Abstract
The conclusions of the European Food Safety Authority (EFSA) following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State, Hungary, and co‐rapporteur Member State, the Netherlands, for the pesticide active substance Clonostachys rosea strain J1446, currently approved as Gliocladium catenulatum strain J1446, are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012. The conclusions were reached on the basis of the evaluation of the representative uses of Clonostachys rosea strain J1446 as a fungicide in agriculture and horticulture. The reliable end points, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: Clonostachys rosea strain J1446, Gliocladium catenulatum strain J1446, peer review, risk assessment, pesticide, fungicide
Summary
Commission Implementing Regulation (EU) No 844/2012 (hereinafter referred to as ‘the Regulation’) lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012. Clonostachys rosea strain J1446 is one of the active substances listed in Regulation (EU) No 686/2012. The strain J1446 was initially identified as Gliocladium catenulatum; however, due to changes in the taxonomic rules it was later transferred to the genus Clonostachys.
In accordance with Article 1 of the Regulation, the rapporteur Member State (RMS), Hungary, and co‐rapporteur Member State (co‐RMS), the Netherlands, received an application from Verdera Oy for the renewal of approval of the active substance Clonostachys rosea strain J1446. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (the Netherlands), the European Commission and the European Food Safety Authority (EFSA) about the admissibility.
The RMS provided its initial evaluation of the dossier on Clonostachys rosea strain J1446 in the renewal assessment report (RAR), which was received by EFSA on 5 July 2016. In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, Verdera Oy for comments on 9 August 2016. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 9 October 2016.
Following consideration of the comments received on the RAR, it was concluded that additional information should be requested from the applicant and that EFSA should conduct an expert consultation in the area of mammalian toxicology. It was also concluded that the organism should now be identified and renamed as C. rosea considering the developments in microorganism species identification and nomenclature that have occurred since the strain was first approved.
In accordance with Article 13(1) of the Regulation, EFSA should adopt a conclusion on whether Clonostachys rosea strain J1446 (approved in Regulation (EU) No 540/2011 as Gliocladium catenulatum strain J1446) can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 of the European Parliament and of the Council.
The conclusions laid down in this report were reached on the basis of the evaluation of the representative uses of Clonostachys rosea strain J1446 as a fungicide in agriculture and horticulture as proposed by the applicant. Full details of the representative uses can be found in Appendix A of this report.
The uses of Clonostachys rosea strain J1446 according to the representative uses proposed result in a sufficient fungicidal efficacy against the target plant pathogenic fungi.
In the section on identity, physical–chemical and technical properties and analytical methods, data gaps were identified for 5‐batch data under good laboratory practice (GLP) for the manufactured product, for validation data to prove the limit of quantification (LOQ) of the method analysing gliotoxin, information to prove the absence of metabolites in the microbial pest control product (MPCP), a study from an officially recognised testing facility for the determination of growth temperature range of the strain and a GLP study of the content of the active substance before and after storage the resistance of Clonostachys rosea strain J1446 to antibiotics/anti‐microbial agents.
For the section on mammalian toxicology, it was concluded, that the production of toxins/secondary metabolites by Clonostachys rosea strain J1446 cannot be excluded. As a consequence, the risk assessment for workers and residents cannot be finalised.
In the area of residues and consumer exposure, a risk assessment cannot be conducted due to the absence of toxicological reference values and the lack of actual concentrations of potential secondary metabolites on the raw agricultural commodities (RACs); however viable cell counts at higher concentrations than applied to RACs have not shown adverse health effects.
Because of the current uncertainties related to the potential production of toxins/secondary metabolites, an inclusion of Clonostachys rosea strain J1446 in Annex IV of Regulation (EC) No 396/2005 cannot be recommended. This may need to be reconsidered in the future if sufficient information on the toxicity of potential metabolites were to be reported.
The information available was insufficient to demonstrate that Clonostachys rosea strain J1446 would respect the uniform principles criterion of not being expected to persist in soil in concentrations considerably higher than the natural background levels, taking into account repeated applications over the years. Satisfactory information to demonstrate that, under the conditions of use, any secondary metabolites produced by Clonostachys rosea strain J1446 or present in the product, if present will not occur in the environmental compartments in concentrations considerably higher than under natural conditions was missing. Consequently, further data on the persistence, transformation and mobility of these compounds may be needed in order to assess the potential level of environmental exposure including the exposure of groundwater. In addition, data gaps were identified for information on behaviour of Clonostachys rosea strain J1446 in soil, growing media, water and movement via air to be available from officially recognised testing organisations and for potential for conidia to transfer from foliage into soil to be addressed for the field crops assessed except strawberry.
In the area of ecotoxicology, two outstanding issues were identified; one for the aquatic organisms (invertebrates and algae) and another regarding the potential risk from any secondary metabolites produced by Clonostachys rosea strain J1446.
Background
Commission Implementing Regulation (EU) No 844/20121 (hereinafter referred to as ‘the Regulation’) lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/20092. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of up to 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3).
In accordance with Article 1 of the Regulation, the RMS Hungary and co‐RMS the Netherlands received an application from Verdera Oy for the renewal of approval of the active substance Clonostachys rosea strain J1446, currently approved as Gliocladium catenulatum strain J1446. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (the Netherlands), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on Clonostachys rosea strain J1446 in the RAR, which was received by EFSA on 5 July 2016 (Hungary, 2016).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, Verdera Oy for consultation and EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 9 October 2016. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant's response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA, the RMS and the co‐RMS on 24 November 2016. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant and that EFSA should conduct an expert consultation in the area of mammalian toxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. One of these conclusions was that the organism should now be identified as Clonostachys rosea strain J1446 considering the developments in microorganism species identification and nomenclature that have occurred since the strain was first approved. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in May–June 2017.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative uses of Clonostachys rosea strain J1446 as a fungicide in agriculture and horticulture as proposed by the applicant. A list of the relevant end points for the active substance and the formulation is provided in Appendix A.
In addition, a key supporting document to this conclusion is the peer review report (EFSA, 2017), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (25 November 2016);
the evaluation table (16 June 2017);
the report(s) of the scientific consultation with Member State experts (where relevant)
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Hungary, 2017), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the European Union (EU) for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Clonostachys rosea strain J1446 is a fungus deposited at the culture collection of the German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen). Germany, ascribed the name Gliocladium catenulatum strain J1446, under the accession number DSM 9212. Clonostachys rosea strain J1446 is a naturally occurring, indigenous wild‐type fungal strain, initially isolated from Finnish field soil.
The strain J1446 was initially identified as Gliocladium catenulatum; however, due to changes in the taxonomic rules it was later transferred to the genus Clonostachys. According to Index Fungorum, the current official name is Clonostachys rosea, and Gliocladium catenulatum is listed as a synonym for C. rosea. The fungus G. catenulatum is the same as C. rosea.
The representative formulated product for the evaluation was ‘PRESTOP’, a wettable powder (WP) containing 230 g/kg (nominal 4.6 × 1011 CFU/kg, minimum content 2 × 1011 CFU/kg, maximum 1 × 1012 CFU/kg) Clonostachys rosea strain J1446.
The representative uses evaluated comprise applications by soil incorporation or drip irrigation against seed‐borne and soil‐borne fungi, such as Fusarium spp., Pythium spp. and Phytophthora spp., applications by foliar spraying against foliar pathogens such as Botrytis spp. and Didymella spp., and seed treatment against seed‐borne and soil‐borne fungi, in agriculture and horticulture. The crops involved were wheat, corn, potato, onion, grape, turf and on ornamentals, vegetables, strawberries grown under protection (greenhouses, tunnels) and/or in open field.
Data were submitted to conclude that the uses of Clonostachys rosea strain J1446 result, according to the representative uses proposed at EU level, in a sufficient fungicidal efficacy against plant pathogenic fungi, following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014).
Conclusions of the evaluation
1. Identity of the microorganism/biological properties/physical and technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: SANCO/12116/2012‐rev. 0 (European Commission, 2012), Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance (EFSA FEEDAP Panel, 2012).
The technical grade microbial pest control agent (MPCA) is only a hypothetical stage in the continuous production process of the end use product. A data gap was identified for 5‐batch data under good laboratory practice (GLP) for the manufactured product.
Methods based on random amplified polymorphic DNA‐polymerase chain reaction (RAPD‐PCR) are available for an unequivocal identification of Clonostachys rosea J1446 at the strain level.
C. rosea has the potential for producing secondary metabolites. No detailed information was available for Clonostachys rosea strain J1446 concerning the production of metabolites and its complete metabolite pattern. As a consequence, a data gap was identified to prove the absence of metabolites in the microbial pest control product (MPCP).
There is no evidence of direct relationships of Clonostachys rosea strain J1446 to known plant, animal or human pathogens. It can produce lytic enzymes like chitinase and β‐1,3‐glucanase. Although some members of the Gliocladium genus can produce gliotoxin, no gliotoxin production was detected in Clonostachys rosea strain J1446; reports were not identified of it being produced by the Clonostachys genus. However the limit of detection of the method analysing gliotoxin was 50 μg/kg, so a data gap was identified for validation data to prove the limit of quantification (LOQ) of the method in view of the a concern with regard to the genotoxic properties of gliotoxin.
The minimum and maximum temperatures for the growth of Clonostachys rosea strain J1446 are 4.6°C and 34.6°C, respectively; however, the study was not carried out in an officially recognised testing facility and as a consequence a data gap was identified. Clonostachys rosea strain J1446 is able to grow at a large pH range (3–8.2). A data gap was identified to address the resistance of Clonostachys rosea strain J1446 to antibiotics/antimicrobial agents. The assessment of the data package revealed no issues that need to be included as critical areas of concern with respect to the identity and physical, chemical and technical properties of the representative formulation; however, a data gap was identified for the determination of the active substance content before and after storage. It should be noted that the formulation cannot be mixed with concentrated solutions of pesticides and fertilisers.
Acceptable methods are available for the determination of the microorganism in the formulation and for the determination of the content of contaminating microorganisms.
2. Mammalian toxicity
2.1. General data
Clonostachys rosea strain J1446 was discussed during the Pesticides Peer Review experts’ meeting 155 on mammalian toxicology in March 2017.
In an appropriate literature review, no evidence was found that G. catenulatum/C. rosea presents any risk to animals or humans. No adverse health effects have been observed or reported in manufacturing plant personnel since 1995. Few cases were reported in the literature where Gliocladium spp. have been identified to infect people, but with very weak evidence of a causal relationship (in one case).
The EFSA Panel on Biological Hazards (EFSA BIOHAZ Panel) could not recommend G. catenulatum/C. rosea for the qualified presumption of safety (QPS) list (EFSA BIOHAZ Panel, 2009).
2.2. Toxicity studies
A detailed analysis of the batches used in the available acute toxicity studies with Gliocladium catenulatum J1446 (currently named Clonostachys rosea strain J1446) was not available. Further information is not required provided that adequate quality control is undertaken on the batches produced, ensuring that toxicologically relevant pathogenic contaminants are kept below levels internationally recognised as safe (see Section 1).
Gliocladium catenulatum strain J1446 (currently named Clonostachys rosea strain J1446) showed no evidence of toxicity, pathogenicity or infectivity following oral, inhalation or intraperitoneal administration to rats. It can be concluded that the microorganism is unlikely to be pathogenic or infective after repeated exposure. The representative formulation Prestop WP was neither a skin nor an eye irritant, and was shown to be of low acute toxicity following oral, inhalation or dermal administration to rats. In a skin sensitisation study (Buehler test), equivocal results were observed; however considering the limitations of such a study for a microorganism and the lack of investigation of the sensitisation by inhalation, the following warning phrase is proposed: ‘Clonostachys rosea J1446 may have the potential to provoke sensitising reactions’.
2.3. Secondary metabolites/toxins
In the literature, several metabolites are reported as produced by Gliocladium spp. and Clonostachys spp. In Ames tests, neither the crude extract of Clonostachys rosea strain J1446 nor gliotoxin had a genotoxic effect. For gliotoxin, this is not considered sufficient evidence to exclude a potential genotoxic concern since this toxin is known as interfering with DNA synthesis, and as having anti‐phagocytic and immune‐modulating effects. It is noted that the LOQ for gliotoxin might not be low enough to exclude any genotoxic effect if the toxin is present. (see also Section 1).
For the other metabolites potentially produced by Gliocladium spp. and Clonostachys spp. (e.g. viridin, gliovirin, glisoprenins, heptelidic acid), see as well Section 4, the available information is insufficient to conclude on their toxicological profile. Further assessment of the potential toxicity of secondary metabolites/toxins is pending on the data gaps for exposure assessment (see Sections 1, 3 and 4).
2.4. Reference values and exposure
It is agreed that the exposure estimates with the usual models (for chemicals) are not applicable to microbials. Considering the toxicological profile of Clonostachys rosea strain J1446, being concluded as not pathogenic, toxic of infective, the derivation of reference values is not necessary. For re‐entry workers and residents, uncertainties should be clarified (and supported by data) with regard to the potential exposure to secondary metabolites produced by Clonostachys rosea strain J1446 after application (see also Sections 3 and 4).
3. Residues
Clonostachys rosea strain J1446 (previously named Gliocladium catenulatum strain J1446) was observed to persist for up to 4 weeks on leaves; however, viable counts of Clonostachys rosea strain J1446 were shown to decline to colony forming units (CFU) below 100 per leave/fruit within 3 weeks after spray application which is lower than fungal background levels, and therefore, significant viable residues were not anticipated to be present on the raw agricultural commodity (RAC) after this period.
It was noted that according to the good agricultural practice (GAP) for fruiting vegetables, berries and grapes last applications can be carried out at BBCH 73‐89. These growth stages imply that the fruit has already developed or has even reached harvest maturity and it can be assumed that viable counts of above 100 might be present at the time of harvest and consumption which is not expected to be above natural background levels. It is nevertheless recommended to specify a preharvest interval (PHI) supported by studies on decline of viable counts following last applications to ensure that viable counts at the time of harvest are negligible because it is currently not clear whether toxic metabolites might be produced.
With regard to potential toxins/secondary metabolites, it was shown that gliotoxin was not present in the technical product however as raised in Section 2.3, the LOQ of the method is considerably high. In addition, no information is available as to whether Clonostachys rosea strain J1446 produces other toxins/secondary metabolites on the plant after application and during decline of viable counts (data gap). Notably meanwhile, G. catenulatum was reclassified to C. rosea and a number of metabolites maybe associated with this species. It is recommended to investigate this aspect to be able to exclude potential risks for the consumer.
It was not necessary to perform a consumer risk assessment for remaining viable cell CFU of the strain, as the latter did not show harmful health effects at higher concentrations and is therefore of no concern.
Because of the uncertainties related to the potential production of toxins/secondary metabolites, an inclusion in Annex IV of Regulation (EC) No 396/2005 can therefore not be recommended (European Commission, 2015). This may need to be reconsidered in the future in case that sufficient information on toxic and/or on toxicity of metabolites would become available.
4. Environmental fate and behaviour
Satisfactory information has been provided in relation to potential interference of Clonostachys rosea strain J1446 with the analytical systems for the control of the quality of drinking water provided for in Directive 98/83/EC3 (see specific Annex VI decision making criteria in Part II Commission Regulation (EU) No 546/20114). As these methods require pathogenic bacteria to be identified and confirmed as absent, it was considered unlikely that filamentous fungi or their conidia would interfere with methodologies used for such determinations.
Being a mitotic asexual fungus (no sexual recombination or meiosis having been observed in its life cycle), in which plasmids are absent from the cell cytoplasm (only mitochondrial plasmids are known), Clonostachys rosea strain J1446 would not be expected to have the potential for transfer of genetic material to other organisms.
4.1. Fate and behaviour in the environment of the microorganism
Specific studies on the persistence and multiplication in peat growing media of Clonostachys rosea strain J1446 indicated that between 8 weeks and 6 months after application of CFU of Clonostachys rosea strain J1446, CFU were present at levels comparable to the natural background levels of Clonostachys spp. The investigations were not carried out by officially recognised organisations. Information regarding this behaviour in soil was not available for strain J1446. These data are considered sufficient to conclude that Clonostachys rosea strain J1446 will respect the uniform principles criterion of not being expected to persist in peat growing media in concentrations considerably higher than the natural background levels, taking into account repeated applications over the years. The information available for soil or for other non‐peat based growing media (that was from investigations published in the peer reviewed scientific literature with Clonostachys rosea strain IK726) indicated the species was not highly competitive (significant decline was observed). However, the experiments were not carried out for sufficient time to reach a definitive conclusion on whether CFU returned to background levels. This is identified as a data gap and an assessment not finalised. Predicted environmental concentrations (PEC) in soil have been calculated in the RAR considering no crop interception. A justification to include canopy interception in refined soil PEC calculations was included for the field use on strawberry; however, a comparable justification for canopy interception and wash off into soil was not available for any of the other outdoor representative uses where foliar applications are recommended. Consequently, a data gap was identified for such information regarding the field crops except strawberry.
With respect to the persistence and multiplication in water, specific studies in the dossier indicated that Clonostachys rosea strain J1446 was able to survive in sea, lake, tap and distilled water but did not proliferate. However, germination and population growth was likely to have been prevented due to the relatively low availability of nutrients in the systems investigated that would be expected to have lower nutrient status than natural edge of field water bodies. The investigations were not carried out by officially recognised organisations. Consequent to these issues, the need for further information on persistence and multiplication in water was identified as a data gap. Due to the method of application assessed via downward hydraulic spraying and air assisted broadcast spraying, the Clonostachys rosea strain J1446 propagules applied have the potential to reach surface water via spray drift. PEC surface water were calculated considering this route of exposure in the RAR including the implementation of no spray buffer zones (using approaches analogous to those for spray drift described in FOCUS, 2001, 2007 guidance). It is likely that exposure via lateral surface/subsurface flow or drainage via field drains will be negligible, as available information indicated that filamentous fungal spp. did not percolate through soil at significant levels and had restricted potential to grow except in the topsoil layers (although it might be expected that mycelial growth occurs in the rhizosphere). Therefore, surface water exposure is expected to be negligible when application is by the techniques of drenching, drip irrigation, soil incorporation and hydroponic systems that recycle nutrient solution.
Investigations included in the dossier indicated that Clonostachys spp. inocula are generally found at limited distances from where used, primarily due to spray drift, indicating movement via air over distances greater than several meters would not be expected. The investigations were not carried out by officially recognised organisations.
Regarding mobility, the available investigations described above in relation to persistence and multiplication in peat indicated that Clonostachys rosea strain J1446 had restricted potential to grow except in the top layers of growing media. Horizontal spread over the growing media surface was indicated to occur but to a limited extent.
4.2. Fate and behaviour in the environment of any relevant metabolite formed by the microorganism under relevant environmental conditions
An appropriate systematic review of the peer‐reviewed scientific literature identified that Clonostachys spp. are able to produce a lot of different metabolites such as epipolythiodioxopiperazines, 1H,1′H‐[3,3′]biindolyl epidithiodioxopiperazine, peptaibiotics, gliocladins A, B, C, D and E, 5‐n‐heneicosylresorcinol, bisorbicillinoids, verticillin A, 11′‐deoxyverticillin A, Sch52900, Sch52901 and polyterpenoid glisoprenins. Some of these are inhibitory to fungi, bacteria, or nematodes.
A data gap is identified since it is not known to what extent Clonostachys rosea strain J1446 will produce any metabolites following their application once the spores/hyphae reach soil. It is not clear if such metabolites should they be formed might fulfil the criteria according to Part B section 7 (iv) of Commission Regulation (EU) No 283/20135 namely:
the relevant metabolite is stable outside the microorganism;
a toxic effect of the relevant metabolite is independent of the presence of the micro‐organism;
the relevant metabolite is expected to occur in the environment in concentrations considerably higher than under natural conditions.
Therefore, data on the potential for Clonostachys rosea strain J1446 to produce metabolites in relation to these criteria (either following application or their presence or absence within the product) are necessary to assess if the further data requirements and the corresponding risk assessment according to Commission Regulation (EU) No 283/2013, part A, section 7 (standard data requirements and assessment mandatory for chemical plant protection active substances) are triggered. See Section 7 of this conclusion regarding this data gap. This leads to the necessary assessment being not finalised.
5. Ecotoxicology
Since it is not known to what extent Clonostachys rosea strain J1446 will produce any toxin/secondary metabolites following its application, a data gap was identified in Section 4. The test item of the dossier studies for the ecotoxicological testing was Clonostachys rosea cell mass (originally Gliocladium catenulatum cell mass); batch number 44/95. The strain has not been stated.
An appropriate laboratory study indicated no signs for pathogenicity and infectiveness for birds. Some supplemental information was available from the open literature confirming that natural exposure of Clonostachys spp. to birds indicating no evidence of infectivity or pathogenicity. Therefore, a low risk for pathogenicity and infectiveness of Clonostachys rosea strain J1446 was concluded for birds for the representative uses. Furthermore, the data that was available for laboratory mammals (rats) also indicated no evidence of pathogenicity and infectivity (see Section 2).
The exposure of surface water via soil processes was concluded as negligible in Section 4. Therefore, a low risk to aquatic organisms was concluded for all the representative uses other than foliar spray uses. As regards the risk assessment for the foliar spray uses, suitable studies were available for fish and aquatic invertebrates (Daphnia). Considering these studies, a low risk for pathogenicity and infectiveness of Clonostachys rosea strain J1446 was concluded for fish. However, the available data for daphnids were not considered satisfactory by the RMS to conclude on a low risk even when spray drift mitigation was taken into consideration. EFSA agrees to the assessment and therefore, a data gap was identified. No suitable study or any robust information was available to conclude a low risk for algae. Therefore, a data gap was identified. No specific information was available for aquatic plants. However, considering the biological properties and the mode of action of Clonostachys rosea strain J1446, a low risk for pathogenicity and infectiveness to aquatic macrophytes was concluded. It is noted that further information on persistence and multiplication of the microorganism in water was requested in Section 4. Once this data gap is addressed, the risk assessment for the foliar spray uses may be reconsidered.
A chronic laboratory study on honeybees and a large set of supplemental information from the open literature was available for honeybees and bumblebees. On the basis of this information, a low risk for pathogenicity and infectiveness to bees was concluded for the representative uses.
On the basis of the available laboratory studies on non‐target arthropods and considering the biological properties of the microorganism, a low risk for pathogenicity and infectiveness was concluded for the representative uses.
An appropriate 14‐day laboratory study indicated no signs of pathogenicity or infectiveness on earthworms. Additionally, some supplemental information was available from the open literature indicating lack of adverse effects on earthworms. Therefore, a low risk for pathogenicity and infectiveness of Clonostachys rosea strain J1446 was concluded for earthworms for the representative uses. Nevertheless, it is noted that a study from the open literature reported some little parasitism towards nematode eggs resulting in reduced hatch.
Relevant information for soil microorganisms was available from the open literature and from studies submitted for the assessment on fate and behaviour of Clonostachys rosea (see Section 4). Considering this information, it was concluded that a temporary change in the natural soil microflora after the application of Clonostachys rosea strain J1446 as a pesticide cannot be excluded. However, it is unlikely that these changes will be long‐lasting. Considering this, the biological properties and the mode of action, the risk for adverse effects on non‐target soil microorganisms from the use of Clonostachys rosea strain J1446 was considered as low.
6. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1–4)
Table 1.
Soil
| Compound (name and/or code) | Persistence | Ecotoxicology |
|---|---|---|
| Clonostachys rosea strain J1446 | Open for soil, declines in peat. (Data gaps) | The risk for pathogenicity and infectiveness for soil macroorganisms or the risk for adverse effects on non‐target soil microorganisms was considered as low |
| Potential secondary metabolites including: epipolythiodioxopiperazines, 1H,1′H‐[3,3]biindolyl epidithiodioxopiperazine, peptaibiotics, gliocladins A, B, C, D & E, 5‐n‐heneicosylresorcinol, bisorbicillinoids, verticillin A, 11′‐deoxyverticillin A, Sch52900, Sch52901 and polyterpenoid glisoprenins | Data gap, pending identification | Data gap, pending identification |
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Clonostachys rosea strain J1446 | Rat LC50 > 6.60−7.98 × 107 CFU/kg bw |
LC50: lethal concentration, median; CFU: colony forming units; bw: body weight.
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| Potential secondary metabolites including: epipolythiodioxopiperazines, 1H,1′H‐[3,3′]biindolyl epidithiodioxopiperazine, peptaibiotics, gliocladins A, B, C, D & E, 5‐n‐heneicosylresorcinol, bisorbicillinoids, verticillin A, 11′‐deoxyverticillin A, Sch52900, Sch52901 and polyterpenoid glisoprenins | Data gap | Data gap | Data gap, pending identification | Data gap, pending identification |
FOCUS scenarios or relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Clonostachys rosea strain J1446 | Data gap |
| Potential secondary metabolites including: epipolythiodioxopiperazines, 1H,1′H‐[3,3′]biindolyl epidithiodioxopiperazine, peptaibiotics, gliocladins A, B, C, D & E, 5‐n‐heneicosylresorcinol, bisorbicillinoids, verticillin A, 11′‐deoxyverticillin A, Sch52900, Sch52901 and polyterpenoid glisoprenins | Data gap, pending identification |
7. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of Regulation (EC) No 1107/2009 concerning information on potentially harmful effects).
7.1. Data gaps identified for the representative uses evaluated
5‐batch data under GLP for the manufactured product (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1).
Validation data to prove the LOQ of the method analysing for gliotoxin content (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1).
Information to prove the absence of toxins/secondary metabolites in the microbial pest control product (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1).
Information to prove the absence of secondary metabolites in the RAC after application of the MPCP (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 1 and 3).
Study for the determination of the growth temperature range of Clonostachys rosea strain J1446 from an officially recognised testing facility/organisation or to GLP as required by the data requirements was not available (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1).
Data to address the resistance of Clonostachys rosea strain J1446 to antibiotics/antimicrobial agents (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1).
Determination of the content of the active substance and levels of contaminants before and after storage (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1).
Information on persistence and multiplication of Clonostachys rosea strain J1446 in soil and growth media including times taken for decline to levels present before J1446 was introduced, competitiveness/ability for J1446 to proliferate in natural surface water from peer‐reviewed scientific literature, an officially recognised testing facility/organisation or to GLP as required by the data requirements was not available (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
Satisfactory information on the ability of Clonostachys rosea strain J1446 to proliferate in natural surface water systems was not available (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
Possible mobility of conidia into soil was not addressed for the representative uses on grape vines, wheat, maize, fruiting vegetables leafy vegetables, onion, potato, leek, raspberry, blueberry or turf. This open issue would be addressed if information would be provided that demonstrates that C. rosea is not present in soil in quantities higher than natural amounts (relevant for use in grape vines, wheat, maize, fruiting vegetables leafy vegetables, onion, potato, leek, raspberry, blueberry or turf; submission date proposed by the applicant: unknown; see Section 4).
Information to demonstrate that when drenching or spraying the plant protection product under the glasshouse conditions movement via air is limited with deposition occurring only within small distances in investigations originating from peer‐reviewed scientific literature, an officially recognised testing facility/organisation or a GLP facility was not available (relevant for all representative glasshouse uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
Satisfactory evidence (measurements) from Clonostachys rosea strain J1446 on its ability or lack of ability to produce toxins/secondary metabolites that have the potential to be present in the product or produced in situ by Clonostachys rosea strain J1446 in soil after application against the fate and behaviour assessment criteria at section 7 introduction (iv) of the data requirements was not available. In particular this information is missing for the potential secondary metabolites: epipolythiodioxopiperazines, 1H,1′H‐[3,3′]biindolyl epidithiodioxopiperazine, peptaibiotics, gliocladins A, B, C, D and E, 5‐n‐heneicosylresorcinol, bisorbicillinoids, verticillin A, 11′‐deoxyverticillin A, Sch52900, Sch52901 and polyterpenoid glisoprenins (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 4 and 5).
Further information and assessments for potential effects of Clonostachys rosea strain J1446 on aquatic invertebrates and algae is missing (relevant for foliar spray uses; submission date proposed by the applicant: unknown; see Section 5).
8. Particular conditions proposed to be taken into account to manage the risk(s) identified
It should be noted that the formulation cannot be mixed with concentrated solutions of pesticides and fertilisers.
9. Concerns
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/20116 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The information available was insufficient to demonstrate that Clonostachys rosea strain J1446 would respect the uniform principles criterion of not being expected to persist in soil in concentrations considerably higher than the natural background levels, taking into account repeated applications over the years and should this not be the case, satisfy the uniform principles associated unless clause, in the context of soil organisms (see Section 4).
The production of toxins/secondary metabolites cannot be excluded by Clonostachys rosea strain J1446. Therefore, the risk assessment could not be finalised for workers, residents, consumers and the environment including the assessment of potential groundwater exposure.
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at the higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
None identified for the representative uses.
9.3. Overview of the concerns identified for each representative use considered
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
Table 5.
Overview of concerns
| Representative use | Spray uses | Non‐spray uses | |
|---|---|---|---|
| Operator risk | Risk identified | ||
| Assessment not finalised | |||
| Worker risk | Risk identified | ||
| Assessment not finalised | X2 | X2 | |
| Resident/bystander risk | Risk identified | ||
| Assessment not finalised | X2 | X2 | |
| Consumer risk | Risk identified | ||
| Assessment not finalised | X2 | X2 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | ||
| Assessment not finalised | |||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | ||
| Assessment not finalised | |||
| Risk to aquatic organisms | Risk identified | X | |
| Assessment not finalised | |||
| Groundwater exposure to active substance | Legal parametric value breached | ||
| Assessment not finalised | |||
| Groundwater exposure to metabolites | Legal parametric value breacheda | ||
| Parametric value of 10 μg/Lb breached | |||
| Assessment not finalised | X2 | X2 | |
Columns are grey if no safe use can be identified. The superscript numbers relate to the numbered points indicated in Section 9.2. Where there is no superscript number, see Sections 2–6 for further information.
When the consideration for classification made in the context of this evaluation under Regulation (EC) No 1107/2009 is confirmed under Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission (2003).
Abbreviations
- a.s.
active substance
- BBCH
growth stages of mono‐ and dicotyledonous plants
- BIOHAZ
EFSA Panel on Biological Hazards
- bw
body weight
- CFU
colony forming units
- EEC
European Economic Community
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
good agricultural practice
- GLP
good laboratory practice
- LOQ
limit of quantification (determination)
- MPCA
microbial pest control agent
- MPCP
microbial pest control product
- PCR
polymerase chain reaction
- PEC
predicted environmental concentration
- PECair
predicted environmental concentration in air
- PECgw
predicted environmental concentration in groundwater
- PECsed
predicted environmental concentration in sediment
- PECsoil
predicted environmental concentration in soil
- PECsw
predicted environmental concentration in surface water
- PHI
pre‐harvest interval
- QPS
qualified presumption of safety
- RAC
raw agricultural commodity
- RAPD
random amplified polymorphic DNA
- RAR
renewal assessment report
- RMS
rapporteur Member State
- SMILES
simplified molecular‐input line‐entry system
- WP
wettable powder
Appendix A – List of end points for the active substance and the representative formulation
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4905
Appendix B – Used compound codes
| Code/trivial namea | Chemical name/SMILES notation | Structural formula |
|---|---|---|
| Gliotoxin |
(3R,5aS,6S,10aR)‐6‐hydroxy‐3‐(hydroxymethyl)‐2‐methyl‐2,3,6,10‐tetrahydro‐5aH‐3,10a‐epidithiopyrazino[1,2‐a]indole‐1,4‐dione O=C1N3[C@@H]4[C@@H](O)C=CC=C4C[C@]32SS[C@@]1(CO)N(C)C2=O |
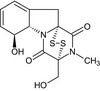
|
| Gliovirin |
(1aS,4R,4aS,8S,9R,11aR,12aS)‐4‐hydroxy‐9‐(3‐hydroxy‐4,5‐dimethoxyphenyl)‐4,4a,8,9‐tetrahydro‐1aH,7H‐8,11a‐(epiminomethano)[1,2,4]dithiazepino[4,3‐b]oxireno[e][1,2]benzoxazine‐7,13(12H)‐dione Oc1cc(cc(OC)c1OC)[C@H]3SS[C@@]42C[C@]65O[C@H]6C=C[C@@H](O)[C@@H]5ON4C(=O)[C@@H]3NC2=O |
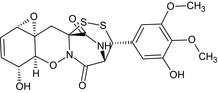
|
| Verticillin A |
(3S,5aR,10bS,11S,11aS)‐11‐hydroxy‐10b‐[(3R,5aS,10bS,11R,11aR)‐11‐hydroxy‐2,3‐dimethyl‐1,4‐dioxo‐1,2,3,4,5a,6‐hexahydro‐3,11a‐epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3‐b]indol‐10b(11H)‐yl]‐2,3‐dimethyl‐2,3,5a,6,10b,11‐hexahydro‐3,11a‐epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3‐b]indole‐1,4‐dione O=C1N3[C@]2(SS[C@]1(C)N(C)C2=O)[C@@H](O)[C@@]4(c5ccccc5N[C@H]34)[C@@]89[C@@H](O)[C@]%106SS[C@@](C)(N(C)C6=O)C(=O)N%10[C@@H]9Nc7ccccc78 |
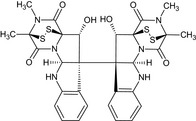
|
| 11′‐deoxyverticillin A |
(3S,5aR,10bR,11aS)‐10b‐[(3R,5aS,10bS,11R,11aR)‐11‐hydroxy‐2,3‐dimethyl‐1,4‐dioxo‐1,2,3,4,5a,6‐hexahydro‐3,11a‐epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3‐b]indol‐10b(11H)‐yl]‐2,3‐dimethyl‐2,3,5a,6,10b,11‐hexahydro‐3,11a‐epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3‐b]indole‐1,4‐dione O=C9N1[C@@]%10(C[C@@]2(c3ccccc3N[C@H]12)[C@@]67[C@@H](O)[C@]84SS[C@@](C)(N(C)C4=O)C(=O)N8[C@@H]7Nc5ccccc56)SS[C@]9(C)N(C)C%10=O |
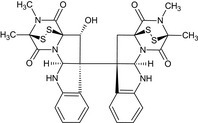
|
| 5‐ n ‐heneicosylresorcinol |
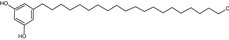
5‐henicosyl‐1,3‐benzenediol Oc1cc(CCCCCCCCCCCCCCCCCCCCC)cc(O)c1 |
|
| Gliocladine A |
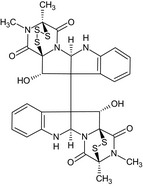
(11bS)‐12‐hydroxy‐11b‐[(3S,5aR,10bR,11R,11aS)‐11‐hydroxy‐2,3‐dimethyl‐1,4‐dioxo‐1,2,3,4,5a,6‐hexahydro‐3,11a‐epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3‐b]indol‐10b(11H)‐yl]‐4,14‐dimethyl‐6a,7,11b,12‐tetrahydro‐4,12a‐(epiminomethano)[1,2,3,5]trithiazepino[5′,4′:1,5]pyrrolo[2,3‐b]indole‐5,13(4H)‐dione O=C1N3[C@]2(SS[C@]1(C)N(C)C2=O)[C@@H](O)C4(c5ccccc5N[C@H]34)C89c%10ccccc%10N[C@@H]9N6C(=O)[C@]7(C)SSS[C@]6(C(=O)N7C)[C@H]8O |
|
| Gliocladine B |
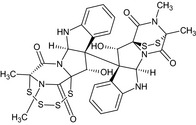
(5R,7aR,12bS,13S)‐13‐hydroxy‐12b‐[(3R,5aR,10bS,11S,11aS)‐11‐hydroxy‐2,3‐dimethyl‐1,4‐dioxo‐1,2,3,4,5a,6‐hexahydro‐3,11a‐epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3‐b]indol‐10b(11H)‐yl]‐5,15‐dimethyl‐7a,8,12b,13‐tetrahydro‐5,13a‐(epiminomethano)[1,2,3,4,6]tetrathiazocino[6′,5′:1,5]pyrrolo[2,3‐b]indole‐6,14(5H)‐dione C[C@@]12C(=O)N3[C@@H]4[C@]([C@@H]([C@@]3(C(=O)N1C)SS2)O)(C5=CC=CC=C5N4)[C@]67[C@@H](C89C(=O)N([C@@](C(=O)N8[C@H]6NC1=CC=CC=C71)(SSSS9)C)C)O |
|
| Gliocladine C |
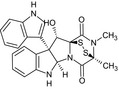
(5aR,10bR,11S,11aS)‐11‐hydroxy‐10b‐(1H‐indol‐3‐yl)‐2,3‐dimethyl‐2,3,5a,6,10b,11‐hexahydro‐3,11a‐epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3‐b]indole‐1,4‐dione O=C5N6[C@H]7Nc1ccccc1[C@@]7(c3cnc2ccccc23)[C@H](O)[C@@]64SS[C@]5(C)N(C)C4=O |
|
| Gliocladine D | (4S,6aR,11bS,12S,12aS)‐12‐hydroxy‐11b‐(1H‐indol‐3‐yl)‐4,14‐dimethyl‐6a,7,11b,12‐tetrahydro‐4,12a‐(epiminomethano)[1,2,3,5]trithiazepino[5′,4′:1,5]pyrrolo[2,3‐b]indole‐5,13(4H)‐dioneC[C@]12C(=O)N3[C@@H]4[C@@]([C@@H]([C@@]3(C(=O)N1C)SSS2)O)(C5=CC=CC=C5N4)C6=CNC7=CC=CC=C76 |
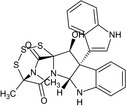
|
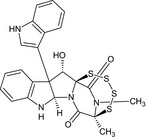
| Gliocladine E |
(5S,7aR,13S,13aS)‐13‐hydroxy‐12b‐(1H‐indol‐3‐yl)‐5,15‐dimethyl‐7a,8,12b,13‐tetrahydro‐5,13a‐(epiminomethano)[1,2,3,4,6]tetrathiazocino[6′,5′:1,5]pyrrolo[2,3‐b]indole‐6,14(5H)‐dione O[C@@H]5[C@]71SSSS[C@@](C)(C(=O)N1[C@H]6Nc2ccccc2C56c4cnc3ccccc34)N(C)C7=O |
|
| Glisoprenin A |
(2E,6E,10E,14E)‐3,7,11,15,19,23,27,31,36‐nonamethyl‐2,6,10,14,35‐heptatriacontapentaene‐1,19,23,27,31‐pentol OC(C)(CCCC(C)(O)CCCC(C)(O)CCCC(\C)=C\CCC(\C)=C\CCC(\C)=C\CCC(\C)=C\CO)CCCC(C)(O)CCC\C=C(/C)C |
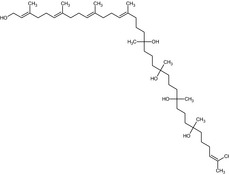
|
| Glisoprenin B |
(2E,6E,10E,14E)‐30‐[5‐(2‐hydroxy‐2‐propanyl)‐2‐methyltetrahydro‐2‐furanyl]‐3,7,11,15,19,23,27‐heptamethyl‐2,6,10,14‐triacontatetraene‐1,19,23,27‐tetrol CC1(CCCC(C)(O)CCCC(C)(O)CCCC(C)(O)CCCC(\C)=C\CCC(\C)=C\CCC(\C)=C\CCC(\C)=C\CO)CCC(O1)C(O)(C)C |
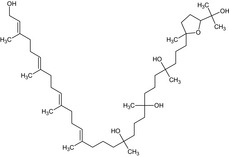
|
| Viridin |
(1S,2S,11bR)‐1‐hydroxy‐2‐methoxy‐11b‐methyl‐1,7,8,11b‐tetrahydrocyclopenta[7,8]phenanthro[10,1‐bc]furan‐3,6,9(2H)‐trione O=C5CCc1c5ccc4c1C(=O)c2occ3c2[C@]4(C)[C@H](O)[C@H](OC)C3=O |
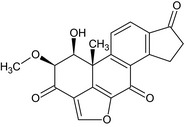
|
| Heptelidic acid |
(5aS,6R,9S,9aS)‐6‐isopropyl‐1‐oxo‐1,5a,6,7,8,9a‐hexahydro‐3H‐spiro[2‐benzoxepine‐9,2′‐oxirane]‐4‐carboxylic acid O=C(O)C2=C[C@@H]3[C@H](CC[C@@]1(CO1)[C@H]3C(=O)OC2)C(C)C |
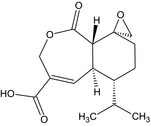
|
SMILES: simplified molecular‐input line‐entry system.
The compound name in bold is the name used in the conclusion.
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Arena M, Auteri D, Barmaz S, Bellisai G, Brancato A, Brocca D, Bura L, Byers H, Chiusolo A, Court Marques D, Crivellente F, De Lentdecker C, De Maglie M, Egsmose M, Erdos Z, Fait G, Ferreira L, Goumenou M, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Magrans JO, Medina P, Miron I, Molnar T, Nougadere A, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Tarazona J, Terron A, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Conclusion on the peer review of the pesticide risk assessment of the active substance Clonostachys rosea strain J1446 (approved in Regulation (EU) No 540/2011 as Gliocladium catenulatum strain J1446). EFSA Journal 2017;15(7):4905, 22 pp. 10.2903/j.efsa.2017.4905
Requestor: European Commission
Question number: EFSA‐Q‐2015‐00582
Approved: 15 June 2017
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.98, p. 32–54.
Commission Regulation (EU) 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Commission Regulation (EU) 283/2013 of 1 March 2013 setting out the data requirements for active substances in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- EFSA (European Food Safety Authority), 2017. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance Clonostachys rosea strain J1446. Available online: www.efsa.europa.eu
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2009. Scientific Opinion on the maintenance of the list of QPS microorganisms intentionally added to food or feed (2009 update). EFSA Journal 2009;7(12):1431, 92 pp. 10.2903/j.efsa.2009.1431 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal 2012;10(6):2740, 10 pp. 10.2903/j.efsa.2012.2740 [DOI] [Google Scholar]
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10‐final 25 February 2003.
- European Commission , 2012. Working Document on Microbial Contaminant Limits for Microbial Pest Control Products. SANCO/12116/2012‐rev. 0. September 2012.
- European Commission , 2014. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- European Commission , 2015. Guidance document on criteria for the inclusion of active substances into Annex IV of Regulation (EC) No 396/2005. SANCO/11188/2013‐rev. 2, 14 September 2015.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2001.FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.1, March 2012.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2007. Landscape and mitigation factors in aquatic risk assessment. Volume 1. Extended summary and recommendations. Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v. 2.0, 169 pp.
- Hungary , 2016. Renewal Assessment Report (RAR) on the active substance Gliocladium catenulatum strain J1446 prepared by the rapporteur Member State Hungary, in the framework of Commission Implementing Regulation (EU) No 844/2012, July 2016. Available online: www.efsa.europa.eu
- Hungary , 2017. Revised Renewal Assessment Report (RAR) on Clonostachys rosea strain J1446 approved in Regulation (EU) No 540/2011 as Gliocladium catenulatum strain J1446 prepared by the rapporteur Member State Hungary in the framework of Regulation (EC) No 844/2012, April 2017. Available online: www.efsa.europa.eu
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


