Abstract
T‐2 toxin (T2) and HT‐2 (HT2) toxin are trichothecenes, which form part of the group of Fusarium mycotoxins. Food and feed samples used to estimate human dietary and animal exposure were reported either as the individual results for T2 and/or, HT2, and/or as the sum of the two. The highest concentrations were reported in oats and oat‐containing commodities. Very high levels were reported in a small number of data on specific plant‐ and herb‐based dietary supplements. In humans, the mean chronic dietary exposure to the sum of T2 and HT2 was highest in ‘Toddlers’ and ‘Infants’, with maximum upper bound (UB) estimates of 64.8 and 62.9 ng/kg body weight (bw) per day, respectively. The 95th percentile dietary exposure was highest in ‘Infants’ with a maximum UB estimate of 146 ng/kg bw per day. UB estimations were on average fourfold higher than lower bound (LB) estimations. Average acute exposure ranged from a minimum of 13.4 ng/kg bw per day, estimated in ‘Elderly’, up to a maximum of 64.7 ng/kg bw per day estimated in ‘Toddlers’. The highest 95th percentile acute dietary exposure was estimated for a dietary survey within the age class ‘Infants’ (170 ng/kg bw per day). Overall, among processed foods the main contributors were cereal flakes, fine bakery wares and, for acute exposure, also bread and rolls. In the elderly and very elderly, dietary supplements made an important contribution. Exposure to the sum of T2 and HT2 in farm and companion animals varied according to the animal species. Exposures considering mean concentration scenarios varied between 0.03–0.08 (LB–UB) μg/kg bw per day in beef cattle and 1.13–1.47 μg/kg bw per day in milking goats. For high concentration scenarios, exposures varied between 0.12–0.16 μg/kg bw per day and 2.37–2.58μg/kg bw per day in the same species. In the absence of data, potential modified form were not included.
Keywords: T‐2, HT‐2, food, feed, dietary exposure
Summary
T‐2 toxin (T2) and HT‐2 toxin (HT2) belong to the group of compounds known as trichothecenes, which are part of the largest group of Fusarium mycotoxins comprising more than 150 compounds. In 2017, the European Food Safety Authority (EFSA) published a scientific opinion on the appropriateness to set a group health‐based guidance value (HBGV) for T2 and HT2 and its modified forms with objective of confirming or revising the previously established tolerable daily intake (TDI) of 0.1 μg/kg body weight (bw) and also to set, if appropriate, an acute reference dose (ARfD). Acute emetic events in mink following exposure to both T2 and HT2 observed in an in vivo acute toxicity study were identified as critical effects for setting an ARfD for T2 and HT2, and benchmark dose (BMD) calculations produced a BMDL10 of 2.97 μg T2 or HT2/kg bw per day. Using an uncertainty factor (UF) of 10, a group ARfD of 0.3 μg T2 and HT2/kg bw was established. Based on an in vivo subchronic toxicity study with rats, a reduction of total leukocyte count was identified as the critical effect for setting a TDI. Using this endpoint, a BMDL10 of 3.33 μg T2/kg bw per day was calculated and by using a UF of 200, a new group TDI for T2 and HT2 of 0.02 μg/kg bw was established.
Following an official request by the European Commission in September 2016, the EFSA Evidence Management Unit (DATA unit) has estimated chronic and acute dietary exposure to T2 and HT2 in humans and animals. A total of 93,335 analytical results reported either as the individual results for T2 (n = 41,922), HT2 (n = 40,236) or as the sum of two (n = 11,177), were submitted to EFSA. The data covered food, feed and unprocessed grains of undefined end‐use. The T2 and HT2 concentration levels on unprocessed grains did not show a robust consistency when compared to those reported as ‘Grains for human consumption’ or when compared to those reported as feed grains. Therefore, these data were not used in the present human or animal exposure assessment.
A total of 19,505 analytical results for food (8,502 results for T2, 6,877 results for HT2 and 4,126 results for the sum of T2 and HT2) and 6,411 analytical results for feed (2,735 results for T2, 2,429 results for HT2 and 1,247 results for the sum of T2 and HT2) were included in the final data set. In addition, more analytical results for the sum of T2 and HT2 were obtained by combining the individual concentrations of the T2 and HT2 for each sample, and consequently merged with those reported as the sum of T2 and HT2. For instances where either one of the individual T2 or HT2 values was not reported, an imputation method using the mean occurrence of the food group in concern was used to simulate the missing results. For food, this resulted in 9,551 analytical results for the sum of T2 and HT2 available for the human dietary exposure assessment. For feed, 3,007 analytical results for the sum of T2 and HT2 were available for the animal exposure assessment.
The food samples were collected between 2011 and 2016 in 20 different European countries, most of them in Germany (50%). The data set was characterised by a high proportion of left‐censored data (results below limit of detection (LOD)/limit of quantification (LOQ)) with 90% of left‐censored data reported for T2, and 87% of left‐censored data reported for HT2 and the sum of T2 and HT2 data. Within the food category ‘Grains and grain‐based products’, the highest levels of the sum of T2 and HT2 were reported in ‘Grains for human consumption’ and ‘Breakfast cereals’, in particular in oat‐containing commodities (e.g. oat grains, 127–128 μg/kg, lower bound–upper bound (LB–UB); oat cereal flakes, 13.9–16.5 μg/kg, LB–UB). Very high concentrations were reported for ‘Dietary supplements’ (592–594 μg/kg, LB–UB) with all high levels related to dietary supplements containing plant extracts and reported by one data provider. Quantified results were also found in other food categories covering grain‐based food products (e.g. snacks, food for infants and young children), while in other non‐grain‐based foods (e.g. legumes, fats and oils) the T2 and HT2 concentrations were quantified only occasionally.
For 34% of the food samples, no information was provided about the analytical methods used to analyse the toxins. In those where information was submitted, the separation method was mostly liquid chromatography (LC) with diverse detection methods. Low variability of the LOQs was observed across food categories as well as across the analytical methods. Although the majority of reported LOQs are in line with those described in recent literature, many of the analytical methods used for this dataset reported relatively high LOQs. This has a significant impact on the UB estimations when dealing with left‐censored data, even though this influence on the UB estimations was reduced by excluding those samples that reported LOQs above 10 μg/kg for the individual T2 and HT2 and above 20 μg/kg for the sum of T2 and HT2.
Also for feed, a high proportion of left‐censored data was observed for all three parameters (91% for T2, and 88% for HT2 and the sum of T2 and HT2). Only the sum of T2 and HT2 occurrence data were considered for the animal exposure. The feed samples were collected in 14 different European countries between 2011 and 2016. The data were mostly available for the feed category ‘Cereal grains, their products and by‐products’ and within that, the highest mean concentrations were observed for oats (LB mean=401 μg/kg; UB mean=405 μg/kg). For other feed categories, quantified values were available for cereal straw, mixed grains, sunflower seed, grass (field dried), maize silage, barley, maize, complementary feed, skimmed milk, rye, complete feed, cotton seed, toasted soya, wheat and triticale, with the mean concentrations being rather low (LB mean concentrations in the range of 0.7 μg/kg measured in triticale to 16.9 μg/kg measured for cereal straw). The most common analytical method reported for the analysis of T2 and HT2 in feed was LC‐tandem mass spectrometry (MS/MS) (69%). The highest sensitivity (median LOQ=3.0 μg/kg) was observed for results analysed by high‐performance liquid chromatography‐fluorescence detection (HPLC‐FD).
In humans, mean chronic dietary exposure to the sum of T2 and HT2 was highest in ‘Toddlers’ and ‘Infants’ with maximum UB estimates of 64.8 and 62.9 ng/kg bw per day, respectively. The 95th percentile dietary exposure was highest in ‘Infants’ with a maximum UB estimate of 146 ng/kg bw per day. Overall, chronic dietary exposure to the sum of T2 and HT2 in the young population (‘Infants’, ‘Toddlers’, and ‘Other children’) was 2–3 times higher than that estimated for the adult population (‘Adults’, ‘Elderly’, and ‘Very elderly’). UB estimations were on average fourfold higher than LB estimations. Overall, ‘Grains and grain‐based products’ made the largest contribution to the LB mean chronic dietary exposure to the sum of T2 and HT2 in all age classes, in particular ‘Cereal flakes’ and ‘Fine bakery wares’.
Average UB acute exposure to the sum of T2 and HT2 ranged from a minimum of 13.4 ng/kg bw per day, estimated in ‘Elderly’, up to a maximum of 64.7 ng/kg bw per day, estimated in ‘Toddlers’. The highest 95th percentile acute dietary exposure was estimated for a dietary survey within the age class ‘Infants’ (170 ng/kg bw per day). The food mostly contributing to the acute exposure to the sum of T2 and HT2 was ‘Bread and rolls’. Considering the 95th percentile acute exposure, for ‘Bread and rolls’ the exposure reached values of up to 72.8 ng/kg bw per day (95% CI = 71.0–74.6). The contribution of this food category resulted to be rather low for the chronic exposure to the sum of T2 and HT2. It is important to mention that different approaches for the chronic and acute exposure assessment were followed. For the chronic exposure, the main contributors to the mean exposure were based on the LB approach, which is considered to be less influenced by results below LOD/LOQ, while the acute exposure and the most relevant food contributing to that was based on the UB approach, which is considered more appropriate to capture an exposure to a maximum possible dose. This is likely a reason of discrepancy as for foods mainly contributing to the exposure observed between the chronic and acute exposures.
Compared to the 2011 EFSA opinion, the present data set contained a much higher proportion of left‐censored occurrence data, particularly in foods making the biggest contribution to dietary exposure (i.e. grain‐based food commodities). As a consequence, the chronic exposure estimates are at UB approximately threefold higher for ‘Infants’ and approximately 30% higher for other age classes (relative to the maximum UB exposure levels) in the present assessment when compared to that estimated in 2011.
Although in animal nutrition compound feeds (complementary or complete feeds) represent a very large proportion of the feed consumed by farm animals, the available data on the occurrence of the sum of T2 and HT2 in these feeds are difficult to use for exposure calculations due to the low number of samples available for each target species or category or feeds not sufficiently characterised to allow a proper utilisation in diet formulations. Therefore, only the occurrence data on cereal grains and on some forages and roughage were used to calculate animal exposure. Farm and companion animal dietary exposure to the sum of T2 and HT2 varied according to the animal species. The exposure for the mean concentration scenario varied between 0.03–0.08 (LB–UB) μg/kg bw per day in beef cattle and 1.13–1.47 μg/kg bw per day in milking goats, while for a high concentration scenario this varied between 0.12–0.16 μg/kg bw per day and 2.37–2.58 μg/kg bw per day for the same species. The exposure estimates are lower when compared to those reported in the previous scientific opinions of the EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) published in 2011 and 2014, which corresponds to the lower T2 and HT2 concentrations in feed reported in the last 5 years.
Dietary exposure estimates to T2 and HT2 have uncertainties relating to the representativeness of the food and feed samples across Europe. The large proportion of samples with left‐censored data introduced considerable uncertainties to the exposure assessment as the use of the LB tends to underestimate, while UB tends to overestimate the dietary exposure. Furthermore, by applying the imputation method, the real concentrations may have been under‐ or overestimated. Extremely high concentrations on very specific food products (i.e. plant extract formula), reported by only one data provider, may have led to overestimation. Due to lack of data, a potential presence of other modified forms of T2 and HT2 was not considered, which could have resulted in an underestimation of the exposure. The uncertainty related to the consumption data mainly concerns the eating occasions reported as raw agricultural or minimally processed commodities derived from consumption data on processed foods that were disaggregated (e.g. bread disaggregated into flour). Therefore, exposure estimations derived from using these commodities are most probably overestimates.
Overall, the chronic dietary exposure to T2 and HT2 presented in this report is likely to overestimate the exposure levels of the European population, in particular at the UB estimation. Acute exposure estimates based only on UB occurrence data are also likely to be overestimates. Both chronic and acute exposure estimates are affected by the high proportion of left‐censored data.
Efforts should continue to collect analytical data on T2 and HT2, including potential modified forms, in relevant food and feed commodities, with a particular focus on analysing both individual toxins within the same sample. Analytical methods with the appropriate sensitivity should be used allowing the reduction of the uncertainty associated to dietary exposure estimations, probably leading to a large overestimation when using UB scenarios. It would be desirable to encourage further research for the occurrence of T2 and HT2 in dietary supplements in order to evaluate a possible important source of exposure from these products.
1. Introduction
T‐2 toxin (T2) and HT‐2 toxin (HT2) (Figure 1) are trichothecenes, which are the largest group of Fusarium mycotoxins. They comprise of more than 150 compounds. The basic structure of all trichothecenes is a tetracyclic sesquiterpene with a spiro‐epoxide group at C‐12 and C‐13, and an olefinic double bond between C‐9 and C‐10. According to the substituents of the tetracyclic ring system, trichothecenes are grouped into different types (A–D). T2 and HT2 belong to the type A trichothecenes, which are characterised by an esterified or free hydroxyl group at C‐8, or an unsubstituted C‐8. Type B trichothecenes, e.g. nivalenol and deoxynivalenol, carry a keto group at C‐8. Type A and B compounds constitute the majority of trichothecene contaminants in food and feed.
Figure 1.
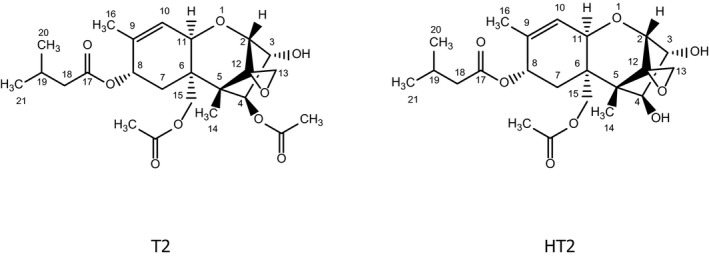
Chemical structures of T2 and HT2
The analytical methods for analysis of T2 and HT2 are well established and quantification is mainly carried out by liquid chromatography (LC) coupled with (multistage) mass spectrometry (MS) usually within a multianalyte approach. For rapid screening, immunochemical methods are used but have the disadvantage of cross‐reactivity. The existing immunochemical methods are not validated in interlaboratory studies.
In 2011, the European Food Safety Authority (EFSA) published a scientific opinion on the risks to human and animal health related to the presence of T‐2 toxin and HT‐2 toxin in food and feed (EFSA CONTAM Panel, 2011). In that risk assessment, the highest chronic human dietary exposure was estimated for toddlers, and was 12–43 ng/kg body weight (bw) per day for average consumers, and 23–91 ng/kg bw for 95th percentile consumers. Grains and grain‐based foods, in particular bread, fine bakery wares, grain milling products and breakfast cereals contributed most to the exposure. Based on the limited data available, no significant difference in exposures was found between vegetarians and the general population.
In their opinion of 2011, the EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) concluded that T2 inhibits protein, RNA and DNA synthesis and there are indications that T2 induces apoptosis, and in some cell types necrosis, as well as lipid peroxidation affecting cell membrane integrity. T2 induces haematotoxicity and myelotoxicity associated with impairment of haematopoiesis in bone marrow. A group tolerable daily intake (TDI) of 0.1 μg/kg bw was established for the sum of T2 and HT2 based on reduced antibody response to a specific antigen seen in a subchronic study with pigs.
All exposure estimates were below the group TDI of 0.1 μg/kg bw, and consequently, the Panel concluded that there was no health concern (EFSA CONTAM Panel, 2011).
Exposure of livestock and companion animals is mainly due to consumption of cereal grains and cereal by‐products, since levels in forages and oilseed meals were low. Species considered in the exposure assessment were dairy cows, beef cattle, sheep and goats, pigs and piglets, hens, broiler chickens, turkeys, ducks, rabbits, fish, dogs, cats and horses. The highest upper bound (UB) exposure estimates to the sum of T2 and HT2 was 3.3 μg/kg bw per day in milking goats, while the lowest was for farmed fish at 0.19 μg/kg bw per day.
For ruminants, a lowest observed adverse effect level (LOAEL) of 300 μg T2/kg bw per day was identified, based on findings of altered serum proteins and haematological and gastrointestinal parameter alterations. A no observed adverse effect level (NOAEL) could not be identified. For pigs, a LOAEL of 29 μg T2/kg bw per day was established based on observations of immunological and haematological effects from that dose onwards. No NOAEL could be established. In broiler chickens and turkeys, T2 caused mucosal oral damage (LOAELs of 40 and 48 μg/kg bw per day, respectively), while in ducks at 40 μg/kg bw per day reduced bw gain was observed. For laying hens, a LOAEL of 120 μg T2/kg bw per day was set based on infertility/reduced egg production at that dose. For rabbits, a NOAEL of 100 μg T2/kg bw per day was identified based on moderate haematological and hormonal effects seen at higher doses. For fish, a NOAEL of 13 μg T2/kg bw per day was identified (reduced feed intake, growth and increased mortality at higher doses). Due to the limited data and severity of effects observed (i.e. mortality), no reference points could be established for cats which are among the most sensitive species (EFSA CONTAM Panel, 2011). Due to absent/limited data, no NOAELs/LOAELs could be identified for dogs or horses.
The Panel concluded that the exposure of ruminants, fish and rabbits to the sum of T2 and HT2 is well below respective LOAELs and NOAELs and thus unlikely of health concern. For pigs, poultry, dogs and horses, the BMDL05 for pigs (10 μg/kg bw per day) was used as the reference point in the absence of NOAELs/LOAELs for these species. When comparing estimated exposures to the sum of T2 and HT2 with this value, it was concluded that the health risk for these species was low. For cats, health risks could not be assessed.
In 2014, EFSA published a scientific opinion on the risks for human and animal health related to modified forms of zearalenone, nivalenol, T‐2 and HT‐2 toxins and fumonisins (EFSA CONTAM Panel, 2014). The Panel concluded that modified forms may add another 10% to the concentration of T2 and HT2 in food and feed. As a pragmatic approach it was assumed that modified forms of T2 and HT2 have equal toxicity as their parent compounds. To assess risks to T2 and HT2 and their modified forms, 10% was added to the exposures to the sum of T2 and HT2 for the different consumer groups as calculated in the previous opinion (EFSA CONTAM Panel, 2011) and EFSA compared these exposure levels with the TDI of 100 ng/kg bw established previously (EFSA CONTAM Panel, 2011). Exposure to the sum of T2 and HT2 and their modified forms was not of concern because lower bound (LB) and UB exposures across dietary surveys were lower than the TDI, except for the highest UB exposure for toddlers which was similar to the TDI. As in the previous opinion, exposure to T2 and HT2 of farm and companion animals were based on levels in feed ingredients. For risk characterisation, 10% was added to total UB exposures to T2 and HT2 and compared with NOAELs/LOAELs established in the previous opinion or indicative or guidance values where these were not available. Briefly, not only the mean but also the 95th percentile (P95) (not estimated in 2011) exposure to T2 and HT2 and their modified forms were lower than the respective effect/indicative/guidance levels and thus not of concern for pigs, ruminants, poultry, rabbits and dogs. For fish, the risk could not be characterised and for cats not ruled out, although for both this was considered unlikely.
In 2017, EFSA published a scientific opinion on the appropriateness to set a group health‐based guidance value (HBGV) for T2 and HT2 toxin and its modified forms (EFSA CONTAM Panel, 2017). For this opinion, the Panel reviewed new data on T2 and HT2 (published after the previous assessment) to confirm or revise the previously established TDI and also to set, if appropriate, an acute reference dose (ARfD). Any information on modified T2 and HT2 (i.e. metabolites of T2 and HT2) was evaluated to see if these could be included in a group HBGV with T2 and HT2.
New in vivo acute toxicity studies showed that T2 and HT2 have anorectic effects upon short‐term exposure. Acute emetic events in mink upon exposure to both T2 and HT2 were identified as critical effects for setting an ARfD for T2 and HT2, and calculations for benchmark dose (BMD) resulted in a BMDL10 of 2.97 μg T2 or HT2/kg bw per day. Using a uncertainity factor (UF) of 10, a group ARfD of 0.3 μg T2 and HT2/kg bw was established. An interspecies factor was not applied as it was assumed that humans are not more sensitive than mink towards this effect.
A new in vivo subchronic toxicity study with rats confirmed that that immune‐ and haematotoxicity are the critical effects of T2 upon repeated exposure. In this new study, a reduction of total leukocyte count was observed which was identified as the critical effect for setting a TDI. Using this endpoint a BMDL10 of 3.33 T2/kg bw per day was calculated and by using a UF of 200 (adding to the standard UF an additional factor of 2 because a subchronic study was used and by noting that the toxic effect reached no plateau at the end of the study), a new group TDI for T2 and HT2 of 0.02 (rounded from 0.017) μg/kg bw was established.
Modified forms of T2 and HT2 arise from phase I and phase II metabolism of T2 and HT2 in fungi, plants and mammals. In order to include the modified forms in a group HBGV, the modified forms occurring in food and/or feed have been considered. Phase I metabolites identified as relevant were: 19‐HO‐T2, neosolaniol (NEO) and 19‐HO‐HT2, T2‐triol and T2‐tetraol. Phase II metabolites identified as relevant were: T2‐3‐glucose (T2‐3‐Glc), T2‐3‐diglucose (T2‐3‐diGlc), T2‐3‐sulfate (T2‐3‐Sulf), T2‐3‐glucuronic acid (T2‐3‐GlcA), 3‐acetyl‐T2 (3‐Ac‐T2), 3‐feruolyl‐T2 (3‐Fer‐T2), HT2‐3‐glusose (HT2‐3‐Glc), HT2‐diglucose (HT2‐diGlc), HT2‐glucuronic acid (HT2‐GlcA) and HT2‐malonylglucose (HT2‐MalGlc).
NEO showed equal emetic potency and was therefore included in a group ARfD with T2 and HT2 with the same molarity‐based relative potency factor (RPF) of 1. In the absence of toxicity data on phase II metabolites of T2, HT2 and NEO, the CONTAM Panel assumed that they are hydrolysed to their aglycones in the intestine and therefore included them in the group ARfD and used the same RPFs of 1 as for their parent compound/phase I metabolites.
Haematotoxicity, the underlying mode of action being protein synthesis inhibition, induction of ribotoxic stress and apoptosis, are the critical effects of T2. Since T2 is rapidly metabolised to HT2, the toxicity of T2 might partly be attributed to HT2. No in vivo studies on haematotoxicity of modified forms of T2 and HT2 have been identified, but the Panel assumed that their phase I metabolites work via a similar mode of action, as some have been shown to cause protein synthesis inhibition. The CONTAM Panel concluded that the phase I metabolites of NEO, T2‐triol and T2‐tetraol should be included in a group TDI with T2 and HT2. Because phase I metabolites show different potencies in inhibition of protein synthesis and other toxic effects, it was decided to assign molarity‐based relative potency factors (RPFs) for their inclusion in any risk assessment. These RPFs are 1 for T2 and HT2 and 19‐HO‐T2, 0.3 for NEO and 19‐HO‐HT2, and 0.1 for T2‐triol and T2‐tetraol. Since it was assumed that their phase II metabolites are hydrolysed to their aglycones after ingestion, they should also be included in a group TDI. Thus, T2‐3‐Glc, T2‐3‐diGlc, T2‐3‐Sulf, T2‐3‐GlcA, 3‐Ac‐T2, 3‐Fer‐T2, HT2‐3‐Glc, HT2‐diGlc, HT2‐GlcA and HT2‐MalGlc were included in the group TDI by applying an RPF of 1. NEO‐Glc was included by using a factor 0.3 and T2‐triol‐Glc and T2‐tetraol‐Glc by applying a factor of 0.1.
1.1. Background and Terms of Reference as provided by the requestor
Following the outcome of EFSA's scientific opinion on T‐2 and HT‐2 toxin in food and feed in 2011,1 the Commission adopted on 27 March 2013 Commission Recommendation 2013/165/EU on the presence of T‐2 and HT‐2 toxin in cereals and cereal products2 to collect more occurrence data on T‐2 and HT‐2 in cereals and cereal products, and more information on the effects of feed and food processing and agronomic factors on the presence of T‐2 and HT‐2 toxin. In view of the consideration of the need of further regulatory measures to prevent and reduce the presence of T‐2 and HT‐2 in feed and food to ensure a high level of animal and human protection, it is appropriate to perform an updated animal and human exposure assessment taking into account the new occurrence data. Furthermore, a scientific opinion on the appropriateness to set a group health based guidance value, including the assessment of a need to set an acute reference dose, for T‐2 and HT‐2 toxin is envisaged (EFSA‐Q‐2015‐02293).
TERMS OF REFERENCE
In accordance with Art. 31 (1) of Regulation (EC) No 178/2002, the Commission asks EFSA for an updated chronic and acute animal and human exposure assessment to T‐2 and HT‐2 toxin taking into account the occurrence data available in the EFSA database.
2. Data and methodologies
2.1. Data
2.1.1. Occurrence data (food and feed)
2.1.1.1. Data collection and validation
Following an European Commission mandate to EFSA, a call for an annual collection of chemical contaminant occurrence data in food and feed, including T‐2 and HT‐2 toxin, was issued in December 2010 with a closing date of 1 October of each year.4 European national authorities and similar bodies, research institutions, academia, food business operators and other stakeholders were invited to submit analytical data on T2 and HT2 in food and feed.
At the time of the closure of the data collection (January 2017), a total of 93,335 analytical results on T2 and HT2 were available in the EFSA database (analysed in 46,354 samples). Analytical results were reported either as individual analytical results for T2, HT2 and as the sum of two.
The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a); occurrence data were managed following the EFSA standard operational procedures (SOPs) on ‘Data collection and validation’ and on ‘Data analysis of food consumption and occurrence data’.
2.1.1.2. Data analysis
In line with the EFSA SOP on ‘Data analysis of food consumption and occurrence data’ to ensure an appropriate quality of the data used in the exposure assessment, the initial data set was evaluated by applying several data cleaning and validation steps. Special attention was paid to different parameters such as ‘Sampling strategy’, ‘Sampling year’, ‘Sampling country’, ‘Analytical methods’, ‘Reporting unit’, ‘Limit of detection’, and the codification of samples under FoodEx classification (EFSA, 2011a) and feed samples according to the catalogue of feed materials described in Commission Regulation 68/20135. The outcome of the data analysis is presented in Section 3.
The left‐censored data (results below limit of detection (LOD) or below limit of quantification (LOQ)) were treated by the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO/IPCS, 2009). The same method is indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b). The guidance suggests that the LB and UB approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). The LB is obtained by assigning a value of zero (minimum possible value) to all samples reported as lower than the LOD (< LOD) or LOQ (< LOQ). The UB is obtained by assigning the numerical value of LOD to values reported as < LOD and LOQ to values reported as < LOQ (maximum possible value), depending on whether LOD or LOQ is reported by the laboratory.
2.1.2. Human consumption data
The EFSA Comprehensive European Food Consumption Database (hereinafter referred as Comprehensive Database) provides a compilation of existing national information on food consumption at the individual levels. It was first established in 2010 (EFSA, 2011b; Huybrechts et al., 2011; Merten et al., 2011). Details on how the Comprehensive Database is used are published in the Guidance of EFSA (2011b). The latest version of the Comprehensive Database, updated in 20156 contains results from a total of 51 different dietary surveys carried out in 23 different Member States covering 94,532 individuals.
Within the dietary studies, subjects are classified in different age classes as follows:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old
Two additional surveys provided information on specific population groups: ‘Pregnant women’ (≥ 15 years to ≤ 45 years old; Latvia) and ‘Lactating women’ (≥ 28 years to ≤ 39 years old; Greece).
Overall, the food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the European Union (EU). Consumption data were collected using single or repeated 24‐ or 48‐h dietary recalls or dietary records covering from three to seven days per subject. Owing to the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
Detailed information on the different dietary surveys used in this report is shown in Appendix A, including the number of subjects and days available for each age class.
2.1.3. Animal consumption data
The feeds consumed (and the feed intake) by the most relevant farm livestock and companion animals can only be based on estimates, since no comprehensive feed consumption database exists covering the EU. The animal species and categories considered were: (i) ruminants (dairy cows (producing approximately 40 kg milk/day) for which non‐forage feeds accounted for 60% of the diet (on a dry matter basis) or different forage based diets, beef cattle (reared on silage and non‐forage feed or cereal based diets), lactating sheep, milking and fattening goats; (ii) pigs (piglets, fattening pigs and lactating sows); (iii) poultry (broilers, laying hens, turkeys for fattening and ducks for fattening); (iv) rabbits; (v) farmed fish; (vi) companion animals (dogs, cats) and (vii) horses.
The average feed intakes used to calculate animals’ exposure to T2 and HT2 are described in Appendix B. They were derived from information extensively described by the CONTAM Panel in its Scientific Opinion on the risks for animal and public health related to the presence of T2 and HT2 toxin in food and feed (EFSA CONTAM Panel, 2011). The estimated feed intakes are based on published guidelines on nutrition and feeding (AFRC, 1993; Carabano and Piquer, 1998; NRC, 2006, 2007a,b; Leeson and Summers, 2008; EFSA, 2009).
2.1.4. Food classification
Consumption data were classified according to the FoodEx classification system (EFSA, 2011a). FoodEx is a food classification system developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances. It contains 20 main food categories (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 end‐points (food names or generic food names) at the fourth level.
2.1.5. Feed classification
Feed samples were classified according to the Catalogue of feed materials as described in Commission Regulation No 68/20137 and recorded according to the FoodEx classification system.
2.2. Methodologies
2.2.1. Human dietary exposure assessment
In the EFSA 2011 CONTAM opinion (EFSA CONTAM Panel, 2011), only chronic dietary exposure to the individual T2 and HT2 and to the sum of T2 and HT2 was assessed. In the present scientific report, in addition to the chronic also acute dietary exposure to the individual T2 and HT2 and to the sum of T2 and HT2 is assessed. In Appendix A, the number of available days for each age class used in the acute exposure assessment is described, together with the number of subjects available for the chronic exposure assessment.
As suggested by the EFSA Working Group on Food Consumption and Exposure, dietary surveys with only 1 day per subject were considered only for acute exposure as they are not adequate to assess repeated exposure (EFSA, 2011b). Similarly, subjects who participated for only 1 day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded for the chronic exposure assessment. Thus, for chronic exposure assessment, food consumption data were used from 35 different – and the most recent – dietary surveys carried out in 19 different European countries present in the latest version of the Comprehensive Database. Not all countries provided consumption information for all age classes, and in some cases, the same country provided more than one consumption survey.
For calculating chronic dietary exposure to the individual T2 and HT2 and to the sum of T2 and HT2, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Occurrence data and consumption data were linked at the lowest (most detailed) FoodEx level possible. In addition, the different food commodities were grouped within each food category to better explain their contribution to the total dietary exposure. The mean and the high (95th percentile) chronic dietary exposures were calculated by combining the LB and UB mean occurrence values of the individual T2 and HT2 and the sum of T2 and HT2 for food samples collected in different countries (pooled European occurrence data) with the average daily consumption for each food at individual level in each dietary survey and age class. Consequently, individual average exposures per day and body weight were obtained for all individuals. On the basis of distributions of individual exposures, the mean and 95th percentile exposures were calculated per survey and per age class.
Acute dietary exposure to the individual T2 and HT2 and the sum of T2 and HT2 was estimated using a probabilistic approach. A total of 41 most recent dietary surveys carried out in 23 different European countries were used (Appendix A). Acute exposure was assessed for each reporting day by multiplying the total consumption amount for each food category by one occurrence level randomly drawn among the individual results available for that food category. Respective intakes of the foods consumed that day were then summed and finally divided by the individual's body weight. This process was iterated 1,000 times for each reporting day. For the calculations, occurrence data estimated using the UB approach were used. For each of these endpoints, the 95% confidence interval was defined as the 2.5th and 97.5th percentiles obtained from the 1,000 iterations.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 5.1), including the modelling of the probabilistic acute exposure.
2.2.2. Animal dietary exposure assessment
Estimated example diets for each animal species and category were used to calculate the exposure to the sum of T2 and HT2. The diets, already presented and extensively described by the CONTAM Panel in the Scientific Opinion on T‐2 and HT‐2 toxin in food and feed (EFSA CONTAM Panel, 2011), are summarised in the Appendix B. Different scenarios were proposed for ruminant animals: high yielding dairy cows are represented by animals with a daily milk production of 40 kg, fed either a diet based on non‐forage feeds (accounting for 60% of the dry matter intake, corresponding to about 12 kg), or different forage‐based diets diet. Regarding beef cattle, two feeding systems were used, one based on cereals and one on forages. For non‐ruminant livestock and companion animals, the assumed inclusion rates of different feed materials are given in Appendix B.
3. Assessment
3.1. Occurrence data on T‐2 and HT‐2 toxin
An initial number of 93,335 analytical results on T2 and HT2 (46,354 samples analysed) from 25 European countries were available for the assessment. The major contributor of the data was Germany which reported 35% of data, followed by the UK and France. Results were reported on samples collected between the years 2001 and 2016 with the majority of the data collected after 2006. The data covered food and feed, but also included unprocessed grains of undefined end‐use (hereinafter referred as ‘Unprocessed grains’).
Analytical results were reported either as the individual results for T2 (n = 41,922), HT2 (n = 40,236) and as the sum of two (n = 11,177).
The occurrence data were carefully evaluated and a list of validation steps was applied before being used to estimate dietary exposure.
As a first step, the data set was checked for duplicates (analytical results transmitted twice) and all duplicates (n = 107) were excluded.
Outdated results may not reflect the current levels of contamination. In order to select the most recent data, only the data sampled since the beginning of 2011 were retained for the further analysis. Consequently, 45,595 analytical results collected between 2001 and 2010 were excluded. The loss of these data did not compromise the availability of (quantified) data for the food categories mainly contributing to the dietary exposure to T2 and HT2 (i.e. grain‐based food/feed products). Particular attention was paid to data reported as suspect samples. Suspect samples are the samples taken repeatedly from the same site as a consequence of evidence or suspicion of contamination, and are often taken as a follow‐up of demonstrated non‐compliance with legislation. As they may lead to an overestimation of the contamination levels, results reported as ‘Suspect sampling’ (n = 7,216) were excluded from further analysis.
The LODs/LOQs of the T2 and HT2 data reported to EFSA varied between laboratories, between food matrices and substances, with higher LODs/LOQs for grain‐based food/feed products as compared to other food/feed categories. Considering the large amount of left‐censored data present in the dataset (91% for food, 90% for feed and 95% for unprocessed grains), the presence of relatively high LODs/LOQs may have a significant influence on the UB scenario. In order to reduce this impact, but without compromising the number of data available on food/feed categories contributing to the exposure to T2 and HT2, a careful evaluation of LOQs was performed. To identify the most appropriate LOQ cut‐off values, the distributions of quantified values (values above LOQ) as well as the reported LOQs were evaluated. A percentile (50th or 75th) derived from the quantified values was selected as a cut‐off value and subsequently applied to the LOQs reported. For food, a value of 10 μg/kg was selected as LOQ cut‐off for the individual T2 and HT2 data and a value of 20 μg/kg as LOQ cut‐off for the sum of T2 and HT2 data. For feed, a value of 50 μg/kg was selected as LOQ cut‐off for the individual T2 and HT2 data and a value of 100 μg/kg as LOQ cut‐off for the sum of T2 and HT2 data. In total, 12,298 analytical results on food (398 quantified, ~ 3%) and 552 data on feed (7 quantified, ~ 1%) were excluded following this criterion. Appendix C shows the effect of these cut‐offs on the occurrence values for the main selected food categories.
In addition, a total of 501 analytical results for which neither LOQ nor LOD were reported, were also excluded from the final data set.
A total of 66,269 analytical results were excluded from the final data set as described in Appendix D.
The majority of the remaining T2 and HT2 data (96%) were obtained for samples collected within official EU or national monitoring programmes, while the remaining samples were collected within other programmes types (e.g. surveys). Some of the analytical results (3%) were obtained from pooled samples, meaning that the result represented an average of a number of samples taken in equal parts from different consignments/batches and pooled together for the laboratory analysis. Since the level of aggregation for pooled samples matched the level of classification of the individual samples (only similar food matrices were pooled together), results from pooled samples were retained for further evaluation. To ensure a proportionate representation of the individual samples and thus an accurate use of occurrence data in assessing the dietary exposure, the mean concentrations per food category were calculated by weighting the reported analytical results for the number of samples pooled.
Based on the commodity classification provided, separate data sets were extracted for each of the three categories: food, feed and unprocessed grains. The final data set available for dietary exposure assessment included 19,505 analytical results on food (corresponding to a total of 9,035 samples), 6,411 analytical data on feed (corresponding to a total of 2,972 samples) and 1,150 analytical data on unprocessed grains (corresponding to a total of 562 samples). As explained later in Section 3.1.2, the data on unprocessed grains were treated separately and were not considered for the exposure assessment.
3.1.1. Occurrence data on food
The analytical results included in the final data set (n = 19,505 analytical results analysed in 9,035 samples) and considered for the dietary exposure of the present scientific report were collected in 20 different European countries, most of them in Germany (n = 9,712), Austria (n = 2,786) and the UK (n = 2,758) (Figure 2). It should be noted that the origin of the data was not always the European country reporting the data, i.e. the data set also contained samples originating from North and South America, Africa, Asia and Australia. The samples were collected between 2011 and 2016 (Figure 3).
Figure 2.
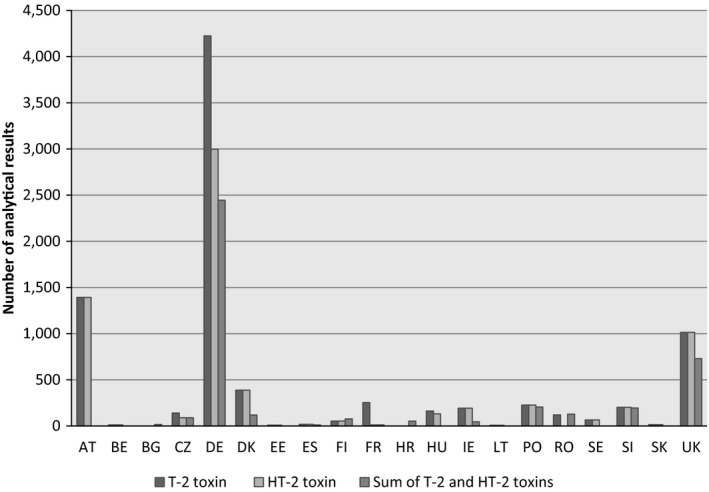
- AT, Austria; BE, Belgium; BG, Bulgaria; CZ, the Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; ES, Spain; FI, Finland; FR, France; HR, Croatia; HU, Hungary; IE, Ireland; LT, Lithuania; PO, Poland; RO, Romania; SE, Sweden; SI, Slovenia; SK, Slovakia; UK, the United Kingdom.
Figure 3.
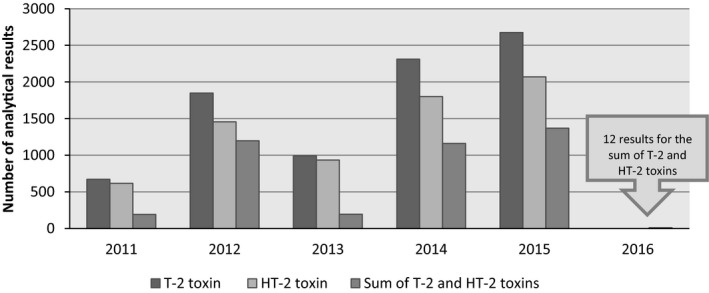
Distribution of the food analytical results for the individual T2, HT2 and the sum of T2 and HT2 over the sampling years (after excluding non‐qualifying data)
Table 1 summarises the number of analytical results and the percentage of left‐censored data per toxin and food category at FoodEx Level 1.
Table 1.
Distribution of analytical results per toxin and food category
| FoodEx Level 1 food category | T‐2 toxin | HT‐2 toxin | Sum of T‐2 and HT‐2 toxins (as reported) | |||
|---|---|---|---|---|---|---|
| N | %LC | N | %LC | N | %LC | |
| Grains and grain‐based products | 7,126 | 90 | 5,711 | 85 | 3,488 | 86 |
| Vegetables and vegetable products | 42 | 100 | 4 | 100 | 39 | 100 |
| Starchy roots and tubers | 5 | 100 | – | – | 5 | 100 |
| Legumes, nuts and oilseeds | 109 | 95 | 31 | 100 | 104 | 95 |
| Fruit and fruit products | 32 | 100 | – | – | 33 | 100 |
| Meat and meat products | 4 | 25 | 4 | 0 | 4 | 0 |
| Sugar and confectionary | 10 | 100 | – | – | 10 | 100 |
| Animal and vegetable fats and oils | 45 | 73 | 45 | 100 | 44 | 73 |
| Non‐alcoholic beverages | 2 | 100 | 2 | 100 | – | – |
| Alcoholic beverages | 604 | 100 | 604 | 99 | 101 | 100 |
| Herbs, spices and condiments | 13 | 100 | – | – | 14 | 93 |
| Food for infants and small children | 448 | 97 | 437 | 96 | 254 | 95 |
| Products for special nutritional use | 25 | 72 | 10 | 30 | 18 | 67 |
| Composite food | 3 | 100 | 2 | 100 | – | – |
| Snacks, desserts, and other foods | 34 | 71 | 27 | 70 | 12 | 100 |
| Total | 8,502 | 90 | 6,877 | 87 | 4,126 | 87 |
N: number of analytical results; LC: left‐censored data.
Most of the analytical results were available for the individual T2 (n = 8,502) and HT2 (n = 6,877), while data reported as a sum of T2 and HT2 were less represented (n = 4,126). A high proportion of left‐censored data was observed for all three substances (90% for the T2, 87% for the HT2 and the sum of T2 and HT2). No data on potential modified forms of T2 and HT2 were available in the EFSA database.
The most frequently analysed food category was ‘Grains and grain‐based products’ with 7,126 results reported for the T2, 5,711 results reported for HT2 and 3,488 results reported for the sum of the T2 and HT2. Other food categories were much less covered and comprised only limited number of quantified values.
As described above (Section 3.1), some of the results obtained by analytical methods with high LOD/LOQ or with the information on LOD/LOQ missing were excluded from the final data set. Information on the analytical methods used to analyse the toxins was only provided for 66% of the samples. In those where information was submitted, the separation method used was mostly liquid chromatography with diverse detection methods (Figure 4).
Figure 4.
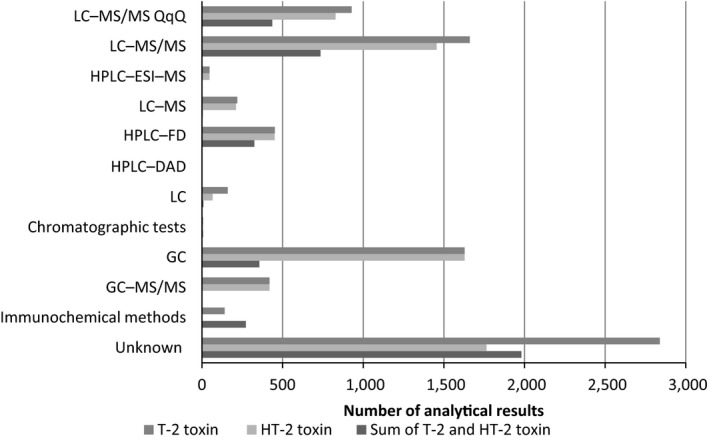
- LC: liquid chromatography; MS/MS: tandem mass spectrometry; QqR: triple quadrupole; HPLC: high‐performance liquid chromatography; ESI: electrospray (ionisation); FD: fluorescence detection; DAD: diode array detection; GC: gas chromatography.
The predominant detection method was tandem mass spectrometry (MS/MS), either reported without information (LC–MS/MS) or with information on the analyser used (triple‐quadrupole (QqQ)) and reported as LC–MS/MS (QqQ). Part of the data set was analysed by the high‐performance LC electrospray (ionisation) mass spectrometry (HPLC‐ESI‐MS). The use of single quadrupole mass spectrometry coupled to LC (LC–MS) was also reported. Other than mass spectrometry, the detection method of choice was fluorescence detection (FD) or diode array detection (DAD) while for other samples only information on the separation method was provided (LC) without further details on the detection technique. For a limited number of analytical data, only the description ‘Chromatographic tests’ was indicated as an analytical method used. Some of the data were reported as obtained using gas chromatography (GC)‐based methods, and when information on the detection method used was available, it was gas chromatography coupled to tandem mass spectrometry or GC‐MS/MS. The remaining set of data was analysed by immunochemical tests, with most of them using enzyme‐linked immunosorbent assays (ELISA).
The distribution of analytical results across the analytical methods used for the analysis of T2, HT2 and the sum of T2 and HT2 in food samples is illustrated in Figure 4.
The distribution of the LOQs for the individual T2, HT2 and the sum of T2 and HT2 and across food categories relevant for the dietary exposure is summarised in Table 2. No particular variability of LOQs was observed within the individual T2 and HT2 across food categories with the median LOQs being around 5 μg/kg. The highest median LOQ for the sum of T2 and HT2, was reported for ‘Snack food’ (15.5 μg/kg) and the lowest median LOQ was reported for ‘Beer and beer‐like beverage’ (3.0 μg/kg). A low variability of the LOQs is likely due to the fact that food commodities prevalently belong to cereal‐based food products even if classified according to food categories different than ‘Grains and grain‐ based products’ (e.g. snack food). Similar low variability of the LOQs was observed when checked across the analytical method used to analyse the toxins.
Table 2.
Distribution of the reported LOQs in the analysis of the individual T2, HT2 and the sum of T2 and HT2 in selected food commodities after applying the LOQ cut‐offs
| N | Percentiles (μg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Min | P5 | P25 | P50 | P75 | P95 | Max | ||
| T‐2 toxin | ||||||||
| Grains and grain‐based products, unspecified | 24 | 4.0 | 4.0 | 5.0 | 5.0 | 10.0 | 10.0 | 10.0 |
| Grains for human consumption | 3,025 | 0.01 | 0.7 | 5.0 | 5.0 | 5.0 | 10.0 | 10.0 |
| Grain milling products | 1,888 | 0.01 | 0.4 | 4.5 | 5.0 | 5.0 | 10.0 | 10.0 |
| Bread and rolls | 451 | 0.4 | 4.5 | 5.0 | 5.0 | 9.0 | 10.0 | 10.0 |
| Pasta (Raw) | 312 | 0.4 | 0.4 | 4.5 | 4.5 | 5.0 | 10.0 | 10.0 |
| Breakfast cereals | 1,161 | 0.2 | 0.4 | 2.0 | 5.0 | 5.0 | 10.0 | 10.0 |
| Fine bakery wares | 265 | 0.4 | 0.4 | 1.0 | 5.0 | 5.0 | 10.0 | 10.0 |
| Oilseeds | 76 | 0.4 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 10.0 |
| Beer and beer‐like beverage | 604 | 2.5 | 3.0 | 3.3 | 3.3 | 3.3 | 10.0 | 10.0 |
| Food for infants and small children, unspecified | 11 | 4.5 | 4.5 | 4.5 | 5.0 | 5.0 | 5.0 | 5.0 |
| Cereal‐based food for infants and young children | 382 | 0.03 | 0.7 | 5.0 | 5.0 | 5.0 | 6.0 | 10.0 |
| Dietary supplements | 15 | 2.0 | 2.0 | 5.0 | 5.0 | 10.0 | 10.0 | 10.0 |
| Snack food | 33 | 0.4 | 0.4 | 5.0 | 6.0 | 8.0 | 10.0 | 10.0 |
| HT‐2 toxin | ||||||||
| Grains and grain‐based products, unspecified | 22 | 5.0 | 5.0 | 5.0 | 6.0 | 10.0 | 10.0 | 10.0 |
| Grains for human consumption | 2,511 | 1.0 | 2.0 | 5.0 | 5.0 | 8.0 | 10.0 | 10.0 |
| Grain milling products | 1,507 | 0.01 | 2.0 | 4.5 | 5.0 | 5.0 | 10.0 | 10.0 |
| Bread and rolls | 293 | 1.0 | 4.5 | 4.5 | 6.7 | 10.0 | 10.0 | 10.0 |
| Pasta (Raw) | 256 | 4.5 | 4.5 | 4.5 | 4.5 | 10.0 | 10.0 | 10.0 |
| Breakfast cereals | 933 | 0.2 | 0.4 | 2.0 | 5.0 | 5.0 | 10.0 | 10.0 |
| Fine bakery wares | 189 | 1.0 | 1.0 | 1.1 | 5.0 | 10.0 | 10.0 | 10.0 |
| Beer and beer‐like beverage | 604 | 2.5 | 3.3 | 3.3 | 3.3 | 6.4 | 10.0 | 10.0 |
| Food for infants and small children, unspecified | 11 | 4.5 | 4.5 | 4.5 | 5.0 | 5.0 | 5.0 | 5.0 |
| Cereal‐based food for infants and young children | 372 | 0.09 | 1.1 | 5.0 | 5.0 | 5.0 | 8.0 | 10.0 |
| Dietary supplements | 7 | 2.0 | 2.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Snack food | 26 | 2.0 | 2.0 | 6.0 | 8.0 | 10.0 | 10.0 | 10.0 |
| Sum of T‐2 and HT‐2 toxins (as reported) | ||||||||
| Grains for human consumption | 1,476 | 0.7 | 4.0 | 5.0 | 10.0 | 10.0 | 20.0 | 20.0 |
| Grain milling products | 968 | 1.1 | 4.0 | 5.0 | 5.0 | 10.0 | 20.0 | 20.0 |
| Bread and rolls | 172 | 2.0 | 5.0 | 5.0 | 10.0 | 20.0 | 20.0 | 20.0 |
| Pasta (Raw) | 132 | 5.0 | 5.0 | 5.0 | 10.0 | 17.5 | 20.0 | 20.0 |
| Breakfast cereals | 542 | 0.4 | 0.4 | 2.0 | 5.0 | 10.0 | 20.0 | 20.0 |
| Fine bakery wares | 196 | 1.1 | 1.1 | 2.0 | 10.0 | 15.0 | 20.0 | 20.0 |
| Oilseeds | 73 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 20.0 | 20.0 |
| Beer and beer‐like beverage | 101 | 3.0 | 3.0 | 3.0 | 3.0 | 10.0 | 10.0 | 10.0 |
| Food for infants and small children, unspecified | 8 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Cereal‐based food for infants and young children | 239 | 0.1 | 1.1 | 5.0 | 5.0 | 5.0 | 10.0 | 20.0 |
| Dietary supplements | 12 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Snack food | 12 | 10.0 | 10.0 | 15.0 | 15.5 | 16.0 | 20.0 | 20.0 |
N: number of analytical results.
Although the majority of the reported LOQs are in line with those described in recent literature (Zachariasova et al., 2010; Rubert et al., 2012), many analytical methods still reported relatively high LOQs. This has a significant impact on the UB estimations when dealing with left‐censored data, even though this influence on the UB estimations was reduced by excluding those samples that reported LOQs above 10 μg/kg for the individual T2 and HT2 and above 20 μg/kg for the sum of T2 and HT2 (Section 3.1).
3.1.1.1. Occurrence data on the individual T2 and HT2
The data reported for the food category ‘Grains and grain‐based products’ (n = 7,126 data points for T2 and n = 5,711 data points for HT2) covered, at the FoodEx Level 2, seven food categories, namely ‘Grains and grain‐based products’ (unspecified), ‘Grains for human consumption’, ‘Breakfast cereals’, ‘Grain milling products’, ‘Fine bakery wares’, ‘Pasta (raw)’ and ‘Bread and rolls’. It is important to note that the majority of samples consisted of raw agricultural commodities, in particular grains and minimally processed commodities (i.e. grain milling products). The effects of the processing, in particular de‐hulling of grains may lead to a marked overall reduction in levels of T2 and HT2. Reductions of up to 98% in the Fusarium toxins, including T2 and HT2 concentration in the final products such as oat flakes, have been reported when compared with the original grains (Scudamore et al., 2007; Schwake‐Anduschus et al., 2010). For the majority of data reported as ‘Grains for human consumption’ information on processing was not available, and therefore no factors to convert from raw agricultural commodities into processed commodities were applied. The 2011 EFSA scientific opinion mentioned that T2 and HT2 are relatively stable during cooking and baking. Although some degradation has been reported, the results were variable and inconclusive (EFSA CONTAM Panel, 2011), and therefore, also the T2 and HT2 concentrations on ‘Grain milling products’ were not converted. This approach might have resulted in an overestimation of the T2 and HT2 exposure from ‘Grains for human consumption’, and ‘Grain milling products’.
The highest mean concentration of the individual T2 and HT2 was observed in ‘Grains for human consumption’ (LB mean = 3.37 μg/kg; UB = 7.51 μg/kg for T2, and LB mean = 8.10 μg/kg; UB = 12.5 μg/kg for HT2). The highest mean concentrations were measured in oats (LB mean = 40.2 μg/kg; UB = 41.7 μg/kg for T2, and LB mean = 91.4 μg/kg; UB = 93.0 μg/kg for HT2). These observations are in line with several recent surveys of cereals and cereal‐based food across Europe that have shown that T2 and HT2 are detected most frequently and at the highest concentrations in oats and oat products (Meng‐Reiterer et al., 2016; Van Der Fels‐Klerx et al., 2012). Very low concentrations were observed for rye, rice, spelt and millet grains. Within the food category ‘Grain milling products’, corn milling products showed the highest mean T2 concentrations (LB mean = 6.93 μg/kg; UB = 9.92 μg/kg), while oat milling products showed the highest mean HT2 concentrations (LB mean = 16.6 μg/kg; UB = 17.9 μg/kg). The latter also was characterised by a high level of contamination with HT2, with 61% of the samples having quantified values. For the grain‐based products including only processed food products, the highest T2 and HT2 contamination frequency was observed for ‘Breakfast cereals’, with 27% and 40% of quantified values, respectively (LB mean = 2.07 μg/kg; UB = 4.47 μg/kg for T2, and LB mean = 5.34 μg/kg; UB = 7.76 μg/kg for HT2). In the food category ‘Bread and rolls’, T2 and HT2 were found in less than 1% of the samples and the mean concentration levels were rather low.
The food category ‘Food for infants and small children’ contained predominantly cereal‐based baby food (n = 448 data points for T2 and n = 437 data points for HT2). The proportion of quantified results was rather low (3% for T2 and 4% for HT2) and the concentration levels ranged from LB mean of 0.11 μg/kg measured for T2 to UB mean of 3.70 μg/kg measured for HT2. In the food categories other than cereal‐based baby food (e.g. fruit juice, follow‐on formulae), T2 and HT2 were not detected or quantified in any of the samples.
The food category ‘Snacks, desserts, and other foods’ comprised only a limited number of results (n = 34 data points for T2 and n = 27 data points for HT2) and was mostly represented by ‘Snack food’, while quantified values were only reported for corn‐based snack food.
Unexpectedly, high T2 and HT2 concentrations were found in a limited number of data reported for ‘Products for special nutritional use’, and in particular in ‘Dietary supplements’ (n = 15 for T2 and n = 7 for HT2; LB mean = 220 μg/kg; UB = 223 μg/kg for T2; and LB mean = 372 μg/kg; UB = 373 μg/kg for HT2). All quantified results were in herbs‐ or plants‐based dietary supplements, classified as ‘Plant extract formula’ according to the FoodEx 1 classification system. As a result, the mean concentration for the food category ‘Dietary supplements’ was strongly influenced by the results obtained on the plant extract formula samples. Most of these products were dietary supplements based on milk thistle believed to have beneficial effects in case of kidney disorders. It is important to note that these data were reported by only one country (the Czech Republic). Veprikova et al. (2015) reported similarly high concentrations of T2 and HT2 in dietary supplements based on various herbs or plants collected from the Czech and US retail markets, and it is possible that a part of their data might have also been submitted to EFSA.
In the following food categories, only a limited number of quantified results were reported: ‘Legumes, nuts and oilseeds’, four sunflower and one chestnut sample (up to 37.3 μg/kg measured for T2), ‘Meat and meat based products’, seven blood sausage samples (up to 8.88 μg/kg measured for T2), ‘Animal and vegetable fats and oils’, nine sunflower oil and three olive oil samples (up to 5.07 μg/kg measured for T2) and ‘Alcoholic beverages’, nine beer samples (up to 20.2 μg/kg measured for T2).
For the remaining food categories, ‘Vegetables and vegetable products (including fungi)’, ‘Starchy roots and tubers’, ‘Fruit and fruit products’, ‘Sugar and confectionary’, Non‐alcoholic beverages’, ‘Herbs, spices and condiments’, ‘Composite food’, T2 or HT2 was not detected or quantified in any of samples.
An overview of the number of data points available for evaluation, the percentage of results below LOD/LOQ and the mean and 95th percentile concentrations of the individual T2 and HT2, are presented in Appendix E, Table E.1.
Before the occurrence data were used to estimate dietary exposure, the data were grouped at different FoodEx levels according to their T2 and HT2 levels and the number of analytical results reported (Appendix E, Table E.2).
3.1.1.2. Occurrence data on the sum of the T2 and HT2
Occurrence data for the sum of T2 and HT2 concentrations were obtained by summing the available individual concentrations of the T2 and HT2 for each sample and subsequently combining them with data reported as sum of the T2 and HT2. In order to avoid the creation of duplicates, this was not done for records already summed by the data providers and reported as both the individual toxins and the sum (n = 4,056). The records for which the reported sum of T2 and HT2 was lower than the effective sum of the individual T2 and HT2 reported for the same sample (n = 70) were not considered reliable, and in those cases the concentrations based on the effective sum of the two individual toxins were used for exposure assessment.
For the UB scenario, the left‐censored data (data below LOD/LOQ) were before the summation specifically handled. It was assumed that the summation of LOD/LOQ values at UB level would lead to overestimation, the middle‐bound (MB) of LOD/LOQ values (obtained by assigning the half of LOD to values reported as < LOD and half of LOQ to values reported as < LOQ) was used. By using this approach, the actual concentrations may have been under‐ or overestimated, and this uncertainty has to be borne in mind when interpreting the exposure assessment to the sum of T2 and HT2.
It should be noted that both T2 and HT2 were only analysed on 74% of food samples and 77% of feed samples, while for the remaining samples either the T2 or HT2 concentration value was missing. In those cases, an imputation method using the mean occurrence of the food group under consideration was used to simulate the missing results. Data below LOD/LOQ were not considered as missing, and therefore were not imputed.
As an outcome of this approach, a total of 9,551 food analytical results were obtained for the sum of T2 and HT2. In comparison, 4,126 analytical data reported as the sum of T2 and HT2 were initially available. The following description of the occurrence data refer to the final dataset as available for exposure assessment.
The majority of the data were in the food category ‘Grains and grain‐based products’ (n = 8,075), followed by ‘Alcoholic beverages’ (n = 604) and ‘Food for infants and small children’ (n = 459). The dataset was characterised by a high proportion of left‐censored data.
Within the ‘Grains and grain‐based products’ all seven food categories at the FoodEx Level 2, namely ‘Grains and grain‐based products’ (unspecified), ‘Grains for human consumption’, ‘Breakfast cereals’, ‘Grain milling products’, ‘Fine bakery wares’, ‘Pasta (raw)’ and ‘Bread and rolls’ were covered. The highest mean concentration levels were measured for ‘Grains for human consumption’ (n = 3,362; LB mean=11.4 μg/kg; UB=15.7 μg/kg) and ‘Breakfast cereals’ (n = 1,270; LB mean = 7.11 μg/kg; UB = 10.1 μg/kg). Among the different food categories classified with additional details at FoodEx Level 3, the highest mean concentrations of the sum of T2 and HT2 were in oat grains (LB mean = 127 μg/kg; UB = 128 μg/kg). Within the ‘Breakfast cereals’, the food category ‘Cereal flakes’, and in particular its subcategories related to oat flakes, had highest mean concentrations of the sum of T2 and HT2 (LB mean = 13.9 μg/kg; UB = 16.5 μg/kg). The food category ‘Breakfast cereals’ was also the category with the highest contamination of the sum of T2 and HT2 accounted (29% of quantified results).
Other FoodEx level 1 food categories containing predominantly results on grain‐based food products were ‘Food for infants and small children’ (n = 459) and ‘Snacks, desserts, and other foods’ (n = 35) with contamination frequency observed at 4% and 26%, respectively. The mean concentration levels of the ‘Food for infants and small children’ ranged from LB mean of 0.84 μg/kg to UB mean of 3.95 μg/kg, and quantified results were only reported for cereal‐based baby foods. The mean concentration levels of the ‘Snacks, desserts, and other foods’ ranged from LB mean of 8.75 μg/kg to UB mean of 13.5 μg/kg, and quantified results were reported only for corn‐based snack food.
As described above (Section 3.1.1.1), within the food category ‘Products for special nutritional use’ very high concentrations of T2 and HT2 were reported for ‘Dietary supplements’, and in particular on herbs‐ or plants‐based dietary supplements. Consequently, the mean concentrations of the sum of T2 and HT2 for the food category ‘Dietary supplements’ resulted in very high concentrations (n = 15; LB mean = 592 μg/kg; UB = 594 μg/kg). It is important to mention that those very high levels were found in very specific products (i.e. plant extract formula samples) and were reported by one country (the Czech Republic).
In the following food categories, only a limited number of quantified results was available for ‘Legumes, nuts and oilseeds’ (up to 119 μg/kg), ‘Meat and meat based products’ (up to 16.0 μg/kg), ‘Animal and vegetable fats and oils’ (up to 5.64 μg/kg) and ‘Alcoholic beverages’ (up to 27.6 μg/kg).
For the remaining food categories, ‘Vegetables and vegetable products (including fungi)’, ‘Starchy roots and tubers’, ‘Fruit and fruit products’, ‘Sugar and confectionary’, Non‐alcoholic beverages’, ‘Herbs, spices and condiments’, ‘Composite food’, no quantified results of the sum of T2 and HT2 were available for exposure assessment.
An overview of the number of data points available for evaluation, the percentage of results below LOD/LOQ and the mean and 95th percentile concentrations of the sum of T2 and HT2, are presented in Appendix E, Table E.3.
Before the occurrence data were used to estimate dietary exposure, the data were grouped at different FoodEx levels according to their levels for the sum of T2 and HT2 and the number of analytical results reported (Appendix E, Table E.4).
3.1.2. Occurrence data on unprocessed grains
Data on unprocessed grains (codified as ‘Grain as crops’ according to FoodEx classification) cannot be considered either for food or feed, and the processing might influence the concentration of the toxins in the end‐product. The T2 and HT2 concentration levels of ‘Grain as crops’ did not show a robust consistency when compared to those reported as ‘Grains for human consumption’ nor when compared to those reported as feed grains. Therefore, data on unprocessed grains were not used in the present human or animal exposure assessment and thus no exclusion criteria based on LOQ was applied to data of this commodity.
Within this subdataset, 474 data points were on the individual T2, 445 data points were on the individual HT2, and 231 data point were on the sum of T2 and HT2. These data were collected between 2011 and 2016 in 11 European countries. The distribution of the analytical results for unprocessed grains across the European countries and across grain categories is presented in Figures 5 and 6, respectively.
Figure 5.
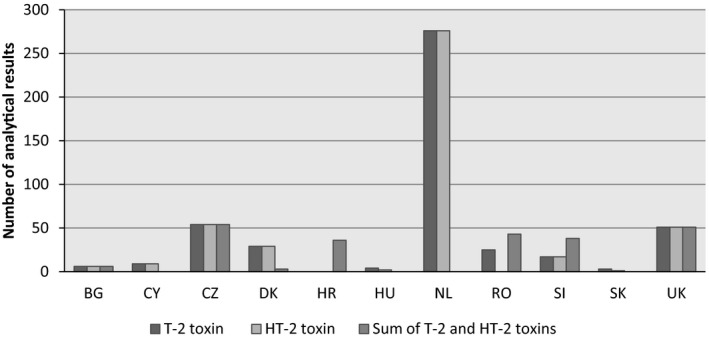
- BG, Bulgaria; CY, Cyprus; CZ, the Czech Republic; DK, Denmark; HR, Croatia; HU, Hungary; NL, the Netherlands; RO, Romania; SI, Slovenia; SK, Slovakia; UK, the United Kingdom.
Figure 6.
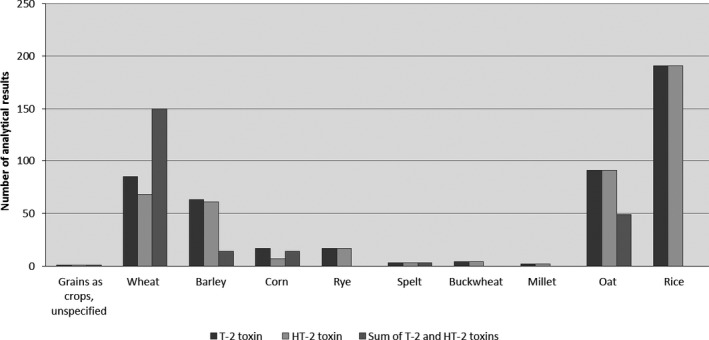
Distribution of the analytical results of unprocessed grains for the individual T2, HT2 and the sum of T2 and HT2 across grain categories (after excluding non‐qualifying data)
Results were reported on whole weight8 (98% of samples) or on an 88% dry matter basis (2% of samples). For consistency, the latter were converted to values expressed on a whole‐weight basis. The conversion was based on the moisture content reported.
The data on unprocessed grains mainly covered ‘Rice (crop)’, ‘Wheat grain crop’ and ‘Oats, grain (crop)’ with the highest mean levels for the individual T2 measured in ‘Corn grain (crop)’ (LB = 8.92 μg/kg, UB = 20.3 μg/kg), followed by oats and barley grains. The highest mean levels of the individual HT2 were measured in ‘Oats, grain (crop)’ (LB = 11.1 μg/kg, UB = 65.4 μg/kg), followed by barley and wheat grains. For the sum of T2 and HT2, the highest mean levels were observed in ‘Corn grain (crop)’ (LB = 40.3 μg/kg, UB = 52.5 μg/kg). No T2 or HT2 toxin was found in spelt, rice, rye, millet and buckwheat grains. An overview of the number of data points, the proportion of left‐censored data as a percentage, the mean, median, 75th percentile (P75) and 95th percentile (P95) concentration values is presented in Table 3.
Table 3.
Summary statistics of the levels of the individual T2, HT2 and the sum of the T2 and HT2 in unprocessed grains
| Commodity | N | %LC | Concentration range (LB – UB) (μg/kg) | |||
|---|---|---|---|---|---|---|
| Mean | Median | P75 | P95a | |||
| T‐2 toxin | ||||||
| Grains as crops, unspecified | 1 | 100 | 0.00–19.0 | 0–19.0 | 0–19.0 | – |
| Wheat grain crop | 85 | 98 | 0.32–19.8 | 0–25.0 | 0–25.0 | 0–25.0 |
| Barley grain (Crop) | 63 | 98 | 0.70–80.8 | 0–100 | 0–100 | 0–100 |
| Corn grain (Crop) | 17 | 76 | 8.92–20.3 | 0–6.40 | 0–24.6 | – |
| Rye grain (Crop) | 17 | 100 | 0.00–91.2 | 0–100 | 0–100 | – |
| Spelt grain (Crop) | 3 | 100 | 0.00–2.00 | 0–2.00 | 0–2.00 | – |
| Buckwheat grain (Crop) | 4 | 100 | 0.00–87.5 | 0–100 | 0–100 | – |
| Millet grain (Crop) | 2 | 100 | 0.00–75.0 | 0–75.0 | 0–100 | – |
| Oats, grain (Crop) | 91 | 87 | 1.52–33.7 | 0–50.0 | 0–50.0 | 10.4–150 |
| Rice (Crop) | 191 | 100 | 0.00–93.2 | 0–100 | 0–100 | 0–100 |
| HT‐2 toxin | ||||||
| Grains as crops, unspecified | 1 | 100 | 0.00–47.5 | 0–47.5 | 0–47.5 | – |
| Wheat grain crop | 68 | 99.5 | 0.13–20.2 | 0–25.0 | 0–25.0 | 0–25.0 |
| Barley grain (Crop) | 61 | 98 | 0.89–95.6 | 0–100 | 0–100 | 0–100 |
| Corn grain (Crop) | 7 | 100 | 0.00–8.29 | 0–7.60 | 0–10.0 | – |
| Rye grain (Crop) | 17 | 100 | 0.00–100 | 0–100 | 0–100 | – |
| Spelt grain (Crop) | 3 | 100 | 0.00–0.00 | 0.00–0.00 | 0.00–0.00 | – |
| Buckwheat grain (Crop) | 4 | 100 | 0.00–100 | 0–100 | 0–100 | – |
| Millet grain (Crop) | 2 | 100 | 0.00–100 | 0–100 | 0–100 | – |
| Oats, grain (Crop) | 91 | 74 | 11.1–65.4 | 0–70.0 | 2.8–100 | 90–250 |
| Rice (Crop) | 191 | 100 | 0.00–100 | 0–100 | 0–100 | 0–100 |
| Sum of T‐2 and HT‐2 toxins | ||||||
| Grains as crops, unspecified | 1 | 100 | 0.00–19.0 | 0–19.0 | 0–19.0 | – |
| Wheat grain crop | 150 | 98 | 1.32–35.8 | 0–50.0 | 0–50.0 | 0–50.0 |
| Barley grain (Crop) | 14 | 100 | 0.00–113 | 0–22.8 | 0–75.0 | – |
| Corn grain (Crop) | 14 | 64 | 40.3–52.5 | 0–25.0 | 35.0–35.0 | – |
| Spelt grain (Crop) | 3 | 100 | 0.00–0.00 | 0.00–0.00 | 0.00–0.00 | – |
| Oats, grain (Crop) | 49 | 90 | 13.4–109 | 0–75.0 | 0–75.0 | – |
N: number of analytical results; LC: left‐censored data; P75: 75th percentile; P95: 95th percentile; LB: lower bound; UB: upper bound.
The 95th percentiles obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and is therefore not reported in the table.
3.1.3. Occurrence data on feed
A total of 6,411 analytical data on feed analysed in 2,972 samples were available in the final data set and considered for the animal exposure. Results were reported on whole weight (42% of samples) or on 88% dry matter (58% of samples). For consistency, the latter were converted to values expressed on a whole‐weight basis. The conversion was based on the moisture content reported.
Data were collected in 14 different European countries, most of them from Bulgaria (n = 2,780), France (n = 1,309) and Hungary (n = 759) (Figure 7). The samples were collected between 2011 and 2016 (Figure 8).
Figure 7.
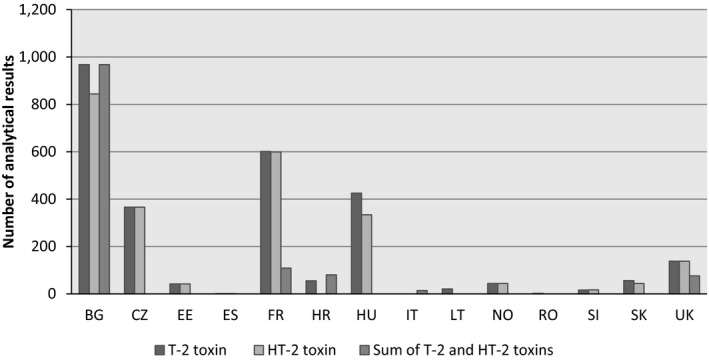
- BG, Bulgaria; CZ, the Czech Republic; EE, Estonia; ES, Spain; FR, France; HR, Croatia; HU, Hungary; IT, Italy; LT, Lithuania; NO, Norway; RO, Romania; SI, Slovenia; SK, Slovakia; UK, the United Kingdom.
Figure 8.
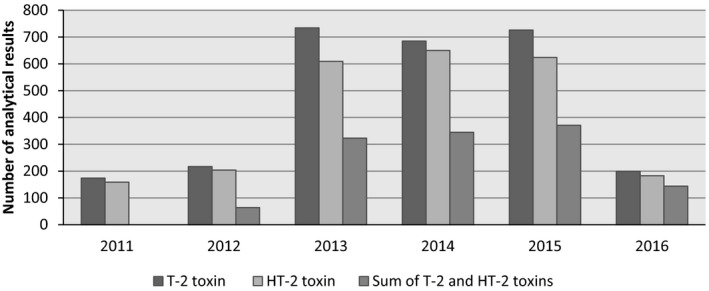
Distribution of the feed analytical results for the individual T2, HT2 and the sum of T2 and HT2 over the sampling years (after excluding non‐qualifying data)
Analytical results of feed were reported for the individual T2 (n = 2,735), HT2 (n = 2,429) and as a sum of T2 and HT2 (n = 1,247). A high proportion of left‐censored data was observed for all three substances (91% for the T2, 88% for the HT2 and the sum of T2 and HT2).
The most frequently analysed feed category at Level 1 was ‘Cereal grains, their products and by‐products’ with 1,381 results reported for the T2, 1,298 results reported for HT2 and 596 results reported for the sum of the T2 and HT2. The feed category ‘Compound feed’ also comprised a substantial number of data (n = 2,386 in total) while other feed categories were much less represented.
Table 4 summarises the number of analytical results and the percentage of left‐censored data per toxin and feed category.
Table 4.
Distribution of analytical results per substance and feed category
| Feed category Level 1 | T‐2 toxin | HT‐2 toxin | Sum of T‐2 and HT‐2 toxins (as reported) | |||
|---|---|---|---|---|---|---|
| N | %LC | N | %LC | N | %LC | |
| Feed terms (unspecified) | 8 | 88 | – | – | 8 | 88 |
| Cereal grains, their products and by‐products | 1,381 | 89 | 1,298 | 85 | 596 | 82 |
| Minerals and products derived thereof | 1 | 100 | 1 | 100 | – | – |
| Fermentation (by‐)products from microorganisms the cells of which have been inactivated or killed | 1 | 100 | 1 | 100 | – | – |
| Miscellaneous | 5 | 100 | 1 | 100 | 1 | 100 |
| Oil seeds, oil fruits, and products derived thereof | 159 | 94 | 36 | 94 | 119 | 98 |
| Legume seeds and products derived thereof | 8 | 100 | 8 | 100 | 1 | 100 |
| Tubers, roots, and products derived thereof | 1 | 100 | 1 | 100 | – | – |
| Other seeds and fruits, and products derived thereof | 2 | 100 | 2 | 100 | – | – |
| Forages and roughage, and products derived thereof | 162 | 90 | 160 | 83 | 58 | 69 |
| Milk products and products derived thereof | 1 | 0 | 1 | 0 | – | – |
| Land animal products and products derived thereof | 2 | 100 | 2 | 100 | – | – |
| Compound feed | 1004 | 94 | 918 | 92 | 464 | 96 |
| Total | 2,735 | 91 | 2,429 | 88 | 1,247 | 88 |
N: number of analytical results; LC: left‐censored data.
The most common analytical method reported for the analysis of T2 and HT2 in feed was LC–MS/MS (69%). As for food, for some samples additional information was provided on the instrumentation used (triple‐quadrupole (QqQ)). Some of data reported the use of LC–MS (22%), while other analytical methods used were HPLC‐FD (1%), LC without detection method reported (0.03%), chromatographic tests (0.1%), GC without detection method reported (4%) and immunochemical methods, mostly ELISA (3%). For approximately, 1% of samples no information on analytical method was provided.
Considering only feed categories with a substantial number of data, the LOQ values varied within the feed category. The highest median LOQs for T2 were observed in ‘Oil seeds, oil fruits, and products derived thereof’ (median LOQ = 20.1 μg/kg), for HT2 in ‘Compound feed’ (median = 15.0 μg/kg) and for the sum of T2 and HT2 in ‘Oil seeds, oil fruits, and products derived thereof’ (median LOQ = 20.1 μg/kg). The highest sensitivity (median LOQ = 3.0 μg/kg) was observed for results analysed by HPLC‐FD. For samples analysed by immunochemical tests, chromatographic tests, GC without detection method reported, LC–MS/MS and LC–MS, a median LOQ was around 10 μg/kg, while for LC–MS/MS QqQ a median LOQ was 20 μg/kg.
Among the Level 1 feed categories, the individual T2 and HT data were most frequently found in ‘Cereal grains, their products and by‐products’ (11% of quantified results for T2 and 15% of quantified results for HT2) and ‘Forages and roughage, and products derived thereof’ (10% of quantified results for T2 and 17% of quantified results for HT2). Quantified data were also reported for ‘Compound feed’, ‘Oil seeds, oil fruits, and products derived thereof’, ‘Milk products and products derived thereof’ (only one result available) and feed samples not further specified (classified as ‘Feed terms’).
Within grains, the highest T2 and HT2 contamination frequency (78% and 80%, respectively) and the highest mean concentrations were measured in oats (n = 76 for both T2 and HT2; LB mean = 124 μg/kg; UB mean = 126 μg/kg for T2, and LB mean = 307 μg/kg; UB mean = 310 μg/kg for HT2). The maximum value of 3,789 μg/kg was reported for HT2. Barley, maize, rye, wheat and triticale were less contaminated (LB mean concentrations in the range of 0.07–6.40 μg/kg), while in rice and spelt no quantified values were found. For ‘Forages and roughage, and products derived thereof’, the highest mean T2 and HT2 concentrations were observed in cereal straw for T2 (n = 43; LB mean = 10.4 μg/kg; UB mean=18.3 μg/kg) and in maize silage for HT2 (n = 52; LB mean =10.6 μg/kg; UB mean=21.6 μg/kg). For ‘Compound feed’ (considering only feed categories with a substantial number of data), complementary feeds for dairy cows was the feed category with the highest T2 and HT2 mean concentration levels reported (n = 49; LB mean = 5.74 μg/kg; UB mean = 11.3 μg/kg for T2, and n = 42; LB mean=7.50 μg/kg; UB mean=19.2 μg/kg for HT2). In all other feed categories, T2 and HT2 were only quantified occasionally.
The proportion of quantified results and mean concentrations for the sum of T2 and HT2 across the feed categories followed generally the same pattern as for the individual T2 and HT2.
As reported for food, in order to obtain more analytical data on the sum of T2 and HT2, the individual T2 and HT2 results were summed within the same sample and merged with those reported as the sum. The imputation method was used in case of missing values either for T2 or HT2. The approach is described in detail in Section 3.1.1.2. A total of 3,007 feed analytical results were obtained for the sum of T2 and HT2 available for the exposure assessment (in comparison to 1,247 analytical data reported as sum of T2 and HT2 and initially available for the present assessment). The vast majority of the data for the sum of T2 and HT2 were on ‘Cereal grains, their products and by‐products’ (n = 1,556). This was also the Level 1 feed category with the highest mean concentration ranging from LB mean of 29.2 μg/kg to UB mean of 44.6 μg/kg. Among grains, the highest mean concentrations were observed for oats (n = 87; LB mean = 401 μg/kg; UB mean = 405 μg/kg) and this feed category was also characterised by high contamination (88% of quantified results). Regarding other feed categories at Level 2, quantified values were available for cereal straw, mixed grains, sunflower seed, grass field dried, maize silage, barley, maize, complementary feed, skimmed milk, rye, complete feed, cotton seed, toasted soya, wheat and triticale. However, the mean concentrations were much lower in comparison to those measured for oats (LB mean concentrations in the range of 0.7 μg/kg measured in triticale to 16.9 μg/kg measured for cereal straw).
An overview of the number of data points available for evaluation, the percentage of results below LOD/LOQ, the mean and 95th percentile concentrations of the individual T2 and HT2 and the sum of T2 and HT2 are presented in Appendix F, Tables F.1 and F.2.
3.2. Human dietary exposure to T‐2 and HT‐2 toxin
Following the Terms of reference (Section 1.1), both chronic and acute dietary exposure to the individual T2, HT2 and to the sum of T2 and HT2 were assessed.
Prior to linking occurrence and consumption data, some adjustments were carried out on both datasets to obtain the most accurate exposure estimates possible. First, consumption data were grouped according to the same food categories as described for the occurrence data (Appendix E, Tables E.2 and E.4). A standard conversion factor of 5 was applied to occurrence data on porridge samples reported as dry instant cereals to be reconstituted with water or milk and as such linked to the consumption amounts of porridge. Other conversion factors were applied to the consumption data when necessary to match the occurrence values in samples of food reported as ready for consumption with their respective dry/powder consumption amounts. Adjustments were also necessary for some of the eating occasions of ‘Cereal‐based food for infants and young children’. Those that were assumed to be dry were multiplied either by a standard factor of 7 or 4 depending on whether they referred to ‘Simple cereals which are or have to be reconstituted with milk or other appropriate nutritious liquids’ or to ‘Cereals with an added high protein food which are or have to be reconstituted with water or other protein‐free liquid’, respectively. It was assumed that low consumption amounts reported for ‘Cereal flakes’ refer to plain cereal flakes (to be prepared with milk) and therefore a standard conversion factor of 7 was applied in order to multiply these eating occasions and link consumption amounts to the occurrence data on cereal flakes already prepared with milk.
It should be noted that few dietary surveys reported all or part of the consumption data disaggregated into minimally processed commodities (e.g. bread disaggregated into flour). Since these consumption data do not specify the originally consumed processed commodity (e.g. bread), it was not possible to apply any yield factor to return to the processed commodities. Likewise, at the level of occurrence data, the lack of conclusive and precise data on the fate of the T2 and HT2 during processing makes it impossible to apply any retention factor for the T2 and HT2. Therefore, the dietary exposure related to the disaggregated consumption events was most likely overestimated assuming that the concentration of T2 and HT2 is not affected by processing.
The food categories represented by either very low number of data (< 6 results) or by all data left‐censored were considered unsuitable and were not used in exposure calculation.
Overall, it should be kept in mind that a high proportion of left‐censored data has a major impact on the exposure estimates, and that the exposure is likely to be underestimated with the LB approach and overestimated with the UB approach.
The contribution (%) of each of the FoodEx Level1 food category to the overall mean exposure of T2 and HT2 was calculated for each age class and dietary survey. Estimations of exposure using the LB approach, which is considered to be less influenced by results below LOD/LOQ, were used to explain the contribution of the different food categories to the overall exposure.
The following chapters dealing with the chronic and acute dietary exposure refer to the exposure to the sum of T2 and HT2, while the exposure estimates to the individual T2 and HT2 are summarised in Appendix G, Tables G.1, G.2, G.3, G.6, G.7 and G.8. For the LB scenario, the exposure estimates of the individual T2 were on average threefold, and for the individual HT2, twofold lower than those estimated for the sum of T2 and HT2.
3.2.1. Chronic dietary exposure to the sum of T2 and HT2
Dietary exposure to the sum of T2 and HT2 was estimated in 35 dietary surveys from 19 different European countries. Table 5 shows summary statistics for the mean and 95th percentile chronic dietary exposure to the sum of T2 and HT2 across different age classes. The detailed mean and 95th percentile dietary exposure estimates calculated for each of the 35 dietary surveys are presented in Appendix G, Table G.4.
Table 5.
Summary statistics of chronic dietary exposure to the sum of T2 and HT2 across European dietary surveys and different age classes
| Age class | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Mean dietary exposure in total population (ng/kg bw per day) | ||||||
| Infants | 4.41 | 19.3 | 14.9 | 31.1 | 18.3 | 62.9 |
| Toddlers | 8.99 | 49.8 | 15.3 | 55.6 | 29.0 | 64.8 |
| Other children | 8.50 | 35.1 | 11.1 | 45.4 | 18.0 | 62.1 |
| Adolescents | 4.37 | 15.4 | 6.59 | 27.9 | 11.3 | 38.9 |
| Adults | 2.54 | 14.3 | 3.62 | 19.8 | 8.82 | 26.4 |
| Elderly | 2.27 | 13.4 | 2.94 | 17.5 | 10.2 | 23.4 |
| Very elderly | 1.82 | 14.5 | 3.05 | 18.4 | 8.23 | 20.7 |
| Pregnant womenb | 5.72 | – | – | – | – | 20.5 |
| Lactating womenb | 4.60 | – | – | – | – | 15.9 |
| 95 th percentile dietary exposure in total population (ng/kg bw per day) | ||||||
| Infantsa | 18.0 | 50.5 | 45.1 | 89.6 | 53.9 | 146 |
| Toddlersa | 23.8 | 95.0 | 43.3 | 107 | 67.3 | 109 |
| Other children | 20.3 | 60.9 | 28.1 | 81.5 | 37.2 | 112 |
| Adolescents | 11.4 | 26.1 | 17.1 | 53.2 | 29.9 | 71.5 |
| Adults | 6.37 | 27.6 | 9.11 | 37.7 | 16.7 | 54.1 |
| Elderly | 5.41 | 26.8 | 7.32 | 33.6 | 20.9 | 41.8 |
| Very elderlya | 4.22 | 29.1 | 7.61 | 32.2 | 20.3 | 41.2 |
| Pregnant womenb | 14.0 | – | – | – | – | 38.5 |
| Lactating womenb | 13.4 | – | – | – | – | 33.3 |
bw: body weight; LB: lower bound; UB: upper bound.
The 95th percentile estimates obtained on dietary surveys/age classes with less than 60 observations may not be statistically robust (EFSA, 2011b) and therefore not included in this table.
Only one dietary survey available.
Overall, the chronic dietary exposure to the sum of T2 and HT2 was estimated to be two‐ to threefold higher in the young population groups (‘Infants’, ‘Toddlers’, and ‘Other children’) than that estimated for the adult population groups (‘Adults’, ‘Elderly’, and ‘Very elderly’). UB estimations were on average fourfold higher than LB estimations.
The highest mean dietary exposure was estimated in toddlers with estimates of 29.0/64.8 ng/kg bw per day (LB/UB). Regarding the 95th percentile exposure, the highest LB exposure estimates were estimated in toddlers with a maximum exposure of 67.3 ng/kg bw per day, while the UB maximum exposure was estimated for infants (146 ng/kg bw per day).
Dietary exposure in specific groups of the population, namely ‘Pregnant women’ and ‘Lactating women’, were within the range of exposure estimates in the adult population.
Differences between the min‐max exposure levels within the same scenario (LB or UB) were observed with estimates up to threefold higher for UB scenario when considering the maximum exposure levels. This indicates different consumption patterns across European countries, although these differences might be partially due to inconsistency in reporting between different dietary surveys (foods disaggregated into minimally processed commodities vs. non‐disaggregated). High maximum exposure levels observed at the 95th percentile exposure estimated in young population groups were due to high consumption of cereal‐based baby food reported by a group of high consumers in one dietary survey of infants, and due to high consumption of fine bakery wares, cereal flakes and snack food reported by a group of high consumers in four dietary surveys of toddlers.
Overall, the food category ‘Grains and grain‐based products’ made the largest contribution to the LB mean chronic dietary exposure to the sum of T2 and HT2 in all age classes. In particular, ‘Cereal flakes’ had the highest contribution to the overall exposure in infants (up to 84%) and toddlers (up to 79%), while ‘Fine bakery wares’ had the highest contribution in the very elderly (up to 68%) and the elderly (up to 53%). Since the concentrations in ‘Fine bakery wares’ were not particularly high (Appendix E, Table E.4), the relevant contribution of this food category is likely to be mainly driven by its relatively high consumption. Other grain‐based food categories making a considerable contribution to the exposure were ‘Oats, grain’, (contributing up to 32% in the very elderly), ‘Porridge’ (contributing up to 63% in the elderly) and ‘Grains for human consumption’ (contributing up to 30% in toddlers). ‘Pasta (raw)’ also resulted to be an important contributor to the LB mean exposure to the sum of T2 and HT2 (up to 27% in toddlers), but usually in dietary surveys from Mediterranean countries, in particular Italy. Given very low LB mean concentrations in ‘Bread and rolls’, the contribution of this food category resulted to be rather low (up to 0.6% in very elderly).
In infants, ‘Cereal‐based food for infants and young children’ also made an important contribution (up to 49%) to the LB mean exposure of the sum of T2 and HT2.
‘Snack food’ was also found to be an important contributor, particularly for adolescents (up to 45%). However, it should be noted that only a limited number of quantified data was available for this food category.
The food category ‘Plant extract formula’ was an important contributor to the overall LB mean exposure to the sum of T2 and HT2 for the elderly and very elderly, contributing up to 46% and 40%, respectively. However, this finding must be interpreted with caution due to a limited number of results on plant extract formula available for the exposure assessment, and because the extremely high concentrations which had a large impact on this result were reported by only one data provider.
‘Sunflower oil’ also played an important role as contributor (up to 26%), but only in countries reporting higher consumption of this food commodity (particularly in Hungary and Romania).
The contribution of other food categories of non‐grain‐based origin was minor (e.g. beer contributed up to 11% in the elderly, olive oil up to 4% in the elderly and sunflower seed up to 8% in adolescents).
Detailed contribution of the different food categories grouped by age classes is shown in Appendix G, Table G.5.
3.2.2. Acute dietary exposure
Acute dietary exposure to the sum of T2 and HT2 was estimated using a probabilistic approach for 41 dietary surveys from 23 different European countries. For the calculations, the UB scenario and the single occurrence data were used. The methodology is explained in detail in Section 2.2.1.
Table 6 summarises the range of average and 95th percentile acute exposures to the sum of T2 and HT2 across different age classes and dietary surveys. Information is also given on the percentage of subjects above the ARfD in all the different iterations (n = 1,000); an ARfD of 300 ng/kg bw per day was established in the 2017 EFSA CONTAM opinion (EFSA CONTAM Panel, 2017). Detailed average and 95th percentile acute exposure estimates for each dietary survey across age classes with their corresponding confidence intervals (2.5th and 97.5th percentiles) are described in Appendix G, Table G.9.
Table 6.
Range of acute exposure assessment (average and 95th percentile)a to the sum of T2 and HT2 across European dietary surveys and percentage of subjects above the acute reference doseb
| Age class | Number dietary surveys | Minimum | Median | Maximum |
|---|---|---|---|---|
| Range of average acute exposure (ng/kg bw per day) | ||||
| Infants | 6 | 15.2 (10.1–29.2) | 27.7 | 54.6 (48.5–69.3) |
| Toddlers | 11 | 47.9 (46.1–50.3) | 55.7 | 64.7 (62.3–69.8) |
| Other children | 20 | 35.1 (33.9–36.7) | 46.3 | 62.1 (59.9–64.7) |
| Adolescents | 20 | 15.3 (14.7–16.1) | 28.1 | 39.5 (38.0–41.2) |
| Adults | 22 | 14.4 (14.1–14.7) | 19.9 | 26.5 (25.6–27.6) |
| Elderly | 16 | 13.4 (13.0–13.9) | 17.8 | 23.5 (21.2–26.5) |
| Very elderly | 14 | 14.5 (13.6–15.8) | 18.7 | 20.7 (19.3–22.4) |
| Pregnant women | 1 | –c | –c | 25.7 (24.7–27.0) |
| Lactating women | 1 | –c | –c | 17.3 (16.1–19.0) |
| Range of 95th percentile acute exposure (ng/kg bw per day) | ||||
| Infants | 5 | 73.0 (65.3–81.1) | –d | 170 (152–193) |
| Toddlers | 10 | 110 (101–121) | 142 | 154 (116–222) |
| Other children | 20 | 84.4 (79.1–89.8) | 116 | 140 (130–151) |
| Adolescents | 20 | 36.2 (33.5–39.4) | 73.2 | 100 (92.1–109) |
| Adults | 22 | 38.0 (36.7–39.3) | 49.8 | 68.4 (63.0–75.0) |
| Elderly | 16 | 34.3 (31.8–37.1) | 43.1 | 55.4 (46.7–66.9) |
| Very elderly | 14 | 37.2 (32.4–42.7) | 43.4 | 55.3 (43.9–69.5) |
| Pregnant women | 1 | –c | –c | 72.0 (66.1–78.7) |
| Lactating women | 1 | –c | –c | 50.2 (43.1–57.6) |
| Number dietary surveys | Minimum | Maximum | |
|---|---|---|---|
| Percentage of subjects above the ARfD b | |||
| Infants | 6 | < 0.01 (0–< 0.01) | 0.07 (0.04–0.10) |
| Toddlers | 11 | < 0.01 (0–0.01) | 0.05 (0.03–0.07) |
| Other children | 20 | < 0.01 (0–< 0.01) | 0.03 (0.01–0.05) |
| Adolescents | 20 | < 0.01 (0–0.01) | 0.01 (< 0.01–0.01) |
| Adults | 22 | < 0.01 (0–< 0.01) | < 0.01 (< 0.01–0.01) |
| Elderly | 16 | < 0.01 (0–< 0.01) | < 0.01 (0–0.02) |
| Very elderly | 14 | < 0.01 (0–< 0.01) | < 0.01 (0–0.02) |
| Pregnant women | 1 | –c | < 0.01 (0–< 0.01) |
| Lactating women | 1 | –c | < 0.01 (0–0.02) |
With their corresponding confidence intervals (2.5th and 97.5th percentiles).
Range of percentage of subjects above the ARfD after 1,000 iterations in each of the dietary surveys and age classes. ARfD = 300 ng/kg bw per day as derived in the 2017 EFSA CONTAM opinion (EFSA CONTAM Panel, 2017).
Only one dietary survey available for ‘Pregnant women’ and ‘Lactating women’.
Minimum number of six dietary surveys are required to estimate a statistically robust median (EFSA, 2011b).
Overall, the young population groups (‘Infants’, ‘Toddlers’, ‘Other children’) showed higher acute exposure to the sum of T2 and HT2 than the other age classes. Acute dietary exposure in the two dietary surveys covering ‘Pregnant women’ and ‘Lactating women’ were within the range of exposure estimates in the adult population, with exception of the 95th percentile exposure which was slightly higher for ‘Pregnant women’.
Average acute exposure ranged from a minimum of 13.4 ng/kg bw per day estimated in the elderly up to a maximum of 64.7 ng/kg bw per day estimated in toddlers. The highest 95th percentile acute dietary exposure were estimated for a dietary survey within the age class ‘Infants’ (170 ng/kg bw per day).
Looking at the acute exposure for consumers only, in very few occasions, the consumption of only one food resulted in mean estimates close to or above 300 ng/kg bw per day. This referred to population groups with very low number of consumers, and above all, to the consumption of plant extract formula and oat grains. It is important to mention that the dietary exposure from plant extract formula is most probably overestimated due to extremely high concentration levels of T2 and HT2 reported by only one data provider.
The food mostly contributing to the acute exposure to the sum of T2 and HT2 was ‘Bread and rolls’ in contrast to the chronic exposure where the contribution of this food commodity was rather low (Section 3.2.1). This inconsistency is explained by the different approaches used for both exposure assessments; the main contributors of the acute exposure were based on the UB approach while the main contributors of the chronic exposure were based on the LB approach. Considering the 95th percentile acute exposure, the exposure from ‘Bread and rolls’ can reach values up to 72.8 ng/kg bw per day (95% CI = 71.0–74.6). Other food categories making a large contribution to the acute exposure were ‘Fine bakery wares’ and for infants and toddlers also ‘Cereal‐based food for infants and young children’ and ‘Cereal flakes’. Detailed information on the exposure levels estimated following the consumption of diverse single commodities is given in Appendix G, Table G.10.
3.3. Animal exposure
In the present assessment, the animal exposure was estimated only for the sum of T2 and HT2.
Although in animal nutrition, compound feeds (complementary or complete feeds) represent a very large proportion of the feed consumed by farm animals, the available data on the occurrence of the sum of T2 and HT2 in these feeds are of little value due to the low number of samples available for each target species or category or feeds insufficiently characterised to allow a proper utilisation in diet formulations. Therefore, only the occurrence data on cereal grains and on some forages and roughages, reported in Appendix F, Table F.2, were used to calculate animal exposure.
Two scenarios were considered in the calculation of animal exposure: a mean occurrence scenario, in which the mean LB and UB values for each feedingstuff were used to estimate T2 and HT2 dietary concentrations; and a high occurrence scenario, in which the high (P90 or P95, depending on the number of data available) LB and UB values were used. The calculated mean and high concentrations of the sum of T2 and HT2 (reported in Appendix F, Table F.2) were combined with the estimated feed intake (also described in Appendix B) to obtain the estimated exposure by the different animal species and categories in the two scenarios. The detailed results, summarised below, are tabulated in the Appendix H, Sections H.1 and H.2.
Dietary exposure to the sum of T2 and HT2 in dairy cows varied between: (i) 0.06 (LB) and 0.24 (UB) μg/kg bw per day using the mean occurrence scenario, and between 0.23 and 0.45 μg/kg bw per day in the high exposure scenario when fed on a grass/silage plus concentrates diet, and (ii) 0.10 (LB) and 1.14 (UB) μg/kg bw per day using the mean occurrence scenario, and between 0.51 and 2.25 μg/kg bw per day in the high exposure scenario when fed different forage based diets.
The corresponding exposures of beef cattle varied between 0.03 and 0.35, and 0.12 and 0.79 μg/kg bw per day in the mean and high exposure scenarios. Sheep exposure varied between 0.05–0.15 and 0.14–0.29 μg/kg bw per day in the mean and high exposure scenarios. For milking goat (1.13–1.47 and 2.37–2.58 μg/kg bw per day) and fattening goat (0.44–0.56 and 0.89–0.96 μg/kg bw per day) the exposure was higher, owing the higher contribution of oat to the diets.
Pigs showed comparable levels of exposure for the three categories at both mean and high exposure scenarios: piglets 0.22–0.59 and 0.59–1.39 μg/kg bw per day, fattening pigs 0.11–0.38 and 0.33–0.89 μg/kg bw per day, and lactating sows 0.09–0.38 and 0.21–0.83 μg/kg bw per day at the LB and UB levels, respectively.
Similar levels of exposure were observed in poultry species, with values for broilers of 0.30–0.92 and 1.14–2.04 μg/kg bw per day, for laying hens 0.31–0.85 and 1.08–1.82 μg/kg bw per day, for turkeys for fattening 0.18–0.39 and 0.61–0.97 μg/kg bw per day, and for ducks for fattening 0.20–0.59 and 0.46–1.27 μg/kg bw per day.
Rabbits showed exposure levels of 0.40–1.02 and 0.90–1.50 μg/kg bw per day at the LB and UB levels, respectively.
Fish (salmonids) showed exposure levels of 0.03–0.14 and 0.07–0.21 μg/kg bw per day.
Low exposure levels were estimated for dogs and cats, being for dogs 0.04–0.14 and 0.19–0.29μg/kg bw per day, and for cats 0.04–0.15 and 0.19–0.30 μg/kg bw per day.
Horses showed relatively high levels of exposure 1.16–1.26 and 2.40–2.50 μg/kg bw per day, respectively, in the two exposure scenarios, due to the high contribution of oats to their diets.
4. Uncertainties
A qualitative evaluation of the inherent uncertainties in the assessment of the dietary exposure to T2 and HT2 was performed following the guidance of the Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment (EFSA, 2007).
The occurrence data were mostly reported by three countries (Germany, Austria and the UK) while other countries submitted only limited number of data. Since the Fusarium species responsible for the production of T2 and HT2 is generally more prevalent in moist climatic conditions, the limited number of data from dryer European countries may have distorted the overall occurrence in the EU. There is an uncertainty in possible regional differences in T2 and HT2 contamination of food and feed commodities and it is evident that the dataset is not fully representative of food for the EU market.
The large proportion of samples with left‐censored data (values below LOD/LOQ) introduced considerable uncertainties to the overall exposure estimate. As a result, the use of the LB in this opinion tends to underestimate, while UB tends to overestimate the dietary exposure. The limited number of available analytical results for particular food and feed subgroups adds uncertainty to the representativeness of the mean concentration values used to estimate exposure.
In order to obtain more analytical data on the sum of T2 and HT2, the individual T2 and HT2 levels within the same sample were summed and combined with those analytical data originally reported as the sum of the two. In cases where either one of the individual T2 or HT2 concentrations were missing, an imputation method was used (for more details see Section 3.1.1.2). It was assumed that the summation of LOD/LOQ values at UB level would lead to overestimation, and therefore, the MB of LOD/LOQ values was used in the UB scenario. By this approach, the actual concentrations may have been under‐ or overestimated, and this uncertainty should be borne in mind when interpreting the exposure assessment to the sum of T2 and HT2.
The limited number of data reporting very high T2 and HT2 concentrations for some specific food products may have led to overestimation. This particularly concerns dietary exposure from plant extract formula, for which extremely high concentrations were reported by only one data provider, which considerably influenced the estimates.
No data were provided on other modified forms of T2 and HT2, and therefore, a potential presence of other modified forms was not considered in the present assessment. This could have resulted in an underestimation of the exposure. Previously, the CONTAM Panel estimated that such forms would contribute 10% to the exposure, but this should be confirmed in future investigations (EFSA CONTAM Panel, 2014).
The EFSA Opinion published earlier in 2017 (EFSA CONTAM Panel, 2017) gave equivalent relative potencies for T2 and HT2 expressed on a molar basis. Since the molecular mass of T2 is 466 and for HT2 it is 424, when converted to a weight basis, the relative potency of HT2 is about 1.1. This means that concentration values for HT2 should be multiplied by that factor before they are summed in order to measure potency. Since some of the data was already summed when submitted to EFSA, and due to the methods used to handle left‐censored data, this has not been done in this exposure assessment, and will result in an underestimate of the relative potency for HT2 of about 10%, and will result in an underestimate for samples where similar amounts of T2 and HT2 are found by about 5%. Overall, it was assumed that the impact of this uncertainty compared to other uncertainties is rather minor.
Uncertainties and limitations related to the use of the EFSA Comprehensive Food Consumption Database have already been described elsewhere (EFSA, 2011b) and are not further discussed here. In addition, there is uncertainty concerning the eating occasions reported as raw agricultural/minimally processed commodities derived from consumption data on processed foods that were disaggregated (e.g. bread disaggregated into flour). Therefore, exposure estimations derived from using these commodities are most probably overestimated.
Table 7 shows a summary of the uncertainty evaluation indicating an estimate of whether the respective source of uncertainty might have led to an over‐ or underestimation of the exposure.
Table 7.
Summary of the qualitative evaluation of the impact of uncertainties on the dietary exposure to T2 and HT2
| Sources of uncertainty | Directiona |
|---|---|
| Extrapolation of occurrence data few Member States to whole Europe | +/− |
| Large proportion of left‐censored data in the final data set | +/− |
| Using the substitution method at the lower bound (LB) scenario | − |
| Using the substitution method at the upper bound (UB) scenario | + |
| Using imputation method | +/− |
| Lack of data on modified forms | − |
| Limited occurrence data from several food groups/limited consumption data for certain population groups | +/− |
| Very high occurrence data for one product reported by only one data provider | + |
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/− |
| Reporting of consumption data on raw agricultural commodities derived from consumption of processed foods | + |
| High variability of feedstuffs used and feeding systems for livestock | +/− |
+ = uncertainty with potential to cause overestimation of exposure; − = uncertainty with potential to cause underestimation of exposure.
Overall, the chronic dietary exposure to T2 and HT2 presented in this report is likely to overestimate the exposure levels of the European population, in particular at the UB estimation. The acute exposure estimates based only on UB occurrence data are also likely to be overestimated. Both chronic and acute exposure estimates are affected by the high proportion of left‐censored data, in particular for the UB scenario.
5. Conclusions
Recent data on T2 and HT2 (2011–2016) in food (19,505 analytical results) and feed (6,411 analytical data) were available for the human and animal dietary exposure assessment. Analytical results were reported either as individual results for T2 (8,502 data for food and 2,735 data for feed), HT2 (6,877 data for food and 2,429 data for feed) and as the sum of two (4,126 data for food and 1,247 data for feed). In order to obtain more analytical data on the sum of T2 and HT2, the individual T2 and HT2 were summed within the sample and merged with those analytical data originally reported as a sum of the two. In cases where the individual T2 or HT2 was missing, an imputation method was used.
In food, the highest levels of the sum of T2 and HT2 were reported in ‘Grains for human consumption’ and ‘Breakfast cereals’, particularly in oat‐containing commodities (e.g. oat grains, 127–128 μg/kg, LB–UB; oat cereal flakes, 13.9–16.5 μg/kg, LB‐UB). Very high concentration levels were reported for ‘Dietary supplements’ (592–594 μg/kg, LB–UB) with all high levels related to specific plant extract formula dietary supplements and reported by one data provider.
The mean chronic dietary exposure to the sum of T2 and HT2 was highest in ‘Toddlers’ and ‘Infants’ with maximum UB estimates of 64.8 and 62.9 ng/kg bw per day, respectively. The 95th percentile dietary exposure was highest in ‘Infants’ with a maximum UB estimate of 146 ng/kg bw per day. Overall, chronic dietary exposure to the sum of T2 and HT2 in the young population (‘Infants’, ‘Toddlers’, and ‘Other children’) was 2–3 times higher than that estimated for the adult population (‘Adults’, ‘Elderly’, and ‘Very elderly’). UB estimations were on average fourfold higher than LB estimations.
Overall, ‘Grains and grain‐based products’ made the largest contribution to the mean chronic dietary exposure to the sum of T2 and HT2 in all age classes, in particular ‘Cereal flakes’ (contributing up to 84% in ‘Infants’) and ‘Fine bakery wares’ (contributing up to 68% in ‘Very elderly’).
Average acute exposure to the sum of T2 and HT2 ranged from a minimum of 13.4 ng/kg bw per day estimated in ‘Elderly’ up to a maximum of 64.7 ng/kg bw per day estimated in ‘Toddlers’. The highest 95th percentile acute dietary exposure was estimated for a dietary survey within the age class ‘Infants’ (170 ng/kg bw per day).
The food mostly contributing to the acute exposure to the sum of T2 and HT2 was ‘Bread and rolls’. Considering the 95th percentile acute exposure, the exposure from ‘Bread and rolls’ can reach values up to 72.8 ng/kg bw per day (95% CI = 71.0–74.6). Other food categories mainly giving high acute exposure were ‘Fine bakery wares’ and in ‘Infants’ and ‘Toddlers’ also ‘Cereal‐based food for infants and young children’ and ‘Cereal flakes’.
Compared to the 2011 EFSA opinion, a much higher proportion of left‐censored occurrence data characterised the present dataset, in particular for foods contributing mostly to the dietary exposure (i.e. grain‐based food commodities). As a consequence, the chronic UB exposure estimates are approximately threefold higher for ‘Infants’ and approximately 30% higher for other age classes (relative to the maximum UB exposure levels) in the present assessment compared to that estimated in 2011.
Exposure to the sum of T2 and HT2 in farm and companion animals varied according to the animal species. Exposures considering mean concentration scenarios varied between 0.03–0.08 (LB–UB) μg/kg bw per day in beef cattle and 1.13–1.47 μg/kg bw per day in milking goats. For high concentration scenarios, exposures varied between 0.12–0.16 μg/kg bw per day and 2.37–2.58 μg/kg bw per day in the same species. The exposure estimates are lower when compared to those reported in the previous scientific opinions of the EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) published in 2011 and 2014 which corresponds to the lower T2 and HT2 concentrations in feed reported in the last 5 years.
6. Recommendations
Collection of analytical data on T2 and HT2, including potential modified forms, in relevant food and feed commodities should continue with efforts to analyse both individual toxins within the same sample.
Analytical methods with the appropriate sensitivity should be used allowing the reduction of the uncertainty associated to dietary exposure estimations, probably leading to a large overestimation when using UB scenarios.
Further research for the occurrence of T2 and HT2 in dietary supplements should be encouraged in order to evaluate a possible important exposure source from these products.
Abbreviations
- ARfD
acute reference dose
- BMD
benchmark dose
- bw
body weight
- CI
confidence interval
- Comprehensive Database
EFSA Comprehensive European Food Consumption Database
- CONTAM Panel
EFSA Panel on Contaminants in the Food Chain
- DAD
diode array detection
- DATA Unit
EFSA Evidence Management Unit
- ELISA
enzyme‐linked immunosorbent assays
- FD
fluorescence detection
- GC
gas chromatography
- GC–MS/MS
gas chromatography coupled to tandem mass spectrometry
- HBGV
health‐based guidance value
- HPLC‐ESI‐MS
high‐performance liquid chromatography‐electrospray (ionisation)‐mass spectrometry
- HPLC‐FD
high‐performance liquid chromatography‐fluorescence detection
- HT2
HT‐2 toxin
- LB
lower bound
- LC
liquid chromatography
- LC–MS
mass spectrometry coupled to liquid chromatography
- LC–MS/MS
liquid chromatography–tandem mass spectrometry
- LOAEL
lowest observed adverse effect level
- LOD
limit of detection
- LOQ
limit of quantification
- MB
middle‐bound
- NEO
neosolaniol
- NOAEL
no observed adverse effect level
- QqQ
triple quadrupole
- RPF
relative potency factor
- SOPs
standard operational procedures
- T2
T‐2 toxin
- TDI
tolerable daily intake
- UB
upper bound
- UF
uncertainty factor
Appendix A – Dietary surveys used for the estimation of chronic and acute dietary exposure to T2 and HT2
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section).
Appendix B – Intakes and composition of diets used estimating animal exposure to T2 and HT2
1.
The feed intake and the diet composition used to estimate the exposure to T2 and HT2 of the animal species considered in this report are those extensively described in the by the CONTAM Panel in the Scientific Opinion on the risks for animal and public health related to the presence of T‐2 and HT‐2 toxin in food and feed (EFSA CONTAM Panel, 2011). They are summarised in this appendix. In addition, the diets for the farm livestock species and companion animals include also the calculated lower bound (LB) and upper bound (UB) mean and high concentrations for T2 and HT2, based on the LB and UB mean and high (P90–P95) concentrations in the feedingstuffs reported in the Appendix F, Table F.2.
B.1. Feed intake
B.1.1. Cattle, sheep, goats and horses
B.1.1.1. Live weights, growth rate/productivity, dry matter intake for cattle, sheep, goats and horses, and the proportions of the diet as non‐forage
| Live weight (kg) | Growth rate or productivity | Dry matter intake (kg/day) | % of diet as non‐forage feed | Reference | |
|---|---|---|---|---|---|
| Dairy cows, lactatinga | 650 | 40 kg milk/day | 20.7 | 60 | AFRC (1993) |
| Beef: fatteningb | 400 | 1 kg/day | 9.6 | 20 | AFRC (1993) |
| Beef: cereal | 400 | 1.4 kg/day | 8.4 | 85 | AFRC (1993) |
| Sheep: lactating | 80 | Feeding twin lambs | 2.8 | 35 | AFRC (1993) |
| Goats: milkingc | 60 | 6 kg milk/day | 3.4 | 75 | NRC (2007a) |
| Goats: fattening | 40 | 0.2 kg/day | 1.5 | 40 | NRC (2007a) |
| Horses | 450 | – | 9 | 50 | NRC (2007b) |
High‐yielding dairy cows fed grass silage plus a complementary (compound) feed.
Housed castrate cattle, medium maturing breed.
Months 2–3 of lactation.
B.1.2. Dairy cows fed different forages and non‐forage feeds
B.1.2.1. Feed intake of dairy cows producing 40 kg milk/day and fed diets based on different forages and non‐forage feeds (From AFSSA, 2009, modified)
| Type of forage | Quantities of feed consumed (kg dry matter/day) | ||
|---|---|---|---|
| Forage | Maize grain | Soybean meal | |
| Maize silage | 15.0 | 9.5 | 2.8 |
| Grass silage | 16.8 | 9.2 | 0.8 |
| Hay | 16.3 | 12.6 | 0.73 |
| Grazed grass | 18.8 | 7.2 | 0 |
B.1.3. Pigs, poultry, fish and rabbit
B.1.3.1. Live weights and feed intake for pigs, poultry, fish and rabbits
| Live weight (kg) | Feed intake (kg/day) | Reference | |
|---|---|---|---|
| Pigs: piglets | 20 | 1.0 | EFSA (2009) |
| Pigs: fattening pigs | 100 | 3.0 | EFSA (2009) |
| Pigs: lactating sows | 200 | 6.0 | EFSA (2009) |
| Poultry: broilers | 2 | 0.12 | EFSA (2009) |
| Poultry: laying hens | 2 | 0.12 | EFSA (2009) |
| Turkeys: fattening turkeys | 12 | 0.40 | EFSA (2009) |
| Ducks: fattening ducks | 3 | 0.14 | Leeson and Summers (2008) |
| Salmonids | 2 | 0.04 | EFSA (2009) |
| Rabbits | 2 | 0.15 | Carabano and Piquer (1998) |
B.1.4. Dogs and cats
B.1.4.1. Live weights and feed intake for dogs and cats
B.2. Diets composition and T‐2 and HT‐2 toxins concentration estimates
B.2.1. Cattle sheep, goats and horses
B.2.1.1. Diet compositions of non‐forage feed for cattle, sheep and goats, and calculated mean and high lower bound and upper bound levels of T‐2 and HT‐2 toxins in these diets
| Feeds | Dairy cow | Beef cattle | Beef cattle | Sheep | Goats | Goats | Horses |
|---|---|---|---|---|---|---|---|
| Cereal beef | Fattening | Lactating | Dairy | Fattening | |||
| Wheat (%) | 15 | 14 | |||||
| Barley (%) | 20 | 60 | 40 | 18 | 25 | 20 | |
| Oats (%) | 35 | 40 | 40 | ||||
| Soybean meal (%) | 5 | 5 | 10 | 10 | |||
| Rapeseed meal (%) | 20 | 5 | 20 | 10 | 10 | 10 | |
| Sunflower meal (%) | 5 | 5 | |||||
| Beans (%) | 5 | 10 | 10 | ||||
| Maize gluten feed (%) | 10 | 10 | 11 | ||||
| Wheat feed (%) | 10 | 4 | 10 | 15 | 10 | 10 | 30 |
| Oat feed (%) | 12 | ||||||
| Sugar beet pulp (%) | 8 | 10 | 12 | 15 | 2 | ||
| Molasses (%) | 3 | 2 | 3 | 4 | 4 | 3 | 5 |
| Vegetable oils (%) | 1 | 1 | 1 | 1 | 2 | 2 | |
| Minerals and vitamins (%) | 3 | 3 | 3 | 3 | 4 | 3 | 3 |
| T‐2 and HT‐2 toxins a | |||||||
| Mean lower bound (μg/kg) | 3.2 | 7.8 | 4.9 | 3.5 | 26.6 | 29.3 | 116 |
| Mean upper bound (μg/kg) | 12.9 | 17.1 | 14.8 | 10.6 | 34.5 | 37.3 | 126 |
| High lower bound (μg/kg) | 12.6 | 32.2 | 22.0 | 10.2 | 55.8 | 59.5 | 240 |
| High upper bound (μg/kg) | 24.6 | 38.5 | 30.3 | 20.9 | 60.7 | 64.2 | 250 |
B.2.2. Pigs and poultry
B.2.2.1. Diet compositions of feed for pigs and poultry, and calculated mean and high lower bound and upper bound levels of T‐2 and HT‐2 toxins in these diets
| Feeds | Piglets | Pigs for fattening | Lactating sow | Broilers | Laying hens | Turkeys for fattening | Ducks for fattening |
|---|---|---|---|---|---|---|---|
| Wheat (%) | 48 | 48 | 50 | 38 | 30 | 30 | 45 |
| Barley (%) | 20 | 20 | 11 | 35 | 15 | ||
| Maize (%) | 38 | 35 | |||||
| Soybean meal (%) | 22 | 11 | 16 | 15 | 22 | 15 | 28 |
| Rapeseed meal (%) | 3 | 4 | |||||
| Lucerne meal (%) | 4 | 9 | 5 | ||||
| Wheat feed (%) | 9 | 14 | 1 | 7 | |||
| Molasses (%) | 3 | 4 | 4 | 3 | 3 | 3 | |
| Vegetable oils (%) | 1 | 1 | 2 | 1 | 2 | 4 | |
| Minerals and vitamins (%) | 3 | 3 | 3 | 4 | 4 | 4 | |
| T‐2 and HT‐2 toxins a | |||||||
| Mean lower bound (μg/kg) | 4.3 | 3.6 | 3.1 | 5.0 | 5.1 | 5.3 | 4.3 |
| Mean upper bound (μg/kg) | 11.7 | 12.7 | 12.7 | 15.3 | 14.2 | 11.6 | 12.5 |
| High lower bound (μg/kg) | 11.8 | 11.1 | 7.2 | 19.0 | 18.1 | 18.4 | 9.9 |
| High upper bound (μg/kg) | 27.9 | 29.7 | 27.7 | 34.0 | 30.3 | 29.2 | 27.2 |
B.2.3. Rabbits
B.2.3.1. Diet composition for rabbits and calculated mean and high lower bound and upper bound levels of T‐2 and HT‐2 toxins
| Feeds | Rabbit |
|---|---|
| Sunflower meal (%) | 20 |
| Dried lucerne (%) | 19 |
| Wheat bran (%) | 19 |
| Barley (%) | 18 |
| Sugar beet pulp (%) | 12 |
| Beans (%) | 11 |
| Minerals and vitamins (%) | 1 |
| T‐2 and HT‐2 toxins a | |
| Mean lower bound (μg/kg) | 5.3 |
| Mean upper bound (μg/kg) | 13.6 |
| High lower bound (μg/kg) | 12.1 |
| High upper bound (μg/kg) | 19.9 |
B.2.4. Fish
B.2.4.1. Diet composition for salmonids and calculated mean and high lower bound and upper bound levels of T‐2 and HT‐2 toxins
| Feeds | Salmonids |
|---|---|
| Fishmeal (%) | 30.5 |
| Wheat (%) | 13.2 |
| Soybean meal (%) | 12.3 |
| Maize gluten feed (%) | 11.5 |
| Fish and vegetable oils (%) | 31.9 |
| Mineral vitamins premix (%) | 0.6 |
| T‐2 and HT‐2 toxins a | |
| Mean lower bound (μg/kg) | 1.5 |
| Mean upper bound (μg/kg) | 6.9 |
| High lower bound (μg/kg) | 3.6 |
| High upper bound (μg/kg) | 10.7 |
B.2.5. Dogs and cats
B.2.5.1. Diet compositions for dogs and cats, and calculated mean and high lower bound and upper bound levels of T‐2 and HT‐2 toxins
| Feeds | Dogs | Cats |
|---|---|---|
| Wheat (%) | 30.5 | 30.5 |
| Maize (%) | 13.2 | 13.2 |
| Barley (%) | 12.3 | 12.3 |
| Rice (%) | 11.5 | 11.5 |
| Maize gluten feed (%) | 31.9 | 31.9 |
| T‐2 and HT‐2 toxins a | ||
| Mean lower bound (μg/kg) | 4.9 | 4.9 |
| Mean upper bound (μg/kg) | 17.9 | 17.9 |
| High lower bound (μg/kg) | 23.5 | 23.5 |
| High upper bound (μg/kg) | 36.4 | 36.4 |
Appendix C – Use of cut‐offs for the LOQs and its effect on the final occurrence values
C.1. Use of cut‐offs for the LOQs and its effect on the final food occurrence values
| Food category (FoodEx Level 1) | Number of data before applying cut‐off | LC % | Mean occurrence values before application of cut‐offs (μg/kg) | Cut‐off applied on LOQ (μg/kg) | Number of data excluded | Number samples after applying cut‐off | Mean occurrence values after application of cut‐offs (μg/kg) | ||
|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | ||||||
| T‐2 toxin | |||||||||
| Grains and grain‐based products | 9,306 | 91 | 2.17 | 12.3 | 10 | 2,180 | 7,126 | 2.46 | 6.02 |
| Vegetables and vegetable products | 120 | 100 | 0.00 | 60.2 | 78 | 42 | 0.00 | 3.19 | |
| Starchy roots and tubers | 7 | 100 | 0.00 | 30.7 | 2 | 5 | 0.00 | 3.00 | |
| Legumes, nuts and oilseeds | 1,031 | 99.5 | 0.11 | 53.9 | 922 | 109 | 1.01 | 3.45 | |
| Fruit and fruit products | 513 | 100 | 0.00 | 47.7 | 481 | 32 | 0.00 | 3.00 | |
| Meat and meat products (including edible offal) | 5 | 40 | 2.55 | 12.8 | 1 | 4 | 3.18 | 3.43 | |
| Sugar and confectionary | 39 | 100 | 0.00 | 32.2 | 29 | 10 | 0.00 | 3.00 | |
| Animal and vegetable fats and oils | 84 | 86 | 0.31 | 23.8 | 39 | 45 | 0.58 | 1.03 | |
| Fruit and vegetable juices | 74 | 100 | 0.00 | 77.0 | 74 | 0 | – | – | |
| Non‐alcoholic beverages | 2 | 100 | 0.00 | 5.00 | 0 | 2 | 0.00 | 5.00 | |
| Alcoholic beverages | 829 | 99.6 | 0.03 | 26.0 | 225 | 604 | 0.05 | 1.74 | |
| Herbs, spices and condiments | 41 | 100 | 0.00 | 38.8 | 28 | 13 | 0.00 | 3.00 | |
| Food for infants and small children | 528 | 97 | 0.14 | 4.96 | 80 | 448 | 0.11 | 3.09 | |
| Products for special nutritional use | 26 | 73 | 127 | 132 | 1 | 25 | 133 | 135 | |
| Composite food | 3 | 100 | 0.00 | 4.33 | 0 | 3 | 0.00 | 4.33 | |
| Snacks, desserts, and other foods | 82 | 88 | 1.89 | 18.7 | 48 | 34 | 4.56 | 8.37 | |
| HT‐2 toxin | |||||||||
| Grains and grain‐based products | 9,224 | 87 | 4.44 | 16.7 | 10 | 3,513 | 5,711 | 5.67 | 9.55 |
| Vegetables and vegetable products (including fungi) | 120 | 100 | 0.00 | 63.5 | 116 | 4 | 0.00 | 4.67 | |
| Starchy roots and tubers | 7 | 100 | 0.00 | 35.7 | 7 | 0 | – | – | |
| Legumes, nuts and oilseeds | 1,034 | 99.9 | 0.09 | 85.8 | 1,003 | 31 | 0.00 | 2.72 | |
| Fruit and fruit products | 513 | 100 | 0.00 | 91.6 | 513 | 0 | – | – | |
| Meat and meat products | 5 | 20 | 3.14 | 23.1 | 1 | 4 | 3.93 | 3.93 | |
| Sugar and confectionary | 21 | 100 | 0.00 | 11.2 | 21 | 0 | – | – | |
| Animal and vegetable fats and oils | 84 | 100 | 0.00 | 47.1 | 39 | 45 | 0.00 | 1.24 | |
| Fruit and vegetable juices | 74 | 100 | 0.00 | 100 | 74 | 0 | – | – | |
| Non‐alcoholic beverages | 2 | 100 | 0.00 | 5.00 | 0 | 2 | 0.00 | 5.00 | |
| Alcoholic beverages | 829 | 99 | 0.04 | 26.2 | 225 | 604 | 0.06 | 2.07 | |
| Herbs, spices and condiments | 41 | 98 | 0.49 | 72.0 | 41 | 0 | – | – | |
| Food for infants and small children | 528 | 96 | 0.75 | 6.04 | 91 | 437 | 0.48 | 3.70 | |
| Products for special nutritional use | 19 | 63 | 139 | 155 | 9 | 10 | 264 | 265 | |
| Composite food | 3 | 100 | 0.00 | 5.00 | 1 | 2 | 0.00 | 5.00 | |
| Snacks, desserts, and other foods | 77 | 90 | 1.56 | 21.1 | 50 | 27 | 4.45 | 9.76 | |
| Sum of T‐2 and HT‐2 toxins | |||||||||
| Grains and grain‐based products | 5,795 | 87 | 8.35 | 19.02 | 20 | 2,307 | 3,488 | 9.12 | 14.9 |
| Vegetables and vegetable products (including fungi) | 40 | 100 | 0.00 | 5.50 | 1 | 39 | 0.00 | 5.18 | |
| Starchy roots and tubers | 5 | 100 | 0.00 | 5.80 | 0 | 5 | 0.00 | 5.80 | |
| Legumes, nuts and oilseeds | 108 | 95 | 1.86 | 7.02 | 4 | 104 | 1.93 | 5.94 | |
| Fruit and fruit products | 33 | 100 | 0.00 | 8.15 | 0 | 33 | 0.00 | 8.15 | |
| Meat and meat products (including edible offal) | 4 | 0.0 | 7.11 | 7.11 | 0 | 4 | 7.11 | 7.11 | |
| Sugar and confectionary | 11 | 100 | 0.00 | 8.31 | 1 | 10 | 0.00 | 7.20 | |
| Animal and vegetable fats and oils | 44 | 73 | 0.64 | 0.88 | 0 | 44 | 0.64 | 0.88 | |
| Alcoholic beverages | 101 | 100 | 0.00 | 3.16 | 0 | 101 | 0.00 | 3.16 | |
| Herbs, spices and condiments | 18 | 83 | 9.76 | 20.2 | 4 | 14 | 1.43 | 11.3 | |
| Food for infants and small children | 327 | 94 | 1.43 | 9.36 | 73 | 254 | 0.65 | 4.63 | |
| Products for special nutritional use | 20 | 70 | 295 | 306 | 2 | 18 | 328 | 333 | |
| Composite food (including frozen products) | 1 | 100 | 0.00 | 8.00 | 1 | 0 | – | – | |
| Snacks, desserts, and other foods | 25 | 88 | 4.74 | 20.3 | 13 | 12 | 0.00 | 13.5 | |
LB: lower bound; LC: left‐censored; LOQ: limit of quantification; UB: upper bound.
C.2. Use of cut‐offs for the LOQs and its effect on the final feed occurrence values
| Feed category (Level 1) | Number of data before applying cut‐off | LC % | Mean occurrence values before application of cut‐offs (μg/kg) | Cut‐off applied on LOQ (μg/kg) | Number of data excluded | Number samples after applying cut‐off | Mean occurrence values after application of cut‐offs (μg/kg) | ||
|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | ||||||
| T‐2 toxin | |||||||||
| Feed terms | 9 | 89 | 3.07 | 24.3 | 50 | 1 | 8 | 3.46 | 21.1 |
| Cereal grains, their products and by‐products | 1,488 | 89 | 7.53 | 25.7 | 107 | 1,381 | 7.89 | 19.2 | |
| Fish, other aquatic animals and products derived thereof | 1 | 100 | 0.00 | 53.5 | 1 | 0 | – | – | |
| Minerals and products derived thereof | 1 | 100 | 0.00 | 7.00 | 0 | 1 | 0.00 | 7.00 | |
| Fermentation (by‐)products from microorganisms the cells of which have been inactivated or killed | 1 | 100 | 0.00 | 5.00 | 0 | 1 | 0.00 | 5.00 | |
| Miscellaneous | 8 | 100 | 0.00 | 26.2 | 3 | 5 | 0.00 | 11.0 | |
| Oil seeds, oil fruits, and products derived thereof | 174 | 95 | 0.10 | 19.2 | 15 | 159 | 0.11 | 15.9 | |
| Legume seeds and products derived thereof | 8 | 100 | 0.00 | 7.50 | 0 | 8 | 0.00 | 7.50 | |
| Tubers, roots, and products derived thereof | 2 | 100 | 0.00 | 53.0 | 1 | 1 | 0.00 | 5.00 | |
| Other seeds and fruits, and products derived thereof | 2 | 100 | 0.00 | 5.00 | 0 | 2 | 0.00 | 5.00 | |
| Forages and roughage, and products derived thereof | 169 | 91 | 4.78 | 13.6 | 7 | 162 | 4.99 | 12.6 | |
| Milk products and products derived thereof | 1 | 0 | 3.00 | 3.00 | 0 | 1 | 3.00 | 3.00 | |
| Land animal products and products derived thereof | 2 | 100 | 0.00 | 7.50 | 0 | 2 | 0.00 | 7.50 | |
| Compound feed | 1,125 | 95 | 1.62 | 24.2 | 121 | 1,004 | 1.67 | 16.3 | |
| HT‐2 toxin | |||||||||
| Feed terms | 9 | 78 | 27.9 | 67.1 | 50 | 9 | 0 | – | – |
| Cereal grains, their products and by‐products | 1,394 | 86 | 19.6 | 49.0 | 96 | 1,298 | 20.9 | 41.3 | |
| Fish, other aquatic animals and products derived thereof | 1 | 100 | 0.00 | 53.5 | 1 | 0 | – | – | |
| Minerals and products derived thereof | 1 | 100 | 0.00 | 10.0 | 0 | 1 | 0.00 | 10.00 | |
| Fermentation (by‐)products from microorganisms the cells of which have been inactivated or killed | 1 | 100 | 0.00 | 5.00 | 0 | 1 | 0.00 | 5.00 | |
| Miscellaneous | 3 | 100 | 0.00 | 39.2 | 2 | 1 | 0.00 | 38.0 | |
| Oil seeds, oil fruits, and products derived thereof | 167 | 99 | 0.29 | 41.5 | 131 | 36 | 1.35 | 6.33 | |
| Legume seeds and products derived thereof | 8 | 100 | 0.00 | 11.5 | 0 | 8 | 0.00 | 11.5 | |
| Tubers, roots, and products derived thereof | 2 | 100 | 0.00 | 53.0 | 1 | 1 | 0.00 | 5.00 | |
| Other seeds and fruits, and products derived thereof | 2 | 100 | 0.00 | 5.00 | 0 | 2 | 0.00 | 5.00 | |
| Forages and roughage, and products derived thereof | 167 | 83 | 6.79 | 21.9 | 7 | 160 | 7.09 | 21.1 | |
| Milk products and products derived thereof | 1 | 0 | 3.40 | 3.40 | 0 | 1 | 3.40 | 3.40 | |
| Land animal products and products derived thereof | 2 | 100 | 0.00 | 7.50 | 0 | 2 | 0.00 | 7.50 | |
| Compound feed | 964 | 93 | 2.19 | 35.0 | 46 | 918 | 2.30 | 28.9 | |
| Sum of T‐2 and HT‐2 toxins | |||||||||
| Feed terms | 8 | 88 | 18.5 | 36.1 | 100 | 0 | 8 | 18.5 | 36.1 |
| Cereal grains, their products and by‐products | 598 | 82 | 39.8 | 81.0 | 2 | 596 | 39.9 | 54.5 | |
| Miscellaneous | 1 | 100 | 0.00 | 15.2 | 0 | 1 | 0.00 | 15.2 | |
| Oil seeds, oil fruits, and products derived thereof | 119 | 98 | 0.66 | 20.4 | 0 | 119 | 0.66 | 20.4 | |
| Legume seeds and products derived thereof | 1 | 100 | 0.00 | 18.8 | 0 | 1 | 0.00 | 18.8 | |
| Forages and roughage, and products derived thereof | 58 | 69 | 14.2 | 22.4 | 0 | 58 | 14.2 | 22.4 | |
| Compound feed | 465 | 96 | 1.44 | 36.5 | 1 | 464 | 1.44 | 19.3 | |
LB: lower bound; LC: left‐censored; LOQ: limit of quantification; UB: upper bound.
Appendix D – Food samples excluded from the final data set used to estimate dietary exposure and the criteria applied for exclusion
1.
| Criteria for exclusion | Number of samples excluded |
|---|---|
| Duplicates | 107 |
| Outdated data (data sampled before 2011) | 45,595 |
| Reported as suspect samples (not random sampling) | 7,216 |
| LOD/LOQ not reported | 501 |
| Food data eliminated due to application of LOQ cut‐offs | 12,298 |
| Feed data eliminated due to application of LOQ cut‐offs | 552 |
| Total | 66,269 |
LOD: limit of detection; LOQ: limit of quantification.
Appendix E – Statistical description of the concentrations of the individual T2, individual HT2 and the sum of T2 and HT2 across the FoodEx food categories.
E.1. Statistical description of the concentrations of the individual T2 and HT2 across the FoodEx food categories (cleaned final data set, data as reported)
Appendix E.1. can be found in the online version of this output (‘Supporting information’ section).
E.2. Statistical description of the concentrations of the individual T2 and HT2 across the FoodEx food categories as used to estimate chronic dietary exposure
Appendix E.2. can be found in the online version of this output (‘Supporting information’ section).
E.3. Statistical description of the concentrations of the sum of T2 and HT2 across the FoodEx food categories (cleaned final data set, after imputation and summation applied)
Appendix E.3. can be found in the online version of this output (‘Supporting information’ section).
E.4. Statistical description of the concentrations of the sum of T2 and HT2 across the FoodEx food categories as used to estimate chronic dietary exposure
Appendix E.4. can be found in the online version of this output (‘Supporting information’ section).
Appendix F – Statistical description of the concentrations of the individual T2, HT2 and the sum of T2 and HT2 across the feed categories
F.1. Statistical description of the concentrations of the individual T2 and HT2 across the feed categories (cleaned final data set, data as reported)
Appendix F.1. can be found in the online version of this output (‘Supporting information’ section).
F.2. Statistical description of the concentrations of the sum of T2 and HT2 across the feed categories (cleaned final data set, after imputation and summation applied)
Appendix F.2. can be found in the online version of this output (‘Supporting information’ section).
Appendix G – Human dietary exposure to T2 and HT2
G.1. Summary statistics of chronic dietary exposure to the individual T2 across European dietary surveys and different age classes
| Age class | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Mean dietary exposure in total population (ng/kg bw per day) | ||||||
| Infants | 0.74 | 12.5 | 4.17 | 19.6 | 5.46 | 47.0 |
| Toddlers | 2.94 | 26.8 | 5.56 | 35.6 | 8.00 | 43.8 |
| Other children | 2.89 | 20.7 | 4.04 | 29.5 | 6.10 | 41.9 |
| Adolescents | 1.35 | 9.8 | 2.42 | 17.7 | 3.73 | 24.4 |
| Adults | 0.96 | 9.6 | 1.28 | 13.5 | 3.55 | 18.3 |
| Elderly | 0.77 | 9.1 | 1.04 | 12.1 | 4.55 | 16.2 |
| Very elderly | 0.63 | 9.8 | 1.10 | 12.8 | 2.96 | 14.2 |
| Pregnant womenb | 1.62 | – | – | – | – | 13.1 |
| Lactating womenb | 1.67 | – | – | – | – | 9.98 |
| 95 th percentile dietary exposure in total population (ng/kg bw per day) | ||||||
| Infantsa | 6.33 | 26.7 | 12.2 | 52.1 | 17.2 | 108 |
| Toddlersa | 9.14 | 47.3 | 15.5 | 63.6 | 19.3 | 71.7 |
| Other children | 7.06 | 33.2 | 9.73 | 51.1 | 15.1 | 72.9 |
| Adolescents | 3.82 | 16.0 | 6.62 | 35.9 | 10.7 | 43.5 |
| Adults | 2.41 | 18.4 | 3.68 | 26.5 | 6.49 | 39.8 |
| Elderly | 2.04 | 17.3 | 2.72 | 23.9 | 10.2 | 32.8 |
| Very elderlya | 1.61 | 19.4 | 2.61 | 21.5 | 9.32 | 26.8 |
| Pregnant womenb | 4.07 | – | – | – | – | 24.7 |
| Lactating womenb | 4.10 | – | – | – | – | 21.4 |
bw: body weight; LB: lower bound; UB: upper bound.
The 95th percentile estimates obtained on dietary surveys/age classes with less than 60 observations may not be statistically robust (EFSA, 2011b) and therefore not included in this table.
Only one dietary survey available.
G.2. Summary statistics of chronic dietary exposure to the individual HT2 across European dietary surveys and different age classes
| Age class | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Mean dietary exposure in total population (ng/kg bw per day) | ||||||
| Infants | 2.32 | 16.7 | 10.2 | 26.4 | 13.2 | 58.7 |
| Toddlers | 5.42 | 40.4 | 9.02 | 47.8 | 20.2 | 54.9 |
| Other children | 5.06 | 28.5 | 7.20 | 38.9 | 11.8 | 52.6 |
| Adolescents | 2.81 | 12.9 | 3.94 | 23.9 | 7.26 | 33.2 |
| Adults | 1.16 | 12.5 | 2.10 | 17.0 | 5.32 | 23.3 |
| Elderly | 1.03 | 11.9 | 1.80 | 15.2 | 6.22 | 20.3 |
| Very elderly | 1.03 | 12.9 | 2.05 | 16.5 | 5.62 | 18.4 |
| Pregnant womenb | 3.59 | – | – | – | – | 17.6 |
| Lactating womenb | 2.94 | – | – | – | – | 13.3 |
| 95 th percentile dietary exposure in total population (ng/kg bw per day) | ||||||
| Infantsa | 9.79 | 38.4 | 32.8 | 76.5 | 37.3 | 130 |
| Toddlersa | 14.5 | 75.2 | 31.6 | 87.2 | 48.7 | 95.5 |
| Other children | 11.5 | 47.7 | 17.9 | 68.5 | 25.8 | 95.5 |
| Adolescents | 6.78 | 21.6 | 10.6 | 46.1 | 19.8 | 57.3 |
| Adults | 3.01 | 23.9 | 5.73 | 33.7 | 11.5 | 48.9 |
| Elderly | 2.88 | 23.8 | 5.06 | 30.1 | 13.3 | 42.6 |
| Very elderlya | 2.36 | 26.0 | 5.26 | 27.5 | 11.7 | 34.8 |
| Pregnant womenb | 9.22 | – | – | – | – | 32.6 |
| Lactating womenb | 8.42 | – | – | – | – | 29.0 |
bw: body weight; LB: lower bound; UB: upper bound.
The 95th percentile estimates obtained on dietary surveys/age classes with less than 60 observations may not be statistically robust (EFSA, 2011b) and therefore not included in this table.
Only one dietary survey available.
G.3. Mean and 95th percentile chronic exposures to the individual T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Appendix G.3. can be found in the online version of this output (‘Supporting information’ section).
G.4. Mean and 95th percentile chronic exposures to the sum of T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Appendix G.4. can be found in the online version of this output (‘Supporting information’ section).
G.5. Number of surveys split according to their percentage contribution to chronic dietary exposure to the sum of T2 and HT2 using LB concentrations across age classes
Appendix G.5. can be found in the online version of this output (‘Supporting information’ section).
G.6. Range of acute exposure assessment (average and 95th percentile)a to the individual T2 across European dietary surveys
| Age class | Number dietary surveys | Minimum | Median | Maximum |
|---|---|---|---|---|
| Range of average acute exposure (ng/kg bw per day) | ||||
| Infants | 6 | 10.2 (7.55–13.0) | 16.9 | 40.6 (38.4–42.7) |
| Toddlers | 11 | 26.8 (25.9–27.9) | 36.2 | 43.8 (42.4–45.6) |
| Other children | 20 | 20.7 (20.2–21.4) | 30.0 | 41.9 (40.7–43.4) |
| Adolescents | 20 | 9.76 (9.47–10.1) | 18.2 | 26.4 (25.5–27.4) |
| Adults | 22 | 9.58 (9.44–9.74) | 13.4 | 18.4 (17.8–19.0) |
| Elderly | 16 | 9.06 (8.80–9.35) | 12.2 | 16.3 (14.9–18.0) |
| Very elderly | 14 | 9.77 (9.26–10.4) | 13.0 | 14.2 (12.8–16.1) |
| Pregnant women | 1 | –b | –b | 15.3 (14.9–15.7) |
| Lactating women | 1 | –b | –b | 11.0 (10.3–11.8) |
| Range of 95th percentile acute exposure (ng/kg bw per day) | ||||
| Infants | 5 | 34.2 (31.5–37.1) | –c | 137 (125–151) |
| Toddlers | 10 | 59.5 (55.8–63.8) | 83.6 | 96.3 (90.8–103) |
| Other children | 20 | 44.3 (42.2–46.6) | 70.9 | 90.9 (85.4–96.6) |
| Adolescents | 20 | 21.8 (20.6–23.0) | 45.7 | 60.4 (55.5–65.6) |
| Adults | 22 | 24.7 (23.9–25.4) | 33.6 | 46.4 (44.7–48.3) |
| Elderly | 16 | 22.4 (20.9–24.0) | 28.9 | 38.9 (36.1–42.0) |
| Very elderly | 14 | 24.7 (21.7–28.6) | 28.5 | 36.5 (32.6–41.4) |
| Pregnant women | 1 | –b | –b | 37.7 (36.0–39.6) |
| Lactating women | 1 | –b | –b | 30.3 (27.0–34.9) |
With their corresponding confidence intervals (2.5th and 97.5th percentiles).
Only one dietary survey available for ‘Pregnant women’ and ‘Lactating women’.
Minimum number of six dietary surveys are required to estimate a statistically robust median (EFSA, 2011b).
G.7. Range of acute exposure assessment (average and 95th percentile)a to the individual HT2 across European dietary surveys
| Age class | Number dietary surveys | Minimum | Median | Maximum |
|---|---|---|---|---|
| Range of average acute exposure (ng/kg bw per day) | ||||
| Infants | 6 | 12.9 (8.66–23.7) | 23.1 | 50.9 (46.8–60.9) |
| Toddlers | 11 | 40.4 (38.6–42.2) | 47.7 | 54.8 (53.0–58.1) |
| Other children | 20 | 28.5 (27.6–29.6) | 39.8 | 52.6 (50.9–55.0) |
| Adolescents | 20 | 12.8 (12.4–13.3) | 24.3 | 34.3 (33.1–35.6) |
| Adults | 22 | 12.5 (12.3–12.8) | 17.1 | 23.4 (22.7–24.0) |
| Elderly | 16 | 11.9 (11.6–12.3) | 15.3 | 20.3 (19.6–21.1) |
| Very elderly | 14 | 12.9 (12.2–13.7) | 16.7 | 18.4 (16.5–20.6) |
| Pregnant women | 1 | – b | – b | 21.6 (20.8–22.5) |
| Lactating women | 1 | – b | – b | 14.6 (13.7–15.6) |
| Range of 95th percentile acute exposure (ng/kg bw per day) | ||||
| Infants | 5 | 55.5 (50.1–61.1) | – c | 165 (151–181) |
| Toddlers | 10 | 97.0 (89.6–105) | 119 | 133 (125–141) |
| Other children | 20 | 65.4 (62.1–69.2) | 96.4 | 117 (110–125) |
| Adolescents | 20 | 29.3 (27.4–31.3) | 61.1 | 81.1 (75.5–87.4) |
| Adults | 22 | 33.2 (32.1–34.3) | 44.9 | 59.3 (56.9–61.9) |
| Elderly | 16 | 30.1 (28.1–32.4) | 38.9 | 51.6 (47.5–56.2) |
| Very elderly | 14 | 32.8 (30.5–35.4) | 38.0 | 49.9 (38.1–63.4) |
| Pregnant women | 1 | – b | – b | 57.9 (53.8–62.6) |
| Lactating women | 1 | – b | – b | 41.0 (36.2–47.3) |
With their corresponding confidence intervals (2.5th and 97.5th percentiles).
Only one dietary survey available for ‘Pregnant women’ and ‘Lactating women’.
Minimum number of six dietary surveys are required to estimate a statistically robust median (EFSA, 2011b).
G.8. Range of acute exposure assessment (average and 95th percentile) with their corresponding confidence intervals (2.5th and 97.5th percentiles) to the individual T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Appendix G.8. can be found in the online version of this output (‘Supporting information’ section).
G.9. Range of acute exposure assessment (average and 95th percentile) with their corresponding confidence intervals (2.5th and 97.5th percentiles) to the sum of T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Appendix G.9. can be found in the online version of this output (‘Supporting information’ section).
G.10. Acute exposure levels of the sum of T2 and HT2 exposure (ng/kg bw per day) that can be reached following the consumption of diverse single commodities
Appendix G.10. can be found in the online version of this output (‘Supporting information’ section).
Appendix H – Exposure assessment to the sum of T2 and HT2 in animals
H.1. Estimated intake of the sum of T2 and HT2 using mean LB and UB T2 and HT2 concentrations in feedingstuffs
H.1.1. Dairy cows
H.1.1.1. Estimated lower bound and upper bound exposure by 650‐kg body weight lactating dairy cows to the sum of T2 and HT2 at a milk production level of 40 kg milk/day and of non‐forage feed intake of 12 kg (DM) (μg/day and μg/kg bw per day)
| Exposure | |
|---|---|
|
Milk Yield: 40 kg non‐forage feed consumed: 12 kg dry matter/day | |
| μg/day | |
| Lower bound | 39 |
| Upper bound | 155 |
| μg/kg bw per day | |
| Lower bound | 0.06 |
| Upper bound | 0.24 |
bw: body weight.
H.1.1.2. Estimates of lower bound and upper bound exposure to the sum of T2 and HT2 by lactating dairy cows producing 40 kg milk/day and fed diets based on different forages (maize silage, grass silage, hay or pasture grass) and non‐forage feeds (μg/day, μg/kg bw per day)
| Exposure | ||||
|---|---|---|---|---|
| Forage type | ||||
| Maize silage | Grass silage | Hay | Pasture grass | |
| μg/day | ||||
| Lower bound | 364 | 90 | 120 | 66 |
| Upper bound | 742 | 229 | 310 | 173 |
| μg/kg bw per day | ||||
| Lower bound | 0.56 | 0.14 | 0.19 | 0.10 |
| Upper bound | 1.14 | 0.35 | 0.48 | 0.27 |
bw: body weight.
H.1.2. Beef cattle
H.1.2.1. Estimated lower bound and upper bound exposure to the sum of T2 and HT2 by 400‐kg body weight fattening beef cattle reared on grass silage plus non‐forage feeds system or a cereal beef system (μg/day and μg/kg bw per day)
| Exposure | ||
|---|---|---|
| Fattening beef | Cereal beef | |
| Non‐forage feeds consumed (kg dry matter/day) | ||
| 1.9 | 7.1 | |
| μg/day | ||
| Lower bound | 11 | 64 |
| Upper bound | 32 | 139 |
| μg/kg bw per day | ||
| Lower bound | 0.03 | 0.16 |
| Upper bound | 0.08 | 0.35 |
bw: body weight.
H.1.3. Sheep and goats
H.1.3.1. Estimated lower bound and upper bound exposure to the sum of T2 and HT2 by lactating sheep, milking goats and fattening goats (μg/day and μg/kg bw per day)
| Exposure | |||
|---|---|---|---|
| 80‐kg lactating sheep | 60‐kg milking goat | 40‐kg fattening goat | |
| μg/day | |||
| Lower bound | 4 | 68 | 18 |
| Upper bound | 12 | 88 | 22 |
| μg/kg bw per day | |||
| Lower bound | 0.05 | 1.13 | 0.44 |
| Upper bound | 0.15 | 1.47 | 0.56 |
bw: body weight.
H.1.4. Pigs
H.1.4.1. Estimated lower bound and upper bound exposure of pigs to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | |||
|---|---|---|---|
| Piglets | Fattening pigs | Lactating sows | |
| μg/day | |||
| Lower bound | 4.3 | 10.8 | 18.5 |
| Upper bound | 11.7 | 38.0 | 76.1 |
| μg/kg bw per day | |||
| Lower bound | 0.22 | 0.11 | 0.09 |
| Upper bound | 0.59 | 0.38 | 0.38 |
bw: body weight.
H.1.5. Poultry
H.1.5.1. Estimated lower bound and upper bound exposure of poultry to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | ||||
|---|---|---|---|---|
| Broilers | Laying hens | Turkeys for fattening | Ducks for fattening | |
| μg/day | ||||
| Lower bound | 0.6 | 0.6 | 2.1 | 0.6 |
| Upper bound | 1.8 | 1.7 | 4.6 | 1.8 |
| μg/kg bw per day | ||||
| Lower bound | 0.30 | 0.31 | 0.18 | 0.20 |
| Upper bound | 0.92 | 0.85 | 0.39 | 0.59 |
bw: body weight.
H.1.6. Rabbits
H.1.6.1. Estimated lower bound and upper bound exposure of a 2‐kg body weight rabbit to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | |
|---|---|
| Rabbit | |
| μg/day | |
| Lower bound | 0.79 |
| Upper bound | 2.05 |
| μg/kg bw per day | |
| Lower bound | 0.40 |
| Upper bound | 1.02 |
bw: body weight.
H.1.7. Farmed fish
H.1.7.1. Estimated lower bound and upper bound exposure of a 2‐kg body weight salmon to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | |
|---|---|
| Salmon | |
| μg/day | |
| Lower bound | 0.06 |
| Upper bound | 0.27 |
| μg/kg bw per day | |
| Lower bound | 0.03 |
| Upper bound | 0.14 |
bw: body weight.
H.1.8. Companion animals
H.1.8.1. Estimated lower bound and upper bound exposure of dogs and cats to the sum of T‐2 and HT‐2 toxins (μg/day and μg/kg bw per day)
| Exposure | ||
|---|---|---|
| Dogs | Cats | |
| μg/day | ||
| Lower bound | 0.96 | 0.16 |
| Upper bound | 3.54 | 0.59 |
| μg/kg bw per day | ||
| Lower bound | 0.04 | 0.04 |
| Upper bound | 0.14 | 0.15 |
bw: body weight.
H.1.9. Horses
H.1.9.1. Estimated lower bound and upper bound exposure of 450‐kg body weight horses to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | |
|---|---|
| Horses | |
| μg/day | |
| Lower bound | 524 |
| Upper bound | 568 |
| μg/kg bw per day | |
| Lower bound | 1.16 |
| Upper bound | 1.26 |
bw: body weight.
H.2. Estimated intake of the sum of T2 and HT2 using high LB and UB T2 and HT2 concentrations in feedingstuffs
H.2.1. Dairy cows
H.2.1.1. Estimated lower bound and upper bound exposure by lactating dairy cows to the sum of T2 and HT2 at a milk production level of 40 kg milk/day and of non‐forage feed intake of 12 kg (DM) (μg/day and μg/kg bw per day)
| Exposure | |
|---|---|
|
Milk Yield: 40 kg non‐forage feed consumed: 12 kg dry matter/day | |
| μg/day | |
| Lower bound | 151 |
| Upper bound | 295 |
| μg/kg bw per day | |
| Lower bound | 0.23 |
| Upper bound | 0.45 |
bw: body weight.
H.2.1.2. Estimates of lower bound and upper bound exposure to the sum of T2 and HT2 by lactating dairy cows producing 40 kg milk/day and fed diets based on different forages (maize silage, grass silage, hay or pasture grass) and non‐forage feeds (μg/day, μg/kg bw per day)
| Exposure | ||||
|---|---|---|---|---|
| Forage type | ||||
| Maize silage | Grass silage | Hay | Pasture grass | |
| μg/day | ||||
| Lower bound | 1,154 | 430 | 586 | 332 |
| Upper bound | 1,461 | 498 | 678 | 383 |
| μg/kg bw per day | ||||
| Lower bound | 1.78 | 0.66 | 0.90 | 0.51 |
| Upper bound | 2.25 | 0.77 | 1.04 | 0.59 |
bw: body weight.
H.2.2. Beef cattle
H.2.2.1. Estimated lower bound and upper bound exposure to the sum of T2 and HT2 by 400‐kg body weight fattening beef cattle reared on grass silage plus non‐forage feeds system or a cereal beef system (μg/day and μg/kg bw per day)
| Exposure | ||
|---|---|---|
| Fattening beef | Cereal beef | |
| Non‐forage feeds consumed (kg dry matter/day) | ||
| 1.9 | 7.1 | |
| μg/day | ||
| Lower bound | 48 | 263 |
| Upper bound | 66 | 314 |
| μg/kg bw per day | ||
| Lower bound | 0.12 | 0.66 |
| Upper bound | 0.16 | 0.79 |
bw: body weight.
H.2.3. Sheep and goats
H.2.3.1. Estimated lower bound and upper bound exposure to the sum of T2 and HT2 by lactating sheep, milking goats and fattening goats (μg/day and μg/kg bw per day)
| Exposure | |||
|---|---|---|---|
| 80‐kg lactating sheep | 60‐kg milking goat | 40‐kg fattening goat | |
| μg/day | |||
| Lower bound | 11 | 142 | 36 |
| Upper bound | 23 | 155 | 38 |
| μg/kg bw per day | |||
| Lower bound | 0.14 | 2.37 | 0.89 |
| Upper bound | 0.29 | 2.58 | 0.96 |
bw: body weight.
H.2.4. Pigs
H.2.4.1. Estimated lower bound and upper bound exposure of pigs to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | |||
|---|---|---|---|
| Piglets | Fattening pigs | Lactating sows | |
| μg/day | |||
| Lower bound | 11.8 | 33.2 | 42.9 |
| Upper bound | 27.9 | 89.2 | 166.0 |
| μg/kg bw per day | |||
| Lower bound | 0.59 | 0.33 | 0.21 |
| Upper bound | 1.39 | 0.89 | 0.83 |
bw: body weight.
H.2.5. Poultry
H.2.5.1. Estimated lower bound and upper bound exposure of poultry to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | ||||
|---|---|---|---|---|
| Broilers | Laying hens | Turkeys for fattening | Ducks for fattening | |
| μg/day | ||||
| Lower bound | 2.3 | 2.2 | 7.4 | 1.4 |
| Upper bound | 4.1 | 3.6 | 11.7 | 3.8 |
| μg/kg bw per day | ||||
| Lower bound | 1.14 | 1.08 | 0.61 | 0.46 |
| Upper bound | 2.04 | 1.82 | 0.97 | 1.27 |
bw: body weight.
H.2.6. Rabbits
H.2.6.1. Estimated lower bound and upper bound exposure of a 2‐kg body weight rabbit to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | |
|---|---|
| Rabbit | |
| μg/day | |
| Lower bound | 1.81 |
| Upper bound | 2.99 |
| μg/kg bw per day | |
| Lower bound | 0.90 |
| Upper bound | 1.50 |
bw: body weight.
H.2.7. Farmed fish
H.2.7.1. Estimated lower bound and upper bound exposure of a 2‐kg body weight to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | |
|---|---|
| Salmon | |
| μg/day | |
| Lower bound | 0.14 |
| Upper bound | 0.43 |
| μg/kg bw per day | |
| Lower bound | 0.07 |
| Upper bound | 0.21 |
bw: body weight.
H.2.8. Companion animals
H.2.8.1. Estimated lower bound and upper bound exposure of dogs and cats to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | ||
|---|---|---|
| Dogs | Cats | |
| μg/day | ||
| Lower bound | 4.65 | 0.77 |
| Upper bound | 7.21 | 1.20 |
| μg/kg bw per day | ||
| Lower bound | 0.19 | 0.19 |
| Upper bound | 0.29 | 0.30 |
bw: body weight.
H.2.9. Horses
H.2.9.1. Estimated lower bound and upper bound exposure of 450‐kg body weight horses to the sum of T2 and HT2 (μg/day and μg/kg bw per day)
| Exposure | |
|---|---|
| Horses | |
| μg/day | |
| Lower bound | 1,081 |
| Upper bound | 1,124 |
| μg/kg bw per day | |
| Lower bound | 2.40 |
| Upper bound | 2.50 |
bw: body weight.
Supporting information
Dietary surveys used for the estimation of chronic and acute dietary exposure to T2 and HT2
Statistical description of the concentrations of the individual T2, individual HT2 and the sum of T2 and HT2 across the FoodEx food categories
Statistical description of the concentrations of the individual T2, HT2 and the sum of T2 and HT2 across the feed categories
Mean and 95th percentile chronic exposures to the individual T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Mean and 95th percentile chronic exposures to the sum of T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Number of surveys split according to their percentage contribution to chronic dietary exposure to the sum of T2 and HT2 using LB concentrations across age classes
Range of acute exposure assessment (average and 95th percentile) with their corresponding confidence intervals (2.5th and 97.5th percentiles) to the individual T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Range of acute exposure assessment (average and 95th percentile) with their corresponding confidence intervals (2.5th and 97.5th percentiles) to the sum of T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Acute exposure levels of the sum of T2 and HT2 exposure (ng/kg bw per day) that can be reached following the consumption of diverse single commodities
Suggested citation: EFSA (European Food Safety Authority) , Arcella D, Gergelova P, Innocenti ML and Steinkellner H, 2017. Scientific report on human and animal dietary exposure to T‐2 and HT‐2 toxin. EFSA Journal 2017;15(8):4972, 57 pp. 10.2903/j.efsa.2017.4972
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00563
Acknowledgements: EFSA specially thanks Bruce Cottrill, Annette Petersen and Martin Rose for providing valuable comments during the preparation of the report and for reviewing the final version. EFSA wishes to acknowledge all European competent institutions, academia, Member State bodies and other organisations that provided occurrence data on T‐2 and HT‐2 toxin, as well as all European competent institutions that supported the data collection for the Comprehensive European Food Consumption Database. This Scientific report was endorsed by the EFSA Panel on Contaminants in the Food Chain.
Approved: 21 July 2017
Notes
EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on the risks for animal and public health related to the presence of T‐2 and HT‐2 toxin in food and feed. EFSA Journal 2011;9(12):2481, 187 pp. https://doi.org/10.2903/j.efsa.2011.2481. Available online: http://www.efsa.europa.eu/efsajournal
Commission Recommendation 2013/165/EU of 27 March 2013 on the presence of T‐2 and HT‐2 toxin in cereals and cereal products. OJ L 91, 3.4.2013, p. 12.
EFSA Register of questions: http://registerofquestions.efsa.europa.eu/roqFrontend/wicket/page?2
Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of feed materials Text with EEA relevance. OJ L 29, 16.1.2013, p. 1.
Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of feed materials. OJ L 29, 30.1.2013, p. 1‐64.
Also referred to as ‘fresh weight’, for which the moisture content may be unknown.
References
- AFRC (Agricultural and Food Research Council), 1993. Energy and Protein Requirements of Ruminants. An advisory manual prepared by the AFRC Technical Committee on Responses to Nutrients. CAB International.
- AFSSA (Agence française de sécurité sanitaire des aliments), 2009. Évaluation des risques liés à la présence de mycotoxines dans les chaînes alimentaires humaine et animale. Available online: http://www.anses.fr/Documents/RCCP-Ra-Mycotoxines2009.pdf
- Carabano R and Piquer J, 1998. The digestive system of the rabbit In: de Blas C, Wiseman J. (eds.). The Nutrition of the Rabbit. CABI Publishing, London: pp. 1–16. [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. 10.2903/j.efsa.2012.2534 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain (CONTAM)), 2011. Scientific Opinion on the risks for animal and public health related to the presence of T‐2 and HT‐2 toxin in food and feed. EFSA Journal 2011;9(12):2481, 187 pp. 10.2903/j.efsa.2011.2481 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain (CONTAM)), 2014. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal 2014;12(12):3916, 107 pp. 10.2903/j.efsa.2014.3916 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain (CONTAM)), 2017. Scientific opinion on the appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA Journal 2017;15(1):4655, 53 pp. 10.2903/j.efsa.2017.4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, Serra‐ Majem L, Volatier JL, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren J, 2011. Dietary Exposure Assessments for Children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 1169–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson S and Summers JD, 2008. Commercial Poultry Nutrition, 3rd edition Nottingham University Press, Nottingham, UK. [Google Scholar]
- Meng‐Reiterer J, Bueschl C, Rechthaler J, Berthiller F, Lemmens M and Schuhmacher R, 2016. Metabolism of HT‐2 Toxin and T‐2 Toxin in Oats. Toxins, 8, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty A, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Additives & Contaminants: Part A, 28, 975–995. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council), 2006. Nutrient requirements of dogs and cats. National Academies Press, Washington, DC. [Google Scholar]
- NRC (National Research Council), 2007a. Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. US National Academy of Science, Washington DC. [Google Scholar]
- NRC (National Research Council), 2007b. Nutrient requirement of horses, 6th Revised Edition. National Academies Press, Washington, DC. [Google Scholar]
- Rubert J, James K, Mañes J and Soler C, 2012. Study of mycotoxin calibration approaches on the example of trichothecenes analysis from flour. Food and Chemical Toxicology, 50, 2034–2041. [DOI] [PubMed] [Google Scholar]
- Schwake‐Anduschus C, Langenkämper G, Unbehend G, Dietrich R, Märtlbauer E and Münzing K, 2010. Occurrence of Fusarium T‐2 and HT‐2 toxins in oats from cultivar studies in Germany and degradation of the toxins during grain cleaning treatment and food processing. Food Additives and Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 27, 1253–1260. [DOI] [PubMed] [Google Scholar]
- Scudamore KA, Baillie H, Patel S and Edwards SG, 2007. Occurrence and fate of Fusarium mycotoxins during commercial processing of oats in the UK. Food Additives and Contaminants, 24, 1374–1385. [DOI] [PubMed] [Google Scholar]
- Van Der Fels‐Klerx HJ, Klemsdal S, Hietaniemi V, Lindblad M, Ioannou‐Kakouri E and Van Asselt ED, 2012. Mycotoxin contamination of cereal grain commodities in relation to climate in North West Europe. Food Additives & Contaminants: Part A, 29, 1581–1592. [DOI] [PubMed] [Google Scholar]
- Veprikova Z, Zachariasova M, Dzuman Z, Zachariasova A, Fenclova F, Slavikova P, Vaclavikova M, Mastovska K, Hengst D and Hajslova J, 2015. Mycotoxins in plant‐based dietary supplements: hidden health risk for consumers. Journal of Agriculture and Food Chemistry, 63, 6633–6643. [DOI] [PubMed] [Google Scholar]
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food, International Programme on Chemical Safety, Environmental Health Criteria 240. Chapter 6: Dietary Exposure Assessment of Chemicals in Food. Available online: http://www.who.int/ipcs/food/principles/en/index1.html
- Zachariasova M, Lacina O, Malachova A, Kostelanska M, Poustka J, Godula M and Hajslova J, 2010. Novel approaches in analysis of Fusarium mycotoxins in cereals employing ultra performance liquid chromatography coupled with high resolution mass spectrometry. Analytica Chimica Acta., 662, 51–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dietary surveys used for the estimation of chronic and acute dietary exposure to T2 and HT2
Statistical description of the concentrations of the individual T2, individual HT2 and the sum of T2 and HT2 across the FoodEx food categories
Statistical description of the concentrations of the individual T2, HT2 and the sum of T2 and HT2 across the feed categories
Mean and 95th percentile chronic exposures to the individual T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Mean and 95th percentile chronic exposures to the sum of T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Number of surveys split according to their percentage contribution to chronic dietary exposure to the sum of T2 and HT2 using LB concentrations across age classes
Range of acute exposure assessment (average and 95th percentile) with their corresponding confidence intervals (2.5th and 97.5th percentiles) to the individual T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Range of acute exposure assessment (average and 95th percentile) with their corresponding confidence intervals (2.5th and 97.5th percentiles) to the sum of T2 and HT2 (ng/kg bw per day) for total population for each dietary survey
Acute exposure levels of the sum of T2 and HT2 exposure (ng/kg bw per day) that can be reached following the consumption of diverse single commodities


