Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the competent national authority in Belgium, Federal Public Service of Health, Food Chain Safety and Environment, submitted a request to modify the existing maximum residue level (MRL) for the active substance cymoxanil in beans without pods. The data submitted in support of the request were found to be sufficient to derive a MRL proposal for beans without pods. Adequate analytical methods for enforcement are available to control the residues of cymoxanil in beans without pods at the validated limit of quantification (LOQ) of 0.01 mg/kg. Based on the risk assessment results, EFSA concluded that the short‐term and long‐term intake of residues resulting from the use of cymoxanil according to the reported agricultural practice is unlikely to present a risk to consumer health.
Keywords: cymoxanil, beans without pods, pesticide, MRL, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, the competent national authority in Belgium, Federal Public Service (FPS) of Public Health, Food Chain Safety and Environment, compiled an application to modify the existing maximum residue level (MRL) for the active substance cymoxanil in beans without pods. Belgium (evaluating Member State (EMS)) drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 8 February 2017. To accommodate for the intended use of cymoxanil, the EMS proposed to raise the existing MRL from the limit of quantification (LOQ) of 0.01 to 0.05 mg/kg.
EFSA based its assessment on the evaluation report submitted by the EMS, the draft assessment report (DAR) (and its addendum) prepared under Council Directive 91/414/EEC, the Commission review report on cymoxanil, the conclusion on the peer review of the pesticide risk assessment of the active substance cymoxanil, as well as the conclusions from the previous EFSA opinion on the review of the existing maximum residue levels for cymoxanil according to Article 12 of Regulation (EC) No 396/2005.
Based on the metabolic pattern identified in primary and rotational crops, the residue definition for plant products was proposed as cymoxanil for enforcement and risk assessment. Studies investigating the nature and magnitude of cymoxanil residues in processed commodities were not provided, but are not required. EFSA concluded that for the use on beans without pods assessed in this application, the previously derived residue definition is applicable.
Adequate analytical methods for enforcement are available to control the residues of cymoxanil in beans without pods at the validated LOQ of 0.01 mg/kg. The available residue trials are sufficient to derive a MRL proposal at the LOQ of 0.05 mg/kg for beans without pods.
Residues of cymoxanil in commodities of animal origin were not assessed since beans without pods are normally not fed to livestock.
The toxicological profile of cymoxanil was assessed in the framework of the EU pesticides peer review under Directive 91/414/EEC and the data were sufficient to derive an acceptable daily intake (ADI) of 0.013 mg/kg body weight (bw) per day and an acute reference dose (ARfD) of 0.08 mg/kg bw.
The consumer risk assessment was performed with revision 2 of the EFSA Pesticide Residues Intake Model (PRIMo). EFSA concludes that the long‐term intake of residues of cymoxanil resulting from the existing and the intended uses is unlikely to present a risk to consumer health. The estimated long‐term dietary intake accounted for up to 4.8% of the ADI for WHO Cluster diet B. The contribution of residues expected in beans without pods according to the intended use to the overall long‐term exposure is up to 0.07% of the ADI. The short‐term exposure is low (international estimated short‐term intake (IESTI) is 0.4% of the ARfD for UK toddler). Based on these calculations, EFSA concludes that the proposed use of cymoxanil on beans without pods is unlikely to pose a risk for the consumers.
EFSA proposes to amend the existing MRL as reported in the summary table below.
Full details of all endpoints and the consumer dietary risk assessment can be found in Appendices Appendix B – List of end points, Appendix C – Pesticide Residue Intake Model (PRIMo), Appendix D – Input values for the exposure calculations.
| Codea | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: cymoxanil | ||||
| 260020 | Beans without pods | 0.01* | 0.05* | The submitted data are sufficient to derive a MRL proposal for the NEU use. Risk for consumers unlikely |
MRL: maximum residue level; NEU: northern Europe.
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Background
Regulation (EC) No 396/20051 (hereinafter referred to as ‘the MRL regulation’) establishes the rules governing the setting of pesticide maximum residue levels (MRLs) at European Union (EU) level. In accordance with Article 6(3) of the MRL regulation, the competent authority in Belgium, Federal Public Service (FPS) Public Health, Food Chain Safety and Environment2 hereafter referred to as the evaluating Member State (EMS), compiled an application to modify the existing MRL for the active substance cymoxanil in beans without pods. This application was notified to the European Commission and the European Food Safety Authority (EFSA) and was subsequently evaluated in accordance with Article 8 of the MRL regulation.
The EMS submitted an evaluation report to the European Commission which was forwarded to EFSA on 8 February 2017. The application was included in the EFSA Register of Questions with the reference number EFSA‐Q‐2017‐00096 and the following subject:
Cymoxanil – MRL in beans without pods
Belgium proposed to raise the existing MRL of cymoxanil in beans without pods from the limit of quantification (LOQ) 0.01 to 0.05 mg/kg.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation.
Terms of Reference
In accordance with Article 10 of Regulation (EC) No 396/2005, EFSA shall assess the application and the evaluation report and give a reasoned opinion on the risks to the consumer and where relevant to animals associated with the setting of the requested MRLs. The opinion shall include:
an assessment of whether the analytical method for routine monitoring proposed in the application is appropriate for the intended control purposes;
the anticipated LOQ for the pesticide/product combination;
an assessment of the risks of the acceptable daily intake (ADI) and acute reference dose (ARfD) being exceeded as a result of the modification of the MRL;
the contribution to the intake due to the residues in the product for which the MRLs was requested;
any other element relevant to the risk assessment.
In accordance with Article 11 of the MRL regulation, EFSA shall give its reasoned opinion as soon as possible and at the latest within 3 months from the date of receipt of the application.
The evaluation report submitted by the EMS (Belgium, 2017) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion.
The active substance and its use pattern
The detailed description of the intended use of cymoxanil in beans without pods, which is the basis for the current MRL application, is reported in Appendix A.
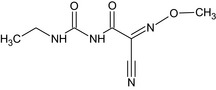
Cymoxanil is the ISO common name for 1‐[(E/Z)‐2‐cyano‐2‐methoxyiminoacetyl]‐3‐ethylurea (IUPAC). The chemical structure of the active substance is reported in Appendix E.
Cymoxanil was evaluated in the framework of Directive 91/414/EEC3 with Austria designated as rapporteur Member State (RMS) for the representative uses as a fungicide on lettuce and potato. The draft assessment report (DAR) prepared by the RMS has been peer reviewed by EFSA (2008). The peer review of renewal of the first approval has not yet been initiated.
Cymoxanil was approved4 for the use as a fungicide on 1 September 2009.
The EU MRLs for cymoxanil are established in Annex II A of Regulation (EC) No 396/2005. The review of existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (MRL review) has been performed (EFSA, 2015) and the proposed modifications have been implemented in the MRL legislation.5
Assessment
EFSA has based its assessment on the evaluation report submitted by the EMS (Belgium, 2017), the DAR (and its addendum) prepared under Directive 91/414/EEC (Austria, 2007, 2008), the European Commission review report on cymoxanil (European Commission, 2008), the conclusion on the peer review of the pesticide risk assessment of the active substance cymoxanil (EFSA, 2008), as well as the conclusions from the previous EFSA opinion on the review of the existing maximum residue levels for cymoxanil according to Article 12 of Regulation (EC) No 396/2005 (EFSA, 2015).
For this application, the data requirements established in Regulation (EU) No 544/20116 and the guidance documents applicable at the date of submission of the application to the EMS are applicable (European Commission, 1997a, b, c, d, e, f, g, 2000, 2010a, b, 2016; OECD, 2011). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20117.
A selected list of end points of the studies assessed by EFSA in the framework of the MRL review, including the end points of studies submitted in support of the current MRL application, are presented in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of cymoxanil following foliar treatment in primary corps belonging to the group of fruit crops (tomato, grapes), root crops (potato) and leafy crops (lettuce) has been investigated in the framework of the EU pesticides peer review (EFSA, 2008) and MRL review (EFSA, 2015). According to these studies, in all crop groups the main component of the residue is parent cymoxanil only.
1.1.2. Nature of residues in rotational crops
Beans can be grown in rotation with other plants. The maximum DT90 observed in the field dissipation studies ranged between 0.5 and 33.3 days (EFSA, 2008). As soil degradation studies demonstrated that cymoxanil is not persistent further investigation was not required. Nonetheless, a rotational crop study was evaluated during the peer review, and based on this study, it was confirmed that significant residues of cymoxanil are not expected in rotational crops (EFSA, 2008). Therefore, it is concluded the possible occurrence of residues in rotational crops resulting from the use on primary crops is not expected.
1.1.3. Nature of residues in processed commodities
Studies investigating the effect of processing on the nature of cymoxanil (hydrolysis studies) are not available. Nevertheless, as residues of cymoxanil exceeding 0.1 mg/kg are not expected in beans without pods and the chronic exposure does not exceed 10% of the ADI (theoretical maximum daily intake (TMDI) is up to 4.8% of the ADI), there is no need to investigate the effect of industrial and/or household processing on the nature of the residues.
1.1.4. Methods of analysis in plants
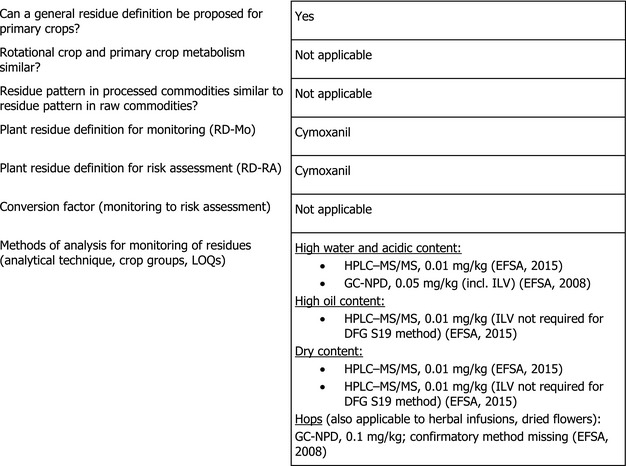
Adequate analytical methods for enforcement are available to control the residues of cymoxanil in beans without pods at the validated LOQ of 0.01 mg/kg (EFSA, 2015).
1.1.5. Stability of residues in plants
The storage stability of cymoxanil in plants stored under frozen conditions was investigated in the framework of the EU pesticides peer review (EFSA, 2008). According to these studies, in beans without pods (high water content matrix) residues are stable at −18°C for a period of 18 months.
1.1.6. Proposed residue definitions
Based on the metabolic pattern identified in primary crops and in rotational crops, the residue definition for plant products was proposed as cymoxanil for enforcement and risk assessment (EFSA, 2015). EFSA concludes that for the use on beans without pods assessed in this application, the proposed residue definition is still applicable.
The residue definition for enforcement set in Regulation (EC) No 396/2005 is identical with the above mentioned residue definition.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
Four residue trials performed in beans without pods were submitted in support of the MRL application. Samples were stored in compliance with the demonstrated storage conditions and analysed for the parent compound. According to the assessment of the EMS, the methods used were sufficiently validated and fit for purpose.
Three trials could not be considered independent as they were carried out in the same month at nearby locations. Therefore, having only two independent residue trials, on their own these trials are not sufficient to derive an MRL. Nevertheless, the following considerations should also be taken into account:
residues were always below the level of quantification (LOQ) of 0.05 mg/kg in the above trials;
residues were below the LOQ of 0.05 mg/kg in residue trials on peas without pods performed according to the same Good Agricultural Practice (GAP) and evaluated in the framework of the MRLs review (EFSA, 2015);
according to the current guidance document extrapolation from peas without pods to beans without pods is possible (European Commission, 2016).
Altogether, the available data are considered sufficient to derive an MRL proposal of 0.05 mg/kg.
1.2.2. Magnitude of residues in rotational crops
Significant residues of cymoxanil are not expected in rotational crops (see Section 1.1.2).
1.2.3. Magnitude of residues in processed commodities
No processing studies were submitted in the present assessment and they are not required as significant residues of cymoxanil are not expected in processed commodities (see Section 1.1.2).
1.2.4. Proposed MRLs
The available residue trials are sufficient to derive a MRL proposal for beans without pods (see Appendix B.1.2.1).
2. Residues in livestock
Not relevant as beans without pods are not used for feed purposes.
3. Consumer risk assessment
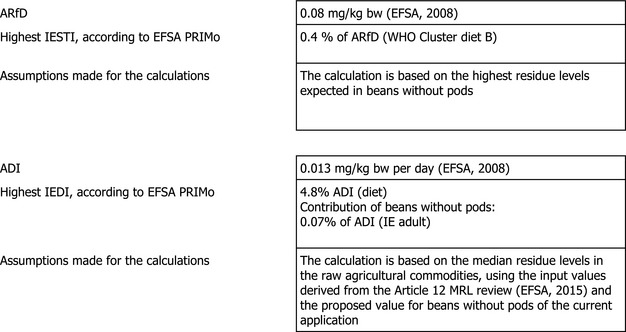
The toxicological profile of cymoxanil was assessed in the framework of the EU pesticides peer review under Directive 91/414/EEC and the data were sufficient to derive an ADI of 0.013 mg/kg body weight (bw) per day and an ARfD of 0.08 mg/kg bw. The consumer risk assessment was performed with revision 2 of the EFSA PRIMo (EFSA, 2007).
3.1. Short‐term (acute) dietary risk assessment
The short‐term exposure assessment was performed for beans without pods in accordance with the internationally agreed methodology. The calculations were based on the highest residue (HR) derived from supervised field trials on beans without pods and peas without pods and the international estimated short‐term intake (IESTI) accounted for 0.4% of the ARfD for UK toddler. The complete list of input values can be found in Appendix D.2.
3.2. Long‐term (chronic) dietary risk assessment
The long‐term exposure assessment was performed, taking into account the existing uses at EU level (EFSA, 2015). EFSA updated the calculation with the supervised trials median residue (STMR) value derived for beans without pods. The input values used in the exposure calculations are summarised in Appendix D.2.
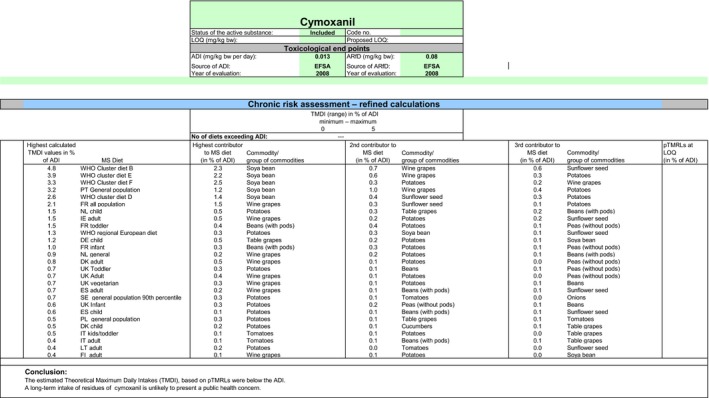
The estimated long‐term dietary intake accounted for up to 4.8% of the ADI for WHO Cluster diet B. The contribution of residues expected in beans without pods according to the intended use to the overall long‐term exposure is up to 0.07% of the ADI for IE adult (see Appendix C).
Based on these calculations, EFSA concludes that the proposed use of cymoxanil on beans without pods is unlikely to pose a risk for the consumers.
Conclusions and recommendations
The data submitted in support of this MRL application were found to be sufficient to derive MRL proposals for beans without pods.
Adequate analytical methods for enforcement are available to control the residues of cymoxanil in beans without pods.
Based on the risk assessment results, EFSA concluded that the short‐term and long‐term intake of residues resulting from the use of cymoxanil according to the reported agricultural practice is unlikely to present a risk to consumer health.
The MRL recommendations are summarised in Appendix B.4.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- DAR
draft assessment report
- DAT
days after treatment
- DT90
period required for 90% dissipation (define method of estimation)
- EMS
evaluating Member State
- FAO
Food and Agriculture Organization of the United Nations
- FPS
Federal Public Service
- GAP
Good Agricultural Practice
- GC‐NPD
gas chromatography with nitrogen/phosphorous detector
- HPLC–MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification
- MRL
maximum residue level
- MS
Member States
- MW
molecular weight
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RA
risk assessment
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern Europe
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TAR
total applied radioactivity
- TMDI
theoretical maximum daily intake
- WG
water‐dispersible granule
- WHO
World Health Organization
Appendix A – Summary of intended GAP triggering the amendment of existing EU MRLs
1.
| Crop and/or situation | NEU, SEU, MS or country | F G or Ia | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages and seasonc | Number min–max | Interval between application (min) | g a.s./hL min–max | Water L/ha min–max | g a.s./ha min–max | ||||||
| Beans, fresh without pods | Belgium (NEU) | F | Mildew | WG | 4% | Spraying | 1 | – | 0.1125 | 14 | ||||
NEU: northern European Union; SEU: southern European Union; MS; Member State; GAP: Good Agricultural Practice; MRL: maximum residue level; a.s.: active substance; WG: water‐dispersible granule.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) |
|---|---|---|---|---|
| Fruit crops | Tomatoes(a) | Foliar, 3 × 0.63 kg a.s./ha | 3 | |
| Foliar, 4 × 0.24 kg a.s./ha | 13 | |||
| Foliar, 7 × 0.14 kg a.s/ha | 7, 14, 21, 35 | |||
| Grapes(a) | Foliar, 8 × 0.21 kg a.s./ha | 0, 1, 4, 10,18 | ||
| Root crops | Potatoes(b) | Foliar, 8 × 0.24 kg a.s./ha | 10 | |
| Foliar, 3 × 0.40 kg a.s./ha | 3 | |||
| Leafy crops | Lettuce(b) | Foliar, 3 × 0.24 kg a.s./ha | 11 | |
| Foliar, 4 × 0.84 kg a.s./ha | 3 | |||
| Reference: a) EFSA (2015); b) EFSA (2008) | ||||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) |
|---|---|---|---|---|
| Root/tuber crops | Sugar beet | Bare soil, 1.2 kg a.s./ha | 30, 120 | |
| Leafy crops | Lettuce | Bare soil, 1.2 kg a.s./ha | 30, 120 | |
| Cereal (small grain) | Wheat | Bare soil, 1.2 kg a.s./ha | 30, 120 | |
|
Comments: A ‘no residue’ situation in rotational crops was established Reference: EFSA (2008) | ||||
| Processed commodities (hydrolysis study) | Conditions | Investigated? |
|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | No | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | No | |
| Sterilisation (20 min, 120°C, pH 6) | No | |
| Comment: Not triggered for the present application | ||
DAT: days after treatment; a.s.: active substance; PBI: plant‐back interval.
B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability (months/years) |
|---|---|---|---|---|
| High water content | Tomatoes(a) | −18 | ≤ 18 months | |
| High oil content | Sunflower seed | −18 | 18 months | |
| High acid content | Grapes | −18 | 24 months | |
|
(a): Study performed on several crops investigating storage periods of up to 873 days. However, an unexplained decline of residues was observed in tomatoes between 18 and 24 months. Reference: EFSA (2015) | ||||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Crop | Region/indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments | MRL proposals (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) |
|---|---|---|---|---|---|---|
| Beans (fresh, without pods) | NEU |
Beans (fresh, without pods): 2× < 0.05* Peas (fresh, without pods): 4× < 0.05 |
The MRL proposal is based on the combined data set of the trials with beans without pods (Belgium, 2017) and peas without pods performed according to the same GAP (EFSA, 2015) | 0.05* | 0.05 | 0.05 |
MRL: maximum residue level; GAP: Good Agricultural Practice.
* Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue.
Supervised trials median residue.
B.1.2.2. Conversion factors for risk assessment in plant products
Not relevant.
B.1.2.3. Residues in succeeding crops

B.1.2.4. Processing factors
Not relevant.
B.2. Residues in livestock
Not relevant.
B.3. Consumer risk assessment
B.4. Recommended MRLs
| Codea | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: cymoxanil | ||||
| 0260020 | Beans without pods | 0.01* | 0.05* | The submitted data are sufficient to derive a MRL proposal for the NEU use. Risk for consumers is unlikely |
MRL: maximum residue level; NEU: northern Europe.
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Appendix C – Pesticide Residue Intake Model (PRIMo)

Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
Not relevant.
D.2. Consumer risk assessment
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Beans (fresh, without pods) | 0.05* | STMR | 0.05* | HR |
| Table grapes | 0.05 | STMR (EFSA, 2015) | Acute risk assessment only for the crops under consideration | |
| Wine grapes | 0.05 | STMR (EFSA, 2015) | ||
| Potatoes | 0.01* | STMR (EFSA, 2015) | ||
| Garlic | 0.01* | STMR (EFSA, 2015) | ||
| Onions | 0.01* | STMR (EFSA, 2015) | ||
| Tomatoes | 0.01* | STMR (EFSA, 2015) | ||
| Aubergines (egg plants) | 0.05* | STMR (EFSA, 2015) | ||
| Cucumbers | 0.01* | STMR (EFSA, 2015) | ||
| Gherkins | 0.01* | STMR (EFSA, 2015) | ||
| Courgettes | 0.01* | STMR (EFSA, 2015) | ||
| Melons | 0.002 | STMR × PF (EFSA, 2015) | ||
| Pumpkins | 0.002 | STMR × PF (EFSA, 2015) | ||
| Watermelons | 0.002 | STMR × PF (EFSA, 2015) | ||
| Broccoli | 0.01* | STMR (EFSA, 2015) | ||
| Cauliflower | 0.01* | STMR (EFSA, 2015) | ||
| Lettuce | 0.01* | STMR (EFSA, 2015) | ||
| Spinach | 0.02 | STMR (EFSA, 2015) | ||
| Beans (fresh, with pods) | 0.05* | STMR (EFSA, 2015) | ||
| Peas (fresh, with pods) | 0.05* | STMR (EFSA, 2015) | ||
| Peas (fresh, without pods) | 0.05* | STMR (EFSA, 2015) | ||
| Globe artichokes | 0.01* | STMR (EFSA, 2015) | ||
| Leek | 0.01* | STMR (EFSA, 2015) | ||
| Beans (dry) | 0.02 | STMR (EFSA, 2015) | ||
| Lentils (dry) | 0.02 | STMR (EFSA, 2015) | ||
| Peas (dry) | 0.02 | STMR (EFSA, 2015) | ||
| Lupins (dry) | 0.02 | STMR (EFSA, 2015) | ||
| Sunflower seed | 0.1 | EU MRL | ||
| Soya bean | 0.5 | EU MRL | ||
| Herbal infusions (dried, flowers) | 0.01* | STMR (EFSA, 2015) | ||
| Hops (dried), including hop pellets and unconcentrated powder | 0.05* | STMR (EFSA, 2015) | ||
STMR: supervised trials median residue; HR: highest residue; PF: processing factor; MRL: maximum residue level.
* Indicates that the input value is proposed at the limit of quantification.
Appendix E – Used compound code(s)
1.
| Code/trivial name | Chemical name/SMILES notationa | Structural formulaa |
|---|---|---|
| Cymoxanil |
1‐[(EZ)‐2‐Cyano‐2‐methoxyiminoacetyl]‐3‐ethylurea N#C\C(=N\OC)C(=O)NC(=O)NCC |
|
SMILES: simplified molecular‐input line‐entry system.
(ACD/ChemSketch, Advanced Chemistry Development, Inc., ACD/Labs Release: 12.00 Product version: 12.00 (Build 29305, 25 Nov 2008).
Suggested citation: EFSA (European Food Safety Authority) , Brancato A, Brocca D, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Nougadere A, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Reasoned opinion on the modification of the existing maximum residue level for cymoxanil in beans without pods. EFSA Journal 2017;15(12):5066, 19 pp. 10.2903/j.efsa.2017.5066
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00096
Approved: 26 October 2017
Notes
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
FPS Public Health, Food Chain Safety and Environment, Place Victor Horta 40 Box 10,1060, Brussels, Belgium.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32.
Commission Directive 2008/125/EC of 19 December 2008 amending Council Directive 91/414/EEC to include aluminium phosphide, calcium phosphide, magnesium phosphide, cymoxanil, dodemorph, 2,5‐dichlorobenzoic acid methylester, metamitron, sulcotrione, tebuconazole and triadimenol as active substances. OJ L 344, 20.12.2008, p. 78–88.
Commission Regulation (EU) 2016/1785 of 7 October 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for cymoxanil, phosphane and phosphide salts and sodium 5‐nitroguaiacolate, sodium o‐nitrophenolate and sodium p‐nitrophenolate in or on certain products (Text with EEA relevance) C/2016/6353. OJ L 273, 8.10.2016, p. 10–30.
Commission Regulation (EU) No 544/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for active substances. OJ L 155, 11.6.2011, p. 1–66.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- Austria , 2007. Draft assessment report on the active substance cymoxanil prepared by the rapporteur Member State Austria in the framework of Council Directive 91/414/EEC, June 2007. Available online: http://www.efsa.europa.eu
- Austria , 2008. Final addendum to the draft assessment report on the active substance cymoxanil prepared by the rapporteur Member State Austria in the framework of Council Directive 91/414/EEC, September 2008.
- Belgium , 2017. Evaluation report on the modification of MRLs for cymoxanil in beans without pods. January 2017, 13 pp.
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers' health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Conclusion on the peer review of the pesticide risk assessment of the active substance cymoxanil. EFSA Journal 2008;6(10):167r, 116 pp. 10.2903/j.efsa.2008.167r [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Review of the existing maximum residue levels for cymoxanil according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2015;13(12):4355, 48 pp. 10.2903/j.efsa.2015.4355 [DOI] [Google Scholar]
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev.2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2008. Review report for the active substance cymoxanil. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 28 October 2008 in view of the inclusion of active substance in Annex I of Council Directive 91/414/EEC. SANCO/179/08 – Final rev. 1, 9 July 2010.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2016. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.2, 23 September 2016.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org


