Abstract
EFSA is committed to assess and communicate the risks occurring in the food and feed chain from farm to fork and to provide other forms of scientific advice. This work, carried out by EFSA since its inception, has resulted in the adoption of thousands of scientific assessments. EFSA is obliged to re‐assess past assessments in specific regulatory contexts such as those on food and feed additives, active substances in plant protection products and genetically modified food and feed. In other sectors, the consideration for updating past EFSA scientific assessments is taken on an ad hoc basis mainly depending on specific requests by risk managers or on EFSA self‐tasking. If safety is potentially at stake in any area within EFSA's remit, the readiness to update past scientific assessments is important to keep EFSA at the forefront of science and to promote an effective risk assessment. Although this task might be very complex and resource demanding, it is fundamental to EFSA's mission. The present EFSA Scientific Committee opinion deals with scientific motivations and criteria to contribute to the timely updating of EFSA scientific assessments. It is recognised that the decision for updating should be agreed following careful consideration of all the relevant elements by the EFSA management, in collaboration with risk managers and stakeholders. The present opinion addresses the scientific approaches through which it would be possible for EFSA to increase the speed and effectiveness of the acquisition of new data, as well as, to improve the consequent evaluations to assess the relevance and reliability of new data in the context of contributing to the better definition of whether to update past scientific assessments.
Keywords: scientific assessment, guidance, methodology
1. Introduction
In the context of its mission, the European Food Safety Authority (EFSA) is committed to assess the risks occurring in the food and feed chain from farm to fork and to provide other forms of scientific advice in an independent, high‐quality and timely manner. EFSA's duties also include open communication of its scientific evaluations to all interested parties, including managers, scientists and the public at large.
Since its inception in 2002, EFSA's work has resulted in the adoption and publication of thousands of scientific assessments1 on different subjects within its remit. In order to increase the transparency of its scientific evaluation procedures, EFSA has also formally developed, adopted and published a number of assessment methodologies (including guidance documents), which have been applied to assess risks in specific segments of the food chain.
All the scientific assessments adopted by EFSA depend on the assessment methodology used and on the data available at the time of the assessment. The availability of new relevant and reliable scientific and/or technical data and the amendments of the assessment methodologies over time to keep track of new scientific developments all contribute to the systematic consideration if the need to update some previous scientific assessments.
EFSA is obliged to re‐assess specific assessments, e.g. after a defined time period, in specific regulatory contexts such as:
Food additives permitted before 20 January 2009 are currently re‐evaluated by EFSA/ANS Panel to be finalised by 2020.
All feed additives currently on the market, authorised under the previous regulatory framework are being re‐evaluated by the EFSA/FEEDAP Panel, in line with current EU legislation on feed additives for use in animal nutrition to ensure that all feed additives in Europe are assessed following the same up‐to‐date guidelines and taking into account the newest scientific developments. Also, the renewal of the authorisation process has started for those feed additives where 10 years has elapsed since the first authorisation.
Plant protection products whose active substances are approved for a maximum of 10 years and it is possible to apply for the renewal of approval under Regulation EC 1107/2009.
Genetically modified (GM) food and feed authorised under Regulation (EC) 1829/2003. The authorisation shall be renewable for 10‐year periods on application to the Commission by the authorisation holder at the latest 1 year before the expiry date of the authorisation.
In other sectors within EFSA's remit, the consideration for updating adopted scientific assessment is based on an ad hoc basis mainly depending on requests by risk managers such as the European Commission, European Parliament and Member States or on EFSA self‐tasking. A potential need for updating an adopted EFSA opinion may also arise due to the prescriptions of Art. 30 of the Regulation (EC) No 178/2002.
If safety is potentially at stake in any one of the areas within the EFSA's remit, the updating of relevant scientific assessments is of fundamental importance for EFSA's mission and reputation regardless of whether it results from an ad hoc basis or within a periodic evaluation framework. EFSA's readiness to update scientific assessments, when needed, is also fundamental to avoid possible risk management actions resulting from assessments carried out on obsolete data and/or methodologies.
In view of the high complexity, many implications and considerable resources required for updating the many scientific assessments adopted by EFSA, the objective of the present opinion is to provide scientific criteria that can be used in identifying and prioritising those scientific assessments, which are more likely to need updating in relation to the emergence of new data and/or risk assessment methodologies.
In order to make more effective and timely the identification of new data and/or methodologies suggesting an updating of specific scientific opinions adopted by EFSA, it is useful to provide scientific criteria and approaches to better structure the work in the context of EFSA's overall work programme. It is recognised that, in any case, the decision for the updating should be discussed, together with legal considerations, potential consequences for stakeholders and implications for EFSA resources, by the EFSA management, risk managers and stakeholders.
The present opinion covers guidance documents and scientific assessments including scientific opinions, statements and other EFSA scientific outputs. It applies to both regulated and non‐regulated products.
1.1. Background and Terms of Reference
The Scientific Committee of EFSA established a Standing Working Group on Guidance Review with the following objectives:
to provide recommendations and to propose priorities for the preparation of new EFSA crosscutting guidance documents produced or endorsed by the Scientific Committee;
to review existing EFSA cross‐cutting guidance documents to confirm whether they are relevant and scientifically up‐to‐date, and to identify whether revision or replacement guidance should be developed;
to develop a plan for and to assist in the implementation and use of the above‐mentioned guidance documents across EFSA;
to assist in the development of advanced scientific training programmes in cross‐cutting scientific issues/guidance documents for experts of the Scientific Committee, Panels and EFSA Scientific Units.
Specifically, the Standing Working Group was asked to propose:
priority topics for the development of risk assessment guidance by the Scientific Committee in 2016–2018;
criteria to update a scientific assessment/guidance when new information becomes available.
This document addresses the second specific request.
2. Methodologies
The Standing Working Group on Guidance Review, consisting of members with expertise in various scientific areas covered by EFSA, developed the draft of this Scientific Opinion for consideration by the Scientific Committee.
3. Assessment
3.1. Motivations for updating EFSA scientific assessments
In addition to complying with the periodic re‐evaluation programmes, based on regulatory prescriptions or on specific requests by the European Institutions, in order to ensure the timely updating of specific scientific assessments, it is also important to carefully consider the availability of new relevant2 and reliable3 data likely to have a significant impact on the EFSA scientific assessments under consideration. This includes new end points, new studies, exposure data (including occurrence and consumption data) and variables (e.g. efficacy and disease risk markers) not taken into account previously which, through an ad hoc screening, are considered likely to influence the conclusions of and to have a significant impact on the previously adopted scientific assessments and imply the need for updating them. The emergence of relevant and reliable new data may derive from investigations and research activities not necessarily motivated by the risk assessment activities and/or carried out in response to specific EFSA recommendations or the adoption of major amendments in a risk assessment methodology (e.g. new targets or microbiological criteria) or completely new methodologies due to prescriptions of EU legislation.
Depending on the context in which the new data are produced, there may be considerable differences in the speed and effectiveness of their transfer to EFSA and EFSA's consequent ability to take them into account to update scientific assessments, when necessary.
The EFSA Scientific Committee addressed also the importance of transparency in the scientific EFSA outputs by elaborating the general principles to be applied in the identification of data sources, criteria for inclusion/exclusion of data, confidentiality of data and assumptions and uncertainties (EFSA, 2006, 2009). Transparency is needed in all parts of a scientific assessment which needs to be understandable and reproducible and, where possible, based on a harmonised, internationally‐ accepted methodology and standards of best practice.
The principles of the transparency guidance document (EFSA, 2009) also apply to the identification of motivations and priorities for the updating of EFSA scientific assessments. Hence, transparency should be ensured by clearly identifying the scientific reasons for updating a previous scientific assessment, together with a clear description of the objective(s) and timetable of the revision procedure. Relevant data linked to the decision to update a scientific assessment should be made publicly available. It is also fundamental that EFSA clearly applies a cut‐off date for data/studies to be considered in the course of an updating (as is done in any scientific assessment).
In this context, the EFSA scientific report on ‘Principles and process for dealing with data and evidence in risk assessment’, produced in the framework of the PROMETHEUS project (Promoting Methods for Evidence Use in Scientific assessments), is relevant (EFSA, 2015). The scientific report states that on the basis of EFSA's core values, the principles for dealing with data and evidence to decide whether updating a specific EFSA scientific assessment are those of: (i) impartiality; (ii) excellence in scientific assessment; (iii) transparency and openness; and (iv) responsiveness. In the light of these principles, the assessment should be structured in four phases: (i) planning the strategy upfront; (ii) conducting the process in line with the strategy; (iii) verifying the process; and (iv) documenting and reporting the process, results and conclusions, and ensuring accessibility of methods and data. Even if the process is structured in a way to minimize subjectivity and maximise transparency, the value of expert judgement in all phases of the process remains fundamental.
EFSA is also expected to achieve an important milestone with the adoption of a new guidance on how to characterise, document and explain uncertainties in the various steps of risk assessment (EFSA Scientific Committee, 2016). In fact, the better understanding of uncertainties in each scientific assessment will considerably improve the possibility of identifying those scientific assessments more likely to need specific attention for updating.
Similar considerations apply in the development of guidance by EFSA Scientific Committee on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017a) and on biological relevance (EFSA Scientific Committee, 2017b).
3.2. Identification of scientific assessments that require a proactive search for new data
It is considered useful to develop a procedure to identify EFSA's adopted scientific assessments that are more likely to require a proactive search for new data with a view to possible updating (Figure 1). The scientific criteria to be used by EFSA to this end are as follows:
identification of essential data gaps resulting in large remaining uncertainties in the previous assessment;
development of new scientific assessment concepts/methods which may significantly affect the conclusions of a previous assessment;
evaluation of the temporal relevance of the data used for the previous assessment;
where the estimated exposure for specific population groups is close to the respective health‐based guidance value or the margin of exposure/safety is small. In such cases, the impact of new data or a modified assessment methodology could be more relevant.
Figure 1.

Operational scheme 1. Updating EFSA's scientific assessments due to the emergence of new data
For each scientific assessment identified in such a context, the Scientific Committee considers important to encourage the agencies of the European Union (EU), international and Member State organisations, and Networks and, possibly, a specific segment of the scientific community to make EFSA, as soon as possible, aware of new potentially relevant data for updating EFSA's scientific evaluations. In fact, collaborating with EFSA to facilitate the identification of new data that are potentially relevant for updating past scientific assessments would be a significant contribution to the improvement of food, feed and environmental safety within the EU. For regulated products, there is also a legal responsibility for the relevant stakeholders to forward to EFSA as soon as possible any new information, which may affect previous EFSA scientific assessments.
An example of an effective approach to collect the scientific information needed for updating a scientific assessment is provided in the Appendix A of the EFSA opinion on the ‘Identification and selection criteria for scientific data consideration for the re‐evaluation of aspartame’ (EFSA ANS Panel, 2013) that is based on several opinions on principles of risk assessments adopted by different EFSA bodies between 2009 and 2012.
A similar consideration also applies to the EFSA Guidance on the submission of scientific peer‐reviewed open literature for the approval of plant protection products (EFSA, 2011) that provides a definition of scientific peer‐reviewed open literature and instructions on how to minimise bias in the identification, selection and inclusion of peer‐reviewed open literature in dossiers, according to the principles of systematic review (i.e. methodological rigor, transparency, reproducibility).
3.3. New or substantially modified assessment methodologies
The adoption of a new scientific methodology or of a major modification to an existing methodology originally used by EFSA for assessing the risk of specific categories of chemical substances or biological agents, may lead to the need to update a considerable number of past scientific assessments. In this case, however, it is possible through an analysis of the impact of the new or modified assessment methodology on already adopted scientific assessments to be carried out by the relevant EFSA SC/Panel and Unit, to evaluate the need for and extent of updating of past assessments and schedule the work plan (Figure 2). This impact assessment should be made preferably before the formal adoption of the new or modified assessment methodology and be discussed with EFSA management, risk managers and stakeholders. It should also consider the requirement for new data and the time needed for their generation.
Figure 2.
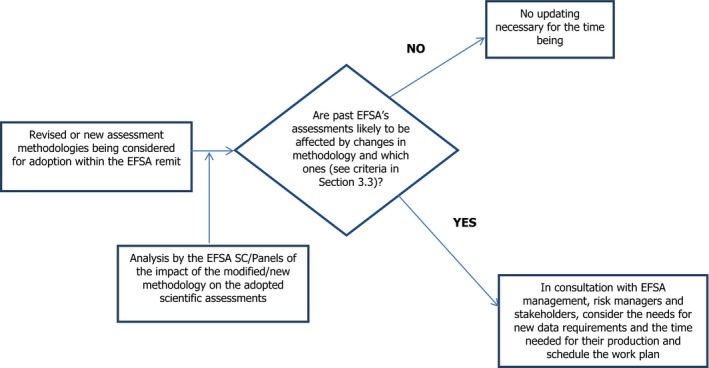
Operational scheme 2. Updating EFSA's scientific assessments due to the adoption of new or modified methodologies for scientific assessments
The principal criteria for considering if the adoption of a new or substantially modified assessment methodology implies the need to update specific scientific assessments include the following:
Are there critical new data requirements (e.g. new toxicity or exposure studies)?
Are there new approaches for hazard and exposure assessment?
Is there an assessment of the implications of a new guidance conducted by the responsible EFSA Panel/Unit?
Is the estimated exposure close to the respective health‐based guidance value?
Is the margin of exposure/safety small?
Similarly, for cross‐cutting guidance, such as those generally formulated by the EFSA Scientific Committee, it is often necessary to consult with the relevant Panels/Units/risk managers/stakeholders before the final adoption of the modified methodology or guidance document. The report on ‘Study on implications on the requirements for submission of toxicological information, restrictions and administrative consequences of the draft revised guideline on Food Contact Material (FCM)’ (Van Hoeck et al., 2016) provides an example of the approach aiming at predicting to what extent the adoption of a revised risk assessment methodology is likely to affect past scientific assessments.
Also, relevant in this context is the guidance document on the use of the benchmark dose approach in risk assessment developed by the Scientific Committee (EFSA Scientific Committee et al., 2017). In this guidance, the Scientific Committee reconfirmed that the Benchmark Dose (BMD) approach is a scientifically more advanced method compared to the No‐Observed‐Adverse‐Effect Level (NOAEL) approach. However, it did not call for a general re‐evaluation of previous assessments based on the NOAEL approach or on the BMD method as described in 2009 by the Scientific Committee, in particular where the exposure is more than one order of magnitude smaller than the health‐based guidance value.
3.4. Approach for screening the relevance and reliability of new data
The timely consideration of whether a previous scientific assessment needs to be updated is crucial for EFSA. Such a decision should be based on a judgement as to whether the new available data identified through the approaches described in Sections 3.2 and 3.3, are relevant, reliable and likely to be of decisive importance for the scientific assessment (Figures 1 and 2). In such a case, it would be appropriate also to check if additional data have become available in the meantime through any other route, which should be taken into consideration in the updating process.
A harmonised approach to decide on whether there is a need to update a scientific assessment, as yet to be formalised by EFSA, should possibly to be based on criteria including the following ones:
Are the new data relevant and reliable?
Does the new scientific evidence address important data gaps previously identified?
Are the new data likely to affect significantly the conclusions of an earlier assessment?
The screening of new data should preferably be carried out by a small working group under the responsibility of the EFSA Scientific Committee/Panels/Units involved in the original assessment.
The decision on whether there is a need to update the assessment should be taken by the EFSA Management and, where required by the applicable legal acts, by the appropriate risk managers.
4. Conclusions
The readiness to update past scientific assessments, when a new or substantially modified assessment methodology is adopted by EFSA or when new relevant, reliable and decisive data become available through a proactive search or any other route is considered by the Scientific Committee as essential for EFSA to stay at the forefront of scientific assessment of food and feed safety. To such an end, it is important for EFSA to take action to increase as much as possible the speed and effectiveness of the acquisition of new available and potentially relevant data and to systematically screen their relevance and reliability.
5. Recommendations
The Scientific Committee recommends that a systematic procedure is developed based on the criteria described in Section 3.2 to identify scientific assessments that are more likely to become out of date and where a proactive search for new data is needed. It is recommended that such a requirement is, whenever possible, clearly stated in the specific opinion or as soon as such a need emerges. For each such scientific assessment identified, a specific monitoring programme should be established by EFSA, in collaboration with relevant partners at European and international level, to identify as soon as possible the emergence of new data, which may require an updating initiative (Figure 1).
In the case of the adoption of a new or significantly modified scientific assessment methodology used by EFSA, the relevant Scientific Committee/Panel/Working Group and Unit should evaluate the implications for already adopted scientific assessments performed using previous methodologies. If the impact evaluation indicates the need for updating past assessments (criteria described in Section 3.3), the updating process should be properly planned in collaboration with EFSA management, risk managers and stakeholders (Figure 2).
Moreover, EFSA should develop and test, by making use of the criteria described in Section 3.4, a harmonised approach to decide on whether there is a need to start the updating of previous scientific assessments.
Abbreviations
- BMD
Benchmark Dose
- FCM
Food Contact Material
- GM
genetically modified
- NOAEL
No‐Observed‐Adverse‐Effect Level
- PROMETHEUS
Promoting Methods for Evidence Use in Scientific assessments
- SC
Scientific Committee
Suggested citation: EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Brock T, Chesson A, Karenlampi S, Lambre C, Sanz Y, Goumperis T, Kleiner J and Maurici D, 2017. Scientific opinion on scientific motivations and criteria to consider updating EFSA scientific assessments. EFSA Journal 2017;15(3):4737, 11 pp. doi: 10.2903/j.efsa.2017.4737
Requestor: EFSA
Question number: EFSA‐Q‐2016‐00326
Scientific Committee members: Diane Benford, Thorhallur Halldorsson, Anthony Hardy, Michael John Jeger, Katrine Helle Knutsen, Simon More, Alicja Mortensen, Hanspeter Naegeli, Hubert Noteborn, Colin Ockleford, Antonia Ricci, Guido Rychen, Josef R. Schlatter, Vittorio Silano, Roland Solecki and Dominique Turck.
Acknowledgements: The Scientific Committee wishes to thank the following for the support provided to this scientific opinion: the members of the Standing Working Group on Guidance Review Diane Benford, Theo Brock, Andrew Chesson, Anthony Hardy, Michael John Jeger, Sirpa Karenlampi, Claude Lambre, Yolanda Sanz, Josef Schlatter and Vittorio Silano and EFSA staff members Tilemachos Goumperis, Juliane Kleiner and Daniela Maurici.
Adopted: 14 February 2017
Notes
Scientific assessments include scientific opinions, statements, conclusions on Pesticides Peer Review and reasoned opinions.
‘Relevant data’ means data, which are likely to substantially influence a scientific assessment within the remit of EFSA. ‘Data relevance’ refers to the appropriateness of the data for the intended purpose of the assessment (Vermeire et al., 2013 and Ruden et al., 2016). In other words, relevance refers to the extent to which available data address the objective of the assessment (e.g. right target population, specific protection goals, hazard of concern, geographic area). Criteria for relevance of data have to be defined before starting the actual review of the data. Then, all the new data and information are evaluated, and only those judged to be relevant should be used in the assessment.
‘Reliable data’ means valid data supported by solid scientific reasoning, preferably obtained according to widely accepted test methodologies. ‘Reliability’ of evidence refers to: (i) precision, i.e. the extent to which random error is minimised and the outcome of the process is reproducible over time, and (ii) accuracy, i.e. the extent to which systematic error (bias) is minimised (IPCS, 2009).
References
- EFSA (European Food Safety Authority), 2006. Transparency in risk assessment carried out by EFSA: guidance document on procedural aspects. EFSA Journal 2006;4(5):353, 16 pp. doi: 10.2903/j.efsa.2006.353 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessment carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(5):1051, 22 pp. doi: 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009 (OJ L 309, 24.11.2009, p. 1–50). EFSA Journal 2011;9(2):2092, 49 pp. doi: 10.2903/j.efsa.2011.2092. Available online: http://www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Scientific report on Principles and process for dealing with data and evidence in scientific assessments. EFSA Journal 2015;13(5):4121, 35 pp. doi: 10.2903/j.efsa.2015.4121 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2013. Scientific Opinion on the re‐evaluation of aspartame (E 951) as a food additive. EFSA Journal 2013;11(12):3496, 263 pp. doi: 10.2903/j.efsa.2013.3496 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2016. Guidance on Uncertainty in EFSA Scientific Assessment (Revised Draft for Internal Testing). Available online: https://www.efsa.europa.eu/sites/default/files/160321DraftGDUncertaintyInScientificAssessment.pdf
- EFSA Scientific Committee , 2017a. Guidance on the use of the weight of evidence approach in scientific assessments. Draft published on 6 March 2017 for public consultation. Available on line: http://www.efsa.europa.eu/sites/default/files/consultation/170306-0.pdf
- EFSA Scientific Committee , 2017b. Guidance on biological relevance. Draft published on 6 March 2017 for public consultation. Available on line: http://www.efsa.europa.eu/sites/default/files/consultation/170306.pdf
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. doi: 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCS (International Programme on Chemical Safety), 2009. Principles and methods for the risk assessment of chemicals in food. Environmental Health Criteria 240. A joint publication of the Food and Agriculture Organization of the United Nations and the World Health Organization. Avaialble online: http://www.inchem.org/documents/ehc/ehc/ehc240_front.pdf
- Ruden C, Adams I, Agerstrand M, Brock TCM, Oulsen V, Schlekat CE and Henry TR, 2016. Assessing the relevance of ecotoxicological studies for regulatory decision‐making. Integrated Environmental Assessment and Management, 9999, 1–11. doi: 10.1002/ieam.1846 [DOI] [PubMed] [Google Scholar]
- Van Hoeck E, Mertens B, Goscinny S and N'Goy T, 2016. Study on implications on the requirements for submission of toxicological information, restrictions and administrative consequences of the draft revised guideline on Food Contact Material (FCM). EFSA supporting publication 2016;13(1):EN‐671, 117 pp. doi: 10.2903/sp.efsa.2016.EN-671 [DOI] [Google Scholar]
- Vermeire T, Aldenberg T, Buist H, Escher S, Mangelsdorf I, Pauné E, Rorije E and Kroese D, 2013. OSIRIS, a quest for proof of principle for integrated testing strategies of chemicals for four human health endpoints. Regulatory Toxicology and Pharmacology, 67, 136–145. [DOI] [PubMed] [Google Scholar]


