Abstract
Following a request from the European Commission, the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to deliver a scientific opinion on the re‐evaluation of soybean hemicellulose (E 426) as a food additive. Soybean hemicellulose is not absorbed intact, but is extensively fermented by the intestinal microflora in animals and humans. No adverse effects were reported in a 90‐day dietary toxicity study in rats at the highest doses tested of 2,430 mg/kg body weight (bw) per day for males and 2,910 mg/kg bw per day for females. Furthermore, soybean hemicellulose is not of genotoxic concern. The highest exposure estimates calculated based on the maximum permitted levels were up to 191 mg/kg bw per day for children (95th percentile). Given the limited uses, if any, reported, the Panel considered it probable that the actual dietary exposure to soybean hemicellulose (E 426) would be negligible. Following the conceptual framework for the risk assessment of certain food additives, the Panel concluded that it is very unlikely that there is a safety concern from the current use of soybean hemicellulose (E 426) as a food additive, and that there is no need for a numerical acceptable daily intake (ADI). The Panel recommended that the amount of residual proteins in E 426 should be reduced as much as possible, and that consumers should be informed of the presence of potentially allergenic proteins in the food additive.
Keywords: soybean hemicellulose, E 426, food additive
Summary
Following a request from the European Commission (EC), the Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to deliver a scientific opinion re‐evaluating the safety of soybean hemicellulose (E 426) when used as a food additive.
Soybean hemicellulose (E 426) is an authorised food additive in the European Union (EU) in accordance with Annex II to Regulation (EC) No 1333/2008 on food additives, and specific purity criteria have been defined in Commission Regulation (EU) No 231/2012.
Soybean hemicellulose (E 426) has been evaluated by the Scientific Committee for Food (SCF) in 2003. No acceptable daily intake (ADI) was specified. The SCF compared the intake of soybean hemicellulose as an additive (E 426) with that of soybean fibre in the diet and concluded that soy‐based dietary fibre may be safely used at a daily oral intake of up to 25–35 g/person per day, much more than two orders of magnitude above the range of intake of the food additive E 426 from the intended applications (up to 0.05 g/person per day).
The Panel noted that the Chemical Abstract Service (CAS) Registry No 9034‐32‐6, corresponding to the EC No 618‐530‐1, is not unique for soybean hemicellulose, but is used to identify hemicelluloses in general, whereas EC No 923‐430‐9 refers to soybean hemicellulose but without a corresponding CAS Registry number. The Panel also noted that, according to EU specifications, impurities of the toxic elements arsenic, lead, mercury and cadmium, are accepted up to concentrations of 2, 5, 1 and 1 mg/kg, respectively.
Data from in vitro studies indicated that hemicelluloses or their major components (xylan, glucomannan) would be fermented during passage through the human colon. The main end products of these colonic anaerobic digestive processes are short‐chain fatty acids (SCFA). The Panel considered that these data indicate that hemicelluloses would be most probably not absorbed intact, but fermented by the intestinal microflora in animals and humans.
An acute oral toxicity study in rats with soybean hemicellulose (E 426) resulted in an oral LD50 > 2,000 mg/kg body weight (bw).
Soluble soybean hemicellulose did not show adverse effects in a well‐conducted (OECD Guideline 408 and good laboratory practice (GLP)) 90‐day dietary study in rats at concentrations up to 2,430 mg/kg bw per day in males and 2,910 mg/kg bw per day in females, the highest doses tested.
No mutagenic activity was detected in the bacterial reverse mutation test in Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537, and Escherichia coli strain WP2uvrA. No other in vitro or in vivo studies were available. Although the data were limited, the Panel concluded that, similarly to other dietary polysaccharides, soybean hemicellulose is not of genotoxic concern.
No chronic toxicity or carcinogenicity studies or reproductive or developmental toxicity studies were available.
Several soy proteins have been shown to have allergenic potential, some having cross‐reactivity with other plant allergens (e.g. with peanuts). Even though the consequences of allergenicity of soy proteins and the associated reported cases of anaphylaxis in children are still a matter of debate, the Panel noted that a significant amount of protein (up to 14% in mass) can be present in the food additive E 426. An unpublished report on the sensitising properties of soy polysaccharides in guinea pigs suggested that the sensitisation potential of orally administered soy polysaccharides may be considered very low compared with that of soy proteins. However, the risk of sensitisation in persons allergic to soy proteins cannot be excluded given the presence of residual proteins in soybean hemicellulose (E 426). Therefore, the Panel considered that the amount of residual proteins in E 426 should be reduced as much as possible, and that consumers should be informed of the presence of potentially allergenic proteins in the food additive.
No information on the actual usage of soybean hemicellulose (E 426) in foodstuffs was provided by the industry. Therefore, the exposure assessment for soybean hemicellulose (E 426) was performed based only on the maximum permitted levels (MPLs), as set in Annex II to Regulation (EC) No 1333/2008 (regulatory maximum level exposure assessment scenario). Exposure estimates derived following this scenario should be considered conservative, as this scenario assumes that the consumer will be exposed to the food additive present in food at the maximum level over a long period of time. Mean estimates ranged from 5.0 mg/kg bw per day in the adult population to 117.0 mg/kg bw per day in toddlers. At the 95th percentile, estimates ranged from 18.6 mg/kg bw per day in the elderly to 191 mg/kg bw per day in children. Children and toddlers were identified as the groups with the highest exposure.
As an additional source of information on the use of soybean hemicellulose (E 426) in foods, the Mintel Global New Products Database (GNPD) was consulted. According to this database, in the EU, soybean hemicellulose (E 426) was labelled on only one product (ice‐cream sandwich) in 2002.
Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the actual exposure to soybean hemicellulose (E 426) as a food additive in European countries for the regulatory maximum level exposure scenario.
Therefore, the Panel considered it likely that the food additive might not be used in the food categories in which it is authorised and that the actual dietary exposure to soybean hemicellulose (E 426) would be negligible.
Mean intakes of soybean hemicellulose from the regular diet, for consumers only, were much lower (up to 5.9 mg/kg bw in toddlers) than the mean estimated exposure to soybean hemicellulose as a food additive (E 426).
Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010, presented by the ANS Panel in 2014, and given that:
soybean hemicellulose is not absorbed intact, but is extensively fermented by the intestinal microflora in animals and humans to SCFA;
no adverse effects were reported in an adequate dietary 90‐day study in rats at the highest dose tested of 2,430 mg/kg bw per day for males and 2,910 mg/kg bw per day for females;
soybean hemicellulose is not of genotoxic concern;
the highest exposure estimates, calculated based on the MPLs to be up to 191 mg/kg bw per day for children (95th percentile), are very conservative;
no uses were reported by industry and only one food product containing soybean hemicellulose (E 426) was found in the Mintel GNPD,
the Panel concluded that it is very unlikely that there is a safety concern from the current use of soybean hemicellulose (E 426) as a food additive for the general population, and that there is no need for a numerical ADI.
Due to the absence of information provided by industry on the usage of E 426 and only a single food product containing soybean hemicellulose (E 426) reported in the Mintel GNPD database, the Panel recommended the collection of data on usage and use levels of soybean hemicellulose (E 426), in order to perform a more realistic exposure assessment.
The Panel recommended that the maximum limits for the impurities of toxic elements (arsenic, lead, mercury and cadmium) in the EU specifications for soybean hemicellulose (E 426) should be revised in order to ensure that soybean hemicellulose (E 426) as a food additive will not be a significant source of exposure to those toxic metals in food.
Furthermore, the Panel recommended that the amount of residual proteins in soybean hemicellulose (E 426) should be reduced as much as possible, and that consumers should be informed of the presence of allergenic proteins in the food additive E 426.
1. Introduction
The present opinion deals with the re‐evaluation of the safety of soybean hemicellulose (E 426) when used as a food additive. Soybean hemicellulose (E 426) is an authorised food additive in the European Union (EU) according to Annex II to Regulation (EC) No 1333/2008.1
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the EU. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the EU before 20 January 2009 has been set up under Regulation (EU) No 257/2010.2 This Regulation also foresees that food additives are re‐evaluated whenever necessary in the light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU3 of 2001. The report ‘Food Additives in Europe 2000’ submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010, the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks EFSA to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.2. Information on existing authorisations and evaluations
Toxicological data for soybean hemicellulose (E 426) were evaluated by the SCF in 2003. The Committee concluded that the proposed use of soybean hemicellulose (E 426) in foods and the levels of use are acceptable. No acceptable daily intake (ADI) was specified.
The SCF compared the intake of soybean hemicellulose as an additive (E 426) with that of soybean fibre in the diet. The SCF concluded that soy‐based dietary fibre may be safely used at a daily oral intake of up to 25–35 g/person per day which is much more than the intake of the food additive soybean hemicellulose (E 426) from the intended applications (up to 0.5 g/person per day) (SCF, 2003). The SCF pointed to the limits for heavy metals and the possible allergenic potential.
Soybean hemicellulose has not been evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA).
2. Data and methodologies
2.1. Data
The Panel on Food Additives and Nutrient Sources added to Food (ANS) was not provided with a newly submitted dossier. EFSA launched public calls for data4 , 5 , 6 to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to November 2016. Attempts were made to retrieve any relevant original study reports on which previous evaluations or reviews were based, however, these were not always available to the Panel.
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database7) was used to estimate the dietary exposure.
The Mintel Global New Products Database (GNPD) is an online resource listing food products and compulsory ingredient information that should be included in labelling. This database was used to verify the use of soybean hemicellulose (E 426) in food products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The ANS Panel assessed the safety of soybean hemicellulose (E 426) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the SCF (2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
When the test substance was administered in the feed or drinking water, but doses were not explicitly reported by the authors as mg/kg body weight (bw) per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as equivalent. When, in human studies in adults (aged above 18 years), the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population, as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012).
Dietary exposure to soybean hemicellulose (E 426) from its use as a food additive was estimated by combining food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum levels according to Annex II to Regulation (EC) No 1333/2008 (see Section 3.4.1). Uncertainties in the exposure assessment were identified and discussed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
In general, hemicelluloses are polysaccharides with β‐(1→4)‐linked backbones with an equatorial (i.e. radiating out from the glucose ring) configuration. Hemicelluloses include xyloglucans, xylans, mannans and glucomannans. The detailed structure of the hemicelluloses and their abundance vary widely between different species and cell types (Scheller and Ulvskov, 2010).
Soy fibre is a mixture of cellulosic and non‐cellulosic structural components of the internal cell wall of soybeans. The major fractions are non‐cellulosic (Aspinall et al., 1967). Soybean hemicellulose (SHC) is extracted from soy fibre.
The Panel noted that the Chemical Abstract Service (CAS) Registry No 9034‐32‐6, corresponding to EC (EINECS) No 618‐530‐1, is not unique to soybean hemicellulose, but is used to identify hemicelluloses in general. EC No 923‐430‐9 refers to soybean hemicellulose but without a corresponding CAS Registry number.
The molecular formula of soybean hemicellulose is unspecified. The backbone chain and the side chains contain as repeating units: rhamnose (Rha), fucose (Fuc), arabinose (Ara), xylose (Xyl), galactose (Gal), glucose (Glc) and galacturonic acid (GalA) (Aspinall et al., 1966; Kikuchi and Sugimoto, 1976; Nakamura et al., 2002a).
For soybean hemicelluloses, based on gel‐permeation chromatography results, four subgroups were identified, with molecular weights indicated by chromatographic peaks at ~ 550 × 103, 150 × 103, 25 × 103 and 5 × 103 g/mol (Furuta and Maeda, 1999).
The chemical structure of soluble soybean hemicellulose is complex and not yet fully elucidated (Figure 1).
Figure 1.
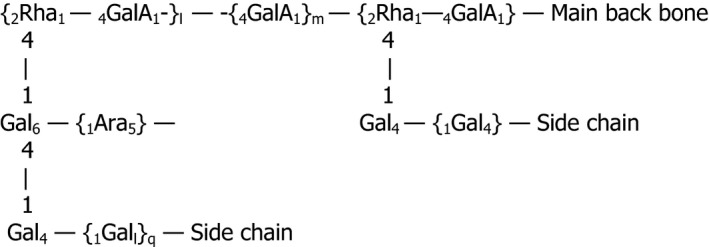
Structural formula of soluble soybean hemicellulose (adapted from Maeda and Nakamura, 2009 copyright (1999) with permission from Elsevier)
According to Maeda and Nakamura (2009), the structure is composed of:
the starting non‐reducing‐end backbone composed of a galacturonan chain: [‐4)‐α‐d‐GalA‐1‐]m (m = 7–9).
-
a first intermediate backbone composed of a rhamnogalacturonan chain: [‐4)‐α‐d‐GalA‐(1→2)‐α‐l‐Rha‐(1→4)‐α‐d‐GalA‐(1‐]n (n = 15–100). This intermediate structure carries the following side chains:
-
1
‐ homoarabinan side chains: [‐3 or 5)‐α‐l‐Ara‐1‐]o (o = undefined)
-
2
‐ homogalactan side chains: [‐4)‐β‐d‐Gal‐1‐]p (p = undefined)
-
3
‐ galacturonans with side chains composed of xylans
-
4
‐ galactans chain branched by neutral sugars (Ara, Xyl, Fuc, Glc)
-
1
a second intermediate backbone composed of a rhamnogalacturonan chain: [‐2)‐α‐l‐Rha‐(1→4)‐α‐d‐GalA‐(1]k→2‐α‐l‐Rha‐(1→4)‐α‐d‐GalA‐(1‐], k = 4–10
The terminal reducing‐end backbone composed of a galacturonan chain: [‐4)‐α‐d‐GalA‐1‐].
The detailed structure of the main component of soybean hemicelluloses has a molecular weight of 550 × 103 g/mol and consists of two types of units in the main backbone: a short‐chain homogalacturonan (degree of polymerisation, DP, 4–10) and a long‐chain rhamnogalacturonan (repeating units 15, 28, 100) (Nakamura et al., 2002a).
The side chains are composed of galactans (DP, 43–47) branched with fucose and arabinose residues, and arabinans; these arabinans are linked to rhamnose residues in the rhamnogalacturonan. Some galacturonic acids are modified xylosyl oligosaccharides (DP, 4–7) (Nakamura et al., 2002b).
Soybean hemicelluloses also contain peptides supposed to be covalently bound to the rhamnogalacturonan main backbone (Matsumura et al., 2003).
Several varieties of commercially available soybean hemicellulose exist but they all are very similar in composition and are composed of the same types of sugars. The composition of three different varieties of soybean hemicellulose is presented in Table 1.
Table 1.
Average composition of different soybean hemicellulose products (%)
| Sugar composition | Soyafibe‐S (Maeda and Nakamura, 2009) | Crude SHC (Nakamura et al., 2001) | SHC (Wang et al., 2005) |
|---|---|---|---|
| Rhamnose (Rha) | 5.0 | 5.1 | 5.5 |
| Fucose (Fuc) | 3.2 | 2.0 | 1.1 |
| Arabinose (Ara) | 22.6 | 18.5 | 20.5 |
| Xylose (Xyl) | 3.7 | 2.9 | 3.5 |
| Galactose (Gal) | 46.1 | 49.7 | 48.5 |
| Glucose (Glc) | 1.2 | 2.0 | 1.0 |
| Galacturonic acid (GalA) | 18.8 | 19.8 | 19.9 |
SHC: soybean hemicellulose.
The composition and physical characteristics of different commercial soybean hemicellulose products were described by the SCF (2003). Furthermore, the SCF reported the main components of soybean hemicellulose to be within the following ranges: moisture, 4.5–6.5%; crude protein, 4.8–13.9%; ash, 6.0–8.6%; dietary fibre content, 60.0–77.0%; carbohydrates, 74.0–83.0%; others, 6.0–14.0%.
SHC is a free‐flowing white or yellowish white powder. It is soluble in hot and cold water without gel formation and the viscosity of a 10% solution must be not more than 200 mPa per second (mPa·s). Depending on the extraction method used, different viscosities have been reported. The viscosity of a 10% solution extracted under weak acidic conditions was 75.8 mPa·s (Furuta and Maeda, 1999), whereas a 9.1% solution extracted with hot water (Morita, 1965) had a viscosity of 209 mPa·s (Thompson et al., 1987). A much lower viscosity of 16.3 mPa·s was measured when extraction under alkaline conditions was carried out (Aspinall et al., 1967).
Synonyms are water‐soluble soybean polysaccharides, water‐soluble soybean fibre, semicellulose or water‐soluble polysaccharide. Examples of commercial names are Soyafibe S‐DN and Soya‐Up M 800.
3.1.2. Specifications
The specifications for soybean hemicellulose (E 426), as defined in Commission Regulation (EU) No 231/20128, are listed in Table 2.
Table 2.
Specifications for soybean hemicellulose (E 426) according to Commission Regulation (EU) No 231/2012
| Commission Regulation (EU) No 231/2012 | |
|---|---|
| Definition | Soybean hemicellulose is a refined water‐soluble polysaccharide obtained from strain soybean fibre by hot water extraction. No organic precipitant shall be used other than ethanol |
| Assay | Not less than 74% carbohydrate |
| Description | Free‐flowing white or yellowish white powder |
| Identification | |
| Solubility | Soluble in hot and cold water without gel formation |
| pH | 5.5 ± 1.5 (1% solution) |
| Purity | |
| Loss on drying | Not more than 7% (105°C, 4 h) |
| Protein | Not more than 14% |
| Viscosity | Not more than 200 mPa·s (10% solution) |
| Total ash | Not more than 9.5% (600°C, 4 h) |
| Arsenic | Not more than 2 mg/kg |
| Ethanol | Not more than 2% |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Microbiological criteria | |
| Total plate count | Not more than 3,000 colonies per gram |
| Yeast and moulds | Not more than 100 colonies per gram |
| Escherichia coli | Absent in 10 g |
The Panel noted that, according to the EU specifications for soybean hemicellulose (E 426), impurities of the toxic elements arsenic, lead, mercury and cadmium are accepted up to concentrations of 2, 5, 1 and 1 mg/kg, respectively. Contamination at those levels could have a significant impact on the exposure to these metals, for which the intake is already close to the health‐based guidance values established by EFSA (EFSA, 2009; EFSA CONTAM Panel, 2009, 2010, 2012).
In view of the botanical origin of soybean hemicellulose, possible contamination with pesticides should be considered. The Panel considered it necessary to pay attention to the compliance of soybean hemicellulose raw material to the existing EU regulation on pesticides.
3.1.3. Manufacturing process
Soybean hemicellulose is located in the fibre of the soybean Glycine max (L.) Merr.
Several studies dealing with the production of soluble soybean polysaccharides are described in the literature (Kawamura et al., 1955; Morita, 1965; Yoshii et al., 1996; Sonda et al., 2002).
In general, soluble soybean hemicellulose is produced from ‘okara’, a by‐product of the production of soy oil, soy protein isolates or tofu.
Furuta et al. (1998) described a method for the extraction of water‐soluble soybean polysaccharides from soybean cotyledons under acidic conditions at different pH values and temperatures (pH 4–6/100–120°C or pH 2–3/40–80°C). It was indicated that the molecular weight was relatively high (main fractions M w > 150 × 103 g/mol) and comparable for both processes, but samples extracted under the higher pH and temperature conditions remained fluid, whereas the others gelled.
During the production process, okara, in diluted acid (pH 5, adjusted with HCl) is autoclaved at 120°C for 1.5 h. After cooling to room temperature, the suspension is centrifuged at 10,000 g for 30 min. The remaining soluble soya fibre in the residue is removed by washing with distilled water and the extract is centrifuged again. The supernatants are combined, concentrated and dried.
The resulting water‐soluble fraction contains a variety of polysaccharides that differ in their physical properties and functionality. According to Maeda and Nakamura (2009), a sub‐fraction of water‐soluble polysaccharides is obtained by ethanol precipitation. The extraction is followed by refining, pasteurisation and spray‐drying.
3.1.4. Methods of analysis in food
There are no methods available for the analysis of soybean hemicellulose in foodstuffs.
Soybean hemicellulose (E 426) has a high content of dietary fibre (60−77%). The standard method for the determination of total dietary fibre was defined by the AOAC 985.29 (AOAC, 1992) and is based on the method of Prosky et al. (1985).
Recently, a new AOAC official enzymatic‐gravimetric method has been validated that describes the analysis of insoluble, soluble and total dietary fibre (AOAC 2011.25; McCleary et al., 2012). This method combines key attributes of AOAC 985.29 and its extension AOAC 991.43. In this method, the test substance is treated with pancreatic α‐amylase and amyloglucosidase for 16 h at 37°C in a sealed container under agitation. The reaction is terminated by pH adjustment and temporary heating. The protein fraction is then digested with proteinase.
For the measurement of insoluble dietary fibre (IDF), the digestate is filtered and the IDF is determined gravimetrically.
For the measurement of water‐soluble, but ethanol‐insoluble, dietary fibre (e.g. soybean hemicellulose), ethanol is added to the filtrate of IDF. The precipitated soluble dietary fibre is captured by filtration and determined gravimetrically. The non‐precipitable water‐ and alcohol‐soluble dietary fibre is recovered by concentrating the filtrate, deionised using ion‐exchange resins and quantified by liquid chromatography.
Huang et al. (2013) described an analytical method for rapid determination of insoluble (indigestible) dietary fibre in soybeans and other types of beans. The method was a modification of AACC method 32–20 (AACC International, 1999) and AOAC method 985.29 (AOAC, 1992) for the determination of total dietary fibre. The procedure included starch gelatinisation (20 min at 100°C in a water bath) and hydrolysis with 2.5% (w/w) α‐amylase, followed by a neutral detergent wash and acetone extraction. The authors stated that the filtration and enzymatic treatment procedures in the improved method were completed within 15 min and 1.5 h, respectively. The length of time required for filtration and enzymatic hydrolysis in the improved method was significantly shortened from 19.5 h (AACC method) to 1.75 h. The recovery yield of microcrystalline cellulose (used as internal standard) was 97.75% (w/w). According to the authors, this was in agreement with the result obtained using the typical AACC method and demonstrated the reliability of the improved method.
3.1.5. Stability of the substance, and reaction and fate in food
Soybean hemicellulose (E 426) itself is very stable against hydrolysis. It is reported that soybean hemicellulose endures sterilisation for 15 min at pH 3–6, 120°C and storage for a long time at pH 3 and at 70°C (Furuta and Maeda, 1999).
3.2. Authorised uses and use levels
Maximum levels of soybean hemicellulose (E 426) have been defined in Annex II to Regulation (EC) No 1333/2008 on food additives, as amended. In this document, these levels are named maximum permitted levels (MPLs).
Currently, soybean hemicellulose (E 426) is an authorised food additive in the EU at levels ranging from 1,500 to 30,000 mg/kg in foods.
The foods permitted to contain soybean hemicellulose (E 426) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008 are summarised in Table 3.
Table 3.
MPLs of soybean hemicellulose (E 426) in foods according to Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | Restrictions/exceptions | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|
| 04.2.6 | Processed potato products | Only prepacked processed potato products | 10,000 |
| 05.2 | Other confectionary, including breath‐freshening microsweets | Only jelly confectionary, except jelly mini‐cups | 10,000 |
| 05.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | Only jelly confectionery (other than jelly mini‐cups) | 10,000 |
| 06.5 | Noodles | Only prepackaged ready‐to‐eat oriental noodles intended for retail sale | 10,000 |
| 06.7 | Pre‐cooked or processed cereals | Only prepackaged ready‐to‐eat rice and rice products intended for retail sale | 10,000 |
| 07.2 | Fine bakery wares | Only prepackaged fine bakery wares intended for retail sale | 10,000 |
| 10.2 | Processed eggs and egg products | Only dehydrated and concentrated frozen and deep‐frozen egg products | 10,000 |
| 12.6 | Sauces | Only emulsified sauces | 30,000 |
| 14.1.4 | Flavoured drinks | Only dairy‐based drinks intended for retail sale | 5,000 |
| 17.1a | Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms | 1,500 | |
| 17.2a | Food supplements supplied in a liquid form | 1,500 | |
| 17.3a | Food supplements supplied in a syrup‐type or chewable form | 1,500 |
MPL: Maximum permitted level.
Food category (FC) 17 refers to food supplements as defined in Directive 2002/46/EC of the European Parliament and of the Council excluding food supplements for infants and young children.
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of soybean hemicellulose (E 426)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a level lower than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment, especially for those food additives for which no MPL is set and which are authorised according to quantum satis (QS).
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued a public call9 for scientific data on food additives including soybean hemicellulose (E 426), on current use and use patterns, i.e. which food categories and subcategories, proportion of food within categories/subcategories in which it is used, actual use levels (typical and maximum), especially for those uses which are only limited by QS. In response to this call, no data on soybean hemicellulose (E 426) were submitted to EFSA.
In October 2015, a public call10 for food additive usage level and/or concentration data in food and beverages intended for human consumption, including soybean hemicellulose (E 426), was launched, with a deadline of May 2016. No information on soybean hemicellulose (E 426) was made available to EFSA in response to this public call.
3.3.2. Summarised data extracted from the Mintel Global New Products Database
The Mintel GNPD is an online database that monitors product introductions in consumer packaged goods markets worldwide. It contains information on over 2 million food and beverage products, of which more than 900,000 are or have been available on the European food market. Mintel started covering the EU's food markets in 1996, currently having 20 out of its 28 Member States and Norway presented in the Mintel GNPD.11
For the purpose of this Scientific Opinion, the Mintel GNPD12 was used to check the labelling of products containing E 426 within the EU's food products, as it shows the compulsory ingredient information presented on the labelling of products.
According to the Mintel GNDP, soybean hemicellulose (E 426) was labelled on only one product, in 2002 (ice‐cream sandwich).
3.3.3. Food consumption data used for exposure assessment
3.3.3.1. EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. EFSA Guidance on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a)). New consumption surveys13 added in the Comprehensive Database14 were also taken into account in this assessment.
The food consumption data gathered by EFSA were collected using different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced due to possible subjects' underreporting and/or misreporting of consumption amounts. Nevertheless, the EFSA Comprehensive Database represents the best available source of food consumption data across Europe at present.
Food consumption data for the following population groups: infants, toddlers, children, adolescents, adults and the elderly were used for the exposure assessment. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 4).
Table 4.
Population groups considered for the exposure estimates of soybean hemicellulose (E 426)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlers | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, the Netherlands, Spain, UK |
| Childrena | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlya | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Sweden, UK |
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merging of ‘elderly’ and ‘very elderly’ in the EFSA Guidance on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS), as presented in Annex II to Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, FoodEx food codes were matched to the FCS food categories.
3.3.3.2. Food categories selected for the exposure assessment of soybean hemicellulose (E 426)
The food categories in which the use of soybean hemicellulose (E 426) is authorised were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Some food categories or their restrictions/exceptions are not referenced in the EFSA Comprehensive Database and could, therefore, not be taken into account in the present estimate. This was the case for three food categories and may have resulted in an underestimation of the exposure. The food categories which were not taken into account are described below (in ascending order of the FCS codes):
05.4 Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 only jelly confectionery (other than jelly mini‐cups),
06.5 Noodles, only prepackaged ready‐to‐eat oriental noodles intended for retail sale,
06.7 Pre‐cooked or processed cereals, only prepackaged ready‐to‐eat rice and rice products intended for retail sale.
For the following food categories, the restrictions/exceptions which apply to the use of soybean hemicellulose (E 426) could not be taken into account, and therefore the whole food category was considered in the exposure assessment. This applies to three food categories and may have resulted in an overestimation of the exposure:
04.2.6 Processed potato products, only prepackaged processed potato product,
07.2 Fine bakery wares, only prepackaged fine bakery wares intended for retail sale,
10.2 Processed eggs and egg products, only dehydrated and concentrated frozen and deep‐frozen egg products.
The restriction ‘only dairy‐based drinks’ related to the food category ‘Flavoured drinks’ (FC 14.1.4) was taken into account in the selection of the foods, whereas the restriction ‘intended for retail sale’, also related to the flavoured drinks, could not be taken into account.
In the EFSA Comprehensive Database, no information is provided on the type of food supplements consumed by infants and young children. In the exposure assessment, it was therefore assumed that the food supplements consumed by these population groups were the same as those consumed in the older population groups, resulting in an overestimation of the exposure to soybean hemicellulose (E 426) in infants and young children.
Overall, for the regulatory maximum level exposure scenario, nine food categories were included in the present exposure assessment to soybean hemicellulose (E 426).
3.4. Exposure estimate
3.4.1. Exposure to soybean hemicellulose (E 426) from its use as a food additive
The Panel estimated chronic exposure to soybean hemicellulose (E 426) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. Dietary exposure to soybean hemicellulose (E 426) was calculated by multiplying soybean hemicellulose (E 426) concentrations for each food category (Table 3) by their respective consumption amount per kilogram of body weight, for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only 1 day per subject were excluded, as they are considered not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 4). On the basis of these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was calculated only for those population groups in which the sample size was sufficiently large to allow this calculation (EFSA, 2011a). Therefore, in the present assessment, 95th percentiles of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not included.
Exposure assessment of soybean hemicellulose (E 426) was carried out by the ANS Panel based on the MPLs as set down in the EU legislation (defined as the regulatory maximum level exposure assessment scenario); this scenario is discussed in detail below.
3.4.1.1. Regulatory maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008 and listed in Table 3.
The Panel considers the exposure estimates derived following this scenario to be conservative, as it is assumed that the population group will be exposed to soybean hemicellulose (E 426) present in food at the MPL over a longer period of time.
3.4.1.2. Dietary exposure to soybean hemicellulose (E 426)
Table 5 summarises the estimated exposure to soybean hemicellulose (E 426) from its use as a food additive in six population groups (Table 4) according to the regulatory maximum level exposure assessment scenario (Section 3.4.4.1). Detailed results per population group and survey are presented in Appendix A.
Table 5.
Summary of dietary exposure to soybean hemicellulose (E 426) from its use as a food additive in the regulatory maximum level exposure assessment scenario in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks to 11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Mean | 6.8–48.2 | 37.1–117.0 | 29.9–98.5 | 11.4–52.1 | 5.0–28.2 | 5.9–31.4 |
| 95th percentile | 70.5–122.2 | 82.9–188.1 | 72.4–190.8 | 31.8–101.1 | 20.6–64.9 | 18.6–69.4 |
3.4.1.3. Main food categories contributing to exposure to soybean hemicellulose (E 426) using the regulatory maximum level exposure assessment scenario (Table 6)
Table 6.
Main food categories contributing to exposure to soybean hemicellulose (E 426) using MPLs (> 5% to the total mean exposure) and number of surveys in which each food category is contributing
| Food category number | Food category name | Infants | Toddlers | Children | Adolescents | Adults | The elderly |
|---|---|---|---|---|---|---|---|
| Range of % contribution to the total exposure (number of surveys)a | |||||||
| 04.2 | Processed fruit and vegetables | 13.1–100 (6) | 5.8–95.9 (10) | 6.5–82.5 (17) | 8.5–77.9 (16) | 10.4–77.4 (14) | 21.7–77.8 (13) |
| 05.2 | Other confectionery including breath‐refreshening microsweets | − | 5.9 (1) | 6.6–10.7 (3) | 5.6–13.2 (4) | − | − |
| 07.2 | Fine bakery wares | 17.9–85.2 (3) | 14.6–81.6 (9) | 8.8–79.4 (17) | 6.3–83.5 (16) | 9.0–85.4 (17) | 8.7–61.6 (14) |
| 12.6 | Sauces | − | 11.6 (1) | 5.6–14.4 (4) | 5.3–19.3 (10) | 7.7–19.7 (14) | 5.3–16.4 (9) |
| 14.1.4 | Flavoured drinks | − | 6.3–21.4 (5) | 5.1–23.1 (15) | 5.7–24.1 (9) | 6.3–58.8 (3) | 5.2–57.0 (2) |
−: Food categories not contributing or contributing less than 5% to the total mean exposure.
The total number of surveys may be greater than the total number of countries as listed in Table 4, because some countries submitted more than one survey for a specific population.
3.4.1.4. Uncertainty analysis
Uncertainties in the exposure assessment of soybean hemicellulose (E 426) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA Scientific Committee, 2007), the following sources of uncertainties have been considered and are summarised in Table 7.
Table 7.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/− |
| Use of data from food consumption survey of few days to estimate long‐term (chronic) exposure for high percentiles (95th percentile) | + |
| Food categories selected for the exposure assessment: exclusion of food categories due to missing FoodEx linkage (n = 3/12 food categories) | − |
| Food categories selected for the exposure assessment: inclusion of food categories without considering the restriction/exception (n = 3/12 food categories) | + |
| Regulatory maximum level exposure assessment scenario: exposure calculations based on the MPL according to Annex II to Regulation (EC) No 1333/2008 | + |
+: uncertainty with potential to cause overestimation of exposure; −: uncertainty with potential to cause underestimation of exposure.
Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to soybean hemicellulose (E 426) as a food additive in European countries for the regulatory maximum level exposure scenario.
Information retrieved from the Mintel GNPD showed that only one food item was labelled with this food additive throughout the EU market. This is in line with the fact that no use levels were reported to EFSA by industry (neither by food industry nor by food additive manufacturers).
Therefore, the Panel considered it likely that the additive might not be used in food categories in which it is authorised and that the actual dietary exposure to soybean hemicellulose (E 426) from its use as a food additive would be negligible.
3.4.2. Exposure via the regular diet
For the calculation of exposure to soybean hemicellulose via the regular diet, it was assumed that all the fibre present in soy‐derived foods is hemicellulose, as only data on the total dietary fibre were available for these products. As other non‐starch polysaccharides are also present in soybean fibre, this assumption may lead to an overestimation of the intake of soybean hemicellulose from natural sources.
Different sources of food composition information were consulted (FOODDATA, DTU‐Food of National Institute15; Ciqual‐ANSES16; BEDCA; Spanish database17; NEVO, Dutch Food Composition Database18). Among these, the NEVO database was considered to be the most complete, and therefore was used to retrieve information on the total dietary fibre content of soy food products.
The amount of total dietary fibre in soybean and soy‐derived foodstuffs (mg fibre/g food) used for calculating the intake of soybean hemicellulose via the regular diet is presented in Table 8.
Table 8.
Composition data on total dietary fibre in soybean and soy‐derived foodstuffs (NEVO database)
| Foods | Total dietary fibre concentration (mg fibre/g food) |
|---|---|
| Soya beans | 2.20 |
| Soya bread | 1.45 |
| Pasta, soy flour | 1.45 |
| Soya beans flour | 1.45 |
| Textured soy protein | 1.45 |
| Soy sauce | 0.16 |
| Soya drink | 0.13 |
| Soya yoghurt | 0.10 |
| Tofu | 0.05 |
| Soya cheese | 0.01 |
| Soya‐based infant formulae | 0 |
| Soya‐based follow‐on formulae | 0 |
| Soybean oil | 0 |
The concentration of total dietary fibre in soya bread and pasta was derived from that of soy flour by applying a factor of 0.719 and 1,20 respectively, to express the conversion from the flour to the final food (soya bread and pasta).
The dietary intake of soybean hemicellulose (in mg/kg bw per day) from soybean and soy‐derived food products via the regular diet for the general population and consumers only, combining composition data on total fibre in soy‐derived foodstuff with consumption data from the EFSA food consumption database, is presented in Table 9.
Table 9.
Intake of soybean hemicellulose (general population and consumers only) in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks to 11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| All population | ||||||
| Mean | < 0.01−0.02 | < 0.01−0.38 | < 0.01−0.11 | < 0.01−0.03 | < 0.01−0.05 | < 0.01−0.04 |
| 95th percentile | 0 | 0−0.01 | 0−0.50 | 0−0.11 | 0−0.30 | 0−0.28 |
| Consumers only | ||||||
| Mean | < 0.01−0.4 | < 0.01−5.9 | < 0.01−1.9 | < 0.01−0.8 | 0.02−0.6 | < 0.01−0.6 |
| 95th percentile | – | 0.1−5.2a | 0.4−1.5a | 0.2−0.8 | 0.1−2.1 | 0.4−0.8 |
| % consumers | 0−4.6 | 0−18.3 | 0−28.4 | 0−28.1 | 0.2−21.6 | 0−15.3 |
Levels at the 95th percentile can be lower than the upper level at the mean, as the estimates can represent different surveys. The 95th percentile estimates are presented only when based on more than 60 consumers.
The intake of soybean hemicellulose for consumers only (mean and high level) from natural sources is much lower than the mean estimated exposure from the food additive (Table 4), by a factor of 20–800 (for toddlers at the lower range of the 95th percentile).
3.5. Biological and toxicological data
3.5.1. Absorption, distribution, metabolism and excretion
There is evidence that certain high molecular weight dietary polysaccharides could be partially broken down by microbiota in the human large intestine. In addition to intermediate metabolites such as lactic, acrylic or fumaric acid, the main end products of this colonic anaerobic digestive process are short‐chain fatty acids (SCFA), such as acetic, propionic and butyric acids, which are absorbed from the colon (Cummings and Englyst, 1987).
3.5.1.1. In vitro studies
In vitro data on the microbial fermentation of hemicelluloses or one of their major components, xylan, were available.
A total of 188 strains of 10 species of Bacteroides found in the human colon were examined for their ability to ferment mucins and plant polysaccharides (Salyers et al., 1977a). Many of the Bacteroides strains tested were able to ferment a variety of plant polysaccharides, including pectin, amylose, dextran and gums. The ability to utilise mucins and plant polysaccharides varied considerably among the Bacteroides species tested. Xylan (Sigma Chemical Co.) was shown to be fermented by three species of Bacteroides.
A total of 154 strains of 22 species of Bifidobacterium, Peptostreptococcus, Lactobacillus, Ruminococcus, Coprococcus, Eubacterium and Fusobacterium, which are present in high concentrations in the human colon, were surveyed for their ability to ferment 21 different complex carbohydrates. Among them, xylan (Sigma Chemical Co.) was fermented by strains of Bifidobacterium adolescentis, Bifidobacterium infantis and Peptostreptococcus productus (Salyers et al., 1977b).
Fermentation of 10 polysaccharides, including xylan (Sigma Chemical Co.), by species of the family Enterobacteriaceae (Klebsielleae and other facultative Gram‐negative bacilli) were examined by Ochuba and Von Riesen (1980). Xylan was not fermented by any of the species tested.
The fibrolytic microbiota of the human large intestine was examined to determine the numbers and types of cellulolytic and hemicellulolytic bacteria present (Wedekind et al., 1988). Faecal samples from each of five individuals contained bacteria capable of degrading larchwood xylan as a substrate. The mean concentration of hemicellulolytic bacteria (1.8 × 1010/mL of faeces) was 100 times higher than that of cellulolytic bacteria. Hemicellulose‐degrading bacteria included Butyrivibrio sp., Clostridium sp., Bacteroides sp. and two unidentified strains, as well as four of the five cellulolytic strains. According to the authors, this work demonstrated that many humans harbour intestinal hemicellulolytic bacteria.
A total of 290 strains of 29 species of bifidobacteria of human and animal origin (mainly of faecal origin) were surveyed for their ability to ferment complex carbohydrates (Crociani et al., 1994). The substrates fermented by the largest number of species were d‐galactosamine, d‐glucosamine, amylose and amylopectin. Xylan (Sigma Chemical Co.) was shown to be fermented by several species (10 out of 29 species tested).
Overall, data from in vitro studies indicated that hemicelluloses or one of their major components, xylan, would be fermented during passage through the large intestine by strains of bacteria found in the human colon. The Panel noted that glucomannans (such as konjac gum), which are other components of hemicelluloses, are also known to be fermented in the human colon. The main end products of these colonic anaerobic digestive processes are SCFA, such as acetic, propionic and butyric acids, which are absorbed from the colon. Based on the available knowledge on the role of SCFA as end products of the fermentation of dietary fibres by the anaerobic intestinal microflora (Topping and Clifton, 2001; Den Besten et al., 2013), the Panel considered that their potential formation as fermentation products from soybean hemicellulose does not raise any safety concern. Despite the absence of in vivo studies in humans, the Panel considered that these data indicated that hemicelluloses would be most probably not absorbed intact, but fermented by the intestinal microflora in animals and humans.
3.5.2. Acute toxicity
In an acute oral toxicity study, five male and five female Wistar rats were dosed via gavage with a soluble soy fibre (soluble soybean hemicellulose, purity 80.6%) at 2,000 mg/kg bw (Prinsen, 1995). The LD50 of soybean hemicellulose exceeded 2,000 mg/kg bw, based on a 14‐day post‐exposure observation period.
3.5.3. Short‐term and subchronic toxicity
3.5.3.1. Rats
In a subacute feeding study (Carson and Smith, 1983), groups of 10 male weanling Wistar rats were given a diet containing 0 or 20% (equivalent to 24,000 mg/kg bw per day) of hemicellulose (not further specified). After an exposure period of 14 days, both the final mean body weight, as well as the food consumption, were decreased (~ 7% and 10%, respectively), while food efficiency was not altered. No other parameters were determined in this study.
Male Sprague–Dawley rats (n = 5) received a diet containing 6% water‐soluble hemicellulose (not further specified) (equivalent to 7,200 mg/kg bw per day) ad libitum for 14 days. All rats showed diarrhoea (no data about control), which could be prevented by adding germinated barley foodstuff to the diet (Kanauchi et al., 1997).
In a study focused on the physiological effects of water‐soluble soybean fibre (WSSF), generally considered to be identical to soybean hemicellulose, groups of six male Wistar rats (5 weeks of age) received a diet containing either no dietary fibre or 3% WSSF (equivalent to 3,600 mg/kg bw per day) and 2% cellulose (equivalent to 2,400 mg/kg bw per day) over 4 weeks (Takahashi et al., 1999). The composition of WSSF was given as: 75.1% total dietary fibre, 6.8% crude protein, 2.9% moisture and 4.8% ash. The growth of rats was not affected throughout the experimental period and none of the rats showed diarrhoea. Apart from significantly (p < 0.05) decreased liver cholesterol levels, no effects on clinical chemistry and haematological parameters were noted. There were also no changes in organ weights. The caecum of dosed rats showed an almost two‐fold dilatation and was significantly heavier (p < 0.05) than that of the other groups.
In a feeding study (Takahashi et al., 2003) conducted in compliance with OECD Guideline 408 and good laboratory practice (GLP), groups of 20 male and 20 female Crl:CD®(SD)IGS BR rats received WSSF, considered soybean hemicellulose (Soyafibe‐S), in the diet for 3 months at concentrations of 20,000, 30,000 or 40,000 mg/kg diet (equal to 1,270, 1,860 and 2,430 mg/kg bw per day for males and 1,305, 2,100 and 2,910 mg/kg bw per day for females). A similarly constituted group received untreated diet and served as the control. The composition of this fibre was given as: 5.3% water, 6.5% crude protein, 8.1% crude ash and 80.1% carbohydrate, with a typical fibre content of 72–77%. There was no evidence of any toxic effect at any of the concentrations administered. The main clinical finding was unformed stool, which was observed in all dose groups. As this finding is an expected physiological response to the amount of fibre in the diets, it was not considered to be a specific adverse effect. During the first half of the treatment period, there was a decrease in body weight gain, which can be attributed to decreased food consumption, while during the rest of the treatment period, the food intake and body weight gain were increased compared with controls (development of tolerance to the test article in the diet). At the end of the treatment period, a decrease in total serum cholesterol concentrations was noted in males dosed with 30,000 mg/kg diet. This is not an adverse effect, as the intake of soluble fibres is known to decrease serum cholesterol levels. From this study, a no‐observed‐adverse‐effect level (NOAEL) of 40,000 mg/kg diet (equal to 2,430 mg/kg bw per day for males and 2,910 mg/kg bw per day for females), the highest dose tested, was derived by the authors. The Panel agreed with this conclusion.
The Panel noted that a well‐conducted 90‐day (OECD Guideline 408 and GLP) study with water‐soluble soybean hemicellulose (Takahashi et al., 2003) did not show adverse effects at concentrations up to 40,000 mg/kg diet, equal to 2,430 mg/kg bw per day for males and 2,910 mg/kg bw per day for females, the highest doses tested.
3.5.4. Genotoxicity
Soluble soy fibre (soluble soybean hemicellulose, purity 80.6%) was examined for mutagenic activity in the bacterial reverse mutation test in Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537, and Escherichia coli strain WP2uvrA (Blijleven, 1995). The substance was tested at five different dose levels ranging from 0.062 to 5 mg/plate (vehicle: Milli‐Q water) both with and without metabolic activation and appropriate positive controls. No mutagenic effect was detected. The Panel noted that the study was compliant with the current OECD Guideline 471 and GLP.
No other in vitro or in vivo studies were available.
Overall, although the data were limited, the Panel concluded that, similarly to other dietary polysaccharides, soybean hemicellulose is not of genotoxic concern.
3.5.5. Chronic toxicity and carcinogenicity
No studies are available.
3.5.6. Reproductive and developmental toxicity
No studies are available.
3.5.7. Allergenicity, hypersensitivity, immunotoxicity
3.5.7.1. Allergenicity
Soy proteins appear to be at least equally antigenic to cow milk proteins (Witherly, 1990). Several soy proteins have allergenic potential (Ito et al., 2011; Sewekow et al., 2012), some having cross‐reactivity with other plant allergens (e.g. with peanuts; Sicherer et al., 2000). Even though the consequence of the allergenicity of soy proteins and the associated reported cases of anaphylaxis in children are still a matter of debate (Cordle, 2004; Osborn and Sinn, 2006; Savage et al., 2010; EFSA NDA Panel, 2014; Katz et al., 2014; Vandenplas et al., 2014), the Panel noted that a significant amount of protein (up to 14% in mass) can be present in the food additive E 426. Therefore, the Panel considered that the amount of residual proteins in E 426 should be reduced as much as possible, and that consumers should be informed of the presence of potentially allergenic proteins in the food additive.
3.5.7.2. Hypersensitivity
Animal studies
The sensitising properties of soy polysaccharides (soluble soy fibre derived from soy bean curd residue, purity not specified) were studied in male guinea pigs of the Hartley strain (n = 5/group) (Shibata, 1996; cited in SCF, 2003). The induction was performed with soy proteins and the challenge with soy polysaccharides via the intravenous route (route less relevant for food additives). The animals challenged with soy polysaccharides via oral administration showed neither active systemic anaphylactic (ASA) nor passive cutaneous anaphylactic (PCA) reactions (no further details). Challenge with soy polysaccharides via the intravenous route resulted in no ASA reaction, but a ‘very weak positive’ PCA reaction (antibody titre increased five‐fold). In further trials, soy protein‐induced animals were challenged via intravenous administration of soy proteins; the ASA reaction was negative, but the PCA reactions gave an antibody titre increase of 5–125‐fold in four out of five animals. The authors of the study stated that soy polysaccharides do not have an important sensitisation potential in comparison with the widely used positive control generating PCA antibody titre increases in the order of 600–3,000.
In the SCF evaluation (2003), the Committee concluded, based on these studies, that the sensitisation potential of orally administered soy polysaccharides may be considered very low, compared with that of soy proteins. However, low sensitisation does not guarantee the absence of risk in persons allergic to soybeans, since the presence of residual proteins in soybean hemicellulose (E 426) is presumed. The Panel noted that the soy polysaccharides used for the studies did contain some protein material (around 25% on a dry basis), and agreed with this conclusion.
3.5.8. Other studies
3.5.8.1. Humans
A comparison with other dietary fibres was performed by the SCF (2003). The committee cited a review (FASEB, 1987) in which the authors evaluated a wide range of dietary fibres, including those with chemical and physical properties similar to soybean hemicellulose. The authors of the review concluded that dietary fibres are safe ‘at levels far higher than the likely dietary intake of soybean hemicellulose in the intended applications’. The beneficial physiological effects of the consumption of soybean cotyledon fibre (Lo, 1990) and soy fibre (Erdman and Fordyce, 1989; Slavin, 1991) were reported in reviews, e.g. improved laxation, improvement of glucose tolerance for diabetics and cholesterol‐lowering ability. The SCF compared the intake of soybean hemicellulose as an additive (E 426) with that of soybean fibre in the diet. The SCF concluded that soy‐based dietary fibre may be safely used at a daily oral intake of up to 25–35 g/person per day, which was much more than the intake of the food additive soybean hemicellulose (E 426) from the intended applications (up to 0.5 g/person per day) (SCF, 2003). The SCF pointed to the limits for heavy metals and the possible allergenic potential.
The effect of soy polysaccharide on gastrointestinal function, nutrient balance, steroid excretion, blood lipid levels, postprandial serum glucose response and other blood parameters was studied in 14 healthy male human volunteers (aged 20−30 years) (Tsai et al., 1983). This diet‐control study with a cross‐over design included two 17‐day study periods with a 2‐week break in between. In the first period, seven subjects were used as controls consuming a low‐fibre basal diet. Seven subjects in the treatment group received a diet supplemented with 25 g/day (equivalent to 360 mg/kg bw per day) of soy polysaccharide. In the second period, treatments were crossed. The typical composition of soy polysaccharide was given as: 75% carbohydrate, 12% crude protein, 6% moisture, 5% ash and 2% fat. The carbohydrate fraction contained 60% total dietary fibre or 30% neutral detergent fibre and comprised components such as cellulose, hemicellulose, lignin, pectin‐like materials and other complex carbohydrates. Blood samples were taken on days −1, 1, 17 and 18 of each period and were used for the determination of total cholesterol and triacylglycerol, and other parameters routinely assayed for clinical screening: total protein, inorganic phosphate, calcium, chloride, magnesium, potassium, sodium, total bilirubin, urea nitrogen, CO2, glucose, uric acid, creatinine, creatine phosphokinase, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, alkaline phosphatase, lactose dehydrogenase and total iron. Amounts of cholesterol and triacylglycerol in chylomicron, very low‐density, low‐density and high‐density lipoproteins were also determined. The intake of diet supplemented with 25 g/day of soy polysaccharide produced no changes in the examined blood parameters. As shown in the nutrient balance and faecal transit studies, there was a significant increase in faecal wet weight (706 vs 561 g in controls) and faecal water content (549 vs 423 g in controls), but there were no changes in total dry weight, faecal neutral steroid, bile salt, protein and mineral contents. In the glucose tolerance test, the addition of soy polysaccharide to the glucose solution (15 g/100 g glucose) caused a significant reduction in the reactive hypoglycaemia 180 min after uptake (70 mg/dL).
3.6. Discussion
The present opinion deals with the re‐evaluation of the safety of soybean hemicellulose (E 426) when used as a food additive.
The Panel noted that soybean hemicellulose cannot be unambiguously identified by the CAS Registry No 9034‐32‐6 and the EC No 618‐530‐1, as those are not unique to soybean hemicellulose. The EC No 923‐430‐9 refers to soybean hemicellulose, but without a corresponding CAS number.
The Panel also noted that, according to the EU specifications, arsenic, lead, mercury and cadmium are accepted up to concentrations of 2, 5, 1 and 1 mg/kg, respectively. The Panel considered that contamination at these levels could have a significant impact on the exposure to these metals for which the intake is already close to the health‐based guidance values established by EFSA.
Despite the absence of studies in humans, the Panel considered that hemicelluloses would be most probably not absorbed intact. Data from in vitro studies indicated that hemicelluloses or their major components (xylan, glucomannans) would be fermented during passage through the large intestine by strains of bacteria found in the human colon. The main end products of these colonic anaerobic digestive processes are short‐chain fatty acids such as acetic, propionic and butyric acids, which are absorbed from the colon and considered of no safety concern by the Panel.
WSSF is generally considered to be identical to soybean hemicellulose. Therefore, the data from the 90‐day dietary rat study (Takahashi et al., 2003) were considered relevant for the evaluation of the food additive soybean hemicellulose (E 426). In this 90‐day study (OECD Guideline 408 and GLP), no adverse effects were reported at concentrations up to 40,000 mg/kg diet, equal to 2,430 mg/kg bw per day in males and 2,910 mg/kg bw per day in females, the highest doses tested.
Although data were limited, the Panel concluded that, similarly to other dietary polysaccharides, soybean hemicellulose is not of genotoxic concern.
Although no chronic toxicity or carcinogenicity studies or reproductive or developmental toxicity studies were available, given the negligible absorption of intact soybean hemicellulose and the absence of genotoxic concern, the Panel considered that these studies were not essential for the risk assessment of soybean hemicellulose (E 426), consistently with the ANS guidance for submission for food additives (EFSA ANS Panel, 2012).
An unpublished report on the sensitising properties of soy polysaccharides in guinea pigs (Shibata, 1996) suggested that the sensitisation potential of orally administered soybean hemicellulose may be considered very low, compared with that of soy proteins. However, this low sensitisation potential does not exclude the absence of risk in persons allergic to soybean proteins, given the presence of residual proteins in soybean hemicellulose (E 426).
No information on the actual usage of soybean hemicellulose (E 426) in foods was provided by the industry. Therefore, the exposure assessment for soybean hemicellulose (E 426) was performed based only on the MPLs, as set in Annex II to Regulation (EC) No 1333/2008 (regulatory maximum level exposure assessment scenario). Exposure estimates derived following this scenario should be considered conservative, as this scenario assumes that the consumer will be exposed to the food additive present in food at the maximum level over a long period of time. Children and toddlers were identified as the groups with the highest exposure (up to 191 mg/kg bw per day for children).
Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the actual exposure to soybean hemicellulose (E 426) as a food additive in European countries for the regulatory maximum level exposure scenario. Given that no usage of soybean hemicellulose (E 426) was reported by industry and that, according to the Mintel GNPD database, in the EU, soybean hemicellulose (E 426) was labelled on only one product in 2002 (ice‐cream sandwich), the Panel considered it likely that the food additive might not be used in the food categories in which it is authorised and that the actual dietary exposure to soybean hemicellulose (E 426) would be negligible.
4. Conclusions
Following the conceptual framework (EFSA ANS Panel, 2014) for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010, and given that:
soybean hemicellulose is not absorbed intact, but is extensively fermented by the intestinal microflora in animals and humans to SCFA;
no adverse effects were reported in an adequate dietary 90‐day study in rats at the highest dose tested of 2,430 mg/kg bw per day for males and 2,910 mg/kg bw per day for females;
soybean hemicellulose is not of genotoxic concern;
the highest exposure estimates, calculated based on the MPLs to be up to 191 mg/kg bw per day for children (95th percentile), are very conservative;
no uses were reported by industry and only one food product containing soybean hemicellulose (E 426) was found in the Mintel GNPD,
the Panel concluded that it is very unlikely that there is a safety concern from the current use of soybean hemicellulose (E 426) as a food additive for the general population, and that there is no need for a numerical ADI.
5. Recommendations
Due to the absence of information provided by the industry on the usage of E 426 and only a single food product containing soybean hemicellulose (E 426) reported in the Mintel GNPD database, the Panel recommended the collection of data on usage and use levels of soybean hemicellulose (E 426) in order to perform a more realistic exposure assessment.
The Panel recommended that the maximum limits for the impurities of toxic elements (arsenic, lead, mercury and cadmium) in the EU specifications for soybean hemicellulose (E 426) should be revised in order to ensure that soybean hemicellulose (E 426) as a food additive will not be a significant source of exposure to those toxic elements in food.
Furthermore, the Panel recommended that the amount of residual proteins in soybean hemicellulose (E 426) should be reduced as much as possible, and that the consumers should be informed of the presence of allergenic proteins in the food additive E 426.
Documentation provided to EFSA
Pre‐evaluation document on soybean hemicellulose (E 426) as a food additive. Prepared by Fraunhofer, March 2013.
Abbreviations
- AACC
American Association of Cereal Chemists
- ADI
acceptable daily intake
- ANS Panel
EFSA Panel on Food Additives and Nutrient Sources added to Food
- AOAC
The Association Of Analytical Chemists
- ASA
active systemic anaphylactic
- bw
body weight
- CAS
Chemical Abstract Service
- DP
degree of polymerisation
- EINECS
European Inventory of Existing Commercial Chemical Substances
- FAO/WHO
Food and Drug Organisation/World Health Organisation
- FCS
food categorisation system
- GLP
good laboratory practice
- GNPD
Global New Products Database
- IDF
insoluble dietary fibre
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LD50
lethal dose, 50%, i.e. dose that causes death among 50% of treated animals
- MPL
maximum permitted level
- NOAEL
no‐observed‐adverse‐effect level
- OECD
Organisation for Economic Co‐operation and Development
- PCA
passive cutaneous anaphylactic
- QS
quantum satis
- SCF
Scientific Committee on Food
- SCFA
short‐chain fatty acids
- SHC
soybean hemicellulose
- WSSF
water‐soluble soybean fibre
Appendix A – Summary of total estimated exposure to soybean hemicellulose (E 426) from its use as a food additive for the regulatory maximum level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)
1.
| Number of subjects | Mean | P95 | |
|---|---|---|---|
| Infants | |||
| Bulgaria (NUTRICHILD) | 659 | 15.7 | 70.5 |
| Germany (VELS) | 159 | 30.7 | 89.2 |
| Denmark (IAT 2006 07) | 826 | 47.0 | 117.4 |
| Finland (DIPP 2001 2009) | 500 | 48.2 | 122.2 |
| United Kingdom (DNSIYC 2011) | 1,369 | 26.6 | 90.1 |
| Italy (INRAN SCAI 2005 06) | 12 | 6.8 | − |
| Toddlers | |||
| Belgium (Regional Flanders) | 36 | 117.0 | − |
| Bulgaria (NUTRICHILD) | 428 | 44.8 | 109.4 |
| Germany (VELS) | 348 | 58.0 | 118.1 |
| Denmark (IAT 2006 07) | 917 | 37.1 | 82.9 |
| Spain (enKid) | 17 | 43.4 | − |
| Finland (DIPP 2001 2009) | 500 | 60.9 | 144.5 |
| United Kingdom (NDNS rolling programme Years 1–3) | 185 | 59.4 | 120.6 |
| United Kingdom (DNSIYC 2011) | 1,314 | 51.3 | 124.7 |
| Italy (INRAN SCAI 2005 06) | 36 | 39.3 | − |
| Netherlands (VCP kids) | 322 | 72.5 | 188.1 |
| Children | |||
| Austria (ASNS Children) | 128 | 51.4 | 105.4 |
| Belgium (Regional Flanders) | 625 | 98.5 | 190.8 |
| Bulgaria (NUTRICHILD) | 433 | 50.5 | 119.5 |
| Czech Republic (SISP04) | 389 | 29.9 | 72.4 |
| Germany (EsKiMo) | 835 | 39.0 | 86.9 |
| Germany (VELS) | 293 | 58.8 | 114.5 |
| Denmark (DANSDA 2005‐08) | 298 | 43.1 | 89.2 |
| Spain (enKid) | 156 | 52.2 | 145.3 |
| Spain (NUT INK05) | 399 | 38.9 | 89.1 |
| Finland (DIPP 2001 2009) | 750 | 32.3 | 77.6 |
| France (INCA2) | 482 | 66.3 | 123.8 |
| United Kingdom (NDNS rolling programme Years 1–3) | 651 | 60.0 | 117.9 |
| Greece (Regional Crete) | 838 | 60.3 | 127.8 |
| Italy (INRAN SCAI 2005 06) | 193 | 43.2 | 101.2 |
| Latvia (EFSA TEST) | 187 | 58.5 | 141.7 |
| Netherlands (VCP kids) | 957 | 67.6 | 138.9 |
| Netherlands (VCPBasis AVL2007 2010) | 447 | 69.8 | 148.7 |
| Sweden (NFA) | 1,473 | 71.9 | 138.0 |
| Adolescents | |||
| Austria (ASNS Children) | 237 | 30.1 | 71.9 |
| Belgium (Diet National 2004) | 576 | 35.8 | 71.9 |
| Cyprus (Childhealth) | 303 | 31.1 | 70.9 |
| Czech Republic (SISP04) | 298 | 23.6 | 60.6 |
| Germany (National Nutrition Survey II) | 1,011 | 23.0 | 62.2 |
| Germany (EsKiMo) | 393 | 28.2 | 62.0 |
| Denmark (DANSDA 2005–08) | 377 | 26.6 | 56.4 |
| Spain (AESAN FIAB) | 86 | 11.4 | 31.8 |
| Spain (enKid) | 209 | 32.4 | 70.7 |
| Spain (NUT INK05) | 651 | 24.3 | 56.7 |
| Finland (NWSSP07 08) | 306 | 19.9 | 41.6 |
| France (INCA2) | 973 | 36.2 | 74.8 |
| United Kingdom (NDNS rolling programme Years 1–3) | 666 | 36.5 | 73.8 |
| Italy (INRAN SCAI 2005 06) | 247 | 26.5 | 66.0 |
| Latvia (EFSA TEST) | 453 | 43.3 | 90.0 |
| Netherlands (VCPBasis AVL2007 2010) | 1,142 | 47.3 | 100.3 |
| Sweden (NFA) | 1,018 | 52.1 | 101.1 |
| Adults | |||
| Austria (ASNS Adults) | 308 | 22.7 | 57.8 |
| Belgium (Diet National 2004) | 1,292 | 27.7 | 60.9 |
| Czech Republic (SISP04) | 1,666 | 11.9 | 35.6 |
| Germany (National Nutrition Survey II) | 10,419 | 20.3 | 49.8 |
| Denmark (DANSDA 2005–08) | 1,739 | 18.6 | 40.4 |
| Spain (AESAN) | 410 | 7.6 | 26.2 |
| Spain (AESAN FIAB) | 981 | 7.5 | 23.9 |
| Finland (FINDIET2012) | 1,295 | 20.0 | 46.4 |
| France (INCA2) | 2,276 | 23.3 | 46.2 |
| United Kingdom (NDNS rolling programme Years 1–3) | 1,266 | 23.0 | 46.6 |
| Hungary (National Repr Surv) | 1,074 | 5.0 | 22.0 |
| Ireland (NANS 2012) | 1,274 | 25.3 | 51.8 |
| Italy (INRAN SCAI 2005 06) | 2,313 | 14.4 | 36.3 |
| Latvia (EFSA TEST) | 1,271 | 26.6 | 62.5 |
| Netherlands (VCPBasis AVL2007 2010) | 2,057 | 28.2 | 64.9 |
| Romania (Dieta Pilot Adults) | 1,254 | 7.2 | 20.6 |
| Sweden (Riksmaten 2010) | 1,430 | 27.1 | 63.6 |
| The elderly | |||
| Austria (ASNS Adults) | 92 | 20.7 | 48.8 |
| Belgium (Diet National 2004) | 1,215 | 27.7 | 57.8 |
| Germany (National Nutrition Survey II) | 2,496 | 22.1 | 49.3 |
| Denmark (DANSDA 2005–08) | 286 | 21.9 | 40.9 |
| Finland (FINDIET 2012) | 413 | 20.2 | 47.1 |
| France (INCA2) | 348 | 23.3 | 49.9 |
| United Kingdom (NDNS rolling programme Years 1–3) | 305 | 23.2 | 46.0 |
| Hungary (National Repr Surv) | 286 | 6.2 | 23.8 |
| Ireland (NANS 2012) | 226 | 25.5 | 51.1 |
| Italy (INRAN SCAI 2005 06) | 518 | 13.4 | 34.2 |
| Netherlands (VCPBasis AVL2007 2010) | 173 | 23.5 | 48.6 |
| Netherlands (VCP‐Elderly) | 739 | 23.0 | 46.6 |
| Romania (Dieta Pilot Adults) | 128 | 5.9 | 18.6 |
| Sweden (Riksmaten 2010) | 367 | 31.4 | 69.4 |
P95: 95th percentile; bw: body weight; −: p95 of exposure was only calculated for those population groups where the sample size was sufficiently large to allow this calculation (EFSA, 2011a).
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Lambré C, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Oskarsson A, Parent‐Massin D, Stankovic I, Waalkens‐Berendsen I, Wright M, Younes M, Tobback P, Tard A, Tasiopoulou S and Woutersen RA, 2017. Scientific opinion on the re‐evaluation of soybean hemicellulose (E 426) as a food additive. EFSA Journal 2017;15(3):4721, 27 pp. doi: 10.2903/j.efsa.2017.4721
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00521
Panel members: Fernando Aguilar, Riccardo Crebelli, Alessandro Di Domenico, Birgit Dusemund, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Claude Lambré, Jean‐Charles Leblanc, Oliver Lindtner, Peter Moldeus, Alicja Mortensen, Pasquale Mosesso, Agneta Oskarsson, Dominique Parent‐Massin, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The ANS Panel wishes to acknowledge all the European competent institutions, the Member State bodies and other organisations that provided data for this scientific opinion.
Adopted: 26 January 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: © Elsevier
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM (2001) 542 final.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Published: 22 November 2009. Available online: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption. Published: 12 October 2015. Available online: http://www.efsa.europa.eu/en/data/call/151012
Call for technical data on certain starches and celluloses authorised as food additives in the EU. Published: 11 February 2016. Available online: https://www.efsa.europa.eu/en/data/call/160211
Available online: http://www.efsa.europa.eu/en/food-consumption/comprehensive-database
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Published 22 November 2009. Available online: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption. Published: 12 October 2015. Available from: https://www.efsa.europa.eu/en/data/call/151012
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home; accessed on 17/10/2016.
Available online: http://www.efsa.europa.eu/en/press/news/150428.htm
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Available online: http://frida.fooddata.dk/index.php?lang=en
Available online: https://pro.anses.fr/TableCIQUAL/
Available online: http://www.bedca.net/
Available online: http://nevo-online.rivm.nl/Default.aspx
Call for input data for the Exposure Assessment of Food Enzymes (Annex: baking and brewing). Available online: http://www.efsa.europa.eu/en/data/call/161110
Technical conversion factors for agricultural commodities. Available online: http://www.fao.org/fileadmin/templates/ess/documents/methodology/tcf.pdf
References
- AACC International (American Association of Cereal Chemists), 1999. Insoluble Dietary Fiber. AACC Method 32‐20.01. AACC International, St. Paul, MN, USA. [Google Scholar]
- AOAC (Association of Official Analytical Chemists), 1992. Official Methods of Analysis. AOAC Method 985.29. Association of Official Analytical Chemists, Arlington, VA, USA. [Google Scholar]
- Aspinall GO, Hunt K and Morrison IM, 1966. Polysaccharides of soy‐beans. Part II. Fractionation of hull cell‐wall polysaccharides and the structure of a xylan. Journal of the Chemical Society C: Organic, 1945–1949. doi: 10.1039/J39660001945 [DOI] [PubMed] [Google Scholar]
- Aspinall GO, Begbie R, Hamilton A and Whyte JNC, 1967. Polysaccharides of soy‐beans. Part III. Extraction and fractionation of polysaccharides from cotyledon meal. Journal of the Chemical Society C: Organic, 1065–1070. [DOI] [PubMed] [Google Scholar]
- Blijleven WGH, 1995. Bacterial mutagenicity test with soluble soya fibre. TNO‐Report V 95.391. Unpublished report. Cited in SCF, 2003 (as Maeda H, 2000c).
- Carson MS and Smith TK, 1983. Effect of feeding alfalfa and refined plant fibers on the toxicity and metabolism of T‐2 toxin in rats. Journal of Nutrition, 113, 304–313. [DOI] [PubMed] [Google Scholar]
- Cordle CT, 2004. Soy protein allergy: incidence and relative severity. Journal of Nutrition, 134, 1213S–1219S. [DOI] [PubMed] [Google Scholar]
- Crociani F, Alessandrini A, Mucci MM and Biavati B, 1994. Degradation of complex carbohydrates by Bifidobacterium spp. International Journal of Food Microbiology, 24, 199–210. [DOI] [PubMed] [Google Scholar]
- Cummings JH and Englyst HN, 1987. Fermentation in the human large intestine and the available substrates. American Journal of Clinical Nutrition, 45, 1243–1255. [DOI] [PubMed] [Google Scholar]
- Den Besten G, van Eunen K, Groen A, Venema K, Reijngoud DJ and Bakker M, 2013. The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. The Journal of Lipid Research, 54, 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Scientific Opinion of the Panel on Contaminants in the Food Chain (CONTAM) on cadmium in food. EFSA Journal 2009;7(3):980, 139 pp. doi: 10.2903/j.efsa.2009.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in exposure assessment. EFSA Journal 2011;9(3):2097, 34 pp. doi: 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. doi: 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. doi: 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. doi: 10.2903/j.efsa.2014.3697 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. doi: 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. doi: 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. doi: 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA NDA Panel (Panel on Dietetic Products, Nutrition and Allergies), 2014. Scientific Opinion on the evaluation of allergenic foods and food ingredients for labelling purposes. EFSA Journal 2014;12(11):3894, 286 pp. doi: 10.2903/j.efsa.2014.3894 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2007. Opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. doi: 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: General principles. EFSA Journal 2009;7(5):1051, 22 pp. doi: 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. doi: 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- Erdman JW Jr and Fordyce EJ, 1989. Soy products and the human diet. American Journal of Clinical Nutrition, 49, 725–737. [DOI] [PubMed] [Google Scholar]
- FASEB (Federation of American Societies for Experimental Biology), 1987. Physiological effects and health consequences of dietary fibre. Contract Number FDA 223‐84‐2059. Cited in SCF, 2003.
- Furuta H and Maeda H, 1999. Rheological properties of water‐soluble soybean polysaccharides extracted under weak acidic condition. Food Hydrocolloids, 13, 267–274. [Google Scholar]
- Furuta H, Takahashi T, Tobe J, Kiwata R and Maeda H, 1998. Extraction of water‐soluble soybean polysaccharides under acidic conditions. Bioscience, Biotechnology, and Biochemistry, 62, 2300–2305. [DOI] [PubMed] [Google Scholar]
- Huang Z, Ye R, Chen J and Xu F, 2013. An improved method for rapid quantitative analysis of the insoluble dietary fiber in common cereals and some sorts of beans. Journal of Cereal Science, 57, 270–274. [Google Scholar]
- Ito K, Sjölander S, Sato S, Movérare R, Tanaka A, Söderström L, Borres M, Poorafshar M and Ebisawa M, 2011. IgE to Gly m 5 and Gly m 6 is associated with severe allergic reactions to soybean in Japanese children. Journal of Allergy and Clinical Immunology, 128, 673–675. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- Kanauchi O, Nakamura T, Agata K and Fushiki T, 1997. Preventive effect of germinated barley foodstuff on diarrhea induced by water‐soluble dietary fiber in rats. Bioscience, Biotechnology and Biochemistry, 61, 449–454. [DOI] [PubMed] [Google Scholar]
- Katz Y, Gutierrez‐Castrellon P, González MG, Rivas R, Lee BW and Alarcon P, 2014. A comprehensive review of sensitization and allergy to soy‐based products. Clinical Reviews in Allergy and Immunology, 46, 272–281. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Kobayashi T, Osima M and Mino M, 1955. Studies on the carbohydrates of soybeans. A hemicellulose fraction soluble in hot water. Isolation and component sugar determinations. Bulletin of the Agricultural Chemical Society of Japan, 19, 69–76. [Google Scholar]
- Kikuchi T and Sugimoto H, 1976. Detailed structure of an acidic polysaccharide in soy sauce, confirmed by use of two kinds of purified pectinases. Agricultural and Biological Chemistry, 40, 87–92. [Google Scholar]
- Lo GS, 1990. Physiological effects and physicochemical properties of soy cotyledon fiber. New Developments in Dietary Fiber, 270, 49–66. [DOI] [PubMed] [Google Scholar]
- Maeda H and Nakamura A, 2009. Soluble soybean polysaccharide In: Phillips GO. and Williams PA. (eds.). Handbook of Hydrocolloids, 2nd Edition, CRC Press, Boca Raton, FL, USA, pp. 693–709. [Google Scholar]
- Matsumura Y, Egami M, Satake C, Maeda Y, Takahashi T, Nakamura A and Mori T, 2003. Inhibitory effects of peptide‐bound polysaccharides on lipid oxidation in emulsions. Food Chemistry, 83, 107–119. [Google Scholar]
- McCleary BV, DeVries JW, Rader JI, Cohen G, Prosky L, Mugford DC and Okuma K, 2012. Determination of insoluble, soluble, and total dietary fiber (CODEX definition) by enzymatic–gravimetric method and liquid chromatography: collaborative study. Journal of AOAC International, 95, 824–844. [DOI] [PubMed] [Google Scholar]
- Morita M, 1965. Polysaccharides of soybean seeds. Part I. Polysaccharide constituents of ‘hot‐water‐extract’ fraction of soybean seeds and an arabinogalactan as its major component. Agricultural and Biological Chemistry, 29, 564–573. [Google Scholar]
- Nakamura A, Furuta H, Maeda H, Nagamatsu Y and Yoshimoto A, 2001. Analysis of structural components and molecular construction of soybean soluble polysaccharides by stepwise enzymatic degradation. Bioscience, Biotechnology and Biochemistry, 65, 2249–2258. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Furuta H, Maeda H, Takao T and Nagamatsu Y, 2002a. Structural studies by stepwise enzymatic degradation of the main backbone of soybean soluble polysaccharides consisting of galacturonan and rhamnogalacturonan. Bioscience, Biotechnology, and Biochemistry, 66, 1301–1313. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Furuta H, Maeda H, Takao T and Nagamatsu Y, 2002b. Analysis of the molecular construction of xylogalacturonan isolated from soluble soybean polysaccharides. Bioscience, Biotechnology, and Biochemistry, 66, 1155–1158. [DOI] [PubMed] [Google Scholar]
- Ochuba GU and Von Riesen VL, 1980. Fermentation of polysaccharides by Klebsielleae and other facultative bacilli. Applied and Environmental Microbiology, 39, 988–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 1981. OECD Guideline for the testing of chemicals, 408. Subchronic Oral Toxicity – Rodent: 90‐day Study. Adopted 12 May 1981.
- OECD (Organisation for Economic Co‐operation and Development), 1997. OECD Guideline for the testing of chemicals, 471. Bacterial Reverse Mutation Test. Adopted 21 July 1997.
- Osborn DA and Sinn J, 2006. Soy formula for prevention of allergy and food intolerance in infants. The Cochrane Database of Systematic Reviews, 18, CD003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinsen MK, 1995. Acute oral toxicity study (limit study) with soluble soya fiber in rats. TNO‐Report V 95.259. Unpublished report. Cited in SCF, 2003 (as Maeda H, 2000b).
- Prosky L, Asp NG, Furda I, DeVries JW, Schweizer TF and Harland BF, 1985. Determination of total dietary fiber in foods and food products: collaborative study. Journal of the Association of Official Analytical Chemists, 68, 677–679. [PubMed] [Google Scholar]
- Salyers AA, Vercellotti JR, West SE and Wilkins TD, 1977a. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Applied and Environmental Microbiology, 33, 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers AA, West SE, Vercellotti JR and Wilkins TD, 1977b. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Applied and Environmental Microbiology, 34, 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JH, Kaeding AJ, Matsui EC and Wood RA, 2010. The natural history of soy allergy. Journal of Allergy and Clinical Immunology, 125, 683–686. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food), 2001. Guidance on submission for food additive evaluations by the Scientific Committee on Food. Opinion expressed on 12 July 2001. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out98_en.pdf
- SCF (Scientific Committee on Food), 2003. Opinion of the Scientific Committee on Food on soybean hemicellulose. Opinion expressed on 4 April 2003. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out187_en.pdf
- Scheller HV and Ulvskov P, 2010. Hemicelluloses. Annual Review of Plant Biology, 61, 263–289. [DOI] [PubMed] [Google Scholar]
- Sewekow E, Bimczok D, Kähne T, Faber‐Zuschratter H, Kessler LC, Seidel‐Morgenstern A and Rothkötter HJ, 2012. The major soyabean allergen P34 resists proteolysis in vitro and is transported through intestinal epithelial cells by a caveolae‐mediated mechanism. British Journal of Nutrition, 108, 1603–1611. [DOI] [PubMed] [Google Scholar]
- Shibata R, 1996. Allergenicity study of soy polysaccharides in guinea pigs. Final Report U‐1182, Kannami Laboratory, Bozo Research Center, Tokyo. Unpublished report. Cited in SCF, 2003 (as Maeda H, 2000e).
- Sicherer SH, Sampson HA and Burks AW, 2000. Peanut and soy allergy: a clinical and therapeutic dilemma. Allergy, 55, 515–521. [DOI] [PubMed] [Google Scholar]
- Slavin J, 1991. Nutritional benefits of soy protein and soy fiber. Journal of the American Dietetic Association, 91, 816–819. [PubMed] [Google Scholar]
- Sonda TS, Kain RJ and Yao H, 2002. Physiochemical properties of polysaccharides extracted from tofu processing wastewater. Journal of Food Science, 67, 1682–1687. [Google Scholar]
- Takahashi T, Maeda H, Aoyama T, Yamamoto T and Takamatsu K, 1999. Physiological effects of water‐soluble soybean fiber in rats. Bioscience, Biotechnology and Biochemistry, 63, 1340–1345. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nakamura A, Kato M, Maeda H, Mandella RC, Broadmeadow A and Ruckman SA, 2003. Soluble soybean fiber: a 3‐month dietary toxicity study in rats. Food and Chemical Toxicology, 41, 1111–1121. [DOI] [PubMed] [Google Scholar]
- Thompson DB, Huang C and Sieglaff C, 1987. Rheological behavior of soluble polysaccharide fractions from soybeans. Food Hydrocolloids, 1, 333–337. [Google Scholar]
- Topping DL and Clifton PM, 2001. Short‐chain fatty acids and human colonic function: roles and resistant starch and nonstarch polysaccharides. Physiological Reviews, 81, 1031–1064. [DOI] [PubMed] [Google Scholar]
- Tsai AC, Mott EL, Owen GM, Bennick MR, Lo GS and Steinke FH, 1983. Effects of soy polysaccharide on gastrointestinal functions, nutrient balance, steroid excretions, glucose tolerance, serum lipids, and other parameters in humans. American Journal of Clinical Nutrition, 38, 504–511. [DOI] [PubMed] [Google Scholar]
- Vandenplas Y, Castrellon PG, Rivas R, Gutiérrez CJ, Garcia LD, Jimenez JE, Anzo A, Hegar B and Alarcon P, 2014. Safety of soya‐based infant formulas in children. British Journal of Nutrition, 111, 1340–1360. [DOI] [PubMed] [Google Scholar]
- Wang Q, Huang X, Nakamura A, Burchard W and Hallett FR, 2005. Molecular characterisation of soybean polysaccharides: an approach by size exclusion chromatography, dynamic and static light scattering methods. Carbohydrate Research, 340, 2637–2644. [DOI] [PubMed] [Google Scholar]
- Wedekind KJ, Mansfield HR and Montgomery L, 1988. Enumeration and isolation of cellulolytic and hemicellulolytic bacteria from human feces. Applied and Environmental Microbiology, 54, 1530–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherly SA, 1990. Soy formulas are not allergenic. Letter to the Editor. American Journal of Clinical Nutrition, 51, 705–706. [DOI] [PubMed] [Google Scholar]
- Yoshii H, Furuta T, Maeda H and Mori H, 1996. Hydrolysis kinetics of okara and characterization of its water‐soluble polysaccharides. Bioscience, Biotechnology and Biochemistry, 60, 1406–1409. [DOI] [PubMed] [Google Scholar]


