenzofurans (PCDD/F)) were < 0.149 ng WHO‐PCDD/F‐TEQ per kg and the sum of dioxins and dioxin‐like PCBs was < 0.291 ng WHO‐PCDD/F‐TEQ per kg. These concentrations are of no concern.19 Data on Aflatoxin B1, B2, G1 and G2 were given; total aflatoxins were below 1.5 μg/kg in both products and did not raise safety concern. Six batches of Sacox® 120 microGranulate and Sacox® 200 microGranulate showed no evidence of Salmonella contamination.20
The absence of living cells of the producing strain S. azureus was demonstrated in samples taken after processing of three successive fermentation batches. Viable cells of the production strain were not detected in any of the test samples.21
Sacox® 120 microGranulate and Sacox® 200 microGranulate consist of beige to brown granules with a tapped density of 0.52–0.62 and 0.40–0.51 kg/L, respectively, and a loose density of 0.46–0.55 and 0.34–0.44 kg/L, respectively, based on measurements of five batches. Five batches of each product were analysed for particle size (sieve analysis). Sacox® 120 microGranulate consisted of 99% and 6% particles (w/w) with a diameter < 800 μm and < 100 μm, respectively; Sacox® 200 microGranulate consisted of 99% and 3% particles (w/w) with a diameter of < 800 μm and < 100 μm, respectively.22
Three batches of each product were analysed for dusting potential (Stauber‐Heubach test). The dusting potential of Sacox® 120 microGranulate was 0.03 g/m3, that of Sacox® 200 microGranulate was 0.01–0.07 g/m3. The SAL content in the dust of Sacox® 120 microGranulate was in the range of 111–143 mg/g, that of Sacox® 200 microGranulate in the range of 168–223 mg/g.23 , 24 The particle‐size distribution of the dust of Sacox® 200 microGranulate was evaluated by laser diffraction, indicating 23–44% particles (v/v) < 10 μm.25
3.1.2. Characterisation of the active substance
Salinomycin sodium, the active substance of Sacox®, is a monocarboxylic polyether ionophore.
The structural formula of SAL‐Na, ethyl‐6‐[5‐{2‐(5‐ethyltetrahydro‐5‐hydroxy‐6‐methyl‐2H‐pyrano‐2‐yl)‐15‐hydroxy‐2,10,12‐trimethyl‐1,6,8‐trioxadispiro [4,1,5,3] pentadec‐13‐en‐9‐yl} 2‐hydroxy‐1,3‐dimethyl‐4‐oxoheptyl]tetrahydroxy‐5‐methyl‐2H‐pyran‐2‐acetic acid, sodium salt (C42H69NaO11; molecular weight 773; CAS number: [55721‐31‐8]) is given in Figure 1.
Figure 1.
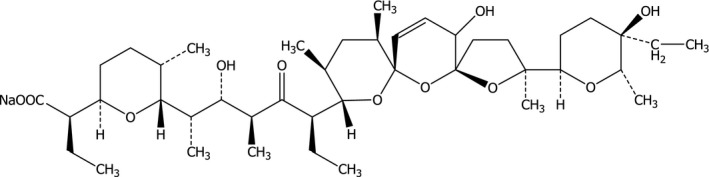
Structural formula of salinomycin sodium
SAL‐Na has a melting point of 140–142°C. It is a weak acid (pKa 6.4), highly soluble in water at pH 7 and 9, less soluble at pH 4.26 It is readily soluble in methanol, acetone, chloroform and benzene.27 The log partition coefficient (n‐octanol/water) is < 1.28
The current authorisations29 set limits for the following related impurities: < 42 mg elaiophylin/kg SAL‐Na and < 40 g 17‐epi‐20‐desoxy‐salinomycin/kg SAL‐Na.
In the current dossier a new study is provided in which the above‐mentioned two substances and 20‐deoxysalinomycin were monitored in three batches of Sacox® 120 microGranulate.30 Elaiophylin was not detected (limit of detection: 10 mg/kg).31 17‐Epi‐20‐desoxy‐salinomycin amounted to 0.9 and 1.6 g/kg SAL‐Na. 20‐Deoxysalinomycin, additionally analysed in six batches of Sacox® 200 microGranulate, was in the range 1.6 and 8.7 g/kg. In an additional study, methylated salinomycin(s) and 18,19‐dihydro salinomycin were also identified in the additive, each amounting ≤ 10 g/kg of total SAL.32
3.1.3. Manufacturing process
The active substance SAL‐Na is produced by a strain of S. azureus by fermentation in a nutrient medium, followed by a salt formation with sodium hydroxide. The manufacturing process of the product is fully described in the technical dossier.
3.1.3.1. Characterisation of the production organism
The active substance SAL‐Na is produced by fermentation of a strain of Streptomyces. The strain was originally identified as Streptomyces albus and deposited as American Type Culture Collection 21838. Data provided based on the 16S rRNA gene sequence analysis allow identifying the production strain as S. azureus.33 The strain has been recently deposited in the Deutsche Sammlung von Mikroorganismen Zellkulturen with the accession number DSM 32267 (former accession number DSM 12217).34 The production strain is not genetically modified, but has been subjected to chemical mutagenesis.35
Genetic stability was demonstrated by comparison of morphological, physiological and biochemical characteristics, and the productivity of SAL between the master cell culture and the working culture.36 No data on the unique identification of the strain were provided, other than the taxonomical identification.37
The absence of antimicrobial compounds relevant to the use of antibiotics in humans or animals, other than the SAL in the mycelial products, was assessed comparing the minimum inhibitory concentrations (MICs) of three batches of the fermentation product with three batches of pure SAL‐Na (75% SAL). The batches were tested against 33 strains of aerobic and anaerobic species of both Gram‐positive and Gram‐negative bacteria.38 The MIC values were determined using a twofold broth dilution in appropriate media for the different bacterial species. SAL‐Na shows an antimicrobial activity in a concentration range of 0.5–16 mg/L against all the tested Gram‐positive bacterial species. Differently, all the Gram‐negative species are resistant to this ionophore, with MIC values higher than 128 mg/L.
Since no differences in the inhibitory spectrum and in the MIC values were observed between the pure and mycelial form for any of the strain tested, the product is considered to be free of antimicrobial activity, other than SAL.20
3.1.4. Stability and homogeneity
3.1.4.1. Shelf‐life of the additive
The stability of three batches of Sacox® 120 microGranulate and Sacox® 200 microGranulate was studied when kept either in multiple layer bags with an internal polyethylene layer or in polypropylene bags at 25°C/60% relative humidity (RH) (up to 24 months) and 40°C/75% RH (up to 6 months).
The SAL‐Na content of both products remained stable under the conditions tested (losses of SAL‐Na < 8% at 25°C after 24 months in both products; losses of SAL‐Na < 5% in Sacox® 120 microGranulate and < 4% in Sacox® 200 microGranulate at 40°C after 6 months; no influence of packaging).39
3.1.4.2. Stability of the additive in premixtures and feedingstuffs
The stability of SAL‐Na from Sacox® 120 microGranulate and Sacox® 200 microGranulate in a vitamin–mineral premixture with choline chloride containing 12 g SAL‐Na/kg was examined at 25°C and 40°C (ambient RH) for 6 months. SAL losses amounted to 2% at 25°C and 4% at 40°C in one premixture batch produced with Sacox® 120 microGranulate. The corresponding data for three premixture batches produced with Sacox® 200 microGranulate were in the range of 2–3% and 4–6%.
Stability was also examined in a complete feed for chickens for fattening at 25°C/60% RH for 3 months and at 40°C/75% RH for 1 month. Three batches each of Sacox® 120 microGranulate and Sacox® 200 microGranulate were incorporated to achieve a SAL‐Na concentration of 60 mg/kg complete feed. Losses at 25°C and 40°C were in the range of 1–9%. No essential differences were observed between the two additives.
Minimal losses (2–3%) of SAL concentration in complete feed were measured during feed processing (pelleting at 85°C). SAL recoveries in pelleted samples under the same storage conditions were in the same range as in mash feed.40 , 41
3.1.4.3. Homogeneity of the additive in premixtures and feedingstuffs
The same premixture and complete feed used for the stability studies were used to assess the capacity of SAL‐Na from Sacox® 120 microGranulate and Sacox® 200 microGranulate to homogeneously distribute. The coefficient of variation (CV) of the SAL concentration in 10 premixture samples was 0.5% and 1.0%, respectively, that of six times 10 feed samples (mash and pelleted each) for both additives below 4.5%.
The same samples as used for homogeneity studies were taken to examine the segregation during transport. The CV of the SAL concentration of five premixture samples after transport was 0.6%.40 , 41
3.1.5. Conditions of use
Sacox® 120 microGranulate and Sacox® 200 microGranulate, containing 120 and 200 g/kg SAL‐Na, respectively, are feed additives for the prevention of coccidiosis in chickens for fattening and chickens reared for laying. The SAL‐Na dose range is 60–70 mg/kg complete feed for chickens for fattening. For chickens reared for laying the dose applied is 50 mg/kg feed up to 12 weeks of age. The applicant proposes a zero‐day withdrawal period.42
3.2. Safety
3.2.1. Safety for the target species
3.2.1.1. Tolerance studies
The applicant submitted the same tolerance study with SAL‐Na from Sacox® 120 microGranulate in chickens for fattening that was already assessed in 2004 by the FEEDAP Panel (EFSA, 2004a).43
A short summary of the study is reported below.
A total of 288 day‐old male Lohmann chickens were allocated to 72 cages with four birds per cage. They were fed a SAL‐Na un‐supplemented diet for 8 days. After that adaptation period, eight cages each were allocated to nine treatment groups.
The treatments were: 0, 0, 60 (1 × recommended dose), 80, 100, 120, 140, 160 and 180 mg SAL‐Na from Sacox® 120 microGranulate/kg feed. Eight days later, the groups receiving SAL‐Na supplemented feed and one group without SAL‐Na were infected with a mixture of Eimeria acervulina and Eimeria tenella. The experiment was completed 35 days after initiating the SAL‐Na treatment.
Birds were monitored daily for health. Body weight and feed consumption were recorded weekly. A total of eight birds per treatment (one fasted bird per cage) were taken for blood sampling at 18–20 and 32–34 days from the start of treatment for clinical chemistry44 and haematology.45 Gross necropsy on the blood‐sampled chickens was done at the end of the study.
For performance data, an analysis of variance was performed. A test for differences between group means was done subsequently. The clinical parameters were tested for independence of the distribution of treatments. The parameters of clinical chemistry and haematology were examined using a non‐parametric test under monotone dose–response conditions.
Mortality was not different between the groups receiving SAL‐Na. SAL‐Na levels up to 120 mg/kg feed were tolerated for entire length of the study without effects on any of the end‐points measured. Supplementation of SAL‐Na at 140 mg/kg feed and above significantly reduced final weight, feed gain, feed intake and serum calcium level compared to the infected control. Moreover, levels of 160 and 180 mg/kg feed significantly increased the mean corpuscular volume and levels of 180 mg resulted in a reduction in haematocrit, cholinesterase activity and sodium concentration in serum.
The FEEDAP Panel noted that the study deviated in two issues from the requirements of Regulation (EC) No 429/200846 and FEEDAP Technical Guidance on Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011b). The age of the animals at start of the treatment was 9 days instead of 1 day, and the animals were infected with Eimeria spp. (which is not required in tolerance study). Nevertheless, the FEEDAP Panel considers the study as a valid tolerance study, since chickens were exposed to SAL for the minimum required duration of 35 days and the infection with Eimeria spp. would not modify tolerance of the target animals.
The FEEDAP Panel concludes that SAL‐Na from Sacox® 120 microGranulate is safe for chickens for fattening at the proposed concentration of 60–70 mg/kg feed, with a margin of safety of 1.7 (120/70).
The FEEDAP Panel would normally extend the conclusion reached for chickens for fattening to chickens reared for laying. However, in a limited study in chickens reared for laying, Rizvi et al. (2008) observed a reduction in body weight after 11 weeks in chickens receiving 60 mg SAL‐Na/kg feed (the lowest dose tested). Therefore, the FEEDAP Panel requested the applicant to perform a (limited) tolerance study on chickens reared for laying for a duration of 12 weeks, in which only performance parameters were measured.47 These parameters were shown to be the most sensitive parameters in tolerance study with chickens for fattening.
The study was performed on a total of 1,600 day‐old pullets (Bovans Brown) allocated to four treatment groups (with 16 replicates each and 25 birds/pen), fed pelleted diets supplemented with 0, 50, 60 and 90 mg SAL‐Na/kg, respectively, for 12 weeks. A starter diet (20.3% crude protein, 12.1 MJ ME/kg) was given for the first 8 weeks, followed by a grower diet (16.1% crude protein (CP), 11.4 MJ ME/kg) until study completion. Body weight and feed intake were measured in 14 day intervals; general health was monitored and mortality was recorded. The data were statistically analysed as a completely randomised design by ANOVA and presented as least square corrected means. The results are summarised in Table 2. Mortality was not observed.
Table 2.
Results of the 12‐week tolerance study with SAL‐Na in chickens reared for laying (cumulative 12‐week data)
| Target dose of SAL‐Na in diet (mg/kg) | Analysed SAL‐Na in starter diet (mg/kg) | Analysed SAL‐Na in grower diet (mg/kg) | Body weight (g) | Feed intake (g) | Feed to gain ratio |
|---|---|---|---|---|---|
| 0 | < 0.5 | < 0.5 | 1,138c | 4,135c | 3.75 |
| 50 | 48 | 51 | 1,129b,c | 4,111b,c | 3.76 |
| 60 | 57 | 62 | 1,117a,b | 4,060a,b | 3.76 |
| 90 | 89 | 90 | 1,105a | 4,017a | 3.76 |
SAL: salinomycin sodium.
a, b, c: Values in the same column with no common superscript are significantly different (p ≤ 0.05).
No effects of SAL‐Na supplementation on growth and feed intake were observed until the age of 56 days. But in the period 56–70 days there was a significant reduction in growth (232 and 226 for the groups with 60 and 90 mg SAL‐Na/kg compared to 248 and 243 g for the groups with 0 and 50 mg SAL‐Na/kg) likely due to a reduced feed intake (1,036 and 1,011 for the groups with 60 and 90 mg SAL‐Na/kg compared to 1,100 and 1,088 g for the groups with 0 and 50 mg SAL‐Na/kg). A significant reduction in the final body weight and feed intake was observed in the 60 and 90 mg SAL‐Na/kg groups compared to the control group.
A lower feed palatability was discussed by the applicant, but is not considered likely by the FEEDAP Panel. A lower feed intake at higher doses is a common and sensitive sign of intolerance to ionophore coccidiostats in poultry (Dowling, 1992). This symptom was observed in pullets already at 60 mg SAL‐Na/kg feed starting after 56 days of age, whereas it was seen in chickens for fattening only at 140 mg/kg at an age of 44 days (no intermediate values available). The difference between the two different chicken categories could be the result of the different age at which observations on adverse effects could be made (Dowling, 1992). It could also be regarded as a follow‐up of the higher feed to gain ratio in chicken reared for laying (about 3.8 compared to 1.9 in chickens for fattening), indicating a smaller tissue compartment available for drug distribution.
3.2.1.2. Interactions
Interactions between ionophores and other drugs (tiamulin, sulphonamides, chloramphenicol, erythromycin, oleandomycin and furazolidone) were already described in the FEEDAP opinion in 2004 (EFSA, 2004a). In 2004, the FEEDAP Panel concluded that:
‘Incompatibilities or interactions with feedingstuffs, carriers, other approved additives are not to be expected given the known history of salinomycin. It could also be shown that SAL‐Na from Sacox is fully compatible with some veterinary drugs.48 On the other hand it is well known from the literature that severe interactions between the ionophore coccidiostats and the diterpen‐antibiotic tiamulin as well as other antibiotic substances (mainly macrolides) may occur. Therefore the simultaneous use of Sacox and certain antibiotic drugs (e.g. tiamulin) is contra‐indicated.'
The applicant performed a literature review49 to update the information available and two papers were identified as relevant.50 A review paper (Islam et al., 2009) confirmed the strong interaction at high dose (even leading to death) between the ionophore anticoccidials monensin, narasin and SAL when tiamulin is used at therapeutic levels. The interaction was found to be dose related, not observed at low dose. Two articles from the same research group substantiated the dose dependency of the interaction between tiamulin and SAL (Islam et al., 2008a,b).
Wang et al. (2013) studied the effect of SAL on the kinetics of florfenicol, a chloramphenicol derivative in chickens for fattening. The authors found lower plasma concentrations of florfenicol when SAL was co‐administered. It should be mentioned that florfenicol is not authorized in the EU for treatment of poultry.
Since no substantially new findings were reported, the FEEDAP Panel reiterates its former conclusion.
3.2.1.3. Microbial studies
The antimicrobial activity of SAL‐Na, as for other ionophoric compounds, is mainly limited to Gram‐positive bacteria. In an earlier study already assessed in the previous opinion (EFSA, 2004a), MICs were determined for bacterial isolates from the gastrointestinal microbiota of broilers. Isolates of Enterococcus faecalis and E. faecium proved to vary with MICs of SAL ranging from 1 to 16 and 4 to 16 mg/L. MICs, respectively; the MICs for Staphylococcus spp. ranged from 4 to 16 mg/L, and those for Clostridium perfringens from 0.5 to 1 mg/L. In a new study51 to assess the antimicrobial spectrum of SAL‐Na, the MICs of three batches of the fermentation product and three batches of purified product (SAL‐Na) were tested against 33 strains of aerobic and anaerobic species of both Gram‐positive and Gram‐negative bacteria (see Section 3.1.3.1).38 SAL‐Na shows antimicrobial activity in the concentration range of 0.5–16 mg/L against all the tested Gram‐positive bacterial species. Differently, all the Gram‐negative species are resistant to this ionophore, with MIC values higher than 128 mg/L.
In 2004 the FEEDAP Panel (EFSA, 2004a) summarised that:
‘SAL‐Na shows a selective antimicrobial activity in a concentration range of 0.5–16 mg/L against many Gram‐positive bacterial species while Enterobacteriaceae are resistant. Induction of resistance and cross‐resistance to other antibiotics except to narasin has not been demonstrated neither in vitro or in vivo. The MICs of SAL for common intestinal bacterial species such as Enterococcus spp. and Clostridium perfringens are basically low but enterococci may develop resistance to SAL, which is not associated with cross resistance to antibiotics used for therapy in human or veterinary medicine. Inhibitory concentrations of salinomycin for susceptible bacterial strains are lower than the dose in supplemented feed. Increased shedding of Salmonella spp., Campylobacter and clostridia was not shown to occur under experimental/practical conditions.'
A literature review52 considering papers published in the years 2000–2015 and focusing on the emergence of resistance to SAL‐Na, on the cross‐resistance to antimicrobials and to the shedding of enteropathogens was made by the applicant.53 Twenty‐seven papers dealing with SAL‐Na have been identified and in none of them evidence of insurgence of resistance to this ionophore or cross‐resistance to antimicrobials used for therapy in human or veterinary medicine was reported. Moreover, the use of SAL‐Na in farmed animals does not affect the shedding of Salmonella, Campylobacter and Escherichia coli.
Consequently, the FEEDAP Panel concludes, in principle agreement with its previous conclusions, that SAL is active against certain Gram‐positive bacteria, while Gram‐negative bacteria are resistant.
The use of SAL‐Na as feed additive is unlikely to increase shedding of Salmonella, E. coli and Campylobacter and to induce resistance and cross‐resistance to antimicrobials used of human and animal relevance.
3.2.1.4. Conclusions on safety for the target species
Salinomycin sodium from Sacox® 120 microGranulate or Sacox® 200 microGranulate is safe for chickens for fattening at a concentration of 70 mg/kg complete feed with a margin of safety of 1.7. For chickens reared for laying, 50 mg SAL‐Na/kg complete feed is considered safe for a feeding period of the first 12 weeks of life; a margin of safety cannot be given.
The simultaneous use of Sacox® microGranulate and certain antibiotic drugs (e.g. tiamulin) is contraindicated.
SAL‐Na is active against certain Gram‐positive bacteria, while Gram‐negative species are resistant. The use of SAL‐Na as a feed additive at the proposed concentration is unlikely to increase shedding of Salmonella, E. coli and Campylobacter and to induce resistance and cross‐resistance to antimicrobials important in human and animal therapy.
3.2.2. Safety for the consumer
3.2.2.1. Metabolic and residue studies
In 2004 the FEEDAP Panel (EFSA, 2004a) concluded that:
‘Salinomycin is absorbed and metabolized by the chicken. Depending on the studies, but using apparently sound analytical methods, unchanged SAL represents between trace amounts and 67% of the SAL‐derived compounds present in the excreta, but the weight of evidence suggests that unchanged salinomycin represent a very small fraction. However, these data have been obtained after gavage of the animals whereas administration through the feed is recommended (Directive 2000/79/EC) and therefore the FEEDAP Panel is not in a position to conclude. By weight of evidence, the lower figures are used for further calculations.
Seventeen metabolites have been separated and identified from the excreta, most of them representing less than 10% of the total SAL‐derived compounds. They correspond to a major oxidative pathway leading to mono‐, di‐ and tri‐hydroxysalinomycins plus keto/hydroxy derivatives. Similar metabolites have been separated and identified in the tissues. A considerable fraction of tissue residues is non extractable, especially in the muscle and fat. Decarboxylation of [14C]‐SAL occurs to a limited but significant extent that leads to the labelling of fatty acids (and possibly proteins).
Even if the correspondence of the metabolic pathways of SAL in the chicken and laboratory animals (rat and mice) cannot be completely assessed due to the analytical methods used, the FEEDAP Panel recognizes that an adequate degree of commonality exists.
Kinetics studies of the whole residues and unchanged SAL in chicken tissues, established with the highest SAL dosage recommended for complete feed, indicate that the liver is the target tissue during the first 3‐day withdrawal period followed by the skin/fat for longer periods. SAL represents a very small rapidly disappearing (1‐day) fraction of tissue residues. A constant ratio between salinomycin and the total residue, qualifying salinomycin as the MR, could not be shown. However, for practical control considerations the skin/fat and salinomycin could be retained as target tissue and MR.
Extractable liver residues have a reduced (20%) ionophoric activity when compared to SAL.'
A new absorption, distribution, metabolism and excretion (ADME) study of SAL‐Na in chicken, including a kinetic study of lasalocid residues in tissues aimed at addressing the uncertainties of the previous assessment (EFSA, 2004a), has been provided.54 An additional study on the characterization and quantification of SAL and metabolites in the excreta of chickens was also provided.55
The results of the ADME study confirmed those from the former assessment (EFSA, 2004a). In the other additional study,54 chickens were administered a single dose of 14C‐SAL (equivalent to 70 mg SAL/kg feed, labelling position not given) and excreta were collected up to 156 h post‐gavage, pooled and aliquots analysed by liquid chromatography‐mass spectrometry (LC‐MS). Unchanged SAL represented about 1% of the whole radioactivity measured in the excreta, supporting the lowest figure mentioned in the former assessment. Ten SAL metabolites were separated and quantified: a cluster of seven metabolites (43%) including two tetrahydroxy‐SAL isomers, two dehydrotetrahydroxy‐SAL isomers and three trihydroxy‐SAL isomers, another cluster (18%) of one dehydrotetrahydroxy‐SAL and one dihydroxy‐SAL and one dihydroxy‐SAL (7%). None of the hydroxy group positions was established. When comparing the metabolic profiles of SAL in the liver54 and excreta55 using a similar analytical approach (radio‐HPLC), it appeared that at steady state excreta contained a much lower proportion of mono‐hydroxylated‐SAL (1% vs 18%) and a higher proportion of multi‐hydroxylated (di‐, tri‐ and tetra‐) SAL‐metabolites (e.g. 43% vs 8% for tetrahydroxy‐salinomycins) than the liver.
In 2004, the FEEDAP Panel assessed and retained as a key study among others a kinetics study56 in which SAL total residues (not MRCs) were measured in tissues of chickens for fattening administered 14C‐SAL for 7 days at a dose corresponding to 70 mg SAL/kg complete feed, after 0.25, 1, 2 and 3 day withdrawal (EFSA, 2004a). In the current submission, the applicant made a critical analysis of that study, highlighting the fact that the animals were overexposed (about 140%) based on the dose administered vs body weight and compared to the dose nominally targeted (7.7 mg/kg bw). However, in the absence of feed consumption data, no equivalence with the feed concentration of SAL (nominally 70 mg/kg) could be established. These uncertainties were addressed in the new study submitted by the applicant.54 Four groups of three male and three female chickens for fattening (Ross 308) each were administered (twice‐daily) 14C‐SAL by gavage for seven consecutive days at a rate nominally equivalent to 70 mg SAL/kg diet. Calculation from the dose administered and feed consumption indicated that the daily dose would correspond to 73, 60 and 60 mg/kg feed for days 5, 6 and 7 of administration, respectively. Birds (three males and three females) were sacrificed 1, 3, 6 and 24 h after the final dose. Total radioactivity and MR (SAL) concentration in tissues were measured; the results are reported in Table 3.
Table 3.
Kinetics of total residues concentration (TRC) and marker residue (SAL) in chicken tissues from animals given by gavage 14C‐SAL equivalent to 70 mg/kg feed. Means of individual values (three males and three females) ± standard deviations for 1 and 6 h withdrawal, pooled samples (males and females) for 3 and 24 h withdrawal. SAL determined by radio‐HPLC (LOD reported to be < 0.001 to < 0.004 mg/kg)
| Withdrawal time (animals) | Liver | Kidney | Muscle | Skin/fat | ||||
|---|---|---|---|---|---|---|---|---|
| TRC | SALa | TRC | SAL | TRC | SAL | TRC | SAL | |
| 1 h (n = 6) | 1.487 ± 0.290 | 0.087 ± 0.027 | 0.183 ± 0.046 | 0.027 ± 0.006 | 0.027 ± 0.003 | 0.008 ± 0.002 | 0.156 ± 0.037 | 0.087 ± 0.029 |
| 3 h (n = 2) | 0.987 ± 0.266 | 0.031 ± 0.026 | 0.144 ± 0.024 | 0.005 ± 0.004 | 0.021 ± 0.004 | 0.004 ± 0.003 | 0.164 ± 0.035 | 0.067 ± 0.033 |
| 6 h (n = 6) | 0.391 ± 0.146 | 0.005b | 0.077 ± 0.023 | 0.002 ± 0.001c | 0.012 ± 0.002 | 0.001b | 0.060 ± 0.008 | 0.011 ± 0.009 |
| 24 h (n = 2) | 0.194 ± 0.072 | < LOD | 0.055 ± 0.011 | 0.001d | 0.006 ± 0.001 | < LOD | 0.045 ± 0.01 | 0.001d |
SAL determined by radio‐HPLC (LOD reported < 0.001 to < 0.004 mg/kg).
Based on one value, other values below the LOQ (0.001 mg/kg).
Based on four values, other values below the LOQ.
Based on one value of two pooled samples, other below LOD.
Conclusion on metabolite and residues studies
New ADME data confirm those from the former assessment. The proportion of mono‐ and multi‐hydroxylated SAL metabolites was shown to be different in the liver and the excreta. Total residues and MR were determined in the same study allowing to set ratios marker to total residue (RMTRs) for all tissues.
3.2.2.2. Residues of toxicological significance
The ionophoric activity of residues of SAL and metabolites (including mono, di‐, tri‐ and tetra‐hydroxy‐SAL) from the liver of chicken administered the additive was studied using a 86Rb radiolabelled binding assay (Dimenna et al., 1989). Day‐old chickens were fed until day 11 unmedicated feed, then administered twice a day 3.37 mg 14C‐SAL/kg bw (equivalent to 75 mg/kg feed) for 20 days. Livers sampled 6 h after the last dose were homogenized, thawed and frozen until analysis. Salinomycin and metabolites were extracted from the homogenate, purified (column chromatography) and converted to the acidic form (cation free). The binding capacity of the extracts from control and experimental livers was evaluated by adding 86Rb chloride and measuring solvent‐extractable 86Rb‐SAL. The affinity of rubidium binding for SAL metabolites was approximately 20% that of the parent compound.
In a study on the biological activity of SAL and SAL‐derivatives (Miyazaki et al., 1976), the monohydroxy 11‐OH‐SAL exhibited no activity against Bacillus subtilis, Staphylococcus aureus, Sarcina lutea and Mycobacterium phlei (minimal antimicrobial activity ≥ 100 μg/mL) in comparison with SAL (3.1, 1.6, 3.1 and 13 μg/mL, respectively).
In a further study (Miyazaki et al., 1978), 20‐deoxysalinomycin that corresponds to the reduction of the hydroxyl group at C‐20 of SAL was tested in comparison with SAL on rat liver mitochondria. 20‐Deoxysalinomycin at low concentration interacted with the alkali metal cation translocating system of mitochondria. It appeared to be five to ten times more effective than SAL for binding ADP and for increasing SAL‐stimulated glutamate oxidation. The authors concluded that 20‐deoxysalinomycin is less polar than SAL and can more easily diffuse across mitochondrial membranes.
Other studies performed with monensin sodium, a polyether ionophore of similar structure as SAL, allowed the Committee for Medicinal Products for Veterinary Use (CVMP) of the European Medicines Agency (EMA, 2007) and the FEEDAP Panel (EFSA, 2008d) to conclude that, following a conservative approach, only 50% of the total monensin‐related residues would be of toxicological relevance.
In the study of Rocha et al. (2014), two monohydroxylated metabolites of monensin sodium (3‐OH and 12‐OH‐monensin, corresponding to in vivo metabolites) were prepared by chemical synthesis using a Jacobsen catalyst as a cytochrome P450 biomimetic model. Monensin A and the two metabolites were tested (1 μM) in a rat liver mitochondrial toxicity model comprising the measurement of the dissipation of mitochondrial membrane potential, of mitochondrial swelling and of mitochondrial production of free radicals. Monensin A decreased by 23% the mitochondrial membrane potential due to the uncoupling effect of the ionophore, increased by 55% the mitochondrial swelling related to the interference with Na+ and Ca2+ mitochondrial regulation and increased by 12% the accumulation of free radicals. The two metabolites showed no effect on any of the studied parameters. Monensin A and the two metabolites were also tested in an antimicrobial bioassay against S. aureus and methicillin‐resistant S. aureus. 3‐OH‐monensin had a reduced antibacterial activity (to about 10%) against S. aureus and methicillin‐resistant S. aureus compared to monensin A (25 and 50 μg/mL vs 3.1 and 6.3 μg/mL, respectively), 12‐OH‐monensin was without activity (≥ 100 μg/mL).
Synopsis
The biological activity of ionophores depends on their ability to form complexes of different strength with cations and to penetrate and move across lipid bilayers of cells to exchange cations. Ionophore complexes exhibit a polar interior insuring the linkage to cations and a non‐polar highly hydrophobic exterior which allows their free movement across lipid bi‐layers (Pressman and Fahim, 1982). The studies performed with SAL indicate that its metabolites have a lesser binding capacity to cations than the parent compound. The studies performed with two different monohydroxy‐monensins indicate that their ability to move across membranes is considerably reduced compared to monensin, due to a decrease in the hydrophobicity of the external part of the complexes. The FEEDAP Panel reasonably assumes that the multi‐hydroxylation (di‐, tri‐ and tetra‐hydroxy) of polyether ionophores would even more drastically impair that property. After 6 h withdrawal corresponding to a practical zero withdrawal time, virtually no SAL was found in liver and muscle; however, in skin/fat, the proportion of SAL to total residues was about 18% (see Table 3). Considering the different contributions of muscle, liver and skin/fat to the food basket (Commission Regulation (EC) No 429/2008) and keeping a conservative approach, the FEEDAP Panel considers that on average SAL residues (SAL + metabolites) would not retain more than 20% of the ionophoric activity of the parent compound, which represents the fraction of toxicological concern.
3.2.2.3. Salinomycin carryover into eggs of laying hens
Forty day‐old pullets were randomly allocated to two pens (groups 1 and 2) and fed complete feed supplemented nominally with 50 mg SAL /kg feed (analytical values: 50.5 mg SAL/kg grower feed for group 1 and 69.5 mg/kg for group 2) for a period of 12 (group 1) or 14 weeks (group 2).57 Blank feed (0 mg SAL/kg, analytically confirmed) was then administered until the end of the study. Eggs were collected in both groups from the onset of laying, every day and for 14 consecutive days. SAL residues were measured in the whole egg using an HPLC‐MS/MS analytical method with a limit of quantification (LOQ) of 1 μg/kg whole egg.
In group 1, the first egg was layed the first day of the 16th week of the experiment (second week on the control feed) and no SAL was quantified; none of the eggs layed during the following 14 days contained measurable amounts of SAL either. In group 2, the first egg was layed on the second day of the 16th week of the experiment also, with a SAL content of 8.6 μg/kg whole egg; in the limited number of eggs layed during the following 2 weeks (1–5 per day from 20 birds), SAL contents were in the range of 8.6–3.1 μg/kg whole egg (average value: 4.8 ± 1.9 μg/kg), with a tendency to decline over that period.
When SAL is administered to chickens reared for laying according to the intended condition of use (50 mg SAL‐Na/kg feed up to 12 weeks of life), no residues are detected in the eggs at the onset of laying. However, increasing the SAL concentration to 70 mg/kg feed and extending the administration period to 14 weeks, traces of SAL were found in the first eggs (3–9 μg/kg whole egg).
3.2.2.4. Toxicology studies
It was noted that the applicant re‐submitted the same toxicological studies assessed in the 2004 opinion (EFSA, 2004a). The conclusions in the 2004 opinion are the following:
Based on the data provided, SAL does not induce gene mutations in vitro and it is not genotoxic in mouse bone marrow studies in vivo.
The SAL biomass was not carcinogenic in studies on mouse and rats.
Reproduction studies (one two‐generation study with Sacox 120 and three developmental studies, two with Sacox® 120 and one with SAL biomass) did not indicate concern, the No Observable Adverse Effect Level (NOAELs) being 1.1 mg SAL/kg bw per day in the two‐generation study (based on decreased pup weight in the F1A generation) and 0.63 mg as the lowest NOAEL in developmental studies (based on rabbit embryo‐foetal toxicity).
‘The lowest NOEL for SAL‐Na from Sacox® 120 microGranulate was 0.5 mg/kg bw per day, identified from the results of a 12‐month dog study. An acceptable daily intake (ADI) can be proposed as 0.005 mg/kg bw, applying a safety factor of 100.’
During the assessment it was noted that the submitted 90‐day toxicity study in rats was incomplete by current standards and particularly did not investigate other endpoints (particularly neurotoxicity) seen in dogs. In consequence, the applicant was requested to provide a new study compliant with good laboratory practice (GLP) and current guidelines taking account of the known toxicity in other species.
Other ionophores are known to have caused positive inotropic effects after dosing to dogs (EFSA, 2004b). Since these effects have been previously accepted as being relevant to consumer risk assessment, particularly when occurring at doses lower than identified as a toxicological NOAEL, the applicant was requested to perform an acute study in dogs, by the oral route, investigating these effects to identify whether they would be critical to the risk assessment for this product.
Following the above requests, the applicant submitted a 90‐day study in rats58 and an acute study of cardiovascular effects in dogs59 (inotropic effect). The applicant also performed, upon request, a literature review60 covering the last 10 years.61 The literature review identified no new data requiring consideration in the latest opinion. Details and results of the requested studies are summarised below:
Three groups of 10 male and 10 female Sprague‐Dawley rats received SAL mycelial concentrate mixed in feed to deliver target dose levels of 0.6, 2.4 and 9.6 mg SAL/kg bw per day for 13 weeks, in a study compliant with Organisation for Economic Co‐operation and Development (OECD) guideline 408.58 Another group of 10 males and 10 females received untreated diet and acted as a control group. The animals were checked twice daily for mortality and once a day for clinical signs. Detailed clinical examinations were performed weekly and functional observation battery (FOB) and motor activity were conducted once in week 12. Body weight and food consumption were recorded three times during the pre‐treatment period, and then at least once a week. Ophthalmological examinations were performed on all animals before the beginning of the treatment period and on control and high‐dose animals in week 13. Haematology, blood biochemistry and urinary investigations were carried out during week 13. On completion of the treatment period, the animals were sacrificed and a full macroscopic post‐mortem examination was performed. A microscopic examination was performed on selected tissues from control and high‐dose animals and on all macroscopic lesions.
Analysis of administered diets confirmed that the target doses of SAL were achieved for both sexes. No unscheduled deaths occurred during the study. Hunched posture was observed in 7/10 females at the highest dose, starting in week 3, 4 or 5 and persisting in some animals up to week 11. Lower body weight gain was recorded in the high‐dose group when compared with controls (−35% in males during the first 5 weeks of the study and −75% in females during the first 4 weeks of the study). These changes were associated with lower food consumption during the same period (males: −16%; females: −17%) and resulted in lower body weight at the end of the treatment period (males: −16%; females: −13%). Both body weight and feed intake of the lower dose groups were consistently lower than that of controls for both sexes, although the difference was small, unrelated to dose and not statistically significant. No effects of treatment were noted on ophthalmology findings, FOB results or results of haematological and urine examination. Blood biochemistry examination results showed lower mean triglyceride levels in animals given 9.6 mg/kg per day (−59 and −35% in males and females, respectively, vs. controls). At necropsy, there were no treatment‐related macroscopic findings, and changes in organ weights were consistent with the lower terminal body weight of high‐dose animals, compared to controls. Considering the histopathological findings at the highest dose, the reduced absolute and relative thymus weight of both intermediate and high‐dose males must be considered as treatment‐related, despite a lack of statistical significance. Microscopic examination of tissues revealed some treatment‐related changes in high‐dose animals (a slightly higher incidence of minimal lymphoid atrophy in the thymus in males, and a slight trend towards less development of some lymphoid compartments in mandibular (males) and mesenteric (females) lymph nodes). At the highest dose the test item induced signs of poor clinical condition in females and lower body weight gains and food consumption in both sexes, associated with changes in blood biochemistry parameters (lower triglyceride levels), reduced thymus weights and at microscopic examination (lymphoid changes in thymus and lymph nodes). At 2.4 mg/kg per day, effects were observed on thymus weight in males. Consequently, under the experimental conditions of the study the FEEDAP Panel concludes that the NOAEL was established at 0.6 mg SAL/kg per day.
A study was performed to evaluate the potential modifications of cardiovascular function, cardiac contractility and electrophysiology following a single oral administration to conscious dogs.59 Four beagle dogs with implanted telemetry devices for monitoring of ECG, arterial blood pressure, left ventricular pressure (LVP) and body temperature were used in this study. After randomization, the vehicle and the test item were administered in a cross‐over design as single oral doses of SAL mycelial concentrate delivering 0, 0.25, 0.5 or 1.0 mg SAL/kg body weight by gavage at constant dosage‐volume of 5 mL/kg. Clinical signs were observed at least twice a day. The body weight was recorded once before the beginning of the treatment period and on the day of each administration. Cardiovascular parameters were recorded before dose administration and up to 24 h after treatment. Measurements obtained at 1, 0.75 and 0.5 h before treatment and 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12, 16, 20 and 24 h after treatment were analysed. At these time points, heart rate (HR), diastolic, systolic and mean arterial pressure (DAP, SAP and MAP), LVP‐derived parameters (systolic LVP, ΔP (systolic LVP − diastolic LVP), dP/dt max and dP/dt min) and body temperature were measured as well as ECG parameters (PQ interval, QRS complex and QT interval durations). QT interval duration was corrected for HR according to the formula of Bazett (QTb) and Van de Water (QTv). Occurrence of arrhythmia was checked at each analysis time point.
No animal died during the study and no clinical signs were observed related to the treatment. No treatment‐related variations in body weight, arterial blood pressure, LVP, HR, core body temperature or ECG parameters were observed after administration of the test item at 0.25 or 0.5 mg/kg when compared with the vehicle. Following treatment at 1 mg/kg, SAL mycelial concentrate produced statistically significant increases in HR, arterial blood pressure parameters (increases in systolic, diastolic and mean arterial blood pressures) as well as in left ventricular contraction parameters (dP/dt max, systolic LVP and ΔP) when compared with the vehicle group. Other observations at this dose level were a transient decrease in left ventricular relaxation (dP/dt min) as well as changes in QT interval and QTb interval, mainly related to the increase in HR. No major alterations in QTv or core body temperature were observed at 1 mg/kg. No treatment‐related arrhythmias were observed at the analysis time points. Under the experimental conditions of the present study SAL mycelial concentrate induced positive inotropic effects at 1 mg SAL/kg from approximately 30 to 240 min after treatment. These effects were associated with increases in HR, mean diastolic and systolic arterial blood pressures, systolic LVP and dP/dt max as well as a decrease in dP/dt min. Changes in QT and QTb duration were also observed with no major changes in QTv. SAL mycelial concentrate at lower doses did not produce any statistically significant changes in the endpoints measured. On the basis of the results of the present study, the NOEL was considered to be 0.5 mg SAL/kg body weight.
Conclusions on toxicology studies
After consideration of the data previously submitted in 2004 and the studies conducted more recently, the FEEDAP Panel concludes that a NOAEL of 0.5 mg/kg bw per day could be derived from a cardiovascular study in dogs (pharmacological NOAEL) as well as a from a 12‐month dog study (toxicological NOAEL). This is further supported by the NOAEL from the recent 90‐day study in rats (0.6 mg/kg bw per day).
3.2.2.5. Assessment of consumer safety
The metabolic fate of SAL in the chicken and laboratory animals is considered to have an adequate degree of commonality (EFSA, 2004a). Consequently, the data on laboratory animals can be used for the toxicological evaluation for the consumer. Applying a safety factor of 100 to the NOAEL of 0.5 mg/kg bw per day, a safe dose of 300 μg SAL per day is derived for a 60‐kg person, corresponding to an ADI of 0.005 mg/kg bw.
When taking a conservative approach, the FEEDAP Panel considers that the whole SAL‐derived residues represent a risk which is at the most equal to an equivalent quantity of SAL. The exposure of the consumer to SAL‐related total residues has been calculated according to daily food consumption values of animal products set in Regulation (EC) No 429/2008,46 and maximized residue concentrations (average plus 2SD, 95% confidence limit).
The FEEDAP Panel proposed in its previous assessment of SAL‐Na a withdrawal time of 1 day (the ADI was exceeded by a withdrawal of 0.25 days) and uniform MRL of 0.005 mg/kg tissue compatible with the LOQ (EFSA, 2004a). The proposal was apparently based on residue data from overexposed birds (140% of the target dose of 70 mg/kg feed). The study was therefore repeated and submitted with the current dossier.54 Withdrawal times of 1, 3, 6, and 24 h were examined, total SAL‐related residue and MR concentrations could be determined for withdrawal time of 1, 3, and 6 h. These experimental withdrawal times are considered equivalent to a practical zero‐day withdrawal.
The total residue at 1 h withdrawal time (Table 4) complied with the ADI (241 mg ~80% of the ADI). In fact, the margin of safety for the consumer considerably exceeds this estimation since the residue of toxicological relevance represents only 20% of the total radioactive residue (see Section 3.2.4.2).
Table 4.
Consumer theoretical exposure to SAL total residue concentrations (TRCs) in chicken tissues after 1 h withdrawal
| Liver | Kidney | Muscle | Skin/fat | |
|---|---|---|---|---|
|
TRC (mg/kg) ± SD (average + 2 SD) |
1.48 ± 0.29 (2.06) |
0.183 ± 0.046 (0.275) |
0.027 ± 0.003 (0.033) |
0.156 ± 0.037 (0.230) |
| DITR a mg | 0.206 | 0.003 | 0.011 | 0.021 |
| % ADI | 68 | 1 | 4 | 7 |
Daily intake of total residues.
Consequently, no MRLs are considered necessary. However, if MRLs are required for control purposes the recent residue study provides all data necessary to derive MRLs.
3.2.2.6. Proposal for maximum residue limits
A calculation based on the proposed tissue‐specific MRLs (liver: 0.150 mg/kg; kidney: 0.040 mg/kg; muscle: 0.015 mg/kg; skin/fat 0.150 mg/kg) and the ratios MR to total residues (after 1 h withdrawal) indicates consumer exposure of 0.292 mg SAL‐related residues, equal to 97% of the ADI (Table 5).
Table 5.
Consumer safety of the proposed MRLs for salinomycin from Sacox® microGranulate, in chicken tissues after 1 h withdrawal
| Liver | Kidney | Muscle | Skin/fat | Sum | |
|---|---|---|---|---|---|
| MRC (mg/kg) a | 0.141 | 0.039 | 0.012 | 0.145 | – |
| RMTR b | 0.06 | 0.15 | 0.30 | 0.56 | – |
| DITR (mg) c | 0.206 | 0.003 | 0.011 | 0.021 | 0.241 |
| MRL proposed (mg/kg) | 0.150 | 0.040 | 0.015 | 0.150 | – |
| DITMRL (mg) d | 0.250 | 0.003 | 0.015 | 0.024 | 0.292 |
Marker residue concentration in individual tissues (average values + 2 SD, mg/kg).
Ratio MRC to TRC for individual tissues at 1 h withdrawal time.
Dietary intake for individual tissues calculated from total residues (mg).
Dietary intake for individual tissues of total residues calculated from the MRLs.
3.2.2.7. Proposal for a withdrawal period
No withdrawal period appears necessary to ensure consumer safety.
3.2.2.8. Conclusions on safety for the consumer
Exposure estimates to SAL from products of SAL‐Na treated chickens for fattening at the highest proposed use level indicate compliance with an ADI of 0.005 mg SAL/kg bw after 1 h withdrawal, equivalent to a practical 0‐h withdrawal time. MRLs are not considered necessary. If MRLs should be provided for control purposes, the following values are proposed: 0.150 mg SAL/kg liver, 0.040 mg SAL/kg kidney; 0.015 mg SAL/kg muscle and 0.150 mg SAL/kg skin/fat.
3.2.3. Safety for the user
3.2.3.1. Effects on eyes and skin
The acute dermal irritation potential of a test material Sacox®120 microGranulate, was investigated in 3 New Zealand White rabbits according to OECD 404.62 Very slight erythema was noted in all animals from day 2 up to day 11. No oedema was noted in any animal throughout the observation period. Additional observations noted on the test sites from day 5 included dry, flaky skin and skin thickening.
Based on these observations Sacox®120 microGranulate, is not to be classified as a skin irritant.
The acute eye irritation potential of a test material, Sacox® 120 microGranulate, was investigated in 3 New Zealand White rabbits according to OECD 405.63 The test material was instilled as supplied, at a dose volume of the weight equivalent or 0.1 mL containing 70 mg. Systemic toxicity was observed but not eye irritation. A second study was conducted with a 10‐fold lower dose. No corneal or iridal responses were noted for any of the animals throughout the observation period. Slight conjunctival redness was observed in all animals up to either 5 or 24 h after instillation of the test material. Slight chemosis was noted in two animals on the day of instillation only. Slight ocular discharge was observed in all animals on the day of instillation only. Miosis was noted in the treated eye on the day of instillation only. On the basis of these observations Sacox® 120 microGranulate is not to be classified as an eye irritant.
The delayed contact hypersensitivity of the test material, Sacox® 120 microGranulate, was investigated by means of a Magnusson‐Kligman Maximisation Test in guinea pigs according to OECD 406.64 Five test group animals were humanely killed prior to challenge due to lesions on the test sites. Fifteen (100%) positive responses were observed after challenge in the remaining Test Group animals, and 3 (30%) positive responses were observed in the control Group animals following challenge with 5% Sacox® 120 microGranulate. Under the conditions of the study, Sacox® 120 microGranulate is considered to be a sensitizer in guinea pigs.
3.2.3.2. Effects on the respiratory system
One study already assessed in the FEEDAP opinion adopted in 2004 was re‐submitted (EFSA, 2004a). In this study Sacox® 120 microGranulate was tested as a powder aerosol in Sprague‐Dawley rats in groups of five male or five female rats according to OECD 403. A concentration of 0.033 mg/L additive was achieved, 30.5% of the particles had a size of 1–4 μm.65 There were no unscheduled mortalities. The rats were observed to be normal during the exposure period. During the observation period the rats were observed to be wet and unkempt and have test material on snouts. These signs were considered related to treatment but not of toxicological significance. There were no effects on body weights. Moreover, there were no findings at necropsy related to treatment with the test material. The lung/body weight ratio was not affected by the treatment. It was concluded that the rats exposed by snout‐only inhalation for 4 h to a test atmosphere containing aerosolised Sacox® 120 microGranulate showed minimal evidence of toxicity. The LC50 for aerosolised Sacox® 120 microGranulate was not established but was considered to be in excess of the maximum technically achievable concentration of 0.033 mg/L.
A new acute inhalation toxicity study was performed with Sacox® 200 microGranulate according to OECD 436.66 The mean attainable atmosphere concentration of SAL produced after grinding the granules was 1.20 mg/L and 35.4% of the particles had a size of < 4 μm. No deaths occurred. No significant influence on bodyweight was observed and no major abnormalities were observed at necropsy. It was concluded that the acute inhalation median lethal concentration (4 h LC50) of Sacox® 200 microGranulate was in excess of 1.20 mg/L (Globally Harmonized Classification System – unclassified).
3.2.3.3. Inhalation exposure
The potential exposure of users by handling the additive to inhaled SAL was calculated according to the Technical Guidance on User safety (Appendices A and B) (EFSA FEEDAP Panel, 2012b). The 8 h exposure to SAL from inhalation would be about 0.6 mg from Sacox® 120 and 2.2 mg for Sacox® 200 microGranulate. These figures do not consider the alveolar fraction. Data for particle < 10 μm in the dust were only available for Sacox® 200 microGranulate, allowing a reduction of the critical exposure to 44% of the total SAL (about 1 mg). The potential for chronic effects is unknown by this route.
3.2.3.4. Conclusions for user safety
Salinomycin from Sacox® 120 microGranulate is not an irritant to skin and eyes. The additive is considered a potential dermal sensitiser and a likely respiratory sensitiser. These conclusions are considered also valid for the Sacox® 200 microGranulate.
The LC50 for acute inhalation toxicity is > 1.2 mg SAL/L. The potential exposure of users by handling the additive to inhaled SAL was calculated. The 8 h exposure to SAL from inhalation would be about 0.6 mg from Sacox® 120 and 2.2 mg for Sacox® 200 microGranulate. These figures do not consider the alveolar fraction. Data for particles < 10 μm in the dust were only available for Sacox® 200 microGranulate, allowing a reduction of the critical exposure to 44% of the total SAL (about 1 mg). Since no data on the chronic inhalation toxicity of SAL were available, a risk from inhalation toxicity cannot be excluded.
3.2.4. Safety for the environment
The active ingredient is not a physiological/natural substance of established safety for the environment. The additive is also not intended for companion animals only. Consequently, according to Regulation (EC) No 429/2008, the Phase I assessment has to be continued to determine the predicted environmental concentration (PEC), according to the proposed conditions of use in chickens for fattening.
3.2.4.1. Phase I
Physical–chemical properties of salinomycin sodium
The physical–chemical properties of SAL sodium are summarised in Table 6.
Table 6.
Physical–chemical properties of salinomycin sodium
| Property | Value | Unit |
|---|---|---|
| Octanol/water partition coefficient (log K ow 25°C)a | 5.12 (pH 7.4) | – |
| Water solubility (20°C)b |
< 5 (pH 4) 622.3 (pH 7) 1371.2 (pH 9) |
mg/L |
| Vapour pressureb | < 5 x 10−5 (25°C) | Pa |
| Dissociation constantb | 6.4 (20°C) | – |
Paulus J, 2002 (to be found).
Technical dossiers/Supplementary information July 2015/Reference 31.
The low vapour pressure indicates that the substance will not volatilise to any great extent.
Salinomycin is a carboxylic acid which is converted to the anionic form below pH 6.4. At low pH the neutral form is insoluble but the anion is soluble. High solubility (at high pH) is generally associated with a low sorption. Low sorption leads to a higher risk of the pollution of groundwater and aquatic ecosystems.
Fate in soil
Adsorption/desorption in soil
The FEEDAP Panel notes that ionophores like SAL are influenced by the cations present in soil and can interact with clay complexes (Hussain and Prasher, 2011) and that the metabolites of SAL can also show different sorption behaviour compared with SAL itself.
The same study as assessed in a previous opinion (EFSA, 2004a) was submitted by the applicant for the characterisation of the adsorption/desorption in soil.67 In that opinion, the soil sorption coefficient (K oc) value of 180 L/kg was used for the assessment.
The sorption of an anionic ionophore like SAL is dependent on a number of factors. The pKa of 6.4 indicates that above pH 6.4 the molecule occurs as a carboxylate anion. Normalising the sorption for the amount of organic carbon (2% according to the EFSA guidance (EFSA, 2008a)) is not appropriate since SAL can also sorb to other soil components. As an ionophore it can also interact with cations like sodium or calcium during a sorption experiment. The addition of phosphate buffer can extract SAL from soil indicating that also other anions can influence sorption (Ramaswamy et al., 2012). These influences explain the wide range of reported Koc values in different studies (Table 7). Since the normalisation to K oc values is not appropriate for SAL, a different approach is used. For SAL a sandy soil with 2% organic carbon and a pH above 7.4 might be a reasonable worst‐case soil for the prediction of sorption. The only study which follows these requirements is referenced in an EFSA opinion (EFSA, 2004c). A sorption study with sandy loam showed a K d of 1 and a K oc of 77.68 This K oc value was selected to determine the porewater concentrations in the present assessment.
Table 7.
Soil sorption coefficients (K oc) of salinomycin in different studies
| Soil | % OC | % Clay | pH | K oc | Reference |
|---|---|---|---|---|---|
| Sandy clay loam | 5.3 | 20 | 6.8 | 415 | Hussain and Prasher (2011) |
| Sandy | 2.7 | 0.8 | 6.9 | 407 | Hussain and Prasher (2011) |
| Clay | 4.31 | 50 | 6.7 | 373 | Ramaswamy et al. (2012) |
| Loamy sand | 3.7 | 5 | 6.8 | 396 | Ramaswamy et al. (2012) |
| Sandy | 2.25 | 7.5 | 6.9 | 494 | Ramaswamy et al. (2012) |
| Clay loam | 1.6 | 30.3 | 7.3 | 180 | Technical dossier/Section III/Ref. III.68 |
| Acid sand | 1.8 | 6.6 | 4.7 | 368 | Technical dossier/Section III/Ref. III.68 |
| Loamy sand | 1.6 | 17.2 | 6 | 1306 | Technical dossier/Section III/Ref. III.68 |
| Silty clay loam | 2.5 | 21 | 6.1 | 120 | EFSA (2004c) |
| Sandy loam | 1.3 | 13 | 7.5 | 77 | EFSA (2004c) |
| Clay loam | 4.3 | 33 | 5.3 | 152 | EFSA (2004c) |
Biodegradation in soil
The degradation of 14C radiolabelled SAL was investigated according to OECD 307 in four fresh field soils at a nominal temperature of 20°C.69 Upon request, the applicant provided recalculated values at 12°C.37 Samples of LUFA‐Speyer standard soils were treated with [14C]‐SAL at a rate approximately equivalent to 1.8 mg/kg. The samples were incubated in the dark for up to 120 days under aerobic conditions. At intervals throughout the incubation period samples were removed for analysis of total radioactivity (1, 4, 14, 35, 58 and 120 days).
The characterisation of the radioactivity in soil extract was carried out by using reverse‐phase HPLC with a radio‐detector. Chromatographic analysis indicated that SAL was rapidly degraded into a number of components in each soil. Two major metabolites where found at levels below 10% but they were not identified. Most of the SAL was degraded to volatile gases (probably 14CO2). The half‐life (DT50) values calculated for SAL‐Na ranged from 8 days up to 19 days, with an average of 14 days at 20°C (31 days at 12°C). A DT50 of 31 days will be used for further evaluation.
Conclusion on fate and behaviour
The FEEDAP Panel considers a K oc value of 77 L/kg for SAL‐Na. An average DT50 of 31 days (at 12°C) was taken for further use.
Predicted environmental concentrations
Based on the proposed use of 67.96 mg SAL (= 70 mg SAL‐Na)/kg feed for chickens for fattening the calculated PECsoil and PECgroundwater were 353 μg/kg and 239 μg/L, respectively. Both values exceed the trigger values of 10 μg/kg and 0.1 μg/L as indicated in the FEEDAP Technical guidance for assessing the safety of feed additives for the environment (EFSA, 2008a). Therefore, the environmental risk assessment of salinomycin requires a Phase II Environmental Risk Assessment.
3.2.4.2. Phase II
Exposure assessment
The FEEDAP Panel noted that SAL‐Na added to chickens feed is not completely mineralised to carbon dioxide and water and therefore degradation products are present in the manure. There is a large number of metabolites which are not well characterised. Therefore, a detailed environmental risk assessment of each of these degradation products is not feasible. However, SAL plus its metabolites in chicken manure may represent up to 20% of the ionophoric activity (see Section 3.2.3.2).
Characterisation of residues in manure
The findings described in Sections 3.2.3.1 and 3.2.3.2 can be summarised as follows:
SAL is rapidly metabolised and excreted and unchanged SAL amounts to 1% of the SAL‐related products in the excreta.
The metabolites are mainly hydroxylated‐SAL. It can be reasonably assumed that with increasing hydroxylation of SAL the hydrophobicity will decrease and consequently the ionophoric activity.
The ionophoric activity of SAL‐related liver residues is about 20% of SAL intrinsic activity (Dimenna et al., 1989). SAL metabolites in excreta show a higher degree of hydroxylation than those in the liver.
It is concluded that equivalent amounts of SAL‐derived substances in the excreta would show less than 20% ionophoric activity of SAL. For further calculation, it is assumed that the SAL‐derived ionophoric activity in manure would not exceed 20% of the SAL administered dose.
PECs calculation refined in Phase II
Assuming that the ionophoric activity of SAL and its metabolites in chicken excreta would not exceed in total 20% of the orally administered dose, the refined dose used for PEC calculations was 13.6 mg/kg feed. The PECsoil, PECsurfacewater and PECsediment are reported in Table 8.
Table 8.
Predicted environmental concentrations (PECs) of salinomycin in soil, groundwater, surfacewater and sediment
| Input | Value |
|---|---|
| Dose (mg SAL/kg feed) | 67.96×20% = 13.6 |
| MW SAL‐Na | 772.99 |
| VP (Pa) | 5E‐05 |
| Solubility (mg/L) | 622 |
| K oc (L/kg) | 77 |
| DT50 at 12°C (days) | 31 |
| Output | |
| PECsoil (μg/kg) | 71 |
| PECgroundwater (μg/L) | 48 |
| PECsurfacewater (μg/L) | 16 |
| PECsediment (μg/L) | 57 |
The FEEDAP Panel noted that the PEC values do not refer to SAL but consider the relative ionophoric activity of salinomycin plus its metabolites. The assumption that the K oc for SAL is the same as the one of metabolites is made. These metabolites can be more mobile than SAL which would cause higher PEC surface water than shown in the table above.
Since in the exposure route from soil via groundwater to surface water there is also ample time for further mineralization of the initial degradation products of salinomycin, the FEEDAP Panel notes that that the trigger value of 0.1 μg/L will not be exceeded in groundwater. This conclusion is supported with the FOCUS groundwater exposure calculation on the parent compound that results in very low predicted concentration of SAL in groundwater (5 × 10−5 μg/L).70
Effect assessment – ecotoxicity studies
Soil microorganisms: nitrogen transformation
In a 28‐day GLP‐compliant study71 according to the OECD guideline 216, the sandy soil was treated with PEC and ten times PEC nominal concentrations at a rate of 353.25 and 3532.5 μg SAL/kg soil dry weight, equivalent to PECsoil dry weight and ten times PECsoil dry weight. The study was valid; variation in nitrate concentration of control replicates was less than 15% (actual ≤ 9.4%) for all time points. Nitrate formation rate deviations from the controls were less than 25% for both treatments at all‐time points.
No effect on soil nitrogen transformation could be expected at PEC or ten times the PEC.
Terrestrial invertebrates: earthworms (Eisenia fetida)
An acute toxicity GLP‐compliant study following OECD guideline 207 was performed on earthworms (Eisenia fetida).72 Earthworms were placed in an artificial soil at nominal concentrations of 12.1, 24.3, 48.5, 97.1 and 194.2 mg of SAL/kg of soil dry weight (dw). The 14 day 50% lethal concentration (LC50) was determined as 102.9 mg of SAL/kg of soil (dw).
Terrestrial plants
Two GLP‐compliant studies following OECD guideline 208 were performed to investigate the effect of SAL on terrestrial plants. Both studies are valid.
In the study from 2003 (EFSA, 2004a), only three treatment concentrations with a factor of 10 between concentrations were tested on a loamy sand soil. The substrate was treated with SAL‐Na at 1, 10 and 100 mg/kg dw (0.97, 9.71 and 97.1 mg SAL/kg of dry soil). Three species (monocotyledon Lolium perenne and dicotyledon species Raphanus sativus and Phaseolus aureus) were tested. The 14 days test endpoints were the effects on emergence, survival and shoot fresh weight biomass. R. sativus was the most sensitive species for emergence with an effective concentration causing 50% of response (EC50) of 2.55 mg of SAL/kg of soil.
In the test form 201573 six species were tested (monocotyledon species Hordeum vulgare (var. Florentine) and Allium cepa (var. Golden Bear) and dicotyledon species Phaseolus vulgaris (var. Annabel), Solanum lycopersicum (var. Money Maker), Cucumis sativus (var. Telegraph Improved) and Raphanus sativus (var. French Breakfast)). The endpoints determined were the effects on emergence/survival shoot length, shoot fresh weight and shoot dry weight biomass. A loamy sand soil was treated with SAL sodium in geometric series of two based on the gravimetric (nominal) values applied.74
The test item was not sufficiently soluble in any suitable solvent; therefore, the test substance was mixed with fine sand then incorporated into the bulk soil for the treatment.
The most sensitive endpoint was fresh weight and dry weight biomass. The lowest EC50 was dry weight biomass for R. sativus of 4.51 mg SAL/kg of soil dry weight.
The more recent study was considered as a reliable assessment of effects of SAL to plants. The endpoint was calculated based on six descending concentrations in geometric series of two, which gives the result sufficient statistical power. The older study (EFSA, 2004a) is considered less reliable as only three concentrations that differ for the factor of 10 were tested.
Algae and cyanobacteria
Two GLP‐compliant studies following OECD guideline 20175,76 were performed to investigate the effect of SAL on algae and cyanobacteria, respectively.
The study on the algae Scenedesmus subspicatus was valid, mean biomass increase in the control cultures was at least a factor of 16 within the 72 h test period. SAL was stable throughout the exposure period with overall measured concentrations within 15% of nominal concentrations. The 72 h ErC50 77 and the 72 h no observable effect concentration (NOEC) based on the inhibition of growth of algae species S. subspicatus were determined to be 28.4 mg/L and 6.25 mg/L of SAL, respectively.
The 72 h study on cyanobacteria Anabaena flos‐aquae was valid, mean biomass increase in the control cultures was at least a factor of 16 within the 72 h test period. The 72 h ErC50 based on the inhibition of growth of cyanobacterial species A. flos‐aquae were determined to be 25.5 mg/L and the 72 h NOEC > 1 mg/L of SAL, respectively.
The submitted test on cyanobacteria A. flos‐aquae cannot be accepted to extrapolate the effects of SAL on algae or phytoplankton. Cyanobacteria are prokaryotic primary producers and not related to eukaryotic green algae. The physiological differences between these two groups do not allow extrapolation of test results from one group to another. However, the effect on cyanobacteria can be extrapolated to primary producers in freshwater plankton. Nevertheless, the ErC50 values for both groups were very similar (the 72 h ErC50 of 28.4 mg/L v.s. 72 h ErC50 of 25.4 mg/L of SAL for algae and cyanobacteria, respectively).
Aquatic invertebrates
A GLP‐compliant study following OECD guideline 20278 was performed on Daphnia magna to investigate and extrapolate the acute effects of SAL on aquatic invertebrates. The study was valid, no immobilised daphnids could be observed in the control and the dissolved oxygen concentration in control and test vessels at the end of the test were > 85%. The evaluation of biological endpoints was performed using nominal concentrations. No statistically significant differences were found between the control and the highest tested concentration. The 48 h EC50 for immobilisation of D. magna was determined to > 12.33 mg/L of SAL.
Fish
A GLP‐compliant study following OECD guideline 203 was performed on the zebra fish Danio rerio (EFSA, 2004a). The study was valid, no mortality could be observed in the controls and dissolved oxygen in control and test vessels was ≥ 67–98% (≥ 60% of the air saturation value), the concentrations of the test item determined in the test media were within 78–96% of nominal values and the conditions were within acceptable limits throughout the duration of the test. The 96 h LC50 was determined to be 6.98 mg/L of SAL.
Sediment‐dwelling invertebrates
GLP study79 was performed on larvae of Chironomus riparius according to the OECD Guideline 218 to assess chronic effects of SAL on sediment‐dwelling organisms. The chironomid larvae were exposed to 1.46, 2.91, 5.83, 11.65, 23.3, 46.6 and 93.2 mg of SAL7/kg dw of sediment.
The study was valid, the emergence in the controls was ≥ 70% at the end of the test, the emergence of adults in the control vessels occurred between 12 and 28 days, the oxygen concentration was > 60 the air saturation value. SAL was not stable during the exposure period with mean measured concentrations ranging between 60.6–84.2% of nominal start concentrations at the end of the test, therefore the evaluation of biological endpoints was performed using mean measured concentrations.
The NOEC was determined for the most sensitive test endpoint (emergence) as 6.6 mg SAL/kg dw of sediment.
Conclusions on the ecotoxic effect on soil, water and sediment
The applicant submitted GLP studies which followed OECD guidelines as proposed in the technical guidance for assessing the safety of feed additives for the environment (EFSA, 2008a). Tests are valid and the test results can be accepted and used for determination of predicted no effect concentrations (PNECs) and to establish the safe values for exposed environmental compartments.
For the terrestrial compartment, data are available for microorganisms, earthworms and plants. Based on the lowest E(L)C50 of 4.51 mg/kg for plants, the PNEC that is used in the risk assessment is 45.1 μg SAL/kg, applying an assessment factor (AF) of 100 (Table 9).
Table 9.
Risk characterisation (PEC/PNEC ratio) for terrestrial compartment
| Taxa | PECsoil (μg/kg) | E(L)C50 /NOEC (mg/kg) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
| Earthworm | 71 | 103/– | 100 | 1030 | 0.06 |
| Plants | 4.51/– | 100 | 45.1 | 1.6 |
For the aquatic compartment, data are available for algae and cyanobacteria, aquatic invertebrates and fish. Based on the lowest E(L,r)C50 of 6.98 mg SAL/L for fish, the PNEC used in the risk assessment is 69.8 μg/L, applying an AF of 100 (Table 10).
Table 10.
Risk characterisation (PEC/PNEC ratio) for freshwater compartment
| Taxa | PECsurfacewater (μg/kg) | E(L, r)C50/NOEC (mg/L) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
|
Algae Scenedesmus subspicatus |
16 | 28.4/6.25 | 100 | 284a | 0.06 |
|
Cyanobacteria Anabaena flos‐aquae |
25.5/> 1 | 100 | 255 | 0.06 | |
|
Aquatic invertebrates Daphnia magna |
> 12.33/– | 100 | / | / | |
|
Fish Danio rerio |
6.98/– | 100 | 69.8 | 0.23 |
Based on the amount of ecotoxicity data on aquatic species and sediment, an AF of 100 is selected.
Ecotoxicological data for sediment‐dwelling invertebrates are provided for the sediment compartment. The calculated PNEC for the risk assessment is 660 μg SAL/kg, applying an AF of 10 to the NOEC of 6.6 mg/kg (Table 11).
Table 11.
Risk characterisation (PEC/PNEC ratio) for sediment
| Taxa | PECsediment (μg/kg) | NOEC (mg/kg) | AF | PNECa (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
|
Sediment‐dwelling invertebrates Chironomus riparius |
57 | 6.6 | 10 | 660 | 0.09 |
PNEC based on the acute endpoint E(r)C is lower than long term NOEC.
Risk characterisation (PEC/PNEC ratio)
The risk characterisation ratios for terrestrial, freshwater and sediment compartments are reported in Tables 9, 10 and 11, respectively. While for the aquatic and sediment compartment the PEC/PNEC ratios are all < 1, the PEC/PNEC ratio for plants is slightly exceeding the value of 1 (1.6), indicating that a risk for the terrestrial compartment cannot be completely excluded.
Bioaccumulation
The FEEDAP Panel noted that the high octanol/water partition coefficient (log K ow) of SAL‐Na allows bioconcentration in environmental food chains (log K ow values smaller than 3 indicate that a substance is unlikely to bioconcentrate or biomagnify in environmental food chains). Considering that SAL is extensively metabolised in the chicken (Section 3.2.2.1), the risk for bioaccumulation is considered very low.
Conclusion on environmental risk assessment
The use of the SAL‐Na in feed for chickens for fattening and chickens reared for laying up to the highest proposed dose will not pose a risk for aquatic environment. Although the PEC/PNEC ratio for plants slightly exceeds the threshold value, a risk for the terrestrial ecosystem is considered unlikely due to metabolisation and the rapid degradation of SAL in the environment.
3.3. Efficacy
For coccidiostats under re‐evaluation, efficacy data should derive from two types of target animal experiments: a) natural/artificial infection to simulate use conditions (e.g. floor pen studies with poultry), at least one of the locations should be in the EU, b) actual use conditions in field trials, all should be done in the EU within the last 5 years. Anticoccidial sensitivity tests (AST) could replace field trials provided they follow the criteria mentioned in the relevant guidance document on coccidiostats and histomonostats (EFSA FEEDAP Panel, 2012a).80
3.3.1. Floor pen studies
Three floor pen studies81 performed with Sacox® microGranulate were submitted.82 The studies showed similar experimental design (Table 12). One‐day‐old male chickens for fattening (Ross 308) were penned and distributed into three treatment groups: an uninfected untreated control group (UUC), an infected untreated control group (IUC), and an infected treated group (IT). The IT group received feed containing 50 mg SAL‐Na/kg feed, dosage was analytically confirmed (see Table 12). Birds were under study from day 1 to day 35 of life. In the second week of life, birds in the IUC and IT group were inoculated with recent field isolates of pathogenic Eimeria species. Animal health and mortality were monitored daily. Feed intake and body weight of the animals were measured throughout the study, feed to gain ratio was calculated. In the three trials samples of excreta were analysed for oocyst excretion and intestinal lesions were scored. In trials 1 and 3, an analysis of variance (ANOVA) was performed with the performance data and oocyst excretion. Group means were compared using Dunnett test. A Kruskal–Wallis test was used to analyse the data on the mortality and intestinal lesion score. In trial 2, an ANOVA was performed with all data, group means were compared applying Bonferroni correction. In all cases, statistical significance was set at p < 0.05.
Table 12.
Summary of floor pen studies with chickens for fattening using Sacox® microGranulate
| Trial | Birds per pen (replicates per treatment) | Inoculum characteristics | Test item | Analysed SAL‐Na (mg/kg feed) | |||
|---|---|---|---|---|---|---|---|
| Month/year and country of isolation | Intended dose per bird | Day and mode of inoculation | |||||
| 1 |
41–42 (16) |
03/2012 Spain |
1.0 x 105 | E. acervulina |
Day 13 via feed |
Sacox 200 | 46–47 |
| 1.0 x 104 | E. tenella | ||||||
| 5.0 x 104 | E. maxima | ||||||
| 2 |
35 (10) |
12/2010 Lithuania |
6.6 x 104 | E. acervulina |
Day 14 orally via syringe |
Sacox 200 | 45–51 |
| 2.4 x 104 | E. tenella | ||||||
| 1.0 x 104 | E. maxima | ||||||
| 4.0 x 103 | E. mitis | ||||||
| 4.0 x 103 | E.necatrix/ E.praecox | ||||||
| 3 |
40 (14) |
01/2014 Spain |
1.0 x 105 | E. acervulina |
Day 14 via feed |
Sacox 120 | 51 |
| 2.0 x 104 | E. tenella | ||||||
| 5.0 x 104 | E. maxima | ||||||
Table 13 shows the results of the mortality and performance of the birds. Mortality was relatively low in trials 2 and 3 (< 4%). In trial 1 a higher mortality was registered due to a very high mortality registered in the IUC group, which showed a mortality of 17% (mostly related to coccidiosis, 107 birds out of 115). In the three trials, the additive increased significantly the body weight and daily weight gain of the birds and improved significantly the feed to gain ratio as compared to the IUC groups. The data showed also improvements of the IT compared to the UUC groups in trials 1 and 2; the statistical analysis did not allow this comparison in the case of trial 3.
Table 13.
Performance parameters and mortality of chickens for fattening in the floor pen studiesa
| Feed intake (g/day) | Body weightb (g) | Daily weight gain (g) | Feed to gain ratio | Mortalityc (n) | |
|---|---|---|---|---|---|
| Trial 1 d | |||||
| UUC | 105.2 | 2,398* | 67.3* | 1.56* | 10 |
| IUC | 100.9 | 2,258* | 63.3* | 1.60* | 115* |
| IT | 104.5 | 2,454 | 68.9 | 1.52 | 13 |
| Trial 2 e | |||||
| UUC | 102.0 | 1,990b | 56.9b | 1.80a | 7 |
| IUC | 102.3 | 1,985b | 56.7b | 1.80a | 3 |
| IT | 101.5 | 2,092a | 59.8a | 1.70b | 3 |
| Trial 3 f | |||||
| UUC | 97.8* | 2,279* | 64.0* | 1.53* | 21 |
| IUC | 94.3 | 2,163 | 60.6 | 1.56 | 25 |
| IT | 99.0* | 2,392* | 67.2* | 1.47* | 16 |
Results refer to the overall study period (35 days).
Values are final body weight in study 1 and 3 and body weight gain in trial 2.
Total number of birds per treatment group at the beginning of the study: 663, 350 and 560 in Trial 1, 2 and 3, respectively.
Means within a column with * are significantly different to IT group (p ≤ 0.05).
Means within a column with different superscript letters are significantly different (p ≤ 0.05).
Means within a column with * are significantly different to IUC group (p ≤ 0.05).
Results of oocyst excretion and intestinal lesion scores are presented in Tables 14 and 15, respectively.
Table 14.
Total number of Eimeria oocysts per gram of excreta (OPG)
| Measurement 1 | Measurement 2 | Measurement 3 | |
|---|---|---|---|
| Trial 1 a | |||
| UUC | 5.89 x 102c | 5.89 x 102b | 1.02 x 102b |
| IUC | 2.69 x 105a | 7.76 x 103a | 5.37 x 103a |
| IT | 1.35 x 104b | 4.47 x 102b | 1.86 x 102a,b |
| Trial 2 b | |||
| UUC | 1.72 x 104 | 4.15 x 104 | 2.71 x 104 |
| IUC | 8.34 x 104 | 5.46 x 104 | 3.08 x 104 |
| IT | 7.14 x 104 | 1.06 x 104 | 2.89 x 104 |
| Trial 3 c | |||
| UUC | ndc | ndb | nd |
| IUC | 6.03 x 104a | 1.91 x 104a | nd |
| IT | 2.29 x 103b | 1.70 x 102b | nd |
nd: not detected.
Means within a column with different superscript letters are significantly different (p ≤ 0.05).
Measurements were performed on day 19, 27 and 35 of life.
Measurements were performed on day 21, 28 and 35 of life.
Measurements were performed on day 20, 26 and 33 of life.
Table 15.
Coccidiosis lesion scores in different regions of the intestinea
| Day of life | Small intestine | Caeca | |||
|---|---|---|---|---|---|
| Upper | Middle | Lower | |||
| Trial 1 b | |||||
| UUC | 19 | 0.2* | 0.1* | – | 0.2* |
| IUC | 1.9* | 1.4* | 2.4* | ||
| IT | 0.5 | 0.4 | 0.8 | ||
| Trial 2 c | |||||
| UUC | 20 | 0.0a | 0.4 | 0.4 | 0.0 |
| IUC | 0.1a,b | 0.6 | 0.5 | 0.0 | |
| IT | 0.2b | 0.4 | 0.4 | 0.1 | |
| UUC | 26 | 0.2b | 1.0 | 0.4b | 0.1a,b |
| IUC | 0.1a,b | 1.0 | 0.3b | 0.2b | |
| IT | 0.0a | 0.9 | 0.1a | 0.0a | |
| Trial 3 d | |||||
| UUC | 20 | 0* | 0* | – | 0* |
| IUC | 1.9 | 1.8 | 2.2 | ||
| IT | 0.5* | 0.3* | 0.6* | ||
| UUC | 26 | 0 | 0 | – | 0 |
| IUC | 0 | 0 | 0 | ||
| IT | 0 | 0 | 0 | ||
–: not determined.
Coccidiosis lesion scoring using the method of Johnson and Reid, 1970 (0 = no lesion, 1 = very mild, 2 = mild, 3 = moderate and 4 = severe) was carried out on three birds per pen from each pen.
Means within a column with * are significantly different to IT group (p ≤ 0.05).
Means within a column with different superscript letters are significantly different (p ≤ 0.05).
Means within a column with * are significantly different to IU group (p ≤ 0.05).
In trial 1 and 3, on day 6 after inoculation the IT groups showed significantly higher counts of oocysts compared to the UUC and significantly lower counts compared to the IUC group. These differences between the IT and IUC groups persisted until day 14 post‐inoculation in trial 1 and day 12 post‐inoculation in Trial 3. No differences in the total number of oocysts excretion were found between the groups in trial 2, however, the data showed a significant lower count of E. maxima in the IT group (7.4 x 103) compared to the IUC group (15.7 x 103).
In trial 1 and 3, the intestinal lesion scoring showed that 6 days post‐inoculation the IT groups had lower score values compared to the IUC group in all the intestinal regions scored. In trial 2 on day 26, a lower score was found in IT group compared to the IUC in the lower sections of the small intestine and caeca.
3.3.1.1. Conclusions on floor pen studies
SAL‐Na at a concentration of 50 mg/kg complete feed is effective in controlling coccidiosis after artificial inoculation. This could be shown in three studies by an improved body weight gain and feed to gain ratio, by a reduction of coccidiosis‐related intestinal lesion scores and in one study by a reduction of coccidiosis‐related mortality.
3.3.2. Anticoccidial sensitivity tests
Three ASTs83 (AST) performed with Sacox® 120 microGranulate were submitted.82 The studies showed similar experimental design (Table 16). Male chickens for fattening (Ross 308) were selected on the second week of life and distributed into three treatment groups: an uninfected untreated control group (UUC), an infected untreated control group (IUC), and an infected treated group (IT). The IT group received feed containing 50 mg SAL‐Na/kg (analytically confirmed, see Table 16) throughout the study duration. Two to three days after selection, birds in the IUC and IT group were inoculated with pathogenic Eimeria species (recent field isolates, see Table 16); the strains underwent not more than two passages before use. Data were subject to ANOVA. In study 1 and 2 group means were compared with least significant difference test while in study 3 group values were compared against IT group. In study 1 and 2, oocysts counts per gram of excreta (OPG) were log transformed to conduct the analysis. In the same studies, the intestinal lesion score was also analysed using a non‐parametric test of the stratified distribution. In all cases, statistical significance was set at p < 0.05.
Table 16.
Summary of anticoccidial sensitivity tests performed with chickens for fattening using Sacox® 120 microGranulate
| Study | Birds per cage (replicates per treatment) | Inoculum characteristics | Period of the study (day of life) | Analysed SAL‐Na (mg/kg feed) | |||
|---|---|---|---|---|---|---|---|
| Month/year and country of isolation | Intended dose per bird | Day and mode of inoculation | |||||
| 1 |
5 (6) |
03/2012 Belgium |
1.89 x 105 | E. acervulina |
Day 16 via syringe |
14–23 | 48 |
| 4.67 x 104 | E. maxima | ||||||
| 3.66 x 104 | E. tenella | ||||||
| 8.00 x 103 | E. mitis | ||||||
| 1.00 x 103 | E.necatrix/E.praecox | ||||||
| 2 a |
5 (6) |
02/2013 Belgium |
8.56 x 104 | E. acervulina |
Day 18 via syringe |
15–25 | 55 |
| 3.50 x 104 | E. tenella | ||||||
| 6.80 x 104 | E. maxima | ||||||
| 6.40 x 103 | E. mitis | ||||||
| 3.20 x 103 | E.necatrix/E.praecox | ||||||
| 3 |
5 (8) |
03/2012 EU |
6.40 x 104 | E. acervulina |
Day 15 via syringe |
13–22 | 55 |
| 1.30 x 104 | E. tenella | ||||||
| 9.00 x 103 | E. maxima | ||||||
| 3.00 x 103 | E. mitis | ||||||
| 3.00 x 103 | E.necatrix/E.praecox | ||||||
In this study, further to Sacox, five other coccidiostats were also tested.
Table 17 shows the results of the mortality, performance and lesion scores in the sensitivity tests. Coccidiosis‐related mortality was not observed in study 1 and 3 and was low and not significantly different between groups in study 2. In study 1 SAL‐Na treatment of infected groups improved performance parameters significantly, up to the level of the uninfected group. In study 2 and 3 daily weight gain and the feed to gain ratio were significantly improved by the SAL‐Na treatment of infected groups (comparison of the IT and the IUC group). In study 1 and 3 the IT group had lower scores of the intestinal lesions while in study 2 no significant difference was observed between the IT and IUC group.
Table 17.
Performance parameters, mortality and lesion score in chickens for fattening in the anticoccidial sensitivity tests
| Feed intake (g/day) | Body weight (g) | Daily weight gain (g) | Feed to gain ratio | Mortality (n)a | Lesion scoreb | |
|---|---|---|---|---|---|---|
| Study 1 c | ||||||
| UUC | 115.0a | 1,187a | 77.0a | 1.50b | 0 | 1.0c |
| IUC | 107.0b | 1,045b | 61.0b | 1.77a | 1 | 4.9a |
| IT | 114.0a | 1,204a | 79.0a | 1.46b | 0 | 3.2b |
| Study 2 d | ||||||
| UUC | 117.3 | 1,301a | 81.3a | 1.30c | 0 | 0.6b |
| IUC | 116.9 | 1,026c | 55.2c | 1.92a | 1 | 3.7a |
| IT | 118.4 | 1,140b | 66.9b | 1.60b | 1 | 3.2a |
| Study 3 e | ||||||
| UUC | 102 | 953 | 71 | 1.44 | 0 | 0.7* |
| IUC | 99 | 858* | 60* | 1.64* | 0 | 4.7* |
| IT | 102 | 928 | 68 | 1.56 | 0 | 2.6 |
Total number of birds per treatment group at the beginning of the study: 30, 30 and 40 in Study 1, 2 and 3, respectively.
Sum of average lesion scores found for the species E. acervulina, E. maxima and E. tenella. Coccidiosis lesion scoring using the method of Johnson and Reid, 1970 (0 = no lesion, 1 = very mild, 2 = mild, 3 = moderate and 4 = severe) was carried out on three birds per cage from each cage.
Performance parameters refer to the period day 14 – day 23; lesion score was performed on day 23.
Performance parameters refer to the period day 15 – day 25; lesion score was performed on day 26.
Performance parameters refer to the period day 15 – day 22; lesion score was performed on day 22.
a, b, c: Means within a column with different superscript letters are significantly different (p ≤ 0.05).
* Means within a column are significantly different to IT group (p ≤ 0.05).
The results on the excretion of oocysts are shown is Table 18. In all studies, as expected, the UUC had the lowest counts. In study 1, E. acervulina, E. maxima, E. mitis and E. praecox/E. necatrix oocyst excretion was found to be significantly lower in the IT group as compared to the IUC group. In study 3, the excretion of E. acervulina and E. maxima in the IT group was significantly lower than in the IUC group. In study 2, there was no significant difference in oocyst excretion between the IT and IUC group.
Table 18.
Eimeria ooocysts counts per gram of excreta (OPG) in the anticoccidial sensitivity studiesa
| E. acervulina | E. maxima | E. tenella | E. mitis | E. praecox/E. necatrix | Total | |
|---|---|---|---|---|---|---|
| Study 1 | ||||||
| UUC | 0c | 0c | 0b | 0c | 0c | 0c |
| IUC | 3.11 x 105a | 1.18 x 105a | 3.17 x 103a | 2.22 x 104a | 1.10 x 104a | 4.65 x 105a |
| IT | 1.26 x 105b | 5.25 x 104b | 3.73 x 103a | 1.11 x 104b | 4.37 x 103b | 1.98 x 105b |
| Study 2 | ||||||
| UUC | 3.00 x 102b | 0b | 0b | 0b | 0b | 3.00 x 102b |
| IUC | 9.05 x 105a | 9.01 x 104a | 7.40 x 104a | 6.80 x 104a | 4.73 x 104a | 1.18 x 106a |
| IT | 4.71 x 105a | 1.04 x 105a | 3.93 x 104a | 2.60 x 104a | 4.87 x 104a | 6.89 x 105a |
| Study 3 | ||||||
| UUC | 7* | 0* | 0* | 0* | 0* | 7* |
| IUC | 3.16 x 105 * | 5.33 x 10* | 1.71 x 102 | 3.21 x 103 | 2.56 x 103 | 3.97 x 105 |
| IT | 4.67 x 104 | 2.68 x 104 | 2.61 x 102 | 1.74 x 103 | 2.62 x 102 | 8.80 x 104 |
Oocyst count was performed on the last of day of the experiment (day 23, 25 and 22 of the three studies, respectively).
a, b, c: Means within a column with different superscript letters are significantly different (p ≤ 0.05).
*Means within a column are significantly different to IT group (p ≤ 0.05).
3.3.2.1. Conclusions on anticoccidial sensitivity tests
Salinomycin sodium at a dietary concentration of 50 mg/kg was effective in three short term studies (AST) in which Eimeria strains from three different sources with a pathogenic background were administered to chickens by syringe. The results allow the conclusion that SAL has the potential to be effective against Eimeria species occurring under field conditions.
3.3.3. Anticoccidial efficacy of salinomycin‐related substances
The SAL analogues 20‐desoxy‐salinomycin and 17‐epi‐20‐desoxy‐salinomycin were tested for their coccidiostatic property. Chickens for fattening (HNL Nick) were infected on the third and fifth day of life, respectively, with Eimeria tenella (100,000 oocysts/bird). Four chicks per group were allocated to an UUC, an IUC and six IT groups supplemented with 120 mg, 90 mg, 75 mg, 60 mg and 45 mg 20‐desoxy‐salinomycin and 60 mg SAL‐Na per kg feed. Eight chicks per group were allocated to an UUC, an IUC and six IT groups supplemented with 120 mg, 90 mg, 75 mg, 60 mg and 45 mg 17‐epi‐20‐desoxy‐salinomycin and 60 mg SAL‐Na per kg feed. Birds received test diets from day −2 to day 7. Results (OPG, mortality, daily weight gain, lesion score) showed that 20‐desoxy‐salinomycinin was effective at concentrations of 120 and 90 mg, comparable to that obtained with 60 mg SAL‐Na. 17‐Epi‐20‐desoxy‐salinomycinin at concentrations of 120, 90 and 75 mg was marginally effective compared to SAL‐Na at the dose of 60 mg per kg feed.32 , 84
3.3.3.1. Conclusions on the efficacy of salinomycin‐related substances
The 20‐desoxy‐salinomycin is likely half as effective as SAL‐Na against Eimeria tenella. 17‐epi‐20‐desoxy‐salinomycin has a very low activity compared to SAL‐Na. Considering the quantity of both salinomycin‐related substances in the additive, they do not substantially contribute to its anticoccidial efficacy.
3.3.4. Conclusions on efficacy for the target species
Salinomycin sodium is effective in the control of coccidiosis in chickens for fattening. This conclusion is based on the results of three floor pen studies and three ASTs. The minimum effective concentration is 50 mg SAL‐Na/kg complete feed. The conclusion on efficacy in chickens for fattening is extended to chickens reared for laying.
Salinomycin sodium in Sacox® 120 microGranulate and Sacox® 200 microGranulate is considered bioequivalent with respect to its anticoccidial effect.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation85 and Good Manufacturing Practice.
4. Conclusions
Salinomycin sodium is active against certain Gram‐positive bacteria, while Gram‐negative species are resistant. The use of SAL‐Na as a feed additive at the proposed concentration is unlikely to increase shedding of Salmonella, E. coli and Campylobacter or to induce resistance and cross‐resistance to antimicrobials important in human and animal therapy.
Salinomycin sodium from Sacox® 120 microGranulate or Sacox® 200 microGranulate is safe for chickens for fattening at a concentration of 70 mg/kg complete feed with a margin of safety of 1.7. For chickens reared for laying, 50 mg SAL‐Na/kg complete feed are considered safe for a feeding period of the first 12 weeks of life; a margin of safety cannot be given. The simultaneous use of Sacox® microGranulate and certain antibiotic drugs (e.g. tiamulin) is contraindicated.
SAL‐Na is absorbed to a certain extent in the chicken and extensively metabolised. Unchanged SAL represents a very small fraction of the metabolites in tissue and excreta. Many metabolites, predominantly mono‐ and multi‐hydroxylated, have been identified in tissues and excreta. The metabolites in excreta show a higher degree of hydroxylation than in the liver. SAL‐related metabolites have a reduced ionophoric activity when compared with SAL. SAL is considered the MR; ratios MR to total residue are available for all relevant tissues for one and 6 h withdrawal. No residues in eggs are expected provided that the proposed maximum dose and duration of administration are respected.
The FEEDAP Panel reiterates its conclusion from 2004 that (i) SAL does not induce gene mutations in vitro and it is not genotoxic in vivo, (ii) SAL is not a carcinogen, and (iii) the findings of the reproduction toxicity studies do not lead to concern. A NOAEL of 0.5 mg/kg bw per day is derived from a cardiovascular study in dogs (pharmacological NOAEL) as well as from a 12‐month dog study (toxicological NOAEL). This value is further supported by the NOAEL from the recent 90‐day study in rats.
Exposure estimates to SAL from products of SAL‐Na treated chickens for fattening at the highest proposed use level indicate compliance with an ADI of 0.005 mg SAL/kg bw after 1 h withdrawal, equivalent to a practical 0 h withdrawal time. MRLs are not considered necessary.
Salinomycin from Sacox® 120 microGranulate is not irritant to skin and eyes, it is considered a potential dermal sensitiser and a likely respiratory sensitiser. These conclusions are considered valid also for the Sacox® 200 microGranulate. The LC50 for acute inhalation toxicity is > 1.2 mg SAL/L. An 8 h exposure to SAL from inhalation is estimated to be about 0.6 mg from Sacox® 120 microGranulate and 2.1 mg from Sacox® 200 microGranulate (1 mg as alveolar fraction). Since no data on the chronic inhalation toxicity of SAL are available, a risk from inhalation toxicity for persons handling the additive cannot be excluded.
The use of the SAL‐Na in feed for chickens for fattening and chickens reared for laying up to the highest proposed dose will not pose a risk for aquatic environment. Although the PEC/PNEC ratio for plants slightly exceeds the threshold value, a risk for the terrestrial ecosystem is considered unlikely due to metabolisation and the rapid degradation of SAL in the environment.
SAL‐Na is effective in the control of coccidiosis in chickens for fattening. This conclusion is based on the results of three floor pens studies and three ASTs. The minimum effective concentration is 50 mg SAL‐Na/kg complete feed. The conclusion on efficacy is extended to chickens reared for laying.
SAL‐Na in Sacox® 120 microGranulate and Sacox® 200 microGranulate is considered bioequivalent with respect to its anticoccidial effect.
5. Recommendations
A recent 16S rRNA gene sequence analysis allowed the production strain to be identified as S. azureus with the accession number DSM 32267.
The related impurities contents in SAL‐Na should be modified as a result of more recent analytical data to: elaiophylin ≤ 10 mg/kg, 20‐deoxysalinomycin ≤ 10 g/kg, 17‐epi‐20‐desoxy‐salinomycin ≤ 2 g/kg, 18,19‐dihydro salinomycin ≤ 10 g/kg, methylated salinomycin(s) ≤ 10 g/kg.
Documentation provided to EFSA
Sacox® microGranulate for chickens reared for laying. November 2012. Submitted by Huvepharma N. V.
Sacox® microGranulate for chickens for fattening. July 2013. Submitted by Huvepharma N. V.
Sacox® microGranulate for chickens for fattening and chickens reared for laying. November 2013. Submitted by Huvepharma N. V.
Sacox® microGranulate for chickens for fattening and chickens reared for laying. Supplementary information. August 2014. Submitted by Huvepharma N. V.
Sacox® microGranulate for chickens reared for laying. Supplementary information. July 2015. Submitted by Huvepharma N. V.
Sacox® microGranulate for chickens for fattening. Supplementary information. July 2015. Submitted by Huvepharma N. V.
Sacox® microGranulate for chickens for fattening and chickens reared for laying. Supplementary information. May 2016. Submitted by Huvepharma N. V.
Sacox® microGranulate for chickens for fattening and chickens reared for laying. Supplementary information. August 2016. Submitted by Huvepharma N. V.
Evaluation report of the European Union Reference Laboratory for Feed Additives on the Methods of Analysis for salinomycin sodium.
Comments from Member States.
Abbreviations
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism and excretion
- AF
assessment factor
- AST
anticoccidial sensitivity test
- bw
body weight
- CP
crude protein
- CV
coefficient of variation
- CVMP
Committee for Medicinal Products for Veterinary Use
- DAP
diastolic arterial pressure
- dw
dry weight
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FOB
functional observation battery
- GLP
good laboratory practice
- HR
heart rate
- LC‐MS
liquid chromatography‐mass spectrometry
- LOQ
limit of quantification
- LVP
left ventricular pressure
- MAP
mean arterial pressure
- MIC
minimum inhibitory concentration
- MR
marker residue
- MRL
maximum residue limit
- NOAEL
no observed adverse effect level
- NOEC
no observable effect concentration
- OECD
Organisation for Economic Co‐operation and Development
- OPG
oocysts per gram of excreta
- PCB
polychlorinated biphenyl
- PEC
predicted environmental concentration
- PNEC
predicted no effect concentration
- RH
relative humidity
- RMTR
ratio marker to total residue
- SAL‐Na
salinomycin sodium‐sodium
- SAP
systolic arterial pressure
- TRC
Total residue concentration
Appendix A – Estimation of user exposure to salinomycin sodium from the additive Sacox® 120 microGranulate, including consideration of using a filter mask FF P2 or FF P3 as a preventative measure
1.
| Calculation | Identifier | Description | Amount | Source |
|---|---|---|---|---|
| a | SAL‐Na in the dust (mg/g) | 143 | Technical dossier | |
| b | Dusting potential (g/m3) | 0.03 | Technical dossier | |
| a × b | c | SAL‐Na in the air (mg/m3) | 4.29 | |
| d | N° of premixture batches prepared/working day | 10 | EFSA Guidance on user safety EFSA FEEDAP Panel (2012b) | |
| e | Time of exposure per production of one batch (s) | 20 | EFSA Guidance on user safety EFSA FEEDAP Panel (2012b) | |
| d × e | f | Total duration of daily exposure/worker (s) | 200 | |
| g | Uncertainty factor | 2 | EFSA Guidance on user safety EFSA FEEDAP Panel (2012b) | |
| f × g | h | Refined total duration of daily exposure/worker (s) | 400 | |
| h/3 600 | i | Refined total duration of daily exposure (h) | 0.11 | |
| j | Inhaled air per hour (m3) | 1.25 | EFSA Guidance on user safety EFSA FEEDAP Panel (2012b) | |
| j × i | k | Inhaled air during exposure (m3) | 0.14 | |
| c × k | l | SAL‐Na inhaled during exposure per 8‐h working day (mg) | 0.60 | |
| l/10 | m | SAL‐Na inhaled per 8‐h working day (mg) reduced by filter mask FF P2 (reduction factor 10) | 0.06 | |
| l/20 | n | SAL‐Na inhaled per 8‐h working day (mg) reduced by filter mask FF P3 (reduction factor 20) | 0.03 |
Appendix B – Estimation of user exposure to salinomycin sodium from the additive Sacox® 200 microGranulate, including consideration of using a filter mask FF P2 or FF P3 as a preventative measure
1.
| Calculation | Identifier | Description | Amount | Source |
|---|---|---|---|---|
| a | SAL‐Na in the dust (mg/g) | 223 | Technical dossier | |
| b | Dusting potential (g/m3) | 0.07 | Technical dossier | |
| a × b | c | SAL‐Na in the air (mg/m3) | 15.61 | |
| d | N° of premixture batches prepared/working day | 10 | EFSA Guidance on user safety EFSA FEEDAP Panel (2012b) | |
| e | Time of exposure per production of one batch (s) | 20 | EFSA Guidance on user safety EFSA FEEDAP Panel (2012b) | |
| d × e | f | Total duration of daily exposure/worker (s) | 200 | |
| g | Uncertainty factor | 2 | EFSA Guidance on user safety EFSA FEEDAP Panel (2012b)) | |
| f × g | h | Refined total duration of daily exposure/worker (s) | 400 | |
| h/3 600 | i | Refined total duration of daily exposure (h) | 0.11 | |
| j | Inhaled air per hour (m3) | 1.25 | EFSA Guidance on user safety EFSA FEEDAP Panel (2012b) | |
| j × i | k | Inhaled air during exposure (m3) | 0.14 | |
| c × k | l | SAL‐Na inhaled during exposure per 8‐h working day (mg) | 2.17 | |
| m | particles below 10 μm in the dust (%) generated during the Stauber‐Heubach measurement | 44 | Technical dossier | |
| l x m/100 | n | SAL‐Na inhaled per 8‐h working day (mg) reduced by respirable fraction | 0.92 | |
| n/10 | o | SAL‐Na inhaled per 8‐h working day (mg) reduced by filter mask FF P2 (reduction factor 10) | 0.09 | |
| n/20 | p | SAL‐Na inhaled per 8‐h working day (mg) reduced by filter mask FF P3 (reduction factor 20) | 0.05 |
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for Sacox
1.
Sacox ® is a feed additive ‐ belonging to the “Coccidiostats and other medicinal substances” group listed in Directive 70/524/EEC ‐ currently authorized for chickens for fattening and reared for laying by Commission Regulations (EC) No 1463/2004 and No 1852/2003. In the current applications authorisation is sought under articles 10(2)1,2 and under article 13(3)3 of the Regulation (EC) No 1831/2003. Sacox ® consists of salinomycin sodium (active substance) at concentrations of 120 and 200 g/kg (Sacox ® 120 and Sacox ® 200), silica dioxide as flowability enhancer and calcium carbonate as structure‐forming agent and diluent. Sacox ® is intended to be incorporated into feedingstuffs directly and/or through premixtures. The Applicant proposed a concentration of salinomycin sodium in feedingstuffs of 50 mg/kg for chickens reared for laying or ranging from 60 to 70 mg/kg for chickens for fattening. Furthermore the Applicant proposed two sets of MRLs for salinomycin in chicken tissues: 5 μg/kg in all wet tissues1,2 (as already established by Commission Regulation (EC) No 167/2008) or ranging from 12 to 145 μg/kg depending on the target tissues3.
For the quantification of salinomycin sodium in premixtures and feedingstuffs the Applicant submitted the ring‐trial validated method (EN ISO 14183) based on High Performance Liquid Chromatography with post‐column derivatisation coupled to spectrophotometric detection (HPLC‐UV‐Vis). Furthermore, the Applicant adapted the EN ISO 14183 with minor experimental modifications and applied it to the feed additive (Sacox ®) providing similar method performance characteristics. Based on the experimental evidence available the EURL recommends for official control the HPLC‐UV‐Vis method for the quantification of salinomycin in the feed additive, premixtures and feedingstuffs.
For the quantification of salinomycin in chicken tissues the Applicant submitted a single laboratory validated and further verified method based on reverse phase HPLC coupled to a triple quadrupole mass spectrometer in electrospray ionisation mode using matrix matched standards (RP‐HPLC‐MS/MS), similar to the one developed and validated by the European Union Reference Laboratory for Pharmacologically Active Substances (BVL, Berlin). The satisfactory performance characteristics provided by the Applicant for the four tissues of concern (i.e. muscle, kidney, skin/fat and liver) demonstrate that (i) the method proposed by the Applicant is equivalent to the BVL method, and (ii) the Applicant method is also applicable to kidney and skin/fat tissues. Based on the performance characteristics presented, the EURL recommends for official control the single‐laboratory validated and further verified RP‐HPLC‐MS/MS method proposed by the Applicant or any equivalent analytical methods complying with the requirements set by Commission Decision 2002/657/EC to enforce the salinomycin MRLs in the relevant tissues.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, de Lourdes Bastos M, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Kolar B, Kouba M, Puente SL, López‐Alonso M, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Brantom P, Halle I, van Beelen P, Holczknecht O, Vettori MV and Gropp J, 2017. Scientific opinion on the safety and efficacy of Sacox® microGranulate (salinomycin sodium) for chickens for fattening and chickens reared for laying. EFSA Journal 2017;15(1):4670, 40 pp. doi: 10.2903/j.efsa.2017.4670
Requestor: European Commission
Question numbers: EFSA‐Q‐2012‐00994, EFSA‐Q‐2013‐00706, EFSA‐Q‐2013‐00998
Panel members: Gabriele Aquilina, Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Georges Bories, Andrew Chesson, Pier Sandro Cocconcelli, Gerhard Flachowsky, Jürgen Gropp, Boris Kolar, Maryline Kouba, Secundino López Puente, Marta López‐Alonso, Alberto Mantovani, Baltasar Mayo, Fernando Ramos, Guido Rychen, Maria Saarela, Roberto Edoardo Villa, Robert John Wallace and Pieter Wester.
Adopted: 6 December 2016
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Huvepharma NV, Uitbreidingstraat 80, 2600 Antwerp, Belgium.
Commission Regulation (EC) No 1852/2003 of 21 October 2003 authorising the use for 10 years of a coccidiostat in feedingstuffs. OJ L 271, 22.10.2003, p. 13. Amended by OJ L 43, 14.2.2006, p. 22.
Commission Regulation (EC) No 1463/2004 of 17 August 2004 concerning the authorisation for 10 years of the additive ‘Sacox120 microGranulate’ in feedingstuffs, belonging to the group of coccidiostats and other medicinal substances. OJ L 270, 18.8.2004, p. 5. Amended by OJ L 43, 14.2.2006, p. 22.
Commission Regulation (EC) No 600/. OJ L 118, 8.5.2007, p. 3. amending OJ L 270, 18.8.2004, p. 5.
OJ L 99, 19.4.2005, p. 5. amended by OJ L 117, 7.5.2007, p. 9. amended by OJ L 38, 11.2.2012, p. 36. amended by OJ L 108, 20.4.2012, p. 6.
OJ L 50, 23.2.2008, p. 14.
FEED dossier references: FAD‐2012‐0041, FAD‐2013‐0029 and FAD‐2013‐0053.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
The full report is available on the EURL website: https://ec.europa.eu/jrc/en/eurl/feed-additives/evaluation-reports/fad-2012-0041-fad-2013-00290053?search&form-return
FAD‐2013‐0029.
FAD‐2012‐0041.
FAD‐2013‐0053.
This section has been amended following the provision of Article 8(6) and Article 18 of Regulation (EC) No 1831/2003.
Technical dossiers/Supplementary information July 2015/Reference 1.
Technical dossiers/Section II/Reference II.02 and II.03 and Supplementary information July 2015/Reference 2 and 3.
Technical dossiers/Section II/Section II Identity.
Technical dossiers/Section II/Reference II.07 and II.08.
Directive 2002/32/EC of the European Parliament and the Council of 7 May 2002 on undesirable substances in animal feed. OJ L 32, 20.10.2006, 006.001, p. 1.
Technical dossiers/Section II/Reference II.09 and II.10.
Technical dossiers/Section II/Reference II.05.
Technical dossiers/Section II/Reference II.11 and II.12.
Technical dossiers/Section II/Reference II.13 and II.14.
Technical dossiers/Section II/Reference II.15 and II.16.
Technical dossiers/Section II/Reference II.17.
Technical dossiers/Supplementary information July 2015/Reference 31.
Technical dossiers/Section II/Reference II.18.
Technical dossiers/Section II/Reference II.19.
OJ L 271, 22.10.2003, p.13. Amended by OJ L 43, 14.2.2006, p. 22. and OJ L 270, 18.8.2004, p. 5. Amended by OJ L 43, 14.2.2006, p. 22.
Technical dossiers/Section II/Reference II.06.
Technical dossiers/Section II/Reference II.52.
Technical dossiers/Supplementary information May 2016/Annex 1.
Technical dossiers/Supplementary information May 2016/Annex 3.
Technical dossiers/Supplementary information May 2016/Annex 4.
Technical dossiers/Supplementary information May 2016/Annex 6.
Technical dossiers/Section II/Reference II.27.
Technical dossiers/Supplementary information August 2016.
Including the following: Escherichia coli (ATCC 25922), Escherichia coli (UK – human), Pseudomonas aeruginosa (ATCC 27853), Pseudomonas aeruginosa (UK – human), Staphylococcus aureus (ATCC 25923), Staphylococcus aureus (UK – human), Enterococcus faecalis (ATCC 29212), Enterococcus faecalis (UK – human), Bacillus subtilis (ATCC 6633), Bacillus spp. (UK – human), Salmonella typhimurium (NCIMB 13034), Salmonella typhimurium (UK – chicken), Campylobacter jejuni (ATCC 33560), Campylobacter jejuni (UK – human), Campylobacter jejuni (German cow isolate), Campylobacter coli (UK – human), Campylobacter coli (German cow isolate), Campylobacter coli (UK cow isolate), Bacteroides spp. (Bacteroides spp.), Bacteroides fragilis (UK – human), Bifidobacterium bifidum (ATCC 29521), Bifidobacterium spp. (UK – human), Clostridium perfringens (NCTC 8237), Clostridium spp. (UK – human), Eubacterium lentum (ATCC 43055), Eubacterium cylindroides (UK – human), Fusobacterium necrophorum (ATCC 25286), Fusobacterium nucleatum (UK – human), Peptostreptococcus anaerobius (ATCC 27337), Peptostreptococcus anaerobius (UK – human), Lactobacillus casei (UK – human), Lactobacillus acidophilus (UK – human), Lactobacillus lactis (DSM 20384).
Technical dossiers/Section II/Reference II.35 and II.36.
Technical dossiers/Section II/Reference II.37 and II.38.
Technical dossiers/Section II/Reference II.39 and II.40.
One day withdrawal was originally proposed in the dossier. However, subsequently an application under Article 13 of Regulation (EC) No 1831/2003 was submitted (FAD‐2013‐0053) in which a zero‐day withdrawal period is proposed.
Technical dossiers/Section III/Reference III.2.
Sodium, potassium, calcium, chloride, iron, magnesium, total bilirubin, creatinine, glutamic oxoloacetic transaminase, aspartate aminotransferase, serum cholinesterase, alkaline phosphatase.
Leucocytes, erythrocytes, haemoglobin, haematocrit, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, heterophiles, eosinophiles, basophiles, lymphocytes, monocytes, undifferentiated cells.
OJ L 133, 22.5.2008, p. 1.
Technical dossiers/Supplementary information August 2016/Annex 3.
Enrofloxacine, CTC‐HCl, sulfadimidine‐Na, colistine, erythromycine (EFSA, 2004a).
Databases: PubMed and Web of Science; key words: salinomycin drug interaction; time span 2000–2015.
Technical dossiers/Supplementary information July 2015/References 4 and 5.
Technical dossiers/Supplementary information May 2016/Annex 5.
Databases: PubMed and Web of Science; key words: salinomycin bacterial resistance; time span 2000–2015.
Technical dossiers/Supplementary information July 2015/References 10 and 11.
Technical dossier FAD‐2013‐0053/Section III/Annex III.2.
Technical dossier FAD‐2013‐0053/Section III/Annex III.3.
Technical dossier FAD‐2013‐0053/Section III/Annex III.4.
Technical dossier FAD‐2012‐0041/Supplementary information July 2015/Reference 69.
Technical dossiers/Supplementary information July 2015/Reference 23.
Technical dossiers/Supplementary information July 2015/Reference 24.
Databases: PubMed, Toxnet and Web of Science; key words: salinomycin toxicity, mutagenicity and genotoxicity; time span 2000–2015.
Technical dossiers/Supplementary information July 2015/Reference 49, 50 and 51.
Technical dossiers/Section III/Reference 37.
Technical dossiers/Section III/Reference 38 and 39.
Technical dossiers/Section III/Reference 40.
Technical dossier FAD‐2012‐0041/Section III/Reference 36.
Technical dossier FAD‐2012‐0041/Supplementary Information July 2015/Reference 25; Technical dossier FAD‐2013‐0029/Section III/Reference 36.
Technical dossiers/Section III/Reference III.68.
The studies of Hussain and Prasher and Ramaswamy et al. did not have sandy soils with a naturally high pH. Adjusting the pH of a soil influences the cations and anions present in the porewater which can have an influence on the sorption of SAL. Therefore soils with an artificially changed pH value are not suitable as a reasonably worst‐case soil for the prediction of sorption.
Technical dossiers/Supplementary information July 2015/Reference 28.
Value obtained with FOCUS calculations with the following parameters (200 g/ha, calculated from 20% of 67.96 as the dose; K oc = 77, DT50 = 31 days at 12°C for the Chateaudun scenario with winter cereals. In the model SAL was incorporated into the soil.
Technical dossiers/Supplementary information July 2015/Reference 27.
Technical dossiers/Section III/Reference III.77.
Technical dossiers/Supplementary information July 2015/Reference 29.
0.31, 0.62, 1.27, 2.50, 5.02 and 10.11 mg/kg dry soil for Cucumis sativus and Allium cepa. 0.62, 1.27, 2.50, 5.02, 10.11 and 20.04 mg/kg dry soil for Raphanus sativus. 1.27, 2.50, 5.02, 10.11, 20.04 and 39.92 mg/kg dry soil for Phaseolus vulgaris and Solanum lycopersicum. 5.02, 10.11, 20.04, 39.92, 79.11 and 158.41 mg/kg dry soil for Hordeum vulgare.
Technical dossiers/Section III/Reference III.81.
Technical dossiers/Supplementary information July 2015/Reference 30.
EC50 in terms of reduction of growth after exposure for 72 h.
Technical dossiers/Section III/Reference III.78.
Technical dossiers/Supplementary information July 2015/Reference 32.
The FEEDAP Panel stated in its guidance for the preparation of dossiers for coccidiostats and histomonostats (EFSA FEEDAP Panel, 2012a) that studies with artificial infection would be preferred over field trials due to their inherent weaknesses. These short term studies should use field strains of Eimeria, recently confirmed as pathogenic/resistant by a sensitivity test or recognised problems in the poultry operation (confirmed by veterinary certificate). The Eimeria field strains should ideally undergo one, but in any case not more than two passage(s) before use in such trials.
Trial 1: Technical dossiers/Section IV/Reference IV.10. Trial 2: Technical dossier FAD‐2012‐0041/Supplementary information July 2015/Reference 70; Technical dossier FAD‐2013‐0029/Section IV/Reference IV. 16. Trial 3: Technical dossiers/Supplementary information May 2016/Annex 7.
The applicant submitted additional studies. These studies were not accepted as they did not follow the requirements of the Guidance for the preparation of dossiers for coccidiostats and histomonostats (EFSA FEEDAP Panel, 2011a).
Study 1: Technical dossiers/Supplementary information July 2015/Reference 40. Study 2: Technical dossiers/Supplementary information July 2015/Reference 41. Study 3: Technical dossiers/Supplementary information May 2016/Annex 8.
Technical dossiers/Supplementary information May 2016/Annex 2.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- Dimenna GP, Lyon FS, Thompson FM, Creegan JA and Wrigt GJ, 1989. Effect of antibiotic combination, dosing period, dose vehicle and assay method on salinomycin residue levels and their ionophoricity in chicken tissues. Journal of Agricultural and Food Chemistry, 37, 668–676. [Google Scholar]
- Dowling L, 1992. Ionophore toxicity in chickens: a review of pathology and diagnosis. Avian Pathology, 21, 355–368. [DOI] [PubMed] [Google Scholar]
- EC (European Commission), 1982, online. Report of the Scientific Committee for Animal Nutrition on the use of salinomycin in feedingstuffs. Available from https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_antibiotics-43.pdf
- EC (European Commission), 1984, online. Second report of the Scientific Committee for Animal Nutrition on the use of salinomycin in feedingstuffs for chicken. Available from https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_antibiotics-44.pdf
- EC (European Commission), 1991, online. Report of the Scientific Committee for Animal Nutrition on the use of Salinomycin Sodium in feedingstuffs for pigs. Available from https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_antibiotics-46.pdf
- EC (European Commission), 1992, online. Report of the Scientific Committee for Animal Nutrition on the use of salinomycin‐sodium in feedingstuffs for rabbits for fattening. Available from https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_antibiotics-47.pdf
- EC (European Commission), 1997, online. Report of the Scientific Committee for Animal Nutrition on the extension of use of salinomycin (E‐766) to the feedingstuffs for chickens reared for laying. Available from https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_antibiotics-54.pdf
- EFSA (European Food Safety Authority), 2004a. Opinion of the scientific Panel on Additives and Products or substances used in Animal Feed on a request from the Commission on the re‐evaluation of Sacox 120 microGranulate in accordance with article 9G of Council Directive 70/524/EEC. EFSA Journal 2004;2(7):76, 49 pp. doi: 10.2903/j.efsa.2004.76 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2004b. Opinion of the scientific Panel on Additives and Products or substances used in Animal Feed on a request from the Commission on the on the evaluation of coccidiostat Kokcisan® 120G. EFSA Journal 2004;2(6):42, 61 pp. doi: 10.2903/j.efsa.2004.63 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2004c. Opinion of the scientific Panel on Additives and Products or substances used in Animal Feed on a request from the Commission on the safety and efficacy of product “BIO‐COX 120G” as feed additive in accordance with article 9G of Council Directive 70/524/EEC. EFSA Journal, 2004;2(7):75, 51 pp. doi: 10.2903/j.efsa.2004.75 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Update of the Opinion of the Scientific Panel on Additives and Products or Substances used in Animal Feed on a new request from the Commission related to the safety of “Bio‐Cox® 120G” based on Salinomycin sodium as a feed additive in accordance with Council Directive 70/524/EEC. EFSA Journal, 2005;3(2):170, 4 pp. doi: 10.2903/j.efsa.2005.170 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Opinion of the Panel on additives and products or substances used in animal feed (FEEDAP) related to the safety and efficacy of “Kokcisan 120G”. EFSA Journal 2006;4(7):378, 12 pp. doi: 10.2903/j.efsa.2006.378 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Safety of Kokcisan 120G as a feed additive for chickens for fattening – Updated Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed. EFSA Journal 2007;5(10):547, 10 pp. doi: 10.2903/j.efsa.2007.547 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. doi: 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008b, revised in 2009. Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for the preparation of dossiers for the re‐evaluation of certain additives already authorised under Directive 70/524/EEC. EFSA Journal 2008;6(9):779, 9 pp. doi: 10.2903/j.efsa.2008.779 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008c. Technical Guidance: Microbial Studies. EFSA Journal 2008;6(10):836, 3 pp. doi: 10.2903/j.efsa.2008.836 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008d. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on a request from the European Commission on the withdrawal period for Coxidin® for chickens and turkeys for fattening and the re‐examination of the provisional Maximum Residue Limit. EFSA Journal 2008;6(7):731, 14 pp. doi: 10.2903/j.efsa.2008.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011a. Guidance for the preparation of dossiers for coccidiostats and histomonostats. EFSA Journal 2011;9(5):2174, 12 pp. doi: 10.2903/j.efsa.2011.2174 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011b. Technical guidance: Tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. doi: 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. doi: 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. doi: 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal 2012;10(6):2740. 10 pp. doi: 10.2903/j.efsa.2012.2740 [DOI] [Google Scholar]
- EMA (European Medicines Agency), 2007. MONENSIN (Cattle, including dairy cows). EMEA/CVMP/185123/2007‐Final. http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500015096.pdf
- Hussain SA and Prasher SO, 2011. Understanding the sorption of ionophoric pharmaceuticals in a treatment wetland. Wetlands, 31, 563–571. [Google Scholar]
- Islam KM, Afrin S, Das PM, Hassan MM, Valks M, Klein U, Burch DG and Kemppainen BW, 2008a. Compatibility of a combination of tiamulin and chlortetracycline with salinomycin in feed during a pulsed medication program coadministration in broilers. Poultry Science, 87, 2528–2534. [DOI] [PubMed] [Google Scholar]
- Islam KM, Afrin S, Khan MJ, Das PM, Hassan MM, Valks M, Burch DG and Pesti GM, 2008b. Compatibility of a combination of tiamulin plus chlortetracycline with salinomycin in feed during a long‐term co‐administration in broilers. Poultry Science, 87, 1565–1568. [DOI] [PubMed] [Google Scholar]
- Islam KM, Klein U and Burch DG, 2009. The activity and compatibility of the antibiotic tiamulin with other drugs in poultry medicine—a review. Poultry Science, 88, 2353–2359. [DOI] [PubMed] [Google Scholar]
- Johnson J and Reid WM, 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor pen experiments with chickens. Experimental Parasitology, 28, 30–36. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Kinashi H, Otake N, Mitani M and Yamamishi T, 1976. Chemical modification and structure‐activity correlation of salinmomycin. Agricultural and Biological Chemistry, 40, 1633–1640. [Google Scholar]
- Miyazaki Y, Mitani M and Otake N, 1978. Ionophorous properties of SY‐1 (20‐Deoxysalimomycin) in rat liver mitochondria. Agricultural and Biological Chemistry, 42, 2133–2138. [Google Scholar]
- Pressman BC and Fahim M, 1982. Pharmacology and toxicology of the monovalent carboxylic ionophores. Annual Review of Pharmacology and Toxicology, 22, 465–490. [DOI] [PubMed] [Google Scholar]
- Ramaswamy J, Prasher SO and Patel RM, 2012. Sorption and desorption of salinomycin sodium in clay, loamy sand, and sandy soils. Environmental Monitoring and Assessment, 184, 5363–5369. [DOI] [PubMed] [Google Scholar]
- Rizvi F, Anjum D, Khan A, Mohsan M and Shazad M, 2008. Pathological and serum biochemical effects of salinomycin on layer chicks. Pakistan Veterinary Journal, 28, 71–75. [Google Scholar]
- Rocha BA, de Oliveira AR, Pazin M, Dorta DJ, Rodrigues AP, Berretta AA, Peti AP, de Moraes LA, Lopes NP, Popisil S, Gates PJ and Assis M, 2014. Jacobsen catalyst as a cytochrome P450 biomimetic model for the metabolism of monensin. A. BioMed Research International, 2014 Available from 10.1155/2014/152102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GY, Tu P, Chen X, Guo YG and Jiang SX, 2013. Effect of three polyether ionophores on pharmacokinetics of florfenicol in male broilers. Journal of Veterinary Pharmacology and Therapeutics, 36, 494–501. [DOI] [PubMed] [Google Scholar]


