Abstract
Deoxynivalenol (DON) is a mycotoxin primarily produced by Fusarium fungi, occurring predominantly in cereal grains. Following the request of the European Commission, the CONTAM Panel assessed the risk to animal and human health related to DON, 3‐acetyl‐DON (3‐Ac‐DON), 15‐acetyl‐DON (15‐Ac‐DON) and DON‐3‐glucoside in food and feed. A total of 27,537, 13,892, 7,270 and 2,266 analytical data for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, respectively, in food, feed and unprocessed grains collected from 2007 to 2014 were used. For human exposure, grains and grain‐based products were main sources, whereas in farm and companion animals, cereal grains, cereal by‐products and forage maize contributed most. DON is rapidly absorbed, distributed, and excreted. Since 3‐Ac‐DON and 15‐Ac‐DON are largely deacetylated and DON‐3‐glucoside cleaved in the intestines the same toxic effects as DON can be expected. The TDI of 1 μg/kg bw per day, that was established for DON based on reduced body weight gain in mice, was therefore used as a group‐TDI for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. In order to assess acute human health risk, epidemiological data from mycotoxicoses were assessed and a group‐ARfD of 8 μg/kg bw per eating occasion was calculated. Estimates of acute dietary exposures were below this dose and did not raise a health concern in humans. The estimated mean chronic dietary exposure was above the group‐TDI in infants, toddlers and other children, and at high exposure also in adolescents and adults, indicating a potential health concern. Based on estimated mean dietary concentrations in ruminants, poultry, rabbits, dogs and cats, most farmed fish species and horses, adverse effects are not expected. At the high dietary concentrations, there is a potential risk for chronic adverse effects in pigs and fish and for acute adverse effects in cats and farmed mink.
Keywords: Deoxynivalenol, 3‐acetyl‐deoxynivalenol, 15‐acetyl‐deoxynivalenol, deoxynivalenol‐3‐glucoside, exposure, toxicity, human and animal risk assessment
Summary
In a request from the European Commission, the Panel on Contaminants in the Food Chain (CONTAM Panel) was asked to assess the risk to animal and human health related to the presence of the mycotoxins deoxynivalenol (DON), metabolites of DON and masked DON in food and feed. Taking into account the availability of data suitable for the risk assessment of DON and its acetylated and modified forms, the CONTAM Panel decided to assess the risk of DON, 3‐acetyl‐DON (3‐Ac‐DON), 15‐acetyl‐DON (15‐Ac‐DON) and DON‐3‐glucoside. The CONTAM Panel was asked to consider all relevant adverse acute and chronic health effects, addressing, in particular, co‐occurrence of DON with its acetylated and modified forms. Furthermore, to estimate the dietary (chronic and acute) exposure of the European population addressing, in particular, the consumption patterns of specific (vulnerable) groups of the population. The risk for different farm and companion animal species from exposure from feed should also be assessed.
The structurally related trichothecene‐mycotoxins, DON, 3‐Ac‐DON and 15‐Ac‐DON, are commonly found in Europe. They are produced by plant pathogenic fungi of the Fusarium genus growing on the cereals in the field, preferably at temperate climates. DON is one of the most widely studied mycotoxins worldwide and it co‐occurs often with other mycotoxins, particularly with other Fusarium toxins. DON‐3‐glucoside is a modified form of DON (also called masked DON) and is the main plant metabolite of DON. However, it was noted that other modified forms of DON have also been reported and more of them might be discovered in upcoming research. These four forms, DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, occur predominantly in cereal grains such as wheat, barley, oats, rye and maize.
Trichothecenes are characterised by a tetracyclic sesquiterpenoid 12,13‐epoxytrichothec‐9‐en ring structure. The epoxide group between C12 and C13 seems to account for many of the typical toxic effects of trichothecenes. They have been classified into four groups (A–D) based on their chemical structures, and type A and type B trichothecenes are predominant in food and feed. DON, 3‐Ac‐DON and 15‐Ac‐DON are type B trichothecenes.
The methods of analysis for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are well established and can be applied for cereals, food, feed and biological samples. Accurate quantification of DON, its acetylated forms and DON‐3‐glucoside is mostly carried out by liquid chromatography coupled with (multistage) mass spectrometry, presently often with a multianalyte approach. However, this methodology has not been formally validated through interlaboratory studies and proficiency tests have shown that considerable analytical variability exists in the determination of DON. Direct approaches (requiring standards) and indirect ones (based on the conversion to DON) have been reported for the determination of DON‐3‐glucoside, for which the direct approach is the preferred method. For DON, performance criteria for methods of analysis and certified reference materials (both reference matrices and reference calibrants) are available. Non‐certified calibrants are available for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. Immunochemical methods for DON provide rapid and economical alternatives to chromatography, but cross‐reactivity and matrix effects have not been fully considered. Recent progress in biomarker research has allowed the determination of DON and its metabolites in urine, primarily as DON‐glucuronides, by using single or multiple biomarker methods. However, the commercial sources for the standards of DON‐glucuronides are scarce and no (certified) reference materials are available for urinary DON biomarkers.
Occurrence data
Within the continuous data collection of EFSA, a total of 21,916, 4,000 and 1,621 analytical results of DON in food, feed and unprocessed grains of undefined end‐use, respectively, fulfilled the required quality criteria. For 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, fewer analytical results, which fulfilled the criteria, were submitted to EFSA. The numbers of results ranged from 430 for 15‐Ac‐DON in unprocessed grains of undefined end‐use to 11,944 for 3‐Ac‐DON in food.
For ‘Grains and grain‐based products’, which was the food category with the majority of analytical results, the proportion of left‐censored data (results below the limit of detection (LOD) or limit of quantification (LOQ)) was 52% for DON and 59% for DON‐3‐glucoside, but above 95% for both acetylated forms. ‘Cereal grains’ was the category with the majority of analytical results for feed and had only 47% of left‐censored data for DON, 80% for 15‐Ac‐DON, and about 95% for DON‐3‐glucoside and 3‐Ac‐DON. The proportions of left‐censored data for unprocessed grains of undefined end‐use ranged from 45% for DON to 96% for the other three forms. The highest mean concentrations of DON and the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were recorded for the categories ‘Products for special nutritional use’ and ‘Grains and grain‐based products’ for food, ‘Cereal straw’ for feed and ‘Grains as crops’ for unprocessed grains of undefined end‐use.
Because DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are mostly attached to the outer hull of the grain, cleaning, sorting, sieving and dehulling of grains lead to marked increases in concentrations of these toxins in cereal by‐products, e.g. bran. During baking and cooking, DON appears to be relatively stable but the results for 3‐Ac‐DON and 15‐Ac‐DON were inconclusive and studies on DON‐3‐glucoside showed inconsistent and varying results. Relevant increases of concentrations were observed during fermentation stages, while relevant reductions occurred during baking. Malting and brewing do not seem to lead to losses of DON and DON‐3‐glucoside and no increased concentrations were observed in by‐products of brewing. However, the ratio between DON and DON‐3‐glucoside concentrations changed during the process due to a notable increase of the concentrations of DON‐3‐glucoside as compared to DON. Studies on the fate of 3‐Ac‐DON and 15‐Ac‐DON during malting and brewing were too limited to draw conclusions. The data on feed processing were limited but since many of the processes applied to cereal grains for food production are also applied to grains used for animal feeds, the findings for food should also apply to feed.
The CONTAM Panel estimated the relative ratios of concentrations of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to DON as 10%, 15% and 20%, respectively, and noted that they concurred with observations in feed and unprocessed grains of undefined end‐use, except for ‘Alcoholic beverages’ (‘Beer and beer‐like beverage’) where the ratio of DON‐3‐glucoside to DON was estimated as 80%. Overall, the concentration ratios varied considerably both for data reported to EFSA and those reported in the literature.
Human exposure assessment
Based on toxicological considerations (see below), the CONTAM Panel decided to assess the exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. The mean acute human exposure across 39 different dietary surveys and all age groups ranged from 0.2 to 2.9 μg/kg body weight (bw) per day for the minimum lower bound (LB) to the maximum upper bound (UB). The 95th percentile acute exposure ranged from 0.7 to 6.7 μg/kg bw per day. Infants showed the highest acute dietary exposure. The mean chronic human exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across 33 different dietary surveys and all age groups ranged from 0.2 to 2.0 μg/kg bw per day (minimum LB–maximum UB), and the 95th percentile chronic exposure ranged from 0.5 to 3.7 μg/kg bw per day (minimum LB–maximum UB). Again infants showed the highest mean chronic dietary exposure. The highest contributions to the exposure of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside came from grain‐based products, especially ‘Bread and rolls’, ‘Fine bakery wares’ and ‘Pasta (raw)’.
The limited consumption data available for vegetarians did not indicate a significant difference in the dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and 3‐DON‐glucoside between the vegetarians and the general population.
The CONTAM Panel noted that the overall human dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was mainly driven by DON.
Toxicokinetics and toxicity in rodents and human data
Rat gut intestinal microflora metabolised DON to the less toxic metabolite DOM‐1 and transformed acetylated DON to DON ex vivo. Gut microfloral transformation of DON or DON‐3‐glucoside into DOM‐1 was less evident in humans than in rats. DON was able to cross the placenta and reach the fetus. The oral bioavailability of DON has not been quantified in mice, but plasma, tissue and urine concentrations indicate that the absorption is high and maximum plasma concentrations are reached rapidly. DON was rapidly distributed to the tissues, e.g. liver, kidney, spleen and heart in rodents, reaching the maximum concentrations at about the same time as in plasma with exception for the brain, where the peak of total concentrations of DON and metabolites was reached later and at a lower concentration than in other organs. In mice, the distribution pattern of DON was independent of age and the tissue concentrations decreased rapidly after the peak concentration was reached. The concentrations were higher in weanling than in young adult mice. In rats, DON was rapidly excreted in faeces and urine. A significant biliary excretion was found in only one study ex vivo. In rat faeces, DON sulfonates were shown to be major metabolites exceeding the contribution from DON glucuronides. There is evidence that 3‐Ac‐DON and 15‐Ac‐DON were largely deacetylated prior to systemic distribution. DON‐3‐glucoside appears to be excreted solely as DON. Limited human data suggested that approximately 70% of ingested DON is excreted via urine, of which about 80% was in conjugated forms, mainly as DON‐15‐glucuronide that was about threefold more efficiently formed than DON‐3‐glucuronide.
After a single oral exposure to DON, feed refusal appeared very quickly in mice. Previous risk assessments of DON conducted by the Scientific Committee on Food (SCF) in 1999 and by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 2001 identified a no‐observed‐adverse‐effect level (NOAEL) of 0.04 mg DON/kg bw from subacute and subchronic toxicity studies. The CONTAM Panel reviewed studies that were published since the previous assessments and did not identify new information that changed this NOAEL of 0.04 mg DON/kg bw. Only one chronic toxicity study on DON was identified from which a NOAEL of 0.1 mg/kg bw per day was derived using data on reduced feed intake and reduced body weight gain in mice. This is in line with previous evaluations from the SCF and JECFA. No subacute, subchronic and chronic toxicity data on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified.
The available data did not show any haemato‐ or myelotoxicity of DON and differs in that respect from other trichothecenes. The data on neurotoxicological effects of DON were limited, and the CONTAM Panel did not identify any dose–response data suitable for hazard characterisation or any link with the data on the mode of action of DON. For carcinogenicity of DON, no new data were identified since the previous assessments in which the only available long‐term study did not indicate carcinogenic properties of DON in mice. For 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, the CONTAM Panel did not identify data on chronic toxicity, haemato‐ and myelotoxicity, neurotoxicity and carcinogenicity.
DON impacts the immune response in vitro and in vivo. Subchronic studies performed in mice and in farm animals indicate that DON exposure induces an increase in the plasmatic level of immunoglobulin A (IgA) but it could not be associated with IgA nephropathy. The effects on the immune response lead to an increased susceptibility to infectious diseases at medium to high doses of DON (8–10 mg/kg bw). Studies on immune response of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were scarce.
Oral exposure to DON exhibits both developmental and reproductive toxicity in experimental animals rats including reduced fertility, embryotoxicity, skeletal abnormalities, effects on body weight and relative epididymal weight and postnatal mortality. No data were identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
DON is genotoxic in vitro and the data available on the genotoxicity of DON in vivo were inconclusive. The available evidence suggests that oxidative stress may be involved in the mechanism of genotoxicity, rather than a direct interaction of DON (adduct formation) with cellular DNA. Similar to DON, 3‐Ac‐DON was inactive in a bacterial mutation assay and no data on in vitro genotoxicity tests with 15‐Ac‐DON or DON‐3‐glucoside were identified. No in vivo genotoxicity studies on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified.
Human outbreaks from acute exposure to DON have been repeatedly reported in Asia, with symptoms including nausea, vomiting, diarrhoea, abdominal pain, headaches, dizziness, fever, and in severe cases, bloody stool. No lethality was reported. However, the evidence of adverse health effects in humans due to chronic exposure to DON is lacking.
The CONTAM Panel noted that DON urinary biomarkers have been frequently investigated in several European populations and that these correlate well with dietary DON exposure. The available human biomarker studies suggested ubiquitous exposure to DON in many populations. The CONTAM Panel concluded that the exposure estimates, derived from the biomarker data from three European countries, were of the same order of magnitude as the exposure estimates for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside derived from the occurrence data reported to the European Food Safety Authority (EFSA) and the dietary surveys from those countries. However, several factors were identified that contributed to the uncertainty of exposure estimates of DON based on human DON biomarker data.
Concerning the mode of action, DON binds to ribosomes, leading to inhibition of protein synthesis and subsequently also RNA and DNA synthesis. This binding also induces ribotoxic stress and activates different mitogen‐activated protein kinases (MAPKs). Activation of MAPKs explains several effects of DON, such as apoptosis or survival of cells, inflammatory effect and oxidative stress. Two major mediators of DON‐induced anorexia/emesis have been described: pro‐inflammatory cytokines and secretion of satiety hormones, which activate receptors in the abdominal vagus afferent. The data on the mechanism of action of 3‐Ac‐DON and 15‐Ac‐DON were scarce but suggested an activation of MAPK and an induction of inflammatory cytokine and satiety hormones. Because of steric hindrance DON‐3‐glucoside cannot bind to the ribosome, and thus, it neither activates MAPKs nor it induces inflammation.
Only few studies compared the toxicity of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in the same experiment. In mink, DON, 3‐Ac‐DON and 15‐Ac‐DON were found to display similar toxicity in terms of anorectic and inflammatory effects, however the emetic capacity of 3‐Ac‐DON was substantially lower compared to DON and 15‐Ac‐DON. In porcine and human intestinal cells, the cytotoxic potency ranked in the order of 15‐Ac‐DON > DON > 3‐Ac‐DON and that was similar for barrier function, MAPK activation and expression of tight junction, and histological alterations. DON‐3‐glucoside was considerably less toxic than DON in both in vitro and in vivo studies.
The available database on effects of combined exposure to DON and other mycotoxins was weak and insufficient for establishing the nature of combined effects. In addition, no in vivo studies on the combined effects of 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside with other mycotoxins were identified.
Accounting all toxicokinetic and toxicity information available, the CONTAM Panel noted that 3‐Ac‐DON and 15‐Ac‐DON were largely deacetylated to DON prior to systemic distribution, such that they might induce the same acute and chronic effects as DON. The available data indicate that DON‐3‐glucoside can be cleaved to DON by bacteria in the gastrointestinal tract and distributed, metabolised and excreted similarly to DON. While the mode of action and the toxicity data for 3‐Ac‐DON and 15‐Ac‐DON indicated a similar toxicity as that of DON, toxicity data for DON‐3‐glucoside were limited and in vivo data on chronic toxicity were missing with the consequence that the CONTAM Panel could not make a firm conclusion on the hazard of DON‐3‐glucoside and could also not compare it with that of DON and the two acetylated forms. Therefore, the CONTAM Panel applied a conservative approach assuming that (1) 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are all metabolised to DON and absorbed at the same extent as DON, (2) the acetylated forms of DON induce the same acute and chronic adverse health effects as DON and (3) similar acute and chronic adverse health effects of DON‐3‐glucoside as DON cannot be excluded. Therefore, the CONTAM Panel decided to characterise the hazard for the group of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside together, both for chronic and for acute adverse health effects in humans and farm and companion animals.
The CONTAM Panel identified vomiting as critical acute effect in humans. Since studies from experimental‐ and farm animals could not provide a basis for an acute reference dose (ARfD) the CONTAM Panel decided to use the human data on vomiting and gastrointestinal effects, collected in a number of epidemiological studies on the outbreaks of acute mycotoxicosis in Asia, for the human hazard characterisation. Despite the limitations in the available human data, the CONTAM Panel decided to use these data to establish a group ARfD for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. In addition, the available human biomarker data were exploited as supporting information.
Based on adverse gastrointestinal effects identified from the human outbreak data in China and noting that vomiting occurred within 30 min after an eating occasion, the CONTAM Panel calculated a NOAEL of 26 μg DON/kg bw for a single eating occasion. By applying a default uncertainty factor of 3.16 for toxicodynamic kinetic differences in the intra‐human population variability, a group ARfD of 8 μg/kg bw per eating occasion was determined. The CONTAM Panel concluded that the dose range calculated from the human biomarker data supported this reference dose.
In the absence of data on chronic effects in humans, the CONTAM Panel identified reduced body weight gain in experimental animals as the critical chronic effect for human risk assessment. A clear dose–response relationship between the exposure to DON and mean body weight was observed both for female and male mice. The CONTAM Panel combined the data from both sexes to calculate a BMDL05 of 0.11 mg/kg bw per day for reduced body weight gain, and established a group tolerable daily intake (TDI) of 1 μg/kg bw per day using the default uncertainty factor of 100 for inter‐ and intraspecies variability for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
Risk characterisation for humans
Based on the available occurrence data, the estimates of acute dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were below the group ARfD of 8 μg/kg bw per eating occasion for all age groups of humans, and considered not an acute health concern.
The estimates of the UB chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside based on the available occurrence data were above the group TDI of 1 μg/kg bw per day for the infants, toddlers and other children, and to some extent also for adolescents and adults. At the LB, estimates only high exposure in toddlers and other children exceeded the group TDI. Regular exceedance of the group TDI indicates a potential health concern, however, the CONTAM Panel noted the uncertainty associated with exposure estimates due to a high fraction of left‐censored data.
There are limited data on dietary habits of vegetarians, with data available for only five European countries, and with very few subjects in four of them. These limited data did not indicate notable differences in acute and chronic dietary exposure between the vegetarians and the general population. Therefore, the conclusions on the general population remain valid also for the subpopulation of vegetarians.
Farm and companion animal exposure assessment
In contrast to the human exposure assessment, the farm and companion animal exposure was presented as dietary concentrations because the animal risk assessment was carried out on a concentration–response basis, except for the farmed mink for which the acute dietary exposure was presented on a dose‐response basis expressed per kg bw. The animal exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was primarily from the consumption of cereal grains, cereal by‐products and forage maize.
For lactating dairy cows and beef cattle, the estimated mean dietary concentrations to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside ranged from 64.2 to 996 μg/kg diet (lowest LB to highest UB). The estimated 95th percentile dietary concentrations ranged from 124 to 3,655 μg/kg diet. For small ruminants, the lowest LB and highest UB mean dietary concentrations were 151 and 414 μg/kg diet, respectively, and the estimated 95th percentile dietary concentrations 561 and 849 μg/kg diet, respectively.
For pigs, the estimated lowest LB and highest UB mean dietary concentrations were 437 and 618 μg/kg diet, respectively, and the estimated 95th percentile dietary concentrations 1,284 and 1,302 μg/kg diet, respectively.
For poultry, the lowest LB and highest UB mean dietary concentrations were 794 and 1,494 μg/kg diet, respectively, and the 95th percentile estimates of dietary concentrations 2,900 and 3,971 μg/kg diet, respectively. For horses, only mean dietary concentrations could be estimated and they were 155 (LB) and 253 (UB) μg/kg diet.
For farmed fish (salmonids and carp), the estimated lowest LB and highest UB mean dietary concentrations were 83.3 and 388 μg/kg diet, respectively, and the 95th percentile estimates of dietary concentrations 362 and 1,151 μg/kg diet, respectively.
For farmed rabbits, the LB and UB mean dietary concentrations were 196 and 282 μg/kg diet, respectively, and the estimated 95th percentile dietary concentrations 1,048 and 1,135 μg/kg diet, respectively.
For farmed mink, the estimated LB and UB mean dietary concentrations were 99.5 and 109 μg/kg diet, respectively, and the 95th percentile dietary concentrations 407 μg/kg diet (equivalent to 14.7 μg/kg bw per day) and 409 μg/kg diet (equivalent to 14.8 μg/kg bw per day), respectively.
For cats, the estimated LB and UB mean dietary concentrations were 229 and 264 μg/kg diet, respectively, and the estimated 95th percentile dietary concentrations 968 and 975 μg/kg diet, respectively. The values for dogs were somewhat lower with the LB and UB mean dietary concentrations being 174 and 214 μg/kg diet, respectively, and the 95th percentile dietary concentrations being 741 and 753 μg/kg diet, respectively.
The CONTAM Panel noted that the overall dietary concentrations of farm and companion animals to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were mainly driven by DON.
Toxicokinetics and toxicity in farm and companion animals
Intestinal absorption and metabolism of DON vary largely between different farm animal species and this may depend on the location of the consecutive intestinal segments, regional pH and activity of bacteria. Localisation of the gut bacteria prior or after the small intestine affects the bioavailability of ingested DON and its metabolites.
In cows, DON is extensively transformed to the less toxic de‐epoxidised metabolite DOM‐1 by the ruminal flora and only very minor amounts (< 1%) of DON reach systemic circulation. The proportion of DOM‐1 glucuronide in serum is high. The urine seems to be the main route of excretion. No relevant toxicokinetic data were identified for 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside. While in healthy ruminants DON is converted into DOM‐1 by the rumen flora, the toxicokinetics could be different in ruminants with acidosis or in young animals such as calves, for which the ruminal system is not fully functioning. In sheep, DON exhibited a systemic bioavailability and was quickly absorbed. DON and its metabolites were excreted primarily via the urinary but also via biliary routes. One study in lambs showed that 3‐Ac‐DON, administrated intraruminal, was rapidly converted to DON and partly excreted in the urine. No data were identified for goats.
The absorption of DON in pigs was generally high and DON was extensively distributed with a plasma elimination half‐life that varied from 1 to 4 h. DON and its metabolites were excreted primarily via the urinary but also via biliary routes. 3‐Ac‐DON was rapidly deacetylated in the upper intestinal tract and absorbed exclusively as DON. The limited data on DON‐3‐glucoside indicated that it had two times lower bioavailability than DON, and it could be concluded that DON‐3‐glucoside was only absorbed as DON. An extensive cleavage of DON‐3‐glucoside is likely to occur, primarily by the microflora in the gastrointestinal tract. No data were identified for 15‐Ac‐DON.
For poultry, a low degree of absorption of DON into plasma and a rapid metabolism and clearance from plasma was observed. The only available study on broiler chickens indicated a nearly complete hydrolysis of 3‐Ac‐DON and a partial hydrolysis of 15‐Ac‐DON to DON. Therefore, the CONTAM Panel assumed that 3‐Ac‐DON is absorbed as DON to a larger extent than 15‐Ac‐DON. The single available study on broiler chickens indicated that DON‐3‐glucoside was not hydrolysed to DON and its oral bioavailability was lower than that of DON.
Limited data on horses indicated a rapid clearance of DON in plasma with a major portion present as glucuronide. DON may reach the systemic circulation only at low concentrations. DOM‐1 was observed in serum and it correlated with DON exposure. No data were identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in horses.
Transfer of DON from feed to food of animal origin was only identified for dairy cows, pigs and poultry, and a substantial contribution of the residues of DON in products of animal origin to human exposure was considered to be unlikely. No information on the transfer of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was identified.
Focussing on studies using the highest DON concentrations in feed combined with the longest periods of time, the CONTAM Panel identified a NOAEL of 5 mg DON/kg feed for dairy cows because no adverse effects on body weight, feed intake, milk yield or any other adverse effect were observed over a period of 13 weeks. In heifers and steers, levels of 10 and 18 mg DON/kg feed, respectively, did not generate any adverse effects on feed intake, average daily weight gain and feed efficiency, and these levels were identified as not leading to effects. For sheep, the CONTAM Panel identified a NOAEL of 16 mg DON/kg feed for reduced feed intake and reduced body weight gain. In the absence of data for goats, the lowest NOAEL of 5 mg DON/kg feed of cows was adopted for them. The CONTAM Panel noted that young animals such as calves with not fully developed rumen and adult animals with a previous history of ruminal acidosis may have less effective de‐epoxidation and, consequently, could be more susceptible to the toxic effects of DON.
Reduced feed intake and reduced body weight gain were the most relevant chronic adverse effects of DON in pigs. However, DON may cause several other adverse effects in pigs including lesions in the oesophageal region of the stomach, in the liver, the lungs and the kidneys and changes in different clinical chemistry parameters (plasma nutrients and plasma enzyme activities). Several studies described also an impact of DON on immune responses in pigs but types and the sizes of those responses could not be associated with relevant adverse immunological effects in pigs. From the available data, the CONTAM Panel identified for the acute adverse health effects a lowest‐observed‐adverse‐effect level (LOAEL) of 2.8 mg DON/kg feed for vomiting. For reduced feed intake and reduced weight gain reduction, identified as the critical chronic adverse health effects of DON in pigs, wide ranges of NOAEL and LOAEL values were reported from which an overall NOAEL of 0.7 mg DON/kg feed was identified by the CONTAM Panel.
In broiler chicken, levels of 4.6–7 mg DON/kg feed did not generate any adverse effects, but levels of 10–12 mg DON/kg feed did, including reduced feed intake and reduced body weight gain. Thus, the CONTAM Panel identified 5 mg DON/kg feed as a NOAEL for broiler chicken. For laying hens, diets with DON concentrations up to 18 mg DON/kg feed did not induce any negative impact on body weight gain, hatchability and egg production in some studies. However, a diet of 10–13 mg DON/kg feed induced a decrease of feed intake at an early stage of the experiment, and a decrease of relative weights of spleen and gizzard and egg fertility. In other studies diets containing 5 mg DON/kg feed did not affect body weight gain, hatchability and egg fertility. Therefore, the CONTAM Panel identified 5 mg DON/kg feed as a NOAEL for laying hens. For ducks and turkeys, the CONTAM Panel identified a NOAEL of 7 mg DON/kg feed because no changes in body weight, weight gain, feed intake or feed conversion ratios were observed at this concentration.
For horses, the CONTAM Panel confirmed the previous NOAEL of 36 mg DON/kg feed for reduced feed intake. In rabbits a concentration of 30 mg DON/kg feed was associated with maternal and fetal body weight reduction, while a concentration of 15 mg DON/kg feed did not induce any adverse effects in rabbit fetuses. Since a concentration of 4.3 mg DON/kg feed did not induce any effects on feed intake, body weight gain and relative organ weights the NOAEL of 4 mg DON/kg feed was identified for rabbits.
The limited data on chronic adverse effects in farmed fish did not allow the identification of specific NOAELs/LOAELs for each fish species. In carp, the concentration of 0.95 mg DON/kg feed induced lipid peroxidation and histopathological changes in the liver and in rainbow trout 0.8 mg DON/kg feed reduced feed intake, weight gain, growth rate and feed efficiency. The lowest NOAEL was 0.6 mg DON/kg feed observed for carps and the CONTAM Panel decided to use 0.6 mg DON/kg feed as an overall NOAEL for farmed fish.
For vomiting as acute effect in farmed mink, BMDL10 values of 0.004, 0.05 and 0.004 mg/kg bw per day were calculated for DON, 3‐Ac‐DON and 15‐Ac‐DON, respectively. From the available subacute toxicity study on mink, a NOAEL of 1 mg DON/kg feed was identified for the reduced feed intake and body weight gain in farmed mink as reference point for chronic adverse effects.
For dogs, one study on acute effects was available. Vomiting was not reported at the concentration of 6 mg DON/kg feed, which was identified as the NOAEL for acute effects, while reduced body weight gain in dogs was observed at this level. Application of the BMD approach resulted in a lowest BMCL10 of 5 mg DON/kg feed for vomiting as reference point of acute effects in dogs. For chronic effects in dogs, the identified NOAEL for reduced body weight gain was 4 mg DON/kg feed.
The same study on dogs was also the only available study for acute and chronic adverse effects in cats. Vomiting was not reported at the concentration of 8 mg DON/kg feed and this was identified as the NOAEL for acute effects, while reduced body weight gain in cats was observed at this level. Application of the BMD approach resulted in a lowest BMCL10 of 1 mg DON/kg feed for vomiting as reference point of acute effects. For chronic effects in cats, the identified NOAEL for reduced body weight gain was 6 mg DON/kg feed.
Risk characterisation for farm and companion animals
For the farm and companion animals, the CONTAM Panel characterised the chronic animal health risk for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside using the dietary concentration estimates at the UB mean and UB 95th percentile based on feed composition and the available occurrence data on feeds and compared them with the identified NOAELs. For characterising the acute animal health risk, the calculated UB 95th percentile dietary concentrations (dietary exposure in case of farmed mink) were used and compared with the respective NOAEL or BMDL/BMCL10 for pigs, farmed mink, dogs and cats, for whom vomiting was reported.
The dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for ruminants and horses were clearly below the identified NOAELs for chronic adverse effects and are therefore considered unlikely to be a health concern, while for poultry and farmed rabbits the estimated dietary concentrations indicated that the risk of chronic adverse health effects from the feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is low.
For pigs, the estimated dietary concentrations indicated that the risk of acute adverse health effects is low, while a possible risk of chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was identified at the 95th percentile dietary concentrations. Also for farmed fish (salmonids and carp), the estimated dietary exposures indicated that possible risk of chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was identified for carp. However, the CONTAM Panel noted that the diet composition of different fish species may majorly differ and some fish species might be more tolerant.
For dogs, the risk of acute and chronic adverse health effects is low. Also for cats and farmed mink, the estimated dietary concentrations indicated that the risk for chronic adverse health effects is low, while a possible risk of acute adverse effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was identified for farmed mink and cats.
Recommendations
The CONTAM Panel concluded that although the impact of the uncertainties in the human risk assessment of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is large, the risk is more likely to be over‐ than under‐estimated. The impact of the uncertainties in the risk assessment of farm and companion animals is large. Therefore, the CONTAM Panel recommends interlaboratory validation and standardisation of liquid chromatography with tandem mass spectrometric (LC–MS/MS) methodology for the simultaneous quantification of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. Certified reference materials should be made available and proficiency tests should be facilitated. The CONTAM Panel also recommends studies on the co‐occurrence of these four forms of DON, where each food and feed sample is analysed for all the four forms, and monitoring of the co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed to acquire knowledge of possible trends, e.g. due to climate change or technological processing. With regard to human biomarkers of exposure, interlaboratory‐validation and standardisation of the methods for the analysis of urinary DON biomarkers are recommended and certified reference materials should be made available, too. Also, well‐designed quantitative studies on DON urinary excretion in different human sub‐population groups should be encouraged to enable the use of DON biomarkers for human exposure assessments. The CONTAM Panel further recommends to conduct well‐designed studies, which take into account practical feeding conditions for farm and companion animals to study toxicokinetics and toxicity of the four forms of DON. In addition, modified forms of DON, other than those covered in this risk assessment, which could be potentially relevant concerning their (co‐)occurrence and toxicological properties, should be investigated to further refine the human and animal risk assessment.
Background as provided by the European Commission
The Scientific Committee on Food (SCF) adopted a scientific opinion on deoxynivalenol (DON)1 on 2 December 1999, establishing a tolerable daily intake (TDI) of 1 μg/kg bw.
At the request of the Commission, the European Food Safety Authority (EFSA) adopted a scientific opinion on DON as undesirable substance in animal feed2 in 2 June 2004.
Maximum levels for DON were set by Commission Regulation (EC) No 1881/20063.
At that time it was considered not necessary, due to co‐occurrence, to consider specific measures for the metabolites 3‐acetyl deoxynivalenol (3‐Ac‐DON) and 15‐acetyl deoxynivalenol (15‐Ac‐DON), as the measures on DON would also protect the human population from an unacceptable exposure from 3‐Ac‐DON and 15‐Ac‐DON.
Guidance values have been established for DON in animal feed by Commission Recommendation 2006/576/EC4.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated DON at its 72nd meeting in 2010.
The JECFA decided to convert the provisional maximum tolerable daily intake (PMTDI) for DON to a group PTMDI of 1 μg/kg bw for DON and its metabolites 3‐Ac‐DON and 15‐Ac‐DON as 3‐Ac‐DON is converted to DON in vivo and therefore contributes to the total DON‐induced toxicity. The Committee considered the toxicity of the metabolites to be equal to that of DON. The JECFA concluded that there was insufficient information to include DON‐3‐glucoside in the group PMTDI.
The JECFA derived a group acute reference dose (ARfD) of 8 μg/kg bw for DON and its metabolites using the lower limit on the benchmark dose for a 10% response (BMDL10) of 210 μg/kg bw per day for emesis in pigs. Limited data from human case reports indicated that dietary exposures to DON up to 50 μg/kg bw per day are not likely to induce emesis.
In view of the recent JECFA evaluation, it is appropriate for EFSA to assess the acute toxicity of DON in addition to the chronic toxicity and to assess the toxicity of the DON metabolites, in particular the acetylated derivatives. In recent years, findings on the presence of masked DON, in particular DON‐3‐glucoside has been reported. The toxicity of these masked forms, in particular DON‐3‐glucoside, has to be assessed.
Terms of Reference as provided by the European Commission
In accordance with Art. 29 (1) (a) of Regulation (EC) No 178/2002, the Commission asks EFSA for a scientific opinion on the risks to animal and human health related to the presence of deoxynivalenol (DON), metabolites of DON and masked DON in food and feed.
The scientific opinion should, inter alia, comprise the:
evaluation of the toxicity of DON, metabolites of DON and masked DON for animals and humans, considering all relevant adverse acute and chronic health effects;
assessment of the co‐occurrence of DON with metabolites of DON and masked DON in feed and food;
estimation of the dietary exposure (chronic and acute dietary exposure) of the European Union (EU) population to DON, metabolites of DON and masked DON, including the consumption patterns of specific (vulnerable) groups of the population (e.g. high consumers, children, people following a specific diet, etc.);
estimation of the exposure of the different animal species to DON, metabolites of DON and masked DON from feed;
assessment of the acute and chronic human health risks for the EU population including for specific (vulnerable) groups of the population as the consequence of the estimated dietary exposure;
assessment of the animal health risks for the different animal species as the consequence of the estimated exposure from animal feed.
Assessment
1. Introduction
Deoxynivalenol (DON) belongs to the large group of mycotoxins called trichothecenes, which represent the main group of Fusarium toxins. DON occurs predominantly in cereal grains such as wheat, barley, oats, rye and maize. DON is predominantly produced by the plant pathogenic fungi of the Fusarium genus, mainly by Fusarium graminearum and Fusarium culmorum. These fungi grow on the cereals in the field, preferably at temperate climates as they are commonly found in Europe (Marin et al., 2013). Crop infection by Fusarium is dependent on the weather and is favoured by high humidity at the time of flowering (WHO, 2001). Cereal grains intended for food and feed may also become contaminated during storage. DON is chemically stable and to some extent resistant to thermal processing (Kabak, 2009). As a result, DON is found in cereal‐based foods (Schothorst et al., 2005; Sirot et al., 2013) and feeds (Döll and Dänicke, 2011; Streit et al., 2012) ready for consumption.
Ingestion of highly contaminated feed by animals can lead to acute gastrointestinal symptoms such as vomiting (emesis), feed refusal and bloody diarrhoea. Because of its ability to induce acute vomiting in pigs (Vesonder et al., 1973), DON has also been assigned the trivial name ‘vomitoxin’. The most common effects of long‐term dietary exposure of animals to DON are weight gain suppression and anorexia. DON has been involved in a number of incidents of human intoxication in Asia and its acute effects in humans are similar to those in animals.
DON can co‐occur in grains and in cereal‐based food and feed together with its acetyl derivatives 3‐acetyl deoxynivalenol (3‐Ac‐DON), 15‐acetyl deoxynivalenol (15‐Ac‐DON) and 3,15‐diacetyl‐deoxynivalenol (3,15‐Ac‐DON). Both DON and its acetylated forms are produced by fungi such as F. graminearum and F. culmorum as toxic secondary metabolites and are therefore regarded as free or unmodified mycotoxins (Berthiller et al., 2013; Varga et al., 2013; Rychlik et al., 2014). Moreover, different Fusarium strains can produce different patterns of mycotoxins (chemotype). As an example, 3‐Ac‐DON and 15‐Ac‐DON chemotypes of F. graminearum produce DON together with 3‐Ac‐DON, and DON together with 15‐Ac‐DON, respectively, and their distribution varies with the geographical location and years of sampling (Gilbert et al., 2014). However, the acetyl derivatives of DON have been reported to occur at much lower levels than the parent compound DON (Usleber et al., 1996; Pestka, 2010a; FAO/WHO, 2011) but they also exert toxicity (Pinton and Oswald, 2014). Furthermore, the CONTAM Panel noted that 3,15‐Ac‐DON has been reported to occur together with DON, however, at much lower levels than 3‐Ac‐DON and 15‐Ac‐DON (Usleber et al., 1996).
Plants infected by mycotoxin‐producing fungi can alter the chemical structure of the mycotoxins resulting in (at least in part) extractable conjugated and/or non‐extractable bound mycotoxins. These altered mycotoxins were usually not detected when analysing food and feed for the mycotoxins they originated from, and therefore they have been commonly referred to as ‘masked mycotoxins’ (Gareis et al., 1990). DON‐3‐glucoside, the main plant metabolite of DON, is considered to be a masked mycotoxin and has been detected in cereal grains and cereal‐based products (Berthiller et al., 2013; Varga et al., 2013). This is an example of detoxification by glycosylation in the plant. Glycosylation converts DON into a glucoside that is unable to inhibit protein synthesis of plant ribosomes and is regarded as non‐toxic for plants (Poppenberger et al., 2003). Plant cultivars differ in their ability to detoxify DON and this depends on genetic and environmental factors (Lemmens et al., 2016; Warth et al., 2016). However, there is concern that DON‐3‐glucoside may be converted in the gastrointestinal tract by humans and animals to DON (FAO/WHO, 2011; Nagl et al., 2012) and thus may contribute to the overall exposure to DON. It is known that mycotoxins can also be modified by living organisms other than plants (e.g. bacteria and mammals), and by further processing of the plants. All the altered mycotoxins are not covered by the term ‘masked mycotoxins’. Therefore, the CONTAM Panel decided to use for this opinion the systematic classification of the various DON forms as proposed by Rychlik et al. (2014) (see Table 1) and interpreted the ‘presence of DON, metabolites of DON and masked DON in food and feed’, as indicated in the TOR, to assess the risks to human and animal health related to the presence of DON and its acetylated forms (free mycotoxins) and DON‐3‐glucoside (modified mycotoxin) based on currently existing knowledge and data published by July 2016. Overall, the CONTAM Panel noted that DON is one of the most widely studied mycotoxins worldwide and therefore more modified forms of DON might be discovered in upcoming research soon and basic molecular research (e.g. using techniques of genomics and gene expression) of DON and its various forms are expected to clarify the biosynthesis pathway of DON and its modified forms.
Table 1.
Categorisation of various forms of deoxynivalenol (DON) classified in four hierarchic levels modified from Rychlik et al. (2014)
| 1st level | 2nd level | 3rd level | 4th level | Examples of DON forms |
|---|---|---|---|---|
| Free mycotoxinsa |
DON 3‐Ac‐DON 15‐Ac‐DON 3,15‐Ac‐DON |
|||
| Food and feed matrix‐associated mycotoxins | DON‐oligosaccharides | |||
| Modified mycotoxins | Biologically modified | Conjugated (phase 2 metabolites) | Conjugated by plantsb |
DON‐3‐glucoside DON‐3/15‐sulfatesc DON‐glutathione |
| Conjugated by animals |
DON‐3‐glucuronide DON‐15‐glucuronide |
|||
| Differently modifiedd | De‐epoxy‐DON (DOM‐1)e, 3‐epi‐DONf | |||
| Chemically modified | Thermally formed | norDON A, B, C, D, F and DON‐lactone; norDON‐3‐glucoside A B, C, D and DON‐ DON‐3‐glucoside lactoneg | ||
| Non‐thermally formed |
DON‐sulfonates norDON A, B, Ch |
Free or unmodified mycotoxins are formed as toxic secondary metabolites by various fungi.
Mycotoxins conjugated by plants are also known as modified mycotoxins.
Both DON‐sulfates were detected in wheatears after treatment with DON. DON‐3‐sulfate was the only detected sulfate conjugate in wheat artificially infected with Fusarium. Their natural occurrence still needs to be investigated (Warth et al., 2015).
Biologically modified mycotoxins that are no (plant, animal or fungal) phase 1 nor phase 2 metabolites.
Intestinal metabolite of DON, which is formed by the microbiota of animals and humans.
Bacterial transformation product, produced by the bacterial strain Devosia mutans 17‐2‐E‐8 in aerobic conditions (He et al., 2015).
Various forms of norDON, nor DON‐3‐glucoside, DON‐lactone and DON‐3‐glucoside lactone have been found as thermal degradation products of DON and DON‐3‐glucoside in heat‐treated wheat and in the crust of experimental breads (Kostelanska et al, 2011b; Wu and Wang, 2015).
DON‐sulfonates (DONS‐1, ‐2 and ‐3) have been found in animal feed treated with sulfur reagents such as sodium bisulfite or sodium metabisulfite. It has been suggested that the latter two sodium‐based compounds could be used for detoxification (Schwartz‐Zimmermann et al., 2014).
Taking into account the availability of data suitable for the risk assessment of DON and its acetylated and modified forms, the CONTAM Panel decided to assess acute and chronic exposure and the risk of DON, the acetylated forms 3‐Ac‐DON and 15‐Ac‐DON, as well as of DON‐3‐glucoside in this opinion for food and feed relevant for the European market. This interpretation of the terms of reference has mutually been agreed with the requestor of the present scientific opinion. The uncertainties rising from the lack of information on the other acetylated and modified forms of DON are described in the uncertainty chapter.
The Appendix A describes the identification and selection of evidence relevant for the present opinion.
1.1. Previous assessments
In 1993, the International Agency for Research on Cancer (IARC, 1993) evaluated the carcinogenic effect of Fusarium mycotoxins including DON, and concluded that DON is ‘not classifiable as to its carcinogenicity to humans’ (Group 3).
In 1998, the Nordic Council of Ministers (NCM, 1998) conducted a risk assessment on Fusarium mycotoxins in cereals and established a temporary tolerable daily intake (t‐TDI) of 1 μg/kg bw per day based on a no‐observed‐adverse‐effect level (NOAEL) of 1 mg/kg feed (0.1 mg/kg bw per day) derived from the 2‐year feeding study in mice of Iverson et al. (1995) and using uncertainty factor of 100. The TDI was declared to be temporary due to the limited long‐term study data and the uncertainty of the mechanisms of action at that time. It was considered to also be protective against the acute vomiting effect.
In 1999, the Scientific Committee on Food (SCF, 1999) evaluated DON for the first time. As no carcinogenicity or mutagenicity evidence was identified, the NOAEL of 0.1 mg/kg bw per day from the 2‐year feeding study of Iverson et al. (1995) was used as reference point. The uncertainty factor of 100 was used to establish a t‐TDI of 1 μg/kg bw per day. The SCF considered this t‐TDI to also protects against the acute vomiting effect and other subchronic or reproductive effects of DON. Later, the SCF (2002) assessed the group‐combined effect of common trichothecenes including T‐2 and HT‐2 toxins, DON and nivalenol, and concluded that the available data were not sufficient to establish a TDI for either the combined effects or the relative potencies of the trichothecenes. Based on its assessment, the SCF decided to turn the t‐TDI of 1 μg/kg bw per day for DON to a full TDI.
The National Institute for Public Health and the Environment (RIVM) in the Netherlands also carried out risk assessments for DON (Pieters et al., 1999). Five toxicological studies (four on mice and one on swine) were examined for deriving a NOAEL. The 2‐year feeding study in mice by Iverson et al. (1995) was used to derive a provisional TDI. An uncertainty factor of 100 (10 for interspecies and 10 for interindividual uncertainty) and a more accurate round‐up approach than in other assessments mentioned above were applied and, therefore, the TDI was reported as 1.1 μg/kg bw per day. The Health Council of the Netherlands also conducted a risk assessment on DON (HCN, 2001). The Council used the data from the same 2‐year feeding study (Iverson et al., 1995) with an uncertainty factor of 210 to establish a TDI of 0.5 μg/kg bw per day. The uncertainty factor comprised the subfactors of 10 for intra‐ and 3 for interspecies differences, and a factor of 7 for differences in energy use and differences in metabolism, possibly affecting body weight changes, between humans and mice. Both TDIs are not anymore in use in the Netherlands, instead a TDI of 1 μg/kg is used now (Janssen et al., 2015).
At its 56th meeting, the Joint Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) Expert Committee on Food Additives (JECFA) evaluated the risk of DON dietary exposure (FAO/WHO, 2001). JECFA considered the same 2‐year feeding study in mice (Iverson et al., 1995) to be appropriate for long‐term effect evaluation as did the SCF. A provisional maximum (PM)TDI of 1 μg/kg bw per day was considered to protect against immunotoxicity, growth reduction and reproductive effects of DON. However, for acute toxicity, JECFA could not establish a level below which no effects would be expected to occur in humans.
The Japanese Food Safety Commission (FSCJ) assessed DON in 2010 (FSCJ, 2010). It considered vomiting, reduced feed intake and reduced body weight gain, and immunotoxicity as the critical effects for the risk assessment of DON, while at high doses fetal toxicity and teratogenicity could be induced. The FSCJ considered that given the absence of any evidential genotoxic or carcinogenic effect, a TDI for DON of 1 μg/kg bw per day could be established based on the same 2‐year feeding study in mice (Iverson et al., 1995).
At its 72nd meeting, JECFA evaluated its previously established PMTDI for DON of 1 μg/kg bw per day (FAO/WHO, 2011). Taking into account few short‐term studies available at that time, the Committee considered the use of the NOAEL of 0.1 mg/kg bw per day to still be appropriate. JECFA noted that the acetylated derivatives (3‐Ac‐DON and 15‐Ac‐DON) are, in general, not frequently detected and constitute less than 10% of the DON concentrations. However, they are considered to be as toxic as DON, and 3‐Ac‐DON is converted to DON in vivo and therefore contributes to the total DON‐induced toxicity. JECFA converted its previous PMTDI for DON to a group PMTDI of 1 μg/kg bw per day for DON and its acetylated derivatives (3‐Ac‐DON and 15‐Ac‐DON). DON‐3‐glucoside was not included in the group PMTDI due to a lack of sufficient data.
For assessing the acute toxicity of DON, JECFA considered that DON‐induced systemic emesis was the critical acute effect. The observations from two studies in pigs with DON exposure through naturally contaminated feed (Young et al., 1983; Pollmann et al., 1985) were considered appropriate and were combined for dose–response modelling to establish the acute reference dose (ARfD). Based on the lowest BMDL10 of 0.21 mg/kg bw per day for emesis in pigs, a group ARfD for DON and its acetylated derivatives (3‐Ac‐DON and 15‐Ac‐DON) of 8 μg/kg bw per day was established. JECFA considered this to be sufficiently protective against human emesis because limited data suggested that dietary exposures to DON up to 50 μg/kg bw per day are not likely to induce emesis in humans (FAO/WHO, 2011).
Details on the exposure assessments from the previous assessments are reported in the Section 6.1.1.
1.2. Chemistry of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
DON, 3‐Ac‐DON and 15‐Ac‐DON (see Figure 1) are mycotoxins belonging to the group of trichothecenes, which are produced by Fusarium species. Trichothecenes are characterised by a tetracyclic sesquiterpenoid 12,13 epoxytrichothec‐9‐en ring structure. They have been classified into four groups (A–D) based on to their chemical structures. The epoxide group between C12 and C13 accounts for many of the typical toxic effects of trichothecenes (Betina, 1989).
Figure 1.
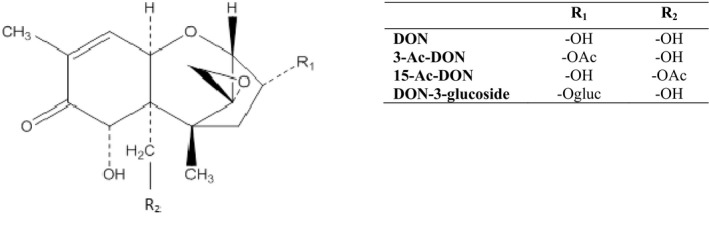
Chemical structures of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
Epidemiological surveys have demonstrated that the predominant type A and B trichothecenes are widely distributed in cereals and feeds as natural pollutants, whereas type C (characterised by a second epoxide at C‐7,8 or C‐9,10) and type D trichothecenes (containing an ester‐linked macrocycle at C‐4,16) occur rarely in food and feed. Type A trichothecenes include T‐2 toxin, HT‐2 toxin and 4,15‐diacetoxyscirpenol, and type B toxins include nivalenol, fusarenon‐X, DON, 3‐Ac‐DON and 15‐Ac‐DON (Figure 1).
The first isolation of DON was reported in 1972 from Japanese Fusarium‐damaged barley (Weidenbörner, 2001). DON is the trivial name for trichothec‐9‐en‐8‐one, 12, 13‐epoxy‐3,7,15‐trihydroxy‐, (3α,7α) (Chemical Abstracts Service (CAS) number 51481‐10‐8). Its molecular formula is C15H20O6 and its molecular weight is 296.32 g/mol. DON crystallises as colourless needles and has a high temperature tolerance (stable at 120°C, moderately stable at 180°C). Ultraviolet (UV) absorption spectra for DON yield an absorption maximum of 217 nm (Krska et al., 2004). For pure commercially available crystalline DON, extinction coefficients were 6,727 L/mol per cm (DON standard from Sigma) and 6,825 L/mol per cm (DON standard from Biopure) (Krska et al., 2004).
3‐Ac‐DON (trichothec‐9‐en‐8‐one, 3‐(acetyloxy)‐12,13‐epoxy‐7,15‐dihydroxy‐,(3α,7α); CAS number 50722‐38‐8) and 15‐Ac‐DON (trichothec‐9‐en‐8‐one, 15‐(acetyloxy)‐12,13‐epoxy‐3,7‐dihydroxy‐, (3α,7α); CAS number 88337‐96‐6) both have the molecular formula of C17H22O7, and a molecular weight of 338.35 g/mol. Their UV absorption maxima are at 217 and 219 nm, respectively (Krska et al., 2005).
DON‐3‐glucoside has been isolated from Zea mays suspension cultures that were treated with DON (Sewald et al., 1992), in maize and wheat (Berthiller et al., 2005), and in beer (carryover from field barley through malt to beer) (Lancova et al., 2008a). In DON‐3‐glucoside, DON is bound with the anomeric carbon of glucose via a glycosidic bond. A UDP‐glucosyltransferase from Arabidopsis thaliana catalyses the transfer of glucose from UDP‐glucose to the hydroxyl group at carbon 3 of DON and 15‐Ac‐DON (Poppenberger et al., 2003).
DON‐3‐glucoside (trichothec‐9‐en‐8‐one, 12,13‐epoxy‐3‐(β‐d‐glucopyranosyloxy)‐7,15‐dihydroxy‐, (3α,7α); CAS number 131180‐21‐7) has the molecular formula C21H30O11 and a molecular weight of 458.46 g/mol.
DON is soluble in water and in some polar solvents (e.g. aqueous methanol, acetonitrile and ethyl acetate) (EFSA, 2004). The presence of an acetyl moiety in 3‐Ac‐DON and 15‐Ac‐DON results in a decrease in the polarity of the molecule compared with the parent toxin. Conversely, the presence of a glucoside in DON‐3‐glucoside leads to an increase in polarity compared with DON (Maresca, 2013).
DON is considered to be a relatively thermostable compound (Kostelanska et al., 2011b). In the study of Wolf and Bullerman (1998), the effects of heat and pH on the stability of DON were investigated in an aqueous buffer solution, and only high pH (10.0) and high heat combinations (170°C, 15 min and 120°C, 30 min) were reported to completely destroy DON.
2. Legislation
Worldwide, at least 40 countries have established maximum levels (MLs) or recommendations for DON in food and animal feed. These countries include the Member States of the EU, the USA and China (FAO, 2004; LFRA, 2010). MLs for acetylated and modified forms of DON have not been reported in any country. In the EU, MLs in food for specific contaminants shall be established if this is necessary to protect public health (Article 2 of Council Regulation (EEC) No 315/93 of February 1993 laying down Community procedures for contaminants in food5). MLs are laid down in Regulation (EC) No 1881/20066, in which legal levels for DON in unprocessed cereals and cereal products for human consumption have been established (Table 2). MLs for undesirable substances in feed are laid down in EU Directive 2002/32/EC7. Annex I of this Directive contains MLs of a number of inorganic and organic contaminants in feed. DON is not regulated under this Directive. DON is regulated, however, within Recommendation 2006/576/EC8, in which guidance levels are given for DON in certain products intended for animal feed. The MLs for cereals and cereal products are given in Table 2 and the guidance levels for products intended for animal feed are given in Table 3.
Table 2.
Maximum levels for DON in foodstuffs in EU Regulation (EC) No 1881/2006
| Category number | Foodstuffsa , b | Maximum levels (μg/kg) |
|---|---|---|
| 1 | Unprocessed cerealsc , d other than durum wheat, oats and maize | 1,250 |
| 2 | Unprocessed durum wheat and oatsc , d | 1,750 |
| 3 | Unprocessed maizec | 1,750 |
| 4 | Cereals intended for direct human consumption, cereal flour (including maize flour, maize meal and maize grits) bran as end product marketed for direct human consumption and germ, with the exception of foodstuffs listed in 7, 8 and 9 | 750 |
| 5 | Pasta (dry)e | 750 |
| 6 | Bread (including small bakery wares), pastries, biscuits, cereal snacks and breakfast cereals | 500 |
| 7 | Processed cereal‐based foods and baby foods for infants and young childrenf , g | 200 |
| 8 | Milling fractions of maize with particle size > 500 μm falling within CN code 1103 13 or 1103 20 40 and other maize milling products with particle size > 500 μm not used for direct human consumption falling within CN code 1904 10 10 | 750 |
| 9 | Milling fractions of maize with particle size ≤ 500 μm falling within CN code 1102 20 and other maize milling products with particle size ≤ 500 μm not used for direct human consumption falling within CN code 1904 10 10 | 1,250 |
CN, Combined Nomenclature (maize milling fractions are classified using the particle size in different headings in the Combined Nomenclature based upon a rate of passage through a sieve with an aperture of 500 μm).
As regards fruits, vegetables and cereals, reference is made to the foodstuffs listed in the relevant category as defined in Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC11 as last amended by Regulation (EC) No 178/200612. This means, inter alia, that buckwheat (Fagopyrum sp.) is included in ‘cereals’ and buckwheat products are included in ‘cereal products’.
Rice is not included in ‘cereals’ and rice products are not included in ‘cereal products’.
The maximum level applies to unprocessed cereals placed on the market for first‐stage processing. ‘First‐stage processing’ shall mean any physical or thermal treatment, other than drying, of or on the grain. Cleaning, sorting and drying procedures are not considered to be ‘first‐stage processing’ insofar as no physical action is exerted on the grain kernel itself and the whole grain remains intact after cleaning and sorting. In integrated production and processing systems, the maximum level applies to the unprocessed cereals if they are intended for first‐stage processing.
The maximum level applies to cereals harvested and taken over, as from the 2005/06 marketing year, in accordance with Commission Regulation (EC) No 824/2000 of 19 April 2000 establishing procedures for the taking over of cereals by intervention agencies and laying down methods of analysis for determining the quality of cereals,13 as last amended by Commission Regulation (EC) No 1068/2005 of 6 July 2005 amending Regulation (EC) No 824/2000 establishing procedures for the taking over of cereals by intervention agencies and laying down methods of analysis for determining the quality of cereals.14
Pasta (dry) means pasta with a water content of approximately 12%.
Foodstuffs listed in this category as defined in Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children.15
The maximum level refers to the dry matter. The dry matter is determined in accordance with Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs.16
Table 3.
Guidance levels for DON in animal feed in EU Recommendation 2006/576/EC11
| Products intended for animal feeda | Guidance value in mg/kg relative to a feedingstuff with a moisture content of 12% |
|---|---|
| Cereals and cereal productsb with the exception of maize by‐products | 8 |
| Maize by‐products | 12 |
| Complementary and complete feedingstuffs with the exception of: | 5 |
| Complementary and complete feedingstuffs for pigs | 0.9 |
| Complementary and complete feedingstuffs for calves (< 4 months), lambs and kids | 2 |
Particular attention has to be paid to cereals and cereal products fed directly to the animals that their use in a daily ration should not lead to the animal being exposed to a higher level of these mycotoxins than the corresponding levels of exposure where only the complete feedingstuffs are used in a daily ration.
The term ‘Cereals and cereal products’ includes not only the feed materials listed under heading 1 ‘Cereal grains, their products and by‐products’ of the non‐exclusive list of main feed materials referred to in part B of the Annex to Council Directive 96/25/EC of 29 April 1996 on the circulation and use of feed materials amending Directives 70/524/EEC, 74/63/EEC, 82/471/EEC and 93/74/EEC and repealing Directive 77/101/EEC10, but also other feed materials derived from cereals in particular cereal forages and roughages.
In Regulation (EC) No 401/20069, as amended by Regulation (EU) No 519/201410, requirements for methods of sampling and analysis for the official control of the levels of mycotoxins are laid down (see Section 3 for further details). In Annex I of this Regulation, general provisions for sampling are stated in part A, specific provisions for the sampling of cereals and cereal products are given in part B, and specific provisions for the sampling of baby foods and processed cereal‐baby foods for infants and young children are stated in part J. Furthermore, in Annex II of this Regulation criteria for sample preparation and methods of analysis are laid down. Performance criteria for methods of analysis for DON used in the official control as mentioned in this Regulation are presented in Table 5 (see Section 3).
Table 5.
Performance criteria for confirmatory methods of analysis for DON
| Concentration (μg/kg) | RSDr (%) | RSDR (%) | Recovery (%) |
|---|---|---|---|
| > 100 to ≤ 500 | ≤ 20 | ≤ 40 | 60–110 |
| > 500 | ≤ 20 | ≤ 40 | 70–120 |
RSDr: relative standard deviation under repeatability conditions; RSDR: relative standard deviation under reproducibility conditions.
Within the Codex Alimentarius Commission, supported by FAO and WHO, the Codex Committee on Food Additives and Contaminants is in the process of drafting MLs for DON in food, which are decisive in trade conflicts. MLs for DON have been proposed for raw cereal grains (wheat, maize and barley); flour, semolina, meal and flakes derived from wheat, maize or barley; and cereal‐based foods for infants and young children (CAC, 2014).
3. Analysis
The analytical methodology described in this section relates to the determination of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed. In addition, there exists analytical methodology to determine DON, and its acetylated and modified forms in human and animal tissues and body fluids such as urine.
3.1. Sampling and storage
Prior to the determination of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, a representative sample must be provided. There are no specific requirements or recommendations for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside that should be followed concerning the sampling and the storage of the samples intended for the determination of these compounds. Due to the possible heterogeneous distribution of these toxins in lots (of grains), sampling may contribute to a substantial extent to the variability in analytical results.
In Commission Regulation (EC) No 519/201413, methods of sampling for the official control of the levels of mycotoxins are laid down. This Regulation No 519/2014 amended Commission Regulation (EC) No 401/200617 in which general provisions for sampling are stated in part A, and specific provisions for the sampling of cereals and cereal products are given in part B. This sampling procedure is also of application for the official control of the maximum levels established for Fusarium toxins. The amendments on sampling concern in particular additional requirements for the sampling of very large lots or lots stored or transported in a way whereby sampling throughout the lot is not feasible. This regulation has to be followed when sampling for the determination of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in cereals. After sampling, the samples are to be stored under appropriate conditions (dry, preferably frozen) until analysis in order to prevent Fusarium fungi growing further and producing toxins.
3.2. Determination of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
In the past, most attention was on the determination of DON only, and therefore analytical methods dedicated to DON are abundant in literature. Later on methods for simultaneous detection and quantification of DON and acetylated and modified forms were developed and described. In general, analytical approaches to determine DON do not differ from those applied for other trichothecenes. They include toxin extraction from samples with an extraction solvent, usually followed by a clean‐up step to eliminate interferences from the sample matrix, and a final detection of the target toxin by suitable methods.
Suitable analytical methodology, summarised also by Ran et al. (2013), relied on high‐performance liquid chromatography (HPLC) with subsequent UV and fluorescence detector (FLD), gas chromatography with subsequent electron capture detection (GC‐ECD) or GC with MS detection (GC–MS). Screening methods based on techniques such as thin‐layer chromatography (TLC) and enzyme‐linked immunosorbent assay (ELISA) have also been published. More recent trends in mycotoxin analysis have led to the development of HPLC coupled with MS with minimal extract clean‐up, with the possibility for multitoxin analysis applicable to a wide variety of matrices.
All analytical methods for DON are potentially also suitable for the acetylated and modified DON forms (Berthiller et al., 2013). However, the structural differences of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside compared with DON induce changes in the physicochemical properties, such as solubility and polarity (see Section 1.2) that require different conditions for analysis.
The main challenge for both acetylated forms was the separation of the 3‐Ac‐DON and the 15‐Ac‐DON isomers in the analytical methods (Ibañez‐Vea et al., 2011; Kostelanska et al., 2011a). As the two acetylated DONs are isomers and the only difference in structure is the position of the acetyl group, chromatographic retention times and most tandem mass spectrometry (MS/MS) product ions are similar for the two compounds (Yoshinari et al., 2013). Some authors therefore take both 3‐Ac‐DON and 15‐Ac‐DONs together for quantification (Kostelanska et al., 2011a). Other authors focus on only one of the two acetylated derivatives (Rasmussen et al., 2012). Monbaliu et al. (2009); De Boevre et al. (2012); Yoshinari et al. (2012, 2013) and Versilovskis et al. (2012) successfully separated 3‐Ac‐DON and 15‐Ac‐DON in their optimised HPLC method. However, Habler and Rychlik (2016) could quantify 3‐Ac‐DON and 15‐Ac‐DON without chromatographic separation, especially by the difference in the intensities of the product ions.
Generally, two different approaches are reported in the literature for the determination of DON‐3‐glucoside. The first one is the analytical technique, which directly detects the modified form as it is (direct methods). This approach is usually based on MS analysis and requires calibrants. The second approach is the conversion into DON by enzymatic and/or chemical treatment before the analysis (indirect methods). In such a way, the sum of DON and DON‐3‐glucoside was simultaneously detected in the sample (Berthiller et al., 2013). The indirect approach works with the common chromatographic or immunochemical techniques and it does not require any calibrant. Its main drawback is the lack of efficacy of the cleavage reaction and therefore, direct methods remain the method of choice for the determination of DON‐3‐glucoside (Malachova et al., 2015) (see Section 3.2.1).
3.2.1. Analyte isolation
As DON is a polar mycotoxin, it can easily be extracted from food and feed by shaking or blending with pure water or mixtures of water with other polar solvents such as acetonitrile or methanol. The most important procedures for clean‐up of extracts and concentration of DON are liquid–liquid extraction, solid‐phase extraction (SPE), immunoaffinity columns (IAC), and multifunctional columns (Ran et al., 2013). However, as DON‐3‐glucoside is more polar than DON, SPE techniques might not be suitable for this modified mycotoxin (De Boevre et al., 2012; Berthiller et al., 2013). As more sensitive HPLC–MS equipments have become available, it has been possible to replace clean‐up by diluting the sample extract (Sulyok et al., 2006) or by evaporating and redissolving the sample extract (Monbaliu et al., 2009; Kostelanska et al., 2011a).
For the isolation of DON together with 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, extraction solvents containing acetonitrile and water in different ratios, eventually acidified with acetic acid and/or formic acid are the most often used (Sulyok et al., 2006; Ibañez‐Vea et al., 2011; Kostelanska et al., 2011a; De Boevre et al., 2012; De Angelis et al., 2013; Juan et al., 2013). In addition, modified QuEChERS (quick, easy, cheap, effective, rugged and safe) sample preparation procedures, based on the partitioning between water or methanol and acetonitrile by the addition of salt mixtures, was proposed in combination with MS (Zachariasova et al., 2010; Desmarchelier and Seefelder, 2011; Cirlini et al., 2012; Pereira et al., 2015). Dall'Asta et al. (2013) used a higher amount of acetonitrile and a lower amount of salts. Acceptable recoveries were also obtained for DON‐3‐glucoside.
For indirect methods, the use of glucosidases is in general not effective to hydrolyse DON‐3‐glucoside (Sewald et al., 1992). However, a promising glucosidase from Bifidobacterium able to hydrolyse DON‐3‐glucoside in cereal matrices was recently identified (Michlmayr et al., 2015). Enzyme treatment with amylolytic, proteolytic or cell wall‐degrading enzymes had an effect on the quantity of DON released from its modified forms in barley (Zhou et al., 2007). An acid hydrolysis procedure based on hot trichloroacetic acid was applied to samples containing 3‐Ac‐DON and 15‐Ac‐DON (Liu et al., 2005). The procedure did not hydrolyse all of the Ac‐DON known to be present. Tran and Smith (2011) determined optimal conditions for hydrolysis of conjugated DON in maize and wheat with trifluoromethanesulfonic acid. However, after a critical evaluation, Malachova et al. (2015) strongly discourage the use of indirect methods based on acidic or alkaline hydrolysis as none of the hydrolytic conditions were found to be suitable for achieving reliable decomposition of modified forms of DON to DON.
3.2.2. Chromatographic methods
Chromatographic methods have been developed for the identification and quantification of several trichothecenes including DON, 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside in various matrices including food and feed as well as samples of human and animal origin.
TLC was the first chromatographic method to be applied for DON determination, and an official method of the Association of Analytical Communities (AOAC) exists for determination of DON in wheat by fluoro‐densitometric quantification at levels ≥ 300 μg/kg (Eppley et al., 1986). TLC is a fast and low‐cost method, but it is laborious and its application is now mainly limited to developing countries (Berthiller et al., 2013). In the past, GC methods were routinely used for the determination of DON, 3‐Ac‐DON and 15‐Ac‐DON (Krska et al., 2001), and an official AOAC method exists for determination of DON in wheat by GC with ECD for quantification at levels ≥ 350 μg/kg (Ware et al., 1986). However, the major limitation is the necessity of a time‐consuming derivatisation of the polar analytes prior to determination. Methods using ECD (Ware et al., 1986; Black et al., 1987; Hallier et al., 2011; Simsek et al., 2012) or MS detection (Black et al., 1987; Krska et al., 2001; Edwards, 2009a; Ibañez‐Vea et al., 2011; Pereira et al., 2015) have been described. Limits of quantification (LOQs) can differ substantially depending on the analytical scheme used (see Table 4 for typical examples). HPLC‐analysis can be performed with fluorescence (FLD), UV, or diode‐array (DAD), or MS detection. For FLD, a pre‐ or post‐column derivatisation is required, which makes this type of analysis time‐consuming. UV detection is often limited by a lack of specificity due to the use of low wavelengths (see Section 1.2). Nevertheless, European Committee for Standardisation (CEN) methods exist for DON in animal feed (EN15791:200918) and for DON in cereals, cereal products and cereal‐based food for infants and young children (EN15891:201019), with HPLC‐UV. Examples of HPLC methods together with LOQs are reported in Table 4.
Table 4.
Typical examples of the method characteristics and limits of quantification (LOQ) of analytical methods used for the determination of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed
| Analytical technique | Method characteristics | LOQ (μg/kg) | References | |||
|---|---|---|---|---|---|---|
| DON | 3‐Ac‐DON | 15‐Ac‐DON | DON‐3‐glucoside | |||
| TLC | Screening (qualitative–semiquantitative) | 300a | n.a. | n.a. | n.a. | Eppley et al. (1986) |
| ELISA | Screening (semiquantitative–quantitative) | 25–300 | n.a. | n.a. | n.a. | Maragos and McCormick (2000), Hiraoka et al. (2013), Dzuman et al. (2014) |
| GC‐ECD |
Confirmation (semiquantitative–quantitative) Possible multianalyte detection |
5–350a 16–28b |
16–23b | 18–23b | n.a. | Ware et al. (1986), Labuda et al. (2005), Valle‐Algarra et al. (2005), Yue et al. (2010) |
| GC–MS(/MS) |
Confirmation (semiquantitative–quantitative) Possible multianalyte detection |
1–120 | 1–57 | 8–25 | n.a. | Edwards (2009a,b,c), Cunha and Fernandez (2010), Ibañez‐Vea et al. (2011), Tran et al. (2012), Rodríguez‐Carrasco et al. (2014b), Pereira et al., (2015) |
| HPLC‐UV or HPLC‐FLD |
Confirmation (semiquantitative–quantitative) Possible multianalyte detection |
5–380 | 60c | 56c | n.a. | Klötzel et al. (2005), Klinglmayr et al. (2010), Soleimany et al. (2011), Heidtmann‐Bemvenuti et al. (2012), Yang et al. (2013) |
| LC–MS(/MS) |
Confirmation (semiquantitative–quantitative) Possible multianalyte detection |
1–260 | 2–46 | 2–250 | 2–30 | Desmarchelier et al. (2010), Desmarchelier and Seefelder (2011), Eckard et al. (2011), Kostelanska et al. (2011a), De Boevre et al. (2012), Juan et al. (2012), Lim et al. (2012), Soleimany et al. (2012), Yoshinari et al. (2012), Habler and Rychlik (2016) |
n.a.: not applicable; LOQ: limit of quantification; TLC: thin‐layer chromatography; GC: gas chromatography; ECD: electron capture detection; MS: mass spectrometry; HPLC: high‐performance liquid chromatography; UV: ultraviolet; FLD: fluorescence detection; n.a.: not applicable.
Association of Analytical Communities (AOAC) official method.
Only reported as limit of detection (LOD).
LOQs based on one reference only (Yang et al., 2013).
High‐ and ultra‐high‐performance liquid chromatography (HPLC/U‐HPLC) coupled with various MS detectors has become the most frequently used hyphenated technique in mycotoxin determination and especially for the simultaneous determination of multiple mycotoxins, including DON and its acetylated and modified forms. Unlike GC–MS, there is no need for derivatisation. The majority of HPLC–MS‐based methods for the quantification of DON and other B‐trichothecenes is based on MS/MS in combination with selected reaction monitoring (SRM) (Gottschalk et al., 2009; Sulyok et al., 2010; Juan et al., 2012). Ionisation methods used are atmospheric pressure chemical ionisation (APCI) and electrospray ionisation (ESI) in positive as well as negative ionisation modes. ESI seems to be more robust than APCI and is thus more frequently employed for analysis of mycotoxins including DON (Monbaliu et al., 2009; Santini et al., 2009; Desmarchelier et al., 2010; Han et al., 2010; Desmarchelier and Seefelder, 2011; De Boevre et al., 2012; Juan et al., 2012; De Angelis et al., 2013). Quantification by means of method matrix‐matched calibration curves (calibration curves constructed with spiked blank matrix) is usually considered as a good option to compensate for both losses during extraction and matrix effects generated during the ionisation of the analytes (Desmarchelier and Seefelder, 2011). Matrix effects, however can be further minimised by adding isotope‐labelled internal standards (Ran et al., 2013). Moreover, multi‐mycotoxin LC–MS methods using of either isotope‐labelled internal standards or matrix‐matched calibration curves depending on the availability of the internal standards for the analytes have also been described (Habler and Rychlik, 2016). LOQs can differ substantially depending on the analytical scheme used, (see Table 4 for typical examples).
3.2.3. Immunochemical methods
Immunochemical methods, based on antigen–antibody reactions, provide rapid and economical alternatives to chromatographic methods and represent one of the most practical options for farmers and industries for a fast and easy control of DON in raw materials and processed food and feed (De Saeger and Van Egmond, 2012; Zachariasova et al., 2014a). Different formats of immunochemical methods for DON detection have been described in literature and/or are commercially available, including ELISA (Ji et al., 2011; Hiraoka et al., 2013), lateral flow test strips (Kolosova et al., 2008; Huang et al., 2012; Albert et al., 2013; Lattanzio et al., 2016), flow‐through tests (Ediage et al., 2012a), fluorescence polarisation immunoassay (Maragos and Plattner, 2002; Lippolis et al., 2006), immunosensors (Romanazzo et al., 2010; Dorokhin et al., 2011; Maragos, 2011; Zhilei et al., 2011) and IAC (the latter for clean‐up) (Brenn‐Struckhofova et al., 2009). However, the ELISA tests still dominate the routine screening practice (Zachariasova et al., 2014a). Recently, a trend towards multi‐mycotoxin detection has been observed for immunochemical methods (Peters et al., 2011; Ediage et al., 2012a; He et al., 2012; Lattanzio et al., 2012; Song et al., 2014).
Different antibodies against DON are (commercially) available and described in the literature (Usleber et al., 1991; Maragos and McCormick, 2000; Maragos et al., 2006; Lee et al., 2013; Sanders et al., 2014). Antibodies against DON can cross‐react with acetylated and/or modified forms of DON. Cross‐reactivity of DON antibodies with 3‐Ac‐DON and/or 15‐Ac‐DON has been frequently reported with levels ranging between 51–770% and 0–65%, respectively (Tangni et al., 2010; Sanders et al., 2014; Zachariasova et al., 2014a). However, very specific 3‐Ac‐DON antibodies have also been described (Sanders et al., 2014). No antibodies have specifically targeted and been selected against DON‐3‐glucoside. In an overview of antibodies and (commercial) ELISA and IAC for DON, with special focus on DON‐3‐glucoside, Berthiller et al. (2013) showed that cross‐reactivity with DON‐3‐glucoside can significantly20 vary from 0% to 157% depending on the antibodies used. Due to these cross‐reactivities to different DON derivatives, discrepancies between the results for DON obtained from ELISA and LC–MS/MS demonstrate a possible risk for biased results when using immunochemical methods. Not only the DON derivatives, but also the unidentified matrix components are responsible for concentration overestimation of the target DON analyte (Kostelanska et al., 2009; Dzuman et al., 2014; Zachariasova et al., 2014a). Cross‐reactivity values have been reported for most of the commercially available products, but several studies in literature reveal that values declared by kit producers are often fairly different from those determined within the in‐house validation (Zachariasova et al., 2014a). A guideline on how to characterise antibodies used in immunochemical methods for mycotoxins analysis has been provided by Fremy and Usleber (2003). Typical examples of immunochemical methods together with LOQs are in Table 4 .
3.2.4. Other approaches
One of the emerging techniques is direct analysis in real time (DART). In this technique, (chromatographic) separation and clean‐up are omitted. Vaclavik et al. (2010) demonstrated the use of DART coupled to high‐resolution MS (HRMS) to quantify DON and 3‐Ac‐DON in QuEChERS‐based extracts prepared from cereals.
A very recent trend is the use of HRMS for untargeted metabolic profiling and metabolomics. Besides the known mycotoxins and metabolites, novel metabolites, biotransformation products and/or modified fungal metabolites can be identified. The stable isotope labelling‐assisted untargeted metabolic profiling method developed by Kluger et al. (2013) revealed novel conjugates of DON in wheat. One of the products was annotated as DON‐glutathione conjugate. Zachariasova et al. (2012) revealed by using HRMS that also oligoglycosylated DON forms were present in cereal‐based products. HRMS was also used to study thermal degradation products of DON in roasted wheat samples and baked bread (Kostelanska et al., 2011b). De Dominicis et al. (2015) developed an U‐HPLC‐HRMS method for simultaneous quantitative determination of pesticides, antibiotics and mycotoxins in different food products (milk, wheat flour and mini‐cakes).
While matrix interferences in extracts can have a significant effect on response in MS detectors, electroactive interferents are relatively rare, and thus electrochemical measurements have been applied for DON (Hsueh et al., 1999; Ricci et al., 2009).
The use of sensors using molecularly imprinted polymers (MIP) as synthetic receptors for DON (as an alternative for natural antibodies) was applied by Choi et al. (2011). The sensor was selective for DON, 3‐Ac‐DON and 15‐Ac‐DON in standard solutions.
In addition to the chemical and immunochemical methods for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, there exist non‐invasive screening methods based on the spectroscopic characteristics of the analytes or on the acoustic characteristics of DON‐contaminated grain kernels. (Kos et al., 2004; Juodeikiene et al., 2008, 2014; Liu et al., 2009; Fox and Manley, 2013). Another alternative screening method that can detect DON contamination of cereal grains with high sample throughput is the electronic nose (Campagnoli et al., 2011; Lippolis et al., 2014).
3.3. Analytical quality assurance: performance criteria, reference materials and proficiency testing for analysis of food
In Annex II of the Regulation (EU) No 401/2006 of 23 February 2006,12 as amended by the Regulation (EU) No 519/2014 of 16 May 201413 criteria for methods of analysis of various mycotoxins are laid down. Performance criteria for confirmatory methods of analysis of DON used in the official control as mentioned in this Regulation are presented in Table 5. For in‐house validated methods, as an alternative, a ‘fitness‐for‐purpose’ approach may be used to assess their suitability for official control. In this approach, certain criteria for the measurement uncertainty must be fulfilled. These requirements are not toxin specific. In Regulation (EU) No 519/201413, specific requirements for semiquantitative screening methods are also laid down. These specific requirements apply to methods of which the result of the measurement is a numerical value, for example a (relative) response from a dip‐stick reader or a signal from LC–MS, and for which normal statistics apply. While numerical performance criteria in terms of precision and recovery specific for DON are given for the confirmatory methods (Table 5), the requirements in Regulation (EU) No 519/201412 for semiquantitative screening methods are limited to instructions and procedures for both single laboratory and interlaboratory validation. These are general requirements and they are not specifically given for DON. In the case of bio‐analytical methods that give a combined response for a certain mycotoxin group applicability must be demonstrated and limitations of the test must be mentioned in the scope of the method. Undesired cross‐reactivity (e.g. DON‐3‐glucoside, 3‐Ac‐DON or 15‐Ac‐DON for immuno‐based methods for DON) is not considered to increase the false‐negative rate of the target mycotoxins, but may increase the false suspect rate. This unwanted increasing will be diminished by confirmatory analysis for unambiguous identification and quantification of the mycotoxins.
The quality of analytical results regarding accuracy, precision and comparability is essentially linked to the use of reference materials and certified reference materials (CRMs). Several test materials (various ground grains with DON) and standards for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, including 13C‐labelled (internal) standards for DON and 3‐Ac‐DON, are offered by commercial suppliers. In addition, de‐epoxy‐DON (DOM‐1) is frequently used as an internal standard and is commercially available. Moreover, CRMs for DON in maize and wheat flour and reference calibrant solutions certified for DON content are available from the Joint Research Center‐Institute for Reference Materials and Measurements (JRC‐IRMM).21 Proficiency tests for the determination of DON in breakfast cereals, biscuits, wheat flour and animal feed are regularly organised by FAPAS® 22 and by the JRC‐IRMM (Stroka et al, 2009; Kunsagi et al., 2012; Kujawski et al., 2014). The studies organised by the JRC‐IRMM showed that the major methodologies used (LC–MS and HPLC‐optical detection) and the minor methodologies used (ELISA and GC) led to comparable results. A proficiency test for multi‐mycotoxin determination, including DON, in maize by using HPLC–MS/MS methodology was conducted within the EC's FP‐6 project Monitoring and Quality Assurance in the Food Supply Chain (MoniQA)23 (De Girolamo et al., 2013; Solfrizzo et al., 2013a). This study revealed a considerable analytical variability in the determination of DON with LC–MS(/MS) methodology. A robust and reliable method for simultaneous determination of 11 mycotoxins (including DON) in maize could not be identified from the results of this proficiency test.
3.4. Analytical methods for urinary DON biomarker
Several analytical methods have been developed for the determination of urinary DON and its metabolites in the past decade (see also Section 7.5.2). DON, DON‐3‐glucuronide and the predominant DON‐15‐glucuronide are the common forms of DON in human urine. In this opinion, the sum of these three forms refers to total DON (tDON). Although urinary DOM‐1 and its conjugates were reported in some studies, the levels varied in different populations and across species (see Section 7.1). Except for the recent studies of Huybrechts et al. (2015) and Heyndrickx et al. (2015) which reported high levels of DOM‐1‐glucuronide in human urine, low levels of urinary DOM‐1 were reported in the other studies. These results do not yet warrant to consider DOM‐1 as a biomarker of exposure of DON (Turner et al., 2010b, 2011a).
During the early stage of biomarker development in animal experiments, it was reported that DON can be detected using HPLC‐UV method in urine samples from rats (Meky et al., 2003) and from pig (Thieu and Pettersson, 2009).
A single biomarker method developed for tDON analysis by Turner et al. (2008a) has been applied in most of the single biomarker studies reviewed in this opinion. For LODs/LOQs of the single biomarker methods, see Table 52. DON‐glucuronide can be detected following digestion with β‐glucuronidase (Turner et al., 2008b). However, DON‐3‐glucuronide and DON‐15‐glucuronide cannot be separated by this approach, and therefore simply DON‐glucuronide or tDON was reported in the study. An adjustment of urinary pH and β‐glucuronidase digestion gave the optimal recovery and repeatability for the urinary DON biomarker method (Turner et al., 2008a). The samples were purified with IAC and analysed by HPLC–MS using an internal standard (13C‐DON). The method was validated using the UK Adult National Diet and Nutrition Survey (ANDNS) data and individual 24‐h urine samples (see Turner et al., 2008a, 2010a).
Table 52.
An outbreak of DON toxicosis from consumption of scabby wheat in China ‐modified from Luo et al. (1987)
| Town | Number of wheat samples tested | DON concentration in scabby wheat (mg/kg) | Number of wheat food consumers | Prevalence of food poisoning (%) | % of vomiting cases |
|---|---|---|---|---|---|
| Haitong (Wumiao) | 12 | 2.0, 10.0, 16.0, 16.0, 20.0, 20.0, 26.7, 26.7, 26.7, 30.0, 30.0, 40.0 | 44 | 100 | 46 |
| Zian | 2 | 16.0, 27.6 | 37 | 24 | n.r. |
| Qingzu | 5 | 1.0, 4.0, 6.0, 6.0, 8.0 | 73 | 0 | 0 |
n.r.: not reported by the author.
Fast advances in LC–MS technology have allowed multiple mycotoxins to be analysed simultaneously. This technique was applied to multiple mycotoxin biomarker development and several methods have been reported recently. For LODs/LOQs of the multi‐biomarker methods see Table 53. Solfrizzo et al. (2011) developed a multi‐biomarker method using UPLC–MS/MS for detection of DON, DOM‐1 and six other mycotoxins and their metabolites following a combined IAC and SPE purification. β‐Glucuronidase/sulfatase were added to deconjugate DON prior to extraction. Wallin et al. (2015) analysed urinary DON and DOM‐1 concentrations using the multiple urinary biomarker LC–MS/MS method following 6‐in‐1 IAC column purification. Using a ‘dilute and shoot’ approach, Warth et al. (2011) reported an analytical method to directly quantify DON‐3‐glucuronide. They further developed a ‘dilute‐and‐shoot’ LC‐ESI‐MS/MS method for the quantitative determination of 15 mycotoxins in human urine including DON and each DON‐glucuronide (Warth et al., 2012a). Also, Gerding et al. (2009) used a ‘dilute‐and‐shoot’ LC–MS/MS method for DON and DON‐glucuronides but were not able to separately identify DON‐3‐glucuronide and DON‐15‐glucuronide by this method.
Table 53.
Summary of literature reports of urinary DON/DON‐glucuronides/DOM‐1 biomarker data using the single biomarker method (DON expressed as ng DON/mL urine or ng DON/mg creatinine) (ordered by publishing year)
| Population | Number of subjects (F/M) | Age (year) | Positive samples (%) | Total DON Mean (range) ± standard deviation (ng/mL) | Total DON Mean (range) ± standard deviation (ng/mg) | Analytical method (LOD/LOQ, ng/mL) | Reference |
|---|---|---|---|---|---|---|---|
| UK | 202 (128/74) | 3–> 65 | DON: 93 | 31.4, median: 19.3 | DON (0.12/0.25) | Brera et al. (2015) | |
| DOM‐1: 0 | DOM‐1 (0.25/0.5) | ||||||
| Italy | 203 (122/81) | DON: 76 | 9.12, median: 6.54 | DON (0.25/0.5) | |||
| DOM‐1: 1.5 | DOM‐1 (0.25/0.5) | ||||||
| Norway | 230 (138/92) | DON: 97 | 8.29, median: 6.12 | DON (0.005/0.015) | |||
| DOM‐1: 12 | DOM‐1 (0.09/0.27) | ||||||
| UK | 15 (7/8) | 22–50 | DON: 100 (Year 1) | 7.1 ± 8.0 | DON (n.r./0.1) | Gratz et al. (2014) | |
| DON: 100 (Year 2) | 13.5 ± 12.0 | ||||||
| Sweden | 326 (n.r.) | 18–80 | DON: 90 | Median: 2.9 (< LOD–65.8) | DON (0.25/0.5) | Wallin et al. (2013) | |
| UK | 5 (3/2) | n.r. | DON: 100 | 3.62 (0.38–10.05) | DON (0.1/n.r.) | Gratz et al. (2013) | |
| UK | 85 (85/0) | 16–44 | DON: 100 | 10.3 (0.5–116.7)a | DON (0.25/0.5) | Hepworth et al. (2012) | |
| DOM‐1: 0 | DOM‐1 (LOQ = 0.06) | ||||||
| UK(b) | 34 (16/18) | Adults | DON: 100 | 17.8 (5–78.2), median: 3.8 | DON (0.25/0.5) | Turner et al. (2011a) | |
| DOM‐1: 0.3 | 0.65 (one sample) | DOM‐1 (LOQ = 0.06) | |||||
| UK | 35 (18/18) | 21–59 | DON: 94 | 11.6 (< LOD–78.2) | 10.1 (< LOD–70.7)a | DON (0.5/n.r.) | Turner et al. (2010a) |
| France | 76 (0/76) | 23–74 | DON: 99 | Median: 6.8 (0.5–28.8) | DON (0.5/n.r.) | Turner et al. (2010b) | |
| DOM‐1: 34 | 0.2 (0.2–2.8) (positive samples) | DOM‐1(LOD = 0.05) | |||||
| UK | 300 (158/142) | 19–64 | DON: 99 | 7.2 (6.2–8.2)a (L)9.2 (8.1–10.5)a (M)10.9 (9.5–12.4)a (H) | DON (0.1/0.6) | Turner et al. (2008a) | |
| UK | 25 (16/9) | 21–59 | DON: 100 | 10.7 (2.4–67.8)a | 7.2 (4.9–10.5)a | DON (n.r./0.6) | Turner et al. (2008b) |
n.r.: not reported; F: female; M: male; LOD: limit of detection; LOQ: limit of quantification; L: low dietary exposure; M: medium dietary exposure; H: high dietary exposure.
All urine samples were treated with enzymatic hydrolysis followed by IAC extraction prior to determination by HPLC‐MS.
geometric mean (95% CI); (b): all are selected positive samples from another study.
Ediage et al. (2012b) reported a multi‐biomarker method for 18 mycotoxins and their metabolites using an LC–MS/MS method. Solvent extraction and SPE clean‐up were used for sample preparation, and DON‐3‐glucuronide was directly measured. A LC–MS/MS method for 32 mycotoxins including DON, DOM‐1, 3‐Ac‐DON, 15‐Ac‐DON and several metabolites such as 3‐Ac‐DON‐15‐glucuronide, 15‐Ac‐DON‐3‐glucuronide, DON‐3‐glucuronide, DON‐15‐glucuronide and DOM‐glucuronide was reported by Huybrechts et al. (2015).
Commercial sources for DON‐glucuronide standards are scarce. The standards described in literature were in‐house produced methods e.g. by using rat and human liver microsomes followed by isolation and purification (Versilovskis et al., 2012; Uhlig et al., 2013) or by chemical synthesis (Fruhmann et al., 2012). CRMs are not available for urinary DON biomarkers.
The performance of analytical methods for DON biomarkers have also been compared in an interlaboratory study. Three laboratories compared their LC–MS mycotoxin biomarker methods: (1) the single biomarker method, (2) the eight mycotoxins biomarker method following β‐glucuronidase digestion and IAC and SPE clean‐up, and (3) the ‘dilute and shoot’ multi‐biomarker method. At first, a pre‐interlaboratory validation revealed that there was satisfactory agreement between laboratories for DON analysis (Solfrizzo et al., 2013b). Then, the comparison of the three methods was further expanded to a human study in South Africa (Shephard et al., 2013) where urine samples from 53 female subjects were collected and distributed to the three laboratories. Creatinine concentrations were measured and urinary DON concentrations both with and without creatinine adjustment were calculated. The single biomarker method reported tDON in all samples (mean concentration 20.4 ± 49.4 ng/mL). The eight mycotoxins biomarker method detected DON in 87% of the samples (mean concentration 11.3 ± 27.1 ng/mL). The ‘dilute and shoot’ method detected DON, DON‐3‐glucuronide and DON‐15‐glucuronide in 13%, 26% and 55% of the samples, respectively with maximum concentrations of DON, DON‐3‐glucuronide and DON‐15‐glucuronide of 21 ng/mL, < LOQ and 109 ng/mL in urine, respectively. The third method was not as sensitive as the two other methods (no urinary tDON results from this method). Overall, the DON biomarker results of the different laboratories were well correlated, suggesting the compatibility between single DON biomarker and the multi‐mycotoxin biomarker approach. However, discrepancy in urinary DON concentrations among methods and the large difference in the detection sensitivity between laboratories were noted.
In contrary to methods above, Rodríguez‐Carrasco et al. (2014b) developed a novel GC–MS/MS method for detection of 15 mycotoxins and metabolites following a salting‐out liquid–liquid extraction without enzyme digestion. A GC–MS method has also been used for tDON analysis using 13C‐DON as internal standard and SPE clean‐up (Cunha and Fernandes, 2012).
3.5. Conclusions
It is important to notice that prior to the determination of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, a representative food and feed sample must be provided.
In the past, most attention has been paid to the determination of DON only, and therefore analytical methods dedicated to DON are abundantly present in the literature. Later on methods for simultaneous detection and quantification of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were developed. The LOQs differ substantially depending on the analytical scheme, matrix and technology used. However, LOQs are in the same range (μg/kg) for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. LC–MS(/MS) is currently the most frequently used method for simultaneous analysis of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. LC–MS(/MS) methods have not been formally validated through interlaboratory studies, however proficiency tests have shown that considerable analytical variability exists in the determination of DON with LC–MS(/MS) methodology.
Two different approaches are reported for the determination of DON‐3‐glucoside. The first is the use of an analytical technique able to directly detect the modified form as it is (direct methods). This approach is usually based on LC–MS analysis and requires standard calibrants. The second approach is the conversion into DON by enzymatic and/or chemical treatment before the analysis (indirect methods). Because of the lack of efficacy of the cleavage reaction, direct methods are the method of choice.
Immunochemical methods for DON, based on antigen‐antibody reactions, provide rapid and economical alternatives to chromatography, but considerable attention should be paid to the cross‐reactivities and matrix effects, which are inherent to this type of analysis.
Performance criteria for methods of analysis (screening and confirmatory) for DON used in the official control of food and feed are laid down in the regulation of the European Commission (see Section 3.3). Furthermore, (certified) reference materials and proficiency tests are part of the necessary quality control systems used in analytical laboratories. These are available for DON, but CRMs and proficiency tests are not (yet) available for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
New trends in high‐resolution MS for untargeted metabolic profiling and metabolomics will unravel and identify novel metabolites, biotransformation products and/or modified DON forms.
Research on DON biomarkers in the past decade has made great progress. Urinary DON and its metabolites can be detected in single or in multiple biomarker approaches. The single biomarker approach was of appropriate sensitivity and precision, and showed a strong correlation between tDON in urine and dietary intake. The multi‐biomarker methods detected not only DON and metabolites of DON, but also many other mycotoxins. Most of the reported analytical methods for DON biomarker analysis in urine were sensitive enough to differentiate exposure levels. However, commercial sources for DON glucuronide standards are scarce and no certified reference materials are available for urinary DON biomarkers.
4. Occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed
4.1. Occurrence data reported in the literature
A large amount of published results on the occurrence of DON for the EFSA FoodEx food categories ‘grains intended for human consumption’ and ‘grain based food products’ has also been reported in the scientific literature. However, papers with information about 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were rare and only small data sets reported analytical results as numerical values on the co‐occurrence separately for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (see Section 4.2). The occurrence data of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for the food category ‘Food for infants and young children’ were particularly scarce. Therefore,the CONTAM Panel decided not to review all available literature on the occurrence of DON, but to review only the more recently published data on DON from 2004 onwards also including data on the occurrence of 3‐Ac‐DON and 15‐Ac‐DON and of DON‐3‐glucoside alone or together with DON for the two FoodEx categories mentioned above. Only data relating to the European market were considered. However, for the category of ‘baby food and infant food’ a full review is provided on all available data in the published literature for DON, 3‐Ac‐DON and 15‐Ac‐DON and of DON‐3‐glucoside. It was noted that some articles provided only limited information, which sometimes made their interpretation difficult. Since the literature occurrence data on food and feed were not systematically checked for possible duplicate occurrence in the data reported in Section 4.2, a partial overlap with the data reported in Section 4.2 cannot be excluded.
4.1.1. Occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in grains and grain‐based products (excluding food for infants and young children) intended for human consumption
Details on published data on the occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in grains and grain products intended for human consumption (except food for infants and young children) are summarised in Table B.1 (Appendix B).
Table B.1.
Published occurrence data on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in grains and food products (excluding food for infants and young children: for them see Table B.2)
| Country | Sampling year | Type of sampling | Product | Toxin | N of samples | LOD μg/kg | LOQ μg/kg | Samples greater than LOQ | Mean of positive samples μg/kg | Mean of all samplesa μg/kg | Mean (not specified)b μg/kg | Median μg/kg | Min μg/kg | Max μg/kg | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria, Germany, Slovakiac | 2005 | Random | Wheat | DON | 23 | 8 | 20 | 23 | 1,500 | n.r. | n.r. | n.r. | 203 | 4,130 | Berthiller et al. (2009) |
| DON‐3‐glucoside | 4 | 10 | 23 | 393 | n.r. | n.r. | n.r. | 76 | 1,070 | ||||||
| Austria | 2006 | Random | Maize | DON | 54 | 16 | 40 | 54 | 753 | n.r. | n.r. | n.r. | 42 | 3,680 | Berthiller et al. (2009) |
| DON‐3‐glucoside | 4 | 10 | 54 | 141 | n.r. | n.r. | n.r. | 10 | 763 | ||||||
| Austria and UKc | n.r. | Random | Wheat bread | DON | 4 | n.r. | 100 | 0 | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | < LOQ | Vendl et al. (2010) |
| 3‐Ac‐DON | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOQ | ||||||
| Rye bread | DON | 4 | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Wheat semolina | DON | 3 | n.r. | 100 | 0 | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | < LOQ | ||||
| 3‐Ac‐DON | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Biscuits | DON | 4 | n.r. | 100 | 0 | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | < LOQ | ||||
| 3‐Ac‐DON | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Beer (including wheat beer) | DON | 4 | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Breakfast cereals (corn flakes) | DON | 3 | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Breakfast cereals (muesli) | DON | 4 | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Quinoa grain or flour | DON | 4 | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Polenta | DON | 3 | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Austria | 2011 | Random | Pale beer | DON | 10 | 2.2 | 5.4 | 4 | 13 | 6.8d | n.r. | 2.7 | < LOD | 30 | Malachova et al. (2012) |
| 3‐Ac‐DON | 2.4 | 6.8 | 0 | < LOD | n.r. | n.r. | < LOD | < LOD | < LOD | ||||||
| DON‐3‐glucoside | 0.4 | 3.5 | 7 | 8.3 | 6.3 | n.r. | 5.5 | < LOQ | 19 | ||||||
| Wheat beer | DON | 10 | 1.0 | 4.5 | 8 | 14 | 12 | n.r. | 9.2 | < LOD | 27 | ||||
| 3‐Ac‐DON | 2.2 | 8.2 | 0 | < LOD | n.r. | n.r. | < LOD | < LOD | < LOD | ||||||
| DON‐3‐glucoside | 0.9 | 3.5 | 7 | 8.6 | 6.4 | n.r. | 5.9 | < LOD | 15 | ||||||
| Dark beer | DON | 10 | 2.9 | 11 | 2 | 11 | 5 | n.r. | 5.4 | < LOD | 11 | ||||
| 3‐Ac‐DON | 4.3 | 11 | 0 | < LOD | n.r. | n.r. | < LOD | < LOD | < LOD | ||||||
| DON‐3‐glucoside | 1.4 | 4.1 | 7 | 9.6 | 7.4 | n.r. | 6.9 | < LOQ | 16 | ||||||
| Bock beer | DON | 10 | 1.2 | 4.1 | 10 | 13 | 13 | n.r. | 13 | n.r. | 22 | ||||
| 3‐Ac‐DON | 3.6 | 9.2 | 0 | < LOD | n.r. | n.r. | < LOD | < LOD | < LOD | ||||||
| DON‐3‐glucoside | 0.5 | 1.5 | 10 | 16 | 16 | n.r. | 12 | n.r. | 32 | ||||||
| Non‐alcoholic beer | DON | 10 | 1.2 | 3.0 | 1 | 3.7 | 0.9 | n.r. | 0.6 | < LOD | 3.7 | ||||
| 3‐Ac‐DON | 2.6 | 6.0 | 0 | < LOD | n.r. | n.r. | < LOD n.r. | < LOD | < LOD | ||||||
| DON‐3‐glucoside | 0.4 | 1.4 | 5 | 2.3 | 1.3 | n.r. | 0.9 | < LOD | 3.1 | ||||||
| Shandy beer | DON | 10 | 1.5 | 3.9 | 1 | 6.4 | 2.1 | n.r. | 1.9 | < LOQ | 6.4 | ||||
| 3‐Ac‐DON | 2.7 | 10 | 0 | < LOD | n.r. | n.r. | < LOD | < LOD | < LOD | ||||||
| DON‐3‐glucoside | 0.4 | 1.3 | 6 | 3.5 | 2.3 | n.r. | 2.3 | < LOQ | 5.5 | ||||||
| Austria, Hungary, Croatia, Serbiae | 2011–2012 | Random | Pale beer | DON | 217 | 2.2 | 5.4 | 118 | 12.0 | 7.5 | n.r. | 5.6 | n.r. | 89.3 | Varga et al. (2013) |
| 3‐Ac‐DON | 2.4 | 6.8 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | 0.4 | 3.5 | 142 | 9.3 | 6.7 | n.r. | 5.2 | n.r. | 81.3 | ||||||
| Wheat beer | DON | 46 | 1 | 4.5 | 36 | 18.4 | 14.6 | n.r. | 9.5 | n.r. | 49.6 | ||||
| 3‐Ac‐DON | 2.2 | 8.2 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | 0.9 | 3.5 | 32 | 11.5 | 8.4 | n.r. | 5.4 | n.r. | 28.4 | ||||||
| Dark beer | DON | 47 | 2.9 | 11 | 14 | 22.4 | 9.0 | n.r. | 5.5 | < LOD | 45.0 | ||||
| 3‐Ac‐DON | 4.3 | 11 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | 1.4 | 4.1 | 28 | 10.7 | 6.9 | n.r. | 4.5 | n.r. | 26.2 | ||||||
| Bock beer | DON | 20 | 1.2 | 4.1 | 18 | 13.8 | 12.4 | n.r. | 10.8 | < LOD | 27.1 | ||||
| 3‐Ac‐DON | 3.6 | 9.2 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | 0.5 | 1.5 | 20 | 14.8 | 14.8 | n.r. | 11.7 | 2.4 | 33.3 | ||||||
| Non‐alcoholic beer | DON | 19 | 1.2 | 3.0 | 5 | 8.7 | 2.7 | n.r. | < LOD | < LOD | 26.1 | ||||
| 3‐Ac‐DON | 2.6 | 6.0 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | 0.4 | 1.4 | 9 | 3.0 | 1.5 | n.r. | < LOD | (< LOD) | 6.6 | ||||||
| Shandy beer | DON | 25 | 1.5 | 3.9 | 13 | 6.9 | 4.4 | n.r. | 4.2 | n.r. | 12.7 | ||||
| 3‐Ac‐DON | 2.7 | 10 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | 0.4 | 1.3 | 20 | 3.8 | 3.2 | n.r. | 2.9 | n.r. | 7.9 | ||||||
| Belgium | 2010–2011 | Random | Fibre‐enriched bread | DON | 52 | 8‐13 | 16‐26 | 44 | n.r. | n.r. | 34 | n.r. | (< LOQ) | 138 | De Boevre et al. (2012) |
| 3‐Ac‐DON | 20 | n.r. | n.r. | 14 | n.r. | (< LOQ) | 74 | ||||||||
| 15‐Ac‐DON | 17 | n.r. | n.r. | 9 | n.r. | (< LOQ) | 45 | ||||||||
| DON‐3‐glucoside | 25 | n.r. | n.r. | 34 | n.r. | (< LOQ) | 425 | ||||||||
| Bran‐enriched bread | DON | 36 | 8‐13 | 16‐26 | 27 | n.r. | n.r. | 25 | n.r. | (< LOQ) | 127 | ||||
| 3‐Ac‐DON | 16 | n.r. | n.r. | 16 | n.r. | (< LOQ) | 59 | ||||||||
| 15‐Ac‐DON | 16 | n.r. | n.r. | 7 | n.r. | (< LOQ) | 45 | ||||||||
| DON‐3‐glucoside | 19 | n.r. | n.r. | 21 | n.r. | (< LOQ) | 103 | ||||||||
| Corn‐flakes | DON | 61 | 7‐12 | 14‐24 | 40 | n.r. | n.r. | 44 | n.r. | (< LOQ) | 718 | ||||
| 3‐Ac‐DON | 43 | n.r. | n.r. | 31 | n.r. | (< LOQ) | 431 | ||||||||
| 15‐Ac‐DON | 39 | n.r. | n.r. | 10 | n.r. | (< LOQ) | 194 | ||||||||
| DON‐3‐glucoside | 31 | n.r. | n.r. | 13 | n.r. | (< LOQ) | 63 | ||||||||
| Popcorn | DON | 12 | n.r. | n.r. | 6 | n.r. | n.r. | 49 | n.r. | (< LOQ) | 442 | ||||
| 3‐Ac‐DON | 10 | n.r. | n.r. | 30 | n.r. | (< LOQ) | 69 | ||||||||
| 15‐Ac‐DON | 10 | n.r. | n.r. | 26 | n.r. | (< LOQ) | 55 | ||||||||
| DON‐3‐glucoside | 8 | n.r. | n.r. | 33 | n.r. | (< LOQ) | 96 | ||||||||
| Oatmeal | DON | 13 | 5‐12 | 10‐24 | 5 | n.r. | n.r. | 18 | n.r. | (< LOQ) | 91 | ||||
| 3‐Ac‐DON | n.r. | n.r. | n.r. | 45 | n.r. | (< LOQ) | 116 | ||||||||
| 15‐Ac‐DON | n.r. | 27 | n.r. | 7 | n.r. | (< LOQ) | 27 | ||||||||
| DON‐3‐glucoside | n.r. | n.r. | n.r. | 28 | n.r. | (< LOQ) | 97 | ||||||||
| Belgium | 2012 | Random | Wheat | DON | 93 | 31 | 62 | 81 | 1,053 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | Vanheule et al. (2014) |
| 3‐Ac‐DON | 40 | 81 | 4 | 38 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||||
| 15‐Ac‐DON | 25 | 49 | 2 | 87 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||||
| DON‐3‐glucoside | 72 | 143 | 54 | 250 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||||
| Barley | DON | 65 | 31 | 62 | 64 | 2,029 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||
| 3‐Ac‐DON | 40 | 81 | 8 | 120 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||||
| 15‐Ac‐DON | 25 | 49 | 10 | 97 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||||
| DON‐3‐glucoside | 72 | 143 | 59 | 390 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||||
| Triticale | DON | 10 | 31 | 62 | 8 | 1,145 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||
| 3‐Ac‐DON | 40 | 81 | 0 | (< LOD) | n.r. | n.r. | n.r. | (< LOD) | (< LOD) | ||||||
| 15‐Ac‐DON | 25 | 49 | 0 | (< LOD) | n.r. | n.r. | n.r. | (< LOD) | (< LOD) | ||||||
| DON‐3‐glucoside | 72 | 143 | 6 | 169 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||||
| Bread | DON | 25 | 31 | 62 | 24 | 316 | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||
| 3‐Ac‐DON | 40 | 81 | 0 | (< LOD) | n.r. | n.r. | n.r. | (< LOD) | (< LOD) | ||||||
| 15‐Ac‐DON | 25 | 49 | 0 | (< LOD) | n.r. | n.r. | n.r. | (< LOD) | (< LOD) | ||||||
| DON‐3‐glucoside | 72 | 143 | 15 | n.r. | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||||
| Breakfast cereals | DON | 20 | 31 | 62 | 5 | n.r. | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||
| 3‐Ac‐DON | 40 | 81 | 0 | (< LOD) | n.r. | n.r. | n.r. | (< LOD) | (< LOD) | ||||||
| 15‐Ac‐DON | 25 | 49 | 0 | (< LOD) | n.r. | n.r. | n.r. | (< LOD) | (< LOD) | ||||||
| DON‐3‐glucoside | 72 | 143 | 5 | n.r. | n.r. | n.r. | n.r. | (< LOQ) | n.r. | ||||||
| Czech Republic | 2010 | Random | White flour products | DON | 17 | n.r. | 12.5 | 16 | n.r. | n.r. | 125 | 96 | < LOQ | 350 | Malachova et al. (2011) |
| DON‐3‐glucoside | n.r. | 5 | 14 | n.r. | n.r. | 15 | 10 | < LOQ | 30 | ||||||
| Mixed flour products | DON | 36 | n.r. | 12.5 | 32 | n.r. | n.r. | 139 | 68 | < LOQ | 431 | ||||
| DON‐3‐glucoside | n.r. | 5 | 28 | n.r. | n.r. | 19 | 14 | < LOQ | 41 | ||||||
| Breakfast cereals | DON | 7 | n.r. | 12.5 | 2 | n.r. | n.r. | 189 | LOQ | < LOQ | 347 | ||||
| DON‐3‐glucoside | n.r. | 5 | 6 | n.r. | n.r. | 35 | 30 | < LOQ | 66 | ||||||
| Snacks | DON | 34 | n.r. | 12.5 | 21 | n.r. | n.r. | 124 | 36 | < LOQ | 320 | ||||
| DON‐3‐glucoside | n.r. | 5 | 28 | n.r. | n.r. | 32 | 19 | < LOQ | 94 | ||||||
| Flours | DON | 22 | n.r. | 12.5 | 16 | n.r. | n.r. | 103 | 41 | < LOQ | 594 | ||||
| DON‐3‐glucoside | n.r. | 5 | 15 | n.r. | n.r. | 15 | 16 | < LOQ | 72 | ||||||
| Czech Republic | 2012 | Random | Malt | DON | 6 | n.r. | n.r. | 6 | 68 | n.r. | n.r. | n.r. | 8.9 | 139 | Zachariasova et al., (2012) |
| DON‐3‐glucoside | n.r. | n.r. | 6 | 105 | n.r. | n.r. | n.r. | 12.9 | 186 | ||||||
| Beer | DON | 15 | n.r. | n.r. | 15 | 21 | n.r. | n.r. | n.r. | 5.6 | 62 | ||||
| DON‐3‐glucoside | n.r. | n.r. | 15 | 34 | n.r. | n.r. | n.r. | 6.0 | 82 | ||||||
| Wheat, wheat‐rye, multicereal and sunflower baguettes | DON | 14 | n.r. | n.r. | 14 | 252 | n.r. | n.r. | n.r. | 43 | 431 | ||||
| DON‐3‐glucoside | n.r. | n.r. | 14 | 25 | n.r. | n.r. | n.r. | 6 | 33 | ||||||
| BIO wheat flakes | DON | 1 | n.r. | n.r. | 1 | n.r. | n.r. | n.r. | n.r. | 347 | 347 | ||||
| DON‐3‐glucoside | n.r. | n.r. | 1 | n.r. | n.r. | n.r. | n.r. | 46 | 46 | ||||||
| Denmark | 2006–2007 | RANDOM | Spring barley | DON | 15 | 18 | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | < 60 | Rasmussen et al. (2012) |
| 3‐Ac‐DON | 40 | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | ||||||
| DON‐3‐glucoside | 35 | n.r. | 0 | n.r. | n.r. | n.r. | (< LOD) | (< LOD) | < LOD | ||||||
| 2007–2008 | Rye | DON | 12 | 18 | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | < 50 | |||
| 3‐Ac‐DON | 40 | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | ||||||
| DON‐3‐glucoside | 35 | n.r. | 0 | n.r. | n.r. | n.r. | (< LOD) | (< LOD) | < LOD | ||||||
| 2007–2010 | Oat | DON | 11 | 18 | n.r. | 9 | (810) | n.r. | n.r. | (683) | < LOD | 2,216 | |||
| 3‐Ac‐DON | 40 | n.r. | 4 | (102) | n.r. | n.r. | (< LOD) | < LOD | 136 | ||||||
| DON‐3‐glucoside | 35 | n.r. | 5 | (224) | n.r. | n.r | (< LOD) | < LOD | 287 | ||||||
| 2007–2010 | Triticale | DON | 5 | 18 | n.r. | 4 | (306) | n.r. | n.r. | (192) | < LOD | 737 | |||
| 3‐Ac‐DON | 40 | n.r. | 0 | (< LOD) | n.r. | n.r. | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | 35 | n.r. | 1 | (109) | n.r. | n.r. | (< LOD) | < LOD | 109 | ||||||
| 2007–2010 | Winter wheat | DON | 6 | 18 | n.r. | 6 | (910) | n.r. | n.r. | (715) | 46 | 2,638 | |||
| 3‐Ac‐DON | 40 | n.r. | 0 | (< LOD) | n.r. | n.r. | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | 35 | n.r. | 3 | (190) | n.r. | n.r. | (65) | (< LOD) | 342 | ||||||
| Europe (25 countries) | 2010–2011 | Random | Beer | DON | 106 | 0.5 | 1.5 | 72 | n.r. | n.r. | 2.1 | n.r. | < LOD | 18.6 | Bertuzzi et al. (2011) |
| 3‐Ac‐DON | 0.5 | 1.4 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | ||||||
| 15‐Ac‐DON | 1.0 | 2.8 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | ||||||
| Finland | 2013 | Random | Barley | DON | 34 | 1.2 | 3.6 | 28f | n.r. | n.r. | 234g | n.r. | (< LOD) | 1,180 | Nathanail et al. (2015) |
| 3‐Ac‐DON | 3.4 | 10.2 | 14f | n.r. | n.r. | < LOQg | n.r. | (< LOD) | 23.6 | ||||||
| DON‐3‐glucoside | 2.8 | 8.4 | 25f | n.r. | n.r. | 148g | n.r. | (< LOD) | 1,300 | ||||||
| Oats | DON | 31 | 1.8 | 5.4 | 31 f | n.r. | n.r. | 2,690g | n.r. | n.r. | 23,800 | ||||
| 3‐Ac‐DON | 5.1 | 15.3 | 24f | n.r. | n.r. | 341g | n.r. | (< LOD) | 2,720 | ||||||
| DON‐3‐glucoside | 3.9 | 11.7 | 27f | n.r. | n.r. | 806g | n.r. | (< LOD) | 6,600 | ||||||
| Wheat | DON | 30 | 1.3 | 3.9 | 29f | n.r. | n.r. | 866g | n.r. | (< LOD) | 5,510 | ||||
| 3‐Ac‐DON | 3.7 | 11.1 | 14 f | n.r. | n.r. | 24.6 g | n.r. | (< LOD) | 71 | ||||||
| DON‐3‐glucoside | 2.2 | 6.6 | 25f | n.r. | n.r. | 174g | n.r. | (< LOD) | 922 | ||||||
| France | 2009 | n.r. | Maize kernels | DON | 5 | 1 | 2 | 5 | (725) | n.r. | n.r. | (223) | 64 | 2,864 | Desmarchelier and Seefelder (2011) |
| DON‐3‐glucoside | 1 | 2 | 5 | (73) | n.r. | n.r. | (25) | 4 | 237 | ||||||
| Germany | 2009 | n.r. | Wheat kernels | DON | 3 | 1 | 2 | 3 | (140) | n.r. | n.r. | (143) | 97 | 181 | Desmarchelier and Seefelder (2011) |
| DON‐3‐glucoside | 1 | 2 | 3 | (10) | n.r. | n.r. | (11) | 8 | 11 | ||||||
| Durum semolina | DON | 1 | 1 | 2 | 1 | (177) | n.r. | n.r. | (177) | 177 | 177 | ||||
| DON‐3‐glucoside | 1 | 2 | 1 | (12) | n.r. | n.r. | (12) | 12 | 12 | ||||||
| Durum grains | DON | 1 | 1 | 2 | 1 | (603) | n.r. | n.r. | (603) | 603 | 603 | ||||
| DON‐3‐glucoside | 1 | 2 | 1 | (74) | n.r. | n.r. | (74) | 74 | 74 | ||||||
| Italy | 2009–2010 | targeted | Durum wheat | DON | 150 | 8 | n.r. | 150 | n.r. | n.r. | n.r. | n.r. | 47 | 3,715 | Dall'Asta et al. (2013) |
| 3‐Ac‐DON | 8 | n.r. | 86 | n.r. | n.r. | n.r. | 134 | < LOD | 203 | ||||||
| 15‐Ac‐DON | 8 | n.r. | 150 | n.r. | n.r. | n.r. | 92 | n.r. | 244 | ||||||
| DON‐3‐glucoside | 8 | n.r. | 127 | n.r. | n.r. | n.r. | n.r. | < LOD | 842 | ||||||
| Italy | 2009–2010 | Random, 7 regions | Durum wheat | DON | 47 | 5 | 15 | 28 | 172 | 102 | n.r. | n.r. | n.r. | 1,230 | Alkadri et al. (2014) |
| 3‐Ac‐DON | 3 | 10 | 4 | 23 | 2 | n.r. | n.r. | n.r. | 33 | ||||||
| 15‐Ac‐DON | 3 | 10 | 15 | 45 | 14 | n.r. | n.r. | n.r. | 105 | ||||||
| Poland | 2009 | n.r. | Barley flour | DON | 1 | 1 | 2 | 1 | (5.5) | n.r. | n.r. | (5.5) | (5.5) | 5.5 | Desmarchelier and Seefelder (2011) |
| DON‐3‐glucoside | 1 | 2 | 0 | (< LOQ) | n.r. | n.r. | < LOQ | < LOQ | < LOQ | ||||||
| Oat flour | DON | 1 | 1 | 2 | 1 | (6.3) | n.r. | n.r. | (6.3) | (6.3) | 6.3 | ||||
| DON‐3‐glucoside | 1 | 2 | 0 | (< LOQ) | n.r. | n.r. | (< LOQ) | (< LOQ) | (< LOQ) | ||||||
| Wheat flour | DON | 2 | 1 | 2 | 2 | (10) | n.r. | n.r. | (10) | (3) | (17) | ||||
| DON‐3‐glucoside | 1 | 2 | 1 | (2.6) | n.r. | n.r. | (2.3) | < LOD | 2.6 | ||||||
| Malt syrup | DON | 1 | 1 | 2 | 0 | (< LOD) | n.r. | n.r. | (< LOD) | (< LOD) | < LOD | ||||
| DON‐3‐glucoside | 1 | 2 | 0 | (< LOD) | n.r. | n.r. | (< LOD) | (< LOD) | < LOD | ||||||
| Romania | 2009 | n.r. | Wheat flour | DON | 1 | 1 | 2 | 1 | (45) | n.r. | n.r. | (45) | (45) | 45 | Desmarchelier and Seefelder (2011) |
| DON‐3‐glucoside | 1 | 2 | 1 | (4.8) | n.r. | n.r. | (4.8) | (4.8) | 4.8 | ||||||
| Spain | 2012 | random | Wheat‐based cereals | DON | 119 | n.r. | 1.25 | 95 | n.r. | n.r. | 12.7 | n.r. | (< LOQ) | 83 | Rodríguez‐Carrasco et al. (2014b) |
| 3‐Ac‐DON | n.r. | 1.25 | 4 | n.r. | n.r. | 4.6 | n.r. | (< LOQ) | 5.3 | ||||||
| Rice‐based cereals | DON | 23 | n.r. | 1.25 | 3 | n.r. | n.r. | 5 | n.r. | (< LOQ) | 5.5 | ||||
| 3‐Ac‐DON | n.r. | 2.5 | 0 | n.r. | n.r. | (< LOQ) | n.r. | (< LOQ) | (< LOQ) | ||||||
| Maize‐based cereals | DON | 17 | n.r. | 1.25 | 5 | n.r. | n.r. | 10.5 | n.r. | (< LOQ) | 22.1 | ||||
| 3‐Ac‐DON | n.r. | 1.25 | 0 | n.r. | n.r. | (< LOQ) | n.r. | (< LOQ) | (< LOQ) | ||||||
| Spelt‐based cereals | DON | 8 | n.r. | n.r. | 5 | n.r. | n.r. | 25.6 | n.r. | (< LOQ) | 56.8 | ||||
| 3‐Ac‐DON | n.r. | n.r | 0 | n.r. | n.r. | (< LOQ) | n.r. | (< LOQ) | (< LOQ) | ||||||
| Oat‐based cereals | DON | 8 | n.r. | n.r. | 0 | n.r. | n.r. | (< LOQ) | n.r. | (< LOQ) | (< LOQ) | ||||
| 3‐Ac‐DON | n.r. | n.r | 0 | n.r. | n.r. | (< LOQ) | n.r. | (< LOQ) | (< LOQ) | ||||||
| Soy‐based cereals | DON | 4 | n.r. | n.r. | 1 | n.r. | n.r. | 34.8 | n.r. | (< LOQ) | 34.8 | ||||
| 3‐Ac‐DON | n.r. | n.r | 0 | n.r. | n.r. | (< LOQ) | n.r. | (< LOQ) | (< LOQ) | ||||||
| Tapioca‐based cereals | DON | 3 | n.r. | n.r. | 1 | n.r. | n.r. | 18.3 | n.r. | (< LOQ) | 18.3 | ||||
| 3‐Ac‐DON | n.r. | n.r | 0 | n.r. | n.r. | (< LOQ) | n.r. | (< LOQ) | (< LOQ) | ||||||
| UK | n.r. | Random | Wheat four | DON | 3 | n.r. | 100 | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | 237 | Vendl et al. (2010) |
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Wheat bread‐ whole meal | DON | 3 | n.r. | 100 | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | < LOQ | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | < LOQ | ||||||
| Maize meal and flour | DON | 3 | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Extruded maize snacks | DON | 3 | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Pasta | DON | 4 | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Canned maize | DON | 4 | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Breakfast cereals – wheat | DON | 4 | n.r. | 100 | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | (< LOQ) | < LOQ | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Breakfast cereals – bran flakes | DON | 3 | n.r. | 100 | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | 254 | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Breakfast cereals – oats | DON | 3 | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Crackers | DON | 3 | n.r. | 100 | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | 248 | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Popcorn | DON | 3 | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Cereal snack bars | DON | 3 | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Buck wheat grain or flour | DON | 3 | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| Extruded oat snacks | DON | 3 | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||
| 3‐Ac‐DON | n.r. | n.r. | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| DON‐3‐glucoside | n.r. | 100 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | ||||||
| UK | 2009 | n.r. | Wheat flour | DON | 3 | 1 | 2 | 3 | (981) | n.r. | n.r. | (873) | 592 | 1,478 | Desmarchelier and Seefelder (2011) |
| DON‐3‐glucoside | 1 | 2 | 3 | (65) | n.r. | n.r. | (60) | 37 | 99 |
n.r.: not reported; LOD: limit of detection; N: number of samples; DON: deoxynivalenol; Ac: acetyl.
Only those studies, which reported DON‐3‐glucoside and/or 3‐Ac‐DON or 15‐Ac‐DON are reported. Values in brackets were calculated by the CONTAM Panel based on the data provided in the papers.
Mean values of all samples are calculated by setting samples in which the compounds were not detected to 0 and trace value below LOQ to LOD.
It was not reported in the paper whether the mean was calculated for all samples or for positive samples only.
Exact origin not indicated.
For the calculation of the mean of all samples, 50% of the determined LOD and of the determined LOQ in the specific beer category were used for values below the LOD and LOQ, respectively.
Beers produced in 38 countries (88% European), purchased in Austria, Hungary, Croatia and Serbia.
Number of samples greater than LOD.
Concentrations below the respective LOQ were assigned a value of LOQ/2; values below LOD were treated as non‐contaminated.
4.1.1.1. Grains intended for human consumption
Nine studies reported the occurrence of DON in combination with 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in grains intended for human consumption. The results are extensively presented in Table B.1 (Appendix B), and summarised data are given in Table 6 below. Several papers provided diverse and sometimes only limited information making comparisons of reports difficult. Since maximum concentrations were reported in most of the studies, these are specifically presented in Table 6. It appeared that maximum concentrations of DON, DON‐3‐glucoside, 3‐Ac‐DON and 15‐Ac‐DON varied widely between studies, which may partly reflect different fungal varieties, and differences in climatic and agricultural conditions during the growing seasons in the various regions, where the grains were grown. The studies also showed that the highest concentrations were found for DON, while maximum concentrations for DON‐3‐glucoside, 3‐Ac‐DON and 15‐Ac‐DON were (much) lower.
Table 6.
Summary of published study results on occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in grains intended for human consumption
| Country | Type of samples | Number of samples | Maximum (μg/kg) DON | Maximum (μg/kg) DON‐3‐glucoside | Maximum (μg/kg) 3‐Ac‐DON | Maximum (μg/kg) 15‐Ac‐DON | Observations and remarks | Reference |
|---|---|---|---|---|---|---|---|---|
| Austria, Germany, Slovakia | Wheat, maize | 77 | 4,130 | 1,070 | n.r. | n.r. | Berthiller et al. (2009) | |
| Belgium | Grains | 168 | 2,029 | 390 | 120 | 97 | Data reported as means of positive samples, instead of maximum values. (All barley) | Vanheule et al. (2014) |
| Denmark | Grains | 49 | 2,638 | 342 | 136 | n.r. |
Maximum value for DON: winter wheat Maximum value for DON‐3‐glucoside: oats Maximum value 3‐Ac‐DON: winter wheat |
Rasmussen et al. (2012) |
| Italy | Durum wheat | 150 | 3,715 | 842 | 203 | 244 | Dall'Asta et al. (2013) | |
| Italy | Durum wheat | 47 | 1,230 | n.r. | 33 | 105 | Alkadri et al. (2014) | |
| Finland | Barley, oats, wheat | 95 | 23,800 | 6,600 | 2,720 | n.r. |
All oats Values reported as unusually high as compared to previous years |
Nathanail et al. (2015) |
| Austria, UK | Grains | 16 | 237 | n.d. | n.d. | n.r. | Vendl et al. (2010) | |
| France, Germany, Poland, Romania, UK | Grains | 18 | 2,864 | 237 | n.r. | n.r. | Desmarchelier and Seefelder (2011) | |
| Poland | Mixtures of oat, wheat, barley | 18 | 21.2 | n.r. | 2.8 | 4.3 | Data reported as means | Ostrowska‐Kołodziejczak et al. (2016) |
n.r.: not reported; n.d.: not detected.
4.1.1.2. Grain‐based food products
Twelve published studies described the occurrence of DON in combination with 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in grain‐based food products. The results are extensively presented in Table B.1 (Appendix B), and summarised data are given in Table 7 below. Maximum concentrations are specifically presented in Table 7 for reasons mentioned in Section 4.1.1.1 varied widely between studies, but the maximum concentration were (much) lower than found for grains intended for human consumption (see Section 4.1.1.1). Interestingly, in the category malt and beer maximum concentrations for DON‐3‐glucoside were found at the same level as maximum concentrations for DON. This might be explained by the formation and release of DON‐3‐glucoside during germination and brewing.
Table 7.
Summary of published study results on occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in grain‐based food products
| Country | Type of samples | Number of samples | Maximum (μg/kg) DON | Maximum (μg/kg) DON‐3‐glucoside | Maximum (μg/kg) 3‐Ac‐DON | Maximum (μg/kg) 15‐Ac‐DON | Observations and remarks | Reference |
|---|---|---|---|---|---|---|---|---|
| Austria, UK | Cereal‐based foods | 62 |
254 248 |
n.d. | n.d. | n.r. | Maximum DON values given for Austria (bran flakes) and UK (crackers), respectively | Vendl et al. (2010) |
| Belgium | Cereal‐based foods | 174 | 718 | 425 | 431 | 194 |
Maximum values for DON, 3‐Ac‐DON and 15‐Ac‐DON: corn flakes Maximum value for DON‐3‐glucoside: fibre‐enriched bread |
De Boevre et al. (2012) |
| Belgium | Bread and breakfast cereals | 45 | 316 | n.d. | n.d. | n.d. | Data reported as means of positive samples instead of maximum values; mean value for DON: bread | Vanheule et al. (2014) |
| Poland | Malt syrup | 1 | n.d. | n.d. | n.r. | n.r. | Desmarchelier and Seefelder (2011) | |
| Italy | Grain‐based cereals | 182 | 83 | n.r. | 5 | n.r. | Maximum values in wheat‐based cereals | Rodríguez‐Carrasco et al. (2014b) |
| Czech Republic | Cereal‐based products | 116 | 594 | 94 | n.r | n.r. |
Maximum value DON: flour Maximum value DON‐3‐glucoside: snacks |
Malachova et al. (2011) |
| Czech Republic | malt, beer, commercially baked goods | 36 |
139 62 431 |
186 82 33 |
n.r. | n.r. | Maximum values given for malt, beer and baked goods respectively | Zachariasova et al. (2012) |
| Germany | Beer | 9 | 28 | 63 | n.r. | n.r. | Habler et al. (2016) | |
| Germany | Barley malt from 2012, 2013, 2014 | 30 | 10,300 | 19,000 | 436 | 287 | Maximum values were all from 2012 | Habler and Rychlik (2016) |
| Produced in 38 countries, purchased in Central Europe | Beer | 374 | 89 | 81 | n.d. | n.r. | Varga et al. (2013) | |
| Austria | Beer | 60 | 30 | 32 | n.d. | n.r. | Malachova et al. (2012) | |
| 25 European countries | Beer | 106 | 19 | n.r. | n.d. | n.d. | Bertuzzi et al. (2011) |
n.r.: not reported; n.d.: not detected.
4.1.2. Occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food for infants and young children
In Table B.2 (Appendix B), published data on the occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in baby food and infant food are summarised. The data have been published in five different papers and they represent four countries from southern, western and central Europe. Altogether, 179 samples were investigated. In all five studies analyses were performed for DON; in one study, 3‐Ac‐DON and DON‐3‐glucoside were also looked for; and in another study, analyses were performed for 3‐Ac‐DON and 15‐Ac‐DON in addition to DON. DON was found in four studies with occurrence percentages ranging from 36% to 76% and in levels up to 286 μg/kg (in baby food from Spain). The levels of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were below the LOD in all samples investigated. Results recently reported by Pereira et al. (2015) for 12 samples of baby food, investigated for DON, 3‐Ac‐DON and 15‐Ac‐DON, were not included in Table B.2 (Appendix B) because of insufficient information on the methodology used.
Table B.2.
Published occurrence data on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food for infants and young children
| Country | Sampling year | Type of sampling | Product | Toxin | N of samples | LOD μg/kg | LOQ μg/kg | Samples greater than LOQ | Mean of positive samples μg/kg | Mean of all samplesa μg/kg | Mean (not mentioned)b μg/kg | Median μg/kg | Min μg/kg | Max μg/kg | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Italy | 2008–2009 | Random | Baby food | DON | 44 | 60 | n.r. | 16 | n.r. | n.r. | n.r. | n.r. | (< LOD) | n.r. | Romagnoli et al. (2010) |
| Italy | n.r. | Random | Cereal‐based baby foods | DON | 25 | 1 | 10 | 19 | 103 | n.r. | n.r. | n.r. | (< LOD) | 268 | Juan et al. (2014) |
| 3‐Ac‐DON | 25 | 2 | 10 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | |||||
| 15‐Ac‐DON | 25 | 2 | 10 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | |||||
| Spain | n.r. | n.r. | Baby food | DON | 30 | n.r. | n.r. | 12 | n.r. | n.r. | 131 | n.r. | (< LOQ) | 286 | Cano‐Sancho et al. (2011) |
| UK and Austriac | n.r. | Random | Wheat‐based baby foods | DON | 3 | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | Vendl et al. (2010) |
| 3‐Ac‐DON | 3 | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | |||||
| DON‐3‐glucoside | 3 | n.r. | 10 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | |||||
| UK | n.r. | Random | Oat‐based baby foods | DON | 3 | n.r. | 100 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | Vendl et al. (2010) |
| 3‐Ac‐DON | 3 | n.r. | n.r. | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | |||||
| DON‐3‐glucoside | 3 | n.r. | 10 | 0 | (< LOD) | (< LOD) | (< LOD) | (< LOD) | (< LOD) | < LOD | |||||
| UK | n.r. (presumably 2011 | Random | Foods for infants and young children | DON | 77 | n.r. | 10 | 28 | 43 | n.r. | n.r. | < LOQ | < LOQ | 217 | FSA (2011) |
n.r.: not reported; LOD: limit of detection; N: number of samples; DON: deoxynivalenol; Ac: acetyl.
Values in brackets were calculated values by the CONTAM Panel based on the data provided in the papers.
Mean values of all samples are calculated by setting samples in which the compounds were not detected to 0 and trace value below LOQ to LOD.
It was not reported in the paper whether the mean was calculated for all samples or for positive samples only.
Exact origin not indicated.
4.1.3. Occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in feed
EFSA reviewed the occurrence of DON in feeds in 2004 (EFSA, 2004), and therefore the CONTAM Panel has only reviewed papers reporting the occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside published from 2004 onwards. In common with many other reviews, EFSA (2004) concluded that the occurrence of DON is predominantly associated with cereals and cereal by‐products. A number of other surveys of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in feeds, particularly on DON, have been undertaken since 2004 but have come to similar conclusions. In this section, more emphasis is given to those studies which reported occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in other feed materials than cereal grains because the occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in cereal grains was well covered by the EFSA occurrence database. The selected studies are briefly summarised below, with details given in Table B.3 (Appendix B).
Table B.3.
Published data on the occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in feed materials since 2004
| Country | Year | Product | Toxin | No. of samples | LOD μg/kg | LOQ μg/kg | Number of positive samplesa | Median of positive samplesa (μg/kg) | Mean of positive samples(b) (μg/kg) | Maximum (μg/kg) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Netherlands | 2002–2004 | Maize silage | DON | 140 | n.r. | 250 | 101 | n.r. | 854 | 3,142 | Driehuis et al. (2008b) |
| Wheat silage | DON | 30 | n.r. | 3 | n.r. | 621 | 1,165 | ||||
| 2005 | Silagec | DON | 47 | n.r. | 125 | 25 | n.r. | 550 | 1,250 | Driehuis et al. (2008a) | |
| Compound feed | DON | 72 | n.r. | 125 | 39 | n.r. | 433 | 2,408 | |||
| Ensiled by‐products d | DON | 29 | n.r. | 125 | 0 | n.r. | n.r. | n.r. | |||
| Feed commodities e | DON | 8 | n.r. | 125 | 3 | n.r. | n.r. | n.r. | |||
| Forage productsf | DON | 13 | n.r. | 125 | 2 | n.r. | n.r. | n.r. | |||
| Italy | n.r. | Maizeg | DON | 35 | n.r. | n.r. | 13 | 810 | 1,000 | 1,900 | Cortinovis et al. (2012) |
| Barleyg | DON | 15 | n.r. | n.r. | 11 | 650 | 600 | 900 | |||
| Oatsg | DON | 12 | n.r. | n.r. | 1 | n.r. | 400 | 400 | |||
| Rice brang | DON | 10 | n.r. | n.r. | 3 | n.r. | 800 | 1,200 | |||
| Germany | 2007–2008 | Muesli and mash‐type horse feedh | DON | 62 | 10 | n.r. | 62 | n.r. | 410 | 4,900 | Liesener et al. (2010) |
| Portugal | n.r. | Compound feeds for pigs | DON | 277 | 100 | n.r. | 47 | n.r. | 223 | 864 | Almeida et al. (2011) |
| Austria | 2007 | Dry dog food | DON | 76 | 10 | n.r. | 63 | n.r. | n.r. | 1,390 | Böhm et al. (2010) |
| EUi | 2008 | Wheat | DON | 8 | n.r. | n.r. | 6 | 1,898 | 2,657 | 8,841 | Monbaliu et al. (2010) |
| Wheat | 3‐Ac‐DON | 8 | n.r. | n.r. | 3 | 18.0 | 33.3 | 67 | |||
| Wheat | 15‐Ac‐DON | 8 | n.r. | n.r. | 5 | 23.0 | 38.2 | 100 | |||
| Maize | DON | 42 | n.r. | n.r. | 35 | 520 | 891 | 9,528 | |||
| Maize | 3‐Ac‐DON | 42 | n.r. | n.r. | 31 | 21.0 | 36.5 | 339 | |||
| Maize | 15‐Ac‐DON | 42 | n.r. | n.r. | 26 | 79.5. | 134 | 1,047 | |||
| Switzerland | 2005–2007 | Maize grain | DON | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | Dorn et al. (2011) |
| Maize plant (minus grain) | DON | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | |||
| n.r. | Maize silage | DON | 20 | n.r. | n.r. | 19 | n.r. | 1,356 | 2,990 | Eckard et al. (2011) | |
| Maize silage | Sum of 3‐Ac‐ and 15‐Ac‐DON | 19 | n.r. | n.r. | 2 | n.r. | 217 | 300 | |||
| Lithuania | 2007 | Winter rapeseed | DON | 8 | n.r. | n.r. | 8 | n.r. | 165 | 176 | Mankeviciene et al. (2011) |
| Spring rapeseed | DON | 8 | n.r. | n.r. | 8 | n.r. | 133 | 181 | |||
| Linseed | DON | 6 | n.r. | n.r. | 6 | n.r. | 155 | 163 | |||
| 2008 | Winter rapeseed | DON | 25 | n.r. | n.r. | 21 | n.r. | 209 | 278 | ||
| Spring rapeseed | DON | 11 | n.r. | n.r. | 2 | n.r. | <LOD | 226 | |||
| Linseed | DON | 19 | n.r. | n.r. | 19 | n.r. | 210 | 226 | |||
| 2009 | Linseed | DON | 10 | n.r. | n.r. | 10 | n.r. | 116 | 124 | ||
| Rapeseed cake | DON | 7 | n.r. | n.r. | 7 | n.r. | 387 | n.r. | |||
| Belgium | 2010–2011 | Poultry feed | DON | 14 | 5–12 | 10–24 | – | – | 197 | 894 | De Boevre et al. (2012) |
| 3‐Ac‐DON | 14 | – | – | 47 | 182 | ||||||
| 15‐Ac‐DON | 14 | – | – | 50 | 190 | ||||||
| DON‐3‐glucoside | 14 | – | – | 30 | 90 | ||||||
| Piglet feed | DON | 8 | 5–12 | 10–24 | – | – | 318 | 892 | |||
| 3‐Ac‐DON | 8 | – | – | 25 | 136 | ||||||
| 15‐Ac‐DON | 8 | – | – | 42 | 86 | ||||||
| DON‐3‐glucoside | 8 | – | – | 43 | 166 | ||||||
| Sow feed | DON | 15 | 5–11 | 10–22 | – | – | 135 | 245 | |||
| 3‐Ac‐DON | 15 | – | – | 11 | 53 | ||||||
| 15‐Ac‐DON | 15 | – | – | 20 | 44 | ||||||
| DON‐3‐glucoside | 15 | – | – | 29 | 96 | ||||||
| Fattening pig feed | DON | 13 | 5–11 | 10–22 | – | – | 291 | 1,250 | |||
| 3‐Ac‐DON | 13 | – | – | 29 | 155 | ||||||
| 15‐Ac‐DON | 13 | – | – | 38 | 144 | ||||||
| DON‐3‐glucoside | 13 | – | – | 121 | 1,304 | ||||||
| Horse feed | DON | 14 | 5–11 | 10–22 | – | – | 200 | 874 | |||
| 3‐Ac‐DON | 14 | – | – | 13 | 87 | ||||||
| 15‐Ac‐DON | 14 | – | – | 44 | 296 | ||||||
| DON‐3‐glucoside | 14 | – | – | 34 | 179 | ||||||
| Dairy cattle and young stock | DON | 3 | 5–11 | 10–22 | – | – | 90 | 151 | |||
| 3‐Ac‐DON | 3 | – | – | 44 | 126 | ||||||
| 15‐Ac‐DON | 3 | – | – | 22 | 57 | ||||||
| DON‐3‐glucoside | 3 | – | – | n.d | n.d. | ||||||
| Croatia | 2011 | Compound feed (fattening pigs) | DON | 30 | 150 | 29 | n.r. | 817 | 1,864 | Pleadin et al. (2012) |
LOD: limit of detection; LOQ: limit of quantification; n.r.: not reported; n: number of samples; –: not reported in the paper; DON: deoxynivalenol; Ac: acetyl.
(a) Number of samples with mycotoxin content greater than the limit of quantification or detection.
(c) Mixture of grass and maize silage.
(d) Pressed sugar beet pulp (n = 12), Brewer's spent grains (n = 15) and potato pulp (n = 2).
(e) Rapeseed/soy meal (n = 4), maize gluten meal (n = 2) and cereal grains (n = 2).
(f) Fresh grass (n = 5), dried grass (n = 2) and grass hay (n = 6).
(g) Feed for horses.
(h) Commercial horse feed preparations (complementary feeds) containing varying proportions of barley, wheat, oats and maize.
(i) Various Member States (the Czech Republic, Hungary, Spain and Portugal). Only data for co‐contaminated samples reported.
Compound (complete) feedingstuffs are important for livestock, particularly in intensive production for pigs and poultry. In a survey of 277 commercially manufactured feeds for fattening pigs in Portugal, DON was detected in only 47 samples, with levels ranging from 100 to 864 μg/kg. Only four samples had DON concentrations above 500 μg/kg and none above 900 μg/kg (Almeida et al., 2011).
De Boevre et al. (2012) reported the results of a survey of 67 compound feeds for poultry, pigs, horses and cattle sampled in Belgium in 2010 and 2011. In summary, DON was found in all feed samples analysed. The feed type with the highest mean concentration of DON (318 μg/kg) was for piglets, while the highest individual level of DON (1,250 μg/kg) was found in one sample of compound feed for fattening pigs. The mean across all groups were less than 400 μg/kg. DON‐3‐glucoside, 3‐Ac‐DON and 15‐Ac‐DON were also found in all samples with the exception of compound feeds for cattle and young stock, where DON‐3‐glucoside was not detected. The highest concentrations of DON‐3‐glucoside, 3‐Ac‐DON and 15‐Ac‐DON were 121, 47 and 50 μg/kg, respectively. Across all samples, levels of DON‐3‐glucoside, 3‐Ac‐DON and 15‐Ac‐DON were approximately 18, 19 and 18% of the concentration of DON, but variation between samples was lower for 15‐Ac‐DON than for the other two forms.
These authors also reported data for 19 feed materials commonly used in the manufacture of compound feeds for livestock. They consisted predominantly of cereals, cereal by‐products and oilseed meals. DON levels were generally low (< 500 μg/kg) but were 1,533 and 1,381 μg/kg in two samples of maize gluten feed and wheat, respectively. DON‐3‐glucoside, 3‐Ac‐DON and 15‐Ac‐DON were also reported for these feed materials. The highest concentrations of DON‐3‐glucoside was reported for a sample of maize gluten feed (542 μg/kg), but was not detected in 5 of the feeds analysed and < LOD in a further 5 samples. For 3‐Ac‐DON, the highest concentration (271 μg/kg) was observed in a sample of maize grain but was not detected or below the level of detection in 13 of the 19 samples analysed. The same sample of maize grain had the highest level of 15‐Ac‐DON (194 μg/kg) but otherwise levels were below 100 μg/kg. It was not detected or below the level of detection in 10 of the 19 samples (De Boevre et al., 2012).
Driehuis et al. (2008a) reported the results of a survey of feedstuffs for dairy cows (comprising silages, feed commodities and compound feeds) from 24 dairy farms in the Netherlands sampled during 2005. The incidence of DON ranged from 38% to 54%, and was particularly high in compound feed and silage samples, where the average concentrations were 433 and 550 μg/kg, respectively. DON was detected in only five samples of other feed classes: two corn gluten samples, one barley grain sample and two grass hay samples. It was not detected in any of the ensiled by‐product samples.
Mitak et al (2011)24 reviewed levels of DON in various farm animal feeds and feed mixtures sampled in Croatia during 2009 and 2010. A total of 94 samples were examined for DON (64 in 2009, 30 in 2010). In 2009 DON was detected in 25% of samples with a mean concentration (in contaminated samples) of 22.07 μg/kg. In contrast, 96% of samples in 2010 were contaminated with DON, with a mean concentration 1,432 μg/kg. For feed mixtures, concentrations greater than 900 μg/kg were detected in seven samples of pig feed (range 1,008–4,055 μg/kg). The levels were elevated also in the complete feeds for poultry (maximum 3,695 μg/kg) and cattle (maximum 384 μg/kg).
Samples of wheat and maize were obtained from four EU countries and analysed for DON, 3‐Ac‐DON and 15‐Ac‐DON (Monbaliu et al., 2010). In the 50 samples analysed (wheat n = 8, maize n = 42), DON, 3‐Ac‐DON and 15‐Ac‐DON were found in 40, 34 and 31 samples, respectively. In only half of the samples were all three mycotoxins detected, while only DON and 3‐Ac‐DON co‐occurred in six samples, and only DON and 15‐Ac‐DON in four samples. Mean DON concentrations were approximately 40 and 10 times higher than those of 3‐Ac‐DON and 15‐Ac‐DON, respectively. The mean concentrations of DON, 3‐Ac‐DON and 15‐Ac‐DON in wheat were 2,657, 33.3 and 38.2 μg/kg, respectively, while for the maize grains they were 891, 36.5 and 134 μg/kg, respectively.
Zachariasova et al., (2014b) analysed a total of 343 different of feed materials (forages, cereals, cereal by‐products oilseed meals and compound feeds) originating from the Czech Republic (256 samples) and the UK (87 samples) over a 4‐year period (2008–2012) for DON, DON‐3‐glucoside, and for the sum of 3‐Ac‐DON and 15‐Ac‐DON. Details are given in Table B.4, Appendix B. The highest concentration of DON was found in dried distiller's grains with solubles (5,981 μg/kg), a fermented feedingstuff. For DON‐3‐glucoside, the highest reported value (773 μg/kg) was in malt sprouts, while 28 samples had a mean concentration of DON‐3‐glucoside 229 μg/kg. The maximum of the sum of 3‐Ac‐DON and 15‐Ac‐DON (458 μg/kg) was similar to that of DON (494 μg/kg). Apart from this feedingstuff, the mean concentration of DON‐3‐glucoside was less than 22% of that of the mean concentration of DON for those feeds for which both were reported. With the exception of maize dried distiller's grains with solubles, the concentrations of DON‐3‐glucoside were higher than those of the sum 3‐Ac‐DON and 15‐Ac‐DON.
Table B.4.
Occurrence of DON, DON‐3‐glucoside and the sum of 3‐Ac‐DON and 15‐Ac‐DON in feedingstuffs for livestock as reported by Zachariasova et al., (2014b) (modified)
| DON | DON‐3‐glucoside | Sum of 3‐Ac‐DON and 15‐Ac‐DON | DON‐3‐glucoside: DON ratiob | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Median | Maximum | LOQ | Mean | Median | Maximum | LOQ | Mean | Median | Maximum | LOQ | ||
| μg/kg | μg/kg | μg/kg | ||||||||||||
| Hay | 4 | 46 | 40 | 62 | 50 | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | 50 | 0.02 |
| Maize silagea | 11 | 867 | 759 | 2,950 | 50 | 27 | 25 | 66 | 50 | 211 | 25 | 1,150 | 50 | 0.09 |
| Feed wheat | 21 | 213 | 118 | 1,038 | 10 | 16 | 5 | 92 | 10 | n.r. | n.r. | n.r. | 10 | 0.27 |
| Feed barley | 16 | 264 | 105 | 1,582 | 10 | 56 | 5 | 433 | 10 | 3 | 3 | 41 | 5 | 0.06 |
| Feed maize | 8 | 624 | 599 | 1,523 | 10 | 49 | 63 | 86 | 10 | n.r. | n.r. | n.r. | 10 | |
| Feed oats | 3 | 102 | 5 | 296 | 10 | n.r. | n.r. | n.r. | 10 | n.r. | n.r. | n.r. | 10 | |
| Soybean meal | 10 | 7 | 5 | 25 | 10 | n.r. | n.r. | n.r. | 10 | n.r. | n.r. | n.r. | 10 | |
| Sugar beet pulp | 6 | 49 | 31 | 128 | 25 | n.r. | n.r. | n.r. | 25 | n.r. | n.r. | n.r. | 50 | 0.05 |
| Extracted oil seeds | 14 | 134 | 40 | 1,285 | 25 | 23 | 20 | 60 | 20 | n.r. | n.r. | n.r. | 25 | 1.57 |
| Malt sprouts | 28 | 154 | 111 | 494 | 25 | 229 | 168 | 773 | 25 | 153 | 171 | 458 | 25 | 0.12 |
| Maize dried distiller's grains with solubles | 71 | 1,675 | 1,393 | 5,981 | 50 | 128 | 20 | 710 | 25 | 297 | 266 | 688 | 25 | 0.22 |
| Wheat dried distillers grains with solubles | 16 | 262 | 506 | 588 | 25 | 31 | 20 | 130 | 25 | 43 | 13 | 102 | 25 | |
| Compound feeds | ||||||||||||||
| Pigs | 26 | 401 | 175 | 1,735 | 25 | 48 | 20 | 219 | 25 | 14 | 1 | 169 | 25 | 0.13 |
| Poultry | 44 | 328 | 374 | 710 | 25 | 25 | 23 | 13 | 39 | 25 | ||||
| Dairy cowsa | 19 | 524 | 447 | 1,262 | 25 | 25 | 20 | 51 | 25 | 4 | 1 | 62 | 25 | 0.04 |
n: number of samples; LOQ: limit of quantification; n.r., not reported; DON: deoxynivalenol; Ac: acetyl.
For calculating the arithmetic means of mycotoxin concentrations, 1⁄2 of LOQ value (g/kg) was used.
Samples oven dried prior to analysis.
Calculated by the CONTAM Panel using the maximum concentrations.
Both grain maize and silage maize are important animal feed sources. While the grain is used widely as a feed for non‐ruminant livestock, the whole plant is used as silage (Dorn et al., 2011). In a 2‐year (2006 and 2007) maize hybrid study from one site in Switzerland, DON was found in all of the maize kernels analysed in both 2006 (range 0.21–8.58 mg/kg, mean 2.30 mg/kg) and 2007 (range 0.02–2.19 mg/kg, mean: 0.97 mg/kg) (Dorn et al., 2011). In the 2 years of the study, 63% and 50% of the samples were above the recommended limit of 0.9 mg/kg in complete feeds for pigs. Mycotoxin concentrations in crop residues were also reported to be very high and ranged from 2.6 to 15.3 mg/kg.
In another Swiss study of Eckard et al. (2011), DON was detected in 19 out of 20 samples of maize silage sampled from farmers’ fields. Levels were between 780 and 2,990 μg/kg. The mean concentrations of DON and the sum of 3‐Ac‐DON and 15‐Ac‐DON in maize silages were 1,356 and 218 μg/kg, respectively. However, while DON was present in all 19 samples analysed, the sum of 3‐Ac‐DON and 15‐Ac‐DON was only detected in two samples (at concentrations of 135 and 300 μg/kg). In a further two samples the concentrations of the sum 3‐Ac‐DON and 15‐Ac‐DON were below the LOQ (46 μg/kg).
A survey of forage crops (140 maize, 120 grass and 30 wheat silages) produced in the Netherlands between 2002 and 2004 was reported by Driehuis et al. (2008b). Although the DON concentrations were all below 8,000 μg/kg,11 it was detected above the LOQ (250 μg/kg) in 72% of maize and 10% of wheat silages. Average DON concentrations were 854 and 621 μg/kg, respectively, and maximum concentrations were 3,142 and 1,165 μg/kg, respectively. A maximum concentration of 3,142 μg/kg of DON was detected in a maize silage sample from 2004. In this study, forages were also analysed for acetylated DON. No 3‐Ac‐DON was detected in maize silage samples from any of the sampling years. Analysis for 15‐Ac‐DON was only undertaken on the 2004 samples, but in that year it was detected in three maize silage samples (5% of the samples analysed) in concentrations of 729, 962 and 1,013 μg/kg. DON was also detected in three (10%) whole‐crop wheat silage samples.
Although grass has been reported to be contaminated with DON, levels are generally low. In a study from the Czech Republic, mean levels of DON in five grass species and sampled between June and December in 2008 and 2009 of 37.6 and 46.3 μg/kg DM, respectively. Differences between species and years were not significant (Skládanka et al., 2011).
Cortinovis et al. (2012) undertook a survey in northern Italy of cereal grains intended specifically as feeds for horses. The barley grains were most frequently contaminated (73% positive samples, median concentration 650 μg/kg) although the highest median concentrations were in the maize and rice bran feeds (37% and 30% positive samples, 810 and 850 μg/kg, respectively). In another survey, Liesener et al. (2010) analysed 62 samples of commercial horse feed preparations (complementary feeds) containing cereal mixtures (‘muesli’ or mash, n = 39; pelleted feeds, n = 12), and plain horse feed grains (maize, n = 5; oats, n = 4; barley, n = 2) purchased from 21 different producers/distributors from the German market. DON was detected in all samples, ranging from 16 to 4,900 μg/kg (mean and standard deviation) 410 ± 660 μg/kg; LOD 10 μg/kg).
In 29 samples of dry dog food sampled in Vienna, 24 (83%) were positive for the presence of DON, with a mean concentration of 409 μg/kg (Böhm et al., 2010). The highest value detected was 1,390 μg/kg.
DON may also be present in other feedingstuffs as a result of inappropriate storage and handling during processing. Rafai et al. (2000) reported DON in soybean (60–720 μg/kg) and sunflower seeds (150 μg/kg) intended for animal feed. Mankeviciene et al. (2011) examined 22 representative samples of oil seeds produced in Lithuania during 2007–2009 (8 winter rapeseed, 8 spring rapeseed, 6 linseed and 7 rapeseed cake). DON was detected in all types of feed, although levels and percentage contamination varied between years and feed type. The maximum reported concentration in oilseeds was 278 and 271 μg/kg in oilseed rape and linseed, respectively. Of particular interest is that DON was detected in all of the samples of rapeseed cake (n = 7; mean concentration 387 μg/kg). Since rapeseed cake is used as a protein source in livestock diets, this represents a potential source of exposure. Palm kernel cake is also used as a feed for livestock, and recently Yibadatihan et al. (2014) reported levels of DON of between 49 and 108 μg/kg in 19 of 25 samples of Malaysian palm kernel cake (LOD 10 μg/kg, LOQ 33 μg/kg).
4.1.4. Co‐occurrence of DON with 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed
While the CONTAM Panel identified several studies on the co‐occurrence of DON with 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food there were only few of them on feed. Co‐occurrence profiles may differ due to different Fusarium chemotypes occurring in various parts of the world as well as different plant cultivars with different detoxification abilities. The CONTAM Panel selected only studies (from 2004 till the end of July 2016) relevant for the European market where the ratios of 3‐Ac‐DON and/or 15‐Ac‐DON and/or DON‐3‐glucoside to DON were reported or could be calculated. See also the co‐occurrence reported for the current occurrence data reported to EFSA in Section 4.3.3.
4.1.4.1. Co‐occurrence in food
A number of published studies investigated co‐occurrence of DON, 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside in the food categories ‘grains intended for human consumption’, ‘grain‐based food products’ and ‘malt and beer’. The analytical methods used were MS based (see Section 3). The CONTAM Panel used the results from these studies (including some from Personal communication, Dall'Asta, 2016) to calculate ratios of DON‐3‐glucoside, 3‐Ac‐DON and 15‐Ac‐DON to DON. Unfortunately, data reported in the literature were often incomplete and highly inconsistent, which hampered calculation of ratios based on reported mean values. Only a limited number of studies reported means (usually of samples being positive) for DON and 3‐Ac‐DON and/or 15‐Ac‐DON and/or DON‐3‐glucoside that allowed the CONTAM Panel to calculate at least ratios of those means. More often only maximum values were reported. For these reasons, the CONTAM Panel summarised the published information on co‐occurrence as ratios of means and/or ratios of maxima. A number of studies on ‘grains intended for human consumption’, ‘grain based food products’ and ‘malt and beer’ could be summarised for the calculation of ratios of DON‐3‐glucoside to DON (Tables 8–10). Much less data were available for the calculation of ratios of the two acetylated forms to DON, and therefore no tables were made for these.
Table 8.
Summary of maximum, mean and ratio values for DON and DON‐3‐glucoside based on published studies on occurrence in grains intended for food consumption from a total of 763 samples
| Country | Type of samples | Number of samples | Maximum DON (μg/kg) | Maximum DON‐3‐glucoside (μg/kg) | Mean DON (μg/kg) | Mean DON‐3‐glucoside (μg/kg) | Ratio DON‐3‐glucoside/DON based on maximum | Ratio DON‐3‐glucoside/DON based on mean | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Austria + Germany + Slovakia |
Wheat Maize |
23 54 |
4,130 3,680 |
1,070 763 |
1,500 753 |
393 141 |
0.26 0.21 |
0.26 0.19 |
Berthiller et al. (2009) |
| Belgium |
Wheat Barley Triticale |
93 65 10 |
n.r. n.r. n.r. |
n.r. n.r. n.r. |
1,053 2,029 1,145 |
250 390 169 |
− − − |
0.24 0.19 0.15 |
Vanheule et al. (2014) |
| Denmark |
Spring barley Rye Oat Triticale Winter wheat |
15 12 11 5 6 |
< 60 < 50 2,216 737 2,638 |
< LOD < LOD 287 109 342 |
n.r. n.r. n.r. n.r. n.r. |
n.r. n.r. n.r. n.r. n.r. |
− − 0.13 0.15 0.13 |
− − − |
Rasmussen et al. (2012) |
| Italy | Durum wheat | 150 | 3,715 | 842 | n.r. | n.r. | 0.23 | − | Dall'Asta et al. (2013) |
|
Wheat (2014) Wheat (2015) |
53 162 |
2,283 1,703 |
841 648 |
1,351 936 |
558 281 |
0.37 0.38 |
0.41 0.30 |
Dall'Asta et al. (2016), personal communicationa | |
| Finland |
Barley Oats Wheat |
34 31 30 |
1,180 23,800 5,510 |
1,300 6,600 922 |
n.r. n.r. n.r. |
n.r. n.r. n.r. |
1.10 0.28 0.17 |
− − − |
Nathanail et al. (2015) |
|
France Germany |
Maize Wheat Durum |
5 3 1 |
2,864 181 603 |
237 11 74 |
n.r. n.r. 603 |
n.r. n.r. 74 |
0.08 0.06 0.12 |
− − 0.12 |
Desmarchelier and Seefelder (2011) |
| Overall mean | 0.26 | 0.23 | |||||||
| Overall median | 0.19 | 0.21 | |||||||
n.r.: not reported; < LOD: not detected; −: could not be calculated by the CONTAM Panel.
Due to scarcity of data also some data obtained via personal communication were included.
Table 10.
Summary of maximum, mean and ratio values for DON and DON‐3‐glucoside based on published studies on occurrence in malt and beers from a total of 459 samples
| Type of samples | Number of samples | Maximum DON (μg/kg) | Maximum DON‐3‐glucoside (μg/kg) | Mean DON (μg/kg) | Mean DON‐3‐glucoside (μg/kg) | Ratio DON‐3‐glucoside/DON based on maximum | Ratio DON‐3‐glucoside/DON based on mean | Referencea | |
|---|---|---|---|---|---|---|---|---|---|
| Austria |
Pale beer Wheat beer Dark beer Bock beer Non‐alcoholic beer Shandy beer |
10 10 10 10 10 10 |
30 27 11 22 3.7 6.4 |
19 15 16 32 3.1 5.5 |
13 14 11 13 3.7 6.4 |
8.3 8.6 9.6 16 2.3 3.5 |
0.63 0.61 1.45 1.45 0.84 0.86 |
0.64 0.61 0.87 1.23 0.62 0.55 |
Malachova et al. (2012) |
| Czech Republic |
Malt Beer |
6 15 |
139 62 |
186 82 |
68 21 |
105 34 |
1.34 1.32 |
1.54 1.61 |
Zachariasova et al. (2012) |
| Germany |
Barley malt 2012 2013 2014 |
10 10 10 |
10,300 406 60 |
19,000 441 86 |
n.r. n.r. n.r. |
n.r. n.r. n.r. |
1.84 1.09 1.43 |
− − − |
Habler and Rychlik (2016) |
| Germany | Beer | 9 | 28 | 63 | 13 | 23 | 2.25 | 1.77 | Habler et al. (2016) |
| Produced in 38 countries, purchased in Central Europe |
Pale beer Wheat beer Dark beer Bock beer Non‐alcoholic beer Shandy beer |
217 46 47 20 19 25 |
89 50 45 27 26 13 |
81 28 26 33 6.6 7.9 |
12 18 22 14 8.7 6.9 |
9.3 12 11 15 3.0 3.8 |
0.91 0.56 0.57 1.22 0.26 0.61 |
0.78 0.67 0.50 1.07 0.34 0.55 |
Varga et al. (2013) |
| Overall mean | 1.07 | 0.93 | |||||||
| Overall median | 1.00 | 0.67 | |||||||
n.r.: not reported; < LOD: not detected; −: could not be calculated by the CONTAM Panel.
Vendl et al. (2010) analysed DON and DON‐3‐glucoside in beer including wheat beer from Austria and the UK. They reported DON and DON‐3‐glucoside concentrations < LOD for the analysed beer samples. Therefore the ratios could not be calculated and are not reported in Table.
For the category ‘grains intended for human consumption’, the concentration ratios of DON‐3‐glucoside to DON roughly varied from 0.12 to 0.26 (overall mean: 0.23; overall median: 0.21) for the reported means and from 0.06 to 1.10 (overall mean: 0.26; overall median: 0.19) for the reported maxima (Table 8). Studies investigating the co‐occurrence of 3‐Ac‐DON and/or 15‐Ac‐DON with DON did often not detect the acetylated forms at concentrations above the LOQ and when those concentrations were above the respective LOQ they were usually much lower than the concentrations of DON. The scarce available data showed that the 3‐Ac‐DON to DON concentration ratios roughly varied from 0.03 to 0.22 for the reported means and from 0.01 to 0.11 for the reported maxima. The 15‐Ac‐DON to DON concentration ratios roughly varied from 0.05 to 0.34 for the reported means and from 0.07 to 0.09 for the reported maxima.
The DON‐3‐glucoside to DON ratios in ‘grain‐based food products’ (except for beer) ranged from 0.07 to 0.13 (overall mean: 0.10; overall median: 0.10) for the reported mean concentrations and from 0.07 to 3.08 (overall mean: 0.43; overall median: 0.12) for the reported maximum concentrations, respectively (Table 9). The reported maximum concentrations for DON and DON‐3‐glucoside in grain‐based food products were lower than in ‘grains intended for human consumption’. The CONTAM Panel noted that co‐occurrence for the acetylated forms of DON were reported in two studies only (De Boevre et al., 2012; Rodríguez‐Carrasco et al., 2014b) and the ratios of 3‐Ac‐DON to DON and 15‐Ac‐DON to DON could only be calculated from the reported maxima, ranging from 0.06 to 1.27 and from 0.12 to 0.35, respectively.
Table 9.
Summary of maximum, mean and ratio values for DON and DON‐3‐glucoside based on published studies on occurrence in grain‐based food products (except beer) from a total of 360 samples
| Country | Type of samples | Number of samples | Maximum DON (μg/kg) | Maximum DON‐3‐glucoside (μg/kg) | Mean DON (μg/kg) | Mean DON‐3‐glucoside (μg/kg) | Ratio DON‐3‐glucoside/DON based on maximum | Ratio DON‐3‐glucoside/DON based on mean | Referencea |
|---|---|---|---|---|---|---|---|---|---|
| Belgium |
Fibre‐enriched bread Bran‐enriched bread Corn flakes Popcorn Oatmeal |
52 36 61 12 13 |
138 127 718 442 91 |
425 103 63 96 97 |
n.r. n.r. n.r. n.r. n.r. |
n.r. n.r. n.r. n.r. n.r. |
3.08 0.81 0.09 0.21 1.07 |
− − − − − |
De Boevre et al. (2012) |
| Belgium |
Bread Breakfast cereals |
25 20 |
n.r. n.r. |
n.r. n.r. |
316 n.r. |
n.r. n.r. |
− − |
− − |
Vanheule et al. (2014) |
| Czech Republic |
White flour products Mixed flour products Breakfast cereals Snacks Flours |
17 36 7 34 22 |
350 431 347 320 594 |
30 41 66 94 72 |
n.r. n.r. n.r. n.r. n.r. |
n.r. n.r. n.r. n.r. n.r. |
0.09 0.10 0.19 0.29 0.12 |
− − − − − |
Malachova et al. (2011) |
| Czech Republic |
Wheat, wheat‐rye, multi cereal and sunflower baguettes BIO wheat flakes |
14 1 |
431 347 |
33 46 |
252 347 |
25 46 |
0.08 0.13 |
0.10 0.13 |
Zachariasova et al. (2012) |
|
Germany Poland Romania UK |
Durum semolina Barley flour Oat flour Wheat flour Malt syrup Wheat flour Wheat flour |
1 1 1 2 1 1 3 |
177 5.5 6.3 n.r. < LOD 45 1,478 |
12 < LOQ n.r. 2.6 < LOD 4.8 99 |
177 5.5 6.3 n.r. < LOD 45 n.r. |
12 < LOQ n.r. n.r. < LOD 4.8 n.r. |
0.07 − − − − 0.11 0.07 |
0.07 − − − − 0.11 − |
Desmarchellier et al. (2011) |
| Overall mean | 0.43 | 0.10 | |||||||
| Overall median | 0.12 | 0.10 | |||||||
n.r.: not reported; < LOD: not detected; < LOQ: not quantified; −: could not be calculated by the CONTAM Panel.
Vendl et al. (2010) analysed DON and DON‐3‐glucoside in a number of different grain products from Austria and the UK. They reported quantified maximum DON concentrations only for a few products and < LOD for DON‐3‐glucoside for all analysed products. Therefore the ratios could not be calculated and are not reported in Table.
For the category ‘malt and beers’, the concentration ratios of DON‐3‐glucoside to DON differed due to the malting process (see Section 4.3.1.4) and were (often much) higher than in grains. Co‐occurrence of 3‐Ac‐DON and 15‐Ac‐DON with DON was only occasionally looked for but no concentrations above the respective LOQ/LOD were identified. The calculated ratios of DON‐3‐glucoside to DON in ‘malt and beer’ ranged from 0.34 to 1.61 (overall mean: 0.93; overall median: 0.67) for the means and from 0.26 to 1.84 (overall mean: 1.07; overall median: 1.00) for the maxima (Table 10).
Janssen et al. (2015) used published data (collected up to 2013, and including some non‐EU studies) on co‐occurring levels of DON‐3‐glucoside and DON to estimate the DON‐3‐glucoside to DON ratio for several food product categories. This resulted in a DON‐3‐glucoside to DON ratio of 0.2 (90% confidence interval (CI), 0.04–0.9) in grains and grain‐milling products, 0.3 (90% CI, 0.03–2.8) in grain‐based products and 0.8 (90% CI, 0.4–1.8) in beer. These ratios are in line with those given for the DON‐3‐glucoside to DON ratios in Tables 8–10.
In summary, the scarce data from the available literature revealed that the 3‐Ac‐DON to DON concentration ratios varied roughly from 0.03 to 0.22 for the reported mean concentrations and from 0.01 to 0.11 for the reported maximum concentrations, while for the ratios of 15‐Ac‐DON to DON were roughly 0.05–0.34 based on mean concentrations and 0.07–0.09 based on maximum concentrations. The overall mean ratio of DON‐3‐glucoside to DON calculated from the ratios of mean concentrations of the samples on ‘Grains intended for food consumption’ from the available literature was 23%. The overall mean ratio of DON‐3‐glucoside to DON calculated from the ratios of maximum concentrations of the samples was similar. The ratios of DON‐3‐glucoside to DON calculated for ‘Grain‐based food products’ were more variable than those for ‘Grains for food consumption’ but were not in disagreement with each other. For category ‘malt and beer’ the concentration ratios of DON‐3‐glucoside to DON differed due to the malting process and were (often much) higher than in grains. Co‐occurrence of 3‐Ac‐DON and 15‐Ac‐DON with DON was only occasionally looked for in malt and beer. The ratios of DON‐3‐glucoside to DON in ‘malt and beer’ ranged from 0.34 to 1.61 based on the mean concentrations and were similar at the maximum concentrations.
4.1.4.2. Co‐occurrence in feed
A number of published studies have examined the co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in feeds, predominantly in grains, intended for animal feed. Monbaliu et al. (2010) reported concentrations of DON, 3‐Ac‐DON and 15‐Ac‐DON in samples of wheat and maize from four EU countries (see Section 4.1.3 and Appendix B, Table B.3). Mean DON concentrations were approximately 40 and 10 times higher than those of 3‐Ac‐DON and 15‐Ac‐DON, respectively. The ratios of the mean concentrations (of positive samples) of 3‐Ac‐DON and 15‐Ac‐DON to DON were 0.013 and 0.014, respectively. However, the authors reported that the relationships were poorly correlated (for 3‐Ac‐DON and DON was r2 = 0.31; for 15‐Ac‐DON and DON was r2 = 0.18).
In a survey of maize silages produced in Switzerland, the ratio of the mean concentrations of the sum of 3‐Ac‐DON and 15‐Ac‐DON to DON (for positive samples) was 0.10 (Section 4.1.3 and Appendix B, Table B.3) (Eckard et al., 2011).
De Boevre et al. (2012) reported levels of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in compound feeds for poultry, pigs, horses and cattle sampled in Belgium in 2010 and 2011 (see details in Section 4.1.3 and Appendix B). The DON‐3‐glucoside to DON ratio, based on the mean concentrations, ranged from 0.15 to 1.04, with the highest value for fattening pig feed. The ratio of 3‐Ac‐DON to DON calculated from the means ranged from 0.06 to 0.49, while for 15‐Ac‐DON to DON the range was 0.13 to 0.25. In the same study, 19 samples of raw feed materials were analysed for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. Only in samples of maize gluten feed (n = 1) and maize grain (n = 3) were all four forms (DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside) found. The DON‐3‐glucoside to DON ratio, based on the mean concentrations, ranged from 0.04 (a sample of maize grain) to 1.39 (soy bean), while the ratios of the mean concentrations of 3‐Ac‐DON to DON and 15‐Ac‐DON to DON ranged from 0.12 to 0.37 and 0.19 to 0.26, respectively.
Zachariasova et al., (2014b) analysed different feed materials originating from the Czech Republic and the UK for DON, DON‐3‐glucoside, and the sum of 3‐Ac‐DON and 15‐Ac‐DON. In those feeds where both DON and DON‐3‐glucoside were measured, the data showed that the concentration ratios for DON‐3‐glucoside to DON varied from 0.05 to 1.49 based on the reported mean concentrations, and from 0.04 to 4.56 for the reported maximum concentrations respectively. The high DON‐3‐glucoside to DON concentration ratio was due to high levels of DON‐3‐glucoside in malt sprouts.
Berthiller (2016, Personal communication) analysed co‐occurrence for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in various feed materials, including compound feed (animal species not specified), barley, wheat, maize silage, hay and straw in nearly 700 samples from 20 European countries and three bordering countries (Russia, Ukraine and Turkey) from the year 2015 harvest. In the vast majority of these samples, 3‐Ac‐DON and 15‐Ac‐DON were not detected. From the concentrations of the positive samples, the CONTAM Panel calculated the mean of the ratios of 3‐Ac‐DON to DON of 0.02 for compound feed (n = 45, mainly from Poland and Italy), 0.01 for maize (n = 16, from Austria, Germany, the Netherlands, Poland and the UK) and 0.05 for oats (n = 3, from Finland). The mean of the ratios of 15‐Ac‐DON to DON was 0.03 for 45 compound feed samples from eight European countries (mostly from Italy, Poland, Austria and Croatia) and 0.27 for 35 maize samples mainly from Germany. The calculated means of the ratios of DON‐3‐glucoside to DON were 0.09 for compound feed (n = 236, across 18 countries), 0.24 for barley (n = 51, mainly from Austria), 0.09 for wheat (n = 8, mainly from Austria), 0.19 for oats (n = 6 from Finland, Sweden and Germany). For an example, for other types of feeds than cereals and/or cereal‐based feeds (compound feed; consisting ~ 50% of cereals), a ratio of means of DON‐3‐glucoside to DON of 0.08 for eight straw samples from Germany and the UK was calculated.
Overall, for the limited number of feed material samples reported in the literature, the means of the ratios calculated from the mean concentrations for 3‐Ac‐DON to DON ranged from 0.01 to 0.49, for 15‐Ac‐DON to DON from 0.01 to 0.25 and for DON‐3‐glucoside to DON from 0.09 to 1.49. A large variation of the ratios was observed across the different feed materials.
4.1.5. Co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside with other Fusarium mycotoxins
DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are found to co‐occur in grains and food products with various other Fusarium mycotoxins, including culmorin, quisetin, fumonisins, fusaproliferin, fusaric acid, fusarenon‐X, moniliformin, beauvericin, enniatins, monocerin, nivalenol, T‐2 and HT‐2 toxins and zearalenone and their derivatives (Malachova et al., 2011; De Boevre et al., 2012; Serrano et al., 2012; Juan et al., 2013; Lindblad et al., 2013; Streit et al., 2013). In the study of Streit et al. (2013), 83 samples of feed and feed raw materials were analysed by a multi‐mycotoxin LC–MS/MS method and the authors concluded that a co‐contamination of mycotoxins is the rule. They indicated that mixtures of Fusarium toxins are detected most frequently and noted the fact that as Fusarium species are plant pathogens of high importance worldwide, especially in cereal production, this co‐occurrence is not surprising. The CONTAM Panel also investigated the co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed with other Fusarium mycotoxins in the occurrence data reported to EFSA (see Section 4.2.7).
4.1.6. Conclusions on occurrence data reported in the literature
Published studies, which investigated the concentrations of DON and at least one of 3‐Ac‐DON, 15‐Ac‐DON and/or DON‐3‐glucoside in grains intended for human consumption, showed frequent occurrence of DON. Concentrations up to 23,800 μg/kg were reported for DON. Lower levels were reported for DON‐3‐glucoside (maximum reported level 6,600 μg/kg) and even lower concentrations were also observed for 3‐Ac‐DON and 15‐Ac‐DON with maximum reported concentrations of 2,720 μg/kg and 244 μg/kg, respectively.
While published data of DON, 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside in grain‐based food products are rather scarce, the general pattern of occurrence was comparable with that in grains intended for human consumption, while the concentrations found were generally lower. This is different though for the category of beer, where only DON and DON‐3‐glucoside were found, at relatively low concentrations, but with DON‐3‐glucoside often present at higher concentrations than DON. The scarce studies investigating food for infants and young children focused mainly at DON, which was found in almost half of the samples at relatively low levels. 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside were not found in these food products.
In general, studies on feeds have focussed mainly on DON, with relatively fewer data on 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside. In feed grains, the maximum reported levels were 9,528, 339 and 1,047 μg/kg for DON, 3‐Ac‐DON and 15‐Ac‐DON, respectively.
In food and feed grains, and grain based food products and feed materials DON concentrations are on average 4–5 times higher than concentrations of DON‐3‐glucoside, and at an order of magnitude higher than concentrations of 3‐Ac‐DON and 15‐Ac‐DON. However, the variation in reported ratios of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to DON is substantial. In beer the average levels for DON and DON‐3‐glucoside are at the same order of magnitude, while 3‐Ac‐DON and 15‐Ac‐DON have not been detected in beer.
DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside have been reported to co‐occur with various other Fusarium mycotoxins.
4.2. Current occurrence data in food, feed and unprocessed grains of undefined end‐use reported to EFSA
Following an European Commission mandate to EFSA, a call for annual collection of chemical contaminant occurrence data in food and feed, including DON, the acetylated derivatives 3‐Ac‐DON and 15‐Ac‐DON, and DON‐3‐glucoside, was issued by the former EFSA Dietary and Chemical Monitoring Unit (now DATA Unit)25 in December 2010 with a closing date of 1 October of each year. European national authorities and similar bodies, research institutions, academia, food business operators and other stakeholders were invited to submit analytical data on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed. The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a).
In addition to the data collected from the Member States within the call for data, the CONTAM Panel also explored the literature and reviewed the studies on occurrence data on acetylated forms and DON‐3‐glucoside in food and feed in order to include additional data in the occurrence data sets submitted to EFSA. As an outcome of this exercise, five European research groups were contacted. The occurrence data on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food, feed and unprocessed grains of undefined end‐use were obtained from two contacted research groups from institutes in Belgium and Italy.
A total of 89,157 analytical results for DON (n = 49,833), 3‐Ac‐DON (n = 18,708), 15‐Ac‐DON (n = 18,286) and DON‐3‐glucoside (n = 2,330) from 28 European countries (Figure 2) were available for the assessment. The major contributor of DON data was Germany, which reported 34% of the DON data, followed by the Netherlands and Hungary. The major contributor of DON acetylated forms and DON‐3‐glucoside data was the Netherlands, reporting 37%, 38% and 42% of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside analytical results, respectively. Other important contributors to results for DON acetylated forms results were Germany, Austria and the UK. DON‐3‐glucoside data were submitted by the Netherlands, Belgium, Italy, the UK and the Czech Republic. The origin of the samples was not always the European country reporting the data, i.e. the data set also contained samples originating from North and South America, Africa, Asia and Australia.
Figure 2.
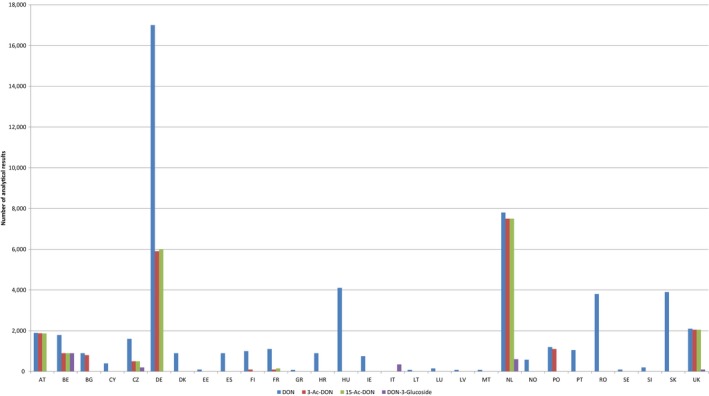
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside collected from the European countries
- Results were reported for food, feed and unprocessed grains of undefined end use. AT, Austria; BE, Belgium; BG, Bulgaria; CY, Cyprus; CZ, the Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; ES, Spain; FI, Finland; FR, France; GR, Greece; HU, Hungary; IE, Ireland; IT, Italy; LT, Lithuania; LU, Luxembourg; LV, Latvia; MT, Malta; NL, the Netherlands; NO, Norway; PO, Poland; PT, Portugal; RO, Romania; SE, Sweden; SI, Slovenia; SK, Slovakia; UK, the United Kingdom.
The left‐censored data (analytical data below the LOD/LOQ) were treated by the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO, 2009). The same method is indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b) as an option in the treatment of left‐censored data. The guidance suggests that the lower bound (LB) and upper bound (UB) approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). At the LB, results below the LOQ or LOD were replaced by zero; at the UB, the results below the LOD were replaced by the LOD and those below the LOQ were replaced by the value reported as LOQ. Additionally, a middle bound (MB) approach was also used by assigning a value of LOD/2 or LOQ/2 to the left‐censored data. The use of different cut‐off values on the reported LOQs was also evaluated in order to reduce the uncertainty associated to the exposure estimations.
The 95th percentile estimates obtained on dietary surveys/age classes with less than 60 observations and food categories with 5 or less samples may not be statistically robust (EFSA, 2011b). Those estimates were not included in the analysis.
The data covered food and feed, but also unprocessed grains of undefined end‐use. After deletion of duplicates (n = 200), separate data sets were extracted and analysed for food, feed and unprocessed grains of undefined end‐use based on product category information. The distribution of data between food, feed and unprocessed grains of undefined end‐use is detailed in Table 11. The data were reported on samples collected between years 2007 and 2014 (Figure 3).
Table 11.
Number of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food, feed and unprocessed grains of undefined end‐use as submitted to EFSA
| Toxin | Food | Feed | Unprocessed grains of undefined end‐use | Total |
|---|---|---|---|---|
| DON | 39,997 | 6,980 | 2,676 | 49,653 |
| 3‐Ac‐DON | 15,525 | 1,649 | 1,514 | 18,688 |
| 15‐Ac‐DON | 15,629 | 1,210 | 1,447 | 18,286 |
| DON‐3‐glucoside | 860 | 932 | 538 | 2,330 |
| Total | 72,011 | 10,771 | 6,175 | 88,957 |
Figure 3.
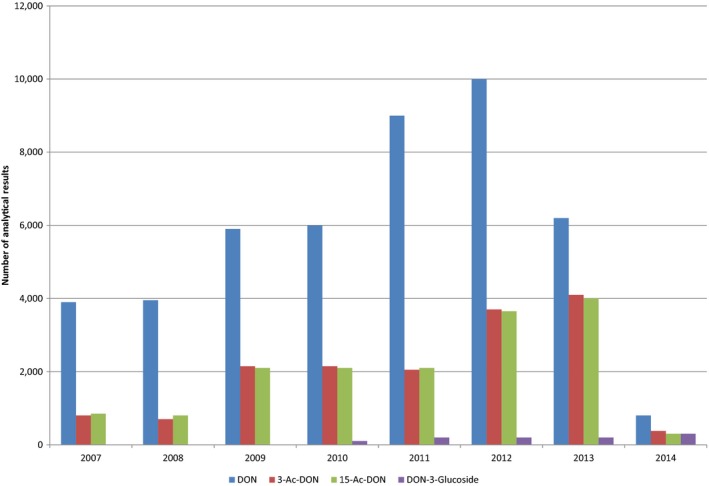
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside over the sampling years
The data providers were asked to codify all food descriptors in accordance with the EFSA FoodEx 1 classification system (EFSA, 2011a). FoodEx 1 is a provisional food classification system that was developed by the EFSA DATA Unit in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing exposure to hazardous substances. It contains 20 main food categories (FoodEx level 1), which are further divided into subgroups having 140 items at the FoodEx level 2, 1,260 items at the FoodEx level 3 and reaching about 1,800 end‐points (food names or generic food names) at the FoodEx level 4. It is based on a hierarchical coding for an easier cross‐checking and it is structured in a child‐parent relationship, where each element (‘child’) depends on upper element (‘parent’).
4.2.1. Cleaning and validation of the occurrence data
To ensure the quality of the occurrence data, several cleaning and validation steps were applied prior to statistical analysis. Only data sampled since the beginning of 2007 were considered in the further assessment. A group of data was excluded because of duplicate submission, or due to incomplete or insufficient level of description (lack of information on analytical method, LOD/LOQ), or due to uncertainties in the unit of expression of the results. If the data providers could not exclude an error in reporting results, the corresponding data or data sets were not further taken into account in the analysis.
Analytical data obtained by ELISA were not included in further analysis because cross‐reactivity of antibodies employed in ELISA‐based methods for DON may lead to biased results (see Sections 4.2.3.3 and 4.2.4.3). Components structurally related to DON, such as the acetyl‐forms of DON, DON‐3‐glucoside and other co‐occurring trichothecenes (e.g. nivalenol), thus may cause overestimation of the DON concentration. TLC methods have only been interlaboratory validated to determine DON at levels ≥ 300 μg/kg matrix (see Table 4 in Section 3.2.3) which is above the cut‐off values as discussed in Sections 4.2.3.1, 4.2.4.1 and 4.2.5.1. Therefore analytical data obtained by TLC were not included in further analysis. In addition, analytical results generated with other methodologies not having a sufficiently low LOQ were excluded from further analyses (see Sections 4.2.3.1, 4.2.4.1 and 4.2.5.1 for further details).
In summary, the cleaning process led to the exclusion of 19,692 results of DON data, 4,074 results of 3‐Ac‐DON data, 4,203 results of 15‐Ac‐DON and 799 results of DON‐3‐glucoside because of:
5.1% sampling before 2007,
0.2% duplicate submission,
2.1% inconsistent and/or incomplete description, uncertainties in the unit of expression,
24% unreliable analytical method or information on analytical method missing.
4.2.2. Calculation of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
The CONTAM Panel decided to consider concentrations for the sum of the concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in its risk assessment. The reasons for this are explained in the beginning of Section 7.8 and details of the calculation are described in Section 4.2.8. In the following Sections 4.2.3, 4.2.3.1, 4.2.3.2, 4.2.3.3, 4.2.3.4, 4.2.3.4.1, 4.2.3.4.2, 4.2.3.4.3, 4.2.3.4.4, 4.2.3.4.5, 4.2.4, 4.2.4.1, 4.2.4.2, 4.2.4.3, 4.2.4.4, 4.2.5, the statistical descriptors of the occurrence data are first calculated separately for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside and then for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for food, feed and unprocessed grains of undefined end‐use, respectively. In addition, the concentrations for DON alone were considered in this opinion (see Appendix F for DON).
It should be noted, that not all samples of the data set were analysed for all four DON forms (DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside) (see Sections 4.2.3.4.5 and 4.2.7). Therefore, the concentrations for the non‐analysed forms of DON (DON or 3‐Ac‐DON or 15‐Ac‐DON or DON‐3‐glucoside) in these samples were estimated using available knowledge from the published scientific literature and the information obtained from the occurrence data submitted to EFSA (see Section 4.2.8).
4.2.3. Data on food
4.2.3.1. Data collection in food
A total of 72,011 results (39,997 analytical results on DON, 15,525 analytical results on 3‐Ac‐DON, 15,629 analytical results on 15‐Ac‐DON and 860 analytical results on DON‐3‐glucoside) on food were obtained from 27 reporting countries and were related to samples collected between 2007 and 2014 (Figures 4 and 5). The major contributing country was Germany (42% of DON data, 37% of 3‐Ac‐DON, 38% of 15‐Ac‐DON), followed by the Netherlands. The major contributor of the DON‐3‐glucoside data was Italy reporting 47% of the data. The original analytical results were reported in mg/kg (14%), μg/L (0.04%) or in μg/kg (86%). All measurements were converted to μg/kg. Results were reported on whole weight (99.8%) or on dry matter (0.2% of samples). For consistency in the assessment, the latter were converted to values expressed on a whole‐weight basis.
Figure 4.
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food collected from the European countries
- AT, Austria; BE, Belgium; BG, Bulgaria; CY, Cyprus; CZ, the Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; ES, Spain; FI, Finland; FR, France; GR, Greece; HU, Hungary; IE, Ireland; IT, Italy; LT, Lithuania; LU, Luxembourg; LV, Latvia; MT, Malta; NL, the Netherlands; NO, Norway; PO, Poland; RO, Romania; SE, Sweden; SI, Slovenia; SK, Slovakia; UK, the United Kingdom.
Figure 5.
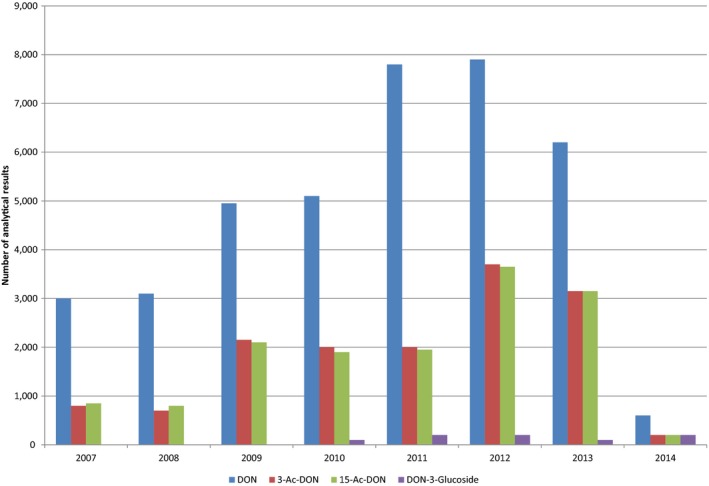
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food over the sampling years
It was noted that the detection capabilities of the methods used for the determination of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were not always sufficient, having high LODs and LOQs (LOQs up to 750 μg/kg) and thus resulting in a large number of left‐censored data (results below LOD or LOQ). Those data would bias the outcome of the assessment and, therefore, only results obtained by methods with LOQs ≤ 50 μg/kg on ‘Food for infants and small children’ and with LOQs ≤ 100 μg/kg for other food categories were included in the assessment. By this approach, 1,985 results of DON data, 9 results of 3‐Ac‐DON data and 5,516 results of 15‐Ac‐DON were not included in the food data set to be used for occurrence analysis and dietary exposure assessment.
After applying the exclusion criteria described in (Section 4.2.3.) and applying the LOQ cut‐off, the final food data set (n = 41,027) included observations on DON (n = 21,916), 3‐Ac‐DON (n = 11,944), 15‐Ac‐DON (n = 6,370) and DON‐3‐glucoside (n = 797).
4.2.3.2. Distribution of samples across food groups
The distribution of analytical results across different food groups for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside classified in accordance with the EFSA FoodEx 1 classification system (see Section 4.2) is illustrated in Figure 6.
Figure 6.
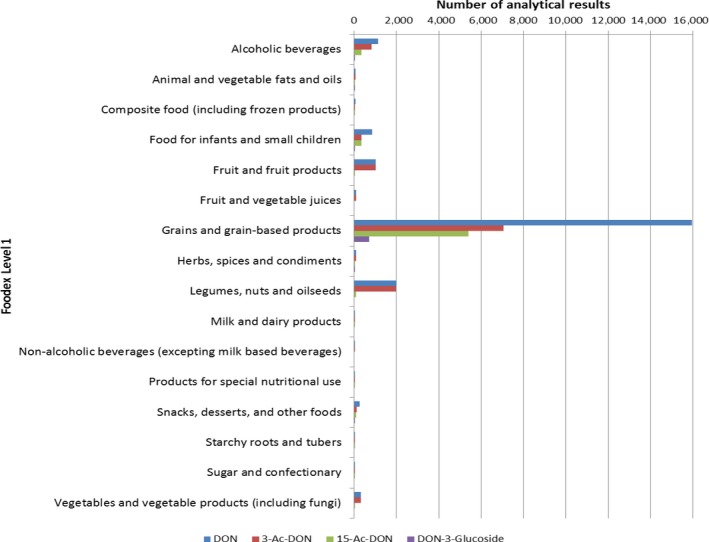
Distribution of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside analytical results across food groups
As described above (Section 4.2), food samples were classified in accordance with the FoodEx 1 classification system. In total, 16 out of 20 food categories were covered at FoodEx level 1 with the vast majority of data on ‘Grains and grain‐based products’, followed by ‘Legumes, nuts and oilseeds’. Only six food categories had data available for all four toxins (DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside). At FoodEx level 2, ‘Grain milling products’ and ‘Grains for human consumption’ dominated the product coverage of all four toxins (DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside). A more detailed distribution and statistical description of concentrations are reported in Tables 12, 13, 14, 15, 16 (Section 4.2.3.4.) up to the third level of the FoodEx.
Table 12.
Statistical description of the concentrations of DON across the food categories (FoodEx Level 1(bold) and Level 2 (regular typeface))
| Food groupsa | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Alcoholic beverages | 1,123 | 90 | 2.21 | 15.9 | 29.7 | 13.2 | 25.0 | 50.0 |
| Beer and beer‐like beverage | 742 | 85 | 3.42 | 11.3 | 19.3 | 21.0 | 25.0 | 50.0 |
| Wine | 381 | 100 | 0 | 25.0 | 50.0 | 0 | 25.0 | 50.0 |
| Animal and vegetable fats and oils | 72 | 100 | 0.00 | 20.1 | 40.1 | 0 | 25.0 | 50.0 |
| Composite food (including frozen products) | 68 | 85 | 19.1 | 32.7 | 46.3 | 108 | 108 | 108 |
| Cereal‐based dishes | 6 | 83.3 | 18.0 | 38.0 | 58.0 | − | − | − |
| Composite food, unspecified | 6 | 83.3 | 55.2 | 66.4 | 77.7 | − | − | − |
| Meat‐based meals | 1 | 100 | 0 | 25.0 | 50.0 | − | − | − |
| Potato based dishes | 4 | 25.0 | 118.6 | 121.1 | 123.6 | − | − | − |
| Ready to eat soups | 43 | 91 | 7.42 | 21.7 | 36.1 | − | − | − |
| Rice‐based meals | 7 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Vegetable‐based meals | 1 | 0.0 | 65.0 | 65.0 | 65.0 | − | − | − |
| Food for infants and small children | 849 | 85 | 8.33 | 21.8 | 35.4 | 57.0 | 57.0 | 57.0 |
| Cereal‐based food for infants and young children | 735 | 83 | 9.32 | 23.2 | 37.1 | 58.3 | 58.3 | 58.3 |
| Follow‐on formulae, powder | 13 | 100 | 0 | 13.0 | 26.0 | − | − | − |
| Food for infants and small children, unspecified | 40 | 98 | 3.61 | 12.4 | 21.3 | − | − | − |
| Fruit juice and herbal tea for infants and young children | 1 | 100 | 0 | 25.0 | 50.0 | − | − | − |
| Infant formulae, powder | 3 | 100 | 0 | 11.7 | 23.3 | − | − | − |
| Ready‐to‐eat meal for infants and young children | 48 | 96 | 0.72 | 13.7 | 26.6 | − | − | − |
| Yoghurt, cheese and milk‐based dessert for infants and young children | 9 | 100 | 0 | 12.8 | 25.6 | − | − | − |
| Fruit and fruit products | 1,019 | 99 | 1.81 | 26.2 | 50.5 | 0 | 25.0 | 50.0 |
| Berries and small fruits | 25 | 100 | 0 | 23.2 | 46.4 | − | − | − |
| Dried fruits | 463 | 99.8 | 0.23 | 25.1 | 49.9 | 0 | 25.0 | 50.0 |
| Fruit and fruit products, unspecified | 37 | 100 | 0 | 25.0 | 50.0 | − | − | − |
| Miscellaneous fruits | 287 | 99.7 | 0.30 | 24.9 | 49.5 | 0 | 25.0 | 50.0 |
| Pome fruits | 13 | 100 | 0 | 23.8 | 47.7 | − | − | − |
| Stone fruits | 194 | 96 | 8.70 | 31.4 | 54.1 | 0 | 25.0 | 50.0 |
| Fruit and vegetable juices | 91 | 100 | 0 | 25.0 | 50.0 | 0 | 25.0 | 50.0 |
| Concentrated fruit juice | 7 | 100 | 0 | 25.0 | 50.0 | − | − | − |
| Fruit juice | 73 | 100 | 0 | 25 | 50 | − | − | − |
| Fruit nectar | 11 | 100 | 0 | 25 | 50 | − | − | − |
| Grains and grain‐based products | 15,943 | 52 | 84.5 | 95.5 | 107 | 357 | 357 | 357 |
| Grains for human consumption | 3,491 | 55 | 122 | 133 | 144 | 560 | 560 | 560 |
| Grain milling products | 4,609 | 50 | 86.8 | 98.0 | 109 | 369 | 369 | 369 |
| Bread and rolls | 2,837 | 44 | 66.3 | 75.8 | 85.6 | 262 | 262 | 262 |
| Breakfast cereals | 2,169 | 64 | 56.0 | 68.5 | 81.1 | 246 | 246 | 246 |
| Fine bakery wares | 975 | 53 | 62.4 | 72.0 | 81.6 | 241 | 241 | 241 |
| Pasta (Raw) | 1,803 | 51 | 81.6 | 93.7 | 104 | 342 | 342 | 342 |
| Grains and grain‐based products, unspecified | 59 | 73 | 68.6 | 84.6 | 101 | − | − | − |
| Herbs, spices and condiments | 96 | 91 | 9.90 | 27.4 | 44.9 | 57.0 | 57.0 | 70.0 |
| Baking ingredients | 1 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Condiment | 51 | 96.1 | 3.10 | 27.1 | 51.1 | − | − | − |
| Flavourings or essences | 2 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Herb and spice mixtures | 7 | 85.7 | 57.1 | 71.4 | 85.7 | − | − | − |
| Herbs | 5 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Herbs, spices and condiments | 4 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Seasoning or extracts | 4 | 25.0 | 49.0 | 51.5 | 54.0 | − | − | − |
| Spices | 22 | 86 | 9.10 | 19.1 | 29.1 | − | − | − |
| Legumes, nuts and oilseeds | 1,990 | 99.6 | 0.70 | 24.9 | 49.2 | 0 | 25.0 | 50.0 |
| Legumes, beans, dried | 365 | 99 | 2.53 | 25.8 | 49.0 | 0 | 25.0 | 50.0 |
| Oilseeds | 390 | 99 | 1.00 | 23.9 | 46.8 | 0 | 25.0 | 50.0 |
| Other seeds | 34 | 100 | 0 | 25.0 | 50.0 | − | − | − |
| Tree nuts | 1,201 | 100 | 0 | 25.0 | 50.0 | 0 | 25.0 | 50.0 |
| Milk and dairy products | 4 | 75 | 12.5 | 17.5 | 22.5 | − | − | − |
| Non‐alcoholic beverages | 1 | 100 | 0 | 25.0 | 50.0 | − | − | − |
| Products for special nutritional use | 39 | 56 | 127 | 137 | 146 | − | − | − |
| Dietary supplements | 9 | 44.4 | 302.8 | 313.9 | 325.0 | − | − | − |
| Dietetic food for diabetics | 3 | 33.3 | 108.1 | 119.8 | 131.5 | − | − | − |
| Food for weight reduction | 1 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Medical food | 23 | 65 | 33.5 | 40.2 | 46.8 | − | − | − |
| Products for special nutritional use, unspecified | 3 | 100 | 0 | 29.2 | 58.3 | − | − | − |
| Snacks, desserts, and other foods | 265 | 49 | 81.5 | 92.8 | 104 | 360 | 360 | 360 |
| Ices and desserts | 1 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Snack food | 262 | 48 | 82.4 | 93.6 | 105 | 360 | 360 | 360 |
| Other foods | 2 | 100 | 0 | 18.8 | 37.5 | 2 | 100.0 | 0.0 |
| Starchy roots and tubers | 6 | 100 | 0 | 17.5 | 35.0 | − | − | − |
| Sugar and confectionery | 23 | 91 | 9.70 | 27.7 | 45.7 | − | − | − |
| Chocolate (Cocoa) products | 8 | 100 | 0 | 24.1 | 48.1 | − | − | − |
| Confectionery (non‐chocolate) | 6 | 66.7 | 37.0 | 48.3 | 59.5 | − | − | − |
| Dessert sauces | 2 | 100 | 0 | 25.0 | 50.0 | − | − | − |
| Sugar and confectionary | 4 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Sugars | 3 | 100 | 0 | 21.7 | 43.3 | − | − | − |
| Vegetables and vegetable products (including fungi) | 327 | 98 | 2.10 | 25.5 | 48.9 | 0 | 25.0 | 50.0 |
| Cocoa beans and cocoa products | 276 | 99 | 0.41 | 25.2 | 50.0 | 0 | 25.0 | 50.0 |
| Coffee imitates (Solid) | 8 | 100 | 0 | 9.1 | 18.1 | − | − | − |
| Fruiting vegetables | 29 | 86 | 20.6 | 39.4 | 58.2 | − | − | − |
| Legume vegetables | 1 | 100 | 0 | 35.0 | 70.0 | − | − | − |
| Vegetables and vegetable products (including fungi) | 2 | 100 | 0 | 18.8 | 37.5 | − | − | − |
| Vegetable products, unspecified | 11 | 100 | 0 | 18.8 | 19.1 | − | − | − |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle Bound; UB: upper bound.
FoodEx Level 1 categories in bold and Food Level 2 categories in regular typeface.
The 95th percentiles obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and are therefore not reported in the table and replaced by a dash.
Table 13.
Statistical description of the concentrations of DON in the grain and grain based products across FoodEx level 3
| Food groupa | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Grains and grain‐based products | 15,943 | 52 | 84.5 | 95.5 | 107 | 357 | 357 | 357 |
| Grains for human consumption | 3,491 | 55 | 122 | 133 | 144 | 560 | 560 | 560 |
| Barley grain | 357 | 62 | 143 | 152 | 162 | 708 | 708 | 708 |
| Buckwheat grain | 63 | 92 | 38.0 | 48.3 | 58.5 | 263 | 263 | 263 |
| Corn grain | 226 | 41 | 41.8 | 50.9 | 60.0 | 320 | 320 | 320 |
| Grains for human consumption, unspecified | 28 | 71 | 140 | 146 | 152 | − | − | − |
| Millet grain | 42 | 76 | 129 | 137 | 144 | − | − | − |
| Oats, grain | 228 | 42 | 2.90 | 20.4 | 37.9 | 13.8 | 34.3 | 62.0 |
| Other grains | 41 | 59 | 53.4 | 68.6 | 84.0 | − | − | − |
| Rice | 140 | 94 | 39.2 | 49.7 | 60.1 | 118 | 118 | 118 |
| Rye grain | 543 | 67 | 181 | 190 | 200 | 751 | 751 | 751 |
| Spelt grain | 200 | 62 | 66.2 | 79.4 | 92.7 | 268 | 268 | 268 |
| Wheat grain | 1,623 | 47 | 6.40 | 21.5 | 36.5 | 50.0 | 50.0 | 70.0 |
| Bread and rolls | 2,837 | 44 | 66.3 | 75.8 | 85.6 | 261 | 262 | 262 |
| Bread and rolls, unspecified | 728 | 44 | 79.7 | 88.9 | 98.3 | 308 | 308 | 308 |
| Bread products | 88 | 40 | 136 | 145 | 155 | 488 | 488 | 488 |
| Mixed wheat and rye bread and rolls | 483 | 45 | 65.5 | 75.3 | 85.1 | 249 | 249 | 249 |
| Multigrain bread and rolls | 217 | 29 | 68.7 | 75.4 | 83.1 | 298 | 298 | 298 |
| Other bread | 114 | 52 | 51.3 | 62.0 | 72.6 | 184 | 184 | 184 |
| Rye bread and rolls | 97 | 59 | 52.8 | 62.1 | 71.4 | 231 | 231 | 231 |
| Unleavened bread, crisp bread and rusk | 424 | 57 | 31.9 | 43.5 | 55.3 | 136 | 136 | 136 |
| Wheat bread and rolls | 686 | 37 | 68.7 | 77.9 | 87.2 | 252 | 252 | 252 |
| Breakfast cereals | 2,169 | 64 | 56.0 | 68.5 | 81.1 | 246 | 246 | 246 |
| Breakfast cereals, unspecified | 278 | 55 | 73.6 | 81.5 | 89.7 | 270 | 270 | 270 |
| Cereal bars | 31 | 77 | 19.1 | 36.0 | 53.0 | − | − | − |
| Cereal flakes | 940 | 60 | 75.0 | 86.7 | 98.3 | 335 | 335 | 335 |
| Grits | 28 | 86 | 99.0 | 117 | 136 | − | − | − |
| Mixed breakfast cereals | 37 | 24 | 80.5 | 85.6 | 90.7 | − | − | − |
| Muesli | 685 | 72 | 25.7 | 40.8 | 55.8 | 132 | 132 | 132 |
| Popped cereals | 126 | 73 | 41.6 | 57.4 | 73.2 | 201 | 201 | 201 |
| Grain milling products | 4,609 | 50 | 86.8 | 98.0 | 109 | 369 | 369 | 369 |
| Buckwheat milling products | 60 | 88 | 12.5 | 32.9 | 53.3 | 133 | 133 | 140 |
| Corn milling products | 884 | 56 | 99.2 | 113 | 126 | 460 | 460 | 460 |
| Grain milling products, unspecified | 209 | 57 | 69.1 | 81.3 | 93.6 | 317 | 317 | 317 |
| Oat milling products | 225 | 47 | 58.5 | 65.4 | 72.7 | 220 | 220 | 220 |
| Other milling products | 76 | 65 | 68.4 | 80.3 | 92.3 | 370 | 370 | 370 |
| Rice milling products | 61 | 98 | 1.21 | 24.8 | 48.3 | 0.00 | 25.0 | 50 |
| Rye milling products | 639 | 56 | 53.4 | 65.6 | 78.1 | 240 | 240 | 240 |
| Spelt milling products | 254 | 56 | 46.0 | 56.7 | 67.7 | 227 | 227 | 227 |
| Wheat milling products | 2,201 | 42 | 105 | 116 | 126 | 410 | 410 | 410 |
| Grains and grain‐based products, unspecified | 59 | 73 | 68.6 | 84.6 | 100 | − | − | − |
| Pasta (Raw) | 1,803 | 51 | 81.6 | 8.80 | 104 | 342 | 342 | 342 |
| Noodle, wheat flour, with eggs | 67 | 48 | 61.0 | 69.2 | 77.4 | 338 | 338 | 338 |
| Noodle, wheat flour, without eggs | 84 | 6 | 220 | 222 | 223 | 730 | 730 | 730 |
| Noodle, rice | 2 | 100 | 0 | 8.8 | 17.5 | − | − | − |
| Pasta (Raw), unspecified | 473 | 69 | 53.8 | 69.6 | 85.5 | 260 | 260 | 260 |
| Pasta, spelt flour | 13 | 46 | 123 | 129 | 135 | − | − | − |
| Pasta, spelt wholemeal | 16 | 63 | 17.8 | 29.0 | 40.2 | − | − | − |
| Pasta, wheat flour, filled | 16 | 44 | 65.4 | 74.2 | 82.9 | − | − | − |
| Pasta, wheat flour, with eggs | 487 | 40 | 92.6 | 102 | 111 | 324 | 324 | 324 |
| Pasta, wheat flour, without eggs | 448 | 47 | 87.0 | 95.3 | 104 | 377 | 377 | 377 |
| Pasta, wheat wholemeal, with eggs | 94 | 73 | 40.3 | 59.3 | 78.2 | 316 | 316 | 316 |
| Pasta, wheat wholemeal, without eggs | 103 | 59 | 80.5 | 92.8 | 105 | 388 | 388 | 388 |
| Fine bakery wares | 975 | 53 | 62.4 | 72.0 | 81.6 | 241 | 241 | 241 |
| Biscuits (cookies) | 626 | 50 | 61.5 | 69.9 | 78.3 | 241 | 241 | 241 |
| Fine bakery wares, unspecified | 187 | 60 | 61.2 | 74.1 | 87.0 | 230 | 230 | 230 |
| Pastries and cakes | 162 | 60 | 67.1 | 77.7 | 88.3 | 288 | 288 | 288 |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle Bound; UB: upper bound.
FoodEx Level 1 categories in bold, Food Level 2 categories in bold first indent, FoodEx level 3 in regular typeface.
The 95th percentiles obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and are therefore not reported in the table and replaced by a dash.
Table 14.
Statistical description of the concentrations of 3‐Ac‐DON across the FoodEx food categories (FoodEx Level 1(bold) and Level 2 (regular typeface))
| Food groupa | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Alcoholic beverages | 835 | 100 | 0 | 30.6 | 61.2 | 0 | 50.0 | 100 |
| Beer and beer‐like beverage | 454 | 100 | 0 | 14.3 | 28.7 | 0 | 50.0 | 100 |
| Wine | 381 | 100 | 0 | 50.0 | 100 | 0 | 50.0 | 100 |
| Animal and vegetable fats and oils | 71 | 100 | 0 | 38.3 | 76.6 | 0 | 50.0 | 100 |
| Vegetable fat | 53 | 100 | 0 | 50.0 | 100 | − | − | − |
| Vegetable oil | 18 | 100 | 0 | 3.9 | 7.8 | − | − | − |
| Composite food (including frozen products) | 11 | 100 | 0 | 14.3 | 28.6 | − | − | − |
| Cereal‐based dishes | 2 | 100 | 0 | 7.5 | 15.0 | − | − | − |
| Meat‐based meals | 1 | 100 | 0 | 50.0 | 100 | − | − | − |
| Rice‐based meals | 7 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Vegetable‐based meals | 1 | 100 | 0 | 5.0 | 10.0 | − | − | − |
| Food for infants and small children | 346 | 100 | 0 | 13.5 | 27.1 | 0 | 25.0 | 50.0 |
| Cereal‐based food for infants and young children | 288 | 100 | 0 | 14.4 | 28.7 | 0 | 25.0 | 50.0 |
| Follow‐on formulae, powder | 12 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Food for infants and small children | 18 | 100 | 0 | 7.1 | 14.2 | − | − | − |
| Infant formulae, powder | 2 | 100 | 0 | 5.0 | 10.0 | − | − | − |
| Ready‐to‐eat meal for infants and young children | 24 | 100 | 0 | 10.4 | 20.8 | − | − | − |
| Yoghurt, cheese and milk‐based dessert for infants and young children | 2 | 100 | 0 | 5.0 | 10.0 | − | − | − |
| Fruit and fruit products | 1,017 | 100 | 0 | 48.9 | 97.8 | 0 | 50.0 | 100 |
| Berries and small fruits | 25 | 100 | 0 | 45.2 | 90.4 | − | − | − |
| Dried fruits | 461 | 100 | 0 | 49.8 | 99.7 | 0 | 50.0 | 100 |
| Fruit and fruit products | 37 | 100 | 0 | 50.0 | 100.0 | − | − | − |
| Miscellaneous fruits | 287 | 100 | 0 | 49.2 | 98.3 | 0 | 50.0 | 100 |
| Pome fruits | 13 | 100 | 0 | 46.9 | 93.8 | − | − | − |
| Stone fruits | 194 | 100 | 0 | 46.7 | 93.4 | 0 | 50.0 | 100 |
| Fruit and vegetable juices | 91 | 100 | 0 | 50.0 | 100 | 0 | 50.0 | 100 |
| Concentrated fruit juice | 7 | 100 | 0 | 50.0 | 100 | − | − | − |
| Fruit juice | 73 | 100 | 0 | 50.0 | 100 | 0 | 50.0 | 100 |
| Fruit nectar | 11 | 100 | 0 | 50.0 | 100 | − | − | − |
| Grains and grain‐based products | 7,044 | 97 | 1.3 | 21.1 | 40.9 | 0 | 50.0 | 100 |
| Grains for human consumption | 1,434 | 98 | 0.4 | 16.6 | 32.7 | 0 | 50.0 | 100 |
| Grain milling products | 2,173 | 99 | 0.3 | 26.5 | 52.6 | 0 | 50.0 | 100 |
| Bread and rolls | 875 | 95 | 2.1 | 13.4 | 24.8 | 0 | 25.0 | 50.0 |
| Breakfast cereals | 1,196 | 92 | 4.7 | 23.3 | 42.0 | 31.2 | 50.0 | 100 |
| Fine bakery wares | 379 | 99 | 0.2 | 11.4 | 22.6 | 0 | 25.0 | 50.0 |
| Pasta (Raw) | 942 | 100 | 0 | 22.6 | 45.1 | 0 | 50.0 | 100 |
| Grains and grain‐based products, unspecified | 45 | 100 | 0 | 47.1 | 94.3 | − | − | − |
| Herbs, spices and condiments | 89 | 100 | 0 | 32.3 | 64.6 | 0 | 50.0 | 100 |
| Baking ingredients | 1 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Condiment | 48 | 100 | 0 | 49.1 | 98.1 | − | − | − |
| Flavourings or essences | 2 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Herb and spice mixtures | 5 | 100 | 0 | 18.0 | 36.0 | − | − | − |
| Herbs | 5 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Herbs, spices and condiments | 3 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Seasoning or extracts | 4 | 100 | 0 | 6.3 | 12.5 | − | − | − |
| Spices | 21 | 100 | 0 | 13.8 | 27.6 | − | − | − |
| Legumes, nuts and oilseeds | 1,982 | 100 | 0.1 | 48.4 | 96.7 | 0 | 50.0 | 100 |
| Legumes, beans, dried | 361 | 100 | 0 | 46.0 | 92.1 | 0 | 50.0 | 100 |
| Oilseeds | 387 | 100 | 0.4 | 45.5 | 90.6 | 0 | 50.0 | 100 |
| Other seeds | 34 | 100 | 0 | 50.0 | 100 | − | − | − |
| Tree nuts | 1,200 | 100 | 0 | 50.0 | 100 | 0 | 50.0 | 100 |
| Milk and dairy products | 2 | 100 | 0 | 5.0 | 10.0 | − | − | − |
| Non‐alcoholic beverages | 1 | 100 | 0 | 50.0 | 100 | − | − | − |
| Products for special nutritional use | 12 | 100 | 0 | 14.4 | 28.8 | − | − | − |
| Dietary supplements | 1 | 100 | 0 | 50.0 | 100 | − | − | − |
| Food for weight reduction | 1 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Medical food | 4 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Medical food (are specially formulated and intended for the dietary management of a disease that has distinctive nutritional needs that cannot be met by normal diet alone; intended to be used under medical supervision) | 5 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Products for special nutritional use | 1 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Snacks, desserts and other foods | 118 | 100 | 0 | 17.7 | 35.3 | 0 | 50.0 | 100 |
| Ices and desserts | 1 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Other foods (foods which cannot be included in any other group) | 1 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Snack food | 116 | 100 | 0 | 17.8 | 35.6 | 0 | 50.0 | 100 |
| Starchy roots and tubers | 6 | 100 | 0 | 30.0 | 60.0 | − | − | − |
| Sugar and confectionery | 14 | 100 | 0 | 32.9 | 65.7 | − | − | − |
| Chocolate (Cocoa) products | 6 | 100 | 0 | 50.0 | 100 | − | − | − |
| Confectionery (non‐chocolate) | 2 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Dessert sauces | 2 | 100 | 0 | 50.0 | 100 | − | − | − |
| Sugar and confectionary | 4 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Vegetables and vegetable products (including fungi) | 305 | 100 | 0 | 47.2 | 94.4 | 0 | 50.0 | 100 |
| Cocoa beans and cocoa products | 276 | 100 | 0 | 50.0 | 100 | 0 | 50.0 | 100 |
| Coffee imitates (Solid) | 8 | 100 | 0 | 8.9 | 17.7 | − | − | − |
| Fruiting vegetables | 8 | 100 | 0 | 45.0 | 90.0 | − | − | − |
| Vegetable products | 11 | 100 | 0 | 9.5 | 19.1 | − | − | − |
| Vegetables and vegetable products (including fungi) | 2 | 100 | 0 | 31.3 | 62.5 | − | − | − |
N: number of samples; % LC: percentage of left‐censored data; LB: lower bound; MB: middle bound; UB: upper bound.
Food Level 1 categories in bold and Food Level 2 categories in regular typeface.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table and replaced by a dash.
Table 15.
Statistical description of the concentrations of 15‐Ac‐DON across the FoodEx food categories (FoodEx Level 1(bold) and Level 2 (regular typeface))
| Food groupa | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Alcoholic beverages | 336 | 99 | 0.1 | 5.5 | 10.9 | 0 | 50.0 | 100 |
| Animal and vegetable fats and oils | 18 | 100 | 0 | 6.9 | 13.9 | − | − | − |
| Composite food (including frozen products) | 10 | 100 | 0 | 10.8 | 21.5 | − | − | − |
| Cereal‐based dishes | 2 | 100 | 0 | 7.5 | 15.0 | − | − | − |
| Rice‐based meals | 7 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Vegetable‐based meals | 1 | 100 | 0 | 5.0 | 10.0 | − | − | − |
| Food for infants and small children | 334 | 100 | 0 | 14.5 | 29.1 | 0 | 25.0 | 50.0 |
| Cereal‐based food for infants and young children | 288 | 100 | 0 | 15.1 | 30.1 | 0 | 25.0 | 50.0 |
| Follow‐on formulae, powder | 12 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Food for infants and small children | 18 | 100 | 0 | 7.1 | 14.2 | − | − | − |
| Infant formulae, powder | 2 | 100 | 0 | 5.0 | 10.0 | − | − | − |
| Ready‐to‐eat meal for infants and young children | 12 | 100 | 0 | 18.3 | 36.7 | − | − | − |
| Yoghurt, cheese and milk‐based dessert for infants and young children | 2 | 100 | 0 | 5.0 | 10.0 | − | − | − |
| Fruit and fruit products | 28 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Berries and small fruits | 3 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Dried fruits | 2 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Miscellaneous fruits | 6 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Pome fruits | 1 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Stone fruits | 16 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Grains and grain‐based products | 5,392 | 96 | 2.2 | 13.9 | 25.7 | 0 | 25.0 | 50.0 |
| Grains for human consumption | 1,151 | 97 | 1.5 | 10.1 | 18.8 | 0 | 25.0 | 50.0 |
| Grain milling products | 1,314 | 95 | 3.8 | 14.8 | 25.9 | 0 | 25.0 | 50.0 |
| Grains and grain‐based products | 3 | 100 | 0.0 | 7.9 | 15.8 | − | − | − |
| Bread and rolls | 855 | 95 | 1.2 | 13.1 | 25.3 | 6.0 | 25.0 | 50.0 |
| Breakfast cereals | 989 | 94 | 3.9 | 18.4 | 32.9 | 4.7 | 25.0 | 50.0 |
| Fine bakery wares | 374 | 99 | 0.6 | 12.2 | 23.8 | 0 | 25.0 | 50.0 |
| Pasta (Raw) | 706 | 100 | 0.0 | 14.2 | 28.4 | 0 | 25.0 | 50.0 |
| Herbs, spices and condiments | 37 | 97 | 0.4 | 17.5 | 34.6 | − | − | − |
| Baking ingredients | 1 | 100 | 0.0 | 12.5 | 25.0 | − | − | − |
| Condiment | 1 | 0 | 16.0 | 16.0 | 16.0 | − | − | − |
| Flavourings or essences | 2 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Herb and spice mixtures | 4 | 100 | 0 | 17.5 | 35.0 | − | − | − |
| Herbs | 5 | 100 | 0 | 25.0 | 50.0 | − | − | − |
| Herbs, spices and condiments | 3 | 100 | 0 | 15.0 | 30.0 | − | − | − |
| Seasoning or extracts | 4 | 100 | 0 | 6.3 | 12.5 | − | − | − |
| Spices | 17 | 100 | 0 | 19.7 | 39.4 | − | − | − |
| Legumes, nuts and oilseeds | 79 | 100 | 0 | 8.6 | 17.1 | 0 | 10.0 | 20.0 |
| Legumes, beans, dried | 34 | 100 | 0 | 7.9 | 15.8 | − | − | − |
| Oilseeds | 45 | 100 | 0 | 9.1 | 18.1 | − | − | − |
| Milk and milk product imitates | 2 | 100 | 0 | 5.0 | 10.0 | − | − | − |
| Products for special nutritional use | 11 | 91 | 5.2 | 17.2 | 29.3 | − | − | − |
| Food for weight reduction | 1 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Medical food | 4 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Medical food (are specially formulated and intended for the dietary management of a disease that has distinctive nutritional needs that cannot be met by normal diet alone; intended to be used under medical supervision) | 5 | 80 | 11.4 | 23.4 | 35.4 | − | − | − |
| Products for special nutritional use | 1 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Snacks, desserts and other foods | 93 | 90 | 3.0 | 12.1 | 21.2 | 23.0 | 25.0 | 50.0 |
| Ices and desserts | 1 | 100 | 0 | 25.0 | 50.0 | − | − | − |
| Other foods (foods which cannot be included in any other group) | 1 | 100 | 0 | 12.5 | 25.0 | − | − | − |
| Snack food | 91 | 90 | 3.1 | 12.0 | 20.9 | 23.0 | 25.0 | 50.0 |
| Starchy roots and tubers | 3 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Sugar and confectionary | 6 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Confectionery (non‐chocolate) | 2 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Sugar and confectionary | 4 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Vegetables and vegetable products (including fungi) | 21 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Coffee imitates (Solid) | 8 | 100 | 0 | 9.2 | 18.4 | − | − | − |
| Fruiting vegetables | 1 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Vegetable products | 11 | 100 | 0 | 9.5 | 19.1 | − | − | − |
| Vegetables and vegetable products (including fungi) | 1 | 100 | 0 | 12.5 | 25.0 | − | − | − |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MD: middle Bound; UB: upper bound.
Food Level 1 categories in bold and Food Level 2 categories in regular typeface.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table and replaced by a dash.
Table 16.
Statistical description of the concentrations of DON‐3‐glucoside across the FoodEx food categories (FoodEx Level 1 (bold) and Level 2 (regular typeface))
| Food groupa | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Alcoholic beverages | 30 | 50 | 15.2 | 20.3 | 25.6 | − | − | − |
| Beer and beer‐like beverage | 30 | 50 | 15.2 | 20.3 | 25.6 | − | − | − |
| Animal and vegetable fats and oils | 1 | 100 | 0.0 | 10.0 | 20.0 | − | − | − |
| Food for infants and small children | 30 | 90 | 6.01 | 19.0 | 32.0 | − | − | − |
| Cereal‐based food for infants and young children | 12 | 75 | 14.9 | 32.4 | 49.9 | − | − | − |
| Ready‐to‐eat meal for infants and young children | 18 | 100 | 0.00 | 10.0 | 20.0 | − | − | − |
| Grains and grain‐based products | 706 | 59 | 93.4 | 101 | 109 | 481 | 481 | 481 |
| Grains for human consumption | 382 | 52.1 | 159 | 165 | 170 | 570 | 570 | 570 |
| Grain milling products | 22 | 32 | 32.2 | 38.0 | 44.4 | − | − | − |
| Fine bakery wares | 3 | 100 | 0.0 | 15.0 | 30.0 | − | − | − |
| Bread and rolls | 131 | 64 | 19.7 | 28.5 | 38.2 | 101 | 101 | 101 |
| Breakfast cereals | 168 | 72 | 10.8 | 21.8 | 33.3 | 63.8 | 63.8 | 63.8 |
| Herbs, spices and condiments | 29 | 100 | 0.00 | 10.0 | 20.0 | − | − | − |
| Herb and spice mixtures | 2 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Herbs | 5 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Herbs, spices and condiments | 4 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Spices | 18 | 100 | 0 | 10.0 | 20.0 | − | − | − |
| Snacks, desserts, and other foods | 1 | 100 | 0 | 10.0 | 20.0 | − | − | − |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle bound; UB: upper bound.
Food Level 1 categories in bold and Food Level 2 categories in regular typeface.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table and replaced by a dash.
4.2.3.3. Analytical methods used for food
The information on analytical methods and/or on LOQ levels was not available for 19% of food analytical results. In addition, the measurements obtained by ELISA and TLC methods were not considered reliable. According to the criteria described in Section 4.2.3, those data did not fulfil the quality criteria and were not included in the further analyses. With this approach, 26% of the analytical data on food were excluded.
For DON in food, 42% of the data were obtained by LC–MS‐based methods (LC–MS/MS 33%, LC–MS quadrupole 9%), 34% by HPLC‐based methods (HPLC‐UV 15%, HPLC with standard detection methods (not specified) 11%, HPLC‐FLD 8%) and 24% by GC‐based methods (GC–MS 9%, GC with standard detection methods (not specified) 7.5%, GC‐ECD 7%, GC‐HRMS 0.5%). The data set on food contained overall 63% of left‐censored data (results below LOD/LOQ) for DON. The LOQs varied with the method used, the food matrix and the laboratory. A range of LOQs was observed with the lowest median LOQ of 15 μg/kg for the food category ‘Milk and dairy products’ and the highest median LOQ of 59 μg/kg for the food category ‘Composite food’.
Regarding the acetylated forms, data on 3‐Ac‐DON in food were obtained by LC–MS‐based methods (66%) and GC‐based methods (34%). Analytical data on 15‐Ac‐DON in food were measured only by LC–MS‐based methods. The data sets comprised 98% and 97% of left‐censored data for 3‐Ac‐DON and 15‐Ac‐DON, respectively. A range of LOQs was observed with the lowest median LOQ of 7 μg/kg for the food category ‘Alcoholic beverages’ obtained for 15‐Ac‐DON and the highest median LOQ of 100 μg/kg observed for 3‐Ac‐DON in nine food categories.
Analytical data on DON‐3‐glucoside in food were obtained by LC–MS‐based methods (49%), HPLC‐based methods (18%) and GC‐based methods (33%). The left‐censored data accounted for 57% of the results. LOQs were observed at a median LOQ of 20 μg/kg for five food categories and at a median LOQ of 26 μg/kg observed for the food category ‘Grains and grain‐based products’.
The distribution of LOQs for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across food categories is illustrated in Figure 7. A detailed presentation of the proportion of left‐censored data across food categories is given in Tables 12, 13, 14, 15, 16–16 (Section 4.2.3.4).
Figure 7.
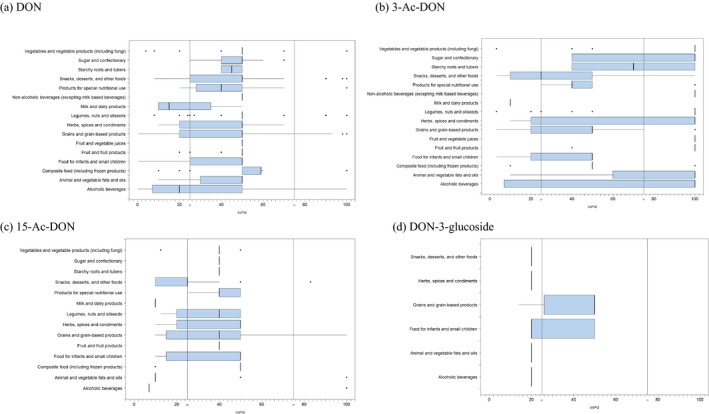
Distribution of the LOQs for (a) DON, (b) 3‐Ac‐DON, (c) 15‐Ac‐DON and (d) DON‐3‐glucoside across food groups
- (Box‐plot: whiskers at minimum and maximum, box at 25th percentile and 75th percentile with line at 50th percentile). When the median is equal to the lower or upper quartile, the median and the quartile overlap and in the graph a bold single line is displayed. This may occur especially when the number of left‐censored data is high. Moreover, when the maximum (minimum) value is equal to the upper (lower) quartile, whiskers overlap with the box and a bold line is displayed.
4.2.3.4. Occurrence data in food
4.2.3.4.1. Currently reported occurrence data on DON in food
Occurrence data on DON were reported in 16 FoodEx level 1 food categories. An overview of the number of data points available for evaluation, the proportion of left‐censored data as a percentage, mean and 95th percentile (P95) concentration values is presented in Table 12. The majority of the data received for DON were on ‘Grains and grain‐based products’ (n = 15,943), with 52% of left‐censored data followed by ‘Legumes, nuts and oilseeds’ (n = 1,990) with 99.6% of left‐censored data. Taking into account this limitation, ‘Products for special nutritional use’ was the food category that recorded the highest values of DON in comparison with all other food categories (LB mean = 127 μg/kg; UB mean = 146 μg/kg) followed by ‘Grains and grain‐based products’ (LB mean = 84.5 μg/kg; UB mean = 107 μg/kg). For the food groups ‘Wine’, ‘Animal and vegetable fats and oils’, ‘Follow‐on formulae, powder’, ‘Berries and small fruits’, ‘Fruit and fruit products’, ‘Pome fruit’, ‘Fruit and vegetable juices’, ‘Other seeds’, ‘Tree nuts’ and ‘Vegetable products’, DON was not detected or quantified. The predominant food category at the FoodEx level 1 ‘Grains and grain‐based products’, covered seven food groups at the FoodEx level 2: ‘Grains for human consumption’, ‘Grain milling products’, ‘Bread and rolls’, ‘Breakfast cereals’, ‘Fine bakery wares’, ‘Pasta (raw)’ and ‘Grains and grain‐based products, unspecified’. Within this category, the highest mean concentrations were measured in ‘Grains for human consumption’, with 55% of left‐censored data and the LB mean at 122 μg/kg and UB mean at 144 μg/kg.
The statistical description of results reported on DON in accordance with FoodEx level 3 food categories is summarised in Tables 12 and 13.
4.2.3.4.2. Currently reported occurrence data on 3‐Ac‐DON in food
Occurrence data on 3‐Ac‐DON covered 13 food categories. An overview of the number of data points available for evaluation, proportion of left‐censored data as a percentage, mean and 95th percentile (P95) concentration values is presented in Table 14. The majority of the data received for 3‐Ac‐DON were on ‘Grains and grain‐based products’ (n = 7,044), with 97% of left‐censored data, followed by ‘Legumes, nuts an oilseeds’ (n = 1,982) with 99.9% of left‐censored data. All remaining food categories comprised only left‐censored data. The mean concentrations in ‘Grains and grain‐based products’ were observed at a level of 1.30 μg/kg for the LB and at a level of 40.9 μg/kg for the UB. The highest mean concentrations according FoodEx level 2 were measured in ‘Breakfast cereals’ (LB mean = 4.71 μg/kg; UB mean = 42.0 μg/kg).
4.2.3.4.3. Currently reported occurrence data on 15‐Ac‐DON in food
Occurrence data on 15‐Ac‐DON covered 11 food categories. An overview of the number of data points available for evaluation, proportion of left‐censored as a percentage, mean and 95th percentile (P95) concentration values is presented in Table 15. The majority of the data received for 15‐Ac‐DON were on ‘Grains and grain‐based products’ (n = 5,392), with 96% of left‐censored data, followed by ‘Alcoholic beverages’ (n = 336) with 99% of left‐censored data. Only a limited number of food categories comprised detected/quantified results of 15‐Ac‐DON. The highest mean concentrations in food groups were observed in ‘Products for special nutritional use’ (LB mean = 5.23 μg/kg; UB mean = 29.3 μg/kg), ‘Snacks, desserts, and other foods’ (LB mean = 3.01 μg/kg; UB mean = 21.2 μg/kg) and ‘Grains and grain‐based products’ (LB mean = 2.20 μg/kg; UB mean = 25.7 μg/kg). Within the food category ‘Products for special nutritional use’, 15‐Ac‐DON was quantified only in ‘Medical food’.
4.2.3.4.4. Currently reported occurrence data on DON‐3‐glucoside in food
Occurrence data on DON‐3‐glucoside were reported for four food categories. An overview of the number of data points available for evaluation, proportion of left‐censored data as a percentage, mean and 95th percentile (P95) concentration values is presented in Table 16. The data received for DON‐3‐glucoside were predominantly on ‘Grains and grain‐based products’ (n = 706) with 59% of left‐censored data and only a limited number of results were reported for other food categories. ‘Grains and grain‐based products’ was also the food category with the highest mean concentrations of DON‐3‐glucoside (LB mean = 93.4 μg/kg; UB mean = 109 μg/kg). According to the FoodEx level 2 classification, the highest DON‐3‐glucoside concentrations were measured in ‘Grains for human consumption’ (LB mean = 159 μg/kg; UB mean = 170 μg/kg), followed by ‘Grain milling products’ (LB mean = 32.2 μg/kg; UB mean = 44.4 μg/kg).
Apart from grain‐based products food categories, DON‐3‐glucoside was quantified in ‘Beer and beer‐like beverage’ (LB mean = 15.2 μg/kg; UB mean = 25.6 μg/kg) and ‘Cereal‐based food for infants and young children (LB mean = 14.9 μg/kg; UB mean = 49.9 μg/kg).
4.2.3.4.5. Currently reported occurrence data on the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food
To assess the exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, the CONTAM Panel reviewed the occurrence data for the sum of concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food with the number of samples available for evaluation and statistical descriptors of the results (mean and 95th percentile for LB, MB and UB results) as shown in Table 17. See Sections 4.2.2 and 4.2.8 for the calculation of the sum of concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. It should be noted that the occurrence data on the sum of concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside refer to the number of samples instead of the number of the analytical results. (The exposure assessment of DON alone is presented in Appendix F).
Table 17.
Statistical description of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food across the FoodEx food categories (FoodEx Level 1 (bold) and Level 2 (regular typeface))
| Food groupa | N | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | ||||||
| LB | MB | UB | LB | MB | UB | ||
| Alcoholic beverages a | 1,123 | 4.51 | 55.8 | 107 | 27.1 | 98.8 | 198 |
| Animal and vegetable fats and oils | 72 | 0 | 66.6 | 133 | 0 | 83.8 | 168 |
| Composite food (including frozen products) | 68 | 27.4 | 50.6 | 73.8 | 157 | 157 | 168 |
| Food for infants and small children | 849 | 11.8 | 40.9 | 70.2 | 80.5 | 84.1 | 160 |
| Fruit and fruit products | 1,019 | 2.53 | 84.4 | 166 | 0 | 83.8 | 168 |
| Fruit and vegetable juices | 91 | 0 | 83.7 | 168 | 0 | 83.8 | 168 |
| Grains and grain‐based products | 15,943 | 117 | 144 | 173 | 493 | 506 | 519 |
| Grains for human consumptiona | 3,491 | 171 | 195 | 220 | 787 | 793 | 797 |
| Grain milling products | 4,609 | 119 | 150 | 181 | 513 | 523 | 539 |
| Bread and rolls | 2,837 | 91.2 | 112 | 133 | 356 | 361 | 371 |
| Breakfast cereals | 2,169 | 77.3 | 112 | 146 | 338 | 357 | 370 |
| Fine bakery wares | 975 | 83.5 | 106 | 128 | 334 | 334 | 343 |
| Pasta (Raw) | 1,803 | 110 | 143 | 175 | 469 | 477 | 489 |
| Grains and grain‐based products, unspecified | 59 | 96.3 | 154 | 212 | − | − | − |
| Herbs, spices and condiments | 96 | 13.4 | 75.6 | 138 | 82.7 | 94.1 | 168 |
| Legumes, nuts and oilseeds | 1,990 | 0.99 | 82.1 | 163 | 0 | 83.8 | 168 |
| Milk and dairy products | 3 | 18.2 | 29.7 | 41.4 | − | − | − |
| Non‐alcoholic beverages (excepting milk based beverages) | 1 | 0 | 83.7 | 168 | − | − | − |
| Products for special nutritional use | 39 | 177 | 198 | 219 | − | − | − |
| Snacks, desserts, and other foods | 265 | 113 | 140 | 167 | 518.4 | 522 | 522 |
| Starchy roots and tubers | 6 | 0 | 57.86 | 116 | − | − | − |
| Sugar and confectionery | 23 | 14.0 | 61.3 | 109 | − | − | − |
| Vegetables and vegetable products (including fungi) | 327 | 3.08 | 79.3 | 156 | 0 | 83.8 | 168 |
N: number of samples; LB: lower bound; MB: middle Bound; UB: upper bound.
Food Level 1 categories in bold and Food Level 2 categories in regular typeface. Left‐censored data not reported because the table reports the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table and replaced by a dash.
4.2.4. Data on feed
4.2.4.1. Data collection in feed
The data set on feed comprised 10,771 analytical results, including 6,980 results for DON, 1,649 for 3‐Ac‐DON, 1,210 for 15‐Ac‐DON and 932 analytical results for DON‐3‐glucoside. A total of 10,771 analytical results were submitted. Data on feed were reported by 18 European countries (Figure 8). The major contributing countries were the Netherlands (19% of the DON data, 50% of the 3‐Ac‐DON data, 68% of the 15‐Ac‐DON data and 86% of the DON‐3‐glucoside data) and Hungary (35% of the DON data). The distribution of the occurrence data over the sampling years is presented in Figure 9. EFSA called data for feed samples between 2004 and 2014 but no data were reported to EFSA before 2007.
Figure 8.
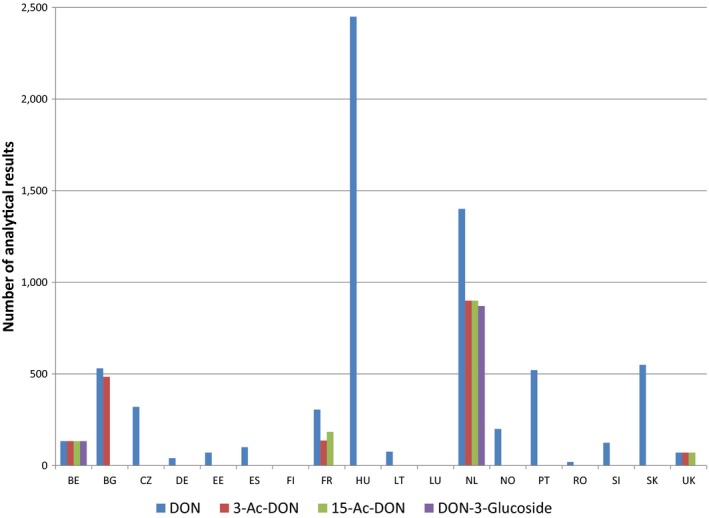
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in feed across the European countries
- BE, Belgium; BG, Bulgaria; CZ, the Czech Republic; DE, Germany; EE, Estonia; ES, Spain; FI, Finland; FR, France; HU, Hungary; LT, Lithuania; LU, Luxembourg; NL, the Netherlands; NO, Norway; PT, Portugal; RO, Romania; SI, Slovenia; SK, Slovakia; UK, the United Kingdom.
Figure 9.
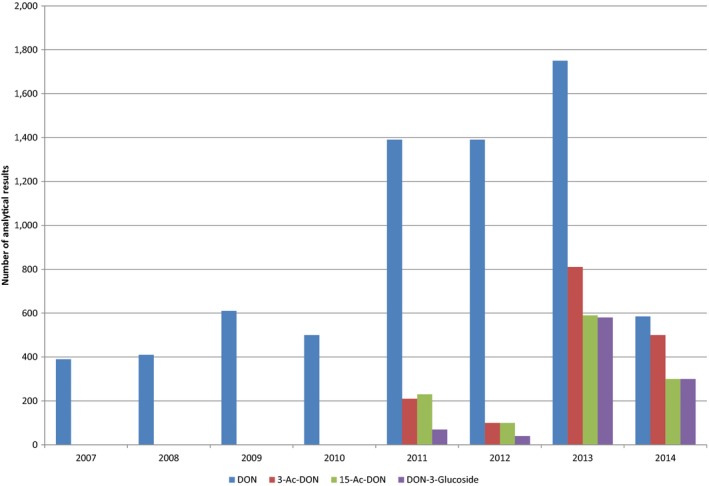
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in feed over the sampling years
Feeds were classified based on the catalogue of feed materials specified in the Commission Regulation (EU) No 575/2011 of 16 June 2011 creating the Catalogue of feed materials.26 Where information was available, compound feedingstuffs were classified in groups based on the species/production categories for which the feed is intended.
‘Compound feed’ was the feed category with the highest number of results reported for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, which accounted for 58%, 37%, 37% and 40%, respectively. In addition, the feed categories ‘Cereal grains’, ‘Forages and roughage, and products derived thereof’ and ‘Oilseeds, oil fruits, and products derived thereof’ were well represented. Only a limited number of occurrence data were submitted for other feed categories. A distribution of the samples in feed groups is shown in Figure 10. Results were reported on whole weight (48% samples) or on 88% dry matter (52% of samples). For consistency in the assessment, the latter were converted to values expressed on a whole‐weight basis.
Figure 10.
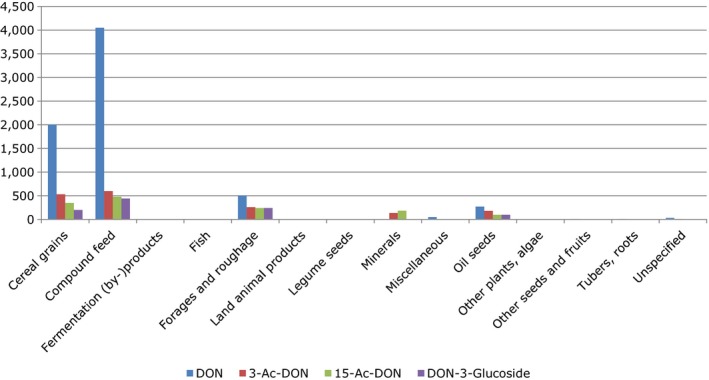
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in the feed categories
In order to include only the results obtained by analytical methods with sufficient sensitivity, LOQ cut‐off value ≤ 250 μg/kg was applied. As a result of this approach, 622 results of the DON data, 736 results of the 3‐Ac‐DON data, 720 results of the 15‐Ac‐DON data and one result of the DON‐3‐glucoside data were not included in the feed data set to be used for occurrence analysis and dietary exposure assessment. After applying the exclusion criteria described in Section 4.2. and applying the LOQ cut‐off, the final feed data set included observations on DON (n = 4,000), 3‐Ac‐DON (n = 894), 15‐Ac‐DON (n = 470) and DON‐3‐glucoside (n = 931).
4.2.4.2. Distribution of samples across feed categories
The distribution of analytical results across different feed groups for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside classified based on the catalogue of feed materials (see Section 4.2.4.1) is illustrated in Figure 11.
Figure 11.
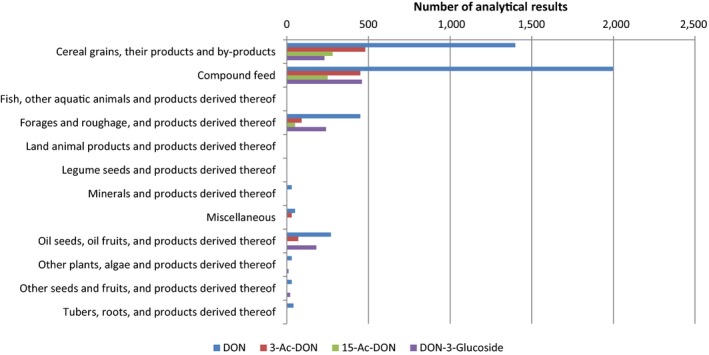
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in the feed categories
‘Compound feed’ was the feed category with the major number of results reported for DON and DON‐3‐glucoside, which accounted for 52% and 37%, respectively. For acetylated forms (3‐Ac‐DON and 15‐Ac‐DON) ‘Cereal grains, their products and by‐products’ was the feed category comprising the major number of results. For DON and DON‐3‐glucoside, ‘Forages and roughage, and products derived thereof’ and ‘Oilseeds, oil fruits, and products derived thereof’ were also well represented. A more detailed distribution and statistical description of concentrations is reported in Tables 18–21 (Section 4.2.4.4).
Table 18.
Statistical description of the concentrations of DON across the feed categories (all results expressed in μg/kg whole‐weight) (Feed Material Catalogue Level 1 (bold) categories and Level 2 categories (regular typeface))
| Feed groupa | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Cereal grains, their products and by‐products | 1,288 | 47 | 427 | 454 | 480 | 1,815 | 1,815 | 1,815 |
| Barley | 209 | 58 | 148 | 187 | 227 | 591 | 591 | 591 |
| Maize | 394 | 44 | 641 | 667 | 689 | 2,470 | 2 470 | 2,470 |
| Oats | 127 | 43 | 516 | 530 | 546 | 2,203 | 2 203 | 2,203 |
| Wheat | 465 | 42 | 401 | 423 | 442 | 1,589 | 1 589 | 1,589 |
| Compound feed | 1,996 | 32 | 401 | 416 | 431 | 1,580 | 1 580 | 1,570 |
| Compound feed, unspecified | 66 | 94 | 16.0 | 40.0 | 63.9 | 69.0 | 69.0 | 115 |
| Complementary feed (incomplete diet) | 215 | 36 | 254 | 277 | 291 | 1,065 | 1,210 | 1,065 |
| Complete feed | 1,715 | 30 | 433 | 448 | 462 | 1,680 | 1,680 | 1,680 |
| Fish, other aquatic animals and products derived thereof | 1 | 0 | 182 | 182 | 182 | − | − | − |
| Forages and roughage and products derived thereof | 412 | 47 | 485 | 510 | 536 | 1,810 | 1,810 | 1,810 |
| Cereal straw | 125 | 44 | 1,074 | 1,101 | 1,128 | 3,372 | 3,372 | 3,372 |
| Grass, field dried, [Hay] | 101 | 78 | 16.0 | 60.0 | 105 | 50.0 | 69.0 | 138 |
| Maize silage | 129 | 15 | 410 | 417 | 423 | 1,340 | 1,340 | 1,340 |
| Land animal products and products derived thereof | 5 | 80 | 10.0 | 17.0 | 24.0 | − | − | − |
| Legume seeds and products derived thereof | 5 | 80 | 10.0 | 74.4 | 137 | − | − | − |
| Minerals and products derived thereof | 2 | 100 | 0.00 | 58.1 | 116 | − | − | − |
| Miscellaneous | 11 | 18 | 441 | 445 | 449 | − | − | − |
| Oil seeds, oil fruits and products derived thereof | 250 | 94 | 7.00 | 80.0 | 153 | 50.0 | 100 | 200 |
| Sunflower seed | 67 | 100 | 0 | 43.2 | 87.0 | 0 | − | 250 |
| Toasted soya (beans) | 146 | 95 | 6.10 | 95.3 | 181 | 0 | − | 200 |
| Other plants, algae and products derived thereof | 4 | 100 | 0.0 | 96.0 | 192 | − | − | − |
| Other seeds and fruits, and products derived thereof | 2 | 100 | 0.0 | 96.0 | 192 | − | − | − |
| Tubers, roots and products derived thereof | 11 | 73 | 98.0 | 164 | 229 | − | − | − |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle bound; UB: upper bound.
The occurrence data at Feed Catalogue Level 2 are reported only for feed categories with more than 60 analytical results.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table and replaced by a dash.
Table 21.
Statistical description of the concentrations of DON‐3‐glucoside across the feed categories (all results expressed in μg/kg whole‐weight). (Feed Material Catalogue Level 1 (bold) and Level 2 (regular typeface))
| Feed groupa | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Cereal grains, their products and by‐products | 222 | 94 | 29.6 | 133 | 235 | 251 | 251 | 257 |
| Barley | 41 | 98 | 9.85 | 130 | 250 | − | − | − |
| Maize | 75 | 99 | 3.34 | 118 | 232 | 0 | 116 | 232 |
| Oats | 41 | 76 | 137 | 171 | 205 | − | − | − |
| Rice, broken | 4 | 100 | 0 | 128 | 257 | − | − | − |
| Rye | 8 | 100 | 0 | 122 | 245 | − | − | − |
| Spelt | 4 | 100 | 0 | 122 | 245 | − | − | − |
| Triticale | 4 | 100 | 0 | 124 | 248 | − | − | − |
| Wheat | 43 | 98 | 6.51 | 128 | 249 | − | − | − |
| Cereal grains, unspecified | 2 | 100 | 0 | 120 | 239 | − | − | − |
| Compound feed | 374 | 84 | 16.6 | 107 | 198 | 89.9 | 126 | 253 |
| Complementary feed (incomplete diet) | 49 | 82 | 37.1 | 109 | 180 | − | − | − |
| Complete feed | 325 | 84 | 13.7 | 107 | 200 | 77.6 | 125 | 250 |
| Forages and roughage, and products derived thereof | 220 | 96 | 60.8 | 144 | 228 | 0.0 | 121 | 241 |
| Cereal straw | 81 | 88 | 165 | 271 | 376 | 665 | 665 | 645 |
| Forage meal | 2 | 100 | 0 | 64.1 | 127 | − | − | − |
| Grass, field dried | 55 | 100 | 0 | 86.1 | 172 | − | − | − |
| Lucerne | 1 | 100 | 0 | 120 | 239 | − | − | − |
| Maize silage | 80 | 100 | 0 | 59.0 | 117 | 0 | 59.0 | 117 |
| Pea straw | 1 | 100 | 0 | 120 | 239 | − | − | − |
| Legume seeds and products derived thereof | 1 | 100 | 0 | 122 | 245 | − | − | − |
| Sweet lupins | 1 | 100 | 0 | 122 | 245 | − | − | − |
| Oil seeds, oil fruits, and products derived thereof | 110 | 100 | 0 | 125 | 249 | 0 | 125 | 250 |
| Linseed | 1 | 100 | 0 | 120 | 239 | − | − | − |
| Palm kernel expeller | 5 | 100 | 0 | 120 | 239 | − | − | − |
| Rape seed | 2 | 100 | 0 | 125 | 249 | − | − | − |
| Sunflower seed | 3 | 100 | 0 | 125 | 249 | − | − | − |
| Toasted soya (beans) | 99 | 100 | 0 | 125 | 250 | 0 | 125 | 250 |
| Other plants, algae and products derived thereof | 2 | 100 | 0 | 120 | 240 | − | − | − |
| Other plants, algae and products derived thereof, unspecified | 2 | 100 | 0 | 120 | 239 | − | − | − |
| Other seeds and fruits, and products derived thereof | 1 | 100 | 0 | 120 | 240 | − | − | − |
| Citrus pulp | 1 | 100 | 0 | 120 | 239 | − | − | − |
| Tubers, roots, and products derived thereof | 1 | 100 | 0.00 | 122 | 245 | − | − | − |
| Sugar beet | 1 | 100 | 0.00 | 122 | 245 | − | − | − |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle bound; UB: upper bound.
The occurrence data at Feed Catalogue Level 2 are reported only for feed categories with more than 60 analytical results.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and is therefore not reported in the table and replaced by a dash.
4.2.4.3. Analytical methods used for feed
Only occurrence data with information on the analytical method and on LOD/LOQ levels fulfilled the inclusion criteria for the present analysis. The measurements obtained by ELISA and TLC methods were not considered reliable. By this approach, about 28% of feed data were excluded.
For DON in feed, 82% of the data were obtained by LC–MS‐based methods (LC–MS/MS 44%, LC–MS quadrupole 38%), 11% by HPLC‐based methods (HPLC‐UV 7%, HPLC with standard detection methods (not specified) 1%, HPLC‐FLD 3%), 4% by GC‐based methods (GC–MS 3%, GC with standard detection methods (not specified) 0.8%, GC‐HRMS 0.2%), and 3% by other methods. The data set was characterised by a proportion of 43% of left‐censored data (results below the LOD/LOQ). The LOQs varied with the method used, the food matrix and the laboratory. A range of LOQs was observed with the lowest median LOQ of 40 μg/kg for the feed categories ‘Compound feed’ and ‘Land animal products and products derived thereof’, while the highest median LOQ of 200 μg/kg was observed for several feed categories, including legume seeds, oilseeds, oil fruits, other plants, algae, other seeds and fruits, tubers and roots.
Regarding acetylated forms, 97% of 3‐Ac‐DON data and 94% of 15‐Ac‐DON data in feed were obtained by LC–MS‐based methods (96% of 3‐Ac‐DON data and 93.6% of 15‐Ac‐DON data by LC–MS/MS) and only a small number of results were measured by GC‐based methods (4% of 3‐Ac‐DON data and 6.4% of 15‐Ac‐DON data). The data sets comprised 92% and 65% of left‐censored data for 3‐Ac‐DON and 15‐Ac‐DON, respectively. The median LOQ of 50 μg/kg was observed in all feed categories for 3‐Ac‐DON. A range of LOQs was observed for 15‐Ac‐DON with the lowest median LOQ of 10 μg/kg for ‘Cereal grains, their products and by‐products’ and the highest median LOQ of 200 μg/kg for ‘Forages and roughage’ and ‘Oilseeds and oil fruits’.
Analytical data on DON‐3‐glucoside in feed were obtained by LC–MS‐based methods (89%), HPLC‐based methods (7%) and GC‐based methods (4%). The left‐censored data accounted for 91% of the results. The median LOQ was at a level of 250 μg/kg for all feed categories.
A detailed presentation of the proportion of left‐censored data across feed categories is given in Tables 18–21 (Section 4.2.4.4).
4.2.4.4. Currently reported occurrence data on feed by feed group
Results below the LOD or LOQ accounted for 43%, 92%, 65% and 91% of the results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, respectively. Apart from ‘Compound feed’, ‘Cereal grains, their products and by‐products’, ‘Forages and roughage, and products derived thereof’ and ‘Oilseeds, oil fruits, and products derived thereof’, only a limited number of data were available for other feed groups, e.g. fish, legume seeds, peas, sweet lupins, products from the bakery and pasta industry, citrus pulp and sugar beet. Therefore, the occurrence data are presented in Tables 18–21 for the aggregated feed groups. For cereal grains, the data for individual grains specified for feed (e.g. wheat, barley etc.) have been combined with those for those unprocessed grains of undefined end‐use.
For DON, the highest mean concentration levels were measured in ‘Forages and roughage, and products derived thereof’ (LB mean = 485 μg/kg, UB mean = 536 μg/kg). Similar DON levels were found also in ‘Cereal grains, their products and by‐products’, ‘Compound feed’ and ‘Miscellaneous’. Within the forages and roughages, the ‘Cereal straw’ had the highest concentrations for DON (LB mean = 1,074 μg/kg, UB mean = 1,128 μg/kg). Within the cereals, the highest values were measured in ‘Maize’ (LB mean = 641 μg/kg, UB mean = 689 μg/kg). ‘Compound feed’ covered by complementary and complete feed was mostly represented by complete feed for breeding pigs, growing/fattening pigs and piglets. The highest DON levels were measured in complementary feed for fattening chickens, fattening sheep and fattening rabbits and unspecified complete and complementary feed (Appendix C). In ‘Oilseeds, oil fruits, and products derived thereof’ covered mostly by toasted soya beans and sunflower seeds, mean concentrations ranged from 7.00 to 153 μg/kg in LB and UB scenarios, respectively.
Similar to DON, also for 3‐Ac‐DON and 15‐Ac‐DON, the highest occurrence levels were observed in ‘Forages and roughage, and products derived thereof’ with mean LB and UB concentrations of 53.2 and 95.9 μg/kg for 3‐Ac‐DON, and 1,054 and 1,116 μg/kg for 15‐Ac‐DON. Within the ‘Compound feed’ category, excluding the categories with all samples non‐detected, the highest 3‐Ac‐DON and 15‐Ac‐DON concentration levels were in complete feed for poultry and piglets and in unspecified complete feed (Appendix C). In ‘Cereal grains, their products and by‐products’ covered mostly by barley, maize, oats and wheat, mean concentrations were 2.20–34.9 μg/kg (LB–UB) for 3‐Ac‐DON and 41.4–64.2 μg/kg (LB–UB) for 15‐Ac‐DON with the highest concentration levels observed in ‘Maize’.
The data set on DON‐3‐glucoside in feed was characterised by a high proportion of left‐censored data, which influenced the mean concentrations at UB level. Taking into account this uncertainty, the highest DON‐3‐glucoside levels were reported in ‘Forages and roughage, and products derived thereof’ (LB mean = 60.8 μg/kg, UB mean = 228 μg/kg. In ‘Cereal grains, their products and by‐products’ covered mostly by barley, maize, oats and wheat, the mean concentrations ranged between the LB of 29.6 and the UB of 235 μg/kg. Limited data on other feed categories do not allow conclusions to be drawn in relation to DON‐3‐glucoside concentrations.
To assess the exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, the CONTAM Panel reviewed the occurrence data for the sum of concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in feed with the number of samples available for evaluation and statistical descriptors of the results (mean and 95th percentile for LB, MB and UB results) as shown in Table 22. See Sections 4.2.2 and 4.2.8 for the calculation of the sum of concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. It should be noted that the occurrence data on the sum of concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside refer to the number of samples instead of the number of the analytical results. (The exposure assessment of DON alone is presented in Appendix F).
Table 22.
Statistical description of the sum of the concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across the feed categories (all results expressed in μg/kg whole‐weight). (Feed Material Catalogue Level 1 (bold) and Level 2 (regular typeface))
| Feed groupa | N | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | ||||||
| LB | MB | UB | LB | MB | UB | ||
| Cereal grains, their products and by‐products | 1,288 | 610 | 654 | 698 | 2,465 | 2,465 | 2,465 |
| Barley | 209 | 212 | 274 | 337 | 857 | 857 | 867 |
| Maize and corn | 394 | 936 | 975 | 1,015 | 3,582 | 3,582 | 3,582 |
| Oats | 127 | 695 | 719 | 747 | 2,644 | 2,654 | 2,664 |
| Wheat | 465 | 570 | 607 | 644 | 2,304 | 2,304 | 2,304 |
| Compound feed | 1,996 | 580 | 605 | 631 | 2,291 | 2,291 | 2,291 |
| Compound feed, unspecified | 66 | 22 | 78 | 134 | 93 | 119 | 167 |
| Complementary feed (incomplete diet) | 215 | 374 | 401 | 428 | 1,755 | 1,755 | 1,755 |
| Complete feed | 1,715 | 628 | 649 | 670 | 2,436 | 2,436 | 2,436 |
| Fish, other aquatic animals and products derived thereof c | 1 | 264 | 264 | 264 | − | − | − |
| Forages and roughage, and products derived thereof | 412 | 750 | 792 | 835 | 2,689 | 2,691 | 2,715 |
| Cereals straw | 125 | 1,717 | 1,765 | 1,813 | 5,541 | 5,541 | 5,541 |
| Grass, field dried, [Hay] | 101 | 23 | 89 | 155 | 73 | 100 | 200 |
| Maize silage | 129 | 588 | 606 | 624 | 1,943 | 1,943 | 1,943 |
| Land animal products and products derived thereof | 5 | 15 | 25 | 35 | − | − | − |
| Legume seeds and products derived thereof | 5 | 15 | 111 | 207 | − | − | − |
| Minerals and products derived thereof | 2 | 0 | 84 | 168 | − | − | − |
| Miscellaneous | 11 | 639 | 646 | 653 | − | − | − |
| Oil seeds, oil fruits, and products derived thereof | 250 | 9 | 123 | 237 | 73 | 181 | 363 |
| Sunflower seed | 67 | 0 | 80 | 161 | 0 | 181 | 363 |
| Toasted soya (beans) | 146 | 9 | 143 | 277 | 0 | 260 | 319 |
| Other plants, algae and products derived thereof | 4 | 0 | 139 | 278 | − | − | − |
| Other seeds and fruits, and products derived thereof | 2 | 0 | 139 | 278 | − | − | − |
| Tubers, roots, and products derived thereof | 11 | 142 | 237 | 333 | − | − | − |
N: number of samples; LB: lower bound; MB: middle bound; UB: upper bound.
The occurrence data at Feed Catalogue Level 2 are reported only for feed categories with more than 60 analytical results. Left‐censored data not reported because the table reports the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table.
Fish used as feed.
Table 19.
Statistical description of the concentrations of 3‐Ac‐DON across the feed categories (all results expressed in μg/kg whole‐weight). (Feed Material Catalogue Level 1 categories (bold) and Level 2 categories (regular typeface))
| Feed groupa | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Cereal grains, their products and by‐products | 402 | 95 | 2.20 | 19.0 | 34.9 | 3.30 | 39.0 | 78.4 |
| Barley | 52 | 98 | 0.41 | 13.2 | 25.9 | 0 | 39.0 | 78.8 |
| Maize | 100 | 93 | 4.60 | 24.1 | 44.0 | 22.5 | 50.1 | 74.6 |
| Oats | 71 | 87 | 5.92 | 11.1 | 16.5 | 34.0 | 34.1 | 49.9 |
| Rice, broken | 3 | 100 | 0 | 24.4 | 47.8 | − | − | − |
| Rye | 1 | 100 | 0 | 39.0 | 78.4 | − | − | − |
| Triticale | 2 | 100 | 0 | 25.1 | 49.7 | − | − | − |
| Wheat | 169 | 100 | 0 | 20.3 | 39.1 | 0 | 25.1 | 49.7 |
| Cereal grains, unspecified | 4 | 100 | 0 | 24.1 | 47.9 | − | − | − |
| Compound feed | 357 | 87 | 7.41 | 25.0 | 43.3 | 52.6 | 53.0 | 100 |
| Compound feed, unspecified | 64 | 100 | 0 | 25.4 | 49.0 | 0 | 25.1 | 50.2 |
| Complementary feed (incomplete diet) | 38 | 97 | 2.00 | 16.2 | 30.1 | − | − | − |
| Complete feed | 255 | 82 | 10.0 | 27.3 | 43.5 | 69.3 | 69.3 | 100 |
| Forages and roughage, and products derived thereof | 71 | 94 | 53.2 | 75.0 | 95.9 | 385 | 385 | 385 |
| Cereal straw | 47 | 91 | 80.4 | 103 | 126 | − | − | − |
| Grass, field, dried | 6 | 100 | 0 | 19.1 | 37.9 | − | − | − |
| Maize silage | 18 | 100 | 0 | 19.1 | 37.5 | − | − | − |
| Legume seeds and products derived thereof | 1 | 100 | 0 | 24.1 | 47.9 | − | − | − |
| Peas | 1 | 100 | 0 | 24.1 | 47.9 | − | − | − |
| Miscellaneous | 1 | 100 | 0 | 17.0 | 34.1 | − | − | − |
| Products from the bakery and pasta industry | 1 | 100 | 0 | 17.0 | 34.1 | − | − | − |
| Oil seeds, oil fruits, and products derived thereof | 59 | 100 | 0 | 27.1 | 55.7 | − | − | − |
| Rape seed | 1 | 100 | 0 | 25.2 | 49.8 | − | − | − |
| Sunflower seed | 48 | 100 | 0 | 25.2 | 50.5 | − | − | − |
| Toasted soya (beans) | 13 | 100 | 0 | 34.0 | 75.3 | − | − | − |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle bound; UB: upper bound.
The occurrence data at Feed Catalogue Level 2 are reported only for feed categories with more than 60 analytical results
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table and replaced by a dash.
Table 20.
Statistical description of the concentrations of 15‐Ac‐DON across the feed categories (all results expressed in μg/kg whole‐weight). (Feed Material Catalogue Level 1 (bold) and Level 2 (regular typeface))
| Feed groupa | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Cereal grains, their products and by‐products | 236 | 80 | 41.4 | 53.0 | 64.2 | 280 | 280 | 280 |
| Barley | 47 | 94 | 1.70 | 19.1 | 35.9 | − | − | − |
| Maize | 62 | 53 | 120 | 132 | 144 | 641 | 641 | 641 |
| Oats | 67 | 81 | 31.3 | 35.1 | 37.8 | 185 | 185 | 185 |
| Rice, broken | 3 | 67 | 48.3 | 84.2 | 120 | − | − | − |
| Rye | 1 | 100 | 0 | 98.1 | 196 | − | − | − |
| Wheat | 46 | 100 | 0 | 13.1 | 26.2 | − | − | − |
| Compound feed | 191 | 47 | 22.7 | 36.0 | 50.0 | 105 | 105 | 200 |
| Complementary feed (incomplete diet) | 27 | 67 | 23.1 | 37.1 | 51.5 | − | − | − |
| Complete feed | 164 | 44 | 22.6 | 36.2 | 49.6 | 85.6 | 100 | 200 |
| Forages and roughage, and products derived thereof | 36 | 58 | 1,054 | 1,085 | 1,116 | − | − | − |
| Cereal straw | 16 | 13 | 2,353 | 2,366 | 2,378 | − | − | − |
| Grass, field dried | 1 | 100 | 0 | 69.2 | 138 | − | − | − |
| Maize silage | 19 | 95 | 15.8 | 60.1 | 105 | − | − | − |
| Oil seeds, oil fruits, and products derived thereof | 7 | 100 | 0.00 | 100 | 200 | − | − | − |
| Sunflower seed | 1 | 100 | 0 | 100 | 199 | − | − | − |
| Toasted soya (beans) | 6 | 100 | 0 | 100 | 200 | − | − | − |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle bound; UB: upper bound.
The occurrence data at Feed Catalogue Level 2 are reported only for feed categories with more than 60 analytical results.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table and replaced by a dash.
4.2.5. Data on unprocessed grains of undefined end‐use
4.2.5.1. Data collection on unprocessed grains of undefined end‐use
In addition to food and feed, 6,175 analytical results were reported for unprocessed grains of undefined end‐use (hereinafter referred to unprocessed grains). As the samples analysed cannot be considered to be either food or feed, and the processing might influence the concentration of the toxin in the end product, these occurrence data are described separately. The data set on unprocessed grains comprised 2,676 analytical results for DON of which 85.5% were analysed by LC–MS‐based methods (LC–MS/MS 74%, LC–MS 11.5%), 8.5% by HPLC methods (HPLC‐UV 4%, HPLC standard detection methods (not specified) 2%, HPLC‐FD 2.56%), and 6% by GC‐methods (GC–MS 5.4%, GC‐ECD 0.3%, GC‐HRMS 0.06%). For the other forms of DON, the main method applied was LC–MS/MS; 1,514 analytical results for 3‐Ac‐DON (LC–MS/MS 70%, LC–MS 21%, GC–MS 8.7%, GC‐ECD 0.3%), 1,447 analytical results for 15‐Ac‐DON (LC–MS/MS 96%, GC–MS 3.3%, GC‐ECD 0.7%) and 538 analytical results for DON‐3‐glucoside (LC–MS/MS 100%).
The distribution of unprocessed grains samples across the European countries and over the sampling years is presented in Figures 12 and 13, respectively. The majority of data were reported by the Netherlands, followed by the UK and Belgium. The sampling period covered the years 2007–2014.
Figure 12.
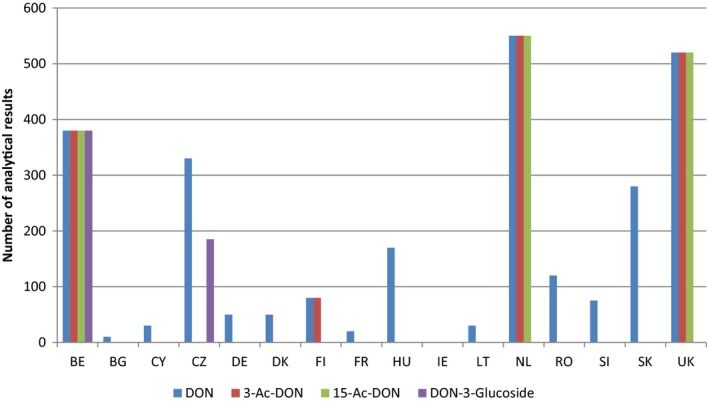
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in unprocessed grains of undefined end‐use across the European countries
- BE, Belgium; BG, Bulgaria; CY, Cyprus; CZ, the Czech Republic; DE, Germany; DK, Denmark; FI, Finland; FR, France; HU, Hungary; IE, Ireland; LT, Lithuania; NL, the Netherlands; RO, Romania; SI, Slovenia; SK, Slovakia; UK, the United Kingdom.
Figure 13.
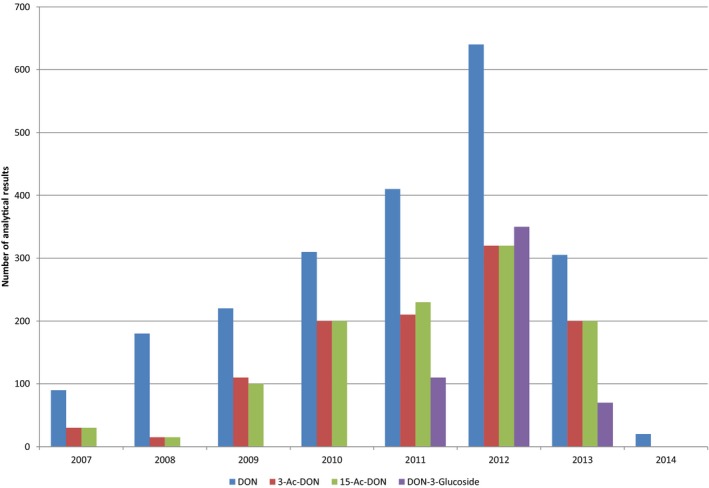
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in unprocessed grains of undefined end‐use over the sampling years
Unprocessed grains of undefined end‐use were classified in accordance with the EFSA FoodEx 1 classification system as described in Section 4.2.3. The majority of data were on unprocessed wheat grains, barley grain and rice (crop). Other types of grains were less represented. A distribution of the samples in unprocessed grains of undefined end‐use is shown in Figure 14.
Figure 14.
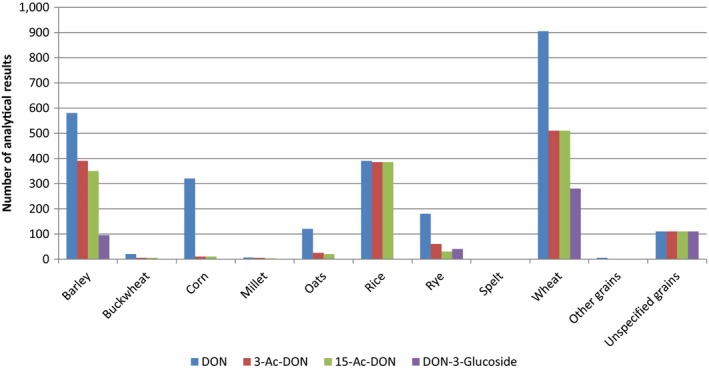
Distribution of analytical results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in unprocessed grains across grain groups
Similar to feed, from the total number of data submitted (n = 6,175), only data obtained by methods with LOQ ≤ 250 μg/kg were considered in the evaluation. As a result, 17 results of DON data and 557 results of 15‐Ac‐DON were not included in the unprocessed grains data set to be used for occurrence analysis and dietary exposure assessment. After applying the exclusion criteria described before (Section 4.2.3) and applying the LOQ cut‐off, the final cleaned data set of unprocessed grains of undefined end‐use included observations on DON (n = 1,621), 3‐Ac‐DON (n = 1,054), 15‐Ac‐DON (n = 430) and DON‐3‐glucoside (n = 538).
The information on analytical methods and/or on LOQ levels was not available for 27% of the analytical results of unprocessed grains of undefined end‐use. In addition, the measurements obtained by ELISA and TLC methods were not considered reliable. According to the criteria described in Section 4.2.3 those data did not fulfil the quality criteria and were not included in the further analyses. With this approach, 40% of the analytical data on unprocessed grains of undefined end‐use were excluded. The data set was characterised by 67% of left‐censored data (results below LOD/LOQ). The LOQs ranged between 3 and 200 μg/kg with a mean of 32.5 μg/kg.
4.2.5.2. Currently reported occurrence data on unprocessed grains of undefined end‐use
The category ‘Unprocessed grains’ comprised grains of undefined end‐use, defined also as ‘Grains as crops’. As the end‐use of the grains at harvest is not established and because normally grains for human and animal consumption undergo several processing steps before being used, it was considered appropriate to report their concentrations separately. Results below the LOD or LOQ accounted for 56%, 91%, 68 and 66% of the results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, respectively.
The highest mean DON concentrations were reported in unspecified grains (‘Grains as crops’ and ‘Other grains’) for which the origin was unknown. ‘Wheat grain’ and ‘Barley grain’ were also categories mostly contaminated with DON with mean LB‐UB concentrations of 432–447 μg/kg in wheat grains and 475–493 μg/kg in barley grains (Table 23).
Table 23.
Statistical description of the concentrations of DON at the FoodEx Level 3 across the unprocessed grains of undefined‐use (all results expressed in μg/kg whole‐weight)
| Type of unprocessed grain | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentilea | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Barley grain | 332 | 45 | 475 | 484 | 493 | 2,934 | 2,934 | 2,934 |
| Buckwheat grain | 20 | 100 | 0 | 18.3 | 36.5 | – | – | – |
| Corn grain | 64 | 41 | 205 | 224 | 243 | 560 | 560 | 660 |
| Millet grain | 7 | 86 | 77 | 136 | 195 | – | – | – |
| Oats grain | 97 | 49 | 130 | 138 | 147 | 670 | 670 | 670 |
| Rice | 280 | 100 | 0 | 25.0 | 50.0 | 0 | 25 | 50.0 |
| Rye grain | 130 | 68 | 37.1 | 48.5 | 59.8 | 200 | 200 | 228 |
| Spelt grain | 2 | 50 | 100 | 108 | 115 | – | – | – |
| Wheat grain | 411 | 36 | 432 | 439 | 447 | 1,663 | 1,663 | 1,663 |
| Other grains | 24 | 17 | 566 | 568 | 570 | – | – | – |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle bound; UB: upper bound.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and is therefore not reported in the table and replaced by a dash.
High concentrations of 3‐Ac‐DON were reported in ‘Grains as crops (unspecified)’ (LB mean = 94.6 μg/kg; UB mean = 101 μg/kg) followed by ‘Barley grain’ (LB mean = 9.12 μg/kg; UB mean = 59.3 μg/kg) (Table 24). It should be noted that 3‐Ac‐DON data of unprocessed grains comprised a high proportion of left‐censored data. For 15‐Ac‐DON, the highest mean concentrations were in ‘Grains as crops (unspecified)’ (LB mean = 107 μg/kg; UB mean = 110 μg/kg), followed by ‘Corn grain’ (LB mean = 53.8 μg/kg; UB mean = 54.1 μg/kg) (Table 25).
Table 24.
Statistical description of the concentrations of 3‐Ac‐DON at the FoodEx Level 3 across the unprocessed grains of undefined‐use (all results expressed in μg/kg whole weight)
| Type of unprocessed grain | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentilea | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Barley grain | 220 | 91 | 9.19 | 34.2 | 59.3 | 55.0 | 58.0 | 100 |
| Buckwheat grain | 8 | 100 | 0 | 50.0 | 100 | – | – | – |
| Corn grain | 15 | 100 | 0 | 5.00 | 10.0 | – | – | – |
| Grains as crops | 115 | 46 | 94.5 | 97.7 | 101 | 357 | 357 | 357 |
| Millet grain | 3 | 100 | 0.00 | 50.0 | 100 | – | – | – |
| Oats grain | 17 | 89 | 2.60 | 44.1 | 85.6 | – | – | – |
| Rice | 279 | 100 | 0 | 50.0 | 100 | 0 | 50.0 | 100 |
| Rye grain | 58 | 98 | 0.23 | 21.0 | 41.8 | – | – | – |
| Wheat grain | 187 | 96 | 1.00 | 5.4 | 9.74 | 0 | 10.0 | 10.0 |
| Other grains | 22 | 100 | 0 | 5.00 | 10.0 | – | – | – |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle bound; UB: upper bound.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table and replaced by a dash.
Table 25.
Statistical description of the concentrations of 15‐Ac‐DON at the FoodEx Level 3 across the unprocessed grains of undefined‐use (all results expressed in μg/kg whole‐weight)
| Type of unprocessed grain | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentilea | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Barley grain | 91 | 80 | 22.5 | 27.3 | 32.1 | 147 | 147 | 147 |
| Corn grain | 15 | 7 | 53.8 | 53.8 | 54.1 | – | – | – |
| Grains as crops | 115 | 27 | 107 | 109 | 110 | 367 | 367 | 367 |
| Wheat grain | 187 | 91 | 6.50 | 11.1 | 15.8 | 60.0 | 60.0 | 60.0 |
| Other grains | 22 | 91 | 15.1 | 20.6 | 26.1 | – | – | – |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle bound; UB: upper bound.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and is therefore not reported in the table and replaced by a dash.
Regarding concentrations of the DON‐3‐glucoside in unprocessed grains, high concentrations were reported in ‘Barley grains’ (LB mean = 218 μg/kg; UB mean = 225 μg/kg) followed by ‘Wheat grain crop’ (LB mean = 67.0 μg/kg; UB mean = 75.2 μg/kg) (Table 26).
Table 26.
Statistical description of the concentrations of DON‐3‐glucoside at the FoodEx Level 3 across the unprocessed grains of undefined‐use (all results expressed in μg/kg whole‐weight)
| Type of unprocessed grain | N | %LC | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95th percentilea | |||||||
| LB | MB | UB | LB | MB | UB | |||
| Barley grain | 91 | 44 | 218 | 222 | 225 | 928 | 928 | 928 |
| Grains as crops | 115 | 64 | 29.5 | 34.7 | 39.8 | 116 | 116 | 116 |
| Rye grain | 33 | 100 | 0 | 5.00 | 10.0 | – | – | – |
| Wheat grain | 277 | 69 | 67.0 | 71.1 | 75.2 | 347 | 347 | 347 |
| Other grains | 22 | 73 | 46.2 | 52.0 | 57.8 | – | – | – |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; MB: middle bound; UB: upper bound.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and is therefore not reported in the table and replaced by a dash.
The frequency of contamination and the concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were higher in ‘unprocessed grains’ than in ‘Grains for human consumption’, with the exception of DON‐3‐glucoside concentrations in wheat grains (Tables 12 and 23–27). However, the high proportion of left‐censored data and different LOQ cut‐off values applied to foods and unprocessed grains should be taken into consideration when interpreting this observation. With regard to the feed cereal grains, a comparison was possible for barley grains, oats and wheat grains only. Concentrations were higher in unprocessed grains for barley and wheat grains, while they were lower for oats than in the feed cereal grains (Tables 18–21 and 23–26).
Table 27.
Statistical description of the sum of the concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across the unprocessed grains of undefined‐use
| Type of unprocessed graina | N | Concentration range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentileb | ||||||
| LB | MB | UB | LB | MB | UB | ||
| Barley grain | 332 | 594 | 625 | 655 | 3,528 | 3,539 | 3,550 |
| Buckwheat grain | 20 | 0 | 43 | 86 | – | – | – |
| Corn grain | 64 | 294 | 323 | 352 | 812 | 812 | 957 |
| Grains as crops | 116 | 1,256 | 1,262 | 1,269 | 5,863 | 5,863 | 5,863 |
| Millet grain | 7 | 112 | 217 | 323 | – | – | – |
| Oats, grain | 97 | 187 | 207 | 226 | 972 | 972 | 972 |
| Rice | 280 | 0 | 84 | 167 | 0 | 84 | 168 |
| Rye grain | 130 | 52 | 77 | 103 | 270 | 275 | 331 |
| Spelt grain | 2 | 145 | 156 | 167 | – | – | – |
| Wheat grain | 411 | 546 | 561 | 576 | 2,057 | 2,068 | 2,079 |
| Other grains | 23 | 723 | 735 | 748 | – | – | – |
N: number of samples; LB: lower bound; MB: middle bound; UB: upper bound.
Left‐censored data not reported because the table reports the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table and replaced by a dash.
To assess the exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, the CONTAM Panel reviewed the occurrence data for the sum of concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in unprocessed grains of undefined‐use with the number of samples available for evaluation and statistical descriptors of the results (mean and 95th percentile for LB, MB and UB results) as shown in Table 27. See Sections 4.2.2 and 4.2.8 for the calculation of the sum of concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. It should be noted that the occurrence data on the sum of concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside refer to the number of samples instead of the number of the analytical results.
4.2.6. Comparison of the occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in foods, feeds and unprocessed grains of undefined end‐use from organic and conventional farming
Due to the limited information on the farming methods of the production on food, feed and unprocessed grains of undefined‐use in the occurrence data submitted to EFSA, the CONTAM Panel also reviewed the literature.
In a review article of Olsen (2008), occurrence of DON in organic and conventional foods were compared, based on 10 published European studies. Most of the studies focused on grains and milling products, in a few studies bread and beer were investigated. In four studies conventionally produced grains yielded lower levels of DON than organically produced grains. In the other six studies (which included bread and beer), no statistically significant differences were found.
In the Norwegian review of the NVK (2014) on the comparison of organic and conventional food and food production, 27 studies were included, comparing DON content in organically and conventionally produced cereals. Some studies compared DON content in more than one cereal species. In the majority of the studies, no significant differences in DON content in cereals from the two cultivation systems were found. Eleven studies reported lower DON content in organic compared to conventional cereals and four studies reported the opposite.
In some of the studies reviewed by the NVK (2014), not only cereals but also grain products resulting from organically and conventionally grown cereals were investigated for DON (Twazur≐k et al., 2013; Vidal et al., 2013; Błajet‐Kosicka et al., 2014). In addition, a few studies were published in which solely grain products derived from organically and conventionally grown cereals were investigated for DON (Cirillo et al., 2003; Schollenberger et al., 2005; Anselme et al., 2006). The studies reviewed in NVK (2014) revealed a tendency towards more frequent occurrence and higher concentrations of DON in conventionally produced food products as compared to organically produced food products but the observed tendency was rather weak and only in some cases statistically significant. This pattern confirmed the findings of Olsen (2008) that DON levels in organically produced grains (and products thereof) were either similar or slightly lower than in conventionally produced grains (and products thereof). In the study of Anselme et al. (2006), which focused specifically on organically and conventionally produced beers sold on the Belgian market, no statistically significant differences were found for DON levels or occurrences. An earlier report of the NVK (2013) about risk assessment of mycotoxins in cereal grains in Norway hypothesised that lower mycotoxin content recorded in organically grown cereals compared with conventionally grown cereals could be due to more widespread use at organic farms of cultivation practices known to reduce the risk of mycotoxin contamination in cereals.
Published studies on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in foods from organic farming were hardly found. Only in the studies of Twazur≐k et al., 2013, and of Błajet‐Kosicka et al. (2014) occurrence of 3‐Ac‐DON was compared between organic and conventional oats/oats products and rye products respectively. 3‐Ac‐DON was more frequently detected in conventional oats/oats products, but comparative levels were not reported. 3‐Ac‐DON was not detected in both types of rye products.
The various literature studies in which occurrence and concentrations of DON in organic and conventionally produced grains and foods were compared, showed a slight tendency of more frequent occurrence and higher concentrations of DON in conventional products as compared to organic products. However, this tendency was only significant in a limited number of studies. No relevant conclusions can be drawn about differences in occurrence and concentrations of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, due to lack of data.
In the food occurrence data submitted to EFSA, a number of results (n = 8,912) with a clear specification of the method of production was available. A comparison of the DON occurrence in food from organic and conventional farming was possible for ‘Food for infants and small children’, ‘Grains and grain‐based products’, ‘Products for special nutritional use’ and ‘Snacks, desserts, and other foods’ (Table 28).
Table 28.
DON concentrations (μg/kg) in food groups coming from organic farming (a) and conventional farming (b) (2007–2013)
| Food group level 1 | N | %LC | Concentrations (μg/kg) | |
|---|---|---|---|---|
| Mean | 95th percentilea | |||
| Lower bound | ||||
| Food for infants and small children (a) | 93 | 66 | 9.47 | 58.0 |
| Food for infants and small children (b) | 58 | 91 | 5.38 | – |
| Grains and grain‐based products (a) | 1,399 | 59 | 41.7 | 180 |
| Grains and grain‐based products (b) | 2,392 | 56 | 92.6 | 400 |
| Products for special nutritional use (a) | 11 | 64 | 40.4 | – |
| Products for special nutritional use (b) | 7 | 29 | 389 | – |
| Snacks, desserts, and other foods (a) | 12 | 25 | 75.5 | – |
| Snacks, desserts, and other foods (b) | 37 | 70 | 39.4 | – |
| Middle bound | ||||
| Food for infants and small children (a) | 93 | 66 | 19.8 | 58.0 |
| Food for infants and small children (b) | 58 | 91 | 26.9 | – |
| Grains and grain‐based products (a) | 1,399 | 59 | 53.9 | 200 |
| Grains and grain‐based products (b) | 2,392 | 56 | 107 | 400 |
| Products for special nutritional use (a) | 11 | 64 | 47.1 | – |
| Products for special nutritional use (b) | 7 | 29 | 400 | – |
| Snacks, desserts, and other foods (a) | 12 | 25 | 81.8 | – |
| Snacks, desserts, and other foods (b) | 37 | 70 | 62.8 | – |
| Upper bound | ||||
| Food for infants and small children (a) | 93 | 66 | 29.7 | 58.0 |
| Food for infants and small children(b) | 58 | 91 | 46.9 | – |
| Grains and grain‐based products (a) | 1,399 | 59 | 64.3 | 200 |
| Grains and grain‐based products (b) | 2,392 | 56 | 122 | 400 |
| Products for special nutritional use (a) | 11 | 64 | 53.8 | – |
| Products for special nutritional use (b) | 7 | 29 | 411 | – |
| Snacks, desserts, and other foods (a) | 12 | 25 | 88.0 | – |
| Snacks, desserts, and other foods (b) | 37 | 70 | 86.2 | – |
N: number of samples; %LC: percentage of left‐censored data.
The 95th percentile obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and therefore not reported in the table and replaced by a dash.
Regarding the feed data, the comparison of the occurrence from organic and conventional farming was possible only for DON concentrations of the ‘Cereal grains, their products and by‐products’ feed category (Table 29). For unspecified grains, the comparison of DON concentrations was possible for barley, oats, rye and wheat grains (Table 30). Overall, the EFSA data showed that the concentrations of DON in food and feed grains tended to be higher in conventionally cultivated cereal grains than in grains from the organic farming, but no firm conclusions could be made due to the heterogenicity in cultivation years, countries of origin, applied analytical methods and high percentage of left‐censored data in the available data sets.
Table 29.
DON concentrations (μg/kg) in feed category ‘Cereal grains, their products and by‐products’ coming from organic farming (a) and conventional farming (b)
| N | %LC | Concentrations (μg/kg) | ||
|---|---|---|---|---|
| Mean | 75th percentilea | |||
| Lower bound | ||||
| Cereal grains, their products and by‐products (a) | 8 | 75 | 239 | 255 |
| Cereal grains, their products and by‐products (b) | 156 | 26 | 324 | 305 |
| Middle bound | ||||
| Cereal grains, their products and by‐products (a) | 8 | 75 | 246 | 268 |
| Cereal grains, their products and by‐products (b) | 156 | 26 | 328 | 293 |
| Upper bound | ||||
| Cereal grains, their products and by‐products (a) | 8 | 75 | 253 | 280 |
| Cereal grains, their products and by‐products (b) | 156 | 26 | 333 | 293 |
N: number of samples; %LC: percentage of left‐censored data.
The 75th percentile concentration was calculated because the number of samples was below 11.
Table 30.
DON concentrations (μg/kg) in unprocessed grains of undefined end‐use coming from organic farming (a) and conventional farming (b)
| N | %LC | Concentrations (μg/kg) | ||
|---|---|---|---|---|
| Mean | 75th percentilea | |||
| Lower bound | ||||
| Barley grain (a) | 11 | 73 | 44.6 | 150 |
| Barley grain (b) | 93 | 74 | 57.3 | 50.0 |
| Oats, grains (a) | 32 | 38 | 62.2 | 98.5 |
| Oats, grains (b) | 41 | 49 | 233 | 110 |
| Rye grain (a) | 9 | 100 | 0.00 | 0.00 |
| Rye grain (b) | 52 | 83 | 44.4 | 0.00 |
| Wheat grain crop (a) | 14 | 50 | 354 | 450 |
| Wheat grain crop (b) | 192 | 59 | 79.5 | 79.4 |
| Middle bound | ||||
| Barley grain (a) | 11 | 73 | 65.0 | 150 |
| Barley grain (b) | 93 | 68 | 76.4 | 50.0 |
| Oats, grains (a) | 32 | 38 | 68.1 | 98.5 |
| Oats, grains (b) | 41 | 49 | 243 | 110 |
| Rye grain (a) | 9 | 100 | 27.8 | 25.0 |
| Rye grain (b) | 52 | 83 | 56.6 | 26.0 |
| Wheat grain crop (a) | 14 | 50 | 383 | 450 |
| Wheat grain crop (b) | 192 | 59 | 87.8 | 92.2 |
| Upper bound | ||||
| Barley grain (a) | 11 | 73 | 85.5 | 150 |
| Barley grain (b) | 93 | 68 | 95.4 | 60.0 |
| Oats, grains (a) | 32 | 38 | 74.0 | 99.5 |
| Oats, grains (b) | 41 | 49 | 253 | 110 |
| Rye grain (a) | 9 | 100 | 55.6 | 50.0 |
| Rye grain (b) | 52 | 83 | 68.8 | 50.0 |
| Wheat grain crop (a) | 14 | 50 | 411 | 450 |
| Wheat grain crop (b) | 192 | 59 | 96.1 | 100 |
N: number of samples; %LC: percentage of left‐censored data; LB: lower bound; UB: upper bound.
The 75th percentile concentration was calculated because the number of samples was below 11.
In summary, based on limited available information the data from the literature on comparative occurrence of DON in organically and conventionally produced cereal grains (used for food and feed) and grain products generally confirmed the pattern as derived from the data on occurrence of DON in food, feed and unprocessed grains of undefined end‐use submitted to EFSA. DON concentrations were either generally at the same level or marginally lower in organically produced grains (and food and feed products) as compared to conventionally produced grains (and food and feed products).
4.2.7. Currently reported co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food, feed and unprocessed grains of undefined end‐use
The occurrence data submitted to EFSA, where analytical results on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in all possible combinations (singletons, pairs, triplets or quadruplets) were reported for the same samples, were extracted from the EFSA database. While all samples complying with the criteria established in Sections 4.2.3–4.2.5 were used to calculate the exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, only those samples for which the analysis resulted in numerical values at the respective LOQs or above were selected to calculate the ratios of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to DON. The number of samples available for each of the three paired combinations and the mean, median and minimum‐maximum values of the ratios were calculated. These current co‐occurrence results were generally consistent with the co‐occurrence data reported in the literature in Section 4.1.4.
From the current occurrence data in food (Section 4.2.3), the CONTAM Panel selected the data of ‘Grains and grain‐based products’ at FoodEx level 1 (and those subtypes at FoodEx level 2 for which ratios were available) and ‘Alcoholic beverages’ (subdivided into FoodEx levels 2 of ‘Beer and beer‐like beverages’) to evaluate the co‐occurrence (Table 31). There were a total of 142, 168 and 83 samples with quantified ratios of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to DON, respectively, for the group of ‘Grains and grain‐based products’. The CONTAM Panel noted that these ratios represented only a very small proportion of the entire database of occurrence in food (see Tables 12–17). The mean ratios were 1.6 for 3‐Ac‐DON to DON, 0.7 for 15‐Ac‐DON to DON and 1.9 for DON‐3‐glucoside to DON for the food group of ‘Grains and grain‐based products’. For ‘Alcoholic beverages’ the number of quantified ratios was too small to draw any conclusions (Table 31).
Table 31.
Concentration ratios of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to DON in different food categories among the occurrence data providers (FoodEx Level 1(bold) and Level 2 (regular typeface))
| Food category | Ratio of 3‐Ac‐DON to DON | Ratio of 15‐Ac‐DON to DON | Ratio of DON‐3‐glucoside to DON | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Median | SD | Min | Max | N | Mean | Median | SD | Min | Max | N | Mean | Median | SD | Min | Max | |
| All countries | ||||||||||||||||||
| Grains and grain‐based products | 142 | 1.6 | 0.3 | 4.7 | 0.0 | 53 | 168 | 0.7 | 0.4 | 1.6 | 0.0 | 16 | 83 | 1.9 | 1.2 | 2.0 | 0.0 | 13 |
| Bread and rolls | 33 | 2.5 | 2.6 | 2.7 | 0.1 | 15 | 37 | 1.6 | 0.9 | 3.1 | 0.0 | 16 | 40 | 2.5 | 1.5 | 2.4 | 0.1 | 13 |
| Breakfast cereals | 75 | 1.8 | 0.3 | 6.2 | 0.1 | 53 | 46 | 0.7 | 0.7 | 0.7 | 0.0 | 4.3 | 35 | 1.4 | 0.9 | 1.6 | 0.0 | 6.0 |
| Fine bakery wares | 3 | 0.3 | 0.3 | 0.3 | 0.0 | 0.7 | 4 | 0.3 | 0.3 | 0.1 | 0.2 | 0.4 | 0 | – | – | – | – | – |
| Grain milling products | 14 | 0.3 | 0.1 | 0.6 | 0.0 | 1.6 | 56 | 0.3 | 0.2 | 0.2 | 0.0 | 1.1 | 8 | 1.1 | 1.1 | 0.8 | 0.1 | 2.4 |
| Grains for human consumption | 17 | 0.1 | 0.1 | 0.1 | 0.0 | 0.3 | 25 | 0.3 | 0.1 | 0.4 | 0.0 | 1.8 | 0 | – | – | – | – | – |
| Alcoholic beverages | 0 | – | – | – | – | – | 2 | 0.4 | 0.4 | 0.2 | 0.3 | 0.5 | 4 | 1.7 | 1.5 | 0.7 | 1.1 | 2.6 |
| Beer and beer‐like beverage | 0 | – | – | – | – | – | 2 | 0.4 | 0.4 | 0.2 | 0.3 | 0.5 | 4 | 1.7 | 1.5 | 0.7 | 1.1 | 2.5 |
| All countries (excluding one MS) | ||||||||||||||||||
| Grains and grain‐based products | 73 | 0.2 | 0.1 | 0.5 | 0.0 | 3.4 | 96 | 0.4 | 0.3 | 0.3 | 0.0 | 1.8 | 11 | 0.8 | 0.9 | 0.5 | 0.2 | 1.6 |
| Bread and rolls | – | – | – | – | – | – | 1 | 0.0 | 0.0 | – | 0.0 | 0.0 | 1 | 1.5 | 1.5 | – | 1.5 | 1.5 |
| Breakfast cereals | 41 | 0.4 | 0.2 | 0.6 | 0.1 | 3.4 | 14 | 0.7 | 0.8 | 0.3 | 0.3 | 1.2 | 6 | 0.5 | 0.4 | 0.3 | 0.2 | 1.0 |
| Fine bakery wares | 3 | 0.3 | 0.3 | 0.4 | 0.0 | 0.7 | 4 | 0.3 | 0.3 | 0.1 | 0.2 | 0.4 | – | – | – | – | – | – |
| Grain milling products | 12 | 0.1 | 0.0 | 0.1 | 0.0 | 0.3 | 52 | 0.3 | 0.2 | 0.3 | 0.0 | 1.1 | 4 | 1.1 | 1.1 | 0.5 | 0.4 | 1.6 |
| Grains for human consumption | 17 | 0.1 | 0.1 | 0.1 | 0.0 | 0.3 | 25 | 0.3 | 0.1 | 0.4 | 0.0 | 1.8 | – | – | – | – | – | – |
| Alcoholic beverages | – | – | – | – | – | – | 2 | 0.4 | 0.4 | 0.2 | 0.3 | 0.5 | 4 | 1.7 | 1.5 | 0.7 | 1.1 | 2.6 |
| Beer and beer‐like beverages | – | – | – | – | – | – | 2 | 0.4 | 0.4 | 0.2 | 0.3 | 0.5 | 4 | 1.7 | 1.5 | 0.7 | 1.1 | 2.6 |
| Excluded MS | ||||||||||||||||||
| Grains and grain‐based products | 69 | 3.0 | 1.5 | 6.5 | 0.1 | 53 | 72 | 1.2 | 0.8 | 2.4 | 0.0 | 16 | 72 | 2.1 | 1.3 | 2.1 | 0.1 | 13 |
| Bread and rolls | 33 | 2.5 | 2.6 | 2.7 | 0.1 | 15 | 36 | 1.7 | 0.9 | 3.2 | 0.1 | 16 | 39 | 2.5 | 1.6 | 2.4 | 0.1 | 13 |
| Breakfast cereals | 34 | 3.5 | 1.2 | 8.9 | 0.1 | 53 | 32 | 0.7 | 0.7 | 0.8 | 0.0 | 4.3 | 29 | 1.6 | 0.9 | 1.7 | 0.1 | 6.0 |
| Grain milling products | 2 | 1.6 | 1.6 | 0.1 | 1.5 | 1.7 | 4 | 0.3 | 0.3 | 0.2 | 0.1 | 0.5 | 4 | 1.2 | 1.2 | 1.2 | 0.1 | 2.4 |
N: number of samples; SD: standard deviation; Min: minimum; Max: maximum; Dash means that there are no data to calculate the ratio.
The CONTAM Panel noted that the ratios in ‘Grain and grain‐based products’ for 3‐Ac‐DON, 15‐Ac‐DON, and DON‐3‐glucoside to DON (i.e. 3.0, 1.2 and 2.1, respectively) were higher for one MS compared to other countries where the ratios were 0.2, 0.4 and 0.8, respectively. It was also noted that acetylated DON concentrations reported in the published literature were typically < 10% of those reported for DON (FAO/WHO, 2011). For DON‐3‐glucoside, concentrations have been reported to be 5–46% of those reported for DON (Berthiller et al., 2009; Desmarchelier and Seefelder, 2011). The deviating results of these food samples led the CONTAM Panel to request further investigations by the analytical laboratory of the MS who submitted the samples and by another MS laboratory.27 Both laboratories had provided large amounts of occurrence data on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to EFSA for this opinion. Therefore, the CONTAM Panel initiated a comparative ad hoc study for which 20 samples (10 from each laboratory) were selected by the two laboratories in 2014 and cross‐evaluated by the two laboratories. It was concluded that the method performance of the two laboratories was similar and gave comparable results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for this set of samples. However, since no samples of ‘Grain and grain‐based products’ were available where DON concentrations were lower than the other DON forms (i.e. ratio > 1) this ad hoc study could not confirm the higher concentrations of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside compared to DON reported by the one MS shown in Table 31.
For feed, there were 44, 69 and 50 samples with quantified ratios of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to DON, respectively, all for complete feed, the largest category of samples of feed. As for food, the CONTAM Panel noted that these ratios represented only a very small proportion of the entire database of occurrence in feed. The mean ratios were 0.3 for 3‐Ac‐DON to DON, 0.3 for 15‐Ac‐DON to DON and 0.4 DON‐3‐glucoside to DON.
For grains of undefined end‐use in grain and grain‐based products, there were 94, 125 and 175 samples with quantified information for the ratios of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to DON, respectively, again only a small proportion of the entire database. The mean ratios were 0.2 for 3‐Ac‐DON to DON, 0.3 for 15‐Ac‐DON to DON and 0.4 for DON‐3‐glucoside to DON.
A comparison of the three ratios of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to DON as derived from the EFSA database with those found in the literature was difficult to make since the ratios derived from data in the EFSA database were means of the ratios, while it was not possible to derive these from the literature data (see Section 4.1) in a reliable way due to the inconsistent way of reporting in the literature (see also Section 4.1.4).
4.2.8. Estimating the occurrence of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food, feed and unprocessed grains of undefined end‐use
This section provides details on the calculation of the concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for the reasons described in Section 4.2.2. The results of the calculation are presented in the Sections 4.2.3.4.5, 4.2.4.4 and 4.2.5.2 for food, feed and unprocessed grains of undefined end‐use, respectively.
Noting that the co‐occurrence data on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in the same sample was scarce, because all four forms of DON were simultaneously analysed for a very small number of samples, the CONTAM Panel explored whether the non‐analysed values could be estimated by %‐ratios calculated from the available ratios of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to DON as described in Section 4.2.7 for samples for the food groups ‘Grains and grain‐based products’ and ‘Alcoholic beverages’ for which analysis results with a numerical values were reported. The data from these food groups were considered the most relevant due to the high number of overall reported results and the sufficient number of quantified results. Noting that the pairs of samples with such quantified co‐occurrence results were only a small proportion of the entire occurrence database, the CONTAM Panel decided to supplement these ratios by ratios calculated from the available literature data for food and feed (see Section 4.1.4). Excluding the data of the one MS (see Section 4.2.7 and Table 31), the ratios for 3‐Ac‐DON and 15‐Ac‐DON to DON for ‘Grains and grain‐based products’ were approximately 0.1 and 0.3 respectively. Accounting furthermore for the ratios reported in the literature (see Section 4.1), the CONTAM Panel concluded that a %‐ratio of 10% for 3‐Ac‐DON to DON was well supported by literature data. In contrast, a %‐ratio of 30% for 15‐Ac‐DON to DON was not at all supported by the ratios reported in the literature, which were ranging from 0.05 to 0.3. Therefore, a %‐ratio of 15% for 15‐Ac‐DON to DON appeared to be a plausible estimate.
For the estimation of a %‐ratio for DON‐3‐glucoside to DON, the CONTAM Panel decided to use only literature data because the amount of samples with quantified co‐occurrence results available to calculate the ratio of DON‐3‐glucoside to DON was much smaller in the EFSA database than in the available literature (see Section 4.1). From the ratios of DON‐3‐glucoside to DON calculated based on available literature data on ‘Grains intended for food consumption’ (see Section 4.1.4.1 and Table 8) and ‘Grains and grain‐based food products’ (see Section 4.1.4.1 and Table 9), the CONTAM Panel concluded that a %‐ratio of 20% for DON‐3‐glucoside to DON would be plausible. The literature data for malt and beer suggested a %‐ratio of 80% for DON‐3‐glucoside to DON as plausible for ‘Alcoholic beverages’ (‘Beer and beer‐like beverage’) (see Section 4.1.4.1 and Table 10). This was supported by the information on the effects of the malting process on the concentrations of DON and DON‐3‐glucoside (see Section 4.3.1.4) and therefore an 80% ratio of DON‐3‐glucoside to DON appeared justified. The CONTAM Panel also noted that the %‐ratio of 80% for DON‐3‐glucoside to DON should be used for the by‐products from ‘Alcoholic beverages’ (‘Beer and beer‐like beverage’) used as animal feed.
Although the data on the concentration ratios on cereal grains for feed were scarce in both the available literature and the EFSA database (see Sections 4.1.4 and 4.2.7), the available calculated concentration ratios for cereal grains for feed were not inconsistent with those calculated for food. Therefore the CONTAM Panel assumed that using the %‐ratios derived for food would also be plausible for feed and unprocessed grains of undefined end‐use.
The CONTAM Panel also explored whether the non‐analysed concentrations of 3‐Ac‐DON and/or 15‐Ac‐DON and/or DON‐3‐glucoside in the food sample could be estimated by the mean concentration of the respective DON‐form of the same food category. Such an approach would then also apply for the non‐analysed concentrations of feed or the concentrations of unprocessed grains of undefined end‐use. Regarding the mean human exposures to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (across the European dietary surveys and age groups at the LB, MB and UB) the same ranges of exposure were obtained as when using the %‐ratio approach described above. However, the ranges of the 95th percentile dietary exposures differed. Particularly, the maximum 95th percentile dietary exposure was substantially higher when the non‐analysed DON‐form concentrations were replaced by the mean concentration of the respective DON‐form of the respective food category. This difference was also observed for calculated exposures of farm and companion animals. Regardless of the uncertainties of the approach, the CONTAM Panel concluded that the use of the %‐ratios to replace the non‐analysed DON‐forms is currently the most appropriate way to estimate the exposures to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for both humans and farm and companion animals. A more accurate exposure estimation may be possible in future when more analytical data on the co‐occurrence of the four DON‐forms are available.
The CONTAM Panel noted that the estimation of the respective %‐ratios and the calculation of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed using the percentages of 10, 15 and 20% of DON, respectively, (with the exception of the 80% used for DON‐3‐glucoside for ‘Alcoholic beverages’ and the by‐products from the production of ‘Alcoholic beverages’ used as feed materials) has to be considered as the outcome of an expert judgement of the CONTAM Panel on the basis of available information on the co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed.
4.2.9. Currently reported co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside with other Fusarium mycotoxins in food, feed and unprocessed grains of undefined end‐use
In order to assess the co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside with other Fusarium toxins, the occurrence data submitted to EFSA (see Section 4.2) which reported all four DON forms and at least one of the other Fusarium toxins in the same sample were extracted from the EFSA database. Co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside with zearalenone, α/β‐zearalenols, nivalenol, sum of T‐2 and HT‐2 toxins or fumonisins (mainly B1) were calculated considering 266,686 samples both with quantified and detected results reported for food, feed and unprocessed grains of undefined end‐use. The highest co‐occurrence with the other Fusarium toxins was observed for DON (58% of the samples) with one of the other Fusarium toxins. The highest co‐occurrence was for DON and nivalenol (63% of the samples) and the second highest for fumonisins (56% of the samples). Co‐occurrence of approximately 50% was observed for DON with zearalenone and for DON with the sum of T‐2 and HT‐2 toxins, whereas 20% of the samples for DON and α/β‐zearalenols co‐occurred at approximately 20% only. The CONTAM Panel noted that these percentages are only rough estimates of the co‐occurrences affected with a high level of uncertainty since the numbers of samples tested for the different Fusarium toxins varied widely and only a limited number of toxins was tested per sample. Therefore these percentages should be compared with caution.
The limited number of data does not allow any detailed analysis of the co‐occurrence of 3‐Ac‐DON and/or 15‐Ac‐DON and/or DON‐3‐glucoside with other Fusarium toxins. However, it seems that DON either alone or together with DON‐3‐glucoside co‐occur regularly with other Fusarium toxins in cereal‐based food products, in particular with zearalenone. The submitted co‐occurrence data confirm the co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside with other Fusarium mycotoxins in food found in the literature (see Section 4.1.5).
4.3. Food and feed processing
4.3.1. Food processing
The extent to which cereals are processed depends on the cereal type and the final feed/food product. In general it is known that processing reduces Fusarium toxin concentrations in products for human consumption but may increase levels in food or feed by‐products. This is because mechanical cleaning of cereals (dehulling) may lead to by‐products (for the feed industry) in which Fusarium toxins concentrate significantly. This may result in (much) higher concentrations of Fusarium toxins in these materials than in the cereals before cleaning. The effects of processing of cereals and cereal products, in ways common to the food and feed industry, on the concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and 3‐DON‐glucoside in food and feed have been investigated in various studies. The focus of most of these investigations was on DON, often in combination with other Fusarium toxins. In some studies, in particular those on the effects of malting and baking processes, the fate of DON and DON‐3‐glucoside was also studied. Only few studies were published where the effects of processing on the concentrations of acetyl‐forms of DON were (also) investigated.
4.3.1.1. Cleaning and sorting
In general, sorting of cereals by removing extensively damaged or infected kernels such as those with visible mould growth or shrivelled kernels, has the effects of lowering the concentrations of mycotoxins in subsequently produced products (Abbas et al., 1985; Ryu et al., 2008; Scudamore and Patel, 2009). Fusarium‐contaminated grains can be recognised using a range of optic, laser, gravity‐based or acoustic analysis methods, and segregated from healthy grains to remove them before they are incorporated into food. The reduction of DON levels by cleaning can be significant in those grains that are heavily contaminated initially, and reductions in DON concentrations up to 74% after sorting of grossly contaminated samples have been reported (Hazel and Patel, 2004).
Delwiche (2005) conducted an experiment on high speed optical sorting of Fusarium‐damaged wheat. Wheat contaminated with DON at a range of 0.6–20 mg/kg was segregated into fractions that contained 0.2–12 mg/kg (accepts) and 3.4–58 mg/kg (rejects) after single sorting. Reductions of 38 and 21% of DON were observed in the cleaning stage of wheat, containing between 1,400 and 1,900 μg/kg, when using industrial (several separators, sieves and aspirator devices) and traditional cleaning methods (a simple screen device and an integrated aspirator), respectively (Lešnik et al., 2008). In a similar study, DON levels of four wheat samples with initial concentrations ranging from approximately 100 to 3,000 μg/kg were reduced by cleaned by sieving, scouring and polishing to roughly 50% (Lancova et al., 2008b).
Matumba et al. (2015) investigated the effectiveness of hand sorting, flotation/washing and dehulling on moulded maize that contained DON, 3‐Ac‐DON and 15‐Ac‐DON at levels of 54 μg/kg of DON and 29 μg/kg of the sum of 3‐Ac‐DON and 15‐Ac‐DON. Hand sorting was most efficient and led to a reduction of the toxins of approximately 96%. Dehulling reduced the levels of the toxins by 50–60% and flotation by 30–60%.
4.3.1.2. Rolling and milling
Milling has the effect of reduction and redistribution of DON. Ryu et al. (2008) described the effect of dry milling as causing a redistribution of DON into separate milling fractions, and causing higher concentrations of DON in particular fractions such as the bran and shorts. During dry milling, DON concentrates in the fractions containing the outer parts of the grain rather than in those fractions containing the inner parts of the grain (Abbas et al., 1985; Lešnik et al., 2008). Thus, the highest levels of DON are observed in the germ, screenings, dust and bran while the flour and grits contain lower levels than those found in the grain before dry milling (Hazel and Patel, 2004). Wet milling results in dissolving and redistribution of DON in by‐products and steep water (Ryu et al., 2008).
Cheli et al. (2013) reviewed 15 studies published in the period 2004–2011 on the repartitioning of DON (and other Fusarium toxins) during wheat milling. Various types of mills were used. Similar trends were reported. The concentrations of DON in semolina and flour ranged from 25–89% as compared to the initial concentrations, while concentrations of DON in bran ranged from 81–340% in comparison with the initial levels. Five studies on the effect of milling on the content of DON of grains (including wheat, maize and barley) published after the review of Cheli et al. (2013), showed similar amounts of reduction in semolina and flour, and an increase of DON concentrations in bran (Brera et al., 2013; Burger et al., 2013; Giménez et al., 2013; Zheng et al., 2014; Tibola et al., 2015).
The effects of three types of milling processes (industrial roller‐grinding, grain hammer crashing and traditional millstone grinding) on the DON levels in wheat (1,400–1,900 μg/kg) were compared in two studies on the distribution of DON in the milling products (Lešnik et al., 2008; Khatibi et al., 2014a). Lešnik et al. (2008).Roller grinding resulted in a 71% reduction in the concentrations of DON from the grain to the flour, while hammer crashing and millstone milling resulted in minor reductions of 0.5% and 9.5%, respectively. Khatibi et al. (2014a) compared roller milling and precision milling (with blades) for their ability to reduce DON levels in the endosperm‐enriched tissue in barley. Roller milling removed a much smaller percentage of DON than precision milling, resulting in 13% and 77% reductions in the concentrations of DON from the grain to the flour, respectively.
In the study of Kostelanska et al. (2011b), the fractionation of DON and DON‐3‐glucoside in milling fractions of wheat was rather similar. White flours contained approximately 60–70% of the concentrations of DON and DON‐3‐glucoside in unprocessed wheat grains, while in bran these concentrations were 160–170% as compared to initial concentrations. Schwake‐Anduschus et al. (2015) investigated the distribution patterns of DON, DON‐3‐glucoside, 3‐Ac‐DON and 15‐Ac‐DON in ten milling fractions of naturally contaminated wheat. DON and DON‐3‐glucoside were found in similar amounts in all fractions. In bran, the levels were only slightly higher than in the endosperm. In contrast, 3‐ and 15‐Ac‐DON were dominantly present in the bran fractions. The authors explained the observed differences in distribution between DON/DON‐3‐glucoside and the acetylated forms by the polarity of the components: 3‐Ac‐DON and 15‐Ac‐DON are less polar, which would reduce their mobility in the aqueous environment of the plant.
Zhang and Wang (2014), detected a reduction of DON and DON‐3‐glucoside concentrations in wheat flour by 79–90% and by 23–39%, respectively. Concentrations of DON and DON‐3‐glucoside in bran were 1.2–2.2 times and 2.9–4.4 times higher respectively than initial concentrations, and they were slightly lower in shorts as compared to bran. The total amount of DON in all fractions decreased by 30–40% compared to the whole wheat, whereas the amount of DON‐3‐glucoside increased by 50–100%. The authors indicated that the temperature becomes increasingly high as milling time continues and they hypothesised that modification of the DON structure by interaction with wheat components may occur leading to a reduction of DON and an increase of DON‐3‐glucoside after milling.
4.3.1.3. Cooking and baking
There have been quite a number of studies to determine the effect of various forms of thermal processing (cooking, pasta preparation and baking) on the fate of DON. In several studies also the stability of DON‐3‐glucoside was investigated. The studies were often carried out with mutually different experimental designs and conditions, which made comparisons sometimes difficult.
A few studies focussed on the fate of DON (and DON‐3‐glucoside) during the preparation of noodles and spaghetti. In the study of Nowicki et al. (1988) wheat naturally contaminated with DON at 12.5 mg/kg was processed to Japanese‐style and Chinese‐style noodles. The amount of DON retained in cooked Japanese and Chinese noodles averaged 52% and 42%, respectively, of the amount of DON in the flour. In the same study, wheat naturally contaminated with DON at 9.6 mg/kg was processed to spaghetti. Retention of DON in cooked spaghetti averaged 43–53% of the amount present before cooking. Overcooking resulted in a slight further decrease. Visconti et al. (2004) investigated the reduction of DON during spaghetti cooking starting from durum wheat, naturally contaminated with DON at levels ranging from 0.3 to 13.1 mg/kg and observed stronger reductions. As compared to the uncleaned wheat, the percentage levels of DON were 33% in spaghetti and 20% in cooked spaghetti.
In the study of Zhang and Wang (2015), the fate of DON and DON‐3‐glucoside was studied during the preparation of Chinese‐style noodles from wheat flour samples naturally contaminated with DON and DON‐3‐glucoside at five different levels, ranging from 0.94–5.89 and 0.11–0.77 μg/kg, respectively. The study revealed a 52% reduction in DON between wheat flour and cooked noodles (not reported whether wet or dry weight), which confirmed the findings of Nowicki et al. (1988). For DON‐3‐glucoside, a stronger decrease of 79% after noodle making was observed. DON and DON‐3‐glucoside were detected in cooking water, but DON‐3‐glucoside at very minor and non‐ quantifiable levels. Moazami et al. (2014) studied the effect of three food additives (l‐ascorbic acid, l‐cysteine and sodium bisulfite) on DON reduction in fried instant noodle strands. The noodles were prepared from wheat flour fortified with DON at 1 μg/g. The authors observed reductions of DON up to 67%.
Vidal et al. (2014) reviewed a dozen of studies on the effect of bakery processing on DON contamination in wheat products (bread, cakes, biscuits). Initial DON levels in wheat flours ranged from 40–1,824 μg/kg. Loaf sizes, fermentation and proofing conditions, use of additives in the dough mixture and baking conditions (time, temperature) varied in these studies. Some of the studies reported a significant increase in DON levels during dough fermentation (up to 99% increase, hypothesised as possibly due to the enzymatic release of native DON from conjugated forms occurring in the raw material). In contrast, other studies showed a reduction of 62% in fermented dough, hypothesised to be due to transformation of DON during dough fermentation into a substance that could not be determined by conventional analysis. Regarding baking, most studies reported that DON concentrations were reduced to a variable extend (up to 79% reduction), with the degree of reduction affected by baking time, temperature, and loaf size. The published studies indicate that DON is largely stable and (partly) survives the bread making process. Reduced levels found in finished products could also be attributed to a dilution of the levels of DON due to the incorporation of ingredients such as fat, sugar and water, rather than to a thermal degradation (Scudamore et al., 2009).
Several recent studies (Kostelanska et al., 2011b; Suman et al., 2012; Zachariasova et al., 2012; de Angelis et al., 2013; Vidal et al., 2014, 2015; Zhang and Wang, 2014; Wu and Wang, 2015; Generotti et al., 2015) on the effect of baking and steaming on DON content included investigations on the fate of DON‐3‐glucoside and (incidentally) on 3‐Ac‐DON and 15‐Ac‐DON.
Kostelanska et al. (2011b) studied the effects of baking technologies on levels of DON and DON‐3‐glucoside as part of a larger study, where also the effects of milling of wheat were investigated (see Section 4.3.1.2) Concentrations of DON and DON‐3‐glucoside in kneaded dough ranged from 43–758 μg/kg for DON and from 7–105 μg/kg for DON‐3‐glucoside. No substantial changes of DON and DON‐3‐glucoside occurred during the dough preparation process, i.e. kneading, fermentation and proofing. However, when bakery improvers enzymes mixtures were employed as a dough ingredient, a distinct increase of DON‐3‐glucoside concentrations up to 145% as compared to initial concentrations in white flour occurred in proofed dough. This was assumed to be due to the release of DON‐3‐glucoside from bonded forms to starch‐based matrix, rather than to glucosidation of DON, since for DON no significant changes in concentrations were noted. Some decrease of both DON and DON‐3‐glucoside compared to proofed dough (decrease of 13% and 10%, respectively) took place during baking. Thermal degradation products of DON and DON‐3‐glucoside were detected in roasted wheat and in baked bread samples, mostly located in the crust.
Zachariasova et al. (2012) compared the fate of DON‐3‐glucoside with the fate of DON during the preparation of bread, starting from wheat flour contaminated with DON and DON‐3‐glucoside at 729 and 160 μg/kg, respectively. The DON level in the final bread decreased during the fermentation and baking to approximately 86% of the initial concentration in the wheat flour. The DON‐3‐glucoside/DON ratio slightly increased during proofing which was assumed to be due to the enzymatic activity after the application of a bakery improver. However, the final DON‐3‐glucoside level in the bread was only approximately 50% of the initial concentration in the wheat flour.
The multitude of contradictory information on the fate of DON during baking prompted De Angelis et al. (2013) to investigate the fate of DON and DON‐3‐glucoside upon baking. Three batches of whole wheat flour were used, naturally contaminated with DON at 1,824, 954 and 816 μg/kg. DON‐3‐glucoside concentrations were at 23% of the DON concentrations. The baking process yielded DON concentrations, approximately 18% higher in bread than in the original flour. Parallel with the increase of DON content during baking, a corresponding decrease of DON‐3‐glucoside was recorded in the same samples analysed, making a possible release of DON from the conjugated form likely during baking. This could have been due to yeast activity during fermentation.
Vidal et al. (2014) studied the fate of DON and DON‐3‐glucoside during the bread making process, starting from 3 wheat flours, contaminated with DON at 2,090, 1,459 and 1,012 μg/kg levels, while DON‐3‐glucoside was at the same level in the flours (45 μg/kg). Loaf breads were made. The concentrations of DON decreased in fermented dough by 22–37% as compared to the concentrations in wheat flour. Subsequently reductions in DON concentrations from proofed dough to bread were observed for baking times of 75 min and over, but the levels of reduction varied (highly significant at high DON concentrations and non‐significant at low DON concentration). However, the data provided did not allow quantitative estimates. For DON‐3‐glucoside a 25% decrease was observed from flour to fermented dough and, in contrast to DON, a subsequent substantial increase from fermented dough to final bread was found, leading to an overall DON‐3‐glucoside concentration increase of 224% during baking, as compared to the concentration in the fermented dough. Its increase could not be linked to baking temperature/time levels and it was hypothesised that glycosidation of DON in the initial stages of baking occurred, before enzyme inactivation.
Vidal et al. (2015) focused on the thermal stability and kinetics of degradation of DON and DON‐3‐glucoside, and in addition on the fate of 3‐Ac‐DON during baking of wheat bakery products. Two wheat flours were used with DON concentrations of 1,042 and 550 μg/kg, while in both flours the DON‐3‐glucoside concentration was 45 μg/kg. DON concentrations reduced during the baking process, depending on the baking temperature and time. Reduction at 40 min baking time varied from 29% at 140°C to 81% at 200°C. In contrast, DON‐3‐glucoside increased significantly during the initial baking phase from 30% after 5 min at 160°C, up to 642% after 10 min at 180°C, while a strong reduction to < LOD (1.6 μg/kg) was observed at the higher temperature/time conditions. The concentration of 3‐Ac‐DON (8.5 μg/kg in the unbaked cake) tended to decrease during baking with increasing temperature and baking time, but the low initial concentration, close to the LOQ (4.5 μg/kg), and contradictory results in some of the analysed samples, made it difficult to draw firm conclusions.
The fate of DON and DON‐3‐glucoside during Chinese steamed bread processing was investigated by Zhang and Wang (2014). Five sets of whole wheat samples were used, naturally contaminated with DON and DON‐3‐glucoside at levels ranging from 4,680 to 3,672 μg/kg for DON and 170 to 1,040 μg/kg for DON‐3‐glucoside, with a constant ratio between DON and DON‐3‐glucoside. The production of steamed bread involved mixing with yeast, dough preparation, fermenting, and steaming for 20 min at 100°C. Up to the stage of fermentation, DON concentrations hardly changed, but they doubled in the steamed bread. In contrast DON‐3‐glucoside concentrations in mixed and fermented dough and in steamed bread were rather similar, but almost 50% lower than in the flour. Bases on the study, dough‐making seemed to decrease the amount of DON‐3‐glucoside, while steaming increased the amount of DON.
Wu and Wang (2015) investigated levels and conversion profiles of DON, 3‐Ac‐DON and 15‐Ac‐DON during bread‐making. Five Fusarium toxin‐free flours were spiked with DON at levels from 100 to 500 μg/kg. Another set of five Fusarium toxin‐free flours were spiked with 3‐Ac‐DON and 15‐Ac‐DON at levels from 100 to 1,500 μg/kg. Bread loaves were baked at 225°C for 20 min. No significant changes of DON levels were observed during dough preparation stages. During bread baking, a modest reduction of DON levels was observed, ranging from 4 to 14%. Decreases of 20–40% for 3‐Ac‐DON and of 28–60% for 15‐Ac‐DON were found during the fermentation stage. Further losses of 3‐Ac‐DON and 15‐Ac‐DON were observed after the proofing process, but the 3‐Ac‐DON and 15‐Ac‐DON levels gained a significant increase after baking. Parallel with the 3‐Ac‐DON and 15‐Ac‐DON concentration decreases a corresponding increase of DON was noted, which pointed at a conversion taking place from 3‐Ac‐DON and 15‐Ac‐DON to DON. The mechanism of this conversion remained unclear to the authors.
Suman et al. (2012) studied the fate of DON and DON‐3‐glucoside throughout the industrial (pilot‐scale) production of wholegrain crackers, starting with wheat bran naturally contaminated with DON and DON‐3‐glucoside at 3,436 and 45 μg/kg, respectively. The process involved dough preparation followed by kneading, fermentation and baking. The authors applied an experimental multifactorial design, allowing to study the influence of fermentation time, fermentation temperature, baking time, baking temperature and sodium bicarbonate on the final levels of DON and DON‐3‐glucoside in the wholegrain crackers. For DON hardly any effect during fermentation and a limited reduction during baking were noted, especially at higher baking temperatures (255 and 270°C) where reductions ranged from 3% to 46%. It was not possible to identify influences of the technological process parameters on the DON‐3‐glucoside levels, because of the relatively low DON‐3‐glucoside values and the associated uncertainties in analytical measurements.
A comprehensive study (Generotti et al., 2015) on the effects of industrial processing focused on the fate of DON and DON‐3‐glucoside along the chain of industrial production of rusks, characterised by three steps: fermentation, baking and toasting. Three batches of bran naturally contaminated with DON at 600, 1,050 and 1,500 μg/kg were used for the study, which showed how concentrations of DON and DON‐3‐glucoside are influenced by modifying ingredients and operative conditions. Ten factors were taken into account: DON concentration of the starting bran, dextrose, yeast and enzyme amounts, fermentation time and temperature, baking time and temperature and toasting time and temperature. It appeared that the evolution of DON is mostly affected by the baking and roasting stages, with decreases in DON levels from 8% to 19%, and from 19% to 65%, respectively. The co‐occurrence of DON‐3‐glucoside in the batches of bran at 32 μg/kg allowed to observe an increase in the concentration of DON‐3‐glucoside up to 48% after the fermentation step, followed by a reduction during baking and toasting.
4.3.1.4. Malting process
Lancova et al. (2008a) investigated the fate of DON, the sum of 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside from field barley through malt to beer. Two batches of barley, naturally and artificially infected with Fusarium species were used for processing experiments. The naturally infected batch contained DON, the sum of 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside at low levels of 12, < 5 and < 10 μg/kg dry weight, respectively, while the artificially infected batch contained these toxins at levels of 234, 14 and 140 μg/kg dry weight, respectively. In addition, two batches of malt grist were prepared, naturally and artificially infected with DON, the sum of 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside at 316, 67, 133 μg/kg, and 1,712, 349 and 933 μg/kg, respectively. During the steeping of barley grains, the toxins were not detectable in the naturally infected series, while in the artificially infected series levels reduced to < 10% of the original amounts in the samples of barley, except for DON‐3‐glucoside, which remained at the initial level. During subsequent stages in the beer brewing process, substantially increases of DON and DON‐3‐glucoside occurred in both series of experiments. In particular, the presence and formation of high levels of DON‐3‐glucoside were documented, leading to DON‐3‐glucoside levels in final beers to exceed those of DON. In sweet wort the relative DON‐3‐glucoside content was ten times higher as compared with that in malt grist taken for the processing experiment. The authors explained the concentration changes observed by de novo growth of Fusarium under certain malting conditions and enzymatic activity during the mashing of malt grists, thus releasing DON‐3‐glucoside from insoluble forms. For 3‐Ac‐DON and 15‐Ac‐DON, the transfer to final beers was found to be slightly above 100%.
Kostelanska et al. (2011b) studied the concentration changes of DON and DON‐3‐glucoside in the brewing process of four beer brands: light, dark tap and two lagers, produced from ground malt mixtures differing in composition and toxin content. In a first monitoring period mixture malts were used, contaminated with DON and DON‐3‐glucoside at average levels of 31.5 and 55.7 μg/kg respectively. A relative increase of DON plus DON‐3‐glucoside content occurred during brewing. In the final beer, the increase of DON and of DON‐3‐glucoside was in the range 195–365 and 275–460%, respectively, depending on the beer brand. These increases confirmed the findings of Lancova et al. (2008a). In a second monitoring period where new malts were used contaminated with DON and DON‐3‐glucoside at average levels of 15.7 μg/kg and 15.6 μg/kg, respectively, these concentration changes ranged from 49% to 248% and 139% to 416%, respectively. No relationship between the contamination level of the malt and the trend of the concentration change could be observed, and both an increase and a decrease of DON was found during brewing. Without any exception, DON‐3‐glucoside levels increased during brewing.
Zachariasova et al. (2012) used barley, artificially infected with Fusarium spp., for the production of malt and beer and studied the fate of DON and DON‐3‐glucoside during that process. The barley contained DON at 2,467 μg/kg and DON‐3‐glucoside at 939 μg/kg and the levels in the final malt were 11,638 μg/kg for DON and 20,912 μg/kg for DON‐3‐glucoside. The increase of DON was explained by Fusarium fungus growth and de novo production of DON. An even much stronger increase of DON‐3‐glucoside during the malting process was observed, leading to concentrations of DON‐3‐glucoside much higher than DON in final malt. thought to result from the glycosidation of DON by glucose during the starch hydrolysis and from the enzyme‐catalysed release from the binding with polysaccharides in the cells. During the subsequent brewing process beers were produced containing DON at 2,760 μg/L and DON‐3‐glucoside at 3,883 μg/L, resulting in a DON‐3‐glucoside/DON ratio of approximately 1.4 which is in line with results obtained from surveys of beer (see Section 4.1.1.2)
4.3.2. Feed processing
4.3.2.1. Cereal grains
Cereal grains intended for use as animal feed are usually subject to some of the processes used for processing grains for human consumption (cleaning, sorting, drying, rolling/grinding and/or extrusion) before being fed to livestock, and therefore many of the effects reported above for food (Section 4.3.1) apply equally to cereal grains for animal feed. In addition, by‐products of processing grains for human consumption are widely used as feeds for livestock.
The initial cleaning of cereal grains is usually a physical process involving the use of screens, to remove smaller particles. Tittlemeier et al. (2015) reported that for Canadian wheat, DON was found in at levels of up to 2.9 mg/kg in the material removed by cleaning.28 The levels of DON in the uncleaned samples were 0.5–4.4 mg/kg.
For long‐term storage, a maximum moisture content of approximately 12% is generally recommended. In order to achieve this, air temperatures of up to 125–130°C may be used, resulting in grain temperatures of up to 45°C. DON is relatively stable when exposed to heat, even at temperatures of 120°C (Hazel and Patel, 2004) or more (Wolf‐Hall et al., 1999; Lancova et al., 2008b) and therefore drying is unlikely to affect DON concentrations in grains dried in this way.
Prior to feeding, grains may be further processed by rolling, milling, extruding or flaking. These processes involve the application of pressure (e.g. rolling, extruding) and/or heat (e.g. cooking, flaking), but DON appears to be stable under these conditions (Scudamore, 2008).
Where whole cereal grains are fed, the application of an alkali has been used as a means of reducing levels of DON present. Abramson et al. (2005) demonstrated that the application of an alkali (1 M Na2CO3) in combination with heat (80°C) resulted in significant reductions in DON, from 18.4 to 4.7 mg/kg after 1 day, and to 0.4 mg/kg after 8 days with the addition of 10% v/w alkali. When 20% v/w alkali was used, DON declined to 1.4 mg/kg after 1 day and near zero levels after 8 days. The effect of alkali therefore appears to be dependent on both the duration of the heat application and the concentration of the alkali used.
4.3.2.2. Cereal by‐products
The by‐products of grain processing for human consumption are widely used in livestock diets. The European Commission Catalogue of Feed Materials29 lists over 80 cereal by‐products used as animal feeds. These include by‐products from the major cereals (wheat, barley, oats and maize) used in the manufacture of foods for human consumption, as well as in the production of alcohol.
In common with other mycotoxins, DON is found predominantly on the outer layer of the grain. Dry milling generally results in a redistribution of DON into separate milling fractions, and an increase of DON in particular fractions such as the bran and grits. Scudamore and Patel (2009) showed that concentrations of DON in maize by‐products could be three to four times higher than in the original grain, with variability attributed to both the source of the grains and the milling process used.
By‐products of the brewing industry are widely used as feeds for livestock, but reports of the transfer of mycotoxins to animal by‐products are somewhat contradictory. A number of studies have demonstrated that mycotoxins are transmitted from contaminated grains to beer (Scott, 1995; Papadopoulou‐Bouraoui et al., 2004). For DON, the transfer from malt grist to finished beer was reported to be between 80% and 93% (Schwarz et al., 1995), and Lancova et al. (2008a) concluded that a relatively high proportion of the DON in contaminated grains is transferred to the beer but not to the by‐products of brewing. In contrast, the carry‐over of DON, 3‐Ac‐DON, 15‐Ac‐DON and 3‐DON‐glucoside to co‐products of bioethanol production has been reported. Distillers dried grains with solubles (DDGS) are a co‐product of bioethanol production and a valuable feed material for farm animals. Schaafsma et al. (2009) and Zhang and Caupert (2012) reported increases of between 3.0 and 3.5 times in the concentration of DON in DDGS relative to starting material from bioethanol plants in the USA. In another survey from the USA, Khatibi et al. (2014b) reported DON concentrations in DDGS following bioethanol fermentation that were about 1.6 to 8.2 times higher than in the starting grain, depending on the barley line/cultivar used in the mash. Recently, a number of trichothecene 3‐O‐acetyltransferases have been evaluated for their ability to modify DON, and small‐scale barley ethanol fermentations using two of these enzymes demonstrated their potential to reduce DON in DDGS (Khatibi et al., 2011b). Pinotti et al. (2016) concluded that contamination, by DON and 3‐Ac‐DON, 15‐Ac‐DON and 3‐DON‐glucoside, of by‐products of the malting and brewing process for animal feeding was influenced by a number of factors, including the initial levels of the mycotoxins in the barley and the brewing process technology.
4.3.2.3. Compound feeds
Compound feeds consist of mixtures of feed materials and additives formulated to meet the specific nutritional requirements of the livestock to which they are fed. They may be complete feeds that provide all of the daily requirements of nutrients, or complementary feeds that provide part of the ration (e.g. protein and energy). For ruminants and horses, compound feeds usually represent part of the ration and are supplemented with forages, while for pigs, poultry, rabbits, fish, cats and dogs they are usually the sole feed. It is estimated that 155 million tonnes of compound feed were manufactured in the EU‐27 in 2013 (FEFAC, 2014).
One of the final stages in the compound feed manufacturing process is the production of feed pellets, which results in an increase in temperature. The extent of the temperature rise will depend on a number of factors, including the types of ingredients used in the formulation, the amount of moisture added and the equipment used, but pellets generally leave the die at temperatures ranging between 60 and 95°C (Thomas et al., 1997). Like most trichothecenes, DON is stable at these temperatures (Schwake‐Anduschus et al., 2010).
4.3.2.4. Forage cereals/silage
Forages, including maize, sorghum and other cereals, may be preserved by ensiling for use when fresh feeds are unavailable. The presence of DON in forages has been regularly observed in both fresh and ensiled form (Driehuis et al., 2008b; Rodrigues et al., 2012; Storm et al, 2014), although there is evidence that the physical, chemical, or microbiological changes associated with ensiling may reduce levels of DON in silage relative to those in the fresh herbage (Mansfield et al., 2005). Although it is generally accepted that contamination with DON is the result of infection with Fusarium pre‐harvest, contamination has been reported to occur post‐harvest in poorly preserved silages (Rasmussen et al., 2010).
4.3.3. Conclusions
Drying after harvest does not affect levels of DON in the grains. Application of alkali to whole grains for feed reduces the DON content significantly. During mechanical cleaning, sorting and milling of grains DON, like other Fusarium toxins, is unevenly redistributed between the grain fractions (bran, endosperm and germ), and is mostly attached to the outer hull of the grains and therefore the toxin occurs at much higher concentrations in the bran than in other parts of the grain. As a result, the dehulling process may result in by‐products used in the food or feed industries in which levels of DON are significantly higher than in the original grain. The scarce studies that also involved DON‐3‐glucoside and 3‐Ac‐DON and 15‐Ac‐DON showed that concentrations of these DON forms were also reduced in the dehulled products, but at varying degrees. The corresponding increase of their concentrations in shorts (consisting of bran, germ and flour) and bran was often more substantial than that of DON.
During cooking and baking DON appears to be a relatively stable compound, for which often some degradation was reported, with more reduction at higher processing temperatures and longer times. Studies on the fate of DON‐3‐glucoside during cooking and baking showed rather inconsistent and varying results, ranging from no substantial changes to significant increase of concentrations during fermentation stages, mostly followed by reductions during baking steps. The incidental studies that also focussed on 3‐Ac‐DON and 15‐Ac‐DON led to contradictory results.
Malting and brewing do not seem to lead to losses of DON/DON‐3‐glucoside or increased concentrations in by‐products of brewing used in the feed industry. However, the ratio between concentrations of these compounds in beer changes significantly, leading to (often) higher concentrations in beer of DON‐3‐glucoside than those of DON. Studies on the fate of the 3‐Ac‐DON and 15‐Ac‐DON during beer preparation are limited, but point at a transfer to final beer of approximately 100%. DON concentrates in distillers’ dried grains with solubles at levels up to eight times higher than in the starting grain.
Overall, CONTAM Panel noted that processing has been reported to reduce DON concentrations in cereals at a range of 10–80% depending on the method of processing.
Some of the processes applied to cereal grains for food production (particularly drying, cleaning, sorting, rolling and milling) are also applied to grains used for animal feeds, and therefore the effects on concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside reported for food also apply to feed. Published studies confirm that milling can minimise concentrations of these toxins in grains, but concentrate them into fractions commonly used as animal feed.
Overall, the literature data on the effects of processing of food and feed are not always consistent, but lead to the conclusion that both increases and decreases of concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in the processed food and feed products occur, depending on the type and intensity of the form of processing.
5. Food and feed consumption
5.1. Food consumption
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data from national information on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with food consumption data at the level of the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a)). New consumption surveys added in 2015 in the Comprehensive Database30 were also taken into account in this assessment.31
Food consumption data included in the Comprehensive Database were collected through different methodologies, and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used in the exposure calculations, uncertainties can be introduced because of possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the food consumption data gathered at EFSA in the Comprehensive Database are the most complete and detailed data currently available in Europe.
Competent authorities in the European countries provided EFSA with data from the most recent national dietary survey in their country at the level of consumption by the individual consumer. This included food consumption data concerning infants (6 surveys from 6 countries), toddlers (11 surveys from 10 countries), children (20 surveys from 17 countries), adolescents (20 surveys from 17 countries), adults (24 surveys from 22 countries), elderly (16 surveys from 15 countries) and very elderly (14 surveys from 14 countries) for a total of 41 different dietary surveys carried out in 23 different countries covering more than 78,400 individuals. Surveys on children were mainly obtained through the Article 36 project ‘Individual food consumption data and exposure assessment studies for children’ (acronym EXPOCHI) (Huybrechts et al., 2011). Two additional surveys provided information on specific population groups of ‘Pregnant women’ (≥ 15 years to ≤ 45 years old) and ‘Lactating women’ (≥ 28 years to ≤ 39 years old) from two countries.
As suggested by EFSA (EFSA, 2011b), dietary surveys with only 1 day per subject were not considered for the calculation of chronic dietary exposure, as they are not adequate to assess repeated exposure. Similarly, subjects who participated for only 1 day in the dietary studies although the protocol prescribed more reporting days per individual, were excluded. Thus, for chronic exposure assessment, food consumption data were available from 35 different dietary surveys carried out in 19 different European countries (Table D.1, Appendix D). In addition to the studies used for chronic exposure, six dietary surveys with only 1 day per subject from six different countries were used for the acute exposure assessment.
Table D.1.
Dietary surveys used for the estimation of acute and chronic dietary exposure
| Country | Survey acronym | Survey period | N of days per subject | N of subjects/N of days | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infants | Toddlers | Other children | Adolescents (mean age) | Adults | Elderly | Very elderly | ||||
| Austria | ASNS – Adults | 2010–2012 | 2 | – | – | – | – | 308/726 | 67/181 | 25/85 |
| ASNS – Children | 2010–2012 | 3 | – | – | 128/384 | 237/706 | – | – | – | |
| Belgium | Regional Flanders | 2002–2002 | 3 | 36/108 | 625/1875 | – | – | – | – | |
| Belgium | Diet National 2004 | 2004 | 2 | – | – | 576/1,187 (16a) | 1,292/2,648 | 511/1,045 | 704/1,408 | |
| Bulgaria | NSFIN | 2004 | 1 | –/162 | –/691 | –/151 | –200 | |||
| Bulgaria | NUTRICHILD | 2007 | 2 | 861/1,720 | 428/856 | 433/867 | – | – | – | – |
| Cyprus | Childhealth | 2003 | 3 | – | – | – | 303/909 (13a) | – | – | – |
| CzechRepublic | SISP04 | 2003–2004 | 2 | – | – | 389/778 | 298/596 (13a) | 1666/3,332 | – | – |
| Denmark | DANSDA 2005‐08 | 2005–2008 | 7 | – | – | 298/2,085 | 377/2,622 (13a) | 1739/12,127 | 274/1,916 | 12/84 |
| Denmark | IAT 2006 07 | 2006–2007 | 7 | 826/5,771 | 917/6,388 | – | – | – | – | – |
| Estonia | NDS 1997 | 1997 | 1 | –/1,866 | – | – | ||||
| Finland | DIPP 2001 2009 | 2001–2009 | 3 | 500/1,500 | 500/1,500 | 750/2,250 | – | – | – | – |
| Finland | NWSSP07 08 | 2007–2008 | 4 | – | – | 306/1,186 (13a) | – | – | – | |
| Finland | FINDIET2012 | 2012 | 2 | – | – | – | 1,295/2,590 | 413/826 | – | |
| France | INCA2 | 2007 | 7 | – | 482/3,315 | 973/6,728 (14a) | 2,276/15,727 | 264/1,824 | 84/571 | |
| Germany | VELS | 2001–2002 | 6 | 159/927 | 348/1,947 | 293/1,610 | – | – | – | – |
| Germany | EsKiMo | 2006 | 3 | – | 835/2,498 | 393/1,179 (11a) | – | – | – | |
| Germany | National Nutrition Survey II | 2007 | 2 | – | – | 1,011/2,022 (16a) | 10,419/20,838 | 2,006/4,012 | 490/980 | |
| Greece | Regional Crete | 2004–2005 | 3 | 838/2,508 | – | – | – | – | ||
| Greece | DIET LACTATION GR | 2005–2007 | 3 | – | – | – | 65/350 | – | – | |
| Hungary | National Repr Surv | 2003 | 3 | – | – | – | 1,074/3,222 | 206/618 | 80/240 | |
| Ireland | NANS 2012 | 2008–2010 | 4 | – | – | – | 1,274/5,096 | 149/596 | 77/308 | |
| Italy | INRAN SCAI 2005 06 | 2005–2006 | 3 | 16/48 | 36/108 | 193/579 | 247/741 (14a) | 2,313/6,939 | 290/870 | 228/684 |
| Latvia | EFSA TEST | 2008 | 2 | 187/377 | 453/979 (14a) | 1,271/2,655 | – | – | ||
| Latvia | FC PREGNANTWOMEN 2011 | 2011 | 2 | – | – | – | 1,002/2,005 | – | – | |
| Netherlands | VCP kids | 2006–2007 | 3 | 322/644 | 957/1,914 | – | – | – | – | |
| Netherlands | VCPBasis AVL2007 2010 | 2007–2010 | 2 | – | 447/894 | 1,142/2,284 (14a) | 2,057/4,114 | 173/346 | ||
| Netherlands | VCP‐Elderly | 2010–2012 | 2 | – | – | – | – | 289/578 | 450/900 | |
| Poland | IZZ FAO 2000 | 2000 | 1 | –/79 | –/409 | –/666 (14a) | –/2,527 | –/329 | –/124 | |
| Romania | Dieta Pilot Children | 2012 | 1 | – | –/205 | –/567 (14a) | – | – | – | |
| Romania | Dieta Pilot Adults | 2012 | 7 | – | – | – | 1,254/8,770 | 83/581 | 45/315 | |
| Slovakia | SK MON 2008 | 2008 | 1 | – | – | – | 2761 | – | – | |
| Slovenia | CRP 2008 | 2007–2008 | 1 | – | – | – | 407 | – | – | |
| Spain | enKid | 1998–2000 | 2 | 17/34 | 156/312 | 209/418 (12a) | – | – | – | |
| Spain | AESAN | 1999–2001 | 3 | – | – | – | 410/828 | – | – | |
| Spain | NUT INK05 | 2004–2005 | 2 | 399/798 | 651/1,302 (14a) | – | – | – | ||
| Spain | AESAN FIAB | 2009 | 3 | – | – | 86/226 (17a) | 981/2,748 | – | – | |
| Sweden | NFA | 2003 | 4 | – | 1,473/5875 | 1018/4047 (12a) | – | – | – | |
| Sweden | Riksmaten 2010 | 2010–2011 | 4 | – | – | – | 1,430/5,680 | 295/1,167 | 72/288 | |
| UnitedKingdom | NDNS‐Rolling Programme Years 1‐3 | 2008–2011 | 4 | 185/737 | 651/2,595 | 666/2,653 (14a) | 1,266/5,040 | 166/662 | 139/552 | |
| UnitedKingdom | DNSIYC 2011 | 2011 | 4 | 1,369/5,446 | 1,314/5,217 | – | – | – | – | – |
Within the dietary studies, subjects were classified in different age classes as defined below:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old
Two additional surveys provided information on specific population groups: ‘Pregnant women’ (≥ 15 years to ≤ 45 years old) and ‘Lactating women’ (≥ 28 years to ≤ 39 years old).
Consumption records were coded in accordance with the FoodEx classification system (see Section 4.2.3), which has been developed by the DATA Unit in 2009 (EFSA, 2011a). Further details on how the Comprehensive Database is used were published in the Guidance of EFSA (EFSA, 2011b).
5.2. Feed consumption
As reported above, DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside occur predominantly in cereal grains and their by‐products following processing. These are widely used as feed for livestock; in the EU more than 91 million tonnes was used in the manufacture of compound feeds in 2012, accounting for 60% of all feed materials used,32 almost all of which (> 95%) are grown or processed in the EU.
In addition to incorporation in compound feeds, cereal grains and by‐products are frequently fed in on‐farm mixes or as single ingredients, particularly to supplement forages for ruminant livestock. Therefore, the total amount of cereal grains and cereal by‐products used as feed for livestock will be considerably greater than that reported for compound feed production. However, there are no industry data on the partition of these cereal grains between livestock species (cattle, pigs, poultry, etc.).
Mycotoxin‐producing Fusarium species may also be present in infect forage crops used as livestock feed. Although not widely reported to be present on fresh grass, the presence of DON in maize and cereal silages has been regularly observed (Driehuis et al., 2008b; Rodrigues et al., 2012; Storm et al., 2014).
There is considerable variation in both the feeds used and the feeding systems adopted for farm livestock, companion animals and fish throughout Europe. This variation is largely due to the availability of feeds and market demands for specific animal products, the quality of the feeds available and nutritional needs of the animals concerned.
Estimating the exposure to DON and to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (Section 6.2) requires estimates of feed intake, and in this opinion two approaches have been adopted. For many livestock, part or all of the daily ration is commonly is provided in the form of manufactured compound feeds, and where data on levels of DON and its acetylated and modified forms in species specific compound feeds are available these have been used to estimate exposure. Because the compound feeds are what the animals receive, this must be the preferred method of calculating exposure. However, for some livestock categories information on levels in compound feeds has not been given, or insufficient data have been provided to allow reliable estimates of exposure to be made. Since data on individual feed materials have been presented (Appendix C), estimates of exposure have also therefore been made using example diets. It should be stressed that these do not represent ‘average’ diets, nor are the feeding systems ‘typical’ for all of Europe. Instead, they are used to estimate levels of exposure to DON and to its acetylated and modified forms that might be indicative. They are based on published guidelines on nutrition and feeding (AFRC, 1993; Carabano and Piquer, 1998; NRC, 2007a,b; Leeson and Summers, 2008; EFSA FEEAP Panel, 2012; OECD, 2009; McDonald et al., 2011), data on EU manufacture of compound feeds (FEFAC, 2012)32 and expert knowledge of production systems in Europe. For companion animals (cats and dogs), information on typical diet formulations have been provided by The European Pet Food Industry.33 Details of feed consumption of farm and companion animals and the rations used are given in Appendix E.
5.2.1. Ruminants
For most ruminants, forages (either fresh or conserved) are the main ingredient in their diet, and in some cases may represent the total diet.
5.2.1.1. Dairy cows
For this scientific opinion, exposure is estimated for a 650‐kg dairy cow, with a milk yield of 40 kg per day (considered as a high milk yield), for which the main forages are either grass or grass silage, maize silage or hay. Assumptions on the amounts of forages and non‐forage feed, and the proportions of cereal grains, their products and their by‐products in the diet, are given in Appendix E, Tables E.1 and E.3.
Table E.1.
Live weights, growth rate/productivity, dry matter intake for cattle, sheep, goats and horses, and the proportions of the diet as non‐forage
| Live weight (kg) | Growth rate or productivity | Dry matter intake (kg/day) | % of diet as non‐forage feed | Reference | |
|---|---|---|---|---|---|
| Dairy cows: lactating | 650 | 40 kg milk/day | 20.7 | 40 | AFRC (1993) |
| Fattening cattle: beefa | 400 | 1 kg/day | 9.6 | 15 | AFRC (1993) |
| Fattening cattle: maize silage‐based ration | 300 | 1.4 kg/day | 6.6 | 25 | Browne et al. (2004) |
| Fattening cattle: cereal straw‐based diet | 300 | 0.9 kg/day | 8.0 | 68 | EBLEX, 2008 |
| Sheep: lactating | 80 | Feeding twin lambs | 2.8 | 50 | OECD (2009) |
| Goats: milkingb | 60 | 6 kg milk/day | 3.4 | 65 | NRC (2007b, 2007a) |
| Horses | 450 | Moderate activity | 9.0 | 50 | NRC (2007b) |
Housed castrate cattle, medium maturing breed
months 2‐3 of lactation.
Table E.3.
Assumed diet compositions and feed intake of lactating dairy cows (40 litres/day) and fattening beef cattle fed diets based on different forages
| Quantities of feed consumed(kg dry matter/day) | Reference | |||||
|---|---|---|---|---|---|---|
| Forage | Maize grain | Soybean meal | Barley grain | Rapeseed meal | ||
| Lactating dairy cows:maize silage‐based diet | 15.0 | 9.5 | 2.8 | ni | ni | AFSSA, 2009 |
| Fattening beef cattle:maize silage‐based diet | 4.9 | ni | ni | ni | 1.5 | EBLEX, 2012 |
| Fattening beef cattle:cereal straw‐based diet | 2.5 | ni | ni | 4.1 | 1.4 | EBLEX, 2008 |
| Fattening beef cattle:intensive cereal‐based diet | 1.5 | ni | ni | 5.5 | 1.5 | EBLEX, 2008 |
ni: Not included in the diet formulations.
5.2.1.2. Beef cattle
In this opinion, exposures are estimated for fattening beef cattle in which the forage is supplemented with species specific compound feed. Two scenarios are considered; in the first the compound feed is fed with grazed grass or grass silage, which are assumed to make no contribution to exposure. In the second scenario, grass hay is assumed to be the forage. Three other feeding systems are also considered, in which the forages are either maize silage or cereal straw, and ‘cereal‐beef’ (intensively reared beef cattle on cereal‐based diet) with, in each case, appropriate supplementation. For exposure estimates, live weights of 300 or 400 kg, and feed intakes of between 6.6 and 10 kg dry matter per day have been assumed, depending on the feeding regime (see details in Appendix E, Tables E.1 and E.3).
5.2.1.3. Sheep and goats
The CONTAM Panel has used a daily dry matter intake of 2.8 kg for an 80‐kg lactating sheep feeding twin lambs to estimate the exposures (Appendix E, Table E.1). Details on the composition of the diets used in estimating the exposure for lactating sheep are given in Appendix E, Table E.4.
Table E.4.
Assumed diet compositions (%) for lactating sheep and goats, and fattening goats, and the calculated mean lower bound and upper bound concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in these diets
| Non‐forage feed materials | Lactating sheep | Lactating goats | Fattening goats |
|---|---|---|---|
| Wheat (%) | 14 | ni | ni |
| Barley (%) | 18 | 25 | 20 |
| Oats (%) | ni | 35 | 40 |
| Soybean meal (%) | 5 | 10 | 10 |
| Rapeseed meal (%) | 10 | 10 | 10 |
| Sunflower meal (%) | 5 | ni | ni |
| Beans (%)b | 10 | ni | ni |
| Maize gluten feed (%) | ni | ni | ni |
| Wheat feed (%)a | 15 | 10 | 10 |
| Oat feed (%)a | ni | ni | ni |
| Sugar beet pulp (%)b | 14 | 1 | 1 |
| Molasses (%)b | 4 | 4 | 4 |
| Vegetable oils (%)b | 5 | 5 | 5 |
| Minerals, vitamins etc. (%)b | ni | ni | ni |
| % of non‐forage feeds in the diet | 50 | 75 | 40 |
| Estimated mean dietary concentration of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucosidec | |||
| Lower bound (μg/kg) | 132 | 262 | 148 |
| Upper bound (μg/kg) | 253 | 367 | 270 |
| Estimated 95th percentile dietary concentration of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucosided | |||
| Lower bound (μg/kg) | 653 | 462 | 559 |
| Upper bound (μg/kg) | 749 | 558 | 666 |
ni: Not included in the diet formulations; DON: deoxynivalenol; Ac: acetyl.
By‐products of processing these grains See Commission Regulation (EU) No 575/2011 of June 2011 for full description.51
No data for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside concentration were available, and therefore no contribution from these feeds has been assumed.
The dry matter intakes of goats reared for meat and fed ad libitum can be as high as 3.8% of body weight (Devendra and Burns, 1983). The CONTAM Panel has used daily dry matter intakes of 3.3 kg for a 60‐kg goat for milking (4 kg milk/day) and 1.5 kg for a 40‐kg goat for fattening to estimate the exposures (Appendix E, Table E.4). Details on the composition of the diets used in estimating the exposure for goats are given in Appendix E, Table E.4.
5.2.2. Pigs
Exposure estimates have been made for piglets (20 kg bw), fattening pigs (100 kg bw) and lactating sows (200 kg bw) using feed intakes proposed by EFSA (2009). The proportions of cereal grains, their products and by‐products used in estimating the exposure for pigs are given in Appendix E, Table E.2.
Table E.2.
Live weights and feed intake for pigs, poultry and farmed fish
| Live weight (kg) | Feed intake (kg dry matter/day) | Reference | |
|---|---|---|---|
| Pigs: piglets | 20 | 1.0 | EFSA FEEDAP Panel (2012) |
| Pigs: fattening pigs | 100 | 3.0 | EFSA FEEDAP Panel (2012) |
| Pigs: lactating sows | 200 | 6.0 | EFSA FEEDAP Panel (2012) |
| Poultry: broilersa | 2 | 0.12 | EFSA FEEDAP Panel (2012) |
| Poultry: laying hens | 2 | 0.12 | EFSA FEEDAP Panel (2012) |
| Turkeys: fattening turkeys | 12 | 0.40 | EFSA FEEDAP Panel (2012) |
| Ducks: fattening ducks | 3 | 0.14 | Leeson and Summers (2008) |
| Salmonids | 2 | 0.04 | EFSA FEEDAP Panel (2012) |
| Carp | 1 | 0.02 | Schultz et al. (2012) |
Chickens for fattening.
5.2.3. Poultry
The CONTAM Panel applied the live weights and feed intakes reported for different poultry (broilers, laying hens and turkeys) by EFSA (2009) and for ducks by Leeson and Summers (2008) for the exposure estimations. The proportions of cereal grains, their products and by‐products used in estimating the exposure for poultry are given in Appendix E, Table E.2.
5.2.4. Horses
The CONTAM Panel estimated the exposure for a 450‐kg horse, with a daily intake of 9 kg dry matter/day, of which half is in the form of grass hay and where cereal grains, their products and by‐products represent 82% of the non‐forage component of the daily ration (Appendix E, Table E.1 and Section E.2.1).
5.2.5. Rabbits
For the exposure estimates, the CONTAM Panel assumed a live weight of 2 kg, and a feed intake of 75 g/kg bw per day. The proportions of cereal grains, their products and by‐products used in estimating the exposure are given in Appendix E, Section E.2.3.
5.2.6. Farmed fish (salmonids and carp)
Berntssen et al. (2010) provided details of the composition of a diet for growing salmonids, and the CONTAM Panel used this feed formulation to estimate the exposures, for salmon (2 kg) with a feed intake of 0.04 kg dry matter/day (EFSA FEEDAP Panel, 2012) (Appendix E, Tables E.2 and E.5).
Table E.5.
Assumed diet composition (%) for farmed rabbits, farmed mink, farmed fish (salmonids and carp) and companion animals (cats and dogs), and the calculated mean lower bound and upper bound levels of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in these diets
| Feed materials | Rabbits | Farmed mink | Farmed fish | Cats/Dogs | ||
|---|---|---|---|---|---|---|
| Salmonids | Carp | Cats | Dogs | |||
| Wheat | ni | 6 | 13.2 | 24 | 10.0 | 10.0 |
| Barley | ni | 1 | ni | ni | ni | ni |
| Maize | 17.6 | 6 | ni | 10 | 5.0 | 6.0 |
| Oats | ni | ni | ni | ni | ni | 0.5 |
| Soybean meal | ni | ni | 12.3 | 32.4 | 8.0 | 4.0 |
| Rapeseed meal | ni | ni | ni | 12.5 | ni | ni |
| Maize gluten meal | ni | ni | 11.5 | ni | 17.0 | 15.0 |
| Sunflower meal | 20.0 | ni | ni | ni | ni | ni |
| Lucerne meal | 19.1 | ni | ni | ni | ni | ni |
| Beans | 10.4 | ni | ni | ni | 1.0 | 2.0 |
| Peas | 7.5 | |||||
| Wheat feeda | 18.3 | ni | ni | ni | 12.0 | 20.0 |
| Sugar beet pulp | 11.9 | ni | ni | ni | ni | ni |
| Fishmeala | ni | ni | 30.5 | 6.7 | 6.0 | ni |
| Meat meala | ni | 40 | ni | ni | ni | ni |
| Molassesa | ni | ni | ni | ni | ni | ni |
| Fish and vegetable oilsa | ni | 8 | 31.9 | 2.3 | ni | ni |
| Others feeds (unspecified)a | ni | ni | ni | 1.0 | 38.0 | 40.0 |
| Minerals, vitamins etc.a | 2.7 | 3 | 0.6 | 3.6 | 3.0 | 2.5 |
| Estimated mean dietary concentration of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucosideb | ||||||
| Lower bound (μg/kg) | 196 | 99.5 | 83.3 | 254 | 229 | 174 |
| Upper bound (μg/kg) | 282 | 109 | 123 | 388 | 264 | 214 |
| Estimated 95th percentile dietary concentration of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucosideb | ||||||
| Lower bound (μg/kg) | 1048 | 407 | 362 | 1084 | 968 | 741 |
| Upper bound (μg/kg) | 1135 | 409 | 380 | 1152 | 975 | 753 |
ni: These feeds have not been included in the diet formulations; DON: deoxynivalenol; Ac: acetyl.
No data for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside concentration were available, and therefore no contribution from these feeds has been assumed.
Diet formulation based on data provided by the Finnish Fur Breeders Association in 2015 and translated from Finnish to English, http://www.profur.fi
For carp, the CONTAM Panel have used the ingredients of commercial compound feeds for carp reported by Schultz et al. (2012) to estimate the exposures for fish of 1 kg live weight and a feed intake of 0.022 kg dry matter/day (Appendix E, Tables E.2 and E.5).
5.2.7. Farmed mink
For estimating exposure, the CONTAM Panel have assumed a live weight of 2.07 kg for a male mink at pelting, and with a feed intake of 227 g/day (75 g dry matter) (NRC, 1982). The proportions of cereal grains, their products and by‐products used in estimating the exposure are given in Appendix E, Section E.2.5.
5.2.8. Dogs and cats
The amounts of food consumed by dogs and cats are influenced by many factors, including breed, size, level of activity and their reproductive state. For estimating the exposure, the CONTAM Panel applied a live weight of 4 kg and a feed intake of 60 g per day of standard‐quality pet food for cats (Appendix E, Section E.1.4). For dogs, a live weight of 25 kg and a feed intake of 360 g per day of standard‐quality pet food were assumed (Appendix E, Table E.5).
6. Exposure assessment of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in humans and animals
6.1. Human exposure assessment
6.1.1. Human exposure assessments reported in the literature
A large number of human exposure assessments have been conducted for DON, with only one study including acetylated DON and metabolites. Below are summarised the evaluations of those conducted by international or national bodies, especially for the European countries (see Table 32).
Table 32.
Previous dietary exposure assessments of DON in different countries and regions
| Country | Population group | Dietary exposure (μg/kg bw per day) | Reference |
|---|---|---|---|
| Europe | Adult |
1.4a 0.22, 1.02 (means for different populations) 0.22, 1.11 (mean, minimum LB, maximum UB) |
FAO/WHO (2001) EFSA (2015) EFSA (2014) |
| All regions | 0.2–14.5 (means for different regions) | FAO/WHO (2011) | |
| Finland | Adult |
0.36, 0.72b 0.14, 0.28b 0.57, 1.14b 0.49, 0.98b 0.40, 0.80b |
NCM (1998) (Based on food balance sheets consumption data) |
| Denmark | Adult | ||
| Iceland | Adult | ||
| Norway | Adult | ||
| Sweden | Adult | ||
|
France Germany Netherland Norway Portugal Sweden United Kingdom Austria Belgium Denmark Finland |
Adult Child Adult Infant 4 months Adult Child 1–6 years Adult (male) Adult (female) Infant 6 months Adult Adult Adult (female) Adult (male) 4–6 years < 1 year Infant Adult 13–18 years old Adult Adult |
0.46, 0.89c 0.73, 1.51c 0.27, 0.38c 0.51, 0.60c 0.34c 0.76c 0.34, 0.61c 0.30, 0.53c 0.29, 0.44c 0.36, 1.00c 0.08, 0.13c 0.14c 0.18c 0.50c 0.48c 0.37c 0.29, 0.66c 0.25, 1.26c 0.17, 0.21c 0.14, 0.25c |
SCOOP (2003) |
| Finland |
EVIRA data MTT data |
0.04, 0.10b 0.08, 0.18b |
Rautala et al. (2008) |
| France |
Adult 15+ years Child 3–14 years Vegetarian |
0.28, 0.57b 0.45, 0.93b 0.32, 0.96b |
Leblanc et al. (2005) |
|
Adult Child |
0.38, 0.72b 0.56, 1.03b |
ANSES (2011) | |
| The Netherlands |
Child 1year old Child Child 1 year old |
0.66a 0.29a 0.30, 0.50b |
Schothorst et al. (2005) Bakker et al. (1994) Boon et al. (2009) |
| Norway |
Child 1–2 years old Adult |
0.89, 1.80d 0.27, 0.55d |
NVK (2013) |
| New Zealand | Not reported | 0.01a | Cressey and Thomson (2006) |
| Japan |
Total (year 2005) Child < 1 year old (year 2003) Child 1–6 years old (year 2003) |
0.01–0.02 0.17a 0.36a |
FSCJ (2010) |
bw, body weight; LB: lower bound; UB: upper bound.
Overall mean.
Average consumer and high consumer (consumption at 95th percentile).
Mean 1 and mean 2 in the SCOOP study: mean food consumption and mean 1 (count for all values, middle bound (MB)) or 2 (count for only positive values, UB) occurrence data. Where only one is shown, mean 1 is used.
Based on occurrence data of earlier and later years. For details see NVK (2013).
In the exposure assessment of the Nordic Council (NCM, 1998) mean concentrations of DON in grains and the consumption data from the food balance sheets and from individual quantitative questionnaires were used. The mean DON exposure for adults in Finland, Denmark, Iceland, Norway and Sweden ranged from 0.14 to 0.57 μg/kg bw per day, and the 95th percentile exposure from 0.28 to 1.14 μg/kg bw per day. The biggest contribution came from wheat, followed by oats.
The SCOOP task 3.2.10 assessed exposure to several Fusarium mycotoxins in 11 EU Member States. Of the 11,022 samples, 57% had detectable DON concentrations (Schothorst and van Egmond, 2004). The average DON exposure ranged from 0.08 to 1.51 μg/kg bw per day across all countries with France having the highest and Sweden the lowest exposure. Wheat flour and bread were the major source of DON exposure.
JECFA (2001) evaluated the dietary exposure to DON using the average concentrations (mostly using pooled data and excluding processed food) and the average food consumption data from the five regional diets in the Global Environment Monitoring System – Food Contamination Monitoring and Assessment Programme (GEMS/Food) (WHO, 1998). DON exposure was estimated to range from 0.77 to 2.4 μg/kg bw per day. Wheat contributed the largest proportion of DON exposure in Europe (79%).
In 1998–1999, high DON concentrations in the cereals were reported in a national exposure assessment of the Netherlands (Pieters et al., 2001) using consumption data of 2 consecutive days were from the National Food Consumption Survey. The median exposure level to DON was 0.3 μg/kg bw per day in 6,247 individuals. Young children had the highest exposure. Other dietary exposure assessments of DON in young Dutch children showed a similar level of exposure (Pieters et al., 2004; Boon et al., 2009).
The first French total diet study measuring various contaminants including DON in ‘ready to eat’ food was conducted in 2000 by the French National Institute for Agricultural Research (INRA) and AFSSA (now ANSES). The mean DON exposure was 0.28 and 0.45 μg/kg bw per day in adults and children, respectively. Depending on the different types of vegetarians, DON average exposure ranged between 0.32 and 0.41 μg/kg bw per day in this subpopulation. Cereal products, particularly bread/rusk contributed over 90% of DON exposure (Leblanc et al., 2005; AFSSA, 2009). The second French Total Diet Study (ANSES, 2011) assessed exposure to DON, 3‐Ac‐DON and 15‐Ac‐DON based on the French Individual and National study of Food Consumption (INCA2) during 2006–2007. The average exposure to the sum of DON, 3‐Ac‐DON and 15‐Ac‐DON ranged from 0.37 to 0.62 (UB) μg/kg bw per day. The DON exposure of the latter study was higher than that of the first one. Acetylated DON constituted less than 10% of DON.
JECFA (FAO/WHO, 2011) estimated the exposure to DON using their diet cluster system from the GEMS/Food and occurrence data reported in the literature or provided by the member states of FAO. DON occurrence data were obtained for barley, maize, wheat, rice, rye and oats. The dietary exposure to DON varied from 0.2 to 14.5 μg/kg bw per day across the different regions. For Europe the average exposure was 1.4 μg/kg bw per day. The acetylated forms of DON were not included in the dietary exposure estimates due to limited data and also because the levels of the acetylated forms of DON were typically less than 10% of those reported for DON. Wheat was the major contributor (56–100%) to DON exposure in the majority of the regions (FAO/WHO, 2011). Acute DON exposure was assessed using the highest mean occurrence data and the 97.5th percentile consumption data for the sum of DON, 3‐Ac‐DON and 15‐Ac‐DON using the GEMS/Food database. For the acute exposure calculation, bread was taken as the representative food type due to the frequent daily consumption (typically 9 g/kg bw per day). Based on the concentration of 1 mg DON/kg, the estimated acute dietary exposure was 9 μg/kg bw per day (FAO/WHO, 2011).
6.1.2. Current mean and 95th percentile dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
The CONTAM Panel considered it appropriate to estimate both acute and chronic exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside and to DON alone (see Appendix F, Table F.2) for all age groups (Section 5.1). The sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was estimated by summing up the individual concentrations of all four forms, DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (see Sections 4.2.2 and 4.2.8).
Table F.2.
Summary statistics of acute dietary exposure assessment of DON alone by age group
| Age groupa | Mean dietary exposure (μg/kg bw per day) | 95th percentile dietary exposure (μg/kg bw per day) | |||
|---|---|---|---|---|---|
| Upper bound | |||||
| n | Minimum | Maximum | Minimum | Maximum | |
| Infantsb | 6 | 0.5 (0.5–0.5) | 1.8 (1.8–1.9) | 1.5 (1.3–1.6) | 4.4 (4.1–4.9) |
| Toddlers | 11 | 0.7 (0.7–0.8) | 1.6 (1.4–1.9) | 1.8 (1.7–1.9) | 4.3 (2.8–6.2) |
| Other children | 20 | 0.5 (0.5–0.5) | 1.7 (1.6–1.8) | 1.1 (1.1–1.2) | 4.5 (4.0–4.9) |
| Adolescents | 20 | 0.2 (0.2–0.2) | 1.1 (0.9–1.0) | 0.6 (0.5–0.6) | 2.5 (2.3–2.7) |
| Adults | 22 | 0.4 (0.3–0.5) | 0.9 (0.8–0.9) | 1.1 (1.0–1.2) | 2.3 (1.9–2.9) |
| Elderly | 16 | 0.4 (0.3–0.5) | 0.7 (0.6–0.8) | 0.9 (0.7–1.2) | 2.1 (1.6–3.2) |
| Very elderly | 14 | 0.4 (0.3–0.5) | 0.7 (0.6–0.7) | 0.9 (0.8–1.1) | 1.8 (1.6–2.0) |
DON: deoxynivalenol; bw: body weight; n: number of surveys.
Section 5.1 describes the age range within each age class.
One the dietary surveys had less than 60 survey participants and therefore could not be included in calculation of the 95th percentile exposure.
The food categories, which were represented by a very low number of samples (≤ 5 samples) or for which all the data were left‐censored, were not considered suitable, and were not used in exposure calculation.
6.1.2.1. Current mean and 95th percentile acute dietary exposure
The acute dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside and the exposure to DON (Appendix F, Table F.2) was calculated on a per day basis, since individual meals are recorded for only a few countries in the consumption database. The preferred option is, therefore, to use individual consuming days. Consuming days offer a conservative estimate of the exposure, since it will sum the contribution of all meals during the same day.
Acute exposure was assessed for each reporting day by multiplying the total consumption amount for each food category by an occurrence level randomly drawn among individual results available for that food category. Respective intakes of the foods consumed that day were summed and finally divided by the individual's body weight. This process was iterated 100 times for each consuming day reported by each participant.
The mean and 95th percentile of acute exposure (LB, MB and UB estimates) to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside obtained for different age groups are shown in Table 33. The distribution of the mean and 95th percentile of acute exposure to DON alone obtained for different age groups is shown in Appendix F, Table F.2.
Table 33.
Summary statistics of probabilistic acute dietary exposure assessment to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (at the lower, middle and upper bound) across European dietary surveys (μg/kg bw per day) by age group
| Age groupa | Mean dietary exposure (μg/kg bw per day) | 95th percentile dietary exposure (μg/kg bw per day) | |||
|---|---|---|---|---|---|
| n | Minimum | Maximum | Minimum | Maximum | |
| Lower bound | |||||
| Infantsb | 6 | 0.3 (0.1–0.5) | 0.5 (0.4–0.9) | 1.7 (1.6–1.8) | 2.2 (1.9–2.7) |
| Toddlers | 11 | 0.6 (0.3–1.1) | 1.0 (0.9–1.1) | 1.8 (1.6–2.0) | 3.2 (2.1–4.5) |
| Other children | 20 | 0.5 (0.5–0.6) | 1.0 (0.9–1.1) | 1.5 (1.4–1.6) | 3.0 (2.7–3.4) |
| Adolescents | 20 | 0.3 (0.3–0.4) | 0.7 (0.6–0.7) | 0.8 (0.7–0.9) | 2.2 (2.2–2.2) |
| Adults | 24 | 0.2 (0.2–0.3) | 0.4 (0.3–0.5) | 0.8 (0.7–1.0) | 1.5 (1.3–1.7) |
| Elderly | 16 | 0.2 (0.2–0.3) | 0.4 (0.3–0.5) | 0.7 (0.6–0.7) | 1.4 (1.1–1.7) |
| Very elderly | 14 | 0.3 (0.2–0.5) | 0.4 (0.3–0.6) | 0.7 (0.5–1.0) | 1.5 (1.1–2.2) |
| Middle bound | |||||
| Infantsb | 6 | 0.6 (0.6–0.7) | 1.7 (1.6–2.1) | 1.8 (1.7–1.9) | 4.1 (4.1–4.2) |
| Toddlers | 11 | 1.0 (0.8–1.5) | 1.6 (1.5–1.7) | 2.5 (2.3–2.7) | 4.1 (3.1–5.6) |
| Other children | 20 | 0.9 (0.8–1.0) | 1.5 (1.4–1.5) | 2.0 (1.9–2.1) | 3.6 (3.4–3.9) |
| Adolescents | 20 | 0.4 (0.4–0.4) | 0.9 (0.8–1.0) | 1.0 (1.0–1.1) | 2.3 (2.0–2.7) |
| Adults | 24 | 0.4 (0.3–0.5) | 0.7 (0.6–0.7) | 1.1 (1.0–1.2) | 2.0 (1.9–2.2) |
| Elderly | 16 | 0.4 (0.4–0.5) | 0.6 (0.5–0.7) | 1.0 (0.9–1.2) | 1.7 (1.4–2.1) |
| Very elderly | 14 | 0.4 (0.4–0.5) | 0.6 (0.6–0.8) | 0.9 (0.9–0.9) | 1.8 (1.4–2.5) |
| Upper bound | |||||
| Infantsb | 6 | 1.0 (0.9–1.0) | 2.9 (2.8–3.2) | 2.7 (2.6–2.8) | 6.7 (6.2–7.1) |
| Toddlers | 11 | 1.5 (1.2–1.9) | 2.2 (2.0–2.6) | 3.5 (3.3–3.6) | 5.4 (4.5–6.5) |
| Other children | 20 | 1.2 (1.1–1.3) | 2.0 (1.9–2.0) | 2.6 (2.5–2.7) | 4.5 (4.2–4.9) |
| Adolescents | 20 | 0.6 (0.6–0.6) | 1.2 (1.1–1.3) | 1.3 (1.3–1.4) | 2.9 (2.7–3.2) |
| Adults | 24 | 0.5 (0.5–0.5) | 1.0 (1.0–1.0) | 1.5 (1.4–1.6) | 2.8 (2.8–2.8) |
| Elderly | 16 | 0.5 (0.5–0.6) | 0.8 (0.8–0.9) | 1.3 (1.1–1.6) | 2.2 (1.9–2.6) |
| Very elderly | 14 | 0.5 (0.5–0.6) | 0.9 (0.8–1.0) | 1.3 (1.2–1.4) | 2.2 (1.7–2.9) |
bw: body weight; n: number of surveys.
The corresponding 95% confidence intervals are presented in the brackets. Estimates are rounded to one decimal place.
Section 5.1 describes the age range within each age class.
One the dietary surveys had less than 60 survey participants and therefore could not be included in calculation of the 95th percentile exposure.
Infants (< 12 months)
Six dietary surveys were available for this age group, one of which had less than 60 survey participants and therefore could not be included in calculation of the 95th percentile exposure. The mean acute dietary exposure was between 0.3 and 2.9 μg/kg bw per day (minimum LB and maximum UB) and the 95th percentile dietary exposure ranged from 1.7 to 6.7 μg/kg bw per day (minimum LB to maximum UB). Infants had the highest estimates of acute dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. This can be explained by the higher intake of food per kg bw in younger age groups.
Toddlers (≥ 12 months to < 36 months old)
There were 11 surveys available reporting food consumption for toddlers. The mean acute dietary exposure ranged from 0.6 to 2.2 μg/kg bw per day (minimum LB to maximum UB), and the 95th percentile dietary exposure ranged from 1.8 to 5.4 μg/kg bw per day (minimum LB to maximum UB).
Other children (≥ 36 months to < 10 years old)
There were 20 surveys available reporting food consumption for other children. The mean acute dietary exposure ranged from 0.5 to 2.0 μg/kg bw per day (minimum LB to maximum UB), and the 95th percentile dietary exposure ranged from 1.5 to 4.5 μg/kg bw per day (minimum LB to maximum UB).
Adolescents (≥ 10 years to < 18 years old)
There were 20 surveys available reporting food consumption for adolescents. The mean acute dietary exposure ranged from 0.3 to 1.2 μg/kg bw per day (minimum LB to maximum UB), and the 95th percentile dietary exposure ranged from 0.8 to 2.9 μg/kg bw per day (minimum LB to maximum UB).
Adults (≥ 18 years to < 65 years)
There were 24 surveys available reporting food consumption for adults including pregnant and lactating women. In the adult population, the mean acute dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside varied from the minimum LB of 0.2 μg/kg bw per day to the maximum UB of 1.0 μg/kg bw per day. The 95th percentile dietary exposure estimate varied from 0.8 to 2.8 μg/kg bw per day (minimum LB to maximum UB).
Elderly and very elderly (≥ 65 years old)
There were 16 surveys available reporting food consumption for elderly and 14 surveys available reporting food consumption for very elderly participants. The dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside varied for the mean between 0.2 and 1.0 μg/kg bw per day (minimum LB to maximum UB) with a 95th percentile ranged between 0.7 and 2.2 μg/kg bw per day (minimum LB to maximum UB).
Table 34 shows the estimates of average % of acute exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside through the daily consumption per age class and individual foods/food groups across European dietary surveys. The values presented are minimum and maximum values across the different surveys and corresponding 95% confidence intervals. The highest contributions came from grain‐based products across all age groups ranging between 65% (in infants) and 94% (in very elderly). Other relevant contributions came from food for infants and small children (maximum 64% in that age group), from fruit and fruit products (maximum 26% and 20% in infants and toddlers, respectively) and from composite foods (maximum 24% in adults).
Table 34.
Estimates of average % contribution (95% confidence intervals) to acute exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across age class and European dietary surveys at FoodEx level 1
| Food group | Infants (n = 6) | Toddlers (n = 11) | Other children (n = 20) | Adolescents (n = 20) | Adults (n = 22) | Elderly (n = 16) | Very elderly (n = 14) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |
| Alcoholic beverages | – | – | – | – | – | – | – | 3 (2–4) | 2 (2–2) | 12 (12–13) | 1 (0–1) | 13 (12–14) | – | 7 (5–9) |
| Composite food (including frozen products) | 4 (3–4) | – | 11 (10–12) | – | 20 (19–21) | – | 18 (17–19) | – | 24 (23–25) | – | 14 (12–16) | – | 16 (14–20) | |
| Food for infants and small children | 22 (22–22) | 64 (63–65) | – | 18 (16–20) | – | 5 (5–5) | – | – | – | – | – | – | – | – |
| Fruit and fruit products | 8 (6–10) | 26 (25–26) | 10 (9–11) | 20 (20–20) | 6 (6–6) | 15 (14–15) | 4 (4–4) | 12 (11–13) | 4 (4–5) | 18 (18–19) | 4 (4–5) | 15 (14–16) | 3 (2–3) | 16 (15–17) |
| Grains and grain‐based products | 15 (11–20) | 65 (64–66) | 56 (54–57) | 78 (76–80) | 65 (63–66) | 84 (83–86) | 60 (58–63) | 88 (85–92) | 54 (51–57) | 89 (87–90) | 61 (56–66) | 92 (87–98) | 67 (58–77) | 94 (88–100) |
| Snacks, desserts, and other foods | – | 1 (1–1) | – | 4 (3–4) | – | 5 (4–6) | 1 (1–1) | 7 (6–7) | – | 3 (3–4) | – | 2 (1–2) | – | 1 (1–1) |
| Vegetables and vegetable products (including fungi) | – | 5 (5–5) | – | 6 (5–8) | – | 5 (5–6) | – | 3 (3–4) | – | 2 (2–2) | – | 1 (1–1) | – | 1 (1–1) |
n: number of surveys; Min: minimum; Max: maximum.
Food category < 5% in all age groups are not reported are represented by a dash.
Section 5.1 describes the age range within each age class.
Given the high number of occurrence data reported for DON alone and the high percentage of left‐censored data for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, the CONTAM Panel calculated that the % of the contribution34 of the acute exposure that could be attributed to DON alone (Appendix F). The acute exposure from DON alone was between 50% and 90% of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across different age groups, and the CONTAM Panel concluded that the overall dietary exposure was driven by DON.
6.1.2.2. Current mean and 95th percentile chronic dietary exposure
For assessing the chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside and DON alone, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Occurrence data and consumption data were linked at the lowest FoodEx code possible.
Exposure estimates were calculated per dietary survey and age class for each of the 35 different dietary surveys carried out in 19 different European countries (see Section 5.1). However, it should be noted that not all countries provided consumption information for all age groups of interest or in some cases the same country provided more than one consumption survey.
The mean and the 95th percentile chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside and to DON alone were calculated separately for each dietary survey using consumption data recorded at the individual level. Individual food consumption data were combined with the mean occurrence values in order to provide mean and the 95th percentile exposure estimates. Exposure estimates were calculated for both LB and UB scenarios. Exposure estimates of DON alone are presented in Appendix F and not further discussed below.
The percentage of left‐censored data, mostly for 3‐Ac‐DON and 15‐Ac‐DON, in the food occurrence data set accounted for more than 90% of the data and may result in a large difference between the dietary exposure estimates in the LB and UB scenarios.
6.1.2.2.1. Current mean and 95th percentile chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
The chronic mean and the 95th percentile dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for all age groups across dietary surveys are summarised in Table 35. Mean and 95th percentile dietary exposure estimates calculated for each of the dietary survey are presented in Appendix F, Table F.3. In accordance with the specifications of the EFSA Guidance on the use of the Comprehensive database (EFSA, 2011b), the 95th percentile estimates for dietary surveys/age classes with less than 60 observations may not be statistically robust and therefore they were not considered in the exposure assessment.
Table 35.
Summary statistics of the chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (μg/kg bw per day) by age group
| Age group | Lower Bound | Middle Bound | Upper Bound | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Min | Median | Max | Min | Median | Max | Min | Median | Max | |
| Mean dietary exposure in total population (μg/kg bw per day) | ||||||||||
| Infants | 6 | 0.2 | 0.4 | 0.5 | 0.5 | 0.7 | 1.3 | 0.6 | 1.0 | 2.0 |
| Toddlers | 10 | 0.6 | 0.9 | 1.1 | 0.9 | 1.2 | 1.4 | 1.1 | 1.6 | 1.7 |
| Other children | 18 | 0.6 | 0.8 | 1.1 | 0.7 | 1.0 | 1.3 | 0.9 | 1.2 | 1.6 |
| Adolescents | 17 | 0.3 | 0.4 | 0.6 | 0.4 | 0.6 | 0.8 | 0.4 | 0.7 | 0.9 |
| Adults | 17 | 0.3 | 0.3 | 0.4 | 0.3 | 0.4 | 0.5 | 0.4 | 0.5 | 0.7 |
| Elderly | 14 | 0.2 | 0.3 | 0.4 | 0.3 | 0.4 | 0.5 | 0.4 | 0.5 | 0.6 |
| Very elderly | 12 | 0.3 | 0.3 | 0.4 | 0.3 | 0.4 | 0.5 | 0.4 | 0.5 | 0.6 |
| 95th percentile dietary exposure in total population (μg/kg bw per day) | ||||||||||
| Infantsa | 5 | 0.7 | 1.2 | 1.5 | 1.0 | 1.7 | 2.6 | 1.4 | 2.2 | 3.7 |
| Toddlersa | 7 | 1.1 | 1.5 | 1.7 | 1.4 | 2.0 | 2.1 | 1.8 | 2.6 | 2.7 |
| Other children | 18 | 0.9 | 1.3 | 1.9 | 1.1 | 1.6 | 2.3 | 1.4 | 2.0 | 2.7 |
| Adolescents | 17 | 0.5 | 0.9 | 1.2 | 0.6 | 1.0 | 1.5 | 0.7 | 1.3 | 1.8 |
| Adults | 17 | 0.5 | 0.6 | 0.8 | 0.7 | 0.8 | 1.0 | 0.8 | 1.0 | 1.4 |
| Elderly | 14 | 0.5 | 0.6 | 0.8 | 0.6 | 0.7 | 1.0 | 0.7 | 0.9 | 1.1 |
| Very elderlya | 9 | 0.5 | 0.5 | 0.7 | 0.6 | 0.7 | 0.9 | 0.8 | 0.8 | 1.0 |
bw: body weight; n: number of surveys; Min: minimum; Max: maximum.
Estimates are rounded to one decimal place.
One dietary survey for infants, and three dietary surveys for toddlers and very elderly had less than 60 survey participants and therefore could not be included in calculation of the 95th percentile exposure.
Table F.3.
Summary statistics of the chronic dietary exposure to DON alone (μg/kg bw per day) across European countries
| Age group | Lower Bound | Middle Bound | Upper Bound | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Min | Median | Max | Min | Median | Max | Min | Median | Max | |
| Mean dietary exposure in total population (μg/kg bw per day) | ||||||||||
| Infants | 6 | 0.2 | 0.2 | 0.4 | 0.3 | 0.5 | 0.9 | 0.4 | 0.7 | 1.4 |
| Toddlers | 10 | 0.4 | 0.6 | 0.7 | 0.6 | 0.8 | 0.9 | 0.8 | 1.1 | 1.2 |
| Other children | 18 | 0.4 | 0.5 | 0.7 | 0.5 | 0.7 | 0.9 | 0.6 | 0.8 | 1.1 |
| Adolescents | 17 | 0.2 | 0.3 | 0.4 | 0.3 | 0.4 | 0.5 | 0.3 | 0.5 | 0.7 |
| Adults | 17 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.4 | 0.3 | 0.4 | 0.5 |
| Elderly | 14 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 |
| Very elderly | 12 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.4 | 0.3 | 0.3 | 0.4 |
| 95th percentile dietary exposure in total population (μg/kg bw per day) | ||||||||||
| Infantsa | 5 | 0.5 | 0.8 | 1.0 | 0.7 | 1.1 | 1.8 | 1.0 | 1.6 | 2.6 |
| Toddlersa | 7 | 0.7 | 1.0 | 1.2 | 1.0 | 1.4 | 1.5 | 1.2 | 1.8 | 1.9 |
| Other children | 18 | 0.6 | 0.9 | 1.3 | 0.7 | 1.1 | 1.6 | 1.0 | 1.4 | 1.9 |
| Adolescents | 17 | 0.4 | 0.6 | 0.9 | 0.4 | 0.7 | 1.0 | 0.5 | 0.9 | 1.2 |
| Adults | 17 | 0.3 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.6 | 0.7 | 1.0 |
| Elderly | 14 | 0.3 | 0.4 | 0.6 | 0.4 | 0.5 | 0.7 | 0.5 | 0.6 | 0.8 |
| Very elderlya | 9 | 0.3 | 0.4 | 0.5 | 0.4 | 0.5 | 0.6 | 0.5 | 0.6 | 0.7 |
DON: deoxynivalenol; bw: body weight.
One dietary survey for infants, and three dietary surveys for toddlers and very elderly had less than 60 survey participants and therefore could not be included in calculation of the 95th percentile exposure.
Given the high number of occurrence data reported for DON alone and the high percentage of left‐censored data for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, the CONTAM Panel calculated that the % of the contribution38 of the chronic exposure to DON alone (Appendix F) to the chronic exposure of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was between 60% and 85% across different age groups and concluded that the overall dietary exposure was driven by DON.
Infants (< 12 months)
Six dietary surveys were available for this age group, one of which had less than 60 survey participants and therefore could not be included in calculation of the 95th percentile exposure. The mean chronic dietary exposure was between 0.2 and 2.0 μg/kg bw per day (minimum LB and maximum UB) and the 95th percentile dietary exposure ranged from 0.7 to 3.7 μg/kg bw per day (minimum LB to maximum UB). Infants had the highest estimates of chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. This can be explained by the higher intake of food per kg bw in younger age groups.
Toddlers (≥ 12 months to < 36 months old)
There were 10 surveys available reporting food consumption for toddlers covering a total of 4,103 survey participants. The mean chronic dietary exposure ranged from 0.6 to 1.7 μg/kg bw per day (minimum LB to maximum UB), and the 95th percentile dietary exposure ranged from 1.1 to 2.7 μg/kg bw per day (minimum LB to maximum UB).
Other children (≥ 36 months to < 10 years old)
There were 18 surveys available reporting food consumption for other children covering a total of 9,534 survey participants. The mean chronic dietary exposure ranged from 0.6 to 1.6 μg/kg bw per day (minimum LB to maximum UB), and the 95th percentile dietary exposure ranged from 0.9 to 2.7 μg/kg bw per day (minimum LB to maximum UB).
Adolescents (≥ 10 years to < 18 years old)
There were 17 surveys available reporting food consumption for adolescents covering a total of 8,946 survey participants. The mean chronic dietary exposure ranged from 0.3 to 0.9 μg/kg bw per day (minimum LB to maximum UB), and the 95th percentile dietary exposure ranged from 0.5 to 1.8 μg/kg bw per day (minimum LB to maximum UB).
Adults (≥ 18 years to < 65 years)
There were 17 surveys available reporting food consumption for adults including pregnant and lactating women covering a total of 33,392 survey participants. In the adult population, the mean chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside varied from the minimum LB of 0.3 μg/kg bw per day to the maximum UB of 0.7 μg/kg bw per day. The 95th percentile dietary exposure estimate varied from 0.5 to 1.4 μg/kg bw per day (minimum LB to maximum UB).
Elderly and very elderly (≥ 65 years old)
There were 14 surveys available reporting food consumption for elderly covering a total of 5,186 survey participants and 12 surveys available reporting food consumption for very elderly covering a total of 2,406 survey participants. The dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside varied for the mean between 0.2 and 0.6 μg/kg bw per day (minimum LB to maximum UB) with a 95th percentile range between 0.5 and 1.1 μg/kg bw per day (minimum LB to maximum UB).
6.1.2.2.2. Contributions of different food groups to DON and the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside chronic dietary exposure
The contribution of individual food groups to chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside and to DON alone (see Appendix F, Section F.5) varied between the dietary surveys. This is explained by the specific food consumption patterns in the individual European countries and even in the different regions of one country. In two dietary surveys, grain‐based products (e.g. bread, fine bakery products) were disaggregated to ingredients (flour) and therefore these studies did not qualify for calculation of the contribution of food groups to the exposure. The contribution to chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside and to DON alone (see Appendix F, Section F.5) for the individual food groups was assessed separately for each survey and age group. The results are reported as a number of surveys for the following contribution ranges: 0–5, 6–10, 11–25, 26–50 and higher than 50%. A summary of the median values calculated from the average contribution of each food group across the dietary surveys and the range of the lowest and highest average contribution is shown in Tables 36 and 37.
Table 36.
Number of surveys split according to their percentage contribution to chronic dietary exposure of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (LB) concentrations for toddlers, other children and adolescents
| Food category | Toddlersa | Other childrena | Adolescents | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤5% | 6‐10% | 11‐25% | 26‐50% | = 50% | ≤5% | 5‐10% | 10‐25% | 26‐50% | = 50% | ≤5% | 5‐10% | 10‐25% | 65‐50% | >50% | |
| Barley grain | 4 | 8 | 6 | ||||||||||||
| Beer and beer‐like beverage | 4 | 12 | 15 | ||||||||||||
| Bread and rolls | 1 | 1 | 4 | 4 | 1 | 12 | 5 | 1 | 11 | 5 | |||||
| Breakfast cereals | 4 | 1 | 4 | 1 | 7 | 8 | 3 | 10 | 7 | ||||||
| Buckwheat grain | 4 | 5 | 2 | ||||||||||||
| Cereal‐based dishes | 4 | 2 | 11 | 1 | 1 | 10 | 2 | 1 | |||||||
| Cereal‐based food for infants and young children | 7 | 1 | 11 | 6 | |||||||||||
| Cocoa beans and cocoa products | 10 | 16 | 17 | ||||||||||||
| Cocoa beverage | 3 | 7 | 8 | ||||||||||||
| Composite food (including frozen products) | 2 | 7 | 5 | ||||||||||||
| Condiment | 9 | 18 | 17 | ||||||||||||
| Corn grain | 2 | 5 | 5 | ||||||||||||
| Dietary supplements | 6 | 8 | 6 | ||||||||||||
| Dried fruits | 8 | 16 | 16 | ||||||||||||
| Fine bakery wares | 2 | 7 | 1 | 2 | 9 | 7 | 2 | 13 | 2 | ||||||
| Food for infants and small children | 1 | ||||||||||||||
| Grain milling products | 5 | 1 | 3 | 1 | 9 | 3 | 4 | ||||||||
| Grains and grain‐based products, unspecified | 1 | ||||||||||||||
| Grains for human consumption | 1 | 3 | 3 | ||||||||||||
| Medical food | 2 | 3 | 2 | ||||||||||||
| Millet grain | 3 | 3 | 5 | ||||||||||||
| Miscellaneous fruits | 10 | 18 | 17 | ||||||||||||
| Oats, grain | 3 | 3 | 2 | ||||||||||||
| Other grains | 2 | 2 | 4 | ||||||||||||
| Pasta (raw) | 3 | 2 | 4 | 1 | 4 | 4 | 9 | 1 | 3 | 3 | 10 | 1 | |||
| Peanut (Arachis hypogea) | 7 | 18 | 17 | ||||||||||||
| Pumpkin seeds (Cucurbita pepo var. oleifera) | 2 | 6 | 6 | ||||||||||||
| Ready to eat soups | 8 | 1 | 14 | 1 | 14 | 1 | |||||||||
| Ready‐to‐eat meal for infants and young children | 7 | 9 | 2 | ||||||||||||
| Rice | 10 | 18 | 17 | ||||||||||||
| Rye grain | 1 | 2 | |||||||||||||
| Snack food | 7 | 3 | 13 | 5 | 10 | 7 | |||||||||
| Soya beans (Glycine max) | 2 | 5 | 8 | ||||||||||||
| Spelt grain | 1 | 1 | 1 | ||||||||||||
| Stone fruits | 9 | 18 | 17 | ||||||||||||
| Sweet corn (Zea mays var. saccharata) | 7 | 16 | 16 | ||||||||||||
| Wheat grain | 4 | 9 | 8 | ||||||||||||
FI/1 survey excluded from calculation of the contribution of ‘Grains and grain‐based products’.
Table 37.
Number of surveys split according to their percentage contribution to chronic dietary exposure of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (LB) concentrations for adults, elderly and very elderly
| Food category | Adultsa | Elderlya | Very elderly | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤5% | 6‐10% | 11‐25% | 26‐50% | > 50% | ≤5% | 6‐10% | 11‐25% | 26‐50% | > 50% | ≤5% | 6‐10% | 11‐25% | 26‐50% | >50% | |
| Barley grain | 9 | 4 | 3 | ||||||||||||
| Beer and beer‐like beverage | 12 | 5 | 12 | 2 | 11 | 1 | |||||||||
| Bread and rolls | 11 | 6 | 5 | 9 | 3 | 9 | |||||||||
| Breakfast cereals | 12 | 3 | 2 | 8 | 2 | 2 | 2 | 5 | 4 | 2 | 1 | ||||
| Buckwheat grain | 3 | 3 | 2 | ||||||||||||
| Cereal‐based dishes | 11 | 3 | 9 | 8 | |||||||||||
| Cereal‐based food for infants and young children | 5 | 1 | 2 | ||||||||||||
| Cocoa beans and cocoa products | 16 | 14 | 11 | ||||||||||||
| Cocoa beverage | 10 | 7 | 5 | ||||||||||||
| Composite food (including frozen products) | 6 | 1 | 1 | ||||||||||||
| Condiment | 17 | 14 | 12 | ||||||||||||
| Corn grain | 4 | 3 | 2 | 1 | |||||||||||
| Dietary supplements | 6 | 5 | 1 | 4 | |||||||||||
| Dried fruits | 17 | 14 | 11 | ||||||||||||
| Fine bakery wares | 3 | 13 | 1 | 2 | 2 | 8 | 2 | 1 | 3 | 5 | 3 | ||||
| Grain milling products | 7 | 5 | 5 | 10 | 4 | 7 | 2 | 2 | |||||||
| Grains and grain‐based products, unspecified | 2 | 2 | 1 | ||||||||||||
| Grains for human consumption | 6 | 1 | 2 | ||||||||||||
| Medical food | 2 | 4 | 4 | ||||||||||||
| Millet grain | 7 | 4 | 2 | ||||||||||||
| Miscellaneous fruits | 17 | 14 | 12 | ||||||||||||
| Oats, grain | 5 | 5 | 2 | ||||||||||||
| Other grains | 7 | 3 | 1 | ||||||||||||
| Pasta (Raw) | 5 | 4 | 7 | 1 | 8 | 4 | 1 | 1 | 6 | 4 | 1 | 1 | |||
| Peanut (Arachis hypogea) | 17 | 12 | 9 | ||||||||||||
| Pumpkin seeds (Cucurbita pepo var. oleifera) | 12 | 9 | 6 | ||||||||||||
| Ready to eat soups | 15 | 1 | 12 | 1 | 10 | 1 | |||||||||
| Ready‐to‐eat meal for infants and young children | 3 | 14 | 1 | ||||||||||||
| Rice | 17 | 1 | 12 | ||||||||||||
| Rye grain | 3 | 14 | 1 | ||||||||||||
| Snack food | 16 | 1 | 5 | 9 | |||||||||||
| Soya beans (Glycine maximum) | 11 | 2 | 3 | ||||||||||||
| Spelt grain | 1 | 14 | 1 | ||||||||||||
| Stone fruits | 17 | 12 | 12 | ||||||||||||
| Sweet corn (Zea mays var. saccharata) | 16 | 6 | 10 | ||||||||||||
| Wheat grain | 13 | 4 | 6 | ||||||||||||
FI/1 survey excluded from calculation of the contribution of ‘Grains and grain‐based products’.
Grains and grain‐based foods made the largest contribution to the dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in all age groups. For DON alone, see Appendix F, Section F.5. The most important contributors within this food category were ‘Bread and rolls’, followed by ‘Fine bakery wares’ and ‘Pasta (raw)’ (only in one country with high consumption of pasta reported). It can be assumed that a high contribution of ‘Bread and rolls’ is more likely driven by high consumption of this food category. Other important contributors were ‘Grain milling products’, ‘Breakfast cereals’ and for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside also ‘Beer and beer‐like beverage’ (only in countries with higher consumption of beer reported).
6.1.2.3. Current acute and chronic dietary exposure the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for specific groups
Dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for vegetarian diets include more cereal and cereal‐based products and therefore it was considered that the exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in this consumer group could be higher. The Comprehensive Database contains only limited data on food consumption of vegetarians. Dietary exposure was calculated and compared to the exposure of all subjects included in the respective dietary study. Generally, for acute exposure, higher mean and 95th percentile exposures were not observed compared to the general population, with the exception of toddlers, other children and adults who appeared to have marginally higher exposures (Table 38). Similarly, for chronic exposure higher mean and 95th percentile exposures were not generally observed in vegetarians compared to the total population within the same dietary survey (Table 39). Thus, the limited data on vegetarians do not indicate a substantial difference in the dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside between the vegetarians and the general population. This finding was supported by the estimated exposure based on the database in the EFSA biomarker study (Brera et al., 2015) (see also Section 7.5.2).
Table 38.
Summary statistics of probabilistic acute dietary exposure assessment to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (at the lower, middle and upper bound) (μg/kg bw per day) by age group in vegetarians. The corresponding 95% confidence intervals are presented in the brackets. Estimates are rounded to one decimal place
| Age groupa | Mean dietary exposure (μg/kg bw per day) | 95th percentile dietary exposure (μg/kg bw per day)b | |||
|---|---|---|---|---|---|
| n | Minimum | Maximum | Minimum | Maximum | |
| Lower bound | |||||
| Infants | 1 | 0.5 (0.2–0.8) | – | – | |
| Toddlers | 3 | 0.6 (0.2–1.1) | 1.2 (0.2–2.9) | – | – |
| Other children | 2 | 0.4 (0.0–1.1) | 1.2 (0.8–1.6) | – | – |
| Adolescents | 4 | 0.3 (0.2–0.4) | 0.6 (0.3–1.0) | – | – |
| Adults | 10 | 0.2 (0.1–0.4) | 0.7 (0.3–1.3) | 1.6 (1.0–2.4) | 2.7 (2.2–3.7) |
| Elderly | 7 | 0.1 (0.0–0.3) | 0.6 (0.3–0.9) | – | – |
| Very elderly | 4 | 0.3 (0.1–0.6) | 0.4 (0.0–1.0) | – | – |
| Middle bound | |||||
| Infants | 1 | 1.1 (0.8–1.5) | – | – | |
| Toddlers | 3 | 1.3 (0.9–2.1) | 2.4 (1.5–4.5) | – | – |
| Other children | 2 | 0.5 (0.2–1.1) | 1.8 (1.5–2.4) | – | – |
| Adolescents | 4 | 0.5 (0.4–0.7) | 0.9 (0.7–1.2) | – | – |
| Adults | 10 | 0.3 (0.2–0.6) | 0.9 (0.5–1.5) | 2.0 (1.3–2.8) | 3.0 (2.5–3.6) |
| Elderly | 7 | 0.2 (0.1–0.4) | 0.8 (0.5–1.3) | – | – |
| Very elderly | 4 | 0.5 (0.3–0.9) | 0.7 (0.5–1.4) | – | – |
| Upper bound | |||||
| Infants | 1 | 1.6 (1.4–2.1) | – | – | |
| Toddlers | 3 | 2.0 (1.5–3.1) | 3.9 (2.7–6.1) | – | – |
| Other children | 2 | 0.6 (0.3–1.2) | 2.4 (2.0–2.7) | – | – |
| Adolescents | 4 | 0.7 (0.6–0.8) | 1.1 (0.9–1.4) | – | – |
| Adults | 10 | 0.4 (0.3–0.6) | 1.2 (1.1–1.3) | 2.4 (1.8–3.4) | 3.4 (2.8–4.1) |
| Elderly | 7 | 0.2 (0.1–0.4) | 1.2 (0.8–1.7) | – | – |
| Very elderly | 4 | 0.6 (0.5–0.9) | 1.1 (0.8–1.8) | – | – |
bw: body weight; n: number of surveys; Min: minimum, Max: maximum.
Section 5.1 describes the age range within each age class.
Not calculated for the other age groups because estimates were only available from one dietary survey.
Table 39.
Summary statistics of the chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (μg/kg bw per day) by age group in vegetarians. Estimates are rounded to one decimal place
| Age group | Lower bound | Middle bound | Upper bound | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Min | Median | Max | Min | Median | Max | Min | Median | Max | |
| Mean dietary exposure in vegetarians (μg/kg bw per day) | ||||||||||
| Infants | 1 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 1.4 | 0.7 | 1.0 | 2.1 |
| Toddlers | 3 | 0.6 | 0.8 | 1.1 | 0.9 | 1.2 | 1.4 | 1.1 | 1.6 | 1.7 |
| Other children | 2 | 0.6 | 0.7 | 1.1 | 0.7 | 1.0 | 1.3 | 0.9 | 1.2 | 1.6 |
| Adolescents | 4 | 0.3 | 0.4 | 0.6 | 0.4 | 0.6 | 0.8 | 0.4 | 0.7 | 0.9 |
| Adults | 8 | 0.3 | 0.3 | 0.4 | 0.3 | 0.4 | 0.5 | 0.4 | 0.5 | 0.7 |
| Elderly | 7 | 0.2 | 0.4 | 0.5 | 0.3 | 0.4 | 0.5 | 0.4 | 0.5 | 0.6 |
| Very elderly | 3 | 0.3 | 0.3 | 0.4 | 0.3 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 |
| 95th percentile dietary exposure in vegetarians (μg/kg bw per day) a | ||||||||||
| Adults | 8 | 0.4 | 0.6 | 0.8 | 0.5 | 0.7 | 0.8 | 0.5 | 0.7 | 0.8 |
n: number of surveys; bw: body weight; Min: minimum, Max: maximum.
Not calculated for the other age groups because estimates were only available from one dietary survey.
6.1.2.4. Conclusions
Acute dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across 23 European countries in different age groups, using LB and UB concentrations, ranged from 0.2 μg/kg bw per day in adults and elderly to 2.9 μg/kg bw per day in infants (the range represents minimum LB to the maximum UB from the different countries). The 95th percentile ranged from 0.7 μg/kg bw per day in the elderly and very elderly to 6.7 μg/kg bw per day in infants (the range represents minimum LB to the maximum UB from the different countries).
Infants had the highest estimates of chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (the mean exposure 0.2–2.0 μg/kg bw per day and the 95th percentile 0.7–3.7 μg/kg bw per day). This can be explained by the higher intake of food per kg bw in younger age groups. Similar or for some age groups slightly lower exposure estimates were reported in the previous exposure assessment from earlier years (see Section 6.1.1), but this was probably due to important differences in the assessment methodologies (e.g. data obtained by methods with different LODs/LOQs, different treatment of left‐censored data, use of aggregated consumption data).
The highest contributions to the exposure of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were from grain‐based products across all age groups. Other relevant contributions were found in food for infants and small children, fruit and fruit products in infants and toddlers, and composite foods in adults.
The CONTAM Panel noted that the overall dietary exposure was mainly driven by DON.
The limited consumption data on vegetarians do not indicate a major difference in the acute and chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and 3‐DON‐glucoside between vegetarians and the general population.
6.2. Potential non‐dietary sources of exposure
Grain dust samples have been reported to contain DON, sometimes even more often than the kernels. Inhalation and occupational exposure to DON has been reviewed by IARC (Pitt et al., 2013). Several studies have reported a high prevalence of DON in grain dust samples in the elevator and surfaces of workplace (Nordby et al., 2004; Krysińska‐Traczyk et al., 2007; Mayer et al., 2007). DON levels in raw wheat materials were correlated with levels in corresponding dust samples (Sanders et al., 2013; Sanders et al., 2014). Straumfors et al. (2015) reported higher levels of DON, 3‐Ac‐DON and DON‐3‐glucoside in grain elevator samples than in compound feed mills. A mean inhalation exposure of 20 ng/m3 of fungal metabolites was estimated. A study in French farmers showed that maize acreage of the farmer is a key determinant of DON exposure biomarker level, indicating the occupational exposure route of DON (see Section 7.1.3) (Turner et al., 2010b).
In summary, current evidence from grain dust in grain elevators suggest the possibility of occupational inhalational exposure to DON, 3‐Ac‐DON, 15‐Ac‐DON and metabolites. However, quantitative comparison of oral and inhalation exposure is not possible due to limited data. This route is not relevant for general population.
6.3. Animal exposure assessment
For all species, the LB and UB of the mean (chronic) and 95th percentile dietary concentrations and exposures have been estimated.35 According to EFSA (2011b), caution is needed when calculating the 95th percentile exposure where data on less than 60 samples are available, since the results may not be statistically robust. Therefore estimates of 95th percentile exposure were only made where data on > 60 samples were available. Where the number of samples reported was ≤ 9, the data provided were not used to estimate dietary concentration and exposure.
For many farm animals in Europe, feeds are supplied in the form of commercially produced blends or compound feeds, and for some of the livestock categories considered in this opinion data on the DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside contents of species‐specific compound feeds were provided by the European countries (see Section 4.2.2 and Appendix C, Table C.2). Where these data were provided in sufficient numbers, the mean and 95th percentile dietary concentrations and exposures have been calculated using the concentrations reported.
Table C.2.
Concentrations (μg/kg) of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across feed groups as used in the animal exposure assessment
| Feed group | Concentration (μg/kg)b | |||||||
|---|---|---|---|---|---|---|---|---|
| Na | Mean | 75th percentile | 95th percentile | Maximum | ||||
| LB | UB | LB | UB | LB | UB | UB | ||
| Feed materials | ||||||||
| Barley | 525 | 442 | 527 | 311 | 363 | 2,761 | 2,783 | 10,447 |
| Maize grain | 456 | 844 | 920 | 660 | 663 | 3,335 | 3,335 | 22,954 |
| Oats | 187 | 518 | 571 | 348 | 363 | 2,452 | 2,462 | 9,249 |
| Oat feed | 29 | 304 | 321 | 404 | 404 | 797 | 797 | 1,766 |
| Wheat | 659 | 542 | 588 | 542 | 563 | 2,173 | 2,195 | 16,801 |
| Wheat feed | 74 | 466 | 528 | 607 | 617 | 2,059 | 2,059 | 3,828 |
| Rape seed meal | 10 | 38 | 211 | 73 | 289 | 116 | 289 | 498 |
| Sunflower seed meal | 11 | 0 | 202 | 0 | 289 | 0 | 289 | 498 |
| Soya (bean) meal | 111 | 8 | 289 | 0 | 290 | 0 | 519 | 729 |
| Pressed (sugar) beet pulp | 10 | 156 | 363 | 363 | 363 | 795 | 795 | 795 |
| Forages and roughage, and products derived thereof | ||||||||
| Cereals straw | 124 | 1,722 | 1,819 | 1,090 | 1,108 | 5,541 | 5,541 | 68,418 |
| Grass, field dried, [Hay] | 93 | 13 | 154 | 0 | 200 | 73 | 200 | 359 |
| Maize silage | 129 | 588 | 624 | 663 | 681 | 1943 | 1,943 | 6,235 |
| Complete/complementary feeds | ||||||||
| Dairy cows | 96 | 191 | 248 | 190 | 195 | 1,148 | 1,148 | 1,885 |
| Fattening cattle | 42 | 377 | 474 | 203 | 363 | 725 | 725 | 10,658 |
| Horses | 15 | 273 | 292 | 378 | 378 | 1,432 | 1,432 | 1,275 |
| Weaning pigs | 193 | 213 | 361 | 336 | 391 | 858 | 957 | 3,944 |
| Growing/fattening pigs | 229 | 385 | 453 | 536 | 537 | 1,130 | 1,130 | 3,799 |
| Breeding pigs | 185 | 453 | 544 | 615 | 637 | 1,146 | 1,146 | 5,757 |
| Laying hens | 89 | 698 | 716 | 1,009 | 1,009 | 2,973 | 2,973 | 4,220 |
| Fattening chickens | 496 | 894 | 909 | 1,404 | 1,404 | 2,900 | 2,900 | 7,540 |
| Fattening ducks | 97 | 905 | 918 | 1,552 | 1,552 | 2,552 | 2,552 | 3,393 |
| Fattening turkeys | 101 | 1,302 | 1,315 | 2,074 | 2,074 | 3,495 | 3,495 | 4,988 |
DON: deoxynivalenol; Ac: acetyl; N: number of samples; LC: left‐censored data (values below the limit of detection or limit of quantification); LB: lower bound; UB: upper bound.
If N < 60 then the calculated 95th percentile concentration should be considered as an indicative value only due to the limited number of data (EFSA, 2011b).
Concentration reported as μg/kg 88% dry matter.
For those farm animal categories for which insufficient data were provided (i.e. for less than 10 samples), the CONTAM Panel identified example diets and feed inclusion rates (see Appendix E for details), and used concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in individual feed materials (Appendix C, Table C.2) to estimate the mean and 95th percentile exposure. Again, where concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were derived from < 60 samples, these have not been used to estimate dietary concentration and exposure.
As previously reported (Section 5), a wide range of feeds and feeding systems are used for livestock in Europe. It must be stressed that the feed intakes or diet compositions used in estimating dietary concentrations and exposures in this scientific opinion are not ‘average’ diets, nor are they an attempt to describe ‘worst case’ scenarios. Rather, they are intended to provide an indication of likely dietary concentration and exposure to DON alone (Appendix F, Section F.4) and to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across a range of feeding systems in Europe. In some situations, dietary concentration and exposure may be higher than described in this opinion.
The CONTAM Panel also estimated the animal dietary concentrations and exposures to DON alone (see Appendix F, Section F.4). The %‐contribution of DON alone (mean and 95th percentile concentrations/exposures) to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside at both the LB and UB levels and for farm and companion animals (across all species) was calculated. The mean value for this contribution was 68% (SD = 7.4%) for all the animal species, both for dietary concentrations/exposures based on species‐specific compound feeds and on the individual feed materials and their relative proportions in diets.
For ruminants and horses, forages are a major component of the diet, and in much of Europe fresh or conserved grass (conserved as silage or hay) is the main or sole feed. As shown in Tables 18–21, no data on levels of DON, 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside were available for fresh grass or grass silage. Furthermore, other authors (Driehuis et al., 2008a; Rodrigues et al., 2012) have reported that they are not normally present in these feeds. Therefore it has been assumed that where fresh grass and/or grass silage are the sole forages, that they make no contribution to dietary concentration and exposure. In other feeding systems, grass hay is the main or sole forage, and therefore data for these Fusarium toxins in grass hay have been used to estimate dietary concentration and exposure for this feeding regime.
The dietary concentrations and exposures of farm and companion animals to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are presented below
6.3.1. Estimates of animal exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
6.3.1.1. Ruminants and horses
Mean (chronic) and 95th percentile dietary concentration and exposure estimates for high yielding dairy cows, fattening beef cattle and horses to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were derived from data on species‐specific compound feed and are presented in Table 40.
Table 40.
Estimated mean and 95th percentile dietary concentration and exposure at the LB and UB to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for dairy cows, fattening beef cattle and horses derived from concentrations in species‐specific compound feeds
| Dietary concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentile | Mean | 95th percentile | Mean | 95th percentile | ||
| Dairy cow high yieldinga | LB | 86.6 | 522 | 1,793 | 10,805 | 2.76 | 16.6 |
| UB | 113 | 522 | 2,337 | 10,805 | 3.60 | 16.6 | |
| Dairy cow high yieldingb | LB | 95.6 | 571 | 1,979 | 11,829 | 3.04 | 18.2 |
| UB | 218 | 658 | 4,510 | 13,629 | 6.94 | 21.0 | |
| Fattening beef cattlea | LB | 64.2 | 124 | 617 | 1,186 | 1.54 | 2.97 |
| UB | 80.8 | 124 | 776 | 1,186 | 1.94 | 2.97 | |
| Fattening beef cattleb | LB | 77.0 | 194 | 739 | 1,859 | 1.85 | 4.65 |
| UB | 230 | 317 | 2,203 | 3,041 | 5.51 | 7.60 | |
| Horsesa | LB | 155 | c | 1,397 | c | 3.11 | c |
| UB | 166 | c | 1,493 | c | 3.32 | c | |
| Horsesb | LB | 163 | c | 1,465 | c | 3.26 | c |
| UB | 253 | c | 2,280 | c | 5.07 | c | |
bw: body weight, LB: lower bound; UB: upper bound.
Fresh grass and/or grass silage‐based diets (i.e. assumes no exposure from forages).
Grass hay‐based diets.
Insufficient number of samples reported (i.e. < 60) to calculate 95th percentile concentration.
As described above, there is a wide range of feeding systems fed different forages but there were insufficient data on compound feeds used in these systems to permit this approach. Therefore, example rations and concentrations in individual feed materials (see Appendix E, Table E.5) were used to estimate dietary concentration and exposure. The livestock categories chosen were dairy cows (maize silage‐based rations), fattening beef cattle (diets where the major ingredient was maize silage, cereals or straw) and sheep, goats and horses (fed grass hay‐based diets). Estimates of dietary concentrations and exposures for these are given in Table 41.
Table 41.
Estimated mean and 95th percentile dietary concentration and exposure at the LB and UB to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside by ruminants derived from concentrations in individual feed materials and their relative proportions in diets
| Dietary concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentile | Mean | 95th percentile | Mean | 95th percentile | ||
| Lactating dairy cows: maize silage‐based diet | LB | 703 | 2,562 | 19,188 | 69,950 | 29.5 | 107 |
| UB | 783 | 2,574 | 21,370 | 70,276 | 32.9 | 108 | |
| Fattening beef cattle: intensive cereal‐based diet | LB | 290 | 1,756 | 2,902 | 17,561 | 7.26 | 43.9 |
| UB | 391 | 1,816 | 3,908 | 18,164 | 9.77 | 45.4 | |
| Fattening beef cattle: maize silage‐based diet | LB | 506 | 1,669 | 3,341 | 11,017 | 11.1 | 36.7 |
| UB | 581 | 1,714 | 3,833 | 11,312 | 12.8 | 37.7 | |
| Fattening beef cattle: cereal straw‐based diet | LB | 878 | 3,608 | 7,022 | 28,866 | 23.4 | 96.2 |
| UB | 996 | 3,655 | 7,966 | 29,239 | 26.5 | 97.5 | |
| Lactating sheepa | LB | 151 | 749 | 421 | 2,098 | 5.27 | 35.0 |
| UB | 285 | 849 | 797 | 2,376 | 9.96 | 39.6 | |
| Lactating goatsa | LB | 298 | 561 | 1,013 | 1,906 | 16.9 | 31.8 |
| UB | 414 | 613 | 1,407 | 2,084 | 23.4 | 34.7 | |
| Fattening goatsa | LB | 168 | 653 | 252 | 979 | 6.31 | 24.5 |
| UB | 305 | 747 | 457 | 1,120 | 11.4 | 28.0 | |
bw: body weight; LB: lower bound; UB: upper bound.
Note that these concentrations/exposures assume that grass hay is the sole forge. Where fresh grass or grass silage are the main or sole forage concentration/exposure are likely to be lower.
The dietary concentrations and exposures by ruminants reported in Table 41 are generally higher than those estimated for high yielding dairy cows derived from data for compound feeds (Table 40). In the latter it was assumed that the forages contributed little (hay‐based diet) or no (grass or grass silage‐based diet) to dietary concentration and exposure, since levels of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are not reported in grass and are markedly lower in grass hay than in cereals, maize silage and cereal straw (Tables 18–21).
6.3.1.2. Pigs and poultry
Estimates of mean and 95th percentile dietary concentration and exposure by pigs and poultry to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were derived from data for species‐specific compound feeds, and are given in Table 42.
Table 42.
Estimates of mean and 95th percentile dietary concentration and exposure at the LB and UB to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for pigs and poultry (fattening chickens, laying hens, fattening turkeys and fattening ducks) derived from concentrations in species‐specific compound feeds
| Dietary concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentile | Mean | 95th percentile | Mean | 95th percentile | ||
| Pig starter | LB | 242 | 975 | 242 | 975 | 12.1 | 48.8 |
| UB | 410 | 1,088 | 410 | 1,088 | 20.5 | 54.4 | |
| Pig finisher | LB | 437 | 1,284 | 1,312 | 3,851 | 13.1 | 38.5 |
| UB | 515 | 1,284 | 1,546 | 3,851 | 15.5 | 38.5 | |
| Lactating sow | LB | 515 | 1,302 | 3,088 | 7,810 | 15.4 | 39.1 |
| UB | 618 | 1,302 | 3,709 | 7,810 | 18.5 | 39.1 | |
| Fattening chickens | LB | 1,016 | 3,295 | 121.9 | 395 | 61.0 | 198 |
| UB | 1,033 | 3,295 | 124.0 | 395 | 62.0 | 198 | |
| Laying hens | LB | 794 | 3,378 | 95.2 | 405 | 47.6 | 203 |
| UB | 814 | 3,378 | 97.6 | 405 | 48.8 | 203 | |
| Fattening turkeys | LB | 1,480 | 3,971 | 592 | 1,588 | 49.3 | 132 |
| UB | 1,494 | 3,971 | 598 | 1,588 | 49.8 | 132 | |
| Fattening ducks | LB | 1,029 | 2,900 | 144 | 406 | 48.0 | 135 |
| UB | 1,043 | 2,900 | 146 | 406 | 48.7 | 135 | |
bw: body weight; LB: lower bound; UB: upper bound.
6.3.1.3. Farmed fish (salmonids and carp), farmed rabbits and farmed mink
In the absence of reliable data on concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in species‐specific compound feeds, estimates of dietary concentration and exposure were made by using example rations and concentrations in individual feed materials (see Appendix E, Table E.5) for details of rations used and are reported in Table 43.
Table 43.
Estimated mean and 95th percentile dietary concentration and exposure at the LB and UB to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for farmed rabbits, farmed fish and farmed mink derived from concentrations in individual feed materials and their relative proportions in diets
| Dietary concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentile | Mean | 95th percentile | Mean | 95th percentile | ||
| Salmonids | LB | 83.3 | 362 | 3.33 | 14.5 | 1.67 | 7.25 |
| UB | 123 | 380 | 4.93 | 15.2 | 2.47 | 7.60 | |
| Carp | LB | 254 | 1,084 | 5.60 | 23.8 | 5.60 | 5.60 |
| UB | 388 | 1,152 | 8.53 | 25.3 | 8.53 | 8.53 | |
| Farmed rabbits | LB | 196 | 1,048 | 29.5 | 157 | 14.7 | 78.6 |
| UB | 282 | 1,135 | 42.2 | 170 | 21.1 | 85.1 | |
| Farmed mink | LB | 99.5 | 407 | 7.46 | 30.52 | 3.61 | 14.7 |
| UB | 109 | 409 | 8.16 | 30.65 | 3.94 | 14.8 | |
bw: body weight; LB: lower bound; UB: upper bound.
6.3.1.4. Companion animals (dogs and cats)
Few data on concentrations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in proprietary feeds for dogs and cats were available, and therefore dietary concentration and exposure were estimated using example rations (see Appendix E, Table E.5 for details) and concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in individual feed materials. The dietary concentrations and exposures are reported in Table 44.
Table 44.
Estimated mean and 95th percentile dietary concentration and exposure at the LB and UB to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside by companion animals (dogs and cats) derived from concentrations in individual feed materials and their relative proportions in diets
| Dietary concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentile | Mean | 95th percentile | Mean | 95th percentile | ||
| Dogs | LB | 174 | 741 | 10.5 | 44.5 | 2.62 | 11.1 |
| UB | 214 | 753 | 12.8 | 45.2 | 3.21 | 11.3 | |
| Cats | LB | 229 | 968 | 82.3 | 348 | 3.29 | 13.9 |
| UB | 264 | 975 | 95.1 | 351 | 3.80 | 14.0 | |
bw: body weight; LB: lower bound; UB: upper bound.
6.3.2. Conclusions
In the absence of data on levels of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in fresh grass or grass silage, data for grass hay have been used in estimating the contribution from forages for lactating dairy cows and beef cattle. As discussed above, this approach may have overestimated dietary concentration and exposure. The estimated dietary concentration and exposure estimates were generally highest for beef and dairy cattle fed diets in which the forages were maize silage or cereal straw, or for fattening beef cattle on cereal grain‐based diets.
Cereal grains are widely used in the diets of pigs and poultry, and in commercially manufactured compound feeds they may represent 60% or more of the total ingredients. However, levels of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are markedly lower in compound feeds for pigs than they are for poultry, and this is reflected in lower estimates of dietary concentration and exposure for these species.
While estimates of dietary concentration and exposure of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for fattening chickens (broilers) were possible using data for compound feeds, there was insufficient information on compound feeds for laying hens, and therefore the alternative approach of estimating dietary concentration and exposure, i.e. using example rations and concentrations of these toxins in individual feed materials was used.
7. Hazard identification and characterisation
This scientific opinion on the risks for human and animal health related to the presence of DON, its acetylated and modified forms in food and feed updates the previous risk assessments of the SCF for DON in food (SCF, 1999) and of the EFSA for DON as undesirable substance in animal feed (EFSA, 2004). While the SCF opinion on DON established a TDI of 1 μg/kg bw per day for humans chronically exposed to DON in food, the EFSA opinion addressed the toxicity of DON and its impact on animal health and also its transfer into animal products. Since these two previous risk assessments on DON reviewed the available data on DON at these times, the current opinion, and in particular this section on the identification and characterisation of the hazard of DON will focus on the description and analysis of the more recent data available since 1999 and 2004 for humans and animals, respectively, except for studies considered of importance for the current risk assessment and which need to be reported in detail even if they have already been discussed in previous opinions. Regarding hazards from the 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed, which were not assessed by the SCF (1999) or EFSA (2004), all available data will be assessed in the next sections of this chapter.
If not otherwise stated quantitative results reported as number ± number are (arithmetic) means plus/minus the standard deviation (SD).
7.1. Toxicokinetics
The toxicokinetics of DON have been reviewed by JECFA (FAO/WHO, 2001, 2011), Pestka and Smolinski (2005), Pestka (2007, 2010a,b), Sobrova et al. (2010), Maresca (2013) and Wu et al. (2014a). While Section 7.1.1 considers primarily ex vivo studies, Section 7.2.1 reports the more recently published in vivo studies.
7.1.1. In vitro studies in experimental animals
7.1.1.1. DON
The role of gut organisms in the metabolism of DON has been studied in vitro using incubations of DON with faeces or gut content from different animal species. The faecal microbiota from several species, including rats, poultry and pigs have been shown to be able to transform DON to DOM‐1 (Swanson et al., 1988; Worrel et al., 1989; He et al., 1992; FAO/WHO 2001, 2011). No phase‐I metabolism was found in incubations with rat liver microsomes (Côté et al., 1997).
In a perfused rat liver from a male Wistar rat, 3 mg DON was added to recirculating perfusion system and samples of bile, perfusate and liver homogenates collected during the 90 min of infusion were analysed for DON with and without the addition of β‐glucuronidase. A total of 40.4% of the added DON was glucuronide‐conjugated. Of the administered DON, 20.4% was found as glucuronide conjugate in the perfusate, 19.2% in the bile and 0.8% in the liver homogenate. Only 1.3% of the total DON was recovered as free DON (1.1% in the perfusate and 0.2% in the bile). The DON glucuronide conjugates in the bile were cleaved when incubated with intestinal content under anaerobic conditions (Gareis et al., 1987). However, the total recovery of the administered dose was below 50%, which according to the authors indicated that unknown metabolites may have been formed or that the remaining fraction may be bound in the liver.
After incubations with liver microsomes from rat, carp, pigs and cows DON was glucuronidated (Maul et al., 2012, 2014; Uhlig et al., 2013). DON‐3‐glucuronide was the dominant glucuronide in microsomal incubations, accompanied by smaller amounts of DON‐15‐glucuronide. In addition, small amounts of a third glucuronide, tentatively assigned as DON‐7‐glucuronide, was present in incubations with carp and rat liver microsomes (Maul et al., 2012) (see also Section 7.1.4.1), while nuclear magnetic resonance studies of a glucuronide formed in incubations with rat microsomes revealed that this was DON‐8‐glucuronide (Uhlig et al., 1990).
7.1.1.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.1.1.3. DON‐3‐glucoside
No data were identified.
7.1.2. In vivo studies in rodents
7.1.2.1. Absorption
7.1.2.1.1. DON
Mice
The toxicokinetics of DON were compared between weanling female mice (3–4 weeks old) and young adult female mice (8–10 weeks old, n ≥ 8/group) given a single oral dose of 5 mg/kg bw and killed after 15, 30, 60 and 120 min. The DON concentrations were measured by ELISA in plasma, spleen, kidney, liver and lung. Maximum plasma concentrations were measured 15–30 min after administration. The maximum concentrations were approximately 1.0 μg/mL in young adults and twice that in weanling mice, indicating that the absorption is higher in very young animals (Pestka and Amuzie, 2008).
A 4‐week feeding study in male mice given 2–20 mg DON/kg diet demonstrated a dose‐dependent increase in plasma concentrations of DON equivalents ranging from 20 ng/mL to 90 ng/mL approximately as measured by Pestka et al. (2008).
Rats
Male Sprague–Dawley rats were given a single oral dose of 5.0 mg radiolabelled DON/kg bw, and plasma samples were collected at 8, 24 and 72 h after dosing. The maximum measured plasma radioactivity occurred after 8 h, at which time approximately 9% was bound to plasma protein. The maximum concentration was 291 ng/mL and decreased to approximately 14% of that level in 24 h post‐dosing. A total of 37% of the administered radioactivity was excreted in the urine and DON‐glucuronides were the major urinary metabolites (Meky et al., 2003). The CONTAM Panel noted that in this study, as well as in many other studies, the authors did not identify the forms of DON‐glucuronides.
Wistar rats (n = 3 males and 3 females/group) were given a single oral dose of 0.5 or 2.5 mg radiolabelled DON/kg bw (Wan et al., 2014). The urinary recovery during a collection period of 96 h in female rats was 57.1 ± 1.2% for the 0.5 mg/kg bw group and 42.3 ± 3.1% in the group given 2.5 mg/kg bw For males, the urinary recovery rates for DON were 27.3 ± 0.7% in the low dose and 24.0 ± 2.3% in the high–dose group. According to the authors, this indicated that the bioavailability may be higher in female than in male rats.
Male Sprague–Dawley rats (n = 6) were given a single oral dose of 2.0 mg DON/kg bw by gavage on days 1, 8 and 15. Urine and faeces were collected after 48 h and samples were analysed for DON, DON‐3‐glucuronide and DOM‐1. In addition, an enzymatic deconjugation was performed to check for other potential DOM‐1 glucuronides. Only 17% of the administered DON was recovered as DON and its metabolites in the urine, indicating a limited oral bioavailability. However, the total recovery was low (28% in total for urine and faeces after 48 h) and unknown metabolites may have been formed (Nagl et al., 2012). Later, the samples from this experiment were reanalysed with a new analytical method (described in the study by Wan et al. (2014) (see above) which could detect additional metabolites (Schwartz‐Zimmermann et al., 2014). When these additional metabolites (see also Section 7.1.2.3.1) were included, the total recovery of DON increased to 75% of the administered dose. Most of the administered DON was recovered in faeces and only a small portion (11%) in urine.
7.1.2.1.2. 3‐Ac‐DON and 15‐Ac‐DON
Rats
The only report identified for rats is a limited study using two Wistar rats (weight 330 and 368 g) which indicated that the acetylated forms are transformed to DON prior to absorption. Each rat was given a single oral dose of a mixture of 25 μg of both 3‐Ac‐DON and 15‐Ac‐DON and sacrificed 55 min after exposure. Different tissues in each rat were analysed for 3‐Ac‐DON and 15‐Ac‐DON, DON, DON‐3‐glucuronide, 3‐Ac‐DON‐glucuronide and 15‐Ac‐DON‐glucuronide (Versilovskis et al., 2012). Absolute amounts of DON (302 ng) and DON‐3‐glucuronide (1,240 ng) were measured in the liver of one rat, while DON (386 ng), DON‐3‐glucuronide (586 ng) and 15‐Ac‐DON‐3‐glucuronide (89 ng) were measured in the bladder of the other rat, indicating that DON and/or the acetylated forms of DON had been absorbed, but the bioavailability could not be quantified.
7.1.2.1.3. DON‐3‐glucoside
Nagl et al. (2012) (see also Section 7.1.2.1.1 for the part of the study on DON) compared the absorption of DON‐3‐glucoside with that of DON in male Sprague–Dawley rats given a single oral equimolar dose of 3.1 mg DON‐3‐glucoside/kg bw (equimolar 2.0 mg DON/kg bw) using the same protocol. DON‐3‐glucoside was found in urine, but the urinary recovery was low and only 6.9 ± 2.2 nmol was recovered in the urine 24 h after exposure, while 26 ± 4.9 nmol was recovered as DON, 24 ± 5.0 nmol as DON‐glucuronides and 15 ± 7.3 nmol as DOM‐1. However, including all analysed metabolites, only 4% of the administered DON‐3 glucoside was recovered in the urine compared to 17% of the DON in rats given a similar dose of DON. The recovery of administered DON‐3‐glucoside from faeces was 17%. The authors concluded that the bioavailability of DON‐3‐glucoside was lower than that of DON. When these samples were reanalysed by Schwartz‐Zimmermann et al. (2014) (see Section 7.1.2.1.1), only traces of the sulfonate conjugates were found in the urine, and even when the additional metabolites were included, only 5% of the administered DON‐3‐glucoside was recovered as metabolites in urine, supporting the assumption that the oral bioavailability of DON‐3‐glucoside is low. With the new metabolites included, the recovery from faeces increased to 63% of the administered DON‐3‐glucoside.
7.1.2.2. Distribution
7.1.2.2.1. DON
Mice
Male B6C3F1 mice 10–12 weeks of age (n = 3/group) were given a single oral dose of 5 or 25 mg radiolabelled DON/kg bw Maximum DON concentrations measured as radioactivity, were found 30 min after administration in spleen, Peyer's patches, kidney, liver and large intestine and plasma and after 1 h in the small intestine. The highest concentrations were found in the liver (radiolabelled compounds equivalent to 4.9 and 18.4 pmol/mg wet weight in the 5 and 25 mg/kg dose groups), kidney (equivalent to 5.6 and 19.8 pmol/mg wet weight, respectively) and plasma (equivalent of 4.4 and 22.3 pmol/mg wet weight, respectively). The concentrations decreased rapidly after 1 h following a two compartment kinetics in all tissues and at both doses (Azcona‐Olivera et al., 1995).
Male B6C3F1 mice (n = 3 per group and 8 weeks old) were given a single oral dose of 25 mg DON/kg bw, and tissue and plasma samples were taken at several time points from 5 min to 24 h after administration. All samples were analysed by an ELISA kit, which according to the producer binds to DON and 3‐Ac‐DON and no other trichothecenes. The recovery of this ELISA in spiked liver samples was 90% or more. The cross reactivity in this ELISA to potential DON metabolites was not reported and the results are reported as DON equivalents per mL plasma or per g tissue. The highest plasma concentrations (12.1 and 11.5 μg DON/mL plasma) were observed at the two‐first sampling times, 5 and 15 min after exposure. The plasma concentrations decreased rapidly thereafter, following two compartment kinetics with a distribution half‐life (t1/2α) of 20.4 min and a clearance half‐life (t1/2β) of 11.8 h. DON was rapidly distributed to all tissues, reaching a maximum tissue concentration 5–15 min after exposure. The concentration decreased rapidly thereafter. A similar pattern, with the highest concentrations observed 5–15 min after the oral administration of DON, followed by a rapid decrease, was observed in liver, kidney, spleen and heart. The maximum DON concentrations were 19.6 μg/g in liver, 9.0 μg/g in kidney, 7.9 μg/g in the spleen, and 6.8 μg/g in the heart. The half‐lives in tissues were in the same range as for plasma (t1/2α = 22–41 min, t1/2β = 9–21 h). Compared to the other examined tissues, DON entered to the brain slower and peaked at lower concentrations, 0.7–1.0 μg/g, lasting from 5 min to 2 h (Pestka et al., 2008).
Using the same analytical method, Pestka and Amuzie (2008) (see Section 7.1.2.1.1) measured DON concentrations in plasma, spleen, kidney, liver and lung of weaning and young adult female mice for up to 2 h after treatment and observed the same kinetics of DON in all five organs. The highest tissue concentrations were found in kidney where approximately 2 μg DON/g after 15 min was measured in adults. The maximum concentrations in the other investigated tissues were approximately half of that. The tissue concentrations were approximately twice as high in weanling mice compared to the young adults. The tissue concentrations decreased rapidly and were reduced by 78% and 81% within 2 h in weanling and young adults, respectively.
In female mice, the tissue distribution was compared between oral or nasal exposure following a single dose of 5 mg DON/kg bw (Amuzie et al., 2008). Independent of exposure route, the concentrations in plasma, spleen, liver, lung and kidney were maximal after 15–30 min and they declined thereafter by 75–90% in 120 min. The maximum plasma concentrations were approximately 1 μg/mL after oral and approximately 3 μg/mL after nasal exposure. The peak DON concentrations in spleen, liver, lung and kidney were 0.77, 1.10, 0.95 and 1.76 μg/g tissue after oral exposure, and 1.87, 2.37, 2.20 and 3.37 μg/g after nasal exposure, respectively.
The tissue concentrations, measured as DON‐equivalents by an ELISA kit, was higher in aged mice (22 months) than in adult mice (3 months) 12 h after an i.p. injection of 1 mg DON/kg bw The findings indicated an age‐difference in distribution and elimination (Clark et al., 2015). When the mice were given either 2.5 or 10 mg DON/kg diet for 2 weeks, a non‐significant tendency towards lower tissue concentrations in the aged mice compared to adult mice was observed. However, since the mice were fed ad libitum, the reduced tissue concentrations might also result from a tendency towards reduced feed consumption in the elderly rather than reflecting an age‐dependent kinetic.
Rats
No detectable radioactivity was retained in any tissue in rats 96 h after a single oral dose of 10 mg radiolabelled DON/kg bw (Lake et al., 1987).
Female and male Wistar rats (n = 15/gender) were given one daily dose of radioactively labelled DON at either 0.5 or 2.5 mg DON/kg bw per day for 5 days and sacrificed at 6, 24, 48, 72 and 96 h after last dosing (Wan et al., 2014) (see also Section 7.1.2.1.1). Total DON (DON and metabolites measured as radioactively labelled substance) was rapidly distributed to all examined tissues, i.e. plasma, muscle, adipose tissue, gastrointestinal tract, bile, liver, kidney, heart, brain, lung, skin, spleen, brain, testes, ovary and adrenals. The highest concentrations were found at the first sampling, 6 h after the last dosing, in the large intestine (5,226 μg DON/kg), followed by stomach (1,795.9 μg DON/kg), small intestine (1,473.7 μg DON/kg), liver (776.6 μg DON/kg), kidney (418.7 μg DON/kg), lung (254 μg DON/kg), spleen 235.4 μg DON/kg), heart (211.3 μg DON/kg), testes (218.4 μg DON/kg), skin (194.0 μg DON/kg), kidney (172.9 μg DON/kg), ovary (171.1 μg DON/kg), plasma (160.4 μg DON/kg), muscle (132.0 μg DON/kg), brain (100.0 μg DON/kg) and adipose tissue (50.1 μg DON/kg). The concentrations thereafter decreased steadily at the following sampling times of 24, 48, 72 and 96 h after the last treatment.
7.1.2.2.2. 3‐Ac‐DON and 15‐Ac‐DON
Rats
In the study by Versilovskis et al. (2012) (see details in Section 7.1.2.1.2) on two rats, 3‐Ac‐DON and 15‐Ac‐DON could not be detected in the liver, but 3‐Ac‐DON‐7‐glucuronide, 3‐Ac‐DON‐15‐glucuronide and 15‐Ac‐DON‐3‐glucuronide were detected at levels below the LOQ. In addition, DON (386 ng), DON‐3‐glucuronide (5,041 ng) and 15‐Ac‐DON‐3‐glucuronide (89 ng) were found in the bladder of the other rat. These findings indicate that these acetylated forms are mainly transformed to DON prior to absorption.
7.1.2.2.3. DON‐3‐glucoside
Rats
Two male Wistar rats (each of 275 g) were given each a single oral dose of a mixture of 25 μg unlabelled DON‐3‐glucoside, unlabelled zearalenone‐glucoside, 13C‐DON and 13C‐zearalenone (Versilovskis et al., 2012). The animals were sacrificed 55 min after exposure. The DON‐3‐glucoside was recovered mainly in the gastrointestinal tract but 2% of the DON‐3‐glucoside was recovered as DON in the stomach. No metabolite of DON‐3‐glucoside was found in spleen and lungs and only traces in the kidneys (< 0.1% of the administered dose). The total recovery of DON‐3‐glucoside was below 60%.
7.1.2.3. Metabolism
7.1.2.3.1. DON
Mice
Since the available studies of the toxicokinetics in mice have used ELISA methods or focussed only on radioactivity in the case of radiolabelled DON, no information on the potential metabolites of DON is available in mice.
Rats
De‐epoxidation and glucuronide conjugation have been considered to be the main metabolic transformations of DON in rats. However, recent studies have shown that sulfonate conjugation is another important metabolic pathway (Schwartz‐Zimmermann et al., 2014; Wan et al., 2014).
The de‐epoxy metabolite DOM‐1 was identified as a metabolite in rat urine already in 1983 (Yoshizawa et al., 1983). DOM‐1 was also found in faeces and urine of rats after a single oral dose of 10 mg DON/kg bw (Worrell et al., 1989). The DOM‐1 was not found in rats treated with antibiotics prior to administration of DON. Furthermore, incubations of DON with gut content resulted in the formation of DOM‐1, while no such transformation was found in incubations with liver homogenate. The authors concluded that the appearance of DOM‐1 in urine and faeces of rats was a result of gut bacterial metabolism.
Male Wistar rats (n = 8) were given feed containing an extract of F. graminearum with a final DON concentration of 33.9 mg/kg feed for 4 days, with a mean DON intake of 3.57 mg/kg bw per day. The urine contained 1.9–4.9 μg/mL DON and 1.6–5.9 μg/mL DOM‐1. In addition, two unidentified DON‐glucuronide metabolites and one unidentified DOM‐1 glucuronide were present but not quantified (Lattanzio et al., 2011).
Wan et al. (2014) gave rats a single dose of either 0.5 or 2.5 mg radioactively labelled DON/kg bw (see Section 7.1.2.1.1) and found the highest concentrations of DON in urine and faeces, followed by an unidentified metabolite and DOM‐1. In addition, DON‐3‐sulfonate and DOM‐3‐sulfonate were detected only in faeces (< 10% of the total radioactivity) while DON‐3‐glucuronide was only detected in the urine (2% of the total radioactivity). DON‐3‐sulfonate and DOM‐1‐sulfonate were only detected in the faeces 24 h after the administration.
In the male Sprague–Dawley rats given a single oral dose of 2.0 mg DON/kg bw (Nagl et al., 2012) (described in Section 7.1.2.1.1), most of DON was recovered from faeces in the forms of DON and DOM‐1, but the total recovery was low (28%), indicating that other metabolites may be formed. The reanalysis by Schwartz‐Zimmermann et al. (2014) (see Section 7.1.2.1.1) identified three different sulfonate conjugates of DON and two forms of DOM‐1 sulfonate conjugates. Only traces of sulfonate conjugates were present in urine (< 1% of the administered dose), while 48% of the administered DON was recovered as sulfonate conjugated forms of DON and DOM‐1 in the faeces within 48 h.
The presence of DOM‐1‐glucuronide was reported in the male Sprague–Dawley rats of the study of Meky et al. (2003) (see Section 7.1.2.2.1) given 5 mg radioactively labelled DON/kg bw by gavage. In this study, a main peak containing approximately 80% of the total urinary radioactivity and a minor peak coeluting with DON‐labelled standard containing approximately 8% were found in rat urine. Incubation with sulfatase did not alter this pattern, but incubation with glucuronidase decreased the main peak to approximately 30% of the total radioactivity while the second peak increased to approximately 43% of the radioactivity. In addition, a minor peak containing 9% of the radioactivity appeared after the deconjugation. The authors indicated that this peak would correspond to DOM‐1, but this was not verified in the study. They concluded that DON‐glucuronide was the main metabolite in rat urine followed by DON and that a third metabolite, assumed to be DOM‐1‐glucuronide, was present. DON glucuronides were determined by enzymatic deconjugation but the different glucuronides in the urine were not specified.
7.1.2.3.2. 3‐Ac‐DON and 15‐Ac‐DON
Rats
In the two Wistar rats receiving a mixture of 25 μg of both 3‐Ac‐DON and 15‐Ac‐DON by gavage (Versilovskis et al., 2012) (see Section 7.1.2.1.2), both 3‐Ac‐DON and 15‐Ac‐DON, DON and their glucuronides were detected in the content of the gastrointestinal tract. DON was the most prevalent form in the stomach, indicating that a hydrolysation of the acetylated forms releasing DON occurs in the stomach. 3‐Ac‐DON disappeared slower than 15‐Ac‐DON. No de‐epoxide was detected, possibly due to the short exposure time. 3‐Ac‐DON was not detected in liver or bladder, but a low level of 15‐Ac‐DON‐3‐glucuronide (89 ng compared to 386 ng DON and 504 ng DON‐3‐glucuronide) was found in the bladder.
7.1.2.3.3. DON‐3‐glucoside
Rats
In the other two male Wistar rats given a single oral dose of a mixture of 25 μg of DON‐3‐glucoside (Versilovskis et al., 2012) (see Section 7.1.2.2.3), the DON‐3‐glucoside was recovered mainly in the gastrointestinal tract as DON‐3‐glucoside (42–56% of the administered dose), but 2% of the DON‐3‐glucoside was recovered as DON in the stomach. The total recovery of DON‐3‐ glucoside was below 60%.
Urine and faeces were collected for 48 h after each single oral dosing and were analysed for DON, DON‐3‐glucuronide and DOM‐1 in the male Sprague–Dawley rats in the study of Nagl et al. (2012) (see Section 7.1.2.1.3). In addition, an enzymatic deconjugation was performed to check for other potential DOM‐1 glucuronides. Most of the recovered DON‐3‐glucoside metabolites were from faeces in the forms of DON (17%) and DOM‐1, but the total recovery was low 20.9 ± 6.6% indicating that other metabolites might have been formed. The reanalysis by Schwartz‐Zimmermann et al. (2014) (see Section 7.1.2.1.3) showed the presence of three different sulfonate conjugates of DON (DONS 1–3) and two forms of DOM‐1 sulfonate conjugates. Only traces of sulfonate conjugates were present in urine (< 1% of the administered dose), while 47% of the administered DON‐3‐glucoside was recovered as sulfonate conjugated forms of DON, DOM and DON‐3‐glucoside.
7.1.2.4. Excretion
7.1.2.4.1. DON
Mice
The plasma elimination rate in mice given one single oral dose follows two‐compartment kinetics with t1/2α of 0.29–0.5 h and a t1/2β of 7.6–11.8 h (Azcona‐Olivera et al., 1995; Amuzie et al., 2008; Pestka et al., 2008). A slower secondary elimination rate was observed in mice given a high dose (25 mg DON/kg bw) compared to a lower dose (5 mg DON/kg bw) (Azcona‐Olivera et al., 1995). This was considered to be due to a minor second plasma peak concentration occurring after 4–8 h in the high‐dose group.
Rats
Following a single oral dose of 10 mg radioactively labelled DON/kg bw, 25 and 64% of the compound was recovered in urine and faeces within 96 h, with no apparent radioactivity retained in the tissues (Lake et al., 1987).
DON was rapidly excreted from Wistar rats in the study of Wan et al. (2014) described above (Section 7.1.2.1.1) in which 73–77% of the radioactive dose was recovered in urine and faeces within 48 h. By 96 h, the total urinary and faecal recovery were accumulated to 83–88%. The urinary excretion of the radioactivity was 40% in the low dose and 33% in the high dose. There was a significant difference in the urinary excretion rate between gender, when the females excreted with 57% and 42% and the males only 27% and 24% at the low and high dose, respectively.
In the male Sprague–Dawley rats given a single oral dose of 2.0 mg DON/kg bw in the study of Nagl et al. (2012) (see Section 7.1.2.1.1), 15% of the applied dose was recovered in the urine and 13% in the faeces with a total recovery of only 28%. When sulfonate conjugates were included in the analytical method in the re‐analysis of Schwartz‐Zimmermann et al. (2014) (see Section 7.1.2.1.1) the total recovery of DON related metabolites was 64% in the faeces and 11% in the urine. The findings indicate that a large proportion is excreted as faecal metabolites.
7.1.2.4.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.1.2.4.3. DON‐3‐glucoside
Only 17% of the administered DON‐3‐glucoside was recovered in faeces within 48 h in male in the Sprague–Dawley rats, while only 4% was recovered from urine (Nagl et al., 2012) (see Section 7.1.2.1.3). The total recovery was only 21%. The re‐analysis of these samples when the additional sulfonate conjugates were detected (see Section 7.1.2.1.3) showed a total recovery of metabolites of DON‐3‐glucoside from faeces of 44% during the first 24 h and another 19% between 24 and 48 h after dosing. Only small amounts of sulfonate conjugates (0.8%) were recovered from the urine and the total recovery from urine was 4.5%. The total recovery of DON increased from 21% to 68% in the second 24‐h period, indicating that DON‐3‐glucoside and its metabolites are mainly excreted in the faeces.
7.1.3. Conclusions on in vitro and in vivo studies on toxicokinetics in experimental animals
In rats, the gut intestinal microflora metabolised DON to DOM‐1 and transformed acetylated DON to DON, while no data were identified on acid hydrolysis, which may contribute to the deacetylation prior to absorption.
In mice, the oral bioavailability of DON has not been quantified. DON was rapidly absorbed after oral exposure and maximum plasma concentrations were reached in 15–30 min. Following absorption, DON was rapidly distributed to liver, kidney, spleen and heart but the total concentrations of DON and metabolites (not specified in the study) peak later and at lower concentrations in the brain than in other organs. The absorption was apparently higher in female rats than in male rats given the same doses. The distribution pattern was independent of age, but tissue concentrations were higher in weanling than in young adult mice given the same single oral dose. DON was glucuronidated at several sites of the molecule and the glucuronidation profile was species‐dependent.
In rats, DON sulfonates were shown to be major metabolites in faeces in addition to the DON glucuronides. The tissue concentrations of DON in mice and rats decreased rapidly after the peak concentrations (t1/2α in the range of 20–40 min in mice) and the decrease in the tissue concentrations followed the decrease in the plasma concentrations. In rats, DON was rapidly (48 h) excreted in faeces and urine with no apparent accumulation in any tissue. A significant biliary excretion was found in one ex vivo rat liver study, but there are no in vivo data from the bile of experimental animals.
The oral bioavailability of 3‐Ac‐DON and 15‐Ac‐DON has not been quantified and a study with only two rats indicates that 3‐Ac‐DON and 15‐Ac‐DON were largely deacetylated prior to systemic distribution, which is in line with ex vivo experiments.
The available studies from rats indicated that the bioavailability of DON‐3‐glucoside was lower than that for DON, but it has not been quantified.
7.1.4. Human studies
7.1.4.1. In vitro studies
The bioaccessibility of DON from cooked pasta was determined in an in vitro model of the human gastrointestinal digestion (Raiola et al., 2012). Bioaccessibility has been defined as the fraction of a compound that is released from its matrix in the gastrointestinal tract and thus becomes available for intestinal absorption (Benito and Miller, 1998). The mean gastric bioaccessibility was 23% and the mean duodenal bioaccessibility was 12% (Raiola et al., 2012).
DON is glucuronidated at several sites of the molecule. Experiments showed that two main metabolites DON‐15‐glucuronide and DON‐3‐glucuronide are formed in the human liver microsomal fraction. These metabolites are also the two major conjugates found in human urine. In addition, small amounts of a third glucuronide, tentatively assigned as DON‐7‐glucuronide by the authors, was present in incubations with human liver microsomes (Maul et al., 2012, 2014) (see below and Section 7.5.2). The microsomal glucuronidation using human liver microsomes was slower than the glucuronidation using liver microsomes from other species.
The role of gut organisms in the metabolism of DON has been studied in vitro using incubations of DON with faeces or gut content from humans. Gut microfloral transformation of DON is less evident in humans than in rats. Gut microflora transformation of DON was not found in incubations with faeces collected from 10 humans (Eriksen and Pettersson, 2003) and only traces of its de‐epoxylated metabolite DOM‐1 were detected in incubations with faeces from nine individuals for 24 h (Dall'Erta et al., 2013). Gratz et al. (2013) found that DON was transformed to DOM‐1 in incubations with faecal samples from one out of five individuals.
Both an in vitro study with cell lines representing the human placenta (BeWO cells, b30 clone) and two ex vivo studies indicate that DON is able to cross the human placenta and reach the fetus (Nielsen et al., 2011; Mose et al., 2012).
The absorption of DON, 3‐Ac‐DON and 15‐Ac‐DON were compared in vitro using a Caco‐2 cell line model (Kadota et al., 2013). All three toxins were transported across the monolayer. The apparent absorption of 15‐Ac‐DON was significantly higher than that of DON and 3‐Ac‐DON. The total recovery of the toxins was 81–108%. Less than 1% of sum of the two acetylated forms of DON was deacetylated to DON. This indicates that the acetylated forms of DON were absorbed across the Caco‐2 cell monolayer without deacetylation.
In the cell medium from HepG2 cells incubated with either 3‐Ac‐DON or 15‐Ac‐DON for 24, 48 or 72 h, products from acetylated DON conjugated with glutathione, sulfuric acid and the amino group from the cysteine conjugates were reported by Juan‐Garcia et al. (2015). No DON or DON related metabolites were reported from any of the toxins. The CONTAM Panel noted that there were no standards for the determination of the conjugates and lack of details given on the analytical methods in this study.
After 24 h incubations of 3‐Ac‐DON with human faeces, DON was the only metabolite detected and no 3‐Ac‐DON was found (Eriksen and Pettersson, 2003). There were no studies identified on 15‐Ac‐DON.
DON‐3‐glucoside added to infant formulae was not hydrolysed in any detectable amount in an in vitro digestion model with addition of enzymes and pH adjustments to simulate the human digestion (De Nijs et al., 2012). Furthermore, DON‐3‐glucoside was not metabolised nor transported from the apical to the basolateral side in a Caco‐2 cell monolayer, while DON was transported across the cell monolayer in this study. Based on these findings, the authors concluded that the bioavailability of DON‐3‐glucoside may be low compared to DON.
The fate of DON‐3‐glucoside during digestion has been investigated in several in vitro assays using different models. When the stability of both DON and DON‐3‐glucoside in naturally contaminated bread was tested in a gastroduodenal in vitro digestion model, an increase in DON‐3‐glucoside concentration was reported together with a decrease in DON concentration. The authors suggested that a conversion of DON to DON‐3‐glucoside is likely to occur during digestion (De Angelis et al., 2014). Berthiller et al. (2011) reported that DON‐3‐glucoside is resistant to both 0.2 M hydrochloric acid and artificial stomach and gut juice, suggesting that DON‐3‐glucoside will not be hydrolysed under the conditions of low gastric pH. DON‐3‐glucoside was also found to be resistant towards human cytosolic glucosidase. The fungal enzymes cellulase and cellobiase were able to cleave 11 and 60% of the DON‐3‐glucosidase after 3 h incubations. Furthermore, DON‐3‐glucoside was incubated with different bacterial strains isolated from guts to test for potential bacterial hydrolysis in the gut. Even though the hydrolysis was low or negligible in most incubations, a significant proportion of the DON‐3‐glucoside was hydrolysed after incubations with some strains, reaching a maximum of 39% after 4 h and 66% after 8 h. The study demonstrated that there was strain‐specific ability of gut microfloral to cleave DON‐3‐glucoside and produce free DON and that this ability may differ between individual, depending on the composition of the gut microflora.
In incubations with human faeces, 25–38% of DON‐3‐glucoside was transformed into DON after 4 h of incubations and 100% in incubations with faeces from four out of five individuals after 6 h (Gratz et al., 2013). Even traces of DOM‐1 have been reported from incubations of DON‐3‐glucoside with human faeces (Dall'Erta et al., 2013; Gratz et al., 2013).
7.1.4.2. In vivo studies
Turner et al. (2010a) estimated the human urinary excretion of DON in 35 UK adults. Based on the individual concentrations of urinary DON and DON‐glucuronides and the total dietary exposure to DON, the geometric mean (95% confidence interval) excretion was calculated to 72% (59–85%). The excretion percentage was not correlated with age, sex, body weight and DON intake (for more details see Section 7.6).
A metabolic study (Warth et al., 2013) was conducted in one human volunteer who consumed a diet naturally contaminated with 138 μg DON, 20 μg 3‐Ac‐DON and 7 μg DON‐3‐glucoside for 4 days. Urinary DON and conjugated DON were measured in urine samples collected over a period of 8 days including 2 days before and 2 days after the 4 exposure days. The daily excretion of total DON was on average 68% (range 60–73%) during the 4 exposure days. The excretion of DON on the 2 days before and the 2 days after the exposure (a mycotoxin reduced diet was taken during this period) was almost always below the LOD. DON‐3‐glucoside and 3‐Ac‐DON were not detected in the urine samples. DON‐15‐glucuronide was the main conjugation product, constituting 73% of total DON‐glucuronides (range 69–76%), while DON‐3‐glucuronide constituted only 27% (range 24–31%). DON‐glucuronides constituted 76% (range 72–80%) of the total DON. The ratios of DON‐15‐glucuronide, DON‐3‐glucuronide and DON‐glucuronides to DON were fairly stable over the 8 days. A third DON‐glucuronide was identified as either DON‐7‐glucuronide or DON‐8‐glucuronide. Research on human metabolites is ongoing and other metabolites of DON may be identified.
7.1.4.3. Conclusions on in vitro and in vivo studies on toxicokinetics in humans
Limited available data suggests that DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐glucoside may be absorbed across intestine monolayer at different efficiency. Gut microfloral transformation of DON or DON‐3‐glucoside into DOM‐1 was less evident in humans than in rats. Individual differences in the gut transformation may depend on the composition of the gut microflora. DON was able to cross the placenta and reach the fetus. Limited human data suggested that approximately 70% of ingested DON would be excreted via urine, of which about 80% were in conjugated forms with DON‐15‐glucuronide as the main conjugation product, about threefold more than DON‐3‐glucuronide.
7.1.5. Farm and companion animals
The toxicokinetics of DON in ruminants, pigs and poultry were reviewed in the previous 2004 EFSA opinion (EFSA, 2004). Since then kinetics and metabolism of DON, 3‐Ac‐DON and 15‐Ac‐DON in farm animals were reviewed by Dänicke and Brezina (2013), Maresca (2013) and specifically in poultry by Guerre (2015). Data on toxicokinetics of DON were available for ruminants, pigs, poultry and horses. No information was identified for other farm and companions animal species. Information on toxicokinetics of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was identified for pigs and poultry only.
7.1.5.1. Ruminants
7.1.5.1.1. DON
As indicated in the EFSA (2004) opinion, DON is metabolised by the ruminal flora to the de‐epoxy metabolite DOM‐1. Swanson et al. (1987a) showed that DON incubated in vitro for 48 h with rumen microorganisms obtained from a fistulated dairy cow was completely metabolised after 48 h. When a single oral dose of 920 mg DON was administered to two lactating cows of similar weight (1.9 mg DON/kg bw), less than 1% of DON was systemically absorbed. Serum DON concentrations reached 90–200 ng/mL, of which 24–46% was a β‐glucuronide with serum half‐life of approximately 4 h (Prelusky et al., 1984b). When dairy cows were fed 66 mg DON/kg feed, about 20% of DON was recovered in urine and faeces as unconjugated metabolites, primarily DOM‐1. Enzymatic (β‐glucuronidase) treatment of the urine released more DOM‐1 than DON (Côté et al., 1986). Low concentrations of DOM‐1 in plasma and urine from lactating Holstein cows were confirmed by MS (Yoshizawa et al., 1986).
Dänicke et al. (2005a) examined the metabolism of DON by feeding contaminated wheat to six dairy cows (five being non‐lactating). Each of two experiments included a control period in which the uncontaminated control wheat was fed and a period in which the control wheat was replaced by Fusarium toxin‐contaminated wheat containing 8.05 mg DON/kg and 0.26 mg zearalenone/kg, and 7.15 mg DON/kg and 0.1 mg zearalenone/kg. The two concentrations of DON corresponded to dietary concentrations of 3.1 and 3.5 mg DON/kg feed (88% dry matter basis), respectively. While the concentrations of DON and its metabolites in the duodenal digesta were either not detectable or marginal during the control periods, distinct concentrations were measured during the feeding with contaminated wheat. DON was nearly completely metabolised to DOM‐1.
Seeling et al. (2006) performed an experiment involving 14 ‘German Friesian’ cows fed a diet containing 60% of complementary feedstuffs and 40% of maize and grass silage (50/50). The only cereal component of the complementary feedstuffs was either 55% of low contaminated wheat (during the control period) or 55% of high contaminated wheat (during the exposure period), the rest was soybean meal, sugar beet pulp, soy oil and minerals. The high contaminated wheat contained 8.21 mg of DON, 0.05 mg of 3‐Ac‐DON, 0.12 mg of 15‐Ac‐DON, 0.09 mg zearalenone and 0.05 mg nivalenol per kg dry matter, while the low contaminated wheat contained 0.25 mg of DON, 0.01 mg of 3‐Ac‐DON, 0.01 mg of 15‐Ac‐DON, 0.05 mg of zearalenone and 0.04 mg of nivalenol per kg dry matter. Each cow was subject for two periods of 4 weeks to the low (control) contaminated diet containing 0.3 mg DON/kg and then to the high contaminated diet containing 3.4 mg DON/kg complete ration, respectively. In serum, no DON but only DOM‐1 was detected, 100% as glucuronide. Excretion of DON and DOM‐1 occurred through both urinary and faecal routes, with the urinal route being the most important one. The authors concluded that DON was almost completely biotransformed to DOM‐1 directly passed to the liver. DON and DOM‐1 residues were only detected in the milk from cows fed the high contaminated diet (see also Section 7.1.5.5).
The metabolism of DON was examined by Keese et al. (2008a) in lactating German Holstein cows in an experiment divided in two periods. During the first period of 11 weeks, cows (n = 13) were fed an isoenergetic total mixed ration with 50% complementary feedstuffs containing 5.3 mg DON/kg dry matter and 0.12 mg zearalenone/kg dry matter (called Myco group) and were compared with control cows (n = 14) fed an isoenergetic total mixed ration containing 0.6 mg DON/kg dry matter and 0.05 mg zearalenone/kg dry matter. In the second period (18 weeks), the same 27 cows plus five additional cows were divided into four groups (n = 8): Control‐30 (30% complementary feedstuffs), Myco‐30 (30% complementary feedstuffs containing 4.4 mg DON/kg dry matter), Control‐60 (60% complementary feedstuffs) and Myco‐60 (60% complementary feedstuffs containing 4.6 mg DON/kg dry matter). DON and DOM‐1 in serum and bile were determined by HPLC‐UV (LODs approximately 2 ng/mL in serum and 4 ng/mL and bile for both DON and DOM‐1). The bile were taken from each cow by percutaneous ultrasound‐guided cholecystocentesis. During both periods, unmetabolised DON was not detected or was close to the LODs in the body fluids. During the first period, the concentrations of DOM‐1 in serum and bile ranged between 22 and 123 ng/mL and between < LOD and 391 ng/mL in the Myco group, respectively. In the second period, cows in Myco‐30 group had significantly higher DOM‐1 concentrations in serum and bile (< LOD–69 ng/mL and 6–114 ng/mL, respectively) compared to cows in Myco‐60 group (4–52 and 4–93 ng/mL, respectively). The authors noted that beside the different complementary feedstuff proportion also the proportion of maize‐ and grass silage differed between the two groups and that the release and bioavailability of DON coming from the maize silage might be different from the kinetics of the contaminated triticale. Transfer into milk investigated in this study is described in Section 7.1.5.5.
In another study of the same group (Winkler et al., 2014), 30 lactating German Holstein cows were divided into three groups (n = 10). The animals received for 13 weeks either a control diet (CON) with 50% grass silage and 50% of the complementary feedstuffs with low mycotoxin‐contaminated maize (approximately 0.07 mg DON/kg and 0.02 mg zearalenone/kg on dry matter basis), a low contaminated diet (FUS‐50) with 50% grass silage, 25% low mycotoxin‐contaminated maize (CON) and 25% highly contaminated maize grain (approximately 2.62 mg DON/kg, 0.33 mg DON‐3‐glucoside/kg, 0.25 mg 15‐Ac‐DON/kg and 0.33 mg zearalenone/kg dry matter) or a high contaminated diet (FUS‐100) with 50% grass silage and 50% highly contaminated maize grain (approximately 5.24 mg DON/kg, 0.66 mg DON‐3‐glucoside/kg, 0.50 mg 15‐Ac‐DON/kg and 0.66 mg zearalenone/kg dry matter). Zearalenone, DON and DOM‐1 were detected in all plasma samples and the authors recorded in the FUS‐50 and FUS‐100 groups a marked increase for all three toxins within the first week. DON concentrations ranged from 0.27 to 0.88 ng/mL in CON, from 0.41 to 1.69 ng/mL in FUS‐50 and from 0.83 to 5.06 ng/mL in FUS‐100. In contrast, DOM‐1 was detected at much higher concentrations than DON: 1.2–5.9 ng/mL in CON, 3.7–22.4 ng/mL in FUS‐50, and 4.6–61.7 ng/mL in FUS‐100, respectively. The calculated mean proportion of DOM‐1 in the sum of DON and DOM‐1 was about 93%. Plasma levels of DOM‐1 were significantly correlated with DON exposure.
In two follow‐up studies of that experiment, DON and DOM‐1 were analysed in serum, follicular fluid and urine. In the first study, the ratio between plasma and follicular fluid concentrations of DON ranged between 0.5 and 2.2 for DON and between 0.6 and 1.1 for DOM‐1. DON and DOM‐1 concentrations significantly correlated both in plasma and in follicular fluid (Winkler et al., 2015a). In the second study, DON and DOM‐1 were detected in all urine samples with the concentration of DOM‐1 being 17.5‐fold higher than the concentration of DON in the two Fusarium contaminated groups (Winkler et al., 2015b). DON was almost completely metabolised to DOM‐1 (83–98%) independently of the level of DON exposure. Moreover, conjugated toxins were the major urinary metabolites. Despite a high variation between the different urine samples, a significant difference existed between the Fusarium contaminated groups and the control group, for both DON and DOM‐1 and there was also a correlation between DON exposure levels and urinary‐DON and DOM‐1 concentrations, respectively. The authors concluded that increased urine‐DON concentrations may indicate DON exposure through the diet and thus the studied mycotoxins and their detected metabolites could be used as biomarkers of exposure.
The kinetic of DON and DOM‐1 was studied in a total of eight non‐pregnant and non‐lactating cows, aged 5 years (two cows), 4 years (three cows) and 3 years (three cows), by Dänicke et al. (2016). During a 6 week pre‐experimental period, their diet changed from a pure straw ration to a total mixed ration containing 60% complementary feedingstuffs, 24% corn silage and 16% grass silage containing 0.19, 0.58 and 0.06 mg DON/kg dry matter and 0.02, 0.05 and 0.02 mg zearalenone/kg dry matter. The composition of the total mixed ration was then kept constant from week 7 to week 16 at 0.274 mg DON/kg bw per day. Blood samples were collected at four weekly intervals and in blood DON was exclusively available as DOM‐1 following the time course of DON exposure over the 16 weeks. The authors concluded that rumen microbes nearly completely metabolised DON to DOM‐1 irrespective of dietary DON concentration and diet composition.
As reviewed by EFSA (2004), in four rumen‐fistulated male sheep (60–70 kg) systemic bioavailability of DON amounted to 7.5% after oral administration of a single dose of 5 mg DON/kg bw (Prelusky et al., 1985). In this study, DON was quickly adsorbed (tmax=4–5.3 h) and efficiently glucuronidated (75%). Elimination half‐live of the glucuronide was considerably longer than that of the parent toxin (t1/2β = 100–125 min and 6.1–7.1 h, respectively). DOM‐1 was detected in plasma only as minor portion of < 0.3% of the orally administered DON. The urinary excretion rates of the major metabolites reached maximum levels 6–9 h after the treatment, and declined then exponentially with t1/2 values of 3.2, 4.0, and 5.0 h for DON, conjugated DON, and conjugated DOM‐1, respectively. In agreement with the low bioavailability, urinary (7.0%) and biliary (0.11%) recovery was generally low. In the urine, 2.1% of the administered dose was detected as DON, 3.6% as DON‐glucuronide, 0.06% as DOM‐1 and 1.2% as DOM‐1‐glucuronide.
Metabolism and elimination of DON in sheep have further been investigated by intravenous administration of 14C‐radiolabelled‐DON (4.0 mg/kg bw) to two 1‐year‐old lactating ewes (70–80 kg) (Prelusky et al., 1987). The plasma clearance of radioactivity was rapid and followed a triphasic decay with a rapid distribution phase (t1/2α = 16.2 min), slower elimination phase (t1/2β = 66.5 min) and the formation and elimination (t1/2β = 188.0 min) of the predominant conjugate in plasma, the DON‐glucuronide, accounting for 13% of the measured radioactivity. The recovered radioactivity after urinary (91%) and biliary (6%) excretion accounted for 54% as DON‐glucuronide, 13% as DOM‐1‐glucuronide, 11% as unmetabolised DON, 6% as unmetabolised DOM‐1, DON‐sulfate conjugate for 3% and unidentified radioactive components for 10%.
The distribution of DON in cerebral spinal fluid has been investigated following i.v. administration of 1 mg DON to four 1‐year‐old wethers (60–70 kg). DON was detected rapidly in the cerebral spinal fluid, peak levels occurring at 5–10 min after injection (Prelusky et al., 1990).
No data were identified for goats.
7.1.5.1.2. 3‐Ac‐DON and 15‐Ac‐DON
Seeling et al. (2006) and Winkler et al. (2014) (see above) exposed cows also to 3‐Ac‐DON and/or 15‐Ac‐DON but did not report any results on toxicokinetics for the two acetylated forms. Radioactively labelled 3‐Ac‐DON (5 mg/kg bw) was administered intraruminally to two female lambs (Brewer et al., 1996). The authors reported the 3‐Ac‐DON was rapidly hydrolysed to DON. Rumen fluid collected only 5 min after administration indicated the presence of 3‐Ac‐DON and DON in the ratio of 1:1. An apparent half‐ life of 46 min was calculated by the authors for DON in the rumen. The maximum radioactivity of the serum was observed 300–400 min after dosing. Twenty‐three per cent of the radioactivity was excreted in the urine.
No data were identified for goats.
7.1.5.1.3. DON‐3‐glucoside
Winkler et al. (2014) (see above) exposed cows also to DON‐3‐glucoside but did not report any results on its toxicokinetics. No data were identified for sheep and goats.
7.1.5.2. Pigs
7.1.5.2.1. DON
Kinetic parameters in pigs measured in experiments administrating single doses of DON were summarised by EFSA (2004). In the sections below, the findings of EFSA (2004) are summarised and relevant studies published since then are described in detail.
DON is rapidly absorbed in the proximal parts of the digestive tract of pigs. After intragastric dosing of radiolabelled DON (600 μg/kg bw), the absorption half‐time was less than 30 min, and DON showed a high bioavailability ranging from 48% to 65% (Prelusky et al., 1989). After feeding pigs with a diet containing naturally DON‐contaminated wheat (4.2 mg DON/kg feed), the maximum serum DON concentration was found after 4.1 h (Dänicke et al., 2004a). Avantaggiato et al. (2004) developed an in vitro model using preparations of gastric, biliary and pancreatic secretions to simulate physiological conditions of the gastrointestinal tract in pigs. The rate of intestinal absorption of DON (2.8 ± 0.3 mg/kg wheat) was measured by pumping dialysis liquid through hollow fibre membranes (method previously described by Minekus et al., 1995). They postulated an absorption rate of 51% for DON from that model.
Dänicke and Brezina (2013) summarised 12 independent published balance experiments with a total of 122 pigs and estimated the systemic absorption of DON as 0.044–0.6 mg/kg bw An average systemic DON absorption of 49% in fattening pigs was postulated.
In an ex vivo‐experiment after slaughtering, Halawa et al. (2013) compared the intestines of animals exposed to 141 μg DON/kg bw for at least 1 week with controls (n = 8 per group) under restricted feeding, and observed a higher jejunal mucosal uptake of DON in the exposed group. They also identified a statistically significant interaction between the change of dietary concentration of DON in feed and the change of rate of absorption.
The influence of wheat grain, straw and chaff on the bioavailability was studied in pigs exposed to DON (53 μg/kg bw) but no matrix‐dependency was observed (Rohweder et al., 2013). Neither the invasion (0.76, 0.77 and 0.48 h), nor the elimination half‐life (3.74, 3.96 and 4.75 h) differed significantly. A DON‐mediated effect on the liver clearance capability (Goyarts and Dänicke, 2006) and on tight junction proteins (Pinton et al., 2010; Diesing et al., 2011) might have contributed to the finding that chronic feeding of a DON‐contaminated diet (68.5 ± 4.9 μg/kg bw, n = 5) for 5–8 weeks resulted in a significantly higher bioavailability when compared to an acute oral single bolus of 77.3 ± 2.4 μg/kg bw (Goyarts and Dänicke, 2006).
Earlier investigations of the distribution to organs in pigs have been performed following a single i.v. injection 1 mg DON/kg bw ((Prelusky and Trenholm, 1991) revealing high initial concentrations in plasma, kidney and liver (1–2 mg/kg organ weight). Measurable concentrations were, however, detected also in the abdominal fat, back fat, lung, adrenals, spleen, testis, heart, brain, muscle tissue, intestines and pancreas (0.02–0.5 mg/kg organ weight), indicating a large volume of distribution (EFSA, 2004).
Since then several studies, reviewed by Dänicke and Brezina (2013) have been performed to characterise the distribution of DON. The highest transfer rate of the sum of DON and DOM‐1 into edible tissues of pigs was found for kidneys (3.4%) by Dänicke et al. (2010). Other maximum transfer rates were lower by the factor of 5, 7, 11 and 33 for liver, muscle, spleen and back fat, respectively (Dänicke and Brezina, 2013) (see also Section 7.1.5.5).
In contrast to ruminants, only a minor metabolisation of DON takes place in pigs. However, DON can be de‐epoxidated by the gastrointestinal microflora (Eriksen et al., 2002). In an experiment of Dänicke et al. (2004a), 11 castrated male pigs (88.1 ± 3.9 kg bw), adapted to a diet containing 52 μg DON/kg bw for 7 days, de‐epoxidation occurred particular in the distal segments of the porcine digestive tract.
In earlier in vitro studies (Côté et al., 1987), no metabolic conversion of DON by hepatic microsomes (1 μmol/mL) was observed. More recently, it has been recognised that DON undergoes extensive conjugation with glucuronic acid in pigs. The DON‐3‐glucuronide was identified as predominant phase‐II‐metabolite in vitro after incubation of a 3.75 μM solution of DON with liver microsomes from six animal species including pigs (Maul et al., 2012). The formation of DON‐15‐glucuronide by porcine liver microsomes has also been shown, while DON‐7‐glucuronide could not be detected after incubation with DON concentrations of 0.4–50 μM (Maul et al., 2014) (see also Section 7.1.1).
In gilts, after administration of up to 163.5 μg/kg bw (n = 9 per group) for 5 weeks, 36–56% of the total DON content in urine was present as glucuronide, while the proportion of glucuronides (glucuronide forms not stated) in serum was 19–45% (Dänicke et al., 2005b), which was similar to the findings in fattening pigs (average of 35%) (Dänicke et al., 2013). Only a small proportion of DOM‐1 was detected in urine (1.2–7.9% of DON detected as DOM‐1) and in blood (average of 7% of DON detected as DOM‐1) in barrows (n = 12, mean live weight 40.1 kg) after oral administration of 104 μg DON/kg bw for 7 days in a balance experiment (Dänicke et al., 2014). These small amounts point to a substantial absorption of DON in pigs in the upper digestive tract preventing microbial de‐epoxidation.
The plasma elimination half‐life varied between 1.2 and 3.9 h in pigs (Coppock et al., 1985; Prelusky and Trenholm, 1991; Eriksen et al., 2003). When radiolabelled DON (600 μg/kg bw) was given by gavage, plasma clearance was found to be 7.14 h (Prelusky et al., 1988). Dänicke et al. (2012) demonstrated that the apparent volume of distribution and the plasma clearance differed significantly between exposure via simultaneous i.v. administration of DON (100 μg/kg per h) and lipopolysaccharides (LPS) (7.5 μg/kg per h) and administration of DON alone (2.14 vs 1.45 L/kg and 11.9 vs 5.87 mL/kg per minute). Interactions between DON and LPS have been revealed in several other studies from the group of Dänicke and collaborators.
Dänicke and Brezina (2013) concluded from their review (see above) the existence of a linear relationship between oral DON exposure and urinary excretion of the metabolites of DON (not further specified) which was 90–95% of total excretion. Only weak relationships were found between DON exposure and the amount of DON or DOM‐1 excreted with faeces. The authors estimated in this review a recovery of only 40% and concluded that the low recovery was probably due to post‐absorptive rather than intestinal effects. That post‐absorptive effects might suggest that a steady‐state was not reached and that post‐renal matrix effects of urine might have occurred Therefore, DON might have further been converted to non‐detectable compounds or it was included in poorly extractable complexes.
7.1.5.2.2. 3‐Ac‐DON and 15‐Ac‐DON
In pigs, 3‐Ac‐DON is rapidly deacetylated in the upper intestinal tract and absorbed exclusively as DON, as the acetylated form could not be detected in plasma, urine and faeces after oral administration of 98 μg/kg bw The absorption half‐time for 3‐Ac‐DON was estimated to be 1.26 h (Eriksen et al., 2003). There is no similar information available for 15‐Ac‐DON.
7.1.5.2.3. DON‐3‐glucoside
The metabolism of DON‐3‐glucoside has been evaluated by the consecutive oral administration of water (control), DON‐3‐glucoside (116 μg/kg bw) and the equimolar amount of DON (75 μg/kg bw) to four piglets per group on day 1, 5 and 9 of the experiment, respectively, followed by an i.v. administration of 15.5 μg DON‐3‐glucoside/kg bw on day 13 (Nagl et al., 2014). As the urinary excretion of metabolites for DON‐3‐glucoside was about two times less compared to DON (40.3 ± 8.5 and 84.8 ± 9.7%, respectively), a lower bioavailability of the conjugated form was concluded in this study. However, only small amounts of DON metabolites were recovered in faeces (1.8 ± 1.6% as DOM‐1). The majority of orally administered DON‐3‐glucoside was excreted via urine as DON, DON‐15‐glucuronide, DOM‐1 and DON‐3‐glucuronide. While urinary DON‐3‐glucoside accounted for only 2.6 ± 1.4% after oral, but for 99.9 ± 8.9% after i.v. administration of DON‐3‐glucoside, it seems that a nearly complete cleavage of the modified mycotoxin occurred, primarily in the intestinal tract before systemic absorption.
In a cross‐over trial with i.v. and oral administration of 55.7 μg DON‐3‐glucoside/kg bw and 36 μg DON/kg bw, respectively, the contribution of DON‐3‐glucoside to DON exposure in pigs (n = 6, 26.3 ± 1.8 kg bw) was studied (Broekaert et al., 2016). The bioavailability, hydrolysis and toxicokinetics of DON‐3‐glucoside were calculated after quantification of the systemic and portal plasma concentrations of DON, DON‐3‐glucoside and DOM‐1. The complete presystemic hydrolysis of the absorbed fraction of DON‐3‐glucoside was indicated by the exclusive detection of DON in plasma after oral administration of DON‐3‐glucoside. After i.v. administration of DON‐3‐glucoside, no hydrolysis to DON was observed, indicating the absence of systemic hydrolysis. After oral administration the absorbed fraction of DON‐3‐glucoside, recovered as DON, was approximately 5 times lower (16.1 ± 5.4%) than that of DON (81.3 ± 17.4%.). The authors concluded that hydrolysis occurs at the site of the gastrointestinal tract (microbiota or gastrointestinal tissues). The observed lag time for DON absorption after oral administration of DON‐3‐glucoside to pigs (83.6 min) further supports this hypothesis, as the higher pH‐values in the distal parts of the small intestine may lead to a more abundant growth of microbiota and therefore a higher probability of DON‐3‐glucoside hydrolysis. Furthermore, the analysis of phase II metabolites in this study revealed that biotransformation of DON and DON‐3‐glucoside in pigs mainly consists of glucuronidation (ratio of DON‐glucuronides/DON equal to 4.98 after oral administration).
7.1.5.3. Poultry
In the previous assessment of EFSA (2004), only two studies, one on laying hens and one on ducks, described the toxicokinetics in poultry.
7.1.5.3.1. DON
In studies performed in chicken a low degree of absorption into plasma and tissues (1%) as well as rapid clearance (Prelusky et al., 1986) was observed. Intestinal microflora can convert DON to DOM‐1 in poultry (Lun et al., 1986, 1988; He et al., 1992).
No DON or DOM‐1 residues could be detected in plasma and bile of broilers in an experiment where the DON concentrations reached approximately 1.5 mg/kg diet (Dänicke et al., 2007a).
Yunus et al. (2010) evaluated the transfer of DON to DOM‐1 in the plasma of chicken. Naturally contaminated oats with 9.5 mg DON/kg were fed to four broilers (35 days of age) at a dose of 20 g per bird. The sum of DON and DOM‐1 appearing in the plasma at 1 and 2 h of post‐feeding in the birds was estimated to be 0.044 and 0.036% of the total DON fed, respectively. The authors concluded that the absorption rate of DON is very low in broilers and that there is also a rapid transformation, and clearance from plasma. However, individual variability in the capacity of birds to de‐epoxidise DON was reported.
In a study by Awad et al. (2011), a total of 45 one‐day‐old broiler chickens (Ross 308 males) were randomly allotted to three dietary treatments (15 birds/treatment) and were fed (1) a control diet with 0.088 mg DON/kg, 0.005 mg 3‐Ac‐DON/kg and 0.014 mg zearalenone/kg feed, (2) a diet contaminated with 0.872 mg DON/kg, 0.018 mg 3‐Ac‐DON/kg and 0.110 mg zearalenone kg feed, and (3) a diet contaminated with 5.017 mg DON/kg, 0.114 mg 3‐Ac‐DON/kg and 0.352 mg zearalenone/kg feed for 5 weeks. DON and DOM‐1 were measured in serum, bile, liver, faeces and digesta from consecutive segments of the digestive tract (gizzard, caecum, and rectum). DON and DOM‐1 concentrations in serum, bile and liver were lower than the LOD of 7.0 ng DON/g for the used LC–MS/MS method. No DOM‐1 was recovered in the large intestine and in faeces DON was recovered to 18–22% in cecum and to about 10–12 and 6% in gizzard and faeces, respectively, not influenced by the amount of DON‐concentration ingested. The authors noted that de‐epoxidation occurred in these birds in the proximal small intestine, where the majority of DON is absorbed, but hardly in the distal segments of the digestive tract. The results showed that the majority of the ingested DON quickly disappears through the gastrointestinal tract.
Yunus et al. (2012b) explored the intestinal absorptive functionality and DON metabolism in broilers. DON was produced by inoculating rice with F. graminearum. Ross broilers at 7 days of age were fed either a control diet (0.265 mg DON/kg, 0.009 mg 3‐Ac‐DON/kg and 0.013 mg zearalenone/kg), a low DON diet (1.68 mg DON/kg, 0.198 mg 3‐Ac‐DON and 0.145 mg zearalenone/kg), or a high DON diet (12.209 mg DON/kg, 1.446 mg 3‐Ac‐DON/kg and 1.094 mg zearalenone/kg). After 5 weeks of exposure the birds were intubated into the crops with a solution of Fusarium mycotoxin concentrate containing DON, 3‐Ac‐DON and zearalenone at concentrations of 616.5, 67.2 and 20.55 μg/mL, respectively. Higher amounts of DON and DOM‐1 were found 5 h of post‐intubation in the gut of birds raised on the low and high DON diets than in that of birds fed the control diet. The plasma level of DON in the birds at 1 h of post‐intubation linearly decreased with increasing dietary exposure during the previous period.
Two experiments of Osselaere et al. (2012, 2013a,b) showed a rapid clearance of DON in plasma. In a first experiment of Osselaere et al. (2012), three groups (n = 8 per group) of 1 day old chickens were treated for 3 weeks after 10 days of acclimatisation. Group 1 (Control) was fed non‐contaminated feed, group 2 artificially contaminated feed (2.44 mg DON, 0.09 mg nivalenol, 0.57 mg 3‐Ac‐DON, 2.05 mg 15‐Ac‐DON and below 0.05 mg (LOD) fumonisins B 1–3 per kg feed, and group 3 naturally contaminated maize (7.54 mg DON, below 0.07 mg (LOD) nivalenol, 1.48 mg 3‐Ac‐DON, below 0.005 mg (LOD) 15‐Ac‐DON and 0.70 mg fumonisin B1, 0.20 mg fumonisin B2, 0.21 mg fumonisin B3 per kg feed). No significant differences of effects on zootechnical parameters (feed intake, body weight gain, final live body weight) were observed between the three groups. No DOM‐1 was detected in the three groups and DON was above the LOD (1.25 μg/mL) only in the plasma of the birds in group 3. Plasma concentrations of DON were higher after week 1 than after week 3 and no DON was detected after week 3 in group 3, but it was above the LOD in bile. DOM‐1 was detected in groups 2 and 3 with concentrations lower in group 2 than in group 3. No residues of DON or DOM‐1 were detected in liver and kidneys in any of the three groups (see also Section 7.1.6.1).
A second experiment of Osselaere et al. (2013a,b) on eight broiler chickens was performed following a two way design where four animals received a single dose i.v. and four animals orally a bolus of 0.75 mg DON/kg bw Blood was collected at several time points. Plasma levels of DON and DOM‐1 were quantified using LC–MS/MS (LOQs 1.0–2.5 ng/mL). After oral administration no plasma concentrations of DOM‐1 were detected above the LOQ. Moreover, from 2 h after administration on DON was below the LOQ. The maximum DON concentration in plasma was reached after approximately 35 min (about 26 ng/mL). The plasma concentration after i.v. administration was much higher than that after oral administration. Oral bioavailability of DON was determined to be 19.3 ± 7.42%. Elimination half times after oral and i.v. were 38.2 ± 11.2 and 27.9 ± 6.9 min, respectively.
Tritium‐labelled DON, 3β‐3H‐DON (1.1 mCi/g) in ethanol was orally administered to broilers at the dose of 2.5 mg DON/kg bw for 5 days and analysed by radio‐HPLC‐TOF‐MS (Wan et al., 2014). DON was widely distributed and quickly eliminated in all tested tissues tested. Its concentration was the highest in the gastrointestinal tract and substantially lower in the kidney, liver, heart, lung, spleen and brain. Amongst the three metabolites identified (10‐DON‐sulfonate, 10‐DOM‐1‐ sulfonate and DON‐3‐sulfate), DON‐3‐sulfate was most prominent one.
Toxicokinetics of DON, 3‐Ac‐DON and 15‐Ac‐DON were investigated separately in a pilot study on three male broiler chickens of 20 days of age (Broekaert et al., 2014) and an experimental study of Broekaert et al. (2015) involving 18 broilers of 3 weeks of age by gavage in a two‐way‐cross‐over design with a washout period lasting 4 days. In the pilot study, the three toxins were administered purified at three doses equivalent to 5 mg DON/kg feed, respectively. The absorbed fractions of DON, 3‐Ac‐DON or 15‐Ac‐DON were 10.6, 18.2 and 42.2%, respectively. No DOM‐1 was detected in any of the plasma samples (LOD 0.51 ng/mL) taken. In a follow up study using the design of Broekaert et al. (2014), this group investigated the metabolism of DON (Devreese et al., (2015). In plasma, the major metabolite of DON was DON‐3‐sulfate, with high ratios of DON‐3‐sulfate to DON ratios ranging between 243–453 and 1,365–29,624 after i.v. and gavage, respectively. Only trace amounts of other metabolites such as DON‐ and DOM‐1‐glucuronides, 10‐DON‐sulfonate, DOM‐1‐ and 10‐DOM‐1‐sulfonate were detected. The authors concluded a low absolute oral bioavailability of DON, a rapid absorption and elimination of DON, and an extensive biotransformation of DON to DON‐3‐sulfate.
Broekaert et al. (2016) studied the toxicokinetics of DON and DON‐3‐glucoside in 6 broiler chickens (Ross 308) treated with either DON or DON‐3‐glucoside by i.v. bolus injection or gavage in a two‐way‐cross‐over design with a washout period lasting 3 days. The administered doses of pure toxins in solution were equivalent to a feed of 5 mg DON/kg feed. After i.v. and after gavage administrations of DON‐3‐glucoside, no hydrolysis to DON was observed. The oral bioavailability of DON‐3‐glucoside was low (3.79 ± 2.68%). No DOM‐1 was detected in the plasma samples. The mean elimination half life for DON‐3‐glucoside in broilers was 34.0 ± 6.2 min. Analysis of phase II metabolites revealed that biotransformation of DON and DON‐3‐glucoside in chickens mainly consisted of conjugation with sulfate compared to glucuronidation.
Schwartz‐Zimmerman (2015) analysed by LC–MS/MS excreta samples from different feeding trials with chickens, pullets and roosters. Control groups were fed with basal poultry feed naturally contaminated with 0.2 mg/kg DON. Five‐week old chickens (Ross 508) of the DON group received basal poultry feed enriched with DON from culture material to a concentration of 1.7 mg DON/kg during 1 day. Excreta were collected every 3 h on the same day and one sample collected the next morning. Twelve‐week old pullets (Lohmann LSL) were exposed to 4.4 mg DON/kg feed during 2 weeks. The feed also contained traces of 3‐Ac‐DON (0.13 mg/kg) and 15‐Ac‐DON (0.03 mg/kg). Excreta were collected every morning and afternoon for 1 week. Adult roosters received diet containing 11 mg DON/kg during 9 days and excreta were collected as for pullets. The authors indicated that DON‐3‐sulfate was the major metabolite of DON found in excreta in all these poultry species.
In the only study mentioned in EFSA (2004) of Prelusky et al. (1986) on laying hens, a single oral dose of radiolabelled DON (2.2 mg per animal) was given. DON was found to be poorly absorbed (< 1% of the administrated dose). Elimination of the radiolabelled DON into excreta occurred rapidly and recovered radioactivity accounted for 98.5% of the dose after 72 h. In the experiment performed by Ebrahem et al. (2014a) 23‐week‐old laying hens (n = 80) were fed low contaminated wheat with 0.054 mg DON and 0.069 15‐Ac‐DON/kg feed and wheat with 0.04 mg DON/kg feed (control). Highly contaminated wheat (previously inoculated with an F. culmorum strain) contained 13.5 mg DON, 4.7 mg aurofusarin, 0.76 mg DON‐3‐glucoside and 0.23 mg 3‐Ac‐DON/kg and was used to prepare the diet containing 9.9 mg DON/kg feed. In the 70th week of the life, all hens were slaughtered and samples of blood and bile were collected and analysed by LC–MS/MS for DON and DOM‐1. DON was detected only in hens given the toxic diet in plasma and bile with levels ranging between 0.2 and 0.6 ng/mL and 1.8 and 4.1 ng/mL, respectively. No DOM‐1 was detected.
As also mentioned in the EFSA (2004), diets with increasing proportions of Fusarium‐toxin‐contaminated wheat were fed to 54 one year old Pekin ducks for 49 days. Dietary DON concentration was 7 mg/kg. Concentrations of DON and DOM‐1 in plasma and bile were lower than the LODs of 6 and 16 ng/mL, respectively, when using HPLC (Dänicke et al., 2004b). No data were identified since then.
Gauvreau (1991) administered DON solutions orally and i.v. at 5 and 1 mg/kg bw, respectively, to 24 one‐day old turkeys at the end of their growing period of 8 weeks (weighing about 3 kg). Orally given DON was poorly absorbed from the gastrointestinal tract (0.96% of the ingested DON) and the majority was eliminated within 3 h in the excreta. A rapid plasma clearance (t1/2 = 44 min) occurred after i.v. injection. In an experiment of Dänicke et al. (2007b), 4 groups of 48 male turkeys were fed with increasing proportions of naturally DON contaminated wheat at approximately 0.1, 2.0, 4.6 and 5.4 mg DON/kg feed from day 21 to day 56 of age. DON and DOM‐1 concentrations in plasma were lower than the LOQ of 2 ng/mL and the DON concentration in bile reached up to 13–23 ng/mL, whereas DOM‐1 concentrations were lower than 4 ng/mL. Devreese et al. (2014) conducted an experiment on 120 one‐day‐old male turkey poults for 12 weeks including starter, grower, developer and finisher phases. Birds were randomly distributed into two groups of three pens each either fed a control diet containing 0.06–0.39 mg DON, below 0.05 (LOD)–0.10 mg 15‐Ac‐DON and below 0.03 mg of other mycotoxins per kg feed or a naturally contaminated diet containing 4.0–6.5 mg DON, 0.45–0.55 mg 15‐Ac‐DON, 0.25–1.2 mg FB1+FB2 and 0.37–0.67 mg zearalenone, per kg feed. No DON or DOM‐1 was detected in any plasma samples of the birds fed the control diet but both were detected in all analysed samples of birds fed the contaminated diet. Plasma concentrations of 1.14 ng DON/mL and 2.45 ng DOM‐1/mL were measured during the starter phase, and increased to a maximum of 3.21 ng DON/mL and 9.51 ng DOM‐1/mL at the end of the grower phase. The authors attributed the increased DON absorption to intestinal damage, as demonstrated with the morphometry indices (see Section 7.3.3.1).
Devreese et al. (2015) administered DON to six turkey poults of 7 weeks of age (body weight around 1.27 kg) at 0.75 mg/kg bw per o.s. and i.v. in a two‐way‐cross‐over design. DON was absorbed rapidly (tmax = 0.57 h) but incompletely as the oral availability was only 20.9%. DON was also rapidly eliminated both after oral (t1/2 elimination = 0.86 h) and i.v. (t1/2 elimination = 0.62 h) administration. Semiquantitative analysis using HRMS (see Broekaert et al., 2014), revealed that the major metabolite of DON in plasma was DON‐3‐sulfate, with DON‐3‐sulfate/DON ratios between 1.3–12.6 and 32.4–140.8 after i.v. and oral administration, respectively. Glucuronidation of DON to DON‐3‐glucuronide was a minor pathway. Only trace amounts of other metabolites were found including 10‐DON‐sulfonate, DOM‐1 and 10‐DOM‐1‐sulfonate. The authors concluded a low absolute oral bioavailability, a rapid absorption and elimination, and an extensive biotransformation to DON‐3‐sulfate.
Schwartz‐Zimmerman (2015) analysed by LC–MS/MS excreta samples from feeding trial with turkeys. Control group was fed with basal poultry feed naturally contaminated with 0.3 mg DON/kg. Eleven‐week old turkeys of the DON group received basal poultry feed enriched with DON from culture material to a concentration of 1.5 mg/kg during 1 day. Excreta were collected every 3 h on the same day and one sample collected the next morning. The authors indicated that DON‐3‐sulfate was the major metabolite of DON found in turkey excreta.
7.1.5.3.2. 3‐Ac‐DON and 15‐Ac‐DON
Broekaert et al. (2014, 2015) reported toxicokinetic data on 3‐Ac‐DON and 15‐Ac‐DON in broiler chickens (see details above). The absorbed fraction after gavage administration of 3‐Ac‐DON and 15‐Ac‐DON was 18.2 and 42.2%, respectively. The absorbed fraction of 3‐Ac‐DON was completely hydrolysed presystemically to DON and 75.4% of the absorbed fraction of 15‐Ac‐DON to DON. No DOM‐1 was detected in any of the plasma samples (LOD 0.51 ng/mL).
7.1.5.3.3. DON‐3‐glucoside
Broekaert et al. (2016) reported toxicokinetic data on DON‐3‐glucoside and DON in broiler chickens (see details above). The results indicated that DON‐3‐glucoside was not hydrolysed to DON. The oral bioavailability of DON‐3‐glucoside was low (mean ± SD: 3.79 ± 2.68%) and comparable to that of DON. The absence of DOM‐1 in all analysed samples of plasma was observed. The mean t1/2 elimination for DON‐3‐glucoside in broilers was 34.0 ± 6.2 min. Analysis of phase II metabolites revealed that biotransformation of DON‐3‐glucoside in chickens mainly consisted of conjugation with sulfate compared to glucuronidation.
7.1.5.4. Horses
7.1.5.4.1. DON
No information on toxicokinetics of DON, its acetylated forms or DON‐3‐glucoside in horses was reported in the previous EFSA (2004) opinion. Two studies on horses have been published since then. Setyabudi et al. (2012) investigated the concentrations of DON and DOM‐1 in plasma samples of five Haflinger mares fed oats contaminated with DON (12 mg DON/kg), total exposure being 36 mg of DON per day during 10 days. The concentrations of DON and DOM‐1 were determined using HPLC‐IAC‐UV. In the plasma samples (without treatment with β‐glucuronidase) collected 24 h after feeding on day 10, concentrations of DON and DOM‐1 ranged from below LOD (4 ng/mL) to just below LOQ (13 ng/mL). In plasma, the majority of DON was present as glucuronides of DON and DOM‐1 representing 53–79% of the sum of DON and detected metabolites. Schulz et al. (2015) fed for 21 days 12 geldings with wheat based feed naturally contaminated with DON (14.6 ± 6.5 mg DON/kg dry matter). The feed intake was adjusted to 4 kg wheat per day and 1.7 kg silage/100 kg bw per day. Three groups of 4 horses were fed either a control diet with 0% contaminated wheat or two wheat mixtures: one with 53 ± 2% of DON‐contaminated wheat (7.7 mg DON/kg feed) and one with 78 ± 4% of DON‐contaminated wheat (11.4 mg DON/kg feed). Blood samples were collected on day 0 and 21 before and 3 h after the wheat intake. Serum DON concentrations increased with higher DON exposure. After the first day of wheat intake, the metabolite DOM‐1 was not detected in serum until day 21 of DON exposure. DOM‐1 was only detected in serum on day 21 after feeding the two DON diets. DOM‐1 concentrations in serum correlated with DON exposures.
7.1.5.4.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.1.5.4.3. DON‐3‐glucoside
No data were identified.
7.1.5.5. Conclusions on toxicokinetics in farm and companion animals
The CONTAM Panel concluded that overall intestinal absorption and metabolism of DON varies largely between different farm animal species and this may depend on the location of the consecutive intestinal segments, regional pH and activity of bacteria. One may distinguish polygastric animals (e.g. ruminants) and birds (e.g. poultry) from monogastric animals (e.g. pigs) when considering the location of high bacterial content which is both before and after the small intestine in the first and only after the small intestine, in particular in colon, in the second case, see e.g. review of Maresca (2013). Localisation of the gut bacteria affects the bioavailability of ingested DON and its metabolites.
In cows, DON is almost completely metabolised by the ruminal flora to DOM‐1 and only minor amounts of DON reach systemic circulation (< 1%). The proportion of DOM‐1 glucuronide conjugate in serum is high. The urine seems to be the main route of excretion, whereas faecal and biliary excretion routes seem to be less important. Relevant toxicokinetic data were not identified for 3‐Ac‐DON and/or 15‐Ac‐DON and/or DON‐3‐glucoside for cows. When orally administered to sheep, DON has systemic bioavailability of 7.5% and is quickly absorbed. Following absorption, DON is efficiently glucuronidated; de‐epoxidation seems to play a minor role (< 0.3% DOM‐1 in plasma). Excretion of DON and metabolites occurred through both urinary and biliary routes, with urinary excretion being most important. One study on lambs showed that radioactively labelled 3‐Ac‐DON administrated intraruminally was rapidly converted to DON. Twenty‐three per cent of the radioactivity was excreted in the urine. No data were identified on the toxicokinetics of the 15‐Ac‐DON or DON‐3‐glucoside for sheep. For goats no data were identified. The CONTAM Panel noted that toxicokinetics could be different in ruminants with acidosis or young animals such as calves for which the ruminal system is not fully functioning.
The absorption of DON in pigs is generally high (48–65%) and may depend on the level of exposure. DON shows an extensive organ distribution and also a rapid renal excretion, partly conjugated to glucuronic acid. The plasma elimination half‐life was found to vary between 1.2 and 3.9 h. Excretion of DON and metabolites occurred through both urinary and biliary routes, with urinary excretion being the most important route in pigs. No data were identified for 15‐Ac‐DON but 3‐Ac‐DON was rapidly deacetylated in the upper intestinal tract and absorbed exclusively as DON. Limited data on DON‐3‐glucoside indicated that its bioavailability was two times lower than that of DON, and it can be concluded that DON‐3‐glucoside was only absorbed as DON. Cleavage of DON‐3‐glucoside may occur extensively and primarily by the microflora in the gastrointestinal tract.
For poultry, a low degree of absorption of DON into plasma up to 10% was observed as well as a rapid metabolism and clearance from plasma. The only available study on broiler chickens indicated a nearly complete hydrolysis of 3‐Ac‐DON to DON and a partial (74%) hydrolysis of 15‐Ac‐DON to DON. Therefore the CONTAM Panel assumed that 3‐Ac‐DON is absorbed as DON to a larger extent than 15‐Ac‐DON. Only one available study in chickens indicated that DON‐3‐glucoside was not hydrolysed to DON in vivo. Furthermore, the absolute oral bioavailability of DON‐3‐glucoside was low (3.8%) and comparable to that of DON. The results of all identified studies in poultry species indicated the absence of DOM‐1 in plasma. Analysis of phase II metabolites revealed that biotransformation of DON and DON‐3‐glucoside in chickens mainly consisted of conjugation with sulfate compared to glucuronidation. In broiler chickens and turkey poults the major metabolite of DON in plasma was DON‐3‐sulfate. Glucuronidation of DON to DON‐3‐glucuronide is a minor pathway. Only trace amounts of other metabolites were found including 10‐DON‐sulfonate, DOM‐1 and 10‐DOM‐1‐sulfonate. DON‐3‐sulfate was also identified as the major metabolite of orally administrated DON in excreta of several poultry species.
In horses, rapid clearance of DON in plasma was observed and it is assumed that only low concentrations (approximately 10 ng/mL) could reach the systemic circulation. One study indicated the presence of DOM‐1 in serum, which was correlated with the amount of DON intake. Consequently, microbial de‐epoxidation of DON could also take place in the horse gut. Another study in horses indicated that 76–79% of the absorbed DON is present in plasma as its glucuronide form. No data were identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in horses.
For dogs and cats, no information on toxicokinetics was identified in the literature for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
7.1.6. Transfer in farm animals
7.1.6.1. DON
The transfer of DON into edible products from livestock was reviewed by EFSA (2004), and subsequently by Pestka and Smolinski (2005), Pestka (2007, 2010a,b), Fink‐Gremmels (2008), Völkel et al. (2011), Dänicke and Brezina (2013) and Flores‐Flores (2015).
For ruminants, a study was performed by Prelusky et al. (1984b) in which a single oral dose of 920 mg DON was administered to two lactating cows of similar weight (1.9 mg/kg bw DON). Both free DON and DON β‐glucuronide were detected in milk at low concentrations (< 4 ng/mL), with an LOD of 1 ng/mL (see Section 7.1.5.1). After the administration of up to 12 mg DON/kg of complementary feed for 10 weeks, neither DON nor DOM‐1 were found in cow milk above the LOD of 1 ng/mL (Charmley et al., 1993). In the study of Seeling et al. (2006) on lactating cows, the transfer from a daily oral DON exposure of 16.6–75.6 mg DON to milk was low in the range of 0.01–0.02%, while for DOM‐1 it varied between 0.04% and 0.24%. Keese et al. (2008a) performed a long‐term study to investigate the metabolism of DON and its transfer into milk (for details see Section 7.1.5.1). DON and DOM‐1 in freeze‐dried milk were determined by HPLC‐UV with a LOD of 4 μg/kg in freeze‐dried milk (corresponding to 0.5 μg/kg milk with 12.5% dry matter). No unmetabolised DON was detected in milk. Concentrations of DOM‐1 in milk of cows fed a contaminated diet with 5.3 mg DON/kg feed ranged between 0.6 and 2.2 μg DOM‐1/kg milk. Milk samples of control cows did not contain measurable amounts of DOM‐1 (< 0.5 μg/kg). The total transfer ranged between 0.02 and 0.1% and tended to be linearly correlated with the milk yield.
Winkler et al. (2015c) studied the transfer rate of zearalenone, DON and their metabolites into milk (see details in Section 7.1.5.1.1). For milk analysis, an LC–MS/MS method was used for the simultaneous determination of zearalenone, DON and their metabolites. The mycotoxin concentrations in milk of cows fed the control diet were significantly lower compared with cows fed the contaminated diet. DOM‐1 showed the highest concentration (5.03 ng/mL milk). In addition, DON concentrations up to 2.5 ng/mL were detected in the milk samples. The calculated transfer ranged between 0% and 0.17% for DON and were independent of the DON exposure.
Prelusky et al. (1987) performed two experimental studies on ewes. When 16.5–18.9 mg DON/kg bw was orally administered, levels up to 0.017 and 0.205 μg/L of DON and DOM‐1 were observed in milk, respectively. In another study, when 4 mg DON/kg bw was administered intravenously, the highest concentrations in milk were 0.061 μg DON/L and 1.220 μg DOM‐1/L, respectively.
In pigs, fed DON both ad libitum or with restrictive diets up to 6.68 mg/kg over a period of 12 weeks, mean transfer rates of the sum of DON and DOM‐1 were 1.5% for kidneys, 0.5% for liver, 0.23% for serum, 0.16% for muscle and 0.02% for back fat. The time period between the end of feeding and slaughter had no consistent effect on concentrations of the sum of DON and DOM‐1 in the analysed specimen (Goyarts et al., 2007). In the review of Dänicke and Brezina (2013), a maximum transfer rates of DON for muscle and back fat were 0.43 and 0.12%, respectively.
For broilers, no DON or DOM‐1 residues were detected (LOD 4 μg/kg) in liver and breast meat of broilers in an experiment where the DON concentration reached approximately 1.5 mg/kg diet (Dänicke et al., 2007a). In an experiment performed by CODA‐CERVA (2011–2012), 80 broilers were fed with a control starter diet during 12 days and then divided in two groups of 40 animals. One group used as control was fed with the slightly contaminated feed as control containing 0.16 mg DON/kg, 0.002 mg 3‐Ac‐DON/kg feed and other Fusarium toxins. The other group was fed with a highly contaminated feed containing 1.71 mg DON/kg, 0.04 mg 3‐Ac‐DON/kg, 0.09 mg 15‐Ac‐DON/kg feed and other Fusarium toxins. The exposure period of 14 days was followed by a depletion period of 14 days during which animals received again the control feed. No DON, 3‐Ac‐DON, 15‐Ac‐DON and their metabolites were detected in the meat, liver and skin of any group. In the experiment of Osselaere et al. (2012) (see Section 7.1.5.3.1) in three groups of 8 broiler chickens none of the residues were detected in liver and kidneys in any groups.
In three experiments performed by Prelusky et al. (1986, 1987, 1989), laying hens were fed contaminated diet with spiked radiolabelled 14C‐DON. In a first experiment, radiolabelled DON given at a single dose of 2.2 mg/bird. Maximum tissue levels were measured at 3 h in liver, kidney, heart, spleen and gizzard, while for muscle and fat the maximum radioactivity was measured after 6 h. Clearance of radioactivity from tissues had an average half‐life of 16.83 ± 8.2 h (Prelusky et al., 1986, 1987). In a second experiment, birds were fed daily over a period of 12 days with a diet spiked with DON (2.2 mg DON/bird and per day during 6 days, followed by 2.2 mg 14C‐DON/bird per day for 6 days). Measured radioactivity levels in eggs increased with each subsequent egg laid up until the end of the exposure. The maximum accounted level was equivalent to 4.2 μg of DON or metabolites/60 g egg, corresponding to 0.19% of the daily dose. Residues declined quickly once the birds were fed non‐contaminated diet (Prelusky et al, 1987). In a third experiment, birds were fed with a diet spiked at 0.55 mg 14C‐DON/bird per day during a 65‐day period followed by a 21‐day period during which birds were fed by a non‐contaminated diet. At day 8 of exposure the maximum level was 1.7 μg of DON or metabolites‐equivalents/60 g egg corresponding to 0.31% of the daily 0.550 mg DON exposure of hen. The yolk, albumen and membrane contributed to 70, 29 and 1% of measured radioactivity, respectively. At the 30th day, levels declined to 25% from the measured maximum level and remained constant until the end of the contamination period and became quickly negligible when birds were fed non‐contaminated diet (Prelusky et al., 1989).
Neither DON nor DOM‐1 were detected in yolk or albumen (concentrations < LODs of 2.5 and 1 μg/kg, respectively) at a dietary DON concentration of 11.9 mg/kg feed (Valenta and Dänicke, 2005). After feeding laying hens diets with DON concentrations between 5 and 10 mg/kg, trace amounts of DON in the range of 0.13 and 0.79 μg/kg whole egg were detected. These trace concentrations corresponded to estimated transfer between 0.003% and 0.007% (Sypecka et al., 2004).
In the study performed by CODA‐CERVA (2011–2012) (see above), 36 laying hens (30–55 weeks old), were selected on zootechnical performance during 4 weeks and then divided in two groups of 18 animals. The first group was fed with the low contaminated feed as control containing 0.46 mg DON/kg, 0.02 mg 3‐Ac‐DON/kg and 0.1 mg 15‐Ac‐DON/kg feed. The second group was fed with the highly contaminated feed containing 2.23 mg DON/kg, 0.07 mg 3‐Ac‐DON and 0.23 mg 15‐Ac‐DON/kg feed. This exposure period of 14 days was followed by a depletion period of 14 days during which animals received again the control feed. No DON or its acetylated forms were detected in eggs (see also Section 7.3.3.1).
In the experiment of Ebrahem et al. (2014a) (see details in Section 7.1.5.3.1) in the 60th week of the hen's life, 10 eggs from each group were collected and analysed by LC–MS/MS for DON and DOM‐1. DON levels in egg yolk and albumen ranged between 0–0.46 μg/kg and 0–0.35 μg/kg, respectively, corresponding to transfer rates of DON into eggs from 0% to 0.0016%. No differences in DON levels or transfer were observed between the two tested breeds.
Male turkeys were fed from day 21 to day 56 of age with increasing proportions of Fusarium toxin‐contaminated wheat containing up to approximately 5.4 mg DON/kg diet. Concentrations of DON and DOM‐1 in liver and breast meat were lower than the LODs of 4 μg/kg (Dänicke et al., 2007b).
No information on transfer of DON in animal species other than dairy cows, pigs and poultry was identified.
7.1.6.2. 3‐Ac‐DON and 15‐Ac‐DON
In the CODA‐CERVA (2011/2012) study, neither 3‐Ac‐DON nor 15‐Ac‐DON were detected in the liver, muscles and skin of the broilers nor in the eggs (see details above) of laying hens. In the experiment of Osselaere et al. (2012) on chickens no residues of 3‐Ac‐DON or 15‐Ac‐DON were detected in liver. No data on other animals than chicken were identified regarding transfer of 3‐Ac‐DON or 15‐Ac‐DON.
7.1.6.3. DON‐3‐glucoside
No data were identified.
7.1.6.4. Conclusions on transfer
The available data on DON in dairy cows, pigs and poultry show that its transfer from feed to food products of animal origin is very low. The CONTAM Panel concluded that residues of DON in products of animal origin are unlikely to contribute substantially to human exposure. No information on transfer of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was identified for dairy cows, pigs or poultry. For other farm animal species, no information on transfer of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was identified.
7.2. Toxicity in experimental animals
Since the assessment of the SCF (1999), the toxicity of DON has been reviewed by JECFA (2001, 2011), Pestka and Smolinski (2005), Pestka (2007, 2010a,b) and Sobrova et al. (2010). Toxicity data available for 3‐Ac‐DON and 15‐Ac‐DON have been reviewed by JECFA (2011). The SCF (1999) characterised acute/subacute toxicity of DON as vomiting, feed refusal, weight loss and diarrhoea. At that time, reduced feed intake, reduced body weight gain and changes of some haematological parameters were identified as subchronic effects of DON and its chronic toxicity was described on the basis of the data reported by Iverson et al. (1995). Since then more studies on the acute, subacute, subchronic and chronic toxicity in experimental animals have been published, a few of them also describe the toxicity of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. For this opinion, the most relevant ones were identified and considered for the hazard characterisation of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (see also Table 45 for the studies).
Table 45.
The selected subacute, subchronic and chronic studies on adverse effects in rodents orally exposed to DON
| Study duration | Animal species | Exposure | Adverse effects observed | No effect dose (mg/kg bw per day) | Reference |
|---|---|---|---|---|---|
| Subacute 14 and 28 days | Mouse | 0, 0.25, 0.5, 1.0, 2.0 mg/kg diet (equivalent to 0.047, 0.094, 0.188, 0.376 mg/kg bw per day (males) and 0.056, 0.112, 0.225, 0.450 mg/kg bw per day (females) for 14 and 28 daysa | Haematological disturbances (14 days) | 0.094 (male) | Wu et al. (2009) |
| Decreased feed intake | Not identified < 0.047 | ||||
|
Subacute 9 weeks |
Rat | 0, 0.25, 0.5, 1 mg/kg bw per daya for 9 weeks |
Decreased DNA synthesis in spleen and thymus Decreased feed intake Decreased body weight gain |
Not identified < 0.25 |
Arnold et al. (1986) |
|
Subacute 28 days |
Rat | 0, 0.5, 1, 2.5, 5 mg/kg bw per daya |
Modification of male reproductive parameters Decreased feed intake |
1 | Sprando et al. (2005) |
|
Subchronic 90 days |
Rat | 20 mg/kg diet, equivalent 1.62 mg/kg bw per dayb for 90 days | Decrease in feed conversion efficiency |
Not identified < 1.62 |
Morissey et al. (1985) |
| Subchronic 80 days | Rat | 0, 0.06, 0.25 mg/kg bw per day for 80 days | Decreased body weight | 0.06 | Li et al. (2011)e |
| Articular lesions |
Not identified < 0.06 |
||||
| Subchronic 26 weeks | Mouse | 0, 0.09, 0.53, 1.57 mg/kg bw per daya for 26 weeks | Decreased body weight | 0.09d | Bondy et al. (2016) |
| Chronic 2 years | Mouse | 0, 0.098, 0.506, 1.126 mg/kg bw per day (males) and 0, 0.115, 0.661, 1.520 mg/kg bw per day (females)c for 2 years |
Decreased feed intake Decreased body weight |
0.1 | Iverson et al. (1995) |
NOAEL: no‐observed‐adverse‐effect; LOAEL: lowest‐observed‐adverse‐effect; bw: body weight; n.a.: not applicable
Dose calculated by the authors
Dose calculated by the CONTAM Panel according to EFSA guidance documents (EFSA Scientific Committee FEEDAP Panel, 2012).
Dose calculated by the CONTAM Panel by using the values reported in the study.
Bench mark dose (BMD)‐interval of 0.11–0.90 mg DON/kg bw per day was calculated by the CONTAM Panel (see Section 7.8.3).
Original paper in Chinese, text based on the translation to English.
7.2.1. Acute toxicity in rodents
This section describes studies on acute effects of an oral exposure to DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in rodents. Acute effects in farm and companion animals are reported under the respective subsections in Section 7.3.8.
7.2.1.1. DON
After oral administration, the LD50 values for DON have been calculated as 78 and 46 mg/kg bw in B6C3F1 and DDY mice, respectively (Yoshizawa et al., 1983).
After the SCF (1999) assessment on DON, two studies were published on mice. In one study, a single oral administration of 6.25, 12.5 and 25 mg DON/kg bw to adult mice (DBA/llac J) revealed that DON profoundly affected and altered the night‐time food intake and meal microstructure measured from 3 to 168 h after treatment (Girardet et al., 2011a,b). Flannery et al. (2011) reported that within 2 h of exposure to 2.5 and 5 mg DON/kg bw by oral gavage, food consumption was reduced by 68 and 77%, respectively, while doses of 0.5 and 1.0 mg DON/kg bw had no effect in female B6C3F1 mice.
7.2.1.2. 3‐Ac‐DON and 15‐Ac‐DON
Schiefer et al. (1985) studied acute toxicity of 3‐Ac‐DON after a single intragastrical administration at doses of 0, 5, 10, 20 or 40 mg/kg bw in male mice ([Crl:CDl (ICR) BR) (n = 175) fasting 16 h before and 6 h after dosing and sacrificing batches of n = 5 after 2, 4, 6, 12, 24, 48 and 96 h. After 12 h, the animals started to show behavioural signs (e.g. restricted moving). Histopathological examination showed duodenal crypt injury (reduced mitosis and necrosis) and necrosis in spleen and thymus post severe at the highest dose of 40 mg 3‐Ac‐DON/kg bw where all animals died until 96 h.
Since the intensity of lesions in the 40 mg/bw group corresponded to lesions known to be caused by 4 mg/kg of T‐2 toxin, the authors concluded that 3‐Ac‐DON was considerably less toxic than T‐2 toxin, but caused acute effects in the dividing cells of the body in a manner characteristic of trichothecenes. The CONTAM Panel noted that no LD50 value for 3‐Ac‐DON could be calculated from this study.
Doses of 1 and 2.5 mg/kg bw of 3‐Ac‐DON and 15‐Ac‐DON, respectively, caused in female B6C3F1 mice feed refusal within 2 h after exposure, but afterwards a compensatory increase in food intake was observed over the next 14 h (Wu et al., 2014a).
7.2.1.3. DON‐3‐glucoside
DON‐3‐glucoside was tested in a nocturnal mouse food consumption model. Oral administration of DON‐3‐glucoside, at doses of 2.5–10 mg/kg bw induced feed refusal that lasted up to 16 and 6 h, respectively. Exposure to 2.5 mg DON‐3‐glucoside/kg bw stimulated plasma elevations of the gut satiety peptides cholecystokinin and to a lesser extent, peptide YY3‐36 that corresponded to reduced feed consumption (Wu et al., 2014d).
7.2.1.4. Conclusions on acute toxicity in rodents
The CONTAM Panel noted that after a single DON exposure feed refusal appeared very quickly in mice. After oral administration, the lowest LD50 values for DON was 46 mg/kg bw in mice. It was also noted that as rodents do not vomit. However, decreased feed intake was identified as relevant endpoint for acute effects in mice.
7.2.2. Subacute toxicity
After the SCF (1999) assessments, the following studies below were identified by the CONTAM Panel.
7.2.2.1. DON
7.2.2.1.1. Mice
Groups of 10 male and 10 female BALB/c mice were fed diets with 0, 0.25, 0.5, 1.0 and 2.0 mg DON/kg feed (equivalent to 0, 0.047, 0.094, 0.188 and 0.376 mg/kg bw per day in males and 0, 0.056, 0.112, 0.225 and 0.450 mg/kg bw per day in female) for 14 and 28 days (Wu et al., 2009). All 200 mice grew normally with no gross signs of illness. There was no significant difference in initial and final body weight and also in feed intake in the four dose groups compared with controls, except that the mean feed intake was significantly lower in female mice fed 2 mg DON/kg for 28 days (3.13 ± 0.05 g per day) compared with control females (3.34 ± 0.05 g per day). Using flow cytometry with staining for leukocyte surface markers, the percentage of CD19+ leukocytes (B cells) in peripheral blood was decreased in both sexes of BALB/c mice after 14 days of exposure to 1.0 or 2.0 mg DON/kg, whereas exposure to DON over 28 days did not reduce B cells at any dose compared to the control diet. The percentage of mononuclear cells in peripheral blood was decreased in female mice fed 1.0 and 2.0 mg DON/kg after 14 days compared to mice under the control diet. The percentage of CD11b(+) leukocytes (monocytes) in peripheral blood and total CD11b(+) splenic leukocytes was decreased in female mice fed 1.0 and 2.0 mg DON/kg only after 28 days compared with control mice. Form these data, the CONTAM Panel concluded that the female BALB/c mice were more sensitive to DON than the males and that BALB/c mice adapted to DON exposure because peripheral blood cellular effects of DON at 14 days disappeared by 28 days with the exception of monocyte changes in females. The CONTAM Panel considered the haematological disturbances as relevant effects to identify a NOAEL from this study (see Section 7.2.6 and Table 45).
The CONTAM Panel noted that Kobayashi‐Hattori et al. (2011) and Clark et al. (2015) also reported subacute toxicity of DON in mice but limitations in the design of the studies did not allow to identify NOAELs and they were not considered further for hazard characterisation.
7.2.2.1.2. Rats
Subacute toxicity in male and female Sprague–Dawley rats (n = 25 rats per dose group and sex) exposed to 0. 0.25, 0.5 or 1 mg DON/kg bw per day approximately for 9 weeks was reported by Arnold et al. (1986) from a substudy of a project which also studied reproductive toxicity (Khera et al., 1984) (see Section 7.2.7.1.1). Body weight gain correlated with reduced feed intake and was significantly reduced in all treated female rats from the lowest dose group of 0.25 mg/kg bw per day and in male rats at the highest dose group of 1.0 mg DON/kg bw compared with controls. No haematological disturbances and histopathological lesions were attributed to DON (see Section 7.2.6), but significant decreases in thymidine labelling occurred in the spleens and jejunums of the male rats, markedly at the highest dose of 1 mg DON/kg bw. No NOAEL could be identified since adverse effects were observed at the lowest dose in this study.
Sprando et al. (2005) studied the effect of DON on male reproductive function (see also Section 7.2.7). Male Sprague–Dawley rats were divided into a control group and four treatment groups (0.5, 1.0, 2.5 and 5.0 mg DON/kg bw per day) exposed to DON daily for 28 days via gastric intubation. Four animals were removed from the 5.0 mg/kg dose group prior to tissue collection because of treatment related effects resulting from DON exposure. These animals were emaciated and presented with an ocular and/or nasal discharge, rapid respiration, diarrhoea and/or oedema in the front and rear legs. Both body weight gain and the final body weight of animals in the 5.0 mg/kg dose group and feed consumption in animals in the 2.5 mg/kg and 5.0 mg/kg dose groups were significantly reduced compared to controls. The body weight gain for the animals receiving 5.0 mg/kg bw DON was decreased during the entire dosing period. The left testis and left epididymis from each of the experimental and control animals were removed and weighed prior to perfusion fixation. The other testis and epididymis, the prostate and seminal vesicles were weighed after tissue fixation. Heart, spleen, liver, kidneys, adrenal and brain were collected and weighed, after perfusion fixation. Statistically significant reduced heart and kidney weights (expressed per gram of brain weight) for experimental animals were reported. A decrease in heart weight/gram of brain weight occurred in the 5.0 mg/kg dose group while effects on paired kidney weight per gram of brain weight occurred in the 2.5 mg/kg bw and 5.0 mg/kg bw Statistically significant differences in organ weights were not observed when expressed per gram of body weight. The animals were examined in detail for reproductive effects, in particular on epididymal and seminal vesicle weights (expressed per gram of body weight and brain weight), prostate weight (expressed per gram of body weight and brain weight), germ cell degeneration, sperm retention, abnormal nuclear morphology, spermatid numbers, cauda epididymal sperm numbers expressed per gram cauda epididymis), sperm tail abnormalities, sperm swimming speed (see Section 7.2.7.1.1), including effects on serum follicle‐stimulating hormone (FSH) and luteinising hormone (LH). Overall, the CONTAM Panel identified a NOAEL of 1 mg DON/kg bw per day at which no reduced feed intake was observed (see also Section 7.2.7.1.1).
7.2.2.1.3. Pigs, dogs and mink
Several studies have been conducted in pigs, dogs and mink to evaluate the subacute effects of dietary levels of DON‐contaminated feed on performance and pathology. These studies were not further used for human hazard characterisation but were used for hazard characterisation of farm and companion animals and are therefore reported in Section 7.3.
7.2.2.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.2.2.3. DON‐3‐glucoside
No data were identified.
7.2.2.4. Conclusions on subacute toxicity in rodents
Subacute toxicity of DON was characterised also as vomiting, feed refusal, weight loss and diarrhoea. No data on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified.
7.2.3. Subchronic toxicity in rodents
7.2.3.1. DON
As reported by the SCF (1999), subchronic oral toxicity studies in mice, rats and pigs showed several effects i.e. reduced feed intake, reduced weight gain, and changed levels in some blood, with NOAELs ranging from 0.04 to 0.06 mg/kg bw per day from the available data.
7.2.3.1.1. Rats
The SCF (1999) did not consider the 90‐day study of Morissey et al. (1985). Purified DON incorporated into the diet at a level of 20 mg/kg (equivalent to 1.62 mg/kg bw per day as calculated by the CONTAM Panel using the data reported by the authors) was provided to male Sprague–Dawley rats ad libitum for 90 days (10 animals each in control and treated groups). Few clinical signs of toxicity were observed. Rats in the DON treatment group were less efficient in converting feed into body mass, but there was no feed refusal. Terminal body weight was reduced in the DON‐treatment group. A few appreciable effects in haematological parameters and differential white blood‐cell counts were observed (Section 7.2.6). In the DON treatment group there were slightly fewer red and white cells, less haemoglobin and more segmented neutrophils than in the control group. None of these changes was statistically significant. Slight but not statistically significant differences in serum enzyme activities and substrates were also reported. No adverse effects were observed in the liver detoxification system, the liver/body weight ratio or the activity of glutathione S‐transferase. The data from this study indicated that DON decreased feed conversion efficiency in rats at the level of 1.62 mg/kg bw per day. The CONTAM Panel noted that this was a 90‐day study but because only one single dose was used it was not considered further for hazard characterisation.
In another study, two groups of healthy male SPF‐grade Wistar rats (24 young rats of 20 days of age) and adults (24 animals) were divided into a young rat control group, young rat low dose group, young rat high‐dose group, and normal adult rat control group, adult rat low dosage group and adult rat high‐dosage group (Li et al., 2011, 36). Each group contained eight animals. Animals in all experimental groups were exposed during 80 days to DON via gavage every day with the low dosage groups given 0.06 mg/kg bw per day, the high dosage groups given 0.25 mg/kg bw per day and the control groups were given an equivalent volume of saline. Following DON exposure, no abnormalities were observed in the control groups, but animals in the highest dose groups showed a slight decrease in body weight and manifested increased sensitivity and irritability in response to external stimuli. In both young and adult rats, the number of lesions increased and lesions on articular cartilage, but also bone development adversely affected primarily in the form of developmental abnormalities and growth stagnation (cessation) in the trabecular bone were more severe as the dose of DON increased. The CONTAM Panel noted that no NOAEL could be identified.
In 2016, Bondy et al. (2016) performed a study on p53+/+ and p53+/− male mice exposed to DON (10 animals per group) via diet during 26 weeks at doses of 0, 1, 5 and 10 mg/kg diet equal to 0, 0.09, 0.53 and 1.57 mg/kg bw per day as calculated by the authors. No toxicologically significant modifications of haematological and biochemical parameters were observed. A statistically significant decrease of body weight compared with controls was observed at the middle and highest doses (0.53 and 1.57 mg/kg bw per day, respectively) for the p53(+/+) and at the highest dose only for p53(+/−) mice, but not at the lowest dose, indicating a NOAEL for reduced body weight of 0.09 mg/kg diet. Food consumption was significantly lower than controls only at the highest dose and only up to week 2 and weeks 2–3 for the two genotypes, respectively, but higher than the controls later on at weeks 6–12 and 5–22, respectively. Therefore the authors calculated no single dose per concentration group but a range of possible doses for each group based on these varying intake. No tumours were observed after the DON treatment (see also Section 7.2.10).
7.2.3.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.2.3.3. DON‐3‐glucoside
No data were identified.
7.2.3.4. Conclusions on subchronic toxicity in rodents
For DON, no NOAEL could be identified from the new studies available after the assessment of the SCF in 1999 where NOAELs of 0.04–0.06 mg/kg bw were indentified. However, the lowest calculated LOAEL was 0.06 mg DON/kg bw per day for decreased body weight in rats. No data on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified.
7.2.4. Chronic toxicity in rodents
7.2.4.1. DON
Only one chronic/carcinogenicity toxicity study was identified in the literature (see also Table 46). The design of this study of Iverson et al. (1995) was very similar to the OECD Guidelines 452 (2009).37 Male and female B6C3F1 mice in groups of 50 (2 mice per cage, male and females separated) were given diets containing DON mixed with 4% corn oil at a concentrations of 0, 1, 5 and 10 mg/kg diet, equal to 0, 0.115, 0.661 and 1.520 mg/kg bw per day in males and 0, 0.098, 0.506 and 1.126 mg/kg bw per day in females for 2 years. Exposure was calculated by the CONTAM Panel using the available information on average body weight and average feed consumption reported by the authors. The purity of DON was > 95%, and it contained no 3‐Ac‐DON, 15‐Ac‐DON or 7‐deoxy‐DON. It was confirmed that control diet contained less than 0.1 mg DON/kg diet. Consumption and body weight was based on 2‐weekly weighing. The 2‐year survival rates were similar in males (68–84%) and females (72–84%) with deaths starting after about 1 year in males and after about 1.5 years in females.
Table 46.
Developmental and reproductive toxicity studies on DON in experimental animals
| Species | n per group | Dose (mg/kg bw per day) | Route | Effect | NOAEL (mg/kg bw per day) | Reference |
|---|---|---|---|---|---|---|
| Reproductive toxicology | ||||||
| Mouse, Swiss Webster, weanling | 7–20 | 0, 0.38, 0.75, 1.5, 2.0 | Diet | Postnatal survival | 0.38 | Khera et al. (1984) |
| Rat, Sprague–Dawley, male/female | 15/15 | 0, 0.25, 0.5, 1.0 | Diet | Embryotoxicity/maternal toxicity | 1.0 | Khera et al. (1984) |
| Rat, Sprague–Dawley, male/female | 10 m,25 f | 2.0 | Diet | Reduced fertility | 2.0 (LOAEL) | Morrissey and Vesonder (1985) |
| Mouse, B6C3F1 IL‐6KO and WT | 3–6 | 2.0 | Diet | Body weight, epididymal weight | 2.0 (LOAEL) | Sprando et al. (1999) |
| Rat, Sprague–Dawley, male | 15–16 | 0, 0.5, 1.0, 2.5, 5.0 | Gavage, 28 days | Reproductive endpoints | 1.0 | Sprando et al. (2005) |
| Developmental toxicity | ||||||
| Mouse, Swiss Webster, pregnant | 15–19 | 0, 0.5, 1.0, 2.5, 5.0, 10.0, 15.0 | Gavage,gestation days 8–11 | Skeletal abnormalities | 0.5 | Khera et al. (1982) |
| Rat, Fischer, female | 22–23 | 0, 0.06,0.24, 0.6 | Diet | No teratogenic/reproductive effects | 0.6 | Morrissey et al. (1984) |
| Rabbit, New Zealand, female | 13–15 | 0, 0.3, 0.6, 1.0, 1.6, 1.8, 2.0 | Diet | Maternal and fetal weight | 0.6 | Khera et al. (1986) |
| Rat, Sprague–Dawley, pregnant | 24 | 0.5, 1.0, 2.5, 5.0 | Gavage, gestation day 6–19 |
Fetal toxicity Maternal toxicity |
1.0 0.5 |
Collins et al. (2006) |
n: number; bw: body weight; LOAEL: lowest‐observed‐adverse‐effect level; m: male; f: female.
There was no significant difference in food consumption between the control and the female treated mice. Male mice at the top two doses consumed significantly less than those on the control diet. Body weight curves differed between males and females. While males reached a saturation by around 1 year and then the curves declined, females continue to gain weight up to about 1.5 years. Lower weight gains were associated with increasing amounts of DON in the diet, and both males and females in the top two doses showed a significant decrease in body weight compared to the control animals. In females there was a decreasing linear dose‐related trend in the absolute liver and kidney weights, but this was not apparent after adjustment for body weight differences.
In male mice, there was a decreasing linear dose‐related trend in absolute and adjusted liver weights, the differences being significant for animals receiving the 5 and 10 mg DON/kg diet Adjusted spleen weights also had a decreasing dose‐related trend and testes weights an increasing dose‐related trend, with a statistical difference in weights at the DON dose of 10 mg/kg diet. There was a linear increasing dose‐related trend in serum IgA and IgG levels in female mice but not in males, and a statistically significant increase in IgA concentrations in female mice treated with 10 mg DON/kg in the diet. Sporadic changes were noted in haematological and clinical chemical end‐points, but the authors considered that these changes were not biologically significant.
From this study, the CONTAM Panel calculated a NOAEL of 0.1 mg/kg bw per day for reduced feed intake and reduced body weight.
7.2.4.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.2.4.3. DON‐3‐glucoside
No data were identified.
7.2.4.4. Conclusions on chronic toxicity in rodents
Only one chronic toxicity study on DON was identified in the literature. From this study, the CONTAM Panel identified a NOAEL of 0.1 mg/kg bw per day for reduced feed intake and reduced body weight. No data on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified.
7.2.5. Immunotoxicity
Both the SCF (1999) and JECFA (2011) have recognised the effect of DON on immune response. The SCF (1999) noted that ‘studies with experimental animals demonstrated effects on the immune system, notably effects on immunoglobulin A (IgA). There are indications for a suppression of humoral and cellular immunity, resulting in an increased susceptibility for infectious diseases (NOAEL of 0.25 mg/kg bw per day and a lowest‐effect level of 0.22 mg/kg bw per day in studies with male Swiss‐Webster and male Balb/C mice, respectively)’. More recently, JECFA (2011) noted that ‘results from studies on immunotoxicity in mice and pigs showed that low doses of DON increase IgA levels in the blood. There were insufficient data with which to establish a threshold for IgA nephropathy. Most mechanistic studies on immunological end‐points in mice and pigs were unsuitable for deriving a NOAEL’.
The first section below focuses on studies performed in vivo (ingestion of contaminated diet) on experimental animals and assessing immune functions. In vitro experiments performed on human immune cells are also described since they may be relevant for hazard characterisation. Due to the large database on in vivo and in vitro studies on immunotoxicity of DON, the CONTAM Panel decided to base the description of the immunotoxicity of DON mainly on review papers (Rotter et al., 1996; Pestka, 2003, 2010b; Pestka et al., 2004; Antonissen et al 2014; Wu et al., 2014c; Payros et al., 2016). The evidence database was far smaller for the 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside and for these compounds the CONTAM Panel reviewed all the identified studies.
7.2.5.1. In vivo studies on rodents
7.2.5.1.1. DON
As reported by the SCF (1999), studies with experimental animals demonstrated effects on the immune system notably effects on IgA. In mice, DON induces a dramatic increase in the level of serum IgA up to 10–15‐fold, as well as a marked elevation of serum IgA‐immune complex and polymeric IgA (as reviewed by Pestka, 2003). A concomitant increase in immunoglobulin E (IgE) and decrease in IgG and IgM levels has also been observed in some experiments (as reviewed by Pestka et al., 2004). In rodents, the threshold for this effect is 2 mg DON/kg diet, with maximal effects occurring in the 10–25 mg DON/kg range (Rotter et al., 1996). A study on growing pigs fed with a semisynthetic diet contaminated with 0, 300, 600, and 1,200 μg pure DON/kg diet indicated a statistically significant increase of IgA levels in the serum in animals receiving levels 600 μg/kg feed (Drochner et al., 2004). An increased IgA level has also been observed in duodenal washing from BALB/c mice orally administered with 0.5 or 2 mg DON/kg bw for 14 days (Islam et al., 2013 ). What is notable is that in this experiment as well as in the 2‐year study of Iverson et al. (1995) the increased serum IgA level was seen only in females exposed to the highest dose of DON (1,600 μg/kg bw).
The capacity of DON to expand IgA‐secreting cells in mice is mediated by increased cytokine production, especially IL‐6, by macrophages and T cells (as reviewed in Pestka et al., 2004).
In mice, oral exposure to DON also induces at the protein and the mRNA levels a diverse array of pro‐inflammatory cytokines and chemokines that can be expressed in spleen, liver, kidney and lung (as reviewed by Pestka, 2010b). The inflammatory response was observed in male B6C3F1 mice after a single oral exposure of 5 and 25 mg/kg bw DON as early as 2 h after exposure, showing the induction of mRNAs that encode for proinflammatory cytokines (IL‐1beta, IL‐6, and TNF‐alpha), T helper 1 cytokines (interferon‐gamma IL‐2 and IL‐12p40) and T helper 2 cytokines (IL‐4 and IL‐10). Lower doses had no marked effect. IL‐12p40 mRNA was also induced, but not IL‐12p35 mRNA. The effects were more pronounced in the spleen than in the Peyer patches. No effect was observed at 1 mg DON/kg bw The same protocol was used to investigate the effect of repeated doses (2, 4 or 7 consecutive days). Upon exposure to 2 and 5 mg/kg bw DON, the relative abundance of IL‐1beta, IL‐6, TNF‐alpha, IL‐12 p35, IL‐12p40, IL‐2 and IL‐10 mRNAs increased with dose frequency, whereas IFN‐gamma and IL‐4 mRNAs were unaffected. From this study the NOAEL for DON reported by the authors was 0.5 mg/kg bw per day (review of Pestka and Smolinski, 2005). The CONTAM Panel considered this to be a NOEL instead of a NOAEL as stated by authors. This DON‐induced ‘cytokine storm’ likely contributes to the shock‐like effects observed in acute high‐dose toxicity. It might also contribute to impaired appetite and weight gain observed during chronic DON exposure by directly acting on the central nervous system or indirectly by interfering with the growth hormone axis (see Section 7.7). Lipopolysaccharide and other toll‐like receptor agonists potentiate DON toxicity in mice (Pestka, 2010b), suggesting that if collateral damage to gut integrity occurs during DON exposure, resultant bacterial translocation would greatly magnify trichothecene toxicity.
In one study, where DON was administrated at very low doses (1, 2.5 or 25 μg/kg bw per day for 10 or 30 days) in C57BL/6 mice a low grade inflammatory response was observed (Tardivel et al., 2015). Plasma IL‐1β levels increased by 1.59 fold after 10 days administration of 25 μg DON/kg bw per day but remained unaffected by a 2.5 μg DON/kg bw per day treatment. This lowest dose increased plasma IL‐1β when administered during 30 days (2.1 fold). At the mRNA level, a 10 days DON administration at 25 μg/kg bw per day resulted in a TNFα and IL‐1β upregulation in the liver (2.9‐fold increase). Transcript quantification showed that DON administered at 2.5 μg/kg bw per day during 10 days did not modify TNFα and IL‐1β gene expression. Interestingly, when the intoxication period was extended to 30 days, an upregulation of TNF‐α and IL‐1β mRNAs expression was detected in the liver at this dose. Contrary to the liver, TNF‐α and IL‐1β mRNA expressions remained unchanged in the spleen. Noticeably, IL‐6 mRNA remained undetectable with qPCR. DON exposure at 1 μg/kg bw per day during 30 days had no effect on systemic IL‐1β concentration and did not induce any significant gene expression modulation. The CONTAM Panel noted an increase at the protein and mRNA levels of inflammatory cytokines but no pathological or other adverse effects in these animals. Therefore the CONTAM Panel did not consider this study further for hazard characterisation.
DON also modulates immune cell numbers and functions. Islam et al. (2013) observed that DON had different effects on T and B cells, in female BALB/c mice orally administered DON at a dose of 0.5 or 2 mg/kg bw for 14 days. DON treatment increased the population of CD8+ and Foxp3+ regulatory T cells in the spleen and CD4+ T cells in the mesenteric lymph node, and decreased the population of CD19+ and CD11c+ immune cells in the spleen and mesenteric lymph nodes, and of F4/80+ cells in the spleen.
The above mentioned effects on the immune response may lead to adverse effects, such as increased susceptibility to infectious diseases, observed at medium to high doses of DON (8 to 10 mg/kg bw) as described in detail below.
In mice, DON in drinking water (0.2 mg DON/L (equivalent to 8 mg/kg bw)) reduced the resistance to oral infection with Salmonella enteritidis by promoting translocation of Salmonella to mesenteric lymph node (MLN), liver and spleen (Hara‐Kudo et al., 1996). Reduction in survival time to death in mice infected i.v. with Listeria monocytogenes was observed by Tryphonas et al. (1986) and Li et al. (2005) observed after a single gavage of 10 mg DON/kg bw inability to clear a reovirus from the intestine, and increased fecal shedding of the virus indicating suppressed host immune response to viral infection. Exposure to DON increased the intestinal viral load, which could increase inflammation and discomfort to the host during the infection process, decreased the cell‐mediated viral clearance by suppressing the gene expression of IFN‐γ in Peyer's patches and enhanced Th2 cytokine expression prior to and after reovirus infection, which potentiates the IgA and IgG responses to reovirus.
7.2.5.1.2. 3‐Ac‐DON and 15‐Ac‐DON
Very few studies have investigated the in vivo effect of 3‐Ac‐DON and 15‐Ac‐DON on the immune response. Kasali et al. (1985) incorporated 2.5, 5, 10 or 20 mg 3‐Ac‐DON/kg feed (corresponding to 0.36, 0.72, 1.44 and 2.88 mg 3‐Ac‐DON/kg bw per day, calculated by the CONTAM Panel using default values of EFSA Scientific Committee (2012) into a semisynthetic diet and fed male mice for up to 48 days. The humoral immune response assessed using the Jerne plaque assay was not significantly affected after seven or 14 days, but at 21 days, a dose‐dependent enhanced response was observed.
The effect of 3‐Ac‐DON was also studied on mitogen‐induced lymphocyte proliferation and antibody production in male CD‐1 mice exposed for 35 days to diets contaminated at 2.5, 5 or 10 mg/kg feed. Mitogen‐induced lymphocyte proliferation and T‐cell‐independent antibody responses to dinitrophenyl‐ficoll or Escherichia coli were not altered by dietary exposure to 3‐Ac‐DON. By contrast, the T‐cell‐dependent antibody response to sheep red blood cells was increased by dietary exposure to 3‐Ac‐DON (Tomar et al., 1987).
Acute oral exposure to 3‐Ac‐DON and 15‐Ac‐DON (gavage with 2.5 mg toxin/kg bw) induced a transient upregulation of TNF‐α, IL‐1β, IL‐6, CXCL‐2, CCL‐2 and CCL‐7 mRNA expression in the spleen of mice (Wu et al., 2014a).
7.2.5.1.3. DON‐3‐glucoside
Only the above mentioned study of Wu et al. (2014d) investigated the effect of DON‐3‐glucoside on the immune response of mice. Mice orally gavaged with 2.5 mg/kg bw DON‐3‐glucoside failed eliciting cytokine or chemokine mRNA responses in the spleen.
7.2.5.2. In vitro studies on human cells
7.2.5.2.1. DON
In vitro studies indicated that DON (100–400 ng/mL) inhibits proliferation of B‐ and T‐cell subsets stimulated by different mitogens (Forsell and Pestka, 1985; Meky et al., 2001). At concentration ranging from 150 to 1,500 ng/mL, DON was also described to inhibit the activation of different immune cells. It altered lymphocyte activation, as measured by the expression of CD71 (Johannisson et al., 1999) and macrophage activation as measured by the expression of CD54, CD14, CD119 and HLA‐DP/DQ/DR (Waché et al., 2009a). It also inhibited the maturation of dendritic cells as measured by the expressions of CD86, HLA‐DR and CCR7, the capacity for endocytosis, and the secretion of interleukins (IL‐10 and IL‐12) (Hymery et al., 2006).
DON was also described to up‐regulate cytokine production. In Jurkat T cells and in primary human lymphocytes prestimulated with mitogens, DON (62.5–500 ng/mL) up‐regulated the production of IL‐2, IFN‐gamma, IL‐4, IL‐6 and IL‐8 (Meky et al., 2001; Pestka et al., 2005; Severino et al., 2006). In human macrophage cell lines (U937 and HL60), DON (500–1,000 ng/mL) upregulated the production of TNF‐α, IL‐6, IL‐8, and the macrophage inflammatory proteins MIP‐1α and MIP‐1β (Sugita‐Konishi and Pestka, 2001; Nagashima et al., 2012).
7.2.5.2.2. 3‐Ac‐DON and 15‐Ac‐DON
Tomar et al. (1986) investigated the effects of 3‐Ac‐DON on the in vitro mitogen responses and the antibody producing ability of human peripheral blood lymphocytes. 3‐Ac‐DON inhibited the proliferative response to pokeweed mitogen and concanavalin A at a lower concentration (100 ng/mL) as compared to phytohemagglutinin (200 ng/mL). The antibody producing ability was inhibited by 3‐Ac‐DON concentrations of greater than 200 ng/mL. Concentrations of 3‐Ac‐DON greater than 300 ng/mL produced severe suppression of plaque forming cell response in vitro and reduced the total yield of lymphocytes without altering cell viability (Tomar et al., 1986).
The previously mentioned studies (Sugita‐Konishi and Pestka, 2001; Pestka et al., 2005) also compared the effect of DON and acetylated forms of DON on the human U937 macrophage and Jurkat T‐cell lines. 3‐Ac‐DON and 15‐Ac‐DON were also capable of upregulating or suppressing TNF‐alpha, IL‐6, and IL‐8 production in U937 macrophages at concentrations similar to that of DON. Similarly 15‐Ac‐DON at 62.5–500 ng/mL and 3‐Ac‐DON at 625–5,000 ng/mL also upregulated IL‐8 production in Jurkat T‐cell line. In contrast, no effect on IL‐2 synthesis was seen at concentrations of 62.5–500 ng/mL 3‐Ac‐DON and 15‐Ac‐DON (Pestka et al., 2005).
7.2.5.2.3. DON‐3‐glucoside
No data were identified.
7.2.5.3. Conclusions on immunotoxicity
DON has an impact on the immune response. Subchronic studies performed in mice and in farm animals indicate that the ingestion of this toxin induces an increase in the plasmatic level of IgA. The DON‐induced elevated IgA level was not associated with IgA nephropathy in humans and rodents. In studies with high doses, DON induced inflammation in mice. An increase in TNF‐α and IL‐1‐β was observed at doses as low as 2.5 μg DON/kg bw per day but this was seen in the absence of an observed adverse effect.
In the one available 2‐year study the increase of IgA was observed in female mice at the highest dose only, and inflammatory responses were not investigated. The effects on the immune response (doses 8–10 mg/kg bw) lead to an increased susceptibility to infectious diseases.
The data concerning the effect of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside on the immune response were scarce and none of them investigated effects on IgA levels. As far as the inflammatory response is concerned, one study observed a lower expression of genes encoding for inflammatory cytokines when compared to DON. The effect of DON‐3‐glucoside on the immune response was reported in only one study and no induction of inflammatory cytokine was observed.
The CONTAM Panel noted that no suitable dose–response data were identified on immunological adverse effects, including increase susceptibility to infection in humans or animals, relevant for risk assessment.
7.2.6. Haematotoxicity and myelotoxicity
Haematotoxicity and myelotoxicity of DON, 3‐Ac‐DON, 15‐Ac‐DON and/or DON‐3‐glucoside were not explicitly considered in the previous risk assessment of the SCF (1999). However, the CONTAM Panel noted that haematological effects have been reported in several in vivo and in vitro studies since then and therefore these are summarised below together with the few older reports.
7.2.6.1. In vivo studies
7.2.6.1.1. DON
Groups of 10 female and 10 male BALB/c mice were fed 0, 0.25, 0.5, 1.0 and 2.0 mg DON/kg feed for 14 and 28 days (Wu et al., 2009). Haematological disturbances appeared after 14 days of exposure at the two highest doses in both sexes, but decreased in male mice after 28 days (see details in Section 7.2.2). From this study, the CONTAM Panel identified the NOAEL of 0.094 mg DON/kg bw per day for haematological disturbances in mice (see also Table 45).
The CONTAM Panel noted that a study of Arnold et al. (1986) on male and female Sprague–Dawley rats (see details in Section 7.2.2) reported that in the haematological assessment no dose‐related haematological disturbances were observed. In addition, the CONTAM Panel noted a more recent study of Chatopadhyay et al. (2011) on haematological effects in mice but did not consider them further in this opinion due to imprecisely reported data. Filimon et al. (2012) observed haematological effects in male Sprague–Dawley rats after the administration of DON with feed. Since the unit of the DON administrated to the animals was not reported precisely enough also this study was not considered for hazard characterisation in this opinion.
Morissey et al. (1985) conducted a single dose study (1.62 mg DON/kg bw per day ad libitum) on male Sprague–Dawley rats for 90 days in which some haematological disturbances are described (see details in Section 7.2.3 and Table 45).
In the 2‐year study by Iverson et al. (1995), described in detail in Section 7.2.4.1, the mean RBC and eosinophil values were significantly higher in males at the highest dose (0.130 mg DON/kg bw per day) than in controls, and there was some evidence for a linear increasing dose‐related trend.
7.2.6.1.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.2.6.1.3. DON‐3‐glucoside
No data were identified.
7.2.6.2. In vitro studies
7.2.6.2.1. DON
Several in vitro studies have been published over the years. DON has been tested on human and rat haematopoietic progenitors and circulating blood cells and the effect has been compared to those of T‐2 toxin, HT‐2 toxin and DAS on the same cells (Rizzo et al., 1992; Lautraite et al., 1997; Rio et al., 1997; Froquet et al., 2001). No severe myelotoxicity or cytotoxicity of DON on circulating blood was detected. A review of Parent‐Massin (2004) on in vitro studies concluded that DON appeared to be the least haematotoxic and myelotoxic toxin of the trichothecenes and the CONTAM Panel adopted this conclusion for the available in vitro studies.
7.2.6.2.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.2.6.2.3. DON‐3‐glucoside
No data were identified.
7.2.6.3. Conclusions on haematotoxicity and myelotoxicity
The CONTAM Panel noted that no neutropenia, agranulocytosis or aplastic anaemia have been described in in vivo studies confirming the low toxicity of DON described in vitro studies. The CONTAM Panel noted that haematotoxicity and myelotoxicity DON is less compared with that from the other trichothecenes, such as T‐2 toxin, HT‐2 toxin and nivalenol, which are known to induce haematological effects. No data on haematotoxicity or myelotoxicity of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified.
7.2.7. Developmental and reproductive toxicity
7.2.7.1. DON
The conclusion of the SCF (1999) on the reproductive toxicity and teratogenicity of DON was that ‘Oral studies in mice, rats and rabbits did not show teratogenic effects. Embryotoxic effects were observed in mice and rabbits at maternally toxic doses, i.e. 1 mg/kg bw Increase in postnatal mortality was observed in mice with a NOAEL = 0.375 mg/kg bw (Khera et al., 1984). Absence and fusions of ribs were also noted in mice at the maternally toxic doses; the NOAEL was 0.5 mg/kg bw (Khera et al., 1982). In one study with rats a slight decrease in fertility was observed at 2 mg/kg bw, the only dose tested, whereas in another rat study doses up to 1 mg/kg bw did not show any effect (Khera et al., 1982, 1984, 1986; Eriksen and Alexander, 1998; Baars et al., 1999)’.38
‘Two studies exposing swine to DON in the diet during gestation are reported. Gilts fed diets containing 0.1‐4.8 mg DON/kg feed did not exhibit overt maternal toxicity or decreased feed consumption, but 1‐2 mg/kg feed (0.03‐0.07 mg DON/kg bw per day) caused reduced weight gain. No effect in number of offspring or survival or any deformities was observed. No effect of DON on reproduction was observed in doses lower than those leading to reduced weight gain (1‐2 mg/kg feed) (Bergsjö et al., 1992, 1993a,b; Eriksen and Alexander, 1998; Baars et al., 1999)’.38
The JECFA assessment of DON (FAO/WHO, 2001) considered the studies of Khera et al. (1982, 1984, 1986), Friend et al. (1983, 1986a,b), Morrissey (1984), Morrissey and Vesonder (1985), Tutel'ian et al. (1991), Bergsjö et al. (1993a,b) and Sprando et al. (1999). The conclusion was that ‘deoxynivalenol was teratogenic but not maternally toxic when given to pregnant mice at 5 mg/kg bw per day by gavage over a short critical period (days 8–11) of gestation, but not when given at 2.5 mg/kg bw per day. When deoxynivalenol was administered in the feed, the NOAEL for maternal toxicity and fetotoxicity was 0.38 mg/kg bw per day’.
Key studies considered by these assessments and the more recent studies of Sprando et al. (2005), Collins et al. (2006) and Hou et al. (2014) are described below. See also Table 46.
7.2.7.1.1. Reproductive toxicity
In the study of Khera et al. (1984), weanling male and female F0 mice were fed diets containing DON at concentrations that resulted in doses of 0 or 2 mg/kg bw per day (experiment 1) or 0, 0.38, 0.75 or 1.5 mg/kg bw per day (experiment 2). After 30 days the mice were allowed to mate. The F1a progeny of 10 dams from the control group and that from the 1.5 mg/kg bw group were cross‐fostered at birth. The F0 mice were then rebred to produce F1b litters and the fetuses examined for malformations. The result of the DON treatment was a reduction in the following parameters: feed and water intakes and body weights of male and female F0 mice, numbers of live pups and postnatal survivors, postnatal body weight of F1a progeny, number of live fetuses, and mean weight of F1b fetuses. Statistically significant effects on the lowering of the number of postnatal survivors were seen at doses of 0.75 mg/kg bw per day and above. The fertility of male and female F0 mice was not adversely affected and the F1b generation had no major malformations. Cross‐fostering of the offspring of control dams and those at 1.5 mg DON/kg bw per day adversely affected postnatal survival and body weight showing that these were affected by prenatal exposure as well as by combined pre‐ and postnatal exposure. On the basis of postnatal survival, the CONTAM Panel concluded that the NOAEL from this experiment is 0.38 mg DON/kg bw per day.
When groups of 15 male and 15 female Sprague–Dawley rats were fed diets in the study of Khera et al. (1984) that resulted in doses of 0, 0.25, 0.5 and 1 mg DON/kg bw per day for 6 weeks before and throughout pregnancy, fertility was not impaired. The fetal viscera were normal, although some dilation of the renal pelvis and urinary bladder, of unknown biological significance, was noted at all dose levels. The CONTAM Panel considered that the NOAEL for this experiment was 1 mg DON/kg bw per day.
The CONTAM Panel noted that Morrissey and Vesonder (1985) reported an adverse effect of DON on the fertility, pregnancy and postnatal development of Sprague–Dawley rats (purified DON at a concentration of 20 mg/kg (approximately 2 mg/kg bw)). Treatment was for 60 days (males) or 15 days (females) before breeding, and female rats then continued on the same diet. The CONTAM Panel also noted that Sprando et al. (1999) reported an adverse effect of DON in a study of testicular morphology and testicular and epididymal sperm counts in three strains of mice (diet containing 10 mg DON/kg feed, equivalent to 2 mg DON/kg bw per day, for 90 days). However, as these studies were single dose studies they were not considered further for hazard characterisation.
Sprando et al. (2005) characterised the effect of DON on selected male reproductive endpoints in the Sprague–Dawley rat (for details see Section 7.2.2.1). Groups were exposed daily to DON for 28 days via gastric intubation at doses of 0.5, 1.0, 2.5 and 5.0 mg/kg bw There were significantly reduced epididymal (right and left) and seminal vesicle weights (expressed per gram of bw and brain weight) in animals treated with 2.5 and 5 mg/kg bw. Sperm swimming speed was increased only in the 2.5 mg/kg bw dose group. In the 2.5 mg/kg and 5.0 mg/kg bw dose groups there was an increase in germ cell degeneration, sperm retention and abnormal nuclear morphology. As treatment‐related effects were not seen in the 1.0 mg/kg bw group, the CONTAM Panel concluded that a NOAEL of 1 mg/kg bw per day can be identified.
Hou et al. (2014) investigated the effects of a diet co‐contaminated with DON, zearalenone and aflatoxin on oocyte quality in ICR mice. Since the extent of the contribution of DON to the effect was unknown, the CONTAM Panel did not consider this study further hazard characterisation.
7.2.7.1.2. Developmental toxicity
Groups of 15–19 pregnant Swiss‐Webster mice were administered DON by oesophageal intubation daily on gestation days 8–11 (Khera et al., 1982). Two experiments were carried out, one at high doses (0, 5.0, 10.0 and 15.0 mg/kg bw per day) and one at lower doses (0, 0.5, 1.0 and 2.5 mg/kg bw per day). On the 19th day of pregnancy, animals were sacrificed and examined. There was a progressive decrease in body weight gain in the dams at doses of 5.0 mg/kg bw and higher. At the 10.0 and 15.0 mg/kg bw doses, the incidence of resorptions was 100%. At the 5.0 mg/kg bw dose, the incidence of resorptions was 80% and there was a reduced number of live fetuses per dam and reduced fetal weight. The resorptions at the dose of 2.5 mg/kg bw were lower (9%) but still statistically significant. At the dose of 5 mg/kg bw there were some external and visceral anomalies including exencephaly, syndactyly and hypoplastic cerebellum. Lower levels of external and visceral anomalies were also observed in the 1.0 and 2.5 mg/kg bw dose groups. A number of skeletal malformations were present in the 1.0, 2.5 and 5.0 mg/kg bw dose groups and these increased with dose. They included lumbar vertebrae with fused arches or partly absent centra, absent or fused ribs, missing, fused or scrambled sternebrae. There were no treatment‐related pathological changes in fetal tissues at any dose of DON. The CONTAM Panel concluded that the NOAEL in this study was 0.5 mg/kg bw per day.
In order to study the teratogenic potential of DON, feed containing DON was fed ad libitum to groups of female Fischer 344 rats during the entire course of pregnancy (Morrissey, 1984). The concentrations of DON were 0, 0.5, 2.0 and 5.0 mg/kg, equivalent to 0, 0.06, 0.24 and 0.6 mg/kg bw per day. No signs of toxicity or statistically significant differences in feed consumption were observed in the treated animals. After removal of the uterus and pups the carcass weights of dams receiving the 2.0 and 5.0 mg/kg concentrations were significantly lower than those of the control animals. No significant differences were observed between the groups on markers of the outcome of pregnancy and fetal development, such as the number of pregnant animals per group, the number of male and female pups per pregnant animal, male and female pup weights, the number of litters with early resorption, the percentage of pre‐implantation or post‐implantation losses and the number of corpora lutea. No statistically significant effect of DON treatment on visceral morphology or skeletal development was found, although some malformations and aberrations were observed. Neither dams nor pups showed any significant histopathological changes. The CONTAM Panel concluded that the NOAEL for developmental toxicity from this study was 0.6 mg/kg bw per day.
A teratology study of DON was carried out in adult female New Zealand white rabbits by Khera et al. (1986). On days 0–30 of gestation, the animals (groups of 13–15) were fed diets containing DON at concentrations of 0, 7.5, 15, 30, 60, 120 or 240 mg/kg, equivalent to 0, 0.3, 0.6, 1.0, 1.6, 1.8 and 2.0 mg/kg bw per day. A pair‐fed group was also included. Maternal body weight and feed consumption were significantly below control levels in groups receiving 1.0, 1.6, 1.8 and 2.0 mg/kg bw per day of DON in their diet. Resorptions were increased to 100% at doses of 1.8 and 2.0 mg DON/kg diet and fetal weight was decreased at doses of 1.0 and 1.6 mg/kg bw and the pair‐fed group. No teratogenic effects were observed in the treated animals. As the lower dose of 0.6 mg/kg bw per day was not maternotoxic or fetotoxic, this dose was considered by the CONTAM Panel as the NOAEL for this study.
Tutel'Yan et al. (1991)39 studied female Wistar rats for fertility and their offspring for developmental toxicity of DON (purity not reported). The CONTAM Panel decided not to consider this study further because of substantial limitations in design and analysis (feed consumption not reported, small number of dams selected, litter effects and intralitter correlation not considered and the numbers of fetuses and dams not reported for the various endpoints tested).
Collins et al. (2006) studied the effect of DON on fetal development in pregnant Charles River Sprague–Dawley rats, who were gavaged daily on gestation days 6–19, in groups of 24, with doses of DON of 0, 0.5, 1.0, 2.5 and 5.0 mg/kg bw. Reproductive and developmental parameters were measured at caesarean section on gestation day 20. A dose‐related increase in salivation was observed in the number of females with excessive salivation, which was statistically significant at doses of 2.5 and 5 mg/kg bw. Feed consumption, body weight gain and gravid uterine weight were significantly reduced in the group dosed with 5 mg/kg bw. Much smaller effects on these parameters were seen at 2.5 mg/kg bw and no effects were observed at doses below this. Liver‐to‐body weight ratios increased dose dependently in all treated groups, and the increases were significant at doses of 1, 2.5 and 5 mg/kg bw DON delayed fetal development. At 2.5 and 5 mg/kg bw, fetal body weight and crown‐rump length were significantly reduced and the incidence of runts was increased (reaching significance at 5 mg/kg bw). DON at 5 mg/kg bw per day significantly increased the average number of fetuses per litter with at least one, at least two or at least three sternebral variations. In addition, the average number of fetuses with at least one sternebral variation was significantly increased at 2.5 mg/kg bw. At 5 mg/kg bw, there were significant decreases in skeletal ossification in centra, dorsal arches, metacarpals, and metatarsals which may be related to maternal toxicity. A decrease was also seen in the average number of ossified vertebrae per fetus at 2.5 and 5 mg/kg bw per day which the authors suggested may be related to maternal toxicity. The authors proposed that, based on anomalous sternebrae development, DON could be considered a teratogen at 5 mg/kg bw per day, and the CONTAM Panel concluded that the NOAEL for this effect was 1 mg/kg bw per day. No adverse developmental effects were observed at 0.5 or 1 mg/kg bw per day, and the CONTAM Panel concluded that a NOAEL for fetal toxicity of 1 mg/kg bw and a NOAEL for maternal toxicity of 0.5 mg/kg bw could be identified from this study.
In conclusion, the most critical effect, already noted by the SCF (1999), was the increase in postnatal mortality in mice treated orally with DON, with a NOAEL of 0.38 mg/kg bw per day. The new studies since that report, do not add further to this conclusion.
7.2.7.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified for developmental or reproductive toxicity.
7.2.7.3. DON‐3‐glucoside
No data were identified for developmental or reproductive toxicity.
7.2.7.4. Conclusions
DON exhibits both developmental and reproductive toxicological effects. Based on studies of rats treated orally with DON, the CONTAM Panel concluded that the NOAEL was 1.0 mg/kg bw per day for reproductive endpoints, 1.0 mg/kg bw per day for fetal toxicity and 0.5 mg/kg bw per day for maternal toxicity. Therefore, the CONTAM Panel agreed with the SCF (1999) that the most critical effect is the increase in postnatal mortality in pups from mice treated orally with DON, with a NOAEL of 0.38 mg/kg bw per day. No data were identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
7.2.8. Neurotoxicity
No neurotoxic effects suitable for hazard characterisation were identified by the SCF (1999, 2002) or JECFA (FAO/WHO, 2001). The conclusion of JECFA (FAO/WHO, 2001) was that decreased food consumption and emesis observed after administration of DON may be linked to increased central serotoninergic activity.
7.2.8.1. DON
Al‐Hazmi and Waggas (2013) investigated the neurophysiological and behavioural effects of orally administered DON in mice but because of some inconsistencies in the reported doses and results, the CONTAM Panel did not consider this study further. Several other studies with rats, cockerels and pigs have demonstrated alterations in biogenic amine levels after treatment with DON, but no neurotoxicological effects were reported (Fitzpatrick et al., 1988a,b; Prelusky et al., 1992; Prelusky, 1993). In a study with adult male Long Evans rats, Ossenkopp et al. (1994) demonstrated that when DON was administered (i.p. 0.125 mg/kg bw) after treatment of the rats with a saccharin solution, a taste aversion to the latter developed, and that this was mediated by the area postrema.
7.2.8.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.2.8.3. DON‐3‐glucoside
No data were identified.
7.2.8.4. Conclusions
The data on neurotoxicological effects of DON were limited. The CONTAM Panel noted that no dose–response data suitable for hazard characterisation were available. No data on neurological effects were identified for 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside. Based on the available studies, a link between the neurotoxicity and mode of action could not be identified by the CONTAM Panel.
7.2.9. Genotoxicity
The assessment of the genotoxicity of DON by the SCF in 1999 and 2002 concluded that DON did not show mutagenic activity in bacteria and mammalian cells, or cause unscheduled DNA synthesis (UDS) in rat primary hepatocytes. DON enhanced cell transformation in mouse embryo cells in vitro, and induced clastogenic effects and inhibited gap‐junctional intercellular communication in Chinese hamster V79 cells. It was noted that DON inhibited protein synthesis in Chinese hamster ovary cells in vitro in the same dose range as that inducing clastogenic effects (as cited in SCF 1999, 2002). FAO/WHO (2001) concluded that DON was not mutagenic in bacteria, but suggested that DON is genotoxic as chromosomal aberrations were observed both in vitro and in vivo. However, the overall significance of the results observed in the only study conducted in vivo was considered to be equivocal as most of the chromosome aberration studies consisted of gaps.
Studies whose results formed the basis of the JECFA (FAO/WHO, 2001) and SCF (1999) assessments are summarised below together with the more recent studies identified since then of Li and Guo (2001), Hsia et al. (2004), Lin and Sun (2004), Bony et al. (2006), Frankič et al. (2006, 2008), Zhang et al. (2009), Bensassi et al (2009), Awad et al. (2012b, 2014), Takakura et al. (2014), Yang et al. (2014) and Abdel‐Wahhab et al. (2015).
The in vitro and in vivo studies identified by the CONTAM Panel are described below and results also shown in Table 47. Additionally, in vitro and in vivo genotoxicity studies of DON have been carried out by Le Hégarat et al. (2014). These were conducted according to the EFSA Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment (EFSA Scientific Committee, 2011) in the context of a grant agreement between EFSA and the authors. The results are not published in a peer‐reviewed journal but as an EFSA external report.
Table 47.
Genotoxicity studies on DON and 3‐Ac‐DON in vitro and in vivo
| Test system | Cells/animals | Concentration/treatment | Results | Comment | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
| Bacterial reverse mutation assay (Ames test) | S. Typhimurium TA98, TA100, TA1535, TA1537 | 0.4–400 μg of DON or 3‐Ac‐DON/plate (−S9) | Negative | Wehner et al. (1978) | |
| 0.4–400 μg of DON or 3‐Ac‐DON/plate (+S9) | Negative | ||||
| Bacterial reverse mutation assay (Ames test) | S. Typhimurium TA98, TA100 | 0.7–500 μg/plate (−S9) | Negative | Knasmüller et al. (1997) | |
| 0.7–500 μg/plate (+S9) | Negative | ||||
| Bacterial reverse mutation assay (Ames test) | S. Typhimurium TA98, TA100, TA102 | 0.03–500 μg of DON/plate (−S9) | Negative | Takakura et al. (2014) | |
| 0.03–500 μg/plate (+S9) | Negative | ||||
| SOS chromotest | E. coli PQ37 | 5–500 μg/assay (−S9) | Negative | Knasmüller et al. (1997) | |
| 5–500 μg/assay (+S9) | Negative | ||||
| DNA repair assay | E. coli K12 (343/753, uvrB/recA and 343/765, uvr+/rec+) | 0.7–500 μg/mL (−S9) | Negative | Knasmüller et al. (1997) | |
| 0.7–500 μg/mL (+S9) | Negative | ||||
| Mammalian cell forward mutation assay | Chinese hamster V79 cells/HGPRT with or without hepatocyte‐mediated activation | 1–3 μg/mL | Negative | Rogers and Héroux‐Metcalf et al. (1983) | |
| In vitro CAs | Rat hepatocytes | 0.001–10.0 μg/mL | Positive at 1 and 10 μg/mL | Mitotic index decreased at 10 μg/mL. Pronounced cytotoxicity at 100 μg/mL | Knasmüller et al. (1997) |
| In vitro CAs | Chinese hamster V79 cells | HPLC‐purified fractions with the retention time of DON from extracts of corn (concentration 0.1–1.0 μg/mL) | Increased CAs |
Purity of DON in the HPLC fraction not clear Insufficient number of samples. Inadequate data for risk assessment |
Hsia et al. (1988) |
| Pure DON and 3‐Ac‐DON (up to 10 μg/mL) | Increased CAs | Insufficient details given of results | |||
| In vitro CAs | Chinese hamster V79 cells | HPLC‐purified fractions with the retention time of DON from extracts of wheat flour (30 ng/mL), barley (200 ng/mL) and corn (300 ng/mL | Increased CAs |
Purity of DON in the HPLC fraction not clear Insufficient number of samples Inadequate data for risk assessment |
Hsia et al. (2004) |
| Micronucleus assay | Rat hepatocytes | 0.001–10.0 μg/mL | Positive at 0.01 μg/mL, but not at higher concentrations |
Positive result not considered biologically relevant as no dose response Mitotic index decreased at 10 μg/mL. Pronounced cytotoxicity at 100 μg/mL |
Knasmüller et al. (1997) |
| Micronucleus assay | Lymphoblastoid TK6 cells | 1.6–25.0 μM, 3 h (+) or (−) rat or human S9 |
(−) S9: positive at 25.0 μM (> 60% cytotoxicity) (+) rat S9: negative (+) human S9: positive at 12.5 μM and 25 μM (> 60% cytotoxicity) |
Induction of micronuclei only at cytotoxic doses | Takakura et al. (2014) |
| 0.4–3.2 μM, 18 or 24 h | Positive at 1.6 μM and 3.2 μM (both >60% cytotoxicity) | ||||
| Hepatoma HepaRG cells | 1.6–50.0 μM, 3 h | Negative | |||
| 5.0–35.0 μM, 24 h | Negative | ||||
| Micronucleus assay | Human peripheral lymphocytes | 6.25, 12.5, 25, 50 ng/mL for 6, 12 or 24 h | Positive |
Dose‐dependent increase in MN DON induced oxidative stress and decreased cell viability |
Yang et al. (2014) |
| Sister chromatid exchange | Human peripheral lymphocytes | 6.25, 12.5, 25, 50 ng/mL for 6, 12 or 24 h | Positive | DON induced oxidative stress and decreased cell viability | Yang et al. (2014) |
| DNA strand breaks (Comet assay) | Human peripheral lymphocytes | 6.25, 12.5, 25, 50 ng/mL for 6, 12 or 24 h | Positive | DON induced oxidative stress and decreased cell viability | Yang et al. (2014) |
| DNA strand breaks (Comet assay) | Rat hepatocytes | 0.01–1 μg/mL for 2 h | Positive | Li and Guo (2001) | |
| DNA strand breaks (Comet assay) | Vero cells | 1, 5 and 10 μM for 4 and 16 ha | Positive | Lin and Sun (2004) | |
| DNA strand breaks (Comet assay) | undifferentiated and differentiated Caco‐2 cells | 0.01–0.5 μM, 24–72 h | Positive | Bony et al. (2006) | |
| DNA strand breaks (Comet assay) | Human colon carcinoma cells (HT‐29) | 10 μM, 2, 4, 8, 12 and 24 h | Positive | Bensassi et al, (2009) | |
| DNA strand breaks (Comet assay) | human hepatoma HepG2 cells | 3.75–30 μM | Positive | After pretreatment with an antioxidant, the DNA migration dramatically decreased. Authors considered that the DNA damage is probably related to oxidative stress | Zhang et al. (2009) |
| DNA strand breaks (Comet assay) |
Lymphoblastoid TK6 hepatoma HepaRG cells |
0.25–1.5 μM, 24 h | Negative | Cytotoxicity > 50% at 1.5 μM | Takakura et al. (2014) |
| 5–35 μM, 24 h | Negative | Cytotoxicity > 50% at 30 μM and 35 μM | |||
| DNA strand breaks (Comet assay) | TK6 cells |
1.56–25 μg/mL for 3 h 0.25–1.5 μg/mL for 24 h Incubation with or without fpg |
Inconclusive |
Lack of some toxicity data. Variations in tail DNA intensity in control samples Effects of BSO and NAC on the oxidation status of the cells not interpretable |
Le Hégarat et al. (2014) |
| In vivo | |||||
| Pig‐a mutation assay | male Swiss mice, peripheral blood cells | 2, 4, and 8 mg/kg bw per day, 3 days | Inconclusive | Inappropriate choice of collection times. | Le Hégarat et al. (2014) |
| Micronucleus assay | six female Balb/c albino mice | Oral, 1,000 mg/kg bw | Increased micronuclei (study not used by the CONTAM Panel) | Micronuclei were increased but the CONTAM Panel could not interpret the results in view of the high dose of DON administered | Singh et al. (2015) |
| Micronucleus assay | Swiss male mice, bone marrow, colon | 4, 8 and 16 mg/kg bw per day for 3 days. Gavage at 0, 24 and 45 h | Negative | In the colon, there was an increase in mitotic cell figures with 4 mg DON/kg bw per day, and of apoptotic cells with 16 mg DON/kg bw per day | Le Hégarat et al. (2014) |
| Micronucleus assay | Male Sprague–Dawley rats, bone marrow | 5 mg/kg bw orally, 3 weeks, with or without supplementation with activated carbon (AC) or Egyptian montmorillonite (EM) | Inconclusive |
Toxicity shown by reduced ratio of PCEs to NCEs. Oxidative stress observed. Single dose study Supplementation with AC or EM reduced the DNA damage caused by DON. |
Abdel‐Wahhab et al. (2015) |
| Chromosome aberrations | Male and female Albino mice, bone marrow cells | 3 mg DON/kg bw two times per week orally for 8 weeks | Increased total number of aberrations, but inconclusive | The CONTAM Panel noted that gaps, precocious chromatid separation and gross aberrations were included in the total number of aberrations, and therefore considered that the study was inconclusive | Bilgrami et al. (1993) |
| 0.4 ± 0.05 mg DON/kg in diet, equivalent to 0.08 mg/kg bw per day | Negative | ||||
| DNA strand breaks (Comet assay) | Male broiler chickens, spleen leukocytes | Feed containing 10 mg/kg DON for 17 days with or without the addition of dietary nucleotides (2 g nucleotides/kg feed) | Positive (without supplementation with nucleotides) | Chickens not normally used in standard genotoxicity screening procedures | Frankič et al. (2006) |
| DNA strand breaks (Comet assay) | Male pigs, lymphocytes | Feed containing 4 mg DON/kg for 2 weeks with or without supplementation with vitamin E | Positive (without supplementation with vitamin E) | Pigs not normally used in standard genotoxicity screening procedures | Frankic et al. (2008) |
| DNA strand breaks (Comet assay) | Broiler chicks, lymphocytes | Feed containing 10 mg DON/kg for 21 days with or without a supplement of feed additive | Positive | Chickens not normally used in standard genotoxicity screening procedures. A microbial feed additive reduced the DNA damage caused by DON | Awad et al. (2012a,b) |
| DNA strand breaks (Comet assay) | Broiler chicks, lymphocytes | Feed containing 10 mg DON/kg for 21 days with or without a supplement of feed additive | Positive | Chickens not normally used in standard genotoxicity screening procedures. A microbial feed additive reduced the DNA damage caused by DON | Awad et al. (2014) |
| DNA strand breaks (Comet assay) | Swiss male mice, blood, bone marrow, liver, kidney, spleen, duodenum, colon. |
4, 8 and 16 mg/kg bw per day, 3 days. Gavage at 0, 24 and 45 h With or without fpg. |
Inconclusive | Inconsistencies in values of % tail DNA in controls | Le Hégarat et al. (2014) |
| DNA strand breaks (Comet assay) | Swiss male mice, blood cells | 4, 8 and 16 mg/kg bw per day for 3 days with or without hOGG1 | Inconclusive |
Inconsistencies in values of % tail DNA in controls and in the effects of DON Reason for the decrease in breaks that was observed at doses of 4 and 8 mg DON/kg bw was not determined 16 mg/kg bw caused death of all animals |
Le Hégarat et al. (2014) |
| 2, 4 and 8 mg/kg bw per day for 3 days with or without fpg | Inconclusive |
Inconsistencies in values of % tail DNA 2 mg/kg bw with fpg was positive, but was not considered biologically significant, as there was no dose response |
|||
bw: body weight.
The unit μmol was used in the paper but the CONTAM Panel assumed that the unit was μM and not the one as stated in the paper.
7.2.9.1. In vitro studies
7.2.9.1.1. DON
Wehner et al. (1978) showed that DON was inactive in the Salmonella Typhimurium bacterial mutation assay (Ames test) using strains TA98, TA100, TA1535 and TA1537 (0.4–400 μg DON/plate) with and without metabolic activation with an induced rat liver S9 fraction. DON was also subsequently tested by Knasmüller et al. (1997) using S. Typhimurium strains TA98 and TA100 (0.7–500 μg DON/plate) and in the SOS‐chromotests with E. coli strain PQ37 (5–500 μg DON per assay) with and without S9, and it did not induce mutations. Negative results were observed by Takakura et al. (2014) in the Ames test performed on S. Typhimurium strains TA98, TA100 and TA102 (0.03–500 μg DON/plate), both with and without Aroclor 1,254‐induced rat liver S9.
DON gave negative results in differential DNA repair assays with E. coli K‐12 strains (343/753, uvrB/recA and 343/765, uvr+/rec+) at concentrations ranging from 0.7 to 500 μg/mL (Knasmüller et al., 1997).
Rogers and Héroux‐Metcalf (1983) reported that DON, at concentrations up to 3 μg/mL, had no mutagenic activity to V79 cells at the hypoxanthine‐guanine phosphoribosyl transferase (HGPRT) locus, with or without hepatocyte‐mediated activation. The number of 6‐thioguanine‐resistant mutants at marginally cytotoxic levels of 6 and 8 μg/mL was not significantly increased.
Knasmüller et al. (1997) observed a dose–response relationship for the effect of DON (0.001–10.0 μg/mL) on chromosome aberrations (CAs) in primary cultures of rat hepatocytes. The CA numbers increased at the lower dose levels studied and the maximal effect on CAs (which was statistically significant) was seen at 1 μg/mL at which the CA rate was approximately sixfold over the background level. CAs declined at the highest concentration evaluated which was 10.0 μg/mL, probably due to inhibition of cell division. The mitotic index decreased at a concentration of 10 μg DON/mL, and DON showed a pronounced cytotoxic effect at a concentration of 100 μg/mL. Micronuclei were also studied in the same cells by Knasmüller et al. (1997). A significant increase was seen at a DON concentration of 0.01 μg/mL. However, this positive result was not considered to be biologically relevant as no clear dose–response relationship was seen and micronuclei levels declined at DON concentrations above 0.1 μg/mL.
Hsia et al. (2004) reported the induction of CAs in Chinese hamster V79 cells by HPLC‐purified fractions with the retention time of DON (concentration 300 ng/mL) derived from extracts of wheat flour, barley and corn from Linxian, China. This confirmed earlier results with HPLC‐purified fractions which had the retention time of DON from a corn extract from Linxian (Hsia et al., 1988). The CONTAM Panel could not use the results from either of these studies for risk assessment as the number of samples was insufficient, and also the purity of DON in the HPLC fractions was not clear. Pure DON was also reported to produce CAs in V79 cells at concentrations up to 1 μg/mL although details of the experiment were not completely reported (Hsia et al., 1988).
Takakura et al. (2014) carried out the micronucleus test in accordance with the Organisation for Economic Co‐operation and Development (OECD) Guideline 487 (with some modifications) in two human cell lines, the lymphoblastoid TK6 and the hepatoma HepaRG cells. At concentrations below 12.5 μM DON failed to induce micronuclei formation in TK6 cells after 3 h of treatment with or without human and rat liver S9. Positive results were seen at 25 μΜ (‐S9) and at 12.5 and 25 μΜ (+ human S9). However, DON was more than 60% cytotoxic at a concentration of 25 μM. After 24 h of treatment, DON significantly increased micronuclei formation at 1.6 and 3.2 μM compared to the negative control. However, these concentrations of DON produced more than 60% cytotoxicity, a higher level of cytotoxicity than that recommended by OECD Guideline 487. DON did not induce micronuclei formation in metabolically competent HepaRG cells.
The induction of oxidative stress and genotoxicity by DON was studied in human peripheral lymphocytes by Yang et al. (2014). Cell viability was tested and DNA damage was determined using the Comet assay. Other assays were for sister chromatid exchange (SCE), micronucleus (MN) formation, ROS generation, lipid peroxidation, levels of GSH and the amounts of 8‐OHdG in DNA. Human lymphocytes were from both male and female volunteers. DON reduced the viability of lymphocytes of both sexes, treated at concentrations of 0, 6.25, 12.5, 25, 50, 100, 250 and 500 ng/mL DON for 6, 12 and 24 h, in a concentration‐ and time‐dependent manner. With concentrations 0, 6.25, 12.5, 25 and 50 ng/mL DON for 6, 12 and 24 h, DNA damage (tail length, tail DNA percentage and tail moment), MN and SCE were significantly increased, compared with controls at all DON concentrations and times of treatment. The effects increased with higher concentrations and times of incubation. Similar responses were seen for increases of lipid peroxidation, ROS generation and 8‐OHdG in DNA. GSH levels were reduced and GSSG levels were increased. In addition, DON was shown to enhance mRNA or protein expressions of DNA repair genes HOGG1, XRCC1 and haem oxygenase 1 at 6 h, the effect then decreased with time. Damage to the cell membrane was also observed, which increased with dose of DON and time of incubation. The CONTAM Panel considered that the genotoxicity might be related to oxidative stress.
In the study of Li and Guo (2001),36 hepatocytes from male SD rats were incubated with DON (0.01 to 1 μg/mL) for 2 h and the cells were analysed using single cell gel electrophoresis (Comet assay). DNA damage, assessed from the Comet tail, was observed at all the studied concentrations of DON and increased with DON concentration.
Lin and Sun (2004)44 incubated Vero cells with DON (1, 5 and 10 μmol for 4 and 16 h, respectively and analysed the cells using the Comet assay. With increase of dose and time, the damage in the cells increased. A short‐term incubation time (4 h) mainly caused the increase of the number of DNA fragments, while a long‐term incubation (16 h) mainly caused the smaller size of DNA fragments. Cells that had been treated with 10 μmol DON were re‐incubated for 15, 30, 60 and 120 min to test the DNA repair. Cells began to repair after removing DON and being re‐incubated. If the cells were incubated with DON for 4 h, it took less time to start repair (< 15 min) than if the cells were incubated with DON for 16 h. Repair was complete after 120 mins.
The DNA damaging potential of DON was assessed in the concentration range 0.01–0.5 μM in undifferentiated and differentiated Caco‐2 cells using the alkaline Comet assay (Bony et al., 2006). Dividing cells were found to be more sensitive to DON than differentiated cells and the lowest 10% inhibitory concentration (IC10) (0.5 μM) obtained for dividing cells exposed for 72 h was used as the highest working concentration in the genotoxicity study. Results were assessed after 24 or 72 h of exposure. Both differentiated and dividing cells responded with a concentration‐dependent relationship to DON in terms of DNA damage in the 0.01–0.5 μM range.
Bensassi et al. (2009) investigated the pathway of DON‐induced apoptosis in human colon carcinoma cells (HT‐29) (Section 7.7.1.3). Cells treated with DON underwent a p53 and caspase‐dependent apoptosis. DNA damage was measured by the alkaline Comet assay, and increased in a time‐dependent manner in HT‐29 cells after treatment with DON (10 μM) for 2, 4, 8, 12, 16 and 24 h. No significant generation of ROS was observed in the cells and the authors concluded that the DNA damage was not a consequence of oxidative injury.
The aim of the study of Zhang et al. (2009) was to assess the role of oxidative stress in DON‐induced DNA damage, using human hepatoma HepG2 cells. Exposure of the cells to DON caused a significant increase of DNA migration in the comet assay at concentrations of 3.75–30 μM, which suggests that DON caused DNA strand breaks. It was found that DNA migration was dramatically decreased after pretreatment with the antioxidant hydroxytyrosol (HT). A significant increase in the level of ROS, assessed using 2,7‐dichlorofluorescein diacetate (DCFH‐DA) assay, was observed in HepG2 cells at a concentration of DON of 60 μM. Confirmation of the involvement of lipid peroxidation in the DNA damage was obtained by immunoperoxidase staining for 8‐hydroxydeoxyguanosine (8‐OHdG) and by measuring levels of thiobarbituric acid‐reactive substances (TBARS). Significant increases in these markers were observed at DON concentrations of 60 μM and 15 μM, respectively. The authors concluded that these results indicate that the DNA damage induced by DON in HepG2 cells is probably related to the oxidative stress. Liu et al. (2016, see supplementary information) confirmed the DNA damaging effects of DON at a concentration of 3 μg/mL, and of the protecting effect of HT, using the same methodology as Zhang et al. (2009).
In the investigation of Takakura et al. (2014), the Comet assay was performed on TK6 and HepaRG cells treated with DON for 24 h (DON concentrations of 0–1.5 μM and 0–35 μM, respectively). No DNA damage was induced by DON, whereas positive controls, methyl methanesulfonate (MMS) and cyclophosphamide (CPA), clearly induced DNA migration in these cells. DON was cytotoxic to both cell lines, although proliferative TK6 cells were more sensitive than quiescent HepaRG cells.
Using the Comet assay, Le Hégarat et al. (2014) investigated the extent of DNA damage induced by DON in TK6 cells. The assay was carried out with or without the presence of formamidopyrimidine DNA glycosylase (fpg). Fpg recognises 7,8‐dihydro‐8‐oxoguanine (8‐oxoguanine), 2,6‐diamino‐4‐hydroxy‐5‐formamidopyrimidine (Fapy‐Gua), 4,6‐diamino‐5‐formamido‐pyrimidine (Fapy‐Ade) and to a smaller extent 7,8‐dihydro‐8‐oxoadenine (8‐oxoadenine) as well as apurinic/apyrimidinic sites. The authors reported that no significant increase of DNA migration in TK6 cells was induced by DON, 1.56–25 μg/mL after 3 h or 0.25–1.5 μg/mL after 24 h incubation with or without fpg. TK6 cells were also pretreated with buthionine sulfoximine (BSO) to deplete glutathione 1 day before treatment with DON. The Comet assay carried out after 24 h exposure was reported not to show any increase in DNA migration with or without fpg. The antioxidant NAC was reported not have any significant effect on the Comet assay responses after DON treatment in TK6 cells. The CONTAM Panel considered that the study was inconclusive, because of lack of some toxicity data, variations in tail DNA intensity in control samples, and the observed effects of BSO and NAC on the oxidation status of the cells.
Overall, based on the available evidence, the CONTAM Panel considered that DON is not mutagenic in bacteria, and is negative in one study of mutagenicity in mammalian cells. In studies on chromosomal effects and DNA damage in mammalian cells, several positive results have been observed. DON is cytotoxic at high doses in some of these tests and increases in oxidative stress have also been observed. The CONTAM Panel considered that DON is genotoxic in vitro, and that the effects may be related to oxidative stress.
7.2.9.1.2. 3‐Ac‐DON and 15‐Ac‐DON
Wehner et al. (1978) showed that 3‐Ac‐DON was inactive in the S. Typhimurium bacterial mutation assay (Ames test) using strains TA98, TA100, TA1535 and TA1537 (0.4–400 μg 3‐Ac‐DON/plate), with and without metabolic activation, with an induced rat liver S9 fraction. Hsia et al. (1988) showed that 3‐Ac‐DON induced chromosome aberrations in Chinese hamster V79 cells with a maximum effect at a concentration of 1 μg/mL. The CONTAM Panel did not use the data from this study for risk assessment as the publication provided only limited experimental details, no information on cytotoxicity and no statistical assessment of results. No data were identified for 15‐Ac‐DON.
7.2.9.1.3. DON‐3‐glucoside
No data were identified.
7.2.9.2. In vivo studies
7.2.9.2.1. DON
In vivo studies on the effect of DON on CAs have been carried out by Bilgrami et al. (1993) who studied mice treated with 3 mg DON/kg bw two times per week by gavage for 8 weeks, or 0.4 ± 0.05 mg DON/kg in diet, equivalent to 0.08 mg/kg bw per day. The total frequency of abnormal chromosomes in bone marrow cells in the gavage study was 10.0 ± 1.7% in the DON‐treated animals, which was significantly higher than the control group (3.3 ± 1.0%). The total frequency of abnormal chromosomes after dietary administration of DON was 6.3 ± 1.4%, a non‐significant increase compared with the control group. The CONTAM Panel noted that gaps, precocious chromatid separation and gross aberrations were included in the total number of aberrations, and considered that the study was inconclusive.
In a limited study by Singh et al. (2015), six female Balb/c albino mice received DON orally at a dose of 1,000 mg/kg bw. The frequency of micronucleated polychromatic erythrocytes in bone marrow cells and DNA damage in primary cultures of hepatocytes were studied. The CONTAM Panel could not interpret the results in view of the high dose of DON administered.
Groups of five Swiss male mice were administered DON by gavage in sterile saline solution at doses of 4, 8 and 16 mg/kg bw (Le Hégarat et al., 2014). A control group was treated with saline and a positive control group with methyl methanesulfonate (80 mg/kg bw). The gavage schedule was at 0, 24 and 45 h with a volume of 10 mL/kg, and animals were sacrificed 3 h after the final administration. The Comet assay of blood, liver, duodenum, kidney, spleen, colon, and bone marrow with and without fpg, and the determination of MN in bone marrow (according to the OECD guideline 474) and the colon, were carried out on the animals. The authors reported that DON did not significantly increase DNA damage measured by the Comet assay in any organ studied, with or without fpg. The CONTAM Panel considered that this study was inconclusive because of inconsistencies in the control value of % tail DNA.
No induction of MN was observed in bone marrow cells or in the colon of mice treated with DON. In the colon MN assay, at the lowest dose of 4 mg/kg bw, there was an increase in mitotic cell figures; at the dose of 8 mg/kg bw, there was a decrease in mitotic figures; and, at the dose of 16 mg/kg bw, there was an absence of mitotic figures and a large increase in apoptosis.
In a follow‐up in vivo study by Le Hégarat et al. (2014) (see above), groups of five male Swiss mice were treated with DON. The Comet assay with and without hOGG1 was carried out in blood collected 3 h after the final treatment with DON, and the same set of animals was used for the Pig‐a assay using blood collected 28 and 45 days after the final treatment with DON. All the animals in the highest dose group died 2 days after the last gavage, and so a repeat study was carried out with doses of 2, 4 and 8 mg/kg bw using the Pig‐a assay and the Comet assay with and without fpg. The authors reported that DON at doses of 4, 8 and 16 mg/kg bw did not increase DNA fragmentation in the presence or in the absence of hOGG1. The CONTAM Panel noted that the reason for a decrease in breaks that was observed at doses of 4 and 8 mg DON/kg bw was not determined. In the subsequent experiment at doses of 2, 4 and 8 mg/kg bw, no decrease in DNA migration was reported in blood cells in the absence of fpg. In the presence of fpg, a significant increase in DNA migration was reported, but only at the dose of 2 mg DON/kg bw. The CONTAM Panel considered that these studies of Le Hégarat et al. (2014) using the Comet assay were inconclusive because of inconsistencies in values of % tail DNA in controls and in the effects of DON.
In the Pig‐a assay of Le Hégarat et al. (2014) in mice, the frequency of mutant phenotype reticulocytes and erythrocytes was measured to examine the genotoxic effect of DON. The only statistically significant increase in mutant phenotype erythrocytes was observed 28 days following the final dose of 2 mg/kg bw per day. No other increases of mutant frequency were seen for any of the tested doses at the two post‐exposure time points. The CONTAM Panel concluded that this substudy was inconclusive because of the choice of collection times used.
The conclusion by the authors of these studies (Le Hégarat et al. (2014)), which were conducted according to the EFSA Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment (EFSA Scientific Committee, 2011), is that DON showed no genotoxic effect in mice using the multiple organ Comet assay, the MN test in bone marrow and the colon, and the Pig‐a assay. However, the CONTAM Panel considered that the results from the Comet assay and the Pig‐a assay were inconclusive.
The study of Abdel Wahhab et al. (2015) investigated if activated carbon (AC) or Egyptian montmorillonite (EM) was able to protect against the toxic effects of DON in male Sprague–Dawley rats. Treatment with DON (oral, 5 mg/kg bw in corn oil for 3 weeks) caused a significant increase in the frequency of micronucleated polychromatic erythrocytes (Mn‐PCEs). Addition of AC or EM to this diet caused a significant reduction in the frequency of Mn‐PCEs. DON also showed a cytotoxic effect as it significantly reduced the ratio of polychromatic erythrocytes to normochromatic erythrocytes. An increase in DNA fragmentation was observed in the livers of DON‐treated rats together with an increase in lipid peroxidation and a decrease in hepatic glutathione, indicating oxidative stress. The CONTAM Panel considered that the study was inconclusive as only one dose was tested, and this was cytotoxic.
Four assays that are not part of the normal standard screening procedures for genotoxicity have been carried out on DNA damage in pigs and chickens.
Chickens (n = 10 per group) were exposed to DON in feed (fortified to 10 mg/kg) with or without the addition of dietary nucleotides (2 g nucleotides/kg feed) (Frankič et al., 2006). The objective was to study if dietary nucleotides protected against this damage. After 17 days of treatment, the Comet assay was used to measure DNA damage in spleen leukocytes. DON was found to significantly induce DNA damage (percentage of DNA in tail of comet), and the addition of nucleotides decreased this non‐significantly. However, DON did not induce a change in DNA damage as assessed by the tail moment.
Pigs (11.7 kg starting weight, n = 9) were fed a diet containing 4 mg DON/kg for a period of 2 weeks. The body mass and the daily live weight gain was significantly reduced compared to that of a control group (n = 12). Using the Comet assay, DON treatment was found to cause a 28% increase in lymphocyte DNA damage, as measured from the proportion of DNA in the tail of the Comet. Vitamin E supplementation of the diet (100 mg/kg) non‐significantly reduced the percentage of DNA in the tail. Markers of oxidative stress were not affected by the treatment but levels of total antioxidant status were lowered by treatment with DON with or without vitamin E (Frankic et al., 2008).
The effect of DON on lymphocyte DNA fragmentation in broilers fed low‐protein diets and the potential of a commercial food additive, that is intended to protect animal health by deactivating mycotoxins found in contaminated feed, was evaluated by Awad et al. (2012b). Thiobarbituric acid reactive substances (TBARS) in the liver were measured as an indicator of lipid peroxidation. Broiler chicks in four groups of eight animals were fed with (1) a non‐contaminated diet, (2) a non‐contaminated diet supplemented with the feed additive (0.25%), (3) a diet contaminated with DON at 10 mg/kg diet, and (4) a diet contaminated with DON (10 mg/kg diet) supplemented with the feed additive (0.25%). Chicks were fed the normal starter diets from day 1 to day 13 and a low protein grower diet from day 14 to day 35. The degree of lymphocyte damage was determined using the Comet assay. The concentration of TBARS in the liver did not differ between the DON‐treated group and the control group, with or without the food additive. The proportion of DNA in the tail of the comet was statistically increased by DON exposure, and the feed additive reduced this increase, with the level reverting to approximately control levels.
In a subsequent paper, Awad et al. (2014) used a similar experimental design using groups of 10 broiler chicks. Birds were fed a starter feed from 1 to 13 days and grower feed from 14 to 35 days. TBARS level was measured in plasma, heart, kidney, duodenum and jejunum. TBARS levels in plasma, heart, kidney, duodenum did not show significant changes due to DON exposure, but the level in jejunal tissue increased (p < 0.001). The commercial microbial feed additive described in Schatzmayr et al. (2006) had no effect on reducing the TBARS level in jejunal tissue. After 5 weeks, DON increased the % of DNA damage in lymphocytes (p < 0.001), and addition of the feed additive prevented this rise, giving a DNA damage level similar to that of controls. The authors concluded that DNA damage induced by DON is probably caused through oxidative stress.
Overall, based on the available evidence, the CONTAM Panel considered that the data on the genotoxicity of DON in vivo are inconclusive.
7.2.9.2.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.2.9.2.3. DON‐3‐glucoside
No data were identified.
7.2.9.3. Conclusions
DON has not shown mutagenicity in bacteria or in mammalian cells in vitro. Studies of the ability of DON to induce chromosome damage or DNA damage in cells in vitro have yielded conflicting results, but several positive results have been reported. The CONTAM Panel noted that there is evidence for oxidative stress in some of these experiments. The CONTAM Panel considers that DON is genotoxic in vitro, and that the effects may be related to oxidative stress. 3‐Ac‐DON was inactive in a bacterial mutation assay. In one study it induced chromosome aberrations in cells in vitro, but this study could not be used for risk assessment because of lack of published information. The CONTAM Panel did not identify genotoxicity data for 15‐Ac‐DON or DON‐3‐glucoside.
Based on the available evidence, the CONTAM Panel considered that the data on the genotoxicity of DON in vivo are inconclusive with the majority of positive results obtained from assays in pigs and chickens that are unusual in standard genotoxicity screening procedures. Overall, the evidence suggests that oxidative stress induced DNA damage, rather than a direct interaction of DON with DNA, underlies the genotoxic effects of DON.
No in vivo genotoxicity studies on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified by the CONTAM Panel.
7.2.10. Carcinogenicity
7.2.10.1. DON
The IARC classified DON as being in Group 3, ‘not classifiable as to its carcinogenicity to humans’ (IARC, 1993). In the SCF assessment in 1999, it was stated that ‘only one long‐term feeding study is available. No increase in tumour frequency or other sign of carcinogenic effect were found in this study (Iverson et al., 1995). There are no indications for carcinogenic and/or mutagenic properties of DON’ (SCF, 1999). The JECFA assessment in 2001 concluded that ‘a study of carcinogenicity in mice showed fewer tumours of the liver in treated male mice than in controls. The Committee concluded that the lower incidence was due to the reduced body weight of the treated animals. No significant difference in tumour incidence was seen in female mice’ (FAO/WHO, 2011).
In a long‐term carcinogenicity study performed by Iverson et al. (1995) (for details of the study design see Section 7.2.4), the majority of lesions observed were of a benign hyperplastic nature. In male mice dose‐related changes were observed for hepatic nodular hyperplasia, hepatic adenoma, hepatocellular carcinoma, soft tissue fibrosarcoma and large islets in the pancreas, with the only significant lesion after adjustment using the Bonferroni procedure being nodular hyperplasia. However, in the liver and pancreas, the correlation with dose was negative, i.e. there was a decrease in preneoplastic and neoplastic lesions with increasing DON doses. The negative trend for the liver might have resulted from the known positive correlation between body weight and the appearance of spontaneous hepatic neoplasms in this strain of mouse. The only positive trend reported was for fibrosarcomas where three were observed in the high‐dose group. Female mice produced no statistically significant differences.
In a subsequent subchronic study, the effects of DON were studied in p53 heterozygous (p53+/−) and p53 homozygous (p53+/+) male mice (Bondy et al., 2016) (see also Section 7.2.4). Groups of animals (n = 10) received a diet containing 0, 1, 5 or 10 mg DON/kg diet for 26 weeks. There was no evidence of carcinogenicity in either the (p53+/−) or the (p53+/+) mice, consistent with the results of the 2‐year feeding study in B6C3F1 mice (Iverson et al., 1995).
Huang et al. (2004)36 studied a potential modulation of the carcinogenicity of sterigmatocystin by DON using an incomplete two‐factorial design with six groups of 30 NIH mice (n = 15 males and 15 females). This included a control group, a group exposed to DON only (1.5 g/kg), two groups exposed to sterigmatocystin only (3 and 30 g/kg) and two groups exposed to sterigmatocystin and DON combined at these doses using intragastrical administration three times per week for a total of 24 weeks. Deterioration of the general condition of the animals in all groups treated with DON and/or sterigmatocystin was reported, and a total of 49 out of 180 animals died after 10 days of treatment (doses not specified). The incidence of glandular stomach mucosal dysplasia and tumours in the lung, specified as adenocarcinoma, was reported for subsets of animals (varying between n = 8–16 per group) of the study. Although this study has been repeatedly cited for evidence of possible carcinogenicity of DON, the CONTAM Panel identified several flaws of the design, conduct and reporting of the results (incomplete factorial design, large intercurrent mortality, no information on the distribution of the animals to the dose groups and the sacrifice times and the treatment of the controls). The CONTAM Panel concluded that the data do neither support carcinogenicity nor a synergistic effect of DON when added to sterigmatocystin, and cannot be used for hazard characterisation.
The CONTAM Panel also noted the studies of Lambert et al. (1995) and Mishra et al. (2014b; 2016) on topical application of DON to skin of rodents but did not consider these studies further for the risk assessment of DON in food.
7.2.10.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.2.10.3. DON‐3‐glucoside
No data were identified.
7.2.10.4. Conclusions
In summary, as was the case for the SCF (1999) assessment, there is only one long‐term feeding study available for consideration of the carcinogenicity of DON, and this showed no indications of carcinogenic properties of DON in mice. The CONTAM Panel did not identify carcinogenicity data for 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside.
7.3. Adverse effects in farm and companion animals
The previous EFSA (2004) opinion reported the available data at that time on the potential adverse effects from diets in pigs, cattle, sheep, poultry, horses, dogs and cats for DON only. Since then new studies on the toxicity of DON have been published including additional farm animals and also including 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside. Since 2004 a number of studies reported specifically the co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and/or DON‐3‐glucoside which is likely due to analytical progress made by using optimised chromatographic methods. However, most studies report almost exclusively data on animals exposed to naturally contaminated feed which may contain also other mycotoxins.
In this section, the CONTAM Panel decided to use the mycotoxin concentration in feed/diet (mg DON/kg feed) to express the NOAEL and LOAEL instead of a dose (mg/kg bw per day) because when decreases in feed intake and vomiting were the reported adverse effects it was considered inappropriate to express exposure on the body weight basis. In particular, the CONTAM Panel noted that the use of dose caused misleading NOAEL/LOAELs for pigs because it has been observed that the first adverse effect seen in pigs when fed DON‐contaminated feed at the low concentrations was reduced feed intake, resulting in reductions in the body weight and body weight gain during the feeding period when pigs consumed less feed. However, over time the hunger in pigs increased and they turned to eat normal rations of the available DON contaminated feed resulting in a recovery and an increase in body weight and body weight gain after some delay. As this observation on adaptation has also been seen in poultry and farmed fish, it may also be relevant for other species than pigs, poultry and farmed fish, the CONTAM Panel decided to use the concentration to express NOAELs/LOAELs for all animals considered below with the exception of farmed mink when vomiting was observed as acute effect in this species (see Section 7.3.8). The CONTAM Panel further remarked that the primary effect of DON in farm and companion animals was a reduced feed intake, resulting in reduced body weight gain or reduced body weight depending on the energy balance of the animal. Therefore, as adult animals have reached a stable weight or have a very slow weight gain, ‘reduced body weight gain’ and ‘reduced body weight’ measure virtually the same effect of DON but of different severity. The CONTAM Panel also noted that vomiting due to DON exposure has only occasionally been observed in farm and companion animals while reduction in feed intake has been regularly reported. Vomiting has typically been reported at higher concentrations of DON than those associated with reduced feed consumption.
7.3.1. Ruminants
A number of studies have reviewed the health effects of DON in cattle (DiConstanzo and Murphy, 1994; Yiannikouris and Jouany, 2002; Pestka, 2007, 2010b; Fink‐Gremmels, 2008; Whitlow et al., 2010). As described in the toxicokinetic section (see Section 7.1.5.1), DON is converted into the less toxic de‐epoxidised metabolite DOM‐1 by the ruminal flora. Therefore from the studies available in the literature, only those performed using the largest number of animals and the highest DON concentrations in feed combined with the longest periods of time were selected for this opinion. However, young ruminants, in which the rumen is not fully developed, could be less active in de‐epoxidation and might therefore be more sensitive than adult ruminants when exposed to DON. For small ruminants (sheep and goats) the database on adverse effects of DON, 3‐Ac‐DON, 15‐Ac‐DON and/or DON‐3‐glucoside was very limited or lacking.
7.3.1.1. Cattle
7.3.1.1.1. DON
In the study of Nelson et al. (1984) reported by Wagner (2002), heifers and steers were fed diets containing 0.2, 2.3 and 10 mg DON/kg feed for 126 days. The low‐DON diet was corn based, while the medium and the high‐DON diets contained wheat contaminated with 13.7 mg DON/kg incorporated in the ration at 5.17% and 65%, respectively. Results reported for the low‐, medium‐ and high‐DON diets were similar regarding feed intake, average daily weight gain and feed efficiency. During a 166‐day experiment conducted by DiConstanzo and Murphy (1994), 180 crossbred steers were fed diets containing 0, 6, 12 or 18 mg DON/kg diet dry matter. Data were analysed separately for the initial 28 days and the remaining 138 day finishing period. Feeding diets containing as much as 18 mg DON/kg did not affect feed intake, average daily weight gain, feed efficiency or carcass characteristics. The CONTAM Panel identified from the above studies that feeding heifers a diet at 10 mg DON/kg feed and feeding steers a diet at 18 mg DON/kg feed did not affect feed intake, average daily weight gain and feed efficiency, and identified these values as NOAELs for heifers and steers for reduced feed intake and reduced body weight gain.
A slight temporary decrease in the consumption of complementary feedstuff by dairy cows was recorded when the concentration of DON was increased from 1.5 to 6.4 mg/kg complementary feeding stuffs (Trenholm et al., 1985). No effect was seen on weight gain or hay consumption. The complementary feedstuff consumption returned to the previous levels when the cows were again fed the complementary feeding stuffs after 6–10 weeks containing 1.5 mg DON/kg. However, DON fed to dairy cows at 66 mg/kg for 5 days (Côté et al., 1986) did not cause performance reduction or signs of illness. No decrease in feed intake and milk production was shown after the administration of 0, 6.0 and 12 mg DON/kg of complementary feedstuff over 10 weeks to lactating cows (Charmley et al., 1993). However, cows given DON at 6.0 mg/kg of complementary feeding stuffs dry matter had the lowest milk fat content and fat output. Later studies by Ingalls (1996) showed that dairy cattle may tolerate diets containing up to 8.5 mg DON/kg for 21 days without major health effects. During two experiments performed by Dänicke et al. (2005a), the pH‐value and the concentration of volatile fatty acids in the rumen fluid were not significantly influenced by feeding with contaminated wheat at dietary DON concentrations of 3.1 and 3.5 mg/kg (88% dry matter basis) to dry cows (see details in Section 7.1.5.1). In contrast, the postprandial ammonia concentration was consistently higher when the mycotoxin‐contaminated wheat was fed. This might be the result of the higher protein intake due to the higher crude protein concentration of the contaminated wheat and/or due to a decreased capacity of rumen microbes to utilise the released ammonium for microbial protein synthesis (Dänicke et al., 2005a).
No adverse effects were observed in feed consumption, body weight, body condition score, milk production, milk composition or milk somatic cell count of Holstein cows exposed to 3.6 mg DON/kg feed (Smith et al., 2006). While the total serum protein and globulin concentrations were increased and albumin–globulin ratio decreased, there was no elevation in other markers of inflammation. A similar observation was reported by Kinoshita et al. (2015) who concluded that a long‐term exposure to DON cannot be associated with an inflammation in cows. Therefore, the CONTAM Panel did not consider these observations as adverse effects. The CONTAM Panel noted in the study of Mendoza et al. (2014)40 on Holstein cows that there was a difference in feed composition between the control and contaminated diets which may have biased the effect of DON observed as lower fat content in milk from treated cows. Therefore, the CONTAM Panel did not consider this study further. Marczuk et al. (2012) reported DON and zearalenone mycotoxicosis in a herd of dairy cows but because the mycotoxin concentrations were not reported, the CONTAM Panel did not consider this study further. The CONTAM Panel noted a study of Dänicke et al. (2016) on whether the change of composition of the diet containing DON could challenge the immune system in cows but did not consider this study further because no effects in the immune system were reported.
In the study of Winkler et al. (2014) on 30 lactating German Holstein cows were divided into three groups (see details in Section 7.1.5.1.1). No clinical signs of acute or chronic DON intoxication were observed. Thus, DON and zearalenone concentrations of the diets up to 5 and 0.5 mg/kg feed respectively did not exert negative effects on performance parameters such as body weight, dry matter intake and milk yield and composition. The CONTAM Panel noted that both control and toxic diet compositions were similar and based on maize as ingredient. The CONTAM Panel noted that a relationship between DON exposure and clinical signs of cows with regard to performance parameters could not be identified due to the absence of adverse effects in this study. Moreover, no effects of mycotoxin contaminated feed were observed on milk production and milk composition in this study. The CONTAM Panel noted that the Winkler et al. (2014) study provided the highest concentration of 5.24 mg DON/kg feed which did not generate any adverse effects during the longest time of experiment and considered this study as pivotal to establish a NOAEL for dairy cows.
7.3.1.1.2. 3‐Ac‐DON and 15‐Ac‐DON
In the experiments performed on dairy cows by Seeling et al. (2006) and Winkler et al. (2014) (see Section 7.1.5.1.1 above) on 3‐Ac‐DON and 15‐Ac‐DON, no specific data were reported on adverse effects associated to the acetylated forms of DON.
7.3.1.1.3. DON‐3‐glucoside
Winkler et al. (2014) (see Section 7.1.5.1.1 above) did not report any specific data on adverse effects associated to DON‐3‐glucoside.
7.3.1.2. Sheep and goats
The only available studies on sheep were previously reported by EFSA (2004). No data on goats were identified.
7.3.1.2.1. DON
Harvey et al. (1986) fed eight lambs (mean body weight = 17.90 kg), wheat contaminated with 15.6 mg DON/kg for 28 days. No differences were observed in feed consumption, body weight gain, feed efficiency or other parameters tested between the lambs fed with DON‐contaminated diet and the control animals. Dänicke and team (Dänicke et al., 2002; Dänicke, 2002) concluded that the sheep rumen fermentation was not impaired (pH, concentration of short chain fatty acids and ammonia) by a diet with 4.6 mg DON and 0.34 mg zearalenone per kg feed although a decreasing trend in the digestibility of slowly degradable straw was observed. In the absence of any other data, the CONTAM Panel decided to use 15.6 mg DON/kg feed as a NOAEL for sheep.
7.3.1.2.2. 3‐Ac‐DON and 15‐Ac‐DON
The CONTAM Panel noted the study of Brewer et al. (1996) on two lambs exposed via intraruminal dosing to radioactively labelled 3‐Ac‐DON at a high dose of 5 mg/kg bw, but did not consider the study further for hazard characterisation due to the use of that high dose. No data were identified for 15‐Ac‐DON.
7.3.1.2.3. DON‐3‐glucoside
No data were identified.
7.3.1.3. Conclusions on adverse effects in ruminants
Data that can be used for hazard characterisation were available for dairy cows, heifers, steers and sheep but not for goats. For cows, from the available studies in the literature, the CONTAM Panel selected the studies performed using the highest DON concentrations in feed combined with the longest periods of time for hazard characterisation. The concentration of 5.24 mg DON/kg feed did not generate adverse effects on body weight, feed intake, milk yield and composition or any other adverse effect in dairy cows over 13 weeks, and therefore, the CONTAM Panel considered this concentration as a NOAEL for dairy cows. The levels of 10 and 18 mg DON/kg feed did not generate any adverse effects on feed intake, average daily weight gain and feed efficiency in heifers and steers, respectively. Therefore, the CONTAM Panel considered a NOAEL of 10 mg DON/kg feed for heifers and 18 mg DON/kg feed for steers. For sheep, the CONTAM Panel identified the NOAEL of 15.6 mg DON/kg feed for reduced feed intake and body weight gain. Acute effects could not be identified for ruminants from the available literature.
In healthy ruminants, DON is converted into the less toxic de‐epoxidised metabolite DOM‐1 by the rumen flora. Dairy cows, cattle and young animals have not been compared directly in the same experiment. However, young animals such as calves with not fully developed rumen and adult animals with a previous history of ruminal acidosis may have less effective de‐epoxidation and, consequently, could be more susceptible to the toxic effects of DON.
Due to the limited or lack of data on adverse effects in ruminants caused by 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, no specific NOAELs/LOAELs could be identified for these forms.
7.3.2. Pigs
Amongst livestock pigs show the highest susceptibility to DON among the animals evaluated (Pestka, 2007). At high doses, DON leads to complete feed refusal, vomiting and haematological disorders. Long‐term exposure results in reduced feed consumption and growth reduction (Rotter et al., 1996; Döll and Dänicke, 2011).
Due to the high number of studies available in the literature for adverse effects on pigs since the recent assessment of EFSA (2004), the CONTAM Panel decided to consider the recent publications since then only when a causal relationship between exposure to DON and/or its acetylated and modified forms was stated or was plausible to conclude from the reported data. Studies which reported substantial co‐contamination with other mycotoxins such as fusaric acid, whose effects are sometimes attributed to the effects of DON, or zearalenone which may contribute to adverse effects (e.g. the reproductive disorders) of DON were not included in this section neither studies of low quality due do limitations in design and reporting or studies which aimed at not primarily to investigate adverse effects of DON (e.g. aiming at mycotoxin degradation or adsorption to feed supplements). An overview of the studies included by the CONTAM Panel in this opinion is presented in Table 48.
Table 48.
Selected studies on adverse effects in pigs
| Breed of pig (body weight or age) | n | Concentration (mg/kg feed) | Toxin source | Exposure timea | Effects | LOAEL (mg/kg feed) | NOAEL (mg/kg feed) | Reference |
|---|---|---|---|---|---|---|---|---|
| Studies reported in EFSA ( 2004 ) | ||||||||
| Yorkshire (6 weeks) | 6 | 0–9 | Pure toxin | 7 days | Reduced feed intake and body weight gain | 4 | n.a. | Prelusky (1997) |
| Yorkshire (6 weeks) | 7 | 0–3 ad libitum and pair fed controls | Contaminated corn | 28 days | First 7 days: Reduced feed intake and body weight gain | 0.75 | n.a. | Rotter et al. (1994) |
|
Days 8–28: Reduced feed intake and body weight gain, decreased thyroid weight (not compared to pair‐fed) Linearly decreased skin temperature and thyroid weight, improved feed efficiency, increased serum T4, albumin and decreased serum α‐globulin compared to ad libitum control, but not to pair‐fed. Reduced anti‐body response to SRBC after 1 and 2 weeks, but not after 3 weeks |
1.5 | n.a. | ||||||
| Landrace X Piet (no age or bw reported) | 9 | 0.3–1.2 | Pure toxin | 8 weeks | No effect on body weight gain. Tendency towards reduced IGF‐1 and IgA | n.a. | 1.2 | Götz‐Schröm et al. (1998) |
| Yorkshire (7–8 weeks) | 8 | 0, 4.0 ad libitum and pair fed controls | Contaminated corn | 42 days | Reduced feed intake (20%) and body weight gain (13%), corrugated stomach compared to controls, temporary decreased serum protein after 2 and 3 weeks, and temporary reduced β‐globulin weeks 2–4. | 4.0 | n.a. | Rotter et al. (1995) |
| Landrace (~ 25 kg) | 8 | 0.1–4.5 | Contaminated oat | 8, 14 weeks | Transient reduced feed consumption and body weight gain (8 weeks) | 2.3 | 1.14 | Bergsjö et al. (1992) |
| Reduced feed consumption (14 weeks) | 4.5 | 2.3 | ||||||
| Landrace (~ 21 kg) | 17–20 | 0.05–3.50 | Contaminated oat | ~ 3 months (2–100 kg) | Decreased feed consumption | 1.68 | 0.7 | Bergsjö et al. (1993b) |
| Transient reduction in PCV, decreased serum calcium and phosphorus, increased relative liver weight | 3.5 | 1.68 | ||||||
| Yorkshire (4–5 weeks) | 3 | 0, 5.9–15.1 | Pure | 2 weeks | Reduced feed intake, reduced bw gain, particularly on the first 3 days (partially recovered at concentrations < 12 mg/kg) | 5.9 | n.a. | Trenholm et al. (1994) |
| 4 | 0, 3.49–19.1 | Contaminated wheat | 2 weeks | Reduced feed intake, reduced body weight gain, particularly on the first 3 days | 3.4 | n.a. | ||
| 4–5 | 0–8.7 | Contaminated wheat | 7 weeks | Reduced feed intake and body weight gain, increased relative liver weight, and increased relative stomach weight | 3.9 | n.a. | ||
| Landrace (12–13 weeks) | 6 | 0, 2.5 | Contaminated corn | 5 weeks | Reduced feed intake and body weight gain. Significant changes in stomach mucosa. | 2.5 | n.a. | Friend et al. (1992) |
| Yorkshire (12–15 weeks) | 5 | 0, 6 | Pure toxin | 21 days | Reduced feed intake, body weight gain and slightly reduced feed efficiency. Lesions in stomach | 6 | n.a. | Rotter et al. (1992) |
| Yorkshire (9–10 weeks) | 3 | 0–5.2 | Contaminated maize and pure DON | 7 weeks | Reduced feed intake and body weight gain, increased relative stomach and urinary bladder weights. Stomach lesions | 5.2 | n.a. | Foster et al. (1986) |
| Breed not reported (16–18 kg) | 15–16 | 0–5.26 | Contaminated wheat | 90–110 days (~ 17–90 kg) | Reduced feed intake and increased age at slaughter weight | 5.26 | 2.89 | Friend et al. (1986a) |
| No significant increase in weight of kidney, liver and uterus. No effect on feed efficiency | n.a. | 5.26 | ||||||
| Yorkshire (~ 25 kg) | 18 | 3.7, 4.2 | Contaminated wheat or inoculated maize | 7 weeks | Decreased feed consumption and body weight gain | 3.7 | n.a. | Friend et al. (1986b) |
| Breed not reported (7–9 kg) | 3–4 | 0.14–875 | Contaminated corn | 4–21 days (4 trials) | Vomiting | 19.7 | 11.9 | Young et al. (1983) |
| Reduced feed intake and body weight gain | 1.3 | n.a. | ||||||
| Increased serum protein, albumin, cholesterol, decreased serum P, glucose and alkaline phosphatase | 1.3 | n.a. | ||||||
| Breed not reported (cross‐breed 5 weeks) | 8–10 | 0.7–5.8 | Contaminated corn | 4 week | Reduced feed intake and body weight gain (male more than female, reversed in females when given control feed), reddening of mucosa in stomach and small intestines and oedema in the mesenteric lymph nodes | 3.1 | n.a. | Côté et al. (1985) |
| Breed not reported (8–9 kg) | 10 | 10.5 ad libitum and pair fed controls | Contaminated corn | 21 days | Reduced feed consumption and body weight gain, reduced feed efficiency (p < 0.07) compared to ad libitum control. Decreased serum haematocrit, haemoglobin, glucose, P compared to ad libitum control | 10.5 | n.a | Lun et al. (1985) |
|
No reduced feed consumption and body weight gain, reduced feed efficiency (p < 0.07) compared to pair‐fed control. Higher haemoglobin and no other significant change compared to pair‐fed control |
n.a. | 10.5 | ||||||
| Breed not reported(~ 9 kg) | 8 | 0–2.8 | Contaminated wheat | 2 or 3 weeks | Reduced feed intake and body weight gain (no difference in body weight gain when fed a clean diet after 4 weeks exposure) | 2.0 | 0.9 | Pollmann et al. (1985) |
| Breed not reported(~ 61 kg) | 4 | 0–4.2 | 42 days | Reduced feed intake and body weight gain. No effect on organ weight | 2.2 | 0.9 | ||
| Vomiting | 2.8 | 2.2 | ||||||
| Breed not reported (45 kg) | 4 | 0, 6.3 | Pure toxin | 4 days | Reduced feed intake | 6.3 | n.a | Forsyth et al. (1977)b |
| 4 | 12.5 | Contaminated corn | Feed refusal | 12.5 | n.a | |||
| Breed not reported (20 kg) | 4 | 0–40 | Pure toxin | 4 days | Dose‐dependent reduction in feed intake | 3.6 | n.a | |
| 3 | 0–16 | Contaminated corn | 3 days | Reduced feed intake and reduced body weight gain. (Pigs receiving 3.6 mg DON/kg naturally contaminated corn had a lower feed intake and average daily body weight gain than all pigs receiving pure toxin) | 3.6 | n.a. | ||
| Breed not reported (~ 9 kg) | 3 | 0.025–0.2 mg/kg bw | Pure toxin | Single dose, i.p | Vomiting | 0.05 mg/kg bw | 0.025 mg/kg bw | |
| 3 | 0–0.4 mg/kg bw | Pure toxin | Single dose, oral (gavage) | Vomiting | 0.1 mg/kg bw | 0.075 mg/kg bw | ||
| Breed not reported(~ 24 kg) | 12 | 0–14.1 | Contaminated wheat | 14 days | Vomiting | 12.7 | 8.3 | Williams et al. (1988) |
| Reduced feed intake and growth rate | 8.3 | 5.0 | ||||||
| 54 | 0–11.0 | Contaminated wheat | 14 weeks | Vomiting | 4.4 | 1.6 | ||
| Reduced feed intake | 4.4 | 1.6 | ||||||
| Reduced growth rate | 6.6 | 4.4 | ||||||
| Reduced feed to body weight gain ratio | 9.1 | 6.6 | ||||||
| Landrace(~ 25 kg) | 8 | 0.6, 1.8, 4.7 | Contaminated oats | 100 days(25–100 kg) | Reduced feed intake, feed conversion and reduced antibody response toward tetanus toxoid (only after 9 weeks, not after 3 and 6) | 1.8 | n.a. | Overnes et al. (1997) |
| Inconclusive lymphocyte response towards PHA mitogen.Interstitial hepatitis in liver (p ~ 0.05) | n.a. | n.a. | ||||||
| Landrace(≥ 22 kg) | 15 | 0.03, 6.0 | Contaminated wheat | 12 weeks | Reduced feed consumption and body weight gain (not in the enhanced diet) | 6.0 | n.a. | Chavez and Rheaume (1986) |
| Breed not reported(80–90 kg) | 11 | 8 | Contaminated wheat | 11 days | Reduced feed consumption compared to previous 5 days. Slowly increasing from day 6Degeneration of hepatocytes, degenerative changes in renal tubular epithelium and eosinophilic infiltration in lymphatic organs | 8 | n.a. | Marpegan et al. (1988) |
| Yorkshire(~ 39 kg) | 6 | 0.05, 0.75 | Contaminated wheat | 21 days | Reduced feed intake and reduced body weight gain in the first 3 days. No difference later, but the loss was never recovered | 0.75 | n.a. | Friend et al. (1982) |
| Yorkshire(~ 75 kg) | 6 | 0.05–0.75 | 21 days | Concentration‐dependent reduction in feed intake and body weight gain in the first 3 days, which were not recovered. Discolouration of oesophageal region of the stomach | 0.38 | n.a. | ||
| Yorkshire(~ 43 kg) | 4 | 0.05–0.75 | 7 weeks | Reduced feed intake and reduced feed to body weight gain ratio | (only trend) | n.a. | ||
| Yorkshire(~ 21 kg) | 4 | 0.05–0.75 | 4 weeks | Reduced weight gain, significant trend for body weight gain and feed intake | (only trend) | n.a. | ||
| Yorkshire(age and weight not specified) | 6 | 0–0.7 | Contaminated wheat | 21 days | Reduced feed intake and average body weight gain | 0.35 | n.a. | Trenholm et al. (1983) |
| Yorkshire(~ 30 kg) | 10 | 0.20, 5.08 | Contaminated wheat | 5 weeks | Reduced feed intake and reduced body weight gain | 5.08 | n.a. | Friend et al. (1984) |
| Yorkshire(9 weeks) | 6 | 0–3.0 | Contaminated corn and pure toxin | 32 days | Reduced feed intake and body weight gain (whole period for naturally contaminated feed, only 2 days for pure toxin). Significant decrease in serum γ‐globulin content and trend towards a decrease in total globulin content. No significant pathological changes in stomach region, but trend towards more mucosal folding and thickening of oesophageal region tissue with increasing DON. | 3.0 | 1.0 | Prelusky et al. (1994) |
| Breed not reported (60 kg) | 6 | 0, 1.0 | Pure toxin | 90 days | No effect on feed intake or body weight gain or other parameters measured. | n.a. | 1.0 | Lusky et al. (1998) |
| Breed not reported(70–83 days) | 6 | 0–1.4 | Contaminated wheat | 100–125 days | No effect on feed intake, body weight gain or other parameters measured | n.a. | 1.4 | Richter (1989) |
| Breed not reported(~ 30 kg) | 12–18 | 0.2–2.8 | 30–100 kg | No effect on feed consumption, body weight gain or other parameters measured | n.a. | 2.8 | ||
| Yorkshire (~ 90 kg) (≥ 178 days, during pregnancy) | 12 | 0.1–3.5 | Contaminated wheat | 50–54 days | Reduced maternal body weight and feed intake | 3.5 | n.a. | Friend et al. (1986a, 1986b) |
| Significant trend towards reduced fetal weights and fetal lengths | 3.5 | n.a. | ||||||
| Four‐strain male and female hybrids (~ 30 kg) | 12 | 2.4–3.1 | Contaminated wheat | 98 days(~ 30–110 kg) | Reduced feed intake and reduced body weight gain, no effects on clinical chemistry | 2.4 | n.a. | Dänicke et al. (2004c) |
| Four‐strain male and female hybrids (14 days)(~ 28–36 kg) | 16 | 2.6, 4.1 | Contaminated wheat | 14 days(~ 28–36 kg) | Pigs fed the highest DON‐concentrations showed reduced feed intake to approximately 50% of the control group within 2 days after the beginning of the experiment. Some individual pigs completely refused feed | 2.6 | n.a. | Dänicke et al. (2004e) |
| Four‐strain male and female hybrids (70 days, ~ 56–103 kg) | 18 | 0.2, 0.7, 1.2, 2.5, 3.7 | Contaminated wheat | 70 days(~ 56–103 kg) |
No significant effects on performance with a tendency of a concentration‐independent decrease in performance due to DON‐presence in the diets No effects on clinical chemistry, linearly related increase of DON‐concentration in serum, and no effects on nutrient digestibility |
n.a. | 3.7 | Dänicke et al. (2004d) |
| Four‐strain female hybrids(~ 12.5 kg) | 20 | 0.2–3.9 | Contaminated corn | 35 days(12.5–32.5 kg) |
Reduced feed intake and reduced body weight gain. Reduced total serum protein at 3.9 mg/kg Serum activity of GLDH significantly decreased at and above 0.8 mg/kg No or no consistent effects on organ weights of the digestive tract or related organs and immune globulin concentration in the serum. Linearly related increase of DON‐concentration in the serum |
3.9 | n.a. | Döll et al. (2003) |
| Four‐strain female hybrids(~ 10 kg) | 20 | 2.3 | Contaminated corn | 35 days(10.5–27.5 kg) |
Reduced feed intake, reduced body weight gain and feed to body weight gain ratio. Increased relative weight of stomach and heart. Decreased serum albumin concentration and activity of GLDH. |
2.3 | n.a. | Döll et al. (2004) |
| 12 | 3.2 | 35 days(9.7–21.4 kg) | Reduced feed intake and body weight gain | 3.2 | n.a. | |||
| Yorkshire (10 kg) | 35 | 4.6 | Contaminated corn and wheat | 21 days |
Reduced feed intake and body weight gain. Decreased relative liver and kidney weights. Significantly decreased concentrations of neurotransmitters in pons and hypothalamus. Significantly increased serum concentrations of immune globulins A and M |
4.6 | n.a. | Swamy et al. (2002) |
| Yorkshire(9.3 kg) | 30 | 3.9 and 5.8 (with pair fedcontrol) | Contaminated corn and wheat | 21 days |
Concentration‐dependent reduced feed intake and body weight gain. Decreased albumin‐globulin ratio, total serum protein and globulin concentrations (as compared to pair fed pigs). No effects on organ weights, serum immune globulines, percentages of peripheral blood lymphocyte subsets and primary response to sheep red blood cells |
3.9 | n.a. | Swamy et al. (2003) |
| Studies identified after EFSA ( 2004 ) | ||||||||
| Male and female hybrids (11.2 kg) | 12 | 0, 0.28, 0.56, 0.84 | Contaminated wheat | 28 days | No effect on 34 haematological, biochemical and immune variables | n.a. | 0.84 | Accensi et al. (2006) |
| Dutch Landrace(7.9 kg) | 10 | 0, 0.9 | Pure toxin | 10 days | Reduced body weight gain | 0.9 | n.a. | Alizadeh et.al. (2015) |
| Histomorphological alterations in the duodenum and jejunum | 0.9 | n.a. | ||||||
| Affected mRNA expression of different tight junction proteins, of inflammatory markers (IL‐1 beta, IL‐10, haem oxygenase). | 0.9 | n.a. | ||||||
| Deutsches Edelschwein × Pietrain (8 kg) | 13,15 | 0, 3.0 | Contaminatedwheat | 8 weeks | Reduced feed intake (−19%) and reduced body weight gain (−14%) | 3.0 | n.a. | Böhm and Razzazi (2003) |
| Male hybrids(10.2 kg) | 6 | 0, 2.8 | Fungal culture | 35 days | Histopathological lesions in the liver, the lungs and the kidney. Morphological and histological changes in the jejunum. Reduced number of immune cells and an upregulation of the expression levels of several cytokines | 2.8 | n.a. | Bracarense et al. (2012) |
| Male hybrids(23 kg) | 5–8 | 5.7 | Contaminated wheat | 4 weeks | Reduced protein synthesis in kidneys, spleen and ileum | 5.7 | n.a. | Dänicke et al. (2006) |
| 83 μg/kg bw | Acute oral | 83 μg/kg bw | n.a. | |||||
| Crossbred piglets (5 weeks) | 10 | 0, 0.5, 1.5 | n.r. | 15 days | Increase of serum concentrations of urea and γ‐glutamyl transferase | 0.5 | n.a. | Dinischiotu et al. (2007) |
| Male hybrids(23 kg) | 5–7 | 5.7 | Contaminated wheat | 4 weeks | No alterations in the number of positive stained cells for IgA+, CD3+, CD4+ and CD8+ from cryosections of spleen and jejunum | n.a. | 5.7 | Döll et al. (2006) |
| 83 μg/kg bw | Acute oral | n.a. | 83 μg/kg bw | |||||
| Female hybrids(9.8 kg) | 34 | 0–1.2 | Pure toxin | 8 weeks | No difference in body weight gain (restricted feeding) | n.a. | 1.2 | Drochner et al. (2004) |
| Depression of glucose levels | 1.2 | n.a. | ||||||
| Cortisol and IGF‐1 levels were not significantly affected | n.a. | 1.2 | ||||||
| Increase of IgA concentration in serum | 0.6 | |||||||
| Female hybrids(9.8 kg) | 9 | 0–1.2 | Pure toxin | 8 weeks | No difference in body weight gain (restricted feeding) | n.a. | 1.2 | Drochner et al. (2006) |
| Blood concentrations of aspartate aminotransferase were elevated | 1.2 | |||||||
| No systematic effect on other enzymes (alanine aminotransferase, γ‐glutamyl transferase, glutamate dehydrogenase and sorbit dehydrogenase) | n.a. | 1.2 | ||||||
| Increasing urea level and declining glucose concentrations | 1.20 | n.a. | ||||||
| Pietrain male(35 days) | 10 | 0.12, 1.5 | Contaminated wheat | 28 days | No difference in body weight gain | n.a. | 1.5 | Gerez et al. (2015) |
| Liver lesions | 1.5 | n.a. | ||||||
| Decrease in villi height (jejunum) | 1.5 | n.a. | ||||||
| Reduction in crypt depth | 1.5 | n.a. | ||||||
| Intestinal lesions | 1.5 | n.a. | ||||||
| Number of Goblet cells decreased | 1.5 | n.a. | ||||||
| Epithelial cell proliferation decreased | 1.5 | n.a. | ||||||
| Spleen and lymph nodes lesions increased | 1.5 | n.a. | ||||||
| German Landrace x Pietrain (26 kg) | 48 | 0, 6.51 | Contaminated wheat | 11 weeks (26–100 kg) | Reduced feed intake (15%), reduced body weight gain (−13%) | 6.51 | n.a. | Goyarts et al. (2005) |
| No effect on feed to body weight gain ratio | n.a. | 6.51 | ||||||
| No effect on body weight gain (restrictive feeding) | n.a. | 6.51 | ||||||
| Increase of metabolisable energy, nitrogen retention, digestibility of organic matter, crude protein and crude fibre diet consuming time (restrictive feeding) increased | 6.51 | n.a. | ||||||
| Serum IgA concentration increased | 6.51 | n.a. | ||||||
| German Landrace × Pietrain (40 kg) | 7–10 | 0, 5.7 | Contaminated wheat | 4–6 weeks | No differences in plasma concentrations of total protein, albumin, fibrinogen and serum enzymes | n.a. | 5.7 | Goyarts et al. (2006) |
| 5.7 | Acute oral | Decreased fractional synthesis rate of albumin and lymphocytes | 5.7 | n.a. | ||||
| German Landrace gilts(180 kg) | 3 | 4.42 | Contaminated triticale | Days 63–70 of gestation | No macroscopic lesions in any organ (sows and piglets) | n.a. | 4.42 | Goyarts et al. (2010) |
| No histopathological alterations of sows liver and spleen | n.a. | 4.42 | ||||||
| No effect on proliferation rate of peripheral blood mononuclear cells | n.a. | 4.42 | ||||||
| No adverse effects on health, fertility, maintenance of pregnancy, and performance of sows and their fetuses | n.a. | 4.42 | ||||||
| Crossbred piglets (7.7 kg) | 5 | 0, 2.0 | Crude toxin extract | 38 days | Reduced body weight gain | 2.0 | n.a. | Changyun et al. (2014) |
| Landrace × Yorkshire (28 days) | 8 | 0, 3.5 | Contaminated wheat | 42 days | IgG antibody response to OVA increased | 3.5 | n.a. | Lessard et al. (2015) |
| Downregulation of claudin, occludin, and vimentin genes | 3.5 | n.a. | ||||||
| Upregulation of expression of IL‐8, CXCL10, interferon‐γ and major antioxidant glutathione peroxidase 2 (GPX‐2) genes | 3.5 | n.a. | ||||||
| Downregulation of GPX‐3, GPX‐4 and superoxide dismutase‐3 genes | 3.5 | n.a. | ||||||
| Reduced body weight gain | 3.5 | n.a. | ||||||
| Crossbred pigs(22.9 kg) | 6, 18 | 0.2, 5 | Contaminated distiller grain | 120 days | No differences in macroscopic or microscopic lesions, bone ash and density | n.a. | 5.0 | Madson et al. (2012) |
| Growing pigs | 5 | 0–2.85 | n.r. | 35 days | Decreased claudin‐4 expression | 2.85 | n.a. | Pinton et al. (2009) |
| Crossbred piglets (8.9 kg) | 25 | 0–4.52 | Contaminated maize | 29 days | Reduced body weight gain | 4.52 | n.a. | Rempe et al. (2013) |
| No effect on visceral organ weights | n.a. | 4.52 | ||||||
| No effect on histopathological organ specimens | n.a. | 4.52 | ||||||
| No effect on total leukocytes, differential blood count and stimulation index of peripheral blood mononuclear cells | n.a. | 4.52 | ||||||
| Crossbred piglets (28 days) | 6 | 0, 2.5, 3.5 | Contaminated wheat | 42 days | Inhibition of vaccination efficiency of PRRSV live attenuated vaccine by impairing viral replication | 2.5 | n.a. | Savard et al. (2015) |
| Crossbred(16.3 kg) | 6 | 0, 3, 6, 12 | Contaminated maize | 21 days | Increased daily body weight gain | 12 | 6 | Wu et al. (2015) |
| Increased relative liver weight | 12 | 6 | ||||||
| Elevated blood urea nitrogen, alkaline phosphatase, alanine aminotransferase and aspartate amino transferase | 3 | n.a. | ||||||
| Decreased serum concentrations of l‐valine, glycine,l‐serine and l‐glutamine, serum total superoxide dismutase, glutathione peroxidase | 3 | n.a. | ||||||
| Decreased microvilli height, increased lymphocyte cell number | 3 | n.a. | ||||||
| Elevated mRNA expression levels of excitatory amino acid transporter‐3, sodium‐glucose transporter‐1, dipeptide transporter‐1, cationic amino acid transporter‐1 and y+L‐type amino acid transporter‐1 | 3 | n.a. | ||||||
n: number of animals per group; bw: body weight, n.r.: not reported; ~: approximately; n.a.: not applicable because not identified by the authors of the study or by the CONTAM Panel; SRBC: sheep red blood cells; IGF: Insulin‐like growth factors; PCV: Packed cell volume; PHA: Phytohaemagglutinin; GLDH: Glutamate dehydrogenase; PRRSV: porcine reproductive and respiratory syndrome virus.
The first part of the table (until the reference of Swamy et al. 2003) has been modified from EFSA (2004). Note that the units are expressed as mg/kg feed unless otherwise stated.
The exposure time is indicated as time period and/or as body weight reached at the of the time period.
Not reported by FAO/WHO (2001, 2010).
7.3.2.1. DON
The feeding studies on the effects of DON in pigs were summarised in EFSA (2004) and are briefly described below (see also Table 48). Among these studies, the adverse effects of either crystalline DON or naturally or artificially Fusarium infected cereals containing DON were distinguished. Naturally infected feed had a stronger effect on the feed intake and weight gain than pure DON, and this lead to the assumption that the presence of other toxins and compounds (e.g. bacterial polysaccharides) in feed affects the toxicity of DON and other trichothecenes (EFSA, 2004). In many studies, particularly those conducted with purified DON, no effect was found at 0.6–0.9 mg DON/kg feed. Feeding trials, where naturally or artificially infected material had been mixed into the diet, showed decreased feed intake and body weight gain in pigs at concentrations as low as 0.35 mg DON/kg feed (Trenholm et al., 1983).
In one of the subtrials of Young et al. (1983) with piglets (n = 20) concentrations from 9.0 mg DON/kg in naturally contaminated feed caused a large depression in performance, and there were indications that at least one pig fed 19.7, 33.5 or 43.4 mg DON/kg feed vomited on the first day of the trial. In another subtrial, 24 piglets were fed 1.3–11.9 mg DON/kg diet for 21 days resulting in reduction of body weight gain, feed consumption and feed conversion but no vomiting was observed. From this study, the CONTAM Panel identified the LOAEL of 1.3 mg DON/kg feed for the reduced feed intake, and the NOAEL of 11.9 mg DON/kg feed and the LOAEL of 19.7 mg DON/kg feed for vomiting.
Feed contaminated with 2.0 mg DON/kg diet fed to piglets (n = 8, average body weight = 7.7 kg) for 3 weeks resulted in reduced feed intake (Pollmann et al., 1985). Another subtrial in the same study (n = 4, average body weight = 8.3 kg) showed a reduced feed intake at 1.4 mg/kg diet for 2 weeks, but not at higher concentrations. A third subtrial was conducted for 6 weeks with grower‐finishing pigs with an average body weight of 60.8 kg. Evidence of vomiting was reported from the third subtrial (6 weeks old grower‐finishing pigs with average body weight of 60.8 kg) at a concentration of 2.8 mg/kg diet, but not at higher concentrations, and reduced feed intake occurred at 2.2 mg/kg feed.
Based on the available studies (see also Table 48), the CONTAM Panel concluded that vomiting was the critical acute effect in pigs, and that the concentration of 2.8 mg/kg feed was considered a LOAEL for vomiting. The CONTAM Panel noted that this value was lower than the NOAEL of 11.9 mg/kg feed observed in another study (Young et al., 1983) in another breed of pigs, but still higher than the lowest NOAEL identified as 0.7 mg/kg feed observed by Forsyth et al. (1977). A NOAEL of 0.7 mg/kg feed was reported by Bergsjö et al. (1993b) for reduced feed intake.
The CONTAM Panel noted that several studies, summarised by EFSA (2004) and a number of more recent studies (see Table 48), investigated the impact of DON on immune responses in pigs. However, no suitable parameter for the risk assessment of DON (nor its acetylated and modified forms) could be identified among the various immune responses reported in these studies, nor could the observed size of the change of investigated immune responses be associated with relevant adverse immunological effects. Therefore, the CONTAM Panel concluded that no suitable concentration–response data on adverse immunological effects were available for the hazard characterisation of pigs.
In the previous assessment of EFSA (2004), various studies reported changes in different clinical chemistry parameters (plasma nutrients and plasma enzyme activities), while in other studies no changes were observed. Reported alterations were often considered as the consequence of the reduced feed intake and not a direct effect of DON since no changes in these parameters were observed when compared to pair‐fed controls (Lun et al., 1985). Lusky et al. (1998) reported changes in kidney tubular epithelium in pigs after 90 days of DON exposure but no such changes were found in the control pigs.
EFSA (2004) also summarised the studies of the effects of DON on pig reproduction and noted that significant trends towards a lower fetal weight and length in fetuses of sows fed DON contaminated feed during pregnancy were reported by Friend et al. (1986a,b) and Chavez (1984), while Friend et al. (1986a) did not find effects of DON on litter size, weight or size at birth, or weight gain during lactation or survival rate of piglets when gilts were fed naturally DON contaminated feed. Later, Alm et al. (2006) reported that a combination of 6.1 mg/kg feed of DON and 0.235 mg/kg feed of zearalenone fed for a period of 35 days resulted in a reduced oocyte quality (reduced proportion having immature chromatin and reaching metaphase II in culture) in gilts. When Stanek et al. (2012) evaluated the combined effect of orally administered DON contaminated diet and LPS on hepatic histopathology and blood clinical parameters in barrows, no impact of DON alone on liver morphology and function was observed. The CONTAM Panel concluded that these findings on reproductive and developmental effects were not convincing to be used for the hazard characterisation of DON in pigs.
Genotoxic effects of DON in pigs are reported in Section 7.2.9 and the CONTAM Panel noted due to the short life time of pig these effects were not relevant for the chronic adverse effects in pigs.
Time‐dependent effects of feed contaminated with low doses of DON and zearalenone were reported in female piglets (initial mean weight = 8.9 kg, n = 4 per group) (Rempe et al., 2013). A significant weight gain reduction after the 5th (last) week of the experiment was observed at the highest dose group only (4.52 mg DON/kg feed, 0.29 mg zearalenone/kg feed) Histopathological examination of the organs did not reveal any toxin‐related lesions, and the haematological and clinical‐chemical parameters showed only minor effects. However, in the EFSA (2004) assessment it was noted that the consumption of DON‐contaminated feed was associated with epithelial lesions in the oesophageal region of the stomach when pigs had been given naturally infected feed containing from about 3–6 mg DON/kg feed. More recently, Grenier et al. (2011) studied the effects of 2.8 mg/kg feed of DON in piglets (5‐week old male, initial body weight of 10.2 ± 1.9 kg, n = 6 per group) and observed induced histopathological lesions in the liver, lungs and kidneys after 35 days, including morphological and histological changes in the jejunum. Furthermore, Gerez et al. (2015) reported liver and intestinal lesions even after 28 days of feeding of 1.5 mg DON/kg feed to 35 days old piglets (n = 5).
Based on the available evidence (see Table 48), the CONTAM Panel concluded that the reduced feed intake and reduced body weight gain were the critical chronic adverse effects of DON in pigs. The CONTAM Panel confirmed what had been noted earlier by EFSA (2004), that although reduced feed intake at the lowest DON concentrations tested was often temporary and the pigs adapted to these concentrations, the reduction in body weight gain observed during the first period was not fully compensated by the time when the pigs reached slaughter weight. The CONTAM Panel noted that in several studies the LOAELs for reduced feed intake and reduced body weight gain were lower than the NOAELs in the other studies; e.g. LOAEL of 0.35 mg DON/kg feed from Trenholm et al. (1983) compared to the NOAEL of 2.8 mg DON/kg feed from Richter et al. (1989). Therefore, a wide range of NOAELs for reduced feed intake and reduced body weight gain (0.7–5.0 mg DON/kg feed) was observed overlapping with even a wider range of LOAELs (0.35–13 mg DON/kg feed).
7.3.2.2. 3‐Ac‐DON and 15‐Ac‐DON
The only identified study on acetylated forms of DON was on intestinal toxicity of DON, 3‐Ac‐DON and 15‐Ac‐DON ex vivo and in vivo (Pinton et al., 2012). In the 4‐week feeding trial, weaned piglets (n = 6 per group) were fed a diet either containing 2.29 mg DON/kg feed only or a mixture of DON and 15‐Ac‐DON 1.24 and 0.935 mg/kg feed, respectively (3‐Ac‐DON at 100 times lower concentrations). The in vivo experiment showed more histological lesions in the group exposed to 15‐Ac‐DON compared to the animals of the DON and the 3‐Ac‐DON group indicating an enhanced intestinal toxicity of 15‐Ac‐DON compared to DON. This effect was correlated with the abilities of DON, 3‐Ac‐DON and 15‐Ac‐DON to lead to an increased phosphorylation of MAPK, which confirmed the observations from the ex vivo experiment (see Section 7.7.2). However, as only one concentration was tested no specific NOAEL/LOAEL for 3‐Ac‐DON or 15‐Ac‐DON could be identified.
7.3.2.3. DON‐3‐glucoside
The only study available on the toxicity of DON‐3‐glucoside was the ex vivo study of Pierron et al. (2016b) on the glucosylation of DON (see Section 7.7) and thus no NOAEL/LOAEL could be identified for DON‐3‐glucoside in pigs.
7.3.2.4. Conclusions on pigs
Reduced feed intake and reduced body weight gain were the most often reported chronic adverse effects of DON in pigs. However, DON may cause several other adverse effects in pigs including lesions in the oesophageal region of the stomach, in the liver, the lungs and the kidneys and changes in different clinical chemistry parameters (plasma nutrients and plasma enzyme activities). Several studies described an impact of DON on immune responses in pigs but the type and the size of those responses could hardly be associated with relevant adverse immunological effects and thus no dose‐concentration/response data were identified to assess adverse immunological effects. While the acute NOAEL for vomiting varied between 0.7 and 12 mg DON/kg feed a lowest LOAEL of 2.8 mg DON/kg feed was identified for vomiting from the available data. Reduced feed intake and weight gain reduction were identified as the critical chronic adverse effects of DON in pigs and wide ranges of NOAEL and LOAEL values were identified by the CONTAM Panel. The overall NOAEL for reduced feed intake and weight gain was 0.7 mg DON/kg feed and the overall LOAEL 0.35 mg DON/kg feed. The CONTAM Panel noted that the LOAELs in some studies on pigs were lower than the NOAELs in other studies and depending on the observation period NOAELs were equal to the LOAELs in some studies, which was likely due to adaptation of the pigs to the DON exposure. However, reiterating EFSA (2004), the CONTAM Panel noted that the reduction in body weight gain observed during the first period was not fully compensated by the time when the pigs reached slaughter weight. Due to the limited or lack of data on adverse effects in pigs caused by 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, no specific NOAELs/LOAELs could be identified for these forms.
7.3.3. Poultry
Health effects of DON on poultry have recently been reviewed by Awad et al. (2008), Awad et al. (2012a, 2013). Several experimental studies using feed naturally contaminated by Fusarium mycotoxins including acetylated forms of DON were identified, while no studies were found using feed containing modified forms of DON.
Due to the high number of studies available for adverse effects on poultry, the CONTAM Panel decided not to consider the publications in which the effects could not directly be linked solely to DON because of the substantial co‐contamination with other mycotoxins such as fusaric acid, zearalenone, fumonisins, enniatins and beauvericin or the details on methods of analysis, presence/absence of other mycotoxins and concentrations in feeds were not reported. The overview of the studies on broiler chickens included by the CONTAM Panel in this opinion is presented in Table 49.
Table 49.
Adverse effects of broiler chickens exposed to DON in feed
| Animals (age) | n | Concentration (mg/kg feed) | Toxin source | Exposure time | Effects | LOAEL (mg/kg feed) | NOAEL (mg/kg feed) | Reference |
|---|---|---|---|---|---|---|---|---|
| Studies reported in EFSA (2004) | ||||||||
| Ross (day 1) | 45 | 1.8–5.4 | Contaminated maize | 37 days | Increased heart weight | n.a. | 3.6 | Leitgeb et al. (1999) |
| Broiler chicks (day 1) | 36 | 16 | Contaminated wheat | 21 days | No effect on feed intake, body weight gain or other parameters measured | n.a. | 16 | Harvey et al. (1997) |
| White Leghorn (day 1) | 100 | 0, 18 | Contaminated wheat | 12 weeks | Reduced body weight after 4 and 8 weeks, but not 12 weeks, increased relative gizzard weight, decreased haemoglobin at 4 weeks, but not after 8 and 12 weeks | 18 | n.a. | Kubena and Harvey (1988) |
| White Plymouth Rock x White Cornish (day 1) | 240 | 0.1–3.4 | Contaminated oats | 35 days | No effect on feed intake, body weight gain or meat quality | n.a. | 3.4 | Bergsjö and Kaldhusdal (1994) |
| Hubbard x Hubbard (day 1) | 0, 16 | 0, 16 | Contaminated wheat | 3 weeks | Reduced body weight gain, increased feed:body weight gain ratio, relative gizzard weight and relative bursa weight | 16 | n.a. | Kubena et al. (1989) |
| Hubbard xHubbard)(day 1) | 60 | 0, 16ad libitum | Contaminated wheat | 3 weeks | Reduced body weight gain, increased feed:body weight gain ratio, increased relative gizzard weight, increased red blood cell count and serum phosphorus, decreased mean corpuscular haemoglobin and glucose | 16 | n.a. | Kubena et al. (1988) |
| White Leghorn(day 1) | 51 | 0, 9, 18 | Contaminated wheat | 35 days | Reduced liver weight, increased gizzard weight, temporary decreased plasma triglycerides, glucose, increased creatinine, increased plasma haemoglobin and temporary red blood cells | 9 | n.a. | Kubena et al. (1985) |
| White Leghorn(day 1 to egg production) | 30 | 0, 18ad libitum | Contaminated wheat | 48 weeks | Small significant increase in shell weight, shell thickness, decrease in serum uric acid, glucose, triglycerides and cholesterol and increase in serum γ‐glutamyltransferase and alkaline phosphatase | 18 | n.a. | Kubena et al. (1987a) |
| White Leghorn(day 7) | 50 | 0–0.7 | Contaminated wheat | 14 days | No significant effect on feed intake, body weight gain or other parameters measured (control feed composition differed from the contaminated diet) | n.a. | 0.7 | Hamilton et al. (1985) |
| White Leghorn(day 7) | 50 | 3.1–4.1 | Contaminated wheat | 28 days | Increased feed intake, body weight gain and feed:gain ratio or no apparent lesions in oral activity or other parameters measured (control feed composition different from the contaminated diet) | n.a. | 4.1 | |
| White Leghorn(day 1) | 60, | 0, 18 | Contaminated wheat | 18 and 9 weeks | Reduced immune response to vaccine reduced mitogen‐induced lymphoblastogenesis | 18 | n.a. | Harvey et al. (1991) |
| Hubbard × Hubbard and white Leghorn(day 1) | 10 | 0, 50 | Pure toxin | 9 weeks | Reduced response to mitogens in female broiler chickens, no effect in male broiler chickens or in leghorn chickens of either gender | 50 | n.a. | |
| White mountain × Hubbard(6 days) | 24 | 0–210 | Contaminated maize | 5 days | Increased feed conversion, reversible concentration‐dependent increase in oral and gizzard epithelial lesions | 49.4 | n.a. | Moran et al. (1982), |
| Reduced feed intake | n.a. | 116.1 | ||||||
| Shaver (day 1) | 18 × 2 | < 0.2–1.87 | Contaminated wheat | 28 days | No effect on feed intake, body weight gain, liver or kidney | n.a. | 1.89 | Hulan and Proudfoot (1982) |
| Hubbard × Hubbard (day 1) | 60 | 0, 16 | Contaminated wheat | 3 weeks | Reduced body weight, increased feed efficiency, increased relative gizzard weight, anaemia, decreased lactate dehydrogenase and serum triglycerides | 16 | n.a. | Huff et al. (1986) |
| White Leghorn × Single Comb (26 weeks) | 10 | 0, 83 | Contaminated wheat | 27 days | Small erosions in the gizzard, no other pathological changes | 83 | n.a. | Lun et al. (1986) |
| Different types | 3 | 0–0.70 | Contaminated wheat | 86 or 135 days | Increased liver triglycerides and total liver lipid at 0.35 mg/kg, not at 0.70 mg/kg | n.a. | Farnworth et al. (1983) | |
| Studies identified after EFSA (2004) | ||||||||
| Lohmann (1 day) | 64 | 0, 3.5, 7, 10.5, 14 | Contaminated wheat | 3–5 weeks | Decreased spleen weight, reduced body weight gain, decreased Newcastle Disease Virus vaccine response | 10.5 | 7 | Dänicke et al. (2003) |
| Ross (1 day) | 95 | 0, 10 | Toxin added to feed | 6 weeks | No effects on feed intake or body weight gain or other zootechnical parameters, alteration of intestinal morphology | 10 | n.a. | Awad et al. (2004, 2006) |
| Ross (1 day) | 8 | 0, 10 | Toxin added to feed | 5 weeks | Reduced feed intake, reduced body weight gain during the first 2 weeks, reduced total lymphocyte count, decreased Infectious Bronchitis Virus vaccine response | 10 | n.a. | Ghareeb et al. (2012, 2014) |
| Ross (1 day) | 15 | 0.9, 5 | Contaminated wheat | 7 weeks | Reduced feed intake, reduced body weight, body weight gain during the first 2 weeks but not later, relative weight of organs not altered, in the jejunum the villi were shorter but the villi width not modified | n.a. | 5 | Awad et al. (2011) |
| Ross (7 day) | 25 | 0.3, 1.7, 12 | Contaminated culture medium added to feed | 5 weeks | Reduced feed intake, reduced of body weight gain and alteration of intestinal morphology during the first 3 weeks, no zootechnical effects at the end of the experiment | 12 | n.a. | Yunus et al. (2012a,2012b) |
| Ross (3 weeks) | 8 | 2.4, 7.5 | Toxin added to feed | 3 weeks | No effects on feed intake or body weight gain or other zootechnical parameters at the end of the experiment | n.a. | 7.5 | Osselaere et al. (2012, 2013a) |
| Ross (1 day) | 56 | 0.2–4.6 | Contaminated culture medium added to feed | 2 weeks | No effects on feed conversion ratio and body weight gain | n.a. | 4.6 | Antonissen et al. (2015) |
7.3.3.1. DON
The CONTAM Panel noted that the studies summarised by EFSA (2004) and more recent studies of Chowdhury et al. (2005), Girgis et al. (2008), Levkut et al. (2009), Ghareeb et al. (2012, 2016) and Yunus et al. (2012a) investigated the impact of DON to immune response or immunocompetence in poultry besides zootechnical parameters such as feed intake and body weight gain (see also Table 49 on broiler chickens). However, no suitable parameter for the risk assessment of DON (nor its acetylated and modified forms) in poultry could be identified among the various immune responses reported in these studies or the observed size of the change of investigated immune responses could not be associated with relevant adverse immunological effects. The CONTAM Panel concluded that no suitable concentration–response data on adverse immunological effects were available for the hazard characterisation of poultry. Chowdhury (2005) studied effects of DON on haematology in poultry but no haematotoxicity was observed.
Acute mycotoxicosis in broiler chickens associated to DON was characterised by extensive ecchymotic haemorrhaging throughout the carcass, widespread deposition of urates, disturbance of the nervous system, and irritation of the upper gastrointestinal tract. An approximate oral LD50 dose of 140 mg/kg bw was reported by the authors (Huff et al., 1981).
The previous EFSA (2004) opinion reported data on the potential adverse effects of DON in chickens. Reduced feed intake and body weight gain were only found when concentrations reached 16–20 mg DON/kg feed (Kubena et al., 1987a, 1988, 1989; Kubena and Harvey, 1988; Harvey et al., 1991). Kubena et al. (1985) describe, however, a decrease in the relative and absolute liver weight and an increase on the relative and absolute gizzard weight in chickens fed 9 or 18 mg DON/kg feed for days 1–35 of age.
Dänicke et al. (2003) used five groups of 64 male broilers from day 1 to day 35 of age to evaluate the effects of different dietary proportions of wheat (0, 16.5, 33, 49.5 and 66%) naturally contaminated with 21.2 mg of DON and 0.4 mg of zearalenone per kg of wheat (0, 3.5, 7, 10.5 and 14 mg DON/kg diet). The feed intake and body weight gain decreased linearly in broilers given DON at concentrations of 10.5 and 14 mg/kg feed. The authors concluded that feeding of diets containing approximately 10 mg DON/kg or more affected the performance of broilers negatively. Based on these data, the CONTAM Panel considered the concentrations of 10 and 7 mg DON/kg feed as LOAEL and NOAEL for chickens, respectively.
Several experiments on broilers chicks were performed by Awad et al. (2004, 2006, 2012b, 2014) and Ghareeb et al. (2012, 2014) using diets supplemented by 10 mg DON/kg feed The first experiment was on broilers chicks (one‐day‐old) fed for 42 days (Awad et al., 2004, 2006). The control group (n = 91) was fed starter and grower diets based on wheat, soybean meal, maize, rapeseed oil, and a premix with vitamins, minerals, amino acids, salt, and dicalcium phosphate. The DON‐group (n = 95) was fed the starter and grower diets supplemented with 10 mg DON/kg feed. The DON‐diet impaired intestinal transfer and uptake of nutrients such as glucose, but it did not impair growth performance such as body weight gain and feed conversion. The absolute or relative organ (liver, gizzard, duodenum, pancreas, heart and spleen) weights were not altered significantly in broilers fed the diet containing DON and no pathological lesions were found in the gut. However, a decrease of the weight and alteration of the morphology of the small intestine were observed: slight villus atrophy and irregular crypts especially in the duodenum and jejunum (shorter and thinner villi) such that the authors concluded that, diets with DON concentrations below the levels that induce a negative impact on performance could alter small intestinal morphology in broilers. In the two follow‐up studies, Awad et al. (2012b, 2014) investigated the genotoxic effects of DON in broiler chickens (see Section 7.2.9) but the CONTAM Panel noted that these effects are not relevant for the health of broiler chickens due to their short life time. Using the design of Awad et. al. (2004, 2006), but with 10 animals per group, Ghareeb et al. (2014) observed an elevation of plasmatic corticosterone and increased the heterophil‐lymphocyte ratio (stress index) at 10 mg DON/kg diet. Body weight and body weight gain were reduced during the starter phase and during the grower phase, respectively. Based on these studies above the CONTAM Panel identified the concentration of 10 mg/kg feed as LOAEL for broiler chickens.
In the review of Ghareeb et al. (2015) on the impact of DON contaminated diet on intestine integrity in chickens, a level around 5 mg DON/kg feed generated shorter villi and a decrease of villi surface area without any decrease of villus height and crypt depth and consequently without any impact on feed intake and body weight. However, at a level of 10–12 mg DON/kg feed the alteration of intestine morphology results in reduced body weight gain and a LOAEL of 10 mg DON/kg feed was identified.
Unlike the above studies using the artificially contaminated diets, Awad et al. (2011) fed broiler chickens with a diet naturally contaminated with DON, 3‐Ac‐DON and zearalenone (see details in Section 7.1.5.3.1). None of the zootechnical parameters (body weight, body weight gain, feed intake and feed conversion) responded to increased DON levels in the diet and the absolute and relative weights of organs (liver, heart, proventriculus, gizzard, small intestine, spleen, pancreas, colon, cecum, bursa of Fabricius and thymus) remained unaltered; also the intestine density (weight/length ratio). However, in the jejunum, the villi were shorter and the villus surface area decreased in DON fed birds compared to controls; however villus width, crypt depth, and villus height/crypt depth ratio in the jejunal mucosa showed no changes. Therefore, the CONTAM Panel indentified the 5 mg DON/kg in feed as a NOAEL.
Yunus et al. (2012b) investigated the effects of DON in male broilers, and in particular body weight gain and intestinal morphology (see details in Section 7.1.5.3.1). DON was negatively (linear and significant over the first 3 weeks only) correlated with body weight gain. No difference in body weight gain was observed after the third week between treated and control birds. A negative correlation was also observed for the relative density (weight/length) of the small intestine, itself correlated with a decrease in villus height. The transport function of the epithelium per unit area was reduced at higher concentrations of DON when investigating the short circuit current of the jejunal epithelium. DON was positively (linear over the first 4 weeks only) correlated with the length of the jejunum. The authors concluded that consumption of contaminated grains resulted in adverse effects on intestinal morphology during early growth phases. Since no differences in body weight between control and DON exposed birds was observed at the end of the experiment, the authors considered these chickens may adapt to a chronic DON exposure by morphological and functional modifications.
Osselaere et al. (2012) fed Ross chickens with control, artificially and naturally DON‐contaminated feed (see details in Section 7.1.5.3.1). No statistically significant effects on zootechnical parameters (feed intake, body weight gain and final live body weight) were observed between the two groups 2 and 3 and controls and the authors interpreted that as development of a tolerance to DON either due to aging or due to an adaptation via metabolic and hormonal compensatory mechanisms. In the follow‐up study (see Section 7.1.5.3.1), Osselaere et al. (2013a) treated of eight 3‐week old Ross broiler chickens in a two‐way cross‐over design: four animals received an oral bolus and four animals received intravenously DON single doses at 0.750 mg/kg bw. After a wash‐out period was applied, the animals that previously received an oral bolus, received at that time an intravenous dose and vice versa. Since no clinical signs of intoxication were observed the authors interpreted this outcome also as tolerance of poultry to DON given the observed high plasma clearance.
Kautzman et al. (2015) fed Ross 308 male broiler chickens (n = 15 per group) for 35 days with naturally contaminated feed at the levels of 0.5–1.5, 0.7–3.7, 1.0–6.9 and 1.2–8.3 mg DON/kg feed. No significant difference in body weight, feed intake and feed conversion ratio was observed between the groups. Since this study did not include a control group, it was not considered for hazard characterisation by the CONTAM Panel.
One‐day‐old broiler chickens (Ross 308, both sexes) were divided into two groups, each consisting of eight pens of seven birds each, and were fed for 2 weeks either a control diet (0.2 mg DON/kg feed) or a DON‐contaminated diet (4.6 mg DON/kg feed), which was a mixture of the control feed with toxic culture medium (Antonissen et al., 2015). No significant differences were observed in body weight and feed conversion ratio between the control group and chicken fed a DON‐contaminated diet. The expression of the gene coding for MUC2 was significantly down‐regulated in the duodenum of broilers fed DON‐contaminated diet (see also Section 7.2.9). However, this genotoxic effect was not considered relevant for broiler chickens by the CONTAM Panel. From this study, the CONTAM Panel identified 4.6 mg DON/kg in feed as a NOAEL for body weight gain and feed conversion ratio.
In a cross‐over trial of Broekaert et al. (2016), 5 mg DON/kg feed was administered orally to broiler chickens (see details in Section 7.1.5.3.3). No adverse effects were observed in broiler chickens but since this was a single dose study it was not considered for hazard characterisation.
Conclusions on broiler chickens exposed to DON. From the above experiments in broiler chickens (see also Table 49), the CONTAM Panel selected the studies performed using the highest DON concentrations in feed combined with the longest periods of time to determined a NOAEL for chicken. Referring in particularly to the data of Dänicke et al. (2003), Awad et al. (2011) and Antonissen et al. (2015), the CONTAM Panel concluded that the range of 4.6–7.0 mg DON/kg feed did not generate adverse effects on feed intake, body weight gain and final live body weight. Based on the available data, particularly from Dänicke et al. (2003), Yunus et al. (2012b), Awad et al. (2012b, 2014) and Ghareeb et al. (2014), the CONTAM Panel noted that concentrations of 10–12 mg DON/kg feed did not only induce modifications in intestine morphology but also generated a reduction of body weight gain. Therefore, the concentration ranges of 4.6–7 and 10–12 mg DON/kg feed were identified by the CONTAM Panel as the ranges for NOAELs and LOAELs, respectively, for chickens.
Potential adverse effects of DON in laying hens, were reported in the previous EFSA (2004) assessment. Diets containing DON up to 83 mg/kg feed did not have a significant effect on the egg production. Hatchability was not affected by levels of up to 18 mg DON/kg feed (Hamilton et al., 1985; Lun et al., 1986; Kubena et al., 1987b; Bergsjö et al., 1993a). However, a small increase in the incidence of minor malformations, considered as delayed fetal maturation (delayed ossification, un‐withdrawn yolk sac) were observed in chick fetuses from hens given a feed containing 2.5 or 3.1 mg DON/kg, but not in the group given 4.9 mg DON/kg feed (Bergsjö et al., 1993a). No abnormalities were recorded in hens given 0–4.9 mg DON/kg feed (Hamilton et al., 1985).
A 16‐week experiment tested 25 Lohmann Brown laying hybrids hens (18‐week‐old) using two types of diets: a contaminated diet containing 17.63 mg DON, 5.5 mg 15‐Ac‐DON, 1.60 nivalenol, 1.58 mg zearalenone and 0.09 mg 3‐Ac‐DON per kg and an uncontaminated diet as control containing 0.21 mg DON and 0.04 mg zearalenone per kg (other mycotoxins were not analysed). Feeding of the contaminated diet depressed feed intake by approximately 5% (Dänicke et al., 2002).
Forty‐two 26‐week old broiler breeder hens were fed a control (0.2 mg DON/kg) and a contaminated (13 mg DON/kg) diet (lower levels were reported for 15‐Ac‐DON and zearalenone) (Smith et al., 2007). Similarly, nine roosters (Ross 308) were fed a control (0.9 mg DON/kg) and a contaminated (7.8 mg DON/kg) diet. Rooster semen volume and sperm concentration, viability, motility and relative weights of testes were not significantly affected by DON. When inseminating the hens three times during the week before egg collection with 50 μL of fresh pooled semen from these roosters, no effects of DON were observed for feed intake, feed efficiency (feed consumed/egg produced), body weight and for liver, spleen and kidney relative weights. There was also no effect on egg weight, yolk weight, albumen height and eggshell deformity and body weight or viability of newly hatched chicks. However, egg shell thickness was reduced after 4 weeks and in early (1–7 day) embryonic mortality was increased. In conclusion, DON concentration at 13 mg DON/kg feed in the diet of Ross breeder hens had a negative impact on fertility.
Ebrahem et al., (2014b) examined diets with three increasing levels of DON in 216 laying hens (23‐week‐old, Lohmann Brown and Lohmann Selected Leghorn) and 24 adult roosters for the reproductive performance and the health of the newly hatched chicks. Very low contaminated wheat with 0.054 mg DON and 0.069 15‐Ac‐DON/kg was used as ingredient to prepare the control diet which contained 0.04 mg DON/kg feed. Highly contaminated wheat (inoculated by a F. culmorum strain) with 13.5 mg DON, 4.7 mg aurofusarin, 0.76 mg DON‐3‐glucoside and 0.23 mg 3‐Ac‐DON was used as ingredient to prepare the other two DON‐containing diets which contained around 5 and 10 mg DON/kg feed. Hatchability was significantly more affected by the presence of DON in Lohmann brown than the Lohmann selected Leghorn chicks. An interaction between the concentration of DON in the diet and the breed was observed for fertility. At 10 mg DON/kg diet the fertility was lower for the eggs from Lohmann brown hens compared with Lohmann Selected Leghorn hens. Compared with controls, chicks hatching from exposed eggs had significantly decreased spleen relative weights, and gizzard relative weight was significantly decreased only in Lohmann Brown chicks at 10 mg DON/kg. No haematological disturbances were observed and no organ histopathology was identified. DON showed no effects on the fertility of the roosters (the percentage of fertile eggs of all laid eggs). However, from the observation at 10 mg DON/kg diet on fertility and hatchability of eggs for the Lohmann Brown strain, the authors concluded that the breed of the hens might modulate the effect of DON on reproductive performance of laying and they considered 5 mg DON/kg feed as no‐effect level for reproductive performance of hens.
Conclusions on laying hens exposed to DON. From the studies on laying hens described above, the CONTAM Panel identified the range of LOAELs of 10–13 mg DON/kg feed for inducing a decrease of feed intake, spleen and gizzard relative weights, egg fertility and hatchability. Since diets of 4.9 and 5 mg DON/kg did not generate any abnormality in zootechnical parameters such as feed intake, hatchability and egg fertility, 5 mg DON/kg feed was considered as overall NOAEL for laying hens.
In Pekin ducks, as reported in the EFSA (2004) opinion, no significant differences in feed intake, body weight gain, and feed to gain ratio was observed at DON concentrations up to 7 mg/kg feed, although during the first week of exposure a slight depression in body weight gain was observed, which was, however, fully compensated later. Gross macroscopical inspection of the upper digestive tract did not reveal any signs of irritation, inflammation or other pathological changes. The relative organ weight of the bursa of Fabricius decreased dose dependently. Activities of glutamate‐dehydrogenase and γ‐glutamyltransferase in serum were not or inconsistently affected by DON exposure (Dänicke et al., 2004). No later data than that reported in the 2004 EFSA opinion were identified in the recent literature. Yoshizawa and Morooka (1974) (not included in the EFSA, 2004) reported for the 10‐day old duckling the minimum emetic dose subcutaneously administered of 10 mg DON/kg bw. Based on these studies above on ducks, the CONTAM Panel identified concentration of 7 mg DON/kg feed from the study of Dänicke et al. (2004) generating no zootechnical parameters change as the NOAEL for ducks in this study.
Grimes et al. (2010) performed a trial on 168 male turkey poults placed into 24 pens (7 birds/pen; 6 pens/treatment) and reared to 3 weeks (21 days) involving four diets with naturally contaminated corn: (1) clean maize as control, and diets containing (2) 0.1 mg aflatoxin B1, (3) 1.7 mg DON and (4) 0.05 mg aflatoxin B1 + 0.9 mg DON per kg feed. Poults fed DON contaminated diets (3 and 4) showed reduced feed intake and reduced body weight gain and had the lowest feed consumption among the four groups. The relative spleen weight of DON fed birds was also reduced. Relative weights of heart, gizzard, bursa of Fabricius and the colours of breast muscle and gizzard were not affected. There were no differences in antibody productions between the four groups. Based on data from the group (3), a diet only contaminated with DON at 1.7 mg/kg feed generated changes in zootechnical parameters (feed intake, body weight gain, feed to gain ratio and organ weight). However, this study was conducted for a short period of time (3 weeks).
Devreese et al. (2014) conducted an experiment on 120 one‐day‐old male turkey poults fed a control diet containing 0.06–0.39 mg DON, below 0.05 (LOD)–0.10 mg 15‐Ac‐DON, and below 0.03 mg of others mycotoxins per kg feed, and a naturally contaminated diet containing 4.0–6.5 mg DON, 0.45–0.55 mg 15‐Ac‐DON, 0.026–0.120 mg fumonisins B1+B2 and 0.37–0.67 zearalenone, per kg feed (see details in Section 7.1.5.3). Except for the starter phase, no significant differences in performance parameters (body weight, body weight gain, feed intake or feed conversion ratios) were observed. The feeding of contaminated diets reduced duodenal villus height and apparent villus surface area. An adaptation to DON exposure was observed by the authors after the starter stage of the trial (see also Section 7.1.5.3.1). The CONTAM Panel noted that Devreese et al. (2014) reported effects in this study for the longest period of time and considered these results as the most relevant for the hazard characterisation of DON in farmed turkeys. Since the concentration of 6.5 mg/kg feed generated no major changes in zootechnical parameters (body weight, body weight gain, feed intake or feed conversion ratios), this value was identified as overall NOAEL for turkeys.
7.3.3.2. 3‐Ac‐DON and 15‐Ac‐DON
Although a number of the studies on chickens, ducks, turkeys or laying hens described above dealt with diets that contained 3‐Ac‐DON/kg and/or 15‐Ac‐DON/kg feed, the described effects could not be causally related to 3‐Ac‐DON and/or 15‐Ac‐DON and such a specific hazard characterisation of 3‐Ac‐DON/kg and/or 15‐Ac‐DON/kg feed could not be performed.
7.3.3.3. DON‐3‐glucoside
The only identified study on DON‐3‐glucoside performed cross‐over animal trials with oral administration of 5 mg DON‐3‐glucoside/kg feed to broiler chickens (Broekaert et al., 2016) (see details in Section 7.1.5.3.3). No adverse effects were observed. However, as only one concentration was tested no NOAEL/LOAEL for DON‐3‐glucoside was identified.
7.3.3.4. Conclusions on poultry
For broiler chickens, the level of 4.6–7 mg DON/kg feed did not generate any adverse effects. The level of 10–12 mg DON/kg feed did not only cause some modifications in intestine but also generated some feed intake and body weight gain changes. The CONTAM Panel identified 4.6–7 mg DON/kg feed as the range of NOAELs and of 10–12 mg DON/kg feed as the range of LOAELs for chickens. For laying hens diets with DON concentrations up to 18 mg DON/kg, feed did not induce any negative impact on as body weight gain, hatchability and egg production. However, a diet of 10–13 mg DON/kg feed induced a decrease of feed intake at an early stage of the experiment, a decrease of spleen and gizzard relative weights and a decrease of egg fertility. The diets of 4.9 and 5 mg DON/kg did not generate any abnormality in body weight gain, hatchability and egg fertility. Therefore the CONTAM Panel identified 10–13 mg DON/kg feed as the range of LOAELs and 5 mg DON/kg feed as NOAEL for laying hens. For ducks, the CONTAM Panel identified the concentration of 7 mg DON/kg feed and for turkeys the concentration of 6.5 mg/kg feed generating no changes in body weight, weight gain, feed intake or feed conversion ratios as the NOAELs for these animals. Due to the limited or no data on adverse effects caused by 3‐Ac‐DON, 15‐Ac‐DON and/or DON‐3‐glucoside, no specific NOAELs/LOAELs could be identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for poultry.
7.3.4. Horses
Only few studies on the toxicity of DON were identified in horses and are described in this section.
7.3.4.1. DON
The previous EFSA (2004) opinion reviewed two studies on the effects of DON in horses of Johnson et al. (1997). Barley contaminated with 36–44 mg/kg DON was fed to five healthy horses for 40 days. Blood for clinical investigations was sampled every 10th day. Haematocrit values decreased slightly in a linear fashion, but changes in the peripheral white blood cell counts, polymorphonuclear leukocytes and lymphocyte counts were not detected. No changes in serum creatinine, sodium, potassium, chloride, total calcium, and inorganic phosphate were detected. Serum enzyme activities of γ‐glutamyltransferase (GGT), aspartate aminotransferase (AST) and creatine kinase decreased slightly in a linear fashion during the experimental period. Total serum protein, serum albumin and globulin, as well as serum IgG and IgA, also decreased but changes were not‐significant and all values were within the normal range. The horses showed no reduced feed intake or any other signs of adverse effects at the concentrations of 36–44 mg/kg feed. The CONTAM Panel identified 36 mg/kg feed as the overall NOAELs from this study.
New data that have been reported since the EFSA (2004) opinion have been published and are described next. Among those were four studies that were not consider further by the CONTAM Panel, namely the two studies of Raymond et al. (2003, 2005) since co‐contamination of the diet with high levels of fusaric acid could have introduced bias in the observed effects of DON, the study of Caloni and Cortinovis (2010) reporting a weight loss and elevated hepatic enzymes in riding horses connected with straw contaminated without quantifying the DON concentrations and the study of Urošević et al. (2011) investigating mycotoxicosis associated to the occurrence of DON in oats where relationship of DON levels in diets and feed intake by animals were imprecise.
Khol‐Parisini et al. (2012) observed no adverse effects on general health in a cross‐over design the impact of DON on cellular and humoral immune effects in horses fed for 14 days naturally contaminated 2 kg oat diets with low (0.49 mg DON/kg diet) and high (20.2 mg DON/kg diet) levels, allowing for a 3‐week wash‐out. Serum haptoglobin concentration was significantly elevated at the high dose, but only minor effects were noted for differential blood counts and serum biochemistry, and no effects were seen for lymphocytes and their proliferation (concanavalin A, phytohaemagglutinin and pokeweed mitogen). The authors concluded that the concentration of 20.2 mg DON/kg feed appeared to have no major impact on the measured immune response of horses and the CONTAM Panel identified this concentration as the NOAEL for horses.
Schulz et al. (2015) fed 12 geldings with control, low DON intake (7.7 mg DON/kg) and high DON intake (11.4 mg DON/kg) diets for 21 days (see details in Section 7.1.5.4.1). None of the horses demonstrated any adverse effects on health status. Although the reported data on body weights were limited they seemed to remain unchanged throughout the experiment. No changes in haematological and serum parameters or serum globulins were observed. From this study the concentration of 11.4 mg DON/kg feed was considered as the NOAEL by the CONTAM Panel.
7.3.4.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.3.4.3. DON‐3‐glucoside
No data were identified.
7.3.4.4. Conclusions on horses
Overall, the CONTAM Panel identified only three studies suitable for the characterisation of hazard of horses, among them the study over 40 days identified previously by EFSA (2004) from which NOAELs of 36–44 mg DON/kg feed for reduced feed intake were derived. Two recent studies allowed the identification of NOAELs of 20.2 and 11.4 mg DON/kg feed based on short‐term studies of only 2 and 3 weeks, respectively. Accounting for the longer period of observation time without generating adverse effects, the CONTAM Panel concluded that the NOAELs of 36–44 mg DON/kg feed were still valid for horses and decided to use 36 mg DON/kg feed as currently most reliable value for the NOAEL of horses. No endpoints for acute effects in horses were identified. Due to the lack of data on adverse effects caused by 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, no specific NOAELs/LOAELs could be identified.
7.3.5. Farmed rabbits
7.3.5.1. DON
The previous EFSA (2004) opinion noted that the available data on the potential adverse effects of DON on rabbits were limited. Khera et al. (1986) carried out a teratology study in which maternal and fetal body weights were decreased at the level of 30 mg/kg feed or higher (see details in Section 7.2.7). That level was considered by the CONTAM Panel as the LOAEL and the next lower dose of 15 mg/kg feed which was not maternotoxic or fetotoxic was considered as the NOAEL for farmed rabbits.
Since then two studies on rabbits exposed to DON were identified. Hewitt et al. (2015) investigated the effects of feeding diets containing grains naturally contaminated with mycotoxins to fryer rabbits. During 21 days, thirty 5‐week old male New Zealand white rabbits (0.4–0.9 kg at the beginning) were fed a control diet that contained DON, aflatoxin B1 and ochratoxin A at a concentration of 0.25, 0.0024 and 0.0011 mg/kg feed, respectively, and a contaminated diet that contained DON, 15‐Ac‐DON, aflatoxin B1 and ochratoxin A at a concentration of 4.3, 0.12, 0.0026 and 0.0009 mg/kg feed, respectively. Concentrations of the other mycotoxins were below their LODs (0.05 mg/kg feed for 3‐Ac‐DON 0.12 mg/kg feed for nivalenol, 0.04–0.07 mg/kg feed for T‐2 toxin, HT‐2 toxin, diacetoxyscirpenol and neosolaniol, and 0.25 mg/kg feed for zearalenone). Body weight gain and water intake were greater in rabbits fed the contaminated diet compared with control. However, the authors suggested that the increase in body weight gain of rabbits fed the contaminated diet was caused by increased water consumption. There was no effect on haematological parameters and relative organ weights, but decreased infiltration of eosinophilic granulocytes in different regions of the intestine was observed. However, the CONTAM Panel did not use this histopathologic observation as an endpoint for hazard characterisation due to the co‐occurrence of several mycotoxins or possible other causes such as infections. Consequently, the concentration of 4.3 mg DON/kg feed was considered by the CONTAM Panel as the NOAEL of this study.
Kachlek et al. (2015) investigated the impact of DON contaminated diet on caecal microbiota and fermentation of growing rabbits. Two groups of 12 Pannon White rabbits were reared for 6 weeks from 35 (after weaning) until 77 days of age and were fed either a control diet or a diet containing 10.1 mg DON/kg feed prepared from a mixture of the control feed with homogenised fungal cultures of a DON producing Fusarium strain. DON increased the number of aerobic bacteria in the caecum of rabbits which is an undesirable situation due to the anaerobic conditions (in physiological state) of caecum. The authors concluded that DON affected adversely the number of aerobic bacteria in caecum. The concentration of 10.1 mg DON/kg feed was considered by the CONTAM Panel as the LOAEL from this study.
7.3.5.2. 3‐Ac‐DON and 15‐Ac‐DON
The CONTAM Panel identified only the study of Hewitt et al. (2012) reporting a diet with 15‐Ac‐DON/kg feed with a concentration of 0.12 mg/kg feed (see details above) which was more than 30 times lower than the DON content. The CONTAM Panel noted that effects caused specifically by 15‐Ac‐DON cannot be identified in this study. Therefore, and since no data on adverse effects in rabbits caused by 3‐Ac‐DON were identified, the hazard of acetylated forms of DON could not be assessed.
7.3.5.3. DON‐3‐glucoside
No data were identified.
7.3.5.4. Conclusions on farmed rabbits
Limited or no data were available on the adverse effects of DON, and acetylated and modified forms of DON in rabbits. Maternal and fetal body weights were decreased from the concentration of 30 mg DON/kg feed in one study on teratology but not at the lower concentration of 15 mg DON/kg feed. From a study in which no adverse effects on body weight gain, average feed intake, relative organ weights and haematological parameters were observed, a NOAEL of 4.3 mg DON/kg feed was identified. In another study, a concentration of 10.1 mg DON/kg feed adversely affected the composition of caecal microbiota, and consequently a LOAEL of 10.1 mg DON/kg feed was identified. No specific endpoints for acute effects in rabbits have been identified. Due to the limited or no data on adverse effects caused by 3‐Ac‐DON, 15‐Ac‐DON and/or DON‐3‐glucoside, no specific NOAELs/LOAELs could be identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for rabbits.
7.3.6. Farmed fish
Among a reasonable number of publications on DON in fish, the CONTAM Panel decided not to consider those studies. where substantial co‐contamination with other mycotoxins (e.g. with zearalenone) and the cause of the adverse effects could not be attributed to DON.
7.3.6.1. DON
Three different levels of purified DON (0.35, 0.62 and 0.95 mg DON/kg feed) were added to a non‐cereal‐based fish feed and given to carp (12–16 cm length, 4 tanks per diet, with 6 fish each) for 4 weeks, at which 2 tanks per diet were sampled while the remaining 2 tanks per diet were given uncontaminated feed for 2 weeks (Pietsch et al., 2014a). DON did not affect growth and mass of fish during the 6‐week long experiment. Only marginal DON concentrations were found in muscle (the highest concentration was 1.4 μg/kg found in the low‐dose group, and in all other groups) and DON was not found in plasma samples. Blood parameters were not influenced although the erythrocytes in fish given feed containing 0.35 mg DON/kg were smaller than in fish from the other groups. Superoxide dismutase and catalase activities were increased in fish fed the low‐dose feed.
In a second study of this group of authors, carp were given a feed containing virtually the same concentrations (0.352, 0.619 or 0. 953 mg pure DON/kg feed for 6 weeks (Pietsch et al., 2014b). DON increased lipid peroxidation in liver, head kidney and spleen in carp given 0.95 mg pure DON/kg feed but not at 0.35 or 0.62 mg DON/kg feed. These effects were mostly reversed after 2 weeks of feeding with uncontaminated feed. Histopathological examinations revealed increased liver damage in DON‐treated fish at all doses, which persisted after the 2‐week recovery phase. More fat was found in the whole‐body homogenates of fish from the highest dose group than in controls. Furthermore, increased lactate dehydrogenase (LDH) activity in the kidneys and decreased LDH in muscle was observed. According to the authors, the results indicated that DON affects metabolism. A NOAEL of 0.62 and a LOAEL of 0.95 mg/kg feed for lipid peroxidation and histopathological damages in the liver were identified in this study by the CONTAM Panel.
The CONTAM Panel also noted a study of He et al. (2010) on reduced body weight gain in carp after exposure to DON in feed, but since the study design was not clear it was not considered further for the risk assessment.
Feed based on the inclusion of contaminated maize containing DON concentrations of 0, 3.3, 5.5, 7.7 or 8.8 mg DON/kg feed (based on ELISA analysis) were used in a study with channel catfish for a total of 7 weeks in a 10 week‐experiment. Twenty catfish (starting weight from 5.9 g/fish) in each of 40 aquaria, giving eight aquaria per treatment, were used in the experiment. Seven weeks of feeding did not affect growth, feed intake or feed conversion ratio. After 7 weeks, the fish were challenged with Edwardsiella ictaluri a gram‐negative bacteria (final concentration of 1.04 × 106 colony‐forming units (CFU)/mL water for 30 min. DON apparently had a protective effect, as the cumulative 21‐day post‐challenge mortality of the fish was significantly lower in fish receiving at least 5.0 mg DON/kg feed (no more than 5% post‐challenge mortality) than the control and fish at 2.5 mg DON/kg feed (61 and 50% post‐challenge mortality, respectively) (Manning et al., 2014). From this study, the CONTAM Panel identified a NOAEL of 8.8 mg DON/kg feed for channel catfish based on unaltered feed intake, growth and feed conversion ratio. A level of 5.5 mg DON/kg feed apparently increased the resistance towards infection in this study.
Rainbow trouts (starting weight from 24 g/fish) were fed diets containing 0.3 (control), 0.8, 1.4, 2.0 or 2.6 mg DON/kg feed (12 fish/tank and three tanks/diet). The contaminated diets were prepared by mixing naturally DON contaminated maize into the control feed (composition of both feeds were comparable) (Hooft et al., 2013). Statistically significant decreasing trends in feed intake, body weight gain, growth rate, feed and efficiency, retained nitrogen, recovered energy, energy retention efficiency and nitrogen retention were observed. In addition, there was a pair‐fed control given the lowest feed consumption. Fish fed with the diet of highest DON concentration had a significantly higher growth rate, feed efficiency and whole‐body crude protein concentration than the other groups. According to the authors, the results indicated that, rainbow trout are sensitive to DON from naturally contaminated grains and that the effects of DON on rainbow trout are not simply related to a reduction of feed intake, but rather are due to metabolic effects. Based on the authors’ statistical trend analysis (no pairwise comparisons between does groups and the controls performed) The CONTAM Panel identified the lowest dose of 0.8 mg DON/kg feed as a LOAEL for in feed intake, body weight gain, growth rate, feed and efficiency, retained nitrogen, recovered energy, energy retention efficiency and nitrogen retention.
Matejova et al. (2014) fed 40 one‐year‐old rainbow trout a commercial feed subdivided into a control (0.23 mg DON/kg feed) and one experimental group with the addition of 1.96 mg DON/kg feed for 23 days with two tanks per group and 10 fish in each tank. There were no differences between the groups in body lengths, body weight, liver weight, Fulton's condition factor or hepatosomatic index. Histopathological examinations revealed severe hyaline droplet degeneration in the tubular epithelial cells of the caudal kidneys in 9 out of 10 fish given the DON‐amended feed and none for the control fish. No histopathological changes were found in the gills, skin, liver, cranial kidney and spleen. However, the mean cell haemoglobin was significantly decreased and a significant decrease in plasma concentrations of glucose, cholesterol and ammonia were recorded in exposed fish compared to the controls at the end of the experiment. No NOAEL or LOAEL could be identified from this study as only one dose was used, but the CONTAM Panel noted that 1.96 mg DON/kg feed caused alterations in the tubular epithelial cells of the caudal kidney and in clinical chemistry compared to fish given 0.23 mg DON/kg feed.
Diets containing < 0.1 (control), 3.1 or 6.4 mg DON/kg feed were fed to rainbow trout for 7 weeks (30 fish/tank, three tanks/treatment) (Ryerse et al., 2014). In addition, there was one pair‐fed control to the high‐dose group. The fish were experimentally infected (i.p.) with the gram‐negative bacterium Flavobacterium psychrophilum after 4 weeks of exposure. A dose‐dependent significant reduction in feed intake was observed during the first 4 weeks, but was not after the infection. After infection, the mortality of rainbow trout fed 6.4 mg DON/kg feed was significantly reduced (p < 0.05) in comparison with trout fed the control diet. However, a reduced mortality after bacterial infection was also observed in pair‐fed control fish, indicating that the effect may be related more to the reduced feed intake than to DON in the feed (mortality: in control 35.0 ± 5.0%, in high‐dose group 13.3 ± 2.8% and in the pair‐fed control 13.3 ± 3.6%). In a second feeding experiment, trouts (35 fish/tank, three tanks/group) were fed either a control diet, a diet containing 3.3 mg DON/kg feed naturally contaminated with DON or 3.8 mg pure DON/kg feed for up to 35 days and a pair‐fed control. Both feed intake and body weight gain were significantly reduced in both DON‐exposed groups compared with the control, while there was no effect on the feed efficiency. Fish given 3.8 mg pure DON/kg feed also had significantly lower feed consumption and body weight gain than fish given 3.3 mg DON/kg feed naturally contaminated with DON. From these studies on rainbow trout a LOAEL of 3.1 mg/kg feed was identified by the CONTAM Panel for reduced feed intake and body weight gain.
Feeding rainbow trouts 2 mg DON/kg feed for 23 or 32 days significantly induced markers of oxidative stress such as glutathione peroxidase in kidney, glutathione reductase in gill and kidney, catalase in kidney and liver and glutathione S‐transferase in gill and liver compared to controls (Sisperova et al., 2015). No other concentrations were used.
No studies on the adverse effects of DON in salmon were identified by the CONTAM Panel.
In zebrafish (Danio rerio) used as a model for farmed fish (13 fish/tank, four to five tanks/group), pure DON (0.1, 0.5, 1.5, 2.0 or 3.0 mg DON/kg feed) did not affect the performance when given in the feed for 45 days starting from 30 days after hatching (Sanden et al., 2012).The liver CYP1A mRNA levels were significantly higher in fish fed 2 mg DON/kg feed than in the other groups. Gene transcripts of CuZn SOD and cyclin G1 in the liver increased with increasing content of dietary DON. In the follow up (up to 8 months), no difference in the levels of 5‐methylcytosine in embryos was found between the groups. The fecundity of fish recorded between three and 8 months in controls and fish fed 0.5, 1.5 and 3.0 mg/kg feed was 22% higher in the 1.5 mg DON/kg group compared with the control group, while the fecundity in fish fed 3.0 mg DON/kg feed was statistically significantly lower than both the control and the group given 1.5 mg/kg feed. A feed concentration of 0.5 mg DON/kg feed did not have any observed effect and was identified as the NOAEL of this study by the CONTAM Panel.
7.3.6.2. 3‐Ac‐DON and 15‐Ac‐DON
No data were identified.
7.3.6.3. DON‐3‐glucoside
No data were identified.
7.3.6.4. Conclusions on farmed fish
The available data on the toxicity of DON to farmed fish were limited. For farmed fish, no specific endpoints for acute effects in any fish species were identified. The CONTAM Panel also noted that the hazard characterisation of DON was overall more difficult than for other farm animals because of the designs and analysis methods chosen by the authors and a large variation in experimental protocols and endpoints measured. Reduced feed intake and/or body weight gain was reported from trout given 0.8 mg DON/kg feed, while no reduction in feed intake was found in carp given up to 0.95 mg DON/kg feed. Channel catfish may be rather tolerant towards this effect of DON as no effect on feed intake was found in catfish given as much as 8.8 mg DON/kg feed. DON was shown to reduce the reproduction in zebrafish at 1.5 mg DON/kg feed (a NOAEL of 0.5 mg DON/kg feed) and to cause lipid peroxidation and histopathological damages in the liver of carp at 0.95 mg DON/kg feed with a NOAEL of 0.62 mg DON/kg feed. No studies on the adverse effects of DON in salmon were identified by the CONTAM Panel. There were no studies on these parameters from other species. Based on the available data, the CONTAM Panel decided to use the NOAELs of 0.6–0.8 DON/kg feed from carp and rainbow trout for farmed fish species. However, the CONTAM Panel noted that the diet composition of different fish species may differ to a major extent between the fish species and that some fish species might be more tolerant for the effects of DON than the others. Due to the lack of data on adverse effects caused by 3‐Ac‐DON, 15‐Ac‐DON and/or DON‐3‐glucoside, no specific NOAELs/LOAELs could be identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for farmed fish species.
7.3.7. Farmed mink
The CONTAM Panel noted that the majority of studies on farmed mink had been designed, in particular by one group of authors, for the risk assessment of DON and its acetylated and modified forms using pure/purified toxin not as concentration in feed but as bolus dose administered by gavage and expressed in mg/kg bw. Therefore, the CONTAM Panel assessed the acute hazard of DON and its acetylated and modified forms using the dose and not the concentration in this section.
7.3.7.1. DON
Gibson et al. (1993) studied in a first of two experiments the adverse effect of DON on 32 adult female pastel mink (Neovison, formerly Mustela vison) randomly allocated into four groups treated for 28 days. A basal diet was given the controls and 0.28, 0.62 and 1.18 mg DON/kg feed added as DON‐contaminated ground wheat to the basal diet (equivalent to 36, 93 and 195 μg DON/kg bw per day, respectively, calculated by the CONTAM Panel from the data reported by the authors). The DON‐contaminated diets had no statistically significant adverse effect on feed intake or body weight in the adult female mink over the 4 weeks. Average feed consumption was lower at the highest dose during the first week since one mink consistently ate very little, whereas the others reduced consumption for no more than 3 days. A second experiment showed that the animals having the choice preferred to eat non‐contaminated feed except one animal which preferred contaminated diet. The study did not consider vomiting and no apparent illness was noted. The CONTAM Panel identified 1.18 mg DON/kg feed, the highest concentration tested, as a NOAEL for reduced feed intake and body weight for farmed mink as chronic adverse effects.
Standard dark 1‐ to 2‐year‐old female mink (Neovison, formerly Mustela Vison) (6 animals per group), bred and housed at an experimental fur farm, were administered orally via gavage 0.01, 0.05, 0.25 and 0.5 mg DON/kg bw or saline (Wu et al., 2013a) gives as bolus. The mink had been fasted for 24 h prior to dosing (water available ad libitum) and provided 50 g of feed 30 min before exposure to DON on the day of experiment.41 Animals were then monitored for emesis (characterised in the publications of this group of authors as either vomiting or retching42) for 3 h during which the incidence of emesis, latency to emesis, emesis duration and number of emetic events were recorded (0/6 animals with emesis in the control and the lowest dose group, 5/6, 6/6 and 6/6 animals with emesis in the next higher dose groups, respectively). Latency to emesis refers to the time from dosing of DON to the first emetic event, whereas emesis duration is the time from the first occurrence of emesis to the end of the last emesis. Plasma DON level returned to basal level 2 h after the exposure. According to the authors, the NOAEL was 0.01 mg/kg bw and the LOAEL 0.05 mg/kg bw for emesis. The effective dose resulting in emetic events in 50% of the animals for oral exposure to DON was estimated to be 0.030 mg/kg bw by the authors. Using the Wu et al. (2013a) data, Male et al. (2016) reported a BMD10 value of 0.024 mg/kg bw for DON. The CONTAM Panel performed a BMD analysis following the EFSA guidance (EFSA Scientific Committee, 2017) and calculated a BMD10 value of 0.013 mg/kg bw and a BMDL10 value of 0.004 mg/kg bw for DON (see Appendix G).
7.3.7.2. 3‐Ac‐DON and 15‐Ac‐DON
In the same oral exposure study in female minks by Wu et al. (2013a,b), 3‐Ac‐DON (0, 0.05, 0.25, 0.5 and 1 mg/kg bw) and 15‐Ac‐DON (0.1, 0.25, 0.5 and 1 mg/kg bw) were tested using the same experimental design as for DON. Emesis was reported in 0/6 animals in the control and in 0/6, 1/6, 5/6 and 6/6 in the dose groups, respectively, for 3‐Ac‐DON and in 0/6 animals in the control and in 0/6, 5/6, 6/6 and 6/6 in the dose groups for 15‐Ac‐DON, respectively. According to the authors the NOAEL for the 3‐Ac‐DON was 0.05 mg/kg bw, the LOAEL 0.25 mg/kg bw, and the effective dose for emetic events in 50% of the animals 0.29 mg/kg bw. For 15‐Ac‐DON, the NOAEL was 0.01 mg/kg bw, the LOAEL 0.1 mg/kg bw and the effective dose for emetic events in 50% of the animals 0.04 mg/kg bw. Using the Wu et al. (2013a) data, Male et al. (2016), reported BMD10 values of 0.198 and 0.040 mg/kg bw for 3‐Ac‐DON and 15‐Ac‐DON, respectively. The BMD analysis of the CONTAM Panel following the EFSA guidance (EFSA Scientific Committee, 2017), resulted in the BMD10 values of 0.14 and 0.007 mg/kg bw and BMDL10 values of 0.05 and 0.004 mg/kg bw for 3‐Ac‐DON and 15‐Ac‐DON, respectively (Appendix G).
7.3.7.3. DON‐3‐glucoside
In another study on mink, Wu et al. (2014d) tested DON‐3‐glucoside for emesis at the doses of 0, 0.05, 0.25, 0.5, 1 and 2 mg/kg bw (2 animals/group) and observed emesis in only one of the two animals at the highest dose. The small number of animals per dose did not allow the CONTAM Panel to conclude on a NOAEL/LOAEL/BMDL10 of DON‐3‐glucoside in mink.
7.3.7.4. Conclusions on farmed mink
For vomiting as acute effect in farmed dark mink, NOAELs of 10, 5 and 10 μg/kg bw per day were identified for DON, 3‐Ac‐DON and 15‐Ac‐DON, respectively. BMDL10 values of 0.004, 0.05 and 0.004 mg/kg bw were calculated for DON, 3‐Ac‐DON and 15‐Ac‐DON, respectively. Due to insufficient data on DON‐3‐glucoside, no NOAEL, LOAEL or BMDL10 was calculated for acute effects. From the available subacute toxicity study on pastel mink (Gibson et al., 1993), a NOAEL of 1.18 mg DON/kg feed was identified for the reduced feed intake and body weight in farmed mink as chronic effects. Due to the lack of data on chronic adverse effects 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside no specific NOAELs/LOAELs could be identified for chronic toxicity of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
7.3.8. Dogs and cats
7.3.8.1. DON
The previous EFSA opinion (2004) reported two studies on cats and dogs conducted by Hughes et al. (1999). Reviews have also been published by Pestka (2007) and Boermans and Leung (2007).
In the subacute study by Hughes et al. (1999) on dogs, wheat naturally contaminated with 37 mg DON/kg was used to prepare diets containing 0, 1, 2, 4, 6, 8 or 10 mg DON/kg feed. The contaminated wheat was examined for co‐contamination with other mycotoxin. It was found to contain 1 mg of 15‐Ac‐DON/kg feed but none of the following mycotoxins (LODs in brackets expressed mg/kg feed): T‐2 toxin (0.1 mg/kg feed), HT‐2 toxin (0.1 mg/kg feed), diacetoxyscirpenol (0.3 mg/kg feed), neosolaniol (0.5 mg/kg feed), fusarenon‐X (0.5 mg/kg feed), 3‐Ac‐DON (0.1 mg/kg feed), nivalenol (0.5 mg/kg feed), zearalenone (0.2 mg/kg feed) and zearalenol (0.3 mg/kg feed). Forty‐nine mature (1 to 7 years of age) Brittany and Beagle dogs (not distinguished in the analysis) weighing from 15 to 20 kg were involved in an experimental period of 14 days. Dogs were weighed at the start of the study and on day 7 and 14. Dogs assigned diets with DON levels of 0, 1, 2 or 4 mg/kg feed consumed 84 to 100% of the food offered during the 14‐day test period and no vomiting was observed. There was no apparent influence of these levels of DON on body weight gain or food intake. The 14 dogs fed diet with DON level of 6 mg/kg feed did not vomit, but food intake was depressed and they lost weight during the study. The CONTAM Panel considered the concentration of 4 mg DON/kg feed and of 6 mg/kg feed as the NOAEL and LOAEL for chronic effects, respectively. The two dogs housed in one pen and fed diet with a DON level of 8 mg/kg feed consumed a total of 544 g on day 1. Both dogs vomited within 2 h after being fed. Overall, vomiting was observed in five of seven pens assigned to diet with DON level of 10 mg/kg feed. However, CONTAM Panel noted that the authors observed a high variability in emetic patterns among individual dogs, with some being highly susceptible and others being very resistant. The CONTAM Panel also noted that dogs are known to vomit easily as a defence mechanism. The CONTAM Panel identified the concentrations of 6 mg DON/kg feed as the NOAEL and 8 mg/kg feed as the LOAEL for vomiting.
Leung et al. (2007) investigated the effects of feeding cereal‐based diets that were naturally contaminated with DON, 3‐Ac‐DON, 15‐Ac‐DON and fusaric acid (highest concentration) together with several other Fusarium toxins and aflatoxins to mature female Beagle dogs for 14 days. The CONTAM Panel concluded that as it is uncertain whether the adverse effects were caused by DON only in this study and did not consider it further for hazard characterisation.
Only one study on cats was identified (Hughes et al., 1999). In this subacute study, 20 mature (1 to 9 years of age) American Shorthair cats (from 2 to 4 kg) were fed the same diets of 0, 1, 2, 4 and 6 mg DON/kg feed as used for dogs (see above) during the same period of 14 days. Vomiting was observed in four of the seven cages only at the highest level of 10 mg/kg feed. Therefore, the CONTAM Panel identified for vomiting a NOAEL of 8 mg/kg feed and a LOAEL of 10 mg/kg feed. Since average daily feed intake was reduced at 8 mg DON/kg feed, the CONTAM Panel identified a NOAEL of 6 mg DON/kg feed and the LOAEL of 8 mg DON/kg feed for reduced feed intake.
7.3.8.2. 3‐Ac‐DON and 15‐Ac‐DON
The study on dogs of Leung et al. (2007) included 3‐Ac‐DON and 15‐Ac‐DON in the treatment diet. However, for the same reasons as given in Section 7.3.8.1, the CONTAM Panel did not consider this study further. No other data on adverse effects in dogs or in cats were identified.
7.3.8.3. DON‐3‐glucoside
No data were identified.
7.3.8.4. Conclusions on dogs and cats
From the available data on vomiting, the concentrations of 6 and 8 mg DON/kg feed in dogs and cats, respectively, were considered by the CONTAM Panel as the NOAELs for acute effects in dogs and cats, respectively, and the levels of 8 and 10 mg DON/kg feed as the LOAELs for acute effects in dogs and cats, respectively. With regard to the reduced body weight,43 the CONTAM Panel identified the NOAELs of 4 and 6 mg DON/kg feed for dogs and cats, respectively, and the LOAELs of 6 and 8 mg DON/kg feed for dogs and cats, respectively. Due to the limited or lack of data on acute and chronic adverse effects in cats and dogs caused by 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, no specific NOAELs/LOAELs could be identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
7.3.9. Overall conclusions on adverse effects in farm and companion animals
Despite the use of naturally contaminated feed containing both DON and its acetylated and modified forms in some of the studies, mainly data on the effects of DON only are available. For most of the animal species the limited or lack of data on adverse effects caused by 3‐Ac‐DON, 15‐Ac‐DON and/or DON‐3‐glucoside, did not allow the identification of the specific NOAELs/LOAELs for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
For cows not subject to rumen acidosis, the concentration of 5.2 mg DON/kg feed did not generate any adverse effects on body weight, dry matter intake and milk yield and milk composition, and therefore the CONTAM Panel considered this level as a NOAEL for cows. For heifers and steers, the levels of 10 and 18 mg DON/kg feed, respectively, did not generate any adverse effects in feed intake, average daily weight gain and feed efficiency, and they were identified as NOAELs by the CONTAM Panel. For sheep, the CONTAM Panel identified the NOAEL of 15.6 mg DON/kg feed for reduced feed intake and body weight gain, while for goats no data were identified.
In healthy ruminants, DON is converted into the less toxic de‐epoxidised metabolite DOM‐1 by the rumen flora. Dairy cows, cattle and young animals have not been compared directly in the same experiment. However, young animals such as calves with not fully developed rumen and adult animals with a previous history of ruminal acidosis may have less effective de‐epoxidation and, consequently, could be more susceptible to the toxic effects of DON.
In pigs, DON may cause several adverse effects including lesions in the oesophageal region of the stomach, in the liver, the lungs and the kidneys and changes in different clinical chemistry parameters (plasma nutrients and plasma enzyme activities). The immune modulating properties of DON have also been confirmed in pigs by several feeding studies. The acute effect of vomiting was observed primarily at higher DON concentrations, but may also occur occasionally at lower concentrations. While the acute NOAEL for vomiting varied between 0.7 and 12 mg DON/kg feed, a lowest LOAEL of 2.8 mg DON/kg feed was identified for vomiting. Reduced feed intake and body weight gain reduction are the critical effects of DON in pigs, and a wide range of NOAELs/LOAELs were identified by the CONTAM Panel. The CONTAM Panel noted that the overall identified NOAEL of 0.7 mg DON/kg feed for reduced feed intake and body weight gain in pigs was higher than the overall LOAEL of 0.35 mg DON/kg feed for these chronic adverse effects.
For broiler chickens, the concentration range of 4.6–7.0 mg DON/kg feed did not generate any adverse effects on feed intake and body weight gain. The level of 10–12 mg DON/kg feed generated some feed intake and body weight gain reductions and also caused some modifications in intestines, and was identified by the CONTAM Panel to be the range of LOAELs for broiler chickens. For the laying hens, the CONTAM Panel identified the NOAEL of 5.0 mg DON/kg feed and LOAELs of range of 10–13 mg DON/kg feed for reduced feed intake and decreased egg fertility. For ducks, the CONTAM Panel identified the concentration of 7.0 mg DON/kg feed and for turkeys, the concentration of 6.5 mg DON/kg feed generating no changes in body weight, body weight gain, feed intake or feed conversion ratios. Thus, these DON concentrations in feed were identified as the NOAELs for these poultry species. Only one recent study allowed to identify the NOAEL of 5.0 mg DON‐3‐glucoside/kg feed for broiler chickens.
For horses, only data from three studies were available. No reduced feed intake or any other signs of adverse effects were observed in horses at the concentrations of 36–44 mg DON/kg feed. Although the studies involved a limited number of animals, the CONTAM Panel considered that the concentration of 36 mg DON/kg feed can be used as the NOAELs for horses.
Data from three studies were used for farmed rabbits. From one study, the concentration of 30 mg DON/kg feed was associated with maternal and fetal body weight reduction, but a concentration of 15 mg DON/kg feed did not appear to be maternotoxic and did not induce any adverse effects in fetuses. From another study, a concentration of 4.3 mg DON/kg feed induced no effects on body weight gain, average feed intake, relative organ weights and haematological parameters. In another study, a diet level of 10.1 mg DON/kg feed adversely affected the composition of caecal microbiota. Therefore, the CONTAM Panel considered that the concentrations of 4.3 and 10.1 mg DON/kg feed can be used as the NOAEL and LOAEL, respectively, for farmed rabbits.
Limited data on farmed fish derived from variable experimental designs and feed concentrations were available. The data were not sufficient to identify specific NOAELs/LOAELs for each fish species. In addition, the diet composition of different fish species may differ markedly between the fish species, and some fish species might be more tolerant to the adverse effects of DON than others. Based on the studies with carp, in which 0.95 mg DON/kg feed induced lipid peroxidation and histopathological changes in the liver, and with rainbow trout in which 0.8 mg DON/kg feed reduced feed intake, body weight gain, growth rate and feed efficiency, the CONTAM Panel considered that 0.6 mg DON/kg feed can be used as a NOAEL for farmed fish.
For farmed mink, NOAELs for vomiting of 10, 5 and 10 μg/kg bw and BMDL10 values of 4, 50 and 4 μg/kg bw were obtained for DON, 3‐Ac‐DON and 15‐Ac‐DON, respectively, (see Section 7.8.4 for dose–response modelling). No sufficient data on acute adverse effects were available for DON‐3‐glucoside but the limited data available indicated a lower toxic potency compared with DON. For adverse effects in a subchronic study, a NOAEL of 1.18 mg DON/kg feed was identified for the reduced feed intake and body weight.
From the available data on vomiting in dogs and cats, the concentrations of 6 and 8 mg DON/kg feed were considered by the CONTAM Panel as the NOAELs for dogs and cats, respectively, and the levels of 8 and 10 mg DON/kg feed as the LOAELs for vomiting in dogs and cats, respectively. With regard to the reduced body weight, the CONTAM Panel identified the NOAELs of 4 and 6 mg DON/kg feed for dogs and cats, respectively, and the LOAELs of 6 and 8 mg DON/kg feed for dogs and cats, respectively.
Due to the lack of data on chronic adverse effects for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, no specific NOAELs/LOAELs could be identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in the farm and companion animals.
7.4. Combined effects of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside with other mycotoxins
Co‐exposure to more than one mycotoxin in animals and humans through food and feed has been reported (Grenier and Oswald, 2011; Streit et al., 2012). In vitro and in vivo experiments were performed to analyse the effect of DON when present with other mycotoxins. This chapter reports only studies in which the experiment design was set to study combined effects and in which the conclusions were drawn on the combined effects (Table 50). Other studies reporting effects due to mixtures of mycotoxins in the feed fed to the animals in the experiments were not considered but it was noted that a few of these studies on adverse effects in farm animals suggested that fusaric acid induced similar effects as DON (Leung et al., 2007; Girish and Smith, 2008; Marczuk et al., 2012) (see Section 7.3).
Table 50.
Possible combined effects between DON and other mycotoxins in vivo
| Tested mycotoxin/DON Species (exposure period) | Concentrations (tested mycotoxin/DON) (mg/kg feed) | Additive or synergistic combined effects | Antagonistic combined effect | Reference |
|---|---|---|---|---|
| Aflatoxin/DON Pig (28 days) | 3.0/3.0 |
‐ cytokines ‐ total proteins ‐ cholesterol, glucose ‐ white blood cells |
‐ body weight gain ‐ gamma‐glutamyltransferase, aspartate aminotransferase ‐ blood urea nitrogen ‐ calcium, magnesium ‐ albumin ‐ alkaline phosphatase ‐ potassium, phosphorus ‐ red blood cell, haemoglobin, prothrombin time |
Harvey et al. (1989) |
| Aflatoxin/DON Chicken (21 days) | 2.5/16 |
‐ body weight gain ‐ haemoglobin ‐ aspartate aminotransferase ‐ liver/gizzard/proventriculus weight ‐ alanine aminotransferase |
‐ spleen/kidney weight ‐ total serum protein, albumin, uric acid, cholesterol, triglyceride ‐ calcium ‐ glucose ‐ lactate dehydrogenase ‐ liver lipid ‐ red blood cell ‐ phosphorus |
Huff et al. (1986) |
| Aflatoxin/DON Mouse (14 days) | 2.5/5 |
‐ total serum protein ‐ liver weight ‐ liver apoptotic gene expression |
‐ alanine aminotransferase ‐ aspartate aminotransferase ‐ serum albumin ‐ liver total antioxidant capacity ‐ liver malondialdehyde |
Sun et al. (2014) |
| Sterigmatocystin/DON Mouse (24 weeks) | 0.3/1.5 | ‐ adenocarcinoma of the lungs | ‐ stomach dysplasia | Huang et al. (2004)(b) |
| Sterigmatocystin/DON Mouse (24 weeks) | 3/1.5 | No parameter tested |
‐ adenocarcinoma of the lungs ‐ stomach dysplasia |
Huang et al. (2004)(b) |
| Ochratoxin A/DON Chicken (21 days) | 2.0/16 |
‐ gizzard weight ‐ red Blood cells |
‐ body weight gain ‐ liver, kidney, proventriculus weight, ‐ glucose, total serum proteins, albumin ‐ blood urea nitrogen ‐ uric acid ‐ creatinine ‐ triglycerides ‐ cholesterol ‐ inorganic phosphorus |
Kubena et al. (1988) |
|
Fumonisins/DON Pig (28 days) |
50/4.0 |
‐ feed intake ‐ lung weight ‐ body weight gain ‐ aspartate aminotransferase, CHL, alanine aminotransferase ‐ lymphocytes stimulation |
‐ creatinine ‐ albumin ‐ cholesterol ‐ gamma‐glutamyltransferase ‐ liver weight |
Harvey et al. (1996) |
|
Fumonisins/DON Chicken (21 days) |
300/15 |
‐ gizzard relative weight ‐ blood urea nitrogen ‐ gamma‐glutamyltransferase ‐ feed intake ‐ mortality ‐ proventriculus weight ‐ cholesterol ‐ aspartate aminotransferase ‐ lactate dehydrogenase ‐ feed intake |
‐ body weight gain ‐ liver/kidney/heart/bursa of Fabricius weight ‐ total serum proteins |
Kubena et al. (1997) |
| Fumonisins/DON Pig (35 days) | 6.0/3.0 |
‐ severity, extent of liver and lung lesions ‐ specific antibody, IgG ‐ cytokines expression ‐ cytokines (IL‐10, TNF‐a) ‐ goblet cells ‐ intestinal eosinophils |
‐ neutrophils ‐ severity, extent of kidney lesions ‐ specific lymphocytes stimulation ‐ creatinine, albumin ‐ specific antibody, immunoglobulin A ‐ severity, extent of intestinal lesions ‐ cytokines (interferon gamma, interleukin‐1beta, interleukin‐6) |
Grenier et al. (2011), Bracarense et al. (2012) |
| Zearalenone/DONMouse (56 days) | 10/5.0 | ‐ no parameter tested | ‐ body weight‐ liver weight‐ immunoglobulin A‐ red blood cell | Forsell et al. (1986) |
| Zearalenone/DONMouse (14–21 days) | 10/25 | ‐ bacteria in spleen | ‐ delayed‐type hypersensitivity reaction | Pestka et al. (1987) |
| Zearalenone/DONMouse (14 days) | 5/5 | ‐ serum albumin‐ liver total antioxidant capacity‐ total serum protein‐ liver apoptotic gene expression | ‐ liver weight‐ alanine aminotransferase‐ aspartate aminotransferase‐ liver malondialdehyde | Sun et al. (2014) |
| T‐2 toxin/DONPig (35 days) | 0.4/2.5 | No parameter tested | ‐ body weight, feed intake | Friend et al. (1992) |
| 0.8/2.5 | No parameter tested | ‐ body weight, feed intake | ||
| 1.6/2.5 | No parameter tested | ‐ body weight, feed intake | ||
| 3.2/2.5 | ‐ body weight, feed intake | ‐ no parameter tested | ||
| T‐2 toxin/DONChicken (21 days) | 4.0/16 | ‐ body weight‐ cholesterol | ‐ gizzard/bursa of Fabricius relative weight‐ total serum protein, albumin‐ lactate dehydrogenase‐ oral lesions | Kubena et al. (1989) |
| Nivalenol/DONMouse (28 days)a | 0.071/0.071 | ‐ total serum proteins‐ PROD‐ uric acid | ‐ immunoglobulin A‐ CDNB‐ total CO2‐ EROD | Gouze et al. (2005) |
| 0.355/0.071 | ‐ total serum proteins‐ immunoglobulin A‐ CDNB‐ uric acid | ‐ total CO2‐ DCNB‐ EROD, PROD‐ feed intake | ||
| 0.071/0.355 | ‐ total CO2‐ total proteins‐ phosphorus‐ uric acid | ‐ immunoglobulin A‐ CDNB‐ EROD, PROD | ||
| 0.355/0.355 | ‐ total serum proteins‐ phosphorus‐ immunoglobulin A‐ DCNB | ‐ total CO2‐ uric acid‐ CDNB‐ EROD, PROD‐ feed intake |
Administration twice a week for 4 weeks (Gouze et al., 2005); (b): Original paper in Chinese, text based on the translation to English.
No parameter tested: In a given study there was no parameter tested that demonstrated synergistic, additive or antagonistic combined effect: CHL: cholinesterase; IL‐10: interleukin‐10; TNF‐α: tumour necrosis factor‐α; PROD: pentoxyresorufin‐O‐depenthylase; CDNB: 1‐chloro‐2,4‐dinitrobenzene; EROD: ethoxyresorufin‐O‐dealkylase.
7.4.1. In vivo experiments
Several in vivo experiments have been performed to analyse the effect of DON when present with other mycotoxins. Only experiments comparing the toxicological effects due to the exposure to a mycotoxin combination with the effects due to an exposure to single mycotoxins have been considered. Most of these experiments were performed on farm animals and few of them used laboratory animals. Grenier and Oswald (2011) have previously reviewed the combined effects of DON with other mycotoxins. Table 50 summarises the data obtained from in vivo experiment and the conclusion drawn by the authors. The CONTAM Panel noted that because of the lack of dose–response data, it is difficult to perform a refined statistical analysis and to draw definitive conclusion.
7.4.2. In vitro experiments
Combined effect of DON and others mycotoxins has also been studied in vitro in many different studies (Thompson and Wannemacher 1986; Thuvander et al., 1999; Kouadio et al., 2007; Malekinejad et al., 2007; Luongo et al., 2008; Ruiz et al., 2011; Ficheux et al., 2012; Wan et al., 2013). Combined effects of DON, 3‐Ac‐DON and 15‐Ac‐DON have only been studied in vitro on intestinal epithelial cells from human and porcine origins (Alassane‐Kpembi et al., 2013, 2015). When human Caco‐2 cells were exposed to low concentrations (cytotoxic effect was between 10 and 30–40% at the concentrations from 0.15 to 0.55 μM), the binary and ternary mycotoxin mixture of DON, 3‐Ac‐DON and 15‐Ac‐DON demonstrated a synergistic cytotoxic effect. At higher concentrations with cytotoxic effect of around 50%, the combinations had an additive or nearly additive effect. Conversely antagonism was observed at 70% cytotoxicity and above. The in vitro synergy between DON and its acetyl derivatives was quantified using the reduction indices and ranged from 2 to 5 (Alassane‐Kpembi et al., 2013). In vitro studies allowed to investigate the dose–response and to use more sophisticated analysis of the combined effects. They indicated that combined effects of DON and other trichothecenes vary with the concentrations chosen, and that at low concentration the in vitro studies showed a synergy for the combined cytotoxic effect of mycotoxins. This type of interaction need to be confirmed in in vivo experiments.
7.4.3. Conclusions
The available database describing possible effects of combined exposure to DON and other mycotoxins was weak and not sufficient for establishing the nature of combined effects. No studies on the combined effects of 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside with other mycotoxins were identified.
7.5. Human data
7.5.1. Observations in humans
While a number of reports on acute intoxications of DON were identified in the scientific literature, human data related to chronic exposure to DON were lacking. Most human studies on intoxications of DON have previously been reviewed by the SCF (1999) and JECFA (FAO/WHO, 2001, 2011) and are summarised below.
Acute outbreaks due to ingestion of DON contaminated food has occurred repeatedly in China and India, as reviewed by IARC (1993), JECFA (FAO/WHO, 2001, 2011) and Pestka and Smolinski (2005). Typically, an acute DON associated toxicosis showed symptoms such as nausea, vomiting, diarrhoea, abdominal pain, headaches, dizziness, fever, and in severe cases, bloody stool. The onset of the symptoms was typically within 30 min after ingestion. There have been no human deaths directly attributed to DON toxicosis. However, in most of the reported cases, specific effects could not be conclusively attributed to DON because exposure was likely from a mixture of trichothecenes in mouldy grains.
Already in the early 1890s, a disease known as ‘taumelgetreide’ was reported in Siberia with symptoms including vomiting, headache and vertigo. This disease was later suspected to be related to ingestion of trichothecenes (as reviewed by Pestka and Smolinski, 2005).
In India, a severe outbreak of gastrointestinal disease following ingestion of bread made of rain‐damaged mouldy wheat occurred in 1989, when 50,000 people in the Kashmir Valley were reported to be ill, with primary symptoms of abdominal pain, a feeling of fullness in the stomach, irritation of the throat and diarrhoea (Bhat et al., 1989) (see Table 51). From wheat and (refined) wheat flour samples collected from the affected area, DON was the most frequently detected trichothecenes. It was found in half of the 24 wheat and wheat flour samples, with levels up to 8 mg/kg wheat flour. Ac‐DON at levels between 0.6 and 2.4 mg/kg were found in 16% of the 24 wheat and wheat flour samples (the type of acetyl form was not reported). From this study, a NOAEL of 0.44 mg/kg bw was calculated by the SCF (1999) using an average consumption of 67 g wheat products and a body weight of 52 kg.
Table 51.
The outbreaks of DON intoxications in humans in China and India – modified from Luo (1994)
| Year | Region | Number of outbreaks | Number of poisoning cases | Number of vomiting cases | DON (mg/kg) | Number of samples | Reference |
|---|---|---|---|---|---|---|---|
| 1961–1981 | Yangzi river, China | 32 | 5,998 | n.r. | n.r. | n.r. | Information from local regional government |
| 1984 | Hebei, China | 1 | 362 | 232 | n.r. | n.r. | Luo and Li (1985) |
| 1985 | Gansu, China | 1 | 1357 | n.r. | n.r. | n.r. | Tang and Su (1988) |
| 1985 | Henan, China | 1 | 101 | n.r. | 2–40 | 14 | Luo et al. (1987) |
| 1988 | Guangxi, China | 1 | 40 | 34 | 1.5–2.2 | 3 | Liu et al. (1989) |
| 1988 | Hebei, China | 1 | 270 | 59 | 20–50 | 3 | Zhao et al. (1989) |
| 1988 | Shanxi, China | 1 | 142 | n.r. | n.r. | n.r. | Information from local regional government |
| 1989 | Guanxi, China | 1 | 10 | n.r. | n.r. | n.r. | Information from local regional government |
| 1989 | Sichuan, China | 1 | 17 | n.r. | n.r. | n.r. | Information from local regional government |
| 1989 | Shanxi, China | 1 | 701 | n.r. | n.r. | n.r. | Information from local regional government |
| 1991 | Anhui, China | 8 | 130 | 141 | 2–50 | 10 | Huang (1992); Yang et al. (1992) |
| 1991 | Jiangsu, China | 4 | 6,560 | n.r. | n.r. | n.r. | Yuan et al. (1992) |
| 1987 | Kashmir, India | 1 | 50,000 | n.r. | Up to 8.4 | 24 | Bhat et al. (1989) |
n.r.: not reported.
Luo (1994) reported 32 outbreaks in China between 1961 and 1981 where 5,998 cases were associated with the consumption of scabby wheat/barley and mouldy corn. However, DON and other mycotoxins were not analysed due to a lack of facilities. Subsequently, in 21 outbreaks occurring between 1984 and 1991, DON levels in wheat and corn samples (range of 0.34–92.8 mg/kg as reported by the authors) were associated with food poisoning. In 1984, there were 362 out of 383 people (95%) intoxicated in the Hebei province of China were and later in 1985 after unseasonal rainfall, the prevalence of scabby wheat in Gansu province was associated with a total of 1,357 cases of food poisoning out of a population of 1,594 people. The typical symptoms were similar as those described above and started usually within 30 min after consumption of food based on scabby wheat. The occurrence was not age or sex dependent, but was associated with consumption of food items prepared from scabby wheat. The largest outbreak in China was in Anhui and Jangsu provinces in 1991, affecting 130,141 and 6,560 people, respectively. These outbreaks are also summarised in Table 51.
The CONTAM Panel noted that amongst these outbreaks the most detailed report of DON acute intoxication outbreak was that of Luo et al. (1987) from 1985 in Puyang, Henan province in China. A total of 246 people from four villages were studied, 217 of them consumed scabby wheat flour, and 101 food poisoning cases were identified. Matching information on patients with food DON concentrations, data were available from three of the four villages affected (see Table 52). Food poisoning prevalence was 100 and 24% in Haitong (Wumiao) and Zian, respectively, but no case was reported from Qingzu. The most common symptoms in the 44 cases from Haitong (Wumiao) were nausea (89%), fatigue (52%) and vomiting (46%). Dizziness and headache were less frequent. The symptoms usually started within 10–30 min after eating contaminated food and most people recovered in about 2 h time. No deaths were reported in all the 101 persons. DON was detected in the scabby wheat samples using TLC method, which was the state‐of‐art method at that time. Levels of DON were found to be the highest in samples from Haitong (Wumiao), and lowest in those from Qingzu, corresponding well with the order of the intoxication percentage. The data from the three villages are detailed in Table 52.
Between 1998 and 1999, scabby wheat was also highly prevalent in Puyang and DON was reported to be the predominant toxin, with levels up to 14.0 mg/kg (mean 2.9 mg/kg) in 30 out of 31 wheat samples collected from this area. 15‐Ac‐DON was detected in 15 out of the 31 samples with a mean concentration of 0.4 mg/kg. In the neighbouring Zhumadian region, which did not have a history of scabby cereal intoxication outbreaks, DON was detected in 25 out of 28 wheat samples at a lower concentration (mean 0.2 mg/kg) than that of Puyang region (Li et al., 2002).
Body weight reduction has not been reported in any human populations in relation to long‐term exposure to DON. However, three studies reported the association between chronic exposure to DON and the occurrence of human cancers including oesophageal cancer, liver cancer and stomach cancer. Gao and Yoshizawa, (1997) collected 54 maize and 40 wheat samples from Chinese counties of Linxian, an area known to have a high oesophageal cancer incidence, and Shangqui, an area known to have a low oesophageal cancer incidence. The concentrations of DON and 15‐Ac‐DON were higher in samples from Linxian than from Shangqui. This finding was supported by another study analysing the urinary DON biomarker in 11 Chinese adults in Linxian (mean 37 ng/mL) and four in Gejiu (mean 12 ng/mL), an area of low levels of oesophageal cancer (Meky et al., 2003). Turner et al. (2012) investigated the exposure to DON in 110 women (aged 39–72 years) from Golestan in Iran, an area with a high risk of oesophageal cancer. Using a single urinary biomarker method described in Turner et al. (2008a), the presence of urinary DON was detected in 72% of the women, with the maximum of 6 ng/mL. Overall, the CONTAM Panel noted that this type of studies were of the ecological nature (IARC, 1993; FAO/WHO, 2001) and did not allow a valid conclusion on the relationship between DON exposure and oesophageal cancer.
A few recent studies utilising a biomarker method reported the association between human DON exposure and other human health outcomes such as child growth (Ediage et al., 2013) and HIV status (Abia et al., 2013) (see Section 7.5.2). However, the CONTAM Panel considered that these studies were of limited value in interpreting the human health risk owing to either the small sample size and immaturity of biomarker development.
Human exposure to DON‐3‐glucoside was not reported in any of the identified studies.
In conclusion, the CONTAM Panel noted that several outbreaks of acute mycotoxicosis, through the consumption of mouldy food in larger populations in Asia with DON concentrations up to 8 mg/kg wheat (flour) in India and varying between non‐detect/non‐reported up to 50 mg/kg scabby wheat. For the acetylated forms of DON concentrations up to 2.4 mg/kg wheat flour were recorded in India, whereas in China the data on acetylated DON forms were scarce and only a mean level of 0.4 mg/kg wheat was reported for 15‐Ac‐DON. Although chronic exposure to DON is common worldwide, data related to adverse health effects due to chronic exposure are lacking.
7.5.2. Biomarkers of exposure
DON, DON‐3‐glucuronide and the predominant DON‐15‐glucuronide are the common forms of DON in human urine, while DOM‐1 appears to be low in human urine and unsuitable as a biomarker. Numerous recent studies have suggested that tDON (total DON) urinary biomarker measured by LC–MS methods is well correlated with dietary DON exposure and allows an integrated exposure assessment for DON and possibly also its acetylated and modified forms present in many foods. The DON urinary biomarker methods (see Section 3.4) have been applied in several exposure studies in many countries suggesting that DON exposure was ubiquitous in many populations. These studies are summarised in Table 53 for the single biomarker methods and in Table 54 for multiple biomarker methods and some of them described below in more detail. It should be noted that the DON urinary biomarker can be expressed either as ng/mL or as ng/mg. In the latter case it has been adjusted for variations caused by fluctuations in the urinary flow rate.44
Table 54.
Summary of literature reports of urinary DON/DON‐glucuronides/DOM‐1 biomarker data using multiple biomarker method (concentration expressed as ng DON/mL urine or ng DON/mg creatinine) (ordered by publishing year)
| Population | Number of subjects (F/M) | Age (year) | Positive samples (%) | Total DON Mean (range) ± standard deviation (ng/mL) | Total DON Mean (range) ± standard deviation (ng/mg) | Analytical method (LOD/LOQ, ng/mL) | Reference |
|---|---|---|---|---|---|---|---|
| Germany | 50 (n.r.) | Adults | DON: 16 | 11.2 ± 13.0 (median 7.5) | 11.7 (SD not reported) | DON: 4/4DON‐G: 4/4 | Gerding et al. (2015) |
| DON‐G: 54 | |||||||
| Sweden | 252 (n.r.) | Adults | Total DON: 63 | 4.40 ± 19.6 | 7.02 ± 24.4 | See Solfrizzo et al. (2014) | Wallin et al. (2015) |
| DOM‐1: 8 | 0.12 ± 0.55 | 1.56 ± 1.26 | |||||
| Spaina | One male person | 26 | one 24‐h urine sample | DON: 18.8 ± 3.5 | DON: 0.1/0.23‐Ac‐DON: 0.2/0.5DOM‐1: 0.2/0.5 | Rodríguez‐Carrasco et al. (2015) | |
| Belgium | 239 (133/106) | 19–65 | DON: 37 | 3.9 (0.5–129.8) | 0.2b for all forms | Heyndrickx et al. (2015) | |
| DON‐15‐G: 100 | 53.8 (1.1–460.8) | ||||||
| DON‐3‐G: 77 | 7.5 (0.5–126.2) | ||||||
| DOM‐1‐G: 22 | 16.9 (0.6–172.0) | ||||||
| 155 (88/67) | 3–12 | DON: 70 | 5.2 (0.5–32.5) | ||||
| DON‐15‐G: 100 | 58.4 (4.3–343.0) | ||||||
| DON‐3‐G: 91 | 10.6 (0.7–43.0) | ||||||
| DOM‐1‐G: 17 | 91.7 (1.1–526.1) | ||||||
| Italy (Southern) | 52 (26/26) | 3–85 | Total DON: 96 | 11.9 ± 10.1 | Total DON: n.r./1.5 | Solfrizzo et al. (2014) | |
| DOM‐1: n.r. | n.r. | DOM‐1: n.r./9.9 | |||||
| Spaina | 16 (n.r.) | Children | DON: 56 | 27.8 (< LOQ–84.5) | DON: 0.12/0.25DOM‐1: 0.25/0.50 | Rodríguez‐Carrasco et al. (2014a) | |
| DOM‐1: 6 | 1.3 (in one sample) | ||||||
| 16 (6/10) | Young adults | DON: 75 | 32.9 (< LOQ–69.1) | ||||
| DOM‐1: 6 | 4.2 (in one sample) | ||||||
| 22 (10/12) | Adults | DON: 73 | 14.8 (< LOQ–56.9) | ||||
| DOM‐1: 0 | 0 | ||||||
| Spaina | 10 (n.r.) | 8–11 | DON: 30 | 7.4 (< LOD–21.1) | DON: 0.12/0.25 | Rodríguez‐Carrasco et al. (2014b) | |
| DOM‐1: 10 | 1.3 | DOM‐1: 0.25/0.50 | |||||
| Belgium | 32 (16/16) | n.r. | DON: 72 | 0.4 (< LOD–3.0) | DON: 0.2/0.5 | Huybrechts et al. (2015) | |
| DON‐15‐G: 100 | 82.6 (3.0–420.0) | DON‐15‐G: 0.2/0.5 | |||||
| DON‐3‐G: 91 | 10.7 (< LOD–55.0) | DON‐3‐G: 0.2/0.5 | |||||
| DOM‐1: 0 | DOM‐1: 0.1/0.3 | ||||||
| Croatia | 40 (40/0) | 26–33 | DON: 76 | 18.3 (< LOD–275.0) | Free DON: 4/13 | Sarkanj et al. (2013) | |
| DON‐15‐G: 98 | 120.4 (< LOD–1,237.7) | DON‐15‐G: 3/11 | |||||
| DON‐3‐G: 83 | 28.8 (< LOD–298.1) | DON‐3‐G: 6/20 | |||||
| Belgium | 40 (n.r.) | n.r. | DON: 13 | < LOD–68.3 | DON: 2.85/5.7 | Ediage et al. (2012b) | |
| DON‐3‐G: 0 | DON‐3‐G: 2.25/4.5 | ||||||
| DOM‐1: 0 | DOM‐1: 0.65/1.3 | ||||||
| Austrian | 27 (n.r.) | 20–63 | DON: 0 | 13.7 | DON: 4/13 | Warth et al. (2012b) | |
| DON‐15‐G: 63 | (< LOD–43.0) | DON‐15‐G: 3/11 | |||||
| DON‐3‐G: 4 | 13 (in one sample) | DON‐3‐G: 6/20 | |||||
| Total DON: 59 | 20.4 (< LOD–63) ± 2.4 | Total DON: 4/13 | |||||
| Portugal | 13 (8/5) | 20–50 | DON: 15 | 1.8, 8.8 (in two samples) | DON: 0.08/0.25 | Cunha and Fernandes (2012) | |
| 15‐Ac‐DON: 0.60/2.0 | |||||||
| 3‐Ac‐DON: 0.30/1.0 | |||||||
| DOM‐1: 0 | DOM‐1: 0.15/0.50 | ||||||
| Total DON: 69 | 16.3 (< LOD–26.2) | ||||||
| Italy | 10 (5/5) | 26–87 | DON: 50 | 1.98 (< LOD–14.0) ± 1.4 | DON: 0.8/1.6 | Solfrizzo et al. (2011) | |
| Total DON: 70 | 3.67 (< LOD–14.2) ± 1.6 | Total DON: 0.8/1.6 | |||||
| Italy | 2 (1/1) | 30–45 | DON: 100 | 3.0, 8.0 | DON: 2.0/6.0 | Lattanzio et al. (2011) | |
| DOM‐1: 0 | DOM‐1: 1.0/3.0 | ||||||
| Spain | 27 (10/17) | 21–77 | DON: 33 | < LOD–35 | DON: 10/35 | Rubert et al. (2011) |
n.r.: not reported; F: female; M: male; LOD: limit of detection; LOQ: limit of quantification; DON‐3‐G: DON‐3‐glucuronide; DON‐15‐G: DON‐15‐glucuronide; DOM‐1‐G: DOM‐1‐glucuronide
All studies were conducted using LC–MS/MS method unless otherwise specified.
using GC–MS detection method;
geometric mean (95% CI);
limit of detection.
Turner et al. (2008a) used a detailed 7‐day weighted food diary data to calculate the total cereal consumption in the UK. The 1,724 women were ranked according to their total cereal consumption as low, medium and high cereal consumers. Urinary tDON was measured in 100 women from each of the three consumption groups and detected in 99% of the urine samples. The urinary tDON had a strong positive correlation with total cereal consumption (p < 0.0005). The correlation analysis suggests that wholemeal made up the largest portion to urinary tDON per unit of consumption, while white bread contributed most to urinary tDON because of its high consumption. The data also showed that urinary tDON was better explained by the cereal consumption in the most recent 2 days than the average cereal intake in the previous 7 days (Turner et al., 2009).
In the intervention study by Turner et al. (2010a), morning urine was collected from 35 UK adult volunteers in 5 consecutive days over 2 consecutive weeks. After a normal diet in week 1, a partially or fully restricted diet on low wheat content food was used in week 2. Urinary tDON levels in the two intervention groups were 54 and 3% of control diet group. The reduction in urinary tDON was consistent with the reduction in the total cereal consumption. Average DON daily exposure was estimated based on average DON concentration and consumption level of bread. A strong correlation between urinary tDON and DON daily exposure was reported especially when the average consumption data from the recent 4 days was used (r2 = 0.83). This study and a similar intervention study (Turner et al., 2008b) clearly demonstrated a close relationship between tDON biomarker and DON exposure, and the major contribution of cereal based food to DON exposure. Using samples of individuals with tDON above 5 ng/mL urine from the intervention study of Turner et al. (2011a), the mean tDON urine concentration was 17.8 ng/mL (range 5.0–78.2 ng/mL). Unconjugated DON was detected in 68% of the samples, with a mean urine concentration of 2.4 ng/mL (range 0.5–9.3 ng/mL). On average, DON‐glucuronides constituted 87% of the tDON with the rest being unconjugated DON. The percentage appeared to be independent from the tDON level. DOM‐1 was detected only in one sample out of 34 samples from 35 persons, at a level approximately 1% of tDON.
Urinary DON and DOM‐1 were also measured in 76 male French farmers (23–74 years old) (Turner et al., 2010b). All but one man had DON detected (range 0.5–28.8 ng/mL). DOM‐1 was detected in 26 (34%) out of 76 samples (range 0.2–2.8 ng/mL). The DOM‐1 positive group had a higher average urinary DON level than those of DOM‐1 negative (geometric mean 8.1 vs 5.5 ng/mL, p = 0.043). However, a correlation between DON and DOM‐1 concentrations in individuals was not reported.
The single urinary DON biomarker was studied in 85 pregnant women (at last trimester of pregnancy, age 16–44 years) from a large multiethnic mother/infant birth cohort resident in the UK (Hepworth et al., 2012). The urinary tDON biomarker was significantly higher in the South Asian group compared to non‐South Asian group with the geometric means (95% CI) of 15.2 (10.7–21.5) vs 8.6 (6.6–11.8) ng/mg creatinine. The higher exposure level in the South Asian group was attributed to a higher consumption of white bread (chapattis) in the South Asian diet. Another study in 98 Egyptian pregnant women (Piekkola et al., 2012) reported only a moderate urinary tDON level, with a 68% detectable rate, and a geometric mean (95% CI) at 2.8 (2.1–3.6) ng/mg creatinine for the positive samples only, possibly reflecting the moderate concentrations of tDON and consumption of, e.g. wheat and maize in the Egyptian diet.
Using the GC–MS method, Cunha and Fernandes (2012) determined tDON in 69% of the 13 samples collected from northern Portuguese, with a mean concentration of 16.3 ng/mL urine (range 1.9–26.2 ng/mL urine).
The multi‐biomarker analytical methods have increasingly been used in various studies. Solfrizzo et al. (2011) reported that seven out of 10 Italian adults had detectable tDON concentrations in urine. The mean level was 3.7 ng/mL (range < LOD–14.2 ng/mL). In another study by Solfrizzo et al. (2014) 96% of the 52 adults in southern Italy had detectable tDON concentrations (mean ± SD) at 11.9 ± 10.1 ng/mL (range < LOQ–67.4 ng/mL).
Warth et al., (2012b) reported that amongst 27 urinary samples from Austria, 22 and 96% of the samples were positive for DON and DON‐glucuronide, respectively. On average, conjugated DON counted for 86% of the tDON (DON + DON‐3‐glucuronide + DON‐15‐glucuronide). DON‐15‐glucuronide represented approximately 75% of total DON‐glucuronide. The mean concentration of tDON was 20.4 ± 2.4 ng/mL (range < LOD–63 ng/mL). No DOM‐1 was detected in any sample.
Ediage et al. (2012b) measured 18 mycotoxins and their metabolites in 40 urine samples from Belgian volunteers. Five samples had DON levels above the LOD, with range between 5.9 and 68.3 ng/mL. DON‐3‐glucuronide was not detected in any sample. Huybrechts et al. (2015) quantified DON, DON‐3‐glucuronide, DON‐15‐glucuronide in 60, 90 and 100% of the 32 urine samples from Belgian adults. The mean concentrations were 0.4 ng/mL (maximum 3 ng/mL), 10.7 ng/mL (maximum 55 ng/mL) and 82.6 ng/mL (maximum 420 ng/mL), respectively. Although DOM‐1 was not detected, DOM‐1‐glucuronide was detected in 25% samples with a mean concentration of 4.6 ng/mL (maximum 16.4 ng/mL). This indicated that the glucuronide metabolites were the main metabolites of DOM‐1.
Gerding et al. (2015) reported that none of the 50 German adults had urinary free DON above LOQ although 16% were reported positive and 54% adults were positive with DON‐glucuronides with mean of 11.2 (SD 13.0) ng/mL and median of 7.5 (maximum 60.9) ng/mL.
In Swedish adults (n = 252) urinary DON and DOM‐1 were detected in 63 and 8% of the total group (Wallin et al., 2015). The mean(SD) was 4.40(19.6) ng/mL ((7.0224.4) ng/mg) for DON and 0.12(0.55) ng/mL (1.56(1.26) ng/mg) for DOM‐1. DON was the most frequently detected mycotoxins. DOM‐1 was only detected when DON was detected. The adults were subsequently grouped into low, medium and high multi‐toxin group according to the number of multiple mycotoxins being detected. No significant association could be established for the three groups and their specific food patterns.
Diet information and urinary biomarker data were studied in Belgian children (n = 155 of age 3–12 years; 67 boys and 88 girls) and adults (n = 239 of age 19–65; 106 men and 133 women) by Heyndrickx et al. (2015) including DON, DON‐3‐glucuronide, DON‐15‐glucuronide, 3‐Ac‐DON, 15‐Ac‐DON, 3‐Ac‐DON‐15‐glucuronide, 15‐Ac‐DON‐3‐glucuronide, DOM‐1 and DOM‐1‐glucuronide. DON was detected in 70% of the children (mean concentration 5.5 ng/mg creatinine) and 37% of the adults (mean concentration 6.1 ng/mg creatinine). DON‐15‐glucuronide, being the most prevalent, was detected in all samples with mean concentration of 65.3 and 50.1 ng/mg creatinine in children and adults, respectively. DON‐3‐glucuronide was detected in 91% of the children (mean concentration 12.3 ng/mg creatinine), and in 77% of adults (mean concentration 6.7 ng/mg creatinine). No DOM‐1 was detected. DOM‐1‐glucuronide was present in 17% of the urine samples from children (mean and median concentration 100.9 and 28.2 ng/mg creatinine, respectively) and in 22% from adults (mean concentration 25.0 ng/mg creatinine).
Rodríguez‐Carrasco et al. (2014a) applied a multi‐biomarker method to measure DON concentrations in urine from 54 Spanish volunteers (DON‐glucuronides not measured). DON and DOM‐1 were detected in 68.5 and 3.7% of the 54 subjects. 3‐Ac‐DON was not detected. The urinary DON was detected in 56, 75 and 73% of the children, young adults and adults, respectively. The mean (maximum) concentration for urinary DON was 27.8 (84.5), 32.9 (69.1) and 14.8 (56.9) ng/mg creatinine for children, young adults and adults, respectively. There was no significant difference in urinary DON concentrations between males and females. Statistical significance was not reported for the difference in DON between age groups nor for the difference in creatinine levels between adults and children.
The highest exposure reported this far, was form the study by Šarkanj et al. (2013) in 40 non‐smoking pregnant women in the last trimester of pregnancy (26–33 years old) from eastern Croatia. DON‐15‐glucuronide, DON‐3‐glucuronide and DON were detected in 98%, 83% and 76% of the samples, respectively. The mean, median and maximum levels were 120.4, 55.2 and 1,238 ng/mL, respectively, for DON‐15‐glucuronide. For DON‐3‐glucuronide the mean, median and maximum levels were 28.8, 10.0 and 298.1 ng/mL, respectively, and for DON 18.3, 6.7 and 275.0 ng/mL, respectively. When daily exposure was estimated based on the sum of DON and DON equivalent concentrations of DON‐glucuronides using an estimated excretion percentage of 72% as reported by Turner et al. (2010b), 48% of the subjects exceeded 1 000 ng/kg bw per day. The highest estimated exposure of 1,238 ng/mL for the sum (estimated DON exposure 33 ng/kg bw per day) was observed in woman with 1,238 ng/mL DON‐15‐glucuronide 298 ng/mL DON‐3‐glucuronide and 275.0 ng/ml DON This maximum urinary DON level was nine times higher than the highest level reported in UK also in a pregnant women (Hepworth et al., 2012).
In the context of a grant agreement between EFSA and the authors, an urinary DON biomarker project was conducted in 2014 to measure and compare the levels and proportions of urinary DON, DON‐glucuronides and DOM‐1 in UK, Italy and Norway, and to investigate the main types of food that contribute to urinary biomarker levels (Brera et al., 2015). Two consecutive morning urine samples were collected approximately from 200 subjects per country (~ 40 children (3–9 years), ~ 40 adolescents (10–17 years), ~ 30 adults (18–64 years), ~ 20 elderly (> 65 years), ~ 30 vegetarians and ~ 40 pregnant women). A total of 635 subjects were recruited, and a total of 1,270 urine samples were collected. The consumption data were obtained through a food frequency questionnaire and harmonised food diary survey. Urinary DON, DON‐glucuronides and DOM‐1 were measured in the UK, Italy and Norway by a single biomarker analytical method following an interlaboratory study (see details in Brera et al. (2015)). The results are not published in a peer‐reviewed journal but as an EFSA external report.
tDON was detected in 93%, 76% and 97% of the samples collected from UK, Italy and Norway, respectively (Brera et al., 2015). The CONTAM Panel noted that the LOQs of the method applied in the three laboratories varied and did not allow to compare the percentage of samples with detectable levels between the countries. The median and mean concentrations of urinary DON and glucuronides are summarised in Table 55 by country, age groups and specific subpopulations (vegetarians and pregnant women). For the samples below LOQ, a lower bound approach was taken, and the value of zero was assigned to these samples when mean concentrations were calculated. Overall the median tDON in the UK population was about threefold higher than that in the Italian and Norwegian populations, and in all three populations the median tDON concentration was the highest in children. There was no significant difference on tDON concentrations between samples collected on day 1 and day 2. DON glucuronide in the 2 days urine samples constituted 82/82, 66/71 and 80/79% of the tDON in the UK, Italian and Norwegian populations, respectively. DOM‐1 was almost completely absent in the UK and Italian populations, whilst a 12% positive rate was reported in Norwegian population, which according to the authors probably owed to the lower LOQ of the method used in Norway.
Table 55.
Mean and median concentrations of the sum of urinary DON and DON‐glucuronides (ng/mg creatinine) in UK, Italy and Norway reported in Brera et al. (2015)
| UK | Italy | Norway | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Population groups | n | Mean (ng/mg) | Median (ng/mg) | n | Mean (ng/mg) | Median (ng/mg) | n | Mean (ng/mg) | Median (ng/mg) |
| Children (3–9 years) | 40 | 41.6 | 33.3 | 40 | 14.0 | 11.3 | 40 | 14.0 | 10.8 |
| Adolescent (10–17 years) | 39 | 21.0 | 18.5 | 40 | 12.2 | 9.3 | 41 | 7.3 | 7.0 |
| Adults (18–64 years) | 31 | 19.3 | 12.9 | 31 | 6.4 | 4.7 | 58 | 5.8 | 4.7 |
| Elderly (> 65 years) | 20 | 25.4 | 19.9 | 20 | 8.3 | 7.0 | 20 | 8.9 | 5.5 |
| Vegetarians | 30 | 36.6 | 22.2 | 30 | 8.3 | 4.9 | 31 | 7.3 | 7.2 |
| Pregnant women | 42 | 39.4 | 14.3 | 42 | 4.6 | 2.0 | 40 | 7.7 | 5.3 |
| Total | 202 | 31.4 | 19.3 | 203 | 9.1 | 6.5 | 230 | 8.3 | 6.1 |
n: number of subjects.
The CONTAM Panel decided to use the available biomarker data from the Brera et al. (2015) study to calculate the exposure and to compare this exposure with the exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside calculated from the diet in Section 6.1.2.2.1 and Appendix F. Therefore, based on the individual urinary tDON concentrations from the study of Brera et al. (2015) and assuming that the average excretion rate is 70%, and that 24‐h urine volume is 0.5 L for children (Haga and Sakata, 2010) and 2 L (EFSA FEEDAP Panel, 2012) for adults, the CONTAM Panel estimated the exposure to DON using the following formula:
In Table 56, these DON exposure estimates reported together with the exposure estimates for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside obtained from the diet‐based calculations in this opinion (Section 6.1) using the country specific food consumption data available for UK and Italy. For Norway the Danish food consumption data were used to due to the lack of Norwegian food consumption data in the EFSA Comprehensive Food Consumption Database. Overall, the CONTAM Panel concluded that the exposure estimates based on the biomarker data were within the same order of magnitude with the estimates based on diet. However, the CONTAM Panel noted that there were several aspects which contributed to the uncertainty45 of the exposure estimates of DON by using biomarker data. These were:
The DON excretion rate was assumed to be 70%, but individual variation exists and the extent of this variation is unknown to date. Further, the contribution of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to this DON biomarker was unknown.
The 24‐h urine volume was not recorded and default values for children and adult were used which contributed to the uncertainty.
Different back‐calculation methods (e.g. whether or not corrected for molar mass) have been proposed in literature (Heyndrickx et al., 2015) for more accurate calculation of the tDON biomarker but they were not all applicable for single biomarker data because the single biomarker method does not measure each DON‐glucuronide individually, hence results may vary depending on the calculation method used.
Table 56.
DON exposure estimates back‐calculated from the biomarker concentrations reported to EFSAa , b and compared to the dietary based exposure estimates for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside at the middle bound presented in Section 6.1.2.2.1 and Appendix F
| Age group(age) | MB exposure estimate from dietc | Urinary DON and glucuronides b | Exposure estimateafrom biomarker | MB exposure estimate from dietc | Urinary DON and glucuronidesb | Exposure estimateafrom biomarker | MB exposure estimate from dietc | Urinary DON and glucuronidesb | Exposure estimateafrom biomarker |
|---|---|---|---|---|---|---|---|---|---|
| μg/kg bw per day | ng/mL | μg/kg bw per day | μg/kg bw per day | ng/mL | μg/kg bw per day | μg/kg bw per day | ng/mL | μg/kg bw per day | |
| UK | Italy | Norway | |||||||
| Children (3–9 years) | 1.15 | 21.2 | 0.70 | 1.33 | 8.4 | 0.25 | 1.10 | 10.8 | 0.34 |
| Adolescent (10–17 years) | 0.60 | 20.0 | 1.14 | 0.80 | 7.7 | 0.40 | 0.60 | 8.5 | 0.50 |
| Adults (18–64 years) | 0.45 | 11.9 | 0.42 | 0.54 | 4.4 | 0.18 | 0.47 | 5.6 | 0.22 |
| Elderly (65 years or >) | 0.42 | 8.1 | 0.31 | 0.50 | 4.1 | 0.18 | 0.42 | 4.4 | 0.18 |
| Pregnant women | na | 12.2 | 0.42 | na | 1.4 | 0.06 | na | 5.0 | 0.19 |
| Vegetarian | na | 10.1 | 0.42 | na | 3.9 | 0.16 | na | 5.1 | 0.22 |
bw: body weight; na: not applicable; MB: middle bound.
Daily exposure is calculated based the DON and glucuronide biomarker and assumption of daily urine volume at 0.5 and 2 L for child and adult, and DON excretion percentage is estimated at 70%.
The individual urinary DON and glucuronide concentrations as ng/mL were extracted from the EFSA database as submitted to EFSA in the DON biomarker project in 2015 (Brera et al., 2015).
The chronic exposures for UK and Italy are the estimates from the diet as presented in this opinion (see Appendix F.1). The food consumption data reported for Denmark were used to compare to the exposure estimate from biomarker in Norway due to the lack of Norwegian food consumption data in the EFSA Comprehensive Food Consumption Database.
7.5.3. Biomarker of effect
A few studies have been published on a biomarker of effect for DON (Hopton et al., 2010; Amuzie and Pestka, 2010; Flannery et al., 2013) but because this area is still under development no studies on humans were identified.
7.5.4. Conclusions
Research on DON biomarkers in the past decade has made great progress. The DON urinary biomarker (DON and DON‐glucuronides) has been frequently detected in several European and non‐European populations, largely attributed to the high urinary excretion and frequent contamination in food. Based on the limited available information the CONTAM Panel estimated that 70% of the exposed DON is excreted via urine within a day or two after the exposure to DON. Typically DON‐glucuronide constitutes a major proportion (70–90%) of the total DON in urine, with DON‐15‐glucuronide being the predominant type of metabolite. DOM‐1 appears to be low in human urine and unsuitable as a biomarker.
The available literature data from DON single and multiple biomarker methods have been shown to correlate well with dietary DON exposure. There was clear evidence that urinary DON excretion varies at different time of the day and between individuals. The CONTAM Panel noted that there was limited knowledge on what determines toxicokinetics of DON in humans, and clear age, gender, regional and seasonal patterns of the biomarker cannot be established. A recent EFSA study showed the UK population had higher levels of DON biomarker when compared to the Italian and the Norwegian populations, although reasons for the different biomarker levels between countries are yet to be elucidated. However, the CONTAM Panel noted that the exposure estimated based on the EFSA biomarker study were somewhat lower but still of the same order of magnitude as the national diet based exposure estimates for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
7.6. Comparative toxicity of DON and 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
Only few studies compared side by side the toxicity of DON, its acetylated forms 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside (Table 57). The toxicity was assessed both on cell lines, organ tissues and animals.
Table 57.
Comparative toxicity studies of DON, its acetylated forms of 3‐Ac‐DON and 15‐Ac‐DON and the modified form of DON‐3‐glucoside
| Toxins | Model | End‐point | Ranking | Reference |
|---|---|---|---|---|
| DON, 3‐Ac‐DON, 15‐Ac‐DON | Mink | Emetic effect | DON = 15‐Ac‐DON > 3‐Ac‐DON | Wu et al. (2013a) |
| DON, 3‐Ac‐DON, 15‐Ac‐DON | Mice | Anorectic effect | DON = 15‐Ac‐DON = 3‐Ac‐DON | Wu et al. (2012) |
| DON, 3‐Ac‐DON, 15‐Ac‐DON |
Human cells Intestinal epithelial cell (Caco‐2) |
Cytotoxicity (MTS and Neutral red assay) | 15‐Ac‐DON > DON ≥ 3‐Ac‐DON | Alassane‐Kpembi et al. (2013) |
| DON, 3‐Ac‐DON, 15‐Ac‐DON |
Pig cells Intestinal epithelial cell (IPEC‐1) |
Cytotoxicity (MTS assay) | 15‐Ac‐DON > DON > 3‐Ac‐DON | Alassane‐Kpembi et al. (2015) |
| DON, 3‐Ac‐DON, 15‐Ac‐DON |
Pig cells Intestinal epithelial cell (IPEC‐1) |
‐ Cell Proliferation (ATP)‐ Barrier function (TEER)‐ Claudins proteins expression‐ MAPK activation | 15‐Ac‐DON > DON > 3‐Ac‐DON | Pinton et al. (2012) |
| DON, 3‐Ac‐DON, 15‐Ac‐DON | Pig intestinal tissues | ‐ Histological alterations‐ MAPK activation | 15‐Ac‐DON > DON > 3‐Ac‐DON | Pinton et al. (2012) |
| DON, 3‐Ac‐DON, 15‐Ac‐DON |
Mice 3T3 fibroblast |
‐ Cytotoxicity (BrdU bioassay) | 15‐Ac‐DON = DON ≫ 3‐Ac‐DON | Sundstøl Eriksen et al. (2004) |
| DON, 3‐Ac‐DON, 15‐Ac‐DON, DON‐3‐glucoside | Mice | Inflammation (mRNA for IL‐1b, IL‐6, TNF‐a, CXCL‐2); CCL‐2; CCL‐7 in the liver | DON = 15‐Ac‐DON = 3‐Ac‐DON ≫ DON‐3‐glucoside | Wu et al. (2014d) |
| DON, DON‐3‐glucoside | Mice | Anorectic effect | DON ≫ DON‐3‐glucoside | Wu et al. (2014d) |
| DON, DON‐3‐glucoside | Mink | Emetic effect | DON ≫ DON‐3‐glucoside | Wu et al. (2014d) |
| DON, DON‐3‐glucoside | Pig intestinal tissues | Inflammation (mRNA for IL‐1α, IL‐1β, IL8, TNF‐α, IL‐17A, IL‐22):Pan‐genomic analysis | DON ≫ DON‐3‐glucoside | Pierron et al. (2016b) |
| DON, DON‐3‐glucoside |
Human cell Intestinal epithelial cell (Caco‐2) |
‐ Cytotoxicity‐ Barrier function (TEER)‐ MAPK activation | DON ≫ DON‐3‐glucoside | Pierron et al. (2016b) |
DON‐3‐glucoside was compared to DON on human intestinal epithelium assessing cytotoxic effect, barrier function and activation of MAP‐Kinase (Pierron et al., 2016b). These toxins were also compared on intestinal tissues for inflammatory effect and pan genomic gene expression (Wu et al., 2014a; Pierron et al., 2016b). Emetic and anorectic effects were also tested on mink and mice, respectively (Wu et al., 2014). For all these parameters DON‐3‐glucoside was substantially less toxic than DON.
When DON was compared to 3‐Ac‐DON and 15‐Ac‐DON the relative toxicity varied. DON, 3‐Ac‐DON and 15‐Ac‐DON were found to display similar toxicity in terms of anorectic and inflammatory effects (Wu et al., 2012, 2014d). DON and 15‐Ac‐DON have the same emetic effect and were more emetic than 3‐Ac‐DON (Wu et al., 2013a). On intestinal epithelial cells from porcine or human origin, the cytotoxicity of DON and acetylated form was ranked in increasing order 3‐Ac‐DON > DON > 15‐Ac‐DON (Pinton et al., 2012; Alassane‐Kpembi et al., 2013, 2015). Similar ranking was observed for barrier function, MAP‐Kinase activation and expression of tight junction, and for histological alteration (Pinton et al., 2012). In mice 3T3 fibroblast, DON and 15‐Ac‐DON were equally cytotoxic and more cytotoxic than 3‐Ac‐DON (Sundstøl Eriksen et al., 2004).
In summary, only few studies compared the toxicity of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside head‐to‐head. DON, 3‐Ac‐DON and 15‐Ac‐DON were found to display similar toxicity in terms of anorectic and inflammatory effects, except for emetic capacity which was substantially lower for 3‐Ac‐DON compared to DON and 15‐Ac‐DON. The potency of cytotoxicity in mammalian intestinal cells ranked in the order of 15‐Ac‐DON > DON > 3‐Ac‐DON and that was similar for barrier function, MAP‐Kinase activation and expression of tight junction, and histological alteration. DON‐3‐glucoside was considerably less toxic than DON both in vitro and in vivo studies.
7.7. Mode of action
Due to the large database on biochemical mode of action on DON, the CONTAM Panel decided to report only the main reviews and the studies relevant with regard to the adverse effects observed in vivo. For 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside all the available studies were reviewed.
7.7.1. DON
7.7.1.1. Induction of a ribotoxic stress
DON as other trichothecenes is known to target the ribosome. It binds to the 60S subunit of this organelle and crystallographic studies indicate that DON more specifically targets the A site of the peptidyl transferase centre. This interaction inhibits the chain elongation step of protein synthesis leading to an inhibition of RNA, DNA and protein synthesis (EFSA 2004; Pestka, 2010a; Garreau de Loubresse et al., 2014).
This binding of DON to ribosome activates also several ribosome‐associated mitogen activated protein kinases (MAPKs), including p38, c‐Jun N‐terminal Kinase (JNK), and extracellular signal‐regulated kinase 1 and 2 (ERK1/2), an effect called ribotoxic stress response (Pestka, 2010a,b). Activation of p38 and ERK1/2 triggers two competing signalling pathways, one down‐stream of p38 favouring apoptosis and one downstream of ERK1/2 favouring cell survival and cytokine expression, the net effect on cell fate depending on the dose and duration of exposure. Leucocytes, notably those of mononuclear lineage, are particularly sensitive to DON. Upon exposure to DON, MAPK activation drives a proinflammatory cytokines response through rapid and strong transcriptional activation and stabilisation of mRNAs (Pestka, 2010a,b) (see Section 7.2.5).
7.7.1.2. Induction of oxidative stress
DON‐dependent production of reactive oxygen species (ROS) has been reported in several cell culture studies as well as in vivo in the intestine (review Mishra et al., 2014a). It should be noted that, DON‐induced ROS generation proved to be an early event (Liu et al., 2016) which might lead to genotoxicity (see Section 7.2.9). Modifications in antioxidant enzyme activities have been used as a biosensor for ROS formation during the oxidative assault in the cell system. In this line, DON has also been reported to alter the antioxidant defence system in various cell lines and in tissues from rodents or farm animals such as chicken and pigs exposed to DON (reviewed by Mishra et al., 2014a). By contrast, DON at doses up to 100 μM has been reported to have negligible effects on the production of nitric oxide (Ji et al., 1998; Graziani et al., 2015).
There was overwhelming evidence for the implication of mitochondria in trichothecene‐induced oxidative stress (Bin‐Umer et al., 2011). DON interferes with oxidative stress by inhibiting mitochondrial translation, however the precise target(s) remain(s) to be characterised.
DON induced oxidative stress leads to enhanced lipid peroxidation, and DNA and protein damage. Several studies demonstrated an increase in lipid peroxidation levels in mice, rat and chicken exposed to DON (84 nM to 84 μM), as well as in cells treated in vitro with DON (Mishra et al., 2014a). Very few studies on DON‐induced carbonylation of protein have been reported (Kalaiselvi et al., 2013; Strasser et al., 2013), however, inhibition of protein synthesis has been reported in a number of studies (Pestka, 2010a,b).
7.7.1.3. Effect on proliferation, cell‐cycle and apoptosis
As already mentioned, DON as other trichothecenes, are known to interfere with DNA synthesis, cell growth, mitosis and cell‐cycle (Brera et al., 2005). When treated with a range of concentrations of DON (84 nM to 84 μM), human intestinal epithelial Caco‐2 cells showed a reduction in proliferation and survival. Dividing Caco‐2 cells were found to be more sensitive compared to differentiated cells. The greater sensitivity of proliferating cells is probably due to the capacity of DON to inhibit protein synthesis and subsequently nucleic acid synthesis (Bony et al., 2006). In these cells, DON induced G2/M phase cell cycle arrest associated with an increase in p21 levels, a cyclin‐dependent kinase (CDK) inhibitor that mainly inhibits cyclin‐CDK2 complex and negatively regulates cell cycle progression. This increase in p21 levels was partly due to up‐regulation of p21 mRNA expression and also to p21 mRNA stabilisation (Yang et al., 2008). DON was also able to promote cell cycle arrest in the G0/G1 phase of J774A.1 murine macrophages (Marzocco et al., 2009). In addition, DON was found to impair oocyte developmental competence by interfering with microtubule dynamics during meiosis and disturbing oocyte maturation (Schoevers et al., 2010).
DON induces apoptosis through mitochondria‐mediated or ‐independent pathways (Shifrin and Anderson, 1999; Pestka, 2010a,b). DON stimulated apoptosis in murine macrophages via caspase 3 pathway with an increased expression of Bax and poly‐ADP‐ribose synthase (PARP) (Marzocco et al., 2009). HT‐29 cells treated with DON underwent a p53 and caspase‐dependent apoptosis (Bensassi et al., 2009). However, other studies demonstrated that DON caused ROS‐dependent apoptosis in HT‐29 along with over‐expression of NF‐kB‐P65 (Krishnaswamy et al., 2010; Kalaiselvi et al., 2013).
7.7.1.4. Cell membrane integrity and permeability
DON as other trichothecenes are amphophilic molecules and are capable of exerting cellular toxicity by affecting cell and organellar membranes including mitochondria (Rocha et al., 2005). A decreased expression of the several tight junction proteins in the gut barrier, especially caludins, was also observed in intestinal epithelial cells from human or porcine origin (Pinton et al., 2009; Van De Walle et al., 2010; Diesing et al., 2011; Akbari et al., 2014). It should be noted that the decrease expression in protein was accompanied by a compensatory up‐regulation of mRNA levels observed both in cell lines and in animals (Osselaere et al., 2013b; Akbari et al., 2014). This reduction of tight junction was mediated by MAPK and accompanied with a decreased trans‐epithelial electrical resistance (TEER) and increased permeability to 4 kDa dextran and pathogenic E. coli (Pinton et al., 2009).
The MAPK activation also lead to the inhibition of the expression of resistin‐like molecule β, and the subsequent decreased the level of mRNA encoding for the intestinal membrane‐associated (MUC1) and the secreted MUCs (MUC2, MUC3). This reduced mucin production in the human goblet cell line HT29‐16E cells (Pinton et al., 2015). A reduced number of goblet cells was also observed in the intestine of pig fed DON contaminated diets (Bracarense et al., 2012).
7.7.1.5. Anorexia and vomiting
Although anorexia and the resultant growth suppression constitute major and relevant adverse effects of DON and other structurally related trichothecenes, the underlying organismal mechanisms are not yet fully understood. Two major mediators of DON‐induced anorexia, i.e. pro‐inflammatory cytokines and satiety hormones, emerged from studies carried out mainly in mice. The suppression of cytokine signalling and interference with pituitary growth hormone (GH) axis has also been proposed (EFSA 2014; Lebrun et al., 2015). It is important to point out that, contrary to humans or pigs, vomiting cannot occur in rodents, but the abnormal food intake behaviour observed in mice (or other rodents) is considered indicative of nausea‐induced anorexia (Yamamoto et al., 2002).
Several studies mentioned in Section 7.2.5 demonstrated that DON induces the synthesis of cytokines in both the peripheral and in the central nervous system. It has also been shown that upon injection, the proinflammatory cytokines IL‐1β, IL‐6 and TNF‐α induce a set of symptoms characteristic of sickness behaviour, including anorexia (Kelley et al., 2003). During acute or chronic inflammation, prostaglandins act downstream of proinflammatory cytokines and induce some of the symptoms of sickness behaviour, notably anorexia (Pecchi et al., 2006). It has been observed that DON induces prostaglandins (Lebrun et al., 2015). It was therefore suggested that DON‐induced anorexia could be triggered by proinflammatory cytokines and/or prostaglandins. Genetically deficient mice and pharmacological inhibitors have been used to test this hypothesis. Mice knockout for either TNF‐α receptor or IL‐6 do not exhibit any reduced susceptibility to DON‐induced anorexia, as compared to wild type mice (Pestka and Zhou 2000, 2002). COX‐2 knockout mice and wild type littermates displayed similar decrease in body weight when exposed to DON (Jia and Pestka, 2005). Similarly, mPGES‐1 KO mice were shown to be as sensitive as their wild‐type littermates to DON‐induced anorexia upon acute oral exposure (Girardet et al., 2011a). Although these results suggest that DON‐induced anorexia is independent of the production of inflammatory mediators, compensatory mechanisms observed in knockout animal models have to be taken into consideration (Lebrun et al., 2015). Using TNF‐α or IL‐1 receptor antagonists, Wu and Zhang (2014) observed a dose‐dependent attenuation of anorexia upon acute oral gavage of mice with DON (5 mg/kg bw). These later data suggest that TNF and IL‐1 could contribute at least in part to the reduced food intake detected after exposure to DON.
The gut satiety hormones cholecystokinin (CCK), secreted by specialised I cells of the duodenum and jejunum, and Peptide YY3‐36 (PYY), secreted by specialised L cells of the distal ileum and colon, were identified as a major mediator of DON‐induced anorexia after oral and i.p. administration respectively (Flannery et al., 2012; Wu et al., 2014b). Plasma levels of CCK and PYY rapidly increased upon an acute i.p. exposure to DON (10 mg/kg bw), concomitantly with DON‐induced anorexia. By contrast, DON does not impact plasma levels in glucagon‐like peptide‐1 (GLP‐1), leptin, amylin, pancreatic polypeptide (PP), gastric inhibitory peptide (GIP) or ghrelin (Flannery et al., 2012). After i.p. administration of DON, the use of a specific receptor antagonist demonstrated the implication of PYY in anorexia in the mouse model (Flannery et al., 2012) and emesis in the mink model (Wu et al 2013b). Oral gavage of mice with DON at the dose of 2.5 mg/kg bw elicited a strong increase in plasma CCK levels that correlated with food refusal in a mouse anorexia model (Wu et al., 2014b). It is not yet known how exactly DON activates the secretion of CCK and PYY. However, that the increased CCK level was responsible for DON‐induced anorexia was demonstrated by the observation that CCK receptor antagonists (SR 27897 or L‐365,260) attenuate this adverse anorectic outcome upon ingestion of DON in a dose‐dependent manner (Wu et al., 2014b). Using a cell line, the same group identified a specific G‐protein‐coupled chemosensor receptor that mediates the CCK response (Zhou and Pestka, 2015). When CCK is released from the gastrointestinal tract, it can act in a paracrine manner by activating CCK receptors in the abdominal vagus afferents, which communicate with the nucleus tractus solitarius (NTS) in the hindbrain (Morton et al., 2006). Neurons of the NTS in turn possess axons that convey information about the status of the viscera to many areas of the brain involved in the regulation of feed or food uptake.
In addition to being secreted by cells of the gastrointestinal tract, CCK is also produced directly in various brain regions (Rehfeld et al., 1985). In this respect, it is important to note that DON penetrates into the central nervous system after oral exposure (Prelusky et al., 1986, 1990; Pestka et al., 2008), raising the possibility that it may stimulate CCK secretion directly in specific brain locations. This view is further supported by the finding that DON (12.5 mg/kg bw) induces c‐Fos expression (a marker of neuronal activity) in several brain areas such as the hypothalamus, the dorsal vagal complex, the pons and the central amygdala (Girardet et al., 2011a,b).
7.7.2. 3‐Ac‐DON and 15‐Ac‐DON
3‐Ac‐DON and 15‐Ac‐DON are able to bind the ribosome. They only formed 2 hydrogen bonds with the ribosome, in contrast to DON that forms 3 hydrogen bonds (Payros et al., 2016; Pierron et al., 2016a).
The ability of 10 and 30 μM 3‐Ac‐DON and 15‐Ac‐DON to induce a ribotoxic stress was examined through the activation of MAPK in differentiated intestinal epithelial cell line, intestinal explant and in the intestine of exposed animals (Pinton et al., 2012). In these three different models, 15‐Ac‐DON activated the MAPKs, ERK1/2, p38, and JNK, at lower dose than DON and 3‐Ac‐DON. As a consequence 15‐Ac‐DON is more toxic than DON. 15‐Ac‐DON induces greater histological intestinal lesions and had a higher impact on barrier intestinal function (Pinton et al., 2012).
No data were identified on induction of oxidative stress.
In relation to the effect on the cell‐cycle and apoptosis, 3‐Ac‐DON and 15‐Ac‐DON were found to induce apoptosis of Jurkat T‐cell at the same concentration as DON (Pestka et al., 2005). The underlying mechanism of apoptosis has not been investigated for these two metabolites.
3‐Ac‐DON and 15‐Ac‐DON induced anorexia and vomiting (see Section 7.2.1). Both of them also induced inflammatory response, but the inflammatory reaction was less pronounced than with DON as indicated by protein and mRNA levels (Wu et al., 2014a) and the comparable synthesis of satiety CCK after oral ingestion (Wu et al., 2014b). No studies with inhibitors and/or deficient mice were performed to determine the underlying mechanism.
7.7.3. DON‐3‐glucoside
Probably due to the presence of the glucosyl group DON‐3‐glucoside is unable to bind to the A site of the ribosome peptidyl transferase centre and to induce a ribotoxic stress. Accordingly, 10 μM DON‐3‐glucoside did not activate MAPKs in treated Caco‐2 cells and did not alter viability and barrier function of intestinal cells. Microarray analysis of 30,000 genes also revealed that treatment of intestinal explants for 4 h with 10 μM DON‐3‐glucoside does not change the expression of any genes (Pierron et al., 2016b). No data were identified on induction of oxidative stress or on effect on the cell‐cycle and apoptosis.
DON‐3‐glucoside showed reduced anorectic and emetic response compared with DON (see Section 7.2.1.3) and the underlying mechanism of this response is unknown but does not appear to involve inflammatory response (Wu et al., 2014d).
7.7.4. Conclusions
The mode of action of DON indicated that DON binds to the ribosome, induces a ribotoxic stress and activates the MAP‐Kinases. Depending on the dose and duration of exposure, activation of different MAP‐Kinases triggered different signalling pathways favouring apoptosis, cell survival or cytokine expression. DON also triggered an oxidative stress through a mitochondria dependent mechanism. Anorexia, emesis and the resultant growth suppression constituted major adverse effects of DON. Two major mediators of DON‐induced anorexia/emesis have been described: pro‐inflammatory cytokines and secretion of satiety hormones, which activate receptors in the abdominal vagus afferent.
The data on the mechanism of action of 3‐Ac‐DON and 15‐Ac‐DON were scarce but suggested an activation of MAP‐Kinase, as well as an induction of inflammatory cytokine and satiety hormones. Because of steric hindrance, DON‐3‐glucoside cannot bind to the ribosome, activate MAP‐Kinases and induce inflammation.
7.8. Identification of critical effects and dose–response/concentration–response modelling for hazard characterisation
Since the previous evaluations of the SCF (1999, 2002) and EFSA (2004) (see Section 1.1) several new studies on DON were reported for experimental, farm and companion animals and few on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. While a wealth of toxicokinetic and toxicity data was available for experimental animals to identify and characterise the hazard of DON, the database on adverse effects and toxicity of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was still scarce for this opinion (see Sections 7.3 and 7.4), and only a limited number of toxicokinetic studies were identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (see Section 7.1). Based on that information, the CONTAM Panel assumed that the acetylated forms were largely deacetylated prior to systemic distribution and rapidly metabolised to DON, such that they might induce the same acute and chronic effects as DON. There was evidence that at least 15‐Ac‐DON elicits similar general chronic toxicity as DON, whereas the immunotoxicity of 3‐Ac‐DON and 15‐Ac‐DON might be less expressed. In contrast, data on the absorption of DON‐3‐glucoside were limited and no evidence was found that it is absorbed as such. The bioavailability of DON‐3‐glucoside was considered low. On the other hand, it is cleaved in the gut of animals (mice, pigs and chicken) with large inter‐ and intraspecies variability. In rats, and similarly in pigs, DON‐3‐glucoside was recovered in the gastrointestinal tract at small amounts and only as traces of metabolites in kidneys. In vitro but not in vivo studies on human gut bacteria demonstrated cleavage of DON‐3‐glucoside to DON. While the available data on the mode of action and toxicity of the acetylated forms indicated a similar toxicity as that of DON, those on the toxicity of DON‐3‐glucoside were limited (see Sections 7.2 and 7.6) and in vivo data on the chronic toxicity were missing. Therefore, no firm conclusions on the toxicity of DON‐3‐glucoside could be drawn and by applying a conservative approach the CONTAM Panel assumed that 1) 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are all metabolised to DON and absorbed at the same extent as DON, 2) the acetylated forms of DON induced the same acute and chronic effects as DON and 3) acute and chronic effects of DON‐3‐glucoside similar to DON cannot be excluded. Therefore, the CONTAM Panel decided to characterise the hazard for the group of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside together, both for chronic and for acute effects in humans and farm and companion animals.
7.8.1. Critical acute effects of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
Gastrointestinal disorders, such as nausea, vomiting, abdominal pain and tensions and diarrhoea, have been observed in humans in a number of acute intoxications associated with the consumption of moulded or scabby cereal grains contaminated primarily by Fusarium mycotoxins where DON was dominant (see Section 7.5.1). Other effects were dizziness and in some cases also headache and fever. The CONTAM Panel identified vomiting as critical acute effect to characterise the acute hazard of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in humans, and decided to use human data collected in a number of epidemiological studies performed after outbreaks of acute mycotoxicosis in Asia for acute hazard characterisation. In addition, the CONTAM Panel used the available human biomarker data as supporting information for the characterisation of acute hazard for humans.
The CONTAM Panel noted that studies on rodents, in particular mice and rats, could not be considered for the acute risk assessment of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in humans because rodents do not vomit. On the other hand, vomiting was identified as critical endpoint for acute effects of DON for some non‐ruminant species of farm and companion animals, in particular, pigs, farmed mink, and dogs and cats. However, when assessing the suitability of this information for human risk assessment, the CONTAM Panel noted essential limitations of the available studies and their data due to a considerable heterogeneity in vomiting (including feed refusal), both between and within animal species (see Section 7.3.2) and also between different breeds, see e.g. the heterogeneity in dose–response evaluation for pigs (Section 7.8.4), some studies on poultry (Section 7.3.3) and also in the one available study on dogs and cats (Section 7.3.7). The CONTAM Panel noted that vomiting/emesis of mink has been used as animal model to study acute effects of DON, 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside (Section 7.8.5), but decided to base their acute hazard characterisation on the available human epidemiological data.
7.8.2. Critical chronic effects of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
In absence of human data on the chronic exposure to DON, 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside, the CONTAM Panel was not able to identify critical chronic effects in humans and considered reduced body weight gain (also reported as reduced body weight, suppression in weight gain or growth reduction) in experimental animals as the most relevant and suitable critical effect to characterise the chronic hazard for humans. In addition, the CONTAM Panel identified reproductive and developmental toxicity of DON in experimental animals (see Sections 7.2.2.1 and 7.2.7) as suitable to characterise the chronic hazard of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in humans.
The CONTAM Panel noted that suppression of growth and anorexia has been identified as a chronic effect in experimental and some farm animal species such as pigs. In particular, feed refusal and reduced feed intake have been associated with hormonal and immunotoxic effects of DON since changes of satiety hormones (e.g. CCK and PYY) and changes of proinflammatory cytokines (e.g. IL‐1β, IL‐6, TNF‐alpha) have been observed to be related to DON‐induced anorexia. However, this database was too weak to identify any critical endpoint based on immunotoxic or hormonal effects in experimental animals for chronic hazards of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in humans.
7.8.3. Dose–response modelling for human chronic hazard characterisation of DON
From the available studies reporting reduced body weight gain, the CONTAM Panel considered the results of the chronic 2‐year study in mice of Iverson et al. (1995) addressing general toxicity of DON as the most appropriate source of data for dose–response modelling (see Section 7.2.4.1). A clear dose–response relationship between the exposure to DON and mean body weight was observed in both female and male mice (see Section 7.2.4.1). In contrast, the dose–response relationship between DON exposure and feed intake was less clearly expressed in females compared to males (see Appendix G). The CONTAM Panel considered the dose–response evaluation of the body weight data available for male and female mice as most appropriate to characterise the hazard of DON and investigated whether the data of both sexes could be combined to determine a reference point for chronic exposure to DON. The CONTAM Panel noted that the shape of the dose–response curves of males and females were not identical but similar enough to be combined in a BMD analysis, and that the combination with an overall much larger sample size would effectively increase the statistical power of the analysis (EFSA Scientific Committee, 2017).
The BMD approach for continuous data was applied using the default benchmark response (BMR) of 5% in the absence of statistical or toxicological considerations supporting a deviation. Applying the PROAST software (Version 62.6) dose–response models for continuous data were fitted to the combination of the data on female and male mice of Iverson et al. (1995) following the EFSA guidance (EFSA Scientific Committee, 2017). The benchmark dose (BMD) confidence interval was determined as 0.11–0.32 mg DON/kg bw per day (see Appendix G and Table 57) and the lowest BMDL05 value was 0.11 mg DON/kg bw per day obtained for female mice when using the exponential model was identified as reference point for the chronic toxicity of DON.
Although the data on reduced feed intake did not show such a clear dose–response relationship and differed substantially between males and females (see Section 7.2.2) the BMD approach was also applicable to the combination of the data on female and male mice of Iverson et al. (1995) and the BMD analysis resulted in a lowest BMDL05 values of 1.6 mg/kg bw per day for females and 0.22 mg/kg bw per day for males. Since both were higher than the lowest BMDL05 value of 0.11 mg DON/kg bw per day from the combined analysis of the body weight data, the CONTAM Panel concluded that the reference point 0.11 mg DON/kg bw per day covers also reduced feed intake as endpoint for the hazard characterisation of DON.
In addition, the CONTAM Panel analysed the dose–response data from the recently published subchronic study of Bondy et al. (2016) on p53+/+ and p53+/− mice (see Section 7.2.3) using the doses of 0, 0.09, 0.53 and 1.57 mg/kg bw per day as calculated by the authors from the same concentrations of DON in the diet (0, 1, 5 and 10 mg DON/kg feed) as used by Iverson et al. (1995). Since the study of Bondy et al. (2016) with a duration of 26 weeks was subchronic, the CONTAM Panel decided to use it to support the evaluation of the chronic study of Iverson et al. (1995). Applying the PROAST software (Version 62.6), dose–response models for continuous data were fitted to the combination of the data on p53+/+ and p53+/− mice following the EFSA guidance (EFSA Scientific Committee, 2017). The calculation resulted in a BMD confidence interval of 0.11–0.90 mg DON/kg bw per day for decreased body weight, and therefore the results obtained for the subchronic (26 weeks) study of Bondy et al. (2016) supported well the reference point of 0.11 mg DON/kg bw per day determined above from the study of Iverson et al. (1995) (see also Section 7.10).
The CONTAM Panel also noted that general toxicity in male and female rats investigated for developmental toxicity study by Sprando et al. (2005) and Collins et al. (2006), exhibited information on reduced feed intake, body weight and body weight gain that could be evaluated for dose–response (see Appendix G). Using the BMD approach, as described above, the lowest BMDL05 values were 0.70, 0.72 and 0.92 mg DON/kg bw per day for reduced feed intake, body weight and body weight gain in male rats, respectively, as reported by Sprando et al. (2005) and 1.80, 2.34 and 1.14 mg DON/kg bw per day for reduced feed intake, body weight and body weight gain in dams, respectively, as reported by Collins et al. (2006). The CONTAM Panel concluded that these BMDL05 values obtained from these short‐term studies also supported the reference point of 0.11 mg/kg bw per day derived from the study of Iverson et al. (1995). For details see Sections 7.2.2 and 7.2.7, and Appendix G.
7.8.4. Vomiting as a critical acute effect of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for farm and companion animals
The CONTAM Panel identified vomiting as the critical effect only for pigs, dogs, cats and farmed mink when exposed to DON (see Section 7.3) and used this endpoint to characterise the acute hazard of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in them. The CONTAM Panel noted that several studies in pigs and farmed mink showed clear concentration–response and dose–response relationships, respectively, suitable to apply the BMC/D (benchmark concentration/dose) approach (see Section 7.8.5 below). However, the concentration–response relationships reported in the only available study for cats and dogs were not so well expressed but still suitable for the application of the BMC analysis (see Appendix G). Therefore the CONTAM Panel identified also the NOAELs and/or LOAELs for the acute exposure of these animal species (see Section 8.2).
7.8.5. Concentration–response modelling for acute toxicity in pigs, dogs and cats, and dose–response modelling for acute toxicity in farmed mink
The CONTAM Panel noted that vomiting was occasionally reported for pigs, while reduced feed intake was consistently (occasionally also reduced weight gain) reported after exposure to DON in feed. From a large database of studies (see Table 48), the CONTAM Panel identified the two studies of Young et al. (1983) and Williams et al. (1988) as most suitable to analyse the concentration–response relationship of vomiting in pigs. Several subtrials reported by Young et al. (1983) contained such concentration–response information. Using the incidence data for immediate vomiting of piglets of JECFA (FAO/WHO, 2011; Personal communication, A. Tritscher, WHO, 2015) a lowest BMDL10 of 1.36 mg DON/kg feed was calculated using the data from two of the four subtrials (Trial 2 and 3, each with 20 piglets in 5 dose groups observed for 4 or 11 days, respectively) (see Appendix G). The CONTAM Panel noted that the co‐occurrence of zearalenone in the feed was low in the two subtrials. Therefore, this co‐occurrence could hardly have confounded the outcome of immediate vomiting, because this effect is not known as relevant effect of zearalenone in pigs. Williams et al. (1988) investigated vomiting in pigs in two experiments, one with a small (n = 12) and another one with a larger number of pigs (n = 54) each with a control and five concentrations. Both experiments showed a clear concentration–response relationship where vomiting was observed at the higher doses at up to 100% incidence. Applying the BMD approach to the data of the two experiments resulted in a lowest BMCL10 of 1.84 mg DON/kg feed per day (see Appendix G). The CONTAM Panel decided to use 1.36 mg DON/kg feed as the overall reference point to characterise the acute risk of pigs when exposed to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. For a summary of NOAELs and LOAELs for the acute exposure to DON in pigs, see Section 7.3.
Concentration‐response data for dogs were identified in the only available study of Hughes et al. (1999) (see Section 7.3.7 and Appendix G) in which a total of 49 dogs were exposed to 6 different concentrations of DON in feed. The number of animals per group varied between n = 2 and n = 14 and vomiting was observed at the two highest concentrations only. Due to the scarcity of the data not all models of the set of the models available in the BMDS software could be fitted to the data. However, when applying the BMD approach following the EFSA guidance (EFSA Scientific Committee, 2017) three models could be selected to calculate a lowest BMDL10 of 5.1 mg DON/kg feed per day for dogs.
Concentration‐response data for cats were identified in the same only available study of Hughes et al. (1998) (see Section 7.3.7 and Appendix G) in which a total of 20 cats were exposed to 5 different concentrations of DON in feed. The number of animals per group varied between n = 2 and n = 8 and vomiting was observed at a mid (1/2) and at the highest (4/8) concentrations only. Due to the scarcity of the data not all models of the set of the models available in the BMDS software could be fitted to the data. However, when applying the BMD approach following the EFSA guidance (EFSA Scientific Committee, 2017) two models could be selected to calculate a lowest BMDL10 of 1.0 mg DON/kg feed per day for cats.
Wu et al. (2013a) reported the incidence of emesis (defined by the authors as vomiting or retching) from an experiment with 1–2 years old female mink exposed either to DON or to 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside (see Section 7.3.7 and Appendix G). The dose–response data showed a clear relationship for DON and the two acetylated forms and were evaluated using the BMD approach for quantal data as described by EFSA Scientific Committee (2017). The lowest BMDL10 values were 0.004 mg DON/kg bw per day, 0.05 mg 3‐Ac‐DON/kg bw per day and 0.004 mg 15‐Ac‐DON/kg bw per day. A dose–response evaluation of vomiting of farmed mink exposed to DON‐3‐glucoside was not possible since vomiting was observed only at highest dose of 2 mg DON‐3‐glucoside/kg bw in the study of Wu et al. (2014) (see also Section 7.3.7). The CONTAM Panel noted that the study of Wu et al. (2013) used intragastric administration with a dosing per kg body weight and decided therefore to use 0.004 mg DON/kg bw per day as reference point to characterise the acute risk of farmed mink when exposed to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
7.8.6. Reduced feed intake and reduced body weight or weight gain as critical chronic adverse effects of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for farm and companion animals
The CONTAM Panel identified reduced feed intake and reduced body weight or weight gain as the critical chronic effects of DON in farm and companion animals (see Section 7.3) to characterise the chronic hazard of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in animals. Reduced feed intake was typically the first adverse effect observed within the first days of the experiments and its frequency and intensity appeared to decline later in several studies. However, there were also many studies where reduced feed intake persisted over the whole treatment period with an intensity varying over time, and differing between individual animals and between species. Therefore, the available data on the changes in feed intake and body weight, and when available also on feed refusal, were considered in addition to the data on the reduction of feed intake and reduction of body weight gain. The CONTAM Panel identified overall NOAELs or LOAELs (only if NOAELs were not identified) for all farm and companion animal species for which exposure data were available (see Section 6.3).
The CONTAM Panel also explored whether the BMD approach can been applied for selected concentration–response data from the chronic studies on pigs, farmed rabbits, farmed fish, dogs and cats. Regarding pigs, considering the vast amount of the available studies, the studies with suitable concentration–response data were scarce and limited by small numbers of experimental groups and numbers of animals per concentration group. In addition, the designs (also the overall outcomes) of the identified studies varied substantially. Regarding the changes in feed intake and body weight in rabbits, cats and dogs only one study was identified that could have been analysed for each of the three species. The few studies on farmed fish with concentration–response data were all based on a specific (ANOVA‐type) statistical design using fish tanks as the experimental unit rather than the individual fish and they reported only limited information on the variability between the tanks and none between individual fish. The CONTAM Panel also noted that the calculated BMCL values differed over an order of magnitude for the same animal species between different models and different data sets. For a number of data sets they were substantially lower than the identified NOAELs and often also orders of magnitude lower than the lowest concentrations tested, probably due to the low number of animals per concentration group or a design chosen for other aims than concentration/dose response evaluation. It was also noted that several quantitative response data decreased rapidly between the control and the low doses and levelled out at the higher doses. This behaviour is analogue to that of supra‐linear curves of incidences with high slopes at low doses where low BMDL values need to be considered with caution. In view of these complications, the CONTAM Panel did not pursue calculating the reference points based on concentration–response modelling for the chronic critical effects of the farm and companion animals.
Overall NOAELs of 5, 10 and 15 mg/kg feed were identified for reduced feed intake and reduced weight gain in dairy cows, heifers and steers, respectively (see Section 7.3.1 and Table 59). Lack of data prevented the identification of a NOAEL/LOAEL for goats but the CONTAM Panel decided to use the NOAEL of dairy cow for goats because the efficiency of de‐epoxidation of DON in goat is similar to that in dairy cows. Based on the only one study available for sheep, the CONTAM Panel identified the NOAEL of 16 mg/kg feed for reduced feed intake and body weight gain for sheep (see Table 59).
Table 59.
The identified overall NOAELs for the chronic adverse effects as detailed in Section 7.8.6 (rounded to the first decimal place or to a whole number) with the calculated upper bound (UB) mean and 95th percentile dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for the farm and companion animals (see also Section 6.3)
| Animal species for which a NOAEL was identified | NOAEL (mg/kg feed) | Animal species for which exposure was estimated | Calculated UB mean dietary concentration (mg/kg DM) | Calculated UB 95th percentile dietary concentration (mg/kg DM) | % of dietary concentration of the NOAEL | |
|---|---|---|---|---|---|---|
| UB meanh (%) | UB 95th percentileh (%) | |||||
| Ruminants | Ruminants | |||||
| Dairy cows | 5 | Dairy cows | ||||
| high yieldingb | 0.11 | 0.52 | 2 | 10 | ||
| high yieldingc | 0.22 | 0.66 | 4 | 13 | ||
| maize silage | 0.78 | 2.6 | 16 | 55 | ||
| Heifers | 10 | Heifers | 0.11 | 0.52 | 1 | 5 |
| Beef cattle/Steers | 18 | Beef cattle | ||||
| fatteningb | 0.08 | 0.19 | 0.4 | 1 | ||
| fatteningc | 0.23 | 0.32 | 1 | 2 | ||
| cereal‐based diet | 0.39 | 1.8 | 2 | 10 | ||
| maize silage | 0.58 | 1.7 | 3 | 9 | ||
| straw‐based diet | 1.0 | 3.7 | 6 | 21 | ||
| Sheep | 16 | Lactating sheep | 0.29 | 0.85 | 2 | 5 |
| Goats | 5a | Lactating goats | 0.41 | 0.61 | 8 | 12 |
| Fattening goats | 0.31 | 0.75 | 6 | 15 | ||
| Pigs | 0.7 | Pigs | ||||
| Fattening pigs | 0.52 | 1.3 | 74 | 186 | ||
| Lactating sow | 0.62 | 1.3 | 89 | 186 | ||
| Poultry | Poultry | |||||
| Broiler chickens | 5 | Broiler chickens | 1.0 | 3.3 | 20 | 66 |
| Laying hens | 5 | Laying hens | 0.81 | 3.4 | 16 | 68 |
| Fattening ducks | 7 | Fattening ducks | 1.0 | 2.9 | 14 | 41 |
| Fattening turkeys | 7 | Fattening turkeys | 1.5 | 4.0 | 21 | 57 |
| Farmed rabbits | 4 | Rabbits | 0.28 | 1.1 | 7 | 28 |
| Farmed fish d | Farmed fish | |||||
| Salmon | 0.6 | Salmonids | 0.12 | 0.38 | 20 | 63 |
| Carp | 0.6 | Carp | 0.39 | 1.2 | 65 | 200 |
| Horses | 36 | Horses e | ||||
| Fresh grass dietf | 0.17 | – | 0.5 | – | ||
| Grass‐hay dietg | 0.25 | – | 0.6 | – | ||
| Dogs | 4 | Dogs | 0.21 | 0.75 | 5 | 19 |
| Cats | 6 | Cats | 0.26 | 1.0 | 7 | 25 |
| Farmed mink | 1 | Farmed mink | 0.11 | 0.41 | 11 | 36 |
NOAEL: no‐observed‐adverse‐effect level; bw: body weight; UB: upper bound; –: insufficient number of samples reported (i.e. < 60) to calculate 95th percentile concentration; DM: dry matter.
NOAEL for the dairy cows used for goats (see Section 7.8.5).
Fresh grass and/or grass silage‐based diets (see Section 6.3).
Grass hay‐based diets (see Section 6.3).
NOAEL for the carp and rainbow trout was used for salmon (see Section 7.8.5).
Horses with moderate activity and hay based diet.
Fresh grass and/or grass silage‐based diets (see Section 6.3).
Grass hay‐based diets (see Section 6.3).
Rounded to the first decimal place or to the whole number.
For pigs the CONTAM Panel noted that ranges for the identified NOAELs and LOAELs for reduced feed intake and reduced weight gain were wide and overlapped (see Section 7.3.2 and Table 47). The CONTAM Panel decided to use the overall NOAEL of 0.7 mg/kg feed identified for reduced feed intake and/or reduced body weight gain in pigs (see Section 7.3.2 and Table 59).
For broiler chickens, laying hens, ducks and turkeys, the CONTAM Panel identified the overall NOAELs of 5, 5, 7 and 7 mg/kg feed, respectively, for reduced feed intake and reduced weight gain (see Section 7.3.3 and Table 59).
Scarceness or lack of data did not allow identification of specific NOAELs/LOAELs for each farmed fish species assessed in Section 7.3.5. However, based on the available data, the CONTAM Panel decided to use the overall NOAEL of 0.6 mg/kg feed for carp and rainbow trout for all farmed fish species (Table 59). The CONTAM Panel noted that salmon is physiologically close to the rainbow trout and thus the NOAEL derived from rainbow trout data was also used for salmon.
Based on a limited number of studies on horses the overall NOAEL of 36 mg/kg feed for reduced feed intake and reduced body weight was identified by the CONTAM Panel (see Section 7.3.4 and Table 59). Similarly, the identified overall NOAEL of 4 mg/kg feed for reduced body weight gain, relative organ weight and haematological parameters in rabbits was based also on a limited number of studies (see Section 7.3.5 and Table 59). Only one study was available on dogs, cats and farmed mink from which the NOAELs of 4, 6 and 1 mg/kg feed, respectively, for the reduced feed intake and reduced body weight were identified (see Sections 7.3.5–7.3.8 and Table 59).
7.9. Derivation of a group ARfD for acute risks of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in humans
Acute DON toxicosis has been associated with symptoms such as nausea, vomiting, diarrhoea, abdominal pain, headaches, dizziness, fever, and in severe cases, bloody stool in humans (see Section 7.5.2). Despite the limitations in the available human data, the CONTAM Panel decided to use the data from the DON toxicosis outbreak reported by Luo et al. (1987) (see Sections 7.5.1 and 7.9), supported by the available biomarker data (see Section 7.5.2), to establish a group acute reference dose (ARfD) for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
From a large number of outbreaks of mycotoxicoses, most of them in China (Table 51), the CONTAM Panel identified the study of Luo et al. (1987), in which primarily gastrointestinal disorders including vomiting in humans were recorded and could be associated with the measured DON concentrations in samples of contaminated grains consumed by the affected subjects (Table 52). In this study none of the 73 persons in the town of Qingzu (one of the three towns studied) became ill after being exposed to DON concentrations ranging between 1 and 8 mg/kg wheat. The CONTAM Panel considered this study as the most suitable to derive a NOAEL for vomiting in humans exposed to DON and identified the highest concentration of 8 mg/kg wheat from this population as a NOAEL for vomiting in humans exposed to DON.
The CONTAM Panel noted that wheat processing has been reported to reduce DON concentrations at a range of 10–80% depending on the method of processing (see Section 4.3), and decided to account for wheat processing in the calculation of a NOAEL for vomiting in humans exposed to DON. Considering that a minimal wheat refining process was likely used at the time in China, the CONTAM Panel assumed a 10% reduction of the concentration of DON when processing wheat to wheat flour and estimated the DON concentration as of 7.2 mg DON/kg wheat flour. Furthermore, a maximum wheat flour consumption of 555 g/day for adults was assumed using the data from the Chinese National Nutrition Survey data in 1982 (CNNS, 1982) that were the closest available data to the situation during the outbreaks. The CONTAM Panel also noted that, instead of using the daily wheat flour consumption, a single meal portion of wheat flour was more appropriate because vomiting was usually reported to occur within 30 min after the eating occasion in the reports from the mycotoxicosis outbreaks. Because wheat flour is typically consumed in three separate eating occasions per day in China (YY Gong, personal communication, 2016), the CONTAM Panel subdivided the maximum daily wheat flour consumption to three eating occasions resulting in an average meal size rounded to 200 g (0.2 kg) per eating occasion.
By multiplying the 0.2 kg wheat flour per meal with the concentration of 7.2 mg DON/kg wheat flour and dividing by a 55 kg body weight the average body weight for an Chinese adult at the time (CNNS, 1982), a NOAEL of 26 μg DON/kg bw per eating occasion for vomiting was calculated. The CONTAM Panel considered that a default uncertainty factor of 3.16 for toxicokinetic differences in the human population was needed and established a group ARfD rounded to 8 μg/kg bw per eating occasion for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
7.9.1. Support of human biomarker data
In addition, the CONTAM Panel decided to investigate to what extent the currently available urinary DON biomarker data (see Section 7.5.2) could support the established group ARfD of 8 μg/kg bw per eating occasion. The CONTAM Panel noted that the urinary biomarker only reflects previous day(s) exposure and therefore it would estimate acute but not chronic exposure. From the available studies on the DON biomarkers, the CONTAM Panel noted that no adverse health effects were reported in any of these studies and concluded that the reported biomarker data were obtained from healthy adults.46 Therefore, the CONTAM Panel considered that the available urinary DON biomarker data can inform on a NOAEL for vomiting in humans exposed to DON. Based on this consideration, the CONTAM Panel searched for the highest tDON biomarker levels observed in the biomarker studies on the healthy subjects and decided to sum up the urinary DON, DON‐3‐glucuronide, DON‐15‐glucuronide and DOM‐1/DOM‐1‐glucuronide concentrations. The CONTAM Panel noted that different mathematical formulae have been proposed in the literature (see e.g. Heyndrickx et al., 2015) for the calculation of tDON urinary biomarker levels accounting for the fact that the DON biomarker in urine comprises both free and conjugated forms of DON (see also Section 3.4) and concluded that the summation calculated by the CONTAM Panel is likely an overestimation. To back‐calculate the highest human exposure to DON from the highest tDON biomarker values, the CONTAM Panel used a linear approach using the equation from Section 7.5.2 assuming an average DON excretion rate of 70%, an average adult body weight of 70 kg and a default 24‐h urine volume of 2 L for adults (EFSA Scientific Committee, 2012). The highest urinary tDON biomarker level was reported for a healthy pregnant woman in Croatia (Šarkanj et al., 2013) (Appendix H) from which the exposure of 74 μg DON/kg bw per day47 was back‐calculated. The next highest urinary biomarker level in healthy adults was observed in a study from Belgium (Heyndrickx et al., 2015), from which 36 μg DON/kg bw per day was back‐calculated. The CONTAM Panel concluded from these calculations that the range from 36 to 74 μg DON/kg bw per day would represent a range of NOAELs at which vomiting is not expected to occur in humans.
However, the CONTAM Panel noted several data gaps and uncertainties in the above described approach which need to be taken into consideration:
the presence of an inconsistency between the urinary tDON biomarker levels measured using single and multiple biomarker methods;
calculated the maximum concentration of the tDON biomarker, based on the summing up of free DON and the glucuronides and DOM‐1, is likely to be an overestimation;
neglecting the variation of DON excretion and urine volume amongst individuals in the used back‐calculation approach;
inconsistent reporting of tDON biomarker results in the literature e.g. when urinary free DON and DON‐3‐glucuronide, DON‐15‐glucuronide and DOM 1 were not always measured or not reported;
being unable to address the contribution of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to the tDON biomarker due to the limited or lack of such data.
Considering these multiple sources of uncertainties, the CONTAM Panel noted that it was not possible to determine an uncertainty factor that would quantify the lack of current knowledge associated with all these uncertainties and that could be applied to adjust the range of NOAELs of 36–74 μg DON/kg bw per day at which vomiting is not expected to occur in humans. Therefore, no such uncertainty factor was identified and the available DON‐biomarker data were only used to support the group ARfD derived from human acute outbreak data in this opinion.
Thus, the CONTAM Panel compared the range of NOAELs of 36–74 μg DON/kg bw per day at which vomiting is not expected to occur in humans according to the tDON urinary biomarker information with the dose range of 26–78 μg DON/kg bw per day that was calculated from the NOAEL of 26 μg DON/kg bw per eating occasion derived from the human outbreak data assuming from one to three eating occasions per day. The CONTAM Panel concluded that these ranges were in the same order of magnitude and supported each other.
The CONTAM Panel also noted that the group ARfD of 8 μg/kg bw per eating occasion for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is based on human outbreak data while the ARfD of 8 μg/kg bw per day for DON and its acetylated forms established by JECFA (FAO/WHO, 2011) was based on the data on vomiting in pigs. Furthermore, the CONTAM Panel noted that FAO/WHO (2011) used the human epidemiological data from the Henan incidence in China from 1985, and of Luo (1988) and Guo et al. (1989), to calculate a level of 50 μg DON/kg bw per eating occasion, which could elicit acute intoxication in humans. The CONTAM Panel noted that its calculated NOAEL of 26 μg DON/kg bw per eating occasion for vomiting is not in disagreement with these calculations of JECFA (FAO/WHO, 2011).
7.10. Derivation of a group TDI for chronic risks of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in humans
The CONTAM Panel decided to establish a group TDI for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. Based on the BMDL05 of 0.11 mg/kg bw per day for reduced body weight gain in mice, the CONTAM Panel established a group TDI of 1 μg/kg bw per day using the default uncertainty factor of 100 for inter‐ and intraspecies variability. Since the BMDL values calculated for developmental and reproductive toxicity were all larger than the BMDL05 of 0.11 mg DON/kg bw per day (see Section 7.8.2), the CONTAM Panel concluded that this was also protective for developmental and reproductive toxicity.
The CONTAM Panel noted that this group TDI of 1 μg/kg bw per day the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is the same as the previously established health base guidance values by the SCF (2002) and by JECFA (FAO/WHO, 2011).
8. Risk characterisation
8.1. Human health risk characterisation
For human risk characterisation, the CONTAM Panel took into account the dietary exposure assessment of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside using the available analytical results on the occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food reported to EFSA (Section 4.2) and the consumption patterns described in recent dietary surveys of European countries available to EFSA (Section 5.1). The estimates of exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are presented in Section 6.1 and exposure to DON alone calculated from the data reported to EFSA in Appendix F.
8.1.1. Acute human health risk from the dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
The CONTAM Panel characterised the human health risk associated with acute dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside by comparing the mean and 95th percentile acute dietary LB and UB exposure estimates across European dietary surveys and the age groups (summarised in Table 33) with the calculated group ARfD of 8 μg/kg bw per eating occasion for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
The estimated mean acute dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food ranged from 0.2 (minimum LB) to 2.9 μg/kg bw per day (maximum UB) across the dietary surveys and age groups.
For ‘infants’, the estimated 95th percentile acute dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food ranged from 1.7 (minimum LB) to 6.7 μg/kg bw per day (maximum UB) for ‘toddlers’ and ‘other children’ from 1.5 to 5.4 μg/kg bw, for adolescents from 0.8 to 2.9 μg/kg bw per day and for adult population (‘adults’, ‘elderly’ and ‘very elderly’) from 0.7 to 2.8 μg/kg bw per day.
All mean and 95th percentile acute dietary exposure were below the group ARfD of 8 μg/kg bw per eating occasion. The CONTAM Panel also noted that the probabilistic confidence intervals at the UB for the maximum 95th percentile dietary exposures were all below the group ARfD, including the highest upper confidence interval bound of 7.1 μg/kg bw per day calculated for ‘infants’. Therefore, the CONTAM Panel concluded that the current acute exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food raises no health concern.
Regarding the vegetarian population, the limited available data on dietary habits of vegetarians with data available for only five European countries and with very few subjects in four of them indicate that mean acute dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is not notably different from the general population. Although the CONTAM Panel noted that the population group of infants could not be fully assessed for vegetarians since only one dietary survey was available, there is no indication that their risk for acute effects would be different from other vegetarian population groups.
8.1.2. Chronic human health risk from the dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
The CONTAM Panel characterised the human health risk associated with chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside by comparing the mean and 95th percentile chronic dietary LB and UB exposure estimates across the European dietary surveys and the age groups (summarised in Table 35) with the group TDI of 1 μg/kg bw per day.
For ‘Infants’, the estimated mean chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside ranged (minimum LB to maximum UB) from 0.2 to 2.0 μg/kg bw per day. The CONTAM Panel noted that for ‘infants’ the means of LB exposure estimates were below the group TDI of 1 μg/kg bw per day, whereas the median UB exposure was equal and the maximum UB exceeded twofold the group TDI. The high (95th percentile) chronic exposures, ranging from 0.7 to 3.7 μg/kg bw per day (minimum LB to maximum UB), exceeded the TDI slightly already at the median LB, and at all UB exposures.
The estimated mean exposures for ‘toddlers’ ranged from 0.6 (minimum LB) to 1.7 (maximum UB) μg/kg bw per day, and the group TDI was exceeded at mean LB exposure in some and at mean UB exposure in all dietary surveys. At the estimated high (95th percentile) exposures, ranging from 1.1 to 2.7 μg/kg bw per day (minimum LB to maximum UB), the TDI was exceeded in all dietary surveys already at the LB exposure.
For ‘other children’, estimated mean chronic dietary exposures ranged from 0.6 to 1.1 μg/kg bw per day at the LB and from 0.9 to 1.6 μg/kg bw per day at the UB. The group TDI was exceeded in one dietary survey at the LB and in the majority at the UB. At the estimates of high (95th percentile) exposure, ranging from 0.9 to 2.7 μg/kg bw per day (minimum LB to maximum UB), the group TDI was exceeded in all except one survey.
For ‘adolescents’, the estimated mean chronic exposures ranged from 0.3 to 0.9 μg/kg bw per day (minimum LB to maximum UB), and were below the group TDI. The estimated high (95th percentile) exposures, ranged from 0.5 to 1.8 μg/kg bw per day (minimum LB to maximum UB) and the group TDI was exceeded slightly at the LB exposure in some and at the UB exposure in the majority of the surveys.
In the adult population groups (‘adults’, ‘elderly’ and ‘very elderly’), the estimated mean exposures ranged from 0.2 to 0.7 μg/kg bw per day (minimum LB to maximum UB) did not exceeded the group TDI, whereas the estimates of high (95th percentile) exposures exceeded slightly the group TDI at the UB in some surveys.
The CONTAM Panel concluded that there is a health concern associated with chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, particularly for ‘infants’ ‘toddlers’ and ‘other children’ at the high (95th percentile) but at least in some European countries also at the mean LB exposure. In ‘adolescents’ and the adult age groups, the group TDI was exceeded only at the UB at the high (95th percentile) chronic exposures.
The limited available data on dietary habits of vegetarians with data available for only five European countries, with very few subjects in four of them indicate that mean chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is not notably different from the general population. Although the CONTAM Panel noted that the population group of infants could not be fully assessed for vegetarians since only one dietary survey was available, there is no indication that their risk for chronic effects would be different from other vegetarian population groups.
8.2. Farm and companion animal health risk characterisation for the exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
For animal risk characterisation, the CONTAM Panel took into account the dietary exposure assessment of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside using recent analytical results on the occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in feed reported to EFSA (Section 4.2) and the diet composition and feed consumption of farm and companion animals described in Sections 5.2 and 6.3, and Appendix E. The estimates of exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are presented in Section 6.3 and exposure to DON alone in Appendix F.
The CONTAM Panel characterised the farm and companion animal health risk associated with chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside by comparing the estimated UB mean and UB 95th percentile, calculated as dietary concentrations for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (see Section 6.3 and Appendix E), with the identified NOAELs (expressed as mg/kg feed) (see Table 58). In addition, for pigs, farmed mink, dogs and cats, for which vomiting was reported as an acute adverse effect, the acute health risk was characterised. For pigs, dogs and cats, this was done by comparing the UB 95th percentile dietary concentrations with the identified NOAELs and calculated BMCLs (see Sections 7.8.4 and 7.8.5), and for farmed mink by comparing the estimated UB 95th percentile exposure (μg DON/kg bw per day) with the calculated BMDL10 (see Section 7.8.5).
Table 58.
Dose–response analysis of critical endpoint of reduced body weight gain based on the body weight data of Iverson et al. (1995) for female and male mice combined using the PROAST software for the BMD approach
| Best fitting model | Body weight | ||
|---|---|---|---|
| BMD05 | BMDL05–BMDL05 | ||
| mg/kg bw per day | |||
| Female mice | |||
| Exponential model family | E3 | 0.19 | 0.11–0.28 |
| Hill model family | H3 | 0.19 | 0.12–0.29 |
| Male mice | |||
| Exponential model family | E3 | 0.22 | 0.15–0.30 |
| Hill model family | H3 | 0.23 | 0.16–0.32 |
BMD05: benchmark dose response of 5%; BMDL05/BMDU05: 95% lower/upper confidence limit for the benchmark dose response of 5%; bw: body weight.
The two sided 90% confidence intervals (BMDL05 – BMDU05) of the BMD05 of the best fitting models defined by the Akaike Information Criterion (AIC) are reported for the exponential and the Hill family following the EFSA guidance (EFSA Scientific Committee, 2017).
8.2.1. Ruminants
For dairy cows, the highest calculated UB mean and UB 95th percentile dietary concentrations were 4 and 13% of the identified NOAEL of 5 mg/kg feed for chronic effects, respectively, except for cows fed on maize silage‐based diets were they were 16% and 55% of the NOAEL, respectively (see Table 59). Therefore, the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was considered low.
In the absence of any specific dietary concentrations for heifers, the CONTAM Panel decided to use the dietary concentrations of fatting cattle fed with fresh grass and/or grass silage‐based diets because their diet rations would be broadly similar. The highest UB mean and UB 95th percentile dietary concentrations were 1% and 5% of the identified NOAEL of 10 mg/kg feed for chronic effects, respectively (see Table 59). Therefore, the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was considered low.
For fattening beef cattle (including steers), the highest exposure was for cattle on straw‐based diets. The calculated UB mean and UB 95th percentile dietary concentrations were 6% and 21% of the NOAEL of 18 mg/kg feed for chronic effects, respectively (see Table 59). For fattening beef cattle with other feeding systems, the percentages were lower. Therefore, the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was considered low.
For lactating sheep, the UB mean and UB 95th percentile dietary concentrations were 2% and 5% of the NOAEL of 16 mg/kg feed for chronic effects, respectively (see Table 59), indicating that the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was low.
For goats, the exposure at the UB mean dietary concentrations was higher for lactating than fattening goats, while it was the opposite at the UB 95th percentile. When comparing the highest dietary concentrations with the NOAEL of 5 mg/kg feed for chronic effects, the percentages were 8% for lactating and 15% for fattening goats (see Table 59). Therefore, the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was considered low.
The CONTAM Panel noted further that the risk might be higher for pre‐ruminant animals such as calves (see also Section 7.4.1), which might be more susceptible to the toxic effects of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. This might also apply for lambs. Overall, the CONTAM Panel noted that the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for different ruminant species was clearly lower than for several other farm animal species considered in this opinion.
8.2.2. Pigs
The CONTAM Panel used the BMCL10 of 1.4 mg DON/kg feed as the reference point for acute risk characterisation for pigs (fattening pigs and lactating sows) (see Section 7.8.4). The highest UB 95th percentile dietary concentration (see Table 59) was 93% of the BMCL10 for acute effects (see Section 7.8.5), indicating that the risk for acute adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was low.
For fattening pigs and lactating sows, the UB mean dietary concentrations were 74% and 89% of the NOAEL of 0.7 mg/kg feed for chronic effects, respectively (see Table 59) and 186% at the UB 95th percentile dietary concentrations for these animals. Therefore, the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was considered low for pigs exposed to the mean dietary concentrations. At the UB 95th percentile dietary concentrations, a possible risk for chronic adverse effects in pigs was identified.
8.2.3. Poultry
Amongst the poultry species, the highest exposure was for broiler chickens and laying hens for which the UB mean and UB 95th percentile dietary concentrations were 16–20% and 66–68% of the NOAEL of 5 mg/kg feed for chronic effects, respectively (see Table 59). For fattening ducks, the percentages were 14% and 41%, respectively, and for fattening turkeys 21% and 57%, respectively. Therefore, the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was considered low.
8.2.4. Horses
The exposures were similar for horses when fed with diets with and without hay. The highest UB mean dietary concentration was only 0.6% of the NOAEL of 36 mg/kg feed for chronic effects (see Table 59), indicating that the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was negligible. An insufficient number of occurrence data prevented the CONTAM Panel from estimating the risk for adverse health effects at the 95th percentile dietary concentrations. However, based on the difference between the mean and the 95th percentile concentrations in feeds in general, it is reasonable to expect that the risk remains negligible also at the 95th percentile for horses.
The CONTAM Panel noted that the characterisation of the risks for horses should be considered indicative owing to the uncertainties associated to the identified NOAEL (see Section 7.8.6).
8.2.5. Farmed rabbits
For rabbits, the calculated UB mean and UB 95th percentile dietary concentrations were 7 and 28% of the NOAEL of 4 mg/kg feed for chronic effects, respectively (see Table 59), indicating that the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was low.
The CONTAM Panel noted that this characterisation of risks for farmed rabbits should be considered indicative owing to the uncertainties associated to the identified NOAEL (see Section 7.8.6).
8.2.6. Farmed fish
For farmed fish (salmon and carp), the UB mean and UB 95th percentile dietary concentrations were 20–65% and 63–200% of the NOAEL of 0.6 mg/kg feed for chronic effects, respectively (see Table 59), indicating that the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was low at the mean dietary concentrations. In contrast, at the UB 95th percentile dietary concentrations, a possible risk for chronic adverse effects at least for carp was identified.
The CONTAM Panel noted that the diet composition of different fish species may differ to a major extent between fish species and that some fish species might be more tolerant for the adverse effects than the others.
8.2.7. Farmed mink
For farmed mink, the UB 95th percentile dietary exposure (410 μg/kg bw per day) (see Table 43 in Section 6.3) was 100‐fold the BMDL10 of 4 μg DON/kg bw per day for acute effects (Section 7.8.5), and therefore, a possible risk for acute adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was identified.
The calculated UB mean and UB 95th percentile dietary concentrations were 11% and 36% of the NOAEL of 1 mg DON/kg feed for chronic effects, respectively (see Table 59), indicating that the estimated risk for chronic adverse health effects from feed containing the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was low.
The CONTAM Panel noted that the characterisation of chronic risks for farmed mink should be considered indicative owing to the uncertainties associated to the identified reference points (see Section 7.8.6).
8.2.8. Dogs
For dogs, the calculated UB 95th percentile dietary concentration (see Table 59) was 15% of the BMCL10 of 5 mg/kg feed for acute effects (Section 7.8.5), indicating that the estimated risk for acute adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was low.
The UB mean and UB 95th percentile dietary concentrations were 5 and 19% of the NOAEL of 4 mg/kg feed for chronic effects, respectively (see Table 59), indicating that the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was low.
The CONTAM Panel noted that this characterisation of acute and chronic risks for dogs should be considered indicative owing to the uncertainties associated to the identified reference points (see Section 7.8.6).
8.2.9. Cats
For cats, the UB 95th percentile dietary concentration (see Table 59) was 100% of the BMCL10 of 1.0 mg/kg feed for acute effects (Section 7.8.5) and therefore a possible risk for acute adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside at the UB 95th percentile dietary concentrations was identified.
The calculated UB mean and UB 95th percentile dietary concentrations were 7% and 25% of the NOAEL of 6 mg/kg feed for chronic effects, respectively (see Table 59), indicating that the estimated risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was low.
The CONTAM Panel noted that this characterisation of acute and chronic risks for cats should be considered indicative owing to the uncertainties associated to the identified reference points (see Section 7.8.6).
9. Uncertainty analysis
Evaluation of the inherent uncertainties in the assessment of exposure to the sum of DON, 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside and DON has been performed following the guidance given in the Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment (EFSA, 2007). In addition, the report on ‘Characterizing and Communicating Uncertainty in Exposure Assessment’ has been considered (WHO/IPCS, 2008). According to the guidance provided by the EFSA opinion (2006) the following sources of uncertainties have been considered: Assessment objectives, exposure scenario, exposure model and model input (parameters). In addition to the EFSA opinion (2006), the CONTAM Panel also considered other uncertainties.
9.1. Assessment objectives
The objectives of the assessment were defined in the terms of reference. In communication with the requestor of the present opinion, the assessment objectives were clarified as described in Section 1 and thereafter existed no uncertainty in addressing the objectives.
9.2. Exposure scenario and model
The calculation of exposure from food and feed was based on the occurrence data reported to EFSA for a period from 2007 to 2014 for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside following the specifications of EFSA (EFSA, 2010a) and on a substitution of ‘non‐analysed’ occurrence data for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. This substitution was based on the estimated ratios of 10, 15 and 20% of 3‐Ac‐DON to DON, 15‐Ac‐DON to DON and DON‐3‐glucoside to DON, respectively, in grains for food consumption and grain‐based products and cereal grains for feed, and on the estimated ratio of 80% of DON‐3‐glucoside to DON for the alcoholic beverages by using the currently available literature data and the occurrence data submitted to EFSA on the co‐occurrence of these four DON‐forms (see Section 4.2.8). The substitution and, particularly the choice of plausible %‐ratios introduced uncertainties in both, the acute and chronic exposure assessments of humans and farm and companion animals.
The concentrations for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed were not corrected for molar masses which added uncertainties on the overall exposure estimates for humans and farm and companion animals. This uncertainty is considered small in comparison to the other uncertainties identified.
Data on occurrence in food, feed and unprocessed grains of undefined end‐use were used as submitted by the data providers, either corrected for recovery or not. Only data with a predefined minimum level of the LOQ of the analytical method (LOQ < 100 μg/kg in food and feed, except data on food for infants where an LOQ < 50 μg/kg was required) were used to estimate exposure. This might have introduced further uncertainties to the exposure estimates.
Although the occurrence data used covered the relevant food and feed groups known for contamination by DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, the numbers of samples were not equally distributed across these groups, and there was a high proportion of left‐censored data in many food and feed categories. These aspects contributed to the overall uncertainty of exposure. The number of the samples that were analysed for DON alone was almost an order of magnitude larger then number of samples also analysed for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. Furthermore, the number of samples analysed simultaneously for DON and at least one of the three other forms was very small compared to the total number of analytical results available and there was only a minute number of samples where DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were quantified simultaneously.
The lack of analytical methods formally validated through interlaboratory studies hampered a reliable conclusion on the uncertainty of the currently available results in EFSA's database or in the cited literature on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed. The lack of certified reference materials for DON‐3‐glucoside, 3‐Ac‐DON and 15‐Ac‐DON (as calibrants and in particular matrix materials) are additional limitations that hamper an uncertainty estimation for individual laboratories. Both issues, as a result, contribute to the overall uncertainty of the occurrence data. These limitations are equally valid for the chemical analysis used in toxicokinetic and toxicity studies and in studies for human urinary biomarkers and their metabolites.
The use of the UB approach for high percentage of occurrence data < LODs/LOQs, even when 100% of the data were left‐censored, is conservative and represented an overestimation of both the acute and the chronic risk, while the use of the LB approach represented an underestimation.
The CONTAM Panel noted that a considerable number of food categories, in particular at EFSA Food Category levels 2 and where used also at level 3 exhibited 100% left‐censored data. Although left‐censored data always adds uncertainty to the exposure assessment, a sensitivity analysis excluding food groups with 100% left‐censored data indicated only a minor impact for the present assessment.
Based on the information from the available literature data, effects of processing of the food and the feed contaminated by DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside can lead to increases or decreases of concentrations of the toxins depending on the type and intensity of the form of processing. This adds to the overall uncertainty.
Limited data on food consumption for vegetarians adds uncertainty in their exposure assessment. There was a lack of dietary surveys reporting consumption data for children younger than 1 year, which led to an uncertainty for this age group. All these aspects added to the overall uncertainty.
For the exposure of farm and companion animals, where species‐specific data on compound feed were not available the CONTAM Panel formulated example feed rations. This and the considerable variability that exists between feeds and feeding systems used for farm animals in Europe add to the uncertainty of animal exposure. Exposure of ruminants may be overestimated when data for grass hay were used for estimating the contribution from forages for lactating dairy cows and beef cattle. These aspects contributed further to overall uncertainty for the animal risk assessment.
9.3. Other uncertainties
The approach taken assumed that DON‐3‐glucoside is metabolised to DON and absorbed at the same extent as DON and might induce the same acute and chronic effects as DON, and is considered conservative. The same assumptions made for the 3‐Ac‐DON and 15‐Ac‐DON also contribute also to the overall uncertainty, but to a lesser extent.
The available data from mice indicated that the absorption is higher in weanling mice than in young adults. This may indicate that young animals are more susceptible than adults and this contributed to the overall uncertainty.
The CONTAM Panel noted that the available toxicity data indicated that DON is genotoxic in vitro, however insufficient evidence was identified to assess whether DON is genotoxic in vivo or not, although no evidence was found that DON is directly DNA reactive and no evidence for carcinogenicity was identified. Currently, available data for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are too limited to draw qualified conclusions on their single or combined genotoxicity and/or carcinogenicity and therefore the genotoxic potential of the exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is not fully characterised. The CONTAM Panel also noted a lack of data on neurotoxicity of DON and on general and gastrointestinal toxicity of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in experimental animals. All these aspects contributed to the uncertainty of the risk characterisation for both humans and farm and companion animals.
Most studies in animals on the adverse effects induced by DON used naturally contaminated feed which contained, in addition to DON and/or 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, other mycotoxins. The presence and/or concentrations of these were often incompletely reported. This contributed to the overall uncertainty for the risk assessment of farm and companion animals.
For the risk assessment on dogs and cats, the CONTAM Panel identified only one study on acute and chronic adverse effects that reported substantial interindividual variability in behaviour of the animals. The numbers of animals per dose group was rather small ranging to 10 at its maximum. This added substantial uncertainty on the identified reference points, and thus further to the risk assessment of these animal species. Similarly, only one study on acute and one study on chronic adverse effects were available on farmed mink introducing uncertainty to the reference points and their risk assessment. For the adverse health effects in farmed rabbits and horses, a limited number of studies were available and this introduced uncertainty to their risk assessment.
For the risk assessment on farmed fish, the CONTAM Panel noted that the design of most studies used the fish tank as experimental unit and therefore interindividual variation was not fully accounted for in the hazard characterisation. This and incomplete reporting of the statistical variability generated substantial uncertainty. The CONTAM Panel also noted a large uncertainty of the hazard characterisation for farmed fish, in general, since only two fish species could be assessed.
Due to the limited information, combined effects of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside and/or with other mycotoxins could not be characterised and this might lead to over‐ or underestimation of the hazard of DON and its acetylated and modified forms in food and feed. This contributed to the overall uncertainty.
The assessment for the acute risk from the exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was based on an ARfD established for vomiting in humans and calculated from a limited number of occurrence data from only one epidemiological study on previous mycotoxicosis outbreaks in Asia. Although the ARfD was well supported by well sized human urinary biomarker data recruited in Europe, the CONTAM Panel noted several data gaps and uncertainties when assessing these biomarker data. Therefore, in the absence of an appropriate and well‐designed acute toxicological study on humans and/or experimental animals the overall uncertainty of the acute risk assessment of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for humans remains high.
The uncertainties related to the application of biomarkers of DON are presented in Section 7.9.
9.4. Summary of uncertainties
In Table 60, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the respective source of uncertainty might have led to an over‐ or underestimation of the exposure or the resulting risk.
Table 60.
Summary of qualitative evaluation of the impact of uncertainties on the risk assessment of the human and animal dietary exposure to the sum of DON, 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside
| Sources of uncertainty | Directiona |
|---|---|
| Uncertainty of the analytical measurements | +/− |
| Considering only occurrence data in food and feed for which the LOQs were reported to be below the cut‐off LOQ values | +/− |
| Effects of food and feed processing | +/− |
| Limited data on the co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in the food and feed samples | +/− |
| Non‐analysed concentrations of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were assigned based on the estimated %‐ratios | +/− |
| Across the different food and feed matrices the same estimated %‐ratios for the non‐analysed concentrations were used | +/− |
| High variability of the composition of feedstuffs used and feeding systems for farm animals in Europe | +/− |
| Use of UB occurrence data in the exposure estimations | + |
| Use of LB occurrence data in the exposure estimations | − |
| Limited data on exposure of infants | +/− |
| Limited data on exposure of vegetarians | +/− |
| Limited or no toxicokinetic data on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for humans, experimental animals a number of farm animal species and companion animal species | +/− |
| Limited or no toxicological data on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for humans, experimental animals, a number of farm animal species and companion animal species | +/− |
| Incomplete data on the mode of action underlying the possible genotoxicity of DON | − |
| Similar toxicity and toxicological potency as associated with DON were applied for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside | + |
| Lack of information on combined effects with other mycotoxins or other toxic substances in food and feed | +/− |
| Use of one limited epidemiological study on an outbreak of acute mycotoxicosis in humans in the absence of an appropriate and well‐designed acute toxicological study on humans and/or experimental animals | +/− |
| Approach and assumptions taken for establishing the ARfD for humans | + |
DON: deoxynivalenol; LOQ: limit of quantification; UB: upper bound; LB: lower bound; ARfD: acute reference dose.
+ = uncertainty with potential to cause over‐estimation of exposure/risk; − = uncertainty with potential to cause under‐estimation of exposure/risk.
The CONTAM Panel concluded that although the impact of the uncertainties in the human risk assessment of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is large, the risk is more likely to be over than underestimated. The impact of the uncertainties in the risk assessment of farm and companion animals is large.
Conclusions and recommendations
Conclusions
Deoxynivalenol, 3‐acetyl‐DON (3‐Ac‐DON), 15‐acetyl‐DON (15‐Ac‐DON) and DON‐3‐glucoside are currently the most relevant forms of DON in Europe occurring in food and feed, mainly in cereal grains and cereal‐based food. While DON, 3‐Ac‐DON and 15‐Ac‐DON are produced by plant pathogenic fungi of the Fusarium genus as toxic secondary metabolites, DON‐3‐glucoside is the main plant metabolite of DON and considered as a modified mycotoxin.
Methods of analysis
Analytical methods for detection and quantification of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are well established.
For DON, performance criteria for methods of analysis and certified reference materials (both reference matrices and reference calibrants) are available. Non‐certified calibrants are available for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
Quantification of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is mostly carried out by liquid chromatography (LC) coupled with (multistage) mass spectrometry (MS). This methodology has not been formally validated through interlaboratory studies, and proficiency studies demonstrated considerable analytical variability in the determination of DON.
Urinary DON and its metabolites in urine, primarily DON‐glucuronides, can be detected using single or multiple biomarker methods. Commercial sources for the standards of DON‐glucuronides are scarce and no (certified) reference materials are available for urinary DON biomarkers.
Occurrence
For food, a total of 41,027 analytical results for DON (n = 21,916), 3‐Ac‐DON (n = 11,944), 15‐Ac‐DON (n = 6,370) and DON‐3‐glucoside (n = 797) fulfilled the quality criteria applied and have been used in the assessment.
For feed, a total of 6,295 analytical results for DON (n = 4,000), 3‐Ac‐DON (n = 894), 15‐Ac‐DON (n = 470) and DON‐3‐glucoside (n = 931) fulfilled the quality criteria applied and have been used in the assessment.
For unprocessed grains of undefined end‐use, a total of 3,643 analytical results for DON (n = 1,621), 3‐Ac‐DON (n = 1,054), 15‐Ac‐DON (n = 430) and DON‐3‐glucoside (n = 538) fulfilled the quality criteria applied and have been used in the assessment.
The proportion of left‐censored data in food (results below the limit of detection (LOD) or limit of quantification (LOQ)) were 63% for DON, 98% for 3‐Ac‐DON, 97% for 15‐Ac‐DON, and 57% for DON‐3‐glucoside. The corresponding values for feed were 43% for DON, 92% for 3‐Ac‐DON, 65% for 15‐AC‐DON, and 91% for DON‐3‐glucoside. The corresponding values for unprocessed grains were 56, 91, 68 and 66% of the results for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, respectively.
The highest mean concentrations of DON and the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were recorded for food in the category of ‘Products for special nutritional use’, followed by ‘Grains and grain‐based products’, for feed, in ‘Cereal straw’ and for unprocessed grains of undefined end‐use in ‘Grains as crops’.
In general, the DON concentrations were either similar to or marginally lower in organically produced grains compared to conventionally produced grains in the data submitted to EFSA. This pattern was generally confirmed by the limited data available in the published literature. Due to lack of data, it was not possible to draw similar conclusions for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
The co‐occurrence ratios of DON with 3‐Ac‐DON, 15‐Ac‐DON and/or DON‐3‐glucoside in food and feed reported to EFSA varied considerably between different food, feed and grain categories, both for data submitted to EFSA and data reported in the literature.
Based on a review of the available information and using expert judgement, the CONTAM Panel estimated the ratios of concentrations of 3‐Ac‐DON to DON, 15‐Ac‐DON to DON and DON‐3‐glucoside to DON as 10%, 15% and 20%, respectively. The exception was ‘Alcoholic beverages’ (‘Beer and beer‐like beverage’) for which the ratio of DON‐3‐glucoside to DON was estimated as 80%. These %‐ratios were used for exposure assessments.
Effects of processing
Because DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are mostly attached to the outer hull of the grain, cleaning, sorting, sieving and dehulling of grains lead to marked increases in concentrations of these toxins in cereal by‐products, e.g. bran.
During baking and cooking, DON appears to be relatively stable. Studies on 3‐Ac‐DON and 15‐Ac‐DON and DON‐3‐glucoside were inconclusive
Malting and brewing do not seem to lead to losses of DON and DON‐3‐glucoside and no increased concentrations were observed in by‐products of brewing. However, the ratio between DON and DON‐3‐glucoside concentrations changed during the process leading to a notable increase of the concentrations of DON‐3‐glucoside as compared to DON. Studies on the fate of 3‐Ac‐DON and 15‐Ac‐DON during malting and brewing were too limited to draw conclusions.
Data on feed processing were too limited to draw conclusions. However, since some of the processes applied to cereal grains for food production are also applied to grains used for animal feeds, the findings for food should also apply to feed.
Human exposure
The estimates of mean acute exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across 39 different dietary surveys and all age groups using the minimum lower bound (LB) and the maximum upper bound (UB) concentrations ranged from 0.2 to 2.9 μg/kg body weight (bw) per day. The estimates of 95th percentile acute exposure ranged from 0.7 to 6.7 μg/kg bw per day. The highest mean and the highest 95th percentile acute dietary exposures were for infants. The estimates of mean chronic exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across 33 different dietary surveys and all age groups using the minimum LB and the maximum UB concentrations ranged from 0.2 to 2.0 μg/kg bw per day. The estimates of 95th percentile chronic exposure ranged from 0.5 to 3.7 μg/kg bw per day. The highest mean and the highest 95th percentile chronic dietary exposures were for infants.
The most important contributors to the chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were ‘Grains and grain‐based products’, especially ‘Bread and rolls’, ‘Fine bakery wares’ and ‘Pasta (raw)’.
The limited consumption data on vegetarians do not indicate a major difference in the dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and 3‐DON‐glucoside between vegetarians and the general population.
Farm and companion animal exposure
The animal exposure was presented as dietary concentrations because the animal risk assessment was carried out on a concentration–response basis, except for the farmed mink for which the acute dietary exposure was presented on a dose‐response basis expressed per kg bw.
Animal exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was primarily from the consumption of cereal grains and cereal byproducts. With the exception of forage maize (and maize silage produced from it), levels in forages were generally low.
For lactating dairy cows and beef cattle, the calculated lowest LB and highest UB mean dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were 64.2 and 996 μg/kg diet, respectively, and the 95th percentile dietary concentrations were 124 and 3,655 μg/kg diet, respectively.
For small ruminants, the calculated lowest LB and highest UB mean dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were 151 and 414 μg/kg diet, respectively, and the 95th percentile dietary concentrations were 561 and 849 μg/kg diet, respectively.
For pigs, the calculated lowest LB and highest UB mean dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were 437 and 618 μg/kg diet, respectively, and the 95th percentile dietary concentrations were 1,284 and 1,302 μg/kg diet, respectively.
For poultry, the calculated lowest LB and highest UB mean dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were 794 and 1,494 μg/kg diet, and the 95th percentile dietary concentrations were 2,900 and 3,971 μg/kg diet, respectively.
For horses, the calculated lowest LB and highest UB mean dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were 155 and 253 μg/kg diet, respectively.
For farmed fish (salmonids and carp), the calculated lowest LB and highest UB mean dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were 83.3 and 388 μg/kg diet, respectively, and the 95th percentile dietary concentrations were 362 and 1,151 μg/kg diet, respectively.
For farmed rabbits, the calculated LB and UB mean dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were 196 and 282 μg/kg diet, respectively, and the 95th percentile dietary concentrations were 1,048 and 1,135 μg/kg diet, respectively.
For farmed mink, the calculated LB and UB mean dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were 99.5 and 109 μg/kg diet, respectively. The 95th percentile dietary concentrations were 407 μg/kg diet (equivalent to 14.7 μg/kg bw per day) and 409 μg/kg diet (equivalent to 14.8 μg/kg bw per day), respectively.
For dogs, the calculated LB and UB mean dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were 174 and 214 μg/kg diet, respectively, and the 95th percentile dietary concentrations were 741 and 753 μg/kg diet, respectively.
For cats, the calculated LB and UB mean dietary concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were 229 and 264 μg/kg diet, respectively, and the 95th percentile dietary concentrations were 968 and 975 μg/kg diet, respectively.
Toxicokinetics in rodents
The oral bioavailability of DON has not been quantified in mice, but plasma, tissue and urine concentrations indicated that the absorption is high and maximum plasma concentrations are reached rapidly.
Following absorption, DON is rapidly distributed to tissues, e.g. liver, kidney, spleen and heart in rodents reaching the maximum concentrations at about the same time as in plasma with exception for the brain, where the total concentrations of DON and metabolites peak later and at lower concentrations than in other organs.
In rats, DON is partially transformed to the less toxic de‐epoxidised metabolite DOM‐1 by gut bacteria prior to absorption.
The main biotransformation of DON is conjugation with glucuronic acid leading to the formation of mainly DON‐3‐glucuronide and DON‐15‐glucuronide. DON‐sulfonates were the major metabolites in rat faeces.
In rats, DON was rapidly excreted in faeces and urine with no apparent accumulation in any tissue. A mean urinary excretion of at least 70% was noted. In faeces, DON was largely metabolised to DOM‐1.
In rats and mice, 3‐Ac‐DON and 15‐Ac‐DON was mainly transformed to DON by gut microflora prior to absorption and systemic distribution. After transformation, 3‐Ac‐DON and 15‐Ac‐DON followed the kinetics of DON.
In rats, DON‐3‐glucoside was also mainly transformed to DON in the gut prior to absorption and followed the kinetics as DON. However, the total bioavailability was considerably lower than for DON. The cleavage of DON‐3‐glucoside in the gastrointestinal tract was species and strain specific.
Toxicokinetics in humans
In humans, an estimated 70% of the ingested DON was excreted to urine, mainly as glucuronide conjugated DON. DON‐15‐glucuronide was the predominant conjugate in human urine, DON‐3‐glucuronide being about three times less.
The transformation of DON to DOM‐1 in humans was less evident than in rats and mice.
DON was transported across the human placenta in both in vitro and ex vivo models and can reach the fetus.
Toxicokinetics in farm animals
In ruminants, DON was extensively transformed to the less toxic de‐epoxidised metabolite DOM‐1. Both are efficiently glucuronidated. The main route of excretion is via urine and the faecal and biliary excretion routes are less important.
Much less of DOM‐1 was generated in ruminants with ruminal acidosis and in young ruminants such as calves in which the ruminal system is not fully functioning.
In dairy cows, DON was almost completely metabolised by the ruminal flora to the de‐epoxidated form DOM‐1 and only minor amounts for DON (< 1%) and DOM‐1 reached systemic circulation.
In sheep, DON was bioavailable at 7.5% and was quickly absorbed.
In goats, no data were identified on the toxicokinetics of DON.
No data were identified on the toxicokinetics of 3‐Ac‐DON, 15‐Ac‐DON and/or DON‐3‐glucoside for ruminants except for sheep where one study indicated that 3‐Ac‐DON was rapidly hydrolysed to DON.
In pigs, the absorption of DON was rapid (less than 30 min) and generally high (48–65%), influenced by the level of exposure. DON showed an extensive organ distribution and a rapid renal excretion and it was partly conjugated to glucuronic acid. The plasma elimination half‐life varied from 1 to 4 h. Excretion of DON and its metabolites was via urinary and biliary routes with urinary excretion being most important. 3‐Ac‐DON was rapidly deacetylated in the upper intestinal tract and absorbed exclusively as DON. DON‐3‐glucoside showed a two times lower bioavailability compared with DON and was available primarily as aglucone, likely after its cleavage in the gastrointestinal tract. No data were identified for 15‐Ac‐DON.
In poultry, the oral bioavailability of DON was low (only up to 10%) and it was rapidly absorbed into plasma with fast metabolism and clearance from plasma. Only low concentrations of DON and DOM‐1 were detected in plasma and bile. The major metabolite of DON in plasma of broiler chickens and turkeys was DON‐3‐sulfate. Formation of DON‐3‐glucuronide appeared to be a minor pathway. In broiler chickens, a nearly complete hydrolysis of 3‐Ac‐DON to DON and a partial hydrolysis of 15‐Ac‐DON to DON were observed. Limited results indicated that DON‐3‐glucoside was not hydrolysed to DON in broiler chickens. The oral bioavailability of DON‐3‐glucoside was low and comparable to that of DON.
In horses, microbial de‐epoxidation of DON might take place in gut. A rapid clearance of DON in plasma was observed in a glucuronide‐conjugated form. No data were identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in horses.
Transfer
The transfer of DON to food of animal origin was generally low and residues of DON in products of animal origin are unlikely substantially to contribute to human exposure. No data on the transfer of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified.
Toxicity of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in rodents
Acute toxicity
Acute toxicity of DON was characterised as feed refusal, weight loss and diarrhoea.
Acute toxicity of 3‐Ac‐DON was considerably lower than that of DON. In mice, both 3‐Ac‐DON and 15‐Ac‐DON showed shortly after start of exposure feed refusal that appeared to be compensated later by increased feed intake.
Subacute and subchronic toxicity
Subacute and subchronic toxicities of DON were characterised as feed refusal, reduced body weight gain and diarrhoea.
In the few available subchronic toxicity studies on DON in mice and rats, a no‐observed‐adverse‐effect level (NOAEL) of 0.1 mg/kg bw per day was identified based on haematological disturbances.
No data on subacute or subchronic toxicity of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified.
Chronic toxicity
The only long‐term chronic toxicity study on DON was designed in mice as a carcinogenicity study. This study did not indicate carcinogenic properties of DON. From the general toxicity reported in this trial, a NOAEL of 0.1 mg/kg bw per day was identified for reduced feed intake and body weight gain in mice.
The available data did not show any haemato‐ or myelotoxicity of DON.
Data on neurotoxicological effects of DON were very limited and no dose–response data were suitable for hazard characterisation or the generation of a link with the data on the mode of action of DON. No data on chronic toxicity, haemato‐ and myelotoxicity, neurotoxicity and carcinogenicity of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified.
Immunotoxicity
DON induced an elevation of IgA in rodents and farm animals. In the available chronic 2‐year study, the increase of IgA was only observed in female mice at the highest dose of 1.6 mg/kg bw per day.
In studies with high doses, DON induced inflammation in mice. An increase in TNF‐α and IL‐1‐β was observed at doses as low as 2.5 μg DON/kg bw per day but this was seen in the absence of an observed adverse effect.
The DON effects on the immune response (doses 8–10 mg/kg bw) led to an increased susceptibility to infectious diseases.
Inflammation was observed after exposure to 3‐Ac‐DON and 15‐Ac‐DON in mice but not for DON‐3‐glucoside. However, data on immune response, were scarce and no effects on IgA levels were reported.
Reproductive and developmental toxicity
Oral exposure to DON exhibited both developmental and reproductive toxicity in experimental animals including reduced fertility, embryotoxicity, skeletal abnormalities, effects on body weight and relative epididymal weight and postnatal mortality.
For mice, no new relevant data were identified since the previous assessment and the CONTAM Panel confirmed the previous NOAELs of 0.38 mg/kg bw per day for increased postnatal mortality in pups and 0.5 mg/kg bw per for skeletal abnormalities.
Studies on rats treated orally with DON, that were carried out since the evaluation of the Scientific Committee of Food (SCF), showed a NOAEL of 1.0 mg/kg bw per day for reproductive endpoints, 1.0 mg/kg bw per day for fetal toxicity and 0.5 mg/kg bw per day for maternal toxicity.
No data were identified for 3‐Ac‐DON, 15‐Ac‐DON and DON‐3 glucoside.
Genotoxicity
DON was genotoxic in vitro, and the data available on the genotoxicity of DON in vivo were inconclusive.
The available evidence suggested that oxidative stress may be involved in the genotoxicity, rather than a direct interaction of DON with DNA (e.g. adduct formation).
3‐Ac‐DON was inactive in a bacterial mutation assay and no in vitro genotoxicity data for 15‐Ac‐DON or DON‐3‐glucoside were identified.
No in vivo genotoxicity studies on 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified.
Human toxicity data
Human outbreaks from acute exposure to DON have been repeatedly reported in Asia, with symptoms including nausea, vomiting, diarrhoea, abdominal pain, headaches, dizziness, fever, and in severe cases, bloody stool; however, no lethality was reported.
Evidence of adverse health effect in humans due to chronic exposure to DON was lacking.
Human biomarkers of exposure
A urinary DON biomarker for total DON comprising DON, DON‐3‐glucuronide and DON‐15‐glucuronide correlated well with dietary exposure to DON.
DON‐glucuronides contribute a major portion eventually up to 90% of total DON in urine with DON‐15‐glucuronide the predominant metabolite. The limited information on DOM‐1 indicated low levels compared to free DON and DON‐glucuronides.
Exposure estimates derived from the biomarker data from three European countries were of the same order of magnitude as the exposure estimates for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside derived from the occurrence data reported to EFSA and the dietary surveys from those countries.
Mode of action
DON, 3‐Ac‐DON and 15‐Ac‐DON bound to the ribosome peptidyl transferase centre, inhibiting protein synthesis. This binding also induces a ribotoxic stress and activates different MAPKs. Activation of MAPKs explains several effects of DON such as apoptosis/survival, inflammatory effect and oxidative stress.
Because of steric hindrance DON‐3‐glucoside could not bind to the ribosome and thus it did not activate MAPKs and induce inflammation.
Both pro‐inflammatory cytokines and satiety hormones have been proposed as major mediators of DON‐induced anorexia/emesis.
The anorectic and emetic potencies of DON‐3‐glucoside were lower than those of DON and underlying mechanisms are so far unknown.
Combined effects of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside with other mycotoxins
The database describing possible effects of combined exposure to DON and other mycotoxins was not sufficient for establishing the nature of combined effects. No in vivo studies on the combined effects of 3‐Ac‐DON, 15‐Ac‐DON or DON‐3‐glucoside with other mycotoxins were identified.
Comparative toxicity of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
Only few studies compared the toxicity of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in the same experiment.
DON, 3‐Ac‐DON and 15‐Ac‐DON were found to display similar toxicity in terms of anorectic and inflammatory effects, except for emetic capacity which was substantially lower for 3‐Ac‐DON compared to DON and 15‐Ac‐DON.
The potency of cytotoxicity in mammalian intestinal cells ranked in the order of 15‐Ac‐DON > DON > 3‐Ac‐DON and that was similar for barrier function, MAP‐Kinase activation and expression of tight junctions, and histological alterations.
When compared to DON, DON‐3‐glucoside was substantially less toxic both in vitro and in vivo.
Consideration for a combined risk assessment for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
The CONTAM Panel noted that 3‐Ac‐DON and 15‐Ac‐DON were largely deacetylated prior to systemic distribution and rapidly metabolised to DON. The available data indicated that DON‐3‐glucoside can be cleaved to DON in the gastrointestinal tract and distributed, metabolised and excreted as DON.
While the mode of action and the toxicity data for 3‐Ac‐DON and 15‐Ac‐DON indicated a similar toxicity as that of DON, toxicity data for DON‐3‐glucoside were limited and in vivo data on the chronic toxicity were missing. Therefore, the CONTAM Panel could not make a firm conclusion on the hazard of DON‐3‐glucoside.
Applying a conservative approach the CONTAM Panel assumed that (1) 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are all metabolised to DON and absorbed at the same extent as DON, (2) 3‐Ac‐DON and 15‐Ac‐DON induced the same acute and chronic adverse health effects as DON and (3) similar acute and chronic adverse health effects of DON‐3‐glucoside as of DON cannot be excluded.
The CONTAM Panel decided to characterise the hazard for the group of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside together, both for chronic and for acute adverse health effects in humans, and farm and companion animals.
Adverse effects and derivation of health based guidance values for risk characterisation in humans
The CONTAM Panel identified vomiting as critical acute effect for human risk assessment for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
Despite the limitations in the available human data, the CONTAM Panel decided to use these data, supported by the available human biomarker data, to establish a group acute reference dose (ARfD) for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
Based on the human outbreak data in China on gastrointestinal effects after ingestion of contaminated food and noting that vomiting occurred within 30 min after an eating occasion, the CONTAM Panel estimated a NOAEL for vomiting in humans of 26 μg DON/kg bw per a single eating occasion.
By applying a default uncertainty factor of 3.16 for toxicodynamic intra‐human variability a group ARfD of 8 μg/kg bw per eating occasion for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was established.
The CONTAM Panel concluded that the dose range back‐calculated from the human biomarker data supported the dose range obtained from the human outbreak data.
The CONTAM Panel identified reduced body weight gain in experimental animals as the most relevant critical chronic effect for human risk assessment.
A clear dose–response relationship between the exposure to DON and reduced mean body weight was observed both for female and male mice and the CONTAM Panel noted that the data from both sexes could be combined to calculate a reference point by using the BMD approach.
The CONTAM Panel calculated the BMDL05‐BMDU05 interval of 0.11–0.28 mg DON/kg bw per day and identified 0.11 mg DON/kg bw per day as a reference point for chronic human risk characterisation.
The CONTAM Panel established a group TDI of 1 μg/kg bw per day for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside by dividing the BMDL05 of 0.11 mg/kg bw per day for reduced body weight gain in mice by the default uncertainty factor of 100 for inter‐ and intraspecies variability.
Adverse effects and identification of reference points for risk characterisation in farm and companion animals
The CONTAM Panel identified vomiting as the critical acute adverse effect for pigs, farmed mink, dogs and cats.
The CONTAM Panel identified reduced feed intake, and reduced body weight and body weight gain as critical chronic adverse effects for farm and companion animals and identified the NOAELs/lowest‐observed‐adverse‐effect levels (LOAELs) for these effects as the reference points of DON in feed.
The concentration of 5.2 mg DON/kg feed did not generate adverse effects on body weight, feed intake or any other adverse effect in healthy dairy cows over 13 weeks and 5 mg DON/kg feed was identified as NOAEL for dairy cows.
NOAELs of 10 and 18 mg DON/kg feed were identified for healthy heifers and steers, respectively.
For sheep, a NOAEL of 16 mg DON/kg feed for reduced feed intake and body weight gain was identified.
In the absence of concentration–response data, the NOAEL of 5 mg DON/kg feed of dairy cows was adopted for goats.
The CONTAM Panel noted that young animals such as calves, in which the rumen is not fully developed and adult animals with previous history of ruminal acidosis, could have deficiency to de‐epoxidase DON to its less toxic metabolite DOM‐1 and consequently, could be more susceptible to toxic effects of DON.
In pigs, vomiting was observed as acute adverse effect at a wide range of concentrations of DON depending on the study design and study duration and the breeds used in the experiments. While the LOAELs for vomiting in pigs varied from 2.8 to 19.7 mg DON/kg feed the NOAELs varied from 1.6 to 11.9 mg DON/kg feed. The CONTAM Panel identified 1.6 DON/kg feed as an overall NOAEL for acute effects and noted that the lowest BMCL10 of 1.4 mg DON/kg feed was almost the same concentration.
Reduced feed intake and body weight gain identified as chronic effects in pigs ranged also over a wide concentration range of DON. The identified overall NOAEL was 0.7 mg DON/kg feed. The CONTAM Panel noted that for pigs the ranges of reported LOAELs and NOAELs overlapped substantially.
The concentration range of 4.6–7.0 mg DON/kg feed did not generate adverse effects such as reduced feed intake or body weight gain in broiler chickens and NOAELs of 4.6–7.0 mg DON/kg feed were identified. At 5.0 mg, DON/kg feed no decrease of feed intake or egg fertility was observed in laying hens. A NOAEL of 5.0 mg DON/kg feed was identified for broiler chickens and laying hens.
The concentration of 7.0 mg DON/kg feed did not induce changes in body weight, body weight gain, feed intake or feed conversion ratios in (fattening) ducks and turkeys and was used the overall NOAEL for these two poultry species.
Based on limited data, no reduced feed intake or any other signs of adverse effects were observed in horses at the concentrations of 36–44 mg DON/kg feed. The concentration of 36 mg DON/kg feed was identified as the overall NOAEL for horses.
Based on limited data, a concentration of 30 mg DON/kg feed was associated with maternal and fetal body weight reduction in farmed rabbits, while a concentration of 15 mg DON/kg feed did not induce any adverse effects in rabbit fetuses. A concentration of 4.3 mg DON/kg feed did not induce any effects on feed intake, body weight gain and relative organ weights and therefore the NOAEL for farmed rabbits was 4 mg DON/kg feed.
Limited data on farmed fish did not allow to identify specific NOAELs/LOAELs for each fish species. In carp, the concentration of 0.95 mg DON/kg feed induced lipid peroxidation and histopathological changes in the liver. In rainbow trout 0.8 mg DON/kg feed induced reduced feed intake, body weight gain, growth rate and feed efficiency. The lowest NOAEL was 0.6 mg DON/kg feed observed for carps and the CONTAM Panel decided to use this value as overall NOAEL for farmed fish.
Farmed mink were among the most sensitive animals based on a study designed as an experimental animal study. For vomiting, NOAELs of 10, 5 and 10 μg/kg bw per day were identified for DON, 3‐Ac‐DON and 15‐Ac‐DON, respectively. The CONTAM Panel calculated BMDL10 values of 0.004 mg DON/kg bw, 0.03 mg 3‐Ac‐DON/kg bw and 0.004 mg 15‐Ac‐DON/kg bw for vomiting as acute effect. For the reduced feed intake and body weight gain in farmed mink, the NOAEL of 1 mg DON/kg feed was identified for chronic effects.
In dogs, vomiting was not reported in the only one available study on dogs at the concentration of 6 mg DON/kg feed and it was identified as the NOAEL for acute effects, while reduced body weight gain in dogs was observed at this level. Applying the BMD approach, a lowest BMCL10 of 5 mg DON/kg feed was calculated for vomiting as reference point of acute effects. For chronic effects, the NOAEL of 4 mg DON/kg feed was identified for reduced body weight gain.
In cats, vomiting was not reported in the only one available study on cats at the concentration of 8 mg DON/kg feed and it was identified as the NOAEL for acute effects, while reduced body weight gain in cats was observed at this level. Applying the BMD approach, a lowest BMCL10 of 5 mg DON/kg feed was calculated for vomiting as reference point of acute effects. For chronic effects, the NOAEL of 6 mg DON/kg feed was identified for reduced body weight gain.
Human health risk characterisation
Based on the available occurrence data, the estimates of acute dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were below the group ARfD of 8 μg/kg bw per eating occasion for all age groups of humans, and considered not an acute health concern.
The estimates of the UB chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside based on the available occurrence data were above the group TDI of 1 μg/kg bw per day for the infants, toddlers and other children, and to some extent also for adolescents and adults. At the LB estimates, only high exposure in toddlers and other children exceeded the group TDI. Regular exceedance of TDI indicates a potential health concern, however, the CONTAM Panel noted the uncertainty associated with exposure estimates due to a high fraction of left‐censored data.
The limited data on dietary habits of vegetarians with data available for only five European countries, and with very few subjects in four of them, did not indicate notable differences in acute and chronic dietary exposure between the vegetarians and the general population. Therefore, the conclusions on the general population remain valid also for the subpopulation of vegetarians.
Farm and companion animal health risk characterisation
Based on the available occurrence data, the calculated UB mean and the 95th percentile dietary concentrations for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for ruminants and horses were clearly below the NOAELs for chronic adverse effects and were therefore considered unlikely to be a health concern. For poultry and farmed rabbits, the UB mean and 95th percentile dietary concentrations were higher but below the NOAELs indicating that the risk of chronic adverse health effects from the feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is low.
For pigs, the calculated UB 95th percentile dietary concentrations for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were lower than the calculated BMCL10 indicating that the risk of acute adverse health effects is low. The UB mean dietary concentrations were below the NOAEL for chronic adverse effects, but above the UB 95th percentile dietary concentrations. Therefore, a possible risk of chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was identified at the UB 95th percentile.
For farmed fish (salmonids and carp), the calculated UB mean dietary concentrations for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were below the NOAEL for chronic adverse effects but above at the UB 95th percentile dietary concentrations for carp. This indicates that a possible risk of chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was identified at the UB 95th percentile dietary concentrations at least for carp. However, the diet composition may majorly differ between the fish species and some fish species might be more tolerant for the adverse effects than others.
For farmed mink, the calculated UB 95th percentile dietary concentration for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was clearly above the BMDL10 for acute adverse health effects, and therefore a possible risk for acute adverse effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside at the UB 95th percentile dietary concentration was identified. The exposure estimates at the UB mean and UB 95th percentile concentrations in feed were below the NOAEL, and indicate that the risk of chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is low.
The calculated UB 95th percentile dietary concentration for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside for dogs was below the BMCL10 for acute adverse health effects and the UB mean and the UB 95th percentile dietary concentrations below the NOAEL for chronic adverse health effects. Therefore, the risk of acute and chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is low.
For cats, the calculated UB 95th percentile dietary concentration for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was above the BMCL10 for acute adverse health effects, and therefore a possible risk for acute adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside at the UB 95th percentile dietary concentration was identified. The UB mean and UB 95th percentile dietary concentrations were lower than the NOAEL for chronic adverse health effects indicating that the risk for chronic adverse health effects from feed containing DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside is low.
Recommendations
Interlaboratory validation and standardisation of LC–MS/MS methodology for the simultaneous quantification of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside are needed for more reliable conclusions on the analytical results and the methods should be sensitive enough to decrease the uncertainty of the risk assessment.
Certified reference materials (both matrix reference materials and calibrants) should be made available and proficiency tests should be facilitated for the determination of 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
Comprehensive studies are needed on co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in grains intended for human consumption, in food products, in grains intended for feed and in feed materials, where each sample is analysed for all these four forms.
Co‐occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside need to be monitored in food and feed, to acquire a knowledge on possible trends due to factors, such as changes in climate and weather, agronomic factors, breeding practices, storage systems and technological processing.
Interlaboratory validation and standardisation of the methods for the analysis of human urinary DON biomarkers, such as DON glucuronides, are needed before they can be reliably applied for human risk assessment. Certified reference materials (both matrix reference materials and calibrants) should be made available for DON glucuronides.
Well‐designed quantitative studies on DON urinary excretion in different population groups should be encouraged to enable the use of DON biomarkers for human exposure assessments.
Well‐designed studies that take account of practical feeding conditions are needed for farm animals, particularly for pigs, poultry, farmed fish and companion animals to study toxicokinetics and toxicity of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside to reduce the uncertainties in the animal risk assessment.
Modified forms of DON, other than those covered in this risk assessment, which could be potentially relevant concerning their (co‐)occurrence and toxicological properties, should be investigated to further refine the human and animal risk assessment.
Abbreviations
- ACE
accelerated solvent extraction
- AFRC
Agricultural and Food Research Council
- AFSSA
French Food Safety Authority/Agence Française de Sécurité des Aliments
- AIC
Akaike Information Criterion
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ANSES
French National Agency for Sanitary Safety of Food, Environment and Labor /Agence Nationale de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail
- AOAC
Association of Official Analytical Chemists
- APC
antigen‐presenting cell
- APCI
atmospheric pressure chemical ionisation
- AST
aspartate aminotransferase
- ATA
alimentary Toxic Aleukia
- BAM
Federal Institute for Materials Research and Testing
- Bax
Bcl‐2 associated X protein
- BE
Belgium
- BG
Bulgaria
- BMD
benchmark dose
- BMDL05
95 % lower confidence limit for the benchmark dose response of 5 %
- bw
body weight
- CAS
Chemical Abstracts Service
- CD
cytotoxicity dose
- CK
creatine kinase
- CONTAM Panel
Panel on Contaminants in the Food Chain
- CRM
certified reference materials
- CY
Cyprus
- CZ
Czech Republic
- DAD
diode‐array detector
- DART
direct analysis in real time
- DCM Unit
Dietary and Chemical Monitoring Unit
- DDGS
Distillers dried grains with solubles
- DE
Germany
- DK
Denmark
- DM
dry matter
- DMSO
dimethylsulfoxide
- DON
deoxynivalenol
- ECD
electron capture detection
- ELISA
enzyme‐linked immunosorbent assay
- ERK
extracellular signal regulated kinase
- ES
Spain
- ESI
electrospray ionization
- EVIRA
The Finnish Food Safety Authority
- EXPOCHI
Article 36 project ’Individual food consumption data and exposure assessment studies for children‘
- FAO/WHO
Food and Agriculture Organization of the United Nations/World Health Organization
- FAPAS
Food Analysis Performance Assessment Scheme
- FEDIAF
European Pet Food Industry Federation
- FEFAC
European Feed Manufacturers Federation
- FI
Finland
- FID
flame ionisation detection
- FLD
fluorescence detector
- fpg
Formamidopyrimidine DNA glycosylase
- FR
France
- FSH
follicle stimulating hormone
- GC
gas chromatography
- GEMS/Food
Global Environment Monitoring System ‐ Food Contamination Monitoring and Assessment Programme
- GMT
gamma glutamyltransferase
- GPX
glutathione peroxide
- GR
Greece
- GSH
glutathione
- HAT
hours after treatment
- HeLa
human cervical carcinoma cells
- HepG2
human hepatoma cells
- HLA‐DR
human leukocyte antigen‐antigen d related
- HPLC
high‐performance liquid chromatography
- HPLC‐FLD
high‐performance liquid chromatography coupled with fluorescence detection
- HPTLC
high‐performance thin layer chromatography
- HRMS
high‐resolution mass spectrometry
- HT‐2
HT‐2 toxin
- HU
Hungary
- IA
immunoaffinity
- IARC
International Agency for Research on Cancer
- IE
Ireland
- IFN‐beta
interferon‐beta
- IFN‐γ
interferon‐γ
- IgA
immunoglobulin A
- IgE
immunoglobulin E
- IGF
insulin‐like growth factor
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IL‐2
interleukin‐2
- IL‐8
interleukin‐8
- IL‐1β
interleukin‐1beta
- INRA
French National Institute for Agricultural Research/Institut national de la recherche agronomique, France
- i.p.
intraperitoneal
- IPCS
International Programme on Chemical Safety
- IT
Italy
- i.v.
intravenous
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- JRC
Joint Research Centre
- LB
lower bound
- LC
liquid chromatography
- LC–MS/MS
liquid chromatography coupled to tandem mass spectrometry
- LDH
lactate dehydrogenase
- LOAEL
lowest‐observed‐adverse‐effect level
- LOD
limit of detection
- LOQ
limit of quantification
- LPS
lipopolysaccharide
- LV
Latvia
- MAPK
mitogen‐activated protein kinase
- MIP
molecularly imprinted polymers
- ML
maximum level
- MPS
mononuclear phagocyte system
- MS
mass spectrometry
- MS/MS
tandem mass spectrometer
- MTT
Agrifood Research Finland
- N
Number of samples
- ND
Not detected
- NED
no‐effect dose
- NL
Netherlands
- NMR
nuclear magnetic resonance
- NO
nitric oxide
- NOAEL
no‐observed‐adverse‐effect level
- NOEL
no‐observed‐ effect level
- NRC
National Research Council
- NS
Not specified
- OTA
ochratoxin A
- P25
25th percentile
- P50
50th percentile
- P75
75th percentile
- P95
95th percentile
- PARP
poly‐adenosine diphosphate (ADP)‐ribose polymerase
- PCBs
polychlorinated biphenyls
- PGF2α
prosthaglandin F2 alpha
- PMTDI
provisional maximum tolerable daily intake
- RIVM
National Institute for Public Health and the Environment
- PT
prothrombin time
- RL
Reference Laboratory
- QuEChERS
Quick, easy, cheap, effective, rugged and safe
- RM
Reference material
- ROS
reactive oxygen species
- RSD
relative standard deviation
- RSDr
relative standard deviation under repeatability conditions
- RSDR
relative standard deviation under reproducibility conditions
- SCF
Scientific Committee on Food
- SCOOP
Scientific co‐operation
- SE
Sweden
- SPE
solid phase extraction
- SRM
Selected reaction monitoring
- SPE
solid phase extraction
- T‐2
T‐2 toxin
- TDI
tolerable daily intake
- tDON
total DON
- TLC
thin‐layer chromatography
- TOF‐MS
time‐of‐flight mass spectrometry
- TT
thrombin time
- t‐TDI
temporary tolerable daily intake
- TNF‐alpha
tumour necrosis factor‐alpha
- UB
upper bound
- UDP
uridine 5’‐diphospho‐glucuronosyltransferase
- UGT
UDP‐Glucuronosyltransferase
- UK
United Kingdom
- USSR
Union of Sovietic Socialist Republics
- UV
ultraviolet
- v/v
volume/volume
- WBC
white blood cells
- WBG
weight body gain
- WHO
World Health Organization
Appendix A – Identification and selection of evidence relevant for the risk assessment of deoxynivalenol, its acetylated and modified forms in food and feed
1.
For its risk assessment of deoxynivalenol, its acetylated and modified forms in food and feed, the CONTAM Panel applied the general principles of the risk assessment procedure as follows:
A human dietary exposure assessment for deoxynivalenol, its acetylated and modified forms in food was performed in order to compute the current level of exposure to deoxynivalenol (DON), 3‐acetylated‐DON (3‐Ac‐DON), 15‐acetylated‐DON (15‐Ac‐DON) and DON‐3‐glucoside in the population and to cover specific consumption habits (see Sections 4, 5 and 6). Dietary exposure assessment for DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was performed by computation of concentrations in indicative feeds for farm and companion animals used in Europe in order estimate their exposure (see Sections 4, 5 and 6). An external scientific report on ‘Experimental study of deoxynivalenol biomarkers in urine’ (GP/EFSA/CONTAM/2013/04 – Brera et al., 2015) on urinary biomarkers was used to support the human dietary exposure assessment and the human hazard characterisation (see Sections 7.5.2 and 7.10).
The potential health effects for humans and farm and companion animals caused by DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside were identified and characterised on the basis of the available scientific studies published in the open literature (hazard identification and characterisation (see Section 7). An external scientific report on ‘The in vivo genotoxicity studies on nivalenol and deoxynivalenol’ (GP/EFSA/CONTAM/2013/05 – Le Hégarat et al., 2014) was used to support the available genotoxicity data (see Section 7.2.9).
The risk for humans and farm and companion animals was characterised by comparing the reference point(s) identified with the exposure estimates to conclude on the likelihood of adverse effects (see Section 8).
The uncertainty analysis was performed by describing the identified uncertainties qualitatively and summarised in a summary table. The direction of the over and/or underestimation was given and the uncertainty for the overall assessment was concluded (see Section 9).
The principles presented in the following EFSA publications were applied in the different steps of the process:
EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Committee on a request from EFSA related to A Harmonised Approach for Risk Assessment of Substances Which are both Genotoxic and Carcinogenic. EFSA Journal 2005;3(10):282, 33 pp. https://doi.org/10.2903/j.efsa.2005.282
EFSA (European Food Safety Authority), 2006. Guidance of the Scientific Committee on a request from EFSA related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2006;4(12):438, 54 pp. https://doi.org/10.2903/j.efsa.2007.2438
EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: General principles. EFSA Journal 2009;7(5):1051, 22 pp. https://doi.org/10.2903/j.efsa.2009.1051
EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. https://doi.org/10.2903/j.efsa.2010.1457
EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. https://doi.org/10.2903/j.efsa.2010.1557
EFSA (European Food Safety Authority), 2011. Overview of the procedures currently used at EFSA for the assessment of dietary exposure to different chemical substances. EFSA Journal 2011;9(12):2490. [33 pp.] https://doi.org/10.2903/j.efsa.2011.2490
EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. https://doi.org/10.2903/j.efsa.2012.2534
EFSA Scientific Committee, 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579,32 pp. https://doi.org/10.2903/j.efsa.2012.2579
EFSA Scientific Committee, 2012b. Scientific Opinion on Risk Assessment Terminology. EFSA Journal 2012;10 (5):2664, 43 pp. https://doi.org/10.2903/j.efsa.2012.2664
EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. https://doi.org/10.2903/j.efsa.2017.4658
In addition, the following guidance was applied in the absence of the EFSA guidance:
OECD (Organisation for Economic Co‐operation and Development) (2010). Guidance for the derivation of an acute reference dose. OECD Environment, Health and Safety Publications, Series of Testing and Assessment, No. 124. IOMC Inter‐Organisation Programme for the Sound Management of Chemicals. Environment Directorate, Organisation for Economic Co‐operation and Development. Pars 2010. ENV/JM/MONO(2010)15. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2010)15&doclanguage=en
A.1. Hazard identification and characterisation
A.1.1. Identification of scientific evidence
For the present human risk assessment, the CONTAM Panel considered the outcome of the assessment of the SCF in 1999 and 2002 and the new evidence that has become available since then. For the present animal risk assessment, the CONTAM Panel considered the outcome of its risk assessment in 2004 and the new evidence that has become available since 2004. The most recent EFSA opinions and statements (EFSA CONTAM Panel, 2013, 2014), were also consulted, noting that they were not full risk assessments on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed.
A.1.1.1. The details on the identification of the evidence
Search in scientific databases aimed at identifying studies published in the open scientific literature and in scientific peer‐reviewed journals. The collection of scientific studies available in the public domain published since 2001 for humans and since 2004 for the farm and companion animals was done through searching the scientific literature database Web of Science. The approach taken for searching was selected in such a way that relevant studies were retrieved for the hazard identification and characterisation of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside. The literature searches were performed at different time points in May 2013, June 2014, October 2014, April 2015, October 2015, August 2016 and September 2016. The key words reported in Table A.1 were used and Boolean operators (AND) were used to connect the terms in the searches:
Table A.1.
Key words used in the literature search
| Key words used | Key words used |
|---|---|
| Deoxynivalenol AND tox* | 3‐Acetyl‐deoxynivalenol |
| Deoxynivalenol AND acute tox* | 15‐Acetyl‐deoxynivalenol |
| Deoxynivalenol AND chronic tox* | Deoxynivalenol AND masked |
| Deoxynivalenol AND cytotox* | Deoxynivalenol AND deoxynivalenol‐3‐glucoside |
| Deoxynivalenol AND development* AND tox* | Deoxynivalenol‐3‐glucoside |
| Deoxynivalenol AND reprod* | Deoxynivalenol AND DOM‐1 |
| Deoxynivalenol AND genotox* AND carcino* | De‐epoxy‐deoxynivalenol |
| Deoxynivalenol AND toxicokinetics | DONS‐1 |
| Deoxynivalenol AND metabolism AND absorption AND distribution | DONS‐2 |
| Deoxynivalenol AND dose response | DONS‐3 |
| Deoxynivalenol AND mode of action | DOM‐1 |
| Deoxynivalenol AND immunotox* | De‐epoxy‐deoxynivalenol |
The search was performed to cover the period since 2001 for humans and since 2004 for the farm and companion animals till the cut‐off date of 31 July 2015 and later extended till 31 July 2016 decided by the CONTAM Panel. In order to update the database for specific questions arising during the discussion, separate searches were conducted when needed. After the cut‐off date, the publication of new papers from the major journals expected to publish articles on mycotoxins was monitored from their email alerts for content of the issue till the end of 2016 in order to verify if new relevant papers, which would change the outcome of the risk assessment were published but no new search was conducted.
As background information, the CONTAM Panel considered the earlier scientific evaluations of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed conducted by other national risk assessment bodies, and other national and international independent expert advisory committees than SCF and EFSA (see Section 1.1).
A.1.2. Study selection process and its results
The obtained literature database for the this opinion, which was retrieved as outlined above, included scientific peer‐reviewed papers published in scientific journals and relevant non‐peer reviewed papers (such as risk assessment reports by national/international bodies and book chapters).
The following criteria were considered for the inclusion of studies in the selection process:
Experimental toxicity studies in vitro (including biological models) and in vivo.
Experimental toxicity studies in laboratory animals (oral route), epidemiological studies in humans addressing associations between exposure to DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in humans and adverse health outcome(s) in the general population (via the diet).
Experimental toxicity studies on farm and companion animals (oral route), addressing associations between exposure to DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in farm and companion animals and adverse health outcome(s).
The following criteria were considered for the exclusion of studies in the selection process:
Studies in the field of ecotoxicology, i.e. not in the field of laboratory animals and human health, and the health of farm and companion animals.
In addition, the following criteria were used for the inclusion/exclusion of scientific papers and reports in the selection process:
Only studies in English and studies in other languages including an abstract in English were considered. These papers with other languages than English were submitted for the translation to the Translation Centre of the Bodies of the European Union in Luxemburg and the received translation was used in this opinion. The translation of the paper is indicated as footnote in this opinion.
Conference proceedings were not considered.
Reviews were considered as source of background information and as an additional source of scientific evidence unless otherwise stated in the body of the text. These were not considered as the pivotal information for the hazard characterisation.
Book chapters were considered as sources of background information. These were not considered as the pivotal information for the hazard characterisation.
A.1.3. Result of the study selection process
The search strategy identified nearly 1,200 studies published in the open literature relevant for the hazard identification and characterisation on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in humans and in the farm and companion animals. The searched literature was grouped as presented in Table A.2 according to topics of interest. The table includes the numbers of papers of which relevance was assessed by the CONTAM Panel working group members (some duplicates may have been included). The selection of the studies for inclusion or non‐inclusion in the hazard identification and characterisation of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside was based on consideration of the extent to which the study was relevant to the assessment and general study quality considerations (e.g. sufficient details on the methodology, performance or outcome of the studies, route of administration, clear dose–response relationship, statistical description of the results EFSA, 2009c), irrespective of whether they yielded positive, negative or null results. The exclusion criteria of the studies are further detailed in the beginning of the sections (see e.g. Sections 7.3.1 and 7.3.2).
Table A.2.
Number of papers retrieved from the database for hazard identification and characterisation and which relevance was assessed for the opinion within different classification group
| Humans ‐ toxicity | n | Farm animals – acute and chronic toxicity and toxicokinetics | n |
|---|---|---|---|
| Toxicokinetics – experimental animals and animals | 57 | Pigs | 151 |
| Acute toxicity – experimental animals | 24 | Poultry | 117 |
| Chronic toxicity – experimental animals | 35 | Ruminants | 52 |
| Genotoxicity and carcinogenicity | 54 | Fish | 30 |
| Reproductive and developmental toxicity | 38 | Horses and rabbits | 10 |
| Neurotoxicity, hepatotoxicity | 16 | Dogs and cats | 8 |
| Cytotoxicity and immunotoxicity – in vivo studies – Rodents | 65 | Minks and ferrets | 17 |
| Cytotoxicity and immunotoxicity – in vivo studies – Farm animals | 47 | Transfer rate | 22 |
| Cytotoxicity and immunotoxicity – in vitro studies | 176 | ||
| Mode of action | 152 | Toxicity assessment – toxicity reviews in humans, experimental and farm animals | 60 |
| Epidemiology | 28 | ||
| Biomarkers | 69 | ||
| Gastrointestinal toxicity | 8 | Sum of all papers | 1,236 |
n: number of papers archived at EFSA, and reviewed and assessed for the opinion.
The application of the selection process described above resulted in the studies cited in this opinion.
A.2. Exposure assessment for humans and farm and companion animals
A.2.1. Identification of evidence
The following sources of evidence were used:
The occurrence data on food and feed collected by the Member States/European Countries submitted in the framework of the EFSA continuous call for data.48
The EFSA Comprehensive European Food Consumption Database as a source of information on food consumption across the EU.49
An external scientific report on ‘Experimental study of deoxynivalenol biomarkers in urine” (GP/EFSA/CONTAM/2013/04 –; Brera et al., 2015) (to support the exposure assessment).50
The catalogue of feed materials specified in the Commission Regulation (EU) No 575/2011 of 16 June 2011 creating the Catalogue of feed materials.51
In the absence of EFSA feed consumption database, external databases and the literature on the public domain were used as outlined in the opinion.
A.2.2. Data selection process and its results
The consideration of the selection criteria for occurrence and consumption data for the dietary exposure assessment of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside and the results thereafter are described in Sections 4, 5 and 6.
A.3. Other sections of the draft scientific opinion
The sections on Introduction (Section 1 and subsections therein), Legislation (Section 2), Sampling and methods of analysis (Section 3 and subsections therein), Previously reported literature data on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed (Section 4.1) and previously reported human exposure assessment (Section 6.3 and subsections therein) provide generic background information to support the overall conclusions. The identification of evidence for these chapters was limited to the most relevant information and the following sources of evidence were used:
Previous scientific evaluations by national agencies, national and international independent expert advisory committees.
Search in scientific databases (Web of Science) aimed at identifying studies and reviews that have appeared in the open scientific literature and published in scientific peer‐reviewed journals.
Respective legislation on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
The selection of the studies for inclusion in the aforementioned sections to give a comprehensive but not exhaustive background information on DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in food and feed was therefore based on consideration of the extent to which the study was informative and relevant to the assessment, and of general quality of the study. For these other sections, approximately 1,000 papers were archived at EFSA.
Appendix B – Occurrence data on food and feed reported in the literature
1.
Appendix C – Current occurrence of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in feeds
1.
The concentrations of DON alone and the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside (μg/kg) across feed groups as used in the animal exposure assessment are presented in Tables C.1 and C.1, respectively. Although data were available for other feeds, they were either poorly described (e.g. ‘grains as crops’) or the number of samples were insufficient (n < 10) to provide reliable estimates (EFSA, 2011b). For cereal grains, the data for individual grains specified for feed (e.g. wheat, barley etc.) have been combined with those for those unprocessed grains of undefined end‐use to increase the number of samples per cereal type.
Table C.1.
Concentrations (μg/kg) of DON across feed groups
| Feed group | Concentration (μg/kg)b | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | %LCc | Mean | 75th percentile | 95th percentile | Maximum | ||||
| LB | UB | LB | UB | LB | UB | UB | |||
| Feed materials | |||||||||
| Barley | 525 | 48.7 | 346 | 387 | 219 | 250 | 2,301 | 2,301 | 8,978 |
| Maize grain | 457 | 42.0 | 580 | 627 | 455 | 455 | 2,300 | 2,300 | 15,830 |
| Oats | 187 | 48.1 | 379 | 408 | 240 | 250 | 1,907 | 1,907 | 6,992 |
| Oat feed | 29 | 20.7 | 234 | 237 | 290 | 290 | 599 | 599 | 1,313 |
| Wheat | 659 | 37.3 | 410 | 436 | 425 | 429 | 1,650 | 1,650 | 14,505 |
| Wheat feed | 74 | 55.4 | 351 | 376 | 506 | 506 | 1,550 | 1,550 | 2,640 |
| Rape seed meal | 10 | 60.0 | 26 | 146 | 50 | 199 | 80 | 199 | 199 |
| Sunflower seed meal | 11 | 100 | 0 | 128 | 0 | 199 | 0 | 199 | 199 |
| Soya bean meal | 111 | 96.4 | 5 | 190 | 0 | 200 | 0 | 200 | 220 |
| Pressed sugar beet pulp | 10 | 76.4 | 108 | 250 | 250 | 250 | 549 | 549 | 549 |
| Forages and roughage, and products derived thereof | |||||||||
| Cereals straw | 124 | 44.4 | 1076 | 1130 | 771 | 771 | 3,373 | 3,373 | 47,218 |
| Grass, field dried, hay | 93 | 81.7 | 9 | 104 | 0 | 138 | 50 | 138 | 138 |
| Maize silage | 129 | 14.7 | 410 | 423 | 469 | 469 | 1,340 | 1,340 | 4,300 |
| Complete (compound)/complementary feedingstuffs | |||||||||
| Dairy cows | 96 | 56.4 | 131 | 159 | 131 | 135 | 792 | 792 | 1,300 |
| Fattening cattle | 42 | 54.8 | 261 | 318 | 140 | 250 | 500 | 500 | 7,350 |
| Horses | 15 | 55.2 | 183 | 189 | 236 | 236 | 874 | 874 | 874 |
| Weaning pigs | 193 | 61.1 | 149 | 238 | 232 | 250 | 589 | 660 | 2,720 |
| Growing/fattening pigs | 229 | 27.3 | 266 | 311 | 370 | 370 | 779 | 779 | 2,620 |
| Breeding pigs (sows) | 185 | 27.0 | 314 | 369 | 435 | 440 | 790 | 790 | 3,970 |
| Laying hens | 89 | 29.2 | 482 | 494 | 696 | 696 | 2,050 | 2,050 | 2,910 |
| Fattening chickens | 496 | 19.2 | 617 | 627 | 969 | 969 | 2,000 | 2,000 | 5,200 |
| Fattening ducks | 97 | 23.7 | 624 | 633 | 1,070 | 1,070 | 1,760 | 1,760 | 2,340 |
| Fattening turkeys | 101 | 20.8 | 898 | 907 | 1,430 | 1,430 | 2,410 | 2,410 | 3,440 |
DON: deoxynivalenol; N: number of samples; LC: left‐censored data (values below the limit of detection or limit of quantification); LB: lower bound; UB: upper bound.
If N < 60 then the calculated 95th percentile concentration should be considered as an indicative value only due to the limited number of data (EFSA, 2011b).
Concentration reported as μg/kg 88% dry matter.
Value represent the left‐censoring limit.
Appendix D – Dietary surveys considered for the acute and chronic human dietary exposure assessment
1.
Appendix E – Intake and composition of diets used in estimating animal exposure to DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
1.
To estimate the farm and companion animal exposure to DON alone and to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, information is required on both the amount of feed consumed and the concentration of these compounds in the feed. This Appendix E gives details of the feed consumption and live weights (Section E.1) and assumed diet compositions (Section E.2) for different farm and companion animals used to estimate exposures. These are based on published guidelines on nutrition and feeding (Carabano and Piquer, 1998; NRC, 2007a, 2007b; Leeson and Summers, 2008; OECD, 2009; McDonald et al., 2011; EFSA FEEDAP Panel, 2012) and information provided by European feed manufacturers. Given the wide range of livestock and feeding systems in Europe, it has not been possible to estimate exposures that encompass all production systems. They are therefore estimates of the Panel on Contaminants in the Food Chain (CONTAM Panel), but agree with common practice. Based on these estimates of intake, the mean and 95th percentile concentrations of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside at the lower bound (LB) and upper bound (UB) in the species‐specific compound feed or in the estimated diets for the farm animal and companion animals, the dietary concentrations and exposures have been calculated, and are given in this Appendix E and in Section 6.3. In Appendix F, Section F.4 the estimated dietary concentrations of DON alone and exposures to DON alone in feed, calculated in the similar manner as for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside, for farm and companion animals are presented.
E.1. Feed intakes
E.1.1. Cattle, sheep, goats and horses
Dairy cows
Feed intakes by lactating dairy cows vary, and influenced principally by the quantity and quality of forages and other feeds available, the milk yield and the size of the cow. Exposures to DON alone and the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside have been estimated for a 650‐kg dairy cow, with a milk yield of 40 kg/day. Where cows are fed a commercially manufactured complementary feed it is assumed that this is fed at the rate of 0.3 kg/kg of milk produced (Nix, 2010), and supplemented as with forage. Assumptions on the amounts of forages and non‐forage feed are given in Appendix E, Table E.1. A second scenario is also considered in which lactating dairy cows are fed a diet based on maize silage and supplemented with maize grain and soybean meal (Appendix E, Table E.2).
Beef cattle
There are a wide variety of beef production and husbandry systems in Europe. They may be categorised broadly as forage‐based or cereal‐based systems, although combinations of these systems are commonly found. In this opinion, several feeding systems have been considered for cattle 300 or 400 kg, and feed intakes of between 6.6 and 10 kg dry matter/day, based on guidelines published by EBLEX (2008, 2012). Details are given in Appendix E, Table E.1.
Sheep and goats
Many breeds and systems of management have been developed for sheep and goats to suit the land, climate and husbandry conditions in Europe. As for other ruminants, forages may be the only feeds used after weaning (NRC, 2007a). Common exceptions to this are pregnant and lactating animals, whose feed is usually supplemented with non‐forage feeds or commercially manufactured compound (complementary) feeds. In this opinion, exposure estimates have been made for lactating sheep and goats, and for fattening goats. Details of live weights and feed intakes assumed, and the proportion of the diet from forages, are given in Appendix E, Table E.1.
Horses
Horses are non‐ruminant herbivores. They generally consume 2–3.5% of their body weight in feed (dry matter) each day, of which a minimum of 50% should be as forage (pasture or hay) (NRC, 2007b). The CONTAM Panel has estimated exposure for a 450‐kg horse, with a daily intake of 9 kg dry matter/day (Appendix E, Table E.1).
E.1.2. Pigs, poultry and farmed fish
Pigs
Although there is a considerable range of pig production systems in Europe, exposure estimates have been made for growing pigs (20‐kg body weight), fattening pigs (100‐kg body weight) and lactating sows (200‐kg body weight) using feed consumption proposed by EFSA FEEDAP Panel (2012) (Appendix E, Table E.2).
Poultry
The CONTAM Panel applied the live weights and feed intakes reported for fattening chickens (broilers), laying hens and turkeys proposed by EFSA FEEDAP Panel (2012) and for ducks by Leeson and Summers (2008) (Appendix E, Table E.2).
Farmed fish (salmonids and carp)
Commercially reared species include Atlantic salmon, rainbow trout, sea bass, sea bream, cod, halibut, tuna, eel and turbot. In this opinion, exposures to DON alone and the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside have been made for farmed salmon and carp. Details of the body weights and feed intakes used are given in Appendix E, Table E.2.
E.1.3. Farmed rabbits
Feed consumptions of 65–80 g/kg bw per day have been reported for farmed rabbits (Carabano and Piquer, 1998). For the exposure estimates, the CONTAM Panel have assumed a live weight of 2 kg, and a daily feed consumption of 75 g/kg bw
E.1.4. Companion animals (dogs and cats)
The amount of food consumed is largely a function of the mature weight of the animal, level of activity, physiological status (e.g. pregnancy or lactation) and the energy content of the diet. In this opinion, the CONTAM Panel assumed body weights (kg) and feed intakes (g dry matter/day) for dogs and cats of 25 kg/360 g dry matter per day and 4 kg/60 g dry matter per day, respectively (derived by the CONTAM Panel from NRC, 2006).
E.1.5. Farmed mink
For estimating exposure, the CONTAM Panel have assumed a live weight of 2.07 kg for a male mink at pelting, and with a feed intake of 227 g/day (75 g dry matter) (NRC, 1982).
E.2. Diet composition and dietary concentration and exposure estimates for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside
Many livestock in the European countries are fed proprietary commercial compound feeds formulated to meet the nutritional needs of the animals for which they are intended. Where sufficient data have been provided on the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in species‐specific compound feeds (see Appendix C, Table C.2), estimates of dietary concentrations and exposures have been made. Where data on proprietary compound feeds were not available, or were available but in insufficient numbers, estimates of dietary concentrations and exposure have been made using dietary inclusion rates of feed materials given in this section. The concentrations for the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in these feed materials are provided in Appendix C, Table C.2). The estimated dietary concentrations and exposures for the different farm and companion animals are presented in Section 6.3.1.
E.2.1. Cattle, sheep, goats and horses
For most ruminants and horses, forages (either fresh or conserved) constitute the largest fraction of their diet, but they are normally supplemented with non‐forage feeds such as cereals, cereal by‐products, oilseed meals and by‐products of human food production. These may be fed either as individual feeds, mixtures of feed materials or as species‐specific complementary feeds in the form of compound feeds. In some situations, however, forages may represent the total diet.
Fresh (grazed) grass or grass silage are the principal forages for many ruminants and horses in Europe. As reported elsewhere in this opinion (Section 5.2) DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside have not been widely reported in these feeds, and therefore, it has been assumed that where they are fed they make no contribution to exposure. Where grass has been conserved as hay, the presence of DON has been widely reported. Therefore, estimates of dietary concentrations and exposures have been reported for lactating dairy cows, fattening beef cattle and horses in which species‐specific compound feeds are supplemented with (a) fresh grass or grass silage or (b) grass hay.
For other forages, particularly maize silage and cereal straw, DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside have been widely reported. Therefore, dietary concentrations and exposures have been estimated for lactating dairy cows and fattening beef cattle where these represent a significant part of the diet. AFSSA (2009) have provided example consumption of dairy cows fed maize silage supplemented with maize grain and soybean meal, while example diets of beef cattle on maize silage or cereal straw‐based diets are taken from EBLEX (2008, 2012), and these are given in Appendix E, Table E.3.
While forages represent the main or in some case the only feed for ruminants, some beef cattle are reared on diets consisting predominantly of cereals to achieve higher growth rates. Therefore, estimates of dietary concentrations and exposures have also been made for ‘cereal beef’ cattle (intensively reared beef cattle on cereal‐based diet). The assumed ration is given in Appendix E, Table E.3.
For lactating sheep, milking goats and fattening goats, no information on levels of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in species‐specific compound feed were available, and therefore, example diets have been used to estimate dietary concentrations and exposures (Appendix E, Table E.4).
E.2.2. Pigs and poultry
Sufficient data for species‐specific compound feeds for pigs, and for most categories of poultry (fattening chickens, ducks and turkeys, and for laying hens), were provided (see Appendix C, Table C.2) and these were used to estimate dietary concentrations and exposures of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
E.2.3. Rabbits
Rabbits are usually fed a pelleted diet (in the form of complete feedingstuffs) consisting of dried forages, cereals and vegetable proteins supplemented with minerals, vitamins and trace elements. Lebas and Renouf (2009) reviewed diet formulations used in experimental studies: in 58 diets, cereals and cereal by‐products (mostly wheat bran) accounted for up to 40% of all ingredients. In these studies, maize was a major cereal grain and was included in more than one‐third of all diets. In northern Europe, however, maize may be replaced by barley and wheat. In this opinion, the feed ingredients used in a typical French commercial rabbit compound, as provided by T. Gidenne, (Personal communication, 2011) have been used, details of which are given in Appendix E, Table E.5.
E.2.4. Farmed fish (salmonids and carp)
Traditionally, the principal raw materials used for the manufacture of fish feeds in Europe have been fishmeal and fish oils, and although alternative sources of oil and protein (e.g. soybean meals and vegetable oils) are increasingly being used fish‐derived feeds still remain the major ingredients.
For many fish species, digestion of complex carbohydrates and the metabolic utilisation of the absorbed glucose is low, reflecting the scarcity of carbohydrates in the aquatic environment (Guillaume et al., 2001). Instead, fish obtain much of their energy from protein in the diet. Where carbohydrates are used, they generally require some form of pretreatment (e.g. cooking, flaking or toasting).
Berntssen et al. (2010) provided details of the composition of a diet for growing salmonids, and the CONTAM Panel used this feed formulation to estimate the dietary concentrations and exposures (Appendix E, Table E.5).
In contrast, studies with the common carp (Cyprinus carpio) have demonstrated greater intestinal amylase activity than in carnivorous fish, which accounts for the better utilisation of carbohydrates by these fish. The optimum level of carbohydrates appears to be 30–40% (Food and Agriculture Organization of the United Nations (FAO), Aquaculture Feed and Fertiliser Resources Information System52), which allows for higher levels of cereals than in diets for salmonids. The CONTAM Panel used the ingredients of commercial compound feeds for carp reported by Schultz et al. (2012) to estimate dietary concentrations and exposures of the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside.
E.2.5. Farmed mink
Mink are carnivorous animals and are fed high protein diets consisting mainly of meat and meat by‐products. Commercially manufactured mink feed consists largely of fish and land animal by‐products, with lesser amounts of cereals and cereal by‐products, and supplemented with mineral/vitamin premixtures. Mink are fed diets high in protein, although their nutritional requirements vary according to the animal's physiological stage (e.g. gestating, lactating and growing) and climatic conditions, particularly temperature. The proportions of cereal grains, their products and by‐products used in estimating the dietary concentrations and exposures are given in Appendix E, Table E.5.
E.2.6. Companion animals (dogs and cats)
Most small companion animals derive their nutritional needs from processed food, and in 2010 EU annual sales of pet food products was approximately 8.3 million tonnes.53 Although a wide range of ingredients is used in commercial diets, most dog and cat diets contain at least some animal protein. Other ingredients include cereals (predominantly wheat, rice or maize), cereal by‐products, vegetable proteins and by‐products of human food production. The ingredients will vary depending both on the availability of feed materials and on the nutrient requirements of the animals.
The European Pet Food Industry Federation (FEDIAF) has provided information on typical inclusion levels of cereals, cereal by‐products and other feed materials in dry cat and dog food.54 In the absence of sufficient data on species‐specific manufactured complete feedingstuffs, the CONTAM Panel has used example diets based on information provided by FEDIAF (details given in Appendix E, Table E.5).
Appendix F – Current chronic exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across the different surveys, and current estimates of exposures to DON for humans and animals
F.1. Current chronic human exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across the different dietary surveys
Table F.1.
Chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside in μg/kg bw per day across the different dietary surveys
| Age class | Country | Survey | Number of subjects | Mean | 95th percentile | ||||
|---|---|---|---|---|---|---|---|---|---|
| Infants | LB | MB | UB | LB | MB | UB | |||
| Bulgaria | NUTRICHILD | 859 | 0.3 | 0.6 | 0.9 | 1.2 | 1.7 | 2.4 | |
| Denmark | IAT 2006_07 | 826 | 0.5 | 0.8 | 1.2 | 1.2 | 1.7 | 2.2 | |
| Finland | DIPP_2001_2009 | 500 | 0.3 | 0.5 | 0.6 | 0.7 | 1.0 | 1.4 | |
| Germany | VELS | 159 | 0.5 | 1.3 | 2.0 | 1.5 | 2.6 | 3.7 | |
| Italy | INRAN_SCAI_2005_06 | 16 | 0.2 | 0.5 | 0.8 | – | – | – | |
| United Kingdom | DNSIYC_2011 | 1,369 | 0.4 | 0.7 | 1.1 | 1.0 | 1.6 | 2.2 | |
| Toddlers | |||||||||
| Belgium | Regional_Flanders | 36 | 1.0 | 1.3 | 1.6 | – | – | – | |
| Bulgaria | NUTRICHILD | 428 | 1.1 | 1.4 | 1.7 | 1.7 | 2.1 | 2.6 | |
| Denmark | IAT 2006_07 | 917 | 0.9 | 1.3 | 1.6 | 1.4 | 1.9 | 2.4 | |
| Finland | DIPP_2001_2009 | 500 | 0.6 | 0.9 | 1.1 | 1.1 | 1.4 | 1.8 | |
| Germany | VELS | 348 | 0.9 | 1.2 | 1.6 | 1.7 | 2.1 | 2.7 | |
| Italy | INRAN_SCAI_2005_06 | 36 | 0.9 | 1.3 | 1.7 | – | – | – | |
| Netherlands | VCP_kids | 322 | 0.8 | 1.1 | 1.4 | 1.5 | 1.9 | 2.4 | |
| Spain | enKid | 17 | 0.6 | 0.9 | 1.1 | – | – | – | |
| United Kingdom | DNSIYC_2011 | 1,314 | 0.8 | 1.2 | 1.6 | 1.5 | 2.1 | 2.7 | |
| United Kingdom | NDNS‐Rolling Programme Years 1‐3 | 185 | 0.8 | 1.2 | 1.6 | 1.5 | 2.0 | 2.7 | |
| Other children | |||||||||
| Austria | ASNS_Children | 128 | 0.9 | 1.1 | 1.4 | 1.6 | 2.0 | 2.4 | |
| Belgium | Regional_Flanders | 625 | 0.8 | 1.1 | 1.3 | 1.3 | 1.7 | 2.2 | |
| Bulgaria | NUTRICHILD | 433 | 1.1 | 1.3 | 1.6 | 1.9 | 2.3 | 2.7 | |
| Czech Republic | SISP04 | 389 | 0.8 | 1.0 | 1.2 | 1.5 | 1.9 | 2.3 | |
| Denmark | DANSDA 2005‐08 | 298 | 0.8 | 1.0 | 1.2 | 1.2 | 1.5 | 1.9 | |
| Finland | DIPP_2001_2009 | 750 | 0.6 | 0.7 | 0.9 | 0.9 | 1.1 | 1.4 | |
| France | INCA2 | 482 | 0.7 | 0.9 | 1.1 | 1.2 | 1.5 | 1.7 | |
| Germany | VELS | 293 | 0.9 | 1.1 | 1.4 | 1.5 | 1.8 | 2.2 | |
| Germany | EsKiMo | 835 | 0.8 | 1.0 | 1.2 | 1.4 | 1.7 | 2.0 | |
| Greece | Regional_Crete | 838 | 0.7 | 0.9 | 1.2 | 1.2 | 1.6 | 2.0 | |
| Italy | INRAN_SCAI_2005_06 | 193 | 0.9 | 1.2 | 1.4 | 1.7 | 2.1 | 2.6 | |
| Latvia | EFSA_TEST | 187 | 0.6 | 0.8 | 1.0 | 1.4 | 1.7 | 2.0 | |
| Netherlands | VCPBasis_AVL2007_2010 | 447 | 0.7 | 0.8 | 1.0 | 1.2 | 1.4 | 1.7 | |
| Netherlands | VCP_kids | 957 | 0.7 | 0.9 | 1.2 | 1.3 | 1.6 | 1.9 | |
| Spain | NUT_INK05 | 399 | 0.7 | 0.9 | 1.1 | 1.1 | 1.5 | 1.8 | |
| Spain | enKid | 156 | 0.7 | 0.8 | 1.0 | 1.3 | 1.5 | 1.8 | |
| Sweden | NFA | 1,473 | 0.7 | 0.9 | 1.2 | 1.1 | 1.5 | 2.0 | |
| United Kingdom | NDNS‐Rolling Programme Years 1‐3 | 651 | 0.8 | 1.0 | 1.3 | 1.3 | 1.7 | 2.2 | |
| Adolescents | |||||||||
| Austria | ASNS_Children | 237 | 0.5 | 0.7 | 0.8 | 1.0 | 1.3 | 1.5 | |
| Belgium | Diet_National_2004 | 576 | 0.4 | 0.6 | 0.7 | 0.8 | 1.1 | 1.3 | |
| Cyprus | Childhealth | 303 | 0.4 | 0.5 | 0.6 | 0.7 | 0.9 | 1.1 | |
| Czech Republic | SISP04 | 298 | 0.6 | 0.8 | 0.9 | 1.2 | 1.5 | 1.8 | |
| Denmark | DANSDA 2005‐08 | 377 | 0.4 | 0.5 | 0.6 | 0.7 | 0.9 | 1.1 | |
| Finland | NWSSP07_08 | 306 | 0.3 | 0.4 | 0.4 | 0.5 | 0.6 | 0.7 | |
| France | INCA2 | 973 | 0.4 | 0.5 | 0.6 | 0.9 | 1.0 | 1.2 | |
| Germany | EsKiMo | 393 | 0.6 | 0.8 | 0.9 | 1.1 | 1.3 | 1.6 | |
| Germany | National_Nutrition_Survey_II | 1,011 | 0.4 | 0.5 | 0.6 | 0.7 | 1.0 | 1.2 | |
| Italy | INRAN_SCAI_2005_06 | 247 | 0.6 | 0.7 | 0.8 | 1.1 | 1.3 | 1.5 | |
| Latvia | EFSA_TEST | 453 | 0.5 | 0.6 | 0.8 | 1.0 | 1.2 | 1.5 | |
| Netherlands | VCPBasis_AVL2007_2010 | 1,142 | 0.5 | 0.6 | 0.7 | 0.9 | 1.1 | 1.2 | |
| Spain | AESAN_FIAB | 86 | 0.3 | 0.4 | 0.5 | 0.8 | 0.9 | 1.1 | |
| Spain | enKid | 209 | 0.5 | 0.6 | 0.7 | 1.0 | 1.3 | 1.5 | |
| Spain | NUT_INK05 | 651 | 0.5 | 0.6 | 0.7 | 0.9 | 1.0 | 1.3 | |
| Sweden | NFA | 1,018 | 0.4 | 0.6 | 0.7 | 0.8 | 1.0 | 1.2 | |
| United Kingdom | NDNS‐Rolling Programme Years 1‐3 | 666 | 0.4 | 0.5 | 0.7 | 0.8 | 1.0 | 1.2 | |
| Adults | |||||||||
| Austria | ASNS_Adults | 308 | 0.4 | 0.5 | 0.7 | 0.8 | 1.0 | 1.4 | |
| Belgium | Diet_National_2004 | 1,292 | 0.4 | 0.5 | 0.6 | 0.7 | 0.9 | 1.1 | |
| Czech Republic | SISP04 | 1,666 | 0.4 | 0.5 | 0.7 | 0.7 | 1.0 | 1.4 | |
| Denmark | DANSDA 2005‐08 | 1,739 | 0.3 | 0.4 | 0.5 | 0.5 | 0.7 | 0.9 | |
| Finland | FINDIET2012 | 1,295 | 0.4 | 0.4 | 0.5 | 0.7 | 0.9 | 1.1 | |
| France | INCA2 | 2,276 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | |
| Germany | National_Nutrition_Survey_II | 10,419 | 0.3 | 0.4 | 0.5 | 0.6 | 0.8 | 1.0 | |
| Greece | DIET LACTATION GR | 65 | 0.3 | 0.4 | 0.6 | 0.6 | 0.8 | 1.1 | |
| Hungary | National_Repr_Surv | 1,074 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | |
| Ireland | NANS_2012 | 1,274 | 0.3 | 0.5 | 0.6 | 0.6 | 0.8 | 1.1 | |
| Italy | INRAN_SCAI_2005_06 | 2,313 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 1.0 | |
| Latvia | EFSA_TEST | 1,271 | 0.3 | 0.4 | 0.5 | 0.7 | 0.9 | 1.1 | |
| Latvia | FC_PREGNANTWOMEN_2011 | 1,002 | 0.3 | 0.5 | 0.6 | 0.6 | 0.8 | 1.1 | |
| Netherlands | VCPBasis_AVL2007_2010 | 2,057 | 0.3 | 0.4 | 0.5 | 0.6 | 0.8 | 1.0 | |
| Romania | Dieta_Pilot_Adults | 1,254 | 0.3 | 0.4 | 0.4 | 0.5 | 0.7 | 0.9 | |
| Spain | AESAN_FIAB | 981 | 0.3 | 0.3 | 0.4 | 0.5 | 0.7 | 0.9 | |
| Spain | AESAN | 410 | 0.3 | 0.4 | 0.5 | 0.5 | 0.7 | 0.9 | |
| Sweden | Riksmaten 2010 | 1,430 | 0.3 | 0.4 | 0.5 | 0.5 | 0.7 | 0.9 | |
| United Kingdom | NDNS‐Rolling Programme Years 1‐3 | 1,266 | 0.3 | 0.4 | 0.5 | 0.5 | 0.7 | 1.0 | |
| Elderly | |||||||||
| Austria | ASNS_Adults | 67 | 0.3 | 0.5 | 0.6 | 0.7 | 0.8 | 1.0 | |
| Belgium | Diet_National_2004 | 511 | 0.3 | 0.4 | 0.5 | 0.6 | 0.8 | 1.0 | |
| Denmark | DANSDA 2005‐08 | 274 | 0.3 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | |
| Finland | FINDIET2012 | 413 | 0.4 | 0.5 | 0.6 | 0.8 | 1.0 | 1.1 | |
| France | INCA2 | 264 | 0.3 | 0.4 | 0.4 | 0.6 | 0.7 | 0.8 | |
| Germany | National_Nutrition_Survey_II | 2,006 | 0.3 | 0.4 | 0.5 | 0.6 | 0.8 | 1.0 | |
| Hungary | National_Repr_Surv | 206 | 0.3 | 0.4 | 0.4 | 0.5 | 0.6 | 0.7 | |
| Ireland | NANS_2012 | 149 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 1.0 | |
| Italy | INRAN_SCAI_2005_06 | 290 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.9 | |
| Netherlands | VCP‐Elderly | 289 | 0.3 | 0.4 | 0.5 | 0.5 | 0.6 | 0.8 | |
| Netherlands | VCPBasis_AVL2007_2010 | 173 | 0.3 | 0.4 | 0.5 | 0.5 | 0.7 | 0.9 | |
| Romania | Dieta_Pilot_Adults | 83 | 0.3 | 0.4 | 0.4 | 0.6 | 0.7 | 0.9 | |
| Sweden | Riksmaten 2010 | 295 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.8 | |
| United Kingdom | NDNS‐Rolling Programme Years 1‐3 | 166 | 0.3 | 0.4 | 0.5 | 0.5 | 0.6 | 0.8 | |
| Very elderly | |||||||||
| Austria | ASNS_Adults | 25 | 0.4 | 0.5 | 0.6 | – | – | – | |
| Belgium | Diet_National_2004 | 704 | 0.3 | 0.4 | 0.5 | 0.5 | 0.7 | 1.0 | |
| Denmark | DANSDA 2005‐08 | 12 | 0.3 | 0.4 | 0.5 | – | – | – | |
| France | INCA2 | 84 | 0.3 | 0.3 | 0.4 | 0.5 | 0.6 | 0.8 | |
| Germany | National_Nutrition_Survey_II | 490 | 0.3 | 0.4 | 0.5 | 0.6 | 0.8 | 1.0 | |
| Hungary | National_Repr_Surv | 80 | 0.4 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | |
| Ireland | NANS_2012 | 77 | 0.4 | 0.5 | 0.5 | 0.7 | 0.8 | 1.0 | |
| Italy | INRAN_SCAI_2005_06 | 228 | 0.4 | 0.5 | 0.6 | 0.6 | 0.9 | 1.0 | |
| Netherlands | VCP‐Elderly | 450 | 0.3 | 0.4 | 0.5 | 0.5 | 0.7 | 0.8 | |
| Romania | Dieta_Pilot_Adults | 45 | 0.4 | 0.4 | 0.5 | – | – | – | |
| Sweden | Riksmaten 2010 | 72 | 0.3 | 0.4 | 0.5 | 0.5 | 0.7 | 0.8 | |
| United Kingdom | NDNS‐Rolling Programme Years 1‐3 | 139 | 0.3 | 0.4 | 0.5 | 0.5 | 0.6 | 0.8 | |
DON: deoxynivalenol; Ac: acetyl.
–: The dietary surveys had less than 60 survey participants and therefore could not be included in calculation of the 95th percentile exposure.
F.2. Current mean and 95th percentile acute human dietary exposure to DON alone and the contributions of the different dietary surveys to chronic DON alone exposure across food groups
For calculating the acute and chronic dietary exposures to DON alone (Tables F.2–F.5, see the details in the Section 6.1.2). The CONTAM Panel also estimated the levels of exposure separately to 3‐Ac‐DON, 15‐Ac‐DON and 3‐DON‐glucoside, and those estimations are available on the request at EFSA. The contributions of the different dietary surveys to chronic DON exposure across the food groups are presented in Table F.5.
Table F.5.
Number of surveys split according to their percentage contribution to chronic dietary exposure of the DON alone (lower bound) concentrations for adults, elderly and very elderly
| Food category | Adults | Elderly | Very elderly | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤5% | 5‐10% | 10‐25% | 25‐50% | ≥ 50% | ≤5% | 5‐10% | 10‐25% | 25‐50% | ≤ 50% | ≥5% | 5‐10% | 10‐25% | 25‐50% | ≥50% | |
| Barley grain | 9 | 4 | 3 | ||||||||||||
| Beer and beer‐like beverage | 12 | 5 | 12 | 2 | 11 | 1 | |||||||||
| Bread and rolls | 11 | 6 | 5 | 9 | 3 | 9 | |||||||||
| Breakfast cereals | 12 | 3 | 2 | 8 | 2 | 2 | 2 | 5 | 4 | 2 | 1 | ||||
| Buckwheat grain | 3 | 3 | 2 | ||||||||||||
| Cereal‐based dishes | 11 | 3 | 9 | 8 | |||||||||||
| Cereal‐based food for infants and young children | 5 | 1 | 2 | ||||||||||||
| Cocoa beans and cocoa products | 16 | 14 | 11 | ||||||||||||
| Cocoa beverage | 10 | 7 | 5 | ||||||||||||
| Composite food (including frozen products) | 6 | 1 | 1 | ||||||||||||
| Condiment | 17 | 14 | 12 | ||||||||||||
| Corn grain | 4 | 3 | 2 | 1 | |||||||||||
| Dietary supplements | 6 | 5 | 1 | 4 | |||||||||||
| Dried fruits | 17 | 14 | 11 | ||||||||||||
| Fine bakery wares | 3 | 13 | 1 | 2 | 2 | 8 | 2 | 1 | 3 | 5 | 3 | ||||
| Grain milling products | 7 | 5 | 5 | 10 | 4 | 7 | 2 | 2 | |||||||
| Grains and grain‐based products | 2 | 2 | 1 | ||||||||||||
| Grains for human consumption | 6 | 1 | 2 | ||||||||||||
| Medical food | 2 | 4 | 4 | ||||||||||||
| Millet grain | 7 | 4 | 2 | ||||||||||||
| Miscellaneous fruits | 17 | 14 | 12 | ||||||||||||
| Oats, grain | 5 | 5 | 2 | ||||||||||||
| Other grains | 7 | 3 | 1 | ||||||||||||
| Pasta (Raw) | 5 | 4 | 7 | 1 | 8 | 4 | 1 | 1 | 6 | 4 | 1 | 1 | |||
| Peanut (Arachis hypogea) | 17 | 12 | 9 | ||||||||||||
| Pumpkin seeds (Cucurbita pepo var. oleifera) | 12 | 9 | 6 | ||||||||||||
| Ready to eat soups | 15 | 1 | 12 | 1 | 10 | 1 | |||||||||
| Ready‐to‐eat meal for infants and young children | 3 | 1 | |||||||||||||
| Rice | 17 | 14 | 12 | ||||||||||||
| Rye grain | 3 | 1 | 1 | ||||||||||||
| Snack food | 16 | 1 | 14 | 9 | |||||||||||
| Soya beans (Glycine max) | 11 | 5 | 3 | ||||||||||||
| Spelt grain | 1 | 2 | 1 | ||||||||||||
| Stone fruits | 17 | 14 | 12 | ||||||||||||
| Sweet corn (Zea mays var. saccharata) | 16 | 12 | 10 | ||||||||||||
| Wheat grain | 13 | 6 | 6 | ||||||||||||
DON: deoxynivalenol.
FI/1 survey excluded from calculation of the contribution of ‘Grains and grain‐based products’.
F.3. Current mean and 95th percentile human chronic dietary exposure to DON alone
The mean and the 95th percentile of chronic dietary exposure to DON alone from all food categories for all age groups across dietary surveys are summarised in Table F.3.
Table F.4.
Number of food dietary surveys split according to their percentage contribution to chronic dietary exposure of DON alone (lower bound) concentrations across age groups
| Food category | Toddlersa | Other childrena | Adolescents | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 5% | 5–10% | 10–25% | 25–50% | ≥ 50% | ≤ 5% | 5–10% | 10–25% | 25–50% | ≥ 50% | ≤ 5% | 5–10% | 10–25% | 25–50% | ≥ 50% | |
| Barley grain | 4 | 8 | 6 | ||||||||||||
| Beer and beer‐like beverage | 4 | 12 | 15 | ||||||||||||
| Bread and rolls | 1 | 1 | 4 | 4 | 1 | 12 | 5 | 1 | 11 | 5 | |||||
| Breakfast cereals | 4 | 1 | 4 | 1 | 7 | 8 | 3 | 10 | 7 | ||||||
| Buckwheat grain | 4 | 5 | 2 | ||||||||||||
| Cereal‐based dishes | 4 | 2 | 11 | 1 | 1 | 10 | 2 | 1 | |||||||
| Cereal‐based food for infants and young children | 7 | 1 | 11 | 6 | |||||||||||
| Cocoa beans and cocoa products | 10 | 16 | 17 | ||||||||||||
| Cocoa beverage | 3 | 7 | 8 | ||||||||||||
| Composite food (including frozen products) | 2 | 7 | 5 | ||||||||||||
| Condiment | 9 | 18 | 17 | ||||||||||||
| Corn grain | 2 | 5 | 5 | ||||||||||||
| Dietary supplements | 6 | 8 | 6 | ||||||||||||
| Dried fruits | 8 | 16 | 16 | ||||||||||||
| Fine bakery wares | 2 | 7 | 1 | 2 | 9 | 7 | 2 | 13 | 2 | ||||||
| Food for infants and small children | 1 | ||||||||||||||
| Grain milling products | 5 | 1 | 3 | 1 | 9 | 3 | 4 | ||||||||
| Grains and grain‐based products | 1 | ||||||||||||||
| Grains for human consumption | 1 | 3 | 3 | ||||||||||||
| Medical food | 2 | 3 | 2 | ||||||||||||
| Millet grain | 3 | 3 | 5 | ||||||||||||
| Miscellaneous fruits | 10 | 18 | 17 | ||||||||||||
| Oats, grain | 3 | 3 | 2 | ||||||||||||
| Other grains | 2 | 2 | 4 | ||||||||||||
| Pasta (Raw) | 3 | 2 | 4 | 1 | 4 | 4 | 9 | 1 | 3 | 3 | 10 | 1 | |||
| Peanut (Arachis hypogea) | 7 | 18 | 17 | ||||||||||||
| Pumpkin seeds (Cucurbita pepo var. oleifera) | 2 | 6 | 6 | ||||||||||||
| Ready to eat soups | 8 | 1 | 14 | 1 | 14 | 1 | |||||||||
| Ready‐to‐eat meal for infants and young children | 7 | 9 | 2 | ||||||||||||
| Rice | 10 | 18 | 17 | ||||||||||||
| Rye grain | 1 | 2 | |||||||||||||
| Snack food | 7 | 3 | 13 | 5 | 10 | 7 | |||||||||
| Soya beans (Glycine max) | 2 | 5 | 8 | ||||||||||||
| Spelt grain | 1 | 1 | 1 | ||||||||||||
| Stone fruits | 9 | 18 | 17 | ||||||||||||
| Sweet corn (Zea mays var. saccharata) | 7 | 16 | 16 | ||||||||||||
| Wheat grain | 4 | 9 | 8 | ||||||||||||
DON: deoxynivalenol.
FI/1 survey excluded from calculation of the contribution of ‘Grains and grain‐based products’.
F.4. Current estimates of the mean and 95th percentile dietary exposure to DON alone for farm and companion animals
The lower bound and upper bound concentrations for DON alone in compound feeds and individual feed materials are represented in Appendix C, Table C.1 and the feed intakes and diet composition applied are presented in Appenidx E. They were used to estimate the dietary concentrations and exposure to DON alone presented below for the farm and companion animals.
F.4.1. Ruminants and horses
Using available occurrence data on compound feeds (Appendix C, Table C.1) for dairy cows, beef cattle and horses, estimates of mean and 95th percentile dietary exposure to DON alone have been estimated and are presented in Table F.6.
Table F.6.
Estimated mean and 95th percentile dietary concentration and exposure to DON alone at the LB and UB by dairy cows, fattening beef cattle and horse based on concentrations in species‐specific compound feeds
| Dietary concentration μg/kg dry matter | Exposureμg/day | Exposure μg/kg bw per day | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentile | Mean | 95th percentile | Mean | 95th percentile | ||
| Dairy cow high yieldinga | LB | 52.4 | 316 | 1,084 | 6558 | 1.67 | 10.09 |
| UB | 63.4 | 316 | 1,313 | 6558 | 2.02 | 10.09 | |
| Dairy cow high yieldingb | LB | 57.8 | 346 | 1,197 | 7179 | 1.84 | 11.04 |
| UB | 126 | 400 | 2,603 | 8271 | 4.01 | 12.72 | |
| Fattening beef cattlea | LB | 39.1 | 316 | 376 | 720 | 0.94 | 1.80 |
| UB | 47.7 | 75.0 | 458 | 720 | 1.14 | 1.80 | |
| Fattening beef cattleb | LB | 46.9 | 346 | 450 | 1128 | 1.12 | 2.82 |
| UB | 135 | 192 | 1,305 | 1846 | 3.26 | 4.61 | |
| Horsesa | LB | 91.4 | c | 823 | c | 1.83 | c |
| UB | 94.5 | c | 851 | c | 1.89 | c | |
| Horsesb | LB | 96.0 | c | 864 | c | 1.92 | c |
| UB | 146 | c | 1,318 | c | 2.93 | c | |
DON: deoxynivalenol; bw: body weight; LB: lower bound; UB: upper bound.
Fresh grass and/or grass silage‐based diets.
Grass hay‐based diets.
Insufficient sample numbers to reliably calculate 95th percentile exposure.
For other categories of ruminant livestock and horses, insufficient data for DON in compound feeds were available, and therefore estimates of dietary concentrations and exposures have been made using the example rations (Appendix E) and concentrations of DON alone in individual feed materials (Appendix C, Table C.1). As reported in Section 5.2.1, there is a wide range of feeding systems for ruminants involving different forages. Since levels of DON in samples of maize silage and cereal straw were available (Appendix C, Table C.1), estimates of dietary concentration and exposure by lactating dairy cows and fattening beef cattle on maize‐based diets, and beef cattle on cereal and straw‐based diets have also been made. Estimates of dietary concentration and exposure for beef cattle on a cereal‐based diet are also presented (Table F.7).
Table F.7.
Estimated mean and 95th percentile dietary concentration and exposure to DON alone at the LB and UB by ruminants derived from concentrations in individual feed materials and their relative proportions in diets
| Dietary concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentile | Mean | 95th percentile | Mean | 95th percentile | ||
| Lactating dairy cows: maize silage‐based diet | LB | 428 | 1,537 | 11,682 | 41,950 | 17.97 | 64.54 |
| UB | 470 | 1,557 | 12,829 | 42,509 | 19.74 | 65.40 | |
| Fattening beef cattle:intensive cereal‐based diet | LB | 199 | 1,274 | 1,987 | 12,736 | 4.97 | 31.84 |
| UB | 251 | 1,300 | 2,513 | 13,003 | 6.28 | 32.51 | |
| Fattening beef cattle: maize silage‐based diet | LB | 311 | 1,013 | 2050 | 6,686 | 6.83 | 22.29 |
| UB | 347 | 1,040 | 2,290 | 6,865 | 7.63 | 22.88 | |
| Fattening beef cattle: straw‐based diet | LB | 519 | 2,254 | 4,156 | 18,033 | 13.85 | 60.11 |
| UB | 578 | 2,275 | 4623 | 18198 | 15.41 | 60.66 | |
| Lactating sheepa | LB | 99.7 | 506 | 279 | 1,417 | 4.65 | 23.62 |
| UB | 178 | 566 | 499 | 1,585 | 8.32 | 26.42 | |
| Lactating goatsa | LB | 196 | 365 | 667 | 1,241 | 11.12 | 20.69 |
| UB | 261 | 411 | 887 | 1,398 | 14.78 | 23.29 | |
| Fattening goatsa | LB | 110 | 386 | 165 | 579 | 4.12 | 14.47 |
| UB | 189 | 451 | 284 | 676 | 7.09 | 16.90 | |
DON: deoxynivalenol; bw: body weight, LB, lower bound; UB: upped bound.
Note that these exposures assume that the sole forge is grass hay. Where fresh grass is the main or sole forage exposure will be lower.
F.4.2. Non‐ruminant farm animals
Estimates of mean and 95th percentile dietary concentration and exposure to DON alone at the LB and UB by pigs and poultry, derived from data on species‐specific compound feeds (Appendix C, Table C.1) are reported in Table F.8.
Table F.8.
Estimated mean and 95th percentile dietary concentration and exposure to DON alone by pigs and poultry based on concentrations in species‐specific compound feeds
| Dietary concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentile | Mean | 95th percentile | Mean | 95th percentile | ||
| Pig starter | LB | 149 | 589 | 149 | 589 | 7.44 | 29.5 |
| UB | 238 | 660 | 238 | 660 | 11.88 | 33.0 | |
| Pig finisher | LB | 266 | 779 | 797 | 2337 | 7.97 | 23.4 |
| UB | 311 | 779 | 932 | 2337 | 9.32 | 23.4 | |
| Lactating sow | LB | 314 | 790 | 1882 | 4740 | 9.41 | 23.7 |
| UB | 369 | 790 | 2214 | 4740 | 11.1 | 23.7 | |
| Fattening chickens | LB | 617 | 2,000 | 74.0 | 240 | 37.0 | 120 |
| UB | 627 | 2,000 | 75.2 | 240 | 37.6 | 120 | |
| Laying hens | LB | 482 | 2,050 | 57.8 | 246 | 28.9 | 123 |
| UB | 494 | 2,050 | 59.3 | 246 | 29.6 | 123 | |
| Fattening turkeys | LB | 898 | 2,410 | 359 | 964 | 29.9 | 80.3 |
| UB | 907 | 2,410 | 363 | 964 | 30.2 | 80.3 | |
| Fattening ducks | LB | 624 | 1,760 | 87.4 | 246 | 29.1 | 82.1 |
| UB | 633 | 1,760 | 88.6 | 246 | 29.5 | 82.1 | |
DON: deoxynivalenol; bw: body weight; LB, lower bound; UB: upped bound.
For farmed fish (salmonids and carp), farmed rabbits and farmed mink insufficient data on levels of DON alone in compound feeds were available with which to estimate dietary concentration and exposure, and therefore example rations and concentrations of DON in individual feed materials (Appendix C, Table C.1) were used (see Appendix E for details of rations used). Dietary concentrations and exposures to DON alone derived using this approach are given in Table F.9 below.
Table F.9.
Estimated mean and 95th percentile dietary concentration and exposure to DON alone at the LB and UB by farmed fish (salmonids and carp), farmed rabbits and farmed mink derived from concentrations in individual feed materials and their relative proportions in diets
| Dietary concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentile | Mean | 95th percentile | Mean | 95th percentile | ||
| Salmonids | LB | 54.7 | 218 | 2.19 | 8.71 | 1.09 | 4.36 |
| UB | 81.0 | 242 | 3.24 | 9.69 | 1.62 | 4.85 | |
| Carp | LB | 161 | 636 | 3.55 | 14.0 | 3.55 | 3.55 |
| UB | 247 | 716 | 5.44 | 15.7 | 5.44 | 5.44 | |
| Farmed rabbits | LB | 131 | 728 | 19.7 | 109 | 9.86 | 54.6 |
| UB | 185 | 768 | 27.8 | 115 | 13.9 | 57.6 | |
| Farmed mink | LB | 62.9 | 260 | 4.71 | 19.5 | 2.28 | 9.42 |
| UB | 67.7 | 260 | 5.07 | 19.5 | 2.45 | 9.42 | |
DON: deoxynivalenol; bw: body weight; LB, lower bound; UB: upped bound.
F.4.3. Companion animals (dogs and cats)
For dogs and cats, insufficient data on concentrations in proprietary feeds were available with which to estimate dietary concentration and exposure, and therefore example rations (Appendix E) and concentrations of DON in individual feed materials were used (Appendix C, Table C.1) for details of rations used). Dietary concentration and exposure to DON derived using this approach are given in Table F.10 below.
Table F.10.
Estimated mean and 95th percentile dietary concentration and exposure to DON at the LB and UB by companion animals (dogs and cats) derived from concentrations in individual feed materials and their relative proportions in diets
| Dietary concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95th percentile | Mean | 95th percentile | Mean | 95th percentile | ||
| Dogs | LB | 113 | 466 | 6.75 | 28.0 | 1.69 | 6.99 |
| UB | 135 | 482 | 8.12 | 28.9 | 2.03 | 7.23 | |
| Cats | LB | 148 | 623 | 53.3 | 224 | 2.13 | 8.96 |
| UB | 166 | 631 | 59.8 | 227 | 2.39 | 9.08 | |
DON: deoxynivalenol; bw: body weight; LB, lower bound; UB: upped bound.
F.5. Contribution of the food groups to the chronic dietary exposure
Estimates of average % contribution (95% confidence intervals) to chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across age class at FoodEx Level 1 in middle‐bound scenario are presented in Table F.11.
Table F.11.
Estimates of average % contribution (95% confidence intervals) to chronic dietary exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside across age class at FoodEx Level 1 in middle‐bound scenario
| Food groupa | Median contribution across dietary surveys (Lowest average contribution–highest average contribution) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infants | Toddlers | Other children | Adolescents | Adults | Elderly | Very elderly | ||||||||
| Median | % | Median | % | Median | % | Median | % | Median | % | Median | % | Median | % | |
| Beer and beer‐like beverage | 0 | (0–0) | 0 | (0–0) | 0 | (0–0) | 3 | (0–5) | 8 | (2–22) | 7 | (1–16) | 4 | (0–13) |
| Bread and rolls | 15 | (0–40) | 30 | (1–55) | 37 | (1–59) | 40 | (1–54) | 40 | (30–56) | 46 | (32–57) | 47 | (31–61) |
| Breakfast cereals | 15 | (0–22) | 8 | (0–29) | 6 | (0–13) | 4 | (1–10) | 3 | (1–24) | 3 | (0–37) | 5 | (0–32) |
| Cereal‐based dishes | 4 | (4–4) | 2 | (0–10) | 2 | (0–16) | 2 | (1–18) | 1 | (0–10) | 1 | (0–5) | 0 | (0–0) |
| Cereal‐based food for infants and young children | 7 | (2–20) | 2 | (0–11) | 0 | (0–2) | 0 | (0–0) | 0 | (0–0) | 0 | (0–0) | 0 | (0–0) |
| Fine bakery wares | 5 | (0–14) | 14 | (1–23) | 21 | (0–37) | 18 | (0–31) | 16 | (3–24) | 16 | (2–24) | 16 | (3–27) |
| Grain milling products | 7 | (1–48) | 6 | (0–47) | 3 | (0–62) | 3 | (0–73) | 4 | (0–19) | 2 | (0–20) | 1 | (0–21) |
| Grains for human consumption | 0 | (0–3) | 0 | (0–4) | 0 | (0–4) | 0 | (0–5) | 0 | (0–4) | 0 | (0–3) | 0 | (0–6) |
| Miscellaneous fruitsb | 9 | (4–14) | 8 | (5–10) | 4 | (2–7) | 2 | (2–5) | 3 | (1–6) | 3 | (1–5) | 3 | (1–6) |
| Pasta (Raw) | 3 | (0–27) | 8 | (3–34) | 10 | (2–28) | 10 | (1–22) | 7 | (1–21) | 4 | (1–23) | 4 | (0–23) |
| Ready to eat soupsb | 0 | (0–12) | 1 | (0–12) | 2 | (0–16) | 1 | (0–13) | 2 | (0–14) | 3 | (0–14) | 5 | (0–16) |
| Ready‐to‐eat meal for infants and young childrenb , c | 26 | (11–35) | 1 | (0–6) | 0 | (0–0) | 0 | (0–0) | 0 | (0–0) | 0 | (0–0) | 0 | (0–0) |
| Snack food | 2 | (0–2) | 3 | (0–6) | 4 | (1–6) | 4 | (1–7) | 2 | (0–6) | 0 | (0–3) | 0 | (0–1) |
| Stone fruitsb | 1 | (0–10) | 1 | (0–5) | 0 | (0–5) | 1 | (0–3) | 1 | (0–8) | 2 | (0–8) | 2 | (0–6) |
| Sweet corn (Zea mays var. saccharata) | 0 | (0–8) | 0 | (0–2) | 0 | (0–1) | 0 | (0–1) | 0 | (0–1) | 0 | (0–0) | 0 | (0–0) |
DON: deoxynivalenol; Ac: acetyl.
Median values across dietary surveys and ranges from the lowest to the highest average contribution are presented.
Calculation based on occurrence data with more than 90% of left‐censored data for 3‐Ac‐DON, 15‐Ac‐DON.
Calculation based on occurrence data with more than 90% of left‐censored data for DON.
Calculation based on occurrence data with more than 90% of left‐censored data for DON‐3‐glucoside.
Appendix G – Dose–response/concentration modelling using the Benchmark Dose approach
1.
This appendix reports details of use and the outcome of the dose/concentration–response modelling using the benchmark dose (BMD) approach on the data sets selected to assess the risk of the acute and chronic toxicity of DON (and when available also of its acetylated forms) for humans, farm and companion animals. It should be noted that for this opinion both dose–response data (usually using the dose as mg toxin/kg bw) and concentration–response data (using the concentration as mg toxin/kg feed) were used. To assess chronic risk in humans dose–response data of experimental animals were used by the CONTAM Panel, in contrast to assessment in animals where concentration–response data were analysed. For the acute risk of the four animal species for which data suitable for the BMD analysis were identified, the CONTAM Panel used concentration–response data for pigs, dogs and cats and dose–response data for mink. For convenience, the term dose–response will be used below when addressing general issues of the application of the BMD approach.
When applying the BMD approach to dose–response data, the methodology used for the selection of the data and the models, the estimation of the BMDs and their lower one‐sided 95% confidence limits (BMDLs) and the determination of a BMD interval followed the new guidance of EFSA from 2017 (EFSA Scientific Committee, 2017) with the editorial modification to use notions BMC and BMCL when analysing concentrations. The BMDS software version 2.6.0.1 (BMDS2601_2015 0629) was applied for quantal data, while the continuous data were analysed using the PROAST software version 62.6. This appendix reports the results of all endpoints and their dose/concentration–response data sets identified as critical and/or relevant for the risk assessment of DON, including the two acetylated forms (see Sections 7 and 8 in the main text of the opinion). The results are summarised in tables including conclusions on the final BMD/BMC confidence interval and the lowest BMDL/BMCL used to determine a reference point. Selected graphics illustrate this. Full details of the modelling are given as Supplementary Information for each data set evaluated in Annex A Supplementary Information (using identifiers, e.g. ‘IVERSONBW’, when reporting the dose/concentration–response modelling of the body weight of mice of Iverson et al. (1995) – see Section G.2.1 below.
The following sections report BMD modelling for assessing the chronic human risks based on the data from rodents as experimental animals, and for the acute risks of farm and companion animals. No chronic or acute human epidemiological dose–response data suitable for the BMD modelling were identified by the CONTAM Panel. Regarding the assessment of the chronic risks of farm and companion animals, the CONTAM Panel examined the dose/concentration–response data available in the literature for the application of the BMD approach and concluded that no consistent dose–response evaluation could be performed to determine reference points for the identified critical endpoints, neither for the chronic dose–response nor for the chronic concentration–response data available (see Section 7.8.6).
G.1. Specific considerations when applying the BMD approach to DON
The EFSA guidance (EFSA Scientific Committee, 2017) recommends to calculate a BMD confidence interval (BMD‐CI), also denoted BMDL–BMDU interval, for each data set and to use the lower limit of this interval as the reference point. Model averaging is considered the preferred method for calculating the BMD‐CI in EFSA Scientific Committee et al. (2017). However, since model averaging software was not available in BMDS and PROAST the alternative approach recommended by EFSA Scientific Committee et al. (2017) and explicated in a flow chart in that guidance was used for this opinion. Similarly to the model averaging, this alternative method is based on the Akaike Information Criterion (AIC) and since the AIC values are directly related to the log‐likelihood values usually used in a BMD analysis these values were also reported together with the results of the goodness‐of‐fit likelihood ratio tests and their p‐values.
The BMD‐CIs for the quantitative data were analysed using PROAST. In the case of quantal data, BMD‐CIs were not determined because BMDU values were only available for some models in the BMDS software. As a consequence, only the BMDL/BMCL values are reported for quantal data to determine the lowest BMDL/BMCL of each data set used to calculate a reference point. Note that the reference point is specific for the data set and differs from an overall reference point determined across all the considered studies similarly as NOAELs of single data set identified for an assessment differ from an overall NOAEL.
Therefore, the summary tables for quantal data report type of models, number of model parameters, negative of the log likelihood value (usually then a positive number), p‐value of the goodness‐of‐fit, AIC value, BMD/BMC estimate and BMDL/BMCL values. In the case of quantal data, the model with the lowest AIC, denoted AICmin and the models with AIC values not larger than AICmin+2 are highlighted as the models selected to determine the lowest BMDL/BMCL. It should be noted that the model with the AICmin belongs by definition to the set of selected models (if no alert is triggered) The reference point is identified as the lowest BMDL of that set of selected models. The AICFull+2 values and the AICNull‐2 values can be obtained from the first two lines of the summary table from the results of the BMDS software and are used as thresholds for alerts as recommended in EFSA Scientific Committee et al. (2017).
In the case of continuous data, the guidance of EFSA Scientific Committee et al. (2017) has been implemented in the PROAST software and therefore the models not selected by the AIC criterion, as described in EFSA Scientific Committee et al. (2017), are not reported. Therefore, the summary table is restricted to the values of the BMD and the BMD‐CI of the Exponential and the Hill models selected by the PROAST software out of the sets of two models E3 and E5 for the Exponential and H3 and H5 for the Hill family, respectively, denoted below as ‘Best fitting model’.
For quantal data, all models available in the BMDS software were selected using the default benchmark response (BMR) of 10% extra risk. For quantitative data, the BMR was defined as a percent change of the magnitude of the response when compared to that predicted at background, i.e. a relative deviation from background. The default value of 5% (BMR = 0.05) has been recommended by EFSA Scientific Committee et al. (2017) to be used in the absence of statistical or toxicological considerations supporting a deviation from that default value.
The BMD analyses of quantitative data were based on means and standard deviations or standard errors available from the reports of the selected studies. Deviations from the published values and calculations of standard deviations or standard errors, in cases where incomplete data were reported, were indicated. For interpreting the graphs and tables obtained by PROAST it should be noted that the data of each dose group are assumed to be log‐normally distributed such that the means are geometric means and the whiskers are based on geometric standard deviations.55
G.2. Dose–response modelling of chronic toxicity data in mice
Reduced feed intake and reduced body weight gain were identified as the most sensitive toxicological endpoints for chronic toxicity in experimental animals.
G.2.1. Reduced body weight gain and reduced feed intake in mice of Iverson et al. (1995)
The results of Iverson et al. (1995) on feed intake and body weight of male and female B6C3F1 mice were identified as pivotal for the dose–response modelling of the general toxicity of DON in rodents. Although this long‐term study had been designed to examine DON for possible carcinogenicity (and being negative for this endpoint), the data reported on general toxicity of the animals, in particular on feed intake and body weight, over 2 years were suitable for a dose–response analysis using the BMD approach to assess the critical effect of reduced body weight gain and reduced intake identified in Section 7.8. The CONTAM Panel approached the authors of the study through Health Canada in October 2016 for the availability of the individual animal data but learned that these data were no more available. Therefore, the CONTAM Panel calculated the doses for the three dose groups for both male and female mice from the published data of average feed intake and average body weight (see Table G.1).
Table G.1.
Calculations of the doses using available data on average body weight and average daily feed consumption per dose group reported in Iverson et al. (1995) for female and male mice
| Iverson et al. (1995) data | |||||
|---|---|---|---|---|---|
| Concentration as reported in the study (mg DON/kg diet) | Average feed consumption (g/day) | Number of animals per group | Average body weight (g) | Number of animals per group | Dose (mg DON/kg bw per day) |
| Female mice data | |||||
| 0 | 4.48 | 22 | 41.54 | 36 | 0 |
| 1 | 4.44 | 24 | 38.71 | 42 | 0.115 |
| 5 | 4.46 | 23 | 33.76 | 37 | 0.661 |
| 10 | 4.34 | 25 | 28.55 | 38 | 1.520 |
| Male mice data | |||||
| 0 | 4.30 | 24 | 43.85 | 37 | 0 |
| 1 | 4.28 | 24 | 43.51 | 35 | 0.098 |
| 5 | 4.05 | 25 | 40.03 | 43 | 0.506 |
| 10 | 3.79 | 25 | 35.09 | 42 | 1.126 |
bw: body weight.
The CONTAM Panel noted that the body weight data differed between the males and females although the shape of the curves of average body weight for the two sexes appeared rather similar. Compared with males, the weights of the females were lower and the dose‐dependent decrease was larger with larger differences between the dose groups. In contrast, feed intake was slightly larger and dose–response was less expressed in the female mice compared with the male mice where the dose‐dependent decrease of average feed consumption was clear. In a situation like this where the dose–response data of two groups can be combined in principle and differ only partially, a dose–response model can be fitted to the combined data set and the BMD approach would result in subgroup specific BMD/Ls or BMD confidence intervals; in this case in sex‐specific outcomes, see the subsection on covariates within the section 2.5.5 of the recent guidance of EFSA (EFSA Scientific Committee, 2017).
The PROAST software version 62.6 applied to the combined body weight data selected the models E3 and H3, and due to a statistical difference between males and females BMD‐CIs were calculated separately for females and males (see Table G.2). The overall BMD‐CIs were 0.1–0.29 and 0.15–0.32 mg/kg bw per day for females and males, respectively. The overall reference point for body weight identified by the CONTAM Panel was 0.11 mg/kg bw per day (see Table G.2). The dose–response curves for the exponential model E3 and Hill model H3 are presented in Figure G.1. For more details, see Annex A Supplementary Information IVERSONBW.
Table G.2.
Dose–response analysis of body weight (see Table G.1) for female and male mice combined of Iverson et al. (1995)
| Best fitting model | BMD05 | BMDL05–BMDU05 | |
|---|---|---|---|
| mg/kg bw per day | |||
| Female mice | |||
| Exponential model family | E3 | 0.19 | 0.11–0.28 |
| Hill model family | H3 | 0.19 | 0.12–0.29 |
| Male mice | |||
| Exponential model family | E3 | 0.22 | 0.15–0.30 |
| Hill model family | H3 | 0.23 | 0.16–0.32 |
BMD05: benchmark dose response of 5%; BMDL05/BMDU05: 95% lower/upper confidence limit for the benchmark dose response of 5%; bw: body weight.
Figure G.1.
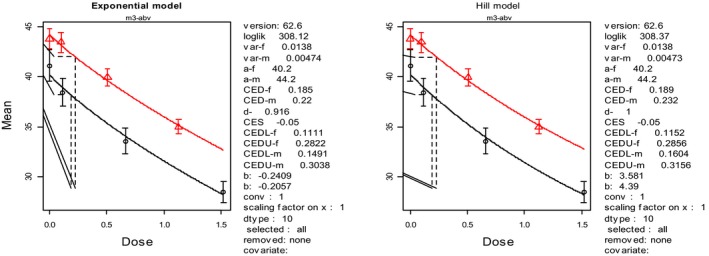
Dose–response curves for the body weight of female and male mice combined (see also Table G.2)
- Mean body weight is expressed as g and dose as mg/kg bw per day. The red (upper) curve is for male and the black (lower) for female mice data.
The CONTAM Panel noted that the feed intake data differed substantially between the males and females, which is also reflected by different shapes of the curves of average feed intake for the two sexes shown in Iverson et al. (1995). The PROAST software version 62.6 applied to the combined feed intake data selected the models E5 and H5, and due to a statistical difference between males and females BMD‐CIs were calculated separately for the two sexes (see Table G.3). The overall BMD‐CIs were 1.57–17.0 and 0.23–0.66 mg/kg bw per day for females and males, respectively. The overall reference point for feed intake identified by the CONTAM Panel was 0.23 mg/kg bw per day (see Table G.3). The dose–response curves for the exponential model E5 and Hill model H5 are presented in Figure G.2. For more details of the analysis for the body weight, see Annex A Supplementary Information IVERSONFDC.
Table G.3.
Dose–response analysis of feed intake (see Table G.1) for female and male mice combined of Iverson et al. (1995)
| Best fitting model | BMD05 | BMDL05–BMDU05 | |
|---|---|---|---|
| mg/kg bw per day | |||
| Female mice | |||
| Exponential model family | E5 | 2.95 | 1.57–17.0 |
| Hill model family | H5 | 2.62 | 1.58–9.71 |
| Male mice | |||
| Exponential model family | E5 | 0.42 | 0.24–0.66 |
| Hill model family | H5 | 0.42 | 0.23–0.63 |
BMD05: benchmark dose response of 5%; BMDL05/BMDU05: 95% lower/upper confidence limit for the benchmark dose response of 5%; bw: body weight.
Figure G.2.
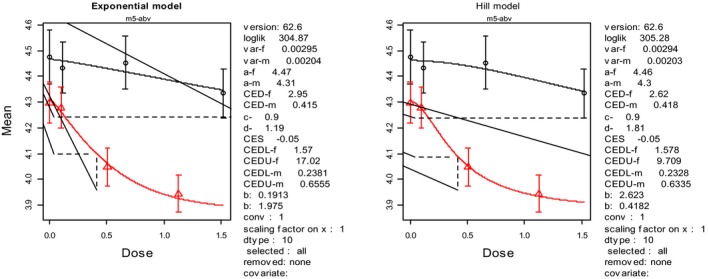
Dose–response curves for the feed intake of female and male mice combined of Iverson et al. (1995) (see Table G.3)
- Mean feed intake is expressed as g feed per day and dose as mg/kg bw per day. The red curve is for female mice data and the black for male mice data.
G.2.2. Reduced body weight gain and reduced feed intake in mice of Iverson et al. (1995) related to the concentration of DON in feed
To allow a comparison with the hazard characterisation of reduced body weight gain and feed intake for farm and companion animals based on concentration data in feed, the results of Iverson et al. (1995) on body weight and feed intake of male and female B6C3F1 mice were also analysed as concentration–response data using the BMD approach using the three concentrations of 1, 5 and 10 mg DON/kg feed (see Table G.1) for which the experiment was designed. The PROAST software version 62.6 applied to the body weight data of female and male mice combined selected the models E5 and H5, and, as above, due to a statistical difference between males and females BMC‐CIs were calculated separately for the two sexes (see Table G.4). The overall BMC‐CIs were 1.04–2.40 and 1.75–3.39 mg DON/kg feed per day for females and males, respectively. The overall reference point for feed intake identified by the CONTAM Panel was therefore 1.0 mg/kg feed per day (see Table G.4). The dose–response curves of body weight for the exponential model E5 and Hill model H5 are presented in Figure G.3. For more details of these results, see Annex A Supplementary Information IVERSON‐concentration–response.
Table G.4.
Concentration–response analysis of body weight (see Table G.1) for female and male mice combined of Iverson et al. (1995)
| Best fitting model | BMC05 | BMCL05– BMCU05 | |
|---|---|---|---|
| mg/kg feed per day | |||
| Female mice | |||
| Exponential model family | E5 | 1.64 | 1.04–2.37 |
| Hill model family | H5 | 1.68 | 1.08–2.40 |
| Male mice | |||
| Exponential model family | E5 | 2.47 | 1.75–3.27 |
| Hill model family | H5 | 2.60 | 1.87–3.39 |
BMC05: benchmark concentration response of 5%; BMCL05/BMCU05: 95% lower/upper confidence limit for the benchmark dose response of 5%.
Figure G.3.
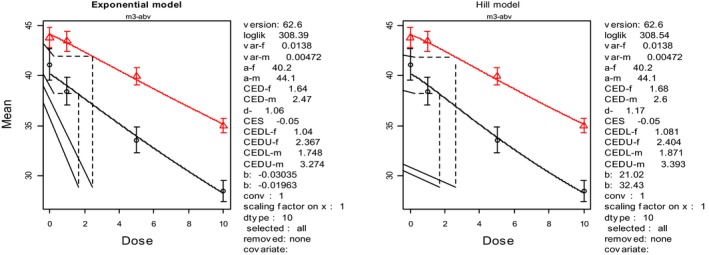
Concentration–response curves for the body weight of female and male mice combined (see also Table G.4)
- Mean body weight is expressed as g and concentration as mg/kg feed per day. The red (upper) curve is for male and the black (lower) for female mice data.
For feed intake, the overall BMC‐CIs were 10.4–100 and 2.39–6.29 mg DON/kg feed per day for females and males, respectively. The overall reference point for feed intake identified by the CONTAM Panel was therefore 2.4 mg DON/kg feed per day (see Table G.5). The dose–response curves of feed intake for the exponential model E5 and Hill model H5 are presented in Figure G.3. For more details of these results, see Annex A Supplementary Information IVERSON‐concentration–response.
Table G.5.
Concentration–response analysis of feed intake (see Table G.1) for female and male mice combined of Iverson et al. (1995)
| Best fitting model | BMC05 | BMCL05–BMCU05 | |
|---|---|---|---|
| mg/kg feed per day | |||
| Female mice | |||
| Exponential model family | E5 | 18.6 | 10.4–100 |
| Hill model family | H5 | 16.5 | 10.4–61.9 |
| Male mice | |||
| Exponential model family | E5 | 4.19 | 2.46–6.29 |
| Hill model family | H5 | 2.39 | 4.24–6.13 |
BMC05: benchmark concentration response of 5%; BMCL05/BMCU05: 95% lower/upper confidence limit for the benchmark dose response of 5%.
Figure G.4.
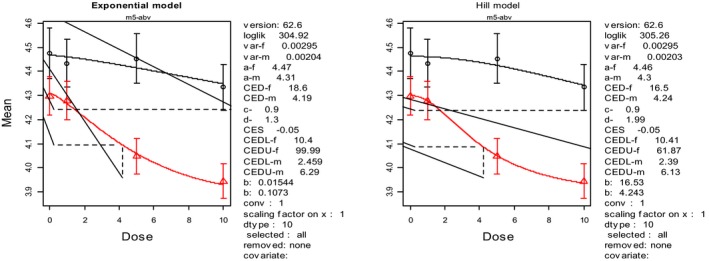
Concentration–response curves for feed intake of female and male mice combined (see also Table G.5)
- Mean feed intake is expressed as g feed per day and concentration as mg/kg feed per day. The red (upper) curve is for male and the black (lower) for female mice data.
G.2.3. Reduced body weight gain in mice of Bondy et al. (2016)
Bondy et al. (2016) performed a 26‐week study in mice using a diet at concentrations of 0, 1, 5 and 10 mg DON/kg feed (the same as used by Iverson et al. (1995)) to study carcinogenicity and general chronic effects. The study investigated effects of DON in two strains of male mice (p53+/+ and p53+/− mice) with initial mean body weight of 27.0 g (SD 2.9 g) and 28.7 g (SD 1.7 g), respectively. Dietary concentrations of DON were converted into mean doses by the authors (see also Table G.6). Mean daily feed consumption was shown in a graphic only and could not be evaluated for dose–response analysis by the CONTAM Panel. The authors noted some uncertainty of the calculation of the dose (See Table 2 in Bondy et al., 2016). The mean doses over the 26 weeks were reported by the authors as of ranging from 0.08 to 0.14, 0.42 to 0.69 and 1.22 to 2.24 mg DON/kg diet per day for the p53+/+ mice, and 0.08 to 0.14, 0.39 to 0.65 and 1.05 to 1.95 mg DON/kg diet per day for the p53+/− mice, respectively. The mean doses calculated by the authors per dose group were used for the modelling. Similarly, as done for the two sexes in the study of Iverson et al. (1995), the dose–response data of two genotypes were combined since they differed only partially and a dose–response model was fitted to the combined data set such that the BMD approach resulted also in subgroup specific BMD/Ls or BMD confidence intervals. Using the PROAST software version 62.6, for the data of the p53+/+ and p53+/− mice combined the overall BMD‐CI was 0.11–0.90 mg/kg bw per day (Table G.7).56 The lowest BMDL was 0.11 mg/kg bw per day as such identical with the reference point derived from the body weight data of Iverson et al. (1995). The dose–response curves for the exponential model E3 and Hill model H3 for the two genotypes are presented in Figure G.5.
Table G.6.
Doses of DON, number of animals per dose group and mean body weights reported for mice by Bondy et al. (2016)
| p53+/+ male mice | p53+/− male mice | ||||
|---|---|---|---|---|---|
| Dose (mg DON/kg bw per day) | Number of animals | Mean bw (SD) g | Dose (mg DON/kg bw per day) | Number of animals | Mean bw (SD) g |
| 0 | 10 | 44.8 (2.9) | 0 | 10 | 43.1 (2.5) |
| 0.09 | 10 | 43.8 (3.7) | 0.09 | 10 | 42.5 (4.9) |
| 0.53 | 10 | 39.9 (3.4) | 0.49 | 10 | 39.7 (2.6) |
| 1.57 | 9 | 32.0 (2.8) | 1.50 | 10 | 32.9 (2.3) |
DON: deoxynivalenol; bw: body weight; SD: standard deviation.
Table G.7.
Dose–response analysis of body weight (see Table G.6) for male p53 +/+ and p53 +/− mice combined (Bondy et al., 2016)
| Best fitting model | BMD05 | BMDL05 − BMDU05 | |
|---|---|---|---|
| mg/kg bw per day | |||
| Exponential model family | E3 | 0.38 | 0.11–0.89 |
| Hill model family | H3 | 0.39 | 0.12–0.90 |
BMD05: benchmark dose response of 5%; BMDL05/BMDU05: 95% lower/upper confidence limit for the benchmark dose response of 5%; bw: body weight.
Figure G.5.
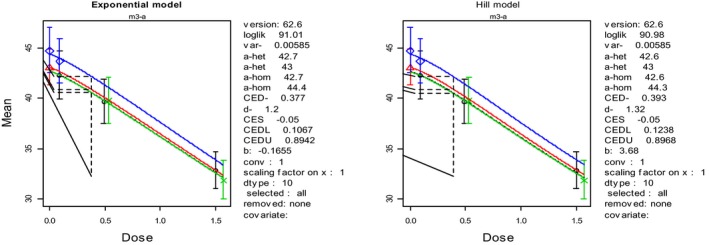
Dose–response curves of body weight for p53+/+ and p53+/− mice combined of Bondy et al. (2016) (see also Table G.7)
- Means are expressed as body weight (g) and dose as mg/kg bw per day. The blue (upper) curve is for the p53+/+ male mice, the green (lower) for the p53+/− male mice and the red (middle) for the combined mice data.
The CONTAM Panel noted that this study with a duration of 26 weeks should be considered as subchronic study. Comparing with the study of Iverson et al. (1995), when a total of 400 male and female mice were investigated, the study of Bondy et al. (2016) investigated only a total of 100 animals including the two p53 types of male mice. If the outcome of the study of Bondy et al. (2016) had been the same as reported by the authors but were based on 50 animals per dose group instead of 10, the BMD‐CI would have been 0.20 and 0.59 mg/kg bw per day for the two p53 types of mice at a higher dose (tentatively calculated by the CONTAM Panel). Therefore, the results of the study by Bondy et al. (2016) appeared to be in a good agreement with the outcome of the Iverson et al. (1995) study, even as a subchronic study. The CONTAM Panel concluded that the BMD‐interval of 0.11–0.90 mg DON/kg bw per day obtained from the data of Bondy et al., (2016) supported the results obtained in the dose–response evaluation of the data of Iverson et al. (1995). For more details of the analysis, see Annex A Supplementary Information BONDY.
G.3. Dose–response modelling of general toxicity in rats observed in the studies on reproductive and developmental toxicity of Sprando et al. (2005) and Collins et al. (2006)
General toxicity data of DON were available also from several recent studies in rodents on the reproductive and developmental toxicity of DON (see Section 7.2.7). Although the duration of these studies in pregnant dams was usually short and resembled in their duration subacute studies, the CONTAM Panel considered these data as relevant and supportive to the results of the long term study on general toxicity of Iverson et al. (1995). Regarding reduced feed intake and body weight gain two studies were identified as relevant and suitable for dose–response evaluations, namely the studies of Sprando et al. (2005) and Collins et al. (2006), both informing reduced feed consumption and reduced body weight gain.
G.3.1. Reduced body weight gain and reduced feed intake in male rats of Sprando et al. (2005)
Mean initial body weight (SD) of male rats was 343.1 (4.2), 341.5 (4.4), 342.1 (4.3), 341.4 (4.9) and 339.9 (5.8) g for the controls and the four dose groups exposed to 0, 0.5, 1.0, 2.5 and 5 mg/kg by Sprando et al. (2005). Since the same group reported in Collins et al. (2006) data on male rats exposed to the same doses of 0, 0.5, 1, 2.5 and 5 in mg DON/kg bw per day, the CONTAM Panel assumed that the unit in Sprando et al. (2005) was also mg/kg bw per day. The CONTAM Panel used the PROAST software version 62.6 for the BMDL analysis. The overall BMD‐CIs were 0.72–3.91 mg/kg bw per day for body weight, 0.59–2.38 mg/kg bw per day for body weight gain and 0.70–4.08 mg/kg bw per day for feed intake, respectively, in male rats (see Table G.8). The dose–response curves for the exponential model E3 and Hill model H3 are presented in Figures G.6–G.8). For more details of the analysis, see Annex A Supplementary Information SPRANDO2005all.
Table G.8.
Dose–response analysis of body weight, body weight gain and feed intake (see Annex A Supplementary Information SPRANDO2005all) for male rats of Sprando et al. (2005)
| Best fitting model | BMD05 | BMDL05–BMDU05 | |
|---|---|---|---|
| mg/kg bw per day | |||
| Body weight | |||
| Exponential model family | E3 | 2.59 | 0.72–3.91 |
| Hill model family | H3 | 2.60 | 0.81–3.90 |
| Body weight gain | |||
| Exponential model family | E3 | 1.23 | 0.59–2.38 |
| Hill model family | H3 | 1.48 | 0.92–2.38 |
| Feed intake | |||
| Exponential model family | E3 | 2.39 | 0.70–4.08 |
| Hill model family | H3 | 2.41 | 0.81–4.06 |
BMD05: benchmark dose response of 5%; BMDL05/BMDU05: 95% lower/upper confidence limit for the benchmark dose response of 5%; bw: body weight.
Figure G.6.
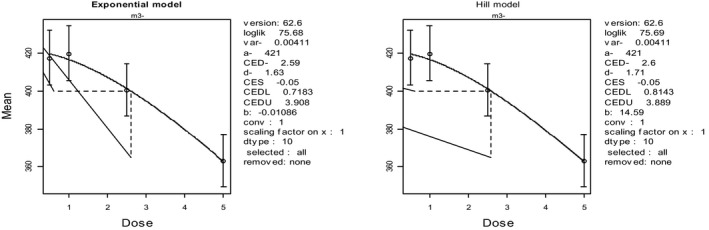
Dose–response curve of mean body weight in male rats of Sprando et al. (2005) (see also Table G.8)
- Mean is expressed as body weight (kg) and dose as mg/kg bw per day.
Figure G.8.
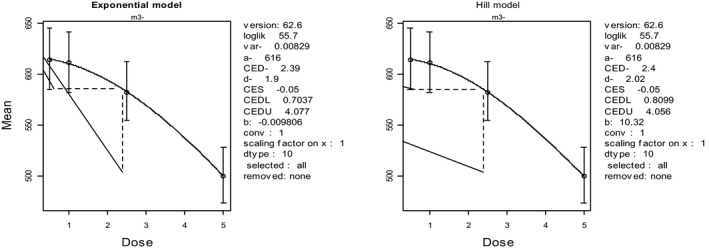
Dose–response curve of feed intake in male rats of Sprando et al. (2005) (see also Table G.8)
- Mean is expressed as body weight (kg) and dose as mg/kg bw per day.
Figure G.7.
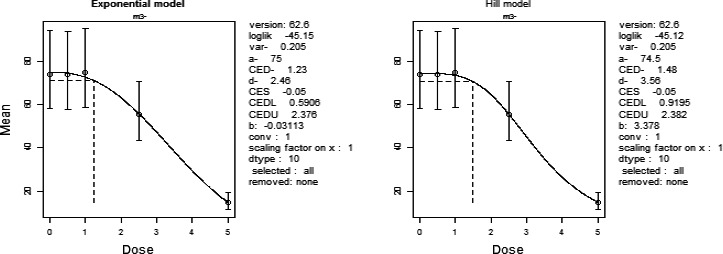
Dose–response curve of body weight gain in male rats of Sprando et al. (2005) (see also Table G.8)
- Mean is expressed as body weight (kg) and dose as mg/kg bw per day.
G.3.2. Body weight, body weight gain and feed intake in female rats of Collins et al. (2006)
The authors clearly stated in their paper that female rats were treated with doses of 0, 0.5, 1, 2.5 and 5 mg DON/kg bw per day at GD6‐19 based on a dose‐ranging study at doses of 0.25–7.5 mg DON/kg bw per day. Initial mean body weights ranged from 326 to 350 g for males and from 201 to 225 g for females, respectively. The overall BMD‐CIs 0.72–3.91 mg/kg bw per day were for body weight, 1.14–3.95 mg/kg bw per day for body weight gain and 1.80–3.62 mg/kg bw per day for feed intake, respectively (see Table G.9). The dose–response curves for the exponential model E3 and Hill model H3 are presented in Figures G.9–G.11. For more details of the analysis, see Annex A Supplementary Information COLLINS.
Table G.9.
Dose–response analysis of body weight, body weight gain and feed intake (see Supplementary Information COLLINS (Annex A)) for female rats of Collins et al. (2006)
| Best fitting model | BMD05 | BMDL05–BMDU05 | |
|---|---|---|---|
| mg/kg bw per day | |||
| Body weight | |||
| Exponential model family | E3 | 3.41 | 2.34–4.43 |
| Hill model family | H3 | 3.39 | 2.37–4.31 |
| Body weight gain | |||
| Exponential model family | E3 | 1.92 | 1.14–3.95 |
| Hill model family | H3 | 2.01 | 1.34–3.49 |
| Feed intake | |||
| Exponential model family | E3 | 2.58 | 1.80–3.62 |
| Hill model family | H3 | 2.57 | 1.82–3.58 |
BMD05: benchmark dose response of 5%; BMDL05/BMDU05: 95% lower/upper confidence limit for the benchmark dose response of 5%; bw: body weight.
Figure G.9.
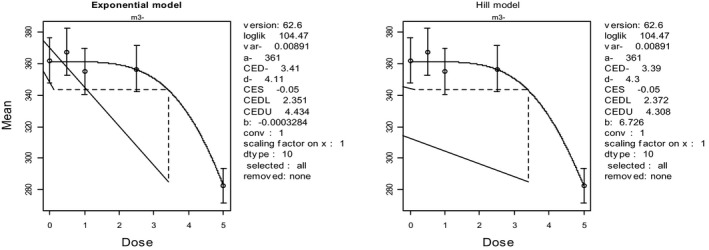
Dose–response curve of body weight in female rats Collins et al. (2006) (see also Table G.9)
- Mean is expressed as body weight (kg) and dose as mg/kg bw per day.
Figure G.11.
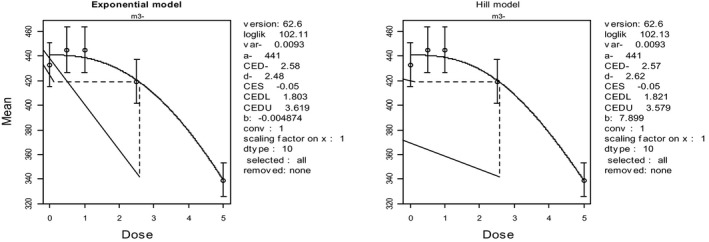
Dose–response curve of feed intake in female rats Collins et al. (2006) (see also Table G.9)
- Mean is expressed as body weight (kg) and dose as mg/kg bw per day.
Figure G.10.
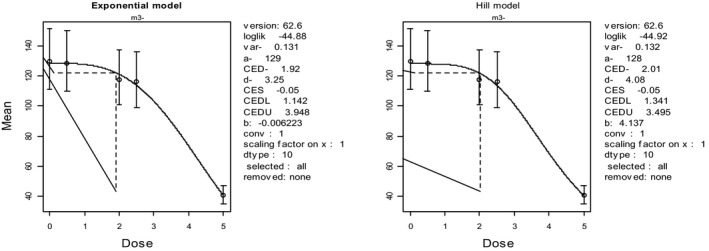
Dose–response curve of body weight gain in female rats Collins et al. (2006) (see also Table G.9)
- Mean is expressed as body weight (kg) and dose as mg/kg bw per day.
G.4. Concentration/dose–response modelling for acute toxicity data on vomiting in farm and companion animals (pigs, dogs, cats and farmed mink)
Vomiting was identified as the critical endpoint to characterise acute toxicity of DON and its acetylated forms of 3‐Ac‐DON and 15‐Ac‐DON (Section 7.10) for those farm animal species, which are known to be able to vomit. Data on vomiting appearing suitable for applying BMD‐approach were available for pigs from Young et al. (1983) and Williams et al. (1988), for dogs and cats from Hughes at al. (1999) and for farmed mink from Wu et al. (2013a). The CONTAM Panel decided to use concentration–response data for pigs, dogs and cats for the reasons outlined in Section 7.3 and dose–response data for the mink due to the design chosen by Wu et al. (2013a).
G.4.1. Concentration–response data on vomiting in pigs
The data on vomiting in piglets from Young et al. (1983) and Williams et al. (1988) (see Section 7.3.4.2) were evaluated using the BMD approach as recommended in EFSA Scientific Committee et al. (2017). Trials 2 and 3 of the study of Young et al. (1983) were identified as suitable for a concentration–response evaluation using the BMD approach with the reported concentrations of 0.79, 44.4, 97.2, 124.9 and 227.5 mg/kg feed for trial 2 and 0.14, 9.0, 19.7, 33.5 and 43.4 mg/kg feed for trial 3 (see Table G.10). Incidences of vomiting were obtained from the evaluation of JECFA (FAO/WHO, 2011). Two experiments of Williams et al. (1988) were evaluated with concentrations of up to 0.05 mg/kg feed in the two control groups and concentrations of 1.6. 4.4. 6.6. 9.1 and 11.0 mg/kg feed in the main trial with 6–12 animals per group and concentrations of 2.6. 5.0, 8.3, 12.7 and 14.0 mg/kg feed in the pretrial with 2 animals per group.
Table G.10.
Concentration–response data on vomiting in pigs exposed to DON identified from the studies of Young et al. (1983) and Williams et al. (1988)
| Young et al. (1983) | Williams et al. (1988) | ||||||
|---|---|---|---|---|---|---|---|
| Concentration (mg DON/kg feed) | N of animals | Incidence | Trial | Concentration (mg DON/kg feed) | N of animals | Incidence | Trial |
| 0.79 | 3 | 0 | 2 | 0.05 | 12 | 0 | 2 |
| 44.4 | 4 | 2 | 2 | 1.6 | 12 | 0 | 2 |
| 97.2 | 4 | 1 | 2 | 4.4 | 12 | 2 | 2 |
| 124.6 | 4 | 4 | 2 | 6.6 | 6 | 3 | 2 |
| 227.5 | 4 | 3 | 2 | 9.1 | 6 | 2 | 2 |
| 11.0 | 6 | 3 | 2 | ||||
| 0.14 | 4 | 0 | 3 | 0.05 | 2 | 0 | 1 |
| 9 | 4 | 0 | 3 | 2.6 | 2 | 0 | 1 |
| 19.7 | 4 | 1 | 3 | 5.0 | 2 | 0 | 1 |
| 33.5 | 4 | 1 | 3 | 8.3 | 2 | 0 | 1 |
| 43.4 | 4 | 1 | 3 | 12.7 | 2 | 1 | 1 |
| 14.1 | 2 | 2 | 1 | ||||
DON: deoxynivalenol; N: number of animals.
G.4.1.1. Concentration–response data on vomiting in pigs of Young et al. (1983)
The CONTAM Panel modelled the data on vomiting in pigs of the two trials of Young et al. (1983) using the BMDS software following the EFSA guidance (EFSA Scientific Committee, 2017) and identified 1.36 mg/kg feed per day of the Weibull model as the BMDL10 of this data set (see Table G.11). No alert regarding the criterion AIC‐Full+2 = 50.04 and AIC‐NULL‐2 = 53.68 was obtained. With AICMIN+2 = 38.92 all models except the probit and the logistic model were selected (see Table G.11). The fit of the Weibull model with the lowest BMDL10 is shown in Figure G.12. The Gamma model could not be fitted in reasonable computing time to these data. For more details of the analysis, see Annex A Supplementary Information YOUNGBMC.
Table G.11.
Summary of concentration–response analysis of vomiting in pigs exposed to DON as reported by Young et al. (1983)
| Models | Number of parameters | Minus Log‐likelihood | p‐value | AIC | BMC10 | BMCL10 | Comment |
|---|---|---|---|---|---|---|---|
| (mg/kg feed) | |||||||
| Full model | 10 | 14.02 | na | 38.04 | na | na | |
| Null (reduced) model | 1 | 24.82 | 0. 01 | 51.64 | na | na | |
| Probit | 2 | 19.21 | 0.24 | 42.42 | 32.60 | 21.80 | Not selected |
| Logistic | 2 | 19.18 | 0.24 | 42.36 | 33.18 | 21.30 | Not selected |
| LogProbit | 2 | 17.27 | 0.59 | 38.54 | 14.11 | 2.42 | Selected |
| LogLogistic | 2 | 17.32 | 0.58 | 38.63 | 13.47 | 2.12 | Selected |
| Multistage | 2 | 17.38 | 0.57 | 38.75 | 10.50 | 5.54 | Selected |
| Multistage Cancer | 1 | 17.46 | 0.65 | 36.92 | 12.22 | 7.78 | Selected |
| Quantal‐Linear | 1 | 17.46 | 0.65 | 36.92 | 12.22 | 7.82 | Selected |
| Weibull | 2 | 17.44 | 0.55 | 38.89 | 10.88 | 1.36 | Selected |
| Gamma | No fit | ||||||
The models selected to identify the lowest BMDL10 following EFSA guidance (EFSA Scientific Committee, 2017) are identified in the column ‘Comments’ and include the model with the lowest AIC.
BMC10: benchmark concentration response of 10%; BMCL10: 95% lower/upper confidence limit for the benchmark concentration response (BMR) of 10%; na: not available, including cases where the BMC/Ls were not calculated, the fit was incomplete, or serious or conflicting comments were noted (e.g. parameters reaching boundary).
Figure G.12.
Concentration–response curve of the incidence of vomiting in pigs reported by Young et al. (1983) (see also Table G.11)
- The (red) curve is the fitted dose–response curve from the Weibull model and the dotted line curve indicates the 95% lower confidence limit to the calculated BMCL.
G.4.1.2. Concentration–response data on vomiting in pigs of Williams et al. (1988)
The CONTAM Panel modelled the data on vomiting in pigs of the two trials of Williams et al. (1988) using the BMDS software following the EFSA guidance (EFSA Scientific Committee, 2017) and identified 1.84 mg/kg feed per day of the Multistage cancer model as the BMDL10 of this data set (see Table G.12). No alert regarding the criterion AIC‐Full+2 = 61.86 and AIC‐NULL‐2 = 67.50 was obtained. With AICMIN+2 = 48.59 all models except the probit, the logistic model, the Quantal linear and the Weibull model were selected (see Table G.12). The fit of the Multistage cancer model with the lowest BMDL10 is shown in Figure G.13. The Gamma model could not be fitted in reasonable computing time to these data. For more details of the analysis see Annex A Supplementary Information WILLIAMSBMC.
Table G.12.
Summary of concentration–response analysis of vomiting in pigs exposed to DON as reported by Williams et al. (1988)
| Models | Number of parameters | Minus Log‐likelihood | p‐value | AIC | BMC10 | BMCL10 | Comment |
|---|---|---|---|---|---|---|---|
| (mg/kg feed) | |||||||
| Full model | 12 | 18.93 | na | 61.86 | na | na | |
| Null (reduced) model | 1 | 32.75 | 0. 004 | 67.50 | na | na | |
| Probit | 2 | 22.84 | 0.65 | 49.68 | 4.64 | 3.31 | Not selected |
| Logistic | 2 | 23.14 | 0.59 | 50.28 | 4.89 | 3.53 | Not selected |
| LogProbit | 2 | 22.18 | 0.77 | 48.35 | 3.93 | 2.07 | Selected |
| LogLogistic | 2 | 22.30 | 0.75 | 48.60 | 3.93 | 1.95 | Selected |
| Multistage | 2 | 22.26 | 0.76 | 48.52 | 4.28 | 1.86 | Selected |
| Multistage Cancer | 1 | 22.29 | 0.82 | 46.59 | 3.97 | 1.84 | AIC minimum |
| Quantal‐Linear | 1 | 23.64 | 0.58 | 49.28 | 2.05 | 1.33 | Not selected |
| Weibull | 2 | 22.84 | 0.65 | 49.68 | 4.46 | 3.31 | Not selected |
| Gamma | No fit | ||||||
DON: deoxynivalenol.
The models selected to identify the lowest BMCL10 following EFSA guidance (EFSA Scientific Committee, 2017) are identified in the column ‘Comments’ and include the model with the lowest AIC.
BMC10: benchmark concentration response of 10%; BMCL10: 95% lower/upper confidence limit for the benchmark concentration response (BMR) of 10%; na: not available, including cases where the BMC/Ls were not calculated, the fit was incomplete, or serious or conflicting comments were noted (e.g. parameters reaching boundary).
Figure G.13.
Concentration–response curve of the incidence of vomiting in pigs reported by Williams et al. (1988) (see also Table G.12)
- The (red) curve is the fitted dose–response curve and the dotted line curve indicates the 95% lower confidence limit to the calculated BMCL.
G.4.2. Concentration–response data on vomiting in dogs and cats of Hughes et al. (1999)
The CONTAM Panel used the concentration–response data on vomiting in dogs and cats of Hughes et al. (1999) (Table 13) for the BMC modelling using the BMDS software following the EFSA Scientific Committee et al. (2017) guidance.
Table G.13.
Incidence of vomiting in dogs and cats exposed to DON reported by Hughes et al. (1999)
| Dogs | Cats | ||||
|---|---|---|---|---|---|
| Concentration (mg DON/kg feed) | Number of animals per group | Incidence | Concentration (mg DON/kg feed) | Number of animals per group | Incidence |
| 0 | 14 | 0 | 0 | 2 | 0 |
| 1 | 2 | 0 | – | 2 | – |
| 2 | 2 | 0 | 2 | 2 | 0 |
| 4 | 2 | 0 | 4 | 2 | 1 |
| 6 | 14 | 0 | 6 | 2 | 0 |
| 8 | 2 | 2 | 8 | 2 | 0 |
| 10 | 13 | 7 | 10 | 8 | 4 |
DON: deoxynivalenol.
–: not reported in the paper.
G.4.2.1. Vomiting in dogs
The CONTAM Panel modelled the data on vomiting in dogs using the BMDS software following the EFSA guidance (EFSA Scientific Committee, 2017) and identified 5.05 mg DON/kg feed from the Gamma model as the BMCL10 of this data set. No alert regarding the criterion AIC‐Full+2 = 33.94 and AIC‐NULL‐2 = 46.74 was obtained. With AIC‐MIN+2 = 28.77 only the logprobit and the Gamma model were selected (see Table G.14). Since the plot of the Gamma model was not available, when applying BMDS software that of the next nearest concentration–response curve of the LogProbit is shown in Figure G.14. For more details of the analysis, see Annex A Supplementary Information DOGSVOMIT.
Table G.14.
Summary of concentration–response analysis of incidence of vomiting in dogs reported by Hughes et al. (1999)
| Models | Number of parameters | Minus Log‐likelihood | p‐value | AIC | BMC10 | BMCL10 | Comment |
|---|---|---|---|---|---|---|---|
| (mg/kg feed) | |||||||
| Full model | 7 | 8.97 | na | 31.94 | na | na | |
| Null (reduced) model | 1 | 23.37 | < 0.001 | 48.74 | na | na | |
| Probit | 2 | 12.62 | 0.20 | 29.23 | 6.91 | 4.90 | Not selected |
| Logistic | 2 | 12.86 | 0.17 | 29.71 | 6.87 | 4.90 | Not selected |
| LogProbit | 2 | 12.28 | 0.25 | 28.56 | 6.85 | 5.17 | Selected |
| LogLogistic | 2 | 12.51 | 0.22 | 29.02 | 6.79 | 4.92 | Not selected |
| Multistage | Failed | ||||||
| Multistage Cancer | 1 | 14.73 | 0.07 | 31.46 | 4.22 | 2.48 | Not selected |
| Quantal‐Linear | 1 | 16.61 | 0.02 | 35.21 | 2.32 | 1.39 | Not selected |
| Weibull | 2 | 12.75 | 0.18 | 29.49 | 6.79 | 4.65 | Selected |
| Gamma | 2 | 12.39 | 0.34 | 26.77 | 6.83 | 5.05 | AIC minimum |
DON: deoxynivalenol.
The models selected to identify the lowest BMCL10 following EFSA guidance (EFSA Scientific Committee, 2017) are identified in the column ‘Comments’ and include the model with the lowest AIC.
BMC10: benchmark concentration response of 10%; BMCL10: 95% lower confidence limit for the benchmark concentration response (BMR) of 10%; na: not available, including cases where the BMC/Ls were not calculated, the fit was incomplete, or serious or conflicting comments were noted (e.g. parameters reaching boundary).
Figure G.14.
Concentration–response curve of the incidence of vomiting in dogs reported by Hughes et al. (1999) (see also Table G.14)
- The (red) curve is the fitted dose–response curve and the dotted line curve indicates the 95% lower confidence limit to the calculated BMCL.
G.4.2.2. Vomiting in cats
The CONTAM Panel modelled the data on vomiting in cats using the BMDS software following the EFSA guidance (EFSA Scientific Committee, 2017) and identified 1.04 mg DON/kg bw from the Quantal linear model as the BMCL10 of this data set (see Table G.15). No alert regarding the criterion AIC‐Full+2 = 27.86 and AIC‐NULL‐2 = 22.64 was obtained. With AIC‐MIN+2 = 22.47 only the Multistage cancer and the Quantal linear model were selected. The fit of the Quantal linear model with the lowest BMDL10 is shown in Figure G.15. For more details of the analysis, see Annex A Supplementary Information CATSVOMIT.
Table G.15.
Summary of dose–response analysis of incidence of vomiting in cats reported by Hughes et al. (1999) using BMDS software
| Models | Number of parameters | Minus Log‐likelihood | p‐value | AIC | BMC10 | BMCL10 | Comment |
|---|---|---|---|---|---|---|---|
| (mg/kg feed) | |||||||
| Full model | 6 | 6.93 | na | 25.86 | na | na | |
| Null (reduced) model | 1 | 10.64 | < 0.001 | 22.64 | na | na | |
| Probit | 2 | 9.25 | 0.33 | 22.49 | 4.21 | 2.36 | Not selected |
| Logistic | 2 | 9.24 | 0.33 | 22.49 | 4.54 | 2.50 | Not selected |
| LogProbit | Failed | ||||||
| LogLogistic | Failed | ||||||
| Multistage | Failed | ||||||
| Multistage Cancer | 2 | 9.19 | 0.34 | 22.38 | 2.80 | 1.05 | Selected |
| Quantal‐Linear | 1 | 9.24 | 0.47 | 20.47 | 2.03 | 1.04 | AIC minimum |
| Weibull | Failed | ||||||
| Gamma | Failed | ||||||
DON: deoxynivalenol.
The models selected to identify the lowest BMCL10 following EFSA guidance (EFSA Scientific Committee, 2017) are identified in the column ‘Comments’ and include the model with the lowest AIC.
BMC10: benchmark concentration response of 10%; BMCL10: 95% lower confidence limit for the benchmark concentration response (BMR) of 10%; na: not available, including cases where the BMC/Ls were not calculated, the fit was incomplete, or serious or conflicting comments were noted (e.g. parameters reaching boundary).
Figure G.15.
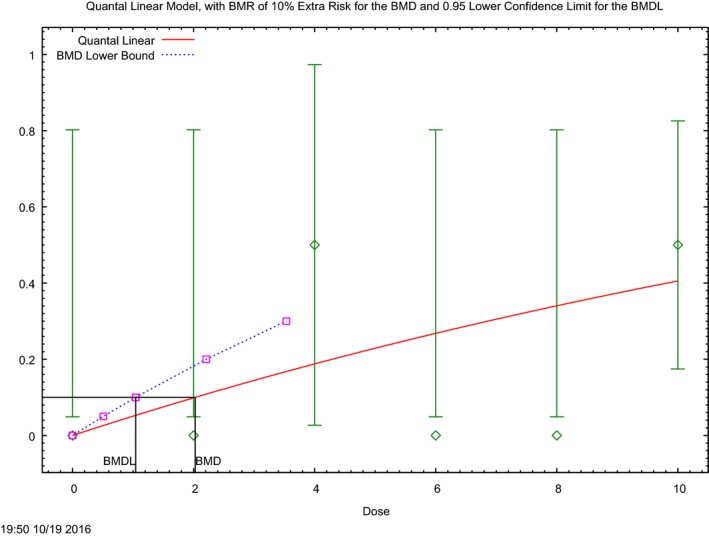
Concentration–response curve of the incidence of vomiting in cats reported by Hughes et al. (1999) (see also Table G.15)
- The (red) curve is the fitted dose–response curve and the dotted line curve indicates the 95% lower confidence limit to the calculated BMCL.
G.4.3. Vomiting in farmed mink
The CONTAM Panel used the data on emesis (vomiting and retching) of Wu et al. (2013a) in mink for the BMD modelling with n = 6 animals in four dose groups and the control group (see Section 7.8.5 for Pestka Personal communication). Tables G.16–G.18 show the data used and the details of the BMD analyses for DON, 3‐Ac‐DON and 15‐Ac‐DON. Doses were applied as mg toxin/kg bw in feed via gavage after fasting, and therefore, the CONTAM Panel could not transfer the doses into concentrations of the toxin in the animal feed.
Table G.16.
Incidence data on emesis (vomiting and/or retching) in mink exposed to DON, 3‐Ac‐DON and 15‐Ac‐DON reported by Wu et al. (2013a)
| DON | 3‐Ac‐DON | 15‐Ac‐DON | ||||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg bw per day) | Number of animals | Incidence | Dose (mg/kg bw per day) | Number of animals | Incidence | Dose (mg/kg bw per day) | Number of animals | Incidence |
| 0 | 6 | 0 | 0 | 6 | 0 | 0 | 6 | 0 |
| 0.01 | 6 | 0 | 0.05 | 6 | 0 | 0.01 | 6 | 0 |
| 0.05 | 6 | 5 | 0.25 | 6 | 1 | 0.1 | 6 | 5 |
| 0.25 | 6 | 6 | 0.5 | 6 | 5 | 0.5 | 6 | 6 |
| 0.5 | 6 | 6 | 1.0 | 6 | 6 | 1.0 | 6 | 6 |
bw: body weight.
Table G.18.
Summary of dose–response analysis of incidence of vomiting/retching and retching in mink exposed to 3‐Ac‐DON reported by Wu et al. (2013a)
| Models | Number of parameters | Minus Log‐likelihood | p‐value | AIC | BMD10 | BMDL10 | Comment |
|---|---|---|---|---|---|---|---|
| (mg/kg bw per day) | |||||||
| Full model | 5 | 5.04 | na | 20.08 | na | na | |
| Null (reduced) model | 1 | 20.19 | na | 42.18 | na | na | |
| Probit | 2 | 5.44 | 0.99 | 14.89 | 0.22 | 0.11 | Selected |
| Logistic | 2 | 5.51 | 0.97 | 15.03 | 0.23 | 0.12 | Selected |
| LogProbit | 2 | 5.41 | 1.00 | 14.83 | 0.22 | 0.11 | Selected |
| LogLogistic | 2 | 5.45 | 0.99 | 14.90 | 0.22 | 0.11 | Selected |
| Multistage | 2 | Faileda | na | ||||
| Multistage Cancer | 1 | 5.89 | 0.91 | 13.78 | 0.14 | 0.054 | AIC minimum |
| Quantal‐Linear | 1 | 8.08 | 0.25 | 18.15 | 0.045 | 0.027 | Not selected |
| Weibull | 2 | 5.41 | 1.00 | 14.62 | 0.21 | 0.081 | Selected |
| Gamma | Failed | ||||||
The models selected to identify the lowest BMDL10 following EFSA guidance (EFSA Scientific Committee, 2017) are identified in the column ‘Comments’ and include the model with the lowest AIC.
AIC: area under the curve; BMD10: benchmark dose response of 10%; BMDL10: 95% lower confidence limit for the benchmark dose response (BMR) of 10%; bw: body weight; na: not available, including cases where the BMD/Ls were not calculated, the fit was incomplete, or serious or conflicting comments were noted (e.g. parameters reaching boundary)
The multistage model was considered as failed since the output, see Suppl. Information Mink gave for model fit a statistically impossible p‐value of 2 which was taken as an indication for problems of model fitting using BMDS (see also the graphic in Supplementary Information WUDON3Ac (Annex A).
G.4.3.1. Vomiting in mink exposed to DON
The CONTAM Panel modelled the data on vomiting/retching in mink using the BMDS software following the EFSA guidance (EFSA Scientific Committee, 2017) and identified 0.0037 mg DON/kg bw per day from the Multistage cancer model as the BMDL10 of this data set (see Table G.17). No alert regarding the criterion AIC‐Full+2 = 17.40 and AIC‐NULL‐2 = 41.06 was obtained. With AIC‐MIN+2 = 9.41 all fitted models, except the Quantal linear model, were selected. It was noted that this model was one of the two models where the fit was not saturated (log‐likelihood equal to that of the full model). The lowest BMDL10 of the data set was three times lower than the lowest dose tested at which the observed incidence was zero among the six mink tested. The fit of the Multistage cancer model with the lowest BMDL10 is shown Figure G.16. For more details of the analysis, see Annex A Supplementary Information WUDON.
Table G.17.
Summary of dose–response analysis of incidence of vomiting/retching in mink exposed to DON reported by Wu et al. (2013a)
| Models | Number of parameters | Minus Log‐likelihood | p‐value | AIC | BMD10 | BMDL10 | Comment |
|---|---|---|---|---|---|---|---|
| (mg/kg bw per day) | |||||||
| Full model | 5 | 2.70 | na | 15.40 | na | na | |
| Null (reduced) model | 1 | 20.53 | 1 | 43.06 | na | na | |
| Probit | 2 | 2.70 | 1 | 9.41 | 0.037 | 0.010 | Selected |
| Logistic | 2 | 2.70 | 1 | 9.41 | 0.043 | 0.012 | Selected |
| LogProbit | 2 | 2.70 | 1 | 9.41 | 0.029 | 0.0067 | Selected |
| LogLogistic | 2 | 2.70 | 1 | 9.41 | 0.037 | 0.0067 | Selected |
| Multistage | 2 | no fit | |||||
| Multistage Cancer | 1 | 3.11 | 0.94 | 8.22 | 0.013 | 0.0037 | Selected |
| Quantal‐Linear | 1 | 4.45 | 0.48 | 10.89 | 0.0044 | 0.0022 | Not selected |
| Weibull | 2 | 2.70 | 1 | 9.41 | 0.032 | 0.0055 | Selected |
| Gamma(a) | 2 | 2.70 | 1 | 7.41 | 0.029 | 0.0057 | AIC minimum |
The models selected to identify the lowest BMDL10 following EFSA guidance (EFSA Scientific Committee, 2017) are identified in the column ‘Comments’ and include the model with the lowest AIC.
AIC: area under the curve; BMD10: benchmark dose response of 10%; BMDL10: 95% lower confidence limit for the benchmark dose response (BMR) of 10%; bw: body weight; na: not available, including cases where the BMD/Ls were not calculated, the fit was incomplete, or serious or conflicting comments were noted (e.g. parameters reaching boundary).
Figure G.16.
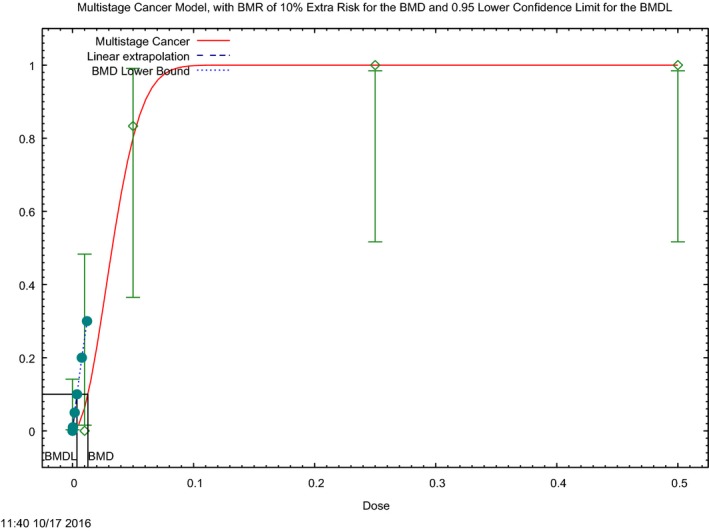
Dose–response curve of the incidence of vomiting/retching in mink exposed to DON reported by Wu et al. (2013) (see also Table G.17)
- The (red) curve is the fitted dose–response curve and the dotted line curve indicates the 95% lower confidence limit to the calculated BMDL.
G.4.3.2. Vomiting in mink exposed to 3‐Ac‐DON
The CONTAM Panel modelled the data on vomiting/retching in mink using the BMDS software following the EFSA guidance (EFSA Scientific Committee, 2017) and identified 0.054 mg 3‐Ac‐DON/kg bw per day from the Multistage cancer model as the BMDL10 of this data set (see Table G.18). This value was practically identical with the lowest dose tested at which the observed incidence of vomiting/retching was zero among the six mink tested. No alert for the criterion AIC‐Full+2 = 22.08 and AIC‐NULL‐2 = 40.18 was obtained. With AIC‐MIN+2 = 15.52 all models, except the Quantal linear model, were selected. The fit of the Multistage cancer model with the lowest BMDL10 is shown in Figure G.17. For details of the analysis, see Annex A Supplementary Information WUDON3Ac.
Figure G.17.
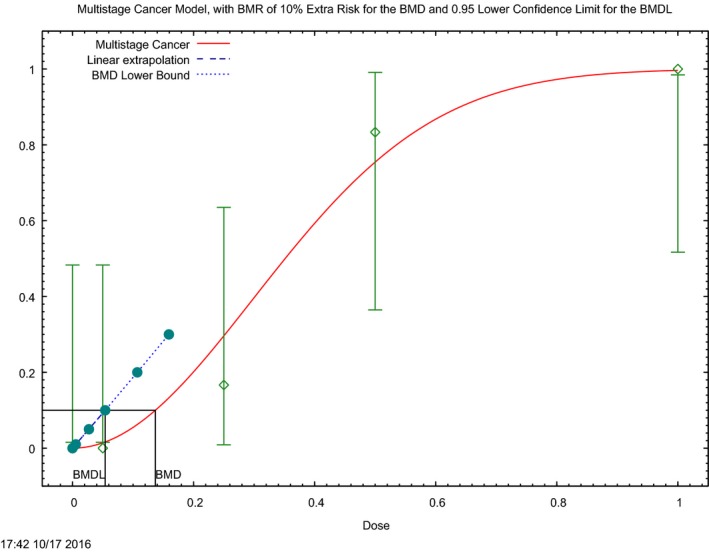
Dose–response curve of the incidence of vomiting/retching in mink exposed to 3‐Ac‐DON reported by Wu et al. (2013a) (see also Table G.18)
- The (red) curve is the fitted dose–response curve and the dotted line curve indicates the 95% lower confidence limit to the calculated BMDL.
G.4.3.3. Vomiting in mink exposed to 15‐Ac‐DON
The CONTAM Panel modelled the data on vomiting/retching in mink using the BMDS software following the EFSA guidance (EFSA Scientific Committee, 2017) and identified 0.0035 mg 15‐Ac‐DON/kg bw per day from the Quantal linear model as the BMCL10 of this data set (see Table G.19). This value was three times lower than the lowest dose tested at which the observed incidence of vomiting/retching was zero among the six mink tested. No alert for the criterion AIC‐Full+2 = 17.50 and AIC‐NULL‐2 = 41.06 was obtained. With AIC‐MIN+2 = 9.62 all models were selected. The fit of the Quantal linear model with the lowest BMDL10 is shown in Figure G.18. For more details of the analysis, see Annex A Supplementary Information WUDON15AC.
Table G.19.
Summary of dose–response analysis of incidence of vomiting/retching in mink exposed to 15‐Ac‐DON reported by Wu et al. (2013a)
| Models | Number of parameters | Minus Log‐likelihood | p‐value | AIC | BMD10 | BMDL10 | Comment |
|---|---|---|---|---|---|---|---|
| (mg/kg bw per day) | |||||||
| Full model | 5 | 2.70 | na | 15.50 | na | na | |
| Null (reduced) model | 2 | 20.53 | na | 43.06 | na | na | |
| Probit | 2 | 2.70 | 1 | 9.41 | 0.070 | 0.018 | Selected |
| Logistic | 2 | 2.70 | 1 | 9.41 | 0.084 | 0.019 | Selected |
| LogProbit | 2 | 2.70 | 1 | 9.41 | 0.047 | 0.0060 | Selected |
| LogLogistic | 2 | 2.70 | 1 | 9.41 | 0.071 | 0.0061 | Selected |
| Multistage | Failed | ||||||
| Multistage Cancer | 1 | 2.81 | 0.99 | 7.62 | 0.025 | 0.0047 | AIC minimum |
| Quantal‐Linear | 1 | 3.66 | 0.75 | 9.32 | 0.0074 | 0.0035 | Selected |
| Weibull | 2 | 2.70 | 1 | 9.41 | 0.057 | 0.0042 | Selected |
| Gamma | 2 | 2.70 | 1 | 9.41 | 0.0053 | 0.0041 | Selected |
The models selected to identify the lowest BMDL10 following EFSA guidance (EFSA Scientific Committee, 2017) are identified in the column ‘Comments’ and include the model with the lowest AIC.
BMD10: benchmark dose response of 10%; BMDL10: 95% lower confidence limit for the benchmark dose response (BMR) of 10%; bw: body weight; na: not available, including cases where the BMD/Ls were not calculated, the fit was incomplete, or serious or conflicting comments were noted (e.g. parameters reaching boundary).
Figure G.18.
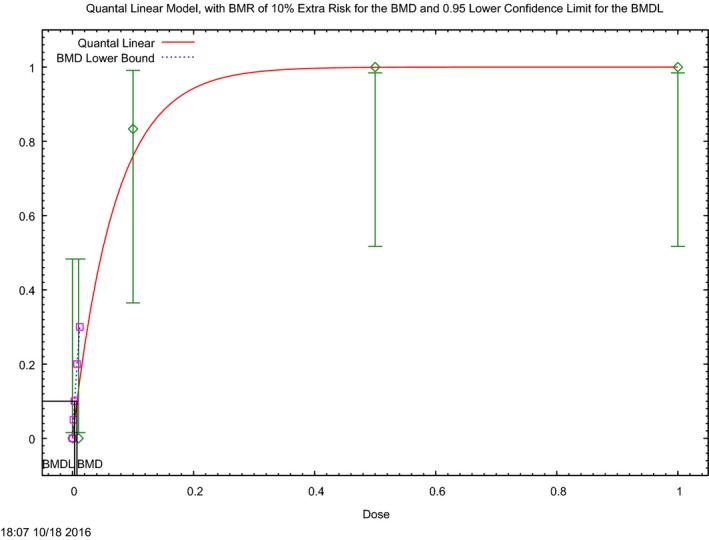
Dose–response curve of the incidence of vomiting/retching in mink exposed to 15‐Ac‐DON reported by Wu et al. (2013a) (see also Table G.19)
- The (red) curve is the fitted dose–response curve and the dotted line curve indicates the 95% lower confidence limit to the calculated BMDL.
Appendix H – Total DON (tDON) exposure in adults estimated by applying back‐calculation based on the maximum biomarker levels in the different studies available
1.
Table H.1.
Total DON (tDON) exposure in adults estimated by applying back‐calculation based on the maximum biomarker levels in the different studies presented in Tables 53 and 54 in this scientific opinion and assuming the average excretion rate of 70% and urine volume of 2 L 24‐h urine volume for adults in Europe
| Country | Age of study subjects (years) | Maximum value of tDON reported in the study (ng/mL urine)a | Maximum value of tDON used for the back calculation (ng/mL urine) | Maximum value of the back calculated exposure to DON (μg/kg bw per day) | Reference |
|---|---|---|---|---|---|
| UK | 18–64 | 436 ng/mL | 436 | 0.4 | EFSA (2015) |
| Italy | 18–64 | 75.9 ng/mL | 75.9 | 0.2 | |
| Norway | 18–64 | 86.9 ng/mL | 86.9 | 0.2 | |
| UK | 22–50 | 43.1 ng/mL | 1.8 | Gratz et al. (2014) | |
| Sweden | 18–80 | 65.8 ng/mL | 65.8 | 2.7 | Wallin et al. (2013) |
| Germany | Adults |
Free DON < 0.4 (LOQ) ng/mL DON‐glucuronide: 60.9 ng/mL |
61.0 | 2.5 | Gerding et al (2015) |
| UK | n.r. | 10.05 ng/mL | 10.1 | 0.4 | Gratz et al. (2013) |
| UK | 16–44 | 116.7 ng/mgb | 116.7 | 4.8 | Hepworth et al. (2012) |
| UK | 21–59 | 78.2 ng/mL | 78.2 | 3.2 | Turner et al. (2011a) |
| UK | 21–59 | 78.2 ng/mL | 78.2 | 3.2 | Turner et al. (2010a) |
| France | 23–74 | 28.8 ng/mL | 28.8 | 1.2 | Turner et al. (2010b) |
| UK | 19–64 | 48.2 ng/mgb | 48.2 | 2.0 | Turner et al. (2008a) |
| UK | 21–59 | 67.8 ng/mL | 67.8 | 2.8 | Turner et al. (2008b) |
| Spain | 26 | 18.8 ng/mL | 18.8 | 0.8 | Rodriguez‐Carrasco et al. (2015) |
| Belgium | 18–65 |
Free DON: 129.8 ng/mL DON‐15‐glucuronide: 460.8 ng/mL DON‐3‐glucuronide: 126.2 ng/mL DOM‐glucuronide: 172.0 ng/mL |
888.8 | 36.3 | Heyndrickx et al. (2015) |
| Italy (Southern) | 3–85c | 67.36 ng/mL | 67.4 | 2.8 | Solfrizzo et al. (2014) |
| Belgium | > 18 |
Free DON: 3.0 ng/mL DON‐15‐glucuronide: 420.0 ng/mL DON‐3‐glucuronide: 35.0 ng/mL DOM‐glucuronide: 11.0 ng/mL |
469.0 | 19.1 | Huybrechts et al. (2015) |
| Croatia | 26–33 |
Free DON: 275.0 ng/mL DON‐15‐glucuronide: 1,237.7 ng/mL DON‐3‐glucuronide: 298.1 ng/mL |
1810.8 | 73.9 | Šarkanj et al. (2013) |
| Belgium | n.r. | DON: 68.3 ng/mL | 68.3 | 2.8 | Ediage et al. (2012b) |
| Austria | 20–63 |
DON‐15‐glucuronide: 43.0 ng/mL DON‐3‐glucuronide: 13.0 ng/mL |
56 | 2.3 | Warth et al. (2012b) |
| Portugal | 20–50 | 26.2 ng/mL | 26.2 | 1.1 | Cunha and Fernandes (2012) |
| Italy | 26–87 | 14.2 ng/mL | 14.2 | 0.6 | Solfrizzo et al. (2011) |
| Italy | 30–45 | 8.0 ng/mL | 8 | 0.3 | Lattanzio et al. (2011) |
| Spain | 21–77 | 35 ng/mL | 35 | 1.4 | Rubert et al. (2011) |
bw: body weight; n.r.: not reported, na: not applicable.
Total DON (tDON) unless specified.
Creatinine adjusted DON biomarker.
Age specific biomarker data were not reported.
Annex A – Supplementary information for the BMD calculations
1.
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4718/abstract
Annex A contains supplementary information for the BMD calculations mentioned in Appendix G.
Supporting information
Supplementary information for the BMD calculations
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot A‐C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, De Saeger S, Eriksen GS, Farmer P, Fremy J‐M, Gong YY, Meyer K, Naegeli H, Parent‐Massin D, Rietjens I, van Egmond H, Altieri A, Eskola M, Gergelova P, Ramos Bordajandi L, Benkova B, Dörr B, Gkrillas A, Gustavsson N, van Manen M and Edler L, 2017. Scientific Opinion on the risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA Journal 2017;15(9):4718, 345 pp. 10.2903/j.efsa.2017.4718
Requestor: European Commission
Question Number: EFSA‐Q‐2013‐00721
Panel members: Jan Alexander, Lars Barregård, Margherita Bignami, Beat Brüschweiler, Sandra Ceccatelli, Bruce Cottrill, Michael Dinovi, Lutz Edler, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Helle Katrine Knutsen, Carlo Stefano Nebbia, Isabelle P. Oswald, Annette Petersen, Martin Rose, Alain‐Claude Roudot, Tanja Schwerdtle, Christiane Vleminckx, Günter Vollmer and Heather Wallace.
Acknowledgements: The CONTAM Panel wishes to acknowledge all European competent authorities and other stakeholders that provided occurrence data on DON, metabolites of DON and masked DON in food and feed, and supported the consumption data collection for the Comprehensive European Food Consumption Database.
Adopted: 26 January 2017
Notes
Opinion of the Scientific Committee on Food on Fusarium‐toxins Part 1: Deoxynivalenol (DON), (expressed on 2 December 1999) http://ec.europa.eu/food/fs/sc/scf/out44_en.pdf
Opinion of the Scientific Panel on contaminants in the Food Chain of the European Food Safety Authority (EFSA) on a request from the Commission related to deoxynivalenol as undesirable substance in animal feed, adopted on 2 June 2004.
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in food OJ L 364, 20.12.2006, p. 5.
Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T‐2 and HT‐2 and fumonisins in products intended for animal feeding. OJ L 229, 23.8.2006, p. 7.
Council Regulation (EEC) No 315/93 of February 1993 laying down Community procedures for contaminants in food. OJ L 37, 13.2.1993, p. 1–3.
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OJ L 364, 20.12.2006, p. 5–24.
Directive 2002/32/EC of the European Parliament and the Council of 7 May 2002 on undesirable substances in animal feed. OJ L 140, 30.5.2002, p. 10–21.
Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T‐2 and HT‐2 and fumonisins in products intended for animal feeding. OJ L 229, 23.8.2006, p. 7–9.
Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. OJ L 70, 9.3.2006, p. 12–34.
Commission Regulation (EU) No 519/2014 of 16 May 2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T‐2, HT‐2 toxin and citrinin and screening methods of analysis. OJ L 147, 17.5.2014, p. 29–43.
OJ L 70, 16.3.2005, p. 1–16.
OJ L 29, 2.2.2006, p. 3–25.
OJ L 100, 20.4.2000, p. 31–50.
OJ L 174, 7.7.2005, p. 65–68.
OJ L 339, 6.12.2006, p. 16–35.
OJ L 70, 9.3.2006, p. 12–34.
Regulation (EC) No 401/2006 of the European Parliament and the Council of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. OJ L 70, 9.3.2006, p. 12−34.
Animal feeding stuff – Determination of Deoxynivalenol in animal feed ‐ HPLC method with UV detection and immunoaffinity column clean‐up. Available online: http://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:28286,6308&cs=1C28E32D46DBFB607F7C58DFA1FA66526
Foodstuffs – Determination of deoxynivalenol in cereals, cereal products and cereal based foods for infants and young children ‐ HPLC method with immunoaffinity column cleanup and UV detection. Available online: http://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:27749,6256&cs=1C5E441A544C20E01B7841833F77E0B7B
In this opinion, if not indicated in the text explicitly the term ‘significant(ly)’ always indicates the presence of statistical significance at the level of 0.05.
Original paper in Croatian, text based on the translation to English.
From 1 January 2014 onwards, Evidence Management Unit (DATA).
Commission Regulation (EU) No 575/2011 of 16 June 2011 on the Catalogue of feed materials, OJ L 195, 17.6.2011, p. 25–65.
The analyses were performed in 2015. A full report of the comparative study was submitted to EFSA and it is available on request.
In Northern America this is known as dockage, and includes husk, rootlets, unthreshed wheat heads or spikelets, chaff, dust, loose hulls, and fragments of stem or rachis.
Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of Feed Materials.
Available online: http://www.efsa.europa.eu/en/press/news/150428.htm
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Source: FEFAC 2012 statistics. Available online: http://www.fefac.eu
FEDIAF, Personal communication by email, May 2016
The %‐contribution of DON alone to the exposure of the sum of DON and its modified forms was calculated as (exposure to DON alone divided by exposure to the sum of DON, 3‐Ac‐DON, 15‐Ac‐DON and DON‐3‐glucoside) × 100, for the different age groups.
Dietary concentrations and exposures assume a 12% moisture content in the feed (i.e. they are expressed on an 88% DM basis).
Original paper in Chinese, text based on the translation to English.
OECD Guidelines for the Testing of Chemicals, section 4. Test No. 452 (2009): Chronic Toxicity Studies. Available from: http://www.oecd-ilibrary.org/environment/test-no-452-chronic-toxicity-studies_9789264071209-en
In this opinion the reference of ‘Eriksen and Alexander, 1998’ is cited as NCM, 1998.
Original paper in Russian, text based on the translation to English.
Original paper in Spanish, text based on the translation to English.
EFSA contacted the authors of Wu et al. (2013a) for further clarifications of the study and received the reply by email on 31.8.2015.
Vomiting is defined as rhythmic abdominal contraction with oral expulsion of either solid or liquid material, whereas retching refers to responses that mimicked vomiting but without any material being expelled.
The term body weight is used for adult dogs and cats as they do not gain weight.
Urine creatinine daily excretion (mg) is generally considered stable for a healthy individual. Urine flow rate variation inter‐ and intra‐individually can influence urinary creatinine and urinary DON concentration in a similar manner. Hence urinary creatinine concentration is used for adjusting urine flow rate in DON biomarker studies. However, creatinine excretion can be influenced by age, diet and health condition. Urinary creatinine mean concentration is typically higher in adults than in children, hence there is controversy when adults and children biomarker levels are compared using creatinine adjusted values.
It should be noted that these uncertainties are not presented in Section 9 on Uncertainty Analysis because the biomarker data were used as supporting information for this opinion and thus they do not influence the outcome of the risk assessment on DON, its acetylated and modified forms in food and feed.
Participants of the biomarker studies had been studied within a clinical study context under the regulation of the Declaration of Helsinki and therefore must have been informed on the aim of the biomarker study and given informed consent. The CONTAM Panel assumed that any illness or acute disease of a participant would have been recognised by the clinical staff and its presence, in particular vomiting in days before the visit would have excluded the proband.
Back‐calculated from a tDON value of 1,810.8 ng/mL, which was a sum of free DON (275.0 ng/mL), DON‐3‐glucuronide (298.1 g/mL) and DON‐15‐glucuronide (1,237.7 ng/mL)
Comission Regulation (EU) No 575/2011 of 16 June 2011 on the Catalogue of feed materials, OJ L 195, 17.6.2011, p. 25‐65.
Available online: http://www.Fediaf.org
The European Pet Food Industry Federation (FEDIAF), Personal communication by email, May 2016.
It should also be noted that all graphics of this Appendix are copied without modification from the output of the software including the terms used by BMDS and PROAST, which are not necessarily the most appropriate terms and may differ from the text. However, the meaning should be clear from the context. As an example, the new BMDS uses as default now ‘Fraction Affected’ for incidence and it uses always ‘Dose’ even when it is a concentration.
References
- Abia WA, Warth B, Sulyok M, Krska R, Tchana A, Njobeh PB, Turner PC, Kouanfack C, Eyongetah M, Dutton M and Moundipa PF, 2013. Bio‐monitoring of mycotoxin exposure in Cameroon using a urinary multi‐biomarker approach. Food and Chemical Toxicology, 62, 927–934. [DOI] [PubMed] [Google Scholar]
- Abbas HK, Mirocha CJ, Pawlosky RJ and Pusch DJ, 1985. Effect of cleaning, milling and baking on deoxynivalenol in wheat. Applied and Environmental Microbiology, 50, 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson D, House JD and Nyachoti CM, 2005. Reduction of deoxynivalenol in barley by treatment with aqueous sodium carbonate and heat. Mycopathologia, 160, 297–301. [DOI] [PubMed] [Google Scholar]
- Abdel‐Wahhab MS, El‐Kady AA, Hassan AM, Abd El‐Moneim OM and Abdel‐Aziem SH, 2015. Effectiveness of activated carbon and Egyptian montmorillonite in the protection against deoxynivalenol‐induced cytotoxicity and genotoxicity in rats. Food and Chemical Toxicology, 83, 174–182. [DOI] [PubMed] [Google Scholar]
- Accensi F, Pinton P, Callu P, Abella‐Bourges N, Guelfi JF, Grosjean F and Oswald IP, 2006. Ingestion of low doses of deoxynivalenol does not affect hematological, biochemical, or immune responses of piglets. Journal of Animal Science, 84, 1935–1942. [DOI] [PubMed] [Google Scholar]
- AFRC (Agricultural and Food Research Council), 1993. Energy and protein requirements of ruminants. An advisory manual prepared by the AFRC Technical Committee on Responses to Nutrients. CAB International.
- AFSSA (Agence française de sécurité sanitaire des aliments), 2009. Étude Individuelle Nationale des Consommations Alimentaires 2 (INCA 2) 2006‐2007. Available online: http://www.anses.fr/Documents/PASER-Ra-INCA2.pdf
- Akbari P, Braber S, Gremmels H, Koelink PJ, Verheijden KA, Garssen J and Fink‐Gremmels J, 2014. Deoxynivalenol: a trigger for intestinal integrity breakdown. The FASEB Journal, 28, 2414–2429. [DOI] [PubMed] [Google Scholar]
- Alassane‐Kpembi I, Kolf‐Clauw M, Gauthier T, Abrami R, Abiola FA, Oswald IP and Puel O, 2013. New insight into mycotoxin mixtures: the toxicity of low doses of type B trichothecenes against intestinal epithelial cells is synergistic. Toxicology and Applied Pharmacology, 272, 191–198. [DOI] [PubMed] [Google Scholar]
- Alassane‐Kpembi I, Puel O and Oswald IP, 2015. Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetyl derivatives in intestinal epithelial cells. Archives of Toxicology, 89, 1337–1346. [DOI] [PubMed] [Google Scholar]
- Albert AL, Champoux PD and Davis AH, 2013. QuickToxTM kit for QuickScan DON (vomitoxin). Journal of AOAC International, 96, 1006–1016. [DOI] [PubMed] [Google Scholar]
- Al‐Hazmi MA and Waggas AM, 2013. Neurophysiological and behavioral effects of mycotoxin deoxynivalenol and fumonisin. African Journal of Microbiology Research, 7, 1371–1377. [Google Scholar]
- Alkadri D, Rubert J, Prodi A, Pisi A, Manes J and Soler C, 2014. Natural co‐occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chemistry, 157, 111–118. [DOI] [PubMed] [Google Scholar]
- Alm H, Brüssow K‐P, Torner H, Vanselow J, Tomek W, Dänicke S and Tiemann U, 2006. Influence of Fusarium‐toxin contaminated feed on initial quality and meiotic competence of gilt oocytes. Reproductive Toxicology, 22, 44–50. [DOI] [PubMed] [Google Scholar]
- Almeida I, Martins HM, Santos S, Costa JM and Bernardo F, 2011. Co‐occurrence of mycotoxins in swine feed produced in Portugal. Mycotoxin Research, 27, 177–181. [DOI] [PubMed] [Google Scholar]
- Amuzie CJ and Pestka JJ, 2010. Suppression of insulin‐like growth factor acid‐labile subunit expression–a novel mechanism for deoxynivalenol‐induced growth retardation. Toxicological Sciences, 113, 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuzie CJ, Harkema JR and Pestka JJ, 2008. Tissue distribution and proinflammatory cytokine induction by the trichothecene deoxynivalenol in the mouse: comparison of nasal vs. oral exposure. Toxicology, 248, 39–44. [DOI] [PubMed] [Google Scholar]
- Antonissen G, Martel A, Pasmans F, Ducatelle R, Verbrugghe E, Vandenbroucke V, Li S, Haesebrouck F, Van Immerseel F and Croubels S, 2014. The impact of fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins, 6, 430–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSES (Agence Nationale de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail), 2011. Second French Total Diet Study (TDS 2) Report 1. Inorganic contaminants, minerals, persistent organic pollutants, mycotoxins and phytoestrogens. Available online: http://www.anses.fr/Documents/PASER2006sa0361Ra1EN.pdf
- Anselme M, Tangni EK, Pussemier L, Motte JC, Van Hove F, Schneider YJ, Van Peteghem C and Larondelle Y, 2006. Comparison of ochratoxin A and deoxynivalenol in organically and conventionally produced beers sold on the Belgian market. Food Additives and Contaminants, 23, 910–918. [DOI] [PubMed] [Google Scholar]
- Arnold DL, Karpinski KF, McGuire PF, Nera EA, Zawidzka ZZ, Lok E, Campbell JS, Tryphonas L and Scott PM, 1986. A short‐term feeding study with deoxynivalenol (vomitoxin) using rats. Fundamental and Applied Toxicology, 6, 691–696. [DOI] [PubMed] [Google Scholar]
- Avantaggiato G, Havenaar R and Visconti A, 2004. Evaluation of the intestinal absorption of deoxynivalenol and nivalenol by an in vitro gastrointestinal model, and the binding efficacy of activated carbon and other adsorbent materials. Food and Chemical Toxicology, 42, 817–824. [DOI] [PubMed] [Google Scholar]
- Awad WA, Böhm J, Razzazi‐Fazeli E, Hulan HW and Zentek J, 2004. Effects of deoxynivalenol on general performance and electrophysiological properties of intestinal mucosa of broiler chickens. Poultry Science, 83, 1964–1972. [DOI] [PubMed] [Google Scholar]
- Awad WA, Böhm J, Razzazi‐Fazeli E and Zentek J, 2006. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poultry Science, 85, 974–979. [DOI] [PubMed] [Google Scholar]
- Awad WA, Ghareeb K and Böhm J, 2012a. The toxicity of Fusarium mycotoxins deoxynivalenol in poultry feeding. World's Poultry Science Journal, 68, 651–668. [Google Scholar]
- Awad WA, Ghareeb K, Böhm J and Zentek J, 2013. The toxicological impacts of the fusarium mycotoxin, deoxynivalenol, in poultry flocks with special reference to immunotoxicity. Toxins, 5, 912–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad WA, Ghareeb K, Dadak A, Gille A, Staniek K, Hess M and Böhm J, 2012b. Genotoxic effects of deoxynivalenol in broiler chickens fed low‐protein feeds. Poultry Science, 91, 550–555. [DOI] [PubMed] [Google Scholar]
- Awad WA, Ghareeb K, Dadak A, Hess M and Böhm J, 2014. Single and combined effects of deoxynivalenol mycotoxin and a microbial feed additive on lymphocyte DNA damage and oxidative stress in broiler chickens. PLoS ONE, 9, e88028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad WA, Hess M, Twarużek M, Grajewski J, Kosicki R, Böhm J and Zentek J, 2011. The impact of the Fusarium mycotoxin deoxynivalenol on the health and performance of broiler chickens. International Journal of Molecular Sciences, 12, 7996–8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcona‐Olivera JI, Ouyang Y, Murtha J, Chu FS and Pestka JJ, 1995. Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): relationship to toxin distribution and protein synthesis inhibition. Toxicology and Applied Pharmacology, 133, 109–120. [DOI] [PubMed] [Google Scholar]
- Baars AJ, Van Apeldoorn M and Wouters M, 1999. Appendix 1 Toxicology, in Pieters MN, Fiolet DCM, Baars AJ. 1999. Deoxynivalenol. Derivation of concentration limits in wheat and wheat containing products. RIVM report 388802008, Rijks Instituut voor Volksgezondheid en Milieu, Bilthoven, The Netherlands. Available online: http://www.rivm.nl/en/Documents_and_publications/Scientific/Reports/1999/November/Deoxynivalenol_Derivation_of_concentration_limits_in_wheat_and_wheat_containing_food_products?sp=cml2bXE9ZmFsc2U7c2VhcmNoYmFzZT0yMjYxMDtyaXZtcT1mYWxzZTs=&pagenr=2262
- Bakker GJI, Sizoo EA, Jekel AA, Pereboom‐de‐Fauw DPKH, Schothorst EC and van Egmond HP, 2009. determination of mean daily intaks of aflatoxin B1, aflatoxin M1, ochratoxin A, trichothecenes and fumonisins in 24‐hour diets of children in the Netherlands. World Mycotoxin Journal, 2, 451–459. [Google Scholar]
- Bensassi F, El Golli‐Bennour E, Abid‐Essefi S, Bouaziz C, Hajlaoui MR and Bacha H, 2009. Pathway of deoxynivalenol‐induced apoptosis in human colon carcinoma cells. Toxicology, 264, 104–109. [DOI] [PubMed] [Google Scholar]
- Bergsjö B, Herstad O and Nafstad I, 1993a. Effects of feeding deoxynivalenol‐contaminated oats on reproduction performance in White Leghorn British. Poultry Science, 34, 147–159. [DOI] [PubMed] [Google Scholar]
- Bergsjö B, Langseth W, Nafstad I, Jansen JH and Larsen HJ, 1993b. The effects of naturally deoxynivalenol‐contaminated oats on the clinical condition, blood parameters, performance and carcass composition of growing pigs. Veterinary Research Communication, 17, 283–294. [DOI] [PubMed] [Google Scholar]
- Bergsjø B, Matre T and Nafstad I, 1992. Effects of diets with graded levels of deoxynivalenol on performance in growing pigs. Journal of Veterinary Medicine, A, 39, 752–758. [DOI] [PubMed] [Google Scholar]
- Berntssen MHG, Olsvik PA, Torstensen BE, Julshamn K, Midtun T, Goksøyr A, Johansen J, Sigholt T, Joerum N, Jakobsen J‐V, Lundebye A‐K and Lock E‐J, 2010. Reducing persistent organic pollutants while maintaining long chain omega‐3 fatty acid in farmed Atlantic salmon using decontaminated fish oils for an entire production cycle. Chemosphere, 81, 242–252. [DOI] [PubMed] [Google Scholar]
- Berthiller F, Dall'Asta C, Schuhmacher R, Lemmens M, Adam G and Krska R, 2005. Masked mycotoxins: determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography‐tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 53, 3421–3425. [DOI] [PubMed] [Google Scholar]
- Berthiller F, Dall'Asta C, Corradini R, Marchelli R, Sulyok M, Krska R, Adam G and Schumacher R, 2009. Occurrence of deoxynivalenol and its 3‐β‐D‐glucoside in wheat and maize. Food Additives and Contaminants, 26, 507–511. [DOI] [PubMed] [Google Scholar]
- Berthiller F, Krska R, Domig KJ, Kneifel W, Juge N, Schuhmacher R and Adam G, 2011. Hydrolytic fate of deoxynivalenol‐3‐glucoside during digestion. Toxicology Letters, 206, 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiller F, Crews C, Dall'Asta C, De Saeger SD, Haesaert G, Karlovsky P, Oswald IP, Seefelder W, Speijers G and Stroka J, 2013. Masked mycotoxins: a review. Molecular and Nutrition Food Research, 57, 165–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuzzi T, Rastelli S, Mulazzi A, Donadini G and Pietri A, 2011. Mycotoxin occurrence in beer produced in several European countries. Food Control, 22, 2059–2064. [Google Scholar]
- Betina V, 1989. Structure‐activity relationship among mycotoxins. Chemico‐Biological Interactions, 71, 105–146. [DOI] [PubMed] [Google Scholar]
- Benito P and Miller D, 1998. Iron absorption and bioavailability: an updated review. Nutrition Research, 18, 581–603. [Google Scholar]
- Bhat RV, Beedu SR, Ramakrishna Y and Munshi KL, 1989. Outbreak of trichothecene mycotoxicosis associated with consumption of mould‐damaged wheat products in Kashmir Valley, India. The Lancet, 333, 35–37. [DOI] [PubMed] [Google Scholar]
- Bin‐Umer MA, McLaughlin JE, Basu D, McCormick S and Tumer NE, 2011. Trichothecene mycotoxins inhibit mitochondrial translation–implication for the mechanism of toxicity. Toxins, 3, 1484–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgrami KS, Rahman MF, Massod A and Sahay SS, 1993. DON induced histopathological abnormalities in mice (Mus musculus). National Academy Science Letters, 16, 161–162. [Google Scholar]
- Black RM, Clarke RJ and Read RW, 1987. Detection of trace levels of trichothecene mycotoxins in environmental residues and foodstuffs using gas chromatography with mass spectrometric or electron‐capture detection. Journal of Chromatography, 388, 365–378. [DOI] [PubMed] [Google Scholar]
- Błajet‐Kosicka A, Twaru≐k M, Kosicki R and Sibiorowska E, 2014. Co‐occurrence and evaluation of mycotoxins in organic and conventional rye grain and products. Food Control, 38, 61–66. [Google Scholar]
- Boermans HJ and Leung MCK, 2007. Mycotoxins and the pet food industry: toxicological evidence and risk assessment. International Journal of Food Microbiology, 119, 95–102. [DOI] [PubMed] [Google Scholar]
- Bondy GS, Coady L, Curran I, Caldwell D, Armstrong C, Aziz SA, Nunnikhoven A, Gannon AM, Liston V, Shenton J and Mehta R, 2016. Effects of chronic deoxynivalenol exposure on p53 heterozygous and p53 homozygous mice. Food and Chemical Toxicology 96, 24–34. [DOI] [PubMed] [Google Scholar]
- Bony S, Carcelen M, Olivier L and Devaux A, 2006. Genotoxicity assessment of deoxynivalenol in the Caco‐2 cell line model using the Comet assay. Toxicology Letters, 166, 67–76. [DOI] [PubMed] [Google Scholar]
- Boon PE, Bakker MI, van Klaveren JD and van Rossumet CTM, 2009. Risk assessment of the dietary exposure to contaminants and pesticide residues in young children in the Netherlands. Report 350070002/2009.
- Böhm J and Razzazi E, 2003. Fütterungseinfluss von Deoxynivalenol‐belastetem Weizen bei Ferkeln (Effects of feeding deoxynivalenol contaminated wheat to piglets). Mycotoxin Research, 19, 176–179. [DOI] [PubMed] [Google Scholar]
- Böhm J, Koinig L, Razzazi‐Fazeli E, Blajet‐Kosicka A, Twaruzek M, Grajewski J and Lang C, 2010. Survey and risk assessment of the mycotoxins deoxynivalenol, zearalenone, fumonisins, ochratoxin A, and aflatoxins in commercial dry dog food. Mycotoxin Research, 26, 147–153. [DOI] [PubMed] [Google Scholar]
- Bracarense AP, Lucioli J, Grenier B, Drociunas‐Pacheco G, Moll WD, Schatzmayr G and Oswald IP, 2012. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. British Journal of Nutrition, 107, 1776–1786. [DOI] [PubMed] [Google Scholar]
- Brenn‐Struckhofova Z, Füreder C, Cichna‐Markl M and Razzazi‐Fazeli E, 2009. Co‐isolation of deoxynivalenol and zearalenone with sol‐gel immunoaffinity columns for their determination in wheat and wheat products. Journal of Chromatography A, 1216, 5828–5837. [DOI] [PubMed] [Google Scholar]
- Brera C, Peduto A, Debegnach F, Pannunzi E, Prantera E, Gregori E, De Giacomo M and De Santis B, 2013. Study of the influence of the milling process on the distribution of deoxynivalenol content from the caryopsis to cooked pasta. Food Control, 32, 309–312. [Google Scholar]
- Brera C, de Santis B, Debegnach F, Miano B, Moretti G, Lanzone A, Del Sordo G, Buonsenso D, Chiaretti A, Hardie L, White K, Brantsæter AL, Knutsen H, Eriksen GS, Sandvik M, Wells L, Allen S and Sathyapalan T, 2015. Experimental study of deoxynivalenol biomarkers in urine. EFSA Supporting Publication 2015;12(6):EN‐818, 136 pp. 10.2903/sp.efsa.2015.en-818 [DOI] [Google Scholar]
- Brewer D, Mcalees AJ and Taylor A, 1996. Ovine ill‐thrift in Nova Scotia. 13. Anorexia and digestibility decline in female lambs given 3,7,11‐3H3‐3‐acetoxy‐7,15‐dihydroxy‐12,3‐epoxytrichothec‐9‐en‐8‐one. Proceedings of the Nova Scotian Institute of Science, 41, 39–47. [Google Scholar]
- Broekaert N, Devreese M, De Mil T, Fraeyman S, De Baere S, De Saeger S, De Backer P and Croubels S, 2014. Development and validation of an LC–MS/MS method for the toxicokinetic study of deoxynivalenol and its acetylated derivatives in chicken and pig plasma. Journal of Chromatography B, 971, 43–51. [DOI] [PubMed] [Google Scholar]
- Broekaert N, Devreese M, De Mil T, Fraeyman S, Antonissen G, De Baere S, De Backer P, Vermeulen A and Croubels S, 2015. Oral bioavailability, hydrolysis, and comparative toxicokinetics of 3‐acetyldeoxynivalenol and 15‐acetyldeoxynivalenol in broiler chickens and pigs. Journal of Agriculture and Food Chemistry, 63, 8734–8742. [DOI] [PubMed] [Google Scholar]
- Broekaert N, Devreese M, van Bergen T, Schauvliege S, De Boevre M, De Saeger S, Vanhaecke L, Berthiller F, Michmayr H, Malachová A and Adam G, 2016. In vivo contribution of deoxynivalenol‐3‐β‐d‐glucoside to deoxynivalenol exposure in broiler chickens and pigs: oral bioavailability, hydrolysis and toxicokinetics. Archives of Toxicology, 91, 1–14. [DOI] [PubMed] [Google Scholar]
- Burger H‐M, Shepherd GS, Louw W, Rheeder JP and Gelderblom WCA, 2013. The mycotoxin distribution in maize milling fractions under experimental conditions. International Journal of Food Microbiology 165, 57–64. [DOI] [PubMed] [Google Scholar]
- CAC (Codex Alimentarius Commission), 2014. Report of the Eighth Session of the Codex Committee on Contaminants in Foods, the Hague, the Netherlands, 31 March – 4 April 2014. Available online: http://www.codexalimentarius.org/meetings-reports/en/?sortingDate=012014
- Caloni F and Cortinovis C, 2010. Effects of fusariotoxins in the equine species. The Veterinary Journal, 186, 157–161. [DOI] [PubMed] [Google Scholar]
- Campagnoli A, Cheli F, Polidori C, Zaninelli M, Zecca O, Savoini G, Pinotti L and Dell'Orto V, 2011. Use of the electronic nose as a screening tool for the recognition of durum wheat naturally contaminated by deoxynivalenol: a preliminary approach. Sensors, 11, 4899–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano‐Sancho G, Gauchi J‐P, Sanchis V, Marín S and Ramos AJ, 2011. Quantitative dietary exposure assessment of the Catalonian population (Spain) to the mycotoxin, Deoxynivalenol. Food Additives and Contaminants A 28, 1098–1109. [DOI] [PubMed] [Google Scholar]
- Carabano R and Piquer J, 1998. The digestive system of the rabbit In: de Blas C. and Wiseman J. (eds.). The Nutrition of the Rabbit. CABI Publishing, Oxfordshire: pp. 1–16. [Google Scholar]
- Cheli F, Pinotti L, Rossi L and dell‘Orto V, 2013. Effect of milling procedures on mycotoxin distribution in wheat fractions: A review. LWT Food Science and Technology, 54, 307–314. [Google Scholar]
- Charmley E, Trenholm HL, Thompson BK, Vudathala D, Nicholson JWG, Prelusky DB and Charmeley LL, 1993. Influence of level of deoxynivalenol in the diet of dairy cows on feed intake, milk production, and its composition. Journal of Dairy Science, 76, 3580–3587. [DOI] [PubMed] [Google Scholar]
- Chatopadhyay P, Gupta V, Gogoi HK and Singh L, 2011. Hematotoxicity of deoxynivalenol in BALB/c mice. Journal of Pharmacology and Pharmacotherapeutics, 2, 115–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez ER, 1984. Vomitoxin‐contaminated wheat in pig diets: pregnant and lactating gilts and weaners. Canadian Journal of Animal Science, 64, 717–723. [Google Scholar]
- Choi S‐W, Chang H‐J, Lee N and Chun HS, 2011. A surface plasmon resonance sensor for the detection of deoxynivalenol using a molecularly imprinted polymer. Sensors, 11, 8654–8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirlini M, Dall'Asta C and Galaverna G, 2012. Hyphenated chromatographic techniques for structural characterization and determination of masked mycotoxins. Journal of Chromatography A, 1255, 145–152. [DOI] [PubMed] [Google Scholar]
- Cirillo T, Ritieni A, Visone M and Cocchieri RA, 2003. Evaluation of conventional and organic Italian foodstuffs for deoxynivalenol and fumonisins B1 and B2. Journal of Agricultural and Food Chemistry, 51, 8128–8131. [DOI] [PubMed] [Google Scholar]
- Clark ES, Flannery BM, Gardner EM and Pestka JJ, 2015. High sensitivity of aged mice to deoxynivalenol (vomitoxin)‐induced anorexia corresponds to elevated proinflammatory cytokine and satiety hormone responses. Toxins (Basel), 7, 4199–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CNNS , 1982. Report of the 1882 National Nutrition Survey data by China Academy of Preventive Medicine.
- Cortinovis C, Battini M and Caloni F, 2012. Deoxynivalenol and T‐2 toxin in raw feed for horses. Journal of Equine Veterinary Science, 32, 72–74. [Google Scholar]
- Côté LM, Dahlem AM, Yoshizawa T, Swanson SP and Buck WB, 1986. Excretion of deoxynivalenol and its metabolite in milk, urine, and faeces of lactating dairy cows. Journal of Dairy Science, 69, 2416–2423. [DOI] [PubMed] [Google Scholar]
- Côté L‐M, Buck W and Jeffery E, 1997. Lack of hepatic microsomal metabolism of deoxynivalenol and its metabolite DOM‐1. Food and Chemical Toxicology, 25, 291–295. [DOI] [PubMed] [Google Scholar]
- Collins TFX, Sprando RL, Black TN, Olejnik N, Eppley RM, Hines FA, Rorie J and Ruggles DI, 2006. Effects of deoxynivalenol (DON, vomitoxin) on in utero development in rats. Food and Chemical Toxicology, 44, 747–757. [DOI] [PubMed] [Google Scholar]
- Coppock RW, Swanson SP, Gelberg SP, Gelberg HB, Koritz GD, Hoffman WE, Buck WB and Vesonder RF, 1985. Preliminary study on the pharmacokinetics and toxicopathy of deoxynivalenol (vomitoxin) in swine. American Journal of Veterinary Research, 46, 169–174. [PubMed] [Google Scholar]
- Cressey PJ and Thomson BM, 2006. Risk profile: mycotoxins in the New Zealand food supply. ESR Client Report FW0617, Wellington, New Zealand: New Zealand Food Safety Authority. Available online: http://www.mpi.govt.nz/document-vault/12924
- Cunha SC and Fernandez JO, 2010. Development and validation of a methods based on a QuEChERS procedure and heart‐cutting GC‐MS for determination of five mycotoxins in cereals products. Journal of Separation Science, 33, 600–609. [DOI] [PubMed] [Google Scholar]
- Cunha SC and Fernandes JO, 2012. Development and validation of a gas chromatography‐mass spectrometry method for determination of deoxynivalenol and its metabolites in human urine. Food and Chemical Toxicology, 50, 1019–1026. [DOI] [PubMed] [Google Scholar]
- Dänicke S, 2002. Effects of Fusarium toxin contaminated wheat grain and of a detoxifying agent on rumen physiological parameters and in sacco dry matter degradation of wheat straw and lucerne hay in wethers. Journal of Animal Feed Sciences, 11, 437–451. [Google Scholar]
- Dänicke S, Gädeken D, Ueberschär KH, Meyer U and Scholz H, 2002. Effects of fusarium toxin contaminated wheat and of a detoxifying agent on performance of growing bulls, on nutrient digestibility in wethers and on the carry‐over of zearalenone. Archives of Animal Nutrition, 56, 245–261. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Matthes S, Halle I, Ueberschär KH, Döll S and Valenta H, 2003. Effects of graded levels of Fusarium toxin‐contaminated wheat and of a detoxifying agent in broiler diets on performance, nutrient digestibility and blood chemical parameters. British Poultry Science, 44, 113–126. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Valenta H and Döll S, 2004a. On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. Archives of Animal Nutrition, 58, 169–180. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Valenta H, Döll S, Ganter M and Flachowsky G, 2004c. On the effectiveness of a detoxifying agent in preventing fusario‐toxicosis in fattening pigs. Animal Feed Science and Technology, 114, 141–157. [Google Scholar]
- Dänicke S, Valenta H, Goyarts T, Razzazi E and Böhm J, 2004d. On the effects of increasing deoxynivalenol (DON) concentrations in pig feed on growth performance and utilization of nutrients and on DON metabolism. Journal of Animal and Feed Sciences, 13, 539–556. [Google Scholar]
- Dänicke S, Valenta H, Klobasa F, Döll S, Ganter M and Flachowsky G, 2004e. Effects of graded levels of Fusarium toxin contaminated wheat in diets for fattening pigs on growth performance, nutrient digestibility, deoxynivalenol balance and clinical serum characteristics. Archives of Animal Nutrition, 58, 1–17. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Matthäus K, Lebzien P, Valenta H, Stemme K, Ueberschär KH, Razzazi‐Fazeli E, Böhm J and Flachowski G, 2005a. Effects of Fusarium toxin‐contaminated wheat grain on nutrient turnover, microbial protein synthesis and metabolism of deoxynivalenol and zearalenone in the rumen of dairy cows. Journal of Animal Physiology and Animal Nutrition, 89, 303–315. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Valenta H, Gareis M, Lucht HW and von Reichenbach H, 2005b. On the effects of a hydrothermal treatment of deoxynivalenol (DON)‐contaminated wheat in the presence of sodium metabisulphite (Na2S2O5) on DON reduction and on piglet performance. Animal Feed Science and Technology, 118, 93–108. [Google Scholar]
- Dänicke S, Goyarts T, Döll S, Grove N, Spolders M and Flachowsky G, 2006. Effects of the Fusarium toxin deoxynivalenol on tissue protein synthesis in pigs. Toxicology Letters, 165, 297–311. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Valenta H and Matthes S, 2007a. On the interactions between Fusarium toxin‐contaminated wheat and nonstarch polysaccharide hydrolyzing enzymes in diets of broilers on performance, intestinal viscosity, and carryover of deoxynivalenol. Poultry Science, 86, 291–298. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Valenta H, Ueberschär K‐H and Matthes S, 2007b. On the interactions between Fusarium toxin‐contaminated wheat and non‐starch‐polysaccharide hydrolysing enzymes in turkey diets on performance, health and carry‐over of deoxynivalenol and zearalenone. British Poultry Science, 48, 39–48. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Hegewald A‐K, Kahlert S, Kluess S, Rothkötter H‐J, Breves G and Döll S, 2010. Studies on the toxicity of deoxynivalenol (DON), sodium metabisulfite, DON‐sulfonate (DONS) and de‐epoxy‐DON for porcine peripheral blood mononuclear cells and the Intestinal Porcine Epithelial Cell lines IPEC‐1 and IPEC‐J2, and on effects of DON and DONS on piglets. Food and Chemical Toxicology, 48, 2154–2162. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Brosig B, Kahlert S, Panther P, Reinhardt N, Diesing AK, Kluess J, Kersten S, Valenta H and Rothkötter H‐J, 2012. The plasma clearance of the Fusarium toxin deoxynivalenol (DON) is decreased in endotoxemic pigs. Food and Chemical Toxicology, 50, 4405–4411. [DOI] [PubMed] [Google Scholar]
- Dänicke S and Brezina U, 2013. Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: consequences for diagnosis of exposure and intoxication and carry over. Food and Chemical Toxicology, 60, 58–75. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Brosig B, Kersten S, Kluess J, Kahlert S, Panther P, Diesing A‐K and Rothkötter H‐J, 2013. The Fusarium toxin deoxynivalenol (DON) modulates the LPS induced acute phase reaction in pigs. Toxicology Letters, 220, 172–180. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Valenta H, Ganter M, Brosig B, Kersten S, Diesing A‐K, Kahlert S, Panther P, Kluess J and Rothkötter H‐J, 2014. Lipopolysaccharides (LPS) modulate the metabolism of deoxynivalenol (DON) in the pig. Mycotoxins Research, 30, 161–170. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Winkler J, Meyer U, Frahm J and Kersten S, 2016. Haematological, clinical–chemical and immunological consequences of feeding Fusarium toxin contaminated diets to early lactating dairy cows. Mycotoxin Research 33, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Asta C, Dall'Erta A, Mantovani P, Massi A and Galaverna G, 2013. Occurrence of deoxynivalenol and deoxynivalenol‐3‐glucoside in durum wheat. World Mycotoxin Journal, 6, 83–91. [Google Scholar]
- Dall'Erta A, Cirlini M, Dall'Asta M, Del Rio D, Galaverna G and Dall'Asta C, 2013. Masked mycotoxins are efficiently hydrolysed by human colonic microbiota releasing their aglycones. Chemical Research in Toxicology, 26, 305–312. [DOI] [PubMed] [Google Scholar]
- De Angelis E, Monaci L, Pascale M and Visconti A, 2013. Fate of deoxynivalenol, T‐2 and HT‐2 toxins and their glucoside conjugates from flour to bread: an investigation by high‐performance liquid chromatography high‐resolution mass spectrometry. Food Additives and Contaminants Part A Chemistry, analysis, control, exposure and risk assessment, 30, 345–355. [DOI] [PubMed] [Google Scholar]
- De Angelis E, Monaci L and Visconti A, 2014. Investigation on the stability of deoxynivalenol and DON‐3 glucoside during gastro‐duodenal in vitro digestion of a naturally contaminated bread model food. Food Control, 43, 270–275. [Google Scholar]
- De Boevre M, Di Mavungu JD, Maene P, Audenaert K, Deforce D, Haesaert G, Eeckhout M, Callebaut A, Berthiller F, Van Peteghem C and De Saeger S, 2012. Development and validation of an LC‐MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T‐2‐toxin and some masked metabolites in different cereals and cereal‐derived food. Food Additives and Contaminants Part A, 29, 819–835. [DOI] [PubMed] [Google Scholar]
- De Dominicis E, Commissati I, Gritti E, Catellani D and Suman M, 2015. Quantitative targeted and retrospective data analysis of relevant pesticides, antibiotics and mycotoxins in bakery products by liquid chromatography‐single‐stage Orbitrap mass spectrometry. Food Additives and Contaminants Part A, 32, 1617–1627. 10.1080/19440049.2015.1061703 [DOI] [PubMed] [Google Scholar]
- De Girolamo A, Solfrizzo M, Lattanzio VMT, Stroka J, Alldrick A, van Egmond HP and Visconti A, 2013. Critical evaluation of LC‐MS‐based methods for simultaneous determination of deoxynivalenol, ochratoxin A, zearalenone, aflatoxins, fumonisins and T‐2/HT‐2 toxins in maize. World Mycotoxin Journal, 6, 317–334. [Google Scholar]
- De Nijs M, Van den Top HJ, Portier L, Oehema G, Kramer E, Van Egmond HP and Hoogenboom LAP, 2012. Digestibility and absorption of deoxynivalenol‐3‐β‐glucoside in in vitro models. World Mycotoxin Journal, 5, 319–324. [Google Scholar]
- De Saeger S and Van Egmond HP, 2012. Foreword special issue masked mycotoxins. World Mycotoxin Journal, 5, 203–206. [Google Scholar]
- De Walle JV, Sergent T, Piront N, Toussaint O, Schneider YJ and Larondelle Y, 2010. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicology and Applied Pharmacology, 245, 291–298. [DOI] [PubMed] [Google Scholar]
- Delwiche SR, 2005. High‐speed optical sorting of soft wheat for reduction of deoxynivalenol. Plant Disease, 89, 1214–1219. [DOI] [PubMed] [Google Scholar]
- Desmarchelier A, Oberson J‐M, Tella P, Gremaud E, Seefelder W and Mottier P, 2010. Development and comparison of two multiresidue methods for the analysis of 17 mycotoxins in cereals by liquid chromatography electrospray ionization tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 58, 7510–7519. [DOI] [PubMed] [Google Scholar]
- Desmarchelier A and Seefelder W, 2011. Survey of deoxynivalenol and deoxynivalenol‐3‐glucoside in cereal‐based products by liquid chromatography electrospray ionization tandem mass spectrometry. World Mycotoxin Journal, 4, 29–35. [Google Scholar]
- Devendra C and Burns M, 1983. Goat production in the tropics. Technical Communication, Commonwealth Agricultural Bureau. pp. 90–114. [Google Scholar]
- Devreese M, Girgis G, Tran S, De Baere S, De Backer S, Croubels S and Smith T, 2014. The effects of feed‐borne Fusarium mycotoxins and glucomannan in turkey poults based on specific and non‐specific parameters. Food and Chemical Toxicology, 63, 69–75. [DOI] [PubMed] [Google Scholar]
- Devreese M, Antonissen T, Broekaert N, De Mil T, De Baere S, Vanhaeke L, De Backer S and Croubels S, 2015. Toxicokinetic study and oral bioavailability of DON in turkey poults, and comparative biotransformation between broilers and turkeys. World Mycotoxin Journal, 8, 533–539. [Google Scholar]
- DiConstanzo A and Murphy M, 1994. Strategies for feeding mycotoxin and mold contaminated grains to cattle. In: Beef Management update. University extension service, September 1994, Issue 32: 1–11. Available online: http://www.extension.umn.edu/agriculture/beef/components/docs/strategies_for_feeding_mycotoxin_and_mold_contaminated_grain.pdf
- Diesing AK, Nossol C, Dänicke S, Walk N and Post A, 2011. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol. PLoS ONE, 6, e17472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinischiotu A, Dinu D, Rebedea M, Stoian G, Taranu I, Marin D and Costache M, 2007. Biochemical effects induced by deoxynivalenol intoxication in piglets. Biotechnology in Animal Husbandry, 23, 245–250. [Google Scholar]
- Döll S, Dänicke S, Ueberschär K‐H, Valenta H and Flachowsky G, 2003. Fusarium toxin residues in phyisiological samples of piglets. Mycotoxin Research, 19, 171–175. [DOI] [PubMed] [Google Scholar]
- Döll S and Dänicke S, 2011. The Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Preventive Veterinary Medicine, 102, 132–145. [DOI] [PubMed] [Google Scholar]
- Dorn B, Forrer HR, Jenny E, Wettstein FE, Bucheli TD and Vogelsang S, 2011. Fusarium species complex and myotoxins in grain maize from maize hybrid trials and from grower's fields. Journal of Applied Microbiology, 111, 693–706. [DOI] [PubMed] [Google Scholar]
- Dorokhin D, Haasnoot W, Franssen MCR, Zuilhof H and Nielen MWF, 2011. Imaging surface plasmon resonance for multiplex microassay sensing of mycotoxins. Analytical and Bioanalytical Chemistry, 400, 3005–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driehuis F, Spanjer MC, Scholten JM and Te Giffel MC, 2008b. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Additives and Contaminants, Part B, 1, 41–50. [DOI] [PubMed] [Google Scholar]
- Driehuis F, Spanjer MC, Scholten JM and te Giffel MC, 2008a. Occurrence of mycotoxins in feedstuffs of dairy cows and estimation of total dietary intakes. Journal of Dairy Science, 91, 4261–4271. [DOI] [PubMed] [Google Scholar]
- Drochner W, Schollenberger M, Piepho HP, Götz S, Lauber U, Tafaj M, Klobasa F, Weiler U, Claus R and Steffl M, 2004. Serum IgA‐promoting effects induced by feed loads containing isolated deoxynivalenol (DON) in growing piglets. Journal of Toxicology and Environmental Health A, 67, 1051–1067. [DOI] [PubMed] [Google Scholar]
- Drochner W, Schollenberger M, Gotz S, Lauber U, Tafaj M and Piepho HP, 2006. Subacute effects of moderate feed loads of isolated Fusarium toxin deoxynivalenol on selected parameters of metabolism in weaned growing piglets. Journal of Animal Physiology and Animal Nutrition, 90, 421–428. [DOI] [PubMed] [Google Scholar]
- Dzuman Z, Vaclavikova M, Polisenska I, Veprikova Z, Fenclova M, Zachariasova M and Hajslova J, 2014. Enzyme‐linked immunosorbent assay in analysis of deoxynivalenol: investigation of the impact of sample matrix on results accuracy. Analytical and Bioanalytical Chemistry, 406, 505–514. [DOI] [PubMed] [Google Scholar]
- EBLEX (Division of the Agriculture and Horticulture Development Board, AHDB), 2008. Feeding growing and finishing cattle for better return. EBLEX BEEF BPR Manual 7 Available online: http://beefandlamb.ahdb.org.uk/about/
- EBLEX (Division of the Agriculture and Horticulture Development Board, AHDB), 2012. Maize in beef system. Available online: http://beefandlamb.ahdb.org.uk/about/
- Ebrahem M, Kersten S, Valenta H, Breves G and Dänicke S, 2014a. Residues of deoxynivalenol (DON) and its metabolite de‐epoxy‐DON in eggs, plasma and bile of laying hens of different genetic backgrounds. Archives of Animal Nutrition, 68, 412–422. [DOI] [PubMed] [Google Scholar]
- Ebrahem M, Kersten S, Valenta H, Reineke A, Hermeyer K and Dänicke S, 2014b. Effects of feeding deoxynivalenol (DON)‐contaminated wheat to laying hens and roosters of different genetic background on the reproductive performance and health of the newly hatched chicks. Mycotoxin Research, 30, 131–140. [DOI] [PubMed] [Google Scholar]
- Eckard S, Wettstein FE, Forrer H‐R and Vogelsang S, 2011. Incidence of Fusarium species and mycotoxins in silage maize. Toxins, 3, 949–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ediage EN, Diana Di Mavungu J, Song S, Wu A, Van Peteghem C and De Saeger S, 2012b. A direct assessment of mycotoxin biomarkers in human urine samples by liquid chromatography tandem mass spectrometry. Analytical Chimica Acta, 5, 58–69. [DOI] [PubMed] [Google Scholar]
- Ediage EN, Diana Di Mavungu J, Goryacheva IY, Van Peteghem C and De Saeger S, 2012a. Multiplex flow‐through immunoassay formats for screening of mycotoxins in a variety of food matrices. Analytical and Bioanalytical Chemistry, 403, 265–278. [DOI] [PubMed] [Google Scholar]
- Ediage EN, Diana Di Mavungu J, Song S, Sioen I and De Saeger S, 2013. Multimycotoxin analysis in urines to assess infant exposure: a case study in Cameroon. Environment International, 57–58, 50–59. [DOI] [PubMed] [Google Scholar]
- Edwards SG, 2009a. Fusarium mycotoxin content of UK organic and conventional oats. Food Additives and Contaminants, 26, 1063–1069. [DOI] [PubMed] [Google Scholar]
- Edwards SG, 2009b. Fusarium mycotoxin content of UK organic and conventional barley. Food Additives and Contaminants, 26, 1185–1190. [DOI] [PubMed] [Google Scholar]
- Edwards SG, 2009c. Fusarium mycotoxin content of UK organic and conventional wheat. Food Additives and Contaminants, 26, 496–506. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Opinion of the Scientific Panel on contaminants in the Food Chain of the European Food Safety Authority (EFSA) on a request from the Commission related to deoxynivalenol as undesirable substance in animal feed. EFSA Journal 2004;2(6):73, 42 pp. 10.2903/j.efsa.2004.73 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. Deoxynivalenol in food and feed: occurrence and exposure. EFSA Journal 2013;11(10):3379, 56 pp. [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Evaluation of the increase of risk for public health related to a possible temporary derogation from the maximum level of deoxynivalenol, zearalenone and fumonisins for maize and maize products. EFSA Journal 2014;12(5):3699, 61 pp. 10.2903/j.efsa.2014.3699 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2013. Statement on the risks for public health related to a possible increase of the maximum level of deoxynivalenol for certain semi‐processed cereal products. EFSA Journal 2013;11(12):3490, 56 pp. 10.2903/j.efsa.2013.3490 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal 2014;12(12):3916, 107 pp. 10.2903/j.efsa.2014.3916 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534 26 pp. 10.2903/j.efsa.2012.2534 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, Mo re S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. doi:10.29 03/j.efsa.2017.4658 [Google Scholar]
- Eppley RM, Trucksess MW, Nesheim S, Thorpe CW and Pohland AE, 1986. Thin layer chromatographic method for determination of deoxynivalenol in wheat: collaborative study. Journal of Association of Official Analytical Chemists, 69, 37–40. [PubMed] [Google Scholar]
- Eriksen GS, Pettersson H, Johnsen K and Lindberg JE, 2002. Transformation of trichothecenes in ileal digesta and faeces from pigs. Archiv für Tierernährung, 56, 263–274. [DOI] [PubMed] [Google Scholar]
- Eriksen GS and Pettersson H, 2003. Lack of de‐epoxidation of type B trichothecenes in incubates with human faeces. Food Additives and Contaminants, 20, 579–582. [DOI] [PubMed] [Google Scholar]
- Eriksen GS, Pettersson H and Lindberg JE, 2003. Absorption, metabolism and excretion of 3‐acetyl DON in pigs. Archives of Animal Nutrition, 57, 335–345. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 2001. Safety evaluation of certain contaminants in food (deoxynivalenol). Prepared by the Fifty‐sixth meeting of the Joint FAO/WHO Expert Committee in Food Additives (JECFA), FAO Food and Nutrition Paper 74, Food and Agriculture Organization of the United Nations, World Health Organization, Geneva, IPCS – International Programme on Chemical Safety. 799 pp. Available online: http://www.inchem.org/documents/jecfa/jecmono/v47je01.htm
- FAO (Food and Agriculture Organization of the United Nations), 2004. Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper 81, Food and Agriculture Organization of the United Nations, Rome, Italy. Available online: http://www.fao.org/3/a-y5499e.pdf
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 2011. Safety evaluation of certain contaminants in food (deoxynivalenol). Prepared by the Seventy‐second meeting of the Joint FAO/WHO Expert Committee in Food Additives (JECFA), WHO Food Additives Series 63, FAO JECFA Monographs 8. World Health Organization, Geneva, Food and Agriculture Organization of the United Nations, Rome. 799 pp. Available online: http://www.inchem.org/documents/jecfa/jecmono/v63je01.pdf
- Farnworth ER, Hamilton RM, Thompson BK and Trenholm HL, 1983. Liver lipid levels in White Leghorn hens fed diets that contained wheat contaminated by deoxynivalenol (vomitoxin). Poultry Science, 62, 832–836. [DOI] [PubMed] [Google Scholar]
- FEFAC (European Feed Manufacturers’ Federation), 2014. The Feed Chain in Action. Animal nutrition ‐ the key to animal performance, health & welfare. Available online: http://www.fefac.eu/files/54738.pdf
- Ficheux AS, Sibiril Y and Parent‐Massin D, 2012. Co‐exposure of Fusarium mycotoxins: in vitro myelotoxicity assessment on human hematopoietic progenitors. Toxicon, 60, 1171–1179. [DOI] [PubMed] [Google Scholar]
- Filimon MN, Sinitean A, Ianovici N, Borozan A, Bordean DM, Dumitrescu G and Popescu R, 2012. The influence of deoxynivalenol (DON) on hemoleucogram components at rats. Analele Universităţii din Oradea ‐ Fascicola Biologie, Tom XIX, issue, 1, 23–28. [Google Scholar]
- Fitzpatrick DW, Boyd KE, Wilson LM and Wilson JR, 1988a. Effect of the trichothecene deoxynivalenol on brain biogenic monoamines concentrations in rats and chickens. Journal of Environmental Science and Health, Part B: Pesticide, Food Contaminants, and Agricultural Wastes, 23, 159–170. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DW, Boyd KE and Watts BM, 1988b. Comparison of the trichothecenes deoxynivalenol and T‐2 toxin for their effects on brain biogenic monoamines in the rat. Toxicology Letters, 40, 241–245. [DOI] [PubMed] [Google Scholar]
- Fink‐Gremmels J, 2008. Mycotoxins in cattle feeds and carry‐over to dairy milk: a review. Food Additives and Contaminants, 25, 172–180. [DOI] [PubMed] [Google Scholar]
- Flannery BM, Wu W and Pestka JJ, 2011. Characterization of deoxynivalenol‐induced anorexia using mouse bioassay. Food and Chemical Toxicology, 49, 1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery BM, Clark ES and Pestka JJ, 2012. Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide YY. Toxicological Sciences, 130, 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery BM, Amuzie CJ and Pestka JJ, 2013. Evaluation of insulin‐like growth factor acid‐labile subunit as a potential biomarker of effect for deoxynivalenol‐induced proinflammatory cytokine expression. Toxicology, 304, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Flores ME, Lizarraga E, López de Cerain A and Gonzàlez‐Peñas E, 2015. Presence of mycotoxins in animal milk: a review. Food Control, 53, 163–176. [Google Scholar]
- Forsell JH, Witt MF, Tai JH, Jensen R and Pestka JJ, 1986. Effects of 8‐week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food and Chemical Toxicology, 24, 213–219. [DOI] [PubMed] [Google Scholar]
- Forsell JH and Pestka JJ, 1985. Relation of 8‐ketotrichothecene and zearalenone analog structure to inhibition of mitogen‐induced human lymphocyte blastogenesis. Applied Environmental Microbiology, 50, 1304–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth DM, Yoshizawa T, Morooka N and Tuite J, 1977. Emetic and refusal activity of deoxynivalenol to swine. Applied and Environmental Microbiology, 34, 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G and Manley M, 2013. Applications of single kernel conventional and hyperspectral imaging near infrared spectroscopy in cereals. Journal of the Science of Food and Agricultural, 94, 174–179. [DOI] [PubMed] [Google Scholar]
- Frankič T, Pajk T, Rezar V, Levart A and Salobir J, 2006. The role of dietary nucleotides in reduction of DNA damage induced by T‐2 toxin and deoxynivalenol in chicken leukocytes. Food and Chemical Toxicology, 44, 1838–1844. [DOI] [PubMed] [Google Scholar]
- Frankič T, Salobir J and Rezar V, 2008. The effect of vitamin E supplementation on reduction of lymphocyte DNS damage induced by T‐2 toxin and deoxynivalenol in weaned pigs. Animal Feed Science and Technology, 141, 274–286. [Google Scholar]
- Fremy JM and Usleber E, 2003. Policy on characterization of antibodies used in immunochemical methods of analysis for mycotoxins and phycotoxins. Journal of AOAC International, 86, 868–871. [PubMed] [Google Scholar]
- Friend DW, Trenholm HL, Fiser PS, Thompson BK and Hartin KE, 1983. Effect of dam performance and fetal development of deoxynivalenol (vomitoxin) contaminated wheat in the diet of pregnant gilts. Canadian Journal of Animal Sciences, 63, 689–698. [Google Scholar]
- Friend DW, Trenholm HL, Thompson BK, Fiser PS and Hartin KE, 1986a. Effect of feeding diets containing deoxynivalenol (vomitoxin)‐contaminated wheat or corn on the feed consumption, weight gain, organ weight and sexual development of male and female pigs. Canadian Journal of Animal Science, 66, 765–775. [Google Scholar]
- Friend DW, Trenholm HL, Thompson BK, Prelusky DB and Hartin KE, 1986b. Effect of deoxynivalenol (DON)‐contaminated diet fed to growing‐finishing pigs on their performance at market weight, nitrogen retention and DON excretion. Canadian Journal of Animal Science, 66, 1075–1085. [Google Scholar]
- Friend DW, Thompson BK, Trenholm HL, Boermans HJ, Hartin KE and Panich PL, 1992. Toxicity of T‐2 toxin and its interaction with deoxynivalenol when fed to young‐pigs. Canadian Journal of Animal Science, 72, 703–711. [Google Scholar]
- Froquet R, Sibiril Y and Parent‐Massin D, 2001. Trichothecene toxicity on human megakaryocyte progenitors (CFU‐MK). Human and Experimental Toxicology, 20, 84–89. [DOI] [PubMed] [Google Scholar]
- Fruhmann P, Warth B, Hametner C, Berthiller F, Horkel E, Adam G, Sulyok M, Krska R and Fröhlich J, 2012. Synthesis of deoxynivalenol‐3‐β‐D‐O‐glucuronide for its use as biomarker for dietary deoxynivalenol exposure. World Mycotoxin Journal, 5, 127–132. [Google Scholar]
- FSCJ (Japanese food safety commission), 2010. Risk Assessment Report Deoxynivalenol and Nivalenol (Mycotoxin), Risk assessment report – veterinary medicines FS/872/2010.
- Gao HP and Yoshizawa T, 1997. Further study on fusarium mycotoxins in corn and wheat from a high‐risk area for human esophageal cancer in China. Mycotoxins, 45, 51–55. [Google Scholar]
- Gareis M, Bauer J, Thiem J, Plank G, Grabley S and Gedek B, 1990. Cleavage of zearalenone‐glycoside, a ‘masked’ mycotoxin, during digestion in swine. Journal of Veterinary Medicine Series B, 37, 236–240. [DOI] [PubMed] [Google Scholar]
- Gareis M, Bauer J and Gedek B, 1987. On the metabolism of the mycotoxin deoxynivalenol in the isolated perfused rat liver. Mycotoxin Research, 3, 25–32. [DOI] [PubMed] [Google Scholar]
- Garreau de Loubresse N, Prokhorova I, Holtkamp W, Rodnina MV, Yusupova G and Yusupov M, 2014. Structural basis for the inhibition of the eukaryotic ribosome. Nature, 513, 517–522. [DOI] [PubMed] [Google Scholar]
- Gauvreau HC, 1991. Toxicokinetics, Tissue Residue, and Metabolic Studies of Deoxynivalenol (Vomitoxin) in Turkeys. PhD thesis Simon Fraser University, Vancouver, BC.
- Generotti S, Cirlini M, Malachova A, Sulyok M, Berthiller F, Dall'Asta C and Suman M, 2015. Deoxynivalenol & deoxynivalenol‐3‐glucoside mitigation through bakery production strategies: effective experimental design within industrial rusk‐making technology. Toxins 7, 2773–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding J, Ali N, Schwartzbord J, Cramer B, Brown DL, Degen GH and Humpf HU, 2015. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany and Haiti using a rapid and sensitive LC‐MS/MS approach. Mycotoxin Research, 31, 127–136. [DOI] [PubMed] [Google Scholar]
- Gerez JR, Pinton P, Callu P, Grosjean P, Oswald IP and Bracarense APFL, 2015. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Experimental and Toxicologic Pathology, 67, 89–98. [DOI] [PubMed] [Google Scholar]
- Ghareeb K, Awad WA and Böhm J, 2012. Ameliorative effect of a microbial feed additive on infectious bronchitis virus antibody titer and stress index in broiler chicks fed deoxynivalenol. Poultry Science, 91, 800–807. [DOI] [PubMed] [Google Scholar]
- Ghareeb K, Awad WA, Böhm J and Zebeli Q, 2015. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine. Journal of Applied Toxicology, 35, 327–337. [DOI] [PubMed] [Google Scholar]
- Ghareeb K, Awad WA, Sid‐Ahmed OE and Böhm J, 2014. Insights on the host stress, fear and growth responses to the deoxynivalenol feed contaminant in broiler chickens. PLoS ONE, 9, e87727 10.1371/journal.pone.0087727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareeb K, Awad WA, Zebeli Q and Böhm J, 2016. Deoxynivalenol in chicken feed alters the vaccinal immune response and clinical biochemical serum parameters but not the intestinal and carcass characteristics. Journal of Animal Physiology and Animal Nutrition, 100, 53–60. [DOI] [PubMed] [Google Scholar]
- Gibson MK, Bursian SJ and Aulerich RJ, 1993. Effects of deoxynivalenol on feed consumption and body weight gains in mink (Mustela vison). Bulletin of Environmental Contamination and Toxicology, 51, 6–11. [DOI] [PubMed] [Google Scholar]
- Gilbert J, Brûlé‐Babel A, Guerrieri AT, Clear RM, Patrick S, Slusarenko K and Wolfe C, 2014. Ratio of 3‐ADON and 15‐ADON isolates of Fusarium graminearum recovered from wheat kernels in Manitoba from 2008 to 2012. Canadian Journal of Plant Pathology, 36, 54–63. [Google Scholar]
- Giménez I, Herrera M, Escobar J, Ferruz E, Loran S, Herrera A and Ariño A, 2013. Distribution of deoxynivalenol and zearalenone in milled germ during wheat milling and analysis of toxin levels in wheat germ and wheat germ oil. Food Control, 34, 268–273. [Google Scholar]
- Girardet C, Bonnet MS, Jdir R, Sadoud M, Thirion S, Tardivel C, Roux J, Lebrun B, Wanaverbecq N, Mounien L, Trouslard J, Jean A, Dallaporta M and Troadec JD, 2011a. Central inflammation and sickness‐like behavior induced by the food contaminant deoxynivalenol: a PGF2‐independent mechanism. Toxicological Sciences, 124, 179–191. [DOI] [PubMed] [Google Scholar]
- Girardet C, Bonnet MS, Jdir R, Sadoud M, Thirion S, Tardivel C, Roux J, Lebrun B, Wanaverbecq N and Mounien L, 2011b. The food‐contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS ONE, 6, e26134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girish CK and Smith TK, 2008. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on small intestinal morphology of turkeys. Poultry Science, 87, 1075–1082. [DOI] [PubMed] [Google Scholar]
- Gottschalk C, Barthel J, Engelhardt G, Bauer J and Meyer K, 2009. Simultaneous determination of type A, B and D trichothecenes and their occurrence in cereals and cereal products. Food Additives and Contaminants, 26, 1273–1289. [Google Scholar]
- Götz‐Schröm S, Schollenberger M, Lauber U, Muller H‐M and Drochner W, 1998. Wirkung von reinem Deoxynivalenol bei Läuferschweinen ‐ Ergebnisse In: Wolff J. and Betsche T. (eds.). Procedings of the 20th German Workshop on Mycotoxins, 8–10 June 1998. Detmold, Germany: pp. 171–175. [Google Scholar]
- Gouze ME, Laffitte J, Dedieu G, Galinier A, Thouvenot JP, Oswald IP and Galtier P, 2005. Individual and combined effects of low oral doses of deoxynivalenol and nivalenol in mice. Cellular and Molecular Biology, 51 Suppl, OL809‐817. [PubMed] [Google Scholar]
- Goyarts T and Danicke S, 2006. Bioavailability of the Fusarium toxin deoxynivalenol (DON) from naturally contaminated wheat for the pig. Toxicology Letters, 163, 171–182. [DOI] [PubMed] [Google Scholar]
- Goyarts T, Dänicke S, Valenta H and Urberschär K‐H, 2007. Carry‐over of Fusarium toxins (deoxynivalenol and zearalenone) from naturally contaminated wheat to pigs. Food Additives and Contaminants, 24, 369–380. 10.1080/02652030600988038 [DOI] [PubMed] [Google Scholar]
- Gratz SW, Duncan G and Richardson AJ, 2013. The human fecal microbiota metabolizes deoxynivalenol and deoxynivalenol‐3‐glucoside and may be responsible for urinary deepoxy‐deoxynivalenol. Applied and Environmental Microbiology, 79, 1821–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani F, Pujol A, Nicoletti C, Pinton P, Armand L, Di Pasquale E, Oswald IP, Perrier J and Maresca M, 2015. The food‐associated ribotoxin deoxynivalenol modulates the expression of the inducible NO synthase by the human intestinal epithelium. Toxicological Sciences, 145, 372–382. [DOI] [PubMed] [Google Scholar]
- Grenier B and Oswald IP, 2011. Mycotoxin co‐contamination of food and feed: meta‐analysis of publications describing toxicological interactions. World Mycotoxin Journal, 4, 285–313. [Google Scholar]
- Grenier B, Loureiro‐Bracarense AP, Lucioli J, Drociunas‐Pacheco G, Cossalter AM, Moll WD, Schatzmayr G and Oswald IP, 2011. Individual and combined effects of subclinical doses of deoxynivalenol and fumonisins in piglets. Molecular Nutrition and Food Research, 55, 761–771. [DOI] [PubMed] [Google Scholar]
- Grimes JL, Koci MD, Stark CR, Smith DP, Nighot PK and Middleton T, 2010. Biological effect of naturally occurring mycotoxins fed to poults reared to 21 days of age. International Journal of Poultry Science, 9, 871–874. [DOI] [PubMed] [Google Scholar]
- Guerra P, 2015. Fusariotoxins in avian species: toxicokinetics, metabolism and persistence in tissues. Toxins, 7, 2289–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H‐W, et al., 1989. The contamination of Fusarium toxins in wheat and the intake of these toxins by farmers. Chinese Journal of Food Hygiene, 1, 20–24. [Google Scholar]
- Habler K and Rychlik M, 2016. Multi‐mycotoxin stable isotope dilution LC‐MS/MS method for Fusarium toxins in cereals. Analytical and Bioanalytical Chemistry, 408, 307–317. [DOI] [PubMed] [Google Scholar]
- Habler K, Hofer K, Geißinger C, Schüler J, Hückelhoven R, Hess M, Gastl M and Rychlik M, 2016. Fate of fusarium toxins during the malting process. Journal of Agricultural and Food Chemistry, 64, 1377–1384. [DOI] [PubMed] [Google Scholar]
- Haga M and Sakata T, 2010. Daily salt intake of healthy Japanese infants of 3‐5 years based on sodium excretion in 24‐hour urine. Journal of Nutritional Science and Vitaminology, 56, 305–310. [DOI] [PubMed] [Google Scholar]
- Halawa A, Dänicke S, Kersten S and Breves G, 2013. Intestinal transport of deoxynivalenol across porcine small intestines. Archives of Animal Nutrition, 67, 134–146. [DOI] [PubMed] [Google Scholar]
- Hallier A, Celette F and David C, 2011. Effects of sampling and extraction on deoxynivalenol quantification. Food Chemistry, 127, 303–307. [Google Scholar]
- Hamilton RMG, Trenholm HL, Thompson BK and Greenhalgh R, 1985. The tolerance of White Leghorn and broiler chicks, and turkey poults to diets that contained deoxynivalenol (vomitoxin)‐contaminated wheat. Poultry Science, 64, 273–286. [DOI] [PubMed] [Google Scholar]
- Han Z, Zheng Y, Luan L, Cai Z, Ren Y and Wu Y, 2010. An ultra‐high‐performance liquid chromatography‐tandem mass spectrometry method for simultaneous determination of aflatoxins B1, B, G1, G2, M1 and M2 in traditional Chinese medicines. Analytica Chimica Acta, 664, 165–171. [DOI] [PubMed] [Google Scholar]
- Hara‐Kudo Y, Sugita‐Konishi Y, Kasuga F and Kumagai S, 1996. Effects of deoxynivalenol on Salmonella Enteritidis infection. Mycotoxins, 42, 51–56. [Google Scholar]
- Harvey RB, Kubena LF, Corrier DE, Witzel DA, Phillips TD and Heidelbaugh ND, 1986. Effects of deoxynivalenol in a wheat ration fed to growing lambs. American Journal of Veterinary Research, 47, 1630–1632. [PubMed] [Google Scholar]
- Harvey RB, Kubena LF, Huff WE, Elissalde MH and Phillips TD, 1991. Hematologic and immunologic toxicity of deoxynivalenol (DON)‐contaminated diets to growing chickens. Bulletin of Environment Contamination and Toxicology, 46, 410–416. [DOI] [PubMed] [Google Scholar]
- Harvey RB, Edrington TS, Kubena LF, Elissalde MH, Casper HH, Rottinghaus GE and Turk JR, 1996. Effects of dietary fumonisin B‐1‐containing culture material, deoxynivalenol‐contaminated wheat, or their combination on growing barrows. American Journal of Veterinary Research, 57, 1790–1794. [PubMed] [Google Scholar]
- Harvey RB, Kubena LF, Huff WE, Corrier DE, Clark DE and Phillips TD, 1989. Effects of aflatoxin, deoxynivalenol, and their combinations in the diets of growing‐pigs. American Journal of Veterinary Research, 50, 602–607. [PubMed] [Google Scholar]
- Hazel CM and Patel S, 2004. Influence of processing on trichothecene levels. Toxicology Letters, 153, 51–59. [DOI] [PubMed] [Google Scholar]
- HCN (Health Council of the Netherlands), 2001. Deoxynivalenol (DON). The Hague: Health Council of the Netherlands, 2001; publication no. 2001/23E. Available online: http://www.gezondheidsraad.nl/en/publications/gezonde-voeding/deoxynivalenol-don
- He C‐H, Fan Y‐H, Wang Y, C‐Y Huang, X‐C Wang and H‐B Zhang, 2010. Combinative effects of aflatoxin B1 and deoxynivalenol in feed on carp. Journal of Nanjing Agricultural University, 33, 85–89. [Google Scholar]
- He JW, Yang R, Zhou T, Boland GJ, Scott PM and Bondy GS, 2015. An epimer of deoxynivalenol: purification and structure identification of 3‐epi‐deoxynivalenol. Food Additives and Contaminants: Part A, 32, 1523–1530. 10.1080/19440049.2015.1072771 [DOI] [PubMed] [Google Scholar]
- He P, Young LG and Forsberg C, 1992. Microbial transformation of deoxynivalenol (vomitoxin). Applied and Environmental Microbiology, 58, 3857–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q‐H, Xu Y, Wang D, Kang M, Huang Z‐B and Li Y‐P, 2012. Simultaneous multiresidue determination of mycotoxins in cereal samples by polyvinylidene fluoride membrane dot immunoassay. Food Chemistry, 134, 507–512. [Google Scholar]
- Heidtmann‐Bemvenuti R, dos Santos Hackbart HC, Moraes de Souza M, Badiale‐Furlong E, Dors GC and Fagundes CA, 2012. Determination of deoxynivalenol and zearalenone in natural and parboiled rice and their fractions using QuEChERS and HPLC‐UV‐Fl. Quimica Nova, 35, 1244–1249. [Google Scholar]
- Hepworth SJ, Hardie LJ, Fraser LK, Burley VJ, Mijal RS, Wild CP, Azad R, McKinney PA and Turner PC, 2012. Deoxynivalenol exposure assessment in a cohort of pregnant women from Bradford, UK. Food Additives and Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 29, 269–276. [DOI] [PubMed] [Google Scholar]
- Hewitt MA, Girgis GN, Brash M and Smith TK, 2015. Effects of feed‐borne Fusarium mycotoxins on performance, serum chemistry, and intestinal histology of New Zealand White fryer rabbits. Journal of Animal Science, 90, 4833–4838. [DOI] [PubMed] [Google Scholar]
- Heyndrickx E, Sioen I, Huybrechts B, Callebaut A, Henauw SD and De Saeger Sarah, 2015. Human biomonitoring of multiple mycotoxins in the Belgian population: results of the BIOMYCO study. Environment International, 84, 82–89. [DOI] [PubMed] [Google Scholar]
- Hiraoka H, Yamamoto K, Mori Y, Asao N, Fukunaka R, Deguchi K, Iida K, Miyazaki S and Goto T, 2013. Modified use of a commercial ELISA kit for deoxynivalenol determination in rice and corn silage. Mycotoxin Research, 29, 79–88. [DOI] [PubMed] [Google Scholar]
- Hooft JM, Elmoor AEHI, Encarnaçao P and Bureau D, 2010. Rainbow trout (Oncorynchus mykiss) is extremely sensitive to the feed‐borne Fisarium mycotoxin deoxynivalenol (DON). Aquaculture, 311, 224–232. [Google Scholar]
- Hooft JM, Elmor AEHI, Encarnação P and Bureau P, 2013. Rainbow trout (Oncorhynchus mykiss) is extremely sensitive to the feed‐borne Fusarium mycotoxin deoxynivalenol (DON). Aquaculture, 311, 224–232. [Google Scholar]
- Hopton RP, Turner E, Burley VJ, Turner PC and Fisher J, 2010. Urine metabolite analysis as a function of deoxynivalenol exposure: a NMR‐based metabolomics investigation. Food Additives and Contaminants, 27, 255–261. [DOI] [PubMed] [Google Scholar]
- Hou Y‐J, Xiong B, Zheng W‐J, Duan X, Cui X‐S, Kim N‐H, Wang Q, Xu Y‐X and Sun S‐C, 2014. Oocyte quality in mice is affected by a mycotoxin‐contaminated diet. Environmental and Molecular Mutagenesis, 55, 354–362. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Wu ZY, Li YS, Zhang F and Sun ZT, 2004. Nivalenol, a main Fusarium toxin in dietary foods from high‐risk areas of cancer of esophagus and gastric cardia in China, induced benign and malignant tumors in mice. Oncology Reports, 14, 449–456. [PubMed] [Google Scholar]
- Hsia CC, Wu JL, Lu XQ and Li YS, 1988. Natural occurrence and clastogenic effects of nivalenol, deoxynivalenol, 3‐acetyl‐deoxynivalenol, 15‐acetyl‐deoxynivalenol, and Zearalenone in corn from a high‐risk area of esophageal cancer. Cancer Detection and Prevention, 13, 79–86. [PubMed] [Google Scholar]
- Hsueh CC, Liu Y and Freund MS, 1999. Indirect electrochemical detection of type‐B trichothecene mycotoxins. Analytical Chemistry, 71, 4075–4080. [DOI] [PubMed] [Google Scholar]
- Huang XH, Zhang XH, Li YH, Yan X, Xing LX and Wang FR, 2004. Carcinogenic effects of sterigmatocystin and deoxynivalenol in NIH mice. Zhonghua Zhong Liu Za Zhi, 26, 705–708. [PubMed] [Google Scholar]
- Huang Z‐B, Xu Y, Li L‐S, Li Y‐P, Zhang H and He Q‐H, 2012. Development of an immunochromatographic strip test for the rapid simultaneous detection of deoxynivalenol and zearalenone in wheat and maize. Food Control, 28, 7–12. [Google Scholar]
- Huff WE, Doerr JA, Hamilton PB and Vesonder RF, 1981. Acute toxicity of vomitoxin (deoxynivalenol) in broiler chickens. Poultry Science, 60, 1412–1414. [DOI] [PubMed] [Google Scholar]
- Huff WE, Kubena LF, Harvey RB, Hagler WM, Swanson SP, Phillips TD and Creger CR, 1986. Individual and combined effects of aflatoxin and deoxynivalenol (don, vomitoxin) in broiler‐chickens. Poultry Science, 65, 1291–1298. [DOI] [PubMed] [Google Scholar]
- Hughes DM, Gahl MJ, Graham CH and Grieb SL, 1999. Overt signs of toxicity to dogs and cats of dietary deoxynivalenol. Journal of Animal Science, 77, 693–700. [DOI] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, Serra‐Majem L, Volatier J‐L, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren JD, 2011. Dietary exposure assessments for children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 1169–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts B, Martins JC, Debongnie P, Uhlig S and Callebaut A, 2015. Fast and sensitive LC‐MS/MS method measuring human mycotoxin exposure using biomarkers in urine. Archives of Toxicology, 89, 1993–2005. [DOI] [PubMed] [Google Scholar]
- Hymery N, Sibiril Y and Parent‐Massin D, 2006. In vitro effects of trichothecenes on human dendritic cells. Toxicology in Vitro, 20, 899–909. [DOI] [PubMed] [Google Scholar]
- IARC (World Health Organization International Agency for Research on Cancer), 1993. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic aromatic amines and mycotoxins. Vol 56. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, IARC Lyon. 609 pp. Available online: http://monographs.iarc.fr/ENG/Monographs/vol56/mono56.pdf
- Ibañez‐Vea M, Lizarraga E and González‐Peñas E, 2011. Simultaneous determination of type‐A and type‐B trichothecenes in barley samples by GC‐MS. Food Control, 22, 1428–1434. [Google Scholar]
- Ingalls JR, 1996. Influence of deoxynivalenol on feed consumption by dairy cows. Animal Feed Science and Technology, 60, 297–300. [Google Scholar]
- Islam MR, Roh YS, Kim J, Lim CW and Kim B, 2013. Differential immune modulation by deoxynivalenol (vomitoxin) in mice. Toxicology Letters, 221, 152–163. [DOI] [PubMed] [Google Scholar]
- Iverson F, Armstrong C, Nera E, Truelove J, Fernie S, Scott P, Stapley R, Hayward S and Gunner S, 1995. Chronic feeding study of deoxynivalenol in B6C3F1 male and female mice. Teratogenesis, Carcinogenesis and Mutagenesis, 15, 283–306. [DOI] [PubMed] [Google Scholar]
- Janssen EM, Sprong RC, Wester PW, De Boevre M and Mengelers MJB, 2015. Risk assessment of chronic dietary exposure to the conjugated mycotoxin deoxynivalenol‐3‐β‐glucoside (D3G) in the Dutch population. World Mycotoxin Journal, 8, 561–572. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 2001. Safety evaluation of certain mycotoxins in food. WHO Food Additives Series, No. 47/FAO Food and Nutrition Paper 74. Available online: http://www.inchem.org/documents/jecfa/jecmono/v47je01.htm
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 2011. Deoxynivalenol. In: Safety evaluation of certain contaminants in food, Prepared by the Seventy‐second meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). World Health Organization, Geneva, 2011, and Food and Agriculture Organization of the United Nations, Rome, 2011. WHO Food Additives Series 63, FAO JECFA MONOGRAPHS 8. Available online: http://apps.who.int/iris/bitstream/10665/44520/1/9789241660631_eng.pdf
- Ji F, Li H, Xu J and Shi J, 2011. Enzyme‐linked immunosorbent‐assay for deoxynivalenol (DON). Toxins, 3, 968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji GE, Park SY, Wong SS and Pestka JJ, 1998. Modulation of nitric oxide, hydrogen peroxide and cytokine production in a clonal macrophage model by the trichothecene vomitoxin (deoxynivalenol). Toxicology, 125, 203–214. [DOI] [PubMed] [Google Scholar]
- Jia Q and Pestka JJ, 2005. Role of cyclooxygenase‐2 in deoxynivalenol‐induced immunoglobulin a nephropathy. Food Chemical Toxicology, 43, 721–728. [DOI] [PubMed] [Google Scholar]
- Johannisson A, Bjökhag B, Hansson W, Gadhasson IL and Thuvander A, 1999. Effects of four trichothecene mycotoxins on activation marker expression and cell proliferation of human lymphocytes in culture. Cell Biology and Toxicology, 15, 203–215. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Casteel SW and Messer NT, 1997. Effect of feeding deoxynivalenol (vomitoxin)‐contaminated barley to horses. Journal of Veterinary Diagnostic Investigation, 9, 219–221. [DOI] [PubMed] [Google Scholar]
- Juan‐Garcia A, Juana C, König S and Ruiz M‐J, 2015. Cytotoxic effects and degradation products of three mycotoxins: alternariol, 3‐acetyl‐deoxynivalenol and 15‐acetyl‐deoxynivalenol in liver hepatocellular carcinoma cells. Toxicology Letters, 235, 8–16. [DOI] [PubMed] [Google Scholar]
- Juan C, Ritieni A and Mañes J, 2012. Determination of trichothecenes and zearalenones in grain cereal, flour and bread by liquid chromatography tandem mass spectrometry. Food Chemistry, 134, 2389–2397. [DOI] [PubMed] [Google Scholar]
- Juan C, Ritieni A and Mañes J, 2013. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chemistry, 141, 1747–1755. [DOI] [PubMed] [Google Scholar]
- Juan C, Raiola A, Mañes A and Ritieni A, 2014. Presence of mycotoxin in commercial infant formulas and baby foods from Italian market. Food Control, 39, 227–236. [Google Scholar]
- Juodeikiene G, Basinskiene L, Vidmantiene D, Bartkiene E and de Koe WJ, 2008. Rapid acoustic screening of deoxynivalenol (DON) in grain. World Mycotoxin Journal, 1, 267–274. [Google Scholar]
- Juodeikiene G, Vidmantiene D, Basinskiene L, Cernauskas D, Klupsaite D, Bartkiene E, Petrauskas A and de Koe WJ, 2014. Recent advances in the rapid acoustic screening of deoxynivalenol in wheat grains. World Mycotoxin Journal, 7, 517–525. [Google Scholar]
- Kabak B, 2009. The fate of mycotoxins during thermal food processing. Journal of the Science of Food and Agriculture, 89, 549–554. [Google Scholar]
- Kadota T, Furusawa H, Hirano S, Tajima O, Kamata Y and Sugita‐Konishi Y, 2013. Comparative study of deoxynivalenol, 3‐acetyldeoxynivalenol, and 15‐acetyldeoxynivalenol on intestinal transport and IL‐8 secretion in the human cell line Caco‐2. Toxicology in Vitro, 27, 1888–1895. [DOI] [PubMed] [Google Scholar]
- Kalaiselvi P, Rajashree K, Bharathi Priya L and Padma VV, 2013. Cytoprotective effect of epigallocatechin‐3‐gallate against deoxynivalenol‐induced toxicity through anti‐oxidative and anti‐inflammatory mechanisms in HT‐29 cells. Food and Chemical Toxicology, 56, 110–118. [DOI] [PubMed] [Google Scholar]
- Kasali OB, Schiefer HB, Hancock DS, Blakley BR, Tomar RS and Greenhalgh R, 1985. Subacute toxicity of dietary 3‐acetyldeoxynivalenol in mice. Canadian Journal of Comparative Medicine, 49, 319–322. [PMC free article] [PubMed] [Google Scholar]
- Kautzman ME, Wickstrom Mark L, Hogan Natacha S and Scott Tom A, 2015. Using near infrared transmittance to generate sorted fractions of Fusarium infected wheat and the impact on broiler performance. Poultry Science, 94, 1619–1628. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW and Broussard SR, 2003. Cytokine‐induced sickness behavior. Brain, Behavior, and Immunity, 17, 112–118. [DOI] [PubMed] [Google Scholar]
- Keese C, Meyer M, Valenta H, Schollenberger M, Starke A, Weber IA, Rehage J, Breves G, Dänicke S, 2008a. No carry over of unmetabolised deoxynivalenol in milk of dairy cows fed high concentrate proportions. Molecular Nutrition Food Research, 52, 1514–1529. [DOI] [PubMed] [Google Scholar]
- Khatibi PA, Montanti J, Nghiem NP, Hicks KB, Berger2 G, Brooks WS, Griffey CA and Schmale DG, 2011b. Conversion of deoxynivalenol to 3‐acetyldeoxynivalenol in barley‐derived fuel ethanol co‐products with yeast expressing trichothecene 3‐O‐acetyltransferases. Biotechnology for Biofuels, 4, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatibi PA, Berger G, Wilson J, Brooks WS, McMaster N, Griffey CA, Hicks KB, Ngiem NP and Schmale G, 2014a. A comparison of two milling strategies to reduce the mycotoxins deoxynivalenol in barley. Journal of Agricultural and Food Chemistry, 62, 4204–4213. [DOI] [PubMed] [Google Scholar]
- Khatibi PA, McMaster NJ and Musser R, 2014b. Survey of mycotoxins in corn distillers’ dried grains with solubles from seventy‐eight ethanol plants in twelve states in the US in 2011. Toxins, 6, 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera KS, Whalen C, Angers G, Vesonder RF and Kuiper‐Goodman T, 1982. Embryotixicity of 4‐deoxynivalenol (vomitoxin) in mice. Bulletin of Environmental Contamination, 29, 487–491. [DOI] [PubMed] [Google Scholar]
- Khera KS, Arnold DL, Whalen C, Angers G and Scott PM, 1984. Vomitoxin (4‐deoxynivalenol): effects on reproduction of mice and rats. Toxicology and Applied Pharmacology, 74, 345–356. [DOI] [PubMed] [Google Scholar]
- Khera KS, Whalen C and Angers F, 1986. A teratology study on vomitoxin (4‐deoxynivalenol) in rabbits. Food and Chemical Toxicology, 24, 421–424. [DOI] [PubMed] [Google Scholar]
- Khol‐Parisini A, Hellweg P, Razzazi‐Fazeli E, Saalmüller Strasser A, Tichy A and Zentek J, 2012. Highly deoxynivalenol contaminated oats and immune function in horses. Archives of Animal Nutrition, 66, 149–161. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Keese C, Beineke A, Meyer U, Starke A, Sauerwein H, Danicke S and Rehage J, 2015. Effects of Fusarium mycotoxins in rations with different concentrate proportions on serum haptoglobin and hepatocellular integrity in lactating dairy cows. Journal of Animal Physiology and Animal Nutrition, 99, 887–892. [DOI] [PubMed] [Google Scholar]
- Kluger B, Bueschl C, Lemmens M, Berthiller F, Häubl G, Jaunecker G, Adam G, Krska R and Schuhmacher R, 2013. Stable isotopic labelling‐assisted untargeted metabolic profiling reveals novel conjugates of the mycotoxin deoxynivalenol in wheat. Analytical and bioanalytical chemistry, 405, 5031–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klötzel M, Schmidt S, Lauber U, Thielert G and Humpf H‐U, 2005. Comparison of different clean‐up procedures for the analysis of deoxynivalenol in cereal‐based food and validation of a reliable HPLC method. Chromatographia, 62, 41–48. [Google Scholar]
- Klinglmayr C, Nöbauer K, Razzazi‐Fazeli E and Cichna‐Markl M, 2010. Determination of deoxynivalenol in organic and conventional food and feed by sol–gel immunoaffinity chromatography and HPLC–UV detection. Journal of Chromatography B, 878, 187–193. [DOI] [PubMed] [Google Scholar]
- Knasmüller S, Bresgen N, Kassie F, Mersch‐Sundermann V, Gelderblom W, Zöhrer E and Eckl P, 1997. Genotoxic effects of three Fusarium mycotoxins, fumonisin B1, moniliformin and vomitoxin in bacteria and in primary cultures of rat hepatocytes. Mutation Research, 391, 39–48. [DOI] [PubMed] [Google Scholar]
- Kobayashi‐Hattori K, Amuzie CJ, Flannery BM and Pestka JJ, 2011. Body composition and hormonal effects following exposure to mycotoxin deoxynivalenol in the high‐fat diet‐induced obese mouse. Molecular Nutrition and Food Research, 55, 1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova AY, Sibanda L, Dumoulin F, Lewis J, Duveiller E, Van Peteghem C and De Saeger S, 2008. Lateral‐flow colloidal gold‐based immunoassay for the rapid detection of deoxynivalenol with two indicator ranges. Analytica Chimica Acta, 616, 235–244. [DOI] [PubMed] [Google Scholar]
- Kos G, Krska R, Lohninger H and Griffiths PR, 2004. A comparative study of mid‐infrared diffuse reflection (DR) and attenuated total reflection (ATR) spectroscopy for the detection of fungal infection on RWA2‐corn. Analytical and Bioanalytical Chemistry, 378, 159–166. [DOI] [PubMed] [Google Scholar]
- Kostelanska M, Dzuman Z, Malachova A, Capouchova I, Prokinova E, Skerikova A and Hajslova J, 2011b. Effects of milling and baking technologies on levels of deoxynivalenol and its masked form deoxynivalenol‐3‐glucoside. Journal Agricultural Food Chemistry, 59, 9303–9312. [DOI] [PubMed] [Google Scholar]
- Kostelanska M, Hajslova J, Zachariasova M, Kalachova K, Poustka J, Fiala J, Scott P, Berthiller F and Krska R, 2009. Occurrence of deoxynivalenol and its major conjugate, deoxynivalenol‐3‐glucoside, in beer and some brewing intermediates. Journal of Agricultural and Food Chemistry, 57, 3187–3194. [DOI] [PubMed] [Google Scholar]
- Kostelanska M, Zachariasova M, Lacina O, Fenclova M, Kollos A‐L and Hajslova J, 2011a. The study of deoxynivalenol and its masked metabolites fate during the brewing process realised by UPLC‐TOFMS method. Food Chemistry, 126, 1870–1876. [DOI] [PubMed] [Google Scholar]
- Kouadio JH, Dano SD, Moukha S, Mobio TA and Creppy EE, 2007. Effects of combinations of Fusarium mycotoxins on the inhibition of macromolecular synthesis, malondialdehyde levels, DNA methylation and fragmentation, and viability in Caco‐2 cells. Toxicon, 49, 306–317. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy R, Devaraj SN and Padma VV, 2010. Lutein protects HT‐29 cells against deoxynivalenol‐induced oxidative stress and apoptosis: prevention of NF‐kappaB nuclear localization and down regulation of NF‐kappaB and cyclo‐oxygenase‐2 expression. Free Radical Biology and Medicine, 49, 50–60. [DOI] [PubMed] [Google Scholar]
- Krska R, Baumgartner S and Josephs R, 2001. The state‐of‐the‐art in the analysis of type‐A and ‐B trichothecene mycotoxins in cereals. Fresenius Journal Analytical Chemistry, 371, 285–299. [DOI] [PubMed] [Google Scholar]
- Krska R, Szente E and Freudenschuss M, 2004. Purity assessment of commercially available crystalline deoxynivalenol. Journal of AOAC International, 87, 909–919. [PubMed] [Google Scholar]
- Krska R, Schothorst RC, van Egmond HP, Josephs RD, Lepschy J, Pettersson H, Chan D, Berthiller F, Schuhmacher R and Kandler W, 2005. Processing and purity assessment of standards for the analysis of type‐B trichothecene mycotoxins. Analytical and bioanalytical chemistry, 382, 1848–1858. [DOI] [PubMed] [Google Scholar]
- Krysińska‐Traczyk E, Perkowski J and Dutkiewicz J, 2007. Levels of fungi and mycotoxins in the samples of grain and grain dust collected from five various cereal crops in Eastern Poland. Annals of Agricultural and Environmental Medicine, 14, 159–167. [PubMed] [Google Scholar]
- Kubena LF and Harvey RB, 1988. Response of growing Leghorn chicks to deoxynivalenol‐contaminated wheat. Poultry Science, 67, 1778–1780. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Swanson SP, Harvey RB, Fletcher OJ, Rowe LD and Phillips TD, 1985. Effects of feeding deoxynivalenol (vomitoxin)‐contaminated wheat to growing chicks. Poultry Science, 64, 1649–1655. [Google Scholar]
- Kubena L, Harvey R, Corrier D, Huff W and Phillips T, 1987a. Effects of feeding deoxynivalenol‐contaminated wheat to female white Leghorn chickens from day old through egg production. Poultry Science, 66, 1612–1618. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Harvey RB, Phillips TD, Holman GM and Creger CR, 1987b. Effects of feeding mature White Leghorn hens diets that contain deoxynivalenol (vomitoxin). Poultry Science, 66, 55–58. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Huff WE, Harvey RB, Corrier DE, Phillips TD and Creger CR, 1988. Influence of ochratoxin A and deoxynivalenol on growing broiler chicks. Poultry Science, 67, 253–260. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Huff WE, Harvey RB, Phillips TD and Rottinghaus GE, 1989. Individual and combined effects of deoxynivalenol and T‐2 toxin in broiler chicks. Poultry Science, 68, 622–626. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Edrington TS, Harvey RB, Phillips TD, Sarr AB and Rottinghaus GE, 1997. Individual and combined effects of fumonisin B‐1 present in Fusarium moniliforme culture material and diacetoxyscirpenol or ochratoxin A in turkey poults. Poultry Science, 76, 256–264. [DOI] [PubMed] [Google Scholar]
- Kunsagi Z, Bouten K, Breidbach A, Mischke C, Bratinova S and Stroka J, 2012. Report on the 2012 Proficiency Test of the European Union Reference Laboratory for Mycotoxins, for the Network of National Reference Laboratories. Determination of DON, ZON, T‐2 and HT‐2 in Cereals. Available online: http://publications.jrc.ec.europa.eu/repository/handle/JRC76638
- Kujawski M, Mischke C, Bratinova S and Stroka J, 2014. Report on the 2014 Proficiency Test of the European Union Reference Laboratory for Mycotoxins, for the Network of National Reference Laboratories ‐ Determination of Aflatoxin B1 in Copra (Coconut powder). Available online: http://publications.jrc.ec.europa.eu/repository/handle/JRC91908
- Labuda R, Parich A, Berthiller F and Tancinova D, 2005. Incidence of trichothecenes and zearalenone in poultry feed mixtures from Slovakia. International Journal of Food Microbiology, 105, 19–25. [DOI] [PubMed] [Google Scholar]
- Lake BG, Phillips JC, Walters DG, Bayley DL, Cook MW, Thomas LV, Gilbert J, Startin JR, Baldwin NC, Bycroft BW and Dewick PM, 1987. Studies on the metabolism of deoxynivalenol in the rat. Food and Chemical Toxicology, 25, 589–592. [DOI] [PubMed] [Google Scholar]
- Lambert LA, Hines FA and Eppley RM, 1995. Lack of initiation and promotion potential of deoxynivalenol for skin tumorigenesis in Sencar mice. Food and Chemical Toxicology, 33, 217–222. [DOI] [PubMed] [Google Scholar]
- Lancova K, Hajslova J, Kostelanska M, Kohoutkova J, Nedelnik J, Moravcova H and Vanova M, 2008b. Fate of trichothecene mycotoxins during the processing: milling and baking. Food Additives and Contaminants Part A, 25, 650–659. [DOI] [PubMed] [Google Scholar]
- Lancova K, Hajslova J, Poustka J, Krplova A, Zachariasova M, Dostalek P and Sachambula L, 2008a. Transfer of Fusarium mycotoxins and ‘masked’ deoxynivalenol (deoxynivalenol‐3‐glucoside) from field barley through malt to beer. Food Additives and Contaminants Part A, 25, 723–744. [DOI] [PubMed] [Google Scholar]
- Lattanzio VMT, Ciasca B, Powers S and von Holst C, 2016. Validation of screening methods according to Regulation 519/2014/EU. Determination of deoxynivalenol in wheat by lateral flow immunoassay: A case study. Trends in Analytical Chemistry, 76, 137–144. [Google Scholar]
- Lattanzio VMT, Nivarlet N, Lippolis V, Della Gatta S, Huet A‐C, Delahaut P, Granier B and Visconti A, 2012. Multiplex dipstick immunoassay for semi‐quantitative determination of Fusarium mycotoxins in cereals. Analytica Chimica Acta, 718, 99–108. [DOI] [PubMed] [Google Scholar]
- Lattanzio VM, Solfrizzo M, De Girolamo A, Chulze SN, Torres AM and Visconti A, 2011. LC–MS/MS characterization of the urinary excretion profile of the mycotoxin deoxynivalenol in human and rat. Journal of Chromatography B, 879, 707–715. [DOI] [PubMed] [Google Scholar]
- Lautraite S, Parent‐Massin D, Rio B and Hoellinger H, 1997. In vitro toxicity induced by deoxynivalenol (DON) on human and rat granolu‐monocytic progenitors. Cell Biology and Toxicology, 13, 175–183. [DOI] [PubMed] [Google Scholar]
- Lebas F and Renouf B, 2009. Nutrition: utilisation des matières premières et techniques d'alimentation. Cuniculture Magazine, 36, 12–64. [Google Scholar]
- Leblanc J‐C, Tard A, Volatier J‐L and Verger P, 2005. Estimated dietary exposure to principal food mycotoxins from the first French total diet study. Food Additives and Contaminants, 22, 652–672. [DOI] [PubMed] [Google Scholar]
- Lebrun B, Tardivel C, Félix B, Abysique A, Troadec JD, Gaigé S and Dallaporta M, 2015. Dysregulation of energy balance by trichothecene mycotoxins: mechanisms and prospects. Neurotoxicology, 49, 15–27. [DOI] [PubMed] [Google Scholar]
- Lee H‐M, Song S‐O, Cha S‐H, Wee S‐B, Bischoff K, Park S‐W, Son S‐W, Kang H‐G and Cho M‐H, 2013. Development of a monoclonal antibody against deoxynivalenol for magnetic nanoparticle‐based extraction and an enzyme‐linked immunosorbent assay. Journal of Veterinary Science, 14, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hégarat L, Takakura N, Simar S, Nesslany F and Fessard V, 2014. The in vivo genotoxicity studies on nivalenol and deoxynivalenol. EFSA Supporting Publication 2014; 11(11):EN‐697, 33 pp. 10.2903/sp.efsa.2014.en-697 [DOI] [Google Scholar]
- Leitgeb R, Lew H, Wetscherek W, Bohm J and Quinz A, 1999. Influence of fusariotoxins on growing and slaughtering performance of broilers. Die Bodenkultur, 50, 57–66. [Google Scholar]
- Lemmens M, Steiner B, Sulyok M, Nicholson P, Mesterhazy A and Buerstmayr H, 2016. Masked mycotoxins: does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol‐3‐glucoside in new wheat varieties ? World Mycotoxin Journal, 9, 741–754. [Google Scholar]
- Leeson S and Summers JD, 2008. Commercial Poultry Nutrition, 3rd Edition Nottingham University Press, Guelph, Ontario, Canada: 412 pp. [Google Scholar]
- Lešnik M, Cencič A, Vajs S and Simončič A, 2008. Milling and bread baking techniques significantly affect the mycotoxin (deoxynivalenol and nivalenol) level in bread. Acta Alimentaria, 37, 471–483. [Google Scholar]
- Leung M, Smith T, Karrow N and Boermans H, 2007. Effects of foodborne Fusarium mycotoxins with and without a polymeric glucomannan mycotoxin adsorbent on food intake and nutrient digestibility, body weight, and physical and clinicopathologic variables of mature dogs. American Journal of Veterinary Research, 68, 1122–1129. [DOI] [PubMed] [Google Scholar]
- LFRA (Leatherhead Food Research Association), 2010. Guide to Contaminants in Foodstuffs. An international overview of maximum limits. Leatherhead Food International Limited, Leatherhead, United Kingdom. ISBN 987‐907895‐09‐8.
- Li B and Guo H‐W, 2001. AFB1 and DON‐induced DNA damage in primary rat hepatocytes. China Public Health, 17, Article ID: 1001‐0580 (2001) 12‐01085‐02 [Google Scholar]
- Li M, Cuff CF and Pestka J, 2005. Modulation of murine host response to enteric reovirus infection by the trichothecene deoxynivalenol. Toxicological Sciences 87, 134–145. [DOI] [PubMed] [Google Scholar]
- Li Q‐W, Li J‐P, Houh H‐F, Ding G‐Y, Li Y‐L, Gao H‐Y and Zhang Q‐L, 2011. Deoxynivalenol induced bone and articular cartilage injuries among young and adult rats. Chinese Preventive Medicine, 12, 813–817. [Google Scholar]
- Li FQ, Li YW, Luo XY and Yoshizawa T, 2002. Fusarium toxins in wheat from an area in Henan Province, PR China, with a previous human red mould intoxication episode. Food Additives and Contaminants, 19, 163–167. [DOI] [PubMed] [Google Scholar]
- Liesener K, Curtui V, Dietrich R, Märtlbauer E and Usleber E, 2010. Mycotoxins in horse feed. Mycotoxin Research, 26, 23–30. [DOI] [PubMed] [Google Scholar]
- Lim CW, Tai SH, Lee LM and Chan SH, 2012. Analytical method for the accurate determination of tricothecenes in grains using LC‐MS/MS: a comparison between MRM transition and MS3 quantitation. Analytical and Bioanalytical Chemistry, 403, 2801–2806. [DOI] [PubMed] [Google Scholar]
- Lin L and Sun S‐Q, 2004. Study on DNA damage and repair induced by deoxynivalenol in cultured Vero cell with Comet Assay. Chinese Journal of Control of Endemic Diseases, 19, 139–141. [Google Scholar]
- Lindblad M, Gidlund A, Sulyok M, Börjesson T, Krska R, Olsen M and Fredlund E, 2013. Deoxynivalenol and other selected Fusarium toxins in Swedish wheat ‐ occurrence and correlation to specific Fusarium species. International Journal of Food Microbiology, 167, 284–291. [DOI] [PubMed] [Google Scholar]
- Lippolis V, Pascale M and Visconti A, 2006. Optimization of a fluorescence polarization immunoassay for rapid quantification of deoxynivalenol in durum wheat‐based products. Journal of Food Protection, 69, 2712–2719. [DOI] [PubMed] [Google Scholar]
- Lippolis V, Pascale M, Cervellieri S, Damascelli A and Visconti A, 2014. Screening of deoxynivalenol contamination in durum wheat by MOS‐based electronic nose and identification of the relevant pattern of volatile compounds. Food Control, 37, 263–271. [Google Scholar]
- Liu Y, Ran R, Hu C, Cui B, Xu Y, Liu H, Quan S, Li D, Li X, Wu Y, Zhang D and Shi J, 2016. The metabolic responses of HepG2 cells to the exposure of mycotoxin deoxynivalenol. World Mycotoxin Journal, 9, 577–586. [Google Scholar]
- Liu Y, Walker F, Hoeglinger B and Buchenauer H, 2005. Solvolysis procedures for the determination of bound residues of the mycotoxin deoxynivalenol in fusarium species infected grain of two winter wheat cultivars preinfected with barley yellow dwarf virus. Journal of Agricultural and Food Chemistry, 53, 6864–6869. [DOI] [PubMed] [Google Scholar]
- Liu Y, Delwiche SR and Dong Y, 2009. Feasibility of FT–Raman spectroscopy for rapid screening for DON toxin in ground wheat and barley. Food Additives and Contaminants, 26, 1396–1401. [DOI] [PubMed] [Google Scholar]
- Lun AK, Young LG and Lumsden JH, 1985. The effects of vomitoxin and feed intake on the performance and blood characteristics of young pigs. Journal of Animal Science, 61, 1178–1185. [DOI] [PubMed] [Google Scholar]
- Lun AK, Young LG, Moran ET, Hunter DB and Rodriguez JP, 1986. Effects of feeding hens a high level of vomitoxin‐contaminated corn on performance and tissue residues. Poultry Science, 65, 1095–1099. [DOI] [PubMed] [Google Scholar]
- Lun AK, Moran ET Jr, Young LG and McMillan EG, 1988. Disappearance of deoxynivalenol from digesta progressing along the chicken's gastrointestinal tract after intubation with feed containing contaminated corn. Bulletin of Environmental Contamination and Toxicology, 40, 317–324. [DOI] [PubMed] [Google Scholar]
- Luongo D, De Luna R, Russo R and Severino L, 2008. Effects of four Fusarium toxins (fumonisin B(1), alpha‐zearalenol, nivalenol and deoxynivalenol) on porcine whole‐blood cellular proliferation. Toxicon, 52, 156–162. [DOI] [PubMed] [Google Scholar]
- Luo XY, Li YW, Wen SF and Hu X, 1987. Food poisoning caused by scabby wheat and the detection of Fusarium mycotoxins. Journal of Hygiene Research, 16, 33–37. [Google Scholar]
- Luo XY, 1988. Outbreaks of moldy cereals poisoning in China. Issues in Food Safety. A joint meeting of the Toxicology Forum and the Chinese Academy of Preventive Medicine in cooperation with the International Life Sciences Institute and the World Health Organization. Washington DC, USA, 56–63. [Google Scholar]
- Luo X, 1994. Food poisoning caused by Fusarium toxins. Proceedings of the Second Asian Conference on Food Safety, pp. 129–136.
- Lusky K, Goebel R, Tesch D, Tenner G, Haider W, Krueger M and Lippert A, 1998. Studies on the effects of ochratoxin A and deoxynivalenol toxicity on the health of pigs and tissue residue concentrations. Tierärztliche Umschau, 53, 623–630. [Google Scholar]
- Malekinejad H, Schoevers EJ, Daemen IJJM, Zijlstra C, Colenbrander B, Fink‐Gremmels J and Roelen BAJ, 2007. Exposure of oocytes to the fusarium toxins zearalenone and deoxynivalenol causes aneuploidy and abnormal embryo development in pigs. Biology of Reproduction, 77, 840–847. [DOI] [PubMed] [Google Scholar]
- Malachova A, Dzuman Z, Veprikova Z, Vaclavikova M, Zachariasova M and Hajslova J, 2011. Deoxynivalenol, deoxynivalenol‐3‐glucoside, and enniatins: the major mycotoxins found in cereal‐based products on the Czech market. Journal of Agricultural and Food Chemistry, 59, 12990–12997. [DOI] [PubMed] [Google Scholar]
- Malachova A, Varga E, Schwartz H, Krska R and Berthiller F, 2012. Development, validation and application of an LC‐MS/MS based method for the determination of deoxynivalenol and its conjugates in different types of beer. World Mycotoxin Journal, 5, 261–270. [Google Scholar]
- Malachova A, Stockova L, Wakker A, Varga E, Krska R, Michlmayr H, Adam G and Berthiller F, 2015. Critical evaluation of indirect methods for the determination of deoxynivalenol and its conjugated forms in cereals. Analytical and Bioanalytical Chemistry, 407, 6009–6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male D, Wu W, Mitchell N, Bursian S, Pestka J and Wu F, 2016. Modeling the emetic potencies of food‐borne trichothecenes by benchmark dose methodology. Food and Chemical Toxicology, 94, 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BB, Abbas HK, Wise DJ and Greenway T, 2014. The effect of feeding diets containing deoxynivalenol contaminated corn on channel catfish (Ictalurus punctatus) challenged with Edwardsiella ictaluri. Aquaculture Research, 45, 1782–1786. [Google Scholar]
- Mankeviciene A, Suproniene S, Brazauskiene I and Gruzdeviene E, 2011. Natural occurrence of Fusarium mycotoxins in oil crop seed. Plant Breeding and Seed Science, 63, 109–116. [Google Scholar]
- Maragos CM and McCormick SP, 2000. Monoclonal antibodies for the mycotoxins deoxynivalenol and 3‐acetyl‐deoxynivalenol. Food and Agricultural Immunology, 12, 181–192. [Google Scholar]
- Maragos CM and Plattner RD, 2002. Rapid fluorescence polarization immunoassay for the mycotoxin deoxynivalenol in wheat. Journal of Agricultural and Food Chemistry, 50, 1827–1832. [DOI] [PubMed] [Google Scholar]
- Maragos C, Busman M and Sugita‐Konishi Y, 2006. Production and characterization of a monoclonal antibody that cross‐reacts with the mycotoxins nivalenol and 4‐deoxynivalenol. Food Additives and Contaminants, 23, 816–825. [DOI] [PubMed] [Google Scholar]
- Maragos CM, 2011. Detection of deoxynivalenol using biolayer interferometry. Mycotoxin Research, 27, 157–165. [DOI] [PubMed] [Google Scholar]
- Mansfield MA, De Wolf ED and Kuldau GA, 2005. Relationships between weather conditions, agronomic practices, and fermentation characteristics with deoxynivalenol content in fresh and ensiled maize. Plant Disease, 89, 1151–1157. [DOI] [PubMed] [Google Scholar]
- Marczuk J, Obremski K, Lutnicki K, Gajecka M and Gajecki M, 2012. Zearalenone and deoxynivalenol mycotoxicosis in dairy cattle herds. Polish Journal of Veterinary Science, 15, 365–372. [DOI] [PubMed] [Google Scholar]
- Maresca M, 2013. From the gut to the brain: journey and pathophysiological effects of the food‐associated trichothecene mycotoxin deoxynivalenol. Toxins (Basel), 5, 784–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzocco S, Russo R, Bianco G, Autore G and Severino L, 2009. Pro‐apoptotic effects of nivalenol and deoxynivalenol trichothecenes in J774A.1 murine macrophages. Toxicological Letters, 189, 21–26. [DOI] [PubMed] [Google Scholar]
- Marin S, Ramos AJ, Cano‐Sancho SG and Sanchis V, 2013. Mycotoxins: occurrence, toxicology, and exposure assessment. Food and Chemical Toxicology, 60, 218–237. [DOI] [PubMed] [Google Scholar]
- Matejova I, Modra H, Blahova J, Franc A, Fictum P, Sevcikova M and Svobodova Z, 2014. The effect of mycotoxin deoxynivalenol on haematological and biochemical indicators and histopathological changes in rainbow trout (Oncorhynchus mykiss). BioMed Research International, 10.1155/2014/310680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matumba L, Van Poucke C, Njumbe Ediage E, Jacobs B and De Saeger S, 2015. Effectiveness of hand sorting, flotation/washing, dehulling and combinations thereof on the decontamination of mycotoxin‐contaminated white maize. Food Additives and Contaminants; Part A, 32, 960–969. [DOI] [PubMed] [Google Scholar]
- Maul R, Warth B, Kant JS, Schebb NH, Krska R, Koch M and Sulyok M, 2012. Investigation of the hepatic glucuronidation pattern of the Fusarium mycotoxin deoxynivalenol in various species. Chemical Research in Toxicology, 25, 2715–2717. [DOI] [PubMed] [Google Scholar]
- Maul R, Warth B, Schebb NH, Krska R, Koch M and Sulyok M, 2014. In vitro glucuronidation kinetics of deoxynivalenol by human and animal microsomes and recombinant human UGT enzymes. Archives of toxicology, 89, 949–960. [DOI] [PubMed] [Google Scholar]
- Mayer S, Curtui V, Usleber E and Gareis M, 2007. Airborne mycotoxins in dust of grain elevators. Mycotoxin Research, 23, 94–100. [DOI] [PubMed] [Google Scholar]
- McDonald P, Greenhalgh JFD, Morgan CA, Edwards R, Sinclair L and Wilkinson R, 2011. Animal Nutrition. 7th Edition, Benjamin Cummings. 692 pp. [Google Scholar]
- Meky FA, Turner PC, Ashcroft AE, Miller JD, Qiao YL, Roth MJ and Wild CP, 2003. Development of a urinary biomarker of human exposure to deoxynivalenol. Food and Chemical Toxicology, 41, 265–273. [DOI] [PubMed] [Google Scholar]
- Meky FA, Hardie LJ, Evans SW and Wild CP, 2001. Deoxynivalenol‐induced immunomodulation of human lymphocyte proliferation and cytokine production. Food and Chemical Toxicology, 39, 827–836. [DOI] [PubMed] [Google Scholar]
- Mendoza A, La Manna A, Mieres J and Acosta Y, 2014. Evaluation of deoxynivalenol intake and a commercial adsorbent of mycotoxins in grazing dairy cows. Agrociencia Uruguay, 18, 133–140. [Google Scholar]
- Michlmayr H, Varga E, Malachova A, Nguyen NT, Lorenz C, Haltrich D, Berthiller F and Adam G, 2015. A versatile family of glycoside hydrolase from Bifidobacterium adolescentis hydrolyzes β‐glucosides of the Fusarium mycotoxins deoxynivalenol, nivalenol, and HT‐2 toxin in cereal matrices. Applied and Environmental Microbiology, 81, 4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minekus M, Marteau P, Havenaar R and Huis in't Veld JHJ, 1995. A multi compartmental dynamic computer‐controlled model simulating the stomach and small intestine. Alternatives to Laboratory Animals (ATLA), 23, 197–209. [Google Scholar]
- Mishra S, Dwivedi PD, Pandey HP and Das M, 2014a. Role of oxidative stress in deoxynivalenol induced toxicity. Food Chemistry and Toxicology, 72, 20–29. [DOI] [PubMed] [Google Scholar]
- Mishra S, Dixit S, Dwivedi PD, Pandey HP and Das M, 2014b. Influence of temperature and pH on the degradation of deoxynivalenol (DON) in aqueous medium: comparative cytotoxicity of DON and degraded product. Food Additives and Contaminants, Part. A, 31, 121–131. [DOI] [PubMed] [Google Scholar]
- Mishra S, Tewari P, Chaudhari BP, Dwivedi PD, Pandey HP and Das M, 2016. Deoxynivalenol induced mouse skin tumor initiation: elucidation of molecular mechanisms in human HaCaT keratinocytes. International Journal on Cancer, 139, 2033–2046. [DOI] [PubMed] [Google Scholar]
- Mitak M, Pleadin J, Perši N, Vulić A and Zadravec M, 2011. Mikotoksini u krmnim sirovinama I smjesama tjekom 2009. I 2010. Godine (translated to Mycotoxins in feed ingredients and mixes during 2009 and 2010). Veterinarska Stanica, 42, 139–145. [Google Scholar]
- Moazami E, Jinap S, Mousa W and Hajeb P, 2014. Effect of Food Additives on deoxynivalenol (DON) reduction and quality attributes in steamed‐and‐fried instant noodles. Cereal Chemistry, 91, 88–94. [Google Scholar]
- Monbaliu S, Van Poucke C, Van Peteghem C, Van Poucke K, Heungens K and De Saeger S, 2009. Development of a multi‐mycotoxin liquid chromatography/tandem mass spectrometry method for sweet pepper analysis. Rapid Communications in Mass Spectrometry, 23, 3–11. [DOI] [PubMed] [Google Scholar]
- Monbaliu S, Van Poucke C, Detavernier C, Dumoulin F, Van De Velde M, Schoeters E, Van Dyck S, Averkieva O, Van Peteghem C and De Saeger S, 2010. Occurrence of mycotoxins in feed as analyzed by a multi‐mycotoxin LC‐MS/MS method. Journal of Agricultural and Food Chemistry, 58, 66–71. [DOI] [PubMed] [Google Scholar]
- Morrissey RE, 1984. Teratological study of Fischer rats fed diet containing added vomitoxin. Food and Chemical Toxicology, 22, 453–457. [DOI] [PubMed] [Google Scholar]
- Morrissey RE and Vesonder RF, 1985. Effect of Deoxynivalenol (Vomitoxin) on Fertility. Pregnancy, and Postnatal Development of Sprague‐Dawley Rats, Applied and Environmental Microbiology, 49, 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisey RE, Norred WP and Vesonder RF, 1985. Subchronic toxicity of vomitoxin in Sprague‐Dawley rats. Food and Chemical Toxicology, 23, 959–999. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS and Schwartz MW, 2006. Central nervous system control of food intake and body weight. Nature, 443, 289–295. [DOI] [PubMed] [Google Scholar]
- Mose T, Mathiesen L, Karttunen V, Nielsen JK, Sieppi E, Kummu M, Mørck TA, Myöhänen K, Partanen H, Vähäkangas K, Knudsen LE and Myllynen P, 2012. Meta‐analysis of data from human ex vivo placental perfusion studies on genotoxic and immunotoxic agents within the integrated European project NewGeneris. Placenta, 33, 433–439. [DOI] [PubMed] [Google Scholar]
- Nagashima H, Nakagawa H and Kushiro M, 2012. Opposite effects of two trichothecene mycotoxins, deoxynivalenol and nivalenol, on the levels of macrophage inflammatory protein (MIP)‐1α and MIP‐1β in HL60 cells. Environmental Toxicology and Pharmacology, 34, 1014–1017. [DOI] [PubMed] [Google Scholar]
- Nagl V, Schwartz H, Krska R, Moll WD, Knasmüller S, Ritzmann M, Adam G and Berthiller F, 2012. Metabolism of the masked mycotoxin deoxynivalenol‐3‐glucoside in rats. Toxicology Letters, 213, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl V, Woechtl B, Schwartz‐Zimmermann HE, Hennig‐Pauka I, Moll WD, Adam G and Berthiller F, 2014. Metabolism of the masked deoxynivalenol‐3‐glucoside in pigs. Toxicology Letters, 229, 190–197. [DOI] [PubMed] [Google Scholar]
- Nathanail AV, Syvähuoko J, Malachova A, Jestoi M, Varga E, Michlmayr H, Adam G, Sieviläinen E, Berthiller F and Peltonen K, 2015. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography‐tandem mass spectrometric method. Analytical and Bioanalytical Chemistry, 407, 4745–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCM (Nordic Council of Ministers), 1998. Fusarium toxins in cereals – risk assessment. Nordic Council of Ministers, TemaNord Food, Copenhagen. TemaNord, 502, 147. ISBN 92‐893‐0149‐X [Google Scholar]
- Nelson M, Schneider NR, Doster AR, Carlson MP and Klopfenstein T, 1984. Vomitoxin contaminated wheat‐pathology, toxicity in cattle. Nebraska Beef Cattle Reports, MP‐47, 3. [Google Scholar]
- Nielsen JK, Vikström AC, Turner P and Knudsen LE, 2011. Deoxynivalenol transport across the human placental barrier. Food and Chemical Toxicology, 49, 2046–2052. [DOI] [PubMed] [Google Scholar]
- Nix JS, 2010. The John Nix Farm Management Pocketbook. 40th Edition The Anderson Centre. [Google Scholar]
- Nordby K‐C, Straumfors Halstense A, Elen O, Clasen P‐E, Langseth W, Kristensen P and Eduard W, 2004. Trichothecene mycotoxins and their determinants in settled dust related to grain production. Annals of Agricultural and Environmental Medicine, 11, 75–83. [PubMed] [Google Scholar]
- Nowicki TW, Gaba DG, Dexter JE, Matsuo RR and Clear RM, 1988. Retention of the Fusarium mycotoxin deoxynivalenol in wheat during processing and cooking of spaghetti and noodles. Journal of Cereal Science, 8, 189–202. [Google Scholar]
- NRC (National Research Council), 1982. Nutrient requirements of Mink and Foxes. 2nd, Revised edition. National Academies Press, Washington, DC. [Google Scholar]
- NRC (National Research Council), 2006. Nutrient requirements of dogs and cats. National Academies Press, Washington, DC. [Google Scholar]
- NRC (National Research Council), 2007a. Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. National Academies Press, Washington, DC. [Google Scholar]
- NRC (National Research Council), 2007b. Nutrient requirement of horses. 6th Revised Edition National Academies Press, Washington, DC. [Google Scholar]
- NVK , Norwegian Scientific Committee for Food Safety, 2013. Risk assessment of mycotoxins in cereal grain in Norway. Opinion of the Scientific Steering Committee of the Norwegian Scientific Committee for Food Safety. Doc. 10‐004‐4‐Final. 287 pp. Available online: http://www.vkm.no/dav/eee04d10c4.pdf
- NVK , Norwegian Scientific Committee for Food Safety, 2014. Comparison of the Panel on Plant Health and the Steering Committee of the Norwegian Scientific Committee for food Safety. Part I: Plant health and plant production. Opinion of the Panel on Plant Health and the Steering Committee of the Norwegian Scientific Committee for Food Safety. Doc. No. 11/007‐1‐final.
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document on overview of residue chemistry studies (as revised in 2009). Series on Testing and Assessment number 64 and Series on Pesticides number 32, OECD Environment, Health and Safety Publications, Paris, ENV/JM/MONO (2009) 31, 93 pp.
- Olsen M, 2008. Mycotoxins in organic and conventional food and effects of the environment In: Givens DI, Baxter S, Minihane AM. and Shaw E. (eds), Health Benefits of Organic Food: Effects of the Environment. University of Reading, United Kingdom, Available online: http://www.cabi.org [Google Scholar]
- Ostrowska‐Kołodziejczak A, Stuper‐Szablewska K, Kulik T, Buśko M, Rissmann I, Wiwart M and Perkowski J, 2016. Concentration of fungal metabolites, phenolic acids and metals in mixtures of cereals grown in organic and conventional farms. Journal of Animal and Feed Sciences, 25, 74–81. [Google Scholar]
- Osselaere A, Devreese M, Watteyn A, Vandenbroucke V, Goossens J, Hautekiet V, Eeckhout M, De Saeger S, De Baere S, De Backer P and Croubels S, 2012. Efficacy and safety testing of mycotoxin‐detoxifying agents in broilers following the European Food Safety Authority guidelines. Poultry Science, 91, 2046–2054. [DOI] [PubMed] [Google Scholar]
- Osselaere A, Devreese M, Goossens J, Vandenbroucke V, De Baere S, De Backer P and Croubels S, 2013a. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T‐2 toxin and zearalenone in broiler chickens. Food and Chemical Toxicology, 51, 350–355. [DOI] [PubMed] [Google Scholar]
- Osselaere A, Santos R, Hautekiet V, De Backer P, Chiers K, Ducatelle R and Croubels S, 2013b. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS ONE 8, e69014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkopp K‐P, Hirst M and Rapley M, 1994. Deoxynivalenol (vomitoxin)‐induced conditioned taste aversions in rats are mediated by the chemosensitive area postrema. Pharmacology, Biochemistry and Behavior, 47, 363–367. [DOI] [PubMed] [Google Scholar]
- Papadopoulou‐Bouraoui A, Vrabcheva T, Valzacchi S, Stroka J and Anklam E, 2004. Screening survey of deoxynivalenol in beer from the European market by an enzyme‐linked immunosorbent assay. Food Additives and Contaminants, 21, 607–617. [DOI] [PubMed] [Google Scholar]
- Parent‐Massin D, 2004. Haemetotoxicity of trichothecenes. Toxicology Letters, 153, 75–81. [DOI] [PubMed] [Google Scholar]
- Payros D, Alassane‐Kpembi I, Pierron A, Loiseau N, Pinton P and Oswald IP, 2016. Toxicology of deoxynivalenol and its acetylated and modified forms. Archives of Toxicology, 90, 2931–2957. [DOI] [PubMed] [Google Scholar]
- Pecchi E, Dallaporta M, Thirion S, Salvat C, Berenbaum F, Jean A and Troadec JD, 2006. Involvement of central microsomal prostaglandin E synthase‐1 in IL‐1beta‐induced anorexia. Physiological Genomics, 25, 485–492. [DOI] [PubMed] [Google Scholar]
- Pereira VL, Fernandez JO and Cunha SC, 2015. Comparative assessment of three cleanup procedures after QuEChERS extraction for determination of trichothecenes (type A and type B) in processed cereal‐based baby foods by GC‐MS. Food Chemistry, 182, 143–149. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, 2003. Deoxynivalenol‐induced IgA production and IgA nephropathy‐aberrant mucosal immune response with systemic repercussions. Tox Letters, 140–141, 287–295. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Tai JH, Witt MF, Dixon DE and Forsell JH, 1987. Suppression of immune‐response in the B6c3f1 mouse after dietary exposure to the fusarium mycotoxins deoxynivalenol (vomitoxin) and zearalenone. Food and Chemical Toxicology, 25, 297–304. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Uzarski RL and Islam Z, 2005. Induction of apoptosis and cytokine production in the Jurkat human T cells by deoxynivalenol: role of mitogen‐activated protein kinases and comparison to other 8‐ketotrichothecenes. Toxicology, 206, 207–219. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, 2007. Deoxynivalenol: toxicity, mechanisms and animal health risks. Animal Feed Science and Technology, 137, 283–298. [Google Scholar]
- Pestka JJ, 2010a. Deoxynivalenol: mechanisms of action, human exposure and toxicological relevance. Archives of Toxicology, 84, 663–679. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, 2010b. Deoxynivalenol‐induced proinflammatory gene expression: mechanisms and pathological sequelae. Toxins, 2, 1300–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ and Amuzie CJ, 2008. Tissue distribution and proinflammatory cytokine gene expression following acute oral exposure to deoxynivalenol: comparison of weanling and adult mice. Food and Chemical Toxicology, 46, 2826–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ, Islam Z and Amuzie CJ, 2008. Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse. Toxicology Letters, 178, 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ and Smolinski AT, 2005. Deoxynivalenol: Toxicology and Potential Effects on Humans. Journal of Toxicology and Environmental Health, Part B: Critical Reviews, 8, 39–69. [DOI] [PubMed] [Google Scholar]
- Pestka JJ and Zhou H‐R, 2000. Interleukin‐6‐deficient mice refractory to IgA dysregulation but not anorexia induction by vomitoxin (deoxynivalenol) ingestion. Food Chemical Toxicology, 38, 565–575. [DOI] [PubMed] [Google Scholar]
- Pestka JJ and Zhou HR, 2002. Effects of tumor necrosis factor type 1 and 2 receptor deficiencies on anorexia, growth and IgA dysregulation in mice exposed to the trichothecene vomitoxin. Food Chemical Toxicology, 40, 1623–1631. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Zhou HR, Moon Y and Chung YJ, 2004. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicology Letters, 153, 61–73. [DOI] [PubMed] [Google Scholar]
- Peters J, Bienenmann‐Ploum M, de Rijk T and Haasnoot W, 2011. Development of a multiplex flow cytometric microsphere immunoassay for mycotoxins and evaluation of its application in feed. Mycotox Research, 27, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekkola S, Turner PC, Abdel‐Hamid M, Ezzat S, El‐Daly M, El‐Kafrawy S, Savchenko E, Poussa T, Woo JC, Mykkänen H and El‐Nezami H, 2012. Characterisation of aflatoxin and deoxynivalenol exposure among pregnant Egyptian women. Food Additives and Contaminants, Part A‐Chem Anal Control Expo Risk Assess, 29, 962–971. [DOI] [PubMed] [Google Scholar]
- Pierron A, Mimoun S, Murate LS, Loiseau N, Lippi Y, Bracarense APFL, Schatzmayr G, Berthiller F, Moll WD and Oswald IP, 2016b. Intestinal toxicity of the masked mycotoxin deoxynivalenol‐3‐β‐d‐glucoside. Archives of Toxicology, 90, 1–2037−2046. [DOI] [PubMed] [Google Scholar]
- Pierron A, Mimoun S, Murate LS, Loiseau N, Lippi Y, Bracarense APFL, Schatzmayr G, He J, Zhou T, Moll WD and Oswald IP, 2016a. Microbial biotransformation of DON: molecular basis for reduced toxicity. Scientific Reports, 6, 29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters MN, Freijer J, Baars AJ and Slob W, 2001. Risk Assessment of Deoxynivalenol in Food, An assessment of exposure and effects in the Netherlands. RIVM report 388802 022, National Institute of Public Health and the Environment. 33 pp.
- Pieters MN, Bakker M and Slob W, 2004. Reduced intake of deoxynivalenol in The Netherlands: a risk assessment update. Toxicology Letters, 153, 145–153. [DOI] [PubMed] [Google Scholar]
- Pieters MN, Fiolet DCM and Baars AJ, 1999, Deoxynivalenol. Derivation of concentration limits in wheat and wheat containing food products, National Institute of Public Health and the Environment, RIVM report 388802 018. Bilthoven, the Netherlands.
- Pietsch C, Michel C, Kersten S, Valenta H, Dänicke S, Schulz C, Kloas W and Burkhardt‐Holm P, 2014a. In vivo effects of deoxynivalenol (DON) on innate immune responses of carp (Cyprinus carpio L.). Food and Chemical Toxicology, 68, 44–52. [DOI] [PubMed] [Google Scholar]
- Pietsch C, Schulz C, Rovira P, Kloas W and Burkhardt‐Holm P, 2014b. “Organ Damage and Hepatic Lipid Accumulation in Carp (Cyprinus carpio L.) after Feed‐Borne Exposure to the Mycotoxin, Deoxynivalenol (DON).” Toxins 6, 756–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinotti L, Ottoboni M, Giromini C, Dell'Orto V and Cheli F, 2016. Mycotoxin contamination in the EU feed supply chain: a focus on cereal byproducts. Toxins, 8, 45 10.3390/toxins8020045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Graziani F, Pujol A, Nicoletti C, Paris O, Ernouf P, Di Pasquale E, Perrier J, Oswald IP and Maresca M, 2015. Deoxynivalenol inhibits the expression by goblet cells of intestinal mucins through a PKR and MAP kinase‐dependent repression of the resistin‐like molecule β. Molecular Nutrition and Food Research, 59, 1076–1087. [DOI] [PubMed] [Google Scholar]
- Pinton P, Nougayrede JP, del Rio JC, Moreno C, Marin D, Ferrier L, Bracarense AP, Kolf‐Clauw M and Oswald IP, 2009. The food contaminant, deoxynivalenol, decreases intestinal barrier function and reduces claudin expression. Toxicology and Applied Pharmacology, 237, 41–48. [DOI] [PubMed] [Google Scholar]
- Pinton P, Braicu C, Nougayrede J‐P, Laffitte J, Taranu I and Oswald IP, 2010. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin‐4 through a mitogen‐activated protein kinase‐dependent mechanism. Journal of Nutrition, 140, 1956–1962. [DOI] [PubMed] [Google Scholar]
- Pinton P and Oswald IP, 2014. Effect of deoxynivalenol and other type B trichothecenes on the intestine: a review. Toxins, 6, 1615–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Tsybulskyy D, Lucioli J, Laffitte J, Callu P, Lyazhri F, Grosjean F, Bracarense AP, Kolf‐Clauw M and Oswald IP, 2012. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: differential effects on morphology, barrier function, tight junctions proteins and mitogen‐activated protein kinases. Toxicological Sciences, 130, 180–190. [DOI] [PubMed] [Google Scholar]
- Pitt JI, Taniwaki MH and Cole MB, 2013. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control, 32, 205–215. [Google Scholar]
- Pollmann DS, Koch BA, Seitz LM, Mohr HE and Kennedy GA, 1985. Deoxynivalenol‐contaminated wheat in swine diets. Journal of Animal Science, 60, 239–247. [DOI] [PubMed] [Google Scholar]
- Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R, Krska R, Kuchler K, Glossl J, Luschnig C and Adam G, 2003. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP‐glucosyltransferase from Arabidopsis thaliana. Journal of Biological Chemistry, 278, 47905–47914. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, 1993. The effect of low‐level deoxynivalenol on neurotransmitter levels measured in pig cerebral spinal fluid. Journal of Environmental Science and Health, Part B, 28, 731–761. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, 1997. Effect of intraperitoneal infusion of deoxynivalenol on feed consumption and weight gain in the pig. Natural Toxins, 5, 121–125. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Trenholm HL, Lawrence GA and Scott PM, 1984b. Nontransmission of deoxynivalenol (vomitoxin) to milk following oral administration to dairy cows. Journal of Environmental Science and Health B, 19, 593–609. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Veira DM and Trenholm HL, 1985. Plasma pharmacokinetics of the mycotoxin deoxynivalenol following oral intravenous administration to sheep. Journal of Environmental Science and Health. Part B, 20, 603–624. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Hamilton RM, Trenholm HL and Miller JD, 1986. Tissue distribution and excretion of radioactivity following administration of 14C‐labeled deoxynivalenol to White Leghorn hens. Fundamental and Applied Toxicology, 7, 635–645. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Veira DM, Trenholm HL and Foster BC, 1987. Metabolic fate and elimination in milk, urine and bile of deoxynivalenol following administration to lactating sheep. Journal of Environmental Science and Health B, 22, 125–148. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Hartin KE, Trenholm HL and Miller JD, 1988. Pharmacokinetic fate of 14C‐labeled deoxynivalenol in swine. Fundamental and Applied Toxicology, 10, 276–286. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Hamilton RM and Trenholm HL, 1989. Transmission of residues to eggs following long‐term administration of 14C‐labelled deoxynivalenol to laying hens. Poultry Science, 68, 744–748. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Hartin KE and Trenholm HL, 1990. Distribution of deoxynivalenol in cerebral spinal fluid following administration to swine and sheep. Journal of Environmental Science and Health Part. B, 25, 395–413. [DOI] [PubMed] [Google Scholar]
- Prelusky DB and Trenholm HL, 1991. Tissue distribution of deoxynivalenol in swine dosed intravenously. Journal of Agricultural and Food Chemistry, 39, 748–751. [Google Scholar]
- Prelusky DB, Yeung JM, Phompson BK and Trenholm HL, 1992. Effect of deoxynivalenol on neurotransmitters in discrete regions of swine brains. Archives of Environmental Contamination and Toxicology, 22, 36–40. [DOI] [PubMed] [Google Scholar]
- Rafai P, Bata R, Jakab L and Vanyi A, 2000. Evaluation of mycotoxin‐contaminated cereals for their use in animal feeds in Hungary. Food Additives and Contaminants, 17, 799–808. [DOI] [PubMed] [Google Scholar]
- Raiola A, Meca G, Mañes J and Ritieni A, 2012. Bioaccessibility of deoxynivalenol and its natural co‐occurrence with ochratoxin A and aflatoxin B1 in Italian commercial pasta. Food and Chemical Toxicology, 50, 280–287. [DOI] [PubMed] [Google Scholar]
- Ran R, Wang C, Han Z, Wu A, Zhang D and Shi J, 2013. Determination of deoxynivalenol (DON) and its derivatives: Current status of analytical methods. Food Control, 34, 138–148. [Google Scholar]
- Rasmussen PH, Nielsen KF, Ghorbani F, Spliid NH, Nielsen GC and Jørgensen LN, 2012. Mycotoxin Research, 28, 181–190. [DOI] [PubMed] [Google Scholar]
- Rasmussen RR, Storm IMLD, Rasmussen PH, Smedsgaard J and Nielsen KF, 2010. Multi‐mycotoxin analysis of maize silage by LC‐MS/MS. Analytical and Bioanalytical Chemistry, 397, 765–776. [DOI] [PubMed] [Google Scholar]
- Rautala T, Hietaniemi V, Rämö S, Koivisto T, Ovaskainen M‐L, Sinkko H, Kronberg‐Kippilä C, Hirvonen T, Liukkonen K‐H, Kartio M and Hallikainen A, 2008. Fusarium toxins: adult intake from cereals and cereal‐based products in Finland. Evira Research Reports 5. Finnish Food Safety Authority Evira. 44 pp.
- Raymond SL, Smith TK and Swamy HVLN, 2003. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on feed intake, serum chemistry and haematology of horses and the efficacy of a polymeric glucomannan mycotoxin adsorbent. Journal of Animal Science, 81, 2123–2130. [DOI] [PubMed] [Google Scholar]
- Raymond SL, Smith TK and Swamy HVLN, 2005. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on feed intake, metabolism, and indices of athletic performance of exercised horses. Journal of Animal Science, 83, 1267–1273. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF, Hansen HF, Marley PD and Stengaardpedersen K, 1985. Molecular forms of cholecystokinin in the brain and the relationship to neuronal gastrins. Annals of the New York Academy of Sciences, 448, 11–23. [DOI] [PubMed] [Google Scholar]
- Rempe I, Kersten S, Brezina U, Hermeyer K, Beineke A and Dänicke S, 2013. Time dependent effects of graded levels of Fusarium toxin contaminated maize in diets for female piglets. World Mycotoxin Journal, 6, 51–63. [Google Scholar]
- Ricci F, Flavio P, Abagnale M, Messia MC, Marconi E, Volpe G, Moscone D and Palleschi G, 2009. Direct electrochemical detection of trichothecenes in wheat samples using a 96‐well electrochemical plate coupled with microwave hydrolysis. World Mycotoxin Journal, 2, 239–245. [Google Scholar]
- Rio B, Lautraite S and Parent‐Massin D, 1997. In vitro toxicity of trichothecenes on human erythroblastic progenitors. Human and Experimental Toxicology, 16, 673–679. [DOI] [PubMed] [Google Scholar]
- Rizzo AF, Atroshi F, Hirvi T and Saloniemi H, 1992. The hemolytic activity of deoxynivalenol and T‐2 toxin. Natural Toxins, 1, 106–110. [DOI] [PubMed] [Google Scholar]
- Rocha O, Ansari K and Doohan FM, 2005. Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Additives and Contaminants Part A, 22, 369–378. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Carrasco Y, Moltó JC, Mañes J and Berrada H, 2014a. Exposure assessment approach through mycotoxin/creatinine ratio evaluation in urine by GC–MS/MS. Food and Chemical Toxicology, 72, 69–75. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Carrasco Y, Moltó JC, Mañes J and Berrada H, 2014b. Development of a GC–MS/MS strategy to determine 15 mycotoxins and metabolites in human urine. Talanta, 128, 125–131. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Carrasco Y, Mañes J, Berrada H and Font G, 2015. Preliminary estimation of deoxynivalenol excretion through a 24 h pilot study. Toxins, 7, 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CG and Héroux‐Metcalf C, 1983. Cytotoxicity and absence of mutagenic activity of vomitoxin (4‐deoxynivalenol) in a hepatocytes‐mediated mutation assay with V79 Chinese hamster lung cells. Cancer Letters, 20, 29–35. [DOI] [PubMed] [Google Scholar]
- Rohweder D, Kersten S, Valenta H, Sondermann S, Schollenberger M, Drochner W and Dänicke S, 2013. Bioavailability of the Fusarium toxin deoxynivalenol (DON) from wheat straw and chaff in pigs. Archives of Animal Nutrition, 67, 37–47. [DOI] [PubMed] [Google Scholar]
- Romanazzo D, Ricci F, Volpe G, Elliott CT, Vesco S, Kroeger K, Moscone D, Stroka J, Van Egmond H, Vehniäinen M and Palleschi G, 2010. Development of a recombinant Fab‐fragment based electrochemical immunosensor for deoxynivalenol detection in food samples. Biosensors and Bioelectronics, 25, 2615–2621. [DOI] [PubMed] [Google Scholar]
- Rotter BA, Prelusky DB and Pestka JJ, 1996. Invited review: Toxicology of deoxynivalenol (vomitoxin). Journal of Toxicology and Environmental Health, 48, 1–34. 10.1080/009841096161447 [DOI] [PubMed] [Google Scholar]
- Rubert J, Mañes J, James KJ and Soler C, 2011. Application of hybrid linear ion trap‐high resolution mass spectrometry to the analysis of mycotoxins in beer. Food Additives and Contaminants A, 28, 1438–1446. [DOI] [PubMed] [Google Scholar]
- Ruiz MJ, Franzova P, Juan‐Garcia A and Font G, 2011. Toxicological interactions between the mycotoxins beauvericin, deoxynivalenol and T‐2 toxin in CHO‐K1 cells in vitro. Toxicon, 58, 315–326. [DOI] [PubMed] [Google Scholar]
- Rychlik M, Humpf H‐U, Marko D, Dänicke S, Mally A, Berthiller F, Klaffke H and Lorenz N, 2014. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxins Research, 30, 197–205. 10.1007/s12550-014-0203-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryerse IA, Hooft JM, Bureau DP, Hayes MA and Lumsden JS, 2014. Purified deoxynivalenol or feed restriction reduces mortality in rainbow trout, Oncorhynchus mykiss (Walbaum), with experimental bacterial coldwater disease but biologically relevant concentrations of deoxynivalenol do not impair the growth of Flavobacterium psychrophilum. Journal of Fish Diseases, 38, 809–819. 10.1111/jfd.12295 [DOI] [PubMed] [Google Scholar]
- Ryu D, Bianchini A and Bullerman LB, 2008. Effects of processing on mycotoxins. Stewart Postharvest Review, 4, 1–7. [Google Scholar]
- Sanden M, Jørgensen S, Hemre GI, Ørnsrud R and Sissener NH, 2012. Zebrafish (Danio rerio) as a model for investigating dietary toxic effects of deoxynivalenol contamination in aquaculture feeds. Food and Chemical Toxicology, 50, 4441–4448. [DOI] [PubMed] [Google Scholar]
- Sanders M, Guo Y, Iyer A, Ruiz Garcia Y, Galvita A, Heyerick A, Deforce D, Risseeuw MDP, Van Calenbergh S, Bracke M, Eremin S, Madder A and De Saeger S, 2014. An immunogen synthesis strategy for the development of specific anti‐deoxynivalenol monoclonal antibodies. Food Additives and Contaminants part A, 31, 1751–1759. [DOI] [PubMed] [Google Scholar]
- Santini A, Ferracane R, Somma MC, Aragon A and Ritieni A, 2009. Multitoxin extraction and detection of trichothecenes in cereals: an improved LC‐MS/MS approach. Journal of the Science of Food and Agriculture, 89, 1145–1153. [Google Scholar]
- Šarkanj B, Warth B, Uhlig S, Abia WA, Sulyok M, Klapec T, Krska R and Banjari I, 2013. Urinary analysis reveals high deoxynivalenol exposure in pregnant women from Croatia. Food and Chemical Toxicology, 62, 231–237. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food), 1999. Opinion on Fusarium toxins, Part 1: Deoxynivalenol (DON). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_catalogue_out44_en.pdf
- SCF (Scientific Committee on Food), 2002. Opinion of the Scientific Committee on Food on Fusarium toxins. Part 6: Group evaluation of T‐2 toxin, HT‐2 toxin, nivalenol and deoxynivalenol (adopted on 26 February 2002)SCF/CS/CNTM/MYC/27 Final 27 February 2002. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_catalogue_fusarium_out123_en.pdf
- Schaafsma AW, Limay‐Rios V, Paul DE and Miller JD, 2009. Mycotoxins in fuel ethanol co‐products derived from maize: a mass balance for deoxynivalenol. Journal of The Science of Food and Agriculture, 89, 1574–1580. [Google Scholar]
- Schatzmayr G, Zehner F, Tãubel M, Schatzmayr D, Klimitsch A, Loibner AP and Binder EM, 2006. Microbiologicals for deactivating mycotoxins. Molecular Nutrition and Food Research, 50, 543–551. [DOI] [PubMed] [Google Scholar]
- Schiefer HB, Nicholson S, Kasali OB, Hancock DS and Greenhalgh R, 1985. Pathology of acute 3‐acetyldeoxynivalenol toxicity in mice. The Canadian Journal of Comparative Medicine, 49, 315–318. [PMC free article] [PubMed] [Google Scholar]
- Schoevers EJ, Fink‐Gremmels J, Colenbrander B and Roelen BA, 2010. Porcine oocytes are most vulnerable to the mycotoxin deoxynivalenol during formation of the meiotic spindle. Theriogenology, 74, 968–978. [DOI] [PubMed] [Google Scholar]
- Schothorst RC and van Egmond HP, 2004. Report from SCOOP task 3.2.10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states” Subtask: trichothecenes. Toxicology Letters, 153, 133–143. [DOI] [PubMed] [Google Scholar]
- Schothorst RC, Jekel AA, van Egmond HP, de Mul A, Boon PE and van Klaveren JD, 2005. Determination of trichothecenes in duplicate diets of young children by capillary gas chromatography with mass spectrometric detection. Food Additives and Contaminants, 22, 48–55. [DOI] [PubMed] [Google Scholar]
- Schollenberger H, Müller M and Drochner W, 2005. Fusarium toxin contents of maize and maize products purchased in the years 2000 and 2001 in Germany. Mycotoxin Research, 21, 26–28. [DOI] [PubMed] [Google Scholar]
- Schulz AK, Kersten S, Dänicke S, Coenen M and Vervuert I, 2015. Effects of deoxynivalenol in naturally contaminated wheat on feed intake and health status of horses. Mycotoxin Research, 31, 209–216. [DOI] [PubMed] [Google Scholar]
- Schultz S, Vallant B, andKainz MJ, 2012. Preferential feeding on high quality diets decreases methyl mercury of farm‐raised common carp (Cyprinus carpio L.). Aquaculture, 338–341, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake‐Anduschus C, Langenkämper G, Unbehend G, Dietrich R, Märtlbauer E and Münzing K, 2010. Occurrence of fusarium T‐2 and HT‐2 toxins in oats from cultivar studies in Germany and degradation of the toxins during grain cleaning treatment and food processing. Food Additives and Contaminants Part A, 27, 1253–1260. [DOI] [PubMed] [Google Scholar]
- Schwake‐Anduschus C, Proske M, Sciurba E, Muenzing K, Koch M and Maul R, 2015. Distribution of deoxynivalenol, zearalenone, and their respective modified analogues in milling fractions of naturally contaminated wheat grains. World Mycotoxin Journal, 8, 433–443. [Google Scholar]
- Schwarz PB, Casper HH and Beattie S, 1995. Fate and development of naturally occurring Fusarium mycotoxins during malting and brewing. Journal of American Society of Brewing Chemistry, 53, 121–127. [Google Scholar]
- Schwartz‐Zimmermann HE, Paulick M, Dänicke S, Schatzmayr D and Berthiller F, 2014. Determination of deoxynivalenol sulphonates in cereal samples: method development, validation and application. World Mycotoxin Journal, 7, 233–245. [Google Scholar]
- Schwartz‐Zimmermann HE, Fruhman P, Dänicke S, Wiesenberger G, Caha S, Weber J and Berthiller F, 2015. Metabolism of deoxynivalenol and deepoxy‐deoxynivalenol in broiler chickens, pullets, roosters and turkeys. Toxins, 7, 4706–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOOP (Scientific Co‐Operation), 2003. Collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU Member States. Report of experts participati9ng in Task 3.2.10, April 2003. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_catalogue_fusarium_task3210.pdf
- Scott PM, 1995. Mycotoxins transmitted into beer from contaminated grains during brewing. Food Chemical Contaminants, 79, 875–882. [PubMed] [Google Scholar]
- Scudamore KA, 2008. Fate of Fusarium mycotoxins in the cereal industry, 2008: recent studies. World Mycotoxin Journal, 1, 315–325. [Google Scholar]
- Scudamore KA and Patel S, 2009. Fusarium mycotoxins in milling streams from the commercial milling of maize imported to the UK, and relevance to current legislation. Food Additives and Contaminants, 26, 744–753. [DOI] [PubMed] [Google Scholar]
- Scudamore KA, Hazel CM, Parel S and Scriven F, 2009. Deoxynivalenol and other Fusarium mycotoxins in bread, cake and biscuits produced from UK‐grown wheat under commercial and pilot scale conditions. Food Additives and Contaminants, 26, 1191–1198. [Google Scholar]
- Seeling K, Dänicke S, Valenta H, van Egmond H, Schothorst R, Jekel A, Lebzien B, Schollenbergen M, Razzazi‐Fazelli E and Flachowsky G, 2006. Effects of Fusarium toxin‐contaminated wheat and feed intake level on the biotransformation and carry‐over of deoxynivalenol in dairy cows. Food Additives and Contaminants, 23, 1008–1020. [DOI] [PubMed] [Google Scholar]
- Serrano AN, Font G, Ruiz MJ and Ferrer F, 2012. Co‐occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chemistry, 135, 432–429. [DOI] [PubMed] [Google Scholar]
- Severino L, Luongo D, Bergamo P, Lucisano A and Rossi M, 2006. Mycotoxins nivalenol and deoxynivalenol differentially modulate cytokine mRNA expression in Jurkat T cells. Cytokine 36, 75–82. [DOI] [PubMed] [Google Scholar]
- Setyabudi FM, Böhm J, Mayer HK and Razazzi FE, 2012. Analysis of deoxynivalenol and de‐epoxy‐ deoxynivalenol in horse blood through liquid chromatography after clean‐up with immunoaffinifty column. Journal of Veterinary and Animal Science, 2, 21–31. [Google Scholar]
- Sewald N, Von Gleissenthall JL, Schuster M, Müller G and Aplin RT, 1992. Structure elucidation of a plant metabolite of 4‐desoxynivalenol. Tetrahedron‐Asymmetry, 3, 953–960. [Google Scholar]
- Shephard GS, Burger HM, Gambacorta L, Gong YY, Krska R, Rheeder JP, Solfrizzo M, Srey C, Sulyok M, Visconti A, Warth B and van der Westhuizen L, 2013. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food and Chemical Toxicology, 62, 217–225. [DOI] [PubMed] [Google Scholar]
- Shifrin VI and Anderson P, 1999. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c‐Jun N‐terminal kinase and p38 mitogen‐activated protein kinase and induces apoptosis. The Journal of Biological Chemistry, 274, 13985–13992. [DOI] [PubMed] [Google Scholar]
- Simsek S, Burgess K, Whitney KL, Gu Y and Qian SY, 2012. Analysis of deoxynivalenol and deoxynivalenol‐3‐glucoside in wheat. Food Control, 26, 287–292. [Google Scholar]
- Singh S, Banerjee S, Chattopadhyay P, Borthakur SK and Veer V, 2015. Deoxynivalenol induces cytotoxicity and genotoxicity in animal primary cell culture. Toxicology Mechanisms and Methods, 25, 184–191. [DOI] [PubMed] [Google Scholar]
- Sirot V, Fremy J‐M and Leblanc J‐C, 2013. Dietary exposure to mycotoxins and health risk assessment in the second French total diet study. Food and Chemical Toxicology, 52, 1–11. [DOI] [PubMed] [Google Scholar]
- Skládanka J, Nedělník J, Adam V, Doležal P, Moravcová H and Dohna V, 2011. Forage as a primary source of mycotoxins in animal diets. International Journal of Environmental Research and Public Health, 8, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Diaz‐Llano G, Korosteleva SN and Yegani M, 2006. The effect of feed‐borne Fusarium mycotoxins on reproductive efficiency in dairy cows, sows and broiler breeders In: Lyons TP, Jacques KA, Hower JM. Nutritional biotechnology in the feed and food industries: Proceedings of Alltech's 22nd Annual Symposium, Lexington, Kentucky, USA, 23–26 April 2006. pp. 367–372 ref. 9. [Google Scholar]
- Sobrova P, Adam V, Vasatkova A, Beklova M, Zeman L and Kizek R, 2010. Deoxynivalenol and its toxicity. Interdisciplinary Toxicology, 3, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimany F, Jinap S and Abas F, 2012. Determination of mycotoxins in cereals by liquid chromatography tandem mass spectrometry. Food Chemistry, 130, 1055–1060. [Google Scholar]
- Soleimany F, Jinap S, Rahmani A and Khatib A, 2011. Simultaneous detection of 12 mycotoxins in cereals using RP‐HPLC‐PDA‐FLD with PHRED and a post‐column derivatization system. Food Additives and Contaminants Part A, 28, 494–501. [DOI] [PubMed] [Google Scholar]
- Solfrizzo M, Gambacorta L, Lattanzio VM, Powers S and Visconti A, 2011. Simultaneous LC‐MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de‐epoxydeoxynivalenol, α and β‐zearalenols and fumonisin B1 in urine as a multi‐biomarker method to assess exposure to mycotoxins. Analytical and Bioanalytical Chemistry, 401, 2831–2841. [DOI] [PubMed] [Google Scholar]
- Solfrizzo M, Gambacorta L, Warth B, White K, Srey C, Sulyok M, Krska R and Gong YY, 2013b. Comparison of single and multi‐analyte methods based on LC‐MS/MS for mycotoxin biomarker determination in human urine. World Mycotoxin Journal, 6, 355–366. [Google Scholar]
- Solfrizzo M, De Girolamo A, Lattanzio VMT, Visconti A, Stroka J, Alldrick A and Van Egmond HP, 2013a. Results of a proficiency test for multi‐mycotoxin determination in maize by using methods based on LC‐MS/MS. Quality Assurance and Safety of Crops and Foods, 5, 15–48. [Google Scholar]
- Solfrizzo M, Gambacorta L and Visconti A, 2014. Assessment of multi‐mycotoxin exposure in southern Italy by urinary multi‐biomarker determination. Toxins (Basel), 6, 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Na L, Zhiyong Z, Njumbe Ediage E, Songling W, Changpo S, De Saeger S and Wu A, 2014. Multiplex lateral flow immunoassay for mycotoxin determination. Analytical Chemistry, 86, 4995–5001. [DOI] [PubMed] [Google Scholar]
- Sprando RL, Pestka J, Collins TFX, Ririe J, O'donnell M, Hinton D and Chirtel S, 1999. The effects of vomitoxin (deoxynivalenol) on testicular morphology, testicular spermatid counts and epididymal sperm counts in IL‐KO [B6129‐IL6 TmlKopf (IL‐6 gene deficient)] and WT [B6129F2 (wild type to B9129‐IL6 with an intact IL‐6 gene)] mice. Food and Chemical Toxicology, 37, 1073–1079. [DOI] [PubMed] [Google Scholar]
- Sprando RL, Collins TFX, Black TM, Olejnik N, Rorie JI, Eppley RM and Ruggleset DI, 2005. Characterization of the effect of deoxynivalenol on selected male reproductive endpoints. Food and Chemical Toxicology, 43, 623–635. [DOI] [PubMed] [Google Scholar]
- Stanek C, Reinhardt N, Diesing AK, Nossol C, Kahlert S, Panther P, Kluess J, Rothkotter HJ, Kuester D, Brosig B, Kersten S and Danicke S, 2012. A chronic oral exposure of pigs with deoxynivalenol partially prevents the acute effects of lipopolysaccharides on hepatic histopathology and blood clinical chemistry. Toxicology letters, 215, 193–200. [DOI] [PubMed] [Google Scholar]
- Storm IMLD, Rasmussen RR and Rasmussen PH, 2014. Occurrence of pre‐ and post‐harvest mycotoxins an other secondary metabolites in Danish maize silage. Toxins, 6, 2256–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Carra M, Ghareeb K, Awad W and Böhm J, 2013. Protective effects of antioxidants on deoxynivalenol‐induced damage in murine lymphoma cells. Mycotoxin Research, 29, 203–208. [DOI] [PubMed] [Google Scholar]
- Straumfors A, Uhlig S, Eriksen GS, Heldal KK, Eduard W, Krska R and Sluyok M, 2015. Mycotoxins and other fungal metabolites in grain dust from Norwegian grain elevators and coumpound feed mills. World Mycotoxin Journal, 8, 361–373. [Google Scholar]
- Streit E, Schatzmayr G, Tassis P, Tzika E, Marin D, Taranu I, Tabuc C, Nicolau A, Aprodu I, Puel O and Oswald IP, 2012. Current situation of mycotoxin contamination and co‐occurrence in animal feed ‐ focus on Europe. Toxins, 4, 788–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit E, Schwab C, Sulyok M, Naehrer K, Krska R and Schatzmayr C, 2013. Multi‐mycotoxin screening reveals the occurrence of 139 different secondary metabolites in feed and feed ingredients. Toxins, 5, 504–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroka J, Doncheva I, Breidbach A and Mischke C, 2009. Report on the 2008 Proficiency Test of the Community Reference Laboratory for Mycotoxins, for the Network of National Reference Laboratories, regarding the Determination of Deoxynivalenol in a Cereal Product and a Test Solution. Available online: http://publications.jrc.ec.europa.eu/repository/handle/JRC51226
- Sugita‐Konishi Y and Pestka JJ, 2001. Differential upregulation of TNF‐alpha, IL‐6, and IL‐8 production by deoxynivalenol (vomitoxin) and other 8‐ketotrichothecenes in a human macrophage model. Journal of Toxicology Environmental Health A 64, 619–636. [DOI] [PubMed] [Google Scholar]
- Sulyok M, Berthiller F, Krska R and Schuhmacher R, 2006. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Communications in Mass Spectrometry, 20, 2649–2659. [DOI] [PubMed] [Google Scholar]
- Sulyok M, Krska R and Schuhmacher R, 2010. Application of an LC‐MS/MS based multi‐mycotoxin method for the semi‐quantitative determination of mycotoxins occurring in different types of food infected by moulds. Food Chemistry, 119, 408–416. [Google Scholar]
- Suman M, Manzitti A and Catellani D, 2012. A design of experiments approach to studying deoxynivalenol and deoxynivalenol‐3‐glucoside evolution throughout industrial production of wholegrain crackers exploiting LC‐MS/MS techniques. World Mycotoxin Journal, 5, 241–249. [Google Scholar]
- Sun L‐H, Lei M‐Y, Zhang N‐Y, Zhao L, Krumm CS and Qi D‐S, 2014. Hepatotoxic effects of mycotoxin combinations in mice. Food and Chemical Toxicology, 74, 289–293. [DOI] [PubMed] [Google Scholar]
- Swamy HVLN, Smith TK, MacDonald EJ, Boermans HJ and Squires EJ, 2002. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on swine performance, brain regional neurochemistry, and serum chemistry and the efficacy of a polymeric glucomannan mycotoxin adsorbent. Journal of Animal Science, 80, 3257–3267. [DOI] [PubMed] [Google Scholar]
- Swanson SP, Helaszek C, Buck WB, Rood HD and Haschek WM, 1988. The role of intestinal microflora in the metabolism of trichothecene mycotoxins. Food and Chemical Toxicology, 26, 823–829. [DOI] [PubMed] [Google Scholar]
- Swanson S, Nicoletti J, Rood H, Buck W, Côté L and Yoshizawa T, 1987a. Metabolism of three trichothecene mycotoxins, T‐2 toxin, diacetoxyscirpenol and deoxynivalenol, by bovine rumen microorganisms. Journal of Chromatography, 414, 324–330. [DOI] [PubMed] [Google Scholar]
- Sypecka Z, Kelly M and Brereton P, 2004. Deoxynivalenol and zearalenone residues in eggs of laying hens fed with a naturally contaminated diet: effects on egg production and estimation of transmission rates from feed to eggs. Journal of Agricultural and Food Chemistry, 52, 5463–5471. [DOI] [PubMed] [Google Scholar]
- Takakura N, Nesslany F, Fessard V and Le Hegarat L, 2014. Absence of in vitro genotoxicity potential of the mycotoxin deoxynivalenol in bacteria and in human TK6 and HepaRG cell lines. Food Chemical Toxicology, 66, 113–121. [DOI] [PubMed] [Google Scholar]
- Tangni EK, Motte J‐C, Callebaut A and Pussemier L, 2010. Cross‐reactivity of antibodies in some commercial deoxynivalenol test kits against some fusariotoxins. Journal of Agricultural and Food Chemistry, 58, 12625–12633. [DOI] [PubMed] [Google Scholar]
- Tardivel C, Airault C, Djelloul M, Guillebaud F, Barbouche R, Troadec JD, Gaigé S and Dallaporta M, 2015. The food born mycotoxin deoxynivalenol induces low‐grade inflammation in mice in the absence of observed‐adverse effects. Toxicology Letters, 232, 601–611. [DOI] [PubMed] [Google Scholar]
- Tittlemier SA, Sobering D, Bowler K, Zirdum T, Gaba D, Chan JM, Roscoe M, Blagden R and Campbell L, 2015. By‐products of grain cleaning: an opportunity for rapid sampling and screening of wheat for mycotoxins. World Mycotoxin Journal, 8, 45–53. [Google Scholar]
- Thieu NQ and Pettersson H, 2009. Zearalenone, deoxynivalenol and aflatoxin B1 and their metabolites in pig ruien as biomarkers for mycotoxin exposure. Mycotoxin Research, 25, 59–66. [DOI] [PubMed] [Google Scholar]
- Thomas M, van Zuilichem DJ and van der Poel AFB, 1997. Physical quality of pelleted animal feed. 2. Contribution of processes and its conditions. Animal Feed Science and Technology, 64, 173–192. [Google Scholar]
- Thompson WL and Wannemacher RW Jr, 1986. Structure‐function relationships of 12,13‐epoxytrichothecene mycotoxins in cell culture: comparison to whole animal lethality. Toxicon, 24, 985–994. [DOI] [PubMed] [Google Scholar]
- Thuvander A, Wikman C and Gadhasson I, 1999. In vitro exposure of human lymphocytes to trichothecenes: individual variation in sensitivity and effects of combined exposure on lymphocyte function. Food and Chemical Toxicology, 37, 639–648. [DOI] [PubMed] [Google Scholar]
- Tibola CS, Fernandes JMC and Guariente EM, 2015. Distribution of Fusarium mycotoxins in wheat milling process. Food Control, 53, 91–95. [Google Scholar]
- Tomar RS, Blakley BR, Schiefer HB and DeCoteau WE, 1986. In vitro effects of 3‐acetyl‐deoxynivalenol on the immune response of human peripheral blood lymphocytes. Internationl Journal of Immunopharmacology, 8, 125–130. [DOI] [PubMed] [Google Scholar]
- Tomar RS, Blakley BR and DeCoteau WE, 1987. Immunological responsiveness of mouse spleen cells after in vivo or in vitro exposure to 3‐acetyldeoxynivalenol. Food Chemistry and Toxicology, 25, 393–398. [DOI] [PubMed] [Google Scholar]
- Tran ST and Smith TK, 2011. Determination of optimal conditions for hydrolysis of conjugated deoxynivalenol in corn and wheat with trifluoromethanesulfonic acid. Animal Feed Science and Technology, 163, 84–92. [Google Scholar]
- Tran S‐T, Smith TK and Girgis GN, 2012. A survey of free and conjugated deoxynivalenol in the 2008 corn crop in Ontario, Canada. Journal of the Science of Food and Agriculture, 92, 37–41. [DOI] [PubMed] [Google Scholar]
- Trenholm HL, Cochrane WP, Cohen H, Elliot JI, Farnworth ER, Friend DW, Hamilton RM, Standish JF and Thompson BK, 1983. Survey of vomitoxin contamination of 1980 Ontario white winter wheat crop: results of survey and feeding trials. Journal of the Association of Official Analytical Chemists, 66, 92–97. [PubMed] [Google Scholar]
- Trenholm HL, Thomson BK, Hartin KE, Greenhalgh R and Mc Allister AJ, 1985. Ingestion of vomitoxin (deoxynivalenol)‐contaminated wheat by non lactating cows. Journal of Dairy Science, 68, 1000–1005. [DOI] [PubMed] [Google Scholar]
- Tryphonas H, Iverson F, So Y, Nera EA, McGuire PF, O'Grady L, Clayson DB and Scott PM, 1986. Effects of deoxynivalenol (vomitoxin) on the humoral and cellular immunity of mice. Toxicology Letters, 30, 137–150. [DOI] [PubMed] [Google Scholar]
- Turner PC, Rothwell JA, White KL, Gong YY, Cade JE and Wild CP, 2008a. Urinary deoxynivalenol is correlated with cereal intake in individuals from the United kingdom. Environmental Health Perspectives, 116, 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PC, Burley VJ, Rothwell JA, White KL, Cade JE and Wild CP, 2008b. Dietary wheat reduction decreases the level of urinary deoxynivalenol in UK adults. Journal of Exposure Science and Environmental Epidemiology, 18, 392–399. [DOI] [PubMed] [Google Scholar]
- Turner PC, Taylor EF, White KL, Cade JE and Wild CP, 2009. A comparison of 24 h urinary deoxynivalenol with recent v. average cereal consumption for UK adults. British Journal of Nutrition, 102, 1276–1279. [DOI] [PubMed] [Google Scholar]
- Turner PC, White KL, Burley VJ, Hopton RP, Rajendram A, Fisher J, Cade JE and Wild CP, 2010a. A comparison of deoxynivalenol intake and urinary deoxynivalenol in UK adults. Biomarkers, 15, 553–562. [DOI] [PubMed] [Google Scholar]
- Turner PC, Hopton RP, Lecluse Y, White KL, Fisher J and Lebailly P, 2010b. Determinants of urinary deoxynivalenol and de‐epoxy deoxynivalenol in male farmers from Normandy, France. Journal of Agricultural and Food Chemistry, 58, 5206–5212. [DOI] [PubMed] [Google Scholar]
- Turner PC, Hopton RP, White KL, Fisher J, Cade JE and Wild CP, 2011a. Assessment of deoxynivalenol metabolite profiles in UK adults. Food and Chemical Toxicology, 49, 132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PC, Gong YY, Pourshams A, Jafari E, Routledge MN, Malekzadeh R, Wild CP, Boffetta P and Islami F, 2012. A pilot survey for Fusarium mycotoxin biomarkers in women from Golestan, northern Iran. World Mycotoxin Journal, 5, 195–199. [Google Scholar]
- Tutel'ian VA, Krinitskaia NA, Avreneva LI, Kuzmina EE, Levitskaia AB and Kravchenko LV, 1991. [Embryotoxic effects of deoxynivalenol mycotoxin (vomitoxin) in rats]. Gigiena i Sanitariia, 10, 49–51 (in Russian). [PubMed] [Google Scholar]
- Twazur≐k M, Błajet‐Kosicka A, Wenda‐Piesik A and Pałubicki J, 2013. Statistical comparison of Fusarium mycotoxins content in oat grain and related products from two agricultural systems. Food Control, 34, 291–295. [Google Scholar]
- Uhlig S, Ivanova L and Fæste CK, 2013. Enzyme‐assisted synthesis and structural characterization of the 3‐, 8‐, and 15‐glucuronides of deoxynivalenol. Journal of Agriculture and Food Chemistry, 61, 2006–2012. [DOI] [PubMed] [Google Scholar]
- Urošević MI, Jajic IM and Milicic ZG, 2011. Mycotoxins in Horse Feed: incidence of deoxynivalenol in oats samples from stud farms. Proc Nat. Sci, Matica Srpska Novi Sad, No, 120, 33–40. [Google Scholar]
- Usleber E, Märtlbauer E, Dietrich R and Terplan G, 1991. Direct enzyme‐linked immunosorbent assays for the detection of the 8‐ketotrichothecene mycotoxins deoxynivalenol, 3‐acetyldeoxynivalenol, and 15‐acetyldeoxynivalenol in buffer solutions. Journal of Agricultural and Food Chemistry, 39, 2091–2095. [Google Scholar]
- Usleber E, Abramson D, Gessler R, Smith DM, Clear RM and Maertlbauer E, 1996. Natural contamination of Manitoba barley by 3,15‐diacetyldeoxynivalenol and its detection by immunochromatography. Applied and Environmental Microbiology, 62, 3858–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta H and Dänicke S, 2005. Study on the transmission of deoxynivalenol and de‐epoxy‐deoxynivalenol into eggs of laying hens using a high‐performance liquid chromatography‐ultraviolet method with clean‐up by immunoaffinity columns. Molecular Nutrition and Food Research, 49, 779–785. [DOI] [PubMed] [Google Scholar]
- Vaclavik L, Zachariasova M, Hrbek V and Hajslova J, 2010. Analysis of multiple mycotoxins in cereals under ambient conditions using direct analysis in real time (DART) ionization coupled to high resolution mass spectrometry. Talanta, 82, 1950–1957. [DOI] [PubMed] [Google Scholar]
- Valle‐Algarra F, Medina A, Gimeno‐Adelantadoa JV, Llorens A, Jimenez M and Mateo R, 2005. Comparative assessment of solid‐phase extraction clean‐up procedures, GC columns and perfluoroacylation reagents for determination of type B trichothecenes in wheat by GC–ECD. Talanta, 66, 194–201. [DOI] [PubMed] [Google Scholar]
- Vanheule A, Audenaert K, De Boevre M, Landschoot S, Bekaert B, Munaut F, Eeckhout M, Höfte M, De Saeger S and Haesaert G, 2014. The compositional mosaic of Fusarium species and their mycotoxins in unprocessed cereals, food and feed products in Belgium. International Journal of Food Microbiology, 181, 28–36. [DOI] [PubMed] [Google Scholar]
- Van De Walle J, Sergent T, Piront N, Toussaint O, Schneider Y‐J and Larondelle Y, 2010. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicology and Applied Pharmacology, 245, 291–298. [DOI] [PubMed] [Google Scholar]
- Varga E, Malachova A, Schwartz H, Krska R and Berthiller F, 2013. Survey of deoxynivalenol and its conjugates deoxynivalenol‐3‐glucoside and 3‐acetyl‐deoxynivalenol in 374 beer samples. Food Additives and Contaminants, 30, 137–146. [DOI] [PubMed] [Google Scholar]
- Vendl O, Crews C, MacDonald S, Krska R and Berthiller F, 2010. Occurrence of free and conjugated Fusarium mycotoxins in cereal‐based food. Food Additives and Contaminants, 27, 1148–1152. [DOI] [PubMed] [Google Scholar]
- Versilovskis A, Geys J, Huybrechts B, Goosens E, De Saeger S and Callebaut A, 2012. Simultanous determination of masked forms of deoxynivalenol and zearalenone after oral dosing in rats by LC‐MS/MS. World Mycotoxin Journal, 5, 303–318. [Google Scholar]
- Vesonder RF, Ciegler A and Jensen AH, 1973. Isolation of the emetic principle from fusarium‐infected corn. Applied Microbiology, 26, 1008–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal A, Marín S, ramos AJ, Cano‐Sancho G and Sanchis V, 2013. Determination of aflatoxins, deoxynivalenol, ochratoxins A and zearalenone in wheat and oat based bran supplements sold in the Spanish market. Food and Chemical Toxicology, 53, 133–138. [DOI] [PubMed] [Google Scholar]
- Vidal A, Morales H, Sanchis V, Ramos AJ and Marín S, 2014. Stability of DON and OTA during the breadmaking process and determination of process and performance criteria. Food Control, 40, 234–242. [Google Scholar]
- Vidal A, Sanchis V, Ramos AJ and Marín S, 2015. Thermal stability and kinetics of degradation of deoxynivalenol conjugates and ochratoxin A during baking of wheat bakery products. Food Chemistry, 178, 276–286. [DOI] [PubMed] [Google Scholar]
- Visconti A, Haidukowski EM, Pascale M and Silvestri M, 2004. Reduction of deoxynivalenol during durum wheat processing and spaghetti cooking. Toxicology Letters, 153, 181–189. [DOI] [PubMed] [Google Scholar]
- Völkel I, Schröer‐Merker E and Czerny CP, 2011. The carry‐over of mycotoxins in products of animal origin with special regard to its implications for the European food safety legislation. Food and Nutrition Sciences, 2, 852–867. [Google Scholar]
- Waché YJ, Hbabi‐Haddioui L, Guzylack‐Piriou L, Belkhelfa H, Roques C and Oswald IP, 2009a. The mycotoxin deoxynivalenol inhibits the cell surface expression of activation markers in human macrophages. Toxicology, 262, 239–244. [DOI] [PubMed] [Google Scholar]
- Wagner J, 2002. Scab‐Infected Wheat or Barley for Feedlot Cattle or Sheep. College of Agriculture and Biological Sciences, South Dakota State University, USDA, Extension Extra 2017 Sept 1997, updated 2002.
- Wallin S, Gambacorta L, Kotova N, Lemming EW, Nälsén C, Solfrizzo M and Olsen M, 2015. Biomonitoring of concurrent mycotoxin exposure among adults in Sweden through urinary multi‐biomarker analysis. Food and Chemical Toxicology, 83, 133–139. [DOI] [PubMed] [Google Scholar]
- Wallin S, Hardie LJ, Kotova N, Lemming EW, Nalsen C, Ridefelt P, Turner PC, White KLM and Olsen M, 2013. Biomonitoring study of deoxynivalenol exposure and association with typical cereal consumption in Swedish adults. World Mycotoxin Journal, 6, 439–448. [Google Scholar]
- Wan LY, Woo CS, Turner PC, Wan JM and El‐Nezami H, 2013. Individual and combined effects of Fusarium toxins on the mRNA expression of pro‐inflammatory cytokines in swine jejunal epithelial cells. Toxicology Letters, 220–238‐246. [DOI] [PubMed] [Google Scholar]
- Wan M LY, Poon VWL, Allen KJ, Turner PC and El‐Nezamy H, 2014. Lactobacillus rhamnosus GG modulates intestinal barrier function and inflammation in BALB/C mice following dietary exposure to deoxynivalenol and zearalenone through changes in gut. In International Conference on Food Safety (ICFS). The 1st International Conference on Food Safety (ICFS), The University of Hong Kong, Hong Kong, 16‐18 June 2014. In Programme Book, 2014, p. 75, abstract OS2.
- Ware GM, Francis OJ, Carman AS and Kuan SS, 1986. Gas chromatographic determination of deoxynivalenol in wheat with electron capture detection: collaborative study. Journal of Association of Official Analytical Chemists, 69, 899–901. [PubMed] [Google Scholar]
- Warth B, Sulyok M, Berthiller F, Schuhmacher R, Fruhmann P, Hametner C, Adam G, Fröhlich J and Krska R, 2011. Direct quantification of deoxynivalenol glucuronide in human urine as biomarker of exposure to the Fusarium mycotoxin deoxynivalenol. Analytical and Bioanalytical Chemistry, 401, 195–200. [DOI] [PubMed] [Google Scholar]
- Warth B, Sulyok M, Fruhmann P, Mikula H, Berthiller F, Schuhmacher R, Hametner C, Abia WA, Adam G, Fröhlich J and Krska R, 2012a. Development and validation of a rapid multi‐biomarker liquid chromatography/tandem mass spectrometry method to assess human exposure to mycotoxins. Rapid Communications in Mass Spectrometry, 26, 1533–1540. [DOI] [PubMed] [Google Scholar]
- Warth B, Sulyok M, Fruhmann P, Berthiller F, Schuhmacher R, Hametner C, Adam G, Fröhlich J and Krska R, 2012b. Assessment of human deoxynivalenol exposure using an LC‐MS/MS based biomarker method. Toxicology Letters, 211, 85–90. [DOI] [PubMed] [Google Scholar]
- Warth B, Sulyok M, Berthiller F, Schuhmacher R and Krska R, 2013. New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicology Letters, 220, 88–94. [DOI] [PubMed] [Google Scholar]
- Warth B, Fruhmann P, Wiesenberger G, Kluger B, Sarkanj B, Lemmens M, Hametner C, Fröhlich J, Adam G, Krska R and Schuhmacher R, 2015. Deoxynivalenol‐sulfates: identification and quantification of novel conjugated (masked) mycotoxins in wheat. Analytical and Bioanalytical Chemistry, 407, 1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth B, Siegwart G, Lemmens M, Krska R, Adam G and Schuhmacher R, 2016. Hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry for the quantification of uridine diphosphateglucose, uridine diphosphate‐glucuronic acid, deoxynivalenol and its glucoside: In‐house validation and application to wheat. Journal of Chromatography A, 1423, 183–189. [DOI] [PubMed] [Google Scholar]
- Wehner FC, Marasas WFO and Thiel PG, 1978. Lack of mutagenicity to Salmonella typhimurium of some Fusarium toxins. Applied and Environmental Microbiology, 35, 659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenbörner M, 2001. Encyclopedia of Food Mycotoxins. Springer, Berlin Heidelberg: 294 p. ISBN 978‐3‐662‐04464‐3 (online), 978‐3‐642‐08703‐5 (print). [Google Scholar]
- Whitlow L, Hagler W Jr and Diaz D, 2010. Mycotoxins in fields. Feedstuffs, September 15, 2010, 74–85. [Google Scholar]
- WHO (World Health Organization), 1998. GEMS/Food Regional Diets. Regional per capita consumption of raw and semi‐processed agricultural commodities. Available online: http://apps.who.int/iris/bitstream/10665/63989/1/WHO_FSF_FOS_98.3.pdf
- WHO (World Health Organization), 2001. Evaluation of certain mycotoxins in food. Fifty‐sixth report of the Join FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 906, Geneva. [PubMed]
- WHO (World Health Organization), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. Environment Health Criteria 240. ISBN: 978 92 4 157240 8. Available online: http://www.who.int/foodsafety/publications/chemical-food/en/
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2008. Guidance document on characterizing and communicating uncertainty in exposure assessment. Harmonisation project document No. 6. ISBN 978 92 4 156376 5.U.
- Winkler J, Kersten S, Meyer U, Engelhardt U and Dänicke S, 2014. Residues of zearalenone, deoxynivalenol and their metabolites in plasma of dairy cows fed Fusarium contaminated maize and their relationships to performance parameters. Food and Chemical Toxicology, 65, 196–204. [DOI] [PubMed] [Google Scholar]
- Winkler J, Kersten S, Meyer U, Stinshoff H, Locher L, Rehage J, Wrenzycki C, Engelhardt UH and Danicke S, 2015a. Diagnostic opportunities for evaluation of the exposure of dairy cows to the mycotoxins deoxynivalenol (DON) and zearalenone (ZEN): reliability of blood plasma, bile and follicular fluid as indicators. Journal of Animal Physiology and Animal Nutrition, 99, 847–850. [DOI] [PubMed] [Google Scholar]
- Winkler J, Kersten S, Valenta H, Huther L, Meyer U, Engelhardt U and Danicke S, 2015b. Simultaneous determination of zearalenone, deoxynivalenol and their metabolites in bovine urine as biomarker of exposure. World Mycotoxin Journal, 8, 63–74. [Google Scholar]
- Winkler J, Kersten S, Valenta H, Meyer U, Engelhardt U and Dänicke S, 2015c. Development of a multi‐toxin method for investigating the carryover of zearalenone, deoxynivalenol and their metabolites into milk of dairy cows. Food additives and Contaminants: Part A, 32, 371–380. [DOI] [PubMed] [Google Scholar]
- Williams KC, Blaney BJ and Magee MH, 1988. Responses of pigs fed wheat naturally infected with Fusarium graminearum and containing the mycotoxins 4‐deoxynivalenol and zearalenone. Australian Journal of Agricultural Research, 39, 1095–1105. [Google Scholar]
- Wolf CE and Bullerman LB, 1998. Heat and pH alter the concentration of deoxynivalenol in an aqueous environment. Journal of Food Protection, 61, 365–367. [DOI] [PubMed] [Google Scholar]
- Wolf‐Hall CE, Hanna MA and Bullerman LB, 1999. Stability of deoxynivalenol in heat‐treated foods. Journal of Food Protection, 8, 962–964. [DOI] [PubMed] [Google Scholar]
- Worrell NR, Mallett AK, Cook WM, Baldwin NCP and Shepherd MJ, 1989. The role of gut micro‐organisms in the metabolism of deoxynivalenol administered to rats. Xenobiotica, 19, 25–32. [DOI] [PubMed] [Google Scholar]
- Wu X, Kohut M, Cunnick J, Bailey T and Hendrich S, 2009. Deoxynivalenol suppresses circulating and splenic leukocyte subpopulations in BALB/c mice: dose response, time course and sex differences. Food Additives and Contaminants, 26, 1070–1080. [DOI] [PubMed] [Google Scholar]
- Wu F, Groopman JD and Pestka JJ, 2014c. Public health impacts of foodborne mycotoxins. Annual Review of Food Science and Technology, 5, 351–372. [DOI] [PubMed] [Google Scholar]
- Wu W, Flannery BM, Sugita‐Konishi Y, Watanabe M, Zhang H and Pestka JJ, 2012. Comparison of murine anorectic responses to the 8‐ketotrichothecenes 3‐acetyldeoxynivalenol, 15‐acetyldeoxynivalenol, fusarenon X and nivalenol. Food and Chemical Toxicology, 50, 2056–2061. [DOI] [PubMed] [Google Scholar]
- Wu W, Bates MA, Bursian SJ, Link JE, Flannery BM, Sugita‐Konishi Y, Watanabe M, Zhnag H and Pestka JJ, 2013b. Comparison of emetic potencies of the 8‐ketotrichothecenes deoxynivalenol, 15‐acetyldeoxynivalenol, 3‐acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicological Sciences, 131, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Bates MA, Bursian SJ, Flannery B, Zhou HR, Link JE, Zhang H and Pestka JJ, 2013a. b Peptide YY3‐36 and 5‐hydroxytryptamine mediate emesis induction by trichothecene deoxynivalenol (vomitoxin). Toxicological Sciences, 133, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W and Zhang H, 2014. Role of tumor necrosis factor‐α and interleukin‐1β in anorexia induction following oral exposure to the trichothecene deoxynivalenol (vomitoxin) in the mouse. The Journal of Toxicological Sciences, 9, 875–886. [DOI] [PubMed] [Google Scholar]
- Wu W, Zhou H‐R, He K, Pan X, Sugita‐Konishi Y, Watanabe M, Zhang H and Pestka JJ, 2014b. Role of cholecystokinin in anorexia induction following oral exposure to the 8‐ketotrichothecenes deoxynivalenol, 15‐acetyldeoxynivalenol, 3‐acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicological Sciences, 138, 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, He K, Zhou H‐R, Berthiller F, Adam G, Sugita‐Konishi Y, Watanabe M, Krantis A, Durst T, Zhang H and Pestka JJ, 2014a. Effects of oral exposure to naturally‐occurring and synthetic deoxynivalenol congeners on proinflammatory cytokine and chemokine mRNA expression in the mouse. Toxicology and Applied Pharmacology, 278, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Zhou H‐R, Bursian SJ, Pan X, Link JE, Berthiller F, Adam G, Krantis A, Durst T and Pestka JJ, 2014d. Comparison of anorectic and emetic potencies of deoxynivalenol (vomitoxin) to the plant metabolite deoxynivalenol‐3‐glucoside and synthetic deoxynivalenol derivatives EN139528 and EN139544. Toxicological Sciences, 142, 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L and Wang B, 2015. Evaluation on levels and conversion profiles of DON, 3‐ADON, and 15‐ADON during bread‐making process. Food Chemistry, 185, 509–516. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Matsunaga S, Matsui M, Takeda N and Yamatodani A, 2002. Pica in mice as a new model for the study of emesis. Methods and Findings in Experimental and Clinical Pharmacology, 24, 135–138. [DOI] [PubMed] [Google Scholar]
- Yang W, Yu M, Fu J, Bao W, Wang D, Hao L, Yao P, Nüssler AK, Yan H and Liu L, 2014. Deoxynivalenol induced oxidative stress and genotoxicity in human peripheral blood lymphocytes. Food and Chemical Toxicology, 64, 383–396. [DOI] [PubMed] [Google Scholar]
- Yang D, Geng ZM, Yao JB, Zhang X, Zhang PP and Ma HX, 2013. Simultaneous determination of deoxynivalenol, and 15‐ and 3‐acetyldeoxynivalenol in cereals by HPLC‐UV detection. World Mycotoxin Journal, 6, 117–125. [Google Scholar]
- Yang H, Chung DH, Kim YB, Choi YH and Moon Y, 2008. Ribotoxic mycotoxin deoxynivalenol induces G2/M cell cycle arrest via p21Cip/WAF1 mRNA stabilization in human epithelial cells. Toxicology, 243, 145–154. [DOI] [PubMed] [Google Scholar]
- Yiannikouris A and Jouany J‐P, 2002. Mycotoxins in feeds and their fate in animals: a review. Animal Research, 51, 81–99. [Google Scholar]
- Yibadatihan S, Jinap S and Mahyudin NA, 2014. Simultaneous determination of multi‐mycotoxins in palm kernel cake (PKC) using liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). Food Additives and Contaminants, Part A, 31, 2071–2079. [DOI] [PubMed] [Google Scholar]
- Yoshinari T, Tanaka T, Ishikuro E, Horie M, Nagayama T, Nakajima M, Naito S, Ohnishi T and Sugita‐Konishi Y, 2013. Inter‐laboratory study of an LC‐MS/MS method for simultaneous determination of deoxynivalenol and its acetylated derivatives, 3‐acetyl‐deoxynivalenol and 15‐acetyl‐deoxynivalenol in wheat. Journal of the Food Hygienic Society of Japan, 54, 75–82. [DOI] [PubMed] [Google Scholar]
- Yoshinari T, Ohnishi T, Kadota T and Sugita‐Konishi Y, 2012. Development of a purification method for simultaneous determination of deoxynivalenol and its acetylated and glycosylated derivatives in corn grits and corn flour by liquid chromatography‐tandem mass spectrometry. Journal of Food Protection, 75, 1355–1358. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T and Morooka N, 1974. Studies on the toxic substances in infected cereals; acute toxicities of new trichothecene mycotoxins: deoxynivalenol and its monoacetate. Journal of the Food Hygiene Society of Japan, 15, 261–269. [Google Scholar]
- Yoshizawa T, Takada H and Ohi T, 1983. Structure of a novel metabolite from deoxynivalenol, a trichothecene mycotoxin, in animals. Agricultural and Biological Chemistry, 47, 2133–2135. [Google Scholar]
- Yoshizawa T, Cote LM, Swanson SP and Buck WB, 1986. Confirmation of DOM‐1, a deepoxidation metabolite of deoxynivalenol, in biological fluids of lactating cows. Agricultural and Biological Chemistry, 50, 227–229. [Google Scholar]
- Young LG, McGirr L, Valli VE, Lumdsen JH and Lun A, 1983. Vomitoxin in corn fed to young pigs. Journal of Animal Science, 57, 655–664. [DOI] [PubMed] [Google Scholar]
- Yue Y‐T, Zhang X‐F, Pan J, Ou‐Yang Z, Wu J and Yang M‐H, 2010. Determination of deoxynivalenol in medicinal herbs and related products by GC–ECD and confirmation by GC–MS. Chromatographia, 71, 533–538. [Google Scholar]
- Yunus AW, Valenta H, Abdel‐Raheem SM, Döll S, Dänicke S and Böhm J, 2010. Blood plasma levels of deoxynivalenol and its de‐epoxy metabolite in broilers after a single oral dose of the toxin. Mycotoxin Research, 26, 217–220. [DOI] [PubMed] [Google Scholar]
- Yunus AW, Ghareeb K, Twaruzek M, Grajewski J and Böhm J, 2012a. Deoxynivalenol as a contaminant of broiler feed: Effects on bird performance and response to common vaccines. Poultry Science, 91, 844–851. [DOI] [PubMed] [Google Scholar]
- Yunus AW, Błajet‐Kosicka A, Kosicki R, Khan MZ, Rehman H and Böhm J, 2012b. Deoxynivalenol as a contaminant of broiler feed: intestinal development, absorptive functionality, and metabolism of the mycotoxin. Poultry Science, 91, 852–861. [DOI] [PubMed] [Google Scholar]
- Zachariasova M, Lacina O, Malachova A, Kostelanska M, Poustka J, Godula M and Hajslova J, 2010. Novel approaches in analysis of Fusarium mycotoxins in cereals employing ultra performance liquid chromatography coupled with high resolution mass spectrometry. Analytica Chimica Acta, 662, 51–61. [DOI] [PubMed] [Google Scholar]
- Zachariasova M, Cuhra P and Hajslova J, 2014a. Cross‐reactivity of rapid immunochemical methods for mycotoxins detection towards metabolites and masked mycotoxins: the current state of knowledge. World Mycotoxin Journal, 7, 449–464. [Google Scholar]
- Zachariasova M, Dzuman Z, Veprikova Z, Hajkova K, Jiru M, Vaclavikoa M, Zachariasova A, Pospichalova M, Floria N and Hajslova A, 2014b. Occurrence of multiple mycotoxins in European feedingstuffs, assessment of dietary intake by farm animals. Animal Feed Science and Technology, 193, 124–140. [Google Scholar]
- Zachariasova M, Vaclavikova M, Lacina O, Vaclavik L and Hajslova J, 2012. Deoxynivalenol oligoglycosides: new “masked” fusarium toxins occurring in malt, beer, and breadstuff. Journal of Agricultural and Food Chemistry, 60, 9280–9291. [DOI] [PubMed] [Google Scholar]
- Zhang Y and Caupert J, 2012. Survey of mycotoxins in U.S. distiller's dried grains with solubles from 2009 to 2011. Journal of Agricultural and Food Chemistry, 60, 539–543. [DOI] [PubMed] [Google Scholar]
- Zhang H and Wang B, 2014. Fate of deoxynivalenol and deoxynivalenol‐3‐glucoside during wheat milling and Chinese steamed bread processing. Food Control, 44, 86–91. [Google Scholar]
- Zhang H and Wang B, 2015. Fate of deoxynivalenol and deoxynivalenol‐3‐glucoside during bread and noodle processing. Food Control, 50, 754–757. [Google Scholar]
- Zhang X, Jiang L, Geng C, Cao J and Zhong L, 2009. The role of oxidative stress in deoxynivalenol‐induced DNA damage in HepG2 cells. Toxicon, 54, 513–518. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Hossen SM, Sago Y, Yoshida M, Nakagawa H, Nagashima H, Okadome H, Nakajima T and Kushiro M, 2014. Effect of milling on the content of deoxynivalenol, nivalenol, and zearalenone in Japanese wheat. Food Control, 40, 193–197. [Google Scholar]
- Zhilei W, Xiulan S, Zaijun L, Yinjun F, Guoxiao R, Yaru H and Junkang L, 2011. Highly sensitive deoxynivalenol immunosensor based on a glassy carbon electrode modified with a fullerene/ferrocene/ionic liquid composite. Microchimica Acta, 172, 365–371. [Google Scholar]
- Zhou HR and Pestka JJ, 2015. Deoxynivalenol (vomitoxin)‐induced cholecystokinin and glucagon‐like peptide‐1 release in the STC‐1 enteroendocrine cell model is mediated by calcium‐sensing receptor and transient receptor potential ankyrin‐1 channel. Toxicological Sciences, 145, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Li Y, Gillespie J, He G‐Q, Horsley R and Schwarz P, 2007. Doehlert matrix design for optimization of the determination of bound deoxynivalenol in barley grain with trifluoroacetic acid (TFA). Journal of Agricultural and Food Chemistry, 55, 10141–10149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information for the BMD calculations


