Abstract
The Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids of the European Food Safety Authority was requested to deliver a scientific opinion on the implications for human health of the flavouring rum ether [FL‐no: 21.001] in the Flavouring Group Evaluation 500 (FGE.500), according to Regulation (EC) No 1331/2008 and Regulation (EC) No 1334/2008 of the European Parliament and of the Council. Rum ether is a complex mixture of volatile substances obtained by distillation of the reaction products of pyroligneous acid and ethyl alcohol under oxidative conditions in the presence of manganese dioxide and sulfuric acid. A total of 84 volatile constituents have been reported by the applicant. It is a colourless liquid with a rum‐like odour and flavour. Its major uses are in the food categories beverages, confectionery and baked goods. The Panel decided to apply a congeneric group‐based approach. The 84 reported constituents were allocated to 12 congeneric groups, based on structural and metabolic similarity. For eight of the congeneric groups, the Panel concluded that there is no safety concern at the intended conditions of use. However, the Panel concluded that substances in congeneric group 1 (ethanol and acetaldehyde) and congeneric group 12 (furan) are carcinogenic and genotoxic. The Panel also identified genotoxicity concerns for substances in congeneric group 3 (3‐pentene‐2‐one). The exposure for congeneric group 10 (ethers of various structures) was above the Threshold of Toxicological Concern (TTC) applicable for this group, but a point of departure or health based guidance value that covers all the substances in this group could not be identified. The Panel concluded that according to the overall strategy for the risk assessment of flavouring substances, the presence of genotoxic substances as process‐derived constituents of rum ether is of safety concern.
Keywords: rum ether, FGE.500, FL‐no: 21.001, other flavouring, complex flavouring mixture, congeneric group approach
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The use of flavourings is regulated under Regulation (EC) No 1334/20081 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of article 9(e) of this Regulation, an evaluation and approval are required for ‘other flavourings’ referred to in Article 3(2)(h).
Regulation (EC) No 1331/20082 shall apply for the evaluation and approval of ‘other flavourings’.
The Commission has received from the European Flavour Association an application for an authorisation of a new ‘other flavouring’, named rum ether.
In order for the Commission to be able to consider its inclusion in the Union list of flavourings and source materials (Annex I of Regulation (EC) No 1334/2008), the European Food Safety Authority (EFSA) should carry out a safety assessment of this substance.
1.1.2. Terms of Reference
The European Commission requests EFSA to carry out a safety assessment on rum ether as ‘other flavouring’ in accordance with Regulation (EC) No 1331/2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings.
1.2. Existing authorisations and evaluations
In the US, the status ‘Generally Recognised As Safe’ (GRAS) has been allocated to rum ether by the industrial ‘Flavour and Extract Manufactures Association’ (FEMA) expert Panel (FEMA no 2996). The Panel is not aware of any official evaluations of rum ether performed by national or international authorities.
2. Data and methodologies
A dossier with information on the flavouring rum ether has been submitted by the European Flavour Association (EFFA).
The safety assessment of rum ether [FL‐no: 21.001] has been carried out by EFSA in accordance with Commission Regulations (EC) No 1331/2008 and 1334/2008 as well as the procedures outlined in the EFSA scientific opinion: ‘Guidance on the data required for the risk assessment of flavourings to be used in or on foods’ (EFSA CEF Panel, 2010a), Part B. IV. ‘Information to be supplied with an application for the authorisation of Other Flavourings’ (see Appendix C).
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
The flavouring is a complex mixture of volatile substances obtained by distillation of the esterification products of pyroligneous acid and ethyl alcohol, under oxidative conditions in the presence of sulfuric acid and manganese dioxide. Pyroligneous acid, also known as wood vinegar, is obtained by pyrolysis of wood as a by‐product of charcoal production.
Chemical name
There is no single chemical name for the flavouring. The commonly used trivial name is ‘rum ether’. Other names are ‘ethyl oxyhydrate’ and ‘ZV8‐253’. The chemical names of the substances reported by the applicant to be present in rum ether are given in Table 4 and Appendix A.
Table 4.
Rum ether constituents (in total 84) reported in the submissions from February to September 2016
| Congeneric groupa | Cramer Classb | Chemical name | Structural formula | MAX (% of peak area)c | Estimated chronic APET μg/kg bw per dayd | |
|---|---|---|---|---|---|---|
| Adult | Child | |||||
| 1 | III | Saturated linear primary aliphatic alcohols/aldehydes/acids/esters and acetals, including a cyclic acetal | ||||
| 1 | I | Ethyl alcohol |
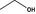
|
83.000 | 745 | 929 |
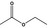
| 1 | I | Ethyl acetate |
|
49.000 | 440 | 548 |
| 1 | I | Ethyl formate |

|
12.210 | 110 | 137 |
| 1 | I | Ethyl propionate |

|
7.470 | 67 | 84 |
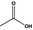
| 1 | I | Acetic acid |
|
5.060 | 45 | 57 |
| 1 | I | Methyl acetate |

|
3.740 | 34 | 42 |
| 1 | I | Methyl alcohol | CH3OH | 1.070 | 9.6 | 12.0 |
| 1 | I | Acetaldehyde dimethylacetal |

|
0.600 | 5.4 | 6.7 |
| 1 | I | Ethyl valerate |

|
1.610 | 14 | 18 |
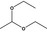
| 1 | I | Acetaldehyde diethylacetal |
|
2.107 | 19 | 24 |
| 1 | I | Formaldehyde diethylacetal |

|
1.640 | 15 | 18 |
| 1 | I | Ethyl butyrate |
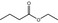
|
1.390 | 12 | 16 |
| 1 | I | Formic acid |

|
0.320 | 2.9 | 3.6 |
| 1 | I | Acetaldehyde |

|
0.361 | 3.2 | 4.0 |
| 1 | I | Methyl propionate |
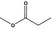
|
0.360 | 3.2 | 4.0 |
| 1 | I | 1‐Butanol |

|
0.120 | 1.1 | 1.3 |
| 1 | I | Butanal diethyl acetal |

|
0.100 | 0.9 | 1.1 |
| 1 | I | Propanoic acid |

|
0.154 | 1.4 | 1.7 |
| 1 | I | Methyl formate |
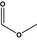
|
0.052 | 0.47 | 0.58 |
| 1 | III | Acetic anhydride |
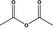
|
0.017 | 0.15 | 0.19 |
| 1 | I | Butyl acetate |

|
0.017 | 0.15 | 0.19 |
| 1 | I | Butanoic acid |

|
0.020 | 0.18 | 0.22 |
| 1 | I | Propyl acetate |

|
0.005 | 0.045 | 0.056 |
| 1 | I | Acetaldehyde ethyl methyl acetal |

|
0.003 | 0.027 | 0.034 |
| 1 | I | Diethyl succinate |

|
0.003 | 0.027 | 0.034 |
| 1 | I | Ethyl hexanoate |
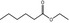
|
0.002 | 0.018 | 0.022 |
| 1 | I | Propanal diethyl acetal |
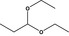
|
0.001 | 0.009 | 0.011 |
| 1 | I | Ethyl nonanoate |
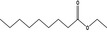
|
0.001 | 0.009 | 0.011 |
| 1 | I | Hexanal diethyl acetal |

|
0.001 | 0.009 | 0.011 |
| 1 | III | 2‐Ethoxytetrahydrofuran |
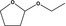
|
0.023 | 0.21 | 0.26 |
| 2 | I | Saturated aliphatic, acyclic, branched‐chain primary alcohols, aldehydes, carboxylic acids and related esters and acetals | ||||
| 2 | I | Isobutanal diethyl acetal |
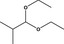
|
0.001 | 0.009 | 0.011 |
| 2 | I | 2‐Methylbutanal diethyl acetal |
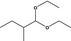
|
0.001 | 0.009 | 0.011 |
| 2 | I | 3‐Methylbutanal diethyl acetal |

|
0.001 | 0.009 | 0.011 |
| 2 | I | Ethyl isovalerate |

|
1.630 | 15 | 18 |
| 2 | I | Ethyl isobutyrate |
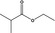
|
0.480 | 4.3 | 5.4 |
| 2 | I | Ethyl 4‐methylpentanoate |

|
0.070 | 0.63 | 0.78 |
| 2 | I | Ethyl 2‐methylbutanoate |

|
0.070 | 0.63 | 0.78 |
| 2 | I | Ethyl 3‐methylpentanoate |

|
0.003 | 0.027 | 0.034 |
| 2 | I | Isobutyl acetate |

|
0.002 | 0.018 | 0.022 |
| 3 | III | α,β‐Unsaturated linear and branched aliphatic primary alcohols/ketones/esters (excluding esters of α,β‐unsaturated carboxylic acids | ||||
| 3 | III | Allyl alcohol |

|
0.017 | 0.15 | 0.19 |
| 3 | II | 2‐Propenyl acetate |

|
0.459 | 4.1 | 5.1 |
| 3 | I | 3‐Penten‐2‐one |

|
0.051 | 0.46 | 0.57 |
| 3 | II | 2‐Methyl‐2‐cyclopentenone |
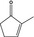
|
0.034 | 0.31 | 0.38 |
| 3 | II | 2‐Cyclopenten‐1‐one |

|
0.006 | 0.054 | 0.067 |
| 4 | II | Ester of an alicyclic carboxylic acid | ||||
| 4 | II | Ethyl cyclopropanecarboxylate |

|
0.080 | 0.72 | 0.90 |
| 5 | III | Esters of unsaturated linear and branched aliphatic carboxylic acids | ||||
| 5 | I | Ethyl crotonate |

|
0.200 | 1.8 | 2.2 |
| 5 | III | Ethyl acrylate |
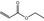
|
0.280 | 2.5 | 3.1 |
| 5 | III | Ethyl methacrylate |

|
0.100 | 0.90 | 1.1 |
| 5 | I | Ethyl 2‐methyl‐2‐butenoate |

|
0.068 | 0.61 | 0.76 |
| 5 | I | Ethyl 4‐pentenoate |

|
0.070 | 0.63 | 0.78 |
| 5 | I | Ethyl pent‐3‐enoate |

|
0.010 | 0.090 | 0.11 |
| 5 | I | Ethyl but‐3‐enoate |
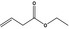
|
0.060 | 0.54 | 0.67 |
| 5 | I | Ethyl 2‐pentenoate |

|
0.050 | 0.45 | 0.56 |
| 5 | I | Ethyl 3‐methyl‐but‐3‐enoate |

|
0.008 | 0.072 | 0.090 |
| 6 | I | Aliphatic primary alcohols, aldehydes, carboxylic acids, acetals and esters containing additional oxygenated functional groups | ||||
| 6 | I | 1,1‐Diethoxyhexan‐2‐one |

|
0.007 | 0.063 | 0.078 |
| 6 | I | Ethyl levulinate |

|
0.007 | 0.063 | 0.078 |
| 6 | I | Acetol |
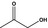
|
0.039 | 0.35 | 0.44 |
| 6 | I | 1,1‐Diethoxyacetone |
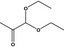
|
0.045 | 0.40 | 0.50 |
| 6 | I | Glyceraldehyde diethyl acetal |

|
0.026 | 0.23 | 0.29 |
| 6 | I | Ethyl lactate |

|
0.027 | 0.24 | 0.30 |
| 6 | I | Ethyl glycolate |

|
0.008 | 0.072 | 0.090 |
| 6 | I | Ethyl pyruvate |

|
0.007 | 0.063 | 0.078 |
| 6 | I | Hydroxyacetaldehyde diethyl acetal |

|
0.014 | 0.13 | 0.16 |
| 7 | I | Saturated aliphatic acyclic ketones | ||||
| 7 | I | Acetone |

|
0.176 | 1.6 | 2.0 |
| 7 | I | 2‐Butanone |

|
0.167 | 1.5 | 1.9 |
| 7 | I | 2‐Pentanone |

|
0.017 | 0.15 | 0.19 |
| 8 | III | Aliphatic α‐diketones and related α‐hydroxyketones | ||||
| 8 | II | Diacetyl |

|
0.520 | 4.7 | 5.8 |
| 8 | III | 2,3‐Pentanedione |

|
0.015 | 0.13 | 0.17 |
| 8 | I | 1‐Hydroxy‐2‐butanone |

|
0.011 | 0.099 | 0.12 |
| 9 | II | Alicyclic ketones and secondary alcohols | ||||
| 9 | II | Cyclopentanone |
|
0.017 | 0.15 | 0.19 |
| 9 | II | 2‐Methylcyclopentanone |

|
0.034 | 0.31 | 0.38 |
| 10 | III | Aliphatic and alicyclic ethers | ||||
| 10 | I | 1,1,3‐Triethoxy‐butane |

|
0.006 | 0.054 | 0.067 |
| 10 | I | Diethylether |
|
0.318 | 2.9 | 3.6 |
| 10 | III | 2,5‐Diethoxy‐tetrahydropyran |
|
0.015 | 0.13 | 0.17 |
| 10 | III | Diethoxytetrahydrofuran (mixture of 2,5‐; 2,4‐ and 2,3 positional isomers) |
|
0.005 | 0.045 | 0.056 |
| 11 | III | Furfural and related substances | ||||
| 11 | III | Furfural |

|
0.220 | 2.0 | 2.5 |
| 11 | III | 3‐Furaldehyde |

|
0.017 | 0.15 | 0.19 |
| 11 | III | 5‐Methyl‐2‐furfural |
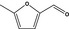
|
0.002 | 0.018 | 0.022 |
| 11 | III | Ethyl 2‐furoate |

|
0.003 | 0.027 | 0.034 |
| 11 | III | Ethyl 5‐methyl furoate |

|
0.002 | 0.018 | 0.022 |
| 11 | III | 2‐Furfural diethyl acetal |

|
0.001 | 0.009 | 0.011 |
| 12 | III | Furan derivatives | ||||
| 12 | III | 2‐Methylfuran |

|
0.080 | 0.72 | 0.90 |
| 12 | III | 2‐Acetylfuran |

|
0.030 | 0.27 | 0.34 |
| 12 | III | Furan |
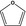
|
0.040 | 0.36 | 0.45 |
| Total identified volatiles (sum of averages) | 176.084 | |||||
| Water e | H2O | 22.600 | ||||
| Total maximised GC peak area including water | 176.285 | |||||
APET: added portions exposure technique; bw: body weight.
Distribution of rum ether components into congeneric groups.
Cramer Class according to TOXTREE version v 2.6.13.
Highest ratio (%) of the peak area of the component in the CG‐chromatogram, compared to the sum of the peak areas of all components.
For the calculation of the estimated chronic APET calculation of individual rum ether components, refer to Section 3.4.4. ‘Exposure assessment to rum ether individual components’. The individual APET values have been rounded to 2 significant digits with a maximum of 3 decimals.
The water content (determined by Karl Fisher titration) is expressed as percentage [%] of the total mass of rum ether rather than as percentage of GC peak area.
Identification numbers
CAS‐number: 8030‐89‐5 (Unspecified. Pyroligneous acids, reaction products with ethyl alcohol, distillates)
Chemical and structural formula, molecular weight
The structures of the substances reported by the applicant to be present in rum ether are given in Table 4.
3.1.2. Organoleptic characteristics
The flavouring has a rum‐like odour and flavour. It is a colourless liquid (caramel is sometimes added to the final distillate for colouring purposes).
3.1.3. Manufacturing process
Source materials
The wood used to produce pyroligneous acid is hardwood of primarily white oak (Quercus alba and Quercus robur) and beech (Fagus sylvatica), and less commonly of hickory (Carya ovata). The trees have not been genetically modified.
As reported by the applicant, the materials typically employed in the process are: 95% ethyl alcohol, pyroligneous acid, 93–96% sulfuric acid, manganese dioxide and for some preparations acetic acid.
Production process
Pyroligneous acid is added slowly with agitation to ethyl alcohol and manganese dioxide, along with acid (sulfuric acid, in some cases supplemented with acetic acid), with the temperature maintained below 40–50°C during the course of the reaction. The mixture is then distilled at atmospheric pressure. The fraction distilling between 60 and 100°C is collected and subjected to a rectification. The resulting product exhibits a final boiling range of 65–87°C.
Alterations of this standard production process can include the use of different amounts of acetic acid. Furthermore, the ‘head’‐ and ‘tail’‐fractions obtained during the rectification step may be partly readded to the distillate in amounts up to 20%. According to the applicant, the resulting final products still exhibit boiling points below 100°C, which is in line with the proposed specifications.
3.1.4. Composition
In the course of the development of this opinion, the applicant provided several data sets on the composition of rum ether upon EFSA requests. The submission of February 2016 was the first that was considered suitable for assessment. It provided information on the volatile constituents in a total of 22 batches (Table 1). The volatile constituents were analysed using gas chromatography/flame ionisation detector (GC/FID) and GC/mass spectrometry (MS). The contents of the volatile constituents were determined on the basis of GC‐peak area percentages relative to the total peak area in the chromatogram. No information on the consideration of individual, substance‐specific GC‐response factors has been provided. A total of 83 constituents were reported; on average 0.53% of the total peak area detected in the chromatograms remained unidentified. Despite shortcomings of the applied semiquantitative approach, this data set was considered for the safety assessment.
Table 1.
Compositional data of 22 commercial rum ether batches (submission from February 2016)3 sorted according to maximum percentage peak areas as determined by GC/MS
| Chemical name | MIN (% of peak area)a | MAX (% of peak area)b | # of batches |
|---|---|---|---|
| Ethyl alcohol | 27.320 | 83.000 | 22 |
| Ethyl acetate | 2.190 | 49.000 | 22 |
| Ethyl formate | 0.379 | 12.210 | 20 |
| Ethyl propionate | 0.090 | 7.470 | 22 |
| Acetic acid | 0.011 | 5.060 | 20 |
| Methyl acetate | 0.024 | 3.740 | 11 |
| Acetaldehyde diethylacetal | 0.058 | 2.107 | 13 |
| Formaldehyde diethylacetal | 0.100 | 1.640 | 20 |
| Ethyl isovalerate | 0.002 | 1.630 | 11 |
| Ethyl valerate | 0.011 | 1.610 | 9 |
| Ethyl butyrate | 0.014 | 1.390 | 14 |
| Methyl alcohol | 0.083 | 1.070 | 4 |
| Diacetyl | 0.011 | 0.520 | 9 |
| Ethyl isobutyrate | 0.003 | 0.480 | 13 |
| 2‐Propenyl acetate | 0.440 | 0.459 | 2 |
| Acetaldehyde | 0.018 | 0.361 | 15 |
| Methyl propionate | 0.006 | 0.360 | 7 |
| Formic acid | 0.160 | 0.320 | 3 |
| Diethylether | 0.003 | 0.318 | 10 |
| Ethyl acrylate | 0.008 | 0.280 | 10 |
| Furfural | 0.012 | 0.220 | 9 |
| Ethyl crotonate | 0.001 | 0.200 | 20 |
| Acetone | 0.004 | 0.176 | 14 |
| 2‐Butanone | 0.167 | 0.167 | 1 |
| Propanoic acid | 0.003 | 0.154 | 8 |
| 1‐butanol | 0.100 | 0.120 | 2 |
| Butanal diethyl acetal | 0.001 | 0.100 | 3 |
| Ethyl methacrylate | 0.003 | 0.100 | 4 |
| Ethyl cyclopropanecarboxylate | 0.080 | 0.080 | 1 |
| 2‐Methylfuran | 0.001 | 0.080 | 5 |
| Ethyl 4‐methylpentanoate | 0.001 | 0.070 | 4 |
| Ethyl 2‐methylbutanoate | 0.003 | 0.070 | 8 |
| Ethyl 4‐pentenoate | 0.001 | 0.070 | 8 |
| Ethyl 2‐methyl‐2‐butenoate | 0.051 | 0.068 | 2 |
| Ethyl but‐3‐enoate | 0.005 | 0.060 | 5 |
| Methyl formate | 0.008 | 0.052 | 8 |
| 3‐Penten‐2‐one | 0.051 | 0.051 | 2 |
| Ethyl 2‐pentenoate | 0.005 | 0.050 | 4 |
| 1,1‐Diethoxyacetone | 0.005 | 0.045 | 4 |
| Furan | 0.001 | 0.040 | 14 |
| Acetol | 0.008 | 0.039 | 2 |
| 2‐Methylcyclopentanone | 0.034 | 0.034 | 2 |
| 2‐Methyl‐2‐cyclopentenone | 0.003 | 0.034 | 8 |
| 2‐Acetylfuran | 0.002 | 0.030 | 10 |
| Ethyl lactate | 0.007 | 0.027 | 2 |
| Glyceraldehyde diethyl acetal | 0.026 | 0.026 | 2 |
| 2‐Ethoxytetrahydrofuran | 0.008 | 0.023 | 6 |
| Butanoic acid | 0.004 | 0.020 | 3 |
| Acetic anhydride | 0.017 | 0.017 | 2 |
| Allyl alcohol | 0.017 | 0.017 | 2 |
| Butyl acetate | 0.017 | 0.017 | 2 |
| 3‐Furaldehyde | 0.017 | 0.017 | 2 |
| 2‐Pentanone | 0.017 | 0.017 | 2 |
| Cyclopentanone | 0.001 | 0.017 | 4 |
| 2,3‐Pentanedione | 0.015 | 0.015 | 2 |
| 2,5‐Diethoxy‐tetrahydropyran | 0.002 | 0.015 | 4 |
| Hydroxyacetaldehyde diethyl acetal | 0.014 | 0.014 | 2 |
| 1‐Hydroxy‐2‐butanone | 0.011 | 0.011 | 1 |
| Ethyl pent‐3‐enoate | 0.008 | 0.010 | 2 |
| Ethyl 3‐methyl‐but‐3‐enoate | 0.007 | 0.008 | 2 |
| Ethyl glycolate | 0.008 | 0.008 | 1 |
| 1,1‐Diethoxyhexan‐2‐one | 0.001 | 0.007 | 4 |
| Ethyl pyruvate | 0.006 | 0.007 | 2 |
| Ethyl levulinate | 0.004 | 0.007 | 2 |
| 1,1,3‐Triethoxy‐butane | 0.006 | 0.006 | 1 |
| 2‐Cyclopenten‐1‐one | 0.002 | 0.006 | 4 |
| Propyl acetate | 0.005 | 0.005 | 2 |
| Diethoxytetrahydrofuran | 0.004 | 0.005 | 2 |
| Acetaldehyde ethyl methyl acetal | 0.003 | 0.003 | 1 |
| Diethyl succinate | 0.003 | 0.003 | 1 |
| Ethyl 3‐methylpentanoate | 0.003 | 0.003 | 2 |
| Ethyl 2‐furoate | 0.003 | 0.003 | 3 |
| Ethyl hexanoate | 0.002 | 0.002 | 2 |
| Isobutyl acetate | 0.002 | 0.002 | 2 |
| 5‐Methyl‐2‐furfural | 0.002 | 0.002 | 1 |
| Ethyl 5‐methyl furoate | 0.002 | 0.002 | 1 |
| Propanal diethyl acetal | 0.001 | 0.001 | 1 |
| Isobutanal diethyl acetal | 0.001 | 0.001 | 1 |
| 2‐Methylbutanal diethyl acetal | 0.001 | 0.001 | 1 |
| 3‐Methylbutanal diethyl acetal | 0.001 | 0.001 | 1 |
| Ethyl nonanoate | 0.001 | 0.001 | 2 |
| Hexanal diethyl acetal | 0.001 | 0.001 | 1 |
| 2‐Furfural diethyl acetal | 0.001 | 0.001 | 1 |
| Waterc | 0.174 | 22.600 | 20 |
GC/MS: gas chromatography/mass spectrometry.
Lowest reported ratio (%) of the peak area of the component in the GC‐chromatogram of the rum ether, compared to the sum of the peak areas of all components.
Highest reported ratio (%) of the peak area of the component in the GC‐chromatogram of the rum ether, compared to the sum of the peak areas of all components.
[%], determined via Karl Fisher.
In order to get information on the representativeness of the data shown in Table 1, the Panel asked the applicant to assign the batches to producers of rum ether in the European Union (EU) and to provide information on the reproducibility of the composition of individual products from these producers and on their production volumes in the EU. Upon this request, additional compositional data have been provided (Table 2), and this latest submission (September 2016) contained information on 27 commercial batches of rum ether, produced by four companies. According to the applicant, the analysed rum ether batches are representative products. The reported annual production volumes for flavouring purposes covered by this submission amount to 35 tonnes for company 1 (corresponding to 47.2% of the combined production volume of the four companies), 1.9 tonnes for company 2 (2.6%), 11.3 tonnes for company 3 (15.2%) and 26 tonnes for company 4 (35%).
Table 2.
Compositional data of 27 commercial rum ether batches (submission from September 2016)4
| Company 1 | Company 2 | Company 3 | Company 4 | ||||
|---|---|---|---|---|---|---|---|
| 2 batches, (2014) | 2 batches, (2011) | 7 batches, (2009/2010) | 3 batches, (2015) | 7 batches, (2016) | 4 batches (‘normal’) | 2 batches (‘10‐fold’) | |
| Water (%) | 0.02–0.11 | 0.17 | 10.14 ± 0.68 | 9.83 ± 0.38 | 8.79 ± 0.34 | 7.92 ± 0.58 | 2.8–2.6 |
| Volatile components (%) a , b | |||||||
| Ethanol | 83.00 | 81.16–81.99 | 41.23 ± 2.75 | 42.97 ± 0.49 | 43.55 ± 0.67 | 45.57 ± 0.88 | 27.09–27.55 |
| Ethyl acetate | 9.69–8.86 | 15.84–16.6 | 44.99 ± 2.69 | 44.01 ± 0.29 | 43.21 ± 0.60 | 31.41 ± 0.16 | 48.06–49.95 |
| Ethyl formate | –c | 0.52–0.61 | 10.49 ± 1.08 | 9.66 ± 0.37 | 9.96 ± 0.30 | 3.44 ± 0.36 | 7.15–7.82 |
| Ethyl propionate | 1.63 | 0.79–0.81 | 1.45 ± 0.15 | 1.67 ± 0.13 | 1.62 ± 0.08 | 4.96 ± 0.48 | 7.23–7.7 |
| Ethyl butyrate | 0.02 | 0.11–0.12 | 0.16 ± 0.07 | 0.19 ± 0.01 | 0.17 ± 0 | 0.99 ± 0.05 | 1.39 |
| Ethyl isobutyrate | 0.07 | 0.03 | – | – | – | 0.23 ± 0.01 | 0.47–0.50 |
| Ethyl valerate | 1.21–1.61 | 0.01 | – | – | – | 0.11 ± 0.01 | 0.14–0.16 |
| Ethyl isovalerate | 1.12–1.63 | 0.01 | – | – | – | 0.06 ± 0 | 0.13 |
| Ethyl 2‐methylbutanoate | 0.03 | – | – | – | – | 0.10 ± 0 | 0.07 |
| Ethyl 4‐methylpentanoate | – | – | – | – | – | 0.07 ± 0.01 | 0.10 |
| Ethyl but‐3‐enoate | – | – | – | – | – | 0.10 ± 0 | 0.06 |
| Ethyl crotonate | 0.09 | 0.03 | 0.16 ± 0.07 | 0.19 ± 0.01 | 0.17 ± 0 | 0.16 ± 0 | 0.14–0.19 |
| Ethyl acrylate | – | 0.02 | – | – | – | 0.14 ± 0.01 | 0.27–0.29 |
| Ethyl 4‐pentenoate | 0.02 | – | – | – | – | 0.07 ± 0.01 | 0.06–0.08 |
| Ethyl 2‐pentenoate | – | – | – | – | – | 0.08 ± 0.03 | 0.10 |
| Ethyl methacrylate | 0.02 | – | – | – | – | – | – |
| Methanol | 1.04–1.07 | – | – | – | – | – | – |
| Methyl formate | – | – | 0.04 ± 0.01 | 0.01 ± 0 | 0.03 ± 0.01 | – | – |
| Methyl acetate | 0.88–0.97 | 0.02 | – | – | – | – | – |
| Methyl propionate | 0.03 | – | – | – | – | 0.29 ± 0.13 | 0.08–0.13 |
| Propyl acetate | – | – | 0.01 ± 0.02 | 0.05 ± 0 | 0.05 ± 0 | – | – |
| 1‐Butanol | 0.1–0.12 | – | – | – | – | – | |
| Butyl acetate | 0.02 | – | – | – | – | – | – |
| Formaldehyde diethylacetal | 0.10 | 0.12 | 0.32 ± 0.05 | 0.31 ± 0.01 | 0.30 ± 0.02 | 1.45 ± 0.22 | 0.31–0.34 |
| Acetaldehyde | – | 0.08 | 0.22 ± 0.09 | 0.43 ± 0.03 | 0.43 ± 0.08 | – | – |
| Acetaldehyde diethylacetal | – | 0.08 | 0.60 ± 0.73 | 0.11 ± 0.02 | 0.23 ± 0.15 | – | – |
| Acetaldehyde dimethyl acetal | 0.60 | – | – | – | – | ||
| Formic acid | – | – | – | – | – | 0.17 ± 0.05 | 0.1–0.32 |
| Acetic acid | – | 0.06–0.12 | 0.05 ± 0.06 | 0.14 ± 0.02 | 0.19 ± 0.03 | 1.66 ± 1.42 | 0.29–1.87 |
| Acetone | – | 0.01 | 0.14 ± 0.03 | 0.11 ± 0.01 | 0.13 ± 0.01 | – | 0.09–0.11 |
| 2‐Pentanone | 0.02 | – | – | – | – | – | – |
| Cyclopentanone | 0.02 | – | – | – | – | – | – |
| 2‐Methyl‐2‐cyclopentenone | 0.02–0.03 | 0.01 | – | – | – | – | – |
| Diacetyl | 0.02 | 0.01 | – | – | – | 0.09 ± 0.01 | 0.51–0.53 |
| Furfural | – | 0.01 | – | – | – | 0.20 ± 0.04 | 0.10 |
| 3‐Furaldehyde | 0.02 | – | – | – | – | – | – |
| Diethoxytetrahydrofuran | – | 0.004–0.005 | – | – | – | – | – |
| Diethylether | – | 0.01–0.02 | 0.20 ± 0.07 | 0.13 ± 0.02 | 0.1 ± 0.02 | – | – |
| Furan | 0.017 | 0.003–0.004 | 0.003 ± 0.001 | 0.003 ± 0.002 | 0.004 ± 0 | 0.006 ± 0.001 | 0.036–0.048 |
| 2‐Methylfuran | 0.07 | – | – | – | – | 0.078 ± 0.103 | 0.10 |
| 2‐Acetylfuran | 0.02 | – | – | – | – | – | – |
| Total (%) – excluding water | 99.98–99.89 | 99.83 ± 0 | 100.05 ± 0.40 | 99.99 ± 0.05 | 100.15 ± 0.18 | 91.22 ± 0.57 | 96.08–96.74 |
Regarding the analytic methodology for the volatile constituents, the following information was provided: company 1: GC/TOF‐MS; companies 2 and 3: GC/FID; company 4: GC/MS, use of 1,2,3‐trimethoxybenzene as an internal standard.
Average ratio (%) of the peak area of the component in the CG‐chromatogram of the rum ether, compared to the sum of the peak areas of all components. In case of 2 batches analysed, ranges of ratios are indicated instead of averages.
Not identified.
In the September 2016 submission, a total of 41 constituents have been reported by the applicant (Table 2). For the batches of company 4, only approximately 91% and 97%, respectively, of the detected GC peaks were identified and quantified. According to the total (%), excluding water, reported for the batches of companies 1–3, all peaks in the chromatograms have been identified and quantified with a detection limit of 0.01% (apart from furan) which is a factor of 10 higher than the minimum area of 0.001% reported in the February 2016 submission of compositional data (Table 1). The grey‐shaded constituents in Table 1 are not present in the compositional overview that was submitted in September 2016 (Table 2).
For the quantitation of furan, specific approaches based on the use of isotopically labelled internal standards have been reported by two companies.
The investigated batches for which data were provided in the second submission differ in their compositions. Only seven volatile constituents (ethanol, ethyl acetate, ethyl propionate, ethyl butyrate, ethyl crotonate, formaldehyde diethylacetal and furan) have been reported in all investigated batches. On the other hand, it is noteworthy that the batches show a common compositional feature: ethanol, three esters (ethyl acetate, formate and propionate) and acetic acid constitute on average 95.4% (87.1–99.3%) of all batches. Also for the 22 batches analysed for the first submission (Table 1), ethanol, these three esters (ethyl acetate, formate and propionate) and acetic acid represent most of the material (sum of average concentrations 92%). In addition, the standard deviations for rum ether constituents between batches of individual producers are small.
In the submission of September 2016, all batches presented contained furan. For the rum ether batches of three companies, the average furan content amounted to 0.006% (0.003–0.017%). Company 4 produces two types of rum ether: For the so‐called ‘normal type’ rum ether (25 tonnes per year; corresponding to 96% of the total production volume of this company), the content of furan (0.006%) was comparable to the data provided by the other companies. For the so‐called ‘rum ether 10‐fold’ (production of 1 tonne per year) the content of furan (0.042%) is seven times higher than in the other batches. According to information provided for company 4, this ‘rum ether 10‐fold’ is used to impart a typical spirit drink flavour (‘Inländerrum’) and at lower dosages for example to pralines or bakery wares. It has been stated that it may be possible to reduce the concentration of furan in a ‘rum ether 10‐fold’, however not below 0.01% if the typical flavour is to be maintained.
Because the submission of February 2016 is based on a higher GC sensitivity (down to 0.001 peak area %) than the submission of September 2016, the compositional data for the first submission were considered to provide a more complete insight into the presence of substances occurring at low concentrations. Therefore, these data will be used as basis for the subsequent risk assessment. However, there was also one constituent (i.e. acetaldehyde dimethylacetal, grey‐shaded in Table 2) that has been reported in the submission of September 2016 which has not been listed in the submission of February 2016 (Table 1). This substance will also be included in the assessment. The evaluation will thus be based on a total of 84 constituents (83 reported in the submission from February 2016 and one additional substance in the submission from September 2016).
Considering that rum ether is a mixture of volatiles, which are all anticipated to be amenable to GC analysis, the Panel considered the applied procedure involving the conversion of GC‐peak areas to concentrations of volatile constituents as acceptable for the purpose of this evaluation. Despite the analytical shortcomings, the Panel considered the data sufficient: (a) to identify and semi‐quantitate rum ether constituents and (b) to demonstrate the reproducibility of the production process.
3.1.5. Stability and reaction and fate in food
No information has been provided on the stability of the flavouring, but shelf lives up to 6 months have been given by company 4 (submission from September 2016).
No information has been provided on the interaction with food components.
3.1.6. Specifications
The following specifications have been proposed by the applicant:
Rum ether is the distillate produced by the treatment of pyroligneous acid (wood vinegar) with ethyl alcohol under acidic, oxidative and heating conditions.
Raw materials for the production of the pyroligneous acid are white oak, beech, and hickory hardwoods.
Rum ether shall all distil at a temperature not exceeding 100°C, at atmospheric pressure, and shall leave no residue on evaporation.
The furan content shall not exceed 0.02%.
Average ethanol and ethanol derivatives, expressed as ethanol, acetaldehyde, acetic acid, and their corresponding acetals and ethyl esters, minimum content 93%.
Average methanol and methanol derivatives, expressed as methanol, formaldehyde, and their ester and acetal derivatives) not to exceed 2.5%.
The Panel considered that the provision of limits for ethanol and methanol equivalents as proposed by the applicant is not sufficient and proposes that the maximum levels of the constituents listed in Table 2, expressed as mg/L, should be included in the specifications to define the composition of rum ether.
3.2. Structural/metabolic similarity of substances according to the congeneric group approach
The applicant suggested to perform the evaluation of rum ether using the congeneric group approach as developed by Smith et al. (2005) for complex flavouring mixtures.
Despite the described shortcomings, the Panel considered rum ether sufficiently defined to use the compositional data as basis for the congeneric group approach.
The Panel decided to apply the approach as developed by Smith et al. (2005) for the assessment of rum ether with a number of modifications. The procedure used by Panel is as follows:
The components are allocated to 12 groups of related substances (‘congeneric groups’) based on chemical structure and other information (e.g. considerations with respect to metabolism), if available (Table 3).
Each component is allocated to a structural class according to Cramer et al. (1978).
For each congeneric group, the ‘generalised Cramer class’ is determined on the basis of that group member which has the highest Cramer class number (I, II or III). In other words, the toxicity of the congeneric group is determined by the substance for which the highest toxicity may be anticipated, based on its chemical structure.
For each component, the highest peak area % in any of the batches analysed is taken, and combined with the ‘Added Portions Exposure Technique’ (APET) exposure estimate for rum ether to obtain a maximised exposure estimate for each individual component.
For each congeneric group, the exposure estimates are summed to obtain a maximised summed exposure estimate for the congeneric group.
Subsequently, each congeneric group is evaluated as if it were a single substance. The exposure to the congeneric group does not raise a safety concern at the intended levels of use if the exposure to the group is below the Threshold of Toxicological Concern (TTC), for the respective Cramer structural class assigned to the congeneric group (i.e. 1,800 μg/person per day for Cramer structural class I, 540 μg/person per day for Cramer structural class II and 90 μg/person per day for Cramer structural class III). If the exposure is above the TTC, a margin of safety is calculated based on available toxicity data. This margin of safety should be sufficiently large to conclude that there is no safety concern for this congeneric group.
Table 3.
Assignment of rum ether components to congeneric groups
| Congeneric group | Congeneric group no | Compounds in rum ether distributed into congeneric groups | Supporting substances in FGEs |
|---|---|---|---|
| Saturated linear primary aliphatic alcohols/aldehydes/acids/esters and acetals, including a cyclic acetal | 1 | Ethanol, Ethyl acetate, Ethyl propionate, Ethyl butyrate, Ethyl formate, Acetic acid, Acetaldehyde, Propyl acetate, Methyl formate, Ethyl valerate, Methyl propionate, Propanoic acid, Methyl acetate, Formic acid, Methanol, 1‐Butanol, Butyl acetate, Ethyl nonanoate, Ethyl hexanoate, Butanoic acid, Acetic anhydride, Diethyl succinate, Butanal diethyl acetal, Acetaldehyde diethyl acetal, Formaldehyde diethylacetal, Propanal diethyl acetal, Acetaldehyde ethyl methyl acetal, Acetaldehyde dimethyl acetal, Hexanal diethyl acetal, 2‐Ethoxytetrahydrofuran | FGE.02Rev1 (EFSA, 2008a), FGE.03Rev2 (EFSA CEF Panel, 2011a) |
| Saturated aliphatic, acyclic, branched‐chain primary alcohols, aldehydes, carboxylic acids and related esters and acetals | 2 | Ethyl isobutyrate, Ethyl isovalerate, Ethyl 2‐methylbutanoate, Isobutanal diethyl acetal, 2‐Methylbutanal diethyl acetal, 3‐Methylbutanal diethyl acetal, Isobutyl acetate, Ethyl 3‐methylpentanoate, Ethyl 4‐methylpentanoate | FGE.01Rev2 (EFSA CEF Panel, 2010c), FGE.03Rev2 |
| α,β‐Unsaturated linear and branched aliphatic primary alcohols/ketones/esters (excluding esters of α,β‐unsaturated carboxylic acids) | 3 | 2‐Propenyl acetate, Allyl alcohol, 3‐Penten‐2‐one, 2‐Methyl‐2‐cyclopentenone, 2‐Cyclopenten‐1‐one | FGE.05Rev2 (EFSA CEF Panel, 2010b), FGE.07Rev5 (EFSA CEF Panel, 2017), FGE.212Rev3 (EFSA CEF Panel, 2015c), FGE.09Rev6 (EFSA CEF Panel, 2015c), FGE.51Rev2 (EFSA CEF Panel, 2016) |
| Ester of an alicyclic carboxylic acid | 4 | Ethyl cyclopropanecarboxylate | FGE.44 (EFSA, 2008b) |
| Esters of unsaturated linear and branched aliphatic carboxylic acids | 5 | Ethyl 4‐pentenoate, Ethyl but‐3‐enoate, Ethyl methacrylate, Ethyl crotonate, Ethyl acrylate, Ethyl 2‐pentenoate, Ethyl 2‐methyl‐2‐butenoate, Ethyl pent‐3‐enoate, Ethyl 3‐methyl‐but‐3‐enoate | FGE.05Rev2 |
| Aliphatic primary alcohols, aldehydes, carboxylic acids, acetals and esters containing additional oxygenated functional groups | 6 | 1,1‐Diethoxyacetone, 1,1‐Diethoxyhexan‐2‐one, Acetol, Ethyl glycolate, Ethyl lactate, Ethyl levulinate, Ethyl pyruvate, Glyceraldehyde diethyl acetal, Hydroxyacetaldehyde diethyl acetal | FGE.10Rev3 (EFSA CEF Panel, 2016) |
| Saturated aliphatic acyclic ketones | 7 | Acetone, 2‐Pentanone, 2‐Butanone | FGE.07Rev5 |
| Aliphatic α‐diketones and related α‐hydroxyketones | 8 | Diacetyl, 1‐Hydroxybutanone, 2,3‐Pentanedione | FGE.11Rev3 (EFSA CEF Panel, 2014) |
| Alicyclic ketones and secondary alcohols | 9 | Cyclopentanone, 2‐Methylcyclopentanone | FGE.09Rev6, FGE.51Rev2 |
| Aliphatic and alicyclic ethers | 10 | Diethylether, 1,1,3‐Triethoxybutane, Diethoxytetrahydrofuran (3 isomers), 2,5‐Diethoxytetrahydropyran | FGE.23Rev4 (EFSA CEF Panel, 2013) |
| Furfural and related substances | 11 | Furfural, 3‐Furaldehyde, 2‐Furfural diethyl acetal, 5‐Methyl‐2‐furfural, Ethyl‐2‐furoate, Ethyl 5‐methyl furoate | FGE.13Rev2 (EFSA CEF Panel, 2011b), FGE.67Rev2 (EFSA CEF Panel, 2015a) |
| Furan derivatives | 12 | Furan, 2‐Methylfuran, 2‐Acetylfuran | FGE.13Rev2, FGE.67Rev2 |
FGE: Flavouring Group Evaluation.
If specific data are available that contraindicate the use of the TTC concept for the group (e.g. genotoxicity data) then that group cannot be evaluated in this way, in line with the decision tree for genotoxic substances described in the EFSA Guidance on the data required for the risk assessment of flavourings (EFSA CEF Panel, 2010a), and the entire flavouring cannot be concluded to be of no safety concern.
In Table 4, the constituents reported by the applicant in rum ether (either in the February 2016 submission or in the September 2016 submission) as well as their structural formulas and their highest reported estimated concentrations are listed.
There are many individual constituents in rum ether for which structurally similar substances can be found in different Flavouring Group Evaluations (FGEs), as indicated in Table 3. In Appendix A, it is indicated in which FGE individual constituents have been evaluated as chemically defined flavouring substances. In Table A.1, the evaluation status of the components of rum ether which have been evaluated as individual chemically defined flavouring substances is given.
Table A.1.
Summary of evaluation of exposure and evaluation status of rum ether components which have been evaluated as flavouring substances
| FL‐no FGE | EU Register name | Structural formula | Cramer class | α,β subgroup | EFSA Evaluation status | JECFA no CoE no CAS no | SCF statusa JECFA statusb CoE statusc | Comments |
|---|---|---|---|---|---|---|---|---|
| 02.004– | Butan‐1‐ol |
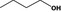
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
85 52 71‐36‐3 |
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
02.078 – |
Ethanol |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
41 1189164‐17‐5 |
Category 1 (SCF, 1995) No safety concern (JECFA, 1997) |
At the forty‐sixth JECFA meeting (JECFA, 1997), the Committee concluded that ethanol posed no safety concern at its current level of intake when ethyl esters are used as flavouring agents |
|
05.001 – |
Acetaldehyde |
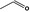
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
80 89 75‐07‐0 |
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
06.001 61 |
1,1‐Diethoxyethane |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
941 35 105‐57‐7 |
– No safety concern (JECFA, 2002a) Category A (CoE, 1992) |
|
|
06.015 61 |
1,1‐Dimethoxyethane |
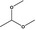
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
940 510 534‐15‐6 |
– No safety concern (JECFA, 2002a) Category A (CoE, 1992) |
|
|
06.023 – |
1,1‐Diethoxyhexane |
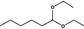
|
– | No safety concern at the estimated level of intake based on the MSDI approach |
– 557 3658‐93‐3 |
– – Category A (CoE, 1992) |
||
|
06.057 03 |
1,1‐Diethoxy‐2‐methylbutane |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 10013 3658‐94‐4 |
||
|
06.058 03 |
1,1‐Diethoxy‐2‐methylpropane |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 10015 1741‐41‐9 |
||
|
06.059 03 |
1,1‐Diethoxy‐3‐methylbutane |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
1730 10014 3842‐03‐3 |
||
|
06.061 03 |
1,1‐Diethoxybutane |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 10009 3658‐95‐5 |
||
|
06.064 03 |
Diethoxymethane |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 10012 462‐95‐3 |
||
|
06.069 03 |
1,1‐Diethoxypropane |
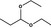
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 10018 4744‐08‐5 |
||
|
06.084 03 |
1‐Ethoxy‐1‐methoxyethane |
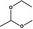
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 10039 10471‐14‐4 |
||
|
07.044 204 |
3‐Penten‐2‐one |
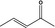
|
Class I | 1.2.1 | Evaluated in FGE.204, additional genotoxicity data required | |||
|
07.050 – |
Acetone |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
139 737 67‐64‐1 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
07.052 – |
Diacetyl |

|
Class II | – | No safety concern at the estimated level of intake based on the MSDI approach |
408 752 431‐03‐8 |
– No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
07.053 – |
Butan‐2‐one |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
278 753 78‐93‐3 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
07.054 – |
Pentan‐2‐one |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
279 754 107‐87‐9 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
07.060 – |
Pentan‐2,3‐dione |
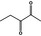
|
Class II | – | No safety concern at the estimated level of intake based on the MSDI approach |
410 2039 600‐14‐6 |
– No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
07.090 92 |
1‐Hydroxybutan‐2‐one |
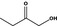
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
1717 11102 5077‐67‐8 |
||
|
07.149 51 |
Cyclopentanone |

|
Class II | – | No safety concern at the estimated level of intake based on the MSDI approach |
1101 11050 120‐92‐3 |
– No safety concern (JECFA, 2002b) – |
|
|
07.169 10 |
1‐Hydroxypropan‐2‐one |
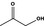
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 11101 116‐09‐6 |
||
|
08.001 – |
Formic acid |
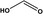
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
79 1 64‐18‐6 |
Category 1 (SCF, 1995)No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
08.002 – |
Acetic acid |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
81 2 64‐19‐7 |
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
08.003 – |
Propionic acid |
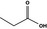
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
84 3 79‐09‐4 |
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
ADI not limited (JECFA, 1974) Evaluated by JECFA before 2000 – No EFSA consideration required |
|
08.005 – |
Butyric acid |
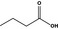
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
87 5 107‐92‐6 |
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.001 – |
Ethyl acetate |
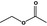
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
27 191 141‐78‐6 |
– No safety concern (JECFA, 1997) Category A (CoE, 1992) |
ADI: 0–25 (JECFA, 1968) Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.002 – |
Propyl acetate |
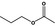
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
126 192 109‐60‐4 |
– No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.004 – |
Butyl acetate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
127 194 123‐86‐4 |
– No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.005 – |
Isobutyl acetate |
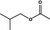
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
137 195 110‐19‐0 |
– No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.023 – |
Methyl acetate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
125 213 79‐20‐9 |
– No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.037 71 |
Ethyl acrylate |
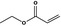
|
Class III | – | No safety concern at the estimated level of intake based on the MSDI approach |
1351 245 140‐88‐5 |
– No safety concern (JECFA, 2005) Category A (CoE, 1992) |
|
|
09.039 – |
Ethyl butyrate |
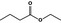
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
Category 1 (SCF, 1995) No safety concern (JECFA, 1997) Category A (CoE, 1992) |
ADI: 0–15 (JECFA, 1968). Evaluated by JECFA before 2000 – No EFSA consideration required |
|
|
09.060 – |
Ethyl hexanoate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
31 310 123‐66‐0 |
– No safety concern (JECFA, 1997) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.072 – |
Ethyl formate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
26 339 109‐94‐4 |
– No safety concern (JECFA, 1997) Category A (CoE, 1992) |
GrADI: 0–3 (JECFA, 1980). Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.107 – |
Ethyl nonanoate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
34 388 123‐29‐5 |
Category 1 (SCF, 1995) No safety concern (JECFA, 1997) Category A (CoE, 1992) |
ADI: 0–2.5 (JECFA, 1980). Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.121 – |
Ethyl propionate |
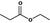
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
28 402 105‐37‐3 |
– No safety concern (JECFA, 1997) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.124 – |
Butyl propionate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
143 405 590‐01‐2 |
– No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 ‐ No EFSA consideration required |
|
09.134 – |
Methyl propionate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
141 415 554‐12‐1 |
– No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration require |
|
09.147 – |
Ethyl valerate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
30 465 539‐82‐2 |
Category 1 (SCF, 1995) No safety concern (JECFA, 1997) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.248 05 |
Ethyl trans‐2‐butenoate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 2244 623‐70‐1 |
– – Category B (CoE, 1992) |
|
|
09.375 05 |
Ethyl methacrylate |
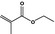
|
Class III | – | No safety concern at the estimated level of intake based on the MSDI approach |
– – 97‐63‐2 |
||
|
09.379 05 |
Ethyl pent‐2‐enoate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 10623 2445‐93‐4 |
||
|
09.409 – |
Ethyl 2‐methylbutyrate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
206 265 7452‐79‐1 |
– No safety concern (JECFA, 1999) Category B (CoE, 1992) |
(R) or (S) enantiomer not specified by CASrn in Register Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.413 – |
Ethyl isobutyrate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
186 288 97‐62‐1 |
– No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.433 64 |
Ethyl lactate |
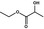
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
931 371 97‐64‐3 |
– No safety concern (JECFA, 1999) Category A (CoE, 1992) |
|
|
09.435 – |
Ethyl 4‐oxovalerate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
607 373 539‐88‐8 |
– No safety concern (JECFA, 2000b) Category B (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.442 64 |
Ethyl pyruvate |
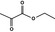
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
938 430 617‐35‐6 |
– No safety concern (JECFA, 2002a) Category B (CoE, 1992) |
|
|
09.444 – |
Diethyl succinate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
617 438 123‐25‐1 |
– No safety concern (JECFA, 2000b) Category B (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.447 – |
Ethyl isovalerate |

|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach | 196442108‐64‐5 |
– No safety concern (JECFA, 1999) Category B (CoE, 1992) |
Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.541 – |
Ethyl 3‐methylvalerate |
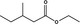
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
215 – 5870‐68‐8 |
– No safety concern (JECFA, 1999) – |
(R) or (S) enantiomer not specified by CASrn in Register Evaluated by JECFA before 2000 – No EFSA consideration required |
|
09.642 02 |
Methyl formate |
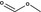
|
Class I | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 10795 107‐31‐3 |
||
|
13.001 218/66 |
5‐Methylfurfural |

|
Class III | 4.2 | No safety concern at the estimated level of intake based on the MSDI approach. Genotoxicity concern could be ruled out (FGE.218Rev1). |
745 119 620‐02‐0 |
– No safety concern (JECFA, 2001) Category B (CoE, 1992) |
|
|
13.018 218/66 |
Furfural |

|
Class III | 4.2 | No safety concern at the estimated level of intake based on the MSDI approach |
450 2014 98‐01‐1 |
Category 4 (SCF, 1995) No safety concern (JECFA, 2001) Category B (CoE, 1992) |
GrADI: 0–0.5 (JECFA, 2001), (EFSA, 2004) |
|
13.030 – |
2‐Methylfuran |
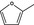
|
Class III | – | No longer supported by Industry (DG SANCO, 2013b) |
1487 2209 534‐22‐5 |
– No evaluation (JECFA, 2009) Category B (CoE, 1992) |
|
|
13.054 221/67 |
2‐Acetylfuran |

|
Class III | 4.5 | Evaluated in FGE.67Rev1, additional genotoxicity data are required |
1503 11653 1192‐62‐7 |
– No evaluation (JECFA, 2009) – |
|
|
13.122 13 |
Ethyl 2‐furoate |
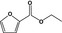
|
Class III | – | No safety concern at the estimated level of intake based on the MSDI approach |
– 10588 614‐99‐3 |
||
|
13.126 – |
Furfural diethyl acetal |

|
Class III | – | Not in the Union List. EFSA Opinion ‐ Group ADI with furfural |
– – 13529‐27‐6 |
ADI: 0.5 mg/kg bw for furfural and the furfural component of furfural diethylacetal (EFSA, 2004) |
FL‐no: FLAVIS number; FGE: Flavouring Group Evaluation; MSDI: maximised Survey‐derived Daily Intake; ADI: acceptable daily intake.
Category 1: Considered safe in use, Category 2: Temporarily considered safe in use, Category 3: Insufficient data to provide assurance of safety in use, Category 4: Not acceptable due to evidence of toxicity.
No safety concern at estimated levels of intake.
Category A: Flavouring substance, which may be used in foodstuffs, Category B: Flavouring substance which can be used provisionally in foodstuffs.
Fifty‐eight of the substances are in the Union List of flavouring substances. These have been evaluated either by EFSA, by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) or by the Council of Europe (CoE) to be of no safety concern from use as flavouring substances.
Amongst these 58 substances, for 3‐penten‐2‐one [FL‐no: 07.044], 2‐acetylfuran [FL‐no: 13.054] and 2‐methylfuran [FL‐no: 13.030] (which is no longer supported by the industry), additional genotoxicity data have been requested in order to evaluate their genotoxic potential (FGE.204, FGE.67Rev2 and FGE.13Rev2). Twenty‐five of the constituents in rum ether are not used in the EU as flavouring substances (Table A.2). One (ethyl 4‐pentenoate) has been evaluated by JECFA as flavouring substance.
Table A.2.
Constituents of rum ether which have not been evaluated as flavouring substances
| Chemical name | Structural formula | CAS no | Comments |
|---|---|---|---|
| Methyl alcohol | CH3OH | 67‐56‐1 | |
| Allyl alcohol |

|
107‐18‐6 | |
| 1,1‐Diethoxyacetone |
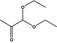
|
5774‐26‐5 | |
| Acetic anhydride |

|
108‐24‐7 | |
| Ethyl 4‐pentenoate |

|
1968‐40‐7 | |
| 2‐Propenyl acetate |

|
108‐22‐5 | |
| Ethyl 3‐methyl‐but‐3‐enoate |

|
1617‐19‐2 | |
| Ethyl pent‐3‐enoate |

|
1617‐05‐6 | |
| Ethyl glycolate |

|
623‐50‐7 | |
| Ethyl cyclopropanecarboxylate |
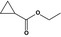
|
4606‐07‐9 | |
| Ethyl 3‐butenoate |

|
1617‐18‐1 | |
| Ethyl 4‐methylpentanoate |

|
25415‐67‐2 | |
| Glyceraldehyde diethyl acetal |

|
10487‐05‐5 | |
| 1,1,3‐Triethoxy‐butane |

|
5870‐82‐6 | |
| 1,1‐Diethoxyhexan‐2‐one |

|
35523‐34‐3 | |
| Hydroxyacetaldehyde diethyl acetal |

|
621‐63‐6 | |
| 2‐Methyl‐2‐cyclopentenone |

|
1120‐73‐6 | |
| 2‐Cyclopenten‐1‐one |
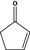
|
930‐30‐3 | |
| 2‐Methylcyclopentanone |
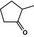
|
1120‐72‐5 | |
| Diethylether |

|
60‐29‐7 | |
| 2,5‐diethoxy‐tetrahydropyran |
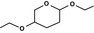
|
n.a. | |
| Diethoxytetrahydrofuran |

|
3320‐90‐9 | Not fully identified; may occur as three positional isomers (i.e. 2,5‐; 2,4‐ and 2,3‐diethoxytetrahydrofuran) |
| 2‐Ethoxytetrahydrofuran |

|
13436‐46‐9 | |
| 3‐Furaldehyde |

|
498‐60‐2 | |
| Ethyl 5‐methylfuroate |

|
14003‐12‐4 | |
| Furan |

|
110‐00‐9 |
Furan has been evaluated by the EFSA Scientific Panel on Contaminants in the Food Chain in 2004 (EFSA CONTAM Panel, 2004) and it is currently under re‐evaluation by the EFSA CONTAM Panel. The current opinion is that furan is carcinogenic, probably attributable to genotoxicity.
3.3. Information on existing evaluations from EFSA
Rum ether has not been evaluated by EFSA before.
3.4. Exposure assessment (details are reported in Appendix B)
3.4.1. Intended use
According to EFFA and the International Organisation of the Flavor Industry (IOFI), the annual production volume in Europe is 74.2 tonnes, and major uses are in the food categories ‘beverages’, ‘confectionery’, and ‘baked goods’ (Appendix B, Table B.1) (EFFA, 2016b).
Table B.1.
Normal and maximum occurrence levels for refined categories of foods and beverages
| CODEX code | Food categoriesa | Standard portionsb (g) | Occurrence level as added flavouring substance (mg/kg) | Occurrence level from other sourcesc (mg/kg) | Combined occurrence level from all sourcese (mg/kg) | |||
|---|---|---|---|---|---|---|---|---|
| Normal | Maximum | Averaged | Maximum | Normal | Maximum | |||
| 03.0 | Edible ices, including sherbet and sorbet | 50 | 150 | 180 | 150 | 180 | ||
| 05.0 | Confectionery | 40 | 180 | 385 | 180 | 385 | ||
| 05.3 | Chewing gum | 3 | 260 | 850 | 260 | 850 | ||
| 06.0 | Cereal and cereal products derived from cereal grains, roots and tubers, and pulses and legumes, excluding bakery wares of food category 7.0 | 200 | 12 | 22 | 12 | 22 | ||
| 07.0 | Bakery wares | 80 | 220 | 420 | 220 | 420 | ||
| 08.0 | Meat and meat products, including poultry and game | 200 | 160 | 200 | 160 | 200 | ||
| 12.0 | Salts, spices, soups, sauces, salads, protein products (including soya bean protein products) and fermented soya bean products | 200 | 175 | 175 | 175 | 175 | ||
| 14.1 | Non‐alcoholic beverages | 300 | 40 | 75 | 40 | 75 | ||
| 14.2.1 | Alcoholic beverages | 300 | 200 | 600 | 200 | 600 | ||
Most of the categories reported are the subcategories of Codex GSFA (General Standard for Food Additives) used by the JECFA in the SPET technique (FAO/WHO, 2008). In the case of category 13.2 (complementary foods for infants and young children), further refined categories have been created so that a specific assessment of dietary exposure can be performed in young children.
- 1/25 for powder used to prepare water‐based drinks such as coffee, containing no additional ingredients,
- 1/10 for powder used to prepare water‐based drinks containing additional ingredients such as sugars (ice tea, squashes, etc.),
- 1/7 for powder used to prepare milk, soups and puddings,
- 1/3 for condensed milk.
As natural constituent and/or developed during the processing and/or as carry over resulting from their use in animal feed.
In order to estimate normal values in each category, only foods and beverages in which the substance is present in significant amount will be considered (e.g. for the category ‘Fresh fruit’ 04.1.1., the normal concentration will be the median concentration observed in all kinds of fruit where the flavouring substance is known to occur).
As added flavouring or from other sources. The normal and maximum combined occurrence levels of the substance will be assessed by the applicant either by adding up occurrence levels from added use to that from other sources or by expert judgment based on the likelihood of their concomitant presence. This will be done both for normal use levels and for maximum use levels.
3.4.2. Chronic dietary exposure
For the safety evaluation, exposure to the flavouring is assessed by the chronic added portions exposure technique (APET; EFSA CEF Panel, 2010a), which is based on the combined normal occurrence levels (Appendix B). The chronic APET for rum ether [FL‐no: 21.001] has been calculated for adults and children (Table 5). For adults, a value of 1,583 μg/kg body weight (bw) per day or 95 mg/person per day was obtained (maximum intake of 60 mg/person per day from alcoholic beverages and 35 mg/person per day from soups and broths). For children (3 years of age), an APET of 1,974 μg/kg bw per day or 30 mg/person per day was calculated. The Panel is aware that these values probably overestimate real exposure due to the broad food categories used.
Table 5.
APET – chronic dietary exposure
| Chronic APETa | Addedb (μg/kg bw per day) | Added (μg/person per day) | Other dietary sourcesc (μg/kg bw per day) | Combined (μg/kg bw per day) | Combinedd (μg/person per day) |
|---|---|---|---|---|---|
| Adults | 1,583 | 95,000e | 0 | 1,583 | 95,000 |
| Children | 1,974 | 30,000f | 0 | 1,974 | 30,000 |
APET: added portions exposure technique; bw: body weight: the chronic APET calculation is based on the combined normal occurrence level.
APET Added is calculated on the basis of the normal amount of flavouring added to a specific food category.
APET Other Dietary Sources is calculated based on the natural occurrence of the flavouring in a specified food category.
APET Combined is calculated based on the combined amount of added flavouring and naturally occurring flavouring in a specified food category.
For the adult, APET calculation a 60‐kg person is considered representative.
For the child, APET calculation a 3‐year‐old child with a 15‐kg bw is considered representative.
Although the flavouring is not intended to be used in food categories specifically intended for infants and toddlers, these could still be exposed through consumption of foods from the general food categories, which may contain the substance. However, at present, there is no generally accepted methodology to estimate exposure in these age groups resulting from consumption of foods from the general categories. Exposure of infants and toddlers is currently under consideration by EFSA.
3.4.3. Acute dietary exposure
The acute APET calculation for rum ether [FL‐no: 21.001] (Table 5) is based on the combined maximum occurrence level and large portion size, i.e. three times standard portion size (Appendix B).
Although the flavouring is not intended to be used in food categories specifically intended for infants and toddlers, these could still be exposed through consumption of foods from the general food categories, which may contain the substance. However, at present, there is no generally accepted methodology to estimate exposure in these age groups resulting from consumption of foods from the general categories. Exposure of infants and toddlers is currently under consideration by EFSA.
3.4.4. Exposure assessment to individual constituents of rum ether
APETs for individual constituents are calculated based on the following assumptions:
Based on the use levels provided by the applicant, the chronic APET for adults is 1,583 μg/kg bw per day and for children 1,974 μg/kg bw per day for rum ether (including the water fraction, 0.174%), which based on the summed maximum GC peak areas (176.084%) plus water is represented by a total percentage of 176.258% (Table 4). The lower amount of water as mentioned in Table 1 was included in the calculation as this is more conservative.
The component is present in rum ether at the maximum level, based on the peak areas compared to the total peak area detected in the gas chromatogram.
The individual GC peak areas are normalised for total summed maximum GC peak areas of volatiles (i.e. 176.084%).
GC peak areas are transformed into concentrations, assuming that the total peak area of the chromatogram corresponds to the total mass of the injected volatiles.
The APET of rum ether has to be corrected for the fraction of water, i.e. 0.174%. Therefore, the corrected APET for volatiles only is 1,583 x 176.084/176.258 (0.999)= 1,581 μg/kg bw per day.
APET values for the single components of rum ether have then been estimated based on the ratio of the single component compared to the total amount of volatiles in rum ether. For example the calculated APET for ethyl alcohol (ethanol) for an adult is (83.000/176.084) ×1,581 = 745 μg/kg bw per day.
3.4.5. Exposure assessment to congeneric groups of rum ether
Based on the calculated APETs for the individual constituents, acute and chronic summed maximised APET estimates for the 12 congeneric groups for adults and children have been calculated (Table 7).
Table 7.
Maximised summed exposure for adults and children to congeneric groups in rum ether, based on the APET estimates for rum ether components at maximum reported concentrations
| Congeneric group | Chronic summed maximised APETs (μg/kg bw per day) | Highest Cramer Class identified for the congeneric group | Lowest TTC applicable for the congeneric group (μg/kg bw per day) | |
|---|---|---|---|---|
| Adults | Children | |||
| 1 | 1,529 | 1,907 | III | n.a.a |
| 2 | 20 | 25 | I | 30 |
| 3 | 5.1 | 6.3 | III | n.a. |
| 4 | 0.72 | 0.90 | II | 9 |
| 5 | 7.6 | 9.5 | III | 1.5 |
| 6 | 1.6 | 2.0 | I | 30 |
| 7 | 3.2 | 4.0 | I | 30 |
| 8 | 4.9 | 6.1 | III | 1.5 |
| 9 | 0.46 | 0.57 | II | 9 |
| 10 | 3.1 | 3.8 | III | 1.5 |
| 11 | 2.2 | 2.7 | III | 1.5 |
| 12 | 1.3 | 1.7 | III | n.a. |
APET: added portions exposure technique; bw: body weight; TTC: Threshold of Toxicological Concern.
n.a.: not applicable; there is a concern for genotoxicity for one or more constituents in this congeneric group.
3.5. Biological and toxicological data
3.5.1. Absorption, distribution, metabolism and elimination
Rum ether as such has not been tested in any toxicity studies. However, many of the components that have been identified in rum ether have been previously evaluated in various FGEs. An overview of the components and the FGEs in which these have been considered is given in Appendix A. The components in rum ethers, which have not been evaluated as chemically defined flavouring substances have structures that in general match closely to those that have been evaluated before. In Table 3 and Appendix A, the respective FGEs where these structurally similar flavouring substances have been discussed are indicated. In these FGEs, some information on metabolism is provided.
Most of the constituents of rum ether are readily metabolised to innocuous substances. Esters and acetals will be hydrolysed after ingestion, either in the gastrointestinal (GI) tract or in liver or plasma. The liberated primary alcohols and aldehydes will be further oxidised, similar to those which are already present in rum ether as free constituents, to give the corresponding carboxylic acids, which will be further oxidised to carbon dioxide and water. Secondary alcohols (also those resulting from keto‐reduction) can be conjugated to glucuronic acid or sulfate and subsequently excreted. Furfural and related substances of CG 11 can be conjugated at the side chain (either directly for the furoic acid derivatives or after oxidation of the furaldehyde moiety to furoic acid) with glycine and subsequently excreted.
The constituents in CG 12 (furan, 2‐methylfuran and 2‐acetylfuran) are known or suspected to be metabolised to very reactive ring‐opening products (e.g. 2,4‐but‐2‐enedial).
For more details, the reader is referred to Appendix D and to the previous evaluations by EFSA or JECFA (Appendix A).
3.5.2. Toxicity data
Rum ether as such has not been tested in any toxicity studies. The Panel decided to carry out the safety assessment for rum ether by using the congeneric group approach and to use TTCs as surrogate toxicity parameters, where applicable. As explained in Section 3.2, toxicity data are needed if the exposure to a congeneric group is higher than the TTC, applicable for that group. Toxicity data for individual constituents will not be summarised here; if necessary reference will be made to evaluations by EFSA, JECFA or other bodies.
3.6. Safety assessment
3.6.1. Safety assessment for acute exposure
The highest acute exposure estimate for rum ether is 8 mg/kg bw per day, which would approximately correspond to 500 mg/person per day in an adult. For children (15‐kg body weight), the level of acute exposure would be approximately 65 mg/person per day. Even if all the rum ether consisted of ethanol, this amount would still be less than that consumed from a 300 mL bottle of ‘alcohol free’‐beer (0.5% of ethanol = 0.5/100 × 300 g = 1.5 g).
Data from the evaluations of the components in rum ether, that are used as chemically defined flavouring substances (see Appendix A for references) do not indicate that these substances either consumed alone or in rum ether as a complex mixture would represent a risk for acute toxicity at their levels of intake from rum ether.
3.6.2. Safety assessment for long‐term exposure per congeneric group
Classifications into congeneric groups for rum ether and intake data for the congeneric groups are presented in Tables 3 and 4, and in Sections 3.2 and 3.4.
Within each congeneric group, metabolic data for one or more members of the group or for structurally related substances have been reported (Appendix D). For more details, reference is made to the JECFA or EFSA evaluations indicated in Appendix A. The established TTC for each congeneric group is compared to total intake for that congeneric group in rum ether, where applicable (Table 7).
Congeneric group 1:
Saturated linear primary aliphatic alcohols/aldehydes/acids/esters and acetals, including a cyclic acetal
The substances in this congeneric group (Table 4) may be readily metabolised. Ethanol and acetaldehyde are two of the constituents in this congeneric group. IARC (1988) has classified consumption of alcoholic beverages as class 1 carcinogenic to humans. In 2012, IARC has published an update of their previous evaluation in which the body of evidence was further expanded and the conclusions were reiterated and extended to cover also ethanol and acetaldehyde (IARC, 2012). IARC presented evidence to show that this carcinogenicity may be related to mutagenic activity caused by acetaldehyde, the primary metabolite of ethanol (IARC, 2010, 2012). IARC determined that the occurrence of malignant tumours of the oral cavity, pharynx, larynx, oesophagus, liver, colorectum and female breast is related to the consumption of alcoholic beverages. IARC did not estimate an indicator of carcinogenic potency (e.g. a BMDL10) that can be used in a risk assessment.
For flavouring purposes and for use as, e.g. extraction solvent, ethanol was evaluated by JECFA in 1970. No numerical acceptable daily intake (ADI) was derived, but the ADI was stated to be ‘limited by Good Manufacturing Practices (as a solvent)’ and this conclusion was accepted by the SCF (1992). Ethanol, resulting from the use of ethyl esters as flavouring substances, was evaluated at the 46th JECFA meeting and found to be of no safety concern (JECFA, 1997). Commission Regulation (EU) No 231/20125 lays down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/20086 of the European Parliament and of the Council. If ethanol is used in the manufacturing of certain food additives (e.g. food colours, gums, sweeteners, etc.), different restrictions on the levels of ethanol per food additive are reported in the Regulation (e.g. from 50 mg/kg to 2% of ethanol in the food additive).
No safety concern has been identified for 28 constituents in this group. However, given the presence of free ethanol and free acetaldehyde for which a concern for genotoxicity has been identified, it is not justified to compare the exposure estimate for this congeneric group with a TTC value. Therefore, for this congeneric group, a safety concern has been identified.
Congeneric group 2:
Saturated aliphatic, acyclic, branched‐chain primary alcohols, aldehydes, carboxylic acids and related esters and acetals
This group consists of nine constituents in rum ether (Table 4), all of which are ethyl (or one isobutyl) esters or acetals of short chain branched saturated carboxylic acids or aldehydes. These substances are readily metabolised. The highest chronic exposure to this congeneric group amounts to 25 μg/kg bw per day (for children), which is below the TTC of 30 μg/kg bw per day (see also Table 7). Consequently, the Panel concludes that there is no safety concern for this congeneric group under the intended conditions of use for rum ether.
Congeneric group 3:
α,β‐Unsaturated linear and branched aliphatic primary alcohols/ketones/esters (excluding esters of α,β‐unsaturated carboxylic acids)
The five substances in CG 3 are α,β‐unsaturated carbonyls or precursors for such, for which the Panel has identified a concern for genotoxicity. For allyl alcohol (which is also formed upon hydrolysis from 2‐propenyl acetate), equivocal data on genotoxicity and carcinogenicity have been reported (OECD SIDS, 2005). JECFA allocated a group ADI of 0–50 μg allyl alcohol equivalents/kg bw per day to three allyl alcohol esters (hexanoate, heptanoate and isovalerate). Allyl alcohol and 2‐propenyl acetate can be converted to acrolein (2‐propenal). IARC (1995) has evaluated the carcinogenicity data on acrolein and concluded that there was inadequate evidence for carcinogenicity of acrolein in animals or humans (‘not classifiable’). In a more recent review paper (Abraham et al., 2011), it was argued that acrolein may form adducts with glutathione and other cellular components among which DNA, and that it is genotoxic in vitro, but that mutagenicity and carcinogenicity have not been demonstrated after oral exposure. For 3‐penten‐2‐one, the genotoxicity is still under consideration in EFSA (FGE.204). Two substances 2‐methyl‐2 cyclopentenone and 2‐cyclopenten‐1‐one have not been evaluated for genotoxicity. In FGE.212Rev3, a number of substances structurally related to the latter two have been considered for genotoxic properties; the Panel concluded that they were not of concern with respect to genotoxicity (EFSA CEF Panel, 2015c).
Given the reservations of the Panel with regards to the genotoxic potential of at least one constituent in this congeneric group, it is not adequate to compare the exposure for congeneric group 3 to a TTC value. For this congeneric group, a safety concern is identified.
Congeneric group 4
An ester of an alicyclic carboxylic acid
This congeneric group consists of only one member (ethyl cyclopropanecarboxylate, Table 4). For an analogous substance in FGE.44 (cis‐2‐heptyl‐cyclopropanecarboxylic acid [FL‐no: 08.131]), no concern for genotoxicity was identified (EFSA, 2008b), based on metabolism considerations and comparison with hazardous properties from cyclopropanecarboxylate‐derived pyrethroid insecticides. The highest chronic APET for this group 0.90 is μg/kg bw per day, which is below the TTC of 9 μg/kg bw per day for this group (see also Table 7). The Panel concludes that there is no safety concern for this congeneric group under the intended conditions of use for rum ether.
Congeneric group 5
Esters of unsaturated linear and branched aliphatic carboxylic acids
This congeneric group includes nine substances (Table 4). The highest chronic APET for this group is 9.5 μg/kg bw per day (children), which is higher than the TTC of 1.5 μg/kg bw per day for this group (Table 7). In FGE.05Rev2 (EFSA CEF Panel, 2010b), a no observed adverse effect level (NOAEL) of 100 mg/kg bw per day has been identified for ethyl methacrylate in a 2‐year oral toxicity study in rats (Borzelleca et al., 1964), which provides a margin of safety of 10,530 for this group. The Panel concludes that there is no safety concern for this congeneric group under the intended conditions of use for rum ether.
Congeneric group 6
Aliphatic primary alcohols, aldehydes, carboxylic acids, acetal and esters containing additional oxygenated functional groups
This congeneric group comprises nine substances (Table 4). The highest chronic APET for this group is 2.0 μg/kg bw per day (children), which is below the TTC of 30 μg/kg bw per day for this group (Table 7). The Panel concludes that there is no safety concern for this congeneric group under the intended conditions of use for rum ether.
Congeneric group 7
Saturated aliphatic acyclic ketones
This congeneric group comprises three substances (Table 4). The highest chronic APET for this group is 4.0 μg/kg bw per day (children), which is below the TTC of 30 μg/kg bw per day for this group (Table 7). The Panel concludes that there is no safety concern for this congeneric group under the intended conditions of use for rum ether.
Congeneric group 8
Aliphatic α‐diketones and related α‐hydroxyketones
This congeneric group comprises three substances (Table 4). The highest chronic APET for this group is 6.1 μg/kg bw per day (children), which is higher than the TTC of 1.5 μg/kg bw per day for this group (see Table 7). In FGE.07Rev5 for acetone, one of the members of this group, a no observed adverse effect level (NOAEL) of 1,000 mg/kg bw per day has been identified in 13 weeks drinking water study in rats (EFSA CEF Panel et al., 2017), which provides a margin of safety for this group of 164,000. The Panel concludes that there is no safety concern for this congeneric group under the intended conditions of use for rum ether.
Congeneric group 9
Alicyclic ketones, and secondary alcohols
This congeneric group comprises four substances (Table 4). The highest chronic APET for this group is 0.57 μg/kg bw per day (children), which is below the TTC of 9 μg/kg bw per day for this group (see also Table 7). The Panel concludes that there is no safety concern for this congeneric group under the intended conditions of use for rum ether.
Congeneric group 10
Aliphatic and alicyclic ethers
This congeneric group comprises four substances (Table 4), which are very different in structure, and the ether moiety is the only common structural element. The highest chronic APET for this group is 3.8 μg/kg bw per day (children), which is above the TTC (1.5 μg/kg bw per day) for this congeneric group. For diethylether, the member showing the simplest structure, the US‐EPA has derived an oral reference dose from a 13 weeks oral toxicity study in rats, which provided a NOAEL of 500 mg/kg bw per day (USEPA, 1986). By application of an uncertainty factor of 3,000, a reference dose of 200 μg/kg bw per day was derived.
Considering the structural diversity in this group, the Panel considered that the availability of a point of departure (PoD) or health‐based guidance value, for diethylether only, is not sufficient to perform a safety assessment for the other three members of the group. Therefore, the safety of this congeneric group cannot be assessed.
Congeneric group 11
Furfural and Related Substances
This congeneric group comprises six substances (Table 4). Hydrolysis of the ester and acetal constituents in this group will result in the formation of furaldehyde or furoic acid. In FGE.13Rev2, several related substances have been evaluated using the ADI of 500 μg/kg bw per day for furaldehyde (EFSA CEF Panel, 2011b), which is more relevant for the safety assessment of this congeneric group than the TTC indicated in Table 7. For this congeneric group, the highest chronic APET is 2.7 μg/kg bw per day (children), which well below the ADI for furfural. The Panel concludes that there is no safety concern for this congeneric group under the intended conditions of use for rum ether.
Congeneric group 12
Furan derivatives
The data on genotoxicity of furan will not be extensively discussed here, since they were also included in the EFSA opinion on furan (EFSA CONTAM Panel, 2004), where it was concluded that the weight of evidence indicates that furan‐induced carcinogenicity is probably attributable to a genotoxic mechanism. For the two other constituents in rum ether that were also used as individual flavouring substances, the Panel has requested additional genotoxicity data, i.e. 2‐methylfuran [FL‐no: 13.030], and 2‐acetylfuran [FL‐no: 13.054]. 2‐Methylfuran is no longer supported by industry for use in Europe as a chemically defined flavouring substance, and submission of additional information on the genotoxicity of this substance is not anticipated. The evaluation of the substance 2‐acetylfuran is on hold, awaiting further information on its genotoxic potential (FGE.67Rev2 (EFSA CEF Panel, 2015a)). Up to now a PoD for the risk assessment for furan has not been derived by EFSA. From the literature, suggestions for a PoD can be extracted, e.g. a BMDL10 of 1,230 μg furan/kg bw per day for hepatocellular tumours (Carthew et al., 2010) or a BMDL10 of 140 μg furan/kg bw per day for cholangiocarcinomas (VKM, 2012).
The Panel is aware that the amount of information on the toxicity of furan has increased tremendously over the last decade, among which there are 90‐day oral toxicity studies on furan in rats and mice (Gill et al., 2010, 2011) and a new chronic oral toxicity study in rats (Von Tungeln et al., 2017). Also, for 2‐methyfuran, new data have become available, e.g. a 28‐day oral toxicity study on 2‐methylfuran in the rat (Gill et al., 2014). This new information is currently under evaluation by EFSA's Panel on Contaminants in the food chain. Information on natural occurrence of furan is presented in Appendix E.
Therefore, the Panel concluded that there is a safety concern for the members of this congeneric group. The assessment of the toxicologically relevance of the levels of furan as proposed by the applicant in the specifications should take into account the results of the ongoing evaluation on furan and furan derivatives by the CONTAM Panel.
4. Discussion
The Panel used the congeneric group approach for the evaluation of rum ether.
Several uncertainties have been identified in different steps of the risk assessment:
The analytical methods leave room for the presence of as yet unidentified constituents, which could lead to underestimation of the risk.
The provided semiquantitative data only allow a rough estimate of exposure to rum ether.
The information on use and use levels in combination with APET technique is anticipated to produce an overestimation of exposure to rum ether constituents.
Read across to FGEs was applied to accommodate for the absence of full genotoxicity and toxicity data in all CG.
The Panel noted that the final conclusion is determined by the hazards identified for some of the rum ether constituents and is not dependent on the above mentioned uncertainties.
5. Conclusions
For eight of the congeneric groups in rum ether, the Panel concluded that there is no safety concern at the intended conditions of use for rum ether.
For four of the congeneric groups, there is a safety concern, because:
no PoD or health‐based guidance value is available to cover all members of the congeneric group 10; this information is needed since the exposure to this congeneric group was estimated to be higher than its TTC;
a concern for genotoxicity cannot be ruled out due to insufficient data (congeneric group 3);
substances in congeneric groups are carcinogenic and genotoxic (congeneric groups 1 and 12).
According to the overall strategy for the risk assessment of flavouring substances (EFSA CEF Panel, 2010a), the presence of genotoxic substances as process‐derived constituents of rum ether is of safety concern.
Documentation provided to EFSA
EFFA (European Flavour Association), 2011. EFFA dossier on: The safety evaluation of flavourings other than flavouring substances, flavouring complexes (FCs): Rum ether. dated 09 September 2011. Unpublished report submitted by EFFA to EFSA.
EFFA (European Flavour Association), 2014. Rum ether: additional data and clarifications. 19 March 2014. Unpublished data submitted by EFFA to EFSA.
EFFA (European Flavour Association), 2015. Updated EFFA dossier on: The safety evaluation of flavourings other than flavouring substances, flavouring complexes (FCs): Rum ether. dated 18 August 2015. Unpublished report submitted by EFFA to EFSA.
EFFA (European Flavour Association), 2016a. Updated EFFA dossier on: The safety evaluation of flavourings other than flavouring substances, flavouring complexes (FCs): Rum ether. dated 22 February 2016. Unpublished report submitted by EFFA to EFSA.
EFFA (European Flavour Association), 2016b. Rum ether: additional data and clarifications from four companies. 20 September 2016. Unpublished data submitted by EFFA to EFSA.
Abbreviations
- ADI
acceptable daily intake
- APET
Added Portions Exposure Technique
- BMDL
benchmark dose lower confidence limit
- bw
body weight
- CAS
Chemical Abstract Service
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CoE
Council of Europe
- EFFA
European Flavour Association
- FAO
Food and Agriculture Organization of the United Nations
- FEMA
Flavour and Extract Manufactures Association
- FGE
Flavouring Group Evaluation
- FID
flame ionisation detector
- FLAVIS (FL)
Flavour Information System (database)
- GC
gas chromatography
- GI
gastrointestinal
- GRAS
Generally Recognised As Safe
- IOFI
The International Organization of the Flavor Industry
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- MS
mass spectrometry
- MSDI
maximised survey‐derived daily intake
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PoD
Point of Departure
- SCF
Scientific Committee on Food
- SPET
single portion exposure technique
- TCA
tricarboxylic acid cycles
- TTC
threshold of toxicological concern
- WHO
World Health Organization
Appendix A – Composition of rum ether
1.
Appendix B – Use levels and exposure calculations
1.
Calculation of the dietary exposure ‐ ‘Added Portions Exposure Technique' (APET)7
1.
Chronic dietary exposure
The chronic APET calculations are based on the normal combined occurrence level by adding the highest contributing portion of food and highest contributing portion of beverages (either among soft drinks or alcoholic beverages) (see Table 5). For APET calculation for children is by adding the highest contributing portion of food and highest contributing portion of beverages (among soft drinks). Furthermore, in the APET calculation for children the portion sizes listed in Table B.1 is adjusted by a factor 0.63 to take into account the smaller portion sizes consumed by the child.
Adults
On the basis of normal occurrence level from added flavourings
Solid Food: The maximum intake will be from category 12.0 (Salts, spices, soups, sauces, salads, protein products (including soya bean protein products) and fermented soya bean products) with the normal combined occurrence level of 35 mg/adult per day.
Beverage: The maximum intake will be from category 14.2.1 (Alcoholic beverages) with the normal combined occurrence level of 60 mg/adult per day.
The total APET will be 95 mg/adult per day corresponding to 1.6 mg/kg bw per day for a 60 kg person.
Children (3‐year‐old child of 15‐kg body weight) 8
Solid Food: The maximum intake will be from category 12.0 (Salts, spices, soups, sauces, salads, protein products (including soya bean protein products) and fermented soya bean products) with the normal combined occurrence level of 35 x 0.63 = 22 mg/child per day.
Beverage: The maximum intake will be from category 14.1 (Non‐alcoholic beverages) with the normal combined occurrence level of 12 x 0.63 = 7.6 mg/child per day.
The total APET will be 30 mg/child per day corresponding to 2 mg/kg bw per day for a 15 kg child.
Conclusion
The higher of the two values among adults and children, expressed per kg/bw per day, should be used as the basis for the safety evaluation of the candidate substance, i.e. the value of 2 mg/kg bw per day for a 15 kg child should be compared to the appropriate NOAEL for the candidate substance.
Infants and young children
The estimate to infant exposure is currently under revision in the DATA Unit of EFSA.
Acute dietary exposure
The calculation was based on the maximum use levels and large portion size, i.e. three times standard portion size (see Table 6). Although the substance is not intended to be used in food categories specifically intended for infants and toddlers, these could still be exposed through consumption of foods from the general food categories, which may contain the substance. However, at present there is no generally accepted methodology to estimate exposure in these age groups resulting from consumption of foods from the general categories. The APET calculation for children the portion sizes listed in Table B.1 is adjusted by a factor 0.63 to take into account the smaller portion sizes consumed by the child.
Table 6.
APET – acute dietary exposure
| Acute APETa | Addedb (μg/kg bw per day) | Added (μg/person per day) | Other dietary sourcesc (μg/kg bw per day) | Combined (μg/kg bw per day) | Combinedd (μg/person per day) |
|---|---|---|---|---|---|
| Adults | 9,000 | 540,000e | 0 | 9,000 | 540,000 |
| Children | 5,040 | 75,600f | 0 | 5,040 | 75,600 |
APET: added portions exposure technique; bw: body weight: the acute APET calculation is based on the combined maximum occurrence level.
APET Added is calculated on the basis of the maximum amount of flavouring added to a specific food category.
APET Other Dietary Sources is calculated based on the natural occurrence of the flavouring in a specified food category.
APET Combined is calculated based on the combined amount of added flavouring and naturally occurring flavouring in a specified food category.
For the adult, APET calculation a 60‐kg person is considered representative.
For the child, APET calculation a 3‐year‐old child with a 15‐kg bw is considered representative.
Adults
The highest contribution comes from three portions of category 14.2.1 (Alcoholic beverages) and is (3 × 300 g) × 600 mg/kg = 540 mg/adult.
Children 8
The highest contribution comes from three portions of category 08.0 (Meat and meat products, including poultry and game) and is (3 × 200 g) × 0.63 × 200 mg/kg = 75.6 mg/child.
Infants and young children (0–1 year)
Acute dietary exposure is not calculated for infants and young children.
Appendix C – Methodology
1.
The definition of a complex flavour is ‘a flavouring added or intended to be added to food in order to impart odour and/or taste and which does not fall under the definitions of Article 3(2)(b)–(g) of Regulation (EC) No 1334/2008’, and the data requirements for its safety evaluation can be found in the EFSA scientific opinion: ‘Guidance on the data required for the risk assessment of flavourings to be used in or on foods’ (EFSA CEF Panel, 2010a), Part B. IV. ‘Information to be supplied with an application for the authorisation of Other Flavourings’.
It is difficult to anticipate what kind of materials will undergo an evaluation as ‘Other Flavourings’, which suggests that the standard evaluation template is flexible. As a general approach, the following data should be provided:
full description of the production process, with emphasis on the parameters that might influence the composition of the flavouring;
identification and quantification of the substances present in the flavouring;
specifications of the flavouring;
exposure and toxicological data required to perform a risk assessment of the flavouring.
Appendix D – Congeneric groups metabolism and detoxification pathways
1.
| Congeneric group number | Congeneric group description | Metabolism | Detoxification pathways |
|---|---|---|---|
| 1 | Saturated linear primary aliphatic alcohols/aldehydes/acids/esters and acetals, including a cyclic acetal | Oxidation to corresponding acid, aldehydes may be reduced to alcohols, which may be conjugated to glucuronic acid. Medium‐chain carboxylic acids may condense with acetyl CoA to form fatty acids or omega‐oxidise to form diacids. Ultimately, these substances will be metabolised into carbon dioxide and water, or will be excreted via the urine | Fatty acid, beta‐oxidation, conjugation, TCA |
| 2 | Saturated aliphatic, acyclic, branched‐chain primary alcohols, aldehydes, carboxylic acids and related esters and acetals | Hydrolysis (esters and acetals). Oxidation to the corresponding aldehyde and carboxylic acid followed by beta‐oxidation predominantly in the longer branched chain to yield beta‐hydroxyacids which may be further oxidised (beta‐oxidation) and cleaved to yield short‐chain acids that are completely metabolised via the fatty acid pathway or tricarboxylic acid (TCA) cycles | Fatty acid, beta‐ oxidation, TCA |
| 3 | α,β‐Unsaturated linear and branched aliphatic primary alcohols/ketones/esters (excluding esters of α,β‐unsaturated carboxylic acids) | Oxidation to the corresponding aldehyde and carboxylic acid followed by beta‐oxidation predominantly in the longer branched chain to yield beta‐ hydroxyacids, which may be further oxidised (beta‐oxidation) and cleaved to yield short‐chain acids that are completely metabolised via the fatty acid pathway or tricarboxylic acid cycles. Part of the α,β‐unsaturated aldehydes formed from oxidation of corresponding alcohols may react with macromolecules (forming adducts). Cyclic ketones may be reduced to corresponding secondary alcohol and conjugated. Also, ring oxidation or oxidation of the ring substituent, followed by conjugation is an option. Allyl alcohol and 2‐propenyl acetate are converted to acrolein (i.e. 2‐propenal), which can be further oxidised or undergo reaction with cellular nucleophiles. Part of acrolein polymerises in vivo. The mercapturic acid of acrolein can be bioactivated by sulfoxidation to form nephrotoxic metabolites (Hashmi et al., 1992; Parent et al., 1998) | Fatty acid, TCA, ketoreduction or ring‐oxidation followed by conjugation |
| 4 | Ester of alicyclic carboxylic acid | Hydrolysis into ethanol and cyclopropane carboxylic acid. Ethanol will be converted into carbon dioxide. The acid moiety will most likely be conjugated with glucuronic acid/or glycine | Hydrolysis, conjugation |
| 5 | Esters of unsaturated linear and branched aliphatic carboxylic acids | Hydrolysis followed by conversion into carbon dioxide, conjugation and excretion | Hydrolysis, conjugation |
| 6 | Aliphatic primary alcohols, aldehydes, carboxylic acids, acetals and esters containing additional oxygenated functional groups | Esters and acetals will be hydrolysed; into the corresponding alcohols, aldehydes and carboxylic acid. In subsequent oxidation steps, these substances can be converted into carbon dioxide. Conjugation is also possible | Conjugation |
| 7 | Saturated aliphatic acyclic ketones | Oxidation to a carboxylic acid, which can be converted into carbon dioxide. Ketoreduction to secondary alcohols which will be conjugated and excreted | Conjugation, fatty acid metabolism; TCA |
| 8 | Aliphatic Aα‐diketones and Related α‐hydroxyketones | Oxidation to the corresponding keto‐carboxylic acids that can be converted into carbon dioxide; ketoreduction followed by conjugation | Conjugation, fatty acid metabolism; TCA |
| 9 | Alicyclic ketones and secondary alcohols | Ketones can be reduced to secondary alcohols which will be conjugated and excreted. Oxidation of ring substituents to give the corresponding alcohol which can also be conjugated and excreted | Conjugation |
| 10 | Aliphatic and alicyclic ethers | Metabolised by cytochrome P450 catalysed O‐dealkylation to the corresponding alcohol and aldehyde followed by complete oxidation in the fatty acid pathway and tricarboxylic acid cycle | Fatty acid, TCA |
| 11 | Furfural and related substances | Oxidation to furoic acid, conjugation and excretion | Conjugation |
| 12 | Furan derivatives | Metabolised by cytochrome P450 catalysed ring opening to the corresponding dialdehyde followed by non‐enzymatic conjugation with glutathione and amino‐groups and elimination in the urine. The (unsaturated) dialdehyde may form DNA adducts | Conjugation, oxidation to carbon dioxide |
Appendix E – information on occurrence of furan in food
1.
Furan has been reported to occur naturally in beef, beer (0–0.1 mg/L), cocoa, coffee, hazelnuts, honey (0–0.3 mg/kg), lamb and mutton, pork, potatoes, soybeans, tea, wheaten bread, tequila and truffles (Triskelion, 2017). Furan is reported most often as contaminant in heat‐processed foods (coffee, canned vegetables and fruits, beer, juice, potted meats, canned soups and sauces, soy sauce and cereals). Further information on the exposure to furan and 2‐methylfuran is anticipated to be included in the opinion of the EFSA CONTAM Panel, which will be published in the near future.
At a level of addition of rum ether to food of 250 mg/kg for example (Table B.1), a 0.02% content of furan (as proposed in the specifications) could give rise to a concentration of 50 μg/kg of furan in the food. This is similar to the upper concentration of furan found in food such as brewed coffee and foods that are heat‐processed in jars and cans.
Suggested citation: EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , Silano V, Bolognesi C, Castle L, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Gürtler R, Husøy T, Kärenlampi S, Milana MR, Penninks A, Tavares Poças MF, Smith A, Tlustos C, Wölfle D, Zorn H, Zugravu C‐A, Beckman Sundh U, Benigni R, Brimer L, Mulder G, Oskarsson A, Svendsen C, Martino C and Mennes W, 2017. Scientific Opinion of Flavouring Group Evaluation 500 (FGE.500): rum ether. EFSA Journal 2017;15(8):4897, 53 pp. 10.2903/j.efsa.2017.4897
Requestor: European Commission
Question number: EFSA‐Q‐2012‐00904
Panel members: Claudia Bolognesi, Laurence Castle, Jean‐Pierre Cravedi, Karl‐Heinz Engel, Paul Fowler, Roland Franz, Konrad Grob, Rainer Gürtler, Trine Husøy, Sirpa Kärenlampi, Wim Mennes, Maria Rosaria Milana, André Penninks, Maria de Fátima Tavares Poças, Vittorio Silano, Andrew Smith, Christina Tlustos, Detlef Wölfle, Holger Zorn and Corina‐Aurelia Zugravu.
Acknowledgements: The Panel wishes to thank the members of the Working Group on Flavourings: the hearing experts Vibe Beltoft and Karin Nørby for the support provided to this scientific opinion.
Adopted: 14 June 2017
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings. OJ L 354, 31.12.2008, p. 1–6.
EFFA (European Flavour Association), 2016a. Updated EFFA dossier on: The safety evaluation of flavourings other than flavouring substances, flavouring complexes (FCs): Rum ether. dated 22 February 2016. Unpublished report submitted by EFFA to EFSA.
EFFA (European Flavour Association), 2016b. Rum ether: additional data and clarifications from four companies. 20 September 2016. Unpublished data submitted by EFFA to EFSA.
Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
The APET has been calculated based on the occurrence levels in the food sub‐categories reported in the above table, with the exclusion of categories 13.2 (complementary foods for infants and young children).
Based on the same considerations as for adults but using a factor of 0.63 for children.
References
- Abraham K, Andres S, Palavinskas R, Berg K, Appel KE and Lampen A, 2011. Toxicology and risk assessment of acrolein in food. Molecular Nutrition & Food Research, 55, 1277–1290. 10.1002/mnfr.201100481 [DOI] [PubMed] [Google Scholar]
- Borzelleca JF, Larson PS, Hennigar GR Jr, Huf EG, Crawford EM and Smith RB Jr, 1964. Studies on the chronic oral toxicity of monomeric ethyl acrylate and methyl methacrylate. Toxicology and Applied Pharmacology, 6, 29–36. [DOI] [PubMed] [Google Scholar]
- Carthew P, Dinovi M and Setzer RW, 2010. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic: example: Furan (CAS No. 110‐00‐9). Food and Chemical Toxicology, 48 Suppl 1, S69–S74. [DOI] [PubMed] [Google Scholar]
- CoE , 1992. Flavouring substances and natural sources of flavourings. 4th Ed. vol. I. Chemically defined flavouring substances. Council of Europe, partial agreement in the social and public health field; Strasbourg. [Google Scholar]
- Cramer GM, Ford RA and Hall RL, 1978. Estimation of toxic hazard – a decision tree approach. Food and Cosmetics Toxicology, 16, 255–276. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with food on a request from the Commission related to furfural and furfural diethylacetal. Question number EFSA‐Q‐2003‐236. Adopted by written procedure on 2 June 2004. EFSA Journal 2004;2(7):67, 27 pp. 10.2903/j.efsa.2004.67 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission Flavouring Group Evaluation 2, Revision 1 (FGE.02Rev1): Branched‐ and straight‐chain aliphatic saturated primary alcohols and related esters of primary alcohols and straight‐chain carboxylic acids and one straight‐chain aldehyde from chemical groups 1 and 2. EFSA Journal 2008;6(5):709, 60 pp. 10.2903/j.efsa.2008.709 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008b. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from Commission on FGE.44: cis‐2–Heptyl‐cyclopropanecarboxylic Acid from Chemical Group 30. EFSA Journal 2008;6(9):805, 18 pp. 10.2903/j.efsa.2008.805 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2010a. Guidance on the data required for the risk assessment of flavourings to be used in or on foods. EFSA Journal 2010;8(6):1623, 38 pp. 10.2903/j.efsa.2010.1623 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2010b. Flavouring Group Evaluation 5, Revision 2 (FGE.05Rev2): branched‐ and straight‐chain unsaturated carboxylic acids and esters of these with aliphatic saturated alcohols from chemical groups 1, 2, 3 and 5. EFSA Journal 2010; 8(10):1400, 84 pp. 10.2903/j.efsa.2010.1400 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2010c. Flavouring Group Evaluation 01, Revision 2 (FGE.01Rev2): branched‐chain aliphatic saturated aldehydes, carboxylic acids and related esters of primary alcohols and branched‐chain carboxylic acids from chemical groups 1 and 2. EFSA Journal 2010;8(11):1843, 46 pp. 10.2903/j.efsa.2010.1843 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2011a. Scientific Opinion on Flavouring Group Evaluation 3, Revision 2 (FGE.03Rev2): acetals of branched‐ and straight‐chain aliphatic saturated primary alcohols and branched‐ and straight‐chain saturated or unsaturated aldehydes, an ester of a hemiacetal and an orthoester of formic acid, from chemical groups 1, 2 and 4. EFSA Journal 2011;9(10):2312, 65 pp. 10.2903/j.efsa.2011.2312 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2011b. Scientific Opinion on Flavouring Group Evaluation 13, Revision 2 (FGE.13 Rev2) Furfuryl and furan derivatives with and without additional side‐chain substituents and heteroatoms from chemical group 14. EFSA Journal 2011;9(8):2313, 126 pp. 10.2903/j.efsa.2011.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2012. Scientific Opinion on Flavouring Group Evaluation 10, Revision 3 (FGE.10Rev3): aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30. EFSA Journal 2012;10(3):2563, 127 pp. 10.2903/j.efsa.2012.2563 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2013. Scientific Opinion Flavouring Group Evaluation 23, Revision 4 (FGE.23Rev4): aliphatic, alicyclic and aromatic ethers including anisole derivatives from chemical groups 15, 16, 22, 26 and 30. EFSA Journal 2013;11(2):3092, 77 pp. 10.2903/j.efsa.2013.3092 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2014. Scientific Opinion on Flavouring Group Evaluation 11, Revision 3 (FGE.11Rev3): aliphatic dialcohols, diketones, and hydroxyketones from chemical groups 8 and 10. EFSA Journal 2014;12(11):3888, 60 pp. 10.2903/j.efsa.2014.3888 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2015a. Scientific Opinion on Flavouring Group Evaluation 67, Revision 2 (FGE.67Rev2): Consideration of 28 furan‐substituted compounds evaluated by JECFA at the 55th, 65th and 69th meeting (JECFA, 2001, 2006a and 2009b). EFSA Journal 2015;13(5):4155, 107 pp. 10.2903/j.efsa.2015.4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2015b. Scientific Opinion on Flavouring Group Evaluation 212 Revision 3 (FGE.212Rev3): α,β‐Unsaturated alicyclic ketones and precursors from chemical subgroup 2.6 of FGE.19. EFSA Journal 2015;13(5):4116, 39 pp. 10.2903/j.efsa.2015.4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2015c. Scientific Opinion on Flavouring Group Evaluation 09, Revision 6 (FGE.09Rev6): secondary alicyclic saturated and unsaturated alcohols, ketones and esters containing secondary alicyclic alcohols from chemical group 8 and 30, and an ester of a phenol derivative from chemical group 25. EFSA Journal 2015;13(9):4243, 81 pp. 10.2903/j.efsa.2015.4243 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2016. Scientific Opinion on Flavouring Group Evaluation 51, Revision 2 (FGE.51Rev2): Consideration of alicyclic ketones and secondary alcohols and related esters evaluated by the JECFA (59th meeting) structurally related to alicyclic ketones secondary alcohols and related esters in FGE.09Rev6 (2015b). EFSA Journal 2016;14(1):4338, 57 pp. 10.2903/j.efsa.2016.4338 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), Silano V, Bolognesi C, Castle L, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Gürtler R, Husøy T, Kärenlampi S, Milana MR, Penninks A, Tavares Poças MF, Smith A, Tlustos C, Wölfle D, Zorn H, Zugravu C‐A, Beckman Sundh U, Brimer L, Mosesso P, Mulder G, Anastassiadou M and Mennes W, 2017. Scientific Opinion on Flavouring Group Evaluation 7, Revision 5 (FGE.07Rev5): saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched‐chain carboxylic acids from chemical group 5. EFSA Journal 2017;15(3):4725, 81 pp. 10.2903/j.efsa.2017.4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2004. Report of the CONTAM Panel on provisional findings on furan in food. EFSA Journal 2004;2(12):137, 43 pp. 10.2903/j.efsa.2004.137 [DOI] [Google Scholar]
- FAO/WHO , 2008. Evaluation of certain food additives. Sixty‐ninth report of the joint FAO/WHO expert committee on food additives. Rome, 17–26 June 2008. WHO technical report series, No 952.
- Gill S, Bondy G, Lefebvre DE, Becalski A, Kavanagh M, Hou Y, Turcotte AM, Barker M, Weld M, Vavasour E and Cooke GM, 2010. Subchronic oral toxicity study of furan in Fischer‐344 rats. Toxicologic Pathology, 38, 619–630. [DOI] [PubMed] [Google Scholar]
- Gill S, Kavanagh M, Barker M, Weld M, Vavasour E, Hou Y and Cooke GM, 2011. Subchronic oral toxicity study of furan in B6C3F1 Mice. Toxicologic Pathology, 39, 787–794. [DOI] [PubMed] [Google Scholar]
- Gill S, Kavanagh M, Cherry W, Barker M, Weld M and Cooke GM, 2014. A 28‐day Gavage Toxicity Study in Male Fischer 344 Rats with 2‐methylfuran. Toxicologic Pathology, 42, 352–360. [DOI] [PubMed] [Google Scholar]
- Hashmi M, Vamvakas S and Anders MW, 1992. Bioactivation mechanism of S‐(3‐oxopropyl)‐N‐acetyl‐L‐cysteine, the mercapturic acid of acrolein. Chemical Research in Toxicology, 5(3), 360–365. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 1988. Alcohol drinking. IARC monographs on the evaluation of carcinogenic risks to humans, 44, 1–378. Available online: https://monographs.iarc.fr/ENG/Monographs/vol44/mono44.pdf [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 1995. Acrolein. IARC monographs on the evaluation of carcinogenic risks to humans, 63, 337–372. Available online: http://www.inchem.org/documents/iarc/vol63/acrolein.html [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 2010. Alcohol consumption and ethyl carbamate. IARC monographs on the evaluation of carcinogenic risks to humans, 96, 1–1428. Available online: https://monographs.iarc.fr/ENG/Monographs/vol96/mono96.pdf [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 2012. Consumption of alcoholic beverages. IARC monographs on the evaluation of carcinogenic risks to humans, 100E, 373–499. Available online: http://monographs.iarc.fr/ENG/Monographs/vol100E/mono100E-11.pdf [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1968. Specifications for the identity and purity of food additives and their toxicological evaluation. Eleventh Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 383. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974. 17. Report: Seventeenth Meeting of the Joint FAO/WHO Expert Committee on Food Additives. Report: Technical Report Series, no. 539. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1980. Evaluation of certain food additives. Twenty‐third report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 648, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1997. Evaluation of certain food additives and contaminants. Forty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 6–15 February 1996. WHO Technical Report Series, no. 868. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999. Evaluation of certain food additives and contaminants. Forty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Rome, 17–26 June 1997. WHO Technical Report Series, no. 884. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000a. Evaluation of certain food additives. Fifty‐first Meeting of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 9–18 June 1998. WHO Technical Report Series, no. 891. Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000b. Evaluation of certain food additives and contaminants. Fifty‐third meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series no. 896. Geneva, 1–10 June 1999.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001. Evaluation of certain food addtives and contaminants. Fifty‐fifth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 901. Geneva, 6–15 June 2000.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002a. Evaluation of certain food additives and contaminants. Fifty‐seventh report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 909. Geneva, 5–14 June 2001.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002b. Evaluation of certain food additives. Fifty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 913. Geneva, 4–13 June 2002.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2005. Evaluation of certain food additives. Sixty‐third report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 928. Geneva, 8–17 June 2004.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2009. Evaluation of certain food additives. Sixty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 952. Rome, 17–26 June 2008. http://whqlibdoc.who.int/trs/WHO_TRS_952_eng.pdf (May 2009)
- OECD SIDS , 2005. Initial Assessment Report For SIAM 21. 2‐Propen‐1‐ol Category. Berlin, Germany, 18–21 October 2005. Available online: http://www.inchem.org/documents/sids/sids/107186.pdf
- Parent R, Paust DE, Schrimpf MK, Talaat RE, Doane RA, Caravello HE, Lee SJ and Sharp DE, 1998. Metabolism and Distribution of [2,3‐14C] Acroleinin Sprague‐Dawley rats: II. Identification of Urinary and Fecal Metabolites Toxicological Sciences, 43, 110–120. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1992. Reports of the scientific committee for food (twenty‐ninth series) Food ‐ science and techniques series ISBN 92‐826‐4745‐5. Available online: http://aei.pitt.edu/40838/1/29th_food.pdf
- SCF (Scientific Committee for Food), 1995. First annual report on chemically defined flavouring substances. May 1995, 2nd draft prepared by the SCF Working Group on Flavouring Substances (Submitted by the SCF Secretariat, 17 May 1995). CS/FLAV/FL/140‐Rev2. Annex 6 to Document III/5611/95, European Commission, Directorate‐General III, Industry.
- Smith RL, Cohen SM, Doull J, Feron VJ, Goodman JI, Marnett LJ, Portoghese PS, Waddell WJ, Wagner BM, Hall RL, Higley NA, Lucas‐Gavin C and Adams TB. 2005. A procedure for the safety evaluation of natural flavour complexes used as ingredients in food: essential oils. Food and Chemical Toxicology, 43, 345–363, 10.1016/j.fct.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Triskelion , 2017. VCF online. Volatile compounds in food. Nijssen B, van Ingen‐Visscher K, Donders J. (eds.). Database Version 16.3, Triskelion, Zeist, Netherlands.
- USEPA (U.S. Environmental Protection Agency National Center for Environmental Assessment), 1986. Integrated Risk Information System (IRIS) Chemical Assessment Summary on Ethyl Ether; CASRN 60‐29‐7. Available on line: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0423_summary.pdf
- VKM (Norwegian Scientific Committee for Food Safety), 2012. Risk assessment of furan exposure in the Norwegian population; Opinion of the Panel on Food Additives, Flavourings, Processing Aids, Materials in Contact with Food and Cosmetics and the Panel on Contaminants of the Norwegian Scientific Committee for Food Safety. 2012;107 pp.
- Von Tungeln LS, Walker NJ, Olson GR, Mendoza MC, Felton RP, Thorn BT, Marques MM, Pogribny IP, Doerge DR and Beland FA, 2017. Low dose assessment of the carcinogenicity of furan in male F344/N Nctr rats in a 2‐year gavage study. Food and Chemical Toxicology, 99, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]


