Abstract
Council Regulation (EC) No 1099/2009 on the protection of animals at the time of killing lists in Annex I the stunning interventions currently allowed in the EU, together with the related conditions under which those interventions can be implemented. The regulation allows the Commission to amend Annex I, listing additional stunning interventions, provided they ensure a level of animal welare at least equivalent to that ensured by the one already approved. EFSA was requested to perform such assessment with regard to the implementation of the low atmospheric pressure stunning (LAPS) system on broiler chickens. The ad hoc Working Group (WG) set up by EFSA performed the assessment in three main steps, i.e. checking the data provided against the criteria laid down in the EFSA Guidance (EFSA AHAW Panel, 2013); running an extensive literature search, followed by data extraction and performing a judgemental ranking exercise based on expert opinion. As main outcome, the LAPS intervention was found to be able to provide a level of animal welfare not lower than that provided by at least one of the currently allowed methods. The overall assessment of EFSA is valid ONLY under the technical conditions described in the submission and for broiler chickens, intended for human consumption, weighting less than 4 kg. Deviations from these conditions might have different consequences for animal welfare which were not assessed in this exercise. The LAPS method may, in addition to commercial slaughter, be suitable for depopulation, respecting the technical conditions defined in the present conclusions. The WG considers that a revision of the present version of the EFSA Guidance could be beneficial.
Keywords: broilers, stunning, animal welfare, low atmospheric pressure
Summary
Council Regulation (EC) No 1099/2009 on the protection of animals at the time of killing lists in its Annex I the stunning interventions currently allowed in the European Union (EU), together with the related conditions under which those interventions can be implemented. With the aim of constantly improving animal welfare, the Commission can amend the list of the approved methods in Annex I, taking into account scientific and technical progress. However, in order to be listed in Annex I, a new stunning intervention has to provide evidences that it ensures a level of animal welfare at least equivalent to that ensured by the currently approved methods. The European Food Safety Authority (EFSA) was requested to perform such assessment with regard to the implementation of the low atmospheric pressure stunning (LAPS) system on broiler chickens.
An ad hoc Working Group (WG) was set up by EFSA to address the Terms of Reference of the mandate received by the Commission. As a first step, the WG assessed the scientific papers and the related annexes based on the criteria described in the EFSA Guidance (EFSA AHAW Panel, 2013). The outcome was that, individually, no paper was able to pass the criteria. Nevertheless, the most relevant data and information on the stunning methodology under evaluation were provided, although distributed over the different scientific papers. For this reason, the WG decided to evaluate the data and information distributed over the different scientific papers as a unique set of evidences. However, some important aspects, considered crucial for the welfare assessment, were not available in the dossier from the applicant. EFSA, therefore, requested the applicant to provide an additional set of data and statistical analysis as well as access to the raw data underpinning the scientific publications. The most critical phase of the assessment was to compare the LAPS method with the existing stunning interventions, in terms of impact on animal welfare, with a quantitative approach. In fact, an Extensive Literature Search followed by data extraction was performed, but it was not possible to retrieve quantitative data (i.e. quantitative parameters to assess the welfare implications associated with the interventions) from stunning interventions other than LAPS. This is partly due to the fact that the stunning methods currently available in EU were approved before the publication of the EFSA Guidance (EFSA AHAW Panel, 2013) and partly because, to a certain degree, recognised standards of animal welfare are still lacking. Therefore, the EFSA WG undertook another approach, based on expert opinion. As a first step, the WG experts identified the main hazards related to each stunning intervention, i.e. electrical water‐bath, gas stunning methods, excluding hypoxia induced with inert gases, and LAPS. A pool of field experts, with different background and responsibilities, was set up and asked to rank these hazards in terms of impact on animal welfare.
The LAPS procedure, leads to loss of consciousness followed by death in all birds. The LAPS procedure does not induce immediate unconsciousness. During the first 50 s of the LAPS procedure the broiler chickens are likely to fall into a state of drowsiness. When oxygen concentration drops to a low level (about 7% atmospheric equivalent), the broilers show electroencephalography (EEG) signs of arousal, indicating capacity to experience potential aversive stimuli (on average at 50 s from the start of the LAPS process). The mean time to induction of unconsciousness, based on the mean time to loss of posture, as a proxy, varies between 58 and 80 s in different studies.
As main conclusion, the LAPS intervention was found to be able to provide a level of animal welfare at least equivalent to that provided by at least one of the currently allowed methods.
It is important to stress that this assessment was performed under the conditions described in the submitted dossier and, for this reason, its conclusions are valid ONLY under those conditions, i.e. (i) the technical specifications (e.g. rate of decompression, duration of each phase, total exposure time); (ii) the animal characteristics (e.g. broiler chickens weighting less than 4 kg, dry vs wet chickens) and (iii) the ambient conditions (e.g. temperature, humidity). Deviation from the conditions might have different consequences for animal welfare which were not assessed in this exercise and will need a dedicated assessment.
Considering the important lack of comparable data on the stunning interventions other than LAPS, EFSA recommends dedicated studies to be performed to enable a proper assessment in case the EC needs more support from EFSA on this subject. The emergency procedures associated with system failures should be included by the manufacturer in the manufacturer's instructions for the use of the equipment and Food Business Operators should follow the manufacturer's instructions and include them in the standard operating procedures. Finally, based on the evidences provided, the LAPS method may, in addition to commercial slaughter, be suitable for depopulation of farms, respecting the technical conditions defined in the present conclusions.
1. Introduction
1.1. Background and terms of reference as provided by the requestor
Council Regulation (EC) No 1099/2009 on the protection of animals at the time of killing defines “stunning” in Article 2(f) as “any intentionally induced process which causes loss of consciousness and sensibility without pain, including any process resulting in instantaneous death”. Annex I of the Regulation lists the stunning interventions and related specifications. Article 3(1) of the Regulation on the general requirements for killing and related operations requires that animals shall be spared any avoidable pain, distress or suffering during their killing and related operations. Article 4 on stunning interventions regulates that “animals shall only be killed after stunning in accordance with the methods and specific requirements related to the application of those methods set out in Annex I of the Regulation” and “that the loss of consciousness and sensibility shall be maintained until the death of the animal”. Article 4(1) requires that Article 4(2) of this Regulation allows the Commission to amend Annex I to this Regulation as to take into account scientific and technical progress on the basis of an opinion of the EFSA. Any such amendments shall ensure a level of animal welfare at least equivalent to that ensured by the existing methods.
At present, low atmospheric pressure systems are not allowed for stunning poultry.
In 2013, the Commission received a request from a private business operator to allow the use of a low atmospheric pressure system for stunning poultry. The EFSA in its opinion of 2013 considered that the assessed studies did not pass the eligibility criteria and, therefore, no further assessment was undertaken.
In 2016, the Commission received a series of publications and further technical information from the same operator in order to obtain a full assessment of the method. To reply to this request, the Commission requested the EFSA to review the scientific publications provided and possibly other sources if available and assess to what extent the system proposed for stunning poultry is able to provide a level of animal welfare at least equivalent to that ensured by the currently allowed methods and, in case of a favourable reply, under which conditions.
The Terms of Reference are as follows:
The scope of this request is limited to the stunning of broiler chicken for slaughter (i.e. killing for human consumption).
-
EFSA will give its view on the scientific and technical information with a focus on the following issues:
-
1
1 ‐ the extent to which the use of a low atmosphere pressure system is, in principle, an acceptable method for the stunning of broiler chicken meeting the requirements of Article 3(1) and Article 4(1) of Council Regulation (EC) No 1099/2009;
-
2
2 ‐ the extent to which the findings are consistent with other sources of information;
-
3
3 ‐ requirements attached to the use of a low atmosphere pressure system;
-
4
4 ‐ the extent to which the findings may be valid under commercial conditions in the EU.
-
1
1.2. Interpretation of the Terms of Reference
Considering the background and the Terms of Reference as provided by the European Commission, this assessment of EFSA:
Is based on the documents provided by the applicant, and will focus on broiler chickens only;
-
Will evaluate to what extent the use of a low atmospheric pressure system is, in principle, an acceptable method for the stunning of broiler chickens, meeting the following two criteria of Council Regulation (EC) 1099/2009:
-
–
Article 3(1): Animals shall be spared any avoidable pain, distress or suffering during their killing and related operations.
-
–
Article 4(1): Animals shall only be killed after stunning in accordance with the methods and specific requirements related to the application of those methods set out in Annex I. The loss of consciousness and sensibility shall be maintained until the death of the animal. The methods referred to in Annex I which do not result in instantaneous death (hereinafter referred to as simple stunning) shall be followed as quickly as possible by a procedure ensuring death [redacted];
-
–
-
Will evaluate to what extent the system proposed for stunning poultry is able to provide a level of animal welfare at least equivalent to that ensured by the methods currently allowed in the European Union (EU);
The evaluation will be conducted following the approach as outlined in the EFSA guidance on the assessment criteria for studies evaluating the effectiveness of stunning interventions regarding animal protection at the time of killing (EFSA AHAW Panel, 2013).
It has to be noted that the EFSA guidance covers only part of the assessment as required by the European Commission and further steps will be needed to ascertain equivalence. Further details can be found in the methodological section of this Scientific Opinion.
The basis of the evaluation is represented by the set of 5 published scientific papers and related annexes provided to EFSA by the applicant (Data Part 1, hereinafter). This first set of data and information was eventually supplemented with additional details and analysis following a specific EFSA request (Data Part 2, hereinafter).
Will detail under which technical conditions the intervention that shall be performed; should the level of animal welfare provided by the low atmospheric pressure stunning (LAPS) be at least equivalent to that ensured by the currently allowed methods;
Will evaluate to which extent the findings are consistent with other sources of information; by means of an extensive literature search and review of this literature, including the scientific papers published on the LAPS itself and submitted on the occasion of the first application;
Will consider potential requirements linked to the use of a low atmosphere pressure system by involving experts from different domains, i.e. engineering and/or physics, should this be required;
Will evaluate to which extent the findings may be valid under commercial conditions in the EU.
1.3. Additional information
In the course of the assessment process, as defined in the EFSA guidance, it appeared that some of the information, as provided by the applicant, was not sufficiently detailed to give a clear picture of the potential welfare concerns. In addition, some inconsistencies in the terminology used to define the different behaviours recorded in the ethogram were also detected.
For these reasons, and with the specific aim of gathering all necessary information and data to assess the exact sequence of the events during the LAPS process, EFSA asked the applicant for additional data and information. See Section 2.1 for more information.
2. Data and methodology
2.1. Data
The applicant provided EFSA with five scientific studies, either already published or submitted for publication. During the assessment process, all papers were accepted for publication. It was decided by EFSA to consider only the final version of the five studies, updating the evidences provided originally in the dossier with the new information contained in the published papers. The list of the five scientific papers can be consulted in Table 1.
Table 1.
List of papers submitted to EFSA
| ID | Author | Title | Journal | Status |
|---|---|---|---|---|
| Paper 5a | Nikki Mackie, Dorothy E. F. McKeegan | Behavioural responses of broiler chickens during low atmospheric pressure stunning | Applied Animal Behaviour Science 174 (2016) 90–98 | Published |
| Paper 6 | Jessica E. Martin, Karen Christensen, Yvonne Vizzier‐Thaxton, Dorothy E. F. McKeegan | Effects of analgesic intervention on behavioural responses to Low Atmospheric Pressure Stunning | Applied Animal Behaviour Science 180 (2016) 157–165 | Published |
| Paper 7 | Jessica E. Martin, Karen Christensen, Yvonne Vizzier‐Thaxton, Malcolm Mitchell, Dorothy E. F. McKeegan | Behavioural, brain and cardiac responses to hypobaric hypoxia in chickens | Physiology and Behaviour 163 (2016) 25–36 | Published |
| Paper 8 | Jessica E Martin, Karen Christensen, Yvonne Vizzier‐Thaxton, Dorothy E. F. McKeegan | Effects of light on responses to low atmospheric pressure stunning in broilers | British Poultry Science 57 (2016) 585–600 | Published |
| Paper 9 | Paul H. Holloway, David G. Pritchard | Effects of ambient temperature and water vapor on chamber pressure and oxygen level during low atmospheric pressure stunning of poultry | Poultry Science (2017) 0 1–12 | Epub ahead of print |
Papers 1–4 are the ones submitted in the first application and are considered, in this assessment, under Section 3.3.
The five scientific papers were accompanied by some annexes reporting additional information, including a self‐evaluation performed by the applicant on the five studies based on the criteria listed in the EFSA guidance (EFSA AHAW Panel, 2013).
As stated above (Section 1.3) EFSA asked the applicant for additional material. In detail, EFSA requested:
A revised description of the following behaviours: ataxia, loss of posture, clonic convulsions (i.e. wing flapping), lying, motionless, head shaking, open bill breathing, jump, escape. The revised description was requested to be unambiguous and harmonised across the different studies and accompanied by a sound discussion on the relevance of those behaviours for animal welfare regarding pain, distress and suffering;
A more appropriate (for the scope of this assessment) statistical analysis, together with the raw data at individual bird level;
Some complementary information about the electroencephalography (EEG) parameters reported in the papers. In detail: onset and offset of high amplitude, low frequency (HALF) electrical activity (if observed), time of onset and offset of convulsions, onset of EEG suppression, time to the loss of somatosensory evoked potentials (SEP) and onset of isoelectric EEG;
Any additional available data/information on organ lesions recorded after the stunning process (necropsy).
These requests of EFSA were explained in detail in a subsequent web‐conference held with the applicant.
In response to this request, the applicant provided EFSA with the following additional data and information:
A set of EEG and electrocardiography (ECG) raw traces (ASCII comma delimited text files) from birds undergoing LAPS in papers 7 and 8;
A set of video footages of some birds undergoing the LAPS process (observed by infrared camera) from papers 5 and 6;
A final set of behavioural indicators, selected by the applicant as relevant for an understanding of animal welfare in the context of LAPS, together with a definition and a suggested interpretation for each of those behaviours;
Standard Kaplan–Mayer survival analysis, comparative survival analysis and survival analysis corrected for the individual timing of loss of posture on behavioural, EEG and ECG data;
The results of an ad hoc study which produced necropsy data from birds subjected to LAPS. The study (Experiment 2017) was performed by comparing a group of animals undergoing LAPS with a group of birds euthanised with barbiturate.
A set of raw data at bird level (‘csv’ and ‘xlsx’ format) from papers reported in Papers 6, 7 and 8. Data from Paper 5 were not included and the applicant justified this choice stating that the data from Papers 6, 7 and 8 were their ‘most accurate and detailed data’. It has also to be noted that the data set provided has 202 records (i.e. 202 strings of information, each string related to one bird for a total of 202 broilers). The theoretical total number of records should have been 220 (90 broilers from study 6 and 7 and 40 broilers from Paper 8). No justification was explicitly provided for this discrepancy (202 instead of 220), but it was assumed that the 18 missing broilers were the ones that ‘went out of sight’ for a time period above the threshold set by the scientists;
A technical description of the statistical models used to analyse the data from Papers 6, 7 and 8;
A discussion on the methodology for the assessment of consciousness.
A discussion on the rationale for the chosen analgesic treatment in Paper 6.
2.2. Methodology
2.2.1. EFSA guidance on the assessment criteria for studies evaluating the effectiveness of stunning interventions regarding animal protection at the time of killing (EFSA AHAW Panel, 2013)
The first part of the assessment process involved checking the submitted documentation against the criteria laid down in the EFSA guidance (EFSA AHAW Panel, 2013).
If the criteria regarding eligibility, reporting quality and methodological quality are fulfilled, i.e. the study on the new method provides sufficient detail regarding the intervention to make conclusions about the suitability (or lack thereof) of the intervention, a full assessment of the animal welfare implications would be carried out at the next level of the assessment.
In case the criteria are not fulfilled, the assessment report has to highlight the shortcomings and indicate where improvements are required before the study can be further assessed.
It should be noted that the EFSA Guidance is applicable to the individual studies. As mentioned in Section 2.1, the Working Group (WG) experts identified some lack of information and inconsistencies in each of the submitted papers. As a consequence, considering these limitations with regard to at least one of the criteria laid down in the guidance (see following sections), strictly speaking none of them would have passed the first assessment phase. Therefore, as explained above, the assessment process should have stopped; and EFSA would then have indicated the required improvements. However, the WG experts and the AHAW Panel agreed that, to promote development of potential improvements of existing methods or encourage innovation leading to the introduction of improved stunning methods, the WG would assess the entire information combined, provided as a unique set of evidences originating from the combination of the different submitted papers, rather than applying the criteria to the individual studies. Additionally, the AHAW Panel and the WG experts decided to ask the applicant for additional data in order to perform ad hoc analyses (e.g. EEG quantitative analysis, extensive literature search, expert opinion elicitation, statistical analysis) with the aim of improving clarity of understanding and to facilitate the assessment.
2.2.1.1. Eligibility criteria
The eligibility criteria, as described in the EFSA guidance concerning the modified atmosphere stunning intervention and more precisely the low atmosphere pressure methods (EFSA AHAW Panel, 2013 – Section 3), comprise technical information and information on the outcome of the intervention, here below briefly reported.
Regarding the technical information, the data required pertain to:
Animal stocking density
Duration of the intervention
Rate of decompression
Rate of changes in partial pressure of oxygen
Temperature/humidity/illumination of the chamber
Maximum stun‐to‐stick/kill interval(s)
Calibration of the LAPS equipment and monitoring system
Regarding the outcome of the intervention, the information needed by EFSA to ascertain if the animal welfare requirements are fulfilled pertains to:
-
Onset of unconsciousness and insensibility, by means of:
-
–
Electroencephalogram
-
–
appearance of slow waves,
-
–
reduction of EEG total power content to less than 10% of the pre‐stun EEG power content,
-
–
abolition of evoked electrical activity in the brain
-
–
-
–
Arterial partial pressure of blood oxygen or pulse oximetry (in addition to EEG)
-
–
Ethogram
-
–
loss of posture
-
–
-
–
Other parameters
-
–
dilated pupils,
-
–
absence of palpebral, corneal and pupillary reflexes,
-
–
apnoea,
-
–
relaxed body/lack of muscle tone
-
–
absence of response to painful stimuli
-
–
-
–
-
Absence of pain, distress and suffering until the loss of consciousness and sensibility by means of combined indirect animal‐based measures, such as:
-
–
Behavioural responses (vocalisation, posture and movements, general behaviour)
-
2
AND
-
–
Physiological responses (hormone concentrations, blood metabolites, autonomic responses)
-
4
OR
-
–
Neurological response (brain activity)
-
–
Duration of unconsciousness and insensibility
The relevant data and information were extracted from the evidence provided in the dossier and the results can be consulted in Section 3.1.1.
2.2.1.2. Reporting Quality
Once the eligibility assessment was performed, the dossier underwent the reporting quality assessment. As recommended in the EFSA guidance, the parameters to be taken into consideration for this step are the outcome of a review and adaptation of the parameters from the checklists of the REFLECT1 and STROBE2 statements (see summary in Table 2).
Table 2.
Parameters used to assess the reporting quality of studies on stunning interventions, per section of the study report
| Parameter | Description |
|---|---|
| Introduction | |
| Background and rationale | |
| Objective | |
| Materials and methods | |
| Study population | |
| Number of animals (sample size) | |
| Intervention | |
| Outcome | |
| Bias and confounding | |
| Blinding (masking) | |
| Statistical methods | |
| Results | |
| Numbers analysed | |
| Outcomes and estimations | |
| Adverse events | |
| Ancillary analyses | |
| Discussion | |
| Key results and interpretation | |
| External validity | |
| Other | |
| Funding | |
As explained in the EFSA guidance, the reporting quality of the dossier submitted had to be evaluated against the criteria described in Table 2, but the decision whether the overall reporting quality was sufficient was finally based upon the experts’ judgement.
It is important to record that the applicant specified that all the studies were conducted using the ARRIVE3 guidelines as in the applicant's opinion, they were better suited to experiments relating to assessment of stunning effectiveness than the STROBE and REFLECT statements. This element has to be evaluated in case the AHAW Panel decides to revise and update the existing EFSA guidance, with the purpose to ensure that the information conforms to international reporting guidelines (i.e. as outlined in the Equator Network.4 ARRIVE is among these, specifically focusing on animal pre‐clinical studies).
2.2.1.3. Methodological quality
As outlined in the EFSA guidance, the methodological quality of a research study and related information will be determined by assessing its precision and its internal and external validity. These elements are related to the extent to which the study design, implementation, data acquisition, analysis and interpretation of results:
minimise systematic errors (biases) that compromise the study's internal validity;
minimise random errors that reduce the precision of the measurements made in the study;
allow broad applicability of the results beyond any single study (i.e. external validity).
The methodological quality criteria assessment in the EFSA guidance focuses on elements in the report that allow the assessment of the internal validity of the individual submitted papers. The parameters to be evaluated for each paper are selection bias, attrition bias, performance bias and confounding (see EFSA guidance for more details).
Appraisal of a study's external validity (i.e. its applicability beyond the study population) requires that its results are compared with those of comparable studies. As the present EFSA guidance is only applicable to individual studies, assessing the external validity of those studies exceeds its mandate. The following section (Section 2.2.2) aims to cover the present lack of standards for assessing the external validity of the studies provided, i.e. to what extent the system proposed for stunning broilers can provide a level of animal welfare at least equivalent to that ensured by the currently allowed methods.
2.2.2. Assessment of the level of animal welfare provided by the LAPS
After eligibility, reporting quality and methodological quality have been assessed, the results provided are analysed in detail regarding the animal welfare implication of the LAPS (external validity).
One of the main aims of this assessment was indeed to establish whether the LAPS fulfils the requirements of the relevant EU regulation, and whether the level of animal welfare provided is at least equivalent to that ensured by the methods currently allowed in the EU. At first, EFSA undertook a full quantitative analysis, as described in the next section (see Section 2.2.2.1), aiming at comparing LAPS with the currently available stunning methods on the basis of quantitative parameters (e.g. time to onset of welfare indicators, proportion of birds showing a given behaviour, etc.). It emerged clearly, however, that the data needed to perform such a quantitative assessment are not fully available, as explained further. Consequently, a different approach was explored based on expert knowledge, as described in Section 3.2.2.
2.2.2.1. Quantitative assessment
The first step was to identify the words used in the background and the terms of reference, as provided by the European Commission, and to give each key word a clear definition as regards the understanding of animal welfare issues potentially related to stunning procedures. For the purposes of this assessment, the key words identified as relevant to animal welfare were:
pain,
distress,
suffering,
loss of consciousness,
loss of sensibility and
onset death
(referred to as Welfare Outcomes hereafter). An additional term was considered crucial to the full application and the interpretation and assessment: the term avoidable (also in the mandate from the European Commission), with reference to the potential pain, distress and suffering. All proposed definitions can be found in Section 3.2 (Table 4).
Table 4.
Definition of the relevant terminology having an impact on the assessment (welfare outcomes)
| Definition | Source | |
|---|---|---|
| Terminology | ||
| Pain | An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Pain may be caused by tissue lesions or by mechanical, chemical or thermal stimulation | The International Association for the Study of Pain (IASP) |
| Distress | An aversive, negative state in which coping and adaptation processes fail to return an organism to physiological and/or psychological homoeostasis |
Carstens E, Moberg GP, 2000. Recognizing pain and distress in laboratory animals. ILAR Journal, 41, 62–71. Moberg GP, 1987. Problems in defining stress and distress in animals. Journal of the American Veterinary Medical Association, 191, 1207–1211. NRC (National Research Council), 1992. Recognition and Alleviation of Pain and Distress in Laboratory Animals. National Academy Press, Washington, DC. |
| Suffering | An unpleasant or aversive experience associated with the perception of harm or threat of harm against the physiological or psychological integrity of an individual | Animal pain: Identifying, understanding and minimising pain in farm animals, INRA, 2009 |
| Loss of Consciousness | Unconsciousness is a state of unawareness (loss of consciousness) in which there is temporary or permanent damage to brain function and the individual is unable to perceive external stimuli (which is referred to as insensibility) and control its voluntary mobility and, therefore, respond to normal stimuli, including pain (EFSA, 2004) | EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2013. Scientific Opinion on monitoring procedures at slaughterhouses for poultry. EFSA Journal 2013;11(12):3521, 65 pp. https://doi.org/10.2903/j.efsa.2013.3521 |
| Loss of sensibility |
See definition of unconsciousness: ‘inability to perceive external stimuli’ – ‘According to the Regulation 1099/2009, the sensibility of an animal is essentially its ability to feel pain. In general, an animal can be presumed to be insensible when it does not show any reflexes or reactions to stimuli such as sound, odour, light or physical contact’ In this specific context of a methodology for stunning and killing animals, there is no reason to distinguish between loss of sensibility and loss of consciousness |
EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2013. Scientific Opinion on monitoring procedures at slaughterhouses for poultry. EFSA Journal 2013;11(12):3521, 65 pp. https://doi.org/10.2903/j.efsa.2013.3521 Council Regulation (EC) No 1099/2009 on the protection of animals at the time of killing |
| Death | A physiological state of an animal, where respiration and blood circulation have ceased as the respiratory and circulatory centres in the Medulla Oblongata are irreversibly inactive. Due to the permanent absence of nutrients and oxygen in the brain, consciousness is irreversibly lost. In the context of application of stunning and stun/kill methods, the main clinical signs seen are the absence of respiration (and no gagging), absence of pulse and absence of corneal and palpebral reflex and presence of pupillary dilatation | Opinion of the Scientific Panel on Animal Health and Welfare on a request from the Commission related to welfare aspects of the main systems of stunning and killing the main commercial species of animals. EFSA Journal 2004; 45, 1–29. |
The EFSA guidance provides a non‐exhaustive set of welfare indicators that could be recorded and reported by applicants when submitting a dossier related to any new stunning methodology to be evaluated (or related to a modification to one of the currently allowed stunning methods). The applicant provided EFSA with a set of behavioural welfare indicators as proposed in the guidance, selecting the ones considered relevant and informative for a welfare assessment. Each behavioural welfare indicator was accompanied by a proposed interpretation in terms of welfare implications. The set of welfare indicators and interpretations proposed by the applicant can be found in Section 3.2.1.1 (Table 5).
Table 5.
Set of behavioural welfare indicators as provided by the applicant. The first column lists the behavioural welfare indicators selected and recorded by the applicant. The second column reports the definition and interpretation of the indicators provided by the applicant. The third column reports EFSA's comments. The last column associates each welfare indicator with the relevant welfare outcome
| Applicant's definition and interpretation | EFSA's comments | Welfare outcomesa | |
|---|---|---|---|
| Behavioural indicators | |||
| Ataxia | Reflects the start of the process of loss of consciousness. Welfare impact of ataxia during LAPS is low, as its duration is relatively short and the birds may be considered not to be fully conscious at this time, however there are likely to be sensations of disorientation during ataxia which may be unpleasant |
Defined as an ‘inability to coordinate muscle activity during voluntary (i.e. conscious) movement; most often results from disorders of the cerebellum or the posterior columns of the spinal cord; may involve the limbs, head, or trunk’b In agreement with applicant definition |
Distress and/or Suffering |
| Clonic/Tonic convulsions | Never before LOP. Minimal relevance to welfare |
Clonic convulsion is defined as a convulsion in which the contractions are intermittent, the muscles alternately contracting and relaxingc Tonic convulsion is defined as convulsion with sustained muscle contractiond Not relevant for welfare as occurring only after LOP |
Loss of Consciousness and Sensitivity |
| Wing flapping | Included in clonic convulsions | – | – |
| Deep inhalation |
Deep non‐rhythmic inspiration with bill open, may be accompanied by extension of the neck Deep inhalation has also been termed ‘gasping’ and relates to the birds taking (usually single) deep breaths, often with neck extension. We consider this behaviour to reflect dyspnoea and probably air hunger, and thus reduced welfare during stunning Deep inhalation has been particularly associated with hyperventilation during CO2 stunning, but it is also seen with inert gases (e.g. McKeegan et al., 2007) and after electrical stunning (Verhoeven et al., 2015). We note that during LAPS, as in CO2 stunning, this behaviour continues after loss of posture indicating that consciousness is not required for its performance (Verhoeven et al., 2014). There is evidence that some dyspnoea occurs in all control atmosphere stunning (CAS) mixtures that have been investigated, including inert gases, however behavioural responses reflecting these have been variously described as ‘gasping’, ‘deep breathing’, ‘respiratory disruption’ (e.g. Gerritzen et al., 2004; Abeyesinghe et al., 2007; McKeegan et al., 2007) making direct comparison with our two behavioural categories more difficult. However, the numbers of bouts of deep inhalation seen during LAPS are in the range of previously reported equivalent values for CAS. In general, we consider that open bill breathing and deep inhalation relate to dyspnoea and associated reduced welfare during hypoxia, as opposed to being responses to reduced atmospheric pressure |
In agreement In agreement The evidences provided are misleading because McKeegan et al. report deep inhalation to occur mainly in birds that were mostly exposed to CO2 (in agreement with Gerritzen et al., 2004) and more importantly Gerritzen et al. did not investigate effect of hypoxia to make direct comparison with LAPS EFSA's interpretation is that: (i) the cause of respiratory distress during LAPS is likely to be air hunger; (ii) the cause of respiratory distress during CAS is hypercapnia or hypercapnic hypoxia; (iii) this behaviour has not been described when birds were exposed to hypoxia only. For example, McKeegan et al., (2007) reported 1 or 2 breathing patterns similar to this when chickens were exposed to hypoxia when compared with 9–16 in chickens exposed to CO2 mixtures Deep inhalation after the electrical water‐bath stunning is the evidence of poor stunning or recovery of consciousness, unless ‘deep breathing’ here refers to agonic gasping |
Distress and/or Suffering if it occurs before loss of consciousness |
| Head shaking | Less preferred/novel environment. Awareness by the birds of atmospheric pressure reduction and/or reducing oxygen concentration while conscious. May also relate to auditory stimulation from increased noise levels in the chamber. Reduced welfare during LAPS. Seen in sham LAPS treatments |
In addition: possible reaction of the birds in response to pressure reduction recorded by the cochlea and to lack of humidity (dryness of the nasal mucosae) Based on the video footage: birds started headshaking just after the fogging cleared in the chamber (no more humidity) |
Distress and/or Suffering |
| Jump/Jumping | Jumping was generally seen after the onset of ataxia and did not seem to be an entirely voluntary behaviour. The explosive upward movement we termed ‘jump’ appeared to be related to attempts to regain posture during ataxia and may have be caused by involuntary muscle contractions (myoclonic jerks) as loss of muscle control progressed |
Because jumping occurs mainly prior to loss of consciousness, it has implications on animal welfare The wording is ambiguous or imprecise. A movement cannot be partly voluntary: either it is voluntary or it is not. The difficulty might be in differentiating between voluntary and involuntary. An attempt to regain posture cannot be caused by involuntary muscle contractions Based on the above and considering that during ataxia the animals are conscious, ‘jump’ is a behaviour of welfare concern |
Distress and/or Suffering |
| Loss of posture | During LAPS, we considered loss of posture to be the earliest indicator of potential loss of consciousness | In agreement | Loss of Consciousness and Sensitivity |
| Lying | Lying was adopted after loss of posture, and was never seen prior to this. It reflects the inability of the animal to control its posture and ventral, lateral and dorsal lying was observed | In agreement | Loss of Consciousness and Sensitivity |
| Motionless | Motionless refers to a limp carcass with the bird being completely still including the cessation of visible breathing movements; it reflects complete and irreversible loss of muscle tone. We considered motionless to confirm the non‐recovery state induced by LAPS |
In agreement Broadly accepted as indicator of death |
Death |
| Open bill breathing | Rhythmic breathing with an open bill (distinguished from panting by the fact that the tongue did not protrude) was routinely seen during LAPS and is a response to hypoxia | Open bill breathing has never been reported as a response to hypoxia (no reference provided), but rather to hypercapnia (Gerritzen et al., 2000) | Distress and/or Suffering if it occurs before loss of consciousness |
Green = no welfare concern; orange = some degree of welfare concern; red = serious welfare concern (not present in the table).
Farlex Partner Medical Dictionary © Farlex 2012 (<a href=“http://medical-dictionary.thefreedictionary.com/ataxia”>ataxia</a>)
Farlex Partner Medical Dictionary © Farlex 2012 (<a href=“http://medical-dictionary.thefreedictionary.com/clonic+convulsion”>clonic convulsion</a>)
Farlex Partner Medical Dictionary © Farlex 2012 (<a href=“http://medical-dictionary.thefreedictionary.com/tonic+convulsion”>tonic convulsion</a>)
Regarding the physiological responses, a set of ECG traces were provided to EFSA and analytical attention was mainly focussed on heart rate variability as a welfare indicator (in terms of stress leading to an increase of the heart rate and to a decrease of the heart rate variability (HRV)). The data on HRV were not explicitly presented in the dossier and EFSA tentatively analysed the ECG traces with the aim of gathering useful information. In relation to the neurological responses, a set of EEG traces were provided by the applicant, and the EFSA, based on the quality of the material, was able to identify two indicators which allowed conclusions to be made on the welfare status of the animals: (i) a reduction of the total power content to less than 10% of the pre‐stunning value (i.e. recorded prior to the intervention), as suggested in the EFSA guidance and (ii) a decrease of the F50 (i.e. frequency splitting EEG power spectrum into two equal parts) compared to the pre‐stunning level (see Table 16).
Table 16.
Set of neurological welfare indicators as provided by the applicant. The first column lists the neurological welfare indicators selected and recorded by the applicant. The second column reports the definition and interpretation of the indicators provided by the applicant. The third column reports EFSA's comments. The last column associates each welfare indicator with the relevant welfare parameter
| Applicant's definition and interpretation | EFSA's comments | Welfare outcomesa | |
|---|---|---|---|
| Neurological indicators | |||
| < 10% of pre‐stun EEG Power | In accordance with Raj (2006) and Lukatch et al. (1997), 10% or less of total pre‐stun EEG power content in three consequent one‐second epochs is an indicator of isoelectric EEG. Isoelectric EEG is interpreted as an indicator of an unrecoverable, definitely unconscious state that leads to death | Unfortunately, because of a strong equipment background noise in many records, the power of EEG signal does not fall below the threshold of 10% of the pre‐stun EEG power at the end of stunning procedure (i.e. 280 s and this criterion is difficult to apply without additional assumptions. For this reason, the estimated background noise power was subtracted from the signal power, assuming stationarity of the background noise | Loss of Consciousness and Sensitivity |
| Reaching by F50 thresholds of the state of non‐responsiveness (F50 < 12 Hz) and general anaesthetic plane (F50 < 7 Hz) | Following article Sandercock et al. (2014, abstract), Martin et al. (2016) and PhD thesis of J.E. Martin (2015), F50a has been compared with three previously validated thresholds: < 14 Hz – sedation; < 12 Hz – non‐responsive to toe pinch after rapid anaesthetic ‘knock down’; and < 7 Hz – surgical plane of general anaesthesia. More precisely, Sandercock et al. determined sedation threshold as 14 ± 4 Hz and general anaesthesia threshold as 7 ± 2 Hz (for chickens, Mean ± SD) | An increase of F50a is produced by an increase of high‐frequency brain activity usually associated with arousal and excitement, and/or decrease of slow‐wave activity, the latter usually linked with sleep and drowsiness. However, at the late stages of LAPS, due to general degradation of EEG power at all frequencies, the equipment high‐frequency noise becomes dominant, shifting the F50 to higher frequencies. Thus, this indicator is reliable only when EEG amplitude essentially exceeds background noise amplitude | Pain and/or Distress and/or Suffering |
F50 = median frequency that splits power spectrum in two equal parts.
Green = no welfare concern; orange = some degree of welfare concern (absent in this table); red = serious welfare concern.
Based on the definition and interpretation of the recorded behaviours provided by the applicant, and confirmed by the available scientific literature, in the second step EFSA classified them as indicators of distress and suffering rather than pain or unconsciousness and death. The outcome of this exercise can be found in Sections 3.2.1.1, 3.2.1.2 and 3.2.1.3 (Tables 5, 15 and 16).
Table 15.
Set of physiological welfare indicators as provided by the applicant. The first column lists the physiological welfare indicators selected and recorded by the applicant. The second column reports the definition and interpretation of the indicators provided by the applicant. The third column reports EFSA's comments. The last column associates each welfare indicator with the relevant welfare parameter
| Applicant's definition and interpretation | EFSA's comments | Welfare outcomesa | |
|---|---|---|---|
| Physiological indicators | |||
| Increase in heart rate variability | NA | A stress response in birds is manifested by an increase in HR and a decrease in HRV in all frequency bands (Carravieri et al., 2016). Thus, one can predict that HRV in LAPS will go down. However, even if it was not analysed in the Paper 7 of the applicant, it is visible in Figure 5 that arrhythmia is observed after LAPS application. It is known that the autonomous nervous system (ANS) that controls the heart also controls breathing. Thus, the observed HRV can be interpreted not as a stress response, but as a respiratory arrhythmia. Taking into account highly abnormal respiratory conditions in LAPS, the heart rate measurements can be strongly biased and can't be used as reliable indicators | Pain and/or Distress and/or Suffering |
| Decrease in heart rate variability | NA | (see above) | Loss of Consciousness and Sensitivity |
Green = no welfare concern; orange = some degree of welfare concern (absent in this table); red = serious welfare concern.
Once the association between the welfare indicators and the welfare outcomes was established, EFSA collected relevant quantitative data (third step) as a basis for a comparison with the currently allowed stunning methods. In detail, for each item of each group of welfare indicators (behavioural, physiological and neurological response), EFSA gathered three main quantitative parameters from the evidence provided by the applicant: the fraction of birds affected, the latency (time to onset of the welfare indicator) and the total duration of the response.
In further detail, the main descriptive statistics related to the behavioural welfare parameters of LAPS were extracted from the outcomes of the survival analysis performed by the applicant following the specific request of EFSA (see results in Section 3.2.1.1). Regarding the physiological response, the ECG traces provided by the applicant were analysed using custom‐written Matlab scripts and the RHRVTool 0.98 for Matlab. The outcomes of this analysis can be seen in Section 3.2.1.2. In relation to the neurological response, the EEG traces were processed and analysed with the help of EEGLAB 14.0.0 package for Matlab (see results in Section 3.2.1.3).
Further to the extraction of the main statistical parameters as described above, to permit better understanding of the sequence of the events during the LAPS intervention, the information originating from the ethogram, the ECG and the EEG traces were combined at the individual bird level. The aim was to explore the potential to understand how the different behaviours (e.g. loss of posture) were correlated with the changes occurring in the ECG and the EEG, but also, conversely, the potential to better understand a specific behaviour, given the information recorded in the ECG and/or the EEG. The outcome considerations, based on the quality of the submitted data, are described in Section 3.2.1.4.
As a fourth step, by means of an Extensive Literature Search, EFSA aimed at gathering values/figures, for all of the quantitative parameters identified for each welfare indicator reported for the LAPS, originating from scientific studies performed on other stunning methods for broiler chicken currently available in the EU (see Appendix E).
2.2.2.2. Qualitative assessment and hazard ranking
As explained further in the document (see Section 3.2.1.5), it was not possible to perform a full quantitative comparison between the LAPS and the currently allowed methods (i.e. gas stunning methods, either with CO2, inert gases or a mixture of CO2 and inert gases, and electrical water‐bath stunning). Therefore, another approach had to be adopted.
As a first step, EFSA identified three main phases of the stunning procedure, common to all methods. These are:
Prestunning phase (preparation of animals for stunning)
-
Stunning phase, subdivided into an
-
–
Induction phase (the period from the start of the intervention until the onset of loss of consciousness) and
-
–
Unconsciousness phase (the period between the onset of loss of consciousness until the killing intervention)
-
–
Death onset phase (the period from the start of the killing intervention until onset of death)
Each phase was described in detail for each of the methods, i.e. for LAPS, and for the currently available methods.
For each phase and for each method, an overview of the available quantitative parameters was outlined.
The second step consisted of identifying the welfare hazards for each phase and method, i.e. every possible event (or sequence of events) linked to the stunning process that can occur during the stunning process itself and having an impact on the welfare of the animals undergoing the procedure (see Section 3.2.2.1). EFSA identified a set of potential hazards, based on the available scientific literature and expert opinion and under the assumption that the different stunning procedures were carried out in the best way possible and fulfilling completely the legislation in force. In other words, the hazards deriving from potential fraud, or ineffective operator activity, were excluded from this assessment. This first set of hazards was then submitted to two external experts who were asked to check the list for exhaustiveness and for the quality of the descriptions associated with each hazard.
As a third step, all the identified welfare hazards were pooled together, and disentangled from the stunning method in which they were identified. Each welfare hazard was associated with two additional features: (i) the percentage of birds affected (or the probability for a single bird to be affected by the occurrence of the adverse event/sequence of events); (ii) the time period the birds are subjected to the event (sequence of events) in case it occurs (worst‐case scenario). The full list of welfare hazards and related information are presented in Section 3.2.2.1 (Table 20).
Table 20.
List and description of the relevant hazards with related estimation of frequency and duration of exposure
| Description | Percentage of birds exposed (or probability of a single bird being exposed to it) – worst‐case scenario | MAXIMUM duration of the exposure to the hazard – worst‐case scenario – (NB: direct exposure, not related to the persistence of the welfare consequences) | |
|---|---|---|---|
| Fact/Operation/Process IN CONSCIOUS ANIMALS | |||
| Handling | Being caught by an operator by the legs and being held upside down | 100% | 00:00:05 |
| Hanging and compression of the legs | Being suspended upside‐down by the legs and being conveyed and being subject to compression of the legs by metal bars | 100% | 00:01:00 |
| Removal of air | Exposure to an environment with progressive depletion of air | 100% | 00:01:20 |
| Potential gas expansion in body cavities/internal organs | Potential expansion of the gases contained in the intestine, the air sacs and the internal ear due to a reduction of the atmospheric pressure in the environment | 100% | 00:01:20 |
| Acidic gas or gas mixture | Inhalation of an acidic gas or gas mixture | 100% | 00:00:30 |
| Respiratory stimulant gas or gas mixture | Exposure to a gas or gas mixture that leads to increasing depth and rate of breathing | 100% | 00:00:30 |
| Unintended electric shock | Being subject to a pre‐stunning electric shock in any part of the body | 25% | 00:00:01 |
| Neck cutting | Cutting the neck while still conscious (severance of the tissues around the neck) | 5% | 00:00:01 |
| Bleeding | Bleeding to death while recovering consciousness | 5% | 00:00:15 |
| High stocking density | High stocking density reduces space allowance and increase the probability of collision between the neighbouring animals | 100% | 00:01:00 |
| Tipping/Tilting | Sudden fall in groups onto a moving conveyor | 100% | 00:00:20 |
| Noise | Being exposed to a sudden unexpected loud noise | 100% | 00:01:18 |
| Decreasing air humidity | Exposure to an environment with progressive depletion of humidity | 100% | 00:01:20 |
A panel of field experts was established, selected from different domains, i.e. research, National Contact Points, Competent Authorities, welfare monitoring officials at the slaughterhouse and private welfare consultants. This panel of experts was asked to perform a ranking exercise on the list of welfare hazards, based on the principles of a judgemental rank‐ordering method based on expert knowledge (fourth step). In more detail, the list of hazards identified by EFSA was validated by external experts and subsequently subjected to expert opinion. These experts were asked to rank the hazards by the expected welfare consequences, based on the hazard itself, the probability of occurrence and its duration, such that the magnitude of the consequences were taken into account. The sought outcome was an ordinal scale of the hazards based on the associated welfare consequences.
The ordinal scale of the hazards retrospectively was reassigned to the associated stunning method (electrical water‐bath, gas methods [excluding inert gases alone], LAPS). Hence rank positions relative to the stunning method could be subjected to hypothesis testing by means of a non‐parametric pairwise multiple ranking test (step five) as represented in Equation 1.
Equation 1
Null Hypothesis (H 0) and Alternative Hypothesis (H 1) of the non‐parametric pairwise multiple ranking test. Rk = Rank; “WATERBATH” = electrical water bath stunning methodology; “CO2” = gas stunning method with carbon‐dioxide; “CO2 + IG” = gas stunning method with a mixture of carbon‐dioxide and inert gases.
In detail, the Null Hypothesis is that LAPS has a greater impact on animal welfare than each of the currently allowed stunning methods. In case the Null Hypothesis is rejected, it is possible to conclude that LAPS ensures a level of welfare at least equivalent to at least one of the currently allowed stunning methods. See Annex A for more details.
3. Assessment
3.1. Eligibility, reporting quality and methodological quality assessment
3.1.1. Eligibility assessment
The aim of the eligibility assessment, as in the current EFSA guidance, is to retrieve information on the LAPS process and related key parameters (see Sections 3.1.1.1) and information capable of ascertaining whether LAPS results in immediate unconsciousness or not, whether the stun is reversible until death and if the induction phase brings pain, distress and or suffering to the animals (see Section 3.1.1.2). In addition, potential causes of failure need to be characterised (see Section 3.1.1.3).
3.1.1.1. Intervention
The information on parameters of the LAPS process from all papers and from the complementary dossier has been summarised in the tables included in the relevant appendices (from Appendices Appendix A – Eligibility criteria Part 1: intervention, Appendix B – Eligibility criteria Part 2: Outcome, Appendix C – Reporting quality, Appendix D – Methodological quality).
Work by Mackie et al. (2016), Paper 5, records and reports complete and detailed information on the study population (e.g. sample size, genotype, bodyweight, animal stocking density in the module). The LAPS process, as for Papers 6, 7 and 8, is described in a generic way: only the total duration of the process and the target pressure are reported, but no detailed information is provided about the rate of decompression, the rate of change in partial pressure of oxygen. The trial conditions (illumination, humidity, ambient temperature and temperature setting) are also missing.
Considering the quality and the quantity of the information, the paper as such does not meet the EFSA guidance requirements.
The paper of Martin et al. (2015 ), Paper 6, records and reports complete and detailed information on the study population (e.g. sample size, genotype, bodyweight, animal stocking density in the module). The LAPS process, as for Papers 5, 7 and 8, is described in a generic way. The rate of decompression and the changes in partial pressure of oxygen over time are not described in the paper but reported in the submitted annexes. However, it has to be highlighted that the same information is repeated in all annexes of Papers 6, 7 and 8 and therefore not paper‐specific. The trial conditions (illumination, humidity, ambient temperature) are reported adequately, except for the temperature setting. The chamber used is not a commercial one but an experimental one with reduced size.
Considering the quality and the quantity of the information, the paper sufficiently meets the EFSA guidance requirements.
The work from Martin et al. (2016b), Paper 7, records and reports complete and detailed information on the study population (e.g. sample size, genotype, bodyweight, animal stocking density in the module). The LAPS process, as for Papers 5, 6 and 8, is described in a generic way. The rate of decompression and the changes in partial pressure of oxygen over time are not described in the paper but reported in the submitted annexes. However, it has to be highlighted that the same information is repeated in all annexes of Papers 6, 7 and 8 and therefore not paper‐specific. The trial conditions (illumination, humidity, ambient temperature and temperature setting) are reported adequately.
Overall, the paper meets the EFSA guidance requirements.
Martin et al. (2016c), Paper 8, records and reports complete and detailed information on the study population (e.g. sample size, genotype, bodyweight, animal stocking density in the module). The LAPS process, as for Papers 5, 6 and 7, is described in a generic way. The rate of decompression and the changes in partial pressure of oxygen over time are not described in the paper but reported in the submitted annexes. However, it has to be highlighted that the same information is repeated in all annexes of Papers 6, 7 and 8 and therefore not paper‐specific. The trial conditions (illumination, humidity, ambient temperature and temperature setting) are reported adequately.
Overall, the paper meets the EFSA guidance requirements.
Holloway et al. (2017), Paper 9, records and reports unclear and incomplete information on the study population (e.g. sample size, genotype, bodyweight, animal stocking density in the module): it is stated that the results are based on the same study populations used in experiments reported in Papers 6, 7 and 8. However, another group of birds is mentioned and described. It is not clear if the results included the aggregated data also from this group of birds. The LAPS process is described in an extensive and detailed way: the paper provides a description of the apparatus, rate of change in partial pressure and per cent atmospheric equivalent oxygen concentrations with or without birds during trials carried out at different environmental temperatures and changes in temperature and relative humidity in the chamber. The trial conditions (illumination, humidity, ambient temperature and temperature setting) are reported, but it is not clear to which study population they refer to (a = population study from Papers 6, 7 and 8; b = population ‘a’ + new group of birds; c = new group of birds only).
Considering the quality and the quantity of the information, the paper as such does not meet the EFSA guidance requirements.
3.1.1.2. Outcome
3.1.1.2.1. Onset of unconsciousness and insensibility
Stunning intervention should disrupt the neuronal function and thereby render animals unconscious and insensible. The EFSA guidance says that it is acceptable that studies on alternative stunning methods (such as LAPS) assess only the onset of unconsciousness as this state is always accompanied by the onset of insensibility.
Martin et al. (2016b,c), Papers 7 and 8, respectively, examine the response to LAPS by recording behaviours and EEG. The behaviours reported as indicators of unconsciousness and/or insensibility were LOP and motionless. Regarding the neurological indicators, the onset of isoelectric EEG signal was determined in two ways: (i) by visual inspection, and (ii) by identification of spectral characteristics (Ptot < 170 mv and F50 > 22 Hz). Latency variables to unconsciousness were defined as time for F50 < 12.7 Hz (non‐responsive state) and < 6.8 Hz (general anaesthetic (GA) plane).
The result from the EEG analysis are based on parameters that are not validated. The two papers, as such, do not meet the EFSA guidance requirements.
Mackie et al. (2016) (Paper 5) and Martin et al. ( 2015 ) (Paper 6) report only data on behavioural indicators. The behaviours reported as indicators of unconsciousness and/or insensibility were LOP and motionless. Regarding the neurological indicators, no EEG was recorded.
Holloway et al. (2017) (Paper 9) was not designed to record any indicator related to animal welfare but it was a technical description of the process.
3.1.1.2.2. Absence of pain, distress and suffering until loss of consciousness and sensibility
If a stunning intervention does not induce immediate unconsciousness and insensibility (as is the case of LAPS), the absence of pain, distress and suffering until the onset of unconsciousness and insensibility should be assessed (EFSA AHAW Panel, 2013).
Mackie et al. (2016) and Martin et al. ( 2015 ,b,c), i.e. Papers from 5 to 8, all report a complete set of behavioural indicators with some parameters associated (e.g. number of bouts, latency, etc.).
Regarding the physiological response, in the experiment reported in Paper 7 (Martin et al., 2016b) ECG traces were recorded.
Paper 7 and 8 Martin et al. (2016b,c), as described above, report data on neurological response: EEG traces and EEG quantitative analysis results.
3.1.1.2.3. Duration of unconsciousness and insensibility
Council regulation No 1999/2009 states that unconsciousness and insensibility induced by stunning should last until the moment of death. As for the onset of unconsciousness and insensibility, the EFSA guidance considers it acceptable that studies on alternative stunning interventions assess only the duration of unconsciousness.
Regarding the outcome, the information on the different behavioural indicators provided in the work of Mackie et al. (2016), Paper 5, is sufficient for a quantitative assessment. Neurological responses were not recorded.
In Martin et al. ( 2015 ), Paper 6, the authors did not record the neurological responses, but provided an important set of behavioural observations. Some of the animal‐based indicators provided can be used to assess the onset of unconsciousness and insensibility (LOP, motionless) and the onset of death (rhythmic breathing, nictitating membrane).
The work of Martin et al. (2016b), Paper 7, records the neurological responses (EEG) and reports the results of the quantitative analysis. This work also provides information on behavioural indicators used to detect the onset of death, making possible to ascertain what happens between the loss of consciousness and the death.
In Martin et al. (2016c), Paper 8 records the neurological responses (EEG) and reports the results of the quantitative analysis. This work also provides information on behavioural indicators used to detect the onset of death, making possible to ascertain what happens between the loss of consciousness and the death.
Holloway et al. (2017), Paper 9, did not report any behavioural or neurological responses. It is a description of the LAPS technical process.
3.1.1.3. Identification of potential critical points and causes of failure
The applicant identified a set of hazards potentially affecting the LAPS system and provided safeguards to prevent poor animal welfare outcomes. Table 3 provides the relevant information on this subject as submitted by the applicant. It appears clearly that the procedures associated with system failures are aimed at preventing failures, rather than putting in place emergency procedures to face a potential failure. Therefore, it becomes crucial that the manufacturer includes in the manufacturer's instructions for the use of the equipment, all of the maintenance procedures and security checks identified. In addition, Food Business Operators should follow the manufacturer's instructions and include them in the standard operating procedures.
Table 3.
Potential causes of failure and related countermeasures/safeguards
| Issue | Technical details and safeguard | Comments |
|---|---|---|
| Failure of control and management system and/or electromechanical system | The process is coordinated by a programmable logic controller (PLC) using a digital computer for automation of electromechanical processes. In LAPS, sensors, pumps, valves, doors and conveyors are controlled in this way. PLCs are designed for multiple arrangements of digital and analogue inputs and outputs, extended temperature ranges, immunity to electrical noise, and resistance to vibration and impact. Programs to control the machine operation are stored in battery‐backed‐up or non‐volatile memory. A human–machine interface (HMI) is employed to allow interaction with people for the purpose of routine monitoring of pressure curves and bird behaviour. The HMI screen (built using LookoutDirect software) displays a graphical animation of process flow, event driven controls, and lights to give warnings. Real time changes are shown on the display screen thereby the status of safety features, position of transport modules on conveyors and in chamber, chamber door status and the pressure within of chamber, status of electromechanical devices, valves and pumps and hydraulic and air services chamber are all available | The HMI provides clear information of each process involved with handling and stunning the birds. The user manual provides information on interpretation and actions required in case of alerts. The user manual describes the maintenance procedures for all the equipment and also the calibration of the pressure gauge |
| Electrical failure | Many poultry processing plants are fitted with back‐up generators but in addition the LAPS system is fitted with its own battery back system, which ensures sufficient power for all systems needed to complete the LAPS cycle and move modules | The user manual required regular checks and tests of electrical back facilities |
| Failure of Chamber integrity and operation | Specialist certified manufactures to international standards for low‐pressure vacuum chambers construct the chamber walls and doors and portals using high‐quality steel. The machine withstand pressures greater than those incurred during LAPS. The metal parts of the chamber and doors and portals have a low risk of damage or wear during operation. The door seals are made of high quality rubber and over several months are liable to wear. To detect such wear, the operational procedures require a leak test of each chamber each day of operation. The LAPS system cannot be used if a leak test has not been made in the previous 24 h | High‐quality materials are used in construction and production techniques are used in manufacture that ensure high reliability in operation similar to those used for submarine construction. The door seals have potential for wear and their efficiency is checked daily and replaced when necessary. The user manual describes the maintenance procedures for all the equipment and also the calibration of the pressure gauge. To minimise risk of failure of monitoring equipment, the HMI has alarms to alert the operators of any equipment failure from movement of transport modules to door closure etc. Various pumps, valves, doors and conveyors use electrical motors or compressed air or hydraulic systems and these are monitored for operational state and effectiveness and maintained as described in the owner's manual |
3.1.1.4. Eligibility: conclusions
Should the criteria in the EFSA guidance be strictly applied to each submitted paper, none of them would have passed the eligibility assessment. Each paper is lacking one or more item to fulfil the requirements on its own (see Appendices A and B for more details). On the other hand, it was clear that a complete picture of the process could be derived by the joint evaluation of the single papers, e.g. the details of the intervention, to a certain degree incomplete in Papers from 5 to 8, was fully detailed in Paper 9. The latter lacking completely of any indicator on the animal welfare outcome of the intervention, which were available in Papers from 5 to 8.
For this reason, EFSA decided to consider all information from the 5 publications to pass the eligibility criteria and to proceed with the next step in the assessment process as outlined in the EFSA guidance.
3.1.2. Reporting quality assessment (see Appendix C)
In general, the objective and the scope of the single experiment was clearly defined and described in the introduction of all papers.
The ‘result’ section also reported important criticisms and the most important was that the results were all presented as aggregated values, making impossible to ascertain the sequence of the events at bird level.
The ‘discussion’ section, in the different papers, did not show any major problem, except for the lack of proper argumentation on:
the possible relationship between the recorded behaviours and the animal welfare consequences;
the relationship between neurological and behavioural data which is not discussed or reported.
3.1.2.1. Reporting quality: conclusions
Considering that one of the most important goals of this assessment was to fully understand the effects of the LAPS intervention on the birds and the possible impact on animal welfare, the lack of proper statistical analysis (survival analysis) would have made the papers not fit for the next step of the assessment. However, EFSA, based on the quantity and the quality, including the level of detail of the information provided, decided to ask for complementary data instead of stopping the assessment process.
For this reason, EFSA decided to proceed with the next step in the assessment process as outlined in the EFSA guidance.
3.1.3. Methodological quality assessment (Appendix D)
It can be seen from Section 3.1 that the methodological quality criteria assessment as described in the EFSA guidance focuses on elements in the report that allow the assessment of the internal validity of the individual submitted studies.
The internal validity of each study has been checked, mainly by evaluating the different existing biases that are summarised in Appendix D. It appeared that no specific bias has been identified in any of the studies, ensuring a correct internal validity.
The ‘material and methods’ sections across Papers 5, 6, 7 and 8 presented some issues and the most important are summarised here:
the definitions and the proposed interpretation, i.e. suggested impact on animal welfare, of the different reported behavioural indicators were not always clear and not consistent across the different experiments/papers;
the analysis of the EEG traces was done in a qualitative way and the choice of some of the parameters used are not validated;
although proper statistical models were implemented to take into account the potential effect of external factors (humidity, temperature, etc.) on the main events (e.g. LOP, motionless), a proper survival analysis was missing in all studies.
3.1.3.1. Methodological quality: conclusions
Overall, the methodological quality of the submitted papers does not represent major issues.
Appraisal of a study's external validity (i.e. its applicability beyond the study population) requires that its results are compared with those of comparable studies. As reported above, the present EFSA guidance is only applicable to individual studies and assessing the external validity of those studies exceeds its mandate. In the following section, the external validity assessment of the studies is provided, i.e. to what extent the system proposed for stunning broilers can provide a level of animal welfare at least equivalent to that ensured by the currently allowed methods.
3.2. Assessment of the level of animal welfare provided by LAPS
The first step in this assessment process was to identify the words used in the ‘background’ and in the ‘terms of reference’ as provided by the European Commission, which may be regarded key words for the understanding of animal welfare issues potentially related to stunning procedures, and to give each key word a clear definition. For the purposes of this assessment, the key words identified as relevant to animal welfare were: pain, distress, suffering, loss of consciousness, loss of sensibility and death (Welfare Outcomes). Table 4 shows the definitions and the related source of information.
A definition of the key word ‘avoidable’ is not given as it was impossible to move away from the well‐known dictionary definition (‘to keep away from’/’to prevent the occurrence of’) and find a tailored definition which was appropriately applicable for this specific context. In fact, each attempt to link this key word to a general or specific aspect in this context entailed a reference to some management aspects, which are specifically outside EFSA's remit. In other words, it was clear that in principle each event is ‘avoidable’ but with different consequences from minor to major, e.g. suffering from bleeding can be avoided by stunning but decompression cannot be avoided in LAPS (as part of the procedure). Every action undertaken to ‘avoid’ one of those events has of course an impact on one or more of the practices normally in place which are, indeed, based on management decision. As a consequence, EFSA dedicated a section (see Section 3.3) to the identification of the potentially critical points concerning LAPS and a possible solution when relevant. In this way, the European Commission will be able to understand the context and make specific decisions if relevant.
3.2.1. Quantitative assessment
In order to address the request of the European Commission to assess whether the LAPS methodology ensures a level of animal welfare (restricted to pain, distress and suffering in this assessment) at least equivalent to the one provided by the currently approved methods, EFSA identified a set of quantitative parameters as the basis for the required comparison. These quantitative parameters were obtained from the data submitted by the applicant (Parts 1 and 2) for aspects which concerns the LAPS methodology and by means of a data extraction from the scientific literature for aspects which concern the other methods (see Appendix E).
Additional statistics, either provided by the applicant or performed by EFSA are presented and discussed (see Sections 3.2.1.1, 3.2.1.2 and 3.2.1.3).
3.2.1.1. Behaviours
The applicant provided a final list of behaviours considered to be relevant, each accompanied by a definition and a possible interpretation in terms of animal welfare. Each behaviour has been considered as an indicator of one or more welfare outcomes. The details of these measures can be seen in Table 5.
The set of behavioural indicators was then subdivided into two groups: a first one including all indicators for loss of consciousness and sensibility (see Table 6) and a second one for indicators of either distress and/or suffering (see Tables 8 and 9).
Table 6.
Quantitative parameters with reference to the behaviours indicative of loss of consciousness and sensibility and death (merged data set from Paper 6,7 and 8 for a theoretical total number of 220 birds)
| Number of birds affected/total number of observed birds | Missing recordsa | Proportion of birds affected (exact binomial 95% CI) | Median latency to behavioural event (observed birds), sec | |
|---|---|---|---|---|
| Behavioural event | ||||
| Loss of posture | 194/194 | 26 | 1 (0.98–1) | 58.4 (194) |
| Lying | 198/198 | 22 | 1 (0.98–1) | 60.3 (198) |
| Motionless | 194/194 | 26 | 1 (0.98–1) | 145.8 (194) |
e.g. out of sight.
Table 8.
Main statistical parameters for the reported behavioural welfare indicators. The ‘number of birds affected’ report the number of birds showing the behaviour (merged data set from Papers 6, 7 and 8 for a total number of 220 birds)
| Behavioural events | Number of birds affected/total number of observed birds | Fraction of birds affected (exact binomial 95% CI) |
|---|---|---|
| Ataxia | 187/188 | 0.99 (0.97–1) |
| Clonic/tonic convulsions | 203/203 | 1 (0.98–1) |
| Wing flapping | – | – |
| Deep inhalation | 87/201 | 0.43 (0.36–0.5) |
| Head shaking | 109/202 | 0.54 (0.47–0.61) |
| Jump | 102/201 | 0.51 (0.44–0.58) |
| Open bill breathing | 148/202 | 0.73 (0.67–0.79) |
Table 9.
Detailed summary statistics on the LATENCY of the different behaviours (see also Figure 1)
| LATENCY | |||||||
|---|---|---|---|---|---|---|---|
| Min. | 1st quartile | Median | Mean | 3rd quartiles | Max. | Obs.a | |
| Behavioural events | |||||||
| Ataxia | 16.8 | 36.6 | 41.9 | 41.6 | 47.2 | 65.2 | 187 |
| Headshake | 1.3 | 27.4 | 45.6 | 42.2 | 56.7 | 107.1 | 108 |
| Jump | 35.4 | 47.5 | 53.3 | 53.4 | 57.8 | 78.31 | 54 |
| LOP | 40.1 | 53.7 | 58.4 | 58.5 | 62.3 | 78.2 | 194 |
| Lying | 41.7 | 55.2 | 60.3 | 61.6 | 66.2 | 130.4 | 198 |
| Open bill | 11.1 | 57.0 | 63 | 64.9 | 70.1 | 154.4 | 148 |
| Convulsions | 41.4 | 61.5 | 69.4 | 72.2 | 77.2 | 147.0 | 201 |
| Deep inhalation | 4.7 | 71.8 | 89.4 | 87.6 | 103 | 157.5 | 87 |
| Motionless | 86.1 | 136.3 | 145.8 | 146.1 | 155.9 | 191.6 | 194 |
Number of observations used for the computation from the raw data as submitted by the applicant with reference to Papers 6, 7 and 8 (total number of birds = 202).
Before proceeding with the estimation of the quantitative parameters, some preliminary checks were performed. In detail, a Kruskal–Wallis rank sum test was performed based on the raw data provided to EFSA to check if there was a statistically significant difference in the onset of the LOP between the groups used in Papers 6, 7 and 8. In fact, one of the tests gave a p‐value below the significance level (0.002) with reference to the comparison between the groups of animals, reported in Paper 7, undergoing LAPS with temperature setting 3 (median = 61.8 s) and the one undergoing LAPS with a temperature setting 4 (median = 58.2 s).
In any case, although statistically significant, the difference in the time to onset between the two groups was considered not to be biologically relevant (EFSA Guidance on Statistical Significance & Biological Relevance) for the purposes of the present assessment, and all birds were included for the estimation of the quantitative parameters of interest. No other statistically significant difference between groups (butorphanol/placebo and light/dark) were identified.
Table 7 shows the latency after which LOP, lying and motionless appeared among birds in experiments described in Papers 6, 7 and 8. One of the important outcomes is that all observed birds showed all three behavioural indicators in sequence. The fact that all behaviours are recorded and that they appear in this sequence indicates that, according to these publications, the LAPS cycle applied, as defined there, led to loss of consciousness followed by death in all of the birds.
Table 7.
Detailed summary statistics on the LATENCY of the different behaviours indicating loss of consciousness and sensibility and death
| LATENCY in seconds | ||||||||
|---|---|---|---|---|---|---|---|---|
| Behavioural events | Min. | 1st quartiles | Median | Mean | 3rd quartiles | Max. | Missing | Obs.a |
| Loss of Posture | 40.1 | 53.7 | 58.4 | 58.5 | 62.3 | 78.2 | 8 | 194 |
| Lying | 41.7 | 55.2 | 60.3 | 61.6 | 66.2 | 130.4 | 4 | 198 |
| Motionless | 86.1 | 136.3 | 145.8 | 146.1 | 155.9 | 191.6 | 8 | 194 |
Number of observations used for the computation from the raw data as submitted by the applicant with reference to Papers 6, 7 and 8 (total number of birds = 202).
It is important to note that the range of time to onset of LOP reported in Paper 5 and ‘Experiment 2017’ is higher when compared to the ones recorded for Papers 6, 7 and 8 (cf. Tables 7 and 13).
Table 13.
Minimum, mean and maximum values of latency for each behaviour. Comparison between Ross and Cobb
| PAPER 6, 7, 8a (Cobb) BWb6 = 2.3 ± 0.12 BW7 = 2.36 ± 0.38 BW8 = 2.96 ± 0.41 | PAPER 5 (Ross) BW = 3.4 ± 0.5 | Experiment 2017e (Ross) BW = 3.65 ± 0.5f | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Behavioural events | Min. | Mean ± SD | Max. | Min. | Mean ± SD | Max. | Min. | Meanf | Max. |
| Ataxia | 16.82 | 41.61 | 65.16 |
17.8c 39.6d |
57.3 ± 11.5 58.3 ± 8.9 |
77.2 78.6 | |||
| Headshake | 1.27 | 42.21 | 107.1 |
3.3 4.1 |
58.5 ± 29.6 57.3 ± 11.5 |
167.3147.8 | |||
| Jump | 35.43 | 53.43 | 78.31 | NA | |||||
| LOP | 40.07 | 58.47 ± 6.9 | 78.24 |
58.8 50.0 |
80.7 ± 17.7 80.4 ± 11.1 |
182.5117.8 | 56 | 78 ± 11.7 | 106 |
| Lying | 41.67 | 61.56 | 130.4 | NA | |||||
| Open bill | 11.11 | 64.91 | 154.4 |
4.3 5.4 |
57.4 ± 35.8 64.4 ± 29.3 |
187.9162.3 | |||
| Convulsions | 41.41 | 72.21 | 147 |
63.3 66.7 |
110.5 ± 37.6 128.2 ± 38.3 |
208.2204.6 | |||
| Deep inhalation | 4.67 | 87.61 | 157.5 | NA | |||||
| Motionless | 86.12 | 146.1 ± 16.8 | 191.6 |
158.2 180.1 |
199.4 ± 21.3 207.5 ± 12.0 |
245.6235.3 | 131 | 206 ± 29.8 | 260g |
Number of observations used for the computation from the raw data as submitted by the applicant with reference to Papers 6, 7 and 8 (total number of birds = 202).
BW = bodyweight in kg ± SD.
First row (relevant for Paper 5) = experiments with individual birds.
Second row (relevant for Paper 5) = experiments with group of birds.
Results from the experiment conducted by the applicants to address EFSA's request for additional data and information.
In the report, it is not specified what type of statistical measurement of the variation around the mean was used.
The orange cell indicates the proximity to the end of the LAPS process.
Unfortunately, it was not possible to perform any modelling to assess if the two populations (Papers 5 and 10 vs Papers 6, 7 and 8) are significantly different and which are the potential covariates having an influence on the behavioural events (LOP, motionless). No explanation, comment or discussion could be found in the submitted scientific papers on the matter, with the exception of Paper 5, in which the correlation between the body weight of the birds and the duration of ataxia was studied. However, this information was not considered sufficiently relevant for animal welfare assessment (the correlation between body weight and latency/time to onset of LOP and/or motionless would have been much more informative) and the raw data for additional analysis were not made available.
Considering that the main parameters of the LAPS process are controlled by a computer (i.e. the rate of decompression is reported to be similar in all studies carried out under different experimental conditions), the reason for this difference must have a biological ground which EFSA was not in the position to rule out, due to the lack of a complete set of raw data from Papers 5 and 10 (i.e. data on individual body weight for Ross type broilers was not available). However, EFSA hypothesised that some factors may play a role. These include: (i) the different genotype used in the different experiments (i.e. Cobb 500 in Papers 6, 7 and 8 and Ross 708 in Papers 5 and 10); (ii) body weight; (iii) age at slaughter.
The animal welfare consequences of this would be that there would or could be variable times to onset of unconsciousness and death depending of the genotype/weight/age of bird. Indeed, in Paper 5 and Experiment 2017, the maximum latency observed for motionless was 235 and 260 s (Table 13), respectively, i.e. 20 s before the end of the LAPS process. These results question the use of LAPS with heavier birds (including broiler breeders) that might not be motionless before the end of the LAPS process. This could be of major concern in the EU where the Ross genotypes accounts for a large proportion of all chickens reared and slaughtered.
The different behaviours are shown in sequence in Figure 1 where the range of the latency, estimated for each bird individually (from Papers 6, 7 and 8), compared to the LOP (blue line), can be seen (see also summary statistics in Table 9). All behaviours appearing before the LOP are below the blue line and represent the point of concern as the animal is still conscious at this stage. It can be seen that the latest appearance of motionless was at 192 s, i.e. 88 s before the end of the LAPS process. This indicates that all birds are dead before the end of the process. The detailed statistics of the different behaviours can be consulted in Table 9.
Figure 1.
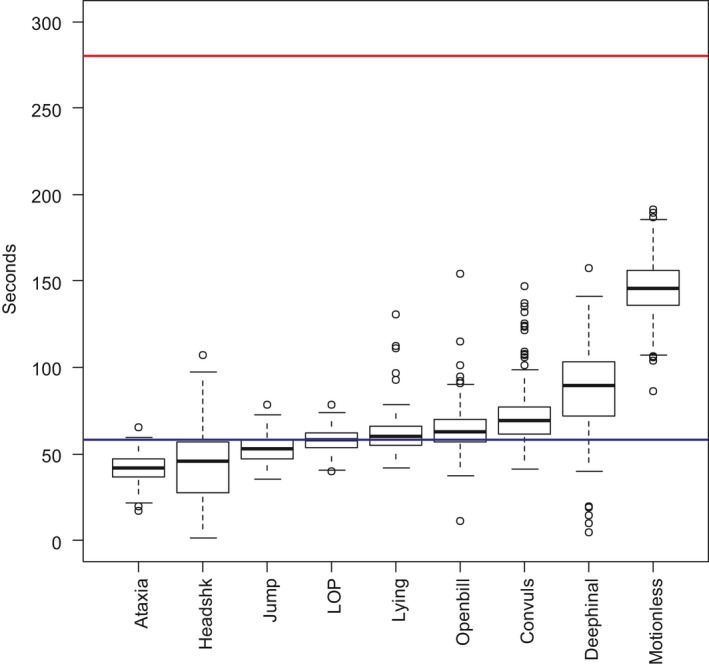
Boxplot of the latency (time of onset) of the different behaviours in sequence. Blue line = median of the onset of LOP Red line = end of the LAPS process Number of observations = see Tables 8 and 9
In order to better understand the sequence and the occurrence of the events before the LOP, as this is the period of major concern as the animals are still conscious, further investigations were performed. For each bird, the boxplots in Figure 2 describe the data relative to the difference between the first appearance of each behaviour and the onset of LOP. In this manner, it is possible to visualise which behaviour is recorded before the LOP (below the blue line). It can be seen that ataxia, headshaking and jump behaviours almost always appear before the LOP, while open bill breathing, convulsions and deep inhalation appear after LOP. In this regard, it is curious to note that five birds out of 194 showing clonic convulsions were recorded to show the behaviour before the LOP (see Table 10). However, as those five animals are part of the same experiment (see Paper 8), it is likely that a systematic bias affected this information, in the sense that wing flapping, occurring in conscious birds, was erroneously recorded as clonic convulsions (which, by definition, take place when the animal is unconscious).
Figure 2.
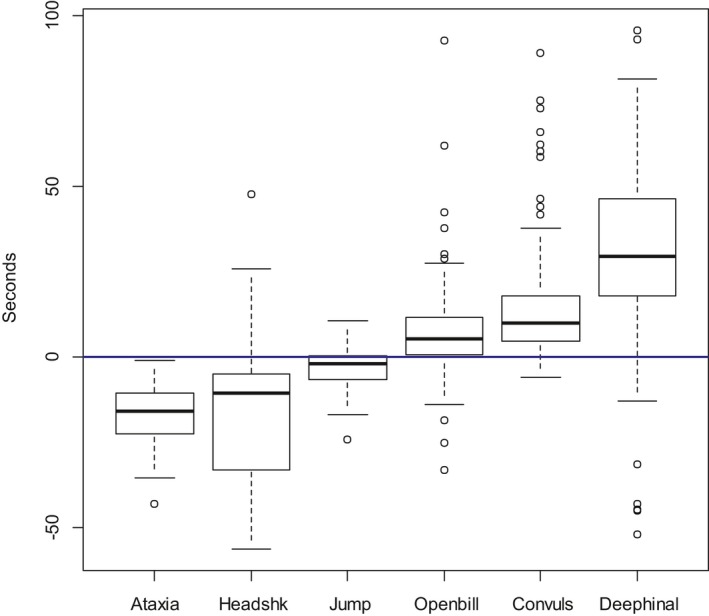
The boxplots describe the difference in time (seconds) between the onset of each behaviour against the loss of posture (LOP, blue line) calculated for each bird from Papers 6, 7 and 8. Below the blue line = before the LOP; above the blue line = after the LOP
Table 10.
Number and fraction of birds showing the respective behaviour BEFORE the LOP
| Behavioural events | Number of birds showing the respective behaviour BEFORE the LOP/number of birds performing the behaviour | Fraction of birds showing the respective behaviour BEFORE the LOP (95% CI) |
|---|---|---|
| Ataxia | 185/185 | 1 (0.98–1) |
| Head shaking | 95/107 | 0.89 (0.81–0.94) |
| Jump | 36/53 | 0.68 (0.54–0.8) |
| Open bill breathing | 31/145 | 0.21 (0.15–0.29) |
| Clonic/tonic convulsions | 5/194 | 0.03 (0.01–0.06) |
| Deep inhalation | 12/83 | 0.14 (0.08–0.24) |
| Wing flapping | – | – |
The detailed statistics of the different behaviours can be seen in Table 11.
Table 11.
Main statistical parameters for the reported behavioural welfare indicators describing the latency (time to onset) adjusted for the onset of the loss of posture
| LATENCY (time to onset) | ||||||
|---|---|---|---|---|---|---|
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |
| Behavioural events | ||||||
| Ataxia | −42.84 | −22.42 | −15.88 | −16.85 | −10.68 | −1.03 |
| Headshake | −56.29 | −33 | −10.41 | −16.47 | −5.005 | 47.61 |
| Jump | −24.17 | −6.51 | −1.83 | −2.988 | 0.44 | 10.58 |
| Open bill | −33.1 | 0.63 | 5.41 | 6.888 | 11.71 | 92.75 |
| Convulsions | −5.89 | 4.612 | 9.84 | 13.55 | 18.03 | 89.22 |
| Deep inhalation | −51.79 | 17.86 | 29.5 | 29.29 | 46.45 | 95.86 |
The behavioural events expressed by the chickens submitted to LAPS showed a wide variability in terms of the number of animals showing different behaviours and the proportion of animals showing them prior to LOP (Table 10). The least frequent behaviours exhibited before LOP were tonic‐clonic convulsions, deep inhalation and open bill breathing.
It has to be pointed out that according to the definitions provided by the applicant (see Table 5) tonic‐clonic convulsions always occur, as a spinal reflex, when the animals are rendered unconscious by the intervention (i.e. after the LOP). However, the data presented in Table 10 show a small proportion of birds (5/194) exhibiting this behaviour prior to LOP. It is difficult at this stage to understand if the reason for this is misclassification of the event, observer bias, or miscalculation of the time to onset of the event. However, the welfare impact of this proportion can be considered low.
The relatively high fraction of birds showing the behaviour Jump before the LOP (of the 51% of birds showing jumping behaviour, 68% did this before LOP) makes it a matter of concern and has to be evaluated against the other stunning methods currently approved.
Regarding open bill breathing and deep inhalation, the proportion of birds showing this behaviour before the LOP is slightly higher (21% and 14% of the birds showing the behaviour, respectively). As these behaviours clearly indicate respiratory discomfort related to distress and suffering, when they occur before the LOP, they are a matter of concern. These behaviours have also been reported before LOP in other stunning methods using gas mixtures containing CO2 (Lambooij et al., 1999; Gerritzen et al., 2000).
Many studies involving exposure of poultry to carbon dioxide have reported the occurrence of gasping (described as ‘deep inhalation’ by the applicant) as the initial reaction (Raj, 1996; Lambooij et al., 1999; Coenen et al., 2000; Webster and Fletcher, 2004). Mckeegan et al. (2003) demonstrated by recording the firing rate of trigeminal nerve to inhalation of CO2 in chickens that 11% by volume of CO2 in air substantially increased the rate of firing. McKeegan et al. (2005) provided further evidence to this effect of hypercapnia and other noxious gases in poultry.
In another study, the number of gasping (described as ‘deep inhalation’ by the applicant) and headshaking events per bird prior to the onset of LOP (considered as a behavioural indicator of onset of unconsciousness) was recorded from a set of 75 broilers exposed to different gas mixtures (Raj, 1997 – see also Table 12).
Table 12.
Number of ‘gasping’ and ‘headshaking’ events per broiler chicken (study population = 15 birds/treatment) prior to loss of posture undergoing gas stunning with different gas mixtures (Raj, 1997)
| Treatment | Gasping | Headshaking | Latency (time to onset) to loss of posture (seconds) |
|---|---|---|---|
| 90% argon in air | 0 | 0 | 13 |
| 30% carbon dioxide and 60% argon in air | 3 | 3 | 12 |
| 30% carbon dioxide and 30% oxygen in air | 12 | 5 | 47 |
| 40% carbon dioxide and 30% oxygen in air | 9 | 4 | 35 |
| 40% carbon dioxide in air | 8 | 3 | 29 |
It is important to note that no gasping occurred during exposure to hypoxia induced with 90% argon in air (2% residual oxygen) and gasping occurred in broilers exposed to hypercapnia induced with gas mixtures containing CO2. These results are in agreement with the findings reported by Gerritzen et al. (2000). Inevitably, birds will have to endure any pain, suffering and/or distress which is associated with the induction of unconsciousness with hypercapnia prior to loss of consciousness. By analogy, the animals showing open bill breathing in LAPS might experience such pain, suffering and distress. The time to LOP, as a behavioural indicator of onset of unconsciousness, during exposure to different gas mixtures, recorded shorter times compared to LAPS (Raj, 1997, see also Table 12). Unfortunately, neither the live weight of the birds nor the range of values was reported and no considerations on this regard was possible in the study reported.
Ataxia, head shaking and jump are the behaviours recurring more frequently before LOP (100%, 89% and 68%, respectively), to lesser extent open bill breathing and deep inhalation (see Table 10).
Ataxia is observed as motor incoordination in conscious birds and the welfare consequences are not clear. All the birds might be subjected to distress due to ataxia before LOP.
The interpretation and the impact on animal welfare of head shaking is highly debated in the scientific literature: some papers link this behaviour to the presence of CO2 in the gas mixture in control atmosphere stunning (CAS) methods (Raj, 2006; Gerritzen et al., 2000) and some others report the absence of this behaviour when the gas mixture includes CO2 (McKeegan et al., 2007, Abeyesinghe et al., 2004). Highly debated is also the cause for this behaviour (acidic gas stimulation of the trigeminal nerve in the nasal mucosa, reaction to aversive stimuli, breathlessness, air hunger) and it is believed that this is a multifactorial response. In any case, in the LAPS method, head shaking can be used as an indicator of distress and/or suffering in response to an unpleasant stimulus. The relatively high fraction of birds showing this behaviour before the LOP makes it a matter of animal welfare concern and has to be evaluated against the other stunning methods currently approved.
Based on the definition provided by the applicant, jump includes more causes (attempts to regain posture, involuntary muscle contractions) and as a considerable fraction of individuals show this behaviour before LOP, the impact on animal welfare cannot be considered negligible.
Based on the sample of video clips of birds subjected to LAPS provided by the applicant, it is observed that all birds in the video files defecated and the latency (time to onset) for this event seems to be to a certain extent correlated to the clearing of the fog in the chamber (observation by the WG from the small set of video samples), which occurs when the chamber pressure is reduced to less than 450 Torr. In this regard, the range of time to defecation lies between 45 and 57 s from the start of LAPS. It is worth mentioning that birds exposed to normobaric (i.e. at atmospheric pressure) hypoxia induced with argon do not defecate (Raj M, personal communication). Together, these observations seem to suggest that defecation is likely to be due to gas expansion in the distal part of the digestive tract, as described as ‘degassing of birds’ in Paper 9. Further dedicated experiments should be performed to clarify this potential critical point.
Under commercial slaughter conditions, chickens are fasted prior to catching and crating on farms prior to transport to the slaughterhouse. It is known that the amount of gas produced in the duodenum increases with the duration of fasting, especially after 5 h of feed withdrawal (Savage, 1998). It is likely that such gas present in the gut will expand during LAPS. The consequences of gas expansion for the welfare of poultry has not been elucidated. Although rupture of intestine due to the gas expansion is not occurring during LAPS, no data are available to rule out the possibility of colic‐like pain occurring in conscious poultry due to gas expansion.
3.2.1.2. Physiological response
HR and HRV as measures for assessing stress and welfare in farm animals have been described in detail in the review published by von Borell et al. (2007). In healthy broilers, HR represents the net interactions at any point in time between parasympathetic regulation (vagal regulation, which reduces HR) and sympathetic regulation (which increases HR). At rest, vagal regulation dominates whereas increasing physical activity is frequently characterised by decreasing vagal and increasing sympathetic influences.
It is difficult to access the functional regulatory characteristics of the autonomous nervous system (ANS) with simple measurements of HR. However, HR is a single measure of heart activity quantitatively documented in alternative stunning methods with which LAPS should be compared. The HRV has not been investigated by the applicant. In order to maximise the information useful for this assessment, other types of analysis were performed by EFSA.
Comparison of HR dynamics in CAS and LAPS is given in Table 14. Data from hypercapnic CAS taken from McKeegan et al. (2007) were compared with the data from Papers 7 and 8 of the current application.
Table 14.
Main statistical parameters for the reported physiological welfare indicators
| CAS (McKeegan et al., BPS 2007) | LAPS | ||||
|---|---|---|---|---|---|
| Condition or data source | CO2 + O2 | Ar + CO2 | Paper 7 | Paper 8 | 7 & 8 combined |
| Number of birds | 45 | 45 | 25 | 17 | 42 |
| Pre‐stun HR (BPM) | 350 | 350 | 400 | 400 | 400 |
| Rapid HR decline start (s) | 0 | 0 | 25 | 15 | 21 |
| Rapid HR decline end (s) | 10 | 10 | 65 | 55 | 61 |
| Duration of rapid HR decline (s) | 10 | 10 | 40 | 40 | 40 |
| Post‐decline HR (BPM) | 236 | 204 | 225 | 250 | 235 |
| HR at the end of stunning (BPM) | 230 | 150 | 150 | 150 | 150 |
Pre‐stun HR in LAPS was somewhat higher comparatively to hypercapnic CAS. It was not possible to ascertain if this difference was somehow related to the genotype and/or the weight of the birds as in McKeegan (2007) the birds are described as ‘mature broiler chickens’, without further details. However, these pre‐stun HR values reported in these studies are within the physiological range (Korte et al., 1999).
Both hypercapnic CAS and LAPS caused a rapid decline in HR from 350–400 BPM to 204–250 BPM. However, HR decline in CAS was much faster than in LAPS and lasted 10 seconds compared to 40 seconds, respectively. HRs at the end of stunning were also similar. There is no clear information in the scientific literature about the impact on animal welfare of the bradycardia that we observe here, especially during the induction of unconsciousness with gas mixtures or LAPS. Consequently, it is not possible to draw conclusions based on this information regarding HR.
HRV analysis, on the other hand, allows a much more accurate and detailed determination of the functional regulatory characteristics of the ANS. Carravieri et al. (2016) in a study employing selective pharmacological blocking of two branches of the ANS, has shown that the stress response in birds is dominated by a withdrawal of the activity of the parasympathetic (vagal) nervous system. Thus, the stress response in birds is manifested by an increase in HR and a decrease in HRV (see Figure A1 in Carravieri et al., 2016). The data provided in Paper 8 (Table 14) clearly indicates a significant decrease in HR during LAPS, but there is no information concerning HRV which would be expected to increase.
The applicant reported the onset of bradycardia, often associated with arrhythmia, starting on average around 50 s and lasting until 60 s into the LAPS process, after which the HR levelled. The applicant reported that there is a consistency between behavioural indicators, EEG and ECG with reference to loss of consciousness. The LAPS authors stated that bradycardia and arrhythmia are indicative of compromised cardiovascular physiology. It is worth noting that the onset of bradycardia and arrhythmia precede loss of consciousness by 10 s and the welfare consequences are not adequately discussed. The EEG data analysis carried out by EFSA showed EEG arousal during this time (50–60 s into LAPS) but it is not clear whether these events are related and/or their welfare significance for birds in this period before LOP.
3.2.1.3. Neurological response
EEG data have been analysed by the applicant to determine the time to loss of consciousness using criteria listed in Table 16. Because of high noise in the EEG records, and concerns about the quality of the signal artefacts rejection procedure carried out by the applicant, EFSA decided to request raw data and to perform additional analysis. To increase the robustness of the data analysis, Papers 7 and 8 were pooled together.
Table 17.
Main statistical parameters for the reported neurological welfare indicators
| Number of birds | Latency/time to onset (mean ± SEM, s) | |
|---|---|---|
| Welfare indicator | ||
| < 10% of pre‐stun EEG power | 38 | 140.6 ± 5.5 |
| Arousala detected by EEG | 38 | 51.2 ± 5.3 |
Increase in fast/high frequency or, decrease in slow/low frequency electrical activity recorded in the EEG.
Application of LAPS leads to some increase of slow‐wave activity during approximatively the first 50 s, of the procedure followed by a relatively rapid decline around the LOP event (Figure 3a). This can be interpreted as falling asleep/drowsiness presumably due to decreased oxygen concentration, and awakening when oxygen concentration drops to a life‐critical level (around 50 s after LAPS onset). It has to be noted that falling into sleep or drowsiness with following awakening was induced by LAPS. Such dynamics are absent in control birds that were subject to similar conditions except decompression (Figure 3b). Because of the similar dynamics of activity in different frequency bands, we pooled all of the bands together to compute the total EEG power Ptot (Figure 4a). The peaks of EEG activity falling in the time interval 60–140 s can be linked with a non‐complete rejection of locomotor artefacts. To assess locomotor activity, we computed the percentage of rejected epochs for each time point (Figure 4b). Assuming that a fixed percentage of events with motor activity leads to detectable artefacts, the number of rejected epochs is roughly proportional to amount of animal movements. As seen in Figures 3a and 4a, the EEG power does not decline to zero at the end of LAPS although all animals are supposed to be dead at that time. Especially, high levels of activity are detected in the Gamma (30–200 Hz) frequency band. Investigation of the EEG power spectrum (Figure 5) indicates that the majority of power of the signal at the end of laps was not biological. Such conclusions can be derived from the very regular ‘spiky shape’ of the spectrum that is not observed in biological systems. Thus, the influence of this technical noise should be taken into account (i.e. subtracted) to achieve non‐biased estimates of EEG properties.
Figure 3.
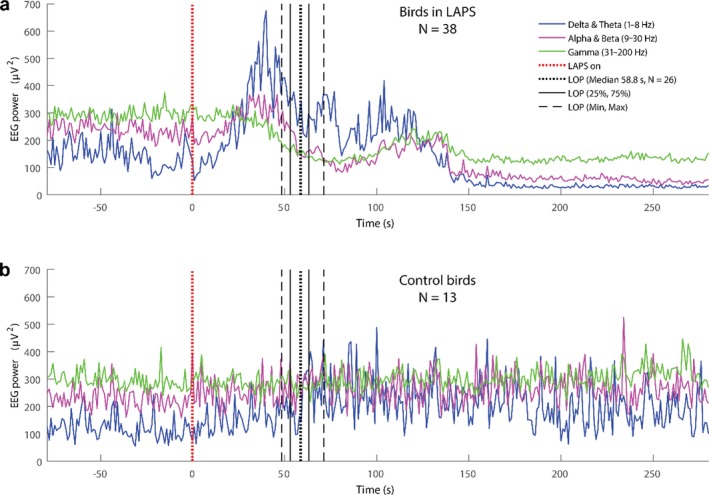
Dynamics of EEG power of selected frequency bands (drawn in different colours) in birds undergoing LAPS and in control birds. LAPS onset is marked by vertical red dotted line at time zero. Loss of posture (LOP) is marked by vertical black lines (median [dotted], quartiles [solid] and range [dashed]). (a) Dynamics of power in Delta & Theta (1–8 Hz), Alpha & Beta (9–30 Hz) and Gamma (31–200 Hz) bands in birds undergoing LAPS. Median values for 38 birds are plotted. (b) Dynamics of power in the same frequency bands in control birds
Figure 4.
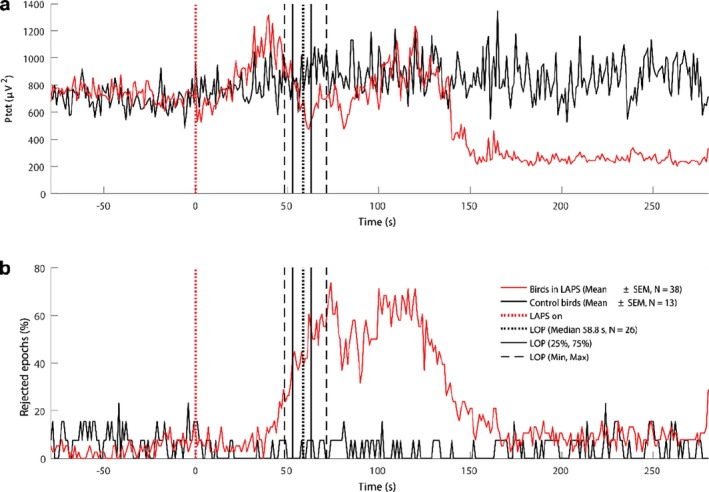
Dynamics of total EEG power (Ptot, a) and percentage of rejected epochs (b) in birds undergoing LAPS (red line) and in control birds (black line). Ptot represents power of the signal in the frequency range (1–200) Hz. LAPS onset is marked by vertical red dotted line at time zero. Loss of posture (LOP) is marked by vertical black lines (median (dotted), quartiles (solid) and range (dashed)). Percentage of rejected epochs is an indirect measure of birds’ motor activity (including clonic convulsions). Note that an onset of an increase of motor activity at around 50 s coincides with animal arousal manifested by a drop in total EEG power (see also Figure 4a)
Figure 5.
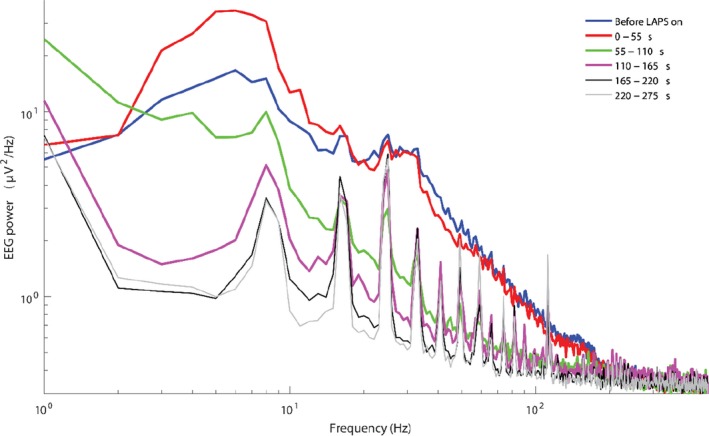
EEG power spectrum (median) in birds (n = 38) during LAPS. The BLUE line describes the pre‐stun EEG power spectrum. The other lines represent temporal dynamics of EEG spectrum during five sequential 55‐s intervals in LAPS. Regular peaks in the power spectrum at the end of LAPS procedure are technical artefacts. During beginning of LAPS they are masked by high‐amplitude EEG
Figure 6a shows smoothed dynamics of Ptot as shown earlier in Figure 4a. The proximity of the time point of the steepest decline of total EEG power interpreted as arousal (51.2 ± 5.3 s, mean ± SEM) to the detected in video records LOP (58.8 ± 1.3 s, mean ± SEM), suggests dependence of these two events. After subtraction of background technical noise, the total EEG power with the removed background technical noise (Pto − Pnoise, Figure 6b) declines to 10% of pre‐stun level in 140.6 ± 5.5 s (mean ± SEM). This time point is interpreted as the onset of an isoelectric EEG, a point of no‐return, with good approximation to the point, after which an animal cannot recover.
Figure 6.
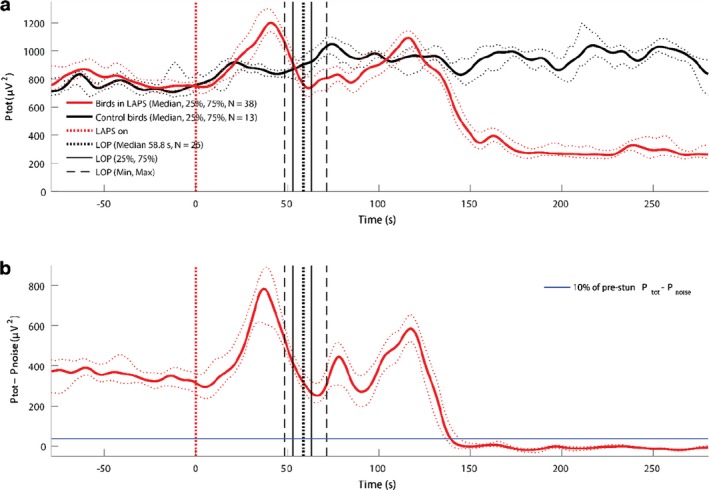
Dynamics of Ptot (total EEG power) in chickens undergoing LAPS and in control birds. Median values and quartiles for the median values of the total EEG power are shown for birds undergoing LAPS (N = 38, red line) and in control animals (N = 13, black line). (a) Dynamics of total EEG power Ptot in birds during LAPS and control animals smoothed by convolution with the Gaussian curve (see Appendix G for details). Note an increase of Ptot during the, approximatively, 50 first seconds of LAPS with a rapid decline at T = 51.2 ± 5.3 s (mean ± SEM of the time point of maximal decline rate). (b) Total EEG power in birds during LAPS with subtraction of the noise of the recorder estimated from the last 80 s of the recording session, i.e. when the birds are supposed to be dead. The EEG power decreases below 10% of pre‐stun EEG power at time T = 140.6 ± 5.5 s (mean ± SEM) assumed to be an isoelectric state. The 10% level of pre‐stun EEG power is marked by blue horizontal line
The LOP can be suggested as a rough proxy for a progressive fall into an unconscious state and for the loss of sensitivity, including loss of nociception. However, at which time point the birds stop feeling pain, and have no response to sensory stimuli in their usual way (that is an indicator of unconsciousness) was not, unfortunately, accessed by the applicant in LAPS. Thus, to what extent the LOP coincides with the loss of nociception in LAPS is not completely clear. From experience of anaesthetic practice during surgery it is known that loss of sensitivity is observed when the so‐called GA plane is reached. This happens after the LOP. It is worth noting that published scientific literature concerning exposure of broilers to normobaric hypoxia (2% residual oxygen) induced with argon indicated that somatosensory evoked potentials in the brain were abolished on average at 32 s (Raj et al., 1998), whereas, another study showed LOP occurred at 13 s after exposure to hypoxia (Raj, 1997).
The GA plane is preceded by a sedative state and then a non‐responsive state. Thus, in LAPS, the loss of pain sensitivity is expected somewhere after the LOP (58.8 ± 1.3 s) but before the onset of an isoelectric state (140.6 ± 5.5 s). To estimate at which time point the GA plane might be reached in LAPS the applicant proposed to extrapolate the data of sevoflurane anaesthetised hens (Sandercock et al., 2014, Physiology & Behavior, https://doi.org/10.1016/j.physbeh.2014.05.030) to LAPS. In this publication, the medial frequency F50 in awake hens was 24 ± 5 Hz (mean ± SD, N = 12), but dropped down to 7 ± 2 Hz during GA. Thus, the level 7 Hz of F50 was proposed as a neurological criterion of an anaesthetic state. According to Figure 2 of Paper 7, this level was reached at a time point 38 or 44 s, but this looks a little bit early because this is before the LOP which takes place at 60.8 s. In addition, these changes are also reported in sham birds that were not subjected to LAPS. Therefore, this EEG criterion is not considered to be valid. Figure 2 of Paper 8 gives larger latencies to reach the 7 Hz level of F50. For the birds in the darkness, the earliest crossing of this level took place at 56 s, but this might be due to random fluctuations of the signal. If one plots a regressive line over time interval [0–56] s it will cross 7 Hz level at time 68 s (Figure 2a of Paper 8). This time coincides with the time point of crossing of 7 Hz level in light conditions, where variability of the signal is somewhat smaller. Thus, the most likely estimate of unconsciousness in LAPS is 68 s (Figure 2a of Paper 8). Thus, the most trustful estimate of the mean value of unconsciousness in LAPS is 68 s. This looks reasonable because this value falls between LOP (58.8 ± 1.3 s) and onset of an isoelectric state (140.6 ± 5.5 s).
The suggested reason for the different estimates presented in Paper 7 may be that there was a somewhat smaller extent of technical background noise in this article when compared to Sandercock et al. (2014) and Paper 8. To check whether background noise affects F50, we computed F50 for the data set of 38 birds with and without subtraction of the spectrum of the background noise estimated from the last 80 s of the LAPS procedure (Figure 7). We smoothed the curve by convolving it with Gaussian smoothing to minimise the influence of random fluctuations (see Methods). As can be seen from the chart, F50 did not reach 7 Hz level at all in Figure 7a showing F50 computed for a signal without noise subtraction. However, when the noise is subtracted the F50 of the pre‐stun period is about 15 Hz, and not 24 Hz described in Sandercock et al. (2014) and Papers 7, 8. However, as seen in Figure 2 of Paper 8, there is a linear trend in F50 at the beginning of LAPS. If we scale the 7 Hz level by the factor (‘pre‐stun F50 with noise subtraction’)/(‘pre‐stun F50 original’) we get a new threshold for GA level for the signal without noise shown by the blue line in Figure 7b. This level will be crossed by the curve at time 68.2 ± 11.0 s (mean ± SEM) that is perfectly matched to 68 s estimated earlier for slightly different data set. Variance was estimated by using a bootstrap methodology. The GA level at 7 Hz constituted 25% from the original pre‐stun level 24 Hz. While, after noise subtraction this ratio remains the same, it seems to be reasonable to identify a GA level not precisely at ‘7 Hz’, but as 25% from the level observed in an awake animal (during the pre‐stun period). In this way, this criterion will be also applicable to a signal with noise subtraction whose baseline F50 level is about 15 Hz. Supposing that there is independency of these two measurements, the duration of the period of concern, from an animal welfare perspective as the animal is awake and conscious, that lasts from arousal 51.2 ± 5.3 to GA plane 68.2 ± 11.0 s can be estimated as 17.0 ± 12.2 s.
Figure 7.
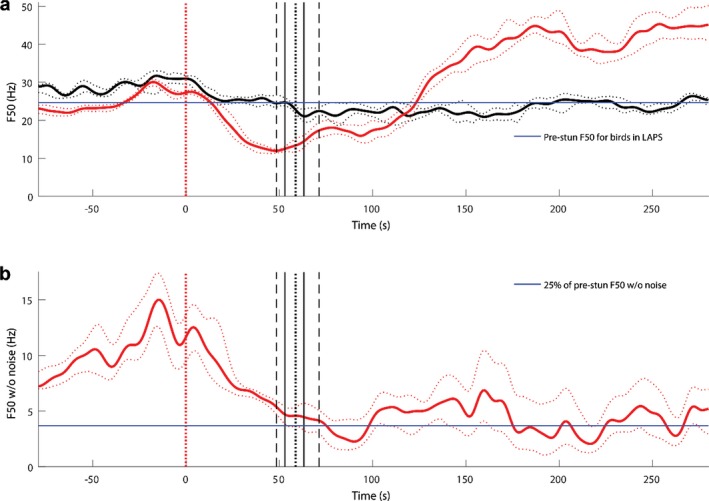
Dynamics of F50 (median frequency that splits the power spectrum in two equal parts) in chickens undergoing LAPS and in control birds. Median values and quartiles for the median values of the F50 are shown for birds undergoing LAPS (N = 38, red line) and in control animals (N = 13, black line). (a) Dynamics of median frequency F50. Note an increase of F50 in the experimental birds towards the end of LAPS. An essential part of this effect is caused by noise in the recording system. The spectrum of this noise should be subtracted from the signal for a non‐biased F50 estimate. (b) Median frequency F50 in birds during LAPS with subtraction of the noise spectrum of the recorder estimated from the last 80 s of the recording session when the birds are supposed to be dead. Median frequency F50 decreases below 25% of pre‐stun F50 at time T = 68.2 ± 11.0 s (mean ± SEM) assumed to be associated to a state of unconsciousness. The 25% level of pre‐stun F50 is marked by blue horizontal line
Necropsy data
EFSA requested specific information on anatomo‐pathological data from broilers undergoing the LAPS process in order to gather more information about potential critical points, namely the impact of decompression on the intestines (due to trapped gas expansion) and on the eardrum. The applicant performed a dedicated experiment which provided data indicating the absence of macroscopic lesions in the ear and intestines, which is reassuring from the animal welfare point of view.
On the other hand, haemorrhagic lesions of different intensities were observed in the calvarium (skull), brain, heart and lungs, which were either not observed or occurred at a lower intensity in control broilers killed with an overdose of barbiturate. The applicant suggested possible causes including decompression, recompression, hypoxia, agonal haemorrhage and trauma. The authors concluded that these lesions were not indicative of pain or distress. In fact, the results provided did not allow clear assessment of the link between these causes and the lesions observed (due to a lack of an appropriate control group, i.e. hypoxia in normobaric conditions). In addition, it was not possible to discriminate between changes occurring prior to loss of consciousness, and those occurring in an unconscious state. Together, these limitations did not permit the assessment of animal welfare outcomes (absence/presence of pain, distress and suffering) to be carried out.
3.2.1.4. Correlation between behavioural, physiological, neurological responses and necropsy findings
The following considerations, drawn from the combination of all the information available in the submitted papers and data, relate to broilers of Cobb genotypes only (see Figure 8). The measurements are reported as statistical means or medians for the recorded variables.
Figure 8.
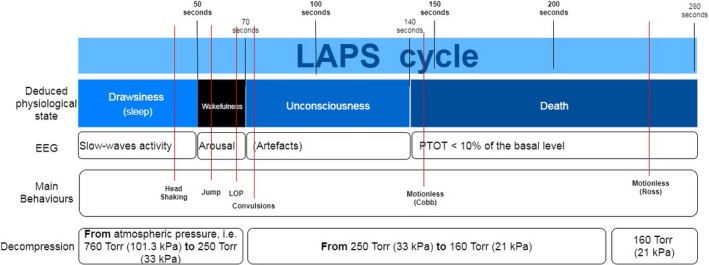
Graphical representation of one LAPS cycle combining different sources of information, i.e. seconds elapsed, EEG, ethogram, decompression and deduced physiological state of the animal
Based on EEG quantitative analysis, the decline of power content starts at t40 (40 s) of the LAPS process when the broilers are believed to progressively fall into a state of drowsiness (increase in low frequency activity suggestive of a sleep‐like state possibly induced by low availability of oxygen and, to a lesser extent, the darkness in the chamber).
Around t50, the EEG quantitative analysis showed arousal, indicating awakening of the birds. The possible explanation for this EEG arousal could be: (i) dryness of the mucosae due to rapidly decreasing humidity in the chamber; (ii) expansion of gases in the intestine; (iii) the level of oxygen below a life‐threatening level.
At t72, the birds start showing tonic/clonic convulsions, interpreted in the literature as a sign of spinal reflexes occurring as a consequence of liberation of the spinal cord from the inhibition of higher brain centres indicative of unconsciousness. It is worth noting that just before this event (t68) the F50 goes below the threshold of 25% of the pre‐stun level, which, although this is not a scientifically validated parameter, could be seen as evidence of unconsciousness.
For this reason, the experts considered the time between t51 and t72 (around 20 s) to be the period of major concern from an animal welfare perspective, as the animals are awake and conscious, while the environment is rapidly deviating further and further from a natural pressure, partial pressure in oxygen, humidity and gas tension condition. During this period, three main behaviours possibly indicative of suffering and/or distress can occur: headshaking, open bill breathing and jumping. However, it has to be noted that during the very same period, at t58, the birds show LOP, broadly accepted as the earliest behavioural indicator of the induction of loss of consciousness. Nevertheless, as unconsciousness is a progressive process, LOP may not be, on its own, considered as the most robust indicator of unconsciousness as such.
Headshaking, jumping and open bill breathing are shown before LOP (at t58) by 48%, 35% and 15% of the chickens, respectively. Headshaking has been observed across all three phases (starting phase, drowsiness, arousal) while jumping ‘peaks’ (latency in relation to LOP less variable and median close to LOP) shortly before LOP and open bill breathing mainly starts after LOP.
The quantitative EEG analysis also showed that at t140, the Ptot is reduced to below the threshold of 10% of the pre‐stun level. This is also consistent with the time to occurrence of motionless (t146) in the ethogram. These two parameters are indicators of death of the animal.
The lack of a proper control group in the necropsy study, does not allow identification, with clarity, the aetiology of the recorded lesions. In addition, it is not possible to discriminate between changes occurring prior to loss of consciousness and during unconsciousness.
3.2.1.5. Comparison with other methods
The main statistical parameters (proportion of broilers and measure of central tendency) reported or estimated by EFSA from the submitted raw data, were listed for each behavioural indicator recorded in LAPS. For the same behavioural indicators, the same statistical parameters reported for the stunning methods currently available, were gathered from the scientific literature, by means of an extensive literature search, and reported for a quantitative comparison (Table 18).
Table 18.
Comparison with other methods: mean/median latency (seconds)
| LAPS | CO2 | CO2 + Inert gases | Inert gases | Electrical Water‐bath | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ar | N2 | Ar | N2 | |||||||||||
| Behavioural event | Mean − Median latency | Range | Mean of mean values | Range | Mean of mean values | Range | Mean of mean values | Range | Mean of mean values | Range | Mean of mean values | Range | Mean of mean values | Range |
| Loss of posture | 58.47 – 58.42 | 40.07–106a | 26 | NA | 15 | NA | 7.5 | NA | 16.8 | NA | 11.5 | NA | NA | NA |
| Lying | 61.56 – 60.31 | 41.67–130.4 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Motionless | 146.1 – 145.8 | 86.12–260a | 129.25 | NA | 82.5 | NA | 80.71 | NA | 107.5 | NA | 94.25 | NA | NA | NA |
| Ataxia | 41.61 – 41.91 | 16.82–65.16 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Clonic/Tonic convulsions | 72.21 – 69.4 | 41.41–147 | NA | NA | 15 | NA | NA | NA | 25 | NA | NA | NA | NA | NA |
| Deep inhalation | 87.61 – 89.35 | 4.67–157.5 | 7 | NA | 4 | NA | NA | NA | 9 | NA | NA | NA | NA | NA |
| Head shaking | 42.21 – 45.61 | 1.27–107.1 | 3 | NA | 4.6 | NA | NA | NA | 8.5 | NA | NA | NA | NA | NA |
| Jump | 53.43 – 53.3 | 35.43–78.31 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Open bill breathing | 64.91 – 63 | 11.11–154.4 | 7 | NA | 4 | NA | NA | NA | 9 | NA | NA | NA | NA | NA |
Max values from the scientific report on the experiment run by the applicant to detect potential anatomo‐pathological lesions due to decompression.
The data extraction process identified that, for the currently available methods, (i) the definition of the behavioural indicators and their interpretation were different in different studies (e.g. motionless, respiratory disruption); (ii) the composition of the gas mixtures were described differently; (iii) the experimental conditions and methodologies used were different in the different studies; (iv) the reporting quality was often not fit for the purpose (see the reporting quality criteria in the EFSA guidance). In addition, no reliable information, when present, was available.
The final data extraction table for the frequency (number of birds showing the behaviour out of the total number) could not be used because of the lack of completeness and reliability and therefore is not considered in this assessment. However, the latency to LOP was found to be useful for assessment between LAPS and other gas methods (see Table 18). In fact, the time to LOP is the earliest behavioural indicator of onset of unconsciousness and it appears to be shorter in CAS methods owing to the fact that these gas methods were designed to induce rapid unconsciousness and onset of death. On the other hand, LAPS was designed to produce a slower rate of induction of unconsciousness in order to avoid the known poor animal welfare consequences linked to rapid decompression (AVMA, [Link]).
Considering the degree of incompleteness of the comparative table, it emerges clearly that it is not possible to perform a full quantitative statistical comparison. The reasons for the lack of relevant information are mainly linked to;
the fact that all the currently available stunning methods have been approved by the European Commission before the publication of the EFSA guidance. Consequently, the data and the information recorded in the scientific literature are not consistent across the different studies, are not in line with the quality requirements laid down in the EFSA guidance and therefore are not harmonised.
the fact that the stunning methodologies currently available are very heterogeneous. As a consequence, if a comparison between the LAPS and the gas stunning methods is conceptually feasible as being very similar from a procedural point of view, a comparison with the electrical water‐bath stunning methods is almost impossible as LAPS and electrical water‐bath stunning are based on completely different stunning principles which are reflected in the procedure.
This exercise, however, is of great value as:
it was possible to compare the time to LOP as one of the most useful indicators of loss of consciousness.
this analysis has provided a well‐defined picture of the LAPS, describing the process in detail and highlighting the aspects that deserve further investigation or that represent a matter of welfare concern.
this assessment highlights the need for harmonised data and information related to the currently allowed methods which would allow similar analysis as that which has been performed for LAPS.
3.2.2. Qualitative assessment: ranking hazards
Data driven comparisons of the stunning methods have been demonstrated to be limited because only for the LAPS method (L, hereinafter) were sufficient quantitative data provided. For the alternatives of electrical water‐bath (W) and gas methods (G) only few data aspects had comparable quality (see Table 18). Therefore, it was agreed to use expert knowledge external to EFSA in order to evaluate the list of hazards to broiler chicken welfare at stunning.
3.2.2.1. Identification of the hazards
As a first step, the main phases of the stunning and killing process were identified. For each phase, a description of the events that take place during the process was provided for each method (see Appendix F for more information).
Tables 19 and 20 summarise the final list of hazards, potentially occurring when the animals are conscious, and the stunning methods they pertain to.
Table 19.
List of identified hazards, potentially occurring when the animals are conscious, associated with alternative stunning methods in industrial stunning of broiler chicken assuming full compliance with technical protocol i.e. no fault. (L) LAPS, (W) Electrical water‐bath, (G) gas mixtures incl. CO2
| L | Gas expansion in body cavities/internal organs |
| L | Removal of air |
| L | Decreasing air humidity |
| L | Noise |
| W | Unintended electric shock |
| W | Neck cutting |
| W | Bleeding |
| W | Handling |
| W | Hanging and compression of the legs |
| G | Acidic gas or gas mixture |
| G | Respiratory stimulant gas or gas mixture |
| G | Tipping/Tilting |
3.2.2.2. Methodology in brief
The list of hazards identified by EFSA was subjected to individual expert consideration for a ranking by the expected consequence given that the animal would be exposed to a certain hazard of the list. In line with Animal Welfare (AW) science, the ranking was performed taking the magnitude of the consequences in to account. Therefore, the experts involved in the study were provided with information on both the frequency and duration of exposure to the hazard during standard commercial stunning of broiler chicken. The experts were expected to integrate these data to assess the magnitude of the consequences resulting from these exposure scenarios and to rank the hazards accordingly. As the outcome, an ordinal scale was sought for the hazards, ranking these according to the associated welfare consequence. This outcome was expected to facilitate the statistical testing of the hypothesis that LAPS hazards rank worse than the hazards involved in other stunning methods. Rejection of the hypothesis would imply that LAPS is at least not worse than the already practiced methods as regards the associated risks of poor welfare.
Secondary data were recorded during the expert judgement procedure. These were; the rank position assigned to each hazard item in the final ordered list of each individual expert, plus any additional verbal comments.
The data recording involved three methodological parts: (i) a web‐based elicitation platform, allowing the ranking of the identified hazards blinded by stunning methods (Roodle‐Webinterface, see Annex A), (ii) expert invitation and conduct, (iii) data analysis including data aggregation by stunning method, estimation of summary ranks and testing of differences in ranking tendency between stunning methods, i.e. calculation of the median rank using the Wilcoxon rank sum test.
The ordinal scale of the hazards was retrospectively reassigned with the associated stunning method (W, G, L). Hence, rank positions relative to the stunning method could be subjected to hypothesis testing. The rank test identified systematic deviations in the median ranks between the cohorts. The hypothesis tested assumed that LAPS hazards more often rank higher (worse) than the hazards of other stunning methods. Rejection of the hypothesis would imply that LAPS is at least not worse than the already practiced methods.
Ranking associated with the items resulted in an ordinal scale. However, the distance between the ranked items was not measurable. Thus, the differences in severity between two items ranked nearby to each other would differ when pairs of hazards were considered. In other words, it could not be assumed that the difference between ranks was equidistant, even though the numbers assigned were. This was in contrast to interval data, in which the difference between responses could be calculated and the numbers would then refer to a measured ‘something’ (Sullivan and Artino, 2013).
Per hazard item, the individual expert ratings were summed over all experts. The hazard‐wise rank sums were associated with the group identification L, W and G. The respective vectors of stratified rank sums were used as input in the non‐parametric Wilcoxon rank sum test (R function Wilcoxon.test). Nonparametric tests do not make an assumption about the ‘shape’ of the distribution from which the study data have been drawn. Nonparametric tests are less powerful than parametric tests and usually require a larger sample size (n value) to have the same power as parametric tests to find a difference between groups when a difference actually exists (Sullivan and Artino, 2013). However, the non‐parametric tests can allow tendency testing even if data are non‐normal and values have intrinsically no metric meaning.
3.2.2.3. Results of the ranking exercise
The study involved 12 hazards (Table 20) and 32 experts were invited. The elicitation resulted in 19 valid sets of response data from the 19 experts who submitted their evaluation. The EFSA WG on LAPS did not contribute to the ranking.
The descriptive results are shown in Table 21. The resulting ranking is robust across the experts and their background. Single deletion of experts did only interchange the order of the first three, ‘Acidic gas or gas mixture’ vs ‘Gas expansion in body cavities/internal organs’ and the last two hazards.
Table 21.
Summary of the survey data (see Annex Table A.1 for full details). The hazards are ordered by the value of the rank sum taken over all 19 responses per hazard item. The column Min and Max reflect the sensitivity of the overall outcome regarding the step‐wise exclusion of one individual experts
| Method | Hazard | Rank estimate (n = 19) | Min | Max |
|---|---|---|---|---|
| W | Unintended electric shock | 12 | 11 | 12 |
| W | Neck cutting | 11 | 11 | 12 |
| W | Bleeding | 10 | 10 | 10 |
| G | Acidic gas or gas mixture | 9 | 8 | 9 |
| W | Hanging and compression of the legs | 8 | 8 | 9 |
| G | Respiratory stimulant gas or gas mixture | 7 | 6 | 7 |
| L | Gas expansion in body cavities/internal organs | 6 | 6 | 7 |
| L | Removal of air | 5 | 4 | 5 |
| W | Handling | 4 | 4 | 5 |
| G | Tipping/Tilting | 3 | 3 | 3 |
| L | Noise | 2 | 1 | 2 |
| L | Decreasing air humidity | 1 | 1 | 2 |
The ranking of hazards according to the rank sum over all experts is shown in the table above and indicative of L ≤ G < W. Median hazard are W = 10; G = 6; L = 3.5.
Further testing was carried out using the Wilcoxon test against the hypothesis that L hazards are equally or worse ranked by the experts than the W and G hazards. Testing may be understood as checking for median difference between the two set of rank values of e.g. L vs W hazards. The null hypothesis was that the probability of observing a randomly selected value from the first group (e.g. L hazard's rank) that was larger than a randomly selected value from the second group (e.g. W hazard's rank) equals one half (i.e. by chance). We applied the hypothesis testing for the alternatives ‘laps’ (Gas expansion in body cavities/internal organs; Removal of air; Decreasing air humidity, Noise), ‘noLaps’ (‘water’ + ‘gas’) with ‘water’ (Unintended electric shock; Neck cutting; Bleeding; Hanging and compression of the legs; Handling) and ‘gas’ (Acidic gas or gas mixture; Respiratory stimulant gas or gas mixture; Tipping/Tilting).
Wilcoxon rank sum test
data: laps and exist: W = 28, p‐value = 0.025
difference in location 5; 95% CI: (1, Inf)
The main contribution is by the hazards related to W. Limiting the test to L > W still rejects the hypothesis of Hazards‐L ranked > Hazards‐W.
data: laps and water: W = 18, p‐value = 0.032
difference in location 6; 95% CI: (2, Inf)
Any other test is not providing statistical support for the apparent observation of ordered median ranked hazards W > G (>) L
data: laps and gas: W = 10, p‐value = 0.115
data: gas and water: W = 12, p‐value = 0.125
The hypothesis of homogeneous ranking of the hazards between LAPS and electrical water‐bath; or LAPS and any existing method was rejected, confirming the subordinate ranking of LAPS hazards as statistically significant at 95% probability. The limitation to differentiate between laps and gas is due to the small sample size of 3 vs 4 hz.
Noteworthy the ranking of particular hazards was rather homogeneous throughout the majority of experts (Annex A – Figures 3 and 4). There were three experts that did show a lower level of agreement on the level of individual rankings of hazard items (expert J, M, N in Table A.1 of the Annex).
Table A.1.
Summarised information for the eligibility assessment regarding the intervention parameters as provided in the five scientific papers in the dossier
| Parameter | Data provided | Reference |
|---|---|---|
| Animal density |
Commercial broiler chickens. Type: Ross 708 (Gallus gallus domesticus). 50 individuals (Age = 49 days) + 50 groups of 20 individuals (Age = 50 days) Weight (kg): Mean = 3.4; Range = 2.6–4.3 Trial density:
|
Mackie et al. (2016) (Paper 5) |
|
Commercial broiler chickens. Type: Cobb 500 (Gallus gallus domesticus). Age = 36–37 days Weight (kg): Mean = 2.3 kg; Range = NA Trial density: 2 birds in a reduced scale transport module (0.76 m × 1.21 m) = 2.2 birds/m2; body weight (kg)/m2: Mean = 5.0; Range = NA |
Martin et al. (2016a) (Paper 6) | |
|
Commercial male broiler chickens. Type: Cobb 500 (Gallus gallus domesticus). Age = 38–39 days Weight (kg): Mean = 2.36; Range = NA Trial density: 3 birds in a reduced scale transport module (0.76 m × 1.21 m) = 3.3 birds/m2; body weight(kg)/m2: Mean = 7.7; Range = NA |
Martin et al. (2016b) (Paper 7) | |
|
Commercial broiler chickens. Type: Cobb 500 (Gallus gallus domesticus). Age = 44–45 days Weight (kg): Mean = 2.96 ± 0.41; Range = NA; 95% CI = 2.14–3.78 Trial density: 2 birds in a reduced scale transport module (0.76 m × 1.21 m) = 2.2 birds/m2; body weight(kg)/m2: Mean = 6.4; Range = NA; 95% CI = 4.7–8.2 |
Martin et al. (2016c) (Paper 8) | |
|
See Papers 6, 7 and 8 It is not clear if additional birds were used (for the experiment using a not further defined ‘chamber 6’). Should this be the case, see below for the details: Commercial broiler chickens. Type: Ross Age = data not available in the paper – Reported as ~ 42 days in the annex submitted in the dossier Weight (kg): Mean = 2.5; Range = NA; 95% CI = NA Trial density: data not available in the paper – Reported as ~ 2 birds/m2; body weight(kg)/m2: 5.0 (average) in the annex submitted in the dossier |
Holloway et al. (2017) (Paper 9) | |
| Duration of intervention a | Total LAPS evacuation process = 280 s, followed by return to atmospheric pressure (recompression cycle is about 20 s) | Mackie et al. (2016) (Paper 5), Martin et al. (2016a) (Paper 6), Martin et al. (2016b) (Paper 7), Martin et al. (2016c) (Paper 8), Holloway et al. (2017) (Paper 9) |
| Rate of decompression |
Rate of decompression changes over time as a function of the temperature (see ‘Temperature’ in this table). Six decompression curves (‘temperature settings’) are created automatically by a computer program based on ambient temperature to control the extraction of O2 from the environment. The pressure curves of all temperature settings are identical in the first phase of LAPS (up to the 67th sec) and all the curves converge on a final pressure of 20.7 kPa (after 280 s). The process consists of two phases: in the first phase, the vacuum chamber pressure is reduced from atmospheric pressure to an absolute vacuum pressure of ~ 250 Torr (~ 33 kPa) in ~ 67 s. In the second phase, a sliding gate valve is partially closed, gradually reducing the effective pumping speed by ‘choke flow’, to a minimum chamber pressure of ~ 150 Torr (~ 20 kPa). The rate of reduction of chamber pressure in the second phase is varied in relation to starting ambient temperature. There is a gradual decrease in pressure in the vacuum chamber from ∼ 760 Torr (101.3 kPa, 29.92 in. Hg) to ∼250 Torr (33.3 kPa, 9.8 in. Hg) in about 67 seconds, i.e. at an average rate of −7.5 Torr/sec (1 kPa/s). The first region of decompression refers to that pumping time period when the vacuum gate valve was fully open. The average pressure and time from 10 and 5 runs without and with birds, respectively, plus the rates of pressure decrease over the increments between data points (data were taken every 5 inches of Hg gauge pressure in region 1) |
Mackie et al. (2016) (Paper 5), Martin et al. (2016a) (Paper 6), Martin et al. (2016b) (Paper 7),Martin et al. (2016c) (Paper 8), Holloway et al. (2017) (Paper 9) |
| Rate of changes in partial pressure of oxygen |
Ambient temperature affects air density and water vapour pressure and thereby oxygen levels and the time at the minimum total pressure of ~ 160 Torr (~ 21 kPa) varied from ~ 120 to ~ 220 s to ensure an effective stun within the 280 s of each cycle. The system maintains an oxygen concentration of < 5% for at least 2 minutes, which ensures that birds are irreversibly stunned. To obtain a similar absolute concentration of oxygen during stunning at low and high temperatures (see Annex of Paper 9, Figure A9/3b), stunning at high temperatures is performed with a higher pressure. The reduction in total atmospheric pressure results in a reduced oxygen partial pressure. A range of pressure curves based on temperature setting are created automatically by a computer program to control the extraction of O2 from the environment. The fractional concentration of oxygen was measured for each of nine different pump downs and the average oxygen fraction vs pump down time is shown in Table 3 |
Martin et al. (2016a) (Paper 6), Martin et al. (2016b) (Paper 7), Martin et al. (2016c) (Paper 8), Holloway et al. (2017) (Paper 9) |
| Temperature/humidity/illumination of the chamber |
Light: absent Temperature setting: single temperature setting (not specified) Ambient Temperature: N/A Humidity: N/A |
Mackie et al. (2016) (Paper 5) |
|
Light: absent Temperature setting: single temperature setting (not specified) Ambient temperature: 13.5 ± 0.5°C (average over the cycles, cycles run over 2 days) Humidity: 76.3 ± 0.6% (average over the cycles, cycles run over 2 days) |
Martin et al. (2016a) (Paper 6) | |
|
Light: absent Temperature setting: 3 (applied between 13 and 18°C) – 16 cycles Temperature setting: 4 (applied between 5 and 12°C) – 14 cycles Ambient temperature: 16 ± 0.3°C (average over the cycles) Humidity: 63.8 ± 0.5% (average over the cycles) |
Martin et al. (2016b) (Paper 7) | |
|
Light: 20 cycles (2 birds/cycle) absent + 20 cycles (2 birds/cycle) present – six 17 W LED lights resulting in 500 lux Temperature setting: 4 (applied between 5 and 12°C) – 40 cycles Ambient temperature: 11.6 ± 0.3°C (average over the cycles, over 2 days) Humidity: 51.8 ± 1.8% |
Martin et al. (2016c) (Paper 8) | |
|
Temperature setting 1 ‘Very hot’: applied > 27.2°C in Papers 1 and 2 Temperature setting 2 ‘Hot’: applied 18.9 to 26.7°C in Papers 5 and 9 Temperature setting 3 ‘Middle’: applied 13.3 to −18.3°C in Papers 5, 6, 7 and 9; Temperature setting 4 ‘Cold’: applied 5.0 to 12.8°C in Papers 5, 7, 8 and 9 Temperature setting 5 ‘Very cold: −3.3 to 4.4°C, not used in papers Temperature setting 6 ‘Freezing: −45.5 to –3.9, not used in papers Light: see Papers 6, 7 and 8 Temperature setting: see Papers 6, 7 and 8 Ambient temperature: see Papers 6, 7 and 8 Humidity: see Paper 6, 7 and 8 It is not clear if an additional experiment was run and if so, under which conditions |
Holloway et al. (2017) (Paper 9) and related annexes | |
| Maximum stun‐to‐stick/kill interval(s) | NOT RELEVANT | NOT RELEVANT |
| Calibration of the LAPS equipment and monitoring system |
The system is managed by a programmable logic controller (PLC), which results in precise and accurate monitoring and control of pump down (pressure vs time). The pressure was measured by a direct reading digital diaphragm gauge (type P62 from Kaeser) under microprocessor control. This Kaeser gauge was calibrated with an Ashcroft precision bellows mechanical total pressure gauge with 0.25% accuracy (type P62 from Kaeser) under microprocessor control. This Kaeser gauge was calibrated with an Ashcroft precision bellows mechanical total pressure gauge with 0.25% accuracy. Gradual reduction of oxygen partial pressure that was measured by a solid‐state electrochemical oxygen sensor. The oxygen fraction was measured using a PureAire Oxygen Sensor based upon solid‐state electrochemistry |
Holloway et al. (2017) (Paper 9) |
Referring to the legal parameter ‘duration of exposure’ of other stunning methods.
3.3. Consistency with other findings
The only published papers on the LAPS methodology reporting behavioural indicators were by the same research group. The consistency of the findings in the submitted dossier of the second application was compared with the results of the papers submitted the first time that consisted of four publications:
STUDY 1 – ‘Physiological responses to low atmospheric pressure stunning (LAPS) and implications for welfare’ – McKeegan et al. (2013);
STUDY 2 – ‘A new humane method of stunning broilers using low atmospheric pressure’ – Vizzier‐Thaxton et al. (2010)’;
STUDY 3 – ‘The effects of low atmosphere stunning and deboning time on broiler breast meat quality’ – Schilling et al. (2012);
STUDY 4 – ‘The Effects of Low‐Atmosphere Stunning and Deboning Time on Broiler Breast Meat Quality’ – Battula et al. (2008).
In study 1, the estimated time to loss of consciousness was reported to be approximately 40 s, but it is not clear whether this is the minimum, maximum or average, and the range of time to LOP was reported to be from 20 to 69 s. These times are lower than those presented in the current studies. In this study, no information on animal‐based measures associated with pain, distress and suffering during the induction of unconsciousness and insensibility was provided.
In study 2, the average time to LOP was reported to be 123.6 s (58.7 + 64.9 s), which is considerably longer than the time to LOP reported in Study 1 and in the current findings. The histopathological evidence reported of haemorrhagic lesions in the lungs and other organs are consistent with the data provided in the current submission.
No data on the measures ‘motionless’ or ‘lying’ were reported in those papers.
In the other two studies (3 and 4) no information about behavioural, EEG nor ECG data were provided.
In summary, there are actually discrepancies between those four papers and the ones included in the current submission (e.g. ethogram data, description of the intervention). However, the four previously published papers were not included in this assessment because they have failed previous eligibility assessment by EFSA. For this reason, EFSA does not consider these discrepancies against the conclusions drawn.
3.4. Current application of LAPS under commercial conditions
LAPS was installed by TechnoCatch, LLC at a Fort Smith, AR, USA poultry processing plant in the fourth quarter of 2010 following reception of ‘No Objection’ status by the USDA in May 2010. The system at this particular plant consists of four chambers which operate concurrently to provide a throughput of 21,600 birds per hour (180 birds/minute per 2 lines for a total of 360 birds/minute). The plant operates two 8‐h shifts per day for 5 days per week. The system has been used to irreversibly stun birds ranging in weight from 2.2 to 4.1 kg. This system is described in detail in Paper 1 (McKeegan et al., 2013), Paper 2 (Vizzier Thaxton et al., 2010), Paper 9 (Holloway et al., 2017) and in the UDSA ‘No Objection’ document. (Pritchard D., 2017. Personal communication).
3.5. Physical aspects involved in LAPS
A review of the details related to the technical aspects of the LAPS machinery related to the physical principles underpinning the decompression operated was performed in order to identify potential critical points during the process, as described in Paper 9.
The theoretical model of the decompression curve (pressure over time) is probably a simplification of a more complex system. In fact, only decompression of the chamber is considered, and water evaporation inside it is neglected. This is why there are differences between the data presented in the model (24 Torr at 144 s) and the reality (150 Torr at 153 s). (Holleville, 2017, personal communication).
The evolution/progression of the partial pressure changes of oxygen along the total pressure gradient seems to be coherent: the latter is divided by 5 (i.e. to approximately 20% of its original) in 160 s, while the former is divided by 5.4. This also means that the partial pressure of oxygen decreases faster than the total pressure, due to outgassing of water vapour, which presumably comes from the walls of the chamber and from the animals inside the chamber. It is estimated that the machinery pumps out a volume of water vapour of around 230 L (at atmospheric pressure, i.e. around 1–2 L of liquid water) per decompression cycle from a chamber volume of 18,000 L (Holleville, 2017, personal communication). It was not possible to estimate the amount of water originating from the birds.
4. Conclusions
4.1. Summary results of the assessment of the LAPS procedure
The LAPS procedure, when applied as described in the different published papers, leads to loss of consciousness followed by death in all birds.
The LAPS procedure does not induce immediate unconsciousness.
During the first 50 s of the LAPS procedure, based on the EEG quantitative analysis in broilers weighing on average 2.9 kg, the broiler chickens are likely to fall into a state of drowsiness, presumably due to decreased oxygen concentration and, to a lesser extent, the darkness in the chamber.
When oxygen concentration drops to a low level (about 7% atmospheric equivalent), the broilers show EEG signs of arousal (on average at 50 s from the start of the LAPS process).
The mean time to induction of unconsciousness, based on the mean time to LOP, as a proxy, varies between 58 and 80 s in different studies.
The estimated time to unconsciousness, based on quantitative analysis of EEG data and the onset of tonic‐clonic convulsion (wing flapping, as a proxy), is on average around 70 s for broilers of 2.9 kg average bodyweight.
Time to onset of isoelectric EEG as an indicator of loss of spontaneous brain activity is on average at 140 s for broilers of 2.9 kg average bodyweight.
The mean time to death, based on time to become motionless as a proxy, varies between 146 and 200 s in different studies.
4.2. Extent to which LAPS is an acceptable method (ToR 1)
During the period of time from the onset of the LAPS procedure and the time at which the broilers are rendered unconscious, the animals show signs (headshaking, jump, open‐bill breathing) potentially linked to distress and/or suffering due to, e.g. air‐hunger.
The duration of distress and/or suffering is on average 20 s, which is the period between start of arousal (˜ 50 s from the onset of LAPS) and onset of tonic‐clonic convulsions (˜ 70 s from the onset of LAPS).
The possibility that during this period the birds experience pain, possibly linked to, e.g. colic‐like pain due to gas expansion in the gut, cannot be ruled out.
The data available in the scientific literature and their quality did not allow for a direct comparison between the LAPS and the currently allowed stunning methods (electrical water‐bath and gas stunning methods).
The time to LOP is shorter in gas stunning methods than in LAPS.
According to the expert ranking of hazards, the risk of poor animal welfare is considered to be lower under the LAPS method when compared to use of electrical water‐bath stunning.
The expert ranking of hazards could not statistically demonstrate a difference between LAPS and the gas stunning methods (excluding inert gases alone which were not included in the ranking exercise).
Therefore, the LAPS method can be considered to be at least equivalent, in terms of animal welfare outcomes, to at least one of the currently available stunning methods.
4.3. Extent to which findings are consistent with other information (ToR 2)
The only previously published papers on the LAPS were by the same research group. There are discrepancies between those four papers and the ones included in the current submission (e.g. ethogram data, description of the intervention). However, the four previously published papers were not included in this assessment because they have failed previous eligibility assessment by EFSA.
The technical parameters linked to the LAPS methodology are consistent with the principles of fluid dynamics including the behaviour of gases (including water vapour) during decompression.
EFSA considered the opportunity of exploring available information on the effect of rapid decompression in species other than birds, e.g. self‐report on human sensations. However, the extrapolation of such information would have implied the assumption of a certain similarity between the different species. This assumption would be very difficult to defend as birds have a very peculiar anatomo‐physiology which makes them almost unique. An example is given by the respiratory system, which is open‐ended and it is likely to react very differently from any other respiratory system to decompression.
4.4. Requirement related to the use of LAPS (ToR 3)
-
The overall assessment of EFSA is valid ONLY under the conditions described in the submissions, i.e.:
-
–
Technical specification (i.e. rate of decompression, duration of each phase, total exposure time)
-
–
Ambient conditions (i.e. temperature, humidity)
-
–
The LAPS methodology was assessed based on the data generated from the studies submitted by the applicant, which used broiler chickens for slaughter (i.e. killed for human consumption) weighting less than 4 kg in the different experiments.
Deviations from the conditions listed above might have different consequences for animal welfare which were not assessed in this exercise.
The animal welfare consequences of system failure are not assessed. However, the applicant has identified a set of potential hazards affecting the system (e.g. electrical failure) and has described safeguards to prevent poor animal welfare outcomes.
4.5. Extent to which the findings may be valid under commercial conditions (ToR 4)
-
The LAPS intervention shall be applied under the following technical requirement with regard to movement of the animals in containers/transport cages/modules:
-
–
The containers are moved in to the equipment in a smooth way.
-
–
Bunching of live birds should be avoided.
-
–
Based on the description provided in the submitted papers and in the additional information, the system appears to be able to fulfil these requirements.
The speed of the process is comparable to the one recorded for the currently allowed stunning methods.
According to the information provided by the applicant, the LAPS is currently in use in one poultry processing plant in USA, with four operating chambers which provide a throughput of 21,600 birds per hour (180 birds/minute per 2 lines for a total of 360 birds/minute). The system has been used to irreversibly stun broilers weighing up to 4 kg.
The requirements for gas stunning under the existing regulation, e.g. the key parameters and the monitoring procedures, are relevant to LAPS as well.
The finding of the assessment and recommendations of this opinion are not intended to limit options to the use of one specific commercial product or device. The findings of this opinion are applicable to any device or mechanism which can provide conditions in line with those which have been assessed.
5. Recommendations
-
The AHAW Panel should consider an update of the EFSA guidance published at present on the basis of the experience acquired during this assessment. Some elements that need to be taken into consideration are:
-
–
The need for greater focus on the data generated and related statistical analysis in the different studies rather than on individual scientific papers. The submission of a unique dossier which includes raw data and a report providing all the relevant information linked to the experiment performed would make the assessment process faster than it is now;
-
–
A check as to whether the REFLECT and STROBE reporting guidelines are the most suitable for this type of experiment, or if other available reporting guidelines might be better suited to the purpose (e.g. ARRIVE). However, EFSA can decide to go beyond standards proposed for good quality studies when performing an assessment, bearing in mind the specific context of the animal welfare;
-
–
As it is, the EFSA guidance provides indications on how to assess the internal validity of the studies/dossiers submitted (i.e. eligibility, reporting and methodological quality). Guidance on assessment of the external validity of the studies performed to produce the data should be considered.
-
–
The methodological approach described in this assessment is recommended as the basis for an additional section in the guidance on how to perform an assessment of equivalence between methodologies with different welfare hazards.
-
–
This assessment has highlighted that, although there are relevant scientific studies on the current stunning methods, the data provided are heterogeneous in terms of neurological, behavioural and physiological welfare indicators. For this reason, the parameters to assess pain, distress and/or suffering and the reporting should be harmonised.
Emergency procedures associated with system failures should be included by the manufacturer in the manufacturer's instructions for the use of the equipment.
Food business operators should follow the manufacturer's instructions and include them in the standard operating procedures.
The LAPS method may, in addition to commercial slaughter, be suitable for depopulation, respecting the technical conditions defined in the present conclusions.
The effectiveness of the LAPS method in killing the broilers needs to be monitored, in line with EC Regulation 1099/2009.
The conclusions of this assessment cannot be extended to other production types of Gallus gallus (i.e. layers, breeders and chicks). For example, if the LAPS methodology is intended to be used for the stunning of layers, further studies would be required to determine the effect of decompression on intra‐abdominal shell eggs.
Glossary and Abbreviations
- AHAW
EFSA Panel on Animal Health and Welfare
- ANS
autonomous nervous system
- Arousal
Increase in fast/high frequency or decrease in slow/low frequency electrical activity recorded in the EEG
- Attrition bias
Attrition bias is a kind of selection bias caused by attrition (loss of participants) discounting trial subjects/tests that did not run to completion. It is closely related to the survivorship bias, where only the subjects that ‘survived’ a process are included in the analysis. It includes dropout, nonresponse (lower response rate), withdrawal and protocol deviators. It gives biased results where it is unequal in regard to exposure and/or outcome (Jüni, P, Egger, Matthias, 2005. ‘Empirical evidence of attrition bias in clinical trials’. International Journal of Epidemiology, 34, 87–88. https://doi.org/10.1093/ije/dyh406)
- AW
Animal Welfare
- CAS
control atmosphere stunning
- CO2
Carbon dioxide
- ECG
electrocardiography
- EEG
electroencephalography
- FFT
Fast Fourier Transformation
- GA
general anaesthetic
- General Anaesthetic Plane
Phase (plane) of induced state of unconsciousness with loss of protective reflexes
- HALF
high amplitude, low frequency
- HMI
human–machine interface
- HR
heart rate
- HRV
heart rate variability
- IASP
International Association for the Study of Pain
- LAPS
low atmospheric pressure stunning
- LOP
loss of posture
- PLC
programmable logic controller
- Selection bias
Selection bias results from the fact that the composition of the study groups differs from that in the source population and this biases the associations between the exposure group and the outcomes (Dohoo et al., 2010)
- SEP
somatosensory evoked potentials
- Time to onset/Latency
Time elapsed until the occurrence of a given event
- ToR
Terms of Reference
- WG
Working Group
Appendix A – Eligibility criteria Part 1: intervention
1.
Appendix B – Eligibility criteria Part 2: Outcome
1.
Table B.1.
Summarised information for the eligibility assessment regarding the outcome parameters (onset of unconsciousness and insensibility) as provided in the five scientific papers in the dossier
| Parameter | Data provided | |
|---|---|---|
| EEG |
In this study, EEG signals were obtained from unrestrained birds undergoing LAPS. As outlined in the EFSA guidance (EFSA AHAW Panel, 2013), such recordings allow identification of reliable criteria to identify loss of consciousness including the presence of slow waves (high amplitude, low frequency activity) and profoundly suppressed or quiescent EEG. Detailed spectral analysis of the EEG response were performed to the entire LAPS cycle and latencies to recently validated species‐specific thresholds for characteristics based on median frequency and total power were determined (Sandercock et al., 2014, Physiol. Behav. 133, 252–259). Latencies to established thresholds for complete loss of spontaneous brain activity (total power less than 10% of baseline power, Raj et al., 1998, Brit. Poult. Sci. 39, 686–695) were also determined and isoelectric EEG using both visual and spectral techniques. Recording behaviour and EEG in the same individuals enabled to fully examine (and quantify with correlation analysis) the relationships between EEG responses and animal based indicators. Spectral analysis of the EEG response to the LAPS cycle were performed to derive two spectral variables: total power (Ptot) – the total area under the frequency spectrum and median frequency (F50) – the frequency below which 50% of the EEG power resides (Johnson et al., 2005 Vet. Anaes. Anal. 62:61‐71; Murrell and Johnson, 2006 J. Vet. Pharm. Therap. 29:325–335; Murrell et al., 2008. Lab. Anim., 42, 161–170; Tonner, 2006. Best Practice & Res. Clin. Anes., 20, 147–159). The use of this analysis has been well documented and has allowed detailed evaluation of EEG activity (Becker et al., 2010. Anaes. Monit., 5, 1–6; Delorme and Makeig, 2004. J. Neurosci. Methods, 134, 9–21; Johnson et al., 2005. Vet. Anaes. Anal., 62, 61–67). Changes in EEG activity resulting from the transition from a conscious to an unconscious state are indicated by a decreasing F50 and a sharp increase in Ptot (Sandercock et al., 2014 Physiol. Behav. 133: 252–259) |
Martin et al. (2016b) (Paper 7), Martin et al. (2016c) (Paper 8) |
| Behavioural indicators to detect onset of unconsciousness and insensibility | Loss of posture (LOP) and Motionless – Tables 1, 2 and 4 | Mackie et al. (2016) (Paper 5) |
| LOP and Motionless – Tables 1, 3 and 5 | Martin et al. (2016a) (Paper 6) | |
| LOP and Motionless – Tables 1, 2 and 3 | Martin et al. (2016b) (Paper 7) | |
| LOP and Motionless – Tables 1, 2, 4 and 5 | Martin et al. (2016c) (Paper 8) |
Table B.2.
Summarised information for the eligibility assessment regarding the outcome parameters (absence of pain, distress and suffering until the loss of consciousness and sensibility) as provided in the five scientific papers in the dossier
| Category | Parameter | Data provided | |
|---|---|---|---|
| Behavioural response | Vocalisations | Tables 1, 3 and 5 | Martin et al. (2016a) (Paper 6) |
| Tables 1, 2 and 3 | Martin et al. (2016b) (Paper 7) | ||
| Tables 1, 2 and 4 | Martin et al. (2016c) (Paper 8) | ||
| Postures and movements | Tables 1, 2, 3, 4 | Mackie et al. (2016) (Paper 5) | |
| Tables 1, 2, 3, 4 | Martin et al. (2016a) (Paper 6) | ||
| Tables 1, 3 and 5 | Martin et al. (2016b) (Paper 7) | ||
| Tables 1, 2, 3, 4, 5, 6 | Martin et al. (2016c) (Paper 8) | ||
| General behaviour | Table 1, 2, 3, 4 | Mackie et al. (2016) (Paper 5) | |
| Tables 1, 3 and 5 | Martin et al. (2016a) (Paper 6) | ||
| Tables 1, 2, 3, 4 | Martin et al. (2016b) (Paper 7) | ||
| Tables 1, 2, 3, 4, 5, 6 | Martin et al. (2016c) (Paper 8) | ||
| Physiological response | Hormone concentration | (Data not provided) | N/A |
| Blood metabolites | (Data not provided) | N/A | |
| Autonomic responses | ECG was measured before and during LAPS and was used to generate heart rate data at regular intervals. Heart rate is a well‐established parameter indicating autonomic fear and stress responses. No elevation of heart rate was seen after start of LAPS. The response of heart rate in broilers to hypoxia is well established from studies with inert gases (e.g. McKeegan et al., 2007, Anim Welf. 16, 409–426). A similar decrease and irregularity in heart rate following onset of hypoxia was seen during LAPS | Martin et al. (2016b) (Paper 7) | |
| Clear ECG waveforms were obtained from all birds during baseline measurements and during the process. Figure 4 shows mean heart rate before, during LAPS or sham treatment based on available data at each time point | Martin et al. (2016c) (Paper 8) | ||
| Neurological response | Brain activity |
In this study, EEG was used primarily to determine time to loss of consciousness, and a range of parameters was measured (see Table Annex A7/3). Apart from visual interpretation and total power ratios, spectral analysis was employed, which provides a quantitative approach to more objectively characterise changes in the EEG pattern throughout LAPS. Fast Fourier Transformation (FFT) is a mathematical tool, which calculates how much power each frequency band contributes to the EEG waveform. Derived variables from this analysis include the total power and the median frequency (F50), which is the frequency below which half of the total power resides. This approach has been recently widely adopted to interpret EEG responses in a range of species and contexts (e.g. Murrell and Johnson 2006, J. Vet. Pharm. Therap., 29, 325–335). A novel filtering and analysis program (Martin et al., 2016 Novel analysis of EEG data on farm killed poultry, British Poultry Abstracts January 2016) was used. This has been shown to improve the number of useable epochs in the data set from of EEG traces and thereby improve the reliability of the loss of consciousness measurements. Synchronisation of the EEG pattern early in the LAPS process following onset of LAPS suggest that the birds are not challenged by any noxious stimulus (see Gentle, 1975. Brit. Poult. Sci. 17, 151–156). As reported in the paper there were statistically significant correlations between the various EEG indicators used and behavioural indicators of loss of consciousness (e.g. LOP). High‐quality EEG signals were recorded for 24 birds, 22 of these traces represented the entire duration of LAPS and the baseline. The overall pattern of EEG response to LAPS in terms of changes in total power and median frequency is shown. The EEG was not recorded with the purpose of assessing pain |
Martin et al. (2016b) (Paper 7) |
| High quality EEG signals were recorded for 24 birds, 22 of these traces provided data for the first 150 s of LAPS. The overall pattern of EEG response to LAPS in terms of changes in total power and median frequency is shown in Figures 1 and 2 | Martin et al. submitted (Paper 8) |
Table B.3.
Summarised information for the eligibility assessment regarding the outcome parameters (Duration of unconsciousness and insensibility) as provided in the five scientific papers in the dossier
| Parameter | Data provided | |
|---|---|---|
| EEG | Summary statistics and graphs were produced at bird level. F50, Ptot and visual interpretation over time reported | Martin et al. (2016b) (Paper 7), Martin et al. (2016c) (Paper 8) |
| Behavioural indicators to detect duration of unconsciousness and insensibility | Derived from LOP and Motionless – Tables 1, 2 and 4 | Mackie et al. (2016) (Paper 5) |
| Derived from LOP and Motionless – Tables 1, 3 and 5 | Martin et al. (2016a) (Paper 6) | |
|
Derived from LOP and Motionless – Tables 1, 2 and 3 Absence of rhythmic breathing, absence of corneal or palpebral reflex |
Martin et al. (2016b) (Paper 7) | |
| Derived from LOP and Motionless – Tables 1, 2, 4 and 5 | Martin et al. (2016c) (Paper 8) |
Appendix C – Reporting quality
1.
Table C.1.
Assessment of the reporting quality against the EFSA guidance criteria
| Paper/study | Information as provided in the dossier and related comments |
|---|---|
| Mackie et al. (2016) (Paper 5) |
Introduction The primary objective of the study was to carry out a detailed behavioural analysis of broiler chickens undergoing LAPS, both in groups and individually, with a focus on behaviour occurring during induction to unconsciousness. The secondary objectives were to investigate the effects of bird weight, and whether slightly adjusted decompression settings (automatically applied in relation to ambient temperature) had any effect on behavioural responses. Materials and methods Commercial broilers, Ross 708, aged 49–50 days. Weighed 3.4 ± 0.5 kg (range 2.6–4.3 kg). The birds were reared in a commercial flock. Before undergoing LAPS, the birds were feed restricted for 8 h and water restricted for 2 h to mimic commercial practice. Missing data resulted from birds being out of view during behavioural observations – see results for details. Experimental units were 50 individual birds and 50 focal birds in groups of 20. Each individual/group was exposed to LAPS in a different run, giving true replication. Sample sizes were determined using power calculations (Cohen J, 1992. A Power Primer. Psychological Bulletin, 112, 155–159) based on differences in behaviour durations and variances reported using similar measurement methodologies in related studies on controlled atmosphere stunning and emergency killing using anoxic gas mixtures (e.g. Abeyesinghe et al., 2007. Brit. Poult. Sci., 48, 406–423; Coenen et al., 2009. Poult. Sci., 88, 10–19; McKeegan et al., 2007a. Brit. Poult. Sci, 48, 430–442; McKeegan et al., 2007b. Anim. Welf. 16, 409–426). The birds all underwent an identical intervention, except that three temperature settings were applied in relation to ambient temperature (see methods). Outcomes recorded in this study were behaviour (postures and movements and general behaviour). These are described in detail in Table 1 of the paper. As appropriate, behavioural latencies, durations and counts were recorded. Birds were randomly selected from a commercial flock and treated in similar manner during transportation, care and husbandry and during handling prior to exposure to the intervention. Birds were randomly allocated to numbered groups and randomly allocated to individual or group killing. Application of temperature setting treatments was sequential and unbalanced, this was unavoidable based on ambient temperature change throughout the two trial days. A single observer conducted behavioural measurements using a specialised behavioural recording program (Noldus Observer). No blinding took place. Variables were created relating to the latencies, durations, bout numbers and bout durations (where appropriate) of the behaviours. Following testing for normality with the Anderson Darling test, using the nortest R package version 1.0‐2 (Grossand Ligges, 2012), and checking normality with a histogram of the data, a one‐way analysis of variance (ANOVA) or Kruskal–Wallis tests were carried out with temperature setting applied as a factor. In individuals, correlations between behavioural parameters and body weight were computed using Pearson's correlation and Spearman's rank correlation, using the pspearson test R package version 0.3‐0 (Savicky, 2014). Where temperature setting did not have an effect, data was pooled for further analysis, but if temperature setting was significant then weight correlations were carried out within each temperature setting. To compare results between individuals and groups, Mann–Whitney U tests and independent two sample t‐tests were used where appropriate. When comparing individuals and groups, if temperature setting had a significant effect, analysis was carried out within temperature setting.
Results Means (and standard deviation) of continuous variables are presented in the results. Numbers are provided in results tables. Results are stated in absolute numbers when feasible. We report the results for each behaviour outcome for individuals and groups, in both table and graphical form. These include means, standard deviations and ranges. No adverse effects were observed. Discussion The discussion summarises the key results with reference to the study objectives and provides an interpretation of results considering objectives and limitations and previous studies. The sequence of behaviours seen at the different temperature settings was similar to those seen previously during LAPS (Thaxton et al., 2010) and also to those seen in experimental and field studies of controlled atmosphere stunning using anoxic gas mixtures. There were variations in the latencies and duration of behaviours with LAPS; in general, latencies were longer than those seen on some gas anoxic systems. The results are relevant to the LAPS procedure as applied to slaughter weight broiler chickens at these temperature settings. Although this study provided more detailed information using precise definitions of behavioural activities, the sequence of behaviours seen at the different temperatures was broadly similar to those seen previously during LAPS (Thaxton et al., 2010). The results also suggest that LAPS produces a sequence of behaviours which are equivalent to those seen with CAS. Other |
| Martin et al. (2016a) (Paper 6) |
Introduction There are concerns that birds undergoing LAPS could experience discomfort or pain. Here, it is investigated whether subjecting birds to LAPS with and without administration of an opioid analgesic (butorphanol) affected behavioural responses, with the rationale that abolition of suspected pain related behaviour with analgesic is circumstantial evidence of pain. The primary objective of this study was to investigate whether subjecting birds to LAPS with and without administration of an opioid analgesic would affect their behavioural responses, especially those that have been previously thought to relate to pain and discomfort. Materials and methods Commercial broilers, Cobb 500, aged 36–37 days (mean bodyweight 2.30 ± 0.12 kg). The birds were reared in a research facility at the University of Arkansas. Before undergoing LAPS, the birds were feed and water restricted for 2–6 h before LAPS, dependent on the pair kill order. Missing data resulted from birds being out of view during behavioural observations. Experimental units were 45 pairs of birds arranged in three types of blocks in relation to treatment (analgesic/analgesic, analgesic/sham, or sham/sham). Each individual/group was exposed to LAPS in a different run, giving true replication. There were 15 replications of each block (AA, AS, and SS), each containing a pair of birds. The birds underwent LAPS in 45 consecutive pairs over 2 days (day 1 = 23 pairs; day 2 = 22 pairs). Sample sizes were determined using power calculations (Cohen J, 1992. A Power Primer. Psychological Bulletin, 112, 155–159, Snedecor and Cochran Statistical Methods 1967 Iowa State University) based on differences in the range of behaviour durations and variances reported using similar measurement methodologies in related studies on controlled atmosphere stunning and emergency killing using anoxic gas mixtures (e.g. Abeyesinghe et al., 2007. Brit. Poult. Sci., 48, 406–423; Coenen et al., 2009. Poult. Sci., 88, 10–19; McKeegan et al., 2007a. Brit. Poult. Sci, 48, 430–442; McKeegan et al., 2007b. Anim. Welf., 16, 409–426; McKeegan et al., 2013. Poult. Sci., 92, 1145–1154). Outcomes recorded in this study were behaviour (postures and movements and general behaviour). These are described in detail in Table 1 of the paper. As appropriate, behavioural latencies, durations and counts were recorded and related to analgesic treatment. Birds were randomly selected from a larger flock reared for a series of research trials. All birds were treated in similar manner with regard to husbandry and during handling prior to exposure to the intervention. Birds were randomly allocated by individual wing tag number into three types of blocked pairs (analgesic/analgesic (AA), analgesic/sham (AS), and sham/sham (SS)) and pair kill order following a Graeco‐Latin square design (Martin and Bateson, 2007. Measuring Behaviour: An Introductory Guide First published 2007. Printed in the United Kingdom at the University Press, Cambridge. ISBN‐978‐0‐521‐82868‐0 hardback ISBN‐978‐0‐521‐53563‐2 paperback). A single observer conducted behavioural measurements using a specialized behavioural recording program (Noldus Observer). The observer was blinded to both individual bird treatment (analgesic/sham) and pair type (analgesic/analgesic, analgesic/sham, or sham/sham). All data were summarised in Microsoft Excel (2010) spreadsheets and analysed using Genstat (14th Edition). Statistical significance was based on F statistics and p < 0.05 threshold level. Summary graphs and statistics were produced at bird level. Statistical comparisons of behavioural variables were conducted via generalised linear mixed models (GLMM) (Poisson distribution) or linear mixed models (LLM) (normal distribution) dependent on the data distributions for each variable. Data transformations were attempted when necessary via logarithm function. All models included bird ID, companion bird ID and pair block type as random effects. All fixed effects were treated as factors and all interactions between factors were included in maximal models. All models included treatment, pair order, and marked bird as fixed effects and bird weight, ambient temperature, ambient humidity as covariates. Correlations between variables and fixed effects were performed as Pearson's Correlations for parametric data, and Spearman's rank correlations for non‐transformable non parametric data. For behaviours which were not exhibited by all birds, the effect of treatment on the proportions of birds showing the behaviour was compared with chi‐square tests using two by two contingency tables.
Results Means, standard deviations and ranges of continuous variables are presented in the results. Numbers analysed are provided in results tables. Results are stated in absolute numbers when feasible. The results are reported for each behaviour outcome by analgesic treatment, in table. These include means, standard deviations and ranges. For behaviours which were not exhibited by all birds, the effect of treatment on the proportions of birds showing the behaviour was compared with chi‐square tests using two by two contingency tables.
Discussion The discussion summarises the key results with reference to the study objectives and provides an interpretation of results considering objectives and limitations and previous studies. The sequence of behaviours seen at the different temperature settings was similar to those seen previously during LAPS (Mackie et al., 2016) and also to those during controlled atmosphere stunning using anoxic gas mixtures. Administration of butorphanol had no effect on the range and patterning of behavioural responses during LAPS. Latencies to ataxia, mandibulation and deep inhalation were slightly delayed by analgesic treatment but the duration of ataxia and other behaviours related to loss of consciousness were unaffected. These effects appear to be most readily explained by potential sedative, dysphoric and physiological side effects of butorphanol. The results are relevant to the LAPS procedure as applied to slaughter weight broiler chickens at this temperature setting. Although this study provided more detailed information using precise observation techniques and detailed behavioural definitions, the patterning and range of behaviours seen was very similar to those reported previously during LAPS (Mackie and McKeegan, 2015). Other N/A |
| Martin et al. (2016b) (Paper 7) |
Introduction To date, no studies on LAPS have been carried out in which EEG and behaviour have been recorded in the same individual and the timings for loss of posture have not been consistent between studies (ranging between 40 and 80 s, McKeegan et al., 2013; Mackie and McKeegan, 2015). There is a need for brain and behavioural measures in the same bird to allow a more robust assessment of the welfare impact of the process and corroborate indicators of loss of consciousness.
Scientific background and rationale for the investigation are reported adequately. The primary objective of the paper is “to comprehensively examine responses to LAPS by recording behaviour, EEG and ECG in individual broiler chickens, and interpret these with regard to the welfare of birds undergoing the process”. However, behavioural indicators are not used to assess aversion before loss of consciousness. Materials and methods Commercial broilers, Cobb 500, aged 34–35 days. The birds were reared in a research facility at the University of Arkansas. Before undergoing LAPS, the birds were feed restricted for 2–6 h before LAPS, dependent on the triplet kill order. Missing data resulted from birds being out of view during behavioural observations – see results for details. Missing data in EEG and ECG traces resulted from movement artefacts and these were excluded from analysis. During the experiments, 31 birds underwent EEG implantation surgery – one dislodged its implant soon after surgery and was humanely euthanised. An additional bird underwent surgery to replace this loss (bird ID 217 replaced bird ID 385). Ninety birds (30 sets of three) were exposed to LAPS over 2 days (day 1 = 15 triplets; day 2 = 15 triplets. Each group was exposed to LAPS in a different run, giving true replication. The aim was to apply each temperature setting to 15 replicates, but changes in ambient temperature resulted in 16 replicates for setting 3 and 14 replicates for setting 4. Sample sizes were determined using power calculations (Cohen J, 1992. A Power Primer. Psychological Bulletin, 112, 155–159; Snedecor and Cochrane (1967) Statistical methods Iowa State University Press) based on differences in the variables reported using similar measurement methodologies in related studies on controlled atmosphere stunning and emergency killing using anoxic gas mixtures (e.g. Abeyesinghe et al., 2007. Brit. Poult. Sci., 48, 406–423; Coenen et al., 2009. Poult. Sci., 88, 10–19; McKeegan et al., 2007a. Brit. Poult. Sci, 48, 430–442; McKeegan et al., 2007b. Anim. Welf., 16, 409–426; McKeegan et al., 2013. Poult. Sci., 92, 1145–1154). According to ambient temperature, two of six possible temperature settings were applied in this study (settings 3 – applied between 13 and 18°C – and 4 – applied between 5 and 12°C). Outcomes recorded in this study were behaviour, electroencephalogram and electrocardiogram (see Annex table A7/4). For the behaviour observations, postures and movements and general behaviour were recorded, described in detail in Table 1 of the paper. The EEG was analysed in non‐overlapping 2 s epochs to produce latencies indicating unconsciousness, including F50 < 12.7 Hz (non‐responsive state) and < 6.8 Hz (general anaesthetic plane) (Martin, 2015; Sandercock et al., 2014), latency to total power equal to 10% of baseline and the onset of isoelectric EEG signal by visual interpretation and by identification of validated spectral characteristics. ECG signal was used to determine heart rate (bpm derived from the number of QRS complexes in a 5s epoch) at six baseline time points before LAPS (three outside chamber, three inside chamber with door open) and every 5 s during the LAPS cycle. Birds were randomly selected from a larger flock reared for a series of research trials. All birds were treated in similar manner with regard to husbandry and during handling prior to exposure to the intervention. The experimental birds were randomly selected from a larger flock by a random number generator (Microsoft Excel 2010) based on wing tag number. The birds underwent LAPS in triplets where one bird was implanted and instrumented to record EEG and ECG; behavioural observations were carried out on all birds. The triplet treatment order was generated by a Graeco‐Latin square design (Martin and Bateson, 2007) to balance day, temperature treatment and source pen for EEG implanted birds. A single observer conducted behavioural measurements using a specialised behavioural recording program (Noldus Observer). The observer was blinded to temperature setting treatment. It was not possible to fully blind the observer, as the physiological recording equipment was visible on birds wearing it. All data were summarised in Microsoft Excel (2010) spread sheets and analysed using Genstat (14th Edition). Statistical significance was based on F statistics and 5% threshold level (i.e. p value < 0.05). Summary graphs and statistics were produced at bird level. Statistical comparisons of behavioural variables were conducted via generalised linear mixed models (GLMM) (Poisson distribution) or linear mixed models (LLM) (normal distribution) dependent on the data distributions for each variable. Data transformations were attempted when necessary via logarithm function. All models included bird ID and triplet number as random effects. All fixed effects were treated as factors and all interactions between factors were included in maximal models. All models included treatment, triplet order, implanted and marked bird as fixed effects and bird weight, ambient temperature, ambient humidity as covariates. Correlations between variables and fixed effects were performed as Pearson's Correlations for parametric data, and Spearman's Rank Correlations for non‐transformable non‐parametric data. Summary statistics and graphs were produced at bird level, while statistical comparisons focussed on estimated means and differences between means. GLMMs (Poisson distribution) or LLMs (normal distribution) dependent on the data distributions for latency variables to unconsciousness (F50 b 12.7 Hz (non‐responsive state); and b6.8 Hz (general anaesthetic plane); latencies to visual inspection characteristics (presence of slow‐wave and three consecutive isoelectric 2 s epochs); latencies for the signal to have a total power equal to 10% of baseline; and finally, latencies to isoelectric (Ptot b170 mv and F50 N22 Hz). All models included bird ID and triplet number as random effects. All fixed effects were treated as factors and all interactions between factors were included in maximal models. All models included treatment and triplet order as fixed effects and bird weight, ambient temperature, ambient humidity as covariates. GLMMs (Poisson distribution) or LLMs (normal distribution) were carried out, dependent on the data distributions for each heart rate interval, including the six baseline intervals and latencies to bradycardia. All models included bird ID and triplet number as random effects. All fixed effects were treated as factors and all interactions between factors were included in maximal models. All models included treatment and triplet order as fixed effects and bird weight, ambient temperature, ambient humidity as covariates. A Pearson's correlation matrix was produced to examine associations between latencies to key behavioural responses (latency to ataxia, loss of posture, loss of jaw tone and motionless), EEG (latency to slow wave based on visual inspection, latency to isoelectric EEG based on visual inspection and spectral characteristics, latency to Ptot b10% of baseline, latency to F50 b 7 Hz and F50 b 12 Hz) and ECG (latency to bradycardia) events during LAPS.
The rate of change in partial pressure of oxygen in relation to time is provided in a Figure in the dossier. It is not clear why two different temperature settings are used when the ambient temperature falls only in Tset_3. There are discrepancies between the annex and the paper about temperature and humidity as well as in terms of replicates No description of the meaning of each behaviour. E.g. L260 ‘On completion of the LAPS cycle, the birds were removed from the chamber and reflexes were immediately assessed (e.g. presence of rhythmic breathing, nictitating membrane) to confirm death’. Some definitions are difficult to replicate and seem subjective (e.g. apparently conscious). The rationale behind the behaviours assessed is lacking. Some EEG variables are not scientifically supported. The onset of isoelectric EEG signal was determined in two ways, by visual interpretation, and by identification of validated spectral characteristics (Ptot less than 170 mv and F50 > 22 Hz). Latency variables to unconsciousness were defined as time for F50 < 12.7 Hz (non‐responsive state) and < 6.8 Hz (general anaesthetic (GA) plane). However, the reference of the validated spectral characteristics of Ptot less than 170 mv and F50 > 22 Hz is not scientifically supported in any the three references provided in the paper. Furthermore, the statement that the latency variables to unconsciousness of F < 12.7 Hz (non‐responsive state) and < 6.8 Hz (GA plane) is supported mainly by the thesis of the first author, which have not scientifically validated. The main scientific support to this statement is based on the paper of Sandercock et al. (2014) that reported that ‘A conservative threshold for F50 in hens and turkeys when unconscious (equivalent to a surgical plane of anaesthesia) was estimated at 7 Hz’. In the same studies the authors reported a F50 < 14 for the semi‐conscious (sedated) state. The relationship between sedation and non‐responsive state is not described. Results Means, standard deviation and ranges of continuous variables are presented in the results. Numbers analysed are provided in results tables. Results are stated in absolute numbers when feasible. The results are reported for each outcome by temperature treatment, in tables and figures. Individual bird data is provided in the form of scatter plots for latency to ataxia, loss of posture, jaw tone and wing flapping. The data presented include means, standard deviations and ranges.
Discussion The discussion summarises the key results with reference to the study objectives and provides an interpretation of results considering objectives and limitations and previous studies. Birds showed a consistent sequence of behaviours during LAPS (ataxia, loss of posture, clonic convulsions and motionless), which were observed in all birds. Leg paddling, tonic convulsions, slow wing flapping, mandibulation, head shaking, open bill breathing, deep inhalation, jumping and vocalisation were observed in a proportion of birds. Spectral analysis of EEG responses at 2 s intervals throughout LAPS revealed progressive decreases in median frequency at the same time as corresponding progressive increases in total power, followed later by decreases in total power as all birds exhibited isoelectric EEG and died. There was a very pronounced increase in total power at 50–60 s into the LAPS cycle, which corresponded to dominance of the signal by high amplitude slow waves, indicating loss of consciousness. ECG recordings showed a pronounced bradycardia during LAPS. There was a good correlation between behavioural, EEG and cardiac measures in relation to loss of consciousness. There were some effects of temperature adjusted pressure curves on behavioural latencies and ECG responses, but in general, responses were consistent and very similar to those reported in previous research on controlled atmosphere stunning with inert gases. The results are relevant to the LAPS procedure as applied to slaughter weight broiler chickens at these temperature settings. This study provides detailed information on behavioural, EEG and ECG responses to LAPS. There is an apparent discrepancy or at least a lack of clarity regarding the effect of the temperature settings on the latencies of the different behaviours. Considering, in particular, the time to onset of LOP, it appears that under temperature setting 4 latency is shorter. The authors make some hypothesis to explain this phenomenon, spotting, in addition, that it is a contradictory result when compared to previous studies where longer latencies were recorded with colder temperatures. However, no mention is made on the fact that in the Material and Methods section it is reported that the ambient temperature has a mean value of 16 ± 0.3°C, which does not justify the use of two different temperature settings. In theory, only temperature setting 3 should have been used and surprisingly enough, the shortest latencies were found in temperature setting 4. It is not clear if this difference in latency in the LOP of almost 3 s is due to the apparent forcing of the use of temperature setting 4, normally used when the ambient temperature ranges between 5 and 12 degrees, while the actual ambient temperature was reported to be 16 ± 0.3°C. It is curious, though, that the shortest latency to LOP was recorded for the temperature setting that should be used when the actual ambient temperature is lower (setting 4). A possible explanation for this phenomenon is that when the LAPS system is set to temperature setting 4, it assumes that the atmosphere is denser (i.e. higher oxygen concentration), compared to situations when the ambient temperature is higher, and as a consequence the pump runs more intensively, reducing the actual available oxygen concentration quicker than specified in the protocols. No discussion about the behavioural indicators of aversion is provided. Other N/A |
| Martin et al. (2016c) (Paper 8) |
Introduction The primary aim of this study was to determine how behavioural, electroencephalogram and electrocardiogram responses to LAPS are influenced by illumination of the decompression chamber. The standard LAPS procedure is done in darkness. It has been noted that slow‐wave EEG patterns are seen early in the LAPS process, before behavioural evidence of loss of consciousness such as ataxia and loss of posture (McKeegan et al., 2013; Martin et al., 2016b). It is well known that birds being placed in darkness fall asleep rapidly and demonstrate similar slow‐wave brain activity (Ookawa and Gotoh, 1965; Gentle and Richardson, 1972). Thus, darkness in LAPS might introduce a confounding factor changing animal behaviour. To disentangle the influence of decompression from the effect of darkness, the LAPS process has been performed in light conditions. A secondary aim was to determine the influence of the decompression chamber itself on birds without submitting them to decompression. Materials and methods The birds were reared in a research facility at the University of Arkansas. Before undergoing LAPS, the birds were feed restricted for 2–8 h before LAPS, dependent on the pair kill order. Missing data resulted from birds being out of view during behavioural observations – see results for details. Missing data in EEG and ECG traces resulted from movement artefacts and these were excluded from analysis. Eighty birds (40 pairs) were exposed to LAPS over 2 days (day 1 = 20 pairs; day 2 = 20 pairs). Each pair was exposed to LAPS/SHAM in a different run, giving true replication. A two by two‐factorial design was employed, with LAPS/light, LAPS/dark, sham/light and sham/dark treatments (10 pairs in each). Sample sizes were determined using power calculations (Cohen J, 1992. A Power Primer. Psychological Bulletin, 112, 155–159; Snedecor and Cochrane (1967) Statistical methods Iowa State University Press) based on differences in the variables reported using similar measurement methodologies in related studies on controlled atmosphere stunning and emergency killing using anoxic gas mixtures (e.g. Abeyesinghe et al., 2007. Brit. Poult. Sci. 48, 406–423; Coenen et al., 2009. Poult. Sci., 88, 10–19; McKeegan et al., 2007a. Brit. Poult. Sci, 48, 430–442; McKeegan et al., 2007b. Anim. Welf. 16, 409–426; McKeegan et al., 2013. Poult. Sci., 92, 1145–1154). The four treatments applied were LAPS/light, LAPS/dark, sham/light and sham/dark treatments. See details in intervention table above. One of six possible temperature settings was applied in this study 4, applied between 5 and 12°C. According to treatment, illumination was applied at 500 lux and in sham treatments birds were identically handled but remained undisturbed in the LAPS chamber without decompression for 280 s. Outcomes recorded in this study were behaviour, electroencephalogram and electrocardiogram (see Annex table A8/4). For the behaviour observations, postures and movements and general behaviour were recorded, described in detail in Table 1 of the paper. The EEG was analysed in non‐overlapping 2s epochs to produce latencies indicating unconsciousness, including F50 < 12.7 Hz (non‐responsive state) and < 6.8 Hz (general anaesthetic plane) (Martin, 2015; Sandercock et al., 2014), latency to total power equal to 10% of baseline and the onset of isoelectric EEG signal by visual interpretation and by identification of validated spectral characteristics. ECG signal was used to determine heart rate (bpm derived from the number of QRS complexes in a 5s epoch) at six baseline time points before LAPS (three outside chamber, three inside chamber with door open) and every 5 s during the LAPS cycle. Birds were randomly selected from a larger flock reared for a series of research trials. All birds were treated in similar manner with regard to husbandry and during handling prior to exposure to the intervention. The experimental birds were randomly selected from a larger flock by a random number generator (Microsoft Excel, 2010) based on wing tag number. The birds underwent LAPS in pairs where one bird was implanted and instrumented to record EEG and ECG; behavioural observations were carried out on both birds. The pair treatment order was generated by a Graeco‐Latin square design (Martin and Bateson, 2007) to balance day, temperature treatment and source pen for EEG implanted birds. A single observer conducted behavioural measurements using a specialized behavioural recording program (Noldus Observer). It was not possible to blind the observer, as the physiological recording equipment was visible on birds wearing it and it could be seen on the video recording whether the lights were on or not. All data were summarised in Microsoft Excel (2010) spread sheets and analysed using Genstat (14th Edition). Statistical significance was based on F statistics and p < 0.05 significance level. Summary graphs and statistics were produced at bird and treatment level. Statistical comparisons were conducted via generalised linear mixed models (GLMM) (Poisson distribution) or linear mixed models (LLM) (normal distribution) dependent on the data distributions for each variable. Data transformations were attempted when necessary via logarithm function. All models included bird identification number (ID) and pair number as random effects. All fixed effects were treated as factors and all interactions between factors were included in maximal models. All models included LAPS/sham treatment, light/dark treatment and whether the bird was implanted as fixed effects and bird weight, ambient temperature, ambient humidity and feed withdrawal time as covariates. It was necessary to group behavioural data for analysis dependent on treatment (LAPS/sham) due to the majority of behaviours not being exhibited when birds did not undergo LAPS. The complete data set was analysed for some behaviours shown in all treatments (notice, standing, sitting, headshake, mandibulation, vigilance and vocalisations). Spearman correlations were used to determine directional associations between temperature and humidity (ambient and within chamber) and behavioural measures. EEG summary statistics and graphs were produced at bird level, while statistical comparisons focussed on estimated means and differences between means. GLMMs (Poisson distribution) or LLMs (normal distribution) were performed dependent on the data distributions for latency variables to unconsciousness (F50 < 12.7 Hz (non‐responsive state); and < 6.8 Hz (general anaesthetic plane); latencies to visual inspection characteristics (presence of slow‐wave and three consecutive isoelectric 2 s epochs); latencies for the signal to have a total power equal to 10% of baseline; and finally latencies to isoelectric (Ptot less than 170 mv and F50 greater than 22 Hz). These spectral variable thresholds were never reached in sham treatment groups, therefore as with behavioural observations data were split into subsets for modelling of other effects. The ECG data were analysed by carrying out GLMMs (Poisson distribution) or LLMs (normal distribution), dependent on the data distributions for each heart rate interval, including the 6 baseline intervals and latencies to bradycardia. Latencies to bradycardia and bpm < 100 were never reached in sham treatment groups, therefore as before subsets of data were analysed. Paired t‐tests were used to do comparisons within treatment groups at individual bird level to compare heart rate at specific time points. The rate of decompression is described in detail but the use of the ‘tilde’ (i.e. ‘approximatively’) provides uncertainty to the range of variation of the chamber pressure by the end of each phase. A two by two factorial design was employed, with LAPS/dark, LAPS/light, sham/dark and sham/light treatments. The design of the experiment is reasonable and the procedures are adequately described. However, instead of visual inspection and elimination of movement artefacts from EEG used in the current study, one could apply automated tools for artefacts detection and rejection. These tools provide somewhat better purification of the data. Also, in case of large amount of artefacts, it might be helpful to use short epochs (1 s) instead of 2 s in the current study. This would increase the duration of non‐rejected episodes at a cost of slightly decreased spectral resolution that is not truly important in this case. It is a pity that heart rate variability (HRV) as indicated in EFSA guidelines was not analysed in the current study. One can note that it was not analysed in alternative stunning methods by other researchers as well. This precludes comparative analysis of HRV in the current and previously described stunning methods. However, inclusion of such analysis might be useful for future applicant and for science in general. Results Means, standard deviation and ranges of continuous variables are presented in the results. Numbers analysed are provided in results tables. Results are stated in absolute numbers when feasible. The results are reported for each outcome by LAPS/SHAM and light/dark treatment, in tables and figures. The data presented include means, standard deviations and ranges. Within the sham treatments, illumination increased active behaviour and darkness induced sleep. The time to loss of consciousness in the two groups of birds was similar (54.7 ± 1.3 s vs 55.9 ± 1.19 s, mean ± SEM, P = 0.25). Electrophysiological measures such as F50 and Ptot were similar under light and dark conditions in LAPS birds as well, although large amount of non‐rejected artefacts especially seen after 60 s after LAPS onset make a comparison difficult. The cardiac response in LAPS was unaffected by illumination. Discussion The discussion summarises the key results with reference to the study objectives and provides an interpretation of results considering objectives and limitations and previous studies. Birds which underwent the sham treatment exhibited standing, slow wing flapping, vigilance, mandibulation, headshakes, vocalisations, sitting, pecking and panting behaviours, while those exposed to LAPS exhibited these plus ataxia, open bill breathing, deep inhalation, jumping, loss of posture, convulsions, leg paddling and motionless. Behavioural latencies and durations were generally increased in the sham treatments, since the whole 280 s cycle time was available (during LAPS birds were motionless by 145 s on average). Within the sham treatments, illumination increased active behaviour and darkness induced sleep but slow wave EEG was seen in both light and dark sham treatments. Exposure to LAPS was associated with increased headshaking, probably relating to increased noise levels in the chamber and the hypoxic environment. The pattern of EEG response to LAPS (steep reduction in median frequency in the first 60 s and increased total power) was similar with and without illumination, though birds in darkness had shorter latencies to reach a non‐responsive state (F50 < 12.7 Hz), GA plane (F50 < 6.8 Hz) and isoelectric EEG. Cardiac responses to LAPS, such as pronounced bradycardia, closely matched those reported previously and were not affected by light treatment. Collectively, these results add to a growing body of evidence that behavioural, ECG and EEG responses to LAPS are consistent and indicative of a process that is largely equivalent to controlled atmosphere stunning with anoxic gases. The LAPS/dark results are relevant to the LAPS procedure as applied to slaughter weight broiler chickens at this temperature setting. This study provides detailed information on behavioural, EEG and ECG responses to LAPS dependent on lighting conditions and on sham exposure to the LAPS chamber, also dependent on lighting conditions.
In general, the given set of data provides a clear impression about the absence of significant influences of light on the LAPS process. Other |
| Holloway et al. (2017) (Paper 9) |
Introduction The primary objective of this study was to define the characteristics of the vacuum used in LAPS in terms of pressure time and to define the dynamics of chamber parameters such as pressure, temperature and relative humidity and their interrelationship with oxygen level. A second objective was to characterise the role of water vapour, which at low pressures can have an increased impact in reducing on oxygen levels. A third objective was to characterise the measured chamber parameters for pressure curves used for several temperature ranges.
Materials and methods The trial was a grab sample conducted during commercial operations and to reduce bias from temperature serial effects. Runs with the chamber empty were recorded both before and after the runs with the birds. Due to operational constraints the runs with and without birds were not balanced. Sample size was estimated using Snedecor and Cochrane (1967) Statistical methods Iowa State University Press) based on variances in the pressure recorded from previous plant records of LAPS runs. A full description of the apparatus is given in detail. These observational studies were summarized using mean values and standard error and compared using Student t test and ANOVA using p < 0.05 (XLSTATBASE, Addinsoft Limited, Paris, France). The description of the system and its component is clear and well detailed. The information on the sample size reported in the annex to the paper are not clear to which animals it refers to. Results The annex to Paper 9 in the dossier provides all the data used for the parallel chamber fore pipe study, for and presence of bird's study and the chamber parameters. Means, standard errors deviation and ranges of continuous variables are presented in the results. Numbers analysed are provided in charts. Results are stated in absolute numbers when feasible.
The effect of altitude, hence environmental barometric pressure, was tested at 141 m, 434 m or 1048 m above the sea level and it is reported that the local barometric pressure was used to automatically compensate for changes in the starting pressure due to weather. The physics of gas flow regime and calculated pump‐down curve are presented, and these have been submitted for evaluation to a physicist. The performance of multiple parallel systems, e.g. four chambers operated simultaneously in this study, were reported to be equal. Effects of environmental temperature on LAPS was also studied. It is reported that temperature inside the chamber decreases approximately 4°C during the first 67 s of the LAPS cycle, when the pressure is reduced from 760 Torr (101.3 kPa) to 250 Torr (33.3 kPa), and then remains almost constant. Fogging inside the chamber is also reported, in this sense, fog is first observed during Region I, when the chamber pressure is reduced to approximately 660 Torr, and then clears when the chamber pressure decreases below 450 Torr. However, data presented in one of the figures indicate that the relative humidity in the chamber falls from about 85% at the start to about 40% at 67 s, and subsequently increases to 50% which is attributed to “outgassing from the birds”. The welfare consequences of this sudden decrease in relative humidity have not been elucidated or discussed. However, latency to some behavioural events (e.g. head shaking, gasping) observed during the period of reduced relative humidity would be of interest. It is also reported that the atmospheric equivalent oxygen concentration decreased from 20.68% to 3.77% at a final vacuum pressure of 150 Torr. Discussion The results/discussion summarise the key results with reference to the study objectives and provides an interpretation of results considering relevant atmospheric physics. The performance of the chamber matches that specified by the temperature based set curves, The layout of the four chambers with different designs and lengths of fore pipe did not affect the pump down curves. Normal loading with tow‐palletized crates of birds did not affect the pump down curve. Chamber temperature was found to fall about 4.5°C due to adiabatic cooling and RH first dropped and then rose which may relate to preferential pumping of water vapour/droplets during the short period of fog formation which occurs in some combinations of temperature and humidity. Measurements of oxygen levels showed a small reduction in fractional oxygen, which may relate to interactions with water vapour. Oxygen levels need to be adjusted to atmosphere equivalent to reflect the physiological impact. The oxygen levels measured were similar to those calculated and reflect those used by Purswell et al., 2007 which found that less than 5% oxygen for 2 minutes was effective to irreversibly stunning poultry from 160 to 280s of pump down. Observations presented in Papers 6, 7 and 8 show that the mean time to motionless is around 145 s and maximum times vary up to 191 s. Thus, most of the birds have fully succumbed to the effects of Hypoxia are motionless before the 5% oxygen level is reached at around 160 s depending on the pressure curve used. As has been shown in mountain medicine and aviation medicine the impact of hypobaric hypoxia is the cumulative effect of both the hypoxic deficit experienced and oxygen consumption. In man, alcohol and smoking have major effects on sensitivity to hypoxia. In broilers oxygen consumption varies with the energy costs of maintaining homeostasis, Blood glucose, pH, oxygen levels and body temperature as well as response to feeding, physical activity and levels of stress (See Bias J 2015 Stress in Sturkie's Avian Physiology Elsevier. The two‐minute period after almost all the birds are motionless is used to ensure that no birds exit the chamber, which are not irreversibly stunned. The results are relevant to the LAPS procedure as applied to poultry. This study provides detailed information on chamber parameters, temperature relative humidity and oxygen levels. The reduction in oxygen in LAPS is controlled by the pressure reduction, which accurately predicts oxygen concentration. There is no operational need to have oxygen meters on LAPS installations for killing poultry. However, there may be a case for using oxygen meters for monitoring purposes. EFSA 2014 suggested that oxygen meters should be used for LAPS and if they could be provided. If oxygen meters were used, the evidence presented by Paper 9 and this annex strongly suggests that the fractional meter reading needs to be adjusted to equivalent atmosphere before displayed to the operative on the HMI.
Other N/A |
Appendix D – Methodological quality
1.
Table D.1.
Assessment of the methodological quality related to the five papers
| Paper/study | Assessment |
|---|---|
| Mackie et al. (2016) (Paper 5) |
Selection bias These trials included random selection of commercially reared broilers so selection bias is unlikely but unknown. This approach has the benefit relevance to LAPS as carried out commercially; target animal in appropriate target conditions were used. The LAPS treatment is carefully controlled and selection bias only possible due to different pressure curves applied to different birds. The impact of different pressure curves which are applied to adjust for ambient temperature is examined and described in the paper. Attrition bias Potential problems due to t birds out of sight if not properly analysed (e.g. survival analysis). Performance bias Husbandry, transport and food restriction were standardized in these trials across replicates. Confounding Effects of hypoxia may be confounded with effect of hypobaria as treatment includes simultaneous application of both as hypobaric hypoxia. This unavoidable issue is considered in the discussion of the paper by comparison of responses to LAPS results with result from studies of normobaric hypoxia. Other confounding factors could be breed, age, and sex and body weight. Breed and age were controlled for in the study (birds were not sexed so it was not possible to examine effects of gender). Effects of bodyweight were examined and found to be minimal. Disease state is another possible confounding factor; all birds in this study were considered to be healthy or at least representative of the health status of typical slaughter weight broilers. We note that the majority of disease states would compromise cardio/respiratory efficiency and therefore reduce time to loss of consciousness. Other N/A |
| Martin et al. (2016a) (Paper 6) |
Selection bias These trials involved research facility reared broilers which were randomly allocated by wing tag number to treatment and block type, so there was no selection bias. Attrition bias Potential problems due to t birds out of sight if not properly analysed (e.g. survival analysis). Performance bias Husbandry and handling was standardized in these trials across replicates. The LAPS treatment is carefully controlled and selection bias only possible due to different pressure curves applied to different birds. All birds received the same depressurization curve and the effects of temperature and humidity were independently examined. Confounding Effects of hypoxia may be confounded with effect of hypobaria as treatment includes simultaneous application of both as hypobaric hypoxia. This unavoidable issue is considered in the discussion of the paper by comparison of responses to LAPS results with result from studies of normobaric hypoxia. Other confounding factors could be breed, age, and sex and body weight. Breed, age and gender were controlled for in the study. Effects of bodyweight were examined and found to be minimal. Disease state is another possible confounding factor; all birds in this study were considered to be healthy or at least representative of the health status of typical slaughter weight broilers. We note that the majority of disease states would compromise cardio/respiratory efficiency and therefore reduce time to loss of consciousness. Other It is claimed that ‘butorphanol is used in this trial because it is a κ‐opioïd receptor agonist and a μ‐opioïd receptor antagonist with characterised pharmacokinetics and is the currently recommended opioid for use in birds’. Opioid analgesics are still under investigation (ongoing studies) concerning their analgesic properties in birds. They are also behaviour modifiers (sedation or increased locomotor activity) and as such evaluation of their potential analgesic effects is often hindered due to behaviour‐based analgesic assessment parameters (Guzman et al., 2011; Paul‐Murphy and Fialkowski, 2001). Morphine has been shown to be more sedative than butorphanol in chicken (Singh et al., 2017) so choosing butorphanol seems a reasonable choice. The choice of an opioid analgesic appears not ideal due to its behavioural effects, potential respiratory depression and central effect on brain response to hypoxia. However, it is a traditional choice to test the occurrence of an acute pain stimulus. Other classes of analgesics with a central effect would present the same challenge. Butorphanol has been shown at 0.5 mg/kg IM to improve activity behaviour in lame turkeys (Buchwalder et al., 2005) without being able to separate a pure analgesic effect from a behaviour modification. At 2 mg/kg IV, it may provide some analgesia in lame chicken (Singh et al., 2017). However, studies in American Kestrels have failed to demonstrate an analgesic effect at 1 and 3 mg/kg IM. The chosen dose of 1 mg/kg IM appears to be on the safe side concerning the risk of having a sedative effect but may be not high enough to provide significant analgesia. Absorption of IM butorphanol seems to be reliable and rapid across bird species and to be overall good (Guzman et al., 2011, 2014) so the route of administration seems adequate in regards to drug availability and bird tolerance. The time elapsed between the injection and the test is also appropriate to insure adequate plasma concentration that is to say long enough for peak effect and short enough to be within the effect duration (less than 2 h). Fewer birds receiving butorphanol performed jump (20 vs 27), authors claimed that butorphanol may act as a pre medication, which we agree with, and that it may demonstrated that jump is pain related. But decrease of jump can be due to sedation also. As a conclusion, the failure of butorphanol to impact the behaviour related to pain/distress/suffering can be due to: (i) the LAPS having no implication in terms of pain/distress/suffering (what is claimed by the applicant), or (ii) the butorphanol having no impact on these outcomes on broilers (no conclusion possible). Authors are claiming that exact location and nature of pain during LAPS is not known, but knowing the process, it can be assumed that the pain will be mainly linked to decompression and lack of air. Decompression may lead to an expansion of the gas trapped in cavitary organs such as internal ear, gut, lung and air sac. The experiment could have included a CAS treatment as a control for comparison and other parameters of pain and welfare assessment (e.g. corticosterone measurement, but the sampling might be not easy). The hypothesis given by the authors is that headshake is more due to dysphoria and disorientation than pain. As the authors say, headshaking is observed in CAS in proportion that looks similar, even if statistic should be performed to check if the behaviour is not more prevalent in LAPS (median of head shake in different CAS mixture are from 0 to 1 depending on treatment (Mc Keegan et al., 2007) vs mean of 1.7 (2.4 with butorphanol) on LAPS). The latter paper of McKeegan tested different CAS (Argon and Nitrogen anoxia, hypercapnic argon and nitrogen anoxia, …) and authors explained that some birds showed head shake at the onset of all gas treatments suggesting that is was not a specific response but a behaviour related to novel or alerting stimuli. Results are similar in the paper of Abeyesinghe et al., 2007 where mean numbers of bouts and durations of head shake observed were higher for Argon (ca. 1 bout) and lower for Ar + CO2 and CO2 + O2. Although head shake has been interpreted as an aversive reaction to CO2 (Raj, 1996), it was there most common in Argon and authors interpret it as a non‐specific response to CO2 and that birds detect Ar (or lack of O2). Anyway, the LAPS results cannot exclude that it might be a distress/suffering behaviour related to decompression discomfort and in any case, it is unclear why it increased for treated animals. The fact that behavioural response, such as head shaking, is also present in CAS is used as an argument to affirm that observed behaviours are linked more with hypoxia than hypobaric conditions. |
| Martin et al. (2016b) (Paper 7) |
Selection bias These trials involved research facility reared broilers, which were randomly allocated by wing tag number to triplet and temperature treatment, so there was no selection bias. Attrition bias Potential problems due to t birds out of sight if not properly analysed (e.g. survival analysis). Performance bias Husbandry and handling was standardized in these trials across replicates. The LAPS treatment is carefully controlled and selection bias only possible due to different pressure curves applied to different birds. Two temperature curves were used and their effects are analysed. The effects of temperature and humidity on the outcomes are independently examined. Confounding Effects of hypoxia may be confounded with effect of hypobaria as treatment includes simultaneous application of both as hypobaric hypoxia. This unavoidable issue is considered in the discussion of the paper by comparison of responses to LAPS results with result from studies of normobaric hypoxia. Other confounding factors could be breed, age, and sex and body weight. Breed, age and gender were controlled for in the study Disease state is another possible confounding factor; all birds in this study were considered to be healthy or at least representative of the health status of typical slaughter weight broilers. We note that the majority of disease states would compromise cardio/respiratory efficiency and therefore reduce time to loss of consciousness. Other The description of the implantation of EEG electrodes is vague and the references cited are inappropriate. Visual inspection was used to eliminate severe movement artefacts, but the criteria to define artefacts are not defined. Furthermore, the criteria to assess isoelectric EEG either by visual interpretation or validated spectral characteristics are not defined. The reference to the validated spectral characteristics of Ptot < 170 mv and F50 > 22 Hz is not supported in any of the three journal references provided referred to in the paper. The reliability of both parameters is questionable for the following two reasons. First, the EEG amplitude depends on the location of placement of the electrodes (e.g. scalp or on the brain). Probably, it would have been more appropriate to express the threshold, not as an absolute amplitude, but in percentage of pre‐stun EEG power (i.e. relative to the pre stunning values). Secondly, when the animal approaches isoelectric EEG, the EEG amplitude is low, and the inherent technogenic noise (background electrical ‘noise’) of the recording system may have a significant impact on signal characteristics. For this reason, the declared F50 threshold of isoelectric EEG detection may be unreliable. The main scientific support for the thresholds F50 < 12.7 Hz (non‐responsive state) and < 6.8 Hz (general anaesthetic plane (GA)) is are based on the work of Sandercock et al. (2014) involving known anaesthetic, and have not been validated under hypoxic conditions. The correlation between EEG and behaviours has been performed on a visual basis only. |
| Martin et al. (2016c) (Paper 8) |
Selection bias These trials involved research facility reared broilers, which were randomly allocated by wing tag number to pair and one of four treatments in a two by two factorial design, so there was no selection bias. The LAPS treatment is carefully controlled and selection bias only possible due to different pressure curves applied to different birds. Effects of sham exposure to the LAPS chamber in the light and the dark are also analysed. The effects of temperature and humidity on the outcomes are independently examined. Attrition bias Potential problems due to t birds out of sight if not properly analysed (e.g. survival analysis). Performance bias Husbandry and handling was standardized in these trials across replicates. Confounding Effects of hypoxia may be confounded with effect of hypobaria as treatment includes simultaneous application of both as hypobaric hypoxia. This unavoidable issue is considered in the discussion of the paper by comparison of responses to LAPS results with result from studies of normobaric hypoxia. Other confounding factors could be breed, age, and sex and body weight. Breed, age and gender were controlled for in the study. Disease state is another possible confounding factor; all birds in this study were considered to be healthy or at least representative of the health status of typical slaughter weight broilers. We note that the majority of disease states would compromise cardio/respiratory efficiency and therefore reduce time to loss of consciousness.
Other |
| Holloway et al. (2017) (Paper 9) |
Selection bias Not relevant Attrition bias Not relevant Performance bias The LAPS treatment is carefully controlled and selection bias only possible due to the different pressure curves applied to different birds. Confounding Effects of chamber pressure, ambient temperature and water vapour are all intimately linked. This observational study used established principles of weather science, surface and gas physics and aviation medicine to characterise the response of chamber parameters namely pressure, temperature, relative humidity and oxygen to the vacuum pump down. Other N/A |
Appendix E – Extensive Literature Search
1.
Table E.1.
Sources of information
| Source | Databases |
|---|---|
| Web of Science (including the following databases) |
|
| Pubmed | |
| DART‐Europe E‐theses Portal |
Table E.2.
Search strategies
| Search parameters | Description |
|---|---|
| Date of the search | 25/5/2017 |
| Limits |
|
Table E.3.
Results
| Parameter | Set | Query | Results |
|---|---|---|---|
| Web of Science | # 8 |
#5 Refined by: [excluding] DOCUMENT TYPES: (ABSTRACT OR MEETING OR EDITORIAL OR NEWS OR PATENT OR LETTER) AND LANGUAGES: (ENGLISH OR UNSPECIFIED) Timespan=1980‐2017 Search language=Auto |
971 |
| # 7 |
#5 Refined by: LANGUAGES: (ENGLISH OR UNSPECIFIED) Timespan=1980‐2017 Search language=Auto |
1,268 | |
| # 6 |
#5 Timespan=1980‐2017 Search language=Auto |
1,365 | |
| # 5 |
#4 AND #1 Timespan=All years Search language=Auto |
1,579 | |
| # 4 |
#3 OR #2 Timespan=All years Search language=Auto |
26,680 | |
| # 3 |
TS=((gas OR gases OR electric* OR waterbath OR “water bath” OR CO2 OR “carbon dioxide”) AND (kill* OR cull* OR slaughter* OR abattoir*) AND (behaviour* OR behavior* OR physiolog* OR neruolog* OR “wing flap*” OR ataxia OR convulsion* OR “deep inhalation” OR “head shak*” OR seizure* OR jump* OR “loss of posture” OR lying OR motion* OR move* OR breath* OR ecg OR eeg OR electrocardiography OR electroencephalography OR “total power content” OR “brain activity” OR “heart rate” OR “leg paddl*” OR welfare)) Timespan=All years Search language=Auto |
12,504 | |
| # 2 |
TS=(stun OR stunn* OR stuning OR stuned OR prestunn* OR prestun* OR electronarcos* OR “electro narcos*”) Timespan=All years Search language=Auto |
15,413 | |
| # 1 |
TS=(poultry OR chicken OR chickens OR broiler* OR Chick OR chicks OR “Gallus gallus” OR “Gallus domesticus”) Timespan=All years Search language=Auto |
691,256 | |
| PubMed | # 7 | Search #6 NOTNOT (“Editorial” [Publication Type] OR “Letter” [Publication Type] OR “News” [Publication Type]) | 213 |
| # 6 | Search #5 Filters: Publication date from 1980/01/01; English | 215 | |
| # 5 | Search #1 AND #4 | 231 | |
| # 4 | Search #2 OR #3 | 5,356 | |
| # 3 | Search (“gases”[Mesh] OR gas[tiab] OR gases[tiab] OR electric*[tiab] OR waterbath[tiab] OR “water bath”[tiab] OR CO2[tiab] OR “carbon dioxide”[tiab]) AND (“Animal Culling”[Mesh] OR “Abattoirs”[Mesh] OR kill*[tiab] OR cull*[tiab] OR slaughter*[tiab] OR abbatoir*[tiab]) AND (“Animal Welfare”[Mesh] OR “Ataxia”[Mesh] OR “Behavior”[Mesh] OR “Physiology”[Mesh] OR “Neurology”[Mesh] OR “Seizures”[Mesh] OR welfare[tiab] OR behaviour*[tiab] OR behavior*[tiab] OR wing flapp*[tiab] OR ataxia[tiab] OR convulsion*[tiab] OR seizure*[tiab] OR “deep inhalation”[tiab] OR (head[tiab] AND shak*[tiab]) OR (loss[tiab] AND posture[tiab]) OR lying[tiab] OR motion*[tiab] OR move*[tiab] OR breath*[tiab] OR ecg[tiab] OR electrocardiography[tiab] OR electroencephalography[tiab] OR “power content”[tiab] OR “brain activity”[tiab] OR “heart rate”[tiab] OR “leg paddling”[tiab]) | 882 | |
| # 2 | Search stun[tiab] OR stunn*[tiab] OR stuning[tiab] OR stuned[tiab] OR prestunn*[tiab] OR prestun*[tiab] OR electronarcos*[tiab] OR electro narcos*[tiab] | 4,631 | |
| # 1 | Search “Chickens”[Mesh] OR “Poultry”[Mesh] OR Poultry[tiab] OR chicken*[tiab] OR Broiler*[tiab] OR cock[tiab] OR chicks[tiab] OR “Gallus gallus”[tiab] OR “Gallus domesticus”[tiab] | 179,600 | |
| DART‐Europe E‐theses Portal | # 3 | #1 OR #2 | 3 |
| # 2 | Keywords = (Poultry OR Chicken OR chickens OR Broiler OR Fowl OR Fowls OR Hen OR Hens OR cock OR chicks OR “Gallus gallus” OR “Gallus domesticus”) AND (gas OR gases OR electric* OR waterbath OR “water bath”) AND (kill* OR cull* OR slaughter*) AND (behaviour* OR behavior* OR physiolog* OR neruolog* OR “wing flap*” OR ataxia OR convulsion* OR “deep inhalation” OR “head shaking” OR seizure* OR jump* OR “loss of posture” OR lying OR motion* OR move* OR breath* OR ecg OR eeg OR electrocardiography OR electroencephalography OR “total power content” OR “brain activity” OR “heart rate” OR “leg paddling” OR welfare) | 1 | |
| # 1 | Keywords = (Poultry OR Chicken OR chickens OR Broiler* OR Fowl OR Fowls OR Hen OR Hens OR cock OR chicks OR “Gallus gallus” OR “Gallus domesticus”) AND (stun OR Stunn* OR Stuning OR Stuned OR prestunn* OR prestun* OR electronarcos* OR “electro narcose” OR “electro narcosis” OR “electro narcoses”) | 3 |
Appendix F – Hazard identification over the phases in different stunning methods
1.
Table F.1.
Description of the different phases involved in each stunning methodology and related hazards
| LAPS | CO2 | CO2 + Inert gas | Inert gas | Water‐bath | ||
|---|---|---|---|---|---|---|
|
Pre‐intervention phase Preparation of animals for stunning |
Description | Birds in the transport module Birds conveyed into the decompression chamber in transport modules will remain in the lairage conditions The module is then moved into the decompression chamber. The chamber is closed and the birds are in the dark | Birds in the transport module
|
Birds in the transport module
|
||
| Hazard | High stocking density | High stocking density |
Handling Hanging U/D by legs Compression of the leg bones Hurting (wing‐flapping occurring in neighbouring birds affecting consciousness) in shackling line |
|||
|
Induction phase Since the start of the intervention until the onset of loss of consciousness |
Description | Birds are exposed to a progressive decrease of atmospheric pressure and air during decompression (hypobaric hypoxia) | Birds are exposed to carbon dioxide (normobaric hypercapnic hypoxia) or inert gases (normobaric hypoxia) | Birds are immersed in a basin containing electrified water at a specified tension and intensity prescribed by the law. The unconsciousness is immediate | ||
| Hazard |
Lack of air Gas expansion in cavitary organs (intestine, air sacs, ears) Hurting (wing‐flapping occurring in neighbouring unconscious birds affecting conscious) in crates |
Acidic gas Respiratory stimulant Hurting (wing‐flapping occurring in neighbouring unconscious birds affecting conscious) in crates/tunnel |
Hurting (wing‐flapping occurring in neighbouring unconscious birds affecting conscious) in crates/tunnel | Pre‐stunning shock (occurring under complete fulfilment of the regulation, which foresees that operators have prevention measures in place), incomplete immersion of head, inadequate exposure to current | ||
|
Unconscious phase Period between the onset of loss of consciousness until killing intervention |
Description | Birds are kept in hypobaric hypoxia until the end of the process (i.e. 280 s in total) | Birds are kept in the gas mixture until they are dead, as foreseen by the legislation | Birds are conveyed to the neck‐cutting equipment situated close to the basin | ||
| Hazard | Under assumption of legal fulfilment, all identified hazards were common to all methods | |||||
|
Death onset Period between killing intervention until onset of death |
Description | Not relevant | Not relevant | Birds undergo neck‐cutting and die through blood loss before reaching the scalding tank | ||
| Hazard | Not relevant | Not relevant | Improper neck‐cutting/bleeding | |||
1.1.
1.1.1.
Discarded hazards
High stocking density/Vicinity to other birds: the closer the animals to each other, the higher the probability that the movements of an individual (e.g. wing flapping) may contact, disturb or even injure the neighbouring animals. Reasons for exclusion: common to all methods (in cage or hanging in line); no clear definition of an optimal density/distance between birds; in LAPS partly avoidable by setting up a specific condition (max density in the transport module used in the LAPS machine).
Size variability between individuals: the higher the heterogeneity, the greater the probability of an improper/ineffective stunning. This problem applies to all stunning methods. In LAPS and in gas methods, heavy birds may need more time to lose consciousness as they might be more resilient or may have a larger stored body oxygen volume relative to oxygen usage. In a water‐bath stunning, small birds may find it possible to avoid the water‐bath by lifting their head upward. The legislation, however, clearly prescribes that in the case of high heterogeneity in the flock, the animals should be properly managed and grouped in clusters of homogeneous size. Under the assumption of complete fulfilment of the legislation, this hazard was removed from the final list, although EFSA acknowledges that in practice this specific rule is frequently ignored in order not to slow down the slaughtering process.
System failure: this hazard was discarded for two reasons: first, the hazard ranking was intended to be performed under the assumption of an ideal situation where the equipment had no failures and the operators were adopting all measures to avoid any type of procedural mistake and, second, in case a certain probability of failure was considered, this would have been common to all methods and therefore it would have not influenced the results.
Appendix G – Methodology applied for the EEG quantitative analysis
1.
The general methodology of EEG data analysis was adopted from Vyssotski et al. (Current Biology, 2009, https://doi.org/10.1016/j.cub.2009.05.070). The data set for the current analysis was compiled from 38 records of animals that underwent LAPS and from 13 records of control animals. Only complete records of sufficient quality were selected for analysis from 70 recording attempts (articles 7 and 8 of the applicant). We did not find essential differences in EEG between the slightly different conditions described in these articles. For this reason, we aggregated the records in two groups for comparison (‘Birds in LAPS’ and ‘Control birds’). Data from 26 observed animals from the same publications were used to plot the loss of posture (LOP) latency.
The data analysis was carried out in EEGLAB package (v. 14.0.0) in Matlab, 2016. The EEG fragments starting from 80 s before LAPS onset lasting to the end of LAPS (280 s) were split in 1‐s epochs. The artefact‐contaminated epochs were identified by methods of EEGLAB with the following parameters: ‘Find abnormal values’ [−400 400] μV, ‘Find improbable data’ 5 standard deviations for single channels and for all channels, ‘Find abnormal distributions’ 5 standard deviations for single channels and for all channels, ‘Find abnormal spectra’ [−50 50] dB in frequency range [20 80] Hz. Values of rejected artefact‐contaminated epochs were substituted for values obtained by linear interpolation between values of two nearest good epochs. Median estimates were used in between groups comparison because of their low sensitivity to outliers. However, for averaging within the time intervals (‘before LAPS onset’ and ‘last 80 s of LAPS’) the mean values were computed.
To compute the latency of the end of the period of drowsiness (~ 50 s after LAPS onset), the latency to reach 10% of the pre‐stun EEG power (Figure 6b), and the latency decreases below 25% level of pre‐stun median frequency F50 (Figure 7a), we smoothed the original data (like in Figure 3) with a Gaussian function with the standard deviation σ = 5 s. At the first step, the temporal dynamics of measures from individual birds was smoothed by convolution with the same Gaussian window function (σ = 5 s, span 13 s) by processing the data in forward and reverse directions to have zero time shift (using Matlab function ‘filtfilt’). Then, the median values were computed for the group (of 38 or 13 birds). These median values were then additionally smoothed by the same method described above using the Gaussian function. The resulting smoothed medians are presented in Figures 6 and 7. Quartiles for median values were estimated by using a bootstrap with 104 iterations. In each iteration, a subset of animals was randomly taken (with return) from the original set and processed in the way described above. The accuracy of latencies (i.e. SEM) was estimated by bootstrap as well.
The EEG power at the end of LAPS session, when all animals were supposed to be dead, did not decline to zero but contained some measurable power as a result of technical noise (see Figure 5). To avoid biasing of the estimate of latency of the appearance of the 10% pre‐stun EEG power, the value was estimated from the last 80 s of LAPS procedure, and noise had been subtracted from total EEG power individually for each animal. The residuals were used for comparison of the group median with the 10% pre‐stun EEG power level (Figure 6b). The level of technical noise also affects the estimate of F50. To diminish this bias we subtracted the averaged power spectrum of the last 80 s of the LAPS procedure from the power spectrum of each epoch. The procedure that lead to the final chart Figure 7b was the following: in the first step, spectra of rejected epochs were substituted by spectra obtained by linear interpolation between the two nearest good epochs as has been noted earlier. Then, to compute the average spectrum of the last 80 s, each frequency bin of sequential 1‐sec epochs was smoothed by convolving with the Gaussian function with the standard deviation σ = 5 s (in both directions as described before). Then, a mean value was computed using data from the last 80 s for each frequency (i.e. mean spectrum). Then this mean spectrum was subtracted from the spectrums of individual 1‐s epochs smoothed by the method mentioned above (Gaussian function) along time axes as described earlier. The value for F50 was then computed. Median values for F50 of different animals are shown in Figure 7b after smoothing (by double convolving with the Gaussian as described before).
Annex – Welfare hazards associated with alternative stunning method in broiler chicken ‐ expert judgement and statistical analysis
1.
Annex A contains the details on the methodology and the results of the expert judgement exercise for the ranking of a set of hazards identified by the ad hoc EFSA working group on the LAPS system and can be found in the online version of this output (in the ‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.5056
Supporting information
Welfare hazards associated with alternative stunning method in broiler chicken ‐ expert judgement and statistical analysis
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More S, Bicout D, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortazar Schmidt C, Miranda MA, Nielsen SS, Sihvonen L, Spoolder H, Willeberg P, Raj M, Thulke H‐H, Velarde A, Vyssotski A, Winckler C, Cortiñas Abrahantes J, Garcia A, Muñoz Guajardo I, Zancanaro G and Michel V, 2017. Scientific Opinion on the low atmospheric pressure system for stunning broiler chickens. EFSA Journal 2017;15(12):5056, 86 pp. 10.2903/j.efsa.2017.5056
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00327
Panel members: Dominique Bicout, Anette Bøtner, Andrew Butterworth, Paolo Calistri, Klaus Depner, Sandra Edwards, Bruno Garin‐Bastuji, Margaret Good, Christian Gortazar Schmidt, Virginie Michel, Miguel Angel Miranda, Simon More, Søren Saxmose Nielsen, Mohan Raj, Liisa Sihvonen, Hans Spoolder, Jan Arend Stegeman, Hans‐Hermann Thulke, Antonio Velarde, Preben Willeberg and Christoph Winckler.
Acknowledgements: The Panel wishes to thank the members of the Working Group on LAPS: Mohan Raj, Hans‐Hermann Thulke, Antonio Velarde, Alexei Vyssotski, Christoph Winckler and Virginie Michel for the preparatory work on this scientific output and the hearing expert Gwenola Touzot‐Jourde for the support provided to this scientific output.
Adopted: 25 October 2017
Notes
References
- Abeyesinghe SM, McKeegan DE, McLeman MA, Lowe JC, Demmers TG, White RP, Kranen RW, vanBemmel H , Lankhaar JA and Wathes CM, 2007. Controlled atmosphere stunning of broiler chickens. I. Effects on behaviour, physiology and meat quality in a pilot scale system at a processing plant. British Poultry Science, 48, 406–423. [DOI] [PubMed] [Google Scholar]
- AVMA Guidelines for the Humane Slaughter of Animals : 2016 Edition. Available at https://www.avma.org/KB/Resources/Reference/AnimalWelfare/Documents/Humane-Slaughter-Guidelines.pdf
- von Borell E, Langbein J, Després G, Hansen S, Leterrier C, Marchant‐Forde J, Marchant‐Forde R, Minero M, Mohr E, Prunier A, Valance D and Veissier I, 2007. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals — a review. Physiology and Behavior, 92, 293–316. [DOI] [PubMed] [Google Scholar]
- Battula V, Schilling MV, Vizzier‐Thaxton Y, Behrends JM, Williams JB and Schmidt TB, 2008. The Effects of Low‐Atmosphere Stunning and Deboning Time on Broiler Breast Meat Quality. Poultry Science, 87, 1202–1210. 10.3382/ps.2007-00454. [DOI] [PubMed] [Google Scholar]
- Buchwalder T and Huber‐Eicher B, 2005. Effect of the analgesic butorphanol on activity behaviour in turkeys (Meleagris gallopavo). Research in Veterinary Science, 79, 239–244 Epub 2005 Feb 2. [DOI] [PubMed] [Google Scholar]
- Carravieri A, Müller MS, Yoda K, Hayama SI and Yamamoto M, 2016. Dominant Parasympathetic Modulation of Heart Rate and Heart Rate Variability in a Wild‐Caught Seabird. Physiological and Biochemical Zoology, 89, 263–276. 10.1086/686894, Epub 2016 May 11. [DOI] [PubMed] [Google Scholar]
- Coenen A, Smit A, Zhonghua L and Van Luijtelaar G, 2000. Gas mixtures for anaesthesia and euthanasia in broiler chickens. World's Poultry Science Journal, 56, 225–234. [Google Scholar]
- Dohoo I, Martin W and Stryhn H, 2010. Veterinary Epidemiologic Research. VER Inc. ISBN 978‐0‐919013‐60‐5. [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Opinion of the Scientific Panel on Animal Health and Welfare on a request from the Commission related to welfare aspects of the main systems of stunning and killing the main commercial species of animals. EFSA Journal 2004;2(7):45, 29 pp. 10.2903/j.efsa.2004.45 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2013. Guidance on the assessment criteria for studies evaluating the effectiveness of stunning methods regarding animal protection at the time of killing. EFSA Journal 2013;11(12):3486, 41 pp. 10.2903/j.efsa.2013.3486 [DOI] [Google Scholar]
- First published , 2007. Printed in the United Kingdom at the University Press, Cambridge. ISBN‐978‐0‐521‐82868‐0 hardback ISBN‐978‐0‐521‐53563‐2 paperback
- Gentle MJ and Richardson A, 1972. Changes in the electroencephalogram of the chicken produced by stimulation of the crop. British Poultry Science, 13, 163–170. 10.1080/00071667208415930 [DOI] [PubMed] [Google Scholar]
- Gerritzen MA, Lambooij E, Hillebrand SJW, Lankhaar JAC and Pieterse C, 2000. Behavioral responses of broilers to different gaseous atmospheres. Poultry Science, 79, 928–933. [DOI] [PubMed] [Google Scholar]
- Gerritzen MA, Lambooij B, Reimert H, Stegeman A and Spruijt B, 2004. On‐farm euthanasia of broiler chickens: effects of different gas mixtures on behavior and brain activity. Poultry Science, 83, 1294–1301. [DOI] [PubMed] [Google Scholar]
- Guzman DS, Flammer K, Paul‐Murphy JR, Barker SA and Tully TN, 2011. Pharmacokinetics of butorphanol after intravenous, intramuscular, and oraladministration in Hispaniolan Amazon parrots (Amazona ventralis). Journal of Avian Medicine and Surgery, 25, 185–191. [DOI] [PubMed] [Google Scholar]
- Guzman DS, Drazenovich TL, KuKanich B, Olsen GH, Willits NH and Paul‐Murphy JR, 2014. Evaluation of thermal antinociceptive effects andpharmacokinetics after intramuscular administration of butorphanol tartrateto American kestrels (Falco sparverius). American Journal of Veterinary Research, 75, 11–18. 10.2460/ajvr.75.1.11 [DOI] [PubMed] [Google Scholar]
- INRA , 2009. Animal pain: Identifying, understanding and minimising pain in farm animals; INRA Expert Scientific Assessment (ESCo) Report; October 2009. Available at http://institut.inra.fr/en/Objectives/Informing-public-policy/Scientific-Expert-Reports/All-the-news/Pain-in-farm-animals
- Korte SM, Sgoifo A, Ruesink W, Kwakernaak C, van Voorst S, Scheele CW and Blokhuis HL, 1999. High carbon dioxide tension (PCO2) and the incidence of cardiac arrhythmias in rapidly growing broiler chickens. The Veterinary Record, 145, 40–43. [DOI] [PubMed] [Google Scholar]
- Lambooij E, Gerritzen MA, Engel B, Hillebrand SJW, Lankhaar J and Pieterse C, 1999. Behavioral responses during exposure of broiler chickens to different gas mixtures. Applied Animal Behaviour Science, 62, 255–265. [Google Scholar]
- Lukatch HS, Echon RM, Maciver MB and Werchan PM, 1997. G‐force induced alterations in rat EEG activity: a quantitative analysis. Electroencephalography and Clinical Neurophysiology, 103, 563–573. [DOI] [PubMed] [Google Scholar]
- Martin JE, 2015. Humane mechanical methods for killing poultry on farm. PhD Thesis. College of Medical Veterinary and Life Sciences, University of Glasgow
- MATLAB and Statistics Toolbox Release , 2016. The MathWorks, Inc., Natick, Massachusetts, United States
- Mckeegan DEF, Demmers TGM, Wathers CM and Jones RB, 2003. Chemosensitivity responses to gaseous pollutants and carbon dioxide: implications for poultry welfare. Poultry Science, 82(supplement 1), 16. [Google Scholar]
- McKeegan DEF, Smith FS, Theo GM, Demmers TGM and Jones RB, 2005. Behavioral correlates of olfactory and trigeminal gaseous stimulation in chickens, Gallus domesticus. Physiology and Behavior, 84, 761–768. [DOI] [PubMed] [Google Scholar]
- McKeegan DEF, Abeyesinghe SM, McLeman MA, Lowe JC, Demmers TGM, White RP, Kranen RW, van Bemmel H, Lankhaar JAC and Wathes CM, 2007. Controlled atmosphere stunning of broiler chickens. II. Effects on behaviour, physiology and meat quality in a commercial processing plant. British Poultry Science, 48, 430–442. [DOI] [PubMed] [Google Scholar]
- McKeegan DEF, Sandercock DA and Gerritzen MA, 2013. Physiological responses to low atmospheric pressure stunning and the implications for welfare. Poultry Science, 92, 858–868. 10.3382/ps.2012-02749 [DOI] [PubMed] [Google Scholar]
- Ookawa T and Gotoh J, 1965. Electroencephalogram of the chicken recorded from the skull under various conditions. The Journal of Comparative Neurology, 124, 1–14. 10.1002/(ISSN)1096-9861 [DOI] [PubMed] [Google Scholar]
- Paul‐Murphy J and Fialkowski J, 2001. Injectable anaesthesia and analgesia of birds In: Gleed RD, Ludders JW. (eds.). Recent Advances in Veterinary Anaesthesiaand Analgesia: Companion Animals. International Veterinary InformationService, Ithaca, New York, USA: http://www.ivis.org [Google Scholar]
- Purswell JL, Thaxton JP and Branton SL, 2007. Identifying process variables for a low atmospheric pressure stunning‐killing system. Journal of Applied Poultry Research, 16, 509–513. [Google Scholar]
- Raj AB, 1996. Aversive reactions of turkeys to argon, carbon dioxide and a mixture of carbon dioxide and argon. Vet Rec. 1996 Jun 15, 138, 592–593. [DOI] [PubMed] [Google Scholar]
- Raj ABM, 1997. Gas stunning broilers: welfare and product quality In: Lambooij E. (ed.). Alternative Stunning Methods for Poultry. ID‐DLO Institute for Animal Science and Health publication number 97.037. [Google Scholar]
- Raj ABM, Wotton SB, McKinstry JL, Hillebrand SJW and Pieterse C, 1998. Changes in the somatosensory evoked potentials and spontaneous electroencephalogram of broiler chickens during exposure to gas mixtures. British Poultry Science, 39, 686–695. [DOI] [PubMed] [Google Scholar]
- Raj ABM, 2006. Recent developments in stunning and slaughter of poultry. World's Poultry Science Journal, 62, 467–484. 10.1079/WPS2005109 [DOI] [Google Scholar]
- Sandercock DA, Auckburally A, Flaherty D, Sandilands V and McKeegan DEF, 2014. Avian reflex and electroencephalogram responses in different states of consciousness. Physiology and Behavior, 133, 252–259. [DOI] [PubMed] [Google Scholar]
- Savage S, 1998. Feed withdrawal: a practical look at its effect on intestine emptying, contamination and yield. Pfizer Inc. Carlyle Bennett Animal Industry Branch, Manitoba Agriculture and Food August, 2002. Available online: https://www.gov.mb.ca/agriculture/livestock/production/commercial-poultry/print,a-picture-guide-of-chicken-feed-withdrawal.html
- Schilling MV, Radhakrishnan V, Vizzier‐Thaxton Y, Christensen K, Joseph P, Williams JB and Schmidt TB, 2012. The effects of low atmosphere stunning and deboning time. Poultry Science, 91, 3214–3222. 10.3382/ps.2012-02266 on broiler breast meat quality [DOI] [PubMed] [Google Scholar]
- Singh PM, Johnson CB, Gartrell B, Mitchinson S, Jacob A and Chambers P, 2017. Analgesic effects of morphine and butorphanol in broiler chickens. Veterinary Anaesthesia and Analgesia, 44, 538–545. 10.1016/j.vaa.2016.05.006, Epub 2017 Jan 11. [DOI] [PubMed] [Google Scholar]
- Sullivan GM and Artino AR, 2013. Analyzing and interpreting data from likert‐type scales. Journal of Graduate Medical Education, 5, 541–542. 10.4300/JGME-5-4-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven MTW, Gerritzen MA, Hellebrekers LJ and Kemp B, 2015. Indicators used in livestock to assess unconsciousness after stunning: a review. Animal, 9, 320–330. 10.1017/S1751731114002596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzier‐Thaxton Y, Christensen KD, Schilling MV, Buhr RJ and Thaxton JP, 2010. A new humane method of stunning broilers using low atmospheric pressure. Journal of Applied Poultry Research, 19, 341–348. 10.3382/japr.2010-00184 [DOI] [Google Scholar]
- Webster AB and Fletcher D, 2004. Assessment of the aversion of hens to different gas atmospheres using an approach‐avoidance test. Applied Animal Behaviour Science, 88, 275–287. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Welfare hazards associated with alternative stunning method in broiler chicken ‐ expert judgement and statistical analysis


