Abstract
The Panel on Plant Health performed a pest categorisation of Cercospora angolensis, the fungus responsible for Pseudocercospora fruit and leaf spot of citrus, for all territories except of the Union territories defined in Article 1 point 3 of Regulation (EU) 2016/2031. C. angolensis is listed in Annex IIAI of Directive 2000/29/EC and is not known to be present in the EU. The pathogen, which has recently been reclassified as Pseudocercospora angolensis, is a well‐defined, distinguishable fungal species affecting all cultivated Citrus spp. and Fortunella japonica plants. It is currently distributed in sub‐Saharan Africa (altitudes 80–1,800 m) and Yemen. Although the epidemiology of P. angolensis is not well understood, infection is favoured by warm temperatures and humidity. The current distribution of the pathogen and climate matching suggests that it might not be well adapted to Mediterranean climates. However, the pathogen is also present in arid areas of Yemen and can infect young fruit with short wetness durations. Uncertainty exists on whether and at which extent the irrigation applied to EU citrus orchards can make the microclimate favourable for P. angolensis. There are no eco‐climatic factors limiting the potential spread of the pathogen in the EU. Long‐distance spread occurs by wind‐disseminated conidia and movement of infected plants for planting and fruit. Short‐distance spread occurs via water splash and/or wind‐driven rain. In the infested areas, the disease causes premature abscission of young leaves and fruit resulting in yield losses up to 50–100%. Cultural practices and chemical measures applied in the infested areas reduce inoculum but they cannot eliminate the pathogen. All criteria assessed by EFSA for consideration as a potential Union quarantine pest are met. As P. angolensis is not known to occur in the EU, this criterion assessed by EFSA to consider it as a Union regulated non‐quarantine pest is not met.
Keywords: Citrus spp., climate, pest distribution, European Union, Pseudocercospora fruit and leaf spot, impacts
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips amitinus Eichhof |
| Cephalcia lariciphila (Klug) | Ips cembrae Heer |
| Dendroctonus micans Kugelan | Ips duplicatus Sahlberg |
| Gilphinia hercyniae (Hartig) | Ips sexdentatus Börner |
| Gonipterus scutellatus Gyll. | Ips typographus Heer |
| Sternochetus mangiferae Fabricius | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa) such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Cercospora angolensis is one of a number of pests listed in the Appendices to the ToR to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest for the area of the European Union (EU) excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A search of literature (1997–2017) in Web of Science and Scopus was conducted at the beginning of the categorisation. The search focused on C. angolensis and its geographic distribution, life cycle, host plants and the damage it causes. The following search terms (TS) and combinations were used: TS=(“Cercospora angolensis” OR “Pseudocercospora angolensis” OR “Phaeoramularia angolensis” OR “citrus leaf spot” OR “citrus fruit spot”) AND TS=(geograph* OR distribution OR “life cycle” OR lifecycle OR host OR hosts OR plant* OR damag*).
Further references and information were obtained from experts, from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO, 2017).
Data about import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT.
The Europhyt database (Europhyt, online) was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Consumers (DG SANCO), and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the MSs and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for C. angolensis following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No. 11 (FAO, 2013) and No. 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union‐regulated non‐quarantine pest in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required as per the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a regulated non‐quarantine pest. If one of the criteria is not met, the pest will not qualify. Note that a pest that does not qualify as a quarantine pest may still qualify as a regulated non‐quarantine pest which needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone, thus the criteria refers to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The protected zone system aligns with the pest‐free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in and spread within the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in and spread within the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6 ) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
YES, the identity of the pest is well established.
Cercospora angolensis T. Carvalho & O. Mendes (1953) is a fungus of the family Mycosphaerellaceae. The Index Fungorum database (www.indexfungorum.org) provides the following taxonomic identification:
Current name: Pseudocercospora angolensis (T. Carvalho & O. Mendes) Crous & U. Braun
Family – Mycosphaerellaceae
Genus – Pseudocercospora
Species – angolensis
Other reported synonyms: Phaeoramularia angolensis (T. Carvalho & O. Mendes) P.M. Kirk, Pseudophaeoramularia angolensis (T. Carvalho & O. Mendes) U. Braun.
Although the request for pest categorisation refers to C. angolensis, the Panel decided to use the current taxonomic name of the organism, i.e. P. angolensis, throughout the opinion.
3.1.2. Biology of the pest
The biology of P. angolensis and the epidemiology of Pseudocercospora fruit and leaf spot (PFLS) disease are poorly understood. Although not documented, P. angolensis is likely to survive in infected host plant tissues in the tree canopy and on plant debris, similarly to other Cercospora species. Only asexual reproduction through conidia has been reported so far, but if sexual reproduction exists, it is likely to be in the form of ascospores belonging to a species of Mycosphaerellaceae. Conidia are formed on leaf and fruit lesions and can be disseminated by water splash and/or air currents. Airborne conidia would be dispersed to longer distances than water‐splashed conidia (Seif and Hillocks, 1993). The optimum temperature for infection and symptom development on citrus leaves and fruit was shown to be 25°C, whereas no symptoms developed at 35°C (Ndzoumba, 1985; Seif and Hillocks, 1998). PFLS has been reported at altitudes between 80 and 1,800 m (Kuate et al., 2002; Ndo et al., 2010). In Ghana, Cameroon and Guinea, PFLS severity was greater at higher altitudes characterised by cooler (22–26°C) and more humid conditions (Kuate et al., 2002; Ndo et al., 2010; Diallo et al., 2011; Lawson et al., 2017). Field observations in Cameroon, Kenya, Ethiopia and Guinea indicated that infections by P. angolensis occurred mainly during the rainy seasons (Seif and Hillocks, 1993; Kuate et al., 1997; Yesuf, 2007; Diallo et al., 2011). Nevertheless, studies have shown that, at the optimum temperature of 25°C, 3 h of leaf wetness were adequate for the infection of detached young (1‐ and 3‐week‐old) sweet orange fruit by P. angolensis (Seif and Hillocks, 1998).
3.1.3. Detection and identification of the pest
Are detection and identification methods available for the pest?
YES, the organism can be detected by visual examination of symptoms produced on leaves and fruit of infected hosts. Identification is primarily based on morphological characteristics of the fungus, pending confirmation by pathogenicity tests and/or molecular methods.
Pseudocercospora angolensis can be identified based on host association, symptomatology and morphological characteristics of the fungus, such as conidial size and septation, conidiophores as well as colony characteristics in different agar media. However, pathogenicity tests and/or molecular methods are necessary for confirming the identification based on morphology. Nevertheless, no harmonised PCR detection protocols specific for P. angolensis are available to date. A combination of sequence data from different loci, such as the 28S nuclear ribosomal RNA gene, ITS, LSU, EF‐1α, and ACT, have also been used for the identification of P. angolensis (Quaedvlieg et al., 2012; Crous et al., 2013).
Symptoms
Pseudocercospora angolensis affects leaves and fruit of Citrus species, hybrids and varieties causing spots and lesions of varying sizes (Brun, 1972; Kuate et al., 1994). Leaf symptoms initially appear as greenish‐yellow patches. At maturity, the leaf spots are amphigenous, mainly hypophyllous, 4–10 mm or more in diameter, pale brown to brown, blackish brown when sporulation is dense, surrounded by a dark‐brown margin and a yellow halo, the centre often becoming detached resulting in a shot‐hole spot. The leaf spots, especially on younger leaves, often coalesce resulting in generalised chlorosis and necrosis followed by premature defoliation (Seif and Hillocks, 1993). During wet weather, the lesions sporulate and become black. On fruit, the spots are circular to irregular, discrete or coalescent and mostly up to 10 mm in diameter (Seif and Hillocks, 1993). On young fruit, symptoms often commence with hyperplasia producing tumour‐like lesions surrounded by a yellow halo. Lesions on mature fruit are normally flat, but sometimes they have a slightly sunken brown centre with a surrounding ring of raised epicarp, giving the fruit a blistered appearance (Seif and Hillocks, 1993). Affected fruit ripen prematurely and drop (Seif and Hillocks, 1993). Fruit and leaves are much more susceptible than stems, on which symptoms are rare. When infection of stems occurs, the lesions are dark brown and usually occur as an extension of the lesions on the petioles. They may coalesce resulting in stem dieback or formation of corky internodal regions (Seif and Hillocks, 1993).
Morphology
Conidiophores solitary, fasciculate or forming loose synnemata, 12–45 μm wide, unbranched, septate, smooth, pale brown to brown, (60)−120 × 240 × 4.5–7 μm, usually arising from a dark stroma 30–60 μm in diameter. Conidia solitary or in simple or branched chains of 2–4, cylindrical to narrowly obclavate, straight or slightly flexuous to more or less curved, smooth, hyaline to very pale brown, (1)3–4(−6)‐septate, 24–79 × 4–5(−6.5) μm, apex rounded, base truncate (Kirk, 1986).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU (Figure 1) (Table 2)
Figure 1.
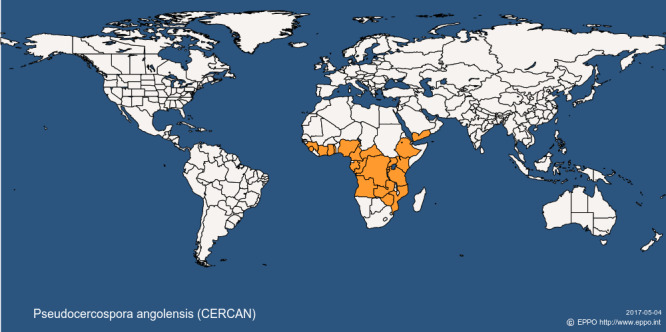
Global distribution map for Pseudocercospora angolensis (extracted from EPPO Global Database accessed on 4 May 2017)
Table 2.
Current distribution of Pseudocercospora angolensis based on information from the EPPO Global Database (last updated: 30/9/2016; last accessed: 04/5/2017)
| Continent | Country | Pest status |
|---|---|---|
| Africa | Angola | Present, no details |
| Africa | Burundi | Present, no details |
| Africa | Cameroon | Present, restricted distribution |
| Africa | Central African Republic | Present, no details |
| Africa | Comoros | Present, no details |
| Africa | Congo | Present, no details |
| Africa | Congo, Democratic republic of the | Present, no details |
| Africa | Cote d'Ivoire | Present, no details |
| Africa | Ethiopia | Present, no details |
| Africa | Gabon | Present, no details |
| Africa | Gambia | Present, no details |
| Africa | Ghana | Present, restricted distribution |
| Africa | Guinea | Present, no details |
| Africa | Kenya | Present, no details |
| Africa | Mozambique | Present, no detailsa |
| Africa | Nigeria | Present, no details |
| Africa | Rwanda | Present, no details |
| Africa | Sierra Leone | Present, restricted distribution |
| Africa | Tanzania | Present, no details |
| Africa | Togo | Present, no details |
| Africa | Uganda | Present, no details |
| Africa | Zambia | Present, no details |
| Africa | Zimbabwe | Present, restricted distribution |
| Asia | Yemen | Present, no details |
De Carvalho and Mendes (1952) are often quoted to indicate the presence of P. angolensis in Mozambique. However, in their publication these authors referred only to the presence of this pathogen in the province of Bié, Angola, without making any reference to Mozambique. No direct references of the presence of P. angolensis in Mozambique were found in the literature. Moreover, Pretorious and Holtz (2008) did not find symptoms of PFLS during a survey conducted in 2003 in several citrus areas in Mozambique. Therefore, the status of P. angolensis in Mozambique needs to be reassessed.
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
NO, P. angolensis is not known to occur in the EU.
P. angolensis is not known to be present in the EU (EPPO Global Database, last updated 30/9/2016).
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
Pseudocercospora angolensis is regulated as a harmful organism in the EU and is listed as C. angolensis in Council Directive 2000/29/EC. Details are shown in Table 3.
Table 3.
Pseudocercospora angolensis (syn. Cercospora angolensis) in Council Directive 2000/29/EC
| Annex II, Part A | Harmful organisms whose introduction into, and spread within, all Member States shall be banned if they are present on certain plants or plant products | |
| Section I | Harmful organisms not known to occur in the community and relevant for the entire community | |
| (c) | Fungi | |
| Species | Subject of contamination | |
| 6. | Cercospora angolensis Carv. and Mendes | Plants of Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids other than seeds |
3.3.2. Legislation addressing plants and plant parts on which C. angolensis is regulated (Table 4)
Table 4.
Regulated hosts and commodities that may involve Pseudocercospora angolensis (syn. Cercospora angolensis) in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
| Description | Country of origin | |
| 16 | Plants of Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids other than fruit and seeds | Third countries |
| Annex IV, Part A | Special requirements which must be laid down by all Member States for the introduction and movement of plants, plant products and other objects into and within all Member States | |
| Section I | Plants, plant products and other objects originating outside the community | |
| Plant, plant products and other objects | Special requirements | |
| 16.1 | Fruits of Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids originating in third countries | The fruits shall be free from peduncles and leaves and the packaging shall bear an appropriate origin mark |
| 16.3 | Fruits of Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids originating in third countries |
Without prejudice to the provisions applicable to the fruits in Annex IV(A)(I)(16.1), (16.2), (16.4) and (16.5), official statement that: (a) the fruits originate in a country recognised as being free from C. angolensis Carv. et Mendes in accordance with the procedure referred to in Article 18(2) or (b) the fruits originate in an area recognised as being free from C. angolensis Carv. et Mendes, in accordance with the procedure referred to in Article 18(2) and mentioned on the certificates referred to in Articles 7 or 8 of this Directive, or (c) no symptoms of C. angolensis Carv. et Mendes have been observed in the field of production and in its immediate vicinity since the beginning of the last cycle of vegetation, and none of the fruits harvested in the field of production has shown, in appropriate official examination, symptoms of this organism. |
| Annex IV, Part A | Special requirements which must be laid down by all Member States for the introduction and movement of plants, plant products and other objects into and within all Member States | |
| Section II | Plants, plant products and other objects originating in the community | |
| Plant, plant products and other objects | Special requirements | |
| 30.1 | Fruits of Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids | The packaging shall bear an appropriate origin mark. |
|
Annex IV, Part B |
Special requirements which must be laid down by all Member States for the introduction and movement of plants, plant products and other objects into and within certain protected zones | ||
| Plants, plant products and other objects | Special requirements | Protected zone(s) | |
| 31. | Fruits of Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids originating in BG, HR, SI, EL (regional units of Argolida and Chania), P (Algarve and Madeira), E, F, CY and I |
Without prejudice to the requirement in Annex IV Part A Section II point 30.1 that packaging should bear an origin mark: (a) the fruits shall be free from leaves and peduncles or (b) in the case of fruits with leaves or peduncles, official statement that the fruits are packed in closed containers which have been officially sealed and shall remain sealed during their transport through a protected zone, recognised for these fruits, and shall bear a distinguishing mark to be reported on the passport. |
EL (except the regional units of Argolida and Chania), M, P (except Algarve and Madeira) |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the community, before being moved within the community – in the country of origin or the consignor country, if originating outside the community) before being permitted to enter the community |
| Part A | Plants, plant products and other objects originating in the community |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire community and which must be accompanied by a plant passport |
| 1.5 | Without prejudice to point 1.6, plants of Citrus L. and their hybrids other than fruit and seeds |
| 1.6 | Fruits of Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids with leaves and peduncles |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the community, before being moved within the community – in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community |
| Part B | Plants, plant products and other objects originating in territories, other than those referred to in Part A |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community |
| 1. | Plants, intended for planting, other than seeds but including seeds of Cruciferae, Gramineae, Trifolium spp., originating in Argentina, Australia, Bolivia, Chile, New Zealand and Uruguay; genera Triticum, Secale and X Triticosecale from Afghanistan, India, Iran, Iraq, Mexico, Nepal, Pakistan, South Africa and the USA; Citrus L., Fortunella Swingle and Poncirus Raf. and their hybrids Capsicum spp., Helianthus annuus L., Solanum lycopersicum L., Medicago sativa L., Prunus L., Rubus L., Oryza spp., Zea mais L., Allium ascalonicum L., Allium cepa L., Allium porrum L., Allium schoenoprasum L. and Phaseolus L. |
| 3. |
Fruits of: Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids Momordica L. and Solanum melongena L. |
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
In the infested areas, the disease has been observed on all cultivated Citrus species and varieties. However, different levels of susceptibility have been reported in the literature among the various Citrus species and among varieties within the same species, with some of this information being contradictory (Bella et al., 1999; Ndo et al., 2010; Diallo et al., 2011; Lawson et al., 2017).
Fortunella japonica (kumquat) is reported to be very tolerant to the infection by P. angolensis in Guinea (Diallo et al., 2011).
No information was found in the literature on other Fortunella species, Poncirus and their hybrids being hosts of P. angolensis.
3.4.2. Entry
Is the pest able to enter into the EU territory?
YES, under the current EU legislation, P. angolensis could potentially enter the risk assessment area via the citrus fruit without leaves pathway.
The PLH Panel identified the following pathways for the entry of the pathogen from infested third countries into the EU territory:
Host plants for planting, excluding seeds, and
Citrus fruit (with or without leaves)
Nevertheless, under the current EU legislation, only the citrus fruit without leaves pathway is relevant, as the import into the EU territory of plants of Citrus, Poncirus and Fortunella and their hybrids and citrus fruit with leaves is prohibited.
Based on the above data, during the period 2011–2015, less than 0.5% of the total volume of citrus fruit imported by the 28 EU Member States from third countries originated in areas where P. angolensis is reported as present.
There is no record of interception of P. angolensis (or C. angolensis or Phaeoramularia sp.) in the Europhyt database (search performed on 3 May 2017).
3.4.3. Establishment
Is the pest able to become established in the EU territory?
YES, biotic factors (host availability) and abiotic factors (climate suitability) suggest that P. angolensis could establish in the EU territory.
3.4.3.1. EU distribution of main host plants
As shown in Figure 2, the greatest density of citrus production occurs in the southern EU Member States. Around 700,000 ha are allocated to citrus production in the EU. Table 5 provides further details on the area of citrus harvested in each EU Member State: four Member States (i.e. Spain, Italy, Greece and Portugal) concentrate 98% of the total EU citrus‐growing area (Table 6).
Figure 2.
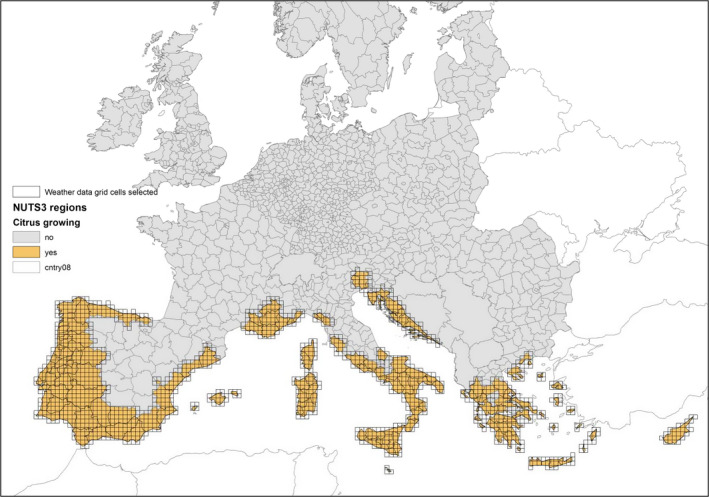
EU map of NUTS3 citrus‐growing regions based on citrus production data extracted from national statistical databases of Portugal, Spain, France, Italy, Malta, Croatia, Greece and Cyprus (EFSA PLH Panel, 2014)
Table 5.
Volume (in tonnes) of citrus fruit imported during the period 2011–2015 into the EU Member States from non‐EU countries and from countries where Pseudocercospora angolensis is reported as present (Source: Eurostat, extracted on 6 June 2017)
| 2011 | 2012 | 2013 | 2014 | 2015 | |
|---|---|---|---|---|---|
| Total EU 28 citrus fruit import (in tonnes) from non‐EU countries | 763,870 | 736,992 | 796,767 | 710,798 | 791,366 |
| Total EU 28 citrus fruit import (in tonnes) from countries where P. angolensis is reported as present by EPPO | 3,527 | 2,480 | 2,831 | 2,557 | 2,472 |
Table 6.
Area cultivated with citrus in the EU between 2011 and 2015 (in 1,000 ha) – Source: EUROSTAT, extracted on 7/6/2017
| Countries | 2011 | 2012 | 2013 | 2014 | 2015 | Mean of EU citrus‐growing area (in 1,000 ha) |
|---|---|---|---|---|---|---|
| European Union (28 countries) | 726.56 | 702.30 | 712.35 | 684.32 | 685.94 | 702.29 |
| Spain | 437.82 | 426.26 | 420.39 | 415.67 | 410.19 | 422.07 |
| Italy | 198.30 | 182.97 | 198.51 | 174.93 | 183.47 | 187.64 |
| Greece | 59.10 | 57.43 | 57.24 | 57.67 | 55.45 | 57.38 |
| Portugal | 21.93 | 22.26 | 22.17 | 22.21 | 22.71 | 22.26 |
| France | 5.69 | 5.78 | 6.61 | 6.26 | 6.32 | 6.13 |
| Croatia | NA | 3.70 | 4.26 | 4.32 | 4.36 | 4.16* |
| Cyprus | 3.72 | 3.90 | 3.17 | 3.25 | 3.44 | 3.50 |
Only citrus‐producing Member States are reported above.
NA: not available.
* Calculated on 4 years (2012–2015).
3.4.3.2. Climatic conditions affecting establishment
The current geographic distribution of P. angolensis and the greater disease severity observed in areas with moderately warm (22–26°C) and humid conditions suggest that the pathogen might not be well adapted to the Mediterranean climatic conditions (EPPO Global Database).
Based on the Köppen–Geiger climate classification as indicated by Peel et al. (2007), the EU area where citrus are mainly grown belongs to five climate types: Csa (temperate, dry and hot summer), Csb (temperate, dry and warm summer), Cfa (temperate, without dry season, hot summer), Cfb (temperate, without dry season, warm summer) and BSk (arid, steppe, cold) (Figure 3A). The pathogen is known to be present in countries having a rather wide range of climate types, which include Af (tropical, rainforest), Am (tropical, monsoon), Aw (tropical, savannah), Cwa (temperate, dry winter, hot summer), Cwb (temperate dry winter, warm summer), BSh (arid, steppe, hot), BWh (arid, desert, hot), Csa and Csb (Figure 3B), with the two latter climate types being present in both the EU and small areas of Ethiopia. However, there is no information whether citrus are grown and P. angolensis is present in those Ethiopian areas having Csa or Csb climate types.
Figure 3.
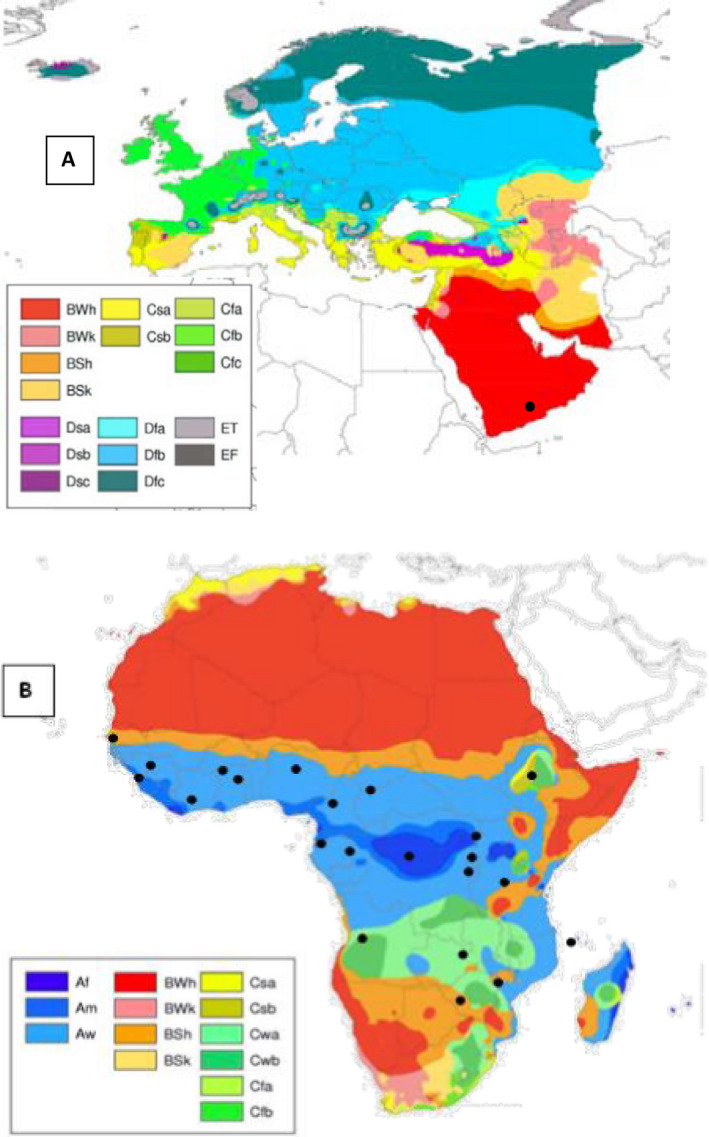
Köppen–Geiger climate‐type map of Europe and Arabian Peninsula (A) and Africa (B); climatic maps are from Peel et al. (2007); dots represent the distribution of Pseudocercospora angolensis (from CABI; http://www.cabi.org/isc/datasheet/12184; accessed on May 12, 2017)
The above climate matching suggests that P. angolensis might not be well adapted to the Mediterranean climates. However, the fact that P. angolensis is not known to be present in some climate types does not imply that the pathogen cannot establish in those climates. It should be also noted that P. angolensis has been reported to be present in Yemen, in the Arabic Peninsula, under arid desert conditions.
Seif and Hillocks (1998) found that artificial inoculation of sweet orange leaves (cv. Washington Navel) with P. angolensis caused infection at 15, 20, 25, and 30°C, but not at 35°C, with optimum at 25°C. The disease severity on leaves increased between 24 and 144 h of wetness, while no disease was observed when leaves were kept in dry conditions. Three hours of wetness were, however, sufficient for causing infection on 1‐ and 3‐week‐old fruits of Washington Navel; older fruits required longer wet periods to be infected (e.g. 48 h of wetness were necessary to infect 18‐week‐old fruits).
Based on the above evidence, the panel cannot exclude that P. angolensis can cause infection and establish under the climatic conditions prevailing in the EU citrus‐growing areas. Nevertheless, there is some uncertainty around this and further studies are required. In addition, the extensive use of surface, sprinkle and micro‐sprinkle irrigation in the EU citrus‐growing areas might add to the suitability of the environment since irrigation has the potential to lengthen the periods of leaf wetness aiding infection (EFSA PLH Panel, 2014). Therefore, there is also uncertainty exists on whether and at which extent the irrigation applied to EU citrus‐growing areas can make the microclimate in citrus orchards more favourable for P. angolensis.
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment? YES
How? By natural and human‐assisted means
Once established in the EU territory, the pathogen can spread efficiently by both natural and human‐assisted means.
Spread by natural means. Long‐distance spread of the pathogen is likely by means of wind‐borne conidia produced on symptomatic host plant tissues, whereas short‐distance spread (within a tree or between trees) is primarily by water splash and/or wind‐driven rain carrying conidia (Seif and Hillocks, 1993). Since leaf lesions produce more conidia than those on fruit, symptomatic leaves are most probably the main source of inoculum for the spread of the pathogen by natural means in an infested area (Seif and Hillocks, 1993).
No information was found in the literature on the distance over which conidia of the pathogen can be carried by air currents. Nevertheless, according to Diallo (2001), in Guinea the average rate of disease progression was estimated to be 23 km/year.
Spread by human assistance. The pathogen can spread over long distances via the movement of infected or contaminated host plants for planting (rootstocks, grafted plants, scions, etc.) and fruits.
3.5. Potential or observed impacts in the EU
Would the pest's introduction have an economic or environmental impact on the EU territory?
YES, the introduction of P. angolensis could cause yield and quality losses to citrus crops.
3.5.1. Potential pest impacts
3.5.1.1. Direct impacts of the pest
PFLS is a leaf and fruit spotting disease causing premature abscission of young leaves and fruit resulting in large and even complete yield loss (Seif and Hillocks, 1993). Affected fruits often show longitudinal and transversal cracks in the rind with the internal locules exposed. Juice content of diseased fruit is greatly reduced, making them unsuitable for fresh consumption or processing. Yield losses of 50–100% due to PFLS have been reported in several African countries, such as Ghana, Cameroon and Kenya, especially on susceptible host species and varieties (Brun, 1972; Seif and Hillocks, 1993; Kuate et al., 1994; Lawson et al., 2017). The disease has been considered as the most important factor limiting citrus production wherever it has been reported in tropical Africa (Seif and Hillocks, 1993; Lawson et al., 2017). PFLS is reported to be more serious at altitudes over 600 m. However, more recent studies in Ghana showed that commercial production under 200 m was also severely affected by the disease (Lawson et al., 2017).
Potential environmental consequences of the introduction of P. angolensis into the EU territory may be associated with the additional fungicide treatments required for disease control. Some of the fungicides used in the infested areas for the control of P. angolensis, like copper compounds and mancozeb, have been associated with environmental concerns (Alva et al., 1993; Houeto et al., 1995) and, in fact, the use of copper in organic production in the EU is strictly limited (Regulation EC/473/2002) to reduce environmental pollution of soil and changes in microbial communities (Zhou et al., 2011). Moreover, increasing the frequency of fungicide applications in citrus orchards may interfere with the current integrated pest management programmes aimed at reducing the use of chemical pesticides, in line with the Directive 2009/128/EC to achieve sustainable use of pesticides in the EU.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
YES, the likelihood of pest entry can be mitigated if host plants for planting and fruit are sourced from pest‐free areas or pest‐free places of production and are inspected both at the place of origin and the EU entry point. In infested areas, agricultural practices and fungicide sprays, although not fully effective, are the only options available for disease management.
3.6.1. Biological or technical factors affecting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
-
Inspection and detection are measures to prevent entry, but there are some factors that may limit their effectiveness:
-
–
Inspection difficulties due to the period of incubation (time between infection and appearance of symptoms): at optimal temperature and on detached leaves, 9–12 days are necessary for the appearance of symptoms on 50% of the leaflets, depending on the citrus species (Seif and Hillocks, 1999).
-
–
So far, harmonised molecular protocols are not available for a fast detection and identification of P. angolensis. The detection and identification of the pathogen is, so far, mainly based on cultural and morphometric characteristics, which require special expertise, as well as on general molecular methods used for the identification of fungi.
-
–
The use of crop protection products is a measure to reduce the risk of establishment, but commercial citrus crops in the EU are not always subject to regular fungicide applications.
Defining buffer zones around disease foci may reduce the spread of the pathogen. However, the effectiveness of this measure may be reduced due to the airborne dispersion of P. angolensis, particularly in EU areas with high density of citrus orchards.
3.6.2. Control methods
Cultural practices for disease management were recommended in PFLS‐affected areas to reduce inoculum in the orchards by removing affected plant tissues, to improve ventilation by increasing tree spacing, pruning and avoiding intercrops as well as synchronising fruit set by irrigation (Seif and Hillocks, 1993; Kuate et al., 1994). Several fungicide field trials for the control of PFLS on sweet orange have been conducted in Africa, although the overall performance of fungicide programs has been erratic. Reductions of 54–90% in PFLS incidence on leaves were obtained in Kenya with monthly applications of flusilazole or benomyl, from the onset of rains to 1 month prior to harvest (Seif and Hillocks, 1997). Chlorothalonil was evaluated in Ethiopia, with a 23% reduction of PFLS incidence on fruit and a threefold increase in total yield with two sprays after fruit set (Derso, 1999). Two‐week fungicide spray schedules with benomyl, difenoconazole, copper or mancozeb were also evaluated in Ethiopia with 11–43% reduction of PFLS incidence on fruit (Yesuf, 2007). Benomyl, chlorothalonil, copper and their mixtures were evaluated in Ethiopia by Kassahun et al. (2006), with 43–92% reduction of PFLS incidence on leaves. In Guinea, mixtures of mancozeb and benomyl were evaluated with increasing concentrations and spray timings, with a 41–64% average reduction of PFLS incidence on fruit (Diallo et al., 2011). Likewise, spray programs with a mixture of carbendazim and mancozeb reduced fruit drop due to PLFS by 59–99% in Ghana (Lawson et al., 2017). A mixture of trifloxystrobin + mancozeb + mineral spray oil was shown to be also effective for the control of PFLS on foliage in Zimbabwe (Pretorius and Holtz, 2008).
3.7. Uncertainty
Presence of the pest in Mozambique: Mozambique is reported in the EPPO PQR database as being infested by the pathogen. Nevertheless, no documented information was found in the literature of the pathogen being present in Mozambique.
Host range: no information was found in the literature on Poncirus, Fortunella and their hybrids being hosts of the pathogen, except for F. japonica (kumquat).
Climate suitability in the EU: climate matching suggests that P. angolensis might not be well adapted to Mediterranean climates, though it has been also reported from areas with arid desert conditions. Nevertheless, the temperature range and the humidity requirements for infection may be compatible with the climates in the citrus‐growing areas in southern EU Member States. Moreover, uncertainty exists on whether and at which extent the irrigation applied to EU citrus‐growing areas can make the microclimate in citrus orchards more favourable for P. angolensis.
Establishment: it is unknown whether cultural practices and disease control methods, currently applied in the EU, would be effective in preventing the establishment of P. angolensis.
Spread: lack of data regarding the distance the airborne inoculum of P. angolensis can travel.
Impacts: it is unknown whether agronomic practices and climatic conditions in the EU will lead to similar levels of impact as in the places of origin.
The Panel considers that these uncertainties do not affect the validity of the conclusions of this pest categorisation.
4. Conclusions (Table 7)
Table 7.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the pest is clearly defined and there are reliable methods for its detection and identification | The identity of the pest is clearly defined and there are reliable methods for its detection and identification | None |
| Absence/presence of the pest in the EU territory (Section 3.2 ) | The pest is not known to occur in the EU | The pest is not known to occur in the EU. Therefore, it does not meet this criterion to qualify as a Union RNQP | None |
| Regulatory status (Section 3.3 ) | The pest is not known to occur in the EU and is currently officially regulated on Citrus L., Fortunella Swingle, Poncirus Raf. plants and their hybrids other than seeds (Directive 2000/29/EC) | The criteria on pest presence, to be considered as potential regulated non‐quarantine pest, is not met, therefore other criteria for consideration as RNQP do not need to be assessed. | None |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | The pest could potentially enter, establish and spread in the EU. Pathways of entry:
|
The pest is not present in the EU. Therefore, other criteria for consideration as an RNQP do not need to be assessed | See uncertainties 1 to 5 |
| Potential for consequences in the EU territory (Section 3.5 ) | The introduction and spread of the pest in the EU could cause yield and quality losses in citrus production as well as environmental impacts | The pest is not present in the EU. Therefore, other criteria for consideration as an RNQP do not need to be assessed | See uncertainty 6 |
| Available measures (Section 3.6 ) | Phytosanitary measures are available to prevent the entry of the pathogen into the EU, e.g. sourcing host plants for planting and fruit from pest‐free areas or pest‐free places of production, inspection at the place of origin and the EU entry point. There are no fully effective measures to prevent establishment and spread. | The pest is not present in the EU. Therefore, other criteria for consideration as an RNQP do not need to be assessed. | See uncertainties 1 to 5 |
| Conclusion on pest categorisation (Section 4 ) | P. angolensis meets all the criteria assessed by EFSA above for consideration as a potential Union quarantine pest | P. angolensis is not known to occur in the EU. Therefore, it does not meet at least one of the criteria assessed by EFSA for consideration as a Union regulated non‐quarantine pest | None |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | The most important knowledge gap concerns the biology and epidemiology of P. angolensis with regard to the climate suitability in the EU citrus‐growing areas. Given that all the data available in the literature have been explored, the Panel considers that a full PRA is unlikely to reduce the uncertainties related to this gap in knowledge. Uncertainties can only be reduced by additional research | ||
Abbreviations
- DG SANCO
Directorate General for Health and Consumers
- IPPC
International Plant Protection Convention
- PFLS
Pseudocercospora fruit and leaf spot
- PLH
EFSA Panel on Plant Health
- TFEU
Treaty on the Functioning of the European Union
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Anton J, Miret J, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Gonzalez‐Dominguez E, Vicent A, Vloutoglou I, Bottex B and Rossi V, 2017. Scientific opinion on the pest categorisation of Pseudocercospora angolensis . EFSA Journal 2017;15(7):4883, 24 pp. 10.2903/j.efsa.2017.4883
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00298
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Gregoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Acknowledgements: The Panel wishes to thank the members of the Working Group on Agricultural Fungal Pathogens: Elisa Gonzalez‐Dominguez, Vittorio Rossi, Antonio Vicent and Irene Vloutoglou for the preparatory work on this pest categorisation, and EFSA Staff Bernard Bottex for the support provided to this scientific output.
Adopted: 24 May 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO; Figure 3: CABI
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, pp. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, pp. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, pp. 1–24.
References
- Alva AK, Graham JH and Tucker DPH, 1993. Role of calcium in amelioration of copper phytotoxicity for citrus. Soil Science, 155, 211–218. [Google Scholar]
- Bella M, Dubois C, Kuate J, Ngbwa MM and Rey JY, 1999. Sensibilité à Phaeoramularia angolensis de divers agrumes cultivés en zone forestière humide au Cameroun. Fruits, 54, 167–176. [Google Scholar]
- Brun J, 1972. Citrus leaf spot caused by Cercospora angolensis . Fruits, 27, 539–541. [Google Scholar]
- Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJM, Shin HD, Nakashima C and Groenewald JZ, 2013. Phylogenetic lineages in Pseudocercospora . Studies in Mycology, 75, 37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho T and Mendes O, 1952. Una Cercosporiose em citrinos. Notícia preliminar. Moçambique: Documentário Trimestral, 72, 5–8. [Google Scholar]
- Derso E, 1999. Occurrence, prevalence and control methods of Phaeoramularia leaf and fruit spot disease of citrus in Ethiopia. Fruits, 54, 225–232. [Google Scholar]
- Diallo MTS, 2001. Progression de la cercosporiose des agrumes (Phaeoramularia angolensis) en Guinée. Fruits, 56, 37–43. [Google Scholar]
- Diallo MTS, Camara M, Diane MY, Bah AS, Pivi AM and Traore L, 2011. Vers une lutte contre la cercosporiose des agrumes en Guinée. Fruits, 58, 329–344. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2093/j.efsa.2010.1495 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2014. Scientific opinion on the risk of Phyllosticta citricarpa (Guignardia citricarpa) for the EU territory with identification and evaluation of risk reduction options. EFSA Journal 2014;12(2):3557. 243 pp. 10.2093/j.efsa.2014.3557 [DOI] [Google Scholar]
- EPPO , 2017. EPPO Global Database (available online). https://gd.eppo.int
- Europhyt , online. The European Network of Plant Health Information System. EUROPHYT database. Available online: https://europhyt.ec.europa.eu
- EUROSTAT . http://ec.europa.eu/eurostat/web/agriculture/data/database [Accessed: 07/06/2017]
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21 – Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11 – Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf [Google Scholar]
- Houeto P, Bindoula G and Hoffman JR, 1995. Ethylenebisdithiocarbamates and Ethylenethiourea: possible human health‐hazards. Environmental Health Perspectives, 103, 568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassahun T, Temam H and Sakhuja PK, 2006. Management of Phaeoramularia fruit and leaf spot disease of citrus in Ethiopia. Agricultura Tropica et Subtropica, 39, 241–247. [Google Scholar]
- Kirk PM, 1986. Phaeoramularia angolensis. CMI Descriptions of Pathogenic Fungi and Bacteria No. 843. Mycopathologia, 94, 177–178. [Google Scholar]
- Kuate J, Bella M, Damesse F, Fouré E and Rey JY, 1994. La cercosporiose des agrumes due à Phaeoramularia angolensis. Evolution de la maladie sur fruits en zone forestière humide. Fruits, 49, 93–101. [Google Scholar]
- Kuate J, Bella M, Damesse F, Foure E and Rey JY, 1997. Évolution de la cercosporiose à Phaeoramularia angolensis sur feuilles d'agrumes en zone forestière humide du Cameroun. Fruits, 52, 297–306. [Google Scholar]
- Kuate J, Foure E, Foko J, Ducelier D and Tchio F, 2002. La phaeoramulariose des agrumes au Cameroun due à Phaeoramularia angolensis: expression parasitaire à différentes altitudes. Fruits, 57, 207–218. [Google Scholar]
- Lawson LEV, Brentu FC, Cornelius EW, Oduro KA, Sedano ME and Vicent A, 2017. Crop loss and control of Pseudocercospora fruit and leaf spot of citrus in Ghana. European Journal of Plant Pathology, 147, 167–180. [Google Scholar]
- Ndo EGD, Bella M, Ndindeng SA, Ndoumbe‐Nkeng M, Fontem AD and Cilas C, 2010. Altitude, tree species and soil type are the main factors influencing the severity of Phaeoramularia leaf and fruit spot disease of citrus in the humid zones of Cameroon. European Journal of Plant Pathology, 128, 385–397. [Google Scholar]
- Ndzoumba B, 1985. Inoculations expérimentales de Cercospora angolensis sur jeunes plantules d'agrumes. Fruits, 40, 191–195. [Google Scholar]
- Peel MC, Finlayson BL and McMahon TA, 2007. Updated world map of the Köppen‐Geiger climate classification. Hydrology and Earth System Sciences, 11, 1633–1644. [Google Scholar]
- Pretorius MC and Holtz G, 2008. Distribution and control of Pseudocercospora angolensis on citrus in Zimbabwe and Mozambique. Phytopathology, 98, S127. [Google Scholar]
- Quaedvlieg W, Groenewald JZ, Yáñez‐Morales MJ and Crous PW, 2012. DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia, 29, 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif AA and Hillocks RJ, 1993. Phaeoramularia fruit and leaf spot of citrus with special reference to Kenya. International Journal of Pest Management, 39, 44–50. [Google Scholar]
- Seif AA and Hillocks RJ, 1997. Chemical control of Phaeoramularia fruit and leaf spot of citrus in Kenya. Crop Protection, 16, 141–145. [Google Scholar]
- Seif AA and Hillocks RJ, 1998. Some factors affecting infection of citrus by Phaeoramularia angolensis . Journal of Phytopathology, 146, 385–391. [Google Scholar]
- Seif AA and Hillocks RJ, 1999. Reaction of some citrus cultivars to Phaeoramularia fruit and leaf spot in Kenya. Fruits, 54, 323–329. [Google Scholar]
- Yesuf M, 2007. Distribution and management of Phaeoramularia leaf and fruit spot disease of citrus in Ethiopia. Fruits, 62, 99–106. [Google Scholar]
- Zhou XX, He ZL, Liang ZB, Stoffela PJ, Fan JH, Yang YG and Powell CA, 2011. Long‐term use of copper‐containing fungicide affects microbial properties of citrus grove soils. Soil Science Society of America Journal, 75, 898–906. [Google Scholar]


