Abstract
Following a request from the European Commission, the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) derives dietary reference values (DRVs) for vitamin K. In this Opinion, the Panel considers vitamin K to comprise both phylloquinone and menaquinones. The Panel considers that none of the biomarkers of vitamin K intake or status is suitable by itself to derive DRVs for vitamin K. Several health outcomes possibly associated with vitamin K intake were also considered but data could not be used to establish DRVs. The Panel considers that average requirements and population reference intakes for vitamin K cannot be derived for adults, infants and children, and therefore sets adequate intakes (AIs). The Panel considers that available evidence on occurrence, absorption, function and content in the body or organs of menaquinones is insufficient, and, therefore, sets AIs for phylloquinone only. Having assessed additional evidence available since 1993 in particular related to biomarkers, intake data and the factorial approach, which all are associated with considerable uncertainties, the Panel maintains the reference value proposed by the Scientific Committee for Food (SCF) in 1993. An AI of 1 μg phylloquinone/kg body weight per day is set for all age and sex population groups. Considering the respective reference body weights, AIs for phylloquinone are set at 70 μg/day for all adults including pregnant and lactating women, at 10 μg/day for infants aged 7–11 months, and between 12 μg/day for children aged 1–3 years and 65 μg/day for children aged 15–17 years.
Keywords: vitamin K, phylloquinone, menaquinones, Adequate Intake, Dietary Reference Value
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1220/full
Summary
Following a request from the European Commission, the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) was asked to deliver a Scientific Opinion on dietary reference values (DRVs) for the European population, including vitamin K.
Vitamin K represents a family of fat‐soluble compounds with the common chemical structure of 3‐substituted 2‐methyl‐1,4‐napthoquinone. It naturally occurs in food as phylloquinone (vitamin K1) and menaquinones (vitamin K2). Phylloquinone has a phytyl side chain and is the primary dietary form of vitamin K in Europe: it is mainly found in dark green leafy vegetables (e.g. spinach, lettuce and other salad plants) and Brassica. Menaquinones are a group of compounds with an unsaturated side chain from 4 to 13 isoprenyl units (vitamin K2 or MK‐n) and are found mainly in animal products such as meat, cheese and eggs. Apart from MK‐4 that is formed via metabolic conversion of phylloquinone during its absorption in the intestinal mucosa and in other organs, menaquinones are produced by bacteria capable of food fermentation and specific anaerobic bacteria of the colon microbiota. In this Opinion, the Panel considers vitamin K to comprise both phylloquinone and menaquinones.
Vitamin K acts as a cofactor of γ‐glutamyl carboxylase (GGCX) that catalyses the carboxylation of glutamic acid (Glu) residues into γ‐carboxyglutamic acid (Gla) residues in vitamin K‐dependent proteins (Gla‐proteins), which convert them into their active forms. These Gla‐proteins are involved in different physiological processes, including blood coagulation or bone mineralisation. MK‐7 may have a greater bioactivity compared to phylloquinone in stimulating γ‐carboxylation, but the available data are insufficient to set different activity coefficients for phylloquinone and menaquinones.
In adults, vitamin K deficiency is clinically characterised by a bleeding tendency in relation to a low activity of blood coagulation factors, resulting in an increase in prothrombin time (PT) or partial thromboplastin time (or activated partial thromboplastin time). Symptomatic vitamin K deficiency and impairment of normal haemostatic control in healthy adults may take more than 2–3 weeks to develop at a ‘low’ phylloquinone intake (i.e. < 10 μg/day). Exclusively breastfed infants are susceptible to bleeding, due to the low vitamin K content of human milk and their small body pool of vitamin K. Administration of phylloquinone at a pharmacological dose, either orally or by intramuscular injection, is usual practice for prevention of haemorrhagic disease in newborn infants. No tolerable upper intake level has been set for vitamin K by the Scientific Committee on Food (SCF).
Phylloquinone is absorbed in the intestine in the presence of dietary fat. Studies on absorption of phylloquinone in healthy adults show widely variable results. The data for absorption of some dietary menaquinones (MK‐4, MK‐7 or MK‐9) in comparison with phylloquinone are also limited. Absorption of menaquinones produced by gut bacteria in the distal intestine remains uncertain, and therefore their contribution to vitamin K status is unclear. The Panel considers that it is not possible to estimate precisely an average absorption of phylloquinone, menaquinones, and thus vitamin K from the diet.
After intestinal absorption, phylloquinone and individual menaquinones are transported into the blood by lipoproteins. The clearance of MK‐7 and MK‐9 from serum/plasma is slower than for phylloquinone. Vitamin K accumulates primarily in the liver, but is also present in bones and other tissues and has a fast turnover in the body. The liver contains widely variable concentrations of phylloquinone and menaquinones, which are catabolised to the same metabolites and excreted in bile and urine. Phylloquinone crosses the placenta in small quantities, while for menaquinones, this is unclear.
PT is the only vitamin K biomarker for which a change (increase) has been associated with vitamin K deficiency. Possible changes in the other biomarkers (concentration/activity of coagulation factors, of the undercarboxylated forms of vitamin‐K dependent proteins, or of vitamin K in blood; urinary concentration of Gla residues or of the 5C‐ and 7C‐metabolites) according to phylloquinone intake are difficult to interpret, as no cut‐off value to define adequate vitamin K status is available. There is no biomarker for which a dose–response relationship with phylloquinone intake has been established. Studies investigating the relationship between biomarkers and intake of different individual menaquinones often used doses that are much higher than the limited observed intake data of these individual menaquinones available in Europe. There is no reference level for γ‐carboxylation that can be considered as ‘optimal’ related to functions controlled by vitamin K status and the dietary intakes of phylloquinone or menaquinones required for maximal or ‘optimal’ urinary Gla excretion have not been determined. Thus, the Panel concludes that none of these biomarkers is suitable by itself to assess vitamin K adequacy. The Panel also concludes that data are insufficient for deriving the requirement for vitamin K according to sex or for ‘younger’ and ‘older’ adults.
The Panel notes the uncertainties in the food composition data and available consumption data related to phylloquinone, individual menaquinones or vitamin K. The Panel concludes that available data on intake of phylloquinone or menaquinones and health outcomes in healthy subjects cannot be used to derive DRVs for vitamin K. Data on vitamin K biomarkers and health outcomes with no quantitative data on vitamin K intake were not considered. The Panel considers a total body pool of phylloquinone of about 0.55 μg/kg body weight in healthy adults at steady state not to be associated with signs of vitamin K deficiency and to be a desirable body pool size for phylloquinone. The Panel notes that available data do not allow the estimation of the daily dietary intake of phylloquinone required to balance total phylloquinone losses through urine and bile and to maintain an adequate body pool of phylloquinone. There is no data on the total body pool of menaquinones.
The Panel considers that average requirements and population reference intakes for vitamin K cannot be derived for adults, infants and children, and therefore sets adequate intakes (AIs). The Panel considers that available evidence on intake, absorption, function and content in the body or organs of menaquinones is insufficient, and thus sets AIs for phylloquinone only. Having assessed additional evidence available since 1993 related to biomarkers, intake data and the factorial approach, the Panel concludes that all possible approaches investigated to set DRVs for vitamin K are associated with considerable uncertainties and that the available scientific evidence is insufficient to update the previous reference value. Therefore, the Panel maintains the reference value proposed by the SCF in 1993. Thus, an AI of 1 μg phylloquinone/kg body weight per day is set for all age and sex population groups.
For adults, the Panel considers the respective reference body weights of men and women and after rounding up, sets the same AI of 70 μg phylloquinone/day. The Panel notes that the proposed AI in adults is close to the median phylloquinone intake of 76 μg/day in the 2012 German National Nutrition Survey II that used updated phylloquinone composition data. The Panel considers that there is no evidence of different vitamin K absorption and different losses according to age in adults; thus sets the same AI for ‘younger’ and ‘older’ adults.
For infants and children, the Panel considers that the requirement for growth would be covered by an intake of 1 μg phylloquinone/kg body weight per day. Considering the respective reference body weights, and after rounding up, AIs for phylloquinone are set at 10 μg/day for infants aged 7–11 months, and between 12 μg/day for children aged 1–3 years and 65 μg/day for children aged 15–17 years.
For pregnant women, taking into account the mean gestational increase in body weight and the reference body weight of non‐pregnant women, the AI is the same as that for non‐pregnant women obtained after rounding. For lactating women, the Panel considers that the AI of 1 μg/kg body weight per day of phylloquinone set for non‐lactating women covers the small excretion of vitamin K in breast milk. Thus, the AI for pregnant or lactating women is set at 70 μg phylloquinone/day.
Background as provided by the European Commission
The scientific advice on nutrient intakes is important as the basis of Community action in the field of nutrition, for example such advice has in the past been used as the basis of nutrition labelling. The Scientific Committee for Food (SCF) report on nutrient and energy intakes for the European Community dates from 1993. There is a need to review and if necessary to update these earlier recommendations to ensure that the Community action in the area of nutrition is underpinned by the latest scientific advice.
In 1993, the SCF adopted an opinion on the nutrient and energy intakes for the European Community.1 The report provided Reference Intakes for energy, certain macronutrients and micronutrients, but it did not include certain substances of physiological importance, for example dietary fibre.
Since then new scientific data have become available for some of the nutrients, and scientific advisory bodies in many European Union (EU) Member States and in the United States have reported on recommended dietary intakes. For a number of nutrients these newly established (national) recommendations differ from the reference intakes in the SCF (1993) report. Although there is considerable consensus between these newly derived (national) recommendations, differing opinions remain on some of the recommendations. Therefore, there is a need to review the existing EU Reference Intakes in the light of new scientific evidence, and taking into account the more recently reported national recommendations. There is also a need to include dietary components that were not covered in the SCF opinion of 1993, such as dietary fibre, and to consider whether it might be appropriate to establish reference intakes for other (essential) substances with a physiological effect.
In this context, the European Food Safety Authority (EFSA) is requested to consider the existing population reference intakes for energy, micro‐ and macronutrients and certain other dietary components, to review and complete the SCF recommendations, in the light of new evidence, and in addition advise on a population reference intake for dietary fibre.
For communication of nutrition and healthy eating messages to the public, it is generally more appropriate to express recommendations for the intake of individual nutrients or substances in food‐based terms. In this context, EFSA is asked to provide assistance on the translation of nutrient based recommendations for a healthy diet into food based recommendations intended for the population as a whole.
Terms of Reference as provided by the European Commission
In accordance with Article 29 (1)(a) and Article 31 of Regulation (EC) No 178/20022, the Commission requests EFSA to review the existing advice of the SCF on population reference intakes for energy, nutrients and other substances with a nutritional or physiological effect in the context of a balanced diet which, when part of an overall healthy lifestyle, contribute to good health through optimal nutrition.
In the first instance, EFSA is asked to provide advice on energy, macronutrients and dietary fibre. Specifically advice is requested on the following dietary components:
Carbohydrates, including sugars;
Fats, including saturated fatty acids, polyunsaturated fatty acids and monounsaturated fatty acids, trans fatty acids;
Protein;
Dietary fibre.
Following on from the first part of the task, EFSA is asked to advise on population reference intakes of micronutrients in the diet and, if considered appropriate, other essential substances with a nutritional or physiological effect in the context of a balanced diet which, when part of an overall healthy lifestyle, contribute to good health through optimal nutrition.
Finally, EFSA is asked to provide guidance on the translation of nutrient‐based dietary advice into guidance, intended for the European population as a whole, on the contribution of different foods or categories of foods to an overall diet that would help to maintain good health through optimal nutrition (food‐based dietary guidelines).
Assessment
1. Introduction
In 1993, the SCF adopted an opinion on the nutrient and energy intakes for the European Community (1993). For vitamin K, SCF (1993) did not set any average requirement (AR) or population reference intake (PRI). The SCF considered that an intake of 1 μg/kg body weight per day, provided by a usual mixed diet, is adequate.
The purpose of this Opinion is to review dietary reference values (DRVs) for vitamin K. Vitamin K naturally occurs in food as phylloquinone (vitamin K1) and menaquinones (vitamin K2, MK‐n). The Panel notes that DRVs set by other authorities and bodies (Section 4) are mainly related to data on phylloquinone and that the role of MK‐n in meeting vitamin K requirement is often not considered. However, some new data are available on both types of components. Therefore, the Panel considers that MK‐n should be included, in addition to phylloquinone, in this assessment. In this Scientific Opinion, the Panel considers that vitamin K comprises both phylloquinone and menaquinones.
2. Definition/category
The data discussed in this Opinion not only include data on vitamin K administered orally, but also parenterally when the data provide additional information on the role of vitamin K in the body.
2.1. Chemistry
Vitamin K represents a family of fat‐soluble compounds with the common chemical structure 3‐substituted 2‐methyl‐1,4‐napthoquinone (Figure 1).
Figure 1.
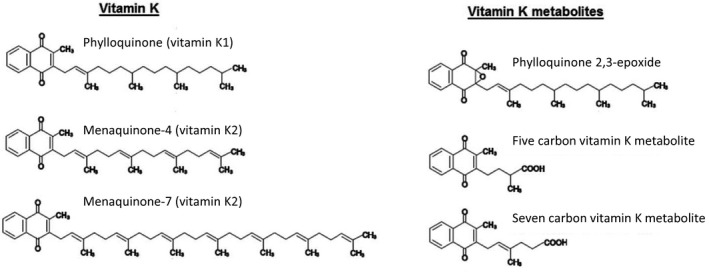
Chemical structures of vitamin K and metabolites
- Molecular masses – Phylloquinone: 450.7 g/mol; MK‐4: 444.7 g/mol; MK‐7: 648.9 g/mol; 5C‐metabolite: 272.3 g/mol; 7C‐metabolite: 298.3 g/mol (see above).
Phylloquinone (also called phytonadione or phytomenadione) is from plant origin. It contains a phytyl group and is the primary dietary form of vitamin K, mainly found in green leafy vegetable plants and Brassica (Section 3.1).
Menaquinones are a group of compounds with unsaturated side chains of varying length (MK‐n)3 from 4 to 13 isoprenyl units at the 3‐position of the 2‐methyl‐1,4‐napthoquinone group and found in animal products such as meat, cheese and egg (Section 3.1).
Most menaquinones, i.e. the medium‐chain and long‐chain MK‐n (MK‐6 or higher) but not the short‐chain MK‐4 (also called menatetrenone), are produced by bacteria, including bacteria capable of food fermentation, gut bacteria in animals and anaerobic bacteria of the human colon microbiota (Conly and Stein, 1992). In breastfed infants, the production of menaquinones by gut microbiota is probably low, as most bacteria of their microbiota, including Bifidobacterium, Lactobacillus and Clostridium species, do not produce menaquinones; and with weaning, there is a progressive colonisation of the gut by MK‐producing bacteria such as Bacteroides fragilis and Escherichia coli (Greer, 2010; Shearer et al., 2012). In humans, MK‐4 is produced via metabolic conversion of phylloquinone during its absorption in the intestinal mucosa and in other organs (Section 2.3.5).
Menadione (unsubstituted 2‐methyl‐1,4‐napthoquinone, a chemical analogue of 1,4‐naphthoquinone with a methyl group in the 2‐position, and that is also called vitamin K3) is a water‐soluble synthetic form of vitamin K that plays a role as an intermediate in the metabolic conversion of phylloquinone to MK‐4 (Section 2.3.5). Menadiol sodium phosphate (also called vitamin K4) is a synthetic water‐soluble form derived from menadione by reduction. Dihydrophylloquinone is present in foods made with partially hydrogenated fat like hydrogenated soybean oil (Section 3.1).
2.2. Function of vitamin K
2.2.1. Biochemical functions
Vitamin K (i.e. either phylloquinone or menaquinones) acts as a cofactor of the enzyme γ‐glutamyl carboxylase (GGCX) that catalyses the post‐translational carboxylation of glutamic acid (Glu) residues into γ‐carboxyglutamic acid (Gla) residues in the amino‐terminal domain of different vitaminK‐dependent proteins. This reaction converts these proteins, also called Gla‐proteins, into their active form (Stafford, 2005). These proteins all display calcium‐mediated actions, with the Gla residues located at their specific calcium‐binding sites (Ferland, 1998; Litwack, 2008).
During the γ‐glutamyl carboxylation of vitamin K‐dependent proteins, the active (reduced) form of vitamin K (hydroquinone) is converted to vitamin K epoxide (Figure 1), the oxidised form of vitamin K, that is subsequently reduced back to hydroquinone (Furie et al., 1999; Tie et al., 2005). This redox cycle, called vitamin K cycle, takes place in different tissues, particularly in the liver and bone. It involves the integral membrane enzymes GGCX and vitamin K epoxide reductase (VKOR), acting on membrane‐bound vitamin K (Stafford, 2005; Tie et al., 2005; Oldenburg et al., 2008; Tie and Stafford, 2008; Wu et al., 2011). VKOR controls a critical step of the vitamin K cycle that is blocked by warfarin and is at the bottom of warfarin's anticoagulant activity (Garcia and Reitsma, 2008). Unlike in adults, vitamin K epoxide is detectable in newborn cord plasma, and may reflect ‘low’ concentrations of VKOR (Bovill et al., 1993). Infants born with a rare genetic deficiency of VKOR may present with severe coagulopathy and/or skeletal defects (Oldenburg et al., 2000).
One group of vitamin K‐dependent proteins comprises blood coagulation factors, including factors II (prothrombin), VII, IX and X, and the anticoagulant proteins C, S and Z. These proteins are synthesised by the liver and the endothelial cells in inactive forms (with Glu residues), converted for a part to their active forms (with Gla residues) in the presence of vitamin K by GGCX found in the endoplasmic reticulum of the cells, and then secreted as both the inactive and active forms to the blood (Hansson and Stenflo, 2005). The protein induced by vitamin K absence or antagonism‐II (PIVKA‐II), the precursor of the active coagulation protein prothrombin, has 10 Glu residues that are carboxylated to Gla residues, leading to the formation of prothrombin. After the formation of Gla residues and in the presence of calcium ions, the clotting factors bind to phospholipids at the surface of the membrane of platelets, where they form membrane‐bound complexes with other clotting cofactors, and these complexes are cleaved after coagulation is initiated in the plasma. This process is sensitive to vitamin K availability in the cells for carboxylation of the blood coagulation factors.
Another important group of vitamin K‐dependent proteins include, e.g. osteocalcin (OC), matrix γ‐carboxyglutamic acid protein (MGP) and growth arrest‐specific protein 6 (GAS6), synthesised by osteoblasts or other tissues (e.g. vascular smooth muscle cells for GAS6 and MGP, chondrocytes for MGP), and Gla‐rich protein (GRP). Osteocalcin, one of the most abundant non‐collagenous proteins in bone, is involved in bone mineralisation (Ferland, 1998; Booth, 2009; Walther et al., 2013). Some authors suggest that carboxylated forms of MGP, GAS6 and GRP may be involved in the control of soft tissue calcification (Proudfoot and Shanahan, 2006; Bellido‐Martin and de Frutos, 2008; Danziger, 2008; Shiozawa et al., 2010; Viegas and Simes, 2016).
Data on in vitro and in vivo animal experiments also suggest that vitamin K is involved in the down‐regulation of expression of genes involved in acute inflammatory response (Ohsaki et al., 2006). The activity of TAM receptors, that are a component of the immune system, is dependent on carboxylated GAS6 and protein S in order to function (Lemke, 2013). However the precise mechanisms (Hanck and Weiser, 1983; Reddi et al., 1995; Li et al., 2003), the required level of carboxylation, and the relevance of this possible role of vitamin K in humans (Juanola‐Falgarona et al., 2013) are unclear.
MK‐n have the same function as phylloquinone (γ‐carboxylation), but MK‐7 may have a greater bioactivity compared to phylloquinone in stimulating γ‐carboxylation. A cross‐over study (n = 18), using equimolar doses of either phylloquinone or MK‐7 (0.22 ?mol/day4) as supplements consumed with a meal for 6 weeks (with a wash‐out period of 12 weeks) showed that MK‐7 induced a higher ratio of serum γ‐carboxylated OC/undercarboxylated OC (cOC/ucOC) compared to phylloquinone (Schurgers et al., 2007). Another cross‐over study in the same paper (n = 12), which used the vitamin K γ‐carboxylation antagonist acenocoumarol with weekly‐increasing oral doses of either phylloquinone or MK‐7 as supplements (0–500 and 0–285 μg/day, respectively, with a wash‐out period of 2 weeks), showed that MK‐7 was about 2.5 times more potent than phylloquinone to counter‐act the effect of acenocoumarol (i.e. 130 vs 315 μg/day, respectively, to obtain a comparable effect).
The Panel notes that dietary vitamin K (i.e. either phylloquinone or menaquinones) acts as cofactor of the enzymatic conversion of vitamin K‐dependent proteins (Gla‐proteins) into their active form, by carboxylation of Glu residues to Gla residues in the amino‐terminal domain. These proteins are involved in different physiological processes, including blood coagulation, bone mineralisation and possibly control of soft tissue calcification. The Panel also notes that MK‐7 may have a greater bioactivity compared to phylloquinone in stimulating γ‐carboxylation, but that the available data are insufficient to set different activity coefficients for phylloquinone and menaquinones.
2.2.2. Health consequences of deficiency and excess
2.2.2.1. Deficiency
In adults, vitamin K deficiency is clinically characterised by a bleeding tendency in relation to a low activity of the blood coagulation factors. This can be demonstrated by a vitamin K‐responsive increase in prothrombin time (PT) or partial thromboplastin time (PTT also called activated partial thromboplastin time, APTT). PT and PTT are indicators of the activity of the extrinsic and intrinsic coagulation pathways, respectively, assessed by the time it takes for a fibrin clot to form. More information on the sensitivity of the PT test compared to other biomarkers, as well as other references discussing these tests, are provided in Section 2.4.
In 10 healthy subjects fed for 3 weeks a diet considered as free of vitamin K by the authors (and that probably contained less than 10 μg/day vitamin K), there was an increase in average weekly PT (from 14.8 to 16 s, p < 0.05) (Udall, 1965). Other depletion/repletion studies, however, showed that healthy adults fed diets containing 5–10 μg phylloquinone/day for 2 weeks showed no change in coagulation time, either measured by PT or PTT (Allison et al., 1987; Ferland et al., 1993) (n = 33 and 32, respectively). A study in 10 adult patients with apoplexy unable to eat and with parenteral administration of vitamins without vitamin K, showed after 21–28 days prolonged PTs (assessed by % Quick test) in seven patients treated with antibiotics (‘affecting the intestinal flora’) but not in the three subjects not treated with antibiotics (Frick et al., 1967). This induced deficiency responded to increasing phylloquinone doses administered intravenously, from which the authors concluded that the amount of phylloquinone needed to restore a normal Quick value is between 0.03 and 1.5 μg/kg body weight per day phylloquinone. The Panel notes that these studies suggest that symptomatic vitamin K deficiency and impairment of normal haemostatic control in healthy adults may take more than 2–3 weeks to develop at ‘low’ phylloquinone intake (i.e. < 10 μg/day).
Exclusively breastfed infants are more susceptible to bleeding than formula‐fed infants (Shearer, 2009), due to the low phylloquinone content of human milk (Section 2.3.6.3) compared to infant formulae (Greer et al., 1991). Phylloquinone concentrations were undetectable in cord blood of infants of unsupplemented mothers unless the pregnant women received phylloquinone intravenously before delivery (Shearer et al., 1982). Liver tissue contents of phylloquinone and of menaquinones in neonates are low (MK‐n were undetectable until 14 days post‐partum), although these low vitamin K stores seem to be sufficient to maintain normal haemostasis during fetal life (von Kries et al., 1988) (Section 2.3.4.3). Incidence rates of vitamin K deficiency bleeding (VKDB) in infants not given vitamin K prophylaxis have been reviewed (Sutor et al., 1999; Zipursky, 1999; Shearer, 2009). Studies cited in these reviews reported that the incidence of early VKDB (< 24 h of life) ranged from less than 6% to 12% of births and that the incidence of classical VKDB (first week of life) ranged from 5.4/105 births to 1.7% of births in Western European countries, and between 25/105 births and 0.9% in Africa and South‐East Asia. The incidence of late VKDB (after the first week of life, up to 6 months, with a peak at 3–8 weeks of life) was reported to range from 4.4 to 7.2/105 births in Western European countries, and from 10.5 to 72/105 births in South‐East Asia (Japan and Thailand). The relative risk (RR) for developing late VKDB is estimated to be 81 times greater for infants not given vitamin K prophylaxis (McNinch and Tripp, 1991). The incidence of VKDB declines at 12 weeks of age, and spontaneous bleeding beyond that age is rare and as a rule limited to lipid malabsorption syndromes.
Administration of phylloquinone at a pharmacological dose, either orally or by intramuscular injection, is usual practice for prevention of haemorrhagic disease in newborn infants (Clarke et al., 2006; Busfield et al., 2007; Strehle et al., 2010; Mihatsch et al., 2016). Oral pharmacological doses of MK‐4 (2 mg at birth, and 4 mg at 1 week of age, n = 72,000) have been successfully used in newborns for prophylaxis of haemorrhagic diseases in Japan (Matsuzaka et al., 1987).
Studies have investigated possible relationships between ‘low’ vitamin K intake and abnormal calcification including osteoporosis or arterial calcification (as reviewed in Kaneki et al. (2006) and Vermeer and Braam (2001)) and possible associations between plasma phylloquinone and the risk of osteoarthritis (Neogi et al., 2006). This is discussed further in Sections 2.4 and 5.2.
2.2.2.2. Excess
The SCF (2003b) reviewed data on phylloquinone and identified two studies in humans (Craciun et al., 1998; Booth et al., 1999b), which showed no evidence of adverse effects associated with supplementation up to 10 mg/day for 1 month. The SCF considered that these limited human data are supported by animal studies, which showed no adverse effect after daily administration of 2,000 mg/kg body weight for 30 days. The SCF concluded that there was no appropriate evidence to derive a tolerable upper intake level (UL) for vitamin K. The Panel notes that revising the UL for vitamin K is not within the scope of the present Opinion.
A review showed that prophylactic vitamin K administration to newborns of supraphysiological parenteral doses (ranging from 0.2 mg/kg body weight to a 1 mg bolus dose) can induce mean/median serum phylloquinone concentrations in the first week of life up to 1,000‐fold higher than non‐fasting adult ‘normal’ values (Clarke, 2010). However, in studies in term or preterm infants investigating different doses of parenteral vitamin K prophylaxis, the increase in production of vitamin K metabolites, of vitamin K recycling and of vitamin K catabolic pathways (Sections 2.2.1 and 2.3.5), showed that infants are capable of metabolising large vitamin K doses (Clarke et al., 2006; Harrington et al., 2010). No adverse effect has been reported with these high prophylactic doses.
2.3. Physiology and metabolism
The way dietary vitamin K is absorbed and transported in the body is complex (Figure 2).
Figure 2.
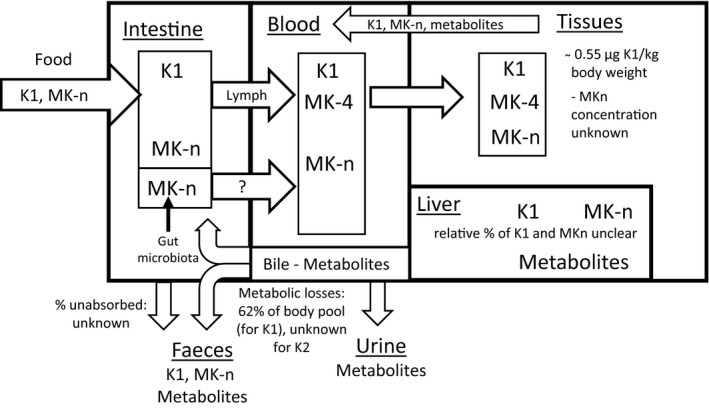
Metabolism of vitamin K in adults
- K1: phylloquinone; K2: MK‐n: menaquinones. Absorption of menaquinones synthesised from gut microbiota in the large intestine remains uncertain (hence the question mark in the figure) (Section 2.3.1).
2.3.1. Intestinal absorption
2.3.1.1. Intestinal absorption of phylloquinone
Phylloquinone is absorbed in the intestine, together with lipophilic compounds, and in the presence of dietary fat in a process that includes bile salts and requires proper pancreatic function for uptake of mixed micelles into the enterocytes and packaging with dietary lipids into nascent chylomicron particles (Blomstrand and Forsgren, 1968; Shearer et al., 1974, 2012). Absorption of phylloquinone depends on the food/meal matrix, as shown by differences in absorption of 13C‐labelled phylloquinone from a supplement consumed with different types of meals (Jones et al., 2009).
Studies investigating phylloquinone absorption in (usually small) samples of healthy adults, generally based on measurements of phylloquinone concentration in blood, differ in design. They used a variety of forms of phylloquinone (free or naturally present in various plant foods), of modes of preparation and administration (foods either cooked or fresh, with or without fat, supplements consumed with or without a meal), of phylloquinone intakes, or of experimental methods (isotope‐labelled or unlabelled phylloquinone, kinetic model, area‐under‐the‐curve (AUC)).
Absorption of free phylloquinone from a supplement was 13 ± 9% (mean ± standard deviation (SD), range 2–26%) or about 80% of the ingested dose in two studies. The lower value was calculated from a kinetic study using labelled phylloquinone in oil and given as gelatine capsules without a meal, and measuring plasma phylloquinone concentration (Jones et al., 2008) (Section 2.3.4). The higher value was obtained from the measurement of unchanged phylloquinone out of the total amount of radioactivity (unchanged form and metabolites) recovered from the faeces, after ingestion of labelled phylloquinone mixed with detergent solubilised phylloquinone and given as a supplement consumed with a meal containing fat, as discussed in the review by Shearer et al. (1974).
Mean relative absorption of unlabelled phylloquinone naturally present in plant foods (broccoli, spinach or lettuce; fresh or cooked, with or without fat), assessed as plasma AUC, ranged from approximately 4% to about 60–64% of the absorption of free phylloquinone in three studies. These studies used a variety of comparators (exogenous free phylloquinone added to the oil consumed with a baseline diet that also contained phylloquinone from foods, detergent‐solubilised free phylloquinone supplement or free phylloquinone from a tablet) that were all efficiently absorbed as indicated by their respective AUCs. The lower mean relative absorption of 4.1% referred to the absorption of 1 mg phylloquinone from cooked spinach without butter (Gijsbers et al., 1996), while the higher mean relative absorption of about 60–64% referred to the absorption of 377 μg phylloquinone/day from cooked broccoli (consumed daily for 5 days) in a baseline diet, in different age groups (Booth et al., 2002). A third study provided intermediate mean relative absorption values (Garber et al., 1999). Compared to a tablet providing 500 μg phylloquinone consumed with fat (27% energy), mean relative absorptions were about 17% for 150 g fresh spinach (450 μg phylloquinone) but about 9% for 50 g fresh spinach (165 μg phylloquinone) both consumed with fat (about 25% of energy) (significant difference between their respective AUC, p < 0.05). Mean relative absorptions were about 14% for fresh broccoli (214 μg phylloquinone) and about 23% for the same amount of cooked broccoli (184 μg phylloquinone) both consumed with fat in a meal (about 30% energy) (no significant difference in their respective AUC). Mean relative absorptions were about 11% for fresh romaine lettuce (179 μg phylloquinone) consumed with fat in a meal (30% of energy) and about 16% for the same amount of fresh lettuce (179 μg phylloquinone) consumed with more fat (45% of energy) (no significant difference in their respective AUC).
Absorption of phylloquinone (70 μg) present in intrinsically labelled cooked kale consumed with 30 g oil was calculated to be 4.7 ± 4.8% (mean ± SD, range 1–14%) or 7% in two studies. The first value was obtained from a kinetic study in subjects who consumed a diet providing daily 119 μg phylloquinone per 8.4 MJ during 1 week prior to kale ingestion and during the blood collection of about 4 weeks (Novotny et al., 2010) (Sections 2.3 and 5.1.1.2), while the second value was obtained from a study in one man who consumed a controlled diet of unknown phylloquinone content (Kurilich et al., 2003).
Relative absorption of phylloquinone (1 mg) from cooked spinach was enhanced up to about three times (i.e. to 13.3%) by dietary fat (butter) (Gijsbers et al., 1996), but this was not observed with fresh lettuce consumed with different fat intakes (Garber et al., 1999).
No significant sex differences (Jones et al., 2009) or age differences in adults (Booth et al., 2002) in phylloquinone absorption were observed (no data on phylloquinone absorption in infants or children are available).
2.3.1.2. Intestinal absorption of menaquinones
The contribution of medium and long‐chain menaquinones produced by gut microbiota to vitamin K status is unclear, as they are probably not easily absorbed from the distal bowel (Conly and Stein, 1992; Shearer, 1992). Menaquinones produced by the gut microbiota are not utilised in sufficient amounts to compensate for experimental dietary phylloquinone depletion in subjects not using antibiotics, as demonstrated by observed changes in vitamin K biomarkers during phylloquinone depletion (Paiva et al., 1998; Booth et al., 2001, 2003b) (Section 2.4).
In healthy adults, absorption of MK‐4, MK‐7 or MK‐9 has been studied in comparison with phylloquinone (either free or in plant food), based on measurements of peak serum concentration and/or AUC. As phylloquinone in plant foods is tightly bound to chloroplasts in plant cells (Manzotti et al., 2008; Reumann, 2013), thus not easily available for absorption when plant foods are ingested, the description below focusses on the results of the comparison with free phylloquinone.
MK‐4 and MK‐9 are less absorbed than free phylloquinone (Gijsbers et al., 1996; Schurgers and Vermeer, 2002). The designs of these studies differed, as e.g. MK‐4 and MK‐9 were provided as free forms (consumed with fat and with or without a meal) and free phylloquinone was either consumed with fat within a meal or from a supplement containing detergent‐solubilised phylloquinone consumed without a meal.
MK‐7 is more absorbed than free phylloquinone (Schurgers and Vermeer, 2000; Schurgers et al., 2007). The designs of these studies differed, as e.g. MK‐7 was consumed either in a food (natto) or as a supplement, free phylloquinone was consumed either in a detergent‐solubilised form within a meal with fat, or as a supplement in a meal of unspecified fat content, and vitamin K was given as a single dose or over several weeks.
MK‐7 is more absorbed than MK‐4, each provided as a single supplement dose (gelatine capsules) consumed with a meal containing fat (Sato et al., 2012).
2.3.1.3. Conclusions on intestinal absorption
The Panel notes that data on phylloquinone absorption in healthy adults, measured from different food sources and matrices, are variable, that absorption of phylloquinone from cooked plant foods may be enhanced by dietary fat by up to threefold, and that limited data suggest no significant sex or age differences in phylloquinone absorption in adults.
The Panel notes that all the studies that used the AUC approach to assess relative absorption of phylloquinone naturally present in cooked or fresh plant foods (with or without fat) had a sufficient duration of serum/plasma phylloquinone measurements to calculate the AUC (9–24 h) (Gijsbers et al., 1996; Garber et al., 1999; Booth et al., 2002). Assuming, as reference, 80% absorption for free phylloquinone (as a supplement consumed with fat (Shearer et al., 1974)), the Panel estimated from these three studies an absolute value of mean absorption of about 3–50%. The Panel also notes that absorption assessed by AUC of plasma concentration or assessed by the peak concentration can be underestimated, as the peak concentration value is influenced not only by absorption, but also by disposal and elimination rate. The Panel also notes that the results do not allow a direct measurement of an absolute value of phylloquinone absorption as no fractional absorption rate can be calculated from these studies. Other data on intrinsically labelled cooked kale consumed with fat showed that absorption of phylloquinone from plant food was about 5–7% (Kurilich et al., 2003; Novotny et al., 2010). Mean absorption of free phylloquinone from a supplement ranges from 13% (provided in oil in a hydrophilic matrix, i.e. gelatin, without a meal (Jones et al., 2008)) to about 80% (mixed with detergent solubilised phylloquinone and given as a supplement consumed with a meal containing fat (Shearer et al., 1974)).
The Panel notes that absorption of menaquinones produced by gut bacteria in the distal intestine remains uncertain, and therefore, the contribution of medium and long‐chain menaquinones produced by gut microbiota to vitamin K status is unclear. For dietary menaquinones, the Panel considers that available results indicate that MK‐4 and MK‐9 are less efficiently absorbed, and MK‐7 is more efficiently absorbed, than synthetic free phylloquinone; however, MK‐7 does not contribute much to MK‐n intake in Europe (Section 3.2.2). The Panel notes that these results are based on studies using serum concentrations (peak concentration or AUC) of menaquinones and phylloquinone that are known to have different kinetics in plasma (Section 2.3.2), and that these results do not allow to directly quantify MK‐4, MK‐7 or MK‐9 absorption as, again, no fractional absorption rate can be calculated.
The Panel considers that it is not possible to estimate precisely an average absorption of phylloquinone, menaquinones, and thus vitamin K from the diet.
2.3.2. Transport in blood
The predominant circulating form of vitamin K in blood is phylloquinone (Hodges et al., 1993a; Thijssen et al., 2002; Gentili et al., 2014), except in populations with high intakes of MK‐7 as in Japan (Tsugawa et al., 2006).
After intestinal absorption, radiolabeled phylloquinone first appears in the lymph (Blomstrand and Forsgren, 1968) and then enters the blood stream incorporated in chylomicrons (Shearer et al., 1970b). No specific carrier protein for phylloquinone in blood has been identified. Its main transporters during the post‐prandial phase of absorption are triglyceride (TG)‐rich lipoproteins (TRL) (about 75–90% of plasma phylloquinone), primarily chylomicron remnants and very low‐density lipoproteins (VLDL) (Kohlmeier et al., 1996; Lamon‐Fava et al., 1998; Schurgers and Vermeer, 2000, 2002; Erkkila et al., 2004). The remainder is approximately equally distributed between low‐ and high‐density lipoproteins (LDL and HDL), with lesser amounts in the intermediate‐density lipoprotein (IDL) fraction.
Studies on ingestion of labelled or unlabelled phylloquinone show that it peaks in plasma/serum about 4–10 h after ingestion and it peaks in the TRL fraction 3 h later than the TG present in the test meal (Shearer et al., 1970b; Lamon‐Fava et al., 1998; Schurgers and Vermeer, 2000, 2002; Dolnikowski et al., 2002; Kurilich et al., 2003; Erkkila et al., 2004; Fu et al., 2009; Novotny et al., 2010). Phylloquinone half‐life (t1/2) in plasma has been determined to range between 0.22 and 8.80 h, depending on studies, study durations and methodologies (Shearer et al., 1972, 1974; Bjornsson et al., 1979; Schurgers and Vermeer, 2000; Olson et al., 2002; Jones et al., 2008; Novotny et al., 2010) (Section 2.3.5).
After ingestion of equimolar doses (2 μmol5) of phylloquinone, MK‐4 and MK‐9, all dissolved in a meal containing fat, serum MK‐4 peaked at 2 h at the same time as the peak of TGs from the test meal, then was transferred to LDL and then to HDL (Schurgers and Vermeer, 2002). Serum phylloquinone and MK‐9 peaked at 4 and 5 h, respectively. MK‐9 was found only with LDL but not in HDL. Phylloquinone or MK‐4 disappeared from the circulation overnight, while MK‐9 serum concentration after 24 h was still about 25% of the peak value and remained detectable until the last measurement at 48 h (Schurgers and Vermeer, 2002). After ingestion of 3.1 μmoles of MK‐7 in the form of natto compared to 3.5 μmoles phylloquinone in the form of spinach and consumed with fat,6 serum phylloquinone and MK‐7 peaked at 6 h following consumption and a quick disappearance of phylloquinone from serum was observed within 24 h while MK‐7 showed complex (biphasic) pharmacokinetics in serum and remained detectable for at least 72 h (Schurgers and Vermeer, 2000). After ingestion of equal quantities of phylloquinone and MK‐7 (1 mg of each) in oil within a meal containing fat, the peak values were seen at about 4 h after the meal, and serum phylloquinone declined by 86% in the following 4 h, while MK‐7 showed a biphasic decline and was still present at 96 h (Schurgers et al., 2007).
The Panel notes that the main transporters of phylloquinone are TRL, and that menaquinones are also transported by lipoproteins. The Panel also notes that phylloquinone and individual menaquinones have different kinetics in serum/plasma, and that the clearance of MK‐7 and MK‐9 from serum/plasma is slower (48–96 h) than for phylloquinone.
2.3.3. Distribution to tissues
The liver is the primary organ that efficiently accumulates absorbed phylloquinone transported in chylomicrons (Section 2.3.4). The uptake of chylomicron remnants by the liver involves different apolipoproteins and high‐affinity lipoprotein receptors that mediate internalisation of the lipoprotein particles (Cooper, 1997). There is no conclusive information on the mechanism of uptake of menaquinones by the liver.
Bone matrix contains several vitamin K‐dependent proteins synthesised by the osteoblasts (Section 2.2.1), and vitamin K (phylloquinone and menaquinones) needs to be transported to osteoblasts for the γ‐glutamyl carboxylation of these proteins. Osteoblasts and osteoblast‐like cells are able to internalise phylloquinone from various lipoprotein fractions, as shown with human cell lines (Newman et al., 2002; Niemeier et al., 2005) and reviewed by Kohlmeier et al. (1996). The mechanism of cellular uptake of phylloquinone associated with TRL in the bone is dependent on both heparan sulfate proteoglycans (HSPG) and apolipoprotein E (ApoE) (Newman et al., 2002) and human osteoblasts express several receptors: the LDL receptor, the LDL receptor‐related protein 1, and to a lesser degree the VLDL receptor (Niemeier et al., 2005). There is no information on the mechanism of uptake of menaquinones by bones.
During pregnancy, only small quantities of phylloquinone cross the placenta from mother to fetus (Greer, 1995). Blood concentrations of phylloquinone in the full‐term newborn are about half of that of the mothers and the phylloquinone concentration in cord blood is low (< 0.1 nmol/L) (Shearer et al., 1982; Pietersma‐de Bruyn and van Haard, 1985; Greer et al., 1988; Mandelbrot et al., 1988). Little information is available on the amount of menaquinones crossing the placenta (Iioka et al., 1991).
2.3.4. Storage
2.3.4.1. Kinetic studies on the total body pool of phylloquinone
A kinetic study involved seven healthy US adults (3 women and 4 men; mean ± SD: 46 ± 14 years, 71 ± 8 kg mean body weight), who received a controlled diet providing daily 119 μg phylloquinone per 8.4 MJ (Novotny et al., 2010) (Section 2.3.1). Blood samples were taken on the intervention day and then for about 4 weeks. Intervention consisted of a single serving of labelled kale (equivalent to 70 μg unlabelled phylloquinone). A modelling of phylloquinone kinetics was developed, considering three compartments (for the gastrointestinal tract, the plasma and a body tissue pool). The authors used this compartmental modelling to determine the vitamin K utilisation rate and tissue storage pool, considering US mean body weights of 86 and 74 kg, and plasma phylloquinone concentrations of 1.43 and 1.47 nmol/L for men and women respectively (as reported in Booth et al. (1997); McDowell et al. (2005)). The model indicated ‘tissue storage pools’ of 46 and 41 μg phylloquinone for men and women, respectively (or 0.53 and 0.55 μg/kg body weight, respectively).
In another kinetic study (Olson et al., 2002), seven healthy subjects (six men including five followed as in‐patients in a metabolic unit, and one woman, aged 22–49 years) consumed a diet (control period) providing a mean phylloquinone intake of 75 μg/day for 1–2 weeks. Then, they consumed a ‘low‐vitamin K’ diet providing a mean of 8 μg phylloquinone/day (n = 5 out of 7 subjects7) for 3 weeks (n = 2) to 8 weeks (n = 3, whose average body weight was about 72 kg (read on figure)). Both diets provided a mean energy intake of about 8–12.8 MJ/day. Subjects received 0.3 μg isotopic‐labelled phylloquinone administered intravenously at the end of each period, and provided blood, urine and faeces samples for 6 days after each injection (Section 2.3.6). Based on a two‐compartment model, dilution of labelled phylloquinone indicated that the mean (± SD) total body pool of phylloquinone in the control or ‘low‐vitamin K’ periods were 87.6 (± 55.6) μg and 44.7 (± 25.1) μg, respectively. However, according to the authors, plasma phylloquinone (used in the calculation of the body pool) was overestimated8 due to the presence of an interference inherent to the analytical method used (method of Ueno and Suttie (1983)). Taking into account the ‘lower’ values for plasma phylloquinone, considered by the authors as more accurate, and the body weights of the participants (not reported for all), the authors calculated that the mean ‘exchangeable body pool size’ in subjects on the control diet would drop from 1.14 (SD 0.64) μg/kg to 0.57 (SD 0.32) μg/kg body weight. The Panel notes that the results were similar to the results by Novotny et al. (2010) and that the study has several limitations.
Ten healthy men and women (aged 22–31 years, mean body weight of 61 ± 10.7 kg), consumed 13C‐labelled phylloquinone (three times 3 μg/day) with food (phylloquinone intake from food not provided) for 6 days and then received a single intravenous dose of either 6 μg (n = 6) or 30 μg (n = 4) phylloquinone plus an oral dose of 4 μg 2H‐labelled phylloquinone (Jones et al., 2008) (Section 2.3.1). Blood samples were collected the day before and on the day of the intravenous phylloquinone injection over 6 h post‐dose. Phylloquinone in plasma was measured by high‐performance liquid chromatography (HPLC) and isotope ratios by gas chromatography/mass spectrometry (GC/MS). The use of a two‐compartment model to calculate the total body pool size of phylloquinone resulted in a mean of 2.3 μg (or 0.04 μg/kg body weight). The Panel notes the shorter length of measurements (6 h post‐dose) compared to the other studies, the different design, the absence of information on phylloquinone intake from food, and that this ‘total body pool size’ of phylloquinone appears to be underestimated.
Another study aimed to investigate, in four men receiving intravenous doses of radiolabelled phylloquinone, the potential interaction between clofibrate and warfarin on vitamin K disposition (Bjornsson et al., 1979). The authors indicate that the pool size of vitamin K in the body is ‘small’ but could not be calculated for these subjects.
The Panel notes the uncertainties and methodological limitations of the studies by Jones et al. (2008) and Bjornsson et al. (1979), and considers that no conclusion can be drawn from these two studies to assess the total body pool of phylloquinone.
2.3.4.2. Measurements of phylloquinone and menaquinones in the liver of adults
In livers obtained by autopsy (Rietz et al., 1970; Duello and Matschiner, 1972), MK‐7, MK‐8, MK‐10 and MK‐11 were identified (as well as MK‐4 and MK‐9 in Duello and Matschiner (1972)). The authors approximated phylloquinone content to be about 50% of the total amount of vitamin K in the liver on a weight basis, visually from relative intensity of thin‐layer chromatographic detection (Rietz et al., 1970) or ‘nearly one‐half’ of vitamin K in the liver, i.e. about 60 ng/g of wet liver weight, as assessed by thin‐layer chromatography and mass spectrometry (Duello and Matschiner, 1972). The Panel notes that the method in these two studies does not allow a quantitative estimation of phylloquinone and menaquinone concentrations in the liver.
In livers obtained by autopsy or donated for transplantation (thus with no information on previous intake), vitamin K concentration was assessed by HPLC in three studies. Concentration in ng/g, and the ratio between phylloquinone and MK‐n on a molar basis, were either reported or recalculated:
The phylloquinone concentration in livers of 32 adults showed a wide range between 1.1 and 21.3 ng/g wet liver weight, while the medians of 5.5 ng/g for men and 5.4 ng/g for women were quite similar (Shearer et al., 1988). The same authors also describe a semiquantitative analysis of menaquinones (i.e. by HPLC and comparison of peak area with that of phylloquinone) of 10 liver samples of adults. Menaquinones accounted for (median, range) 92% (75–97%) of the total amount of vitamin K in the liver on a molar basis. Chromatographic profiles of 17 livers of adults showed MK‐6, MK‐7, and MK‐8 to MK‐11 to be present.
The mean concentration of phylloquinone in livers of three adults was 34 ng/g liver (range: about 8–83 ng/g) and that of menaquinones (MK‐4 and MK‐7 to MK‐11 in most samples) was 21 ng/g liver (range: about 12–36 ng/g) (Kayata et al., 1989). Phylloquinone accounted for (mean, range) 74% (33–90%) of the total amount of vitamin K in the liver on a molar basis.
The mean concentration of phylloquinone in liver samples of three men and three women was about 7 ng/g wet liver weight (range: about 2–23 ng/g) (Thijssen and Drittij‐Reijnders, 1996). The mean concentration of menaquinones (MK‐4 and MK‐6 to MK‐11) was about 50 ng/g (range: about 21–87 ng/g wet liver). Phylloquinone accounted for (mean, range) about 21% (about 4–48%) of the total amount of vitamin K in the liver on a molar basis.
Fresh liver specimens (n = 15) were obtained by biopsy in patients who underwent gastrointestinal surgery, with known phylloquinone and menaquinone intake (Usui et al., 1990). Seven patients had been put on a standard diet (150–450 μg phylloquinone/day, < 2 μg/day each of MK‐4 to MK‐8), and eight on a low phylloquinone diet (per day 5 μg phylloquinone, 16 μg of MK‐9, and MK‐4, ‐5, ‐7, ‐8 and ‐10 each about 1–3 μg), for 3 days before operation. Concentrations of phylloquinone and menaquinones (MK‐4 to MK‐13) were measured by HPLC. The mean liver concentration of phylloquinone was about 13 ng/g and 3 ng/g of wet liver weight with the standard and low phylloquinone diets, respectively (significantly different, p < 0.01). Phylloquinone accounted for (mean, range) about 10% (about 9–12%) of the total amount of vitamin K in the liver on a molar basis with the standard diet, while the mean percentage was 2.4% (about 2–4%) on the low phylloquinone diet. Total MK‐n concentrations in the liver were not significantly different between the two groups, and were (mean, range) about 205 ng/g (137–409 ng/g liver) on the standard diet and about 239 ng/g (166–321 ng/g) on the low phylloquinone diet. Mean total concentrations of vitamin K in the liver were about 217 ng/g with the standard diet and 242 ng/g with the low phylloquinone diet, which are higher than the values reported by Thijssen and Drittij‐Reijnders (1996) and Kayata et al. (1989). The Panel notes that, while plasma phylloquinone was decreased by a low phylloquinone diet (and by pre‐operative fasting) and liver phylloquinone was decreased by 3 days of a low phylloquinone diet, the total concentration of vitamin K in the liver was not. The Panel notes that this study conducted in patients suggests that phylloquinone in the liver may be more rapidly depleted and catabolised than MK‐n.
The Panel notes that the mean/median phylloquinone concentration ranged between about 3 and 34 ng/g of liver, that the mean concentration of menaquinones (MK‐4 up to MK‐13 according to the studies considered) ranged from about 21 to 239 ng/g of liver, and that the mean/median percentage of phylloquinone in the total content of vitamin K of the liver ranged, on a molar basis, from 2.4% to 74%. The Panel also notes that the range of the content of phylloquinone in the human liver is large, due to possible variability in phylloquinone intake and status, but also to possible conversion of phylloquinone to MK‐4 (Sections 2.1 and 2.3.5) and degradation of phylloquinone during tissue handling and storage. The Panel notes that the reason for the high concentration of menaquinones in the liver in the study by Usui et al. (1990) in view of their dietary intake remains unclear.
2.3.4.3. Measurements of phylloquinone and menaquinones in the liver of fetuses and newborns
Phylloquinone concentration was in the range 0.4–3.7 ng/g in 21 fetal livers at 10–27 weeks of gestation (median of 1.3 ng/g in n = 18 at 19–27 weeks of gestation), and in the range 0.1–8.8 ng/g liver for 10 term newborns (median 1.0 ng/g) (Shearer et al., 1988) (Section 2.3.4.2). Median phylloquinone concentrations in the liver of fetuses and neonates did not significantly differ, but were significantly lower than those observed in adults in this study (p < 0.01). The authors could not identify any menaquinones in livers of fetuses or neonates.
Liver samples from autopsies of full‐term infants who died from sudden infant death syndrome, who were formula‐fed and received a phylloquinone intramuscular injection at birth were also analysed (Kayata et al., 1989) (Section 2.3.4.2). Mean concentrations were 36 ng/g liver for phylloquinone and 5.5 ng/g liver for menaquinones in infants aged less than 2 weeks (n = 2), and were 45 ng/g liver for phylloquinone and 36 ng/g liver for menaquinones (MK‐4 and MK‐7 to MK‐10 in most samples) in infants aged 2–4 months (n = 5). The statistical difference with adult values (mean of 34 ng phylloquinone/g liver, Section 2.3.4.2) was not tested.
The Panel notes that data are limited on phylloquinone concentration in the liver of fetuses, neonates and infants, and that these studies suggest that, at birth, the concentration of menaquinones is low in the liver (compared to adults) and increases during the first year of life. This increase could be related to the addition of complementary foods to the diet of infants and/or to the progressive colonisation of the gut by MK‐producing bacteria (Section 2.1).
2.3.4.4. Measurements of phylloquinone and menaquinones in extra‐hepatic tissues
Phylloquinone and MK‐n occur not only in liver and plasma, but data on tissue content in humans are limited. In tissue samples from autopsies (Thijssen and Drittij‐Reijnders, 1996) (Section 2.3.4.2), apart from the liver, the concentrations of phylloquinone were highest in the heart and pancreas, and lowest in the lung, kidney and brain. In this study, MK‐4 concentrations were highest in pancreas, kidney and brain and lowest in heart and lung. Molar ratios of MK4:phylloquinone showed that there was more MK‐4 than phylloquinone in the kidney and brain, similar amounts of both forms in pancreas and more phylloquinone than MK‐4 in the heart. In a study on six men and women who had a hip replacement (mean age: 69.7 ± 8.8 years) (Hodges et al., 1993b), concentrations in cortical and trabecular bone taken from the femoral neck ranged between 0.06 and 8.37 ng/g dry weight for phylloquinone and between 0.25 and 7.24 ng/g dry weight for MK‐6 to MK‐8.
2.3.4.5. Conclusions on storage
The total body pool of phylloquinone depends on phylloquinone intake, and is small, according to kinetic analyses. The Panel notes the limitations of available data from studies on total body pool of phylloquinone in adults (Bjornsson et al., 1979; Olson et al., 2002; Jones et al., 2008) (Section 2.3.4.1). The Panel considers that the most accurate values of the body pool of phylloquinone come from a compartmental analysis of phylloquinone kinetics in women and men (Novotny et al., 2010), as it takes into account the fast kinetics of phylloquinone. This study found ‘tissue storage pools’ of 46 and 41 μg for men and women, respectively, or 0.53 and 0.55 μg/kg body weight. The Panel also notes that the study by Olson et al. (2002), when taking into account the value for plasma phylloquinone considered as more accurate by the authors, provides a mean body pool of phylloquinone of 0.57 μg/kg body weight, a value which is close to the values of 0.53–0.55 μg/kg body weight obtained by Novotny et al. (2010). The Panel considers that a total body pool of phylloquinone of about 0.55 μg/kg body weight in healthy adults at steady state is associated with no signs of vitamin K deficiency.
The Panel notes that there is no data on the total body pool of menaquinones. Various organs contain phylloquinone and different menaquinones. The Panel notes that the liver is the organ that contains the highest concentration of vitamin K, as a mixture of phylloquinone and menaquinones (MK‐4 up to MK‐13 according to the studies considered), which contents are widely variable. The Panel also notes that relatively small amounts of vitamin K are reported in the liver of the newborn, in which phylloquinone predominates over menaquinones.
2.3.5. Metabolism
The turnover of phylloquinone in the body proceeds through two phases. The first phase of fast turnover of phylloquinone has been associated with a plasma/serum half‐life (t1/2) in the range of 0.22–8.80 h (Section 2.3.2), and the second phase of slower turnover has been associated with a tissue t1/2 in the range of 1.8–215 h, depending on studies and methodologies (Shearer et al., 1972, 1974; Bjornsson et al., 1979; Schurgers and Vermeer, 2000; Olson et al., 2002; Erkkila et al., 2004; Jones et al., 2008; Novotny et al., 2010). The value of 215 h was obtained in the study of longest duration (3 weeks) (Novotny et al., 2010), but studies of shorter duration provided smaller values (Olson et al., 2002; Erkkila et al., 2004) or a few h in the remaining studies). In the kinetic study by Olson et al. (2002) (Sections 2.3.4 and 2.3.6), the mean turnover times were 39.7 and 36.1 h on the control and low phylloquinone diets, respectively.
Phylloquinone is converted to menadione (Section 2.1) that is converted by cellular alkylation to MK‐4, which is not commonly produced by bacteria in contrast to other MK‐n (Section 2.1). This tissue‐specific conversion from phylloquinone has been observed in animals (e.g. rats, chicken), independently of gut bacteria since it occurs in germ‐free rats (Will et al., 1992; Thijssen and Drittij‐Reijnders, 1994; Davidson et al., 1998; Ronden et al., 1998; Al Rajabi et al., 2012). Data in human cells/humans are more limited and often refer to high doses of vitamin K. MK‐4 epoxide accumulated in human kidney cells incubated in the presence of 2.2 and 22 μmol/L of phylloquinone (Davidson et al., 1998) and menadione was converted into MK‐4 in cultures of several human cell lines (Thijssen et al., 2006). Authors believe the conversion of phylloquinone to menadione and MK‐4 occurs also in humans, during absorption in the intestinal mucosa and/or in other organs (Thijssen and Drittij‐Reijnders, 1996; Thijssen et al., 2002, 2006). Urinary excretion of menadione increased following single oral phylloquinone supplementation (10 mg) in healthy men, but not after a subcutaneous injection (Thijssen et al., 2006). Urinary excretion of menadione was also stimulated by the intake of single doses of MK‐4 (15 mg), MK‐7 (1 mg) or menadione (10 mg). The authors calculated that daily urinary excretion of menadione corresponded on a molar basis to 1.6–5.6% of the phylloquinone oral dose and 1–2.5% of the MK‐4 oral dose. In lactating women, the site of the conversion from phylloquinone to MK‐4 was suggested to be the mammary tissue, as MK‐4 concentration in breast milk was significantly correlated with phylloquinone concentration and increased with phylloquinone supplementation of the mothers (0.8, 2 or 4 mg/day compared with an unsupplemented group) (Thijssen et al., 2002). The enzyme UbiA prenyltransferase domain‐containing protein 1 (UBIAD1) has been identified in humans and catalyses the initial side chain cleavage of phylloquinone to release menadione and the prenylation of menadione to form MK‐4 (Nakagawa et al., 2010).
The hepatic and extra‐hepatic metabolism of menadione has been assessed in isolated rat livers perfused with menadione (Losito et al., 1967) or in rats administered menadione orally (Hoskin et al., 1954; Losito et al., 1967; Thompson et al., 1972), but no data on menadione metabolism in humans are available.
Phylloquinone in the liver has a fast turnover and is catabolised to metabolites that are rapidly transferred to plasma, urine and mainly bile, according to studies using radiolabelled tracer and unlabelled pharmacological doses of phylloquinone in humans (Shearer and Barkhan, 1973; Shearer et al., 1974; McBurney et al., 1980) (Section 2.3.6).
The catabolism of phylloquinone and menaquinones in the liver proceeds through a common degradative pathway. The side chain is metabolised by an initial ω‐hydroxylation, followed by a progressive side‐chain shortening via the β‐oxidation pathway (Shearer and Newman, 2014), until the side chain is shortened to two major metabolites with 7‐ and 5‐carbon side chains. The 5C‐metabolite has the structure 2‐methyl‐3‐(3′‐3′‐carboxymethylpropyl)‐1,4‐naphthoquinone and the 7C‐metabolite has the structure 2‐methyl‐3‐(5′‐carboxy‐3′‐methyl‐2′‐pentenyl)‐1,4‐naphthoquinone (Figure 1 in Sections 2.1 and 2.4). These two metabolites are conjugated with glucuronic acid and excreted in the bile (Shearer et al., 1972, 1974) and the urine (Shearer et al., 1970a, 1974; Shearer and Barkhan, 1973; McBurney et al., 1980) (Section 2.3.6). The ingestion of a large single pharmacological dose of phylloquinone (400 mg) by subjects treated with warfarin (Section 2.2.1) resulted in the isolation of a third aglycone metabolite in urine, identified as 2‐methyl‐3‐(7′‐carboxy‐3′,7′‐dimethyl‐2′‐heptenyl)‐1,4‐naphthoquinone (10C‐metabolite) (McBurney et al., 1980).
The Panel notes that vitamin K has a fast turnover in the body. Phylloquinone can be converted in humans to menadione and MK‐4, independently of the gut microflora. In the liver, phylloquinone and menaquinones are efficiently catabolised. The metabolism of phylloquinone and menaquinones produces the same metabolites, excreted in urine (5C, 7C or 10C) and bile (5C, 7C).
2.3.6. Elimination
2.3.6.1. Faeces
In the review by Shearer et al. (1974) (Section 2.3.1), in healthy subjects (n = 3) who ingested 1 mg of radioactive phylloquinone with a meal, the radioactivity recovered from the faeces over a period of 3 days was 54–60% of the dose. From this, 15–23% was identified by the authors as unmodified, presumably unabsorbed phylloquinone and the remaining lipid‐soluble radioactivity consisted of more polar metabolites that were separated by thin‐layer chromatography.
The radioactivity in faeces after 5 days from an intravenous dose of 1 mg radioactive phylloquinone represented 34% and 38% of the dose in two subjects, respectively (Shearer et al., 1972, 1974). No detectable faecal levels of radioactivity were present in a patient who also received this intravenous dose and whose total bile was collected for a period of 3 days, which indicates that the biliary route is the major route by which vitamin K metabolites pass into the intestinal lumen and are excreted in the faeces (Shearer et al., 1972). Shearer et al. (1974) also reported that, in one study in a subject injected with 45 μg radioactive phylloquinone, 51% of the dose was excreted in the faeces.
In the study by Olson et al. (2002) (Sections 2.3.4.1 and 2.3.5), in seven adults on the control diet providing a mean intake of 75 μg phylloquinone/day and receiving 0.3 μg isotope‐labelled phylloquinone administered intravenously, the total losses, measured by the excretion of radioactive products of phylloquinone during 6 days following the injection, accounted for (mean ± standard error of the mean (SEM)) 61.8 ± 2% of the isotopic dose, with 31.8 ± 0.8% excreted in faeces through the bile. This decreased to a mean (± SEM) of 13.3 ± 0.5% (p < 0.001) excreted in faeces when on the low phylloquinone diet (providing 8 μg/day).
Both phylloquinone and menaquinones are more prevalent in the stools of formula‐fed infants compared to breastfed infants (Greer et al., 1988; Fujita et al., 1993).
2.3.6.2. Urine
After a 1 mg intravenous dose of tritiated phylloquinone, in three adults, the cumulative excretion within 3 days was 19–26% of the dose via the urine (Shearer et al., 1972). In healthy adults who received an injection of 1 mg labelled phylloquinone with a meal, the urinary excretion of the ‘polar metabolites’ was found to be virtually complete after 3 days, accounting for 8–26% of the administered dose (mean of 19%) (Shearer et al., 1974). Shearer et al. (1974) also reported that, in one study in a subject injected with 45 μg radioactive phylloquinone, 18% of the dose was excreted in the urine. The major urinary metabolites are glucuronide conjugates.
In the study by Olson et al. (2002) (Sections 2.3.4.1, 2.3.5, and 2.3.6.1), in seven adults consuming the control diet providing 75 μg/day and receiving 0.3 μg isotope‐labelled phylloquinone administered intravenously, losses of phylloquinone metabolites in urine, measured by the excretion of radioactive products of phylloquinone (24 h urinary samples) during 6 days following the injection, were (mean ± SEM) 30 ± 1.8% of the isotopic dose. This value was 38.8 ± 9.8% on the low phylloquinone diet providing 8 μg/day. As plasma showed no detectable radioactivity after 6 days, the authors hypothesised that the radioactivity unaccounted for in faeces (Section 2.3.6.1) and urine remained in the adipose tissue.
The 5C‐ and 7C‐metabolites are common products of the metabolism of phylloquinone and menaquinones (Figure 1 and Section 2.3.5). The 5C‐metabolite was shown as the main urinary vitamin K metabolite in adults either unsupplemented or consuming various doses/intakes of phylloquinone, MK‐4 or MK‐7 (Harrington et al., 2005, 2007) (Section 2.4) and in term infants before or after vitamin K prophylaxis (Harrington et al., 2010). Urinary excretion of the 5C‐ and 7C‐metabolites increases in adults also in response to supplementation with menadione and reflects the process of interconversion of menadione to MK‐4 (Harrington et al., 2005).
In term infants, only 0.03% of a parenterally administered phylloquinone dose was excreted as urinary metabolites within the first 24 h post‐prophylaxis (Harrington et al., 2010), which suggests that the rate of phylloquinone clearance to the urine in neonates is slower than in adults. This is supported by the prolonged presence of phylloquinone in term neonate blood after its oral administration up to 4 days (Schubiger et al., 1993, 1997).
2.3.6.3. Breast milk
The SCF (2003c) noted that breast milk contains ‘low’ concentrations of vitamin K (mostly phylloquinone), between about 0.6 and 10 μg/L (von Kries et al., 1987a; Fomon, 2001). The SCF (2003c) also noted that the supply of vitamin K in breast milk is not sufficient to meet the requirements of all young infants. The SCF concluded that vitamin K supplementation is generally recommended in young infants in addition to the supply with breast milk. Based on data reported by IOM (2001), mean phylloquinone concentrations in breast milk around 2.5 μg/L, but varying from 0.85 to 9.2 μg/L, were noted (EFSA NDA Panel, 2013a).
Phylloquinone concentrations in (mostly mature) breast milk of lactating women either not supplemented or supplemented with phylloquinone, and menaquinone concentrations in mature breast milk, in countries of the EU, the USA and Japan, are described in Appendix A, with details on stage of lactation.
In the EU, mean/median concentration of phylloquinone in breast milk of unsupplemented mothers of full‐term infants was 1.2 μg/L in Germany (von Kries et al., 1987b), about 1.7 μg/L in Austria (Pietschnig et al., 1993), 2.1 μg/L in the UK (Haroon et al., 1982), about 2.2 μg/L in the Netherlands (Thijssen et al., 2002), and 9.18 μg/L in France (Fournier et al., 1987). The concentration of phylloquinone in breast milk is affected by maternal oral supplementation (about 0.1–5 mg phylloquinone/day or up to 20 mg as one dose) in the EU and US studies, with mean concentration reaching up to about 130 μg/L. When available, Appendix A reports on maternal vitamin K intake (Pietschnig et al., 1993) or status (Thijssen et al., 2002).
Limited data are available on menaquinone concentration in breast milk. In unsupplemented women in the Netherlands (Thijssen et al., 2002), mean MK‐4 concentration in breast milk was about 0.8–1 μg/L at 16–19 days post‐partum, and increased with phylloquinone supplementation (2 or 4 mg/day, p < 0.05 compared with the unsupplemented group). Mean concentrations in breast milk in two Japanese studies (Kojima et al., 2004; Kamao et al., 2007a) were in the range of about 1.2–1.9 μg/L for MK‐4 and about 0.8–1.7 μg/L for MK‐7.
2.3.6.4. Conclusions on elimination
The Panel notes that, with high oral doses of phylloquinone (e.g. 1 mg), non‐absorbed phylloquinone plus phylloquinone metabolites excreted via the bile are eliminated via faeces in large amounts, up to 60%. The Panel notes that the study by Olson et al. (2002), which measured losses both through collection of urine and faeces over 6 days, considered a lower intake (mean of 75 μg phylloquinone/day) that is closer to observed intake estimates (Section 3.2). Based on this study, the Panel considers that a mean of about 62% of injected phylloquinone is excreted as radioactive metabolites in urine (mean of 30%) and faeces (mean of about 32%). No similar experiment was available to assess losses of metabolites in urine and faeces after menaquinone ingestion. The Panel also notes that the 5C‐metabolite was the main urinary vitamin K metabolite in studies in adults and term infants.
The Panel notes that breast milk of unsupplemented women in the EU contains ‘low’ mean/median concentration of phylloquinone, varying from about 1.2 to 9.2 μg/L. The concentration of phylloquinone in breast milk is increased by maternal oral supplementation. Data on menaquinone concentration in breast milk in the EU are limited.
2.3.7. Interaction with other nutrients
Vitamin K intake is associated with changes in calcium balance that can positively influence bone calcium content (EFSA NDA Panel, 2015b). The vitamin D metabolite 1,25(OH)2 D (together with vitamin K) is needed for the synthesis of osteocalcin in the osteoblasts, and it regulates the expression of osteocalcin (EFSA NDA Panel, 2016).
Vitamin K and α‐tocopherol (vitamin E) share common metabolic pathways, including blood transport via lipoproteins, catabolism and biliary excretion (Schmolz et al., 2016). Up‐regulation of these pathways in response to increased α‐tocopherol intake can increase the rate of vitamin K catabolism and/or urinary and faecal excretion (Traber, 2008). α‐Tocopherol can also interfere with the vitamin K‐activation of the pregnane X receptor, leading to modulation of the expression of oxidative and conjugation enzymes (Landes et al., 2003). A cross‐sectional study suggested that about 10% of the variation in plasma phylloquinone concentrations could be explained by plasma concentrations of other fat‐soluble vitamins, particularly α‐tocopherol (Thane et al., 2006b). A competitive inhibition was described between tocopherol quinone and the phylloquinone hydroquinone for the vitamin K‐dependent γ‐carboxylase (EFSA NDA Panel, 2015a). In its assessment of the UL for vitamin E, the SCF (2003a) concluded that ‘high’ intakes of ‘vitamin E’ in subjects with ‘low’ vitamin K status (caused by malabsorption, impairment of the gut microbiota or therapy with anticoagulants) can cause impairment of blood coagulation. The SCF indicated that this would be a result of a reduction of the cyclooxygenase pathway, therefore of the thromboxane synthesis, thus impairing the thromboxane‐dependent blood coagulation and decreasing the coagulation factor II and VII. In healthy adults, ‘high’ intake of α‐tocopherol or α‐tocopherol given intravenously can result in bleeding, prolonged PT, lowered vitamin K‐dependent coagulation factors and appearance of undercarboxylated prothrombin in the blood (Booth et al., 2004a). α‐Tocopherol supplementation during 10 years had a mild anti‐thrombotic effect (Glynn et al., 2007). Doses of RRR‐α‐tocopherol above the UL can result in an increase in PIVKA‐II in adults in blood with normal coagulation status (Booth et al., 2004a).
The Panel notes that ‘high’ intakes of α‐tocopherol in subjects with ‘low’ vitamin K status can cause impairment of blood coagulation, and considers that data on interactions of vitamin K with other nutrients are limited.
2.4. Biomarkers
2.4.1. Prothrombin time (PT) test and partial thromboplastin time (PTT) test
The PT and PTT tests can reflect vitamin K deficiency (Section 2.2.2.1). PT has a usual range of 10–16 s for infants and 11–14 s for adults; and PTT is 25.4–59.8 s in healthy full‐term infants aged 5 days and 26.6–40.3 s in adults, according to reviews (Andrew, 1997; Greer and Zachman, 1998).
The review by Suttie (1992) reports on an experiment in which ‘normal’ human plasma was mixed with plasma from a warfarin‐treated patient (25% of the ‘normal’ concentration of prothrombin) in varying amounts. The curve of PT according to the percentage of ‘normal’ prothrombin shows that PT was still ‘normal’9 in samples with only 50% of the ‘normal’ prothrombin, and that it increases only at lower percentages (Suttie, 1992; IOM, 2001), suggesting a low sensitivity of the PT test.
From patients with apoplexy fed parenterally without receiving vitamin K, some of them also treated with antibiotics (Frick et al., 1967) (Section 2.2.2.1), the authors estimated that the amount of phylloquinone needed to recover a normal PT is between 0.03 and 1.5 μg/kg body weight per day in adults (body weight not given in the paper). The Panel notes that the results of this study showed a large range of values (that may be explained by methodological limitations in measuring small differences in phylloquinone concentrations).
Depletion/repletion studies in healthy individuals who consumed diets ‘low’ in phylloquinone, i.e. < 10 μg/day for 2–3 weeks, showed an increased coagulation time measured as PT in some subjects (Udall, 1965), but not in others (Allison et al., 1987; Ferland et al., 1993; Paiva et al., 1998), measured either as PT or PTT (Section 2.2.2.1). Dietary restriction of phylloquinone to 18 μg/day for 28 days (Booth et al., 2003b) or to about 35 μg/day for 40 days (Suttie et al., 1988)10 did not affect PT (Suttie et al., 1988) or PT and PTT (Booth et al., 2003b). Increasing phylloquinone intake from 100 μg/day to around 400 μg/day did not induce any change in PT or PTT (Booth et al., 1999b).
PT and PTT cannot be considered as biomarkers of all the functions controlled by vitamin K (Section 2.2.1). A disturbed coagulation time (increase of PT or PTT) may also indicate hepatic dysfunction or haematological disease not related to vitamin K deficiency and several other acute or chronic conditions, as reviewed by Booth and Al Rajabi (2008). Thus, PT and the PTT are markers of vitamin K status, but they are not specific.
The Panel considers that the PT and the PTT are not sensitive markers of vitamin K intake and status and non‐specific indicators of vitamin K deficiency. PT and PTT cannot be considered as markers of all the functions controlled by vitamin K. The Panel also notes that depletion/repletion studies show that vitamin K intakes sufficient for an adequate PT (e.g. equal or above 10 μg phylloquinone/day) may not be enough for the other functions controlled by vitamin K (as suggested by results on e.g. plasma phylloquinone, urinary Gla excretion, serum PIVKA‐II, %ucOC) (Sections 2.4.2, 2.4.3, 2.4.3.1, 2.4.3.2, 2.4.3.3, 2.4.4, 2.4.5, 2.4.6, 2.4.7).
2.4.2. Plasma concentration and activity of blood coagulation factors
Among the vitamin K‐dependent blood coagulation factors, i.e. factor II (prothrombin), VII, IX and X, synthesised by the liver as inactive forms (Section 2.2.1), factor VII (FVII) is the most frequently used, on the basis of its relatively short half‐life (approximately 6 h) (Ferland et al., 1993; Kamali et al., 2001). Normal laboratory ranges of FVII reported in studies in adults were about 70–130% of ‘normal’ values, with 100% corresponding to the FVII value observed in normal pooled plasma, i.e. 0.011 μM, or as Unit/mL (Allison et al., 1987; Andrew et al., 1988; Ferland et al., 1993). Authors considered values of FVII less than 60% as abnormal (Allison et al., 1987).
The depletion study of Allison et al. (1987) (Sections 2.2.2.1 and 2.4.1) was undertaken in 11 groups of three men each (aged 21–49 years, as inpatients in a ward) fed a diet containing less than 5 μg phylloquinone/day) for 2 weeks, with different antibiotics given orally or intravenously during the last 10 days in 10 of these groups. FVII concentration decreased after antibiotics treatment and was < 60% of ‘normal’ value on at least 1 day in 2/3 or 1/3 of treated subjects depending on the type of antibiotic, but not in individuals without antibiotics. The Panel notes that, in the subjects without antibiotics, a phylloquinone intake of 5 μg/day for 2 weeks did not lead to a decrease in FVII concentration. The Panel also notes that it is unknown if the antibiotics tested, some being well absorbable or given intravenously, decreased menaquinone production by the gut microbiota.
In the depletion/repletion study of Ferland et al. (1993) (Sections 2.2.2.1, 2.4.1 and 4), 32 healthy adults aged 20–40 and 60–80 years, in a metabolic unit, not receiving antibiotics, were subjected to a 4‐day baseline diet, a 13‐day depletion diet (about 10 μg phylloquinone/day) and a 16‐day repletion period (additional phylloquinone of 5, 15, 25 and 45 μg/day). No statistically different changes in the production of FVII were observed during the study as mean FVII ‘functional activity’ remained between 103% and 105%, while in both age‐groups, PIVKA‐II antigen concentration (Section 2.4.3) was increased significantly (p < 0.05) at the end of depletion compared to baseline.
Another depletion/repletion study was undertaken on nine younger (20–28 years) and nine older (55–75 years) men on their normal diet restricted in phylloquinone‐rich foods and providing 83 μg phylloquinone/day (younger adults, about 1 μg/kg body weight per day) and 164 μg/day (older adults, ‘about twice’ the amount consumed by younger adults) (Bach et al., 1996) (Section 4). Subjects received after 3 days, and for 14 days, 1 mg/day warfarin (‘acquired vitamin K‐deficiency’), and thereafter for 5 days 1 mg/day phylloquinone. Mean FVII activity was not affected by warfarin treatment whilst PIVKA‐II concentrations (Section 2.4.3) increased by > 30% by day 10 of warfarin treatment (exact increase depending on analytical method used to assess PIVKA‐II and age group), and while %ucOC (Section 2.4.3) increased continuously with time during depletion.
These studies in adults suggest that the depletion phase of about 2 weeks was too short for a change in FVII concentration/activity to occur. Both plasma concentration and functional activity of blood coagulation factors (in particular FVII) have a low sensitivity as biomarkers of vitamin K intake. FVII activity can be modified by other causes than vitamin K deficiency, e.g. genetic or liver diseases (Green et al., 1976; Mariani et al., 2003), thus is not a specific marker of vitamin K status.
Prophylactic administration of phylloquinone to pregnant women (20 mg/day orally for at least 3 days, during the second trimester or at birth) led to total prothrombin (factor II) activity in the fetuses (n = 41) or full‐term neonates (n = 33) that were comparable to that of fetuses or neonates from unsupplemented mothers. The values were lower than ‘normal’ adult values (pool of 30 healthy donors) (difference not tested) (Mandelbrot et al., 1988). Thus, phylloquinone administered to the mother does not change factor II activity in newborns. At day 1 in full‐term infants who all received 1 mg intramuscular ‘vitamin K’ at birth (n = 59–61 depending on the clotting factor), factor II, VII, IX, and X average activities were about 40–60% of adult values (n = 29) (Andrew et al., 1987). The authors report that the activity of these four factors at 6 months were in the adult range.
The Panel notes that FVII concentration/activity is not a sensitive biomarker of phylloquinone intake: for a change in FVII concentration/activity, the depletion phase of about 2 weeks in available studies may have been too short. The Panel also notes that FVII concentration/activity is not a specific marker of vitamin K status. FVII concentration/activity does not represent all functions that are controlled by vitamin K (as shown in studies indicating no change in FVII activity during depletion while PIVKA‐II concentration increased).
2.4.3. Circulating concentration of the undercarboxylated form of vitamin K‐dependent proteins
Insufficient availability of vitamin K results in the secretion into plasma of biologically inactive undercarboxylated vitamin K‐dependent proteins (Ferland et al., 1993; Booth et al., 2000b, 2003b) (Section 2.2.1). Their concentrations have been proposed as biomarkers of vitamin K status/stores for certain tissues (liver, bone, vessels (vascular calcification)) (Liska and Suttie, 1988; Szulc et al., 1993; Rennenberg et al., 2010; Schurgers et al., 2010).
2.4.3.1. Protein induced by vitamin K absence or antagonism‐II (PIVKA‐II) and S:E ratio
Normal blood concentration of PIVKA‐II (Section 2.2.1) has been defined as ≤ 2 μg/L (Booth et al., 2000b, 2001, 2003b). A review by Shea and Booth (2016) indicates that commercially available PIVKA‐II assays have low sensitivity for detecting variation in usual vitamin K intakes in healthy populations. The result of the assay for plasma concentration of functionally active prothrombin is also expressed as the Simplastin:Ecarin (S:E) ratio, which compares the amount of prothrombin generated in the test sample by action of a commercial thromboplastin preparation (Simplastin) with that generated with a protease (Ecarin) derived from the venom of the snake Echis carinatus.
PIVKA‐II blood concentration changes according to vitamin K intake. In metabolic11 depletion/repletion studies in adults (Section 2.4.1), it increases significantly in response to dietary restriction of phylloquinone (restriction to 10–18 μg/day) (Ferland et al., 1993; Booth et al., 2001, 2003b) and decreases significantly in response to dietary repletion with phylloquinone (Booth et al., 2000b, 2001, 2003b).
In particular, PIVKA‐II significantly dropped between end of depletion (at 10–11 μg phylloquinone/day) and end of repletion (at 200 μg/day for 10 days), and was restored to a value of ≤ 2 μg/L (Booth et al., 2000b, 2001). In the study by Booth et al. (2003b) in post‐menopausal women, that comprised a baseline diet (90 μg phylloquinone/day for 14 days) followed by a dietary depletion phase (18 μg/day for 28 days), mean PIVKA‐II decreased during the three consecutive phases of repletion (86, 200 and 450 μg phylloquinone/day for 14 days each) compared to the end of depletion. The decrease was not statistically significant with 86 μg phylloquinone/day (concentration above 2 μg/L) but became significant with 200 μg/day (concentration below 2 μg/L), until it attained the baseline value with 450 μg/day (concentration of about 1.4 μg/L, read on figure). The Panel notes the discrepancy in the results of this study, in that PIVKA‐II concentration did not return to normal with an intake of 86 μg phylloquinone/day for 14 days, while it was normal with the baseline diet corresponding to a similar intake of 90 μg/day for 14 days that is a finding indicating vitamin K sufficiency.
In the depletion/repletion study by Suttie et al. (1988) (Section 2.4.1), used by the SCF to set DRVs for vitamin K (Section 4), 10 young men (mean ± SD: 72 ± 9 kg body weight) followed a ‘normal’ diet with an intake of 82 μg phylloquinone/day for 7 days and continued with a restricted diet for 21 days. Median phylloquinone intake at day 9 and 27 was 40 and 32 μg/day, respectively, analytically measured in duplicate portions of all foods and beverages consumed. Subjects were then supplemented with either 50 or 500 μg phylloquinone/day from day 29 to 40 in addition to the same restricted diet, then with 1 mg/day from day 41 to 47. The mean S:E ratio was significantly lower (p < 0.01) at the end of the restriction period compared with the ‘normal’ diet (0.9111 vs 1.024, respectively), and was restored to normal with either 50 or 500 μg/day supplementation.
Most infants with vitamin K deficiency have ‘high’ PIVKA‐II concentrations, although it is not necessarily a predictor of haemorrhagic disease. Detection rates of PIVKA‐II in cord blood ranged from about 10–30% of full‐term infants (Motohara et al., 1985, 1990; von Kries et al., 1992; Bovill et al., 1993). In full‐term newborns (n = 156 enrolled), 47% of cord blood samples had PIVKA‐II blood concentrations ≥ 0.1 AU/mL (Greer et al., 1998).
2.4.3.2. Undercarboxylated osteocalcin (OC) and matrix γ‐carboxyglutamic acid protein (MGP)
The serum concentrations or proportions of ucOC or desphospho‐uncarboxylated MGP (dp‐ucMGP) (Sections 2.2.1 and 2.3.3) expressed as percentage of the total form (e.g. %ucOC), have been proposed as biomarkers for the extra‐hepatic vitamin K status. The Panel notes that this expression as percentage is more precise, because of the variability in the concentration of the total form. The relationship between vitamin K supplementation (phylloquinone or MK‐4 or MK‐7) and absolute concentration of dp‐ucMGP has been investigated (Cranenburg et al., 2010; Shea et al., 2011; Dalmeijer et al., 2012), showing a decrease in its concentration in the supplemented subjects compared to placebo. In addition, concentration or % ucOC in serum have been proposed as a biomarker of bone vitamin K status, as described below.
A randomised cross‐over metabolic depletion/repletion study compared the effects of phylloquinone or dihydrophylloquinone (dK) on a number of markers in 15 healthy adults (20–40 years) (Booth et al., 2001) (Section 2.4.3.1). The two residency periods of 30 days each, separated by at least 4 weeks, consisted of: (1) a 5‐day control diet (mean: 93.1 μg phylloquinone/day, no dK), (2) a 15–day depletion diet (mean: 11.0 μg phylloquinone/day, no dK) and (3) a 10‐day repletion diet (mean: 206 μg phylloquinone/day with no dK, or 240 μg dK/day with 11.0 μg phylloquinone/day). Mean %uOC was about 28–29% during the control diet, significantly increased (p < 0.01) after the depletion period (to about 42–47%), then significantly decreased (p < 0.01) during the phylloquinone repletion (to about 20%, not significantly different from the control diet), but not during the dihydrophylloquinone repletion. The Panel notes that this study showed not significantly different mean %ucOC with the daily intakes of about 90 μg and about 200 μg phylloquinone.
In the randomised cross‐over metabolic study by Booth et al. (1999b) (Section 2.4.1) with three residency periods of 15 days each, 36 healthy younger and older adults (20–40 and 60–80 years) consumed a mixed diet containing 100 μg phylloquinone/day or the same diet supplemented for days 6–10 with broccoli or fortified oil, thus providing 377 or 417 μg phylloquinone/day, respectively. Younger adults had significantly higher %ucOC than older adults on a mixed diet (p = 0.001, about 23% vs about 18% (read on figures), respectively), but there was no difference between sexes. In both age‐groups, mean %ucOC significantly decreased 5 days after the start of the supplemented diets (no difference between supplemented diets), while it did not significantly change on the mixed diet (i.e. about 20% (older adults) or 25% (younger adults) over the 15 days (read on figure)).
In a cross‐sectional study in 396 healthy Japanese women (30–88 years) with high natto consumption (phylloquinone or menaquinone intake not reported), women older than 70 years (n = 136) had significantly higher (p < 0.003) %ucOC in blood than women < 70 years (Tsugawa et al., 2006). This is in contrast to the previous study by Booth et al. (1999b).
In randomised controlled trials (RCTs) (Binkley et al., 2002; Bolton‐Smith et al., 2007; Kanellakis et al., 2012), in adult populations with mean baseline phylloquinone intake in the range of about 80–120 μg/day, different high doses of phylloquinone (100–1,000 μg/day, from supplements or fortified foods) compared to placebo/control significantly decreased mean % ucOC.
In a prospective cohort study of 245 healthy girls aged 3–16 years (Kalkwarf et al., 2004) (Section 5.2), baseline median phylloquinone intake (assessed by 3‐day food records, from food and supplements) was 45 μg/day (range: 6–275 μg/day). There was no association between phylloquinone intake and %uOC after adjustment for energy intake or energy intake and age.
Cross‐sectional analyses on 766 men and 925 women, either premenopausal or post‐menopausal with or without current oestrogen use (all groups having similar vitamin K intake), showed that post‐menopausal women not using hormonal replacement therapy had higher mean %ucOC in blood (23.5%) compared to the other groups (14–16%, difference not tested) (Booth et al., 2004b). Untreated early post‐menopausal women (n = 19, 40–52 years), also had significantly higher mean %ucOC than premenopausal women (n = 40 women aged 20–30 or 40–52 years) (21.9 vs 17.4%, p = 0.02) (Lukacs et al., 2006). These authors note that whether oestrogen directly modulates carboxylation of OC remains unclear. In addition to vitamin K intake, %ucOC is influenced by non‐genetic factors such as TG and smoking status (Shea et al., 2009).
A number of RCTs designed to investigate bone health (Section 5.2) have been done in Japanese or European adult populations using MK‐4 or MK‐7 supplementation (Schurgers et al., 2007; Koitaya et al., 2009; Emaus et al., 2010; Bruge et al., 2011; Kanellakis et al., 2012; Nakamura et al., 2014; Inaba et al., 2015), and using MK‐7 in children (van Summeren et al., 2009). The Panel notes the observed changes in the ratio between carboxylated and ucOC according to menaquinone intake. However, doses used were much higher (45–360 μg/day for MK‐7, 300–1,500 μg/day for MK‐4) than the limited habitual intake of MK‐4 or MK‐7 in European populations (Section 3), and baseline vitamin K intake was not always reported (Schurgers et al., 2007; Emaus et al., 2010; Bruge et al., 2011; Nakamura et al., 2014). The Panel considers that these data are not relevant to conclude on the relationship of this biomarker with usual dietary menaquinone intake in European populations, thus, that no conclusion can be drawn from these studies for setting DRVs for vitamin K.
Based on data that used the same assay for %ucOC (Booth et al., 1999b, 2001), a cut‐off of 20% has been proposed by McKeown et al. (2002) as the %ucOC above which the risk for dietary vitamin K insufficiency (defined in relation to US DRVs for phylloquinone, see Section 4) increases. In this observational study (Section 2.4.4), the lowest quintile of phylloquinone intake (i.e. median of 64 μg/day in women, 54 μg/day in men) was associated with a significantly higher risk of having a %ucOC above or equal to 20% (odds ratio (OR) (95% confidence interval (CI)): 2.51 (1.23–5.11), p = 0.01 in women; 2.75 (1.29–5.87), p = 0.009 in men), compared to the highest quintile (median of 307 μg/day in women and of 254 μg/day in men) (McKeown et al., 2002). However, the Panel notes that there is no reference level of γ‐carboxylation that can be considered as optimal related to functions controlled by vitamin K status. The Panel also notes that the relationship between %ucOC and bone mineral density (BMD) or risk of hip fracture has been investigated (Szulc et al., 1993, 1994; Schaafsma et al., 2000; Booth et al., 2004b), but the relevance of the 20% cut‐off for %ucOC with regard to these outcomes remains to be established.
Observational studies evaluated the association between circulating concentration of dp‐ucMGP or ucOC and the risk of hip fracture (Szulc et al., 1996; Vergnaud et al., 1997), risk of elevated aortic pulse wave velocities (Pivin et al., 2015; Mayer et al., 2016), risk of fatal or non‐fatal cardiovascular disease (van den Heuvel et al., 2014), cardiovascular mortality or the risk of coronary events (Liu et al., 2015), extent of coronary artery calcification (Dalmeijer et al., 2013), estimated glomerular filtration and risk of chronic kidney disease (Wei et al., 2016) and the risk of metabolic syndrome (Dam et al., 2015). The Panel notes that there were only one or a few observational study (studies) for each health outcome investigated and that vitamin K intake was not reported. The Panel also notes that the heterogeneity of these studies does not allow identifying a cut‐off for dp‐ucMGP or ucOC that is generally associated with adverse health outcomes. Overall, the Panel considers that the available data on the relationship between circulating concentration of dp‐ucMGP or ucOC and health outcomes cannot be used to derive an adequate vitamin K status.
2.4.3.3. Conclusions on circulating concentration of the undercarboxylated form of vitamin K‐dependent proteins
The Panel notes that concentrations of circulating undercarboxylated forms of vitamin K‐dependent proteins (in particular PIVKA‐II and ucOC) have been proposed as biomarkers of vitamin K status/stores for certain tissues (in particular liver and bone). They are sensitive to phylloquinone over a certain range of intake. Data on concentrations of circulating ucOC and menaquinone intake (MK‐4 or MK‐7) have been obtained with doses much higher than the limited observed intake data of MK‐4 or MK‐7 in Europe.
Normal blood concentration of PIVKA‐II has been defined as ≤ 2 μg/L but commercially available PIVKA‐II assays have low sensitivity for detecting variation in usual vitamin K intakes in healthy populations. The Panel notes that oestrogen status may be one determinant of vitamin K status assessed as %ucOC independent of the diet in women, while the limited data on the influence of age on %ucOC in adults are contradictory. The Panel notes that dietary intakes of phylloquinone or menaquinones required for full γ‐carboxylation of PIVKA‐II or OC or MGP have not been determined and that the ‘optimal’ extent of carboxylation is not known.
2.4.4. Circulating concentration of vitamin K
Most of the data on plasma vitamin K concentration are related to phylloquinone, and data on circulating menaquinone concentration (MK‐4, MK‐5 and MK‐7) are limited, as reviewed by Shea and Booth (2016). This review reports that 31 English post‐menopausal women (48–84 years) had a mean value of MK‐7 of 1.44 nmol/L (commented in Kaneki et al. (2001)) and 62 Italian healthy subjects (53–61 years) had mean values of 0.41 nmol/L for MK‐4, 0.58 nmol/L for MK‐5, 0.50 nmol/L for MK‐6 and 0.88 nmol/L for MK‐7 (Fusaro et al., 2012).
Because of the fast turnover of vitamin K (Section 2.3.4), plasma phylloquinone concentration reflects recent intake of phylloquinone, and responds to an increase in phylloquinone intake within 24 h (Sokoll et al., 1997) or to phylloquinone depletion within 3 days (Allison et al., 1987). In adults, circadian variation in the circulating mean vitamin K concentration (mainly phylloquinone) shows minimal and maximal levels at 10:00 and 22:00 h, respectively (Kamali et al., 2001), and plasma TG mirrored changes in plasma vitamin K concentration. In healthy adults, fasting plasma phylloquinone concentrations (not adjusted for TG) have a higher intra‐individual than interindividual variability (Booth et al., 1997).
Circulating phylloquinone concentration decreased with phylloquinone restriction and increased with phylloquinone supplementation (doses up to 1,000 μg/day) (Ferland et al., 1993; Booth et al., 2000b, 2003b; Binkley et al., 2002; Bolton‐Smith et al., 2007). Considering phylloquinone absorption and transport, and the correlation observed between plasma phylloquinone and TG concentration (Booth et al., 2004b; Tsugawa et al., 2006; Azharuddin et al., 2007), plasma phylloquinone concentration should be adjusted for TGs (nmol phylloquinone/mmol TG) (Shea and Booth, 2016). This is often not the case in available studies (Ferland et al., 1993; Booth et al., 1999b, 2000b, 2003b; Binkley et al., 2002; Bolton‐Smith et al., 2007).
After phylloquinone restriction (18 μg/day for 28 days or 10 μg/day for about 2 weeks) (Sections 2.4.1, 2.4.2 and 2.4.3) (Ferland et al., 1993; Booth et al., 2003b), plasma phylloquinone concentration significantly increased after repletion with 450 μg phylloquinone/day for 2 weeks (but not with 86 or 200 μg/day), although it did not return to initial levels (Booth et al., 2003b). However, in the other study (Ferland et al., 1993), it started to increase slightly only within the last repletion phase (additional 45 μg phylloquinone/day for 4 days) without reaching baseline values.
Mean plasma phylloquinone not adjusted for TGs was significantly higher in older than in younger adults (Ferland et al., 1993; Booth et al., 1999b) (Sections 2.4.2 and 2.4.3.2). However, in an observational study, plasma phylloquinone concentrations adjusted for TGs were significantly lower in older adults (65–92 years, n = 195) compared to younger adults (20–49 years, n = 131) (Sadowski et al., 1989). In younger and older adults (Booth et al., 2002) (Section 2.3.1), whose plasma phylloquinone was measured for 24 h, and who consumed diets providing on average 100, 377 or 417 μg phylloquinone/day, there was a significant overall age effect when comparing plasma phylloquinone concentration, either unadjusted or adjusted for TG, at 0 and 24 h, although there were no age differences in the 24 h‐AUC for plasma phylloquinone adjusted for TGs.
A significant positive relationship between phylloquinone intake (from food or food and supplements) and phylloquinone plasma concentration was also observed in large observational studies in adults, over a large range of intake (5–1,000 μg/day measured by seven‐day food record (Thane et al., 2006b); 50–200 μg/day measured by a food frequency questionnaire (FFQ) (McKeown et al., 2002)).
In full‐term infants (Greer et al., 1991), mean plasma phylloquinone concentrations were lower in exclusively breastfed compared to formula‐fed infants (range: 0.29–0.53 nmol/L in 23 breastfed infants between 6 and 26 weeks, vs 9.8–13.3 nmol/L in 11 formula‐fed infants), in relation to their different phylloquinone intake.12
The Panel notes that the circulating concentration of phylloquinone in blood is a biomarker of short‐term phylloquinone intake in adults. Circulating phylloquinone decreases during phylloquinone dietary depletion and increases with phylloquinone supplementation. Plasma phylloquinone concentration needs to be adjusted for TG concentration, which is often not done in available data. The exact dose‐response relationship is unclear. Data on circulating menaquinone concentrations are limited. The Panel also notes that no cut‐off value of plasma phylloquinone or menaquinone concentration has been set to define vitamin K adequacy (Shea and Booth, 2016).
2.4.5. Urinary concentration of γ‐carboxyglutamic acid (Gla) residues
In protein catabolism, Gla residues contained in the vitamin K‐dependent proteins are not further metabolised and are excreted in the urine (Shea and Booth, 2016). As a result, urinary Gla excretion has been used as an indicator of vitamin K status in adults. Urinary Gla excretion is a measure of the overall body content of vitamin K‐dependent proteins, including proteins whose functions are not related to haemostasis but have not been clearly established, as reviewed by Ferland (1998).
In the randomised cross‐over metabolic depletion/repletion study in young men and women by Booth et al. (2001) (Section 2.4.3), mean urinary Gla concentration (measured in 24 h urine samples) significantly decreased during phylloquinone depletion (about 10 μg/day for 15 days) compared with the control diet (about 100 μg phylloquinone/day for 5 days), then significantly increased with phylloquinone repletion (about 200 μg/day for 10 days) without returning to baseline values within the time frame of repletion (and it did not react to dK supplementation). In the depletion/repletion study in young adults by Suttie et al. (1988) (Sections 2.4.1 and 2.4.3), urinary Gla concentration was measured in 3‐day composite urine samples and expressed as a percentage of the ‘normal’ diet period (i.e. a diet with a median intake of 82 μg phylloquinone/day). Mean urinary Gla excretion at the end of the phylloquinone depletion period was significantly decreased (i.e. about 78% of the value of the normal diet period, p < 0.01), then significantly increased with phylloquinone supplementation (50 or 500 μg/day) compared to the depletion phase (p < 0.01, to reach about 97% of the value of the ‘normal’ diet, the two supplemented groups were combined as not significantly different).
In the depletion‐repletion metabolic study in younger and older adults (Ferland et al., 1993) (Sections 2.2.2.1, 2.4.1, 2.4.2 and 4), mean urinary Gla concentration (measured in 24 h urine samples) significantly decreased in response to dietary phylloquinone depletion (~ 10 μg/day for 13 days) in young adults compared to baseline (100 μg phylloquinone/day for 4 days). This was not observed in the older adults (significant difference between age group, p < 0.03). Urinary Gla concentration increased after phylloquinone supplementation (with additional 15, 25 and 45 μg/day, days 22–33, but not with additional 5 μg/day during days 18–21) in adults, respectively, with urinary Gla excretion reaching 96% of baseline values in young adults even with the supplementation at 45 μg phylloquinone/day (i.e. about 55 μg/day in total for 4 days). Twenty‐four hours urine concentrations (μM, mean ± SEM) at baseline, at end of depletion and at end of repletion were 38.5 ± 1.5, 35.2 ± 1.4, and 36.7 ± 1.1 for the young adults and 38.2 ± 2.6, 38.0 ± 2.4, and 39.4 ± 2.7 for the older adults, respectively.
In the randomised cross‐over study by Booth et al. (1999b) (Sections 2.4.1 and 2.4.3), urinary Gla concentration (measured in 24‐h urine samples) did not change significantly during the 15‐day mixed‐diet period (100 μg/day) in younger and older adults. Urinary Gla concentration was expressed as percentage of baseline and the mixed diet was compared with the supplemented diets (377 or 417 μg phylloquinone/day from days 6 to 10): there was no significant difference in urinary Gla concentration between the three diets on day 10 (i.e. mean of about 101% of baseline values for each diet). As well, in the metabolic depletion/repletion study in post‐menopausal women by Booth et al. (2003b) (Sections 2.4.1 and 2.4.3), mean urinary Gla concentration (measured in 24‐h urine samples) was significantly lower (p < 0.05) at the end of the dietary depletion phase (18 μg/day for 28 days) compared to the start of the baseline diet (90 μg/day for 14 days), but did not significantly change during the three consecutive phases of dietary repletion (86, 200 and 450 μg phylloquinone/day for 14 days each).
The Panel notes that urinary concentration of Gla residues, that is a measure of the overall body content of vitamin K‐dependent proteins, is sensitive to phylloquinone dietary depletion and may be sensitive to phylloquinone supplementation over several days in studies in adults, but data on a possible relationship between urinary Gla concentration and phylloquinone supplementation are conflicting. Thus, a dose–response relationship between urinary concentrations of Gla residues with phylloquinone intake cannot be precisely established. The Panel is not aware of any data on the relationship between urinary Gla concentration and menaquinone intake in the range of observed intake in Europe (Section 3). The Panel notes that dietary intakes of phylloquinone or menaquinones required for maximal or ‘optimal’ urinary Gla excretion have not been determined. The Panel also notes that there are no agreed cut‐offs values for urinary Gla concentration that would indicate vitamin K adequacy. The available data suggest that the response of urinary Gla excretion to these dietary changes is age‐specific.
2.4.6. Urinary concentration of vitamin K metabolites 5C and 7C
The measurement of the urinary concentrations of the 5C‐ and 7C‐metabolites, common to the metabolism of both phylloquinone and menaquinones (Sections 2.3.5 and 2.3.6), has also been proposed as a marker of the total body pool of vitamin K in adults, as reviewed by Card et al. (2014). The 5C‐ and 7C‐metabolites have been measured in 24 h or spot urine samples in unsupplemented healthy adults on two consecutive days, and these concentrations respond to high‐dose supplementation with phylloquinone (2 or 50 mg), MK‐4 (45 mg), MK‐7 (1 mg) or menadione (20 mg) in adults or in neonates (intramuscular phylloquinone, 1 mg) (Harrington et al., 2005).
In a randomised cross‐over study in nine adults residing in a metabolic unit for two 30‐day‐periods (separated by at least 4 weeks), subjects consumed a control diet (93 μg phylloquinone/day for 5 days), then a phylloquinone‐restricted diet (11 μg/day for 15 days), then a repletion diet with either 206 μg phylloquinone/day or 240 μg dK/day for 10 days in separate residency periods (Harrington et al., 2007). Urinary 5C‐ and 7C‐ metabolites concentrations, measured in 24‐h urine samples,13 reacted differently to phylloquinone restriction. The urinary 5C‐metabolite concentration significantly decreased (p = 0.001) after phylloquinone restriction while the urinary 7C‐metabolite concentration did not. Both significantly increased after phylloquinone repletion to reach a plateau after 4 days.14 The Panel notes that only one level of intake of phylloquinone was investigated during repletion.
The Panel notes that urinary concentrations of the 5C‐ and 7C‐metabolites, which have been proposed as biomarkers of total vitamin K status, are sensitive to phylloquinone or menaquinone supplementation, but limited data showed that only the urinary 5C‐metabolite concentration decreased during phylloquinone dietary depletion. The usefulness of the measurement of urinary concentrations of the 5C‐ and 7C‐metabolites to assess vitamin K status is limited by the proportion of these metabolites also excreted in the bile (Sections 2.3.5 and 2.3.6). The Panel considers that the dose–response relationship with vitamin K intake (phylloquinone or menaquinones) is not established, and notes that no agreed cut‐off for vitamin K adequacy has been identified.
2.4.7. Conclusions on biomarkers
Vitamin K deficiency leads to an increased PT, which is the only vitamin K biomarker that has been associated with adverse clinical symptoms. Symptomatic vitamin K deficiency and impairment of normal haemostatic control in healthy adults may take more than 2–3 weeks to develop at ‘low’ phylloquinone intake (i.e. < 10 μg/day) (Section 2.2.1).
The other biomarkers (concentration/activity of blood coagulation factors, blood concentrations of undercarboxylated forms of vitamin‐K dependent proteins or of vitamin K, urinary concentrations of Gla residues or of vitamin K metabolites 5C and 7C) may change according to vitamin K dietary intake (biomarker of intake). In the available studies, dietary vitamin K restriction results in lower phylloquinone plasma concentration, higher plasma concentration of undercarboxylated vitamin K dependent proteins, lower urinary Gla excretion, and mostly not in PT increase (possibly in relation to the short study duration). The Panel concludes that there are no biomarkers for which a dose‐response relationship with phylloquinone intake has been established. The available studies generally assessed whether the biomarkers returned to baseline values with phylloquinone supplementation/dietary repletion after phylloquinone depletion. However, for these biomarkers, no cut‐off value to define adequate vitamin K status is available, so these changes in biomarkers are difficult to interpret. Studies investigating the relationship between biomarkers and intake of individual menaquinones often used doses much higher than the limited observed intake data of these individual menaquinones in Europe (Section 3). There is no reference level of γ‐carboxylation that can be considered as ‘optimal’ related to functions controlled by vitamin K status and the dietary intakes of phylloquinone or menaquinones required for maximal or ‘optimal’ urinary Gla excretion have not been determined. Thus, the Panel considers that none of these biomarkers is suitable by itself to assess vitamin K adequacy. The Panel also considers that data on the effect of age and sex on vitamin K status in adults are insufficient for deriving the requirement for vitamin K according to sex or for ‘younger’ and ‘older’ adults.
2.5. Effects of genotypes
The response of biomarkers to vitamin K intake varies among healthy individuals (Shea and Booth, 2016). Meta‐analysis of genome‐wide association studies for single nucleotide polymorphisms (SNPs) associated with circulating phylloquinone concentrations identified multiple candidate genes related to lipoprotein and phylloquinone metabolism (Dashti et al., 2014).
A common polymorphism of the gene for the enzyme GGCX (Section 2.2.1) in human populations has been associated with transcriptional activity and sensitivity to warfarin (Shikata et al., 2004; Wadelius et al., 2005; Vecsler et al., 2006). The GGCX rs699664 SNP induces an increased carboxylase activity (Kinoshita et al., 2007). In community‐dwelling older adults, significant cross‐sectional association was observed between plasma phylloquinone concentration and/or plasma %ucOC and polymorphisms of GGCX (Crosier et al., 2009). In an observational study investigating GGCX polymorphism (974G>A) in healthy young Japanese subjects (Haraikawa et al., 2013), there was a statistically significant negative correlation between the ratio of ucOC to cOC and the intake of total vitamin K in GG‐type homozygotes (r2 = 0.294, p < 0.001) and heterozygotes (GA‐type, r2 = 0.160, p < 0.001), but not in AA‐type homozygotes.
In the VKOR protein structure (Section 2.2.1), the VKOR complex subunit 1 (VKORC1) is involved in enzymatic activity (Goodstadt and Ponting, 2004) and common polymorphisms of the VKORC1 gene are associated with variability in the effect of warfarin (Li et al., 2006; Montes et al., 2006; Obayashi et al., 2006; Rettie and Tai, 2006; Garcia and Reitsma, 2008; Owen et al., 2010). In community‐dwelling older adults, significant cross‐sectional association was observed between plasma phylloquinone concentration and/or plasma %ucOC and polymorphisms of VKORC1 (Crosier et al., 2009). In a Chinese cohort, SNPs and haplotypes within the VKORC1 locus were significantly associated with ucOC and PIVKA‐II concentrations (Wang et al., 2006). Genetic polymorphisms in the coagulation factor FVII (F7‐323Ins10) and VKORC1 were found to have an impact on the coagulation profile and the risk to develop intraventricular haemorrhage in a cohort (n = 90) of preterm infants (Schreiner et al., 2014).
Among the three common alleles of the gene encoding ApoE (i.e. E2, E3 and E4), the ability to clear intestinal lipoproteins rich in vitamin K from the blood is greatest with E4 and lowest with E2 (Kohlmeier et al., 1995; Newman et al., 2002). However, the magnitude of the effect of ApoE genotype on vitamin K status remains unclear, because in some studies, the highest frequency of E4 allele was associated with lower %ucOC in blood but also with higher or no different plasma phylloquinone concentration (Beavan et al., 2005; Yan et al., 2005).
Cytochrome P450 4F2 (CYP4F2) is involved in the hydroxylation of tocopherols and acts as a phylloquinone oxidase to produce the phylloquinone metabolite ω‐hydroxyvitamin K1 (McDonald et al., 2009). A CYP4F2 DNA variant (rs2108622; V433M) is present with a minor allele frequency of 5.8–26.7% in different ethnic groups (American, Chinese, Japanese and African subjects) (Caldwell et al., 2008). Carriers of this polymorphism need an increased warfarin dose for the anticoagulation activity (Caldwell et al., 2008), have lower CYP4F2 protein concentrations in liver and a reduced capacity to metabolise phylloquinone and may require lower dietary intakes of vitamin K compared to non‐carriers to maintain an equivalent vitamin K status (McDonald et al., 2009).
The Panel notes that potential genetic determinants of vitamin K status include polymorphisms in the genes involved in the activity, transport, uptake, metabolism, tissue‐specific availability and recycling of vitamin K, but considers that data on the effect of genotypes are insufficient to be used for deriving the requirement for vitamin K according to genotype variants.
3. Dietary sources and intake data
3.1. Dietary sources
Phylloquinone, present in all photosynthetic plants (Gross et al., 2006), is the predominant dietary form of vitamin K in the human diet. The primary sources of phylloquinone include dark green leafy vegetables (e.g. spinach, lettuce and other salad plants) and Brassica (flowering, head or leafy), with contents of about 60–365 μg and about 80–585 μg/100 g, respectively, according to the Nutrient composition database (Section 3.2.1) of EFSA. Other sources of phylloquinone include some seed oils, spreadable vegetable fats and blended fats/oils (Piironen et al., 1997; Peterson et al., 2002), with content of about 25–60 μg/100 g, based on this EFSA database.
For (total or individual) menaquinones, food composition data are limited in the EU (Schurgers et al., 1999; Koivu‐Tikkanen et al., 2000; Schurgers and Vermeer, 2000; Anses/CIQUAL, 2013; Manoury et al., 2013), in the US (Elder et al., 2006; Ferreira et al., 2006; USDA, 2015; Fu et al., 2016) and in Japan (Hirauchi et al., 1989; Kamao et al., 2007b).
Menaquinones are found in animal‐based foods, in particular in liver products: mostly MK‐4 in the range 0.3–369 μg/100 g in the EU, MK‐9 to MK‐11 in the range 0.4–492 μg/100 g in the USA, and MK‐6 to MK‐14 in the range 0.03–44 μg/100 g in Japan. Menaquinones are also found in meat and meat products (mostly MK‐4, in the range 0.1–42 μg/100 g in the available data), and in poultry products that are particularly rich in MK‐4, as poultry feed is a rich source of menadione, subsequently converted to MK‐4 in certain tissues of the poultry (in the range 5.8–60 μg/100 g in the EU, and 9–39 μg/100 g in the USA and Japan). Menaquinones are also present in some cheese and other dairy products: EU data on MK‐4 to MK‐10 (in particular MK‐9) are in the range 0.1–94 μg/100 g, while US and Japanese data, mainly on MK‐4, are in the range 1–21 μg/100 g. In natto, the most abundant menaquinone is MK‐7, in the range of about 850–1,000 μg/100 g (EU and Japanese data). Limited data on menaquinones are also available in a number of other products: in eggs (in particular in egg yolk) the most abundant menaquinone is MK‐4, in the range 10–30 μg/100 g in the EU, or 9–64 μg/100 g according to Japanese and US data, in fish, spices, chocolate, oil or bread, pies and pie crusts, fast food composite dishes (MK‐4 to MK‐8 and total menaquinones in EU and US data).
For dihydrophylloquinone (Section 2.1), the highest contents (about 60–165 μg/100 g) are reported in products such as some shortenings, some margarines, some snacks and crackers, some pie crusts and some pop‐corns (USDA, 2015).
Currently, phylloquinone (phytomenadione) and menaquinone (menaquinone occurring principally as MK‐7 and, to a minor extent, MK‐6) may be added to foods15 and food supplements.16 The vitamin K content of infant and follow‐on formulae and of processed cereal‐based foods and baby foods for infants and children is regulated.17
3.2. Dietary intake in Europe
The Panel aimed at presenting in this Section observed intakes of vitamin K (both forms) or of phylloquinone or (total or individual) menaquinones in Europe estimated using the EFSA Comprehensive European Food Consumption Database (EFSA, 2011b) and the EFSA Nutrient composition database compiled during a procurement project (Roe et al., 2013) involving several national food database compiler organisations. However, the EFSA Nutrient composition database did not contain data on phylloquinone or menaquinones, most of the involved national food composition databases did not contain any vitamin K data and the estimates for ‘total vitamin K’ also have limitations as described below (Section 3.2.1). In view of these limitations, the Panel also collected published data on estimated intake of phylloquinone and menaquinones (Section 3.2.2).
3.2.1. Dietary intake of ‘total vitamin K’ estimated by EFSA
3.2.1.1. Methodology
The Panel presents in this Section observed ‘total vitamin K’ intakes in Europe, estimated by EFSA using the EFSA Comprehensive European Food Consumption Database and the EFSA Nutrient composition database. Data presented as ‘total vitamin K’ in the EFSA Nutrient composition database were available originally only from three countries (Denmark, Germany and Sweden). Involved food database compiler organisations were allowed to borrow food composition data from other countries in case no original composition data were available in their own national database. As a result, Germany and Sweden borrowed, respectively, 2.5% and 30% of the ‘total vitamin K’ values they reported in the composition database, while Finland, Italy, the UK, the Netherlands and France borrowed 100% of the values reported. In addition, further research on the websites of the Danish,18 German19 and Swedish20 food composition databases suggests that only the data originally provided by Sweden may correspond to amounts of both phylloquinone and menaquinones, while data originally provided by Denmark and Germany concern phylloquinone only. This means that phylloquinone data and vitamin K data (i.e. phylloquinone and menaquinones) may have been listed under the term ‘total vitamin K’ in the composition data provided to EFSA. For intake estimates of Ireland and Latvia, food composition data from the UK and Germany, respectively, were used by EFSA, because no specific composition data from these countries were available. The Panel notes that these methodological limitations induce considerable uncertainty in the ‘total vitamin K’ intake estimates for the included European countries.
This assessment includes food consumption data from 13 dietary surveys (Appendix B) from nine countries (Finland, France, Germany, Ireland, Italy, Latvia, the Netherlands, Sweden and the UK). Individual data from these nationally representative surveys (except for the Finnish surveys in children) undertaken between 2000 and 2012 were available to EFSA, and classified according to the FoodEx2 food classification system (EFSA, 2011a). Intake calculations were performed only on subjects with at least two reporting days. For EFSA's assessment, it was assumed that the best intake estimate would be obtained when both the consumption data and the composition data are provided to EFSA for the same country. EFSA intake estimates are based on consumption of foods, either fortified or not, but without taking dietary supplements into account.
The data covers all age groups from infants to adults. Data on infants 1–11 months old were available from Finland, Germany, Italy and the UK. The proportions of breastfed infants were between 21% and 58% according to the survey considered and most breastfed infants were partially breastfed (see table footnotes of Appendices C–D). The Panel notes the limitations in the methods used for assessing breast milk consumption in infants (table footnotes of Appendices C–D) and related uncertainties in the intake estimates for infants.
3.2.1.2. Results
Taking into account the uncertainties mentioned above, ‘total vitamin K’ intake mean estimates ranged between 23 and 61 μg/day in infants (< 1 year), between 36 and 53 μg/day in children aged 1 to < 3 years, between 42 and 93 μg/day in children aged 3 to < 10 years, between 68 and 143 μg/day in children aged 10 to < 18 years (Appendices C–D). ‘Total vitamin K’ intake mean estimates ranged between 72 and 196 μg/day in adults (≥ 18 years). The main food group contributing to ‘total vitamin K’ intakes was vegetables and vegetable products (Appendices E–F). Leafy vegetables followed by Brassica vegetables were the most important contributors to ‘total vitamin K’ intakes for all age classes apart from infants, for whom the group ‘food products for young population’ was the main source of ‘total vitamin K’. Also, composite dishes were contributors to ‘total vitamin K’ intakes, probably at least partly due to vegetable‐based ingredients in the dishes, as well as (to a lower extent) the groups ‘animal and vegetable fats and oils’ and ‘legumes, nuts, oilseeds and spices’.
Mean intake estimates in adults for two countries (Italy, the Netherlands) were generally higher than the others (and higher than about 150 μg/day) in the different age ranges investigated in adults (Section 3.2.2 for other published Dutch intake data). This may be explained by a particular high contribution of leafy vegetables and aromatic herbs (Italy) and Brassica vegetables (the Netherlands) compared to the other countries, while composition data for these food categories were generally in line among countries.
3.2.1.3. Discussion
EFSA intake estimates were compared with published intake estimates from the same included national surveys. Published data were available for comparison only in Finland, i.e. for children aged 10 to < 18 years (Hoppu et al., 2010) and adults (FINDIET 2012 ( Helldán et al., 2013)), and in Germany for children aged 3 to < 18 years (Mensink et al., 2007)).
EFSA mean intake estimates for Finnish adults differed by about 5–12% from the published values (Helldán et al., 2013). The comparison of EFSA intake estimates with the published intake estimates of Finnish children (Hoppu et al., 2010) (i.e. different by 12–14%) have inherent limitations as they were for two consecutive days of dietary recall, while EFSA data comprised 2 × 48‐h dietary recall. The sources of ‘total vitamin K’ in the diet were not presented in this publication, and therefore could not be compared with EFSA's estimates. Considering the uncertainties of this intake assessment by EFSA (discussed above), a difference of up to 14% can be considered acceptable.
Difference between the ‘total vitamin K’ intake calculated by EFSA and the published estimates for German children (Mensink et al., 2007) (different by 63–65%, EFSA estimates being lower than the published values) is large. The published intake estimates for children are high even in comparison with intakes reported for older age classes in a different study in Germany (DGE, 2012) (Appendix G). One possible explanation could be a different version of the German Nutrient composition database used for this last publication and for the publication on children, which was confirmed by a personal communication.21 This communication indicated that phylloquinone intake data in children were calculated on the basis of version II.3 of the German Nutrient composition database (Bundeslebensmittelschlüssel (BLS)) of the Max Rubner Institut (MRI),22 and were higher than adult data, calculated with the newer version of the BLS (3.02). The EFSA Nutrient composition database contained German data that were also from an earlier version (BLS 3.01), but these vitamin K data were identical to the current BLS version 3.02. In the newer version of the BLS (3.02), 120 more recent and better data have been introduced. With the introduction of the new data, 77 items had 74% lower phylloquinone content, and 43 items had 67% higher phylloquinone content. In conclusion, the ‘older’ data are too high, but, on the other hand, the new data have flaws and may yield some underestimation, due to the lack of data source (thus the values were considered as ‘missing’ by the national food database compiler and ‘0’ for intake calculations).
Uncertainties on the nature of the ‘total vitamin K’ composition data (i.e. phylloquinone only or the sum of phylloquinone and menaquinones) and on the assessment of the intake data in infants (see table footnotes of Appendices C–D) have been discussed above. In addition, uncertainties in the estimates of all countries may be caused by inaccuracies in mapping food consumption data according to the FoodEx2 classification, analytical errors or errors in estimating ‘total vitamin K’ composition for the food composition table, due to the use of borrowed ‘total vitamin K’ values from other countries and the replacement of missing ‘total vitamin K’ values by values of similar foods or food groups in the intake estimation process. These uncertainties may, in principle cause both too high and too low estimates of ‘total vitamin K’ intake.
3.2.2. Dietary intake of phylloquinone and menaquinones as reported in the literature
The Panel performed an additional literature search on vitamin K intake estimates (i.e. phylloquinone, total or individual menaquinones) in observational studies/surveys undertaken in Europe, mainly in adults (Appendix G). Appendix G reports estimated dietary intakes as reported in the literature (national cross‐sectional surveys, large prospective cohorts and one case control study), with available information on, e.g. the number of subjects, the intake assessment method (FFQ, food records on several days, several 24‐h dietary recalls) or the source of the composition data for vitamin K. Comparison of EFSA's ‘total vitamin K’ intake estimates in EU countries with the published intakes of vitamin K from studies undertaken outside Europe (Korea, USA and Japan) (Booth et al., 1996b, 2003a; Feskanich et al., 1999; Kamao et al., 2007b; Kim et al., 2013) was not undertaken, as consumption patterns are significantly different.
Published studies on intake of phylloquinone or menaquinones used different designs, dietary intake assessments and food composition data, which limit direct comparisons between them (Appendix G). However, the intake estimates of ‘vitamin K’ or phylloquinone of these publications are variable and not completely in line with EFSA's calculations (Section 3.2.1). This can be explained by difference in the methods to assess intake (dietary recalls or record for at least two reporting days for EFSA's calculations, vs e.g. dietary history or FFQ), the methods of statistical analysis, the sources of composition data, the adjustments of intake values, or the size and characteristics of the samples of subjects that were smaller and/or not nationally representative. These differences make these published values not directly comparable with EFSA's intake estimates.
Six studies estimated the intake of phylloquinone and menaquinones separately using FFQs, including one (Geleijnse et al., 2004) being on subjects from the same Dutch prospective cohort as in another study (Schurgers et al., 1999) but considering more publications on composition data; and other Dutch, Norwegian and German prospective cohorts (Appendix G). The individual menaquinones investigated in these studies were not all the same. Estimated median intake of total menaquinones in Germany (34.7 μg/day) (Nimptsch et al., 2008) and Norway (10.8 and 11.9 μg/day in men and women respectively) (Apalset et al., 2011) represented about 30% and about 15%, respectively, of estimated median phylloquinone intake (93.6 μg/day in Germany and 67 and 78.4 μg/day in men and women in Norway, respectively). Estimated mean total menaquinone intake (about 27–31 μg/day) was about 10–13% of the sum of the mean intake of phylloquinone and the mean intake of menaquinone in the Netherlands (about 230–288 μg/day according to sex and study) (Schurgers et al., 1999; Geleijnse et al., 2004; Gast et al., 2009; Vissers et al., 2013).
Among individual menaquinones, MK‐4, MK‐8 and MK‐9 had the highest contributions to total menaquinone intakes in one Dutch and one German studies in adults (Nimptsch et al., 2008; Gast et al., 2009). MK‐7 is used in the EU for fortification and supplementation (Section 3.2.1) but no data were available to EFSA to assess its intake via these sources.
In addition, personal communication23 suggested that ‘older’ published vitamin K intake data from the Netherlands, like the German data for phylloquinone intake calculated with the older version of the BLS (II.3) (Sections 3.2.1.3), are an overestimate of the actual vitamin K intake. This may be due to the fact that both Germany and the Netherlands used the same ‘old’ composition data from Schurgers (in both cases the intakes were 200 μg/day or more), that the current analytical methods may be more precise than in the past, and that different food consumption measurements have been used (FFQ in the Dutch studies mentioned above, vs 2 × 24‐h recall in the recent Dutch food consumption survey). Personal communication also confirmed that the Dutch National Food Composition tables for vitamin K (phylloquinone and MK‐4 to MK‐10) are being updated, with analytical values from Dutch analysis and new literature values are used (e.g. from the database of the US Department of Agriculture USDA) whenever possible. Vitamin K intake data estimated from the Dutch National Food Consumption Survey 2007–2010 were calculated with partially updated composition data from 2013, which cover the most relevant sources of vitamin K but are not complete. This may lead to a possible underestimation of the vitamin K intake. In a recently published memo on this Dutch National Survey,24 the median (mean, IQR) intake estimates for vitamin K (phylloquinone and MK‐n) for children are 62 (70, 43–89) and 72 (80, 51–99) μg/day for girls (n = 857) and boys (n = 856) aged 7–18 years, respectively. For adults aged 19–69 years, these values were 100 (111, 70–140) and 117 (128, 85–159) μg/day in women (n = 1,051) and men (n = 1,055), respectively. Of note, according to the German National Nutrition Survey II (DGE, 2012 ) using a recently updated version of the German Nutrient composition database (BLS 3.02, MRI, Section 3.2.1.3), median phylloquinone intake, assessed by 2 × 24‐h recall, was 76 μg/day (95% CI: 75–77) for subjects aged 15–80 years (n = 6,160) (mean intake was not reported).
3.2.3. Conclusions on dietary intake in Europe
The Panel notes that ‘total vitamin K’ mean intake estimated by EFSA for nine EU countries ranged between 72 and 196 μg/day in adults (≥ 18 years). The Panel notes the uncertainties in this intake assessment, in particular with regard to the nature of the ‘total vitamin K’ composition data (i.e. phylloquinone only or the sum of phylloquinone and menaquinones) and on the assessment of the intake data in infants, and that intake of phylloquinone or menaquinones in these countries could not be estimated by EFSA with the available databases.
Published data on intake of phylloquinone and menaquinones in Europe show that phylloquinone is the major form consumed although the exact proportion of phylloquinone in vitamin K intake remains uncertain, and suggest that MK‐4, MK‐8 and MK‐9 have the highest contributions to the intake of total menaquinones.
The Panel also notes the updated food composition database and intake estimates for the Netherlands (vitamin K, i.e. phylloquinone and menaquinones, in children and adults) and for Germany (phylloquinone, in adults). These updated median intake estimates are in line with the lower bound of the range of mean intakes in adults in nine EU countries estimated by EFSA, mentioned above.
4. Overview of Dietary Reference Values and recommendations
4.1. Adults
D‐A‐CH (2015) derived an adequate intake (AI) for vitamin K of 1 μg/kg body weight per day for adults, based on the influence of vitamin K (phylloquinone) on blood coagulation (Frick et al., 1967; National Research Council, 1989; Suttie, 1996).25 Expressed in μg/day, the AIs were 70 and 60 μg/day for men and women aged 19–50 years, respectively. As a precaution, the AIs for older adults were increased, i.e. 80 μg/day for men and 65 μg/day for women, to take into account possible malabsorption and medication at that age.
For the NNR 2012, due to a lack of additional evidence, the NCM (2014) kept the provisional recommended intake of 1 μg/kg body weight per day previously set for adults, taking into account that phylloquinone intakes of about 60–80 μg/day (i.e. 1 μg/kg body weight per day) are adequate to prevent vitamin K deficiency in healthy subjects (Suttie et al., 1988; National Research Council, 1989; Jones et al., 1991; Bach et al., 1996). The Council considered that the available evidence on the relationship between intake of phylloquinone or menaquinones and health consequences (bone health, atherosclerosis and other health outcomes) could not be used to set reference values for vitamin K. The Council noted the low prevalence of vitamin K deficiency in the general population, the impossibility to induce deficiency symptoms with a vitamin K depleted diet, and the insufficient bacterial synthesis of vitamin K in the intestine to maintain serum concentrations of vitamin K. The Council considered that data on biomarkers (concentration of coagulation factors, plasma/serum concentrations of phylloquinone, degree of carboxylation of vitamin K‐dependent proteins, urinary vitamin K metabolites) (Suttie et al., 1988; Ferland et al., 1993; Booth and Suttie, 1998; Booth et al., 2001, 2003b; Binkley et al., 2002; Bugel et al., 2007; Harrington et al., 2007; Schurgers et al., 2007; Booth, 2009; McCann and Ames, 2009) were insufficient to change the previously set reference value.
The World Health Organization WHO/FAO (2004) derived a Recommended Nutrient Intake (RNI) of 1 μg/kg body weight per day of phylloquinone, corresponding to 55 μg/day for adult women and 65 μg/day for adult men. WHO/FAO (2004) set this value considering the function of vitamin K in blood coagulation and the average intakes (mainly of phylloquinone) in adults that are close to UK and US reference values of this period (National Research Council, 1989; DH, 1991; Suttie, 1992; Booth et al., 1996a). WHO/FAO (2004) considered that available data on γ‐carboxylation of OC could not be used to set reference values (Sokoll et al., 1997).
The US Institute of Medicine (IOM, 2001) considered that data on biomarkers of vitamin K status, including PT, FVII activity, plasma/serum concentrations of phylloquinone, the degree of carboxylation of vitamin K‐dependent proteins (prothrombin, OC) and urinary vitamin K metabolite concentrations could not be used to assess the requirements for vitamin K. The IOM considered that only PT has been associated with adverse clinical effects and that the significance of changes observed in the other biomarkers following changes in vitamin K intake is unclear. The IOM considered that data on the relationship between vitamin K intake and chronic diseases (osteoporosis, atherosclerosis) could not be used as well. The IOM reported on data showing abnormal PIVKA‐II concentrations for intakes (phylloquinone) below 40–60 μg/day and lack of signs of deficiency to intakes above 80 μg/day (Suttie et al., 1988; Jones et al., 1991; Ferland et al., 1993; Bach et al., 1996). IOM (2001) took into account the lack of sufficient dose–response data between vitamin K intake and biomarkers of status, the uncertainty surrounding the interpretation of these biomarkers and the low prevalence of vitamin K deficiency in the general population. Thus, IOM (2001) derived an AI of 120 μg/day for men and of 90 μg/day for women, based on the highest median intake of dietary ‘vitamin K’ 26 in apparently healthy subjects (NHANES III, 1988–1994) (highest intake chosen to take into account possible underestimation by dietary intake assessment methods), rounded up to the nearest five.
The French Food Safety Agency (Afssa, 2001) considered that the requirement for vitamin K in adults is probably low due to the efficient vitamin K recycling in the liver. AFSSA (2001) considered that this requirement may be between 0.1 and 1 μg/kg body weight per day based on data on maintenance of normal coagulation reviewed in Shearer et al. (1988), as data on the need for complete γ‐carboxylation of vitamin K‐dependent protein were insufficient for DRV‐setting (Shearer, 1995). AFSSA (2001) set a reference value of 45 μg phylloquinone/day for younger adults (< 75 years). For older adults (≥ 75 years), the reference value was set at 70 μg phylloquinone/day, based on data on vitamin K and bone health in older adults or suggesting a role of vitamin K to maintain sufficient concentration of carboxylated osteocalcin (cOC) in bone tissues (Knapen et al., 1998; Liu and Peacock, 1998; Tamatani et al., 1998; Feskanich et al., 1999; Cynober et al., 2000).
The SCF (1993) did not set an AR or a PRI for vitamin K, but considered that an intake of 1 μg/kg body weight per day, which would be provided by a usual diet, was adequate. To set this value, the SCF (1993) considered the effect of depletion at about 50 μg phylloquinone/day (with no effect on PT) and supplementation with 50 μg phylloquinone/day, on prothrombin biosynthesis and Gla urinary excretion (Suttie et al., 1988) and a previous review (Suttie, 1987).
The Netherlands Food and Nutrition Council (1992) did not consider vitamin K when setting reference values for the whole population.
DH (1991) concluded that, for adults, 1 μg/kg body weight per day phylloquinone is ‘safe and adequate’ (Suttie, 1985), since it maintains vitamin K‐dependent coagulation factors. DH (1991) did not derive an AR or a PRI for vitamin K for adults.
An overview of DRVs for vitamin K for adults is presented in Table 1.
Table 1.
Overview of dietary reference values for vitamin K (expressed as phylloquinone) for adults
| D‐A‐CH (2015)a , b | NCM (2014)c | WHO/FAO (2004)a , b | AFSSA (2001)a | IOM (2001)a | SCF (1993)c | Netherlands Food and Nutrition Council (1992) | DH (1991)c | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 19–50 | ≥ 18 | 19 – ≥ 65 | 19–74 | 19 – ≥ 70 | ≥ 18 | – | ≥ 18 |
| Men | 70 | 1 | 65 | 45 | 120 | 1 | – | 1 |
| Women | 60 | 1 | 55 | 45 | 90 | 1 | – | 1 |
| Age (years) | 51 – ≥ 65 | ≥ 75 | ||||||
| Men | 80 | 70 | ||||||
| Women | 65 | 70 |
D‐A‐CH: Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährung; NCM: Nordic Council of Ministers; WHO/FAO: World Health Organization/Food and Agriculture Organization of the United Nations; Afssa: Agence française de sécurité sanitaire des aliments; IOM: US Institute of Medicine; SCF: Scientific Committee on Food; NL: Health Council of the Netherlands; DH: UK Department of Health.
μg/day.
Derived considering an intake of 1 μg/kg body weight per day.
μg/kg body weight per day.
4.2. Infants and Children
D‐A‐CH (2015) also set an AI for vitamin K of 1 μg/kg body weight per day (Section 4.1) for children. Expressed in μg/day, AIs for children range from 10 μg/day in infants 4–12 months, to 60 (girls) and 70 (boys) μg/day in adolescents 15–19 years.
The NCM (2014) could not set ARs or PRIs for vitamin K in μg/day for children, due to a lack of sufficient evidence. For children, NNR 2012 kept the provisional recommended intake of 1 μg/kg body weight per day (Section 4.1) previously set. NNR 2012 also reported on prophylactic vitamin K administration to newborns (IOM, 2001; Hansen et al., 2003; Van Winckel et al., 2009).
For infants aged 7–12 months and children, WHO/FAO (2004) set RNIs ranging between 10 μg/day (7–12 months) and 35–55 μg/day (10–18 years), based on an intake of phylloquinone of 1 μg/kg body weight per day as for adults (Section 4.1). WHO/FAO (2004) also mentioned prophylactic vitamin K administration to newborns.
For infants aged 7–12 months, IOM (2001) set an AI of 2.5 μg/day based on the extrapolation from the phylloquinone intake of infants aged 0–6 months, estimated considering a mean breast milk intake of 0.78 L/day and an average phylloquinone concentration of 2.5 μg/L in human milk (Haroon et al., 1982; von Kries et al., 1987a; Hogenbirk et al., 1993; Greer et al., 1997). This upward extrapolation was done by allometric scaling (body weight to the power of 0.75, using reference body weights). No adverse clinical outcome was observed in older infants at that intake (Greer et al., 1991). Data on vitamin K in weaning foods were lacking and downward extrapolation from adults was not used to set an AI for older infants. AIs for children aged 1–18 years were set on basis of the highest median intake reported (NHANES III, 1988) (and rounding), since age‐specific data on vitamin K requirement were lacking. The AIs ranged between 30 and 75 μg ‘vitamin K’/day,21 for children aged 1–3 years and 14–18 years respectively. IOM (2001) noted that the methods used to establish AIs for older infants and children and the increased consumption of vitamin K sources (vitamin K‐rich fruits and vegetables) with age may explain the difference in AI values for infants and children.
AFSSA (2001) set the reference value for infants at 5–10 μg phylloquinone/day, and reference values for children based on an estimated requirement of 1 μg/kg body weight per day, leading to reference values between 15 (children 1–3 years) and 65 (children 16–19 years) μg phylloquinone/day. AFSSA (2001) also mentioned prophylactic vitamin K administration to newborns.
The SCF (1993) did not discuss specifically the requirement for vitamin K in children, did not set ARs or PRIs, but generally considered the intake of 1 μg/kg body weight per day (Section 4.1) to be adequate.
The Netherlands Food and Nutrition Council (1992) did not consider vitamin K when setting reference values for the whole population.
After rounding up, the UK COMA (DH, 1991) proposed a ‘safe intake’ of 10 μg/day for infants (about 2 μg/kg body weight), derived from the highest and rounded phylloquinone concentration in human milk (10 μg/L) in the available data (von Kries et al., 1987a; Canfield and Hopkinson, 1989) and a breast milk consumption of 0.85 L/day. They noted the low hepatic reserves of phylloquinone and the absence of hepatic menaquinones at birth (Shearer et al., 1988), as well as the association between haemorrhagic disease of the newborn and exclusive breastfeeding (von Kries et al., 1988). They supported prophylactic vitamin K administration to all newborns. No specific reference value was mentioned for older children.
An overview of DRVs for vitamin K for infants and children is presented in Table 2.
Table 2.
Overview of dietary reference values for vitamin K (expressed as phylloquinone) for infants and children
| D‐A‐CH (2015)a , b | NCM (2014)c | WHO/FAO (2004)a , b | AFSSA (2001)a , b | IOM (2001)a | SCF (1993)c | DH (1991)a | |
|---|---|---|---|---|---|---|---|
| Age (months) | 4–12 | All children | 7–12 | ‘Infants’ | 7–12 | All children | ‘Infants’ |
| Infants (μg/day) | 10 | 1 | 10 | 5–10 | 2.5 | 1 | 10 |
| Age (years) | 1 – < 4 | 1–3 | 1–3 | 1–3 | |||
| All (μg/day) | 15 | 15 | 15 | 30 | – | ||
| Age (years) | 4 – < 7 | 4–6 | 4–6 | 4–8 | |||
| All (μg/day) | 20 | 20 | 20 | 55 | – | ||
| Age (years) | 7 – < 10 | 7–9 | 7–9 | ||||
| All (μg/day) | 30 | 25 | 30 | – | |||
| Age (years) | 10 – < 13 | 10–12 | 9–13 | ||||
| All (μg/day) | 40 | 40 | 60 | – | |||
| Age (years) | 13 – < 15 | 10–18 | 13–15 | 14–18 | |||
| All (μg/day) | 50 | 35–55 | 45 | 75 | – | ||
| Age (years) | 15 – < 19 | 16–19 | |||||
| Boys (μg/day) | 70 | 65 | – | ||||
| Girls (μg/day) | 60 |
D‐A‐CH: Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährung; NCM: Nordic Council of Ministers; WHO/FAO: World Health Organization/Food and Agriculture Organization of the United Nations; Afssa: Agence française de sécurité sanitaire des aliments; IOM: US Institute of Medicine; SCF: Scientific Committee on Food; DH: UK Department of Health.
μg/day.
Derived considering an intake of 1 μg/kg body weight per day.
μg/kg body weight per day.
4.3. Pregnancy and lactation
D‐A‐CH (2015) set the same AI for vitamin K for healthy pregnant or lactating women as for other women, as it is unknown whether pregnant women need additional vitamin K and as the possibly small additional need in lactation is fully covered by a healthy and balanced diet. WHO/FAO (2004) and AFSSA (2001) also proposed for pregnant or lactating women the same reference value as for other women (Section 4.1).
The NCM (2014), SCF (1993) and DH (1991) mentioned no specific information or reference values for vitamin K for pregnant or lactating women. The Netherlands Food and Nutrition Council (1992) did not consider vitamin K when setting reference values for the whole population.
IOM (2001) noted that studies on pregnant women reported no signs of vitamin K deficiency and comparable blood vitamin K concentrations to those of non‐pregnant women (Mandelbrot et al., 1988; von Kries et al., 1992). There was no data on vitamin K content of fetal tissue, and studies on vitamin K supplementation in pregnant women (Morales et al., 1988; Kazzi et al., 1990; Anai et al., 1993; Dickson et al., 1994) could not be used for establishing additional requirements during pregnancy. Median intakes in pregnant or non‐pregnant women ((NHANES III, 1988), Total Diet Study (TDS) 1991–1997) and Booth et al. (1999a)) were noted. IOM (2001) set the same AI for pregnant adolescent or women as for other adolescent girls or women, based on median intakes21 in non‐pregnant female subjects. Data suggested comparable phylloquinone intake in lactating or non‐lactating women and no significant correlation between phylloquinone intake from a usual diet and breast milk concentration (NHANES III, 1988; Greer et al., 1991). As vitamin K concentration in human milk is low, the AI was the same as for non‐pregnant women.
An overview of DRVs for vitamin K for pregnant or lactating women is presented in Table 3.
Table 3.
Overview of dietary reference values for vitamin K (expressed as phylloquinone) for pregnant and lactating women
| D‐A‐CH (2015)a | NCM (2014) | WHO/FAO (2004)a | AFSSA (2001)a | IOM (2001)a | SCF (1993) | DH (1991) | |
|---|---|---|---|---|---|---|---|
| Pregnant women (μg/day) | 75b | ||||||
| 60 | – | 55 | 45 | 90c | – | – | |
| Lactating women (μg/day) | 75b | ||||||
| 60 | – | 55 | 45 | 90c | – | – |
D‐A‐CH: Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährung; NCM: Nordic Council of Ministers; WHO/FAO: World Health Organization/Food and Agriculture Organization of the United Nations; Afssa: Agence française de sécurité sanitaire des aliments; IOM: US Institute of Medicine; SCF: Scientific Committee on Food; DH: UK Department of Health.
Derived considering an intake of 1 μg/kg body weight per day.
Girls aged 14–18 years.
Adults.
5. Criteria (endpoints) on which to base Dietary Reference Values
5.1. Indicators of vitamin K requirement
5.1.1. Adults
5.1.1.1. Use of biomarkers
As discussed in Sections 2.2.2.1 and 2.4, vitamin K deficiency leads to an increased PT and eventually associated adverse clinical symptoms. However, PT and the PTT are not sensitive markers of vitamin K intake and status and non‐specific indicators of vitamin K deficiency, and symptomatic vitamin K deficiency and impairment of normal haemostatic control in healthy adults may take more than 2–3 weeks to develop at ‘low’ phylloquinone intake (i.e. < 10 μg/day) (Sections 2.2.2.1 and 2.4).
For the other biomarkers investigated (Section 2.4), even if they may change with changes in vitamin K (phylloquinone or menaquinone) dietary intake, no dose–response relationship has been established with intake of phylloquinone or of individual menaquinones within the dietary range in Europe. The available metabolic studies generally assessed whether the biomarkers returned to baseline values with phylloquinone supplementation/dietary repletion after phylloquinone depletion. However, for these biomarkers, no cut‐off value to define adequate vitamin K status is available, so these changes in biomarkers are difficult to interpret. The Panel considers that none of these biomarkers is suitable by itself to assess vitamin K adequacy (Section 2.4).
The SCF (1993) considered that an intake of phylloquinone of 1 μg/kg body weight per day was adequate, mainly based on the depletion/repletion study in young men (mean ± SD: 72 ± 9 kg body weight) by Suttie et al. (1988), which showed that supplementation with 50 μg phylloquinone/day in addition to a restricted diet (median of about 32–40 μg phylloquinone/day) restored the S:E ratio (a measure of functionally active prothrombin) and urinary Gla concentration to their baseline values (Section 2.4). The Panel notes that phylloquinone intake from the diet was analytically measured in duplicate portions of all foods and beverages consumed (and not estimated using a food composition database). The Panel also notes that this study was previously used to support a reference value of 1 μg/kg per body weight, based on a mean body weight of subjects that is slightly higher than the reference body weight for adult men for this Opinion (68.1 kg, Section 6). The Panel however considers that the physiological relevance of the changes in biomarkers observed in this study is unclear.
The Panel notes that the SCF (1993) set a reference value of 1 μg/kg per day based on data on biomarkers from Suttie et al. (1988). The Panel considers that none of the new data on the biomarkers reviewed (Section 2.4) are suitable as such to derive DRVs for vitamin K.
5.1.1.2. Factorial approach
The maintenance of an adequate body pool of phylloquinone can be considered as a criterion for establishing the requirement for vitamin K, assuming that it is associated with fulfilling the function of vitamin K as cofactor of GGCX in the different target tissues (Section 2.2.1).
As explained in Section 2.3.4, there is no data on the total body pool of menaquinones and the Panel considers the most accurate values for the total body pool of phylloquinone, obtained from a compartmental analysis of phylloquinone kinetics in adults (46 and 41 μg for men and women) (Novotny et al., 2010), and that can be expressed as 0.53 and 0.55 μg/kg body weight, respectively. The Panel also notes that the study of Olson et al. (2002), when taking into account the value for plasma phylloquinone considered as most accurate by the authors, identifies a body pool of phylloquinone of 0.57 μg/kg body weight, which is a value close to the values obtained from the study by Novotny et al. (2010). The Panel thus considers a body pool of phylloquinone of about 0.55 μg/kg body weight in healthy adults at steady state not to be associated with signs of vitamin K deficiency (Section 2.3.4). The Panel considers this value as a desirable body pool size for phylloquinone.
Turnover of phylloquinone can be determined from kinetic studies. Based on the 6‐day kinetic study by Olson et al. (2002) on seven adults (six men and one woman) consuming 75 μg/day and receiving 0.3 μg isotope‐labelled phylloquinone administered intravenously, the authors found that a mean of about 62% of injected phylloquinone is catabolised and excreted as radioactive metabolites in urine (mean of 30%) and faeces through the bile (mean of 31.8%) (Section 2.3.6).
In view of the fast turnover of phylloquinone in the body (Section 2.3.5), the Panel applied these percentages to the desirable body pool size of phylloquinone calculated above. Thus, assuming a total body pool of phylloquinone of 0.55 μg/kg body weight in adults, the Panel estimates that 0.340 μg phylloquinone/kg body weight would be excreted in the form of phylloquinone metabolites in urine (30% of 0.55 μg/kg body weight, i.e. 0.165 μg/kg) and in bile (31.8% of 0.55 μg/kg body weight, i.e. 0.175 μg/kg body weight). The Panel assumes that 0.340 μg phylloquinone/kg body weight could be considered as the daily losses via faeces and urine. The Panel notes that the daily losses of menaquinones cannot be estimated.
The Panel considered to estimate the daily dietary intake of phylloquinone required to balance total phylloquinone losses through urine and faeces (bile) and to maintain an adequate body pool of phylloquinone (factorial approach). This approach to derive DRVs for vitamin K would require taking into account phylloquinone absorption. However, as explained in Section 2.3.1, the Panel considers that data on phylloquinone absorption in healthy adults, measured from different food sources and matrices, consumed with or without fat, are widely variable. The Panel also considers that it is not possible from the available data in healthy adults to estimate precisely an average absorption of phylloquinone, menaquinones, and thus vitamin K from the diet that would be valid for all dietary conditions.
The Panel noted in Section 2.3.1 the limitations of the available studies and that the observed mean phylloquinone absorption ranged between about 3–80%. In particular, taking into account the reported absolute value of absorption of phylloquinone from kale and assuming, as reference, maximum reported absorption of 80% for free phylloquinone (as a supplement consumed with fat) to convert the relative absorption observed for other plant foods into absolute values, the range of mean absorption from spinach, kale, broccoli or romaine lettuce (fresh or cooked, with or without fat) would be equivalent to about 3–50%.
On the assumption that absorption of phylloquinone from the European diet would be about 35% and that the assumed metabolic losses of phylloquinone mentioned above would be 0.340 μg phylloquinone/kg body weight, an intake of phylloquinone of 1 μg/kg body weight per day would balance the losses.
Although this value agrees with the AI set by the SCF, in view of the limitations associated with deriving the figures for absorption and losses, the Panel considers that the factorial approach cannot be used as such to set DRVs.
5.1.1.3. Intake data
The Panel considers that average/median intakes of vitamin K could be used to estimate an AI. Available data for vitamin K intake mean estimates in adults vary considerably among EU countries (between 72 and 196 μg/day) and suffer from limitations and uncertainties of food composition data with regard to both phylloquinone and menaquinones (Section 3.2.1). Although two national surveys applied partially updated food composition data, the impact of the remaining uncertainty in the composition data on the results median intake estimates for adults for vitamin K (phylloquinone and menaquinones) of 100–117 μg/day (Dutch National Survey) and for phylloquinone of 76 μg/day for subjects aged 15–80 years (German National Nutrition Survey II) (Section 3.2.2) is still not entirely clear.
5.1.1.4. Conclusions on indicators of vitamin K requirement for adults
The Panel concludes that available data on biomarkers do not allow to estimate an average requirement (AR) for either phylloquinone or vitamin K.
The Panel also concludes that, due to the limitations of the data on absorption and excretion of phylloquinone and menaquinone, it is not possible to use the factorial approach to derive DRVs for vitamin K.
Due to the uncertainty associated with available data on average daily level of intake in Europe, the Panel concludes that an AI established from these data cannot be sufficiently reliable.
5.1.2. Infants and children
The Panel considers that there are no studies in infants aged 7–11 months and children that can be used for deriving the requirement for vitamin K in infants and children.
5.1.3. Pregnant or lactating women
During pregnancy, only small quantities of phylloquinone cross the placenta from mother to fetus, and there is no correlation between maternal and cord blood concentrations (Section 2.3.3). Little information is available in relation to placental transfer of menaquinones (Section 2.3.3). Studies on phylloquinone supplementation in pregnant women cannot be used to set DRVs (Morales et al., 1988; Kazzi et al., 1990; Dickson et al., 1994) (Section 4.3). Human milk contains ‘low’ concentrations of vitamin K (mostly phylloquinone) but the concentration of phylloquinone in human milk is affected by maternal oral supplementation of phylloquinone (Section 2.3.6.3).
The Panel considers that there are no studies that can be used for deriving the requirement for vitamin K in pregnant or lactating women and that would suggest that the requirement for vitamin K in pregnant or lactating women is different from non‐pregnant non‐lactating adults.
5.2. Vitamin K intake and health consequences
The relationship between intake of vitamin K (phylloquinone and/or menaquinones) and chronic disease outcomes has been investigated in RCTs, and also in observational studies where associations between intake and disease outcomes may be confounded by uncertainties inherent to the methodology used for the assessment of vitamin K intake and by the effect of dietary, lifestyle or other undefined factors on the disease outcomes investigated. RCTs, as well as prospective cohort studies in populations free of the investigated health outcome/disease(s) at baseline, are discussed in this Section. Taking into account the uncertainty about the relationship between vitamin K intake and biomarkers (Section 2.4), the Panel only considered studies that include either one or longitudinal assessment of vitamin K intake, whereas studies on the relationship of levels of vitamin K biomarkers and health outcomes with no quantitative data on vitamin K intake are not considered.
A comprehensive search of the literature published between 1990 and 2011 was performed as preparatory work to this assessment in order to identify data on relevant health outcomes upon which DRVs for vitamin K may potentially be based (Heinonen et al., 2012). This provided individual studies that are described below. An additional literature search (in PubMed) was performed to identify more recent data published until 2016 on vitamin K intake and health outcomes.
Since the reports by SCF (1993), more data have become available on the relationship between phylloquinone or menaquinone intake and diabetes mellitus (one observational study (Beulens et al., 2010)), metabolic syndrome (one observational study (Dam et al., 2015)), cancer (Nimptsch et al., 2008, 2010)), all‐cause‐mortality, cardiovascular‐related outcomes or bone health. The Panel considers that evidence from only one observational study on a particular outcome is not sufficient to provide strong evidence of a relationship and thus cannot be used for setting DRVs for vitamin K. The Panel thus considers that available data on phylloquinone or menaquinones intake and the risk of diabetes mellitus, metabolic syndrome, various types of cancer cannot be used to derive DRVs for vitamin K. The Panel also noted three studies that investigated the relationship between intake of phylloquinone, menaquinones or both and the risk of all‐cause mortality (Geleijnse et al., 2004; Juanola‐Falgarona et al., 2014; Zwakenberg et al., 2016) with inconsistent results and therefore are not considered to derive DRVs for vitamin K. In this Section, the Panel does not report on studies (Cockayne et al., 2006; Knapen et al., 2007, 2013, 2015; Emaus et al., 2010; Ronn et al., 2016) using doses of phylloquinone, MK‐4 or MK‐7 much higher (1–10 mg/day phylloquinone, 45 mg/day MK‐4, 15–45 μg/day MK–4, 180–375 μg/day MK‐7) than the observed dietary intakes of phylloquinone, MK‐4 or MK‐7 in Europe (Section 3.2).
5.2.1. Cardiovascular‐related outcomes
The seven prospective cohort studies below assessed the association between several cardiovascular‐related outcomes and vitamin K intake from food only or from food and supplements as assessed by an FFQ administered mostly solely at baseline, or also repeatedly during follow‐up. These studies were undertaken in men and women or in one sex only, mostly included large populations (about 4,800–73,000 subjects) and with a mean follow‐up ranging between 7.2 and 16 years, except for one smaller study (Villines et al., 2005) that investigated 807 active‐duty army members with a shorter follow‐up (less than 1.5 year). Results after adjustments for potential confounders are described below.
In one study, the risk of coronary heart disease (CHD) events (total CHD, non‐fatal myocardial infarction (MI), or fatal CHD) was not significantly associated with quintiles of phylloquinone intake, even when comparing quintile Q5 ≥ 249 μg/day to Q1 ≤ 107 μg/day (Erkkila et al., 2007). In another study, the risks of total CHD and of non‐fatal MI were significantly lower only in quintiles Q2 and Q4 of phylloquinone intake compared to Q1 (Q2: 110–144 μg/day, e.g. for total CHD, RR: 0.83 (95% CI: 0.71–0.97); Q4: 183–241 μg/day, e.g. for total CHD, RR 0.82 (95% CI: 0.69–0.96), but p for trend was not statistically significant (Erkkila et al., 2005). In the same study, the risk of fatal CHD was not associated with quintiles of phylloquinone intake. In a third study, the risk of coronary events (incident CHD, non‐fatal MI, CHD mortality) was not associated with energy‐adjusted tertiles of phylloquinone intake even when comparing the highest tertile > 278 μg/day to the lowest < 200 μg/day (Geleijnse et al., 2004). In a fourth study, the risk of CHD was not significantly associated with phylloquinone intake (per 10 μg/day increment in intake) (Gast et al., 2009).
In a study mentioned above (Geleijnse et al., 2004), only in the upper tertile of energy‐adjusted intake of menaquinone (MK‐4 to MK‐10) (> 32.7 μg/day) compared to the lower one (< 21.6 μg/day), there was a significantly reduced risk of incident CHD (RR 0.59, 95% CI: 0.40–0.86) and CHD mortality (RR 0.43, 95% CI: 0.24–0.77), p trend 0.007 and 0.005, respectively, but no significant association was observed for non‐fatal MI. In another study mentioned above (Gast et al., 2009), the risk of CHD was not significantly associated with menaquinone intake (MK‐4 to MK‐9) (per 10 μg/day increment in intake).
Thus, there was no significant (linear or non‐linear) association with phylloquinone intake and the risk of CHD events (four studies); while either a significant non‐linear or no significant linear association was reported between menaquinone intake and the risk of CHD events (two studies).
In one study mentioned above (Erkkila et al., 2007), the risk of strokes (total or ischaemic) was not significantly associated with quintiles of phylloquinone intake, even when comparing Q5 ≥ 249 μg/day to Q1 ≤ 107 μg/day. In another study (Vissers et al., 2013), there was no association between risk of stroke and energy‐adjusted phylloquinone or menaquinone intake (MK‐4 to MK‐10), either per 50 μg/day increment in intake or comparing the highest to the lowest quartiles (mean phylloquinone intake: 96.6 μg/day (q1), 332.7 μg/day (q4); mean MK‐n intake: 15.6 μg/day (q1), 49.3 μg/day (q4). These results did not change when analysing separately haemorrhagic and ischemic stroke, or separately total vitamin K or MK‐4 through MK‐6 and MK‐7 through MK‐10.
Thus, intakes of phylloquinone (two studies) or menaquinones (one study) were not significantly associated (linearly or non‐linearly) with the risk of stroke.
The risk of peripheral arterial disease (PAD) (e.g. atherosclerosis, arterial embolism and thrombosis, aortic aneurysm) was not significantly associated with energy‐adjusted phylloquinone intake, either per 50 μg increment in intake or comparing the highest to the lowest quartiles (mean: 97 μg/day in q1, 333 μg/day in q4) (Vissers et al., 2016). In this study, there was a significant (linear) inverse association between the risk of PAD and intake of menaquinones (per 10 μg increment in intake of MK‐4 to 10) (hazard ratio (HR), 0.92, 95% CI: 0.85–0.99, p = 0.03). The risk of PAD was also significantly reduced when comparing the highest to the lowest quartiles of energy‐adjusted intake of menaquinones, (HR 0.71, 95% CI: 0.53–0.95 (mean: 15.5 μg/day in q1, 49.2 μg/day in q4), but p for trend (0.06) was not significant. Such relationships were not observed among participants without hypertension.
Thus, there was no significant (linear or non‐linear) association between intake of phylloquinone or menaquinones and the risk of PAD in subjects without hypertension (one study).
In one study, there was no significant association between the presence of coronary artery calcification (CAC) (assessed by computed tomography) and phylloquinone intake (either per μg/day increment in intake or comparing quartile q4 > 143.5 μg/day phylloquinone to q1 < 69.5 μg/day phylloquinone) (Villines et al., 2005). In this study, there was no significant linear association of phylloquinone intake with severity of CAC in a bivariate analysis. In another study mentioned above, there was no significant association between energy‐adjusted tertiles of phylloquinone intake and moderate or severe aortic calcification (assessed by a lateral radiography) (Geleijnse et al., 2004).
In the same study, there was no association between energy‐adjusted tertiles of intake of menaquinones (MK‐4 to MK‐10) and moderate aortic calcification, but an association was observed for severe calcification when comparing the highest to the lowest tertiles of intake (OR: 0.48, 95% CI: 0.32–0.71, p trend < 0.001) (Geleijnse et al., 2004).
Thus, there was no significant (linear or non‐linear) association between phylloquinone intake and aortic/coronary calcification (two studies), while a significant (non‐linear) association was observed between menaquinone intake and severe (but not moderate) calcification (one study).
The Panel considers that the available data from these prospective cohort studies on associations between the intake of phylloquinone or menaquinones and the risk of cardiovascular‐related outcomes cannot be used to derive DRVs for vitamin K.
5.2.2. Bone health
Results of two available RCTs and of eight prospective observational studies after adjustments for potential confounders, are described below. These observational studies generally assessed vitamin K (from food only or from food and supplements) through a FFQ at baseline, whereas a few among them assessed intake at different time points or used other methods (three, four or seven‐day food records). They were all in adults except one in children (Kalkwarf et al., 2004), with follow‐up between 2 and 10 years and population size between 200 and about 72,000 subjects.
A 12‐months RCT on 173 healthy women (mean age 62 years) investigated the effect on BMD of the intake of phylloquinone or MK‐7,27 calcium and vitamin D through fortified milk or yogurt (Kanellakis et al., 2012). The subjects received either 800 mg/day of calcium and 10 μg/day of vitamin D3 (n = 38), or the same amounts of these nutrients with 100 μg/day of phylloquinone (n = 38) or MK‐7 (n = 39), or continued with their usual diet during the study (control group, n = 39). BMD of total body and lumbar spine (LS) were measured at baseline and follow‐up with dual‐emission X‐ray absorptiometry (DXA), the BMD of other regional skeletal sites was extracted from the total body scans and data analysis was done on the subjects with compliance of at least 75% (n = 115). Baseline mean phylloquinone intake, assessed by three 24‐h recalls, was between 80.2 and 121.2 μg/day among groups (not statistically different). After adjustments for 25(OH)D concentrations, dietary calcium intake and physical activity, changes (increases) in total‐body BMD in the intervention groups were not significantly different from that (decrease) in the control group. However, there was an increase in BMD of the LS in the vitamin K‐supplemented groups, which was still significantly different, after adjustments, from the change (decrease) observed in control group (p = 0.002).
In a 2‐year double‐blind RCT of the effect of phylloquinone on BMD, 244 healthy women aged ≥ 60 years (Bolton‐Smith et al., 2007) were allocated to: (1) placebo, (2) 200 μg/day phylloquinone, (3) 1,000 mg calcium plus 10 μg/day vitamin D3, or (4) combined supplementation with the three nutrients at the levels in groups 2 and 3. Baseline mean phylloquinone intake (from food and supplements) assessed by FFQ was about 82–87 μg/day among the 209 completers. Bone mineral content (BMC) and BMD were measured by DXA of the femur and radius every 6 months. After adjustments for potential confounders, there was no significant difference of the two‐year changes in BMD or BMC between groups at any site.
Thus, two available RCTs with phylloquinone intake at levels comparable to the observed dietary intakes in Europe do not provide consistent results on the effect of phylloquinone intake on BMD and/or BMC in post‐menopausal women (Bolton‐Smith et al., 2007; Kanellakis et al., 2012).
In one observational study, in either men or women aged 65 years and older, there was no significant association between risk of hip fracture (assessed from hospital records) and energy‐adjusted log‐transformed phylloquinone intake (per SD increment in intake) (Chan et al., 2012). In a second study (Booth et al., 2000a), the risk of hip fracture (assessed from hospital records including X‐rays) was also not significantly associated with phylloquinone intake, even when comparing the highest to the lowest quartiles (median intake according to sexes: 60–64 μg/day in q1 and 234–268 μg/day in q4). In the largest observational study (Feskanich et al., 1999) undertaken among women (nurses), only women in quintile Q3 of baseline phylloquinone intake (146–183 μg/day) had a significantly lower RR of hip fractures (self‐reported), i.e. 0.67 (95% CI: 0.46–0.99), compared to those in Q1 (< 109 μg/day), and p for trend (= 0.32) was not significant. In this study, the RR of hip fracture was significantly lower in Q2–Q5 combined of baseline phylloquinone intake (109 to > 242 μg/day) compared to Q1, with a RR (95% CI) of 0.70 (0.53, 0.93), but this result did not remain statistically significant when using updated dietary data during follow‐up (secondary analyses). In a fourth study (Apalset et al., 2011), the risk of hip fracture (assessed from hospital records) was significantly higher in the lowest quartile of phylloquinone intake when compared to the highest quartile (q1 < 42.2 (women) or 52.9 (men) μg/day; q4 > 108.7 (women) or 113.9 (men) μg/day; HR 1.63, 95% CI: 1.06–2.49, p for trend: 0.015), but findings were not significant for the intermediate quartiles. In this study, the HR of hip fractures was 0.98 (95% CI: 0.95–1.00, p = 0.030) per 10 μg/day increment in phylloquinone intake.
In the same study (Apalset et al., 2011), the risk of hip fractures was not significantly associated with intake of menaquinones (forms not specified), either per 1 μg increment in intake or comparing the lowest to the highest quartiles (q1 < 7.2 (women) or 8.5 (men) μg/day, q4 > 14.5 (women) or 16.2 (men) μg/day).
Thus, the results on the association between phylloquinone intake and the risk of hip fractures, are inconsistent (four studies), while there was no significant (linear or non‐linear) association with menaquinone intake (one study).
In either men or women aged 65 years and older from a study mentioned above, there was no significant association between risk of non‐vertebral fracture and energy‐adjusted log‐transformed phylloquinone intake (per SD increment in intake) (Chan et al., 2012). In perimenopausal women (nested case–control study), receiving or not hormonal replacement therapy and some having already sustained a fracture at baseline, there was no significant association between the risk of vertebral fracture (assessed from hospital records and X‐rays) and phylloquinone intake, even when comparing the highest to the lowest quartiles (> 105 vs < 25 μg/day) or the 95th to the 5th percentiles (> 210 vs < 25 μg/day) (Rejnmark et al., 2006).
Thus, there was no association between phylloquinone intake, and the risk of either non‐vertebral (one study) or vertebral fractures (one study).
In a study mentioned above (Rejnmark et al., 2006), changes in BMD of the LS or femoral neck (FN) (measured by DXA) were not significantly associated with phylloquinone intake expressed either continuously or categorically (in quartiles). In another study mentioned above (Booth et al., 2000a), there was also no significant difference in changes in BMD at any site (hip, FN, trochanter, Ward's area, LS and arm, measured by different methods28) across quartiles of phylloquinone intake, for either men or women (median intake according to sexes of 60–64 μg/day in q1 and 234–268 μg/day in q4). In a third study (Macdonald et al., 2008), in which phylloquinone intake data was available for 898 women at baseline and final visits and 2,340 only at final visit only, there was again no significant difference in the yearly change in BMD at the FN or LS between quartiles of energy‐adjusted phylloquinone at visit 2 (mean intake: 64 (q1) and 181 μg/day (q4)). In this study, energy‐adjusted intake of phylloquinone assessed as a continuous variable was not a significant predictor of BMD at LS or FN. In a fourth study (Bullo et al., 2011), 362 participants of the larger PREDIMED trial were enrolled in a parallel study on bone metabolism. At baseline, participants provided a FFQ. After 2 years of follow‐up, 200 participants provided a second dietary assessment and quantitative ultrasound bone‐related assessments. The study investigated the relationship between change in phylloquinone intake (between beginning and end of follow‐up) and change in BMD or bone structure quality (speed of sound (SOS)), broadband ultrasound attenuation (BUA) and quantitative ultrasound index (QUI) assessed by quantitative ultrasound at the calcaneus. The mean (± SE) phylloquinone intake at baseline was 333.6 ± 17.3 μg/day in men (n = 162) and 299.8 ± 11.6 μg/day in women (n = 200). After two years follow‐up, those who increased their phylloquinone intake (mean change ± SD: +104.1 ± 10.9 μg/day, n = 74) had a statistically significant lower loss of BMD (mean change ± SD: –0.009 ± 0.006 g/cm2) compared to those who decreased their phylloquinone intake (mean change ± SD: −155.8 ± 17.57 μg/day, n = 126) during the follow‐up (mean change in BMD ± SD: –0.023 ± 0.004 g/cm2), p = 0.049. There was no significantly different change in BUA, SOS and QUI. No information was provided on why subjects changed their phylloquinone intake during follow‐up.
Thus, the results on the association between phylloquinone intake and changes in BMD are inconsistent (four studies). For most of the sites investigated, however, the (linear or non‐linear) associations were not significant.
In 245 healthy girls aged 3–16 years at baseline (median 9.8 years) (Kalkwarf et al., 2004) (Section 2.4), BMC (total body, total body minus head, LS, hip, assessed by DXA) was not significantly associated with phylloquinone intake, except for the hip (1.0% decrease when increasing from the 10th percentile of phylloquinone intake i.e. 21 μg/day to the 90th percentile i.e. 89 μg/day, p < 0.01).
Thus, there was no significant association between phylloquinone intake and BMC for most of the sites investigated (one study in children). Menaquinone intake was not investigated.
The Panel considers that the available data on intake of phylloquinone or menaquinones and bone‐related health outcomes cannot be used to derive DRVs for vitamin K.
5.2.3. Conclusions on vitamin K intake and health consequences
The Panel considers that the available data on intake of phylloquinone or menaquinones and health outcomes cannot be used to derive DRVs for vitamin K.
6. Data on which to base Dietary Reference Values
The Panel reviewed the recent information on vitamin K (phylloquinone and menaquinones) with the aim of updating the DRV of 1 μg/kg body weight per day of phylloquinone that was previously set by SCF (1993) (Section 4) based on data on biomarkers and phylloquinone intake (Suttie et al., 1988). The Panel came to the conclusion that the uncertainties pointed out by SCF (1993) have not been resolved.
The Panel considers that all possible approaches investigated to set DRVs (biomarker, factorial approach, intake data) have considerable uncertainties (Sections 5.1.1.1, 5.1.1.2, 5.1.1.3–5.1.1.4). The Panel considers that there is no scientific evidence to update the previous reference value. The Panel notes that there is no indication that 1 μg/kg body weight per day phylloquinone would be associated with a risk of deficiency in the general population and is above the intake at which an increase in PT has been observed in healthy subjects (Sections 2.2.2.1 and 2.4).
In view of the uncertainties and limited data, the Panel considers that an AR and PRI cannot be set for vitamin K, but instead set an adequate intake (AI), at 1 μg/kg body weight per day phylloquinone.
The Panel tried to take menaquinones into account in setting DRVs for vitamin K, as this vitamin is defined as phylloquinone and menaquinones (Section 2.1). The Panel however came to the conclusion that the knowledge on MK‐n, i.e. their intake (Section 3.2), absorption (Section 2.3.1), function (Sections 2.2.1 and 2.4) and content in the body or organs (Section 2.3.4), is limited and contradictory. It is not established that when the requirement for phylloquinone is met, there is still a requirement for menaquinones. It has not been proven that menaquinones have effects that are unrelated to phylloquinone intake. From the available data, it is not possible to conclude on specific activities/effects of menaquinones compared to phylloquinone. Phylloquinone and menaquinones act as a cofactor of GGCX for carboxylation of the same proteins (Section 2.2.1 and 2.4). Thus, the Panel considers that, at present, there are not enough data to take menaquinones into account to set DRVs for vitamin K. There is also no data that would justify to set a separate AI for total or individual MK‐n.
The Panel also considers that the available data on intake of phylloquinone or menaquinones and health outcomes cannot be used to derive DRVs for vitamin K (Section 5.2).
6.1. Adults
The reference body weights of 18‐ to 79‐year‐old men and women were calculated by the measured body heights of 16,500 men and 19,969 women in 13 EU Member States and assuming a body mass index (BMI) of 22 kg/m2 (see Appendix 11 in EFSA NDA Panel (2013b)). Considering these reference body weights and the AI of 1 μg/kg body weight per day of phylloquinone, the daily phylloquinone intake would be 68.1 μg for men and 58.5 μg for women, rounded up to 70 μg/day for all adults.
The Panel notes that the proposed AI is close to the median phylloquinone intake of 76 μg/day (for subjects aged 15–80 years, n = 6,160) in the German National Nutrition Survey II that used updated phylloquinone composition data (Section 3.2.2, mean intake not reported). The Panel also considers that there was no evidence of different vitamin K absorption and different losses according to age in adults, thus, sets the same AI for ‘younger’ and ‘older’ adults.
6.2. Infants aged 7–11 months
The Panel decided to use for infants aged 7–11 months the same AI of 1 μg/kg body weight per day of phylloquinone obtained in adults. Considering the uncertainties associated with the setting of this value, and the small size of the body pool of phylloquinone, the Panel decided not to use growth factors (calculated in EFSA NDA Panel (2014)), considering that the requirement for growth would be covered by such an intake of 1 μg/kg body weight per day.
The Panel calculated averages of the median body weights of male and female infants, aged 9 months (8.6 kg) from the WHO Growth Standards (WHO Multicentre Growth Reference Study Group, 2006). Considering a reference body weight of 8.6 kg for infants aged 7–11 months and the AI of 1 μg/kg body weight per day phylloquinone, the daily phylloquinone intake would be 8.6 μg/day, rounded up to 10 μg/day.
The Panel notes that low vitamin K stores at birth may predispose to haemorrhages in healthy neonates and young infants (EFSA NDA Panel, 2013a). The Panel also notes that European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition (Mihatsch et al., 2016) recommends supplementation with phylloquinone of healthy newborn infants, according to national recommendations on the regimen, which may differ between countries.
6.3. Children
As for infants, the Panel decided not to use growth factors, considering that the requirement for growth would be covered by an intake of 1 μg/kg body weight per day. Considering median body weights of boys and girls, the daily phylloquinone intake in children is indicated in Table 4.
Table 4.
Daily phylloquinone intake in boys and girls based on an AI of 1 μg/kg body weight per day and reference body weights
| Boys | Girls | AIs for both sexes (rounded value) | |
|---|---|---|---|
| 1–3 years | 12.2a | 11.5a | 12 |
| 4–6 years | 19.2b | 18.7b | 20 |
| 7–10 years | 29.0c | 28.4c | 30 |
| 11–14 years | 44.0d | 45.1d | 45 |
| 15–17 years | 64.1(e) | 56.4e | 65 |
Average of the median weight‐for‐age of male or female children aged 24 months according to the WHO Growth Standards (WHO Multicentre Growth Reference Study Group, 2006).
Average of the median weight of male or female children aged 5 years (van Buuren et al., 2012).
Average of the median weight of male or female children aged 8.5 years (van Buuren et al., 2012).
Average of the median weight of male or female children aged 12.5 years (van Buuren et al., 2012).
Average of the median weight of male or female children aged 16 years (van Buuren et al., 2012).
The Panel notes that the median (mean, IQR) intake estimates for vitamin K (phylloquinone and MK–n) for children are 62 (70, 43–89) and 72 (80, 51–99) μg/day for girls (n = 857) and boys (n = 856) aged 7–18 years, in the Dutch National Survey that used updated composition data for phylloquinone and menaquinones (Section 3.2).
6.4. Pregnancy
The Panel notes that, during pregnancy, only small quantities of phylloquinone cross the placenta from mother to fetus, that there is no correlation between maternal and cord blood phylloquinone concentrations, and that little information is available in relation to placental transfer of menaquinones (Section 2.3.3). The Panel considers that the AI of 1 μg/kg body weight per day of phylloquinone set for non‐pregnant women also applies to pregnant women.
A mean gestational increase in body weight of 12 kg, for women with a singleton pregnancy and a pre‐pregnancy BMI in the range between 18.5 and 24.9 kg/m2, was also previously considered (EFSA NDA Panel, 2013b). In view of the increase in blood volume during pregnancy, and considering a mean gestational increase in body weight of 12 kg to the reference body weight of 58.5 kg for non‐pregnant women, the daily phylloquinone intake in pregnant women would be 70.5 μg/day.
As the Panel set an AI of 70 μg/day for all adults after rounding (Section 6.1), the Panel concludes that there is no need for a specific AI for vitamin K for pregnant women. The AI for pregnant women is thus the same as for non‐pregnant women (i.e. 70 μg phylloquinone/day)
6.5. Lactation
The Panel considers that the AI of 1 μg/kg body weight per day of phylloquinone set for non‐lactating women covers the small excretion of vitamin K (mainly phylloquinone) in breast milk, thus that no compensation for this excretion is required in setting DRVs for lactating women. The AI for lactating women is thus the same as for non‐lactating women (i.e. 70 μg phylloquinone/day).
Conclusions
The Panel considers vitamin K as phylloquinone and menaquinones. The Panel concludes that none of the biomarkers of vitamin K intake or status is suitable by itself to derive DRVs for vitamin K and that available data on intake of phylloquinone or menaquinones and health outcomes cannot be used to derive DRVs for vitamin K. The Panel concludes that ARs and PRIs for vitamin K cannot be derived for adults, infants and children, and therefore sets AIs. The Panel also concludes that available evidence on intake, absorption, function and content in the body or organs of menaquinones is insufficient, thus sets AIs for phylloquinone only.
After having considered several possible approaches, based on biomarkers, intake data and the factorial approach, which all are associated with considerable uncertainties, the reference value proposed by the SCF in 1993 is maintained. The same AI for phylloquinone of 1 μg/kg body weight per day is set for all age and sex population groups. For infants and children, the Panel decided not to use growth factors, considering that the requirement for growth would be covered by such an intake. The Panel considers the respective reference body weights for adults, infants and children to set AIs for phylloquinone expressed in μg/day. The Panel notes that the proposed AI in adults (70 μg/day) is close to the median phylloquinone intake of 76 μg/day in the German National Nutrition Survey II that used updated phylloquinone composition data. The mean gestational increase in body weight and the reference body weight of non‐pregnant women were taken into account by the Panel in its calculations, but the AI set for pregnant women is finally the same as for non‐pregnant women obtained after rounding. In view of the small excretion of vitamin K in breast milk, the AI set for lactating women is the same as the one for non‐lactating women obtained after rounding (Table 5).
Table 5.
Summary of Dietary Reference Values for vitamin K (based on phylloquinone only)
| Age | AI (μg/day) |
|---|---|
| 7–11 months | 10 |
| 1–3 years | 12 |
| 4–6 years | 20 |
| 7–10 years | 30 |
| 11–14 years | 45 |
| 15–17 years | 65 |
| ≥ 18 yearsa | 70 |
Including pregnancy and lactation.
Recommendations for research
The Panel suggests to undertake further research on:
more extensive and precise analytical data for phylloquinone and menaquinones in food.
the measurement of absorption of phylloquinone and menaquinones, including the absorption of menaquinones produced by gut bacteria, and the influence of foods and the diet on these processes.
the intake of menaquinones and phylloquinone in Europe and their metabolism, storage and functions, including during pregnancy.
the specific activity of different menaquinones in relation to phylloquinone functions.
cut‐off values for biomarkers for vitamin K status to derive DRVs for vitamin K for infants, children, adults, pregnant and lactating women, through studies specifically designed for this purpose
vitamin K and long‐term health outcomes.
the influence of sex and genotype on vitamin K requirement.
Abbreviations
- 1,25(OH)2D
1,25‐hydroxyvitamin D
- 25(OH)D
25‐hydroxyvitamin D
- %ucOC
percentage of undercarboxylated osteocalcin
- Afssa
Agence française de sécurité sanitaire des aliments
- AI
adequate intake
- ApoE
apolipoprotein E
- APTT
activated partial thromboplastin time
- AR
average requirement
- AU
arbitrary unit
- AUC
area under the curve
- BLS
Bundeslebensmittelschlüssel
- BMC
bone mineral content
- BMD
bone mineral density
- BMI
body mass index
- BUA
broadband ultrasound attenuation
- CAC
coronary artery calcification
- CHD
coronary heart disease
- CI
confidence interval
- cOC
carboxylated osteocalcin
- COMA
Committee on Medical Aspects of Food Policy
- CYP4F2
cytochrome P450 4F2
- D‐A‐CH
Deutschland‐Austria‐Confoederatio Helvetica
- DH
UK Department of Health
- DIPP
Type 1 Diabetes Prediction and Prevention survey
- dK
dihydrophylloquinone
- DNFCS
Dutch National Food Consumption Survey
- DNSIYC
Diet and Nutrition Survey of Infants and Young Children
- DRV
dietary reference values
- dp‐ucMGP
desphospho‐uncarboxylated MGP
- DXA
dual‐emission X‐ray absorptiometry
- EsKiMo
Ernährungsstudie als KIGGS‐Modul
- ESPGHAN
European Society for Paediatric Gastroenterology, Hepatology and Nutrition
- FAO
Food and Agriculture Organization
- FC_PREGNANTWOMEN
Food consumption of pregnant women in Latvia
- FFQ
food frequency questionnaire
- FINDIET
National dietary survey of Finland
- FN
femoral neck
- FVII
factor VII
- GAS6
growth arrest‐specific protein 6
- GC/MS
gas chromatography/mass spectrometry
- GGCX
γ‐glutamyl carboxylase
- Gla
γ‐carboxyglutamic acid
- Glu
glutamic acid
- HDL
high‐density lipoproteins
- HPLC
high performance liquid chromatography
- HR
hazard ratio
- HSPG
heparan sulfate proteoglycans
- IDL
intermediate‐density lipoprotein
- INCA
Étude Individuelle Nationale des Consommations Alimentaires
- INRAN‐SCAI
Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione – Studio sui Consumi Alimentari in Italia
- IOM
US Institute of Medicine of the National Academy of Sciences
- IQR
interquartile range
- IU
international units
- LDL
low‐density lipoproteins
- LS
lumbar spine
- MGP
matrix Gla‐protein or matrix γ‐carboxyglutamic protein
- MI
myocardial infarction
- MK
menaquinone
- MRI
Max Rubner Institut
- NANS
National Adult Nutrition Survey
- NDNS
National Diet and Nutrition Survey
- NHANES
National Health and Nutrition Examination Survey
- NNR
Nordic Nutrition Recommendations
- NWSSP
Nutrition and Wellbeing of Secondary School Pupils
- OC
osteocalcin
- OR
odds ratio
- PAD
peripheral arterial disease
- PIVKA‐II
protein induced by vitamin K absence or antagonism‐II
- PRI
population reference intake
- PT
prothrombin time
- PTT
partial thromboplastin time
- Q
quintile
- q
quartile
- QUI
quantitative ultrasound index
- RCT
randomised controlled trial
- RNI
recommended nutrient intake
- RR
relative risk
- SCF
Scientific Committee for Food
- SD
standard deviation
- S:E
Simplastin:Ecarin
- SEM
standard error of the mean
- SNP
single nucleotide polymorphism
- SOS
speed of sound
- TAM receptors
Tyro3, Axl and Mer receptors
- TDS
Total Diet Study
- TG
triglyceride
- TRL
triglyceride‐rich lipoproteins
- UBIAD1
enzyme UbiA prenyltransferase domain‐containing protein 1
- ucOC
undercarboxylated osteocalcin
- UL
tolerable upper intake level
- USDA
United States Department of Agriculture
- VELS
Verzehrsstudie zur Ermittlung der Lebensmittelaufnahme von Säuglingen und Kleinkindern für die Abschätzung eines akuten Toxizitätsrisikos durch Rückstände von Pflanzenschutzmitteln
- VKDB
vitamin K deficiency bleeding
- VKOR
vitamin K epoxide reductase
- VKORC1
vitamin K epoxide reductase complex subunit 1
- VLDL
very low‐density lipoproteins
- WHO
World Health Organization
Appendix A – Concentrations of phylloquinone and menaquinones in breast milk of healthy mothers
| Reference | Number of women (number of samples) | Country | Maternal dietary intake (mean ± SD) | Maternal serum/plasma (phylloquinone/menaquinone) concentration yes/n.a. | Stage of lactation | Phylloquinone concentration in breast milk (μg/L) (mean ± SD) | Menaquinone concentration in breast milk (μg/L) (mean ± SD) | Comments | |
|---|---|---|---|---|---|---|---|---|---|
| Haroon et al. (1982) |
20 (unsupplemented) 1 (supplemented) |
UK |
n.a. 20 mg (one dose) |
n.a. n.a. |
n.a. ~ 6 months post‐partum |
2.1 (1.1–6.5) 140 |
n.a. n.a. |
No information was given as to whether infants were full‐term or not | |
| Fournier et al. (1987) | 10 | FR | n.a. | n.a. | 21 days post‐partum | 9.18 (4.85–12.76) (median (range)) | n.a. | Full‐term infants | |
| von Kries et al. (1987b) |
9 (unsupplemented) 1 (supplemented) |
DE |
n.a. 100 μg (one dose) |
a. n.a. |
8–36 days post‐partum |
1.2 (median) 4.9 |
n.a. n.a. |
Full‐term infants The authors considered transitional (days 8–15) and mature (days 22–36) milk as one group (days 8–36) as there were no significant differences in phylloquinone concentration Breast milk phylloquinone concentration of the supplemented woman at baseline (before supplementation) was 2.5 μg/L Breast milk phylloquinone concentration is given for one supplemented mother for whom phylloquinone administration and milk sampling techniques were standardised |
|
| Canfield et al. (1990) |
7 (16) 15 |
US | n.a. | n.a. | 1 month post‐partum |
2.94 ± 1.94 (pooled samples) 3.15 ± 2.87 (mean of individuals) |
n.a. |
Infants were growing within normal limits and free of illness No explicit information was given as to whether infants were full‐term or not |
|
| Canfield et al. (1991) | 15 (45) | US | n.a. | n.a. | 1–6 months post‐partum | 2.87 ± 2.40 (mean of all determinations) | n.a. |
No explicit information was given as to whether infants were full‐term or not Samples assayed in triplicate at each time point (1, 3 and 6 months) |
|
| Greer et al. (1991) |
11 (study part 1) 23 (study part 2) |
US |
Supplementation, 20 mg (one dose) Unsupplemented (μg/day) 302 ± 361 296 ± 169 436 ± 667 |
Yes Yes |
2–6 months post‐partum Weeks post‐partum 6 12 26 |
130 ± 188 0.86 ± 0.52 1.14 ± 0.72 0.87 ± 0.5 |
n.a. n.a. |
No information was given as to whether infants were full‐term or not Breast milk phylloquinone concentration at baseline (before supplementation) was 1.11 ± 0.82 μg/L Maternal intakes of phylloquinone exceeded the DRV of 1 μg/kg body weight per day Full‐term infants |
|
| Pietschnig et al. (1991) |
20 (supplemented) 16 (unsupplemented) |
AT |
Mean (range) from food and supplement (μg/day) 442 (226–778) 386 (223–687) Supplementation (μg/day) 88 ± 40 (from 4 through 91 days post‐partum) Mean (range) (μg/day) 417 (134–1,224) 391 (209–695) |
n.a. n.a. |
Days post‐partum 27–29 89–91 Days post‐partum 25–29 87–91 |
Mean (range) 1.36 (0.40–3.81) 1.67 (0.56–8.61) Mean (range) 1.68 (0.64–2.91) 1.78 (0.80–4.11) |
n.a. n.a. |
Full‐term infants Average maternal intake exceeded the DRV for lactating women (55 μg/day) by 670% The supplemental intake of 88 ± 40 μg/day was calculated on average over the whole study period Full‐term infants |
|
| Greer et al. (1997) |
Phase 1 –preliminary investigation) 10 10 Phase 2 (supplementation study) 11 11 |
US |
Supplementation (daily for 6 weeks, starting within 3 days of delivery) 2.5 mg 5 mg Supplementation (daily for 12 weeks (starting time not reported)) 0 (placebo) 5 mg |
Yes Yes |
Weeks post‐partum 2 6 2 6 Weeks post‐partum 2 6 12 Weeks post‐partum 2 6 12 |
27.12 ± 12.18 22.43 ± 16.62 58.96 ± 25.39 44.1 ± 24.10 1.17 ± 0.7 1.14 ± 0.46 1.17 ± 0.40 76.53 ± 26.98 75.27 ± 46.23 82.10 ± 40.10 |
n.a. n.a. |
Full term infants Breast milk phylloquinone concentration at baseline (before supplementation) was 0.63 ± 0.58 μg/L (2.5 mg group) and 0.92 ± 0.62 μg/L (5 mg/day) No information was given as to whether infants were full‐term or not Breast milk phylloquinone concentration at baseline (before supplementation) was 0.69 ± 0.39 μg/L (5 mg group) and 1.10 ± 0.75 μg/L (placebo) |
|
| Thijssen et al. (2002) |
8 8 8 7 |
NL |
(Dietary intake not reported) Daily supplementation (from day 4 to day 16 post‐partum) 0 (control) 0.8 mg 2 mg 4 mg |
Yes Yes Yes Yes |
Days post‐partum 16 19 Days post‐partum 16 19 Days post‐partum 16 19 Days post‐partum 16 19 |
2.2 ± 0.64 2.2 ± 1.33 11.05 ± 4.57 5.57 ± 5.64 27.33 ± 14.24 5.44 ± 2.09 62.93 ± 20.66 20.23 ± 17.95 |
MK‐4 0.96 ± 0.4 0.79 ± 0.28 1.55 ± 1.15 1.44 ± 1.14 2.46 ± 1.5 1.34 ± 0.6 7.33 ± 4.07 4.40 ± 2.30 |
Full‐term infants | |
| Kojima et al. (2004) | (416) | JP | n.a. | n.a. |
Days post‐partum 21–89 90–179 180–365 |
1.95 ± 0.88 2.21 ± 4.29 1.55 ± 0.88 |
MK‐4 1.85 ± 0.41 1.35 ± 0.35 1.28 ± 0.31 |
No explicit information was given as to whether infants were full‐term or not Infants with birth weight higher than 2.5 kg |
|
| Kamao et al. (2007a) |
43 18 8 5 |
JP | n.a. | n.a. |
Days post‐partum 11–30 31–90 91–180 181–270 |
3.94 ± 2.45 3.53 ± 1.45 2.30 ± 1.22 3.41 ± 1.46 |
MK‐4 1.80 ± 0.66 1.78 ± 0.55 1.19 ± 0.34 1.51 ± 0.42 |
MK‐7 1.67 ± 2.73 0.80 ± 0.75 1.36 ± 1.29 0.92 ± 0.92 |
No information on the health status of the infants or if they were born at term or not |
AT: Austria; DE: Germany; DRV: dietary reference value; FR: France; JP: Japan; MK: menaquinone; n.a.: not applicable; NL: the Netherlands; SD: standard deviation; UK: United Kingdom; US: United States.
Appendix B – Dietary surveys in the EFSA Comprehensive European Food Consumption Database included in EFSA's nutrient intake calculation for ‘total vitamin K’
| Country | Dietary survey (year) | Year | Method | Days | Age | Number of subjects | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) |
Infantsa < 1 year |
Children 1 – < 3 years |
Children 3 – < 10 years |
Adolescents 10 – < 18 years |
Adults 18 – < 65 years |
Adults 65 – < 75 years |
Adults ≥ 75 years |
|||||
| Finland/1 | NWSSP | 2007–2008 | 48‐h dietary recallb | 2 × 2b | 13–15 | 306 | ||||||
| Finland/2 | FINDIET2012 | 2012 | 48‐h dietary recallb | 2b | 25–74 | 1,295 | 413 | |||||
| Finland/3 | DIPP | 2000–2010 | Dietary record | 3 | 0.5–6 | 499 | 500 | 750 | ||||
| France | INCA2 | 2006–2007 | Dietary record | 7 | 3–79 | 482 | 973 | 2,276 | 264 | 84 | ||
| Germany/1 | EsKiMo | 2006 | Dietary record | 3 | 6–11 | 835 | 393 | |||||
| Germany/2 | VELS | 2001–2002 | Dietary record | 6 | < 1–4 | 158 | 348c | 296c | ||||
| Ireland | NANS | 2008–2010 | Dietary record | 4 | 18–90 | 1,274 | 149 | 77 | ||||
| Italy | INRAN‐SCAI 2005–06 | 2005–2006 | Dietary record | 3 | < 1–98 | 16d | 36d | 193 | 247 | 2,313 | 290 | 228 |
| Latvia | FC_PREGNANT WOMEN 2011 | 2011 | 24‐h dietary recall | 2 | 15–45 | 12d | 991c | |||||
| Netherlands | DNFCS2007_2010 | 2007–2010 | 24‐h dietary recall | 2 | 7–69 | 447 | 1,142 | 2,057 | 173 | |||
| Sweden | RISKMATEN | 2010–2011 | Dietary records (Web)e | 4 | 18–80 | 1,430 | 295 | 72 | ||||
| UK/1 | DNSIYC‐2011 | 2011 | Dietary record | 4 | 0.3–1.5 | 1,369 | 1,314 | |||||
| UK/2 | NDNS Rolling Programme (Years 1–3) | 2008–2011 | Dietary record | 4 | 1–94 | 185 | 651 | 666 | 1,266 | 166 | 139 | |
Molecular masses: phylloquinone: 450.7 g/mol; MK‐4: 444.7 g/mol; MK‐7: 648.9 g/mol.
DIPP: type 1 Diabetes Prediction and Prevention survey; DNFCS: Dutch National Food Consumption Survey; DNSIYC: Diet and Nutrition Survey of Infants and Young Children; EsKiMo: Ernährungsstudie als KIGGS‐Modul; FC_PREGNANTWOMEN: food consumption of pregnant women in Latvia; FINDIET: the national dietary survey of Finland; INCA: étude Individuelle Nationale des Consommations Alimentaires; INRAN‐SCAI: Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione – Studio sui Consumi Alimentari in Italia; NANS: National Adult Nutrition Survey; NDNS: National Diet and Nutrition Survey; NWSSP: Nutrition and Wellbeing of Secondary School Pupils; VELS: Verzehrsstudie zur Ermittlung der Lebensmittelaufnahme von Säuglingen und Kleinkindern für die Abschätzung eines akuten Toxizitätsrisikos durch Rückstände von Pflanzenschutzmitteln.
Infants 1–11 months of age.
A 48‐h dietary recall comprising two consecutive days.
Four children from the VELS study (one aged 1–< 3 and three aged 3–< 10 years) and one adult from the Latvian study were not considered in the assessment as only one 24‐h dietary recall day was available.
5th or 95th percentile intakes calculated from fewer than 60 subjects require cautious interpretation as the results may not be statistically robust (EFSA, 2011b) and, therefore, for these dietary surveys/age classes, the 5th and 95th percentile estimates are not presented in the intake results.
The Swedish dietary records were introduced through the Internet.
Appendix C – ‘Total vitamin K’ intakes in males in different surveys, estimated by EFSA according to age class and country
| Age class | Country | Survey | Intakesb expressed in μg per day | Intakesb expressed in μg per MJ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nc | Average | Median | P5 | P95 | Average | Median | P5 | P95 | |||
| < 1 year a | Finland | DIPP | 247 | 34 | 35 | 4 | 67 | 18d | 16d | 7d | 34d |
| Germany | VELS | 84 | 43 | 39 | 7 | 111 | 13 | 12 | 2 | 34 | |
| Italy | INRAN_SCAI_2005_06 | 9 | 23 | 14 | –b | –b | 8 | 4 | –b | –b | |
| United Kingdom | DNSIYC_2011 | 699 | 61 | 56 | 20 | 116 | 18 | 17 | 6 | 31 | |
| 1–< 3 years | Finland | DIPP | 245 | 42 | 39 | 15 | 74 | 12 | 11 | 4 | 20 |
| Germany | VELS | 174 | 51 | 36 | 12 | 137 | 11 | 8 | 3 | 30 | |
| Italy | INRAN_SCAI_2005_06 | 20 | 51 | 41 | –b | –b | 11 | 9 | –b | –b | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 107 | 51 | 45 | 19 | 106 | 12 | 11 | 4 | 21 | |
| United Kingdom | DNSIYC_2011 | 663 | 53 | 43 | 18 | 106 | 11 | 10 | 5 | 25 | |
| 3–< 10 years | Finland | DIPP | 381 | 45 | 40 | 21 | 81 | 8 | 7 | 4 | 13 |
| France | INCA2 | 239 | 62 | 52 | 17 | 139 | 10 | 8 | 3 | 26 | |
| Germany | EsKiMo | 426 | 67 | 51 | 21 | 157 | 9 | 6 | 3 | 21 | |
| Germany | VELS | 146 | 47 | 36 | 16 | 122 | 9 | 6 | 3 | 21 | |
| Italy | INRAN_SCAI_2005_06 | 94 | 91 | 68 | 30 | 235 | 13 | 9 | 4 | 37 | |
| Netherlands | DNFCS2007 | 231 | 93 | 54 | 19 | 364 | 11 | 6 | 3 | 39 | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 326 | 68 | 60 | 20 | 144 | 11 | 9 | 4 | 26 | |
| 10–< 18 years | Finland | NWSSP07_08 | 136 | 73 | 70 | 29 | 129 | 9 | 8 | 4 | 15 |
| France | INCA2 | 449 | 80 | 62 | 22 | 183 | 10 | 8 | 3 | 24 | |
| Germany | EsKiMo | 197 | 69 | 55 | 21 | 171 | 9 | 7 | 3 | 21 | |
| Italy | INRAN_SCAI_2005_06 | 108 | 143 | 85 | 43 | 367 | 16 | 9 | 4 | 45 | |
| Netherlands | DNFCS2007 | 566 | 112 | 69 | 28 | 377 | 11 | 6 | 3 | 35 | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 340 | 80 | 66 | 26 | 178 | 10 | 8 | 4 | 22 | |
| 18–< 65 years | Finland | FINDIET2012 | 585 | 92 | 81 | 30 | 180 | 10 | 9 | 3 | 22 |
| France | INCA2 | 936 | 103 | 89 | 28 | 228 | 12 | 10 | 4 | 27 | |
| Ireland | NANS_2012 | 634 | 84 | 71 | 26 | 182 | 9 | 7 | 3 | 19 | |
| Italy | INRAN_SCAI_2005_06 | 1,068 | 161 | 115 | 40 | 440 | 18 | 13 | 5 | 53 | |
| Netherlands | DNFCS2007 | 1,023 | 157 | 93 | 35 | 637 | 14 | 8 | 3 | 56 | |
| Sweden | Riksmaten 2010 | 623 | 91 | 77 | 31 | 184 | 9 | 8 | 4 | 20 | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 560 | 103 | 84 | 32 | 244 | 12 | 9 | 4 | 28 | |
| 65–< 75 years | Finland | FINDIET2012 | 210 | 94 | 81 | 32 | 200 | 12 | 10 | 4 | 26 |
| France | INCA2 | 111 | 130 | 116 | 42 | 240 | 16 | 14 | 5 | 30 | |
| Ireland | NANS_2012 | 72 | 96 | 84 | 23 | 212 | 11 | 9 | 4 | 24 | |
| Italy | INRAN_SCAI_2005_06 | 133 | 196 | 152 | 52 | 531 | 24 | 15 | 7 | 74 | |
| Netherlands | DNFCS2007 | 91 | 155 | 89 | 43 | 553 | 17 | 11 | 4 | 53 | |
| Sweden | Riksmaten 2010 | 127 | 92 | 80 | 37 | 167 | 11 | 10 | 5 | 19 | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 75 | 119 | 104 | 39 | 230 | 15 | 13 | 5 | 26 | |
| ≥ 75 years | France | INCA2 | 40 | 135 | 104 | –b | –b | 18 | 16 | –b | –b |
| Ireland | NANS_2012 | 34 | 72 | 57 | –b | –b | 9 | 9 | –b | –b | |
| Italy | INRAN_SCAI_2005_06 | 69 | 157 | 110 | 52 | 360 | 18 | 13 | 5 | 42 | |
| Sweden | Riksmaten 2010 | 42 | 104 | 87 | –b | –b | 12 | 10 | –b | –b | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 56 | 88 | 82 | –b | –b | 12 | 11 | –b | –b | |
DIPP: type 1 Diabetes Prediction and Prevention survey; DNFCS: Dutch National Food Consumption Survey; DNSIYC: Diet and Nutrition Survey of Infants and Young Children; EsKiMo: Ernährungsstudie als KIGGS‐Modul; FC_PREGNANTWOMEN: food consumption of pregnant women in Latvia; FINDIET: the national dietary survey of Finland; INCA: étude Individuelle Nationale des Consommations Alimentaires; INRAN‐SCAI: Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione – Studio sui Consumi Alimentari in Italia; NANS: National Adult Nutrition Survey; NDNS: National Diet and Nutrition Survey; NWSSP: Nutrition and Wellbeing of Secondary School Pupils; VELS: Verzehrsstudie zur Ermittlung der Lebensmittelaufnahme von Säuglingen und Kleinkindern für die Abschätzung eines akuten Toxizitätsrisikos durch Rückstände von Pflanzenschutzmitteln.
Infants between 1 and 11 months. The proportions of breastfed infants were 58% in the Finnish survey, 40% in the German survey, 44% in the Italian survey, and 21% in the UK survey. Most infants were partially breastfed. The consumption of breast milk was taken into account if the consumption was reported as human milk (Italian survey) or if the number of breast milk consumption events was reported (German and UK surveys). For the German study, the total amount of breast milk was calculated based on the observations by Paul et al. (1988) on breast milk consumption during one eating occasion at different age groups: the amount of breast milk consumed on one eating occasion was set to 135 g/eating occasion for infants between 6 and 7 months of age and to 100 g/eating occasion for infants between 8–12 months of age (Kersting and Clausen, 2003). For the UK survey, the amount of breast milk consumed was either directly quantified by the mother (expressed breast milk) or extrapolated from the duration of each breastfeeding event. As no information on the breastfeeding events were reported in the Finnish survey, breast milk intake was not taken into consideration in the intake estimates of Finnish infants.
5th or 95th percentile intakes calculated from fewer than 60 subjects require cautious interpretation as the results may not be statistically robust (EFSA, 2011b) and, therefore, for these dietary surveys/age classes, the 5th and 95th percentile estimates are not presented in the intake results.
n: number of subjects.
The intake expressed as μg/MJ is referring to 245 male subjects of the Finnish DIPP study as energy intake was not reported for two subjects.
Appendix D – ‘Total vitamin K’ intakes in females in different surveys, estimated by EFSA according to age class and country
| Age class | Country | Survey | Intakesb expressed in μg/day | Intakesb expressed in μg/MJ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nc | Average | Median | P5 | P95 | Average | Median | P5 | P95 | |||
| < 1 yeara | Finland | DIPP | 253 | 33 | 32 | 5 | 69 | 22e | 17e | 8e | 45e |
| Germany | VELS | 75 | 36 | 33 | 10 | 77 | 12 | 12 | 3 | 24 | |
| Italy | INRAN_SCAI_2005_06 | 7 | 31 | 32 | –b | –b | 10 | 9 | –b | –b | |
| United Kingdom | DNSIYC_2011 | 670 | 53 | 50 | 11 | 100 | 17 | 17 | 4 | 31 | |
| 1–< 3 years | Finland | DIPP | 255 | 36 | 34 | 12 | 72 | 11 | 10 | 4 | 20 |
| Germany | VELS | 174 | 46 | 37 | 12 | 120 | 11 | 8 | 3 | 29 | |
| Italy | INRAN_SCAI_2005_06 | 16 | 50 | 37 | –b | –b | 10 | 7 | –b | –b | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 78 | 52 | 47 | 18 | 103 | 12 | 11 | 5 | 22 | |
| United Kingdom | DNSIYC_2011 | 651 | 50 | 44 | 16 | 102 | 13 | 11 | 4 | 26 | |
| 3–< 10 years | Finland | DIPP | 369 | 42 | 37 | 19 | 84 | 8 | 7 | 4 | 15 |
| France | INCA2 | 243 | 63 | 50 | 19 | 160 | 11 | 9 | 4 | 28 | |
| Germany | EsKiMo | 409 | 65 | 50 | 19 | 166 | 10 | 7 | 3 | 23 | |
| Germany | VELS | 147 | 50 | 37 | 14 | 137 | 10 | 7 | 3 | 28 | |
| Italy | INRAN_SCAI_2005_06 | 99 | 85 | 65 | 20 | 223 | 12 | 9 | 3 | 30 | |
| Netherlands | DNFCS2007 | 216 | 70 | 49 | 22 | 164 | 9 | 6 | 3 | 21 | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 325 | 65 | 57 | 22 | 139 | 11 | 10 | 4 | 23 | |
| 10–< 18 years | Finland | NWSSP07_08 | 170 | 71 | 68 | 34 | 115 | 11 | 10 | 6 | 18 |
| France | INCA2 | 524 | 70 | 57 | 19 | 178 | 12 | 9 | 3 | 30 | |
| Germany | EsKiMo | 196 | 74 | 56 | 20 | 200 | 10 | 8 | 3 | 29 | |
| Italy | INRAN_SCAI_2005_06 | 139 | 111 | 79 | 30 | 322 | 15 | 10 | 4 | 51 | |
| Latviad | FC_PREGNANTWOMEN_2011 | 12 | 88 | 67 | –b | –b | 9 | 7 | –b | –b | |
| Netherlands | DNFCS2007 | 576 | 95 | 60 | 26 | 336 | 12 | 7 | 3 | 42 | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 326 | 68 | 57 | 24 | 140 | 10 | 9 | 4 | 22 | |
| 18–< 65 years | Finland | FINDIET2012 | 710 | 90 | 80 | 27 | 176 | 13 | 11 | 4 | 28 |
| France | INCA2 | 1,340 | 105 | 86 | 27 | 244 | 17 | 14 | 5 | 41 | |
| Ireland | NANS_2012 | 640 | 81 | 68 | 25 | 187 | 11 | 9 | 4 | 25 | |
| Italy | INRAN_SCAI_2005_06 | 1,245 | 157 | 114 | 40 | 432 | 23 | 15 | 6 | 64 | |
| Latviad | FC_PREGNANTWOMEN_2011 | 990 | 88 | 76 | 32 | 171 | 11 | 9 | 4 | 20 | |
| Netherlands | DNFCS2007 | 1,034 | 135 | 78 | 26 | 516 | 17 | 10 | 3 | 60 | |
| Sweden | Riksmaten 2010 | 807 | 98 | 82 | 33 | 213 | 13 | 11 | 5 | 28 | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 706 | 101 | 86 | 27 | 218 | 16 | 13 | 5 | 36 | |
| 65–< 75 years | Finland | FINDIET2012 | 83 | 75 | 32 | 154 | 14 | 12 | 6 | 25 | 83 |
| France | INCA2 | 125 | 105 | 44 | 268 | 21 | 17 | 9 | 43 | 125 | |
| Ireland | NANS_2012 | 96 | 81 | 24 | 200 | 15 | 12 | 4 | 33 | 96 | |
| Italy | INRAN_SCAI_2005_06 | 169 | 120 | 38 | 392 | 25 | 17 | 7 | 62 | 169 | |
| Netherlands | VCPBasis_AVL2007_2010 | 151 | 82 | 22 | 505 | 23 | 12 | 4 | 66 | 151 | |
| Sweden | Riksmaten 2010 | 89 | 75 | 39 | 186 | 13 | 12 | 6 | 25 | 89 | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 107 | 97 | 28 | 240 | 18 | 15 | 5 | 42 | 107 | |
| ≥ 75 years | France | INCA2 | 44 | 120 | 102 | –b | –b | 20 | 17 | –b | –b |
| Ireland | NANS_2012 | 43 | 89 | 76 | –b | –b | 14 | 12 | –b | –b | |
| Italy | INRAN_SCAI_2005_06 | 159 | 164 | 121 | 33 | 466 | 25 | 16 | 6 | 74 | |
| Sweden | Riksmaten 2010 | 30 | 111 | 108 | –b | –b | 16 | 16 | –b | –b | |
| United Kingdom | NDNS‐Rolling Programme Years 1–3 | 83 | 88 | 79 | 32 | 177 | 15 | 13 | 6 | 31 | |
DIPP: type 1 Diabetes Prediction and Prevention survey; DNFCS: Dutch National Food Consumption Survey; DNSIYC: Diet and Nutrition Survey of Infants and Young Children; EsKiMo: Ernährungsstudie als KIGGS‐Modul; FC_PREGNANTWOMEN: food consumption of pregnant women in Latvia; FINDIET: the national dietary survey of Finland; INCA: étude Individuelle Nationale des Consommations Alimentaires; INRAN‐SCAI: Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione – Studio sui Consumi Alimentari in Italia; NANS: National Adult Nutrition Survey; NDNS: National Diet and Nutrition Survey; NWSSP: Nutrition and Wellbeing of Secondary School Pupils; VELS: Verzehrsstudie zur Ermittlung der Lebensmittelaufnahme von Säuglingen und Kleinkindern für die Abschätzung eines akuten Toxizitätsrisikos durch Rückstände von Pflanzenschutzmitteln.
Infants between 1 and 11 months. The proportions of breastfed infants were 58% in the Finnish survey, 40% in the German survey, 44% in the Italian survey and 21% in the UK survey. Most breastfed infants were partially breastfed. The consumption of breast milk was taken into account if the consumption was reported as human milk (Italian survey) or if the number of breast milk consumption events was reported (German and UK surveys). For the German study, the total amount of breast milk was calculated based on the observations by Paul et al. (1988) on breast milk consumption during one eating occasion at different age groups: the amount of breast milk consumed on one eating occasion was set to 135 g/eating occasion for infants between 6 and 7 months of age and to 100 g/eating occasion for infants between 8 and 12 months of age (Kersting and Clausen, 2003). For the UK survey, the amount of breast milk consumed was either directly quantified by the mother (expressed breast milk) or extrapolated from the duration of each breastfeeding event. As no information on the breastfeeding events were reported in the Finnish survey, breast milk intake was not taken into consideration in the intake estimates of Finnish infants.
5th or 95th percentile intakes calculated from fewer than 60 subjects require cautious interpretation as the results may not be statistically robust (EFSA, 2011b) and, therefore, for these dietary surveys/age classes, the 5th and 95th percentile estimates are not presented in the intake results.
n: number of subjects.
Pregnant women only.
The intake expressed as μg/MJ is referring to 251 female subjects of the Finnish DIPP study as energy intake was not reported for two subjects.
Appendix E – Minimum and maximum percentage contributions of different food groups (FoodEx2 level 1) to ‘total vitamin K’ intake estimates in males
| Food groups | Age | ||||||
|---|---|---|---|---|---|---|---|
| < 1 year | 1 – < 3 years | 3 – < 10 years | 10 – < 18 years | 18 – < 65 years | 65 – < 75 years | ≥ 75 years | |
| Additives, flavours, baking and processing aids | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alcoholic beverages | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Animal and vegetable fats and oils | 1–12 | 3–15 | 5–31 | 5–36 | 5–26 | 6–28 | 5–13 |
| Coffee, cocoa, tea and infusions | 0 | 0 | < 1 | < 1 | < 1 | < 1 | < 1 |
| Composite dishes | < 1–6 | < 1–10 | < 1–10 | < 1–13 | < 1–34 | < 1–34 | < 1–34 |
| Eggs and egg products | < 1 | < 1–1 | < 1–1 | < 1–2 | < 1–4 | < 1–5 | < 1–4 |
| Fish, seafood, amphibians, reptiles and invertebrates | 0 | < 1 | < 1 | < 1 | < 1–2 | < 1–3 | < 1–2 |
| Food products for young population | 48–62 | 5–30 | < 1–1 | < 1 | < 1 | – | – |
| Fruit and fruit products | 3–14 | 5–12 | 4–10 | 3–9 | 2–6 | 3–8 | 3–8 |
| Fruit and vegetable juices and nectars | < 1–1 | < 1–2 | 1–4 | < 1–3 | < 1–2 | < 1–1 | < 1–1 |
| Grains and grain‐based products | < 1–3 | 3–8 | 3–9 | 2–9 | 1–12 | 1–13 | 1–18 |
| Human milk | 0 | 0 | – | – | – | – | – |
| Legumes, nuts, oilseeds and spices | < 1–6 | 2–24 | 1–23 | 1–21 | 1–18 | 2–13 | 3–15 |
| Meat and meat products | 0–1 | < 1–2 | < 1–5 | 1–5 | 1–4 | 1–3 | 1–3 |
| Milk and dairy products | < 1–2 | 1–6 | 2–4 | 1–3 | < 1–3 | < 1–2 | 1–2 |
| Products for non‐standard diets, food imitates and food supplements or fortifying agents | 0 | 0 | 0 | < 1 | < 1 | 0 | 0 |
| Seasoning, sauces and condiments | 0 | 0–2 | < 1–2 | < 1–3 | < 1–7 | < 1–2 | < 1–2 |
| Starchy roots or tubers and products thereof, sugar plants | < 1–2 | 1–4 | 1–5 | 1–7 | 1–5 | 1–4 | 1–4 |
| Sugar, confectionery and water‐based sweet desserts | 0 | < 1–1 | < 1–1 | < 1–1 | < 1 | < 1 | < 1 |
| Vegetables and vegetable products | 12–37 | 25–62 | 32–64 | 31–71 | 25–75 | 22–77 | 23–73 |
| Water and water‐based beverages | 0 | 0 | 0–1 | < 1–1 | < 1 | 0 | 0 |
‘–’ means that there was no consumption event of the food group for the age and sex group considered, while ‘0’ means that there were some consumption events, but that the food group does not contribute to the intake of the nutrient considered, for the age and sex group considered.
Appendix F – Minimum and maximum percentage contributions of different food groups (FoodEx2 level 1) to ‘total vitamin K’ intake estimates in females
| Food groups | Age | ||||||
|---|---|---|---|---|---|---|---|
| < 1 year | 1 – < 3 years | 3 – < 10 years | 10 – < 18 years | 18 – < 65 years | 65 – < 75 years | ≥ 75 years | |
| Additives, flavours, baking and processing aids | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alcoholic beverages | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Animal and vegetable fats and oils | 1–10 | 3–17 | 4–31 | 5–31 | 4–21 | 3–21 | 4–9 |
| Coffee, cocoa, tea and infusions | 0 | < 1 | < 1 | 0 | 0 | 0 | < 1 |
| Composite dishes | < 1–2 | 0–11 | < 1–12 | < 1–15 | < 1–32 | < 1–34 | < 1–35 |
| Eggs and egg products | < 1 | < 1–1 | < 1–1 | < 1–1 | < 1–4 | < 1–5 | < 1–4 |
| Fish, seafood, amphibians, reptiles and invertebrates | 0 | < 1 | < 1 | < 1 | < 1–1 | < 1–2 | < 1–2 |
| Food products for young population | 38–61 | 5–28 | < 1–1 | < 1 | < 1 | – | < 1 |
| Fruit and fruit products | 3–14 | 5–11 | 6–11 | 4–11 | 3–9 | 4–11 | 4–9 |
| Fruit and vegetable juices and nectars | < 1–1 | < 1–2 | 1–4 | < 1–5 | < 1–2 | < 1–1 | < 1–1 |
| Grains and grain‐based products | 0–2 | 3–8 | 3–9 | 2–8 | 1–12 | 1–12 | 1–13 |
| Human milk | 0 | 0 | – | – | – | – | – |
| Legumes, nuts, oilseeds and spices | 1–5 | 2–23 | 2–20 | 2–18 | 1–15 | 1–12 | 2–9 |
| Meat and meat products | 0 | < 1–3 | 1–4 | < 1–4 | < 1–2 | < 1–3 | < 1–2 |
| Milk and dairy products | < 1–4 | 1–6 | 2–3 | 1–4 | 1–2 | < 1–2 | 1–2 |
| Products for non‐standard diets, food imitates and food supplements or fortifying agents | 0 | 0 | 0 | 0 | < 1 | 0 | 0 |
| Seasoning, sauces and condiments | 0 | < 1–2 | < 1–3 | < 1–4 | < 1–6 | < 1–3 | < 1–2 |
| Starchy roots or tubers and products thereof, sugar plants | 1–2 | 1–4 | 1–5 | 1–8 | 1–4 | 1–3 | 1–3 |
| Sugar, confectionery and water‐based sweet desserts | 0 | < 1–1 | < 1–1 | < 1–1 | < 1 | < 1 | < 1 |
| Vegetables and vegetable products | 26–28 | 25–59 | 32–64 | 34–68 | 33–76 | 27–76 | 30–77 |
| Water and water‐based beverages | 0 | 0 | 0–1 | 0–2 | < 1 | 0 | < 1 |
‘–’ means that there was no consumption event of the food group for the age and sex group considered, while ‘0’ means that there were some consumption events, but that the food group does not contribute to the intake of the nutrient considered, for the age and sex group considered.
Appendix G – Estimated dietary intakes of phylloquinone and menaquinones in European countries as reported in the literature
| Reference | Type of study | Country | Subjects | n | Source of the vitamin K composition data | Intake assessment method | Value of intake (μg/day) | Mean/median/range/IQR |
|---|---|---|---|---|---|---|---|---|
| Phylloquinone | ||||||||
| Jie et al. (1995)a | Case–control study | NL | Post‐menopausal women |
113 79 females without aortic calcifications 34 females with aortic calcifications |
Shearer et al. (1980), Booth et al. (1993) | FFQ |
243.6 (women without aortic calcifications, n = 79) 189.9 (women with aortic calcifications, n = 34) |
Mean |
| Schurgers et al. (1999) | Prospective cohort | NL | Adults (≥ 55 years) | 5,435 | Ferland and Sadowski (1992), Booth et al. (1993), Shearer et al. (1996) and unpublished data | FFQ |
249 ± 2 (all) 257 ± 3 (men) 244 ± 2 (women) |
Mean ± SE |
| Geleijnse et al. (2004) | Prospective cohort (same cohort as in Schurgers et al. (1999)) | NL | Adults (≥ 55 years) |
4,807 (after exclusion of 613 subjects with a history of myocardial infarction diagnosed at baseline, from the 5,435 investigated in Schurgers et al. (1999) |
Suttie (1992), Ferland et al. (1992), Booth et al. (1993), Olson (1994), Booth et al. (1995), Ferland et al. (1992), Shearer et al. (1996), data from the laboratory analysed following Schurgers and Vermeer (2000) and Gijsbers et al. (1996) | FFQ |
257.1 ± 116.1 (men) 244.3 ± 131.9 (women) |
Mean ± SD |
| Prynne et al. (2005) | On‐going prospective cohort | UK | Adults | 5,362 included initially (in 1946); data analysis on 1,253 | Bolton‐Smith et al. (2000) and unpublished data | 5‐day diary (data analysis on subjects with at least 3 reporting days) |
59–81 (women, 81 μg/day in year 1999) 72–77 (men; 77 μg/day in year 1999) |
Range of means (adjusted for social class and region of residence) for the years 1982, 1989 and 1999 |
| Rejnmark et al. (2006) | Prospective cohort, four study centres | DK | Perimenopausal women (43–58 years) | 2,016 | Danish Food composition tables (Moller, 1989) | 4‐day or 7‐day food record | 67 (45–105) | Median (IQR) |
| Thane et al. (2006a) | Nationally representative sample | UK | Adults (19–64 years) | 1,423 | Bolton‐Smith et al. (2000), FSA (2002) and unpublished data (MJ Shearer and C Bolton‐Smith) | 7‐day‐weighed food record | 67 (65–69)b | Geometric mean (95% CI) |
| Nimptsch et al. (2008) | Prospective cohort | DE | Men (40–65 years) | 11,319 | Bolton‐Smith et al. (2000) and unpublished data | Semiquantitative FFQ | 93.6 (70.9–123.5) | Median (IQR) |
| Macdonald et al. (2008) | Prospective cohort | UK | Women (49–54 years) | 3,199 | UK database, compiled by Bolton‐Smith et al. (2000) | FFQ | 109 ± 55c | Mean ± SD |
| Gast et al. (2009) | Prospective cohort | NL | Post‐menopausal women (49–70 years) | 16,057 | Mainly Schurgers and Vermeer (2000), also: Ferland and Sadowski (1992), Suttie (1992), Booth et al. (1993), Booth et al. (1995), Shearer et al. (1996) | FFQ |
211.7 ± 100.3 (9.1 ± 991.1) |
Mean ± SD |
| Apalset et al. (2011) | Prospective cohort | NO | Adults (71–73 years) | 2,582 |
Described in Apalset et al. (2010): (Koivu‐Tikkanen et al., 2000; Schurgers and Vermeer, 2000); Finnish food composition database (National Institute for Health and Welfare, 2009) |
FFQ |
67.0 ± 66.6 (women with no hip fracture) 78.4 ± 61.7 (men with no hip fracture) 57.9 ± 64.3 (women with hip fracture) 65.2 ± 46.1 (men with hip fracture) |
Median (IQR) |
| Bullo et al. (2011) | Prospective cohort | ES | Adults (55–80 years) | 200 | USDA (2009) | Semiquantitative FFQ |
333.6 ± 17.3 (men) 299.8 ± 11.6 (women) |
Mean ± SE |
| DGE (2012) | National survey, Cross‐sectional | DE | Adults (15–80 years) | 6,160 | German food composition database (BLS 3.02) (MRI) | Two 24‐h recalls | 76 | Median |
| Elmadfa et al. (2012) | National survey, cross‐sectional | AT | Children (7–14 years) | 332 (children) | Elmadfa et al. (1994) (using the German food composition database BLS 2.1. (MRI) completed with food composition tables of typical Austrian dishes and nutrient‐enriched foods) | 3‐day dietary record | 59–75 (children) | Range of means depending on sex and age range |
| Adults (18–80 years) |
380 (18–64 years) 176 (65–80 years) |
Jakob and Elmadfa (1996) | Two 24‐h recalls | 89–117 (adults) | Range of means depending on sex and age range | |||
| Vissers et al. (2013) | Prospective cohort | NL | Adults (49 ± 12 years), including the cohort of women investigated by Gast et al. (2009) | 35,476 | Mainly Schurgers and Vermeer (2000), also: Ferland and Sadowski (1992), Suttie (1992), Booth et al. (1993), Booth et al. (1995), Shearer et al. (1996) | FFQ | 199 ± 97.8 | Mean ± SD |
| Ortega Anta et al. (2014)e | Cross‐sectional, nationally representative sample | ES | Mostly adults (17–60 years) | 1,068 | Spanish database: Ortega et al. (2010) | 3‐day food record | 174.2 (males), 166.4 (females) 170.2 (all) | Mean (adjusted for energy intake) |
| Weber et al. (2014)f | Prospective cohort | DE | Children (8–12 years) | 268 | German food composition database BLS II.3 (MRI) | Dietary history over 4 weeks | 292.3 | Median |
| Hayes et al. (2016) | National survey, cross‐sectional | IE | Adults (18–90 years) | 1,500 | Mainly UK food composition table (FSA, 2002), which vitamin K data are largely based on Bolton‐Smith et al. (2000), and data from the previous version of the UK table; also recipe calculations, and USDA (2015) | 4‐day semiweighted food diary |
85.2 ± 59.1 (all) 86.0 ± 57.4 (men) 84.4 ± 60.7 (women) |
Mean ± SD |
| Menaquinones | ||||||||
| Schurgers et al. (1999) | Prospective cohort | NL | Adults (≥ 55 years) | 5,435 | Unpublished data | FFQ |
Total menaquinones (MK‐4 to MK‐10) 28.4 (all) MK‐4 6.8 ± 0.04 (all) 7.5 ± 0.1 (men) 6.3 ± 0.1 (women) MK‐5 to MK‐10 21.6 ± 0.2 (all) 22.9 ± 0.3 (men) 20.6 ± 0.3 (women) |
Mean Mean ± SE Mean ± SE |
| Geleijnse et al. (2004) | Prospective cohort | NL | Adults (≥ 55 years) | 4,807 | Data from the laboratory analysed following Schurgers and Vermeer (2000) and Gijsbers et al. (1996) | FFQ |
Total menaquinones (MK‐4 to MK‐10) 30.8 ± 18 (men) 27 ± 15.1(women) MK‐4 7.7 ± 3.4 (men) 6.3 ± 2.8 (women)MK‐5 to MK‐10 23.1 ± 16.3 (men) 20.7 ± 13.8 (women) |
Mean ± SD Mean ± SD Mean ± SD |
| Nimptsch et al. (2008) | Prospective cohort | DE | Men (40–65 years) | 11,319 | Hirauchi et al. (1989), Schurgers and Vermeer (2000) | FFQ |
Total menaquinones (MK‐4 to MK‐14) 34.7 (25.7–45.7) MK‐4 14.4 (10.9–18.7) MK‐5 0.3 (0.2–0.5) MK‐6 0.3 (0.2–0.5) MK‐7 0.8 (0.5–1.1) MK‐8 4.6 (3.1–6.7) MK‐9 11.9 (7.4–18.4) MK‐10 0.06 (0.01–0.13) MK‐11 0.12 (0.03–0.27) MK‐12 0.20 (0.04–0.42) MK‐13 0.40 (0.08–0.85) MK‐14 0.02 (0.00–0.05) |
Median (IQR) |
| Gast et al. (2009) | Prospective cohort | NL | Post‐menopausal women (49–70 years) | 16,057 | Schurgers and Vermeer (2000) | FFQ |
Total menaquinones (MK‐4 to MK‐9) 29.1 ± 12.8 (0.9–128) MK‐4 7.1 ± 2.1 (0.5–28.2) MK‐5 0.3 ± 0.2 (0–2.1) MK‐6 0.3 ± 0.2 (0–1.5) MK‐7 0.3 ± 0.2 (0–2.2) MK‐8 6.0 ± 3.4 (0–32.8) MK‐9 14.7 ± 8.1 (0–81.9) |
Mean ± SD (range) |
| Apalset et al. (2011) | Prospective cohort | NO | Adults (71–73 years) | 2,582 | Schurgers and Vermeer (2000) | FFQ |
Total menaquinonesg 10.8 ± 7.4 (women) 11.9 ± 7.6 (men) 10.2 ± 7.2 (women with hip fracture) 12.6 ± 8.6 (men with hip fracture) |
Median (IQR) |
| Vissers et al. (2013) | Prospective cohort | NL | Adults (49 ± 12 years) including the cohort of women investigated by Gast et al. (2009) | 35,476 | Schurgers and Vermeer (2000) | FFQ |
Total menaquinones (MK‐4 to MK‐10) 30.7 ± 13.8 |
Mean ± SD |
AT: Austria; BLS: Bundeslebensmittelschlüssel; CI: confidence interval; DE: Germany; DK: Denmark; ES: Spain; FFQ: food frequency questionnaire; IE: Ireland; IQR: interquartile range; MK: menaquinone; MRI: Max Rubner Institut; NL: the Netherlands; NO: Norway; SD: standard deviation; SE: standard error; USDA: US Department of Agriculture; UK: United Kingdom.
Presented as ‘vitamin K’ in the reference by Jie et al., but assumed to be phylloquinone based on the two references cited as source of composition data.
2000–2001 data.
Intake at visit 2 (1997–2000).
Version of 23.1.2009. Current version available at: https://www.livsmedelsverket.se/en/food-and-content/naringsamnen/livsmedelsdatabasen
Presented as ‘vitamin K’ in the reference, but personal communication from one of the authors confirmed that composition data were on phylloquinone.
Presented as ‘vitamin K’ in the reference, but assumed to be phylloquinone, based on information from Section 3.2.1.
No information of the forms of menaquinones.
Suggested citation: EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , Turck D, Bresson J‐L, Burlingame B, Dean T, Fairweather‐Tait S, Heinonen M, Hirsch‐Ernst KI, Mangelsdorf I, McArdle HJ, Naska A, Nowicka G, Pentieva K, Sanz Y, Siani A, Sjödin A, Stern M, Tomé D, Van Loveren H, Vinceti M, Willatts P, Lamberg‐Allardt C, Przyrembel H, Tetens I, Dumas C, Fabiani L, Ioannidou S and Neuhäuser‐Berthold M, 2017. Scientific Opinion on the dietary reference values for vitamin K. EFSA Journal 2017;15(5):4780, 78 pp. 10.2903/j.efsa.2017.4780
Requestor: European Commission
Question number: EFSA‐Q‐2011‐01232
Panel members: Jean‐Louis Bresson, Barbara Burlingame, Tara Dean, Susan Fairweather‐Tait, Marina Heinonen, Karen‐Ildico Hirsch‐Ernst, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Monika Neuhäuser‐Berthold, Grażyna Nowicka, Kristina Pentieva, Yolanda Sanz, Alfonso Siani, Anders Sjödin, Martin Stern, Daniel Tomé, Dominique Turck, Henk Van Loveren, Marco Vinceti and Peter Willatts.
Acknowledgements: The Panel wishes to thank EFSA staff: Krizia Ferrini, Joaquim Maia, Christos Stefanidis and Olga Vidal Pariente for the support provided to this Scientific Opinion.
Adopted: 5 April 2017
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1220/full
Notes
Scientific Committee for Food, Nutrient and energy intakes for the European Community, Reports of the Scientific Committee for Food 31st series, Office for Official Publication of the European Communities, Luxembourg, 1993.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
MK‐5 = 512.8 g/mol; MK‐6 = 580.9 g/mol; MK‐8 = 717.1 g/mol; MK‐9: 785.2 g/mol; MK‐10 = 853.4 g/mol; MK‐11 = 921.5 g/mol; MK‐12 = 989.6 g/mol; MK‐13 = 1,057.7 g/mol
99 and 143 µg/day, respectively.
i.e. 0.90 mg phylloquinone, 0.89 mg MK‐4 and 1.57 mg MK‐9.
i.e. about 1.6 mg phylloquinone and 2 mg MK‐7.
Two subjects dropped‐out before the end of the phylloquinone restriction.
Plasma phylloquinone concentration in the range of 0.82–3.33 nmol/L on the control diet.
i.e. 10–11 s according to Suttie (1992).
Used by the SCF to set DRVs for vitamin K, see Section 4.
Well‐controlled studies in which participants were housed in a metabolic unit are termed metabolic studies.
Mean at 6, 12 and 26 weeks: 0.07–0.12 µg/kg body weight per day in breastfed infants, and 7–9.3 µg/kg body weight per day in formula‐fed infants.
Mean of 3.55 and 1.33 µg/day after the control period, respectively.
5‐C: mean of 2.89 µg/day at the end of depletion and of 8.48 µg/day at the end of repletion; 7‐C: mean of 1.10 µg/day at the end of depletion, and of 2.71 µg/day at the end of repletion.
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJ L 404, 30.12.2006, p. 26.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. OJ L 183, 12.7.2002, p. 51.
Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow‐on formulae and amending Directive 1999/21/EC, OJ L 401, 30.12.2006, p. 1. and Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children, OJ L 339, 6.12.2006, p. 16–35.
http://www.foodcomp.dk/v7/fcdb_aboutfooddata_vitamins.asp#Vitaminand http://frida.fooddata.dk/CntList.php
From a member of the team in charge of the German Nutrient composition database (BLS) at the Max Rubner Institute.
From members of the National Institute for Public Health and the Environment in the Netherlands, and a member of the team in charge of the German Nutrient composition database (BLS) at the Max Rubner Institute as mentioned in Section 3.2.1.3.
The conclusion of the NRC (1989) was mainly based on Frick et al. (1967) and Suttie et al. (1988), which both dealt with phylloquinone.
Assumed by the Panel to be probably phylloquinone.
In view of the high dose investigated (100 µg/day MK‐7) much higher than observed intakes of MK‐7 in Europe (Section 3.2), the results for MK‐7 are not discussed.
Dual‐photon absorptiometry, single‐photon absorptiometry and DXA.
References
- AFSSA (Agence française de sécurité sanitaire des aliments), 2001. Apports nutritionnels conseillés pour la population française. Editions Tec & Doc, Paris, France. 605 pp. [Google Scholar]
- Al Rajabi A, Booth SL, Peterson JW, Choi SW, Suttie JW, Shea MK, Miao B, Grusak MA and Fu X, 2012. Deuterium‐labeled phylloquinone has tissue‐specific conversion to menaquinone‐4 among Fischer 344 male rats. Journal of Nutrition, 142, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PM, Mummah‐Schendel LL, Kindberg CG, Harms CS, Bang NU and Suttie JW, 1987. Effects of a vitamin K‐deficient diet and antibiotics in normal human volunteers. Journal of Laboratory and Clinical Medicine, 110, 180–188. [PubMed] [Google Scholar]
- Anai T, Hirota Y, Yoshimatsu J, Oga M and Miyakawa I, 1993. Can prenatal vitamin K1 (phylloquinone) supplementation replace prophylaxis at birth? Obstetrics and Gynecology, 81, 251–254. [PubMed] [Google Scholar]
- Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM and Powers P, 1987. Development of the human coagulation system in the full‐term infant. Blood, 70, 165–172. [PubMed] [Google Scholar]
- Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Castle V and Powers P, 1988. Development of the human coagulation system in the healthy premature infant. Blood, 72, 1651–1657. [PubMed] [Google Scholar]
- Andrew M, 1997. The relevance of developmental hemostasis to hemorrhagic disorders of newborns. Seminars in Perinatology, 21, 70–85. [DOI] [PubMed] [Google Scholar]
- Anses/CIQUAL (Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail/Centre d'information sur la qualité des aliments), 2013. French food composition table version 2013. Available online: https://pro.anses.fr/TableCIQUAL/
- Apalset E, Gjesdal C, Eide G, Johansen A‐M, Drevon C and Tell G, 2010. Dietary vitamin K and K2 and bone mineral density: The Hordaland Health Study. Archives of Osteoporosis, 5, 73–81. [Google Scholar]
- Apalset EM, Gjesdal CG, Eide GE and Tell GS, 2011. Intake of vitamin K1 and K2 and risk of hip fractures: The Hordaland Health Study. Bone, 49, 990–995. [DOI] [PubMed] [Google Scholar]
- Azharuddin MK, O'Reilly DS, Gray A and Talwar D, 2007. HPLC method for plasma vitamin K1: effect of plasma triglyceride and acute‐phase response on circulating concentrations. Clinical Chemistry, 53, 1706–1713. [DOI] [PubMed] [Google Scholar]
- Bach AU, Anderson SA, Foley AL, Williams EC and Suttie JW, 1996. Assessment of vitamin K status in human subjects administered “minidose” warfarin. American Journal of Clinical Nutrition, 64, 894–902. [DOI] [PubMed] [Google Scholar]
- Beavan SR, Prentice A, Stirling DM, Dibba B, Yan L, Harrington DJ and Shearer MJ, 2005. Ethnic differences in osteocalcin gamma‐carboxylation, plasma phylloquinone (vitamin K1) and apolipoprotein E genotype. European Journal of Clinical Nutrition, 59, 72–81. [DOI] [PubMed] [Google Scholar]
- Bellido‐Martin L and de Frutos PG, 2008. Vitamin K‐dependent actions of Gas6. Vitamins and Hormones, 78, 185–209. [DOI] [PubMed] [Google Scholar]
- Beulens JW, van der ADL, Grobbee DE, Sluijs I, Spijkerman AM and van der Schouw YT, 2010. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care, 33, 1699–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley NC, Krueger DC, Kawahara TN, Engelke JA, Chappell RJ and Suttie JW, 2002. A high phylloquinone intake is required to achieve maximal osteocalcin gamma‐carboxylation. American Journal of Clinical Nutrition, 76, 1055–1060. [DOI] [PubMed] [Google Scholar]
- Bjornsson TD, Meffin PJ, Swezey SE and Blaschke TF, 1979. Effects of clofibrate and warfarin alone and in combination on the disposition of vitamin K1. Journal of Pharmacology and Experimental Therapeutics, 210, 322–326. [PubMed] [Google Scholar]
- Blomstrand R and Forsgren L, 1968. Vitamin K1‐3H in man. Its intestinal absorption and transport in the thoracic duct lymph. Internationale Zeitschrift für Vitaminforschung, 38, 45–64. [PubMed] [Google Scholar]
- Bolton‐Smith C, Price RJ, Fenton ST, Harrington DJ and Shearer MJ, 2000. Compilation of a provisional UK database for the phylloquinone (vitamin K1) content of foods. British Journal of Nutrition, 83, 389–399. [PubMed] [Google Scholar]
- Bolton‐Smith C, McMurdo ME, Paterson CR, Mole PA, Harvey JM, Fenton ST, Prynne CJ, Mishra GD and Shearer MJ, 2007. Two‐year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. Journal of Bone and Mineral Research, 22, 509–519. [DOI] [PubMed] [Google Scholar]
- Booth SL, Madabushi HT, Davidson KW and Sadowski JA, 1995. Tea and coffee brews are not dietary sources of vitamin K‐1 (phylloquinone). Journal of the American Dietetic Association, 95, 82–83. [DOI] [PubMed] [Google Scholar]
- Booth SL, Pennington JA and Sadowski JA, 1996a. Food sources and dietary intakes of vitamin K‐1 (phylloquinone) in the American diet: data from the FDA Total Diet Study. Journal of the American Dietetic Association, 96, 149–154. [DOI] [PubMed] [Google Scholar]
- Booth SL, Pennington JA and Sadowski JA, 1996b. Dihydro‐vitamin K1: primary food sources and estimated dietary intakes in the American diet. Lipids, 31, 715–720. [DOI] [PubMed] [Google Scholar]
- Booth SL, Tucker KL, McKeown NM, Davidson KW, Dallal GE and Sadowski JA, 1997. Relationships between dietary intakes and fasting plasma concentrations of fat‐soluble vitamins in humans. Journal of Nutrition, 127, 587–592. [DOI] [PubMed] [Google Scholar]
- Booth SL and Suttie JW, 1998. Dietary intake and adequacy of vitamin K. Journal of Nutrition, 128, 785–788. [DOI] [PubMed] [Google Scholar]
- Booth SL, Sadowski JA, Weihrauch JL and Ferland G, 1993. Vitamin K1 (phylloquinone) content of foods: a provisional table. Journal of Food Composition and Analysis, 6, 109–120. [Google Scholar]
- Booth SL, Webb DR and Peters JC, 1999a. Assessment of phylloquinone and dihydrophylloquinone dietary intakes among a nationally representative sample of US consumers using 14‐day food diaries. Journal of the American Dietetic Association, 99, 1072–1076. [DOI] [PubMed] [Google Scholar]
- Booth SL, O'Brien‐Morse ME, Dallal GE, Davidson KW and Gundberg CM, 1999b. Response of vitamin K status to different intakes and sources of phylloquinone‐rich foods: comparison of younger and older adults. American Journal of Clinical Nutrition, 70, 368–377. [DOI] [PubMed] [Google Scholar]
- Booth SL, Tucker KL, Chen H, Hannan MT, Gagnon DR, Cupples LA, Wilson PW, Ordovas J, Schaefer EJ, Dawson‐Hughes B and Kiel DP, 2000a. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. American Journal of Clinical Nutrition, 71, 1201–1208. [DOI] [PubMed] [Google Scholar]
- Booth SL, McKeown NM, Lichtenstein AH, Morse MO, Davidson KW, Wood RJ and Gundberg C, 2000b. A hydrogenated form of vitamin K: its relative bioavailability and presence in the food supply. Journal of Food Composition and Analysis, 13, 311–317. [Google Scholar]
- Booth SL and Al Rajabi A, 2008. Determinants of vitamin K status in humans. Vitamins and Hormones, 78, 1–22. [DOI] [PubMed] [Google Scholar]
- Booth SL, 2009. Roles for vitamin K beyond coagulation. Annual Review of Nutrition, 29, 89–110. [DOI] [PubMed] [Google Scholar]
- Booth SL, Lichtenstein AH, O'BrienMorse M, McKeown NM, Wood RJ, Saltzman E and Gundberg CM, 2001. Effects of a hydrogenated form of vitamin K on bone formation and resorption. American Journal of Clinical Nutrition, 74, 783–790. [DOI] [PubMed] [Google Scholar]
- Booth SL, Lichtenstein AH and Dallal GE, 2002. Phylloquinone absorption from phylloquinone‐fortified oil is greater than from a vegetable in younger and older men and women. Journal of Nutrition, 132, 2609–2612. [DOI] [PubMed] [Google Scholar]
- Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, Dawson‐Hughes B, Wilson PW, Cupples LA and Kiel DP, 2003a. Vitamin K intake and bone mineral density in women and men. American Journal of Clinical Nutrition, 77, 512–516. [DOI] [PubMed] [Google Scholar]
- Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE and Wood RJ, 2003b. Dietary phylloquinone depletion and repletion in older women. Journal of Nutrition, 133, 2565–2569. [DOI] [PubMed] [Google Scholar]
- Booth SL, Golly I, Sacheck JM, Roubenoff R, Dallal GE, Hamada K and Blumberg JB, 2004a. Effect of vitamin E supplementation on vitamin K status in adults with normal coagulation status. American Journal of Clinical Nutrition, 80, 143–148. [DOI] [PubMed] [Google Scholar]
- Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson‐Hughes B, Gundberg CM, Cupples LA, Wilson PW and Kiel DP, 2004b. Associations between vitamin K biochemical measures and bone mineral density in men and women. Journal of Clinical Endocrinology and Metabolism, 89, 4904–4909. [DOI] [PubMed] [Google Scholar]
- Bovill EG, Soll RF, Lynch M, Bhushan F, Landesman M, Freije M, Church W, McAuliffe T, Davidson K and Sadowski J, 1993. Vitamin K1 metabolism and the production of des‐carboxy prothrombin and protein C in the term and premature neonate. Blood, 81, 77–83. [PubMed] [Google Scholar]
- Bruge F, Bacchetti T, Principi F, Littarru GP and Tiano L, 2011. Olive oil supplemented with menaquinone‐7 significantly affects osteocalcin carboxylation. British Journal of Nutrition, 106, 1058–1062. [DOI] [PubMed] [Google Scholar]
- Bugel S, Sorensen AD, Hels O, Kristensen M, Vermeer C, Jakobsen J, Flynn A, Molgaard C and Cashman KD, 2007. Effect of phylloquinone supplementation on biochemical markers of vitamin K status and bone turnover in postmenopausal women. British Journal of Nutrition, 97, 373–380. [DOI] [PubMed] [Google Scholar]
- Bullo M, Estruch R and Salas‐Salvado J, 2011. Dietary vitamin K intake is associated with bone quantitative ultrasound measurements but not with bone peripheral biochemical markers in elderly men and women. Bone, 48, 1313–1318. [DOI] [PubMed] [Google Scholar]
- Busfield A, McNinch A and Tripp J, 2007. Neonatal vitamin K prophylaxis in Great Britain and Ireland: the impact of perceived risk and product licensing on effectiveness. Archives of Disease in Childhood, 92, 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell MD, Awad T, Johnson JA, Gage BF, Falkowski M, Gardina P, Hubbard J, Turpaz Y, Langaee TY, Eby C, King CR, Brower A, Schmelzer JR, Glurich I, Vidaillet HJ, Yale SH, Qi Zhang K, Berg RL and Burmester JK, 2008. CYP4F2 genetic variant alters required warfarin dose. Blood, 111, 4106–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield LM and Hopkinson JM, 1989. State of the art vitamin K in human milk. Journal of Pediatric Gastroenterology and Nutrition, 8, 430–441. [DOI] [PubMed] [Google Scholar]
- Canfield LM, Hopkinson JM, Lima AF, Martin GS, Sugimoto K, Burr J, Clark L and McGee DL, 1990. Quantitation of vitamin K in human milk. Lipids, 25, 406–411. [DOI] [PubMed] [Google Scholar]
- Canfield LM, Hopkinson JM, Lima AF, Silva B and Garza C, 1991. Vitamin K in colostrum and mature human milk over the lactation period–a cross‐sectional study. American Journal of Clinical Nutrition, 53, 730–735. [DOI] [PubMed] [Google Scholar]
- Card DJ, Gorska R, Cutler J and Harrington DJ, 2014. Vitamin K metabolism: current knowledge and future research. Molecular Nutrition and Food Research, 58, 1590–1600. [DOI] [PubMed] [Google Scholar]
- Chan R, Leung J and Woo J, 2012. No association between dietary vitamin K intake and fracture risk in Chinese community‐dwelling older men and women: a prospective study. Calcified Tissue International, 90, 396–403. [DOI] [PubMed] [Google Scholar]
- Clarke P, 2010. Vitamin K prophylaxis for preterm infants. Early Human Development, 86(Suppl 1), 17–20. [DOI] [PubMed] [Google Scholar]
- Clarke P, Mitchell SJ, Wynn R, Sundaram S, Speed V, Gardener E, Roeves D and Shearer MJ, 2006. Vitamin K prophylaxis for preterm infants: a randomized, controlled trial of 3 regimens. Pediatrics, 118, e1657–e1666. [DOI] [PubMed] [Google Scholar]
- Cockayne S, Adamson J, Lanham‐New S, Shearer MJ, Gilbody S and Torgerson DJ, 2006. Vitamin K and the prevention of fractures: systematic review and meta‐analysis of randomized controlled trials. Archives of Internal Medicine, 166, 1256–1261. [DOI] [PubMed] [Google Scholar]
- Conly JM and Stein K, 1992. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Progress in Food and Nutrition Science, 16, 307–343. [PubMed] [Google Scholar]
- Cooper AD, 1997. Hepatic uptake of chylomicron remnants. Journal of Lipid Research, 38, 2173–2192. [PubMed] [Google Scholar]
- Craciun AM, Wolf J, Knapen MH, Brouns F and Vermeer C, 1998. Improved bone metabolism in female elite athletes after vitamin K supplementation. International Journal of Sports Medicine, 19, 479–484. [DOI] [PubMed] [Google Scholar]
- Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O and Vermeer C, 2010. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thrombosis and Haemostasis, 104, 811–822. [DOI] [PubMed] [Google Scholar]
- Crosier MD, Peter I, Booth SL, Bennett G, Dawson‐Hughes B and Ordovas JM, 2009. Association of sequence variations in vitamin K epoxide reductase and gamma‐glutamyl carboxylase genes with biochemical measures of vitamin K status. Journal of Nutritional Science and Vitaminology, 55, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynober L, Alix E, Arnaud‐Battandier F, Bonnefoy M, Brocker P, Cals MJ, Cherbut C, Coplo C, Ferry M, Ghisolfi‐Marque A, Kravtchenko T, Lesourd B, Mignot C and Mirand PP, 2000. Apports nutritionnels conseillés chez la personne âgée. Nutrition Clinique et Métabolisme, 14(Supplement 1), 3–60. [Google Scholar]
- D‐A‐CH (Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährung), 2015. Referenzwerte für die Nährstoffzufuhr. 2. Auflage, 1. Ausgabe. DGE, Bonn, Germany.
- Dalmeijer GW, van der Schouw YT, Magdeleyns E, Ahmed N, Vermeer C and Beulens JW, 2012. The effect of menaquinone‐7 supplementation on circulating species of matrix Gla protein. Atherosclerosis, 225, 397–402. [DOI] [PubMed] [Google Scholar]
- Dalmeijer GW, van der Schouw YT, Vermeer C, Magdeleyns EJ, Schurgers LJ and Beulens JW, 2013. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. Journal of Nutritional Biochemistry, 24, 624–628. [DOI] [PubMed] [Google Scholar]
- Dam V, Dalmeijer GW, Vermeer C, Drummen NE, Knapen MH, van der Schouw YT and Beulens JW, 2015. Association between vitamin K and the metabolic syndrome: a 10‐year follow‐up study in adults. Journal of Clinical Endocrinology and Metabolism, 100, 2472–2479. [DOI] [PubMed] [Google Scholar]
- Danziger J, 2008. Vitamin K‐dependent proteins, warfarin, and vascular calcification. Clinical Journal of the American Society of Nephrology, 3, 1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti HS, Shea MK, Smith CE, Tanaka T, Hruby A, Richardson K, Wang TJ, Nalls MA, Guo X, Liu Y, Yao J, Li D, Johnson WC, Benjamin EJ, Kritchevsky SB, Siscovick DS, Ordovas JM and Booth SL, 2014. Meta‐analysis of genome‐wide association studies for circulating phylloquinone concentrations. American Journal of Clinical Nutrition, 100, 1462–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RT, Foley AL, Engelke JA and Suttie JW, 1998. Conversion of dietary phylloquinone to tissue menaquinone‐4 in rats is not dependent on gut bacteria. Journal of Nutrition, 128, 220–223. [DOI] [PubMed] [Google Scholar]
- DGE (Deutsche Gesellschaft für Ernährung e. V.), 2012. Ernährungsbericht 2012. Bonn, Germany. 432 pp. [Google Scholar]
- DH (Department of Health), 1991. Dietary Reference Values for food energy and nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. HMSO, London, UK, 212 pp. [PubMed]
- Dickson RC, Stubbs TM and Lazarchick J, 1994. Antenatal vitamin K therapy of the low‐birth‐weight infant. American Journal of Obstetrics and Gynecology, 170, 85–89. [DOI] [PubMed] [Google Scholar]
- Dolnikowski GG, Sun Z, Grusak MA, Peterson JW and Booth SL, 2002. HPLC and GC/MS determination of deuterated vitamin K (phylloquinone) in human serum after ingestion of deuterium‐labeled broccoli. Journal of Nutritional Biochemistry, 13, 168–174. [DOI] [PubMed] [Google Scholar]
- Duello TJ and Matschiner JT, 1972. Characterization of vitamin K from human liver. Journal of Nutrition, 102, 331–335. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Report on the development of a food classification and description system for exposure assessment and guidance on its implementation and use. EFSA Journal 2011;9(12):2489, 84 pp. 10.2903/j.efsa.2011.2489 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Use of the EFSA Comprehensive European Food Consumption Database in exposure assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013a. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA Journal 2013;11(10):3408, 103 pp. 10.2903/j.efsa.2013.3408 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products Nutrition and Allergies), 2013b. Scientific Opinion on Dietary Reference Values for energy. EFSA Journal 2013;11(1):3005, 112 pp. 10.2903/j.efsa.2013.3005 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014. Scientific Opinion on Dietary Reference Values for selenium. EFSA Journal 2014;12(10):3846, 66 pp. 10.2903/j.efsa.2014.3846 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015a. Scientific Opinion on Dietary Reference Values for vitamin E as α‐tocopherol. EFSA Journal 2015;13(7):4149, 72 pp. 10.2903/j.efsa.2015.4149 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015b. Scientific Opinion on Dietary Reference Values for calcium. EFSA Journal 2015;13(5):4101, 82 pp. 10.2903/j.efsa.2015.4101 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016. Scientific Opinion on Dietary Reference Values for vitamin D. EFSA Journal 2016;14(10):4547, 145 pp. 10.2903/j.efsa.2016.4547 [DOI] [Google Scholar]
- Elder SJ, Haytowitz DB, Howe J, Peterson JW and Booth SL, 2006. Vitamin k contents of meat, dairy, and fast food in the U.S. Diet. Journal of Agricultural and Food Chemistry, 54, 463–467. [DOI] [PubMed] [Google Scholar]
- Elmadfa I, Godina‐Zarfl B, Dichtl M and König JS, 1994. The Austrian Study on nutritional status of 6‐ to 18‐year‐old pupils. Bibliotheca Nutritio et Dieta, 62–67. [DOI] [PubMed] [Google Scholar]
- Elmadfa I, Hasenegger V, Wagner K, Putz P, Weidl NM, Wottawa D, Kuen T, Seiringer G, Meyer AL, Sturtzel B, Kiefer I, Zilberszac A, Sgarabottolo V, Meidlinger B and Rieder A, 2012. Österreichischer Ernährungsbericht 2012. Institut für Ernährungswissenschaften, Universität Wien, Bundesministerium für Gesundheit, Österreichischer Agentur für Gesundheit und Ernährungssicherheit GmbH (AGES), 412 pp.
- Emaus N, Gjesdal CG, Almas B, Christensen M, Grimsgaard AS, Berntsen GK, Salomonsen L and Fonnebo V, 2010. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double‐blind placebo‐controlled trial. Osteoporosis International, 21, 1731–1740. [DOI] [PubMed] [Google Scholar]
- Erkkila AT, Lichtenstein AH, Dolnikowski GG, Grusak MA, Jalbert SM, Aquino KA, Peterson JW and Booth SL, 2004. Plasma transport of vitamin K in men using deuterium‐labeled collard greens. Metabolism: Clinical and Experimental, 53, 215–221. [DOI] [PubMed] [Google Scholar]
- Erkkila AT, Booth SL, Hu FB, Jacques PF, Manson JE, Rexrode KM, Stampfer MJ and Lichtenstein AH, 2005. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. European Journal of Clinical Nutrition, 59, 196–204. [DOI] [PubMed] [Google Scholar]
- Erkkila AT, Booth SL, Hu FB, Jacques PF and Lichtenstein AH, 2007. Phylloquinone intake and risk of cardiovascular diseases in men. Nutrition Metabolism and Cardiovascular Diseases, 17, 58–62. [DOI] [PubMed] [Google Scholar]
- Ferland G and Sadowski JA, 1992. Vitamin K1 (phylloquinone) content of edible oils: effects of heating and light exposure. Journal of Agricultural and Food Chemistry, 40, 1869–1873. [Google Scholar]
- Ferland G, MacDonald DL and Sadowski JA, 1992. Development of a diet low in vitamin K‐1 (phylloquinone). Journal of the American Dietetic Association, 92, 593–597. [PubMed] [Google Scholar]
- Ferland G, Sadowski JA and O'Brien ME, 1993. Dietary induced subclinical vitamin K deficiency in normal human subjects. Journal of Clinical Investigation, 91, 1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland G, 1998. The vitamin K‐dependent proteins: an update. Nutrition Reviews, 56, 223–230. [DOI] [PubMed] [Google Scholar]
- Ferreira DW, Haytowitz DB, Tassinari MA, Peterson JW and Booth SL, 2006. Vitamin K contents of grains, cereals, fast‐food breakfasts, and baked goods. Journal of Food Science, 71, S66–S70. [Google Scholar]
- Feskanich D, Weber P, Willett WC, Rockett H, Booth SL and Colditz GA, 1999. Vitamin K intake and hip fractures in women: a prospective study. American Journal of Clinical Nutrition, 69, 74–79. [DOI] [PubMed] [Google Scholar]
- Fomon SJ, 2001. Feeding normal infants: rationale for recommendations. Journal of the American Dietetic Association, 101, 1002–1005. [DOI] [PubMed] [Google Scholar]
- Fournier B, Sann L, Guillaumont M and Leclercq M, 1987. Variations of phylloquinone concentration in human milk at various stages of lactation and in cow's milk at various seasons. American Journal of Clinical Nutrition, 45, 551–558. [DOI] [PubMed] [Google Scholar]
- Frick PG, Riedler G and Brogli H, 1967. Dose response and minimal daily requirement for vitamin K in man. Journal of Applied Physiology, 23, 387–389. [DOI] [PubMed] [Google Scholar]
- FSA (Food Standards Agency), 2002. McCance & Widdowson's the composition of foods. 6th summary Edition. Royal Society of Chemistry, Cambridge, UK, 568 pp. [Google Scholar]
- Fu X, Peterson JW, Hdeib M, Booth SL, Grusak MA, Lichtenstein AH and Dolnikowski GG, 2009. Measurement of deuterium‐labeled phylloquinone in plasma by high‐performance liquid chromatography/mass spectrometry. Analytical Chemistry, 81, 5421–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Shen X, Finnan EG, Haytowitz DB and Booth SL, 2016. Measurement of multiple vitamin K forms in processed and fresh‐cut pork products in the U.S. food supply. Journal of Agricultural and Food Chemistry, 64, 4531–4535. [DOI] [PubMed] [Google Scholar]
- Fujita K, Kakuya F and Ito S, 1993. Vitamin K1 and K2 status and faecal flora in breast fed and formula fed 1‐month‐old infants. European Journal of Pediatrics, 152, 852–855. [DOI] [PubMed] [Google Scholar]
- Furie B, Bouchard BA and Furie BC, 1999. Vitamin K‐dependent biosynthesis of gamma‐carboxyglutamic acid. Blood, 93, 1798–1808. [PubMed] [Google Scholar]
- Fusaro M, Noale M, Viola V, Galli F, Tripepi G, Vajente N, Plebani M, Zaninotto M, Guglielmi G, Miotto D, Dalle Carbonare L, D'Angelo A, Naso A, Grimaldi C, Miozzo D, Giannini S, Gallieni M and Investigators VIKIDS, 2012. Vitamin K, vertebral fractures, vascular calcifications, and mortality: VItamin K Italian (VIKI) dialysis study. Journal of Bone and Mineral Research, 27, 2271–2278. [DOI] [PubMed] [Google Scholar]
- Garber AK, Binkley NC, Krueger DC and Suttie JW, 1999. Comparison of phylloquinone bioavailability from food sources or a supplement in human subjects. Journal of Nutrition, 129, 1201–1203. [DOI] [PubMed] [Google Scholar]
- Garcia AA and Reitsma PH, 2008. VKORC1 and the vitamin K cycle. Vitamins and Hormones, 78, 23–33. [DOI] [PubMed] [Google Scholar]
- Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH and van der Schouw YT, 2009. A high menaquinone intake reduces the incidence of coronary heart disease. Nutrition Metabolism and Cardiovascular Diseases, 19, 504–510. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A and Witteman JC, 2004. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. Journal of Nutrition, 134, 3100–3105. [DOI] [PubMed] [Google Scholar]
- Gentili A, Cafolla A, Gasperi T, Bellante S, Caretti F, Curini R and Fernandez VP, 2014. Rapid, high performance method for the determination of vitamin K(1), menaquinone‐4 and vitamin K(1) 2,3‐epoxide in human serum and plasma using liquid chromatography‐hybrid quadrupole linear ion trap mass spectrometry. Journal of Chromatography A, 1338, 102–110. [DOI] [PubMed] [Google Scholar]
- Gijsbers BL, Jie KS and Vermeer C, 1996. Effect of food composition on vitamin K absorption in human volunteers. British Journal of Nutrition, 76, 223–229. [DOI] [PubMed] [Google Scholar]
- Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY and Buring JE, 2007. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women's Health Study. Circulation, 116, 1497–1503. [DOI] [PubMed] [Google Scholar]
- Goodstadt L and Ponting CP, 2004. Vitamin K epoxide reductase: homology, active site and catalytic mechanism. Trends in Biochemical Sciences, 29, 289–292. [DOI] [PubMed] [Google Scholar]
- Green G, Poller L, Thomson JM and Dymock IW, 1976. Factor VII as a marker of hepatocellular synthetic function in liver disease. Journal of Clinical Pathology, 29, 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer FR, Mummah‐Schendel LL, Marshall S and Suttie JW, 1988. Vitamin K1 (phylloquinone) and vitamin K2 (menaquinone) status in newborns during the first week of life. Pediatrics, 81, 137–140. [PubMed] [Google Scholar]
- Greer FR and Zachman RD, 1998. Neonatal vitamin metabolism: fat soluble. In: Cowett RM (ed.). Principles of Perinatal‐Neonatal Metabolism. Springer–Verlag, New York, USA. pp. 943–975. [Google Scholar]
- Greer FR, 2010. Vitamin K the basics–what's new? Early Human Development, 86(Suppl 1), 43–47. [DOI] [PubMed] [Google Scholar]
- Greer FR, Marshall S, Cherry J and Suttie JW, 1991. Vitamin K status of lactating mothers, human milk, and breast‐feeding infants. Pediatrics, 88, 751–756. [PubMed] [Google Scholar]
- Greer FR, 1995. The importance of vitamin K as a nutrient during the first year of life. Nutrition Research, 15, 289–310. [Google Scholar]
- Greer FR, Marshall SP, Foley AL and Suttie JW, 1997. Improving the vitamin K status of breastfeeding infants with maternal vitamin K supplements. Pediatrics, 99, 88–92. [DOI] [PubMed] [Google Scholar]
- Greer FR, Marshall SP, Severson RR, Smith DA, Shearer MJ, Pace DG and Joubert PH, 1998. A new mixed micellar preparation for oral vitamin K prophylaxis: randomised controlled comparison with an intramuscular formulation in breast fed infants. Archives of Disease in Childhood, 79, 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Cho WK, Lezhneva L, Falk J, Krupinska K, Shinozaki K, Seki M, Herrmann RG and Meurer J, 2006. A plant locus essential for phylloquinone (vitamin K1) biosynthesis originated from a fusion of four eubacterial genes. Journal of Biological Chemistry, 281, 17189–17196. [DOI] [PubMed] [Google Scholar]
- Hanck A and Weiser H, 1983. Physiological and pharmacological effects of vitamin K. International Journal for Vitamin and Nutrition Research, Supplement, 24, 155–170. [PubMed] [Google Scholar]
- Hansen KN, Minousis M and Ebbesen F, 2003. Weekly oral vitamin K prophylaxis in Denmark. Acta Paediatrica, 92, 802–805. [PubMed] [Google Scholar]
- Hansson K and Stenflo J, 2005. Post‐translational modifications in proteins involved in blood coagulation. Journal of Thrombosis and Haemostasis, 3, 2633–2648. [DOI] [PubMed] [Google Scholar]
- Haraikawa M, Tsugawa N, Sogabe N, Tanabe R, Kawamura Y, Okano T, Hosoi T and Goseki‐Sone M, 2013. Effects of gamma‐glutamyl carboxylase gene polymorphism (R325Q) on the association between dietary vitamin K intake and gamma‐carboxylation of osteocalcin in young adults. Asia Pacific Journal of Clinical Nutrition, 22, 646–654. [DOI] [PubMed] [Google Scholar]
- Haroon Y, Shearer MJ, Rahim S, Gunn WG, McEnery G and Barkhan P, 1982. The content of phylloquinone (vitamin K1) in human milk, cows’ milk and infant formula foods determined by high‐performance liquid chromatography. Journal of Nutrition, 112, 1105–1117. [DOI] [PubMed] [Google Scholar]
- Harrington DJ, Soper R, Edwards C, Savidge GF, Hodges SJ and Shearer MJ, 2005. Determination of the urinary aglycone metabolites of vitamin K by HPLC with redox‐mode electrochemical detection. Journal of Lipid Research, 46, 1053–1060. [DOI] [PubMed] [Google Scholar]
- Harrington DJ, Booth SL, Card DJ and Shearer MJ, 2007. Excretion of the urinary 5C‐ and 7C‐aglycone metabolites of vitamin K by young adults responds to changes in dietary phylloquinone and dihydrophylloquinone intakes. Journal of Nutrition, 137, 1763–1768. [DOI] [PubMed] [Google Scholar]
- Harrington DJ, Clarke P, Card DJ, Mitchell SJ and Shearer MJ, 2010. Urinary excretion of vitamin K metabolites in term and preterm infants: relationship to vitamin K status and prophylaxis. Pediatric Research, 68, 508–512. [DOI] [PubMed] [Google Scholar]
- Hayes A, Hennessy A, Walton J, McNulty BA, Lucey AJ, Kiely M, Flynn A and Cashman KD, 2016. Phylloquinone intakes and food sources and vitamin K status in a nationally representative sample of Irish adults. Journal of Nutrition, 146, 2274–2280. [DOI] [PubMed] [Google Scholar]
- Heinonen M, Kärkkäinen M, Riuttamäki MA, Piironen V, Lampi AM, Ollilainen V and Lamberg‐Allardt C, 2012. Literature search and review related to specific preparatory work in the establishment of Dietary Reference Values. Preparation of an evidence report identifying health outcomes upon which Dietary Reference Values could potentially be based for vitamins A, C, E, and K. Project developed on the procurement project CT/EFSA/NDA/2010/02. EFSA Supporting publication 2012:EN‐256, 331 pp. [Google Scholar]
- Helldán A, Raulio S, Kosola M, Tapanainen H, Ovaskainen ML and Virtanen S, 2013. Finravinto 2012 ‐ tutkimus ‐ The National FINDIET 2012 Survey. THL. Raportti 16/2013, 217 pp.
- Hirauchi K, Sakano T, Notsumoto S, Nagaoka T, Morimoto A, Fujimoto K, Masuda S and Suzuki Y, 1989. Measurement of K vitamins in animal tissues by high‐performance liquid chromatography with fluorimetric detection. Journal of Chromatography, 497, 131–137. [DOI] [PubMed] [Google Scholar]
- Hodges SJ, Akesson K, Vergnaud P, Obrant K and Delmas PD, 1993a. Circulating levels of vitamins K1 and K2 decreased in elderly women with hip fracture. Journal of Bone and Mineral Research, 8, 1241–1245. [DOI] [PubMed] [Google Scholar]
- Hodges SJ, Bejui J, Leclercq M and Delmas PD, 1993b. Detection and measurement of vitamins K1 and K2 in human cortical and trabecular bone. Journal of Bone and Mineral Research, 8, 1005–1008. [DOI] [PubMed] [Google Scholar]
- Hogenbirk K, Peters M, Bouman P, Sturk A and Buller HA, 1993. The effect of formula versus breast feeding and exogenous vitamin K1 supplementation on circulating levels of vitamin K1 and vitamin K‐dependent clotting factors in newborns. European Journal of Pediatrics, 152, 72–74. [DOI] [PubMed] [Google Scholar]
- Hoppu U, Lehtisalo J, Kujala J, Keso T, Garam S, Tapanainen H, Uutela A, Laatikainen T, Rauramo U and Pietinen P, 2010. The diet of adolescents can be improved by school intervention. Public Health Nutrition, 13, 973–979. [DOI] [PubMed] [Google Scholar]
- Hoskin FC, Spinks JW and Jaques LB, 1954. Urinary excretion products of menadione (vitamin K3). Canadian Journal of Biochemistry and Physiology, 32, 240–250. [PubMed] [Google Scholar]
- Iioka H, Moriyama IS, Morimoto K, Akada S, Hisanaga H, Ishihara Y and Ichijo M, 1991. Pharmacokinetics of vitamin K in mothers and children in the perinatal period: transplacental transport of vitamin K2 (MK‐4). Asia‐Oceania Journal of Obstetrics and Gynaecology, 17, 97–100. [DOI] [PubMed] [Google Scholar]
- Inaba N, Sato T and Yamashita T, 2015. Low‐dose daily intake of vitamin K(2) (menaquinone‐7) improves osteocalcin gamma‐carboxylation: a double‐blind, randomized controlled trials. Journal of Nutritional Science and Vitaminology, 61, 471–480. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine), 2001. Dietary reference intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press, Washington, DC, USA. 773 pp. [PubMed] [Google Scholar]
- Jakob E and Elmadfa I, 1996. Application of a simplified HPLC assay for the determination of phylloquinone (vitamin K1) in animal and plant food items. Food Chemistry, 56, 87–91. [Google Scholar]
- Jie KS, Bots ML, Vermeer C, Witteman JC and Grobbee DE, 1995. Vitamin K intake and osteocalcin levels in women with and without aortic atherosclerosis: a population‐based study. Atherosclerosis, 116, 117–123. [DOI] [PubMed] [Google Scholar]
- Jones DY, Koonsvitsky BP, Ebert ML, Jones MB, Lin PY, Will BH and Suttie JW, 1991. Vitamin K status of free‐living subjects consuming olestra. American Journal of Clinical Nutrition, 53, 943–946. [DOI] [PubMed] [Google Scholar]
- Jones KS, Bluck LJ, Wang LY and Coward WA, 2008. A stable isotope method for the simultaneous measurement of vitamin K1 (phylloquinone) kinetics and absorption. European Journal of Clinical Nutrition, 62, 1273–1281. [DOI] [PubMed] [Google Scholar]
- Jones KS, Bluck LJ, Wang LY, Stephen AM, Prynne CJ and Coward WA, 2009. The effect of different meals on the absorption of stable isotope‐labelled phylloquinone. British Journal of Nutrition, 102, 1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juanola‐Falgarona M, Salas‐Salvado J, Estruch R, Portillo MP, Casas R, Miranda J, Martinez‐Gonzalez MA and Bullo M, 2013. Association between dietary phylloquinone intake and peripheral metabolic risk markers related to insulin resistance and diabetes in elderly subjects at high cardiovascular risk. Cardiovascular Diabetology, 12, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juanola‐Falgarona M, Salas‐Salvado J, Martinez‐Gonzalez MA, Corella D, Estruch R, Ros E, Fito M, Aros F, Gomez‐Gracia E, Fiol M, Lapetra J, Basora J, Lamuela‐Raventos RM, Serra‐Majem L, Pinto X, Munoz MA, Ruiz‐Gutierrez V, Fernandez‐Ballart J and Bullo M, 2014. Dietary intake of vitamin K is inversely associated with mortality risk. Journal of Nutrition, 144, 743–750. [DOI] [PubMed] [Google Scholar]
- Kalkwarf HJ, Khoury JC, Bean J and Elliot JG, 2004. Vitamin K, bone turnover, and bone mass in girls. American Journal of Clinical Nutrition, 80, 1075–1080. [DOI] [PubMed] [Google Scholar]
- Kamali F, Edwards C, Wood P, Wynne HA and Kesteven P, 2001. Temporal variations in plasma vitamin K and lipid concentrations and clotting factor activity in humans. American Journal of Hematology, 68, 159–163. [DOI] [PubMed] [Google Scholar]
- Kamao M, Tsugawa N, Suhara Y, Wada A, Mori T, Murata K, Nishino R, Ukita T, Uenishi K, Tanaka K and Okano T, 2007a. Quantification of fat‐soluble vitamins in human breast milk by liquid chromatography‐tandem mass spectrometry. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 859, 192–200. [DOI] [PubMed] [Google Scholar]
- Kamao M, Suhara Y, Tsugawa N, Uwano M, Yamaguchi N, Uenishi K, Ishida H, Sasaki S and Okano T, 2007b. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. Journal of Nutritional Science and Vitaminology, 53, 464–470. [DOI] [PubMed] [Google Scholar]
- Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Y, Mizuno Y, Hoshino S, Miyao M, Inoue S, Horiki K, Shiraki M, Ouchi Y and Orimo H, 2001. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip‐fracture risk. Nutrition, 17, 315–321. [DOI] [PubMed] [Google Scholar]
- Kaneki M, Hosoi T, Ouchi Y and Orimo H, 2006. Pleiotropic actions of vitamin K: protector of bone health and beyond? Nutrition, 22, 845–852. [DOI] [PubMed] [Google Scholar]
- Kanellakis S, Moschonis G, Tenta R, Schaafsma A, van den Heuvel EG, Papaioannou N, Lyritis G and Manios Y, 2012. Changes in parameters of bone metabolism in postmenopausal women following a 12‐month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K(1)) or menaquinone‐7 (vitamin K (2)): the Postmenopausal Health Study II. Calcified Tissue International, 90, 251–262. [DOI] [PubMed] [Google Scholar]
- Kayata S, Kindberg C, Greer FR and Suttie JW, 1989. Vitamin K1 and K2 in infant human liver. Journal of Pediatric Gastroenterology and Nutrition, 8, 304–307. [DOI] [PubMed] [Google Scholar]
- Kazzi NJ, Ilagan NB, Liang KC, Kazzi GM, Grietsell LA and Brans YW, 1990. Placental transfer of vitamin K1 in preterm pregnancy. Obstetrics and Gynecology, 75, 334–337. [PubMed] [Google Scholar]
- Kersting M and Clausen K, 2003. Ernährungsphysiologische Auswertung einer repräsentativen Verzehrsstudie bei Säuglingen und Kleinkindern VELS mit dem Instrumentarium der DONALD Studie. Forschungsinstitut für Kinderernährung Dortmund, 103 pp.
- Kim ES, Kim MS, Na WR and Sohn CM, 2013. Estimation of vitamin K intake in Koreans and determination of the primary vitamin K‐containing food sources based on the fifth Korean National Health and Nutrition Examination Survey (2010–2011). Nutrition Research and Practice, 7, 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Nakagawa K, Narusawa K, Goseki‐Sone M, Fukushi‐Irie M, Mizoi L, Yoshida H, Okano T, Nakamura T, Suzuki T, Inoue S, Orimo H, Ouchi Y and Hosoi T, 2007. A functional single nucleotide polymorphism in the vitamin‐K‐dependent gamma‐glutamyl carboxylase gene (Arg325Gln) is associated with bone mineral density in elderly Japanese women. Bone, 40, 451–456. [DOI] [PubMed] [Google Scholar]
- Knapen MH, Nieuwenhuijzen Kruseman AC, Wouters RS and Vermeer C, 1998. Correlation of serum osteocalcin fractions with bone mineral density in women during the first 10 years after menopause. Calcified Tissue International, 63, 375–379. [DOI] [PubMed] [Google Scholar]
- Knapen MH, Schurgers LJ and Vermeer C, 2007. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporosis International, 18, 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen MH, Drummen NE, Smit E, Vermeer C and Theuwissen E, 2013. Three‐year low‐dose menaquinone‐7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporosis International, 24, 2499–2507. [DOI] [PubMed] [Google Scholar]
- Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP and Vermeer C, 2015. Menaquinone‐7 supplementation improves arterial stiffness in healthy postmenopausal women. A double‐blind randomised clinical trial. Thrombosis and Haemostasis, 113, 1135–1144. [DOI] [PubMed] [Google Scholar]
- Kohlmeier M, Saupe J, Drossel HJ and Shearer MJ, 1995. Variation of phylloquinone (vitamin K1) concentrations in hemodialysis patients. Thrombosis and Haemostasis, 74, 1252–1254. [PubMed] [Google Scholar]
- Kohlmeier M, Salomon A, Saupe J and Shearer MJ, 1996. Transport of vitamin K to bone in humans. Journal of Nutrition, 126, 1192S–1196S. [DOI] [PubMed] [Google Scholar]
- Koitaya N, Ezaki J, Nishimuta M, Yamauchi J, Hashizume E, Morishita K, Miyachi M, Sasaki S and Ishimi Y, 2009. Effect of low dose vitamin K2 (MK‐4) supplementation on bio‐indices in postmenopausal Japanese women. Journal of Nutritional Science and Vitaminology, 55, 15–21. [DOI] [PubMed] [Google Scholar]
- Koivu‐Tikkanen TJ, Ollilainen V and Piironen VI, 2000. Determination of phylloquinone and menaquinones in animal products with fluorescence detection after postcolumn reduction with metallic zinc. Journal of Agricultural and Food Chemistry, 48, 6325–6331. [DOI] [PubMed] [Google Scholar]
- Kojima T, Asoh M, Yamawaki N, Kanno T, Hasegawa H and Yonekubo A, 2004. Vitamin K concentrations in the maternal milk of Japanese women. Acta Paediatrica, 93, 457–463. [PubMed] [Google Scholar]
- Kurilich AC, Britz SJ, Clevidence BA and Novotny JA, 2003. Isotopic labeling and LC‐APCI‐MS quantification for investigating absorption of carotenoids and phylloquinone from kale (Brassica oleracea). Journal of Agricultural and Food Chemistry, 51, 4877–4883. [DOI] [PubMed] [Google Scholar]
- Lamon‐Fava S, Sadowski JA, Davidson KW, O'Brien ME, McNamara JR and Schaefer EJ, 1998. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. American Journal of Clinical Nutrition, 67, 1226–1231. [DOI] [PubMed] [Google Scholar]
- Landes N, Pfluger P, Kluth D, Birringer M, Ruhl R, Bol GF, Glatt H and Brigelius‐Flohe R, 2003. Vitamin E activates gene expression via the pregnane X receptor. Biochemical Pharmacology, 65, 269–273. [DOI] [PubMed] [Google Scholar]
- Lemke G, 2013. Biology of the TAM receptors. Cold Spring Harbor Perspectives in Biology, 5, a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ and Rosenberg PA, 2003. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. Journal of Neuroscience, 23, 5816–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lange LA, Li X, Susswein L, Bryant B, Malone R, Lange EM, Huang TY, Stafford DW and Evans JP, 2006. Polymorphisms in the VKORC1 gene are strongly associated with warfarin dosage requirements in patients receiving anticoagulation. Journal of Medical Genetics, 43, 740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska DJ and Suttie JW, 1988. Location of gamma‐carboxyglutamyl residues in partially carboxylated prothrombin preparations. Biochemistry, 27, 8636–8641. [DOI] [PubMed] [Google Scholar]
- Litwack G, 2008. Vitamin K. Vitamins and hormones. Academic Press, Elsevier, New York, NY, USA. 300 pp. [Google Scholar]
- Liu G and Peacock M, 1998. Age‐related changes in serum undercarboxylated osteocalcin and its relationships with bone density, bone quality, and hip fracture. Calcified Tissue International, 62, 286–289. [DOI] [PubMed] [Google Scholar]
- Liu YP, Gu YM, Thijs L, Knapen MH, Salvi E, Citterio L, Petit T, Carpini SD, Zhang Z, Jacobs L, Jin Y, Barlassina C, Manunta P, Kuznetsova T, Verhamme P, Struijker‐Boudier HA, Cusi D, Vermeer C and Staessen JA, 2015. Inactive matrix Gla protein is causally related to adverse health outcomes: a Mendelian randomization study in a Flemish population. Hypertension, 65, 463–470. [DOI] [PubMed] [Google Scholar]
- Losito R, Owen CA Jr and Flock EV, 1967. Metabolism of [14C]menadione. Biochemistry, 6, 62–68. [DOI] [PubMed] [Google Scholar]
- Lukacs JL, Booth S, Kleerekoper M, Ansbacher R, Rock CL and Reame NE, 2006. Differential associations for menopause and age in measures of vitamin K, osteocalcin, and bone density: a cross‐sectional exploratory study in healthy volunteers. Menopause, 13, 799–808. [DOI] [PubMed] [Google Scholar]
- Macdonald HM, McGuigan FE, Lanham‐New SA, Fraser WD, Ralston SH and Reid DM, 2008. Vitamin K1 intake is associated with higher bone mineral density and reduced bone resorption in early postmenopausal Scottish women: no evidence of gene‐nutrient interaction with apolipoprotein E polymorphisms. American Journal of Clinical Nutrition, 87, 1513–1520. [DOI] [PubMed] [Google Scholar]
- Mandelbrot L, Guillaumont M, Leclercq M, Lefrere JJ, Gozin D, Daffos F and Forestier F, 1988. Placental transfer of vitamin K1 and its implications in fetal hemostasis. Thrombosis and Haemostasis, 60, 39–43. [PubMed] [Google Scholar]
- Manoury E, Jourdon K, Boyaval P and Fourcassie P, 2013. Quantitative measurement of vitamin K2 (menaquinones) in various fermented dairy products using a reliable high‐performance liquid chromatography method. Journal of Dairy Science, 96, 1335–1346. [DOI] [PubMed] [Google Scholar]
- Manzotti P, De Nisi P and Zocchi G, 2008. Vitamin K in plants. Functional Plant Science and Biotechnology, 2, 29–35. [Google Scholar]
- Mariani G, Herrmann FH, Schulman S, Batorova A, Wulff K, Etro D, Dolce A, Auerswald G, Astermark J, Schved JF, Ingerslev J and Bernardi F and International Factor VIIDSG, 2003. Thrombosis in inherited factor VII deficiency. Journal of Thrombosis and Haemostasis, 1, 2153–2158. [DOI] [PubMed] [Google Scholar]
- Matsuzaka T, Yoshinaga M and Tsuji Y, 1987. Prophylaxis of intracranial hemorrhage due to vitamin K deficiency in infants. Brain and Development, 9, 305–308. [DOI] [PubMed] [Google Scholar]
- Mayer O Jr, Seidlerova J, Wohlfahrt P, Filipovsky J, Vanek J, Cifkova R, Windrichova J, Topolcan O, Knapen MH, Drummen NE and Vermeer C, 2016. Desphospho‐uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. Journal of Human Hypertension, 30, 418–423. [DOI] [PubMed] [Google Scholar]
- McBurney A, Shearer MJ and Barkhan P, 1980. Preparative isolation and characterization of the urinary aglycones of vitamin K1 (phylloquinone) in man. Biochemical Medicine, 24, 250–267. [DOI] [PubMed] [Google Scholar]
- McCann JC and Ames BN, 2009. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? American Journal of Clinical Nutrition, 90, 889–907. [DOI] [PubMed] [Google Scholar]
- McDonald MG, Rieder MJ, Nakano M, Hsia CK and Rettie AE, 2009. CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433M variant. Molecular Pharmacology, 75, 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell MA, Fryar CD, Hirsch R and Ogden CL, 2005. Anthropometric reference data for children and adults: U.S. population, 1999–2002. Advance Data, 1–5. [PubMed] [Google Scholar]
- McKeown NM, Jacques PF, Gundberg CM, Peterson JW, Tucker KL, Kiel DP, Wilson PW and Booth SL, 2002. Dietary and nondietary determinants of vitamin K biochemical measures in men and women. Journal of Nutrition, 132, 1329–1334. [DOI] [PubMed] [Google Scholar]
- McNinch AW and Tripp JH, 1991. Haemorrhagic disease of the newborn in the British Isles: two year prospective study. BMJ (Clinical Research Edition), 303, 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensink GB, Heseker H, Richter A, Stahl A and Vohmann C (Robert Koch‐Institut & Universität Paderborn), 2007. Ernährungsstudie als KIGGS‐Modul (EsKiMo). 143 pp.
- Mihatsch WA, Braegger C, Bronsky J, Campoy C, Domellöf M, Fewtrell M, Mis NF, Hojsak I, Hulst J, Indrio F, Lapillonne A, Mlgaard C, Embleton N, van Goudoever J, ESPGHAN Committee on Nutrition , 2016. Prevention of vitamin K deficiency bleeding in newborn infants: a position paper by the ESPGHAN Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 63, 123–129. [DOI] [PubMed] [Google Scholar]
- Moller A, 1989. Food composition tables. Publication No. SC3. The Danish National Food Agency, Copenhagen.
- Montes R, Ruiz de Gaona E, Martinez‐Gonzalez MA, Alberca I and Hermida J, 2006. The c.‐1639G > A polymorphism of the VKORC1 gene is a major determinant of the response to acenocoumarol in anticoagulated patients. British Journal of Haematology, 133, 183–187. [DOI] [PubMed] [Google Scholar]
- Morales WJ, Angel JL, O'Brien WF, Knuppel RA and Marsalisi F, 1988. The use of antenatal vitamin K in the prevention of early neonatal intraventricular hemorrhage. American Journal of Obstetrics and Gynecology, 159, 774–779. [DOI] [PubMed] [Google Scholar]
- Motohara K, Endo F and Matsuda I, 1985. Effect of vitamin K administration on acarboxy prothrombin (PIVKA‐II) levels in newborns. Lancet, 2, 242–244. [DOI] [PubMed] [Google Scholar]
- Motohara K, Takagi S, Endo F, Kiyota Y and Matsuda I, 1990. Oral supplementation of vitamin K for pregnant women and effects on levels of plasma vitamin K and PIVKA‐II in the neonate. Journal of Pediatric Gastroenterology and Nutrition, 11, 32–36. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y and Okano T, 2010. Identification of UBIAD1 as a novel human menaquinone‐4 biosynthetic enzyme. Nature, 468, 117–121. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Aoki M, Watanabe F and Kamimura A, 2014. Low‐dose menaquinone‐4 improves gamma‐carboxylation of osteocalcin in young males: a non‐placebo‐controlled dose‐response study. Nutrition Journal, 13, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Welfare , 2009. Fineli. Finnish food composition database. Release 9. Helsinki. Nutrition Unit. Available online: http://www.fineli.fi/index.php
- NRC (National Research Council), 1989. Recommended Dietary Allowances: 10th Edition. Subcommittee on the Tenth Edition of the RDAs Food and Nutrition Board Commission on Life Sciences National Research Council. National Academy Press, Washington, DC, USA, 302 pp.
- NCM (Nordic Council of Ministers), 2014. Nordic Nutrition Recommendations 2012. Integrating nutrition and physical activity, Copenhagen, Denmark. 627 pp. [Google Scholar]
- Neogi T, Booth SL, Zhang YQ, Jacques PF, Terkeltaub R, Aliabadi P and Felson DT, 2006. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis and Rheumatism, 54, 1255–1261. [DOI] [PubMed] [Google Scholar]
- Netherlands Food and Nutrition Council , 1992. Recommended Dietary Allowances 1989 in the Netherlands. 115 pp.
- Newman P, Bonello F, Wierzbicki AS, Lumb P, Savidge GF and Shearer MJ, 2002. The uptake of lipoprotein‐borne phylloquinone (vitamin K1) by osteoblasts and osteoblast‐like cells: role of heparan sulfate proteoglycans and apolipoprotein E. Journal of Bone and Mineral Research, 17, 426–433. [DOI] [PubMed] [Google Scholar]
- NHANES III , 1988. –1994. Vitamin and mineral supplements data file documentation. Third National Health and Nutrition Examination Survey, 87 pp.
- Niemeier A, Kassem M, Toedter K, Wendt D, Ruether W, Beisiegel U and Heeren J, 2005. Expression of LRP1 by human osteoblasts: a mechanism for the delivery of lipoproteins and vitamin K1 to bone. Journal of Bone and Mineral Research, 20, 283–293. [DOI] [PubMed] [Google Scholar]
- Nimptsch K, Rohrmann S and Linseisen J, 2008. Dietary intake of vitamin K and risk of prostate cancer in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC‐Heidelberg). American Journal of Clinical Nutrition, 87, 985–992. [DOI] [PubMed] [Google Scholar]
- Nimptsch K, Rohrmann S, Kaaks R and Linseisen J, 2010. Dietary vitamin K intake in relation to cancer incidence and mortality: results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC‐Heidelberg). American Journal of Clinical Nutrition, 91, 1348–1358. [DOI] [PubMed] [Google Scholar]
- Novotny JA, Kurilich AC, Britz SJ, Baer DJ and Clevidence BA, 2010. Vitamin K absorption and kinetics in human subjects after consumption of 13C‐labelled phylloquinone from kale. British Journal of Nutrition, 104, 858–862. [DOI] [PubMed] [Google Scholar]
- Obayashi K, Nakamura K, Kawana J, Ogata H, Hanada K, Kurabayashi M, Hasegawa A, Yamamoto K and Horiuchi R, 2006. VKORC1 gene variations are the major contributors of variation in warfarin dose in Japanese patients. Clinical Pharmacology and Therapeutics, 80, 169–178. [DOI] [PubMed] [Google Scholar]
- Ohsaki Y, Shirakawa H, Hiwatashi K, Furukawa Y, Mizutani T and Komai M, 2006. Vitamin K suppresses lipopolysaccharide‐induced inflammation in the rat. Bioscience, Biotechnology, and Biochemistry, 70, 926–932. [DOI] [PubMed] [Google Scholar]
- Oldenburg J, von Brederlow B, Fregin A, Rost S, Wolz W, Eberl W, Eber S, Lenz E, Schwaab R, Brackmann HH, Effenberger W, Harbrecht U, Schurgers LJ, Vermeer C and Muller CR, 2000. Congenital deficiency of vitamin K dependent coagulation factors in two families presents as a genetic defect of the vitamin K‐epoxide‐reductase‐complex. Thrombosis and Haemostasis, 84, 937–941. [PubMed] [Google Scholar]
- Oldenburg J, Marinova M, Muller‐Reible C and Watzka M, 2008. The vitamin K cycle. Vitamins and Hormones, 78, 35–62. [DOI] [PubMed] [Google Scholar]
- Olson RE, 1994. Vitamin K. In: Shils ME, Olson JA, Shilke M (eds.). Modern Nutrition in Health and Disease. Vol. I. Lea & Febiger, Malvern, PA, USA. pp. 342–347. [Google Scholar]
- Olson RE, Chao J, Graham D, Bates MW and Lewis JH, 2002. Total body phylloquinone and its turnover in human subjects at two levels of vitamin K intake. British Journal of Nutrition, 87, 543–553. [DOI] [PubMed] [Google Scholar]
- Ortega RM, Requejo AM, Navia B and López‐Sobaler AM, 2010. Tablas de composición de alimentos por ración media y tamaño de raciones medias. In: Ortega RM, López‐Sobaler AM, Requejo AM, Andrés P (eds.). La composición de los alimentos. Herramienta básica para la valoración nutricional. Complutense, Madrid, Spain. pp. 50–81. [Google Scholar]
- Ortega Anta RM, González‐Rodríguez LG, Navia Lombán B and López‐Sobaler AM, 2014. [Vitamin K adequacy in a representative sample of Spanish adults. Dietary determinants]. Nutrición Hospitalaria, 29, 187–195. [DOI] [PubMed] [Google Scholar]
- Owen RP, Gong L, Sagreiya H, Klein TE and Altman RB, 2010. VKORC1 pharmacogenomics summary. Pharmacogenetics and Genomics, 20, 642–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva SA, Sepe TE, Booth SL, Camilo ME, O'Brien ME, Davidson KW, Sadowski JA and Russell RM, 1998. Interaction between vitamin K nutriture and bacterial overgrowth in hypochlorhydria induced by omeprazole. American Journal of Clinical Nutrition, 68, 699–704. [DOI] [PubMed] [Google Scholar]
- Paul AA, Black AE, Evans J, Cole TJ and Whitehead RG, 1988. Breastmilk intake and growth from two to ten months. Journal of Human Nutrition and Dietetics, 1, 437–450. [Google Scholar]
- Peterson JW, Muzzey KL, Haytowitz D, Exler J, Lemar L and Booth SL, 2002. Phylloquinone (vitamin K1) and dihydrophylloquinone content of fats and oils. Journal of the American Oil Chemists Society, 79, 641–646. [Google Scholar]
- Pietersma‐de Bruyn AL and van Haard PM, 1985. Vitamin K1 in the newborn. Clinica Chimica Acta, 150, 95–101. [DOI] [PubMed] [Google Scholar]
- Pietschnig B, Haschke F, Vanura H, Shearer M, Veitl V, Kellner S and Schuster E, 1993. Vitamin K in breast milk: no influence of maternal dietary intake. European Journal of Clinical Nutrition, 47, 209–215. [PubMed] [Google Scholar]
- Piironen V, Koivu T, Tammisalo O and Mattila P, 1997. Determination of phylloquinone in oils, margarines and butter by high‐performance liquid chromatography with electrochemical detection. Food Chemistry, 59, 473–480. [Google Scholar]
- Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu YP, Drummen NE, Knapen MH, Pechere‐Bertschi A, Paccaud F, Mohaupt M, Vermeer C, Staessen JA, Vogt B, Martin PY, Burnier M and Bochud M, 2015. Inactive matrix Gla‐protein is associated with arterial stiffness in an adult population‐based study. Hypertension, 66, 85–92. [DOI] [PubMed] [Google Scholar]
- Proudfoot D and Shanahan CM, 2006. Molecular mechanisms mediating vascular calcification: role of matrix Gla protein. Nephrology (Carlton), 11, 455–461. [DOI] [PubMed] [Google Scholar]
- Prynne CJ, Paul AA, Mishra GD, Greenberg DC and Wadsworth ME, 2005. Changes in intake of key nutrients over 17 years during adult life of a British birth cohort. British Journal of Nutrition, 94, 368–376. [DOI] [PubMed] [Google Scholar]
- Reddi K, Henderson B, Meghji S, Wilson M, Poole S, Hopper C, Harris M and Hodges SJ, 1995. Interleukin 6 production by lipopolysaccharide‐stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine, 7, 287–290. [DOI] [PubMed] [Google Scholar]
- Rejnmark L, Vestergaard P, Charles P, Hermann AP, Brot C, Eiken P and Mosekilde L, 2006. No effect of vitamin K1 intake on bone mineral density and fracture risk in perimenopausal women. Osteoporosis International, 17, 1122–1132. [DOI] [PubMed] [Google Scholar]
- Rennenberg RJ, van Varik BJ, Schurgers LJ, Hamulyak K, Ten Cate H, Leiner T, Vermeer C, de Leeuw PW and Kroon AA, 2010. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood, 115, 5121–5123. [DOI] [PubMed] [Google Scholar]
- Rettie AE and Tai G, 2006. The pharmocogenomics of warfarin: closing in on personalized medicine. Molecular Interventions, 6, 223–227. [DOI] [PubMed] [Google Scholar]
- Reumann S, 2013. Biosynthesis of vitamin K1 (phylloquinone) by plant peroxisomes and its integration into signaling molecule synthesis pathways. Sub‐Cellular Biochemistry, 69, 213–229. [DOI] [PubMed] [Google Scholar]
- Rietz P, Gloor U and Wiss O, 1970. Menaquinones from human liver and sludge. Internationale Zeitschrift für Vitaminforschung, 40, 351–362. [PubMed] [Google Scholar]
- Roe MA, Bell S, Oseredczuk M, Christensen T, Westenbrink S, Pakkala H, Presser K and Finglas PM, 2013. Updated food composition database for nutrient intake. Project developed on the procurement project CFT/EFSA/DCM/2011/03. EFSA Supporting publication 2013:EN‐355, 21 pp.
- Ronden JE, Drittij‐Reijnders MJ, Vermeer C and Thijssen HH, 1998. Intestinal flora is not an intermediate in the phylloquinone‐menaquinone‐4 conversion in the rat. Biochimica et Biophysica Acta, 1379, 69–75. [DOI] [PubMed] [Google Scholar]
- Ronn SH, Harslof T, Pedersen SB and Langdahl BL, 2016. Vitamin K2 (menaquinone‐7) prevents age‐related deterioration of trabecular bone microarchitecture at the tibia in postmenopausal women. European Journal of Endocrinology, 175, 541–549. [DOI] [PubMed] [Google Scholar]
- Sadowski JA, Hood SJ, Dallal GE and Garry PJ, 1989. Phylloquinone in plasma from elderly and young adults: factors influencing its concentration. American Journal of Clinical Nutrition, 50, 100–108. [DOI] [PubMed] [Google Scholar]
- Sato T, Schurgers LJ and Uenishi K, 2012. Comparison of menaquinone‐4 and menaquinone‐7 bioavailability in healthy women. Nutrition Journal, 11, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food), , 1993. Nutrient and energy intakes for the European Community. Reports of the Scientific Committee for Food, 31st Series. Food ‐ Science and Technique, European Commission, Luxembourg. 248 pp. [Google Scholar]
- SCF (Scientific Committee on Food), 2003a. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Levels of vitamin E. SCF/CS/NUT/UPPLEV/39 Final, 20 pp.
- SCF (Scientific Committee on Food), 2003b. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Vitamin K. SCF/CS/NUT/UPPLEV/32 Final, 12 pp.
- SCF (Scientific Committee on Food), 2003c. Report of the Scientific Committee on Food on the revision of essential requirements of infant formulae and follow‐on formulae. SCF/CS/NUT/IF/65 Final, 211 pp.
- Schaafsma A, Muskiet FA, Storm H, Hofstede GJ, Pakan I and Van der Veer E, 2000. Vitamin D(3) and vitamin K(1) supplementation of Dutch postmenopausal women with normal and low bone mineral densities: effects on serum 25‐hydroxyvitamin D and carboxylated osteocalcin. European Journal of Clinical Nutrition, 54, 626–631. [DOI] [PubMed] [Google Scholar]
- Schmolz L, Birringer M, Lorkowski S and Wallert M, 2016. Complexity of vitamin E metabolism. World Journal of Biological Chemistry, 7, 14–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner C, Suter S, Watzka M, Hertfelder HJ, Schreiner F, Oldenburg J, Bartmann P and Heep A, 2014. Genetic variants of the vitamin K dependent coagulation system and intraventricular hemorrhage in preterm infants. BMC Pediatrics, 14, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiger G, Tonz O, Gruter J and Shearer MJ, 1993. Vitamin K1 concentration in breast‐fed neonates after oral or intramuscular administration of a single dose of a new mixed‐micellar preparation of phylloquinone. Journal of Pediatric Gastroenterology and Nutrition, 16, 435–439. [DOI] [PubMed] [Google Scholar]
- Schubiger G, Gruter J and Shearer MJ, 1997. Plasma vitamin K1 and PIVKA‐II after oral administration of mixed‐micellar or cremophor EL‐solubilized preparations of vitamin K1 to normal breast‐fed newborns. Journal of Pediatric Gastroenterology and Nutrition, 24, 280–284. [DOI] [PubMed] [Google Scholar]
- Schurgers LJ, Geleijnse JM, Grobbee DE, Pols HAP, Hofman A, Witteman JCM and Vermeer C, 1999. Nutritional intake of vitamins K1 (phylloquinone) and K2 (menaquinone) in The Netherlands. Journal of Nutritional and Environmental Medicine, 9, 115–122. [Google Scholar]
- Schurgers LJ and Vermeer C, 2000. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis, 30, 298–307. [DOI] [PubMed] [Google Scholar]
- Schurgers LJ and Vermeer C, 2002. Differential lipoprotein transport pathways of K‐vitamins in healthy subjects. Biochimica et Biophysica Acta, 1570, 27–32. [DOI] [PubMed] [Google Scholar]
- Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H and Vermeer C, 2007. Vitamin K‐containing dietary supplements: comparison of synthetic vitamin K1 and natto‐derived menaquinone‐7. Blood, 109, 3279–3283. [DOI] [PubMed] [Google Scholar]
- Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G and Massy ZA, 2010. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clinical Journal of the American Society of Nephrology, 5, 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D'Agostino RB Sr, Ordovas JM, O'Donnell CJ, Dawson‐Hughes B, Vasan RS and Booth SL, 2009. Genetic and non‐genetic correlates of vitamins K and D. European Journal of Clinical Nutrition, 63, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea MK, O'Donnell CJ, Vermeer C, Magdeleyns EJ, Crosier MD, Gundberg CM, Ordovas JM, Kritchevsky SB and Booth SL, 2011. Circulating uncarboxylated matrix gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. Journal of Nutrition, 141, 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea MK and Booth SL, 2016. Concepts and controversies in evaluating vitamin K status in population‐based studies. Nutrients, 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer MJ and Barkhan P, 1973. Studies on the metabolites of phylloquinone (vitamin K1) in the urine of man. Biochimica et Biophysica Acta, 297, 300–312. [DOI] [PubMed] [Google Scholar]
- Shearer MJ, 1992. Vitamin K metabolism and nutriture. Blood Reviews, 6, 92–104. [DOI] [PubMed] [Google Scholar]
- Shearer MJ, 1995. Vitamin K. Lancet, 345, 229–234. [DOI] [PubMed] [Google Scholar]
- Shearer MJ, Rahim S, Barkhan P and Stimmler L, 1982. Plasma vitamin K1 in mothers and their newborn babies. Lancet, 2, 460–463. [DOI] [PubMed] [Google Scholar]
- Shearer MJ, McCathy PT, Crampton OE and Mattock MB, 1988. The assessment of human vitamin K status from tissue measurements. In: Suttie JW (ed.). Current advances in vitamin K research. New York, NY, USA. pp. 437–452. [Google Scholar]
- Shearer MJ and Newman P, 2014. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK‐4 biosynthesis. Journal of Lipid Research, 55, 345–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer MJ, Barkhan P and Webster GR, 1970a. Absorption and excretion of an oral dose of tritiated vitamin K1 in man. British Journal of Haematology, 18, 297–308. [DOI] [PubMed] [Google Scholar]
- Shearer MJ, Mallinson CN, Webster GR and Barkhan P, 1970b. Absorption of tritiated vitamin K1 in patients with fat malabsorption. Gut, 11, 1063–1064. [PubMed] [Google Scholar]
- Shearer MJ, Mallinson CN, Webster GR and Barkhan P, 1972. Clearance from plasma and excretion in urine, faeces and bile of an intravenous dose of tritiated vitamin K 1 in man. British Journal of Haematology, 22, 579–588. [DOI] [PubMed] [Google Scholar]
- Shearer MJ, McBurney A and Barkhan P, 1974. Studies on the absorption and metabolism of phylloquinone (vitamin K1) in man. Vitamins and Hormones, 32, 513–542. [DOI] [PubMed] [Google Scholar]
- Shearer MJ, Allan V, Haroon Y and Barkhan P, 1980. Nutritional aspects of vitamin K in the human. In: Suttie JW (ed.). Vitamin K metabolism and vitamin K‐dependent protein. University Park Press, Baltimore, MD, USA. pp. 317–327. [Google Scholar]
- Shearer MJ, Bach A and Kohlmeier M, 1996. Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. Journal of Nutrition, 126, 1181S–1186S. [DOI] [PubMed] [Google Scholar]
- Shearer MJ, 2009. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Reviews, 23, 49–59. [DOI] [PubMed] [Google Scholar]
- Shearer MJ, Fu X and Booth SL, 2012. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Advances in Nutrition, 3, 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata E, Ieiri I, Ishiguro S, Aono H, Inoue K, Koide T, Ohgi S and Otsubo K, 2004. Association of pharmacokinetic (CYP2C9) and pharmacodynamic (factors II, VII, IX, and X; proteins S and C; and gamma‐glutamyl carboxylase) gene variants with warfarin sensitivity. Blood, 103, 2630–2635. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, Wang J, Zalucha S, Loberg RD, Pienta KJ and Taichman RS, 2010. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia, 12, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoll LJ, Booth SL, O'Brien ME, Davidson KW, Tsaioun KI and Sadowski JA, 1997. Changes in serum osteocalcin, plasma phylloquinone, and urinary gamma‐carboxyglutamic acid in response to altered intakes of dietary phylloquinone in human subjects. American Journal of Clinical Nutrition, 65, 779–784. [DOI] [PubMed] [Google Scholar]
- Stafford DW, 2005. The vitamin K cycle. Journal of Thrombosis and Haemostasis, 3, 1873–1878. [DOI] [PubMed] [Google Scholar]
- Strehle EM, Howey C and Jones R, 2010. Evaluation of the acceptability of a new oral vitamin K prophylaxis for breastfed infants. Acta Paediatrica, 99, 379–383. [DOI] [PubMed] [Google Scholar]
- Sutor AH, von Kries R, Cornelissen EA, McNinch AW and Andrew M, 1999. Vitamin K deficiency bleeding (VKDB) in infancy. ISTH Pediatric/Perinatal Subcommittee. International Society on Thrombosis and Haemostasis. Thrombosis and Haemostasis, 81, 456–461. [PubMed] [Google Scholar]
- Suttie JW, 1985. Vitamin K. In: Diplock AT (ed.). Fat‐Soluble Vitamins: Their Biochemistry and Applications. Heinemann, London, UK. pp. 225–311. [Google Scholar]
- Suttie JW, 1987. Recent advances in hepatic vitamin K metabolism and function. Hepatology, 7, 367–376. [DOI] [PubMed] [Google Scholar]
- Suttie JW, Mummah‐Schendel LL, Shah DV, Lyle BJ and Greger JL, 1988. Vitamin K deficiency from dietary vitamin K restriction in humans. American Journal of Clinical Nutrition, 47, 475–480. [DOI] [PubMed] [Google Scholar]
- Suttie JW, 1992. Vitamin K and human nutrition. Journal of the American Dietetic Association, 92, 585–590. [PubMed] [Google Scholar]
- Suttie JW, 1996. Vitamin K. In: Ziegler EE and Filer LJ (eds.). Present Knowledge of Nutrition. ILSI Press, Washington DC, USA. pp. 137–145. [Google Scholar]
- Szulc P, Chapuy MC, Meunier PJ and Delmas PD, 1993. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. Journal of Clinical Investigation, 91, 1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc P, Arlot M, Chapuy MC, Duboeuf F, Meunier PJ and Delmas PD, 1994. Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly women. Journal of Bone and Mineral Research, 9, 1591–1595. [DOI] [PubMed] [Google Scholar]
- Szulc P, Chapuy MC, Meunier PJ and Delmas PD, 1996. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture: a three year follow‐up study. Bone, 18, 487–488. [DOI] [PubMed] [Google Scholar]
- Tamatani M, Morimoto S, Nakajima M, Fukuo K, Onishi T, Kitano S, Niinobu T and Ogihara T, 1998. Decreased circulating levels of vitamin K and 25‐hydroxyvitamin D in osteopenic elderly men. Metabolism: Clinical and Experimental, 47, 195–199. [DOI] [PubMed] [Google Scholar]
- Thane CW, Bolton‐Smith C and Coward WA, 2006a. Comparative dietary intake and sources of phylloquinone (vitamin K1) among British adults in 1986‐7 and 2000‐1. British Journal of Nutrition, 96, 1105–1115. [DOI] [PubMed] [Google Scholar]
- Thane CW, Wang LY and Coward WA, 2006b. Plasma phylloquinone (vitamin K1) concentration and its relationship to intake in British adults aged 19–64 years. British Journal of Nutrition, 96, 1116–1124. [DOI] [PubMed] [Google Scholar]
- Thijssen HH and Drittij‐Reijnders MJ, 1994. Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone‐4. British Journal of Nutrition, 72, 415–425. [DOI] [PubMed] [Google Scholar]
- Thijssen HH and Drittij‐Reijnders MJ, 1996. Vitamin K status in human tissues: tissue‐specific accumulation of phylloquinone and menaquinone‐4. British Journal of Nutrition, 75, 121–127. [DOI] [PubMed] [Google Scholar]
- Thijssen HH, Drittij MJ, Vermeer C and Schoffelen E, 2002. Menaquinone‐4 in breast milk is derived from dietary phylloquinone. British Journal of Nutrition, 87, 219–226. [DOI] [PubMed] [Google Scholar]
- Thijssen HH, Vervoort LM, Schurgers LJ and Shearer MJ, 2006. Menadione is a metabolite of oral vitamin K. British Journal of Nutrition, 95, 260–266. [DOI] [PubMed] [Google Scholar]
- Thompson RM, Gerber N, Seibert RA and Desiderio DM, 1972. Identification of 2‐methyl‐1,4‐naphthohydroguinone monoglucuronide as a metabolite of 2‐methyl‐1,4‐naphthoquinone (menadione) in rat bile. Research Communications in Chemical Pathology and Pharmacology, 4, 543–552. [PubMed] [Google Scholar]
- Tie JK, Nicchitta C, von Heijne G and Stafford DW, 2005. Membrane topology mapping of vitamin K epoxide reductase by in vitro translation/cotranslocation. Journal of Biological Chemistry, 280, 16410–16416. [DOI] [PubMed] [Google Scholar]
- Tie JK and Stafford DW, 2008. Structure and function of vitamin K epoxide reductase. Vitamins and Hormones, 78, 103–130. [DOI] [PubMed] [Google Scholar]
- Traber MG, 2008. Vitamin E and K interactions–a 50‐year‐old problem. Nutrition Reviews, 66, 624–629. [DOI] [PubMed] [Google Scholar]
- Tsugawa N, Shiraki M, Suhara Y, Kamao M, Tanaka K and Okano T, 2006. Vitamin K status of healthy Japanese women: age‐related vitamin K requirement for gamma‐carboxylation of osteocalcin. American Journal of Clinical Nutrition, 83, 380–386. [DOI] [PubMed] [Google Scholar]
- Udall JA, 1965. Human sources and absorption of vitamin K in relation to anticoagulation stability. Journal of the American Medical Association, 194, 127–129. [PubMed] [Google Scholar]
- Ueno T and Suttie JW, 1983. High‐pressure liquid chromatographic‐reductive electrochemical detection analysis of serum trans‐phylloquinone. Analytical Biochemistry, 133, 62–67. [DOI] [PubMed] [Google Scholar]
- USDA (US Department of Agriculture), 2007. USDA National Nutrient Database for Standard Reference, Release 21. Agricultural Research Service. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/sr21-home-page/
- USDA (US Department of Agriculture), 2009. USDA National Nutrient Database for Standard Reference, Release 22. Agricultural Research Service. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/sr22-home-page/
- USDA (US Department of Agriculture), 2015. USDA National Nutrient Database for Standard Reference, Release 28. Agricultural Research Service. Available online: https://ndb.nal.usda.gov/ndb/
- Usui Y, Tanimura H, Nishimura N, Kobayashi N, Okanoue T and Ozawa K, 1990. Vitamin K concentrations in the plasma and liver of surgical patients. American Journal of Clinical Nutrition, 51, 846–852. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Schönbeck Y and van Dommelen P, 2012. Collection, collation and analysis of data in relation to reference heights and reference weights for female and male children and adolescents (0‐18 years) in the EU, as well as in relation to the age of onset of puberty and the age at which different stages of puberty are reached in adolescents in the EU. Project developed on the procurement project CT/EFSA/NDA/2010/01. EFSA Supporting publication 2012:EN‐255, 59 pp.
- van den Heuvel EG, van Schoor NM, Lips P, Magdeleyns EJ, Deeg DJ, Vermeer C and den Heijer M, 2014. Circulating uncarboxylated matrix Gla protein, a marker of vitamin K status, as a risk factor of cardiovascular disease. Maturitas, 77, 137–141. [DOI] [PubMed] [Google Scholar]
- van Summeren MJ, Braam LA, Lilien MR, Schurgers LJ, Kuis W and Vermeer C, 2009. The effect of menaquinone‐7 (vitamin K2) supplementation on osteocalcin carboxylation in healthy prepubertal children. British Journal of Nutrition, 102, 1171–1178. [DOI] [PubMed] [Google Scholar]
- Van Winckel M, De Bruyne R, Van De Velde S and Van Biervliet S, 2009. Vitamin K, an update for the paediatrician. European Journal of Pediatrics, 168, 127–134. [DOI] [PubMed] [Google Scholar]
- Vecsler M, Loebstein R, Almog S, Kurnik D, Goldman B, Halkin H and Gak E, 2006. Combined genetic profiles of components and regulators of the vitamin K‐dependent gamma‐carboxylation system affect individual sensitivity to warfarin. Thrombosis and Haemostasis, 95, 205–211. [DOI] [PubMed] [Google Scholar]
- Vergnaud P, Garnero P, Meunier PJ, Breart G, Kamihagi K and Delmas PD, 1997. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. Journal of Clinical Endocrinology and Metabolism, 82, 719–724. [DOI] [PubMed] [Google Scholar]
- Vermeer C and Braam L, 2001. Role of K vitamins in the regulation of tissue calcification. Journal of Bone and Mineral Metabolism, 19, 201–206. [DOI] [PubMed] [Google Scholar]
- Viegas CSB and Simes DC, 2016. Gla‐rich protein (GRP): a new player in the burden of vascular calcification. Journal of Cardiovascular Diseases and Diagnosis, 4, 245. [Google Scholar]
- Villines TC, Hatzigeorgiou C, Feuerstein IM, O'Malley PG and Taylor AJ, 2005. Vitamin K1 intake and coronary calcification. Coronary Artery Disease, 16, 199–203. [DOI] [PubMed] [Google Scholar]
- Vissers LE, Dalmeijer GW, Boer JM, Monique Verschuren WM, van der Schouw YT and Beulens JW, 2013. Intake of dietary phylloquinone and menaquinones and risk of stroke. Journal of American Heart Association, 2, e000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers LE, Dalmeijer GW, Boer JM, Verschuren WM, van der Schouw YT and Beulens JW, 2016. The relationship between vitamin K and peripheral arterial disease. Atherosclerosis, 252, 15–20. [DOI] [PubMed] [Google Scholar]
- von Kries R, Kreppel S, Becker A, Tangermann R and Göbel U, 1987a. Acarboxyprothrombin activity after oral prophylactic vitamin K. Archives of Disease in Childhood, 62, 938–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kries R, Shearer M, McCarthy PT, Haug M, Harzer G and Gobel U, 1987b. Vitamin K1 content of maternal milk: influence of the stage of lactation, lipid composition, and vitamin K1 supplements given to the mother. Pediatric Research, 22, 513–517. [DOI] [PubMed] [Google Scholar]
- von Kries R, Shearer MJ and Gobel U, 1988. Vitamin K in infancy. European Journal of Pediatrics, 147, 106–112. [DOI] [PubMed] [Google Scholar]
- von Kries R, Shearer MJ, Widdershoven J, Motohara K, Umbach G and Gobel U, 1992. Des‐gamma‐carboxyprothrombin (PIVKA II) and plasma vitamin K1 in newborns and their mothers. Thrombosis and Haemostasis, 68, 383–387. [PubMed] [Google Scholar]
- Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, Wallerman O, Melhus H, Wadelius C, Bentley D and Deloukas P, 2005. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics Journal, 5, 262–270. [DOI] [PubMed] [Google Scholar]
- Walther B, Karl JP, Booth SL and Boyaval P, 2013. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Advances in Nutrition, 4, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang W, Zhang Y, Yang Y, Sun L, Hu S, Chen J, Zhang C, Zheng Y, Zhen Y, Sun K, Fu C, Yang T, Wang J, Sun J, Wu H, Glasgow WC and Hui R, 2006. VKORC1 haplotypes are associated with arterial vascular diseases (stroke, coronary heart disease, and aortic dissection). Circulation, 113, 1615–1621. [DOI] [PubMed] [Google Scholar]
- Weber KS, Raab J, Haupt F, Aschemeier B, Wosch A, Ried C, Kordonouri O, Ziegler AG and Winkler C, 2014. Evaluating the diet of children at increased risk for type 1 diabetes: first results from the TEENDIAB study. Public Health Nutrition, 18, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei FF, Drummen NE, Schutte AE, Thijs L, Jacobs L, Petit T, Yang WY, Smith W, Zhang ZY, Gu YM, Kuznetsova T, Verhamme P, Allegaert K, Schutte R, Lerut E, Evenepoel P, Vermeer C and Staessen JA, 2016. Vitamin K dependent protection of renal function in multi‐ethnic population studies. EBioMedicine, 4, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group (World Health Organization), 2006. WHO Child Growth Standards: Length/height‐for‐age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age: Methods and development. 312 pp.
- WHO/FAO (World Health Organization/Food and Agriculture Organization of the United Nations), 2004. Vitamin and mineral requirements in human nutrition: report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21–30 September 1998. 341 pp.
- Will BH, Usui Y and Suttie JW, 1992. Comparative metabolism and requirement of vitamin K in chicks and rats. Journal of Nutrition, 122, 2354–2360. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu S, Davis CH, Stafford DW, Kulman JD and Pedersen LG, 2011. A hetero‐dimer model for concerted action of vitamin K carboxylase and vitamin K reductase in vitamin K cycle. Journal of Theoretical Biology, 279, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Zhou B, Nigdikar S, Wang X, Bennett J and Prentice A, 2005. Effect of apolipoprotein E genotype on vitamin K status in healthy older adults from China and the UK. British Journal of Nutrition, 94, 956–961. [DOI] [PubMed] [Google Scholar]
- Zipursky A, 1999. Prevention of vitamin K deficiency bleeding in newborns. British Journal of Haematology, 104, 430–437. [DOI] [PubMed] [Google Scholar]
- Zwakenberg SR, den Braver NR, Engelen AI, Feskens EJ, Vermeer C, Boer JM, Verschuren WM, van der Schouw YT and Beulens JW, 2016. Vitamin K intake and all‐cause and cause specific mortality. Clinical Nutrition, Aug 30 pii: S0261‐5614(0216)30216‐30213. 30210.31016/j.clnu.32016.30208.30017 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


