Abstract
The EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids was requested to evaluate 53 flavouring substances attributed to the Flavouring Group Evaluation 07, including four new substances but‐3‐en‐2‐ol, non‐1‐en‐e‐ol, hex‐1‐en‐3‐one and 1‐nonene‐3‐one [FL‐nos: 02.131, 02.187, 07.161 and 07.210] in this Revision 5, using the Procedure in Commission Regulation (EC) No 1565/2000. None of the 53 substances was considered to have genotoxic potential. The substances were evaluated through a stepwise approach that integrates information on the structure–activity relationships, intake from current uses, toxicological threshold of concern (TTC), and available data on metabolism and toxicity. The Panel concluded that all 53 substances do not give rise to safety concerns at their levels of dietary intake, estimated on the basis of the ‘Maximised Survey‐derived Daily Intake’ (MSDI) approach. Besides the safety assessment of the flavouring substances, the specifications for the materials of commerce have also been considered and found adequate. For 50 substances, further information is required based on comparison of the ‘modified Theoretical Added Maximum Daily Intakes’ (mTAMDIs) with the TTCs. This would include more reliable intake data and then, if required, additional toxicological data.
Keywords: flavouring substances, aliphatic secondary alcohols, ketones, esters, FGE.07, FGE.63
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The use of flavourings is regulated under Regulation (EC) No 1334/2008 of the European Parliament and Council of 16 December 20081 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union list of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/20122. The list contains flavouring substances for which the scientific evaluation should be completed in accordance with Commission Regulation (EC) No 1565/20003.
On 27 September 2012, the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids adopted an opinion on Flavouring Group Evaluation 205 (FGE.205): consideration of genotoxic potential on α,β‐unsaturated aliphatic ketones with terminal double bonds and precursors from chemical subgroup 1.2.2 of FGE.19.
The Panel concluded that for the two representative substances, oct‐1‐en‐3‐one [FL‐no: 07.081] and pent‐1‐en‐3‐one [FL‐no: 07.102], the positive effects in the bacterial mutagenicity assays cannot be overruled by one negative and one equivocal gene mutation test in mammalian cells. Accordingly, an in vivo Comet assay on the first site of contact (e.g. the stomach or duodenum) and on the liver is requested for the most potent substance, pent‐1‐en‐3‐one. As an alternative, a transgenic animal assay would also be acceptable.
On 10 March 2015, the applicant submitted additional studies on the representative substances [FL‐no: 07.102] and [FL‐no: 07.081]. These studies are intended to cover the substances in this group, namely: FL‐nos: 02.023, 02.099, 02.104, 02.131, 02.136, 02.155, 02.187, 07.161, 07.210, 09.281 and 09.282.
1.1.2. Terms of Reference
The European Commission requests the European Food Safety Authority (EFSA) to evaluate the new information and, depending on the outcome, proceed to the full evaluation on the flavouring substances in accordance with Commission Regulation (EC) No 1565/20003.
1.1.3. Interpretation of the terms of reference
In FGE.205 Revision 1, EFSA evaluated the additional data on genotoxicity submitted for the substances, oct‐1‐en‐3‐one [FL‐no: 07.081] and pent‐1‐en‐3‐one [FL‐no: 07.102], by the flavour industry. For these substances, being the representative of four of the flavouring substances in FGE.07, the Panel concluded that the concern for a genotoxic potential could be ruled out. As a follow up to this conclusion, the substances, but‐3‐en‐2‐ol [FL‐no: 02.131], non‐1‐en‐e‐ol [FL‐no: 02.187], hex‐1‐en‐3‐one [FL‐no: 07.161] and 1‐nonene‐3‐one [FL‐no: 07.210], were evaluated by the Panel in accordance with the Procedure described in Commission Regulation (EC) No 1565/20003, in line with the background and terms of references as provided by the European Commission.
1.1.4. History of the evaluation
The first version of the Flavouring Group Evaluation 07, FGE.07, dealt with 35 saturated and unsaturated aliphatic secondary alcohols, ketones and esters with secondary alcohol moiety.
The first revision of FGE.07, FGE.07Rev1, included the assessment of six additional flavouring substances [FL‐nos: 02.190, 07.162, 07.201, 07.236, 07.676 and 09.926]. No new data on toxicity were provided. For two of the new substances, [FL‐nos: 07.162 and 07.201], data on metabolism were provided. Additional information on 20 flavouring substances [FL‐nos: 02.124, 02.142, 02.148, 02.177, 02.182, 02.183, 07.156, 07.157, 07.182, 07.185, 07.205, 09.304, 09.323, 09.325, 09.328, 09.332, 09.386, 09.388, 09.391 and 09.880] was made available since the FGE.07 was published.
The second revision of FGE.07, FGE.07Rev2, included the assessment of two additional flavouring substances [FL‐nos: 02.255 and 07.239]. No new data on toxicity and metabolism were provided.
The third revision of FGE.07, FGE.07Rev3, included the assessment of one additional candidate substance [FL‐nos: 07.262]. Toxicity data (acute toxicity, 28‐days study and an Ames test) were submitted. No metabolism data were provided for this substance. A search in open literature did not provide any further data on toxicity or metabolism for this substance. Furthermore additional information on the specifications for eight candidate substances requested in FGE.07Rev2 had been submitted by industry and included in this FGE.
The fourth revision of FGE.07, FGE.07Rev4, included the assessment of five additional candidate substances [FL‐nos: 02.145, 02.194, 02.211, 07.198 and 07.204]. These substances had been considered with respect to genotoxicity in FGE.206 (EFSA CEF Panel, 2011) and the Panel concluded that the data available ruled out the concern for genotoxicity and accordingly the substances could be evaluated through the Procedure.
| FGE | Adopted | Link | Substances |
|---|---|---|---|
| FGE.07 | 9.12.2004 | http://www.efsa.europa.eu/en/scdocs/scdoc/164.htm | 35 |
| FGE.07Rev1 | 26.9.2007 | http://www.efsa.europa.eu/en/scdocs/scdoc/722.htm | 41 |
| FGE.07Rev2 | 26.3.2009 | http://www.efsa.europa.eu/en/scdocs/scdoc/1020.htm | 43 |
| FGE.07Rev3 | 30.9.2010 | http://www.efsa.europa.eu/en/efsajournal/pub/1845.htm | 44 |
| FGE.07Rev4 | 27.9.2012 | http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2012.2899/full | 49 |
| FGE.07Rev5 | 1.2.2017 | http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4725/full | 53 |
The present revision of FGE.07, FGE.07Rev5 includes the assessment of four additional candidate substances [FL‐nos: 02.131, 02.187, 07.161 and 07.210]. These substances had been considered with respect to genotoxicity in FGE.205Rev1 (EFSA CEF Panel, 2016). Based on new genotoxicity data submitted by the flavour industry, the Panel concluded that the concern for genotoxicity could be ruled out, and therefore, the four substances could be evaluated through the Procedure in FGE.07Rev5.
A search in open literature for these four new substances conducted for metabolism and toxicity did not reveal any pertinent new information.
2. Assessment
2.1. Presentation of the substances in FGE.07Rev5
2.1.1. Identity of the substances
The present Flavouring Group Evaluation 7, Revision 5 (FGE.07Rev5), using the Procedure as referred to in the Commission Regulation (EC) 1565/2000 (the Procedure – shown in schematic form in Appendix A), deals with 53 saturated and unsaturated aliphatic acyclic secondary alcohols, ketones and esters with a secondary alcohol moiety. These 53 flavouring substances belong to the chemical group 5 of Annex I of Commission Regulation (EC) No 1565/20003.
The 53 flavouring substances (candidate substances) are closely related to 67 flavouring substances (supporting substances) evaluated at the 51st, 59th and 69th meetings of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA) in the group ‘Saturated Aliphatic Acyclic Secondary Alcohols, Ketones, and Related Saturated and Unsaturated Esters’ (JECFA, 2000a, 2002a, 2003, 2009b).
The 53 candidate substances under consideration in the present evaluation are listed in Table 1, as well as their chemical Register names, FLAVIS‐ (FL‐), Chemical Abstract Service‐ (CAS‐), Council of Europe‐ (CoE‐) and Flavor and Extract Manufacturers Association‐ (FEMA‐) numbers, and structures.
Table 1.
Specification summary of the substances in the flavouring group evaluation 7, Revision 5
| FL‐no | EU register name | Structural formula | FEMA no CoE no CAS no | Phys. form Mol. formula Mol. weight | Solubilitya Solubility in ethanolb | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refrac. Indexd Spec. gravitye | Specification comments |
|---|---|---|---|---|---|---|---|---|
| 02.077 | Pentan‐3‐ol |

|
2349 584‐02‐1 |
Liquid C5H12O 88.15 |
Slightly soluble Freely soluble |
115 MS 98% |
1.407–1.413 0.815–0.822 |
|
| 02.124 | 6‐Methylhept‐5‐en‐2‐ol |

|
10264 1569‐60‐4 |
Liquid C8H16O 128.21 |
Slightly soluble Freely soluble |
77 (20 hPa) MS 95% |
1.447–1.453 0.848–0.854 |
Racemate (EFFA, 2002a) |
| 02.131 | But‐3‐en‐2‐ol |

|
598‐32‐3 |
Liquid C4H8O 72.11 |
Slightly soluble Freely soluble |
90 MS 95% |
1.409–1.415 0.831–0.837 |
Racemate (EFFA, 2016) |
| 02.142 | 3,3‐Dimethylbutan‐2‐ol |
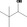
|
464‐07‐3 |
Liquid C6H14O 102.18 |
Slightly soluble Freely soluble |
120 MS 95% |
1.410–1.416 0.814–0.820 |
Racemate (EFFA, 2002a) |
| 02.145 | 2,6‐Dimethylocta‐1,5,7‐trien‐3‐ol |
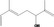
|
29414‐56‐0 |
Liquid C10H16O 152.24 |
Slightly soluble Freely soluble |
240 MS 95% |
1.484–1.490 0.895–0.901 |
Racemate Mixture of E/Z stereoisomers: 50–80% (E) (EFFA, 2012) |
| 02.148 | Dodecan‐2‐ol |

|
11760 10203‐28‐8 |
Liquid C12H26O 186.34 |
Insoluble Freely soluble |
129 (15 hPa) 19 MS 95% |
1.438–1.444 0.829–0.835 |
Racemate (EFFA, 2002a) |
| 02.177 | 2‐Methylhexan‐3‐ol |
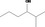
|
10266 617‐29‐8 |
Liquid C7H16O 116.20 |
Slightly soluble Freely soluble |
144 MS 95% |
1.418–1.424 0.820–0.826 |
Racemate (EFFA, 2002a) |
| 02.182 | 3‐Methylpentan‐2‐ol |
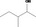
|
10276 565‐60‐6 |
Liquid C6H14O 102.18 |
Insoluble Freely soluble |
134 MS 95% |
1.415–1.421 0.827–0.833 |
Racemate (EFFA, 2010) |
| 02.183 | 4‐Methylpentan‐2‐ol |
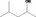
|
10279 108‐11‐2 |
Liquid C6H14O 102.18 |
Slightly soluble Freely soluble |
132 MS 99% |
1.407–1.414 0.802–0.808 |
Racemate (EFFA, 2002a) |
| 02.187 | Non‐1‐en‐3‐ol |

|
10291 21964‐44‐3 |
Liquid C9H18O 142.24 |
Practically insoluble or insoluble Freely soluble |
195 MS 98% |
1.438–1.444 0.835–0.845 |
Racemate (EFFA, 2016) |
| 02.190 | Nonan‐3‐ol |

|
10290 624‐51‐1 |
Liquid C9H20O 144.26 |
Practically insoluble or insoluble Freely soluble |
195 MS 95% |
1.425–1.431 0.818–0.824 |
Racemate (EFFA, 2010) |
| 02.194 | Octa‐1,5‐dien‐3‐ol |

|
83861‐74‐9 |
Liquid C8H14O 126.20 |
Practically insoluble or insoluble Freely soluble |
187 MS 95% |
1.441–1.447 0.832–0.838 |
Racemate (EFFA, 2017) Mixture of E/Z stereoisomers: 60–90% (E) (EFFA, 2012) |
| 02.211 | Undeca‐1,5‐dien‐3‐ol |

|
56722‐23‐7 |
Liquid C11H20O 168.28 |
Practically insoluble or insoluble Freely soluble |
244 NMR 95% |
1.456–1.462 0.872–0.878 |
Racemate (EFFA, 2017) Mixture of E/Z stereoisomers: 60–90% (E) (EFFA, 2012) CASrn refers to the (Z)‐isomer only. CASrn in the Union List to be changed to 319497‐21‐7 |
| 02.255 | (Z)‐4‐Hepten‐2‐ol |

|
66642‐85‐1 |
Liquid C7H14O 114.19 |
Insoluble Freely soluble |
154 MS 92% |
1.433–1.453 0.832–0.852 |
Racemate (EFFA, 2017) Mixture of E/Z stereoisomers: (Z)‐isomer (approx. 92%), (E)‐isomer (approx. 4%). Minor constituents 2‐heptanol (< 1), trans‐3‐hepten‐2‐ol (< 1%), cis‐3‐hepten‐2‐ol (< 1%) (EFFA, 2010) CAS nr does not specify the geometrical isomer Name in the Union List to be changed to 4‐Hepten‐2‐ol |
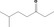
| 07.072 | 6‐Methylheptan‐3‐one |
|
2143 624‐42‐0 |
Liquid C8H16O 128.21 |
Insoluble Freely soluble |
162 MS 95% |
1.412–1.418 0.813–0.819 |
|
| 07.084 | Pentan‐3‐one |

|
2350 96‐22‐0 |
Liquid C5H10O 86.13 |
Partly soluble Freely soluble |
102 MS 99% |
1.389–1.395 0.812–0.818 |
|
|
07.150 2074 |
Decan‐2‐one |

|
4271 11055 693‐54‐9 |
Liquid C10H20O 156.27 |
Insoluble Freely soluble |
210 MS 98% |
1.423–1.429 0.821–0.827 |
|
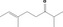
| 07.156 | 2,6‐Dimethyloct‐6‐en‐3‐one (mixture of E and Z) |
|
90975‐15‐8 |
Liquid C10H18O 154.25 |
Insoluble Freely soluble |
80 (13 hPa) NMR 95% |
1.442–1.448 0.823–0.829 |
Mixture of E/Z isomers: 50–80% (E) (EFFA, 2017) |
| 07.157 | 6,10‐Dimethylundecan‐2‐one |

|
11068 1604‐34‐8 |
Liquid C13H26O 198.35 |
Insoluble Freely soluble |
121 (16 hPa) MS 95% |
1.433–1.439 0.828–0.834 |
Racemate (EFFA, 2002a) |
| 07.158 | Dodecan‐2‐one |

|
11069 6175‐49‐1 |
Liquid C12H24O 184.32 |
Insoluble Freely soluble |
119 (13 hPa) 20 MS 99% |
1.431–1.437 0.825–0.835 |
|
| 07.160 | Heptadecan‐2‐one |

|
11089 2922‐51‐2 |
Solid C17H34O 254.46 |
Insoluble Freely soluble |
144 (1 hPa) 48 MS 95% |
n.a. n.a. |
|
| 07.161 | Hex‐1‐en‐3‐one |

|
1629‐60‐3 |
Liquid C6H10O 98.14 |
Practically insoluble or insoluble Freely soluble |
128 MS 95% |
1.420–1.426 0.849–0.855 |
|
| 07.162 | Hex‐5‐en‐2‐one |

|
109‐49‐9 |
Liquid C6H10O 98.14 |
Slightly soluble Freely soluble |
128 MS 95% |
1.418–1.424 0.839–0.845 |
|
| 07.178 | 3‐Methylbutan‐2‐one |

|
11131 563‐80‐4 |
Liquid C5H10O 86.13 |
Slightly soluble Freely soluble |
94 MS 95% |
1.387–1.393 0.801–0.807 |
|
| 07.181 | 6‐Methylheptan‐2‐one |

|
11146 928‐68‐7 |
Liquid C8H16O 128.21 |
Insoluble Freely soluble |
167 MS 95% |
1.412–1.418 0.813–0.819 |
|
| 07.182 | 5‐Methylheptan‐3‐one |

|
541‐85‐5 |
Liquid C8H16O 128.21 |
Insoluble Freely soluble |
158 MS 95% |
1.418–1.424 0.816–0.824 |
Racemate (EFFA, 2002a) |
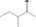
| 07.185 | 3‐Methylpentan‐2‐one |
|
11157 565‐61‐7 |
Liquid C6H12O 100.16 |
Insoluble Freely soluble |
117 MS 95% |
1.398–1.404 0.810–0.816 |
Racemate (EFFA, 2002a) |
| 07.189 | Nonan‐4‐one |
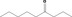
|
11161 4485‐09‐0 |
Liquid C9H18O 142.24 |
Insoluble Freely soluble |
188 MS 95% |
1.416–1.422 0.821–0.827 |
|
| 07.198 | Pseudo‐ionone |

|
4299 11191 141‐10‐6 |
Liquid C13H20O 192.30 |
Insoluble Freely soluble |
144 (16 hPa) MS 95% |
1.529–1.535 0.894–0.903 |
Mixture of E/Z stereoisomers: > 50% EE (EFFA, 2012) |
| 07.199 | Tetradecan‐2‐one |

|
11192 2345‐27‐9 |
Solid C14H28O 212.37 |
Insoluble Freely soluble |
146 (16 hPa) 33 MS 95% |
n.a. n.a. |
|
| 07.201 | Tridec‐12‐en‐2‐one |

|
60437‐21‐0 |
Liquid C13H24O 196.33 |
Insoluble Freely soluble |
129 (13 hPa) NMR 95% |
1.441–1.447 0.815–0.821 |
|
| 07.204 | 3,3,6‐Trimethylhepta‐1,5‐dien‐4‐one |
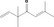
|
546‐49‐6 |
Liquid C10H16O 152.24 |
Practically insoluble or insoluble Freely soluble |
181 MS 95% |
1.462–1.468 0.867–0.873 |
|
| 07.205 | 6,10,14‐Trimethylpentadecan‐2‐one |

|
11205 502‐69‐2 |
Liquid C18H36O 268.48 |
Insoluble Freely soluble |
174 (13 hPa) MS 95% |
1.445–1.451 0.834–0.840 |
Racemate (EFFA, 2002a) |
| 07.210 | 1‐Nonene‐3‐one |

|
24415‐26‐7 |
Liquid C9H16O 140.22 |
Insoluble Freely soluble |
80 (16 hPa) MS 95% |
1.436–1.442 0.826–0.830 |
|
| 07.236 | (Z)‐5‐Octen‐2‐one |

|
11171 22610‐86‐2 |
Liquid C8H14O 126.20 |
Practically insoluble or insoluble Freely soluble |
115 NMR 95% |
1.431–1.437 0.842–0.848 |
|
|
07.239 1840 |
[R‐e]‐5‐Isopropyl‐8‐methylnona‐6,8‐dien‐2‐one |
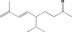
|
4331 2278‐53‐7 |
Liquid C13H22O 194.31 |
Practically insoluble or insoluble Freely soluble |
238 MS 95% |
1.471–1.477 0.846–0.852 |
|
| 07.262 | 9‐Decen‐2‐one |
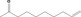
|
4706 35194‐30‐0 |
Liquid C10H18O 154 |
Slightly soluble Soluble |
206.3 IR NMR MS 99% |
1.426–1.446 0.834–0.854 |
|
| 09.304 | sec‐Heptyl isovalerate |
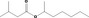
|
10806 238757‐71‐6 |
Liquid C12H24O2 200.32 |
Insoluble Freely soluble |
235 NMR 95% |
1.423–1.429 0.867–0.873 |
Racemate (EFFA, 2002a) |
| 09.323 | sec‐Butyl acetate |
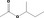
|
10527 105‐46‐4 |
Liquid C6H12O2 116.16 |
Slightly soluble Freely soluble |
111 MS 95% |
1.385–1.391 0.867–0.873 |
Racemate (EFFA, 2002a) |
| 09.325 | sec‐Butyl butyrate |

|
10528 819‐97‐6 |
Liquid C8H16O2 144.21 |
Slightly soluble Freely soluble |
152 MS 95% |
1.399–1.405 0.858–0.864 |
Racemate (EFFA, 2002a) |
| 09.328 | sec‐Butyl formate |
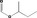
|
10532 589‐40‐2 |
Liquid C5H10O2 102.13 |
Slightly soluble Freely soluble |
94 MS 95% |
1.386–1.392 0.877–0.883 |
Racemate (EFFA, 2002a) |
| 09.332 | sec‐Butyl hexanoate |

|
10533 820‐00‐8 |
Liquid C10H20O2 172.27 |
Insoluble Freely soluble |
82 (21 hPa) NMR 95% |
1.408–1.414 0.861–0.867 |
Racemate (EFFA, 2002a) |
| 09.386 | sec‐Hept‐4(cis)‐enyl acetate |

|
94088‐33‐2 |
Liquid C9H16O2 156.22 |
Insoluble Freely soluble |
185 MS 95% |
1.412–1.418 0.854–0.860 |
Racemate (EFFA, 2002a) |
| 09.388 | sec‐Heptyl acetate |

|
10802 5921‐82‐4 |
Liquid C9H18O2 158.24 |
Insoluble Freely soluble |
172 MS 95% |
1.406–1.412 0.862–0.868 |
Racemate (EFFA, 2002a) |
| 09.391 | sec‐Heptyl hexanoate |

|
10805 6624‐58‐4 |
Liquid C13H26O2 214.35 |
Insoluble Freely soluble |
126 (20 hPa) MS 95% |
1.421–1.427 0.851–0.857 |
Racemate (EFFA, 2002a) |
| 09.604 | Isopropyl decanoate |

|
10730 2311‐59‐3 |
Liquid C13H26O2 214.35 |
Insoluble Freely soluble |
88 (3 hPa) MS 95% |
1.421–1.427 0.851–0.857 |
|
| 09.605 | Isopropyl dodecanoate |

|
10233‐13‐3 |
Liquid C15H30O2 242.40 |
Insoluble Freely soluble |
105 (1 hPa) MS 95% |
1.427–1.433 0.851–0.857 |
|
| 09.606 | Isopropyl hexadecanoate |

|
10732 142‐91‐6 |
Liquid C19H38O2 298.51 |
Insoluble Freely soluble |
342 13 MS 95% |
1.433–1.439 0.852–0.858 |
|
| 09.608 | Isopropyl octanoate |

|
10731 5458‐59‐3 |
Liquid C11H22O2 186.29 |
Insoluble Freely soluble |
124 (53 hPa) MS 95% |
1.414–1.420 0.853–0.859 |
|
| 09.609 | Isopropyl valerate |

|
18362‐97‐5 |
Liquid C8H16O2 144.21 |
Insoluble Freely soluble |
165 MS 95% |
1.398–1.404 0.855–0.861 |
|
| 09.676 | sec‐Octyl acetate |

|
10799 2051‐50‐5 |
Liquid C10H20O2 172.27 |
Practically insoluble or insoluble Freely soluble |
193 MS 95% |
1.409–1.415 0.857–0.863 |
Racemate (EFFA, 2010) |
| 09.880 | (Z)‐Hept‐4‐enyl‐2 butyrate |
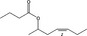
|
94088‐12‐7 |
Liquid C11H20O2 184.28 |
Practically insoluble or insoluble Freely soluble |
224 MS 95% |
1.414–1.420 0.852–0.858 |
Racemate (EFFA, 2010) |
|
09.926 2070 |
Octan‐3‐yl formate |

|
4009 84434‐65‐1 |
Liquid C9H18O2 158.24 |
Practically insoluble or insoluble Freely soluble |
71 (9 hPa) IR NMR MS 98% |
1.413–1.417 0.865–0.875 |
Racemate (EFFA, 2010) |
FL‐no: FLAVIS number; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; ID: identity; MS: mass spectrometry; NMR: nuclear magnetic resonance; IR: infrared spectroscopy.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1 atm (1,013.25 hPa), if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
Seven flavouring substances are saturated aliphatic acyclic secondary alcohols [FL‐nos: 02.077, 02.142, 02.148, 02.177, 02.182, 02.183 and 02.190]; seven are unsaturated aliphatic secondary alcohols [FL‐nos: 02.124, 02.131, 02.145, 02.187, 02.194, 02.211 and 02.255] of which five contain a terminal double bond [FL‐nos: 02.131, 02.145, 02.187, 02.194 and 02.211]; 13 are saturated aliphatic ketones [FL‐nos: 07.072, 07.084, 07.150, 07.157, 07.158, 07.160, 07.178, 07.181, 07.182, 07.185, 07.189, 07.199 and 07.205]; 10 are unsaturated aliphatic ketones [FL‐nos: 07.156, 07.161, 07.162, 07.198, 07.201, 07.204, 07.210, 07.236, 07.239 and 07.262] of which seven contain a terminal double bond [FL‐nos: 07.161, 07.162, 07.201, 07.204, 07.210, 07.239 and 07.262] and 16 are esters of aliphatic acyclic secondary alcohols and linear or branched‐chain aliphatic carboxylic acids [FL‐nos: 09.304, 09.323, 09.325, 09.328, 09.332, 09.386, 09.388, 09.391, 09.604, 09.605, 09.606, 09.608, 09.609, 09.676, 09.880 and 09.926].
The hydrolysis products of the candidate esters are listed in Appendix B, Table B.2.
Table B.2.
Evaluation status of hydrolysis products of candidate esters
| FL‐no | EU register name JECFA no | Structural formula | SCF statusa JECFA statusb CoE statusc EFSA status | Structural classd Procedure path (JECFA)e | Comments |
|---|---|---|---|---|---|
| 4‐Hepten‐2‐ol |
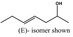
|
Not evaluated as flavouring substance | Not in EU‐Register | ||
| Hexadecanoic acid |

|
Not evaluated as flavouring substance | Not in EU‐Register | ||
| 02.022 |
Octan‐2‐ol 289 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 02.045 |
Heptan‐2‐ol 284 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 02.079 |
Isopropanol 277 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
Class I A3: Intake above threshold, A4: Endogenous |
|
| 02.098 |
Octan‐3‐ol 291 |

|
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) |
Class I A3: Intake below threshold |
|
| 02.121 | Butan‐2‐ol |

|
Category 1 (SCF, 1995) |
Class I No evaluation |
|
| 08.001 |
Formic acid 79 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999b) Deleted (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 08.002 |
Acetic acid 81 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999b) Category A (CoE, 1992) |
Class I A3: Intake above threshold, A4: Endogenous |
|
| 08.005 |
Butyric acid 87 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999b) Category A (CoE, 1992) |
Class I A3: Intake above threshold, A4: Endogenous |
|
| 08.007 |
Valeric acid 90 |
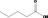
|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999b) Category A (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 08.008 |
3‐Methylbutyric acid 259 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999b) Category A (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 08.009 |
Hexanoic acid 93 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999b) Category A (CoE, 1992) |
Class I A3: Intake above threshold, A4: Endogenous |
|
| 08.010 |
Octanoic acid 99 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999b) Category A (CoE, 1992) |
Class I A3: Intake above threshold, A4: Endogenous |
|
| 08.011 |
Decanoic acid 105 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999b) Category A (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 08.012 |
Dodecanoic acid 111 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999b) Category A (CoE, 1992) |
Class I A3: Intake below threshold |
SCF: Scientific Committee on Food; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; CoE: Council of Europe.
Category 1: Considered safe in use, Category 2: Temporarily considered safe in use, Category 3: Insufficient data to provide assurance of safety in use, Category 4: Not acceptable due to evidence of toxicity.
No safety concern at estimated levels of intake.
Category A: Flavouring substance, which may be used in foodstuffs, Category B: Flavouring substance which can be used provisionally in foodstuffs.
Threshold of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
The names and structures of the 67 supporting substances are listed in Appendix B, Table B.3, together with their evaluation status (CoE, 1992; SCF, 1995; JECFA, 1999a, 2002a, 2003, 2009b).
Table B.3.
Supporting substances summary
| FL‐no | EU register name | Structural formula | FEMA no CoE no CAS no | JECFA no Specification available | MSDI (EU)a (μg/capita per day) | SCF statusb JECFA statusc CoE statusd | Comments |
|---|---|---|---|---|---|---|---|
| 02.022 | Octan‐2‐ol |

|
2801 71 123‐96‐6 |
289 JECFA specification (JECFA, 1998) |
11 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
JECFA evaluated 2‐octanol (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 02.023 | Oct‐1‐en‐3‐ol |

|
2805 72 3391‐86‐4 |
1152 JECFA specification (JECFA, 2002b) |
240 |
No safety concern (JECFA, 2002a) Category A (CoE, 1992) |
|
| 02.044 | Heptan‐3‐ol |

|
3547 544 589‐82‐2 |
286 JECFA specification (JECFA, 1998) |
0.12 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
JECFA evaluated 3‐heptanol (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 02.045 | Heptan‐2‐ol |
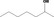
|
3288 554 543‐49‐7 |
284 JECFA specification (JECFA, 1998) |
6.8 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
JECFA evaluated 2‐heptanol (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 02.079 | Isopropanol |

|
2929 67‐63‐0 |
277 JECFA specification (JECFA, 1998) |
84,000 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
|
| 02.081 | 2,6‐Dimethylheptan‐4‐ol |

|
3140 11719 108‐82‐7 |
303 JECFA specification (JECFA, 1998) |
ND |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) |
|
| 02.086 | Undecan‐2‐ol |
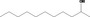
|
3246 11826 1653‐30‐1 |
297 JECFA specification (JECFA, 1998) |
0.24 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 2‐undecanol (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 02.087 | Nonan‐2‐ol |

|
3315 11803 628‐99‐9 |
293 JECFA specification (JECFA, 1998) |
0.61 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 2‐nonanol (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 02.088 | Pentan‐2‐ol |

|
3316 11696 6032‐29‐7 |
280 JECFA specification (JECFA, 1998) |
5.4 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 2‐pentanol (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 02.089 | Hexan‐3‐ol |

|
3351 11775 623‐37‐0 |
282 JECFA specification (JECFA, 1998) |
11 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 3‐hexanol (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 02.098 | Octan‐3‐ol |

|
3581 11715 589‐98‐0 |
291 JECFA specification (JECFA, 1998) |
4.7 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 3‐octanol (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 02.099 | Pent‐1‐en‐3‐ol |

|
3584 11717 616‐25‐1 |
1150 JECFA specification (JECFA, 2002b) |
2.1 | No safety concern (JECFA, 2002a) | |
| 02.103 | Decan‐3‐ol |

|
3605 10194 1565‐81‐7 |
295 JECFA specification (JECFA, 1998) |
ND |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 3‐decanol (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 02.104 | Hex‐1‐en‐3‐ol |

|
3608 10220 4798‐44‐1 |
1151 JECFA specification (JECFA, 2002b) |
0.55 | No safety concern (JECFA, 2002a) | |
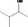
| 02.111 | 3‐Methylbutan‐2‐ol |
|
3703 598‐75‐4 |
300 JECFA specification (JECFA, 2000b) |
0.49 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 3‐methyl‐2‐butanol (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 02.136 | Dec‐1‐en‐3‐ol |

|
3824 51100‐54‐0 |
1153 JECFA specification (JECFA, 2002b) |
ND | No safety concern (JECFA, 2002a) | |
| 02.155 | 1‐Hepten‐3‐ol |
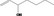
|
4129 10218 4938‐52‐7 |
1842 | 0.13 | No safety concern (JECFA, 2009b) | |
| 02.252 | 4,8‐Dimethyl‐3,7‐nonadien‐2‐ol |

|
4102 67845‐50‐5 |
1841 JECFA specification (JECFA, 2009a). |
3 | No safety concern (JECFA, 2009b) | |
| 07.002 | Heptan‐2‐one |
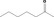
|
2544 136 110‐43‐0 |
283 JECFA specification (JECFA, 1998) |
96 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 07.003 | Heptan‐3‐one |
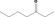
|
2545 137 106‐35‐4 |
285 JECFA specification (JECFA, 1998) |
3.3 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
| 07.015 | 6‐Methylhept‐5‐en‐2‐one |
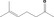
|
2707 149 110‐93‐0 |
1120 JECFA specification (JECFA, 2002b). |
100 | No safety concern (JECFA, 2002a)Category B (CoE, 1992) | |
| 07.016 | Undecan‐2‐one |
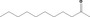
|
3093 150 112‐12‐9 |
296 JECFA specification (JECFA, 1998) |
330 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 07.017 | 4‐Methylpentan‐2‐one |

|
2731 151 108‐10‐1 |
301 JECFA specification (JECFA, 1998) |
6.1 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
| 07.019 | Octan‐2‐one |

|
2802 153 111‐13‐7 |
288 JECFA specification (JECFA, 1998) |
93 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 07.020 | Nonan‐2‐one |

|
2785 154 821‐55‐6 |
292 JECFA specification (JECFA, 1998) |
320 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 07.050 | Acetone |

|
3326 737 67‐64‐1 |
139 JECFA specification (JECFA, 1998) |
6,100 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 07.053 | Butan‐2‐one |

|
2170 753 78‐93‐3 |
278 JECFA specification (JECFA, 1998) |
96 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 07.054 | Pentan‐2‐one |
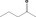
|
2842 754 107‐87‐9 |
279 JECFA specification (JECFA, 1998) |
120 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 07.058 | Heptan‐4‐one |

|
2546 2034 123‐19‐3 |
287 JECFA specification (JECFA, 1998) |
1.9 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
| 07.062 | Octan‐3‐one |

|
2803 2042 106‐68‐3 |
290 JECFA specification (JECFA, 1998) |
2.8 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
| 07.069 | Tetrahydro‐pseudo‐ionone |

|
3059 2053 4433‐36‐7 |
1121 JECFA specification (JECFA, 2002b) |
0.012 |
No safety concern (JECFA, 2002a) Category B (CoE, 1992) |
JECFA evaluated 3,4,5,6‐tetrahydropseudoionone (CASrn as in Register). CASrn refers to the racemate |
| 07.081 | Oct‐1‐en‐3‐one |

|
3515 2312 4312‐99‐6 |
1148 JECFA specification (JECFA, 2002b) |
1.2 |
No safety concern (JECFA, 2002a) Category B (CoE, 1992) |
|
| 07.096 | Hexan‐3‐one |

|
3290 11097 589‐38‐8 |
281 JECFA specification (JECFA, 1998) |
0.37 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) |
|
| 07.099 | 6‐Methylhepta‐3,5‐dien‐2‐one |

|
3363 11143 1604‐28‐0 |
1134 JECFA specification (JECFA, 2002b) |
13 | No safety concern (JECFA, 2002a) | |
| 07.100 | 5‐Methylhex‐5‐en‐2‐one |
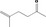
|
3365 11150 3240‐09‐3 |
1119 JECFA specification (JECFA, 2002b). |
0.24 | No safety concern (JECFA, 2002a) | |
| 07.102 | Pent‐1‐en‐3‐one |

|
3382 11179 1629‐58‐9 |
1147 JECFA specification (JECFA, 2002b) |
0.29 | No safety concern (JECFA, 2002a) | |
| 07.103 | Tridecan‐2‐one |

|
3388 11194 593‐08‐8 |
298 JECFA specification (JECFA, 2000b) |
62 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
|
| 07.113 | Nonan‐3‐one |

|
3440 11160 925‐78‐0 |
294 JECFA specification (JECFA, 1998) |
0.12 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) |
|
| 07.114 | 6,10,14‐Trimethylpentadeca‐5,9,13‐trien‐2‐one |

|
3442 11206 762‐29‐8 |
1123 JECFA specification (JECFA, 2002b). |
0.085 | No safety concern (JECFA, 2002a) | JECFA evaluated 2,6,10‐trimethyl‐2,6,10‐pentadecatrien‐14‐one (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 07.122 | 2,6‐Dimethylheptan‐4‐one |

|
3537 11914 108‐83‐8 |
302 JECFA specification (JECFA, 1998) |
0.18 | No safety concern (JECFA, 2000a) | |
| 07.123 | Geranylacetone |

|
3542 11088 3796‐70‐1 |
1122 JECFA specification (JECFA, 2002b). |
41 | No safety concern (JECFA, 2002a) | JECFA evaluated 6,10‐dimethyl‐5,9‐undecadien‐2‐one (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 07.137 | Pentadecan‐2‐one |

|
3724 11808 2345‐28‐0 |
299 JECFA specification (JECFA, 2000b) |
18 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
|
| 07.151 | Decan‐3‐one |

|
3966 11056 928‐80‐3 |
1118 JECFA specification (JECFA, 2002b). |
3 | No safety concern (JECFA, 2002a) | |
| 07.190 | Octa‐1,5‐dien‐3‐one |

|
4405 65213‐86‐7 |
1848 JECFA specification (JECFA, 2009a). |
0.061 | No safety concern (JECFA, 2009b) | |
| 07.240 | 2‐Methylheptan‐3‐one |
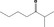
|
4000 13019‐20‐0 |
1156 JECFA specification (JECFA, 2002b). |
3 | No safety concern (JECFA, 2002a) | |
| 07.247 | (E,E)‐3,5‐Octadien‐2‐one |
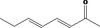
|
4008 30086‐02‐3 |
1139 JECFA specification (JECFA, 2002b). |
3 | No safety concern (JECFA, 2002a) | JECFA evaluated (E,E)‐3,5‐Octadien‐2‐one (CASrn as in Register). CASrn in Register to be verified |
| 07.249 | Undecan‐6‐one |
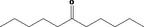
|
4022 927‐49‐1 |
1155 JECFA specification (JECFA, 2002b). |
3 | No safety concern (JECFA, 2002a) | |
| 07.256 | (E) & (Z)‐4,8‐Dimethyl‐3,7‐nonadiene‐2‐ one |
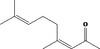
|
3969 817‐88‐9 |
1137 JECFA specification (JECFA, 2002b). |
6.1 | No safety concern (JECFA, 2002a) | |
| 09.003 | Isopropyl acetate |
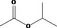
|
2926 193 108‐21‐4 |
305 JECFA specification (JECFA, 1998) |
35 |
No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
No ADI allocated (JECFA, 1980) |
| 09.041 | Isopropyl butyrate |
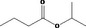
|
2935 267 638‐11‐9 |
307 JECFA specification (JECFA, 1998) |
6 |
No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 09.062 | Isopropyl hexanoate |
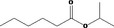
|
2950 312 2311‐46‐8 |
308 JECFA specification (JECFA, 2001) |
3.2 |
No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 09.105 | Isopropyl tetradecanoate |

|
3556 386 110‐27‐0 |
311 JECFA specification (JECFA, 2000b) |
19 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
| 09.123 | Isopropyl propionate |
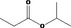
|
2959 404 637‐78‐5 |
306 JECFA specification (JECFA, 2001) |
0.012 | No safety concern (JECFA, 2000a)Category A (CoE, 1992) | |
| 09.165 | Isopropyl formate |
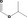
|
2944 503 625‐55‐8 |
304 JECFA specification (JECFA, 2001) |
0.45 |
No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 09.254 | 3‐Octyl acetate |
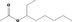
|
3583 2347 4864‐61‐3 |
313 JECFA specification (JECFA, 1998) |
0.61 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
JECFA evaluated 3‐octyl acetate (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 09.281 | Oct‐1‐en‐3‐yl acetate |
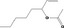
|
3582 11716 2442‐10‐6 |
1836 | 2.1 | No safety concern (JECFA, 2009b) | |
| 09.282 | Oct‐1‐en‐3‐yl butyrate |
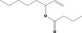
|
3612 16491‐54‐6 |
1837 | 0.0012 | No safety concern (JECFA, 2009b) | |
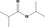
| 09.415 | Isopropyl isobutyrate |
|
2937 290 617‐50‐5 |
309 JECFA specification (JECFA, 1998) |
0.49 |
No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 09.450 | Isopropyl isovalerate |

|
2961 445 32665‐23‐9 |
310 JECFA specification (JECFA, 2002b) |
0.24 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
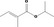
| 09.513 | Isopropyl 2‐methylcrotonate |
|
3229 10733 1733‐25‐1 |
312 JECFA specification (JECFA, 1998) |
0.012 | No safety concern (JECFA, 2000a) | JECFA evaluated isopropyl tiglate (CASrn 6284‐46‐4). CASrn in Register refers to (E)‐isomer |
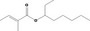
| 09.539 | Oct‐3‐yl 2‐methylcrotonate |
|
3676 94133‐92‐3 |
448 JECFA specification (JECFA, 2001) |
0.012 | No safety concern (JECFA, 2000a) | JECFA evaluated 1‐ethylhexyl tiglate (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
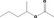
| 09.657 | 1‐Methylbutyl acetate |
|
4012 10761 626‐38‐0 |
1146 JECFA specification (JECFA, 2002b) |
2.9 | No safety concern (JECFA, 2002a) | JECFA evaluated 2‐pentyl acetate (CASrn as in Register). (R)‐ or (S)‐enantiomer not specified by CASrn in Register |
| 09.658 | 1‐Methylbutyl butyrate |

|
3893 10763 60415‐61‐4 |
1142 JECFA specification (JECFA, 2002b) |
0.47 | No safety concern (JECFA, 2002a) | JECFA evaluated 2‐pentyl butyrate (CASrn as in Register). CASrn refers to the racemate |
| 09.923 | Hept‐2‐yl butyrate |
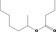
|
3981 39026‐94‐3 |
1144 JECFA specification (JECFA, 2002b) |
3 | No safety concern (JECFA, 2002a) | |
| 09.924 | 3‐Heptyl acetate (mixture of R and S) |
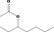
|
3980 5921‐83‐5 |
1143 JECFA specification (JECFA, 2002b) |
3 | No safety concern (JECFA, 2002a) | |
| 09.925 | Nonan‐3‐yl acetate |

|
4007 60826‐15‐5 |
1145 JECFA specification (JECFA, 2002b) |
3 | No safety concern (JECFA, 2002a) | |
| 09.936 | 4,8‐Dimethyl‐3,7‐nonadien‐2‐yl acetate |
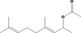
|
4103 91418‐25‐6 |
1847 JECFA specification (JECFA, 2009a). |
3 | No safety concern (JECFA, 2009b) |
FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; MSDI: Maximised Survey‐derived Daily Intake; SCF: Scientific Committee on Food.
EU MSDI: Amount added to food as flavouring substance in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day.
Category 1: Considered safe in use, Category 2: Temporarily considered safe in use, Category 3: Insufficient data to provide assurance of safety in use, Category 4: Not acceptable due to evidence of toxicity.
No safety concern at estimated levels of intake.
Category A: Flavouring substance, which may be used in foodstuffs, Category B: Flavouring substance which can be used provisionally in foodstuffs.
ND: no intake data reported.
2.1.2. Stereoisomers
It is recognised that geometrical and optical isomers of substances may have different properties. Their flavour may be different; they may have different chemical properties resulting in possible variability in their absorption, distribution, metabolism, elimination and toxicity. Thus, information must be provided on the configuration of the flavouring substance, i.e. whether it is one of the geometrical/optical isomers, or a defined mixture of stereoisomers. The available specifications of purity will be considered in order to determine whether the safety evaluation carried out for candidate substances for which stereoisomers may exist can be applied to the materials of commerce. Flavouring substances with different configurations should have individual chemical names and codes (CAS number, FLAVIS number, etc.).
Twenty‐seven candidate substances possess a chiral centre [FL‐nos: 02.124, 02.131, 02.142, 02.145, 02.148, 02.177, 02.183, 02.187, 02.190, 02.194, 02.211, 02.255, 07.157, 07.182, 07.185, 07.239, 09.304, 09.323, 09.325, 09.328, 09.332, 09.386, 09.388, 09.391, 09.676, 09.880 and 09.926] and two of the candidate substances possess two chiral centres [FL‐nos: 02.182 and 07.205] (see Table 1).
Due to the presence and the position of double bonds, 10 candidate substances can exist as geometrical isomers [FL‐nos: 02.145, 02.194, 02.211, 02.255, 07.156, 07.198, 07.236, 07.239, 09.386 and 09.880]. (EFFA, 2010; EFFA, 2012) (see Table 1).
2.1.3. Specifications
Purity criteria for all 53 candidate substances have been provided by the flavour industry (EFFA, 2002a,c, 2007c; Flavour Industry, 2006,2009). Judged against the requirements in Annex II of Commission Regulation (EC) No 1565/20003, the information is adequate for all candidate substances (EFFA, 2010, 2012, 2016, 2017) (see Section 2.1.2 and Table 1). Adequate specifications including purity and identity for the materials of commerce have been provided for all the candidate substances.
2.1.4. Natural occurrence in food
Forty‐five of the candidate substances have been reported to occur naturally. These occurrences include among others: milk and milk products as cheese of various types, beef, chicken, guinea hen, lamb and mutton, fish, oysters, scallops and shrimps, passion fruit, plum, papaya, strawberry, citrus fruits, apples, hop oil, camomile, tomatoes and potatoes, cocoa and tea, maize, nuts and different alcoholic beverages. The highest quantified natural occurrences in foods are presented in Table 2 (full data set are available in Appendix F).
Table 2.
Candidate substances reported to occur naturally in food (VCF online 2016)
| FL‐no | Name | Quantitative data reported |
|---|---|---|
| 02.077 | Pentan‐3‐ol | Up to 34 mg/kg in tea |
| 02.124 | 6‐Methylhept‐5‐en‐2‐ol | Up to 50 mg/kg in citrus fruits |
| 02.145 | 2,6‐Dimethylocta‐1,5,7‐trien‐3‐ol | Up to 100 mg/kg in sage |
| 07.084 | Pentan‐3‐one | Up to 14 mg/kg in mushroom |
| 09.323 | sec‐Butyl acetate | Up to 67 mg/kg in vinegar |
| 09.391 | sec‐Heptyl hexanoate | Up to 6,634 mg/kg in passion fruit |
For eight candidate substances listed in Table 3 no natural occurrence data have been identified (VCF online, 2016).
Table 3.
Candidate substances for which no natural occurrence data in food are available (VCF online, 2016)
| FL‐no | Name |
|---|---|
| 07.162 | Hex‐5‐en‐2‐one |
| 07.201 | Tridec‐12‐en‐2‐one |
| 07.210 | 1‐Nonene‐3‐one |
| 07.239 | [R‐(E)]‐5‐Isopropyl‐8‐methylnona‐6,8‐dien‐2‐one |
| 07.262 | 9‐Decen‐2‐one |
| 09.332 | sec‐Butyl hexanoate |
| 09.880 | (Z)‐Hept‐4‐enyl‐2 butyrate |
| 09.926 | Octan‐3‐yl formate |
2.2. Intake data
Annual production volumes of the flavouring substances as surveyed by industry can be used to calculate the ‘Maximised Survey‐derived Daily Intake’ (MSDI) by assuming that the production figure only represents 60% of the use in food due to underreporting and that 10% of the total European Union (EU) population are consumers (SCF, 1999).
However, the Panel noted that due to year‐to‐year variability in production volumes, to uncertainties in the underreporting correction factor and to uncertainties in the percentage of consumers, the reliability of intake estimates on the basis of the MSDI approach is difficult to assess.
The Panel also noted that in contrast to the generally low per capita intake figures estimated on the basis of this MSDI approach, in some cases, the regular consumption of products flavoured at use levels reported by the flavour industry in the submissions would result in much higher intakes. In such cases, the human exposure thresholds below which exposures are not considered to present a safety concern might be exceeded.
Considering that the MSDI model may underestimate the intake of flavouring substances by certain groups of consumers, the Scientific Committee on Food (SCF) recommended also taking into account the results of other intake assessments (SCF, 1999).
One of the alternatives is the ‘Theoretical Added Maximum Daily Intake’ (TAMDI) approach, which is calculated on the basis of standard portions and upper use levels (SCF, 1995) for flavourable beverages and foods in general, with exceptional levels for particular foods. This method is regarded as a conservative estimate of the actual intake by most consumers because it is based on the assumption that the consumer regularly eats and drinks several food products containing the same flavouring substance at the upper use level.
One option to modify the TAMDI approach is to base the calculation on normal rather than upper use levels of the flavouring substances. This modified approach is less conservative (e.g. it may underestimate the intake of consumers being loyal to products flavoured at the maximum use levels reported). However, it is considered as a suitable tool to screen and prioritise the flavouring substances according to the need for refined intake data (EFSA, 2004).
2.2.1. Estimated daily per capita intake (MSDI approach)
The intake estimation is based on the MSDI approach, which involves the acquisition of data on the amounts used in food as flavourings (SCF, 1999). These data are derived from surveys on annual production volumes in Europe. These surveys were conducted in 1995 by the International Organization of the Flavour Industry (IOFI), in which flavour manufacturers reported the total amount of each flavouring substance incorporated into food sold in the EU during the previous year (IOFI, 1995). The intake approach does not consider the possible natural occurrence in food.
Average per capita intake (MSDI) is estimated on the assumption that the amount added to food is consumed by 10% of the population4 (Eurostat, 1998). This is derived for candidate substances from estimates of annual volume of production provided by industry and incorporates a correction factor of 0.6 to allow for incomplete reporting (60%) in the industry surveys (SCF, 1999).
In the present (FGE.07Rev5, the total annual volume of production of the 53 candidate substances for use as flavouring substances in Europe has been reported to be approximately 690 kg (EFFA, 2002b,c, 2007c; Flavour industry, 2009) and for 64 of the 67 supporting substances approximately 750,000 kg (isopropyl alcohol accounts for 690,000 kg and acetone for 50,000 kg) (cited by the JECFA (1999a). For three supporting substances, no EU annual volumes of production are available (JECFA, 2003; IOFI, 1995) (Tables 4 and B.3).
Table 4.
Tonnage data and MSDI for candidate and supporting substances
| Tonnage (kg/year) | MSDI (μg/capita per day) | |||
|---|---|---|---|---|
| Class I | Class II | Class I | Class II | |
| FGE.07Rev5 | 49.7 (28 substances) | 639.0 (25 substances) | 5.9 (28 substances) | 77.7 (25 substances) |
| FGE.07Rev5supp | 742,832 (34 substances) | 11,096 (30 substances) | 90,441 (34 substances) | 1,351 (30 substances) |
| Total | 742,882 | 11,735 | 90,450 | 1,430 |
MSDI: Maximised Survey‐derived Daily Intake.
On the basis of the annual volumes of production reported for the 53 candidate substances, the daily per capita intakes for each of these flavourings have been estimated (Table B.1). Approximately 90% of the total annual volume of production for the candidate substances (EFFA, 2002b, 2007c) is accounted for by one candidate substance, 9‐decen‐2‐one [FL‐no: 07.262]. The estimated daily per capita intake of this candidate substance from use as a flavouring substance is 73 μg/capita per day. The daily per capita intakes for each of the remaining substances is less than 2 μg/capita per day (Table B.1).
Table B.1.
Summary of safety evaluation applying the Procedure (based on intakes calculated by the MSDI approach)
| FL‐no | EU register name | Structural formula | MSDIa (μg/capita per day) | Classb Evaluation procedure pathc | Outcome on the named compoundd , e | Outcome on the material of commercef , g , h | Evaluation remarks |
|---|---|---|---|---|---|---|---|
| 02.077 | Pentan‐3‐ol |

|
0.19 |
Class I A3: intake below threshold |
d | f | |
| 02.124 | 6‐Methylhept‐5‐en‐2‐ol |
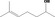
|
0.0061 |
Class I A3: intake below threshold |
d | f | |
| 02.142 | 3,3‐Dimethylbutan‐2‐ol |
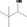
|
0.24 |
Class I A3: intake below threshold |
d | f | |
| 02.148 | Dodecan‐2‐ol |

|
0.35 |
Class I A3: Intake below threshold |
d | f | |
| 02.177 | 2‐Methylhexan‐3‐ol |
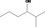
|
0.12 |
Class I A3: Intake below threshold |
d | f | |
| 02.182 | 3‐Methylpentan‐2‐ol |
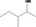
|
0.12 |
Class I A3: Intake below threshold |
d | f | |
| 02.183 | 4‐Methylpentan‐2‐ol |
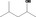
|
0.0012 |
Class I A3: Intake below threshold |
d | f | |
| 02.190 | Nonan‐3‐ol |

|
0.011 |
Class I A3: Intake below threshold |
d | f | |
| 02.255 | (Z)‐4‐Hepten‐2‐ol |
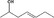
|
0.03 |
Class I A3: Intake below threshold |
d | f | |
| 07.084 | Pentan‐3‐one |

|
0.24 |
Class I A3: Intake below threshold |
d | f | |
| 07.178 | 3‐Methylbutan‐2‐one |
|
0.073 |
Class I A3: Intake below threshold |
d | f | |
| 07.239 | [R‐(E)]‐5‐Isopropyl‐8‐methylnona‐6,8‐dien‐2‐one |
|
0.24 |
Class I A3: Intake below threshold |
d | f | |
| 09.304 | sec‐Heptyl isovalerate |
|
0.0012 |
Class I A3: Intake below threshold |
d | f | |
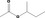
| 09.323 | sec‐Butyl acetate |
|
0.0012 |
Class I A3: Intake below threshold |
d | f | |
| 09.325 | sec‐Butyl butyrate |

|
1.3 |
Class I A3: Intake below threshold |
d | f | |
| 09.328 | sec‐Butyl formate |

|
0.12 |
Class I A3: Intake below threshold |
d | f | |
| 09.332 | sec‐Butyl hexanoate |

|
0.024 |
Class I A3: Intake below threshold |
d | f | |
| 09.386 | sec‐Hept‐4(cis)‐enyl acetate |

|
0.024 |
Class I A3: Intake below threshold |
d | f | |
| 09.388 | sec‐Heptyl acetate |

|
0.12 |
Class I A3: Intake below threshold |
d | f | |
| 09.391 | sec‐Heptyl hexanoate |

|
0.12 |
Class I A3: Intake below threshold |
d | f | |
| 09.604 | Isopropyl decanoate |

|
0.12 |
Class I A3: Intake below threshold |
d | f | |
| 09.605 | Isopropyl dodecanoate |

|
0.12 |
Class I A3: Intake below threshold |
d | f | |
| 09.606 | Isopropyl hexadecanoate |

|
0.012 |
Class I A3: Intake below threshold |
d | f | |
| 09.608 | Isopropyl octanoate |

|
1.3 |
Class I A3: Intake below threshold |
d | f | |
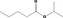
| 09.609 | Isopropyl valerate |
|
0.012 |
Class I A3: Intake below threshold |
d | f | |
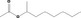
| 09.676 | sec‐Octyl acetate |
|
0.011 |
Class I A3: Intake below threshold |
d | f | |
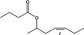
| 09.880 | (Z)‐Hept‐4‐enyl‐2 |
|
0.79 |
Class I A3: Intake below threshold |
d | f | |
| 09.926 | Octan‐3‐yl formate |

|
0.24 |
Class I A3: Intake below threshold |
d | f | |
| 02.131 | But‐3‐en‐2‐ol |

|
0.0012 |
Class II A3: Intake below threshold |
d | f | |
| 02.145 | 2,6‐Dimethylocta‐1,5,7‐trien‐3‐ol |
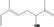
|
0.0085 |
Class II A3: Intake below threshold |
d | f | |
| 02.187 | Non‐1‐en‐3‐ol |

|
0.58 |
Class II A3: Intake below threshold |
d | f | |
| 02.194 | Octa‐1,5‐dien‐3‐ol |

|
0.061 |
Class II A3: Intake below threshold |
d | f | |
| 02.211 | Undeca‐1,5‐dien‐3‐ol |

|
0.061 |
Class II A3: Intake below threshold |
d | f | |
| 07.072 | 6‐Methylheptan‐3‐one |
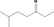
|
0.19 |
Class II A3: Intake below threshold |
d | f | |
| 07.150 | Decan‐2‐one |

|
0.52 |
Class II A3: Intake below threshold |
d | f | |
| 07.156 | 2,6‐Dimethyloct‐6‐en‐3‐one (mixture of E and Z) |
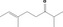
|
0.0012 |
Class II A3: Intake below threshold |
d | f | |
| 07.157 | 6,10‐Dimethylundecan‐2‐one |

|
0.085 |
Class II A3: Intake below threshold |
d | f | |
| 07.158 | Dodecan‐2‐one |

|
0.73 |
Class II A3: Intake below threshold |
d | f | |
| 07.160 | Heptadecan‐2‐one |
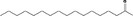
|
0.12 |
Class II A3: Intake below threshold |
d | f | |
| 07.161 | Hex‐1‐en‐3‐one |

|
0.012 |
Class II A3: Intake below threshold |
d | f | |
| 07.162 | Hex‐5‐en‐2‐one |

|
0.049 |
Class II A3: Intake below threshold |
d | f | |
| 07.181 | 6‐Methylheptan‐2‐one |
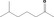
|
0.0012 |
Class II A3: Intake below threshold |
d | f | |
| 07.185 | 3‐Methylpentan‐2‐one |
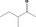
|
1.2 |
Class II A3: Intake below threshold |
d | f | |
| 07.189 | Nonan‐4‐one |
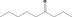
|
0.52 |
Class II A3: Intake below threshold |
d | f | |
| 07.198 | Pseudo‐ionone |

|
0.12 |
Class II A3: Intake below threshold |
d | f | |
| 07.199 | Tetradecan‐2‐one |

|
0.073 |
Class II A3: Intake below threshold |
d | f | |
| 07.201 | Tridec‐12‐en‐2‐one |

|
0.024 |
Class II A3: Intake below threshold |
d | f | |
| 07.204 | 3,3,6‐Trimethylhepta‐1,5‐dien‐4‐one |
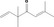
|
0.012 |
Class II A3: Intake below threshold |
d | f | |
| 07.205 | 6,10,14‐Trimethylpentadecan‐2‐one |
|
0.0073 |
Class II A3: Intake below threshold |
d | f | |
| 07.210 | 1‐Nonene‐3‐one |

|
0.0012 |
Class II A3: Intake below threshold |
d | f | |
| 07.236 | (Z)‐5‐Octen‐2‐one |

|
0.0097 |
Class II A3: Intake below threshold |
d | f | |
| 07.262 | 9‐Decen‐2‐one |

|
73 |
Class II A3: Intake below threshold |
d | f | |
| 07.182 | 5‐Methylheptan‐3‐one |

|
0.32 |
Class II B3: Intake below threshold B4: Adequate NOAEL exists |
d | f | NOAEL for neurotoxicity: 82 mg/kg bw per day |
MSDI: Maximised Survey‐derived Daily Intake; NOAEL: no observed adverse effect level; bw: body weight.
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day.
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
No safety concern at the estimated level of intake of the material of commerce meeting the specification requirement (based on intake calculated by the MSDI approach).
Tentatively regarded as presenting no safety concern (based on intake calculated by the MSDI approach) pending further information on the purity of the material of commerce and/or information on stereoisomerism.
No conclusion can be drawn due to lack of information on the purity of the material of commerce.
2.2.2. Intake estimated on the basis of the modified TAMDI (mTAMDI)
The method for calculation of modified Theoretical Added Maximum Daily Intake (mTAMDI) values is based on the approach used by SCF up to 1995 (SCF, 1995). The assumption is that a person may consume a certain amount of flavourable foods and beverages per day.
For the present evaluation of the 53 candidate substances, information on food categories and normal and maximum use levels,5 , 6 , 7 were submitted by the flavour industry (EFFA, 2002a,c, 2007a,b,c; Flavour Industry, 2006, 2009, EFFA, 2016). The 53 candidate substances are used in flavoured food products divided into the food categories, outlined in Annex III of the Commission Regulation (EC) No 1565/20003, as summarised in Table 5. For the present calculation of mTAMDI, the reported normal use levels were used. In the case where different use levels were reported for different food categories, the highest reported normal use level was used.
Table 5.
Use in various food categories for 53 candidate substances for which data on use have been provided
| Food category | Description | Flavourings used |
|---|---|---|
| 01.0 | Dairy products, excluding products of category 2 | All |
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) | All except [FL‐no: 07.262] |
| 03.0 | Edible ices, including sherbet and sorbet | All |
| 04.1 | Processed fruits | All |
| 04.2 | Processed vegetables (including mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds | Only [FL‐no: 07.262] |
| 05.0 | Confectionery | All except [FL‐no: 07.205] |
| 06.0 | Cereals and cereal products, including flours & starches from roots & tubers, pulses & legumes, excluding bakery | All except [FL‐nos: 02.255 & 07.262] |
| 07.0 | Bakery wares | All except [FL‐no: 07.262] |
| 08.0 | Meat and meat products, including poultry and game | All except [FL‐nos: 02.255 & 07.262] |
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms | All except [FL‐nos: 09.608, 02.255 & 07.262] |
| 10.0 | Eggs and egg products | None |
| 11.0 | Sweeteners, including honey | None |
| 12.0 | Salts, spices, soups, sauces, salads, protein products etc. | All except [FL‐nos: 07.156, 02.255 & 07.262] |
| 13.0 | Foodstuffs intended for particular nutritional uses | All |
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products | All |
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts | All except [FL‐no: 07.205] |
| 15.0 | Ready‐to‐eat savouries | All except [FL‐nos: 02.255, 07.157, 09.609 & 07.262] |
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) – foods that could not be placed in categories 1–15 | All |
According to the flavour industry, the normal use levels for the 53 candidate substances are in the range of 1–30 mg/kg food, and the maximum use levels are in the range of 5–150 mg/kg (EFFA, 2002a,b,c,d, 2007a,b,c, 2016; Flavour Industry, 2006, 2009).
The mTAMDI values for the 28 candidate substances from structural class I (see Section 2.4) range from 1,600 to 3,900 μg/person per day. For the 25 candidate substance from structural class II, the mTAMDI range from 1,500 to 6,600 μg/person per day.
For detailed information on use levels and intake estimations based on the mTAMDI approach, see Appendix C.
2.3. Absorption, distribution, metabolism and excretion
In general, aliphatic secondary alcohols and ketones are expected to be rapidly absorbed in the gastrointestinal tract. The candidate aliphatic esters are expected to be hydrolysed enzymatically to their component secondary alcohols and carboxylic acids. The carboxylic acids are completely oxidised in the fatty acid pathway and the tricarboxylic acid pathway (see Appendix D).
Secondary alcohols may undergo oxidation to the corresponding ketone; however, in the in vivo situation, the alcohol is removed from the equilibrium by conjugation to glucuronic acid, which represents the major pathway of metabolism for secondary alcohols. The glucuronides of the candidate secondary alcohols are expected to be eliminated via the urine (Felsted and Bachur, 1980; Kasper and Henton, 1980; JECFA, 1999a).
In general, the major metabolic pathway for aliphatic ketones is reduction of the ketone to the corresponding secondary alcohol and subsequent excretion as glucuronic acid conjugate (Felsted and Bachur, 1980; JECFA, 1999a).
Short‐chain ketones (C < 5) that contain a carbonyl function at the C2 position may undergo oxidation to yield an alpha‐keto carboxylic acid, which through decarboxylation will be oxidised to carbon dioxide and a simple aliphatic carboxylic acid that will enter the fatty acid pathway and citric acid cycle (Dietz et al., 1981). Ketones may also be metabolised by omega‐ or omega‐1‐oxidation yielding a hydroxy‐ketone that may be further reduced to a diol and excreted in the urine as glucuronic acid conjugate. Longer chain aliphatic ketones (C ≥ 5) are primarily metabolised via reduction, but omega‐ and omega‐1‐oxidation are competing pathways at high concentrations (Dietz et al., 1981; Topping et al., 1994).
Omega‐1‐oxidation of certain aliphatic ketones may yield gamma‐diketones, which may give rise to neuropathy of giant‐axonal type. The metabolic pathway includes oxidation of the omega‐1‐carbon, first to a hydroxy‐ketone and then to a diketone. The gamma‐spacing of the carbonyl functions has been shown to be a prerequisite for neurotoxic effects, thus, only ketones with this structural feature may yield the neurotoxic metabolites. Neurotoxic effects are, however, only observed at relatively high dosages (Topping et al., 1994). One of the candidate substances, 5‐methylheptan‐3‐one [FL‐no: 07.182], may potentially be oxidised to a gamma‐diketone.
Twelve of the candidate substances, but‐3‐en‐2‐ol, 2,6‐dimethylocta‐1,5,7‐triene‐3‐ol, non‐1‐en‐3‐ol, octa‐1,5‐dien‐3‐ol, undeca‐1,5‐dien‐3‐ol, hex‐1‐en‐3‐one, hex‐5‐en‐2‐one, tridec‐12‐en‐2‐one, 3,3,6‐trimethylhepta‐1,5‐dien‐4‐one, 1‐nonene‐3‐one, ([R‐(E)]‐5‐isopropyl‐8‐methylnona‐6,8‐dien‐2‐one and 9‐decen‐2‐one [FL‐nos: 02.131, 02.145, 02.187, 02.194, 02.211, 07.161, 07.162, 07.201, 07.204, 07.210, 07.239 and 07.262] have terminal double bonds. These double bonds may be oxidised to the corresponding epoxides. Epoxides are highly reactive molecules, due to the large strain associated with this three‐membered ring structure, and they react easily with nucleophilic sites of cellular macromolecules. However, epoxides will be conjugated with glutathione by glutathione S‐transferases or hydrolysed to diols by epoxide hydrolases. These two reactions can be considered to be detoxications (Sanchez and Kauffman, 2010). 1‐Alkenes are metabolised by P450 through both double bond oxidation to the corresponding epoxide and allylic oxidation (Chiappe et al., 1998). The rates of the two reactions measured with different P450 isoforms indicate that epoxide formation is generally favoured (Chiappe et al., 1998).
Based on the low levels of intake of alkenones and alkenols characterised by a carbonyl or an alcohol group in addition to the terminal double bond, it is expected that the detoxication reactions of the formed epoxides (conjugation with glutathione or epoxide hydrolase mediated hydrolysis) would not be saturated and would outweigh the rate of epoxide formation. The presence of the terminal double bond is therefore not considered of concern under the intended conditions of use.
In addition to reduction and oxidation pathways, low molecular weight alcohols and ketones may be excreted unchanged in expired air (Brown et al., 1987).
2.3.1.
Concluding remarks on metabolism
Fifty‐two of the candidate substances, seven saturated aliphatic acyclic secondary alcohols, seven unsaturated aliphatic secondary alcohols, 12 saturated aliphatic ketones, 10 unsaturated aliphatic ketones and 16 esters of aliphatic acyclic secondary alcohols and linear and branched‐chain aliphatic carboxylic acids, may be expected to be metabolised to innocuous substances at the estimated levels of intake, based on the MSDI approach, as flavouring substances.
One candidate substance, 5‐methylheptan‐3‐one [FL‐no: 07.182], may be oxidised to a potentially neurotoxic gamma‐diketone. Therefore, this substance will be evaluated via the B‐side of the Procedure (see section 2.4).
More detailed information on the metabolism of candidate substances is given in Appendix D.
2.4. Application of the procedure for the safety evaluation of flavouring substances
The application of the Procedure is based on intakes estimated on the basis of the MSDI approach. Where the mTAMDI approach indicates that the intake of a flavouring substance might exceed its corresponding threshold of concern, a formal safety assessment is not carried out using the Procedure. In these cases, the Panel requires more precise data on use and use levels. For comparison of the intake estimations based on the MSDI approach and the mTAMDI approach, see Section 2.5.
For the safety evaluation of the 53 candidate substances the Procedure as outlined in Appendix A was applied, based on the MSDI approach. The stepwise evaluations of the substances are summarised in Table B.1.
Step 1
Twenty‐eight of the candidate substances [FL‐nos: 02.077, 02.124, 02.142, 02.148, 02.177, 02.182, 02.183, 02.190, 02.255, 07.084, 07.178, 07.239, 09.304, 09.323, 09.325, 09.328, 09.332, 09.386, 09.388, 09.391, 09.604, 09.605, 09.606, 09.608, 09.609, 09.676, 09.880 and 09.926] are classified in structural class I, according to the decision tree approach presented by Cramer et al. (Cramer et al., 1978). The remaining 25 candidate substances [FL‐nos: 02.131, 02.145, 02.187, 02.194, 02.211, 07.072, 07.150, 07.156, 07.157, 07.158, 07.160, 07.161, 07.162, 07.181, 07.182, 07.185, 07.189, 07.198, 07.199, 07.201, 07.204, 07.205, 07.210, 07.236 and 07.262], which are unsaturated aliphatic secondary alcohols or acyclic aliphatic saturated or unsaturated ketones, are in structural class II.
Step 2
Fifty‐two candidate substances were considered to be metabolised to innocuous products and would not be expected to saturate available detoxification pathways at estimated levels of intake, based on the MSDI approach, from use as flavouring substances. Therefore, these 52 substances proceed via the A‐side of the Procedure scheme (Appendix A). One candidate substance, 5‐methylheptan‐3‐one [FL‐no: 07.182], cannot be predicted to be metabolised to innocuous products and therefore, proceeds to step B3.
Step A3
The 28 candidate substances assigned to structural class I, have estimated European daily per capita intakes ranging from 0.0012 to 1.3 μg (Table 6). These intakes are below the threshold of concern of 1,800 μg/person per day for structural class I. The 24 unsaturated aliphatic secondary alcohols and ketones, which have been assigned to structural class II, have estimated European daily per capita intakes ranging from 0.0012 to 73 μg (Table 6). These intakes are below the threshold of concern of 540 μg/person per day for structural class II. Based on results of the safety evaluation sequence, the 52 candidate substances proceeding via the A‐side of the Procedure do not pose a safety concern when used as flavouring substances at the estimated levels of intake, based on the MSDI approach.
Table 6.
Estimated intakes based on the MSDI approach and the mTAMDI approach
| FL‐no | EU register name | MSDI (μg/capita per day) | mTAMDI (μg/person per day) | Structural class | TTC (μg/person per day) |
|---|---|---|---|---|---|
| 02.077 | Pentan‐3‐ol | 0.19 | 3,900 | Class I | 1,800 |
| 02.124 | 6‐Methylhept‐5‐en‐2‐ol | 0.0061 | 3,900 | Class I | 1,800 |
| 02.142 | 3,3‐Dimethylbutan‐2‐ol | 0.24 | 3,900 | Class I | 1,800 |
| 02.148 | Dodecan‐2‐ol | 0.35 | 3,900 | Class I | 1,800 |
| 02.177 | 2‐Methylhexan‐3‐ol | 0.12 | 3,900 | Class I | 1,800 |
| 02.182 | 3‐Methylpentan‐2‐ol | 0.12 | 3,900 | Class I | 1,800 |
| 02.183 | 4‐Methylpentan‐2‐ol | 0.0012 | 3,900 | Class I | 1,800 |
| 02.190 | Nonan‐3‐ol | 0.011 | 3,900 | Class I | 1,800 |
| 02.255 | (Z)‐4‐Hepten‐2‐ol | 0.03 | 2,500 | Class I | 1,800 |
| 07.084 | Pentan‐3‐one | 0.24 | 1,600 | Class I | 1,800 |
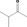
| 07.178 | 3‐Methylbutan‐2‐one | 0.073 | 1,600 | Class I | 1,800 |
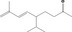
| 07.239 | [R‐(E)]‐5‐Isopropyl‐8‐methylnona‐6,8‐dien‐2‐one | 0.24 | 1,600 | Class I | 1,800 |
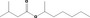
| 09.304 | sec‐Heptyl isovalerate | 0.0012 | 3,900 | Class I | 1,800 |
| 09.323 | sec‐Butyl acetate | 0.0012 | 3,900 | Class I | 1,800 |
| 09.325 | sec‐Butyl butyrate | 1.3 | 3,900 | Class I | 1,800 |
| 09.328 | sec‐Butyl formate | 0.12 | 3,900 | Class I | 1,800 |
| 09.332 | sec‐Butyl hexanoate | 0.024 | 3,900 | Class I | 1,800 |
| 09.386 | sec‐Hept‐4(cis)‐enyl acetate | 0.024 | 3,900 | Class I | 1,800 |
| 09.388 | sec‐Heptyl acetate | 0.12 | 3,900 | Class I | 1,800 |
| 09.391 | sec‐Heptyl hexanoate | 0.12 | 3,900 | Class I | 1,800 |
| 09.604 | Isopropyl decanoate | 0.12 | 3,900 | Class I | 1,800 |
| 09.605 | Isopropyl dodecanoate | 0.12 | 3,900 | Class I | 1,800 |
| 09.606 | Isopropyl hexadecanoate | 0.012 | 3,900 | Class I | 1,800 |
| 09.608 | Isopropyl octanoate | 1.3 | 3,900 | Class I | 1,800 |
| 09.609 | Isopropyl valerate | 0.012 | 3,500 | Class I | 1,800 |
| 09.676 | sec‐Octyl acetate | 0.011 | 3,900 | Class I | 1,800 |
| 09.880 | (Z)‐Hept‐4‐enyl‐2 butyrate | 0.79 | 3,900 | Class I | 1,800 |
| 09.926 | Octan‐3‐yl formate | 0.24 | 3,900 | Class I | 1,800 |
| 02.131 | But‐3‐en‐2‐ol | 0.0012 | 3,900 | Class II | 540 |
| 02.145 | 2,6‐Dimethylocta‐1,5,7‐trien‐3‐ol | 0.0085 | 3,900 | Class II | 540 |
| 02.187 | Non‐1‐en‐3‐ol | 0.58 | 3,900 | Class II | 540 |
| 02.194 | Octa‐1,5‐dien‐3‐ol | 0.061 | 3,900 | Class II | 540 |
| 02.211 | Undeca‐1,5‐dien‐3‐ol | 0.061 | 3,900 | Class II | 540 |
| 07.072 | 6‐Methylheptan‐3‐one | 0.19 | 1,600 | Class II | 540 |
| 07.150 | Decan‐2‐one | 0.52 | 1,600 | Class II | 540 |
| 07.156 | 2,6‐Dimethyloct‐6‐en‐3‐one (mixture of E and Z) | 0.0012 | 1,600 | Class II | 540 |
| 07.157 | 6,10‐Dimethylundecan‐2‐one | 0.085 | 1,500 | Class II | 540 |
| 07.158 | Dodecan‐2‐one | 0.73 | 1,600 | Class II | 540 |
| 07.160 | Heptadecan‐2‐one | 0.12 | 1,600 | Class II | 540 |
| 07.161 | Hex‐1‐en‐3‐one | 0.012 | 1,600 | Class II | 540 |
| 07.162 | Hex‐5‐en‐2‐one | 0.049 | 1,600 | Class II | 540 |
| 07.181 | 6‐Methylheptan‐2‐one | 0.0012 | 1,600 | Class II | 540 |
| 07.182 | 5‐Methylheptan‐3‐one | 0.32 | 1,600 | Class II | 540 |
| 07.185 | 3‐Methylpentan‐2‐one | 1.2 | 1,600 | Class II | 540 |
| 07.189 | Nonan‐4‐one | 0.52 | 1,600 | Class II | 540 |
| 07.198 | Pseudo‐ionone | 0.12 | 1,600 | Class II | 540 |
| 07.199 | Tetradecan‐2‐one | 0.073 | 1,600 | Class II | 540 |
| 07.201 | Tridec‐12‐en‐2‐one | 0.024 | 1,600 | Class II | 540 |
| 07.204 | 3,3,6‐Trimethylhepta‐1,5‐dien‐4‐one | 0.012 | 1,600 | Class II | 540 |
| 07.210 | 1‐Nonene‐3‐one | 0.0012 | 1,600 | Class II | 540 |
| 07.205 | 6,10,14‐Trimethylpentadecan‐2‐one | 0.0073 | 1,500 | Class II | 540 |
| 07.236 | (Z)‐5‐Octen‐2‐one | 0.0097 | 1,600 | Class II | 540 |
| 07.262 | 9‐Decen‐2‐one | 73 | 6,600 | Class II | 540 |
MSDI: Maximised Survey‐derived Daily Intake; mTAMDI: modified Theoretical Added Maximum Daily Intake; TTC: toxicological threshold of concern.
Step B3
The estimated per capita intake of 5‐methylheptan‐3‐one [FL‐no: 07.182] of 0.32 μg/capita per day does not exceed the threshold of concern for structural class II of 540 μg/person per day. Accordingly, the candidate substance proceeds to step B4 of the Procedure.
Step B4
On the basis of a study on the neurotoxic effects of orally administered 5‐methylheptan‐3‐one [FL‐no: 07.182] to male rats, a no observed adverse effect level (NOAEL) of 82 mg/kg body weight (bw) per day was established (IBM Corp., 1989). This NOAEL provides a margin of safety of 1.5 × 107 based on the estimated intake of the candidate substance of 0.32 μg/capita per day. Based on results of the safety evaluation sequence, this candidate substance does not pose a safety concern when used as flavouring substance at the estimated level of intake, based on the MSDI approach.
2.5. Comparison of the intake estimations based on the MSDI and the mTAMDI approach
The estimated intakes for the 28 candidate substances in structural class I based on the mTAMDI approach range from 1,600 to 3,900 μg/person per day. For three [FL‐nos: 07.084, 07.178 and 07.239] of these 28 substances, the mTAMDI is below the threshold of concern of 1,800 μg/person per day.
The estimated intake for the 21 candidate substances assigned to structural class II based on the mTAMDI range from 1,500 to 6,600 μg/person per day, which are all above the threshold of concern for structural class II substances of 540 μg/person per day.
Therefore, for 50 candidate substances, further information is required. This would include more reliable intake data and then, if required, additional toxicological data.
For comparison of the MSDI and mTAMDI values, see Table 6.
2.6. Considerations of combined intakes from use as flavouring substances
Because of structural similarities of candidate and supporting substances, it can be anticipated that many of the flavourings are metabolised through the same metabolic pathways and that the metabolites may affect the same target organs. Furthermore, in case of combined exposure to structurally related flavourings, the pathways could be overloaded. Therefore, combined intake should be considered. As flavourings not included in this FGE may also be metabolised through the same pathways, the combined intake estimates presented here are only preliminary. Currently, the combined intake estimates are only based on MSDI exposure estimates, although it is recognised that this may lead to underestimation of exposure. After completion of all FGEs, this issue should be readdressed. The total estimated combined daily per capita intake of structurally related flavourings is estimated by summing the MSDI for individual substances.
On the basis of the reported annual production volumes in Europe (EFFA, 2002b, c, 2007c; Flavour Industry, 2009), the total estimated daily per capita intake as flavourings of the 28 candidate flavouring substances assigned to structural class I is 6 μg, which does not exceed the threshold of concern for a substance belonging to structural class I of 1,800 μg/person per day. For the combined intake of the 25 candidate flavouring substances assigned to structural class II is 78 μg, which does not exceed the threshold of concern for a substance belonging to structural class II of 540 μg/person per day.
The 53 candidate substances are structurally related to 67 supporting substances evaluated by the JEFCA at its 51st, 59th and 69th meetings in the groups ‘Saturated aliphatic acyclic secondary alcohols, ketones, and related saturated and unsaturated esters’ (JECFA, 2000a, 2002a, 2003, 2009b). The total combined intake of candidate and supporting substances of structural class I and II would be 90,450 μg/capita per day and 1,430 μg/capita per day, respectively. Both intakes exceed the threshold of their structural class of 1,800 and 540 μg/person per day. However, the major contribution (> 99%) was provided by two supporting substances, namely acetone [FL‐no: 07.050] (6,100 μg/capita per day) and isopropanol [FL‐no: 02.079] (84,000 μg/capita per day). These are present in the body as endogenous compounds, which are easily eliminated, either by excretion into the urine and exhaled air or after enzymatic metabolism (Morgott, 1993). Therefore, they would not be expected to give rise to perturbations outside the physiological range (JECFA, 1999a). Excluding the two major contributors, the estimated total combined intake (in Europe) for the candidate (Table 6) and supporting substances (Table B.3) belonging to structural class I would be 350 μg/capita per day, which does not exceed the threshold of concern for the corresponding structural class (1,800 μg person per day); the estimated total combined intake (in Europe) for the candidate (Table 6) and supporting substances (Table B.3) belonging to structural class II would be 1,430 μg/capita per day, which is 2.6 fold higher than the threshold of concern for the corresponding structural class (540 μg/person per day). Five of the supporting substances from structural class II, oct‐1‐en‐3‐ol, heptan‐2‐one, undecan‐2‐one, nonan‐2‐one and tridecan‐2‐one [FL‐nos: 02.023, 07.002, 07.016, 07.020 and 07.103], contribute with 1,050 μg/capita per day to the combined MSDI of 1,430 μg/capita per day (Table B.3). A 90‐day study for nonan‐2‐one [FL‐no: 07.020] (O'Donoghue and Krasavage, 1980) provides a NOAEL of 2,000 mg/kg bw per day. Based on this NOAEL, a margin of safety of 11.5 × 104 can be derived for the combined intake of [FL‐nos: 02.023, 07.002, 07.016, 07.020 and 07.103]. For the remaining substances from structural class II, the estimated combined intake of 380 μg/capita per day is below the threshold of structural class II of 540 μg/capita per day.
If the candidate substance 5‐methylheptan‐3‐one [FL‐no: 07.182] and the two supporting substances heptan‐3‐ol [FL‐no: 02.044] and 3‐heptanone [FL‐no: 07.003], which can all be metabolised to neurotoxic gamma‐diketones, were consumed concomitantly on a daily basis, the estimated combined intake (in Europe) would be 3.7 μg/capita per day, corresponding to 0.06 μg/kg bw per day. This value does not exceed the threshold of concern for the corresponding structural class II (540 μg/person per day) and is also much lower than the NOAEL for 5‐methylheptan‐3‐one [FL‐no: 07.182] of 82 mg/kg bw per day for neurotoxicity in the rat. Therefore, it can be concluded that there is no safety concern for human health for the combined exposure to these three neurotoxic substances at the estimated level of intake as flavourings.
2.7. Genotoxicity
2.7.1.
In vitro
In vitro genotoxicity data have been reported for nine candidate substances. Negative results were obtained in bacterial systems (+/− metabolic activation) with six candidate substances, one saturated aliphatic acyclic secondary alcohol [FL‐no: 02.183], two saturated ketones [FL‐nos: 07.181 and 07.205], two unsaturated ketones [FL‐nos: 07.198 and 07.262] and the ester isopropyl hexadecanoate [FL‐no: 09.606]. Negative results were also obtained for the candidate substances pseudo‐ionone [FL‐no: 07.198], pentan‐3‐ol [FL‐no: 02.077] and methyl‐3‐butan‐2‐one [FL‐no: 07.178], the two‐first mentioned being tested for chromosomal aberrations in mammalian cells and the latter for induction of aneuploidy in yeast cells, respectively.
Induction of aneuploidy in yeast cells has been demonstrated for pentan‐3‐one [FL‐no: 07.084]. The effect, measured only at high concentrations, approaching cytotoxic levels, can be considered to be a threshold effect, not mediated by direct interaction with DNA. In addition, induction of aneuploidy described in the paper is strongly potentiated by ice treatments included in the experimental protocol, consistently with tubulin dissociation at low temperature in vitro; in the absence of this passage the effect is very weak. Therefore, the effect could be considered as an effect occurring only under unrealistic experimental conditions and the extrapolation of this result to the in vivo situation in humans is questionable. Furthermore, it is well recognised that the relevance of fungal systems is limited when induction of aneuploidy in mammalian systems has to be evaluated.
Pseudo‐ionone [FL‐no: 07.198] was considered with respect to genotoxicity in FGE.206 (EFSA CEF Panel, 2011) where the Panel concluded that the data available ruled out the concern for genotoxicity. Pseudo‐ionone was tested in Salmonella Typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 in the presence or absence of S9 and it is concluded that under the test conditions applied pseudo‐ionone is not mutagenic in bacteria. Pseudo‐ionone was also evaluated in an in vitro micronucleus assay in human peripheral blood lymphocytes for its ability to induce chromosomal damage or aneuploidy in the presence and absence of rat S9 fraction as an in vitro metabolising system. Under the conditions of this study, pseudo‐ionone was not clastogenic and/or aneugenic in cultured human lymphocytes. As pseudo‐ionone [FL‐no: 07.198] is a representative with respect to genotoxicity evaluation for [FL‐nos: 02.145, 02.194, 02.211 and 07.204] in FGE.206 the safety concern for genotoxicity can also be ruled out for these four substances and they can be evaluated using the Procedure in the present FGE.
In vitro genotoxicity data are also available for 10 supporting substances.
No evidence of mutagenicity obtained with either bacterial or mammalian cells systems was reported for one saturated aliphatic acyclic secondary alcohol [FL‐no: 02.079], five saturated [FL‐nos: 07.002, 07.050, 07.017, 07.053 and 07.122], two unsaturated [FL‐nos: 07.015 and 07.099] aliphatic acyclic ketones and two esters of an aliphatic acyclic secondary alcohol with linear aliphatic carboxylic acids [FL‐nos: 09.003 and 09.105]. 4‐Methyl‐2‐pentanone [FL‐no: 07.017] gave negative results also when tested for chromosomal aberration activity.
Besides the negative results in in vitro bacterial point mutation tests, acetone [FL‐no: 07.050] showed no evidence of increased sister chromatid exchanges in several cytogenetic assays on different mammalian cells, as well as no induction of chromosomal aberrations in Chinese hamster ovary cells up to very high concentrations. Only one test on hamster lung fibroblasts (conducted at an unspecified acetone concentration) and an aneuploidy induction test on Saccharomyces cerevisiae (about 7% acetone) gave positive results. However, these two studies were considered not relevant on the basis of their poor quality and taking into account all the other negative genotoxicity results obtained with acetone, including results in vivo (see below).
6‐Methylhepta‐3,5‐dien‐2‐one [FL‐no: 07.099] was considered with respect to genotoxicity in FGE.206 (EFSA CEF Panel, 2011) where the Panel concluded that the data available ruled out the concern for genotoxicity. 6‐Methylhepta‐3,5‐dien‐2‐one was tested in S. Typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 in the presence or absence of S9 and it was concluded that under the test conditions applied 6‐methylhepta‐3,5‐dien‐2‐one is not mutagenic in bacteria. 6‐Methylhepta‐3,5‐dien‐2‐one was also evaluated in an in vitro micronucleus assay in human peripheral blood lymphocytes for its ability to induce chromosomal damage or aneuploidy in the presence and absence of rat S9 fraction as an in vitro metabolising system. Under the conditions of this study, 6‐methylhepta‐3,5‐dien‐2‐one was not clastogenic and/or aneugenic in cultured human lymphocytes. As 6‐methylhepta‐3,5‐dien‐2‐one [FL‐no: 07.099] is a representative with respect to genotoxicity evaluation for [FL‐nos: 02.145, 02.194, 02.211 and 07.204] in FGE.206, the safety concern for genotoxicity can also be ruled out for these four substances and they can be evaluated using the Procedure in the present FGE.
In vivo
In vivo data are available for four supporting substances: one saturated aliphatic secondary alcohol [FL‐no: 02.079] and three saturated aliphatic ketones [FL‐nos: 07.017, 07.050 and 07.053], which exhibited no genotoxic potential in the micronucleus cytogenetic assay at doses approaching the LD20 and the LD50 of the tested substances.
Candidate substances with alpha‐beta‐unsaturated carbonyl structural alert
Oct‐1‐en‐3‐one [FL‐no: 07.081] and pent‐1‐en‐3‐one [FL‐no: 07.102] were evaluated with respect to genotoxicity in FGE.205 (EFSA CEF Panel, 2012a) and FGE.205Rev1 (EFSA CEF Panel, 2016). Due to positive effects in the bacterial mutagenicity assays of the two representative substances pent‐1‐en‐3‐one [FL‐no: 07.102] and oct‐1‐en‐3‐one [FL‐no: 07.081], an in vivo Comet assay on the first site of contact (e.g. the stomach or duodenum) and on the liver was requested on the most potent substance, pent‐1‐en‐3‐one (EFSA CEF Panel, 2012a). In response to the data request in FGE.205, the industry submitted in vivo data on both pent‐1‐en‐3‐one and oct‐1‐en‐3‐one. Pent‐1‐en‐3‐one [FL‐no: 07.102] was tested for its potential to induce micronuclei in the polychromatic erythrocytes of the bone marrow of treated rats and to induce DNA damage in the liver and duodenum of the same animals (Keig‐Shevlin, 2015b,c). Oct‐1‐en‐3‐one [FL‐no: 07.081] was tested in a Comet assay for its potential to induce DNA damage in the liver of rats (Keig‐Shevlin, 2015a). Furthermore, to investigate the mechanism of action of the mutagenic activity observed in the bacterial reverse mutation tests of previous studies, a new Ames test with oct‐1‐en‐3‐one was performed with strain TA100 (Bowen, 2013). Pent‐1‐en‐3‐one [FL‐no: 07.102] tested in vivo in a combined micronucleus and comet assay did not show genotoxic effects in either the liver or duodenum of treated rats. The negative results of the bone marrow micronucleus assay are considered inconclusive because there is no evidence of bone marrow exposure to the tested substance. However, as results of the in vitro micronucleus assay were negative, no additional in vivo follow‐up studies on clastogenicity and aneugenicity were needed. The bacterial mutation assay provided for oct‐1‐en‐3‐one [FL‐no: 07.081] confirms the weak mutagenic effect in bacteria shown in previous studies, but does not clarify the mechanism of action. The liver comet assay is considered of limited validity due to low values of mean tail intensity and tail moment. However, based on the data available on the most potent of the two representative substances for the other substances of FGE.205, pent‐1‐en‐3‐one [FL‐no: 07.102], the Panel concluded that there is no concern for genotoxicity. As pent‐1‐en‐3‐one [FL‐no: 07.102] is a representative with respect to genotoxicity evaluation for [FL‐nos: 02.131, 02.187, 07.161 and 07.210] in FGE.205, the safety concern for genotoxicity can also be ruled out for these four substances and they will be evaluated using the Procedure in the present FGE.
Overall conclusion on genotoxicity
From the available in vitro and in vivo tests on candidate and supporting substances and on the basis of the results for substances evaluated in FGE.205, FGE.206 and FGE.205rev1 (EFSA CEF Panel, 2011, 2012a, 2016), no concern is raised with respect to genotoxicity. Consequently the candidate substances can be evaluated using the Procedure.
The genotoxicity data are summarised in Appendix E, Tables E.4–E.7.
Table E.4.
Genotoxicity (in vitro)
| Chemical name [FL‐no] | Test system | Test object | Concentration | Result | Reference | Comments |
|---|---|---|---|---|---|---|
| (Acetone [07.050]) | Rec assay | B. subtilis | NR | Negativea | Kawachi et al. (1980) | f |
| Rec assay | B. subtilis | NR | Negative | Ishizaki et al. (1979) | f | |
| Ames test | S. Typhimurium TA100 | 0.1–1,000 μg/plate | Negative | Rapson et al. (1980) | f | |
| Ames test | S. Typhimurium TA98, TA100, TA1535, TA1537 | 174 μg/plate | Negativea | Florin et al. (1980) | f | |
| Ames test | S. Typhimurium TA98, TA100 | NR | Negativea | Kawachi et al. (1980) | f | |
| Ames testb | S. Typhimurium TA98, TA100 | 30 μL/plate | Negativec | Yamaguchi (1985) | f | |
| Ames test | S. Typhimurium TA97, TA98, TA100, TA1535, TA1537 | Up to 10,000 μg/plate | Negativea | McCann et al. (1975) | f | |
| Ames testb | S. Typhimurium TA97, TA98, TA100, TA1535, TA1537 | Up to 10,000 μg/plate | Negativea | Zeiger et al. (1992) | f | |
| Ames test | S. Typhimurium TA100 | 500 μg/plate | Negativea | Yamaguchi (1982) | f | |
| Ames test | S. Typhimurium TA97, TA98, TA100 | 20–40 μg | Negativea | Azizan and Blevins (1995) | f | |
| Sister chromatid exchange | Human embryo fibroblasts | NR | Negativec | Kawachi et al. (1980) | f | |
| Sister chromatid exchange | Hamster lung fibroblasts | NR | Negativec | Kawachi et al. (1980) | f | |
| Sister chromatid exchange | Chinese hamster ovary cells | Up to 10 μg/mL | Negative | Sasaki et al. (1980) | f | |
| Sister chromatid exchange | Chinese hamster ovary cells | Up to 5,020 μg/mL | Negativea | Loveday et al. (1990) | f | |
| Sister chromatid exchange | Diploid human fibroblasts | 5 μg/mL | Negative | Sasaki et al. (1980) | f | |
| Sister chromatid exchange | Human lymphocytes | 395 μg/mL | Negative | Norppa et al. (1983) | f | |
| Sister chromatid exchange | Human lymphocytes | 0.1–1 mM | Negative | Zarani et al. (1999) | f | |
| Chromosomal aberrations | Chinese hamster ovary cells | Up to 5,020 μg/mL | Negativea | Loveday et al. (1990) | f | |
| Chromosomal aberrations | Hamster lung fibroblasts | NR | Positivec | Kawachi et al. (1980) | f | |
| Aneuploidy induction | S. cerevisiae | 6.98–7.83% | Positivec | Zimmermann et al. (1985) | i | |
| (Isopropyl alcohol [02.079]) | Ames test | S. Typhimurium TA98, TA100, TA1535, TA1537 | 174 μg/plate | Negativea | Florin et al. (1980) | f |
| Ames testb | S. Typhimurium TA98, TA100, TA1535, TA1537, E. coli WP2uvrA | 5–5,000 μg/plate | Negativea | Shimizu et al. (1985) | f | |
| Ames testb | S. Typhimurium TA97, TA98, TA100, TA102, TA104, TA1535, TA1537 | Up to 10 mg/plate5 | Negativea | Zeiger et al. (1992) | f | |
| Forward mutation | Chinese hamster ovary cellsd | 0.5–5.0 mg/mL | Negativea | CMA (1990) | f | |
| Forward mutation | Chinese hamster ovary cellsd | 0.5–5.0 mg/mL | Negativea | Kapp et al. (1993) | f | |
| (2‐Butanone [07.053]) | Ames test | S. Typhimurium TA98, TA100, TA1535, TA1537, TA1538 | 10,000 μg/plate | Negativea | Douglas et al. (1980) | f |
| Ames test | S. Typhimurium TA102, TA104 | 1 mg/plate | Negative | Marnett et al. (1985) | f | |
| (2‐Butanone [07.053]) continued | Ames testb | S. Typhimurium TA98, TA100, TA1535, TA1537, TA1538 | 5–5,000 μg/plate | Negativea | Shimizu et al. (1985) | f |
| Ames test | S. Typhimurium TA98, TA100, TA1535, TA1537, TA1538 | 0.04–26 μg/plate | Negativea | O'Donoghue et al. (1988) | f | |
| Ames testb | S. Typhimurium TA97, TA98, TA100, TA104, TA1535, TA1537 | Up to 10,000 μg/plate | Negativea 1 | Zeiger et al. (1992) | f | |
| Ames test | S. Typhimurium TA102 | 5,000 μg/plate | Negativec | Müller et al. (1993) | f | |
| Ames test | S. Typhimurium TA98, TA100, TA1535, TA1537, TA1538, E. coli WP2uvrA | 4,000 μg/plate | Negative | Brooks et al. (1988) | f | |
| Gene conversion | S. cerevisiae | 5 mg/mL | Negativea | Brooks et al. (1988) | f | |
| Forward Mutation | L5178Y/TL+/− mouse lymphoma cells | 0.67–12 μg/mL | Negativea | O'Donoghue et al. (1988) | f | |
| Unscheduled DNA synthesis | Human lymphocytes | 0.72 mg/mL | Negativea | Perocco et al. (1983) | f | |
| Unscheduled DNA synthesis | Rat hepatocytes | 7.2–360 mg/mL | Negative | O'Donoghue et al. (1988) | f | |
| Chromosomal aberrations | Rat hepatocytes | 1,000 μg/mL | Negative | Brooks et al. (1988) | f | |
| Chromosomal aberrations | Chinese hamster ovary cells | 1,000 μg/mL | Negativea | Brooks et al. (1988) | f | |
| Cell transformation assaya | BALB/3T3 cells (clone A31‐1) | 6–18 μL/mL | Negative | O'Donoghue et al. (1988) | ||
| Aneuploidy induction | S. cerevisiae | 3.38% | Positivec | Zimmermann et al. (1985) | i | |
| Pentan‐3‐one [07.084] | Aneuploidy induction | S. cerevisiae | 1.48% | Positivec | Zimmermann et al. (1985) | i |
| Pentan‐3‐ol [02.077] | Chromosomal aberrations | Chinese hamster ovary cells | 0.5–10% | Negativea | Abbondandolo et al. (1980) | |
| Forward mutation | S. pombe | 0.5–10% | Negativea | Abbondandolo et al. (1980) | ||
| (2‐Heptanone [07.002]) | Unscheduled DNA synthesis | Rat hepatocytes | 1,000 ppm | Negative | Barber et al. (1999) | |
| Methyl‐3‐butan‐2‐one [07.178] | Aneuploidy induction | S. cerevisiae | 1.23–1.36% | Negativec | Zimmermann et al. (1985) | i |
| Aneuploidy induction | S. cerevisiae | 0.84–1.23% | Negativec | Zimmermann et al. (1985) | i | |
| (4‐Methyl‐2‐pentanone [07.017]) | Ames test | S. Typhimurium TA98, TA100, TA1535, TA1537, TA1538 | 0.03–3 mg/plate | Negativea | O'Donoghue et al. (1988) | f |
| Ames testb | S. Typhimurium TA97, TA98, TA100, TA1535 | Up to 6,667 μg/plate | Negativea | Zeiger et al. (1992) | f | |
| Ames test | E. coli WP2uvrA | 8,000 μg/plate | Negativec | Brooks et al. (1988) | f | |
| Gene conversion | S. cerevisiae | 5 mg/mL | Negativea | Brooks et al. (1988) | f | |
| Forward mutation | L5178Y/TL+/– mouse lymphoma cells | 0.26–4.2 μg/mL | Negativea | O'Donoghue et al. (1988) | f | |
| Unscheduled DNA synthesis | Rat hepatocytes | 8–80 μg/mL | Negative | O'Donoghue et al. (1988) | f | |
| Chromosomal aberrations | Rat hepatocytes | 1,000 μg/mL | Negative | Brooks et al. (1988) | f | |
| Cell transformation assaya | BALB/3T3 cells (clone A31‐1) | 1–7 μL/mL | Negative | O'Donoghue et al. (1988) | ||
| Chromosomal aberrations | Chinese hamster ovary cells | 1,000 μg/mL | Negativea | Brooks et al. (1988) | f | |
| Methyl‐4‐pentan‐2‐ol [02.183] | Ames testb | S. Typhimurium TA98, TA100, TA1535, TA1537, TA1538, E. coli WP2uvrA | 5,000 μg | Negativea | Shimizu et al. (1985) | |
| Methyl‐6‐heptan‐2‐one [07.181] | Ames test | S. Typhimurium TA98, TA100, TA1535, TA1537 | 5,000 μg/plate | Negativea | BASF (1989a) | |
| (2,6‐Dimethyl‐4‐heptanone [07.122]) | Ames testb | S. Typhimurium TA98, TA100, TA1535, TA1537 | 1–333 μg/plate | Negativea | Mortelmans et al. (1986) | f |
| Trimethyl‐6,10,14‐pentadecan‐2‐one [07.205] | Ames test | S. Typhimurium TA98, TA100, TA1535, TA1537 | 5,000 μg/plate | Negativea | BASF (1989b) | |
| (6‐Methyl‐5‐hepten‐2‐one [07.015]) | Reverse mutation | S. Typhimurium TA98, TA100, TA1535, TA1537 | 380 μg/plate | Negativea | (Florin et al. 1980) | g |
| (Isopropyl acetate [09.003]) | Ames testb | S. Typhimurium TA97, TA98, TA100, TA1537, TA1538 | Up to 10 mg/plate | Negativea | Zeiger et al. (1992) | f |
| (Isopropyl myristate [09.105]) | Ames teste | S. Typhimurium TA98, TA100, TA1535, TA1537, TA1538 | 50 μg/plate | Negativea | Blevins and Taylor (1982) | f |
| Isopropyl hexadecanoate [09.606] | Ames teste | S. Typhimurium TA98, TA100, TA1535, TA1537, TA1538 | 50 μg/plate | Negativea | Blevins and Taylor (1982) | |
| 9‐Decen‐2‐one [07.262] | Ames testh | S. Typhimurium TA98, TA100, TA1535, TA1537 | Up to 5 μL/plate | Negativea | Flavour Industry (2009) | |
| Ames testh | E. coli WP2 (pKM 101) | Up to 5 μL/plate | Negativea | Flavour Industry (2009) | ||
| (6‐Methylhepta‐3,5‐dien‐2‐one [07.099]) | Reverse mutation | S. Typhimurium TA98, TA100, TA1535, TA1537 | 370 μg/plate | Negativea | Florin et al. (1980) | |
| Reverse Mutation | S. Typhimurium TA98, TA100, TA1535, TA1537, TA 102 | 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate | Negativea | Williams (2009) | Toxicity observed in all strains at 2,000 μg/plate or greater in the absence of S9 and at 800 μg/plate in the presence of S9. Study design complied with current recommendations. Acceptable top concentration was achieved | |
| Micronucleus induction | Human peripheral blood lymphocytes | 225, 325 and 450 μg/mLk 225, 300 and 350 μg/mLl | Negative | Whitwell (2010) | Complies with draft OECD guideline 487. Acceptable levels of cytotoxicity achieved at the top concentrations used in all parts of the study | |
| Pseudo‐ionone [07.198] | Ames test | S. Typhimurium TA98, TA100, TA1535, TA1537 | 20.48, 51.2, 128, 320, 800, 2000 and 5,000 μg/plate j | Negativea | Florin et al. (1980) | |
| Reverse Mutation | S. Typhimurium TA98, TA100, TA1535, TA1537, TA 102 | 0.128, 0.64, 3.2, 16, 80, 400 and 2,000 μg/plate | Negativea | (Beevers, 2009) | Toxicity was observed in all strains at 400 μg/plate and greater in the presence and absence of S9 in this experiment | |
| 0, 12.5, 25, 50, 100, 200 and 400 μg/platej | Negativea | Precipitation was observed in the 400 μg/plate concentration in the presence and absence of S9 in this experiment. Study design complies with current recommendations. Acceptable top concentrations were achieved | ||||
| Micronucleus induction | Human peripheral blood lymphocytes | 30, 50 and 60 μg/mLk; 100, 110 and 120 μg/mLl | Negative | Lloyd (2010) | Complies with draft OECD guideline 487. Acceptable levels of cytotoxicity achieved at the top concentrations used in all parts of the study | |
| Micronucleus induction | Human peripheral blood lymphocytes | 10, 15 and 20 μg/mLm | Negative | Lloyd (2010) | Complies with draft OECD guideline 487. Acceptable levels of cytotoxicity achieved at the top concentrations used in all parts of the study |
FL‐no: FLAVIS number.
Assay performed with and without metabolic activation.
Modified Ames (pre‐incubation) protocol.
Assay performed with S9 metabolic activation.
Assay performed without S9 metabolic activation.
Maximum non‐toxic dose.
HGPRT locus.
Spot test.
Summarised by JECFA, 51st meeting (JECFA, 1999a).
Summarised by JECFA 59th meeting (JECFA, 2003).
Direct incorporation method.
Unusual experimental protocol for detection of aneuploidy, which can be considered a threshold effect not mediated by a direct interaction with DNA. Positive results were obtained at concentrations approaching cytotoxic levels and are very likely due to the presence of technical artefacts (low temperature treatment inducing tubulin dissociation). Indeed, absence of effect was recorded when the ice treatment was skipped. – The limited relevance of fungal systems together with the uncertain quality of these results make questionable their extrapolation to the in vivo situation in humans.
Assay modified with pre‐incubation in the presence of S9.
Without metabolic activation, 3 h treatment + 21 h recovery.
With metabolic activation, 3 h treatment + 21 h recovery.
Without metabolic activation, 24 h + 0 h recovery.
Table E.7.
Summary of in vivo genotoxicity data considered by the Panel in FGE.205Rev1
| Chemical name FL‐no | Test system in vivo | Test object | Route | Dose | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
|
Pent‐1‐en‐3‐one [07.102] |
Micronucleus Assay | Han Wistar Rat; M | Gavage | 0, 10, 20 and 40 mg/kg bw per day | Negative | Keig‐Shevlin (2015b,c) | |
| Comet assay | Han Wistar Rat; M | Gavage | Negativea , b | ||||
|
Oct‐1‐en‐3‐one [07.081] |
Comet assay | Han Wistar Rat; M | Gavage | 0, 45, 90 and 180 mg/kg bw per day | Negativea | Keig‐Shevlin (2015a) |
FL‐no: FLAVIS number; bw: body weight.
Scored in liver cells.
Scored in duodenum cells.
2.8. Toxicity
2.8.1. Acute toxicity
Data are available for 12 candidate substances under consideration and for 23 supporting substances. Most of the candidate and supporting substances have rat and/or mouse oral LD50 values exceeding 2,000 mg/kg bw indicating that their oral acute toxicity is low.
The acute toxicity data are summarised in Appendix E, Table E.1.
Table E.1.
Acute toxicity
| Chemical name [FL‐no] | Species | Sex | LD50 (mg/kg bw) | Reference |
|---|---|---|---|---|
| (Acetone [07.050]) | Rat | M | 8,452 | Smyth et al. (1970) |
| Rat | NR | 8,930 | Smyth et al. (1969) | |
| Rat | NR | 9,750 | FDA (1993) | |
| Rat | NR | 6,800 | Kimura et al. (1971) | |
| Rat | NR | 3,465 | Kohli et al. (1967) | |
| Mouse | M | 5,250 | Tanii et al. (1986) | |
| Rabbit | NR | 5,300 | Krasavage et al. (1982) | |
| (Isopropyl alcohol [02.079]) | Rat | NR | 5,840 | Smyth and Carpenter (1948) |
| Rat | NR | 5,280 | Lehman and Chase (1944) | |
| Rat | NR | 5,300 | Kimura et al. (1971) | |
| Rat | NR | 5,330 | FDA (1993) | |
| Mouse | NR | 5,070 | FDA (1993) | |
| Rabbit | NR | 5,040 | Lehman and Chase (1944) | |
| Rabbit | NR | 7,990 | Munch (1972) | |
| Dog | NR | 4,830 | Lehman and Chase (1944) | |
| (2‐Butanone [07.053]) | Rat | M | 5,490 | Smyth et al. (1962) |
| Rat | NR | 2,730 | Kimura et al. (1971) | |
| Rat | NR | 3,980 | Union Carbide Corp. (1956) | |
| Rat | F | 5,525 | Pozzani et al. (1959) | |
| Mouse | M | 3,137 | Zakhari et al. (1977) | |
| Mouse | M | 4,050 | Tanii et al. (1986) | |
| (2‐Pentanone [07.054]) | Rat | M | 3,730 | Smyth et al. (1962) |
| Mouse | M | 2,205 | Tanii et al. (1986) | |
| (2‐Pentanol [02.088]) | Rabbit | NR | 2,820 | Munch (1972) |
| Pentan‐3‐one [07.084] | Rat | NR | 2,900 | BASF (1969) |
| Rat | NR | 2,140 | Panson and Winek (1980) | |
| Rat | NR | 2,140 | Eder et al. (1982) | |
| Rat | NR | 2,140 | Kennedy and Graepel (1991) | |
| Rat | NR | 3,100 | Ibatullina and Larionova (1997) | |
| Pentan‐3‐ol [02.077] | Rat | NR | 1,870 | Eder et al. (1982) |
| (3‐Hexanone [07.096]) | Rat | NR | 2,727 | Carpenter et al. (1974) |
| (2‐Heptanone [07.002]) | Rat | M | 1,670 | Smyth et al. (1962) |
| Mouse | M | 2,407 | Tanii et al. (1986) | |
| Mouse | NR | 1,088 | Schafer and Bowles (1985) | |
| Mouse | NR | 730 | Srepel and Akacic (1962) | |
| (2‐Heptanol [02.045]) | Rat | M, F | 2,580 | Eder et al. (1982) |
| (3‐Heptanone [07.003]) | Rat | NR | 2,760 | Smyth et al. (1949) |
| (3‐Heptanol [02.044]) | Rat | NR | 1,870 | Smyth et al. (1951) |
| (4‐Heptanone [07.058]) | Rat | NR | 3,049 | Carpenter et al. (1974) |
| (2‐Octanone [07.019]) | Rat | NR | > 5,000 | Katz et al. (1980) |
| Mouse | M | 3,823 | Tanii et al. (1986) | |
| Mouse | NR | 3,870 | Tanii et al. (1986) | |
| (2‐Octanol [02.022]) | Rat | NR | 3,200 | Patty et al. (1935) |
| (3‐Octanone [07.062]) | Rat | NR | 5,000 | Shelanski and Moldovan (1973) |
| (2‐Nonanone [07.020]) | Mouse | M | 7,992 | Tanii et al. (1986) |
| Decan‐2‐one [07.150] | Mouse | M | 7,936 | Tanii et al. (1986) |
| (2‐Undecanone [07.016]) | Mouse | NR | 950 | Schafer and Bowles (1985) |
| Mouse | M | 5,460 | Tanii et al. (1986) | |
| Methyl‐3‐butan‐2‐one [07.178] | Mouse | M | 2,572 | Tanii et al. (1986) |
| Rat | NR | 148 | Kennedy and Graepel (1991) | |
| (4‐Methyl‐2‐pentanone [07.017]) | Rat | NR | 2,080 | Smyth et al. (1951) |
| Mouse | M | 2,670 | Tanii et al. (1986) | |
| Mouse | NR | 1,200 | McOmie and Anderson (1949) | |
| Methyl‐4‐pentan‐2‐ol [02.183] | Rat | NR | 2,590 | Smyth et al. (1951) |
| Mouse | NR | 1,500 | McOmie and Anderson (1949) | |
| Methyl‐6‐heptan‐2‐one [07.181] | Rat | NR | 6,700 | BASF (1975) |
| Methyl‐5‐heptan‐3‐one [07.182] | Rat | NR | 3,500 | Kennedy and Graepel (1991) |
| (2,6‐Dimethyl‐4‐heptanone [07.122]) | Rat | NR | 5,750 | Smyth et al. (1949) |
| Mouse | NR | 2,800 | McOmie and Anderson (1949) | |
| Mouse | NR | 1,416 | RTECS (1975) | |
| Trimethyl‐6,10,14‐pentadecan‐2‐one [07.205] | Rat | NR | > 2,000 | BASF (1988) |
| (6‐Methyl‐5‐hepten‐2‐one [07.015]) | Mouse | M, F | 3,609 | Colaianni (1967) |
| Rat | M, F | 4,100 | Keating (1972) | |
| (3,4,5,6‐Tetra‐hydropseudoionone [07.069]) | Mouse | M, F | 5,200 | Moreno (1982) |
| Rat | M, F | > 5,000 | Moreno (1977) | |
| (6,10‐Dimethyl‐5,9‐undecadien‐2‐one [07.123]) | Mouse | M, F | 8,650 | Moreno (1976) |
| Rat | M, F | > 6,800 | Hofmann (1978) | |
| (2,6,10‐Trimethyl‐2,6,10‐pentadecatrien‐14‐one [07.114]) | Rat | M, F | > 5,000 | deGroot et al. (1974) |
| (Isopropyl formate [09.165]) | Rat | NR | 4,300 | FDA (1993) |
| Rabbit | NR | 2,500 | FDA (1993) | |
| Guinea Pig | NR | 2,700 | FDA (1993) | |
| Chicken | NR | 2,100 | FDA (1993) | |
| (Isopropyl acetate [09.003]) | Rat | M, F | 6,750 | Eder et al. (1982) |
| Rat | NR | 3,000 | FDA (1993) | |
| Rabbit | NR | 6,945 | Munch (1972) | |
| Isopropyl hexadecanoate [09.606] | Rat | M, F | > 40,000 | Food and Drug Research Laboratories, Inc. (1976) |
| Rat | M, F | > 8,000 | Kolmar Research Center (1972) | |
| Rat | M, F | > 64,000 | Bio‐Toxicology Laboratories (1982) | |
| Rat | NR | > 5,000 | Moreno (1978) | |
| sec‐Butyl formate [09.328] | Rat | NR | 11,300 | Union Carbide Corp. (1980) |
| 9‐Decen‐2‐one [07.262] | Rat | F | 2,500 | Flavour Industry (2009) |
| (6‐Methylhepta‐3,5‐dien‐2‐one [07.099]) | Mouse | M, F | 3,200 | Colaianni (1967) |
| Pseudo‐ionone [07.198] | Rat | NR | > 5,000 | Moreno (1976) |
FL‐no: FLAVIS number; LD50: lethal dose, 50%; bw: body weight.
NR: Not Reported; M = Male; F = Female.
2.8.2. Short‐term and subchronic toxicity
Data on oral subchronic toxicity are available for three candidate substances, pentan‐3‐one [FL‐no: 07.084], 5‐methylheptan‐3‐one [FL‐no: 07.182] and 9‐decen‐2‐one [FL‐no: 07.262] with identification of a NOAEL. Data on subacute and subchronic oral toxicity are also available for ten supporting substances, one saturated aliphatic secondary alcohol [FL‐no: 02.079], seven saturated [FL‐nos: 07.002, 07.003, 07.017, 07.020, 07.050, 07.058, 07.122] and two unsaturated [FL‐nos: 07.100 and 07.114] aliphatic ketones evaluated by JEFCA (JECFA, 1999a, 2003).
During the application of the Procedure (Appendix A), the following study on 5‐methylheptan‐3‐one [FL‐no: 07.182], which possesses structural alerts for neurotoxicity, has been used to calculate the NOAEL: 5‐Methylheptan‐3‐one [FL‐no: 07.182] (purity 98.9%) dissolved in distilled water was administered by gavage to groups of five adult male Sprague–Dawley rats at dose levels 0, 82, 410 and 820 mg/kg bw per day, five days/week for 13 weeks. In the high‐dose group, clinical signs, including depression of activity, gait disturbances, reductions in food consumption and body weight gain were observed; moreover, results of the Functional Observational Battery (FOB) indicated peripheral neuropathy. Similar clinical signs and functional deficits were observed less frequently and with reduced severity in the mid‐dose group. No functional deficits were observed in the low‐dose group animals. Microscopic histopathological examinations of the sciatic and tibial nerves from high‐dose animals revealed lesions typical of the ‘giant’ axonal neuropathy produced by gamma‐diketones. Changes observed in the mid‐dose group animals reflected the occurrence of reparative processes in the nerves. Nerves from the low‐dose group animals did not show any evidence of pathology attributable to treatment. Based on behavioural effects and microscopic changes occurring at 410 and 820 mg/kg bw per day, the NOAEL for 5‐ methylheptan‐3‐one induced neurotoxicity was 82 mg/kg bw per day (IBM Corp., 1989).
The short‐term and subchronic toxicity data are summarised in Appendix E, Table E.2.
Table E.2.
Subacute and subchronic toxicity studies
| Chemical name [FL‐no] | Species/sex No/group | Route | Dose levels (mg/kg per day) | Duration | NOAEL (mg/kg per day) | Reference | Comments |
|---|---|---|---|---|---|---|---|
| (Acetone [07.050]) |
Rat/M,F 10 |
Drinking water | 0, 250, 500, 1,000, 2,000, 5,000 | 13 weeks | 1,000a | Diet (1991) |
NTP study |
|
Mouse/M,F 10 |
Drinking water |
0, 312, 625, 1,250, 2,500, 5,000 (M) 0, 625, 1,250, 2,500, 5,000, 12,500 (F) |
13 weeks | 2,500a | Dietz (1991) |
NTP study |
|
|
Rat/M,F 30 |
Gavage | 0, 100, 500, 2,500 | 90 days | 100 | Sonawane et al. (1986) |
Meeting abstract |
|
|
Rat/NR 3 |
Drinking water | 1,000 | 4 weeks | 1,000a , b | Spencer et al. (1978) |
Examinations were limited to specific neurotoxic effects. No other parameter was monitored |
|
| (Isopropyl alcohol [02.079]) |
Human/M 8 |
Oral | 0, 2.6, 6.4 | 6 weeks | 6.4b | Wills et al. (1969) |
Paper published in a peer‐reviewed journal |
|
Rat/M 22 |
Drinking water | 0, 870, 1,280, 1,680, 2,520 | 12 weeks | 870 | Pilegaard and Ladefoged (1993) |
Good quality study |
|
| Pentan‐3‐one [07.084] |
Rat/F 5 |
Drinking water | 0, 1,860 | 120 days | Not detected (< 1,860) | Union Carbide Corp. (1977) | Good quality unpublished report. Focused on neurotoxic effect |
| (2‐Heptanone [07.002]) |
Rat/M,F 15 |
Gavage (dissolved in corn oil) | 0, 20, 100, 500 | 13 weeks | 20 | Gaunt et al. (1972) |
Good quality study – peer‐reviewed journal |
|
Rat/NR 5 |
Drinking Water | 0, 500 | 12 weeks | 500a , b | Spencer et al. (1978) |
Good quality study – peer‐reviewed journal |
|
| (3‐Heptanone [07.003]) |
Rat/M 2 |
Gavage | 0, 250, 500, 1,000, 2,000, 4,000 | 14 weeks | 1,000 | O'Donoghue et al. (1984) |
Good quality study – peer‐reviewed journal |
|
Rat/F NR |
Drinking Water | 1,000 | 120 days | 1,000a | Homan and Maronpot (1978) |
Meeting abstract |
|
|
Rat/F 5 |
Drinking water | 0, 27 | 120 days | 27b | Union Carbide Corp. (1977) | Good quality unpublished report. Focused on neurotoxic effect | |
| (4‐Heptanone [07.058]) |
Rat/M 8 |
Gavage | 0, 1,000 | 90 days |
Not detected (< 1,000) |
O'Donoghue and Krasavage (1980) |
Good quality unpublished report |
|
Rat/M 3 |
Gavage (undiluted) | 0, 1,000, 2,000, 4,000 | 3 weeks |
Not detected (< 1,000) |
Krasavage and O'Donoghue (1979) |
Good quality unpublished report |
|
| (2‐Nonanone [07.020]) |
Rat/M 3 |
Gavage (undiluted) | 0, 1,000, 2,000, 4,000 | 3 weeks |
Not detected (< 1,000) |
Krasavage and O'Donoghue (1979) |
Good quality unpublished report |
|
Rat/M 8 |
Gavage | 0, 2,000 | 90 days |
Not detected (< 2,000) |
O'Donoghue and Krasavage (1980) |
Good quality unpublished report |
|
| (4‐Methyl‐2‐pentanone [07.017]) |
Rat/M,F 5 |
Drinking water | 0, 1,040 | 120 days |
Not detected (< 1,040) |
Union Carbide Corp. (1977) | Good quality unpublished report. Focused on neurotoxic effect |
|
Rat/F NR |
Drinking water | 1,000 | 120 days | 1,000b | Homan and Maronpot (1978) |
Meeting abstract |
|
| Methyl‐5‐heptan‐3‐one [07.182] |
Rat/M 5 |
Gavage (in distilled water) | 82, 410, 820 |
13 weeks (5 days/week) |
82 | IBM Corp. (1989) | Good quality unpublished report submitted to EPA |
| (2,6‐Dimethyl‐4‐heptanone [07.122]) |
Rat/M 8 |
Gavage | 0, 2,000 | 90 days |
Not detected (< 2,000) |
O'Donoghue and Krasavage (1980) |
Good quality unpublished report |
| (5‐Methyl‐5‐hexen‐2‐one [07.100]) |
Rat/M,F 5 |
Diet | 0, 10 | 14 days | 10b | Gill and Van Miller (1987) |
GLP study – unpublished report |
| (2,6,10‐Trimethyl‐2,6,10‐pentadecatrien‐14‐one [07.114]) |
Rat/M,F 5 |
Oral (gavage in maize oil) | 0, 0.35, 3.5 | 14 days | 3.5 | deGroot et al. (1974) |
TNO Unpublished Report |
| 9‐Decen‐2‐one [07.262] |
Rat/M,F 5 |
Oral (gavage in corn oil) | 0, 250, 500, 1,000 | 28 days | 1,000e | Flavour Industry (2009) | Good study, OECD 407 |
FL‐no: FLAVIS number; NOAEL: no observed adverse effect level.
NR = sex not reported; M = Male; F = Female.
Concentrations converted to mg/kg bw per day using conversion table for test chemical treatment doses used in PAFA (FDA, 1993).
This study was performed at a single dose level that produced no adverse effects.
Summarised by JECFA, 51st meeting (JECFA, 1999a).
Summarised by JECFA 59th meeting (JECFA, 2003).
The highest dose tested.
2.8.3. Reproductive and developmental toxicity
Data on reproductive toxicity are available for pentan‐3‐one [FL‐no: 07.084] and data on developmental toxicity are available for pseudo‐ionone [FL‐no: 07.198]. For one supporting substance, isopropyl alcohol [FL‐no: 02.079], data are available on both developmental and reproductive toxicity. With a NOAEL of 50 mg/kg bw per day for intraperitoneal administration in mice for [FL‐no: 07.084] and of 960 mg/kg bw per day for oral administration of [FL‐no: 07.198], it was concluded that the developmental/reproductive toxicity was low after oral exposure.
The developmental/reproductive toxicity data are summarised in Appendix E, Table E.3.
Table E.3.
Developmental and reproductive toxicity studies
| Chemical name [FL‐no] | Study type/duration | Species/sex No/group | Route | NOAEL mg/kg per day including information on possible maternal toxicity | Reference | Comments |
|---|---|---|---|---|---|---|
| (Isopropyl alcohol [02.079]) | Reproductive toxicity: two generations with 10 weeks of dosing prior to mating |
Rat/M, F 4/60 |
Gavage | 500 | Bevan et al. (1995) |
EPA Guideline compliance |
| Developmental toxicity: gestation days 6–15 |
Rat/F 4/25 |
Gavage |
400 (maternal) 400 (fetal) |
Tyl et al. (1994) |
EPA Guideline compliance |
|
| Developmental toxicity: gestation days 6–18 |
Rabbit/F 4/15 |
Gavage |
240 (maternal) 480 (fetal) |
Tyl et al. (1994) |
EPA Guideline compliance |
|
| Pentan‐3‐one [07.084] | Fertility screen: 28 daily doses with mating starting on day 10 |
Mouse/F 2/8 |
IP | 50 | Carlson et al. (1975) | Few details given in the paper |
| Pseudo‐ionone [07.198] | Developmental toxicity: gestation days 8 |
Hamster/F 3/20 (control) and 7 or 10 |
Oral | 960 | Willhite (1986) |
FL‐no: FLAVIS number; NOAEL: no observed adverse effect level.
M = Male; F = Female.
Summarised by JECFA, 51st meeting (JECFA, 1999a).
2.8.4. Other studies
Pseudo‐ionone [FL‐no: 07.198] has been subjected to investigations concerning its potential as a dermal sensitiser as follows:
A guinea pig study (Csato and Chubb, 1996) performed as a GLP OECD 406 maximisation test. There were some problems with reading the result after challenge because of intense red‐brown skin staining. Therefore a rechallenge was performed 7 days later, when skin staining was much reduced and did not prevent assessment of the skin reaction. Test agent concentrations were 3.125% and 1.563% in water, scoring was performed after 24 and 48 h. None of the animals in the control (n = 10) or test (n = 20) groups showed a reaction. Based on this guinea pig maximisation test performed under GLP conditions according to OECD guidelines, pseudo‐ionone is not a dermal sensitiser. However, the problems with skin staining and delayed challenge possibly may bring in some uncertainty (contribution toward false negative results).
Four maximisation test series with pseudo‐ionone were carried out on a total of 108 human volunteers by Kligman (1976) [unpublished] and Epstein (1978) [unpublished]. Test concentration was 8% in petrolatum. The outcome was ‘2/25 (Kligman, 1976), 4/25 (Epstein, 1978), 2/25 (Kligman, 1976), and 1/33 (Epstein, 1978) sensitisation reactions’, as reported by Ford et al. (1988). Thus, there were altogether nine positive out of 108 subjects (8.3%). No further details are given by Ford and the original reports never were published. The fact that pseudo‐ionone is an irritant still may bring in some uncertainty (contribution towards false positive results).
Based on the human studies, there is evidence that pseudo‐ionone may be a weak dermal sensitiser. In accordance with this and as based on the report by Ford et al. (Ford et al., 1988), both the International Fragrance Association (IFRA, 2002) and subsequently the European Union Scientific Committee on Cosmetic Products and Non‐Food Products (SCCNFP, 2000) recommended a ban on the use of pseudo‐ionone as a fragrance ingredient but tolerated it as an impurity at ≤ 2% in various ionones.
Considering that allergic contact sensitisation in the mouth to components in ingested food is extremely rare (EFSA CEF Panel, 2012b), that worsening of skin manifestations of contact dermatitis after ingestion of foods with relatively high levels of the allergen appears to be an uncommon occurrence, and that contact allergic manifestations in the gut although claimed in rare cases have not been well described, it is unlikely that pseudo‐ionone used as a flavouring substance will cause allergic reactions.
3. Conclusions
Following a request from the European Commission, the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) was asked to deliver a scientific opinion on the implications for human health of chemically defined flavouring substances used in or on foodstuffs in the Member States. In particular, the Panel was requested to evaluate 53 flavouring substances allocated to the FGE.07Rev5, using the Procedure as referred to in the Commission Regulation (EC) No 1565/20003. These flavouring substances are listed in the Union List, which was adopted by Commission Regulation (EU) No 872/20122 and its consecutive amendments.
The present Revision of FGE.07, FGE.07Rev5, includes the assessment of four additional candidate substances [FL‐nos: 02.131, 02.187, 07.161 and 07.210]. These substances possess an α,β‐unsaturated structure, which is considered a structural alert for genotoxicity. They have been evaluated by EFSA in FGE.205Rev1, and the genotoxicity concern could be ruled out. The Panel concluded that the substances with [FL‐nos: 02.131, 02.187, 07.161 and 07.210] can be evaluated through the Procedure.
The 53 candidate substances are saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched‐chain saturated carboxylic acids from chemical group 5.
Twenty‐seven candidate substances possess one chiral centre [FL‐nos: 02.124, 02.131, 02.142, 02.145, 02.148, 02.177, 02.183, 02.187, 02.190, 02.194, 02.211, 02.255, 07.157, 07.182, 07.185, 07.239, 09.304, 09.323, 09.325, 09.328, 09.332, 09.386, 09.388, 09.391, 09.676, 09.880 and 09.926], and two of the candidate substances possess two chiral centres [FL‐nos: 02.182 and 07.205].
Due to the presence and the position of double bonds, 10 candidate substances can exist as geometrical isomers [FL‐nos: 02.145, 02.194, 02.211, 02.255, 07.156, 07.198, 07.236, 07.239, 09.386, and 09.880].
Twenty‐eight candidate substances belong to structural class I, and 25 candidate substances belong to structural class II.
Forty‐five of the flavouring substances in the present group of 53 flavouring substances have been reported to occur naturally in a wide range of food items.
According to the default MSDI approach, 53 candidate substances have European daily per capita intakes ranging from 0.0012 to 73 μg, which are below the threshold of concern for structural class I and class II substances (1,800 and 540 μg/person per day, respectively). On the basis of the reported annual production in Europe (MSDI approach), the combined intakes of the 28 of the candidate substances belonging to structural class I and of the 25 candidate substances belonging to structural class II would result in total intakes of 6 and 78 μg/capita per day, respectively.
These values are lower than the thresholds of concern for structural class I or class II substances. The total combined estimated levels of intake of the candidate and supporting substances is approximately 350 μg/capita per day (without acetone and isopropanol) for structural class I substances and 1,430 μg/capita per day for structural class II substances. This latter value does exceed the threshold of concern for the structural class. For the structural class II substances, ca 70% of the combined exposure estimate is represented by five supporting substances [FL‐nos: 02.023, 07.002, 07.016, 07.020 and 07.103]. For [FL no: 07.020], a NOAEL of 2,000 mg/kg bw per day has been reported, which provides an adequate margin of safety of 11.5 × 104. For the remaining 20 structural class II substances, the combined exposure estimate (380 μg/capita per day) remains below the TTC for this structural class.
From the available in vitro and in vivo tests on candidate and supporting substances, no concern is raised with respect to genotoxicity. Fifty‐two candidate substances would be expected to be metabolised to innocuous substances at the estimated levels of intake as flavouring substances. One candidate substance, 5‐methylheptan‐3‐one [FL‐no: 07.182], may be oxidised to a potential neurotoxic gamma‐diketone. However, this metabolic path does not pose a safety concern at the estimated level of intake as a flavouring substance. Indeed, for this substance, a NOAEL for neurotoxicity of 82 mg/kg bw per day was established in a subchronic study on adult male rats dosed with 0, 82, 410 and 820 mg/kg bw per day for 13 weeks. This NOAEL provides a margin of safety of 1.5 × 107 based on the estimated intake (MSDI) of the candidate substance of 0.32 μg/capita per day.
Otherwise, it was noted that where toxicity data were available on single flavouring substances they were consistent with the conclusions in the present FGE using the Procedure.
It is considered that on the basis of the default MSDI approach none of the 53 candidate substances would give rise to safety concerns at the estimated levels of intake arising from their use as flavouring substances.
In order to determine whether the conclusion for the 53 candidate substances evaluated through the Procedure can be applied to the materials of commerce, it is necessary to consider the available specifications. Adequate specifications including purity and identity for the materials of commerce have been provided for all the candidate substances.
The Panel concluded that all 53 flavouring substances [FL‐nos: 02.077, 02.124, 02.131, 02.142, 02.145, 02.148, 02.177, 02.182, 02.183, 02.187, 02.190, 02.194, 02.211, 02.255, 07.072, 07.084, 07.150, 07.156, 07.157, 07.158, 07.160, 07.161, 07.162, 07.178, 07.181, 07.182, 07.185, 07.189, 07.198, 07.199, 07.201, 07.204, 07.205, 07.210, 07.236, 07.239, 07.262, 09.304, 09.323, 09.325, 09.328, 09.332, 09.386, 09.388, 09.391, 09.604, 09.605, 09.606, 09.608, 09.609, 09.676, 09.880 and 09.926] evaluated in this FGE would not be expected to present a safety concern at their estimated levels of intake based on the MSDI approach.
When the estimated intakes were based on the mTAMDI, they ranged from 1,600 to 3,900 μg/person per day for the 28 candidate substances from structural class I. The intakes were all above the threshold of concern for structural class I of 1,800 μg/person per day, except for three flavouring substances [FL‐nos: 07.084, 07.178 and 07.239]. These three substances have mTAMDI intake estimates below the threshold of concern for the structural class, and are also expected to be metabolised to innocuous products. The estimated intakes of the 25 candidate substances assigned to structural class II, based on the mTAMDI, range from 1,500 to 6,600 μg/person per day, which are all above the threshold of concern for structural class II of 540 μg/person per day.
In conclusion, for all candidate substances except [FL‐nos: 07.084, 07.178 and 07.239] further information is required. This would include more reliable intake data and then, if required, additional toxicological data.
Documentation provided to EFSA
EFFA (European Flavour and Fragrance Association), 2002a. Submission 2001‐3. Flavouring group evaluation of 38 flavouring substances (candidate chemicals) of the chemical group 5 (Annex I of 1565/2000/EC), structurally related to saturated aliphatic acyclic secondary alcohols, ketones, and related saturated and unsaturated esters [FAO/WHO JECFA 42/51], or aliphatic secondary alcohols, ketones and related esters [under consideration during the 59th meeting of JECFA] used as flavouring substances. April 5, 2002. SCOOP/FLAV/8.9.
EFFA (European Flavour and Fragrance Association), 2002b. Submission 2001‐3. Flavouring group evaluation of 38 flavouring substances (candidate chemicals) of the chemical group 5 (Annex I of 1565/2000/EC), structurally related to saturated aliphatic acyclic secondary alcohols, ketones, and related saturated and unsaturated esters [FAO/WHO JECFA 42/51], or aliphatic secondary alcohols, ketones and related esters [under consideration during the 59th meeting of JECFA] used as flavouring substances April 5, 2002. SCOOP/FLAV/8.9. European inquiry on volume of use. IOFI, International Organization of the Flavor Industry, 1995. Private communication to FEMA. Unpublished report submitted by EFFA to SCF.
EFFA (European Flavour and Fragrance Association), 2002c. Submission 2002‐addenda 5. Supplement of 11 flavouring substances (candidate chemicals) of the chemical group 5 (Annex I of 1565/2000/EC) structurally related to saturated and unsaturated aliphatic secondary alcohols, ketones and esters containing secondary alcohols used as flavouring substances. December 27, 2002. FLAVIS/8.148.
EFFA (European Flavour and Fragrance Association), 2002d. Letter from EFFA to Dr. Joern Gry, Danish Veterinary and Food Administration. Dated 31 October 2002. Re.: Second group of questions. FLAVIS/8.26.
EFFA (European Flavour and Fragrance Association), 2004. Intake ‐ Collection and collation of usage data for flavouring substances. Letter from Dan Dils, EFFA to Torben Hallas‐Møller, EFSA. May 31, 2004.
EFFA (European Flavour and Fragrance Association), 2007a. E‐mail from Jan Demyttenaere, EFFA to FLAVIS Secretariat, National Food Institute, Technical University of Denmark. Dated 8 February 2007. RE: FLAVIS submissions ‐ use levels for Category 14.2 ‐ Alcoholic beverages. FLAVIS/8.70.
EFFA (European Flavour and Fragrance Association), 2007b. Addendum of 1 flavouring substance to the flavouring group evaluation of the chemical group 5 (Annex I of 1565/2000/EC) structurally related to saturated aliphatic acyclic secondary alcohols, ketones, and related saturated and unsaturated esters, or aliphatic secondary alcohols, ketones and related esters from chemical group 5 used as flavouring substances. Addendum to EFFA submission 2001‐3. 21 December 2006. Unpublished report submitted by EFFA to FLAVIS Secretariat. FLAVIS/8.78.
EFFA (European Flavour and Fragrance Association), 2007c. Submission 2007‐05. Safety evaluation of aliphatic secondary alcohols, ketones and related esters used as flavouring agents (S20‐J37). Submission 2007_05_EFSA S20‐J37. Unpublished report submitted by EFFA to FLAVIS Secretariat. FLAVIS/8.102.
EFFA (European Flavour Association), 2010. EFFA Letters to EFSA for clarification of specifications and isomerism for which data were requested in published FGEs.
EFFA (European Flavour Association), 2012. E‐mail from EFFA to FLAVIS Secretariat, Danish Food Institute, Technical University of Denmark, dated 16 February 2012. Information on isomerism of substances evaluated in FGE.206 and FGE.209 and allocated FGE.07Rev4: [FL‐no: 02.145, 02.194, 02.211, 07.198 and 07.204] and FGE.63Rev1 [FL‐no: 02.252, 07.099, 07.190, 07.247, 07.256 and 09.936]. FLAVIS/8.144.
EFFA (European Flavour Association), 2016. EFFA correspondence to EFSA for clarification of specifications and isomerism, use levels and updated tonnage data for six substances for which additional data were requested.
EFFA (European Flavour Association), 2017. EFFA correspondence to EFSA for clarification of specifications and isomerism. FDA (Food and Drug Administration), 1975. Additional data on oral LD50's for FEMA preparation of SLR's on flavours. Unpublished report submitted by EFFA to SCF.
Flavour Industry, 2006. Unpublished information submitted by Flavour Industry to DG SANCO and forwarded to EFSA. A‐07Rev2.
Flavour Industry, 2009. Unpublished information submitted by Flavour Industry to DG SANCO and forwarded to EFSA. A‐07Rev3.
IOFI (International Organization of the Flavor Industry), 1995. European inquiry on volume of use. IOFI, International Organization of the Flavor Industry, 1995.
Keig‐Shevlin Z, 2015a. 1‐Octen‐3‐one: Rat alkaline Comet assay. Covance Laboratories Ltd. Study no. 8302486. 04 March 2015. Unpublished final report submitted by EFFA to DG SANTE.
Keig‐Shevlin Z, 2015b. Pent‐1‐en‐3‐one: Rat micronucleus and alkaline Comet assay. Covance Laboratories Ltd. Study no. 8302945. 12 February 2015. Unpublished final report submitted by EFFA to DG SANTE.
Keig‐Shevlin Z, 2015c. Pent‐1‐en‐3‐one: Analysis of duodenum Comet slides from Covance study 8302945. Covance Laboratories Ltd. Study no. 8326425. 07 October 2015. Unpublished final report submitted by EFFA to EFSA.
Union Carbide Corp., 1956. Toxicity studies. Methyl ethyl ketone. Unpublished data.
Union Carbide Corp., 1977. Comparative toxicity to rats of methoxyacetone and five other aliphatic ketones in their drinking water with cover letter. Methyl isobutyl ketone. EPA Doc ID 878212140, microfiche no. OTS206068. Unpublished data submitted by EFFA to SCF.
Union Carbide Corp., 1980. Initial submission: 2‐Butyl formate (mixture): Range finding toxicity studies (final report) with cover letter dated 011492. EPA Doc ID 88‐920000662, microfiche no. OTS0535261. October 10, 1980. Unpublished data submitted by EFFA to SCF.
Abbreviations
- AUC
area under curve
- BW
body weight
- CAS
Chemical Abstract Service
- CEF
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CoE
Council of Europe
- EFFA
European Flavour and Fragrance Association
- FAO
Food and Agriculture Organization of the United Nations
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS
(FL) Flavour Information System (database)
- FOB
Functional Observational Battery
- HGPRT
hypoxanthine‐guanine phosphoribosyltransferase
- ID
identity
- IFRA
International Fragrance Association
- IOFI
International Organization of the Flavour Industry
- IP
intraperitoneal
- IR
infrared spectroscopy
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- LD50
lethal dose, 50%; Median lethal dose
- MS
mass spectrometry
- MSDI
Maximised Survey‐derived Daily Intake
- mTAMDI
Modified Theoretical Added Maximum Daily Intake
- NAD
nicotinamide adenine dinucleotide
- NADH
nicotinamide adenine dinucleotide – reduced form
- NADP
nicotinamide adenine dinucleotide phosphate
- NADPH
nicotinamide adenine dinucleotide phosphate – reduced form
- NOAEL
no observed adverse effect level
- NMR
nuclear magnetic resonance
- NTP
National Toxicology Program
- SCCNFP
European Union Scientific Committee on Cosmetic Products and Non‐Food Products
- SCE
sister chromatid exchange
- SCF
Scientific Committee on Food
- TAMDI
Theoretical Added Maximum Daily Intake
- TTC
toxicological threshold of concern
- UGAC
average urinary output of glucuronide
- VCF
Volatile Compounds in Food
- WHO
World Health Organisation
Appendix A – A Procedure for the safety evaluation
1.
The approach for a safety evaluation of chemically defined flavouring substances as referred to in Commission Regulation (EC) No 1565/20003, named the ‘Procedure’, is shown in schematic form in Figure A.1. The Procedure is based on the Opinion of the Scientific Committee on Food expressed on 2 December 1999 (SCF, 1999), which is derived from the evaluation Procedure developed by the Joint FAO/WHO Expert Committee on Food Additives at its 44th, 46th and 49th meetings (JECFA, 1995, 1996, 1997, 1999b).
Figure A.1.
Procedure for safety evaluation of chemically defined flavouring substances
The Procedure is a stepwise approach that integrates information on intake from current uses, structure–activity relationships, metabolism and, when needed, toxicity. One of the key elements in the Procedure is the subdivision of flavourings into three structural classes (I, II and III) for which thresholds of concern (human exposure thresholds) have been specified. Exposures below these thresholds are not considered to present a safety concern.
Class I contains flavourings that have simple chemical structures and efficient modes of metabolism, which would suggest a low order of oral toxicity. Class II contains flavourings that have structural features that are less innocuous, but are not suggestive of toxicity. Class III comprises flavourings that have structural features that permit no strong initial presumption of safety, or may even suggest significant toxicity (Cramer et al., 1978). The thresholds of concern for these structural classes of 1,800, 540 or 90 μg/person per day, respectively, are derived from a large database containing data on subchronic and chronic animal studies (JECFA, 1996).
In Step 1 of the Procedure, the flavourings are assigned to one of the structural classes. The further steps address the following questions:
Can the flavourings be predicted to be metabolised to innocuous products8 (Step 2)?
Do their exposures exceed the threshold of concern for the structural class (Steps A3 and B3)?
Are the flavourings or their metabolites endogenous9 (Step A4)?
Does a NOAEL exist on the flavourings or on structurally related substances (Steps A5 and B4)?
In addition to the data provided for the flavouring substances to be evaluated (candidate substances), toxicological background information available for compounds structurally related to the candidate substances is considered (supporting substances), in order to assure that these data are consistent with the results obtained after application of the Procedure.
The Procedure is not to be applied to flavourings with existing unresolved problems of toxicity. Therefore, the right is reserved to use alternative approaches if data on specific flavourings warranted such actions.
Appendix B – Summary of safety evaluations
1.
Appendix C – Use levels/mTAMDI
Normal and maximum use levels
For each of the 18 food categories (Table C.1) in which the candidate substances are used, flavour industry reports a ‘normal use level’ and a ‘maximum use level’.1 According to the flavour industry, the ‘normal use’ is defined as the average of reported usages and ‘maximum use’ is defined as the 95th percentile of reported usages (EFFA, 2002d). The normal and maximum use levels in different food categories have been extrapolated from figures derived from 12 model flavouring substances (EFFA, 2004).
Table C.1.
Food categories according to Commission Regulation (EC) No 1565/20003
| Food category | Description |
|---|---|
| 01.0 | Dairy products, excluding products of category 02.0 |
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) |
| 03.0 | Edible ices, including sherbet and sorbet |
| 04.1 | Processed fruit |
| 04.2 | Processed vegetables (including mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds |
| 05.0 | Confectionery |
| 06.0 | Cereals and cereal products, including flours & starches from roots & tubers, pulses & legumes, excluding bakery |
| 07.0 | Bakery wares |
| 08.0 | Meat and meat products, including poultry and game |
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms |
| 10.0 | Eggs and egg products |
| 11.0 | Sweeteners, including honey |
| 12.0 | Salts, spices, soups, sauces, salads, protein products, etc. |
| 13.0 | Foodstuffs intended for particular nutritional uses |
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products |
| 14.2 | Alcoholic beverages, including alcohol‐free and low‐alcoholic counterparts |
| 15.0 | Ready‐to‐eat savouries |
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) ‐ foods that could not be placed in categories 01.0–15.0 |
The ‘normal and maximum use levels’ are provided by industry for all 49 candidate substances in the present flavouring group (Table C.3).
Table C.3.
Distribution of the 18 food categories listed in Commission Regulation (EC) No 1565/20003 into the seven SCF food categories used for TAMDI calculation (SCF, 1995)
| Food categories according to Commission Regulation (EC) No 1565/20003 | Distribution of the seven SCF food categories | |||
|---|---|---|---|---|
| Key | Food category | Food | Beverages | Exceptions |
| 01.0 | Dairy products, excluding products of category 02.0 | Food | ||
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) | Food | ||
| 03.0 | Edible ices, including sherbet and sorbet | Food | ||
| 04.1 | Processed fruit | Food | ||
| 04.2 | Processed vegetables (including mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds | Food | ||
| 05.0 | Confectionery | Exception a | ||
| 06.0 | Cereals and cereal products, including flours & starches from roots & tubers, pulses & legumes, excluding bakery | Food | ||
| 07.0 | Bakery wares | Food | ||
| 08.0 | Meat and meat products, including poultry and game | Food | ||
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms | Food | ||
| 10.0 | Eggs and egg products | Food | ||
| 11.0 | Sweeteners, including honey | Exception a | ||
| 12.0 | Salts, spices, soups, sauces, salads, protein products, etc. | Exception d | ||
| 13.0 | Foodstuffs intended for particular nutritional uses | Food | ||
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products | Beverages | ||
| 14.2 | Alcoholic beverages, including alcohol‐free and low‐alcoholic counterparts | Exception c | ||
| 15.0 | Ready‐to‐eat savouries | Exception b | ||
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) ‐ foods that could not be placed in categories 01.0–15.0 | Food | ||
mTAMDI calculations
The method for calculation of modified Theoretical Added Maximum Daily Intake (mTAMDI) values is based on the approach used by SCF up to 1995 (SCF, 1995). The assumption is that a person may consume the amount of flavourable foods and beverages listed in Table C.2. These consumption estimates are then multiplied by the reported use levels in the different food categories and summed up.
Table C.2.
Estimated amount of flavourable foods, beverages, and exceptions assumed to be consumed per person per day (SCF, 1995)
| Class of product category | Intake estimate (g/day) |
|---|---|
| Beverages (non‐alcoholic) | 324.0 |
| Foods | 133.4 |
| Exception a: Candy, confectionery | 27.0 |
| Exception b: Condiments, seasonings | 20.0 |
| Exception c: Alcoholic beverages | 20.0 |
| Exception d: Soups, savouries | 20.0 |
| Exception e: Others, e.g. chewing gum | e.g. 2.0 (chewing gum) |
The mTAMDI calculations are based on the normal use levels reported by industry. The seven food categories used in the SCF TAMDI approach (SCF, 1995) correspond to the 18 food categories as outlined in Commission Regulation (EC) No 1565/20003 and reported by the flavour industry in the following way (see Table C.2):
Beverages (SCF, 1995) correspond to food category 14.1
Foods (SCF, 1995) correspond to the food categories 1, 2, 3, 4.1, 4.2, 6, 7, 8, 9, 10, 13, and/or 16
Exception a (SCF, 1995) corresponds to food category 5 and 113
Exception e (SCF, 1995) corresponds to others, e.g. chewing gum.
The mTAMDI values (see Table C.5) are presented for each of the 49 flavouring substances in the present flavouring group, for which industry has provided use and use levels (EFFA, 2002a,c, 2007a,b,c; Flavour Industry, 2006, 2009). The mTAMDI values are only given for highest reported normal use levels (see Table C.4).
Table C.5.
Estimated intakes based on the mTAMDI approach
| FL‐no | EU register name | mTAMDI (μg/person per day) | Structural class | Threshold of concern (μg/person per day) |
|---|---|---|---|---|
| 02.077 | Pentan‐3‐ol | 3,900 | Class I | 1,800 |
| 02.124 | 6‐Methylhept‐5‐en‐2‐ol | 3,900 | Class I | 1,800 |
| 02.142 | 3,3‐Dimethylbutan‐2‐ol | 3,900 | Class I | 1,800 |
| 02.148 | Dodecan‐2‐ol | 3,900 | Class I | 1,800 |
| 02.177 | 2‐Methylhexan‐3‐ol | 3,900 | Class I | 1,800 |
| 02.182 | 3‐Methylpentan‐2‐ol | 3,900 | Class I | 1,800 |
| 02.183 | 4‐Methylpentan‐2‐ol | 3,900 | Class I | 1,800 |
| 02.190 | Nonan‐3‐ol | 3,900 | Class I | 1,800 |
| 02.255 | (Z)‐4‐Hepten‐2‐ol | 2,500 | Class I | 1,800 |
| 07.084 | Pentan‐3‐one | 1,600 | Class I | 1,800 |
| 07.178 | 3‐Methylbutan‐2‐one | 1,600 | Class I | 1,800 |
| 07.239 | [R‐(E)]‐5‐Isopropyl‐8‐methylnona‐6,8‐dien‐2‐one | 1,600 | Class I | 1,800 |
| 09.304 | sec‐Heptyl isovalerate | 3,900 | Class I | 1,800 |
| 09.323 | sec‐Butyl acetate | 3,900 | Class I | 1,800 |
| 09.325 | sec‐Butyl butyrate | 3,900 | Class I | 1,800 |
| 09.328 | sec‐Butyl formate | 3,900 | Class I | 1,800 |
| 09.332 | sec‐Butyl hexanoate | 3,900 | Class I | 1,800 |
| 09.386 | sec‐Hept‐4(cis)‐enyl acetate | 3,900 | Class I | 1,800 |
| 09.388 | sec‐Heptyl acetate | 3,900 | Class I | 1,800 |
| 09.391 | sec‐Heptyl hexanoate | 3,900 | Class I | 1,800 |
| 09.604 | Isopropyl decanoate | 3,900 | Class I | 1,800 |
| 09.605 | Isopropyl dodecanoate | 3,900 | Class I | 1,800 |
| 09.606 | Isopropyl hexadecanoate | 3,900 | Class I | 1,800 |
| 09.608 | Isopropyl octanoate | 3,900 | Class I | 1,800 |
| 09.609 | Isopropyl valerate | 3,500 | Class I | 1,800 |
| 09.676 | sec‐Octyl acetate | 3,900 | Class I | 1,800 |
| 09.880 | (Z)‐Hept‐4‐enyl‐2 butyrate | 3,900 | Class I | 1,800 |
| 09.926 | Octan‐3‐yl formate | 3,900 | Class I | 1,800 |
| 02.145 | 2,6‐Dimethylocta‐1,5,7‐trien‐3‐ol | 3,900 | Class II | 540 |
| 02.194 | Octa‐1,5‐dien‐3‐ol | 3,900 | Class II | 540 |
| 02.211 | Undeca‐1,5‐dien‐3‐ol | 3,900 | Class II | 540 |
| 07.072 | 6‐Methylheptan‐3‐one | 1,600 | Class II | 540 |
| 07.150 | Decan‐2‐one | 1,600 | Class II | 540 |
| 07.156 | 2,6‐Dimethyloct‐6‐en‐3‐one (mixture of E and Z) | 1,600 | Class II | 540 |
| 07.157 | 6,10‐Dimethylundecan‐2‐one | 1,500 | Class II | 540 |
| 07.158 | Dodecan‐2‐one | 1,600 | Class II | 540 |
| 07.160 | Heptadecan‐2‐one | 1,600 | Class II | 540 |
| 07.162 | Hex‐5‐en‐2‐one | 1,600 | Class II | 540 |
| 07.181 | 6‐Methylheptan‐2‐one | 1,600 | Class II | 540 |
| 07.185 | 3‐Methylpentan‐2‐one | 1,600 | Class II | 540 |
| 07.189 | Nonan‐4‐one | 1,600 | Class II | 540 |
| 07.198 | Pseudo‐ionone | 1,600 | Class II | 540 |
| 07.199 | Tetradecan‐2‐one | 1,600 | Class II | 540 |
| 07.201 | Tridec‐12‐en‐2‐one | 1,600 | Class II | 540 |
| 07.204 | 3,3,6‐Trimethylhepta‐1,5‐dien‐4‐one | 1,600 | Class II | 540 |
| 07.205 | 6,10,14‐Trimethylpentadecan‐2‐one | 1,500 | Class II | 540 |
| 07.236 | (Z)‐5‐Octen‐2‐one | 1,600 | Class II | 540 |
| 07.262 | 9‐Decen‐2‐one | 6,600 | Class II | 540 |
| 07.182 | 5‐Methylheptan‐3‐one | 1,600 | Class II | 540 |
| 02.131 | But‐3‐en‐2‐ol | 3,900 | Class II | 540 |
| 02.187 | Non‐1‐en‐3‐ol | 3,900 | Class II | 540 |
| 07.161 | Hex‐1‐en‐3‐one | 1,600 | Class II | 540 |
| 07.210 | 1‐Nonene‐3‐one | 1,600 | Class II | 540 |
mTAMDI: modified Theoretical Added Maximum Daily Intake.
Table C.4.
Normal and maximum use levels (mg/kg) for the candidate substances in FGE.07Rev4 (EFFA, 2002a,c, 2007a,b,c, 2016; Flavour Industry, 2006, 2009)
| FL‐no | Food categories | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal use levels (mg/kg) Maximum use levels (mg/kg) | ||||||||||||||||||
| 01.0 | 02.0 | 03.0 | 04.1 | 04.2 | 05.0 | 06.0 | 07.0 | 08.0 | 09.0 | 10.0 | 11.0 | 12.0 | 13.0 | 14.1 | 14.2 | 15.0 | 16.0 | |
| 02.077 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.124 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.131 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.142 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.145 |
7 35 |
8 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.148 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.177 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.182 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.183 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.187 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.190 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.194 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.211 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.255 |
5 20 |
– – |
10 50 |
– – |
– – |
10 60 |
– – |
10 60 |
– – |
– – |
– – |
– – |
– – |
5 20 |
2 10 |
10 40 |
– – |
– – |
| 07.072 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.084 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.150 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.156 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
– – |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.157 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
5 25 |
2 10 |
4 20 |
– – |
2 10 |
| 07.158 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.160 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.161 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.162 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.178 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.181 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.182 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.185 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.189 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.198 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.199 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.201 |
3 15 |
2 10 |
3 10 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.204 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.205 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
– – |
4 20 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
– – |
5 25 |
2 10 |
| 07.210 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.236 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.239 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.262 |
10 30 |
– – |
5 15 |
10 30 |
10 30 |
30 150 |
– – |
– – |
– – |
– – |
– – |
– – |
– – |
10 50 |
5 25 |
10 50 |
– – |
30 150 |
| 09.304 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.323 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.325 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.328 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
2 25 |
| 09.332 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.386 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.388 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.391 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.604 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.605 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.606 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.608 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
– – |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.609 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
– – |
5 25 |
| 09.676 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.880 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.926 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
Appendix D – Metabolism
General information
The present flavouring group evaluation consists of 53 candidate substances of which seven are saturated aliphatic acyclic secondary alcohols [FL‐nos: 02.077, 02.142, 02.148, 02.177, 02.182, 02.183 and 02.190]; seven are unsaturated aliphatic secondary alcohols [FL‐nos: 02.124, 02.131, 02.145, 02.187, 02.194, 02.211 and 02.255] of which five contain a terminal double bond [FL‐nos: 02.131, 02.145, 02.187, 02.194 and 02.211]; 13 are saturated aliphatic ketones [FL‐nos: 07.072, 07.084, 07.150, 07.157, 07.158, 07.160, 07.178, 07.181, 07.182, 07.185, 07.189, 07.199 and 07.205], 10 are unsaturated aliphatic ketones [FL‐nos: 07.156, 07.161, 07.162, 07.198, 07.201, 07.204, 07.210, 07.236, 07.239 and 07.262] of which seven contain a terminal double bond [FL‐nos: 07.161, 07.162, 07.201, 07.204, 07.210, 07.239 and 07.262] and 16 are esters of aliphatic acyclic secondary alcohols and linear aliphatic carboxylic acids [FL‐nos: 09.304, 09.323, 09.325, 09.328, 09.332, 09.386, 09.388, 09.391, 09.604, 09.605, 09.606, 09.608, 09.609, 09.676, 09.880 and 09.926]. The general metabolic reactions that the candidate substances may be expected to undergo, and which are discussed below, are one or several of the following:
conjugation of secondary alcohols with glucuronic acid
oxidation of secondary alcohols
reduction of ketones
oxidation of ketones
oxidation of double bonds
oxidation of terminal double bonds
hydrolysis of esters.
A general discussion on the biotransformation of Saturated Aliphatic Acyclic Secondary Alcohols, Ketones, and Related Saturated and Unsaturated Esters may be found in the reports from the 51st, 59th and 69th meetings of the JECFA (1999a, 2002a, 2009b). The discussions and conclusions related to these supporting substances essentially apply also to the candidate substances. There is one candidate substance 5‐methylheptan‐3‐one [FL‐no: 07.182] that may be oxidised to yield a neurotoxic gamma‐diketone and therefore it may potentially give rise to concern.
Absorption
In general, aliphatic secondary alcohols and ketones are expected to be rapidly absorbed in the gastrointestinal tract (JECFA, 1999a).
Peak blood levels were obtained 1–2 h after dosing when isopropanol was given orally to rats as well as when the same substance was administered intravenously to dogs (Lehman et al., 1945; Nordmann et al., 1973). Peak blood levels were also obtained within 2 h when 1‐ and 2‐propanol, or 1‐ and 2‐isobutanol were given orally to human volunteers together with ethanol (Bonte et al., 1981).
In a pharmacokinetic experiment, 2‐butanol (2.2 mL/kg bw or 1,776 mg/kg bw), 2‐butanone (2.1 mL/kg bw or 1,690 mg/kg bw) and 2,3‐butanediol (0.68 mL/kg bw or 676 mg/kg bw), respectively, were administered orally in aqueous solutions to male Sprague–Dawley rats. Peak blood concentrations after administration of 0.95 mg/L 2‐butanone were detected after 4 h and declined to 0.07 mg/mL after 18 h. The concentrations of the metabolites 2,3‐butanediol, 2‐butanol and 3‐hydroxy‐2‐butanone peaked at 0.26, 0.033 and 0.027 mg/L at 18, 6 and 8 h, respectively, after 2‐butanone administration. Total area under the curve (AUC) values for 2‐butanone, 2,3‐butanediol, 2‐butanol and 3‐hydroxy‐2‐butanone were 10,899 ± 824, 3,863 ± 238, 414 ± 38 and 382 ± 38 mg h/L, respectively. Blood concentration after administration of 2‐butanol peaked after 2 h at 0.59 mg/L and declined to 0.05 mg/L after 16 h. The blood concentrations of 2‐butanone, 3‐hydroxy‐2‐butanone and 2,3‐butanediol rose to maximums after 8, 12 and 18 h and were 0.78, 0.04 and 0.21 mg/L, respectively. Total AUC values were 3,254 ± 258 mg h/L for 2‐butanol, 9,868 ± 566 for 2‐butanone, 443 ± 93 for 3‐hydroxy‐2‐butanone and 3,167 ± 503 mg h/L for 2,3‐butanediol (Dietz et al., 1981).
Rats were administered 1 g/kg bw 2‐pentanol, 3‐pentanol and 3‐methyl‐2‐butanol, via intraperitoneal (IP) injection. The alcohols were eliminated within 13–16 h (Haggard et al., 1945).
Metabolism and elimination
Secondary alcohols
Oxidation and glucuronic acid conjugation: Secondary alcohols may undergo oxidation to the corresponding ketone. However, this reaction is generally unfavoured in vivo, since the alcohol is removed from the equilibrium by conjugation with glucuronic acid, which represents the major biotransformation pathway for secondary alcohols (Kasper and Henton, 1980; JECFA, 1999a). Glucuronidation is a phase‐II‐reaction, which involves the transfer of glucuronic acid in an activated form to functional groups of the substrate, in this case to the hydroxyl groups of the molecules. This renders highly polar products, for which excretion is facilitated. The reaction is catalysed by UDP‐glucuronyl transferase, which exists in several isoforms with different substrate specificities. The enzymes are located in the endoplasmic reticulum, and are found in most tissues including the liver. The glucuronic acid conjugates are primarily excreted in the urine or bile, depending on the relative molecular mass and the animal species. For the candidate secondary alcohols, the urine is expected to be the main route of elimination.
Ketones
In addition to reduction and oxidation pathways, low molecular weight ketones (carbon chain length < 5) may be excreted unchanged in expired air (Brown et al., 1987). In mammals, oral doses of volatile ketones or their corresponding alcohols are mainly eliminated as the ketone in expired air. Lower amounts are excreted in the urine (Haggard et al., 1945; Scopinaro et al., 1947; Schwartz, 1989).
In the rat, 2‐pentanone in expired air was the major metabolite following administration of 2‐pentanol by IP injection. Lower amounts of 2‐pentanol were also exhaled and both metabolites were detected in the urine (Haggard et al., 1945). Similarly, unchanged 2‐pentanone administered orally to dogs has been identified in the expired air (Schwartz, 1989).
Reduction of ketones: In general, the major metabolic pathway for the detoxification and excretion of aliphatic ketones involves reduction of the ketone to the corresponding secondary alcohol with subsequent excretion as conjugate of glucuronic acid. This reaction is reversible under physiologic conditions, but in vivo the secondary alcohols are removed from the equilibrium by conjugation to glucuronic acid, as is stated above, and the reaction proceeds to form further secondary alcohols (Felsted and Bachur, 1980; JECFA, 1999a). Reduction of aliphatic ketones is mediated by alcohol dehydrogenase and NADH/NADPH‐dependent cytosolic carbonyl reductases (Bosron and Li, 1980). According to Felsted and Bachur (1980), the reaction catalysed by carbonyl reductase is stereoselective and favours formation of the (S)‐enantiomer of the alcohol (Felsted and Bachur, 1980).
In studies limited to the identification of urinary glucuronide, relatively high single dose levels of a homologous series of aliphatic secondary alcohols and ketones were administered individually by gavage to rabbits. The urinary excretion of glucuronic acid conjugates was determined after 24 h (Kamil et al., 1953). The substances, dose levels and average urinary output of glucuronide (UGAC) are shown below in Table D.1.
Table D.1.
The urinary excretion of glucuronic acid conjugates (UGAC, determined after 24 h) of aliphatic secondary alcohols and ketones after administration by gavage to rabbits (Kamil et al., 1953)
| Substance | Dose (mg/kg bw) | UGAC (%) |
|---|---|---|
| 2‐Pentanol | 735 | 44.8 |
| 2‐Heptanone | 950 | 41.0 |
| 2‐Heptanol | 965 | 54.6 |
| 3‐Heptanol | 965 | 61.9 |
| 2‐Octanol | 1,081 | 15.5 |
bw: body weight; UGAC: average urinary output of glucuronide.
Oxidation of ketones: Ketones may also be metabolised via omega‐ or omega‐1‐oxidation. Participation in these pathways depends on chain length, position of the carbonyl function and dose (Dietz et al., 1981; Topping et al., 1994).
Short chain ketones (C < 5) that contain a carbonyl function at the C‐2 may undergo oxidation of the terminal methyl group and subsequent oxidation to yield an alpha‐keto carboxylic acid. As intermediary metabolites, alpha‐keto acids undergo oxidative decarboxylation to yield carbon dioxide and a simple aliphatic carboxylic acid, which may be completely metabolised in the fatty acid pathway and citric acid cycle. Alternatively, omega‐oxidation may occur to yield a hydroxy‐ketone, which may be further reduced to a diol, e.g. 2,3‐butanediol from butanone, and excreted in the urine as a glucuronic acid conjugate.
Longer chain aliphatic ketones (carbon chain length ≥ 5) are primarily metabolised via reduction, but omega‐ and omega‐1‐oxidation are competing pathways at high concentrations (Dietz et al., 1981; Topping et al., 1994).
Studies with specific substances: 4‐Methylpentan‐2‐ol [FL‐no: 02.183] and 4‐hydroxy‐4‐methylpentan‐2‐one were detected in serum after IP injection of 4‐methylpentan‐2‐one in guinea pigs. The half‐life and clearance times of 4‐methylpentan‐2‐one were 66 min and 6 h, respectively. 4‐Hydroxy‐4‐methylpentan‐2‐one was the principal metabolite and was cleared in 16 h. The concentration of 4‐methylpentan‐2‐ol [FL‐no: 02.183] was too low for quantification. 4‐Methylpentan‐2‐one is metabolised by reduction of the carbonyl group to form the secondary alcohol, 4‐methylpentan‐2‐ol [FL‐no: 02.183], and by oxidation at the omega‐1 carbon atom to form the hydroxylated ketone, 4‐hydroxy‐4‐methylpentan‐2‐one (DiVincenzo et al., 1976).
Gamma‐Diketone formation: Omega‐1‐oxidation of aliphatic ketones with special structural features may yield neurotoxic gamma‐diketones. The metabolic pathway includes oxidation of the omega‐1‐carbon, first to a hydroxy‐ketone and then to a diketone. The gamma‐spacing of the carbonyl functions has been shown to be a prerequisite for neurotoxic effects, only ketones with this structural feature may yield the neurotoxic metabolites. One of the candidate substances 5‐methyl‐3‐heptanone [FL‐no: 07.182], may potentially be oxidised to a gamma‐diketone 3‐methyl‐2,5‐heptanedione.
Studies have shown that neurotoxicity of selected ketones is related to a common metabolic pathway leading to the formation of a gamma‐diketone, which is the metabolite that produces neuropathy. The neurotoxic effects show a specific anatomic and morphological type of nerve degeneration characterised by large multifocal axonal swellings, referred to as ‘giant‐axonal’ neuropathy. Clinical symptomatology in humans includes bilaterally symmetrical paraesthesia, ‘pins and needles’ feeling, and muscle weakness, primarily in arms and legs. Except for 3,6‐octanedione, all metabolic interconversions are oxidation of the omega‐1‐carbon, first to a hydroxy‐ketone and then to a gamma‐diketone. When the omega‐carbon is oxidised in preference to the omega‐1‐carbon, no gamma‐diketone is formed (Topping et al., 1994).
Induction of clear and typical signs of neurotoxicity in male rats dosed with 5‐methyl‐3‐heptanone [FL‐no: 07.182] in a subchronic study (IBM Corp., 1989) supported the hypothesis that a gamma‐diketone may be formed as toxic metabolite.
Data suggest that the neurotoxicity of the diketone decreases as chain length increases, possibly owing to steric hindrance. However, chain length may not be important to some materials, as in the case of 5‐nonanone. Another factor modifying the neurotoxic potential of these substances is the number and size of substituent groups located between the gamma‐spaced carbonyls. Single methyl groups on the carbons located between the carbonyl groups increase the potential neurotoxicity, whereas two methyl groups positioned on one of the carbon atoms between the carbonyls eliminate neurotoxicity (Topping et al., 1994).
Among the supporting substances, 3‐heptanone [FL‐no: 07.003], 2‐methylheptan‐3‐one [FL‐no: 07.240], 3‐heptanol [FL‐no: 02.044] and 3‐heptyl acetate [FL‐no: 09.924] are the only substances that may be metabolised to yield neurotoxic gamma‐diketones (Topping et al., 1994). The neurotoxicity for these substances is observed only at high doses.
In a study reported as a meeting abstract, aliphatic ketones (hexane‐2‐one, pentane‐3‐one, heptane‐3‐one, 4‐methyl‐2‐pentanone and 3,3‐dimethyl‐2‐butanone) were administered in drinking water to female Wistar rats. It was concluded that administration of approximately 1 g/kg bw per day of hexane‐2‐one for 120 days produced muscle weakness, atrophy and peripheral neuropathy. None of the other ketones produced significant neurological alterations (Homan and Maronpot, 1978).
In an oral gavage study Crl rats, two per group, were given 3‐heptanone [FL‐no: 07.003] (0.25, 0.5, 1 or 2 g/kg bw per day, for 5 days/week for 14 weeks. The highest dose group (approaching the LD50 value in rats = 2,760 mg/kg bw) was the only one developing treatment‐related neuropathologic lesions of typical ‘giant‐axonal’ type. No neuropathology was observed in the lower dose groups (O'Donoghue et al., 1984). This study determined that 3‐heptanone has a low neurotoxic potential; however, when its intake was combined with‐methyl ethyl ketone, neurotoxic effects were potentiated, by stimulating 3‐heptanone metabolism to 2,5‐heptandione, a neurotoxic gamma‐diketone (O'Donoghue et al., 1984).
Oxidation of terminal double bonds in secondary alcohols and in ketones
Twelve of the candidate substances, but‐3‐en‐2‐ol, 2,6‐dimethylocta‐1,5,7‐triene‐3‐ol, non‐1‐en‐3‐ol, octa‐1,5‐dien‐3‐ol, undeca‐1,5‐dien‐3‐ol, hex‐1‐en‐3‐one, hex‐5‐en‐2‐one, tridec‐12‐en‐2‐one, 3,3,6‐trimethylhepta‐1,5‐dien‐4‐one, 1‐nonene‐3‐one, ([R‐(E)]‐5‐isopropyl‐8‐methylnona‐6,8‐dien‐2‐one and 9‐decen‐2‐one [FL‐nos: 02.131, 02.145, 02.187, 02.194, 02.211, 07.161, 07.162, 07.201, 07.204, 07.210, 07.239 and 07.262] have terminal double bonds. These double bonds may be oxidised to the corresponding epoxides. Epoxides are highly reactive molecules, due to the large strain associated with this three‐membered ring structure, and they react easily with nucleophilic sites of cellular macromolecules. However, epoxides will be conjugated with glutathione by glutathione S‐transferases or hydrolysed to diols by epoxide hydrolases. These two reactions can be considered to be detoxications (Sanchez and Kauffman, 2010). 1‐Alkenes are metabolised by P450 through both double bond oxidation to the corresponding epoxide and allylic oxidation (Chiappe et al., 1998). The rates of the two reactions measured with different P450 isoforms indicate that epoxide formation is generally favoured (Chiappe et al., 1998).
Based on the low levels of intake of alkenones and alkenols characterised by a carbonyl or an alcohol group in addition to the terminal double bond, it is expected that the detoxication reactions of the formed epoxides (conjugation with glutathione or epoxide hydrolase mediated hydrolysis) would not be saturated and would outweigh the rate of epoxide formation. The presence of the terminal double bond is therefore not considered of concern under the intended conditions of use.
In addition to reduction and oxidation pathways, low molecular weight alcohols and ketones may be excreted unchanged in expired air (Brown et al., 1987).
Ester hydrolysis
The aliphatic esters among the candidate substances are expected to be hydrolysed to their component secondary alcohols and carboxylic acids. The carboxylesterase or esterase classes of enzymes, the most important of which are the beta‐esterases, catalyse ester hydrolysis (Heymann, 1980). In mammals, these enzymes occur within the body in most tissues including the gut lumen and intestinal wall, but predominate in the hepatocytes (Heymann, 1980). The wide range of tissue distribution and the multiplicity of esterases generally give rise to rapid hydrolysis of esters in vivo.
There are no hydrolysis studies on the candidate substances, but there are in vitro hydrolysis data for structurally related esters.
In vitro hydrolysis studies of esters have been performed with specific carboxylesterase isoenzymes isolated from pig and rat livers (Arndt and Krisch, 1973; Junge and Heymann, 1979). The isoenzyme I exhibits an increase in enzyme binding (lower K m) and maximum velocity (V max) as the carbon chain length of either the alcohol or carboxylic acid component of the substrate increases. It is also shown that different isoenzymes show great differences in the hydrolysis rates. Isoenzyme V had an optimum for the C‐5 compound, while this isoenzyme exhibited a minimum activity with the butyl and pentyl acetates. Results of in vitro studies indicate that the rate of hydrolysis of straight chain esters is approximately 100 times faster than the rate of hydrolysis of branched‐chain esters.
Incubation of isopropyl butanoate, isopropyl phenylacetate, isoamyl acetate and isoamyl phenylacetate with pancreatin produced 40%, 50%, 20% and 100% hydrolysis, respectively, after 2 h (Leegwater and van Straten, 1974a; Grundschober, 1977). Also, isoamyl acetate incubated with intestinal mucosa homogenates obtained from pigs demonstrated complete hydrolysis (Leegwater and van Straten, 1974b; Grundschober, 1977).
Esters formed from aliphatic secondary alcohols were hydrolysed to their corresponding alcohols and carboxylic acids when incubated with liver homogenates or small intestinal homogenates obtained from male Wistar albino rats, artificial gastric juice or artificial pancreatic juice with half‐lives ranging from less than one‐second to several hours depending on the incubation medium (Gangolli and Shilling, 1968; Longland et al., 1977). Rat liver homogenates and small intestinal preparations were found to be much more efficient than artificial pancreatic juice for hydrolysis of a variety of aliphatic esters. Also, hydrolysis in simulated intestinal fluid with pancreatin was much faster than in simulated gastric juice (Longland et al., 1977).
The data on substances structurally related to the candidate substances indicate that hydrolysis is the major pathway for the candidate substances that are esters of secondary alcohols, and that they will be hydrolysed to their component alcohols and carboxylic acids within a relatively short time.
Conclusion
In conclusion, it may be anticipated that 52 of the candidate substances (the seven saturated aliphatic acyclic secondary alcohols [FL‐nos: 02.077, 02.142, 02.148, 02.177, 02.182, 02.183 and 02.190], the seven unsaturated aliphatic secondary alcohols [FL‐nos: 02.124, 02.131, 02.145, 02.187, 02.194, 02.211 and 02.255], the 12 of the 13 saturated aliphatic ketones [FL‐nos: 07.072, 07.084, 07.150, 07.157, 07.158, 07.160, 07.178, 07.181, 07.185, 07.189, 07.199 and 07.205], the 10 unsaturated aliphatic ketones [FL‐nos: 07.156, 07.161, 07.162, 07.198, 07.201, 07.204, 07.210, 07.236, 07.239 and 07.262] and the 16 esters of aliphatic acyclic secondary alcohols and linear aliphatic carboxylic acids [FL‐nos: 09.304, 09.323, 09.325, 09.328, 09.332, 09.386, 09.388, 09.391, 09.604, 09.605, 09.606, 09.608, 09.609, 09.676, 09.880 and 09.926]) will be metabolised to innocuous substances at the estimated levels of intake, based on the MSDI approach, as flavouring substances.
One candidate substance, 5‐methyl‐3‐heptanone [FL‐no: 07.182], may be oxidised to a potentially neurotoxic gamma‐diketone, 3‐methyl‐2,5‐heptanedione.
Appendix E – Toxicity summary tables
1.
Oral acute toxicity data are available for 12 candidate substances of the present Flavouring Group Evaluation from chemical group 5, and for 25 supporting substances evaluated by JECFA at the 51st and 59th meetings (JECFA, 1999a, 2003). The supporting substances are listed in brackets.
Subacute and subchronic toxicity data are available for three candidate substances and for 10 supporting substances of the present flavouring group. They were evaluated at the 51st and 59th JECFA meetings (JECFA, 1999a, 2003). No carcinogenicity data are available. The supporting substances are listed in brackets.
Developmental and reproductive toxicity data are available for two candidate substance of the present Flavouring Group Evaluation from chemical group 5 and for one supporting substance evaluated by JECFA at the 51st meetings (JECFA, 1999a). The supporting substance is listed in brackets.
In vitro mutagenicity/genotoxicity data are available for nine candidate substances of the present flavouring group evaluation from chemical group 5 and for 10 supporting substances evaluated at the 51st and 59th JECFA meetings. The supporting substances are listed in brackets.
In vivo mutagenicity/genotoxicity data available for four supporting substances evaluated at the 51st and 59th JECFA meetings. The supporting substances are listed in brackets.
Table E.5.
Genotoxicity in vivo
| Chemical name | Test system | Test object | Route | Dose | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
| (Isopropyl alcohol [02.079]) | Micronucleus test | ICR Mouse (15 M & 15 F) | IP injection in 0.9% NaCl | 350–2,500 mg/kg | Negative | Kapp et al. (1993) | a |
| (Acetone [07.050]) | Micronucleus test | Chinese hamster (5 M & 5 F) | IP injection in corn oil | 865 mg/kg | Negative | Basler (1986) | a |
| (2‐Butanone [07.053]) | Micronucleus test | CD‐1 mice (5 M & 5 F) | IP injection in corn oil | LD20 (1.96 mL/kg) | Negative | O'Donoghue et al. (1988) | a |
| Micronucleus test | Chinese hamster (5 M & 5 F) | IP injection in corn oil | 411 mg/kg | Negative | Basler (1986) | a | |
| (4‐Methyl‐2‐pentanone [07.017]) | Micronucleus test | CD‐1 mice (5 M & 5 F) | IP injection in corn oil | LD20 (0.73 mL/kg) | Negative | Basler (1986) | a |
M = Male; F = Female; IP: intraperitoneal; LD20: lethal dose, 20%.
Summarised by JECFA, 51st meeting (JECFA, 1999a).
Table E.6.
Summary of in vitro mutagenicity study considered by the Panel in FGE.205Rev1
| Chemical name FL‐no | Test | Test object | Concentration tested and test conditions | Result | Reference | Comments |
|---|---|---|---|---|---|---|
|
Oct‐1‐en‐3‐one [07.081] |
Bacterial reverse mutation assay | S. Typhimurium TA100 | 7.8–500 μg/platea , b | Positive | Bowen (2013) |
FL‐no: FLAVIS number.
With and without metabolic activation.
The following free radical/electrophile scavengers were added: glutathione, N‐acetyl cysteine, catalase, 2,5‐dimethylfuran.
Appendix F – Natural food occurrence
1.
| FL‐no | EU register name | Structural formula | CAS no | VCF* online search 15‐11‐2016 |
|---|---|---|---|---|
| 02.077 | Pentan‐3‐ol |

|
584‐02‐1 | Quantified in apricot, grape, grape brandy, guinea hen, loquat, milk and milk products, mushroom, olive, papaya, red currants, rum, shrimps from trace amount up to 1.3 mg/kg and up to 34 mg/kg in tea. Has been identified in a further 36 food items |
| 02.124 | 6‐Methylhept‐5‐en‐2‐ol |
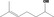
|
1569‐60‐4 | Quantified in annatto, litchi, macadamia nut, tomato from 0.0125 mg/kg and up to 50 mg/kg in citrus fruits. Has been identified in a further 18 food items |
| 02.131 | But‐3‐en‐2‐ol |

|
598‐32‐3 | Identified in citrus fruits |
| 02.142 | 3,3‐Dimethylbutan‐2‐ol |
|
464‐07‐3 | Identified in melon |
| 02.145 | 2,6‐Dimethylocta‐1,5,7‐trien‐3‐ol |
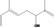
|
29414‐56‐0 | Quantified in Salvia species up to 100 mg/kg |
| 02.148 | Dodecan‐2‐ol |
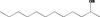
|
10203‐28‐8 | Quantified in mastic up to 1,300 mg/kg. Identified in apple, banana, beer and cheddar cheese |
| 02.177 | 2‐Methylhexan‐3‐ol |
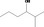
|
617‐29‐8 | Quantified in tomato up to 2.5 mg/kg |
| 02.182 | 3‐Methylpentan‐2‐ol |
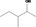
|
565‐60‐6 | Quantified in pineapple up to 0.009 mg/kg. Identified in Capsicum species, date, shrimps and tea |
| 02.183 | 4‐Methylpentan‐2‐ol |
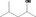
|
108‐11‐2 | Quantified in annatto and citrus fruits from 0.027 up to 0.111 mg/kg. Identified in apple brandy, bantu beer, cocoa, peanut and peas |
| 02.187 | Non‐1‐en‐3‐ol |
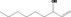
|
21964‐44‐3 | Identified in banana, beef, chervil, date and rambutan |
| 02.190 | Nonan‐3‐ol |

|
624‐51‐1 | Identified in banana, beef, cherimoya, chervil, date, guava and feyoa, mentha oils and passion fruit |
| 02.194 | Octa‐1,5‐dien‐3‐ol |

|
83861‐74‐9 | Quantified in cheese (various types), fish and oysters from 0.025 up to 0.26 mg/kg. Identified in chicken, scallop and tea |
| 02.211 | Undeca‐1,5‐dien‐3‐ol |

|
56722‐23‐7 | Identified in fish and katsuobushi |
| 02.255 | (Z)‐4‐Hepten‐2‐ol |

|
66642‐85‐1 | Identified in maize |
| 07.072 | 6‐Methylheptan‐3‐one |
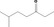
|
624‐42‐0 | Identified in melon and potato |
| 07.084 | Pentan‐3‐one |

|
96‐22‐0 | Quantified in guava and feyoa, Mangifera species, milk and milk products, mushroom, olive, passion fruit and shrimps from 0.0007 up to 14 mg/kg. Identified in a further 41 food items |
| 07.150 | Decan‐2‐one |

|
693‐54‐9 | Quantified in blue cheeses, cheese various types, chicken, milk and milk products, mountain papaya and shrimps from trace amounts up to 2.5 mg/kg and up to 2,600 mg/kg hop oil. Identified in a further 42 food items |
| 07.156 | 2,6‐Dimethyloct‐6‐en‐3‐one (mixture of E and Z) |
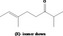
|
90975‐15‐8 Search on substance name |
Quantified up to 0.05 mg/kg in citrus fruits |
| 07.157 | 6,10‐Dimethylundecan‐2‐one |

|
1604‐34‐8 Search on substance name |
Quantified in up to 0.002 mg/kg in Vaccinium species. Identified in buckwheat, coffee, mate, rooibos tea and tea |
| 07.158 | Dodecan‐2‐one |
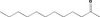
|
6175‐49‐1 | Quantified in blue cheeses, chicken, cocoa category and milk and milk products from 0.0014 up to 1.8 mg/kg and up to 2,700 mg/kg in hop oil |
| 07.160 | Heptadecan‐2‐one |

|
2922‐51‐2 | Quantified in blue cheeses, cocoa category, Mangifera species and milk and milk products from trace amount up to 8.7 mg/kg and up to 100 mg/kg in hop oil |
| 07.161 | Hex‐1‐en‐3‐one |

|
1629‐60‐3 | Quantified in artichoke up to 0.00014 mg/kg. Identified in cocoa category, dill, honey, milk and milk products and passion fruit |
| 07.162 | Hex‐5‐en‐2‐one |

|
109‐49‐9 | No product occurrence data |
| 07.178 | 3‐Methylbutan‐2‐one |
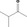
|
563‐80‐4 | Quantified in cheese various types, guava and feyoa, guinea hen, honey, milk and milk products, passion fruit, peanut and strawberry from trace amount up to 1.56 mg/kg and up to 14 mg/kg in hog plum. Identified in a further 23 food items |
| 07.181 | 6‐Methylheptan‐2‐one |
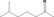
|
928‐68‐7 | Quantified in chicken, guinea hen and wine from 0.001 up to 0.1 mg/kg. Identified in beef, buckwheat, mate, peas and tea |
| 07.182 | 5‐Methylheptan‐3‐one |

|
541‐85‐5 | Quantified in lemon grass oil (14,300 mg/kg), mentha oils (1 mg/kg) and papaya (0.02 mg/kg). Identified in tomato |
| 07.185 | 3‐Methylpentan‐2‐one |
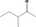
|
565‐61‐7 | Quantified in beer, dill, Filbert hazelnut, plum and tea from trace amount up to 1.7 mg/kg and up to 100 mg/kg in hop oil. Identified in apple brandy, beef, blue cheeses, cheese various types, egg, grape and peanut |
| 07.189 | Nonan‐4‐one |

|
4485‐09‐0 | Quantified in passion fruit up to 0.01 mg/kg and identified in beef |
| 07.198 | Pseudo‐ionone |

|
141‐10‐6 | Quantified in licorice, tea and tomato from trace amount up to 5 mg/kg. Identified in mate, passion fruit and tamarind |
| 07.199 | Tetradecan‐2‐one |

|
2345‐27‐9 | Quantified in milk and milk products, mountain papaya and passion fruit from 0.01 up to 2.5 mg/kg and up to 1,600 mg/kg in hop. Identified in beef, cherimoya, ginger, lamb and mutton and mate |
| 07.201 | Tridec‐12‐en‐2‐one |

|
60437‐21‐0 | No product occurrence data |
| 07.204 | 3,3,6‐Trimethylhepta‐1,5‐dien‐4‐one |
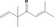
|
546‐49‐6 | Quantified in camomile from 500 up to 5,100 mg/kg and identified in tarragon |
| 07.205 | 6,10,14‐Trimethylpentadecan‐2‐one |

|
502‐69‐2 | Quantified in camomile, grape, lemon balm, mastic, tea and Vaccinium species from 0.007 up to 2,000 mg/kg and up to 50,000 mg/kg in maize. Identified in a further 12 food items |
| 07.210 | 1‐Nonene‐3‐one |

|
24415‐26‐7 | No product occurrence data |
| 07.236 | (Z)‐5‐Octen‐2‐one |

|
22610‐86‐2 | Identified in beans |
| 07.239 | [R‐(E)]‐5‐Isopropyl‐8‐methylnona‐6,8‐dien‐2‐one |
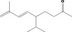
|
2278‐53‐7 | No product occurrence data |
| 07.262 | 9‐Decen‐2‐one |
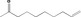
|
35194‐30‐0 | No product occurrence data |
| 09.304 | sec‐Heptyl isovalerate |
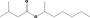
|
238757‐71‐6 | Identified in banana |
| 09.323 | sec‐Butyl acetate |
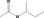
|
105‐46‐4 | Quantified in vinegar from 43 up to 67 mg/kg. Identified in banana, beans, beer, cheddar cheese, cheese various types, cocoa category, coffee, potato and walnut |
| 09.325 | sec‐Butyl butyrate |

|
819‐97‐6 | Quantified in strawberry from 0.0054 up to 0.0086 mg/kg and identified in cheddar cheese, cheese various types, custard apple, atemoya, plum and tomato |
| 09.328 | sec‐Butyl formate |
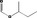
|
589‐40‐2 | Identified in apple fresh and cheese various types |
| 09.332 | sec‐Butyl hexanoate |
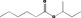
|
820‐00‐8 | No product occurrence data |
| 09.386 | sec‐Hept‐4(cis)‐enyl acetate |

|
94088‐33‐2 | Quantified in banana up to 0.18 mg/kg |
| 09.388 | sec‐Heptyl acetate |

|
5921‐82‐4 | Quantified in guava, feyoa and passion fruit from 0.01 up to 0.563 mg/kg and up to 400 mg/kg in cloves. Identified in banana, beans, soybean and strawberry |
| 09.391 | sec‐Heptyl hexanoate |

|
6624‐58‐4 | Quantified in passion fruit from 0.036 up to 6,634 mg/kg and identified in banana and strawberry |
| 09.604 | Isopropyl decanoate |

|
2311‐59‐3 | Identified in blue cheeses, citrus fruits and strawberry |
| 09.605 | Isopropyl dodecanoate |

|
10233‐13‐3 | Identified in blue cheeses and melon |
| 09.606 | Isopropyl hexadecanoate |
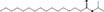
|
142‐91‐6 | Quantified in macadamia nut up to 0.04 mg/kg and identified in buckwheat and citrus fruits |
| 09.608 | Isopropyl octanoate |

|
5458‐59‐3 | Identified in blue cheeses, nectarine and strawberry |
| 09.609 | Isopropyl valerate |

|
18362‐97‐5 | Identified in cashew apple, cheddar cheese and vanilla |
| 09.676 | sec‐Octyl acetate |

|
2051‐50‐5 | Identified in chicken |
| 09.880 | (Z)‐Hept‐4‐enyl‐2 butyrate |
|
233666‐01‐8 | No product occurrence data |
| 09.926 | Octan‐3‐yl formate |

|
84434‐65‐1 | No product occurrence data |
* Triskelion, VCF online, Volatile Compounds in Food. Version 16.2 released by 16 January 2016.
Suggested citation: EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) , Silano V, Bolognesi C, Castle L, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Gürtler R, Husøy T, Kärenlampi S, Milana MR, Penninks A, Tavares Poças MF, Smith A, Tlustos C, Wölfle D, Zorn H, Zugravu C‐A, Beckman Sundh U, Brimer L, Mosesso P, Mulder G, Anastassiadou M and Mennes W, 2017. Scientific Opinion on Flavouring Group Evaluation 7, Revision 5 (FGE.07Rev5): saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched‐chain carboxylic acids from chemical group 5. EFSA Journal 2017;15(3):4725, 81 pp. doi: 10.2903/j.efsa.2017.4725
Requestor: European Commission
Question numbers: EFSA‐Q‐2016‐00467, 00470, 00471, 00472
Panel members: Claudia Bolognesi, Laurence Castle, Jean‐Pierre Cravedi, Karl‐Heinz Engel, Paul Fowler, Roland Franz, Konrad Grob, Rainer Gürtler, Trine Husøy, Sirpa Kärenlampi, Wim Mennes, Maria Rosaria Milana, André Penninks, Maria de Fátima Tavares Poças, Vittorio Silano, Andrew Smith, Christina Tlustos, Detlef Wölfle, Holger Zorn and Corina‐Aurelia Zugravu.
Acknowledgements: The Panel wishes to thank the members of the Working Group on Flavourings: Ulla Beckman Sundh, Leon Brimer, Karl‐Heinz Engel, Paul Fowler, Rainer Gürtler, Trine Husøy, Wim Mennes and Gerard Mulder for the preparatory work on this scientific opinion and the Working Group on Genotoxicity: Mona‐Lise Binderup, Claudia Bolognesi, Riccardo Crebelli, Rainer Gürtler, Francesca Marcon, Daniel Marzin and Pasquale Mosesso for the preparatory work on this scientific opinion and the hearing experts: Vibe Beltoft and Karin Nørby, and EFSA staff: Maria Anastassiadou, Maria Carfi and Annamaria Rossi for the support provided to this scientific opinion.
Adopted: 1 February 2017
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. Official Journal of the European Communities 19.7.2000, L 180, p. 8–16.
EU figure 375 million. This figure relates to EU population at the time for which production data are available, and is consistent (comparable) with evaluations conducted prior to the enlargement of the EU. No production data are available for the enlarged EU (Eurostat, 1998).
’Normal use’ is defined as the average of reported usages and'maximum us’ is defined as the 95th percentile of reported usages (EFFA, 2002d).
The normal and maximum use levels in different food categories have been extrapolated from figures derived from 12 model flavouring substances (EFFA, 2004).
The use levels from food category 5'Confectionery’ have been inserted as default values for food category 14.2'Alcoholic beverages’ for substances for which no data have been given for food category 14.2 (EFFA, 2007a).
‘Innocuous metabolic products’: Products that are known or readily predicted to be harmless to humans at the estimated intakes of the flavouring agent (JECFA, 1997).
‘Endogenous substances’: Intermediary metabolites normally present in human tissues and fluids, whether free or conjugated; hormones and other substances with biochemical or physiological regulatory functions are not included (JECFA, 1997).
References
- Abbondandolo A, Bonatti S, Corsi C, Corti G, Fiorio R, Leporini C, Mazzaccaro A, Nieri R, Barale R and Loprieno N, 1980. The use of organic solvents in mutagenicity testing. Mutation Research, 79, 141–150. [DOI] [PubMed] [Google Scholar]
- Arndt R and Krisch K, 1973. Catalytic properties of an unspecific carboxylesterase (E1) from rat‐liver microsomes. European Journal of Biochemistry, 36, 129–134. [DOI] [PubMed] [Google Scholar]
- Azizan A and Blevins RD, 1995. Mutagenicity and antimutagenicity testing of six chemicals associated with the pungent properties of specific spices as revealed by the Ames Salmonella/microsomal assay. Archives of Environmental Contamination and Toxicology, 28, 248–258. [DOI] [PubMed] [Google Scholar]
- Barber ED, Miller KR, Banton MI and Reddy MV, 1999. The lack of binding of methyl‐n‐amyl ketone (MAK) to rat liver DNA as demonstrated by direct binding measurements, and 32P‐postlabeling techniques. Mutation Research, 442, 133–147. [DOI] [PubMed] [Google Scholar]
- BASF (Baden Aniline and Soda Factory), 1969. Abteilung Toxikologie, unveroeffentlichte Untersuchung (XIX/60). Cited in European Commission ‐ European Chemicals Bureau, 1996. IUCLID Dataset, CAS no. 96‐22‐0. Section 5.1.1 Acute oral toxicity.
- BASF (Baden Aniline and Soda Factory), 1975. Abteilung Toxikologie, unveroeffentlichte Untersuchung (XXIV/164). Cited in European Commission ‐ European Chemicals Bureau, 1996. IUCLID Dataset, CAS no. 928‐68‐7. Section 5.1.1 Acute toxicity.
- BASF (Baden Aniline and Soda Factory), 1988. Abteilung Toxikologie, unveroeffentlichte Untersuchung (88/596). Cited in European Commission ‐ European Chemicals Bureau, 1996. IUCLID Dataset, CAS no. 502‐69‐2. Section 5.1.1 Acute toxicity.
- BASF (Baden Aniline and Soda Factory), 1989a. Abteilung Toxikologie, unveroeffentlichte Untersuchung (88/61). Cited in European Commission ‐ European Chemicals Bureau, 1996. IUCLID Dataset, CAS no. 928‐68‐7. Section 5.5 Genetic toxicity ‘in Vitro’.
- BASF (Baden Aniline and Soda Factory), 1989b. Abteilung Toxikologie, unveroeffentlichte Untersuchung (88/596). Cited in European Commission ‐ European Chemicals Bureau, 1996. IUCLID Dataset, CAS no. 502‐69‐2. Section 5.5 Genetic toxicity ‘in Vitro’.
- Basler A, 1986. Aneuploidy‐inducing chemicals in yeast evaluated by the micronucleus test. Mutation Research, 174, 11–13. [DOI] [PubMed] [Google Scholar]
- Beevers C, 2009. Reverse mutation in five histidine‐requiring strains of Salmonella typhimurium. Pseudo‐lonone. Covance Laboratories Ltd, England. Study no. 8200454. July 2009. Unpublished report submitted by EFFA to FLAVIS Secretariat.
- Bevan C, Tyler TR, Gardiner TH, Kapp RW Jr, Andrews L and Beyer BK, 1995. Two‐generation reproduction toxicity study with isopropanol in rats. Journal of Applied Toxicology, 15, 117–123. [DOI] [PubMed] [Google Scholar]
- Blevins RD and Taylor DE, 1982. Mutagenicity screening of twenty‐five cosmetic ingredients with the Salmonella/microsome test. Journal of Environmental Science and Health, Part A, 17, 217–239. [Google Scholar]
- Bonte W, Rüdell E, Sprung R, Frauenrath C, Blanke E, Kupilas G, Wochnik J and Zah G, 1981. Experimental investigations concerning the blood‐analytical detection of small doses of higher aliphatic alcohols in man. Blutalkohol, 18, 399–411. [Google Scholar]
- Bosron WF and Li TK, 1980. Alcohol dehydrogenase In: Jakoby WB. (ed.). Enzymatic Basis of Detoxification. Vol 1 Academic Press, New York: pp. 231–248. [Google Scholar]
- Bowen R, 2013. Oct‐1‐en‐3‐one: Investigation into the mechanism of mutagenicity: reverse mutation in one histidine‐requiring strain of Salmonella typhimurium. Covance Laboratories Ltd. Study no. 8281446. 7 August 2013. Unpublished report submitted to DG SANTE.
- Brooks TM, Meyer AL and Hutson DH, 1988. The genetic toxicology of some hydrocarbon and oxygenated solvents. Mutagenesis, 3, 227–232. [DOI] [PubMed] [Google Scholar]
- Brown WD, Setzer JV, Dick RB, Phipps FC and Lowry LK, 1987. Body burden profiles of single and mixed solvent exposures. Journal of Occupational Medicine, 29, 877–883. [PubMed] [Google Scholar]
- Carlson GL, Hall IH and Piantadosi C, 1975. Cycloalkanones. VII. Hypocholesterolemic activity of aliphatic compounds related to 2,8‐dibenzylcyclooctane. Journal of Medicinal Chemistry, 18, 1024–1026. [DOI] [PubMed] [Google Scholar]
- Carpenter CP, Weil CS and Smyth HF, 1974. Range‐finding toxicity data. List VIII. Toxicology and Applied Pharmacology, 28, 313–319. [DOI] [PubMed] [Google Scholar]
- Chiappe C, De Rubertis A, Amato G and Gervasi PG, 1998. Stereochemistry of the biotransformation of 1‐hexene and 2‐methyl‐1‐hexene with rat liver microsomes and purified P450s of rats and humans. Chemical Research in Toxicology, 11, 1487–1493. [DOI] [PubMed] [Google Scholar]
- CMA (Chemical Manufacturers Association), 1990. Submission to EPA ‐ mutagenicity test on isopropanol in the CHO/HGPRT forward mutation assay with independent repeat. Chemical Manufacturers Association. Cox GV. Project no. 22207. June 1, 1990. Unpublished report submitted by EFFA to SCF.
- CoE (Council of Europe), 1992. Flavouring substances and natural sources of flavourings. 4th Ed. vol. I. Chemically defined flavouring substances. Council of Europe, partial agreement in the social and public health field. Strasbourg.
- Colaianni LJ, 1967. Acute toxicity, eye and skin irritation tests on aromatic compounds. Unpublished data submitted by EFFA to SCF.
- Cramer GM, Ford RA and Hall RL, 1978. Estimation of toxic hazard ‐ a decision tree approach. Food and Cosmetics Toxicology, 16, 255–276. [DOI] [PubMed] [Google Scholar]
- Csato M and Chubb DR, 1996. Skin sensitization with Ro 02‐2438 (Pseudoionon) in the guinea pig. Quintiles England Ltd. No. A/K/42470. 28 November 1996. Study summarized in OECD SIDS, UNEP Publications 2004.
- Dietz D, 1991. Toxicity studies of acetone in F344/N rats and B6C3F1 mice (drinking water studies). National Toxicology Program. NIH Publication no. 91‐3122, January 1991. Unpublished report submitted by EFFA to SCF.
- Dietz FK, Rodriguez‐Giaxola M, Traiger GJ, Stella VJ and Himmelstein KJ, 1981. Pharmacokinetics of 2‐butanol and its metabolites in the rat. Journal of Pharmacokinetics and Biopharmaceutics, 9, 553–576. [DOI] [PubMed] [Google Scholar]
- DiVincenzo GD, Kaplan CJ and Dedinas J, 1976. Characterization of the metabolites of methyl n‐butyl ketone, methyl iso‐butyl ketone, and methyl ethyl ketone in guinea pig serum and their clearance. Toxicology and Applied Pharmacology, 36, 511–522. [DOI] [PubMed] [Google Scholar]
- Douglas GR, Nestmann ER, Betts JL, Mueller JC, Lee EGH, Stich HF, San HC, Brouzes RJP, Chmelauskas AL, Paavila HD and Walden CC, 1980. Mutagenic activity in pulp mill effluents In: Jolley RL, Brungs WA, Cumming RB. and Jacobs VA. (eds.). Water Chlorination: environmental Impact and Health Effects. Vol 3 Ann Arbor Science Publishers Inc., Ann Arbor, MI: pp. 865–880. [Google Scholar]
- Eder E, Henschler D and Neudecker T, 1982. Mutagenic properties of allylic and a, ß‐unsaturated compounds: consideration of alkylating mechanisms. Xenobiotica, 12, 831–848. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Minutes of the 7th Plenary Meeting of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food, Held in Brussels on 12‐13 July 2004. Brussels. Available online: http://www.efsa.europa.eu/en/events/event/afc040712-m.pdf/ [Accessed: 28 September 2004].
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2011. Scientific Opinion on Flavouring Group Evaluation 206 (FGE.206): consideration of genotoxicity data on representatives for 12 α,β‐unsaturated ketones and precursors from chemical subgroup 1.2.3 of FGE.19 by EFSA. EFSA Journal 2011;9(3):1922, 16 pp. doi: 10.2903/j.efsa.2011.1922 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2012a. Scientific Opinion on Flavouring Group Evaluation 205 (FGE.205): consideration of genotoxicity data on representatives for 13 alpha, beta‐unsaturated aliphatic ketones with terminal double bonds and precursors from chemical subgroup 1.2.2 of FGE.19 by EFSA. EFSA Journal 2012;10(10):2902, 22 pp. doi: 10.2903/j.efsa.2012.2902 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2012b. Minutes of the 28th Plenary meeting of the CEF Panel. Held in Parma. Available online: http://www.efsa.europa.eu/en/events/event/120703b.htm [Accessed: 3–5 July 2012].
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2016. Scientific opinion of Flavouring Group Evaluation 205 Revision 1 (FGE.205Rev1): consideration of genotoxicity data on representatives for 13 α,β‐unsaturated aliphatic ketones with terminal double bonds and precursors from chemical subgroup 1.2.2 of FGE.19. EFSA Journal 2016;14(7):4535. doi: 10.2903/j.efsa.2016.4535 [DOI] [Google Scholar]
- Epstein WL, 1978. Report to RIVM. 25 August and 7 September. Cited in Ford RA, 1988. Food and Chemical Toxicology, 26, 311. [Google Scholar]
- Eurostat , 1998. Total population. Available online: http://ec.europa.eu/eurostat/web/population-demography-migration-projections/population-data/database
- FDA (Food and Drug Administration), 1993. Priority‐based assessment and food additives (PAFA) database. Center for food safety and applied nutrition, p. 58. [Google Scholar]
- Felsted RL and Bachur NR, 1980. Ketone reductases In: Jakoby WB. (ed.). Enzymatic Basis of Detoxification. Vol I Academic Press, New York: pp. 281–293. [Google Scholar]
- Florin I, Rutberg L, Curvall M and Enzell CR, 1980. Screening of tobacco smoke constituents for mutagenicity using the Ames’ test. Toxicology, 18, 219–232. [DOI] [PubMed] [Google Scholar]
- Food and Drug Research Laboratories Inc , 1976. Submission of data by CTFA. Unpublished data on octyl palmitate. Acute oral toxicity in rats. Cited in Anonymous, 1982 Final report on the safety assessment of octyl palmitate, cetyl palmitate and isopropyl palmitate. Journal of the American College of Toxicology, 1, 13–35. [Google Scholar]
- Ford RA, Letizia C and Api AM, 1988. Monographs on fragrance raw materials. 6,10‐dimethyl‐3,5,9‐undecatriene‐2‐one. Food and Chemical Toxicology, 26, 311. [DOI] [PubMed] [Google Scholar]
- Gangolli SD and Shilling WH, 1968. Hydrolysis of esters by artificial gastric and pancreatic juices. Research report no. 11/1968. Unpublished report submitted by EFFA to SCF.
- Gaunt IF, Carpanini FMB, Wright MG, Grasso P and Gangolli SD, 1972. Short‐term toxicity of methyl amyl ketone in rats. Food and Cosmetics Toxicology, 10, 625–636. [DOI] [PubMed] [Google Scholar]
- Gill MW and Van Miller JP, 1987. Fourteen‐day dietary minimum toxicity screen (MTS) in albino rats. 4(2‐Furyl)‐3‐buten‐2‐one, 3‐oxotetradecanoic acid ester of hydrogenated palm oil, 3‐oxooctanoic acid ester of hydrogenated palm oil, pentadean‐2‐one, O‐methoxybenzaldehyde. Bushy Run Research Center. Project report 50‐528. August 31, 1987. Unpublished data submitted by EFFA to SCF.
- deGroot AP, Spanjers MT and van der Heijden CA, 1974. Acute and sub‐acute oral toxicity studies in rats with five flavour compounds. Central Institute for Nutrition and Food Research. Report no. R 4284. January 1974. Unpublished report submitted by EFFA to SCF.
- Grundschober F, 1977. Toxicological assessment of flavouring esters. Toxicology, 8, 387–390. [DOI] [PubMed] [Google Scholar]
- Haggard HW, Miller DP and Greenberg LA, 1945. The amyl alcohols and their ketones: their metabolic fates and comparative toxicities. Journal of Industrial Hygiene and Toxicology, 27, 1–14. [Google Scholar]
- Heymann E, 1980. Carboxylesterases and amidases In: Jakoby WB. (ed.). Enzymatic Basis of Detoxication, 2nd Edition Academic Press, New York: pp. 291–323. [Google Scholar]
- Hofmann W, 1978. Acute Oral Toxicity in Rat (Goranylaceton R). BASF. Substance no. 77/274. 30.10.78. Unpublished report submitted by EFFA to FLAVIS Secretariat. (In German).
- Homan ER and Maronpot RR, 1978. Neurotoxic evaluation of some aliphatic ketones. Toxicology and Applied Pharmacology, 45, 312. [Google Scholar]
- Ibatullina RB and Larionova TK, 1997. Toxicity of diethyl ketone. Meditsina Truda I Promyshlennaya Ekologiya, 4, 41–42 (In Russian). [Google Scholar]
- IBM Corp. , 1989. A subchronic oral toxicity study of 5‐methyl‐3‐heptanone in the rat utilizing a functional observational battery and neuropathology to detect neurotoxicity with cover letter 121589. EPA Doc ID 89‐900000074, microfiche no. OTS0521291‐1. November 15, 1989. Unpublished data submitted by EFFA to SCF.
- IFRA (International Fragrance Association), 2002. Pseudoionone. [Online]. Available online: http://www.ifraorg.org/en-us/search/s/Pseudoionone [Accessed: 18 September 2012].
- Ishizaki M, Oyamada N, Ueno S, Katsumura K and Hosogai Y, 1979. Mutagenicity of degradation and reaction products of butyl hydroxy anisol with sodium nitrite or potassium nitrate by irradiation of ultra violet ray. Journal of the Food Hygienic Society of Japan, 20, 143–146. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1980. Evaluation of certain food additives. Twenty‐third report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 648, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1995. Evaluation of certain food additives and contaminants. Forty‐fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives. 14–23 February 1995. WHO Technical Report Series, no. 859. Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996. Toxicological evaluation of certain food additives. Forty‐fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives and contaminants. WHO Food Additives Series: 35. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1997. Evaluation of certain food additives and contaminants. Forty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 6–15 February 1996. WHO Technical Report Series, no. 868. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1998. Compendium of food additive specifications. Addendum 6. Joint FAO/WHO Expert Committee of Food Additives 51st session. Geneva, 9–18 June 1998. FAO Food and Nutrition paper 52 Add. 6. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999a. Safety evaluation of certain food additives. Fifty‐first Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series: 42. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999b. Evaluation of certain food additives and contaminants. Forty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Rome, 17–26 June 1997. WHO Technical Report Series, no. 884. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000a. Evaluation of certain food additives. Fifty‐first Meeting of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 9–18 June 1998. WHO Technical Report Series, no. 891. Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000b. Compendium of food additive specifications. Addendum 8. Joint FAO/WHO Expert Committee of Food Additives. Fifty‐fifth Meeting. Geneva, 6–15 June 2000. FAO Food and Nutrition paper 52 Add. 8.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001. Compendium of food additive specifications. Addendum 9. Joint FAO/WHO Expert Committee of Food Additives 57th session. Rome, 5–14 June 2001. FAO Food and Nutrition paper 52 Add. 9.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002a. Evaluation of certain food additives. Fifty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 913. Geneva, 4–13 June 2002.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002b. Compendium of food additive specifications. Addendum 10. Joint FAO/WHO Expert Committee of Food Additives 59th session. Geneva, 4–13 June 2002. FAO Food and Nutrition paper 52 Addition 10.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2003. Safety evaluation of certain food additives. Fifty‐ninth Meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 50. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2009a. JECFA Online Edition “Specification for Flavourings”. Available online: http://www.fao.org/ag/agn/jecfa-flav/search.html [Accessed: May, 2009].
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2009b. Evaluation of certain food additives. Sixty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 952. Rome, 17‐26 June 2008. Available online: http://whqlibdoc.who.int/trs/WHO_TRS_952_eng.pdf [Accessed: May 2009].
- Junge W and Heymann E, 1979. Characterization of the isoenzymes of pig liver esterase. II. Kinetic studies. European Journal of Biochemistry, 95, 519–525. [DOI] [PubMed] [Google Scholar]
- Kamil IA, Smith JN and Williams RT, 1953. Studies in detoxication. 46. The metabolism of aliphatic alcohols. The glucuronic acid conjugation of acyclic aliphatic alcohols. Biochemical Journal, 53, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp RW, Marino DJ, Gardiner TH, Maston LW, McKee RH, Tyler TR, Ivett JL and Young RR, 1993. In vitro and in vivo assays of isopropanol for mutagenicity. Environmental and Molecular Mutagenesis, 22, 93–100. [DOI] [PubMed] [Google Scholar]
- Kasper CB and Henton D, 1980. Glucuronidation In: Jakoby WB. (ed.). Enzymatic Basis of Detoxification. Vol 2 Academic Press, New York: pp. 4–36. [Google Scholar]
- Katz G, O'Donoghue JL, DiVincenzo GD and Terhaar CJ, 1980. Comparative neurotoxicity and metabolism of ethyl n‐butyl ketone and methyl n‐buthyl ketone in rats. Toxicology and Applied Pharmacology, 52, 153–158. [DOI] [PubMed] [Google Scholar]
- Kawachi T, Yahagi T, Kada T, Tazima Y, Ishidate M, Sasaki M and Sugiyama T, 1980. Cooperative programme on short‐term assays for carcinogenicity in Japan. IARC Scientific Publications, 27, 323–330. [PubMed] [Google Scholar]
- Keating JW, 1972. Acute oral toxicity (rat‐5 gms/kg body weight dose). Dermal toxicity (rabbit‐5 gms/kg body weight dose). Amyris acetylated, Bois de rose acetylated, Cadinene, Castoreum, Lavandin acetylated, Dihydrojasmone, Trans‐2‐hexenol, Methyl isoeugenol, Methyl eugenol, Santalyl acetate, Phenyl propyl cinnamate, Phenylacetic acid, 1‐Carveol, Santatol, Methyl heptenone. Biological Science Laboratories. Unpublished report submitted by EFFA to SCF.
- Kennedy GL Jr and Graepel GJ, 1991. Acute toxicity in the rat following either oral or inhalation exposure. Toxicology Letters, 56, 317–326. [DOI] [PubMed] [Google Scholar]
- Kimura ET, Ebert DM and Dodge PW, 1971. Acute toxicity and limits of solvent residue for sixteen organic solvents. Toxicology and Applied Pharmacology, 19, 699–704. [DOI] [PubMed] [Google Scholar]
- Kligman AM, 1976. Report to RIVM, 11 May and 23 July. Cited in Ford RA, 1988. Food and Chemical Toxicology, 26, 311. [Google Scholar]
- Kohli RP, Kishor K, Dua PR and Saxena RC, 1967. Anticonvulsant activity of some carbonyl containing compounds. Indian Journal of Medical Research, 55, 1221–1225. [PubMed] [Google Scholar]
- Kolmar Research Center , 1972. The toxicological examination of wickhen isopropyl palmiatae (Wickenol 111). In: Anonymous, 1982. Final report on the safety assessment of octyl palmitate, cetyl palmitate and isopropyl palmitate. Journal of the American College of Toxicology 1(2), 13–35.
- Krasavage WJ and O'Donoghue JL, 1979. Repeated oral administration of five ketones and n‐heptane to rats. March 1, 1979. Unpublished report submitted by EFFA to SCF.
- Krasavage WJ, O'Donoghue JL and DiVincenzo GD, 1982. Ketones In: Clayton GD. and Clayton FE. (eds.). Patty's Industrial Hygiene and Toxicology, 3rd Edition Vol IIC John Wiley and Sons, New York: pp. 4709–4800. [Google Scholar]
- Bio‐Toxicology Laboratories , 1982. Final report on the safety assessment of octyl palmitate, cetyl palmitate and isopropyl palmitate. Unpublished data on octyl palmitate. Journal of the American College of Toxicology, 1, 13–35. [Google Scholar]
- Leegwater DC and van Straten S, 1974a. In vitro study of the hydrolysis of twenty‐six organic esters by pancreatin. Central Institute for Nutrition and Food Research. Report no. R 4319. Project no. 8.33.01. February, 1974.
- Leegwater DC and van Straten S, 1974b. In vitro study on the hydrolysis of eight carboxylic esters by intestinal and liver enzymes. Central Institute for Nutrition and Food Research. Report no. R 4414. Project no. 8.33.06. August, 1974.
- Lehman AJ and Chase HF, 1944. The acute and chronic toxicity of isopropyl alcohol. Journal of Laboratory and Clinical Medicine, 29, 561–567. [Google Scholar]
- Lehman AJ, Schwerma H and Rickards E, 1945. Isopropyl alcohol. Acquired tolerance in dogs, rate of disappearance from the blood stream in various species, and effects on successive generations of rats. Journal of Pharmacology and Experimental Therapeutics, 85, 61–69. [PubMed] [Google Scholar]
- Lloyd M, 2010. Induction of micronuclei in cultured human peripheral blood lymphocytes. Pseudo‐ionone. Covance Laboratories Ltd. Study no. 8218056. April 2010. Unpublished report submitted by EFFA to FLAVIS Secretariat.
- Longland RC, Shilling WH and Gangolli SD, 1977. The hydrolysis of flavouring esters by artificial gastrointestinal juices and rat tissue preparations. Toxicology, 8, 197–204. [DOI] [PubMed] [Google Scholar]
- Loveday KS, Anderson BE, Resnick MA and Zeiger E, 1990. Chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. V. Results with 46 chemicals. Environmental and Molecular Mutagenesis, 16, 272–303. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H and Ames BN, 1985. Naturally‐occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutation Research, 148, 25–34. [DOI] [PubMed] [Google Scholar]
- McCann J, Choi E, Yamasaki E and Ames BN, 1975. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proceedings of the National Academy of Sciences of the United States of America, 72, 5135–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOmie WA and Anderson HH, 1949. Comparative toxicologic effects of some isobutyl carbinols and ketones. University of California Publications in Pharmacology, 2, 217–230. [Google Scholar]
- Moreno OM, 1976. Report to RIFM, 13 May. Cited in Ford RA, Letizia C and Api AM, 1988. 6,10‐ dimethyl‐3,5,9‐undecatriene‐2‐one. Food and Chemical Toxicology 26, 311–312. [Google Scholar]
- Moreno OM, 1977. Acute toxicity study in rats. Dermal toxicity in rabbits. Tetrahydro pseudo ionone. MB Research Laboratories, Inc. Project no. MB 77‐1711. July 6, 1977. Unpublished report submitted by EFFA to SCF.
- Moreno OM, 1978. Report to RIFM, 1 February. Isopropyl palmitate. Cited in Opdyke DLJ and Letizia C, 1982. Monographs on fragrance raw materials. Food and Chemical Toxicology 20(Suppl.), 727–728. [DOI] [PubMed] [Google Scholar]
- Moreno OM, 1982. Oral toxicity in mice. Dermal toxicity in guinea pigs. MB Research Laboratories, Inc. Project no. MB 81‐5688. Date 2/22/82. Unpublished data submitted by EFFA to SCF.
- Morgott DA, 1993. Acetone In: Clayton GD, Clayton FE. (eds.). Patty's Industrial Hygiene and Toxicology. 4th Ed. Vol. II, Part A, John Wiley & Sons, New York, pp. 149–281. [Google Scholar]
- Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B and Zeiger E, 1986. Salmonella mutagenicity tests II. Results from the testing of 270 chemicals. Environmental and Molecular Mutagenesis, 8(Suppl. 7), 1–119. [PubMed] [Google Scholar]
- Müller W, Engelhart G, Herbold B, Jäckh R and Jung R, 1993. Evaluation of mutagenicity testing with Salmonella typhimurium TA102 in three different laboratories. Environmental Health Perspectives (Suppl. 101(3)), 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch JC, 1972. Aliphatic alcohols and alkyl esters: narcotic and lethal potencies to tadpoles and to rabbits. Industrial Medicine, 41, 31–33. [PubMed] [Google Scholar]
- Nordmann R, Ribiere C, Rouach H, Beauge F, Giudicelli Y and Nordmann J, 1973. Metabolic pathways involved in the oxidation of isopropanol into acetone by the intact rat. Life Sciences, 13, 919–932. [DOI] [PubMed] [Google Scholar]
- Norppa H, Vainio H and Sorsa M, 1983. Metabolic activation of styrene by erythrocytes detected as increased sister chromatid exchanges in cultured human lymphocytes. Cancer Research, 43, 3579–3582. [PubMed] [Google Scholar]
- O'Donoghue JL and Krasavage WJ, 1980. 90‐Day repeated oral administration of five ketones and n‐heptane to rats. January 21, 1980. Unpublished report submitted by EFFA to SCF.
- O'Donoghue JL, Krasavage WJ, DiVincenzo GD and Katz GV, 1984. Further studies on ketone neurotoxicity and interactions. Toxicology and Applied Pharmacology, 72, 201–209. [DOI] [PubMed] [Google Scholar]
- O'Donoghue JL, Haworth SR, Curren RD, Kirby PE, Lawlor T, Moran EJ, Phillips RD, Putnam DL, Rogers‐Back AM, Slesinski RS and Thilagar A, 1988. Mutagenicity studies on ketone solvents: methyl ethyl ketone, methyl isobutyl ketone, and isophorone. Mutation Research, 206, 149–161. [DOI] [PubMed] [Google Scholar]
- Panson RD and Winek CL, 1980. Aspiration toxicity of ketones. Clinical Toxicology, 17, 271–317. [DOI] [PubMed] [Google Scholar]
- Patty FA, Schrenk HH and Yant WP, 1935. Acute response of guinea pigs to vapors of some new commercial organic compounds. VIII. Butanone. Public Health Reports, 50, 1217–1228. [Google Scholar]
- Perocco P, Bolognesi S and Alberghini W, 1983. Toxic activity of seventeen industrial solvents and halogenated compounds on human lymphocytes cultured in vitro . Toxicology Letters, 16, 69–75. [DOI] [PubMed] [Google Scholar]
- Pilegaard K and Ladefoged O, 1993. Toxic effects in rats of twelve weeks dosing of 2‐propanol, and neurotoxicity measured by densitometric measurements of glial fibrillary acidic protein in the dorsal hippocampus. In Vivo, 7, 325–330. [PubMed] [Google Scholar]
- Pozzani UC, Weil CS and Carpenter CP, 1959. The toxicological basis of threshold limit values: 5. The experimental inhalation of vapor mixtures by rats, with notes upon the relationship between single dose inhalation and single dose oral data. American Industrial Hygiene Association Journal, 20, 364–369. [DOI] [PubMed] [Google Scholar]
- Rapson WH, Nazar MA and Butzky VV, 1980. Mutagenicity produced by aqueous chlorination of organic compounds. Bulletin of Environmental Contamination and Toxicology, 24, 590–596. [DOI] [PubMed] [Google Scholar]
- RTECS (Registry of Toxic Effects of Chemical Substances), 1975. 2,6‐Dimethyl‐4‐heptanone. National Institute of Occupational Safety & Health (U.S.).
- Sanchez RI and Kauffman FC, 2010. Regulation of Xenobiotic Metabolism in the Liver In: MCQueen CA, Roth RA, Ganey P. (eds.). Comprehensive Toxicology 2nd Edition. Vol 9, pp. 109–128. Elsevier, Amsterdam: Availabel online: 10.1016/B978-0-08-046884-6.01005-8 [DOI] [Google Scholar]
- Sasaki M, Sugimura K, Yoshida MA and Abe S, 1980. Cytogenetic effects of 60 chemicals on cultured human and Chinese hamster cells. Kromosomo, 20, 574–584. [Google Scholar]
- SCCNFP (Scientific Committee on Cosmetic Products and Non‐Food Products), 2000. Opinion of the Scientific Committee on Cosmetic Products and Non‐food Products intended for Consumers concerning an initial list of perfumery materials which must not form part of fragrance compounds used in cosmetic products. Adopted by the SCCNFP during the 12th Plenary meeting. 3 May, 2000. SCCNFP/0320/00, final. Health and Consumers Scientific Committees. Available online: https://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out116_en.pdf
- SCF (Scientific Committee for Food), 1995. Scientific Committee for Food. First annual report on chemically defined flavouring substances. May 1995, 2nd draft prepared by the SCF Working Group on Flavouring Substances (Submitted by the SCF Secretariat, 17 May 1995). CS/FLAV/FL/140‐Rev2. Annex 6 to Document III/5611/95, European Commission, Directorate‐General III, Industry.
- SCF (Scientific Committee for Food), 1999. Opinion on a programme for the evaluation of flavouring substances (expressed on 2 December 1999). Scientific Committee on Food. SCF/CS/FLAV/TASK/11 Final 6/12/1999. Annex I to the minutes of the 119th Plenary meeting. European Commission, Health & Consumer Protection Directorate‐General.
- Schafer EW and Bowles WA, 1985. The acute oral toxicity and repellency of 933 chemicals to house and deer mice. Archives of Environmental Contamination and Toxicology, 14, 111–129. [DOI] [PubMed] [Google Scholar]
- Schwartz L, 1989. On the oxidation of acetones and homologous ketones from fatty acids. Archiv für Experimentelle Pathologie und Pharmakologie, 40, 168–194 (In German). [Google Scholar]
- Scopinaro D, Ghiani P and De Cecco A, 1947. Ketolytic fate of acetone. II. Acetone metabolism in normal subjects. Policlinico ‐ Sezione Medica, 54, 70–84 (In Italian). [PubMed] [Google Scholar]
- Shelanski MV and Moldovan M, 1973. Acute oral toxicity (rats ‐ 5 gms/kg body weight dose). Dermal toxicity (rabbits ‐ 5 gms/kg body weight dose). Geranyl isobutyrate. Food and Drug Research Laboratories, Inc. IBL no. 12207‐F. 30 January 1973. Unpublished report submitted by EFFA to SCF.
- Shimizu H, Suzuki Y, Takemura N, Goto S and Matsushita H, 1985. The results of microbial mutation test for forty‐three industrial chemicals. Japanese Journal of Industrial Health, 27, 400–419. [DOI] [PubMed] [Google Scholar]
- Smyth HF Jr and Carpenter CP, 1948. Further experience with the range‐finding test in the industrial toxicology laboratory. Journal of Industrial Hygiene and Toxicology, 30, 63–68. [PubMed] [Google Scholar]
- Smyth HF Jr, Carpenter CP and Weil CS, 1949. Range‐finding toxicity data. List III. Journal of Industrial Hygiene and Toxicology, 31, 60–62. [PubMed] [Google Scholar]
- Smyth HF Jr, Carpenter CP and Weil CS, 1951. Range finding toxicity data: list IV. Archives of Industrial Hygiene and Occupational Medicine, 4, 119–122. [PubMed] [Google Scholar]
- Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC and Striegel JA, 1962. Range‐finding toxicity data: list VI. American Industrial Hygiene Association Journal, 23, 95–107. [DOI] [PubMed] [Google Scholar]
- Smyth HF Jr, Weil CS, West JS and Carpenter CP, 1969. An exploration of joint toxic action: twenty‐seven industrial chemicals intubated in rats in all possible pairs. Toxicology and Applied Pharmacology, 14, 340–347. [DOI] [PubMed] [Google Scholar]
- Smyth HF Jr, Weil CS, West JS and Carpenter CP, 1970. An exploration of joint toxic action. II. Equitoxic versus equivolume mixtures. Toxicology and Applied Pharmacology, 17, 498–503. [DOI] [PubMed] [Google Scholar]
- Sonawane B, de Rosa C, Rubenstein R, Mayhew D, Becker SV and Dietz D, 1986. Estimation of reference dose (RfD) for oral exposure of acetone. Journal of the American College of Toxicology, 5, 605. [Google Scholar]
- Spencer PS, Bischoff MC and Schaumburg HH, 1978. On the specific molecular configuration of neurotoxic aliphatic hexacarbon compounds causing central‐peripheral distal axonopathy. Toxicology and Applied Pharmacology, 44, 17–28. [DOI] [PubMed] [Google Scholar]
- Srepel B and Akacic B, 1962. Ispitivanje anthelmintièkog djelovanja eteriènih ulja roda Ruta. Acta Pharmaceutica Jugoslavica, 12, 79–87 (In Yugoslav). [Google Scholar]
- Tanii H, Tsuji H and Hashimoto K, 1986. Structure‐toxicity relationship of monoketones. Toxicology Letters, 30, 13–17. [DOI] [PubMed] [Google Scholar]
- Topping DC, Morgott DA, David RM and O'Donoghue JL, 1994. Ketones In: Clayton GD, Clayton FE. (eds.). Patty's Industrial Hygiene and Toxicology, 4th Edition. vol. 2C, John Wiley & Sons, Inc., New York, pp. 1739–1878. [Google Scholar]
- Tyl RW, Masten LW, Marr MC, Myers CB, Slauter RW, Gardiner TH, Strother DE, McKee RH and Tyler TR, 1994. Developmental toxicity evaluation of isopropanol by gavage in rats and rabbits. Fundamental and Applied Toxicology, 22, 139–151. [DOI] [PubMed] [Google Scholar]
- VCF online 2016. Triskelion, VCF online, Volatile Compounds in Food. Version 16.2 released by 16 January 2016.
- Whitwell J, 2010. Induction of micronuclei in cultured human peripheral blood lymphocytes. 6‐Methylhepta‐3,5‐dien‐2‐one. Covance Laboratories Ltd, England. Study no. 8218055. March 2010. Unpublished report submitted by EFFA to FLAVIS Secretariat.
- Willhite CC, 1986. Structure‐activity relationships of retinoids in developmental toxicology. II. Influence of the polyene chain of the Vitamin A molecule. Toxicology and Applied Pharmacology, 83, 563–575. [DOI] [PubMed] [Google Scholar]
- Williams L, 2009. Reverse mutation in five histidine‐requiring strains of Salmonella typhimurium. 6‐Methyl hepta‐3,5‐dien‐2‐one. Covance Laboratories Ltd, England. Study no. 8200456. August 2009. Unpublished report submitted by EFFA to FLAVIS Secretariat.
- Wills JH, Jameson EM and Coulston F, 1969. Effects on man of daily ingestion of small doses of isopropyl alcohol. Toxicology and Applied Pharmacology, 15, 560–565. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, 1982. Mutagenicity of trioses and methyl glyoxal on Salmonella typhimurium. Agricultural and Biological Chemistry, 46, 849–851. [Google Scholar]
- Yamaguchi T, 1985. Stimulating effects of organic solvents on the mutagenicities of sugar‐degradation compounds. Agricultural and Biological Chemistry, 49, 3363–3368. [Google Scholar]
- Zakhari S, Leibowitz M, Levy P and Aviado DM, 1977. Hemodynamic effects of methyl ethyl ketone inhalation in the dog In: Goldberg L. (ed.). Isopropanol and Ketones in the Environment. CRC Press Inc, Cleveland, Ohio: pp. 79–89. [Google Scholar]
- Zarani F, Papazafiri P and Kappas A, 1999. Induction of micronuclei in human lymphocytes by organic solvents in vitro . Journal of Environmental Pathology, Toxicology and Oncology, 18, 21–28. [PubMed] [Google Scholar]
- Zeiger E, Anderson B, Haworth S, Lawlor T and Mortelmans K, 1992. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environmental and Molecular Mutagenesis, 19, 2–141. [DOI] [PubMed] [Google Scholar]
- Zimmermann FK, Mayer VW, Scheel I and Resnick MA, 1985. Acetone, methyl ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic solvents are strong inducers of aneuploidy in Saccharomyces cerevisiae. Mutation Research, 149, 339–351. [DOI] [PubMed] [Google Scholar]


