Abstract
The Panel on Plant Health performed a pest categorisation of Elsinoë fawcettii and E. australis, the causal agents of citrus scab diseases, for the EU. The identities of the pests are well‐established and reliable methods exist for their detection/identification. The pests are listed in Annex IIAI of Directive 2000/29/EC as Elsinoë spp. and are not known to occur in the EU. Species and hybrids of citrus (Family Rutaceae) are affected by E. fawcettii and E. australis, with the latter having a more restricted host range and geographical distribution compared to the former. The status of Simmondsia chinensis (jojoba) as a host of E. australis is uncertain. The pests could potentially enter the EU on host plants for planting and fruit originating in infested Third countries. The current distribution of the pests, climate matching and the use of irrigation in the EU citrus‐growing areas suggest that the pests could establish and spread in the EU citrus‐growing areas. Uncertainty exists on whether cultural practices and control methods, currently applied in the EU, would prevent the establishment of the pests. In the infested areas, the pests cause scab pustules on host leaves and fruit resulting in yield/quality losses. It is expected that the introduction and spread of the pests in the EU could impact citrus production. Cultural practices and chemical control measures may reduce the inoculum sources and to some extent the disease incidence, but they cannot eliminate the pests. Phytosanitary measures are available to mitigate the risk of introduction and spread of the pests in the EU. E. fawcettii and E. australis meet all the criteria assessed by EFSA for consideration as potential Union quarantine pests. As those pests are not known to occur in the EU, this criterion to consider them as Union regulated non‐quarantine pests is not met.
Keywords: citrus scab, climate, European Union, impacts, pest distribution, quarantine, sour orange scab
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above‐mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/ pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L.. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pest categorisations should be delivered by end 2020.
For the above‐mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoë spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches' broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L.,Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches' broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbac |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Elsinoë spp. are listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether they fulfil the criteria of quarantine pests or those of regulated non‐quarantine pests for the area of the European Union (EU) excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores. Two species of Elsinoë, E. fawcettii Bitanc. & Jenkins and E. australis Bitanc. & Jenkins, have been described causing scab diseases on citrus and are both considered in this pest categorisation.
A third new species, Elsinoë citricola Fan, R.W. Barreto & Crous, originating from a re‐classification (based on four loci) of some E. fawcettii isolates from citrus collected in Brazil (Fan et al., 2017), is not considered in this pest categorisation because of lack of information on the pathogenicity, host range and biology of this new species.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A search of literature (1997–2017) in the ISI Web of Science bibliographic database was conducted at the beginning of the categorisation. The search focussed on Elsinoë fawcettii and Elsinoë australis and their geographic distribution, life cycle, host plants and the damage they cause. The following search terms (TS) and combinations were used: TS =(“Elsinoë fawcettii” OR “Elsinoë australis” OR “citrus scab”) AND TS=(geograph* OR distribution OR “life cycle” OR lifecycle OR host OR hosts OR plant* OR damag*).
Further references and information were obtained from experts, from citations within the references and grey literature
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO, 2017).
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (online).
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Consumers (DG SANCO), and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States (MSs) and the phytosanitary measures taken to eradicate or avoid their spread (Europhyt, online).
2.2. Methodologies
The Panel performed the pest categorisation for E. fawcettii and E. australis, following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union regulated non‐quarantine pest in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required as per the specific ToR received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a regulated non‐quarantine pest. If one of the criteria is not met, the pest will not qualify. A pest that does not qualify as a quarantine pest may still qualify as a regulated non‐quarantine pest which needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone, thus the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism. | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area). |
| Regulatory status (Section 3.3) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future. |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone). |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests' introduction have an economic or environmental impact on the EU territory? | Would the pests' introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met. |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pests
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes, Elsinoë fawcettii and E. australis are two well‐identified fungal pathogens
Elsinoë fawcettii and E. australis are well‐identified fungi of the family Elsinoaceae. According to Index Fungorum database (http://www.indexfungorum.org), the pathogens have the following taxonomical identification:
| 1) Preferred scientific name: |
Elsinoë fawcettii Bitanc. and Jenkins, 1936 Family – Elsinoaceae Genus – Elsinoë Species – fawcettii |
| Preferred common name: citrus scab | |
| Other common names: citrus common scab, sour orange scab | |
| 2) Preferred scientific name: |
Elsinoë australis Bitanc. and Jenkins, 1936 Family – Elsinoaceae Genus – Elsinoë Species – australis |
| Preferred common name: sweet orange scab |
3.1.2. Biology of the pests
Elsinoë fawcettii and E. australis share the same biology. New infections are caused by conidia formed in acervuli on the surface of scab pustules (Timmer, 2000). Conidia are spread to new susceptible host tissues (leaves or fruit) primarily by rain splash. Hyaline conidia die quickly if exposed to dry conditions or direct sunlight. In addition to hyaline conidia, E. fawcettii produces coloured, spindle‐shaped conidia on scab lesions following periods of dew. This second type of conidia can be airborne for short distances. Germination of conidia and infection do not require rainfall; both processes can take place in the presence of free water from dew or fog. Conidial germination occurs at temperatures 13–32°C, but infection does not take place at temperatures below 14°C or above 25°C (Whiteside, 1975). The optimum temperature for infection and disease development is 24–27°C (Timmer, 2000). Infection may occur at lower or higher temperatures, but requires longer periods of wetness. A wet period of 2.5–3.5 h is required for infection by conidia, whereas the minimum wetness period for sporulation is only 1–2 h (Timmer, 2000). Wetness periods up to 24 h increase the severity of infection. Thus, infection can occur during dew periods or short periods of irrigation (Timmer, 2000). The incubation period is at least 5 days.
Leaves are most susceptible to infection just after emergence and become tolerant by the time they reach one half of full expansion (Whiteside, 1975). Fruits are susceptible to infection for 6–8 weeks after petal fall (Timmer, 2000).
Both pathogens survive in scab pustules on fruits remaining on the tree, providing the inoculum for next season. Even in resistant cultivars, the pathogens can survive on diseased shoots emerging from susceptible rootstocks (Whiteside, 1988).
The teleomorphs of both Elsinoë species have been reported only from Brazil (Bitancourt and Jenkins, 1936, 1937).
3.1.3. Intraspecific diversity
Several pathotypes have been described in E. fawcettii and E. australis based primarily on a set of differential citrus hosts (Timmer et al., 1996; Hyun et al., 2001; Hou et al., 2014; Miles et al., 2015). Furthermore, cryptic pathotypes have been reported for both, E. fawcettii and E. australis (Hyun et al., 2009; Wang et al., 2009). Since the EU legislation refers to citrus, this intraspecific diversity does not affect the conclusions of this pest categorisation.
3.1.4. Detection and identification of the pests
Are detection and identification methods available for the pest?
Yes, Elsinoë spp. can be detected and identified based on symptomatology, cultural and morphological characteristics. Molecular methods and pathogenicity tests have been developed for the differentiation of E. fawcettii and E. australis.
Semi‐selective culture media are available for isolating Elsinoë spp. from scab lesions in citrus (Whiteside, 1988). However, as E. fawcettii and E. australis have similar morphological characteristics, they cannot be reliably identified in cultures (Timmer, 2000). Pathogenicity tests can be used for species and pathotype identification (Timmer et al., 1996). A molecular method (rapid amplified polymorphic DNAs (RAPDs)) to identify E. fawcettii and E. australis was developed by Hyun et al. (2007). However, recently, Fan et al. (2017) showed that, when single genetic loci were used, as in the case of Hyun et al. (2007) studies, problems of specificity were encountered between E. fawcettii or E. australis and other Elsinoë species. Nevertheless, when a combination of four genetic loci was used, E. fawcettii and E. australis were clearly identified (Fan et al., 2017).
Symptoms
Lesions on young leaves begin as minute water‐soaked spots, which subsequently evolve into amphigenous, creamy‐yellowish or variously bright‐coloured pustules (EPPO/CABI, 1992). These grow as irregular, globose or conical excrescences which coalesce and extend mostly along the main veins to cover a large part of the blade, particularly on the lower surface. The central area of these wart‐like outgrowths is depressed and becomes drab, greyish and velvety when the fungus is fruiting. Old scab lesions have a rough surface, are dusky‐coloured and become cracked and fissured. Affected leaves become stunted, malformed, wrinkled or puckered, with irregular torn margins. Defoliation often follows severe infections. Similar warty lesions and corky eruptions are formed on young twigs, tender shoots and stems of nursery plants which can grow bushy and stunted. Blossom pedicels and buttons may also be attacked.
Fruits are infected in the early stages of their development, grow misshapen and are subject to premature drop (CABI, 2017). On the rind of developed fruits, raised lesions are formed with different shape, size and colour depending on citrus species and cultivar affected. They appear as scattered protuberances, conical projections or crater‐like outgrowths or they coalesce to give scabby patches or extensive areas of fine eruptions. Scab symptoms, however, do not extend to the flesh.
E. fawcettii scabs are typically irregular, warty and deeply fissured, while E. australis forms larger, smoother and more circular scabs.
Citrus scab may be confused with other diseases, e.g. bacterial canker (Xanthomonas citri) and melanose (Diaporthe citri), or with injuries caused by various agents (e.g. wind).
Morphology
Ascomata are pulvinate, globose, dark, pseudoparenchymatous, multilocular, up to 80–120 μm thick. Asci up to 20 per locule are subglobose or ovoid, bitunicate with the inner wall thickened at the top, 12–16 μm in diameter, eight‐spored (EPPO, 1992). Ascospores are hyaline, ellipsoidal or oblong‐ellipsoidal, with two to four cells, usually constricted at the central septum, 10–12 × 5–6 μm in diameter for E. fawcettii and 12–20 × 4–8 μm for E. australis. The teleomorphs of E. fawcettii and E. australis are only known from Brazil.
The anamorphs of E. fawcettii and E. australis are practically identical. Acervuli intraepidermal or subepidermal, scattered or confluent, pseudoparenchymatous (CABI, 2017). Conidia hyaline, one celled, elliptical, 3–4 × 4–8 μm (Timmer, 2000). Mycelium hyaline, scanty, septate, short‐branched. In addition to hyaline conidia, E. fawcettii produces on scab lesions coloured, spindle‐shaped conidia, which germinate to produce hyaline conidia (Timmer, 2000).
Colonies in culture very slow‐growing, rose to purple, well raised above the agar surface and covered by tufts of short erect hyphae.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
The EPPO Global Database provides the geographical distribution for Elsinoë fawcettii (Figure 1, Table 2) and Elsinoë australis (Figure 2, Table 3) reported worldwide.
Figure 1.

Global distribution map for Elsinoë fawcettii extracted from the EPPO Global Database (last updated 13/9/2017; last accessed on 22/11/2017). Dots indicate the presence of the pathogen in the respective areas
Table 2.
Global distribution of Elsinoë fawcettii based on information extracted from the EPPO Global Database (last updated: 13/9/2017; last accessed: 22/11/2017)
| Continent | Country | Status | Sources |
|---|---|---|---|
| Africa | Congo, Democratic Republic of the | Present, no details | EPPO |
| Ethiopia | Present, no details | EPPO | |
| Gabon | Present, no details | EPPO | |
| Ghana | Present, no details | EPPO | |
| Kenya | Present, no details | EPPO | |
| Madagascar | Present, no details | EPPO | |
| Malawi | Present, no details | EPPO | |
| Mozambique | Present, no details | EPPO | |
| Nigeria | Present, no details | EPPO | |
| Sierra Leone | Present, no details | EPPO | |
| Somalia | Present, no details | EPPO | |
| South Africa | Present, widespread | EPPO | |
| Tanzania | Present, no details | EPPO | |
| Uganda | Present, no details | EPPO | |
| Zambia | Present, no details | EPPO | |
| Zimbabwe | Present, few occurrences | EPPO | |
| America | Argentina | Present, no details | EPPO |
| Barbados | Present, no details | EPPO | |
| Belize | Present, no details | EPPO | |
| Bermuda | Present, no details | EPPO | |
| Bolivia | Present, no details | EPPO | |
| Brazil | Present, no details | EPPO | |
| Cayman Islands | Present, no details | EPPO | |
| Colombia | Present, no details | EPPO | |
| Costa Rica | Present, no details | EPPO | |
| Cuba | Present, no details | EPPO | |
| Dominica | Present, no details | EPPO | |
| Dominican Republic | Present, no details | EPPO | |
| Ecuador | Present, restricted distribution | EPPO | |
| El Salvador | Present, no details | EPPO | |
| French Guiana | Present, no details | EPPO | |
| Grenada | Present, widespread | EPPO | |
| Guadeloupe | Present, no details | EPPO | |
| Guatemala | Present, no details | EPPO | |
| Guyana | Present, no details | EPPO | |
| Haiti | Present, no details | EPPO | |
| Honduras | Present, no details | EPPO | |
| Jamaica | Present, no details | EPPO | |
| Martinique | Present, widespread | EPPO | |
| Mexico | Present, restricted distribution | EPPO | |
| Nicaragua | Present, no details | EPPO | |
| Panama | Present, widespread | EPPO | |
| Paraguay | Present, widespread | EPPO | |
| Peru | Present, no details | EPPO | |
| Puerto Rico | Present, no details | EPPO | |
| Saint Lucia | Present, no details | EPPO | |
| Suriname | Present, no details | EPPO | |
| Trinidad and Tobago | Present, widespread | EPPO | |
| United States of America | Present, restricted distribution | EPPO | |
| Uruguay | Present, restricted distribution | EPPO | |
| Venezuela | Present, no details | EPPO | |
| Asia | Bangladesh | Present, widespread | EPPO |
| Brunei Darussalam | Present, no details | EPPO | |
| Cambodia | Present, no details | EPPO | |
| China | Present, no details | EPPO | |
| India | Present, widespread | EPPO | |
| Indonesia | Present, no details | EPPO | |
| Japan | Present, no details | EPPO | |
| Korea Dem. People's Republic | Present, no details | EPPO | |
| Korea, Republic | Present, no details | EPPO | |
| Lao | Present, no details | EPPO | |
| Lebanon | Present, no details | EPPO | |
| Malaysia | Present, widespread | EPPO | |
| Maldives | Present, no details | EPPO | |
| Myanmar | Present, no details | EPPO | |
| Nepal | Present, no details | EPPO | |
| Pakistan | Present, no details | EPPO | |
| Philippines | Present, no details | EPPO | |
| Sri Lanka | Present, no details | EPPO | |
| Taiwan | Present, restricted distribution | EPPO | |
| Thailand | Present, no details | EPPO | |
| Vietnam | Present, no details | EPPO | |
| Europe (non‐EU countries) | Georgia | Present, no details | EPPO |
| Oceania | American Samoa | Present, no details | EPPO |
| Australia | Present, restricted distribution | EPPO | |
| Cook Islands | Present, no details | EPPO | |
| Fiji | Present, no details | EPPO | |
| French Polynesia | Present, no details | EPPO | |
| Guam | Present, no details | EPPO | |
| Micronesia | Present, no details | EPPO | |
| New Caledonia | Present, no details | EPPO | |
| New Zealand | Present, no details | EPPO | |
| Papua New Guinea | Present, no details | EPPO | |
| Samoa | Present, no details | EPPO | |
| Solomon Islands | Present, no details | EPPO | |
| Vanuatu | Present, no details | EPPO |
Figure 2.

Global distribution map for Elsinoë australis extracted from the EPPO Global Database (last updated 13/9/2017; last accessed on 22/11/2017). Dots indicate the presence of the pathogen in the respective areas
Table 3.
Global distribution of Elsinoë australis based on information extracted from the EPPO Global Database (last updated: 13/9/2017; last accessed: 22/11/2017)
| Continent | Country | Status | Sources |
|---|---|---|---|
| America | Argentina | Present, no details | EPPO |
| Bolivia | Present, no details | EPPO | |
| Brazil | Present, no details | EPPO | |
| Paraguay | Present, no details | EPPO | |
| United States of America | Present, restricted distribution | EPPO | |
| Uruguay | Present, no details | EPPO | |
| Asia | Japan | Present, restricted distribution | EPPO |
| Korea, Republic | Present, no details | EPPO | |
| Oceania | Australia | Present, restricted distribution | EPPO |
| Cook Islands | Present, no details | EPPO | |
| Fiji | Present, no details | EPPO | |
| Niue | Present, no details | EPPO | |
| Samoa | Present, no details | EPPO |
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
No, Elsinoë fawcettii and E. australis are not known to occur in the risk assessment area.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
Elsinoë fawcettii and E. australis are regulated as harmful organisms in the EU and are listed as Elsinoë spp. in Council Directive 2000/29/EC. Details are presented in Tables 4 and 5.
Table 4.
Elsinoë fawcettii and E. australis (as Elsinoë spp.) in Council Directive 2000/29/EC
| Annex II, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned if they are present on certain plants or plant products | |
| Section I | Harmful organisms not known to occur in the community and relevant for the entire community | |
| (c) | Fungi | |
| Species | Subject of contamination | |
| 9. | Elsinoë spp. Bitanc. and Jenk. Mendes | Plants of Fortunella Swingle, Poncirus Raf., and their hybrids, other than fruit and seeds and plants of Citrus L. and their hybrids, other than seeds and other than fruits, except fruits of Citrus reticulata Blanco and of Citrus sinensis (L.) Osbeck originating in South America |
Table 5.
Regulated hosts and commodities that may involve Elsinoë fawcettii and E. australis in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
| Description | Country of origin | |
| 16 | Plants of Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids, other than fruit and seeds | Third countries |
| Annex IV, Part A | Special requirements which must be laid down by all Μember States for the introduction and movement of plants, plant products and other objects into and within all member states | |
| Section I | Plants, plant products and other objects originating outside the community | |
| Plant, plant products and other objects | Special requirements | |
| 16.1 | Fruits of Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids, originating in third countries | The fruits shall be free from peduncles and leaves and the packaging shall bear an appropriate origin mark |
| Annex IV, Part A | Special requirements which must be laid down by all Member States for the introduction and movement of plants, plant products and other objects into and within all member states | |
| Section II | Plants, plant products and other objects originating in the community | |
| Plant, plant products and other objects | Special requirements | |
| 30.1 | Fruits of Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids | The packaging shall bear an appropriate origin mark. |
|
Annex IV, Part B |
Special requirements which must be laid down by all member states for the introduction and movement of plants, plant products and other objects into and within certain protected zones | ||
| Plants, plant products and other objects | Special requirements | Protected zone(s) | |
| 31. | Fruits of Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids originating in BG, HR, SI, EL (Regional Units of Argolida and Chania), P (Algarve and Madeira), E, F, CY and I |
Without prejudice to the requirement in Annex IV Part A Section II point 30.1 that packaging should bear an origin mark: (a) the fruits shall be free from leaves and peduncles; or (b) in the case of fruits with leaves or peduncles, official statement that the fruits are packed in closed containers which have been officially sealed and shall remain sealed during their transport through a protected zone, recognised for these fruits, and shall bear a distinguishing mark to be reported on the passport. |
EL (except the Regional Units of Argolida and Chania), M, P (except Algarve and Madeira) |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community—in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community |
| Part A | Plants, plant products and other objects originating in the Community |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community and which must be accompanied by a plant passport |
| 1.5 | Without prejudice to point 1.6, plants of Citrus L. and their hybrids other than fruit and seeds. |
| 1.6 | Fruits of Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids with leaves and peduncles. |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community—in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community |
| Part B | Plants, plant products and other objects originating in territories, other than those referred to in Part A |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community |
| 1. | Plants, intended for planting, other than seeds but including seeds of Cruciferae, Gramineae, Trifolium spp., originating in Argentina, Australia, Bolivia, Chile, New Zealand and Uruguay, genera Triticum, Secale and xTriticosecale from Afghanistan, India, Iran, Iraq, Mexico, Nepal, Pakistan, South Africa and the USA, Citrus L., Fortunella Swingle and Poncirus Raf., and their hybrids, Capsicum spp., Helianthus annuus L., Solanum lycopersicum L., Medicago sativa L., Prunus L., Rubus L., Oryza spp., Zea mais L., Allium ascalonicum L., Allium cepa L., Allium porrum L., Allium schoenoprasum L. and Phaseolus L. |
| 3. |
Fruits of: — Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids, Momordica L. and Solanum melongena L. |
3.3.2. Legislation addressing the hosts of Elsinoë fawcettii and E. australis
Additional movement restrictions for the hosts exist in relation to other pests, such as Cercospora angolensis, Xanthomonas campestris (all strains pathogenic to citrus), Citrus variegated chlorosis, etc.
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
Elsinoë fawcettii and E. australis affect cultivated and ornamental species and hybrids of the family Rutaceae (EPPO, 2017). The principal host of E. fawcettii is Citrus aurantium, but C. paradisi, C. limon, C. reticulata and some cultivars of C. sinensis can also be affected (Table 6).
Table 6.
Hosts of Elsinoë fawcettii and E. australis according to EPPO Global Database (last updated: 13/9/2017; last accessed: 24/9/2017)
| Hosta | Elsinoë fawcettii | Elsinoë australis |
|---|---|---|
| Citrus aurantium | Major | Minor |
| Citrus aurantifolia | Minor | |
| Citrus limon | Major | Minor |
| Citrus paradisi | Major | Minor |
| Citrus reticulata | Major | Minorb |
| Citrus x tangelo | Major | |
| Citrofortunella microcarpa | Minor | Minor |
| Citroncirus | Minor | Minor |
| Citrus deliciosa | Minor | |
| Citrus jambhiri | Minor | |
| Citrus medica | Minor | |
| Citrus sinensis | Minor | Major |
| Citrus unshiu | Minor | Minor |
| Citrus x limonia | Minor | |
| Citrus x nobilis | Minor | Minor |
| Poncirus trifoliata | Minor | Minor |
| Citrus aurantiifolia | Incidental | |
| Fortunella spp. | Incidental | Minor |
| Fortunella margarita | Minor | |
| Citrus hystrix | Incidental |
All these hosts are regulated except for Simmondsia chinensis (jojoba), the status of which as a host of E. australis is uncertain.
Considered as a major host of E. australis by CABI (2017).
E. australis has a more restricted host range compared to E. fawcettii. Its major host is C. sinensis, although C. limon, C. reticulata, C. unshiu, C. aurantifolia, C. paradisi and Fortunella spp. are also affected (Table 6).
All the above‐mentioned hosts of E. fawcettii and E. australis are regulated.
EPPO Global Database indicates Simmondsia chinensis (jojoba) as a host of E. australis. However, in the supporting phylogenetic studies (Ash et al., 2012; Miles et al., 2015), only a few loci were used, which, according to Fan et al. (2017) are not adequate for a reliable identification of Elsinoë species (see section 3.1.4). Therefore, the status of S. chinensis as a host of E. australis is uncertain.
3.4.2. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways!
Yes, under the current EU legislation, E. fawcettii and E. australis could potentially enter the risk assessment area on the citrus fruit without leaves and peduncles pathway
The PLH Panel identified the following pathways for the entry of E. fawcettii and E. australis into the EU territory:
Host plants for planting, excluding seeds, and
Citrus fruit (with or without leaves and peduncles) originating in infested Third countries.
No evidence exists for Elsinoë spp. to be seedborne.
Nevertheless, under the current EU legislation, only the citrus fruit without leaves and peduncles pathway is relevant for both the pathogens, as the import into the EU territory of plants of Citrus, Poncirus and Fortunella and their hybrids, and citrus fruit with leaves and peduncles, is prohibited.
The volume of citrus fruit imported into the EU from non‐EU countries and non‐EU countries infested with E. fawcettii or E. australis is presented in Table 7.
Table 7.
Volume (in tons) of citrus fruit imported during the period 2011–2015 into the EU Member States from non‐EU countries and from countries where Elsinoë fawcettii and E. australis are reported as present (Source: Eurostat, extracted on 9 November 2017)
| Total EU 28 citrus fruit import (in tons) from | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| Non‐EU countries | 434,811 | 425,786 | 444,879 | 365,897 | 445,339 |
| Infested non‐EU28 countries | 307,294 | 335,569 | 336,090 | 270,017 | 315,612 |
Based on the above data, during the period 2011–2015, 71–79% of the total volume of citrus fruit imported by the 28 EU Member States from Third countries originated in areas where the pests are reported as present.
From 2001 to May 2017, there were 64 interceptions of Elsinoë spp. on citrus in the Europhyt database (search performed on 8 November 2017).
3.4.3. Establishment
Is the pest able to become established in the EU territory?
Yes, both the biotic (host availability) and abiotic (climate suitability) factors suggest that E. fawcettii and E. australis could potentially establish in the risk assessment area
3.4.3.1. EU distribution of main host plants
As shown in Figure 3, the greatest density of citrus production occurs in the southern EU Member States. Around 700,000 ha are allocated to citrus production in the EU. Table 8 provides further details on the area of citrus harvested in each EU Member State: four Member States (i.e. Spain, Italy, Greece and Portugal) concentrate 98% of the total EU citrus‐growing area.
Figure 3.

EU map of NUTS3 citrus‐growing regions based on citrus production data extracted from national statistical databases of Portugal, Spain, France, Italy, Malta, Croatia, Greece and Cyprus (EFSA PLH Panel, 2014)
Table 8.
Area cultivated with citrus in the EU between 2011 and 2015 (in 1,000 ha) – Source: Eurostat, extracted on 7/6/2017
| Countries | 2011 | 2012 | 2013 | 2014 | 2015 | Mean of EU citrus‐growing area (in 1,000 ha) |
|---|---|---|---|---|---|---|
| European Union (28 Member States) | 726.56 | 702.30 | 712.35 | 684.32 | 685.94 | 702.29 |
| Spain | 437.82 | 426.26 | 420.39 | 415.67 | 410.19 | 422.07 |
| Italy | 198.30 | 182.97 | 198.51 | 174.93 | 183.47 | 187.64 |
| Greece | 59.10 | 57.43 | 57.24 | 57.67 | 55.45 | 57.38 |
| Portugal | 21.93 | 22.26 | 22.17 | 22.21 | 22.71 | 22.26 |
| France | 5.69 | 5.78 | 6.61 | 6.26 | 6.32 | 6.13 |
| Croatia | NA | 3.70 | 4.26 | 4.32 | 4.36 | 4.16a |
| Cyprus | 3.72 | 3.90 | 3.17 | 3.25 | 3.44 | 3.50 |
NA, not available.
Only citrus‐producing Member States are reported above.
Calculated on 4 years (2012–2015).
3.4.3.2. Climatic conditions affecting establishment
Citrus scab diseases are widespread in areas where suitable conditions of temperature and rainfall or high humidity prevail (wet subtropics and cooler tropics). Elsewhere, it occurs when new flush and fruit set coincide with spells of relatively warm, humid weather (CABI, 2017). Citrus scab is also favoured by damp, low‐lying areas and dense, shaded citrus groves.
The citrus‐growing regions in the risk assessment area (EFSA PLH Panel, 2014) are mainly characterised by the following climate types (Peel et al., 2007): Csa (temperate, dry and hot summer), Csb (temperate, dry and warm summer), BSk (arid, steppe, cold) and Cfa (temperate, without dry season, hot summer); Cfb (temperate, without dry season, warm summer) and Bwk (arid, desert, cold) are also present, but to a lesser extent. Considering the distribution of E. fawcettii (Figure 1), climate types Csa, Cfa and Cfb are present in South America; BSk and Cfb in Africa; Cfa in Asia; Bsk and Cfa in North and Central America; Csa, Cfa and Cfb in Australia; Csa and Csb in the affected areas in the Middle East (Figures 4 and 5). The current distribution of E. australis (Figure 2) also includes the above‐mentioned climate types (Figures 4 and 5). In addition, the extensive use of surface, sprinkle and micro‐sprinkle irrigation in the EU citrus‐growing areas might add to the suitability of the environment, since irrigation has the potential to lengthen the periods of leaf wetness favouring infection (EFSA PLH Panel, 2014).
Figure 4.
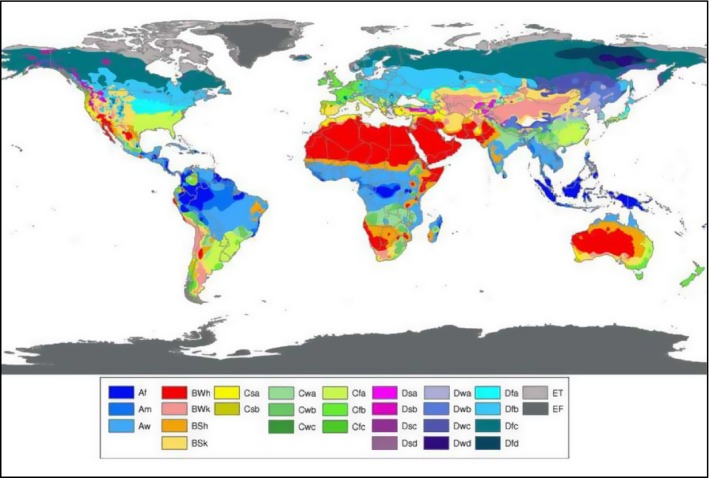
Köppen–Geiger climate type world map from Peel et al. (2007)
Figure 5.

Köppen–Geiger climate type map of Europe from Peel et al. (2007)
Based on the above, E. fawcettii and E. australis could cause infection and establish under the climatic conditions prevailing in the EU citrus‐growing areas.
3.4.4. Spread
3.4.4.1. Vectors and their distribution in the EU (if applicable)
Is the pest able to spread within the EU territory following establishment? Yes
How? By natural and human‐assisted means
Elsinoë fawcettii and E. australis can spread in the risk assessment area by both natural and human‐assisted means.
Spread by natural means. Both Elsinoë species can spread locally by water droplets (rain or irrigation water). Insects and wind‐driven rain may also contribute to the spread of the pathogens (CABI, 2017). Short–distance wind‐dispersal has been reported for the spindle‐shaped conidia of E. fawcettii (Timmer, 2000). There is uncertainty with respect to the maximum distance of spore dispersal by natural means, as no information was found in the literature on this aspect.
Spread by human assistance. In trade, the pathogens can spread via the movement of infected host plants for planting and fresh fruit with or without leaves and peduncles.
3.5. Impacts
Would the pests' introduction have an economic or environmental impact on the EU territory?
Yes, the introduction of the pests could potentially cause yield and quality losses to citrus grown in the risk assessment area
Elsinoë fawcettii is more widespread than E. australis, but the latter is more economically important as it affects widely grown citrus species. The damage caused on fruit by scab (scarred and distorted fruit) does not affect the internal fruit quality but it reduces its marketability.
Citrus scab caused by E. fawcettii may be serious in the nursery on susceptible rootstocks, such as sour oranges, rough lemons, Poncirus trifoliata and Citrus limonia (CABI, 2017). It may stunt seedlings or make them bushy and difficult to bud. E. australis differs in only causing fruit scab, mainly on oranges and mandarins.
Citrus scab is important only in areas where susceptible species or cultivars of citrus fruit are grown for the fresh market and where young plants or new growth develop under favourable conditions of temperature, moisture and shade (CABI, 2017). Losses largely depend on seasonal and local weather conditions.
In Uruguay, Bernal (2000) reported incidence up to 98% of culled fruit due to scab (Elsinoë sp.) in untreated plots. The disease incidence was reduced to 0.7–7.4% after applying fungicides. In Florida, Whiteside (1974, 1981) indicated a scab incidence caused by E. fawcettii from 15% to 78% of affected fruit in untreated control plots. The disease incidence was reduced to 0.4–9.7% after fungicide treatment.
It is not known if agronomic practices and climatic conditions in the risk assessment area will lead to similar levels of impact as in the places of origin.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
YES, the likelihood of pest entry can be mitigated if citrus plants for planting are produced under a certification scheme or host plants for planting and fresh fruit are sourced from pest‐free areas or pest‐free places of production and are inspected and lab tested both at the place of origin and the EU entry point. In infested areas, sanitation, agricultural practices and fungicide sprays are available for disease management.
Measures for preventing the entry of the pathogens into the risk assessment area include:
sourcing host plants for planting and fruit, from pest‐free areas or pest‐free places of production
import citrus planting material produced under a certification scheme
phytosanitary certificate for the export of host plants for planting and fruit from infested Third countries
inspection and lab testing of host plants for planting and fruit prior to export to the EU and at the EU entry point.
Measures for preventing the establishment and spread of the pathogens in the risk assessment area include:
use of sanitary measures (e.g. removal of infected plants or plant parts and pruning residues, disinfection of pruning and grafting tools)
application of fungicide sprays
crop residue management
restrict the movement of infected plant material.
3.6.1. Biological or technical factors limiting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
The following biological and technical factors could potentially limit the feasibility and effectiveness of measures to prevent the entry into, establishment and spread within the risk assessment area of E. fawcettii and E. australis:
the presence of latent infections: incubation period on leaves (i.e. for E. fawcettii, 3 days after infection at 20°C and 4 days at 30°C) (Timmer, 1999) and fruit (i.e. for E. fawcettii, 7 days after infection (Chung, 2011); for E. australis, 10 days (Bitancourt and Jenkins, 1937))
similarity of symptoms with those of other citrus diseases (e.g. citrus canker) or abiotic agents (e.g. mechanical injuries).
3.6.2. Control methods
In the infested areas, the following agricultural practices as well as sanitary and chemical measures are used for the management of the citrus scab diseases caused by E. fawcettii and E. australis:
-
Agricultural measures:
-
1
‐ Use of resistant citrus species and cultivars.
-
2
‐ Improve orchard ventilation by adequate tree spacing, row orientation and pruning.
-
1
Sanitation measures to reduce inoculum sources in the orchards (e.g. burial of fallen infected leaves, removal of symptomatic fruit, etc)
Application of protectant and/or systemic fungicides. In Argentina, two chemical sprays are applied for the control of citrus scab; the first one when 25% of the flowers are open and the second one 7–10 days after the first (Timmer, 2000; Schultz et al., 2013). In Florida, a control programme with two fungicide sprays, one at petal fall followed by a second one 2–3 weeks later, is used. Benzimidazole‐tolerant strains of E. fawcettii have been detected in the USA (Florida) and Uruguay (Whiteside, 1980; Bernal, 2000).
In the risk assessment area, agricultural practices and sanitary and chemical measures are applied to commercial citrus orchards for the control of other fungal diseases. However, it is not known if those measures would be effective in preventing the establishment and spread of E. fawcettii and E. australis in the EU territory.
3.7. Uncertainty
Host range: the status of S. chinensis as a host of E. australis is uncertain because the method used for the characterisation of the pathogen is not considered adequate for a reliable identification of Elsinoë species.
Establishment: it is unknown whether cultural practices and disease control methods, currently applied in the EU, would be effective in preventing the establishment of E. fawcettii and E. australis.
Spread: lack of data regarding the distance the airborne inoculum of E. fawcettii can travel.
Impacts: it is unknown whether agronomic practices and climatic conditions in the EU will lead to similar levels of impact as in the places of origin.
4. Conclusions
Elsinoë fawcettii and E. australis meet all the criteria assessed by EFSA for consideration as potential EU quarantine pests. As the pests are not known to occur in the EU, they do not meet at least one of the criteria assessed by EFSA for consideration as Union regulated non‐quarantine pests (see Table 9).
Table 9.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1) | The identity of the pests (E. fawcettii and E. australis) is clearly defined and there are reliable methods for their detection and identification | The identity of the pests (E. fawcettii and E. australis) is clearly defined and there are reliable methods for their detection and identification | None |
| Absence/presence of the pest in the EU territory (Section 3.2) | The pests are not known to occur in the EU | The pests are not known to occur in the EU | None |
| Regulatory status (Section 3.3) | The pests are currently officially regulated as quarantine pests on plants of Poncirus and Fortunella and their hybrids, other than fruit and seeds and plants of Citrus (Dir 2000/29/EC) | The pests are currently officially regulated as quarantine pests on plants of Poncirus and Fortunella and their hybrids, other than fruit and seeds and plants of Citrus (Dir 2000/29/EC) | It is uncertain whether Simmondsia chinensis (jojoba) is a host of E. australis (Uncertainty 1) |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) |
The pests could potentially enter, establish and spread in the EU. Pathways of entry: 1. Host plants for planting, excluding seeds, and 2. Citrus fruit (with or without leaves and peduncles) originating in infested Third countries |
The pests could potentially spread in the EU through movement of host plants for planting, fresh fruits of host plants, and natural means. Therefore, plants for planting is a main pathway, but not the only one. |
It is uncertain whether Simmondsia chinensis (jojoba) is a host of E. australis (Uncertainty 1) It is unknown whether cultural practices and disease control methods, currently applied in the EU, would be effective in preventing the establishment of Elsinoë spp. (Uncertainty 2) There is lack of data regarding the distance the airborne inoculum of Elsinoë fawcettii can travel. (Uncertainty 3) |
| Potential for consequences in the EU territory (Section 3.5) | The introduction and spread of the pests in the EU could cause yield and quality losses in citrus production | The spread of the pests in the EU could cause losses as regards the intended use of citrus plants for planting | It is unknown whether agronomic practices and climatic conditions in the EU will lead to similar levels of impact as in the places of origin (Uncertainty 4). |
| Available measures (Section 3.6) | Phytosanitary measures are available to prevent the entry of the pests into the EU (e.g. sourcing host plants for planting and fruit from pest‐free areas or pest‐free places of production). There are no fully effective measures to prevent establishment and spread. | There are no fully effective measures to prevent the spread of the pests in the risk assessment area. | The distance the conidia of Elsinoë fawcettii can travel by air currents is unknown (Uncertainty 3) |
| Conclusion on pest categorisation (Section 4) | E. fawcettii and E. australis meet all the criteria assessed by EFSA above for consideration as potential Union quarantine pests. | E. fawcettii and E. australis are not known to occur in the EU. Therefore, they do not meet at least one of the criteria assessed by EFSA for consideration as Union regulated non‐quarantine pests | None |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | None | ||
Abbreviations
- DG SANCO
Directorate General for Health and Consumers
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- MS
Member State
- PLH
EFSA Panel on Plant Health
- RAPD
rapid amplified polymorphic DNA
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Grégoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Vicent A, Vloutoglou I, Bottex B and Rossi V, 2017. Scientific Opinion on the pest categorisation of Elsinoë fawcettii and E. australis . EFSA Journal 2017;15(12):5100, 27 pp. 10.2903/j.efsa.2017.5100
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00330
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Grégoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Adopted: 22 November 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figures 1 and 2: © EPPO; Figures 4 and 5: © CABI
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
References
- Ash GJ, Stodart B and Hyun J‐W, 2012. Black scab of jojoba (Simmondsia chinensis) in Australia caused by a putative new pathotype of Elsinoë australis . Plant Disease, 96, 629–634. [DOI] [PubMed] [Google Scholar]
- Bernal RP, 2000. Occurrence of citrus scab strains resistant to benzimidazoles in the northern part of Uruguay and the evaluation of new fungicides to control this disease. In: Proceedings of the International Society of Citriculture IX Congress, 3–7 December 2000, Orlando, Florida, USA, pp. 984–986.
- Bitancourt AA and Jenkins AE, 1936. Elsinoë fawcettii, the perfect stage of the citrus scab fungus. Phytopathology, 26, 393–396. [Google Scholar]
- Bitancourt AA and Jenkins AE, 1937. Sweet orange fruit scab caused by Elsinoë australis . Journal of Agricultural Research, 54, 1–18. [Google Scholar]
- CABI , 2017. Invasive Species Compendium. Available online: http://www.cabi.org/isc/
- Chung KR, 2011. Elsinoë fawcettii and Elsinoë australis. The fungal pathogens causing citrus scab. Molecular Plant Pathology, 12, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2903/j.efsa.2010.1495 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2014. Phyllosticta citricarpa risk assessment. EFSA Journal 2014;12(2):3557, 243 pp. 10.2903/j.efsa.2014.3557. [DOI] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization)/CABI, 1992. Data Sheets on Quarantine Pests: Elsinoë fawcettii and E. australis In: Smith IM, McNamara DG, Scott PR. and Harris KM. (eds.). Quarantine pests for Europe. CAB International, Wallingford, UK. 6 pp. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2017. EPPO Global Database. Available online: https://gd.eppo.int
- Europhyt , online. The European Network of Plant Health Information System. EUROPHYT database. Available online: https://europhyt.ec.europa.eu
- EUROSTAT, online . Eurostat agricultural statistics. Available online: http://ec.europa.eu/eurostat/web/agriculture/data/database
- Fan XL, Barreto RW, Groenewald JZ, Bezerra JDP, Pereira OL, Cheewangkoon R, Mostert L, Tian CM and Crous PW, 2017. Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Studies in Mycology, 87, 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- Hou X, Huang F, Zhang T, Xu J, Hyde DK and Li H, 2014. Pathotypes and genetic diversity of Chinese collections of Elsinoë fawcettii causing citrus scab. Journal of Integrative Agriculture, 13, 1293–1302. [Google Scholar]
- Hyun J‐W, Timmer LW, Lee SC, Yun SH, Ko SW and Kim KS, 2001. Pathological characterization and molecular analysis of Elsinoë isolates causing scab diseases of citrus in Jeju island in Korea. Plant Disease, 85, 1013–1017. [DOI] [PubMed] [Google Scholar]
- Hyun J‐W, Peres NA, Yi SY, Timmer LW, Kim KS, Kwon HM and Lim HC, 2007. Development of PCR assays for the identification of species and pathotypes of Elsinoë causing scab on citrus. Plant Disease, 91, 865–870. [DOI] [PubMed] [Google Scholar]
- Hyun J‐W, Yi SH, MacKenzie SJ, Timmer LW, Kim KS, Kang SK, Kwon HM and Lim HC, 2009. Pathotypes and genetic relationship of worldwide collections of Elsinoë spp. causing scab diseases of citrus. Phytopathology, 99, 721–728. [DOI] [PubMed] [Google Scholar]
- Miles AK, Tan YP and Shivas RG, 2015. Novel pathotypes of Elsinoë australis associated with Citrus australasica and Simmondsia chinensis in Australia. Tropical Plant Pathology, 40, 26–34. [Google Scholar]
- Peel MC, Finlayson BL and McMahon TA, 2007. Updated world map of the Köppen‐Geiger climate classification. Hydrology and Earth System Sciences, 11, 1633–1644. [Google Scholar]
- Schultz D, Rybak M and French RD, 2013. Citrus scab and sweet orange scab. Texas A&M Agrilife Extension, Amarillo, TX, USA: 2 pp. [Google Scholar]
- Timmer LW, 1999. Diseases of fruit and foliage In: Timmer LW. and Duncan LW. (eds.). Citrus Health Management. APS Press Inc., St. Paul: pp. 107–115. [Google Scholar]
- Timmer LW, 2000. Scab diseases In: Timmer LW, Garnsey SM. and Graham JH. (eds.). Compendium of Citrus Diseases, 2nd Edition The American Phytopathological Society, St Paul, MN, USA: pp. 31–32. [Google Scholar]
- Timmer LW, Priest M, Broadbent P and Tan M‐K, 1996. Morphological and pathological characterization of species of Elsinoë causing scab diseases of citrus. Phytopathology, 86, 1032–1038. [Google Scholar]
- Wang LY, Liao HL, Bau HJ and Chung K‐R, 2009. Characterization of pathogenic variants of Elsinoë fawcettii of citrus implies the presence of new pathotypes and cryptic species in Florida. Canadian Journal of Plant Pathology, 31, 28–37. [Google Scholar]
- Whiteside JO, 1974. Evaluation of fungicides for citrus scab control. Proceedings of the Florida State Horticultural Society, 87, 9–14. [Google Scholar]
- Whiteside JO, 1975. Biological characteristics of Elsinoë fawcettii pertaining to the epidemiology of sour orange scab. Phytopathology, 65, 1170–1175. [Google Scholar]
- Whiteside JO, 1980. Detection of benomyl‐tolerant strains of Elsinoë fawcettii in Florida citrus groves and nurseries. Plant Disease, 64, 871–872. [Google Scholar]
- Whiteside JO, 1981. Evolution of current methods for citrus scab control. Proceedings of the Florida State Horticultural Society, 94, 5–8. [Google Scholar]
- Whiteside JO, 1988. Factors contributing to the rare occurrence of scab of sweet orange in Florida. Plant Disease, 72, 626–628. [Google Scholar]


