Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Palm lethal yellowing phytoplasmas for the EU territory. This name is used to describe diseases that share the same succession of symptoms in palms that are caused by a number of strains of phytoplasma, for which efficient molecular detection assays are available. The pest is not known to occur in the EU and therefore does not meet one of the criteria for being a Union regulated non‐quarantine pest. For ‘Candidatus Phytoplasma palmae’, the planthopper Haplaxius crudus, which is not known to be present in the EU, is the confirmed vector, but for the other strains, the vectors are unknown. The host range of the pest is restricted to Arecaceae species, in particular coconut. The pest is regulated on all known hosts in Annex IIAI of Directive 2000/29/EC. It could potentially enter the EU via plants for planting or through infected vectors. The phytoplasmas could become established in the EU as host plants are present. It is unknown whether arthropods present in the EU could be vectors. The potential impact of the pest if introduced into the EU is difficult to assess given this uncertainty but is estimated to be limited. The main knowledge gaps concern the status of potential vector insects in the EU; the possibility for seed transmission of the phytoplasmas; the origin and volume of the trade in palm seeds and plants for planting; the host status and susceptibility of many palm species grown in the EU and the potential new assignments of phytoplasmas to this categorisation that might have associated alternate hosts. Palm lethal yellowing phytoplasmas meet the criteria assessed by EFSA for consideration as Union quarantine pest.
Keywords: Palm lethal yellowing phytoplasmas, Candidatus Phytoplasma palmae, Haplaxius crudus, Arecaceae, coconut, pest categorisation, quarantine pest
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/ pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L.. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A Section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips amitinus Eichhof |
| Cephalcia lariciphila (Klug) | Ips cembrae Heer |
| Dendroctonus micans Kugelan | Ips duplicatus Sahlberg |
| Gilphinia hercyniae (Hartig) | Ips sexdentatus Börner |
| Gonipterus scutellatus Gyll. | Ips typographus Heer |
| Sternochetus mangiferae Fabricius | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L.,Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Palm lethal yellowing mycoplasm is one of a number of pests listed in the appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest (RNQP) for the area of the European Union (EU) excluding Ceuta, Melilla and the outermost regions of Member States (MSs) referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
Palm lethal yellowing mycoplasm is not the name of a single pest, but it describes a set of symptoms that can be attributed to different phytoplasma strains (note, the causal agents for all these diseases are now classified as phytoplasmas, which has superseded the term mycoplasm/mycoplasma) (IRPCM, 2004). From here on, these will be referred to as the Palm lethal yellowing phytoplasmas and this pest categorisation considers all the Palm lethal yellowing‐type disease (LYD) strains of phytoplasma. Collectively, these diseases share the same succession of symptoms on palms as the originally described palm lethal yellowing, a name first used by Nutman and Roberts (1955) to describe a fatal phytoplasma‐associated disease of coconuts in Jamaica, and include diseases that are also often known by various alternative local or regional names.
Some strains of Palm lethal yellowing phytoplasmas are transmitted by the plant hopper M. crudus that is already listed in Directive 2000/29/EC and the Commission requested the EFSA PLH Panel for a pest categorisation. That insect is nevertheless now reclassified taxonomically as Haplaxius crudus.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on Palm lethal yellowing phytoplasmas was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name (Palm lethal yellowing) of the pest as search term. Relevant papers were reviewed, and further references and information were obtained from experts, from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO 2017).
Data about import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT.
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Consumers (DG SANCO) and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation as well as notifications of plant pests detected in the territory of the MSs and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for Palm lethal yellowing phytoplasmas, following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union RNQP in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required as per the specific ToR received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a RNQP. If one of the criteria is not met, the pest will not qualify. Note that a pest that does not qualify as a quarantine pest may still qualify as a RNQP which needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone; thus, the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/ presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism. | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area). |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future. |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone). |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6 ) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met. |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? Yes
Palm lethal yellowing phytoplasmas is the name used to describe diseases that share the same succession of symptoms in palms and are caused by phytoplasmas. However, while these seemingly similar aetiologies and shared symptoms initially supported the view of a common phytoplasma, it has now been confirmed that there are phytoplasmas from distinct taxonomic groups associated with this disease in different parts of the world (Harrison et al., 2014).
Because no complete genome sequences are currently available for any of these phytoplasmas, their taxonomy is based on 16S rRNA sequences, and two parallel classification systems have been developed, the 16Sr group system, based on restriction enzyme digest profiles of the 16S rDNA, and the ‘Candidatus Phytoplasma’ species system, in which phytoplasmas sharing less than 97.5% similarity of their 16S rRNA gene sequence may be ascribed to different ‘Ca. Phytoplasma’ species when they are characterised by distinctive biological, phytopathological and genetic properties (IRPCM, 2004). It is also the case that the taxonomic status is undergoing constant revision, and the historic literature contains out‐dated classifications that have changed. The summary below is primarily based on the most recent and internationally accepted reclassification as detailed in Harrison et al. (2014), a system that has reference GenBank sequence accession numbers for the phytoplasma‐type strains listed below (see Figure 1).
Figure 1.
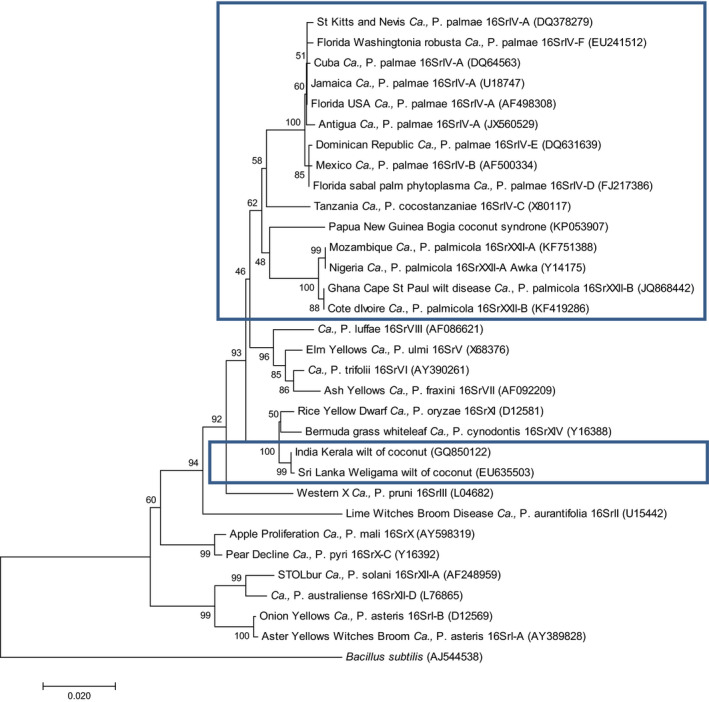
Dendrogram, constructed by the Neighbour‐Joining method, showing the phylogenetic relationships among the coconut phytoplasmas compared to other phytoplasmas based on publicly available sequences of the 16S rRNA gene. GenBank accession numbers are shown in parenthesis. Note the relatively close grouping of the strains considered to fit within the Palm lethal yellowing phytoplasmas description at the top of the tree (upper box), whilst the Indian and Sri Lankan coconut wilt phytoplasmas are grouped elsewhere (lower box). Bootstrap values (expressed as percentages of 1000 replications) are shown, and branch lengths are proportional to the number of inferred character state transformations. Bar, substitutions per base
The origin of the disease is unknown, but localised outbreaks have probably occurred in certain Caribbean islands since the early 19th Century (Harrison et al., 1999). The disease was first recognised as a serious problem when coconut became widely cultivated as a plantation crop in Jamaica, where the causal agent was first identified in 1972 (Plavsic‐Banjac et al., 1972). This original Palm lethal yellowing phytoplasma from Jamaica is referred to as 16SrIV‐A, ‘Candidatus Phytoplasma palmae’, and this strain has been recorded in coconuts (Cocus nucifera) in Florida (Harrison et al., 2008), Saint Martin, Saint Barthelemy, Saint Kitts and Nevis (Myrie et al., 2006, 2012), Antigua, Cuba (where the disease is locally known as Amarilles letal de las palmeras, pudricón del cogollo) (Llauger et al., 2002), Haiti (where the disease is known as ‘Jaunisse létale des palmiers’, ‘pourriture du bourgeon terminal’), Honduras (Ashburner et al., 1996) and Mexico (Vázquez‐Euán et al., 2011). This subgroup has also been detected at a low frequency occurrence in Roystonea regia and Acromonia mexicana palms in Mexico (Narvaez et al., 2016). The very closely grouped 16SrIV‐F subgroup of this ‘Ca. Phytoplasma’ species has been found in Washingtonia robusta palms in Florida, while subgroup 16SrIV‐B was originally identified in coconut in Oaxaca, on the Pacific coast of Mexico, and is the same as Yucatan lethal decline. Subgroup 16SrIV‐D is associated with Carludovica palmata leaf yellowing and Pritchardia pacifica lethal yellowing from Mexico, the Texas Phoenix palm phytoplasma and the Florida sabal palm phytoplasma, and a 16SrIV‐E subgroup has been found associated with coconut in the Dominican Republic (Martinez et al., 2008).
In Africa, there is the 16SrIV‐C group on coconut in Tanzania and Kenya (Schuilling et al., 1992), but this is taxonomically very distinct from the other 16SrIV phytoplamas so has been placed in its own ‘Ca. Phytoplasma’ species, called ‘Ca. Phytoplasma cocostanzania’.
The other phytoplasma strains in Africa are in the 16SrXXII group. The 16SrXXII‐A strain referred to as ‘Ca. Phytoplasma cocosnigeriae’ in Hogenhout et al. (2008) but now renamed as ‘Ca. Phytoplasma palmicola’ (Harrison et al., 2014) was first recorded in Nigeria in 1917, where it is called Awka disease (Osagie et al., 2015), and it is also known as Kaïncopé in Togo and Kribi disease in the Cameroon (Dollet et al., 1977). This is also the strain now found in Mozambique (Bonnot et al., 2010). In Ghana and Cote d'Ivoire, the strain that now occurs is slightly different and is referred to as 16SrXXII‐B or as ‘Ca. Phytoplasma palmicola’ – related strain (also known locally as Cape St Paul wilt disease) (Dabek et al., 1976; Arocha‐Rosete et al., 2014).
In Papua New Guinea, a new Palm lethal yellowing phytoplasma, locally termed ‘Bogia coconut syndrome’ has recently been identified (Kelly et al., 2011), and in the literature, this is being classified as a 16SrIV group phytoplasma, but based on its 16S rRNA sequence, it is quite clearly in a new, previously undescribed ‘Ca. Phytoplasma’ species. However, this syndrome shows the same progression of symptoms as the other Palm lethal yellowing phytoplasmas, so it is included in this categorisation.
It is important to note that the phytoplasmas that have been identified associated with coconut in India, Sri Lanka, Malaysia and Indonesia, reported in Gurr et al. (2016) as being lethal yellowing type diseases, do not follow the typical progression of symptoms of the Palm lethal disease phytoplasmas and are generally non‐lethal, causing wilt symptoms, so these should be excluded from this categorisation. These phytoplasmas are in the very different taxonomic groups of 16SrXI and 16SrXIV (see Figure 1).
3.1.2. Biology of the pest
No single symptom is diagnostic of the Palm lethal yellowing phytoplasma diseases; instead it is the appearance of progression of a series of symptoms in a chronological order that defines the disease, with the whole process usually taking 3–5 months from the initial onset of symptoms to death of the palm. The first obvious symptom in mature palms is premature drop of most or all fruits within a few days. Inflorescence necrosis will then develop, male flowers abscise and fruit set cease. Foliar yellowing will then develop and as discoloration of the foliage advances up through the canopy, the spear leaf will collapse and die. Eventually, the entire canopy of the palm withers and falls off leaving a bare trunk standing (the characteristic ‘telegraph pole’ stage). Currently, because of the lack of knowledge of vector species, it is unknown how long it takes for the initial symptoms to develop following introduction of the phytoplasma into the palm by the vector, but it is suspected that there may be an initial symptomless infection period of a few months (Harrison et al., 2014).
In general, where vectors have been identified for phytoplasmas, they have been found to be species of leafhoppers, planthoppers and psyllids. For the Palm lethal yellowing group of phytoplasmas, the only vector species that has been confirmed is the plant hopper M. crudus (now reclassified taxonomically as H. crudus) (Howard et al., 1983). This has only been confirmed as the vector for the 16SrIV‐A ‘Candidatus Phytoplasma palmae’ strain, where transmission studies have confirmed that it can transmit the phytoplasma between coconut palms. For the other 16SrIV subgroups and for the 16SrXXII ‘Ca. Phytoplasma palmicola’ and ‘Ca. Phytoplasma palmicola’ – related strains, no insect vectors have been confirmed, and H. crudus is not believed to occur in Africa. There are some reports in the literature of phytoplasma DNA from these other groups being found associated with collected insect samples, but even where vector transmission studies have been attempted with these insects, none have been confirmed to transmit the phytoplasmas between plants, so the vector(s) must for now be described as unknown.
A number of studies have been undertaken to determine whether these phytoplasmas can be transmitted through seed or pollen. Phytoplasma DNA has occasionally been detected in coconut embryos, but studies in Ghana (Nipah et al., 2007) and Mexico (Oropeza et al., 2011) found no evidence that nuts from infected palms developed into infected seedlings and there was no confirmation that this DNA in the embryos was associated with viable phytoplasmas. However, recent in vitro studies in Mexico (Oropeza et al., 2017) has found evidence for the presence of the lethal yellowing phytoplasma in plantlets obtained through in vitro germination of zygotic embryos from the seed of infected palms, although the authors conclude that they cannot say for certain whether this phenomenon occurs in nature. In previous studies, Ogle and Harries (2005) concluded that the most likely means of transmission of the disease between Caribbean islands has been by the unintentional introduction of infected vectors on pasture grasses or animal fodder rather than through movement of infected seeds and seedlings, while anecdotal evidence suggests that the supposed movement of the 16SrXXII ‘Ca. Phytoplasma palmicola’ from West Africa to Mozambique was through the movement of young infected seedlings from the Cameroon, with the disease subsequently being transmitted once it had reached Mozambique through indigenous (unknown) insect vector species.
The evidence of any alternate hosts for these phytoplasmas beyond palm species remains controversial. Brown et al. (2008) reported that the 16SrIV ‘Ca. Phytoplasma palmae’ phytoplasma had been found in the weeds Emilia fosbergii and Synedrella nodiflora in Jamaica and Brown and McLaughlin (2011) have reported it in Stachytarpheta jamaicensis, Macroptilium lathyroides and Cleome rutidosperma in Jamaica. Arocha‐Rosete et al. (2016) reported detection of the 16SrXXII‐B ‘Ca. Phytoplasma palmicola’ – related strain in plant species from the families Poaceae (Paspalum vaginatum, Pennisetum pedicellatum), Verbenaceae (Stachytarpheta indica), Plantaginaceae (Scoparia dulcis), Phyllanthaceae (Phyllanthus muellerianus) and Cyperaceae (Diplacrum capitatum) in Cote d'Ivoire and Kra et al. (2017) reported this same phytoplasma in cassava in Cote d'Ivoire, but these studies in alternate hosts have only been done using the nested PCR technique, which is notorious for generating contamination problems and false positives, and previous extensive studies in the USA and in Ghana found no evidence of these phytoplasmas associated with any alternate host species (Yankey et al., 2009).
It is important to note that the recently identified Bogia coconut syndrome from Papua New Guinea, unlike the other coconut phytoplasmas, does appear to have a confirmed alternate host, in that it appears to be the same phytoplasma as that associated with Banana wilt disease in Papua New Guinea (Davis et al., 2012) and the Solomon Islands (Davis et al., 2015). The vector for this phytoplasma is unproven.
3.1.3. Intraspecific diversity
Phytoplasmas cannot be grown under axenic conditions, and therefore, their classification is based on the sequence of their 16S rRNA genes. Palm lethal yellowing phytoplasmas belong to a number of 16Sr groups and subgroups (Harrison et al., 2014) as noted above. Phylogenetic analysis based on other phytoplasma genes, such as the leucyl tRNA synthetase gene (leuS) (Abeysinghe et al., 2016), have confirmed this separation into a number of different and distinct ‘Ca. Phytoplasma’ species. Currently, these different ‘Ca. Phytoplasma’ species are regionally distributed, with ‘Ca. Phytoplasma palmae’ restricted to the Americas and the Caribbean, ‘Ca. Phytoplasma palmicola’ restricted to West Africa and Mozambique, ‘Ca. Phytoplasma cocostanzaniae’ restricted to Kenya and Tanzania and the newly identified Bogia coconut syndrome restricted to Papua New Guinea and the Solomon Islands.
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest? Yes
Palm lethal yellowing phytoplasma detection can be achieved by polymerase chain reaction (PCR) or (loop‐mediated isothermal amplification (LAMP) from total plant DNA extracts. A range of different primers are available and have been published/validated, some of which are specific for particular ‘Ca. Phytoplasma’ species, and some of which will detect phytoplasmas more generally. The use of trunk borings is the preferred method for DNA extraction from palms (see Harrison et al., 2013 for a detailed protocol) and real‐time PCR (Hodgetts et al., 2009; Cordova et al., 2014) or real‐time LAMP (Dickinson, 2015) are the preferred methods for reliable detection.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
The pest is reported from a few countries in America plus Africa and is not known to occur in the EU (Figure 2).
Figure 2.
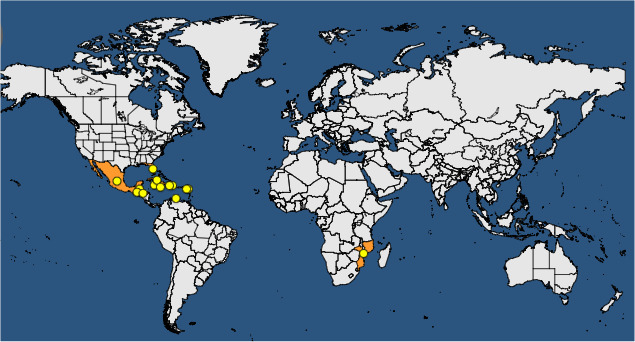
Global distribution of Palm lethal yellowing phytoplasmas (according to EPPO Global Database, accessed September 14 2017). Please note that this distribution map does not include all of the phytoplasmas listed as Palm lethal yellowing phytoplasmas in the EPPO datasheet that accompanies the distribution map on the EPPO Global Database at https://gd.eppo.int/taxon/PHYP56/documents and which are listed below in Table 2
Last updated: 2017‐9‐13
Table 2.
Global distribution Palm lethal yellowing phytoplasmas (according to EPPO Global Database, accessed September 14 2017) and completed using recent references in the scientific literature, including from the EPPO datasheet in the EPPO Global Database at https://gd.eppo.int/taxon/PHYP56/documents
| Continent | Country | Status ‐ EPPO GD | Other sources |
|---|---|---|---|
| Africa | Mozambique | Present, restricted distribution | |
| Africa | Nigeria | Present, EPPO datasheet, Osagie et al. (2015) | |
| Africa | Togo | Present, EPPO datasheet, Dollet et al. (1977) | |
| Africa | Cameroon | Present, EPPO datasheet, Dollet et al. (1977) | |
| Africa | Benin | Present, EPPO datasheet, Dollet et al. (1977) | |
| Africa | Ghana | Present, EPPO datasheet, Dabek et al. (1976) | |
| Africa | Cote d'Ivoire | Present, EPPO datasheet, Arocha‐Rosete et al. (2014) | |
| Africa | Kenya | Present, EPPO datasheet, Schuilling et al. (1992) | |
| Africa | Tanzania | Present, EPPO datasheet, Schuilling et al. (1992) | |
| America | Antigua and Barbuda | Present, no details | GenBank Accession Numbers are available |
| America | Bahamas | Absent, pest no longer present | |
| America | Belize | Present, restricted distribution | |
| America | Cayman Islands | Present, no details | |
| America | Cuba | Present, no details | Llauger et al. (2002) |
| America | Dominican Republic | Present, no details | Martinez et al. (2008) |
| America | Guatemala | Present, no details | |
| America | Guyana | Absent, invalid record | |
| America | Haiti | Present, widespread | |
| America | Honduras | Present, restricted distribution | Ashburner et al. (1996) |
| America | Jamaica | Present, no details | GenBank Accession Numbers are available |
| America | Mexico | Present, restricted distribution | Widespread distribution |
| America | Netherlands Antilles | Present, no details | |
| America | St Kitts‐Nevis | Present, restricted distribution | Myrie et al. (2006, 2012) |
| America | USA |
Present, restricted distribution Present, no details |
Harrison et al. (2014) |
| Oceania | Australia | Absent, confirmed by survey | |
| Oceania | Papua New Guinea and Solomon Islands | Present, restricted distribution Kelly et al. (2011) |
As described in Section 3.1.1, it has been confirmed that there are phytoplasmas from distinct taxonomic groups associated with this disease in different parts of the world (Harrison et al., 2014). The Panel, therefore, underlines that because the phytoplasma in Mozambique marked on the map is the same as that in the West African countries of Nigeria (Osagie et al., 2015), Togo, Benin, the Cameroon (Dollet et al., 1977), Ghana (Dabek et al., 1976) and Cote d'Ivoire (Arocha‐Rosete et al., 2014), these should be added to the map along with Kenya and Tanzania (Schuilling et al., 1992). The Panel also highlights that the recently identified Bogia coconut phytoplasma in Papua New Guinea and the Solomon Islands (Kelly et al., 2011) should be included (see Section 3.1.1 for rationale).
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? No
Palm lethal yellowing phytoplasmas are not known to occur in the EU and as a consequence, they do not meet one of the criteria to qualify as a Union RNQP.
3.3. Regulatory status
3.3.1. Legislation addressing Palm lethal yellowing phytoplasmas (Directive 2000/29/EC)
Palm lethal yellowing phytoplasmas are listed in Council Directive 2000/29/EC under the name Palm lethal yellowing phyoplasmas. Details are presented in Tables 3 and 4.
Table 3.
Palm lethal yellowing phytoplasmas in Council Directive 2000/29/EC
| Annex II, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned if they are present on certain plants or plant products | |
| Section I | Harmful organisms not known to occur in the community and relevant for the entire community | |
| (d) | Virus and virus‐like organisms | |
| Species | Subject of contamination | |
| 11 | Palm lethal yellowing mycoplasm | Plants of Palmae, intended for planting, other than seeds, originating in non‐European countries |
Table 4.
Regulated hosts and commodities that may involve Palm lethal yellowing phytoplasmas in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
| Description | Country of origin | |
| 17. | Plants of Phoenix spp. other than fruit and seeds | Algeria, Morocco |
| Annex IV, Part A | Special requirements which must be laid down by all member states for the introduction and movement of plants, plant products and other objects into and within all member states | |
| Section I | Plants, plant products and other objects originating outside the community | |
| Plants, plant products and other objects | Special requirements | |
| 37. | Plants of Palmae intended for planting other than seeds, originating in non‐European countries | Without prejudice to the prohibitions applicable to the plants listed in Annex III(A)(17), where appropriate, official statement that:
|
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the community, before being moved within the community — in the country of origin or the consignor country, if originating outside the community) before being permitted to enter the community | |
| Part A | Plants, plant products and other objects originating in the Community | |
| I. | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community and which must be accompanied by a plant passport | |
| 2.3.1 | Plants of Palmae, intended for planting, having a diameter of the stem at the base of over 5 cm and belonging to the following genera: Brahea Mart., Butia Becc., Chamaerops L., Jubaea Kunth, Livistona R. Br., Phoenix L., Sabal Adans., Syagrus Mart., Trachycarpus H. Wendl., Trithrinax Mart., Washingtonia Raf | |
| Part B | Plants, plant products and other objects originating in territories, other than those territories referred to in part a | |
| I. | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community | |
| 2. |
Parts of plants, other than fruits and seeds, of: — Castanea Mill., Dendranthema (DC.) Des Moul., Dianthus L., Gypsophila L., Pelargonium l'Herit. ex Ait, Phoenix spp., Populus L., Quercus L., Solidago L. and cut flowers of Orchidaceae, |
|
3.3.2. Legislation addressing plants and plant parts on which Palm lethal yellowing phytoplasmas are regulated (Directive 2000/29/EC)
Council Directive 2000/29/EC also regulates the introduction from third countries and the circulation within the EU of plants from susceptible palm trees. Such measures, detailed in Table 4, contribute to reduction of risks of introduction and spread of Palm lethal yellowing phytoplasmas. The introduction of palm plants is prohibited from certain origins.
3.3.3. Legislation addressing potential vectors of Palm lethal yellowing phytoplasmas (Directive 2000/29/EC)
Council Directive 2000/29/EC also regulates the introduction from third countries of H. crudus (previously named M. crudus). This insect is the only known vector of certain strains and only occurs in the Americas (Table 5).
Table 5.
Regulated vector of Palm lethal yellowing phytoplasmas in Annex II of Council Directive 2000/29/EC
|
Annex I, Part A Section I |
Harmful organisms whose introduction into, and spread within, all member states shall be banned Harmful organisms not known to occur in any part of the community and relevant for the entire community (a) Insects, mites and nematodes, at all stages of their development |
| Species | |
| (a) | Insects, mites and nematodes, at all stages of their development |
| 14. | Myndus crudus Van Duzee |
3.3.4. Other measures
No marketing directive is in force to regulate production and trade of palm trees.
Emergency measures are currently in place to prevent the introduction and spread in the EU of the harmful insect Rhynchophorus ferrugineus (Olivier) that causes damages on palm trees (Commission Decision 2007/365/EC4).
Commission Decision 2007/365/EC (amended by Commission decisions 2008/776/EC of 6 October 2008 and 2010/467/EU of 17 August 2010) sets rules to prevent the introduction and spread in the European Community of that insect. These emergency measures include among others specific requirements for the imports into the EU and for the internal movements within the EU of susceptible plants.
Among the specific requirements for the import into the EU, one states that ‘plants (…) (c) have, during a period of at least one year prior to export, been growing in a place of production (…) where the plants were placed in a site with complete physical protection against the introduction of the specified organism or an application of appropriate preventive treatments takes place’. Such a measure taken against the insect R. ferrugineus may nevertheless not prevent nursery palm trees to be visited by other insects of smaller size that may be vectors of Palm lethal yellowing phytoplasma. Considering the specific requirements for the movement of palm trees within the EU, one option is that palm trees have been growing in a nursery in a MS during a period of 2 years prior to the movement, during which plants are placed in a site with complete physical protection against the introduction of R. ferrugineus or an application of appropriate preventive treatments takes place. That option may limit the probability that nursery palm trees are visited by insects that are potential vectors of Palm lethal yellowing phytoplasmas. For imported palm trees, they should have been growing since their introduction into the Community in a place of production in a MS during a period of at least 1 year prior to the movement during which plants are placed in a site with complete physical protection against the introduction of R. ferrugineus. Such measures allow symptoms caused by Palm lethal yellowing phytoplasmas to develop and limit possibilities of visits of nursery palm trees by insects that could be vectors of the disease.
However, it should be noted that due to the presence of a symptomless phase of unknown length in the Palm lethal yellowing phytoplasma life cycle (see Section 3.1.2), it is unlikely these emergency measures will completely close all pathways for entry of Palm lethal yellowing phytoplasmas in the EU.
In addition, on May 2017, the Standing Committee on Plants, Animals, Food and Feed – section ‘Plant Health’ (PAFF Committee) exchanged views on the draft Commission Implementing Decision repealing Decision 2007/365/EC so that there exist some doubts about the long term status of these emergency measures.
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
All hosts of Palm lethal yellowing phytoplasmas belong to the Arecaceae family (owing to historical usage, the family is also referred to as Palmae as in Directive 2000/29/EC)5, a large family of approximately 181 genera and 2,600 perennial species of trees and shrubs (Christenhusz and Byng, 2016). There are unverified reports of the presence of DNA of the pathogen in some alternate hosts (Brown et al., 2008; Brown and McLaughlin, 2011; Arocha‐Rosete et al., 2016; Kra et al., 2017), but the Panel believes that these reports should be treated with caution. However, the recently identified Bogia coconut syndrome from Papua New Guinea, unlike the other coconut phytoplasmas, does appear to have a confirmed alternate host, in that it appears to be the same phytoplasma as that associated with Banana wilt disease in banana (Musa spp.) in Papua New Guinea (Davis et al., 2012) and the Solomon Islands (Davis et al., 2015).
C. nucifera (coconut palm) is the main host of Palm lethal yellowing phytoplasmas, with most commercial varieties cultivated being susceptible. A number of Arecaceae species including Allagoptera, Arenga, Arikuryroba, Borassus, Caryota, Chrysalidocarpus, Cocos, Corypha, Dictyosperma, Gaussia, Hyophorbe, Latania, Livistona, Mascarena, Nannorrhops, Phoenix, Pritchardia, Trachycarpus, Veitchia (Harrison et al., 1999), Carludovica palmata, Phoenix canariensis, Pritchardia pacifica, Sabal palmetto, W, robusta (Harrison et al., 2002, 2009, 2014), Roystonia regia and A. cromonia mexicana (Narvaez et al., 2016) are natural hosts for some of the strains of phytoplasmas, but the most susceptible palms are C. nucifera, Phoenix dactylifera and different Pritchardia species (McCoy et al., 1983). The oilpalm Elaeis guineensis is not known to be a host (McCoy et al., 1983).
Palm lethal yellowing phytoplasmas are regulated in all of their Arecaceae hosts (Palmae species, see Section 3.3.1).
3.4.2. Entry
Is the pest able to enter into the EU territory? (Yes or No) If yes, identify and list the pathways!
Yes – Palm lethal yellowing phytoplasmas could potentially enter the EU via live plants for planting imported for commercial use (date palm) or as ornamentals. The disease may also be introduced through the introduction of infected vector species but as yet only one species (M. crudus/H. crudus) has been confirmed as a vector and for many of the Palm lethal yellowing phytoplasmas the insect vectors are unknown.
Between 1995 and 2017, there were no records of interception of Palm lethal yellowing phytoplasmas in the Europhyt database.
The main pathway for entry identified by the Panel is the trade of palm plants for planting of susceptible Arecaceae species. Within the EU, many nurseries commercialise young palms for ornamental use and it is likely that those are either imported as small plants or grown in the EU from imported seeds. According to the ISEFOR database,6 between 2000 and 2011 among several Arecaceae species, coconut plants were imported from some regions where the disease occurs, particularly the Caribbean (156 plants; Cayman Islands).
3.4.3. Establishment
Is the pest able to become established in the EU territory?
Yes – The pest is able to become established in the EU territory as host plants are present. The only identified vector (M. crudus/H. crudus) is not known to occur within the EU, but it is unknown whether other arthropods present in the EU could be vectors.
3.4.3.1. EU distribution of main host plants
The only native palm species that grow on the European mainland are the European fan palm (Chamaerops humilis, with a distribution mainly in coastal areas of the western half of the Mediterranean basin) and the Cretan date palm (Phoenix theophrasti, endemic to Crete (Greece) and a few east Aegean islands) (Vamvoukakis, 1988). Many other palm species and mainly Chamaerops species, Canary palm (Phoenix canariensis) and date palm (Phoenix dactylifera) as well as W. robusta and Washingtonia filifera are widely used as ornamentals for landscaping in Southern Europe (Cohen, 2017). The only known palm commercial cultivation for non‐ornamental purposes in Europe is that of date palm (P. dactylifera) in Elche, Spain (38°17′N) (Ferry et al., 2002) an area in the extreme northern latitude for its distribution (Abdelouahhab and Arias‐Jimenez, 1999).
Several palm species are widely grown in the EU under protected cultivation conditions for ornamental purposes. Spain produces about 2 million palm trees annually with P. canariensis (1.2 million plants) being the predominant species, followed by other species such as P. dactylifera, Phoenix reclinata, W. filifera, W. robusta, C. humilis and Trachycarpus fortunei (Armengol et al., 2005). There is also a significant ornamental palm production in nurseries in the Marche region of Italy (Nardi et al., 2009). In addition, the species T. fortunei is an ornamental species that is sometime grown in the open up to more northern latitudes (e.g. southern Switzerland)
3.4.3.2. Climatic conditions affecting establishment
The pathogen is mainly associated with tropical and subtropical climates (Harrison et al., 2014). In the EU, hosts are widespread and favourable climatic conditions for the hosts are found particularly in Mediterranean countries.
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment?
YES ‐ Through currently unidentified insect vector species and vegetative propagation or tissue culture
While the only identified vector for the Palm lethal yellowing phytoplasmas, Myndus (Haplaxius) crudus, is currently reported to be absent from the EU and Africa (EPPO quarantine pest data sheet), the vectors for most strains of the Palm lethal yellowing phytoplasmas remain unidentified, and when the phytoplasmas have been introduced into new regions (e.g. the 16SrXII ‘Ca. Phytoplasma palmicola’ introduction into Mozambique from West Africa), indigenous vector species have been able to spread the disease. Therefore, currently, unidentified insect vectors may be present in the EU. In addition, some palm species are propagated by use of offshoots and tissue culture, and if the phytoplasma was present in the parent material, it could be spread by this route.
The main alternative possible route of spread would be in plants for planting, but this is considered to be unlikely since there is no evidence of mechanical transmission between plants. However, the possibility remains that seedlings may be distributed within the EU as plantlets whilst still in the symptomless phase after having become infected via insects.
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory? YES
Palm lethal yellowing phytoplasmas are considered to be a serious economic threat for coconut, causing their premature decline and death. Estimates of crop losses are hard to obtain (although it was reported to have killed 4.5 million palms in Jamaica between 1961 and 1983 and 8 million palms in Tanzania since the 1960s) and it is known that the disease has killed millions of palm plants in coconut growing regions of the Caribbean and Americas, in West and East Africa, and a newly discovered variant has recently been discovered causing devastation in Papua New Guinea.
A number of other species in the Arecaceae family are susceptible to the disease, including the date palm (Phoenix dactylifera) and the Canary Island date palm (Phoenix canariensis), although symptoms vary, and the pathogen does not appear to be as widespread or severe in these other palm species.
Palm lethal yellowing phytoplasmas are not present in the EU; therefore, no impact is observed. For some species grown in the EU, such as the date palm (P. dactylifera) and the Canary Island date palm (P. canariensis), susceptibility has been observed and some symptoms and damage could be expected should the pathogen be introduced. For other species grown in the EU, and in particular for the two species growing in the EU (European fan palm and Cretan date palm), no information on susceptibility is available so that impact, if any, remains highly uncertain.
None of the known hosts of Palm lethal yellowing phytoplasmas represent an important EU agricultural crop; however, a few of them are of high ornamental, landscape or cultural importance in the Mediterranean countries of the EU (MacLeod and Hussein, 2017). As stated in the Coconut Cadang‐Cadang Viroid pest categorisation (EFSA PLH Panel, 2017), a large number of those ornamental palms are produced in EU countries such as Spain and Italy (see Section 3.4.3.1) to be traded to the European markets; therefore, they can be of considerable economic importance. On the other hand, three major heritage palm groves exist in the Mediterranean European countries, in Elche in Spain, Bordighera in Italy and Crete in Greece. The major one is that of Elche (Spain) that is made up of about 180,000 adult date palms, in an area of almost 400 ha. The total date fruit production in Elche is estimated to be 5,000 tonnes per year, of which only about 100 tonnes are sold for human consumption (Ferry et al. 1997 – cited in Ferry et al., 2002). However, the grove of date palm in Elche (Valencia) trees known as ‘Palmeral of Elche’ was designated in 2000 as a World Heritage Site (http://whc.unesco.org/en/list/930). There are also a couple of additional historical groves in the same area of Spain, in Orihuela and Alicante, but they are not as large as the one in Elche (Suárez, 2010; Jacas et al. 2011). In Bordighera, in Italy, date palms have been cultivated since at least the 16th century for religious purposes, and even though their number has significantly dropped since the last century, they remain of high landscape significance. Other threatened native species may include the Cretan date palm (P. theophrasti) that is present only in Crete (Greece) and a few east Aegean islands (Vamvoukakis, 1988) and is a species with a near threatened status (2006 IUCN Red List of Threatened Species).
Overall, while several species grown in Europe and of commercial or cultural importance are known to be susceptible to Palm lethal yellowing phytoplasmas, information is lacking for other species, in particular the two European native palms. In addition, there are important uncertainties about how efficiently Palm lethal yellowing phytoplasmas would be able to spread in European palms. The potential impact of Palm lethal yellowing phytoplasmas if introduced in the EU is therefore very difficult to assess; however, the impact on the environment/landscape could be severe. Given that the spread potential is likely to be limited, the potential impact is estimated to be limited in extent, but this judgement is affected by large uncertainties.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? YES
Palm lethal yellowing phytoplasmas are listed in Council Directive 2000/29/EC and are regulated in all of their hosts7 (see Section 3.4.1). The present legislation imposes several conditions on imported plants for planting of Arecaceae (Palmae) species.
First, import of palm trees for planting is banned from certain countries.
For other countries, these may be imported on the condition that either Palm lethal yellowing phytoplasmas are not present in the area of origin or either that ‘no symptoms of Palm lethal yellowing phytoplasmas have been observed on the plants since the beginning of the last complete cycle of vegetation and that plants at the place of production which have shown symptoms giving rise to the suspicion of contamination by the organisms have been rogued out at that place’ (Council Directive 2000/29/EC, Annex IV, Part A, Section I, 37b).
In addition, the import into and the movement within the EU of palm tree species are subjected to specific requirements according to emergency measures set by Commission Decision 2007/365/EC (and its amendments) for the insect R. ferrugineus (see Section 3.4.1). Those measures have indirect effects for Palm lethal yellowing phytoplasma.
3.6.1. Biological or technical factors limiting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
Existence of a symptomless phase of unknown length in the field that may make inspection and laboratory tests for detection unsuccessful. The Palm lethal yellowing phytoplasmas may have an incubation period of several months in the field before symptoms develop and roguing is not effective in controlling the spread of the phytoplasma. Therefore, due to its reliance on the short‐term observation of symptoms, the current plant health legislation is not considered fully efficient.
Symptoms, especially the early ones, that resemble those caused by abiotic stress or other pests and therefor make inspection difficult.
The current legislation does not take into account seeds, and although there is currently no evidence that the phytoplasmas can be transmitted in seed (Oropeza et al., 2017), this possibility cannot be completely ruled out.
Imperfect knowledge on the natural means of spread with no known vectors for many strains.
No clear ecoclimatic limitations besides those applying to the host.
Unavailability of genetic resistance.
While there is uncertainty about alternate hosts for the phytoplasmas, it is possible that these exist. For example, the phytoplasma in Musa species in Papua New Guinea and the Solomon Islands may be the same as that causing the Bogia coconut syndrome
3.6.2. Biological or technical factors limiting the ability to prevent the presence of the pest on plants for planting
Palm lethal yellowing phytoplasmas do not occur in the EU, so it does not fulfil one of the criteria required to be a Union RNQP; hence, this section is to be deleted.
3.6.3. Control methods
In countries where the disease is present, the following control strategies have proven successful:
removal of infected palms with clear symptoms;
chemical or mechanical control of weeds;
vector control with systemic insecticides, where vector species are known.
3.7. Uncertainty
The Panel identified five mains sources of uncertainty in the present opinion:
For many of the Palm lethal yellowing phytoplasmas, the insect vector species have not been identified and it is not known if these exist in the EU and/or if other insects capable of transmitting the diseases exist in Europe;
Seed transmission is still unproven for phytoplasmas, but this remains a possibility, and a possible route of entry into the EU;
There is limited information on the origin and volume of the trade in palm seeds and plants for planting imported into the EU;
Lack of information on host status and susceptibility of many palm species grown in the EU and, in particular, on susceptibility of the two native species growing in the EU;
The newly identified Palm lethal yellowing phytoplasma named Bogia coconut syndrome, present in Papua New Guinea and the Solomon Islands, may be the same species that occurs in banana in these countries, which may open up an alternative route of entry for the pathogen into the EU;
These uncertainties primarily affect three aspects of the present pest categorisation; the potential alternative routes of entry of the Palm lethal yellowing phytoplasmas into the EU, the efficiency and extent to which the disease would be able to spread, and the impact it would have if introduced in the EU.
4. Conclusions
Palm lethal yellowing phytoplasmas meet the criteria assessed by EFSA for consideration as Union quarantine pest (Table 6).
Table 6.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the pest is well established; it can be identified with reliable and sensitive molecular diagnostic techniques. | The identity of the pest is well established; it can be identified with reliable and sensitive molecular diagnostic techniques. | The key uncertainty is precisely which phytoplasmas should be included in this pest categorisation (e.g. Bogia, wilt‐inducing phytoplasmas etc.) |
| Absence/presence of the pest in the EU territory (Section 3.2 ) | The pest is not known to occur in the EU territory. | The pest is not known to occur in the EU territory, therefore it does not qualify as a RNQP. | |
| Regulatory status (Section 3.3 ) | Palm lethal yellowing phytoplasmas are currently regulated on Palmae (Arecaceae) plants for planting by 2000/29/EC. | Palm lethal yellowing phytoplasmas are currently regulated on Palmae (Arecaceae) plants for planting by 2000/29/EC. | |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Palm lethal yellowing phytoplasmas could enter the EU, e.g. in palm trees for planting destined for commercial plantations or ornamental uses, and establish, in all areas where palm trees are already grown in the EU. It may spread either through trade of nursery plants or by insect vectors | Plants for planting and seeds of Arecaceae species represent the main entry pathways and potential vector insects present in the EU represent the main likelihood of spread. |
Uncertainties on the origin and volume of the trade in palm seeds and plants for planting imported in the EU Uncertainties about vector insects and the efficiency of spread under EU conditions Lack of information on host status of many palm species grown in the EU and, in particular on susceptibility of the two native species growing in the EU Uncertainty about seed transmission Uncertainty about alternate hosts |
| Potential for consequences in the EU territory (Section 3.5 ) | The potential impact of Palm lethal yellowing phytoplasmas if introduced in the EU is very difficult to assess. Given that the spread potential is, likely to be limited, the potential impact to agriculture is estimated to be minimal but the impact to environment/landscape could be significant. | The presence on plants for planting could influence subsequent yield and quality. |
Lack of knowledge on presence of vector species in the EU Difficulties to assess environment/ landscape impact |
| Available measures (Section 3.6 ) | Exclusion in the only method considered to be effective in controlling the spread of the pest. | There are no efficient methods for controlling Palm lethal yellowing phytoplasma spread after its introduction in an area. | |
| Conclusion on pest categorisation (Section 4 ) | Palm lethal yellowing phytoplasmas meet the criteria assessed by EFSA for consideration as Union quarantine pest. | Palm lethal yellowing phytoplasmas do not meet the presence on the territory criterion and therefore do not qualify as a Union RNQP. | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate |
The main knowledge gaps concern status of potential vector insects in the EU; (2) Seed transmission of the phytoplasmas; (3) the origin and volume of the trade in palm seeds and plants for planting imported in the EU; (4) host status and susceptibility of many palm species grown in the EU; (5) potential new assignments of phytoplasmas to this categorisation that might have associated alternate hosts. |
||
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- EU MS
European Union Member State
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- LAMP
loop‐mediated isothermal amplification
- LYD
Lethal yellowing‐type disease
- PCR
Polymerase chain reaction
- PLH
EFSA Panel on Plant Health
- RA
Risk assessment
- RNQP
Regulated non‐quarantine pest
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Dickinson M, Marzachi C, Hollo G and Caffier D, 2017. Scientific Opinion on pest categorisation of Palm lethal yellowing phytoplasmas. EFSA Journal 2017;15(10):5028, 27 pp. 10.2903/j.efsa.2017.5028
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00333
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Gregoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Adopted: 28 September 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
Commission Decision 2007/365/EC on emergency measures to prevent the introduction into and the spread within the Community of Rhynchophorus ferrugineus (Olivier).
Database developed within the FP7 Project ‘Increasing Sustainability of European Forests: Modelling for Security Against Invasive Pests and Pathogens under Climate Change’.
Chloris has been reported as a host but this assessment is associated with significant uncertainties.
References
- Abdelouahhab Z and Arias‐Jimenez EJ, 1999. Date palm cultivation (No. 156). Food and Agriculture Organization (FAO).
- Abeysinghe S, Abeysinghe PD, Kanatiwela‐de Silva C, Udagama P, Warawichanee K, Aljafar N, Kawicha P and Dickinson M, 2016. Refinement of the taxonomic structure of 16SrXI and 16SrXIV phytoplasmas of gramineous plants using Multilocus Sequence Typing. Plant Disease, 100, 2001–2010. [DOI] [PubMed] [Google Scholar]
- Armengol J, Moretti A, Perrone G, Vicent A, Bengoechea JA and García‐Jiménez J, 2005. Identification, incidence and characterization of Fusarium proliferatumon ornamental palms in Spain. European Journal of Plant Pathology, 112, 123–131. [Google Scholar]
- Arocha‐Rosete Y, Konan‐Konan JL, Diallo AH, Allou K and Scott JA, 2014. Identification and molecular characterization of the phytoplasma associated with a lethal yellowing‐type disease of coconut in Côte d'Ivoire. Canadian Journal of Plant Pathology, 36, 141–150. [Google Scholar]
- Arocha‐Rosete Y, Diallo HA, Konan‐Konan JL, Kouame AEP, Seka K, Kra KD, Toualy MN, Kwadjo KE, Daramcoum WAMP, Beugré NI, Ouattara BWM, Kouadjo Zaka CG, Allou K, Fursy‐Rodelec ND, Doudjo‐Ouattara ON, Yankey N, Dery S, Maharaj A, Saleh M, Summerbell R, Contaldo N, Paltrinieri S, Bertaccini A and Scott J, 2016. Detection and identification of the coconut lethal yellowing phytoplasma in weeds growing in coconut farms in Cote d'Ivoire. Canadian Journal of Botany, 38, 164–173. [Google Scholar]
- Ashburner G, Cardova I, Oropeza C, Illingworth R and Harrison N, 1996. First report of coconut lethal yellowing disease in Honduras. Plant Disease, 80, 960. [Google Scholar]
- Bonnot F, de Franqueville H and Lourenço E, 2010. Spatial and spatiotemporal pattern analysis of coconut lethal yellowing in Mozambique. Phytopathology, 100, 300–312. [DOI] [PubMed] [Google Scholar]
- Brown SE and McLaughlin WA, 2011. Identification of lethal yellowing group (16SrIV) of phytoplasmas in the weeds Stachytarpheta jamaicensis, Macroptiliumla thyroides and Cleomerutido sperma in Jamaica. Phytopathogenic Mollicutes, 1, 27–34. [Google Scholar]
- Brown SE, Been BO and McLaughlin WA, 2008. First report of the presence of the lethal yellowing group (16SrIV) of phytoplasmas in the weeds Emilia fosbergii and Synedrella nodiflora in Jamaica. Plant Pathology, 57, 770–770. [Google Scholar]
- Christenhusz MJM and Byng JW, 2016. The number of known plants species in the world and its annual increase”. Phytotaxa. Magnolia Press., 261, 201–217. [Google Scholar]
- Cohen Y, 2017. Morphology and Physiology of Palm trees as related to the Rhynchophorus ferruginrus and Paysandisia archon infestation and management. In: Soroker V, Colazza S. Handbook of Major Palm Pests: Biology and Management. Wiley & Sons Ltd, Chichester, UK. [Google Scholar]
- Cordova I, Oropeza C, Puch‐Hau C, Harrison N, Colli‐Rodriguez A, Narvaez M, Nic‐Matos G, Reyes C and Saenz L, 2014. A real‐time PCR assay for detection of coconut lethal yellowing phytolasmas of group 16SrIV subgroups A, D and E found in the Americas. Journal of Plant Pathology, 96, 343–352. [Google Scholar]
- Dabek AJ, Johnson CG and Harries HC, 1976. Mycoplasma‐like organisms associated with Kaincopé and Cape St. Paul Wilt Diseases of coconut palms in West Africa. PANS, 22, 354–358. [Google Scholar]
- Davis RI, Kokoa P, Jones LM, Mackie J, Constable FE, Rodoni B, Gunua TB and Rossel JB, 2012. A new wilt disease of banana plants associated with phytoplasmas in Papua New Guinea (PNG). Australasian Plant Disease Notes, 7, 91–97. [Google Scholar]
- Davis RI, Henderson J, Jones LM, McTaggart AR, O'Dwyer C, Fl Tsatsia, Fanai C and Rossel JB, 2015. First record of a wilt disease of banana plants associated with phytoplasmas in Solomon Islands. Australasian Plant Disease Notes, 10, 14. [Google Scholar]
- Dickinson M, 2015. Loop‐mediated isothermal amplification (LAMP) for detection of phytoplasmas in the field. In: Lacomme C (ed.). Plant Pathology: Techniques and Protocols, Vol. 1302, Humana Press, New York, NY, USA. pp. 99–112. [DOI] [PubMed] [Google Scholar]
- Dollet M, Giannotti J, Renard JL and Ghosh SK, 1977. Study of lethal yellowing of coconuts in Cameroon: Kribi disease. Observations of mycoplasma like organisms. Oleagineux, 32, 317–322. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2903/j.efsa.2010.1495. [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2017. Pest categorisation of Cadang‐Cadang viroid. EFSA Journal 2017;15(7):4928, 23 pp. 10.2903/j.efsa.2017.4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2017. EPPO Global Database. Available online: https://gd.eppo.int
- FAO (Food and Agriculture Organization of the United Nations), 2004.ISPM (International Standards for Phytosanitary Measures) 21—Pest Risk Analysis of Regulated Non‐Quarantine Pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk Analysis for Quarantine Pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf [Google Scholar]
- Ferry M, Gómez S, Jimenez E, Navarro J, Ruiperez E and Vilella J, 2002. The date palm grove of Elche, Spain: research for the sustainable preservation of a world heritage site. PALMS‐LAWRENCE‐, 46, 139–148. [Google Scholar]
- Gurr GM, Johnson AC, Ash GJ, Wilson BAL, Ero MM, Pilotti CA, Dewhurst CF and You MS, 2016. Coconut lethal yellowing diseases: A phytoplasma threat to palms of global economic and social significance. Frontiers in Plant Science, 7, 1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Cordova I, Richardson P and Dibonito R, 1999. Detection and diagnosis of lethal yellowing. In: Oropeza C, Verdeil JL, Ashburner GR, Cardeña R and Santamaría JM (eds). Current Advances in Coconut Biotechnology. Kluwer Academic Publishers, Dordrecht, The Netherlands. pp. 183–196. [Google Scholar]
- Harrison NA, Womack M and Carpio ML, 2002. Detection and characterization of a lethal yellowing (16SrIV) group phytoplasma in Canary Island date palms affected by lethal decline in Texas. Plant Disease, 86, 676–681. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Helmick EE and Elliott ML, 2008. Lethal yellowing‐type diseases of palms associated with phytoplasmas newly identified in Florida, USA. Annals of Applied Biology, 153, 85–94. [Google Scholar]
- Harrison NA, Helmick EE and Elliott ML, 2009. First report of a phytoplasma‐associated lethal decline of Sabal palmetto in Florida, USA. Plant Pathology, 58, 792. [Google Scholar]
- Harrison NA, Davis RE and Helmick EE, 2013. DNA extraction from arborescent monocots and how to deal with other challenging hosts. Phytoplasma: Methods and Protocols, pp. 147–158. [DOI] [PubMed] [Google Scholar]
- Harrison N, Davis RE, Oropeza C, Helmick E, Narvaez M, Eden‐Green S, Dollet M and Dickinson M, 2014. ‘Candidatus Phytoplasma palmicola’, a novel taxon associated with a lethal yellowing‐type disease (LYD) of coconut (Cocos nucifera L.) in Mozambique. International Journal of Systematic and Evolutionary Microbiology, 64, 1890–1899. [DOI] [PubMed] [Google Scholar]
- Hodgetts J, Boonham N, Mumford R and Dickinson M, 2009. A panel of real‐time PCR assays for improved universal and group specific detection of phytoplasmas, based on the 23S rRNA gene. Applied and Environmental Microbiology, 75, 2945–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenhout SA, Oshima K, Ammar ED, Kakizawa S, Kingdom HN and Namba S, 2008. Phytoplasmas: bacteria that manipulate plants and insects. Molecular Plant Pathology, 9, 403–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard FW, Norris R and Thomas D, 1983. Evidence of transmission of palm lethal yellowing agent by a planthopper, Myndus crudus (Homoptera, Cixiidae). Tropical Agriculture, 60, 168–171. [Google Scholar]
- IRPCM , 2004. ‘Candidatus Phytoplasma’, a taxon for the wall‐less, non‐helical prokaryotes that colonize plant phloem and insects. IRPCM Phytoplasma/Spiroplasma Working Team – Phytoplasma Taxonomy Group. International Journal of Systematic and Evolutionary Microbiology, 54, 1243–1255. [DOI] [PubMed] [Google Scholar]
- Jacas JA, Dembilio O and Llacer E, 2011. Research activities focused on management of red palm weevil at the UJI‐IVIA Associated Unit (Region of Valencia, Spain). EPPO Bulletin, 41, 122–127. [Google Scholar]
- Kelly PL, Reeder R, Kokoa P, Arocha Y, Nixon T and Fox A, 2011. First report of a phytoplasma identified in coconut palms (Cocos nucifera) with lethal yellowing‐like symptoms in Papua New Guinea. New Disease Reports, 23, 9. [Google Scholar]
- Kra KD, Toualy YMN, Kouamé AC, Diallo HA and Arocha‐Rosete Y, 2017. First report of a phytoplasma affecting cassava orchards in Cote d'Ivoire. New Disease Reports, 35, 21. [Google Scholar]
- Llauger R, Becker D, Cueto J, Peralta E, Gonzalez V, Rodriguez M and Rohde W, 2002. Detection and molecular characterization of phytoplasma associated with Lethal Yellowing disease of coconut palms in Cuba. Journal of Phytopathology, 150, 390–395. [Google Scholar]
- MacLeod A and Hussein M. 2017. Economic and social impacts of Rhynchophorus ferrugineus and Paysandisia archon on palms. Chapter 3 (p54‐68). In: Soroker V, Colazza S (eds.). Handbook of Major Palm Pests. Wiley‐Blackwell, Chichester, UK. xxvii +316 pp [Google Scholar]
- Martinez RT, Narvaez M, Fabre S, Harrison N, Oropeza C, Dollet M and Hitchez E, 2008. Coconut lethal yellowing on the southern coast of the Dominican Republic is associated with a new 16SrIV group phytoplasma. Plant Pathology, 57, 366. [Google Scholar]
- McCoy RE, Howard FW, Tsai JH, Donselman HM, Thomas DL, Basham HG, Atilano RA, Eskafi FM, Britt L and Collins ME, 1983. Lethal yellowing of palms. University of Florida Agricultural Experiment Stations Bulletin 834, Gainesville, USA.
- Myrie W, Paulraj L, Dollet M, Wray D, Been B and McLaughlin W, 2006. First report of lethal yellowing disease of coconut palms caused by phytoplasma on Nevis Island. Plant Disease, 90, 834. [DOI] [PubMed] [Google Scholar]
- Myrie W, Douglas CJ, Harrison NA, McLaughlin WA and James M, 2012. First report of lethal yellowing disease associated with subgroup 16SrIV, a phytoplasma on St.Kitts in the Lesser Antilles. New Disease Reports, 26, 25. [Google Scholar]
- Nardi S, Ricci E, Lozzi R, Marozzi F, Ladurner E, Chiabrando F, Isidoro N and Riolo P, 2009. Use of entomopathogenic nematodes for the control of Paysandisia archon Burmeister. IOBC/WPRS Bulletin, 45, 375–378. [Google Scholar]
- Narvaez M, Cordova‐Lara I, Reyes‐Martinez C, Puch‐Hau C, Mota‐Narvaez L, Colli A, Caamal G, Harrison N, Saenz L and Oropeza C, 2016. Occurrence of 16SrIV Subgroup A phytoplasmas in Roystonea regia and Acrocomia mexicana palms with Lethal Yellowing‐like syndromes in Yucatan, Mexico. Journal of Phytopathology, 164, 1111–1115. [Google Scholar]
- Nipah JO, Jones P and Dickinson MJ, 2007. Detection of lethal yellowing in embryos from coconut palms infected with Cape St Paul wilt disease in Ghana. Plant Pathology, 56, 777–784. [Google Scholar]
- Nutman FJ and Roberts FM, 1955. Lethal yellowing: the ‘unknown disease’ of coconut palms in Jamaica. Emp J Exp Agric, 23, 257–267. [Google Scholar]
- Ogle L and Harries H, 2005. Introducing the vector: how coconut lethal yellowing disease may have reached the Caribbean. Ethnobot. Res. Appl., 3, 139–142. [Google Scholar]
- Oropeza C, Cordova I, Chumba A, Narváez M, Sáenz L, Ashburner R and Harrison N, 2011. Phytoplasma distribution in coconut palms affected by lethal yellowing disease. Annals of Applied Biology, 159, 109–117. [Google Scholar]
- Oropeza C, Cordova I, Puch‐Hau C, Castillo R, Chan JL and Saenz L, 2017. Detection of lethal yellowing phytoplasma in coconut plantlets obtained through in vitro germination of zygotic embryos from the seeds of infected palms. Annals of Applied Biology, 171, 28–36. [Google Scholar]
- Osagie IJ, Ojomo EE and Pilet F, 2015. Occurrence of Awka wilt disease of coconut in Nigeria for one century. Phytopathogical Mollicutes, 5, S61. [Google Scholar]
- Plavsic‐Banjac B, Hunt P and Maramorosch K, 1972. Mycoplasma like bodies associated with lethal yellowing disease of coconut palms. Phytopathology, 62, 298–299. [Google Scholar]
- Schuilling M, Mpunami A and Kaiza D, 1992. Lethal disease of coconut palm in Tanzania. II. History, distribution and epidemiology. Oleagineux, 47, 516–522. [Google Scholar]
- Suárez JMC. 2010. Situation of R. ferrugineus in Spain. Red palm weevil Control Strategy for Europe: International Conference. Valencia, Spain 5‐6 May 2010.
- Vamvoukakis JA, 1988. Phoenix theophrasti on Crete. Principes, 32, 82–83. [Google Scholar]
- Vázquez‐Euán R, Harrison N, Narvaez M and Oropeza C, 2011. Occurrence of a 16SrIV group phytoplasma not previously associated with palm species in Yucatan, Mexico. Plant Disease, 95, 256–262. [DOI] [PubMed] [Google Scholar]
- Yankey EN, Pilet F, Quaicoe RN, Dery SK, Dollet M and Dzogbefia VP, 2009. Search for alternate hosts of the coconut Cape Saint Paul Wilt Disease pathogen. Oléagineux, 16, 123–126. [Google Scholar]


