Abstract
The EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) was requested to evaluate the genotoxic potential of flavouring substances from subgroup 2.2 of FGE.19 in the Flavouring Group Evaluation 208 Revision 2 (FGE.208Rev2). In FGE.208Rev1, the CEF Panel evaluated genotoxicity studies on p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117], the representative substance for FGE.19 subgroup 2.2. The Comet assay performed in liver showed a positive result, and therefore, the Panel concluded that p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is genotoxic in vivo and that, accordingly, there is a safety concern for its use as flavouring substance. Since p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is representative for the nine remaining substances of subgroup 2.2 (p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], 2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde [FL‐no: 05.121], myrtenyl formate [FL‐no: 09.272], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278], myrtenyl acetate [FL‐no: 09.302], myrtenyl‐2‐methylbutyrate [FL‐no: 09.899] and myrtenyl‐3‐methylbutyrate [FL‐no: 09.900]), the Panel concluded in the previous revision of FGE.208 (FGE.208Rev1) that there is a potential safety concern for these substances. Subsequently, the industry has submitted genotoxicity studies on five substances of FGE.19 subgroup 2.2: p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] and myrtenyl acetate [FL‐no: 09.302], which are evaluated in the present revision of FGE.208 (FGE.208Rev2). The Panel concluded that the concern for genotoxicity could be ruled out for p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] and myrtenyl acetate [FL‐no: 09.302], which will be evaluated through the Procedure. Genotoxicity data on myrtenal [FL‐no: 05.106] were considered equivocal, therefore, it cannot be evaluated through the Procedure, presently. p‐Mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] and four substances not supported by industry (2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde [FL‐no: 05.121], myrtenyl formate [FL‐no: 09.272], myrtenyl‐2‐methylbutyrate [FL‐no: 09.899] and myrtenyl‐3‐methylbutyrate [FL‐no: 09.900]) have been deleted from the Union List.
Keywords: FGE.19; subgroup 2.2; alicyclic aldehydes; α,β‐unsaturated
Summary
Following a request from the European Commission, the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) was asked to deliver a scientific opinion on the implications for human health of chemically defined flavouring substances used in or on foodstuffs in the Member States. In particular, the Panel was asked to evaluate flavouring substances using the Procedure as referred to in the Commission Regulation (EC) No 1565/2000 (hereafter ‘the Procedure’).
The Union List of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/2012. The list contains flavouring substances for which the scientific evaluation should be completed in accordance with Commission Regulation (EC) No 1565/2000.
The Flavouring Group Evaluation 208 (FGE.208), corresponding to subgroup 2.2 of FGE.19, concerns three alicyclic aldehydes with the α,β‐unsaturation in ring/side‐chain and seven precursors for such. The α,β‐unsaturated aldehyde structure, which is a structural alert for genotoxicity and the data on genotoxicity previously available for these 10 substances, did not rule out the concern for genotoxicity.
Among the 10 flavouring substances in FGE.19 subgroup 2.2, the Panel identified p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117], for which appropriate genotoxicity data could be used for reading across to the other substances in the subgroup; therefore, genotoxicity data have been requested for p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] according to the testing strategy worked out by the Panel.
In 2012, the Flavour Industry submitted new genotoxicity data: a bacterial reverse mutation assay, a hypoxanthine‐guanine phosphoribosyl transferase (HPRT) assay and an in vitro micronucleus assay. These new data were evaluated in FGE.208 where the Panel concluded that the available data still gave rise to concern for the genotoxic potential of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117]. Therefore, the Panel asked to provide an in vivo Comet assay performed on the first site of contact (e.g. stomach or duodenum) and on liver.
The Flavour Industry submitted a combined study in rats: a bone marrow micronucleus test and Comet assay in liver and duodenum. This study was evaluated in Revision 1 of FGE.208 (FGE.208Rev1). p‐Mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] was administered to rats by oral gavage at three dose levels: 175, 350 and 700 mg/kg body weight (bw) per day (which is an estimate of the maximum tolerated dose). The results of the micronucleus assay suggest that p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] did not induce any increase in micronucleated polychromatic erythrocytes.
In the same animals, results of the Comet assay suggest that p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] did not induce any DNA damage in duodenum, but a statistically significant increase in DNA strand breaks was observed in the liver at the highest dose tested (700 mg/kg bw per day).
The Panel noted that the results observed at the highest dose were more than threefold higher than the concurrent negative control value and statistically significant different from the negative control value, and a statistically significant positive linear trend was observed. The Panel considered that since there was a wide range of historical control data with an overlap of the positive and negative historical control values, the historical control data could not be used as a criterion to interpret the data.
Overall, the Panel concluded that p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is genotoxic in vivo and that, accordingly, there is a safety concern for its use as flavouring substance.
Since p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is representative for the nine remaining substances of this subgroup 2.2 (p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], 2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde [FL‐no: 05.121], myrtenyl formate [FL‐no: 09.272], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278], myrtenyl acetate [FL‐no: 09.302], myrtenyl‐2‐methylbutyrate [FL‐no: 09.899] and myrtenyl‐3‐methylbutyrate [FL‐no: 09.900]), the Panel concluded, in the previous revision of FGE.208 (FGE.208Rev1), that there is a potential safety concern for these substances.
Subsequently, the industry has submitted in vitro genotoxicity studies on p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] and myrtenyl acetate [FL‐no: 09.302], which are evaluated in the present revision of FGE.208 (FGE.208Rev2).
The Panel considered that the newly submitted in vitro genotoxicity studies on p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] and myrtenyl acetate [FL‐no: 09.302] were adequately performed and that the results were negative. Therefore, the concern for genotoxicity could be ruled out for these flavouring substances that could be evaluated through the Procedure.
Myrtenal [FL‐no: 05.106] did not induce gene mutations in a bacterial reverse mutation assay. The first in vitro micronucleus assay provided was equivocal and had several weaknesses; therefore, a repetition of the study was requested. The second study is considered more reliable than the first one, but the result is still not fully adequate to rule out the concern for genotoxicity. In this second study, weak statistically significant increases of the micronuclei frequency were observed at the lowest and highest concentrations (without statistically significant trend) in the absence of S9‐mix after long treatment, while after short treatment, there was a statistically significant trend (without statistically significant differences between single concentrations tested and the concurrent control). The Panel considered that also the result of this second study was equivocal and that this was not adequately investigated by the applicant. Therefore, myrtenal cannot be evaluated through the Procedure, presently.
The Panel also considered two recent publications on the evaluation of genotoxicity studies on p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117], which were published after the publication of the scientific opinion on Flavouring Group Evaluation 208 Revision 1 (FGE.208Rev1). The Panel concluded that these two publications do not give reason to modify the conclusion drawn on the genotoxicity of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] in FGE.208Rev1.
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The use of flavourings is regulated under Regulation (EC) No 1334/20081 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union list of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/20122. The list contains flavouring substances for which the scientific evaluation should be completed in accordance with Commission Regulation (EC) No 1565/20003.
On 27 July 2015, the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) adopted an opinion on Flavouring Group Evaluation 208 Revision 1 (FGE.208Rev1): Consideration of genotoxicity data on representatives for 10 alicyclic aldehydes with the α,β‐unsaturation in ring/side‐chain and precursors from chemical subgroup 2.2 of FGE.19.
The Panel concluded that p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is genotoxic in vivo and as that substance was regarded as the representative of the group, there is a potential safety concern for the other substances in this group. Following this opinion, the Commission withdrew from the Union List of flavourings the representative substance FL‐no: 05.1174 and also the non‐supported substances 2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde [FL‐no: 05.121], myrtenyl formate [FL‐no: 09.272], myrtenyl‐2‐methylbutyrate [FL‐no: 09.899] and myrtenyl‐3‐methylbutyrate [FL‐no: 09.900].5
On 25 November 2015, the applicant submitted an in vitro micronucleus assay and a bacterial reverse mutation assay on the substance myrtenol [FL‐no: 02.091]. It also submitted additional studies and data on the other four remaining substances p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] and myrtenyl acetate [FL‐no: 09.302] at later stages at the beginning of 2016.
Also, following the EFSA opinion of 2015, the Commission amended the conditions of use of these other five substances of this group in another Regulation6 and put a footnote ‘under evaluation by EFSA’ to them in the Union List of flavourings, pending the evaluation of the additional data.
1.1.2. Terms of Reference
The European Commission requests the European Food Safety Authority (EFSA) to evaluate the studies in the submissions on the following five substances of FGE.19 subgroup 2.2: p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] and myrtenyl acetate [FL‐no: 09.302], taking into account also the uses reported, the Commission Regulations adopted following the EFSA opinion of July 2015 and any new other safety information relevant available, and, depending on the outcome, proceed to the full evaluation on these five flavouring substances, taking into account the requirements of the Commission Regulation (EC) No 1565/2000 and of Regulation (EU) No 1334/2008. The authority is also asked to characterise the hazards and also quantify the exposure also in case its concern on genotoxicity cannot be ruled out and the EFSA CEF Panel procedure cannot be applied for any of the substances of the group.
1.2. Interpretation of the Terms of Reference
This revision concerns the evaluation of p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], myrtenyl acetate [FL‐no: 09.302] and p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] for which industry submitted new data on genotoxicity.
During the evaluation process, the industry sent to EFSA two publications on the genotoxicity evaluation of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] which has been evaluated in FGE.208Rev1 and these two recent publications are discussed in the present opinion (FGE.208Rev2).
2. Data and methodologies
2.1. History of the evaluation of FGE.19 substances
Flavouring Group Evaluation 19 (FGE.19) contains 360 flavouring substances from the EU Register being α,β‐unsaturated aldehydes or ketones and precursors which could give rise to such carbonyl substances via hydrolysis and/or oxidation (EFSA, 2008a).
The α,β‐unsaturated aldehyde and ketone structures are structural alerts for genotoxicity (EFSA, 2008a). The Panel noted that there were limited genotoxicity data on these flavouring substances but that positive genotoxicity studies were identified for some substances in the group.
The α,β‐unsaturated carbonyls were subdivided into subgroups on the basis of structural similarity (EFSA, 2008a). In an attempt to decide which of the substances could go through the Procedure, a (quantitative) structure–activity relationship (Q)SAR prediction of the genotoxicity of these substances was undertaken considering a number of models (DEREKfW, TOPKAT, DTU‐NFI‐MultiCASE Models and ISS‐Local Models, (Gry et al., 2007)).
The Panel noted that for most of these models internal and external validation has been performed, but considered that the outcome of these validations was not always extensive enough to appreciate the validity of the predictions of these models for these α,β‐unsaturated carbonyls. Therefore, the Panel considered it inappropriate to totally rely on (Q)SAR predictions at this point in time and decided not to take substances through the Procedure based on negative (Q)SAR predictions only.
The Panel took note of the (Q)SAR predictions by using two ISS Local Models (Benigni and Netzeva, 2007a,b) and four DTU‐NFI MultiCASE Models (Gry et al., 2007; Nikolov et al., 2007) and the fact that there are available data on genotoxicity, in vitro and in vivo, as well as data on carcinogenicity for several substances. Based on these data the Panel decided that 15 subgroups (1.1.1, 1.2.1, 1.2.2, 1.2.3, 2.1, 2.2, 2.3, 2.5, 3.2, 4.3, 4.5, 4.6, 5.1, 5.2 and 5.3) (EFSA, 2008a) could not be evaluated through the Procedure due to concern with respect to genotoxicity. Corresponding to these subgroups, 15 FGEs were established: FGE.200, 204, 205, 206, 207, 208, 209, 211, 215, 219, 221, 222, 223, 224 and 225.
For 11 subgroups, the Panel decided, based on the available genotoxicity data and (Q)SAR predictions, that a further scrutiny of the data should take place before requesting additional data from the Flavouring Industry on genotoxicity. These subgroups were evaluated in FGE.201, 202, 203, 210, 212, 213, 214, 216, 217, 218 and 220. For the substances in FGE.202, 214 and 218, it was concluded that a genotoxic potential could be ruled out and accordingly these substances were evaluated using the Procedure. For all or some of the substances in the remaining FGEs, FGE.201, 203, 210, 212, 213, 216, 217 and 220, the genotoxic potential could not be ruled out.
To ease the data retrieval of the large number of structurally related α,β‐unsaturated substances in the different subgroups for which additional data are requested, EFSA worked out a list of representative substances for each subgroup (EFSA, 2008c). Likewise, an EFSA genotoxicity expert group has worked out a test strategy to be followed in the data retrieval for these substances (EFSA, 2008b).
The Flavouring Industry has been requested to submit additional genotoxicity data according to the list of representative substances and test strategy for each subgroup.
2.2. History of the evaluation of the substances in subgroup 2.2
Subgroup 2.2 was one of the FGE.19 subgroups for which the Panel concluded that, based on the available data, additional genotoxicity data were necessary to perform the risk assessment for these substances (EFSA, 2008a).
The Panel identified one substance in subgroup 2.2 of FGE.19, p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117], which represents the other nine substances in this subgroup (EFSA, 2008c). For this substance, genotoxicity data according to the test strategy (EFSA, 2008b) have been requested. The representative substance is shown in Table 1.
Table 1.
Representative substance for subgroup 2.2 of FGE.19 (EFSA, 2008c)
| FL‐no JECFA‐no | Subgroup | EU Register name | Structural formula | FEMA no CoE no CAS no |
|---|---|---|---|---|
|
05.117 973 |
2.2 | p‐Mentha‐1,8‐dien‐7‐al |
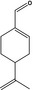
|
3557 11788 2111‐75‐3 |
FGE: Flavouring Group Evaluation; FL‐no: FLAVIS number; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service.
In 2012, the industry submitted new genotoxicity data: a bacterial gene mutation assay (Ames test), a gene mutation assay in mammalian cells (hypoxanthine‐guanine phosphoribosyl transferase (HPRT) assay) and an in vitro micronucleus assay. These data were evaluated in FGE.208 (EFSA CEF Panel, 2013), where the Panel concluded that the available data still gave rise to concern for the genotoxic potential of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117]. Therefore, the Panel asked to provide an in vivo Comet assay performed on the first site of contact (e.g. stomach or duodenum) and on liver.
Revision 1 of FGE.208 (FGE.208Rev1) concerned the evaluation of a combined bone marrow micronucleus test and Comet assay in the liver and duodenum of rats. These data have been submitted by industry (Beevers, 2014a,b) in response to the requested genotoxicity data in FGE.208 on the representative substance for subgroup 2.2, p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117]. The Panel concluded that p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is genotoxic in vivo and that, accordingly, there is a safety concern for its use as flavouring substance. Since p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is representative for the nine remaining substances of this subgroup 2.2 (p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], 2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde [FL‐no: 05.121], myrtenyl formate [FL‐no: 09.272], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278], myrtenyl acetate [FL‐no: 09.302], myrtenyl‐2‐methylbutyrate [FL‐no: 09.899] and myrtenyl‐3‐methylbutyrate [FL‐no: 09.900]), the Panel concluded in revision 1 of FGE.208 that there is a potential safety concern for these substances (EFSA CEF Panel, 2015).
p‐Mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] and four substances not supported by industry (2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde [FL‐no: 05.121], myrtenyl formate [FL‐no: 09.272], myrtenyl‐2‐methylbutyrate [FL‐no: 09.899] and myrtenyl‐3‐methylbutyrate [FL‐no: 09.900]) have been deleted from the Union List (Commission Regulation (EU) 2015/17604, Commission Regulation (EU) 2016/6375).
| FGE | Adopted by the CEF Panel | Link | No. Substances |
|---|---|---|---|
| FGE.208 | 19 March 2013 | http://www.efsa.europa.eu/en/efsajournal/pub/3151.htm | 10 |
| FGE.208Rev1 | 24 June 2015 | http://www.efsa.europa.eu/en/efsajournal/pub/4173.htm | 10 |
| FGE.208Rev2 | 22 March 2017 | http://www.efsa.europa.eu/en/efsajournal/pub/4766.htm | 5 |
FGE: Flavouring Group Evaluation.
Industry has submitted genotoxicity data for the five remaining substances of subgroup 2.2. Genotoxicity studies on p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] and myrtenyl acetate [FL‐no: 09.302] are evaluated in the present revision of FGE.208 (FGE.208Rev2).
During the evaluation process, the industry sent to EFSA two publications on the genotoxicity evaluation of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] which has been evaluated in FGE.208Rev1 and these two recent publications are discussed in the present opinion (FGE.208Rev2, Appendix F).
Exposure data on substances for which the concern for genotoxicity cannot be ruled out are reported in Appendix E.
2.3. Presentation of the substances belonging to FGE.208Rev2
The Flavouring Group Evaluation 208, corresponding to subgroup 2.2 of FGE.19, concerned three alicyclic aldehydes with α,β‐unsaturation in ring/side‐chain and seven precursors for such aldehydes. The 10 substances evaluated in FGE.208 and FGE.208Rev1 are listed in Table 2.
Table 2.
Summary of specifications for the Substances in the Flavouring Group Evaluation 208 (JECFA, 2002b)
| FL‐no JECFA‐no | EU Register name | Structural formula | FEMA no CoE no CAS no | Phys. form Mol. formula Mol. weight | Solubilitya Solubility in ethanolb | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refrac. indexd Spec. gravitye |
|---|---|---|---|---|---|---|---|
|
02.060 974 |
p‐Mentha‐1,8‐dien‐7‐ol |
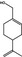
|
2664 2024 536‐59‐4 |
Liquid C10H16O 152.24 |
Slightly soluble Miscible |
119 (14 hPa) – NMR 96% |
1.495–1.505 0.956–0.963 |
|
02.091 981 |
Myrtenol |
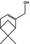
|
3439 10285 515‐00‐4 |
Liquid C10H16O 152.24 |
Insoluble Miscible |
221 – IR NMR 95% |
1.490–1.500 0.976–0.983 |
|
05.106 980 |
Myrtenal |
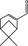
|
3395 10379 564‐94‐3 |
Liquid C10H14O 150.22 |
Insoluble Miscible |
220 – NMR 98% |
1.496–1.507 0.984–0.990 |
|
05.117 973 |
p‐Mentha‐1,8‐dien‐7‐alf |
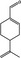
|
3557 11788 2111‐75‐3 |
Liquid C10H14O 150.22 |
Insoluble Miscible |
104 (13 hPa) – NMR 97% |
1.504–1.513 0.948–0.956 |
|
05.121 979 |
2,6,6‐Trimethyl‐1‐cyclohexen‐1‐carboxaldehydeg , h |
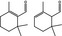
|
3639 2133 432‐25‐7 |
Liquid C10H16O 152.23 |
Insoluble Miscible |
62 (4 hPa) – IR 99% |
1.476–1.483 0.950–0.957 |
|
09.272 983 |
Myrtenyl formateg |
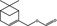
|
3405 10858 72928‐52‐0 |
Liquid C11H16O2 180.25 |
Insoluble Miscible |
127‐130 (52 hPa) – NMR 96% |
1.477–1.483 1.004–1.010 (20°) |
|
09.278 975 |
p‐Mentha‐1,8‐dien‐7‐yl acetate |
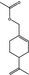
|
3561 10742 15111‐96‐3 |
Liquid C12H18O2 194.27 |
Insoluble Miscible |
218‐223 – NMR 97% |
1.476–1.487 0.972–0.980 |
|
09.302 982 |
Myrtenyl acetate |
|
3765 10887 1079‐01‐2 |
Liquid C12H18O2 194.28 |
Miscible |
134 (49 hPa) – IR NMR MS 98% |
1.470–1.477 0.987–0.996 |
| 09.899 | Myrtenyl‐2‐methylbutyrateg |
|
– – 138530‐44‐6 |
Liquid C15H24O2 236.35 |
Practically insoluble or insoluble Freely soluble |
345 – MS 95% |
1.466–1.470 0.964–0.970 |
| 09.900 | Myrtenyl‐3‐methylbutyrateg |
|
– – 33900‐84‐4 |
Liquid C15H24O2 236.35 |
Practically insoluble or insoluble Freely soluble |
98 (1 hPa) – MS 95% |
1.470–1.476 0.967–0.973 |
FL‐no: FLAVIS number; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; ID: identity; NMR: nuclear magnetic resonance; IR: infrared spectroscopy; MS: mass spectrometry; –: not reported.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
Deleted from the Union List by Commission Regulation (EU) 2015/1760.
Deleted from the Union List by Commission Regulation (EU) 2016/637.
It is not clear which substance was evaluated by JECFA, the CAS number applies to 2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde only. Since [FL‐no: 05.121] has been withdrawn from the Union List by Commission Regulation (EU) 2016/637, its identification will be no longer necessary.
Eight of the flavouring substances have been previously evaluated by JECFA (2002a). A summary of their current evaluation status by JECFA and the outcome of this consideration are presented in Appendix A, Table A.1.
Table A.1.
Summary of Safety Evaluation of the JECFA Substances in the Present Group (JECFA, 2002a)
| FL‐no JECFA‐no | EU Register name | Structural formula | EU MSDIa US MSDI (μg/capita per day) | Classb Evaluation procedure pathc | Outcome on the named compound (d or e) | EFSA conclusion on the named compound (genotoxicity) |
|---|---|---|---|---|---|---|
|
02.060 974 |
p‐Mentha‐1,8‐dien‐7‐ol |
|
1.6 1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.208Rev2, as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.091 981 |
Myrtenol |
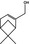
|
0.37 0.03 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.208Rev2, as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.106 980 |
Myrtenal |
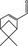
|
4 7 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.208Rev2 but concern for genotoxicity could not be ruled out because of equivocal study results. The substance cannot be evaluated through the Procedure, presently |
|
05.117 973 |
p‐Mentha‐1,8‐dien‐7‐alf |
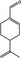
|
2.1 2 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.208Rev1, as of genotoxicity concern |
|
05.121 979 |
2,6,6‐Trimethyl‐1‐cyclohexen‐1‐carboxaldehydeg , h |
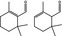
|
0.37 ND |
Class I A3: Intake below threshold |
d | No longer supported by Industry |
|
09.272 983 |
Myrtenyl formateg |
|
0.3 ND |
Class I A3: Intake below threshold |
d | No longer supported by Industry |
|
09.278 975 |
p‐Mentha‐1,8‐dien‐7‐yl acetate |
|
0.35 0.07 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.208Rev2, as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.302 982 |
Myrtenyl acetate |
|
0.37 0.04 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.208Rev2, as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 09.899 | Myrtenyl‐2‐methylbutyrateg |
|
0.012 |
Class I No evaluation |
Not evaluated by JECFA | No longer supported by Industry |
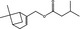
| 09.900 | Myrtenyl‐3‐methylbutyrateg |
|
0.061 |
Class I No evaluation |
Not evaluated by JECFA | No longer supported by Industry |
ND: not determined; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FL‐no: FLAVIS number; MSDI: maximised survey‐derived daily intake; FGE: Flavouring Group Evaluation.
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day.
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A, substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
Deleted from the Union List by Commission Regulation (EU) 2015/1760.
Deleted from the Union List by Commission Regulation (EU) 2016/637.
It is not clear which substance was evaluated by JECFA, the CAS number applies to 2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde only. Since [FL‐no: 05.121] has been withdrawn from the Union List by Commission Regulation (EU) 2016/637, its identification will be no longer necessary.
The α,β‐unsaturated aldehyde structure is a structural alert for genotoxicity (EFSA, 2008a) and data on genotoxicity previously available did not rule out the concern for genotoxicity for these 10 flavouring substances.
In FGE.208Rev1 (EFSA CEF Panel, 2015), the Panel concluded that p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is genotoxic in vivo and that, accordingly, there is a safety concern for the use of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] as a flavouring substance. Since p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is representative for the nine remaining substances of this subgroup 2.2 (p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], 2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde [FL‐no: 05.121], myrtenyl formate [FL‐no: 09.272], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278], myrtenyl acetate [FL‐no: 09.302], myrtenyl‐2‐methylbutyrate [FL‐no: 09.899] and myrtenyl‐3‐methylbutyrate [FL‐no: 09.900]), the Panel concluded in revision 1 of FGE.208 that there is a potential safety concern for these substances (EFSA CEF Panel, 2015). After publication of FGE.208.Rev1, EFSA received information that four of these substances (2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde [FL‐no: 05.121], myrtenyl formate [FL‐no: 09.272], myrtenyl‐2‐methylbutyrate [FL‐no: 09.899] and myrtenyl‐3‐methylbutyrate [FL‐no: 09.900]) are no longer supported for use as flavouring substances in the EU, and have been removed from the Union List5 (see Section 1.1.1). Therefore, these four substances will not be further discussed in the newly added section in the present revision of this FGE. Since new in vitro genotoxicity data have been submitted for p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] and myrtenyl acetate [FL‐no: 09.302], these five substances will be considered in the present revision of FGE.208 (FGE.208Rev2).
Sections 2.4 and 2.5 of this opinion report the same information that was presented in FGE.208 and FGE.208Rev1, respectively. Section 3 reports the evaluation of the new data submitted by industry.
2.4. Additional genotoxicity data evaluated by the Panel in FGE.2087
The industry has submitted additional data concerning genotoxicity studies for the representative substance p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] for this subgroup (EFFA, 2012). The data for p‐mentha‐1,8‐dien‐7‐al are one in vitro test in bacteria and two in vitro tests in mammalian cell systems.
2.4.1. In vitro data
2.4.1.1. Bacterial reverse mutation assay
An Ames assay was conducted in Salmonella Typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 to assess the mutagenicity of p‐mentha‐1,8‐dien‐7‐al, both in the absence and in the presence of metabolic activation by an Aroclor 1254‐induced rat liver post mitochondrial fraction (S9‐mix) in three experiments (Bowen, 2011). A batch of 93.1% purity was used for the first and second experiment, while a batch of 91.9% purity was used for the third experiment. An initial toxicity range finding experiment was carried out using the plate incorporation method in the presence and absence of S9‐mix for the TA100 strain only at concentrations of 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate, plus negative (solvent) and positive controls. Evidence of toxicity in the form of complete killing of the background lawn was observed at 5,000 μg/plate in the absence and presence of S9‐mix. Precipitation was also seen at this concentration. As valid mutation data were available from five different test concentrations, the data from these treatments were considered to be acceptable for mutation analysis as part of the first main experiment. This study complies with Good Laboratory Practice (GLP) and OECD Test Guideline 471 (OECD, 1997a).
In the first experiment, treatments of all the remaining tester strains were performed in the absence and presence of S9‐mix at concentrations of 0.32, 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate, plus negative (solvent) and positive controls. Evidence of toxicity was observed in all strains in the absence and presence of S9‐mix at 5,000 μg/plate, and in some strains also at 1,000 μg/plate. Precipitation was also seen at 5,000 μg/plate. Valid mutation data were obtained from five or six different test concentrations in each strain. Following experiment 1 treatments, a statistically significant and concentration‐related increase in revertant numbers was observed in strain TA98 at 200 μg/plate (1.8‐fold increase) and 1,000 μg/plate (3.2‐fold increase) in the absence of S9‐mix, when data were analysed at the 1% level using Dunnett's test.
In a second experiment, treatments of the strains assayed in experiment 1 were performed in the absence and presence of S9‐mix at 8.192, 20.48, 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate. Treatments in the presence of S9‐mix were further modified by the inclusion of a pre‐incubation step (60 min). Evidence of toxicity ranging from a marked reduction in revertant numbers and/or slight thinning of the bacterial lawn to a complete killing of the test bacteria was observed at 320, 800 and/or 2,000 μg/plate and above in most of the strains in the absence and presence of S9‐mix. Precipitation was again seen at 5,000 μg/plate, particularly in the presence of S9‐mix. However, valid mutation data were obtained from at least five test concentrations in each strain. Following experiment 2 treatments, a statistically significant and concentration‐related increase in revertant numbers was again observed in strain TA98 in the absence of S9‐mix at 320 μg/plate (2.3‐fold increase) and 800 μg /plate (2.9‐fold increase), when data were analysed at 1% level using Dunnett's test.
Following the treatments in experiments 1 and 2, p‐mentha‐1,8‐dien‐7‐al increased the frequency of revertants in strain TA98 by at least twofold in the absence of S9‐mix activation. These results were in contrast with what had been observed for p‐mentha‐1,8‐dien‐7‐al in previous Ames assays described further below. One possible explanation for the varying pattern of behaviour was that the material tested (93.1% purity) in experiments 1 and 2, due to impurities, gave positive results. A third experiment was conducted in strain TA98, with a different batch of the test article (91.9% purity), but with the same treatment conditions as in experiment 1. In the absence of S9‐mix, toxicity was observed at 5,000 μg/plate, while in the presence of S9‐mix toxicity was observed at all concentrations tested. Additionally, while precipitation was observed on all test plates at 5,000 μg/plate in experiments 1 and 2, no precipitation was observed at this concentration in experiment 3. Following the treatments in experiment 3, statistically significant and concentration‐related increases in revertant numbers for strain TA98 in the absence of S9‐mix were observed at 8 μg/plate and above when the data were analysed at 1% level using Dunnett's test. Therefore, the increases observed in strain TA98 were reproduced and are considered to be evidence of mutagenic activity in this strain. No other statistically significant increases in revertant numbers were observed in all other strains when the data were analysed at the 1% level using Dunnett's test (Appendix B, Table B.1).
Table B.1.
Summary of in vitro Genotoxicity Data Evaluated by the Panel in FGE.208
| Chemical name [FL‐no] | Test system | Test object | Concentration | Results | Reference | Comments |
|---|---|---|---|---|---|---|
| p‐Mentha‐1,8‐dien‐7‐al [05.117] | Bacterial reverse mutation assay | Salmonella Typhimurium TA100 | 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate | Negativeb | Bowen (2011) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 471 |
| S. Typhimurium TA98, TA102, TA1535, TA1537 | 0.32, 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate | Positiveb | All strains were negative except TA98 without S9‐mix treatment | |||
| S. Typhimurium TA98, TA102, TA1535, TA1537 | 8.192, 20.48, 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate | Positivea , c | All strains were negative except TA98 without S9‐mix treatment | |||
| S. Typhimurium TA98 | 0.32, 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate | Positiveb | A different batch of test article was used and positive results in TA98 without S9‐ mix were confirmed | |||
| Bacterial reverse mutation assay | S. Typhimurium TA97, TA102 | Up to 100 μg/plate | Negativea , b | Fujita et al. (1994) | Not assignable. Low concentrations; only two strains used, one of which (TA97) not routinely used | |
| Bacterial reverse mutation assay | S. Typhimurium TA92, TA1535, TA100, TA1537, TA94, TA98 | Up to 1,000 μg/plate | Negativea | Ishidate et al. (1984) | Reliable with restriction. Results reported as − or + | |
| Bacterial reverse mutation assay | Escherichia coli WP2 | Up to 0.4 mg/plate | Negativec | Yoo (1986) | Not assignable. Probably only performed in the absence of S9‐mix. Low concentrations tested; only few details available | |
| DNA damage | Bacillus subtilis M45 and H17 | 2.5 μL/disk (probably equivalent to 2,500 μg/disk) | Weak positive | Not assignable. Details difficult to obtain. Endpoint not relevant | ||
| DNA damage | B. subtilis M45 and H17 | 0.16–0.63 μL/plate (0.15–0.6 μg/plate | Negative | Kuroda et al. (1984) | Not assignable. Details difficult to obtain. Endpoint not relevant | |
| 1.25 and 2.5 μL/plate (1.2 and 2.4 μg/plate) | Positive | |||||
| DNA repair | E. coli PQ37 | Not reported | Negative | Eder et al. (1993) | SOS chromotest. Endpoint not relevant | |
| Sister chromatid exchange | Chinese hamster ovary cells | 150 μg/mL | Positivec | Tayama et al. (1990) | Reliable with restriction; genetic endpoint of limited relevance | |
| 100–300 μg/mL | Positivee | |||||
| Chromosomal aberration | Chinese hamster fibroblasts | Up to 50 μg/mL | Positivec | Ishidate et al. (1984) | Reliable with restriction. No concurrent measure of cytotoxicity. Performed only in the absence of S9 | |
| Chromosomal aberration | Chinese hamster ovary cells | 300 μg/mL | Positivee , f | Tayama et al. (1990) | Reliable with restriction. Moderate toxicity at 300 μg/mL (+S9) | |
| 150 μg/mL | Negativec , d , f | Reliable with restriction. No detectable cell division at 150 μg/mL (−S9) | ||||
| Mutagenicity | Chinese hamster ovary cells | 10 μg/mL | Negativec , g | Sasaki et al. (1990) | Not assignable. Ouabain resistance measured. Only one concentration tested without S9; insufficient details | |
| Mutagenicity | Human fetus cells (Rsa) | Up to 0.025 μg/mL | Positiveh | Suzuki et al. (1990) | Unreliable; ouabain resistance measured in Rsa cells not routinely used; insufficient details | |
| 0.010 μg/mL | Negativeh | |||||
| Mutagenicity | Human fetus cells (Rsa) | > 10 ng/mL | Positivei | Suzuki and Suzuki (1994) | Japanese paper quoted but not available | |
| Micronucleus Induction | Primary human lymphocytes | Up to 140 μg/mLj | Negativeb | Lloyd (2009) | Reliable without restriction. Complies with GLP and OECD Guideline 487 | |
| HPRT assay | Mouse lymphoma L5178Y cells | Up to 180 μg/mLk | Equivocalb | Lloyd (2012) | Reliable without restriction. Complies with GLP and OECD Test Guideline 476 |
GLP: Good Laboratory Practice; OECD: Organisation for Economic Co‐operation and Development.
Preincubation with exogenous metabolic system from rat liver.
Assay performed with and without metabolic activation.
Assay performed without metabolic activation.
Cytotoxic at 150 μg/mL.
Assay performed with metabolic activation.
Positive only at cytotoxic concentrations.
Cytotoxic at 12 μg/mL.
Cytotoxic at 0.025 μg/mL.
Cytotoxic at > 20 ng/mL.
Cytotoxic ≥ 160 μg/mL.
Cytotoxic ≥ 180 μg/mL (3 h treatment in the presence of S9‐mix); cytotoxic ≥ 100 μg/mL (3 h treatment in the absence of S9‐mix); cytotoxic ≥ 21 μg/mL (24 h treatment in the absence of S9‐mix).
2.4.1.2. Hypoxanthine‐guanine phosphoribosyl transferase (HPRT) assay
To assess mutagenic potential in a mammalian system, mouse lymphoma L5178Y cells were treated with p‐mentha‐1,8‐dien‐7‐al in the absence and presence of S9‐mix to study the induction of forward mutations at the hprt locus (Lloyd, 2012). A batch of 92.5% purity was used. Across three different experiments, treatments were carried out for 3 h in the absence of S9‐mix, 3 h in the presence of S9‐mix and 24 h in the absence of S9‐mix, and each treatment regime was independently repeated. Concentrations for the main experiments were established in preliminary range finding cytotoxicity experiments. This GLP study complies with OECD Test Guideline 476 (OECD, 1997b).
In the first mutation experiment, cells were treated with p‐mentha‐1,8‐dien‐7‐al for 3 h at 10, 20, 40, 60, 70, 80, 90 and 100 μg/mL in the absence of S9‐mix and at 40, 60, 80, 100, 120, 140, 160 and 180 μg/mL in the presence of S9‐mix. Per cent relative survival (% RS) decreased to 13% at 100 μg/mL in the absence of S9‐mix and to 16% at 180 μg/mL in the presence of S9‐mix. Negative control mutant frequencies were normal, and were significantly increased by treatment with the positive control. No significant increases in mutation frequency were observed at any concentration analysed in the presence or absence of S9‐mix in this experiment, and no statistically significant linear trends were observed.
In a second experiment, cultures were treated with p‐mentha‐1,8‐dien‐7‐al for 3 h at 20, 40, 50, 60, 70, 80, 90, 100 and 120 μg/mL in the absence of S9‐mix and at 25, 50, 75, 100, 120, 140, 160, 170 and 180 μg/mL in the presence of S9‐mix. Per cent RS decreased to 7% at 120 μg/mL in the absence and to 10% at 180 μg/mL in the presence of S9‐mix. Also, in this experiment, 24‐h treatments were carried out with p‐mentha‐1,8‐dien‐7‐al in the absence of S9‐mix at 4, 8, 12, 15, 18 and 21 μg/mL of p‐mentha‐1,8‐dien‐7‐al. Per cent RS decreased to 9% at the highest concentration. Negative control mutant frequencies were normal and were significantly increased by treatment with the positive control. In the absence and presence of S9‐mix, there were no statistically significant increases in mutant frequency relative to control at any concentration analysed, although in the absence of S9‐mix (both 3‐ and 24‐h treatments), there were statistically significant linear trends.
In a third experiment, cultures were treated with p‐mentha‐1,8‐dien‐7‐al for 24 h at 4, 8, 12, 14, 16, 18 and 20 μg/mL in the absence of S9‐mix. Per cent RS decreased to 14% at the highest concentration. Negative control mutant frequencies were normal, and were significantly increased by treatment with the positive control. There were no significant or dose‐related increases in mutant frequency following p‐mentha‐1,8‐dien‐7‐al treatments. The observations made with the 24‐h treatments in the second experiment were not reproduced at similar concentrations and extents of toxicity and were considered not to be biologically relevant by the authors (Lloyd, 2012).
However, it is not clear why the 3‐h treatment was not repeated. Overall, the results in the HPRT assay in the absence of S9‐mix should be considered, differently from the authors’ opinion, as equivocal instead of negative, based on the statistically significant trends in both 3‐ and 24‐h treatments in the second experiment (Appendix B, Table B.1).
2.4.1.3. In vitro micronucleus assays
p‐Mentha‐1,8‐dien‐7‐al (94.9% purity) was assayed for the induction of chromosome damage in mammalian cells in vitro by examining its effect on the frequency of micronuclei in cultured human peripheral blood lymphocytes (whole blood cultures pooled from two healthy male volunteers) treated in the absence and presence of S9‐mix (Lloyd, 2009). p‐Mentha‐1,8‐dien‐7‐al was added at 48 h following culture initiation (stimulation by phytohaemagglutinin) either for 3 h in the absence or presence of S9‐mix followed by 21 h recovery, or for 24 h in the absence of S9‐mix. Cytochalasin B (6 μg/mL) was added either at the start of treatment (24‐h treatment) or at the start of recovery (following 3‐h treatment) in order to block cytokinesis and generate binucleate cells for analysis. It remained in the cultures until they were harvested 24 h after the start of treatment. A range‐finding experiment had been conducted with and without S9‐mix treatment in order to provide toxicity information (reduction in replication index, RI) that could be used as a basis for choosing a range of concentrations to be evaluated in the main micronucleus analysis (Appendix B, Table B.1).
In the main assay, micronuclei were analysed from at least three concentrations for each treatment condition. For 3‐h treatment without S9‐mix, the concentrations were 80, 100, 110 and 120 μg/mL, for 3‐h treatment with S9‐mix the concentrations were 100, 120 and 140 μg/mL, and for 24‐h treatment without S9‐mix the concentrations were 20, 25 and 35 μg/mL. The levels of cytotoxicity (reduction in RI) at the top concentrations reached 58% and 45% in the 3‐h treatment in the absence and presence of S9‐mix, and 58% in the 24‐h treatment in the absence of S9‐mix, respectively. These levels of cytotoxicity therefore reached, or were very close to, the recommended (50–60%) range of cytotoxicity. One thousand binucleate cells per culture from two replicate cultures per concentration were scored for micronuclei. This GLP study complies with OECD Test Guideline 487.
The frequencies of micronucleated binucleate (MNBN) cells in negative control cultures were normal, and were significantly increased by treatment with positive control chemicals. Treatment of cells with p‐mentha‐1,8‐dien‐7‐al in the absence and presence of S9‐mix under all treatment conditions resulted in frequencies of MNBN cells that were similar to and not significantly different from those observed in concurrent vehicle controls for all concentrations analysed. The MNBN cell frequency of all p‐mentha‐1,8‐dien‐7‐al treated cultures fell within (or slightly below) normal ranges. It was concluded that p‐mentha‐1,8‐dien‐7‐al did not induce micronuclei in cultured human peripheral blood lymphocytes when tested at toxic concentrations in both the absence and presence of S9‐mix (Lloyd, 2009).
2.4.2. Previously available data
2.4.2.1. In vitro data
Several in vitro mutagenicity/genotoxicity tests have been performed on the FGE.19 subgroup 2.2 representative substance p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117]. The quality of most of them could not be adequately evaluated, either because they are in Japanese and therefore details are difficult to obtain or because of limitations in the experimental design. Negative results were reported by Ishidate et al. (1984) for an Ames test in which S. Typhimurium strains TA92, TA1535, TA100, TA1537, TA94 and TA98 were used. Duplicate plates were used for each of the six concentrations up to 1,000 μg/plate with S9‐mix. The sample used had the same purity (93.1%) of the batch used by Bowen (2011). The results were only reported as − or + (a + would be given if revertant numbers exceeded 2× concurrent control) and therefore weaker responses may have been observed but cannot be verified. Fujita et al. (1994) also reported negative results for an Ames assay in strains TA97 and TA102 performed both with and without S9‐mix. The top concentration of p‐mentha‐1,8‐dien‐7‐al was less than in the Ishidate study, namely 100 μg/plate. Negative results were reported in mutation tests in which p‐mentha‐1,8‐dien‐7‐al was incubated with Escherichia coli WP2 cells at 50–400 μg/plate (Yoo, 1986). Few details can be obtained from the paper, but it appears that the maximum increase in revertants was 1.3‐fold, which is considered negative. However, only one result was given, so the test was probably only conducted in the absence of S9‐mix.
p‐Mentha‐1,8‐dien‐7‐al was considered to be weakly positive in the rec‐assay with Bacillus subtilis strains M45 and H17 at a concentration of 2.5 μL p‐mentha‐1,8‐dien‐7‐al/disk, probably equivalent to 2,500 μg/disk (Yoo, 1986). This study is a very short paper, with very few details. Another study using the same strains reported negative results for p‐mentha‐1,8‐dien‐7‐al at concentrations between 0.16 and 0.63 μL/plate (corresponding to 0.15 and 0.6 μg/plate) and positive results at higher concentrations of 1.25 and 2.5 μL/plate (1.2 and 2.4 μg/plate) (Kuroda et al., 1984). It should be noted that these DNA damage assays in bacteria do not detect mutation, are non‐standard and not requested by regulatory agencies. The results cannot therefore be considered to carry as much weight as results from recommended, standard assays.
In a study by Eder et al. (1993), p‐mentha‐1,8‐dien‐7‐al gave negative results in a SOS chromotest with genetically engineered E. coli. The maximum induction factor (I max) with p‐mentha‐1,8‐dien‐7‐al was calculated to be 1.0. Positive results are considered to be significant if the I max is at least 1.5. The SOS chromotest is also not a mutation test. It measures induction of the SOS repair system, and this is interpreted as indicating DNA damage. The results cannot therefore be considered to carry as much weight as results from recommended standard assays.
Standard chromosomal aberration (CA) assays for p‐mentha‐1,8‐dien‐7‐al have yielded positive results. In a CA study by Ishidate et al. (1984), Chinese hamster lung (CHL) fibroblasts were only treated in the absence of S9‐mix for 24 or 48 h with a batch of 93.1% purity. There were no treatments in the presence of S9‐mix. Concentrations for the main CA test were selected from a preliminary experiment in which cell density (a crude and subjective measure) on the culture dishes was assessed, but there was no concurrent measure of cytotoxicity in the CA test. Only single cultures of CHL cells were treated with each of three concentrations, and therefore only 100 cells/concentration were scored for CA. CA (including gaps) frequencies of 4.9% or less were considered negative, 5.0–9.9% were equivocal, and 10% or higher were considered positive. p‐Mentha‐1,8‐dien‐7‐al gave a strong positive response (39% cells with CA, and also an increase in polyploid cells to 31%) at 50 μg/mL. In particular, structural CAs were detected at 40 μg/mL at 24 h (20.0%) and at 48 h (28.0%); the strongest effect was observed at 50 μg/mL at 24 h. An increase in polyploidy cells was also detected at 40 μg/mL (15%) and 50 μg/mL (31%) after 48 h. As there was no concurrent measure of cytotoxicity, and the results at the other concentrations tested were not given, these results should be considered with caution; however, they cannot be completely dismissed. In the CA study of Tayama et al. (1990) in CHO‐K1 cells, a significant increase in CA at 150 μg/mL in the absence of S9‐mix was associated with no detectable cell division. This result can probably be dismissed as likely to be an artefact of high levels of cell killing. However, a significant increase in CA at 300 μg/mL in the presence of S9‐mix was associated with 62% proliferating cells, which does not indicate excessive toxicity. Most of the chromosome aberrations were chromatid exchanges. These results are clearly in contrast to the negative micronucleus results obtained in human lymphocytes in the recent GLP study (Lloyd, 2009). The reasons of such discrepancy are unclear.
A sister chromatid exchange (SCE) assay was performed with and without metabolic activation in CHO‐K1 cells at concentrations up to 300 μg p‐mentha‐1,8‐dien‐7‐al/ml (Tayama et al., 1990). Cytotoxicity was determined by the percentage of cells that showed differentially stained chromatids, i.e. had divided. A doubling of SCE/cell would usually be considered biologically relevant, and in the absence of S9‐mix, there was a doubling of SCE/cell at 150 μg/ml, where there was little toxicity, whereas in the presence of S9‐mix, there was a doubling of SCE/cell at all concentrations from 100–300 μg/mL, where there was low or moderate toxicity. However, SCE assays also only provide limited information for assessment of genotoxicity. The mechanism of induction of SCE, and its relevance for mutation and cancer is not understood.
Studies for induction of ouabain resistant mutants conducted in human fetus cells (Rsa) at concentrations of 0, 0.010, 0.015, 0.020 or 0.025 μg/mL gave negative results for p‐mentha‐1,8‐dien‐7‐al at the lowest concentration, positive results (8–16‐fold increases) for concentrations ranging from 0.015 to 0.02 μg/mL (where toxicity was slight to moderate), and showed p‐mentha‐1,8‐dien‐7‐al to be cytotoxic at the highest concentration (Suzuki et al., 1990). In another mutagenicity study with Rsa cells (Suzuki and Suzuki, 1994), induction of ouabain resistance was reported at concentrations above 10 ng p‐mentha‐1,8‐dien‐7‐al/ml with apparent cytotoxicity at 20 ng/mL or higher. Also, in this study, mutagenicity was detected (K‐ras codons) at concentrations of 2–200 ng/mL. Human fetal (Rsa) cells are not routinely used for genotoxicity testing, so evaluation of the quality of the data is difficult. The concentrations used in these tests are much lower than in other mammalian cell tests, and possible reasons for the discrepancy are not clear. Sasaki et al. (1990) tested p‐mentha‐1,8‐dien‐7‐al for induction of ouabain‐resistant mutants in CHO‐K1 cells. The mutant frequency at the only concentration of p‐mentha‐1,8‐dien‐7‐al tested (10 μg/mL, which reduced survival to 83.5% of controls) appears to be low (0.7 mutants per 106 cells, compared to zero in controls) and the result would probably be considered negative. The study of ouabain resistance in all of these studies makes interpretation difficult. Ouabain resistance is generally considered not to be a sensitive mutagenic target (spontaneous frequencies very low; frame‐shift mutations not detected), and it is difficult to conclude negative results when there is a zero incidence of effects in controls. The biological significance of large increase in ouabain resistant mutants at very low concentrations is equally difficult to interpret. This endpoint is no longer used in regulatory testing.
The in vitro studies described above are listed in Appendix B, Table B.1.
2.4.2.2. In vivo data
In vivo mutagenicity/genotoxicity testing has been performed on the FGE.19 subgroup 2.2 representative substance p‐mentha‐1,8‐dien‐7‐al (Appendix B, Table B.2). Eight‐week‐old male ddY mice were administered a single intraperitoneal injection of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] at doses of 75, 150, 300 or 600 mg/kg body weight (bw) for a mouse micronucleus assay (six mice/group). The dosing regimen and the maximum dose were based on a pilot experiment with two mice/group. In the main experiment, after 24 h, the mice were killed and femoral bone marrow cells were collected, fixed and stained with Giemsa. One thousand polychromatic erythrocytes (PCE) were scored per mouse. No indication of micronucleus induction was reported at any dose level (Hayashi et al., 1988). However, the study does not comply with current guidelines, because, after a single administration, groups of animals should be sacrificed 24 and 48 h later. Also, only 1,000 PCE were scored per animal, whereas the current recommendation is for 2,000 PCE/animal.
Table B.2.
Summary of in vivo Genotoxicity Data Evaluated by the Panel in FGE.208
| Chemical Name FL‐no | Test System in vivo | Test Object | Route | Dose | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
|
p‐Mentha‐1,8‐dien‐7‐al [05.117] |
Micronucleus Assay | Mouse bone marrow cells | Intraperitoneal | 75, 150, 300 or 600 mg/kg bw | Negative | Hayashi et al. (1988) | Unreliable; sampling time only at 24 h; only 1,000 PCE per animal scored; poor presentation of data |
bw: body weight; PCE: polychromatic erythrocytes.
2.4.3. Discussion
EFFA has submitted three valid, new in vitro studies, one in bacteria (Ames test) and two in mammalian cells (micronucleus (MN) in human lymphocytes and HPRT in mouse lymphoma cells). The Ames test resulted positive, in the absence of metabolic activation with strain TA98, able to detect gene mutations of frameshift type (insertions/deletions). Equivocal results were reported in the HPRT assay (negative according to the authors) and negative results were reported in the MN test. Equivocal or negative results in the HPRT assay cannot dismiss the positive findings in the new Ames test, positive in the TA98 strain. The different results may be due to a different sensitivity of the two tests to detect frameshift mutations. In this respect, the Panel noted that the molecular analysis of mutational spectra at the hprt locus show a prevalence of GC to AT transitions and AT to CG transversions among spontaneous mutants, with less than 10% of frameshifts (Chen et al., 2002). Thus, given the prevailing contribution of mutations different from frameshift to the baseline incidence of hprt mutant colonies, it is expected that a many‐fold increase in frameshift mutations is needed to give raise to an overall increase in mutation frequency which is detectable and significant on statistical grounds. The Ames test is generally considered as the most sensitive in vitro test for the prediction of genotoxic carcinogens and ‘false positive results’ are rare; in this case, the positivity in the TA98 cannot be considered as a ‘false positive’ without any explanation.
Negativity in mammalian cells ‘per se’ cannot be considered more relevant than positivity in bacteria, simply on the basis of the complexity of cells. Among the previously supplied data, several in vitro and one in vivo mutagenicity/genotoxicity published studies are available. For most of them, performed not in compliance with current guidelines, the quality of data was limited. Negative results were reported in a study with the Ames test; however, the results were only reported as + or −, and therefore could not be verified. Both positive and negative results were reported for induction of ouabain gene mutations in mammalian cells, in limited studies. Ouabain resistance is generally considered of low sensitivity, compared with other gene mutation assays and is unable to detect mutations of frameshift type; it is no longer routinely used for regulatory purposes. Strong clastogenic effects in the absence of S9‐mix were reported in Chinese hamster cell lines in two papers. Notwithstanding some limitations of the study, these positive results cannot be completely dismissed by the negative results in the new in vitro MN assay. The different types of cells used (Chinese hamster cell lines and human lymphocytes) and the different concentrations used can only partially explain the different results, which remain unclear. Negative results were reported in a mouse MN assay, in a study of limited validity for inadequate experimental design and insufficient presentation of data. Other published results, both positive and negative for DNA‐damage/repair (rec‐assay) in bacteria, negative for SOS and positive for SCE in mammalian cells, are not considered as relevant for the assessment of the genotoxic potential of p‐mentha‐1,8‐dien‐7‐al.
2.4.4. Conclusion
Overall, the presently available data raise some concern for the genotoxic potential of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117]. In order to clarify the genotoxic potential of this substance, the Panel considered that further in vivo testing should be performed. To address this, an in vivo Comet assay, considering the first site of contact (e.g. stomach or duodenum) and liver, should be carried out according to the Scientific Report of EFSA on Minimum Criteria for the acceptance of in vivo alkaline Comet Assay Reports (EFSA, 2012).
2.5. Additional genotoxicity data evaluated by the Panel in FGE.208Rev18
In response to the EFSA request, in FGE.208, to provide in vivo genotoxicity data for the representative substance p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117], industry has submitted a combined in vivo bone marrow micronucleus test and Comet assay with scoring in the liver and duodenum (Appendix C, Table C.1).
Table C.1.
Summary of Additionally In Vivo Genotoxicity Data Submitted for FGE.208Rev1
| Chemical Name FL‐no | Test System in vivo | Test Object | Route | Dose | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
|
p‐Mentha‐1,8‐dien‐7‐al [05.117] |
Micronucleus Assay | Male Han Wistar rats | Gavage | 175, 350 and 700 mg/kg bw per day | Negative | Beevers (2014a,b) | Reliable with restriction. Complies with GLP and mainly with OECD Test Guideline 474 (not clear if the bone marrow was exposed) |
| Comet assay | Male Han Wistar rats | Gavage | Positive | Reliable without restriction. Complies with GLP. The study was performed shortly before publication of OECD Test Guideline 489; however, it is consistent with this guideline. Positive in liver, negative in duodenum |
bw: body weight; GLP: Good Laboratory Practice; OECD: Organisation for Economic Co‐operation and Development.
p‐Mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] (purity 94.2%) was tested for its ability to induce micronuclei in the PCE of the bone marrow of treated rats and the potential to induce DNA damage in the liver and duodenum of the same animals in a combined in vivo micronucleus and Comet assay (Beevers, 2014a,b).
Based on results from a range finding study, where no substantial inter‐sex differences in toxicity were observed in rats, a dose of 700 mg/kg bw per day was considered as the maximum tolerated dose (MTD). Groups of six male out‐bred Han Wistar rats were administered doses of 175, 350 and 700 mg/kg bw of p‐mentha‐1,8‐dien‐7‐al by oral gavage at time 0, 24 and 45 h. All doses were administered at a dose volume of 10 mL/kg. Rats were sacrificed and sampled at 48 h post the initial dose. Negative (corn oil) and positive control groups (ethyl methanesulfonate (EMS) 150 mg/kg, dosed at 0, 24 and 45 h) were included in the main study.
Clinical signs of toxicity were limited to animals dosed at 700 mg/kg bw per day, where reduced levels of activity were observed in five out of six animals dosed with p‐mentha‐1,8‐dien‐7‐al. In addition, one animal displayed symptoms of ataxia and one animal had piloerection. Dose‐related decreases in body weight gain, or weight loss were observed at all dose levels. No clinical signs of toxicity were seen in the vehicle or the positive control (EMS).
During clinical chemistry assessment of blood samples, it was noted that a high number of samples were lipaemic. This was attributed to the corn oil used as a vehicle control and for test article formulation, which was administered just 3 h prior to blood sampling. As a consequence, many samples were deemed unsuitable for the analysis of certain parameters and the data were interpreted with caution. There was a slight increase in aspartate aminotransferase and alanine aminotransferase at the 700 mg/kg bw per day dose.
The anatomical pathology examination showed that there were no gross lesions in tissues of exposed animals related to administration of p‐mentha‐1,8‐dien‐7‐al; however, histopathology revealed hepatocyte vacuolation at the dose of 700 mg/kg bw per day.
In line with the requirement of the OECD test guideline 474 (OECD, 1997c), the plasma samples were collected. However, analysis of these samples was not conducted since in this case, this is not relevant for the interpretation of the study.
2.5.1. Micronucleus assay
An in vitro micronucleus assay in human peripheral blood lymphocytes (Lloyd, 2009) was evaluated by the Panel as negative in FGE.208. Although not requested, the applicant has submitted an in vivo micronucleus assay in bone marrow of rats (Beevers, 2014a). In this in vivo study, the proportion of immature among total (immature + mature) erythrocytes was determined for each animal by counting a total of at least 500 cells and then at least 2,000 immature erythrocytes per animal were scored for the incidence of micronucleated polychromatic erythrocytes (MNPCE). Rats treated with p‐mentha‐1,8‐dien‐7‐al exhibited %PCE values that were similar to the concurrent vehicle control group and which were within the laboratory′s historical negative control data, thus indicating that the test substance was not toxic to the bone marrow. Rats treated with p‐mentha‐1,8‐dien‐7‐al exhibited group mean frequencies of MNPCE that were similar to and not statistically different (chi‐square calculation) from those observed in concurrent vehicle controls for all dose groups and were also within the historical control values (Beevers, 2014a).
The Panel concluded that in this study p‐mentha‐1,8‐dien‐7‐al did not induce micronucleated erythrocytes in rat bone marrow cells following administration by oral gavage at the test conditions performed. There was no indication that the test substance reached the target organ. Negative results were observed in an in vitro micronucleus test (Lloyd, 2009). Therefore, there is no need to validate the negative result of the in vivo micronucleus assay and to investigate the target tissue exposure.
2.5.2. Comet assay
2.5.2.1. Duodenum analysis
There was no dose‐related increase in % clouds in duodenum cells following treatment with p‐mentha‐1,8‐dien‐7‐al, thus demonstrating that treatment did not cause excessive DNA damage that could have interfered with Comet analysis. Measurements of tail intensity (% DNA in tail) and tail moment were obtained from 150 cells/animal.
Group mean tail intensity and tail moment values for all groups of animals treated with p‐mentha‐1,8‐dien‐7‐al at 175, 350 and 700 mg/kg bw per day were comparable with the group mean vehicle control data. There were no marked differences in tail intensity between the treated and control groups. All individual animal data at all dose levels were consistent with the vehicle control animal data (Beevers, 2014b).
The Panel concluded that p‐mentha‐1,8‐dien‐7‐al did not induce DNA damage in the duodenum of treated male rats under the test conditions performed.
2.5.2.2. Liver analysis
There was no dose‐related increase in % clouds or % cells with halos in liver cells following treatment with p‐mentha‐1,8‐dien‐7‐al, thus demonstrating that treatment did not cause excessive DNA damage that could have interfered with Comet analysis. However, clinical chemistry of blood showed a slight increase in aspartate aminotransferase and alanine aminotransferase at the highest dose tested, indicating that the liver was exposed to the test substance.
Measurements of tail intensity (% DNA in tail) and tail moment were obtained from 150 cells/animal.
Group mean % tail intensity and tail moment values for animals treated with p‐mentha‐1,8‐dien‐7‐al at the low and medium dose (175 and 350 mg/kg bw per day, respectively) were comparable with the group mean vehicle control data and there were no statistically significant differences in % tail intensity between treated and control groups. In groups treated with the low and medium dose, all individual animal data were consistent with the values of the vehicle control animals and fell within the laboratory's historical control data.
At the highest dose (animals exposed to 700 mg/kg bw per day), a 3.4‐fold and statistically significant increase in tail intensity was observed. A statistically significant linear trend was also apparent. Five out of the six animals treated with the highest dose had tail intensities that exceeded the values observed in the concurrent vehicle control animals, however, the tail intensity values for all animals fell within the laboratory's historical vehicle control values (Beevers, 2014a).
The Panel noted that the range for both the negative and positive historical control values were extremely wide for this test laboratory. In addition, there was an overlap of the negative (95% range: 0.02–11.39) and positive (95% range: 7.15–65.07) control values.
The Comet arm of this study indicates that p‐mentha‐1,8‐dien‐7‐al induces DNA damage in liver.
2.5.3. Conclusion
The data submitted by the applicant were considered to be in accordance with the data requested by the Panel in FGE.208. Industry submitted a Comet assay on the liver and duodenum, and in addition (although not requested), a micronucleus assay in the bone marrow of the same animals (combined bone marrow micronucleus test and Comet assay).
p‐Mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] did not induce any increase in micronucleated PCE of the bone marrow of male rats following oral gavage administration up to 700 mg/kg bw per day (an estimate of the MTD for this study). There was no indication in the study that the test substance reached the bone marrow. Negative results were observed in an in vitro micronucleus assay on human peripheral blood lymphocytes performed according to OECD test guideline 487. Therefore, there is no need to validate the negative result of the in vivo micronucleus assay and to investigate the target tissue exposure.
p‐Mentha‐1,8‐dien‐7‐al did not induce DNA damage in the duodenum of the same animals as analysed by the Comet assay.
In the same animals, a statistically significant increase in DNA strand breaks was observed in the liver at the highest tested dose (700 mg/kg bw per day).The observed values for tail intensity (2.20 ± 0.6) and tail moment (0.24 ± 0.07) fell within the test laboratories historical vehicle control range values for tail intensity (0.02–11.39) and tail moment (0.01–1.45); however, five of the six high‐dose animals had tail intensities that exceeded the values of the concurrent vehicle control animals.
The Panel noted that the results observed at the highest dose were more than threefold higher than the concurrent negative control value and statistically significant different from the negative control value. In addition, a statistically significant positive linear trend was observed. The Panel considered that, since there was a wide range of historical control data with an overlap of the positive and negative historical control values, the historical control data could not be used as a criterion to interpret the data.
Overall, the Panel concluded that p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is genotoxic in vivo and that, accordingly, there is a safety concern for the use of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] as a flavouring substance.
Since p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] is representative for the nine remaining substances of this subgroup 2.2 (p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], 2,6,6‐trimethyl‐1‐cyclohexen‐1‐carboxaldehyde [FL‐no: 05.121], myrtenyl formate [FL‐no: 09.272], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278], myrtenyl acetate [FL‐no: 09.302], myrtenyl‐2‐methylbutyrate [FL‐no: 09.899] and myrtenyl‐3‐methylbutyrate [FL‐no: 09.900]), there is a potential safety concern for these substances.
3. Assessment
3.1. Additional genotoxicity data evaluated by the Panel in FGE.208Rev2
The applicant has submitted in vitro genotoxicity studies for p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], myrtenal [FL‐no: 05.106], myrtenyl acetate [FL‐no: 09.302] and p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] listed in Table 3. These studies are evaluated in the present revision of FGE.208 (FGE.208Rev2). A summary of results is reported in Appendix D, Table D.1.
Table 3.
List of in vitro genotoxicity studies evaluated in FGE.208Rev2
| Substance name | FL‐no | Study |
|---|---|---|
| p‐Mentha‐1,8‐dien‐7‐ol | 02.060 | Bacterial reverse mutation assay (Wagner, 2016) |
| Micronucleus assay in human peripheral blood lymphocytes (Roy, 2016) | ||
| Myrtenol | 02.091 | Bacterial reverse mutation assay (Bhalli and Phil, 2015a) |
| Micronucleus assay in human peripheral blood lymphocytes (Bhalli and Phil, 2015b) | ||
| BlueScreen™ HC assay (Birrell, 2013a) | ||
| Myrtenal | 05.106 | Bacterial reverse mutation assay (Mc Garry, 2016a) |
| Micronucleus assay in human peripheral blood lymphocytes (Mc Garry, 2016b; Lloyd, 2017) | ||
| p‐Mentha‐1,8‐dien‐7‐yl acetate | 09.278 | Bacterial reverse mutation assay (Lloyd, 2016a) |
| Micronucleus assay in human peripheral blood lymphocytes (Lloyd, 2016b) | ||
| Myrtenyl acetate | 09.302 | Bacterial reverse mutation assay (Mc Garry, 2016c) |
| Micronucleus assay in human peripheral blood lymphocytes (Mc Garry, 2016d) | ||
| BlueScreen™ HC assay (Birrell, 2013b) |
Table D.1.
Summary of in vitro genotoxicity data evaluated in FGE.208Rev2
| Chemical name [FL‐no] | Test system | Test object | Concentration | Results | Reference | Comments |
|---|---|---|---|---|---|---|
| p‐Mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060] | Bacterial reverse mutation assay |
Salmonella Typhimurium TA98, TA100, TA1535, TA1537 Escherichia coli WP2uvrA |
10, 33.3, 100, 333, 1,000, 3,333 μg/platea , b | Negativea , b | Wagner (2016) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 471. Toxicity at concentrations ≥ 3,333 μg/plate |
| 1, 3.33, 10, 33.3, 100, 333, 1,000, 3,333 μg/platea , f | Negativea , f | Toxicity at concentrations ≥ 100 or 333 μg/plate | ||||
| Micronucleus assay | Human peripheral blood lymphocytes | 25, 50 and 100 μg/mLc | Negative | Roy (2016) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 487 | |
| 100, 250 and 325 μg/mLg | ||||||
| 100, 25, 275 μg/mLh | ||||||
| Myrtenol [02.091] | Bacterial reverse mutation assay | S. Typhimurium TA98, TA100, TA1535, TA1537 | 5, 16, 50, 160, 500, 1,600, 5,000 μg/platea , b | Negativea , b | Bhalli and Phil (2015a) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 471 |
| E. coli WP2uvrA | 5, 16, 50, 160, 500, 1,600, 5,000 μg/platea , b | Negativea , b | ||||
| S. Typhimurium TA98, TA100, TA1535, TA1537 | 16, 50, 160, 500, 1,600, 5,000 μg/plate | Negativea , b | ||||
| E. coli WP2uvrA | 16, 50, 160, 500, 1,600, 5,000 μg/plate | Negativea , b | ||||
| BluScreen™ HC | Human lymphoblastoid TK6 cells | 9.77, 19.53, 39.06, 78.13, 156.25, 312.50, 625, 1,250 μM | Negativea | Birrell (2013a) | The reliability was not evaluated since this assay does not belong to the assays recommended by the Scientific Committee for regulatory purposes (EFSA 2011) | |
| Micronucleus assay | Human peripheral blood lymphocytes |
30.6, 47.2 and 52.5 μg/mLc 368, 387, 451 and 475 μg/mLd 407, 451 and 475 μg/mLe |
Negativec , d , e | Bhalli and Phil (2015b) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 487 | |
| Myrtenal [05.106] | Bacterial reverse mutation assay | S. Typhimurium TA98, TA100, TA102, TA1535, TA1537 | 5, 16, 50, 160, 500, 1,600, and 5,000 μg/platea , b | Negativea , b | Mc Garry (2016c) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 471 |
| 80, 160, 300, 625, 1,250, 2,500 and 5,000 μg/plate | Negativea , b , f | |||||
| Micronucleus assay | Human peripheral blood lymphocytes |
15, 25 and 34 μg/mLc 50, 130 and 180 μg/mLd 25, 200 and 350 μg/mLe |
Equivocalc , d , e | Mc Garry (2016d) | Reliable with restriction. GLP study in compliance with OECD Test Guideline 487 | |
| Micronucleus assay | Human peripheral blood lymphocytes | Equivocalc , d , e , i | Lloyd (2017) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 487 | ||
| p‐Mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] | Bacterial reverse mutation assay | S. Typhimurium TA98, TA100, TA102, TA1535, TA1537 | 5, 16, 50, 160, 500, 1,600, and 5,000 μg/platea , b | Negativea , b | Lloyd (2016a) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 471 |
| 1.6, 5, 16, 50, 160, 500 and 1,600 μg/platea , b , f | Negativea , b , f | |||||
| Micronucleus assay | Human peripheral blood lymphocytes |
30, 60 and 80 μg/mLc 100, 120 and 130 μg/mLd 150, 240 and 280 μg/mLe |
Negativec , d , e | Lloyd (2016b) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 487 | |
| Myrtenyl acetate [09.302] | Bacterial reverse mutation assay | S. Typhimurium TA98, TA100, TA102, TA1535, TA1537 | 5, 16, 50, 160, 500, 1,600 and 5,000 μg/platea , b | Negativea , b | Mc Garry (2016a) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 471 |
| 3.28, 8.2, 20.5, 51.2, 128, 320, 800 and 2,000 μg/plate | Negativea , b , f | |||||
| BluScreen™ HC | Human lymphoblastoid TK6 cells | 4.88, 9.77, 19.53, 39.06, 78.13, 156.25, 312.50, 625 μM | Negativea | Birrell (2013b) | The reliability was not evaluated since this assay does not belong to the assays recommended by the Scientific Committee for regulatory purposes (EFSA, 2011) | |
| Micronucleus assay | Human peripheral blood lymphocytes |
10, 20 and 40 μg/mLc 20, 60, 80 and 90 μg/mLd 150, 250 and 325 μg/mLe |
Negativec , d , f | Mc Garry (2016b) | Reliable without restriction. GLP study in compliance with OECD Test Guideline 487 |
GLP: Good Laboratory Practice; OECD: Organisation for Economic Co‐operation and Development.
Assay performed with and without metabolic activation.
Plate incorporation method.
24 h treatment without metabolic activation, with no recovery.
3 h treatment without metabolic activation, with 21 h recovery.
3 h treatment with metabolic activation, with 21 h recovery.
Pre‐incubation method applied in the presence of metabolic activation.
4 h treatment without metabolic activation, with 20 h recovery.
4 h treatment with metabolic activation, with 20 h recovery.
24 h treatment, without metabolic activation, with 24 h recovery.
3.1.1. p‐Mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060]
3.1.1.1. Reverse bacterial mutation assay
In order to investigate the potential of p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060] (purity ≥ 90.3%) and/or its metabolites to induce gene mutations in bacteria, an Ames test was performed according to OECD Test Guideline 471 (OECD, 1997a) and following GLP in four strains of S. Typhimurium (TA98, TA100, TA1535 and TA1537) and E. coli WP2uvrA, in the presence or absence of metabolic activation in two separate experiments. The test article was evaluated in the initial mutagenicity assay at concentrations of 10, 33.3, 100, 333, 1,000 and 3,333 μg/plate with and without S9‐mix, applying the plate incorporation method. Toxicity was observed at 3,333 μg/plate both in the presence and absence of S9‐mix in most of the strains, except TA100 and TA1535, showing slightly reduced background at ≥ 1,000 μg/plate, with and without S9‐mix. In the confirmatory assay, p‐mentha‐1,8‐dien‐7‐ol was tested at concentrations of 1, 3.33, 10, 33.3, 100, 333, 1,000 and 3,333 μg/plate with and without S9‐mix, applying the pre‐incubation method. Toxicity was observed at concentrations ≥ 333 μg/plate without S9 activation and at concentrations ≥ 1,000 μg/plate in the presence of S9 activation. No precipitate was observed at any tested concentration in any tester strain with or without S9‐mix. Appropriate positive control chemicals and dimethyl sulfoxide (DMSO, as vehicle control) were evaluated concurrently and all test and control articles were evaluated in triplicate plates. All positive control chemicals induced significant increases in revertant colony numbers, confirming the sensitivity of the tests and the efficacy of the S9‐mix, while negative controls were within the historical control ranges. No increase in the mean number of revertant colonies was observed at any tested concentration in any tester strains with or without S9‐mix (Wagner, 2016).
The Panel considered the results of this assay as negative.
3.1.1.2. In vitro micronucleus assay
The in vitro micronucleus assay was carried out according to OECD Test Guideline 487 (OECD, 2014) and following GLP. Human peripheral blood lymphocytes from healthy donors, stimulated with phytohaemagglutinin (PHA), were treated with p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060] (purity ≥ 90.3%) (Roy, 2016) in a dose‐range finding assay performed at concentrations ranging from 1 to 1,520 μg/mL for 4 h with and without S9‐mix and 24 h without S9‐mix. At the termination of the treatment period, precipitate and hemolysis were observed at concentrations ≥ 1,000 μg/mL and ≥ 400 μg/mL, respectively, in all three treatment conditions.
Based on the dose‐range finding results, duplicate cultures of lymphocytes were treated with the test article 44–48 h after culture initiation at concentrations ranging from 100 to 375 μg/mL for 4 h with and without S9‐mix.
Cytochalasin B (final concentration of 6 μg/mL) was added to each culture after the 4‐h treatment period, while in the 24‐h treatment cultures were treated with the test article in the presence of cytochalasin B.
Appropriate vehicle (DMSO) and positive controls were used (mitomycin C and vinblastine in the absence of S9‐mix, cyclophosphamide in the presence of S9‐mix). All positive control compounds induced a statistically significant increase of MN frequency and the system was considered sensitive and valid.
Two thousand cells were scored per concentration. Based on the level of cytotoxicity observed, three concentration levels were selected for MN analysis in each experimental condition: i) 25, 50 and 100 μg/mL, 24 h treatment (16%, 31% and 58% cytotoxicity, respectively); ii) 100, 250 and 325 μg/mL, 4 h treatment without S9‐mix (16%, 24% and 58% cytotoxicity, respectively); iii) 100, 225 and 275 μg/mL, 4 h treatment with S9‐mix (3%, 18% and 51% cytotoxicity, respectively). No statistically significant increase in the frequency of micronuclei was observed after treatment with the test article at any concentration analysed (Roy, 2016).
The Panel considered the results of this assay as negative.
3.1.2. Myrtenol [FL‐no: 02.091]
3.1.2.1. Reverse bacterial mutation assay
In order to investigate the potential of myrtenol (purity ≥ 97%) and/or its metabolites to induce gene mutations in bacteria, an Ames test was performed according to OECD Test Guideline 471 (OECD, 1997a) and following GLP in four strains of S. Typhimurium (TA98, TA100, TA1535 and TA1537) and E. coli WP2uvrA, in the presence or absence of metabolic activation applying the plate incorporation method. The test article was evaluated in the initial mutagenicity assay at concentrations of 5, 16, 50, 160, 500, 1,600 and 5,000 μg/plate with and without S9‐mix. A confirmatory assay was subsequently performed at concentrations of 16, 50, 160, 500, 1,600 and 5,000 μg/plate with and without S9‐mix. Appropriate positive control chemicals and DMSO (as vehicle control) were evaluated concurrently, and all test and control articles were evaluated in triplicate plates. All positive control chemicals induced significant increases in revertant colony numbers, confirming the sensitivity of the tests and the efficacy of the S9‐mix, while negative controls were within the historical control ranges. No precipitate was observed at any tested concentration in any tester strain with or without S9‐mix. Toxicity, as evident by the absence or reduction in the mean number of revertant colonies and the absence or reduction in the background bacterial lawn, was observed in both experiments at 5,000 μg/plate in all tester strains with and without S9‐mix, except WP2uvrA, where toxicity was observed at concentrations ≥ 1,600 μg/plate without S9‐mix. No increase in the mean number of revertant colonies was observed at any tested concentration in any tester strains with or without S9‐mix (Bhalli and Phil, 2015a).
The Panel considered the results of this assay as negative.
3.1.2.2. In vitro micronucleus assay
The in vitro micronucleus assay was carried out according to OECD Test Guideline 487 (OECD, 2010) and following GLP. Human peripheral blood lymphocytes from healthy donors, stimulated with PHA, were treated with myrtenol (purity ≥ 97%) in a dose‐range finding assay performed in single cultures at concentrations ranging from 28.2 to 1,000 μg/mL for 3 h with and without S9‐mix and 24 h without S9‐mix. No precipitate was observed at the end of treatment and/or harvest at any tested concentration in any treatment condition. In the 3‐h treatment, haemolysis was observed at 1,000 μg/mL at the end of treatment.
Based on the dose‐range finding results, duplicate cultures of lymphocytes were treated with the test article 48 h after culture initiation at concentrations ranging from 15.3 to 80.0 μg/mL in the 24‐h treatment. The test article was also evaluated in the 3‐h treatment at 224–500 μg/mL with and without S9‐mix.
Cytochalasin B (final concentration of 6 μg/mL) was added to each culture after the 3‐h treatment period, while in the 24‐h treatment cultures were treated with the test article in the presence of cytochalasin B. Appropriate vehicle (DMSO) and positive controls were used (mitomycin C in the absence of S9‐mix, cyclophosphamide in the presence of S9‐mix). All positive control compounds induced a statistically significant increase of MN frequency and the system was considered sensitive and valid.
Two thousand cells were scored per concentration. Based on the level of cytotoxicity observed, at least three concentration levels were selected for MN analysis in each experimental condition: i) 30.6, 47.2 and 52.5 μg/mL with the 24‐h treatment (26%, 41% and 54% cytotoxicity, respectively); ii) 407, 451 and 475 μg/mL with the 3‐h treatment with S9‐mix (15%, 34% and 46% cytotoxicity, respectively); iii) 368, 387, 451 and 475 μg/mL with the 3‐h treatment without S9‐mix (19%, 35%, 43% and 64% cytotoxicity, respectively). No statistically significant increase in the frequency of micronuclei was observed after treatment with the test article at any concentration analysed compared to the respective concurrent vehicle controls (Bhalli and Phil, 2015b).
The Panel considered the results of this assay as negative.
3.1.2.3. BlueScreen™ HC assay
Myrtenol [FL‐no: 02.091] was tested in a BlueScreen™ HC assay for cytotoxicity and genotoxicity using a genetically modified strain of cultured human lymphoblastoid TK6 cells, both in the presence and absence of metabolic activation. The study authors concluded that myrtenol did not induce genotoxicity at the concentrations tested (Birrell, 2013a).
3.1.3. Myrtenal [FL‐no: 05.106]
3.1.3.1. Reverse bacterial mutation assay
In order to investigate the potential of myrtenal (purity 97.7%) and/or its metabolites to induce gene mutations in bacteria, an Ames test was performed according to OECD Test Guideline 471 (OECD, 1997a) and following GLP in five strains of S. Typhimurium (TA98, TA100, TA1535, TA1537 and TA102), in the presence or absence of metabolic activation, in two separate experiments. In the first experiment, myrtenal was tested at concentrations of 5, 16, 50, 160, 500, 1,600 and 5,000 μg/plate with and without S9‐mix, applying the plate incorporation assay. In the second experiment, myrtenal was tested at concentrations of 80, 160, 300, 625, 1,250, 2,500 and 5,000 μg/plate with and without S9‐mix, applying the pre‐incubation method. Appropriate positive control chemicals and DMSO (as vehicle control) were evaluated concurrently. All test and positive control articles were evaluated in triplicate plates; the vehicle control was evaluated in quintuplicate.
All positive control chemicals induced significant increases in revertant colony numbers, confirming the sensitivity of the tests and the efficacy of the S9‐mix, while negative controls were within the historical control ranges.
No precipitate was observed at any tested concentration in any tester strain with or without S9‐mix.
In the first experiment, toxicity, as evident by the absence or reduction in the mean number of revertant colonies and the absence or reduction in the background bacterial lawn, was observed at 1,600 and/or 5,000 μg/plate in all tester strains in the absence and in the presence of S9‐mix.
In the second experiment, toxicity was observed at concentrations of 1,250 and/or 2,500 μg/plate and above in all strains in the absence of S9‐mix. Toxicity was observed at 300 and/or 625 μg/plate and above for all strains in the presence of S9‐mix.
No increase in the mean number of revertant colonies was observed at any tested concentration in any tester strains with or without S9‐mix (Mc Garry, 2016c).
The Panel considered the results of this assay as negative.
3.1.3.2. In vitro micronucleus assay (Mc Garry, 2016b)
The in vitro micronucleus assay was carried out according to OECD Test Guideline 487 (OECD, 2014) and following GLP. Human peripheral blood lymphocytes from healthy donors, stimulated with PHA, were treated with myrtenal (purity 97.7%). Based on the level of cytotoxicity observed in a preliminary dose‐range finding assay, at least three concentration levels were selected for MN analysis in each experimental condition: i) for the 24‐h treatment with no recovery period (24 + 0 h), the concentrations of 15, 25 and 34 μg/mL (0, 25% and 55% cytotoxicity, respectively) were selected; ii) for the 3‐h treatment with 21 h recovery period (3 + 21 h) with S9‐mix, the concentrations of 25, 200 and 350 μg/mL (0, 26% and 53% cytotoxicity, respectively) were selected; iii) for the 3‐h treatment with 21 h recovery period (3 + 21 h) without S9‐mix, the concentrations of 50, 130 and 180 μg/mL (8%, 25% and 51% cytotoxicity, respectively) were selected. In the treatment of 3 + 21 h with S9‐mix, precipitate was observed at 350 μg/mL.
Cytochalasin B (final concentration of 6 μg/mL) was added to each culture after the 3‐h treatment period, while in the 24‐h treatment cultures were treated with the test article in the presence of cytochalasin B. Appropriate vehicle (DMSO) and positive controls were used (mitomycin C and noscapine in the absence of S9‐mix, cyclophosphamide in the presence of S9‐mix). Two thousand cells were scored per concentration.
In the absence of S9‐mix, the positive control compounds induced a statistically significant increase of MN frequency and the authors of the study report considered the system as sensitive and valid. However, the Panel noted that the positive control cyclophosphamide used for the experiment performed in the presence of S9‐mix resulted in a mean frequency of 1.50% MNBN cells (1.60% on slide A and 1.40% on slide B). The authors of the study report considered that both replicate cultures demonstrated MNBN cell frequencies that were statistically significantly different from the concurrent vehicle control and clearly exceeded the normal range of negative control data. The Panel, however, noted that the effects observed with the two replicate cultures of the positive control did not clearly exceed the normal range (observed range 0.00–1.40%, 95th percentile range 0.10–0.90%) which raises some concern about the validity of the study. In addition, the Panel observed that the historical negative control data given in the study report were from August 2012 to August 2013, while the experiments were performed from October to December 2015. The Panel considered that historical control data covering the time preceding the current experiments would be more appropriate and noted that historical data for positive control substances were not reported.
After short treatment (3 + 21 h) in the presence of S9‐mix at 350 μg/mL of myrtenal, an elevated MNBN cells frequency (4.9%) was observed in one replicate culture. According to the study authors, bacterial contamination was reported on this slide, which may have affected the frequency of MNBN cells and, thus, the slide was excluded from the analysis. The Panel, however, noted that a bacterial contamination in only one slide from the same culture was unlikely. In addition, the MNBN cell frequency in a vehicle replicate culture fell outside the historical negative control range.
After continuous treatment (24 + 0 h) without metabolic activation at 15 μg/mL of myrtenal, a statistically significant increase in the frequency of micronuclei was observed but not at 25 and 34 μg/mL. The MNBN cell frequency of one replicate culture (1.8%) was outside the historical negative control range (95% range from 0.1% to 1.5%). Based on data on cytotoxicity and MNBN cells, the Panel noted that a cell cycle delay influencing the appearance of MNBN cells might have occurred.
In any other treatment conditions and concentrations analysed, myrtenal did not induce a statistically significant increase of MNBN cells.
As described above, the Panel noted that the in vitro micronucleus assay presented some limitations; therefore, it was requested to repeat the study changing experimental conditions and concentrations tested (in particular for the continuous treatment (24 h) in the absence of metabolic activation, to treat cells for 24 h with no recovery period (24 + 0 h) and for 24 h with 24 h recovery period (24 + 24 h); the concentrations analysed should include a lower range with narrower spacing). Appropriate historical negative and positive control data should also be included in the study report. Following the request from the Panel, industry submitted new data on historical controls. However, also considering these data, the outcome of this study is still equivocal. In addition, industry submitted a new in vitro MN assay (Lloyd, 2017) that is described below.
3.1.3.3. In vitro micronucleus assay (Lloyd, 2017)
The in vitro micronucleus assay was carried out according to OECD Test Guideline 487 (OECD, 2014) and following GLP. Whole blood cultures from healthy donors were treated with myrtenal (purity 98.1%) 48 h after culture initiation following two experimental conditions: a short treatment with and without S9‐mix (3 + 21 h recovery) and a continuous treatment without S9‐mix (24 + 0 h and 24 + 24 h recovery). Cytochalasin B (final concentration of 6 μg/mL) was added to each culture after treatment periods, while in the 24‐h treatment without recovery cultures were treated with the test article in the presence of cytochalasin B (Lloyd, 2017). Appropriate vehicle (DMSO) and positive controls were used (mitomycin C, noscapine and vinblastine in the absence of S9‐mix, in the short and continuous treatments (24 + 0 h and 24 + 24 h), respectively; cyclophosphamide in the short treatment in the presence of S9‐mix). All positive control compounds induced a statistically significant increase of MN frequency and the system was considered sensitive and valid. Two thousand cells were scored per concentration and, at least, three concentration levels were selected for MN analysis in each experimental condition: i) 100, 160 and 200 μg/ml at 3 + 21 h treatment without S9‐mix (10, 34 and 52% cytotoxicity, respectively); ii) 100, 200, 300 and 350 μg/mL at 3 + 21 h treatment with S9‐mix (4%, 17%, 31% and 54% cytotoxicity, respectively;); iii) 10, 20, 30 and 32 μg/mL at 24 + 0 h treatment without S9‐mix (0, 15%, 48% and 60% cytotoxicity, respectively); iv) 30, 45, 65 and 75 μg/ml at 24 + 24 h treatment without S9‐mix (9%, 29%, 35% and 50% cytotoxicity, respectively). Following 3 + 21 h treatment with S9‐mix, precipitation was observed at 300 μg/mL and above. No statistically significant increase in the frequency of MNBN cells was observed at any concentration and treatment condition except at 24 + 0 h treatment, where a statistically significant increase in the frequency of MNBN cells was observed at 10 and 32 μg/mL (p < 0.05), but not at 20 and 30 μg/mL. The MNBN cell frequency of one replicate culture at 32 μg/mL (1.3%) exceeded the 95% historical vehicle control range (0.1–1.19%); however, there was 60% cytotoxicity and these increases were weak (up to 2.1‐fold compared to control). In addition, the effects were not concentration related and at some concentrations the effects observed between the two replicate cultures were not fully consistent. Since such deviations between replicate cultures have been observed in both studies and since the effects obtained with single cultures exceeded the historical 95% vehicle control range in both studies, it is not fully clear if this is due to normal variability. The mean of MNBN cell frequencies were, however, within the 95% historical vehicle control range at all concentrations analysed.
The second study (Lloyd, 2017) is considered more reliable than the first one (Mc Gary, 2016), but also this one is not fully adequate to rule out the concern for genotoxicity. The Panel considered that generally only one of the three criteria for a positive result was fulfilled. In the experiment in the absence of S9‐mix after short treatment, there was a statistically significant trend test, but there were no statistically significant differences between single concentrations tested and the concurrent control, while in the experiment in the absence of S9‐mix after long treatment, there was no statistically significant trend, however, two concentrations were statistically significantly different from the concurrent control.
The Panel considered that the results of the two in vitro studies (Mc Garry 2016b and Lloyd 2017) are equivocal and require further clarification.
3.1.4. p‐Mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278]
3.1.4.1. Reverse bacterial mutation assay
In order to investigate the potential of p‐mentha‐1,8‐dien‐7‐yl acetate (purity 96.5%) and/or its metabolites to induce gene mutations in bacteria, an Ames test was performed according to OECD Test Guideline 471 (OECD, 1997a) and following GLP in five strains of S. Typhimurium (TA98, TA100, TA1535, TA1537 and TA102), in the presence or absence of metabolic activation, in two separate experiments. In the first experiment, p‐mentha‐1,8‐dien‐7‐yl acetate was tested at concentrations of 5, 16, 50, 160, 500, 1,600 and 5,000 μg/plate with and without S9‐mix, applying the plate incorporation assay. In the second experiment, p‐mentha‐1,8‐dien‐7‐yl acetate was tested at concentrations of 1.6, 5, 16, 50, 160, 500 and 1,600 μg/plate with and without S9‐mix, applying the pre‐incubation method. Appropriate positive control chemicals and DMSO (as vehicle control) were evaluated concurrently. All test and positive control articles were evaluated in triplicate plates; the vehicle control was evaluated in quintuplicate.
All positive control chemicals induced significant increases in revertant colony numbers, confirming the sensitivity of the tests and the efficacy of the S9‐mix, while negative controls were within the historical control ranges.
No precipitate was observed at any tested concentration in any tester strain with or without S9‐mix.
In the first experiment, toxicity, as evident by the absence or reduction in the mean number of revertant colonies and absence or reduction in the background bacterial lawn, was observed at 500 μg/plate and above in all tester strains in the absence of S9‐mix and for strain TA1537 in the presence of S9‐mix. For all other strains, in the presence of S9‐mix, toxicity was observed at concentrations above 1,600 μg/plate.
In the second experiment, toxicity was observed at 500 μg/plate and above in all strains in the absence of S9‐mix and in strain TA1535 and TA102 in the presence of S9‐mix. Toxicity was observed at 160 μg/plate and above in strains TA98, TA100 and TA1537 in the presence of S9‐mix.
No increase in the mean number of revertant colonies was observed at any tested concentration in any tester strains with or without S9‐mix (Lloyd, 2016a).
The Panel considered the results of this assay as negative.
3.1.4.2. In vitro micronucleus assay
The in vitro micronucleus assay was carried out according to OECD Test Guideline 487 (OECD, 2014) and following GLP. Human peripheral blood lymphocytes from healthy donors, stimulated with PHA, were treated with p‐mentha‐1,8‐dien‐7‐yl acetate (purity 96.5%) in a dose‐range finding assay performed in single cultures at concentrations ranging from 7.05 to 1943 μg/mL for 3 h with and without S9‐mix and 24 h without S9‐mix. At the time of treatment, precipitate was observed at concentrations ≥ 151.1 μg/mL in all three treatment conditions.
Based on the dose‐range finding results, duplicate cultures of lymphocytes were treated with the test article 48 h after culture initiation at concentrations ranging from 25 to 250 μg/mL for treatments at 3 + 21 h without metabolic activation. Concentrations ranging from 50 to 500 μg/mL were tested in the treatment at 3 + 21 h with metabolic activation. Concentrations ranging from 10 to 150 μg/mL were tested in the treatment at 24 h without metabolic activation. Cytochalasin B (final concentration of 6 μg/mL) was added to each culture at the time of treatment. Appropriate vehicle (DMSO) and positive controls were used (mitomycin C and noscapine in the absence of S9‐mix, cyclophosphamide in the presence of S9‐mix). All positive control compounds induced a statistically significant increase of MN frequency and the system was considered sensitive and valid. Two thousand cells were scored per concentration. Based on the level of cytotoxicity observed, at least three concentration levels were selected for MN analysis in each experimental condition: i) 30, 60 and 80 μg/mL with the 24‐h treatment (12%, 39% and 55% cytotoxicity, respectively); ii) 150, 240 and 280 μg/mL with the 3‐h treatment with S9‐mix (11%, 41% and 55% cytotoxicity, respectively); iii) 100, 120 and 130 μg/mL with the 3‐h treatment without S9‐mix (9%, 44% and 67% cytotoxicity, respectively). In the treatment of 3 + 21 h with S9‐mix, precipitate was observed at 150 μg/mL and above. In the treatment of 3 + 21 h without S9‐mix, precipitate was observed at 120 and 130 μg/mL. p‐Mentha‐1,8‐dien‐7‐yl acetate did not induce a statistically significant increase of MNBN cells at any concentration analysed(Lloyd, 2016b).
The Panel considered the results of this assay as negative.
3.1.5. Myrtenyl acetate [FL‐no: 09.302]
3.1.5.1. Reverse bacterial mutation assay
In order to investigate the potential of myrtenyl acetate (purity 97.6%) and/or its metabolites to induce gene mutations in bacteria, an Ames test was performed according to OECD Test Guideline 471 (OECD, 1997a) and GLP in five strains of S. Typhimurium (TA98, TA100, TA1535, TA1537 and TA102), in the presence or absence of metabolic activation, in two separate experiments. In the first experiment, myrtenyl acetate was tested at concentrations of 5, 16, 50, 160, 500, 1,600, and 5,000 μg/plate with and without S9‐mix, applying the plate incorporation assay. In the second experiment, myrtenyl acetate was tested at concentrations of 3.28, 8.2, 20.5, 51.2, 128, 320, 800 and 2,000 μg/plate with and without S9‐mix, applying the pre‐incubation method. Appropriate positive control chemicals and DMSO (as vehicle control) were evaluated concurrently. All test and positive control articles were evaluated in triplicate plates; the vehicle control was evaluated in quintuplicate.
All positive control chemicals induced significant increases in revertant colony numbers, confirming the sensitivity of the tests and the efficacy of the S9‐mix, while negative controls were within the historical control ranges.
No precipitate was observed at any tested concentration in any tester strain with or without S9‐mix.
In the first experiment, toxicity, as evident by the absence or reduction in the mean number of revertant colonies and the absence or reduction in the background bacterial lawn, was observed in both experiments at 500 μg/plate and above in all tester strains in the absence of S9‐mix and strain TA1535 in the presence of S9‐mix. For the other four strains, in the presence of metabolic activation, toxicity was observed at 1,600 μg/plate and above.
In the second experiment, toxicity was observed at 320 μg/plate and above in strains TA98, TA100, TA1535 and TA1537 in the presence and absence of S9‐mix. In strain TA102, toxicity was observed at 2,000 μg/plate in the presence and absence of S9‐mix.
No increase in the mean number of revertant colonies was observed at any tested concentration in any tester strains with or without S9‐mix (Mc Garry, 2016a).
The Panel considered the results of this assay as negative.
3.1.5.2. In vitro micronucleus assay
The in vitro micronucleus assay was carried out according to OECD Test Guideline 487 (OECD, 2014) and following GLP. Human peripheral blood lymphocytes from healthy donors, stimulated with PHA, were treated with myrtenyl acetate (purity 97.6%) in a dose‐range finding assay performed in single cultures at concentrations ranging from 7.05 to 1,943 μg/mL for 3 h with and without S9‐mix and 24 h without S9‐mix. At the time of treatment, precipitate was observed at concentrations ≥ 250 μg/mL.
Based on the dose‐range finding results, duplicate cultures of lymphocytes were treated with the test article 48 h after culture initiation at concentrations ranging from 5 to 200 μg/mL for treatments without metabolic activation. Concentrations ranging from 25 to 500 μg/mL were tested in the treatment at 3 + 21 h with metabolic activation. Cytochalasin B (final concentration of 6 μg/mL) was added to each culture after the 3‐h treatment period, while in the 24‐h treatment cultures were treated with the test article in the presence of cytochalasin B. Appropriate vehicle (DMSO) and positive controls were used (mitomycin C and noscapine in the absence of S9‐mix, and cyclophosphamide in the presence of S9‐mix). All positive control compounds induced a statistically significant increase of MN frequency and the system was considered sensitive and valid. Two thousand cells were scored per concentration. Based on the level of cytotoxicity observed, at least three concentration levels were selected for MN analysis in each experimental condition: i) 10, 20 and 40 μg/mL with the 24‐h treatment (2%, 21% and 52% cytotoxicity, respectively); ii) 150, 250 and 325 μg/mL with the 3‐h treatment with S9‐mix (7%, 36% and 53% cytotoxicity, respectively); iii) 20, 60, 80 and 90 μg/mL with the 3‐h treatment without S9‐mix (0%, 12%, 45% and 48% cytotoxicity, respectively). No statistically significant increase in the frequency of micronuclei was observed after treatment with the test article at any concentration analysed (Mc Garry, 2016b).
The Panel considered the results of this assay as negative.
3.1.5.3. BlueScreen™ HC assay
Myrtenyl acetate [FL‐no: 09.302] was tested in a BlueScreen™ HC assay for cytotoxicity and genotoxicity using a genetically modified strain of cultured human lymphoblastoid TK6 cells, both in the presence and absence of metabolic activation. The study authors concluded that myrtenyl acetate did not induce genotoxicity at the concentrations tested (Birrell, 2013b).
4. Conclusions
The Panel considered that the newly submitted bacterial reverse mutation assays and the in vitro micronucleus assays on p‐mentha‐1,8‐dien‐7‐ol [FL‐no: 02.060], myrtenol [FL‐no: 02.091], p‐mentha‐1,8‐dien‐7‐yl acetate [FL‐no: 09.278] and myrtenyl acetate [FL‐no: 09.302] were adequately performed and that the results were negative. Therefore, the concern for genotoxicity could be ruled out for these four substances. Accordingly, they could be evaluated through the Procedure.
Myrtenal [FL‐no: 05.106] did not induce gene mutations in a bacterial reverse mutation assay. The first in vitro micronucleus assay provided was equivocal and had several weaknesses; therefore, a repetition of the study was requested. The second study is considered more reliable than the first one, but the result is still not fully adequate to rule out the concern for genotoxicity. In this second study, weak statistically significant increases of the micronuclei frequency were observed at the lowest and highest concentrations (without statistically significant trend) in the absence of S9‐mix after long treatment, while after short treatment, there was a statistically significant trend (without statistically significant differences between single concentrations tested and the concurrent control). The Panel considered that the result of this second study was also equivocal and that this was not adequately investigated by the applicant. Therefore, myrtenal cannot be evaluated through the Procedure, presently.
The Panel also considered two publications on the evaluation of genotoxicity studies on p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] (Cohen et al., 2016; Hobbs et al., 2016) which were published after the publication of the scientific opinion on FGE.208Rev1. The authors presented the same data as those reported in FGE.208Rev1, but reached different conclusions compared to the CEF Panel in relation to the evaluation of the in vivo Comet assay in liver. The Panel considered the reasons provided by the authors to substantiate their conclusions as not convincing and concluded that these two publications do not give reason to modify the conclusion drawn on the genotoxicity of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] in FGE.208Rev1.
Documentation provided to EFSA
Beevers C, 2014a. p‐Mentha‐1,8‐dien‐7‐al: Combined bone marrow micronucleus test and Comet assay in the liver of treated rats. Covance Laboratories Ltd. Study no. 8288455. 20 January 2014. Unpublished final report submitted by EFFA to EFSA.
Beevers C, 2014b. p‐Mentha‐1,8‐dien‐7‐al: Analysis of Comet slides from Covance Study 8288455. Covance Laboratories Ltd. Study no. 8307194. 05 September 2014. Unpublished final report submitted by EFFA to EFSA.
Benigni R and Netzeva T, 2007a. Report on a QSAR model for prediction of genotoxicity of α,β‐unsaturated aldehydes in S. typhimurium TA100 and its application for predictions on α,β‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
Benigni R and Netzeva T, 2007b. Report on a QSAR model for prediction of genotoxicity of α,β‐unsaturated ketones in S. typhimurium TA100 and its application for predictions on α,β‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
Bhalli J and Phil M, 2015a. Myrtenol (CAS # 515‐00‐4): Bacterial reverse mutation assay: plate incorporation method with a confirmatory assay. Covance Laboratories Inc. Study no. 8301943. 11 February 2015. Unpublished final report submitted by EFFA to EFSA.
Bhalli J and Phil M, 2015b. Myrtenol (CAS # 515‐00‐4): in vitro micronucleus assay in human peripheral blood lymphocytes. Covance Laboratories Inc. Study no. 8302022. 27 March 2015. Unpublished final report submitted by EFFA to EFSA.
Birrell L, 2013a. Report on the Testing of 1 Compound (myrtenol) in the BlueScreen™ HC Assay (−/+ S9 Metabolic Activation). Gentronix Limited. Unpublished study report submitted by EFFA to EFSA.
Birrell L, 2013b. Report on the Testing of 1 Compound (myrtenyl acetate) in the BlueScreen™ HC Assay (−/+ S9 Metabolic Activation). Gentronix Limited. Unpublished study report submitted by EFFA to EFSA.
Bowen R, 2011. Reverse mutation in five histidine‐requiring strains of Salmonella typhimurium. p‐Mentha‐1,8‐dien‐7‐al. Covance Laboratories Ltd. Study no. 8221207. May 2011. Unpublished report submitted by EFFA to FLAVIS Secretariat.
EFFA (European Flavour Association), 2012. Submission by the European Flavour Association to the European Food Safety Authority. Flavouring Group Evaluation 19 Subgroup 2.2 Flavouring Substance (Flavouring Substances) of the Chemical Group 7 (Annex I of 1565/2000/EC) structurally related to alicyclic primary alcohols, aldehydes, acids and related esters used as flavouring substances. Alicyclic α,β‐unsaturated aldehydes with the α,β‐conjugation in the ring or in the side chain. July 2012. FLAVIS/8.161.
EFFA (European Flavour Association), 2016. EFFA Letters to Commission for clarification of use levels and updated tonnage data for five substances in FGE.208Rev2.
Gry J, Beltoft V, Benigni R, Binderup M‐L, Carere A, Engel K‐H, Gürtler R, Jensen GE, Hulzebos E, Larsen JC, Mennes W, Netzeva T, Niemelä J, Nikolov N, Nørby KK and Wedebye EB, 2007. Description and validation of QSAR genotoxicity models for use in evaluation of flavouring substances in Flavouring Group Evaluation 19 (FGE.19) on 360 α,β‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
Lloyd M, 2009. Induction of micronuclei in cultured human peripheral blood lymphocytes. p Mentha‐1,8‐dien‐7‐al. Covance Laboratories Ltd. Study no. 8200450. July 17, 2009. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Lloyd M, 2012. Mutation at the hprt locus of mouse lymphoma L5178Y cells (MLA) using the microtitre(R) fluctuation technique. p‐Mentha‐1,8‐dien‐7‐al. Covance Laboratories Ltd. Study no. 8250474. June, 2012. Audited draft report. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Lloyd M, 2016a. p‐Mentha‐1,8‐dien‐7‐yl acetate: bacterial reverse mutation assay. Covance Laboratories Ltd. Study no. 8332790. February 2016. Unpublished study report submitted by EFFA to EFSA.
Lloyd M, 2016b. p‐Mentha‐1,8‐dien‐7‐yl acetate: in vitro human lymphocyte micronucleus assay. Covance Laboratories Ltd. Study no. 8332794. March 2016. Unpublished study report submitted by EFFA to EFSA.
Lloyd M, 2017. Myrtenal: in vitro human lymphocyte micronucleus assay. Covance Laboratories Ltd. Study no. 8351223. January 2017. Unpublished study report submitted by EFFA to EFSA.
Mc Garry S, 2016a. Myrtenal: bacterial reverse mutation assay. Covance Laboratories Ltd. Study no. 8332788. February 2016. Unpublished study report submitted by EFFA to EFSA.
Mc Garry S, 2016b. Myrtenal: in vitro human lymphocyte micronucleus assay. Covance Laboratories Ltd. Study no. 8332792. February 2016. Unpublished study report submitted by EFFA to EFSA.
Mc Garry S, 2016c. Myrtenyl acetate: bacterial reverse mutation assay. Covance Laboratories Ltd. Study no. 8332789. January 2016. Unpublished study report submitted by EFFA to EFSA.
Mc Garry S, 2016d. Myrtenyl acetate: in vitro human lymphocyte micronucleus assay. Covance Laboratories Ltd. Study no. 8332793. February 2016. Unpublished study report submitted by EFFA to EFSA.
Nikolov N, Jensen GE, Wedebye EB and Niemelä J, 2007. Report on QSAR predictions of 222 α,β‐unsaturated aldehydes and ketones from Flavouring Group Evaluation 19 (FGE.19) on 360 α,β‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
Roy S, 2016. In Vitro Mammalian Cell Micronucleus Assay in Human Peripheral Blood Lymphocytes (HPBL) with para‐mentha‐1,8‐dien‐7‐ol, aka p‐mentha‐1,8‐dien‐7‐ol (CAS Number: 536‐59‐4). BioReliance Corporation. Study no. AE43RY.348.BTL. Unpublished study report submitted by EFFA to EFSA.
Wagner VO, 2016. Bacterial Reverse Mutation with an Independent Repeat Assay with para‐mentha‐1,8‐dien‐7‐ol, aka p‐mentha‐1,8‐dien‐7‐ol (CAS Number: 536‐59‐4). BioReliance Corporation. Study no. AE43RY.502002.BTL. Unpublished study report submitted by EFFA to EFSA.
Abbreviations
- bw
body weight
- CA
chromosomal aberration
- CAS
Chemical Abstract Service
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CHL
Chinese hamster lung (cells)
- CoE
Council of Europe
- DMSO
dimethyl sulfoxide
- EFFA
European Flavour and Fragrance Association
- EMS
ethyl methanesulfonate
- FAO
Food and Agriculture Organization of the United Nations
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS (FL)
Flavour Information System (database)
- GLP
Good Laboratory Practice
- HPRT
hypoxanthine‐guanine phosphoribosyl transferase
- ID
Identity
- Imax
maximum induction factor
- IR
infrared spectroscopy
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- MN
micronucleus
- MNBN
micronucleated binucleated (cells)
- MNPCE
micronucleated polychromatic erythrocytes
- MS
mass spectrometry
- MSDI
maximised survey‐derived daily intake
- mTAMDI
modified theoretical added maximum daily intake
- MTD
maximum tolerated dose
- NMR
nuclear magnetic resonance
- No
Number
- OECD
Organisation for Economic Co‐operation and Development
- PCE
polychromatic erythrocytes
- PHA
phytohaemagglutinin
- (Q)SAR
(quantitative) structure–activity relationship
- RI
replication index
- RS
relative survival
- SCE
sister chromatid exchange
- WHO
World Health Organization
Appendix A – Summary of safety evaluation applying the procedure
1.
Appendix B – Genotoxicity Data Considered by the Panel in FGE.208
1.
Appendix C – Genotoxicity Data Considered by the Panel in FGE.208Rev1
1.
Appendix D – Genotoxicity Data Considered by the Panel in FGE.208Rev2
1.
Appendix E – Exposure
E.1. Presence of myrtenal in food and in other sources
Myrtenal [FL‐no: 05.106] is an aromatic constituent of several plant species. Quantitative data are available for 21 natural sources (including three natural sources, in which myrtenal was found only at trace level). For further 11 natural sources, only qualitative data are available (Table E.1).
Table E.1.
Occurrence of myrtenal [FL‐no: 05.106] in natural sources as reported by (Triskelion, 2017)
| Natural source | Quantity (mg/kg) |
|---|---|
| Calabash nutmeg (Monodora myristica Dunal) | 600 |
| Camomile | < 500–4,600 |
| Citrus fruitsa | < 5–340 |
| Eucalyptus oil (Eucalyptus globulus Labill) | 700 |
| Ginger (Zingiber species) | 600 |
| Lamb's lettuce (Valerianella locusta) | 0.2–0.5 |
| Laurel (Laurus nobilis L.) | 1,700 |
| Licorice (Glycyrrhiza species) | 0.5 |
| Mastic (Pistacia lentiscus) | 1,300–7,200 |
| Melon | 0.04 |
| Mentha oils | 2 |
| Myrtle (Myrtus communis L.) | 2,200 |
| Parsley (Petroselinum species) | 10 |
| Pistachio oil (Pistacia vera) | 2,200 |
| Pistacia atlantica | 9,000–12,000 |
| Thyme (Thymus species) | < 1,000 |
| Turpentine oil (Pistacia terebinthus) | 11,000–41,000 |
| Xylopia species | 15,000 |
| Calamus (sweet flag) (Acorus calamus L.) | Qualitative |
| Cherimoya (Annona cherimolia Mill.) | Qualitative |
| Cumin seed (Cuminum cyminum L.) | Trace (ppm) |
| Custard apple, atemoya (Annona atemoya) | Qualitative |
| Juniperus communis | Qualitative |
| Lemon balm (Melissa officinalis L.) | Qualitative |
| Mace (Myristica fragrans Houttuyn) | Trace (ppm) |
| Mangifera species | Qualitative |
| Nutmeg (Myristica fragrans Houttuyn) | Trace (ppm) |
| Pepper (Piper nigrum L.) | Qualitative |
| Raspberry, blackberry and boysenberry | Qualitative |
| Walnut (Juglans species) | Qualitative |
Quantitative data on citrus fruits are reported mainly from peel.
It should be mentioned that not all of the sources reported in Table E.1 are used as foodstuffs or are added to products sold in the EU market (GNPD, 2017) for flavouring purposes: turpentine oil (Pistacia terebinthus) is used as a solvent, cleaning and sanitary product; Pistacia atlantica is used in traditional medicine in Iran, it can also be used in local (Iranian) foods, such as curd, instead of walnuts (Bahmani et al., 2015).
According to the Global New Products Database (GNPD) (2017),9 some plant sources, i.e. Calabash nutmeg (Monodora myristica Dunal) and Xylopia species reported in Table E.1, are not reported to be used in food products placed on the market in the EU.
Myrtle (Myrtle communis L.) berries and leaves are used to make alcoholic‐beverages in Italy, especially in Sardinia, where the smoke of myrtle is also used for flavouring purposes in traditional cooking. Moreover, myrtle leaves and berries can be used by the food industry for flavouring purposes and for the production of sweet liquors (Aleksic and Knezevic, 2014). In the EU, myrtle is used not only in the production of alcoholic beverages, but also in a few other products, e.g. in soothing gum, tea and jelly in France; in a carbonated soft drink, pear preserve and a juice drink in Italy (GNPD, 2017).
E.1.1. Intended use and use levels of myrtenal as provided by the Flavour Industry
Use levels in the different food categories reported in Annex I of Reg. (EC) 1565/200010 have been submitted by the flavour industry and are reported in Table E.2 (EFFA, 2016).
Table E.2.
Use levels of myrtenal [FL‐no: 05.106] in food categories listed in Annex I of Reg. (EC) 1565/2000 (EFFA, 2016)
| FL‐no | Food Categories | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal use levels (mg/kg)a Maximum use levels (mg/kg) | ||||||||||||||||||
| 01.0 | 02.0 | 03.0 | 04.0 | 05.0 | 05.3 | 06.0 | 07.0 | 08.0 | 09.0 | 10.0 | 11.0 | 12.0 | 13.0 | 14.1 | 14.2 | 15.0 | 16.0 | |
| 05.106 |
1 5 |
8 50 |
2 10 |
1 5 |
5 20 |
20 100 |
1 5 |
5 20 |
1 5 |
1 5 |
1 5 |
1 5 |
5 20 |
– |
1 5 |
1 5 |
1 5 |
1 5 |
‘Normal use’ is defined as the average of reported usages and ‘maximum use’ is defined as the 95th percentile of reported usages (EFFA, 2002).
E.2. Intake data from intended use8
Annual production volumes of the flavouring substances as surveyed by industry are used to calculate the ‘Maximised Survey‐derived Daily Intake’ (MSDI) assuming that the production figure only represents 60% of the use in food, due to underreporting and that 10% of the total EU population are consumers (SCF, 1999).
Use levels for myrtenal provided by industry (EFFA, 2016) listed in Table E.2, have been used to calculate the ‘modified Theoretical Added Maximum Daily Intake’ (mTAMDI).11
The MSDI and mTAMDI exposure estimates are given in Table E.3.
Table E.3.
Exposure to the flavouring substance
| FL‐no | Name | EU MSDI μg/capita per day | mTAMDI μg/person per day |
|---|---|---|---|
| 05.106 | Myrtenal | 2.21 | 2,100 |
FL‐no: FLAVIS number; MSDI: maximised survey‐derived daily intake; mTAMDI: modified Theoretical Added Maximum Daily Intake.
Appendix F – Publications on the evaluation of genotoxicity studies on p‐Mentha‐1,8‐dien‐7‐al [FL‐no: 05.117]
1.
p‐Mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] was evaluated by the Panel as genotoxic in vivo and, accordingly, there is a safety concern for the use of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] as a flavouring substance (FGE.208Rev1). After the publication of this opinion (EFSA CEF Panel, 2015), two articles on the genotoxicity of p‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117] were published (Cohen et al., 2016; Hobbs et al., 2016), that industry sent to EFSA. The authors presented the same data reported in EFSA opinion FGE.208Rev1 (EFSA CEF Panel, 2015), but reached different conclusions compared to the CEF Panel in relation to the evaluation of the in vivo Comet assay in liver. Topics in disagreement are summarised below.
-
1
Relevance of in vivo study in liver as follow‐up of in vitro genotoxicity tests on p ‐mentha‐1,8‐dien‐7‐al [FL‐no: 05.117]
Hobbs et al. (2016) considered that ‘if the positive test in the liver, the major site of metabolism in vivo, is assumed to reflect a biologically relevant result, then it is surprising that in vitro mutagenicity and chromosome damage tests performed in the presence of metabolic activation did not provide any indication of genotoxic effects’ (Hobbs et al., 2016).
The Panel noted that there was some evidence of positive results in vitro also in the presence of S9‐mix (Tayama et al., 1990). While Tayama et al. (1990) observed a positive result in a chromosomal aberration assay at 300 μg/mL (in the absence of excessive toxicity), Lloyd (2009) observed a negative result with p‐mentha‐1,8‐dien‐7‐al in an in vitro micronucleus assay up to 140 μg/mL (accompanied by 45% cytotoxicity which is less than the 50–60% cytotoxicity as recommended by the OECD TG 487). The inconsistent results obtained by Tayama et al. (1990) and Lloyd (2009) might therefore be due to the different maximum concentrations tested.
-
2
Liver toxicity
In the analysis of the Comet assay in liver, the CEF Panel noted:
‘at the highest dose (animals exposed to 700 mg/kg bw per day) a 3.4‐fold and statistically significant increase in tail intensity was observed. A statistical significant linear trend was also apparent. Five out of the six animals treated with the highest dose had tail intensities that exceeded the values observed in the concurrent vehicle control animals’.
The two articles indicate:
‘While this statement is correct, it should be pointed out that only 2 of the 6 animals in this dose group, are driving the statistically significant increase in group mean tail intensity, compared to the concurrent vehicle control animals. More importantly, these two animals were also among the three most affected by liver toxicity (animals 27 and 23), indicating a direct association between liver toxicity and increased DNA tail intensities’. (Cohen et al., 2016).
Referring to ‘A statistical significant linear trend was also apparent’, Cohen et al. (2016) indicate:
‘While this statement is accurate, this is also consistent with the reported dose‐dependent toxicity’ (Cohen et al., 2016).
‘The lack of dose‐related increases in % clouds or % cells with halos in liver cells indicates that treatment did not cause excessive DNA damage that could have created artefacts and interfered with comet analysis. However, other endpoints reveal evidence of dose‐dependent general liver toxicity in the test substance‐exposed animals, and this effect was particularly pronounced at the highest dose employed (700 mg/kg bw per day), including a loss of body weight in the high dose group over the period of exposure to the test substance in 5 of the 6 rats in the group, elevated aspartate aminotransferase and alanine aminotransferase and altered clinical biochemistry parameters (cholesterol, potassium, chloride, urea and glucose); three animals (numbered 27, 23 and 22) in the high dose group were particularly affected. Histopathological examination corroborated the clinical pathology findings (..) with observations of hepatocyte vacuolation in all 6 animals in the high dose group. Additionally, 5 of the 6 animals in the high dose group showed overt signs of toxicity reflected in their behaviour (reduced activity), particularly animals 27 and 23’ (Cohen et al., 2016).
‘In this study, there was no dose‐related increase in % hedgehogs or % cells with halos in liver cells of exposed animals. Five out of the six animals in the 700 mg/kg per day dose group had % tail intensity values that exceeded those of the concurrent vehicle control animals.’ (..) ‘Measurements were obtained for at least five animals/group for aspartate aminotransferase (AST) and alanine aminotransferase (ALT); although mean serum values for these enzymes were not statistically significant, three of the six rats dosed with 700 mg/kg per day perillaldehyde exhibited high activity of both ALT and AST, indicative of hepatic toxicity’ (..) ‘Hepatocyte cytoplasmic vacuolation was observed in liver sections from the rats exposed to 700 mg/kg per day perillaldehyde. The small vacuoles are morphologically consistent with microvesicular fat’ (Hobbs et al., 2016).
‘The international effort to validate the in vivo comet assay for the detection of genotoxic carcinogens, coordinated by the Japanese Center for the Validation of Alternative Methods (JaCVAM), concluded that histopathology remains the “gold standard” for assessing tissue cytotoxicity, and changes in % tail DNA require careful interpretation when measured in conjunction with severe histopathological changes (Uno et al., 2015). (..) The perillaldehyde comet findings (both the group mean and individual animal data) are well within this upper limit for acceptable vehicle control values further supporting the conclusion that the small increase in tail intensity observed in the liver following administration at 700 mg/kg per day was not biologically relevant and was most likely an artefact of the observed hepatic cytotoxicity’ (Hobbs et al., 2016).
The Panel noted that histopathology has been taken into account in the evaluation of results, as recommended by OECD TG 489. The authors of the study report indicated ‘In the liver, minimal or slight hepatocyte vacuolation was present in animals given 700 mg/kg/day’ (Beveers, 2014a). The Panel noted that vacuolisation is not considered an indication for strong hepatotoxicity. The Panel considered also clinical chemistry data, which showed a slight increase in aspartate aminotransferase and alanine aminotransferase at the highest dose that was not statistically significant.
Moreover, the Panel noted the lack of dose‐related increase of percent of cells with clouds and halos (which according to OECD TG489 are key parameters for the interpretation of the comet assay) confirming the absence of severe liver toxicity. Actually, also the study authors (Beevers, 2014a) considered that there was no excessive liver damage and liver toxicity at the top dose and that this did not interfere with the validity of the assay. The Panel noted that if liver toxicity was so severe, as claimed by the authors of the two articles, the acceptance criteria for the study should not have been considered as being fulfilled. In this respect, the view of the authors of the two articles (Cohen et al., 2016; Hobbs et al., 2016) is not consistent with the view of the authors of the study report, since the acceptance criteria have actually been considered as being fulfilled by the authors of the study report and the study was evaluated by them.
-
3
Historical controls
In the analysis of the Comet assay in liver, the CEF Panel indicated:
‘… however, the tail intensity values for all animals fell within the laboratory's historical control values. The Panel noted that the range for both the negative and positive historical control values were extremely wide for this test laboratory. In addition there was an overlap of the negative (95% range: 0.02–11.39) and positive (95% range: 7.15–65.07) control values’.
The two articles indicate:
‘The observation that tail intensity values for all animals fell within the laboratory's historical control values is pertinent and under OECD TG 489 guidelines cannot be dismissed when considering the outcome of a comet assay. (..) This is indeed the case, but it is important to consider that the tail intensity for the negative and positive controls of the specific assay fell comfortably within the range of the historical control values and near the means of the respective ranges: the positive control tail intensity is close to the historical positive control mean (35.55 ± 14.86). More importantly, the mean tail intensity of the high dose group (700 mg/kg bw per day) of 2.20 ± 0.60 is comparable to the historical negative control mean (2.22 ± 2.58), despite the skew effected by animals 27 and 23’ (Cohen, 2016).
‘However, all six animals fell well within the laboratory's historical vehicle control 95th percentile range (0.02–11.39; n = 165) and only two of the six animals had % tail intensity values that exceeded the mean % tail intensity of the historical control data (2.22%). (..) In that regard, it is useful to consider the perillaldehyde positive % tail intensity value in the context of broadened historical datasets collated by the testing laboratory, in which the vehicle and positive control reference ranges are clearly distinct (Supplemental Data Table II) (2016). Comparison of the positive % tail intensity value (2.20%) to historical data that included the studies performed immediately prior to the perillaldehyde study (n = 230) or historical data spanning the period immediately prior and subsequent to the perillaldehyde study (n = 400) confirms that the perillaldehyde data are close to the means of both vehicle control data sets (2.31% and 1.60% respectively), supporting the arguments that the positive comet result for perillaldehyde is not biologically relevant (Hobbs et al., 2016)’.
The Panel noted that on the basis of OECD TG 489, the evaluation should be based on a comparison between treatment‐induced values and the concurrent vehicle control, the consideration of a potential dose‐response relationship and on a comparison of treatment‐induced values with (appropriate) historical negative control data, not primarily on a comparison between vehicle/positive control experimental data and historical controls. Hobbs et al. (2016) report three different ranges of historical control, two of the reported ranges are new and not overlapping. However, the range of historical negative control is still wide and no justification on the overlapping range is provided. The Panel did not have access to the broadened dataset when it evaluated p‐mentha‐1,8‐dien‐7‐al in FGE.208Rev1 (EFSA CEF Panel, 2015). As noted in the opinion (FGE.208Rev1), the Panel considered that a comparison with concurrent control is more relevant than a comparison with historical control data, especially if the range of historical control data is broad as it is the case here.
The authors of the two articles actually consider like the CEF Panel that p‐mentha‐1,8‐dien‐7‐al induced an increase in DNA damage in the in vivo comet assay. The difference between EFSA and the authors is that they consider the effect due to liver toxicity. However, in the same paper, the authors noted that liver toxicity, at the top dose, does not interfere with the validity of the assay.
Suggested citation: EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), Silano V, Bolognesi C, Castle L, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Husøy T, Kärenlampi S, Mennes W, Milana MR, Penninks A, Smith A, de Fátima Tavares Poças M, Tlustos C, Wölfle D, Zorn H, Zugravu C‐A, Binderup M‐L, Marcon F, Marzin D, Mosesso P, Anastassiadou M, Carfì M, Saarma S and Gürtler R, 2017. Scientific Opinion on Flavouring Group Evaluation 208 Revision 2 (FGE.208Rev2): Consideration of genotoxicity data on alicyclic aldehydes with α,β‐unsaturation in ring/side‐chain and precursors from chemical subgroup 2.2 of FGE.19. EFSA Journal 2017;15(5):4766, 44 pp. 10.2903/j.efsa.2017.4766
Requestor: European Commission
Question numbers: EFSA‐Q‐2015‐00843, EFSA‐Q‐2016‐00146, EFSA‐Q‐2016‐00151, EFSA‐Q‐2016‐00228, EFSA‐Q‐2016‐00277
Panel members: Claudia Bolognesi, Laurence Castle, Jean‐Pierre Cravedi, Karl‐Heinz Engel, Paul Fowler, Roland Franz, Konrad Grob, Rainer Gürtler, Trine Husøy, Sirpa Kärenlampi, Wim Mennes, Maria Rosaria Milana, André Penninks, Vittorio Silano, Andrew Smith, Maria de Fátima Tavares Poças, Christina Tlustos, Detlef Wölfle, Holger Zorn and Corina‐Aurelia Zugravu.
Acknowledgements: The Panel wishes to thank the hearing experts: Vibe Beltoft and Karin Nørby and EFSA staff members: Annamaria Rossi for the support provided to this scientific output.
Adopted: 22 March 2017
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
Commission Regulation (EU) 2015/1760 of 1 October 2015 amending Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council as regards removal from the Union list of the flavouring substance p‐mentha‐1,8‐dien‐7‐al. OJ L 257, 2.10.2015, p. 27–29.
Commission Regulation (EU) 2016/637 of 22 April 2016 amending Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council as regards removal from the Union list of certain flavouring substances. OJ L 108, 23.4.2016, p. 24–27.
Commission Regulation (EU) 2016/1244 of 28 July 2016 amending Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council as regards certain flavouring substances from a group related with an alpha beta unsaturation structure. OJ L 204, 29.7.2016, p. 7–10.
The data presented in Section 2.4 are cited from the first version of FGE.208. These data are the basis for the conclusions in FGE.208 requesting additional genotoxicity data.
The data presented in Section 2.5 are cited from the Scientific Opinion FGE.208Rev1.
The Global New Products Database monitors product innovation and retail success in consumer packaged goods markets, worldwide.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
mTAMDI estimation is based in an approach used by the SCF up to 1995 (SCF, 1995) and is calculated on the basis of standard portions and normal use levels for flavoured beverages and foods in general, with exceptional levels for particular foods.
References
- Aleksic V and Knezevic P, 2014. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiological Research, 169, 240–254. [DOI] [PubMed] [Google Scholar]
- Bahmani M, Saki K, Asadbeygi M, Adineh A, Saberianpour SH, Rafieian‐Kopaei M, Bahmani F and Bahmani E, 2015. The effects of nutritional and medicinal mastic herb (Pistacia atlantica). Journal of Chemical and Pharmaceutical Research, 7, 646–653. [Google Scholar]
- Chen T, Harrington‐Brock K and Moore MM, 2002. Mutant frequency and mutational spectra in the Tk and Hprt genes of N‐ethyl‐N‐nitrosourea‐treated mouse lymphoma cellsdagger. Environmental and Molecular Mutagenesis, 39, 296–305. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Fukushima S, Gooderham NJ, Guengerich FP, Hecht SS, Rietjens IM, Smith RL, Bastaki M, Harman CL, McGowen MM and Taylor SV, 2016. FEMA expert panel review of p‐mentha‐1,8‐dien‐7‐al genotoxicity testing results. Food and Chemical Toxicology, 98, 201–209. [DOI] [PubMed] [Google Scholar]
- Eder E, Scheckenbach S, Deininger C and Hoffman C, 1993. The possible role of α, β‐unsaturated carbonyl compounds in mutagenesis and carcinogenesis. Toxicology Letters, 67, 87–103. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Minutes of the 26th Plenary meeting of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food, Held in Parma on 27–29 November 2007. Parma, 7 January 2008. Available online: http://www.efsa.europa.eu/EFSA/Event_Meeting/afc_minutes_26thplen_en.pdf
- EFSA (European Food Safety Authority), 2008b. Genotoxicity Test Strategy for Substances belonging to Subgroups of FGE.19 ‐ Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). EFSA Journal 2008;6(12):854, 5 pp. doi: 10.2903/j.efsa.2008.854 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008c. Scientific Opinion. List of α,β‐unsaturated aldehydes and ketones representative of FGE.19 substances for genotoxicity testing ‐ Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). EFSA Journal 2008;6(12):910, 5 pp. doi: 10.2903/j.efsa.2008.910 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Scientific Report of EFSA. Minimum Criteria for the acceptance of in vivo alkaline Comet Assay Reports. EFSA Journal 2012;10(11):2977, 12 pp. doi: 10.2903/j.efsa.2012.2977 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2013. Scientific Opinion on Flavouring Group Evaluation 208 (FGE.208): Consideration of genotoxicity data on representatives for 10 alicyclic aldehydes with the α,β‐unsaturation in ring/side‐chain and precursors from chemical subgroup 2.2 of FGE.19 by EFSA. EFSA Journal 2013;11(4):3151, 25 pp. doi: 10.2903/j.efsa.2013.3151 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2015. Scientific Opinion on Flavouring Group Evaluation 208 Revision 1 (FGE.208Rev1): Consideration of genotoxicity data on representatives for 10 alicyclic aldehydes with the α,β‐unsaturation in ring/side‐chain and precursors from chemical subgroup 2.2 of FGE.19. EFSA Journal 2015;13(7):4173, 28 pp. doi: 10.2903/j.efsa.2015.4173 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. doi: 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- Fujita H, Aoki N and Sasaki M, 1994. [Mutagenicity test of food additives with Salmonella typhimurium TA97 and TA102 (IX*)]. Annual Report of Tokyo Metropolitan Research Laboratory of Public Health, 45, 191–199. (In Japanese) [Google Scholar]
- GNPD (Global New Products Database), March 2017. Mintel, Available online: http://www.gnpd.com/sinatra/home/
- Hayashi M, Kishi M, Sofuni T and Ishidate M Jr, 1988. Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Food and Chemical Toxicology, 26, 487–500. [DOI] [PubMed] [Google Scholar]
- Hobbs CA, Taylor SV, Beevers C, Lloyd M, Bowen R, Lillford L, Maronpot R and Hayashi SM, 2016. Genotoxicity assessment of the flavouring agent, perillaldehyde. Food and Chemical Toxicology, 97, 232–242. [DOI] [PubMed] [Google Scholar]
- Ishidate M Jr, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M and Matsuoka A, 1984. Primary mutagenicity screening of food additives currently used in Japan. Food and Chemical Toxicology, 22, 623–636. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002a. Evaluation of certain food additives. Fifty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 913. Geneva, 4–13 June 2002.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002b. Compendium of food additive specifications. Addendum 10. Joint FAO/WHO Expert Committee of Food Additives 59th session. Geneva, 4‐13 June 2002. FAO Food and Nutrition paper 52 Addition 10.
- Kuroda K, Tanaka S, Yu YS and Ishibashi T, 1984. [Rec‐assay of food additives]. Nippon Koshu Eisei Zasshi [Japanese Journal of Public Health], 31, 277–281. (In Japanese) [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 1997a. Test No. 471: Bacterial reverse mutation test. OECD Guideline for testing of chemicals. Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 1997b. Test No. 476: In vitro Mammalian Cell Gene Mutation Test. OECD Guidelines for the Testing of Chemicals. Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 1997c. Test No. 474: Mammalian erythrocyte micronucleus test. OECD Guidelines for the Testing of Chemicals. Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2010. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2014. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. OECD Guidelines for the Testing of Chemicals, Section 4.
- Sasaki M, Yoshida S, Ichikawa H, Takano I, Yasuda I and Akiyama K, 1990. [Cytological activities of perilla extracts]. Annual Report of Tokyo Metropolitan Research Laboratory of Public Health, 41, 275–281. (In Japanese) [Google Scholar]
- SCF (Scientific Committee for Food), 1999. Opinion on a programme for the evaluation of flavouring substances (expressed on 2 December 1999). Scientific Committee on Food. SCF/CS/FLAV/TASK/11 Final 6/12/1999. Annex I to the minutes of the 119th Plenary meeting. European Commission, Health & Consumer Protection Directorate‐General.
- Suzuki N and Suzuki H, 1994. Induction of K‐ras codon 12 mutation by saccharin sodium in cultured human cells: [Comparison with perillaldehyde‐induced mutagenicity]. Hen'igensei Shiken, 3, 155–160. (In Japanese) [Google Scholar]
- Suzuki H, Ichikawa H and Sasaki M, 1990. [Mutagenicity of perillaldehyde in a human cell strain]. Annual Report of Tokyo Metropolitan Research Laboratory of Public Health, 41, 265–266. (In Japanese) [Google Scholar]
- Tayama S, Ichikawa H and Sasaki M, 1990. [Effects of perillaldehyde on induction of chromosomal aberrations and sister‐chromatid exchanges (SCEs) in CHO‐KI‐cells]. Annual Report of Tokyo Metropolitan Research Laboratory of Public Health, 41, 267–269. (In Japanese) [Google Scholar]
- Triskelion , 2017. VCF online. Volatile Compounds in Food. In: Nijssen B, van Ingen‐Visscher K, Donders J (eds.). Database Version 16.3. Triskelion, Zeist, The Netherlands. 1992–2017. [Google Scholar]
- Yoo YS, 1986. Mutagenic and antimutagenic activities of flavoring agents used in foodstuffs. Osaka City Medical Journal, 34, 267–288. [Google Scholar]


