Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the evaluating Member State (EMS), the Netherlands, received two applications from ISK Biosciences Europe N.V. to modify the existing maximum residue levels (MRLs) for the active substance flonicamid in various commodities. EFSA concludes that the data submitted were sufficient to derive MRL proposals of 0.3 mg/kg for apricots, 0.5 mg/kg for head cabbage, and 1.5 mg/kg for beans and peas with pods. No change of the existing MRL set at the limit of quantification (LOQ) of 0.03 mg/kg was required for sugar beet. Furthermore, EFSA derived new MRL proposals for some commodities of animal origin. Adequate analytical enforcement methods are available to control the residues of flonicamid on the commodities under consideration. Based on the risk assessment results, EFSA concludes that the proposed use of flonicamid on the commodities under consideration will not result in a consumer exposure exceeding the toxicological reference values and therefore is unlikely to pose a consumer health risk.
Keywords: flonicamid, apricot, various vegetables, sugar beet, MRL application, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, the evaluating Member State (EMS) the Netherlands received two applications from ISK Biosciences Europe N.V. to modify the existing maximum residue levels (MRLs) for the active substance flonicamid in various commodities. To accommodate for the intended uses of flonicamid, the Netherlands proposed to raise the existing MRLs from the limit of quantification (LOQ) of 0.03 to 0.5 mg/kg in head cabbage and to 1.5 mg/kg in beans and peas with pods, to maintain the existing MRLs in sugar beet at LOQ of 0.03 mg/kg and to raise the existing MRL of 0.03 mg/kg in poultry muscle and liver to 0.04 mg/kg. For apricots, the Netherlands proposed to raise the existing MRL from the LOQ of 0.03 to 0.3 mg/kg. The Netherlands drafted the evaluation reports in accordance with Article 8 of Regulation (EC) No 396/2005, which were submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 7 July 2015 and on 5 September 2016.
EFSA bases its assessment on the evaluation reports, the draft assessment report (DAR) and its addendum prepared under Directive 91/414/EEC, the Commission review report on flonicamid, the conclusion on the peer review of the pesticide risk assessment of the active substance flonicamid, as well as the conclusions from previous EFSA reasoned opinions on flonicamid, including the review of the existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (hereafter MRL review).
The toxicological profile of flonicamid was assessed in the framework of the peer review under Directive 91/414/EEC and the data were sufficient to derive an acceptable daily intake (ADI) of 0.025 mg/kg body weight (bw) per day and an acute reference dose (ARfD) of 0.025 mg/kg bw.
The metabolism of flonicamid in primary crops was evaluated in the framework of the peer review under Directive 91/414/EEC and the MRL review in the fruit, root and cereal/grass crop groups. From these studies, the peer review established the residue definition for enforcement and risk assessment as ‘sum of flonicamid, TFNA and TFNG, expressed as flonicamid’. Adequate analytical methods exist to simultaneously analyse all compounds included in the residue definitions. For the use on the crops under consideration, EFSA concludes that the metabolism of flonicamid in primary crops has been sufficiently addressed and that the residue definitions derived are applicable.
Studies investigating the nature of flonicamid residues under standard hydrolysis conditions were assessed during the peer review and showed the active substance to be hydrolytically stable under standard processing conditions. Therefore, for processed commodities, the same residue definition as for raw agricultural commodities (RAC) is applicable.
Due to fast degradation of flonicamid and its metabolites in the soil, further investigation of residues in rotational crops is not required.
As the crops under consideration are used as feed products, a potential carry‐over into food of animal origin was assessed. The nature of flonicamid residues in livestock has been investigated during the peer review of the active substance and the residue definition for enforcement and for risk assessment was proposed as ‘sum of flonicamid and TFNA‐AM, expressed as flonicamid’. The calculated livestock dietary burden exceeded the trigger value of 0.1 mg/kg dry matter (DM) for all relevant animal species. Therefore, the possible occurrence of flonicamid residues in commodities of animal origin was investigated.
The additional crops under consideration in this MRL application and the new calculation methodology increase the dietary burden compared to dietary intake calculations performed in the framework of the MRL review for flonicamid, and therefore, new MRLs were proposed by EFSA for certain tissues of ruminants, swine and poultry and eggs.
The consumer risk assessment was performed with revision 2 of the EFSA Pesticide Residues Intake Model (PRIMo). The chronic consumer risk assessment performed in the framework of the MRL review was already revised twice to include median residue levels (STMRs) assessed in EFSA reasoned opinions issued after the MRL review. The calculation was revised further with the STMRs derived for the crops under consideration and the products of animal origin. The acute exposure assessment was performed only with regard to the commodities under consideration.
A long‐term consumer intake concern was not identified for any of the European diets incorporated in the EFSA PRIMo. The highest chronic intake was calculated to be 18% of the ADI (WHO cluster diet B, DK child). The contribution of residues to the total consumer exposure accounted for a maximum of 11.9% of the ADI for wheat (WHO cluster diet B), and less than 10% of the ADI for the remaining commodities.
An acute consumer risk was not identified in relation to the MRL proposals for the crops under consideration. The highest acute consumer exposure was calculated to be 48.4% of the ARfD for head cabbage, 30.4% of the ARfD for beans with pods, 15.7% of the ARfD for apricots, 9.3% of the ARfD for peas with pods, 8.4% of the ARfD for milk, 7.7% of the ARfD for sugar beet and 5% for bird's eggs. For the remaining commodities, the exposure accounted for less than 3% of the ARfD.
EFSA concludes that the intended uses of flonicamid on the commodities under consideration will not result in a consumer exposure exceeding the toxicological reference values and therefore are unlikely to pose a health risk to consumers.
EFSA proposes to amend the existing MRLs as reported in the summary table below.
| Codea | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition for commodities of plant origin: sum of flonicamid, TFNA and TFNG, expressed as flonicamid | ||||
| 0140010 | Apricots | 0.03* | 0.3 | The NEU GAP is sufficiently supported by data. No consumer risk was identified |
| 0242990 | Head cabbage | 0.03* | 0.5 | NEU and SEU GAPs are sufficiently supported; the MRL was derived from the more critical SEU use. No consumer risk was identified |
| 0260010 | Beans with pods | 0.03* | 1.5 | NEU and SEU GAPs are sufficiently supported. The MRL was derived from the more critical SEU data. No consumer risk was identified |
| 0260030 | Peas with pods | 0.03* | 1.5 | |
| 0900010 | Sugar beet | 0.03* | 0.03* | The NEU GAP is sufficiently supported. A change of the existing MRL is not required. No consumer risk was identified |
| Enforcement residue definition for commodities of animal origin: sum of flonicamid and TFNA‐AM, expressed as flonicamid | ||||
| 1011010 | Swine muscle | 0.02* | 0.03 | The proposed MRLs were derived from the updated dietary burden calculation including the relevant feed items |
| 1011020 | Swine fat | 0.02* | 0.02* | |
| 1011030 | Swine liver | 0.03 | 0.03 | |
| 1011040 | Swine kidney | 0.03 | 0.03 | |
| 1012010 | Bovine muscle | 0.03 | 0.05 | |
| 1012020 | Bovine fat | 0.02* | 0.02* | |
| 1012030 | Bovine liver | 0.04 | 0.05 | |
| 1012040 | Bovine kidney | 0.04 | 0.05 | |
| 1013010 | Sheep muscle | 0.03 | 0.05 | |
| 1013020 | Sheep fat | 0.02* | 0.02* | |
| 1013030 | Sheep liver | 0.04 | 0.05 | |
| 1013040 | Sheep kidney | 0.04 | 0.05 | |
| 1014010 | Goat muscle | 0.03 | 0.05 | |
| 1014010 | Goat fat | 0.02* | 0.02* | |
| 1014030 | Goat liver | 0.04 | 0.05 | |
| 1014040 | Goat kidney | 0.04 | 0.05 | |
| 1016010 | Poultry muscle | 0.03 | 0.04 | |
| 1016020 | Poultry fat | 0.03 | 0.03 | |
| 1016030 | Poultry liver | 0.03 | 0.05 | |
| 1020010 | Cattle milk | 0.02* | 0.02* | |
| 1020020 | Sheep milk | 0.02* | 0.02* | |
| 1020030 | Goat milk | 0.02* | 0.02* | |
| 103000 | Birds eggs | 0.04 | 0.1 | |
MRL: maximum residue level; NEU: northern Europe; GAP: Good Agricultural Practice; SEU: southern Europe.
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Background
Regulation (EC) No 396/20051 (hereinafter referred to as ‘the MRL regulation’) establishes the rules governing the setting of pesticide maximum residue levels (MRLs) at European Union (EU) level. Article 6 of the Regulation lays down that any party having a legitimate interest or requesting an authorisation for the use of a plant protection product in accordance with Council Directive 91/414/EEC2, repealed by Regulation (EC) No 1107/20093, shall submit to a Member State, when appropriate, an application to modify a MRL in accordance with the provisions of Article 7 of the MRL regulation.
The Netherlands, hereafter referred to as the evaluating Member State (EMS), received two applications from the company ISK Biosciences Europe N.V.4 to modify the existing MRLs for the active substance flonicamid in various commodities. These applications were notified to the European Commission and the European Food Safety Authority (EFSA) and were subsequently evaluated by the EMS in accordance with Article 8 of the Regulation.
After completion, the evaluation reports were submitted to the European Commission and to EFSA on 7 July 2015 and on 5 September 2016, respectively.
The applications were included in the EFSA Register of Questions with the reference numbers and the following subject:
EFSA‐Q‐2015‐00424: Flonicamid – Modification of existing MRLs in various crops
EFSA‐Q‐2016‐00548: Flonicamid – Modification of existing MRLs in apricots
The Netherlands proposed to raise the existing MRLs from the LOQ of 0.03 to 0.5 mg/kg in head cabbage and to 1.5 mg/kg in beans with pods and peas with pods. The Netherlands proposed to maintain the existing MRL in sugar beet at the LOQ of 0.03 mg/kg and to raise the existing MRL of 0.03 mg/kg in poultry muscle and liver to 0.04 mg/kg. For apricots, the Netherlands proposed to raise the existing MRL from the LOQ of 0.03 to 0.3 mg/kg.
EFSA proceeded with the assessment of the application and the evaluation reports as required by Article 10 of the Regulation. For reasons of efficiency, EFSA assessed both applications in a single reasoned opinion.
In accordance with Article 10 of Regulation (EC) No 396/2005, EFSA shall, based on the evaluation report provided by the EMS, provide a reasoned opinion on the risks to the consumer associated with the application.
The evaluation reports submitted by the EMS (Netherlands, 2015, 2016) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available.
In accordance with Article 11 of the Regulation, the reasoned opinion shall be provided as soon as possible and at the latest within 3 months (which may be extended to 6 months if more detailed evaluations need to be carried out) from the date of receipt of the application. If EFSA requests supplementary information, the time limit laid down shall be suspended until that information has been provided.
The active substance and its use pattern
Flonicamid is the ISO common name for N‐cyanomethyl‐4‐(trifluoromethyl)nicotinamide (IUPAC). The chemical structures of the active substance and its main metabolites are reported in Appendix B. Flonicamid has been approved for the uses as insecticide.
The details of the intended Good Agricultural Practices (GAPs) for flonicamid triggering the MRL application are given in Appendix A.
Flonicamid was evaluated in the framework of Directive 91/414/EEC2 with France designated as the rapporteur Member State (RMS). The representative uses evaluated in the peer review were foliar spray applications on potatoes, wheat, apples and pears in all EU countries and on peaches in Southern Europe. The draft assessment report (DAR) has been peer reviewed by EFSA (EFSA, 2010).
Flonicamid was included in Annex I of this Directive by Directive 2010/29/EU5 which entered into force on 1 September 2010 for use as insecticide only. In accordance with Commission Implementing Regulation (EU) No 540/20116, flonicamid is approved under Regulation (EC) No 1107/20093, repealing Council Directive 91/414/EEC.
Following the MRL review under Article 12 of Regulation (EC) No 396/2005, which was completed in 2014 (EFSA, 2014), the EU MRLs for flonicamid have been revised by Regulation (EU) No 2016/717. Since that, EFSA issued one reasoned opinion on the modification of the existing MRLs for flonicamid in herbs and edible flowers (EFSA, 2016).
Assessment
EFSA has based its assessment on the evaluation reports submitted by the EMS (Netherlands, 2015, 2016), the DAR and its addendum prepared under Directive 91/414/EEC (France, 2005, 2009), the Commission review report on flonicamid (European Commission, 2010a), the conclusion on the peer review of the pesticide risk assessment of the active substance flonicamid (EFSA, 2010) as well as the conclusions from previous EFSA opinions on flonicamid including Article 12 MRL review (EFSA, 2014, 2015, 2016). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20118 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1996, 1997a, b, c, d, e, f, g, 2000, 2010b, c, 2016; OECD, 2011).
1. Method of analysis
1.1. Methods for enforcement of residues in food of plant origin
Analytical methods for the determination of flonicamid residues in plant commodities were assessed in the framework of the peer review and the Article 12 MRL review. Sufficiently validated methods to control residues of flonicamid and its metabolites TFNG and TFNA in high water, high acid and high oil content matrices and in dry commodities were provided. The methods allow quantifying residues for each analyte included in the residue definition at the LOQ of 0.01 mg/kg (combined LOQ of 0.03 mg/kg) (EFSA, 2014).
EFSA concludes that sufficiently validated analytical methods are available for enforcing the proposed MRL for flonicamid in the crops under consideration.
1.2. Methods for enforcement of residues in food of animal origin
The analytical methods for the determination of flonicamid residues in commodities of animal origin were evaluated during the peer review under Directive 91/414/EEC (EFSA, 2010) and in the framework of the MRL review (EFSA, 2014). EFSA concluded that validated analytical methods are available to enforce flonicamid and its metabolite TFNA‐AM in milk, eggs, bovine muscle, fat, kidney and liver with an LOQ of 0.01 mg/kg for each analyte (combined LOQ of 0.02 mg/kg) (EFSA, 2014).
EFSA concludes that sufficiently validated analytical methods for enforcing the proposed MRLs for flonicamid in food of animal origin are available.
2. Mammalian toxicology
The toxicological profile of the active substance flonicamid was assessed in the framework of the peer review under Directive 91/414/EEC (EFSA, 2010). The data were sufficient to derive toxicological reference values compiled in Table 1.
Table 1.
Overview of the toxicological reference values
| Source | Year | Value | Study | Safety factor | |
|---|---|---|---|---|---|
| Flonicamid | |||||
| ADI | EFSA | 2010 | 0.025 mg/kg bw per day | Rabbit development | 100 |
| ARfD | EFSA | 2010 | 0.025 mg/kg bw | Rabbit development | 100 |
ADI: acceptable daily intake: ARfD: acute reference dose; bw: body weight.
The toxicological endpoints set for flonicamid are also applicable to its metabolites TFNA, TFNG and TFNA‐AM, which have been included in the residue definition for risk assessment of plant and animal origin commodities (EFSA, 2010).
3. Residues
3.1. Nature and magnitude of residues in plant
3.1.1. Primary crops
3.1.1.1. Nature of residues
The metabolism of flonicamid in primary crops was evaluated in the framework of the peer review under Directive 91/414/EEC (EFSA, 2010) and reviewed in the Article 12 MRL review (EFSA, 2014) in the fruit, root/tuber and cereal crop groups. An overview of the key features of the available metabolism studies is presented in Table 2.
Table 2.
Summary of available metabolism studies in plants
| Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comments |
|---|---|---|---|---|
| Fruit | Peach | Foliar: 2 × 100 and 2 × 500 g/ha | 21 | Radiolabel position: 3‐14C‐phenyl |
| Pepper | Foliar: 1 × 100 g/ha | 7, 14 | ||
| Root | Potato | Foliar: 2 × 100 and 2 × 500 g/ha | 14 | |
| Cereals/grass | Wheat | Foliar: 2 × 100 and 2 × 500 g/ha | 21 |
DAT: days after treatment.
Based on these metabolism studies, the residue definition for monitoring and risk assessment was proposed as ‘sum of flonicamid, TFNA and TFNG, expressed as flonicamid’. The toxicological reference values set for flonicamid were concluded to be also applicable to the metabolites and adequate analytical methods exist to simultaneously analyse all compounds included in the residue definitions (EFSA, 2010).
The MRL review confirmed the residue definitions proposed by the peer review and identified a need to clarify whether the TFNA metabolite could be a common metabolite to other active substances (EFSA, 2014). The proposed residue definition has been implemented in the MRL Regulation.
For the uses on the crops under consideration, EFSA concludes that the metabolism of flonicamid is sufficiently addressed and the residue definitions for enforcement and risk assessment agreed during the peer review are applicable.
3.1.1.2. Magnitude of residues
All samples of the supervised residue trials submitted with the MRL application were analysed individually and the total residue was expressed as sum of flonicamid and its metabolites TFNA and TFNG. According to the EMS, the analytical methods used to analyse the residue trial samples have been sufficiently validated and were proven to be fit for the purpose (Netherlands, 2015, 2016).
The stability of flonicamid residues in plant matrices under storage conditions prior to analysis was assessed during the peer review under Directive 91/414/EEC (EFSA, 2010). Residues of flonicamid were found to be stable at ≤ −18°C for up to 18 months in high water (apple), high starch (potatoes) and dry/starch commodities (wheat grain) and in wheat straw. As the trial samples provided in support of the MRL application were stored for periods shorter than 18 months under conditions for which integrity of the samples was demonstrated, it is concluded that the residue data are valid with regard to storage stability.
a) Apricots (northern Europe (NEU), GAP: 2 × 70 g/ha, preharvest interval (PHI) 21 days)
In support of the MRL application, four supervised residue trials in apricot conducted in Hungary and Germany in 2011 and 2014 were provided. One trial in Hungary was not compliant with the GAP in terms of PHI, so it was not considered as acceptable. To complete the data set, two Hungarian trials on peaches were provided which is in line with the EU guidance document (European Commission, 2016). Thus, overall five GAP‐compliant residue trials are available to derive an MRL proposal.
Based on this data set, EFSA derived a MRL proposal of 0.3 mg/kg for apricots.
b) Head cabbage (NEU, southern Europe (SEU), GAP: 2 × 70 g/ha, PHI 14 days)
Eight supervised GAP‐compliant residue trials were performed in northern Europe (Germany, north France, the United Kingdom) and four in southern Europe (south France, Italy). Trials were performed in two seasons (2012 and 2013). All trials were performed with a 50WG formulation with and without adjuvant in side‐by‐side trials. A tendency for higher residue levels was observed in the trials with the adjuvant (all trials except one). Thus, the trials with the higher residue values were selected to be used for supporting the MRL request.
Since the NEU and SEU data differed significantly (Mann–Whitney U test), the data sets were assessed separately. Supervised residue trials in southern Europe result in worst‐case residue levels. The data set of four trials performed in countries of the southern European residue zone is acceptable for setting an MRL since head cabbage is a minor crop in southern Europe. The proposed MRL also covers the residue situation in northern Europe.
EFSA concludes that sufficient data are available to support the intended use on head cabbage; a MRL proposal of 0.5 mg/kg was derived.
c) Beans with pods (NEU, SEU, GAP: 1 × 70 g/ha, PHI 14 days)
Four GAP‐compliant supervised residue trials were performed in northern Europe (Belgium, Germany, the United Kingdom, the Netherlands) and four in Southern Europe (Italy, Spain). The trials were performed in 2013.
In accordance with the EU guidance document, beans with pods are a major crop. Thus, at least eight residue trials are required. To complete the data set for beans with pods, the applicant provided four trials on peas with pods for each residue zone.
All trials were performed with a 50WG formulation, with one plot treated in combination with and one plot without adjuvant. The highest residue value of both parallel experiments (with and without adjuvant) was used for MRL setting and risk assessment. Higher residue levels were observed when an adjuvant was added (all trials).
The trials in southern Europe resulted in higher residue levels than trials performed in northern Europe, based on the Mann–Whitney U‐test.
EFSA concludes that sufficient data are available to support the intended use on beans with pods; EFSA derived a MRL proposal of 1.5 mg/kg based on the SEU data set.
d) Peas with pods (NEU, SEU, GAP: 1 × 70 g/ha, PHI 14 days)
The combined data set described above was used to derive the MRL proposal for peas with pods.
e) Sugar beet (NEU, GAP: 1 × 70 g/ha, PHI 60 days)
Eight GAP‐compliant residue trials performed in two seasons (2010 and 2011) in northern Europe (France, Belgium) have been submitted. The trials were performed without adjuvant. Four of the trials were decline studies.
EFSA concludes that sufficient data are available to support the intended use on sugar beet and based on this data set, EFSA derived a MRL proposal of 0.03 mg/kg (LOQ) for sugar beet roots.
A complete overview of the results of the residue trials, the related risk assessment input values (highest residue, median residue) and the MRL proposals are summarised in Table 3. Since the residue data on sugar beet tops are relevant for livestock dietary burden calculation, the results of these trials are also reported in this table.
Table 3.
Overview of the available residues trials data
| Crop (trial conditions) | Region/indoora | Residue levels observed in the supervised residue trialsb (mg/kg) | Recommendations/commentsc | MRL proposal (mg/kg) | HRd (mg/kg) | STMRe (mg/kg) |
|---|---|---|---|---|---|---|
| Apricot and peach (2 × 70 g/ha, PHI 21 days) | NEU (field) |
Trials on apricot: 0.145, 0.05, 0.127 Trials on peach: 0.05, 0.07 |
MRL proposal for apricots: MRLOECD: 0.283/0.3 Extrapolation from peach to apricot is in line with the EU guidance document |
0.3 | 0.127 | 0.099 |
| Head cabbage (2 × 70 g/ha, PHI 14 days) + adjuvant | NEU (field) | 0.03*, 0.04, 0.05, 0.06, 0.07, 0.08, 0.08, 0.11 | MRLOECD: 0.179/0.2 | 0.2 | 0.11 | 0.065 |
| SEU (field) | 0.07, 0.12, 0.16; 0.23 |
MRLOECD: 0.435/0.5 Minor crop in SEU. The proposed MRL is based on worst‐case SEU, which covers also NEU |
0.5 | 0.23 | 0.14 | |
| Peas and beans with pods (1 × 70 g/ha, PHI 14 days) + adjuvant | NEU (field) |
Trials on peas with pods: 0.15, 0.17, 0.25, 0.29 Trials on beans with pods: 0.15, 0.26, 0.26, 0.29 |
MRLOECD: 0.683/0.7 Combined data sets of peas and beans with pods. Populations are similar (U < 5%). The proposed MRL is based on worst‐case SEU, which covers also NEU |
0.7 | 0.29 | 0.255 |
| SEU (field) |
Trials on peas with pods: 0.31, 0.31, 0.51, 0.67 Trials on beans with pods: 0.19, 0.22, 0.37, 0.39 |
MRLOECD: 1.114/1.5 Combined data sets of peas and beans with pods. Populations are similar (U < 5%). The proposed MRL is based on worst‐case SEU, which covers also NEU |
1.5 | 0.67 | 0.34 | |
| Sugar beet root (1 × 70 g/ha, PHI 60 days) No adjuvant | NEU (field) | 8 × < 0.03 | MRLOECD: 0.03/0.03 | 0.03 * | 0.03 | 0.03 |
| Sugar beet top (1 × 70 g/ha, PHI 60 days) No adjuvant | NEU | 0.03, 0.03, 0.04, 0.06, 0.11, 0.13, 0.16, 0.20 | No MRL required. Residue data relevant for livestock dietary burden | – | 0.20 | 0.085 |
MRL: maximum residue level; PHI: preharvest interval; OECD: Organisation for Economic Co‐operation and Development.
* Indicates that the MRL is proposed at the limit of analytical quantification (LOQ).
Bold values indicate the proposed MRL.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Individual residue levels considered for MRL calculation are reported in ascending order.
Any information/comment supporting the decision and OECD MRL calculation (unrounded/rounded values).
HR: Highest residue level according to the residue definition for risk assessment.
STMR: Median residue level according to residue definition for risk assessment.
3.1.1.3. Effect of industrial processing and/or household preparation
Standard hydrolysis studies simulating processing conditions representative of pasteurisation, boiling and sterilisation were assessed in the conclusion of the peer review and the MRL review (EFSA, 2010, 2014). It was concluded that the compound is hydrolytically stable under the representative conditions. Thus, for processed commodities, the same residue definition as for raw agricultural commodities (RAC) is applicable. No studies were submitted for the metabolites TFNG and TFNA (EFSA, 2014).
Studies investigating the effect of processing on the magnitude of flonicamid residues in the crops under consideration have not been provided and are not necessary, considering the low contribution of the crops under consideration to the overall consumer intakes (see Section 4).
3.1.2. Rotational crops
All crops under consideration, except permanent crops (apricots) can be grown in rotation with other plants, and therefore, the possible occurrence of residues in succeeding crops resulting from the use on primary crops has to be assessed. The soil degradation studies demonstrated that the degradation rate of flonicamid and its metabolites is rapid, with a maximum period required for 90% dissipation (DT90) of 1.5–8.7 days (EFSA, 2014), which is far below the trigger value of 100 days. Thus, further studies on rotational crops are not required (European Commission, 1997c).
3.2. Nature and magnitude of residues in livestock
The use of flonicamid resulted in significant residue levels in cabbage and sugar beet tops. Since these commodities might be fed to livestock, the potential transfer of residues to food of animal was investigated.
3.2.1. Dietary burden of livestock
Both cabbage and sugar beet (root/tops) residues increase the dietary burden compared to dietary intake calculation performed in the framework of the MRL review for flonicamid (EFSA, 2014).
Based on the expected residue concentrations for feed items assessed during the MRL review (EFSA, 2014) and in the framework of this MRL application, the median and maximum dietary burden values for livestock were calculated in accordance with the OECD guidance document (OECD, 2009) and the animal dietary burden calculator developed by EFSA. Thus, in the calculation also, the relevant by‐products of citrus, apples, cotton, potato and cereal were included. Since no processing factors (PF) were available for several by‐products, the default PFs were used to estimate the residue level (reported in brackets). The input values for the dietary burden calculation are summarised in Table 4.
Table 4.
Input values for the dietary burden calculation
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input (mg/kg) | Commenta | Input (mg/kg) | Commenta | |
| Cabbage | 0.14 | STMR | 0.23 | HR |
| Sugar beet tops | 0.085 | STMR | 0.2 | HR |
| Sugar beet, dry pulp | 1.53 | STMR × PF (18) | 1.53 | STMR × PF (18) |
| Sugar beet, ensiled pulp | 0.26 | STMR × PF (3) | 0.26 | STMR × PF (3) |
| Sugar beet, molasses | 2.38 | STMR × PF (28) | 2.38 | STMR × PF (28) |
| Citrus, dried pulp | 0.4 | STMR (EFSA, 2014) × PF (10) | 0.4 | STMR (EFSA, 2014) × PF (10) |
| Apple pomace | 0.30 | STMR (EFSA, 2014) × PF (5) | 0.30 | STMR (EFSA, 2014) × PF (2.5) |
| Wheat/Rye grain | 0.35 | STMR (EFSA, 2014) | 0.35 | STMR (EFSA, 2014) |
| Wheat milled by products | 2.45 | STMR (EFSA, 2014) × PF (7) | 2.45 | STMR (EFSA, 2014) × PF (7) |
| Wheat/Rye straw | 0.18 | STMR (EFSA, 2014) | 0.48 | HR (EFSA, 2014) |
| Potatoes | 0.03 | STMR (EFSA, 2014) | 0.06 | HR (EFSA, 2014) |
| Potato process waste | 0.6 | STMR (EFSA, 2014) × PF (20) | 0.6 | STMR (EFSA, 2014) × PF (20) |
| Potato dried pulp | 1.14 | STMR (EFSA, 2014) × PF (38) | 1.14 | STMR (EFSA, 2014) × PF (38) |
| Cotton meal | 0.05 | STMR (EFSA, 2015) × PF (1.3) | 0.05 | STMR (EFSA, 2015) × PF (1.3) |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor.
The processing factors used in the dietary burden calculation are reported in brackets.
The estimated animal dietary intakes are summarised in Table 5. To compare the impact of the commodities assessed in the framework of the MRL review (EFSA, 2014), EFSA included the result of the previous dietary burden recalculated according to new animal feedstuff table in the last column of Table 5.
Table 5.
Results of the dietary burden calculation
| Animal | Median burden (mg/kg bw) | Maximum burden (mg/kg bw) | > 0.1 mg/kg DM | Maximum burden (mg/kg DM) | Highest contributing commoditya | Previously calculated maximum burden (mg/kg DM) (EFSA, 2014) |
|---|---|---|---|---|---|---|
| Beef cattle | 0.056 | 0.059 | Yes | 2.47 | Potato (process waste) | 1.10 |
| Dairy cattle | 0.072 | 0.077 | Yes | 2.00 | Potato (process waste) | 0.92 |
| Ram/Ewe | 0.076 | 0.077 | Yes | 2.30 | Potato (process waste) | – |
| Lamb | 0.07 | 0.07 | Yes | 1.65 | Wheat (milled by‐products) | – |
| Pigs (breeding) | 0.038 | 0.039 | Yes | 1.70 | Wheat (milled by‐products) | 0.87 |
| Pigs (finishing) | 0.048 | 0.048 | Yes | 1.59 | Wheat (milled by‐products) | 0.87 |
| Poultry broiler | 0.06 | 0.061 | Yes | 0.87 | Wheat (milled by‐products) | 0.55 |
| Poultry layer | 0.061 | 0.063 | Yes | 0.92 | Wheat (milled by‐products) | 0.55 |
| Turkey | 0.059 | 0.061 | Yes | 0.86 | Wheat (milled by‐products) | – |
bw: body weight; DM: dry matter.
Considering the maximum dietary animal burden.
The additional crops under consideration in this MRL application and the new calculation methodology have an impact on the previous estimated dietary burden (EFSA, 2014), and therefore, a modification of the MRLs would be appropriate. In the next section, the expected flonicamid residues in livestock have to be investigated.
3.2.2. Nature of residues
The metabolism of flonicamid in goat and poultry was investigated in the framework of the peer review (EFSA, 2010). The residue definitions for enforcement and risk assessment in all commodities of animal origin were defined as the ‘sum of flonicamid and TFNA‐AM, expressed as flonicamid’. Validated analytical methods for enforcement of the proposed residue definition are available. In the framework of the peer review, the proposed residue definitions were considered to be non‐fat soluble.
EFSA concluded that the metabolism of flonicamid in livestock was sufficiently elucidated.
3.2.3. Magnitude of residues
Livestock feeding studies were submitted and assessed in the framework of the peer review and the MRL review (EFSA, 2010, 2014).
Lactating cows were dosed at levels of 2.50, 6.89 and 23.69 mg/kg body weight (bw) per day for 28 consecutive days (equivalent to 0.086, 0.252 and 0.839 mg/kg in the diet), representing 1.1N, 3.3N and 10.9N dose rate levels, respectively, when compared to the maximum dietary burden estimated for dairy cattle and ram/ewe, whereas for swine the feeding levels represented 2.2N, 6.4N and 21.3N dose rate levels, respectively, when compared to the maximum dietary burden values.
Laying hens were dosed at levels of 0.017, 0.169, 0.507 and 1.7 mg/kg bw for 28 consecutive days (equivalent to 0.2326, 2.326, 6.978 and 23.26 mg/kg in the diet) representing 0.3N, 2.7N, 8.0N and 10.9N dose rate levels, respectively.
The MRL proposals in animal matrices were derived from the livestock feeding studies according to the OECD and FAO recommendations (OECD, 2013; FAO, 2016):
The highest residue levels observed in each animal matrix at the different feeding levels and the highest dietary burden (Table 5) were considered for the estimation of the expected highest residue level (HR) (except for milk where the mean levels calculated at the different feeding levels were taken into account).
The mean residue levels calculated at the different feeding levels for each animal matrix and the median dietary burden were considered to derive the median residue level (STMR).
The estimated highest (HR), the median (STMR) residue levels and the MRL proposals are summarised in Table 6.
Table 6.
STMR, HR and MRL values derived from the livestock feeding studies
| Animal | Residues at closest feeding levela (mg/kg) | Estimated value at 1N levelb | MRL proposal (mg/kg) | ||
|---|---|---|---|---|---|
| Mean | Highest | STMR (mg/kg) | HR (mg/kg) | ||
| Bovine | Closest feeding level: | 0.086 mg/kg bw | |||
| 1.1 | N Dairy cow | 1.5 N Beef cow | |||
| Meat | 0.040 | 0.043 | |||
| Muscle | 0.055 | 0.055 | 0.046 | 0.049 | 0.05 |
| Fat | 0.020 | 0.020 | 0.017 | 0.018 | 0.02 * |
| Liver | 0.055 | 0.055 | 0.046 | 0.049 | 0.05 |
| Kidney | 0.055 | 0.055 | 0.046 | 0.049 | 0.05 |
| Milk | 0.02 | 0.02 | 0.017 | 0.018 | 0.02 * |
| Sheep | Closest feeding level: | 0.086 mg/kg bw | |||
| 1.1 | N Lamb | 1.5 N Ram/Ewe | |||
| 0.042 | 0.043 | ||||
| Muscle | 0.055 | 0.055 | 0.048 | 0.049 | 0.05 |
| Fat | 0.020 | 0.020 | 0.018 | 0.018 | 0.02 * |
| Liver | 0.055 | 0.055 | 0.048 | 0.049 | 0.05 |
| Kidney | 0.055 | 0.055 | 0.048 | 0.049 | 0.05 |
| Milk | 0.02 | 0.02 | 0.018 | 0.018 | 0.02 * |
| Swine | Closest feeding level: | 0.086 mg/kg bw | |||
| 2.6 | N Breeding | 4.4 N Finishing | |||
| 0.027 | 0.027 | ||||
| Muscle | 0.055 | 0.055 | 0.031 | 0.031 | 0.03 |
| Fat | 0.020 | 0.020 | 0.011 | 0.011 | 0.02 * |
| Liver | 0.055 | 0.055 | 0.031 | 0.031 | 0.03 |
| Kidney | 0.055 | 0.055 | 0.031 | 0.031 | 0.03 |
| Poultry | Closest feeding level: | 0.02 mg/kg bw | |||
| 0.3 | N Layer | 0.3 N Turkey | |||
| Meat | – | – | 0.034 | 0.040 | – |
| Muscle | 0.022 | 0.022 | 0.035 | 0.041 | 0.04 |
| Fat | 0.022 | 0.022 | 0.026 | 0.030 | 0.03 |
| Liver | 0.022 | 0.022 | 0.037 | 0.042 | 0.05 |
| Eggs | 0.024 | 0.027 | 0.084 | 0.100 | 0.1 |
MRL: maximum residue level; STMR: supervised trials median residue; HR: highest residue; bw: body weight.
* Indicates that the MRL is proposed at the limit of quantification.
n.a.: not applicable.
Closest feeding level and N dose rate related to the maximum dietary burden (Table 5).
Residues of TFNA‐AM recalculated as flonicamid equivalent by molecular weight conversion factor of 1.2 prior to be summed up.
Based on these calculations, it is concluded that there is no need to modify the existing MRLs in fat and milk of ruminants and in fat, liver and kidney of swine and in fat of poultry as set under Regulation (EU) No 2016/19029.
Based on the results of the feeding studies and considering the estimated animal burden values reported in Table 5, the existing EU MRLs for muscle, liver and kidney of ruminants, muscle of swine, muscle and liver of poultry and eggs should be amended as proposed in Table 6. The amendment is to reflect the LOQs of analytical methods used in the feeding study and the new feeding table (OECD, 2009).
4. Consumer risk assessment
In the framework of the review of the existing MRLs for flonicamid according to Article 12 of Regulation (EC) No 396/2005, a comprehensive long‐term exposure assessment was performed taking into account the existing uses at the EU level (EFSA, 2014).
EFSA updated this risk assessment with the median residue levels (STMR) derived from the residue trials conducted on the crops under consideration in this MRL application (Table 4) and the STMR values derived for animal products. Additionally, the exposure assessment was updated with STMR values for crops reported in the Article 10 reasoned opinion (EFSA, 2016) issued after the MRL review.
The acute exposure assessment was performed only with regard to the commodities under consideration assuming the consumption of a large portion of the food items as reported in the national food surveys and that these items contained residues at the HR level as observed in supervised field trials (Table 4). A variability factor accounting for the inhomogeneous distribution on the individual item consumed was included in the calculation for apricots and cabbage.
The consumer risk assessment was performed with revision 2 of the EFSA PRIMo. This exposure assessment model contains the relevant European food consumption data for different sub‐groups of the EU population10 (EFSA, 2007).
The input values used for the dietary exposure calculation are summarised in Table 7.
Table 7.
Input values for the consumer dietary exposure assessment
| Commodity | Chronic exposure assessment | Acute exposure assessment | ||
|---|---|---|---|---|
| Input (mg/kg) | Comment | Input (mg/kg) | Comment | |
| Risk assessment residue definition (commodities of plant origin): sum of flonicamid, TFNA and TFNG, expressed as flonicamid | ||||
| Apricots | 0.099 | STMR | 0.127 | HR |
| Cabbage | 0.14 | STMR | 0.23 | HR |
| Beans with pods | 0.34 | STMR | 0.67 | HR |
| Peas with pods | 0.34 | STMR | 0.67 | HR |
| Sugar beet | 0.03* | STMR | 0.03* | HR |
| Other plant commodities | STMR | See table 4–1 (EFSA, 2016) | Acute risk assessment undertaken only with regard to the crops under consideration | |
| Risk assessment residue definition (commodities of animal origin): sum of flonicamid and TFNA‐AM, expressed as flonicamid | ||||
| Bovine meat | 0.04 | STMR | 0.043 | HR |
| Bovine fat | 0.017 | STMR | 0.018 | HR |
| Bovine liver, kidney | 0.046 | STMR | 0.049 | HR |
| Bovine milk | 0.017 | STMR | 0.017 | STMR |
| Sheep meat | 0.042 | STMR | 0.043 | HR |
| Sheep fat | 0.018 | STMR | 0.018 | HR |
| Sheep liver, kidney | 0.048 | STMR | 0.049 | HR |
| Sheep milk | 0.018 | STMR | 0.018 | STMR |
| Swine meat | 0.027 | STMR | 0.027 | HR |
| Swine fat | 0.011 | STMR | 0.011 | HR |
| Swine liver, kidney | 0,031 | STMR | 0.031 | HR |
| Poultry meat | 0.034 | STMR | 0.040 | HR |
| Poultry fat | 0.026 | STMR | 0.03 | HR |
| Poultry liver | 0.037 | STMR | 0.042 | HR |
| Bird's eggs | 0.084 | STMR | 0.1 | HR |
| Other animal commodities | MRL | Regulation (EU) 2016/1902 | Acute risk assessment undertaken only with regard to commodities under consideration | |
MRL: maximum residue level; STMR: supervised trials median residue; HR: highest residue.
The estimated exposure was then compared with the toxicological reference values derived for flonicamid (Table 1). The results of the intake calculation using the EFSA PRIMo is a key supporting document and is made publicly available as a background document to this reasoned opinion.
A long‐term consumer intake concern was not identified for any of the European diets incorporated in the EFSA PRIMo. The highest chronic intake was calculated to be 18% of the ADI (WHO cluster diet B, DK child). The contribution of residues to the total consumer exposure accounted for a maximum of 11.9% of the acceptable daily intake (ADI) for wheat (WHO cluster diet B), and less than 10% of the ADI for the remaining commodities.
An acute consumer risk was not identified in relation to the MRL proposals for the crops under consideration. The highest acute consumer exposure was calculated to be 48.4% of the acute reference dose (ARfD) for head cabbage, 30.4% of the ARfD for beans with pods, 15.7% of the ARfD for apricots, 9.3% of the ARfD for peas with pods, 8.4% of the ARfD for milk, 7.7% of the ARfD for sugar beet and 5% for bird's eggs. For the remaining commodities, the exposure accounted for less than 3% of the ARfD.
EFSA concludes that the intended uses of flonicamid on the commodities under consideration will not result in a consumer exposure exceeding the toxicological reference values and therefore are unlikely to pose a health concern to consumers.
Conclusions and recommendations
The information submitted was sufficient to propose the MRLs summarised in the table below:
| Codea | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition for commodities of plant origin: sum of flonicamid, TFNA and TFNG, expressed as flonicamid | ||||
| 0140010 | Apricots | 0.03* | 0.3 | The NEU GAP is sufficiently supported by data. No consumer risk was identified |
| 0242990 | Head cabbage | 0.03* | 0.5 | NEU and SEU GAPs are sufficiently supported; the MRL was derived from the more critical SEU use. No consumer risk was identified |
| 0260010 | Beans with pods | 0.03* | 1.5 | NEU and SEU GAPs are sufficiently supported. The MRL was derived from the more critical SEU data. No consumer risk was identified |
| 0260030 | Peas with pods | 0.03* | 1.5 | |
| 0900010 | Sugar beet | 0.03* | 0.03* | The NEU GAP is sufficiently supported. A change of the existing MRL is not required. No consumer risk was identified |
| Enforcement residue definition for commodities of animal origin: sum of flonicamid and TFNA‐AM, expressed as flonicamid | ||||
| 1011010 | Swine muscle | 0.02* | 0.03 | The proposed MRLs were derived from the updated dietary burden calculation including the relevant feed items. There is no need to modify the existing MRLs in fat and milk of ruminants and in fat, liver and kidney of swine and in fat of poultry |
| 1011020 | Swine fat | 0.02* | 0.02* | |
| 1011030 | Swine liver | 0.03 | 0.03 | |
| 1011040 | Swine kidney | 0.03 | 0.03 | |
| 1012010 | Bovine muscle | 0.03 | 0.05 | |
| 1012020 | Bovine fat | 0.02* | 0.02* | |
| 1012030 | Bovine liver | 0.04 | 0.05 | |
| 1012040 | Bovine kidney | 0.04 | 0.05 | |
| 1013010 | Sheep muscle | 0.03 | 0.05 | |
| 1013020 | Sheep fat | 0.02* | 0.02* | |
| 1013030 | Sheep liver | 0.04 | 0.05 | |
| 1013040 | Sheep kidney | 0.04 | 0.05 | |
| 1014010 | Goat muscle | 0.03 | 0.05 | |
| 1014010 | Goat fat | 0.02* | 0.02* | |
| 1014030 | Goat liver | 0.04 | 0.05 | |
| 1014040 | Goat kidney | 0.04 | 0.05 | |
| 1016010 | Poultry muscle | 0.03 | 0.04 | |
| 1016020 | Poultry fat | 0.03 | 0.03 | |
| 1016030 | Poultry liver | 0.03 | 0.05 | |
| 1020010 | Cattle milk | 0.02* | 0.02* | |
| 1020020 | Sheep milk | 0.02* | 0.02* | |
| 1020030 | Goat milk | 0.02* | 0.02* | |
| 103000 | Birds eggs | 0.04 | 0.1 | |
MRL: maximum residue level; NEU: northern Europe; GAP: Good Agricultural Practice; SEU: southern Europe.
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DT90
period required for 90% dissipation (define method of estimation)
- dw
dry weight
- EC
emulsifiable concentrate
- EMS
evaluating Member State
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- GLP
Good Laboratory Practice
- GS
growth stage
- HR
highest residue
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification
- MRL
maximum residue level
- MS
Member States
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RAC
raw agricultural commodity
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern Europe
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- WG
water‐dispersible granule
- WHO
World Health Organization
Appendix A – Good Agricultural Practice
| Crop | NEU, SEU, MS or country | F G or Ia | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages & seasonc | Number min–max | Interval between application | g/hL min–max | Water L/ha min–max | g/ha min–max | ||||||
| Apricots | NEU | F | Aphids | WG | 500 g/kg | Foliar application | BBCH 11 till PHI (spring; April–June) | 2 | 21 days | 7–35 | 200–1,000 | 70 | 21 | Target countries: AT, CZ, HU, RO, SI, SK, PL |
| Head cabbage | NEU, SEU (field) | F | Aphids (Myzus persicae, Aphis fabae), MYZUPE, APHFA | WG | 500 g/kg | Foliar application | BBCH 16/18 till PHI (spring; May–June) | 2 | 14 days | 17.5–35 | 200–400 | 70 | 14 | |
| Beans (green with pods) | NEU, SEU (field) | F | Aphids (Myzus persicae, Aphis fabae), MYZUPE, APHFA | WG | 500 g/kg | Foliar application | BBCH 16/18 till PHI (spring; May–June) | 1 | – | 17.5–35 | 200–400 | 70 | 14 | |
| Peas (green with pods) | NEU, SEU (field) | F | Aphids (Myzus persicae, Aphis fabae), MYZUPE, APHFA | WG | 500 g/kg | Foliar application | BBCH 16/18 till PHI (spring; May–June) | 1 | – | 17.5–35 | 200–400 | 70 | 14 | |
| Sugar beet | NEU (field) | F | Aphids (Myzus persicae, Aphis fabae), MYZUPE, APHFA | WG | 500 g/kg | Foliar application | < BBCH 49 till PHI (spring; May–June) | 1 | – | 17.5–35 | 200–400 | 70 | 60 | |
NEU: northern European Union; SEU: southern European Union; MS; Member State; a.s.: active substance; WG: water‐dispersible granule.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
Appendix B – Used compound codes
| Code/Trivial name | Chemical name/SMILES notation | Structural formula |
|---|---|---|
| Flonicamid |
N‐Cyanomethyl‐4‐(trifluoromethyl)nicotinamide O=C(NCC#N)c1cnccc1C(F)(F)F |

|
| TFNA |
4‐(Trifluoromethyl)nicotinic acid or 4‐(Trifluoromethyl)pyridine‐3‐carboxylic acid OC(=O)c1cnccc1C(F)(F)F |

|
| TFNG |
N‐[4‐(Trifluoromethyl)nicotinoyl]glycine or N‐{[4‐(Trifluoromethyl)pyridin‐3‐yl]carbonyl}glycine O=C(NCC(=O)O)c1cnccc1C(F)(F)F |

|
| TFNA‐AM |
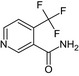
4‐(Trifluoromethyl)nicotinamide or 4‐(Trifluoromethyl)pyridine‐3‐carboxamide O=C(N)c1cnccc1C(F)(F)F |
|
SMILES: simplified molecular‐input line‐entry system.
Suggested citation: EFSA (European Food Safety Authority) , Brancato A, Brocca D, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Nougadere A, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Reasoned opinion on the modification of existing maximum residue levels for flonicamid in various commodities. EFSA Journal 2017;15(3):4748, 20 pp. doi: 10.2903/j.efsa.2017.4748
Requestor: European Commission
Question numbers: EFSA‐Q‐2015‐00424, EFSA‐Q‐2016‐00548
Approved: 9 March 2017
Notes
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
ISK Biosciences Europe N.V, De Kleetlaan 12B, box 9, 1831, Diegem, Belgium.
Commission Directive 2010/29/EU of 27 April 2010 amending Council Directive 91/414/EEC to include flonicamid (IKI‐220) as active substance, OJ L 106, 28.4.2010, p. 9–11.
Commission Implementing Regulation (EU) No 540/2011 of 23 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Regulation (EU) No 2016/71 of 26 January 2016 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for 1‐methylcyclopropene, flonicamid, flutriafol, indolylacetic acid, indolylbutyric acid, pethoxamid, pirimicarb, prothioconazole and teflubenzuron in or on certain products, OJ L 20, 27.1.2016, p. 1–47.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Commission Regulation (EU) 2016/1902 of 27 October 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for acetamiprid, ametoctradin, azoxystrobin, cyfluthrin, difluoroacetic acid, dimethomorph, fenpyrazamine, flonicamid, fluazinam, fludioxonil, flupyradifurone, flutriafol, fluxapyroxad, metconazole, proquinazid, prothioconazole, pyriproxyfen, spirodiclofen and trifloxystrobin in or on certain products. OJ L 298, 4.11.2016, p. 1–60.
The calculation of the long‐term exposure (chronic exposure) is based on the mean consumption data representative for 22 national diets collected from MS surveys plus one regional and four cluster diets from the WHO GEMS Food database; for the acute exposure assessment, the most critical large portion consumption data from 19 national diets collected from Member States surveys are used. The complete list of diets incorporated in the EFSA PRIMo is given in its reference section (EFSA, 2007).
References
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. doi: 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Conclusion on the peer review of the pesticide risk assessment of the active substance flonicamid. EFSA Journal 2010;8(5):1445, 63 pp. doi: 10.2903/j.efsa.2010.1445 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for flonicamid according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(6):3740, 49 pp. doi: 10.2903/j.efsa.2014.3740 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Reasoned opinion on the modification of the existing MRLs for flonicamid in several crops. EFSA Journal 2015;13(5):4103, 26 pp. doi: 10.2903/j.efsa.2015.4103 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Reasoned opinion on the modification of the MRL for flonicamid in herbs and edible flowers. EFSA Journal 2016;14(4):4467, 19 pp. doi: 10.2903/j.efsa.2016.4467 [DOI] [Google Scholar]
- European Commission , 1996. Appendix G. Livestock Feeding Studies. 7031/VI/95‐rev.4.
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev.3.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realisation of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev.6.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev.2.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev.5.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev.3.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev.5.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals. 7039/VI/95.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414). SANCO/3029/99‐rev.4.
- European Commission , 2010a. Review report for the active substance flonicamid (IKI‐220). Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 12 March 2010 in view of the inclusion of flonicamid in Annex I of Council Directive 91/414/EEC. SANCO/10479/2010 final, 12 March 2010, 8 pp.
- European Commission , 2010b. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010c. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev.8.1, 16 November 2010, 28 pp.
- European Commission , 2016. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev.10.2.
- FAO (Food and Agriculture Organization of the United Nations), 2016. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Edition. FAO Plant Production and Protection Paper 225, 286 pp.
- France , 2005. Draft assessment report on the active substance flonicamid prepared by the rapporteur Member State France in the framework of Council Directive 91/414/EEC, February 2005.
- France , 2009. Final addendum to the draft assessment report on the active substance flonicamid prepared by the rapporteur Member State France in the framework of Council Directive 91/414/EEC, October 2009.
- Netherlands , 2015. Evaluation report on the modification of MRLs for flonicamid in cabbage, beans with pods, peas with pods, sugar beet, poultry meat and poultry liver prepared by the evaluating Member State the Netherlands under Article 8 of Regulation (EC) No 396/2005, 9 April 2015, 60 pp.
- Netherlands , 2016. Evaluation report on the modification of MRL for flonicamid in apricots prepared by the evaluating Member State the Netherlands under Article 8 of Regulation (EC) No 396/2005, October 2016, 32 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document on overview of residue chemistry studies. In: Series on Pesticides No 32. ENV/JM/MONO(2009)31, 28 July 2009. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 4 September 2013.


