Abstract
Ovine epididymitis (Brucella ovis) has been assessed according to the criteria of the Animal Health Law (AHL), in particular criteria of Article 7 on disease profile and impacts, Article 5 on the eligibility of ovine epididymitis to be listed, Article 9 for the categorisation of ovine epididymitis according to disease prevention and control rules as in Annex IV and Article 8 on the list of animal species related to ovine epididymitis. The assessment has been performed following a methodology composed of information collection and compilation, expert judgement on each criterion at individual and, if no consensus was reached before, also at collective level. The output is composed of the categorical answer, and for the questions where no consensus was reached, the different supporting views are reported. Details on the methodology used for this assessment are explained in a separate opinion. According to the assessment performed, ovine epididymitis can be considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL. The disease would comply with the criteria as in Sections 3, 4 and 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in points (c), (d) and (e) of Article 9(1). The animal species to be listed for ovine epididymitis according to Article 8(3) criteria are mainly sheep and other species of the families Bovidae and Cervidae as susceptible and sheep and deer as reservoirs.
Keywords: Ovine epididymitis, Brucella ovis, Animal Health Law, listing, categorisation, impact
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The background and Terms of Reference (ToR) as provided by the European Commission for the present document are reported in Section 1.2 of the scientific opinion on the ad hoc methodology followed for the assessment of the disease to be listed and categorised according to the criteria of Article 5, Annex IV according to Article 9, and 8 within the Animal Health Law (AHL) framework (EFSA AHAW Panel, 2017).
1.2. Interpretation of the Terms of Reference
The interpretation of the ToR is as in Section 1.2 of the scientific opinion on the ad hoc methodology followed for the assessment of the disease to be listed and categorised according to the criteria of Article 5, Annex IV according to Article 9, and 8 within the AHL framework (EFSA AHAW Panel, 2017).
The present document reports the results of assessment on ovine epididymitis (Brucella ovis) according to the criteria of the AHL articles as follows:
Article 7: ovine epididymitis profile and impacts
Article 5: eligibility of ovine epididymitis to be listed
Article 9: categorisation of ovine epididymitis according to disease prevention and control rules as in Annex IV
Article 8: list of animal species related to ovine epididymitis
2. Data and methodologies
The methodology applied in this opinion is described in detail in a dedicated document about the ad hoc method developed for assessing any animal disease for the listing and categorisation of diseases within the AHL framework (EFSA AHAW Panel, 2017).
3. Assessment
3.1. Assessment according to Article 7 criteria
This section presents the assessment of ovine epididymitis (Brucella ovis) according to the Article 7 criteria of the AHL and related parameters (see Table 2 of the opinion on methodology (EFSA AHAW Panel, 2017)), based on the information contained in the fact sheet as drafted by the selected disease scientist (see Section 2.1 of the scientific opinion on the ad hoc methodology) and amended by the AHAW Panel.
3.1.1. Article 7(a) Disease Profile
The disease affects sheep exclusively, causing genital lesions and overall reproductive failure. It is caused by Brucella ovis, a Gram‐negative and naturally rough (R) bacterium belonging to the genus Brucella. It has been historically misnamed as ‘ovine epididymitis’ and also ‘ram epididymitis’ or ‘contagious epididymitis’. However, as B. ovis infection can produce epididymitis in rams but also other clinical signs in both male and female sheep (as for example placentitis and abortion), and moreover, epididymitis in rams can be caused by a large variety of pathogenic agents (for a review see Blasco (1990, 2010), the term ‘Brucella ovis infection’1 is the preferred notation to properly denominate this disease. Accordingly, this latter denomination will be used throughout the document.
3.1.1.1. Article 7(a)(i) Animal species concerned by the disease
Susceptible animal species
Parameter 1 – Naturally susceptible wildlife species (or family/orders)
Natural infection has been mainly proven in domestic sheep; however, outbreaks have also been reported in farmed white‐tailed deer (Odocoileus virginianus) reared in direct contact with infected sheep (Bailey, 1997).
Parameter 2 – Naturally susceptible domestic species (or family/orders)
The natural infection appears mainly in sheep; however, experimental inoculation of B. ovis in male goats leads to both genital and extragenital colonisation in some animals, inducing subsequent pathological lesions like those produced in infected rams (García‐Carrillo et al., 1977; Burgess et al., 1985). In extensive breeding systems, goats and sheep are frequently managed together and, therefore, transmission from sheep to goats and vice versa could occur. However, isolation of B. ovis from natural cases of infection in goats has never been reported.
Parameter 3 – Experimentally susceptible wildlife species (or family/orders)
Transmission to red deer (Cervus elaphus elaphus) has been proven experimentally (Ridler et al., 2000), but since infection seems to be self‐limiting in the majority of stags exposed, it remains unclear if B. ovis infection can be sustained naturally in wild deer populations (Ridler et al., 2012).
Moreover, the infection has been suspected in bighorn sheep (Ovis canadensis canadensis), and these animals have proven susceptible to experimental infection, developing pathological signs characteristics of B. ovis infection including abortion, epididymitis and testicular swelling (McCollum et al., 2013). Moreover, mouflon (Ovis musimon) have been suspected of being affected, and experimental infection studies have been conducted. However, bacterial cultures after exposure were always negative, and no pathological lesions were evidenced after both clinical and histological examinations (Cerri et al., 2002).
Parameter 4 – Experimentally susceptible domestic species (or family/orders)
There are few references on experimental infections of domestic animals other than sheep. As commented above, experimental inoculation of B. ovis in male goats leads to genital and extragenital colonisation and development of lesions similar to those observed in naturally infected rams.
Several small laboratory animal species have been experimentally inoculated with B. ovis by a variety of routes, and doses, with varying success. At least rabbits, rats, gerbils, hamsters, guinea pigs and mice can be experimentally infected with B. ovis (for a review see Blasco (2010)).
Reservoir animal species
Parameter 5 – Wild reservoir species (or family/orders)
No wild species have been proven as a natural reservoir of B. ovis. Despite that, white‐tailed and red deer sharing infected environments and reared under captivity with domestic sheep can result in infection, it is unclear if these wild species could sustain the infection and spread B. ovis in the environment in the absence of sheep.
Parameter 6 – Domestic reservoir species (or family/orders)
No domestic species other than sheep have been proven as a reservoir of B. ovis.
3.1.1.2. Article 7(a)(ii) The morbidity and mortality rates of the disease in animal populations
Morbidity
Parameter 1 – Prevalence/ Incidence
B. ovis is found in sheep‐raising regions worldwide and successful examples of eradication (in the whole country – see Section 3.1.4) have never been reported (Blasco, 2010). As B. ovis infection is not submitted to compulsory eradication programmes in any country of the world (perhaps with the exception of important areas of Australia and New Zealand), the official reporting data are scanty and largely incomplete.
The precise situation and distribution of this infection worldwide are largely unknown. B. ovis infection has been reported in most countries in America, Europe, Africa and Asia as well as Australia and New Zealand, but probably occurs in most – if not all – sheep‐raising countries. Only few countries report suitable data proving that the infection has never been reported in Belgium, Denmark, Finland, Greece, Ireland, Luxembourg, Portugal, Sweden, the Netherlands and the UK (AQIS, 2000). Nevertheless, there is no officially free status in EU directives for B. ovis, only some testing requirements for movements between Member States for some animal categories (e.g. animals for breeding) exist.
The prevalence and incidence in domestic naïve sheep populations which are neither vaccinated nor submitted to any official sanitary intervention are usually very high and depend largely on the time elapsed after the onset of disease in a given flock. The prevalence, both apparent (serological) and bacteriologically proven, is highly variable when the disease is first reported in a country, with 2–67% of rams infected and 9–50% of flocks affected (Blasco, 1990; Sergeant, 1994). The collective and individual prevalence reported in the Basque Country in Spain, before implementing any control programme were 9.66% (3695 flocks tested) and 5.33% (9805 rams tested), respectively (Blasco, 2002). In these initial stages, yearly incidences approach prevalence values or can be slightly lower. In countries applying some degree of control programme (based essentially on vaccination with the Rev.1 vaccine and/or testing and culling), the prevalence is much lower, but complete eradication is extremely difficult to achieve. In fact, no country has succeeded in a complete eradication of this infection (see Section 3.1.4.6 Parameter 2). When control interventions are applied, the prevalence/incidence figures are lower but also highly variable depending on the countries and the characteristics and degree of application of the control programmes implemented. Suitable information on these parameters is not available in Member States (MSs) in which no official control and eradication programmes are performed regularly. However, partial data (See Section 3.1.4.6 Parameter 2) prove that control measures can reduce significantly the prevalence.
B. ovis natural infection has never been reported in wildlife species (see Section 3.1.1); thus, the prevalence is considered null (zero).
Parameter 2 – Case morbidity rate (% clinically diseased animals out of infected ones)
Suitable data on the natural morbidity rate of B. ovis infection in sheep are scanty. In naturally infected naïve flocks, the proportion of clinical lesions in infected rams can be 20–50% and abortions in ewes 25–60%. Affected flocks are also affected by an overall reduced fertility (Blasco, 1990, 2010). The morbidity rate depends mainly on the route of infection, the infecting dose, the assessment procedure, and of intrinsic characteristics of the animals such as the age and breed. Experimental doses of around 5 × 109 colony forming units (CFU) applied via intraconjunctival route and/or intrapreputially induce infection rates close to 100% among inoculated animals (Blasco, 1990, 2010). In that artificially infected population, the percentage of clinically affected rams detected by testicular palpation ranges from 30% to 50%. However, when the main target organs (epididymides, seminal vesicles and bulbourethral glands) are inspected microscopically, 90–100% of rams are found pathologically affected (Blasco, 2010).
The infection has been demonstrated in young lambs, suggesting that animals at, or soon after puberty, are susceptible to B. ovis. However, it has been reported repeatedly that the incidence of both testicular alterations and B. ovis prevalence increases with the age of rams, being this related to sexual experience (Blasco, 1990). It has been hypothesised that susceptibility to infection may vary among breeds of sheep, with Merino breeds less frequently infected than British breeds reared in the same environment (Clapp et al., 1962). Moreover, Spanish native and Merino‐derived breeds seem to be more resistant to infection than other European breeds. Although genetic resistance could be important, it has been suggested that susceptibility to infection could also relate to differences in growth rates and sexual precocity and activity (Blasco, 1990).
It is a widespread misconception that only rams are involved in maintaining and spreading the infection and that ewes do not play a relevant role in the epidemiology. In fact, it has been reported that after being mated by infected rams, only few ewes develop an active infection leading to abortion and dead or weak lambs (Clapp et al., 1962). However, in contrast with experimental infection trials in ewes in which only few lymph nodes and organs were found infected (Muhammed et al., 1975), a widespread infection has been induced after experimental challenge in ewes (Grilló et al., 1999). In this latter study, the uterus and the iliac and supramammary lymph nodes were the main target organs of B. ovis and related to the high percentage of ewes that persistently excreted B. ovis by the vaginal route (over 80%) and milk (over 60%). Surprisingly, despite the severe endometritis induced in most of exposed ewes, none aborted and only few gave birth to stillborn lambs (Grilló et al., 1999). These clinical findings concur with those obtained in similar trials conducted in pregnant ewes demonstrating that, despite the induction of severe endometritis, B. ovis seems to have a relatively low capacity to induce abortion in sheep (for a review see Blasco (2010)).
Mortality
Parameter 3 – Case fatality rate
The case fatality rate in naturally or experimentally induced B. ovis infections in domestic, wild or laboratory animals is considered to be very low or null. No mortality cases have been evidenced when naturally or experimentally infected animals are maintained for a given period of time (usually short). However, no naturally or experimentally infected animals have been maintained for significantly long periods – i.e. years – to determine if the infection can produce lethal complications as a consequence of abscess in testicles and secondary infections by other bacteria. In any case, it is assumed and widely accepted to be null or very low.
3.1.1.3. Article 7(a)(iii) The zoonotic character of the disease
Presence
Parameter 1 – Report of zoonotic human cases (anywhere)
Human cases due to B. ovis have never been reported, and this infection is considered as non‐zoonotic.
3.1.1.4. Article 7(a)(iv) The resistance to treatments, including antimicrobial resistance
Parameter 1 – Resistant strain to any treatment even at laboratory level
The absence of plasmids and lysogenic phages in the Brucella genus (Moreno, 1998) explains probably why antibiotics do not play a significant selective role in any Brucella species as compared with other bacterial pathogens. Moreover, due to economical, epidemiological and public health reasons, brucellosis treatment has been precluded in domestic animals; this has also probably limited the potential development of antibiotic resistance. Accordingly, resistance is not considered a significant issue in treating both animal and human brucellosis (Maves et al., 2011).
3.1.1.5. Article 7(a)(v) The persistence of the disease in an animal population or the environment
Animal population
Parameter 1 – Duration of infectious period in animals
B. ovis remains confined to the lymph nodes close to entry sites for 2–3 weeks and then reach blood via the efferent lymph, and bacteraemia leads to a generalised infection in reticuloendothelial organs, lymph nodes distant from entry sites, and the genital and extragenital organs and accessory sexual glands. The precise duration of B. ovis infection has not been properly established and it is accepted that only a low proportion of infected animals develop a self‐cure mechanism, but most remain infected for life, excreting the bacteria intermittently and spreading the infection (Blasco, 1990, 2010).
Parameter 2 – Presence and duration of latent infection period
While the existence of latent infections has been evidenced in other Brucella species, this phenomenon has not been yet proven in the case of B. ovis infection in sheep.
Parameter 3 – Presence and duration of the pathogen in healthy carriers
Latent infections have never been reported in the case of B. ovis infection. Although many lambs are born to ewes with severe placental damage and suckle B. ovis‐infected milk throughout lactation, only very few are found to be heavily infected (Grilló et al., 1999). Several factors can be considered to explain this relatively low infective capacity of B. ovis for lambs and the absence of induced latent infections. First, it is widely accepted that newborn lambs are relatively non‐reactive to brucellae. Second, it could be possible that the specific anti‐B. ovis antibodies present in colostrum help to abrogate B. ovis infection in suckling lambs. Finally, the low pathogenicity of B. ovis relative to that of smooth (S) brucellae could also be considered (Blasco, 2010).
Environment
Parameter 4 – Length of survival (dpi) of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment (scenarios: high and low T)
In contrast with some S Brucella species, no studies have been conducted to determine the precise environmental persistence of B. ovis. Taking this caveat into account, some S Brucella species can survive for long periods under environmental conditions, and this could also be the case of B. ovis. Dryness, high temperatures and direct sunlight exposure are very unfavourable for Brucella survival. On the other hand, in favourable conditions like pH >4, cool temperature, high humidity and the absence of direct sunlight, Brucella spp. may survive for relatively long periods in aborted fetuses and fetal membranes, faeces and liquid manure, water, wool and hay as well as on equipment and clothes. Brucella spp. are able to withstand drying, particularly in the presence of organic material, and can then remain viable in dust and soil for relatively long periods. Survival is prolonged at low temperatures, especially in snow and ice.
Since the presence of DNA in selected matrixes or the environment is not representative of the true survival ability of Brucella, no comments will be made on this particular topic.
3.1.1.6. Article 7(a)(vi) The routes and speed of transmission of the disease between animals and, when relevant, between animals and humans
Routes of transmission
Parameter 1 – Types of routes of transmission from animal to animal (horizontal, vertical)
Passive ram‐to‐ram venereal transmission via the ewe is considered the most important way of spreading the infection. However, ram‐to‐ram transmission can also happen by direct contact between infected and healthy rams through sodomy and mucosal routes (Blasco, 1990, 2010), via orogenital transmission by bucks (rams, stags) licking the preputial area of infected rams (Ridler et al., 2000).
Direct ewe‐to‐ram venereal transmission is considered infrequent. Although uterine infection is characteristic in both natural and experimental infections in ewes (Collier and Molello, 1964; Marco et al., 1994; Grilló et al., 1999), it has been considered classically that only few ewes develop an active infection leading to abortion and dead or weak lambs after being mated by infected rams, thus playing a minor role in transmission. However, in contrast with previous trials in which only few (Muhammed et al., 1975) or no (Collier and Molello, 1964) lymph nodes or extragenital organs became infected, a widespread infection has been reported after suitable B. ovis experimental exposure in ewes (Grilló et al., 1999). In this latter study, the uterus and iliac and supramammary lymph nodes were the main target organs, which explains the high percentage of ewes excreting B. ovis by the vaginal route. This represents a potential risk of transmission from ewe to ewe and also from ewes to rams in the field. Surprisingly, but as reported previously, despite the high challenge dose and the severe endometritis induced in most ewes, none aborted and only few gave birth to stillborn lambs (Grilló et al., 1999). Environmental contamination with B. ovis due to abortions or the vaginal secretions may facilitate the spreading to both ewes and rams, nonetheless because of the low frequency of these events and the minimal infectious dose required, there is a very low probability that the environment (e.g. water) could be a source of infection. In addition, lambs born to infected ewes seldom develop active infection and they do not do so even after nursing on contaminated milk (Grilló et al., 1999). Milk excretion of B. ovis has also been considered classically as a very rare event. However, most experimentally infected ewes develop a mammary infection characterised by a heavy and persistent (at least two successive lactations in some animals) excretion of B. ovis in milk (Grilló et al., 1999). Placental damage and milk excretion could be of relevance in the maintenance of infection via vertical route to the fetus and through perinatal transmission to suckling lambs with the possibility of developing latent infections (possible but never reported). Altogether, the role played by the ewes in spreading the infection from ram to ram and potentially from ewe to ewe may explain in part the failure of control programmes based exclusively on testing and culling the seropositive rams (Marco et al., 1994).
Parameter 2 – Types of routes of transmission between animals and humans (direct, indirect, including food‐borne)
B. ovis has never been proven to be transmitted to humans.
Speed of transmission
Parameter 3 – Incidence between animals and, when relevant, between animals and humans
Data on the incidence of animal brucellosis have been commented above (see Section 3.1.1.2) and are extended in Section 3.1.1.7, Parameter 2. Despite the widespread absence of data, it has been proven that in infected environments and in the absence of suitable control programmes, the risk of transmission of B. ovis between animals includes high transmissibility.
Parameter 4 – Transmission rate (beta) (from R0 and infectious period) between animals and, when relevant, between animals and humans
Several R0 values ranging between 1 and 3 have been reported empirically or hypothesised for brucellosis in ruminants caused by S Brucella species, but these figures are not straightforward because transmission dynamics is complicated by multiple interactions (Beauvais et al., 2016; Hou and Sun, 2016). No precise R0 figures have been established for B. ovis infection.
3.1.1.7. Article 7(a)(vii) The absence or presence and distribution of the disease in the Union, where the disease is not present in the Union, the risk of its introduction into the Union
Presence and distribution
Parameter 1 – Map where the disease is present in the EU
Since control and eradication programmes are not compulsory, precise and officially updated data on the distribution and current prevalence of B. ovis infection are lacking in the MS countries. The infection has been reported in at least Austria, Bulgaria, Croatia, the Czech Republic, France, Germany, Hungary, Italy, Romania, Serbia and Spain, but it is probable that other European countries have been or are currently affected also (Blasco, 2010). The infection has never been reported in Belgium, Denmark, Finland, Greece, Ireland, Luxembourg, Portugal, Sweden, the Netherlands and the UK (AQIS, 2000).
Parameter 2 – Type of epidemiological occurrence (sporadic, epidemic, endemic) at MS level
As indicated above, no officially updated reports exist in the MS on the presence, distribution and epidemiological occurrence of ovine brucellosis due to B. ovis. However, the infection by B. ovis is probably endemic in many MS countries, causing sometimes epidemic outbreaks. After many years of implementation of control programmes against Brucella melitensis infection in sheep (most based on the use in both male and female sheep of the live B. melitensis Rev.1 vaccine – which confers cross‐protection against B. ovis), the epidemiological occurrence of B. ovis infection was becoming sporadic at least in several MS (for example, France and Spain). However, once the infection by B. melitensis has been controlled or eradicated in these MS, and then, the Rev.1 vaccination banned; the prevalence of B. ovis infection has increased dramatically, causing important epidemic outbreaks in some areas. As an example, the number of B. ovis infected flocks has increased significantly in some regions in France, since Rev.1 vaccination was forbidden in 2008 (Praud et al., 2012; Picard‐Hagen et al., 2015). Thus, it can be concluded that there is a clear official under‐reporting and an overall lack of awareness of this infection in the MS. Unless official control programmes be implemented, it is expected that the overall prevalence in the currently infected EU MS will increase exponentially.
Risk of introduction
The disease is already present in the EU.
3.1.1.8. Article 7(a)(viii) The existence of diagnostic and disease control tools
Diagnostic tools
Parameter 1 – Existence of diagnostic tools
Brucella ovis infection lacks pathognomonic symptoms and its diagnosis is based on the existing direct and indirect tests, as indicated by OIE (Table 1), the latter being those applied routinely in surveillance and control and eradication programmes. Detailed information of the availability, feasibility and effectiveness of the diagnostic tests is given below in Section 3.1.4.1.
Table 1.
Main purpose of OIE methods available for the diagnosis of B. ovis infection in sheep (adapted from OIE (2016))
| Method | Purpose | |||||
|---|---|---|---|---|---|---|
| Population freedom from infection | Individual animal freedom from infection prior to movementa | Contribute to eradication policiesb | Confirmation of clinical casesc | Confirmation of suspect casesd | Flock prevalence of infection – surveillance | |
| Agent identification | ||||||
| Staining methods | − | – | – | + | – | – |
| Culture | – | – | – | +++ | +++ | – |
| PCR e | – | – | – | + | + | – |
| Detection of immune response | ||||||
| Complement fixation test (CFT) | +++ | ++ | ++ | ++ | ++ | ++ |
| ELISA | +++ | ++ | ++ | ++ | ++ | ++ |
| Agar gel immune diffusion (AGID) | ++ | ++ | ++ | ++ | ++ | ++ |
+++ = recommended method; ++ = suitable method; + = may be used in some situations, but cost, reliability or other factors severely limits its application; – = not appropriate for this purpose; n/a = not applicable. PCR = polymerase chain reaction; CFT = complement fixation test; ELISA = enzyme‐linked immunosorbent assay; AGID = agar gel immunodiffusion test.
This applies only to flocks, countries or zones free from infection with Brucella ovis.
To improve the efficiency of eradication policies in infected flocks, it is recommended to associate tests in parallel to increase the sensitivity of the diagnosis, i.e. two serological tests at least, e.g. CFT (or AGID) and I‐ELISA.
In low‐prevalence or almost free zones, the predictive value of positive results to serological tests may be very low. In such situation, the agent identification is usually needed for confirming clinical cases.
In infected flocks, any reactor in any serological test should be considered as infected.
No internationally accepted method exists and both false‐positive and false‐negative results may occur.
Control tools
Parameter 2 – Existence of control tools
Selective breeding for generating disease‐resistant genotypes could be theoretically feasible as a control strategy over a prolonged time (Morris, 2007). However, no specific control programmes have been yet developed for animal brucellosis on this genetic basis.
Two possible strategies can be applied to control B. ovis infection. One could be based on a mass vaccination with Rev.1 vaccine (applied to both males and females). Although feasible theoretically, this programme has never been applied regularly in any country, probably due to: (i) the banning of Rev.1 in B. melitensis officially free countries (most EU MS), (ii) the serological interference caused in B. melitensis diagnostic tests and (iii) the unclear cost–benefit ratio. The second strategy (that currently applied in most countries) is an eradication programme based on test and slaughter, combined (infrequently) or not with vaccination (applied in young replacements exclusively). Rev.1 is the only effective vaccine available against B. ovis. Extended comments on feasibility, availability and efficacy of this vaccine are made in Section 3.1.4.2.
It is believed that the simplest way to reduce B. ovis prevalence is the culling of rams showing palpable testicular alterations. However, this is not adequate because of the existence of infected rams lacking lesions and of infected ewes as both constitute a risk. Eradication can be achieved by the combined use of scrotal palpation and complement fixation test (CFT) serological testing, both tests performed every 6 months, then culling both the clinically affected and the serologically positive rams (Blasco, 2010). Of similar diagnostic value, the agar gel immunodiffusion (AGID) test is more practical than the CFT for such purpose. Although there is limited information, when the enzyme‐linked immunosorbent assay (ELISA) is combined with either the CFT or the AGID, an improvement of the efficacy of control programmes based on a single test would be expected (Praud et al., 2012). Although effective in most cases, this culling programme applied in rams exclusively has failed in some cases (Marco et al., 1994). However, B. ovis can be present in a certain proportion of ewes in flocks subjected to exclusive ram culling programmes, and in which B. ovis could not be eradicated (Marco et al., 1994). Although the precise mechanism of transmission could not be established properly, the maintenance of infection in these flocks was most probably due to the infected ewes (Marco et al., 1994). Therefore, in these ‘problem flocks’, ewes should also be included in any control programme against B. ovis. The same caveat should be considered when applying any control programme based on vaccination.
No compulsory control/eradication policy is currently applied in any EU MS, and the only control tests applied officially are those directly related to the international trade of live sheep.
3.1.2. Article 7(b) The impact of diseases
3.1.2.1. Article 7(b)(i) The impact of the disease on agricultural and aquaculture production and other parts of the economy
The level of presence of the disease in the Union
Parameter 1 – Number of MSs where the disease is present
This topic has been commented in Section 3.1.1.7.
The loss of production due to the disease
Parameter 2 – Proportion of production losses (%) by epidemic/endemic situation
There are very few well‐documented studies on the production losses and the economic impact of brucellosis that take into account all aspects of the disease susceptible of affecting the animal industry. In general, the direct production losses due to animal brucellosis have been estimated empirically and focused essentially for brucellosis in cattle (B. abortus infection). However, precise economic information in the case of B. ovis infection is scanty and incomplete.
Direct financial losses are principally due to a drop in fertility, with a high replacement rate of both rams and ewes, and which are commonly reported in infected flocks (Blasco, 1990, 2010). One study conducted in New Zealand (Liberona and Christiansen, 1983) estimated the cost of infection in 359 New Zealand dollars (NZD) per infected ram (124 NZD corresponding to fertility loss, 39 NZD to decreased useful life and 196 NZD to increased proportion ram/ewes).
This estimated amount has been even far exceeded (Table 2) in another study conducted also in New Zealand (Carpenter et al., 1987).
Table 2.
Main costs per ram and per year (New Zealand dollars, NZD) estimated in New Zealand in an average B. ovis‐infected flock composed of 2,500 ewes and 100 rams (Carpenter et al., 1987)
| Main costs | NZD estimate/infected ram/year |
|---|---|
| Decrease of the useful life of each ram | 155 |
| Decrease of production (fecundity) | 302 |
| Transmission of new cases | 605 |
| Premature culling | 82 |
| TOTAL | 1,144 |
However, the above studies excluded the costs due to the infection in ewes as well as other costs in lambs. The abortion rate in ewes and the perinatal mortality varies from 0% to 8% according the different experimental studies (Blasco, 2010). Furthermore, it has been estimated that lambs born in the second and third cycles as a consequence of infertility are 10–20 pounds lighter at weaning, which can represent a loss of 10–20 USD/lamb for each cycle missed (Lamb Epididymitis: Kimberling et al. (2010)).
3.1.2.2. Article 7(b)(ii) The impact of the disease on human health
Human cases due to B. ovis have never been reported, and this infection is considered as non‐zoonotic.
3.1.2.3. Article 7(b)(iii) The impact of the disease on animal welfare
Parameter 1 – Severity of clinical signs at case level and related level and duration of impairment
As it happens in other species brucellosis caused by other Brucella species, ovine brucellosis caused by B. ovis is considered as a major contributor to animal suffering, causing fever, genital lesions, abortions, stillbirths and the birth of weak offspring, with the ensuing increase of perinatal mortality. Despite its relatively low abortifacient ability, B. ovis‐aborted ewes may retain the placenta and develop endometritis and infertility. Orchitis, epididymitis and inflammation of accessory sexual glands are the most frequent clinical signs affecting rams and may result also in total or partial infertility (Blasco, 1990, 2010). Deaths are very rare, except in the fetus or newborn lambs, as a consequence of fetal subnutrition due to placentitis. Studies on the precise impact of B. ovis infection on sheep welfare beyond the clinical signs described above are lacking.
3.1.2.4. Article 7(b)(iv) The impact of the disease on biodiversity and the environment
Biodiversity
Parameter 1 – Endangered wild species affected: listed species as in CITES and/or IUCN list
In the EU, B. ovis infection has never been reported in wild animals. The infection has been reported exclusively in non‐Member States (see Section 3.1.1) in white‐tailed deer and red deer reared in direct contact with infected sheep. However, these wild animals are considered occasional dead‐end hosts of a disease transmitted from infected sheep rather than a true reservoir (Ridler et al., 2012). Moreover, the infection is non‐lethal in these wild species, which appear as of ‘least concern’ in the IUCN list.
Parameter 2 – Mortality in wild species
Brucellosis is a non‐fatal disease; thus, this issue is of minor concern.
Environment
Parameter 3 – Capacity of the pathogen to persist in the environment and cause mortality in wildlife
With the exception of the white‐tailed deer and red deer species reported to be affected in exceptional circumstances, B. ovis infection has never been reported in wild animals. Thus, the capacities of this pathogen to persist in the environment and cause mortality in wildlife have to be considered as negligible.
3.1.3. Article 7(c) Its potential to generate a crisis situation and its potential use in bioterrorism
Brucella ovis can be easily cultured from infected animals or obtained from culture type strain international suppliers. Also, these bacteria can be transferred, multiplied and stored easily. However, an intentional or accidental contamination of food or water with B. ovis should not pose any significant threat for human beings and could cause only a moderate economic impact in the local sheep industry. Altogether, these characteristics make this pathogen a non‐attractive candidate to be used as a potential agent for biological warfare purposes.
Parameter 1 – Listed in OIE/CFSPH classification of pathogens
The main species included in the Brucella genus (including B. ovis) are listed in OIE/CFSPH classification (CFSPH, 2016).
Parameter 2 – Listed in the Encyclopaedia of Bioterrorism Defence of Australia Group
The pathogenic brucellae for humans are included in the Encyclopaedia of Bioterrorism Defence of Australia Group. However, as B. ovis has not been proven pathogenic for humans, it is not included (The Australia Group, 2017).
Parameter 3 – Included in any other list of potential bio‐agroterrorism agents
The pathogenic brucellae for humans are specified in the select agent rules implemented by USDA APHIS and the Centers for Disease Control and Prevention (CDC) of the Department of Health and Human Services (HHS) of the US. However, B. ovis is not included in that list (CRS, 2007).
3.1.4. Article 7(d) The feasibility, availability and effectiveness of the following disease prevention and control measures
3.1.4.1. Article 7(d)(i) Diagnostic tools and capacities
Availability
Parameter 1 – Officially/internationally recognised diagnostic tool, OIE certified
Several diagnostic tests are available, recognised by the OIE and the EU, and used for surveillance/eradication worldwide (OIE, 2016). These OIE tests and their main applications have been summarised above in Table 1.
Since only a moderate proportion of infected rams show palpable genital lesions, and many other infectious agents can induce epididymitis in rams (Actinobacillus seminis, Actinobacillus actinomycetemcomitans, Histophilus ovis, Haemophilus spp., Corynebacterium pseudotuberculosis ovis and Chlamydia psittaci, among others – for a review see Blasco (2010)), the disease cannot be diagnosed on the exclusive basis of testicular palpation. Direct bacteriological isolation, if positive, is the most specific diagnostic test for the confirmation of the disease. Despite their usefulness for identification and typing, molecular tests are not fully suitable for direct diagnosis from field samples, and serology is the most adequate diagnostic alternative at population level, and used regularly for a presumptive diagnosis or for surveillance. The most effective and widely used serological tests are the CFT, the double AGID test and the indirect ELISA. The CFT is the only test currently recognised by the OIE and the EU for certifying individual animals for international trade. However, it has been proven that the AGID test shows similar sensitivity to the CFT, and it is a much simpler test to perform (Blasco, 1990, 2010). Moreover, although international standardisation is lacking, numerous independent validation studies have shown that the ELISA is more sensitive than either the CFT or AGID test (Blasco, 2010; Praud et al., 2012). In the absence of internationally agreed standardisation rules, the ELISA and AGID tests should be validated against a panel of appropriate positive and negative gold standard sera (OIE, 2016).
Effectiveness
Parameter 2 – Sensitivity and Specificity of diagnostic test
The bacteriological culture (if positive) is the only unequivocal method to identify an infected animal, but is cumbersome and needs a suitable combination of selective media and samples for optimal sensitivity (Blasco, 2010; De Miguel et al., 2011; OIE, 2016). Both molecular (polymerase chain reaction (PCR)) and classical microbiological tests (OIE, 2016) can be used for identifying bacterial isolates. The only vaccine strain available (B. melitensis Rev.1) can be readily distinguished from their corresponding B. melitensis field counterparts as well as from B. ovis by both PCR and classical tests. However, since only the indirect tests are used for surveillance/eradication, comments on diagnostic performance (diagnostic sensitivity (DSe) and diagnostic specificity (DSp)) will be focused to the immunological tests.
B. ovis serological tests differ in practical aspects as well as in DSe and DSp (for a review see Blasco (1990, 2010)). The most effective and widely used are the CFT, the AGID and the I‐ELISA. The hot saline (HS) extract of B. ovis is the recommended antigen for these B. ovis tests (OIE, 2016). Its water solubility and high content in relevant epitopes explain its good performance. However, in areas where B. melitensis infection also exists or Rev.1 vaccination is applied, the specificity of the OIE/EU B. ovis tests with HS antigen has to be carefully interpreted taking into account the results of B. melitensis tests (Blasco, 2010). Despite being the only OIE/EU officially recognised test, CFT has important disadvantages such as complexity, obligatory serum inactivation, anticomplementary activity in some sera, the impossibility of testing haemolysed sera and prozones. Moreover, comparative studies have shown that, provided an adequate validation is performed, the ELISA has a better sensitivity than either the AGID or the CFT (Gall et al., 2003; Blasco, 2010; Praud et al., 2012; OIE, 2016). However, due to the existence of ELISA‐negative but CFT‐ (or AGID)‐positive sera and vice versa, the parallel combination of the CFT (or AGID) and ELISA results in optimal diagnostic performance (Blasco, 2010; Praud et al., 2012). Because of their sensitivity, simplicity and easy interpretation, both the ELISA and AGID test would be preferred for surveillance in low‐prevalence zones.
Little is known about false‐positive results in these HS B. ovis tests. The foot rot agent (Dichelobacter nodosus) has been described as cross‐reacting with B. ovis, but the extent and practical consequences in B. ovis tests are not well understood (Blasco, 2010; OIE, 2016). In addition, Arcanobacterium pyogenes and Corynebacterium ovis soluble extracts cross‐react with sera from B. ovis‐infected rams, being both pathogens isolated from rams resulting positive in B. ovis tests (Blasco, 2010). The brucellin skin test could also be used to test unvaccinated sheep against B. ovis (Velasco et al., 1997). This allergen test could be adequate when suspecting false positive serological reactions, but suitable studies proving its DSe and DSp for B. ovis diagnosis are lacking.
Feasibility
Parameter 3 – Type of sample matrix to be tested (blood, tissue, etc.)
A large variety of samples can be collected for culture. Milk samples and vaginal swabs from aborted sheep, and semen and preputial swabs taken from rams are particularly useful for bacteriological diagnosis in live animals. B. ovis can also be cultured from aborted fetuses or the placenta. The spleen, whole lymph nodes and late pregnant or early post‐parturient uterus, testis/epididymides, and accessory ram sex glands are the most reliable samples to collect at necropsy (De Miguel et al., 2011). All these samples are also suitable theoretically for direct PCR diagnostic procedures. Several PCRs have been reported to result in good diagnostic performance when applied to semen samples from B. ovis‐infected rams (Manterola et al., 2003; Xavier et al., 2010). However, none of these PCRs outperform the classical culture, and moreover, their diagnostic performance remains to be properly determined on other clinical samples. Thus, the culture should be considered as the reference standard for the bacteriological diagnosis of B. ovis (De Miguel et al., 2011; OIE, 2016).
Blood serum samples can be collected for serological diagnosis and are the preferred samples for surveillance at large population level.
3.1.4.2. Article 7(d)(ii) Vaccination
Availability
Parameter 1 – Types of vaccines available on the market (live, inactivated, DIVA, etc.)
Vaccination is considered the most effective method of control in high incidence areas (Blasco, 1990, 2010). The B. abortus S19 live‐attenuated vaccine was applied many years ago to prevent B. ovis, but it was later on abandoned because of the important side effects induced (Blasco, 1990; Ridler and West, 2011). The B. melitensis Rev.1, a live attenuated vaccine developed for the control of B. melitensis infection, is the only available vaccine against B. ovis infection. The vaccine is administered via subcutaneous injection or conjunctival inoculation. Its general characteristics are described in Table 3.
Table 3.
General characteristics of the B. melitensis Rev.1 vaccine in sheep (adapted from Blasco et al. (2016))
| Advantages | Disadvantages | Comments |
|---|---|---|
|
Proved efficacy in B. melitensis control/eradication programmes (France, Italy, Portugal, Spain). Effective against both B. melitensis and B. ovis. Safe in young replacements (males and females). Single dose affords useful protection for life. Biological quality control feasible (OIE accepted) |
Highly abortifacient when used in pregnant ewes. Serological interference in classical serological tests (RBT, CFT), indirect and competitive ELISAs, fluorescence polarisation assay and other B. melitensis tests. Serological interference in HS B. ovis tests Virulent (low) for humans; streptomycin resistant |
Safety issues minimised by avoiding use in mid‐pregnancy ewes by the conjunctival route. Serological interference minimised when applied exclusively to young replacement animals by the conjunctival route. Human Rev 1 infections can be diagnosed using simple standard serological tests; treatment requires regimes avoiding streptomycin |
Parameter 2 – Availability/production capacity (per year)
The Rev.1 vaccine is currently produced worldwide with important regional differences. The different manufacturing companies are shown in Table 4.
Table 4.
Current B. melitensis Rev.1 vaccine manufacturing companies
| MANUFACTURER | COUNTRY | COMMERCIAL NAME | ROUTE | WEBSITE |
|---|---|---|---|---|
| AGROVET | RUSSIA | ND | SC | http://www.agrovet.ru/ |
| ATA FEN INC | TURKEY | Rev.1 CJ | CJ | http://www.egevet.com.tr/ |
| BIOCOMBINANT | MONGOLIA | ND | CJ/SC | |
| CEVA | FRANCE | COGLAREV | CJ | http://www.ceva.com/fr/ |
| CZ VETERINARIA | SPAIN | OCUREV | CJ | http://www.czveterinaria.com/ |
| CZ VETERINARIA | CZV REV‐1 | SC | http://www.czveterinaria.com/ | |
| DOLLVET | TURKEY | Brudoll M | SC | http://www.dollvet.com.tr/ |
| INDIAN IMMUNOLOGICALS | INDIA | BRUVAX REV1 | SC | https://www.indimmune.com/ |
| JINYU | CHINA | REV1 | ||
| JOVAC | JORDAN | Brucevac CJ | CJ | http://www.jovaccenter.com/ |
| Brucevac (reduced dose) | SC | |||
| ONDERSTEPOORT | Brucella Rev. 1 | SC | http://www.obpvaccines.co.za/ | |
| OVEJERO | SPAIN | OVERVAC OC | CJ/SC | http://www.labovejero.com/ |
| PENDIK | TURKEY | BR.REV‐1 | CJ/SC | http://penvet.gov.tr/ |
| PRONAVIBE | MELIREV N | SC | http://www.pronabive.gob.mx/ | |
| RAZI | IRAN | ND | CJ/SC | http://www.rvsri.com |
| SYVA | SPAIN | Lio Vac Rev‐1 | SC/CJ | http://www.syva.es/ |
| VETAL | TURKEY | ABORVAC R CS | CJ | http://www.vetal.com.tr/ |
| VETAL | ABORVAC R | SC | http://www.vetal.com.tr/ | |
| VETERINARY SERUM INSTITUTE | EGYPT | Rev‐1 | SC | http://www.vsvri-eg.com/ |
ND: not available; CJ: conjunctival administration; SC: subcutaneous administration.
As a consequence of the effective B. melitensis official eradication programme in sheep and goats in most EU MS, these European manufacturing companies have reduced significantly the overall production capacity, which is essentially maintained for the exterior market. However, manufacturing technology is currently well implemented and the production capacity of these companies could be significantly increased to cover an emergency situation, at least in the medium term. Since Rev.1 vaccine is not protected by patents and the master seed strain can be easily obtained from several sources (e.g. OIE or EU reference laboratories), it is marketed at relatively low cost (EUR 0.05–0.20 per dose, depending on the manufacturing country).
Effectiveness
Parameter 3 – Field protection as reduced morbidity (as reduced susceptibility to infection and/or to disease)
The vaccine is safe enough for use in rams. After being inoculated by subcutaneous or conjunctival routes in either young or adult rams, the Rev.1 strain colonises the spleen and several lymph nodes, but despite persisting for 2–3 months in the vaccinated animals, the genital organs are not colonised by Rev 1 (Muñoz et al., 2008). Experimentally, 56–100% of Rev.1‐vaccinated rams are protected against a B. ovis challenge able to infect 80–100% of unvaccinated controls (Blasco, 2010). Moreover, the genital lesions produced after challenge are significantly less severe than in unvaccinated animals. However, the evidence about the protection conferred by the vaccine in the field is scanty. In a study conducted in France between 1981 and 1989, it was reported that the vaccination with Rev.1 applied in young replacement male and female lambs was able to reduce significantly both the flock (from 16.8% to 5%) and individual (11.9% to 4.3%) prevalences, with a vaccination coverage around 70% of target animals (Sanchis et al., 1991). The same vaccination strategy, applied only in around 50% of flocks, was also able to reduce both flock (from 32% to 14%) and individual (20% to 7%) prevalences in only 3 years of application in other areas of France (AFSSA, 2008). Finally, it has been clearly proven in France (Table 5) that the prevalence of B. ovis infection increases significantly and in a very short interval once the Rev.1 vaccination is abandoned.
Table 5.
Evolution of the prevalence (assessed by serological tests, CFT and ELISA) of B. ovis infection in rams in the Pyrénées‐Atlantiques Département in France, once vaccination with Rev.1 was abandoned in the year 2000 (adapted from AFSSA (2008))
| Years | ||||
|---|---|---|---|---|
| 2001a | 2004b | 2005b | 2006b | |
| Flocks tested | 352 | 280 | 640 | 730 |
| Rams tested | (n.a.) | 2,183 | 3,075 | 5,360 |
| % flocks infected (IC 95%) | 8 (5.4–11.3) | 34 (38.8–52.7) | 38 (34.2–41.9) | 30 (26.7–33.5) |
| % rams infected (IC 95%) | 4 (n.a.) | 16 (14.5–17.6) | 18 (16.7–19.4) | 22 (20.9–23.1) |
Aleatory sampling covering 10% of flocks.
Data obtained from voluntary owners.
(n.a.: figures not available).
The disadvantages of Rev.1 vaccine (Table 3) include the development of both B. melitensis and B. ovis antibodies, which could interfere with serologic diagnosis (Blasco, 1990, 2010). Moreover, this vaccine is prohibited for use in B. melitensis officially free countries. Innovative vaccine approaches are currently being investigated (for a review see Blasco et al. (2016)), which raises the possibility of more effective vaccines in the future. For the moment, none of these new vaccine candidates are available on the market.
Parameter 4 – Duration of protection
The precise duration of protection conferred by Rev.1 against B. ovis infection has never been properly assessed. However, it has been proven experimentally that Rev 1 induces suitable protection against B. melitensis in sheep for at least two consecutive pregnancies (Verger et al., 1995). The protection lapse span of Rev.1 against B. melitensis in goats is very long going from 4 years and a half (Alton, 1990) to 5 years (Díaz‐Aparicio et al., 2004). It is unclear whether revaccination improves the immunity achieved with a single dose, and it is thus widely accepted that a single dose of Rev.1 confers whole‐life immunity against B. melitensis (Blasco et al., 2016). It could be then accepted that this also happens in the case of B. ovis, but field or experimental evidences are lacking.
Feasibility
Parameter 5 – Way of administration
The B. melitensis Rev.1 vaccine can be administered to sheep (both males and females) in a single standard dose (0.5–2 × 109 CFU) by either the subcutaneous or the conjunctival routes (OIE, 2016). Conjunctival administration is safer than the subcutaneous route for vaccinating adult animals and reduces significantly the intensity and duration of the immunological response, then minimising the interference in diagnostic tests (Blasco and Molina‐Flores, 2011; Blasco et al., 2016).
3.1.4.3. Article 7(d)(iii) Medical treatments
Availability
Due to economical, epidemiological and public health reasons, treatment with antibiotics has been generally precluded in animals infected with brucellosis. However, several therapeutic regimens have been evaluated successfully for treating brucellosis in cattle, sheep, pigs and dogs. Several have been applied experimentally also to treat B. ovis infection in sheep.
Parameter 1 – Types of drugs available on the market
In spite of successful antibiotic treatment of human brucellosis, this therapy has seldom been used in animal brucellosis. The existence of eradication programmes and the high treatment costs are the main reasons precluding the use of antibiotics in animals. There are, however, some reports on the successful use of antibiotherapy in B. ovis infection (see below). Suitable antibiotics are largely available on the market.
Parameter 2 – Availability/production capacity (per year)
All effective antibiotics against B. ovis (see below) are readily and sufficiently available worldwide.
Effectiveness
Parameter 3 – Therapeutic effects on the field (effectiveness)
It was reported many years ago that the combined use of chlortetracycline and streptomycin was able to cure epididymitis lesions and stop semen excretion of B. ovis in artificially infected rams, but semen quality after treatment was poor. However, streptomycin given alone or combined with sulphamethazine failed to cure these experimentally infected rams (Kuppuswamy, 1954). In another study, 27 out of 32 B. ovis naturally infected rams were treated with a combination of chlortetracycline and streptomycin and became serologically negative and showed no epididymitis lesions by eight months after treatment (Giauffret and Sanchis, 1974). Likewise, a combination of long‐acting oxytetracycline (seven inoculations of 20 mg/kg body weight (BW) at three‐day intervals) with dihydrostreptomycin (20 mg/kg BW daily for 21 days) was able to abrogate B. ovis semen excretion soon after treatment (Table 6; Marín et al. (1989)). However, treatment with oxytetracycline alone did not avoid semen excretion in a relevant proportion of infected rams. This combined treatment cured B. ovis infection in 11 out of 12 artificially infected rams (Marín et al., 1989). In contrast, only 4 of 12 infected rams were cured when treated with oxytetracycline alone. Additional studies should be conducted to assess the efficacy of these antibiotic treatments at field level and to determine the full recovery of fertility in bacteriologically cured animals. The costs of treatment and the follow‐up testing schedules are very expensive, and thus, this antibiotic therapy is only recommended for valuable rams of exceptional genetic value or belonging to endangered breeds. Successfully treated rams remain seropositive for some time after resolution of infection, which needs to be considered if a testing and culling programme is implemented in these animals.
Table 6.
Outcome of the expert judgement on the Article 5 criteria for ovine epididymitis (Brucella ovis)
|
Criteria to be met by the disease: According to AHL, a disease shall be included in the list referred to in point (b) of paragraph 1 of Article 5 if it has been assessed in accordance with Article 7 and meets all of the following criteria |
Final outcome | |
| A(i) | The disease is transmissible | Y |
| A(ii) | Animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union | Y |
| A(iii) | The disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character | Y |
| A(iv) | Diagnostic tools are available for the disease | Y |
| A(v) | Risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union | Y |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at points A(i)–A(v), the disease needs to fulfil at least one of the following criteria | ||
| B(i) | The disease causes or could cause significant negative effects in the Union on animal health or poses or could pose a significant risk to public health due to its zoonotic character | Y |
| B(ii) | The disease agent has developed resistance to treatments and poses a significant danger to public and/or animal health in the Union | N |
| B(iii) | The disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union | Y |
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | N |
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | N |
Colour code: green = consensus (Yes/No).
Feasibility
Parameter 4 – Way of administration
Oxytetracycline long‐acting solutions and the suitable aminoglycosides (streptomycin) are given intramuscularly. Oxytetracycline is also available in soluble forms to be given orally in water or pelleted feed; but due to potential interferences with rumen flora, this approach is not recommendable for ruminants.
3.1.4.4. Article 7(d)(iv) Biosecurity measures
Availability
Parameter 1 – Available biosecurity measures
B. ovis is mainly spread via venereal transmission. Key risk factors include:
Animals moving between and within farms and, in particular, the introduction of new animals without ascertaining their B. ovis‐free status.2
Direct contact with neighbours' animals/farms infected with B. ovis.
Thus, all measures avoiding these risk factors should contribute to minimise B. ovis spread between infected and healthy flocks. Biosecurity would be focused essentially on controlling and reducing movements of animals (particularly the purchase of replacements). In addition, attention should be paid to movement of feed, water, bedding manure, people and equipment to and from areas where sheep are kept.
Effectiveness
Parameter 2 – Effectiveness of biosecurity measures in preventing the pathogen introduction
While the above measures are feasible and effective for minimising brucellosis spread in highly intensified farming systems, are very difficult to implement in outdoor or fully extensive/transhumant sheep breeding systems (widespread in most sheep raising countries). At least in the EU MS, rules on zoning (see Section 3.1.4.5 Parameter 1 below and Section 3.1.5) and on restriction of animal movements, according the available EU Directives, have been proven instrumental to minimise the spread of B. abortus and B. melitensis infections and are also equally effective to minimise the spread of B. ovis infection in sheep.
Feasibility
Parameter 3 – Feasibility of biosecurity measures
Biosecurity measures for avoiding the spread of brucellosis in domestic ruminants based on the current rules on zoning and on restriction of animal movements, according the available EU Directives, have been proven feasible and very effective.
3.1.4.5. Article 7(d)(v) Restrictions on the movement of animals and products
Availability
Parameter 1 – Available movement restriction measures
EU Legislation affecting animal movements and dealing with Brucella ovis infection is covered by Council Directive 91/68/EEC3 and Council Directive 92/65/EEC4.
For importation, additional animal health requirements are set out in specific Commission Decisions that lay down conditions applying to imports of live animals and products from third countries. The EU legislation is fully harmonised and compliant with its international obligations and, in particular, the requirements of the Sanitary and Phytosanitary Agreement of the WTO. This legislation imposes a series of requirements designed to ensure that imported animals and products meet standards at least equivalent to those required for production in and trade between MS. These lay down on health certificates which must accompany all animal imports. On arrival in the EU, the animals and the accompanying certificates must be verified and checked by EU official veterinarians at a designated Border Inspection Post. Further checks on the animals may also be carried out at the final destination.
Effectiveness
Parameter 2 – Effectiveness of restriction of animal movement in preventing the between‐farm spread
The existing EU Directives on the restriction of movements has been proven highly effective to prevent the spread of Brucella infection between farms in the MS in which the disease yet exists.
Feasibility
Parameter 3 – Feasibility of restriction of animal movement
The Council Directives dealing with restrictions of animal movement (see Section 3.1.4.5 Parameter 1 above) have been successfully and feasibly implemented by MS many years ago, and proven of paramount importance in the successful eradication of brucellosis and to reduce the spread of the disease in the countries in which the disease is yet present. Computerised management of livestock national and international movements constitutes a further step in the management of health hazards associated with the movement of animals.
3.1.4.6. Article 7(d)(vi) Killing of animals
Availability
Parameter 1 – Available methods for killing animals
Brucellosis eradication requires the identification of infected animals, their progressive elimination from the herd/flock and replacement with non‐infected animals (Crespo Léon et al., 2012). In the case of identifying and confirming B. ovis infection in holdings belonging to MS, the slaughter of infected animals is recommended. However, the eradication of this infection is not compulsory in MS, and no official eradication programmes have been implemented compulsorily in the EU.
Effectiveness
Parameter 2 – Effectiveness of killing animals (at farm level or within the farm) for reducing/stopping spread of the disease
The killing of B. ovis‐infected rams (identified by serological tests and clinical palpation) has been implemented for eradicating B. ovis in sheep in several countries. Using this test and slaughter approach, the infection has been eradicated from the Falkland Islands (Reichel et al., 1994), the Flinders and King Islands of Australia and selected areas of New Zealand (Ridler and West, 2011).
This programme (using both the CFT and ELISA as diagnostic tests) has been also applied since 1986 in the Basque Country Autonomous Community of Spain. While initial flock seroprevalence was very high (60%), the number of infected flocks was reduced significantly although without getting a full elimination of the disease. The number of B. ovis‐infected flocks decreased by 96% during the first 10 years of the programme, but in the last 5 years reported, the flock seroprevalence has remained constant at levels of around 0.7% (Juste et al., 2013) (Figure 1 ).
Figure 1.
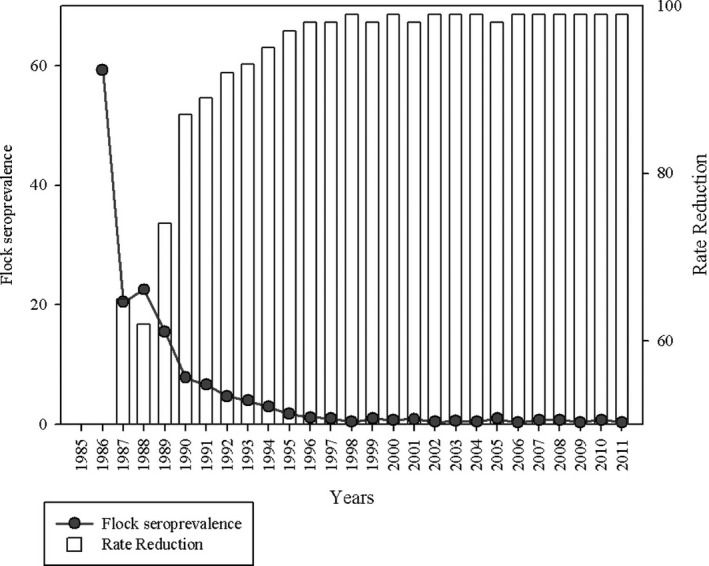
Evolution of the prevalence of B. ovis infection, the Basque Country Autonomous Community in Spain, after several years of application of a testing and culling programme (Juste et al., 2013)
Feasibility
Parameter 3 – Feasibility of killing animals
These killing measures have a wide acceptance in the EU and have been proven highly effective and feasible after many years of application of the compulsory brucellosis eradication programmes in the MS.
3.1.4.7. Article 7(d)(vii) Disposal of carcasses and other relevant animal by‐products
Depending on the age and health of the animal, carcasses and by‐products may be disposed of through the abattoir system or by rendering. Currently, available disposal options are considered effective. Disposal via abattoir or rendering is already routine.
3.1.5. Article 7(e) The impact of disease prevention and control measures
3.1.5.1. Article 7(e)(i) The direct and indirect costs for the affected sectors and the economy as a whole
There are very few well‐documented studies on the production losses and ensuing economic impact of brucellosis considering all disease aspects (see Section 3.1.2 Parameter 1). Since its real prevalence is mostly unknown in the MS, these costs are impossible to calculate for B. ovis infection.
Nevertheless, having consideration for the epidemiology of B. ovis (non‐zoonotic and having low capacity of spreading in the short term), any unexpected local outbreak should not have important consequences for the sector involved.
3.1.5.2. Article 7(e)(ii) The societal acceptance of disease prevention and control measures
The currently applied culling and disposal systems used in the compulsory EU eradication programmes for animal brucellosis (B. abortus and B. melitensis infection) were implemented many years ago and have been widely accepted by the affected owners and the overall society. In the event that a B. ovis eradication programme based on testing and culling was introduced compulsorily in the EU MS, it should be well accepted by the owners and the general society.
3.1.5.3. Article 7(e)(iii) The welfare of affected subpopulations of kept and wild animals
Parameter 1 – Welfare impact of control measures on domestic animals
Whenever properly managed by competent veterinarians, the measures implemented for controlling brucellosis (usually through vaccination) do not pose any relevant issue from the animal welfare standpoint. Moreover, eradication measures (based on the partial culling of infected animals combined or not with vaccination) have been and continue being instrumental for the successful brucellosis eradication programmes applied in the EU and elsewhere. Since 2013 in the EU is of compulsory application the Council Regulation (EC) No 1099/20095, which establishes the protection of animals at the moment of slaughter. This rule indicates that in the case of depopulation of holdings, it is the obligation of competent authorities to preserve the welfare of the affected animals.
Parameter 2 – Wildlife depopulation as control measure
This is not an issue in the case of B. ovis infection since wild animals are very rarely affected (Section 3.1.1) and, in the case of sporadic infections, this does not have any epidemiological significance.
3.1.5.4. Article 7(e)(iv) The environment and biodiversity
Environment
Parameter 1 – Use and potential residuals of biocides or medical drugs in environmental compartments (soil, water, feed, manure)
No drugs/chemicals other than the common and legally accepted antibiotics and disinfectants are used in the current control/eradication campaigns in the EU.
Biodiversity
Parameter 2 – Mortality in wild species
The preventive and control measures for B. ovis infection do not have any impact on wildlife.
3.2. Assessment according to Article 5 criteria
This section presents the results of the expert judgement on the criteria of Article 5 of the AHL about ovine epididymitis (B. ovis) (Table 6). The expert judgement was based on Individual and Collective Behavioural Aggregation (ICBA) approach described in detail in the opinion on the methodology (EFSA AHAW Panel, 2017). Experts have been provided with information of the disease fact sheet mapped into Article 5 criteria (see supporting information, Annex A), based on that the experts indicate their Y/N or ‘na’ judgement on each criterion of Article 5, and the reasoning supporting their judgement.
The minimum number of judges in the judgement was 12. The expert judgement was conducted as described in the methodological opinion (EFSA AHAW Panel, 2017). For details on the interpretation of the questions, see Appendix B of the methodological opinion (EFSA AHAW Panel, 2017).
3.2.1. Outcome of the assessment of ovine epididymitis (Brucella ovis) according to criteria of Article 5(3) of the AHL on its eligibility to be listed
As from the legal text of the AHL, a disease is considered eligible to be listed as laid down in Article 5 if it fulfils all criteria of the first set from A(i) to A(v) and at least one of the second set of criteria from B(i) to B(v). According to the assessment methodology (EFSA AHAW Panel, 2017), a criterion is considered fulfilled when the outcome is ‘Yes’. According to the results shown in Table 6, ovine epididymitis (B. ovis) complies with all criteria of the first set and with two criteria of the second set; therefore, it is considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL.
3.3. Assessment according to Article 9 criteria
This section presents the results of the expert judgement on the criteria of Annex IV referring to categories as in Article 9 of the AHL about ovine epididymitis (B. ovis) (Tables 7–11). The expert judgement was based on ICBA approach described in detail in the opinion on the methodology. Experts have been provided with information of the disease fact sheet mapped into Article 9 criteria (see supporting information, Annex A), based on that the experts indicate their Y/N or ‘na’ judgement on each criterion of Article 9, and the reasoning supporting their judgement.
Table 7.
Outcome of the expert judgement related to the criteria of Section 1 of Annex IV (category A of Article 9) for ovine epididymitis (Brucella ovis) (CI: current impact; PI: potential impact)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Final outcome | |
| 1 | The disease is not present in the territory of the Union or present only in exceptional cases (irregular introductions) or present only in a very limited part of the territory of the Union | N |
| 2.1 | The disease is highly transmissible | N |
| 2.2 | There are possibilities of airborne or waterborne or vector‐borne spread | N |
| 2.3 | The disease affects multiple species of kept and wild animals or single species of kept animals of economic importance | Y |
| 2.4 | The disease may result in high morbidity and significant mortality rates | N |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at points 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, including epidemic or pandemic potential or possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 4(PI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | Y |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | Y |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | N |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
Colour code: green = consensus (Yes/No).
Table 11.
Outcome of the expert judgement related to the criteria of Section 5 of Annex IV (category E of Article 9) for ovine epididymitis (Brucella ovis)
| Diseases in category E need to fulfil criteria of Sections 1, 2 or 3 of Annex IV of AHL and/or the following: | Final outcome | |
| E |
Surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment (If a disease fulfils the criteria as in Article 5, thus being eligible to be listed, consequently category E would apply.) |
Y |
Colour code: green = consensus (Yes/No).
The minimum number of judges in the judgement was 12. The expert judgement was conducted as described in the methodological opinion (EFSA AHAW Panel, 2017). For details on the interpretation of the questions, see Appendix B of the methodological opinion (EFSA AHAW Panel, 2017).
Table 8.
Outcome of the expert judgement related to the criteria of Section 2 of Annex IV (category B of Article 9) for ovine epididymitis (Brucella ovis) (CI: current impact; PI: potential impact)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Final outcome | |
| 1 | The disease is present in the whole or part of the Union territory with an endemic character and (at the same time) several Member States or zones of the Union are free of the disease | Y |
| 2.1 | The disease is moderately to highly transmissible | Y |
| 2.2 | There are possibilities of airborne or waterborne or vector‐borne spread | N |
| 2.3 | The disease affects single or multiple species | Y |
| 2.4 | The disease may result in high morbidity with in general low mortality | Y |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at points 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, including epidemic potential or possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 4(PI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | Y |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | Y |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | N |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
Colour code: green = consensus (Yes/No).
Table 9.
Outcome of the expert judgement related to the criteria of Section 3 of Annex IV (category C of Article 9) for ovine epididymitis (Brucella ovis) (CI: current impact; PI: potential impact)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Final outcome | |
| 1 | The disease is present in the whole or part of the Union territory with an endemic character | Y |
| 2.1 | The disease is moderately to highly transmissible | Y |
| 2.2 | The disease is transmitted mainly by direct or indirect transmission | Y |
| 2.3 | The disease affects single or multiple species | Y |
| 2.4 | The disease usually does not result in high morbidity and has negligible or no mortality and often the most observed effect of the disease is production loss | Y |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at points 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, or possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of parts of the Union, mainly related to its direct impact on certain types of animal production systems | N |
| 4(PI) | The disease has a significant impact on the economy of parts of the Union, mainly related to its direct impact on certain types of animal production systems | N |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | Y |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | N |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
Colour code: green = consensus (Yes/No).
Table 10.
Outcome of the expert judgement related to the criteria of Section 4 of Annex IV (category D of Article 9) for ovine epididymitis (Brucella ovis)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Final outcome | |
| D | The risk posed by the disease in question can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread | Y |
| The disease fulfils criteria of Sections 1, 2, 3 or 5 of Annex IV of AHL | Y | |
Colour code: green = consensus (Yes/No).
3.3.1. Outcome of the assessment of criteria in Annex IV for ovine epididymitis (Brucella ovis) for the purpose of categorisation as in Article 9 of the AHL
As from the legal text of the AHL, a disease is considered fitting in a certain category (A, B, C, D or E corresponding to point (a) to point (e) of Article 9(1) of the AHL) if it is eligible to be listed for Union intervention as laid down in Article 5(3) and fulfils all criteria of the first set from 1 to 2.4 and at least one of the second set of criteria from 3 to 5(d) as shown in Tables 7–11. According to the assessment methodology (EFSA AHAW Panel, 2017), a criterion is considered fulfilled when the outcome is ‘Yes’. With respect to different type of impact where the assessment is divided into current and potential impact, a criterion will be considered fulfilled if at least one of the two outcomes is ‘Y’ and, in case of no ‘Y’, the assessment is inconclusive if at least one outcome is ‘NC’.
A description of the outcome of the assessment of criteria in Annex IV for ovine epididymitis (B. ovis) for the purpose of categorisation as in Article 9 of the AHL is presented in Table 12.
Table 12.
Outcome of the assessment of criteria in Annex IV for ovine epididymitis (Brucella ovis) for the purpose of categorisation as in Article 9 of the AHL
| Category | Article 9 criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° set of criteria | 2° set of criteria | ||||||||||
| 1 | 2.1 | 2.2 | 2.3 | 2.4 | 3 | 4 | 5a | 5b | 5c | 5d | |
| Geographical distribution | Transmissibility | Routes of transmission | Multiple species | Morbidity and mortality | Zoonotic potential | Impact on economy | Impact on society | Impact on animal welfare | Impact on environment | Impact on biodiversity | |
| A | N | N | N | Y | N | N | Y | N | Y | N | N |
| B | Y | Y | N | Y | Y | N | Y | N | Y | N | N |
| C | Y | Y | Y | Y | Y | N | N | N | Y | N | N |
| D | Y | ||||||||||
| E | Y | ||||||||||
According to the assessment here performed, ovine epididymitis (B. ovis) complies with the following criteria of the Sections 1–5 of Annex IV of the AHL for the application of the disease prevention and control rules referred to in points (a)–(e) of Article 9(1):
To be assigned to category A, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment ovine epididymitis (B. ovis) complies only with criterion 2.3. To be eligible for category A, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and ovine epididymitis (B. ovis) complies with criteria 4 and 5b, but not with criteria 3, 5a, 5c and 5d.
To be assigned to category B, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment, ovine epididymitis (B. ovis) complies with criteria 1, 2.1, 2.3 and 2.4, but not with criterion 2.2. To be eligible for category B, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and ovine epididymitis (B. ovis) complies with criteria 4 and 5b, but not with criteria 3, 5a, 5c and 5d.
To be assigned to category C, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment, ovine epididymitis (B. ovis) complies with all of them. To be eligible for category C, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and ovine epididymitis (B. ovis) complies with criterion 5b, but not with criteria 3, 4, 5a, 5c and 5d.
To be assigned to category D, a disease needs to comply with criteria of Sections 1, 2, 3 or 5 of Annex IV of the AHL and with the specific criterion D of Section 4, with which ovine epididymitis (B. ovis) complies.
To be assigned to category E, a disease needs to comply with criteria of Sections 1, 2 or 3 of Annex IV of the AHL and/or the surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment. The latter is applicable if a disease fulfils the criteria as in Article 5, with which ovine epididymitis (B. ovis) complies.
3.4. Assessment of Article 8
This section presents the results of the assessment on the criteria of Article 8(3) of the AHL about ovine epididymitis (B. ovis). The Article 8(3) criteria are about animal species to be listed, as it reads below:
‘3. Animal species or groups of animal species shall be added to this list if they are affected or if they pose a risk for the spread of a specific listed disease because:
they are susceptible for a specific listed disease or scientific evidence indicates that such susceptibility is likely; or
they are vector species or reservoirs for that disease, or scientific evidence indicates that such role is likely'.
For this reason, the assessment on Article 8 criteria is based on the evidence as extrapolated from the relevant criteria of Article 7, i.e. the ones related to susceptible and reservoir species or routes of transmission, which cover also possible role of biological or mechanical vectors.6 According to the mapping, as presented in Table 5, Section 3.2 of the scientific opinion on the ad hoc methodology (EFSA AHAW Panel, 2017), the main animal species to be listed for ovine epididymitis (B. ovis) according to the criteria of Article 8(3) of the AHL are as displayed in Table 13.
Table 13.
Main animal species to be listed for ovine epididymitis (Brucella ovis) according to criteria of Article 8 (source: data reported in Section 3.1.1.1)
| Class | Order | Family | Genus/Species | |
|---|---|---|---|---|
| Susceptible | Mammalia | Artiodactyla | Bovidae | Sheep (Ovis aries), goat (Capra hircus), bighorn sheep (Ovis canadensis canadensis), mouflon (Ovis musimon) |
| Cervidae | White‐tailed deer (Odocoileus virginianus), red deer (Cervus elaphus elaphus) | |||
| Lagomorpha | Leporidae | not specified | ||
| Rodentia | Muridae | Rattus spp., gerbils (Gerbillinae), Mus spp. | ||
| Cricetidae | Hamsters (Cricetinae) | |||
| Caviidae | Guinea pig (Cavia porcellus) | |||
| Reservoir | Mammalia | Artiodactyla | Bovidae | Sheep (Ovis aries) |
| Cervidaea | White‐tailed deer (Odocoileus virginianus), red deer (Cervus elaphus elaphus) | |||
| Vectors | none | |||
Possibly.
4. Conclusions
TOR 1: for each of those diseases an assessment, following the criteria laid down in Article 7 of the AHL, on its eligibility of being listed for Union intervention as laid down in Article 5(3) of the AHL;
According to the assessment here performed, ovine epididymitis (B. ovis) complies with all criteria of the first set and with two criteria of the second set and therefore can be considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL.
TOR 2a: for each of the diseases which was found eligible to be listed for Union intervention, an assessment of its compliance with each of the criteria in Annex IV to the AHL for the purpose of categorisation of diseases in accordance with Article 9 of the AHL;
According to the assessment here performed, ovine epididymitis (B. ovis) meets the criteria as in Sections 3, 4 and 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in points (c), (d) and (e) of Article 9(1) of the AHL.
TOR 2b: for each of the diseases which was found eligible to be listed for Union intervention, a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL.
According to the assessment here performed, the animal species that can be considered to be listed for ovine epididymitis (B. ovis) according to Article 8(3) of the AHL are mainly sheep and other species of the families Bovidae and Cervidae as susceptible and sheep and deer as reservoirs, as reported in Table 13 in Section 3.4 of the present document.
Abbreviations
- AGID
agar gel immunodiffusion test
- AHAW
EFSA Panel on Animal Health and Welfare
- AHL
Animal Health Law
- BW
body weight
- CFSPH
Center for Food Security and Public Health
- CFT
complement fixation test
- CFU
colony forming units
- CITES
Convention on International Trade in Endangered Species
- DSe
diagnostic sensitivity
- DSp
diagnostic specificity
- ELISA
enzyme‐linked immunosorbent assay
- HS
hot saline
- ICBA
Individual and Collective Behavioural Aggregation
- I‐ELISA
indirect enzyme‐linked immunosorbent assay
- IUCN
International Union for Conservation of Nature
- MS
Member State
- OIE
World Organization for Animal Health
- PCR
polymerase chain reaction
- ToR
Terms of Reference
- WTO
World Trade Organization
Annex A – Mapped fact‐sheet used in the individual judgement on ovine epididymitis (Brucella ovis)
1.
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4994
Supporting information
Mapped fact‐sheet used in the individual judgement on ovine epididymitis (Brucella ovis)
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Beltrán‐Beck B, Kohnle L and Bicout D, 2017. Scientific opinion on the assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): ovine epididymitis (Brucella ovis). EFSA Journal 2017;15(10):4994, 30 pp. 10.2903/j.efsa.2017.4994
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00148
Panel members: Dominique Bicout, Anette Bøtner, Andrew Butterworth, Paolo Calistri, Klaus Depner, Sandra Edwards, Bruno Garin‐Bastuji, Margaret Good, Christian Gortázar Schmidt, Virginie Michel, Miguel Angel Miranda, Simon More, Søren Saxmose Nielsen, Mohan Raj, Liisa Sihvonen, Hans Spoolder, Jan Arend Stegeman, Hans‐Hermann Thulke, Antonio Velarde, Preben Willeberg and Christoph Winckler.
Acknowledgements: The Panel wishes to thank Josè Maria Blasco, for the support provided to this scientific output.
Adopted: 14 September 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Table 1 and Table 1 (Annex): © World Organization for Animal Health (OIE); Table 2 and Table 2 (Annex): © American Veterinary Medical Association; Table 5 and Table 5 (Annex): © ANSES; Figure 1 and Figure 1 (Annex): © 2012 Elsevier B.V.
Notes
‘Brucella ovis infection’ is preferred to ‘Ovine epididymitis’ a non‐specific lesion that can be due to many other organisms.
There is only an OIE definition of the B. ovis‐free status of flocks, but there is no EU definition of freedom. The OIE definition is only based on the absence of clinical signs during the past year (OIE, 2017). Other countries (Australia and New Zealand for example) certify free flocks or areas.
Council Directive 91/68/EEC of 28 January 1991 on animal health conditions governing intracommunity trade in ovine and caprine animals. OJ L 46, 19.2.1991, p. 19–36.
Council Directive 92/65/EEC of 13 July 1992 laying down animal health requirements governing trade in and imports into the Community of animals, semen, ova and embryos not subject to animal health requirements laid down in specific Community rules referred to in Annex A (I) to Directive 90/425/EEC. OJ L 268, 14.9.1992, p. 54–72.
Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. OJ L 303, 18.11.2009, p. 1–30.
A vector is a living organism that transmits an infectious agent from an infected animal to a human or another animal. Vectors are frequently arthropods. Biological vectors may carry pathogens that can multiply within their bodies and be delivered to new hosts, usually by biting. In mechanical vectors, the pathogens do not multiply within the vector, which usually remains infected for shorter time than in biological vectors.
References
- AFSSA (Agence française de sécurité sanitaire des aliments), 2008. Avis de l'Agence française de sécurité sanitaire des aliments sur un protocole de lutte contre l'épididymite contagieuse ovine (Brucella ovis) dans les Pyrénées Atlantiques. Saisine no. 2007‐SA‐0405. 14 pp. Available online: https://www.anses.fr/fr/system/files/SANT2007sa0405.pdf
- Alton G, 1990. Brucella melitensis 1887 to 1987. In: Nielsen KH, Duncan JR (eds.). Animal Brucellosis. CRC Press, Boca Raton, FL. pp. 379–409. [Google Scholar]
- AQIS (Australian Quarantine and Inspection Service), 2000. An analysis of the disease risks, other than Scrapie, associated with the importation of ovine and caprine semen and embryos from Canada, the USA and member states of the European Union. Final Report. 67 pp. Available online: http://www.agriculture.gov.au/SiteCollectionDocuments/ba/memos/2000/animal/00-038b.pdf.
- Bailey K, 1997. Naturally acquired Brucella ovis infection in a deer. Surveillance, 24, 10–11. [Google Scholar]
- Beauvais W, Musallam I and Guitian J, 2016. Vaccination control programs for multiple livestock host species: an age‐stratified, seasonal transmission model for brucellosis control in endemic settings. Parasites & Vectors, 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco JM, 1990. Brucella ovis . In: Nielsen KH and Duncan JR (eds). Animal Brucellosis. CRC Press, Boca Raton, FL. pp. 352–378. [Google Scholar]
- Blasco JM, 2002. Brucelosis ovina. Ovis, 82, 113. [Google Scholar]
- Blasco JM, 2010. Brucella ovis infection. In: Lefèvre PC, Blancou J, Chermette R and Uilenberg G (eds). Infectious and Parasitic Diseases of Livestock. Lavoisier, Paris, France. pp. 1047–1063. [Google Scholar]
- Blasco JM and Molina‐Flores B, 2011. Control and eradication of Brucella melitensis infection in sheep and goats. Veterinary Clinics of North America: Food Animal Practice, 27, 95–104. [DOI] [PubMed] [Google Scholar]
- Blasco JM, Moreno E and Moriyon I, 2016. Brucellosis vaccines and vaccine candidates. In: Metwally S, Viljoen GJ, El Idrissi A (eds.). Veterinary Vaccines for Developing Countries. FAO, Rome, Italy. pp. 1–33. [Google Scholar]
- Burgess GW, Spencer TL and Norris MJ, 1985. Experimental infection of goats with Brucella ovis . Australian Veterinary Journal, 62, 262–264. [DOI] [PubMed] [Google Scholar]
- Carpenter T, Berry SL and Glenn JS, 1987. Economics of Brucella ovis control in sheep: computerized decision‐tree analysis. Journal of the American Veterinary Medical Association, 190, 983–987. [PubMed] [Google Scholar]
- Cerri D, Ambrogi C, Ebani VV, Poli A, Cappelli F, Cardini G and Andreani E, 2002. Experimental Brucella ovis infection in mouflon (Ovis musimon). Journal of Wildlife Diseases, 38, 287–290. [DOI] [PubMed] [Google Scholar]
- CFSPH (Center for Food Security & Public Health), 2016. Animal Disease From Potential Bioterrorist Agent. Available online: http://www.cfsph.iastate.edu/Products/resources/WallChart.pdf
- Clapp KH, Keogh J and Richards MH, 1962. Epidemiology of ovine brucellosis in South Australia. Australian Veterinary Journal, 38, 482–486. [Google Scholar]
- Collier JR and Molello JA, 1964. Comparative distribution of Brucella abortus, Brucella melitensis and Brucella ovis in experimentally infected pregnant sheep. American Journal of Veterinary Research, 25, 930–934. [PubMed] [Google Scholar]
- Crespo Léon F, Sáez Llorente JL, Reviriego Gordejo FJ, Rodríguez Ferri EF and Durán Ferrer M, 2012. Complementary tools for the control and eradication of caprine and ovine brucellosis in the European Union. Revue Scientifique et Technique (International Office of Epizootics), 31, 985–996. [DOI] [PubMed] [Google Scholar]
- CRS (Congressional Research Service), 2007. CRS Report for Congress. Agroterrorism: Threats and Preparedness. 63 pp. Available online: https://fas.org/sgp/crs/terror/RL32521.pdf.
- De Miguel MJ, Marín CM, Muñoz PM, Dieste L, Grilló MJ and Blasco JM, 2011. Development of a selective culture medium for primary isolation of the main Brucella species. Journal of Clinical Microbiology, 49, 1458–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz‐Aparicio E, Hernández L and Suárez‐Güemes F, 2004. Protection against brucellosis in goats, five years after vaccination with reduced‐dose of Brucella melitensis Rev 1 vaccine. Tropical Animal Health and Production, 36, 117. [DOI] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Gervelmeyer A, Zancanaro G, Kohnle L, Morgado J and Bicout D, 2017. Scientific opinion on an ad hoc method for the assessment on listing and categorisation of animal diseases within the framework of the Animal Health Law. EFSA Journal 2017;15(5):4783, 42 pp. 10.2903/j.efsa.2017.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall D, Nielsen K, Vigliocco A, Smith P, Perez B, Rojas X and Robles C, 2003. Evaluation of an indirect enzyme‐linked immunoassay for presumptive serodiagnosis of Brucella ovis in sheep. Small Ruminant Research, 48, 173–179. [Google Scholar]
- García‐Carrillo C, Casas‐Olascoaga R, Cuba‐Caparó A, Lucero N and Szyfres B, 1977. Infección experimental de caprinos machos con Brucella ovis. Estudio bacteriológico, serológico e histopatológico. Revista Argentina de Microbiología, 9, 101–108. [PubMed] [Google Scholar]
- Giauffret A and Sanchis R, 1974. Etude d'un foyer d'epididymite contagieuse du bélier. Éradication de la maladie. Bulletin ‐ Office International Des Epizooties, 82, 581–586. [Google Scholar]
- Grilló MJ, Marín CM, Barberán M and Blasco JM, 1999. Experimental Brucella ovis infection in pregnant ewes. Veterinary Record, 144, 555–558. [DOI] [PubMed] [Google Scholar]
- Hou Q and Sun X‐D, 2016. Modelling sheep brucellosis transmission with a multi‐stage model in Changling County of Jilin Province, China. Journal of Applied Mathematics and Computing, 51, 227–244. [Google Scholar]
- Juste RA, Leginagoikoa I, Villoria M, Minguijon E, Elguezabal N, Boix C, Arrazola I, Perez K and González L, 2013. Control of brucellosis and of respiratory Small Ruminant Lentivirus infection in small ruminants in the Basque country, Spain. Small Ruminant Research, 110, 115–119. [Google Scholar]
- Kimberling CV, Parsons GA, Parsons J and Cunningham W, 2010. Ram Epididymitis. Available online: http://www.optimalag.com/cleonscorner/Article002.aspx
- Kuppuswamy PB, 1954. Chemotherapy of brucellosis in rams. New Zealand Veterinary Journal, 2, 110–118. [Google Scholar]
- Liberona H and Christiansen H, 1983. A cost‐benefit study of an eradication scheme for Brucella ovis in sheep. OIE Technical Series, 3, 215–223. [Google Scholar]
- Manterola L, Tejero‐Garcés A, Ficapal A, Shopayeva G, Blasco JM, Marín CM and López‐Goñi I, 2003. Evaluation of a PCR test for the diagnosis of Brucella ovis infection in semen samples from rams. Veterinary Microbiology, 92, 65–72. [DOI] [PubMed] [Google Scholar]
- Marco J, González L, Cuervo LA, Beltrán de Heredia F, Barberán M, Marín C and Blasco JM, 1994. Brucella ovis infection in two flocks of sheep. Veterinary Record, 135, 254–256. [DOI] [PubMed] [Google Scholar]
- Marín CM, Jiménez de Bagués MP, Barberán M and Blasco JM, 1989. Efficacy of long‐acting oxytetracycline alone or in combination with streptomicin for treatment of Brucella ovis infection of rams. American Journal of Veterinary Research, 50, 560–563. [PubMed] [Google Scholar]
- Maves RC, Castillo R, Guillen A, Espinosa B, Meza R, Espinoza N, Núñez G, Sánchez L, Chacaltana J, Cepeda D, González S and Hall ER, 2011. Antimicrobial susceptibility of Brucella melitensis isolates in Peru. Antimicrobial Agents and Chemotherapy, 55, 1279–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum M, Rhyan J, Coburn S, Ewalt D, Lahr C, Nol P, Keefe T, Kimberling C and Salman M, 2013. Clinical, culture, serology and histopathology outcomes of bighorn sheep experimentally infected with Brucella ovis . Journal of Wildlife Diseases, 49, 900–910. [DOI] [PubMed] [Google Scholar]
- Moreno E, 1998. Genome evolution within the alpha Proteobacteria: why do some bacteria not possess plasmids and others exhibit more than one different chromosome? FEMS Microbiology Reviews, 22, 255–275. [DOI] [PubMed] [Google Scholar]
- Morris CA, 2007. A review of genetic resistance to disease in Bos taurus cattle. Veterinary Journal, 174, 481–491. [DOI] [PubMed] [Google Scholar]
- Muhammed SI, Lauerman LH, Mesfin GM and Otim CP, 1975. Duration of Brucella ovis infection in ewes. The Cornell Veterinarian, 65, 221–227. [PubMed] [Google Scholar]
- Muñoz PM, De Miguel MJ, Grilló MJ, Marín CM, Barberán M and Blasco JM, 2008. Immunopathological responses and kinetics of Brucella melitensis Rev 1 infection after subcutaneous or conjunctival vaccination in rams. Vaccine, 26, 2562–2569. [DOI] [PubMed] [Google Scholar]
- OIE (World Organization for Animal Health), 2016. Infection with Brucella ovis. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals.
- OIE (World Organization for Animal Health), 2017. Ovine epididymitis (Brucella ovis). In: Terrestrial Animal Health Code.
- Picard‐Hagen N, Berthelot X, Champion JL, Eon L, Lyazrhi F, Marois M, Peglion M, Schuster A, Trouche C and Garin‐Bastuji B, 2015. Contagious epididymitis due to Brucella ovis: relationship between sexual function, serology and bacterial shedding in semen. BMC Veterinary Research, 11, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant JW, Eamens GJ and Seaman JT, 1986. Serological, bacteriological and pathological changes in rams following different routes of exposure to Brucella ovis . Australian Veterinary Journal, 63, 409–412. [DOI] [PubMed] [Google Scholar]
- Praud A, Champion JL, Corde Y, Drapeau A, Meyer L and Garin‐Bastuji B, 2012. Assessment of the diagnostic sensitivity and specificity of an indirect ELISA kit for the diagnosis of Brucella ovis infection in rams. BMC Veterinary Research, 8, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel MP, Baber DJ, Armitage PW, Lampard D, Whitley RS and Hilbink F, 1994. Eradication of Brucella ovis from the Falkland Islands 1977–1993. Veterinary Record, 134, 595–597. [DOI] [PubMed] [Google Scholar]
- Ridler AL and West DM, 2011. Control of Brucella ovis infection in sheep. Veterinary Clinics of North America: Food Animal Practice, 27, 61–66. [DOI] [PubMed] [Google Scholar]
- Ridler AL, West DM, Stafford KJ, Wilson PR and Fenwick SG, 2000. Transmission of Brucella ovis from rams to red deer stags. New Zealand Veterinary Journal, 48, 57–59. [DOI] [PubMed] [Google Scholar]
- Ridler AL, West DM and Collett MG, 2012. Pathology of Brucella ovis infection in red deer stags (Cervus elaphus). New Zealand Veterinary Journal, 60, 146–149. [DOI] [PubMed] [Google Scholar]
- Sanchis R, Abadie G and Polveroni G, 1991. Evolution de l'épididymite contagieuse du bélier à Brucella ovis dans les Alpes‐Maritimes. Influence de l'utilisation du vaccin Rev. 1. Bulletin De l'Academie Véterinaire De France, 23, 35–40. [Google Scholar]
- Sergeant ES, 1994. Seroprevalence of Brucella ovis infection in commercial ram flocks in the Tamworth area. New Zealand Veterinary Journal, 42, 97–100. [DOI] [PubMed] [Google Scholar]
- The Australia Group , online, 2017. List of human and animal pathogens and toxins for export control. Available online: http://www.australiagroup.net/en/human_animal_pathogens.html
- Velasco J, Díaz R, Grilló MJ, Barberán M, Marín C, Blasco JM and Moriyón I, 1997. Antibody and delayed‐type hypersensitivity responses to Ochrobactrum anthropi cytosolic and outer membrane antigens in infections by smooth and rough Brucella spp. Clinical and Diagnostic Laboratory Immunology, 4, 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger JM, Grayon M, Zundel E, Lechopier P and Olivier‐Bernardin V, 1995. Comparison of the efficacy of Brucella suis strain 2 and Brucella melitensis Rev 1 live vaccines against a Brucella melitensis experimental infection in pregnant ewes. Vaccine, 13, 191–196. [DOI] [PubMed] [Google Scholar]
- Xavier MN, Silva TM, Costa EA, Paixão TA, Moustacas VS, Carvalho CA, Sant'Anna FM, Robles CA, Gouveia AM, Lage AP, Tsolis RM and Santos RL, 2010. Development and evaluation of a species‐specific PCR assay for the detection of Brucella ovis infection in rams. Veterinary Microbiology, 145, 158–164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mapped fact‐sheet used in the individual judgement on ovine epididymitis (Brucella ovis)


