Abstract
The European Food Safety Authority asked the Panel on Biological Hazards (BIOHAZ) to deliver a scientific opinion providing: (i) a review of the approaches used by the BIOHAZ Panel to address requests from risk managers to suggest the establishment of microbiological criteria; (ii) guidance on the required scientific evidence, data and methods/tools necessary for considering the development of microbiological criteria for pathogenic microorganisms and indicator microorganisms; (iii) recommendations on methods/tools to design microbiological criteria and (iv) guidelines for the requirements and tasks of risk assessors, compared to risk managers, in relation to microbiological criteria. This document provides guidance on approaches when: (i) a quantitative microbial risk assessment (QMRA) is available, (ii) prevalence and concentration data are available, but not a QMRA model, and (iii) neither a QMRA nor prevalence and/or concentration data are available. The role of risk assessors should be focused on assessing the impact of different microbiological criteria on public health and on product compliance. It is the task of the risk managers to: (1) formulate unambiguous questions, preferably in consultation with risk assessors, (2) decide on the establishment of a microbiological criterion, or target in primary production sectors, and to formulate the specific intended purpose for using such criteria, (3) consider the uncertainties in impact assessments on public health and on product compliance and (4) decide the point in the food chain where the microbiological criteria are intended to be applied and decide on the actions which should be taken in case of non‐compliance. It is the task of the risk assessors to support risk managers to ensure that questions are formulated in a way that a precise answer can be given, if sufficient information is available, and to ensure clear and unambiguous answers, including the assessment of uncertainties, based on available scientific evidence.
Keywords: indicator microorganisms, food safety targets, microbiological criteria, product compliance, public health, risk assessment
Summary
The European Food Safety Authority (EFSA) asked the Panel on Biological Hazards (BIOHAZ) to deliver a scientific opinion providing guidance for risk assessors on which information and assessments could be provided to risk managers to support them in their decision‐making on microbiological criteria, as defined in Regulation (EC) No 2073/2005, or on targets in primary production sectors, as defined in Regulation (EC) No 2160/2003 (see glossary). Within this opinion, when reference is made to microbiological criteria it includes both Food Safety Criteria (FSC) and Process Hygiene Criteria (PHC) as defined in the EC legislation. FSC are applicable for food products placed on the market during their shelf‐life, while PHC are applicable typically for food during processing or at the end of a production line before putting a product on the market. If a FSC is not met, then the food has to be removed from the market or reprocessed (if not at retail). If a process hygiene criterion, or a target in primary production sectors, is not met, the food business operator (FBO) sometimes in collaboration with the competent authority (CA), in the case of national prevalence targets, should take corrective actions. Currently within the EU legislation, FSC are set for pathogenic microorganisms but also, in a few cases, for indicator microorganisms. In addition, PHC are set for non‐pathogenic indicator microorganisms but also, in some cases, for pathogenic microorganisms.
In this opinion, the guidance on estimation of the effect of microbiological criteria is based on the assumption that all foods/batches comply with the microbiological criteria regardless of the action taken to meet this level of compliance. For these reasons, PHC and FSC are described together and are not considered separately in this document.
In particular, this scientific opinion addresses four terms of reference; namely to provide: (i) a review of the approaches used by the BIOHAZ Panel to address requests from risk managers to suggest the establishment of microbiological criteria; (ii) guidance on the required scientific evidence, data and methods/tools necessary for considering the development of microbiological criteria, including both PHC and FSC. These approaches should take into account the different purposes of applying microbiological criteria; (iii) recommendations on methods/tools to design microbiological criteria (limits, sampling plans, stage of the food chain, method, etc.) and (iv) guidelines for the requirements and tasks of risk assessors, compared to risk managers, in relation to microbiological criteria.
The establishment of microbiological criteria, targets in primary production sectors and/or food safety targets (e.g. Appropriate level of protection (ALOP), Food Safety Objective (FSO), Performance Objective (PO) and Performance Criterion (PC)) is a risk management activity where governments agree on the maximum level of a food safety hazard in a food animal population or food that is technically achievable and appropriate for consumer protection.
The role of risk assessors should be focused on assessing the impact of different microbiological criteria on public health and on the product compliance according to the needs of the risk managers, and, if relevant, to link different microbiological criteria with food safety targets (e.g. ALOP, FSO, PO and PC values). It is the task of the risk managers to (1) formulate unambiguous questions, preferably in consultation with risk assessors, (2) decide on the establishment of a microbiological criterion, or target in primary production sectors, and to specify the intended purpose for using the microbiological criteria (i.e. indicator of process failure, indicator of faecal contamination or general improved food safety), (3) consider the uncertainties in impact assessments on public health and on product compliance (performed by the risk assessors) and (4) decide the point in the food chain where the microbiological criteria are intended to be applied and decide on the actions which should be taken in case of non‐compliance. It is the task of the risk assessors to support risk managers to ensure that questions are formulated in a way that a precise answer can be given, if sufficient information is available, and to ensure clear and unambiguous answers, including the assessment of uncertainties, based on available scientific evidence.
In this document, former BIOHAZ scientific opinions related to microbiological criteria, and targets in primary production sectors, as part of their Terms of Reference (TOR) were reviewed and discussed giving examples of best practices in relation to phrasing of TOR's and addressing lack of data and/or incomplete knowledge.
Following this, the present guidance focuses on the required scientific evidence and data relevant for considering the development of microbiological criteria for pathogenic microorganisms and indicator microorganisms (depending on the requests from risk managers) without taking into account actions taken in case of unsatisfactory results.
The estimated public health risk related to a specific food/pathogen combination is a function of the hazard characterisation (i.e. the pathogenicity of the pathogen including the dose/response relationships) and the exposure assessment (i.e. the prevalence and concentration of the pathogen in the food at the time of consumption, combined with the consumption frequency and serving size). Information from risk assessors to risk managers in relation to decision‐making on microbiological criteria in specific foods includes: (1) evidence linking a food or animal reservoir/pathogen combination to human disease (hazard identification), (2) risk assessment (hazard characterisation, exposure assessment and risk characterisation) of the food or animal reservoir/pathogen (may be quantitative or qualitative), (3) the impact of different microbiological criteria/limits on the public health and product compliance and (4) uncertainties of the above evidence and assessments, including the main sources of such uncertainties. This document provides guidance on approaches when a quantitative microbial risk assessment (QMRA) is available, when prevalence and concentration data are available, but not a QMRA model and in situations when neither a QMRA nor prevalence and/or concentration data are available. In the latter case the risk assessors can only provide data and expert opinion on available epidemiological studies, including outbreak data, dose/response data (if available), and other relevant scientific data i.e. in the format of a risk profile.
This document also deals with the data needed to use indicator microorganisms like Enterobacteriaceae, coliform bacteria, enterococci or Escherichia coli in microbiological criteria, including data needed to evaluate the usefulness of an indicator microorganism and its concentration as the basis for monitoring adequate process hygiene and data needed to evaluate whether an indicator microorganism and its concentration could serve as a marker for a pathogen. If a relationship between an indicator microorganism and the pathogen of concern is found, a risk assessment approach may be applied. But it is concluded that even if data are available, caution should be taken in extrapolation of relationships between indicator microorganisms and pathogenic microorganisms as defined in a particular study to situations very different from those encountered in the initial data collection. In addition, it is concluded that the estimation of the impact of microbiological criteria on public health/food safety using indicator microorganisms is, if at all possible, more complicated, demanding of data, and with more uncertainty and variability, than when performed for pathogenic microorganisms.
The importance of addressing the uncertainties in the assessments is emphasised with reference to EFSA's draft guidance on uncertainty in EFSA scientific assessment (EFSA Scientific Committee, 2016), and finally, the document also deals with available online technical tools to operationalise microbiological criteria.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
A microbiological criterion is a risk management tool based on the outcome of sampling and testing for microorganisms, their toxins/metabolites or markers associated with pathogenicity or other traits at a specified point of the food chain that indicates the acceptability of a food, or the performance of either a process or a food safety control system. The Codex Alimentarius Commission (CAC) has established in 1997 general principles and considerations for the establishment of microbiological criteria which were revised in 2013 (CAC, 2013) as follows:
A microbiological criterion should be appropriate to protect the health of the consumer and, where appropriate, also ensure fair practices in food trade.
A microbiological criterion should be practical and feasible and established only when necessary.
The purpose of establishing and applying a microbiological criterion should be clearly articulated.
The establishment of microbiological criteria should be based on scientific information and analysis and follow a structured and transparent approach.
Microbiological criteria should be established based on knowledge of the microorganisms and their occurrence and behaviour along the food chain.
The intended, as well as the actual, use of the final product by consumers needs to be considered when setting a microbiological criterion.
The required stringency of a microbiological criterion used should be appropriate to its intended purpose.
Periodic reviews of microbiological criteria should be conducted, as appropriate, in order to ensure that microbiological criteria continue to be relevant to the stated purpose under current conditions and practices.
There may be multiple reasons for establishing and applying microbiological criteria. The purposes of microbiological criteria include, but are not limited to, the following:
Evaluating a specific lot of food to determine its acceptance or rejection, in particular, if its history is unknown.
Verifying the performance of a food safety control system or its elements along the food chain, e.g. prerequisite programmes and/or HACCP systems.
Verifying the microbiological status of foods in relation to acceptance criteria specified between food business operators.
Verifying that the selected control measures are meeting Performance Objectives (POs) and/or Food Safety Objectives (FSOs).
Providing information to food business operators on microbiological levels, which should be achieved when applying best practices (CAC, 2013).
The microbiological safety of foods is managed by the effective implementation of control measures within a food safety management system (FSMS) including prerequisite programme (PRP) and hazard analysis and critical control points (HACCP) that have been validated, where appropriate, throughout the food chain to minimise contamination and improve food safety. This preventative approach offers more advantages than sole reliance on microbiological testing through acceptance sampling of individual lots of the final product to be placed on the market. The establishment of microbiological criteria may also be appropriate for verifying that these FSMS are implemented correctly (EFSA, 2007; CAC, 2013).
When recommending the establishment of microbiological criteria, a variety of approaches can be used, depending on the risk management objectives and the available level of knowledge and data. These approaches can range from developing microbiological criteria based on empirical knowledge related to Good Hygienic Practices (GHP), to using scientific knowledge of FSMS such as PRP and HACCP, or by conducting risk assessments. The choice of the approach should be aligned with the risk management objectives and decisions relating to food safety and suitability. The need for a microbiological criterion should be demonstrated, e.g. by epidemiological evidence and/or as the result of a risk assessment indicating that the food under consideration represents a significant public health risk and that a criterion is meaningful for consumer protection. Although meeting microbiological criteria offers some assurance that particular pathogens are not present at unacceptably high concentrations, these do not guarantee ‘absence’ of those pathogens (EFSA, 2007). However, microbiological criteria can also be used as a way to communicate the level of hazard control that should be achieved.
Regulation (EC) No 2073/20051 on microbiological criteria for foodstuffs introduces two different criteria: Food Safety Criteria (FSC) and Process Hygiene Criteria (PHC). An advantage of establishing food safety criteria for pathogenic microorganisms is that harmonised standards on the acceptability of food are provided for both authorities and industry within the EU and for products imported from third countries. Food safety criteria will impact the entire food chain, as they are set for products placed on the market. Risk of recalls and the economic losses, as well as loss of consumer confidence, will be a strong motivation to meet the criteria. Food Safety Criteria are assumed to have an impact on food safety and public health where there is an actual or perceived risk. However, it has proved difficult to evaluate the extent of public health protection provided by a specific food safety criterion (EFSA, 2007).
The EFSA Panel on Biological Hazards (BIOHAZ Panel) previously undertook a self‐tasking activity to: (i) provide an overview of FSMS including microbiological criteria; (ii) provide a short description of the current Codex concepts, viz. Appropriate Level of Protection (ALOP), Food Safety Objective (FSO), Performance Objective (PO), Performance Criteria (PC) and microbiological criteria; (iii) describe the types of microbiological criteria (food safety criteria, process hygiene criteria) and targets contained in the EU legislation in regard to public health and (iv) consider the application of microbiological criteria and targets in the food chain at the EU level based on risk analysis (EFSA, 2007). This Opinion envisaged that there would be the need for periodic review as subsequent advances in this field have taken place, both in the technologies available for the detection of a wider range of hazards as well as in understanding their prevalence and distribution in foods, and the evolution of the science of risk assessment, including the estimation of variabilities and uncertainties.
EFSA's BIOHAZ Panel has previously received mandates where risk managers ask the Panel to provide suggestions, where relevant, for the establishment of microbiological criteria. However, despite the existing CAC document (CAC, 2013) and EFSA Opinion (EFSA, 2007) on general principles and terminology for microbiological criteria, as described above, addressing mandates to the BIOHAZ Panel have proved problematic. Thus these requests generated much discussion on what would be an appropriate approach and end‐point to address questions from risk managers, and how to deal with data gaps, variability and uncertainties in an open and transparent way.
In order to increase clarity and transparency in EFSA's future work, as well as to capitalise on advances in the application of risk assessment, it is suggested to initiate a BIOHAZ self‐tasking mandate on the required scientific evidence, data and methods/tools for supporting decision‐making on microbiological criteria in the future. This should include a clear framework/agreement of the tasks of risk assessors and what are the tasks of risk managers.
1.1.1. Terms of reference (TOR)
The BIOHAZ Panel is asked to issue an opinion that specifies a framework to increase the transparency and clarity of EFSA's future BIOHAZ opinions where considerations of microbiological criteria are a part of the mandate.
The mandate should provide:
A review of the approaches used by the BIOHAZ Panel to address requests from risk managers to suggest the establishment of microbiological criteria.
Guidance on the required scientific evidence, data and methods/tools necessary for considering the development of microbiological criteria, including both Process Hygiene Criteria and Food Safety Criteria. These approaches should take into account the different purposes of applying microbiological criteria.
Recommendations on methods/tools to design microbiological criteria (limits, sampling plans, stage of the food chain, method, etc.).
Guidelines for the requirements and tasks of risk assessors, compared to risk managers, in relation to microbiological criteria.
1.2. Interpretation of the Terms of Reference (TOR)
This scientific opinion aims to provide guidance for risk assessors on which information and assessments could be provided to risk managers to support them in their decision‐making on microbiological criteria, as defined in Regulation (EC) No 2073/20051, or on targets in primary production sectors, as defined in Regulation (EC) No 2160/20032 (see Glossary). This guidance takes into account the different degrees of evidence and tools available for specific assessments and also aims to provide guidance on the different roles of risk managers and risk assessors.
Within this opinion, when reference is made to microbiological criteria, it includes both FSC and PHC as defined in the EC legislation. FSC are applicable for food products placed on the market during their shelf‐life, while PHC are applicable typically for food during processing or at the end of a production line before putting a product on the market. If a FSC is not met, then the food has to be removed from the market or reprocessed (if not at retail). If a process hygiene criterion, or a target in primary production sectors, is not met, the food business operator (FBO) sometimes in collaboration with the competent authority (CA) in the case of national prevalence targets, should take corrective action.
To be consistent with the European legislation, this opinion considers targets in primary production sectors as well as PHC and FSC further up in the food chain using both pathogenic and indicator microorganisms. Currently within the EU legislation, FSC are set for pathogenic microorganisms but also, in a few cases, for indicator microorganisms. In addition, PHC are set for non‐pathogenic indicator microorganisms but also, in some cases, for pathogenic microorganisms.
In this opinion, the guidance on estimation of the effect of microbiological criteria is based on the assumption that, no matter which stage in the food chain, all foods/batches comply with the microbiological criteria regardless of the action taken to meet this level of compliance. For these reasons, PHC and FSC are described together and are not considered separately in this document.
In addition, this opinion also addresses briefly the food safety targets (ALOP, FSO, PO and PC), although these are not yet used in the current EU legislation.
This scientific opinion does not include evaluations of microbiological criteria included in the existing legislation (Commission Regulation (EC) No 2073/2005) but provides a framework for a structured approach to be used by risk assessors when risk managers (e.g. the European Commission or the national competent authorities) seek advice on microbiological criteria. The establishment of microbiological criteria by FBOs is outside the scope of this Opinion.
1.3. Additional information
Microbiological criteria are used worldwide as one of several risk management tools to ensure the safety of foods. The CAC, International Commission on Microbiological Specifications for Foods (ICMSF) and others have provided guidelines to Food Safety Authorities and industries and all these guidelines emphasise that establishment of microbiological criteria should be risk based. Several scientific publications in the last decade have shown how a microbiological criterion can be linked to, or be derived from, a quantitative microbiological risk assessment (QMRA) (Uyttendaele et al., 2006; Nauta et al., 2012; Andersen et al., 2015; Lee et al., 2015; Zwietering et al., 2015; Seliwiorstow et al., 2016) but little information or guidance is provided on how to deal with microbiological criteria in the absence of such QMRAs. Older literature provides information to set, in particular, PHC to evaluate hygiene during meat animal slaughtering or in food processing. The objective of many of these publications was to assess baseline data on hygiene indicators and the relationship between the indicators and zoonotic agents to support the basic assumption that adherence to Good agricultural practices (GAP), Good manufacturing practices (GMP) and GHP (being PRPs) throughout the food chain will contribute to a reduction in public health risks (Zeitoun et al., 1994; Mossel et al., 1998; Ghafir et al., 2008).
A microbiological criterion consists of the following components: (i) the purpose of the microbiological criterion; (ii) the food, process or food safety control system to which the microbiological criterion applies; (iii) the specified point in the food chain where the microbiological criterion applies; (iv) the microorganism(s) and the reason for its selection; (v) the microbiological limits (m, M) or other limits (e.g. a level of risk); (vi) a sampling plan defining the number of sample units to be taken (n), the size of the analytical unit and where appropriate, the acceptance number (c); (vii) depending on its purpose, an indication of the statistical performance of the sampling plan; and (viii) analytical methods and their performance characteristics. Consideration should be given to the action to be taken when the microbiological criterion is not met and the action should be specified (CAC, 2013).
2. Data and methodologies
Data used for this opinion are tools and methods for risk assessments and establishment of microbiological criteria that have been published as stand‐alone scientific reports or guidance documents by international organisations (Codex Alimentarius, FAO/WHO, EFSA, ICMSF, ILSI) or in peer‐reviewed scientific journals.
Scientific opinions from the BIOHAZ Panel that have been adopted between 2003 and 2016 where questions related to microbiological criteria, or to targets in primary production sectors, have been part of the TORs were searched. These opinions were reviewed to summarise, compare and evaluate the different approaches used to answer the TORs in the past.
The general EFSA guidelines on ‘the general principles of transparency in the scientific aspects of risk assessments carried out by EFSA’ (EFSA, 2009a) and ‘uncertainty in EFSA scientific assessment’ (EFSA Scientific Committee, 2016) from EFSA's scientific committee have been taken into account in answering the TOR of this scientific opinion document.
3. Requirements and tasks of risk assessors, compared to risk managers, in relation to Microbiological Criteria (TOR 4)
The impact of application of microbiological criteria on public health is not straightforward, but with the help of recent advantages in QMRA and the development of tools, risk‐based microbiological criteria can be derived from the level of protection deemed appropriate by risk managers (e.g. European Commission or national competent authorities) to protect human life or health within its territory, also known as the ALOP. The format of an ALOP is most often referred to as the number of human cases of illness per 100,000 people per year. Competent authorities and food business operators may use microbiological criteria to operationalise the ALOP, either directly, or through other food safety targets such as FSO, PO and PC (EFSA, 2007; Zwietering et al., 2015). The FSOs and POs only represent targets set as microbial concentration and/or prevalence, whereas a microbiological criterion consists of more specific elements such as the analytical method, the sampling plan, microbiological limit(s), the specified point of the food chain where the limit(s) apply, the number of analytical units that should confirm to the limit(s) and the actions to be taken when the criterion is not met (EFSA, 2007). The FSO concept translates the ALOP into a definable goal with a specified maximum frequency and/or concentration of a microbiological hazard in a food at the time of consumption, which can be defined as the acceptable level of the exposure (ICMSF, 2002) (Cole and Tompkin, 2005). Although the competent authorities are in charge of defining the ALOP, this is very seldom performed in an explicit way.
The establishment of microbiological criteria and food safety targets (e.g. ALOP, FSO, PO and PC values), as defined in Codex Alimentarius, is a risk management activity where governments agree on the maximum level of a food safety hazard in a food animal population or food that is technically achievable and tolerable for consumer protection.
The role of risk assessors should be focused on assessing the impact of different microbiological criteria on public health and on the product compliance, and if relevant to link different microbiological criteria with food safety targets (e.g. ALOP, FSO, PO and PC values). In addition, it is also the task of the risk assessors to: (i) support risk managers to ensure that questions are formulated in a way that a precise answer can be given, if sufficient information is available; and (ii) ensure clear and unambiguous answers, including the assessment of uncertainties, based on available scientific evidence.
Figure 1 represents a schematic overview of the different tasks and processes performed by the risk assessors and risk managers, respectively, in relation to microbiological criteria.
Figure 1.
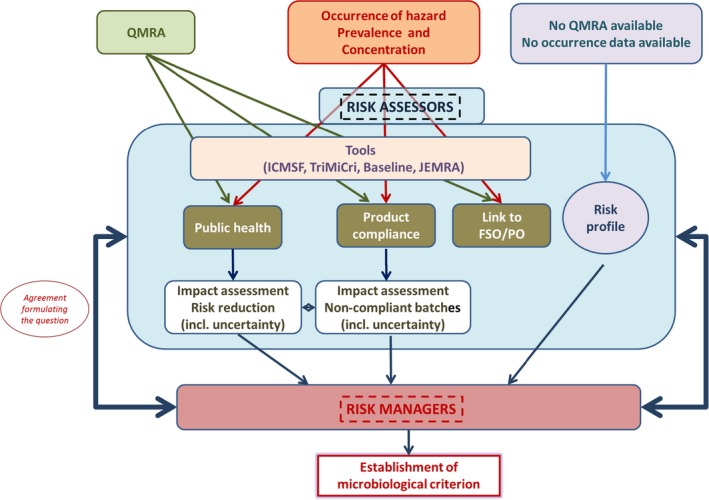
- FSO/PO: Food Safety Objective/Performance Objective; ICMSF: International commission on microbiological specifications on foods; JEMRA: Joint FAO/WHO expert meetings on microbiological risk assessment; QMRA: Quantitative microbial risk assessment; TRiMiCri: Tool for risk‐based microbiological criteria; Baseline: Baseline tool.
Risk managers always have the possibility to establish a microbiological criterion, as long as a method to detect the relevant microorganism is available. But before deciding on the establishment of a microbiological criterion they may approach risk assessors with specific questions. Questions for risk assessment must be specified in precise terms. Imprecise or ambiguous questions make it difficult for assessors to focus their efforts efficiently, and may result in the answer being less useful to managers, or even being misleading. If the meaning of the question is imprecise or ambiguous (could be interpreted in diverse ways by different people), more answers become possible, hence adding to the overall uncertainty about the response. Assessors and decision‐makers should therefore aim to agree on a formulation of the question such that a precise answer can be given if sufficient information is available (EFSA Scientific Committee, 2016). This will optimise the possibility that risk assessors will focus their work and produce a useful result in a timely manner.
Ambiguous questions include questions related to risk management tasks like asking for what is safe, acceptable, appropriate etc. When questions are ambiguous it is more likely that risk assessors may step into risk management judgements. Thus, risk assessors should bear in mind that judgements about what is acceptable in relation to both risk and costs are judgements that should be made by risk managers.
Although the overall purpose of establishing microbiological criteria is improvement of food safety, it is recommended that the more specific objectives are formulated by the risk managers before the risk assessors start assessing the efficacy and establishment of a specific microbiological criterion, or target in primary production sectors. Risk managers may ask questions related to microbiological criteria (FSC and/or PHC) using both pathogenic microorganisms and indicator microorganisms. Questions from risk managers about using indicator microorganisms may arise, either with the purpose of using these as an indicator of a failure in a process, or, if possible, as a marker for a pathogen, for example, in situations where methods for detecting a specific pathogenic microorganism in a food commodity are lacking.
FSC are, e.g. used for Escherichia coli in ‘live bivalve mussels and live echinoderms, tunicates and marine gastropods’ as an indicator for faecal contamination but not linked directly to food safety. On the other hand, pathogenic microorganisms (e.g. Salmonella spp. and Campylobacter spp.) are used for carcases as PHC where there is a direct link to food safety. So, although defined as such in the EC Reg. 2073/2005, PHC do not necessarily relate only to process hygiene and FSC in legislation are not necessarily linked directly to public health. It is the risk managers who decide the point in the food chain where the microbiological criteria are intended to be applied and decide on the actions which should be taken in case of non‐compliance. Whether it is cost‐effective to establish targets in primary production sectors and/or microbiological criteria (including PHC and or FSC) is a management decision and the risk assessors should mainly assess the possible impact of these on public health and product compliance.
As mentioned in Section 1.2, the assessment approaches described in this opinion do not take into account either the point in the food chain where the microbiological criterion is established or the actions which will be taken if the microbiological criterion is not met and therefore FSC and PHC are described and considered together.
Since the consequences of not complying with a FSC and a PHC differ significantly and may impact both public health and the food industry, risk managers need to consider carefully whether one or the other is the most appropriate to use in a given situation, taking into account the evidence related to impact on public health and product compliance, as provided by the risk assessors.
Since product testing for microbiological criteria (both FSC and PHC) represents a very small proportion of the food produced, testing as such and the removal of the tested batch from the market/or reprocessing of products not yet placed on the market does not often contribute significantly to food safety in itself. The public health impact of microbiological criteria (both PHC and FSC) lies in the FSMS and actions taken by the food business operators to ensure compliance with the microbiological criteria.
4. Approaches used by the BIOHAZ Panel to address requests from risk managers related to the establishment of Microbiological Criteria (TOR 1)
From 2003 to 2016, 14 BIOHAZ scientific opinions addressed questions related to microbiological criteria, and to targets in primary production sectors, as part of their TOR. These opinions were reviewed regarding the approach followed by the BIOHAZ Panel to answer those TOR. An overview of this exercise can be seen in Table A.1 in Appendix A where the title of the opinions, framing of the TOR, the approach and the answers to the TOR are summarised. In the text below, some examples of appropriate and inappropriate phrasing and reasoning are given in order to illustrate challenges in separating risk assessment and risk management.
Table A.1.
Overview of EFSA Opinions where microbiological criteria are considered in relation to specific food/pathogen combinations
| Title of scientific opinion (Year of publication, Reference) | Specific TOR related to microbiological criteria (FSC or PHC) | Assessment of impact on: (i) public health, (ii) product compliance | Approach used for the assessment | Answers to TOR (extracts from the Opinions) |
|---|---|---|---|---|
|
Microbiological risks of infant formulae and follow‐on formulae (2004) (EFSA, 2004) |
Identify the best control options, special attention should be paid to assessing the possible use of microbiological testing, through guidelines or standards, as well as measures applicable at the time of preparation and storage of these foods until their consumption | Assessment of impact on public health and product compliance was not performed | Based on epidemiological evidence |
It is recommended that a Performance Objective (PO) for powdered infant formula and follow‐on formula, aiming at very low levels of Salmonella and E. sakazakii (e.g. absence in 1, 10 or 100 kg) is introduced and that verification of compliance with the PO is confirmed by testing for Enterobacteriaceae in the environment and in the product. …. In some situations, in order to ensure that a Performance Objective is reached, microbiological testing might be an option. In the case of E. sakazakii and Salmonella in infant formula, the introduction of a microbiological criterion for these specific pathogen organisms is not recommended. |
|
Biological Hazards on Bacillus cereus and other Bacillus spp. in foodstuffs (2005) (EFSA, 2005) |
List and evaluate specific control measures, including microbiological testing and temperature requirements, to manage the risk caused by Bacillus cereus, other Bacillus spp. and their toxins in foodstuffs | Assessment of impact on public health was performed | Based on epidemiological evidence |
For the development of new food product, or food product that support growth of B. cereus, either by their nature or their conditions of storage (e.g. extended shelf life), processors should ensure that numbers of B. cereus between 103 and 105 per g are not reached at the stage of consumption under anticipated conditions of storage and handling. This should also apply for dehydrated foods reconstituted by hot water before consumption. The maximum limit at consumption described in the above bullet point should be used as a target for food business operators to verify their HACCP system and could be considered as microbiological criteria to test the acceptability of a process. |
|
Updating the former SCVPH opinion on Listeria monocytogenes risk related to RTE foods and scientific advice on different levels of L. monocytogenes in RTE foods and the related risk for human illness (2008) (EFSA, 2008b) |
To provide scientific advice on different levels of L. monocytogenes in ready‐to‐eat foods and the related risk for human illness | Assessment of impact on public health and product compliance was performed |
Based on risk assessment (qualitative MRA) and epidemiological evidence |
Microbiological criteria will assist in controlling the levels of L. monocytogenes, e.g. absence in 25 g or ≤100 CFU/g at the point of consumption. The most recent Codex document on microbiological criteria for L. monocytogenes in ready‐to‐eat foods suggests a zero tolerance throughout the shelf life of the product for ready‐to‐eat foods in which growth of this microorganism can occur. Applying this criterion throughout the shelf life may prevent consumption of ready‐to‐eat foods representing a high risk. However, applying this criterion close to the end of shelf life could classify products as unsatisfactory, although they are of low risk. An additional option proposed in this Codex document is therefore to tolerate 100 CFU/g throughout the shelf life provided that the manufacturer is able to demonstrate that the product will not exceed this limit throughout the shelf life. For ready‐to‐eat foods that support growth of L. monocytogenes, it is impossible to predict with high degree of certainty that the level will or will not exceed 100 CFU/g during the shelf life of these products. Thus, applying this option may result in accepting a probability that foods with more than 100 CFU/g will be consumed. The impact on public health would depend whether the levels markedly above 100 CFU/g are reached. |
|
Risk based control of biogenic amine formation in fermented foods (2011) (EFSA BIOHAZ Panel, 2011b) |
Characterise concentration levels of biogenic amine in relevant fermented foods that are not associated with adverse health effects of defined consumer groups including susceptible consumers | Assessment of impact on public health was performed | Based on risk assessment |
Consumption data and the exposure assessment were used to define the concentrations in food that would be allowable; however, this will vary between individuals, regions and countries. No adverse health effects have been observed in healthy volunteers to a level of 25 to 50 mg of histamine per person per meal. This level may be occasionally exceeded by consumption of one or more food items containing high amounts of histamine during the same meal. Further research is needed on the evaluation of the need for and, if/where necessary, development of process hygiene criteria for histamine and tyramine in fermented foods, as well as food safety criteria for histamine in fermented foods other than fish. |
|
Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain (2011) (EFSA BIOHAZ Panel, 2011a) |
To evaluate potential performance objectives and/or targets at different stages of the food chain in order to obtain, e.g. 50% and 90% reductions of the prevalence of human campylobacteriosis in the EU caused by broiler meat consumption | Assessment of impact on public health and product compliance was performed | Based on risk assessment |
Compliance with microbiological criteria is effective to reduce risks for Campylobacter on broiler meat because of high within‐batch prevalence and low within‐batch variability enabling detection of highly contaminated batches even when taking a limited number of samples. They stimulate improved control of Campylobacter during slaughter. The public health benefits of setting microbiological criteria were evaluated using data from the 2008 EU baseline survey. These estimates are average values for the whole EU; the impact could be very different between MSs. Theoretically, a public health risk reduction > 50% at the EU level could be achieved if all batches that are sold as fresh meat would comply with microbiological criteria with a critical limit of 1000 CFU/gram of neck and breast skin. Theoretically, a public health risk reduction > 90% at the EU level could be achieved if all batches that are sold as fresh meat would comply with microbiological criteria with a critical limit of 500 CFU/gram of neck and breast skin. |
|
Shiga toxin‐producing Escherichia coli and other pathogenic bacteria in seeds and sprouted seeds (2011) (EFSA BIOHAZ Panel, 2011b) |
To recommend, if considered relevant, microbiological criteria for seeds and sprouted seeds, water and other material that may contaminate the seeds and sprouts throughout the production chain | Assessment of impact on public health was performed | Based on epidemiological evidence (outbreak data) and microbiological data |
It is currently not possible to evaluate the extent of public health protection provided by specific microbiological criteria for seeds and sprouted seeds. This highlights the need for data collection to conduct quantitative risk assessment. Consideration should be given to the development of new or revision of the existing microbiological criteria for pathogens most frequently associated with outbreaks involving sprouts – Salmonella spp. and pathogenic E. coli. Currently, there are no criteria for pathogenic E. coli. If such criteria were to be proposed serotypes of concern and associated with severe human disease should be considered. Microbiological criteria for Salmonella, pathogenic E. coli and L. monocytogenes could be considered for seeds before the start of the production process, during sprouting and in the final product. During the industrial sprouting process testing spent irrigation water for pathogenic bacteria has been proposed as an alternative strategy to the analysis of a large number of sprout samples. However, there are some uncertainties regarding the sensitivity of this strategy. Sampling could be conducted on sprouted seed production environments. It could be applied for pathogenic bacteria such as L. monocytogenes as well as indicator bacteria. There are currently no indicator organisms that can effectively substitute for the testing of pathogens in seeds, sprouted seeds or irrigation water. Testing for E. coli, Enterobacteriaceae and Listeria spp. can inform process hygiene control. Further work may be required to assess the value of tests for these indicator organisms. |
|
Update on the present knowledge on the occurrence and control of foodborne viruses (2011) (EFSA BIOHAZ Panel, 2011d) |
To discuss the scientific reasons for and against the establishment of food safety criteria and process hygiene criteria for viruses for certain food categories (e.g. fresh produce, bivalve molluscs etc.). | Assessment of impact on public health and product compliance was not performed | Based on epidemiological evidence (outbreak data) |
Microbiological criteria for HAV and NoV are useful for validation and verification of HACCP‐based processes and procedures, and can be used to communicate to food business operators what is an acceptable or unacceptable viral load. Regulation (EC) 2073/2005 indicates that criteria for pathogenic viruses in live bivalve molluscs should be established when the analytical methods are developed sufficiently. Furthermore, regulation (EC) No 853/200410 provides a possibility to lay down additional health standards for live bivalve molluscs including virus testing procedures, and virological standards. Assuming that quantitative data on viral load is available, it would be possible to establish criteria for NoV in bivalve molluscs, while considering the impact of a given criteria on the exposure of the consumer. Viruses can be detected in fresh produce, but prevalence studies are limited, and quantitative data on viral load is scarce making establishment of microbiological criteria for these food categories difficult. Although there are documented cases of derived illness, the relative contribution of fresh produce to the overall public health FBV risk has not been established. |
|
Norovirus (NoV) in oysters: methods, limits and control options (2012) (EFSA BIOHAZ Panel, 2012a) |
Limits that do not pose an unacceptable risk to consumers for NoV genogroups GI and GII in oysters as determined by real‐time PCR (e.g. copy number per gram) |
Assessment of impact on public health and product compliance was performed | Based on exposure assessment |
Compliance with any of the above NoV limits would reduce the number of contaminated oysters placed on the market and therefore the risk for consumers to become infected. The lower the limit the greater the consumer protection achieved. However, it is not currently possible to quantify the public health impact of establishment of different limits. Microbiological criteria for NoV in oysters are useful for validation and verification of HACCP‐based processes and procedures, and can be used to communicate to food business operators and other stakeholders what is an acceptable or unacceptable viral load for oysters to be placed on the market. Microbiological criteria for NoV in oysters could also be used by competent authorities as an additional control to improve risk management in production areas, during processing and retail. On the basis of the data presented in this Opinion, risk managers should consider establishing an acceptable limit for NoV in oysters to be harvested and placed on the market. |
|
Risk posed by Salmonella and Norovirus in leafy greens (2014) (EFSA BIOHAZ Panel, 2014a) |
To recommend, if considered relevant, microbiological criteria throughout the production chain | Assessment of impact on public health was performed | Based on epidemiological evidence (outbreak data) |
The current legal framework does not include microbiological criteria applicable at the primary production stage. It is here proposed to define criteria to validate and verify Good Agricultural Practices (GAP) and Good Hygiene Practices (GHP). These criteria will be designated as Hygiene Criteria and are defined as criteria indicating the acceptable functioning at pre‐harvest, harvest and on farm post‐harvest production prior to processing. E. coli was identified as suitable for a Hygiene Criterion at primary production of leafy greens and could be considered for validation and verification of Good Agricultural Practices (GAP) and Good Hygiene Practices (GHP) and on the basis of this, growers should take appropriate corrective actions. A Process Hygiene Criterion for E. coli in leafy green packaging plants or fresh cutting plants will give an indication of the degree to which collectively GAP, GHP, GMP or HACCP programmes have been implemented. A Food Safety Criterion for Salmonella in leafy greens intended to be eaten raw as salads could be used as a tool to communicate to producers and processors that Salmonella should not be present in the product. Noroviruses can be detected in leafy greens, but prevalence studies are limited, and quantitative data on viral load are scarce making establishment of microbiological criteria for these foods difficult. Information is lacking on the relationships between the occurrence of Norovirus as detected by real time RT‐PCR, infectivity and the actual risk to public health. |
|
Risk posed by Salmonella and Norovirus in berries (2014) (EFSA BIOHAZ Panel, 2014b) |
To recommend, if considered relevant, microbiological criteria throughout the production chain | Assessment of impact on public health was performed | Based on epidemiological evidence (outbreak data) |
It is currently not possible to assess the suitability of an EU‐wide E. coli Hygiene Criterion at primary production for berries. However, using E. coli as an indicator of recent human or animal faecal contamination is likely to be useful for verification of GAP and GHP when applied to berries in individual production sites. It is currently not possible to assess the suitability of an EU‐wide Norovirus Hygiene Criterion at primary production for raspberries and strawberries, but this should be considered for the future, as well as for other berry fruits if additional public health risks are identified. Currently, there are no Process Hygiene criteria covering whole frozen berries and for these products there are no available data on occurrence of E. coli or Salmonella. It is therefore not possible to assess the suitability of an EU‐wide E. coli Process Hygiene Criterion for whole frozen berries. However, using E. coli as an indicator for verification of GMP and food safety management systems (including HACCP) might be useful for frozen berries in individual processing premises. Microbiological criteria for Norovirus in berries are useful for validation and verification of food safety management systems, including HACCP‐based processes and procedures, and can be used to communicate to food business operators and other stakeholders what is acceptable or unacceptable viral load for berries to be placed on the market It is currently not possible to provide a risk base for establishing a Process Hygiene Criterion for these foods. However, on the basis of the emerging public health risk, the collection of appropriate data and subsequent development of a Norovirus Process Hygiene Criterion for frozen raspberries and strawberries should be considered as a priority. On the basis of public health risk, there is currently insufficient evidence to justify the establishment of a Food Safety Criterion for Salmonella for fresh and minimally processed berries (including frozen berries). It is currently not possible to provide a risk base for establishing a Norovirus Food Safety Criterion for these foods. |
|
Risk posed by Salmonella and Norovirus in tomatoes (2014) (EFSA BIOHAZ Panel, 2014c) |
To recommend, if considered relevant, microbiological criteria throughout the production chain. | Assessment of impact on public health was performed | Based on epidemiological evidence (outbreak data) |
The current lack of data does not allow the proposal of a Hygiene Criterion for E. coli at primary production of tomatoes. There is insufficient information available on the occurrence and levels of E. coli in pre‐cut, mashed and other minimally processed tomatoes and therefore the suitability of this criterion cannot be assessed. For this reason, it is therefore not possible to assess the suitability of an EU‐wide E. coli Process Hygiene Criterion for these products. Using E. coli as an indicator for verification of GMP and food safety management systems (including HACCP) might be useful for tomatoes in individual processing premises. A Food Safety Criterion for Salmonella in whole tomatoes could be considered as a tool to communicate to producers and processors that Salmonella should not be present in the product. Testing of whole tomatoes for Salmonella could be limited to instances where other factors indicate breaches in GAP, GHP, GMP or HACCP programmes. Although Noroviruses have been detected in tomatoes, occurrence studies are limited, and quantitative data on viral load are scarce. For Norovirus, there is very limited occurrence data in the world‐wide literature and only one outbreak was reported in the EU between 2007 and 2012, due to a (vomiting) food handler during buffet preparation in catering, thus it is currently not possible to provide a risk base for establishing a Food Safety Criterion for these foods. |
|
Risk posed by Salmonella in melons (2014) (EFSA BIOHAZ Panel, 2014d) |
To recommend, if considered relevant, microbiological criteria throughout the production chain | Assessment of impact on public health was performed | Epidemiological evidence (outbreak data) |
There are limited studies available on the presence and levels of enteric bacteria such as E. coli on melons and watermelons and therefore it is currently not possible to assess the suitability of an EU‐wide E. coli Hygiene Criterion at primary production. Using E. coli as an indicator of recent human or animal faecal contamination is likely to be useful for verification of GAP and GHP at individual production sites. The existing Process Hygiene Criterion for E. coli in pre‐cut melons and watermelons aims to indicate the degree to which GAP, GHP, GMP or HACCP programmes have been implemented. There is insufficient information available on the occurrence and levels of E. coli in pre‐cut melons and watermelons and therefore the suitability of this criterion cannot be assessed. There are Food Safety Criteria for the absence of Salmonella in 25 g samples of ready‐to‐eat pre‐cut fruit and vegetables which is applicable to cut melon and watermelon placed on the market during their shelf life (Regulation (EC) No 2073/2005). This regulation is also applicable to unpasteurised melon and watermelon juices placed on the market during their shelf life. A Food Safety Criterion for Salmonella in whole melons and watermelons could be considered as a tool to communicate to producers and processors that Salmonella should not be present in the product. Since the occurrence of Salmonella is likely to be low, testing of whole melons or watermelons for this bacterium could be limited to instances where other factors indicate breaches in GAP, GHP, GMP or HACCP programmes. |
|
Risk posed by Salmonella, Yersinia, Shigella and Norovirus in bulb and stem vegetables, and carrots (2014) (EFSA BIOHAZ Panel, 2014e) |
To recommend, if considered relevant, microbiological criteria throughout the production chain | Assessment of impact on public health was performed | Based on epidemiological evidence (outbreak data) |
Considering the limited evidence for both the occurrence and public health risks from contamination of Salmonella, Shigella, Yersinia and Norovirus in the primary production and minimal processing of bulb and stem vegetables and carrots, no conclusions can be made on the impact of the establishment of microbiological Hygiene Criteria, Process Hygiene Criteria or Food Safety Criteria on public health. There is a lack of data on the occurrence and levels of E. coli in bulb and stem vegetables as well as carrots. Thus, the effectiveness of E. coli criteria to verify compliance to Good Agricultural Practices (GAP), Good Hygiene Practices (GHP), Good Manufacturing Practices (GMP) and food safety management systems (including HACCP) in the production and minimal processing of bulb and stem vegetables as well as carrots cannot be assessed. |
|
Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including B. thuringiensis in foodstuffs (2016) (EFSA BIOHAZ Panel, 2016) |
Indicate, if possible, the maximum levels of Bacillus, and specifically of B. thuringiensis, in food that could be regarded as safe for human consumption. | Assessment of impact on public health was performed | Based on epidemiological evidence (outbreak data) |
Most cases of food‐borne outbreaks caused by the B. cereus group have been associated with concentrations above 105 CFU/g. However, cases of both emetic and diarrhoeal illness have been reported involving between 103 and 105 CFU/g of B. cereus. Recently, in some food‐borne outbreaks associated with emetic B. cereus, the level of contamination of food ranged from less than 102 CFU/g to 6 × 107 CFU/g. The levels of B. cereus group posing a health risk to consumers are highly strain‐dependent due to the highly diverse pathogenic potential. The possibility of multiplication in foods after storage and/or handling must be taken into account when defining safe levels for human consumption, as well as the composition of the food, which can affect toxin production. All these factors can be responsible for the large variation in the estimated infectious dose, which makes a valid dose–response relationship hard to establish. Taking the enterotoxigenic potential into account, as well as the fact that B. thuringiensis cannot be distinguished from B. cereus at the chromosomal level, the levels of enterotoxigenic B. cereus that can be considered as a risk for consumers are also likely to be valid for B. thuringiensis. |
CFU: colony‐Forming Unit(s); FSC: Food Safety Criterion(a); GAP: Good Agricultural Practices; GHP: Good Hygienic Practices; GMP: Good Manufacturing Practices; HACCP: Hazard Analysis and Critical Control Points; HAV: Hepatitis A virus; NoV: Norovirus; PHC: Process Hygiene Criterion(a); RTE: ready‐to‐eat; RT‐PCR: reverse transcription polymerase chain reaction; SCVPH: Scientific Committee on Veterinary Measures relating to Public Health; TOR: Terms of reference.
Two general issues which are important to flag, in the review of the opinions dealing with targets in primary production sectors, and microbiological criteria, are (1) the phrasing of the TOR and (2) how EFSA addresses lack of data and/or incomplete knowledge.
When reviewing the phrasing of the TORs in the opinions in Table A.1 (Appendix A), the best practice was for the opinions on Listeria monocytogenes from 2008 and the Campylobacter spp. opinion from 2011 (EFSA, 2008b; EFSA BIOHAZ Panel, 2011c). EFSA was asked to assess either the risk related to: (i) different levels of a specific pathogenic microorganism in specific foods (L. monocytogenes opinion from 2008) or (ii) the performance objectives/microbiological criteria related to specific risk reductions (Campylobacter spp. opinion from 2011). In these cases, the phrasing used illustrates a clear separation between risk assessment and risk management.
An example of an inappropriate phrasing of a TOR was the opinion from 2012 on Norovirus (NoV) in oysters (EFSA BIOHAZ Panel, 2012a) where EFSA was asked to assess ‘… limits that do not pose an unacceptable risk for Norovirus …’. In this case, EFSA was actually asked to make judgements on what is acceptable and not acceptable and this is not the task of the risk assessors but of the risk managers. In the assessment, however, this was addressed by not answering the TOR directly but by assessing only the impact of different limits on the consumer exposure and impact on the market (percentage of compliance if a specific limit was chosen). In addition, the phrasing of the TOR in all the opinions related to the food of non‐animal origin (fresh produce including e.g. leafy greens and berries among others) was not optimal (EFSA BIOHAZ Panel, 2014a–2014b, 2014c, 2014d, 2014e) as EFSA was asked: ‘To recommend, if considered relevant, microbiological criteria’ and with no definition of what is meant by relevant. Without specification of or relation to a certain level of protection/risk this question could not be answered without stepping into the task of risk managers.
When reviewing the answers to the TOR, i.e. how lack of data and/or incomplete knowledge were addressed, there are also lessons to be learnt. When the TOR had been properly and unambiguously phrased, the answers were also objective and unambiguous. However, some examples of ambiguous phrasing of answers are seen in the opinions on Salmonella spp. and NoV in leafy greens eaten raw as salads where phrasings are used like ‘…prevalence studies are limited, and quantitative data on viral load are scarce making establishment of microbiological criteria for these foods difficult’ (EFSA BIOHAZ Panel, 2014a). The message is ambiguous because it is not clear what is meant by stating that it is difficult to establish a microbiological criterion. In addition, it is a risk manager task to decide how to deal with lack of data/uncertainties in relation to management options. Having said that these opinions still provide useful information for risk managers. Generally, it can be concluded that in most cases where questions have been asked about microbiological criteria, there is limited availability of quantitative data related both to prevalence and concentration of the relevant microorganisms in foods, consumption data and dose/response relationship, which makes the assessment of risks related to different criteria highly uncertain. Thus, in only three of the reviewed opinions, the microbiological criteria were linked to a QMRA and in one case to an exposure assessment, while in the rest of the opinions the approach was qualitative, based on epidemiological data and evidence. When linking the assessment of a microbiological criterion to exposure or to risk and when assessing the expected level of product compliance, it is very important also to include the uncertainty in this assessment since this information is important for the risk managers in their decision‐making. The reviewed opinions have not addressed uncertainties in an explicit way.
In addition to the 14 reviewed scientific opinions, the BIOHAZ Panel has issued six opinions related to Salmonella spp. in different animal species. Four dealt with the establishment of targets in poultry (i.e. breeding hens, laying hens, broilers and turkeys) (EFSA, 2009b; EFSA BIOHAZ Panel, 2010a, 2011a, 2012a). These opinions were based on analysis of the data from the EU baseline surveys (BLSs) and harmonised monitoring programmes in order to investigate the potential public health benefit of introducing more strict flock Salmonella spp. prevalence targets at the 3 year review point of the statutory control programmes. The BIOHAZ Panel also provided the EC with knowledge relevant for decision‐making on establishment of other targets in primary production sectors in the EU, as summarised in Messens et al. (2013). Member States are expected to meet such targets on an annual basis, and although no sanctions against countries that have failed to meet targets have so far been imposed by the European Commission, the possibility of trade restrictions exists and the publication of monitoring data may influence commercial decisions made by importing countries. Both introduction of a more strict prevalence target for the Salmonella spp. serovars already included in the target and widening the range of serovars, including the possibility of including all serovars, was investigated by means of literature and surveillance data review, expert opinion and analytical modelling. Although in all cases a benefit in terms of reduced human cases relating to each specific poultry reservoir could be anticipated if the target was tightened, or more relevant serovars included, the individual contribution of each reservoir, apart from laying hens, to the total number of human cases was relatively small, so the benefit compared to the complications of implementation was likely to be limited at that time. Furthermore, other measures such as more sensitive sampling protocols, beginning sampling of laying hens earlier in lay and more effective use of official validation testing in relation to existing targets was considered to potentially offer equivalent or greater additional public health benefits compared to extending the targets. It was also emphasised that Member States (MS) should consider the impact of additional targets applying to their specific national situations in cases where additional Salmonella spp. serovars are contributing to an important proportion of human cases.
One opinion also dealt with a QMRA on Salmonella spp. in slaughter and breeding pigs and includes the impact of reductions of prevalence and numbers on human risk (EFSA BIOHAZ Panel, 2010b). Although European Commission and MS decided, on the basis of a cost‐benefit analysis, not to introduce targets for breeding or commercial pig herds in primary production sectors, the QMRA was useful for exploration of the contribution of carcass contamination to human Salmonella spp. cases and thereby informed the decision of European Commission to tighten the PHC relating to the proportion of carcass swabs testing positive for Salmonella spp. Another Opinion dealt with the link between Salmonella spp. criteria at different stages of the poultry production chain (EFSA BIOHAZ Panel, 2010c). Using different scenarios, it was possible to simulate the implementation of the monitoring procedures at slaughterhouse level and to relate carcass Salmonella spp. prevalence to the probability of meeting the criteria and to calculate the actual level of carcass contamination from PHC data, assuming sensitive sampling and test methodology. Studies such as these, although not defining microbiological criteria as such, provide risk managers with options based on data from bespoke analyses and expert opinion that serve as useful background for regulatory decision‐making and prioritisation.
5. Required scientific evidence and data relevant for considering the development of Microbiological Criteria (TOR 2)
This section focuses on the required scientific evidence and data relevant for considering the development of microbiological criteria for pathogenic microorganisms and indicator microorganisms (depending on the requests from risk managers) without taking into account the stage in the food chain where the microbiological criterion applies and actions taken in case of unsatisfactory results.
5.1. Microbiological criteria for pathogenic microorganisms in food
The estimated public health risk related to a specific food/pathogen combination is a function of the hazard characterisation (i.e. the pathogenicity of the pathogenic microorganism including the dose/response relationships) and the exposure assessment (i.e. the prevalence and concentration of the pathogenic microorganism in the food at the time of consumption, combined with the consumption frequency and serving size).
Information from risk assessors to risk managers in relation to decision‐making on microbiological criteria in specific foods includes:
evidence linking a food or animal reservoir/pathogen combination to human disease (hazard identification);
risk assessment (hazard characterisation, exposure assessment and risk characterisation) of the food or animal reservoir/pathogen (may be quantitative or qualitative);
the impact of different microbiological criteria/limits on the public health and product compliance;
uncertainties in the assessments for points 1‐3, including the main sources of such uncertainties.
In the sections below, the information to be considered by risk assessors to provide answers to risk managers is described, discussed and exemplified.
5.1.1. Evidence linking a food or animal reservoir/pathogen combination to human disease (Hazard identification)
A specific human food‐borne illness may be linked to different sources i.e. foods, water, environmental or animal contact or human‐to‐human contact based on monitoring and surveillance data. Attribution to such sources can be achieved using different methods such as microbial subtyping, outbreak data, other epidemiological studies, comparative exposure assessment, and structured expert opinion. Each of these methods has different strengths and weaknesses and addresses different points in the food chain that have been previously described (EFSA, 2008a).
The importance of a specific food for human illness caused by the specific pathogen in question should be assessed (qualitatively or quantitatively) and compared with other food commodities to provide the risk managers with options to decide on the establishment of a target in primary production sectors, or microbiological criterion, for a specific hazard on the most important primary production sources or food commodities, respectively. One way of doing this is through source attribution models (EFSA, 2008a–2008b, 2008c; EFSA BIOHAZ Panel, 2011a) or risk ranking, either looking into multiple pathogens per food commodity or one pathogen for various commodities (EFSA BIOHAZ Panel, 2012b).
Data on prevalence and concentrations of a pathogenic microorganism in a food may be scarce since random sampling and testing for many pathogens in many foods are not regularly carried out. There will be several situations where the evidence arises only through investigations of food‐borne outbreaks. Examples are the Opinions on Shiga toxin‐producing Escherichia coli (STEC) in sprouted seeds and on Norovirus in berries (EFSA BIOHAZ Panel, 2011d, 2014b).
5.1.2. Risk assessment (hazard characterisation, exposure assessment and risk characterisation) of the food/pathogen combination
5.1.2.1. Hazard characterisation
The public health relevance of any pathogen/food or animal reservoir combination, relates both to the incidence and the severity of the human illness and both should be estimated to the greatest extent possible. However, as public health relevance includes other aspects than incidence and severity, the concept of DALYs (Disability Adjusted Life Years) has been introduced. The DALY is calculated by adding (a) the number of years of life lost (YLL) due to mortality to (b) the time spent in less than perfect health due to morbidity and disability, expressed in healthy year equivalents lost due to disability (YLD) (Murray and Acharya, 1997; Haagsma et al., 2013). The DALYs metric enables comparison between the public health impact of various hazards and has been used to evaluate different intervention options (Havelaar et al., 2000). However, within specific pathogen/food commodity risk assessment studies, the DALYs metric is often not applied to express public health impact. More information on DALYs can be found in EFSA BIOHAZ Panel (2012b) and WHO (2015).
Knowledge of the dose–response relationship is important when considering different limits in a microbiological criterion, and to evaluate the risk related to these different limits. The dose–response relationship describes the link between the dose of a hazard and the magnitude and type of biological response occurring (for microbiological hazards usually defined as infection or defined symptoms of disease). The dose–response relationship is preferably constructed based upon human epidemiological studies including food‐borne outbreaks or, exceptionally, from well‐controlled human volunteer studies. An alternative is to derive the relationship based on animal bioassays. However, usually data to construct dose–response curves are only available for a few biological hazards. In dose–response models used in QMRAs, it is often assumed that different exposure events are independent, hence there is no protective immunity in the target population, e.g. as mentioned by (Ayuso‐Gabella et al., 2011). In addition, in many microbial risk assessments, the ‘default’ assumption used for dose–response does not account for strain or serotype variability in pathogenicity and virulence, other than perhaps, recognising the existence of avirulent strains (Coleman et al., 2004). Also, it is known that the dose–response relationship may differ for healthy adults vs those persons being part of a more susceptible population, which is particularly well documented for L. monocytogenes (Goulet et al., 2012; Pouillot et al., 2016). For example, in EFSA Panel on Biological Hazards (BIOHAZ) et al. (2017), the dose–response relationship for L. monocytogenes was derived for 14 age/gender groups, while Pérez‐Rodríguez et al. (2017) considered three groups, >65 years old, pregnant women and <65 years old.
In such cases, it is suggested to consider using models for groups with different susceptibilities and to discuss the needs for this with the risk managers.
5.1.2.2. Exposure assessment
To assess the risks of human disease following exposure to a specific pathogen in the food, it is necessary to know the prevalence and concentration of the pathogen in the food, the factors that affect it (potential for microbial growth, inactivation, e.g. during cooking, seasonal effects, etc.) as well as knowledge of the factors that have a direct effect on consumer exposure to the hazard, including frequency of consumption of the food (product or commodity) and serving size. Thus, exposure assessments will usually include as a first step the definition and description of the food chain, or sections of it relevant to the particular food and hazard combination and the country or region of interest.
The prevalence and concentration data are usually derived from surveys either published in scientific literature or preferably from dedicated BLSs set up at regional, national or European level.
Data from the annual European Union summary reports on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks produced in collaboration by EFSA and ECDC (e.g. from EFSA and ECDC (2016)) are particularly relevant for hazard identification, but may not be fully representative or most appropriate to serve as an input for risk assessment (Banach et al., 2016). Prevalence data from the EFSA/ECDC annual summary reports are often not derived from sampling plans that are statistically designed, and results are generally not directly comparable between Member States and sometimes not even between different years in one country.
In those cases that a sampling plan is statistically designed, reported prevalence or concentration data can be also influenced by the objectives and how the sampling plan was designed (e.g. food categories, sampling region), which might not be representative for the scope of the risk assessment. In addition, reported data could be strongly biased by the microbiological techniques used to generate them.
The use of validated standard methods, such as ISO/CEN methods, or national standard methods with known performance characteristics is recommended for surveys and to provide confidence in, and acceptance of, the data collected.
ISO/CEN methods are available for most of the established food‐borne pathogens and hygiene indicators under consideration to serve as microbiological parameters in a target in primary production sectors, or microbiological criterion. However, for some food‐borne pathogens that were in the scope of some of the prior opinions of the EFSA BIOHAZ Panel, some reflections were made on the interpretation of data collected from published surveys due to the limitations of the (standard) methods of analysis used.
Increasingly, DNA‐based detection methods are used as alternative methods to the standard culture methods for detection of pathogenic microorganisms in foods. In some cases, the standard methods also include, although sometimes only as an optional confirmation step, the use of DNA techniques to detect virulence factors or suggest an additional typing method to distinguish pathogenic strains of concern for public health among these isolates. However, in many published surveys, it is not mentioned to which extent the prevalence estimates relate to the overall target species or to the pathogenic isolates being detected.
In case of food‐borne viruses, such as NoV and Hepatitis A virus (HAV), as well as for food‐borne parasites such as Cryptosporidium, standard methods in specific commodities such as fresh produce have been only recently established. These methods are specialised and labour intensive, and are still in the process of continuous improvement by specialist reference laboratories. For this reason, few surveys for these emerging pathogens in at‐risk foods are available, and published reports on prevalence and concentration may still be using a variety of test methods, which may create bias or high uncertainty in the prevalence estimates.
Standard methods for detection of microorganisms are progressively based on the use of molecular techniques. This complicates the interpretation of results, as molecular methods detect genomic fragments and may not necessarily indicate the presence of viable or functional (e.g. infectious) intact cells being present in the sample (Stals et al., 2013; Ceuppens et al., 2014). It is clear that using data on estimated prevalence from any source, one should be aware or acknowledge the uncertainties, including the limitations of the sampling plan (including frequency and number of samples), the sampling methods, and the performance characteristics of the analytical methods used.
The possibilities for growth or decline of pathogenic microorganisms in a food, after the point in the food chain where the microbiological criterion is supposed to be applied, should be taken into account when estimating the impact on setting a possible microbiological criterion. Predictive models can be useful in providing an estimation of microbial concentration together with its uncertainty and variability, which can be further used to assess the impact of different microbial limits.
Regarding consumption data, the information usually included in the exposure assessment phase is the serving size and the number of servings. The serving size is defined as the portion of food consumed in a single eating event and is often referred in grams. The number of servings refers to the number of food portions of a specific food (sub)category consumed in a specified time period by a specific (risk‐based) population group in a region or country. The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been built from existing national information on food consumption at a detailed level (EFSA, 2011). EFSA has developed a standardised hierarchical food classification and description system called FoodEx23 to codify all foods and beverages present in the Comprehensive Database. Although the database is potentially useful for performing risk assessment at EU level, some limitations can be found regarding methodological differences in the collection of the food consumption data (Merten et al., 2011). This mainly affects dietary assessment methods, the number of assessment days per subject, sampling design or quantification of portion sizes. Other limitations are related with the sparseness in the number of data per EU country, seasonality and description of food ingredients and culinary preparations reported in the Comprehensive Database. If consumption data is missing, standard serving sizes and frequencies of use may be considered.
If no or few data are available for the pathogen/food combination of concern, a qualitative or semi‐quantitative exposure assessment may be undertaken based on the information available. To provide the decision support needed by the risk manager, it is necessary in all cases to specify the limitations of the study, the assumptions made and the uncertainty in the outcome of the estimate of exposure.
5.1.2.3. Risk characterisation
In risk characterisation, the results from hazard characterisation and exposure assessment are combined.
The public health risk upon exposure to a contaminated food can be expressed in several ways. Some risk assessment studies calculate the risk for a population, i.e. the number of illnesses to be expected from the consumption of a particular food item among the population in a specific area and time period (Danyluk and Schaffner, 2011; Ottoson et al., 2011); others calculate the public health impact as an individual risk, i.e. the probability of illness per serving of a food item (Domenech et al., 2013). Acknowledging that risk estimates are prone to uncertainty and bias, risk assessments often do not aim to estimate absolute risk and public health impact, but are rather used as a tool to assess the relative impact of different risk mitigation strategies (Ottoson et al., 2011). Once the ‘baseline’ risk assessment model is constructed, different scenarios, including different microbiological criteria, can be evaluated and their relative impact on illness can be calculated (CAC, 1999).
5.1.3. The impact of different microbiological criteria on public health and product compliance
The most useful/complete response to risk managers for decision‐making would be to provide information, based on available data of occurrence, on the impact of a target in primary production sectors, and/or microbiological criterion on public health as well as on product compliance (quantity of foods that would have to be removed from the market, reprocessed or undergo other corrective actions).
By providing (i) data on the occurrence of the target microorganism in food/batches of the food commodity under consideration and (ii) a QMRA to estimate the effect of the microbiological criterion on the level of risk, risk managers can be informed about estimations on corresponding levels of possible risk reduction and the likelihood of food/batches meeting microbiological criteria with different levels of stringency.
In the EFSA opinion on Campylobacter spp. in broiler meat production (EFSA BIOHAZ Panel, 2011a), both the public health benefits and the product compliance of setting different targets in primary production sectors, and microbiological criteria in broiler flocks and carcasses were evaluated using data from the 2008 EU BLS on the prevalence of Campylobacter spp. in broiler flocks and broiler carcases (EFSA, 2010a,b). It was concluded that, theoretically, a public health risk reduction > 50% or > 90% at the EU level could be achieved if all batches sold as fresh meat complied with a microbiological criterion with a critical limit of 1,000 colony forming units (CFU)/g of neck and breast skin for all tested n (1, 3, 5 or 10) and c values (0, 1 or 2) or 500 CFU/g of neck and breast skin for n = 10 and c = 0 or 1 and n = 5 and c = 0, respectively. A total of 15% and 45% of all batches tested in the BLS did not comply with these criteria. It was emphasised, however, that the estimates of public health impact and product compliance refer to EU level, but the impact could be very different between MSs.
Figure 2 is an illustration of the estimated effect of different stringencies of a microbiological criterion on the public health impact that may be achieved as well as the likelihood of foods/batches that will not meet the limit, and therefore will require corrective action by the food manufacturer. The latter is expressed as the expected percentage of non‐compliant batches. The first (public health impact) is expressed as the maximum risk reduction through the Minimal Relative Residual Risk (MRRR). The MRRR is the remaining relative risk that may be achieved if all batches are tested and the meat from non‐compliant batches is diverted away from the fresh meat chain and not consumed, or is treated to eliminate Campylobacter spp.
Figure 2.
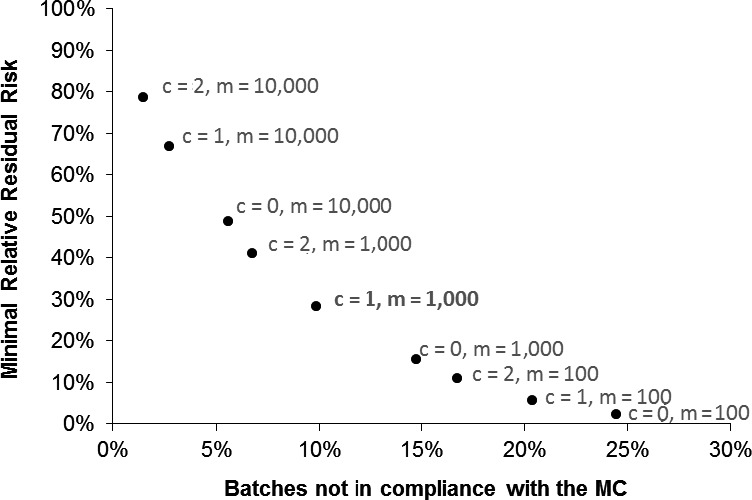
- The Minimal Relative Residual Risk is the remaining relative risk that may be achieved if all batches are tested and the meat from non‐compliant batches is diverted away from the fresh meat chain and not consumed or treated to eliminate Campylobacter spp.
- MC = microbiological criterion, m = microbiological limit, c = number of units that should conform to the limits.
The results shown in Figure 2 are obtained by using the above‐mentioned BLS data for one MS (Denmark) and by applying the model for Campylobacter spp. microbiological criteria (CAMC) as described by EFSA BIOHAZ Panel (2011c). A similar illustration may be produced for other MSs. It appears that by modifying the attributes of the microbiological criterion, by varying the acceptable limit m, and the number allowed to exceed this limit c, it is possible to modify the stringency of the microbiological criterion, thereby moving the balance between the risk reduction that may be achieved and the probability of batches not meeting the criterion. In the case where the limit is set at 1,000 CFU/g and allowing one of five samples to exceed this limit (example marked with bold in Figure 2) it appears that this is likely to infer action in 10% of batches sampled and tested, and is estimated to reduce the consumer risk to about 30% of the current risk (which is equivalent to reducing the risk by ca. 70%). Increasing the stringency of the microbiological criterion could be done by reducing the limit to 100 CFU/g and still allow 1 out of 5 samples to exceed this level. This would likely have the effect that action would be taken in about 20% of batches sampled and tested, but would correspondingly improve consumer safety to 5% of the current risk (which is equivalent to reducing the risk by ca. 95%). Thus, risk managers can modify the microbiological criterion to balance between consumer safety and cost for the industry.
The impact could be very different between MSs, which constitutes an added complication to the situation as illustrated in Figure 2. As the baseline occurrence of Campylobacter spp. on broiler meat varies between the MSs, the effect of a microbiological criterion will have different consequences for different MSs. Figure 3 (EFSA BIOHAZ Panel, 2011c) illustrates the estimated effect of applying a specific microbiological criterion with the stringency achieved by setting m = 1,000 and c = 1 when five samples are collected (example marked in bold in Figure 2). In some MSs, it is estimated that this microbiological criterion will have little effect on the public health risk and a corresponding low frequency of non‐compliant batches. In other MSs, however, the same microbiological criterion will have a huge effect on the public health risk, but also a higher proportion of batches will be non‐compliant with the result that the food business operator will have to implement corrective measures.
Figure 3.
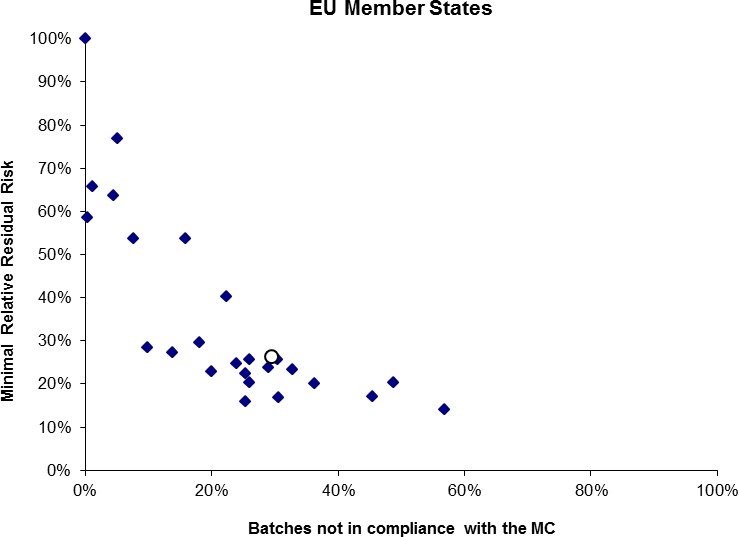
- The Minimal Relative Residual Risk is the remaining relative risk that may be achieved if all batches are tested and the meat from non‐compliant batches is diverted away from the fresh meat chain and not consumed or treated to eliminate Campylobacter spp.
- The point marked in a small circle is the EU mean. The diamonds shown in the figure represent the MRRR and BNMC values for different Member States.
- MC = microbiological criterion, n = number of samples examined, m = microbiological limit, c = number of units that should conform to the limits.
The estimation of risk reduction vs product compliance as illustrated above for Campylobacter spp. in broiler meat in Denmark requires expertise in risk modelling and is the result of a quite resource‐intensive task requiring a multidisciplinary approach. However, web‐based tools can be developed and for Campylobacter spp. in broiler meat the software tool TRiMiCri (tool for risk‐based microbiological criteria – see Section 6.2) has been developed that provide the possibility to assess the simultaneous effect of different microbiological criteria on possible risk reduction and the likelihood of product compliance (Seliwiorstow et al., 2016). The tool applies a published QMRA (Nauta et al., 2012), and requires the availability of data on prevalence and concentration of the numbers of Campylobacter spp. in broiler meat or neck skin taken after industrial processing. Uncertainty in the effect of the microbiological criteria is related both to the QMRA model and the available data. With the QMRA in Nauta et al. (2012), a Bayesian approach was used to evaluate the impact of different national data reflecting Campylobacter spp. in broiler carcasses and flocks, respectively, on the performance of microbiological criteria in terms of relative risk (Ranta et al., 2015).
Alternative multifactor approaches based on a QMRA have been developed for other pathogen/food commodities to evaluate the impact of setting different microbiological criteria on the percentage of risk reduction, e.g. STEC in raw milk soft cheeses (Perrin et al., 2015).
In traditional microbiological criteria, the acceptable limit is a level of microorganisms in a specified quantity of food. The limit of the microbiological criterion could however in some cases be a level of risk, provided that a QMRA is available (CAC, 2013). The risk may be expressed in different ways, as an absolute risk (such as the number of cases per 100,000 servings), or it could be expressed as a relative risk, when compared to the average risk (or baseline risk) of identical foods (such as a risk of 50% compared to the estimated average risk for a given population in a certain year) (Andersen et al., 2015; Nauta et al., 2015).
Above examples of impact assessment are provided when a QMRA model is available. But even, without a QMRA model, it is possible to provide useful information to the risk managers in the format of an exposure assessment followed by a qualitative risk assessment or even by a qualitative assessment alone.
If data are scarce, it may be difficult or impossible for the risk assessors to assess the impact of establishment of different criteria on public health and/or product compliance, but to the extent possible, it should be attempted including an assessment of the uncertainties associated.
If data are scarce, an option could be to express the impact of microbiological criteria, or targets in primary production sectors, on public health or food compliance as a binary proposition, or in the form of a question. An example of such a question could be whether meeting the criterion will reduce (i) the exposure of consumers to the hazard (e.g. via contaminated batches) in view of the expected distribution of the pathogen in batches, pathogen growth and consumption and/or (ii) what would be the expected number of non‐complying products/batches. Based on existing evidence and tools, such as extensive literature search and expert knowledge elicitation, an assessment could be made by scientific experts with relevant reasoning in order to answer the above question, and the uncertainty about this assessment could be described as a distribution of how confident the assessor is about the answer.
If quantitative data on the hazard in the food exists, it is possible to compare the impact of different microbiological criteria/limits (again assuming 100% product compliance) on the level of contamination to which the consumers are exposed. Such an impact assessment was done in an earlier EFSA opinion on Norovirus in oysters (EFSA BIOHAZ Panel, 2012c). In this case, a QMRA model for NoV in oysters was not available and data on NoV polymerase chain reaction (PCR) copies in randomly sampled oyster batches from specific surveys in three MSs was used to assess the impact of different limits on consumer risk and compliance qualitatively and semi‐quantitatively. Thus, it was concluded that quantitative data on viral load during January–March 2010 in three selected MSs, showed that a viral limit of 100, 200, 500, 1,000 or 10,000 NoV PCR copies would result in non‐compliance percentages at 33.6–88.9%, 24.4–83.3%, 10.0–72.2%, 7.7–44.4% or 0–11.1%, respectively. Furthermore, it was concluded that compliance with any of the above NoV limits would reduce the number of contaminated oysters placed on the market and therefore reduce the risk of consumers being infected with NoV. The lower the limit, the greater the consumer protection achieved. However, it was not possible to quantify the public health impact of establishment of different limits.
Considerations on impact of different microbiological criteria on the public health and the challenge for the industry may also be addressed qualitatively. This has been done previously in EFSA opinions, i.e. in the scientific opinion on L. monocytogenes in ready‐to‐eat (RTE) foods to provide information on different levels of L. monocytogenes in RTE foods and the related risk for human illness (EFSA, 2008b). In this opinion, a descriptive approach has been used in the elaboration of a risk assessment using literature review including several QMRAs available at the time and it illustrated the usefulness of predictive models for exposure assessment to estimate the behaviour of L. monocytogenes during distribution and storage of foods. As a starting point, risk assessments published between the SCVPH opinion of 1999 and the EFSA opinion of 2007 were reviewed to conclude that compliance of RTE foods to limits of ‘below 100 CFU/g’ or ‘absence in 25 g’ at consumption would both lead to a very low number of human listeriosis cases. Furthermore, it was shown that growth of L. monocytogenes is a function of the type of food, the storage time and the storage temperature. Thus, predictive modelling tools can be used to determine if the product will or will not support growth of L. monocytogenes and to estimate the extent of growth during the shelf life. However, it was also recommended that the use of predictive models should be combined with validation studies, especially for foods close to the growth/no growth boundaries. It was concluded that most human cases were due to consumption of a small proportion of RTE products able to support growth and thus, contained levels markedly above the regulated limits by Regulation (EC) No 2073/2005.
5.1.4. Summarising remarks
According to the available type of risk assessment data, the following options can be deployed by risk assessors in order to support managers in making informed decisions about the establishment of microbiological criteria:
When a QMRA is available, then the quantitative impact of different microbiological criteria may be assessed both on public health and product compliance. This would enable managers to define the stringency of a criterion, balancing public health protection and the required mitigation strategies.
When prevalence and concentration data are available, but not a QMRA model, then the quantitative impact of different limits may be assessed only on occurrence and product compliance, whereas the impact on public health can be only qualitatively expressed, anticipating that a reduced occurrence would also result in reduced risk.
When neither a QMRA nor prevalence and/or concentration data are available, then it may not be possible to assess either qualitatively or quantitatively the impact of microbiological criteria, or targets in primary production sectors. In this case, the risk assessors can only provide data and expert opinion on available epidemiological studies including outbreak data, dose/response data (if available), and other relevant scientific data, e.g. in the format of a risk profile.
5.2. Microbiological criteria using indicator microorganisms in food
The term ‘indicator microorganisms’ was introduced for those microorganisms whose presence in given numbers indicate failure to comply with applying GAP, GMP or GHP. Usually these ‘good practices’ aim to reduce the negative impact of stages in food processing prone to the increase of microbial contamination of foods, or aim at avoiding the spread or further growth of microorganisms if contamination has occurred.
Several bacteria can be selected as process hygiene indicators. The indicators typically consist of Enterobacteriaceae, coliform bacteria, enterococci or E. coli 1, (Baylis and Petitt, 1997; Mossel et al., 1998; Busta et al., 2003; Suslow et al., 2003), but also aerobic colony count is sometimes used to evaluate hygienic working conditions or failures in control measures. E. coli is most widely used as an indicator of faecal contamination in food.
To evaluate the usefulness of the occurrence and concentration of an indicator microorganism as the basis for monitoring adequate process hygiene, it is necessary to have relevant data on the variability of the counts of indicator microorganisms at one or more defined points in the food chain at representative food business operators. Apart from visual inspection, information on the status of food hygiene implementation can be obtained by a tailored questionnaire on adoption of various aspects of food safety management practices (Sampers et al., 2010) or by using results of hygiene inspections conducted by competent authorities (Habib et al., 2012).
All these data can then be used as input for statistical analysis to investigate the effect of the level of process hygiene in the food premises in question and the food batch variables on the counts of the indicator microorganisms. Such baseline data from empirical research on hygiene indicators and the relationship with food hygiene implementation or visual faecal contamination can then be used by risk managers to set PHC (ICMSF, 2002).
Often the objective of using indicator microorganisms in a microbiological criterion is to improve hygiene. For example, Cibin et al. (2014) performed an experimental study to assess the usefulness of E. coli and Enterobacteriaceae as indicator of hygiene in poultry slaughterhouses, concluding that measuring Enterobacteriaceae and/or E. coli on poultry carcasses is an effective tool to detect faecal contamination in the slaughterhouse.
Barco et al. (2015) reviewed the relationship between indicator bacteria (E. coli and Enterobacteriaceae) counts and visual faecal contamination of cattle and beef carcasses. It was concluded that slaughterhouse characteristics influence bacterial load of beef carcasses, although it is difficult to determine which factors (i.e. slaughterhouse throughput, design of the plant, surveillance system in place) have the greatest effect. Carcasses from faecally contaminated animals were shown to harbour higher E. coli and Enterobacteriaceae counts than clean animals.
In some cases, a slaughter plant or a food processing unit may have a relative high degree of faecal contamination or levels of indicator microorganisms, but may still be able to produce products with low level of pathogens, if the prevalence of pathogens in the animals, the production environment or from food handlers is low. Thus a possible correlation between an increase in the prevalence and/or numbers of an indicator microorganism and the possible presence of pathogenic microorganisms may vary between industries and over time. Therefore, the assessment of the public health impact of microbiological criteria using indicator microorganisms is much more complex than assessing the impact of a microbiological criterion using a specific pathogenic microorganism and the uncertainties would often be higher.
To evaluate whether an indicator microorganism and its concentration could serve as a marker for a pathogen, it is necessary to have comparable data on the prevalence and concentration of the indicator microorganism versus data on the prevalence and concentrations of the pathogenic microorganism under consideration at one or more defined points in the food chain, and at representative food business operators. With such data, it may be possible to evaluate correlations between the indicator microorganism and the pathogen under consideration.
For pork, beef and poultry samples, it has been shown that E. coli counts were significantly higher in samples contaminated with Salmonella spp., thus elevated E. coli counts seem to be correlated with increased prevalence of Salmonella spp. on beef and pork meat (Ghafir et al., 2008). In the processing of broiler carcasses, E. coli was also shown to be useful as an indicator of faecal contamination and Campylobacter spp. contamination of broiler meat during the processing steps up to the point of chilling, but not of chilled broiler meat (Boysen et al., 2016).
In a survey on fresh produce commodities, the presence of elevated levels of E. coli increased the probability of the presence of Salmonella spp. and human pathogenic STECs, but had a low to moderate predictive value on the presence of Campylobacter spp. in the same batch (Ceuppens et al., 2015).
If a relationship between an indicator microorganism and the pathogen of concern is found, a risk assessment approach may be applied to evaluate the impact on public health of setting a microbiological criterion.
Some examples of this type of risk assessment have been elaborated, in particular, in assessing the water quality to be used in fresh produce. Usually in this situation, the information on prevalence of pathogens is scarce. But even if data are available, caution should be taken in extrapolation of relationships between indicator microorganisms and pathogenic microorganisms as defined in a particular study to situations very different from those encountered in the initial data collection (De Keuckelaere et al., 2015). For example, the diversity of pathogens present and their concentrations in animals, human or (sewage) water depends upon the origin of faecal input and the epidemiological status of the contributing populations (Hamilton et al., 2007), both of which can differ by region and time frame. In addition, it is important to take into account whether the behaviour of the indicator microorganism is comparable with that of the pathogen in terms of survival and growth under the conditions of food processing. For example, it is known that in general bacteria are poor indicators of the presence of viruses and parasitic protozoa (De Keuckelaere et al., 2015). In the case of using E. coli as an indicator microorganism for human NoV, E. coli levels might be associated with a non‐human source of faecal contamination (thus the presence of the indicator microorganism overestimates the presence of these viruses), but there is also evidence that NoV (and other enteric viruses, and parasites) may persist for a long time, and also survive some disinfection treatments that eliminate bacteria.
As such, if indicator microorganisms are considered to be used to assess the impact on public health for the pathogen under consideration there is a need to consider the above issues. The estimation of the impact of microbiological criteria on public health/food safety using indicator microorganisms is, if at all possible, therefore more complicated, demanding of data, and with more uncertainty and variability, than when performed for pathogenic microorganisms.
6. Technical tools to operationalise microbiological criteria (TOR 3)
Several tools have been established to support the decision regarding construction of microbiological criteria. Computational tools for decision‐making on sampling strategies, and the impact of microbiological criteria on consumer risk and the likely proportion of non‐compliant batches are freely available on the internet.
Microbiological criteria rely on the performance of sampling plans being a risk management tool used to evaluate whether a food safety or quality system is correctly implemented. Sampling plans have been conventionally derived from theoretical sampling concepts reported by international organisations (i.e. ICMSF, Codex Alimentarius). Each sampling unit is categorised according to some type of attribute (a characteristic of interest), such as the presence/absence of a pathogen, which gives rise to two‐class attributes sampling plans. When there are three categories, such as acceptable/marginal/not acceptable, this gives rise to three‐class attributes sampling plans.
More information on the performance of sampling plans is available in Appendix B. These methods are mainly based on the simplistic assumptions of data normality and homogenous contamination of foods. However, more sophisticated approaches do account for bacterial clumping related to heterogeneous contamination (i.e. negative binomial, Poisson, log‐normal distributions).
In relation to the definition of a microbiological criterion, various phenomena are known to impact the ability of a microbiological criterion to discriminate a defective batch: (i) the spatial distribution of bacterial cells in foods, (ii) how microbial contamination is distributed in a food; (iii) the analytical method; and (iv) the level of confidence which the risk managers will require for a specific use. To address these issues, some efforts have been made in the investigation of suitable statistical distributions to better represent bacterial clustering, and a high proportion of zero counts in samples, especially when contamination levels are low. To make this knowledge more accessible to risk assessors and risk managers, computational tools are available based on different approaches, both probabilistic and deterministic.
In Section 6.1, a selection of existing methods and tools available for the design of microbiological criteria are presented with special focus on how the generated information can be implemented in a realistic food chain scenario. In Section 6.2, practical examples of the use of these tools are provided. These tools may be used in different situations depending on the questions from risk managers and the type of data available (see Appendices C and D).
6.1. Review on currently existing technical tools
Four technical tools to evaluate microbiological criteria were found to be publicly available online: The ICMSF’ Sampling Tool, the JEMRA (Joint FAO/WHO Expert Meeting on Microbiological Risk Assessment) Tool, the Baseline tool and the TRiMiCri. These tools are fully developed applications useful in designing microbiological criteria in food.
A brief summary of these four technical tools and their usefulness for different purposes is described in Table C.1 of Appendix C considering (i) the purpose of application, (ii) the microbial contamination profile, (iii.) the sampling plans and MC, (iv) the software outputs, (v) additional characteristics, (vi) the generation of reports and data exportation and (vii) the usefulness for risk assessors and managers. These tools are subsequently summarised below.
Table C.1.
Summary table on the four technical tools available online to operationalise microbiological criteria and their applications
| ICMSF | JEMRA | Baseline | TRiMiCri | |
|---|---|---|---|---|
| 1. Purpose of Application | Evaluation of the performance of sampling plans and compliance with microbiological criteria in foods based on acquired knowledge on underlying distributions of microbial contamination in a food lot | Establishment and application of risk‐ based microbiological criteria, with a focus on Campylobacter in broiler meat | ||
| 2. Microbial contamination profile | Defined by the mean microbial concentration and standard deviation. Arithmetic and geometric means are included allowing for the estimation of variability in lots | Defined by the range or standard deviation of within‐lot hazard concentrations, and the mean and range (or standard deviation) of the between‐lot distribution | Defined by the mean microbial concentration and standard deviation in a food lot | Quantitative and semi‐quantitative concentration data (define as baseline‐risk) found in samples taking from a food lot |
| 2.1. Definition of within‐lot variability (required inputs) | Mean log concentration and standard deviation. Poisson‐log normal and log‐normal distributions to consider within‐lot variability | Standard deviation for log‐normal, triangular and uniform distributions. Mean is not required to define the within‐lot distribution for the purposes of this tool. OC curves provide the probability of acceptance for every possible hazard concentration | Mean log concentration and standard deviation. Poisson‐log normal and log‐normal distributions to consider between and within‐lot variability | Quantitative concentration data (CFU/g) can be added as integer values per lot. Prevalence, arithmetic and geometric means are given to consider within‐lot variability |
| 2.2. Definition of between‐lot variability (required inputs) | Mean log concentration and standard deviation. Poisson‐log normal and log‐normal distributions to consider between‐lot variability | Mean and standard deviation for log normal, minimum, mode and maximum values for triangular distributions. If empirical, pairs of concentration/cumulative probability values (log CFU/g) | Mean log concentration and standard deviation. Poisson‐log normal and log‐normal distributions to consider between‐lot variability | Variability between lots can be included by introducing quantitative integer values (CFU/g) for individual lots. Prevalence, arithmetic and geometric means are given to consider between‐batch variability |
| 3. Sampling plans and microbiological criteria | Two‐class (enrichment and counts), three‐class | Two‐class (enrichment and counts), three‐class | Two‐class (enrichment and counts), three‐class | Microbiological criteria are those defined by a microbiological limit (c of n samples could have a concentration larger than m CFU/g) and those defined by a relative risk limit (RRL) characterised by the result of risk assessment based on n samples and compared to a baseline risk |
| 3.1. Qualitative (presence/absence) sampling plans | Estimation of the proportion of defectives (Pd) according to the number of samples (n) and maximum allowable positive samples (c) for a given food lot |
|
Estimation of the proportion of defectives (Pd) according to the number of samples (n) and maximum allowable positive samples (c) for a given food lot | |
| 3.2. Concentration‐based sampling plans |
|
|
Mean log and standard deviation for log‐normal and Poisson‐log normal distributions. Further inputs required: number of samples (n), maximum allowable positive samples (c) for a given food lot, microbial limit (m, M log CFU/g). For two‐class enrichment SP, microbial limit is set as the detection limit of the technique (based on the sample size (g or mL)) | |
| 4. Software outputs |
|
|
|
|
| 5. Additional characteristics |
|
|
|
|
| 6. Generation of reports and data exportation | Data and results cannot be exported as csv or Excel files. PDF reports cannot be generated. Several scenarios are not comparable at once | PDF Reports can be generated at any time. The tool displays the list of contamination profiles, and the list of sampling plans, and the user may choose up to 10 combinations to be included | Data and results can be exported as csv format. PDF reports can be generated showing the results of SP performance and derivation of microbiological criteria basing on the establishments of risk‐based metrics. Several scenarios are not comparable at once | Files can be imported/exported in specific TRiMiCri format. The user can load and save models for further use |
| 7. Usefulness for risk assessors and managers | Evaluation of the performance of sampling plans and compliance with microbiological criteria | Estimation of the microbial load after sampling among tested lots. Estimation of the impact of sampling plans parameters can be evaluated through the sensitivity analysis tool | Derivation of microbiological criteria using FSO/PO values. Selection of the most appropriate sampling plans according to the results of the decision support tool | Definition of the most suitable microbiological criteria as a result of comparison of RRL estimates |
FSO: Food Safety Objective; OC: Operating Characteristic; PO: Performance Objective; RRL: Relative Risk Limit; SP: Sampling Plan.
6.1.1. The International Commission on Microbiological Specifications for Foods (ICMSF)’ Sampling Tool
The ICMSF tool is useful for the evaluation of the performance of a sampling plan, both in qualitative and quantitative terms. Risk assessors and managers can evaluate practical examples about the probability of rejection of a food batch with a certain level of confidence. This can be achieved by introducing preliminary information on how microorganisms are distributed in a batch; i.e. mean concentration, standard deviation, number of samples, or microbiological limit to be assessed. Performance of sampling plans can be assessed for microbial counts (two‐ and three‐class sampling plans) and for presence/absence (i.e. when actual concentration in the lot is below the limit of detection of the analytical techniques and enrichment is necessary). It is a free downloadable Excel spreadsheet, which was firstly developed in 1998 and consisted on the evaluation of the performance of sampling plans (Microbiological Sampling Plans: A Tool to Explore ICMSF Recommendations4). The current version (v8) was released in November 2016 including an additional tab with the effect of sensitivity and specificity of the applied analytical method.
6.1.2. The JEMRA (Joint FAO/WHO Expert Meeting on Microbiological Risk Assessment) Tool
The Food and Agriculture Organization and the World Health Organization (FAO/WHO) of the United Nations published a document entitled ‘Risk Manager's Guide to the Statistical Aspects of Microbiological Criteria Related to Foods’ (FAO/WHO, 2016). This guide is useful and accessible to a wide audience. A companion spreadsheet has been developed to illustrate some of the concepts discussed. This web‐based sampling tool (Microbiological Sampling Plan Analysis Tool,5 Beta version 56) allows investigation of two‐ and three‐class sampling plans for presence/absence as well as concentration‐based sampling plans. From the initial version developed in 2007, several features have been introduced such as specification of within‐batch and between‐batch variability, sensitivity and specificity of analytical techniques among others. With this tool, various distributions to describe microbial concentration can be selected (lognormal, uniform, and triangular). Acceptable number of positives and analytical sample size are also available to vary.
Specific applications are able to integrate performance of microbiological criteria within a risk assessment and risk management framework, such as the risk management tool for the control of Campylobacter spp. and Salmonella spp. in chicken meat7 and the risk assessment model for Cronobacter sakazakii in powdered infant formula (PIF).8
6.1.3. Baseline tool
The Baseline tool (v1.0 released in February 2013) is both a predictive modelling and a sampling plan tool that allows establishment of the link between microbiological criteria and other food safety targets (ALOP, FSO, PO, PC, etc.). Predictive microbiology models either for growth or inactivation can be applied and/or introduced to the tool by advanced users. In such a way, microbial concentration can be estimated as a function of environmental factors and processing conditions. The sampling plan module allows the estimation of attributes and concentration‐based sampling plans and different scenarios can be evaluated. Implementation of tailored sampling plans can be achieved based on a food/pathogen combination as well as pre‐existing sampling plans. Furthermore, it contains a decision support tool to direct the selection of an appropriate sampling plan and a tool to relate sampling plans to POs and FSOs. It is a web‐based tool9 developed by the University of Cordoba within the framework of an EU FP7 Baseline project.
6.1.4. TRiMiCri – tool for risk‐based microbiological criteria
The TRiMiCri allows the user to analyse the performance of their batches of broiler meat against user defined microbiological criteria for Campylobacter spp., on the basis of enumeration data of skin or meat samples after industrial processing. It estimates the risk reduction that may be achieved by microbiological criteria for Campylobacter spp. in broiler meat, as well as the likely proportion of batches that are non‐compliant. The tool enables the user to enter semi‐quantitative data found in representative samples so that a suitable baseline risk may be defined and applied for the estimation of Relative Risk Limit microbiological criteria as well as traditional microbiological criteria (Nauta et al., 2015; Seliwiorstow et al., 2016). The Campylobacter spp. risk assessment model used in TRiMiCri has been developed and applied by the EFSA BIOHAZ Panel (2011c) and Nauta et al. (2012). The criteria are evaluated on the basis of analysis of Campylobacter spp. on usually neck skin samples taken after slaughter. Additionally, it is assumed that the risk assessment model is valid in all countries. TRiMiCri v1.3 (released in September 2015) is a free downloadable software tool with an accompanying tutorial created by the National Food Institute of the Technical University of Denmark (DTU) as part of a project subsidised by the Nordic Council of Ministries in 2013.10
6.2. Practical examples of the use of the technical tools
To make feasible the implementation of food safety targets, the routine and successful use of software applications by food operators, governments or educational agencies should be promoted. In the text below, some examples of the technical tools referring to published studies are shown. Furthermore, in the Appendix D, some examples on how to use the sampling tools described in Section 6.1 are illustrated.
6.2.1. Using the ICMSF tool
An example of using the ICMSF tool to determine the performance characteristics of sampling plans in the framework of development of microbiological criteria was described by Scott et al. (2015) in the Special Issue of Food Control on the development of illustrative examples for the establishment and application of microbiological criteria for foods (Caipo et al., 2015).
In the illustrative example of development of microbiological criteria to assess the acceptability of milk powder, Scott et al. (2015) used epidemiological data, information from the literature and expert opinion to define significant hazards and levels of concern. Example criteria (size of analytical unit, sampling plan and limits) were specified for mesophilic aerobic colony count and Enterobacteriaceae as indicators of the adequacy of Good Hygienic Practices and for Salmonella spp. as a food safety criterion. Next, performance characteristics of sampling plans could be calculated using the downloadable spreadsheets provided by ICMSF.
Scott et al. (2015) assumed that the average concentration of the microorganism of concern in the milk powder is lognormally distributed and in case of a pathogen such as Salmonella spp. in milk powder (expected to be present at low levels) that the number of CFU in an analytical unit varies randomly according to the Poisson distribution (i.e. Poisson‐log normal distribution). The performance characteristics of the sampling plans were determined for each microbiological parameter using four different values of the standard deviation of the microbial counts to illustrate how the sampling plan performance depends on the within‐lot standard deviation, which is uncertain for any given lot and varies among lots.
6.2.2. Using the web‐based tools for evaluation of sampling plans developed by FAO and WHO through JEMRA
An example of using the Web‐based tools for evaluation of sampling plans developed by FAO and WHO through JEMRA11 is found in the JEMRA FAO/WHO Risk Analysis tool for risk assessment of C. sakazakii in PIF.12 The latter tool can be used to assess the impact of different end product sampling regimes on risk reduction and product rejection for this specific food‐pathogen commodity. The tool presents a QMRA model developed for C. sakazakii in PIF. This risk assessment considers the preparation, storage and feeding of PIF to infants. In developing the risk assessment, consideration was also given as to whether it was possible to evaluate the impact of testing for Enterobacteriaceae (as indicator microorganisms) on risk reduction of Enterobacter sakazakii 13 illness in infants (Paoli and Hartnett, 2006).
The risk assessment model estimates the risk of C. sakazakii illness posed to infants from PIF. A number of options are provided for the user of the model (and the web‐based tool), which define the variables and the specifications of the exposure pathway described by the model. It is possible to calculate the risk reduction that would be achieved when applying a microbiological criterion alone in the risk assessment model. The calculation of the achieved risk reduction by decisions based on microbiological criteria requires assumptions regarding the relationship between the distribution of pathogens in the accepted product and the assumed risk. To calculate the sampling risk reduction factor, the risk assessment simulates the lot‐by‐lot implementation of decisions based on microbiological criteria. Then the average concentration of accepted lots is calculated and compared to the average concentration of the pre‐sampling powder supply to calculate the net risk reduction effect of the sampling programme (Paoli and Hartnett, 2006).
At the same time as calculating the risk reduction associated with microbiological criteria, it is important to keep track of the proportion of the lots of powder that are rejected by the sampling scheme. Efficiency measures can be calculated, such as risk reduction per lot rejected. As such, a plan that reduces risk indiscriminately will have a relatively low efficiency measure. A plan that reduces risk by selectively rejecting highly contaminated lots will yield a higher efficiency score. An appropriate sampling plan would strike a balance between maximising risk reduction and minimising powder lot rejection, presumably by being most selective for highly contaminated lots of powder (Paoli and Hartnett, 2006).
6.2.3. How a microbiological criterion (MC) can be derived from a performance objective or from a food safety objective
An example illustrating how a Microbiological Criterion (MC) can be derived from a Performance Objective or from a Food Safety Objective, i.e. using a risk‐based approach was described by Zwietering et al. (2015) in the Special Issue of Food Control on the development of illustrative examples for the establishment and application of microbiological criteria for foods (Caipo et al., 2015).
A FSO is defined as the maximum frequency and/or concentration of a hazard in a food at the moment of consumption that provides or contributes to reach an ALOP for human health. A PO allows government risk managers and food business operators to quantify the stringency of a food safety management system in a particular point in the food chain. ALOP, FSO and PO are usually proposed by the competent authority although they can also be set by the food business operators as a part of their management systems. In any case, actions are taken throughout the food process to meet with such objectives. The ICMSF (2002) established the link between a public health measures and food safety management concepts throughout the food chain.
Zwietering et al. (2015) stated that to articulate a MC derived from a PO or FSO, several decisions must be taken:
the assumptions made regarding the distribution (between and within‐lot) of the pathogen in the lot of food;
the ‘maximum frequency or concentration’ of the hazard that is used in the FSO/PO including the proportion (e.g. 95%, 99%, 99.9%) of the distribution of possible concentrations that satisfies the limit, such that the FSO/PO is met;
the level of assurance needed to ensure that a non‐conforming lot is detected and rejected (e.g. with 95% or 99% confidence) by the specific number and size of samples taken.
the analytical method that should be used (including knowledge of its actual test performance characteristics).
Usually data/knowledge of microbiological characteristics of a typical lot can come from sources such as surveillance studies, scientific literature or obtained from dedicated baseline studies or calls for data collection. If information on the distribution of microorganisms and their variability within or between lots is not available, assumptions can be made. The numerical limit of the MC together with the acceptance percentage would probably be decided by the risk managers, since it is this combination that relates to the associated public health impact. However, various scenarios can be exploited and suitable sampling plans for the microbiological criteria can be designed and evaluated, and, given probability of acceptance of a (just) compliant lot, or e.g. how many samples would be needed to achieve the selected probability of rejection of a non‐compliant lot, can be provided.
In Valero (2015), some examples on how to elucidate microbiological criteria based on established PO or FSO targets set as numerical limit of pathogen concentration (i), frequency or proportion terms (ii) and in qualitative to non‐detectable values (iii). These concepts, previously defined by van Schothorst et al. (2009), were exemplified using the Baseline software tool.
As an example regarding the establishment of a MC from a PO set in concentration terms, a log‐normal distribution of microbial contamination was assumed to estimate the allowable number of samples to meet the suggested PO. Different sampling options were provided to reject food lots (95% CL) by setting various microbiological limits (m) for a two‐class attributes sampling plan (Valero, 2015).
In relation to the establishment of a MC from a PO set in proportion terms, the absence of the pathogen in a tested sample after an enrichment technique is considered. In this case, PO can be defined as the maximum allowable percentage of units non‐compliant with a specific target (i.e. x% of positive samples) (Valero, 2015).
Finally, the establishment of a MC from a FSO set in qualitative terms to non‐detectable concentration values was exemplified by Valero (2015) through the use of a Poisson‐log normal distribution. In the present case, the objective was to determine the mean log concentration in the lot in such a way a certain percentage of units would comply with a FSO. Performance criteria were estimated assuming an initial microbial contamination (H0), a certain number of reductions (R) and increments (I) (Zwietering et al., 2010).
In the examples provided, data needs and risk management decisions are required when operationalising a PO/FSO. In all three cases, MCs could successfully be established, but to do so required specific data.
When such data were not available, estimations or informed risk management decisions/assumptions were made regarding key parameters. In addition, risk management decisions relating to the discriminatory power of a MC should be made. For some specific cases, underlying distribution of the microbial contamination is needed and information regarding variability within and between lots (Valero, 2015).
7. Lack of data, how to deal with uncertainties
7.1. In the assessment of the impact on public health and on product compliance related to microbiological criteria and targets in primary production sectors
Like all other risk assessments, assessments performed according to this guidance will be subject to uncertainties. If existing quantitative or qualitative risk assessments are used to perform the assessment of the public health impact and product compliance related to different microbiological criteria, or targets in primary production sectors, many of the uncertainties may already be identified and quantified and can be referred to. If ‘new’ risk assessments are performed, the general guidelines for addressing uncertainties apply. These will also be applicable in the assessment of the relative impact of different microbiological criteria. For many microorganisms and food commodities, there will be limited data on exposure assessment and in many cases also on dose–response relationships, making specific numerical values of public health impact and product compliance highly uncertain. For these reasons, the approach recommended in this opinion is to investigate the relative impact of different Microbiological Criteria and, to the extent possible, to express the uncertainties in these impact assessments including the main sources of uncertainties. Based on this knowledge, risk managers may decide (together with risk assessors and other stakeholders) whether it is worthwhile to invest in further data generation to reduce the uncertainty. As previously mentioned, it is the task of the risk managers to take into account the uncertainties in their decision‐making. Further guidance in relation to different tools to assess uncertainty can be found in EFSA guidance on uncertainty (EFSA Scientific Committee, 2016).
7.2. In the implementation of microbiological criteria
Based on the impact assessment of different microbiological criteria (on public health and product compliance) and the accompanied uncertainties about this assessment as described in these guidance, risk managers may decide to introduce a specific microbiological criterion including a sampling plan, limits, an analytical method, and the stage where the criterion applies.
A guidance document on official control under Regulation EC No 882/200414 concerning microbiological sampling and testing of foodstuffs (EC, 2006) describes that ‘food business operators should always regard all test results above the limits as unacceptable regardless of the measurement uncertainty (MU) involved, whereas in the official controls the MU could be taken into account in the verification of FSC in order to be sure beyond reasonable doubt that the batch in question does not comply with the criterion’.
Currently, there is no agreed way on how to express MU of qualitative determinations. Therefore, there is no guidance on how to take into account the MU in the context of qualitative microbiological results at European Commission level. For quantitative analyses, ISO/TS 19036 Microbiology of food and animal feeding stuffs – Guide on estimation of measurement uncertainty for quantitative determinations should be used (ISO, 2006, 2009).
This ISO Technical specification provides guidance on the estimation and expression of MU attached to results of quantitative food microbiology. MU is based on a standard deviation of reproducibility of the final result of the measurement process in this ISO Technical specification. Each accredited laboratory must calculate the MU in relation to each quantitative microbiological determination and, if requested by the competent authority, to provide it in the test report. In Regulation (EC) No 2073/2005, only one quantitative limit is fixed for a pathogen as a food safety criterion, i.e. L. monocytogenes (100 CFU/g).
8. Conclusions
TOR 4. Guidelines for the requirements and tasks of risk assessors, compared to risk managers, in relation to microbiological criteria.
The establishment of microbiological criteria, targets in primary production sectors and/or food safety targets (e.g. ALOP, FSO, PO and PC) is a risk management activity where governments agree on the maximum level of a food safety hazard in a food animal population or food that is technically achievable and appropriate for consumer protection.
The role of risk assessors should be focused on assessing the impact of different microbiological criteria on public health and on product compliance according to the needs of the risk managers, and, if relevant, to link different microbiological criteria with food safety targets (e.g. ALOP, FSO, PO and PC values).
The following are tasks of risk managers that need to be clearly distinct from those of risk assessors:
To formulate unambiguous questions, preferably in consultation with risk assessors.
To decide on the establishment of a microbiological criterion, or target in primary production sectors, and to formulate the specific intended purpose for using such criteria (e.g. indicator of process failure, indicator of faecal contamination or general improved food safety).
To consider the uncertainties in impact assessments on public health and on product compliance (performed by the risk assessors).
To decide the point in the food chain where the microbiological criteria are intended to be applied and decide on the actions which should be taken in case of non‐compliance.
The following are tasks of risk assessors:
To support risk managers to ensure that questions are formulated in a way that a precise answer can be given, if sufficient information is available.
To ensure clear and unambiguous answers, including the assessment of uncertainties, based on available scientific evidence.
TOR 1. A review of the approaches used by the BIOHAZ Panel to address requests from risk managers to suggest the establishment of microbiological criteria
Fourteen BIOHAZ scientific opinions from 2003 to 2016, have addressed questions related to microbiological criteria, and to targets in primary production sectors, as part of their TOR. Important critical issues found were related to (1) the phrasing of the TOR, and (2) the way EFSA has been addressing lack of data and/or incomplete knowledge:
Phrasing of TORs
Good examples of phrasing a TOR include:
Assess the risk related to different limits of a specific pathogenic microorganism in specific foods, or
Assess the risk reduction that could be obtained by introducing specific performance objectives/microbiological criteria
In these cases, the phrasing used illustrates a clear separation between risk assessment and risk management.
Inappropriate phrasing of a TOR includes:
Assess limits related to a specific pathogenic microorganism that ‘do not pose an unacceptable risk for consumers’.
‘Recommend, if considered relevant, microbiological criteria’ for specific microorganisms in specific foods.
Such phrasings are inappropriate when the words ‘unacceptable’ and ‘relevant’ are not defined. Without specification of, or relation to, a certain level of protection/risk these questions could not be answered without interfering in the role of risk managers.
Addressing lack of data and/or incomplete knowledge
The reviewed opinions have not addressed uncertainties in an explicit way.
When linking the assessment of a microbiological criterion to exposure or to risk and when assessing the expected level of product compliance, it is very important also to include the uncertainty in this assessment since this information is important for the risk managers in their decision making.
TOR 2. Guidance on the required scientific evidence, data and methods/tools necessary for considering the development of microbiological criteria including both Process Hygiene Criteria and Food Safety Criteria. These approaches should take into account the different purposes of applying microbiological criteria.
This guidance focuses on the required scientific evidence and data relevant for considering the development of microbiological criteria for pathogenic microorganisms and indicator microorganisms (depending on the requests from risk managers) without taking into account actions taken in case of unsatisfactory results.
Microbiological criteria for pathogenic microorganisms in food
The estimated public health risk related to a specific food/pathogen combination is a function of the hazard characterisation (i.e. the pathogenicity of the pathogenic microorganism including the dose/response relationships) and the exposure assessment (i.e. the prevalence and concentration of the pathogenic microorganism in the food at the time of consumption, combined with the consumption frequency and serving size).
Information from risk assessors to risk managers in relation to decision‐making on microbiological criteria in specific foods includes:
evidence linking a food or animal reservoir/pathogen combination to human disease (hazard identification);
risk assessment (hazard characterisation, exposure assessment and risk characterisation) of the food or animal reservoir/pathogen (may be quantitative or qualitative);
the impact of different microbiological criteria/limits on the public health and product compliance;
uncertainties about the above evidence and assessments, including the main sources of such uncertainties.
According to the available type of risk assessment data, the following options can be deployed by risk assessors in order to support managers in making informed decisions about the establishment of microbiological criteria:
When a QMRA is available, then the quantitative impact of different microbiological criteria may be assessed both on public health and product compliance. This would enable managers to define the stringency of a criterion, balancing public health protection and the required mitigation strategies.
When prevalence and concentration data are available, but not a QMRA model, then the quantitative impact of different limits may be assessed only on occurrence and product compliance, whereas the impact on public health can be only qualitatively expressed, anticipating that a reduced occurrence would also result in reduced risk.
When neither a QMRA nor prevalence and/or concentration data are available, then it may not be possible to assess either qualitatively or quantitatively the impact of microbiological criteria, or targets in primary production sectors. In this case, the risk assessors can only provide data on available epidemiological studies including outbreak data, dose/response data (if available), and other relevant scientific data, e.g. in the format of a risk profile.
Microbiological criteria using indicator microorganisms in food
Several bacteria can be selected as process hygiene indicators. The indicators typically consist of Enterobacteriaceae, coliform bacteria, enterococci or Escherichia coli.
To evaluate the usefulness of the occurrence of an indicator microorganism and its concentration as the basis for monitoring adequate process hygiene, it is necessary to have relevant data on the variability of the counts of indicator microorganisms at one or more defined points in the food chain at representative food business operators together with data on adoption of various aspects of food safety management practices or results of hygiene inspections conducted by competent authorities. Such baseline data from empirical research on hygiene indicators and the relationship with food hygiene implementation or visual faecal contamination can then be used by risk managers to set PHC.
To evaluate whether an indicator microorganism and its concentration could serve as a marker for a pathogen, it is necessary to have comparable data on the prevalence and concentration of the indicator microorganism versus data on the prevalence and concentrations of the pathogenic microorganism under consideration at one or more defined points in the food chain, and at representative food business operators. With such data, it may be possible to evaluate correlations between the indicator microorganism and the pathogen under consideration.
If a relationship between an indicator microorganism and the pathogen of concern is found, a risk assessment approach may be applied. But even if data are available, caution should be taken in extrapolation of relationships between indicator microorganisms and pathogenic microorganisms as defined in a particular study to situations very different from those encountered in the initial data collection.
The estimation of the impact of microbiological criteria on public health/food safety using indicator microorganisms is, if at all possible, more complicated, demanding of data, and with more uncertainty and variability, than when performed for pathogenic microorganisms.
TOR 3. Recommendations on methods/tools to design microbiological criteria (limits, sampling plans, stage of the food chain, method, etc.)
Four technical tools to operationalise microbiological criteria were found to be publically available online: The ICMSF’ Sampling Tool, the JEMRA Tool, the Baseline tool and the TRiMiCri. These tools are fully developed applications useful for designing microbiological criteria in food.
These tools may be used in different situations depending on the questions from risk managers and the type of data available (see Appendices C and D).
Glossary and Abbreviations
- Batch
as defined in the Regulation (EC) No 2073/2005, means a group or set of identifiable products obtained from a given process under practically identical circumstances and produced in a given place within one defined production period.
- Food safety criterion
as defined in the Regulation (EC) No 2073/2005, means a criterion defining the acceptability of a product or a batch of foodstuff applicable to products placed on the market.
- Food safety target
refers to the maximum allowable numerical values and/or proportions which can be used by competent authorities or food business operators (FBO) to derive a microbiological criterion, e.g. Appropriate Level of Protection (ALOP), Food Safety Objective (FSO), Performance Objective (PO) and Performance Criterion (PC).
- Microbiological criterion
as defined in the Regulation (EC) No 2073/2005, means a criterion defining the acceptability of a product, a batch of foodstuffs or a process, based on the absence, presence or number of microorganisms, and/or on the quantity of their toxins/metabolites, per unit(s) of mass, volume, area or batch. It should be noted that in the Regulation (EC) No 2073/2005 microorganisms means bacteria, viruses, yeasts, moulds, algae, parasitic protozoa, microscopic parasitic helminths, and their toxins and metabolites.
- Process hygiene criterion
as defined in the Regulation (EC) No 2073/2005, means a criterion indicating the acceptable functioning of the production process. Such a criterion is not applicable to products placed on the market. It sets an indicative contamination value above which corrective actions are required in order to maintain the hygiene of the process in compliance with food law.
- Product compliance
refers to the extent to which food products meet a microbiological criterion.
- Target
as defined in the Regulation (EC) No 2160/2003, shall consist at least of: (a) a numerical expression of: (i) the maximum percentage of epidemiological units remaining positive; and/or (ii) the minimum percentage of reduction in the number of epidemiological units remaining positive; (b) the maximum time limit within which the target must be achieved; (c) the definition of the epidemiological units referred to in (a); (d) the definition of the testing schemes necessary to verify the achievement of the target; and (e) the definition, where relevant, of serotypes with public health significance or of other subtypes of zoonoses or zoonotic agents listed in Annex I, column 1, having regard to the general criteria listed in paragraph 6(c) and any specific criteria laid down in Annex III. Community targets shall be established for the reduction of the prevalence of zoonoses and zoonotic agents listed in Annex I, column 1, in the animal populations listed in Annex I, column 2.
- ALOP
Appropriate level of protection
- BLS
baseline survey
- BNMC
batches not complying with the microbiological criterion
- CA
competent authority
- CAC
Codex Alimentarius Commission
- CAMC
Campylobacter spp. microbiological criteria
- CFU
colony forming unit(s)
- DALY
disability adjusted life years
- FAO
Food and Agriculture Organization
- FBO
Food business operator
- FSC
Food safety criterion(a)
- FSMS
Food safety management system
- FSO(s)
Food safety objective(s)
- GAP
Good agricultural practices
- GCP
Good Commercial Practices
- GHP
Good hygienic practices
- GMP
Good manufacturing practices
- HACCP
Hazard analysis and critical control points
- HAV
Hepatitis A virus
- ICMSF
International commission on microbiological specifications on foods
- ILSI
International life sciences institute
- ISO
International Organization for Standardization
- JEMRA
Joint FAO/WHO expert meetings on microbiological risk assessment
- MC
microbiological criterion(a)
- ML(s)
microbial Limit(s)
- MPN
most probable number
- MRRR
Minimal Relative Residual Risk
- MS(s)
Member State(s)
- MU
measurement uncertainty
- NoV
Norovirus
- OC
operating characteristic (curve)
- PC
Performance criterion(a)
- PCR
polymerase chain reaction
- Pd
Proportion of defectives
- PHC
Process hygiene criterion(a)
- PIF
powdered infant formula
- PO(s)
Performance objective(s)
- PRP
Prerequisite programme
- QMRA
quantitative microbial risk assessment
- RTE
ready‐to‐eat
- RRL(s)
relative risk limit(s)
- RT‐PCR
Reverse transcription polymerase chain reaction
- SCVPH
Scientific Committee on Veterinary Measures relating to Public Health
- SP
Sampling plan
- STEC
Shiga toxin‐producing Escherichia coli
- TOR
Terms of reference
- TRiMiCri
Tool for risk‐based microbiological criteria
- WHO
World Health Organization
- YLD
years lost due to disability
- YLL
years of life lost
Appendix A – Summary table of the review performed on how the BIOHAZ Panel answered the Terms of Reference (TOR) related to microbiological criteria in previous scientific opinions
Appendix B – Performance of sampling plans
The design of microbiological criteria
The establishment of MC has been traditionally based on calculating a probability of acceptance or rejection of food batches, given some preliminary specifications. Performance of sampling plans is evaluated as a function of their ability to discriminate positive batches (i.e. capacity of detecting the presence of a hazard in a food lot). Batch acceptance /rejection has been well described as a binomial process where the number of samples (n) and the maximum allowable number of samples not exceeding a certain limit (c) are established. In the text below, performance of attributes sampling plans is exemplified using various operating characteristic (OC) curves.
The microbiological limit‐based criteria are associated with the occurrence of the hazard in a given number of analytical units in a lot. According to FAO/WHO (2016), the analytical unit is a single unit of food, from which a predetermined amount of analytical unit is removed and tested for microorganisms. All or part of the sample unit may be used as the analytical unit. The acceptability of a lot depends on the detection probability, which is the proportion of analytical units that contain the target microorganism or contain the target microorganism above a predetermined microbiological limit, assuming the microbiological test is 100% specific and sensitive. This detection probability is an estimate of the prevalence (of food units containing the target microorganism in a lot) and depends also on the amount of the analytical unit, i.e. how much of the food is tested. Given an expected or known prevalence and concentration of the hazard in the food of concern, sampling plans may derive, illustrating the probability of accepting a batch depending on the microbiological limit and the number of analytical units tested. The tolerable probability of accepting a batch is a risk management decision and once this is set, then the analytical units needed to meet this probability may be defined. A sampling plan is a scheme that defines the number of sample units to collect, the amount of food that constitutes a sample unit, the size of the analytical units tested, and the number of marginal and/or non‐acceptable items allowed in a sample to evaluate the compliance status of a lot. A sampling plan in which each selected sample unit is classified according to the quality characteristics of the product and in which there are only two or three grades of quality, e.g. (i) acceptable vs defective, (ii) absent vs present, (iii) acceptable, marginally acceptable and defective or (iv) low count, medium count, high count, are called attribute plans.
The two‐class sampling plans are characterised by the number of analytical units and the microbiological limit, since no positive (unacceptable) units are allowable. In contrast, the three‐class sampling plans include the so‐called maximum number of unacceptable analytical units in two‐class sampling plans, or marginally acceptable analytical units in three‐class sampling plans that can be tolerated in a sample while still accepting the lot. The lower microbiological limit m in a two‐class plan, separates good quality from defective quality, while, in a three‐class plan, separates good quality from marginally acceptable quality. In general, values equal to m, or below, represent an acceptable product and values above it is either marginally acceptable or unacceptable. The upper microbiological limit M in a three‐class plan, separates marginally acceptable quality from defective quality. Values above M are unacceptable. In such a case, the number of sample is unrealistic; additional requirements may be defined before establishing a practical sampling plan. If the concentration of the pathogen is relatively high, it can be detected by using traditional enumeration methods (i.e. ISO). For that specific case, a two‐class sampling plan can be applied. If this sampling plan is too stringent (i.e. it has a very high discriminatory power to accept/reject batches), the value of c should be different from 0; or alternatively, a three‐class sampling plan can be formulated.
According to sampling plan theory, when the underlying distribution of the safety variable, (e.g. levels of hazard) is known, there is possibility to derive sampling plans exploiting microbial concentration data (i.e. variables sampling plans) rather than ascribing them to classes (i.e. attributes sampling plans) (Gonzales‐Barron et al., 2013). Although variable sampling plans can be sometimes more efficient than those based on attributes (i.e. lesser number of samples are needed to obtain the same confidence), they are scarcely applied at national/international levels since information on how microorganisms are distributed in a food batch is not often available.
For three‐class attributes plans, it is necessary to establish m values (associated with Good Commercial Practices (GCP)) as well as M values, related to the safety/’quality’ limit (ICMSF, 1986). The latter are based on expert opinion as to the acceptable limit, but the former should be based on firm data obtained from producers and retailers operating according to Good Manufacturing Practice (GMP) and GCP. For certain commodities, there is not sufficient information to establish m values on this basis. When information is available, expert opinion may be inquired to establish appropriate m values for three‐class plans together with the collection of further microbiological data. Where such values cannot be derived, two‐class plans are adopted.
The stringency of microbiological criteria, defined by the parameters n (number of analytical units, or else samples), c (number of allowable unacceptable or marginally acceptable samples with hazard level between m and M), m (the lower microbiological limit) and M (the maximum level of hazard allowable in a positive [marginally acceptable] sample, when c≠0), is determined by the sampling plan and the established limit. This can also influence the discriminatory power of the applied microbiological criteria, which is defined as the ability of a given microbiological criterion to accept batches at a set confidence level (p = 0.05). In an idealised situation, the performance characteristic of a sampling plan (OC curve) would fall down from a 100% probability of acceptance to a 100% probability of rejection just at the limit proportion defective that distinguishes between conforming and non‐conforming batch quality. In practice, no sampling plan can achieve this ideal, but the steeper the OC curve, the closer the sampling plan comes to approaching the ideal. In general, this can be achieved by increasing the number of sample units (n) to be drawn from a batch (Figure B.1). This should be distinguished from a shift of the OC curve that is achieved by decreasing the acceptance number c. A lower value for c will result in a general reduction of the probability of accepting non‐compliant batches (Dahms, 2014).
Figure B.1.
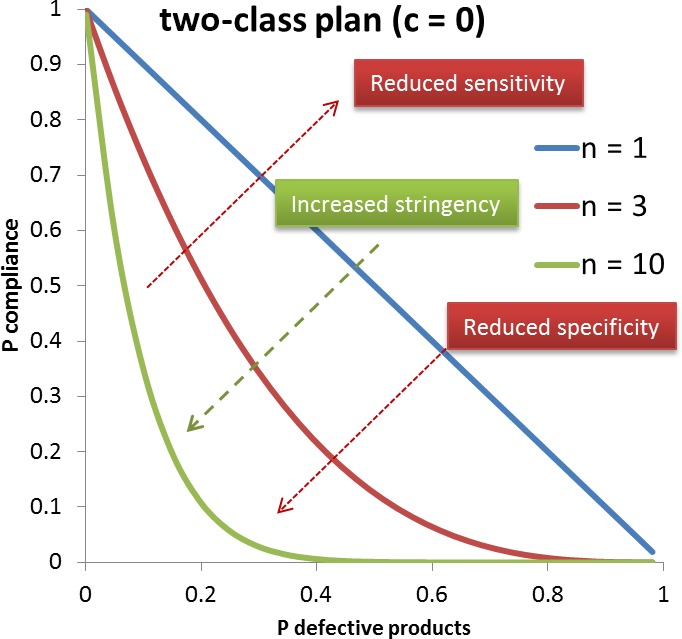
Typical operating characteristic curves of two‐class sampling plans that show the impact of sensitivity and specificity of the analytical method and the stringency of the criterion on the probability of acceptance of a lot (Zwietering and den Besten, 2016)
There is always a chance to detect microorganisms in contaminated samples. However, when contamination is low, the analytical method applied will impact the likelihood of detection of the target microorganism in levels above the critical limit. This largely depends on the sample weight and microbial concentration in the sample. The sensitivity of the test is also called the ‘true positive rate’, and is the proportion of actual positives which is correctly identified as such. The specificity is also described as the ‘true negative rate’, and is the proportion of actual negatives which are correctly identified as such (Zwietering and den Besten, 2016).
Figure B.1 shows the impact of the performance of the analytical method for a two‐class sampling plan. It is shown that, if the sensitivity of the method decreases, (i.e. it underestimates the levels or fails to detect the hazard) then the probability of finding the product in compliance with the criteria increases (the probability of accepting a defective batch increases) (type II error). Conversely, if the specificity of the method decreases (i.e. it overestimates the level or detects a hazard in true negative samples), then the probability of rejecting an acceptable batch increases (type I error).
Figure B.2 shows the operating characteristic curves of various three‐class sampling plans that describe the probability of compliance (Pcompliance) of batches as a function of the % of defective products (Pdefective). By increasing the maximum allowable number of samples exceeding a certain limit (value of c), then the sampling plan will accept defective batches at a higher probability. Defective products are those where the target hazard is present in the analytical unit, or products where the level of the hazard exceeds the microbiological limit ‘m’ (for c = 0). A batch is rejected also when the number of products that contain a hazard at level between m and M (also termed marginally acceptable products) is greater than c, or when a sample is found to contain hazard at a level exceeding M. The stringency of the sampling plan increases by increasing the number of samples (n), or decreasing the number of acceptable (tolerable or marginally acceptable) positive products (c), or by decreasing the limit (m) or the range between m and M.
Figure B.2.
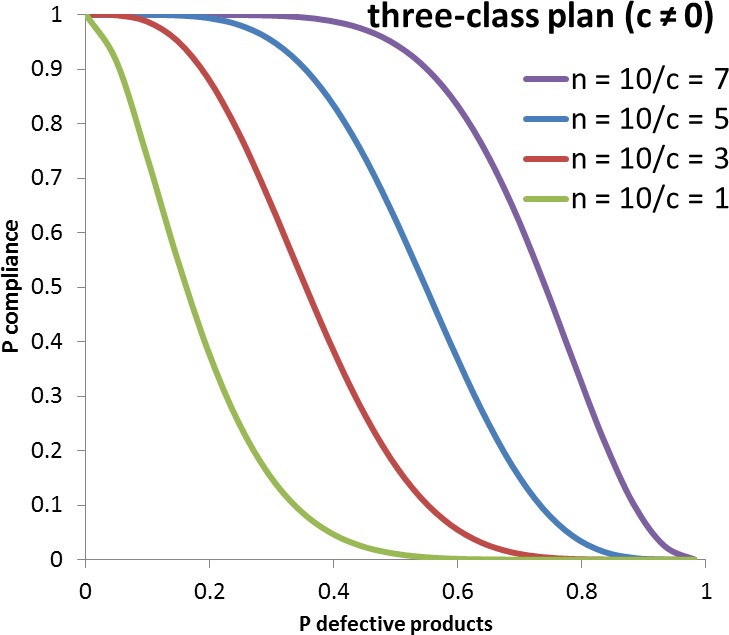
Typical operating characteristic curves of three‐class sampling plans that show the impact of sensitivity and specificity of the analytical method and the stringency of the criterion on the probability of acceptance of a lot
One important aspect of the use of microbiological criteria and the underlying statistics is that application is highly different for quantifying high levels of microorganisms versus detecting low levels by presence/absence tests, due to the differences in the sensitivity of the methods (limit of detection and limit of quantification) and the statistical treatment of the data (e.g. presence/absence data vs numerical data).
In practice, most microbiological sampling plans designed for batch acceptance are attributes sampling plans. For these, to assess the probability of acceptance as a function of the percentage of non‐conforming units, no knowledge or assumption about the underlying distribution of the microorganisms is required. However, for these plans to assess the probability of acceptance as a function of the level of the target microorganism, it is necessary to know or estimate the distribution of microorganisms (CAC (2013) revised in 2013 in Section 4.5 point 25). Regarding concentration‐based sampling plans, the OC curve has two scales, the horizontal scale showing a measure of batch quality (i.e. mean concentration) like the fraction or percentage of positive (‘defective’) units in the batch being tested, and the vertical scale giving the probability of acceptance (Figure B.3).
Figure B.3.
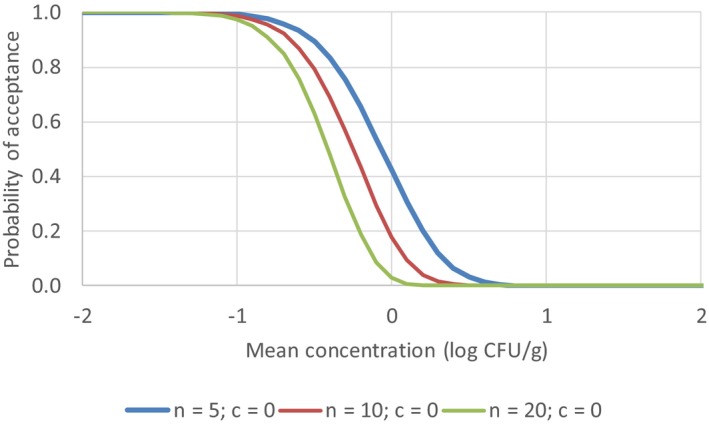
OC curve showing the performance of different two‐class attributes sampling plans based on a microbial log‐normal distribution with mean = 2 log CFU/g and standard deviation = 0.5 log CFU/g
Batches with acceptable or excellent levels of microbial contamination, should be accepted most of the time and rejected only infrequently. Such batches should have a large probability of acceptance and hence a small probability of rejection, (i.e. often referred to as the producer's risk). Similarly, batches with unacceptable levels of microbial contamination, should be rejected most of the time and accepted infrequently (i.e. often referred to as the consumer's risk) (FAO/WHO, 2016). In any case, whichever scheme is adopted, the degree of acceptable risk by producers and consumers should be previously defined. Producer's risk is defined as the ‘risk of wrongly rejecting good quality product’ whilst the consumer's risk relates to ‘wrongly accepting unsatisfactory product’. It should be noted that although producer's risk and consumer's risks are traditional terms in the acceptance sampling literature including the Codex Guidelines on Sampling (CAC, 1999), these terms do not refer strictly to risks, but probabilities, as they do not take into account the resulting severity. Acceptance or rejection of food batches performed by the application of microbiological criteria is a statistically based approach where there is no guarantee that the product compliance is 100% in all cases.
Appendix C – Summary table characterising the four technical tools available online to operationalise microbiological criteria
1.
Appendix D – Practical examples of the use of the technical tools to operationalise microbiological criteria
D.1. Example of compliance with a microbiological criterion based on microbial counts
Concentration‐based sampling plans can be defined as either two‐ or three‐class plans, the distinction being the inclusion of an additional threshold of concentration in three‐class plans. This additional threshold distinguishes ‘marginal’ from ‘outright’ unacceptable concentrations. In this example, compliance of a microbiological criterion using a two‐class concentration‐based sampling plan is shown using the JEMRA tool.
For a first‐time use, a contamination profile must be introduced in the tool. The variability in microbial concentration can be either defined within or between a food lot. For this example, standard deviation of a lognormal within‐lot distribution is established at 0.8 log CFU/g as a default value.
Variability between lots can be described by a lognormal distribution with a mean concentration μ = 1.5 log CFU/g and a standard deviation σ = 1 log CFU/g.
Setting of a sampling plan is referred to the conditions where a fraction of products will be tested. In this example, it is assumed that 5% of lots are sampled, through collecting 10 samples of 25 g each, applying a direct count method and assuming an analytical sample size of 10 g.

Regarding the resolution of the analytical technique, test sensitivity could be also specified. In the next figure, it is assumed that microbial concentrations of 2 log CFU/g can be detected with a sensitivity of 1 (100% of samples analysed are truly positive). It could set a value of c = 0; a limit of m = 10 CFU/g / M = 100 CFU/g; and a target probability of rejection of 95%.
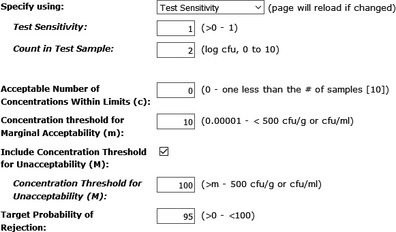
Finally, the tool allows the design of a plan to meet a specified target. For the present example, it is desired to reject lots with concentrations above 2 log CFU/g at 95% level. The JEMRA tool calculates the number of samples needed (4) to implement such sampling plan.
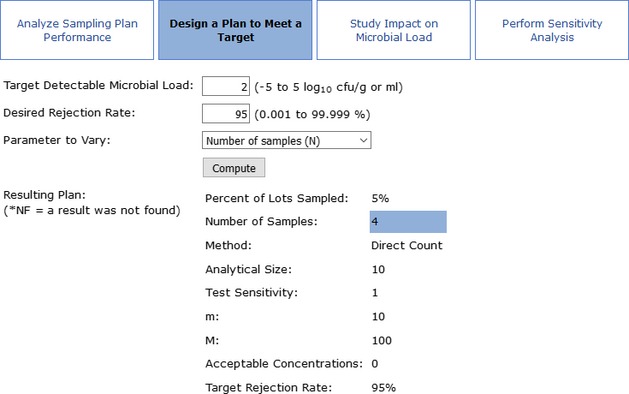
D.2. Example of compliance with a two‐class microbiological criterion
The establishment of a microbiological criterion based on microbial enrichment (detection of the microorganism in a tested sample) is based on the assignment of ‘positive’ or ‘negative’ samples. This result largely depends on the performance of the applied analytical technique to detect a certain microorganism in a defined sample size. Moreover, concentration is not measured, so it is not appropriate to refer to any concentration measurement threshold when specifying the plan.
In such a plan, there is no operational difference between a sample that contains one CFU and another sample containing higher concentration levels since both are considered positive. Sampling plans based on enrichment are mainly used for those commodities with low or very low microbial concentration or prevalence and where current enumeration techniques are not feasible for routine testing.
In this example, compliance with a microbiological criterion based on enrichment will be achieved using the JEMRA tool. As in the previous example, the variability in microbial concentration can be either defined within and between a food lot. For this example, the standard deviation of a lognormal within‐lot distribution is established at 1.5 log CFU/g.
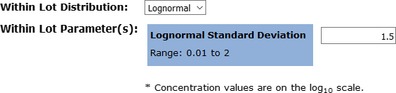
Variability between lots can be described by a lognormal distribution with a mean concentration μ = −3 log CFU/g and a standard deviation σ = 1.2 log CFU/g.
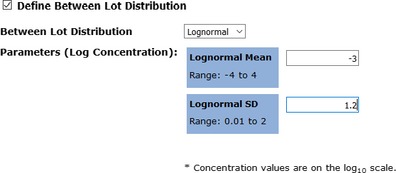
To define a presence/absence sampling plan, the needed inputs are the number of samples taken (n) and the number of positive samples (c) which will be tolerated while still accepting the lot. The size of each sample (s) in terms of the mass or volume of product must also be defined. In this example, it is assumed that 5% of lots are tested, by collecting 10 samples of 250 g each and considering an analytical sample size of 25 g.

The probability of detection is an alternative measure to the test sensitivity and it indicates the probability that the pathogen is detected with a specific analytical technique. It could be assumed that the pathogen is detected in 95% of the cases, with a c value of 0 and a target probability of rejection of 95%.

The performance of the sampling plan can be then tested through the visualisation of OC curves relating the microbial load (in arithmetic units) and the probability of acceptance.

To obtain a desired detectable microbial load of 0 log CFU/g (=1 CFU/g) the resulting probability of acceptance would be 4% (i.e. lots are rejected with a 96% probability if microbial concentration is equal or above 1 CFU/g. Conversely, for a desired probability of acceptance at 5%, the resulting detectable microbial load would be ‐0.0606 log CFU/g (=0.87 CFU/g). In that case, the analytical technique should be able to detect such low concentrations.
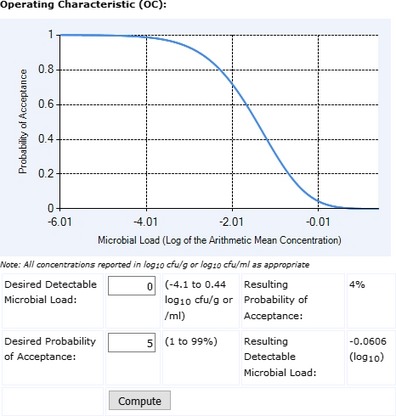
D.3. Evaluation of the performance of microbiological criteria aimed at establishing a relationship between microbiological criteria and risk‐based metrics (FSO, PO, PC)
For the purpose of this example, it can be assumed that the competent authority has established a PO for the concentration of a microbial food‐borne pathogen in a specific food matrix. Then, a suitable microbiological criterion could be established in order to meet this PO. This PO can be established at different points in the food chain.
For illustration purposes, microbial contamination in the lot can be described by a lognormal distribution defined by a mean value μ = 1.75 log CFU/g and a standard deviation σ = 0.8 log CFU/g. Also, a PO could be stated as a pathogen level lower than 4 log CFU/g for 99.75% of the samples comprising the lot. This can be understood as ‘no more than 0.25% of the sampling units in the lot will have a concentration higher than 4 log CFU/g’.
The next step is to decide the most suitable microbiological criterion so that the PO is accomplished. This microbiological criterion should be based on the establishment of a microbiological limit (m) such that the sampling plan is feasible. This decision corresponds to food safety managers and food operators, in such a way the sampling procedure can be effectively done and PO is accomplished.
The use of Baseline software tool allows the evaluation of the compliance with the required PO. Initial values can be entered in the software as shown below.
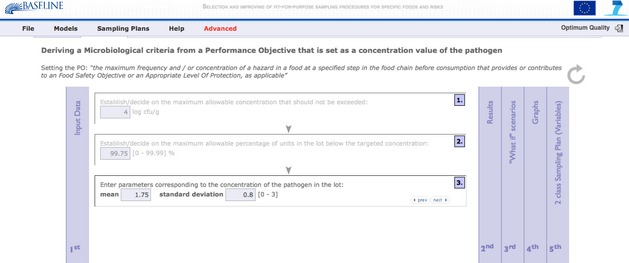
Considering a microbial distribution of mean=1.75 log CFU/g and S.D. = 0.8 log CFU/g, it can be seen that 99.75% of the units would comply the specifications of the established PO. Thus, the microbial contamination of the lot fulfils with the PO specifications. Alternative correctives measures could have been applied instead.

Finally, microbiological criteria can be derived by setting a microbial limit (i.e. m = 10 CFU/g). Note that several other aspects of a microbiological criterion and the underlying sampling plan need to be additionally defined, such as the microbiological characteristics of the food/lot concerned, the analytical method used etc. In this example, a microbiological criterion of n = 5; c = 2; m = 1 log CFU/g could be applied to obtain a probability of acceptance lower than 0.05. The microbiological criterion implemented is presented below.
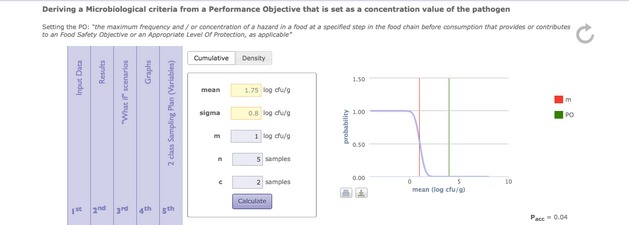
D.4. Evaluation of the performance of microbiological criteria using a risk assessment based on Relative Risk Limit microbiological criteria (RRL)
Here, compliance is defined on the basis of the relative risk estimate associated to the number of samples taken. Such measures would include calculation of Minimal Relative Residual Risk (MRRR); or the percentage of batches not complying with a given microbiological criterion (BNMC). EFSA opinions on Campylobacter spp. and Salmonella spp. in broiler meat are good examples.
The use of TRiMiCri could help to exemplify the risk‐based performance of MC. In the main screen the user can introduce different sample concentrations per batch. The tool calculates the number of samples per batch, the mean prevalence and concentration (CFU/g and log CFU/g).
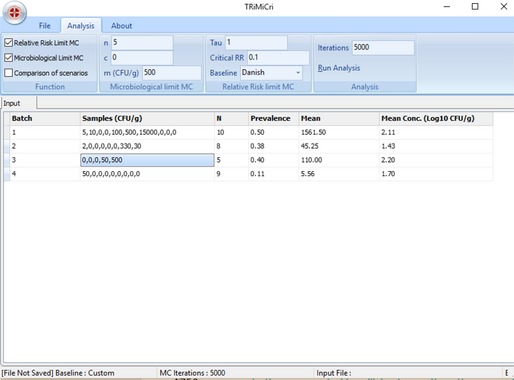
A MC can be set in the function bar by setting the number of samples (n) and maximum allowable number of samples (c) not exceeding a microbial limit (m).
The Relative Risk Limit (RRL) MC includes different parameters:
-
–
Tau: the transition factor which expresses the difference between the observed concentration (e.g. on a skin sample or from a carcass wash) and the concentration per gram of meat in log CFU. As a default value, TRiMiCri proposes to use Tau = 1.0, which implies that the concentration per g of meat is one log lower than the concentration found in the sample taken.
-
–
Critical RR: The value of RRcrit that represents the ‘acceptable risk’. If RRcrit = 0.2 it implies that the relative risk of a batch may be up to 20% as large as the baseline risk for compliance to the MC. Increase of this value gives a less stringent MC, and more batches will comply.
-
–
Baseline: The Danish baseline (which is based on broiler meat data collected 2007–2009 in Denmark), can be used in absence of additional information. Otherwise, a custom baseline can also be set. In any case, a baseline risk (for instance, mean annual risk in a country) should be set.
An in silico simulation can be run and the output window will be shown below:
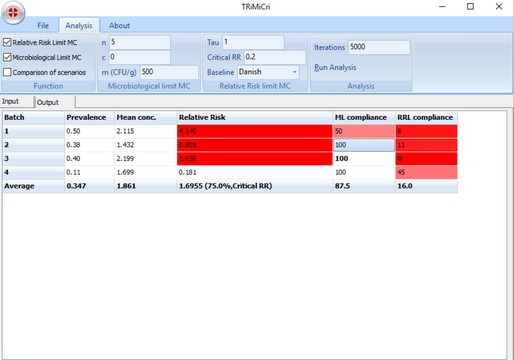
For this example, it can be seen that only for batch No. 4 the relative risk (risk of the batch divided by the baseline risk) is below 0.2. The Microbial Limit (ML) compliance is related with the probability that the batch is complying with the MC, while the RRL compliance is the probability that the batch is complying with the specified RRL.
The tool offers a comparison of scenarios to advanced users to evaluate the expected effect of implementation of a specified MC (in relation to a RRL or a ML).
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Andersen JK, Uyttendaele M, Valero A, Da Silva Felício MT, Messens W and Nørrung B, 2017. Scientific Opinion on the guidance on the requirements for the development of microbiological criteria. EFSA Journal 2017;15(11):5052, 60 pp. 10.2903/j.efsa.2017.5052
Requestor: EFSA
Question number: EFSA‐Q‐2016‐00227
Panel members: Ana Allende, Declan Bolton, Marianne Chemaly, Robert Davies, Pablo Salvador Fernández Escámez, Rosina Girones, Lieve Herman, Konstantinos Koutsoumanis, Roland Lindqvist, Birgit Nørrung, Antonia Ricci, Lucy Robertson, Giuseppe Ru, Moez Sanaa, Marion Simmons, Panagiotis Skandamis, Emma Snary, Niko Speybroeck, Benno Ter Kuile, John Threlfall and Helene Wahlström.
Acknowledgements: The Panel wishes to thank the members of the Working Group on ‘Guidance on the requirements for the development of microbiological criteria’: Ana Allende, Jens Kirk Andersen, Birgit Nørrung, Panagiotis Skandamis, Mieke Uyttendaele and Antonio Valero for the preparatory work on this scientific output and, the EFSA staff members: Maria Teresa Da Silva Felício and Winy Messens for the support provided to this scientific output.
Adopted: 19 October 2017
Notes
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs, OJ L 338, 22.12.2005, p. 1–26.
Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the control of salmonella and other specified food‐borne zoonotic agents, OJ L 325, 12.12.2003, p. 1–15.
A reclassification of Enterobacter sakazakii has been proposed in 2007 and it has been named Cronobacter species, which consists of a total of seven species/subspecies including Cronobacter sakazakii.
Regulation (EC) No 882/2004 of the European Parliament and of the Council of 29 April 2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules, OJ L 165, 30.4.2004, p. 1–141.
References
- Andersen JK, Nørrung B and da Costa Alves Machado S and Nauta M, 2015. A risk‐based microbiological criterion that uses the relative risk as the critical limit. Food Control, 58, 29–32. 10.1016/j.foodcont.2015.04.011 [DOI] [Google Scholar]
- Ayuso‐Gabella N, Page D, Masciopinto C, Aharoni A, Salgot M and Wintgens T, 2011. Quantifying the effect of managed aquifer recharge on the microbiological human health risks of irrigating crops with recycled water. Agricultural Water Management, 99, 93–102. 10.1016/j.agwat.2011.07.014 [DOI] [Google Scholar]
- Banach JL, Stratakou I, van der Fels‐Klerx HJ, Besten HMWd and Zwietering MH, 2016. European alerting and monitoring data as inputs for the risk assessment of microbiological and chemical hazards in spices and herbs. Food Control, 69, 237–249. 10.1016/j.foodcont.2016.04.010 [DOI] [Google Scholar]
- Barco L, Belluco S, Roccato A and Ricci A, 2015. A systematic review of studies on Escherichia coli and Enterobacteriaceae on beef carcasses at the slaughterhouse. International Journal of Food Microbiology, 207, 30–39. 10.1016/j.ijfoodmicro.2015.04.027 [DOI] [PubMed] [Google Scholar]
- Baylis CL and Petitt SB, 1997. The significance of coliforms Escherichia coli and the Enterobacteriaceae in raw and processed foods In: Kay D, Fricker C. (eds.). In Coliforms and E. coli problem or solution? Royal Society of Chemistry, Cambridge, UK: pp. 49–53. [Google Scholar]
- Boysen L, Nauta M and Rosenquist H, 2016. Campylobacter spp. and Escherichia coli contamination of broiler carcasses across the slaughter line in Danish slaughterhouses. Microbial Risk Analysis, 2, 63–67. 10.1016/j.mran.2016.05.005 [DOI] [Google Scholar]
- Busta FF, Suslow TV, Parish ME, Beuchat LR, Farber JN, Garrett EH and Harris LJ, 2003. The use of indicators and surrogate microorganisms for the evaluation of pathogens in fresh and fresh‐cut produce. Comprehensive Reviews in Food Science and Food Safety, 2, 179–185. 10.1111/j.1541-4337.2003.tb00035.x [DOI] [Google Scholar]
- CAC (Codex Alimentarius Commission), 1999. CAC/GL 30‐1999. Principles and guidelines for the conduct of MRA. Available online: http://www.fao.org/docrep/005/y1579e/y1579e05.htm
- CAC (Codex Alimentarius Committee), 2013. Principles and guidelines for the establishment and application of microbiological criteria related to foods. CAC/GL 21 1997, revised and renamed 2013. Available online: http://www.codexalimentarius.org/input/download/standards/394/CXG_021e.pdf
- Caipo M, Cahill S, Kenny M, Wachsmuth K, Toyofuku H, Hielm S, Carolissen V, Bruno A, Mulholland C, Kojima M and Esteban E, 2015. The development of illustrative examples for the establishment and application of microbiological criteria for foods and their role in international standard development. Food Control, 58, 1–6. 10.1016/j.foodcont.2015.06.043 [DOI] [Google Scholar]
- Ceuppens S, Li D, Uyttendaele M, Renault P, Ross P, Ranst MV, Cocolin L and Donaghy J, 2014. Molecular methods in food safety microbiology: interpretation and implications of nucleic acid detection. Comprehensive Reviews in Food Science and Food Safety, 13, 551–577. 10.1111/1541-4337.12072 [DOI] [PubMed] [Google Scholar]
- Ceuppens S, Johannessen GS, Allende A, Tondo EC, El‐Tahan F, Sampers I, Jacxsens L and Uyttendaele M, 2015. Risk factors for Salmonella, Shiga Toxin‐Producing Escherichia coli and Campylobacter occurrence in primary production of leafy greens and strawberries. International Journal of Environmental Research and Public Health, 12, 9809–9831. 10.3390/ijerph120809809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibin V, Mancin M, Pedersen K, Barrucci F, Belluco S, Roccato A, Cocola F, Ferrarini S, Sandri A, Lau Baggesen D and Ricci A, 2014. Usefulness of Escherichia coli and Enterobacteriaceae as Process Hygiene Criteria in poultry: experimental study. EFSA Supporting Publication 2014, 11(8), EN‐635, 121 pp. 10.2903/sp.efsa.2014.en-635 [DOI] [Google Scholar]
- Cole MB and Tompkin RB, 2005. Microbiological performance objectives and criteria In: Sofos J. (ed.). Improving the safety of fresh meat. Woodhead Publishing Ltd, Cambridge, England, pp. 673–695. [Google Scholar]
- Coleman ME, Marks HM, Golden NJ and Latimer HK, 2004. Discerning strain effects in microbial dose‐response data. Journal of Toxicology and Environmental Health. Part A, 67, 667–685. 10.1080/15287390490428134. [DOI] [PubMed] [Google Scholar]
- Dahms S, 2014. Microbiological sampling plans –Statistical aspects. Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und Hygiene, 95, 32–44. [Google Scholar]
- Danyluk MD and Schaffner DW, 2011. Quantitative assessment of the microbial risk of leafy greens from farm to consumption: preliminary framework, data, and risk estimates. Journal of Food Protection, 74, 700–708. 10.4315/0362-028x.Jfp-10-373 [DOI] [PubMed] [Google Scholar]
- De Keuckelaere A, Jacxsens L, Amoah P, Medema G, McClure P, Jaykus L‐A and Uyttendaele M, 2015. Zero risk does not exist: lessons learned from microbial risk assessment related to use of water and safety of fresh produce. Comprehensive Reviews in Food Science and Food Safety, 14, 387–410. 10.1111/1541-4337.12140 [DOI] [Google Scholar]
- Domenech E, Botella S, Ferrus MA and Escriche I, 2013. The role of the consumer in the reduction of Listeria monocytogenes in lettuces by washing at home. Food Control, 29, 98–102. 10.1016/j.foodcont.2012.05.074 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Opinion of the Scientific Panel on Biological Hazards on a request from the Commission related to the microbiological risks in infant formulae and follow‐on formulae. EFSA Journal 2004;2(11):113, 35 pp. 10.2903/j.efsa.2007.462 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on Biological Hazards on Bacillus cereus and other Bacillus spp in foodstuffs. EFSA Journal 2005;3(4):175, 48 pp. 10.2903/j.efsa.2007.462 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Panel on biological hazards (BIOHAZ) on microbiological criteria and targets based on risk analysis. EFSA Journal 2007;5(3):462, 29 pp. 10.2903/j.efsa.2007.462 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Scientific Opinion of the Panel on Biological hazards on a request from the European Commission on a quantitative microbiological risk assessment on Salmonella in meat: Source attribution for human salmonellosis from meat. EFSA Journal, 625, 1–32. [Google Scholar]
- EFSA (European Food Safety Authority), 2008b. Overview of methods for source attribution for human illness from food‐borne microbiological hazards ‐ Scientific Opinion of the Panel on Biological Hazards. EFSA Journal 2008;6(7):764, 43 pp. 10.2903/j.efsa.2008.764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008c. Scientific Opinion of the Panel on Biological Hazards on a request from the European Commission on Request for updating the former SCVPH opinion on Listeria monocytogenes risk related to ready‐to‐eat foods and scientific advice on different levels of Listeria monocytogenes in ready‐to‐eat foods and the related risk for human illness. EFSA Journal 2008;6(1):599, 42 pp. 10.2903/j.efsa.2008.764 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009a. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: General Principles. EFSA Journal 2009;7(5):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009b. Scientific Opinion of the Panel on Biological hazards on a quantitative estimation of the impact of setting a new target for the reduction of Salmonella in breeding hens of Gallus gallus . EFSA Journal, 1036, 1–68. [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008 ‐ Part A: Campylobacter and Salmonella prevalence estimates. EFSA Journal, 8, 1831–4732, 1503. 10.2903/j.efsa.2010.1503 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses, in the EU, 2008 ‐ Part B: Analysis of factors associated with Campylobacter colonisation of broiler batches and with Campylobacter contamination of broiler carcasses; and investigation of the culture method diagnostic characteristics used to analyse broiler carcass samples. EFSA Journal, 8, 1831–4732, 1522. 10.2903/j.efsa.2010.1522 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Use of the EFSA comprehensive European food consumption database in exposure assessment. EFSA Journal, 9, 1831–4732, 2097. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010a. Scientific Opinion on a quantitative estimate of the public health impact of setting a new target for the reduction of Salmonella in laying hens. EFSA Journal 2010;8(4):1546, 86 pp. 10.2903/j.efsa.2010.1546 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010b. Scientific Opinion on a quantitative microbiological risk assessment of Salmonella in slaughter and breeder pigs. EFSA Journal 2010;8(4):1547, 90 pp. 10.2903/j.efsa.2010.1547 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010c. Scientific Opinion on the link between Salmonella criteria at different stages of the poultry production chain. EFSA Journal 2010;8(3):1545, 66 pp. 10.2903/j.efsa.2010.1545 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011a. Scientific Opinion on a quantitative estimation of the public health impact of setting a new target for the reduction of Salmonella in broilers. EFSA Journal 2011;9(7):2106, 94 pp. 10.2903/j.efsa.2011.2106 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011b. Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA Journal 2011;9(4):2105, 141 pp. 10.2903/j.efsa.2011.2105 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011c. Scientific Opinion on Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA Journal 2011;9(10):2393, 93 pp. 10.2903/j.efsa.2011.2393 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011d. Scientific Opinion on the risk posed by Shiga toxin‐producing Escherichia coli (STEC) and other pathogenic bacteria in seeds and sprouted seeds. EFSA Journal 2011;9(11):2424, 101 pp. 10.2903/j.efsa.2011.2424 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011e. Scientific Opinion on an update on the present knowledge on the occurrence and control of foodborne viruses. EFSA Journal 2011;9(7):2190, 96 pp. 10.2903/j.efsa.2011.2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2012a. Scientific Opinion on an estimation of the public health impact of setting a new target for the reduction of Salmonella in turkeys. EFSA Journal 2012;10(4):2616, 89 pp. 10.2903/j.efsa.2012.2616 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2012b. Norovirus (NoV) in oysters: methods, limits and control options. EFSA Journal 2012;10(1):2500, 39 pp. 10.2903/j.efsa.2012.2500 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2012c. Scientific Opinion on the development of a risk ranking framework on biological hazards. EFSA Journal 2012;10(6):2724, 88 pp. 10.2903/j.efsa.2012.2724 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014a. Scientific Opinion on the risk posed by pathogens in food of non‐animal origin. Part 2 (Salmonella and Norovirus in leafy greens eaten raw as salads). EFSA Journal 2014;12(3):3600, 118 pp. 10.2903/j.efsa.2014.3600 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014b. Scientific Opinion on the risk posed by pathogens in food of non‐animal origin. Part 2 (Salmonella and Norovirus in berries). EFSA Journal 2014;12(6):3706, 95 pp. 10.2903/j.efsa.2014.3706 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014c. Scientific Opinion on the risk posed by pathogens in food of non‐animal origin. Part 2 (Salmonella in tomatoes). EFSA Journal 2014;12(10):3832, 75 pp. 10.2903/j.efsa.2014.3832 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014d. Scientific Opinion on the risk posed by pathogens in food of non‐animal origin. Part 2 (Salmonella in melons). EFSA Journal 2014;12(10):3831, 77 pp. 10.2903/j.efsa.2014.3831 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014e. Scientific Opinion on the risk posed by pathogens in food of non‐animal origin. Part 2 (Salmonella, Yersinia, Shigella and Norovirus in bulb and stem vegetables, and carrots). EFSA Journal 2014;12(12):3937, 91 pp. 10.2903/j.efsa.2014.3937 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2016. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA Journal 2016;14(7):4524, 93 pp. 10.2903/j.efsa.2016.4524 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority, European Centre for Disease Prevention), 2016. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2015. EFSA Journal 2016;14(12):4634, 231 pp. 10.2903/j.efsa.2016.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ) , Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, Girones R, Herman L, Koutsoumanis K, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Takkinen J, Wagner M, Arcella D, Da Silva Felicio MT, Georgiadis M, Messens W and Lindqvist R, 2017. Draft Scientific Opinion on Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. Draft Opinion endorsed for public consultation. 189 pp. Available online: https://www.efsa.europa.eu/sites/default/files/engage/170724-0.pdf, 2017.
- EFSA Scientific Committee , 2016. Revised draft guidance on uncertainty in EFSA scientific assessment. DRAFT. Available online: https://www.efsa.europa.eu/sites/default/files/160321DraftGDUncertaintyInScientificAssessment.pdf
- European Commission , 2006. Guidance document on official controls, under Regulation (EC) No 882/2004, concerning microbiological sampling and testing of foodstuffs.
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 2016. Statistical aspects of microbiological criteria related to foods. A risk managers guide. Microbiological Risk Assessment Series, no 24. Rome, 120 pp.
- Ghafir Y, China B, Dierick K, De Zutter L and Daube G, 2008. Hygiene indicator microorganisms for selected pathogens on beef, pork, and poultry meats in Belgium. Journal of Food Protection, 71, 35–45. [DOI] [PubMed] [Google Scholar]
- Gonzales‐Barron U, Zwietering MH and Butler F, 2013. A novel derivation of a within‐batch sampling plan based on a Poisson‐gamma model characterising low microbial counts in foods. International Journal of Food Microbiology, 161, 84–96. 10.1016/j.ijfoodmicro.2012.11.026 [DOI] [PubMed] [Google Scholar]
- Goulet V, Hebert M, Hedberg C, Laurent E, Vaillant V, De Valk H and Desenclos J‐C, 2012. Incidence of listeriosis and related mortality among groups at risk of acquiring listeriosis. Clinical Infectious Diseases, 54, 652–660. [DOI] [PubMed] [Google Scholar]
- Haagsma JA, Polinder S, Stein CE and Havelaar AH, 2013. Systematic review of foodborne burden of disease studies: quality assessment of data and methodology. International Journal of Food Microbiology, 166, 34–47. 10.1016/j.ijfoodmicro.2013.05.029 [DOI] [PubMed] [Google Scholar]
- Habib I, De Zutter L, Van Huffel X, Geeraerd AH and Uyttendaele M, 2012. Potential of Escherichia coli as a surrogate indicator for postchill broiler carcasses with high Campylobacter counts. Food Control, 25, 96–100. 10.1016/j.foodcont.2011.10.022 [DOI] [Google Scholar]
- Hamilton AJ, Stagnitti F, Xiong X, Kreidl SL, Benke KK and Maher P, 2007. Wastewater irrigation: the state of play. Vadose Zone Journal, 6, 823. [Google Scholar]
- Havelaar AH, De Hollander AE, Teunis PF, Evers EG, Van Kranen HJ, Versteegh JF, Van Koten JE and Slob W, 2000. Balancing the risks and benefits of drinking water disinfection: disability adjusted life‐years on the scale. Environmental Health Perspectives, 108, 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods), 1986. Microorganisms in foods 2 Sampling for microbiological analysis: principles and specific applications. Second edition. Blackwell Scientific Publications. Available online: http://www.icmsf.org/pdf/icmsf2.pdf
- ICMSF (International Commission on Microbiological Specifications for Foods), 2002. Microorganisms in Foods 7. Microbiological testing in food safety management. KluwerAcademic/Plenum Publisher, New York,. ISBN: 0306472627. 362 pp. [Google Scholar]
- ISO (International Organization for Standardization), 2006. ISO/TS 19036:2006 Microbiology of food and animal feeding stuffs ‐ Guidelines for the estimation of measurement uncertainty for quantitative determinations.
- ISO (International Organization for Standardization), 2009. ISO/TS 19036:2006/Amd 1:2009 Measurement uncertainty for low counts.
- Lee J, Castle M, Duncan G, Hathaway S, van der Logt P, Wagener S, LassoCruz A, Gichia M, Tebwe T and Silva U, 2015. Example of a microbiological criterion (MC) for verifying the performance of a food safety control system: Campylobacter performance target at end of processing of broiler chickens. Food Control, 58, 23–28. 10.1016/j.foodcont.2014.07.012 [DOI] [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty A, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) comprehensive European food consumption database. Food Additives & Contaminants. Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment, 28, 975–995. 10.1080/19440049.2011.576440 [DOI] [PubMed] [Google Scholar]
- Messens W, Vivas‐Alegre L, Bashir S, Amore G, Romero‐Barrios P and Hugas M, 2013. Estimating the public health impact of setting targets at the European level for the reduction of zoonotic Salmonella in certain poultry populations. International Journal of Environmental Research and Public Health, 10, 4836–4850. 10.3390/ijerph10104836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossel DA, Weenk GH, Morris GP and Struijk CB, 1998. Identification, assessment and management of food‐related microbiological hazards: historical, fundamental and psycho‐social essentials. International Journal of Food Microbiology, 39, 19–51. [DOI] [PubMed] [Google Scholar]
- Murray CJ and Acharya AK, 1997. Understanding DALYs (disability‐adjusted life years). Journal of Health Economics, 16, 703–730. [DOI] [PubMed] [Google Scholar]
- Nauta MJ, Sanaa M and Havelaar AH, 2012. Risk based microbiological criteria for Campylobacter in broiler meat in the European Union. International Journal of Food Microbiology, 158, 209–217. 10.1016/j.ijfoodmicro.2012.07.018 [DOI] [PubMed] [Google Scholar]
- Nauta M, Andersen JK, Tuominen P, Ranta J and Lindqvist R, 2015. Risk‐based microbiological criteria for Campylobacter in broiler meat: a comparison of two approaches. Food Control, 53, 177–184. 10.1016/j.foodcont.2015.01.019 [DOI] [Google Scholar]
- Ottoson JR, Nyberg K, Lindqvist R and Albihn A, 2011. Quantitative microbial risk assessment for Escherichia coli O157 on lettuce, based on survival data from controlled studies in a climate chamber. Journal of Food Protection, 74, 2000–2007. 10.4315/0362-028x.Jfp-10-563 [DOI] [PubMed] [Google Scholar]
- Paoli MG and Hartnett E, 2006. Overview of a risk assessment model for Enterobacter sakazakii in powdered infant formula. Available online: http://www.who.int/foodsafety/publications/micro/RA_Overview.pdf
- Pérez‐Rodríguez F, Carrasco E, Bover‐Cid S, Jofré A and Valero A, 2017. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 2, a quantitative risk characterization on L. monocytogenes in RTE foods; starting from the retail stage. EFSA Supporting Publication 2017:EN‐1252, 211 pp. 10.2903/sp.efsa.2017.en-1252 [DOI]
- Perrin F, Tenenhaus‐Aziza F, Michel V, Miszczycha S, Bel N and Sanaa M, 2015. Quantitative risk assessment of haemolytic and uremic syndrome linked to O157:H7 and non‐O157:H7 Shiga‐toxin producing Escherichia coli strains in raw milk soft cheeses. Risk Analysis, 35, 109–128. 10.1111/risa.12267 [DOI] [PubMed] [Google Scholar]
- Pouillot R, Klontz KC, Chen Y, Burall LS, Macarisin D, Doyle M, Bally KM, Strain E, Datta AR, Hammack TS and Van Doren JM, 2016. Infectious dose of Listeria monocytogenes in outbreak linked to ice cream, United States, 2015. Emerging Infectious Diseases, 22, 2113–2119. 10.3201/eid2212.160165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta J, Lindqvist R, Hansson I, Tuominen P and Nauta M, 2015. A Bayesian Approach to the Evaluation of Risk‐Based Microbiological Criteria for Campylobacter in Broiler Meat. Annals of Applied Statistics, 9, 1415–1432. 10.1214/15-Aoas845. [DOI] [Google Scholar]
- Sampers I, Jacxsens L, Luning PA, Marcelis WJ, Dumoulin A and Uyttendaele M, 2010. Performance of food safety management systems in poultry meat preparation processing plants in relation to Campylobacter spp. contamination. Journal of Food Protection, 73, 1447–1457. [DOI] [PubMed] [Google Scholar]
- van Schothorst M, Zwietering MH, Ross T, Buchanan RL and Cole MB, 2009. Relating microbiological criteria to food safety objectives and performance objectives. Food Control, 20, 967–979. 10.1016/j.foodcont.2008.11.005. [DOI] [Google Scholar]
- Scott VN, Powell M, Cabrera J, Carullo ME, Martinez I and Lohachoompol V, 2015. Development of microbiological criteria to assess the acceptability of a food lot – an example for milk powder. Food Control, 58, 12–16. 10.1016/j.foodcont.2014.09.026 [DOI] [Google Scholar]
- Seliwiorstow T, Uyttendaele M, De Zutter L and Nauta M, 2016. Application of TRiMiCri for the evaluation of risk based microbiological criteria for Campylobacter on broiler meat. Microbial Risk Analysis, 2, 78–82. 10.1016/j.mran.2016.05.001 [DOI] [Google Scholar]
- Stals A, Van Coillie E and Uyttendaele M, 2013. Viral genes everywhere: public health implications of PCR‐based testing of foods. Current Opinion in Virology, 3, 69–73. 10.1016/j.coviro.2012.11.003 [DOI] [PubMed] [Google Scholar]
- Suslow TV, Oria MP, Beuchat LR, Garrett EH, Parish ME, Harris LJ, Farber JN and Busta FF, 2003. Production practices as risk factors in microbial food safety of fresh and fresh‐cut produce. Comprehensive Reviews in Food Science and Food Safety, 2, 38–77. 10.1111/j.1541-4337.2003.tb00030.x [DOI] [Google Scholar]
- Uyttendaele M, Baert K, Ghafir Y, Daube G, De Zutter L, Herman L, Dierick K, Pierard D, Dubois JJ, Horion B and Debevere J, 2006. Quantitative risk assessment of Campylobacter spp. in poultry based meat preparations as one of the factors to support the development of risk‐based microbiological criteria in Belgium. International Journal of Food Microbiology, 111, 149–163. 10.1016/j.ijfoodmicro.2006.05.023 [DOI] [PubMed] [Google Scholar]
- Valero P, 2015. Predictive tools and strategies for establishing risk‐based microbiological criteria in foods. Food Safety Challenges for Mediterranean Products. Options Méditerranéennes (Series A: Mediterranean Seminars 2015‐ Number 111), ISSN: 1016‐1121‐X. ISBN: 1012‐85352‐85547‐85353.
- WHO (World Health Organization), 2015. WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007‐2015. Geneva, Switzerland.
- Zeitoun AAM, Debevere JM and Mossel DAA, 1994. Significance of Enterobacteriaceae as index organisms for hygiene on fresh untreated poultry, poultry treated with lactic acid and poultry stored in a modified atmosphere. Food Microbiology, 11, 169–176. 10.1006/fmic.1994.1020 [DOI] [Google Scholar]
- Zwietering MH and den Besten HMW, 2016. Microbial testing in food safety: effect of specificity and sensitivity on sampling plans—how does the OC curve move. Current Opinion in Food Science, 12, 42–51. 10.1016/j.cofs.2016.06.007 [DOI] [Google Scholar]
- Zwietering MH, Stewart CM and Whiting RC and International Commission on Microbiological Specifications for Foods (ICMSF) , 2010. Validation of control measures in a food chain using the FSO concept. Food Control, 21, 1716–1722. 10.1016/j.foodcont.2010.05.019 [DOI] [Google Scholar]
- Zwietering MH, Gorris LGM and Farber JM, 2015. Operationalising a performance objective with a microbiological criterion using a risk‐based approach. Food Control, 58, 33–42. 10.1016/j.foodcont.2014.07.042 [DOI] [Google Scholar]


