Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, BASF Agro SAS submitted an application to the competent national authority in France to modify the existing maximum residue levels (MRLs) for the active substance pyraclostrobin in celeriacs, spinaches, chards/beet leaves, witloofs, beans and peas with pods, peas without pods, celeries, fennels and leeks. On the basis of the French evaluation report, EFSA concluded that the data are sufficient to derive MRL proposals for all the crops under consideration. Adequate analytical enforcement methods are available to control the residues of pyraclostrobin in the commodities under consideration. EFSA performed a risk assessment in which potential acute consumer health risks for the intended use on celeries and fennels in Northern EU were identified. The intended uses of pyraclostrobin on the crops under consideration, including the Southern EU uses in celeries and fennels, will not result in a consumer exposure exceeding the toxicological reference values and therefore are unlikely to pose a public health risk. For leeks, a refined acute risk assessment was proposed by the French authority, using a processing factor. Since the available data do not provide sufficient evidence that the short‐term exposure is below the acute reference dose, EFSA does not propose to raise the existing MRL.
Keywords: pyraclostrobin, vegetables, MRL application, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, the evaluating Member State (EMS) France, received an application from BASF Agro SAS to modify the existing maximum residue levels (MRLs) for the active substance pyraclostrobin in various crops. France drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA). To accommodate for the intended uses of pyraclostrobin, France proposed to raise the existing MRLs in celeriacs, spinaches, chards/beet leaves, witloofs, beans and peas with pods, peas without pods and leeks. Due to acute intake concerns, a modification of the existing MRL was not proposed for celeries and fennels.
EFSA bases its assessment on the revised evaluation report submitted by the EMS, the draft assessment report (DAR) and its addendum prepared under Directive 91/414/EEC, the Commission review report on pyraclostrobin, the Joint Meeting on Pesticide Residues (JMPR) evaluation report as well as the conclusions from previous EFSA opinions on pyraclostrobin, including the review of the existing MRLs for pyraclostrobin under Article 12 (hereafter Article 12 MRL review).
The toxicological profile of pyraclostrobin was assessed in the framework of the peer review under Directive 91/414/EEC and the data were sufficient to derive an acceptable daily intake (ADI) of 0.03 mg/kg bodyweight (bw) per day and an acute reference dose (ARfD) of 0.03 mg/kg bw.
The metabolism of pyraclostrobin in primary crops was investigated in the fruit, root and cereal/grass crop groups following foliar applications and the general residue definition for enforcement and risk assessment as pyraclostrobin parent compound was established.
EFSA concluded that the submitted residue trials are sufficient to derive MRL proposals for celeriacs (0.5 mg/kg), spinaches, beans and peas with pods (0.6 mg/kg), witloofs (0.09 mg/kg), peas without pods (0.15 mg/kg), celeries and fennels (5 mg/kg) and leeks (1 mg/kg). For chards/beet leaves, EFSA has recently proposed a higher MRL value than the MRL requested by the applicant. Thus, the MRL proposed in the recently published opinion (1.5 mg/kg) covers the intended uses submitted with this MRL application. Adequate analytical enforcement methods are available to monitor the residues of pyraclostrobin in the commodities under consideration at the validated limit of quantification (LOQ) of 0.01 mg/kg.
Under standard hydrolysis processing conditions no degradation of pyraclostrobin was observed; therefore, for processed commodities, the same residue definition as for raw agricultural commodities is applicable. Studies investigating the magnitude of pyraclostrobin residues in the crops under consideration were not submitted and are not required. However, processing studies were made available for other vegetables (cooked spinaches and head cabbages).
EFSA concluded that significant residue levels are unlikely to occur in rotational crops under the proposed uses. Residues of pyraclostrobin in commodities of animal origin were not assessed since none of the crops under consideration in this MRL application was used as feed item for livestock.
The consumer risk assessment was performed with revision 2 of the EFSA Pesticide Residues Intake Model (PRIMo). The long‐term consumer risk assessment performed under the Article 12 MRL review was updated with the STMR values derived for the crops under consideration and reported in previous EFSA reasoned opinions carried out after the Article 12 MRL review. Acute risk assessment was performed only on the crops under consideration.
No long‐term consumer intake concerns were identified for any of the European diets incorporated in the EFSA PRIMo. The total calculated intake accounted for up to 15% of the ADI (German child diet). No acute consumer risk was identified for celeriacs, spinaches, witloofs, beans and peas with pods and peas without pods. While for the less critical southern EU use in celeries and fennels and the northern EU use in leeks no intake concerns were identified, a consumer health risk could not be excluded for the southern EU use in leek. The applicant proposed to refine the acute risk assessment, extrapolating the processing factor for boiled spinaches to leeks. In this refined risk assessment, no consumer intake concerns were identified for the southern EU use in leeks. However, EFSA is of the opinion that the refined exposure scenario for leeks is affected by uncertainties (lack of information on the consumption of leek as cooked, robustness of the processing factor for spinach because of the limited and contradictory results).
The dietary risk assessment demonstrated that the proposed uses of pyraclostrobin on the crops under consideration, except the intended northern use on celeries and fennels and the southern use on leeks, will not result in a consumer exposure exceeding the toxicological reference values and therefore are unlikely to pose a public health risk. The available data do not provide sufficient evidence to conclude that the short‐term dietary exposure for leeks would be below the ARfD.
EFSA proposes to amend the existing MRLs as reported in the summary table below.
| Codeb | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Pyraclostrobinc | ||||
| 213030 | Celeriac | 0.3 | 0.5 | The submitted data are sufficient to derive a MRL proposal for the NEU use. The SEU use is not supported by data. No consumer health concern was identified |
| 252010 | Spinaches | 0.5 | 0.6 |
The MRL proposal reflects the more critical residue situation for the NEU use. No consumer health concern was identified. For the GAP reported by the EMS, a MRL of 0.6 mg/kg would be required (MRL for NEU and SEU use derived by extrapolation from data on spinaches). In a recent assessment, EFSA derived a higher MRL proposal of 1.5 mg/kg for a more critical indoor use which did not pose a consumer health concern (EFSA, 2016). This MRL proposal has not yet been implemented in the MRL legislation |
| 252030 | Chards/beet leaves | 0.5 | 1.5 (0.6) | |
| 255000 | Witloofs/Belgian endives | 0.02a | 0.09 | The MRL proposal reflects indoor use. No consumer health concern was identified |
| 260010 | Beans (with pods) | 0.02a | 0.6 | The MRL proposal covers the intended uses in NEU and SEU (MRL derived from dataset on beans with pods). No consumer health concern was identified |
| 260030 | Peas (with pods) | 0.02a | 0.6 | |
| 260040 | Peas (without pods) | 0.02a | 0.15 | NEU and SEU uses are sufficiently supported by data. The MRL proposal was derived from the more critical residue situation in NEU use. No consumer health concern was identified |
| 270030 | Celeries | 0.02a | 1.5 |
The proposed MRL reflects the intended use in SEU. The MRL proposal based on the residue trials in celeries was extrapolated to fennels. No consumer health risk was identified for this use The intended use in NEU would require a MRL of 5 mg/kg. However, a consumer health risk could not be excluded (celery: 402% of the ARfD; fennel: 178% of the ARfD) |
| 270040 | Florence fennels | 0.02a | 1.5 | |
| 270060 | Leeks | 0.7 | No change |
The intended use in NEU would not require a modification of the existing MRL. Although the SEU seems to be less critical, the supporting residue trials suggest a higher MRL of 1 mg/kg. Based on the SEU residue trials, the short‐term dietary exposure slightly exceeded the ARfD (104%). Although processing studies in head cabbage give an indication that cooking may reduce the residues, other studies (i.e. the standard hydrolysis studies and one processing study in spinach) do not support the assumption that cooking would significantly reduce the residues. Since the available data do not provide sufficient evidence that the short‐term exposure is below the ARfD, EFSA does not propose to raise the existing MRL |
MRL: maximum residue level; EMS: evaluating Member State; NEU: northern Europe; SEU: southern Europe; ARfD: acute reference dose; GAP: good agricultural practices.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Fat soluble.
Background
Regulation (EC) No 396/20051 (hereinafter referred to as ‘the Regulation’) establishes the rules governing the setting of pesticide maximum residue levels (MRLs) at European Union (EU) level. Article 6 of the Regulation lays down any party having a legitimate interest or requesting an authorisation for the use of a plant protection product in accordance with Council Directive 91/414/EEC2 repealed by Regulation (EC) No 1107/20093, shall submit to a Member State, when appropriate, an application to modify a MRL in accordance with the provisions of Article 7 of the Regulation.
The company BASF Agro SAS4 submitted an application to the competent authorities in France, hereafter referred to as the evaluating Member State (EMS) to modify the existing MRLs for the active substance pyraclostrobin in celeriacs, spinaches, chard/beet leaves, witloofs/Belgian endives, beans and peas with pods, peas without pods, leeks, celeries and fennels. This application was notified to the European Commission and the European Food Safety Authority (EFSA) and was subsequently evaluated by the EMS in accordance with Article 8 of the Regulation. The EMS prepared the evaluation report which was submitted to the European Commission and to EFSA on 28 January 2015. The application was included in the EFSA Register of Questions with the reference number EFSA‐Q‐2015‐00079 and the following subject:
Pyraclostrobin – Setting new MRLs in various crops
France proposed to raise the existing MRLs of pyraclostrobin for celeriacs to 0.5 mg/kg, for spinaches and chards/beet leaves to 0.7 mg/kg, for witloofs/Belgian endives to 0.09 mg/kg, for beans and peas with pods to 0.7 mg/kg, for peas without pods to 0.15 mg/kg and for leeks to 1 mg/kg. Due to acute risk concerns identified for celeries and fennels a modification of the existing MRL set at the limit of quantification (LOQ) of 0.02 mg/kg was not proposed by the EMS.
EFSA assessed the application and the evaluation report as required by Article 10 of the Regulation. Since EFSA identified some data gaps or points which needed further clarifications, the EMS was contacted. On 10 June 2016, the EMS submitted the reply in a revised evaluation report (France, 2016), which replaces the previous version of the document dated 16 January 2015. The revised evaluation report submitted by the EMS (France, 2016) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available.
In accordance with Article 10 of Regulation (EC) No 396/2005, EFSA shall, based on the evaluation report provided by the EMS, provide a reasoned opinion on the risks to the consumer associated with the application.
In accordance with Article 11 of the Regulation, the reasoned opinion shall be provided as soon as possible and at the latest within 3 months (which may be extended to 6 months if more detailed evaluations need to be carried out) from the date of receipt of the application. If EFSA requests supplementary information, the time limit laid down shall be suspended until that information has been provided.
The active substance and its use pattern
Pyraclostrobin is the ISO common name for methyl 2‐[1‐(4‐chlorophenyl)pyrazol‐3‐yloxymethyl]‐N‐methoxycarbanilate (IUPAC). The chemical structures of the active substance and its main metabolites are reported in Appendix B.
The details of the intended GAPs for pyraclostrobin which are the basis for the MRL application are given in Appendix A.
Pyraclostrobin was evaluated in the framework of Directive 91/414/EEC with Germany designated as rapporteur Member State (RMS). It was included in Annex I of this Directive by Commission Directive 2004/30/EC5 which entered into force on 1 June 2004 for use as fungicide. In 2009, pyraclostrobin was also authorised for the uses as plant growth regulator (Regulation (EU) No 2009/256). In accordance with Commission Implementing Regulation (EU) No 540/20117 pyraclostrobin is approved under Regulation (EC) No 1107/20093, repealing Directive 91/414/EEC. The representative uses evaluated in the peer review were foliar applications on grapes. The draft assessment report (DAR) was not peer reviewed by EFSA; therefore, no EFSA conclusion is available.
The EU MRLs for pyraclostrobin are established in Annexes II of Regulation (EC) No 396/2005. EFSA has issued several opinions on the modification of MRLs for pyraclostrobin, including a reasoned opinion on the review of the existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (hereafter Article 12 MRL review). The proposals have been considered in the EU legislation. The MRL changes that were reported in the EU legislation after the Article 12 MRL review are summarised in Table 1.
Table 1.
Overview of the MRL changes after Article 12 MRL review
| Procedurea | Considered by Regulation | Remarks |
|---|---|---|
| Art. 12 (EFSA, 2011b) | (EU) No 668/2013 | Review existing MRLs |
| Art. 10 (EFSA, 2011a) | (EU) No 668/2013 | Various crops |
| Art. 10 (EFSA, 2012) | (EU) No 668/2013 | Leafy brassica and various cereals |
| Art. 10 (EFSA, 2013) | (EU) No 51/2014 | Cucumbers, Jerusalem artichokes |
| Art. 10 (EFSA, 2014a) | (EU) 2015/401 | Chicory roots |
| Art. 10 (EFSA, 2014b) | (EU) 2015/846 | Swedes and turnips |
| Art. 10 (EFSA, 2016) | Not yet legally implemented | Beet leaves (chards) |
MRL: maximum residue level.
Art. 10: Assessment of MRL application according to Article 6 to 10 of Regulation (EC) No 396/2005.
Art. 12: Review of the existing MRLs according to Article 12 of Regulation (EC) No 396/2005.
Art. 43: EFSA scientific opinion according to Article 43 of Regulation (EC) No 396/2005.
Codex Alimentarius has established MRLs (codex maximum residue limits; CXLs) for a wide range of commodities. Among the crops under consideration, a CXL is set for leeks at the same level (0.7 mg/kg) as in the current EU MRL.
Assessment
EFSA has based its assessment on the revised evaluation report submitted by the EMS (France, 2016), the DAR and its addendum prepared under Directive 91/414/EEC (Germany, 2001, 2003), the Commission review report on pyraclostrobin (European Commission, 2004), the JMPR evaluation report (FAO, 2006, 2011), as well as the conclusions from previous EFSA opinions on pyraclostrobin (EFSA, 2011a, 2012, 2013, 2014a,b, 2016) including the review of the existing MRLs for pyraclostrobin under Article 12 of Regulation (EC) No 396/2005 (EFSA, 2011b). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20118 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1996, 1997a, b, c, d, e, f, g, European Commission 2000, European Commission, 2010a,b, 2015; OECD, 2011).
1. Method of analysis
1.1. Methods for enforcement of residues in food of plant origin
Adequate analytical methods are available to monitor pyraclostrobin residues in high water, high acid and high fat content commodities and in dry commodities with a LOQ of at least 0.02 mg/kg (Germany, 2001; EFSA, 2011b). The multi‐residue Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) analytical method is also applicable for the determination of residues on high water and acidic content and on dry commodities with a LOQ of 0.01 mg/kg (EFSA, 2011b).
Since the commodities under consideration belong to the group of high water content commodities, EFSA concludes that sufficiently validated analytical methods for enforcing the proposed MRLs are available.
1.2. Methods for enforcement of residues in food of animal origin
Analytical methods for the determination of residues in food of animal origin are not assessed in the current application and are not required since no change of existing MRLs is proposed.
2. Mammalian toxicology
The toxicological profile of pyraclostrobin was assessed in the framework of the peer review under Directive 91/414/EEC (Germany, 2001; European Commission, 2004). The data were sufficient to derive toxicological reference values compiled in Table 2.
Table 2.
: Overview of the toxicological reference values
| Source | Year | Value | Study | Safety factor | |
|---|---|---|---|---|---|
| Pyraclostrobin | |||||
| ADI | European Commission | 2004 | 0.03 mg/kg bw per day | Chronic rat study | 100 |
| ARfD | 2004 | 0.03 mg/kg bw | Rabbit, developmental toxicity study (maternal toxicity | 100 | |
ADI: acceptable daily intake; ARfD: acute reference dose; EC: European Commission; bw: body weight.
3. Residues
3.1. Nature and magnitude of residues in plant
3.1.1. Primary crops
3.1.1.1. Nature of residues
The metabolism of pyraclostrobin in primary crops following foliar applications was evaluated in three different crop groups (Germany, 2001; EFSA, 2011b). An overview of the key parameters of the available metabolism studies is presented in Table 3.
Table 3.
Summary of available metabolism studies in plants
| Crop groups | Crops | Applications | Sampling |
|---|---|---|---|
| Fruit | Grape | Foliar: 6 × 130 to 480 g/ha, from BBCH 53‐55 to 81 | 40 DALA |
| Root | Potato | Foliar: 6 × 300 g/ha, from BBCH 31 to maturity | 7 DAT3, 7 DALA |
| Cereals/grass | Wheat | Foliar: 2 × 300 g/ha, from BBCH 32 to 61 | 0 DAT1, 31 DAT1, 41 DALA |
BBCH: growth stages of mono‐ and dicotyledonous plants; DALA: days after last application; DAT1 or DAT3: days after first or third treatment.
The Article 12 MRL review confirmed the conclusion of the peer review that the relevant residue for enforcement and risk assessment in all plant commodities treated by foliar application is pyraclostrobin (EFSA, 2011b). The current residue definition set in Regulation (EC) No 396/2005 is identical and applies to the crops under consideration.
EFSA concludes that the metabolism of pyraclostrobin in the crops for which modifications of the existing MRLs were requested has been sufficiently investigated; the residue definitions derived previously are applicable.
3.1.1.2. Magnitude of residues
The EMS assessed the results of supervised residue trials on celeriacs, spinaches, witloof chicory, beans with pods, peas without pods, and celeries and leeks (France, 2016).
a) Celeriacs Good agricultural practices (GAP): 2 × 100 g/ha, interval 10 days, pre‐harvest interval (PHI) 14 days (northern Europe (NEU), southern Europe (SEU))
Five GAP‐compliant residue trials conducted in the NEU over two seasons support a MRL proposal of 0.5 mg/kg for celeriac. No residue trials conducted in the SEU were submitted to support the SEU use.
b) Spinaches, chards/beet leaves GAP: 2 × 100 g/ha, interval 10 days, PHI 14 days (NEU, SEU)
In total, eight residue trials conducted on spinaches over two seasons were submitted (four trials each in the NEU and the SEU). Additional four NEU trials reflecting the intended GAP submitted to EFSA in the framework of the Article 12 MRL review (EFSA, 2011b) were used by EFSA to derive the MRL proposal and the risk assessment values. Although NEU and SEU residue levels showed to belong to a similar population (U‐test, 5%), the two datasets were not merged as the MRLs calculated individually differed significantly.9 Based on the NEU dataset the MRL proposal of 0.6 mg/kg10 is derived.
Extrapolation of residue data to chards/beet leaves would be acceptable. It is noted that a more critical indoor use (2 × 100 kg/ha, PHI 14 days) has been recently assessed where EFSA proposed a higher MRL of 1.5 mg/kg (EFSA, 2016).
c) Witloof chicory GAP: indoor GAP: 1 × 5L/m2, PHI 21 days (NEU)
Four indoor residue trials conducted over two seasons were submitted. The trials combined a dipping application of pyraclostrobin prior to root storage followed, 7 to 11 days after, by a spraying application at the intended rate of the roots shortly before forcing with leaves collected after 21 days. Although using a more critical experimental design, EFSA agrees with the EMS that the prior dip application at storage is not expected to significantly impact residues due to the low degree of the translaminar movement expected for pyraclostrobin. The submitted data support the MRL proposal of 0.09 mg/kg.
d) Beans and peas with pods GAP: 2 × 100 g/ha, interval 7 days, PHI 7 days (NEU, SEU)
Eight NEU and nine SEU GAP‐compliant trials (with application rates within the 25% tolerance) conducted on beans with pods over at least two seasons were submitted. The data are sufficient to derive a MRL proposal for beans with pods in both NEU and SEU. Since the residues showed to belong to a similar population (U‐test, 5%) and lead to comparable MRL values, the data were pooled to derive a more robust MRL proposal of 0.6 mg/kg.11
Extrapolation of residue data to peas with pods is acceptable (European Commission, 2015).
e) Peas without pods GAP: 2 × 100 g/ha, interval 7 days, PHI 7 days (NEU, SEU)
Eight NEU and eight SEU GAP‐compliant trials conducted over two seasons were submitted. The data are sufficient to derive a MRL proposal for peas without pods in both NEU and SEU. Although residues showed to belong to a similar population (U‐test, 5%), the statistical test has limited power due to the high number of values below the LOQ. Since the MRLs calculated individually differ significantly,9 the two datasets were not merged. A MRL of 0.15 mg/kg is derived from the more critical residue situation in NEU.
f) Celeries, fennels GAP: 2 × 100 g/ha, interval 10 days, PHI 14 days (NEU, SEU)
Six NEU and four SEU GAP‐compliant residue trials conducted on celery over two seasons were submitted. Although the residue trials performed in NEU and SEU showed to belong to a similar population (U‐test, 5%), the two datasets were not merged as the MRLs calculated individually differ by more than one MRL class (European Commission, 2015). For the NEU dataset the MRL proposal of 5 mg/kg is derived; for SEU the calculated MRL proposal is 1.5 mg/kg.
Extrapolation of residue data to fennel is acceptable (European Commission, 2015).
g) Leeks GAP: 3 (NEU) or 2 (SEU) × 100 g/ha, interval 10 days, PHI 14 days
NEU: Eleven GAP‐compliant residue trials conducted over at least two seasons were submitted. The same trials were already assessed in the framework of the Article 12 MRL review which derived a MRL proposal of 0.7 mg/kg (EFSA, 2011b).
SEU: Four GAP‐compliant residue trials conducted over two seasons were submitted. The number of trials is sufficient to derive a MRL of 1 mg/kg for the intended use in SEU, where leek is a minor crop (European Commission, 2015).
Since the SEU use resulted in higher residues, a MRL of 1 mg/kg is proposed for leeks.
The results of the residue trials, the related risk assessment input values (highest residue, median residue) and the MRL proposals are summarised in Table 4.
Table 4.
Overview of the available residue trial data
| Crop (GAPs) | Region/Indoora | Residue levels observed in the supervised residue trialsb (mg/kg) | Comments; result of MRL calculation based on OECD calculatorc | MRL proposal (mg/kg) | HRd (mg/kg) | STMRe (mg/kg) |
|---|---|---|---|---|---|---|
| Celeriacs | NEU | 0.07; 0.09; 0.16; 0.19; 0.23 | MRLOECD: 0.44/0.50 | 0.5 | 0.23 | 0.16 |
| SEU | – | Intended use in SEU not supported | – | – | – | |
| Spinaches | NEU | < 0.01; 0.02; 0.02; 0.04; 0.05; 0.13; 0.13; 0.28; 0.31 |
Part of NEU trials (in italics) already assessed (EFSA, 2011b). MRLOECD: NEU 0.57/0.60 MRLOECD: SEU 0.17/0.20 Extrapolation to chards/beet leaves |
0.6 | 0.31 | 0.05 |
| SEU | 0.02; 0.05; 0.05; 0.09 | 0.2 | 0.09 | 0.05 | ||
| Witloof/Belgian endives | Indoor | 0.02; 0.03; 0.03; 0.04 | MRLOECD: 0.09/0.09 | 0.09 | 0.04 | 0.03 |
| Beans with pods | NEU | 0.03; 0.06; 0.07; 0.12; 0.13; 0.24; 0.26; 0.37 |
Data pooled (U‐test, 5%). MRLOECD: NEU 0.63/0.70; SEU 0.51/0.60 MRLOECD: NEU/SEU 0.56/0.60 Extrapolation to peas with pods |
0.6 | 0.37 | 0.13 |
| SEU | 2 × 0.03; 0.04; 0.06; 0.13; 0.14; 0.21; 0.24; 0.28 | |||||
| Peas without pods | NEU | 4 × < 0.01; 3 × 0.01; 0.01 | MRLOECD: 0.02/0.03 | 0.03 | 0.01 | 0.01 |
| SEU | 5 × < 0.01; 0.01; 0.02; 0.07 | MRLOECD: 0.11/0.15 | 0.15 | 0.07 | 0.01 | |
| Celeries | NEU | 0.05; 0.11; 0.12; 0.21; 0.24; 2.63 |
MRLOECD: NEU 4.63/5.00 MRLOECD: SEU 1.37/1.50 Extrapolation to fennels |
5 | 2.63 | 0.17 |
| SEU | 0.15; 0.21; 0.59; 0.61 | 1.5 | 0.61 | 0.40 | ||
| Leeks | NEU | 0.05; 0.12; 0.16; 0.16; 0.19; 2 × 0.22; 0.24; 0.25; 0.29; 0.42 |
NEU trials already assessed (EFSA, 2011b). MRLOECD: 0.63/0.70 |
0.7 | 0.42 | 0.22 |
| SEU | 0.14; 0.18; 0.20; 0.53 | MRLOECD: 0.98/1.00 | 1 | 0.53 | 0.19 |
NEU: outdoor trials conducted in northern Europe; SEU: outdoor trials conducted in southern Europe; Indoor: indoor EU trials or Country code: if non‐EU trials.
Individual residue levels considered for MRL calculation are reported in ascending order.
Underlined values: samples taken at a PHI (20–21 days for celeriac, spinach and leek; 14 days for bean) longer than the intended PHI.
Result of OECD MRL calculation (unrounded/rounded values).
HR: highest residue level according to the residue definition for risk assessment.
STMR: Median residue level according to residue definition for risk assessment.
Residues of pyraclostrobin were found to be stable at stored deep frozen conditions up to 18 months in high water, high acid and high oil content matrices as well as in dry/starch and dry/protein matrices (Germany, 2001; EFSA, 2011b). As the trial samples were stored for a maximum period of 8 months under conditions for which integrity of the samples was demonstrated, it is concluded that the residue data are valid with regard to storage stability.
According to the EMS, the analytical methods used to analyse the residue trial samples have been sufficiently validated and were proven to be fit for the purpose (France, 2016).
EFSA concludes that the submitted residue trials are sufficient to derive MRL proposals of 0.5 mg/kg in celeriacs (NEU use only), 0.6 mg/kg in spinaches (NEU and SEU uses), 0.09 mg/kg in witloof (NEU use), 0.6 mg/kg in beans and peas with pods (NEU and SEU uses), 0.15 mg/kg in peas without pods (NEU and SEU uses), 5 mg/kg in celeries and fennels (NEU and EU uses) and 1 mg/kg for leeks (NEU and SEU). Regarding chards/beet leaves, the intended NEU and SEU field uses are sufficiently supported, but in the meantime EFSA has proposed a higher MRL of 1.5 mg/kg derived from a more critical indoor use (EFSA, 2016). The intended use on celeriac in SEU is not supported by residue trials.
3.1.1.3. Effect of industrial processing and/or household preparation
Standard hydrolysis studies simulating the effect on the nature of pyraclostrobin residues under processing conditions representative of pasteurisation, boiling and sterilisation were assessed in the framework of the peer review and in the Article 12 MRL review (Germany, 2001; EFSA, 2011b). It was concluded that the compound is hydrolytically stable under the representative conditions. Therefore, the residue definition derived for raw agricultural commodities (RAC) (i.e. pyraclostrobin) is also applicable for processed commodities.
Studies investigating the magnitude of pyraclostrobin residues in processed products were evaluated in the Article 12 MRL review and processing factors (PF) were proposed for several processed products which are not relevant for this application (EFSA, 2011b). Studies investigating the magnitude of pyraclostrobin residues in the crops under consideration were not submitted but some studies for other processed vegetables were provided (i.e. processing studies for cooked spinaches and head cabbages) (France, 2016). Considering limited number of valid processing studies and the contradictive results, a clear conclusion on the effect of cooking on the residue levels in cooked spinach cannot be derived. The processing studies in head cabbage give an indication of reduction of the residues in processed products; however, the reduction is mainly attributed to the removal of the outer leaves. According to EFSA, this study does not allow to conclude whether cooking of vegetables would lead to a significant reduction of the residues in the processed product.
The PFs derived from these studies are summarised in Table 5.
Table 5.
Overview of the available processing studies
| Crop, processed | Number of studies | Processing factor (PF) | Comments | |
|---|---|---|---|---|
| Individual values | Median PF | |||
| Spinach, cooked | 2 | 0.45; 1.27 | n.a. | Individual PFs differ by more than 50% |
| Head cabbage, cooked | 4 | < 0.01; < 0.02; < 0.03; <0.04 | < 0.03 | |
n.a.: not appropriate; PF: processing factor.
3.1.2. Rotational crops
The crops under consideration can be grown in rotation with other plants, therefore the possible occurrence of residues in succeeding crops resulting from the proposed use of pyraclostrobin has to be assessed (European Commission, 1997c).
The nature and magnitude of pyraclostrobin residues in rotational crops was investigated in radish, lettuce and wheat sown into treated soil (900 g/ha) at intervals of 30, 120 and 365 days. These studies showed that the metabolism in rotational crops was comparable to the one in primary crops and that residues in rotational crops were very low (radish root, lettuce ≤ 0.04 mg/kg and wheat grain ≤ 0.09 mg/kg) for all plant back intervals (Germany, 2001). For the uses considered under the Article 12 MRL review (application rates up to 670 g), EFSA concluded that the residues of pyraclostrobin resulting from the soil uptake are not expected to exceed 0.01 mg/kg (EFSA, 2011b).
Since the intended uses of pyraclostrobin assessed in this reasoned opinion (seasonal application rates up to 300 g/ha) are not more critical than the existing uses assessed in the framework of the Article 12 MRL review, EFSA concludes that relevant residue levels are unlikely to occur in rotational crops provided that the compound is applied on the crops under consideration according to the proposed GAPs.
3.2. Nature and magnitude of residues in livestock
Since the application was submitted before bean and pea vines and their forages were considered as feed items (EFSA, 2015), the assessment of the nature and magnitude of pyraclostrobin residues in livestock is not required (European Commission, 1996).
4. Consumer risk assessment
The consumer risk assessment was performed with revision 2 of the EFSA PRIMo. This exposure assessment model contains the relevant European food consumption data for different subgroups of the EU population12 (EFSA, 2007).
In the framework of the review of the Article 12 MRL review, a comprehensive long‐term exposure assessment was performed taking into account the existing uses at the EU level and the acceptable CXLs (EFSA, 2011b). EFSA updated this risk assessment with the median residue levels (STMRs) derived from the residue trials conducted on the crops under consideration in this MRL application (Table 4) and the STMRs reported in previous EFSA reasoned opinions carried out after the Article 12 MRL review (EFSA, 2012, 2013, 2014a,b, 2016). The food commodities, for which no uses were reported in the framework of the Article MRL 12 review or in subsequent EFSA opinions, were excluded from the exposure calculation, assuming that there is no use of pyraclostrobin on these crops.
The acute exposure assessment was performed only with regard to the commodities under consideration assuming the consumption of a large portion of the food items as reported in the national food surveys. The input values for the exposure assessment are summarised in Table 4. A variability factor accounting for the inhomogeneous distribution on the individual items consumed was included in the calculation (EFSA, 2007). For celeries, fennels and leeks, EFSA calculated two separate scenarios, presenting the acute exposure for the more critical NEU use in celeries, fennels and the more critical SEU use in leeks. In the second scenario the results for the less critical SEU uses for celeries and fennel and the NEU use in leeks are provided.
The input values used for the dietary exposure calculation are summarised in Table 6.
Table 6.
Input values for the consumer dietary exposure assessment
| Commodity | Chronic exposure assessment | Acute exposure assessment | |||
|---|---|---|---|---|---|
| Input (mg/kg) | Comment | Input (mg/kg) | Comment | ||
|
Risk assessment residue definition for product of plant origin: Pyraclostrobin Risk assessment residue definition for product of animal origin: Sum of pyraclostrobin and its metabolites containing the 1‐(4‐chlorophenyl)‐1H‐pyrazole moiety or the 1‐(4‐chloro‐2‐hydroxyphenyl)‐1H‐pyrazole moiety, expressed as pyraclostrobin | |||||
| Celeriacs | 0.16 | STMR | 0.23 | HR | |
| Spinaches | 0.05 | STMR (NEU) | 0.31 | HR (NEU) | |
| Witloof | 0.03 | STMR | 0.04 | HR | |
| Beans with pods | 0.13 | STMR | 0.37 | HR | |
| Peas with pods | 0.13 | STMR | 0.37 | HR | |
| Peas without pods | 0.01 | STMR (SEU) | 0.07 | HR (SEU) | |
| Celeries | Scenario 1 | 0.17 | STMR (NEU) | 2.63 | HR (NEU) |
| Scenario 2 | 0.40 | STMR (SEU) | 0.61 | HR (SEU) | |
| Fennels | Scenario 1 | 0.17 | STMR (NEU) | 2.63 | HR (NEU) |
| Scenario 2 | 0.40 | STMR (SEU) | 0.61 | HR (SEU) | |
| Leeks | Scenario 1 | 0.19 | STMR (SEU) | 0.53 | HR (SEU) |
| Scenario 2 | 0.22 | STMR (NEU, Art 12) | 0.42 | HR (NEU, Art 12) | |
| Beet leaves (chards) | 0.26 | STMR (EFSA, 2016) | 0.81 | HR (EFSA, 2016). | |
| Other plant and animal origin commodities | See table 4.1 of the Reasoned Opinion on the modification of the existing MRLs for pyraclostrobin in swedes and turnips (EFSA, 2014b) | Acute risk assessment performed only for the crops under consideration | |||
HR: highest residue; STMR: supervised trials median residue; NEU: northern Europe; SEU: southern Europe.
The estimated exposure was then compared with the toxicological reference values derived for pyraclostrobin (Table 2). The result of the intake calculation using the EFSA PRIMo is a key supporting document and is made publicly available as a background document to this reasoned opinion.
Neither in scenario 1 nor in scenario 2, a long‐term consumer intake concern was identified for any of the European diets incorporated in the EFSA PRIMo. The highest chronic intake was calculated to be less than 15% of the ADI (German child diet). The contribution of residues in the crops under consideration to the total consumer exposure accounted for about 0.5% for beans with pods and leeks, 0.2% for celeries and 0.1% of the ADI or less for the remaining commodities under evaluation.
The short‐term exposure, expressed as percentage of the ARfD, did not identify a consumer health concern for celeriacs (42%), spinaches (23%), beans with pods (14%), witloof (6%), peas with (4%) and peas without (2%) pods. In scenario 1, a consumer health risk could not be excluded for the NEU use on celeries (402%, Dutch child diet), the NEU use in fennels (178%, German child diet) and the SEU use on leeks (104%, Belgian child diet). Considering that celeries and fennel are frequently consumed without processing, a refinement of the short‐term exposure calculations taking into account PFs is not appropriate, lacking reliable consumption data for unprocessed and processed products. In contrast to celeries and fennel, leeks are more likely to be consumed after processing. Thus, reliable processing studies would allow to perform a refined intake calculation. However, processing studies on leek or on a crop belonging to the crop group of stem vegetables are not available. The EMS proposed to use the mean cooking factor of spinaches in a refined intake calculation (France, 2016), which resulted in an acute consumer exposure of 90% of the ARfD.13 EFSA is of the opinion that the refined exposure scenario for leeks is affected by uncertainties (lack of information on the consumption of leek as cooked, robustness of the PF for spinach because of the limited and contradictory results).
Although one processing study in spinach gave an indication that cooking may reduce the residues, other studies (i.e. the standard hydrolysis studies and one processing study in spinach) do not support the assumption that cooking would significantly reduce the residues. Since the available data do not provide sufficient evidence that the short‐term exposure is below the ARfD, EFSA does not propose to raise the existing MRL.
In scenario 2, no acute consumer concerns were identified for the less critical intended SEU use on celeries and fennels and NEU use on leeks (93%, 41% and 83% of the ARfD, respectively).
In this refined risk assessment, no consumer intake concerns were identified for the southern EU use in leeks. However, EFSA is of the opinion that the refined exposure scenario for leeks is affected by uncertainties (lack of information on the consumption of leek as cooked, robustness of the PF for spinach because of the limited and contradictory results).
Conclusions and recommendations
The information submitted was sufficient to propose the MRLs summarised in the table below:
| Codeb | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Pyraclostrobinc | ||||
| 213030 | Celeriac | 0.3 | 0.5 | The submitted data are sufficient to derive a MRL proposal for the NEU use. The SEU use is not supported by data. No consumer health concern was identified |
| 252010 | Spinaches | 0.5 | 0.6 | The MRL proposal reflects the more critical residue situation for the NEU use. No consumer health concern was identified |
| 252030 | Chards/beet leaves | 0.5 | 1.5 (0.6) |
For the GAP reported by the EMS, a MRL of 0.6 mg/kg would be required (MRL for NEU and SEU use derived by extrapolation from data on spinaches). In a recent assessment, EFSA derived a higher MRL proposal of 1.5 mg/kg for a more critical indoor use which did not pose a consumer health concern (EFSA, 2016). This MRL proposal has not yet been implemented in the MRL legislation |
| 255000 | Witloofs/Belgian endives | 0.02a | 0.09 | The MRL proposal reflects indoor use. No consumer health concern was identified |
| 260010 | Beans (with pods) | 0.02a | 0.6 | The MRL proposal covers the intended use in NEU and SEU (MRL derived from dataset on beans with pods). No consumer health concern was identified |
| 260030 | Peas (with pods) | 0.02a | 0.6 | |
| 260040 | Peas (without pods) | 0.02a | 0.15 | NEU and SEU uses are sufficiently supported by data. The MRL proposal was derived from the more critical residue situation in NEU use. No consumer health concern was identified |
| 270030 | Celeries | 0.02a | 1.5 |
The proposed MRL reflects the intended use in SEU. The MRL proposal based on the residue trials in celeries was extrapolated to fennels. No consumer health risk was identified for this use. The intended use in NEU would require a MRL of 5 mg/kg. However, a consumer health risk could not be excluded (celery: 402% of the ARfD; fennel: 178% of the ARfD) |
| 270040 | Florence fennels | 0.02a | 1.5 | |
| 270060 | Leeks | 0.7 | No change |
The intended use in NEU would not require a modification of the existing MRL. Although the SEU seems to be less critical, the supporting residue trials suggest a higher MRL of 1 mg/kg. Based on the SEU residue trials, the short‐term dietary exposure slightly exceeded the ARfD (104%). Although processing studies in head cabbage give an indication that cooking may reduce the residues, other studies (i.e. the standard hydrolysis studies and one processing study in spinach) do not support the assumption that cooking would significantly reduce the residues. Since the available data do not provide sufficient evidence that the short‐term exposure is below the ARfD, EFSA does not propose to raise the existing MRL |
MRL: maximum residue level; EMS: evaluating Member State; NEU: northern Europe; SEU: southern Europe; ARfD: acute reference dose; GAP: good agricultural practices; PF: processing factor.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Fat soluble.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAS
Chemical Abstract Service
- CXL
Codex maximum residue limit
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- EMS
evaluating Member State
- FAO
Food and Agriculture Organization of the United Nations
- GAP
good agricultural practice
- HR
highest residue
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint FAO/WHO Meeting on Pesticide Residues
- LOQ
limit of quantification
- MRL
maximum residue level
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PF
processing factor
- PHI
pre‐harvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- RAC
raw agricultural commodity
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern Europe
- STMR
supervised trials median residue
- WG
water‐dispersible granule
- WHO
World Health Organization
Appendix A – Good agricultural practice
| Crop | NEU, SEU, MS or country |
F G or Ia |
Pests or Group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages & seasonc |
Number min‐max |
Interval between application |
g/hL min–max |
Water L/ha min–max |
g/ha min–max |
||||||
| Celeriacs | NEU | F | Fungal diseases | WG | 67 g/kg | spray | 15–49 | 2 | 10 days | – | 400–1,000 | 100 | 14 | – |
| SEU | ||||||||||||||
| Spinaches, Chards/beet leaves | NEU | F | Fungal diseases | WG | 67 g/kg | spray | 14–49 | 2 | 7 days | – | 400–1,000 | 100 | 14 | – |
| SEU | ||||||||||||||
| Witloof chicory | SEU | I | Fungal diseases | WG | 67 g/kg | spray | Shortly before forcing, 49 | 1 | – | – | 5 L/m2 |
0.42 g/m2 |
21 | Treatment in station |
| NEU | ||||||||||||||
| Beans and peas with pods | NEU | F | Fungal diseases | WG | 67 g/kg | spray | 15–89 | 2 | 7 days | – | 400–1,000 | 100 | 7 | – |
| SEU | ||||||||||||||
| Peas without pods | NEU | F | Fungal diseases | WG | 67 g/kg | spray | 15–89 | 2 | 7 days | – | 400–1,000 | 100 | 7 | – |
| SEU | ||||||||||||||
| Celeries, Fennels | NEU | F | Fungal diseases | WG | 67 g/kg | spray | 15–49 | 2 | 10 days | – | 400–1,000 | 100 | 14 | – |
| SEU | ||||||||||||||
| Leeks | NEU | F | Fungal diseases | WG | 67 g/kg | spray | 41–49 | 3 | 10 days | – | 200–1,000 | 100 | 14 | GAP Art 12 MRL review |
| SEU | 2 | 100 | – | |||||||||||
NEU: northern European Union; SEU: southern European Union; MS: Member State.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum pre‐harvest interval.
Appendix B – Used compound codes
| Code/trivial name | Chemical name | Structural formula |
|---|---|---|
| Pyraclostrobin |
methyl 2‐[1‐(4‐chlorophenyl)pyrazol‐3‐yloxymethyl]‐N‐methoxycarbanilate O=C(OC)N(OC)c3ccccc3COc1ccn(n1)c2ccc(Cl)cc2 |
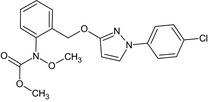
|
|
Desmethoxy metabolite (500M07, BF 500‐3) |
methyl [2‐({[1‐(4‐chlorophenyl)‐1H‐pyrazol‐3‐yl]oxy}methyl)phenyl]carbamate O=C(OC)Nc3ccccc3COc1ccn(n1)c2ccc(Cl)cc2 |
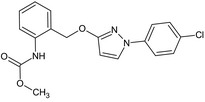
|
Suggested citation: EFSA (European Food Safety Authority) , 2017. Reasoned opinion on the modification of the existing maximum residue levels for pyraclostrobin in various crops. EFSA Journal 2017;15(1):4686, 19 pp. doi: 10.2903/j.efsa.2017.4686
Requestor: European Commission
Question number: EFSA‐Q‐2015‐00079
Adopted: 12 December 2016
Notes
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
BASF Agro SAS, 21 chemin de la Sauvegarde, 69134, Ecully cedex, France.
Commission Directive 2004/30/EC of 10 March 2004 amending Council Directive 91/414/EEC to include benzoic acid, flazasulfuron and pyraclostrobin as active substances, OJ L 77, 13.3.2004, p. 50–53.
Commission Directive 2009/25/EC of 2 April 2009 amending Council Directive 91/414/EEC as regards an extension of the use of the active substance pyraclostrobin. OJ L 91, 3.4.2009, p. 20–22.
Commission Implementing Regulation (EU) No 540/2011 of 23 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
The calculated MRLs did not fall in the same MRL or in neighbouring MRL classes (European Commission, 2015).
The EMS proposed the MRL of 0.7 mg/kg based on the single dataset of the four NEU trials submitted in the framework of the MRL application (France, 2016).
The EMS proposed the MRL of 0.7 mg/kg derived from the single NEU dataset (France, 2016).
The calculation of the long‐term exposure (chronic exposure) is based on the mean consumption data representative for 22 national diets collected from MS surveys plus one regional and four cluster diets from the WHO GEMS Food database; for the acute exposure assessment the most critical large portion consumption data from 19 national diets collected from Member State surveys are used. The complete list of diets incorporated in EFSA PRIMo is given in its reference section (EFSA, 2007).
Input values: HR in SEU (0.53 mg/kg) × tentative PF of 0.86 in spinaches derived from the two individual PFs reported in Table 5.
References
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. doi: 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Reasoned opinion on the modification of the existing MRLs for pyraclostrobin in various crops. EFSA Journal 2011;9(3):2120, 41 pp. doi: 10.2903/j.efsa.2011.2120 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for pyraclostrobin according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2011;9(8):2344, 92 pp. doi: 10.2903/j.efsa.2011.2344 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Reasoned opinion on the modification of the existing MRLs for pyraclostrobin in leafy brassica and various cereals. EFSA Journal 2012;10(3):2606, 36 pp. doi: 10.2903/j.efsa.2012.2606 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. Reasoned opinion on the modification of the existing MRLs for pyraclostrobin in cucumbers and Jerusalem artichokes. EFSA Journal 2013;11(2):3109, 27 pp. doi: 10.2903/j.efsa.2013.3109 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. Reasoned opinion on the modification of the existing MRL for pyraclostrobin in chicory roots. EFSA Journal 2014;12(5):3685, 23 pp. doi: 10.2903/j.efsa.2014.3685 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Reasoned opinion on the modification of the existing MRLs for pyraclostrobin in swedes and turnips. EFSA Journal 2014;12(10):3872, 19 pp. doi: 10.2903/j.efsa.2014.3872 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Estimation of animal intakes and HR, STMR and MRL calculations for products of animal origin. September 2015, 9 pp.
- EFSA (European Food Safety Authority), 2016. Reasoned opinion on the modification of the existing MRLs for pyraclostrobin in beet leaves (chards). EFSA Journal 2016;14(8):4552, 14 pp. doi: 10.2903/j.efsa.2016.4552 [DOI] [Google Scholar]
- European Commission , 1996. Appendix G. Livestock Feeding Studies. 7031/VI/95‐rev.4.
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev.3.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realisation of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev.6.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev.2.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev.5.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev.3.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev.5.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals. 7039/VI/95.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414). SANCO/3029/99‐rev.4.
- European Commission , 2004. Review report for the active substance pyraclostrobin. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 28 November 2003 in view of the inclusion of pyraclostrobin in Annex I of Council Directive 91/414/EEC. SANCO/1420/2001‐Final, 8. September 2004, 24 pp.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev.8.1.
- European Commission , 2015. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev.10.1
- FAO (Food and Agriculture Organization of the United Nations), 2006. Pyraclostrobin. In: Pesticide residues in food – 2016. Evaluatios. Part I – Residues. Joint meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group. FAO Plant Production and Protection Paper 189/1, pp. 821–866.
- FAO (Food and Agriculture Organization of the United Nations), 2011. Pyraclostrobin. In: Pesticide residues in food – 2011. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues. FAO Plant Production and Protection Paper 211, pp. 223–237.
- France , 2016. Evaluation report on the modification of MRLs for pyraclostrobin in various crops prepared by the evaluating Member State France under Article 8 of Regulation (EC) No 396/2005, January 2015 revised in June 2016, 100 pp.
- Germany , 2001. Draft assessment report on the active substance pyraclostrobin prepared by the rapporteur Member State Germany in the framework of Council Directive 91/414/EEC, August 2001.
- Germany , 2003. Addendum to the draft assessment report on the active substance pyraclostrobin prepared by the rapporteur Member State Germany in the framework of Council Directive 91/414/EEC, October 2003.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org


